User login
COMMENT & CONTROVERSY
Medical library access
During most of my clinical career I had an affiliation with a local medical school as a “Clinical Instructor” and then “Assistant Clinical Professor.” In addition to teaching medical students and residents from that institution that rotated through my hospital, it also gave me certain privileges, the most important of which was access to that institution’s electronic medical library. Using that access, even as an “LMD,” I have been able to contribute to the medical literature on subjects of interest to me and to others in my specialty.
Recently, now as an older clinician, I gave up my hospital privileges, although I continue my office practice. Giving up my hospital privileges meant that I no longer qualified as a faculty member—and therefore lost online access to the medical library. Still wishing to continue my medical writing, I have attempted to attain access to the medical literature by special request to that library, by contacting my state medical society, by contacting my national specialty organization, by contacting the department chair at the institution to which I had been affiliated, and by calling the Dean of the medical school to which my hospital was affiliated. Although meaning well, none was able to get me access to an online medical library. Thus, I am greatly hampered in my attempts to do research and to continue to write further papers on those areas in which I have previously published.
Is there no remedy for this? Should all clinicians who “age out” of institutional affiliations no longer be able to pursue research interests? And what about community physicians who have no academic affiliations? Can they not access the latest information they need to practice evidence-based, up-to-date medicine?
It makes no sense to me that access to the latest and most current aspects of medical care should be withheld from any clinician. For every clinician not to have access to such medical knowledge does a disservice to all those practicing medicine who wish to keep up to date and to all patients of American clinicians whose providers are prevented from practicing the best, evidence-based care.
Henry Lerner, MD
Boston, Massachusetts
Medical library access
During most of my clinical career I had an affiliation with a local medical school as a “Clinical Instructor” and then “Assistant Clinical Professor.” In addition to teaching medical students and residents from that institution that rotated through my hospital, it also gave me certain privileges, the most important of which was access to that institution’s electronic medical library. Using that access, even as an “LMD,” I have been able to contribute to the medical literature on subjects of interest to me and to others in my specialty.
Recently, now as an older clinician, I gave up my hospital privileges, although I continue my office practice. Giving up my hospital privileges meant that I no longer qualified as a faculty member—and therefore lost online access to the medical library. Still wishing to continue my medical writing, I have attempted to attain access to the medical literature by special request to that library, by contacting my state medical society, by contacting my national specialty organization, by contacting the department chair at the institution to which I had been affiliated, and by calling the Dean of the medical school to which my hospital was affiliated. Although meaning well, none was able to get me access to an online medical library. Thus, I am greatly hampered in my attempts to do research and to continue to write further papers on those areas in which I have previously published.
Is there no remedy for this? Should all clinicians who “age out” of institutional affiliations no longer be able to pursue research interests? And what about community physicians who have no academic affiliations? Can they not access the latest information they need to practice evidence-based, up-to-date medicine?
It makes no sense to me that access to the latest and most current aspects of medical care should be withheld from any clinician. For every clinician not to have access to such medical knowledge does a disservice to all those practicing medicine who wish to keep up to date and to all patients of American clinicians whose providers are prevented from practicing the best, evidence-based care.
Henry Lerner, MD
Boston, Massachusetts
Medical library access
During most of my clinical career I had an affiliation with a local medical school as a “Clinical Instructor” and then “Assistant Clinical Professor.” In addition to teaching medical students and residents from that institution that rotated through my hospital, it also gave me certain privileges, the most important of which was access to that institution’s electronic medical library. Using that access, even as an “LMD,” I have been able to contribute to the medical literature on subjects of interest to me and to others in my specialty.
Recently, now as an older clinician, I gave up my hospital privileges, although I continue my office practice. Giving up my hospital privileges meant that I no longer qualified as a faculty member—and therefore lost online access to the medical library. Still wishing to continue my medical writing, I have attempted to attain access to the medical literature by special request to that library, by contacting my state medical society, by contacting my national specialty organization, by contacting the department chair at the institution to which I had been affiliated, and by calling the Dean of the medical school to which my hospital was affiliated. Although meaning well, none was able to get me access to an online medical library. Thus, I am greatly hampered in my attempts to do research and to continue to write further papers on those areas in which I have previously published.
Is there no remedy for this? Should all clinicians who “age out” of institutional affiliations no longer be able to pursue research interests? And what about community physicians who have no academic affiliations? Can they not access the latest information they need to practice evidence-based, up-to-date medicine?
It makes no sense to me that access to the latest and most current aspects of medical care should be withheld from any clinician. For every clinician not to have access to such medical knowledge does a disservice to all those practicing medicine who wish to keep up to date and to all patients of American clinicians whose providers are prevented from practicing the best, evidence-based care.
Henry Lerner, MD
Boston, Massachusetts
Cervical Cancer Screening in the Post-Vaccine Era – Confronting a New Clinical Reality
To read more click here
To read more click here
To read more click here
Mycoplasma genitalium: A Pathogen We Can Finally Begin to Understand

Riding alongside known STIs, this historically elusive microbe may have often been the underlying cause of a variety of symptoms. In this supplement to OBG Management Dr. Kyle Bukowski discusses how to meet the challenge presented by this not-so-new microbe while helping foster regular STI testing, and encourage patients to seek care when symptoms occur.

Riding alongside known STIs, this historically elusive microbe may have often been the underlying cause of a variety of symptoms. In this supplement to OBG Management Dr. Kyle Bukowski discusses how to meet the challenge presented by this not-so-new microbe while helping foster regular STI testing, and encourage patients to seek care when symptoms occur.

Riding alongside known STIs, this historically elusive microbe may have often been the underlying cause of a variety of symptoms. In this supplement to OBG Management Dr. Kyle Bukowski discusses how to meet the challenge presented by this not-so-new microbe while helping foster regular STI testing, and encourage patients to seek care when symptoms occur.
Looking at CGRP-Related Medications for Migraine, April 2023
Since 2018, the field of headache medicine has changed significantly. The development of calcitonin gene-related peptide (CGRP)-targeting preventive medications has led to the ability to treat migraine in a much more specific manner. The development of CGRP acute oral medications over the past 2 years has allowed people with migraine the ability to use well-tolerated, migraine-specific, abortive treatments. Triptan medications were the first migraine-specific acute treatments developed, some of which were nonoral, such as injectable sumatriptan and intranasal sumatriptan and zolmitriptan. The study by Lipton and colleagues assesses the safety and tolerability of a novel acute CGRP antagonist nonoral treatment, zavegepant.
In this double-blind, randomized, multicentered trial, nearly 2000 participants were enrolled with a diagnosis of episodic migraine with or without aura; they were excluded if they had previously used another CGRP antagonist, either an injectable or oral medication, before enrolling in this study. In addition to assessing migraine pain, participants were asked to identify their otherwise most bothersome symptom, specifically photophobia, phonophobia, or nausea. They were given a nasal spray to self-administer and were assessed at 15 minutes after treatment and at multiple additional intervals, up to 48 hours after the initial dosing. The primary endpoints were freedom from pain and freedom from the most bothersome symptom at 2 hours after treatment onset. There were 17 secondary endpoints.
At 2 hours after treatment onset, a statistically significant group had achieved freedom from pain. The percentage, however, did remain somewhat low: 24%. Freedom from the most bothersome symptom was also statistically significant but was up to 40%. For 13 of the 17 endpoints, the results were also statistically significant, including pain relief at 2 hours, sustained pain relief at 2-24 hours and 48 hours, functional improvement, and freedom from photophobia and phonophobia. The most common adverse effects were poor taste, nasal discomfort, and throat irritation. No serious adverse events were noted.
Zavegepant has been FDA approved for the acute treatment of migraine on the basis of these data. This is a novel, well-tolerated, nonoral acute treatment for migraine. We can now treat patients with very severe nausea or more sudden-onset pain with a CGRP option that can potentially treat their attacks more quickly.
One early finding in many of the CGRP studies was that a certain subpopulation of migraine patients have a robust and rapid preventive response to monoclonal antibody treatment. Raffaelli and colleagues sought to evaluate potential characteristics that would better predict the efficacy of CGRP antagonist monoclonal antibodies for the prevention of migraine.
In this study, the definition of a superresponse to CGRP antagonist treatment was a >75% reduction in monthly headache days after 3 months of treatment. Nonresponse was defined as <25% reduction over this same period. This was a retrospective cohort study at one headache center in Berlin, Germany. A total of 260 patients were enrolled, all with a diagnosis of migraine and all given a preventive CGRP monoclonal antibody.
There was no significant difference between nonresponders and superresponders when compared for sex, age, or time since migraine diagnosis. Erenumab was the most commonly prescribed CGRP antagonist medication, but all CGRP antagonists were included. There was no significant difference when CGRP receptor or ligand targeting antibodies were compared. Nonresponders were seen as more likely to have chronic migraine and higher monthly headache day and monthly migraine day frequencies. Superresponders were seen to have more "typical" migraine characteristics, such as unilateral or localized migraines or migraines with pulsating/throbbing characteristics, as well as the presence of photophobia and nausea; however, this was not statistically significant. Of note, superresponders were also significantly more likely to report improvement of their acute migraine attacks with triptan medications as compared with nonresponders.
Patients with less frequent migraine attacks and more classic migraine attacks appear to be much more likely to respond quickly and effectively to many preventive options; this appears to be most robust with the CGRP antibody class. Although the reason for this robust response is not entirely clear, it would certainly be best for providers to consider the initiation of CGRP antagonist preventive treatment in patients with these characteristics.
The newest generation of migraine-specific medications targets either the inflammatory neurotransmitter CGRP or the CGRP receptor. Erenumab is a CGRP receptor blocker, whereas both fremanezumab and galcanezumab block the CGRP ligand. Erenumab has been associated with constipation and high blood pressure, whereas the other CGRP antagonist medications are not associated with these side effects. Whether this is due to the difference in mechanism of action, and specifically whether the antibodies block the CGRP receptor or are an antagonist, is under consideration. Schiano di Cola and colleagues specifically sought to investigate the subtle differences between these two subclasses of treatment.
Patients with high-frequency episodic and chronic migraine were enrolled in this retrospective study; 6 months of data were included. The researchers here specifically looked at efficacy after 1, 3, and 6 months of treatment. They examined, as a primary outcome, monthly headache and migraine days, and migraine disability as based on the Migraine Disability Assessment Scale (MIDAS) and Headache Impact Test (HIT-6) score. Concomitant analgesic medication consumption and response rate relative to baseline were also compared.
A total of 152 patients were enrolled, 68 with CGRP ligand-targeting therapy and 84 with CGRP receptor-blocking therapy. Medication overuse was present in 73% of patients. Although a significant improvement from baseline was noted in monthly headache days, monthly migraine days, severity, analgesic consumption, and disability, MIDAS scores were significantly lower in the CGRP ligand-blocking group compared with the CGRP receptor group at 1 and 3 months. Number of monthly migraine days was also lower in the CGRP ligand-blocking group, but only after 3 months. The other variables, including monthly headache days per month, analgesic consumption, severity, and disability, were not statistically different.
Adverse events were not compared between the two groups, even though this was a prior noted difference between these two classes of medications. Although there are some slight differences in efficacy, the majority of outcome metrics did not appear to be significantly different in either group. One would be hard-pressed to choose a specific CGRP medication on the basis of these data.
Since 2018, the field of headache medicine has changed significantly. The development of calcitonin gene-related peptide (CGRP)-targeting preventive medications has led to the ability to treat migraine in a much more specific manner. The development of CGRP acute oral medications over the past 2 years has allowed people with migraine the ability to use well-tolerated, migraine-specific, abortive treatments. Triptan medications were the first migraine-specific acute treatments developed, some of which were nonoral, such as injectable sumatriptan and intranasal sumatriptan and zolmitriptan. The study by Lipton and colleagues assesses the safety and tolerability of a novel acute CGRP antagonist nonoral treatment, zavegepant.
In this double-blind, randomized, multicentered trial, nearly 2000 participants were enrolled with a diagnosis of episodic migraine with or without aura; they were excluded if they had previously used another CGRP antagonist, either an injectable or oral medication, before enrolling in this study. In addition to assessing migraine pain, participants were asked to identify their otherwise most bothersome symptom, specifically photophobia, phonophobia, or nausea. They were given a nasal spray to self-administer and were assessed at 15 minutes after treatment and at multiple additional intervals, up to 48 hours after the initial dosing. The primary endpoints were freedom from pain and freedom from the most bothersome symptom at 2 hours after treatment onset. There were 17 secondary endpoints.
At 2 hours after treatment onset, a statistically significant group had achieved freedom from pain. The percentage, however, did remain somewhat low: 24%. Freedom from the most bothersome symptom was also statistically significant but was up to 40%. For 13 of the 17 endpoints, the results were also statistically significant, including pain relief at 2 hours, sustained pain relief at 2-24 hours and 48 hours, functional improvement, and freedom from photophobia and phonophobia. The most common adverse effects were poor taste, nasal discomfort, and throat irritation. No serious adverse events were noted.
Zavegepant has been FDA approved for the acute treatment of migraine on the basis of these data. This is a novel, well-tolerated, nonoral acute treatment for migraine. We can now treat patients with very severe nausea or more sudden-onset pain with a CGRP option that can potentially treat their attacks more quickly.
One early finding in many of the CGRP studies was that a certain subpopulation of migraine patients have a robust and rapid preventive response to monoclonal antibody treatment. Raffaelli and colleagues sought to evaluate potential characteristics that would better predict the efficacy of CGRP antagonist monoclonal antibodies for the prevention of migraine.
In this study, the definition of a superresponse to CGRP antagonist treatment was a >75% reduction in monthly headache days after 3 months of treatment. Nonresponse was defined as <25% reduction over this same period. This was a retrospective cohort study at one headache center in Berlin, Germany. A total of 260 patients were enrolled, all with a diagnosis of migraine and all given a preventive CGRP monoclonal antibody.
There was no significant difference between nonresponders and superresponders when compared for sex, age, or time since migraine diagnosis. Erenumab was the most commonly prescribed CGRP antagonist medication, but all CGRP antagonists were included. There was no significant difference when CGRP receptor or ligand targeting antibodies were compared. Nonresponders were seen as more likely to have chronic migraine and higher monthly headache day and monthly migraine day frequencies. Superresponders were seen to have more "typical" migraine characteristics, such as unilateral or localized migraines or migraines with pulsating/throbbing characteristics, as well as the presence of photophobia and nausea; however, this was not statistically significant. Of note, superresponders were also significantly more likely to report improvement of their acute migraine attacks with triptan medications as compared with nonresponders.
Patients with less frequent migraine attacks and more classic migraine attacks appear to be much more likely to respond quickly and effectively to many preventive options; this appears to be most robust with the CGRP antibody class. Although the reason for this robust response is not entirely clear, it would certainly be best for providers to consider the initiation of CGRP antagonist preventive treatment in patients with these characteristics.
The newest generation of migraine-specific medications targets either the inflammatory neurotransmitter CGRP or the CGRP receptor. Erenumab is a CGRP receptor blocker, whereas both fremanezumab and galcanezumab block the CGRP ligand. Erenumab has been associated with constipation and high blood pressure, whereas the other CGRP antagonist medications are not associated with these side effects. Whether this is due to the difference in mechanism of action, and specifically whether the antibodies block the CGRP receptor or are an antagonist, is under consideration. Schiano di Cola and colleagues specifically sought to investigate the subtle differences between these two subclasses of treatment.
Patients with high-frequency episodic and chronic migraine were enrolled in this retrospective study; 6 months of data were included. The researchers here specifically looked at efficacy after 1, 3, and 6 months of treatment. They examined, as a primary outcome, monthly headache and migraine days, and migraine disability as based on the Migraine Disability Assessment Scale (MIDAS) and Headache Impact Test (HIT-6) score. Concomitant analgesic medication consumption and response rate relative to baseline were also compared.
A total of 152 patients were enrolled, 68 with CGRP ligand-targeting therapy and 84 with CGRP receptor-blocking therapy. Medication overuse was present in 73% of patients. Although a significant improvement from baseline was noted in monthly headache days, monthly migraine days, severity, analgesic consumption, and disability, MIDAS scores were significantly lower in the CGRP ligand-blocking group compared with the CGRP receptor group at 1 and 3 months. Number of monthly migraine days was also lower in the CGRP ligand-blocking group, but only after 3 months. The other variables, including monthly headache days per month, analgesic consumption, severity, and disability, were not statistically different.
Adverse events were not compared between the two groups, even though this was a prior noted difference between these two classes of medications. Although there are some slight differences in efficacy, the majority of outcome metrics did not appear to be significantly different in either group. One would be hard-pressed to choose a specific CGRP medication on the basis of these data.
Since 2018, the field of headache medicine has changed significantly. The development of calcitonin gene-related peptide (CGRP)-targeting preventive medications has led to the ability to treat migraine in a much more specific manner. The development of CGRP acute oral medications over the past 2 years has allowed people with migraine the ability to use well-tolerated, migraine-specific, abortive treatments. Triptan medications were the first migraine-specific acute treatments developed, some of which were nonoral, such as injectable sumatriptan and intranasal sumatriptan and zolmitriptan. The study by Lipton and colleagues assesses the safety and tolerability of a novel acute CGRP antagonist nonoral treatment, zavegepant.
In this double-blind, randomized, multicentered trial, nearly 2000 participants were enrolled with a diagnosis of episodic migraine with or without aura; they were excluded if they had previously used another CGRP antagonist, either an injectable or oral medication, before enrolling in this study. In addition to assessing migraine pain, participants were asked to identify their otherwise most bothersome symptom, specifically photophobia, phonophobia, or nausea. They were given a nasal spray to self-administer and were assessed at 15 minutes after treatment and at multiple additional intervals, up to 48 hours after the initial dosing. The primary endpoints were freedom from pain and freedom from the most bothersome symptom at 2 hours after treatment onset. There were 17 secondary endpoints.
At 2 hours after treatment onset, a statistically significant group had achieved freedom from pain. The percentage, however, did remain somewhat low: 24%. Freedom from the most bothersome symptom was also statistically significant but was up to 40%. For 13 of the 17 endpoints, the results were also statistically significant, including pain relief at 2 hours, sustained pain relief at 2-24 hours and 48 hours, functional improvement, and freedom from photophobia and phonophobia. The most common adverse effects were poor taste, nasal discomfort, and throat irritation. No serious adverse events were noted.
Zavegepant has been FDA approved for the acute treatment of migraine on the basis of these data. This is a novel, well-tolerated, nonoral acute treatment for migraine. We can now treat patients with very severe nausea or more sudden-onset pain with a CGRP option that can potentially treat their attacks more quickly.
One early finding in many of the CGRP studies was that a certain subpopulation of migraine patients have a robust and rapid preventive response to monoclonal antibody treatment. Raffaelli and colleagues sought to evaluate potential characteristics that would better predict the efficacy of CGRP antagonist monoclonal antibodies for the prevention of migraine.
In this study, the definition of a superresponse to CGRP antagonist treatment was a >75% reduction in monthly headache days after 3 months of treatment. Nonresponse was defined as <25% reduction over this same period. This was a retrospective cohort study at one headache center in Berlin, Germany. A total of 260 patients were enrolled, all with a diagnosis of migraine and all given a preventive CGRP monoclonal antibody.
There was no significant difference between nonresponders and superresponders when compared for sex, age, or time since migraine diagnosis. Erenumab was the most commonly prescribed CGRP antagonist medication, but all CGRP antagonists were included. There was no significant difference when CGRP receptor or ligand targeting antibodies were compared. Nonresponders were seen as more likely to have chronic migraine and higher monthly headache day and monthly migraine day frequencies. Superresponders were seen to have more "typical" migraine characteristics, such as unilateral or localized migraines or migraines with pulsating/throbbing characteristics, as well as the presence of photophobia and nausea; however, this was not statistically significant. Of note, superresponders were also significantly more likely to report improvement of their acute migraine attacks with triptan medications as compared with nonresponders.
Patients with less frequent migraine attacks and more classic migraine attacks appear to be much more likely to respond quickly and effectively to many preventive options; this appears to be most robust with the CGRP antibody class. Although the reason for this robust response is not entirely clear, it would certainly be best for providers to consider the initiation of CGRP antagonist preventive treatment in patients with these characteristics.
The newest generation of migraine-specific medications targets either the inflammatory neurotransmitter CGRP or the CGRP receptor. Erenumab is a CGRP receptor blocker, whereas both fremanezumab and galcanezumab block the CGRP ligand. Erenumab has been associated with constipation and high blood pressure, whereas the other CGRP antagonist medications are not associated with these side effects. Whether this is due to the difference in mechanism of action, and specifically whether the antibodies block the CGRP receptor or are an antagonist, is under consideration. Schiano di Cola and colleagues specifically sought to investigate the subtle differences between these two subclasses of treatment.
Patients with high-frequency episodic and chronic migraine were enrolled in this retrospective study; 6 months of data were included. The researchers here specifically looked at efficacy after 1, 3, and 6 months of treatment. They examined, as a primary outcome, monthly headache and migraine days, and migraine disability as based on the Migraine Disability Assessment Scale (MIDAS) and Headache Impact Test (HIT-6) score. Concomitant analgesic medication consumption and response rate relative to baseline were also compared.
A total of 152 patients were enrolled, 68 with CGRP ligand-targeting therapy and 84 with CGRP receptor-blocking therapy. Medication overuse was present in 73% of patients. Although a significant improvement from baseline was noted in monthly headache days, monthly migraine days, severity, analgesic consumption, and disability, MIDAS scores were significantly lower in the CGRP ligand-blocking group compared with the CGRP receptor group at 1 and 3 months. Number of monthly migraine days was also lower in the CGRP ligand-blocking group, but only after 3 months. The other variables, including monthly headache days per month, analgesic consumption, severity, and disability, were not statistically different.
Adverse events were not compared between the two groups, even though this was a prior noted difference between these two classes of medications. Although there are some slight differences in efficacy, the majority of outcome metrics did not appear to be significantly different in either group. One would be hard-pressed to choose a specific CGRP medication on the basis of these data.
Premedical Student Interest in and Exposure to Dermatology at Howard University
Diversity of health care professionals improves medical outcomes and quality of life in patients. 1 There is a lack of diversity in dermatology, with only 4.2% of dermatologists identifying as Hispanic and 3% identifying as African American, 2 possibly due to a lack of early exposure to dermatology among high school and undergraduate students, a low number of underrepresented students in medical school, a lack of formal mentorship programs geared to underrepresented students, and implicit biases. 1-4 Furthermore, the field is competitive, with many more applicants than available positions. In 2022, there were 851 applicants competing for 492 residency positions in dermatology. 5 Thus, it is important to educate young students about dermatology and understand root causes as to why the number of u nderrepresented in medicine (UiM) dermatologists remains stagnant.
According to Pritchett et al,4 it is crucial for dermatologists to interact with high school and college students to foster an early interest in dermatology. Many racial minority students do not progress from high school to college and then from college to medical school, which leaves a substantially reduced number of eligible UiM applicants who can progress into dermatology.6 Increasing the amount of UiM students going to medical school requires early mediation. Collaborating with pre-existing premedical school organizations through presentations and workshops is another way to promote an early interest in dermatology.4 Special consideration should be given to students who are UiM.
Among the general medical school curriculum, requirements for exposure to dermatology are not high. In one study, the median number of clinical and preclinical hours required was 10. Furthermore, 20% of 33 medical schools did not require preclinical dermatology hours (hours done before medical school rotations begin and in an academic setting), 36% required no clinical hours (rotational hours), 8% required no dermatology hours whatsoever, and only 10% required clinical dermatology rotation.3 Based on these findings, it is clear that dermatology is not well incorporated into medical school curricula. Furthermore, curricula have historically neglected to display adequate representation of skin of color.7 As a result, medical students generally have limited exposure to dermatology3 and are exposed even less to presentations of dermatologic issues in historically marginalized populations.7
Given the paucity of research on UiM students’ perceptions of dermatology prior to medical school, our cross-sectional survey study sought to evaluate the level of interest in dermatology of UiM premedical undergraduates. This survey specifically evaluated exposure to dermatology, preconceived notions about the field, and mentorship opportunities. By understanding these factors, dermatologists and dermatology residency programs can use this information to create mentorship opportunities and better adjust existing programs to meet students’ needs.
Methods
A 19-question multiple-choice survey was administered electronically (SurveyMonkey) in May 2020 to premedical students at Howard University (Washington, DC). One screening question was used: “What is your major?” Those who considered themselves a science major and/or with premedical interest were allowed to complete the survey. All students surveyed were members of the Health Professions Society at Howard University. Students who were interested in pursuing medical school were invited to respond. Approval for this study was obtained from the Howard University institutional review board (FWA00000891).
The survey was divided into 3 sections: Demographics, Exposure to Medicine and Dermatology, and Perceptions of Dermatology. The Demographics section addressed gender, age, and race/ethnicity. The Exposure to Medicine and Dermatology section addressed interest in attending medical school, shadowing experience, exposure to dermatology, and mentoring. The Perceptions of Dermatology section addressed preconceived notions about the field (eg, “dermatology is interesting and exciting”).
Statistical Analysis—The data represented are percentages based on the number of respondents who answered each question. Answers in response to “Please enter any comments” were organized into themes, and the number of respondents who discussed each theme was quantified into a table.
Results
A total of 271 survey invitations were sent to premedical students at Howard University. Students were informed of the study protocol and asked to consent before proceeding to have their responses anonymously collected. Based on the screening question, 152 participants qualified for the survey, and 152 participants completed it (response rate, 56%; completion rate, 100%). Participants were asked to complete the survey only once.
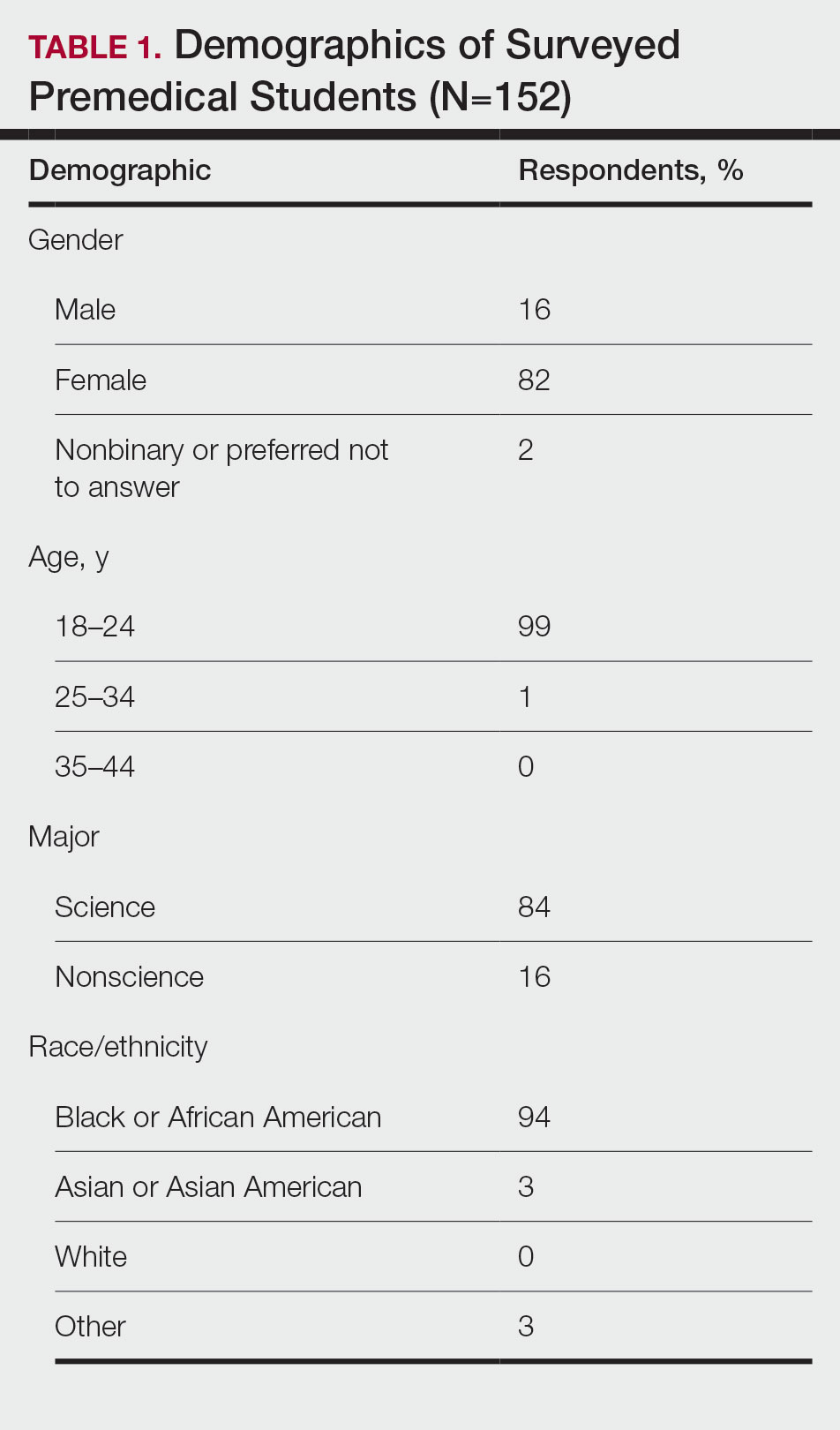
Demographics—Eighty-four percent of respondents identified as science majors, and the remaining 16% identified as nonscience premedical. Ninety-four percent of participants identified as Black or African American; 3% as Asian or Asian American; and the remaining 3% as Other. Most respondents were female (82%), 16% were male, and 2% were either nonbinary or preferred not to answer. Ninety-nine percent were aged 18 to 24 years, and 1% were aged 25 to 34 years (Table 1).
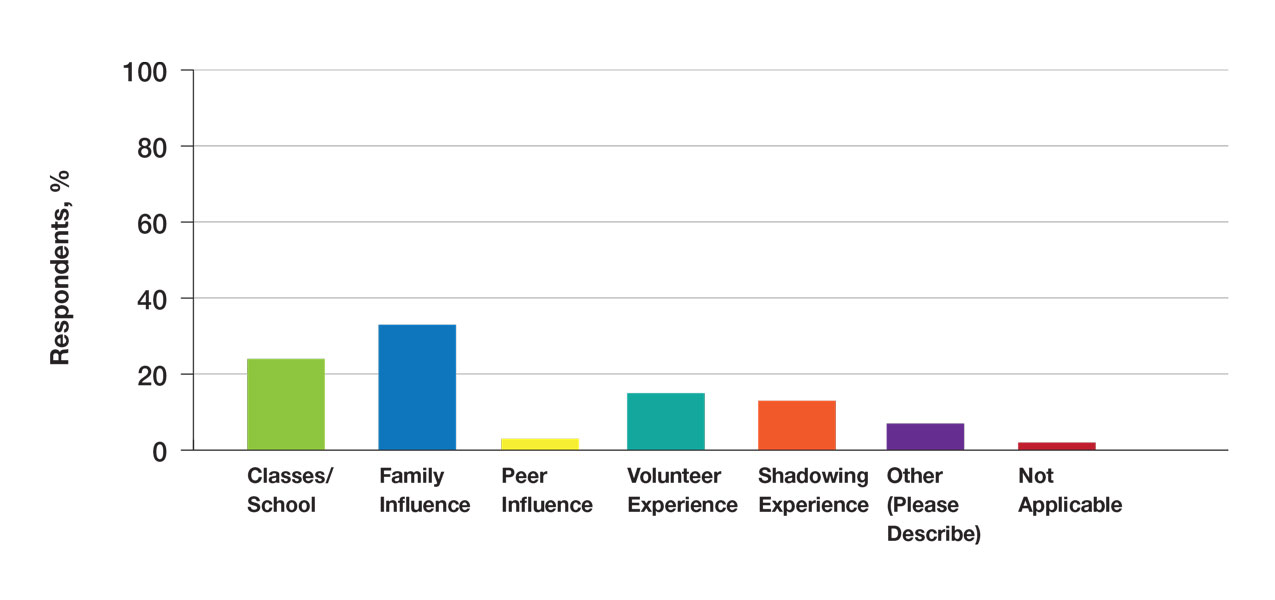
Exposure to Medicine and Dermatology—Ninety-three percent of participants planned on attending medical school, and most students developed an interest in medicine from an early age. Ninety-six percent cited that they became interested in medicine prior to beginning their undergraduate education, and 4% developed an interest as freshmen or sophomores. When asked what led to their interest in medicine, family influence had the single greatest impact on students’ decision to pursue medicine (33%). Classes/school were the second most influential factor (24%), followed by volunteering (15%), shadowing (13%), other (7%), and peer influence (3%)(Figure 1).
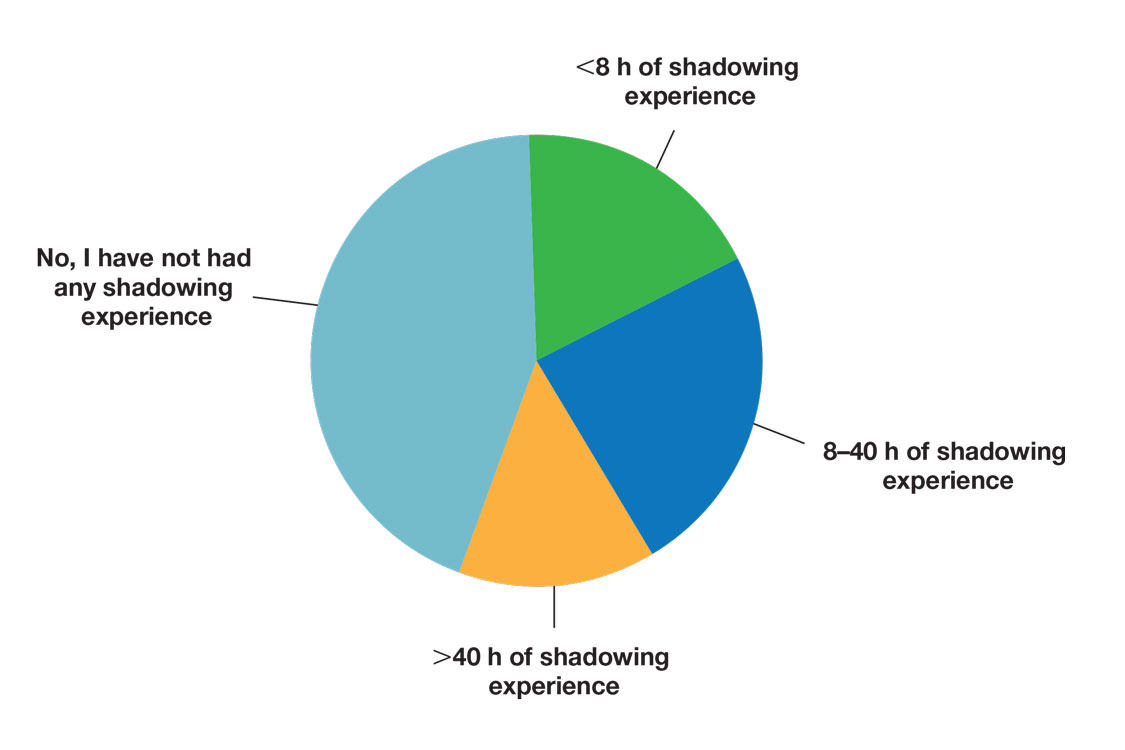
Many (56%) premedical students surveyed had shadowing experience to varying degrees. Approximately 18% had fewer than 8 hours of shadowing experience, 24% had 8 to 40 hours, and 14% had more than 40 hours. However, many (43%) premedical students had no shadowing experience (Figure 2). Similarly, 30% of premedical students responded to having a physician as a mentor.
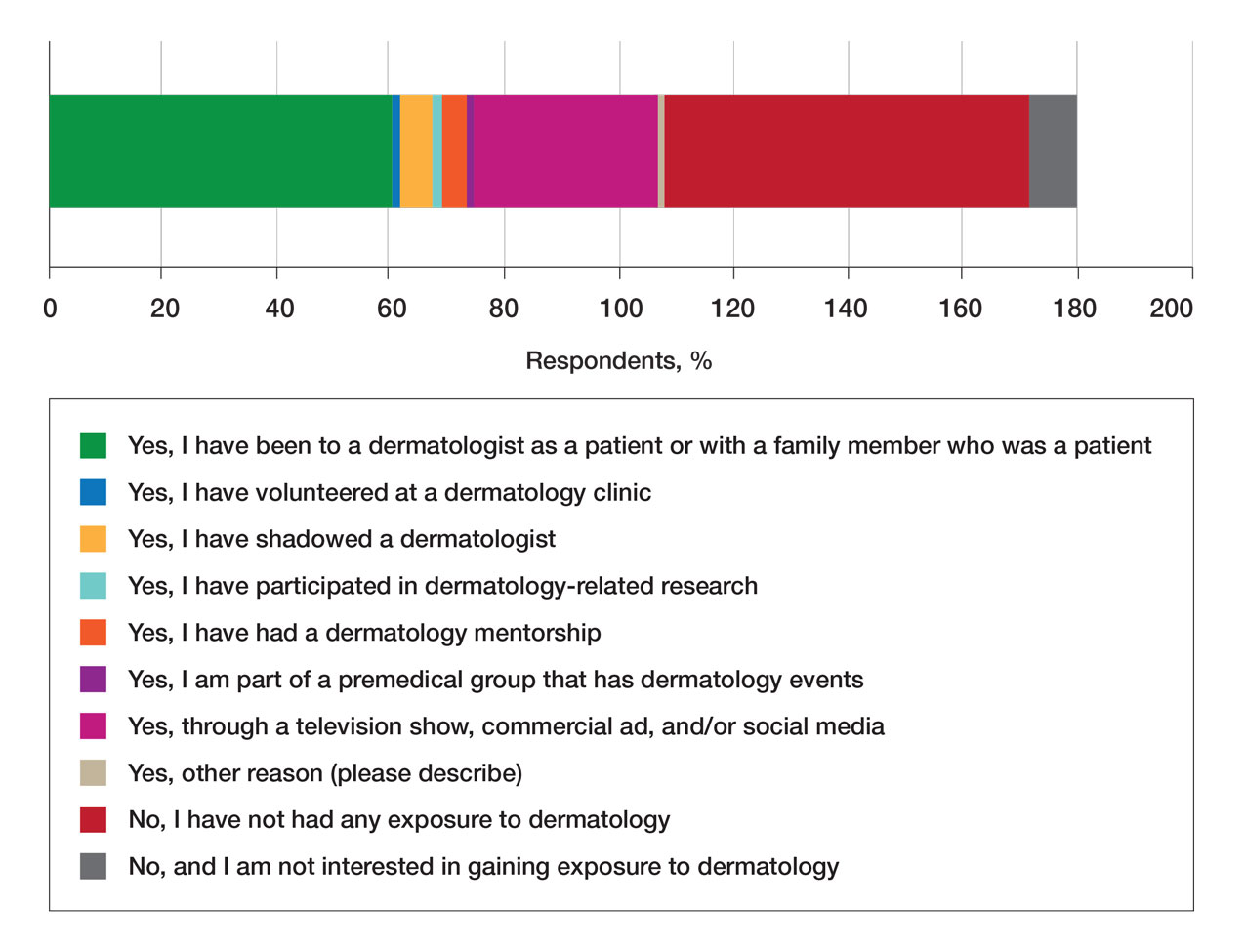
Regarding exposure to dermatology, 42% of premedical students had none. However, 58% of students had exposure to dermatology by being a patient themselves, 40% through seeing a dermatologist with a family member, 21% through seeing a dermatologist on television or social media, 5% through shadowing or volunteering, 3% through mentorship, and 1% through dermatology research (Figure 3).
Of students who said they were interested in dermatology (32%), 16% developed their interest before undergraduate education, while 9% developed interest in their freshman or sophomore year and 7% in their junior or senior year of undergraduate education. Three percent of respondents indicated that they had a dermatology mentorship.
Perceptions of Dermatology—To further evaluate the level of interest that UiM premedical students have in the field of dermatology, students were asked how much they agree or disagree on whether the field of dermatology is interesting. Sixty-three percent of the students agreed that the field of dermatology is interesting, 34% remained uncertain, and 3% disagreed. Additionally, students were asked whether they would consider dermatology as a career; 54% of respondents would consider dermatology as a career, 30% remained uncertain, and 16% would not consider dermatology as a career choice.
Nearly all (95%) students agreed that dermatologists do valuable work that goes beyond the scope of cosmetic procedures such as neuromodulators, fillers, chemical peels, and lasers. Some students also noted they had personal experiences interacting with a dermatologist. For example, one student described visiting the dermatologist many times to get a treatment regimen for their eczema.
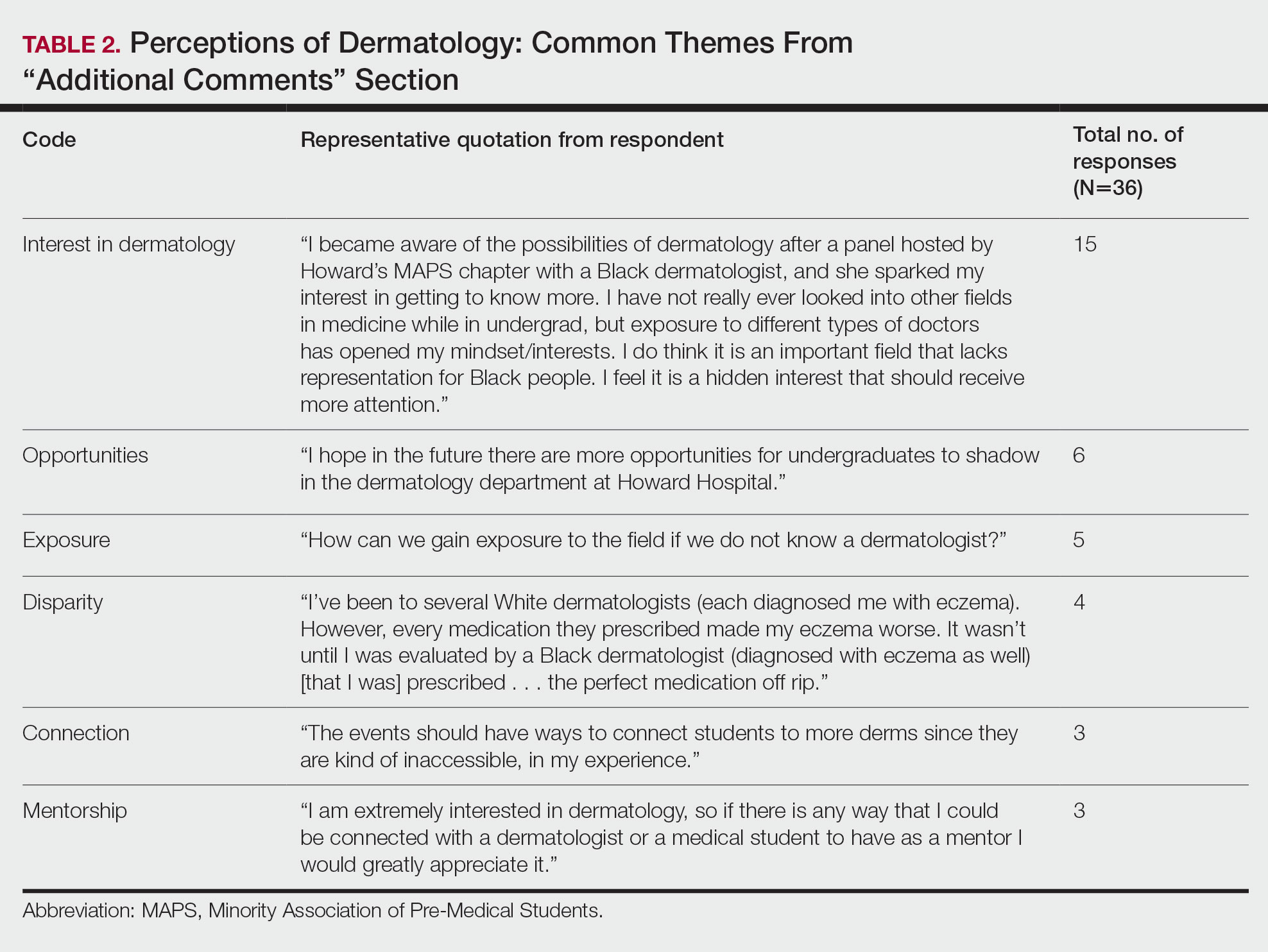
Overall themes from the survey are depicted in Table 2. Major themes found in the comments included the desire for more dermatology-related opportunities, mentorship, exposure, connections, and a discussion of disparities faced by Black patients and students within dermatology. Students also expressed an interest in dermatology and the desire to learn more about the specialty.
Comment
Interest in Dermatology—In this cross-sectional survey study of 152 UiM undergraduate students, it was found that many students were interested in dermatology as a career, and more than 70% would be interested in attending events that increased exposure to the field of dermatology. Of the students who had any exposure to dermatology, less than 5% had shadowed an actual dermatologist. The survey showed that there is great potential interest in exposing UiM undergraduate students to the field of dermatology. We found that UiM students are interested in learning more about dermatology, with 80% indicating that they would be willing to participate in dermatology-focused events if they were available. Overall, students mentioned a lack of opportunities, mentorship, exposure, and connections in dermatology despite their interest in the field.
Racial Disparities in Dermatology—Additionally, students discussed disparities they encountered with dermatology due to a lack of patient-provider race concordance and the perceived difference in care when encountering a race-concordant dermatologist. One student noted that they went to multiple White dermatologists for their eczema, and “it wasn’t until I was evaluated by a Black dermatologist (diagnosed with eczema as well) [that I was] prescribed . . . the perfect medication.” Another student noted how a Black dermatologist sparked their interest in getting to know more about the field and remarked that they “think it is an important field that lacks representation for Black people.” This research stresses the need for more dermatology mentorship among UiM undergraduates.
Family Influence on Career Selection—The majority of UiM students in our study became interested in medicine because of family, which is consistent with other studies. In a cross-sectional survey of 300 Pakistani students (150 medical and 150 nonmedical), 87% of students stated that their family had an influence on their career selection.8 In another study of 15 junior doctors in Sierra Leone, the most common reasons for pursuing medicine were the desire to help and familial and peer influence.9 This again showcases how family can have a positive impact on career selection for medical professionals and highlights the need for early intervention.
Shadowing—One way in which student exposure to dermatology can be effectively increased is by shadowing. In a study evaluating a 30-week shadowing program at the Pediatric Continuity Clinic in Los Angeles, California, a greater proportion of premedical students believed they had a good understanding of the job of a resident physician after the program’s completion compared to before starting the program (an increase from 78% to 100%).10 The proportion of students reporting a good understanding of the patient-physician relationship after completing the program also increased from 33% to 78%. Furthermore, 72% of the residents stated that having the undergraduates in the clinic was a positive experience.10 Thus, increasing shadowing opportunities is one extremely effective way to increase student knowledge and awareness of and exposure to dermatology.
Dermatology Mentors—Although 32% of students were interested in dermatology, 3% of students had mentorship in dermatology. In prior studies, it has been shown that mentorship is of great importance in student success and interest in pursuing a specialty. A report from the Association of American Medical Colleges 2019 Medical School Graduation Questionnaire found that the third most influential factor (52.1%) in specialty selection was role model influence.11 In fact, having a role model is consistently one of the top 3 influences on student specialty choice and interest in the last 5 years of survey research. Some studies also have shown mentorship as a positive influence in specialty interest at the undergraduate and graduate levels. A study on an undergraduate student interest group noted that surgeon mentorship and exposure were positive factors to students’ interests in surgery.12 In fact, the Association of American Medical Colleges noted that some surgical specialties, such as orthopedic surgery, had 45% of respondents who were interested in the specialty before medical school pursue their initial preference in medical school.13 Another survey corroborated these findings; more orthopedic-bound students compared with other specialties indicated they were more likely to pursue their field because of experiences prior to medical school.14
One of the reasons students might not have been exposed to as many opportunities for mentorship in dermatology is because the specialty is one of the smaller fields in medicine and tends to be concentrated in more well-resourced metropolitan areas.15 Dermatologists make up only 1.3% of the physician workforce.16 Because there might not be as much exposure to the field, students might also explore their interests in dermatology through other fields, such as through shadowing and observing primary care physicians who often treat patients with dermatologic issues. Skin diseases are a common reason for primary care visits, and one study suggested dermatologic diseases can make up approximately 8.4% of visits in primary care.17
Moreover, only 1% of medical schools require an elective in dermatology.18 With exposure being a crucial component to pursuing the specialty, it also is important to pursue formal mentorship within the specialty itself. One study noted that formal mentorship in dermatology was important for most (67%) respondents when considering the specialty; however, 39% of respondents mentioned receiving mentorship in the past. In fact, dermatology was one of the top 3 specialties for which respondents agreed that formal mentorship was important.19
Mentorship also has been shown to provide students with a variety of opportunities to develop personally and professionally. Some of these opportunities include increased confidence in their personal and professional success, increased desire to pursue a career in a field of interest, networking opportunities, career coaching, and support and research guidance.20 A research study among medical students at Albert Einstein College of Medicine in New York, New York, found that US Medical Licensing Examination Step 1 scores, clinical grades, and the chance of not matching were important factors preventing them from applying to dermatology.21
Factors in Dermatology Residency Selection—A survey was conducted wherein 95 of 114 dermatology program directors expressed that among the top 5 criteria for dermatology resident selection were Step 1 scores and clinical grades, supporting the notion that academic factors were given a great emphasis during residency selection.22 Furthermore, among underrepresented minority medical students, a lack of diversity, the belief that minority students are seen negatively by residencies, socioeconomic factors, and not having mentors were major reasons for being dissuaded from applying to dermatology.21 These results showcase the heightened importance of mentors for underrepresented minority medical students in particular.
In graduate medical education, resources such as wikis, social networking sites, and blogs provide media through which trainees can communicate, exchange ideas, and enhance their medical knowledge.23,24 A survey of 9606 osteopathic medical students showed that 35% of 992 respondents had used social media to learn more about residencies, and 10% believed that social media had influenced their choice of residency.25 Given the impact social media has on recruitment, it also can be employed in a similar manner by dermatologists and dermatology residency programs to attract younger students to the field.
Access to More Opportunities to Learn About Dermatology—Besides shadowing and mentorship, other avenues of exposure to dermatology are possible and should be considered. In our study, 80% of students agreed that they would attend an event that increases exposure to dermatology if held by the premedical group, which suggests that students are eager to learn more about the field and want access to more opportunities, which could include learning procedures such as suturing or how to use a dermatoscope, attending guest speaker events, or participating in Learn2Derm volunteer events.
Learn2Derm was a skin cancer prevention fair first organized by medical students at George Washington University in Washington, DC. Students and residents sought to deliver sunscreens to underserved areas in Washington, DC, as well as teach residents about the importance of skin health. Participating in such events could be an excellent opportunity for all students to gain exposure to important topics in dermatology.26
General Opinions of Dermatology—General opinions about dermatology and medicine were collected from the students through the optional “Additional Comments” section. Major themes found in the comments included the desire for more opportunities, mentorship, exposure, connections, and a discussion of disparities faced by Black patients/students within dermatology. Students also expressed an interest in dermatology and the desire to learn more about the specialty. From these themes, it can be gleaned that students are open to and eager for more opportunities to gain exposure and connections, and increasing the number of minority dermatologists is of importance.
Limitations—An important limitation of this study was the potential for selection bias, as the sample was chosen from a population at one university, which is not representative of the general population. Further, we only sampled students who were premedical and likely from a UiM racial group due to the demographics of the student population at the university, but given that the goal of the survey was to understand exposure to dermatology in underrepresented groups, we believe it was the appropriate population to target. Additionally, results were not compared with other more represented racial groups to see if these findings were unique to UiM undergraduate students.
Conclusion
Among premedical students, dermatology is an area of great interest with minimal opportunities available for exposure and learning because it is a smaller specialty with fewer experiences available for shadowing and mentorship. Although most UiM premedical students who were surveyed were exposed to the field through either the media or being a dermatology patient, fewer were exposed to the field through clinical experiences (such as shadowing) or mentorship. Most respondents found dermatology to be interesting and have considered pursuing it as a career. In particular, race-concordant mentoring in dermatologic care was valued by many students in garnering their interest in the field.
Most UiM students wanted more exposure to dermatology-related opportunities as well as mentorship and connections. Increasing shadowing, research, pipeline programs, and general events geared to dermatology are some modalities that could help improve exposure to dermatology for UiM students, especially for those interested in pursuing the field. This increased exposure can help positively influence more UiM students to pursue dermatology and help close the diversity gap in the field. Additionally, many were interested in attending potential dermatology informational events.
Given the fact that dermatology is a small field and mentorship may be hard to access, increasing informational events may be a more reasonable approach to inspiring and supporting interest. These events could include learning how to use certain tools and techniques, guest speaker events, or participating in educational volunteer efforts such as Learn2Derm.26
Future research should focus on identifying beneficial factors of UiM premedical students who retain an interest in dermatology throughout their careers and actually apply to dermatology programs and become dermatologists. Those who do not apply to the specialty can be identified to understand potential dissuading factors and obstacles. Ultimately, more research and development of exposure opportunities, including mentorship programs and informational events, can be used to close the gap and improve diversity and health outcomes in dermatology.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Bae G, Qiu M, Reese E, et al. Changes in sex and ethnic diversity in dermatology residents over multiple decades. JAMA Dermatol. 2016;152:92-94.
- McCleskey PE, Gilson RT, DeVillez RL. Medical student core curriculum in dermatology survey. J Am Acad Dermatol. 2009;61:30-35.e4.
- Pritchett EN, Pandya AG, Ferguson NN, et al. Diversity in dermatology: roadmap for improvement. J Am Acad Dermatol. 2018;79:337-341.
- National Resident Matching Program. Results and Data: 2022 Main Residency Match. National Resident Matching Program; 2022. Accessed March 19, 2023. https://www.nrmp.org/wp-content/uploads/2022/11/2022-Main-Match-Results-and-Data-Final-Revised.pdf
- 6. Akhiyat S, Cardwell L, Sokumbi O. Why dermatology is the second least diverse specialty in medicine: how did we get here? Clin Dermatol. 2020;38:310-315.
- Perlman KL, Williams NM, Egbeto IA, et al. Skin of color lacks representation in medical student resources: a cross-sectional study. Int J Womens Dermatol. 2021;7:195-196.
- Saad SM, Fatima SS, Faruqi AA. Students’ views regarding selecting medicine as a profession. J Pak Med Assoc. 2011;61:832-836.
- Woodward A, Thomas S, Jalloh M, et al. Reasons to pursue a career in medicine: a qualitative study in Sierra Leone. Global Health Res Policy. 2017;2:34.
- Thang C, Barnette NM, Patel KS, et al. Association of shadowing program for undergraduate premedical students with improvements in understanding medical education and training. Cureus. 2019;11:E6396.
- Murphy B. The 11 factors that influence med student specialty choice. American Medical Association. December 1, 2020. Accessed March 14, 2023. https://www.ama-assn.org/residents-students/specialty-profiles/11-factors-influence-med-student-specialty-choice
- Vakayil V, Chandrashekar M, Hedberg J, et al. An undergraduate surgery interest group: introducing premedical students to the practice of surgery. Adv Med Educ Pract. 2020;13:339-349.
- 2021 Report on Residents Executive Summary. Association of American Medical Colleges; 2021. Accessed March 14, 2023. https://www.aamc.org/data-reports/students-residents/data/report-residents/2021/executive-summary
- Johnson AL, Sharma J, Chinchilli VM, et al. Why do medical students choose orthopaedics as a career? J Bone Joint Surg Am. 2012;94:e78.
- Feng H, Berk-Krauss J, Feng PW, et al. Comparison of dermatologist density between urban and rural counties in the United States. JAMA Dermatol. 2018;154:1265-1271.
- Active Physicians With a U.S. Doctor of Medicine (U.S. MD) Degree by Specialty, 2019. Association of American Medical Colleges; 2019. Accessed March 14, 2023. https://www.aamc.org/data-reports/workforce/interactive-data/active-physicians-us-doctor-medicine-us-md-degree-specialty-2019
- Rübsam ML, Esch M, Baum E, et al. Diagnosing skin disease in primary care: a qualitative study of GPs’ approaches. Fam Pract. 2015;32:591-595.
- Cahn BA, Harper HE, Halverstam CP, et al. Current status of dermatologic education in US medical schools. JAMA Dermatol. 2020;156:468-470.
- Mylona E, Brubaker L, Williams VN, et al. Does formal mentoring for faculty members matter? a survey of clinical faculty members. Med Educ. 2016;50:670-681.
- Ratnapalan S. Mentoring in medicine. Can Fam Physician. 2010;56:198.
- Soliman YS, Rzepecki AK, Guzman AK, et al. Understanding perceived barriers of minority medical students pursuing a career in dermatology. JAMA Dermatol. 2019;155:252-254.
- Gorouhi F, Alikhan A, Rezaei A, et al. Dermatology residency selection criteria with an emphasis on program characteristics: a national program director survey. Dermatol Res Pract. 2014;2014:692760.
- Choo EK, Ranney ML, Chan TM, et al. Twitter as a tool for communication and knowledge exchange in academic medicine: a guide for skeptics and novices. Med Teach. 2015;37:411-416.
- McGowan BS, Wasko M, Vartabedian BS, et al. Understanding the factors that influence the adoption and meaningful use of social media by physicians to share medical information. J Med Internet Res. 2012;14:e117.
- Schweitzer J, Hannan A, Coren J. The role of social networking web sites in influencing residency decisions. J Am Osteopath Assoc. 2012;112:673-679.
- Medical students lead event addressing disparity in skin cancer morbidity and mortality. Dermatology News. August 19, 2021. Accessed March 14, 2023. https://www.mdedge.com/dermatology/article/244488/diversity-medicine/medical-students-lead-event-addressing-disparity-skin
Diversity of health care professionals improves medical outcomes and quality of life in patients. 1 There is a lack of diversity in dermatology, with only 4.2% of dermatologists identifying as Hispanic and 3% identifying as African American, 2 possibly due to a lack of early exposure to dermatology among high school and undergraduate students, a low number of underrepresented students in medical school, a lack of formal mentorship programs geared to underrepresented students, and implicit biases. 1-4 Furthermore, the field is competitive, with many more applicants than available positions. In 2022, there were 851 applicants competing for 492 residency positions in dermatology. 5 Thus, it is important to educate young students about dermatology and understand root causes as to why the number of u nderrepresented in medicine (UiM) dermatologists remains stagnant.
According to Pritchett et al,4 it is crucial for dermatologists to interact with high school and college students to foster an early interest in dermatology. Many racial minority students do not progress from high school to college and then from college to medical school, which leaves a substantially reduced number of eligible UiM applicants who can progress into dermatology.6 Increasing the amount of UiM students going to medical school requires early mediation. Collaborating with pre-existing premedical school organizations through presentations and workshops is another way to promote an early interest in dermatology.4 Special consideration should be given to students who are UiM.
Among the general medical school curriculum, requirements for exposure to dermatology are not high. In one study, the median number of clinical and preclinical hours required was 10. Furthermore, 20% of 33 medical schools did not require preclinical dermatology hours (hours done before medical school rotations begin and in an academic setting), 36% required no clinical hours (rotational hours), 8% required no dermatology hours whatsoever, and only 10% required clinical dermatology rotation.3 Based on these findings, it is clear that dermatology is not well incorporated into medical school curricula. Furthermore, curricula have historically neglected to display adequate representation of skin of color.7 As a result, medical students generally have limited exposure to dermatology3 and are exposed even less to presentations of dermatologic issues in historically marginalized populations.7
Given the paucity of research on UiM students’ perceptions of dermatology prior to medical school, our cross-sectional survey study sought to evaluate the level of interest in dermatology of UiM premedical undergraduates. This survey specifically evaluated exposure to dermatology, preconceived notions about the field, and mentorship opportunities. By understanding these factors, dermatologists and dermatology residency programs can use this information to create mentorship opportunities and better adjust existing programs to meet students’ needs.
Methods
A 19-question multiple-choice survey was administered electronically (SurveyMonkey) in May 2020 to premedical students at Howard University (Washington, DC). One screening question was used: “What is your major?” Those who considered themselves a science major and/or with premedical interest were allowed to complete the survey. All students surveyed were members of the Health Professions Society at Howard University. Students who were interested in pursuing medical school were invited to respond. Approval for this study was obtained from the Howard University institutional review board (FWA00000891).
The survey was divided into 3 sections: Demographics, Exposure to Medicine and Dermatology, and Perceptions of Dermatology. The Demographics section addressed gender, age, and race/ethnicity. The Exposure to Medicine and Dermatology section addressed interest in attending medical school, shadowing experience, exposure to dermatology, and mentoring. The Perceptions of Dermatology section addressed preconceived notions about the field (eg, “dermatology is interesting and exciting”).
Statistical Analysis—The data represented are percentages based on the number of respondents who answered each question. Answers in response to “Please enter any comments” were organized into themes, and the number of respondents who discussed each theme was quantified into a table.
Results
A total of 271 survey invitations were sent to premedical students at Howard University. Students were informed of the study protocol and asked to consent before proceeding to have their responses anonymously collected. Based on the screening question, 152 participants qualified for the survey, and 152 participants completed it (response rate, 56%; completion rate, 100%). Participants were asked to complete the survey only once.
Demographics—Eighty-four percent of respondents identified as science majors, and the remaining 16% identified as nonscience premedical. Ninety-four percent of participants identified as Black or African American; 3% as Asian or Asian American; and the remaining 3% as Other. Most respondents were female (82%), 16% were male, and 2% were either nonbinary or preferred not to answer. Ninety-nine percent were aged 18 to 24 years, and 1% were aged 25 to 34 years (Table 1).

Exposure to Medicine and Dermatology—Ninety-three percent of participants planned on attending medical school, and most students developed an interest in medicine from an early age. Ninety-six percent cited that they became interested in medicine prior to beginning their undergraduate education, and 4% developed an interest as freshmen or sophomores. When asked what led to their interest in medicine, family influence had the single greatest impact on students’ decision to pursue medicine (33%). Classes/school were the second most influential factor (24%), followed by volunteering (15%), shadowing (13%), other (7%), and peer influence (3%)(Figure 1).

Many (56%) premedical students surveyed had shadowing experience to varying degrees. Approximately 18% had fewer than 8 hours of shadowing experience, 24% had 8 to 40 hours, and 14% had more than 40 hours. However, many (43%) premedical students had no shadowing experience (Figure 2). Similarly, 30% of premedical students responded to having a physician as a mentor.

Regarding exposure to dermatology, 42% of premedical students had none. However, 58% of students had exposure to dermatology by being a patient themselves, 40% through seeing a dermatologist with a family member, 21% through seeing a dermatologist on television or social media, 5% through shadowing or volunteering, 3% through mentorship, and 1% through dermatology research (Figure 3).

Of students who said they were interested in dermatology (32%), 16% developed their interest before undergraduate education, while 9% developed interest in their freshman or sophomore year and 7% in their junior or senior year of undergraduate education. Three percent of respondents indicated that they had a dermatology mentorship.
Perceptions of Dermatology—To further evaluate the level of interest that UiM premedical students have in the field of dermatology, students were asked how much they agree or disagree on whether the field of dermatology is interesting. Sixty-three percent of the students agreed that the field of dermatology is interesting, 34% remained uncertain, and 3% disagreed. Additionally, students were asked whether they would consider dermatology as a career; 54% of respondents would consider dermatology as a career, 30% remained uncertain, and 16% would not consider dermatology as a career choice.
Nearly all (95%) students agreed that dermatologists do valuable work that goes beyond the scope of cosmetic procedures such as neuromodulators, fillers, chemical peels, and lasers. Some students also noted they had personal experiences interacting with a dermatologist. For example, one student described visiting the dermatologist many times to get a treatment regimen for their eczema.
Overall themes from the survey are depicted in Table 2. Major themes found in the comments included the desire for more dermatology-related opportunities, mentorship, exposure, connections, and a discussion of disparities faced by Black patients and students within dermatology. Students also expressed an interest in dermatology and the desire to learn more about the specialty.

Comment
Interest in Dermatology—In this cross-sectional survey study of 152 UiM undergraduate students, it was found that many students were interested in dermatology as a career, and more than 70% would be interested in attending events that increased exposure to the field of dermatology. Of the students who had any exposure to dermatology, less than 5% had shadowed an actual dermatologist. The survey showed that there is great potential interest in exposing UiM undergraduate students to the field of dermatology. We found that UiM students are interested in learning more about dermatology, with 80% indicating that they would be willing to participate in dermatology-focused events if they were available. Overall, students mentioned a lack of opportunities, mentorship, exposure, and connections in dermatology despite their interest in the field.
Racial Disparities in Dermatology—Additionally, students discussed disparities they encountered with dermatology due to a lack of patient-provider race concordance and the perceived difference in care when encountering a race-concordant dermatologist. One student noted that they went to multiple White dermatologists for their eczema, and “it wasn’t until I was evaluated by a Black dermatologist (diagnosed with eczema as well) [that I was] prescribed . . . the perfect medication.” Another student noted how a Black dermatologist sparked their interest in getting to know more about the field and remarked that they “think it is an important field that lacks representation for Black people.” This research stresses the need for more dermatology mentorship among UiM undergraduates.
Family Influence on Career Selection—The majority of UiM students in our study became interested in medicine because of family, which is consistent with other studies. In a cross-sectional survey of 300 Pakistani students (150 medical and 150 nonmedical), 87% of students stated that their family had an influence on their career selection.8 In another study of 15 junior doctors in Sierra Leone, the most common reasons for pursuing medicine were the desire to help and familial and peer influence.9 This again showcases how family can have a positive impact on career selection for medical professionals and highlights the need for early intervention.
Shadowing—One way in which student exposure to dermatology can be effectively increased is by shadowing. In a study evaluating a 30-week shadowing program at the Pediatric Continuity Clinic in Los Angeles, California, a greater proportion of premedical students believed they had a good understanding of the job of a resident physician after the program’s completion compared to before starting the program (an increase from 78% to 100%).10 The proportion of students reporting a good understanding of the patient-physician relationship after completing the program also increased from 33% to 78%. Furthermore, 72% of the residents stated that having the undergraduates in the clinic was a positive experience.10 Thus, increasing shadowing opportunities is one extremely effective way to increase student knowledge and awareness of and exposure to dermatology.
Dermatology Mentors—Although 32% of students were interested in dermatology, 3% of students had mentorship in dermatology. In prior studies, it has been shown that mentorship is of great importance in student success and interest in pursuing a specialty. A report from the Association of American Medical Colleges 2019 Medical School Graduation Questionnaire found that the third most influential factor (52.1%) in specialty selection was role model influence.11 In fact, having a role model is consistently one of the top 3 influences on student specialty choice and interest in the last 5 years of survey research. Some studies also have shown mentorship as a positive influence in specialty interest at the undergraduate and graduate levels. A study on an undergraduate student interest group noted that surgeon mentorship and exposure were positive factors to students’ interests in surgery.12 In fact, the Association of American Medical Colleges noted that some surgical specialties, such as orthopedic surgery, had 45% of respondents who were interested in the specialty before medical school pursue their initial preference in medical school.13 Another survey corroborated these findings; more orthopedic-bound students compared with other specialties indicated they were more likely to pursue their field because of experiences prior to medical school.14
One of the reasons students might not have been exposed to as many opportunities for mentorship in dermatology is because the specialty is one of the smaller fields in medicine and tends to be concentrated in more well-resourced metropolitan areas.15 Dermatologists make up only 1.3% of the physician workforce.16 Because there might not be as much exposure to the field, students might also explore their interests in dermatology through other fields, such as through shadowing and observing primary care physicians who often treat patients with dermatologic issues. Skin diseases are a common reason for primary care visits, and one study suggested dermatologic diseases can make up approximately 8.4% of visits in primary care.17
Moreover, only 1% of medical schools require an elective in dermatology.18 With exposure being a crucial component to pursuing the specialty, it also is important to pursue formal mentorship within the specialty itself. One study noted that formal mentorship in dermatology was important for most (67%) respondents when considering the specialty; however, 39% of respondents mentioned receiving mentorship in the past. In fact, dermatology was one of the top 3 specialties for which respondents agreed that formal mentorship was important.19
Mentorship also has been shown to provide students with a variety of opportunities to develop personally and professionally. Some of these opportunities include increased confidence in their personal and professional success, increased desire to pursue a career in a field of interest, networking opportunities, career coaching, and support and research guidance.20 A research study among medical students at Albert Einstein College of Medicine in New York, New York, found that US Medical Licensing Examination Step 1 scores, clinical grades, and the chance of not matching were important factors preventing them from applying to dermatology.21
Factors in Dermatology Residency Selection—A survey was conducted wherein 95 of 114 dermatology program directors expressed that among the top 5 criteria for dermatology resident selection were Step 1 scores and clinical grades, supporting the notion that academic factors were given a great emphasis during residency selection.22 Furthermore, among underrepresented minority medical students, a lack of diversity, the belief that minority students are seen negatively by residencies, socioeconomic factors, and not having mentors were major reasons for being dissuaded from applying to dermatology.21 These results showcase the heightened importance of mentors for underrepresented minority medical students in particular.
In graduate medical education, resources such as wikis, social networking sites, and blogs provide media through which trainees can communicate, exchange ideas, and enhance their medical knowledge.23,24 A survey of 9606 osteopathic medical students showed that 35% of 992 respondents had used social media to learn more about residencies, and 10% believed that social media had influenced their choice of residency.25 Given the impact social media has on recruitment, it also can be employed in a similar manner by dermatologists and dermatology residency programs to attract younger students to the field.
Access to More Opportunities to Learn About Dermatology—Besides shadowing and mentorship, other avenues of exposure to dermatology are possible and should be considered. In our study, 80% of students agreed that they would attend an event that increases exposure to dermatology if held by the premedical group, which suggests that students are eager to learn more about the field and want access to more opportunities, which could include learning procedures such as suturing or how to use a dermatoscope, attending guest speaker events, or participating in Learn2Derm volunteer events.
Learn2Derm was a skin cancer prevention fair first organized by medical students at George Washington University in Washington, DC. Students and residents sought to deliver sunscreens to underserved areas in Washington, DC, as well as teach residents about the importance of skin health. Participating in such events could be an excellent opportunity for all students to gain exposure to important topics in dermatology.26
General Opinions of Dermatology—General opinions about dermatology and medicine were collected from the students through the optional “Additional Comments” section. Major themes found in the comments included the desire for more opportunities, mentorship, exposure, connections, and a discussion of disparities faced by Black patients/students within dermatology. Students also expressed an interest in dermatology and the desire to learn more about the specialty. From these themes, it can be gleaned that students are open to and eager for more opportunities to gain exposure and connections, and increasing the number of minority dermatologists is of importance.
Limitations—An important limitation of this study was the potential for selection bias, as the sample was chosen from a population at one university, which is not representative of the general population. Further, we only sampled students who were premedical and likely from a UiM racial group due to the demographics of the student population at the university, but given that the goal of the survey was to understand exposure to dermatology in underrepresented groups, we believe it was the appropriate population to target. Additionally, results were not compared with other more represented racial groups to see if these findings were unique to UiM undergraduate students.
Conclusion
Among premedical students, dermatology is an area of great interest with minimal opportunities available for exposure and learning because it is a smaller specialty with fewer experiences available for shadowing and mentorship. Although most UiM premedical students who were surveyed were exposed to the field through either the media or being a dermatology patient, fewer were exposed to the field through clinical experiences (such as shadowing) or mentorship. Most respondents found dermatology to be interesting and have considered pursuing it as a career. In particular, race-concordant mentoring in dermatologic care was valued by many students in garnering their interest in the field.
Most UiM students wanted more exposure to dermatology-related opportunities as well as mentorship and connections. Increasing shadowing, research, pipeline programs, and general events geared to dermatology are some modalities that could help improve exposure to dermatology for UiM students, especially for those interested in pursuing the field. This increased exposure can help positively influence more UiM students to pursue dermatology and help close the diversity gap in the field. Additionally, many were interested in attending potential dermatology informational events.
Given the fact that dermatology is a small field and mentorship may be hard to access, increasing informational events may be a more reasonable approach to inspiring and supporting interest. These events could include learning how to use certain tools and techniques, guest speaker events, or participating in educational volunteer efforts such as Learn2Derm.26
Future research should focus on identifying beneficial factors of UiM premedical students who retain an interest in dermatology throughout their careers and actually apply to dermatology programs and become dermatologists. Those who do not apply to the specialty can be identified to understand potential dissuading factors and obstacles. Ultimately, more research and development of exposure opportunities, including mentorship programs and informational events, can be used to close the gap and improve diversity and health outcomes in dermatology.
Diversity of health care professionals improves medical outcomes and quality of life in patients. 1 There is a lack of diversity in dermatology, with only 4.2% of dermatologists identifying as Hispanic and 3% identifying as African American, 2 possibly due to a lack of early exposure to dermatology among high school and undergraduate students, a low number of underrepresented students in medical school, a lack of formal mentorship programs geared to underrepresented students, and implicit biases. 1-4 Furthermore, the field is competitive, with many more applicants than available positions. In 2022, there were 851 applicants competing for 492 residency positions in dermatology. 5 Thus, it is important to educate young students about dermatology and understand root causes as to why the number of u nderrepresented in medicine (UiM) dermatologists remains stagnant.
According to Pritchett et al,4 it is crucial for dermatologists to interact with high school and college students to foster an early interest in dermatology. Many racial minority students do not progress from high school to college and then from college to medical school, which leaves a substantially reduced number of eligible UiM applicants who can progress into dermatology.6 Increasing the amount of UiM students going to medical school requires early mediation. Collaborating with pre-existing premedical school organizations through presentations and workshops is another way to promote an early interest in dermatology.4 Special consideration should be given to students who are UiM.
Among the general medical school curriculum, requirements for exposure to dermatology are not high. In one study, the median number of clinical and preclinical hours required was 10. Furthermore, 20% of 33 medical schools did not require preclinical dermatology hours (hours done before medical school rotations begin and in an academic setting), 36% required no clinical hours (rotational hours), 8% required no dermatology hours whatsoever, and only 10% required clinical dermatology rotation.3 Based on these findings, it is clear that dermatology is not well incorporated into medical school curricula. Furthermore, curricula have historically neglected to display adequate representation of skin of color.7 As a result, medical students generally have limited exposure to dermatology3 and are exposed even less to presentations of dermatologic issues in historically marginalized populations.7
Given the paucity of research on UiM students’ perceptions of dermatology prior to medical school, our cross-sectional survey study sought to evaluate the level of interest in dermatology of UiM premedical undergraduates. This survey specifically evaluated exposure to dermatology, preconceived notions about the field, and mentorship opportunities. By understanding these factors, dermatologists and dermatology residency programs can use this information to create mentorship opportunities and better adjust existing programs to meet students’ needs.
Methods
A 19-question multiple-choice survey was administered electronically (SurveyMonkey) in May 2020 to premedical students at Howard University (Washington, DC). One screening question was used: “What is your major?” Those who considered themselves a science major and/or with premedical interest were allowed to complete the survey. All students surveyed were members of the Health Professions Society at Howard University. Students who were interested in pursuing medical school were invited to respond. Approval for this study was obtained from the Howard University institutional review board (FWA00000891).
The survey was divided into 3 sections: Demographics, Exposure to Medicine and Dermatology, and Perceptions of Dermatology. The Demographics section addressed gender, age, and race/ethnicity. The Exposure to Medicine and Dermatology section addressed interest in attending medical school, shadowing experience, exposure to dermatology, and mentoring. The Perceptions of Dermatology section addressed preconceived notions about the field (eg, “dermatology is interesting and exciting”).
Statistical Analysis—The data represented are percentages based on the number of respondents who answered each question. Answers in response to “Please enter any comments” were organized into themes, and the number of respondents who discussed each theme was quantified into a table.
Results
A total of 271 survey invitations were sent to premedical students at Howard University. Students were informed of the study protocol and asked to consent before proceeding to have their responses anonymously collected. Based on the screening question, 152 participants qualified for the survey, and 152 participants completed it (response rate, 56%; completion rate, 100%). Participants were asked to complete the survey only once.
Demographics—Eighty-four percent of respondents identified as science majors, and the remaining 16% identified as nonscience premedical. Ninety-four percent of participants identified as Black or African American; 3% as Asian or Asian American; and the remaining 3% as Other. Most respondents were female (82%), 16% were male, and 2% were either nonbinary or preferred not to answer. Ninety-nine percent were aged 18 to 24 years, and 1% were aged 25 to 34 years (Table 1).

Exposure to Medicine and Dermatology—Ninety-three percent of participants planned on attending medical school, and most students developed an interest in medicine from an early age. Ninety-six percent cited that they became interested in medicine prior to beginning their undergraduate education, and 4% developed an interest as freshmen or sophomores. When asked what led to their interest in medicine, family influence had the single greatest impact on students’ decision to pursue medicine (33%). Classes/school were the second most influential factor (24%), followed by volunteering (15%), shadowing (13%), other (7%), and peer influence (3%)(Figure 1).

Many (56%) premedical students surveyed had shadowing experience to varying degrees. Approximately 18% had fewer than 8 hours of shadowing experience, 24% had 8 to 40 hours, and 14% had more than 40 hours. However, many (43%) premedical students had no shadowing experience (Figure 2). Similarly, 30% of premedical students responded to having a physician as a mentor.

Regarding exposure to dermatology, 42% of premedical students had none. However, 58% of students had exposure to dermatology by being a patient themselves, 40% through seeing a dermatologist with a family member, 21% through seeing a dermatologist on television or social media, 5% through shadowing or volunteering, 3% through mentorship, and 1% through dermatology research (Figure 3).

Of students who said they were interested in dermatology (32%), 16% developed their interest before undergraduate education, while 9% developed interest in their freshman or sophomore year and 7% in their junior or senior year of undergraduate education. Three percent of respondents indicated that they had a dermatology mentorship.
Perceptions of Dermatology—To further evaluate the level of interest that UiM premedical students have in the field of dermatology, students were asked how much they agree or disagree on whether the field of dermatology is interesting. Sixty-three percent of the students agreed that the field of dermatology is interesting, 34% remained uncertain, and 3% disagreed. Additionally, students were asked whether they would consider dermatology as a career; 54% of respondents would consider dermatology as a career, 30% remained uncertain, and 16% would not consider dermatology as a career choice.
Nearly all (95%) students agreed that dermatologists do valuable work that goes beyond the scope of cosmetic procedures such as neuromodulators, fillers, chemical peels, and lasers. Some students also noted they had personal experiences interacting with a dermatologist. For example, one student described visiting the dermatologist many times to get a treatment regimen for their eczema.
Overall themes from the survey are depicted in Table 2. Major themes found in the comments included the desire for more dermatology-related opportunities, mentorship, exposure, connections, and a discussion of disparities faced by Black patients and students within dermatology. Students also expressed an interest in dermatology and the desire to learn more about the specialty.

Comment
Interest in Dermatology—In this cross-sectional survey study of 152 UiM undergraduate students, it was found that many students were interested in dermatology as a career, and more than 70% would be interested in attending events that increased exposure to the field of dermatology. Of the students who had any exposure to dermatology, less than 5% had shadowed an actual dermatologist. The survey showed that there is great potential interest in exposing UiM undergraduate students to the field of dermatology. We found that UiM students are interested in learning more about dermatology, with 80% indicating that they would be willing to participate in dermatology-focused events if they were available. Overall, students mentioned a lack of opportunities, mentorship, exposure, and connections in dermatology despite their interest in the field.
Racial Disparities in Dermatology—Additionally, students discussed disparities they encountered with dermatology due to a lack of patient-provider race concordance and the perceived difference in care when encountering a race-concordant dermatologist. One student noted that they went to multiple White dermatologists for their eczema, and “it wasn’t until I was evaluated by a Black dermatologist (diagnosed with eczema as well) [that I was] prescribed . . . the perfect medication.” Another student noted how a Black dermatologist sparked their interest in getting to know more about the field and remarked that they “think it is an important field that lacks representation for Black people.” This research stresses the need for more dermatology mentorship among UiM undergraduates.
Family Influence on Career Selection—The majority of UiM students in our study became interested in medicine because of family, which is consistent with other studies. In a cross-sectional survey of 300 Pakistani students (150 medical and 150 nonmedical), 87% of students stated that their family had an influence on their career selection.8 In another study of 15 junior doctors in Sierra Leone, the most common reasons for pursuing medicine were the desire to help and familial and peer influence.9 This again showcases how family can have a positive impact on career selection for medical professionals and highlights the need for early intervention.
Shadowing—One way in which student exposure to dermatology can be effectively increased is by shadowing. In a study evaluating a 30-week shadowing program at the Pediatric Continuity Clinic in Los Angeles, California, a greater proportion of premedical students believed they had a good understanding of the job of a resident physician after the program’s completion compared to before starting the program (an increase from 78% to 100%).10 The proportion of students reporting a good understanding of the patient-physician relationship after completing the program also increased from 33% to 78%. Furthermore, 72% of the residents stated that having the undergraduates in the clinic was a positive experience.10 Thus, increasing shadowing opportunities is one extremely effective way to increase student knowledge and awareness of and exposure to dermatology.
Dermatology Mentors—Although 32% of students were interested in dermatology, 3% of students had mentorship in dermatology. In prior studies, it has been shown that mentorship is of great importance in student success and interest in pursuing a specialty. A report from the Association of American Medical Colleges 2019 Medical School Graduation Questionnaire found that the third most influential factor (52.1%) in specialty selection was role model influence.11 In fact, having a role model is consistently one of the top 3 influences on student specialty choice and interest in the last 5 years of survey research. Some studies also have shown mentorship as a positive influence in specialty interest at the undergraduate and graduate levels. A study on an undergraduate student interest group noted that surgeon mentorship and exposure were positive factors to students’ interests in surgery.12 In fact, the Association of American Medical Colleges noted that some surgical specialties, such as orthopedic surgery, had 45% of respondents who were interested in the specialty before medical school pursue their initial preference in medical school.13 Another survey corroborated these findings; more orthopedic-bound students compared with other specialties indicated they were more likely to pursue their field because of experiences prior to medical school.14
One of the reasons students might not have been exposed to as many opportunities for mentorship in dermatology is because the specialty is one of the smaller fields in medicine and tends to be concentrated in more well-resourced metropolitan areas.15 Dermatologists make up only 1.3% of the physician workforce.16 Because there might not be as much exposure to the field, students might also explore their interests in dermatology through other fields, such as through shadowing and observing primary care physicians who often treat patients with dermatologic issues. Skin diseases are a common reason for primary care visits, and one study suggested dermatologic diseases can make up approximately 8.4% of visits in primary care.17
Moreover, only 1% of medical schools require an elective in dermatology.18 With exposure being a crucial component to pursuing the specialty, it also is important to pursue formal mentorship within the specialty itself. One study noted that formal mentorship in dermatology was important for most (67%) respondents when considering the specialty; however, 39% of respondents mentioned receiving mentorship in the past. In fact, dermatology was one of the top 3 specialties for which respondents agreed that formal mentorship was important.19
Mentorship also has been shown to provide students with a variety of opportunities to develop personally and professionally. Some of these opportunities include increased confidence in their personal and professional success, increased desire to pursue a career in a field of interest, networking opportunities, career coaching, and support and research guidance.20 A research study among medical students at Albert Einstein College of Medicine in New York, New York, found that US Medical Licensing Examination Step 1 scores, clinical grades, and the chance of not matching were important factors preventing them from applying to dermatology.21
Factors in Dermatology Residency Selection—A survey was conducted wherein 95 of 114 dermatology program directors expressed that among the top 5 criteria for dermatology resident selection were Step 1 scores and clinical grades, supporting the notion that academic factors were given a great emphasis during residency selection.22 Furthermore, among underrepresented minority medical students, a lack of diversity, the belief that minority students are seen negatively by residencies, socioeconomic factors, and not having mentors were major reasons for being dissuaded from applying to dermatology.21 These results showcase the heightened importance of mentors for underrepresented minority medical students in particular.
In graduate medical education, resources such as wikis, social networking sites, and blogs provide media through which trainees can communicate, exchange ideas, and enhance their medical knowledge.23,24 A survey of 9606 osteopathic medical students showed that 35% of 992 respondents had used social media to learn more about residencies, and 10% believed that social media had influenced their choice of residency.25 Given the impact social media has on recruitment, it also can be employed in a similar manner by dermatologists and dermatology residency programs to attract younger students to the field.
Access to More Opportunities to Learn About Dermatology—Besides shadowing and mentorship, other avenues of exposure to dermatology are possible and should be considered. In our study, 80% of students agreed that they would attend an event that increases exposure to dermatology if held by the premedical group, which suggests that students are eager to learn more about the field and want access to more opportunities, which could include learning procedures such as suturing or how to use a dermatoscope, attending guest speaker events, or participating in Learn2Derm volunteer events.
Learn2Derm was a skin cancer prevention fair first organized by medical students at George Washington University in Washington, DC. Students and residents sought to deliver sunscreens to underserved areas in Washington, DC, as well as teach residents about the importance of skin health. Participating in such events could be an excellent opportunity for all students to gain exposure to important topics in dermatology.26
General Opinions of Dermatology—General opinions about dermatology and medicine were collected from the students through the optional “Additional Comments” section. Major themes found in the comments included the desire for more opportunities, mentorship, exposure, connections, and a discussion of disparities faced by Black patients/students within dermatology. Students also expressed an interest in dermatology and the desire to learn more about the specialty. From these themes, it can be gleaned that students are open to and eager for more opportunities to gain exposure and connections, and increasing the number of minority dermatologists is of importance.
Limitations—An important limitation of this study was the potential for selection bias, as the sample was chosen from a population at one university, which is not representative of the general population. Further, we only sampled students who were premedical and likely from a UiM racial group due to the demographics of the student population at the university, but given that the goal of the survey was to understand exposure to dermatology in underrepresented groups, we believe it was the appropriate population to target. Additionally, results were not compared with other more represented racial groups to see if these findings were unique to UiM undergraduate students.
Conclusion
Among premedical students, dermatology is an area of great interest with minimal opportunities available for exposure and learning because it is a smaller specialty with fewer experiences available for shadowing and mentorship. Although most UiM premedical students who were surveyed were exposed to the field through either the media or being a dermatology patient, fewer were exposed to the field through clinical experiences (such as shadowing) or mentorship. Most respondents found dermatology to be interesting and have considered pursuing it as a career. In particular, race-concordant mentoring in dermatologic care was valued by many students in garnering their interest in the field.
Most UiM students wanted more exposure to dermatology-related opportunities as well as mentorship and connections. Increasing shadowing, research, pipeline programs, and general events geared to dermatology are some modalities that could help improve exposure to dermatology for UiM students, especially for those interested in pursuing the field. This increased exposure can help positively influence more UiM students to pursue dermatology and help close the diversity gap in the field. Additionally, many were interested in attending potential dermatology informational events.
Given the fact that dermatology is a small field and mentorship may be hard to access, increasing informational events may be a more reasonable approach to inspiring and supporting interest. These events could include learning how to use certain tools and techniques, guest speaker events, or participating in educational volunteer efforts such as Learn2Derm.26
Future research should focus on identifying beneficial factors of UiM premedical students who retain an interest in dermatology throughout their careers and actually apply to dermatology programs and become dermatologists. Those who do not apply to the specialty can be identified to understand potential dissuading factors and obstacles. Ultimately, more research and development of exposure opportunities, including mentorship programs and informational events, can be used to close the gap and improve diversity and health outcomes in dermatology.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Bae G, Qiu M, Reese E, et al. Changes in sex and ethnic diversity in dermatology residents over multiple decades. JAMA Dermatol. 2016;152:92-94.
- McCleskey PE, Gilson RT, DeVillez RL. Medical student core curriculum in dermatology survey. J Am Acad Dermatol. 2009;61:30-35.e4.
- Pritchett EN, Pandya AG, Ferguson NN, et al. Diversity in dermatology: roadmap for improvement. J Am Acad Dermatol. 2018;79:337-341.
- National Resident Matching Program. Results and Data: 2022 Main Residency Match. National Resident Matching Program; 2022. Accessed March 19, 2023. https://www.nrmp.org/wp-content/uploads/2022/11/2022-Main-Match-Results-and-Data-Final-Revised.pdf
- 6. Akhiyat S, Cardwell L, Sokumbi O. Why dermatology is the second least diverse specialty in medicine: how did we get here? Clin Dermatol. 2020;38:310-315.
- Perlman KL, Williams NM, Egbeto IA, et al. Skin of color lacks representation in medical student resources: a cross-sectional study. Int J Womens Dermatol. 2021;7:195-196.
- Saad SM, Fatima SS, Faruqi AA. Students’ views regarding selecting medicine as a profession. J Pak Med Assoc. 2011;61:832-836.
- Woodward A, Thomas S, Jalloh M, et al. Reasons to pursue a career in medicine: a qualitative study in Sierra Leone. Global Health Res Policy. 2017;2:34.
- Thang C, Barnette NM, Patel KS, et al. Association of shadowing program for undergraduate premedical students with improvements in understanding medical education and training. Cureus. 2019;11:E6396.
- Murphy B. The 11 factors that influence med student specialty choice. American Medical Association. December 1, 2020. Accessed March 14, 2023. https://www.ama-assn.org/residents-students/specialty-profiles/11-factors-influence-med-student-specialty-choice
- Vakayil V, Chandrashekar M, Hedberg J, et al. An undergraduate surgery interest group: introducing premedical students to the practice of surgery. Adv Med Educ Pract. 2020;13:339-349.
- 2021 Report on Residents Executive Summary. Association of American Medical Colleges; 2021. Accessed March 14, 2023. https://www.aamc.org/data-reports/students-residents/data/report-residents/2021/executive-summary
- Johnson AL, Sharma J, Chinchilli VM, et al. Why do medical students choose orthopaedics as a career? J Bone Joint Surg Am. 2012;94:e78.
- Feng H, Berk-Krauss J, Feng PW, et al. Comparison of dermatologist density between urban and rural counties in the United States. JAMA Dermatol. 2018;154:1265-1271.
- Active Physicians With a U.S. Doctor of Medicine (U.S. MD) Degree by Specialty, 2019. Association of American Medical Colleges; 2019. Accessed March 14, 2023. https://www.aamc.org/data-reports/workforce/interactive-data/active-physicians-us-doctor-medicine-us-md-degree-specialty-2019
- Rübsam ML, Esch M, Baum E, et al. Diagnosing skin disease in primary care: a qualitative study of GPs’ approaches. Fam Pract. 2015;32:591-595.
- Cahn BA, Harper HE, Halverstam CP, et al. Current status of dermatologic education in US medical schools. JAMA Dermatol. 2020;156:468-470.
- Mylona E, Brubaker L, Williams VN, et al. Does formal mentoring for faculty members matter? a survey of clinical faculty members. Med Educ. 2016;50:670-681.
- Ratnapalan S. Mentoring in medicine. Can Fam Physician. 2010;56:198.
- Soliman YS, Rzepecki AK, Guzman AK, et al. Understanding perceived barriers of minority medical students pursuing a career in dermatology. JAMA Dermatol. 2019;155:252-254.
- Gorouhi F, Alikhan A, Rezaei A, et al. Dermatology residency selection criteria with an emphasis on program characteristics: a national program director survey. Dermatol Res Pract. 2014;2014:692760.
- Choo EK, Ranney ML, Chan TM, et al. Twitter as a tool for communication and knowledge exchange in academic medicine: a guide for skeptics and novices. Med Teach. 2015;37:411-416.
- McGowan BS, Wasko M, Vartabedian BS, et al. Understanding the factors that influence the adoption and meaningful use of social media by physicians to share medical information. J Med Internet Res. 2012;14:e117.
- Schweitzer J, Hannan A, Coren J. The role of social networking web sites in influencing residency decisions. J Am Osteopath Assoc. 2012;112:673-679.
- Medical students lead event addressing disparity in skin cancer morbidity and mortality. Dermatology News. August 19, 2021. Accessed March 14, 2023. https://www.mdedge.com/dermatology/article/244488/diversity-medicine/medical-students-lead-event-addressing-disparity-skin
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Bae G, Qiu M, Reese E, et al. Changes in sex and ethnic diversity in dermatology residents over multiple decades. JAMA Dermatol. 2016;152:92-94.
- McCleskey PE, Gilson RT, DeVillez RL. Medical student core curriculum in dermatology survey. J Am Acad Dermatol. 2009;61:30-35.e4.
- Pritchett EN, Pandya AG, Ferguson NN, et al. Diversity in dermatology: roadmap for improvement. J Am Acad Dermatol. 2018;79:337-341.
- National Resident Matching Program. Results and Data: 2022 Main Residency Match. National Resident Matching Program; 2022. Accessed March 19, 2023. https://www.nrmp.org/wp-content/uploads/2022/11/2022-Main-Match-Results-and-Data-Final-Revised.pdf
- 6. Akhiyat S, Cardwell L, Sokumbi O. Why dermatology is the second least diverse specialty in medicine: how did we get here? Clin Dermatol. 2020;38:310-315.
- Perlman KL, Williams NM, Egbeto IA, et al. Skin of color lacks representation in medical student resources: a cross-sectional study. Int J Womens Dermatol. 2021;7:195-196.
- Saad SM, Fatima SS, Faruqi AA. Students’ views regarding selecting medicine as a profession. J Pak Med Assoc. 2011;61:832-836.
- Woodward A, Thomas S, Jalloh M, et al. Reasons to pursue a career in medicine: a qualitative study in Sierra Leone. Global Health Res Policy. 2017;2:34.
- Thang C, Barnette NM, Patel KS, et al. Association of shadowing program for undergraduate premedical students with improvements in understanding medical education and training. Cureus. 2019;11:E6396.
- Murphy B. The 11 factors that influence med student specialty choice. American Medical Association. December 1, 2020. Accessed March 14, 2023. https://www.ama-assn.org/residents-students/specialty-profiles/11-factors-influence-med-student-specialty-choice
- Vakayil V, Chandrashekar M, Hedberg J, et al. An undergraduate surgery interest group: introducing premedical students to the practice of surgery. Adv Med Educ Pract. 2020;13:339-349.
- 2021 Report on Residents Executive Summary. Association of American Medical Colleges; 2021. Accessed March 14, 2023. https://www.aamc.org/data-reports/students-residents/data/report-residents/2021/executive-summary
- Johnson AL, Sharma J, Chinchilli VM, et al. Why do medical students choose orthopaedics as a career? J Bone Joint Surg Am. 2012;94:e78.
- Feng H, Berk-Krauss J, Feng PW, et al. Comparison of dermatologist density between urban and rural counties in the United States. JAMA Dermatol. 2018;154:1265-1271.
- Active Physicians With a U.S. Doctor of Medicine (U.S. MD) Degree by Specialty, 2019. Association of American Medical Colleges; 2019. Accessed March 14, 2023. https://www.aamc.org/data-reports/workforce/interactive-data/active-physicians-us-doctor-medicine-us-md-degree-specialty-2019
- Rübsam ML, Esch M, Baum E, et al. Diagnosing skin disease in primary care: a qualitative study of GPs’ approaches. Fam Pract. 2015;32:591-595.
- Cahn BA, Harper HE, Halverstam CP, et al. Current status of dermatologic education in US medical schools. JAMA Dermatol. 2020;156:468-470.
- Mylona E, Brubaker L, Williams VN, et al. Does formal mentoring for faculty members matter? a survey of clinical faculty members. Med Educ. 2016;50:670-681.
- Ratnapalan S. Mentoring in medicine. Can Fam Physician. 2010;56:198.
- Soliman YS, Rzepecki AK, Guzman AK, et al. Understanding perceived barriers of minority medical students pursuing a career in dermatology. JAMA Dermatol. 2019;155:252-254.
- Gorouhi F, Alikhan A, Rezaei A, et al. Dermatology residency selection criteria with an emphasis on program characteristics: a national program director survey. Dermatol Res Pract. 2014;2014:692760.
- Choo EK, Ranney ML, Chan TM, et al. Twitter as a tool for communication and knowledge exchange in academic medicine: a guide for skeptics and novices. Med Teach. 2015;37:411-416.
- McGowan BS, Wasko M, Vartabedian BS, et al. Understanding the factors that influence the adoption and meaningful use of social media by physicians to share medical information. J Med Internet Res. 2012;14:e117.
- Schweitzer J, Hannan A, Coren J. The role of social networking web sites in influencing residency decisions. J Am Osteopath Assoc. 2012;112:673-679.
- Medical students lead event addressing disparity in skin cancer morbidity and mortality. Dermatology News. August 19, 2021. Accessed March 14, 2023. https://www.mdedge.com/dermatology/article/244488/diversity-medicine/medical-students-lead-event-addressing-disparity-skin
Practice Points
- Many premedical students desire more exposure to dermatology than they have been receiving, particularly in mentorship and shadowing. Most exposure has been through social media or as patients in a dermatology clinic.
- Diverse mentorship and diversity of dermatology care are important to underrepresented in medicine premedical students and needs to be further incorporated.
Frontline STI Screening Starts with Primary Care
In this supplement to Family Practice, Heather M. Territo, MD, and Gale R. Burstein, MD, MPH discuss how primary care physicians play an essential role in screening for STIs in young patients.
In this supplement to Family Practice, Heather M. Territo, MD, and Gale R. Burstein, MD, MPH discuss how primary care physicians play an essential role in screening for STIs in young patients.
In this supplement to Family Practice, Heather M. Territo, MD, and Gale R. Burstein, MD, MPH discuss how primary care physicians play an essential role in screening for STIs in young patients.
Subcutaneous Panniculitic T-cell Lymphoma Presenting With Anasarca in a Patient With Known Chronic Lymphocytic Leukemia
To the Editor:
Subcutaneous panniculitic T-cell lymphoma (SPTCL) is a rare cutaneous T-cell lymphoma that was first described in 19911 and comprises less than 1% of all non-Hodgkin lymphomas (NHLs). It most commonly occurs in young adults, with a median patient age of 36 years and a slight female predominance.2 Patients typically present with skin nodules or deep-seated plaques involving the legs, arms, and/or trunk. Presentation on the face is less common.2,3 Paraneoplastic edema has been reported in several cases of SPTCL with facial and periorbital swelling.4-9
Diagnosis of SPTCL is achieved via analysis of a deep tissue skin biopsy and close clinicopathologic correlation. Histopathology demonstrates lobular panniculitis with an atypical lymphoid infiltrate in the subcutaneous tissue with predominantly CD8+ T cells without overlying epidermotropism or interface dermatitis.3 The degree of cellular atypia, fat necrosis, karyorrhexis, cytophagia, and lack of angioinvasion can help to distinguish SPTCL from other panniculitides.2,3
The prognosis of SPTCL is good, with a 5-year survival rate of 82%, and many patients are able to achieve remission.2 However, SPTCL can progress to a fatal hemophagocytic syndrome, which has been reported in 17% of cases, making early diagnosis and treatment of this malignancy imperative.1,2 Treatment varies depending on the progression and extent of disease and can include the use of steroids, multidrug chemotherapy regimens, radiotherapy, and stem cell transplant in refractory cases.2-4,10,11
Subcutaneous panniculitic T-cell lymphoma with edema has been reported in a 2-year-old child.12 We present a case of SPTCL in an adult patient with known stage IV chronic lymphocytic leukemia (CLL) who also had full-body edema.
A 60-year-old woman with a 7-year history of stage IV CLL presented with anasarca of 3 months’ duration. At the time of presentation to dermatology, physical examination revealed erythematous tender nodules on the arms and legs. She had no other medical conditions and was undergoing treatment with ibrutinib for the CLL. The patient reported profound fatigue but no fever, chills, night sweats, cough, or dyspnea. The swelling had begun initially in the legs and progressively worsened to involve the arms, face, and body. She was hospitalized and treated with intravenous steroids and antihistamines, which led to minor improvement in the swelling. The patient’s preliminary diagnosis of erythema nodosum was thought to be related to the CLL or ibrutinib; therefore, treatment subsequently was discontinued and she was discharged from the hospital.
The swelling continued to worsen over the following 3 months, and the patient gained approximately 25 pounds. She presented to our office again with severe periorbital, facial, and lip edema as well as diffuse edema of the torso, arms, and legs (Figure 1). Erythematous tender subcutaneous nodules were noted on the right proximal thigh, left lateral calf, and forearms. She was again hospitalized, and extensive evaluation was performed to exclude other causes of anasarca, including a complete blood cell count; comprehensive metabolic profile; hepatitis panels; HIV test; C3 and C4, complement CH50, C1 esterase inhibitor, IgE, and angiotensin-converting enzyme levels; urine protein to creatinine ratio; computed tomography of the chest, abdomen, and pelvis; and allergy evaluation. The analyses failed to reveal the cause of the anasarca.
During hospitalization, the patient underwent a lymph node biopsy, bone marrow biopsy, and a 6-mm punch biopsy of the right thigh nodule. The lymph node and bone marrow biopsy results were consistent with the known diagnosis of CLL, and the patient was started on intravenous chemotherapy with bendamustine. The skin biopsy demonstrated a predominant T-cell infiltrate consistent with a lobular panniculitis with variable amounts of adipocytes rimmed by lymphocytes, nuclear debris, and karyorrhexis (Figure 2). CD3+, CD8+, and CD4− T cells were positive for T-cell receptor (TCR) βF1 and negative for TCR-γ with strong expression of cytotoxic markers including granzyme B, perforin, and T-cell intracytoplasmic antigen 1. Rare CD56+ cells also were noted. The biopsy did not demonstrate any notable interface dermatitis, epidermotropism, or angioinvasion. T-cell receptor gene rearrangement studies did not show clonality for γ- or β-chain probes. Subcutaneous panniculitic T-cell lymphoma was diagnosed, making this case unique with the presentation of anasarca. This case also is noteworthy due to the rare diagnosis of the secondary malignancy of SPTCL in a patient with known CLL. The patient opted to pursue hospice and comfort measures due to the effects of persistent pancytopenia and the progression of CLL. She died 2 months later.
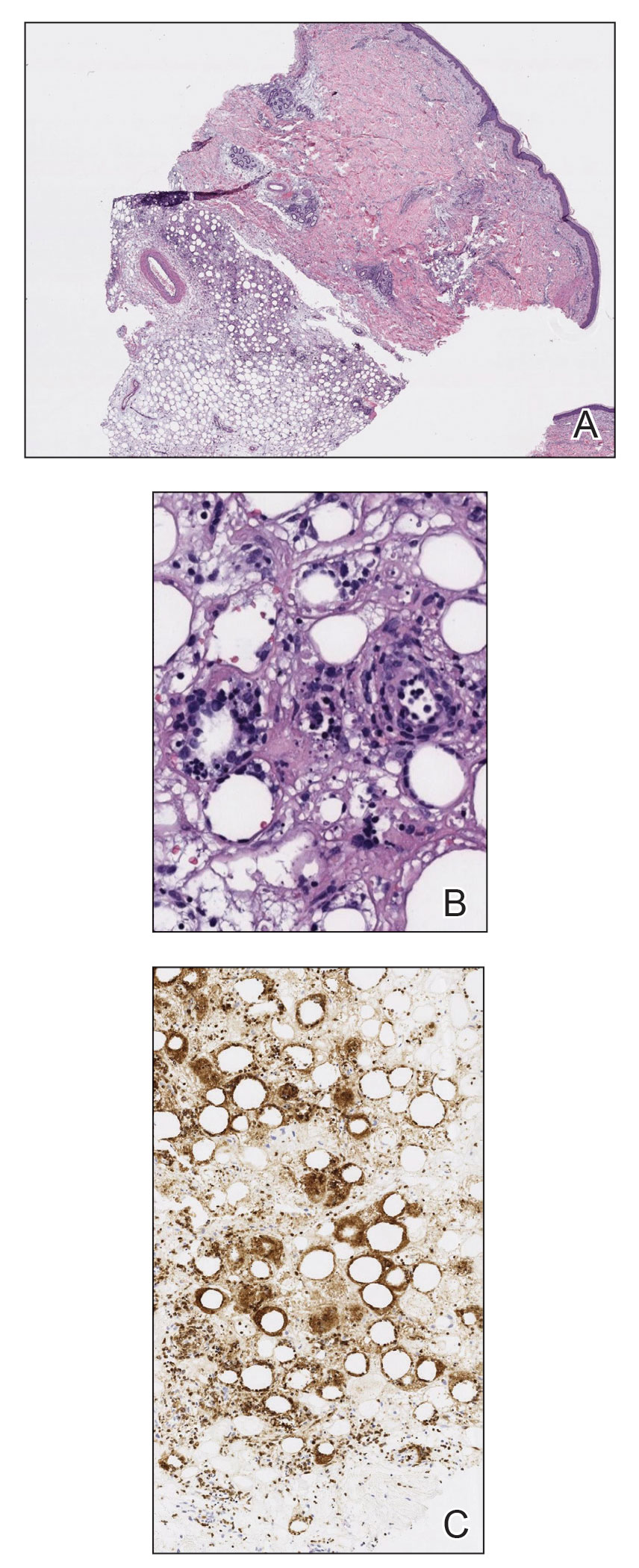
Clinical courses of SPTCL vary based on the TCR phenotype and immunophenotypic characteristics of the tumor cells. The TCR-αβ phenotype, as described in this case, typically is CD4−, CD8+, and CD56– and leads to a more indolent disease course. Lymphomas with the TCR-γδ phenotype typically are CD4−, CD8−, and CD56+; they often are associated with hemophagocytic syndrome and thus a worse prognosis. In 2009, the World Health Organization–European Organization for Research and Treatment of Cancer classification of primary cutaneous lymphomas restricted the category of SPTCL to the TCR-αβ phenotype due to the stark differences between the 2 types. The TCR-γδ phenotype was given its own diagnostic category—primary cutaneous γδ T-cell lymphoma.3
Patients with SPTCL commonly present with nodular skin lesions or deep-seated plaques on the legs, arms, and/or trunk; presentation on the face is rare.2,3 Fever, chills, night sweats, and/or weight loss were present in approximately 50% of recorded cases. Underlying autoimmune disease was present in 12 of 63 (19%) patients in a 2008 study.2 Facial and periorbital swelling with SPTCL has been reported.4-9 The presentation of anasarca, as seen in our adult patient, has been reported in a 2-year-old child.12 Anasarca as a presenting symptom of NHL is a rare phenomenon proposed to be induced by malignant cells secreting a cytokine that causes a vascular leak syndrome.13 Specifically, tumor necrosis factor α was found to be elevated in at least 2 patients with NHL presenting with anasarca in a prior study. Tumor necrosis factor α is known to cause increased capillary permeability, vascular leakage, and development of edema.13 In retrospect, obtaining cytokine levels in our patient would have been useful to support or refute tumor necrosis factor α as a possible cause of anasarca in the setting of NHL. This case continues to highlight that a diagnosis of SPTCL and analysis of a skin biopsy should be considered in cases of sudden unremitting facial and/or body swelling that cannot be explained by other more common causes.
Subcutaneous panniculitic T-cell lymphoma can be diagnosed and distinguished from other panniculitides via analysis of a deep tissue skin biopsy. Multiple biopsies may be required to ensure an adequate sample is obtained.4 Histopathology displays an atypical lymphoid infiltrate with a predominant presence of T cells. Neoplastic cells show CD3+, CD8+, and CD4− T cells, which strongly express cytotoxic proteins such as granzyme B, T-cell intracellular antigen 1, and perforin.3 The degree of cellular atypia, fat necrosis, karyorrhexis, and cytophagia, as well as the lack of angioinvasion, interface dermatitis, and epidermotropism help to distinguish SPTCL from other panniculitides.2,3 According to a previous study, clonal TCR gene rearrangement was identified in 50% to 80% of cases, but the absence of this clonal rearrangement does not exclude the diagnosis.14
This case also highlights the occurrence of secondary malignancies in patients with CLL, an NHL that is classified as a low-grade lymphoproliferative malignancy with clonal expansion of B cells.15 Secondary CTCLs in patients with CLL are rare, but they have been previously described. In 2017, Chang et al16 identified 12 patients with CLL who subsequently developed CTCL between 1992 and 2008. Of the 12 patients, 7 developed mycosis fungoides, 3 had CTCL not otherwise specified, 1 had mature T-cell lymphoma not otherwise specified, and 1 had primary cutaneous CD30+ T-cell lymphoma.16 The proliferation of 2 separate lymphocytic lineages is rare, but this study demonstrated an increased risk for CTCL to develop in patients with CLL. One possible explanation is that malignant cells come from a common stem cell progenitor or from genetic events. They occur secondary to carcinogens, viruses, or cytokines from T-cell or B-cell clones; they evolve due to treatment of the preexisting lymphoproliferative disease; or they occur simply by coincidence. The behavior of the CTCL may be more aggressive in patients with CLL due to immunosuppression, which may have contributed to the extreme presentation in our patient.16 Subcutaneous panniculitic T-cell lymphoma also has been reported in a patient with CLL that was thought to be associated with prior rituximab treatment.17
Treatment of SPTCL depends on the severity and course of the disease. In patients with more indolent disease, systemic steroids have been the most frequently used initial treatment.2,3,10 However, the disease often will progress after steroid tapering and require further intervention. Localized lesions may be treated with radiation alone or in combination with other systemic therapies.3,10 In refractory, aggressive, or relapsing cases, polychemotherapeutic regimens have proven to produce long-term remission in 30% of patients, with an overall response rate of 50%.10 These regimens most commonly have included cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) or CHOP-like treatment (EPOCH regimen [etoposide, prednisone, oncovin, cyclophosphamide, and doxorubicin hydrochloride]).3,10 A stem cell transplant can be considered in patients with recurrent and refractory disease, and it also has been shown to induce remission.4,17 In patients with a good response to therapy, the disease often can be controlled for long periods of time, with an estimated 5-year survival rate of 80%.15
This case highlights the diagnostic challenges and variable presentations of SPTCL. Dermatologists, oncologists, and dermatopathologists should be aware of this condition and consider it in the differential diagnosis of a patient with a hematologic malignancy and unremitting facial and/or body swelling without any other cause. The possibility of a secondary hematologic cancer in a patient with CLL also must be taken into consideration. Early diagnosis and treatment can minimize morbidity and induce remission in most patients.
- Gonzalez CL, Medeiros LJ, Braziel RM, et al. T-cell lymphoma involving subcutaneous tissue. a clinicopathologic entity commonly associated with hemophagocytic syndrome. Am J Surg Pathol. 1991;15:17-27.
- Willemze R, Jansen P, Cerroni L, et al.
Subcutaneous panniculitis-like T-cell lymphoma: definition, classification, and prognostic factors: an EORTC Cutaneous Lymphoma Group Study of 83 cases. Blood. 2008;111:38-45. - Parveen Z, Thompson K. Subcutaneous panniculitis-like T-cell lymphoma: redefinition of diagnostic criteria in the recent World Health Organization–European Organization for Research and Treatment of Cancer classification for cutaneous lymphomas. Arch Pathol Lab Med. 2009;133:303-308.
- Velez N, Ishizawar R, Dellaripa P, et al. Full facial edema: a novel presentation of subcutaneous panniculitis-like T-cell lymphoma. J Clin Oncol. 2012;30:e233-236.
- Asati D, Ingle V, Joshi D, et al. Subcutaneous panniculitis-like T-cell lymphoma with macrophage activation syndrome treated by cyclosporine and prednisolone. Indian Dermatol Online J. 2016;7:529-532.
- Fricker M, Dubach P, Helbing A, et al. Not all facial swellings are angioedemas! J Investig Allergol Clin Immunol. 2015;25:146-147.
- Kosari F, Akbarzadeh H. Local facial edema: a novel presentation of subcutaneous panniculitis-like T-cell lymphoma in a 30-year-old Iranian woman. Acta Med Iran. 2014;52:950-953.
- Bhojaraja M, Kistampally P, Udupa K, et al. Subcutaneous panniculitis-like T-cell lymphoma: a rare tumour. J Clin Diagn Res. 2016;10:OD29-OD30.
- Hashimoto R, Uchiyama M, Maeno T. Case report of subcutaneous panniculitis-like T-cell lymphoma complicated by eyelid swelling. BMC Ophthalmol. 2016;16:117.
- Chinello MN, Naviglio S, Remotti D, et al. Subcutaneous panniculitis-like T-cell lymphoma presenting with diffuse cutaneous edema in a 2-year-old child. J Pediatr Hematol Oncol. 2015;37:329-330.
- Chang TW, Weaver AL, Shanafelt TD, et al. Risk of cutaneous T-cell lymphoma in patients with chronic lymphocytic leukemia and other subtypes of non-Hodgkin lymphoma. Int J Dermatol. 2017;56:1125-1129.
- Chinello MN, Naviglio S, Remotti D, et al. Subcutaneous panniculitis-like T-cell lymphoma presenting with diffuse cutaneous edema in a 2-year-old child. J Pediatr Hematol Oncol. 2015;37:329-330.
- Jillella A, Day D, Severson K, et al. Non-Hodgkin’s lymphoma presenting as anasarca: probably mediated by tumor necrosis factor alpha (TNF-α). Leuk Lymphoma. 2000;38:419-422.
- Lee D-W, Yang J-H, Lee S-M, et al. Subcutaneous panniculitis-like T-cell lymphoma: a clinical and pathologic study of 14 Korean patients. Ann Dermatol. 2011;23:329-337.
- Jaffe ES. The 2008 WHO classification of lymphomas: implications for clinical practice and translational research [published online January 1, 2009]. Hematology Am Soc Hematol Educ Program. https://doi.org/10.1182/asheducation-2009.1.523
- Chang TW, Weaver AL, Shanafelt TD, et al. Risk of cutaneous T-cell lymphoma in patients with chronic lymphocytic leukemia and other subtypes of non-Hodgkin lymphoma. Int J Dermatol. 2017;56:1125-1129.
- Hall M, Sluzevich J, Snow J. Generalized subcutaneous panniculitis-like T-cell lymphoma following rituximab for hemolytic anemia in a patient with chronic lymphocytic leukemia. J Am Acad Dermatol. 2010;62(suppl 1):AB96.
To the Editor:
Subcutaneous panniculitic T-cell lymphoma (SPTCL) is a rare cutaneous T-cell lymphoma that was first described in 19911 and comprises less than 1% of all non-Hodgkin lymphomas (NHLs). It most commonly occurs in young adults, with a median patient age of 36 years and a slight female predominance.2 Patients typically present with skin nodules or deep-seated plaques involving the legs, arms, and/or trunk. Presentation on the face is less common.2,3 Paraneoplastic edema has been reported in several cases of SPTCL with facial and periorbital swelling.4-9
Diagnosis of SPTCL is achieved via analysis of a deep tissue skin biopsy and close clinicopathologic correlation. Histopathology demonstrates lobular panniculitis with an atypical lymphoid infiltrate in the subcutaneous tissue with predominantly CD8+ T cells without overlying epidermotropism or interface dermatitis.3 The degree of cellular atypia, fat necrosis, karyorrhexis, cytophagia, and lack of angioinvasion can help to distinguish SPTCL from other panniculitides.2,3
The prognosis of SPTCL is good, with a 5-year survival rate of 82%, and many patients are able to achieve remission.2 However, SPTCL can progress to a fatal hemophagocytic syndrome, which has been reported in 17% of cases, making early diagnosis and treatment of this malignancy imperative.1,2 Treatment varies depending on the progression and extent of disease and can include the use of steroids, multidrug chemotherapy regimens, radiotherapy, and stem cell transplant in refractory cases.2-4,10,11
Subcutaneous panniculitic T-cell lymphoma with edema has been reported in a 2-year-old child.12 We present a case of SPTCL in an adult patient with known stage IV chronic lymphocytic leukemia (CLL) who also had full-body edema.
A 60-year-old woman with a 7-year history of stage IV CLL presented with anasarca of 3 months’ duration. At the time of presentation to dermatology, physical examination revealed erythematous tender nodules on the arms and legs. She had no other medical conditions and was undergoing treatment with ibrutinib for the CLL. The patient reported profound fatigue but no fever, chills, night sweats, cough, or dyspnea. The swelling had begun initially in the legs and progressively worsened to involve the arms, face, and body. She was hospitalized and treated with intravenous steroids and antihistamines, which led to minor improvement in the swelling. The patient’s preliminary diagnosis of erythema nodosum was thought to be related to the CLL or ibrutinib; therefore, treatment subsequently was discontinued and she was discharged from the hospital.
The swelling continued to worsen over the following 3 months, and the patient gained approximately 25 pounds. She presented to our office again with severe periorbital, facial, and lip edema as well as diffuse edema of the torso, arms, and legs (Figure 1). Erythematous tender subcutaneous nodules were noted on the right proximal thigh, left lateral calf, and forearms. She was again hospitalized, and extensive evaluation was performed to exclude other causes of anasarca, including a complete blood cell count; comprehensive metabolic profile; hepatitis panels; HIV test; C3 and C4, complement CH50, C1 esterase inhibitor, IgE, and angiotensin-converting enzyme levels; urine protein to creatinine ratio; computed tomography of the chest, abdomen, and pelvis; and allergy evaluation. The analyses failed to reveal the cause of the anasarca.

During hospitalization, the patient underwent a lymph node biopsy, bone marrow biopsy, and a 6-mm punch biopsy of the right thigh nodule. The lymph node and bone marrow biopsy results were consistent with the known diagnosis of CLL, and the patient was started on intravenous chemotherapy with bendamustine. The skin biopsy demonstrated a predominant T-cell infiltrate consistent with a lobular panniculitis with variable amounts of adipocytes rimmed by lymphocytes, nuclear debris, and karyorrhexis (Figure 2). CD3+, CD8+, and CD4− T cells were positive for T-cell receptor (TCR) βF1 and negative for TCR-γ with strong expression of cytotoxic markers including granzyme B, perforin, and T-cell intracytoplasmic antigen 1. Rare CD56+ cells also were noted. The biopsy did not demonstrate any notable interface dermatitis, epidermotropism, or angioinvasion. T-cell receptor gene rearrangement studies did not show clonality for γ- or β-chain probes. Subcutaneous panniculitic T-cell lymphoma was diagnosed, making this case unique with the presentation of anasarca. This case also is noteworthy due to the rare diagnosis of the secondary malignancy of SPTCL in a patient with known CLL. The patient opted to pursue hospice and comfort measures due to the effects of persistent pancytopenia and the progression of CLL. She died 2 months later.

Clinical courses of SPTCL vary based on the TCR phenotype and immunophenotypic characteristics of the tumor cells. The TCR-αβ phenotype, as described in this case, typically is CD4−, CD8+, and CD56– and leads to a more indolent disease course. Lymphomas with the TCR-γδ phenotype typically are CD4−, CD8−, and CD56+; they often are associated with hemophagocytic syndrome and thus a worse prognosis. In 2009, the World Health Organization–European Organization for Research and Treatment of Cancer classification of primary cutaneous lymphomas restricted the category of SPTCL to the TCR-αβ phenotype due to the stark differences between the 2 types. The TCR-γδ phenotype was given its own diagnostic category—primary cutaneous γδ T-cell lymphoma.3
Patients with SPTCL commonly present with nodular skin lesions or deep-seated plaques on the legs, arms, and/or trunk; presentation on the face is rare.2,3 Fever, chills, night sweats, and/or weight loss were present in approximately 50% of recorded cases. Underlying autoimmune disease was present in 12 of 63 (19%) patients in a 2008 study.2 Facial and periorbital swelling with SPTCL has been reported.4-9 The presentation of anasarca, as seen in our adult patient, has been reported in a 2-year-old child.12 Anasarca as a presenting symptom of NHL is a rare phenomenon proposed to be induced by malignant cells secreting a cytokine that causes a vascular leak syndrome.13 Specifically, tumor necrosis factor α was found to be elevated in at least 2 patients with NHL presenting with anasarca in a prior study. Tumor necrosis factor α is known to cause increased capillary permeability, vascular leakage, and development of edema.13 In retrospect, obtaining cytokine levels in our patient would have been useful to support or refute tumor necrosis factor α as a possible cause of anasarca in the setting of NHL. This case continues to highlight that a diagnosis of SPTCL and analysis of a skin biopsy should be considered in cases of sudden unremitting facial and/or body swelling that cannot be explained by other more common causes.
Subcutaneous panniculitic T-cell lymphoma can be diagnosed and distinguished from other panniculitides via analysis of a deep tissue skin biopsy. Multiple biopsies may be required to ensure an adequate sample is obtained.4 Histopathology displays an atypical lymphoid infiltrate with a predominant presence of T cells. Neoplastic cells show CD3+, CD8+, and CD4− T cells, which strongly express cytotoxic proteins such as granzyme B, T-cell intracellular antigen 1, and perforin.3 The degree of cellular atypia, fat necrosis, karyorrhexis, and cytophagia, as well as the lack of angioinvasion, interface dermatitis, and epidermotropism help to distinguish SPTCL from other panniculitides.2,3 According to a previous study, clonal TCR gene rearrangement was identified in 50% to 80% of cases, but the absence of this clonal rearrangement does not exclude the diagnosis.14
This case also highlights the occurrence of secondary malignancies in patients with CLL, an NHL that is classified as a low-grade lymphoproliferative malignancy with clonal expansion of B cells.15 Secondary CTCLs in patients with CLL are rare, but they have been previously described. In 2017, Chang et al16 identified 12 patients with CLL who subsequently developed CTCL between 1992 and 2008. Of the 12 patients, 7 developed mycosis fungoides, 3 had CTCL not otherwise specified, 1 had mature T-cell lymphoma not otherwise specified, and 1 had primary cutaneous CD30+ T-cell lymphoma.16 The proliferation of 2 separate lymphocytic lineages is rare, but this study demonstrated an increased risk for CTCL to develop in patients with CLL. One possible explanation is that malignant cells come from a common stem cell progenitor or from genetic events. They occur secondary to carcinogens, viruses, or cytokines from T-cell or B-cell clones; they evolve due to treatment of the preexisting lymphoproliferative disease; or they occur simply by coincidence. The behavior of the CTCL may be more aggressive in patients with CLL due to immunosuppression, which may have contributed to the extreme presentation in our patient.16 Subcutaneous panniculitic T-cell lymphoma also has been reported in a patient with CLL that was thought to be associated with prior rituximab treatment.17
Treatment of SPTCL depends on the severity and course of the disease. In patients with more indolent disease, systemic steroids have been the most frequently used initial treatment.2,3,10 However, the disease often will progress after steroid tapering and require further intervention. Localized lesions may be treated with radiation alone or in combination with other systemic therapies.3,10 In refractory, aggressive, or relapsing cases, polychemotherapeutic regimens have proven to produce long-term remission in 30% of patients, with an overall response rate of 50%.10 These regimens most commonly have included cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) or CHOP-like treatment (EPOCH regimen [etoposide, prednisone, oncovin, cyclophosphamide, and doxorubicin hydrochloride]).3,10 A stem cell transplant can be considered in patients with recurrent and refractory disease, and it also has been shown to induce remission.4,17 In patients with a good response to therapy, the disease often can be controlled for long periods of time, with an estimated 5-year survival rate of 80%.15
This case highlights the diagnostic challenges and variable presentations of SPTCL. Dermatologists, oncologists, and dermatopathologists should be aware of this condition and consider it in the differential diagnosis of a patient with a hematologic malignancy and unremitting facial and/or body swelling without any other cause. The possibility of a secondary hematologic cancer in a patient with CLL also must be taken into consideration. Early diagnosis and treatment can minimize morbidity and induce remission in most patients.
To the Editor:
Subcutaneous panniculitic T-cell lymphoma (SPTCL) is a rare cutaneous T-cell lymphoma that was first described in 19911 and comprises less than 1% of all non-Hodgkin lymphomas (NHLs). It most commonly occurs in young adults, with a median patient age of 36 years and a slight female predominance.2 Patients typically present with skin nodules or deep-seated plaques involving the legs, arms, and/or trunk. Presentation on the face is less common.2,3 Paraneoplastic edema has been reported in several cases of SPTCL with facial and periorbital swelling.4-9
Diagnosis of SPTCL is achieved via analysis of a deep tissue skin biopsy and close clinicopathologic correlation. Histopathology demonstrates lobular panniculitis with an atypical lymphoid infiltrate in the subcutaneous tissue with predominantly CD8+ T cells without overlying epidermotropism or interface dermatitis.3 The degree of cellular atypia, fat necrosis, karyorrhexis, cytophagia, and lack of angioinvasion can help to distinguish SPTCL from other panniculitides.2,3
The prognosis of SPTCL is good, with a 5-year survival rate of 82%, and many patients are able to achieve remission.2 However, SPTCL can progress to a fatal hemophagocytic syndrome, which has been reported in 17% of cases, making early diagnosis and treatment of this malignancy imperative.1,2 Treatment varies depending on the progression and extent of disease and can include the use of steroids, multidrug chemotherapy regimens, radiotherapy, and stem cell transplant in refractory cases.2-4,10,11
Subcutaneous panniculitic T-cell lymphoma with edema has been reported in a 2-year-old child.12 We present a case of SPTCL in an adult patient with known stage IV chronic lymphocytic leukemia (CLL) who also had full-body edema.
A 60-year-old woman with a 7-year history of stage IV CLL presented with anasarca of 3 months’ duration. At the time of presentation to dermatology, physical examination revealed erythematous tender nodules on the arms and legs. She had no other medical conditions and was undergoing treatment with ibrutinib for the CLL. The patient reported profound fatigue but no fever, chills, night sweats, cough, or dyspnea. The swelling had begun initially in the legs and progressively worsened to involve the arms, face, and body. She was hospitalized and treated with intravenous steroids and antihistamines, which led to minor improvement in the swelling. The patient’s preliminary diagnosis of erythema nodosum was thought to be related to the CLL or ibrutinib; therefore, treatment subsequently was discontinued and she was discharged from the hospital.
The swelling continued to worsen over the following 3 months, and the patient gained approximately 25 pounds. She presented to our office again with severe periorbital, facial, and lip edema as well as diffuse edema of the torso, arms, and legs (Figure 1). Erythematous tender subcutaneous nodules were noted on the right proximal thigh, left lateral calf, and forearms. She was again hospitalized, and extensive evaluation was performed to exclude other causes of anasarca, including a complete blood cell count; comprehensive metabolic profile; hepatitis panels; HIV test; C3 and C4, complement CH50, C1 esterase inhibitor, IgE, and angiotensin-converting enzyme levels; urine protein to creatinine ratio; computed tomography of the chest, abdomen, and pelvis; and allergy evaluation. The analyses failed to reveal the cause of the anasarca.

During hospitalization, the patient underwent a lymph node biopsy, bone marrow biopsy, and a 6-mm punch biopsy of the right thigh nodule. The lymph node and bone marrow biopsy results were consistent with the known diagnosis of CLL, and the patient was started on intravenous chemotherapy with bendamustine. The skin biopsy demonstrated a predominant T-cell infiltrate consistent with a lobular panniculitis with variable amounts of adipocytes rimmed by lymphocytes, nuclear debris, and karyorrhexis (Figure 2). CD3+, CD8+, and CD4− T cells were positive for T-cell receptor (TCR) βF1 and negative for TCR-γ with strong expression of cytotoxic markers including granzyme B, perforin, and T-cell intracytoplasmic antigen 1. Rare CD56+ cells also were noted. The biopsy did not demonstrate any notable interface dermatitis, epidermotropism, or angioinvasion. T-cell receptor gene rearrangement studies did not show clonality for γ- or β-chain probes. Subcutaneous panniculitic T-cell lymphoma was diagnosed, making this case unique with the presentation of anasarca. This case also is noteworthy due to the rare diagnosis of the secondary malignancy of SPTCL in a patient with known CLL. The patient opted to pursue hospice and comfort measures due to the effects of persistent pancytopenia and the progression of CLL. She died 2 months later.

Clinical courses of SPTCL vary based on the TCR phenotype and immunophenotypic characteristics of the tumor cells. The TCR-αβ phenotype, as described in this case, typically is CD4−, CD8+, and CD56– and leads to a more indolent disease course. Lymphomas with the TCR-γδ phenotype typically are CD4−, CD8−, and CD56+; they often are associated with hemophagocytic syndrome and thus a worse prognosis. In 2009, the World Health Organization–European Organization for Research and Treatment of Cancer classification of primary cutaneous lymphomas restricted the category of SPTCL to the TCR-αβ phenotype due to the stark differences between the 2 types. The TCR-γδ phenotype was given its own diagnostic category—primary cutaneous γδ T-cell lymphoma.3
Patients with SPTCL commonly present with nodular skin lesions or deep-seated plaques on the legs, arms, and/or trunk; presentation on the face is rare.2,3 Fever, chills, night sweats, and/or weight loss were present in approximately 50% of recorded cases. Underlying autoimmune disease was present in 12 of 63 (19%) patients in a 2008 study.2 Facial and periorbital swelling with SPTCL has been reported.4-9 The presentation of anasarca, as seen in our adult patient, has been reported in a 2-year-old child.12 Anasarca as a presenting symptom of NHL is a rare phenomenon proposed to be induced by malignant cells secreting a cytokine that causes a vascular leak syndrome.13 Specifically, tumor necrosis factor α was found to be elevated in at least 2 patients with NHL presenting with anasarca in a prior study. Tumor necrosis factor α is known to cause increased capillary permeability, vascular leakage, and development of edema.13 In retrospect, obtaining cytokine levels in our patient would have been useful to support or refute tumor necrosis factor α as a possible cause of anasarca in the setting of NHL. This case continues to highlight that a diagnosis of SPTCL and analysis of a skin biopsy should be considered in cases of sudden unremitting facial and/or body swelling that cannot be explained by other more common causes.
Subcutaneous panniculitic T-cell lymphoma can be diagnosed and distinguished from other panniculitides via analysis of a deep tissue skin biopsy. Multiple biopsies may be required to ensure an adequate sample is obtained.4 Histopathology displays an atypical lymphoid infiltrate with a predominant presence of T cells. Neoplastic cells show CD3+, CD8+, and CD4− T cells, which strongly express cytotoxic proteins such as granzyme B, T-cell intracellular antigen 1, and perforin.3 The degree of cellular atypia, fat necrosis, karyorrhexis, and cytophagia, as well as the lack of angioinvasion, interface dermatitis, and epidermotropism help to distinguish SPTCL from other panniculitides.2,3 According to a previous study, clonal TCR gene rearrangement was identified in 50% to 80% of cases, but the absence of this clonal rearrangement does not exclude the diagnosis.14
This case also highlights the occurrence of secondary malignancies in patients with CLL, an NHL that is classified as a low-grade lymphoproliferative malignancy with clonal expansion of B cells.15 Secondary CTCLs in patients with CLL are rare, but they have been previously described. In 2017, Chang et al16 identified 12 patients with CLL who subsequently developed CTCL between 1992 and 2008. Of the 12 patients, 7 developed mycosis fungoides, 3 had CTCL not otherwise specified, 1 had mature T-cell lymphoma not otherwise specified, and 1 had primary cutaneous CD30+ T-cell lymphoma.16 The proliferation of 2 separate lymphocytic lineages is rare, but this study demonstrated an increased risk for CTCL to develop in patients with CLL. One possible explanation is that malignant cells come from a common stem cell progenitor or from genetic events. They occur secondary to carcinogens, viruses, or cytokines from T-cell or B-cell clones; they evolve due to treatment of the preexisting lymphoproliferative disease; or they occur simply by coincidence. The behavior of the CTCL may be more aggressive in patients with CLL due to immunosuppression, which may have contributed to the extreme presentation in our patient.16 Subcutaneous panniculitic T-cell lymphoma also has been reported in a patient with CLL that was thought to be associated with prior rituximab treatment.17
Treatment of SPTCL depends on the severity and course of the disease. In patients with more indolent disease, systemic steroids have been the most frequently used initial treatment.2,3,10 However, the disease often will progress after steroid tapering and require further intervention. Localized lesions may be treated with radiation alone or in combination with other systemic therapies.3,10 In refractory, aggressive, or relapsing cases, polychemotherapeutic regimens have proven to produce long-term remission in 30% of patients, with an overall response rate of 50%.10 These regimens most commonly have included cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) or CHOP-like treatment (EPOCH regimen [etoposide, prednisone, oncovin, cyclophosphamide, and doxorubicin hydrochloride]).3,10 A stem cell transplant can be considered in patients with recurrent and refractory disease, and it also has been shown to induce remission.4,17 In patients with a good response to therapy, the disease often can be controlled for long periods of time, with an estimated 5-year survival rate of 80%.15
This case highlights the diagnostic challenges and variable presentations of SPTCL. Dermatologists, oncologists, and dermatopathologists should be aware of this condition and consider it in the differential diagnosis of a patient with a hematologic malignancy and unremitting facial and/or body swelling without any other cause. The possibility of a secondary hematologic cancer in a patient with CLL also must be taken into consideration. Early diagnosis and treatment can minimize morbidity and induce remission in most patients.
- Gonzalez CL, Medeiros LJ, Braziel RM, et al. T-cell lymphoma involving subcutaneous tissue. a clinicopathologic entity commonly associated with hemophagocytic syndrome. Am J Surg Pathol. 1991;15:17-27.
- Willemze R, Jansen P, Cerroni L, et al.
Subcutaneous panniculitis-like T-cell lymphoma: definition, classification, and prognostic factors: an EORTC Cutaneous Lymphoma Group Study of 83 cases. Blood. 2008;111:38-45. - Parveen Z, Thompson K. Subcutaneous panniculitis-like T-cell lymphoma: redefinition of diagnostic criteria in the recent World Health Organization–European Organization for Research and Treatment of Cancer classification for cutaneous lymphomas. Arch Pathol Lab Med. 2009;133:303-308.
- Velez N, Ishizawar R, Dellaripa P, et al. Full facial edema: a novel presentation of subcutaneous panniculitis-like T-cell lymphoma. J Clin Oncol. 2012;30:e233-236.
- Asati D, Ingle V, Joshi D, et al. Subcutaneous panniculitis-like T-cell lymphoma with macrophage activation syndrome treated by cyclosporine and prednisolone. Indian Dermatol Online J. 2016;7:529-532.
- Fricker M, Dubach P, Helbing A, et al. Not all facial swellings are angioedemas! J Investig Allergol Clin Immunol. 2015;25:146-147.
- Kosari F, Akbarzadeh H. Local facial edema: a novel presentation of subcutaneous panniculitis-like T-cell lymphoma in a 30-year-old Iranian woman. Acta Med Iran. 2014;52:950-953.
- Bhojaraja M, Kistampally P, Udupa K, et al. Subcutaneous panniculitis-like T-cell lymphoma: a rare tumour. J Clin Diagn Res. 2016;10:OD29-OD30.
- Hashimoto R, Uchiyama M, Maeno T. Case report of subcutaneous panniculitis-like T-cell lymphoma complicated by eyelid swelling. BMC Ophthalmol. 2016;16:117.
- Chinello MN, Naviglio S, Remotti D, et al. Subcutaneous panniculitis-like T-cell lymphoma presenting with diffuse cutaneous edema in a 2-year-old child. J Pediatr Hematol Oncol. 2015;37:329-330.
- Chang TW, Weaver AL, Shanafelt TD, et al. Risk of cutaneous T-cell lymphoma in patients with chronic lymphocytic leukemia and other subtypes of non-Hodgkin lymphoma. Int J Dermatol. 2017;56:1125-1129.
- Chinello MN, Naviglio S, Remotti D, et al. Subcutaneous panniculitis-like T-cell lymphoma presenting with diffuse cutaneous edema in a 2-year-old child. J Pediatr Hematol Oncol. 2015;37:329-330.
- Jillella A, Day D, Severson K, et al. Non-Hodgkin’s lymphoma presenting as anasarca: probably mediated by tumor necrosis factor alpha (TNF-α). Leuk Lymphoma. 2000;38:419-422.
- Lee D-W, Yang J-H, Lee S-M, et al. Subcutaneous panniculitis-like T-cell lymphoma: a clinical and pathologic study of 14 Korean patients. Ann Dermatol. 2011;23:329-337.
- Jaffe ES. The 2008 WHO classification of lymphomas: implications for clinical practice and translational research [published online January 1, 2009]. Hematology Am Soc Hematol Educ Program. https://doi.org/10.1182/asheducation-2009.1.523
- Chang TW, Weaver AL, Shanafelt TD, et al. Risk of cutaneous T-cell lymphoma in patients with chronic lymphocytic leukemia and other subtypes of non-Hodgkin lymphoma. Int J Dermatol. 2017;56:1125-1129.
- Hall M, Sluzevich J, Snow J. Generalized subcutaneous panniculitis-like T-cell lymphoma following rituximab for hemolytic anemia in a patient with chronic lymphocytic leukemia. J Am Acad Dermatol. 2010;62(suppl 1):AB96.
- Gonzalez CL, Medeiros LJ, Braziel RM, et al. T-cell lymphoma involving subcutaneous tissue. a clinicopathologic entity commonly associated with hemophagocytic syndrome. Am J Surg Pathol. 1991;15:17-27.
- Willemze R, Jansen P, Cerroni L, et al.
Subcutaneous panniculitis-like T-cell lymphoma: definition, classification, and prognostic factors: an EORTC Cutaneous Lymphoma Group Study of 83 cases. Blood. 2008;111:38-45. - Parveen Z, Thompson K. Subcutaneous panniculitis-like T-cell lymphoma: redefinition of diagnostic criteria in the recent World Health Organization–European Organization for Research and Treatment of Cancer classification for cutaneous lymphomas. Arch Pathol Lab Med. 2009;133:303-308.
- Velez N, Ishizawar R, Dellaripa P, et al. Full facial edema: a novel presentation of subcutaneous panniculitis-like T-cell lymphoma. J Clin Oncol. 2012;30:e233-236.
- Asati D, Ingle V, Joshi D, et al. Subcutaneous panniculitis-like T-cell lymphoma with macrophage activation syndrome treated by cyclosporine and prednisolone. Indian Dermatol Online J. 2016;7:529-532.
- Fricker M, Dubach P, Helbing A, et al. Not all facial swellings are angioedemas! J Investig Allergol Clin Immunol. 2015;25:146-147.
- Kosari F, Akbarzadeh H. Local facial edema: a novel presentation of subcutaneous panniculitis-like T-cell lymphoma in a 30-year-old Iranian woman. Acta Med Iran. 2014;52:950-953.
- Bhojaraja M, Kistampally P, Udupa K, et al. Subcutaneous panniculitis-like T-cell lymphoma: a rare tumour. J Clin Diagn Res. 2016;10:OD29-OD30.
- Hashimoto R, Uchiyama M, Maeno T. Case report of subcutaneous panniculitis-like T-cell lymphoma complicated by eyelid swelling. BMC Ophthalmol. 2016;16:117.
- Chinello MN, Naviglio S, Remotti D, et al. Subcutaneous panniculitis-like T-cell lymphoma presenting with diffuse cutaneous edema in a 2-year-old child. J Pediatr Hematol Oncol. 2015;37:329-330.
- Chang TW, Weaver AL, Shanafelt TD, et al. Risk of cutaneous T-cell lymphoma in patients with chronic lymphocytic leukemia and other subtypes of non-Hodgkin lymphoma. Int J Dermatol. 2017;56:1125-1129.
- Chinello MN, Naviglio S, Remotti D, et al. Subcutaneous panniculitis-like T-cell lymphoma presenting with diffuse cutaneous edema in a 2-year-old child. J Pediatr Hematol Oncol. 2015;37:329-330.
- Jillella A, Day D, Severson K, et al. Non-Hodgkin’s lymphoma presenting as anasarca: probably mediated by tumor necrosis factor alpha (TNF-α). Leuk Lymphoma. 2000;38:419-422.
- Lee D-W, Yang J-H, Lee S-M, et al. Subcutaneous panniculitis-like T-cell lymphoma: a clinical and pathologic study of 14 Korean patients. Ann Dermatol. 2011;23:329-337.
- Jaffe ES. The 2008 WHO classification of lymphomas: implications for clinical practice and translational research [published online January 1, 2009]. Hematology Am Soc Hematol Educ Program. https://doi.org/10.1182/asheducation-2009.1.523
- Chang TW, Weaver AL, Shanafelt TD, et al. Risk of cutaneous T-cell lymphoma in patients with chronic lymphocytic leukemia and other subtypes of non-Hodgkin lymphoma. Int J Dermatol. 2017;56:1125-1129.
- Hall M, Sluzevich J, Snow J. Generalized subcutaneous panniculitis-like T-cell lymphoma following rituximab for hemolytic anemia in a patient with chronic lymphocytic leukemia. J Am Acad Dermatol. 2010;62(suppl 1):AB96.
Practice Points
- Subcutaneous panniculitic T-cell lymphoma (SPTCL) is a rare type of cutaneous T-cell lymphoma that may be complicated by fatal hemophagocytic syndrome.
- Patients typically present with deep-seated plaques or nodules that may be masked by localized edema.
- A biopsy is necessary to diagnose SPTCL, as well as to assess the degree of cellular atypia, fat necrosis, karyorrhexis, cytophagia, and angioinvasion to distinguish it from other panniculitides.
- In patients with a known hematologic malignancy, a secondary malignancy must be considered in the differential diagnosis of paraneoplastic edema.
Progressive Primary Cutaneous Nocardiosis in an Immunocompetent Patient
To the Editor:
The organisms of the genus Nocardia are gram-positive, ubiquitous, aerobic actinomycetes found worldwide in soil, decaying organic material, and water.1 The genus Nocardia includes more than 50 species; some species, such as Nocardia asteroides, Nocardia farcinica, Nocardia nova, and Nocardia brasiliensis, are the cause of nocardiosis in humans and animals.2,3 Nocardiosis is a rare and opportunistic infection that predominantly affects immunocompromised individuals; however, up to 30% of infections can occur in immunocompetent hosts.4 Nocardiosis can manifest in 3 disease forms: cutaneous, pulmonary, or disseminated. Cutaneous nocardiosis commonly develops in immunocompetent individuals who have experienced a predisposing traumatic injury to the skin,5 and it can exhibit a diverse variety of clinical manifestations, making diagnosis difficult. We describe a case of serious progressive primary cutaneous nocardiosis with an unusual presentation in an immunocompetent patient.
A 26-year-old immunocompetent man presented with pain, swelling, nodules, abscesses, ulcers, and sinus drainage of the left arm. The left elbow lesion initially developed at the site of a trauma 6 years prior that was painless but was contaminated with mossy soil. The condition slowly progressed over the next 2 years, and the patient experienced increased swelling and eventually developed multiple draining sinus tracts. Over the next 4 years, the lesions multiplied, spreading to the forearm and upper arm; associated severe pain and swelling at the elbow and wrist joint developed. The patient sought medical care at a local hospital and subsequently was diagnosed with suspected cutaneous tuberculosis. The patient was empirically treated with a 6-month course of isoniazid, rifampicin, pyrazinamide, and ethambutol; however, the lesions continued to progress and worsen. The patient had to stop antibiotic treatment because of substantially elevated alanine aminotransferase and aspartate aminotransferase levels.
He subsequently was evaluated at our hospital. He had no notable medical history and was afebrile. Physical examination revealed multiple erythematous nodules, abscesses, and ulcers on the left arm. There were several nodules with open sinus tracts and seropurulent crusts along with numerous atrophic, ovoid, stellate scars. Other nodules and ulcers with purulent drainage were located along the lymphatic nodes extending up the patient’s left forearm (Figure 1A). The yellowish-white pus discharge from several active sinuses contained no apparent granules. The lesions were densely distributed along the elbow, wrist, and shoulder, which resulted in associated skin swelling and restricted joint movement. The left axillary lymph nodes were enlarged.

Laboratory analyses revealed a hemoglobin level of 9.6 g/dL (reference range, 13–17.5 g/dL), platelet count of 621×109/L (reference range, 125–350×109/L), and leukocyte count of 14.3×109/L (reference range, 3.5–9.5 ×109/L). C-reactive protein level was 88.4 mg/L (reference range, 0–10 mg/L). Blood, renal, and liver tests, as well as tumor marker, peripheral blood lymphocyte subset, immunoglobulin, and complement results were within reference ranges. Results for Treponema pallidum and HIV antibody tests were negative. Hepatitis B virus markers were positive for hepatitis B surface antigen, hepatitis B e antigen, and hepatitis B core antibody, and the serum concentration of hepatitis B virus DNA was 3.12×107 IU/mL (reference range, <5×102 IU/mL). Computed tomography of the chest and cranium were unremarkable. Ultrasonography of the left arm revealed multiple vertical sinus tracts and several horizontal communicating branches that were accompanied by worm-eaten bone destruction (Figure 2).

Additional testing included histopathologic staining of a skin tissue specimen—hematoxylin and eosin, periodic acid–Schiff, and acid-fast staining—showed nonspecific, diffuse, inflammatory cell infiltration suggestive of chronic suppurative granuloma (Figure 3) but failed to reveal any special strains or organisms. Gram stain examination of the purulent fluid collected from the subcutaneous tissue showed no apparent positive bacillus or filamentous granules. The specimen was then inoculated on Sabouraud dextrose agar and Lowenstein-Jensen medium for fungus and mycobacteria culture, respectively. After 5 days, chalky, yellow, adherent colonies were observed on the Löwenstein-Jensen medium, and after 26 days, yellow crinkled colonies were observed on Sabouraud dextrose agar. The colonies were then inoculated on Columbia blood agar and incubated for 1 week to aid in the identification of organisms. Growth of yellow colonies that were adherent to the agar, moist, and smooth with a velvety surface, as well as a characteristic moldy odor resulted. Gram staining revealed gram-positive, thin, and beaded branching filaments (Figure 4). Based on colony characteristics, physiological properties, and biochemical tests, the isolate was identified as Nocardia. Results of further investigations employing polymerase chain reaction analysis of the skin specimen and bacterial colonies using a Nocardia genus 596-bp fragment of 16S ribosomal RNA primer (forward primer NG1: 5’-ACCGACCACAAGGGG-3’, reverse primer NG2: 5’-GGTTGTAACCTCTTCGA-3’)6 were completely consistent with the reference for identification of N brasiliensis. Evaluation of these results led to a diagnosis of cutaneous nocardiosis after traumatic inoculation.
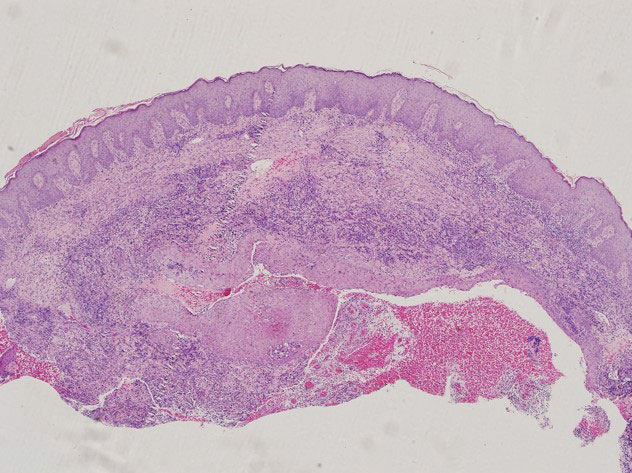
Because there was a high suspicion of actinophytosis or nocardiosis at admission, the patient received a combination antibiotic treatment with intravenous aqueous penicillin (4 million U every 4 hours) and oral trimethoprim-sulfamethoxazole (160/800 mg twice daily). Subsequently, treatment was changed to a combination of oral trimethoprim-sulfamethoxazole (160/800 mg twice daily) and moxifloxacin (400 mg once daily) based on pathogen identification and antibiotic sensitivity testing. After 1 month of treatment, the cutaneous lesions and left limb swelling dramatically improved and purulent drainage ceased, though some scarring occurred during the healing process. In addition, the mobility of the affected shoulder, elbow, and wrist joints slightly improved. Notable improvement in the mobility and swelling of the joints was observed at 6-month follow-up (Figure 1B). The patient continues to be monitored on an outpatient basis.
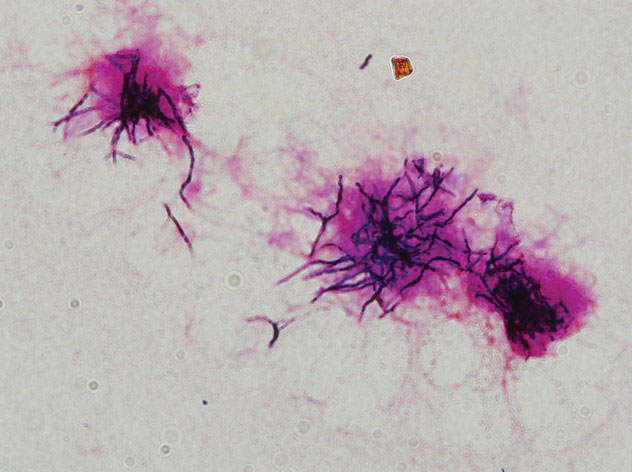
Cutaneous nocardiosis is a disfiguring granulomatous infection involving cutaneous and subcutaneous tissue that can progress to cause injury to viscera and bone.7 It has been called one of the great imitators because cutaneous nocardiosis can present in multiple forms,8,9 including mycetoma, sporotrichoid infection, superficial skin infection, and disseminated infection with cutaneous involvement. The differential diagnoses of cutaneous nocardiosis are broad and include tuberculosis; actinomycosis; deep fungal infections such as sporotrichosis, blastomycosis, phaeohyphomycosis, histoplasmosis, and coccidioidomycosis; other bacterial causes of cellulitis, abscess, or ecthyma; and malignancies.10 The principle method of diagnosis is the identification of Nocardia from the infection site.
Our patient ultimately was diagnosed with primary cutaneous nocardiosis resulting from a traumatic injury to the skin that was contaminated with soil. The clinical manifestation pattern was a compound type, including both mycetoma and sporotrichoid infections. Initially, Nocardia mycetoma occurred with subcutaneous infection by direct extension10,11 and appeared as dense, predominantly painless, swollen lesions. After 4 years, the skin lesions continued to spread linearly to the patient’s upper arm and forearm and manifested as the sporotrichoid infection type with painful swollen lesions at the site of inoculation and painful enlargement of the ipsilateral axillary lymph node.
Although nocardiosis is found worldwide, it is endemic to tropical and subtropical regions such as India, Africa, Southeast Asia, and Latin America.12 Nocardiosis most often is observed in individuals aged 20 to 40 years. It affects men more than women, and it commonly occurs in field laborers and cultivators whose occupations involve direct contact with the soil.13 Most lesions are found on the lower extremities, though localized nocardiosis infections can occur in other areas such as the neck, breasts, back, buttocks, and elbows.
Our patient initially was misdiagnosed, and treatment was delayed for several reasons. First, nocardiosis is not common in China, and most clinicians are unfamiliar with the disease. Second, the related lesions do not have specific features, and our patient had a complex clinical presentation that included mycetoma and sporotrichoid infection. Third, the characteristic grain of Nocardia species is small but that of N brasiliensis is even smaller (approximately 0.1–0.2 mm in diameter), which makes visualization difficult in both histopathologic and microbiologic examinations.14 The histopathologic examination results of our patient in the local hospital were nonspecific. Fourth, our patient did not initially go to the hospital but instead purchased some over-the-counter antibiotic ointments for external application because the lesions were painless. Moreover, microbiologic smear and culture examinations were not conducted in the local hospital before administering antituberculosis treatment to the patient. Instead, a polymerase chain reaction examination of skin lesion tissue for tubercle bacilli and atypical mycobacteria was negative. These findings imply that the traditional microbial smear and culture evaluations cannot be omitted. Furthermore, culture examinations should be conducted on multiple skin tissue and purulent fluid specimens to increase the likelihood of detection. These cultures should be monitored for at least 2 to 4 weeks because Nocardia is a slow-growing organism.10
The optimal antimicrobial treatment regimens for nocardiosis have not been firmly established.15 Trimethoprim-sulfamethoxazole is regarded as the first-line antimicrobial agent for treatment of nocardial infections. The optimal duration of antimicrobial therapy for nocardiosis also has not been determined, and the treatment regimen depends on the severity and extent of the infection as well as on the presence of infection-related complications. The main complication is bone involvement. Notable bony changes include periosteal thickening, osteoporosis, and osteolysis.
We considered the severity of skin lesions and bone marrow invasion in our patient and planned to treat him continually with oral trimethoprim-sulfamethoxazole according to the in vitro drug susceptibility test. The patient showed clinical improvement after 1 month of treatment, and he continued to improve after 6 months of treatment. To prevent recurrence, we found it necessary to treat the patient with a long-term antibiotic course over 6 to 12 months.16
Cutaneous nocardiosis remains a diagnostic challenge because of its nonspecific and diverse clinical and histopathological presentations. Diagnosis is further complicated by the inherent difficulty of cultivating and identifying the clinical isolate in the laboratory. A high degree of clinical suspicion followed by successful identification of the organism by a laboratory technologist will aid in the early diagnosis and treatment of the infection, ultimately reducing the risk for complications and morbidity.
- McNeil MM, Brown JM. The medically important aerobic actinomycetes: epidemiology and microbiology. Clin Microbiol Rev. 1994;7:357-417.
- Brown-Elliott BA, Brown JM, Conville PS, et al. Clinical and laboratory features of the Nocardia spp. based on current molecular taxonomy. Clin Microbiol Rev. 2006;19:259-282.
- Fatahi-Bafghi M. Nocardiosis from 1888 to 2017. Microb Pathog. 2018;114:369-384.
- Beaman BL, Burnside J, Edwards B, et al. Nocardial infections in the United States, 1972-1974. J Infect Dis. 1976;134:286-289.
- Lerner PI. Nocardiosis. Clin Infect Dis. 1996;22:891-903.
- Laurent FJ, Provost F, Boiron P. Rapid identification of clinically relevant Nocardia species to genus level by 16S rRNA gene PCR. J Clin Microbiol. 1999;37:99-102.
- Nguyen NM, Sink JR, Carter AJ, et al. Nocardiosis incognito: primary cutaneous nocardiosis with extension to myositis and pleural infection. JAAD Case Rep. 2018;4:33-35.
- Sharna NL, Mahajan VK, Agarwal S, et al. Nocardial mycetoma: diverse clinical presentations. Indian J Dermatol Venereol Leprol. 2008;74:635-640.
- Huang L, Chen X, Xu H, et al. Clinical features, identification, antimicrobial resistance patterns of Nocardia species in China: 2009-2017. Diagn Microbiol Infect Dis. 2019;94:165-172.
- Bonifaz A, Tirado-Sánchez A, Calderón L, et al. Mycetoma: experience of 482 cases in a single center in Mexico. PLoS Negl Trop Dis. 2014;8:E3102.
- Welsh O, Vero-Cabrera L, Salinas-Carmona MC. Mycetoma. Clin Dermatol. 2007;25:195-202.
- Nenoff P, van de Sande WWJ, Fahal AH, et al. Eumycetoma and actinomycetoma—an update on causative agents, epidemiology, pathogenesis, diagnostics and therapy. J Eur Acad Dermatol Venereol. 2015;29:1873-1883.
- Emmanuel P, Dumre SP, John S, et al. Mycetoma: a clinical dilemma in resource limited settings. Ann Clin Microbiol Antimicrob. 2018;17:35.
- Reis CMS, Reis-Filho EGM. Mycetomas: an epidemiological, etiological, clinical, laboratory and therapeutic review. An Bras Dermatol. 2018;93:8-18.
- Wilson JW. Nocardiosis: updates and clinical overview. Mayo Clin Proc. 2012;87:403-407.
- Welsh O, Vera-Cabrera L, Salinas-Carmona MC. Current treatment for Nocardia infections. Expert Opin Pharmacother. 2013;14:2387-2398.
To the Editor:
The organisms of the genus Nocardia are gram-positive, ubiquitous, aerobic actinomycetes found worldwide in soil, decaying organic material, and water.1 The genus Nocardia includes more than 50 species; some species, such as Nocardia asteroides, Nocardia farcinica, Nocardia nova, and Nocardia brasiliensis, are the cause of nocardiosis in humans and animals.2,3 Nocardiosis is a rare and opportunistic infection that predominantly affects immunocompromised individuals; however, up to 30% of infections can occur in immunocompetent hosts.4 Nocardiosis can manifest in 3 disease forms: cutaneous, pulmonary, or disseminated. Cutaneous nocardiosis commonly develops in immunocompetent individuals who have experienced a predisposing traumatic injury to the skin,5 and it can exhibit a diverse variety of clinical manifestations, making diagnosis difficult. We describe a case of serious progressive primary cutaneous nocardiosis with an unusual presentation in an immunocompetent patient.
A 26-year-old immunocompetent man presented with pain, swelling, nodules, abscesses, ulcers, and sinus drainage of the left arm. The left elbow lesion initially developed at the site of a trauma 6 years prior that was painless but was contaminated with mossy soil. The condition slowly progressed over the next 2 years, and the patient experienced increased swelling and eventually developed multiple draining sinus tracts. Over the next 4 years, the lesions multiplied, spreading to the forearm and upper arm; associated severe pain and swelling at the elbow and wrist joint developed. The patient sought medical care at a local hospital and subsequently was diagnosed with suspected cutaneous tuberculosis. The patient was empirically treated with a 6-month course of isoniazid, rifampicin, pyrazinamide, and ethambutol; however, the lesions continued to progress and worsen. The patient had to stop antibiotic treatment because of substantially elevated alanine aminotransferase and aspartate aminotransferase levels.
He subsequently was evaluated at our hospital. He had no notable medical history and was afebrile. Physical examination revealed multiple erythematous nodules, abscesses, and ulcers on the left arm. There were several nodules with open sinus tracts and seropurulent crusts along with numerous atrophic, ovoid, stellate scars. Other nodules and ulcers with purulent drainage were located along the lymphatic nodes extending up the patient’s left forearm (Figure 1A). The yellowish-white pus discharge from several active sinuses contained no apparent granules. The lesions were densely distributed along the elbow, wrist, and shoulder, which resulted in associated skin swelling and restricted joint movement. The left axillary lymph nodes were enlarged.

Laboratory analyses revealed a hemoglobin level of 9.6 g/dL (reference range, 13–17.5 g/dL), platelet count of 621×109/L (reference range, 125–350×109/L), and leukocyte count of 14.3×109/L (reference range, 3.5–9.5 ×109/L). C-reactive protein level was 88.4 mg/L (reference range, 0–10 mg/L). Blood, renal, and liver tests, as well as tumor marker, peripheral blood lymphocyte subset, immunoglobulin, and complement results were within reference ranges. Results for Treponema pallidum and HIV antibody tests were negative. Hepatitis B virus markers were positive for hepatitis B surface antigen, hepatitis B e antigen, and hepatitis B core antibody, and the serum concentration of hepatitis B virus DNA was 3.12×107 IU/mL (reference range, <5×102 IU/mL). Computed tomography of the chest and cranium were unremarkable. Ultrasonography of the left arm revealed multiple vertical sinus tracts and several horizontal communicating branches that were accompanied by worm-eaten bone destruction (Figure 2).

Additional testing included histopathologic staining of a skin tissue specimen—hematoxylin and eosin, periodic acid–Schiff, and acid-fast staining—showed nonspecific, diffuse, inflammatory cell infiltration suggestive of chronic suppurative granuloma (Figure 3) but failed to reveal any special strains or organisms. Gram stain examination of the purulent fluid collected from the subcutaneous tissue showed no apparent positive bacillus or filamentous granules. The specimen was then inoculated on Sabouraud dextrose agar and Lowenstein-Jensen medium for fungus and mycobacteria culture, respectively. After 5 days, chalky, yellow, adherent colonies were observed on the Löwenstein-Jensen medium, and after 26 days, yellow crinkled colonies were observed on Sabouraud dextrose agar. The colonies were then inoculated on Columbia blood agar and incubated for 1 week to aid in the identification of organisms. Growth of yellow colonies that were adherent to the agar, moist, and smooth with a velvety surface, as well as a characteristic moldy odor resulted. Gram staining revealed gram-positive, thin, and beaded branching filaments (Figure 4). Based on colony characteristics, physiological properties, and biochemical tests, the isolate was identified as Nocardia. Results of further investigations employing polymerase chain reaction analysis of the skin specimen and bacterial colonies using a Nocardia genus 596-bp fragment of 16S ribosomal RNA primer (forward primer NG1: 5’-ACCGACCACAAGGGG-3’, reverse primer NG2: 5’-GGTTGTAACCTCTTCGA-3’)6 were completely consistent with the reference for identification of N brasiliensis. Evaluation of these results led to a diagnosis of cutaneous nocardiosis after traumatic inoculation.

Because there was a high suspicion of actinophytosis or nocardiosis at admission, the patient received a combination antibiotic treatment with intravenous aqueous penicillin (4 million U every 4 hours) and oral trimethoprim-sulfamethoxazole (160/800 mg twice daily). Subsequently, treatment was changed to a combination of oral trimethoprim-sulfamethoxazole (160/800 mg twice daily) and moxifloxacin (400 mg once daily) based on pathogen identification and antibiotic sensitivity testing. After 1 month of treatment, the cutaneous lesions and left limb swelling dramatically improved and purulent drainage ceased, though some scarring occurred during the healing process. In addition, the mobility of the affected shoulder, elbow, and wrist joints slightly improved. Notable improvement in the mobility and swelling of the joints was observed at 6-month follow-up (Figure 1B). The patient continues to be monitored on an outpatient basis.

Cutaneous nocardiosis is a disfiguring granulomatous infection involving cutaneous and subcutaneous tissue that can progress to cause injury to viscera and bone.7 It has been called one of the great imitators because cutaneous nocardiosis can present in multiple forms,8,9 including mycetoma, sporotrichoid infection, superficial skin infection, and disseminated infection with cutaneous involvement. The differential diagnoses of cutaneous nocardiosis are broad and include tuberculosis; actinomycosis; deep fungal infections such as sporotrichosis, blastomycosis, phaeohyphomycosis, histoplasmosis, and coccidioidomycosis; other bacterial causes of cellulitis, abscess, or ecthyma; and malignancies.10 The principle method of diagnosis is the identification of Nocardia from the infection site.
Our patient ultimately was diagnosed with primary cutaneous nocardiosis resulting from a traumatic injury to the skin that was contaminated with soil. The clinical manifestation pattern was a compound type, including both mycetoma and sporotrichoid infections. Initially, Nocardia mycetoma occurred with subcutaneous infection by direct extension10,11 and appeared as dense, predominantly painless, swollen lesions. After 4 years, the skin lesions continued to spread linearly to the patient’s upper arm and forearm and manifested as the sporotrichoid infection type with painful swollen lesions at the site of inoculation and painful enlargement of the ipsilateral axillary lymph node.
Although nocardiosis is found worldwide, it is endemic to tropical and subtropical regions such as India, Africa, Southeast Asia, and Latin America.12 Nocardiosis most often is observed in individuals aged 20 to 40 years. It affects men more than women, and it commonly occurs in field laborers and cultivators whose occupations involve direct contact with the soil.13 Most lesions are found on the lower extremities, though localized nocardiosis infections can occur in other areas such as the neck, breasts, back, buttocks, and elbows.
Our patient initially was misdiagnosed, and treatment was delayed for several reasons. First, nocardiosis is not common in China, and most clinicians are unfamiliar with the disease. Second, the related lesions do not have specific features, and our patient had a complex clinical presentation that included mycetoma and sporotrichoid infection. Third, the characteristic grain of Nocardia species is small but that of N brasiliensis is even smaller (approximately 0.1–0.2 mm in diameter), which makes visualization difficult in both histopathologic and microbiologic examinations.14 The histopathologic examination results of our patient in the local hospital were nonspecific. Fourth, our patient did not initially go to the hospital but instead purchased some over-the-counter antibiotic ointments for external application because the lesions were painless. Moreover, microbiologic smear and culture examinations were not conducted in the local hospital before administering antituberculosis treatment to the patient. Instead, a polymerase chain reaction examination of skin lesion tissue for tubercle bacilli and atypical mycobacteria was negative. These findings imply that the traditional microbial smear and culture evaluations cannot be omitted. Furthermore, culture examinations should be conducted on multiple skin tissue and purulent fluid specimens to increase the likelihood of detection. These cultures should be monitored for at least 2 to 4 weeks because Nocardia is a slow-growing organism.10
The optimal antimicrobial treatment regimens for nocardiosis have not been firmly established.15 Trimethoprim-sulfamethoxazole is regarded as the first-line antimicrobial agent for treatment of nocardial infections. The optimal duration of antimicrobial therapy for nocardiosis also has not been determined, and the treatment regimen depends on the severity and extent of the infection as well as on the presence of infection-related complications. The main complication is bone involvement. Notable bony changes include periosteal thickening, osteoporosis, and osteolysis.
We considered the severity of skin lesions and bone marrow invasion in our patient and planned to treat him continually with oral trimethoprim-sulfamethoxazole according to the in vitro drug susceptibility test. The patient showed clinical improvement after 1 month of treatment, and he continued to improve after 6 months of treatment. To prevent recurrence, we found it necessary to treat the patient with a long-term antibiotic course over 6 to 12 months.16
Cutaneous nocardiosis remains a diagnostic challenge because of its nonspecific and diverse clinical and histopathological presentations. Diagnosis is further complicated by the inherent difficulty of cultivating and identifying the clinical isolate in the laboratory. A high degree of clinical suspicion followed by successful identification of the organism by a laboratory technologist will aid in the early diagnosis and treatment of the infection, ultimately reducing the risk for complications and morbidity.
To the Editor:
The organisms of the genus Nocardia are gram-positive, ubiquitous, aerobic actinomycetes found worldwide in soil, decaying organic material, and water.1 The genus Nocardia includes more than 50 species; some species, such as Nocardia asteroides, Nocardia farcinica, Nocardia nova, and Nocardia brasiliensis, are the cause of nocardiosis in humans and animals.2,3 Nocardiosis is a rare and opportunistic infection that predominantly affects immunocompromised individuals; however, up to 30% of infections can occur in immunocompetent hosts.4 Nocardiosis can manifest in 3 disease forms: cutaneous, pulmonary, or disseminated. Cutaneous nocardiosis commonly develops in immunocompetent individuals who have experienced a predisposing traumatic injury to the skin,5 and it can exhibit a diverse variety of clinical manifestations, making diagnosis difficult. We describe a case of serious progressive primary cutaneous nocardiosis with an unusual presentation in an immunocompetent patient.
A 26-year-old immunocompetent man presented with pain, swelling, nodules, abscesses, ulcers, and sinus drainage of the left arm. The left elbow lesion initially developed at the site of a trauma 6 years prior that was painless but was contaminated with mossy soil. The condition slowly progressed over the next 2 years, and the patient experienced increased swelling and eventually developed multiple draining sinus tracts. Over the next 4 years, the lesions multiplied, spreading to the forearm and upper arm; associated severe pain and swelling at the elbow and wrist joint developed. The patient sought medical care at a local hospital and subsequently was diagnosed with suspected cutaneous tuberculosis. The patient was empirically treated with a 6-month course of isoniazid, rifampicin, pyrazinamide, and ethambutol; however, the lesions continued to progress and worsen. The patient had to stop antibiotic treatment because of substantially elevated alanine aminotransferase and aspartate aminotransferase levels.
He subsequently was evaluated at our hospital. He had no notable medical history and was afebrile. Physical examination revealed multiple erythematous nodules, abscesses, and ulcers on the left arm. There were several nodules with open sinus tracts and seropurulent crusts along with numerous atrophic, ovoid, stellate scars. Other nodules and ulcers with purulent drainage were located along the lymphatic nodes extending up the patient’s left forearm (Figure 1A). The yellowish-white pus discharge from several active sinuses contained no apparent granules. The lesions were densely distributed along the elbow, wrist, and shoulder, which resulted in associated skin swelling and restricted joint movement. The left axillary lymph nodes were enlarged.

Laboratory analyses revealed a hemoglobin level of 9.6 g/dL (reference range, 13–17.5 g/dL), platelet count of 621×109/L (reference range, 125–350×109/L), and leukocyte count of 14.3×109/L (reference range, 3.5–9.5 ×109/L). C-reactive protein level was 88.4 mg/L (reference range, 0–10 mg/L). Blood, renal, and liver tests, as well as tumor marker, peripheral blood lymphocyte subset, immunoglobulin, and complement results were within reference ranges. Results for Treponema pallidum and HIV antibody tests were negative. Hepatitis B virus markers were positive for hepatitis B surface antigen, hepatitis B e antigen, and hepatitis B core antibody, and the serum concentration of hepatitis B virus DNA was 3.12×107 IU/mL (reference range, <5×102 IU/mL). Computed tomography of the chest and cranium were unremarkable. Ultrasonography of the left arm revealed multiple vertical sinus tracts and several horizontal communicating branches that were accompanied by worm-eaten bone destruction (Figure 2).

Additional testing included histopathologic staining of a skin tissue specimen—hematoxylin and eosin, periodic acid–Schiff, and acid-fast staining—showed nonspecific, diffuse, inflammatory cell infiltration suggestive of chronic suppurative granuloma (Figure 3) but failed to reveal any special strains or organisms. Gram stain examination of the purulent fluid collected from the subcutaneous tissue showed no apparent positive bacillus or filamentous granules. The specimen was then inoculated on Sabouraud dextrose agar and Lowenstein-Jensen medium for fungus and mycobacteria culture, respectively. After 5 days, chalky, yellow, adherent colonies were observed on the Löwenstein-Jensen medium, and after 26 days, yellow crinkled colonies were observed on Sabouraud dextrose agar. The colonies were then inoculated on Columbia blood agar and incubated for 1 week to aid in the identification of organisms. Growth of yellow colonies that were adherent to the agar, moist, and smooth with a velvety surface, as well as a characteristic moldy odor resulted. Gram staining revealed gram-positive, thin, and beaded branching filaments (Figure 4). Based on colony characteristics, physiological properties, and biochemical tests, the isolate was identified as Nocardia. Results of further investigations employing polymerase chain reaction analysis of the skin specimen and bacterial colonies using a Nocardia genus 596-bp fragment of 16S ribosomal RNA primer (forward primer NG1: 5’-ACCGACCACAAGGGG-3’, reverse primer NG2: 5’-GGTTGTAACCTCTTCGA-3’)6 were completely consistent with the reference for identification of N brasiliensis. Evaluation of these results led to a diagnosis of cutaneous nocardiosis after traumatic inoculation.

Because there was a high suspicion of actinophytosis or nocardiosis at admission, the patient received a combination antibiotic treatment with intravenous aqueous penicillin (4 million U every 4 hours) and oral trimethoprim-sulfamethoxazole (160/800 mg twice daily). Subsequently, treatment was changed to a combination of oral trimethoprim-sulfamethoxazole (160/800 mg twice daily) and moxifloxacin (400 mg once daily) based on pathogen identification and antibiotic sensitivity testing. After 1 month of treatment, the cutaneous lesions and left limb swelling dramatically improved and purulent drainage ceased, though some scarring occurred during the healing process. In addition, the mobility of the affected shoulder, elbow, and wrist joints slightly improved. Notable improvement in the mobility and swelling of the joints was observed at 6-month follow-up (Figure 1B). The patient continues to be monitored on an outpatient basis.

Cutaneous nocardiosis is a disfiguring granulomatous infection involving cutaneous and subcutaneous tissue that can progress to cause injury to viscera and bone.7 It has been called one of the great imitators because cutaneous nocardiosis can present in multiple forms,8,9 including mycetoma, sporotrichoid infection, superficial skin infection, and disseminated infection with cutaneous involvement. The differential diagnoses of cutaneous nocardiosis are broad and include tuberculosis; actinomycosis; deep fungal infections such as sporotrichosis, blastomycosis, phaeohyphomycosis, histoplasmosis, and coccidioidomycosis; other bacterial causes of cellulitis, abscess, or ecthyma; and malignancies.10 The principle method of diagnosis is the identification of Nocardia from the infection site.
Our patient ultimately was diagnosed with primary cutaneous nocardiosis resulting from a traumatic injury to the skin that was contaminated with soil. The clinical manifestation pattern was a compound type, including both mycetoma and sporotrichoid infections. Initially, Nocardia mycetoma occurred with subcutaneous infection by direct extension10,11 and appeared as dense, predominantly painless, swollen lesions. After 4 years, the skin lesions continued to spread linearly to the patient’s upper arm and forearm and manifested as the sporotrichoid infection type with painful swollen lesions at the site of inoculation and painful enlargement of the ipsilateral axillary lymph node.
Although nocardiosis is found worldwide, it is endemic to tropical and subtropical regions such as India, Africa, Southeast Asia, and Latin America.12 Nocardiosis most often is observed in individuals aged 20 to 40 years. It affects men more than women, and it commonly occurs in field laborers and cultivators whose occupations involve direct contact with the soil.13 Most lesions are found on the lower extremities, though localized nocardiosis infections can occur in other areas such as the neck, breasts, back, buttocks, and elbows.
Our patient initially was misdiagnosed, and treatment was delayed for several reasons. First, nocardiosis is not common in China, and most clinicians are unfamiliar with the disease. Second, the related lesions do not have specific features, and our patient had a complex clinical presentation that included mycetoma and sporotrichoid infection. Third, the characteristic grain of Nocardia species is small but that of N brasiliensis is even smaller (approximately 0.1–0.2 mm in diameter), which makes visualization difficult in both histopathologic and microbiologic examinations.14 The histopathologic examination results of our patient in the local hospital were nonspecific. Fourth, our patient did not initially go to the hospital but instead purchased some over-the-counter antibiotic ointments for external application because the lesions were painless. Moreover, microbiologic smear and culture examinations were not conducted in the local hospital before administering antituberculosis treatment to the patient. Instead, a polymerase chain reaction examination of skin lesion tissue for tubercle bacilli and atypical mycobacteria was negative. These findings imply that the traditional microbial smear and culture evaluations cannot be omitted. Furthermore, culture examinations should be conducted on multiple skin tissue and purulent fluid specimens to increase the likelihood of detection. These cultures should be monitored for at least 2 to 4 weeks because Nocardia is a slow-growing organism.10
The optimal antimicrobial treatment regimens for nocardiosis have not been firmly established.15 Trimethoprim-sulfamethoxazole is regarded as the first-line antimicrobial agent for treatment of nocardial infections. The optimal duration of antimicrobial therapy for nocardiosis also has not been determined, and the treatment regimen depends on the severity and extent of the infection as well as on the presence of infection-related complications. The main complication is bone involvement. Notable bony changes include periosteal thickening, osteoporosis, and osteolysis.
We considered the severity of skin lesions and bone marrow invasion in our patient and planned to treat him continually with oral trimethoprim-sulfamethoxazole according to the in vitro drug susceptibility test. The patient showed clinical improvement after 1 month of treatment, and he continued to improve after 6 months of treatment. To prevent recurrence, we found it necessary to treat the patient with a long-term antibiotic course over 6 to 12 months.16
Cutaneous nocardiosis remains a diagnostic challenge because of its nonspecific and diverse clinical and histopathological presentations. Diagnosis is further complicated by the inherent difficulty of cultivating and identifying the clinical isolate in the laboratory. A high degree of clinical suspicion followed by successful identification of the organism by a laboratory technologist will aid in the early diagnosis and treatment of the infection, ultimately reducing the risk for complications and morbidity.
- McNeil MM, Brown JM. The medically important aerobic actinomycetes: epidemiology and microbiology. Clin Microbiol Rev. 1994;7:357-417.
- Brown-Elliott BA, Brown JM, Conville PS, et al. Clinical and laboratory features of the Nocardia spp. based on current molecular taxonomy. Clin Microbiol Rev. 2006;19:259-282.
- Fatahi-Bafghi M. Nocardiosis from 1888 to 2017. Microb Pathog. 2018;114:369-384.
- Beaman BL, Burnside J, Edwards B, et al. Nocardial infections in the United States, 1972-1974. J Infect Dis. 1976;134:286-289.
- Lerner PI. Nocardiosis. Clin Infect Dis. 1996;22:891-903.
- Laurent FJ, Provost F, Boiron P. Rapid identification of clinically relevant Nocardia species to genus level by 16S rRNA gene PCR. J Clin Microbiol. 1999;37:99-102.
- Nguyen NM, Sink JR, Carter AJ, et al. Nocardiosis incognito: primary cutaneous nocardiosis with extension to myositis and pleural infection. JAAD Case Rep. 2018;4:33-35.
- Sharna NL, Mahajan VK, Agarwal S, et al. Nocardial mycetoma: diverse clinical presentations. Indian J Dermatol Venereol Leprol. 2008;74:635-640.
- Huang L, Chen X, Xu H, et al. Clinical features, identification, antimicrobial resistance patterns of Nocardia species in China: 2009-2017. Diagn Microbiol Infect Dis. 2019;94:165-172.
- Bonifaz A, Tirado-Sánchez A, Calderón L, et al. Mycetoma: experience of 482 cases in a single center in Mexico. PLoS Negl Trop Dis. 2014;8:E3102.
- Welsh O, Vero-Cabrera L, Salinas-Carmona MC. Mycetoma. Clin Dermatol. 2007;25:195-202.
- Nenoff P, van de Sande WWJ, Fahal AH, et al. Eumycetoma and actinomycetoma—an update on causative agents, epidemiology, pathogenesis, diagnostics and therapy. J Eur Acad Dermatol Venereol. 2015;29:1873-1883.
- Emmanuel P, Dumre SP, John S, et al. Mycetoma: a clinical dilemma in resource limited settings. Ann Clin Microbiol Antimicrob. 2018;17:35.
- Reis CMS, Reis-Filho EGM. Mycetomas: an epidemiological, etiological, clinical, laboratory and therapeutic review. An Bras Dermatol. 2018;93:8-18.
- Wilson JW. Nocardiosis: updates and clinical overview. Mayo Clin Proc. 2012;87:403-407.
- Welsh O, Vera-Cabrera L, Salinas-Carmona MC. Current treatment for Nocardia infections. Expert Opin Pharmacother. 2013;14:2387-2398.
- McNeil MM, Brown JM. The medically important aerobic actinomycetes: epidemiology and microbiology. Clin Microbiol Rev. 1994;7:357-417.
- Brown-Elliott BA, Brown JM, Conville PS, et al. Clinical and laboratory features of the Nocardia spp. based on current molecular taxonomy. Clin Microbiol Rev. 2006;19:259-282.
- Fatahi-Bafghi M. Nocardiosis from 1888 to 2017. Microb Pathog. 2018;114:369-384.
- Beaman BL, Burnside J, Edwards B, et al. Nocardial infections in the United States, 1972-1974. J Infect Dis. 1976;134:286-289.
- Lerner PI. Nocardiosis. Clin Infect Dis. 1996;22:891-903.
- Laurent FJ, Provost F, Boiron P. Rapid identification of clinically relevant Nocardia species to genus level by 16S rRNA gene PCR. J Clin Microbiol. 1999;37:99-102.
- Nguyen NM, Sink JR, Carter AJ, et al. Nocardiosis incognito: primary cutaneous nocardiosis with extension to myositis and pleural infection. JAAD Case Rep. 2018;4:33-35.
- Sharna NL, Mahajan VK, Agarwal S, et al. Nocardial mycetoma: diverse clinical presentations. Indian J Dermatol Venereol Leprol. 2008;74:635-640.
- Huang L, Chen X, Xu H, et al. Clinical features, identification, antimicrobial resistance patterns of Nocardia species in China: 2009-2017. Diagn Microbiol Infect Dis. 2019;94:165-172.
- Bonifaz A, Tirado-Sánchez A, Calderón L, et al. Mycetoma: experience of 482 cases in a single center in Mexico. PLoS Negl Trop Dis. 2014;8:E3102.
- Welsh O, Vero-Cabrera L, Salinas-Carmona MC. Mycetoma. Clin Dermatol. 2007;25:195-202.
- Nenoff P, van de Sande WWJ, Fahal AH, et al. Eumycetoma and actinomycetoma—an update on causative agents, epidemiology, pathogenesis, diagnostics and therapy. J Eur Acad Dermatol Venereol. 2015;29:1873-1883.
- Emmanuel P, Dumre SP, John S, et al. Mycetoma: a clinical dilemma in resource limited settings. Ann Clin Microbiol Antimicrob. 2018;17:35.
- Reis CMS, Reis-Filho EGM. Mycetomas: an epidemiological, etiological, clinical, laboratory and therapeutic review. An Bras Dermatol. 2018;93:8-18.
- Wilson JW. Nocardiosis: updates and clinical overview. Mayo Clin Proc. 2012;87:403-407.
- Welsh O, Vera-Cabrera L, Salinas-Carmona MC. Current treatment for Nocardia infections. Expert Opin Pharmacother. 2013;14:2387-2398.
Practice Points
- Although unusual, cutaneous nocardiosis can present with both mycetoma and sporotrichoid infection, which should be treated based on pathogen identification and antibiotic sensitivity testing.
- A high degree of clinical suspicion by clinicians followed by successful identification of the organism by a laboratory technologist will aid in the early diagnosis and treatment of the infection, ultimately reducing the risk for complications and morbidity.
Commentary: Disease activity, JAK inhibitors, and pregnancy risks in PsA, April 2023
Patients with active PsA require early effective therapy to improve long-term outcomes. The choice of therapy should balance effectiveness and potential toxicity. Janus kinase (JAK) inhibitors are a relatively new class of drugs that have been shown to be efficacious in treating PsA, but there are concerns about safety. To evaluate the efficacy and safety of JAK inhibitors in patients with psoriatic disease, Yang and colleagues conducted a systematic review and meta-analysis of 17 phase 2/3 randomized controlled trials including 6802 patients with PsA or moderate to severe plaque psoriasis who received at least one JAK inhibitor. They demonstrated that, compared with placebo, JAK inhibitors were associated with a significantly higher American College of Rheumatology 20 response rate (relative risk [RR] 2.09; P < .00001), with the response being the highest for filgotinib (RR 2.40; P < .00001), followed by upadacitinib, tofacitinib, and deucravacitinib. However, the overall incidence of adverse events was higher with JAK inhibitors vs placebo (RR 1.17; P < .00001) and significantly higher with 10-mg vs 5-mg tofacitinib (P = .03). Thus, JAK inhibitors are efficacious in the treatment of PsA but are associated with adverse effects, particularly at higher doses.
Safety is best assessed in real-world observational studies. Clinical trials have raised concerns about a higher cancer risk in rheumatoid arthritis (RA) patients treated with JAK inhibitors compared with patients treated with tumor necrosis factor (TNF) inhibitors. To evaluate this further, Huss and colleagues conducted an observational cohort study that evaluated prospectively collected data from national Swedish data sources on 4443 patients with PsA and 10,447 patients with RA, all without previous cancer, who received JAK inhibitors, TNF inhibitors, or other non–TNF inhibitor biologic disease-modifying antirheumatic drugs. Overall, use of JAK inhibitors vs TNF inhibitors was not significantly associated with a higher risk for cancer other than nonmelanoma skin cancer, especially in RA. In patients with PsA, there was a trend toward higher risk for nonmelanoma skin cancer, but it was not statistically significant. The study provides reassurance that JAK inhibitors are generally as safe, as are TNF inhibitors in PsA, but continued vigilance is required.
There are limited data on the effect of disease activity on pregnancy outcomes in individuals with PsA. Using data from the Medical Birth Registry of Norway linked to data from a Norwegian nationwide observational register recruiting women with inflammatory rheumatic diseases, Skorpen and colleagues evaluated the association of active disease and cesarean section (CS) rates in singleton births in women with PsA (n = 121), axial spondyloarthritis (n = 312), and controls (n = 575,798). Compared with control individuals, women with PsA had a higher risk for CS (risk difference [RD] 15.0%; P < .001) and for emergency CS (RD 10.6%; P < .001), with active disease in the third trimester further amplifying both risks (CS: RD 17.7%; P = .028; emergency CS: RD 15.9%; P = .015). Thus, although in many patients disease activity decreases during pregnancy, this study highlights the importance of pregestational counseling and disease control along with regular monitoring of PsA during pregnancy such that disease activity remains well controlled.
Patients with active PsA require early effective therapy to improve long-term outcomes. The choice of therapy should balance effectiveness and potential toxicity. Janus kinase (JAK) inhibitors are a relatively new class of drugs that have been shown to be efficacious in treating PsA, but there are concerns about safety. To evaluate the efficacy and safety of JAK inhibitors in patients with psoriatic disease, Yang and colleagues conducted a systematic review and meta-analysis of 17 phase 2/3 randomized controlled trials including 6802 patients with PsA or moderate to severe plaque psoriasis who received at least one JAK inhibitor. They demonstrated that, compared with placebo, JAK inhibitors were associated with a significantly higher American College of Rheumatology 20 response rate (relative risk [RR] 2.09; P < .00001), with the response being the highest for filgotinib (RR 2.40; P < .00001), followed by upadacitinib, tofacitinib, and deucravacitinib. However, the overall incidence of adverse events was higher with JAK inhibitors vs placebo (RR 1.17; P < .00001) and significantly higher with 10-mg vs 5-mg tofacitinib (P = .03). Thus, JAK inhibitors are efficacious in the treatment of PsA but are associated with adverse effects, particularly at higher doses.
Safety is best assessed in real-world observational studies. Clinical trials have raised concerns about a higher cancer risk in rheumatoid arthritis (RA) patients treated with JAK inhibitors compared with patients treated with tumor necrosis factor (TNF) inhibitors. To evaluate this further, Huss and colleagues conducted an observational cohort study that evaluated prospectively collected data from national Swedish data sources on 4443 patients with PsA and 10,447 patients with RA, all without previous cancer, who received JAK inhibitors, TNF inhibitors, or other non–TNF inhibitor biologic disease-modifying antirheumatic drugs. Overall, use of JAK inhibitors vs TNF inhibitors was not significantly associated with a higher risk for cancer other than nonmelanoma skin cancer, especially in RA. In patients with PsA, there was a trend toward higher risk for nonmelanoma skin cancer, but it was not statistically significant. The study provides reassurance that JAK inhibitors are generally as safe, as are TNF inhibitors in PsA, but continued vigilance is required.
There are limited data on the effect of disease activity on pregnancy outcomes in individuals with PsA. Using data from the Medical Birth Registry of Norway linked to data from a Norwegian nationwide observational register recruiting women with inflammatory rheumatic diseases, Skorpen and colleagues evaluated the association of active disease and cesarean section (CS) rates in singleton births in women with PsA (n = 121), axial spondyloarthritis (n = 312), and controls (n = 575,798). Compared with control individuals, women with PsA had a higher risk for CS (risk difference [RD] 15.0%; P < .001) and for emergency CS (RD 10.6%; P < .001), with active disease in the third trimester further amplifying both risks (CS: RD 17.7%; P = .028; emergency CS: RD 15.9%; P = .015). Thus, although in many patients disease activity decreases during pregnancy, this study highlights the importance of pregestational counseling and disease control along with regular monitoring of PsA during pregnancy such that disease activity remains well controlled.
Patients with active PsA require early effective therapy to improve long-term outcomes. The choice of therapy should balance effectiveness and potential toxicity. Janus kinase (JAK) inhibitors are a relatively new class of drugs that have been shown to be efficacious in treating PsA, but there are concerns about safety. To evaluate the efficacy and safety of JAK inhibitors in patients with psoriatic disease, Yang and colleagues conducted a systematic review and meta-analysis of 17 phase 2/3 randomized controlled trials including 6802 patients with PsA or moderate to severe plaque psoriasis who received at least one JAK inhibitor. They demonstrated that, compared with placebo, JAK inhibitors were associated with a significantly higher American College of Rheumatology 20 response rate (relative risk [RR] 2.09; P < .00001), with the response being the highest for filgotinib (RR 2.40; P < .00001), followed by upadacitinib, tofacitinib, and deucravacitinib. However, the overall incidence of adverse events was higher with JAK inhibitors vs placebo (RR 1.17; P < .00001) and significantly higher with 10-mg vs 5-mg tofacitinib (P = .03). Thus, JAK inhibitors are efficacious in the treatment of PsA but are associated with adverse effects, particularly at higher doses.
Safety is best assessed in real-world observational studies. Clinical trials have raised concerns about a higher cancer risk in rheumatoid arthritis (RA) patients treated with JAK inhibitors compared with patients treated with tumor necrosis factor (TNF) inhibitors. To evaluate this further, Huss and colleagues conducted an observational cohort study that evaluated prospectively collected data from national Swedish data sources on 4443 patients with PsA and 10,447 patients with RA, all without previous cancer, who received JAK inhibitors, TNF inhibitors, or other non–TNF inhibitor biologic disease-modifying antirheumatic drugs. Overall, use of JAK inhibitors vs TNF inhibitors was not significantly associated with a higher risk for cancer other than nonmelanoma skin cancer, especially in RA. In patients with PsA, there was a trend toward higher risk for nonmelanoma skin cancer, but it was not statistically significant. The study provides reassurance that JAK inhibitors are generally as safe, as are TNF inhibitors in PsA, but continued vigilance is required.
There are limited data on the effect of disease activity on pregnancy outcomes in individuals with PsA. Using data from the Medical Birth Registry of Norway linked to data from a Norwegian nationwide observational register recruiting women with inflammatory rheumatic diseases, Skorpen and colleagues evaluated the association of active disease and cesarean section (CS) rates in singleton births in women with PsA (n = 121), axial spondyloarthritis (n = 312), and controls (n = 575,798). Compared with control individuals, women with PsA had a higher risk for CS (risk difference [RD] 15.0%; P < .001) and for emergency CS (RD 10.6%; P < .001), with active disease in the third trimester further amplifying both risks (CS: RD 17.7%; P = .028; emergency CS: RD 15.9%; P = .015). Thus, although in many patients disease activity decreases during pregnancy, this study highlights the importance of pregestational counseling and disease control along with regular monitoring of PsA during pregnancy such that disease activity remains well controlled.