User login
HR+/HER2− BC: Adjuvant abemaciclib+ET shows sustained positive benefit-risk profile
Key clinical point: Adjuvant abemaciclib plus endocrine therapy (ET) reduced the risk for recurrence and demonstrated a favorable safety profile in patients with hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2−) early breast cancer (BC) at a high risk for recurrence.
Major finding: Abemaciclib+ET helped sustain the invasive disease-free survival benefit compared with only ET even at 42 months of median follow-up (hazard ratio 0.664; nominal P < .0001). Although the frequency of grade ≥3 adverse events was higher with abemaciclib+ET (49.9%) vs ET alone (16.9%), it was considered manageable and acceptable for patients with high-risk early BC.
Study details: Findings are from the phase 3, monarchE trial including 5637 patients with HR+/HER2−, node-positive, early BC who were randomly assigned to receive adjuvant ET with or without abemaciclib.
Disclosures: This study was funded by Eli Lilly. Five authors declared being employees and shareholders of Eli Lilly, and the other authors reported ties with several sources, including Eli Lilly.
Source: Johnston SRD et al on behalf of the monarchE Committee Members. Abemaciclib plus endocrine therapy for hormone receptor-positive, HER2-negative, node-positive, high-risk early breast cancer (monarchE): Results from a preplanned interim analysis of a randomised, open-label, phase 3 trial. Lancet Oncol. 2022 (Dec 6). Doi: 10.1016/S1470-2045(22)00694-5
Key clinical point: Adjuvant abemaciclib plus endocrine therapy (ET) reduced the risk for recurrence and demonstrated a favorable safety profile in patients with hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2−) early breast cancer (BC) at a high risk for recurrence.
Major finding: Abemaciclib+ET helped sustain the invasive disease-free survival benefit compared with only ET even at 42 months of median follow-up (hazard ratio 0.664; nominal P < .0001). Although the frequency of grade ≥3 adverse events was higher with abemaciclib+ET (49.9%) vs ET alone (16.9%), it was considered manageable and acceptable for patients with high-risk early BC.
Study details: Findings are from the phase 3, monarchE trial including 5637 patients with HR+/HER2−, node-positive, early BC who were randomly assigned to receive adjuvant ET with or without abemaciclib.
Disclosures: This study was funded by Eli Lilly. Five authors declared being employees and shareholders of Eli Lilly, and the other authors reported ties with several sources, including Eli Lilly.
Source: Johnston SRD et al on behalf of the monarchE Committee Members. Abemaciclib plus endocrine therapy for hormone receptor-positive, HER2-negative, node-positive, high-risk early breast cancer (monarchE): Results from a preplanned interim analysis of a randomised, open-label, phase 3 trial. Lancet Oncol. 2022 (Dec 6). Doi: 10.1016/S1470-2045(22)00694-5
Key clinical point: Adjuvant abemaciclib plus endocrine therapy (ET) reduced the risk for recurrence and demonstrated a favorable safety profile in patients with hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2−) early breast cancer (BC) at a high risk for recurrence.
Major finding: Abemaciclib+ET helped sustain the invasive disease-free survival benefit compared with only ET even at 42 months of median follow-up (hazard ratio 0.664; nominal P < .0001). Although the frequency of grade ≥3 adverse events was higher with abemaciclib+ET (49.9%) vs ET alone (16.9%), it was considered manageable and acceptable for patients with high-risk early BC.
Study details: Findings are from the phase 3, monarchE trial including 5637 patients with HR+/HER2−, node-positive, early BC who were randomly assigned to receive adjuvant ET with or without abemaciclib.
Disclosures: This study was funded by Eli Lilly. Five authors declared being employees and shareholders of Eli Lilly, and the other authors reported ties with several sources, including Eli Lilly.
Source: Johnston SRD et al on behalf of the monarchE Committee Members. Abemaciclib plus endocrine therapy for hormone receptor-positive, HER2-negative, node-positive, high-risk early breast cancer (monarchE): Results from a preplanned interim analysis of a randomised, open-label, phase 3 trial. Lancet Oncol. 2022 (Dec 6). Doi: 10.1016/S1470-2045(22)00694-5
Long lasting benefit with dose-dense adjuvant chemotherapy in high-risk early BC
Key clinical point: In patients with high-risk early breast cancer (BC), a dose-dense adjuvant chemotherapy improved disease-free survival (DFS), whereas the addition of fluorouracil to the chemotherapy regimen failed to demonstrate any survival benefits.
Major finding: After a median follow-up of 15.1 years, the median DFS was similar with and without the addition of fluorouracil to the combination therapy of epirubicin, cyclophosphamide, and paclitaxel (EC-P; log-rank P = .11) and was significantly improved in the dose-dense vs standard interval group (hazard ratio 0.77; P = .0004). The most common grade 3-4 adverse events were neutropenia and alopecia.
Study details: Findings are end of study results from the GIM2 trial including 2091 patients with node-positive early BC who were randomly assigned to receive standard-interval EC-P, standard-interval fluorouracil+EC-P (FEC-P), dose-dense EC-P, or dose-dense FEC-P.
Disclosures: This study was funded by Bristol-Myers Squibb, Pharmacia, Dompè Biotec Italy, Italian Ministry of Health, Fondazione Italiana per la Ricerca sul Cancro, and Alliance Against Cancer. The authors declared receiving fees, research grants, honoraria, or support for attending meetings or travel from several sources.
Source: Del Mastro L et al on behalf of the Gruppo Italiano Mammella Investigators. Fluorouracil and dose-dense adjuvant chemotherapy in patients with early-stage breast cancer (GIM2): End-of-study results from a randomised, phase 3 trial. Lancet Oncol. 2022;23(12):1571-1582 (Nov 9). Doi: 10.1016/S1470-2045(22)00632-5
Key clinical point: In patients with high-risk early breast cancer (BC), a dose-dense adjuvant chemotherapy improved disease-free survival (DFS), whereas the addition of fluorouracil to the chemotherapy regimen failed to demonstrate any survival benefits.
Major finding: After a median follow-up of 15.1 years, the median DFS was similar with and without the addition of fluorouracil to the combination therapy of epirubicin, cyclophosphamide, and paclitaxel (EC-P; log-rank P = .11) and was significantly improved in the dose-dense vs standard interval group (hazard ratio 0.77; P = .0004). The most common grade 3-4 adverse events were neutropenia and alopecia.
Study details: Findings are end of study results from the GIM2 trial including 2091 patients with node-positive early BC who were randomly assigned to receive standard-interval EC-P, standard-interval fluorouracil+EC-P (FEC-P), dose-dense EC-P, or dose-dense FEC-P.
Disclosures: This study was funded by Bristol-Myers Squibb, Pharmacia, Dompè Biotec Italy, Italian Ministry of Health, Fondazione Italiana per la Ricerca sul Cancro, and Alliance Against Cancer. The authors declared receiving fees, research grants, honoraria, or support for attending meetings or travel from several sources.
Source: Del Mastro L et al on behalf of the Gruppo Italiano Mammella Investigators. Fluorouracil and dose-dense adjuvant chemotherapy in patients with early-stage breast cancer (GIM2): End-of-study results from a randomised, phase 3 trial. Lancet Oncol. 2022;23(12):1571-1582 (Nov 9). Doi: 10.1016/S1470-2045(22)00632-5
Key clinical point: In patients with high-risk early breast cancer (BC), a dose-dense adjuvant chemotherapy improved disease-free survival (DFS), whereas the addition of fluorouracil to the chemotherapy regimen failed to demonstrate any survival benefits.
Major finding: After a median follow-up of 15.1 years, the median DFS was similar with and without the addition of fluorouracil to the combination therapy of epirubicin, cyclophosphamide, and paclitaxel (EC-P; log-rank P = .11) and was significantly improved in the dose-dense vs standard interval group (hazard ratio 0.77; P = .0004). The most common grade 3-4 adverse events were neutropenia and alopecia.
Study details: Findings are end of study results from the GIM2 trial including 2091 patients with node-positive early BC who were randomly assigned to receive standard-interval EC-P, standard-interval fluorouracil+EC-P (FEC-P), dose-dense EC-P, or dose-dense FEC-P.
Disclosures: This study was funded by Bristol-Myers Squibb, Pharmacia, Dompè Biotec Italy, Italian Ministry of Health, Fondazione Italiana per la Ricerca sul Cancro, and Alliance Against Cancer. The authors declared receiving fees, research grants, honoraria, or support for attending meetings or travel from several sources.
Source: Del Mastro L et al on behalf of the Gruppo Italiano Mammella Investigators. Fluorouracil and dose-dense adjuvant chemotherapy in patients with early-stage breast cancer (GIM2): End-of-study results from a randomised, phase 3 trial. Lancet Oncol. 2022;23(12):1571-1582 (Nov 9). Doi: 10.1016/S1470-2045(22)00632-5
MCL Overview
Overturning Roe: Exacerbating inequities in abortion care and ObGyn training
On a recent overnight shift, our ObGyn on-call team was urgently paged to the emergency room for a patient who was brought in hemorrhaging after having passed out mid-flight from Texas to Boston. She was 12-weeks pregnant. We rushed her to the operating room for surgical removal of the pregnancy by dilation and curettage to stop her bleeding. Landing in Massachusetts had saved her life.
The significance of this patient’s case was not lost on the multidisciplinary teams caring for her, as the—at the time—impending Roe v Wade decision weighed heavily on our minds. One of many, her story foreshadows the harrowing experiences that we anticipate in the coming months and highlights the danger that the Supreme Court has inflicted on pregnant people nationally.
The Supreme Court decision on Dobbs v Jackson condemns us as a nation in which abortion rights are no longer federally protected under Roe v Wade.1 Twenty-six states have been poised to ban abortion, and in at least 12 states, abortion is now illegal.2,3 Political decision making will soon deny pregnant people the right to bodily autonomy, and the United States will lag behind other nations in abortion access.4 As ObGyn resident physicians who practice in tertiary referral hospitals in Massachusetts, where the ROE Act protects abortion beyond 24 weeks’ gestational age, we affirm abortion as essential health care that saves lives.5
Collectively as physician residents, we have provided an abortion for the patient at 22 weeks with a desired pregnancy who would have otherwise died from high blood pressures, the patient who ended her pregnancy to expedite breast cancer treatment, and the 16-year-old who feared for her life after suffering an assault by her partner for disclosing her pregnancy. With the overturn of Roe v Wade, patients like these will suffer dramatically divergent fates as race, class, and, now more than ever, geography will impact who is able to access abortion care.
Ramifications of the overturn of Roe
History foreshadows the grim impact of repealing Roe. Ohio’s 2011 law that requires the use of the restrictive protocol approved by the US Food and Drug Administration for mifepristone administration deepened existing inequities in abortion access.6 Patients with private insurance, higher income, higher level of education, and those who were White were more likely to obtain abortion care.7 In Texas, after the implementation of SB8 and other restrictive laws, Hispanic women whose travel distance increased more than 100 miles had the greatest reduction in abortion rates.8,9 A recent study regarding banning abortion in the United States estimated a 7% increase in pregnancy-related deaths in 1 year, with a 21% increase in subsequent years.10
Inequities in abortion access subsequently will disparately increase deaths of pregnant individuals in certain populations.11,12 Communities with the highest rates of unintended pregnancy, medical comorbidities, and lack of access to abortion, as well as historically marginalized populations—including non-Hispanic Black people, LGBTQIA people, those with limited English proficiency, and undocumented persons—will experience the greatest increase in pregnancy-related deaths due to a total abortion ban.13-15
The US maternal mortality rate is already the highest among developed nations, and it will only climb if ObGyns are not appropriately trained to operate within our full scope of practice and, thus, are unable to provide the highest quality of care.16,17
Continue: Abortion is a medical treatment that requires resident training...
Abortion is a medical treatment that requires resident training
Abortion care must be protected. Uterine evacuation by medical management, suction curettage, or dilation and evacuation is indicated for undesired pregnancy, regardless of reasoning or life circumstance. Pregnancy carries inherent risks that can at times be deadly.18 Abortion serves as first-line treatment for certain life-threatening pregnancy risks, including septic miscarriage, maternal hemorrhage, early-onset severe preeclampsia, and certain health conditions.19 Surgical skills and medical management of abortion are therefore fundamental components of ObGyn care and residency training.20
In choosing to become ObGyns,and particularly in selecting our training program, the ability to provide safe abortion care was a calculated priority. A recent study on the implications of overturning Roe predicted that nearly half of ObGyn residents will likely or certainly lose access to in-state abortion training.21 As demonstrated already in states with restrictive abortion laws, we will lose an entire generation of medical professionals skilled in performing this lifesaving procedure.9,22 While privileged patients may travel across state borders to access care, ObGyn and other medical trainees who are contract bound to residency programs do not have such flexibility to seek out abortion training. Although we hope the reversal of Roe will be fleeting, the consequences of this lost generation are irreparable.23,24 For physicians like ourselves, who fortunately are trained in surgical abortions and safe management of medical terminations, the discrepancy between evidence-based guidelines and impending political restrictions is distressing. We are forced to imagine refusing patients necessary health care—or face incarceration to save their lives.
The idea of watching a patient die, whether by hemorrhage, sepsis, or suicide, while armed with the tools of safe abortion technique is horrific. As authors with roots in Texas, Michigan, and Georgia, where abortion has or will almost certainly become illegal now that Roe v Wade is overturned, this scene is personal. It affects our future patients, our families, our colleagues, and our ability to return to our home states to live and practice.
Political organizing is critical to protect and restore abortion rights and defend against conservative coercive politics.25 Nearly half of pregnancies in the United States are unintended, and more than half of these end in abortion.26,27 Threats to abortion access require action from every one of the 59% of Americans who believe abortion should remain legal.28 This is especially important from a social and racial justice perspective as abortion bans will disproportionately affect marginalized groups and further exacerbate inequities in maternal mortality.13
Call to action
Now is the time for community action for reproductive justice and human rights. We urge everyone to donate to abortion funds, vote for leaders who support reproductive justice, and petition your state legislators to codify Roe into law. Now is the time to expand legislation to protect abortion providers and our patients. To ObGyns, family medicine physicians, internists, and other reproductive health clinicians, now is the time to maximize your abortion training. Now is the time to act; otherwise, pregnant individuals will die and future generations of physicians will not have the training to save their lives. ●
- de Vogue A, Sneed T, Duster C, et al. Supreme Court overturns Roe v Wade. CNN Politics. June 24, 2022. Accessed July 19, 2022. https://www.cnn.com/2022/06/24/politics/dobbs-missis sippi-supreme-court-abortion-roe-wade/index.html
- Nash E, Cross L. 26 States are certain or likely to ban abortion without Roe: here’s which ones and why. Guttmacher Institute. October 28, 2021. Updated April 19, 2022. Accessed July 19, 2022. https://www.guttmacher.org/article/2021/10/26-states-are-certain-or-likely-ban-abortion-without-roe-heres-which-ones-and-why
- Messerly M. Abortion laws by state: where abortions are illegal after Roe v Wade overturned. Politico. June 24, 2022. Accessed July 19, 2022. https://www.politico.com/news/2022/06/24/abortion-laws-by-state-roe-v-wade-00037695
- Archie A. US would lag behind global abortion access if Roe v Wade is undone, advocates say. NPR. May 5, 2022. Accessed July 19, 2022. https://www.npr.org/2022/05/05/1096805490/abortion-access-supreme-court-roe-v-wade-united-nations
- Romo V. Massachusetts senate overrides veto, passes law expanding abortion access. NPR. December 29, 2020. Accessed July 19, 2022. https://www.npr.org/2020/12/29/951259506/massachusetts-senate-overrides-veto-passes-law-expanding-abortion-access
- Upadhyay UD, Johns NE, Combellick SL, et al. Comparison of outcomes before and after Ohio’s law mandating use of the FDA-approved protocol for medication abortion: a retrospective cohort study. PLoS Med. 2016;13:e1002110.
- Upadhyay UD, Johns NE, Cartwright AF, et al. Sociodemographic characteristics of women able to obtain medication abortion before and after Ohio’s law requiring use of the Food and Drug Administration protocol. Health Equity. 2018;2:122-130.
- Goyal V, Brooks IHM, Powers DA. Differences in abortion rates by race-ethnicity after implementation of a restrictive Texas law. Contraception. 2020;102:109-114.
- Noyes E Holder BH, Evans ML. Texas SB8 and the future of abortion care. OBG Manag. 2021;33. doi:12788/obgm.0151.
- Vilda D, Wallace ME, Daniel C, et al. State abortion policies and maternal death in the United States, 2015‒2018. Am J Public Health. 2021;111:1696-1704.
- The Lancet. Why Roe v Wade must be defended. Lancet. 2022;399:1845.
- Nambiar A, Patel S, Santiago-Munoz P, et al. Maternal morbidity and fetal outcomes among pregnant women at 22 weeks’ gestation or less with complications in two Texas hospitals after legislation on abortion. Am J Obstet Gynecol. 2022;227:648-650.e1.
- Stevenson AJ. The pregnancy-related mortality impact of a total abortion ban in the United States: a research note on increased deaths due to remaining pregnant. Demography. 2021;58:20192028.
- Medley S. Gutting abortion rights would be devastating for LGBTQ+ people. Them. September 17, 2021. Accessed July 20, 2022. https://www.them.us/story/gutting-abortion-rights-devastating-lgbtq-people
- Holter L. Detained immigrant women are facing a grueling abortion struggle. National Latina Institute for Reproductive Justice. May 10, 2017. Accessed July 20, 2022. https://www.latinainsti tute.org/es/node/4620
- Haddad LB, Nour NM. Unsafe abortion: unnecessary maternal mortality. Rev Obstet Gynecol. 2009;2:122-126.
- Tikkanen R, Gunja MZ, FitzGerald M, et al. Maternal mortality and maternity care in the United States compared to 10 other developed countries. The Commonwealth Fund. November 18, 2020. Accessed November 17, 2022. https://www .commonwealthfund.org/publications/issue -briefs/2020/nov/maternal-mortality-maternity -care-us-compared-10-countries
- Collier A-RY, Molina RL. Maternal mortality in the United States: updates on trends, causes, and solutions. Neoreviews. 2019;20:e561-e574.
- ACOG practice bulletin no. 135: Second-trimester abortion. Obstet Gynecol. 2013;121:1394-1406.
- Committee on Health Care for Underserved Women. ACOG Committee opinion no. 612: Abortion training and education. Obstet Gynecol. 2014;124:1055-1059.
- Vinekar K, Karlapudi A, Nathan L, et al. Projected implications of overturning Roe v Wade on abortion training in US obstetrics and gynecology residency programs. Obstet Gynecol. 2022;140:146-149.
- Horvath S, Turk J, Steinauer J, et al. Increase in obstetrics and gynecology resident self-assessed competence in early pregnancy loss management with routine abortion care training. Obstet Gynecol. 2022;139:116-119.
- Anderson N. The fall of Roe scrambles abortion training in university hospitals. The Washington Post. June 30, 2022. Accessed July 20, 2022. https://www.washingtonpost.com/educa tion/2022/06/30/abortion-training-upheaval-dobbs/
- Weiner S. How the repeal of Roe v Wade will affect training in abortion and reproductive health. AAMC. June 24, 2022. Accessed July 20, 2022. https://www.aamc.org/news-insights/how-repeal-roe-v-wade-will-affect-training-abortion-and-reproductive-health
- Dreweke J. Coercion is at the heart of social conservatives’ reproductive health agenda. Guttmacher Institute. February 7, 2018. Accessed July 20, 2022. https://www.guttmacher.org/gpr/2018/02/coercion-heart-social-conservatives-reproduc tive-health-agenda
- Unintended pregnancy and abortion worldwide. Guttmacher Institute. March 2022. Accessed July 20, 2022. https://www.guttmacher.org/fact-sheet/induced-abortion-worldwide
- Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008–2011. N Engl J Med. 2016;374:843-852.
- Hartig H. About six-in-ten Americans say abortion should be legal in all or most cases. Pew Research Center. June 13, 2022. Accessed July 20, 2022. https://www.pewresearch.org/fact-tank/2022/06/13/about-six-in-ten-americans-say-abortion-should-be-legal-in-all-or-most-cases-2/
On a recent overnight shift, our ObGyn on-call team was urgently paged to the emergency room for a patient who was brought in hemorrhaging after having passed out mid-flight from Texas to Boston. She was 12-weeks pregnant. We rushed her to the operating room for surgical removal of the pregnancy by dilation and curettage to stop her bleeding. Landing in Massachusetts had saved her life.
The significance of this patient’s case was not lost on the multidisciplinary teams caring for her, as the—at the time—impending Roe v Wade decision weighed heavily on our minds. One of many, her story foreshadows the harrowing experiences that we anticipate in the coming months and highlights the danger that the Supreme Court has inflicted on pregnant people nationally.
The Supreme Court decision on Dobbs v Jackson condemns us as a nation in which abortion rights are no longer federally protected under Roe v Wade.1 Twenty-six states have been poised to ban abortion, and in at least 12 states, abortion is now illegal.2,3 Political decision making will soon deny pregnant people the right to bodily autonomy, and the United States will lag behind other nations in abortion access.4 As ObGyn resident physicians who practice in tertiary referral hospitals in Massachusetts, where the ROE Act protects abortion beyond 24 weeks’ gestational age, we affirm abortion as essential health care that saves lives.5
Collectively as physician residents, we have provided an abortion for the patient at 22 weeks with a desired pregnancy who would have otherwise died from high blood pressures, the patient who ended her pregnancy to expedite breast cancer treatment, and the 16-year-old who feared for her life after suffering an assault by her partner for disclosing her pregnancy. With the overturn of Roe v Wade, patients like these will suffer dramatically divergent fates as race, class, and, now more than ever, geography will impact who is able to access abortion care.
Ramifications of the overturn of Roe
History foreshadows the grim impact of repealing Roe. Ohio’s 2011 law that requires the use of the restrictive protocol approved by the US Food and Drug Administration for mifepristone administration deepened existing inequities in abortion access.6 Patients with private insurance, higher income, higher level of education, and those who were White were more likely to obtain abortion care.7 In Texas, after the implementation of SB8 and other restrictive laws, Hispanic women whose travel distance increased more than 100 miles had the greatest reduction in abortion rates.8,9 A recent study regarding banning abortion in the United States estimated a 7% increase in pregnancy-related deaths in 1 year, with a 21% increase in subsequent years.10
Inequities in abortion access subsequently will disparately increase deaths of pregnant individuals in certain populations.11,12 Communities with the highest rates of unintended pregnancy, medical comorbidities, and lack of access to abortion, as well as historically marginalized populations—including non-Hispanic Black people, LGBTQIA people, those with limited English proficiency, and undocumented persons—will experience the greatest increase in pregnancy-related deaths due to a total abortion ban.13-15
The US maternal mortality rate is already the highest among developed nations, and it will only climb if ObGyns are not appropriately trained to operate within our full scope of practice and, thus, are unable to provide the highest quality of care.16,17
Continue: Abortion is a medical treatment that requires resident training...
Abortion is a medical treatment that requires resident training
Abortion care must be protected. Uterine evacuation by medical management, suction curettage, or dilation and evacuation is indicated for undesired pregnancy, regardless of reasoning or life circumstance. Pregnancy carries inherent risks that can at times be deadly.18 Abortion serves as first-line treatment for certain life-threatening pregnancy risks, including septic miscarriage, maternal hemorrhage, early-onset severe preeclampsia, and certain health conditions.19 Surgical skills and medical management of abortion are therefore fundamental components of ObGyn care and residency training.20
In choosing to become ObGyns,and particularly in selecting our training program, the ability to provide safe abortion care was a calculated priority. A recent study on the implications of overturning Roe predicted that nearly half of ObGyn residents will likely or certainly lose access to in-state abortion training.21 As demonstrated already in states with restrictive abortion laws, we will lose an entire generation of medical professionals skilled in performing this lifesaving procedure.9,22 While privileged patients may travel across state borders to access care, ObGyn and other medical trainees who are contract bound to residency programs do not have such flexibility to seek out abortion training. Although we hope the reversal of Roe will be fleeting, the consequences of this lost generation are irreparable.23,24 For physicians like ourselves, who fortunately are trained in surgical abortions and safe management of medical terminations, the discrepancy between evidence-based guidelines and impending political restrictions is distressing. We are forced to imagine refusing patients necessary health care—or face incarceration to save their lives.
The idea of watching a patient die, whether by hemorrhage, sepsis, or suicide, while armed with the tools of safe abortion technique is horrific. As authors with roots in Texas, Michigan, and Georgia, where abortion has or will almost certainly become illegal now that Roe v Wade is overturned, this scene is personal. It affects our future patients, our families, our colleagues, and our ability to return to our home states to live and practice.
Political organizing is critical to protect and restore abortion rights and defend against conservative coercive politics.25 Nearly half of pregnancies in the United States are unintended, and more than half of these end in abortion.26,27 Threats to abortion access require action from every one of the 59% of Americans who believe abortion should remain legal.28 This is especially important from a social and racial justice perspective as abortion bans will disproportionately affect marginalized groups and further exacerbate inequities in maternal mortality.13
Call to action
Now is the time for community action for reproductive justice and human rights. We urge everyone to donate to abortion funds, vote for leaders who support reproductive justice, and petition your state legislators to codify Roe into law. Now is the time to expand legislation to protect abortion providers and our patients. To ObGyns, family medicine physicians, internists, and other reproductive health clinicians, now is the time to maximize your abortion training. Now is the time to act; otherwise, pregnant individuals will die and future generations of physicians will not have the training to save their lives. ●
On a recent overnight shift, our ObGyn on-call team was urgently paged to the emergency room for a patient who was brought in hemorrhaging after having passed out mid-flight from Texas to Boston. She was 12-weeks pregnant. We rushed her to the operating room for surgical removal of the pregnancy by dilation and curettage to stop her bleeding. Landing in Massachusetts had saved her life.
The significance of this patient’s case was not lost on the multidisciplinary teams caring for her, as the—at the time—impending Roe v Wade decision weighed heavily on our minds. One of many, her story foreshadows the harrowing experiences that we anticipate in the coming months and highlights the danger that the Supreme Court has inflicted on pregnant people nationally.
The Supreme Court decision on Dobbs v Jackson condemns us as a nation in which abortion rights are no longer federally protected under Roe v Wade.1 Twenty-six states have been poised to ban abortion, and in at least 12 states, abortion is now illegal.2,3 Political decision making will soon deny pregnant people the right to bodily autonomy, and the United States will lag behind other nations in abortion access.4 As ObGyn resident physicians who practice in tertiary referral hospitals in Massachusetts, where the ROE Act protects abortion beyond 24 weeks’ gestational age, we affirm abortion as essential health care that saves lives.5
Collectively as physician residents, we have provided an abortion for the patient at 22 weeks with a desired pregnancy who would have otherwise died from high blood pressures, the patient who ended her pregnancy to expedite breast cancer treatment, and the 16-year-old who feared for her life after suffering an assault by her partner for disclosing her pregnancy. With the overturn of Roe v Wade, patients like these will suffer dramatically divergent fates as race, class, and, now more than ever, geography will impact who is able to access abortion care.
Ramifications of the overturn of Roe
History foreshadows the grim impact of repealing Roe. Ohio’s 2011 law that requires the use of the restrictive protocol approved by the US Food and Drug Administration for mifepristone administration deepened existing inequities in abortion access.6 Patients with private insurance, higher income, higher level of education, and those who were White were more likely to obtain abortion care.7 In Texas, after the implementation of SB8 and other restrictive laws, Hispanic women whose travel distance increased more than 100 miles had the greatest reduction in abortion rates.8,9 A recent study regarding banning abortion in the United States estimated a 7% increase in pregnancy-related deaths in 1 year, with a 21% increase in subsequent years.10
Inequities in abortion access subsequently will disparately increase deaths of pregnant individuals in certain populations.11,12 Communities with the highest rates of unintended pregnancy, medical comorbidities, and lack of access to abortion, as well as historically marginalized populations—including non-Hispanic Black people, LGBTQIA people, those with limited English proficiency, and undocumented persons—will experience the greatest increase in pregnancy-related deaths due to a total abortion ban.13-15
The US maternal mortality rate is already the highest among developed nations, and it will only climb if ObGyns are not appropriately trained to operate within our full scope of practice and, thus, are unable to provide the highest quality of care.16,17
Continue: Abortion is a medical treatment that requires resident training...
Abortion is a medical treatment that requires resident training
Abortion care must be protected. Uterine evacuation by medical management, suction curettage, or dilation and evacuation is indicated for undesired pregnancy, regardless of reasoning or life circumstance. Pregnancy carries inherent risks that can at times be deadly.18 Abortion serves as first-line treatment for certain life-threatening pregnancy risks, including septic miscarriage, maternal hemorrhage, early-onset severe preeclampsia, and certain health conditions.19 Surgical skills and medical management of abortion are therefore fundamental components of ObGyn care and residency training.20
In choosing to become ObGyns,and particularly in selecting our training program, the ability to provide safe abortion care was a calculated priority. A recent study on the implications of overturning Roe predicted that nearly half of ObGyn residents will likely or certainly lose access to in-state abortion training.21 As demonstrated already in states with restrictive abortion laws, we will lose an entire generation of medical professionals skilled in performing this lifesaving procedure.9,22 While privileged patients may travel across state borders to access care, ObGyn and other medical trainees who are contract bound to residency programs do not have such flexibility to seek out abortion training. Although we hope the reversal of Roe will be fleeting, the consequences of this lost generation are irreparable.23,24 For physicians like ourselves, who fortunately are trained in surgical abortions and safe management of medical terminations, the discrepancy between evidence-based guidelines and impending political restrictions is distressing. We are forced to imagine refusing patients necessary health care—or face incarceration to save their lives.
The idea of watching a patient die, whether by hemorrhage, sepsis, or suicide, while armed with the tools of safe abortion technique is horrific. As authors with roots in Texas, Michigan, and Georgia, where abortion has or will almost certainly become illegal now that Roe v Wade is overturned, this scene is personal. It affects our future patients, our families, our colleagues, and our ability to return to our home states to live and practice.
Political organizing is critical to protect and restore abortion rights and defend against conservative coercive politics.25 Nearly half of pregnancies in the United States are unintended, and more than half of these end in abortion.26,27 Threats to abortion access require action from every one of the 59% of Americans who believe abortion should remain legal.28 This is especially important from a social and racial justice perspective as abortion bans will disproportionately affect marginalized groups and further exacerbate inequities in maternal mortality.13
Call to action
Now is the time for community action for reproductive justice and human rights. We urge everyone to donate to abortion funds, vote for leaders who support reproductive justice, and petition your state legislators to codify Roe into law. Now is the time to expand legislation to protect abortion providers and our patients. To ObGyns, family medicine physicians, internists, and other reproductive health clinicians, now is the time to maximize your abortion training. Now is the time to act; otherwise, pregnant individuals will die and future generations of physicians will not have the training to save their lives. ●
- de Vogue A, Sneed T, Duster C, et al. Supreme Court overturns Roe v Wade. CNN Politics. June 24, 2022. Accessed July 19, 2022. https://www.cnn.com/2022/06/24/politics/dobbs-missis sippi-supreme-court-abortion-roe-wade/index.html
- Nash E, Cross L. 26 States are certain or likely to ban abortion without Roe: here’s which ones and why. Guttmacher Institute. October 28, 2021. Updated April 19, 2022. Accessed July 19, 2022. https://www.guttmacher.org/article/2021/10/26-states-are-certain-or-likely-ban-abortion-without-roe-heres-which-ones-and-why
- Messerly M. Abortion laws by state: where abortions are illegal after Roe v Wade overturned. Politico. June 24, 2022. Accessed July 19, 2022. https://www.politico.com/news/2022/06/24/abortion-laws-by-state-roe-v-wade-00037695
- Archie A. US would lag behind global abortion access if Roe v Wade is undone, advocates say. NPR. May 5, 2022. Accessed July 19, 2022. https://www.npr.org/2022/05/05/1096805490/abortion-access-supreme-court-roe-v-wade-united-nations
- Romo V. Massachusetts senate overrides veto, passes law expanding abortion access. NPR. December 29, 2020. Accessed July 19, 2022. https://www.npr.org/2020/12/29/951259506/massachusetts-senate-overrides-veto-passes-law-expanding-abortion-access
- Upadhyay UD, Johns NE, Combellick SL, et al. Comparison of outcomes before and after Ohio’s law mandating use of the FDA-approved protocol for medication abortion: a retrospective cohort study. PLoS Med. 2016;13:e1002110.
- Upadhyay UD, Johns NE, Cartwright AF, et al. Sociodemographic characteristics of women able to obtain medication abortion before and after Ohio’s law requiring use of the Food and Drug Administration protocol. Health Equity. 2018;2:122-130.
- Goyal V, Brooks IHM, Powers DA. Differences in abortion rates by race-ethnicity after implementation of a restrictive Texas law. Contraception. 2020;102:109-114.
- Noyes E Holder BH, Evans ML. Texas SB8 and the future of abortion care. OBG Manag. 2021;33. doi:12788/obgm.0151.
- Vilda D, Wallace ME, Daniel C, et al. State abortion policies and maternal death in the United States, 2015‒2018. Am J Public Health. 2021;111:1696-1704.
- The Lancet. Why Roe v Wade must be defended. Lancet. 2022;399:1845.
- Nambiar A, Patel S, Santiago-Munoz P, et al. Maternal morbidity and fetal outcomes among pregnant women at 22 weeks’ gestation or less with complications in two Texas hospitals after legislation on abortion. Am J Obstet Gynecol. 2022;227:648-650.e1.
- Stevenson AJ. The pregnancy-related mortality impact of a total abortion ban in the United States: a research note on increased deaths due to remaining pregnant. Demography. 2021;58:20192028.
- Medley S. Gutting abortion rights would be devastating for LGBTQ+ people. Them. September 17, 2021. Accessed July 20, 2022. https://www.them.us/story/gutting-abortion-rights-devastating-lgbtq-people
- Holter L. Detained immigrant women are facing a grueling abortion struggle. National Latina Institute for Reproductive Justice. May 10, 2017. Accessed July 20, 2022. https://www.latinainsti tute.org/es/node/4620
- Haddad LB, Nour NM. Unsafe abortion: unnecessary maternal mortality. Rev Obstet Gynecol. 2009;2:122-126.
- Tikkanen R, Gunja MZ, FitzGerald M, et al. Maternal mortality and maternity care in the United States compared to 10 other developed countries. The Commonwealth Fund. November 18, 2020. Accessed November 17, 2022. https://www .commonwealthfund.org/publications/issue -briefs/2020/nov/maternal-mortality-maternity -care-us-compared-10-countries
- Collier A-RY, Molina RL. Maternal mortality in the United States: updates on trends, causes, and solutions. Neoreviews. 2019;20:e561-e574.
- ACOG practice bulletin no. 135: Second-trimester abortion. Obstet Gynecol. 2013;121:1394-1406.
- Committee on Health Care for Underserved Women. ACOG Committee opinion no. 612: Abortion training and education. Obstet Gynecol. 2014;124:1055-1059.
- Vinekar K, Karlapudi A, Nathan L, et al. Projected implications of overturning Roe v Wade on abortion training in US obstetrics and gynecology residency programs. Obstet Gynecol. 2022;140:146-149.
- Horvath S, Turk J, Steinauer J, et al. Increase in obstetrics and gynecology resident self-assessed competence in early pregnancy loss management with routine abortion care training. Obstet Gynecol. 2022;139:116-119.
- Anderson N. The fall of Roe scrambles abortion training in university hospitals. The Washington Post. June 30, 2022. Accessed July 20, 2022. https://www.washingtonpost.com/educa tion/2022/06/30/abortion-training-upheaval-dobbs/
- Weiner S. How the repeal of Roe v Wade will affect training in abortion and reproductive health. AAMC. June 24, 2022. Accessed July 20, 2022. https://www.aamc.org/news-insights/how-repeal-roe-v-wade-will-affect-training-abortion-and-reproductive-health
- Dreweke J. Coercion is at the heart of social conservatives’ reproductive health agenda. Guttmacher Institute. February 7, 2018. Accessed July 20, 2022. https://www.guttmacher.org/gpr/2018/02/coercion-heart-social-conservatives-reproduc tive-health-agenda
- Unintended pregnancy and abortion worldwide. Guttmacher Institute. March 2022. Accessed July 20, 2022. https://www.guttmacher.org/fact-sheet/induced-abortion-worldwide
- Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008–2011. N Engl J Med. 2016;374:843-852.
- Hartig H. About six-in-ten Americans say abortion should be legal in all or most cases. Pew Research Center. June 13, 2022. Accessed July 20, 2022. https://www.pewresearch.org/fact-tank/2022/06/13/about-six-in-ten-americans-say-abortion-should-be-legal-in-all-or-most-cases-2/
- de Vogue A, Sneed T, Duster C, et al. Supreme Court overturns Roe v Wade. CNN Politics. June 24, 2022. Accessed July 19, 2022. https://www.cnn.com/2022/06/24/politics/dobbs-missis sippi-supreme-court-abortion-roe-wade/index.html
- Nash E, Cross L. 26 States are certain or likely to ban abortion without Roe: here’s which ones and why. Guttmacher Institute. October 28, 2021. Updated April 19, 2022. Accessed July 19, 2022. https://www.guttmacher.org/article/2021/10/26-states-are-certain-or-likely-ban-abortion-without-roe-heres-which-ones-and-why
- Messerly M. Abortion laws by state: where abortions are illegal after Roe v Wade overturned. Politico. June 24, 2022. Accessed July 19, 2022. https://www.politico.com/news/2022/06/24/abortion-laws-by-state-roe-v-wade-00037695
- Archie A. US would lag behind global abortion access if Roe v Wade is undone, advocates say. NPR. May 5, 2022. Accessed July 19, 2022. https://www.npr.org/2022/05/05/1096805490/abortion-access-supreme-court-roe-v-wade-united-nations
- Romo V. Massachusetts senate overrides veto, passes law expanding abortion access. NPR. December 29, 2020. Accessed July 19, 2022. https://www.npr.org/2020/12/29/951259506/massachusetts-senate-overrides-veto-passes-law-expanding-abortion-access
- Upadhyay UD, Johns NE, Combellick SL, et al. Comparison of outcomes before and after Ohio’s law mandating use of the FDA-approved protocol for medication abortion: a retrospective cohort study. PLoS Med. 2016;13:e1002110.
- Upadhyay UD, Johns NE, Cartwright AF, et al. Sociodemographic characteristics of women able to obtain medication abortion before and after Ohio’s law requiring use of the Food and Drug Administration protocol. Health Equity. 2018;2:122-130.
- Goyal V, Brooks IHM, Powers DA. Differences in abortion rates by race-ethnicity after implementation of a restrictive Texas law. Contraception. 2020;102:109-114.
- Noyes E Holder BH, Evans ML. Texas SB8 and the future of abortion care. OBG Manag. 2021;33. doi:12788/obgm.0151.
- Vilda D, Wallace ME, Daniel C, et al. State abortion policies and maternal death in the United States, 2015‒2018. Am J Public Health. 2021;111:1696-1704.
- The Lancet. Why Roe v Wade must be defended. Lancet. 2022;399:1845.
- Nambiar A, Patel S, Santiago-Munoz P, et al. Maternal morbidity and fetal outcomes among pregnant women at 22 weeks’ gestation or less with complications in two Texas hospitals after legislation on abortion. Am J Obstet Gynecol. 2022;227:648-650.e1.
- Stevenson AJ. The pregnancy-related mortality impact of a total abortion ban in the United States: a research note on increased deaths due to remaining pregnant. Demography. 2021;58:20192028.
- Medley S. Gutting abortion rights would be devastating for LGBTQ+ people. Them. September 17, 2021. Accessed July 20, 2022. https://www.them.us/story/gutting-abortion-rights-devastating-lgbtq-people
- Holter L. Detained immigrant women are facing a grueling abortion struggle. National Latina Institute for Reproductive Justice. May 10, 2017. Accessed July 20, 2022. https://www.latinainsti tute.org/es/node/4620
- Haddad LB, Nour NM. Unsafe abortion: unnecessary maternal mortality. Rev Obstet Gynecol. 2009;2:122-126.
- Tikkanen R, Gunja MZ, FitzGerald M, et al. Maternal mortality and maternity care in the United States compared to 10 other developed countries. The Commonwealth Fund. November 18, 2020. Accessed November 17, 2022. https://www .commonwealthfund.org/publications/issue -briefs/2020/nov/maternal-mortality-maternity -care-us-compared-10-countries
- Collier A-RY, Molina RL. Maternal mortality in the United States: updates on trends, causes, and solutions. Neoreviews. 2019;20:e561-e574.
- ACOG practice bulletin no. 135: Second-trimester abortion. Obstet Gynecol. 2013;121:1394-1406.
- Committee on Health Care for Underserved Women. ACOG Committee opinion no. 612: Abortion training and education. Obstet Gynecol. 2014;124:1055-1059.
- Vinekar K, Karlapudi A, Nathan L, et al. Projected implications of overturning Roe v Wade on abortion training in US obstetrics and gynecology residency programs. Obstet Gynecol. 2022;140:146-149.
- Horvath S, Turk J, Steinauer J, et al. Increase in obstetrics and gynecology resident self-assessed competence in early pregnancy loss management with routine abortion care training. Obstet Gynecol. 2022;139:116-119.
- Anderson N. The fall of Roe scrambles abortion training in university hospitals. The Washington Post. June 30, 2022. Accessed July 20, 2022. https://www.washingtonpost.com/educa tion/2022/06/30/abortion-training-upheaval-dobbs/
- Weiner S. How the repeal of Roe v Wade will affect training in abortion and reproductive health. AAMC. June 24, 2022. Accessed July 20, 2022. https://www.aamc.org/news-insights/how-repeal-roe-v-wade-will-affect-training-abortion-and-reproductive-health
- Dreweke J. Coercion is at the heart of social conservatives’ reproductive health agenda. Guttmacher Institute. February 7, 2018. Accessed July 20, 2022. https://www.guttmacher.org/gpr/2018/02/coercion-heart-social-conservatives-reproduc tive-health-agenda
- Unintended pregnancy and abortion worldwide. Guttmacher Institute. March 2022. Accessed July 20, 2022. https://www.guttmacher.org/fact-sheet/induced-abortion-worldwide
- Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008–2011. N Engl J Med. 2016;374:843-852.
- Hartig H. About six-in-ten Americans say abortion should be legal in all or most cases. Pew Research Center. June 13, 2022. Accessed July 20, 2022. https://www.pewresearch.org/fact-tank/2022/06/13/about-six-in-ten-americans-say-abortion-should-be-legal-in-all-or-most-cases-2/
Does fertility preservation in patients with breast cancer impact relapse rates and disease-specific mortality?

Marklund A, Lekberg T, Hedayati E, et al. Relapse rates and disease-specific mortality following procedures for fertility preservation at time of breast cancer diagnosis. JAMA Oncol. 2022;8:1438-1446. doi:10.1001/jamaoncol.2022.3677.
EXPERT COMMENTARY
Breast cancer is the most diagnosed cancer among US women after skin cancer.1 As of the end of 2020, 7.8 million women were alive who were diagnosed with breast cancer in the past 5 years, making it the world’s most prevalent cancer. Given the wide reach of breast cancer and the increase in its distant stage by more than 4% per year in women of reproductive age (20–39 years), clinicians are urged to address fertility preservation due to reproductive compromise of gonadotoxic therapies and gonadectomy.2 To predict the risk of infertility following chemotherapy, a Cyclophosphamide Equivalent Dose (CED) calculator can be used. A CED of 4,000 mg/m2 has been associated with a significant risk of infertility.3
In 2012, the American Society for Reproductive Medicine removed the experimental label of oocyte cryopreservation then recently endorsed ovarian cryopreservation, thereby providing acceptable procedures for fertility preservation.4 Gonadotropin-releasing hormone agonist use during chemotherapy, which is used to protect the ovary in premenopausal women against the effects of chemotherapy, has been shown to have inconsistent findings and should not replace the established modalities of oocyte/embryo/ovarian tissue cryopreservation.2,5
Details of the study
While studies have been reassuring that ovarian stimulation for fertility preservation in women with breast cancer does not worsen the prognosis, findings are limited by short-term follow-up.6
The recent study by Marklund and colleagues presented an analysis of breast cancer relapse and mortality following fertility preservation with and without hormonal stimulation. In their prospective cohort study of 425 Swedish women who underwent fertility preservation, the authors categorized patients into 2 groups: oocyte and embryo cryopreservation by ovarian hormonal stimulation and ovarian tissue cryopreservation without hormonal stimulation. The control group included 850 women with breast cancer who did not undergo fertility preservation. The cohort and the control groups were matched on age, calendar period of diagnosis, and region. Three Swedish registers for breast cancer were used to obtain the study cohort, and for each participant, 2 breast cancer patients who were unexposed to fertility preservation were used for comparison. The primary outcome was mortality while the secondary outcome was any event of death due to breast cancer or relapse.
Results. A total of 1,275 women were studied at the time of breast cancer diagnosis. After stratification, which included age, parity at diagnosis, tumor size, number of lymph node metastases, and estrogen receptor status, disease-specific mortality was similar in all categories of women, that is, hormonal fertility preservation, nonhormonal fertility preservation, and controls. In the subcohort of 723 women, the adjusted rate of relapse and disease-specific mortality remained the same among all groups.
Study strengths and limitations
This study prompts several areas of criticism. The follow-up of breast cancer patients was only 5 years, adding to the limitations of short-term monitoring seen in prior studies. The authors also considered a delay in pregnancy attempts following breast cancer treatment of hormonally sensitive cancers of 5 to 10 years. However, the long-term safety of pregnancy following breast cancer has shown a statistically significantly superior disease-free survival (DFS) in patients who became pregnant less than 2 years from diagnosis and no difference in those who became pregnant 2 or more years from diagnosis.7
Only 58 women in the nonhormonal fertility preservation group (ovarian tissue cryopreservation) were studied, which may limit an adequate evaluation although it is not expected to negatively impact breast cancer prognosis. Another area of potential bias was the use of only a subcohort to assess relapse-free survival as opposed to the entire cohort that was used to assess mortality.
Strengths of this study include obligatory reporting to the registry and equal access to anticancer treatment and fertility preservation in Sweden. Ovarian stimulating drugs were examined, as letrozole is often used in breast cancer patients to maintain lower estradiol levels due to aromatase inhibition. Nevertheless, this study did not demonstrate a difference in mortality with or without letrozole use. ●
Marklund and colleagues’ findings revealed no increase of breast cancer relapse and mortality following fertility preservation with or without hormonal stimulation. They also propose a “healthy user effect” whereby a woman who feels healthy may choose to undergo fertility preservation, thereby biasing the outcome by having a better survival.8
Future studies with longer follow-up are needed to address the hormonal impact of fertility preservation, if any, on breast cancer DFS and mortality, as well as to evaluate subsequent pregnancy outcomes, stratified for medication treatment type via the CED calculator. To date, evidence continues to support fertility preservation options that use hormonal ovarian stimulation in breast cancer patients as apparently safe for, at least, up to 5 years of follow-up.
MARK P. TROLICE, MD
- Giaquinto AN, Sung H, Miller KD, et al. Breast cancer statistics, 2022. CA Cancer J Clin. 2022;72:524-541. doi:10.3322/caac.21754.
- Oktay K, Harvey BE, Partridge AH, et al. Fertility preservation in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol. 2018;1;36:1994-2001. doi:10.1200/JCO.2018.78.1914.
- Fertility Preservation in Pittsburgh. CED calculator. Accessed November 14, 2022. https://fertilitypreservationpittsburgh.org/fertility-resources/fertility-risk-calculator/
- Practice Committee of the American Society for Reproductive Medicine. Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: a committee opinion. Fertil Steril. 2019;112:1022-1033. doi:10.1016/j.fertnstert.2019.09.013.
- Blumenfeld Z. Fertility preservation using GnRH agonists: rationale, possible mechanisms, and explanation of controversy. Clin Med Insights Reprod Health. 2019;13: 1179558119870163. doi:10.1177/1179558119870163.
- Beebeejaun Y, Athithan A, Copeland TP, et al. Risk of breast cancer in women treated with ovarian stimulation drugs for infertility: a systematic review and meta-analysis. Fertil Steril. 2021;116:198-207. doi:10.1016/j.fertnstert.2021.01.044.
- Lambertini M, Kroman N, Ameye L, et al. Long-term safety of pregnancy following breast cancer according to estrogen receptor status. J Natl Cancer Inst. 2018;110:426-429. doi:10.1093/jnci/djx206.
- Marklund A, Lundberg FE, Eloranta S, et al. Reproductive outcomes after breast cancer in women with vs without fertility preservation. JAMA Oncol. 2021;7:86-91. doi:10.1001/ jamaoncol.2020.5957.

Marklund A, Lekberg T, Hedayati E, et al. Relapse rates and disease-specific mortality following procedures for fertility preservation at time of breast cancer diagnosis. JAMA Oncol. 2022;8:1438-1446. doi:10.1001/jamaoncol.2022.3677.
EXPERT COMMENTARY
Breast cancer is the most diagnosed cancer among US women after skin cancer.1 As of the end of 2020, 7.8 million women were alive who were diagnosed with breast cancer in the past 5 years, making it the world’s most prevalent cancer. Given the wide reach of breast cancer and the increase in its distant stage by more than 4% per year in women of reproductive age (20–39 years), clinicians are urged to address fertility preservation due to reproductive compromise of gonadotoxic therapies and gonadectomy.2 To predict the risk of infertility following chemotherapy, a Cyclophosphamide Equivalent Dose (CED) calculator can be used. A CED of 4,000 mg/m2 has been associated with a significant risk of infertility.3
In 2012, the American Society for Reproductive Medicine removed the experimental label of oocyte cryopreservation then recently endorsed ovarian cryopreservation, thereby providing acceptable procedures for fertility preservation.4 Gonadotropin-releasing hormone agonist use during chemotherapy, which is used to protect the ovary in premenopausal women against the effects of chemotherapy, has been shown to have inconsistent findings and should not replace the established modalities of oocyte/embryo/ovarian tissue cryopreservation.2,5
Details of the study
While studies have been reassuring that ovarian stimulation for fertility preservation in women with breast cancer does not worsen the prognosis, findings are limited by short-term follow-up.6
The recent study by Marklund and colleagues presented an analysis of breast cancer relapse and mortality following fertility preservation with and without hormonal stimulation. In their prospective cohort study of 425 Swedish women who underwent fertility preservation, the authors categorized patients into 2 groups: oocyte and embryo cryopreservation by ovarian hormonal stimulation and ovarian tissue cryopreservation without hormonal stimulation. The control group included 850 women with breast cancer who did not undergo fertility preservation. The cohort and the control groups were matched on age, calendar period of diagnosis, and region. Three Swedish registers for breast cancer were used to obtain the study cohort, and for each participant, 2 breast cancer patients who were unexposed to fertility preservation were used for comparison. The primary outcome was mortality while the secondary outcome was any event of death due to breast cancer or relapse.
Results. A total of 1,275 women were studied at the time of breast cancer diagnosis. After stratification, which included age, parity at diagnosis, tumor size, number of lymph node metastases, and estrogen receptor status, disease-specific mortality was similar in all categories of women, that is, hormonal fertility preservation, nonhormonal fertility preservation, and controls. In the subcohort of 723 women, the adjusted rate of relapse and disease-specific mortality remained the same among all groups.
Study strengths and limitations
This study prompts several areas of criticism. The follow-up of breast cancer patients was only 5 years, adding to the limitations of short-term monitoring seen in prior studies. The authors also considered a delay in pregnancy attempts following breast cancer treatment of hormonally sensitive cancers of 5 to 10 years. However, the long-term safety of pregnancy following breast cancer has shown a statistically significantly superior disease-free survival (DFS) in patients who became pregnant less than 2 years from diagnosis and no difference in those who became pregnant 2 or more years from diagnosis.7
Only 58 women in the nonhormonal fertility preservation group (ovarian tissue cryopreservation) were studied, which may limit an adequate evaluation although it is not expected to negatively impact breast cancer prognosis. Another area of potential bias was the use of only a subcohort to assess relapse-free survival as opposed to the entire cohort that was used to assess mortality.
Strengths of this study include obligatory reporting to the registry and equal access to anticancer treatment and fertility preservation in Sweden. Ovarian stimulating drugs were examined, as letrozole is often used in breast cancer patients to maintain lower estradiol levels due to aromatase inhibition. Nevertheless, this study did not demonstrate a difference in mortality with or without letrozole use. ●
Marklund and colleagues’ findings revealed no increase of breast cancer relapse and mortality following fertility preservation with or without hormonal stimulation. They also propose a “healthy user effect” whereby a woman who feels healthy may choose to undergo fertility preservation, thereby biasing the outcome by having a better survival.8
Future studies with longer follow-up are needed to address the hormonal impact of fertility preservation, if any, on breast cancer DFS and mortality, as well as to evaluate subsequent pregnancy outcomes, stratified for medication treatment type via the CED calculator. To date, evidence continues to support fertility preservation options that use hormonal ovarian stimulation in breast cancer patients as apparently safe for, at least, up to 5 years of follow-up.
MARK P. TROLICE, MD

Marklund A, Lekberg T, Hedayati E, et al. Relapse rates and disease-specific mortality following procedures for fertility preservation at time of breast cancer diagnosis. JAMA Oncol. 2022;8:1438-1446. doi:10.1001/jamaoncol.2022.3677.
EXPERT COMMENTARY
Breast cancer is the most diagnosed cancer among US women after skin cancer.1 As of the end of 2020, 7.8 million women were alive who were diagnosed with breast cancer in the past 5 years, making it the world’s most prevalent cancer. Given the wide reach of breast cancer and the increase in its distant stage by more than 4% per year in women of reproductive age (20–39 years), clinicians are urged to address fertility preservation due to reproductive compromise of gonadotoxic therapies and gonadectomy.2 To predict the risk of infertility following chemotherapy, a Cyclophosphamide Equivalent Dose (CED) calculator can be used. A CED of 4,000 mg/m2 has been associated with a significant risk of infertility.3
In 2012, the American Society for Reproductive Medicine removed the experimental label of oocyte cryopreservation then recently endorsed ovarian cryopreservation, thereby providing acceptable procedures for fertility preservation.4 Gonadotropin-releasing hormone agonist use during chemotherapy, which is used to protect the ovary in premenopausal women against the effects of chemotherapy, has been shown to have inconsistent findings and should not replace the established modalities of oocyte/embryo/ovarian tissue cryopreservation.2,5
Details of the study
While studies have been reassuring that ovarian stimulation for fertility preservation in women with breast cancer does not worsen the prognosis, findings are limited by short-term follow-up.6
The recent study by Marklund and colleagues presented an analysis of breast cancer relapse and mortality following fertility preservation with and without hormonal stimulation. In their prospective cohort study of 425 Swedish women who underwent fertility preservation, the authors categorized patients into 2 groups: oocyte and embryo cryopreservation by ovarian hormonal stimulation and ovarian tissue cryopreservation without hormonal stimulation. The control group included 850 women with breast cancer who did not undergo fertility preservation. The cohort and the control groups were matched on age, calendar period of diagnosis, and region. Three Swedish registers for breast cancer were used to obtain the study cohort, and for each participant, 2 breast cancer patients who were unexposed to fertility preservation were used for comparison. The primary outcome was mortality while the secondary outcome was any event of death due to breast cancer or relapse.
Results. A total of 1,275 women were studied at the time of breast cancer diagnosis. After stratification, which included age, parity at diagnosis, tumor size, number of lymph node metastases, and estrogen receptor status, disease-specific mortality was similar in all categories of women, that is, hormonal fertility preservation, nonhormonal fertility preservation, and controls. In the subcohort of 723 women, the adjusted rate of relapse and disease-specific mortality remained the same among all groups.
Study strengths and limitations
This study prompts several areas of criticism. The follow-up of breast cancer patients was only 5 years, adding to the limitations of short-term monitoring seen in prior studies. The authors also considered a delay in pregnancy attempts following breast cancer treatment of hormonally sensitive cancers of 5 to 10 years. However, the long-term safety of pregnancy following breast cancer has shown a statistically significantly superior disease-free survival (DFS) in patients who became pregnant less than 2 years from diagnosis and no difference in those who became pregnant 2 or more years from diagnosis.7
Only 58 women in the nonhormonal fertility preservation group (ovarian tissue cryopreservation) were studied, which may limit an adequate evaluation although it is not expected to negatively impact breast cancer prognosis. Another area of potential bias was the use of only a subcohort to assess relapse-free survival as opposed to the entire cohort that was used to assess mortality.
Strengths of this study include obligatory reporting to the registry and equal access to anticancer treatment and fertility preservation in Sweden. Ovarian stimulating drugs were examined, as letrozole is often used in breast cancer patients to maintain lower estradiol levels due to aromatase inhibition. Nevertheless, this study did not demonstrate a difference in mortality with or without letrozole use. ●
Marklund and colleagues’ findings revealed no increase of breast cancer relapse and mortality following fertility preservation with or without hormonal stimulation. They also propose a “healthy user effect” whereby a woman who feels healthy may choose to undergo fertility preservation, thereby biasing the outcome by having a better survival.8
Future studies with longer follow-up are needed to address the hormonal impact of fertility preservation, if any, on breast cancer DFS and mortality, as well as to evaluate subsequent pregnancy outcomes, stratified for medication treatment type via the CED calculator. To date, evidence continues to support fertility preservation options that use hormonal ovarian stimulation in breast cancer patients as apparently safe for, at least, up to 5 years of follow-up.
MARK P. TROLICE, MD
- Giaquinto AN, Sung H, Miller KD, et al. Breast cancer statistics, 2022. CA Cancer J Clin. 2022;72:524-541. doi:10.3322/caac.21754.
- Oktay K, Harvey BE, Partridge AH, et al. Fertility preservation in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol. 2018;1;36:1994-2001. doi:10.1200/JCO.2018.78.1914.
- Fertility Preservation in Pittsburgh. CED calculator. Accessed November 14, 2022. https://fertilitypreservationpittsburgh.org/fertility-resources/fertility-risk-calculator/
- Practice Committee of the American Society for Reproductive Medicine. Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: a committee opinion. Fertil Steril. 2019;112:1022-1033. doi:10.1016/j.fertnstert.2019.09.013.
- Blumenfeld Z. Fertility preservation using GnRH agonists: rationale, possible mechanisms, and explanation of controversy. Clin Med Insights Reprod Health. 2019;13: 1179558119870163. doi:10.1177/1179558119870163.
- Beebeejaun Y, Athithan A, Copeland TP, et al. Risk of breast cancer in women treated with ovarian stimulation drugs for infertility: a systematic review and meta-analysis. Fertil Steril. 2021;116:198-207. doi:10.1016/j.fertnstert.2021.01.044.
- Lambertini M, Kroman N, Ameye L, et al. Long-term safety of pregnancy following breast cancer according to estrogen receptor status. J Natl Cancer Inst. 2018;110:426-429. doi:10.1093/jnci/djx206.
- Marklund A, Lundberg FE, Eloranta S, et al. Reproductive outcomes after breast cancer in women with vs without fertility preservation. JAMA Oncol. 2021;7:86-91. doi:10.1001/ jamaoncol.2020.5957.
- Giaquinto AN, Sung H, Miller KD, et al. Breast cancer statistics, 2022. CA Cancer J Clin. 2022;72:524-541. doi:10.3322/caac.21754.
- Oktay K, Harvey BE, Partridge AH, et al. Fertility preservation in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol. 2018;1;36:1994-2001. doi:10.1200/JCO.2018.78.1914.
- Fertility Preservation in Pittsburgh. CED calculator. Accessed November 14, 2022. https://fertilitypreservationpittsburgh.org/fertility-resources/fertility-risk-calculator/
- Practice Committee of the American Society for Reproductive Medicine. Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: a committee opinion. Fertil Steril. 2019;112:1022-1033. doi:10.1016/j.fertnstert.2019.09.013.
- Blumenfeld Z. Fertility preservation using GnRH agonists: rationale, possible mechanisms, and explanation of controversy. Clin Med Insights Reprod Health. 2019;13: 1179558119870163. doi:10.1177/1179558119870163.
- Beebeejaun Y, Athithan A, Copeland TP, et al. Risk of breast cancer in women treated with ovarian stimulation drugs for infertility: a systematic review and meta-analysis. Fertil Steril. 2021;116:198-207. doi:10.1016/j.fertnstert.2021.01.044.
- Lambertini M, Kroman N, Ameye L, et al. Long-term safety of pregnancy following breast cancer according to estrogen receptor status. J Natl Cancer Inst. 2018;110:426-429. doi:10.1093/jnci/djx206.
- Marklund A, Lundberg FE, Eloranta S, et al. Reproductive outcomes after breast cancer in women with vs without fertility preservation. JAMA Oncol. 2021;7:86-91. doi:10.1001/ jamaoncol.2020.5957.
Patient With Severe Headache After IV Immunoglobulin
A 35-year-old woman with a history of hypothyroidism and idiopathic small fiber autonomic and sensory neuropathy presented to the emergency department (ED) 48 hours after IV immunoglobulin (IG) infusion with a severe headache, nausea, neck stiffness, photophobia, and episodes of intense positional eye pressure. The patient reported previous episodes of headaches post-IVIG infusion but not nearly as severe. On ED arrival, the patient was afebrile with vital signs within normal limits. Initial laboratory results were notable for levels within reference range parameters: 5.9 × 109/L white blood cell (WBC) count, 13.3 g/dL hemoglobin, 38.7% hematocrit, and 279 × 109/L platelet count; there were no abnormal urinalysis findings, and she was negative for human chorionic gonadotropin.
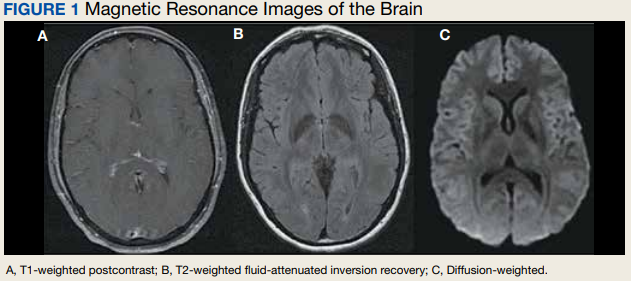
Due to the patient’s symptoms concerning for an acute intracranial process, a brain computed tomography (CT) without contrast was ordered. The CT demonstrated no intracranial abnormalities, but the patient’s symptoms continued to worsen. The patient was started on IV fluids and 1 g IV acetaminophen and underwent a lumbar puncture (LP). Her opening pressure was elevated at 29 cm H2O (reference range, 6-20 cm), and the fluid was notably clear. During the LP, 25 mL of cerebrospinal fluid (CSF) was collected for laboratory analysis to include a polymerase chain reaction (PCR) panel and cultures, and a closing pressure of 12 cm H2O was recorded at the end of the procedure with the patient reporting some relief of pressure. The patient was admitted to the medicine ward for further workup and observations.The patient’s meningitis/encephalitis PCR panel detected no pathogens in the CSF, but her WBC count was 84 × 109/L (reference range, 4-11) with 30 segmented neutrophils (reference range, 0-6) and red blood cell count of 24 (reference range, 0-1); her normal glucose at 60 mg/dL (reference range, 40-70) and protein of 33 mg/dL (reference range, 15-45) were within normal parameters. Brain magnetic resonance images with and without contrast was inconsistent with any acute intracranial pathology to include subarachnoid hemorrhage or central nervous system neoplasm (Figure 1). Bacterial and fungal cultures were negative.
- What is your diagnosis?
- How would you treat this patient?
Discussion
Aseptic meningitis presents with a typical clinical picture of meningitis to include headache, stiffened neck, and photophobia. In the event of negative CSF bacterial and fungal cultures and negative viral PCR, a diagnosis of aseptic meningitis is considered.1 Though the differential for aseptic meningitis is broad, in the immunocompetent patient, the most common etiology of aseptic meningitis in the United States is by far viral, and specifically, enterovirus (50.9%). It is less commonly caused by herpes simplex virus (8.3%), varicella zoster virus, and finally, the mosquito-borne St. Louis encephalitis and West Nile viruses typically acquired in the summer or early fall months. Other infectious agents that can present with aseptic meningitis are spirochetes (Lyme disease and syphilis), tuberculous meningitis, fungal infections (cryptococcal meningitis), and other bacterial infections that have a negative culture.
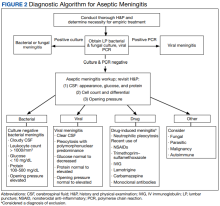
The patient’s history, physical examination, vital signs, imaging, and lumbar puncture findings were most concerning for drug-induced aseptic meningitis (DIAM) secondary to her recent IVIG infusion. An algorithm can be used to work through the diagnostic approach (Figure 2).3,4
Immediate and delayed adverse reactions to IVIG are known risks for IVIG therapy. About 1% to 15% of patients who receive IVIG will experience mild immediate reactions to the infusion.6 These immediate reactions include fever (78.6%), acrocyanosis (71.4%), rash (64.3%), headache (57.1%), shortness of breath (42.8%), hypotension (35.7%), and chest pain (21.4%).
IVIG is an increasingly used biologic pharmacologic agent used for a variety of medical conditions. This can be attributed to its multifaceted properties and ability to fight infection when given as replacement therapy and provide immunomodulation in conjunction with its more well-known anti-inflammatory properties.8 The number of conditions that can potentially benefit from IVIG is so vast that the American Academy of Allergy, Asthma and Immunology had to divide the indication for IVIG therapy into definitely beneficial, probably beneficial, may provide benefit, and unlikely to provide benefit categories.8
Conclusions
We encourage heightened clinical suspicion of DIAM in patients who have recently undergone IVIG infusion and present with meningeal signs (stiff neck, headache, photophobia, and ear/eye pressure) without any evidence of infection on physical examination or laboratory results. With such, we hope to improve clinician suspicion, detection, as well as patient education and outcomes in cases of DIAM.
1. Kareva L, Mironska K, Stavric K, Hasani A. Adverse reactions to intravenous immunoglobulins—our experience. Open Access Maced J Med Sci. 2018;6(12):2359-2362. doi:10.3889/oamjms.2018.513
2. Mount HR, Boyle SD. Aseptic and bacterial meningitis: evaluation, treatment, and prevention. Am Fam Physician. 2017;96(5):314-322.
3. Seehusen DA, Reeves MM, Fomin DA. Cerebrospinal fluid analysis. Am Fam Physician. 2003;68(6):1103-1108.
4. Connolly KJ, Hammer SM. The acute aseptic meningitis syndrome. Infect Dis Clin North Am. 1990;4(4):599-622.
5. Jolles S, Sewell WA, Leighton C. Drug-induced aseptic meningitis: diagnosis and management. Drug Saf. 2000;22(3):215-226. doi:10.2165/00002018-200022030-00005
6. Yelehe-Okouma M, Czmil-Garon J, Pape E, Petitpain N, Gillet P. Drug-induced aseptic meningitis: a mini-review. Fundam Clin Pharmacol. 2018;32(3):252-260. doi:10.1111/fcp.12349
7. Kepa L, Oczko-Grzesik B, Stolarz W, Sobala-Szczygiel B. Drug-induced aseptic meningitis in suspected central nervous system infections. J Clin Neurosci. 2005;12(5):562-564. doi:10.1016/j.jocn.2004.08.024
8. Perez EE, Orange JS, Bonilla F, et al. Update on the use of immunoglobulin in human disease: a review of evidence. J Allergy Clin Immunol. 2017;139(3S):S1-S46. doi:10.1016/j.jaci.2016.09.023
9. Kaarthigeyan K, Burli VV. Aseptic meningitis following intravenous immunoglobulin therapy of common variable immunodeficiency. J Pediatr Neurosci. 2011;6(2):160-161. doi:10.4103/1817-1745.92858
A 35-year-old woman with a history of hypothyroidism and idiopathic small fiber autonomic and sensory neuropathy presented to the emergency department (ED) 48 hours after IV immunoglobulin (IG) infusion with a severe headache, nausea, neck stiffness, photophobia, and episodes of intense positional eye pressure. The patient reported previous episodes of headaches post-IVIG infusion but not nearly as severe. On ED arrival, the patient was afebrile with vital signs within normal limits. Initial laboratory results were notable for levels within reference range parameters: 5.9 × 109/L white blood cell (WBC) count, 13.3 g/dL hemoglobin, 38.7% hematocrit, and 279 × 109/L platelet count; there were no abnormal urinalysis findings, and she was negative for human chorionic gonadotropin.
Due to the patient’s symptoms concerning for an acute intracranial process, a brain computed tomography (CT) without contrast was ordered. The CT demonstrated no intracranial abnormalities, but the patient’s symptoms continued to worsen. The patient was started on IV fluids and 1 g IV acetaminophen and underwent a lumbar puncture (LP). Her opening pressure was elevated at 29 cm H2O (reference range, 6-20 cm), and the fluid was notably clear. During the LP, 25 mL of cerebrospinal fluid (CSF) was collected for laboratory analysis to include a polymerase chain reaction (PCR) panel and cultures, and a closing pressure of 12 cm H2O was recorded at the end of the procedure with the patient reporting some relief of pressure. The patient was admitted to the medicine ward for further workup and observations.The patient’s meningitis/encephalitis PCR panel detected no pathogens in the CSF, but her WBC count was 84 × 109/L (reference range, 4-11) with 30 segmented neutrophils (reference range, 0-6) and red blood cell count of 24 (reference range, 0-1); her normal glucose at 60 mg/dL (reference range, 40-70) and protein of 33 mg/dL (reference range, 15-45) were within normal parameters. Brain magnetic resonance images with and without contrast was inconsistent with any acute intracranial pathology to include subarachnoid hemorrhage or central nervous system neoplasm (Figure 1). Bacterial and fungal cultures were negative.
- What is your diagnosis?
- How would you treat this patient?

Discussion
Aseptic meningitis presents with a typical clinical picture of meningitis to include headache, stiffened neck, and photophobia. In the event of negative CSF bacterial and fungal cultures and negative viral PCR, a diagnosis of aseptic meningitis is considered.1 Though the differential for aseptic meningitis is broad, in the immunocompetent patient, the most common etiology of aseptic meningitis in the United States is by far viral, and specifically, enterovirus (50.9%). It is less commonly caused by herpes simplex virus (8.3%), varicella zoster virus, and finally, the mosquito-borne St. Louis encephalitis and West Nile viruses typically acquired in the summer or early fall months. Other infectious agents that can present with aseptic meningitis are spirochetes (Lyme disease and syphilis), tuberculous meningitis, fungal infections (cryptococcal meningitis), and other bacterial infections that have a negative culture.
The patient’s history, physical examination, vital signs, imaging, and lumbar puncture findings were most concerning for drug-induced aseptic meningitis (DIAM) secondary to her recent IVIG infusion. An algorithm can be used to work through the diagnostic approach (Figure 2).3,4
Immediate and delayed adverse reactions to IVIG are known risks for IVIG therapy. About 1% to 15% of patients who receive IVIG will experience mild immediate reactions to the infusion.6 These immediate reactions include fever (78.6%), acrocyanosis (71.4%), rash (64.3%), headache (57.1%), shortness of breath (42.8%), hypotension (35.7%), and chest pain (21.4%).
IVIG is an increasingly used biologic pharmacologic agent used for a variety of medical conditions. This can be attributed to its multifaceted properties and ability to fight infection when given as replacement therapy and provide immunomodulation in conjunction with its more well-known anti-inflammatory properties.8 The number of conditions that can potentially benefit from IVIG is so vast that the American Academy of Allergy, Asthma and Immunology had to divide the indication for IVIG therapy into definitely beneficial, probably beneficial, may provide benefit, and unlikely to provide benefit categories.8
Conclusions
We encourage heightened clinical suspicion of DIAM in patients who have recently undergone IVIG infusion and present with meningeal signs (stiff neck, headache, photophobia, and ear/eye pressure) without any evidence of infection on physical examination or laboratory results. With such, we hope to improve clinician suspicion, detection, as well as patient education and outcomes in cases of DIAM.
A 35-year-old woman with a history of hypothyroidism and idiopathic small fiber autonomic and sensory neuropathy presented to the emergency department (ED) 48 hours after IV immunoglobulin (IG) infusion with a severe headache, nausea, neck stiffness, photophobia, and episodes of intense positional eye pressure. The patient reported previous episodes of headaches post-IVIG infusion but not nearly as severe. On ED arrival, the patient was afebrile with vital signs within normal limits. Initial laboratory results were notable for levels within reference range parameters: 5.9 × 109/L white blood cell (WBC) count, 13.3 g/dL hemoglobin, 38.7% hematocrit, and 279 × 109/L platelet count; there were no abnormal urinalysis findings, and she was negative for human chorionic gonadotropin.
Due to the patient’s symptoms concerning for an acute intracranial process, a brain computed tomography (CT) without contrast was ordered. The CT demonstrated no intracranial abnormalities, but the patient’s symptoms continued to worsen. The patient was started on IV fluids and 1 g IV acetaminophen and underwent a lumbar puncture (LP). Her opening pressure was elevated at 29 cm H2O (reference range, 6-20 cm), and the fluid was notably clear. During the LP, 25 mL of cerebrospinal fluid (CSF) was collected for laboratory analysis to include a polymerase chain reaction (PCR) panel and cultures, and a closing pressure of 12 cm H2O was recorded at the end of the procedure with the patient reporting some relief of pressure. The patient was admitted to the medicine ward for further workup and observations.The patient’s meningitis/encephalitis PCR panel detected no pathogens in the CSF, but her WBC count was 84 × 109/L (reference range, 4-11) with 30 segmented neutrophils (reference range, 0-6) and red blood cell count of 24 (reference range, 0-1); her normal glucose at 60 mg/dL (reference range, 40-70) and protein of 33 mg/dL (reference range, 15-45) were within normal parameters. Brain magnetic resonance images with and without contrast was inconsistent with any acute intracranial pathology to include subarachnoid hemorrhage or central nervous system neoplasm (Figure 1). Bacterial and fungal cultures were negative.
- What is your diagnosis?
- How would you treat this patient?

Discussion
Aseptic meningitis presents with a typical clinical picture of meningitis to include headache, stiffened neck, and photophobia. In the event of negative CSF bacterial and fungal cultures and negative viral PCR, a diagnosis of aseptic meningitis is considered.1 Though the differential for aseptic meningitis is broad, in the immunocompetent patient, the most common etiology of aseptic meningitis in the United States is by far viral, and specifically, enterovirus (50.9%). It is less commonly caused by herpes simplex virus (8.3%), varicella zoster virus, and finally, the mosquito-borne St. Louis encephalitis and West Nile viruses typically acquired in the summer or early fall months. Other infectious agents that can present with aseptic meningitis are spirochetes (Lyme disease and syphilis), tuberculous meningitis, fungal infections (cryptococcal meningitis), and other bacterial infections that have a negative culture.
The patient’s history, physical examination, vital signs, imaging, and lumbar puncture findings were most concerning for drug-induced aseptic meningitis (DIAM) secondary to her recent IVIG infusion. An algorithm can be used to work through the diagnostic approach (Figure 2).3,4
Immediate and delayed adverse reactions to IVIG are known risks for IVIG therapy. About 1% to 15% of patients who receive IVIG will experience mild immediate reactions to the infusion.6 These immediate reactions include fever (78.6%), acrocyanosis (71.4%), rash (64.3%), headache (57.1%), shortness of breath (42.8%), hypotension (35.7%), and chest pain (21.4%).
IVIG is an increasingly used biologic pharmacologic agent used for a variety of medical conditions. This can be attributed to its multifaceted properties and ability to fight infection when given as replacement therapy and provide immunomodulation in conjunction with its more well-known anti-inflammatory properties.8 The number of conditions that can potentially benefit from IVIG is so vast that the American Academy of Allergy, Asthma and Immunology had to divide the indication for IVIG therapy into definitely beneficial, probably beneficial, may provide benefit, and unlikely to provide benefit categories.8
Conclusions
We encourage heightened clinical suspicion of DIAM in patients who have recently undergone IVIG infusion and present with meningeal signs (stiff neck, headache, photophobia, and ear/eye pressure) without any evidence of infection on physical examination or laboratory results. With such, we hope to improve clinician suspicion, detection, as well as patient education and outcomes in cases of DIAM.
1. Kareva L, Mironska K, Stavric K, Hasani A. Adverse reactions to intravenous immunoglobulins—our experience. Open Access Maced J Med Sci. 2018;6(12):2359-2362. doi:10.3889/oamjms.2018.513
2. Mount HR, Boyle SD. Aseptic and bacterial meningitis: evaluation, treatment, and prevention. Am Fam Physician. 2017;96(5):314-322.
3. Seehusen DA, Reeves MM, Fomin DA. Cerebrospinal fluid analysis. Am Fam Physician. 2003;68(6):1103-1108.
4. Connolly KJ, Hammer SM. The acute aseptic meningitis syndrome. Infect Dis Clin North Am. 1990;4(4):599-622.
5. Jolles S, Sewell WA, Leighton C. Drug-induced aseptic meningitis: diagnosis and management. Drug Saf. 2000;22(3):215-226. doi:10.2165/00002018-200022030-00005
6. Yelehe-Okouma M, Czmil-Garon J, Pape E, Petitpain N, Gillet P. Drug-induced aseptic meningitis: a mini-review. Fundam Clin Pharmacol. 2018;32(3):252-260. doi:10.1111/fcp.12349
7. Kepa L, Oczko-Grzesik B, Stolarz W, Sobala-Szczygiel B. Drug-induced aseptic meningitis in suspected central nervous system infections. J Clin Neurosci. 2005;12(5):562-564. doi:10.1016/j.jocn.2004.08.024
8. Perez EE, Orange JS, Bonilla F, et al. Update on the use of immunoglobulin in human disease: a review of evidence. J Allergy Clin Immunol. 2017;139(3S):S1-S46. doi:10.1016/j.jaci.2016.09.023
9. Kaarthigeyan K, Burli VV. Aseptic meningitis following intravenous immunoglobulin therapy of common variable immunodeficiency. J Pediatr Neurosci. 2011;6(2):160-161. doi:10.4103/1817-1745.92858
1. Kareva L, Mironska K, Stavric K, Hasani A. Adverse reactions to intravenous immunoglobulins—our experience. Open Access Maced J Med Sci. 2018;6(12):2359-2362. doi:10.3889/oamjms.2018.513
2. Mount HR, Boyle SD. Aseptic and bacterial meningitis: evaluation, treatment, and prevention. Am Fam Physician. 2017;96(5):314-322.
3. Seehusen DA, Reeves MM, Fomin DA. Cerebrospinal fluid analysis. Am Fam Physician. 2003;68(6):1103-1108.
4. Connolly KJ, Hammer SM. The acute aseptic meningitis syndrome. Infect Dis Clin North Am. 1990;4(4):599-622.
5. Jolles S, Sewell WA, Leighton C. Drug-induced aseptic meningitis: diagnosis and management. Drug Saf. 2000;22(3):215-226. doi:10.2165/00002018-200022030-00005
6. Yelehe-Okouma M, Czmil-Garon J, Pape E, Petitpain N, Gillet P. Drug-induced aseptic meningitis: a mini-review. Fundam Clin Pharmacol. 2018;32(3):252-260. doi:10.1111/fcp.12349
7. Kepa L, Oczko-Grzesik B, Stolarz W, Sobala-Szczygiel B. Drug-induced aseptic meningitis in suspected central nervous system infections. J Clin Neurosci. 2005;12(5):562-564. doi:10.1016/j.jocn.2004.08.024
8. Perez EE, Orange JS, Bonilla F, et al. Update on the use of immunoglobulin in human disease: a review of evidence. J Allergy Clin Immunol. 2017;139(3S):S1-S46. doi:10.1016/j.jaci.2016.09.023
9. Kaarthigeyan K, Burli VV. Aseptic meningitis following intravenous immunoglobulin therapy of common variable immunodeficiency. J Pediatr Neurosci. 2011;6(2):160-161. doi:10.4103/1817-1745.92858
Oral Therapy for Aerococcus urinae Bacteremia and Thoracic Spondylodiscitis of Presumed Urinary Origin
Aerococcus urinae (A urinae), a gram-positive coccus readily mistaken for a Staphylococcus species, was first identified in 1992.1-3 It now reportedly accounts for 0.2% to 0.8% of clinical urine isolates.4-6 A urinae bacteriuria is typically asymptomatic and mainly occurs in women.7-9 Symptomatic A urinae urinary tract infection (UTI) occurs predominantly in older men with underlying urologic abnormalities.4-10
Serious A urinae infections are rare. The first 2 reported cases involved men with A urinae endocarditis, one of whom died.11,12 To date, only 8 cases of spondylodiscitis due to A urinae have been reported.13-20 Optimal treatment for invasive A urinae infection is undefined; however, the reported cases were treated successfully with diverse antibiotic regimen combinations; all including a β-lactam and beginning with at least 2 weeks of IV antibiotics.13-20 We describe a man with A urinae bacteremia and spondylodiscitis, presumably arising from a urinary source in the setting of bladder outlet obstruction, who was treated successfully.
Case Presentation
A 74-year-old man with morbid obesity, type 2 diabetes mellitus, stage 2 chronic kidney disease, and tobacco use presented to the emergency department after 2 weeks of progressive, nonradiating, midthoracic back pain, lower extremity weakness, gait imbalance, fatigue, anorexia, rigors, and subjective fevers. On presentation, he was afebrile and hemodynamically stable. A physical examination revealed point tenderness of the midthoracic vertebrae, nontender costovertebral angles, diffusely decreased strength, nonsustained clonus in both lower extremities, inguinal intertrigo, and a buried penis with purulent meatal discharge.
Laboratory results indicated a white blood cell (WBC) count of 13.5 K/μL (reference range, 4.0-11.0), absolute neutrophil count of 11.48 K/μL (reference range, 2.0-7.7), C-reactive protein (CRP) level of 225.3 mg/L (reference range, ≤ 5.0), erythrocyte sedimentation rate of 85 mm/h (reference range, 5-15), serum blood urea nitrogen of 76 mg/dL (reference range, 8-26), and serum creatinine (SCr) of 1.9 mg/dL (reference range, 1.1-1.4). A urinalysis showed positive leukocyte esterase, WBC clumps, and little bacteria. Abdominal/pelvic computed tomography showed spondylodiscitis-like changes at T7-T8, bilateral perinephric fat stranding, bladder distension, and bladder wall thickening.
The patient was presumed to have discitis secondary to a UTI, with possible pyelonephritis, and was given empiric vancomycin and ceftriaxone. Spinal magnetic resonance imaging with contrast supported spondylodiscitis at T7-T8, extending to T8-T9. Preliminary results from the admission blood and urine cultures showed gram-positive cocci in clusters, which were presumed initially to be Staphylococcus aureus (S aureus).
The final urine culture report listed multiple organisms, predominantly A urinae (Table 1);
On hospital day 6, the patient’s back pain had resolved, micturition was normal, appetite had normalized, and SCr was minimally above baseline (1.4 mg/dL). He insisted on completing antibiotic treatment at home and had no other medical indication for continued hospitalization. Thus, antibiotic therapy was changed to an all-oral regimen of amoxicillin 1 g 3 times daily for 10 days and levofloxacin 750 mg daily for 6 weeks, and the patient was discharged to home.
The patient returned 5 days postdischarge due to anuria. Investigation showed severe acute kidney injury (SCr, 6.8 mg/dL) and bladder outlet obstruction due to phimosis and urethral meatal stenosis. Urinalysis was unremarkable. His CRP had declined from 225 mg/L (initial admission) to 154 mg/L. A urinae culture and 2 sets of blood cultures were finalized as no growth. He was diagnosed with postrenal acute kidney injury and underwent meatal dilation and Foley catheterization but declined surgical correction. When seen in the clinic 2 months postantimicrobial therapy, the patient had normal micturition, no symptoms or signs of infection, and steadily down-trending inflammatory markers.
Discussion
A urinae, historically considered a rare pathogen, has been identified with increasing frequency in urine cultures due to improved microbiologic diagnostic techniques. However, there are only 8 reported cases of A urinae spondylodiscitis. Urinary pathology is an accepted risk factor for A urinae infections; consequently, we suspect that our patient’s urinary outflow obstruction and poor genitourinary hygiene were related to his invasive A urinae infection.10,21,22 We surmise that he had a chronic urinary outflow obstruction contributing to his infection, as evidenced by imaging findings, while the phimosis and urethral meatal stenosis were most likely infectious sequelae considering his anuria and acute kidney injury 5 days postdischarge. Indeed, the correlation between A urinae and urinary tract pathology may justify an evaluation for urinary pathology in any man with A urinae infection, regardless of the presence of symptoms.
By contrast, the implications of A urinae bacteriuria remain unclear. From a public health perspective, A urinae bacteriuria is rare, but the infectious mechanism remains undetermined with a case report suggesting the possibility of sexual transmission.4-6,23 In our case, the patient was not sexually active and had no clear origin of infection. Considering the potential severity of infection, more studies are needed to determine the infectious mechanism of A urinae.
In terms of infectious morbidity, the results seem to vary by sex. In a retrospective study of about 30,000 clinical urine samples, 62 (58 from women, 4 from men) yielded A urinae. The 62 corresponding patients lacked systemic infectious complications, leading the authors to conclude that A urinae is a relatively avirulent organism.24 Although possibly true in women, we are wary of drawing conclusions, especially regarding men, from a study that included only 62 urine samples were A urinae–positive, with only 4 from men. More evidence is needed to define the prognostic implications of A urinae bacteriuria in men.
As illustrated by the present case and previous reports, severe A urinae infections can occur, and the contributory factors deserve consideration. In our patient, the actual mechanism for bacteremia remains unclear. The initial concern for acute pyelonephritis was prompted by a computed tomography finding of bilateral perinephric fat stranding. This finding was questioned because it is common in older patients without infection, hence, is highly nonspecific. A correlation with urinary outflow obstruction may be an important clue in cases like this one.25,26
Furthermore, whether the urinary tract truly was the source of the patient’s bacteremia is clouded by the differing antimicrobial susceptibility patterns of the A urinae blood and urine isolates. The simplest explanation for this discordance may be that all the isolates shared a common initial origin but adapted to different environments in the host (perhaps over time) or laboratory, producing phenotypic variation. Alternatively, the infection could have been polyclonal from the onset, with sampling error leading to the differing detected susceptibility patterns, or the blood and urine isolates may have represented independent acquisition events, involving distinct A urinae strains. Unfortunately (from an academic perspective), given patient preferences and recommendations from the infectious disease consultant, no bone biopsy was done for histology and culture to confirm infection and to allow comparative strain identification if A urinae was isolated.
Optimal treatment for A urinae spondylodiscitis has yet to be established. β-lactams have shown good clinical efficacy despite being bacteriostatic in vitro.27 Early in vitro studies showed synergistic bactericidal synergistic activity with penicillin plus aminoglycoside combination therapies.27-30 Cases of endocarditis have been successfully treated mainly with the combination of a β-lactam plus aminoglycoside combination therapy.30,31 Previous cases of spondylodiscitis have been treated successfully with diverse antimicrobial agents, including clindamycin, β-lactams, cephalosporins, fluoroquinolones, and aminoglycosides.14
Our patient improved rapidly while receiving empiric therapy with vancomycin and ceftriaxone and tolerated a rapid transition to oral amoxicillin and levofloxacin. This is the shortest IV treatment course for A urinae spondylodiscitis reported to date. We suspect that such rapid IV-to-oral transitions will suffice in most stable patients with A urinae spondylodiscitis or other invasive A urinae infections in line with the results of the OVIVA and POET trials.32,33
Conclusions
We believe A urinae UTI in the absence of obvious predisposing factors should prompt evaluation for urinary outflow obstruction. Despite improved laboratory diagnostic techniques, spondylodiscitis related to A urinae remains a rare entity and thus definitive treatment recommendations are difficult to make. However, we suspect that in many cases it is reasonable to extrapolate from the results of the POET and OVIVA trials and rapidly transition therapy of A urinae spondylodiscitis from IV to oral antibiotics. We suspect a review of the US Department of Veterans Affairs population might uncover a higher incidence of A urinae infection than previously estimated due to the population demographics and the epidemiology of A urinae.
1. Christensen JJ, Korner B, Kjaergaard H. Aerococcus-like organism—an unnoticed urinary tract pathogen. APMIS. 1989;97(6):539-546. doi:10.1111/j.1699-0463.1989.tb00828.x
2. Aguirre M, Collins MD. Phylogenetic analysis of some Aerococcus-like organisms from urinary tract infections: description of Aerococcus urinae sp. nov. J Gen Microbiol. 1992;138(2):401-405. doi:10.1099/00221287-138-2-401
3. Williams RE, Hirch A, Cowan ST. Aerococcus, a new bacterial genus. J Gen Microbiol. 1953;8(3):475-480. doi:10.1099/00221287-8-3-475
4. Kline KA, Lewis AL. Gram-positive uropathogens, polymicrobial urinary tract infection, and the emerging microbiota of the urinary tract. Microbiol Spectr. 2016;4(2). doi:10.1128/microbiolspec.UTI-0012-2012
5. Schuur PM, Kasteren ME, Sabbe L, Vos MC, Janssens MM, Buiting AG. Urinary tract infections with Aerococcus urinae in the south of The Netherlands. Eur J Clin Microbiol Infect Dis. 1997;16(12):871-875. doi:10.1007/BF01700552
6. Grude N, Tveten Y. Aerococcus urinae og urinveisinfeksjon [Aerococcus urinae and urinary tract infection]. Tidsskr Nor Laegeforen. 2002;122(2):174-175.
7. Narayanasamy S, King K, Dennison A, Spelman DW, Aung AK. Clinical characteristics and laboratory identification of Aerococcus infections: an Australian tertiary centre perspective. Int J Microbiol. 2017;2017. doi:10.1155/2017/5684614
8. Hilt EE, McKinley K, Pearce MM, et al. Urine is not sterile: use of enhanced urine culture techniques to detect resident bacterial flora in the adult female bladder. J Clin Microbiol. 2014;52(3):871-876. doi:10.1128/JCM.02876-13
9. Pearce MM, Hilt EE, Rosenfeld AB, et al. The female urinary microbiome: a comparison of women with and without urgency urinary incontinence. mBio. 2014;5(4):e01283-14. doi:10.1128/mBio.01283-14
10. Sahu KK, Lal A, Mishra AK, Abraham GM. Aerococcus-related infections and their significance: a 9-year retrospective study. J Microsc Ultrastruct. 2021;9(1):18-25. doi:10.4103/JMAU.JMAU_61_19
11. Skov RL, Klarlund M, Thorsen S. Fatal endocarditis due to Aerococcus urinae. Diagn Microbiol Infect Dis. 1995;21(4):219-221. doi:10.1016/0732-8893(95)00037-b
12. Kristensen B, Nielsen G. Endocarditis caused by Aerococcus urinae, a newly recognized pathogen. Eur J Clin Microbiol Infect Dis. 1995;14(1):49-51. doi:10.1007/BF02112619
13. Astudillo L, Sailler L, Porte L, Lefevre JC, Massip P, Arlet-Suau E. Spondylodiscitis due to Aerococcus urinae: a first report. Scand J Infect Dis. 2003;35(11-12):890-891. doi:10.1080/00365540310016664
14. Lyagoubi A, Souffi C, Baroiller V, Vallee E. Spondylodiscitis: an increasingly described localization. EJIFCC. 2020;31(2):169-173.
15. Jerome M, Slim J, Sison R, Marton R. A case of Aerococcus urinae vertebral osteomyelitis. J Glob Infect Dis. 2015;7(2):85-86. doi:10.4103/0974-777X.157246
16. Tekin A, Tekin G, Turunç T, Demiroğlu Z, Kizilkiliç O. Infective endocarditis and spondylodiscitis in a patient due to Aerococcus urinae: first report. Int J Cardiol. 2007;115(3):402-403. doi:10.1016/j.ijcard.2006.01.046
17. Rougier E, Braud A, Argemi X, et al. Spondylodiscitis due to Aerococcus urinae and literature review. Infection. 2018;46(3):419-421. doi:10.1007/s15010-017-1106-0
18. Degroote E, Yildiz H, Lecouvet F, Verroken A, Belkhir L. Aerococcus urinae: an underestimated cause of spine infection? Case report and review of the literature. Acta Clin Belg. 2018;73(6):444-447. doi:10.1080/17843286.2018.1443003
19. Torres-Martos E, Pérez-Cortés S, Sánchez-Calvo JM, López-Prieto MD. Spondylodiscitis due to Aerococcus urinae infection in an elderly immunocompetent patient. Enferm Infecc Microbiol Clin. 2017;35(10):682-684. doi:10.1016/j.eimc.2017.02.005
20. Senneby E, Petersson AC, Rasmussen M. Clinical and microbiological features of bacteraemia with Aerococcus urinae. Clin Microbiol Infect. 2012;18(6):546-550. doi:10.1111/j.1469-0691.2011.03609.x
21. Sunnerhagen T, Nilson B, Olaison L, Rasmussen M. Clinical and microbiological features of infective endocarditis caused by aerococci. Infection. 2016;44(2):167-173. doi:10.1007/s15010-015-0812-8
22. de Jong MF, Soetekouw R, ten Kate RW, Veenendaal D. Aerococcus urinae: severe and fatal bloodstream infections and endocarditis. J Clin Microbiol. 2010;48(9):3445-3447. doi:10.1128/JCM.00835-10
23. Babaeer AA, Nader C, Iacoviello V, Tomera K. Necrotizing urethritis due to Aerococcus urinae. Case Rep Urol. 2015;2015:136147. doi:10.1155/2015/136147
24. Sierra-Hoffman M, Watkins K, Jinadatha C, Fader R, Carpenter JL. Clinical significance of Aerococcus urinae: a retrospective review. Diagn Microbiol Infect Dis. 2005;53(4):289-292. doi:10.1016/j.diagmicrobio.2005.06.021
25. Fukami H, Takeuchi Y, Kagaya S, et al. Perirenal fat stranding is not a powerful diagnostic tool for acute pyelonephritis. Int J Gen Med. 2017;10:137-144. doi:10.2147/IJGM.S133685
26. Han NY, Sung DJ, Kim MJ, Park BJ, Sim KC, Cho SB. Perirenal fat stranding on CT: is there an association with bladder outlet obstruction? Br J Radiol. 2016;89(1063):20160195. doi:10.1259/bjr.20160195
27. Hirzel C, Hirzberger L, Furrer H, Endimiani A. Bactericidal activity of penicillin, ceftriaxone, gentamicin and daptomycin alone and in combination against Aerococcus urinae. Int J Antimicrob Agents. 2016;48(3):271-276. doi:10.1016/j.ijantimicag.2016.05.007
28. Zbinden R, Santanam P, Hunziker L, Leuzinger B, von Graevenitz A. Endocarditis due to Aerococcus urinae: diagnostic tests, fatty acid composition and killing kinetics. Infection. 1999;27(2):122-124. doi:10.1007/BF02560511
29. Skov R, Christensen JJ, Korner B, Frimodt-Møller N, Espersen F. In vitro antimicrobial susceptibility of Aerococcus urinae to 14 antibiotics, and time-kill curves for penicillin, gentamicin and vancomycin. J Antimicrob Chemother. 2001;48(5):653-658. doi:10.1093/jac/48.5.653
30. Ebnöther C, Altwegg M, Gottschalk J, Seebach JD, Kronenberg A. Aerococcus urinae endocarditis: case report and review of the literature. Infection. 2002;30(5):310-313. doi:10.1007/s15010-002-3106-x
31. Tai DBG, Go JR, Fida M, Saleh OA. Management and treatment of Aerococcus bacteremia and endocarditis. Int J Infect Dis. 2021;102:584-589. doi:10.1016/j.ijid.2020.10.096
32. Li H-K, Rombach I, Zambellas R, et al; OVIVA Trial Collaborators. Oral versus intravenous antibiotics for bone and joint infection. N Engl J Med. 2019;380(5):425-436. doi:10.1056/NEJMoa1710926
33. Iversen K, Ihlemann N, Gill SU, et al. Partial oral versus intravenous antibiotic treatment of endocarditis. N Engl J Med. 2019;380(5):415-424. doi:10.1056/NEJMoa1808312
Aerococcus urinae (A urinae), a gram-positive coccus readily mistaken for a Staphylococcus species, was first identified in 1992.1-3 It now reportedly accounts for 0.2% to 0.8% of clinical urine isolates.4-6 A urinae bacteriuria is typically asymptomatic and mainly occurs in women.7-9 Symptomatic A urinae urinary tract infection (UTI) occurs predominantly in older men with underlying urologic abnormalities.4-10
Serious A urinae infections are rare. The first 2 reported cases involved men with A urinae endocarditis, one of whom died.11,12 To date, only 8 cases of spondylodiscitis due to A urinae have been reported.13-20 Optimal treatment for invasive A urinae infection is undefined; however, the reported cases were treated successfully with diverse antibiotic regimen combinations; all including a β-lactam and beginning with at least 2 weeks of IV antibiotics.13-20 We describe a man with A urinae bacteremia and spondylodiscitis, presumably arising from a urinary source in the setting of bladder outlet obstruction, who was treated successfully.
Case Presentation
A 74-year-old man with morbid obesity, type 2 diabetes mellitus, stage 2 chronic kidney disease, and tobacco use presented to the emergency department after 2 weeks of progressive, nonradiating, midthoracic back pain, lower extremity weakness, gait imbalance, fatigue, anorexia, rigors, and subjective fevers. On presentation, he was afebrile and hemodynamically stable. A physical examination revealed point tenderness of the midthoracic vertebrae, nontender costovertebral angles, diffusely decreased strength, nonsustained clonus in both lower extremities, inguinal intertrigo, and a buried penis with purulent meatal discharge.
Laboratory results indicated a white blood cell (WBC) count of 13.5 K/μL (reference range, 4.0-11.0), absolute neutrophil count of 11.48 K/μL (reference range, 2.0-7.7), C-reactive protein (CRP) level of 225.3 mg/L (reference range, ≤ 5.0), erythrocyte sedimentation rate of 85 mm/h (reference range, 5-15), serum blood urea nitrogen of 76 mg/dL (reference range, 8-26), and serum creatinine (SCr) of 1.9 mg/dL (reference range, 1.1-1.4). A urinalysis showed positive leukocyte esterase, WBC clumps, and little bacteria. Abdominal/pelvic computed tomography showed spondylodiscitis-like changes at T7-T8, bilateral perinephric fat stranding, bladder distension, and bladder wall thickening.
The patient was presumed to have discitis secondary to a UTI, with possible pyelonephritis, and was given empiric vancomycin and ceftriaxone. Spinal magnetic resonance imaging with contrast supported spondylodiscitis at T7-T8, extending to T8-T9. Preliminary results from the admission blood and urine cultures showed gram-positive cocci in clusters, which were presumed initially to be Staphylococcus aureus (S aureus).
The final urine culture report listed multiple organisms, predominantly A urinae (Table 1);
On hospital day 6, the patient’s back pain had resolved, micturition was normal, appetite had normalized, and SCr was minimally above baseline (1.4 mg/dL). He insisted on completing antibiotic treatment at home and had no other medical indication for continued hospitalization. Thus, antibiotic therapy was changed to an all-oral regimen of amoxicillin 1 g 3 times daily for 10 days and levofloxacin 750 mg daily for 6 weeks, and the patient was discharged to home.
The patient returned 5 days postdischarge due to anuria. Investigation showed severe acute kidney injury (SCr, 6.8 mg/dL) and bladder outlet obstruction due to phimosis and urethral meatal stenosis. Urinalysis was unremarkable. His CRP had declined from 225 mg/L (initial admission) to 154 mg/L. A urinae culture and 2 sets of blood cultures were finalized as no growth. He was diagnosed with postrenal acute kidney injury and underwent meatal dilation and Foley catheterization but declined surgical correction. When seen in the clinic 2 months postantimicrobial therapy, the patient had normal micturition, no symptoms or signs of infection, and steadily down-trending inflammatory markers.
Discussion
A urinae, historically considered a rare pathogen, has been identified with increasing frequency in urine cultures due to improved microbiologic diagnostic techniques. However, there are only 8 reported cases of A urinae spondylodiscitis. Urinary pathology is an accepted risk factor for A urinae infections; consequently, we suspect that our patient’s urinary outflow obstruction and poor genitourinary hygiene were related to his invasive A urinae infection.10,21,22 We surmise that he had a chronic urinary outflow obstruction contributing to his infection, as evidenced by imaging findings, while the phimosis and urethral meatal stenosis were most likely infectious sequelae considering his anuria and acute kidney injury 5 days postdischarge. Indeed, the correlation between A urinae and urinary tract pathology may justify an evaluation for urinary pathology in any man with A urinae infection, regardless of the presence of symptoms.
By contrast, the implications of A urinae bacteriuria remain unclear. From a public health perspective, A urinae bacteriuria is rare, but the infectious mechanism remains undetermined with a case report suggesting the possibility of sexual transmission.4-6,23 In our case, the patient was not sexually active and had no clear origin of infection. Considering the potential severity of infection, more studies are needed to determine the infectious mechanism of A urinae.
In terms of infectious morbidity, the results seem to vary by sex. In a retrospective study of about 30,000 clinical urine samples, 62 (58 from women, 4 from men) yielded A urinae. The 62 corresponding patients lacked systemic infectious complications, leading the authors to conclude that A urinae is a relatively avirulent organism.24 Although possibly true in women, we are wary of drawing conclusions, especially regarding men, from a study that included only 62 urine samples were A urinae–positive, with only 4 from men. More evidence is needed to define the prognostic implications of A urinae bacteriuria in men.
As illustrated by the present case and previous reports, severe A urinae infections can occur, and the contributory factors deserve consideration. In our patient, the actual mechanism for bacteremia remains unclear. The initial concern for acute pyelonephritis was prompted by a computed tomography finding of bilateral perinephric fat stranding. This finding was questioned because it is common in older patients without infection, hence, is highly nonspecific. A correlation with urinary outflow obstruction may be an important clue in cases like this one.25,26
Furthermore, whether the urinary tract truly was the source of the patient’s bacteremia is clouded by the differing antimicrobial susceptibility patterns of the A urinae blood and urine isolates. The simplest explanation for this discordance may be that all the isolates shared a common initial origin but adapted to different environments in the host (perhaps over time) or laboratory, producing phenotypic variation. Alternatively, the infection could have been polyclonal from the onset, with sampling error leading to the differing detected susceptibility patterns, or the blood and urine isolates may have represented independent acquisition events, involving distinct A urinae strains. Unfortunately (from an academic perspective), given patient preferences and recommendations from the infectious disease consultant, no bone biopsy was done for histology and culture to confirm infection and to allow comparative strain identification if A urinae was isolated.
Optimal treatment for A urinae spondylodiscitis has yet to be established. β-lactams have shown good clinical efficacy despite being bacteriostatic in vitro.27 Early in vitro studies showed synergistic bactericidal synergistic activity with penicillin plus aminoglycoside combination therapies.27-30 Cases of endocarditis have been successfully treated mainly with the combination of a β-lactam plus aminoglycoside combination therapy.30,31 Previous cases of spondylodiscitis have been treated successfully with diverse antimicrobial agents, including clindamycin, β-lactams, cephalosporins, fluoroquinolones, and aminoglycosides.14
Our patient improved rapidly while receiving empiric therapy with vancomycin and ceftriaxone and tolerated a rapid transition to oral amoxicillin and levofloxacin. This is the shortest IV treatment course for A urinae spondylodiscitis reported to date. We suspect that such rapid IV-to-oral transitions will suffice in most stable patients with A urinae spondylodiscitis or other invasive A urinae infections in line with the results of the OVIVA and POET trials.32,33
Conclusions
We believe A urinae UTI in the absence of obvious predisposing factors should prompt evaluation for urinary outflow obstruction. Despite improved laboratory diagnostic techniques, spondylodiscitis related to A urinae remains a rare entity and thus definitive treatment recommendations are difficult to make. However, we suspect that in many cases it is reasonable to extrapolate from the results of the POET and OVIVA trials and rapidly transition therapy of A urinae spondylodiscitis from IV to oral antibiotics. We suspect a review of the US Department of Veterans Affairs population might uncover a higher incidence of A urinae infection than previously estimated due to the population demographics and the epidemiology of A urinae.
Aerococcus urinae (A urinae), a gram-positive coccus readily mistaken for a Staphylococcus species, was first identified in 1992.1-3 It now reportedly accounts for 0.2% to 0.8% of clinical urine isolates.4-6 A urinae bacteriuria is typically asymptomatic and mainly occurs in women.7-9 Symptomatic A urinae urinary tract infection (UTI) occurs predominantly in older men with underlying urologic abnormalities.4-10
Serious A urinae infections are rare. The first 2 reported cases involved men with A urinae endocarditis, one of whom died.11,12 To date, only 8 cases of spondylodiscitis due to A urinae have been reported.13-20 Optimal treatment for invasive A urinae infection is undefined; however, the reported cases were treated successfully with diverse antibiotic regimen combinations; all including a β-lactam and beginning with at least 2 weeks of IV antibiotics.13-20 We describe a man with A urinae bacteremia and spondylodiscitis, presumably arising from a urinary source in the setting of bladder outlet obstruction, who was treated successfully.
Case Presentation
A 74-year-old man with morbid obesity, type 2 diabetes mellitus, stage 2 chronic kidney disease, and tobacco use presented to the emergency department after 2 weeks of progressive, nonradiating, midthoracic back pain, lower extremity weakness, gait imbalance, fatigue, anorexia, rigors, and subjective fevers. On presentation, he was afebrile and hemodynamically stable. A physical examination revealed point tenderness of the midthoracic vertebrae, nontender costovertebral angles, diffusely decreased strength, nonsustained clonus in both lower extremities, inguinal intertrigo, and a buried penis with purulent meatal discharge.
Laboratory results indicated a white blood cell (WBC) count of 13.5 K/μL (reference range, 4.0-11.0), absolute neutrophil count of 11.48 K/μL (reference range, 2.0-7.7), C-reactive protein (CRP) level of 225.3 mg/L (reference range, ≤ 5.0), erythrocyte sedimentation rate of 85 mm/h (reference range, 5-15), serum blood urea nitrogen of 76 mg/dL (reference range, 8-26), and serum creatinine (SCr) of 1.9 mg/dL (reference range, 1.1-1.4). A urinalysis showed positive leukocyte esterase, WBC clumps, and little bacteria. Abdominal/pelvic computed tomography showed spondylodiscitis-like changes at T7-T8, bilateral perinephric fat stranding, bladder distension, and bladder wall thickening.
The patient was presumed to have discitis secondary to a UTI, with possible pyelonephritis, and was given empiric vancomycin and ceftriaxone. Spinal magnetic resonance imaging with contrast supported spondylodiscitis at T7-T8, extending to T8-T9. Preliminary results from the admission blood and urine cultures showed gram-positive cocci in clusters, which were presumed initially to be Staphylococcus aureus (S aureus).
The final urine culture report listed multiple organisms, predominantly A urinae (Table 1);
On hospital day 6, the patient’s back pain had resolved, micturition was normal, appetite had normalized, and SCr was minimally above baseline (1.4 mg/dL). He insisted on completing antibiotic treatment at home and had no other medical indication for continued hospitalization. Thus, antibiotic therapy was changed to an all-oral regimen of amoxicillin 1 g 3 times daily for 10 days and levofloxacin 750 mg daily for 6 weeks, and the patient was discharged to home.
The patient returned 5 days postdischarge due to anuria. Investigation showed severe acute kidney injury (SCr, 6.8 mg/dL) and bladder outlet obstruction due to phimosis and urethral meatal stenosis. Urinalysis was unremarkable. His CRP had declined from 225 mg/L (initial admission) to 154 mg/L. A urinae culture and 2 sets of blood cultures were finalized as no growth. He was diagnosed with postrenal acute kidney injury and underwent meatal dilation and Foley catheterization but declined surgical correction. When seen in the clinic 2 months postantimicrobial therapy, the patient had normal micturition, no symptoms or signs of infection, and steadily down-trending inflammatory markers.
Discussion
A urinae, historically considered a rare pathogen, has been identified with increasing frequency in urine cultures due to improved microbiologic diagnostic techniques. However, there are only 8 reported cases of A urinae spondylodiscitis. Urinary pathology is an accepted risk factor for A urinae infections; consequently, we suspect that our patient’s urinary outflow obstruction and poor genitourinary hygiene were related to his invasive A urinae infection.10,21,22 We surmise that he had a chronic urinary outflow obstruction contributing to his infection, as evidenced by imaging findings, while the phimosis and urethral meatal stenosis were most likely infectious sequelae considering his anuria and acute kidney injury 5 days postdischarge. Indeed, the correlation between A urinae and urinary tract pathology may justify an evaluation for urinary pathology in any man with A urinae infection, regardless of the presence of symptoms.
By contrast, the implications of A urinae bacteriuria remain unclear. From a public health perspective, A urinae bacteriuria is rare, but the infectious mechanism remains undetermined with a case report suggesting the possibility of sexual transmission.4-6,23 In our case, the patient was not sexually active and had no clear origin of infection. Considering the potential severity of infection, more studies are needed to determine the infectious mechanism of A urinae.
In terms of infectious morbidity, the results seem to vary by sex. In a retrospective study of about 30,000 clinical urine samples, 62 (58 from women, 4 from men) yielded A urinae. The 62 corresponding patients lacked systemic infectious complications, leading the authors to conclude that A urinae is a relatively avirulent organism.24 Although possibly true in women, we are wary of drawing conclusions, especially regarding men, from a study that included only 62 urine samples were A urinae–positive, with only 4 from men. More evidence is needed to define the prognostic implications of A urinae bacteriuria in men.
As illustrated by the present case and previous reports, severe A urinae infections can occur, and the contributory factors deserve consideration. In our patient, the actual mechanism for bacteremia remains unclear. The initial concern for acute pyelonephritis was prompted by a computed tomography finding of bilateral perinephric fat stranding. This finding was questioned because it is common in older patients without infection, hence, is highly nonspecific. A correlation with urinary outflow obstruction may be an important clue in cases like this one.25,26
Furthermore, whether the urinary tract truly was the source of the patient’s bacteremia is clouded by the differing antimicrobial susceptibility patterns of the A urinae blood and urine isolates. The simplest explanation for this discordance may be that all the isolates shared a common initial origin but adapted to different environments in the host (perhaps over time) or laboratory, producing phenotypic variation. Alternatively, the infection could have been polyclonal from the onset, with sampling error leading to the differing detected susceptibility patterns, or the blood and urine isolates may have represented independent acquisition events, involving distinct A urinae strains. Unfortunately (from an academic perspective), given patient preferences and recommendations from the infectious disease consultant, no bone biopsy was done for histology and culture to confirm infection and to allow comparative strain identification if A urinae was isolated.
Optimal treatment for A urinae spondylodiscitis has yet to be established. β-lactams have shown good clinical efficacy despite being bacteriostatic in vitro.27 Early in vitro studies showed synergistic bactericidal synergistic activity with penicillin plus aminoglycoside combination therapies.27-30 Cases of endocarditis have been successfully treated mainly with the combination of a β-lactam plus aminoglycoside combination therapy.30,31 Previous cases of spondylodiscitis have been treated successfully with diverse antimicrobial agents, including clindamycin, β-lactams, cephalosporins, fluoroquinolones, and aminoglycosides.14
Our patient improved rapidly while receiving empiric therapy with vancomycin and ceftriaxone and tolerated a rapid transition to oral amoxicillin and levofloxacin. This is the shortest IV treatment course for A urinae spondylodiscitis reported to date. We suspect that such rapid IV-to-oral transitions will suffice in most stable patients with A urinae spondylodiscitis or other invasive A urinae infections in line with the results of the OVIVA and POET trials.32,33
Conclusions
We believe A urinae UTI in the absence of obvious predisposing factors should prompt evaluation for urinary outflow obstruction. Despite improved laboratory diagnostic techniques, spondylodiscitis related to A urinae remains a rare entity and thus definitive treatment recommendations are difficult to make. However, we suspect that in many cases it is reasonable to extrapolate from the results of the POET and OVIVA trials and rapidly transition therapy of A urinae spondylodiscitis from IV to oral antibiotics. We suspect a review of the US Department of Veterans Affairs population might uncover a higher incidence of A urinae infection than previously estimated due to the population demographics and the epidemiology of A urinae.
1. Christensen JJ, Korner B, Kjaergaard H. Aerococcus-like organism—an unnoticed urinary tract pathogen. APMIS. 1989;97(6):539-546. doi:10.1111/j.1699-0463.1989.tb00828.x
2. Aguirre M, Collins MD. Phylogenetic analysis of some Aerococcus-like organisms from urinary tract infections: description of Aerococcus urinae sp. nov. J Gen Microbiol. 1992;138(2):401-405. doi:10.1099/00221287-138-2-401
3. Williams RE, Hirch A, Cowan ST. Aerococcus, a new bacterial genus. J Gen Microbiol. 1953;8(3):475-480. doi:10.1099/00221287-8-3-475
4. Kline KA, Lewis AL. Gram-positive uropathogens, polymicrobial urinary tract infection, and the emerging microbiota of the urinary tract. Microbiol Spectr. 2016;4(2). doi:10.1128/microbiolspec.UTI-0012-2012
5. Schuur PM, Kasteren ME, Sabbe L, Vos MC, Janssens MM, Buiting AG. Urinary tract infections with Aerococcus urinae in the south of The Netherlands. Eur J Clin Microbiol Infect Dis. 1997;16(12):871-875. doi:10.1007/BF01700552
6. Grude N, Tveten Y. Aerococcus urinae og urinveisinfeksjon [Aerococcus urinae and urinary tract infection]. Tidsskr Nor Laegeforen. 2002;122(2):174-175.
7. Narayanasamy S, King K, Dennison A, Spelman DW, Aung AK. Clinical characteristics and laboratory identification of Aerococcus infections: an Australian tertiary centre perspective. Int J Microbiol. 2017;2017. doi:10.1155/2017/5684614
8. Hilt EE, McKinley K, Pearce MM, et al. Urine is not sterile: use of enhanced urine culture techniques to detect resident bacterial flora in the adult female bladder. J Clin Microbiol. 2014;52(3):871-876. doi:10.1128/JCM.02876-13
9. Pearce MM, Hilt EE, Rosenfeld AB, et al. The female urinary microbiome: a comparison of women with and without urgency urinary incontinence. mBio. 2014;5(4):e01283-14. doi:10.1128/mBio.01283-14
10. Sahu KK, Lal A, Mishra AK, Abraham GM. Aerococcus-related infections and their significance: a 9-year retrospective study. J Microsc Ultrastruct. 2021;9(1):18-25. doi:10.4103/JMAU.JMAU_61_19
11. Skov RL, Klarlund M, Thorsen S. Fatal endocarditis due to Aerococcus urinae. Diagn Microbiol Infect Dis. 1995;21(4):219-221. doi:10.1016/0732-8893(95)00037-b
12. Kristensen B, Nielsen G. Endocarditis caused by Aerococcus urinae, a newly recognized pathogen. Eur J Clin Microbiol Infect Dis. 1995;14(1):49-51. doi:10.1007/BF02112619
13. Astudillo L, Sailler L, Porte L, Lefevre JC, Massip P, Arlet-Suau E. Spondylodiscitis due to Aerococcus urinae: a first report. Scand J Infect Dis. 2003;35(11-12):890-891. doi:10.1080/00365540310016664
14. Lyagoubi A, Souffi C, Baroiller V, Vallee E. Spondylodiscitis: an increasingly described localization. EJIFCC. 2020;31(2):169-173.
15. Jerome M, Slim J, Sison R, Marton R. A case of Aerococcus urinae vertebral osteomyelitis. J Glob Infect Dis. 2015;7(2):85-86. doi:10.4103/0974-777X.157246
16. Tekin A, Tekin G, Turunç T, Demiroğlu Z, Kizilkiliç O. Infective endocarditis and spondylodiscitis in a patient due to Aerococcus urinae: first report. Int J Cardiol. 2007;115(3):402-403. doi:10.1016/j.ijcard.2006.01.046
17. Rougier E, Braud A, Argemi X, et al. Spondylodiscitis due to Aerococcus urinae and literature review. Infection. 2018;46(3):419-421. doi:10.1007/s15010-017-1106-0
18. Degroote E, Yildiz H, Lecouvet F, Verroken A, Belkhir L. Aerococcus urinae: an underestimated cause of spine infection? Case report and review of the literature. Acta Clin Belg. 2018;73(6):444-447. doi:10.1080/17843286.2018.1443003
19. Torres-Martos E, Pérez-Cortés S, Sánchez-Calvo JM, López-Prieto MD. Spondylodiscitis due to Aerococcus urinae infection in an elderly immunocompetent patient. Enferm Infecc Microbiol Clin. 2017;35(10):682-684. doi:10.1016/j.eimc.2017.02.005
20. Senneby E, Petersson AC, Rasmussen M. Clinical and microbiological features of bacteraemia with Aerococcus urinae. Clin Microbiol Infect. 2012;18(6):546-550. doi:10.1111/j.1469-0691.2011.03609.x
21. Sunnerhagen T, Nilson B, Olaison L, Rasmussen M. Clinical and microbiological features of infective endocarditis caused by aerococci. Infection. 2016;44(2):167-173. doi:10.1007/s15010-015-0812-8
22. de Jong MF, Soetekouw R, ten Kate RW, Veenendaal D. Aerococcus urinae: severe and fatal bloodstream infections and endocarditis. J Clin Microbiol. 2010;48(9):3445-3447. doi:10.1128/JCM.00835-10
23. Babaeer AA, Nader C, Iacoviello V, Tomera K. Necrotizing urethritis due to Aerococcus urinae. Case Rep Urol. 2015;2015:136147. doi:10.1155/2015/136147
24. Sierra-Hoffman M, Watkins K, Jinadatha C, Fader R, Carpenter JL. Clinical significance of Aerococcus urinae: a retrospective review. Diagn Microbiol Infect Dis. 2005;53(4):289-292. doi:10.1016/j.diagmicrobio.2005.06.021
25. Fukami H, Takeuchi Y, Kagaya S, et al. Perirenal fat stranding is not a powerful diagnostic tool for acute pyelonephritis. Int J Gen Med. 2017;10:137-144. doi:10.2147/IJGM.S133685
26. Han NY, Sung DJ, Kim MJ, Park BJ, Sim KC, Cho SB. Perirenal fat stranding on CT: is there an association with bladder outlet obstruction? Br J Radiol. 2016;89(1063):20160195. doi:10.1259/bjr.20160195
27. Hirzel C, Hirzberger L, Furrer H, Endimiani A. Bactericidal activity of penicillin, ceftriaxone, gentamicin and daptomycin alone and in combination against Aerococcus urinae. Int J Antimicrob Agents. 2016;48(3):271-276. doi:10.1016/j.ijantimicag.2016.05.007
28. Zbinden R, Santanam P, Hunziker L, Leuzinger B, von Graevenitz A. Endocarditis due to Aerococcus urinae: diagnostic tests, fatty acid composition and killing kinetics. Infection. 1999;27(2):122-124. doi:10.1007/BF02560511
29. Skov R, Christensen JJ, Korner B, Frimodt-Møller N, Espersen F. In vitro antimicrobial susceptibility of Aerococcus urinae to 14 antibiotics, and time-kill curves for penicillin, gentamicin and vancomycin. J Antimicrob Chemother. 2001;48(5):653-658. doi:10.1093/jac/48.5.653
30. Ebnöther C, Altwegg M, Gottschalk J, Seebach JD, Kronenberg A. Aerococcus urinae endocarditis: case report and review of the literature. Infection. 2002;30(5):310-313. doi:10.1007/s15010-002-3106-x
31. Tai DBG, Go JR, Fida M, Saleh OA. Management and treatment of Aerococcus bacteremia and endocarditis. Int J Infect Dis. 2021;102:584-589. doi:10.1016/j.ijid.2020.10.096
32. Li H-K, Rombach I, Zambellas R, et al; OVIVA Trial Collaborators. Oral versus intravenous antibiotics for bone and joint infection. N Engl J Med. 2019;380(5):425-436. doi:10.1056/NEJMoa1710926
33. Iversen K, Ihlemann N, Gill SU, et al. Partial oral versus intravenous antibiotic treatment of endocarditis. N Engl J Med. 2019;380(5):415-424. doi:10.1056/NEJMoa1808312
1. Christensen JJ, Korner B, Kjaergaard H. Aerococcus-like organism—an unnoticed urinary tract pathogen. APMIS. 1989;97(6):539-546. doi:10.1111/j.1699-0463.1989.tb00828.x
2. Aguirre M, Collins MD. Phylogenetic analysis of some Aerococcus-like organisms from urinary tract infections: description of Aerococcus urinae sp. nov. J Gen Microbiol. 1992;138(2):401-405. doi:10.1099/00221287-138-2-401
3. Williams RE, Hirch A, Cowan ST. Aerococcus, a new bacterial genus. J Gen Microbiol. 1953;8(3):475-480. doi:10.1099/00221287-8-3-475
4. Kline KA, Lewis AL. Gram-positive uropathogens, polymicrobial urinary tract infection, and the emerging microbiota of the urinary tract. Microbiol Spectr. 2016;4(2). doi:10.1128/microbiolspec.UTI-0012-2012
5. Schuur PM, Kasteren ME, Sabbe L, Vos MC, Janssens MM, Buiting AG. Urinary tract infections with Aerococcus urinae in the south of The Netherlands. Eur J Clin Microbiol Infect Dis. 1997;16(12):871-875. doi:10.1007/BF01700552
6. Grude N, Tveten Y. Aerococcus urinae og urinveisinfeksjon [Aerococcus urinae and urinary tract infection]. Tidsskr Nor Laegeforen. 2002;122(2):174-175.
7. Narayanasamy S, King K, Dennison A, Spelman DW, Aung AK. Clinical characteristics and laboratory identification of Aerococcus infections: an Australian tertiary centre perspective. Int J Microbiol. 2017;2017. doi:10.1155/2017/5684614
8. Hilt EE, McKinley K, Pearce MM, et al. Urine is not sterile: use of enhanced urine culture techniques to detect resident bacterial flora in the adult female bladder. J Clin Microbiol. 2014;52(3):871-876. doi:10.1128/JCM.02876-13
9. Pearce MM, Hilt EE, Rosenfeld AB, et al. The female urinary microbiome: a comparison of women with and without urgency urinary incontinence. mBio. 2014;5(4):e01283-14. doi:10.1128/mBio.01283-14
10. Sahu KK, Lal A, Mishra AK, Abraham GM. Aerococcus-related infections and their significance: a 9-year retrospective study. J Microsc Ultrastruct. 2021;9(1):18-25. doi:10.4103/JMAU.JMAU_61_19
11. Skov RL, Klarlund M, Thorsen S. Fatal endocarditis due to Aerococcus urinae. Diagn Microbiol Infect Dis. 1995;21(4):219-221. doi:10.1016/0732-8893(95)00037-b
12. Kristensen B, Nielsen G. Endocarditis caused by Aerococcus urinae, a newly recognized pathogen. Eur J Clin Microbiol Infect Dis. 1995;14(1):49-51. doi:10.1007/BF02112619
13. Astudillo L, Sailler L, Porte L, Lefevre JC, Massip P, Arlet-Suau E. Spondylodiscitis due to Aerococcus urinae: a first report. Scand J Infect Dis. 2003;35(11-12):890-891. doi:10.1080/00365540310016664
14. Lyagoubi A, Souffi C, Baroiller V, Vallee E. Spondylodiscitis: an increasingly described localization. EJIFCC. 2020;31(2):169-173.
15. Jerome M, Slim J, Sison R, Marton R. A case of Aerococcus urinae vertebral osteomyelitis. J Glob Infect Dis. 2015;7(2):85-86. doi:10.4103/0974-777X.157246
16. Tekin A, Tekin G, Turunç T, Demiroğlu Z, Kizilkiliç O. Infective endocarditis and spondylodiscitis in a patient due to Aerococcus urinae: first report. Int J Cardiol. 2007;115(3):402-403. doi:10.1016/j.ijcard.2006.01.046
17. Rougier E, Braud A, Argemi X, et al. Spondylodiscitis due to Aerococcus urinae and literature review. Infection. 2018;46(3):419-421. doi:10.1007/s15010-017-1106-0
18. Degroote E, Yildiz H, Lecouvet F, Verroken A, Belkhir L. Aerococcus urinae: an underestimated cause of spine infection? Case report and review of the literature. Acta Clin Belg. 2018;73(6):444-447. doi:10.1080/17843286.2018.1443003
19. Torres-Martos E, Pérez-Cortés S, Sánchez-Calvo JM, López-Prieto MD. Spondylodiscitis due to Aerococcus urinae infection in an elderly immunocompetent patient. Enferm Infecc Microbiol Clin. 2017;35(10):682-684. doi:10.1016/j.eimc.2017.02.005
20. Senneby E, Petersson AC, Rasmussen M. Clinical and microbiological features of bacteraemia with Aerococcus urinae. Clin Microbiol Infect. 2012;18(6):546-550. doi:10.1111/j.1469-0691.2011.03609.x
21. Sunnerhagen T, Nilson B, Olaison L, Rasmussen M. Clinical and microbiological features of infective endocarditis caused by aerococci. Infection. 2016;44(2):167-173. doi:10.1007/s15010-015-0812-8
22. de Jong MF, Soetekouw R, ten Kate RW, Veenendaal D. Aerococcus urinae: severe and fatal bloodstream infections and endocarditis. J Clin Microbiol. 2010;48(9):3445-3447. doi:10.1128/JCM.00835-10
23. Babaeer AA, Nader C, Iacoviello V, Tomera K. Necrotizing urethritis due to Aerococcus urinae. Case Rep Urol. 2015;2015:136147. doi:10.1155/2015/136147
24. Sierra-Hoffman M, Watkins K, Jinadatha C, Fader R, Carpenter JL. Clinical significance of Aerococcus urinae: a retrospective review. Diagn Microbiol Infect Dis. 2005;53(4):289-292. doi:10.1016/j.diagmicrobio.2005.06.021
25. Fukami H, Takeuchi Y, Kagaya S, et al. Perirenal fat stranding is not a powerful diagnostic tool for acute pyelonephritis. Int J Gen Med. 2017;10:137-144. doi:10.2147/IJGM.S133685
26. Han NY, Sung DJ, Kim MJ, Park BJ, Sim KC, Cho SB. Perirenal fat stranding on CT: is there an association with bladder outlet obstruction? Br J Radiol. 2016;89(1063):20160195. doi:10.1259/bjr.20160195
27. Hirzel C, Hirzberger L, Furrer H, Endimiani A. Bactericidal activity of penicillin, ceftriaxone, gentamicin and daptomycin alone and in combination against Aerococcus urinae. Int J Antimicrob Agents. 2016;48(3):271-276. doi:10.1016/j.ijantimicag.2016.05.007
28. Zbinden R, Santanam P, Hunziker L, Leuzinger B, von Graevenitz A. Endocarditis due to Aerococcus urinae: diagnostic tests, fatty acid composition and killing kinetics. Infection. 1999;27(2):122-124. doi:10.1007/BF02560511
29. Skov R, Christensen JJ, Korner B, Frimodt-Møller N, Espersen F. In vitro antimicrobial susceptibility of Aerococcus urinae to 14 antibiotics, and time-kill curves for penicillin, gentamicin and vancomycin. J Antimicrob Chemother. 2001;48(5):653-658. doi:10.1093/jac/48.5.653
30. Ebnöther C, Altwegg M, Gottschalk J, Seebach JD, Kronenberg A. Aerococcus urinae endocarditis: case report and review of the literature. Infection. 2002;30(5):310-313. doi:10.1007/s15010-002-3106-x
31. Tai DBG, Go JR, Fida M, Saleh OA. Management and treatment of Aerococcus bacteremia and endocarditis. Int J Infect Dis. 2021;102:584-589. doi:10.1016/j.ijid.2020.10.096
32. Li H-K, Rombach I, Zambellas R, et al; OVIVA Trial Collaborators. Oral versus intravenous antibiotics for bone and joint infection. N Engl J Med. 2019;380(5):425-436. doi:10.1056/NEJMoa1710926
33. Iversen K, Ihlemann N, Gill SU, et al. Partial oral versus intravenous antibiotic treatment of endocarditis. N Engl J Med. 2019;380(5):415-424. doi:10.1056/NEJMoa1808312
Therapeutic Approaches in Advanced Breast Cancer
More than 280,000 women in the United States will be diagnosed with invasive breast cancer this year. For those with metastatic breast cancer with distant spread, the 5-year survival rate is approximately 28%. Whether advanced disease is discovered at initial diagnosis or in relapsed disease, it is imperative to understand the molecular characteristics of the metastatic tumor.
Dr Susan Domchek, from the University of Pennsylvania, discusses the importance of retesting for estrogen receptor, progesterone receptor, and HER2/neu on a metastatic tumor focus in order to identify potential discordance between the primary cancer and metastatic disease.
Additionally, Dr Domchek discusses the importance of molecular testing for targetable mutations, including P13K and germline BRCA1/2, for which approved therapies have shown survival benefit.
The list of targetable mutations in breast cancer continues to expand. In the tumor-agnostic studies, pembrolizumab has shown survival benefit in tumors that have mismatch repair deficiency and microsatellite instability, and TRK inhibitors have shown efficacy in tumors positive for NTRK fusions. Numerous clinical trials are available looking at additional molecular-based therapies.
--
Susan M. Domchek, MD, Basser Professor, Department of Oncology; Executive Director, Basser Center for BRCA, Abramson Cancer Center, University of Pennsylvania, Philadelphia
Susan M. Domchek, MD, has disclosed the following relevant financial relationships:
Received income in an amount equal to or greater than $250 from: AstraZeneca; Clovis; Bristol Myers Squibb
More than 280,000 women in the United States will be diagnosed with invasive breast cancer this year. For those with metastatic breast cancer with distant spread, the 5-year survival rate is approximately 28%. Whether advanced disease is discovered at initial diagnosis or in relapsed disease, it is imperative to understand the molecular characteristics of the metastatic tumor.
Dr Susan Domchek, from the University of Pennsylvania, discusses the importance of retesting for estrogen receptor, progesterone receptor, and HER2/neu on a metastatic tumor focus in order to identify potential discordance between the primary cancer and metastatic disease.
Additionally, Dr Domchek discusses the importance of molecular testing for targetable mutations, including P13K and germline BRCA1/2, for which approved therapies have shown survival benefit.
The list of targetable mutations in breast cancer continues to expand. In the tumor-agnostic studies, pembrolizumab has shown survival benefit in tumors that have mismatch repair deficiency and microsatellite instability, and TRK inhibitors have shown efficacy in tumors positive for NTRK fusions. Numerous clinical trials are available looking at additional molecular-based therapies.
--
Susan M. Domchek, MD, Basser Professor, Department of Oncology; Executive Director, Basser Center for BRCA, Abramson Cancer Center, University of Pennsylvania, Philadelphia
Susan M. Domchek, MD, has disclosed the following relevant financial relationships:
Received income in an amount equal to or greater than $250 from: AstraZeneca; Clovis; Bristol Myers Squibb
More than 280,000 women in the United States will be diagnosed with invasive breast cancer this year. For those with metastatic breast cancer with distant spread, the 5-year survival rate is approximately 28%. Whether advanced disease is discovered at initial diagnosis or in relapsed disease, it is imperative to understand the molecular characteristics of the metastatic tumor.
Dr Susan Domchek, from the University of Pennsylvania, discusses the importance of retesting for estrogen receptor, progesterone receptor, and HER2/neu on a metastatic tumor focus in order to identify potential discordance between the primary cancer and metastatic disease.
Additionally, Dr Domchek discusses the importance of molecular testing for targetable mutations, including P13K and germline BRCA1/2, for which approved therapies have shown survival benefit.
The list of targetable mutations in breast cancer continues to expand. In the tumor-agnostic studies, pembrolizumab has shown survival benefit in tumors that have mismatch repair deficiency and microsatellite instability, and TRK inhibitors have shown efficacy in tumors positive for NTRK fusions. Numerous clinical trials are available looking at additional molecular-based therapies.
--
Susan M. Domchek, MD, Basser Professor, Department of Oncology; Executive Director, Basser Center for BRCA, Abramson Cancer Center, University of Pennsylvania, Philadelphia
Susan M. Domchek, MD, has disclosed the following relevant financial relationships:
Received income in an amount equal to or greater than $250 from: AstraZeneca; Clovis; Bristol Myers Squibb

Perspectives on Hypercortisolism Diagnosis and Management in Community and Academic Centers
Lewis Blevins, MD; Richard Auchus, MD, PhD; David Brown, MD, PhD; Amir Hamrahian, MD; and Smita Kargutkar, MD share their insights and real-world perspectives on hypercortisolism diagnosis and management, including:
• The understanding of hypercortisolism has evolved significantly over the past decades to extend beyond classic physical manifestations (e.g., central obesity, facial plethora, buffalo hump, purple striae)
• Early identification of patients with mild autonomous cortisol secretion is important as hypercortisolism can lead to age-inappropriate and treatment-resistant metabolic syndrome
• Patient identification and management approaches for hypercortisolism can differ between academic and community settings due to differences in available resources and multidisciplinary management teams
• Educating primary care providers and community endocrinologists about the consequences of hypercortisolism can be beneficial in bridging the gap between academic and community settings

Lewis Blevins, MD | 
Richard Auchus, MD, PhD
| 
David Brown, MD, PhD
|

Amir Hamrahian, MD | 
Smita Kargutkar, MD |
Click HERE to read the supplement.
©2022 Corcept Therapeutics Incorporated. All Rights Reserved. DSE-00997 DEC 2022
Lewis Blevins, MD; Richard Auchus, MD, PhD; David Brown, MD, PhD; Amir Hamrahian, MD; and Smita Kargutkar, MD share their insights and real-world perspectives on hypercortisolism diagnosis and management, including:
• The understanding of hypercortisolism has evolved significantly over the past decades to extend beyond classic physical manifestations (e.g., central obesity, facial plethora, buffalo hump, purple striae)
• Early identification of patients with mild autonomous cortisol secretion is important as hypercortisolism can lead to age-inappropriate and treatment-resistant metabolic syndrome
• Patient identification and management approaches for hypercortisolism can differ between academic and community settings due to differences in available resources and multidisciplinary management teams
• Educating primary care providers and community endocrinologists about the consequences of hypercortisolism can be beneficial in bridging the gap between academic and community settings

Lewis Blevins, MD | 
Richard Auchus, MD, PhD
| 
David Brown, MD, PhD
|

Amir Hamrahian, MD | 
Smita Kargutkar, MD |
Click HERE to read the supplement.
©2022 Corcept Therapeutics Incorporated. All Rights Reserved. DSE-00997 DEC 2022
Lewis Blevins, MD; Richard Auchus, MD, PhD; David Brown, MD, PhD; Amir Hamrahian, MD; and Smita Kargutkar, MD share their insights and real-world perspectives on hypercortisolism diagnosis and management, including:
• The understanding of hypercortisolism has evolved significantly over the past decades to extend beyond classic physical manifestations (e.g., central obesity, facial plethora, buffalo hump, purple striae)
• Early identification of patients with mild autonomous cortisol secretion is important as hypercortisolism can lead to age-inappropriate and treatment-resistant metabolic syndrome
• Patient identification and management approaches for hypercortisolism can differ between academic and community settings due to differences in available resources and multidisciplinary management teams
• Educating primary care providers and community endocrinologists about the consequences of hypercortisolism can be beneficial in bridging the gap between academic and community settings

Lewis Blevins, MD | 
Richard Auchus, MD, PhD
| 
David Brown, MD, PhD
|

Amir Hamrahian, MD | 
Smita Kargutkar, MD |
Click HERE to read the supplement.
©2022 Corcept Therapeutics Incorporated. All Rights Reserved. DSE-00997 DEC 2022
MS and Emotional Stress: Is There a Relation?
Sir Augustus d’Este (1794-1848) described the circumstances preceding his development of neurological symptoms as follows:1 “I travelled from Ramsgate to the Highlands of Scotland for the purpose of passing some days with a Relation for whom I had the affection of a Son. On my arrival I found him dead. Shortly after the funeral I was obliged to have my letters read to me, and their answers written for me, as my eyes were so attacked that when fixed upon minute objects indistinctness of vision was the consequence: Soon after I went to Ireland, and without any thing having been done to my eyes, they completely recovered their strength and distinctness of vision…" He then described a clinical course of relapsing-remitting neurologic symptoms merging into a progressive stage of unrelenting illness, most fitting with what we know today as multiple sclerosis (MS).1 Why did Sir Augustus d'Este connect the event of the unexpected death to the onset of a lifelong neurologic disease?
Jean-Martin Charcot first described MS in a way close to what we know it as today. Charcot considered stress a factor in MS. He linked grief, vexation, and adverse changes in social circumstances to the onset of MS at that time.2 I, as a practicing MS specialist, am surprised neither by Sir Augustus d'Este's diary nor by Charcot's earlier assessments of MS triggers.3 As I write this narrative, I think of the many times I heard from people diagnosed with MS. "It happened to me because of stress" is a statement not estranged from my daily clinical practice
MS as a multifactorial disease
It is tempting to make a case for emotional stress as a cause of MS, but one must remember that MS is a very complex disease with unclear etiologies. MS, a treatable but not yet curable disease, is the interplay between the genetics of the host and numerous environmental factors that exploit a susceptible immune system leading to unrelenting immune dysregulation.4 Recent studies have brought some pieces of this intricate puzzle together. The role of Epstein-Barr virus (EBV) in the pathogenesis of MS is being dissected.5 The possible synergy between vitamin D deficiency, EBV, and certain genetic variations is being studied.6 The roles of smoking, environmental toxins, obesity, diet, Western lifestyle, and the gut microbiome are some of the top areas of clinical, translational, and basic research.7-11 But what about emotional stress? Where does it fit, if anywhere, in the current research paradigm?
Emotional stress and MS—Causality or not?
In the scientific method, several criteria must be proven for an element to be suspected in the etiology of a disease.12 First, the suspect element must be present before the disease starts—i.e., a temporal association. Second, there must be a plausible biological explanation of how the suspect element acts in the disease's causation. Third, other variables that could confound the picture must be controlled for or dismissed. It is clear that no single factor is the cause of MS. By now, MS is agreed upon as a disease caused by multiple factors, some of which remain to be unraveled.9 The term "cause" has been utilized more recently by many authors when referring to EBV in relation to MS development, reasoning that in one study, in a small number of individuals with MS, EBV infection preceded the MS clinical diagnosis.13 Thus, the temporal association was provided. But does MS start at the onset of clinical symptoms?
For Sir Augustus d'Este, the disease may have started years before he visited the Highlands of Scotland, but only at that visit did MS become clinically apparent. So, the emotional trauma may have acted as a "trigger" for an MS flare-up rather than being a "cause" of MS. This might be a more plausible explanation of the association between emotional trauma and MS development. However, MS pathogenesis is complex, and one could argue that the disease starts many years before the first clinical symptoms that lead to diagnosis.
The MS prodrome has been demonstrated by several studies that suggest that MS may start many years before the clinical diagnosis.14 Radiologically isolated syndrome (RIS) further argues that MS may be clinically dormant for years, and clinical symptoms may not appear until later in the disease process.15 One may think that immune attacks on the optic nerves, spinal cord, or areas of the brainstem might be readily symptomatic compared to attacks on other structures of the central nervous system (e.g., periventricular or juxtacortical brain areas) that may be clinically silent. So, while for Sir Augustus d'Este it seemed that the disease started at the time of his visit to the Highlands of Scotland, it is equally plausible that it started years before the first clinical attack. Nevertheless, how could emotional stress play a role in the pathophysiology of MS?
Stress and the Immune System
At times of chronic stress, one may become more susceptible to infections. Reactivation of certain viruses can lead to oral ulcers, increased common cold symptoms, or other illnesses. For example, stress can reactivate herpes simplex type 1 and interestingly, EBV.16,17 In MS, the immune system is dysregulated and has an autoimmune component. The effect of acute emotional stress differs from that of chronic stress.18 Several studies have examined the immune responses to both forms of stress.19-21
Interestingly, acute stress activates cell-mediated immunity, increases immune cell trafficking to areas of injury, and, importantly, increases blood-brain barrier (BBB) permeability by activating resident mast cells in the brain and other areas, including the optic nerves.22,23 Mast cell activation leads to BBB disruption, which is a key early step in the pathogenesis of MS. Thus, it is plausible that the proinflammatory changes associated with acute stress could be implicated in the pathogenesis of MS. This contrasts with chronic stress, which attenuates various immune responses, including suppressing cell-mediated immunity, but also dysregulate the immune system.
One could establish a biological plausibility for stress playing a role in the proinflammatory responses in MS. Whether it is causal or not, scientists can further explore the potential biologic explanations. While studying the association between acute stress and MS development or disease activity is difficult, several groups have examined the potential association. Many studies, however, have limitations due to the difficult nature of studying such an association, especially in quantifying or defining acute stress in general.
A limited number of studies on MS and stress: What do we know? And what are the challenges?
Rare studies have reported a potential association between MS development and stressful life events, while others reported no association.24-26 Also, some studies observed an increase in MS relapses or the development of new magnetic resonance imaging (MRI) lesions following stressful life events or wartime, while others failed to show such an association.26-30 There are few studies directly addressing the potential association between acute emotional stress and MS. The results of published studies are variable, and limitations are numerous. Limitations include the difficulty in measuring acute emotional stress, difficulty in its prediction, and ethical challenges of experimental design and recruiting participants. So, studies have focused on observational aspects, retrospective reviews, and surveys of memories prone to various biases. Rarely was the design of these clinical studies prospective. A few prospective studies reported an association between stressful life events and increased MS relapses and increased number of brain lesions.27,31,32 Rare clinical trials have attempted to test stress reduction strategies and reported on the modest improvement of patient-reported outcomes and, in one study, a modest improvement in new MRI lesions.33-35
Overall, several lines of evidence support a potential association between acute emotional stress and MS. Yet, the association is challenging to study, and future research might focus on stress-mitigation strategies and improving coping mechanisms in persons living with MS. It is important to note that it will be very difficult to design prospective studies to examine the potential association between acute emotional trauma and the development of de novo MS. Such studies will require a large number of participants (e.g., hundreds of thousands), long durations of follow-up (e.g., decades), and ways to classify repeated stressful events. An alternative approach is to ask persons newly diagnosed with MS at the time of initial diagnosis about any temporal association between their first symptom and stressful life events. However, this approach would provide some information on any association between the two, but not on causality of the disease itself.
Conclusion
The potential association between acute emotional stress and MS dates to the times of early descriptions of MS. Yet, research has been very limited and challenging. To date, the potential association remains elusive. Lines of evidence, while with limitations, have provided possible biologic explanations for the relationship between MS symptom onset and acute emotional stress. Although avoiding acute emotional stress is nearly impossible, incorporating global stress-coping strategies in early childhood education and secondary education might theoretically have potential beneficial effects on the subsequent risk of MS development or symptom flare-up, depending on a variety of factors.
But for now, when patients and colleagues ask me, “Can acute emotional stress be a ‘trigger’ for MS symptomology?,” my answer will remain, “Potentially, until proven otherwise.”
- Firth D. The case of Augustus d'Este (1794-1848): the first account of disseminated sclerosis: (section of the History of Medicine). Proc R Soc Med. 1941;34(7):381-384.
- Lectures on the diseases of the nervous system. Br Foreign Med Chir Rev. 1877;60(119):180-181.
- Obeidat, A, Cope T. Stressful life events and multiple sclerosis: a call for re-evaluation. Paper presented at: Fifth Cooperative Meeting of the Consortium of Multiple Sclerosis Centers; May 13, 2013; Orlando, FL.
- Waubant E, Lucas R, Mowry E, et al. Environmental and genetic risk factors for MS: an integrated review. Ann Clin Transl Neurol. 2019;6(9):1905-1922. doi:10.1002/acn3.50862
- Soldan SS, Lieberman PM. Epstein-Barr virus and multiple sclerosis. Nat Rev Microbiol. 2022;1-14. doi:10.1038/s41579-022-00770-5
- Marcucci SB, Obeidat AZ. EBNA1, EBNA2, and EBNA3 link Epstein-Barr virus and hypovitaminosis D in multiple sclerosis pathogenesis. J Neuroimmunol. 2020;339:57711 doi:10.1016/j.jneuroim.2019.577116
- Alfredsson L, Olsson T. Lifestyle and environmental factors in multiple sclerosis. Cold Spring Harb Perspect Med. 2019;9(4):a028944. doi:10.1101/cshperspect.a028944
- Thompson AJ, Baranzini SE, Geurts J, Hemmer B, Ciccarelli O. Multiple sclerosis. Lancet. 2018;391(10130):1622-1636. doi:10.1016/S0140-6736(18)30481-1
- Dobson R, Giovannoni G. Multiple sclerosis – a review. Eur J Neurol. 2019;26(1):27-40. doi:10.1111/ene.13819
- Arneth B. Multiple sclerosis and smoking. Am J Med. 2020;133(7):783-788. doi:1016/j.amjmed.2020.03.008
- Correale J, Hohlfeld R, Baranzini SE. The role of the gut microbiota in multiple sclerosis. Nat Rev Neurol. 2022;18(9):544-558. doi:10.1038/s41582-022-00697-8
- Gianicolo EAL, Eichler M, Muensterer O, Strauch K, Blettner M. Methods for evaluating causality in observational studies. Dtsch Arztebl Int. 2020;116(7):101-107. doi:10.3238/arztebl.2020.0101
- Bjornevik K, Cortese M, Healy BC, et al. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science. 2022;375(6578):296-301. doi:10.1126/science.abj8222
- Makhani N, Tremlett H. The multiple sclerosis prodrome. Nat Rev Neurol. 2021;17(8):515-521. doi:10.1038/s41582-021-00519-3
- Hosseiny M, Newsome SD, Yousem DM. Radiologically isolated syndrome: a review for neuroradiologists. AJNR Am J Neuroradiol. 2020;41(9):1542-1549. doi:10.3174/ajnr.A6649
- Padgett DA, Sheridan JF, Dorne J, Berntson GG, Candelora J, Glaser R. Social stress and the reactivation of latent herpes simplex virus type 1 [published correction appears in Proc Natl Acad Sci U S A. 1998;95(20):12070]. Proc Natl Acad Sci U S A. 1998;95(12):7231-7235. doi:10.1073/pnas.95.12.7231
- Glaser R, Pearson GR, Jones JF, et al. Stress-related activation of Epstein-Barr virus. Brain Behav Immun. 1991;5(2):219-232. doi:10.1016/0889-1591(91)90018-6
- Dhabhar FS. Enhancing versus suppressive effects of stress on immune function: implications for immunoprotection and immunopathology. Neuroimmunomodulation. 2009;16(5):300-317. doi:10.1159/000216188
- Musazzi L, Tornese P, Sala N, Popoli M. Acute or chronic? A stressful question. Trends Neurosci. 2017;40(9):525-535. doi:10.1016/j.tins.2017.07.002
- Dhabhar FS, McEwen BS. Acute stress enhances while chronic stress suppresses cell-mediated immunity in vivo: a potential role for leukocyte trafficking. Brain Behav Immun. 1997;11(4):286-306. doi:10.1006/brbi.1997.0508
- Maydych V, Claus M, Dychus N, et al. Impact of chronic and acute academic stress on lymphocyte subsets and monocyte function. PLoS One. 2017;12(11):e0188108. Published 2017 Nov 16. doi:10.1371/journal.pone.0188108
- Esposito P, Gheorghe D, Kandere K, et al. Acute stress increases permeability of the blood-brain-barrier through activation of brain mast cells. Brain Res. 2001;888(1):117-127. doi:10.1016/s0006-8993(00)03026-2
- Kempuraj D, Mentor S, Thangavel R, et al. Mast cells in stress, pain, blood-brain barrier, neuroinflammation and Alzheimer's disease. Front Cell Neurosci. 2019;13:54. doi:10.3389/fncel.2019.00054
- Karagkouni A, Alevizos M, Theoharides TC. Effect of stress on brain inflammation and multiple sclerosis. Autoimmun Rev. 2013;12(10):947-953. doi:10.1016/j.autrev.2013.02.006
- Briones-Buixassa L, Milà R, Mª Aragonès J, Bufill E, Olaya B, Arrufat FX. Stress and multiple sclerosis: a systematic review considering potential moderating and mediating factors and methods of assessing stress. Health Psychol Open. 2015;2(2):2055102915612271. doi:10.1177/2055102915612271
- Riise T, Mohr DC, Munger KL, Rich-Edwards JW, Kawachi I, Ascherio A. Stress and the risk of multiple sclerosis. Neurology. 2011;76(22):1866-1871. doi:10.1212/WNL.0b013e31821d74c5
- Burns MN, Nawacki E, Kwasny MJ, Pelletier D, Mohr DC. Do positive or negative stressful events predict the development of new brain lesions in people with multiple sclerosis? Psychol Med. 2014;44(2):349-359. doi:10.1017/S0033291713000755
- Mohr DC, Goodkin DE, Bacchetti P, et al. Psychological stress and the subsequent appearance of new brain MRI lesions in MS. Neurology. 2000;55(1):55-61. doi:10.1212/wnl.55.1.55
- Yamout B, Itani S, Hourany R, Sibaii AM, Yaghi S. The effect of war stress on multiple sclerosis exacerbations and radiological disease activity. J Neurol Sci. 2010;288(1-2):42-44. doi:10.1016/j.jns.2009.10.012
- Artemiadis AK, Anagnostouli MC, Alexopoulos EC. Stress as a risk factor for multiple sclerosis onset or relapse: a systematic review. Neuroepidemiology. 2011;36(2):109-120. doi:10.1159/000323953
- Brown RF, Tennant CC, Sharrock M, Hodgkinson S, Dunn SM, Pollard JD. Relationship between stress and relapse in multiple sclerosis: Part I. Important features. Mult Scler. 2006;12(4):453-464. doi:10.1191/1352458506ms1295oa
- Buljevac D, Hop WCJ, Reedeker W, et al. Self-reported stressful life events and exacerbations in multiple sclerosis: prospective study. BMJ. 2003;327(7416):646. doi:10.1136/bmj.327.7416.646
- Senders A, Hanes D, Bourdette D, Carson K, Marshall LM, Shinto L. Impact of mindfulness-based stress reduction for people with multiple sclerosis at 8 weeks and 12 months: A randomized clinical trial. Mult Scler. 2019;25(8):1178-1188. doi:10.1177/1352458518786650
- Morrow SA, Riccio P, Vording N, Rosehart H, Casserly C, MacDougall A. A mindfulness group intervention in newly diagnosed persons with multiple sclerosis: A pilot study. Mult Scler Relat Disord. 2021;52:103016. doi:10.1016/j.msard.2021.103016
- Mohr DC, Lovera J, Brown T, et al. A randomized trial of stress management for the prevention of new brain lesions in MS. Neurology. 2012;79(5):412-419. doi:10.1212/WNL.0b013e3182616ff9
Sir Augustus d’Este (1794-1848) described the circumstances preceding his development of neurological symptoms as follows:1 “I travelled from Ramsgate to the Highlands of Scotland for the purpose of passing some days with a Relation for whom I had the affection of a Son. On my arrival I found him dead. Shortly after the funeral I was obliged to have my letters read to me, and their answers written for me, as my eyes were so attacked that when fixed upon minute objects indistinctness of vision was the consequence: Soon after I went to Ireland, and without any thing having been done to my eyes, they completely recovered their strength and distinctness of vision…" He then described a clinical course of relapsing-remitting neurologic symptoms merging into a progressive stage of unrelenting illness, most fitting with what we know today as multiple sclerosis (MS).1 Why did Sir Augustus d'Este connect the event of the unexpected death to the onset of a lifelong neurologic disease?
Jean-Martin Charcot first described MS in a way close to what we know it as today. Charcot considered stress a factor in MS. He linked grief, vexation, and adverse changes in social circumstances to the onset of MS at that time.2 I, as a practicing MS specialist, am surprised neither by Sir Augustus d'Este's diary nor by Charcot's earlier assessments of MS triggers.3 As I write this narrative, I think of the many times I heard from people diagnosed with MS. "It happened to me because of stress" is a statement not estranged from my daily clinical practice
MS as a multifactorial disease
It is tempting to make a case for emotional stress as a cause of MS, but one must remember that MS is a very complex disease with unclear etiologies. MS, a treatable but not yet curable disease, is the interplay between the genetics of the host and numerous environmental factors that exploit a susceptible immune system leading to unrelenting immune dysregulation.4 Recent studies have brought some pieces of this intricate puzzle together. The role of Epstein-Barr virus (EBV) in the pathogenesis of MS is being dissected.5 The possible synergy between vitamin D deficiency, EBV, and certain genetic variations is being studied.6 The roles of smoking, environmental toxins, obesity, diet, Western lifestyle, and the gut microbiome are some of the top areas of clinical, translational, and basic research.7-11 But what about emotional stress? Where does it fit, if anywhere, in the current research paradigm?
Emotional stress and MS—Causality or not?
In the scientific method, several criteria must be proven for an element to be suspected in the etiology of a disease.12 First, the suspect element must be present before the disease starts—i.e., a temporal association. Second, there must be a plausible biological explanation of how the suspect element acts in the disease's causation. Third, other variables that could confound the picture must be controlled for or dismissed. It is clear that no single factor is the cause of MS. By now, MS is agreed upon as a disease caused by multiple factors, some of which remain to be unraveled.9 The term "cause" has been utilized more recently by many authors when referring to EBV in relation to MS development, reasoning that in one study, in a small number of individuals with MS, EBV infection preceded the MS clinical diagnosis.13 Thus, the temporal association was provided. But does MS start at the onset of clinical symptoms?
For Sir Augustus d'Este, the disease may have started years before he visited the Highlands of Scotland, but only at that visit did MS become clinically apparent. So, the emotional trauma may have acted as a "trigger" for an MS flare-up rather than being a "cause" of MS. This might be a more plausible explanation of the association between emotional trauma and MS development. However, MS pathogenesis is complex, and one could argue that the disease starts many years before the first clinical symptoms that lead to diagnosis.
The MS prodrome has been demonstrated by several studies that suggest that MS may start many years before the clinical diagnosis.14 Radiologically isolated syndrome (RIS) further argues that MS may be clinically dormant for years, and clinical symptoms may not appear until later in the disease process.15 One may think that immune attacks on the optic nerves, spinal cord, or areas of the brainstem might be readily symptomatic compared to attacks on other structures of the central nervous system (e.g., periventricular or juxtacortical brain areas) that may be clinically silent. So, while for Sir Augustus d'Este it seemed that the disease started at the time of his visit to the Highlands of Scotland, it is equally plausible that it started years before the first clinical attack. Nevertheless, how could emotional stress play a role in the pathophysiology of MS?
Stress and the Immune System
At times of chronic stress, one may become more susceptible to infections. Reactivation of certain viruses can lead to oral ulcers, increased common cold symptoms, or other illnesses. For example, stress can reactivate herpes simplex type 1 and interestingly, EBV.16,17 In MS, the immune system is dysregulated and has an autoimmune component. The effect of acute emotional stress differs from that of chronic stress.18 Several studies have examined the immune responses to both forms of stress.19-21
Interestingly, acute stress activates cell-mediated immunity, increases immune cell trafficking to areas of injury, and, importantly, increases blood-brain barrier (BBB) permeability by activating resident mast cells in the brain and other areas, including the optic nerves.22,23 Mast cell activation leads to BBB disruption, which is a key early step in the pathogenesis of MS. Thus, it is plausible that the proinflammatory changes associated with acute stress could be implicated in the pathogenesis of MS. This contrasts with chronic stress, which attenuates various immune responses, including suppressing cell-mediated immunity, but also dysregulate the immune system.
One could establish a biological plausibility for stress playing a role in the proinflammatory responses in MS. Whether it is causal or not, scientists can further explore the potential biologic explanations. While studying the association between acute stress and MS development or disease activity is difficult, several groups have examined the potential association. Many studies, however, have limitations due to the difficult nature of studying such an association, especially in quantifying or defining acute stress in general.
A limited number of studies on MS and stress: What do we know? And what are the challenges?
Rare studies have reported a potential association between MS development and stressful life events, while others reported no association.24-26 Also, some studies observed an increase in MS relapses or the development of new magnetic resonance imaging (MRI) lesions following stressful life events or wartime, while others failed to show such an association.26-30 There are few studies directly addressing the potential association between acute emotional stress and MS. The results of published studies are variable, and limitations are numerous. Limitations include the difficulty in measuring acute emotional stress, difficulty in its prediction, and ethical challenges of experimental design and recruiting participants. So, studies have focused on observational aspects, retrospective reviews, and surveys of memories prone to various biases. Rarely was the design of these clinical studies prospective. A few prospective studies reported an association between stressful life events and increased MS relapses and increased number of brain lesions.27,31,32 Rare clinical trials have attempted to test stress reduction strategies and reported on the modest improvement of patient-reported outcomes and, in one study, a modest improvement in new MRI lesions.33-35
Overall, several lines of evidence support a potential association between acute emotional stress and MS. Yet, the association is challenging to study, and future research might focus on stress-mitigation strategies and improving coping mechanisms in persons living with MS. It is important to note that it will be very difficult to design prospective studies to examine the potential association between acute emotional trauma and the development of de novo MS. Such studies will require a large number of participants (e.g., hundreds of thousands), long durations of follow-up (e.g., decades), and ways to classify repeated stressful events. An alternative approach is to ask persons newly diagnosed with MS at the time of initial diagnosis about any temporal association between their first symptom and stressful life events. However, this approach would provide some information on any association between the two, but not on causality of the disease itself.
Conclusion
The potential association between acute emotional stress and MS dates to the times of early descriptions of MS. Yet, research has been very limited and challenging. To date, the potential association remains elusive. Lines of evidence, while with limitations, have provided possible biologic explanations for the relationship between MS symptom onset and acute emotional stress. Although avoiding acute emotional stress is nearly impossible, incorporating global stress-coping strategies in early childhood education and secondary education might theoretically have potential beneficial effects on the subsequent risk of MS development or symptom flare-up, depending on a variety of factors.
But for now, when patients and colleagues ask me, “Can acute emotional stress be a ‘trigger’ for MS symptomology?,” my answer will remain, “Potentially, until proven otherwise.”
Sir Augustus d’Este (1794-1848) described the circumstances preceding his development of neurological symptoms as follows:1 “I travelled from Ramsgate to the Highlands of Scotland for the purpose of passing some days with a Relation for whom I had the affection of a Son. On my arrival I found him dead. Shortly after the funeral I was obliged to have my letters read to me, and their answers written for me, as my eyes were so attacked that when fixed upon minute objects indistinctness of vision was the consequence: Soon after I went to Ireland, and without any thing having been done to my eyes, they completely recovered their strength and distinctness of vision…" He then described a clinical course of relapsing-remitting neurologic symptoms merging into a progressive stage of unrelenting illness, most fitting with what we know today as multiple sclerosis (MS).1 Why did Sir Augustus d'Este connect the event of the unexpected death to the onset of a lifelong neurologic disease?
Jean-Martin Charcot first described MS in a way close to what we know it as today. Charcot considered stress a factor in MS. He linked grief, vexation, and adverse changes in social circumstances to the onset of MS at that time.2 I, as a practicing MS specialist, am surprised neither by Sir Augustus d'Este's diary nor by Charcot's earlier assessments of MS triggers.3 As I write this narrative, I think of the many times I heard from people diagnosed with MS. "It happened to me because of stress" is a statement not estranged from my daily clinical practice
MS as a multifactorial disease
It is tempting to make a case for emotional stress as a cause of MS, but one must remember that MS is a very complex disease with unclear etiologies. MS, a treatable but not yet curable disease, is the interplay between the genetics of the host and numerous environmental factors that exploit a susceptible immune system leading to unrelenting immune dysregulation.4 Recent studies have brought some pieces of this intricate puzzle together. The role of Epstein-Barr virus (EBV) in the pathogenesis of MS is being dissected.5 The possible synergy between vitamin D deficiency, EBV, and certain genetic variations is being studied.6 The roles of smoking, environmental toxins, obesity, diet, Western lifestyle, and the gut microbiome are some of the top areas of clinical, translational, and basic research.7-11 But what about emotional stress? Where does it fit, if anywhere, in the current research paradigm?
Emotional stress and MS—Causality or not?
In the scientific method, several criteria must be proven for an element to be suspected in the etiology of a disease.12 First, the suspect element must be present before the disease starts—i.e., a temporal association. Second, there must be a plausible biological explanation of how the suspect element acts in the disease's causation. Third, other variables that could confound the picture must be controlled for or dismissed. It is clear that no single factor is the cause of MS. By now, MS is agreed upon as a disease caused by multiple factors, some of which remain to be unraveled.9 The term "cause" has been utilized more recently by many authors when referring to EBV in relation to MS development, reasoning that in one study, in a small number of individuals with MS, EBV infection preceded the MS clinical diagnosis.13 Thus, the temporal association was provided. But does MS start at the onset of clinical symptoms?
For Sir Augustus d'Este, the disease may have started years before he visited the Highlands of Scotland, but only at that visit did MS become clinically apparent. So, the emotional trauma may have acted as a "trigger" for an MS flare-up rather than being a "cause" of MS. This might be a more plausible explanation of the association between emotional trauma and MS development. However, MS pathogenesis is complex, and one could argue that the disease starts many years before the first clinical symptoms that lead to diagnosis.
The MS prodrome has been demonstrated by several studies that suggest that MS may start many years before the clinical diagnosis.14 Radiologically isolated syndrome (RIS) further argues that MS may be clinically dormant for years, and clinical symptoms may not appear until later in the disease process.15 One may think that immune attacks on the optic nerves, spinal cord, or areas of the brainstem might be readily symptomatic compared to attacks on other structures of the central nervous system (e.g., periventricular or juxtacortical brain areas) that may be clinically silent. So, while for Sir Augustus d'Este it seemed that the disease started at the time of his visit to the Highlands of Scotland, it is equally plausible that it started years before the first clinical attack. Nevertheless, how could emotional stress play a role in the pathophysiology of MS?
Stress and the Immune System
At times of chronic stress, one may become more susceptible to infections. Reactivation of certain viruses can lead to oral ulcers, increased common cold symptoms, or other illnesses. For example, stress can reactivate herpes simplex type 1 and interestingly, EBV.16,17 In MS, the immune system is dysregulated and has an autoimmune component. The effect of acute emotional stress differs from that of chronic stress.18 Several studies have examined the immune responses to both forms of stress.19-21
Interestingly, acute stress activates cell-mediated immunity, increases immune cell trafficking to areas of injury, and, importantly, increases blood-brain barrier (BBB) permeability by activating resident mast cells in the brain and other areas, including the optic nerves.22,23 Mast cell activation leads to BBB disruption, which is a key early step in the pathogenesis of MS. Thus, it is plausible that the proinflammatory changes associated with acute stress could be implicated in the pathogenesis of MS. This contrasts with chronic stress, which attenuates various immune responses, including suppressing cell-mediated immunity, but also dysregulate the immune system.
One could establish a biological plausibility for stress playing a role in the proinflammatory responses in MS. Whether it is causal or not, scientists can further explore the potential biologic explanations. While studying the association between acute stress and MS development or disease activity is difficult, several groups have examined the potential association. Many studies, however, have limitations due to the difficult nature of studying such an association, especially in quantifying or defining acute stress in general.
A limited number of studies on MS and stress: What do we know? And what are the challenges?
Rare studies have reported a potential association between MS development and stressful life events, while others reported no association.24-26 Also, some studies observed an increase in MS relapses or the development of new magnetic resonance imaging (MRI) lesions following stressful life events or wartime, while others failed to show such an association.26-30 There are few studies directly addressing the potential association between acute emotional stress and MS. The results of published studies are variable, and limitations are numerous. Limitations include the difficulty in measuring acute emotional stress, difficulty in its prediction, and ethical challenges of experimental design and recruiting participants. So, studies have focused on observational aspects, retrospective reviews, and surveys of memories prone to various biases. Rarely was the design of these clinical studies prospective. A few prospective studies reported an association between stressful life events and increased MS relapses and increased number of brain lesions.27,31,32 Rare clinical trials have attempted to test stress reduction strategies and reported on the modest improvement of patient-reported outcomes and, in one study, a modest improvement in new MRI lesions.33-35
Overall, several lines of evidence support a potential association between acute emotional stress and MS. Yet, the association is challenging to study, and future research might focus on stress-mitigation strategies and improving coping mechanisms in persons living with MS. It is important to note that it will be very difficult to design prospective studies to examine the potential association between acute emotional trauma and the development of de novo MS. Such studies will require a large number of participants (e.g., hundreds of thousands), long durations of follow-up (e.g., decades), and ways to classify repeated stressful events. An alternative approach is to ask persons newly diagnosed with MS at the time of initial diagnosis about any temporal association between their first symptom and stressful life events. However, this approach would provide some information on any association between the two, but not on causality of the disease itself.
Conclusion
The potential association between acute emotional stress and MS dates to the times of early descriptions of MS. Yet, research has been very limited and challenging. To date, the potential association remains elusive. Lines of evidence, while with limitations, have provided possible biologic explanations for the relationship between MS symptom onset and acute emotional stress. Although avoiding acute emotional stress is nearly impossible, incorporating global stress-coping strategies in early childhood education and secondary education might theoretically have potential beneficial effects on the subsequent risk of MS development or symptom flare-up, depending on a variety of factors.
But for now, when patients and colleagues ask me, “Can acute emotional stress be a ‘trigger’ for MS symptomology?,” my answer will remain, “Potentially, until proven otherwise.”
- Firth D. The case of Augustus d'Este (1794-1848): the first account of disseminated sclerosis: (section of the History of Medicine). Proc R Soc Med. 1941;34(7):381-384.
- Lectures on the diseases of the nervous system. Br Foreign Med Chir Rev. 1877;60(119):180-181.
- Obeidat, A, Cope T. Stressful life events and multiple sclerosis: a call for re-evaluation. Paper presented at: Fifth Cooperative Meeting of the Consortium of Multiple Sclerosis Centers; May 13, 2013; Orlando, FL.
- Waubant E, Lucas R, Mowry E, et al. Environmental and genetic risk factors for MS: an integrated review. Ann Clin Transl Neurol. 2019;6(9):1905-1922. doi:10.1002/acn3.50862
- Soldan SS, Lieberman PM. Epstein-Barr virus and multiple sclerosis. Nat Rev Microbiol. 2022;1-14. doi:10.1038/s41579-022-00770-5
- Marcucci SB, Obeidat AZ. EBNA1, EBNA2, and EBNA3 link Epstein-Barr virus and hypovitaminosis D in multiple sclerosis pathogenesis. J Neuroimmunol. 2020;339:57711 doi:10.1016/j.jneuroim.2019.577116
- Alfredsson L, Olsson T. Lifestyle and environmental factors in multiple sclerosis. Cold Spring Harb Perspect Med. 2019;9(4):a028944. doi:10.1101/cshperspect.a028944
- Thompson AJ, Baranzini SE, Geurts J, Hemmer B, Ciccarelli O. Multiple sclerosis. Lancet. 2018;391(10130):1622-1636. doi:10.1016/S0140-6736(18)30481-1
- Dobson R, Giovannoni G. Multiple sclerosis – a review. Eur J Neurol. 2019;26(1):27-40. doi:10.1111/ene.13819
- Arneth B. Multiple sclerosis and smoking. Am J Med. 2020;133(7):783-788. doi:1016/j.amjmed.2020.03.008
- Correale J, Hohlfeld R, Baranzini SE. The role of the gut microbiota in multiple sclerosis. Nat Rev Neurol. 2022;18(9):544-558. doi:10.1038/s41582-022-00697-8
- Gianicolo EAL, Eichler M, Muensterer O, Strauch K, Blettner M. Methods for evaluating causality in observational studies. Dtsch Arztebl Int. 2020;116(7):101-107. doi:10.3238/arztebl.2020.0101
- Bjornevik K, Cortese M, Healy BC, et al. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science. 2022;375(6578):296-301. doi:10.1126/science.abj8222
- Makhani N, Tremlett H. The multiple sclerosis prodrome. Nat Rev Neurol. 2021;17(8):515-521. doi:10.1038/s41582-021-00519-3
- Hosseiny M, Newsome SD, Yousem DM. Radiologically isolated syndrome: a review for neuroradiologists. AJNR Am J Neuroradiol. 2020;41(9):1542-1549. doi:10.3174/ajnr.A6649
- Padgett DA, Sheridan JF, Dorne J, Berntson GG, Candelora J, Glaser R. Social stress and the reactivation of latent herpes simplex virus type 1 [published correction appears in Proc Natl Acad Sci U S A. 1998;95(20):12070]. Proc Natl Acad Sci U S A. 1998;95(12):7231-7235. doi:10.1073/pnas.95.12.7231
- Glaser R, Pearson GR, Jones JF, et al. Stress-related activation of Epstein-Barr virus. Brain Behav Immun. 1991;5(2):219-232. doi:10.1016/0889-1591(91)90018-6
- Dhabhar FS. Enhancing versus suppressive effects of stress on immune function: implications for immunoprotection and immunopathology. Neuroimmunomodulation. 2009;16(5):300-317. doi:10.1159/000216188
- Musazzi L, Tornese P, Sala N, Popoli M. Acute or chronic? A stressful question. Trends Neurosci. 2017;40(9):525-535. doi:10.1016/j.tins.2017.07.002
- Dhabhar FS, McEwen BS. Acute stress enhances while chronic stress suppresses cell-mediated immunity in vivo: a potential role for leukocyte trafficking. Brain Behav Immun. 1997;11(4):286-306. doi:10.1006/brbi.1997.0508
- Maydych V, Claus M, Dychus N, et al. Impact of chronic and acute academic stress on lymphocyte subsets and monocyte function. PLoS One. 2017;12(11):e0188108. Published 2017 Nov 16. doi:10.1371/journal.pone.0188108
- Esposito P, Gheorghe D, Kandere K, et al. Acute stress increases permeability of the blood-brain-barrier through activation of brain mast cells. Brain Res. 2001;888(1):117-127. doi:10.1016/s0006-8993(00)03026-2
- Kempuraj D, Mentor S, Thangavel R, et al. Mast cells in stress, pain, blood-brain barrier, neuroinflammation and Alzheimer's disease. Front Cell Neurosci. 2019;13:54. doi:10.3389/fncel.2019.00054
- Karagkouni A, Alevizos M, Theoharides TC. Effect of stress on brain inflammation and multiple sclerosis. Autoimmun Rev. 2013;12(10):947-953. doi:10.1016/j.autrev.2013.02.006
- Briones-Buixassa L, Milà R, Mª Aragonès J, Bufill E, Olaya B, Arrufat FX. Stress and multiple sclerosis: a systematic review considering potential moderating and mediating factors and methods of assessing stress. Health Psychol Open. 2015;2(2):2055102915612271. doi:10.1177/2055102915612271
- Riise T, Mohr DC, Munger KL, Rich-Edwards JW, Kawachi I, Ascherio A. Stress and the risk of multiple sclerosis. Neurology. 2011;76(22):1866-1871. doi:10.1212/WNL.0b013e31821d74c5
- Burns MN, Nawacki E, Kwasny MJ, Pelletier D, Mohr DC. Do positive or negative stressful events predict the development of new brain lesions in people with multiple sclerosis? Psychol Med. 2014;44(2):349-359. doi:10.1017/S0033291713000755
- Mohr DC, Goodkin DE, Bacchetti P, et al. Psychological stress and the subsequent appearance of new brain MRI lesions in MS. Neurology. 2000;55(1):55-61. doi:10.1212/wnl.55.1.55
- Yamout B, Itani S, Hourany R, Sibaii AM, Yaghi S. The effect of war stress on multiple sclerosis exacerbations and radiological disease activity. J Neurol Sci. 2010;288(1-2):42-44. doi:10.1016/j.jns.2009.10.012
- Artemiadis AK, Anagnostouli MC, Alexopoulos EC. Stress as a risk factor for multiple sclerosis onset or relapse: a systematic review. Neuroepidemiology. 2011;36(2):109-120. doi:10.1159/000323953
- Brown RF, Tennant CC, Sharrock M, Hodgkinson S, Dunn SM, Pollard JD. Relationship between stress and relapse in multiple sclerosis: Part I. Important features. Mult Scler. 2006;12(4):453-464. doi:10.1191/1352458506ms1295oa
- Buljevac D, Hop WCJ, Reedeker W, et al. Self-reported stressful life events and exacerbations in multiple sclerosis: prospective study. BMJ. 2003;327(7416):646. doi:10.1136/bmj.327.7416.646
- Senders A, Hanes D, Bourdette D, Carson K, Marshall LM, Shinto L. Impact of mindfulness-based stress reduction for people with multiple sclerosis at 8 weeks and 12 months: A randomized clinical trial. Mult Scler. 2019;25(8):1178-1188. doi:10.1177/1352458518786650
- Morrow SA, Riccio P, Vording N, Rosehart H, Casserly C, MacDougall A. A mindfulness group intervention in newly diagnosed persons with multiple sclerosis: A pilot study. Mult Scler Relat Disord. 2021;52:103016. doi:10.1016/j.msard.2021.103016
- Mohr DC, Lovera J, Brown T, et al. A randomized trial of stress management for the prevention of new brain lesions in MS. Neurology. 2012;79(5):412-419. doi:10.1212/WNL.0b013e3182616ff9
- Firth D. The case of Augustus d'Este (1794-1848): the first account of disseminated sclerosis: (section of the History of Medicine). Proc R Soc Med. 1941;34(7):381-384.
- Lectures on the diseases of the nervous system. Br Foreign Med Chir Rev. 1877;60(119):180-181.
- Obeidat, A, Cope T. Stressful life events and multiple sclerosis: a call for re-evaluation. Paper presented at: Fifth Cooperative Meeting of the Consortium of Multiple Sclerosis Centers; May 13, 2013; Orlando, FL.
- Waubant E, Lucas R, Mowry E, et al. Environmental and genetic risk factors for MS: an integrated review. Ann Clin Transl Neurol. 2019;6(9):1905-1922. doi:10.1002/acn3.50862
- Soldan SS, Lieberman PM. Epstein-Barr virus and multiple sclerosis. Nat Rev Microbiol. 2022;1-14. doi:10.1038/s41579-022-00770-5
- Marcucci SB, Obeidat AZ. EBNA1, EBNA2, and EBNA3 link Epstein-Barr virus and hypovitaminosis D in multiple sclerosis pathogenesis. J Neuroimmunol. 2020;339:57711 doi:10.1016/j.jneuroim.2019.577116
- Alfredsson L, Olsson T. Lifestyle and environmental factors in multiple sclerosis. Cold Spring Harb Perspect Med. 2019;9(4):a028944. doi:10.1101/cshperspect.a028944
- Thompson AJ, Baranzini SE, Geurts J, Hemmer B, Ciccarelli O. Multiple sclerosis. Lancet. 2018;391(10130):1622-1636. doi:10.1016/S0140-6736(18)30481-1
- Dobson R, Giovannoni G. Multiple sclerosis – a review. Eur J Neurol. 2019;26(1):27-40. doi:10.1111/ene.13819
- Arneth B. Multiple sclerosis and smoking. Am J Med. 2020;133(7):783-788. doi:1016/j.amjmed.2020.03.008
- Correale J, Hohlfeld R, Baranzini SE. The role of the gut microbiota in multiple sclerosis. Nat Rev Neurol. 2022;18(9):544-558. doi:10.1038/s41582-022-00697-8
- Gianicolo EAL, Eichler M, Muensterer O, Strauch K, Blettner M. Methods for evaluating causality in observational studies. Dtsch Arztebl Int. 2020;116(7):101-107. doi:10.3238/arztebl.2020.0101
- Bjornevik K, Cortese M, Healy BC, et al. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science. 2022;375(6578):296-301. doi:10.1126/science.abj8222
- Makhani N, Tremlett H. The multiple sclerosis prodrome. Nat Rev Neurol. 2021;17(8):515-521. doi:10.1038/s41582-021-00519-3
- Hosseiny M, Newsome SD, Yousem DM. Radiologically isolated syndrome: a review for neuroradiologists. AJNR Am J Neuroradiol. 2020;41(9):1542-1549. doi:10.3174/ajnr.A6649
- Padgett DA, Sheridan JF, Dorne J, Berntson GG, Candelora J, Glaser R. Social stress and the reactivation of latent herpes simplex virus type 1 [published correction appears in Proc Natl Acad Sci U S A. 1998;95(20):12070]. Proc Natl Acad Sci U S A. 1998;95(12):7231-7235. doi:10.1073/pnas.95.12.7231
- Glaser R, Pearson GR, Jones JF, et al. Stress-related activation of Epstein-Barr virus. Brain Behav Immun. 1991;5(2):219-232. doi:10.1016/0889-1591(91)90018-6
- Dhabhar FS. Enhancing versus suppressive effects of stress on immune function: implications for immunoprotection and immunopathology. Neuroimmunomodulation. 2009;16(5):300-317. doi:10.1159/000216188
- Musazzi L, Tornese P, Sala N, Popoli M. Acute or chronic? A stressful question. Trends Neurosci. 2017;40(9):525-535. doi:10.1016/j.tins.2017.07.002
- Dhabhar FS, McEwen BS. Acute stress enhances while chronic stress suppresses cell-mediated immunity in vivo: a potential role for leukocyte trafficking. Brain Behav Immun. 1997;11(4):286-306. doi:10.1006/brbi.1997.0508
- Maydych V, Claus M, Dychus N, et al. Impact of chronic and acute academic stress on lymphocyte subsets and monocyte function. PLoS One. 2017;12(11):e0188108. Published 2017 Nov 16. doi:10.1371/journal.pone.0188108
- Esposito P, Gheorghe D, Kandere K, et al. Acute stress increases permeability of the blood-brain-barrier through activation of brain mast cells. Brain Res. 2001;888(1):117-127. doi:10.1016/s0006-8993(00)03026-2
- Kempuraj D, Mentor S, Thangavel R, et al. Mast cells in stress, pain, blood-brain barrier, neuroinflammation and Alzheimer's disease. Front Cell Neurosci. 2019;13:54. doi:10.3389/fncel.2019.00054
- Karagkouni A, Alevizos M, Theoharides TC. Effect of stress on brain inflammation and multiple sclerosis. Autoimmun Rev. 2013;12(10):947-953. doi:10.1016/j.autrev.2013.02.006
- Briones-Buixassa L, Milà R, Mª Aragonès J, Bufill E, Olaya B, Arrufat FX. Stress and multiple sclerosis: a systematic review considering potential moderating and mediating factors and methods of assessing stress. Health Psychol Open. 2015;2(2):2055102915612271. doi:10.1177/2055102915612271
- Riise T, Mohr DC, Munger KL, Rich-Edwards JW, Kawachi I, Ascherio A. Stress and the risk of multiple sclerosis. Neurology. 2011;76(22):1866-1871. doi:10.1212/WNL.0b013e31821d74c5
- Burns MN, Nawacki E, Kwasny MJ, Pelletier D, Mohr DC. Do positive or negative stressful events predict the development of new brain lesions in people with multiple sclerosis? Psychol Med. 2014;44(2):349-359. doi:10.1017/S0033291713000755
- Mohr DC, Goodkin DE, Bacchetti P, et al. Psychological stress and the subsequent appearance of new brain MRI lesions in MS. Neurology. 2000;55(1):55-61. doi:10.1212/wnl.55.1.55
- Yamout B, Itani S, Hourany R, Sibaii AM, Yaghi S. The effect of war stress on multiple sclerosis exacerbations and radiological disease activity. J Neurol Sci. 2010;288(1-2):42-44. doi:10.1016/j.jns.2009.10.012
- Artemiadis AK, Anagnostouli MC, Alexopoulos EC. Stress as a risk factor for multiple sclerosis onset or relapse: a systematic review. Neuroepidemiology. 2011;36(2):109-120. doi:10.1159/000323953
- Brown RF, Tennant CC, Sharrock M, Hodgkinson S, Dunn SM, Pollard JD. Relationship between stress and relapse in multiple sclerosis: Part I. Important features. Mult Scler. 2006;12(4):453-464. doi:10.1191/1352458506ms1295oa
- Buljevac D, Hop WCJ, Reedeker W, et al. Self-reported stressful life events and exacerbations in multiple sclerosis: prospective study. BMJ. 2003;327(7416):646. doi:10.1136/bmj.327.7416.646
- Senders A, Hanes D, Bourdette D, Carson K, Marshall LM, Shinto L. Impact of mindfulness-based stress reduction for people with multiple sclerosis at 8 weeks and 12 months: A randomized clinical trial. Mult Scler. 2019;25(8):1178-1188. doi:10.1177/1352458518786650
- Morrow SA, Riccio P, Vording N, Rosehart H, Casserly C, MacDougall A. A mindfulness group intervention in newly diagnosed persons with multiple sclerosis: A pilot study. Mult Scler Relat Disord. 2021;52:103016. doi:10.1016/j.msard.2021.103016
- Mohr DC, Lovera J, Brown T, et al. A randomized trial of stress management for the prevention of new brain lesions in MS. Neurology. 2012;79(5):412-419. doi:10.1212/WNL.0b013e3182616ff9