User login
Dupilumab safe and effective in the elderly with moderate-to-severe atopic dermatitis
Key clinical point: Dupilumab is safe and improves atopic dermatitis (AD) signs and symptoms in patients aged ≥60 years with moderate-to-severe AD.
Major finding: At week 16, similar to the <60-year group, a significantly higher proportion of patients receiving dupilumab (every 2 weeks or every week) vs placebo in the ≥60-year group achieved an Investigator’s Global Assessment score of 0 or 1 (44.4% or 39.7% vs 7.1%, respectively; both P < .0001) and a 75% improvement in the Eczema Area and Severity Index (63.0% or 61.6% vs 14.3%, respectively; both P < .0001). Most treatment-emergent adverse events were of mild-to-moderate severity.
Study details: This post hoc pooled analysis of four phase 3 trials included 2444 patients (≥60 years, n = 183; <60 years, n = 2261) with moderate-to-severe AD who were randomly assigned to receive dupilumab or placebo.
Disclosures: This study was funded by Sanofi-Regeneron Pharmaceuticals Inc. Some authors reported various ties, including employment, with Sanofi, Regeneron, or others.
Source: Silverberg JI et al. Efficacy and safety of dupilumab maintained in adults ≥ 60 years of age with moderate-to-severe atopic dermatitis: Analysis of pooled data from four randomized clinical trials. Am J Clin Dermatol. 2023 (Feb 20). Doi: 10.1007/s40257-022-00754-4
Key clinical point: Dupilumab is safe and improves atopic dermatitis (AD) signs and symptoms in patients aged ≥60 years with moderate-to-severe AD.
Major finding: At week 16, similar to the <60-year group, a significantly higher proportion of patients receiving dupilumab (every 2 weeks or every week) vs placebo in the ≥60-year group achieved an Investigator’s Global Assessment score of 0 or 1 (44.4% or 39.7% vs 7.1%, respectively; both P < .0001) and a 75% improvement in the Eczema Area and Severity Index (63.0% or 61.6% vs 14.3%, respectively; both P < .0001). Most treatment-emergent adverse events were of mild-to-moderate severity.
Study details: This post hoc pooled analysis of four phase 3 trials included 2444 patients (≥60 years, n = 183; <60 years, n = 2261) with moderate-to-severe AD who were randomly assigned to receive dupilumab or placebo.
Disclosures: This study was funded by Sanofi-Regeneron Pharmaceuticals Inc. Some authors reported various ties, including employment, with Sanofi, Regeneron, or others.
Source: Silverberg JI et al. Efficacy and safety of dupilumab maintained in adults ≥ 60 years of age with moderate-to-severe atopic dermatitis: Analysis of pooled data from four randomized clinical trials. Am J Clin Dermatol. 2023 (Feb 20). Doi: 10.1007/s40257-022-00754-4
Key clinical point: Dupilumab is safe and improves atopic dermatitis (AD) signs and symptoms in patients aged ≥60 years with moderate-to-severe AD.
Major finding: At week 16, similar to the <60-year group, a significantly higher proportion of patients receiving dupilumab (every 2 weeks or every week) vs placebo in the ≥60-year group achieved an Investigator’s Global Assessment score of 0 or 1 (44.4% or 39.7% vs 7.1%, respectively; both P < .0001) and a 75% improvement in the Eczema Area and Severity Index (63.0% or 61.6% vs 14.3%, respectively; both P < .0001). Most treatment-emergent adverse events were of mild-to-moderate severity.
Study details: This post hoc pooled analysis of four phase 3 trials included 2444 patients (≥60 years, n = 183; <60 years, n = 2261) with moderate-to-severe AD who were randomly assigned to receive dupilumab or placebo.
Disclosures: This study was funded by Sanofi-Regeneron Pharmaceuticals Inc. Some authors reported various ties, including employment, with Sanofi, Regeneron, or others.
Source: Silverberg JI et al. Efficacy and safety of dupilumab maintained in adults ≥ 60 years of age with moderate-to-severe atopic dermatitis: Analysis of pooled data from four randomized clinical trials. Am J Clin Dermatol. 2023 (Feb 20). Doi: 10.1007/s40257-022-00754-4
Antibody-Drug Conjugates: Targeted Treatments Providing Hope for Patients With Breast Cancer
The restrictive therapeutic index of chemotherapy has led to the emergence of antibody-drug conjugates (ADCs), medicines that combine the specificity of monoclonal antibodies (mAbs) with the cytotoxic effects of chemotherapy to deliver cytotoxic payloads to cancer cells. This targeted approach can reduce the side effects of chemotherapy and improve the effectiveness of treatment. Several ADCs, including ado-trastuzumab emtansine (Kadcyla), sacituzumab govitecan-hziy (Trodelvy), and fam-trastuzumab deruxtecan-nxki (Enhertu), are currently approved for treating some types of breast cancer (BC).
The ADC trastuzumab emtansine was approved specifically for treating human epidermal growth factor receptor 2 positive (HER2+) metastatic breast cancer (mBC) in patients who have previously been treated with trastuzumab and a taxane (paclitaxel or docetaxel) and who have already been treated for mBC or have developed tumor recurrence within 6 months of receiving adjuvant therapy. The US Food and Drug Administration (FDA) approval was based on the results of the EMILIA study, a phase 3 clinical trial that compared treatment with trastuzumab emtansine vs capecitabine + lapatinib in participants with HER2+, locally advanced, or metastatic BC. This trial emerged from the need for well-tolerated, HER2-directed therapies for patients with this type of cancer. Trastuzumab emtansine consists of trastuzumab, a mAb that targets HER2 (which is overexpressed in about 20% of BCs), linked to emtansine, a cytotoxic payload that inhibits cell division. The trastuzumab emtansine group had a median overall survival (OS) of 29.9 months vs 25.9 months in the capecitabine + lapatinib group, for a hazard ratio (HR) of 0.75 (95% CI: 0.64, 0.88).
Another ADC, sacituzumab govitecan, targets the Trop-2 protein, which is overexpressed in BC. This ADC includes a mAb that is linked to SN-38, a cytotoxic payload that inhibits DNA replication. Triple-negative breast cancer (TNBC) is a subtype of BC that does not have receptors for estrogen, progesterone, or HER2—making it more difficult to treat than other forms of BC. Sacituzumab govitecan is used to treat patients with metastatic TNBC who have received at least 2 prior therapies for metastatic disease. Sacituzumab govitecan is also approved for the treatment of patients with unresectable locally advanced or metastatic hormone-receptor–positive (HR+), and HER2-negative (HER2−) BC who have received endocrine-based therapy and at least 2 additional systemic therapies in the metastatic setting. Sacituzumab govitecan was the first Trop-2–directed ADC to demonstrate OS benefit in patients with HR+/HER2− mBC who had received prior endocrine-based therapy and at least 2 chemotherapies. It is now also recommended as a Category 1 preferred treatment for metastatic HR+/HER2− BC by the National Comprehensive Cancer Network.
The results of the TROPiCS-02 study, which led to the FDA approval of sacituzumab govitecan, demonstrated a median OS of 14.4 months with sacituzumab govitecan vs 11.2 months with treatment of physician’s choice (HR, 0.79; 95% CI: 0.65, 0.96; P = 0.02). This represents a 3.2-month improvement in survival and a 21% reduction in the risk for patient death. Before this medicine was approved, there were limited options to offer patients with BC after endocrine-based therapy and chemotherapy.
A third ADC, trastuzumab deruxtecan, targets the HER2 protein, like trastuzumab emtansine, but with a different cytotoxic payload. It consists of trastuzumab linked to deruxtecan, whose cytotoxicity inhibits DNA replication. It is approved for the treatment of HER2+ mBC. Its FDA approval was based on the results of the DESTINY-Breast04 phase 3 clinical trial, which demonstrated that treatment with trastuzumab deruxtecan, when compared with standard-of-care chemotherapy, doubles progression-free survival among patients with mBC that expresses low levels of HER2. The median OS for patients in the HR+ group who received trastuzumab deruxtecan was 23.9 months vs 17.5 months for those who received chemotherapy. In the hormone receptor-negative (HR−) group, the median OS for those who took trastuzumab deruxtecan was 16.6 months vs 10.3 months for those treated with chemotherapy.
The emergence of ADCs have demonstrated promising advancements in the treatment of BC, particularly in patients with HER2+ or triple-negative disease. ADCs have given new hope to and prolonged life for patients living with pretreated HR+/HER2− mBC. ADCs also have the potential to provide a more effective and less toxic treatment option for patients with BC. However, further research is needed to fully understand their long-term effects and to develop new ADCs that target other types of BC.
The restrictive therapeutic index of chemotherapy has led to the emergence of antibody-drug conjugates (ADCs), medicines that combine the specificity of monoclonal antibodies (mAbs) with the cytotoxic effects of chemotherapy to deliver cytotoxic payloads to cancer cells. This targeted approach can reduce the side effects of chemotherapy and improve the effectiveness of treatment. Several ADCs, including ado-trastuzumab emtansine (Kadcyla), sacituzumab govitecan-hziy (Trodelvy), and fam-trastuzumab deruxtecan-nxki (Enhertu), are currently approved for treating some types of breast cancer (BC).
The ADC trastuzumab emtansine was approved specifically for treating human epidermal growth factor receptor 2 positive (HER2+) metastatic breast cancer (mBC) in patients who have previously been treated with trastuzumab and a taxane (paclitaxel or docetaxel) and who have already been treated for mBC or have developed tumor recurrence within 6 months of receiving adjuvant therapy. The US Food and Drug Administration (FDA) approval was based on the results of the EMILIA study, a phase 3 clinical trial that compared treatment with trastuzumab emtansine vs capecitabine + lapatinib in participants with HER2+, locally advanced, or metastatic BC. This trial emerged from the need for well-tolerated, HER2-directed therapies for patients with this type of cancer. Trastuzumab emtansine consists of trastuzumab, a mAb that targets HER2 (which is overexpressed in about 20% of BCs), linked to emtansine, a cytotoxic payload that inhibits cell division. The trastuzumab emtansine group had a median overall survival (OS) of 29.9 months vs 25.9 months in the capecitabine + lapatinib group, for a hazard ratio (HR) of 0.75 (95% CI: 0.64, 0.88).
Another ADC, sacituzumab govitecan, targets the Trop-2 protein, which is overexpressed in BC. This ADC includes a mAb that is linked to SN-38, a cytotoxic payload that inhibits DNA replication. Triple-negative breast cancer (TNBC) is a subtype of BC that does not have receptors for estrogen, progesterone, or HER2—making it more difficult to treat than other forms of BC. Sacituzumab govitecan is used to treat patients with metastatic TNBC who have received at least 2 prior therapies for metastatic disease. Sacituzumab govitecan is also approved for the treatment of patients with unresectable locally advanced or metastatic hormone-receptor–positive (HR+), and HER2-negative (HER2−) BC who have received endocrine-based therapy and at least 2 additional systemic therapies in the metastatic setting. Sacituzumab govitecan was the first Trop-2–directed ADC to demonstrate OS benefit in patients with HR+/HER2− mBC who had received prior endocrine-based therapy and at least 2 chemotherapies. It is now also recommended as a Category 1 preferred treatment for metastatic HR+/HER2− BC by the National Comprehensive Cancer Network.
The results of the TROPiCS-02 study, which led to the FDA approval of sacituzumab govitecan, demonstrated a median OS of 14.4 months with sacituzumab govitecan vs 11.2 months with treatment of physician’s choice (HR, 0.79; 95% CI: 0.65, 0.96; P = 0.02). This represents a 3.2-month improvement in survival and a 21% reduction in the risk for patient death. Before this medicine was approved, there were limited options to offer patients with BC after endocrine-based therapy and chemotherapy.
A third ADC, trastuzumab deruxtecan, targets the HER2 protein, like trastuzumab emtansine, but with a different cytotoxic payload. It consists of trastuzumab linked to deruxtecan, whose cytotoxicity inhibits DNA replication. It is approved for the treatment of HER2+ mBC. Its FDA approval was based on the results of the DESTINY-Breast04 phase 3 clinical trial, which demonstrated that treatment with trastuzumab deruxtecan, when compared with standard-of-care chemotherapy, doubles progression-free survival among patients with mBC that expresses low levels of HER2. The median OS for patients in the HR+ group who received trastuzumab deruxtecan was 23.9 months vs 17.5 months for those who received chemotherapy. In the hormone receptor-negative (HR−) group, the median OS for those who took trastuzumab deruxtecan was 16.6 months vs 10.3 months for those treated with chemotherapy.
The emergence of ADCs have demonstrated promising advancements in the treatment of BC, particularly in patients with HER2+ or triple-negative disease. ADCs have given new hope to and prolonged life for patients living with pretreated HR+/HER2− mBC. ADCs also have the potential to provide a more effective and less toxic treatment option for patients with BC. However, further research is needed to fully understand their long-term effects and to develop new ADCs that target other types of BC.
The restrictive therapeutic index of chemotherapy has led to the emergence of antibody-drug conjugates (ADCs), medicines that combine the specificity of monoclonal antibodies (mAbs) with the cytotoxic effects of chemotherapy to deliver cytotoxic payloads to cancer cells. This targeted approach can reduce the side effects of chemotherapy and improve the effectiveness of treatment. Several ADCs, including ado-trastuzumab emtansine (Kadcyla), sacituzumab govitecan-hziy (Trodelvy), and fam-trastuzumab deruxtecan-nxki (Enhertu), are currently approved for treating some types of breast cancer (BC).
The ADC trastuzumab emtansine was approved specifically for treating human epidermal growth factor receptor 2 positive (HER2+) metastatic breast cancer (mBC) in patients who have previously been treated with trastuzumab and a taxane (paclitaxel or docetaxel) and who have already been treated for mBC or have developed tumor recurrence within 6 months of receiving adjuvant therapy. The US Food and Drug Administration (FDA) approval was based on the results of the EMILIA study, a phase 3 clinical trial that compared treatment with trastuzumab emtansine vs capecitabine + lapatinib in participants with HER2+, locally advanced, or metastatic BC. This trial emerged from the need for well-tolerated, HER2-directed therapies for patients with this type of cancer. Trastuzumab emtansine consists of trastuzumab, a mAb that targets HER2 (which is overexpressed in about 20% of BCs), linked to emtansine, a cytotoxic payload that inhibits cell division. The trastuzumab emtansine group had a median overall survival (OS) of 29.9 months vs 25.9 months in the capecitabine + lapatinib group, for a hazard ratio (HR) of 0.75 (95% CI: 0.64, 0.88).
Another ADC, sacituzumab govitecan, targets the Trop-2 protein, which is overexpressed in BC. This ADC includes a mAb that is linked to SN-38, a cytotoxic payload that inhibits DNA replication. Triple-negative breast cancer (TNBC) is a subtype of BC that does not have receptors for estrogen, progesterone, or HER2—making it more difficult to treat than other forms of BC. Sacituzumab govitecan is used to treat patients with metastatic TNBC who have received at least 2 prior therapies for metastatic disease. Sacituzumab govitecan is also approved for the treatment of patients with unresectable locally advanced or metastatic hormone-receptor–positive (HR+), and HER2-negative (HER2−) BC who have received endocrine-based therapy and at least 2 additional systemic therapies in the metastatic setting. Sacituzumab govitecan was the first Trop-2–directed ADC to demonstrate OS benefit in patients with HR+/HER2− mBC who had received prior endocrine-based therapy and at least 2 chemotherapies. It is now also recommended as a Category 1 preferred treatment for metastatic HR+/HER2− BC by the National Comprehensive Cancer Network.
The results of the TROPiCS-02 study, which led to the FDA approval of sacituzumab govitecan, demonstrated a median OS of 14.4 months with sacituzumab govitecan vs 11.2 months with treatment of physician’s choice (HR, 0.79; 95% CI: 0.65, 0.96; P = 0.02). This represents a 3.2-month improvement in survival and a 21% reduction in the risk for patient death. Before this medicine was approved, there were limited options to offer patients with BC after endocrine-based therapy and chemotherapy.
A third ADC, trastuzumab deruxtecan, targets the HER2 protein, like trastuzumab emtansine, but with a different cytotoxic payload. It consists of trastuzumab linked to deruxtecan, whose cytotoxicity inhibits DNA replication. It is approved for the treatment of HER2+ mBC. Its FDA approval was based on the results of the DESTINY-Breast04 phase 3 clinical trial, which demonstrated that treatment with trastuzumab deruxtecan, when compared with standard-of-care chemotherapy, doubles progression-free survival among patients with mBC that expresses low levels of HER2. The median OS for patients in the HR+ group who received trastuzumab deruxtecan was 23.9 months vs 17.5 months for those who received chemotherapy. In the hormone receptor-negative (HR−) group, the median OS for those who took trastuzumab deruxtecan was 16.6 months vs 10.3 months for those treated with chemotherapy.
The emergence of ADCs have demonstrated promising advancements in the treatment of BC, particularly in patients with HER2+ or triple-negative disease. ADCs have given new hope to and prolonged life for patients living with pretreated HR+/HER2− mBC. ADCs also have the potential to provide a more effective and less toxic treatment option for patients with BC. However, further research is needed to fully understand their long-term effects and to develop new ADCs that target other types of BC.
Hair Repigmentation as a Melanoma Warning Sign
To the Editor:
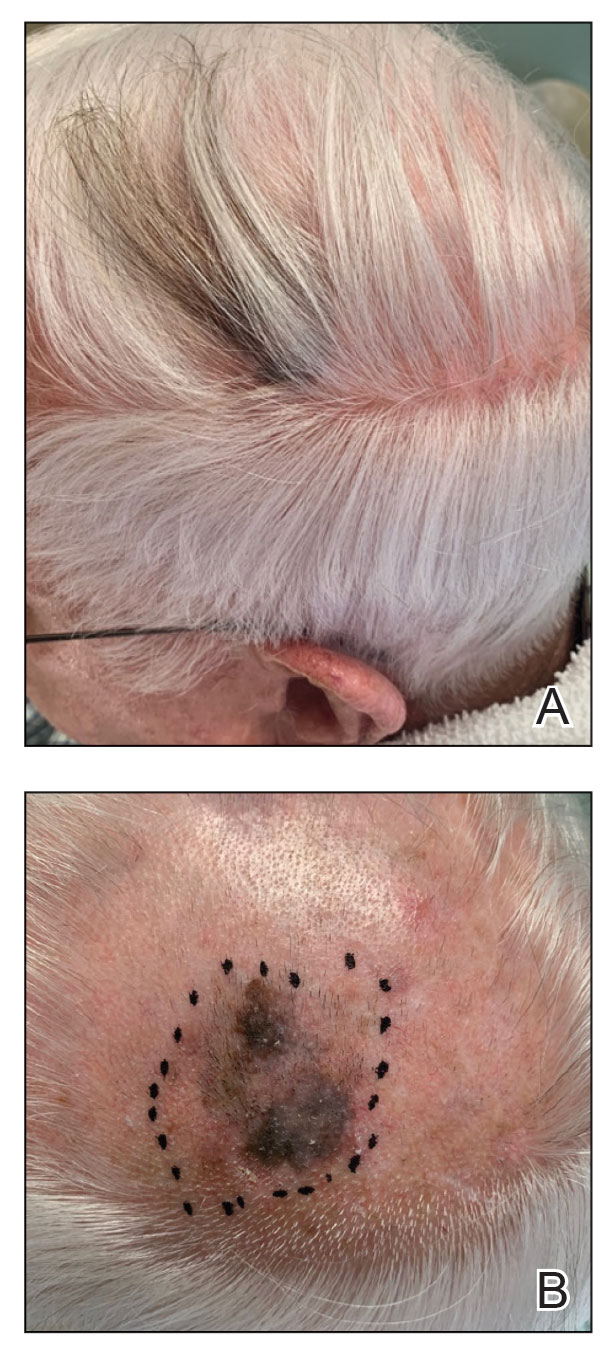
An 85-year-old man with a history of hypertension and chronic kidney disease presented with a localized darkening patch of hair on the left parietal scalp that had progressed over the last 7 years (Figure 1A). He had no prior history of skin cancer. Physical examination revealed the remainder of the hair was gray. There was an irregularly pigmented plaque on the skin underlying the darkened hair measuring 5.0 cm in diameter that was confirmed to be melanoma (Figure 1B). He underwent a staged excision to remove the lesion. The surgical defect was closed via a 5.0×6.0-cm full-thickness skin graft.
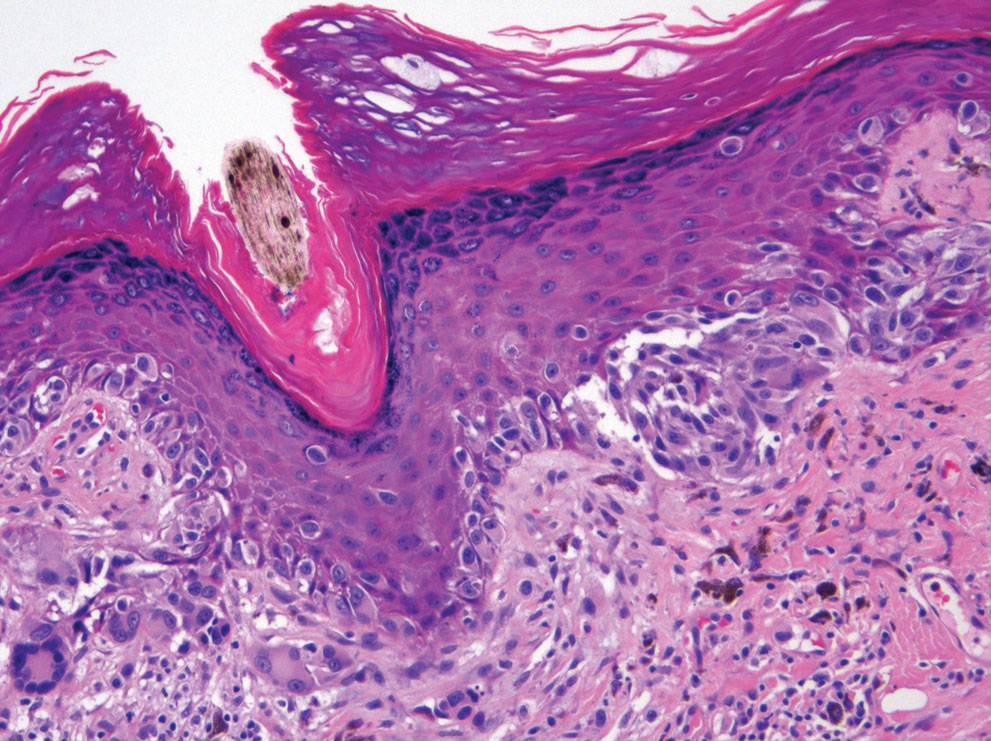
The initial biopsy showed melanoma in situ. However, the final pathology report following the excision revealed an invasive melanoma with a Breslow depth of 1.0 mm (Clark level IV; American Joint Committee on Cancer T1b).1 Histopathology showed pigment deposition with surrounding deep follicular extension of melanoma (Figure 2).
The patient declined a sentinel lymph node biopsy and agreed to a genetic profile assessment.2 The results of the test identified the patient had a low probability of a positive sentinel lymph node and the lowest risk of melanoma recurrence within 5 years. The patient was clear of disease at 12-month follow-up.
Based on a PubMed search of articles indexed for MEDLINE using the terms hair repigmentation and melanoma, there have been 11 other reported cases of hair repigmentation associated with melanoma (Table).3-13 It initially was suspected that this rare phenomenon primarily existed in the female population, as the first 5 cases were reported solely in females,3-7 possibly due to the prevalence of androgenetic alopecia in males.11 However, 6 cases of repigmentation associated with melanoma were later reported in males8-13; our patient represents an additional reported case in a male. It is unknown if there is a higher prevalence of this phenomenon among males or females.

Most previously reported cases of repigmentation were associated with melanoma in situ, lentigo maligna type. Repigmentation also has been reported in malignant melanoma, as documented in our patient, as well as desmoplastic and amelanotic melanoma.5,6 In every case, the color of the repigmentation was darker than the rest of the patient’s hair; however, the repigmentation color can be different from the patient’s original hair color from their youth.4,5,11
The exact mechanism responsible for hair repigmentation in the setting of melanoma is unclear. It has been speculated from prior cases that repigmentation may be caused by paracrine stimulation from melanoma cells activating adjacent benign hair follicle melanocytes to produce melanin.7,14,15 This process likely is due to cytokines or growth factors, such as c-kit ligand.14,15 Several neural and immune networks and mediators activate the receptor tyrosine kinase KIT, which is thought to play a role in activating melanogenesis within the hair bulb.14 These signals also could originate from changes in the microenvironment instead of the melanoma cells themselves.6 Another possible mechanism is that repigmentation was caused by melanin-producing malignant melanocytes.4
Because this phenomenon typically occurs in older patients, the cause of repigmentation also could be related to chronic sun damage, which may result in upregulation of stem cell factor and α-melanocyte–stimulating hormone, as well as other molecules associated with melanogenesis, such as c-KIT receptor and tyrosinase.15,16 Upregulation of these molecules can lead to an increased number of melanocytes within the hair bulb. In addition, UVA and narrowband UVB have been recognized as major players in melanocyte stimulation. Phototherapy with UVA or narrowband UVB has been used for repigmentation in vitiligo patients.17
In cases without invasion of hair follicles by malignant cells, repigmentation more likely results from external signals stimulating benign bulbar melanocytes to produce melanin rather than melanoma cell growth extending into the hair bulb.6 In these cases, there is an increase in the number of hair bulbar melanocytes with a lack of malignant morphology in the hair bulb.8 If the signals are directly from melanoma cells in the hair bulb, it is unknown how the malignant cells upregulated melanogenesis in adjacent benign melanocytes or which specific signals required for normal pigmentation were involved in these repigmentation cases.6
Use of medications was ruled out as an underlying cause of the repigmentation in our patient. Drug-related repigmentation of the hair typically is observed in a diffuse generalized pattern. In our case, the repigmentation was localized to the area of the underlying dark patch, and the patient was not on any medications that could cause hair hyperpigmentation. Hyperpigmentation has been associated with acitretin, lenalidomide, corticosteroids, erlotinib, latanoprost, verapamil, tamoxifen, levodopa, thalidomide, PD-1 inhibitors, and tumor necrosis α inhibitors.18-30 Repigmentation also has been reported after local radiotherapy and herpes zoster infection.31,32
The underlying melanoma in our patient was removed by staged square excision. Excision was the treatment of choice for most similar reported cases. Radiotherapy was utilized in two different cases.3,4 In one case, radiotherapy was successfully used to treat melanoma in situ, lentigo maligna type; the patient’s hair grew back to its original color, which suggests that normal hair physiology was restored once melanoma cells were eliminated.3 One reported case demonstrated successful treatment of lentigo maligna type–melanoma with imiquimod cream 5% applied 6 times weekly for 9 months with a positive cosmetic result.9 The exact mechanism of imiquimod is not fully understood. Imiquimod induces cytokines to stimulate the production of IFN-α via activation of toll-like receptor 7.33 There was complete clearing of the lesion as well as the hair pigmentation,9 which suggests that the treatment also eliminated deeper cells influencing pigmentation. A case of malignant amelanotic melanoma was successfully treated with anti–PD-1 antibody pembrolizumab (2 mg/kg every 3 weeks), with no recurrence at 12 months. Pembrolizumab acts as an immune checkpoint inhibitor by binding to the PD-1 receptor and allowing the immune system to recognize and attack melanoma cells. After 5 doses of pembrolizumab, the patient was clear of disease and his hair color returned to gray.5
In 2022, melanoma was estimated to be the fifth most commonly diagnosed cancer among men and women in the United States.34 Early melanoma detection is a critical factor in achieving positive patient outcomes. Hair repigmentation is a potentially serious phenomenon that warrants a physician visit. Melanoma lesions under the hair may be overlooked because of limited visibility. Physicians must inspect spontaneous hair repigmentation with high suspicion and interpret the change as a possible indirect result of melanoma. Overall, it is important to increase public awareness of regular skin checks and melanoma warning signs.
- Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma staging: evidence‐based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67:472-492.
- Vetto JT, Hsueh EC, Gastman BR, et al. Guidance of sentinel lymph node biopsy decisions in patients with T1–T2 melanoma using gene expression profiling. Futur Oncol. 2019;15:1207-1217.
- Dummer R. Hair repigmentation in lentigo maligna. Lancet. 2001;357:598.
- Inzinger M, Massone C, Arzberger E, et al. Hair repigmentation in melanoma. Lancet. 2013;382:1224.
- Rahim RR, Husain A, Tobin DJ, et al. Desmoplastic melanoma presenting with localized hair repigmentation. Br J Dermatol. 2013;169:1371-1373.
- Tiger JB, Habeshian KA, Barton DT, et al. Repigmentation of hair associated with melanoma in situ of scalp. J Am Acad Dermatol. 2014;71:E144-E145.
- Amann VC, Dummer R. Localized hair repigmentation in a 91-year-old woman. JAMA Dermatol. 2016;152:81-82.
- Chan C, Magro CM, Pham AK, et al. Spontaneous hair repigmentation in an 80-year-old man: a case of melanoma-associated hair repigmentation and review of the literature. Am J Dermatopathol. 2019;41:671-674.
- Lackey AE, Glassman G, Grichnik J, et al. Repigmentation of gray hairs with lentigo maligna and response to topical imiquimod. JAAD Case Rep. 2019;5:1015-1017.
- Chew T, Pannell M, Jeeves A. Focal hair re-pigmentation associated with melanoma of the scalp. ANZ J Surg. 2019;90:1175-1176.
- López-Sánchez C, Collgros H. Hair repigmentation as a clue for scalp melanoma. Australas J Dermatol. 2019;61:179-180.
- Gessler J, Tejasvi T, Bresler SC. Repigmentation of scalp hair: a feature of early melanoma. Am J Med. 2023;136:E7-E8.
- Hasegawa T, Iino S, Kitakaze K, et al. Repigmentation of aging gray hair associated with unrecognized development and progression of amelanotic melanoma of the scalp: a physiological alert underlying hair rejuvenation. J Dermatol. 2021;48:E281-E283. doi:10.1111/1346-8138.15881
- D’Mello SAN, Finlay GJ, Baguley BC, et al. Signaling pathways in melanogenesis. Int J Mol Sci. 2016;17:1144.
- Hachiya A, Kobayashi A, Ohuchi A, et al. The paracrine role of stem cell factor/c-kit signaling in the activation of human melanocytes in ultraviolet-B-induced pigmentation. J Invest Dermatol. 2001;116:578-586.
- Slominski A, Wortsman J, Plonka PM, et al. Hair follicle pigmentation. J Invest Dermatol. 2005;124:13-21.
- Falabella R. Vitiligo and the melanocyte reservoir. Indian J Dermatol. 2009;54:313.
- Seckin D, Yildiz A. Repigmentation and curling of hair after acitretin therapy. Australas J Dermatol. 2009;50:214-216.
- Dasanu CA, Mitsis D, Alexandrescu DT. Hair repigmentation associated with the use of lenalidomide: graying may not be an irreversible process! J Oncol Pharm Pract. 2013;19:165-169.
- Sebaratnam DF, Rodríguez Bandera AI, Lowe PM. Hair repigmentation with anti–PD-1 and anti–PD-L1 immunotherapy: a novel hypothesis. JAMA Dermatol. 2018;154:112-113. doi:10.1001/jamadermatol.2017.4420
- Tintle SJ, Dabade TS, Kalish RA, et al. Repigmentation of hair following adalimumab therapy. Dermatol Online J. 2015;21:13030/qt6fn0t1xz.
- Penzi LR, Manatis-Lornell A, Saavedra A, et al. Hair repigmentation associated with the use of brentuximab. JAAD Case Rep. 2017;3:563-565.
- Khaled A, Trojjets S, Zeglaoui F, et al. Repigmentation of the white hair after systemic corticosteroids for bullous pemphigoid. J Eur Acad Dermatology Venereol. 2008;22:1018-1020.
- Cheng YP, Chen HJ, Chiu HC. Erlotinib-induced hair repigmentation. Int J Dermatol. 2014;53:E55-E57.
- Bellandi S, Amato L, Cipollini EM, et al. Repigmentation of hair after latanoprost therapy. J Eur Acad Dermatology Venereol. 2011;25:1485-1487.
- Read GM. Verapamil and hair colour change. Lancet. 1991;338:1520.
- Hampson JP, Donnelly A, Lewis‐Jones MS, et al. Tamoxifen‐induced hair colour change. Br J Dermatol. 1995;132:483-484.
- Reynolds NJ, Crossley J, Ferguson I, et al. Darkening of white hair in Parkinson’s disease. Clin Exp Dermatol. 1989;14:317-318.
- Lovering S, Miao W, Bailie T, et al. Hair repigmentation associated with thalidomide use for the treatment of multiple myeloma. BMJ Case Rep. 2016;2016:bcr2016215521.
- Rivera N, Boada A, Bielsa MI, et al. Hair repigmentation during immunotherapy treatment with an anti–programmed cell death 1 and anti–programmed cell death ligand 1 agent for lung cancer. JAMA Dermatol. 2017;153:1162-1165.
- Prasad S, Dougheney N, Hong A. Scalp hair repigmentation in the penumbral region of radiotherapy–a case series. Int J Radiol Radiat Ther. 2020;7:151-157.
- Adiga GU, Rehman KL, Wiernik PH. Permanent localized hair repigmentation following herpes zoster infection. Arch Dermatol. 2010;146:569-570.
- Hanna E, Abadi R, Abbas O. Imiquimod in dermatology: an overview. Int J Dermatol. 2016;55:831-844.
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7-33.
To the Editor:
An 85-year-old man with a history of hypertension and chronic kidney disease presented with a localized darkening patch of hair on the left parietal scalp that had progressed over the last 7 years (Figure 1A). He had no prior history of skin cancer. Physical examination revealed the remainder of the hair was gray. There was an irregularly pigmented plaque on the skin underlying the darkened hair measuring 5.0 cm in diameter that was confirmed to be melanoma (Figure 1B). He underwent a staged excision to remove the lesion. The surgical defect was closed via a 5.0×6.0-cm full-thickness skin graft.

The initial biopsy showed melanoma in situ. However, the final pathology report following the excision revealed an invasive melanoma with a Breslow depth of 1.0 mm (Clark level IV; American Joint Committee on Cancer T1b).1 Histopathology showed pigment deposition with surrounding deep follicular extension of melanoma (Figure 2).

The patient declined a sentinel lymph node biopsy and agreed to a genetic profile assessment.2 The results of the test identified the patient had a low probability of a positive sentinel lymph node and the lowest risk of melanoma recurrence within 5 years. The patient was clear of disease at 12-month follow-up.
Based on a PubMed search of articles indexed for MEDLINE using the terms hair repigmentation and melanoma, there have been 11 other reported cases of hair repigmentation associated with melanoma (Table).3-13 It initially was suspected that this rare phenomenon primarily existed in the female population, as the first 5 cases were reported solely in females,3-7 possibly due to the prevalence of androgenetic alopecia in males.11 However, 6 cases of repigmentation associated with melanoma were later reported in males8-13; our patient represents an additional reported case in a male. It is unknown if there is a higher prevalence of this phenomenon among males or females.

Most previously reported cases of repigmentation were associated with melanoma in situ, lentigo maligna type. Repigmentation also has been reported in malignant melanoma, as documented in our patient, as well as desmoplastic and amelanotic melanoma.5,6 In every case, the color of the repigmentation was darker than the rest of the patient’s hair; however, the repigmentation color can be different from the patient’s original hair color from their youth.4,5,11
The exact mechanism responsible for hair repigmentation in the setting of melanoma is unclear. It has been speculated from prior cases that repigmentation may be caused by paracrine stimulation from melanoma cells activating adjacent benign hair follicle melanocytes to produce melanin.7,14,15 This process likely is due to cytokines or growth factors, such as c-kit ligand.14,15 Several neural and immune networks and mediators activate the receptor tyrosine kinase KIT, which is thought to play a role in activating melanogenesis within the hair bulb.14 These signals also could originate from changes in the microenvironment instead of the melanoma cells themselves.6 Another possible mechanism is that repigmentation was caused by melanin-producing malignant melanocytes.4
Because this phenomenon typically occurs in older patients, the cause of repigmentation also could be related to chronic sun damage, which may result in upregulation of stem cell factor and α-melanocyte–stimulating hormone, as well as other molecules associated with melanogenesis, such as c-KIT receptor and tyrosinase.15,16 Upregulation of these molecules can lead to an increased number of melanocytes within the hair bulb. In addition, UVA and narrowband UVB have been recognized as major players in melanocyte stimulation. Phototherapy with UVA or narrowband UVB has been used for repigmentation in vitiligo patients.17
In cases without invasion of hair follicles by malignant cells, repigmentation more likely results from external signals stimulating benign bulbar melanocytes to produce melanin rather than melanoma cell growth extending into the hair bulb.6 In these cases, there is an increase in the number of hair bulbar melanocytes with a lack of malignant morphology in the hair bulb.8 If the signals are directly from melanoma cells in the hair bulb, it is unknown how the malignant cells upregulated melanogenesis in adjacent benign melanocytes or which specific signals required for normal pigmentation were involved in these repigmentation cases.6
Use of medications was ruled out as an underlying cause of the repigmentation in our patient. Drug-related repigmentation of the hair typically is observed in a diffuse generalized pattern. In our case, the repigmentation was localized to the area of the underlying dark patch, and the patient was not on any medications that could cause hair hyperpigmentation. Hyperpigmentation has been associated with acitretin, lenalidomide, corticosteroids, erlotinib, latanoprost, verapamil, tamoxifen, levodopa, thalidomide, PD-1 inhibitors, and tumor necrosis α inhibitors.18-30 Repigmentation also has been reported after local radiotherapy and herpes zoster infection.31,32
The underlying melanoma in our patient was removed by staged square excision. Excision was the treatment of choice for most similar reported cases. Radiotherapy was utilized in two different cases.3,4 In one case, radiotherapy was successfully used to treat melanoma in situ, lentigo maligna type; the patient’s hair grew back to its original color, which suggests that normal hair physiology was restored once melanoma cells were eliminated.3 One reported case demonstrated successful treatment of lentigo maligna type–melanoma with imiquimod cream 5% applied 6 times weekly for 9 months with a positive cosmetic result.9 The exact mechanism of imiquimod is not fully understood. Imiquimod induces cytokines to stimulate the production of IFN-α via activation of toll-like receptor 7.33 There was complete clearing of the lesion as well as the hair pigmentation,9 which suggests that the treatment also eliminated deeper cells influencing pigmentation. A case of malignant amelanotic melanoma was successfully treated with anti–PD-1 antibody pembrolizumab (2 mg/kg every 3 weeks), with no recurrence at 12 months. Pembrolizumab acts as an immune checkpoint inhibitor by binding to the PD-1 receptor and allowing the immune system to recognize and attack melanoma cells. After 5 doses of pembrolizumab, the patient was clear of disease and his hair color returned to gray.5
In 2022, melanoma was estimated to be the fifth most commonly diagnosed cancer among men and women in the United States.34 Early melanoma detection is a critical factor in achieving positive patient outcomes. Hair repigmentation is a potentially serious phenomenon that warrants a physician visit. Melanoma lesions under the hair may be overlooked because of limited visibility. Physicians must inspect spontaneous hair repigmentation with high suspicion and interpret the change as a possible indirect result of melanoma. Overall, it is important to increase public awareness of regular skin checks and melanoma warning signs.
To the Editor:
An 85-year-old man with a history of hypertension and chronic kidney disease presented with a localized darkening patch of hair on the left parietal scalp that had progressed over the last 7 years (Figure 1A). He had no prior history of skin cancer. Physical examination revealed the remainder of the hair was gray. There was an irregularly pigmented plaque on the skin underlying the darkened hair measuring 5.0 cm in diameter that was confirmed to be melanoma (Figure 1B). He underwent a staged excision to remove the lesion. The surgical defect was closed via a 5.0×6.0-cm full-thickness skin graft.

The initial biopsy showed melanoma in situ. However, the final pathology report following the excision revealed an invasive melanoma with a Breslow depth of 1.0 mm (Clark level IV; American Joint Committee on Cancer T1b).1 Histopathology showed pigment deposition with surrounding deep follicular extension of melanoma (Figure 2).

The patient declined a sentinel lymph node biopsy and agreed to a genetic profile assessment.2 The results of the test identified the patient had a low probability of a positive sentinel lymph node and the lowest risk of melanoma recurrence within 5 years. The patient was clear of disease at 12-month follow-up.
Based on a PubMed search of articles indexed for MEDLINE using the terms hair repigmentation and melanoma, there have been 11 other reported cases of hair repigmentation associated with melanoma (Table).3-13 It initially was suspected that this rare phenomenon primarily existed in the female population, as the first 5 cases were reported solely in females,3-7 possibly due to the prevalence of androgenetic alopecia in males.11 However, 6 cases of repigmentation associated with melanoma were later reported in males8-13; our patient represents an additional reported case in a male. It is unknown if there is a higher prevalence of this phenomenon among males or females.

Most previously reported cases of repigmentation were associated with melanoma in situ, lentigo maligna type. Repigmentation also has been reported in malignant melanoma, as documented in our patient, as well as desmoplastic and amelanotic melanoma.5,6 In every case, the color of the repigmentation was darker than the rest of the patient’s hair; however, the repigmentation color can be different from the patient’s original hair color from their youth.4,5,11
The exact mechanism responsible for hair repigmentation in the setting of melanoma is unclear. It has been speculated from prior cases that repigmentation may be caused by paracrine stimulation from melanoma cells activating adjacent benign hair follicle melanocytes to produce melanin.7,14,15 This process likely is due to cytokines or growth factors, such as c-kit ligand.14,15 Several neural and immune networks and mediators activate the receptor tyrosine kinase KIT, which is thought to play a role in activating melanogenesis within the hair bulb.14 These signals also could originate from changes in the microenvironment instead of the melanoma cells themselves.6 Another possible mechanism is that repigmentation was caused by melanin-producing malignant melanocytes.4
Because this phenomenon typically occurs in older patients, the cause of repigmentation also could be related to chronic sun damage, which may result in upregulation of stem cell factor and α-melanocyte–stimulating hormone, as well as other molecules associated with melanogenesis, such as c-KIT receptor and tyrosinase.15,16 Upregulation of these molecules can lead to an increased number of melanocytes within the hair bulb. In addition, UVA and narrowband UVB have been recognized as major players in melanocyte stimulation. Phototherapy with UVA or narrowband UVB has been used for repigmentation in vitiligo patients.17
In cases without invasion of hair follicles by malignant cells, repigmentation more likely results from external signals stimulating benign bulbar melanocytes to produce melanin rather than melanoma cell growth extending into the hair bulb.6 In these cases, there is an increase in the number of hair bulbar melanocytes with a lack of malignant morphology in the hair bulb.8 If the signals are directly from melanoma cells in the hair bulb, it is unknown how the malignant cells upregulated melanogenesis in adjacent benign melanocytes or which specific signals required for normal pigmentation were involved in these repigmentation cases.6
Use of medications was ruled out as an underlying cause of the repigmentation in our patient. Drug-related repigmentation of the hair typically is observed in a diffuse generalized pattern. In our case, the repigmentation was localized to the area of the underlying dark patch, and the patient was not on any medications that could cause hair hyperpigmentation. Hyperpigmentation has been associated with acitretin, lenalidomide, corticosteroids, erlotinib, latanoprost, verapamil, tamoxifen, levodopa, thalidomide, PD-1 inhibitors, and tumor necrosis α inhibitors.18-30 Repigmentation also has been reported after local radiotherapy and herpes zoster infection.31,32
The underlying melanoma in our patient was removed by staged square excision. Excision was the treatment of choice for most similar reported cases. Radiotherapy was utilized in two different cases.3,4 In one case, radiotherapy was successfully used to treat melanoma in situ, lentigo maligna type; the patient’s hair grew back to its original color, which suggests that normal hair physiology was restored once melanoma cells were eliminated.3 One reported case demonstrated successful treatment of lentigo maligna type–melanoma with imiquimod cream 5% applied 6 times weekly for 9 months with a positive cosmetic result.9 The exact mechanism of imiquimod is not fully understood. Imiquimod induces cytokines to stimulate the production of IFN-α via activation of toll-like receptor 7.33 There was complete clearing of the lesion as well as the hair pigmentation,9 which suggests that the treatment also eliminated deeper cells influencing pigmentation. A case of malignant amelanotic melanoma was successfully treated with anti–PD-1 antibody pembrolizumab (2 mg/kg every 3 weeks), with no recurrence at 12 months. Pembrolizumab acts as an immune checkpoint inhibitor by binding to the PD-1 receptor and allowing the immune system to recognize and attack melanoma cells. After 5 doses of pembrolizumab, the patient was clear of disease and his hair color returned to gray.5
In 2022, melanoma was estimated to be the fifth most commonly diagnosed cancer among men and women in the United States.34 Early melanoma detection is a critical factor in achieving positive patient outcomes. Hair repigmentation is a potentially serious phenomenon that warrants a physician visit. Melanoma lesions under the hair may be overlooked because of limited visibility. Physicians must inspect spontaneous hair repigmentation with high suspicion and interpret the change as a possible indirect result of melanoma. Overall, it is important to increase public awareness of regular skin checks and melanoma warning signs.
- Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma staging: evidence‐based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67:472-492.
- Vetto JT, Hsueh EC, Gastman BR, et al. Guidance of sentinel lymph node biopsy decisions in patients with T1–T2 melanoma using gene expression profiling. Futur Oncol. 2019;15:1207-1217.
- Dummer R. Hair repigmentation in lentigo maligna. Lancet. 2001;357:598.
- Inzinger M, Massone C, Arzberger E, et al. Hair repigmentation in melanoma. Lancet. 2013;382:1224.
- Rahim RR, Husain A, Tobin DJ, et al. Desmoplastic melanoma presenting with localized hair repigmentation. Br J Dermatol. 2013;169:1371-1373.
- Tiger JB, Habeshian KA, Barton DT, et al. Repigmentation of hair associated with melanoma in situ of scalp. J Am Acad Dermatol. 2014;71:E144-E145.
- Amann VC, Dummer R. Localized hair repigmentation in a 91-year-old woman. JAMA Dermatol. 2016;152:81-82.
- Chan C, Magro CM, Pham AK, et al. Spontaneous hair repigmentation in an 80-year-old man: a case of melanoma-associated hair repigmentation and review of the literature. Am J Dermatopathol. 2019;41:671-674.
- Lackey AE, Glassman G, Grichnik J, et al. Repigmentation of gray hairs with lentigo maligna and response to topical imiquimod. JAAD Case Rep. 2019;5:1015-1017.
- Chew T, Pannell M, Jeeves A. Focal hair re-pigmentation associated with melanoma of the scalp. ANZ J Surg. 2019;90:1175-1176.
- López-Sánchez C, Collgros H. Hair repigmentation as a clue for scalp melanoma. Australas J Dermatol. 2019;61:179-180.
- Gessler J, Tejasvi T, Bresler SC. Repigmentation of scalp hair: a feature of early melanoma. Am J Med. 2023;136:E7-E8.
- Hasegawa T, Iino S, Kitakaze K, et al. Repigmentation of aging gray hair associated with unrecognized development and progression of amelanotic melanoma of the scalp: a physiological alert underlying hair rejuvenation. J Dermatol. 2021;48:E281-E283. doi:10.1111/1346-8138.15881
- D’Mello SAN, Finlay GJ, Baguley BC, et al. Signaling pathways in melanogenesis. Int J Mol Sci. 2016;17:1144.
- Hachiya A, Kobayashi A, Ohuchi A, et al. The paracrine role of stem cell factor/c-kit signaling in the activation of human melanocytes in ultraviolet-B-induced pigmentation. J Invest Dermatol. 2001;116:578-586.
- Slominski A, Wortsman J, Plonka PM, et al. Hair follicle pigmentation. J Invest Dermatol. 2005;124:13-21.
- Falabella R. Vitiligo and the melanocyte reservoir. Indian J Dermatol. 2009;54:313.
- Seckin D, Yildiz A. Repigmentation and curling of hair after acitretin therapy. Australas J Dermatol. 2009;50:214-216.
- Dasanu CA, Mitsis D, Alexandrescu DT. Hair repigmentation associated with the use of lenalidomide: graying may not be an irreversible process! J Oncol Pharm Pract. 2013;19:165-169.
- Sebaratnam DF, Rodríguez Bandera AI, Lowe PM. Hair repigmentation with anti–PD-1 and anti–PD-L1 immunotherapy: a novel hypothesis. JAMA Dermatol. 2018;154:112-113. doi:10.1001/jamadermatol.2017.4420
- Tintle SJ, Dabade TS, Kalish RA, et al. Repigmentation of hair following adalimumab therapy. Dermatol Online J. 2015;21:13030/qt6fn0t1xz.
- Penzi LR, Manatis-Lornell A, Saavedra A, et al. Hair repigmentation associated with the use of brentuximab. JAAD Case Rep. 2017;3:563-565.
- Khaled A, Trojjets S, Zeglaoui F, et al. Repigmentation of the white hair after systemic corticosteroids for bullous pemphigoid. J Eur Acad Dermatology Venereol. 2008;22:1018-1020.
- Cheng YP, Chen HJ, Chiu HC. Erlotinib-induced hair repigmentation. Int J Dermatol. 2014;53:E55-E57.
- Bellandi S, Amato L, Cipollini EM, et al. Repigmentation of hair after latanoprost therapy. J Eur Acad Dermatology Venereol. 2011;25:1485-1487.
- Read GM. Verapamil and hair colour change. Lancet. 1991;338:1520.
- Hampson JP, Donnelly A, Lewis‐Jones MS, et al. Tamoxifen‐induced hair colour change. Br J Dermatol. 1995;132:483-484.
- Reynolds NJ, Crossley J, Ferguson I, et al. Darkening of white hair in Parkinson’s disease. Clin Exp Dermatol. 1989;14:317-318.
- Lovering S, Miao W, Bailie T, et al. Hair repigmentation associated with thalidomide use for the treatment of multiple myeloma. BMJ Case Rep. 2016;2016:bcr2016215521.
- Rivera N, Boada A, Bielsa MI, et al. Hair repigmentation during immunotherapy treatment with an anti–programmed cell death 1 and anti–programmed cell death ligand 1 agent for lung cancer. JAMA Dermatol. 2017;153:1162-1165.
- Prasad S, Dougheney N, Hong A. Scalp hair repigmentation in the penumbral region of radiotherapy–a case series. Int J Radiol Radiat Ther. 2020;7:151-157.
- Adiga GU, Rehman KL, Wiernik PH. Permanent localized hair repigmentation following herpes zoster infection. Arch Dermatol. 2010;146:569-570.
- Hanna E, Abadi R, Abbas O. Imiquimod in dermatology: an overview. Int J Dermatol. 2016;55:831-844.
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7-33.
- Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma staging: evidence‐based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67:472-492.
- Vetto JT, Hsueh EC, Gastman BR, et al. Guidance of sentinel lymph node biopsy decisions in patients with T1–T2 melanoma using gene expression profiling. Futur Oncol. 2019;15:1207-1217.
- Dummer R. Hair repigmentation in lentigo maligna. Lancet. 2001;357:598.
- Inzinger M, Massone C, Arzberger E, et al. Hair repigmentation in melanoma. Lancet. 2013;382:1224.
- Rahim RR, Husain A, Tobin DJ, et al. Desmoplastic melanoma presenting with localized hair repigmentation. Br J Dermatol. 2013;169:1371-1373.
- Tiger JB, Habeshian KA, Barton DT, et al. Repigmentation of hair associated with melanoma in situ of scalp. J Am Acad Dermatol. 2014;71:E144-E145.
- Amann VC, Dummer R. Localized hair repigmentation in a 91-year-old woman. JAMA Dermatol. 2016;152:81-82.
- Chan C, Magro CM, Pham AK, et al. Spontaneous hair repigmentation in an 80-year-old man: a case of melanoma-associated hair repigmentation and review of the literature. Am J Dermatopathol. 2019;41:671-674.
- Lackey AE, Glassman G, Grichnik J, et al. Repigmentation of gray hairs with lentigo maligna and response to topical imiquimod. JAAD Case Rep. 2019;5:1015-1017.
- Chew T, Pannell M, Jeeves A. Focal hair re-pigmentation associated with melanoma of the scalp. ANZ J Surg. 2019;90:1175-1176.
- López-Sánchez C, Collgros H. Hair repigmentation as a clue for scalp melanoma. Australas J Dermatol. 2019;61:179-180.
- Gessler J, Tejasvi T, Bresler SC. Repigmentation of scalp hair: a feature of early melanoma. Am J Med. 2023;136:E7-E8.
- Hasegawa T, Iino S, Kitakaze K, et al. Repigmentation of aging gray hair associated with unrecognized development and progression of amelanotic melanoma of the scalp: a physiological alert underlying hair rejuvenation. J Dermatol. 2021;48:E281-E283. doi:10.1111/1346-8138.15881
- D’Mello SAN, Finlay GJ, Baguley BC, et al. Signaling pathways in melanogenesis. Int J Mol Sci. 2016;17:1144.
- Hachiya A, Kobayashi A, Ohuchi A, et al. The paracrine role of stem cell factor/c-kit signaling in the activation of human melanocytes in ultraviolet-B-induced pigmentation. J Invest Dermatol. 2001;116:578-586.
- Slominski A, Wortsman J, Plonka PM, et al. Hair follicle pigmentation. J Invest Dermatol. 2005;124:13-21.
- Falabella R. Vitiligo and the melanocyte reservoir. Indian J Dermatol. 2009;54:313.
- Seckin D, Yildiz A. Repigmentation and curling of hair after acitretin therapy. Australas J Dermatol. 2009;50:214-216.
- Dasanu CA, Mitsis D, Alexandrescu DT. Hair repigmentation associated with the use of lenalidomide: graying may not be an irreversible process! J Oncol Pharm Pract. 2013;19:165-169.
- Sebaratnam DF, Rodríguez Bandera AI, Lowe PM. Hair repigmentation with anti–PD-1 and anti–PD-L1 immunotherapy: a novel hypothesis. JAMA Dermatol. 2018;154:112-113. doi:10.1001/jamadermatol.2017.4420
- Tintle SJ, Dabade TS, Kalish RA, et al. Repigmentation of hair following adalimumab therapy. Dermatol Online J. 2015;21:13030/qt6fn0t1xz.
- Penzi LR, Manatis-Lornell A, Saavedra A, et al. Hair repigmentation associated with the use of brentuximab. JAAD Case Rep. 2017;3:563-565.
- Khaled A, Trojjets S, Zeglaoui F, et al. Repigmentation of the white hair after systemic corticosteroids for bullous pemphigoid. J Eur Acad Dermatology Venereol. 2008;22:1018-1020.
- Cheng YP, Chen HJ, Chiu HC. Erlotinib-induced hair repigmentation. Int J Dermatol. 2014;53:E55-E57.
- Bellandi S, Amato L, Cipollini EM, et al. Repigmentation of hair after latanoprost therapy. J Eur Acad Dermatology Venereol. 2011;25:1485-1487.
- Read GM. Verapamil and hair colour change. Lancet. 1991;338:1520.
- Hampson JP, Donnelly A, Lewis‐Jones MS, et al. Tamoxifen‐induced hair colour change. Br J Dermatol. 1995;132:483-484.
- Reynolds NJ, Crossley J, Ferguson I, et al. Darkening of white hair in Parkinson’s disease. Clin Exp Dermatol. 1989;14:317-318.
- Lovering S, Miao W, Bailie T, et al. Hair repigmentation associated with thalidomide use for the treatment of multiple myeloma. BMJ Case Rep. 2016;2016:bcr2016215521.
- Rivera N, Boada A, Bielsa MI, et al. Hair repigmentation during immunotherapy treatment with an anti–programmed cell death 1 and anti–programmed cell death ligand 1 agent for lung cancer. JAMA Dermatol. 2017;153:1162-1165.
- Prasad S, Dougheney N, Hong A. Scalp hair repigmentation in the penumbral region of radiotherapy–a case series. Int J Radiol Radiat Ther. 2020;7:151-157.
- Adiga GU, Rehman KL, Wiernik PH. Permanent localized hair repigmentation following herpes zoster infection. Arch Dermatol. 2010;146:569-570.
- Hanna E, Abadi R, Abbas O. Imiquimod in dermatology: an overview. Int J Dermatol. 2016;55:831-844.
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7-33.
Practice Points
- Localized repigmentation of the hair is a rare phenomenon that may indicate underlying melanoma.
- Careful clinicopathologic correlation is necessary to appropriately diagnose and manage this unusual presentation of melanoma.
Generalized Essential Telangiectasia Treated With Pulsed Dye Laser
To the Editor:
Generalized essential telangiectasia (GET) is a rare, benign, and progressive primary cutaneous disease manifesting as telangiectases of the skin without systemic symptoms. It is unique in that it has widespread distribution on the body. Generalized essential telangiectasia more commonly affects women, usually in the fourth decade of life. The telangiectases most frequently appear on the legs, advancing over time to involve the trunk and arms and presenting in several patterns, including diffuse, macular, plaquelike, discrete, or confluent. Although GET typically is asymptomatic, numbness, tingling, and burning of the involved areas have been reported.1 Treatment modalities for GET vary, though pulsed dye laser (PDL) therapy is most common. We report the case of a 40-year-old woman with a 5-year history of GET who was treated successfully with PDL.
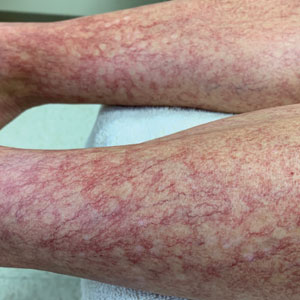
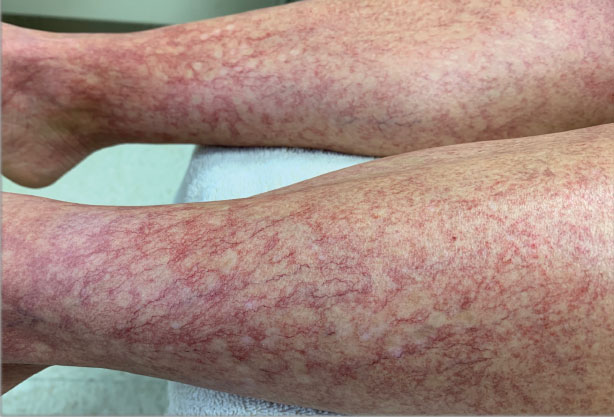
A 40-year-old woman presented to our dermatology clinic with progressive prominence of blood vessels involving the dorsal aspects of the feet of 5 years’ duration. The prominent vessels had spread to involve the legs (Figure 1), buttocks, lower abdomen, forearms, and medial upper arms. The patient denied any personal history of bleeding disorders or family history of inherited conditions associated with visceral vascular malformations, such as hereditary hemorrhagic telangiectasia. Notably, magnetic resonance imaging of the liver approximately 3 weeks prior to initiating treatment with PDL demonstrated multiple hepatic lesions consistent with hemangiomas. The patient reported an occasional tingling sensation in the feet. She was otherwise asymptomatic but did report psychological distress associated with the skin changes.
Punch biopsies from the right lower leg and right buttock demonstrated increased vascularity of the dermis, a mild superficial perivascular lymphocytic infiltrate, and mild edema of the upper dermis without evidence of vasculitis. Autoimmune and coagulopathy workups were negative. The clinical and pathological findings were most consistent with GET.
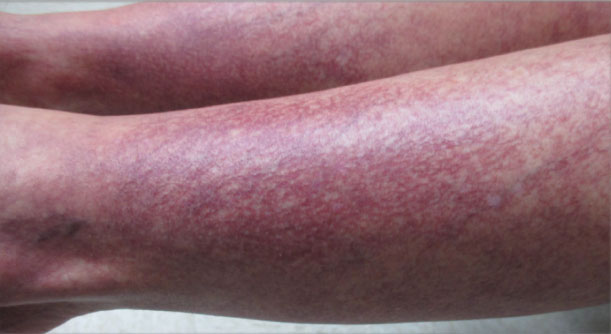
Over the next 2.5 years, the patient underwent treatment with doxycycline and a series of 16 treatments with PDL (fluence, 6–12 J/cm2; pulse width, 10 milliseconds) with a positive cosmetic response. Considerable improvement in the lower legs was noted after 2 years of treatment with PDL (Figure 2).
Recurrence of GET was noted between PDL treatments, which led to progression of the disease process; all treated sites showed slow recurrence of lesions within several months after treatment. After 2 years, doxycycline was discontinued because of a perceived lack of continued benefit and the patient’s desire for alternative therapy. She was started on a 3-month trial of supplementation with ascorbic acid and rutin (or rutoside, a bioflavinoid), without noticeable improvement.
The diffuse distribution of dramatic telangiectases in GET makes treatment difficult. Standard treatments are not well established or studied due to the rarity of the condition. A review of PubMed articles indexed for MEDLINE using the terms treatment and generalized essential telangiectasias demonstrated several attempted treatment modalities for GET with varying success. In 4 cases in which PDL was used,2-5 a positive cosmetic response was noted, similar to what was seen in our patient. In 1 of the 4 cases, conservative management with ascorbic acid and compression stockings was unsuccessful; however, 6-mercaptopurine, used to treat that patient’s ulcerative colitis, incidentally resulted in resolution of GET.2 In 2 cases, response was maintained at 1.5-year follow-up.3,5 Two cases noted successful treatment with acyclovir,6,7 and 2 more demonstrated successful treatment with systemic ketoconazole.6,8 Some improvement was reported with oral doxycycline or tetracycline in 2 cases.9,10 Sclerotherapy improved the cosmetic appearance of telangiectases in one patient but was unsustainable because of the pain associated with the procedure.11 Nd:YAG laser therapy was effective in one case12; however, the patient experienced relapse at 6-month follow-up—similar to what we observed in our patient. Three patients treated with intense pulsed light therapy experienced results that were maintained at 2-year follow-up.13
Generalized essential telangiectasia generally is considered a skin-limited disease without systemic manifestations, but 2 reports11,14 described its association with gastric antral vascular ectasia—known as watermelon stomach. Hepatic hemangiomas are the most common benign liver lesions; however, the findings on magnetic resonance imaging in our patient, in combination with the 2 reported cases of watermelon stomach, suggest that the vascular changes of GET might extend below the skin.
Of the cases we reviewed, our patient had the longest reported duration of PDL treatment and follow-up for GET in which a successful, albeit transient, response was demonstrated. Our review of the literature revealed other reports of success with PDL and intense pulsed light therapy; results were maintained in some patients, while disease relapsed in others. Further studies are needed to understand why results are maintained in some but not all patients.
Although the cost of PDL as a cosmetic procedure must be taken into consideration when planning treatment of GET, we conclude that it is a safe option that can be effective until other treatment options are established to control the disease.
- McGrae JD Jr, Winkelmann RK. Generalized essential telangiectasia: report of a clinical and histochemical study of 13 patients with acquired cutaneous lesions. JAMA. 1963;185:909-913. doi:10.1001/jama.1963.03060120019015
- Glazer AM, Sofen BD, Rigel DS, et al. Successful treatment of generalized essential telangiectasia with 6-mercaptopurine. J Drugs Dermatol. 2017;16:280-282.
- B, M, Boixeda P, et al. Progressive ascending telangiectasia treated with the 585 nm flashlamp-pumped pulsed dye laser. Lasers Surg Med. 1997;21:413-416. doi:10.1002/(sici)1096-9101(1997)21:5<413::aid-lsm1>3.0.co;2-t
- Buscaglia DA, Conte ET. Successful treatment of generalized essential telangiectasia with the 585-nm flashlamp-pumped pulsed dye laser. Cutis. 2001;67:107-108.
- Powell E, Markus R, Malone CH. Generalized essential telangiectasia treated with PDL. J Cosmet Dermatol. 2021;20:1086-1087. doi:10.1111/jocd.13938
- Ali MM, Teimory M, Sarhan M. Generalized essential telangiectasia with conjunctival involvement. Clin Exp Dermatol. 2006;31:781-782. doi:10.1111/j.1365-2230.2006.02217.x
- Shelley WB, Shelley ED. Essential progressive telangiectasia in an autoimmune setting: successful treatment with acyclovir. J Am Acad Dermatol. 1989;21(5 pt 2):1094-1096. doi:10.1016/s0190-9622(89)70303-0
- Shelley WB, Fierer JA. Focal intravascular coagulation in progressive ascending telangiectasia: ultrastructural studies of ketoconazole-induced involution of vessels. J Am Acad Dermatol. 1984;10(5 pt 2):876-887. doi:10.1016/s0190-9622(84)80439-9
- Wiznia LE, Steuer AB, Penn LA, et al. Generalized essential telangiectasia [published online December 15, 2018]. Dermatol Online J. doi:https://doi.org/10.5070/D32412042395
- Shelley WB. Essential progressive telangiectasia. successful treatment with tetracycline. JAMA. 1971;216:1343-1344.
- Checketts SR, Burton PS, Bjorkman DJ, et al. Generalized essential telangiectasia in the presence of gastrointestinal bleeding. J Am Acad Dermatol. 1997;37(2 pt 2):321-325.
- Gambichler T, Avermaete A, Wilmert M, et al. Generalized essential telangiectasia successfully treated with high-energy, long-pulse, frequency-doubled Nd:YAG laser. Dermatol Surg. 2001;27:355-357. doi:10.1046/j.1524-4725.2001.00307.x
- -Torres R, del Pozo J, de la Torre C, et al. Generalized essential telangiectasia: a report of three cases treated using an intense pulsed light system. Actas Dermosifiliogr. 2010;101:192-193.
- Tetart F, Lorthioir A, Girszyn N, et al. Watermelon stomach revealing generalized essential telangiectasia. Intern Med J. 2009;39:781-783. doi:10.1111/j.1445-5994.2009.02048.x
To the Editor:
Generalized essential telangiectasia (GET) is a rare, benign, and progressive primary cutaneous disease manifesting as telangiectases of the skin without systemic symptoms. It is unique in that it has widespread distribution on the body. Generalized essential telangiectasia more commonly affects women, usually in the fourth decade of life. The telangiectases most frequently appear on the legs, advancing over time to involve the trunk and arms and presenting in several patterns, including diffuse, macular, plaquelike, discrete, or confluent. Although GET typically is asymptomatic, numbness, tingling, and burning of the involved areas have been reported.1 Treatment modalities for GET vary, though pulsed dye laser (PDL) therapy is most common. We report the case of a 40-year-old woman with a 5-year history of GET who was treated successfully with PDL.
A 40-year-old woman presented to our dermatology clinic with progressive prominence of blood vessels involving the dorsal aspects of the feet of 5 years’ duration. The prominent vessels had spread to involve the legs (Figure 1), buttocks, lower abdomen, forearms, and medial upper arms. The patient denied any personal history of bleeding disorders or family history of inherited conditions associated with visceral vascular malformations, such as hereditary hemorrhagic telangiectasia. Notably, magnetic resonance imaging of the liver approximately 3 weeks prior to initiating treatment with PDL demonstrated multiple hepatic lesions consistent with hemangiomas. The patient reported an occasional tingling sensation in the feet. She was otherwise asymptomatic but did report psychological distress associated with the skin changes.

Punch biopsies from the right lower leg and right buttock demonstrated increased vascularity of the dermis, a mild superficial perivascular lymphocytic infiltrate, and mild edema of the upper dermis without evidence of vasculitis. Autoimmune and coagulopathy workups were negative. The clinical and pathological findings were most consistent with GET.
Over the next 2.5 years, the patient underwent treatment with doxycycline and a series of 16 treatments with PDL (fluence, 6–12 J/cm2; pulse width, 10 milliseconds) with a positive cosmetic response. Considerable improvement in the lower legs was noted after 2 years of treatment with PDL (Figure 2).

Recurrence of GET was noted between PDL treatments, which led to progression of the disease process; all treated sites showed slow recurrence of lesions within several months after treatment. After 2 years, doxycycline was discontinued because of a perceived lack of continued benefit and the patient’s desire for alternative therapy. She was started on a 3-month trial of supplementation with ascorbic acid and rutin (or rutoside, a bioflavinoid), without noticeable improvement.
The diffuse distribution of dramatic telangiectases in GET makes treatment difficult. Standard treatments are not well established or studied due to the rarity of the condition. A review of PubMed articles indexed for MEDLINE using the terms treatment and generalized essential telangiectasias demonstrated several attempted treatment modalities for GET with varying success. In 4 cases in which PDL was used,2-5 a positive cosmetic response was noted, similar to what was seen in our patient. In 1 of the 4 cases, conservative management with ascorbic acid and compression stockings was unsuccessful; however, 6-mercaptopurine, used to treat that patient’s ulcerative colitis, incidentally resulted in resolution of GET.2 In 2 cases, response was maintained at 1.5-year follow-up.3,5 Two cases noted successful treatment with acyclovir,6,7 and 2 more demonstrated successful treatment with systemic ketoconazole.6,8 Some improvement was reported with oral doxycycline or tetracycline in 2 cases.9,10 Sclerotherapy improved the cosmetic appearance of telangiectases in one patient but was unsustainable because of the pain associated with the procedure.11 Nd:YAG laser therapy was effective in one case12; however, the patient experienced relapse at 6-month follow-up—similar to what we observed in our patient. Three patients treated with intense pulsed light therapy experienced results that were maintained at 2-year follow-up.13
Generalized essential telangiectasia generally is considered a skin-limited disease without systemic manifestations, but 2 reports11,14 described its association with gastric antral vascular ectasia—known as watermelon stomach. Hepatic hemangiomas are the most common benign liver lesions; however, the findings on magnetic resonance imaging in our patient, in combination with the 2 reported cases of watermelon stomach, suggest that the vascular changes of GET might extend below the skin.
Of the cases we reviewed, our patient had the longest reported duration of PDL treatment and follow-up for GET in which a successful, albeit transient, response was demonstrated. Our review of the literature revealed other reports of success with PDL and intense pulsed light therapy; results were maintained in some patients, while disease relapsed in others. Further studies are needed to understand why results are maintained in some but not all patients.
Although the cost of PDL as a cosmetic procedure must be taken into consideration when planning treatment of GET, we conclude that it is a safe option that can be effective until other treatment options are established to control the disease.
To the Editor:
Generalized essential telangiectasia (GET) is a rare, benign, and progressive primary cutaneous disease manifesting as telangiectases of the skin without systemic symptoms. It is unique in that it has widespread distribution on the body. Generalized essential telangiectasia more commonly affects women, usually in the fourth decade of life. The telangiectases most frequently appear on the legs, advancing over time to involve the trunk and arms and presenting in several patterns, including diffuse, macular, plaquelike, discrete, or confluent. Although GET typically is asymptomatic, numbness, tingling, and burning of the involved areas have been reported.1 Treatment modalities for GET vary, though pulsed dye laser (PDL) therapy is most common. We report the case of a 40-year-old woman with a 5-year history of GET who was treated successfully with PDL.
A 40-year-old woman presented to our dermatology clinic with progressive prominence of blood vessels involving the dorsal aspects of the feet of 5 years’ duration. The prominent vessels had spread to involve the legs (Figure 1), buttocks, lower abdomen, forearms, and medial upper arms. The patient denied any personal history of bleeding disorders or family history of inherited conditions associated with visceral vascular malformations, such as hereditary hemorrhagic telangiectasia. Notably, magnetic resonance imaging of the liver approximately 3 weeks prior to initiating treatment with PDL demonstrated multiple hepatic lesions consistent with hemangiomas. The patient reported an occasional tingling sensation in the feet. She was otherwise asymptomatic but did report psychological distress associated with the skin changes.

Punch biopsies from the right lower leg and right buttock demonstrated increased vascularity of the dermis, a mild superficial perivascular lymphocytic infiltrate, and mild edema of the upper dermis without evidence of vasculitis. Autoimmune and coagulopathy workups were negative. The clinical and pathological findings were most consistent with GET.
Over the next 2.5 years, the patient underwent treatment with doxycycline and a series of 16 treatments with PDL (fluence, 6–12 J/cm2; pulse width, 10 milliseconds) with a positive cosmetic response. Considerable improvement in the lower legs was noted after 2 years of treatment with PDL (Figure 2).

Recurrence of GET was noted between PDL treatments, which led to progression of the disease process; all treated sites showed slow recurrence of lesions within several months after treatment. After 2 years, doxycycline was discontinued because of a perceived lack of continued benefit and the patient’s desire for alternative therapy. She was started on a 3-month trial of supplementation with ascorbic acid and rutin (or rutoside, a bioflavinoid), without noticeable improvement.
The diffuse distribution of dramatic telangiectases in GET makes treatment difficult. Standard treatments are not well established or studied due to the rarity of the condition. A review of PubMed articles indexed for MEDLINE using the terms treatment and generalized essential telangiectasias demonstrated several attempted treatment modalities for GET with varying success. In 4 cases in which PDL was used,2-5 a positive cosmetic response was noted, similar to what was seen in our patient. In 1 of the 4 cases, conservative management with ascorbic acid and compression stockings was unsuccessful; however, 6-mercaptopurine, used to treat that patient’s ulcerative colitis, incidentally resulted in resolution of GET.2 In 2 cases, response was maintained at 1.5-year follow-up.3,5 Two cases noted successful treatment with acyclovir,6,7 and 2 more demonstrated successful treatment with systemic ketoconazole.6,8 Some improvement was reported with oral doxycycline or tetracycline in 2 cases.9,10 Sclerotherapy improved the cosmetic appearance of telangiectases in one patient but was unsustainable because of the pain associated with the procedure.11 Nd:YAG laser therapy was effective in one case12; however, the patient experienced relapse at 6-month follow-up—similar to what we observed in our patient. Three patients treated with intense pulsed light therapy experienced results that were maintained at 2-year follow-up.13
Generalized essential telangiectasia generally is considered a skin-limited disease without systemic manifestations, but 2 reports11,14 described its association with gastric antral vascular ectasia—known as watermelon stomach. Hepatic hemangiomas are the most common benign liver lesions; however, the findings on magnetic resonance imaging in our patient, in combination with the 2 reported cases of watermelon stomach, suggest that the vascular changes of GET might extend below the skin.
Of the cases we reviewed, our patient had the longest reported duration of PDL treatment and follow-up for GET in which a successful, albeit transient, response was demonstrated. Our review of the literature revealed other reports of success with PDL and intense pulsed light therapy; results were maintained in some patients, while disease relapsed in others. Further studies are needed to understand why results are maintained in some but not all patients.
Although the cost of PDL as a cosmetic procedure must be taken into consideration when planning treatment of GET, we conclude that it is a safe option that can be effective until other treatment options are established to control the disease.
- McGrae JD Jr, Winkelmann RK. Generalized essential telangiectasia: report of a clinical and histochemical study of 13 patients with acquired cutaneous lesions. JAMA. 1963;185:909-913. doi:10.1001/jama.1963.03060120019015
- Glazer AM, Sofen BD, Rigel DS, et al. Successful treatment of generalized essential telangiectasia with 6-mercaptopurine. J Drugs Dermatol. 2017;16:280-282.
- B, M, Boixeda P, et al. Progressive ascending telangiectasia treated with the 585 nm flashlamp-pumped pulsed dye laser. Lasers Surg Med. 1997;21:413-416. doi:10.1002/(sici)1096-9101(1997)21:5<413::aid-lsm1>3.0.co;2-t
- Buscaglia DA, Conte ET. Successful treatment of generalized essential telangiectasia with the 585-nm flashlamp-pumped pulsed dye laser. Cutis. 2001;67:107-108.
- Powell E, Markus R, Malone CH. Generalized essential telangiectasia treated with PDL. J Cosmet Dermatol. 2021;20:1086-1087. doi:10.1111/jocd.13938
- Ali MM, Teimory M, Sarhan M. Generalized essential telangiectasia with conjunctival involvement. Clin Exp Dermatol. 2006;31:781-782. doi:10.1111/j.1365-2230.2006.02217.x
- Shelley WB, Shelley ED. Essential progressive telangiectasia in an autoimmune setting: successful treatment with acyclovir. J Am Acad Dermatol. 1989;21(5 pt 2):1094-1096. doi:10.1016/s0190-9622(89)70303-0
- Shelley WB, Fierer JA. Focal intravascular coagulation in progressive ascending telangiectasia: ultrastructural studies of ketoconazole-induced involution of vessels. J Am Acad Dermatol. 1984;10(5 pt 2):876-887. doi:10.1016/s0190-9622(84)80439-9
- Wiznia LE, Steuer AB, Penn LA, et al. Generalized essential telangiectasia [published online December 15, 2018]. Dermatol Online J. doi:https://doi.org/10.5070/D32412042395
- Shelley WB. Essential progressive telangiectasia. successful treatment with tetracycline. JAMA. 1971;216:1343-1344.
- Checketts SR, Burton PS, Bjorkman DJ, et al. Generalized essential telangiectasia in the presence of gastrointestinal bleeding. J Am Acad Dermatol. 1997;37(2 pt 2):321-325.
- Gambichler T, Avermaete A, Wilmert M, et al. Generalized essential telangiectasia successfully treated with high-energy, long-pulse, frequency-doubled Nd:YAG laser. Dermatol Surg. 2001;27:355-357. doi:10.1046/j.1524-4725.2001.00307.x
- -Torres R, del Pozo J, de la Torre C, et al. Generalized essential telangiectasia: a report of three cases treated using an intense pulsed light system. Actas Dermosifiliogr. 2010;101:192-193.
- Tetart F, Lorthioir A, Girszyn N, et al. Watermelon stomach revealing generalized essential telangiectasia. Intern Med J. 2009;39:781-783. doi:10.1111/j.1445-5994.2009.02048.x
- McGrae JD Jr, Winkelmann RK. Generalized essential telangiectasia: report of a clinical and histochemical study of 13 patients with acquired cutaneous lesions. JAMA. 1963;185:909-913. doi:10.1001/jama.1963.03060120019015
- Glazer AM, Sofen BD, Rigel DS, et al. Successful treatment of generalized essential telangiectasia with 6-mercaptopurine. J Drugs Dermatol. 2017;16:280-282.
- B, M, Boixeda P, et al. Progressive ascending telangiectasia treated with the 585 nm flashlamp-pumped pulsed dye laser. Lasers Surg Med. 1997;21:413-416. doi:10.1002/(sici)1096-9101(1997)21:5<413::aid-lsm1>3.0.co;2-t
- Buscaglia DA, Conte ET. Successful treatment of generalized essential telangiectasia with the 585-nm flashlamp-pumped pulsed dye laser. Cutis. 2001;67:107-108.
- Powell E, Markus R, Malone CH. Generalized essential telangiectasia treated with PDL. J Cosmet Dermatol. 2021;20:1086-1087. doi:10.1111/jocd.13938
- Ali MM, Teimory M, Sarhan M. Generalized essential telangiectasia with conjunctival involvement. Clin Exp Dermatol. 2006;31:781-782. doi:10.1111/j.1365-2230.2006.02217.x
- Shelley WB, Shelley ED. Essential progressive telangiectasia in an autoimmune setting: successful treatment with acyclovir. J Am Acad Dermatol. 1989;21(5 pt 2):1094-1096. doi:10.1016/s0190-9622(89)70303-0
- Shelley WB, Fierer JA. Focal intravascular coagulation in progressive ascending telangiectasia: ultrastructural studies of ketoconazole-induced involution of vessels. J Am Acad Dermatol. 1984;10(5 pt 2):876-887. doi:10.1016/s0190-9622(84)80439-9
- Wiznia LE, Steuer AB, Penn LA, et al. Generalized essential telangiectasia [published online December 15, 2018]. Dermatol Online J. doi:https://doi.org/10.5070/D32412042395
- Shelley WB. Essential progressive telangiectasia. successful treatment with tetracycline. JAMA. 1971;216:1343-1344.
- Checketts SR, Burton PS, Bjorkman DJ, et al. Generalized essential telangiectasia in the presence of gastrointestinal bleeding. J Am Acad Dermatol. 1997;37(2 pt 2):321-325.
- Gambichler T, Avermaete A, Wilmert M, et al. Generalized essential telangiectasia successfully treated with high-energy, long-pulse, frequency-doubled Nd:YAG laser. Dermatol Surg. 2001;27:355-357. doi:10.1046/j.1524-4725.2001.00307.x
- -Torres R, del Pozo J, de la Torre C, et al. Generalized essential telangiectasia: a report of three cases treated using an intense pulsed light system. Actas Dermosifiliogr. 2010;101:192-193.
- Tetart F, Lorthioir A, Girszyn N, et al. Watermelon stomach revealing generalized essential telangiectasia. Intern Med J. 2009;39:781-783. doi:10.1111/j.1445-5994.2009.02048.x
Practice Points
- Generalized essential telangiectasia (GET) is a primary benign skin condition in which there is progressive development of telangiectases but a lack of systemic symptoms.
- Although patients should be assured that GET is a benign disease, its manifestation on the skin may cause negative psychologic impacts that should not be overlooked.
- Pulsed dye laser therapy does lead to improvement of the condition, but it does not prevent progression.
Rash and edema
Lab work was ordered and came back within normal range, except for an elevated white blood cell count (19,700/mm3; reference range, 4500-13,500/mm3). His mild systemic symptoms, skin lesions without blistering or necrosis, acral edema, and the absence of lymphadenopathy pointed to a diagnosis of urticaria multiforme.
Urticaria multiforme, also called acute annular urticaria or acute urticarial hypersensitivity syndrome, is a histamine-mediated hypersensitivity reaction characterized by transient annular, polycyclic, urticarial lesions with central ecchymosis. It typically manifests in children ages 4 months to 4 years and begins with small erythematous macules, papules, and plaques that progress to large blanchable wheals with dusky blue centers.1-3 Lesions are usually located on the face, trunk, and extremities and are often pruritic (60%-94%).1-3 The rash generally lasts 2 to 12 days.1,3
Patients often report a preceding viral illness, otitis media, recent use of antibiotics, or recent immunizations. Patients also often have associated facial or acral edema (72%).1 Children with significant edema of the feet may find walking difficult, which should not be confused with arthritis or arthralgias.
The diagnosis is made clinically and should not require a skin biopsy or extensive laboratory testing. When performed, laboratory studies—including CBC, erythrocyte sedimentation rate, C-reactive protein, and urinalysis—are routinely normal. The differential diagnosis in this case included erythema multiforme, Henoch-Schönlein purpura, serum sickness-like reaction, and urticarial vasculitis.1,2,4
Treatment consists of discontinuing any offending agent (if suspected) and using systemic H1 or H2 antihistamines for symptom relief. Systemic steroids should only be given in refractory cases.
Our patient’s amoxicillin was discontinued, and he was started on a 14-day course of cetirizine 5 mg bid and hydroxyzine 10 mg at bedtime. He was also started on triamcinolone 0.1% cream to be applied twice daily for 1 week. During his 3-day hospital stay, his fever resolved, and his rash and edema improved.
During an outpatient follow-up visit with a pediatric dermatologist 2 weeks after discharge, the patient’s rash was still present and dermatographism was noted. In light of this, his parents were instructed to continue giving the cetirizine and hydroxyzine once daily for an additional 2 weeks and to return as needed.
This case was adapted from: Cook JS, Angles A, Morley E. Urticaria and edema in a 2-year-old boy. J Fam Pract. 2021;70:353-355.
Photos courtesy of Jeffrey S. Cook, MD, FAAFP
1. Shah KN, Honig PJ, Yan AC. “Urticaria multiforme”: a case series and review of acute annular urticarial hypersensitivity syndromes in children. Pediatrics. 2007;119:e1177-e1183. doi: 10.1542/peds.2006-1553
2. Emer JJ, Bernardo SG, Kovalerchik O, et al. Urticaria multiforme. J Clin Aesthet Dermatol. 2013;6:34-39.
3. Starnes L, Patel T, Skinner RB. Urticaria multiforme – a case report. Pediatr Dermatol. 2011; 28:436-438. doi: 10.1111/j.1525-1470.2011.01311.x
4. Reamy BV, Williams PM, Lindsay TJ. Henoch-Schönlein purpura. Am Fam Physician. 2009;80:697-704.

Lab work was ordered and came back within normal range, except for an elevated white blood cell count (19,700/mm3; reference range, 4500-13,500/mm3). His mild systemic symptoms, skin lesions without blistering or necrosis, acral edema, and the absence of lymphadenopathy pointed to a diagnosis of urticaria multiforme.
Urticaria multiforme, also called acute annular urticaria or acute urticarial hypersensitivity syndrome, is a histamine-mediated hypersensitivity reaction characterized by transient annular, polycyclic, urticarial lesions with central ecchymosis. It typically manifests in children ages 4 months to 4 years and begins with small erythematous macules, papules, and plaques that progress to large blanchable wheals with dusky blue centers.1-3 Lesions are usually located on the face, trunk, and extremities and are often pruritic (60%-94%).1-3 The rash generally lasts 2 to 12 days.1,3
Patients often report a preceding viral illness, otitis media, recent use of antibiotics, or recent immunizations. Patients also often have associated facial or acral edema (72%).1 Children with significant edema of the feet may find walking difficult, which should not be confused with arthritis or arthralgias.
The diagnosis is made clinically and should not require a skin biopsy or extensive laboratory testing. When performed, laboratory studies—including CBC, erythrocyte sedimentation rate, C-reactive protein, and urinalysis—are routinely normal. The differential diagnosis in this case included erythema multiforme, Henoch-Schönlein purpura, serum sickness-like reaction, and urticarial vasculitis.1,2,4
Treatment consists of discontinuing any offending agent (if suspected) and using systemic H1 or H2 antihistamines for symptom relief. Systemic steroids should only be given in refractory cases.
Our patient’s amoxicillin was discontinued, and he was started on a 14-day course of cetirizine 5 mg bid and hydroxyzine 10 mg at bedtime. He was also started on triamcinolone 0.1% cream to be applied twice daily for 1 week. During his 3-day hospital stay, his fever resolved, and his rash and edema improved.
During an outpatient follow-up visit with a pediatric dermatologist 2 weeks after discharge, the patient’s rash was still present and dermatographism was noted. In light of this, his parents were instructed to continue giving the cetirizine and hydroxyzine once daily for an additional 2 weeks and to return as needed.
This case was adapted from: Cook JS, Angles A, Morley E. Urticaria and edema in a 2-year-old boy. J Fam Pract. 2021;70:353-355.
Photos courtesy of Jeffrey S. Cook, MD, FAAFP

Lab work was ordered and came back within normal range, except for an elevated white blood cell count (19,700/mm3; reference range, 4500-13,500/mm3). His mild systemic symptoms, skin lesions without blistering or necrosis, acral edema, and the absence of lymphadenopathy pointed to a diagnosis of urticaria multiforme.
Urticaria multiforme, also called acute annular urticaria or acute urticarial hypersensitivity syndrome, is a histamine-mediated hypersensitivity reaction characterized by transient annular, polycyclic, urticarial lesions with central ecchymosis. It typically manifests in children ages 4 months to 4 years and begins with small erythematous macules, papules, and plaques that progress to large blanchable wheals with dusky blue centers.1-3 Lesions are usually located on the face, trunk, and extremities and are often pruritic (60%-94%).1-3 The rash generally lasts 2 to 12 days.1,3
Patients often report a preceding viral illness, otitis media, recent use of antibiotics, or recent immunizations. Patients also often have associated facial or acral edema (72%).1 Children with significant edema of the feet may find walking difficult, which should not be confused with arthritis or arthralgias.
The diagnosis is made clinically and should not require a skin biopsy or extensive laboratory testing. When performed, laboratory studies—including CBC, erythrocyte sedimentation rate, C-reactive protein, and urinalysis—are routinely normal. The differential diagnosis in this case included erythema multiforme, Henoch-Schönlein purpura, serum sickness-like reaction, and urticarial vasculitis.1,2,4
Treatment consists of discontinuing any offending agent (if suspected) and using systemic H1 or H2 antihistamines for symptom relief. Systemic steroids should only be given in refractory cases.
Our patient’s amoxicillin was discontinued, and he was started on a 14-day course of cetirizine 5 mg bid and hydroxyzine 10 mg at bedtime. He was also started on triamcinolone 0.1% cream to be applied twice daily for 1 week. During his 3-day hospital stay, his fever resolved, and his rash and edema improved.
During an outpatient follow-up visit with a pediatric dermatologist 2 weeks after discharge, the patient’s rash was still present and dermatographism was noted. In light of this, his parents were instructed to continue giving the cetirizine and hydroxyzine once daily for an additional 2 weeks and to return as needed.
This case was adapted from: Cook JS, Angles A, Morley E. Urticaria and edema in a 2-year-old boy. J Fam Pract. 2021;70:353-355.
Photos courtesy of Jeffrey S. Cook, MD, FAAFP
1. Shah KN, Honig PJ, Yan AC. “Urticaria multiforme”: a case series and review of acute annular urticarial hypersensitivity syndromes in children. Pediatrics. 2007;119:e1177-e1183. doi: 10.1542/peds.2006-1553
2. Emer JJ, Bernardo SG, Kovalerchik O, et al. Urticaria multiforme. J Clin Aesthet Dermatol. 2013;6:34-39.
3. Starnes L, Patel T, Skinner RB. Urticaria multiforme – a case report. Pediatr Dermatol. 2011; 28:436-438. doi: 10.1111/j.1525-1470.2011.01311.x
4. Reamy BV, Williams PM, Lindsay TJ. Henoch-Schönlein purpura. Am Fam Physician. 2009;80:697-704.
1. Shah KN, Honig PJ, Yan AC. “Urticaria multiforme”: a case series and review of acute annular urticarial hypersensitivity syndromes in children. Pediatrics. 2007;119:e1177-e1183. doi: 10.1542/peds.2006-1553
2. Emer JJ, Bernardo SG, Kovalerchik O, et al. Urticaria multiforme. J Clin Aesthet Dermatol. 2013;6:34-39.
3. Starnes L, Patel T, Skinner RB. Urticaria multiforme – a case report. Pediatr Dermatol. 2011; 28:436-438. doi: 10.1111/j.1525-1470.2011.01311.x
4. Reamy BV, Williams PM, Lindsay TJ. Henoch-Schönlein purpura. Am Fam Physician. 2009;80:697-704.
Current approaches and challenges to cervical cancer prevention in the United States
CASE Intervention approaches for decreasing the risk of cervical cancer
A 25-year-old woman presents to your practice for routine examination. She has never undergone cervical cancer screening or received the human papillomavirus (HPV) vaccine series. The patient has had 3 lifetime sexual partners and currently uses condoms as contraception. What interventions are appropriate to offer this patient to decrease her risk of cervical cancer? Choose as many that may apply:
1. cervical cytology with reflex HPV testing
2. cervical cytology with HPV cotesting
3. primary HPV testing
4. HPV vaccine series (3 doses)
5. all of the above
The answer is number 5, all of the above.
Choices 1, 2, and 3 are acceptable methods of cervical cancer screening for this patient. Catch-up HPV vaccination should be offered as well.
Equitable preventive care is needed
Cervical cancer is a unique cancer because it has a known preventative strategy. HPV vaccination, paired with cervical screening and management of abnormal results, has contributed to decreased rates of cervical cancer in the United States, from 13,914 cases in 1999 to 12,795 cases in 2019.1 In less-developed countries, however, cervical cancer continues to be a leading cause of mortality, with 90% of cervical cancer deaths in 2020 occurring in low- and middle-income countries.2
Disparate outcomes in cervical cancer are often a reflection of disparities in health access. Within the United States, Black women have a higher incidence of cervical cancer, advanced-stage disease, and mortality from cervical cancer than White women.3,4 Furthermore, the incidence of cervical cancer increased among American Indian and Alaska Native people between 2000 and 2019.5 The rate for patients who are overdue for cervical cancer screening is higher among Asian and Hispanic patients compared with non-Hispanic White patients (31.4% vs 20.1%; P=.01) and among patients who identify as LGBTQ+ compared with patients who identify as heterosexual (32.0% vs 22.2%; P<.001).6 Younger patients have a significantly higher rate for overdue screening compared with their older counterparts (29.1% vs 21.1%; P<.001), as do uninsured patients compared with those who are privately insured (41.7% vs 18.1%; P<.001). Overall, the proportion of women without up-to-date screening increased significantly from 2005 to 2019 (14.4% vs 23.0%; P<.001).6
Unfortunately, despite a known strategy to eliminate cervical cancer, we are not accomplishing equitable preventative care. Barriers to care can include patient-centered issues, such as fear of cancer or of painful evaluations, lack of trust in the health care system, and inadequate understanding of the benefits of cancer prevention, in addition to systemic and structural barriers. As we assess new technologies, one of our most important goals is to consider how such innovations can increase health access—whether through increasing ease and acceptability of testing or by creating more effective screening tests.
Updates to cervical screening guidance
In 2020, the American Cancer Society (ACS) updated its cervical screening guidelines to start screening at age 25 years with the “preferred” strategy of HPV primary testing every 5 years.7 By contrast, the US Preventive Services Task Force (USPSTF) continues to recommend 1 of 3 methods: cytology alone every 3 years; cytology alone every 3 years between ages 21 and 29 followed by cytology and HPV cotesting every 5 years at age 30 or older; or high-risk HPV testing alone every 5 years (TABLE).8
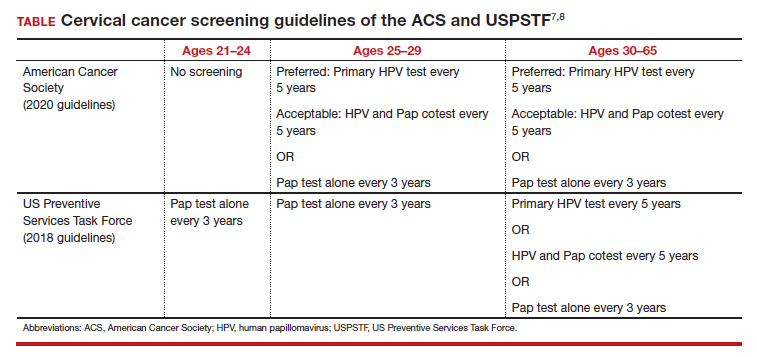
To successfully prevent cervical cancer, abnormal results are managed by performing either colposcopy with biopsy, immediate treatment, or close surveillance based on the risk of developing cervical intraepithelial neoplasia (CIN) 3 or worse. A patient’s risk is determined based on both current and prior test results. The ASCCP (American Society for Colposcopy and Cervical Pathology) transitioned to risk-based management guidelines in 2019 and has both an app and a web-based risk assessment tool available for clinicians (https://www.asccp.org).9
All organizations recommend stopping screening after age 65 provided there has been a history of adequate screening in the prior 10 years (defined as 2 normal cotests or 3 normal cytology tests, with the most recent test within 5 years) and no history of CIN 2 or worse within the prior 25 years.10,11 Recent studies that examined the rate of cervical cancer diagnosed in patients older than 65 years have questioned whether patients should continue screening beyond 65.10 In the United States, 20% of cervical cancer still occurs in women older than age 65.11 One reason may be that many women have not met the requirement for adequate and normal prior screening and may still need ongoing testing.12
Continue to: Primary HPV screening...
Primary HPV screening
Primary HPV testing means that an HPV test is performed first, and if it is positive for high-risk HPV, further testing is performed to determine next steps. This contrasts with the currently used method of obtaining cytology (Pap) first with either concurrent HPV testing or reflex HPV testing. The first HPV primary screening test was approved by the US Food and Drug Administration (FDA) in 2014.13
Multiple randomized controlled trials in Europe have demonstrated the accuracy of HPV-based screening compared with cytology in the detection of cervical cancer and its precursors.14-17 The HPV FOCAL trial demonstrated increased efficacy of primary HPV screening in the detection of CIN 2+ lesions.18 This trial recruited a total of 19,000 women, ages 25 to 65, in Canada and randomly assigned them to receive primary HPV testing or liquid-based cytology. If primary HPV testing was negative, participants would return in 48 months for cytology and HPV cotesting. If primary liquid-based cytology testing was negative, participants would return at 24 months for cytology testing alone and at 48 months for cytology and HPV cotesting. Both groups had similar incidences of CIN 2+ over the study period. HPV testing was shown to detect CIN 2+ at higher rates at the time of initial screen (risk ratio [RR], 1.61; 95% confidence interval [CI], 1.24–2.09) and then significantly lower rates at the time of exit screening at 48 months (RR, 0.36; 95% CI, 0.24–0.54).18 These results demonstrated that primary HPV testing detects CIN 2+ earlier than cytology alone. In follow-up analyses, primary HPV screening missed fewer CIN 2+ diagnoses than cytology screening.19
While not as many studies have compared primary HPV testing to cytology with an HPV cotest, the current most common practice in the United States, one study performed in the United States found that a negative cytology result did not further decrease the risk of CIN 3 for HPV-negative patients (risk of CIN 3+ at 5 years: 0.16% vs 0.17%; P=0.8) and concluded that a negative HPV test was enough reassurance for a low risk of CIN 3+.20
Another study, the ATHENA trial, evaluated more than 42,000 women who were 25 years and older over a 3-year period.21 Patients underwent either primary HPV testing or combination cytology and reflex HPV (if ages 25–29) or HPV cotesting (if age 30 or older). Primary HPV testing was found to have a sensitivity and specificity of 76.1% and 93.5%, respectively, compared with 61.7% and 94.6% for cytology with HPV cotesting, but it also increased the total number of colposcopies performed.21
Subsequent management of a primary HPV-positive result can be triaged using genotyping, cytology, or a combination of both. FDA-approved HPV screening tests provide genotyping and current management guidelines use genotyping to triage positive HPV results into HPV 16, 18, or 1 of 12 other high-risk HPV genotypes.
In the ATHENA trial, the 3-year incidence of CIN 3+ for HPV 16/18-positive results was 21.16% (95% CI, 18.39%–24.01%) compared with 5.4% (95% CI, 4.5%–6.4%) among patients with an HPV test positive for 1 of the other HPV genotypes.21 While a patient with an HPV result positive for HPV 16/18 should directly undergo colposcopy, clinical guidance for an HPV-positive result for one of the other genotypes suggests using reflex cytology to triage patients. The ASCCP recommended management of primary HPV testing is included in the FIGURE.22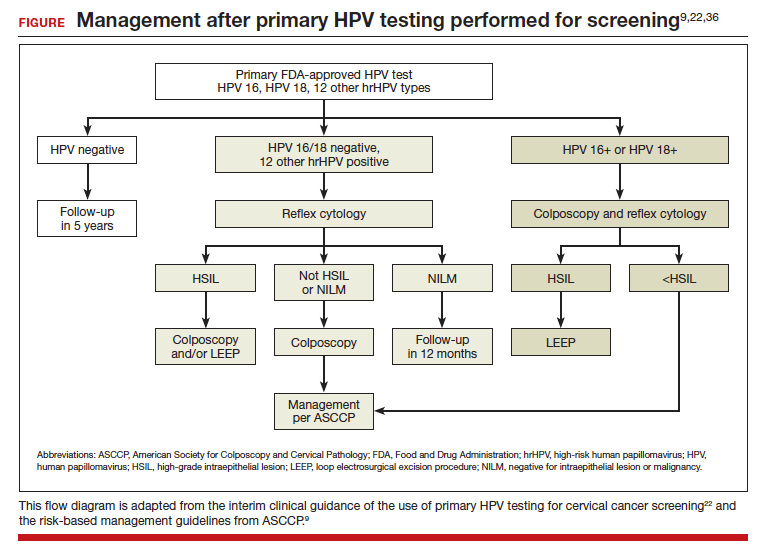
Many barriers remain to transitioning to primary HPV testing, including laboratory test availability as well as patient and provider acceptance. At present, 2 FDA-approved primary HPV screening tests are available: the Cobas HPV test (Roche Molecular Systems, Inc) and the BD Onclarity HPV assay (Becton, Dickinson and Company). Changes to screening recommendations need to be accompanied by patient and provider outreach and education.
In a survey of more than 500 US women in 2015 after guidelines allowed for increased screening intervals after negative results, a majority of women (55.6%; 95% CI, 51.4%–59.8%) were aware that screening recommendations had changed; however, 74.1% (95% CI, 70.3%–77.7%) still believed that women should be screened annually.23 By contrast, participants in the HPV FOCAL trial, who were able to learn more about HPV-based screening, were surveyed about their willingness to undergo primary HPV testing rather than Pap testing at the conclusion of the trial.24 Of the participants, 63% were comfortable with primary HPV testing, and 54% were accepting of an extended screening interval of 4 to 5 years.24
Continue to: p16/Ki-67 dual-stain cytology...
p16/Ki-67 dual-stain cytology
An additional tool for triaging HPV-positive patients is the p16/Ki-67 dual stain test (CINtec Plus Cytology; Roche), which was FDA approved in March 2020. A tumor suppressor protein, p16 is found to be overexpressed by HPV oncogenic activity, and Ki-67 is a marker of cellular proliferation. Coexpression of p16 and Ki-67 indicates a loss of cell cycle regulation and is a hallmark of neoplastic transformation. When positive, this test is supportive of active HPV infection and of a high-grade lesion. While the dual stain test is not yet formally incorporated into triage algorithms by national guidelines, it has demonstrated efficacy in detecting CIN 3+
In the IMPACT trial, nearly 5,000 HPV-positive patients underwent p16/Ki-67 dual stain testing compared with cytology and HPV genotyping.25 The sensitivity of dual stain for CIN 3+ was 91.9% (95% CI, 86.1%–95.4%) in HPV 16/18–positive and 86.0% (95% CI, 77.5%–91.6%) in the 12 other genotypes. Using dual stain testing alone to triage HPV-positive results showed significantly higher sensitivity but lower specificity than using cytology alone to triage HPV-positive results. Importantly, triage with dual stain testing alone would have referred significantly fewer women to colposcopy than HPV 16/18 genotyping with cytology triage for the 12 other genotypes (48.6% vs 56.0%; P< .0001).
Self-sampling methods: An approach for potentially improving access to screening
One technology that may help bridge gaps in access to cervical cancer screening is self-collected HPV testing, which would preclude the need for a clinician-performed pelvic exam. At present, no self-sampling method is approved by the FDA. However, many studies have examined the efficacy and safety of various self-sampling kits.26
One randomized controlled trial in the Netherlands compared sensitivity and specificity of CIN 2+ detection in patient-collected versus clinician-collected swabs.27 After a median follow-up of 20 months, the sensitivity and specificity of HPV testing did not differ between the patient-collected and the clinician-collected groups (specificity 100%; 95% CI, 0.91–1.08; sensitivity 96%; 95% CI, 0.90–1.03).27 This analysis did not include patients who did not return their self-collected sample, which leaves the question of whether self-sampling may exacerbate issues with patients who are lost to follow-up.
In a study performed in the United States, 16,590 patients who were overdue for cervical cancer screening were randomly assigned to usual care reminders (annual mailed reminders and phone calls from clinics) or to the addition of a mailed HPV self-sampling test kit.28 While the study did not demonstrate significant difference in the detection of overall CIN 2+ between the 2 groups, screening uptake was higher in the self-sampling kit group than in the usual care reminders group (RR, 1.51; 95% CI, 1.43–1.60), and the number of abnormal screens that warranted colposcopy referral was similar between the 2 groups (36.4% vs 36.8%).28 In qualitative interviews of the participants of this trial, patients who were sent at-home self-sampling kits found that the convenience of at-home testing lowered barriers to scheduling an in-office appointment.29 The hope is that self-sampling methods will expand access of cervical cancer screening to vulnerable populations that face significant barriers to having an in-office pelvic exam.
It is important to note that self-collection and self-sample testing requires multidisciplinary systems for processing results and assuring necessary patient follow-up. Implementing and disseminating such a program has been well tested only in developed countries27,30 with universal health care systems or within an integrated care delivery system. Bringing such technology broadly to the United States and less developed countries will require continued commitment to increasing laboratory capacity, a central electronic health record or system for monitoring results, educational materials for clinicians and patients, and expanding insurance reimbursement for such testing.
HPV vaccination rates must increase
While we continue to investigate which screening methods will most improve our secondary prevention of cervical cancer, our path to increasing primary prevention of cervical cancer is clear: We must increase rates of HPV vaccination. The 9-valent HPV vaccine is FDA approved for use in all patients aged 9 to 45 years.
The American College of Obstetricians and Gynecologists and other organizations recommend HPV vaccination between the ages of 9 and 13, and a “catch-up period” from ages 13 to 26 in which patients previously not vaccinated should receive the vaccine.31 Initiation of the vaccine course earlier (ages 9–10) compared with later (ages 11–12) is correlated with higher overall completion rates by age 15 and has been suggested to be associated with a stronger immune response.32
A study from Sweden found that HPV vaccination before age 17 was most strongly correlated with the lowest rates of cervical cancer, although vaccination between ages 17 and 30 still significantly decreased the risk of cervical cancer compared with those who were unvaccinated.33
Overall HPV vaccination rates in the United States continue to improve, with 58.6%34 of US adolescents having completed vaccination in 2020. However, these rates still are significantly lower than those in many other developed countries, including Australia, which had a complete vaccination rate of 80.5% in 2020.35 Continued disparities in vaccination rates could be contributing to the rise in cervical cancer among certain groups, such as American Indian and Alaska Native populations.5
Work—and innovations—must continue
In conclusion, the incidence of cervical cancer in the United States continues to decrease, although at disparate rates among marginalized populations. To ensure that we are working toward eliminating cervical cancer for all patients, we must continue efforts to eliminate disparities in health access. Continued innovations, including primary HPV testing and self-collection samples, may contribute to lowering barriers to all patients being able to access the preventative care they need. ●
- Centers for Disease Control and Prevention. United States Cancer Statistics: data visualizations. Trends: changes over time: cervix. Accessed January 8, 2023. https://gis.cdc.gov /Cancer/USCS/#/Trends/
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209-249. doi:10.3322/caac.21660.
- Francoeur AA, Liao CI, Casear MA, et al. The increasing incidence of stage IV cervical cancer in the USA: what factors are related? Int J Gynecol Cancer. 2022;32:ijgc-2022-003728. doi:10.1136/ijgc-2022-003728.
- Abdalla E, Habtemariam T, Fall S, et al. A comparative study of health disparities in cervical cancer mortality rates through time between Black and Caucasian women in Alabama and the US. Int J Stud Nurs. 2021;6:9-23. doi:10.20849/ijsn. v6i1.864.
- Bruegl AS, Emerson J, Tirumala K. Persistent disparities of cervical cancer among American Indians/Alaska natives: are we maximizing prevention tools? Gynecol Oncol. 2023;168:5661. doi:10.1016/j.ygyno.2022.11.007.
- Suk R, Hong YR, Rajan SS, et al. Assessment of US Preventive Services Task Force Guideline–Concordant cervical cancer screening rates and reasons for underscreening by age, race and ethnicity, sexual orientation, rurality, and insurance, 2005 to 2019. JAMA Netw Open. 2022;5:e2143582. doi:10.1001/ jamanetworkopen.2021.43582.
- Fontham ETH, Wolf AMD, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346. doi:10.3322/caac.21628.
- US Preventive Services Task Force; Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force Recommendation statement. JAMA. 2018;320:674-686. doi:10.1001/jama.2018.10897.
- Nayar R, Chhieng DC, Crothers B, et al. Moving forward—the 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors and beyond: implications and suggestions for laboratories. J Am Soc Cytopathol. 2020;9:291-303. doi:10.1016/j.jasc.2020.05.002.
- Cooley JJP, Maguire FB, Morris CR, et al. Cervical cancer stage at diagnosis and survival among women ≥65 years in California. Cancer Epidemiol Biomarkers Prev. 2023;32:91-97. doi:10.1158/1055-9965.EPI-22-0793.
- National Cancer Institute. Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Cervical Cancer. Accessed February 21, 2023. https://seer.cancer.gov /statfacts/html/cervix.html
- Feldman S. Screening options for preventing cervical cancer. JAMA Intern Med. 2019;179:879-880. doi:10.1001/ jamainternmed.2019.0298.
- ASCO Post Staff. FDA approves first HPV test for primary cervical cancer screening. ASCO Post. May 15, 2014. Accessed January 8, 2023. https://ascopost.com/issues/may-15-2014 /fda-approves-first-hpv-test-for-primary-cervical-cancer -screening/
- Rijkaart DC, Berkhof J, Rozendaal L, et al. Human papillomavirus testing for the detection of high-grade cervical intraepithelial neoplasia and cancer: final results of the POBASCAM randomised controlled trial. Lancet Oncol. 2012;13:78-88. doi:10.1016/S1470-2045(11)70296-0.
- Ronco G, Giorgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer Screening (NTCC) Working Group. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol. 2010;11:249-257. doi:10.1016/S1470-2045(09)70360-2.
- Kitchener HC, Almonte M, Thomson C, et al. HPV testing in combination with liquid-based cytology in primary cervical screening (ARTISTIC): a randomised controlled trial. Lancet Oncol. 2009;10:672-682. doi:10.1016/S1470-2045(09)70156-1.
- Bulkmans NWJ, Berkhof J, Rozendaal L, et al. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year followup of a randomised controlled implementation trial. Lancet. 2007;370:1764-1772. doi:10.1016/S0140-6736(07)61450-0.
- Ogilvie GS, Van Niekerk D, Krajden M, et al. Effect of screening with primary cervical HPV testing vs cytology testing on high-grade cervical intraepithelial neoplasia at 48 months: the HPV FOCAL randomized clinical trial. JAMA. 2018;320:43-52. doi:10.1001/jama.2018.7464.
- Gottschlich A, Gondara L, Smith LW, et al. Human papillomavirus‐based screening at extended intervals missed fewer cervical precancers than cytology in the HPV For Cervical Cancer (HPV FOCAL) trial. Int J Cancer. 2022;151:897-905. doi:10.1002/ijc.34039.
- Katki HA, Kinney WK, Fetterman B, et al. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Lancet Oncol. 2011;12:663672. doi:10.1016/S1470-2045(11)70145-0.
- Wright TC, Stoler MH, Behrens CM, et al. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015;136:189-197. doi:10.1016/j.ygyno.2014.11.076
- Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Obstet Gynecol. 2015;125:330-337. doi:10.1097/AOG.0000000000000669.
- Silver MI, Rositch AF, Burke AE, et al. Patient concerns about human papillomavirus testing and 5-year intervals in routine cervical cancer screening. Obstet Gynecol. 2015;125:317-329. doi:10.1097/AOG.0000000000000638.
- Smith LW, Racey CS, Gondara L, et al. Women’s acceptability of and experience with primary human papillomavirus testing for cervical screening: HPV FOCAL trial cross-sectional online survey results. BMJ Open. 2021;11:e052084. doi:10.1136/bmjopen-2021-052084.
- Wright TC, Stoler MH, Ranger-Moore J, et al. Clinical validation of p16/Ki-67 dual-stained cytology triage of HPV-positive women: results from the IMPACT trial. Int J Cancer. 2022;150:461-471. doi:10.1002/ijc.33812.
- Yeh PT, Kennedy CE, De Vuyst H, et al. Self-sampling for human papillomavirus (HPV) testing: a systematic review and meta-analysis. BMJ Global Health. 2019;4:e001351. doi:10.1136/bmjgh-2018-001351.
- Polman NJ, Ebisch RMF, Heideman DAM, et al. Performance of human papillomavirus testing on self-collected versus clinician-collected samples for the detection of cervical intraepithelial neoplasia of grade 2 or worse: a randomised, paired screen-positive, non-inferiority trial. Lancet Oncol. 2019;20:229-238. doi:10.1016/S1470-2045(18)30763-0.
- Winer RL, Lin J, Tiro JA, et al. Effect of mailed human papillomavirus test kits vs usual care reminders on cervical cancer screening uptake, precancer detection, and treatment: a randomized clinical trial. JAMA Netw Open. 2019;2:e1914729. doi:10.1001/jamanetworkopen.2019.14729.
- Tiro JA, Betts AC, Kimbel K, et al. Understanding patients’ perspectives and information needs following a positive home human papillomavirus self-sampling kit result. J Womens Health (Larchmt). 2019;28:384-392. doi:10.1089/ jwh.2018.7070.
- Knauss T, Hansen BT, Pedersen K, et al. The cost-effectiveness of opt-in and send-to-all HPV self-sampling among long-term non-attenders to cervical cancer screening in Norway: the Equalscreen randomized controlled trial. Gynecol Oncol. 2023;168:39-47. doi:10.1016/j.ygyno.2022.10.027.
- ACOG committee opinion no. 809. Human papillomavirus vaccination: correction. Obstet Gynecol. 2022;139:345. doi:10.1097/AOG.0000000000004680.
- St Sauver JL, Finney Rutten LJF, Ebbert JO, et al. Younger age at initiation of the human papillomavirus (HPV) vaccination series is associated with higher rates of on-time completion. Prev Med. 2016;89:327-333. doi:10.1016/j.ypmed.2016.02.039.
- Lei J, Ploner A, Elfström KM, et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383:13401348. doi:10.1056/NEJMoa1917338.
- Pingali C, Yankey D, Elam-Evans LD, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years — United States, 2020. MMWR Morb Mortal Wkly Rep. 2021;70:1183-1190. doi:10.15585/ mmwr.mm7035a1.
- National Centre for Immunisation Research and Surveillance Australia. Annual Immunisation Coverage Report 2020. November 29, 2021. Accessed March 1, 2023. https://ncirs .org.au/sites/default/files/2021-11/NCIRS%20Annual%20 Immunisation%20Coverage%20Report%202020_FINAL.pdf
- Leung SOA, Feldman S. 2022 Update on cervical disease. OBG Manag. 2022;34(5):16-17, 22-24, 26, 28. doi:10.12788/ obgm.0197.
CASE Intervention approaches for decreasing the risk of cervical cancer
A 25-year-old woman presents to your practice for routine examination. She has never undergone cervical cancer screening or received the human papillomavirus (HPV) vaccine series. The patient has had 3 lifetime sexual partners and currently uses condoms as contraception. What interventions are appropriate to offer this patient to decrease her risk of cervical cancer? Choose as many that may apply:
1. cervical cytology with reflex HPV testing
2. cervical cytology with HPV cotesting
3. primary HPV testing
4. HPV vaccine series (3 doses)
5. all of the above
The answer is number 5, all of the above.
Choices 1, 2, and 3 are acceptable methods of cervical cancer screening for this patient. Catch-up HPV vaccination should be offered as well.
Equitable preventive care is needed
Cervical cancer is a unique cancer because it has a known preventative strategy. HPV vaccination, paired with cervical screening and management of abnormal results, has contributed to decreased rates of cervical cancer in the United States, from 13,914 cases in 1999 to 12,795 cases in 2019.1 In less-developed countries, however, cervical cancer continues to be a leading cause of mortality, with 90% of cervical cancer deaths in 2020 occurring in low- and middle-income countries.2
Disparate outcomes in cervical cancer are often a reflection of disparities in health access. Within the United States, Black women have a higher incidence of cervical cancer, advanced-stage disease, and mortality from cervical cancer than White women.3,4 Furthermore, the incidence of cervical cancer increased among American Indian and Alaska Native people between 2000 and 2019.5 The rate for patients who are overdue for cervical cancer screening is higher among Asian and Hispanic patients compared with non-Hispanic White patients (31.4% vs 20.1%; P=.01) and among patients who identify as LGBTQ+ compared with patients who identify as heterosexual (32.0% vs 22.2%; P<.001).6 Younger patients have a significantly higher rate for overdue screening compared with their older counterparts (29.1% vs 21.1%; P<.001), as do uninsured patients compared with those who are privately insured (41.7% vs 18.1%; P<.001). Overall, the proportion of women without up-to-date screening increased significantly from 2005 to 2019 (14.4% vs 23.0%; P<.001).6
Unfortunately, despite a known strategy to eliminate cervical cancer, we are not accomplishing equitable preventative care. Barriers to care can include patient-centered issues, such as fear of cancer or of painful evaluations, lack of trust in the health care system, and inadequate understanding of the benefits of cancer prevention, in addition to systemic and structural barriers. As we assess new technologies, one of our most important goals is to consider how such innovations can increase health access—whether through increasing ease and acceptability of testing or by creating more effective screening tests.
Updates to cervical screening guidance
In 2020, the American Cancer Society (ACS) updated its cervical screening guidelines to start screening at age 25 years with the “preferred” strategy of HPV primary testing every 5 years.7 By contrast, the US Preventive Services Task Force (USPSTF) continues to recommend 1 of 3 methods: cytology alone every 3 years; cytology alone every 3 years between ages 21 and 29 followed by cytology and HPV cotesting every 5 years at age 30 or older; or high-risk HPV testing alone every 5 years (TABLE).8

To successfully prevent cervical cancer, abnormal results are managed by performing either colposcopy with biopsy, immediate treatment, or close surveillance based on the risk of developing cervical intraepithelial neoplasia (CIN) 3 or worse. A patient’s risk is determined based on both current and prior test results. The ASCCP (American Society for Colposcopy and Cervical Pathology) transitioned to risk-based management guidelines in 2019 and has both an app and a web-based risk assessment tool available for clinicians (https://www.asccp.org).9
All organizations recommend stopping screening after age 65 provided there has been a history of adequate screening in the prior 10 years (defined as 2 normal cotests or 3 normal cytology tests, with the most recent test within 5 years) and no history of CIN 2 or worse within the prior 25 years.10,11 Recent studies that examined the rate of cervical cancer diagnosed in patients older than 65 years have questioned whether patients should continue screening beyond 65.10 In the United States, 20% of cervical cancer still occurs in women older than age 65.11 One reason may be that many women have not met the requirement for adequate and normal prior screening and may still need ongoing testing.12

Continue to: Primary HPV screening...
Primary HPV screening
Primary HPV testing means that an HPV test is performed first, and if it is positive for high-risk HPV, further testing is performed to determine next steps. This contrasts with the currently used method of obtaining cytology (Pap) first with either concurrent HPV testing or reflex HPV testing. The first HPV primary screening test was approved by the US Food and Drug Administration (FDA) in 2014.13
Multiple randomized controlled trials in Europe have demonstrated the accuracy of HPV-based screening compared with cytology in the detection of cervical cancer and its precursors.14-17 The HPV FOCAL trial demonstrated increased efficacy of primary HPV screening in the detection of CIN 2+ lesions.18 This trial recruited a total of 19,000 women, ages 25 to 65, in Canada and randomly assigned them to receive primary HPV testing or liquid-based cytology. If primary HPV testing was negative, participants would return in 48 months for cytology and HPV cotesting. If primary liquid-based cytology testing was negative, participants would return at 24 months for cytology testing alone and at 48 months for cytology and HPV cotesting. Both groups had similar incidences of CIN 2+ over the study period. HPV testing was shown to detect CIN 2+ at higher rates at the time of initial screen (risk ratio [RR], 1.61; 95% confidence interval [CI], 1.24–2.09) and then significantly lower rates at the time of exit screening at 48 months (RR, 0.36; 95% CI, 0.24–0.54).18 These results demonstrated that primary HPV testing detects CIN 2+ earlier than cytology alone. In follow-up analyses, primary HPV screening missed fewer CIN 2+ diagnoses than cytology screening.19
While not as many studies have compared primary HPV testing to cytology with an HPV cotest, the current most common practice in the United States, one study performed in the United States found that a negative cytology result did not further decrease the risk of CIN 3 for HPV-negative patients (risk of CIN 3+ at 5 years: 0.16% vs 0.17%; P=0.8) and concluded that a negative HPV test was enough reassurance for a low risk of CIN 3+.20
Another study, the ATHENA trial, evaluated more than 42,000 women who were 25 years and older over a 3-year period.21 Patients underwent either primary HPV testing or combination cytology and reflex HPV (if ages 25–29) or HPV cotesting (if age 30 or older). Primary HPV testing was found to have a sensitivity and specificity of 76.1% and 93.5%, respectively, compared with 61.7% and 94.6% for cytology with HPV cotesting, but it also increased the total number of colposcopies performed.21
Subsequent management of a primary HPV-positive result can be triaged using genotyping, cytology, or a combination of both. FDA-approved HPV screening tests provide genotyping and current management guidelines use genotyping to triage positive HPV results into HPV 16, 18, or 1 of 12 other high-risk HPV genotypes.
In the ATHENA trial, the 3-year incidence of CIN 3+ for HPV 16/18-positive results was 21.16% (95% CI, 18.39%–24.01%) compared with 5.4% (95% CI, 4.5%–6.4%) among patients with an HPV test positive for 1 of the other HPV genotypes.21 While a patient with an HPV result positive for HPV 16/18 should directly undergo colposcopy, clinical guidance for an HPV-positive result for one of the other genotypes suggests using reflex cytology to triage patients. The ASCCP recommended management of primary HPV testing is included in the FIGURE.22
Many barriers remain to transitioning to primary HPV testing, including laboratory test availability as well as patient and provider acceptance. At present, 2 FDA-approved primary HPV screening tests are available: the Cobas HPV test (Roche Molecular Systems, Inc) and the BD Onclarity HPV assay (Becton, Dickinson and Company). Changes to screening recommendations need to be accompanied by patient and provider outreach and education.
In a survey of more than 500 US women in 2015 after guidelines allowed for increased screening intervals after negative results, a majority of women (55.6%; 95% CI, 51.4%–59.8%) were aware that screening recommendations had changed; however, 74.1% (95% CI, 70.3%–77.7%) still believed that women should be screened annually.23 By contrast, participants in the HPV FOCAL trial, who were able to learn more about HPV-based screening, were surveyed about their willingness to undergo primary HPV testing rather than Pap testing at the conclusion of the trial.24 Of the participants, 63% were comfortable with primary HPV testing, and 54% were accepting of an extended screening interval of 4 to 5 years.24
Continue to: p16/Ki-67 dual-stain cytology...
p16/Ki-67 dual-stain cytology
An additional tool for triaging HPV-positive patients is the p16/Ki-67 dual stain test (CINtec Plus Cytology; Roche), which was FDA approved in March 2020. A tumor suppressor protein, p16 is found to be overexpressed by HPV oncogenic activity, and Ki-67 is a marker of cellular proliferation. Coexpression of p16 and Ki-67 indicates a loss of cell cycle regulation and is a hallmark of neoplastic transformation. When positive, this test is supportive of active HPV infection and of a high-grade lesion. While the dual stain test is not yet formally incorporated into triage algorithms by national guidelines, it has demonstrated efficacy in detecting CIN 3+
In the IMPACT trial, nearly 5,000 HPV-positive patients underwent p16/Ki-67 dual stain testing compared with cytology and HPV genotyping.25 The sensitivity of dual stain for CIN 3+ was 91.9% (95% CI, 86.1%–95.4%) in HPV 16/18–positive and 86.0% (95% CI, 77.5%–91.6%) in the 12 other genotypes. Using dual stain testing alone to triage HPV-positive results showed significantly higher sensitivity but lower specificity than using cytology alone to triage HPV-positive results. Importantly, triage with dual stain testing alone would have referred significantly fewer women to colposcopy than HPV 16/18 genotyping with cytology triage for the 12 other genotypes (48.6% vs 56.0%; P< .0001).
Self-sampling methods: An approach for potentially improving access to screening
One technology that may help bridge gaps in access to cervical cancer screening is self-collected HPV testing, which would preclude the need for a clinician-performed pelvic exam. At present, no self-sampling method is approved by the FDA. However, many studies have examined the efficacy and safety of various self-sampling kits.26
One randomized controlled trial in the Netherlands compared sensitivity and specificity of CIN 2+ detection in patient-collected versus clinician-collected swabs.27 After a median follow-up of 20 months, the sensitivity and specificity of HPV testing did not differ between the patient-collected and the clinician-collected groups (specificity 100%; 95% CI, 0.91–1.08; sensitivity 96%; 95% CI, 0.90–1.03).27 This analysis did not include patients who did not return their self-collected sample, which leaves the question of whether self-sampling may exacerbate issues with patients who are lost to follow-up.
In a study performed in the United States, 16,590 patients who were overdue for cervical cancer screening were randomly assigned to usual care reminders (annual mailed reminders and phone calls from clinics) or to the addition of a mailed HPV self-sampling test kit.28 While the study did not demonstrate significant difference in the detection of overall CIN 2+ between the 2 groups, screening uptake was higher in the self-sampling kit group than in the usual care reminders group (RR, 1.51; 95% CI, 1.43–1.60), and the number of abnormal screens that warranted colposcopy referral was similar between the 2 groups (36.4% vs 36.8%).28 In qualitative interviews of the participants of this trial, patients who were sent at-home self-sampling kits found that the convenience of at-home testing lowered barriers to scheduling an in-office appointment.29 The hope is that self-sampling methods will expand access of cervical cancer screening to vulnerable populations that face significant barriers to having an in-office pelvic exam.
It is important to note that self-collection and self-sample testing requires multidisciplinary systems for processing results and assuring necessary patient follow-up. Implementing and disseminating such a program has been well tested only in developed countries27,30 with universal health care systems or within an integrated care delivery system. Bringing such technology broadly to the United States and less developed countries will require continued commitment to increasing laboratory capacity, a central electronic health record or system for monitoring results, educational materials for clinicians and patients, and expanding insurance reimbursement for such testing.
HPV vaccination rates must increase
While we continue to investigate which screening methods will most improve our secondary prevention of cervical cancer, our path to increasing primary prevention of cervical cancer is clear: We must increase rates of HPV vaccination. The 9-valent HPV vaccine is FDA approved for use in all patients aged 9 to 45 years.
The American College of Obstetricians and Gynecologists and other organizations recommend HPV vaccination between the ages of 9 and 13, and a “catch-up period” from ages 13 to 26 in which patients previously not vaccinated should receive the vaccine.31 Initiation of the vaccine course earlier (ages 9–10) compared with later (ages 11–12) is correlated with higher overall completion rates by age 15 and has been suggested to be associated with a stronger immune response.32
A study from Sweden found that HPV vaccination before age 17 was most strongly correlated with the lowest rates of cervical cancer, although vaccination between ages 17 and 30 still significantly decreased the risk of cervical cancer compared with those who were unvaccinated.33
Overall HPV vaccination rates in the United States continue to improve, with 58.6%34 of US adolescents having completed vaccination in 2020. However, these rates still are significantly lower than those in many other developed countries, including Australia, which had a complete vaccination rate of 80.5% in 2020.35 Continued disparities in vaccination rates could be contributing to the rise in cervical cancer among certain groups, such as American Indian and Alaska Native populations.5
Work—and innovations—must continue
In conclusion, the incidence of cervical cancer in the United States continues to decrease, although at disparate rates among marginalized populations. To ensure that we are working toward eliminating cervical cancer for all patients, we must continue efforts to eliminate disparities in health access. Continued innovations, including primary HPV testing and self-collection samples, may contribute to lowering barriers to all patients being able to access the preventative care they need. ●
CASE Intervention approaches for decreasing the risk of cervical cancer
A 25-year-old woman presents to your practice for routine examination. She has never undergone cervical cancer screening or received the human papillomavirus (HPV) vaccine series. The patient has had 3 lifetime sexual partners and currently uses condoms as contraception. What interventions are appropriate to offer this patient to decrease her risk of cervical cancer? Choose as many that may apply:
1. cervical cytology with reflex HPV testing
2. cervical cytology with HPV cotesting
3. primary HPV testing
4. HPV vaccine series (3 doses)
5. all of the above
The answer is number 5, all of the above.
Choices 1, 2, and 3 are acceptable methods of cervical cancer screening for this patient. Catch-up HPV vaccination should be offered as well.
Equitable preventive care is needed
Cervical cancer is a unique cancer because it has a known preventative strategy. HPV vaccination, paired with cervical screening and management of abnormal results, has contributed to decreased rates of cervical cancer in the United States, from 13,914 cases in 1999 to 12,795 cases in 2019.1 In less-developed countries, however, cervical cancer continues to be a leading cause of mortality, with 90% of cervical cancer deaths in 2020 occurring in low- and middle-income countries.2
Disparate outcomes in cervical cancer are often a reflection of disparities in health access. Within the United States, Black women have a higher incidence of cervical cancer, advanced-stage disease, and mortality from cervical cancer than White women.3,4 Furthermore, the incidence of cervical cancer increased among American Indian and Alaska Native people between 2000 and 2019.5 The rate for patients who are overdue for cervical cancer screening is higher among Asian and Hispanic patients compared with non-Hispanic White patients (31.4% vs 20.1%; P=.01) and among patients who identify as LGBTQ+ compared with patients who identify as heterosexual (32.0% vs 22.2%; P<.001).6 Younger patients have a significantly higher rate for overdue screening compared with their older counterparts (29.1% vs 21.1%; P<.001), as do uninsured patients compared with those who are privately insured (41.7% vs 18.1%; P<.001). Overall, the proportion of women without up-to-date screening increased significantly from 2005 to 2019 (14.4% vs 23.0%; P<.001).6
Unfortunately, despite a known strategy to eliminate cervical cancer, we are not accomplishing equitable preventative care. Barriers to care can include patient-centered issues, such as fear of cancer or of painful evaluations, lack of trust in the health care system, and inadequate understanding of the benefits of cancer prevention, in addition to systemic and structural barriers. As we assess new technologies, one of our most important goals is to consider how such innovations can increase health access—whether through increasing ease and acceptability of testing or by creating more effective screening tests.
Updates to cervical screening guidance
In 2020, the American Cancer Society (ACS) updated its cervical screening guidelines to start screening at age 25 years with the “preferred” strategy of HPV primary testing every 5 years.7 By contrast, the US Preventive Services Task Force (USPSTF) continues to recommend 1 of 3 methods: cytology alone every 3 years; cytology alone every 3 years between ages 21 and 29 followed by cytology and HPV cotesting every 5 years at age 30 or older; or high-risk HPV testing alone every 5 years (TABLE).8

To successfully prevent cervical cancer, abnormal results are managed by performing either colposcopy with biopsy, immediate treatment, or close surveillance based on the risk of developing cervical intraepithelial neoplasia (CIN) 3 or worse. A patient’s risk is determined based on both current and prior test results. The ASCCP (American Society for Colposcopy and Cervical Pathology) transitioned to risk-based management guidelines in 2019 and has both an app and a web-based risk assessment tool available for clinicians (https://www.asccp.org).9
All organizations recommend stopping screening after age 65 provided there has been a history of adequate screening in the prior 10 years (defined as 2 normal cotests or 3 normal cytology tests, with the most recent test within 5 years) and no history of CIN 2 or worse within the prior 25 years.10,11 Recent studies that examined the rate of cervical cancer diagnosed in patients older than 65 years have questioned whether patients should continue screening beyond 65.10 In the United States, 20% of cervical cancer still occurs in women older than age 65.11 One reason may be that many women have not met the requirement for adequate and normal prior screening and may still need ongoing testing.12

Continue to: Primary HPV screening...
Primary HPV screening
Primary HPV testing means that an HPV test is performed first, and if it is positive for high-risk HPV, further testing is performed to determine next steps. This contrasts with the currently used method of obtaining cytology (Pap) first with either concurrent HPV testing or reflex HPV testing. The first HPV primary screening test was approved by the US Food and Drug Administration (FDA) in 2014.13
Multiple randomized controlled trials in Europe have demonstrated the accuracy of HPV-based screening compared with cytology in the detection of cervical cancer and its precursors.14-17 The HPV FOCAL trial demonstrated increased efficacy of primary HPV screening in the detection of CIN 2+ lesions.18 This trial recruited a total of 19,000 women, ages 25 to 65, in Canada and randomly assigned them to receive primary HPV testing or liquid-based cytology. If primary HPV testing was negative, participants would return in 48 months for cytology and HPV cotesting. If primary liquid-based cytology testing was negative, participants would return at 24 months for cytology testing alone and at 48 months for cytology and HPV cotesting. Both groups had similar incidences of CIN 2+ over the study period. HPV testing was shown to detect CIN 2+ at higher rates at the time of initial screen (risk ratio [RR], 1.61; 95% confidence interval [CI], 1.24–2.09) and then significantly lower rates at the time of exit screening at 48 months (RR, 0.36; 95% CI, 0.24–0.54).18 These results demonstrated that primary HPV testing detects CIN 2+ earlier than cytology alone. In follow-up analyses, primary HPV screening missed fewer CIN 2+ diagnoses than cytology screening.19
While not as many studies have compared primary HPV testing to cytology with an HPV cotest, the current most common practice in the United States, one study performed in the United States found that a negative cytology result did not further decrease the risk of CIN 3 for HPV-negative patients (risk of CIN 3+ at 5 years: 0.16% vs 0.17%; P=0.8) and concluded that a negative HPV test was enough reassurance for a low risk of CIN 3+.20
Another study, the ATHENA trial, evaluated more than 42,000 women who were 25 years and older over a 3-year period.21 Patients underwent either primary HPV testing or combination cytology and reflex HPV (if ages 25–29) or HPV cotesting (if age 30 or older). Primary HPV testing was found to have a sensitivity and specificity of 76.1% and 93.5%, respectively, compared with 61.7% and 94.6% for cytology with HPV cotesting, but it also increased the total number of colposcopies performed.21
Subsequent management of a primary HPV-positive result can be triaged using genotyping, cytology, or a combination of both. FDA-approved HPV screening tests provide genotyping and current management guidelines use genotyping to triage positive HPV results into HPV 16, 18, or 1 of 12 other high-risk HPV genotypes.
In the ATHENA trial, the 3-year incidence of CIN 3+ for HPV 16/18-positive results was 21.16% (95% CI, 18.39%–24.01%) compared with 5.4% (95% CI, 4.5%–6.4%) among patients with an HPV test positive for 1 of the other HPV genotypes.21 While a patient with an HPV result positive for HPV 16/18 should directly undergo colposcopy, clinical guidance for an HPV-positive result for one of the other genotypes suggests using reflex cytology to triage patients. The ASCCP recommended management of primary HPV testing is included in the FIGURE.22
Many barriers remain to transitioning to primary HPV testing, including laboratory test availability as well as patient and provider acceptance. At present, 2 FDA-approved primary HPV screening tests are available: the Cobas HPV test (Roche Molecular Systems, Inc) and the BD Onclarity HPV assay (Becton, Dickinson and Company). Changes to screening recommendations need to be accompanied by patient and provider outreach and education.
In a survey of more than 500 US women in 2015 after guidelines allowed for increased screening intervals after negative results, a majority of women (55.6%; 95% CI, 51.4%–59.8%) were aware that screening recommendations had changed; however, 74.1% (95% CI, 70.3%–77.7%) still believed that women should be screened annually.23 By contrast, participants in the HPV FOCAL trial, who were able to learn more about HPV-based screening, were surveyed about their willingness to undergo primary HPV testing rather than Pap testing at the conclusion of the trial.24 Of the participants, 63% were comfortable with primary HPV testing, and 54% were accepting of an extended screening interval of 4 to 5 years.24
Continue to: p16/Ki-67 dual-stain cytology...
p16/Ki-67 dual-stain cytology
An additional tool for triaging HPV-positive patients is the p16/Ki-67 dual stain test (CINtec Plus Cytology; Roche), which was FDA approved in March 2020. A tumor suppressor protein, p16 is found to be overexpressed by HPV oncogenic activity, and Ki-67 is a marker of cellular proliferation. Coexpression of p16 and Ki-67 indicates a loss of cell cycle regulation and is a hallmark of neoplastic transformation. When positive, this test is supportive of active HPV infection and of a high-grade lesion. While the dual stain test is not yet formally incorporated into triage algorithms by national guidelines, it has demonstrated efficacy in detecting CIN 3+
In the IMPACT trial, nearly 5,000 HPV-positive patients underwent p16/Ki-67 dual stain testing compared with cytology and HPV genotyping.25 The sensitivity of dual stain for CIN 3+ was 91.9% (95% CI, 86.1%–95.4%) in HPV 16/18–positive and 86.0% (95% CI, 77.5%–91.6%) in the 12 other genotypes. Using dual stain testing alone to triage HPV-positive results showed significantly higher sensitivity but lower specificity than using cytology alone to triage HPV-positive results. Importantly, triage with dual stain testing alone would have referred significantly fewer women to colposcopy than HPV 16/18 genotyping with cytology triage for the 12 other genotypes (48.6% vs 56.0%; P< .0001).
Self-sampling methods: An approach for potentially improving access to screening
One technology that may help bridge gaps in access to cervical cancer screening is self-collected HPV testing, which would preclude the need for a clinician-performed pelvic exam. At present, no self-sampling method is approved by the FDA. However, many studies have examined the efficacy and safety of various self-sampling kits.26
One randomized controlled trial in the Netherlands compared sensitivity and specificity of CIN 2+ detection in patient-collected versus clinician-collected swabs.27 After a median follow-up of 20 months, the sensitivity and specificity of HPV testing did not differ between the patient-collected and the clinician-collected groups (specificity 100%; 95% CI, 0.91–1.08; sensitivity 96%; 95% CI, 0.90–1.03).27 This analysis did not include patients who did not return their self-collected sample, which leaves the question of whether self-sampling may exacerbate issues with patients who are lost to follow-up.
In a study performed in the United States, 16,590 patients who were overdue for cervical cancer screening were randomly assigned to usual care reminders (annual mailed reminders and phone calls from clinics) or to the addition of a mailed HPV self-sampling test kit.28 While the study did not demonstrate significant difference in the detection of overall CIN 2+ between the 2 groups, screening uptake was higher in the self-sampling kit group than in the usual care reminders group (RR, 1.51; 95% CI, 1.43–1.60), and the number of abnormal screens that warranted colposcopy referral was similar between the 2 groups (36.4% vs 36.8%).28 In qualitative interviews of the participants of this trial, patients who were sent at-home self-sampling kits found that the convenience of at-home testing lowered barriers to scheduling an in-office appointment.29 The hope is that self-sampling methods will expand access of cervical cancer screening to vulnerable populations that face significant barriers to having an in-office pelvic exam.
It is important to note that self-collection and self-sample testing requires multidisciplinary systems for processing results and assuring necessary patient follow-up. Implementing and disseminating such a program has been well tested only in developed countries27,30 with universal health care systems or within an integrated care delivery system. Bringing such technology broadly to the United States and less developed countries will require continued commitment to increasing laboratory capacity, a central electronic health record or system for monitoring results, educational materials for clinicians and patients, and expanding insurance reimbursement for such testing.
HPV vaccination rates must increase
While we continue to investigate which screening methods will most improve our secondary prevention of cervical cancer, our path to increasing primary prevention of cervical cancer is clear: We must increase rates of HPV vaccination. The 9-valent HPV vaccine is FDA approved for use in all patients aged 9 to 45 years.
The American College of Obstetricians and Gynecologists and other organizations recommend HPV vaccination between the ages of 9 and 13, and a “catch-up period” from ages 13 to 26 in which patients previously not vaccinated should receive the vaccine.31 Initiation of the vaccine course earlier (ages 9–10) compared with later (ages 11–12) is correlated with higher overall completion rates by age 15 and has been suggested to be associated with a stronger immune response.32
A study from Sweden found that HPV vaccination before age 17 was most strongly correlated with the lowest rates of cervical cancer, although vaccination between ages 17 and 30 still significantly decreased the risk of cervical cancer compared with those who were unvaccinated.33
Overall HPV vaccination rates in the United States continue to improve, with 58.6%34 of US adolescents having completed vaccination in 2020. However, these rates still are significantly lower than those in many other developed countries, including Australia, which had a complete vaccination rate of 80.5% in 2020.35 Continued disparities in vaccination rates could be contributing to the rise in cervical cancer among certain groups, such as American Indian and Alaska Native populations.5
Work—and innovations—must continue
In conclusion, the incidence of cervical cancer in the United States continues to decrease, although at disparate rates among marginalized populations. To ensure that we are working toward eliminating cervical cancer for all patients, we must continue efforts to eliminate disparities in health access. Continued innovations, including primary HPV testing and self-collection samples, may contribute to lowering barriers to all patients being able to access the preventative care they need. ●
- Centers for Disease Control and Prevention. United States Cancer Statistics: data visualizations. Trends: changes over time: cervix. Accessed January 8, 2023. https://gis.cdc.gov /Cancer/USCS/#/Trends/
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209-249. doi:10.3322/caac.21660.
- Francoeur AA, Liao CI, Casear MA, et al. The increasing incidence of stage IV cervical cancer in the USA: what factors are related? Int J Gynecol Cancer. 2022;32:ijgc-2022-003728. doi:10.1136/ijgc-2022-003728.
- Abdalla E, Habtemariam T, Fall S, et al. A comparative study of health disparities in cervical cancer mortality rates through time between Black and Caucasian women in Alabama and the US. Int J Stud Nurs. 2021;6:9-23. doi:10.20849/ijsn. v6i1.864.
- Bruegl AS, Emerson J, Tirumala K. Persistent disparities of cervical cancer among American Indians/Alaska natives: are we maximizing prevention tools? Gynecol Oncol. 2023;168:5661. doi:10.1016/j.ygyno.2022.11.007.
- Suk R, Hong YR, Rajan SS, et al. Assessment of US Preventive Services Task Force Guideline–Concordant cervical cancer screening rates and reasons for underscreening by age, race and ethnicity, sexual orientation, rurality, and insurance, 2005 to 2019. JAMA Netw Open. 2022;5:e2143582. doi:10.1001/ jamanetworkopen.2021.43582.
- Fontham ETH, Wolf AMD, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346. doi:10.3322/caac.21628.
- US Preventive Services Task Force; Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force Recommendation statement. JAMA. 2018;320:674-686. doi:10.1001/jama.2018.10897.
- Nayar R, Chhieng DC, Crothers B, et al. Moving forward—the 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors and beyond: implications and suggestions for laboratories. J Am Soc Cytopathol. 2020;9:291-303. doi:10.1016/j.jasc.2020.05.002.
- Cooley JJP, Maguire FB, Morris CR, et al. Cervical cancer stage at diagnosis and survival among women ≥65 years in California. Cancer Epidemiol Biomarkers Prev. 2023;32:91-97. doi:10.1158/1055-9965.EPI-22-0793.
- National Cancer Institute. Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Cervical Cancer. Accessed February 21, 2023. https://seer.cancer.gov /statfacts/html/cervix.html
- Feldman S. Screening options for preventing cervical cancer. JAMA Intern Med. 2019;179:879-880. doi:10.1001/ jamainternmed.2019.0298.
- ASCO Post Staff. FDA approves first HPV test for primary cervical cancer screening. ASCO Post. May 15, 2014. Accessed January 8, 2023. https://ascopost.com/issues/may-15-2014 /fda-approves-first-hpv-test-for-primary-cervical-cancer -screening/
- Rijkaart DC, Berkhof J, Rozendaal L, et al. Human papillomavirus testing for the detection of high-grade cervical intraepithelial neoplasia and cancer: final results of the POBASCAM randomised controlled trial. Lancet Oncol. 2012;13:78-88. doi:10.1016/S1470-2045(11)70296-0.
- Ronco G, Giorgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer Screening (NTCC) Working Group. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol. 2010;11:249-257. doi:10.1016/S1470-2045(09)70360-2.
- Kitchener HC, Almonte M, Thomson C, et al. HPV testing in combination with liquid-based cytology in primary cervical screening (ARTISTIC): a randomised controlled trial. Lancet Oncol. 2009;10:672-682. doi:10.1016/S1470-2045(09)70156-1.
- Bulkmans NWJ, Berkhof J, Rozendaal L, et al. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year followup of a randomised controlled implementation trial. Lancet. 2007;370:1764-1772. doi:10.1016/S0140-6736(07)61450-0.
- Ogilvie GS, Van Niekerk D, Krajden M, et al. Effect of screening with primary cervical HPV testing vs cytology testing on high-grade cervical intraepithelial neoplasia at 48 months: the HPV FOCAL randomized clinical trial. JAMA. 2018;320:43-52. doi:10.1001/jama.2018.7464.
- Gottschlich A, Gondara L, Smith LW, et al. Human papillomavirus‐based screening at extended intervals missed fewer cervical precancers than cytology in the HPV For Cervical Cancer (HPV FOCAL) trial. Int J Cancer. 2022;151:897-905. doi:10.1002/ijc.34039.
- Katki HA, Kinney WK, Fetterman B, et al. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Lancet Oncol. 2011;12:663672. doi:10.1016/S1470-2045(11)70145-0.
- Wright TC, Stoler MH, Behrens CM, et al. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015;136:189-197. doi:10.1016/j.ygyno.2014.11.076
- Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Obstet Gynecol. 2015;125:330-337. doi:10.1097/AOG.0000000000000669.
- Silver MI, Rositch AF, Burke AE, et al. Patient concerns about human papillomavirus testing and 5-year intervals in routine cervical cancer screening. Obstet Gynecol. 2015;125:317-329. doi:10.1097/AOG.0000000000000638.
- Smith LW, Racey CS, Gondara L, et al. Women’s acceptability of and experience with primary human papillomavirus testing for cervical screening: HPV FOCAL trial cross-sectional online survey results. BMJ Open. 2021;11:e052084. doi:10.1136/bmjopen-2021-052084.
- Wright TC, Stoler MH, Ranger-Moore J, et al. Clinical validation of p16/Ki-67 dual-stained cytology triage of HPV-positive women: results from the IMPACT trial. Int J Cancer. 2022;150:461-471. doi:10.1002/ijc.33812.
- Yeh PT, Kennedy CE, De Vuyst H, et al. Self-sampling for human papillomavirus (HPV) testing: a systematic review and meta-analysis. BMJ Global Health. 2019;4:e001351. doi:10.1136/bmjgh-2018-001351.
- Polman NJ, Ebisch RMF, Heideman DAM, et al. Performance of human papillomavirus testing on self-collected versus clinician-collected samples for the detection of cervical intraepithelial neoplasia of grade 2 or worse: a randomised, paired screen-positive, non-inferiority trial. Lancet Oncol. 2019;20:229-238. doi:10.1016/S1470-2045(18)30763-0.
- Winer RL, Lin J, Tiro JA, et al. Effect of mailed human papillomavirus test kits vs usual care reminders on cervical cancer screening uptake, precancer detection, and treatment: a randomized clinical trial. JAMA Netw Open. 2019;2:e1914729. doi:10.1001/jamanetworkopen.2019.14729.
- Tiro JA, Betts AC, Kimbel K, et al. Understanding patients’ perspectives and information needs following a positive home human papillomavirus self-sampling kit result. J Womens Health (Larchmt). 2019;28:384-392. doi:10.1089/ jwh.2018.7070.
- Knauss T, Hansen BT, Pedersen K, et al. The cost-effectiveness of opt-in and send-to-all HPV self-sampling among long-term non-attenders to cervical cancer screening in Norway: the Equalscreen randomized controlled trial. Gynecol Oncol. 2023;168:39-47. doi:10.1016/j.ygyno.2022.10.027.
- ACOG committee opinion no. 809. Human papillomavirus vaccination: correction. Obstet Gynecol. 2022;139:345. doi:10.1097/AOG.0000000000004680.
- St Sauver JL, Finney Rutten LJF, Ebbert JO, et al. Younger age at initiation of the human papillomavirus (HPV) vaccination series is associated with higher rates of on-time completion. Prev Med. 2016;89:327-333. doi:10.1016/j.ypmed.2016.02.039.
- Lei J, Ploner A, Elfström KM, et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383:13401348. doi:10.1056/NEJMoa1917338.
- Pingali C, Yankey D, Elam-Evans LD, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years — United States, 2020. MMWR Morb Mortal Wkly Rep. 2021;70:1183-1190. doi:10.15585/ mmwr.mm7035a1.
- National Centre for Immunisation Research and Surveillance Australia. Annual Immunisation Coverage Report 2020. November 29, 2021. Accessed March 1, 2023. https://ncirs .org.au/sites/default/files/2021-11/NCIRS%20Annual%20 Immunisation%20Coverage%20Report%202020_FINAL.pdf
- Leung SOA, Feldman S. 2022 Update on cervical disease. OBG Manag. 2022;34(5):16-17, 22-24, 26, 28. doi:10.12788/ obgm.0197.
- Centers for Disease Control and Prevention. United States Cancer Statistics: data visualizations. Trends: changes over time: cervix. Accessed January 8, 2023. https://gis.cdc.gov /Cancer/USCS/#/Trends/
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209-249. doi:10.3322/caac.21660.
- Francoeur AA, Liao CI, Casear MA, et al. The increasing incidence of stage IV cervical cancer in the USA: what factors are related? Int J Gynecol Cancer. 2022;32:ijgc-2022-003728. doi:10.1136/ijgc-2022-003728.
- Abdalla E, Habtemariam T, Fall S, et al. A comparative study of health disparities in cervical cancer mortality rates through time between Black and Caucasian women in Alabama and the US. Int J Stud Nurs. 2021;6:9-23. doi:10.20849/ijsn. v6i1.864.
- Bruegl AS, Emerson J, Tirumala K. Persistent disparities of cervical cancer among American Indians/Alaska natives: are we maximizing prevention tools? Gynecol Oncol. 2023;168:5661. doi:10.1016/j.ygyno.2022.11.007.
- Suk R, Hong YR, Rajan SS, et al. Assessment of US Preventive Services Task Force Guideline–Concordant cervical cancer screening rates and reasons for underscreening by age, race and ethnicity, sexual orientation, rurality, and insurance, 2005 to 2019. JAMA Netw Open. 2022;5:e2143582. doi:10.1001/ jamanetworkopen.2021.43582.
- Fontham ETH, Wolf AMD, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346. doi:10.3322/caac.21628.
- US Preventive Services Task Force; Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force Recommendation statement. JAMA. 2018;320:674-686. doi:10.1001/jama.2018.10897.
- Nayar R, Chhieng DC, Crothers B, et al. Moving forward—the 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors and beyond: implications and suggestions for laboratories. J Am Soc Cytopathol. 2020;9:291-303. doi:10.1016/j.jasc.2020.05.002.
- Cooley JJP, Maguire FB, Morris CR, et al. Cervical cancer stage at diagnosis and survival among women ≥65 years in California. Cancer Epidemiol Biomarkers Prev. 2023;32:91-97. doi:10.1158/1055-9965.EPI-22-0793.
- National Cancer Institute. Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Cervical Cancer. Accessed February 21, 2023. https://seer.cancer.gov /statfacts/html/cervix.html
- Feldman S. Screening options for preventing cervical cancer. JAMA Intern Med. 2019;179:879-880. doi:10.1001/ jamainternmed.2019.0298.
- ASCO Post Staff. FDA approves first HPV test for primary cervical cancer screening. ASCO Post. May 15, 2014. Accessed January 8, 2023. https://ascopost.com/issues/may-15-2014 /fda-approves-first-hpv-test-for-primary-cervical-cancer -screening/
- Rijkaart DC, Berkhof J, Rozendaal L, et al. Human papillomavirus testing for the detection of high-grade cervical intraepithelial neoplasia and cancer: final results of the POBASCAM randomised controlled trial. Lancet Oncol. 2012;13:78-88. doi:10.1016/S1470-2045(11)70296-0.
- Ronco G, Giorgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer Screening (NTCC) Working Group. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol. 2010;11:249-257. doi:10.1016/S1470-2045(09)70360-2.
- Kitchener HC, Almonte M, Thomson C, et al. HPV testing in combination with liquid-based cytology in primary cervical screening (ARTISTIC): a randomised controlled trial. Lancet Oncol. 2009;10:672-682. doi:10.1016/S1470-2045(09)70156-1.
- Bulkmans NWJ, Berkhof J, Rozendaal L, et al. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year followup of a randomised controlled implementation trial. Lancet. 2007;370:1764-1772. doi:10.1016/S0140-6736(07)61450-0.
- Ogilvie GS, Van Niekerk D, Krajden M, et al. Effect of screening with primary cervical HPV testing vs cytology testing on high-grade cervical intraepithelial neoplasia at 48 months: the HPV FOCAL randomized clinical trial. JAMA. 2018;320:43-52. doi:10.1001/jama.2018.7464.
- Gottschlich A, Gondara L, Smith LW, et al. Human papillomavirus‐based screening at extended intervals missed fewer cervical precancers than cytology in the HPV For Cervical Cancer (HPV FOCAL) trial. Int J Cancer. 2022;151:897-905. doi:10.1002/ijc.34039.
- Katki HA, Kinney WK, Fetterman B, et al. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Lancet Oncol. 2011;12:663672. doi:10.1016/S1470-2045(11)70145-0.
- Wright TC, Stoler MH, Behrens CM, et al. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015;136:189-197. doi:10.1016/j.ygyno.2014.11.076
- Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Obstet Gynecol. 2015;125:330-337. doi:10.1097/AOG.0000000000000669.
- Silver MI, Rositch AF, Burke AE, et al. Patient concerns about human papillomavirus testing and 5-year intervals in routine cervical cancer screening. Obstet Gynecol. 2015;125:317-329. doi:10.1097/AOG.0000000000000638.
- Smith LW, Racey CS, Gondara L, et al. Women’s acceptability of and experience with primary human papillomavirus testing for cervical screening: HPV FOCAL trial cross-sectional online survey results. BMJ Open. 2021;11:e052084. doi:10.1136/bmjopen-2021-052084.
- Wright TC, Stoler MH, Ranger-Moore J, et al. Clinical validation of p16/Ki-67 dual-stained cytology triage of HPV-positive women: results from the IMPACT trial. Int J Cancer. 2022;150:461-471. doi:10.1002/ijc.33812.
- Yeh PT, Kennedy CE, De Vuyst H, et al. Self-sampling for human papillomavirus (HPV) testing: a systematic review and meta-analysis. BMJ Global Health. 2019;4:e001351. doi:10.1136/bmjgh-2018-001351.
- Polman NJ, Ebisch RMF, Heideman DAM, et al. Performance of human papillomavirus testing on self-collected versus clinician-collected samples for the detection of cervical intraepithelial neoplasia of grade 2 or worse: a randomised, paired screen-positive, non-inferiority trial. Lancet Oncol. 2019;20:229-238. doi:10.1016/S1470-2045(18)30763-0.
- Winer RL, Lin J, Tiro JA, et al. Effect of mailed human papillomavirus test kits vs usual care reminders on cervical cancer screening uptake, precancer detection, and treatment: a randomized clinical trial. JAMA Netw Open. 2019;2:e1914729. doi:10.1001/jamanetworkopen.2019.14729.
- Tiro JA, Betts AC, Kimbel K, et al. Understanding patients’ perspectives and information needs following a positive home human papillomavirus self-sampling kit result. J Womens Health (Larchmt). 2019;28:384-392. doi:10.1089/ jwh.2018.7070.
- Knauss T, Hansen BT, Pedersen K, et al. The cost-effectiveness of opt-in and send-to-all HPV self-sampling among long-term non-attenders to cervical cancer screening in Norway: the Equalscreen randomized controlled trial. Gynecol Oncol. 2023;168:39-47. doi:10.1016/j.ygyno.2022.10.027.
- ACOG committee opinion no. 809. Human papillomavirus vaccination: correction. Obstet Gynecol. 2022;139:345. doi:10.1097/AOG.0000000000004680.
- St Sauver JL, Finney Rutten LJF, Ebbert JO, et al. Younger age at initiation of the human papillomavirus (HPV) vaccination series is associated with higher rates of on-time completion. Prev Med. 2016;89:327-333. doi:10.1016/j.ypmed.2016.02.039.
- Lei J, Ploner A, Elfström KM, et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383:13401348. doi:10.1056/NEJMoa1917338.
- Pingali C, Yankey D, Elam-Evans LD, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years — United States, 2020. MMWR Morb Mortal Wkly Rep. 2021;70:1183-1190. doi:10.15585/ mmwr.mm7035a1.
- National Centre for Immunisation Research and Surveillance Australia. Annual Immunisation Coverage Report 2020. November 29, 2021. Accessed March 1, 2023. https://ncirs .org.au/sites/default/files/2021-11/NCIRS%20Annual%20 Immunisation%20Coverage%20Report%202020_FINAL.pdf
- Leung SOA, Feldman S. 2022 Update on cervical disease. OBG Manag. 2022;34(5):16-17, 22-24, 26, 28. doi:10.12788/ obgm.0197.
Blisters on arms and legs
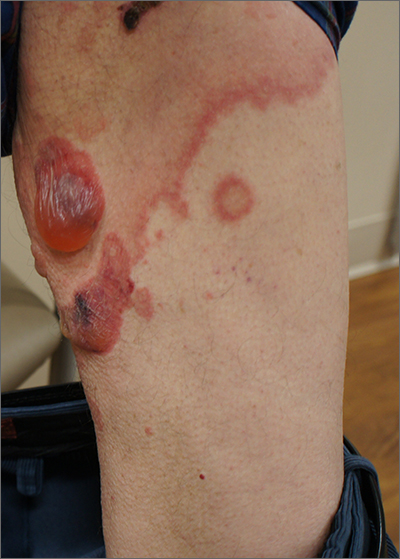
This patient was given a diagnosis of bullous pemphigoid. Although there were a number of clues that pointed to this diagnosis, confirming that this was the case required 2 biopsies and a blood draw. (More on this in a bit.)
Although rare and potentially lethal, bullous pemphigoid is the most common autoimmune blistering disease in the elderly. Patients present with tense bullae over limited or widespread areas of the skin. The pathogenesis includes development of autoimmune antibodies that target important proteins (BP180 and BP230) that bind basal epidermal keratinocytes to the dermis. When weakened by inflammation at these sites, the skin delaminates at the dermal-epidermal junction, while the cells of the epidermis continue to bind to each other. This leads to itching, hive-like wheals, and tense fluid-filled bullae.
The differential diagnosis of an acute or semi-acute bullous disease includes bullous pemphigoid, IgA pemphigoid, linear IgA bullous dermatosis, epidermolysis bullosa acquisita, and Senear-Usher syndrome. In this case, the large tense bullae suggested bullous pemphigoid over the other diagnoses.
Initial diagnosis requires 2 biopsies be performed: One at the edge of a bulla for a standard pathologic exam to identify the skin level at which the bulla is forming, and another biopsy of skin near the site of inflammation (5-10 mm away) to be sent for direct immunofluorescence (DIF) in Michel’s medium or Zeus medium. In bullous pemphigoid, the separation is at the dermal-epidermal junction, and IgG and C3 are found in the DIF in the same location. There are a couple ways to differentiate this disorder from epidermolysis bullosa acquisita—a similar blistering disorder in which autoantibodies attack collagen at the dermal-epidermal junction. A common approach is to send a patient’s serum for indirect immunofluorescence. This is done because it is impossible to distinguish between the 2 clinically.
While bullous pemphigoid has historically been treated with high-dose prednisone, it is more common now to treat with whole-body topical clobetasol and oral doxycycline 100 mg twice a day to avoid the adverse effects of the prednisone. Other immunosuppressive options, such as mycophenolate mofetil and cyclosporine, can provide the potency of prednisone with a more favorable long-term safety profile. Rituximab infusions are another very powerful and durable option in refractory or severe cases.1
This patient was treated with topical clobetasol and doxycycline 100 mg twice a day, but he had incomplete clearance after 2 to 3 weeks. At that point, mycophenolate mofetil was added to the regimen and was titrated up to 1000 mg twice daily. When clearance occurred, the clobetasol was discontinued and the mycophenolate mofetil was titrated down to 250 mg/d; the patient continues to maintain clearance at this dose. He continues on doxycycline 100 mg bid.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Ruggiero A, Megna M, Villani A, et al. Strategies to improve outcomes of bullous pemphigoid: a comprehensive review of clinical presentations, diagnosis, and patients' assessment. Clin Cosmet Investig Dermatol. 2022;15:661-673. doi:10.2147/CCID.S267573

This patient was given a diagnosis of bullous pemphigoid. Although there were a number of clues that pointed to this diagnosis, confirming that this was the case required 2 biopsies and a blood draw. (More on this in a bit.)
Although rare and potentially lethal, bullous pemphigoid is the most common autoimmune blistering disease in the elderly. Patients present with tense bullae over limited or widespread areas of the skin. The pathogenesis includes development of autoimmune antibodies that target important proteins (BP180 and BP230) that bind basal epidermal keratinocytes to the dermis. When weakened by inflammation at these sites, the skin delaminates at the dermal-epidermal junction, while the cells of the epidermis continue to bind to each other. This leads to itching, hive-like wheals, and tense fluid-filled bullae.
The differential diagnosis of an acute or semi-acute bullous disease includes bullous pemphigoid, IgA pemphigoid, linear IgA bullous dermatosis, epidermolysis bullosa acquisita, and Senear-Usher syndrome. In this case, the large tense bullae suggested bullous pemphigoid over the other diagnoses.
Initial diagnosis requires 2 biopsies be performed: One at the edge of a bulla for a standard pathologic exam to identify the skin level at which the bulla is forming, and another biopsy of skin near the site of inflammation (5-10 mm away) to be sent for direct immunofluorescence (DIF) in Michel’s medium or Zeus medium. In bullous pemphigoid, the separation is at the dermal-epidermal junction, and IgG and C3 are found in the DIF in the same location. There are a couple ways to differentiate this disorder from epidermolysis bullosa acquisita—a similar blistering disorder in which autoantibodies attack collagen at the dermal-epidermal junction. A common approach is to send a patient’s serum for indirect immunofluorescence. This is done because it is impossible to distinguish between the 2 clinically.
While bullous pemphigoid has historically been treated with high-dose prednisone, it is more common now to treat with whole-body topical clobetasol and oral doxycycline 100 mg twice a day to avoid the adverse effects of the prednisone. Other immunosuppressive options, such as mycophenolate mofetil and cyclosporine, can provide the potency of prednisone with a more favorable long-term safety profile. Rituximab infusions are another very powerful and durable option in refractory or severe cases.1
This patient was treated with topical clobetasol and doxycycline 100 mg twice a day, but he had incomplete clearance after 2 to 3 weeks. At that point, mycophenolate mofetil was added to the regimen and was titrated up to 1000 mg twice daily. When clearance occurred, the clobetasol was discontinued and the mycophenolate mofetil was titrated down to 250 mg/d; the patient continues to maintain clearance at this dose. He continues on doxycycline 100 mg bid.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.

This patient was given a diagnosis of bullous pemphigoid. Although there were a number of clues that pointed to this diagnosis, confirming that this was the case required 2 biopsies and a blood draw. (More on this in a bit.)
Although rare and potentially lethal, bullous pemphigoid is the most common autoimmune blistering disease in the elderly. Patients present with tense bullae over limited or widespread areas of the skin. The pathogenesis includes development of autoimmune antibodies that target important proteins (BP180 and BP230) that bind basal epidermal keratinocytes to the dermis. When weakened by inflammation at these sites, the skin delaminates at the dermal-epidermal junction, while the cells of the epidermis continue to bind to each other. This leads to itching, hive-like wheals, and tense fluid-filled bullae.
The differential diagnosis of an acute or semi-acute bullous disease includes bullous pemphigoid, IgA pemphigoid, linear IgA bullous dermatosis, epidermolysis bullosa acquisita, and Senear-Usher syndrome. In this case, the large tense bullae suggested bullous pemphigoid over the other diagnoses.
Initial diagnosis requires 2 biopsies be performed: One at the edge of a bulla for a standard pathologic exam to identify the skin level at which the bulla is forming, and another biopsy of skin near the site of inflammation (5-10 mm away) to be sent for direct immunofluorescence (DIF) in Michel’s medium or Zeus medium. In bullous pemphigoid, the separation is at the dermal-epidermal junction, and IgG and C3 are found in the DIF in the same location. There are a couple ways to differentiate this disorder from epidermolysis bullosa acquisita—a similar blistering disorder in which autoantibodies attack collagen at the dermal-epidermal junction. A common approach is to send a patient’s serum for indirect immunofluorescence. This is done because it is impossible to distinguish between the 2 clinically.
While bullous pemphigoid has historically been treated with high-dose prednisone, it is more common now to treat with whole-body topical clobetasol and oral doxycycline 100 mg twice a day to avoid the adverse effects of the prednisone. Other immunosuppressive options, such as mycophenolate mofetil and cyclosporine, can provide the potency of prednisone with a more favorable long-term safety profile. Rituximab infusions are another very powerful and durable option in refractory or severe cases.1
This patient was treated with topical clobetasol and doxycycline 100 mg twice a day, but he had incomplete clearance after 2 to 3 weeks. At that point, mycophenolate mofetil was added to the regimen and was titrated up to 1000 mg twice daily. When clearance occurred, the clobetasol was discontinued and the mycophenolate mofetil was titrated down to 250 mg/d; the patient continues to maintain clearance at this dose. He continues on doxycycline 100 mg bid.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Ruggiero A, Megna M, Villani A, et al. Strategies to improve outcomes of bullous pemphigoid: a comprehensive review of clinical presentations, diagnosis, and patients' assessment. Clin Cosmet Investig Dermatol. 2022;15:661-673. doi:10.2147/CCID.S267573
1. Ruggiero A, Megna M, Villani A, et al. Strategies to improve outcomes of bullous pemphigoid: a comprehensive review of clinical presentations, diagnosis, and patients' assessment. Clin Cosmet Investig Dermatol. 2022;15:661-673. doi:10.2147/CCID.S267573
SGS 2023 Meeting: Daily Reporting from Tucson
Wednesday, March 22. Day 4 of SGS.
Day 4, and the final day of the 49th SGS conference started with a sunrise run up and down the hills surrounding the JW Marriott Starr Resort. After breakfast, I entered the Tucson Ballroom to attend the last 2 scientific sessions of the conference.

Highlights from the first session included a look at postoperative outcomes and complication rates between gynecologic surgeons and general surgeons using the National Surgical Quality Improvement Program (NSQIP) database by Dr. Douglas Luchristt, who showed no difference between the 2 surgical specialties (and even better outcomes by gynecologists in certain operative measures), as well as the work of Dr. Christopher Hong who used 2 separate surgical databases (NSQIP and Michigan Surgical Quality Collaborative) to show that rates of vaginal hysterectomy have been decreasing from 2017 to 2020, even amongst patients who are likely good candidates for a vaginal route of hysterectomy. Dr. Jocelyn Fitzgerald presented her unique mixed methods research on how to better design the gynecologic office to improve the patient experience, using 3,000 Twitter responses to a question on this topic. Lastly, Dr. Emily Aldrich shared her work on better understanding the patient perception of same day discharge after major vaginal reconstructive surgery. An interesting finding of Dr. Aldrich’s study was that the most common response to her question about the “worst part of the surgical experience” was going home with a postoperative catheter, which surgeons often consider a small and temporary discomfort. The first session ended with the passing of the gavel from current SGS president Dr. Cheryl Iglesia, to the incoming SGS president Dr. Rosanne Kho, with much applause and excitement for what Dr. Kho will bring to the table in her new role.
The research presented at the final scientific session of the conference did not disappoint. A retrospective study on the influence of body mass index (BMI) on the time to surgical diagnosis of endometriosis by Dr. Melissa Markowitz found that obesity was associated with a delay of over 1 year in surgical diagnosis of endometriosis compared with normal and underweight patients. Dr. David (Ike) Rahn presented additional findings on his randomized, double-blinded, multicenter trial on perioperative use of vaginal estrogen cream in postmenopausal patients with prolapse. He found that 5 weeks of estrogen cream use was not associated with any improvement in urinary incontinence or sexual function.
Dr. Stephanie Glass Clark used the Premier Healthcare Database to show that that there was no difference in postoperative mesh exposure in patients who underwent a total hysterectomy compared with supracervical hysterectomy at the time of sacrocolpopexy. Dr. Kavita Mishra presented results from the FLOWER trial, which found no difference in postoperative outcomes for transgender women undergoing vaginoplasty for gender affirmation who did and did not undergo preoperative pelvic floor physical therapy. Finally, Dr. Carly Crowder shared her video of anatomy for sacral neuromodulation with some excellent cadaveric dissections to exhibit the peri-sacral and gluteal anatomy.

As the conference ended, raindrops pounded the sandy grounds of the resort as I waited in the lobby for my Uber to the airport. Dr. Rosanne Kho happened to walk by and stopped to speak with me and one of my attendings. She smiled as she asked about our experience at the conference and to wish us safe travels. To me, this moment embodies the spirit of mentorship and connection that is so unique to the SGS conference. I feel incredibly lucky to have met some of the physician leaders of our field, who genuinely want to get to know and help the next generation. This year’s meeting was attended by ObGyn generalists and surgeons of all gynecologic subspecialties and certainly met its goal in addressing topics with an “Impact Factor.” I am inspired by all the work that is happening across the country to move the needle and better our field. This was my first SGS experience, but it certainly won’t be the last. I hope you too will consider attending in the future!
Tuesday, March 21. Day 3 of SGS.
It’s Day 3 of the SGS conference! In addition to the academic roundtables, conference attendees had the option of doing early-morning yoga with Dr. Mireille Truong. Yoga sounded nice, but I spent the morning in bed, catching up on sleep. (My own version of wellness!) The scientific sessions of the day started at 7:30 am, and I especially want to highlight the work of Dr. Amy Askew who performed a randomized controlled trial comparing patient removal of urinary catheters placed for postoperative urinary retention to office removal. She found that patient urinary catheter removal was a feasible and safe option with excellent patient satisfaction and a reduction of in-person postoperative office visits. At the end of the session, Dr. Cheryl Iglesia gave her presidential address, where she shared her journey to becoming the physician, educator, researcher, and leader she is today. She emphasized the importance of being a continual learner and to give back by mentoring and educating the next generation. “Learn it, earn it, and return it.”

This was followed by the Te Linde lecture, given by Dr. Pamela Moalli. An exceptional surgeon-scientist, Dr. Moalli shared about her work on the impact of mesh on tissue, as well as alternative biologic options being developed, such as 3D printed membranes, extracellular matrix scaffolds, and living tissue grafts to create new ligamentous supports for the vagina. She discussed novel research using stem cell transplantation to harness the power of regeneration in the urethra or vagina following injury. I think it is safe to say that the entire room was in awe of the work she has done, and what she continues to do to find better therapeutic options for girls and women with pelvic floor disorders. Her talk ended in a standing ovation. Afterwards, all the University of Pittsburgh Medical Center-Magee Womens Hospital trainees, faculty, and several alumni took a picture with Dr. Moalli (fifth from the right in the picture).
Lunch followed, which included a brief walk around the industry exhibition hall. I then returned back to the Tucson Ballroom to listen in on the next scientific session on surgical ergonomics. Organized by Dr. Amy Park who herself suffered from work-related musculoskeletal injuries, the session was composed of an excellent video by Dr. Abby Stork on stretches to prevent and reduce the risk of surgeon-associated musculoskeletal injuries, especially in vaginal surgeons. There was then a panel of 3 experts, Dr. Noor Abu-Alnadi, Dr. Ladin Yurteri-Kaplan, and Dr. Susan Hallbeck (PhD ergonomics expert), moderated by Dr. Amanda Fader and Dr. Kimberly Kho. In particular, Dr. Hallbeck developed a timer app as a reminder for surgeons to stop every 40 minutes to stretch for 1.5 minutes (orstretch.mayoclinic.org). This has been studied and found to reduce musculoskeletal pain after surgery and improve physical performance without increasing total operating time. If you would like to see some of these between- and in-OR stretches yourself, an informative handout can be accessed at mcforms.mayo.edu.
Tuesday afternoon was left open. I joined Dr. Veronica Lerner, Dr. Kelly Wright, and Dr. Louise Perkins King on a 7.5-mile hike into the surrounding desert hills. We marveled at the many Saguaro cacti, some over 100 years old and towering many feet high, as well as the beautiful yellow, purple, and magenta flowers that were scattered among the desert brush. Several rabbits and deer wandered by us during our hike. On one of the trails, the stone skeleton of an old house stood, once a home to the Bowen family who had moved to Arizona for health reasons. I could see why they would want to move here—I felt such a peace looking at the gorgeous view from what was once their doorway.

After a shower and a lot of stretching, I got ready for the evening event, A Taste and Toast with SGS: Under the Arizona Skies. The food and drink were delicious, and I got to spend the evening catching up with a good friend. We watched as conference attendees assigned to different color teams (red, green, blue, and yellow), fought for the hallowed Golden Uterus Trophy in several competitive gynecology-themed games (eg, throwing sacral neuromodulation needle “darts” at balloons and removing small pom poms from a water bottle with a disposable operative hysteroscope). As the evening progressed, the DJ turned up the music and people made their way to the dance floor. The event served as a fundraiser for the SGS Pelvic Anatomy Group and successfully raised $35,000.
Monday, March 20. Day 2 of SGS.
Day 2 of the SGS meeting started off with a gentle sunrise over the cacti-covered hills surrounding the JW Marriot Starr Pass Hotel, the venue for the 49th SGS annual scientific meeting. The first official event of the day after some engaging academic round tables was the recognition of the new SGS members. Much celebration was had over the 18 gynecologic surgeons who were inducted.

The second day included the first 3 scientific sessions of the conference. Some highlights include the work of Dr. Shawn Menefee on a randomized trial of sacral colpopexy, transvaginal mesh, and native tissue apical repair for posthysterectomy vault prolapse; a video by Dr. Matthew Fallon on a robotic-assisted laparoscopic approach to repairing a chronic uterine inversion; and the impact of age on regret following hysterectomy by Dr. Nathan King. Dr. Candace Parker-Autry also presented her work on the impact of perineorrhaphy on both female and male sexual function, and Dr. Cassie (Clarissa) Niino spoke elegantly on the “red bag problem” that exists in all of our operating rooms, which increases pollution and cost unnecessarily.
There were also several excellent talks given. Dr. Jason Wright spoke about the importance of surgical volume on gynecologic surgery. In particular, he noted that surgical volume needs to be considered not only at the surgeon level but also at the hospital level. Higher-volume hospitals will provide better care, in the same way that general, high-volume surgeons have less complications and better long-term outcomes. Of note, volume is not the whole picture. We need to also consider measurements of surgery and hospital quality and surgeon skill in addition to volume, as Dr. Shawn Menefee insightfully commented.
Dr. Beri Ridgeway gave the Mark D. Walters Lecture about surgeons in the c-suite and the importance of having a seat at the leadership table as surgeons and medical providers. In her words: “If we aren’t at the table, then we are on the menu.” Overworked and underpaid, burned out doctors feel powerless because they are managed by leaders with a business and not a medical background, and we need to have physicians in leadership who understand how medicine is practiced and to ensure equitable care
Dr. Kelly Wright gave a talk on the environmental impact of gynecologic care—from OR to clinic. She gave examples of how metal, reusable speculums become more cost-effective and produce less waste after only 2-3 uses and how there is no evidence that bouffants reduce surgical site infections (and a reusable scrub cap could work just as well without creating waste). Finally, Dr. Ebony Carter gave an impassioned talk on the need for equity in publication and grant funding in our field. She shared about her initiative through the Green Journal (Obstetrics and Gynecology) to create an issue focused on furthering equity and dispelling racism in medical research.
Later in the afternoon, I attended the Fellows’ Pelvic Research Network (FPRN) meeting, which includes AUGS-SGS (urogynecology fellows) and FMIGS-SGS (fellows of all other gynecologic subspecialities, including minimally invasive gynecologic surgery, family planning, reproductive endocrinology and infertility, and pediatric and adolescent gynecology). Dr. John Gebhart gave an excellent lecture with some impressive photos and videos on how to manage mesh exposure and erosion.
Afterwards, updates were given on the current FPRN projects, and 4 new projects were proposed and underwent audience feedback for improvement. It was exciting to see the multicenter collaborations fostered through the FPRN, and I look forward to seeing which projects will get funded for this upcoming year!

The evening ended with the President's Award Ceremony led by Dr. Cheryl Iglesia, the 49th SGS President, as well as the President's Reception. I also wantd to highlight the winner of the Distinguished Surgeon Award: Dr. Dee Fenner. The remaining awardees are listed on the SGS website (https://sgsonline.org).
Sunday, March 19. Day 1 of SGS.
Last night around midnight, bleary-eyed from the long flight from Pittsburgh, I walked out into the dimly lit, mild air of Tucson, Arizona. The Saguaro cacti that lined the entrance to the airport stood tall and tree-like, with welcoming green arms. It was as if they too knew that the next 4 days would be filled with the building of new relationships and the strengthening of old ones, as well as with education, innovation, and the sharing of research. That spirit of collegiality, approachability, and connection in an intimate and vibrant meeting is what the Society of Gynecologic Surgeons (SGS) meeting has been known for and why it draws people to come back, year after year.
The first day of the conference was fantastic. As a first-time attendant at SGS, I was excited to have the opportunity to meet and rub elbows with mentors and role models from across the country. My day started off with the SGS Fellows and Young Attendings Course, moderated by 3 incredible faculty: Dr. Matthew Barker, Dr. Sadikah Behbehani, and Dr. Traci Ito. Some high-yield topics such as contract negotiation, developing a urogynecology- or MIGS-based practice, billing, academic promotion, and taking advantage of relationships with industry were discussed at length, and the session ended with a roundtable, where the experts had time to answer questions in smaller groups. One of the quotes that will ring true for many fellows about to embark on the job search was from Dr. Amanda Ecker: “Up until now, you were told where to go and what your schedule is. This is the first time you have flexibility and power to decide for yourself.” Therefore, it is important to reflect on what you really desire and/or prioritize in a job, whether it is location, compensation, protected time, or opportunities for advancement.

In the afternoon, I attended a postgraduate course led by Dr. Veronica Lerner and Dr. Mireille Truong called “The Sim Factor: Making an Impact in Surgical Education.” Several other excellent postgraduate courses were available, including “Advanced Endometriosis Surgery and Pelvic Pain Patient-Centered Approach,” “Social Media Workshop- #Gynfluencing: Using Social Media to Find Your Digital Voice,” and “Urologic Surgery for the Gynecologic Surgeon: GU Injury, Ureteral Stents, Complex Fistula and More.” I was grateful for the hands-on and tangible tools that Drs. Lerner and Truong left the group with—including ideas such as Zoom-based virtual coaching for trainees learning fundamentals of laparoscopic surgery types of tasks, table-top simulation for high-stakes scenarios (eg, operative vascular injury), and the importance of grounding educational activity in objectives and evaluation. I even got to make and take home my own myomectomy model. (Fun fact: The myoma is actually a stress ball wrapped in an Ace bandage and then Glad Press n’ Seal!

The late afternoon transitioned to an opportunity for trainees to interact with senior SGS members and a welcome reception. The indoor and outdoor spaces were filled with laughing and talking as people connected over drinks and snacks. Finally, the evening ended with a session presented by the SGS Equity Council, “What your Patients REALLY Want to Know.” Patty Brisben, of the Patty Brisben Foundation and founder of the company Pure Romance, was interviewed by Dr. Christine Vaccaro. It was heartwarming to hear how Patty took the stories of women suffering from sexual pain and dissatisfaction and chose to make it her life’s mission to improve women’s sexual health.
Wednesday, March 22. Day 4 of SGS.
Day 4, and the final day of the 49th SGS conference started with a sunrise run up and down the hills surrounding the JW Marriott Starr Resort. After breakfast, I entered the Tucson Ballroom to attend the last 2 scientific sessions of the conference.

Highlights from the first session included a look at postoperative outcomes and complication rates between gynecologic surgeons and general surgeons using the National Surgical Quality Improvement Program (NSQIP) database by Dr. Douglas Luchristt, who showed no difference between the 2 surgical specialties (and even better outcomes by gynecologists in certain operative measures), as well as the work of Dr. Christopher Hong who used 2 separate surgical databases (NSQIP and Michigan Surgical Quality Collaborative) to show that rates of vaginal hysterectomy have been decreasing from 2017 to 2020, even amongst patients who are likely good candidates for a vaginal route of hysterectomy. Dr. Jocelyn Fitzgerald presented her unique mixed methods research on how to better design the gynecologic office to improve the patient experience, using 3,000 Twitter responses to a question on this topic. Lastly, Dr. Emily Aldrich shared her work on better understanding the patient perception of same day discharge after major vaginal reconstructive surgery. An interesting finding of Dr. Aldrich’s study was that the most common response to her question about the “worst part of the surgical experience” was going home with a postoperative catheter, which surgeons often consider a small and temporary discomfort. The first session ended with the passing of the gavel from current SGS president Dr. Cheryl Iglesia, to the incoming SGS president Dr. Rosanne Kho, with much applause and excitement for what Dr. Kho will bring to the table in her new role.
The research presented at the final scientific session of the conference did not disappoint. A retrospective study on the influence of body mass index (BMI) on the time to surgical diagnosis of endometriosis by Dr. Melissa Markowitz found that obesity was associated with a delay of over 1 year in surgical diagnosis of endometriosis compared with normal and underweight patients. Dr. David (Ike) Rahn presented additional findings on his randomized, double-blinded, multicenter trial on perioperative use of vaginal estrogen cream in postmenopausal patients with prolapse. He found that 5 weeks of estrogen cream use was not associated with any improvement in urinary incontinence or sexual function.
Dr. Stephanie Glass Clark used the Premier Healthcare Database to show that that there was no difference in postoperative mesh exposure in patients who underwent a total hysterectomy compared with supracervical hysterectomy at the time of sacrocolpopexy. Dr. Kavita Mishra presented results from the FLOWER trial, which found no difference in postoperative outcomes for transgender women undergoing vaginoplasty for gender affirmation who did and did not undergo preoperative pelvic floor physical therapy. Finally, Dr. Carly Crowder shared her video of anatomy for sacral neuromodulation with some excellent cadaveric dissections to exhibit the peri-sacral and gluteal anatomy.

As the conference ended, raindrops pounded the sandy grounds of the resort as I waited in the lobby for my Uber to the airport. Dr. Rosanne Kho happened to walk by and stopped to speak with me and one of my attendings. She smiled as she asked about our experience at the conference and to wish us safe travels. To me, this moment embodies the spirit of mentorship and connection that is so unique to the SGS conference. I feel incredibly lucky to have met some of the physician leaders of our field, who genuinely want to get to know and help the next generation. This year’s meeting was attended by ObGyn generalists and surgeons of all gynecologic subspecialties and certainly met its goal in addressing topics with an “Impact Factor.” I am inspired by all the work that is happening across the country to move the needle and better our field. This was my first SGS experience, but it certainly won’t be the last. I hope you too will consider attending in the future!
Tuesday, March 21. Day 3 of SGS.
It’s Day 3 of the SGS conference! In addition to the academic roundtables, conference attendees had the option of doing early-morning yoga with Dr. Mireille Truong. Yoga sounded nice, but I spent the morning in bed, catching up on sleep. (My own version of wellness!) The scientific sessions of the day started at 7:30 am, and I especially want to highlight the work of Dr. Amy Askew who performed a randomized controlled trial comparing patient removal of urinary catheters placed for postoperative urinary retention to office removal. She found that patient urinary catheter removal was a feasible and safe option with excellent patient satisfaction and a reduction of in-person postoperative office visits. At the end of the session, Dr. Cheryl Iglesia gave her presidential address, where she shared her journey to becoming the physician, educator, researcher, and leader she is today. She emphasized the importance of being a continual learner and to give back by mentoring and educating the next generation. “Learn it, earn it, and return it.”

This was followed by the Te Linde lecture, given by Dr. Pamela Moalli. An exceptional surgeon-scientist, Dr. Moalli shared about her work on the impact of mesh on tissue, as well as alternative biologic options being developed, such as 3D printed membranes, extracellular matrix scaffolds, and living tissue grafts to create new ligamentous supports for the vagina. She discussed novel research using stem cell transplantation to harness the power of regeneration in the urethra or vagina following injury. I think it is safe to say that the entire room was in awe of the work she has done, and what she continues to do to find better therapeutic options for girls and women with pelvic floor disorders. Her talk ended in a standing ovation. Afterwards, all the University of Pittsburgh Medical Center-Magee Womens Hospital trainees, faculty, and several alumni took a picture with Dr. Moalli (fifth from the right in the picture).
Lunch followed, which included a brief walk around the industry exhibition hall. I then returned back to the Tucson Ballroom to listen in on the next scientific session on surgical ergonomics. Organized by Dr. Amy Park who herself suffered from work-related musculoskeletal injuries, the session was composed of an excellent video by Dr. Abby Stork on stretches to prevent and reduce the risk of surgeon-associated musculoskeletal injuries, especially in vaginal surgeons. There was then a panel of 3 experts, Dr. Noor Abu-Alnadi, Dr. Ladin Yurteri-Kaplan, and Dr. Susan Hallbeck (PhD ergonomics expert), moderated by Dr. Amanda Fader and Dr. Kimberly Kho. In particular, Dr. Hallbeck developed a timer app as a reminder for surgeons to stop every 40 minutes to stretch for 1.5 minutes (orstretch.mayoclinic.org). This has been studied and found to reduce musculoskeletal pain after surgery and improve physical performance without increasing total operating time. If you would like to see some of these between- and in-OR stretches yourself, an informative handout can be accessed at mcforms.mayo.edu.
Tuesday afternoon was left open. I joined Dr. Veronica Lerner, Dr. Kelly Wright, and Dr. Louise Perkins King on a 7.5-mile hike into the surrounding desert hills. We marveled at the many Saguaro cacti, some over 100 years old and towering many feet high, as well as the beautiful yellow, purple, and magenta flowers that were scattered among the desert brush. Several rabbits and deer wandered by us during our hike. On one of the trails, the stone skeleton of an old house stood, once a home to the Bowen family who had moved to Arizona for health reasons. I could see why they would want to move here—I felt such a peace looking at the gorgeous view from what was once their doorway.

After a shower and a lot of stretching, I got ready for the evening event, A Taste and Toast with SGS: Under the Arizona Skies. The food and drink were delicious, and I got to spend the evening catching up with a good friend. We watched as conference attendees assigned to different color teams (red, green, blue, and yellow), fought for the hallowed Golden Uterus Trophy in several competitive gynecology-themed games (eg, throwing sacral neuromodulation needle “darts” at balloons and removing small pom poms from a water bottle with a disposable operative hysteroscope). As the evening progressed, the DJ turned up the music and people made their way to the dance floor. The event served as a fundraiser for the SGS Pelvic Anatomy Group and successfully raised $35,000.
Monday, March 20. Day 2 of SGS.
Day 2 of the SGS meeting started off with a gentle sunrise over the cacti-covered hills surrounding the JW Marriot Starr Pass Hotel, the venue for the 49th SGS annual scientific meeting. The first official event of the day after some engaging academic round tables was the recognition of the new SGS members. Much celebration was had over the 18 gynecologic surgeons who were inducted.

The second day included the first 3 scientific sessions of the conference. Some highlights include the work of Dr. Shawn Menefee on a randomized trial of sacral colpopexy, transvaginal mesh, and native tissue apical repair for posthysterectomy vault prolapse; a video by Dr. Matthew Fallon on a robotic-assisted laparoscopic approach to repairing a chronic uterine inversion; and the impact of age on regret following hysterectomy by Dr. Nathan King. Dr. Candace Parker-Autry also presented her work on the impact of perineorrhaphy on both female and male sexual function, and Dr. Cassie (Clarissa) Niino spoke elegantly on the “red bag problem” that exists in all of our operating rooms, which increases pollution and cost unnecessarily.
There were also several excellent talks given. Dr. Jason Wright spoke about the importance of surgical volume on gynecologic surgery. In particular, he noted that surgical volume needs to be considered not only at the surgeon level but also at the hospital level. Higher-volume hospitals will provide better care, in the same way that general, high-volume surgeons have less complications and better long-term outcomes. Of note, volume is not the whole picture. We need to also consider measurements of surgery and hospital quality and surgeon skill in addition to volume, as Dr. Shawn Menefee insightfully commented.
Dr. Beri Ridgeway gave the Mark D. Walters Lecture about surgeons in the c-suite and the importance of having a seat at the leadership table as surgeons and medical providers. In her words: “If we aren’t at the table, then we are on the menu.” Overworked and underpaid, burned out doctors feel powerless because they are managed by leaders with a business and not a medical background, and we need to have physicians in leadership who understand how medicine is practiced and to ensure equitable care
Dr. Kelly Wright gave a talk on the environmental impact of gynecologic care—from OR to clinic. She gave examples of how metal, reusable speculums become more cost-effective and produce less waste after only 2-3 uses and how there is no evidence that bouffants reduce surgical site infections (and a reusable scrub cap could work just as well without creating waste). Finally, Dr. Ebony Carter gave an impassioned talk on the need for equity in publication and grant funding in our field. She shared about her initiative through the Green Journal (Obstetrics and Gynecology) to create an issue focused on furthering equity and dispelling racism in medical research.
Later in the afternoon, I attended the Fellows’ Pelvic Research Network (FPRN) meeting, which includes AUGS-SGS (urogynecology fellows) and FMIGS-SGS (fellows of all other gynecologic subspecialities, including minimally invasive gynecologic surgery, family planning, reproductive endocrinology and infertility, and pediatric and adolescent gynecology). Dr. John Gebhart gave an excellent lecture with some impressive photos and videos on how to manage mesh exposure and erosion.
Afterwards, updates were given on the current FPRN projects, and 4 new projects were proposed and underwent audience feedback for improvement. It was exciting to see the multicenter collaborations fostered through the FPRN, and I look forward to seeing which projects will get funded for this upcoming year!

The evening ended with the President's Award Ceremony led by Dr. Cheryl Iglesia, the 49th SGS President, as well as the President's Reception. I also wantd to highlight the winner of the Distinguished Surgeon Award: Dr. Dee Fenner. The remaining awardees are listed on the SGS website (https://sgsonline.org).
Sunday, March 19. Day 1 of SGS.
Last night around midnight, bleary-eyed from the long flight from Pittsburgh, I walked out into the dimly lit, mild air of Tucson, Arizona. The Saguaro cacti that lined the entrance to the airport stood tall and tree-like, with welcoming green arms. It was as if they too knew that the next 4 days would be filled with the building of new relationships and the strengthening of old ones, as well as with education, innovation, and the sharing of research. That spirit of collegiality, approachability, and connection in an intimate and vibrant meeting is what the Society of Gynecologic Surgeons (SGS) meeting has been known for and why it draws people to come back, year after year.
The first day of the conference was fantastic. As a first-time attendant at SGS, I was excited to have the opportunity to meet and rub elbows with mentors and role models from across the country. My day started off with the SGS Fellows and Young Attendings Course, moderated by 3 incredible faculty: Dr. Matthew Barker, Dr. Sadikah Behbehani, and Dr. Traci Ito. Some high-yield topics such as contract negotiation, developing a urogynecology- or MIGS-based practice, billing, academic promotion, and taking advantage of relationships with industry were discussed at length, and the session ended with a roundtable, where the experts had time to answer questions in smaller groups. One of the quotes that will ring true for many fellows about to embark on the job search was from Dr. Amanda Ecker: “Up until now, you were told where to go and what your schedule is. This is the first time you have flexibility and power to decide for yourself.” Therefore, it is important to reflect on what you really desire and/or prioritize in a job, whether it is location, compensation, protected time, or opportunities for advancement.

In the afternoon, I attended a postgraduate course led by Dr. Veronica Lerner and Dr. Mireille Truong called “The Sim Factor: Making an Impact in Surgical Education.” Several other excellent postgraduate courses were available, including “Advanced Endometriosis Surgery and Pelvic Pain Patient-Centered Approach,” “Social Media Workshop- #Gynfluencing: Using Social Media to Find Your Digital Voice,” and “Urologic Surgery for the Gynecologic Surgeon: GU Injury, Ureteral Stents, Complex Fistula and More.” I was grateful for the hands-on and tangible tools that Drs. Lerner and Truong left the group with—including ideas such as Zoom-based virtual coaching for trainees learning fundamentals of laparoscopic surgery types of tasks, table-top simulation for high-stakes scenarios (eg, operative vascular injury), and the importance of grounding educational activity in objectives and evaluation. I even got to make and take home my own myomectomy model. (Fun fact: The myoma is actually a stress ball wrapped in an Ace bandage and then Glad Press n’ Seal!

The late afternoon transitioned to an opportunity for trainees to interact with senior SGS members and a welcome reception. The indoor and outdoor spaces were filled with laughing and talking as people connected over drinks and snacks. Finally, the evening ended with a session presented by the SGS Equity Council, “What your Patients REALLY Want to Know.” Patty Brisben, of the Patty Brisben Foundation and founder of the company Pure Romance, was interviewed by Dr. Christine Vaccaro. It was heartwarming to hear how Patty took the stories of women suffering from sexual pain and dissatisfaction and chose to make it her life’s mission to improve women’s sexual health.
Wednesday, March 22. Day 4 of SGS.
Day 4, and the final day of the 49th SGS conference started with a sunrise run up and down the hills surrounding the JW Marriott Starr Resort. After breakfast, I entered the Tucson Ballroom to attend the last 2 scientific sessions of the conference.

Highlights from the first session included a look at postoperative outcomes and complication rates between gynecologic surgeons and general surgeons using the National Surgical Quality Improvement Program (NSQIP) database by Dr. Douglas Luchristt, who showed no difference between the 2 surgical specialties (and even better outcomes by gynecologists in certain operative measures), as well as the work of Dr. Christopher Hong who used 2 separate surgical databases (NSQIP and Michigan Surgical Quality Collaborative) to show that rates of vaginal hysterectomy have been decreasing from 2017 to 2020, even amongst patients who are likely good candidates for a vaginal route of hysterectomy. Dr. Jocelyn Fitzgerald presented her unique mixed methods research on how to better design the gynecologic office to improve the patient experience, using 3,000 Twitter responses to a question on this topic. Lastly, Dr. Emily Aldrich shared her work on better understanding the patient perception of same day discharge after major vaginal reconstructive surgery. An interesting finding of Dr. Aldrich’s study was that the most common response to her question about the “worst part of the surgical experience” was going home with a postoperative catheter, which surgeons often consider a small and temporary discomfort. The first session ended with the passing of the gavel from current SGS president Dr. Cheryl Iglesia, to the incoming SGS president Dr. Rosanne Kho, with much applause and excitement for what Dr. Kho will bring to the table in her new role.
The research presented at the final scientific session of the conference did not disappoint. A retrospective study on the influence of body mass index (BMI) on the time to surgical diagnosis of endometriosis by Dr. Melissa Markowitz found that obesity was associated with a delay of over 1 year in surgical diagnosis of endometriosis compared with normal and underweight patients. Dr. David (Ike) Rahn presented additional findings on his randomized, double-blinded, multicenter trial on perioperative use of vaginal estrogen cream in postmenopausal patients with prolapse. He found that 5 weeks of estrogen cream use was not associated with any improvement in urinary incontinence or sexual function.
Dr. Stephanie Glass Clark used the Premier Healthcare Database to show that that there was no difference in postoperative mesh exposure in patients who underwent a total hysterectomy compared with supracervical hysterectomy at the time of sacrocolpopexy. Dr. Kavita Mishra presented results from the FLOWER trial, which found no difference in postoperative outcomes for transgender women undergoing vaginoplasty for gender affirmation who did and did not undergo preoperative pelvic floor physical therapy. Finally, Dr. Carly Crowder shared her video of anatomy for sacral neuromodulation with some excellent cadaveric dissections to exhibit the peri-sacral and gluteal anatomy.

As the conference ended, raindrops pounded the sandy grounds of the resort as I waited in the lobby for my Uber to the airport. Dr. Rosanne Kho happened to walk by and stopped to speak with me and one of my attendings. She smiled as she asked about our experience at the conference and to wish us safe travels. To me, this moment embodies the spirit of mentorship and connection that is so unique to the SGS conference. I feel incredibly lucky to have met some of the physician leaders of our field, who genuinely want to get to know and help the next generation. This year’s meeting was attended by ObGyn generalists and surgeons of all gynecologic subspecialties and certainly met its goal in addressing topics with an “Impact Factor.” I am inspired by all the work that is happening across the country to move the needle and better our field. This was my first SGS experience, but it certainly won’t be the last. I hope you too will consider attending in the future!
Tuesday, March 21. Day 3 of SGS.
It’s Day 3 of the SGS conference! In addition to the academic roundtables, conference attendees had the option of doing early-morning yoga with Dr. Mireille Truong. Yoga sounded nice, but I spent the morning in bed, catching up on sleep. (My own version of wellness!) The scientific sessions of the day started at 7:30 am, and I especially want to highlight the work of Dr. Amy Askew who performed a randomized controlled trial comparing patient removal of urinary catheters placed for postoperative urinary retention to office removal. She found that patient urinary catheter removal was a feasible and safe option with excellent patient satisfaction and a reduction of in-person postoperative office visits. At the end of the session, Dr. Cheryl Iglesia gave her presidential address, where she shared her journey to becoming the physician, educator, researcher, and leader she is today. She emphasized the importance of being a continual learner and to give back by mentoring and educating the next generation. “Learn it, earn it, and return it.”

This was followed by the Te Linde lecture, given by Dr. Pamela Moalli. An exceptional surgeon-scientist, Dr. Moalli shared about her work on the impact of mesh on tissue, as well as alternative biologic options being developed, such as 3D printed membranes, extracellular matrix scaffolds, and living tissue grafts to create new ligamentous supports for the vagina. She discussed novel research using stem cell transplantation to harness the power of regeneration in the urethra or vagina following injury. I think it is safe to say that the entire room was in awe of the work she has done, and what she continues to do to find better therapeutic options for girls and women with pelvic floor disorders. Her talk ended in a standing ovation. Afterwards, all the University of Pittsburgh Medical Center-Magee Womens Hospital trainees, faculty, and several alumni took a picture with Dr. Moalli (fifth from the right in the picture).
Lunch followed, which included a brief walk around the industry exhibition hall. I then returned back to the Tucson Ballroom to listen in on the next scientific session on surgical ergonomics. Organized by Dr. Amy Park who herself suffered from work-related musculoskeletal injuries, the session was composed of an excellent video by Dr. Abby Stork on stretches to prevent and reduce the risk of surgeon-associated musculoskeletal injuries, especially in vaginal surgeons. There was then a panel of 3 experts, Dr. Noor Abu-Alnadi, Dr. Ladin Yurteri-Kaplan, and Dr. Susan Hallbeck (PhD ergonomics expert), moderated by Dr. Amanda Fader and Dr. Kimberly Kho. In particular, Dr. Hallbeck developed a timer app as a reminder for surgeons to stop every 40 minutes to stretch for 1.5 minutes (orstretch.mayoclinic.org). This has been studied and found to reduce musculoskeletal pain after surgery and improve physical performance without increasing total operating time. If you would like to see some of these between- and in-OR stretches yourself, an informative handout can be accessed at mcforms.mayo.edu.
Tuesday afternoon was left open. I joined Dr. Veronica Lerner, Dr. Kelly Wright, and Dr. Louise Perkins King on a 7.5-mile hike into the surrounding desert hills. We marveled at the many Saguaro cacti, some over 100 years old and towering many feet high, as well as the beautiful yellow, purple, and magenta flowers that were scattered among the desert brush. Several rabbits and deer wandered by us during our hike. On one of the trails, the stone skeleton of an old house stood, once a home to the Bowen family who had moved to Arizona for health reasons. I could see why they would want to move here—I felt such a peace looking at the gorgeous view from what was once their doorway.

After a shower and a lot of stretching, I got ready for the evening event, A Taste and Toast with SGS: Under the Arizona Skies. The food and drink were delicious, and I got to spend the evening catching up with a good friend. We watched as conference attendees assigned to different color teams (red, green, blue, and yellow), fought for the hallowed Golden Uterus Trophy in several competitive gynecology-themed games (eg, throwing sacral neuromodulation needle “darts” at balloons and removing small pom poms from a water bottle with a disposable operative hysteroscope). As the evening progressed, the DJ turned up the music and people made their way to the dance floor. The event served as a fundraiser for the SGS Pelvic Anatomy Group and successfully raised $35,000.
Monday, March 20. Day 2 of SGS.
Day 2 of the SGS meeting started off with a gentle sunrise over the cacti-covered hills surrounding the JW Marriot Starr Pass Hotel, the venue for the 49th SGS annual scientific meeting. The first official event of the day after some engaging academic round tables was the recognition of the new SGS members. Much celebration was had over the 18 gynecologic surgeons who were inducted.

The second day included the first 3 scientific sessions of the conference. Some highlights include the work of Dr. Shawn Menefee on a randomized trial of sacral colpopexy, transvaginal mesh, and native tissue apical repair for posthysterectomy vault prolapse; a video by Dr. Matthew Fallon on a robotic-assisted laparoscopic approach to repairing a chronic uterine inversion; and the impact of age on regret following hysterectomy by Dr. Nathan King. Dr. Candace Parker-Autry also presented her work on the impact of perineorrhaphy on both female and male sexual function, and Dr. Cassie (Clarissa) Niino spoke elegantly on the “red bag problem” that exists in all of our operating rooms, which increases pollution and cost unnecessarily.
There were also several excellent talks given. Dr. Jason Wright spoke about the importance of surgical volume on gynecologic surgery. In particular, he noted that surgical volume needs to be considered not only at the surgeon level but also at the hospital level. Higher-volume hospitals will provide better care, in the same way that general, high-volume surgeons have less complications and better long-term outcomes. Of note, volume is not the whole picture. We need to also consider measurements of surgery and hospital quality and surgeon skill in addition to volume, as Dr. Shawn Menefee insightfully commented.
Dr. Beri Ridgeway gave the Mark D. Walters Lecture about surgeons in the c-suite and the importance of having a seat at the leadership table as surgeons and medical providers. In her words: “If we aren’t at the table, then we are on the menu.” Overworked and underpaid, burned out doctors feel powerless because they are managed by leaders with a business and not a medical background, and we need to have physicians in leadership who understand how medicine is practiced and to ensure equitable care
Dr. Kelly Wright gave a talk on the environmental impact of gynecologic care—from OR to clinic. She gave examples of how metal, reusable speculums become more cost-effective and produce less waste after only 2-3 uses and how there is no evidence that bouffants reduce surgical site infections (and a reusable scrub cap could work just as well without creating waste). Finally, Dr. Ebony Carter gave an impassioned talk on the need for equity in publication and grant funding in our field. She shared about her initiative through the Green Journal (Obstetrics and Gynecology) to create an issue focused on furthering equity and dispelling racism in medical research.
Later in the afternoon, I attended the Fellows’ Pelvic Research Network (FPRN) meeting, which includes AUGS-SGS (urogynecology fellows) and FMIGS-SGS (fellows of all other gynecologic subspecialities, including minimally invasive gynecologic surgery, family planning, reproductive endocrinology and infertility, and pediatric and adolescent gynecology). Dr. John Gebhart gave an excellent lecture with some impressive photos and videos on how to manage mesh exposure and erosion.
Afterwards, updates were given on the current FPRN projects, and 4 new projects were proposed and underwent audience feedback for improvement. It was exciting to see the multicenter collaborations fostered through the FPRN, and I look forward to seeing which projects will get funded for this upcoming year!

The evening ended with the President's Award Ceremony led by Dr. Cheryl Iglesia, the 49th SGS President, as well as the President's Reception. I also wantd to highlight the winner of the Distinguished Surgeon Award: Dr. Dee Fenner. The remaining awardees are listed on the SGS website (https://sgsonline.org).
Sunday, March 19. Day 1 of SGS.
Last night around midnight, bleary-eyed from the long flight from Pittsburgh, I walked out into the dimly lit, mild air of Tucson, Arizona. The Saguaro cacti that lined the entrance to the airport stood tall and tree-like, with welcoming green arms. It was as if they too knew that the next 4 days would be filled with the building of new relationships and the strengthening of old ones, as well as with education, innovation, and the sharing of research. That spirit of collegiality, approachability, and connection in an intimate and vibrant meeting is what the Society of Gynecologic Surgeons (SGS) meeting has been known for and why it draws people to come back, year after year.
The first day of the conference was fantastic. As a first-time attendant at SGS, I was excited to have the opportunity to meet and rub elbows with mentors and role models from across the country. My day started off with the SGS Fellows and Young Attendings Course, moderated by 3 incredible faculty: Dr. Matthew Barker, Dr. Sadikah Behbehani, and Dr. Traci Ito. Some high-yield topics such as contract negotiation, developing a urogynecology- or MIGS-based practice, billing, academic promotion, and taking advantage of relationships with industry were discussed at length, and the session ended with a roundtable, where the experts had time to answer questions in smaller groups. One of the quotes that will ring true for many fellows about to embark on the job search was from Dr. Amanda Ecker: “Up until now, you were told where to go and what your schedule is. This is the first time you have flexibility and power to decide for yourself.” Therefore, it is important to reflect on what you really desire and/or prioritize in a job, whether it is location, compensation, protected time, or opportunities for advancement.

In the afternoon, I attended a postgraduate course led by Dr. Veronica Lerner and Dr. Mireille Truong called “The Sim Factor: Making an Impact in Surgical Education.” Several other excellent postgraduate courses were available, including “Advanced Endometriosis Surgery and Pelvic Pain Patient-Centered Approach,” “Social Media Workshop- #Gynfluencing: Using Social Media to Find Your Digital Voice,” and “Urologic Surgery for the Gynecologic Surgeon: GU Injury, Ureteral Stents, Complex Fistula and More.” I was grateful for the hands-on and tangible tools that Drs. Lerner and Truong left the group with—including ideas such as Zoom-based virtual coaching for trainees learning fundamentals of laparoscopic surgery types of tasks, table-top simulation for high-stakes scenarios (eg, operative vascular injury), and the importance of grounding educational activity in objectives and evaluation. I even got to make and take home my own myomectomy model. (Fun fact: The myoma is actually a stress ball wrapped in an Ace bandage and then Glad Press n’ Seal!

The late afternoon transitioned to an opportunity for trainees to interact with senior SGS members and a welcome reception. The indoor and outdoor spaces were filled with laughing and talking as people connected over drinks and snacks. Finally, the evening ended with a session presented by the SGS Equity Council, “What your Patients REALLY Want to Know.” Patty Brisben, of the Patty Brisben Foundation and founder of the company Pure Romance, was interviewed by Dr. Christine Vaccaro. It was heartwarming to hear how Patty took the stories of women suffering from sexual pain and dissatisfaction and chose to make it her life’s mission to improve women’s sexual health.
Papular Rash in a New Tattoo
The Diagnosis: Allergic Contact Dermatitis
This patient’s history and physical examination were most consistent with a diagnosis of allergic contact dermatitis, likely from an additive or diluent solution within the tattoo ink. Her history of a similar transient reaction following tattooing 2 weeks prior lent credence to an allergic etiology. She was treated with triamcinolone cream 0.1% as well as mupirocin ointment 2% for use as both an emollient and for precautionary antimicrobial coverage. The rash resolved within 2 days, and she reported no recurrence at a 6-month follow-up. The cosmesis of her tattoo was preserved.
Acute cellulitis may follow tattooing, but the absence of warmth, pain, or purulence on physical examination made this diagnosis less likely in this patient. Sarcoidosis or other granulomatous reactions may present as papules or nodules arising within a tattoo but would be unlikely to occur the next day. Nontuberculous mycobacterial infection likewise tends to present subacutely or chronically rather than immediately following tattoo application.
Tattooing has existed for millennia and is becoming increasingly popular.1,2 The tattooing process entails introduction of insoluble pigment compounds into the dermis to create a permanent design on the skin, which most often is accomplished via needling. As a result, tattooed skin is susceptible to both acute and chronic complications. Acute complications prominently include allergic hypersensitivity reactions and pyogenic bacterial infections. Chronic granulomatous, inflammatory, or infectious complications also can occur.
Allergic eczematous reactions to tattooing are well documented in the literature and are thought to originate from sensitization to pigment molecules themselves or alternatively to ink diluent compounds.3 Although reactions to ink diluent chemicals typically are self-resolving, allergic reactions to pigment can persist beyond the acute phase, as these insoluble compounds intentionally remain embedded in the dermis. The mechanism of action may involve haptenization of pigment molecules that then induces allergic hypersensitivity.3,4 Black pigment typically is derived from carbon black (ie, amorphous combustion byproducts such as soot). Colored inks historically consisted of inorganic heavy metal–containing salts prior to the modern introduction of synthetic azo and polycyclic dyes. These newer colored pigments appear to be less allergenic than their metallic predecessors; however, epidemiologic studies have suggested that allergic reactions still occur more commonly in colored tattoos than black tattoos.1 Overall, these reactions may occur in as many as one-third of individuals who receive tattoos.2,4
As with any process that disrupts skin integrity, tattooing carries a risk for transmitting various infectious pathogens. Microbes may originate from adjacent skin, contaminated needles, ink bottles, or nonsterile ink diluents. Although tattoo parlors and artists may undergo licensing to demonstrate adherence to hygienic standards, regulations vary between states and do not include testing of ink or ink additives to ensure sterility.4,5 Staphylococci and streptococci commonly are implicated in acute pyogenic skin infections following tattooing.5,6 Nontuberculous mycobacteria increasingly are being recognized as causative organisms for granulomatous lesions developing subacutely or even months after receiving a new tattoo.5,7 Local and systemic viral infections also may be transmitted during tattooing; cases of tattoo-transmitted viral warts, molluscum contagiosum, and hepatitis B and C viruses all have been observed.5,6,8 Herpes simplex virus transmission (colloquially termed herpes compunctorum) and HIV transmission through tattooing also are hypothesized to be possible, though there is a paucity of known cases for each.8,9
Chronic inflammatory, granulomatous, or neoplastic lesions may arise within tattooed skin months or years after tattooing. Foreign body granulomas, sarcoidosis, pseudolymphoma, pseudoepitheliomatous hyperplasia, and keratoacanthoma are some representative entities.3,5 Cases of cancerous lesions in tattooed skin have been documented, but their incidence appears similar to nontattooed skin.3 These broad categories of lesions are clinically diverse but may be difficult to definitively diagnose on examination alone; therefore, a biopsy should be strongly considered for any subacute to chronic skin lesions within a tattoo. Patients may be hesitant to disrupt the cosmesis of a tattoo but should be counseled on the attendant risks and benefits to make an informed decision regarding biopsy.
- Wenzel SM, Rittmann I, Landthaler M, et al. Adverse reactions after tattooing: review of the literature and comparison to results of a survey. Dermatology. 2013;226:138-147.
- Liszewski W, Kream E, Helland S, et al. The demographics and rates of tattoo complications, regret, and unsafe tattooing practices: a crosssectional study. Dermatol Surg. 2015;41:1283-1289.
- Islam PS, Chang C, Selmi C, et al. Medical complications of tattoos: a comprehensive review. Clin Rev Allergy Immunol. 2016;50:273-286.
- Serup J, Carlsen KH, Sepehri M. Tattoo complaints and complications: diagnosis and clinical spectrum. Curr Probl Dermatol. 2015;48:48-60.
- Simunovic C, Shinohara MM. Complications of decorative tattoos: recognition and management. Am J Clin Dermatol. 2014;15:525-536.
- Kazandjieva J, Tsankov N. Tattoos: dermatological complications. Clin Dermatol. 2007;25:375-382.
- Sergeant A, Conaglen P, Laurenson IF, et al. Mycobacterium chelonae infection: a complication of tattooing. Clin Exp Dermatol. 2013;38:140-142.
- Cohen PR. Tattoo-associated viral infections: a review. Clin Cosmet Investig Dermatol. 2021;14:1529-1540.
- Doll DC. Tattooing in prison and HIV infection. Lancet. 1988;1:66-67.
The Diagnosis: Allergic Contact Dermatitis
This patient’s history and physical examination were most consistent with a diagnosis of allergic contact dermatitis, likely from an additive or diluent solution within the tattoo ink. Her history of a similar transient reaction following tattooing 2 weeks prior lent credence to an allergic etiology. She was treated with triamcinolone cream 0.1% as well as mupirocin ointment 2% for use as both an emollient and for precautionary antimicrobial coverage. The rash resolved within 2 days, and she reported no recurrence at a 6-month follow-up. The cosmesis of her tattoo was preserved.
Acute cellulitis may follow tattooing, but the absence of warmth, pain, or purulence on physical examination made this diagnosis less likely in this patient. Sarcoidosis or other granulomatous reactions may present as papules or nodules arising within a tattoo but would be unlikely to occur the next day. Nontuberculous mycobacterial infection likewise tends to present subacutely or chronically rather than immediately following tattoo application.
Tattooing has existed for millennia and is becoming increasingly popular.1,2 The tattooing process entails introduction of insoluble pigment compounds into the dermis to create a permanent design on the skin, which most often is accomplished via needling. As a result, tattooed skin is susceptible to both acute and chronic complications. Acute complications prominently include allergic hypersensitivity reactions and pyogenic bacterial infections. Chronic granulomatous, inflammatory, or infectious complications also can occur.
Allergic eczematous reactions to tattooing are well documented in the literature and are thought to originate from sensitization to pigment molecules themselves or alternatively to ink diluent compounds.3 Although reactions to ink diluent chemicals typically are self-resolving, allergic reactions to pigment can persist beyond the acute phase, as these insoluble compounds intentionally remain embedded in the dermis. The mechanism of action may involve haptenization of pigment molecules that then induces allergic hypersensitivity.3,4 Black pigment typically is derived from carbon black (ie, amorphous combustion byproducts such as soot). Colored inks historically consisted of inorganic heavy metal–containing salts prior to the modern introduction of synthetic azo and polycyclic dyes. These newer colored pigments appear to be less allergenic than their metallic predecessors; however, epidemiologic studies have suggested that allergic reactions still occur more commonly in colored tattoos than black tattoos.1 Overall, these reactions may occur in as many as one-third of individuals who receive tattoos.2,4
As with any process that disrupts skin integrity, tattooing carries a risk for transmitting various infectious pathogens. Microbes may originate from adjacent skin, contaminated needles, ink bottles, or nonsterile ink diluents. Although tattoo parlors and artists may undergo licensing to demonstrate adherence to hygienic standards, regulations vary between states and do not include testing of ink or ink additives to ensure sterility.4,5 Staphylococci and streptococci commonly are implicated in acute pyogenic skin infections following tattooing.5,6 Nontuberculous mycobacteria increasingly are being recognized as causative organisms for granulomatous lesions developing subacutely or even months after receiving a new tattoo.5,7 Local and systemic viral infections also may be transmitted during tattooing; cases of tattoo-transmitted viral warts, molluscum contagiosum, and hepatitis B and C viruses all have been observed.5,6,8 Herpes simplex virus transmission (colloquially termed herpes compunctorum) and HIV transmission through tattooing also are hypothesized to be possible, though there is a paucity of known cases for each.8,9
Chronic inflammatory, granulomatous, or neoplastic lesions may arise within tattooed skin months or years after tattooing. Foreign body granulomas, sarcoidosis, pseudolymphoma, pseudoepitheliomatous hyperplasia, and keratoacanthoma are some representative entities.3,5 Cases of cancerous lesions in tattooed skin have been documented, but their incidence appears similar to nontattooed skin.3 These broad categories of lesions are clinically diverse but may be difficult to definitively diagnose on examination alone; therefore, a biopsy should be strongly considered for any subacute to chronic skin lesions within a tattoo. Patients may be hesitant to disrupt the cosmesis of a tattoo but should be counseled on the attendant risks and benefits to make an informed decision regarding biopsy.
The Diagnosis: Allergic Contact Dermatitis
This patient’s history and physical examination were most consistent with a diagnosis of allergic contact dermatitis, likely from an additive or diluent solution within the tattoo ink. Her history of a similar transient reaction following tattooing 2 weeks prior lent credence to an allergic etiology. She was treated with triamcinolone cream 0.1% as well as mupirocin ointment 2% for use as both an emollient and for precautionary antimicrobial coverage. The rash resolved within 2 days, and she reported no recurrence at a 6-month follow-up. The cosmesis of her tattoo was preserved.
Acute cellulitis may follow tattooing, but the absence of warmth, pain, or purulence on physical examination made this diagnosis less likely in this patient. Sarcoidosis or other granulomatous reactions may present as papules or nodules arising within a tattoo but would be unlikely to occur the next day. Nontuberculous mycobacterial infection likewise tends to present subacutely or chronically rather than immediately following tattoo application.
Tattooing has existed for millennia and is becoming increasingly popular.1,2 The tattooing process entails introduction of insoluble pigment compounds into the dermis to create a permanent design on the skin, which most often is accomplished via needling. As a result, tattooed skin is susceptible to both acute and chronic complications. Acute complications prominently include allergic hypersensitivity reactions and pyogenic bacterial infections. Chronic granulomatous, inflammatory, or infectious complications also can occur.
Allergic eczematous reactions to tattooing are well documented in the literature and are thought to originate from sensitization to pigment molecules themselves or alternatively to ink diluent compounds.3 Although reactions to ink diluent chemicals typically are self-resolving, allergic reactions to pigment can persist beyond the acute phase, as these insoluble compounds intentionally remain embedded in the dermis. The mechanism of action may involve haptenization of pigment molecules that then induces allergic hypersensitivity.3,4 Black pigment typically is derived from carbon black (ie, amorphous combustion byproducts such as soot). Colored inks historically consisted of inorganic heavy metal–containing salts prior to the modern introduction of synthetic azo and polycyclic dyes. These newer colored pigments appear to be less allergenic than their metallic predecessors; however, epidemiologic studies have suggested that allergic reactions still occur more commonly in colored tattoos than black tattoos.1 Overall, these reactions may occur in as many as one-third of individuals who receive tattoos.2,4
As with any process that disrupts skin integrity, tattooing carries a risk for transmitting various infectious pathogens. Microbes may originate from adjacent skin, contaminated needles, ink bottles, or nonsterile ink diluents. Although tattoo parlors and artists may undergo licensing to demonstrate adherence to hygienic standards, regulations vary between states and do not include testing of ink or ink additives to ensure sterility.4,5 Staphylococci and streptococci commonly are implicated in acute pyogenic skin infections following tattooing.5,6 Nontuberculous mycobacteria increasingly are being recognized as causative organisms for granulomatous lesions developing subacutely or even months after receiving a new tattoo.5,7 Local and systemic viral infections also may be transmitted during tattooing; cases of tattoo-transmitted viral warts, molluscum contagiosum, and hepatitis B and C viruses all have been observed.5,6,8 Herpes simplex virus transmission (colloquially termed herpes compunctorum) and HIV transmission through tattooing also are hypothesized to be possible, though there is a paucity of known cases for each.8,9
Chronic inflammatory, granulomatous, or neoplastic lesions may arise within tattooed skin months or years after tattooing. Foreign body granulomas, sarcoidosis, pseudolymphoma, pseudoepitheliomatous hyperplasia, and keratoacanthoma are some representative entities.3,5 Cases of cancerous lesions in tattooed skin have been documented, but their incidence appears similar to nontattooed skin.3 These broad categories of lesions are clinically diverse but may be difficult to definitively diagnose on examination alone; therefore, a biopsy should be strongly considered for any subacute to chronic skin lesions within a tattoo. Patients may be hesitant to disrupt the cosmesis of a tattoo but should be counseled on the attendant risks and benefits to make an informed decision regarding biopsy.
- Wenzel SM, Rittmann I, Landthaler M, et al. Adverse reactions after tattooing: review of the literature and comparison to results of a survey. Dermatology. 2013;226:138-147.
- Liszewski W, Kream E, Helland S, et al. The demographics and rates of tattoo complications, regret, and unsafe tattooing practices: a crosssectional study. Dermatol Surg. 2015;41:1283-1289.
- Islam PS, Chang C, Selmi C, et al. Medical complications of tattoos: a comprehensive review. Clin Rev Allergy Immunol. 2016;50:273-286.
- Serup J, Carlsen KH, Sepehri M. Tattoo complaints and complications: diagnosis and clinical spectrum. Curr Probl Dermatol. 2015;48:48-60.
- Simunovic C, Shinohara MM. Complications of decorative tattoos: recognition and management. Am J Clin Dermatol. 2014;15:525-536.
- Kazandjieva J, Tsankov N. Tattoos: dermatological complications. Clin Dermatol. 2007;25:375-382.
- Sergeant A, Conaglen P, Laurenson IF, et al. Mycobacterium chelonae infection: a complication of tattooing. Clin Exp Dermatol. 2013;38:140-142.
- Cohen PR. Tattoo-associated viral infections: a review. Clin Cosmet Investig Dermatol. 2021;14:1529-1540.
- Doll DC. Tattooing in prison and HIV infection. Lancet. 1988;1:66-67.
- Wenzel SM, Rittmann I, Landthaler M, et al. Adverse reactions after tattooing: review of the literature and comparison to results of a survey. Dermatology. 2013;226:138-147.
- Liszewski W, Kream E, Helland S, et al. The demographics and rates of tattoo complications, regret, and unsafe tattooing practices: a crosssectional study. Dermatol Surg. 2015;41:1283-1289.
- Islam PS, Chang C, Selmi C, et al. Medical complications of tattoos: a comprehensive review. Clin Rev Allergy Immunol. 2016;50:273-286.
- Serup J, Carlsen KH, Sepehri M. Tattoo complaints and complications: diagnosis and clinical spectrum. Curr Probl Dermatol. 2015;48:48-60.
- Simunovic C, Shinohara MM. Complications of decorative tattoos: recognition and management. Am J Clin Dermatol. 2014;15:525-536.
- Kazandjieva J, Tsankov N. Tattoos: dermatological complications. Clin Dermatol. 2007;25:375-382.
- Sergeant A, Conaglen P, Laurenson IF, et al. Mycobacterium chelonae infection: a complication of tattooing. Clin Exp Dermatol. 2013;38:140-142.
- Cohen PR. Tattoo-associated viral infections: a review. Clin Cosmet Investig Dermatol. 2021;14:1529-1540.
- Doll DC. Tattooing in prison and HIV infection. Lancet. 1988;1:66-67.
A healthy 21-year-old woman presented with a pruritic papulovesicular rash on the left arm of 2 days’ duration. The day before rash onset, she received a black ink tattoo on the left arm to complete the second half of a monochromatic sleevestyle design. She previously underwent initial tattooing of the left arm by the same artist 2 weeks prior and experienced a similar but less extensive rash that self-resolved after 1 week. She had 8 older tattoos on various other body parts and denied any reactions. Physical examination showed numerous scattered papules and papulovesicles confined to areas of newly tattooed skin throughout the left arm. In the larger swaths of the tattoo, the papules coalesced into well-defined plaques. There was a discrete rim of faint erythema bordering the newly tattooed skin. No erosions, ulcerations, or purulent areas were observed, and there was no tenderness or excess warmth of the affected skin. Adjacent previously tattooed areas of the left arm were unaffected.