User login
Mpox (Monkeypox) Clinical Pearls
The 2022 mpox (monkeypox) virus outbreak represents the latest example of how infectious diseases with previously limited reach can spread in a globalized society. More than 86,000 cases have been reported worldwide, with more than 30,000 cases in the United States as of March 15, 2023.1 Herein, we summarize the key features of mpox infection for the dermatologist.
Mpox Transmission
The mpox virus is a double-stranded DNA virus of the Orthopoxvirus genus and Poxviridae family.2,3 There are 2 types of the mpox virus: clade I (formerly the Congo Basin clade) and clade II (formerly the West African clade). Clade I causes more severe disease (10% mortality rate), while clade II is associated with lower mortality (1%–3%) and has been split into subclades of IIa (exhibits zoonotic transmission) and IIb (exhibits human-to-human spread).3,4 The current outbreak is caused by clade IIb, and patients typically have no travel history to classic endemic regions.5,6
In endemic countries, mpox transmission is zoonotic from small forest animals. In nonendemic countries, sporadic cases rarely have been reported, including a cluster in the United States in 2003 related to pet prairie dogs. In stark contrast, human-to-human transmission is occurring in the current epidemic mainly via intimate skin-to-skin contact and possibly via sexual fluids, meeting the criteria for a sexually transmitted infection. However, nonsexual transmission does still occur, though it is less common.7 Many of the reported cases so far are in young to middle-aged men who have sex with men (MSM).2,8 However, it is crucial to understand that mpox is not exclusive to the MSM population; the virus has been transmitted to heterosexual males, females, children, and even household pets of infected individuals.2,9,10 Labeling mpox as exclusive to the MSM community is both inaccurate and inappropriately stigmatizing.
Cutaneous Presentation and Diagnosis of Mpox
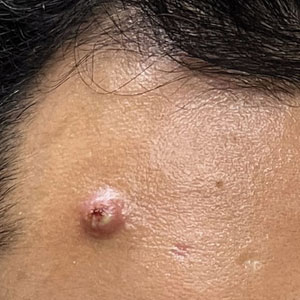
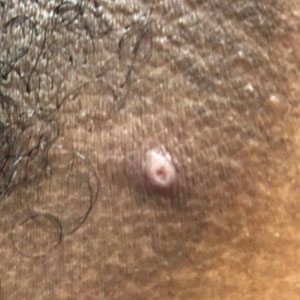
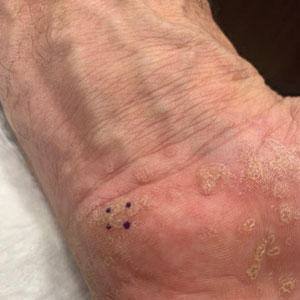
Mpox has an incubation time of approximately 9 days (range, 7–21 days), after which affected persons develop macular lesions that evolve over 2 to 4 weeks into papules, vesicles, and deep-seated pustules before crusting over and resolving with possible residual scarring.2,3,5,9,11,12 Palmoplantar involvement is a key feature.11 Although in some cases there will be multiple lesions with centrifugal progression, the lesions also may be few in number, with some patients presenting with a single lesion in the anogenital region or on the face, hand, or foot (Figure).6,9 Systemic symptoms such as prodromal fever, lymphadenopathy, and headache are common but not universal.9,13 Potential complications include penile edema, proctitis, bacterial superinfection, tonsillitis, conjunctivitis, encephalitis, and pneumonia.5,9,13
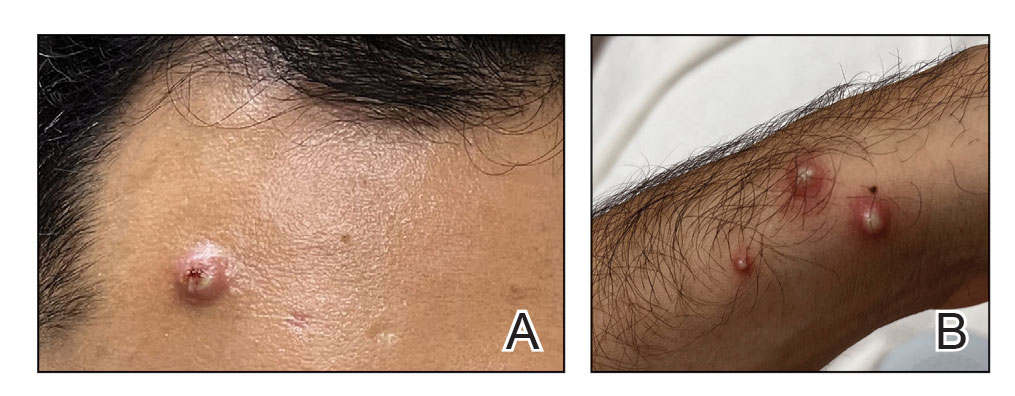
A high index of suspicion is needed to diagnose mpox infection. The differential diagnosis includes smallpox; varicella-zoster virus (primary or reactivation); secondary syphilis; measles; herpes simplex virus; molluscum contagiosum; hand, foot, and mouth disease; and disseminated gonococcal infection.2,3 For lesions confined to the genital area, sexually transmitted infections (eg, chancroid, lymphogranuloma venereum) as well as non–sexually related acute genital ulcers (Lipschütz ulcers) should be considered.2
Certain clinical features may help in distinguishing mpox from other diseases. Mpox exhibits synchronous progression and centrifugal distribution when multiple lesions are present; in contrast, the lesions of primary varicella (chickenpox) appear in multiple different stages, and those of localized herpes zoster (shingles) exhibit a dermatomal distribution. When these features are present, mpox causes a greater degree of lymphadenopathy and systemic symptoms than primary varicella.3Clinical diagnosis of mpox is more than 90% sensitive but only 9% to 26% specific.3 To confirm the diagnosis, a viral swab vigorously obtained from active skin lesions should be sent in viral transport media for mpox DNA-specific polymerase chain reaction testing, which is available from major laboratories.2,3 Other supportive tests include serum studies for anti–mpox virus immunoglobulins and immunohistochemical staining for viral antigens on skin biopsy specimens.2 When evaluating suspected and confirmed mpox cases, dermatologists should wear a gown, gloves, a fitted N95 mask, and eye protection to prevent infection.5
Treating Mpox
Symptomatic mpox infection can last for up to 2 to 5 weeks.3 The patient is no longer infectious once the lesions have crusted over.3,11 The majority of cases require supportive care only.2,3,5,14 However, mpox remains a potentially fatal disease, with 38 deaths to date in the current outbreak.1 High-risk populations include children younger than 8 years, pregnant women, and individuals who are immunocompromised.15 Tecovirimat, an antiviral medication approved by the US Food and Drug Administration (FDA) for smallpox, is available via the expanded access Investigational New Drug (EA-IND) protocol to treat severe mpox cases but is not widely available in the United States.6,16-18 Brincidofovir, a prodrug of the antiviral cidofovir, possesses single-patient emergency use Investigational New Drug (e-IND) status for treatment of mpox but also is not widely available in the United States.17 Intravenous vaccinia immune globulin is under consideration for high-risk individuals, but little is known regarding its efficacy against mpox.5,16,17
Two smallpox vaccines—JYNNEOS (Bavarian Nordic) and ACAM2000 (Emergent Bio Solutions)—are available for both preexposure and postexposure prophylaxis against mpox virus.19 At this time, only JYNNEOS is FDA approved for the prevention of mpox; ACAM2000 can be used against mpox under the FDA’s EA-IND protocol, which involves additional requirements, including informed consent from the patient.20 ACAM2000 is a live, replication-competent vaccine that carries a warning of increased risk for side effects in patients with cardiac disease, pregnancy, immunocompromise, and a history or presence of eczema and other skin conditions.3,21,22 JYNNEOS is a live but replication-deficient virus and therefore does not carry these warnings.3,21,22
Final Thoughts
Mpox is no longer an obscure illness occurring in limited geographic areas. Dermatologists must remain highly vigilant when evaluating any patient for new-onset vesicular or pustular eruptions to combat this ongoing public health threat. This issue of Cutis® also features a thorough mpox update on the clinical presentation, vaccine guidance, and management.23
- Centers for Disease Control and Prevention. Mpox: 2022 Outbreak Cases and Data. Updated March 15, 2023. Accessed March 121, 2023. https://www.cdc.gov/poxvirus/monkeypox/response/2022/
- Srivastava G. Human monkeypox disease [published online August 10, 2022]. Clin Dermatol. doi:10.1016/j.clindermatol.2022.08.009
- Bryer J, Freeman EE, Rosenbach M. Monkeypox emerges on a global scale: a historical review and dermatologic primer [published online July 8, 2022]. J Am Acad Dermatol. doi:10.1016/j.jaad.2022.07.007
- Americo JL, Earl PL, Moss B. Virulence differences of mpox (monkeypox) virus clades I, IIa, and IIb.1 in a small animal model. Proc Natl Acad Sci U S A. 2023;120:E2220415120. doi:10.1073 /pnas.2220415120
- Guarner J, Del Rio C, Malani PN. Monkeypox in 2022—what clinicians need to know. JAMA. 2022;328:139-140. doi:10.1001/jama.2022.10802
- Looi MK. Monkeypox: what we know about the 2022 outbreak so far [published online August 23, 2022]. BMJ. doi:10.1136/bmj.o2058
- Allan-Blitz LT, Gandhi M, Adamson P, et al. A position statement on mpox as a sexually transmitted disease [published online December 22, 2022]. Clin Infect Dis. doi:10.1093/cid/ciac960
- Cabanillas B, Murdaca G, Guemari A, et al. A compilation answering 50 questions on monkeypox virus and the current monkeypox outbreak. Allergy. 2023;78:639-662. doi:10.1111/all.15633
- Tarín-Vicente EJ, Alemany A, Agud-Dios M, et al. Clinical presentation and virological assessment of confirmed human monkeypox virus cases in Spain: a prospective observational cohort study [published online August 8, 2022]. Lancet. doi:10.1016/S0140-6736(22)01436-2
- Seang S, Burrel S, Todesco E, et al. Evidence of human-to-dog transmission of monkeypox virus. Lancet. 2022;400:658-659. doi:10.1016 /s0140-6736(22)01487-8
- Ramdass P, Mullick S, Farber HF. Viral skin diseases. Prim Care. 2015;42:517-67. doi:10.1016/j.pop.2015.08.006
- Centers for Disease Control and Prevention. Mpox: Clinical Recognition. Updated August 23, 2022. Accessed March 21, 2023. https://www.cdc .gov/poxvirus/monkeypox/clinicians/clinical-recognition.html
- Mpox Cases by Age and Gender, Race/Ethnicity, and Symptoms. Centers for Disease Control and Prevention. Updated March 15, 2023. Accessed March 21, 2023. https://www.cdc.gov/poxvirus/monkeypox /response/2022/demographics.html
- Kawsar A, Hussain K, Roberts N. The return of monkeypox: key pointers for dermatologists [published online July 29, 2022]. Clin Exp Dermatol. doi:10.1111/ced.15357
- Khanna U, Bishnoi A, Vinay K. Current outbreak of monkeypox— essentials for the dermatologist [published online June 23, 2022]. J Am Acad Dermatol. doi:10.1016/j.jaad.2022.06.1170
- Fox T, Gould S, Princy N, et al. Therapeutics for treating mpox in humans. Cochrane Database Syst Rev. 2023;3:CD015769. doi:10.1002/14651858 .CD015769
- Centers for Disease Control and Prevention. Treatment information for healthcare professionals. Updated March 3, 2023. Accessed March 24, 2023. https://www.cdc.gov/poxvirus/mpox/clinicians /treatment.html#anchor_1666886364947
- Centers for Disease Control and Prevention. Guidance for tecovirimat use. Updated February 23, 2023. Accessed March 24, 2023. https://www .cdc.gov/poxvirus/mpox/clinicians/Tecovirimat.html
- Interim Clinical Considerations for Use of JYNNEOS and ACAM2000 Vaccines During the 2022 U.S. Monkeypox Outbreak. Centers for Disease Control and Prevention. Updated October 19, 2022. Accessed March 21, 2023. https://www.cdc.gov/poxvirus/monkeypox/health-departments/vaccine-considerations.html
- Key Facts About Vaccines to Prevent Monkeypox Disease. US Food and Drug Administration. Updated August 18, 2022. Accessed March 21, 2023. https://www.fda.gov/vaccines-blood-biologics/vaccines/key-facts-aboutvaccines-prevent-monkeypox-disease
- Smallpox: Vaccines. Centers for Disease Control and Prevention. Updated August 8, 2022. Accessed March 21, 2023. https://www.cdc.gov/smallpox/clinicians/vaccines.html
- ACAM2000. Package insert. Emergent Product Development Gaithersburg Inc; 2019.
- Cices A, Prasad S, Akselrad M, et al. Mpox update: clinical presentation, vaccination guidance, and management. Cutis. 2023;111:197-202. doi:10.12788/cutis.0745
The 2022 mpox (monkeypox) virus outbreak represents the latest example of how infectious diseases with previously limited reach can spread in a globalized society. More than 86,000 cases have been reported worldwide, with more than 30,000 cases in the United States as of March 15, 2023.1 Herein, we summarize the key features of mpox infection for the dermatologist.
Mpox Transmission
The mpox virus is a double-stranded DNA virus of the Orthopoxvirus genus and Poxviridae family.2,3 There are 2 types of the mpox virus: clade I (formerly the Congo Basin clade) and clade II (formerly the West African clade). Clade I causes more severe disease (10% mortality rate), while clade II is associated with lower mortality (1%–3%) and has been split into subclades of IIa (exhibits zoonotic transmission) and IIb (exhibits human-to-human spread).3,4 The current outbreak is caused by clade IIb, and patients typically have no travel history to classic endemic regions.5,6
In endemic countries, mpox transmission is zoonotic from small forest animals. In nonendemic countries, sporadic cases rarely have been reported, including a cluster in the United States in 2003 related to pet prairie dogs. In stark contrast, human-to-human transmission is occurring in the current epidemic mainly via intimate skin-to-skin contact and possibly via sexual fluids, meeting the criteria for a sexually transmitted infection. However, nonsexual transmission does still occur, though it is less common.7 Many of the reported cases so far are in young to middle-aged men who have sex with men (MSM).2,8 However, it is crucial to understand that mpox is not exclusive to the MSM population; the virus has been transmitted to heterosexual males, females, children, and even household pets of infected individuals.2,9,10 Labeling mpox as exclusive to the MSM community is both inaccurate and inappropriately stigmatizing.
Cutaneous Presentation and Diagnosis of Mpox
Mpox has an incubation time of approximately 9 days (range, 7–21 days), after which affected persons develop macular lesions that evolve over 2 to 4 weeks into papules, vesicles, and deep-seated pustules before crusting over and resolving with possible residual scarring.2,3,5,9,11,12 Palmoplantar involvement is a key feature.11 Although in some cases there will be multiple lesions with centrifugal progression, the lesions also may be few in number, with some patients presenting with a single lesion in the anogenital region or on the face, hand, or foot (Figure).6,9 Systemic symptoms such as prodromal fever, lymphadenopathy, and headache are common but not universal.9,13 Potential complications include penile edema, proctitis, bacterial superinfection, tonsillitis, conjunctivitis, encephalitis, and pneumonia.5,9,13

A high index of suspicion is needed to diagnose mpox infection. The differential diagnosis includes smallpox; varicella-zoster virus (primary or reactivation); secondary syphilis; measles; herpes simplex virus; molluscum contagiosum; hand, foot, and mouth disease; and disseminated gonococcal infection.2,3 For lesions confined to the genital area, sexually transmitted infections (eg, chancroid, lymphogranuloma venereum) as well as non–sexually related acute genital ulcers (Lipschütz ulcers) should be considered.2
Certain clinical features may help in distinguishing mpox from other diseases. Mpox exhibits synchronous progression and centrifugal distribution when multiple lesions are present; in contrast, the lesions of primary varicella (chickenpox) appear in multiple different stages, and those of localized herpes zoster (shingles) exhibit a dermatomal distribution. When these features are present, mpox causes a greater degree of lymphadenopathy and systemic symptoms than primary varicella.3Clinical diagnosis of mpox is more than 90% sensitive but only 9% to 26% specific.3 To confirm the diagnosis, a viral swab vigorously obtained from active skin lesions should be sent in viral transport media for mpox DNA-specific polymerase chain reaction testing, which is available from major laboratories.2,3 Other supportive tests include serum studies for anti–mpox virus immunoglobulins and immunohistochemical staining for viral antigens on skin biopsy specimens.2 When evaluating suspected and confirmed mpox cases, dermatologists should wear a gown, gloves, a fitted N95 mask, and eye protection to prevent infection.5
Treating Mpox
Symptomatic mpox infection can last for up to 2 to 5 weeks.3 The patient is no longer infectious once the lesions have crusted over.3,11 The majority of cases require supportive care only.2,3,5,14 However, mpox remains a potentially fatal disease, with 38 deaths to date in the current outbreak.1 High-risk populations include children younger than 8 years, pregnant women, and individuals who are immunocompromised.15 Tecovirimat, an antiviral medication approved by the US Food and Drug Administration (FDA) for smallpox, is available via the expanded access Investigational New Drug (EA-IND) protocol to treat severe mpox cases but is not widely available in the United States.6,16-18 Brincidofovir, a prodrug of the antiviral cidofovir, possesses single-patient emergency use Investigational New Drug (e-IND) status for treatment of mpox but also is not widely available in the United States.17 Intravenous vaccinia immune globulin is under consideration for high-risk individuals, but little is known regarding its efficacy against mpox.5,16,17
Two smallpox vaccines—JYNNEOS (Bavarian Nordic) and ACAM2000 (Emergent Bio Solutions)—are available for both preexposure and postexposure prophylaxis against mpox virus.19 At this time, only JYNNEOS is FDA approved for the prevention of mpox; ACAM2000 can be used against mpox under the FDA’s EA-IND protocol, which involves additional requirements, including informed consent from the patient.20 ACAM2000 is a live, replication-competent vaccine that carries a warning of increased risk for side effects in patients with cardiac disease, pregnancy, immunocompromise, and a history or presence of eczema and other skin conditions.3,21,22 JYNNEOS is a live but replication-deficient virus and therefore does not carry these warnings.3,21,22
Final Thoughts
Mpox is no longer an obscure illness occurring in limited geographic areas. Dermatologists must remain highly vigilant when evaluating any patient for new-onset vesicular or pustular eruptions to combat this ongoing public health threat. This issue of Cutis® also features a thorough mpox update on the clinical presentation, vaccine guidance, and management.23
The 2022 mpox (monkeypox) virus outbreak represents the latest example of how infectious diseases with previously limited reach can spread in a globalized society. More than 86,000 cases have been reported worldwide, with more than 30,000 cases in the United States as of March 15, 2023.1 Herein, we summarize the key features of mpox infection for the dermatologist.
Mpox Transmission
The mpox virus is a double-stranded DNA virus of the Orthopoxvirus genus and Poxviridae family.2,3 There are 2 types of the mpox virus: clade I (formerly the Congo Basin clade) and clade II (formerly the West African clade). Clade I causes more severe disease (10% mortality rate), while clade II is associated with lower mortality (1%–3%) and has been split into subclades of IIa (exhibits zoonotic transmission) and IIb (exhibits human-to-human spread).3,4 The current outbreak is caused by clade IIb, and patients typically have no travel history to classic endemic regions.5,6
In endemic countries, mpox transmission is zoonotic from small forest animals. In nonendemic countries, sporadic cases rarely have been reported, including a cluster in the United States in 2003 related to pet prairie dogs. In stark contrast, human-to-human transmission is occurring in the current epidemic mainly via intimate skin-to-skin contact and possibly via sexual fluids, meeting the criteria for a sexually transmitted infection. However, nonsexual transmission does still occur, though it is less common.7 Many of the reported cases so far are in young to middle-aged men who have sex with men (MSM).2,8 However, it is crucial to understand that mpox is not exclusive to the MSM population; the virus has been transmitted to heterosexual males, females, children, and even household pets of infected individuals.2,9,10 Labeling mpox as exclusive to the MSM community is both inaccurate and inappropriately stigmatizing.
Cutaneous Presentation and Diagnosis of Mpox
Mpox has an incubation time of approximately 9 days (range, 7–21 days), after which affected persons develop macular lesions that evolve over 2 to 4 weeks into papules, vesicles, and deep-seated pustules before crusting over and resolving with possible residual scarring.2,3,5,9,11,12 Palmoplantar involvement is a key feature.11 Although in some cases there will be multiple lesions with centrifugal progression, the lesions also may be few in number, with some patients presenting with a single lesion in the anogenital region or on the face, hand, or foot (Figure).6,9 Systemic symptoms such as prodromal fever, lymphadenopathy, and headache are common but not universal.9,13 Potential complications include penile edema, proctitis, bacterial superinfection, tonsillitis, conjunctivitis, encephalitis, and pneumonia.5,9,13

A high index of suspicion is needed to diagnose mpox infection. The differential diagnosis includes smallpox; varicella-zoster virus (primary or reactivation); secondary syphilis; measles; herpes simplex virus; molluscum contagiosum; hand, foot, and mouth disease; and disseminated gonococcal infection.2,3 For lesions confined to the genital area, sexually transmitted infections (eg, chancroid, lymphogranuloma venereum) as well as non–sexually related acute genital ulcers (Lipschütz ulcers) should be considered.2
Certain clinical features may help in distinguishing mpox from other diseases. Mpox exhibits synchronous progression and centrifugal distribution when multiple lesions are present; in contrast, the lesions of primary varicella (chickenpox) appear in multiple different stages, and those of localized herpes zoster (shingles) exhibit a dermatomal distribution. When these features are present, mpox causes a greater degree of lymphadenopathy and systemic symptoms than primary varicella.3Clinical diagnosis of mpox is more than 90% sensitive but only 9% to 26% specific.3 To confirm the diagnosis, a viral swab vigorously obtained from active skin lesions should be sent in viral transport media for mpox DNA-specific polymerase chain reaction testing, which is available from major laboratories.2,3 Other supportive tests include serum studies for anti–mpox virus immunoglobulins and immunohistochemical staining for viral antigens on skin biopsy specimens.2 When evaluating suspected and confirmed mpox cases, dermatologists should wear a gown, gloves, a fitted N95 mask, and eye protection to prevent infection.5
Treating Mpox
Symptomatic mpox infection can last for up to 2 to 5 weeks.3 The patient is no longer infectious once the lesions have crusted over.3,11 The majority of cases require supportive care only.2,3,5,14 However, mpox remains a potentially fatal disease, with 38 deaths to date in the current outbreak.1 High-risk populations include children younger than 8 years, pregnant women, and individuals who are immunocompromised.15 Tecovirimat, an antiviral medication approved by the US Food and Drug Administration (FDA) for smallpox, is available via the expanded access Investigational New Drug (EA-IND) protocol to treat severe mpox cases but is not widely available in the United States.6,16-18 Brincidofovir, a prodrug of the antiviral cidofovir, possesses single-patient emergency use Investigational New Drug (e-IND) status for treatment of mpox but also is not widely available in the United States.17 Intravenous vaccinia immune globulin is under consideration for high-risk individuals, but little is known regarding its efficacy against mpox.5,16,17
Two smallpox vaccines—JYNNEOS (Bavarian Nordic) and ACAM2000 (Emergent Bio Solutions)—are available for both preexposure and postexposure prophylaxis against mpox virus.19 At this time, only JYNNEOS is FDA approved for the prevention of mpox; ACAM2000 can be used against mpox under the FDA’s EA-IND protocol, which involves additional requirements, including informed consent from the patient.20 ACAM2000 is a live, replication-competent vaccine that carries a warning of increased risk for side effects in patients with cardiac disease, pregnancy, immunocompromise, and a history or presence of eczema and other skin conditions.3,21,22 JYNNEOS is a live but replication-deficient virus and therefore does not carry these warnings.3,21,22
Final Thoughts
Mpox is no longer an obscure illness occurring in limited geographic areas. Dermatologists must remain highly vigilant when evaluating any patient for new-onset vesicular or pustular eruptions to combat this ongoing public health threat. This issue of Cutis® also features a thorough mpox update on the clinical presentation, vaccine guidance, and management.23
- Centers for Disease Control and Prevention. Mpox: 2022 Outbreak Cases and Data. Updated March 15, 2023. Accessed March 121, 2023. https://www.cdc.gov/poxvirus/monkeypox/response/2022/
- Srivastava G. Human monkeypox disease [published online August 10, 2022]. Clin Dermatol. doi:10.1016/j.clindermatol.2022.08.009
- Bryer J, Freeman EE, Rosenbach M. Monkeypox emerges on a global scale: a historical review and dermatologic primer [published online July 8, 2022]. J Am Acad Dermatol. doi:10.1016/j.jaad.2022.07.007
- Americo JL, Earl PL, Moss B. Virulence differences of mpox (monkeypox) virus clades I, IIa, and IIb.1 in a small animal model. Proc Natl Acad Sci U S A. 2023;120:E2220415120. doi:10.1073 /pnas.2220415120
- Guarner J, Del Rio C, Malani PN. Monkeypox in 2022—what clinicians need to know. JAMA. 2022;328:139-140. doi:10.1001/jama.2022.10802
- Looi MK. Monkeypox: what we know about the 2022 outbreak so far [published online August 23, 2022]. BMJ. doi:10.1136/bmj.o2058
- Allan-Blitz LT, Gandhi M, Adamson P, et al. A position statement on mpox as a sexually transmitted disease [published online December 22, 2022]. Clin Infect Dis. doi:10.1093/cid/ciac960
- Cabanillas B, Murdaca G, Guemari A, et al. A compilation answering 50 questions on monkeypox virus and the current monkeypox outbreak. Allergy. 2023;78:639-662. doi:10.1111/all.15633
- Tarín-Vicente EJ, Alemany A, Agud-Dios M, et al. Clinical presentation and virological assessment of confirmed human monkeypox virus cases in Spain: a prospective observational cohort study [published online August 8, 2022]. Lancet. doi:10.1016/S0140-6736(22)01436-2
- Seang S, Burrel S, Todesco E, et al. Evidence of human-to-dog transmission of monkeypox virus. Lancet. 2022;400:658-659. doi:10.1016 /s0140-6736(22)01487-8
- Ramdass P, Mullick S, Farber HF. Viral skin diseases. Prim Care. 2015;42:517-67. doi:10.1016/j.pop.2015.08.006
- Centers for Disease Control and Prevention. Mpox: Clinical Recognition. Updated August 23, 2022. Accessed March 21, 2023. https://www.cdc .gov/poxvirus/monkeypox/clinicians/clinical-recognition.html
- Mpox Cases by Age and Gender, Race/Ethnicity, and Symptoms. Centers for Disease Control and Prevention. Updated March 15, 2023. Accessed March 21, 2023. https://www.cdc.gov/poxvirus/monkeypox /response/2022/demographics.html
- Kawsar A, Hussain K, Roberts N. The return of monkeypox: key pointers for dermatologists [published online July 29, 2022]. Clin Exp Dermatol. doi:10.1111/ced.15357
- Khanna U, Bishnoi A, Vinay K. Current outbreak of monkeypox— essentials for the dermatologist [published online June 23, 2022]. J Am Acad Dermatol. doi:10.1016/j.jaad.2022.06.1170
- Fox T, Gould S, Princy N, et al. Therapeutics for treating mpox in humans. Cochrane Database Syst Rev. 2023;3:CD015769. doi:10.1002/14651858 .CD015769
- Centers for Disease Control and Prevention. Treatment information for healthcare professionals. Updated March 3, 2023. Accessed March 24, 2023. https://www.cdc.gov/poxvirus/mpox/clinicians /treatment.html#anchor_1666886364947
- Centers for Disease Control and Prevention. Guidance for tecovirimat use. Updated February 23, 2023. Accessed March 24, 2023. https://www .cdc.gov/poxvirus/mpox/clinicians/Tecovirimat.html
- Interim Clinical Considerations for Use of JYNNEOS and ACAM2000 Vaccines During the 2022 U.S. Monkeypox Outbreak. Centers for Disease Control and Prevention. Updated October 19, 2022. Accessed March 21, 2023. https://www.cdc.gov/poxvirus/monkeypox/health-departments/vaccine-considerations.html
- Key Facts About Vaccines to Prevent Monkeypox Disease. US Food and Drug Administration. Updated August 18, 2022. Accessed March 21, 2023. https://www.fda.gov/vaccines-blood-biologics/vaccines/key-facts-aboutvaccines-prevent-monkeypox-disease
- Smallpox: Vaccines. Centers for Disease Control and Prevention. Updated August 8, 2022. Accessed March 21, 2023. https://www.cdc.gov/smallpox/clinicians/vaccines.html
- ACAM2000. Package insert. Emergent Product Development Gaithersburg Inc; 2019.
- Cices A, Prasad S, Akselrad M, et al. Mpox update: clinical presentation, vaccination guidance, and management. Cutis. 2023;111:197-202. doi:10.12788/cutis.0745
- Centers for Disease Control and Prevention. Mpox: 2022 Outbreak Cases and Data. Updated March 15, 2023. Accessed March 121, 2023. https://www.cdc.gov/poxvirus/monkeypox/response/2022/
- Srivastava G. Human monkeypox disease [published online August 10, 2022]. Clin Dermatol. doi:10.1016/j.clindermatol.2022.08.009
- Bryer J, Freeman EE, Rosenbach M. Monkeypox emerges on a global scale: a historical review and dermatologic primer [published online July 8, 2022]. J Am Acad Dermatol. doi:10.1016/j.jaad.2022.07.007
- Americo JL, Earl PL, Moss B. Virulence differences of mpox (monkeypox) virus clades I, IIa, and IIb.1 in a small animal model. Proc Natl Acad Sci U S A. 2023;120:E2220415120. doi:10.1073 /pnas.2220415120
- Guarner J, Del Rio C, Malani PN. Monkeypox in 2022—what clinicians need to know. JAMA. 2022;328:139-140. doi:10.1001/jama.2022.10802
- Looi MK. Monkeypox: what we know about the 2022 outbreak so far [published online August 23, 2022]. BMJ. doi:10.1136/bmj.o2058
- Allan-Blitz LT, Gandhi M, Adamson P, et al. A position statement on mpox as a sexually transmitted disease [published online December 22, 2022]. Clin Infect Dis. doi:10.1093/cid/ciac960
- Cabanillas B, Murdaca G, Guemari A, et al. A compilation answering 50 questions on monkeypox virus and the current monkeypox outbreak. Allergy. 2023;78:639-662. doi:10.1111/all.15633
- Tarín-Vicente EJ, Alemany A, Agud-Dios M, et al. Clinical presentation and virological assessment of confirmed human monkeypox virus cases in Spain: a prospective observational cohort study [published online August 8, 2022]. Lancet. doi:10.1016/S0140-6736(22)01436-2
- Seang S, Burrel S, Todesco E, et al. Evidence of human-to-dog transmission of monkeypox virus. Lancet. 2022;400:658-659. doi:10.1016 /s0140-6736(22)01487-8
- Ramdass P, Mullick S, Farber HF. Viral skin diseases. Prim Care. 2015;42:517-67. doi:10.1016/j.pop.2015.08.006
- Centers for Disease Control and Prevention. Mpox: Clinical Recognition. Updated August 23, 2022. Accessed March 21, 2023. https://www.cdc .gov/poxvirus/monkeypox/clinicians/clinical-recognition.html
- Mpox Cases by Age and Gender, Race/Ethnicity, and Symptoms. Centers for Disease Control and Prevention. Updated March 15, 2023. Accessed March 21, 2023. https://www.cdc.gov/poxvirus/monkeypox /response/2022/demographics.html
- Kawsar A, Hussain K, Roberts N. The return of monkeypox: key pointers for dermatologists [published online July 29, 2022]. Clin Exp Dermatol. doi:10.1111/ced.15357
- Khanna U, Bishnoi A, Vinay K. Current outbreak of monkeypox— essentials for the dermatologist [published online June 23, 2022]. J Am Acad Dermatol. doi:10.1016/j.jaad.2022.06.1170
- Fox T, Gould S, Princy N, et al. Therapeutics for treating mpox in humans. Cochrane Database Syst Rev. 2023;3:CD015769. doi:10.1002/14651858 .CD015769
- Centers for Disease Control and Prevention. Treatment information for healthcare professionals. Updated March 3, 2023. Accessed March 24, 2023. https://www.cdc.gov/poxvirus/mpox/clinicians /treatment.html#anchor_1666886364947
- Centers for Disease Control and Prevention. Guidance for tecovirimat use. Updated February 23, 2023. Accessed March 24, 2023. https://www .cdc.gov/poxvirus/mpox/clinicians/Tecovirimat.html
- Interim Clinical Considerations for Use of JYNNEOS and ACAM2000 Vaccines During the 2022 U.S. Monkeypox Outbreak. Centers for Disease Control and Prevention. Updated October 19, 2022. Accessed March 21, 2023. https://www.cdc.gov/poxvirus/monkeypox/health-departments/vaccine-considerations.html
- Key Facts About Vaccines to Prevent Monkeypox Disease. US Food and Drug Administration. Updated August 18, 2022. Accessed March 21, 2023. https://www.fda.gov/vaccines-blood-biologics/vaccines/key-facts-aboutvaccines-prevent-monkeypox-disease
- Smallpox: Vaccines. Centers for Disease Control and Prevention. Updated August 8, 2022. Accessed March 21, 2023. https://www.cdc.gov/smallpox/clinicians/vaccines.html
- ACAM2000. Package insert. Emergent Product Development Gaithersburg Inc; 2019.
- Cices A, Prasad S, Akselrad M, et al. Mpox update: clinical presentation, vaccination guidance, and management. Cutis. 2023;111:197-202. doi:10.12788/cutis.0745
Lip Reconstruction After Mohs Micrographic Surgery: A Guide on Flaps
The lip is commonly affected by skin cancer because of increased sun exposure and actinic damage, with basal cell carcinoma typically occurring on the upper lip and squamous cell carcinoma (SCC) on the lower lip. The risk for metastatic spread of SCC on the lip is higher than cutaneous SCC on other facial locations but lower than SCC of the oral mucosa.1,2 If the tumor is operable and the patient has no contraindications to surgery, Mohs micrographic surgery is the preferred treatment, as it allows for maximal healthy tissue preservation and has the lowest recurrence rates.1-3 Once the tumor is removed and margins are confirmed to be negative, one must consider the options for defect closure, including healing by secondary intention, primary/direct closure, full-thickness skin grafts, local flaps, or free flaps.4 Secondary intention may lead to wound contracture and suboptimal functional and cosmetic outcomes. Primary wedge closure can be utilized for optimal functional and cosmetic outcomes when the defect involves less than one-third of the horizontal width of the vermilion. For larger defects, the surgeon must consider a flap or graft. Skin grafts are less favorable than local flaps because they may have different skin color, texture, and hair-bearing properties than the recipient area.3,5 In addition, grafts require a separate donor site, which means more pain, recovery time, and risk for complications for the patient.3 Free flaps similarly utilize tissue and blood supply from a donor site to repair major tissue loss. Radial forearm free flaps commonly are used for large lip defects but are more extensive, risky, and costly compared to local flaps for smaller defects under local anesthesia or nerve blocks.6,7 With these considerations, a local lip flap often is the most ideal repair method.
When performing a local lip flap, it is important to consider the functional and aesthetic aspects of the lips. The lower face is more susceptible to distortion and wound contraction after defect repair because it lacks a substantial supportive fibrous network. The dynamics of opposing lip elevator and depressor muscles make the lips a visual focal point and a crucial structure for facial expression, mastication, oral continence, speech phonation, and mouth opening and closing.2,4,8,9 Aesthetics and symmetry of the lips also are a large part of facial recognition and self-image.9
Lip defects are classified as partial thickness involving skin and muscle or full thickness involving skin, muscle, and mucosa. Partial-thickness wounds less than one-third the width of the horizontal lip can be repaired with a primary wedge resection or left to heal by secondary intention if the defect only involves the superficial vermilion.2 For defects larger than one-third the width of the horizontal lip, local flaps are favored to allow for closely matched skin and lip mucosa to fill in the defect.9 Full-thickness defects are further classified based on defect width compared to total lip width (ie, less than one-third, between one-third and two-thirds, and greater than two-thirds) as well as location (ie, medial, lateral, upper lip, lower lip).2,10
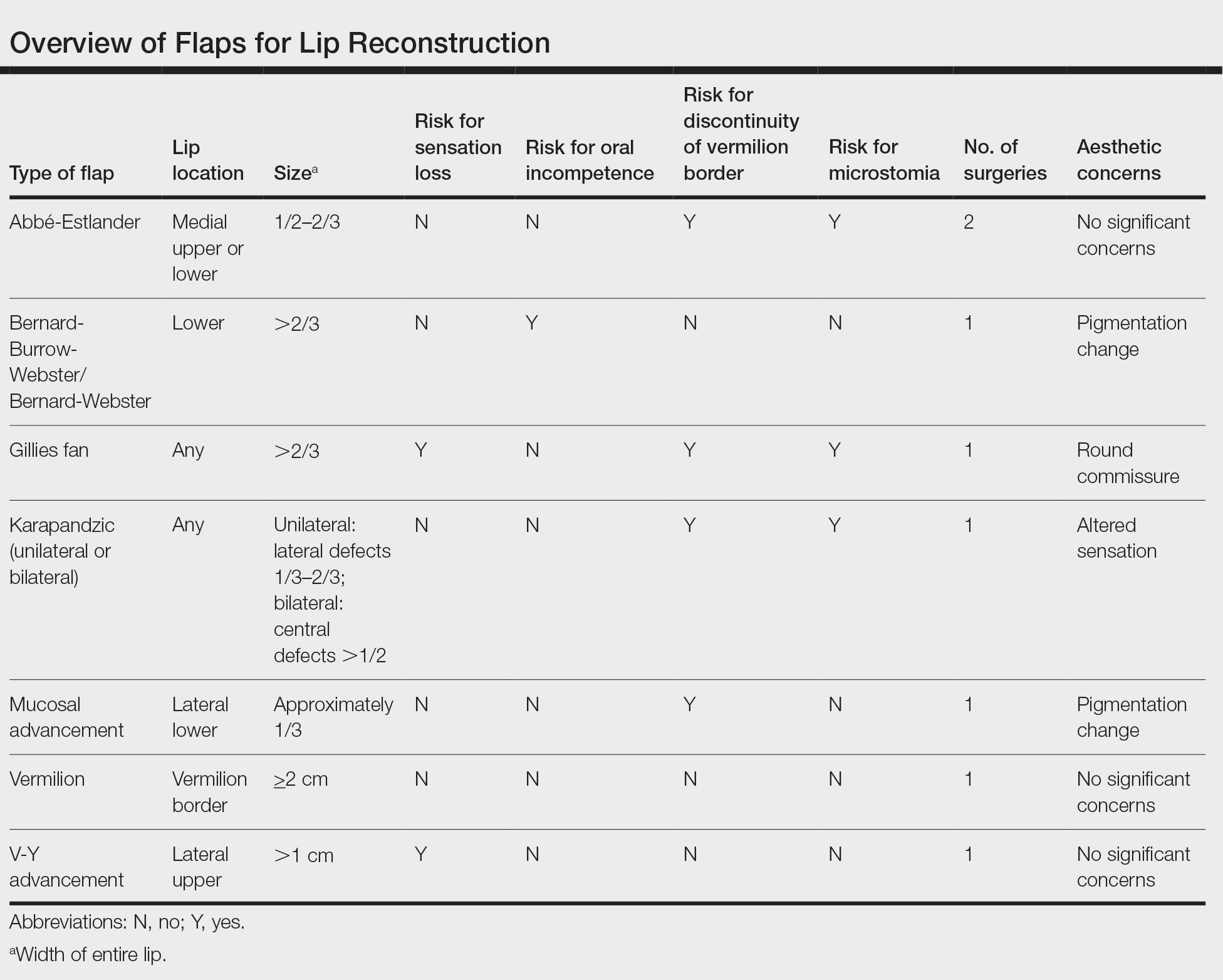
There are several local lip flap reconstruction options available, and choosing one is based on defect size and location. We provide a succinct review of the indications, risks, and benefits of commonly utilized flaps (Table), as well as artist renderings of all of the flaps (Figure).
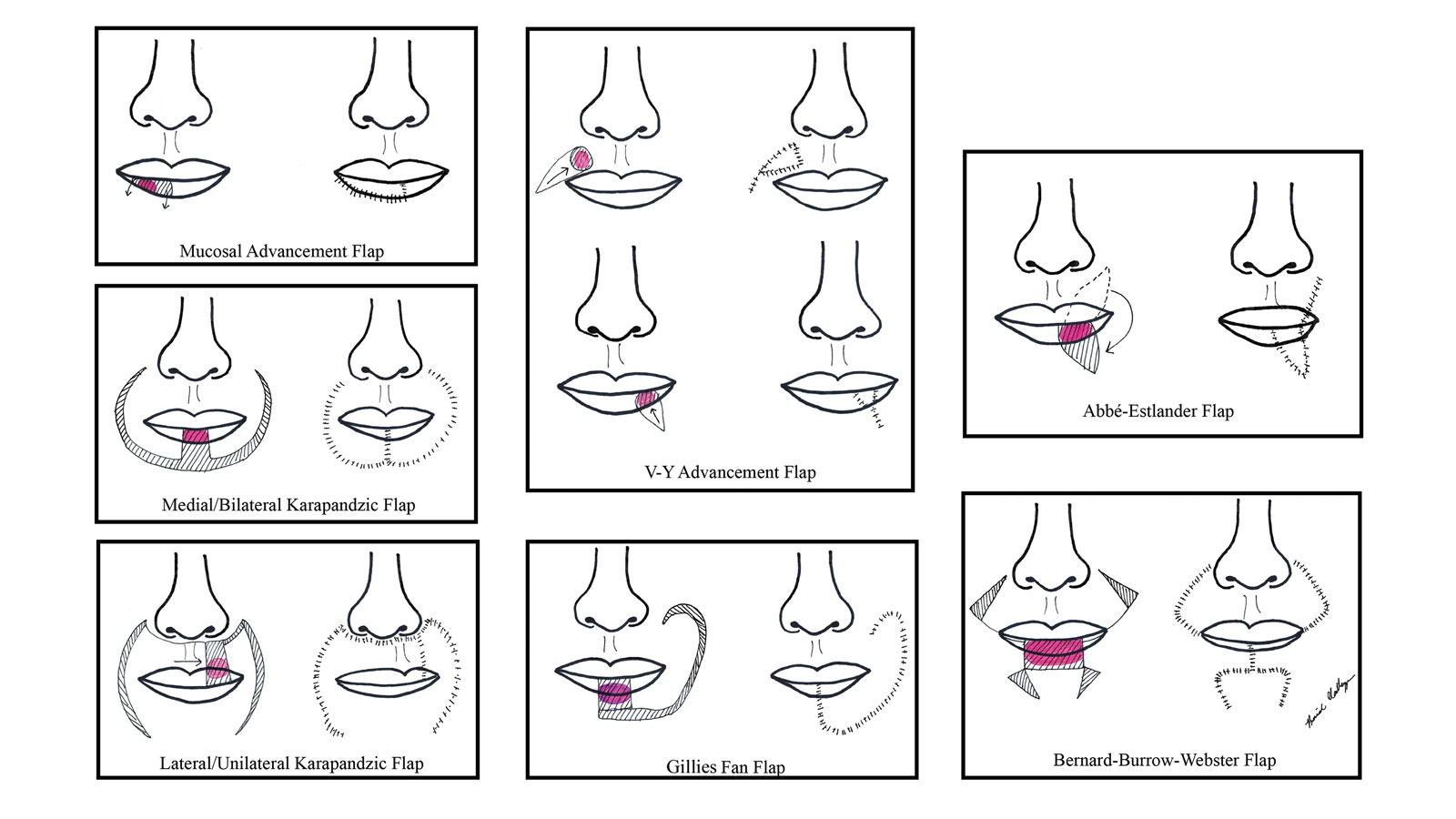
Vermilion Flaps
Vermilion flaps are used to close partial-thickness defects of the vermilion border, an area that poses unique obstacles of repair with blending distant tissues to match the surroundings.8 Goldstein11 developed an adjacent ipsilateral vermilion flap utilizing an arterialized myocutaneous flap for reconstruction of vermilion defects. Later, this technique was modified by Sawada et al12 into a bilateral adjacent advancement flap for closure of central vermilion defects and may be preferred for defects 2 cm in size or larger. Bilateral flaps are smaller and therefore more viable than unilateral or larger flaps, allowing for a more aesthetic alignment of the vermilion border and preservation of muscle activity because muscle fibers are not cut. This technique also allows for more efficient stretching or medial advancement of the tissue while generating less tension on the distal flap portions. Burow triangles can be utilized if necessary for improved aesthetic outcome.1
Mucosal Advancement and Split Myomucosal Advancement Flap
The mucosal advancement technique can be considered for tumors that do not involve the adjacent cutaneous skin or the orbicularis oris muscle; thus, the reconstruction involves only the superficial vermilion area.7,13 Mucosal incisions are made at the gingivobuccal sulcus, and the mucosal flap is elevated off the orbicularis oris muscle and advanced into the defect.10 A plane of dissection is maintained while preserving the labial artery. Undermining effectively advances wet mucosa into the dry mucosal lip to create a neovermilion. However, the reconstructed lip often appears thinner and will possibly be a different shade compared to the adjacent native lip. These discrepancies become more evident with deeper defects.7
There is a risk for cosmetic distortion and scar contraction with advancing the entire mucosa. Eirís et al13 described a solution—a bilateral mucosal rotation flap in which the primary incision is made along the entire vermilion border and tissue is undermined to allow advancement of the mucosa. Because the wound closure tension lays across the entire lip, there is less risk for scar contraction, even if the flap movement is unequal on either side of the defect.13
Although mucosal advancement flaps are a classic choice for reconstruction following a vermilion defect, other techniques, such as primary closure, should be considered in elderly patients and patients taking anticoagulants because of the risks for flap necrosis, swelling, bruising, hematoma, and dysesthesia, as well as a decrease in the anterior-posterior dimension of the lip. These risks can be attributed to trauma of surrounding tissue and stress secondary to longer overall operating times.14
Split myomucosal advancement flaps are used in similar scenarios as myomucosal advancement flaps but for larger red lip defects that are less than 50% the length of the upper or lower lip. Split myomucosal advancement flaps utilize an axial flap based on the labial artery, which provides robust vascular supply to the reconstructed area. This vascularity, along with lateral motor innervation of the orbicularis oris, allows for split myomucosal advancement flaps to restore the resected volume, preserve lip function, and minimize postoperative microstomia.7
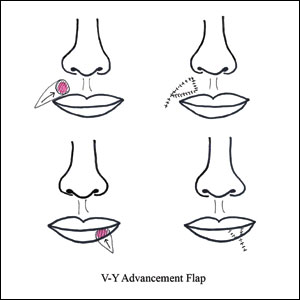
V-Y Advancement Flaps
V-Y advancement flaps are based on a subcutaneous tissue pedicle and are optimal for partial- and full-thickness defects larger than 1 cm on the lateral upper lips, whereas bilateral V-Y advancement flaps are recommended for central lip defects.15-17 Advantages of V-Y advancement flaps are preserved facial symmetry and maintenance of the oral sphincter and facial nerve function. The undermining portions allow for advancement of a skin flap of similar thickness and contour into the upper or lower lip.15 Disadvantages include facial asymmetry with larger defects involving the melolabial fold as well as paresthesia after closure. However, in one study, no paresthesia was reported more than 12 months postprocedure.4 The biggest disadvantage of the V-Y advancement flap is the kite-shaped scar and possible trapdoor deformity.5,15 When working medially, the addition of the pincer modification helps avoid blunting of the philtrum and recreates a Cupid’s bow by curling the lateral flap edges medially to resemble a teardrop shape.17 V-Y advancement flaps for defects of skin and adipose tissue less than 5 mm in size have the highest need for revision surgery; thus, defects of this small size should be repaired primarily.4
When using a V-Y advancement flap to correct large defects, there are 3 common complications that may arise: fullness medial to the commissure, a depressed vermilion lip, and a standing cutaneous deformity along the trailing edge of the flap where the Y is formed upon closure of the donor site. To decrease the fullness, a skin excision from the inferior border of the flap along the vermiliocutaneous border can be made to debulk the area. A vermilion advancement can be used to optimize the vermiliocutaneous junction. Potential standing cutaneous deformity is addressed by excising a small ellipse of skin oriented along the axis of the relaxed skin tension lines.15
Abbé-Estlander Flap
The Abbé-Estlander flap (also known as a transoral cross-lip flap) is a full-thickness myocutaneous interpolation flap with blood supply from the labial artery. It is used for lower lip tumors that have deep invasion into muscle and are 30% to 60% of the horizontal lip.8,9 Abbé transposition flaps are used for defects medial to the oral commissure and are best suited for philtrum reconstruction, whereas Estlander flaps are for defects that involve the oral commissure.9,18 Interpolation flaps usually are performed in 2 stages, but some dermatologic surgeons have reported success with single-stage procedures.1 The second-stage division usually is performed 2 to 3 weeks after flap insetting to allow time for neovascularization, which is crucial for pedicle survival.8,9,19
Advantages of this type of flap are the preservation of orbicularis oris strength and a functional and aesthetic result with minimal change in appearance for defects sized from one-third to two-thirds the width of the lip.20 This aesthetic effect is particularly notable when the donor flap is taken from the mediolateral upper lip, allowing the scarred area to blend into the nasolabial fold.8 Disadvantages of this flap are a risk for microstomia, lip vermilion misalignment, and lip adhesion.21 It is important that patients are educated on the need for multiple surgeries when using this type of flap, as patients favor single-step procedures.1 The Abbé flap requires 2 surgeries, whereas the Estlander flap requires only 1. However, patients commonly require commissuroplasty with the Estlander flap alone.21
Gillies Fan Flap, Karapandzic Flap, Bernard-Webster Flap, and Bernard-Burrow-Webster Flap
The Gillies fan flap, Karapandzic flap, Bernard-Webster (BW) flap, and modified Bernard-Burrow-Webster flap are the likely choices for repair of lip defects that encompass more than two-thirds of the lip.9,10,22 The Karapandzic and BW flaps are the 2 most frequently used for reconstruction of larger lower lip defects and only require 1 surgery.
Upper lip full-thickness defects that are too big for an Abbé-Estlander flap are closed with the Gillies fan flap.18 These defects involve 70% to 80% of the horizontal lip.9 The Gillies fan flap design redistributes the remaining lip to provide similar tissue quality and texture to fill the large defects.9,23 Compared to Karapandzic and Bernard flaps, Gillies fan incision closures are hidden well in the nasolabial folds, and the degree of microstomy is decreased because of the rotation of the flaps. However, rotation of medial cheek flaps can distort the orbicular muscular fibers and the anatomy of the commissure, which may require repair with commissurotomy. Drawbacks include a risk for denervation that can result in temporary oral sphincter incompetence.23 The bilateral Gillies fan flap carries a risk for microstomy as well as misalignment of the lip vermilion and round commissures.21
The Karapandzic flap is similar to the Gillies fan flap but only involves the skin and mucosa.9 This flap can be used for lateral or medial upper lip defects greater than one-third the width of the entire lip. This single-procedure flap allows for labial continuity, preserved sensation, and motor function; however, microstomia and misalignment of the oral commissure are common.1,18,21 In a retrospective study by Nicholas et al,4 the only flap reported to have a poor functional outcome was the Karapandzic flap, with 3 patients reporting altered sensation and 1 patient reporting persistent stiffness while smiling.
The BW flap can be applied for extensive full-thickness defects greater than one-third the lower lip and for defects with limited residual lip. This flap also can be used in cases where only skin is excised, as the flap does not depend on reminiscent lip tissue for reconstruction of the new lower lip. Sensory function is maintained given adequate visualization and preservation of the local vascular, nervous, and muscular systems. Disadvantages of the BW flap include an incision notch in the region of the lower lip; blunting of the alveolobuccal sulcus; and functional deficits, such as lip incontinence to liquids during the postoperative period.21
The Bernard-Burrow-Webster flap is used for large lower lip defects and preserves the oral commissures by advancing adjacent cheek tissue and remaining lip tissue medially.10 It allows for larger site mobilization, but it is possible to see some resulting oral incontinence.1,10 The Burow wedge flap is a variant of the advancement flap, with the Burow triangle located lateral to the oral commissure. Caution must be taken to avoid intraoperative bleeding from the labial and angular arteries. In addition, there also may be downward displacement of the vermilion border.5
How to Choose a Flap
The orbicularis oris is a circular muscle that surrounds both the upper and lower lips. It is pulled into an oval, allowing for sphincter function by radially oriented muscles, all of which are innervated by the facial nerve. Other key anatomical structures of the lips include the tubercle (vermilion prominence), Cupid’s bow and philtrum, nasolabial folds, white roll, hair-bearing area, and vermilion border. The lips are divided into cutaneous, mucosal, and vermilion parts, with the vermilion area divided into dry/external and wet/internal areas. Sensation to the upper lip is provided by the maxillary division of the trigeminal nerve via the infraorbital nerve. The lower lip is innervated by the mandibular division of the trigeminal nerve via the inferior alveolar nerve. The labial artery, a branch of the facial artery, is responsible for blood supply to the lips.3,9 Because of the complex anatomy of the lips, careful reconstruction is crucial for functional and aesthetic preservation.
There are a variety of lip defect repairs, but all local flaps aim to preserve aesthetics and function. The Table summarizes the key risks and benefits of each flap. Local flap techniques can be used in combination for more complex defects.3 For example, Nadiminti et al19 described the combination of the Abbé flap and V-Y advancement flap to restore function and create a new symmetric nasolabial fold. Dermatologic surgeons will determine the most suitable technique based on tumor location, tumor stage or depth of invasion (partial or full thickness), and preservation of function and aesthetics.1
Other factors to consider when choosing a local flap are the patient’s age, tissue laxity, dentition/need for dentures, and any prior treatments.7 Scar revision surgery may be needed after reconstruction, especially with longer vertical scars in areas without other rhytides. In addition, paresthesia is common after Mohs micrographic surgery of the face; however, new neural networks are created postoperatively, and most paresthesia resolves within 1 year of the repair.4 Dermabrasion and Z-plasty also may be considered, as they have been shown to be successful in improving final outcomes.9 Overall, local flaps have risks for infection, flap necrosis, and bleeding, though the incidence is low in reconstructions of the face.
Final Thoughts
There are several mechanisms to repair upper and lower lip defects resulting from surgical removal of cutaneous cancers. This review of specific flaps used in lip reconstruction provides a comprehensive overview of indications, advantages, and disadvantages of available lip flaps.
- Goldman A, Wollina U, França K, et al. Lip repair after Mohs surgery for squamous cell carcinoma by bilateral tissue expanding vermillion myocutaneous flap (Goldstein technique modified by Sawada). Open Access Maced J Med Sci. 2018;6:93-95.
- Faulhaber J, Géraud C, Goerdt S, et al. Functional and aesthetic reconstruction of full-thickness defects of the lower lip after tumor resection: analysis of 59 cases and discussion of a surgical approach. Dermatol Surg. 2010;36:859-867.
- Skaria AM. The transposition advancement flap for repair of postsurgical defects on the upper lip. Dermatology. 2011;223:203-206.
- Nicholas MN, Liu A, Chan AR, et al. Postoperative outcomes of local skin flaps used in oncologic reconstructive surgery of the upper cutaneous lip: a systematic review. Dermatol Surg. 2021;47:1047-1051.
- Wu W, Ibrahimi OA, Eisen DB. Cheek advancement flap with retained standing cone for reconstruction of a defect involving the upper lip, nasal sill, alar insertion, and medial cheek. Dermatol Surg. 2012;38:1077-1082.
- Cook JL. The reconstruction of two large full-thickness wounds of the upper lip with different operative techniques: when possible, a local flap repair is preferable to reconstruction with free tissue transfer. Dermatol Surg. 2013;39:281-289.
- Glenn CJ, Adelson RT, Flowers FP. Split myomucosal advancement flap for reconstruction of a lower lip defect. Dermatol Surg. 2012;38:1725-1728.
- Hahn HJ, Kim HJ, Choi JY, et al. Transoral cross-lip (Abbé-Estlander) flap as a viable and effective reconstructive option in middle lower lip defect reconstruction. Ann Dermatol. 2017;29:210-214.
- Larrabee YC, Moyer JS. Reconstruction of Mohs defects of the lips and chin. Facial Plast Surg Clin North Am. 2017;25:427-442.
- Campos MA, Varela P, Marques C. Near-total lower lip reconstruction: combined Karapandzic and Bernard-Burrow-Webster flap. Acta Dermatovenerol Alp Pannonica Adriat. 2017;26:19-20.
- Goldstein MH. A tissue-expanding vermillion myocutaneous flap for lip repair. Plast Reconstr Surg. 1984;73:768–770.
- Sawada Y, Ara M, Nomura K. Bilateral vermilion flap—a modification of Goldstein’s technique. Int J Oral Maxillofac Surg. 1988;17:257–259.
- Eirís N, Suarez-Valladares MJ, Cocunubo Blanco HA, et al. Bilateral mucosal rotation flap for repair of lower lip defect. J Am Acad Dermatol. 2015;72:E81-E82.
- Sand M, Altmeyer P, Bechara FG. Mucosal advancement flap versus primary closure after vermilionectomy of the lower lip. Dermatol Surg. 2010;36:1987-1992.
- Griffin GR, Weber S, Baker SR. Outcomes following V-Y advancement flap reconstruction of large upper lip defects. Arch Facial Plast Surg. 2012;14:193-197.
- Zhang WC, Liu Z, Zeng A, et al. Repair of cutaneous and mucosal upper lip defects using double V-Y advancement flaps. J Cosmet Dermatol. 2020;19:211-217.
- Tolkachjov SN. Bilateral V-Y advancement flaps with pincer modification for re-creation of large philtrum lip defect. J Am Acad Dermatol. 2021;84:E187-E188.
- García de Marcos JA, Heras Rincón I, González Córcoles C, et al. Bilateral reverse Yu flap for upper lip reconstruction after oncologic resection. Dermatol Surg. 2014;40:193-196.
- Nadiminti H, Carucci JA. Repair of a through-and-through defect on the upper cutaneous lip. Dermatol Surg. 2014;40:58-61.
- Kumar A, Shetty PM, Bhambar RS, et al. Versatility of Abbe-Estlander flap in lip reconstruction—a prospective clinical study. J Clin Diagn Res. 2014;8:NC18-NC21.
- Denadai R, Raposo-Amaral CE, Buzzo CL, et al. Functional lower lip reconstruction with the modified Bernard-Webster flap. J Plast Reconstr Aesthet Surg. 2015;68:1522-1528.
- Salgarelli AC, Bellini P, Magnoni C, et al. Synergistic use of local flaps for total lower lip reconstruction. Dermatol Surg. 2011;37:1666-1670.
- Moreno-Ramirez D, Ferrandiz L, Vasquez-Chinchay F, et al. Uncompleted fan flap for full-thickness lower lip defect. Dermatol Surg. 2009;35:1426-1429.
The lip is commonly affected by skin cancer because of increased sun exposure and actinic damage, with basal cell carcinoma typically occurring on the upper lip and squamous cell carcinoma (SCC) on the lower lip. The risk for metastatic spread of SCC on the lip is higher than cutaneous SCC on other facial locations but lower than SCC of the oral mucosa.1,2 If the tumor is operable and the patient has no contraindications to surgery, Mohs micrographic surgery is the preferred treatment, as it allows for maximal healthy tissue preservation and has the lowest recurrence rates.1-3 Once the tumor is removed and margins are confirmed to be negative, one must consider the options for defect closure, including healing by secondary intention, primary/direct closure, full-thickness skin grafts, local flaps, or free flaps.4 Secondary intention may lead to wound contracture and suboptimal functional and cosmetic outcomes. Primary wedge closure can be utilized for optimal functional and cosmetic outcomes when the defect involves less than one-third of the horizontal width of the vermilion. For larger defects, the surgeon must consider a flap or graft. Skin grafts are less favorable than local flaps because they may have different skin color, texture, and hair-bearing properties than the recipient area.3,5 In addition, grafts require a separate donor site, which means more pain, recovery time, and risk for complications for the patient.3 Free flaps similarly utilize tissue and blood supply from a donor site to repair major tissue loss. Radial forearm free flaps commonly are used for large lip defects but are more extensive, risky, and costly compared to local flaps for smaller defects under local anesthesia or nerve blocks.6,7 With these considerations, a local lip flap often is the most ideal repair method.
When performing a local lip flap, it is important to consider the functional and aesthetic aspects of the lips. The lower face is more susceptible to distortion and wound contraction after defect repair because it lacks a substantial supportive fibrous network. The dynamics of opposing lip elevator and depressor muscles make the lips a visual focal point and a crucial structure for facial expression, mastication, oral continence, speech phonation, and mouth opening and closing.2,4,8,9 Aesthetics and symmetry of the lips also are a large part of facial recognition and self-image.9
Lip defects are classified as partial thickness involving skin and muscle or full thickness involving skin, muscle, and mucosa. Partial-thickness wounds less than one-third the width of the horizontal lip can be repaired with a primary wedge resection or left to heal by secondary intention if the defect only involves the superficial vermilion.2 For defects larger than one-third the width of the horizontal lip, local flaps are favored to allow for closely matched skin and lip mucosa to fill in the defect.9 Full-thickness defects are further classified based on defect width compared to total lip width (ie, less than one-third, between one-third and two-thirds, and greater than two-thirds) as well as location (ie, medial, lateral, upper lip, lower lip).2,10
There are several local lip flap reconstruction options available, and choosing one is based on defect size and location. We provide a succinct review of the indications, risks, and benefits of commonly utilized flaps (Table), as well as artist renderings of all of the flaps (Figure).

Vermilion Flaps
Vermilion flaps are used to close partial-thickness defects of the vermilion border, an area that poses unique obstacles of repair with blending distant tissues to match the surroundings.8 Goldstein11 developed an adjacent ipsilateral vermilion flap utilizing an arterialized myocutaneous flap for reconstruction of vermilion defects. Later, this technique was modified by Sawada et al12 into a bilateral adjacent advancement flap for closure of central vermilion defects and may be preferred for defects 2 cm in size or larger. Bilateral flaps are smaller and therefore more viable than unilateral or larger flaps, allowing for a more aesthetic alignment of the vermilion border and preservation of muscle activity because muscle fibers are not cut. This technique also allows for more efficient stretching or medial advancement of the tissue while generating less tension on the distal flap portions. Burow triangles can be utilized if necessary for improved aesthetic outcome.1
Mucosal Advancement and Split Myomucosal Advancement Flap
The mucosal advancement technique can be considered for tumors that do not involve the adjacent cutaneous skin or the orbicularis oris muscle; thus, the reconstruction involves only the superficial vermilion area.7,13 Mucosal incisions are made at the gingivobuccal sulcus, and the mucosal flap is elevated off the orbicularis oris muscle and advanced into the defect.10 A plane of dissection is maintained while preserving the labial artery. Undermining effectively advances wet mucosa into the dry mucosal lip to create a neovermilion. However, the reconstructed lip often appears thinner and will possibly be a different shade compared to the adjacent native lip. These discrepancies become more evident with deeper defects.7
There is a risk for cosmetic distortion and scar contraction with advancing the entire mucosa. Eirís et al13 described a solution—a bilateral mucosal rotation flap in which the primary incision is made along the entire vermilion border and tissue is undermined to allow advancement of the mucosa. Because the wound closure tension lays across the entire lip, there is less risk for scar contraction, even if the flap movement is unequal on either side of the defect.13
Although mucosal advancement flaps are a classic choice for reconstruction following a vermilion defect, other techniques, such as primary closure, should be considered in elderly patients and patients taking anticoagulants because of the risks for flap necrosis, swelling, bruising, hematoma, and dysesthesia, as well as a decrease in the anterior-posterior dimension of the lip. These risks can be attributed to trauma of surrounding tissue and stress secondary to longer overall operating times.14
Split myomucosal advancement flaps are used in similar scenarios as myomucosal advancement flaps but for larger red lip defects that are less than 50% the length of the upper or lower lip. Split myomucosal advancement flaps utilize an axial flap based on the labial artery, which provides robust vascular supply to the reconstructed area. This vascularity, along with lateral motor innervation of the orbicularis oris, allows for split myomucosal advancement flaps to restore the resected volume, preserve lip function, and minimize postoperative microstomia.7
V-Y Advancement Flaps
V-Y advancement flaps are based on a subcutaneous tissue pedicle and are optimal for partial- and full-thickness defects larger than 1 cm on the lateral upper lips, whereas bilateral V-Y advancement flaps are recommended for central lip defects.15-17 Advantages of V-Y advancement flaps are preserved facial symmetry and maintenance of the oral sphincter and facial nerve function. The undermining portions allow for advancement of a skin flap of similar thickness and contour into the upper or lower lip.15 Disadvantages include facial asymmetry with larger defects involving the melolabial fold as well as paresthesia after closure. However, in one study, no paresthesia was reported more than 12 months postprocedure.4 The biggest disadvantage of the V-Y advancement flap is the kite-shaped scar and possible trapdoor deformity.5,15 When working medially, the addition of the pincer modification helps avoid blunting of the philtrum and recreates a Cupid’s bow by curling the lateral flap edges medially to resemble a teardrop shape.17 V-Y advancement flaps for defects of skin and adipose tissue less than 5 mm in size have the highest need for revision surgery; thus, defects of this small size should be repaired primarily.4
When using a V-Y advancement flap to correct large defects, there are 3 common complications that may arise: fullness medial to the commissure, a depressed vermilion lip, and a standing cutaneous deformity along the trailing edge of the flap where the Y is formed upon closure of the donor site. To decrease the fullness, a skin excision from the inferior border of the flap along the vermiliocutaneous border can be made to debulk the area. A vermilion advancement can be used to optimize the vermiliocutaneous junction. Potential standing cutaneous deformity is addressed by excising a small ellipse of skin oriented along the axis of the relaxed skin tension lines.15
Abbé-Estlander Flap
The Abbé-Estlander flap (also known as a transoral cross-lip flap) is a full-thickness myocutaneous interpolation flap with blood supply from the labial artery. It is used for lower lip tumors that have deep invasion into muscle and are 30% to 60% of the horizontal lip.8,9 Abbé transposition flaps are used for defects medial to the oral commissure and are best suited for philtrum reconstruction, whereas Estlander flaps are for defects that involve the oral commissure.9,18 Interpolation flaps usually are performed in 2 stages, but some dermatologic surgeons have reported success with single-stage procedures.1 The second-stage division usually is performed 2 to 3 weeks after flap insetting to allow time for neovascularization, which is crucial for pedicle survival.8,9,19
Advantages of this type of flap are the preservation of orbicularis oris strength and a functional and aesthetic result with minimal change in appearance for defects sized from one-third to two-thirds the width of the lip.20 This aesthetic effect is particularly notable when the donor flap is taken from the mediolateral upper lip, allowing the scarred area to blend into the nasolabial fold.8 Disadvantages of this flap are a risk for microstomia, lip vermilion misalignment, and lip adhesion.21 It is important that patients are educated on the need for multiple surgeries when using this type of flap, as patients favor single-step procedures.1 The Abbé flap requires 2 surgeries, whereas the Estlander flap requires only 1. However, patients commonly require commissuroplasty with the Estlander flap alone.21
Gillies Fan Flap, Karapandzic Flap, Bernard-Webster Flap, and Bernard-Burrow-Webster Flap
The Gillies fan flap, Karapandzic flap, Bernard-Webster (BW) flap, and modified Bernard-Burrow-Webster flap are the likely choices for repair of lip defects that encompass more than two-thirds of the lip.9,10,22 The Karapandzic and BW flaps are the 2 most frequently used for reconstruction of larger lower lip defects and only require 1 surgery.
Upper lip full-thickness defects that are too big for an Abbé-Estlander flap are closed with the Gillies fan flap.18 These defects involve 70% to 80% of the horizontal lip.9 The Gillies fan flap design redistributes the remaining lip to provide similar tissue quality and texture to fill the large defects.9,23 Compared to Karapandzic and Bernard flaps, Gillies fan incision closures are hidden well in the nasolabial folds, and the degree of microstomy is decreased because of the rotation of the flaps. However, rotation of medial cheek flaps can distort the orbicular muscular fibers and the anatomy of the commissure, which may require repair with commissurotomy. Drawbacks include a risk for denervation that can result in temporary oral sphincter incompetence.23 The bilateral Gillies fan flap carries a risk for microstomy as well as misalignment of the lip vermilion and round commissures.21
The Karapandzic flap is similar to the Gillies fan flap but only involves the skin and mucosa.9 This flap can be used for lateral or medial upper lip defects greater than one-third the width of the entire lip. This single-procedure flap allows for labial continuity, preserved sensation, and motor function; however, microstomia and misalignment of the oral commissure are common.1,18,21 In a retrospective study by Nicholas et al,4 the only flap reported to have a poor functional outcome was the Karapandzic flap, with 3 patients reporting altered sensation and 1 patient reporting persistent stiffness while smiling.
The BW flap can be applied for extensive full-thickness defects greater than one-third the lower lip and for defects with limited residual lip. This flap also can be used in cases where only skin is excised, as the flap does not depend on reminiscent lip tissue for reconstruction of the new lower lip. Sensory function is maintained given adequate visualization and preservation of the local vascular, nervous, and muscular systems. Disadvantages of the BW flap include an incision notch in the region of the lower lip; blunting of the alveolobuccal sulcus; and functional deficits, such as lip incontinence to liquids during the postoperative period.21
The Bernard-Burrow-Webster flap is used for large lower lip defects and preserves the oral commissures by advancing adjacent cheek tissue and remaining lip tissue medially.10 It allows for larger site mobilization, but it is possible to see some resulting oral incontinence.1,10 The Burow wedge flap is a variant of the advancement flap, with the Burow triangle located lateral to the oral commissure. Caution must be taken to avoid intraoperative bleeding from the labial and angular arteries. In addition, there also may be downward displacement of the vermilion border.5
How to Choose a Flap
The orbicularis oris is a circular muscle that surrounds both the upper and lower lips. It is pulled into an oval, allowing for sphincter function by radially oriented muscles, all of which are innervated by the facial nerve. Other key anatomical structures of the lips include the tubercle (vermilion prominence), Cupid’s bow and philtrum, nasolabial folds, white roll, hair-bearing area, and vermilion border. The lips are divided into cutaneous, mucosal, and vermilion parts, with the vermilion area divided into dry/external and wet/internal areas. Sensation to the upper lip is provided by the maxillary division of the trigeminal nerve via the infraorbital nerve. The lower lip is innervated by the mandibular division of the trigeminal nerve via the inferior alveolar nerve. The labial artery, a branch of the facial artery, is responsible for blood supply to the lips.3,9 Because of the complex anatomy of the lips, careful reconstruction is crucial for functional and aesthetic preservation.
There are a variety of lip defect repairs, but all local flaps aim to preserve aesthetics and function. The Table summarizes the key risks and benefits of each flap. Local flap techniques can be used in combination for more complex defects.3 For example, Nadiminti et al19 described the combination of the Abbé flap and V-Y advancement flap to restore function and create a new symmetric nasolabial fold. Dermatologic surgeons will determine the most suitable technique based on tumor location, tumor stage or depth of invasion (partial or full thickness), and preservation of function and aesthetics.1

Other factors to consider when choosing a local flap are the patient’s age, tissue laxity, dentition/need for dentures, and any prior treatments.7 Scar revision surgery may be needed after reconstruction, especially with longer vertical scars in areas without other rhytides. In addition, paresthesia is common after Mohs micrographic surgery of the face; however, new neural networks are created postoperatively, and most paresthesia resolves within 1 year of the repair.4 Dermabrasion and Z-plasty also may be considered, as they have been shown to be successful in improving final outcomes.9 Overall, local flaps have risks for infection, flap necrosis, and bleeding, though the incidence is low in reconstructions of the face.
Final Thoughts
There are several mechanisms to repair upper and lower lip defects resulting from surgical removal of cutaneous cancers. This review of specific flaps used in lip reconstruction provides a comprehensive overview of indications, advantages, and disadvantages of available lip flaps.
The lip is commonly affected by skin cancer because of increased sun exposure and actinic damage, with basal cell carcinoma typically occurring on the upper lip and squamous cell carcinoma (SCC) on the lower lip. The risk for metastatic spread of SCC on the lip is higher than cutaneous SCC on other facial locations but lower than SCC of the oral mucosa.1,2 If the tumor is operable and the patient has no contraindications to surgery, Mohs micrographic surgery is the preferred treatment, as it allows for maximal healthy tissue preservation and has the lowest recurrence rates.1-3 Once the tumor is removed and margins are confirmed to be negative, one must consider the options for defect closure, including healing by secondary intention, primary/direct closure, full-thickness skin grafts, local flaps, or free flaps.4 Secondary intention may lead to wound contracture and suboptimal functional and cosmetic outcomes. Primary wedge closure can be utilized for optimal functional and cosmetic outcomes when the defect involves less than one-third of the horizontal width of the vermilion. For larger defects, the surgeon must consider a flap or graft. Skin grafts are less favorable than local flaps because they may have different skin color, texture, and hair-bearing properties than the recipient area.3,5 In addition, grafts require a separate donor site, which means more pain, recovery time, and risk for complications for the patient.3 Free flaps similarly utilize tissue and blood supply from a donor site to repair major tissue loss. Radial forearm free flaps commonly are used for large lip defects but are more extensive, risky, and costly compared to local flaps for smaller defects under local anesthesia or nerve blocks.6,7 With these considerations, a local lip flap often is the most ideal repair method.
When performing a local lip flap, it is important to consider the functional and aesthetic aspects of the lips. The lower face is more susceptible to distortion and wound contraction after defect repair because it lacks a substantial supportive fibrous network. The dynamics of opposing lip elevator and depressor muscles make the lips a visual focal point and a crucial structure for facial expression, mastication, oral continence, speech phonation, and mouth opening and closing.2,4,8,9 Aesthetics and symmetry of the lips also are a large part of facial recognition and self-image.9
Lip defects are classified as partial thickness involving skin and muscle or full thickness involving skin, muscle, and mucosa. Partial-thickness wounds less than one-third the width of the horizontal lip can be repaired with a primary wedge resection or left to heal by secondary intention if the defect only involves the superficial vermilion.2 For defects larger than one-third the width of the horizontal lip, local flaps are favored to allow for closely matched skin and lip mucosa to fill in the defect.9 Full-thickness defects are further classified based on defect width compared to total lip width (ie, less than one-third, between one-third and two-thirds, and greater than two-thirds) as well as location (ie, medial, lateral, upper lip, lower lip).2,10
There are several local lip flap reconstruction options available, and choosing one is based on defect size and location. We provide a succinct review of the indications, risks, and benefits of commonly utilized flaps (Table), as well as artist renderings of all of the flaps (Figure).

Vermilion Flaps
Vermilion flaps are used to close partial-thickness defects of the vermilion border, an area that poses unique obstacles of repair with blending distant tissues to match the surroundings.8 Goldstein11 developed an adjacent ipsilateral vermilion flap utilizing an arterialized myocutaneous flap for reconstruction of vermilion defects. Later, this technique was modified by Sawada et al12 into a bilateral adjacent advancement flap for closure of central vermilion defects and may be preferred for defects 2 cm in size or larger. Bilateral flaps are smaller and therefore more viable than unilateral or larger flaps, allowing for a more aesthetic alignment of the vermilion border and preservation of muscle activity because muscle fibers are not cut. This technique also allows for more efficient stretching or medial advancement of the tissue while generating less tension on the distal flap portions. Burow triangles can be utilized if necessary for improved aesthetic outcome.1
Mucosal Advancement and Split Myomucosal Advancement Flap
The mucosal advancement technique can be considered for tumors that do not involve the adjacent cutaneous skin or the orbicularis oris muscle; thus, the reconstruction involves only the superficial vermilion area.7,13 Mucosal incisions are made at the gingivobuccal sulcus, and the mucosal flap is elevated off the orbicularis oris muscle and advanced into the defect.10 A plane of dissection is maintained while preserving the labial artery. Undermining effectively advances wet mucosa into the dry mucosal lip to create a neovermilion. However, the reconstructed lip often appears thinner and will possibly be a different shade compared to the adjacent native lip. These discrepancies become more evident with deeper defects.7
There is a risk for cosmetic distortion and scar contraction with advancing the entire mucosa. Eirís et al13 described a solution—a bilateral mucosal rotation flap in which the primary incision is made along the entire vermilion border and tissue is undermined to allow advancement of the mucosa. Because the wound closure tension lays across the entire lip, there is less risk for scar contraction, even if the flap movement is unequal on either side of the defect.13
Although mucosal advancement flaps are a classic choice for reconstruction following a vermilion defect, other techniques, such as primary closure, should be considered in elderly patients and patients taking anticoagulants because of the risks for flap necrosis, swelling, bruising, hematoma, and dysesthesia, as well as a decrease in the anterior-posterior dimension of the lip. These risks can be attributed to trauma of surrounding tissue and stress secondary to longer overall operating times.14
Split myomucosal advancement flaps are used in similar scenarios as myomucosal advancement flaps but for larger red lip defects that are less than 50% the length of the upper or lower lip. Split myomucosal advancement flaps utilize an axial flap based on the labial artery, which provides robust vascular supply to the reconstructed area. This vascularity, along with lateral motor innervation of the orbicularis oris, allows for split myomucosal advancement flaps to restore the resected volume, preserve lip function, and minimize postoperative microstomia.7
V-Y Advancement Flaps
V-Y advancement flaps are based on a subcutaneous tissue pedicle and are optimal for partial- and full-thickness defects larger than 1 cm on the lateral upper lips, whereas bilateral V-Y advancement flaps are recommended for central lip defects.15-17 Advantages of V-Y advancement flaps are preserved facial symmetry and maintenance of the oral sphincter and facial nerve function. The undermining portions allow for advancement of a skin flap of similar thickness and contour into the upper or lower lip.15 Disadvantages include facial asymmetry with larger defects involving the melolabial fold as well as paresthesia after closure. However, in one study, no paresthesia was reported more than 12 months postprocedure.4 The biggest disadvantage of the V-Y advancement flap is the kite-shaped scar and possible trapdoor deformity.5,15 When working medially, the addition of the pincer modification helps avoid blunting of the philtrum and recreates a Cupid’s bow by curling the lateral flap edges medially to resemble a teardrop shape.17 V-Y advancement flaps for defects of skin and adipose tissue less than 5 mm in size have the highest need for revision surgery; thus, defects of this small size should be repaired primarily.4
When using a V-Y advancement flap to correct large defects, there are 3 common complications that may arise: fullness medial to the commissure, a depressed vermilion lip, and a standing cutaneous deformity along the trailing edge of the flap where the Y is formed upon closure of the donor site. To decrease the fullness, a skin excision from the inferior border of the flap along the vermiliocutaneous border can be made to debulk the area. A vermilion advancement can be used to optimize the vermiliocutaneous junction. Potential standing cutaneous deformity is addressed by excising a small ellipse of skin oriented along the axis of the relaxed skin tension lines.15
Abbé-Estlander Flap
The Abbé-Estlander flap (also known as a transoral cross-lip flap) is a full-thickness myocutaneous interpolation flap with blood supply from the labial artery. It is used for lower lip tumors that have deep invasion into muscle and are 30% to 60% of the horizontal lip.8,9 Abbé transposition flaps are used for defects medial to the oral commissure and are best suited for philtrum reconstruction, whereas Estlander flaps are for defects that involve the oral commissure.9,18 Interpolation flaps usually are performed in 2 stages, but some dermatologic surgeons have reported success with single-stage procedures.1 The second-stage division usually is performed 2 to 3 weeks after flap insetting to allow time for neovascularization, which is crucial for pedicle survival.8,9,19
Advantages of this type of flap are the preservation of orbicularis oris strength and a functional and aesthetic result with minimal change in appearance for defects sized from one-third to two-thirds the width of the lip.20 This aesthetic effect is particularly notable when the donor flap is taken from the mediolateral upper lip, allowing the scarred area to blend into the nasolabial fold.8 Disadvantages of this flap are a risk for microstomia, lip vermilion misalignment, and lip adhesion.21 It is important that patients are educated on the need for multiple surgeries when using this type of flap, as patients favor single-step procedures.1 The Abbé flap requires 2 surgeries, whereas the Estlander flap requires only 1. However, patients commonly require commissuroplasty with the Estlander flap alone.21
Gillies Fan Flap, Karapandzic Flap, Bernard-Webster Flap, and Bernard-Burrow-Webster Flap
The Gillies fan flap, Karapandzic flap, Bernard-Webster (BW) flap, and modified Bernard-Burrow-Webster flap are the likely choices for repair of lip defects that encompass more than two-thirds of the lip.9,10,22 The Karapandzic and BW flaps are the 2 most frequently used for reconstruction of larger lower lip defects and only require 1 surgery.
Upper lip full-thickness defects that are too big for an Abbé-Estlander flap are closed with the Gillies fan flap.18 These defects involve 70% to 80% of the horizontal lip.9 The Gillies fan flap design redistributes the remaining lip to provide similar tissue quality and texture to fill the large defects.9,23 Compared to Karapandzic and Bernard flaps, Gillies fan incision closures are hidden well in the nasolabial folds, and the degree of microstomy is decreased because of the rotation of the flaps. However, rotation of medial cheek flaps can distort the orbicular muscular fibers and the anatomy of the commissure, which may require repair with commissurotomy. Drawbacks include a risk for denervation that can result in temporary oral sphincter incompetence.23 The bilateral Gillies fan flap carries a risk for microstomy as well as misalignment of the lip vermilion and round commissures.21
The Karapandzic flap is similar to the Gillies fan flap but only involves the skin and mucosa.9 This flap can be used for lateral or medial upper lip defects greater than one-third the width of the entire lip. This single-procedure flap allows for labial continuity, preserved sensation, and motor function; however, microstomia and misalignment of the oral commissure are common.1,18,21 In a retrospective study by Nicholas et al,4 the only flap reported to have a poor functional outcome was the Karapandzic flap, with 3 patients reporting altered sensation and 1 patient reporting persistent stiffness while smiling.
The BW flap can be applied for extensive full-thickness defects greater than one-third the lower lip and for defects with limited residual lip. This flap also can be used in cases where only skin is excised, as the flap does not depend on reminiscent lip tissue for reconstruction of the new lower lip. Sensory function is maintained given adequate visualization and preservation of the local vascular, nervous, and muscular systems. Disadvantages of the BW flap include an incision notch in the region of the lower lip; blunting of the alveolobuccal sulcus; and functional deficits, such as lip incontinence to liquids during the postoperative period.21
The Bernard-Burrow-Webster flap is used for large lower lip defects and preserves the oral commissures by advancing adjacent cheek tissue and remaining lip tissue medially.10 It allows for larger site mobilization, but it is possible to see some resulting oral incontinence.1,10 The Burow wedge flap is a variant of the advancement flap, with the Burow triangle located lateral to the oral commissure. Caution must be taken to avoid intraoperative bleeding from the labial and angular arteries. In addition, there also may be downward displacement of the vermilion border.5
How to Choose a Flap
The orbicularis oris is a circular muscle that surrounds both the upper and lower lips. It is pulled into an oval, allowing for sphincter function by radially oriented muscles, all of which are innervated by the facial nerve. Other key anatomical structures of the lips include the tubercle (vermilion prominence), Cupid’s bow and philtrum, nasolabial folds, white roll, hair-bearing area, and vermilion border. The lips are divided into cutaneous, mucosal, and vermilion parts, with the vermilion area divided into dry/external and wet/internal areas. Sensation to the upper lip is provided by the maxillary division of the trigeminal nerve via the infraorbital nerve. The lower lip is innervated by the mandibular division of the trigeminal nerve via the inferior alveolar nerve. The labial artery, a branch of the facial artery, is responsible for blood supply to the lips.3,9 Because of the complex anatomy of the lips, careful reconstruction is crucial for functional and aesthetic preservation.
There are a variety of lip defect repairs, but all local flaps aim to preserve aesthetics and function. The Table summarizes the key risks and benefits of each flap. Local flap techniques can be used in combination for more complex defects.3 For example, Nadiminti et al19 described the combination of the Abbé flap and V-Y advancement flap to restore function and create a new symmetric nasolabial fold. Dermatologic surgeons will determine the most suitable technique based on tumor location, tumor stage or depth of invasion (partial or full thickness), and preservation of function and aesthetics.1

Other factors to consider when choosing a local flap are the patient’s age, tissue laxity, dentition/need for dentures, and any prior treatments.7 Scar revision surgery may be needed after reconstruction, especially with longer vertical scars in areas without other rhytides. In addition, paresthesia is common after Mohs micrographic surgery of the face; however, new neural networks are created postoperatively, and most paresthesia resolves within 1 year of the repair.4 Dermabrasion and Z-plasty also may be considered, as they have been shown to be successful in improving final outcomes.9 Overall, local flaps have risks for infection, flap necrosis, and bleeding, though the incidence is low in reconstructions of the face.
Final Thoughts
There are several mechanisms to repair upper and lower lip defects resulting from surgical removal of cutaneous cancers. This review of specific flaps used in lip reconstruction provides a comprehensive overview of indications, advantages, and disadvantages of available lip flaps.
- Goldman A, Wollina U, França K, et al. Lip repair after Mohs surgery for squamous cell carcinoma by bilateral tissue expanding vermillion myocutaneous flap (Goldstein technique modified by Sawada). Open Access Maced J Med Sci. 2018;6:93-95.
- Faulhaber J, Géraud C, Goerdt S, et al. Functional and aesthetic reconstruction of full-thickness defects of the lower lip after tumor resection: analysis of 59 cases and discussion of a surgical approach. Dermatol Surg. 2010;36:859-867.
- Skaria AM. The transposition advancement flap for repair of postsurgical defects on the upper lip. Dermatology. 2011;223:203-206.
- Nicholas MN, Liu A, Chan AR, et al. Postoperative outcomes of local skin flaps used in oncologic reconstructive surgery of the upper cutaneous lip: a systematic review. Dermatol Surg. 2021;47:1047-1051.
- Wu W, Ibrahimi OA, Eisen DB. Cheek advancement flap with retained standing cone for reconstruction of a defect involving the upper lip, nasal sill, alar insertion, and medial cheek. Dermatol Surg. 2012;38:1077-1082.
- Cook JL. The reconstruction of two large full-thickness wounds of the upper lip with different operative techniques: when possible, a local flap repair is preferable to reconstruction with free tissue transfer. Dermatol Surg. 2013;39:281-289.
- Glenn CJ, Adelson RT, Flowers FP. Split myomucosal advancement flap for reconstruction of a lower lip defect. Dermatol Surg. 2012;38:1725-1728.
- Hahn HJ, Kim HJ, Choi JY, et al. Transoral cross-lip (Abbé-Estlander) flap as a viable and effective reconstructive option in middle lower lip defect reconstruction. Ann Dermatol. 2017;29:210-214.
- Larrabee YC, Moyer JS. Reconstruction of Mohs defects of the lips and chin. Facial Plast Surg Clin North Am. 2017;25:427-442.
- Campos MA, Varela P, Marques C. Near-total lower lip reconstruction: combined Karapandzic and Bernard-Burrow-Webster flap. Acta Dermatovenerol Alp Pannonica Adriat. 2017;26:19-20.
- Goldstein MH. A tissue-expanding vermillion myocutaneous flap for lip repair. Plast Reconstr Surg. 1984;73:768–770.
- Sawada Y, Ara M, Nomura K. Bilateral vermilion flap—a modification of Goldstein’s technique. Int J Oral Maxillofac Surg. 1988;17:257–259.
- Eirís N, Suarez-Valladares MJ, Cocunubo Blanco HA, et al. Bilateral mucosal rotation flap for repair of lower lip defect. J Am Acad Dermatol. 2015;72:E81-E82.
- Sand M, Altmeyer P, Bechara FG. Mucosal advancement flap versus primary closure after vermilionectomy of the lower lip. Dermatol Surg. 2010;36:1987-1992.
- Griffin GR, Weber S, Baker SR. Outcomes following V-Y advancement flap reconstruction of large upper lip defects. Arch Facial Plast Surg. 2012;14:193-197.
- Zhang WC, Liu Z, Zeng A, et al. Repair of cutaneous and mucosal upper lip defects using double V-Y advancement flaps. J Cosmet Dermatol. 2020;19:211-217.
- Tolkachjov SN. Bilateral V-Y advancement flaps with pincer modification for re-creation of large philtrum lip defect. J Am Acad Dermatol. 2021;84:E187-E188.
- García de Marcos JA, Heras Rincón I, González Córcoles C, et al. Bilateral reverse Yu flap for upper lip reconstruction after oncologic resection. Dermatol Surg. 2014;40:193-196.
- Nadiminti H, Carucci JA. Repair of a through-and-through defect on the upper cutaneous lip. Dermatol Surg. 2014;40:58-61.
- Kumar A, Shetty PM, Bhambar RS, et al. Versatility of Abbe-Estlander flap in lip reconstruction—a prospective clinical study. J Clin Diagn Res. 2014;8:NC18-NC21.
- Denadai R, Raposo-Amaral CE, Buzzo CL, et al. Functional lower lip reconstruction with the modified Bernard-Webster flap. J Plast Reconstr Aesthet Surg. 2015;68:1522-1528.
- Salgarelli AC, Bellini P, Magnoni C, et al. Synergistic use of local flaps for total lower lip reconstruction. Dermatol Surg. 2011;37:1666-1670.
- Moreno-Ramirez D, Ferrandiz L, Vasquez-Chinchay F, et al. Uncompleted fan flap for full-thickness lower lip defect. Dermatol Surg. 2009;35:1426-1429.
- Goldman A, Wollina U, França K, et al. Lip repair after Mohs surgery for squamous cell carcinoma by bilateral tissue expanding vermillion myocutaneous flap (Goldstein technique modified by Sawada). Open Access Maced J Med Sci. 2018;6:93-95.
- Faulhaber J, Géraud C, Goerdt S, et al. Functional and aesthetic reconstruction of full-thickness defects of the lower lip after tumor resection: analysis of 59 cases and discussion of a surgical approach. Dermatol Surg. 2010;36:859-867.
- Skaria AM. The transposition advancement flap for repair of postsurgical defects on the upper lip. Dermatology. 2011;223:203-206.
- Nicholas MN, Liu A, Chan AR, et al. Postoperative outcomes of local skin flaps used in oncologic reconstructive surgery of the upper cutaneous lip: a systematic review. Dermatol Surg. 2021;47:1047-1051.
- Wu W, Ibrahimi OA, Eisen DB. Cheek advancement flap with retained standing cone for reconstruction of a defect involving the upper lip, nasal sill, alar insertion, and medial cheek. Dermatol Surg. 2012;38:1077-1082.
- Cook JL. The reconstruction of two large full-thickness wounds of the upper lip with different operative techniques: when possible, a local flap repair is preferable to reconstruction with free tissue transfer. Dermatol Surg. 2013;39:281-289.
- Glenn CJ, Adelson RT, Flowers FP. Split myomucosal advancement flap for reconstruction of a lower lip defect. Dermatol Surg. 2012;38:1725-1728.
- Hahn HJ, Kim HJ, Choi JY, et al. Transoral cross-lip (Abbé-Estlander) flap as a viable and effective reconstructive option in middle lower lip defect reconstruction. Ann Dermatol. 2017;29:210-214.
- Larrabee YC, Moyer JS. Reconstruction of Mohs defects of the lips and chin. Facial Plast Surg Clin North Am. 2017;25:427-442.
- Campos MA, Varela P, Marques C. Near-total lower lip reconstruction: combined Karapandzic and Bernard-Burrow-Webster flap. Acta Dermatovenerol Alp Pannonica Adriat. 2017;26:19-20.
- Goldstein MH. A tissue-expanding vermillion myocutaneous flap for lip repair. Plast Reconstr Surg. 1984;73:768–770.
- Sawada Y, Ara M, Nomura K. Bilateral vermilion flap—a modification of Goldstein’s technique. Int J Oral Maxillofac Surg. 1988;17:257–259.
- Eirís N, Suarez-Valladares MJ, Cocunubo Blanco HA, et al. Bilateral mucosal rotation flap for repair of lower lip defect. J Am Acad Dermatol. 2015;72:E81-E82.
- Sand M, Altmeyer P, Bechara FG. Mucosal advancement flap versus primary closure after vermilionectomy of the lower lip. Dermatol Surg. 2010;36:1987-1992.
- Griffin GR, Weber S, Baker SR. Outcomes following V-Y advancement flap reconstruction of large upper lip defects. Arch Facial Plast Surg. 2012;14:193-197.
- Zhang WC, Liu Z, Zeng A, et al. Repair of cutaneous and mucosal upper lip defects using double V-Y advancement flaps. J Cosmet Dermatol. 2020;19:211-217.
- Tolkachjov SN. Bilateral V-Y advancement flaps with pincer modification for re-creation of large philtrum lip defect. J Am Acad Dermatol. 2021;84:E187-E188.
- García de Marcos JA, Heras Rincón I, González Córcoles C, et al. Bilateral reverse Yu flap for upper lip reconstruction after oncologic resection. Dermatol Surg. 2014;40:193-196.
- Nadiminti H, Carucci JA. Repair of a through-and-through defect on the upper cutaneous lip. Dermatol Surg. 2014;40:58-61.
- Kumar A, Shetty PM, Bhambar RS, et al. Versatility of Abbe-Estlander flap in lip reconstruction—a prospective clinical study. J Clin Diagn Res. 2014;8:NC18-NC21.
- Denadai R, Raposo-Amaral CE, Buzzo CL, et al. Functional lower lip reconstruction with the modified Bernard-Webster flap. J Plast Reconstr Aesthet Surg. 2015;68:1522-1528.
- Salgarelli AC, Bellini P, Magnoni C, et al. Synergistic use of local flaps for total lower lip reconstruction. Dermatol Surg. 2011;37:1666-1670.
- Moreno-Ramirez D, Ferrandiz L, Vasquez-Chinchay F, et al. Uncompleted fan flap for full-thickness lower lip defect. Dermatol Surg. 2009;35:1426-1429.
Practice Points
- Even with early detection, many skin cancers on the lips require surgical removal with subsequent reconstruction.
- There are several local flap reconstruction options available, and some may be used in combination for more complex defects.
- The most suitable technique should be chosen based on tumor location, tumor stage or depth of invasion (partial or full thickness), and preservation of function and aesthetics.
Mpox Update: Clinical Presentation, Vaccination Guidance, and Management
The mpox (monkeypox) virus is a zoonotic orthopox DNA virus that results in a smallpoxlike illness.1 Vaccination against smallpox protects against other orthopox infections, including mpox; however, unlike smallpox, mpox is notable for a variety of not-yet-confirmed animal reservoirs.2 Mpox was first identified in Denmark in 1959 among nonhuman primates imported from Singapore, and the first case of human infection was diagnosed in 1970 in a 9-month-old child in the Democratic Republic of Congo.3 Endemic regions of Africa have had sporadic outbreaks with increasing frequency over time since the cessation of smallpox vaccination in 1980.2,4 Infections in nonendemic countries have occurred intermittently, including in 2003 in the Midwest United States. This outbreak was traced back to prairie dogs infected by exotic animals imported from the Republic of Ghana.5
Two genetic clades of mpox that differ in mortality rates have been identified: clade II (formerly the West African clade) generally is self-limited with an estimated mortality of 1% to 6%, whereas clade I (formerly the Congo Basin clade) is more transmissible, with a mortality of approximately 10%.2,6,7 Notably, as of May 2, 2022, all polymerase chain reaction–confirmed cases of mpox in nonendemic countries were identified as clade II.7 Following the continued international spread of mpox, the Director-General of the World Health Organization (WHO) declared the global outbreak a public health emergency of international concern on July 23, 2022.8 As of March 1, 2023, the Centers for Disease Control and Prevention (CDC) reports that there have been more than 86,000 cases of laboratory-confirmed mpox worldwide and 105 deaths, 89 of which occurred in nonendemic regions.9
Transmission of Mpox
In endemic countries, cases have been largely reported secondary to zoonotic spillover from contact with an infected animal.6 However, in nonendemic countries, mpox often results from human-to-human transmission, primarily via skin-to-skin contact with infected skin, but also may occur indirectly via contaminated fomites such as bedding or clothing, respiratory secretions, or vertical transmission.6,10 The indirect transmission of mpox via contaminated fomites is controversial, though some studies have shown the virus can survive on surfaces for up to 15 days.11 In the current outbreak, human-to-human transmission has been strongly associated with close contact during sexual activity, particularly among men who have sex with men (MSM), with notable physical concentration of initial lesions in the genital region.12 Anyone can acquire mpox—infections are not exclusive to MSM populations, and cases have been reported in all demographic groups, including women and children. It is important to avoid stigmatization of MSM to prevent the propagation of homophobia as well as a false sense of complacency in non-MSM populations.13
Clinical Presentation of Mpox
The incubation period of mpox has been reported to last up to 21 days and is posited to depend on the mode of transmission, with complex invasive exposures having a shorter duration of approximately 9 days compared to noninvasive exposures, which have a duration of approximately 13 days.14 In a recent report from the Netherlands, the average incubation time was 8.5 days in 18 men with exposure attributed to sexual encounters with men.12 Following the incubation period, mpox infection typically presents with nonspecific systemic symptoms such as fever, malaise, sore throat, cough, and headache for approximately 2 days, followed by painful generalized or localized lymphadenopathy 1 to 2 days prior to the onset of skin lesions.1,15 In a recent report from Portugal of more than 20 confirmed cases of mpox, approximately half of patients denied symptoms or had mild systemic symptoms, suggesting that many patients in the current outbreak do not endorse systemic symptoms.16
Classic cutaneous lesions are the hallmark feature of mpox.17 Over a period of 1 to 2 weeks, each lesion progresses through morphologic stages of macule, papule (Figure), vesicle, and pustule, which then crusts over, forming a scab that falls off after another 1 to 2 weeks and can result in dyspigmented or pitted scars.1,15 Lesions may be deep-seated or umbilicated; previously they were noted to typically start on the face and spread centrifugally, but recent cases have been notable for a predominance of anogenital lesions, often with the anogenital area as the sole or primary area of involvement.18 Given the high proportion of anogenital lesions in 2022, symptoms such as anogenital pain, tenesmus, and diarrhea are not uncommon.19 A recent study describing 528 international cases of mpox revealed that 95% of patients presented with a rash; nearly 75% had anogenital lesions; and 41%, 25%, and 10% had involvement of mucosae, the face, and palms/soles, respectively. More than half of patients had fewer than 10 lesions, and 10% presented with a single genital lesion.19
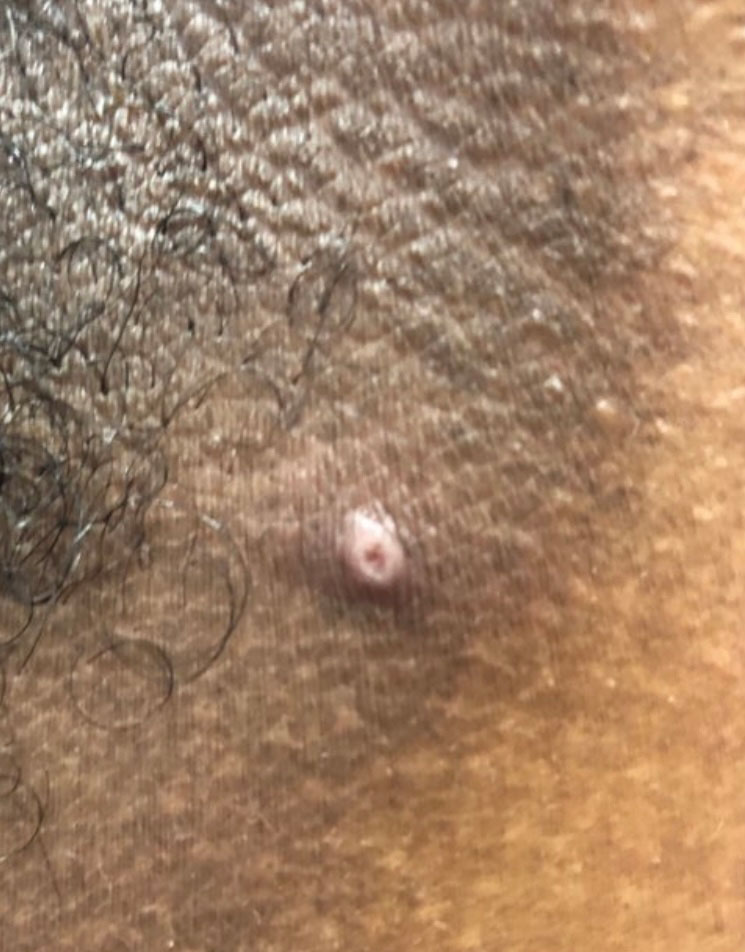
Given the recent predilection of lesions for the anogenital area, the differential diagnosis of mpox should include other common infections localized to these areas. Unlike herpes simplex and varicella-zoster infections, mpox does not exhibit the classic herpetiform clustering of vesicles, and unlike the painless chancre of syphilis, the lesions of mpox are exquisitely painful. Similar to chancroid, mpox presents with painful genital lesions and lymphadenopathy, and the umbilicated papules of molluscum could easily be confused with mpox lesions. Proctitis caused by many sexually transmitted infections (STIs), including chlamydia and gonorrhea, may be difficult to differentiate from proctitis symptoms of mpox. Co-infection with HIV and other STIs is common among patients developing mpox in 2022, which is not surprising given that the primary mechanism of transmission of mpox at this time is through sexual contact, and cases are more common in patients with multiple recent sexual partners.19 Considering these shared risk factors and similar presentation of multiple STIs, patients suspected of having an mpox infection should be tested for other STIs, including HIV.
Complications of Mpox
Although mpox generally is characterized by a mild disease course, there is concern for adverse outcomes, particularly in more vulnerable populations, including immunocompromised, pregnant, and pediatric populations. Complications of infection can include sepsis, encephalitis, bronchopneumonia, and ophthalmic complications that can result in loss of vision.6,17 The most common complications requiring hospitalization in a recent international report of 528 mpox cases were pain management, which was primarily due to severe anogenital pain, followed by soft-tissue superinfection, with other complications including severe pharyngitis limiting oral intake and infection control practices.19 In addition to severe rectal pain, proctitis and even rectal perforation have been reported.19,20
Vertical transmission has been described with devastating outcomes in a case series from the Democratic Republic of Congo, where 4 cases of mpox were identified in pregnant women; 3 of these pregnancies resulted in fetal demise.10 The only fetus to survive was born to a mother with mild infection. In comparison, 2 of 3 mothers with moderate to severe disease experienced spontaneous abortion in the first trimester, and 1 pregnancy ended due to intrauterine demise during the eighteenth week of gestation, likely a complication of mpox. These cases suggest that more severe disease may be linked to worse fetal outcomes.10 Further epidemiologic studies will be crucial, given the potential implications.
Diagnosis
When considering a diagnosis of mpox, clinicians should inquire about recent travel, living arrangements, sexual history, and recent sick contacts.6 A complete skin examination should include the oral and genital areas, given the high prevalence of lesions in these areas. A skin biopsy is not recommended for the diagnosis of mpox, as nonspecific viral changes cannot be differentiated from other viral exanthems, but it often is useful to rule out other differential diagnoses.21 Additionally, immunohistochemistry and electron microscopy can be utilized to aid in a histologic diagnosis of mpox.
Polymerase chain reaction detection of orthopox or mpox DNA is the gold standard for diagnosis.6 Two swabs should be collected from each lesion by swabbing vigorously using sterile swabs made of a synthetic material such as polyester, nylon, or Dacron and placed into a sterile container or viral transport medium.22 Some laboratories may have different instructions for collection of samples, so clinicians are advised to check for instructions from their local laboratory. Deroofing lesions prior to swabbing is not necessary, and specimens can include lesional material or crust. Collection of specimens from 2 to 3 lesions is recommended, preferably from different body areas or lesions with varying morphologies. Anal or rectal swabs can be considered in patients presenting with anal pain or proctitis with clinical suspicion for mpox based on history.19
Infection Prevention
Interim guidance from the WHO on November 16, 2022, reiterated the goal of outbreak control primarily via public health measures, which includes targeted use of vaccines for at-risk populations or postexposure prophylactic vaccination within 4 days, but heavily relies on surveillance and containment techniques, such as contact tracing with monitoring of contacts for onset of symptoms and isolation of cases through the complete infectious period.23 Patients are considered infectious from symptom onset until all cutaneous lesions are re-epithelized and should remain in isolation, including from household contacts and domestic and wildlife animals, for the duration of illness.24,25 Individuals exposed to humans or animals with confirmed mpox should be monitored for the development of symptoms for 21 days following last known exposure, regardless of vaccination status, and should be instructed to measure their temperature twice daily.26 Pets exposed to mpox should be isolated from other animals and humans for 21 days following last known contact.24 Vaccination strategies for preexposure and postexposure prophylaxis (PEP) are discussed below in further detail. Postinfection, the WHO suggests use of condoms for all oral, vaginal, and anal sexual activity for 12 weeks after recovery.7
Patients with suspected or confirmed mpox in a hospital should be in a single private room on special droplet and contact precautions.27 No special air handling or negative pressure isolation is needed unless the patient is undergoing an aerosol-generating procedure (eg, intubation, endoscopy, bronchoscopy). When hospitalized, patients should have a dedicated bathroom, if possible, and at-home patients should be isolated from household members until contagion risk resolves; this includes the use of a separate bathroom, when possible. Health care personnel entering the room of a patient should don appropriate personal protective equipment (PPE), including a disposable gown, gloves, eye protection, and N95 respirator or equivalent. Recommendations include standard practices for cleaning, with wet cleaning methods preferred over dry methods, using a disinfectant that covers emerging viral pathogens, and avoidance of shaking linens to prevent the spread of infectious particles.27 A variety of Environmental Protection Agency–registered wipes with virucidal activity against emerging viruses, including those with active ingredients such as quaternary ammonium, hydrogen peroxide, and hypochlorous acid, should be used for disinfecting surfaces.28
Vaccination
ACAM2000 (Emergent Bio Solutions) and JYNNEOS (Bavarian Nordic)(also known as Imvamune or Imvanex) are available in the United States for the prevention of mpox infection.29 ACAM2000, a second-generation, replication-competent, live smallpox vaccine administered as a single percutaneous injection, is contraindicated in immunocompromised populations, including patients with HIV or on immunosuppressive or biologic therapy, pregnant individuals, people with a history of atopic dermatitis or other exfoliative skin diseases with impaired barrier function, and patients with a history of cardiac disease due to the risk of myocarditis and pericarditis.30
JYNNEOS is a nonreplicating live vaccine approved by the US Food and Drug Administration (FDA) for the prevention of mpox in individuals older than 18 years administered as 2 subcutaneous doses 4 weeks apart. Patients are considered fully vaccinated 2 weeks after the second dose, and JYNNEOS is available to pediatric patients with a single patient expanded access use authorization from the FDA.29,30 More recently, the FDA issued an emergency use authorization (EUA) for administration of the vaccine to patients younger than 18 years who are at high risk of infection after exposure.31 More importantly, the FDA also issued an EUA for the intradermal administration of JYNNEOS at one-fifth of the subcutaneous dose to expand the current vaccine supply. This EUA is based on research by Frey et al,32 which showed that intradermal administration, even at a lower dose, elicited similar immune responses among study participants as the higher dose administered subcutaneously.
JYNNEOS is the preferred vaccine for the prevention of mpox because of its poor ability to replicate in human cells and resultant safety for use in populations that are immunocompromised, pregnant, or have skin barrier defects such as atopic dermatitis, without the risk of myocarditis or pericarditis. However, current supplies are limited. JYNNEOS was specifically studied in patients with atopic dermatitis and has been shown to be safe and effective in patients with a history of atopic dermatitis and active disease with a SCORAD (SCORing Atopic Dermatitis) score of 30 or lower.33 Of note, JYNNEOS is contraindicated in patients allergic to components of the vaccine, including egg, gentamicin, and ciprofloxacin. Although JYNNEOS is safe to administer to persons with immunocompromising conditions, the CDC reports that such persons might be at increased risk for severe disease if an occupational infection occurs, and in the setting of immunocompromise, such persons may be less likely to mount an effective response to vaccination. Therefore, the risk-benefit ratio should be considered to determine if an immunocompromised person should be vaccinated with JYNNEOS.30
The WHO and the CDC do not recommended mass vaccination of the general public for outbreaks of mpox in nonendemic countries, with immunization reserved for appropriate PEP and pre-exposure prophylaxis in intermediate- to high-risk individuals.23,26 The CDC recommends PEP vaccination for individuals with a high degree of exposure that includes unprotected contact of the skin or mucous membranes of an individual to the skin, lesions, body fluids, or contaminated fomites from a patient with mpox, as well as being within 6 feet of a patient during an aerosolization procedure without proper PPE. Following an intermediate degree of exposure, which includes being within 6 feet for 3 or more hours wearing at minimum a surgical mask or contact with fomites while wearing incomplete PPE, the CDC recommends monitoring and shared decision-making regarding risks and benefits of PEP vaccination. Monitoring without PEP is indicated for low and uncertain degrees of exposure, including entering a room without full PPE such as eye protection, regardless of the duration of contact.23,26
Postexposure prophylaxis vaccination should be administered within 4 days of a known high-level exposure to mpox to prevent infection.29 If administered within 4 to 14 days postexposure, vaccination may reduce disease severity but will not prevent infection.34
Pre-exposure prophylaxis is recommended for individuals at high risk for exposure to mpox, including health care workers such as laboratory personnel who handle mpox specimens and health care workers who administer ACAM2000 vaccinations or anticipate providing care for many patients with mpox.34
Management
Most cases of mpox are characterized by mild to moderate disease with a self-limited course. Most commonly, medical management of mpox involves supportive care such as fluid resuscitation, supplemental oxygen, and pain management.6 Treatment of superinfected skin lesions may require antibiotics. In the event of ophthalmologic involvement, patients should be referred to an ophthalmologist for further management.
Currently, there are no FDA-approved therapies for mpox; however, tecovirimat, cidofovir, brincidofovir, and vaccinia immune globulin intravenous are available under expanded access Investigational New Drug protocols.6,35 Human data for cidofovir, brincidofovir, and vaccinia immune globulin intravenous in the treatment of mpox are lacking, while cidofovir and brincidofovir have shown efficacy against orthopoxviruses in in vitro and animal studies, but are available therapeutic options.35
Tecovirimat is an antiviral that is FDA approved for smallpox with efficacy data against mpox in animal studies. It is the first-line treatment for patients with severe disease requiring hospitalization or 1 or more complications, including dehydration or secondary skin infections, as well as for populations at risk for severe disease, which includes immunocompromised patients, pediatric patients younger than 8 years, pregnant or breastfeeding individuals, or patients with a history of atopic dermatitis or active exfoliative skin conditions.36 In this current outbreak, both intravenous and oral tecovirimat are weight based in adult and pediatric patients for 14 days, with the intravenous form dosed every 12 hours by infusion over 6 hours, and the oral doses administered every 8 to 12 hours based on patient weight.37 Tecovirimat generally is well tolerated with mild side effects but is notably contraindicated in patients with severe renal impairment with a creatinine clearance less than 30 mL/min, and renal monitoring is indicated in pediatric patients younger than 2 years and in all patients receiving intravenous treatment.
Conclusion
Given that cutaneous lesions are the most specific presenting sign of mpox infection, dermatologists will play an integral role in identifying future cases and managing future outbreaks. Mpox should be considered in the differential diagnosis for all patients presenting with umbilicated or papulovesicular lesions, particularly in an anogenital distribution. The classic presentation of mpox may be more common among patients who are not considered high risk and have not been exposed via sexual activity. All patients with suspicious lesions should be managed following appropriate infection control precautions and should undergo molecular diagnostic assay of swabbed lesions to confirm the diagnosis. JYNNEOS is the only vaccine that is currently being distributed in the United States and is safe to administer to immunocompromised populations. The risks and benefits of vaccination should be considered on an individual basis between a patient and their provider. Taking into consideration that patients with atopic dermatitis are at risk for severe disease if infected with mpox, vaccination should be strongly encouraged if indicated based on patient risk factors. For atopic dermatitis patients treated with dupilumab, shared decision-making is essential given the FDA label, which recommends avoiding the use of live vaccines.38
The mpox epidemic occurring amidst the ongoing COVID-19 pandemic should serve as a wake-up call to the importance of pandemic preparedness and the global health response strategies in the modern era of globalization. Looking forward, widespread vaccination against mpox may be necessary to control the spread of the disease and to protect vulnerable populations, including pregnant individuals. In the current climate of hesitancy surrounding vaccines and the erosion of trust in public health agencies, it is incumbent upon health care providers to educate patients regarding the role of vaccines and public health measures to control this developing global health crisis.
- Di Giulio DB, Eckburg PB. Human monkeypox: an emerging zoonosis. Lancet Infect Dis. 2004;4:15-25. doi:10.1016/s1473-3099(03)00856-9
- Simpson K, Heymann D, Brown CS, et al. Human monkeypox—after 40 years, an unintended consequence of smallpox eradication. Vaccine. 2020;38:5077-5081. doi:10.1016/j.vaccine.2020.04.062
- Ladnyj ID, Ziegler P, Kima E. A human infection caused by monkeypox virus in Basankusu Territory, Democratic Republic of the Congo. Bull World Health Organ. 1972;46:593-597.
- Alakunle EF, Okeke MI. Monkeypox virus: a neglected zoonotic pathogen spreads globally. Nat Rev Microbiol. 2022;20:507-508. doi:10.1038/s41579-022-00776-z
- Ligon BL. Monkeypox: a review of the history and emergence in the Western hemisphere. Semin Pediatr Infect Dis. 2004;15:280-287. doi:10.1053/j.spid.2004.09.001
- Titanji BK, Tegomoh B, Nematollahi S, et al. Monkeypox: a contemporary review for healthcare professionals. Open Forum Infect Dis. 2022;9:ofac310. doi:10.1093/ofid/ofac310
- Gigante CM, Korber B, Seabolt MH, et al. Multiple lineages of monkeypox virus detected in the United States, 2021-2022. Science. 2022;378:560-565. doi:10.1126/science.add4153
- World Health Organization. WHO Director-General’s statement at the press conference following IHR Emergency Committee regarding the multi-country outbreak of monkeypox—23 July 2022. July 23, 2022. Accessed March 10, 2023. https://www.who.int/director-general/speeches/detail/who-director-general-s-statement-on-the-press-conference-following-IHR-emergency-committee-regarding-the-multi--country-outbreak-of-monkeypox--23-july-2022
- Centers for Disease Control and Prevention. 2022 mpox outbreak global map. Updated March 1, 2023. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/response/2022/world-map.html
- Mbala PK, Huggins JW, Riu-Rovira T, et al. Maternal and fetal outcomes among pregnant women with human monkeypox infection in the Democratic Republic of Congo. J Infect Dis. 2017;216:824-828. doi:10.1093/infdis/jix260
- Centers for Disease Control and Prevention. How to protect yourself. Updated October 31, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/prevention/protect-yourself.html
- Miura F, van Ewijk CE, Backer JA, et al. Estimated incubation period for monkeypox cases confirmed in the Netherlands, May 2022. Euro Surveill. 2022;27:2200448. doi:10.2807/1560-7917.Es.2022.27.24.2200448
- Treisman R. As monkeypox spreads, know the difference between warning and stigmatizing people. NPR. July 26, 2022. Accessed March 10, 2023. https://www.npr.org/2022/07/26/1113713684/monkeypox-stigma-gay-community
- Reynolds MG, Yorita KL, Kuehnert MJ, et al. Clinical manifestations of human monkeypox influenced by route of infection. J Infect Dis. 2006;194:773-780. doi:10.1086/505880
- Centers for Disease Control and Prevention. Clinical recognition. Updated August 23, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/clinical-recognition.html
- Alpalhão M, Frade JV, Sousa D, et al. Monkeypox: a new (sexuallytransmissible) epidemic? J Eur Acad Dermatol Venereol. 2022;36:e1016-e1017. doi:10.1111/jdv.18424
- Reynolds MG, McCollum AM, Nguete B, et al. Improving the care and treatment of monkeypox patients in low-resource settings: applying evidence from contemporary biomedical and smallpox biodefense research. Viruses. 2017;9:380. doi:10.3390/v9120380
- Minhaj FS, Ogale YP, Whitehill F, et al. Monkeypox outbreak—nine states, May 2022. MMWR Morb Mortal Wkly Rep. 2022;71:764-769. doi:10.15585/mmwr.mm7123e1
- Thornhill JP, Barkati S, Walmsley S, et al. Monkeypox virus infection in humans across 16 countries—April-June 2022. N Engl J Med. 2022;387:679-691. doi:10.1056/NEJMoa2207323
- Patel A, Bilinska J, Tam JCH, et al. Clinical features and novel presentations of human monkeypox in a central London centre during the 2022 outbreak: descriptive case series. BMJ. 2022;378:e072410. doi:10.1136/bmj-2022-072410
- Bayer-Garner IB. Monkeypox virus: histologic, immunohistochemical and electron-microscopic findings. J Cutan Pathol. 2005;32:28-34. doi:10.1111/j.0303-6987.2005.00254.x
- Centers for Disease Control and Prevention. Guidelines for collecting and handling of specimens for mpox testing. Updated September 20, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/prep-collection-specimens.html
- Vaccines and immunization for monkeypox: interim guidance, 16 November 2022. Accessed March 15, 2023. https://www.who.int/publications/i/item/WHO-MPX-Immunization
- Centers for Disease Control and Prevention. Pets in the home. Updated December 8, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/specific-settings/pets-in-homes.html
- Centers for Disease Control and Prevention. Isolation andprevention practices for people with monkeypox. Updated February 2, 2023. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/isolation-procedures.html
- Centers for Disease Control and Prevention. Monitoring people who have been exposed. Updated November 25, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/monitoring.html
- Centers for Disease Control and Prevention. Infection prevention and control of monkeypox in healthcare settings. Updated October 31, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/infection-control-healthcare.html
- United States Environmental Protection Agency. EPA releases list of disinfectants for emerging viral pathogens (EVPs) including monkeypox. May 26, 2022. Accessed March 10, 2023. https://www.epa.gov/pesticides/epa-releases-list-disinfectants-emerging-viral-pathogens-evps-including-monkeypox
- Centers for Disease Control and Prevention. Interim clinical considerations for use of JYNNEOS and ACAM2000 vaccines during the 2022 U.S. mpox outbreak. Updated October 19, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/considerations-for-monkeypox-vaccination.html
- Rao AK, Petersen BW, Whitehill F, et al. Use of JYNNEOS (smallpox and monkeypox vaccine, live, nonreplicating) for preexposure vaccination of persons at risk for occupational exposure to orthopoxviruses: recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:734-742. doi: http://dx.doi.org/10.15585/mmwr.mm7122e1
- US Food and Drug Administration. Monkeypox update: FDA authorizes emergency use of JYNNEOS vaccine to increase vaccine supply. August 9, 2022. Accessed March 10, 2023. https://www.fda.gov/news-events/press-announcements/monkeypox-update-fda-authorizes-emergency-use-jynneos-vaccine-increase-vaccine-supply#:~:text=Today%2C%20the%20U.S.%20Food%20and,high%20risk%20for%20monkeypox%20infection
- Frey SE, Wald A, Edupuganti S, et al. Comparison of lyophilized versus liquid modified vaccinia Ankara (MVA) formulations and subcutaneous versus intradermal routes of administration in healthy vaccinia-naïve subjects. Vaccine. 2015;33:5225-5234. doi:10.1016/j.vaccine.2015.06.075
- Greenberg RN, Hurley MY, Dinh DV, et al. A multicenter, open-label, controlled phase II study to evaluate safety and immunogenicity of MVA smallpox vaccine (IMVAMUNE) in 18-40 year old subjects with diagnosed atopic dermatitis. PLoS One. 2015;10:e0138348. doi:10.1371/journal.pone.0138348
- Centers for Disease Control and Prevention. Monkeypox and smallpox vaccine guidance. Accessed March 16, 2023. https://www.cdc.gov/poxvirus/mpox/interim-considerations/overview.html
- Centers for Disease Control and Prevention. Treatment information for healthcare professionals. Updated March 3, 2023. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/treatment.html
- Centers for Disease Control and Prevention. Guidance for tecovirimat use: expanded access investigational new drug protocol during 2022 U.S. mpox outbreak. Updated February 23, 2023. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/Tecovirimat.html
- Expanded access IND protocol: use of tecovirimat (TPOXX®) for treatment of human non-variola orthopoxvirus infections in adults and children. October 24, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/pdf/tecovirimat-ind-protocol-cdc-irb.pdf
- Dupixent (dupilumab). Prescribing information. Regeneron Pharmaceuticals, Inc; 2017. Accessed March 10, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761055lbl.pdf
The mpox (monkeypox) virus is a zoonotic orthopox DNA virus that results in a smallpoxlike illness.1 Vaccination against smallpox protects against other orthopox infections, including mpox; however, unlike smallpox, mpox is notable for a variety of not-yet-confirmed animal reservoirs.2 Mpox was first identified in Denmark in 1959 among nonhuman primates imported from Singapore, and the first case of human infection was diagnosed in 1970 in a 9-month-old child in the Democratic Republic of Congo.3 Endemic regions of Africa have had sporadic outbreaks with increasing frequency over time since the cessation of smallpox vaccination in 1980.2,4 Infections in nonendemic countries have occurred intermittently, including in 2003 in the Midwest United States. This outbreak was traced back to prairie dogs infected by exotic animals imported from the Republic of Ghana.5
Two genetic clades of mpox that differ in mortality rates have been identified: clade II (formerly the West African clade) generally is self-limited with an estimated mortality of 1% to 6%, whereas clade I (formerly the Congo Basin clade) is more transmissible, with a mortality of approximately 10%.2,6,7 Notably, as of May 2, 2022, all polymerase chain reaction–confirmed cases of mpox in nonendemic countries were identified as clade II.7 Following the continued international spread of mpox, the Director-General of the World Health Organization (WHO) declared the global outbreak a public health emergency of international concern on July 23, 2022.8 As of March 1, 2023, the Centers for Disease Control and Prevention (CDC) reports that there have been more than 86,000 cases of laboratory-confirmed mpox worldwide and 105 deaths, 89 of which occurred in nonendemic regions.9
Transmission of Mpox
In endemic countries, cases have been largely reported secondary to zoonotic spillover from contact with an infected animal.6 However, in nonendemic countries, mpox often results from human-to-human transmission, primarily via skin-to-skin contact with infected skin, but also may occur indirectly via contaminated fomites such as bedding or clothing, respiratory secretions, or vertical transmission.6,10 The indirect transmission of mpox via contaminated fomites is controversial, though some studies have shown the virus can survive on surfaces for up to 15 days.11 In the current outbreak, human-to-human transmission has been strongly associated with close contact during sexual activity, particularly among men who have sex with men (MSM), with notable physical concentration of initial lesions in the genital region.12 Anyone can acquire mpox—infections are not exclusive to MSM populations, and cases have been reported in all demographic groups, including women and children. It is important to avoid stigmatization of MSM to prevent the propagation of homophobia as well as a false sense of complacency in non-MSM populations.13
Clinical Presentation of Mpox
The incubation period of mpox has been reported to last up to 21 days and is posited to depend on the mode of transmission, with complex invasive exposures having a shorter duration of approximately 9 days compared to noninvasive exposures, which have a duration of approximately 13 days.14 In a recent report from the Netherlands, the average incubation time was 8.5 days in 18 men with exposure attributed to sexual encounters with men.12 Following the incubation period, mpox infection typically presents with nonspecific systemic symptoms such as fever, malaise, sore throat, cough, and headache for approximately 2 days, followed by painful generalized or localized lymphadenopathy 1 to 2 days prior to the onset of skin lesions.1,15 In a recent report from Portugal of more than 20 confirmed cases of mpox, approximately half of patients denied symptoms or had mild systemic symptoms, suggesting that many patients in the current outbreak do not endorse systemic symptoms.16
Classic cutaneous lesions are the hallmark feature of mpox.17 Over a period of 1 to 2 weeks, each lesion progresses through morphologic stages of macule, papule (Figure), vesicle, and pustule, which then crusts over, forming a scab that falls off after another 1 to 2 weeks and can result in dyspigmented or pitted scars.1,15 Lesions may be deep-seated or umbilicated; previously they were noted to typically start on the face and spread centrifugally, but recent cases have been notable for a predominance of anogenital lesions, often with the anogenital area as the sole or primary area of involvement.18 Given the high proportion of anogenital lesions in 2022, symptoms such as anogenital pain, tenesmus, and diarrhea are not uncommon.19 A recent study describing 528 international cases of mpox revealed that 95% of patients presented with a rash; nearly 75% had anogenital lesions; and 41%, 25%, and 10% had involvement of mucosae, the face, and palms/soles, respectively. More than half of patients had fewer than 10 lesions, and 10% presented with a single genital lesion.19

Given the recent predilection of lesions for the anogenital area, the differential diagnosis of mpox should include other common infections localized to these areas. Unlike herpes simplex and varicella-zoster infections, mpox does not exhibit the classic herpetiform clustering of vesicles, and unlike the painless chancre of syphilis, the lesions of mpox are exquisitely painful. Similar to chancroid, mpox presents with painful genital lesions and lymphadenopathy, and the umbilicated papules of molluscum could easily be confused with mpox lesions. Proctitis caused by many sexually transmitted infections (STIs), including chlamydia and gonorrhea, may be difficult to differentiate from proctitis symptoms of mpox. Co-infection with HIV and other STIs is common among patients developing mpox in 2022, which is not surprising given that the primary mechanism of transmission of mpox at this time is through sexual contact, and cases are more common in patients with multiple recent sexual partners.19 Considering these shared risk factors and similar presentation of multiple STIs, patients suspected of having an mpox infection should be tested for other STIs, including HIV.
Complications of Mpox
Although mpox generally is characterized by a mild disease course, there is concern for adverse outcomes, particularly in more vulnerable populations, including immunocompromised, pregnant, and pediatric populations. Complications of infection can include sepsis, encephalitis, bronchopneumonia, and ophthalmic complications that can result in loss of vision.6,17 The most common complications requiring hospitalization in a recent international report of 528 mpox cases were pain management, which was primarily due to severe anogenital pain, followed by soft-tissue superinfection, with other complications including severe pharyngitis limiting oral intake and infection control practices.19 In addition to severe rectal pain, proctitis and even rectal perforation have been reported.19,20
Vertical transmission has been described with devastating outcomes in a case series from the Democratic Republic of Congo, where 4 cases of mpox were identified in pregnant women; 3 of these pregnancies resulted in fetal demise.10 The only fetus to survive was born to a mother with mild infection. In comparison, 2 of 3 mothers with moderate to severe disease experienced spontaneous abortion in the first trimester, and 1 pregnancy ended due to intrauterine demise during the eighteenth week of gestation, likely a complication of mpox. These cases suggest that more severe disease may be linked to worse fetal outcomes.10 Further epidemiologic studies will be crucial, given the potential implications.
Diagnosis
When considering a diagnosis of mpox, clinicians should inquire about recent travel, living arrangements, sexual history, and recent sick contacts.6 A complete skin examination should include the oral and genital areas, given the high prevalence of lesions in these areas. A skin biopsy is not recommended for the diagnosis of mpox, as nonspecific viral changes cannot be differentiated from other viral exanthems, but it often is useful to rule out other differential diagnoses.21 Additionally, immunohistochemistry and electron microscopy can be utilized to aid in a histologic diagnosis of mpox.
Polymerase chain reaction detection of orthopox or mpox DNA is the gold standard for diagnosis.6 Two swabs should be collected from each lesion by swabbing vigorously using sterile swabs made of a synthetic material such as polyester, nylon, or Dacron and placed into a sterile container or viral transport medium.22 Some laboratories may have different instructions for collection of samples, so clinicians are advised to check for instructions from their local laboratory. Deroofing lesions prior to swabbing is not necessary, and specimens can include lesional material or crust. Collection of specimens from 2 to 3 lesions is recommended, preferably from different body areas or lesions with varying morphologies. Anal or rectal swabs can be considered in patients presenting with anal pain or proctitis with clinical suspicion for mpox based on history.19
Infection Prevention
Interim guidance from the WHO on November 16, 2022, reiterated the goal of outbreak control primarily via public health measures, which includes targeted use of vaccines for at-risk populations or postexposure prophylactic vaccination within 4 days, but heavily relies on surveillance and containment techniques, such as contact tracing with monitoring of contacts for onset of symptoms and isolation of cases through the complete infectious period.23 Patients are considered infectious from symptom onset until all cutaneous lesions are re-epithelized and should remain in isolation, including from household contacts and domestic and wildlife animals, for the duration of illness.24,25 Individuals exposed to humans or animals with confirmed mpox should be monitored for the development of symptoms for 21 days following last known exposure, regardless of vaccination status, and should be instructed to measure their temperature twice daily.26 Pets exposed to mpox should be isolated from other animals and humans for 21 days following last known contact.24 Vaccination strategies for preexposure and postexposure prophylaxis (PEP) are discussed below in further detail. Postinfection, the WHO suggests use of condoms for all oral, vaginal, and anal sexual activity for 12 weeks after recovery.7
Patients with suspected or confirmed mpox in a hospital should be in a single private room on special droplet and contact precautions.27 No special air handling or negative pressure isolation is needed unless the patient is undergoing an aerosol-generating procedure (eg, intubation, endoscopy, bronchoscopy). When hospitalized, patients should have a dedicated bathroom, if possible, and at-home patients should be isolated from household members until contagion risk resolves; this includes the use of a separate bathroom, when possible. Health care personnel entering the room of a patient should don appropriate personal protective equipment (PPE), including a disposable gown, gloves, eye protection, and N95 respirator or equivalent. Recommendations include standard practices for cleaning, with wet cleaning methods preferred over dry methods, using a disinfectant that covers emerging viral pathogens, and avoidance of shaking linens to prevent the spread of infectious particles.27 A variety of Environmental Protection Agency–registered wipes with virucidal activity against emerging viruses, including those with active ingredients such as quaternary ammonium, hydrogen peroxide, and hypochlorous acid, should be used for disinfecting surfaces.28
Vaccination
ACAM2000 (Emergent Bio Solutions) and JYNNEOS (Bavarian Nordic)(also known as Imvamune or Imvanex) are available in the United States for the prevention of mpox infection.29 ACAM2000, a second-generation, replication-competent, live smallpox vaccine administered as a single percutaneous injection, is contraindicated in immunocompromised populations, including patients with HIV or on immunosuppressive or biologic therapy, pregnant individuals, people with a history of atopic dermatitis or other exfoliative skin diseases with impaired barrier function, and patients with a history of cardiac disease due to the risk of myocarditis and pericarditis.30
JYNNEOS is a nonreplicating live vaccine approved by the US Food and Drug Administration (FDA) for the prevention of mpox in individuals older than 18 years administered as 2 subcutaneous doses 4 weeks apart. Patients are considered fully vaccinated 2 weeks after the second dose, and JYNNEOS is available to pediatric patients with a single patient expanded access use authorization from the FDA.29,30 More recently, the FDA issued an emergency use authorization (EUA) for administration of the vaccine to patients younger than 18 years who are at high risk of infection after exposure.31 More importantly, the FDA also issued an EUA for the intradermal administration of JYNNEOS at one-fifth of the subcutaneous dose to expand the current vaccine supply. This EUA is based on research by Frey et al,32 which showed that intradermal administration, even at a lower dose, elicited similar immune responses among study participants as the higher dose administered subcutaneously.
JYNNEOS is the preferred vaccine for the prevention of mpox because of its poor ability to replicate in human cells and resultant safety for use in populations that are immunocompromised, pregnant, or have skin barrier defects such as atopic dermatitis, without the risk of myocarditis or pericarditis. However, current supplies are limited. JYNNEOS was specifically studied in patients with atopic dermatitis and has been shown to be safe and effective in patients with a history of atopic dermatitis and active disease with a SCORAD (SCORing Atopic Dermatitis) score of 30 or lower.33 Of note, JYNNEOS is contraindicated in patients allergic to components of the vaccine, including egg, gentamicin, and ciprofloxacin. Although JYNNEOS is safe to administer to persons with immunocompromising conditions, the CDC reports that such persons might be at increased risk for severe disease if an occupational infection occurs, and in the setting of immunocompromise, such persons may be less likely to mount an effective response to vaccination. Therefore, the risk-benefit ratio should be considered to determine if an immunocompromised person should be vaccinated with JYNNEOS.30
The WHO and the CDC do not recommended mass vaccination of the general public for outbreaks of mpox in nonendemic countries, with immunization reserved for appropriate PEP and pre-exposure prophylaxis in intermediate- to high-risk individuals.23,26 The CDC recommends PEP vaccination for individuals with a high degree of exposure that includes unprotected contact of the skin or mucous membranes of an individual to the skin, lesions, body fluids, or contaminated fomites from a patient with mpox, as well as being within 6 feet of a patient during an aerosolization procedure without proper PPE. Following an intermediate degree of exposure, which includes being within 6 feet for 3 or more hours wearing at minimum a surgical mask or contact with fomites while wearing incomplete PPE, the CDC recommends monitoring and shared decision-making regarding risks and benefits of PEP vaccination. Monitoring without PEP is indicated for low and uncertain degrees of exposure, including entering a room without full PPE such as eye protection, regardless of the duration of contact.23,26
Postexposure prophylaxis vaccination should be administered within 4 days of a known high-level exposure to mpox to prevent infection.29 If administered within 4 to 14 days postexposure, vaccination may reduce disease severity but will not prevent infection.34
Pre-exposure prophylaxis is recommended for individuals at high risk for exposure to mpox, including health care workers such as laboratory personnel who handle mpox specimens and health care workers who administer ACAM2000 vaccinations or anticipate providing care for many patients with mpox.34
Management
Most cases of mpox are characterized by mild to moderate disease with a self-limited course. Most commonly, medical management of mpox involves supportive care such as fluid resuscitation, supplemental oxygen, and pain management.6 Treatment of superinfected skin lesions may require antibiotics. In the event of ophthalmologic involvement, patients should be referred to an ophthalmologist for further management.
Currently, there are no FDA-approved therapies for mpox; however, tecovirimat, cidofovir, brincidofovir, and vaccinia immune globulin intravenous are available under expanded access Investigational New Drug protocols.6,35 Human data for cidofovir, brincidofovir, and vaccinia immune globulin intravenous in the treatment of mpox are lacking, while cidofovir and brincidofovir have shown efficacy against orthopoxviruses in in vitro and animal studies, but are available therapeutic options.35
Tecovirimat is an antiviral that is FDA approved for smallpox with efficacy data against mpox in animal studies. It is the first-line treatment for patients with severe disease requiring hospitalization or 1 or more complications, including dehydration or secondary skin infections, as well as for populations at risk for severe disease, which includes immunocompromised patients, pediatric patients younger than 8 years, pregnant or breastfeeding individuals, or patients with a history of atopic dermatitis or active exfoliative skin conditions.36 In this current outbreak, both intravenous and oral tecovirimat are weight based in adult and pediatric patients for 14 days, with the intravenous form dosed every 12 hours by infusion over 6 hours, and the oral doses administered every 8 to 12 hours based on patient weight.37 Tecovirimat generally is well tolerated with mild side effects but is notably contraindicated in patients with severe renal impairment with a creatinine clearance less than 30 mL/min, and renal monitoring is indicated in pediatric patients younger than 2 years and in all patients receiving intravenous treatment.
Conclusion
Given that cutaneous lesions are the most specific presenting sign of mpox infection, dermatologists will play an integral role in identifying future cases and managing future outbreaks. Mpox should be considered in the differential diagnosis for all patients presenting with umbilicated or papulovesicular lesions, particularly in an anogenital distribution. The classic presentation of mpox may be more common among patients who are not considered high risk and have not been exposed via sexual activity. All patients with suspicious lesions should be managed following appropriate infection control precautions and should undergo molecular diagnostic assay of swabbed lesions to confirm the diagnosis. JYNNEOS is the only vaccine that is currently being distributed in the United States and is safe to administer to immunocompromised populations. The risks and benefits of vaccination should be considered on an individual basis between a patient and their provider. Taking into consideration that patients with atopic dermatitis are at risk for severe disease if infected with mpox, vaccination should be strongly encouraged if indicated based on patient risk factors. For atopic dermatitis patients treated with dupilumab, shared decision-making is essential given the FDA label, which recommends avoiding the use of live vaccines.38
The mpox epidemic occurring amidst the ongoing COVID-19 pandemic should serve as a wake-up call to the importance of pandemic preparedness and the global health response strategies in the modern era of globalization. Looking forward, widespread vaccination against mpox may be necessary to control the spread of the disease and to protect vulnerable populations, including pregnant individuals. In the current climate of hesitancy surrounding vaccines and the erosion of trust in public health agencies, it is incumbent upon health care providers to educate patients regarding the role of vaccines and public health measures to control this developing global health crisis.
The mpox (monkeypox) virus is a zoonotic orthopox DNA virus that results in a smallpoxlike illness.1 Vaccination against smallpox protects against other orthopox infections, including mpox; however, unlike smallpox, mpox is notable for a variety of not-yet-confirmed animal reservoirs.2 Mpox was first identified in Denmark in 1959 among nonhuman primates imported from Singapore, and the first case of human infection was diagnosed in 1970 in a 9-month-old child in the Democratic Republic of Congo.3 Endemic regions of Africa have had sporadic outbreaks with increasing frequency over time since the cessation of smallpox vaccination in 1980.2,4 Infections in nonendemic countries have occurred intermittently, including in 2003 in the Midwest United States. This outbreak was traced back to prairie dogs infected by exotic animals imported from the Republic of Ghana.5
Two genetic clades of mpox that differ in mortality rates have been identified: clade II (formerly the West African clade) generally is self-limited with an estimated mortality of 1% to 6%, whereas clade I (formerly the Congo Basin clade) is more transmissible, with a mortality of approximately 10%.2,6,7 Notably, as of May 2, 2022, all polymerase chain reaction–confirmed cases of mpox in nonendemic countries were identified as clade II.7 Following the continued international spread of mpox, the Director-General of the World Health Organization (WHO) declared the global outbreak a public health emergency of international concern on July 23, 2022.8 As of March 1, 2023, the Centers for Disease Control and Prevention (CDC) reports that there have been more than 86,000 cases of laboratory-confirmed mpox worldwide and 105 deaths, 89 of which occurred in nonendemic regions.9
Transmission of Mpox
In endemic countries, cases have been largely reported secondary to zoonotic spillover from contact with an infected animal.6 However, in nonendemic countries, mpox often results from human-to-human transmission, primarily via skin-to-skin contact with infected skin, but also may occur indirectly via contaminated fomites such as bedding or clothing, respiratory secretions, or vertical transmission.6,10 The indirect transmission of mpox via contaminated fomites is controversial, though some studies have shown the virus can survive on surfaces for up to 15 days.11 In the current outbreak, human-to-human transmission has been strongly associated with close contact during sexual activity, particularly among men who have sex with men (MSM), with notable physical concentration of initial lesions in the genital region.12 Anyone can acquire mpox—infections are not exclusive to MSM populations, and cases have been reported in all demographic groups, including women and children. It is important to avoid stigmatization of MSM to prevent the propagation of homophobia as well as a false sense of complacency in non-MSM populations.13
Clinical Presentation of Mpox
The incubation period of mpox has been reported to last up to 21 days and is posited to depend on the mode of transmission, with complex invasive exposures having a shorter duration of approximately 9 days compared to noninvasive exposures, which have a duration of approximately 13 days.14 In a recent report from the Netherlands, the average incubation time was 8.5 days in 18 men with exposure attributed to sexual encounters with men.12 Following the incubation period, mpox infection typically presents with nonspecific systemic symptoms such as fever, malaise, sore throat, cough, and headache for approximately 2 days, followed by painful generalized or localized lymphadenopathy 1 to 2 days prior to the onset of skin lesions.1,15 In a recent report from Portugal of more than 20 confirmed cases of mpox, approximately half of patients denied symptoms or had mild systemic symptoms, suggesting that many patients in the current outbreak do not endorse systemic symptoms.16
Classic cutaneous lesions are the hallmark feature of mpox.17 Over a period of 1 to 2 weeks, each lesion progresses through morphologic stages of macule, papule (Figure), vesicle, and pustule, which then crusts over, forming a scab that falls off after another 1 to 2 weeks and can result in dyspigmented or pitted scars.1,15 Lesions may be deep-seated or umbilicated; previously they were noted to typically start on the face and spread centrifugally, but recent cases have been notable for a predominance of anogenital lesions, often with the anogenital area as the sole or primary area of involvement.18 Given the high proportion of anogenital lesions in 2022, symptoms such as anogenital pain, tenesmus, and diarrhea are not uncommon.19 A recent study describing 528 international cases of mpox revealed that 95% of patients presented with a rash; nearly 75% had anogenital lesions; and 41%, 25%, and 10% had involvement of mucosae, the face, and palms/soles, respectively. More than half of patients had fewer than 10 lesions, and 10% presented with a single genital lesion.19

Given the recent predilection of lesions for the anogenital area, the differential diagnosis of mpox should include other common infections localized to these areas. Unlike herpes simplex and varicella-zoster infections, mpox does not exhibit the classic herpetiform clustering of vesicles, and unlike the painless chancre of syphilis, the lesions of mpox are exquisitely painful. Similar to chancroid, mpox presents with painful genital lesions and lymphadenopathy, and the umbilicated papules of molluscum could easily be confused with mpox lesions. Proctitis caused by many sexually transmitted infections (STIs), including chlamydia and gonorrhea, may be difficult to differentiate from proctitis symptoms of mpox. Co-infection with HIV and other STIs is common among patients developing mpox in 2022, which is not surprising given that the primary mechanism of transmission of mpox at this time is through sexual contact, and cases are more common in patients with multiple recent sexual partners.19 Considering these shared risk factors and similar presentation of multiple STIs, patients suspected of having an mpox infection should be tested for other STIs, including HIV.
Complications of Mpox
Although mpox generally is characterized by a mild disease course, there is concern for adverse outcomes, particularly in more vulnerable populations, including immunocompromised, pregnant, and pediatric populations. Complications of infection can include sepsis, encephalitis, bronchopneumonia, and ophthalmic complications that can result in loss of vision.6,17 The most common complications requiring hospitalization in a recent international report of 528 mpox cases were pain management, which was primarily due to severe anogenital pain, followed by soft-tissue superinfection, with other complications including severe pharyngitis limiting oral intake and infection control practices.19 In addition to severe rectal pain, proctitis and even rectal perforation have been reported.19,20
Vertical transmission has been described with devastating outcomes in a case series from the Democratic Republic of Congo, where 4 cases of mpox were identified in pregnant women; 3 of these pregnancies resulted in fetal demise.10 The only fetus to survive was born to a mother with mild infection. In comparison, 2 of 3 mothers with moderate to severe disease experienced spontaneous abortion in the first trimester, and 1 pregnancy ended due to intrauterine demise during the eighteenth week of gestation, likely a complication of mpox. These cases suggest that more severe disease may be linked to worse fetal outcomes.10 Further epidemiologic studies will be crucial, given the potential implications.
Diagnosis
When considering a diagnosis of mpox, clinicians should inquire about recent travel, living arrangements, sexual history, and recent sick contacts.6 A complete skin examination should include the oral and genital areas, given the high prevalence of lesions in these areas. A skin biopsy is not recommended for the diagnosis of mpox, as nonspecific viral changes cannot be differentiated from other viral exanthems, but it often is useful to rule out other differential diagnoses.21 Additionally, immunohistochemistry and electron microscopy can be utilized to aid in a histologic diagnosis of mpox.
Polymerase chain reaction detection of orthopox or mpox DNA is the gold standard for diagnosis.6 Two swabs should be collected from each lesion by swabbing vigorously using sterile swabs made of a synthetic material such as polyester, nylon, or Dacron and placed into a sterile container or viral transport medium.22 Some laboratories may have different instructions for collection of samples, so clinicians are advised to check for instructions from their local laboratory. Deroofing lesions prior to swabbing is not necessary, and specimens can include lesional material or crust. Collection of specimens from 2 to 3 lesions is recommended, preferably from different body areas or lesions with varying morphologies. Anal or rectal swabs can be considered in patients presenting with anal pain or proctitis with clinical suspicion for mpox based on history.19
Infection Prevention
Interim guidance from the WHO on November 16, 2022, reiterated the goal of outbreak control primarily via public health measures, which includes targeted use of vaccines for at-risk populations or postexposure prophylactic vaccination within 4 days, but heavily relies on surveillance and containment techniques, such as contact tracing with monitoring of contacts for onset of symptoms and isolation of cases through the complete infectious period.23 Patients are considered infectious from symptom onset until all cutaneous lesions are re-epithelized and should remain in isolation, including from household contacts and domestic and wildlife animals, for the duration of illness.24,25 Individuals exposed to humans or animals with confirmed mpox should be monitored for the development of symptoms for 21 days following last known exposure, regardless of vaccination status, and should be instructed to measure their temperature twice daily.26 Pets exposed to mpox should be isolated from other animals and humans for 21 days following last known contact.24 Vaccination strategies for preexposure and postexposure prophylaxis (PEP) are discussed below in further detail. Postinfection, the WHO suggests use of condoms for all oral, vaginal, and anal sexual activity for 12 weeks after recovery.7
Patients with suspected or confirmed mpox in a hospital should be in a single private room on special droplet and contact precautions.27 No special air handling or negative pressure isolation is needed unless the patient is undergoing an aerosol-generating procedure (eg, intubation, endoscopy, bronchoscopy). When hospitalized, patients should have a dedicated bathroom, if possible, and at-home patients should be isolated from household members until contagion risk resolves; this includes the use of a separate bathroom, when possible. Health care personnel entering the room of a patient should don appropriate personal protective equipment (PPE), including a disposable gown, gloves, eye protection, and N95 respirator or equivalent. Recommendations include standard practices for cleaning, with wet cleaning methods preferred over dry methods, using a disinfectant that covers emerging viral pathogens, and avoidance of shaking linens to prevent the spread of infectious particles.27 A variety of Environmental Protection Agency–registered wipes with virucidal activity against emerging viruses, including those with active ingredients such as quaternary ammonium, hydrogen peroxide, and hypochlorous acid, should be used for disinfecting surfaces.28
Vaccination
ACAM2000 (Emergent Bio Solutions) and JYNNEOS (Bavarian Nordic)(also known as Imvamune or Imvanex) are available in the United States for the prevention of mpox infection.29 ACAM2000, a second-generation, replication-competent, live smallpox vaccine administered as a single percutaneous injection, is contraindicated in immunocompromised populations, including patients with HIV or on immunosuppressive or biologic therapy, pregnant individuals, people with a history of atopic dermatitis or other exfoliative skin diseases with impaired barrier function, and patients with a history of cardiac disease due to the risk of myocarditis and pericarditis.30
JYNNEOS is a nonreplicating live vaccine approved by the US Food and Drug Administration (FDA) for the prevention of mpox in individuals older than 18 years administered as 2 subcutaneous doses 4 weeks apart. Patients are considered fully vaccinated 2 weeks after the second dose, and JYNNEOS is available to pediatric patients with a single patient expanded access use authorization from the FDA.29,30 More recently, the FDA issued an emergency use authorization (EUA) for administration of the vaccine to patients younger than 18 years who are at high risk of infection after exposure.31 More importantly, the FDA also issued an EUA for the intradermal administration of JYNNEOS at one-fifth of the subcutaneous dose to expand the current vaccine supply. This EUA is based on research by Frey et al,32 which showed that intradermal administration, even at a lower dose, elicited similar immune responses among study participants as the higher dose administered subcutaneously.
JYNNEOS is the preferred vaccine for the prevention of mpox because of its poor ability to replicate in human cells and resultant safety for use in populations that are immunocompromised, pregnant, or have skin barrier defects such as atopic dermatitis, without the risk of myocarditis or pericarditis. However, current supplies are limited. JYNNEOS was specifically studied in patients with atopic dermatitis and has been shown to be safe and effective in patients with a history of atopic dermatitis and active disease with a SCORAD (SCORing Atopic Dermatitis) score of 30 or lower.33 Of note, JYNNEOS is contraindicated in patients allergic to components of the vaccine, including egg, gentamicin, and ciprofloxacin. Although JYNNEOS is safe to administer to persons with immunocompromising conditions, the CDC reports that such persons might be at increased risk for severe disease if an occupational infection occurs, and in the setting of immunocompromise, such persons may be less likely to mount an effective response to vaccination. Therefore, the risk-benefit ratio should be considered to determine if an immunocompromised person should be vaccinated with JYNNEOS.30
The WHO and the CDC do not recommended mass vaccination of the general public for outbreaks of mpox in nonendemic countries, with immunization reserved for appropriate PEP and pre-exposure prophylaxis in intermediate- to high-risk individuals.23,26 The CDC recommends PEP vaccination for individuals with a high degree of exposure that includes unprotected contact of the skin or mucous membranes of an individual to the skin, lesions, body fluids, or contaminated fomites from a patient with mpox, as well as being within 6 feet of a patient during an aerosolization procedure without proper PPE. Following an intermediate degree of exposure, which includes being within 6 feet for 3 or more hours wearing at minimum a surgical mask or contact with fomites while wearing incomplete PPE, the CDC recommends monitoring and shared decision-making regarding risks and benefits of PEP vaccination. Monitoring without PEP is indicated for low and uncertain degrees of exposure, including entering a room without full PPE such as eye protection, regardless of the duration of contact.23,26
Postexposure prophylaxis vaccination should be administered within 4 days of a known high-level exposure to mpox to prevent infection.29 If administered within 4 to 14 days postexposure, vaccination may reduce disease severity but will not prevent infection.34
Pre-exposure prophylaxis is recommended for individuals at high risk for exposure to mpox, including health care workers such as laboratory personnel who handle mpox specimens and health care workers who administer ACAM2000 vaccinations or anticipate providing care for many patients with mpox.34
Management
Most cases of mpox are characterized by mild to moderate disease with a self-limited course. Most commonly, medical management of mpox involves supportive care such as fluid resuscitation, supplemental oxygen, and pain management.6 Treatment of superinfected skin lesions may require antibiotics. In the event of ophthalmologic involvement, patients should be referred to an ophthalmologist for further management.
Currently, there are no FDA-approved therapies for mpox; however, tecovirimat, cidofovir, brincidofovir, and vaccinia immune globulin intravenous are available under expanded access Investigational New Drug protocols.6,35 Human data for cidofovir, brincidofovir, and vaccinia immune globulin intravenous in the treatment of mpox are lacking, while cidofovir and brincidofovir have shown efficacy against orthopoxviruses in in vitro and animal studies, but are available therapeutic options.35
Tecovirimat is an antiviral that is FDA approved for smallpox with efficacy data against mpox in animal studies. It is the first-line treatment for patients with severe disease requiring hospitalization or 1 or more complications, including dehydration or secondary skin infections, as well as for populations at risk for severe disease, which includes immunocompromised patients, pediatric patients younger than 8 years, pregnant or breastfeeding individuals, or patients with a history of atopic dermatitis or active exfoliative skin conditions.36 In this current outbreak, both intravenous and oral tecovirimat are weight based in adult and pediatric patients for 14 days, with the intravenous form dosed every 12 hours by infusion over 6 hours, and the oral doses administered every 8 to 12 hours based on patient weight.37 Tecovirimat generally is well tolerated with mild side effects but is notably contraindicated in patients with severe renal impairment with a creatinine clearance less than 30 mL/min, and renal monitoring is indicated in pediatric patients younger than 2 years and in all patients receiving intravenous treatment.
Conclusion
Given that cutaneous lesions are the most specific presenting sign of mpox infection, dermatologists will play an integral role in identifying future cases and managing future outbreaks. Mpox should be considered in the differential diagnosis for all patients presenting with umbilicated or papulovesicular lesions, particularly in an anogenital distribution. The classic presentation of mpox may be more common among patients who are not considered high risk and have not been exposed via sexual activity. All patients with suspicious lesions should be managed following appropriate infection control precautions and should undergo molecular diagnostic assay of swabbed lesions to confirm the diagnosis. JYNNEOS is the only vaccine that is currently being distributed in the United States and is safe to administer to immunocompromised populations. The risks and benefits of vaccination should be considered on an individual basis between a patient and their provider. Taking into consideration that patients with atopic dermatitis are at risk for severe disease if infected with mpox, vaccination should be strongly encouraged if indicated based on patient risk factors. For atopic dermatitis patients treated with dupilumab, shared decision-making is essential given the FDA label, which recommends avoiding the use of live vaccines.38
The mpox epidemic occurring amidst the ongoing COVID-19 pandemic should serve as a wake-up call to the importance of pandemic preparedness and the global health response strategies in the modern era of globalization. Looking forward, widespread vaccination against mpox may be necessary to control the spread of the disease and to protect vulnerable populations, including pregnant individuals. In the current climate of hesitancy surrounding vaccines and the erosion of trust in public health agencies, it is incumbent upon health care providers to educate patients regarding the role of vaccines and public health measures to control this developing global health crisis.
- Di Giulio DB, Eckburg PB. Human monkeypox: an emerging zoonosis. Lancet Infect Dis. 2004;4:15-25. doi:10.1016/s1473-3099(03)00856-9
- Simpson K, Heymann D, Brown CS, et al. Human monkeypox—after 40 years, an unintended consequence of smallpox eradication. Vaccine. 2020;38:5077-5081. doi:10.1016/j.vaccine.2020.04.062
- Ladnyj ID, Ziegler P, Kima E. A human infection caused by monkeypox virus in Basankusu Territory, Democratic Republic of the Congo. Bull World Health Organ. 1972;46:593-597.
- Alakunle EF, Okeke MI. Monkeypox virus: a neglected zoonotic pathogen spreads globally. Nat Rev Microbiol. 2022;20:507-508. doi:10.1038/s41579-022-00776-z
- Ligon BL. Monkeypox: a review of the history and emergence in the Western hemisphere. Semin Pediatr Infect Dis. 2004;15:280-287. doi:10.1053/j.spid.2004.09.001
- Titanji BK, Tegomoh B, Nematollahi S, et al. Monkeypox: a contemporary review for healthcare professionals. Open Forum Infect Dis. 2022;9:ofac310. doi:10.1093/ofid/ofac310
- Gigante CM, Korber B, Seabolt MH, et al. Multiple lineages of monkeypox virus detected in the United States, 2021-2022. Science. 2022;378:560-565. doi:10.1126/science.add4153
- World Health Organization. WHO Director-General’s statement at the press conference following IHR Emergency Committee regarding the multi-country outbreak of monkeypox—23 July 2022. July 23, 2022. Accessed March 10, 2023. https://www.who.int/director-general/speeches/detail/who-director-general-s-statement-on-the-press-conference-following-IHR-emergency-committee-regarding-the-multi--country-outbreak-of-monkeypox--23-july-2022
- Centers for Disease Control and Prevention. 2022 mpox outbreak global map. Updated March 1, 2023. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/response/2022/world-map.html
- Mbala PK, Huggins JW, Riu-Rovira T, et al. Maternal and fetal outcomes among pregnant women with human monkeypox infection in the Democratic Republic of Congo. J Infect Dis. 2017;216:824-828. doi:10.1093/infdis/jix260
- Centers for Disease Control and Prevention. How to protect yourself. Updated October 31, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/prevention/protect-yourself.html
- Miura F, van Ewijk CE, Backer JA, et al. Estimated incubation period for monkeypox cases confirmed in the Netherlands, May 2022. Euro Surveill. 2022;27:2200448. doi:10.2807/1560-7917.Es.2022.27.24.2200448
- Treisman R. As monkeypox spreads, know the difference between warning and stigmatizing people. NPR. July 26, 2022. Accessed March 10, 2023. https://www.npr.org/2022/07/26/1113713684/monkeypox-stigma-gay-community
- Reynolds MG, Yorita KL, Kuehnert MJ, et al. Clinical manifestations of human monkeypox influenced by route of infection. J Infect Dis. 2006;194:773-780. doi:10.1086/505880
- Centers for Disease Control and Prevention. Clinical recognition. Updated August 23, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/clinical-recognition.html
- Alpalhão M, Frade JV, Sousa D, et al. Monkeypox: a new (sexuallytransmissible) epidemic? J Eur Acad Dermatol Venereol. 2022;36:e1016-e1017. doi:10.1111/jdv.18424
- Reynolds MG, McCollum AM, Nguete B, et al. Improving the care and treatment of monkeypox patients in low-resource settings: applying evidence from contemporary biomedical and smallpox biodefense research. Viruses. 2017;9:380. doi:10.3390/v9120380
- Minhaj FS, Ogale YP, Whitehill F, et al. Monkeypox outbreak—nine states, May 2022. MMWR Morb Mortal Wkly Rep. 2022;71:764-769. doi:10.15585/mmwr.mm7123e1
- Thornhill JP, Barkati S, Walmsley S, et al. Monkeypox virus infection in humans across 16 countries—April-June 2022. N Engl J Med. 2022;387:679-691. doi:10.1056/NEJMoa2207323
- Patel A, Bilinska J, Tam JCH, et al. Clinical features and novel presentations of human monkeypox in a central London centre during the 2022 outbreak: descriptive case series. BMJ. 2022;378:e072410. doi:10.1136/bmj-2022-072410
- Bayer-Garner IB. Monkeypox virus: histologic, immunohistochemical and electron-microscopic findings. J Cutan Pathol. 2005;32:28-34. doi:10.1111/j.0303-6987.2005.00254.x
- Centers for Disease Control and Prevention. Guidelines for collecting and handling of specimens for mpox testing. Updated September 20, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/prep-collection-specimens.html
- Vaccines and immunization for monkeypox: interim guidance, 16 November 2022. Accessed March 15, 2023. https://www.who.int/publications/i/item/WHO-MPX-Immunization
- Centers for Disease Control and Prevention. Pets in the home. Updated December 8, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/specific-settings/pets-in-homes.html
- Centers for Disease Control and Prevention. Isolation andprevention practices for people with monkeypox. Updated February 2, 2023. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/isolation-procedures.html
- Centers for Disease Control and Prevention. Monitoring people who have been exposed. Updated November 25, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/monitoring.html
- Centers for Disease Control and Prevention. Infection prevention and control of monkeypox in healthcare settings. Updated October 31, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/infection-control-healthcare.html
- United States Environmental Protection Agency. EPA releases list of disinfectants for emerging viral pathogens (EVPs) including monkeypox. May 26, 2022. Accessed March 10, 2023. https://www.epa.gov/pesticides/epa-releases-list-disinfectants-emerging-viral-pathogens-evps-including-monkeypox
- Centers for Disease Control and Prevention. Interim clinical considerations for use of JYNNEOS and ACAM2000 vaccines during the 2022 U.S. mpox outbreak. Updated October 19, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/considerations-for-monkeypox-vaccination.html
- Rao AK, Petersen BW, Whitehill F, et al. Use of JYNNEOS (smallpox and monkeypox vaccine, live, nonreplicating) for preexposure vaccination of persons at risk for occupational exposure to orthopoxviruses: recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:734-742. doi: http://dx.doi.org/10.15585/mmwr.mm7122e1
- US Food and Drug Administration. Monkeypox update: FDA authorizes emergency use of JYNNEOS vaccine to increase vaccine supply. August 9, 2022. Accessed March 10, 2023. https://www.fda.gov/news-events/press-announcements/monkeypox-update-fda-authorizes-emergency-use-jynneos-vaccine-increase-vaccine-supply#:~:text=Today%2C%20the%20U.S.%20Food%20and,high%20risk%20for%20monkeypox%20infection
- Frey SE, Wald A, Edupuganti S, et al. Comparison of lyophilized versus liquid modified vaccinia Ankara (MVA) formulations and subcutaneous versus intradermal routes of administration in healthy vaccinia-naïve subjects. Vaccine. 2015;33:5225-5234. doi:10.1016/j.vaccine.2015.06.075
- Greenberg RN, Hurley MY, Dinh DV, et al. A multicenter, open-label, controlled phase II study to evaluate safety and immunogenicity of MVA smallpox vaccine (IMVAMUNE) in 18-40 year old subjects with diagnosed atopic dermatitis. PLoS One. 2015;10:e0138348. doi:10.1371/journal.pone.0138348
- Centers for Disease Control and Prevention. Monkeypox and smallpox vaccine guidance. Accessed March 16, 2023. https://www.cdc.gov/poxvirus/mpox/interim-considerations/overview.html
- Centers for Disease Control and Prevention. Treatment information for healthcare professionals. Updated March 3, 2023. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/treatment.html
- Centers for Disease Control and Prevention. Guidance for tecovirimat use: expanded access investigational new drug protocol during 2022 U.S. mpox outbreak. Updated February 23, 2023. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/Tecovirimat.html
- Expanded access IND protocol: use of tecovirimat (TPOXX®) for treatment of human non-variola orthopoxvirus infections in adults and children. October 24, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/pdf/tecovirimat-ind-protocol-cdc-irb.pdf
- Dupixent (dupilumab). Prescribing information. Regeneron Pharmaceuticals, Inc; 2017. Accessed March 10, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761055lbl.pdf
- Di Giulio DB, Eckburg PB. Human monkeypox: an emerging zoonosis. Lancet Infect Dis. 2004;4:15-25. doi:10.1016/s1473-3099(03)00856-9
- Simpson K, Heymann D, Brown CS, et al. Human monkeypox—after 40 years, an unintended consequence of smallpox eradication. Vaccine. 2020;38:5077-5081. doi:10.1016/j.vaccine.2020.04.062
- Ladnyj ID, Ziegler P, Kima E. A human infection caused by monkeypox virus in Basankusu Territory, Democratic Republic of the Congo. Bull World Health Organ. 1972;46:593-597.
- Alakunle EF, Okeke MI. Monkeypox virus: a neglected zoonotic pathogen spreads globally. Nat Rev Microbiol. 2022;20:507-508. doi:10.1038/s41579-022-00776-z
- Ligon BL. Monkeypox: a review of the history and emergence in the Western hemisphere. Semin Pediatr Infect Dis. 2004;15:280-287. doi:10.1053/j.spid.2004.09.001
- Titanji BK, Tegomoh B, Nematollahi S, et al. Monkeypox: a contemporary review for healthcare professionals. Open Forum Infect Dis. 2022;9:ofac310. doi:10.1093/ofid/ofac310
- Gigante CM, Korber B, Seabolt MH, et al. Multiple lineages of monkeypox virus detected in the United States, 2021-2022. Science. 2022;378:560-565. doi:10.1126/science.add4153
- World Health Organization. WHO Director-General’s statement at the press conference following IHR Emergency Committee regarding the multi-country outbreak of monkeypox—23 July 2022. July 23, 2022. Accessed March 10, 2023. https://www.who.int/director-general/speeches/detail/who-director-general-s-statement-on-the-press-conference-following-IHR-emergency-committee-regarding-the-multi--country-outbreak-of-monkeypox--23-july-2022
- Centers for Disease Control and Prevention. 2022 mpox outbreak global map. Updated March 1, 2023. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/response/2022/world-map.html
- Mbala PK, Huggins JW, Riu-Rovira T, et al. Maternal and fetal outcomes among pregnant women with human monkeypox infection in the Democratic Republic of Congo. J Infect Dis. 2017;216:824-828. doi:10.1093/infdis/jix260
- Centers for Disease Control and Prevention. How to protect yourself. Updated October 31, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/prevention/protect-yourself.html
- Miura F, van Ewijk CE, Backer JA, et al. Estimated incubation period for monkeypox cases confirmed in the Netherlands, May 2022. Euro Surveill. 2022;27:2200448. doi:10.2807/1560-7917.Es.2022.27.24.2200448
- Treisman R. As monkeypox spreads, know the difference between warning and stigmatizing people. NPR. July 26, 2022. Accessed March 10, 2023. https://www.npr.org/2022/07/26/1113713684/monkeypox-stigma-gay-community
- Reynolds MG, Yorita KL, Kuehnert MJ, et al. Clinical manifestations of human monkeypox influenced by route of infection. J Infect Dis. 2006;194:773-780. doi:10.1086/505880
- Centers for Disease Control and Prevention. Clinical recognition. Updated August 23, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/clinical-recognition.html
- Alpalhão M, Frade JV, Sousa D, et al. Monkeypox: a new (sexuallytransmissible) epidemic? J Eur Acad Dermatol Venereol. 2022;36:e1016-e1017. doi:10.1111/jdv.18424
- Reynolds MG, McCollum AM, Nguete B, et al. Improving the care and treatment of monkeypox patients in low-resource settings: applying evidence from contemporary biomedical and smallpox biodefense research. Viruses. 2017;9:380. doi:10.3390/v9120380
- Minhaj FS, Ogale YP, Whitehill F, et al. Monkeypox outbreak—nine states, May 2022. MMWR Morb Mortal Wkly Rep. 2022;71:764-769. doi:10.15585/mmwr.mm7123e1
- Thornhill JP, Barkati S, Walmsley S, et al. Monkeypox virus infection in humans across 16 countries—April-June 2022. N Engl J Med. 2022;387:679-691. doi:10.1056/NEJMoa2207323
- Patel A, Bilinska J, Tam JCH, et al. Clinical features and novel presentations of human monkeypox in a central London centre during the 2022 outbreak: descriptive case series. BMJ. 2022;378:e072410. doi:10.1136/bmj-2022-072410
- Bayer-Garner IB. Monkeypox virus: histologic, immunohistochemical and electron-microscopic findings. J Cutan Pathol. 2005;32:28-34. doi:10.1111/j.0303-6987.2005.00254.x
- Centers for Disease Control and Prevention. Guidelines for collecting and handling of specimens for mpox testing. Updated September 20, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/prep-collection-specimens.html
- Vaccines and immunization for monkeypox: interim guidance, 16 November 2022. Accessed March 15, 2023. https://www.who.int/publications/i/item/WHO-MPX-Immunization
- Centers for Disease Control and Prevention. Pets in the home. Updated December 8, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/specific-settings/pets-in-homes.html
- Centers for Disease Control and Prevention. Isolation andprevention practices for people with monkeypox. Updated February 2, 2023. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/isolation-procedures.html
- Centers for Disease Control and Prevention. Monitoring people who have been exposed. Updated November 25, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/monitoring.html
- Centers for Disease Control and Prevention. Infection prevention and control of monkeypox in healthcare settings. Updated October 31, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/infection-control-healthcare.html
- United States Environmental Protection Agency. EPA releases list of disinfectants for emerging viral pathogens (EVPs) including monkeypox. May 26, 2022. Accessed March 10, 2023. https://www.epa.gov/pesticides/epa-releases-list-disinfectants-emerging-viral-pathogens-evps-including-monkeypox
- Centers for Disease Control and Prevention. Interim clinical considerations for use of JYNNEOS and ACAM2000 vaccines during the 2022 U.S. mpox outbreak. Updated October 19, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/considerations-for-monkeypox-vaccination.html
- Rao AK, Petersen BW, Whitehill F, et al. Use of JYNNEOS (smallpox and monkeypox vaccine, live, nonreplicating) for preexposure vaccination of persons at risk for occupational exposure to orthopoxviruses: recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:734-742. doi: http://dx.doi.org/10.15585/mmwr.mm7122e1
- US Food and Drug Administration. Monkeypox update: FDA authorizes emergency use of JYNNEOS vaccine to increase vaccine supply. August 9, 2022. Accessed March 10, 2023. https://www.fda.gov/news-events/press-announcements/monkeypox-update-fda-authorizes-emergency-use-jynneos-vaccine-increase-vaccine-supply#:~:text=Today%2C%20the%20U.S.%20Food%20and,high%20risk%20for%20monkeypox%20infection
- Frey SE, Wald A, Edupuganti S, et al. Comparison of lyophilized versus liquid modified vaccinia Ankara (MVA) formulations and subcutaneous versus intradermal routes of administration in healthy vaccinia-naïve subjects. Vaccine. 2015;33:5225-5234. doi:10.1016/j.vaccine.2015.06.075
- Greenberg RN, Hurley MY, Dinh DV, et al. A multicenter, open-label, controlled phase II study to evaluate safety and immunogenicity of MVA smallpox vaccine (IMVAMUNE) in 18-40 year old subjects with diagnosed atopic dermatitis. PLoS One. 2015;10:e0138348. doi:10.1371/journal.pone.0138348
- Centers for Disease Control and Prevention. Monkeypox and smallpox vaccine guidance. Accessed March 16, 2023. https://www.cdc.gov/poxvirus/mpox/interim-considerations/overview.html
- Centers for Disease Control and Prevention. Treatment information for healthcare professionals. Updated March 3, 2023. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/treatment.html
- Centers for Disease Control and Prevention. Guidance for tecovirimat use: expanded access investigational new drug protocol during 2022 U.S. mpox outbreak. Updated February 23, 2023. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/Tecovirimat.html
- Expanded access IND protocol: use of tecovirimat (TPOXX®) for treatment of human non-variola orthopoxvirus infections in adults and children. October 24, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/pdf/tecovirimat-ind-protocol-cdc-irb.pdf
- Dupixent (dupilumab). Prescribing information. Regeneron Pharmaceuticals, Inc; 2017. Accessed March 10, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761055lbl.pdf
Practice Points
- Mpox (monkeypox) lesions typically present as well-circumscribed, painful, umbilicated papules, vesicles, or pustules, with recent cases having a predilection for an anogenital distribution accompanied by systemic viral symptoms.
- Health care workers treating suspected or confirmed cases of mpox should be familiar with current guidelines for controlling the spread of mpox, including proper personal protective equipment (gloves, disposable gowns, N95 or equivalent respirators, and eye protection) and indications for vaccination.
Habit Reversal Therapy for Skin Picking Disorder
Practice Gap
Skin picking disorder is characterized by repetitive deliberate manipulation of the skin that causes noticeable tissue damage. It affects approximately 1.6% of adults in the United States and is associated with marked distress as well as a psychosocial impact.1 Complications of skin picking disorder can include ulceration, infection, scarring, and disfigurement.
Cognitive behavioral therapy (CBT) techniques have been established to be effective in treating skin picking disorder.2 Although referral to a mental health professional is appropriate for patients with skin picking disorder, many of them may not be interested. Cognitive behavioral therapy for diseases at the intersection of psychiatry and dermatology typically is not included in dermatology curricula. Therefore, dermatologists should be aware of CBT techniques that can mitigate the impact of skin picking disorder for patients who decline referral to a mental health professional.
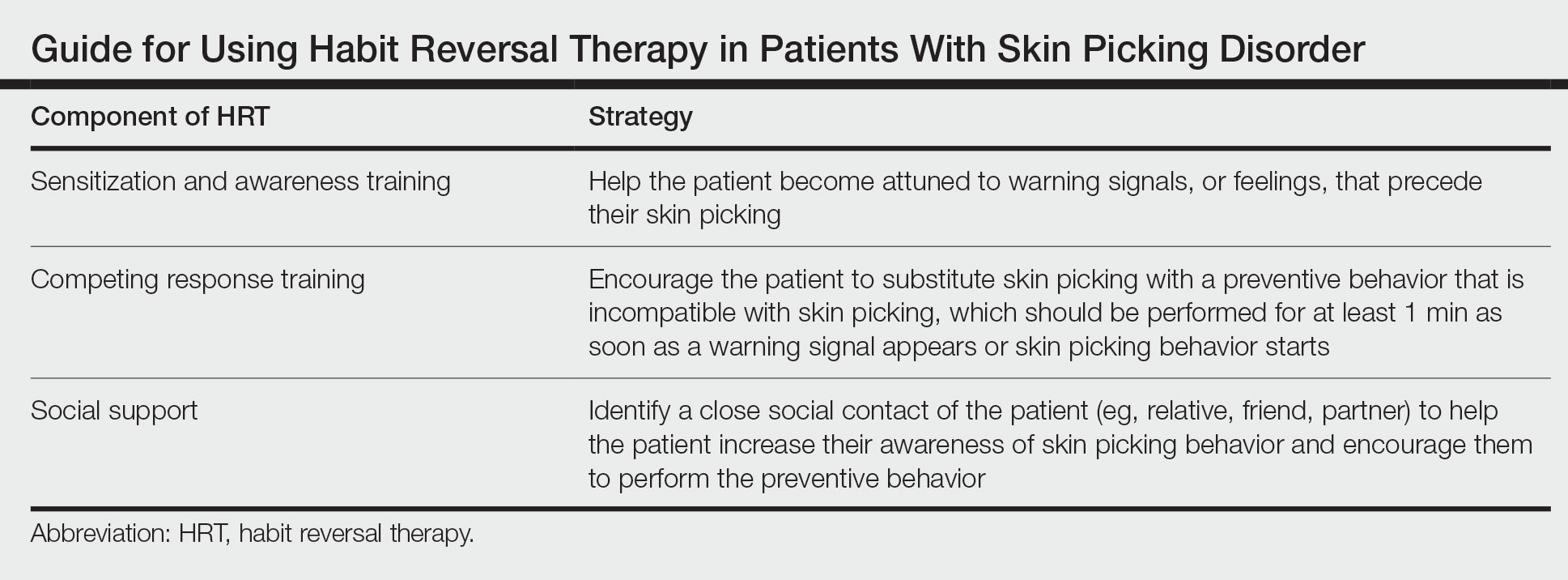
The Technique
Cognitive behavioral therapy is one of the more effective forms of psychotherapy for the treatment of skin picking disorder. Consistent utilization of CBT techniques can achieve relatively permanent change in brain function and contribute to long-term treatment outcomes. A particularly useful CBT technique for skin picking disorder is habit reversal therapy (HRT)(Table). Studies have shown that HRT techniques have demonstrated efficacy in skin picking disorder with sustained impact.3 Patients treated with HRT have reported a greater decrease in skin picking compared with controls after only 3 sessions (P<.01).4 There are 3 elements to HRT:
1. Sensitization and awareness training: This facet of HRT involves helping the patient become attuned to warning signals, or feelings, that precede their skin picking, as skin picking often occurs automatically without the patient noticing. Such feelings can include tingling of the skin, tension, and a feeling of being overwhelmed.5 Ideally, the physician works with the patient to identify 2 or 3 warning signals that precede skin picking behavior.
2. Competing response training: The patient is encouraged to substitute skin picking with a preventive behavior—for example, crossing the arms and gently squeezing the fists—that is incompatible with skin picking. The preventive behavior should be performed for at least 1 minute as soon as a warning signal appears or skin picking behavior starts. After 1 minute, if the urge for skin picking recurs, then the patient should repeat the preventive behavior.5 It can be helpful to practice the preventive behavior with the patient once in the clinic.
3. Social support: This technique involves identifying a close social contact of the patient (eg, relative, friend, partner) to help the patient increase their awareness of skin picking behavior and encourage them to perform the preventive behavior.5 The purpose of identifying a close social contact is to ensure accountability for the patient in their day-to-day life, given the limited scope of the relationship between the patient and the dermatologist.
Other practical solutions to skin picking include advising patients to cut their nails short; using finger cots to cover the nails and thus lessen the potential for skin injury; and using a sensory toy, such as a fidget spinner, to distract or occupy the patient when they feel the urge for skin picking.
Practice Implications
Although skin picking disorder is a challenging condition to manage, there are proven techniques for treatment. Techniques drawn from HRT are quite practical and can be implemented by dermatologists for patients with skin picking disorder to reduce the burden of their disease.
- Keuthen NJ, Koran LM, Aboujaoude E, et al. The prevalence of pathologic skin picking in US adults. Compr Psychiatry. 2010;51:183-186. doi:10.1016/j.comppsych.2009.04.003
- Jafferany M, Mkhoyan R, Arora G, et al. Treatment of skin picking disorder: interdisciplinary role of dermatologist and psychiatrist. Dermatol Ther. 2020;33:E13837. doi:10.1111/dth.13837
- Schuck K, Keijsers GP, Rinck M. The effects of brief cognitive-behaviour therapy for pathological skin picking: a randomized comparison to wait-list control. Behav Res Ther. 2011;49:11-17. doi:10.1016/j.brat.2010.09.005
- Teng EJ, Woods DW, Twohig MP. Habit reversal as a treatment for chronic skin picking: a pilot investigation. Behav Modif. 2006;30:411-422. doi:10.1177/0145445504265707
- Torales J, Páez L, O’Higgins M, et al. Cognitive behavioral therapy for excoriation (skin picking) disorder. Telangana J Psych. 2016;2:27-30.
Practice Gap
Skin picking disorder is characterized by repetitive deliberate manipulation of the skin that causes noticeable tissue damage. It affects approximately 1.6% of adults in the United States and is associated with marked distress as well as a psychosocial impact.1 Complications of skin picking disorder can include ulceration, infection, scarring, and disfigurement.
Cognitive behavioral therapy (CBT) techniques have been established to be effective in treating skin picking disorder.2 Although referral to a mental health professional is appropriate for patients with skin picking disorder, many of them may not be interested. Cognitive behavioral therapy for diseases at the intersection of psychiatry and dermatology typically is not included in dermatology curricula. Therefore, dermatologists should be aware of CBT techniques that can mitigate the impact of skin picking disorder for patients who decline referral to a mental health professional.

The Technique
Cognitive behavioral therapy is one of the more effective forms of psychotherapy for the treatment of skin picking disorder. Consistent utilization of CBT techniques can achieve relatively permanent change in brain function and contribute to long-term treatment outcomes. A particularly useful CBT technique for skin picking disorder is habit reversal therapy (HRT)(Table). Studies have shown that HRT techniques have demonstrated efficacy in skin picking disorder with sustained impact.3 Patients treated with HRT have reported a greater decrease in skin picking compared with controls after only 3 sessions (P<.01).4 There are 3 elements to HRT:
1. Sensitization and awareness training: This facet of HRT involves helping the patient become attuned to warning signals, or feelings, that precede their skin picking, as skin picking often occurs automatically without the patient noticing. Such feelings can include tingling of the skin, tension, and a feeling of being overwhelmed.5 Ideally, the physician works with the patient to identify 2 or 3 warning signals that precede skin picking behavior.
2. Competing response training: The patient is encouraged to substitute skin picking with a preventive behavior—for example, crossing the arms and gently squeezing the fists—that is incompatible with skin picking. The preventive behavior should be performed for at least 1 minute as soon as a warning signal appears or skin picking behavior starts. After 1 minute, if the urge for skin picking recurs, then the patient should repeat the preventive behavior.5 It can be helpful to practice the preventive behavior with the patient once in the clinic.
3. Social support: This technique involves identifying a close social contact of the patient (eg, relative, friend, partner) to help the patient increase their awareness of skin picking behavior and encourage them to perform the preventive behavior.5 The purpose of identifying a close social contact is to ensure accountability for the patient in their day-to-day life, given the limited scope of the relationship between the patient and the dermatologist.
Other practical solutions to skin picking include advising patients to cut their nails short; using finger cots to cover the nails and thus lessen the potential for skin injury; and using a sensory toy, such as a fidget spinner, to distract or occupy the patient when they feel the urge for skin picking.
Practice Implications
Although skin picking disorder is a challenging condition to manage, there are proven techniques for treatment. Techniques drawn from HRT are quite practical and can be implemented by dermatologists for patients with skin picking disorder to reduce the burden of their disease.
Practice Gap
Skin picking disorder is characterized by repetitive deliberate manipulation of the skin that causes noticeable tissue damage. It affects approximately 1.6% of adults in the United States and is associated with marked distress as well as a psychosocial impact.1 Complications of skin picking disorder can include ulceration, infection, scarring, and disfigurement.
Cognitive behavioral therapy (CBT) techniques have been established to be effective in treating skin picking disorder.2 Although referral to a mental health professional is appropriate for patients with skin picking disorder, many of them may not be interested. Cognitive behavioral therapy for diseases at the intersection of psychiatry and dermatology typically is not included in dermatology curricula. Therefore, dermatologists should be aware of CBT techniques that can mitigate the impact of skin picking disorder for patients who decline referral to a mental health professional.

The Technique
Cognitive behavioral therapy is one of the more effective forms of psychotherapy for the treatment of skin picking disorder. Consistent utilization of CBT techniques can achieve relatively permanent change in brain function and contribute to long-term treatment outcomes. A particularly useful CBT technique for skin picking disorder is habit reversal therapy (HRT)(Table). Studies have shown that HRT techniques have demonstrated efficacy in skin picking disorder with sustained impact.3 Patients treated with HRT have reported a greater decrease in skin picking compared with controls after only 3 sessions (P<.01).4 There are 3 elements to HRT:
1. Sensitization and awareness training: This facet of HRT involves helping the patient become attuned to warning signals, or feelings, that precede their skin picking, as skin picking often occurs automatically without the patient noticing. Such feelings can include tingling of the skin, tension, and a feeling of being overwhelmed.5 Ideally, the physician works with the patient to identify 2 or 3 warning signals that precede skin picking behavior.
2. Competing response training: The patient is encouraged to substitute skin picking with a preventive behavior—for example, crossing the arms and gently squeezing the fists—that is incompatible with skin picking. The preventive behavior should be performed for at least 1 minute as soon as a warning signal appears or skin picking behavior starts. After 1 minute, if the urge for skin picking recurs, then the patient should repeat the preventive behavior.5 It can be helpful to practice the preventive behavior with the patient once in the clinic.
3. Social support: This technique involves identifying a close social contact of the patient (eg, relative, friend, partner) to help the patient increase their awareness of skin picking behavior and encourage them to perform the preventive behavior.5 The purpose of identifying a close social contact is to ensure accountability for the patient in their day-to-day life, given the limited scope of the relationship between the patient and the dermatologist.
Other practical solutions to skin picking include advising patients to cut their nails short; using finger cots to cover the nails and thus lessen the potential for skin injury; and using a sensory toy, such as a fidget spinner, to distract or occupy the patient when they feel the urge for skin picking.
Practice Implications
Although skin picking disorder is a challenging condition to manage, there are proven techniques for treatment. Techniques drawn from HRT are quite practical and can be implemented by dermatologists for patients with skin picking disorder to reduce the burden of their disease.
- Keuthen NJ, Koran LM, Aboujaoude E, et al. The prevalence of pathologic skin picking in US adults. Compr Psychiatry. 2010;51:183-186. doi:10.1016/j.comppsych.2009.04.003
- Jafferany M, Mkhoyan R, Arora G, et al. Treatment of skin picking disorder: interdisciplinary role of dermatologist and psychiatrist. Dermatol Ther. 2020;33:E13837. doi:10.1111/dth.13837
- Schuck K, Keijsers GP, Rinck M. The effects of brief cognitive-behaviour therapy for pathological skin picking: a randomized comparison to wait-list control. Behav Res Ther. 2011;49:11-17. doi:10.1016/j.brat.2010.09.005
- Teng EJ, Woods DW, Twohig MP. Habit reversal as a treatment for chronic skin picking: a pilot investigation. Behav Modif. 2006;30:411-422. doi:10.1177/0145445504265707
- Torales J, Páez L, O’Higgins M, et al. Cognitive behavioral therapy for excoriation (skin picking) disorder. Telangana J Psych. 2016;2:27-30.
- Keuthen NJ, Koran LM, Aboujaoude E, et al. The prevalence of pathologic skin picking in US adults. Compr Psychiatry. 2010;51:183-186. doi:10.1016/j.comppsych.2009.04.003
- Jafferany M, Mkhoyan R, Arora G, et al. Treatment of skin picking disorder: interdisciplinary role of dermatologist and psychiatrist. Dermatol Ther. 2020;33:E13837. doi:10.1111/dth.13837
- Schuck K, Keijsers GP, Rinck M. The effects of brief cognitive-behaviour therapy for pathological skin picking: a randomized comparison to wait-list control. Behav Res Ther. 2011;49:11-17. doi:10.1016/j.brat.2010.09.005
- Teng EJ, Woods DW, Twohig MP. Habit reversal as a treatment for chronic skin picking: a pilot investigation. Behav Modif. 2006;30:411-422. doi:10.1177/0145445504265707
- Torales J, Páez L, O’Higgins M, et al. Cognitive behavioral therapy for excoriation (skin picking) disorder. Telangana J Psych. 2016;2:27-30.
Symmetric Palmoplantar Papules With a Keratotic Border
The Diagnosis: Porokeratosis Plantaris Palmaris et Disseminata
A 3-mm punch biopsy of the right upper arm showed incipient cornoid lamellae formation, pigment incontinence, and sparse dermal lymphocytic inflammation (Figure), suggestive of porokeratosis plantaris palmaris et disseminata (PPPD). The dermatopathologist recommended a second biopsy to confirm the diagnosis and to confirm that the lesions on the palms and soles also were suggestive of porokeratosis. A second 4-mm punch biopsy of the left palm was consistent with PPPD.
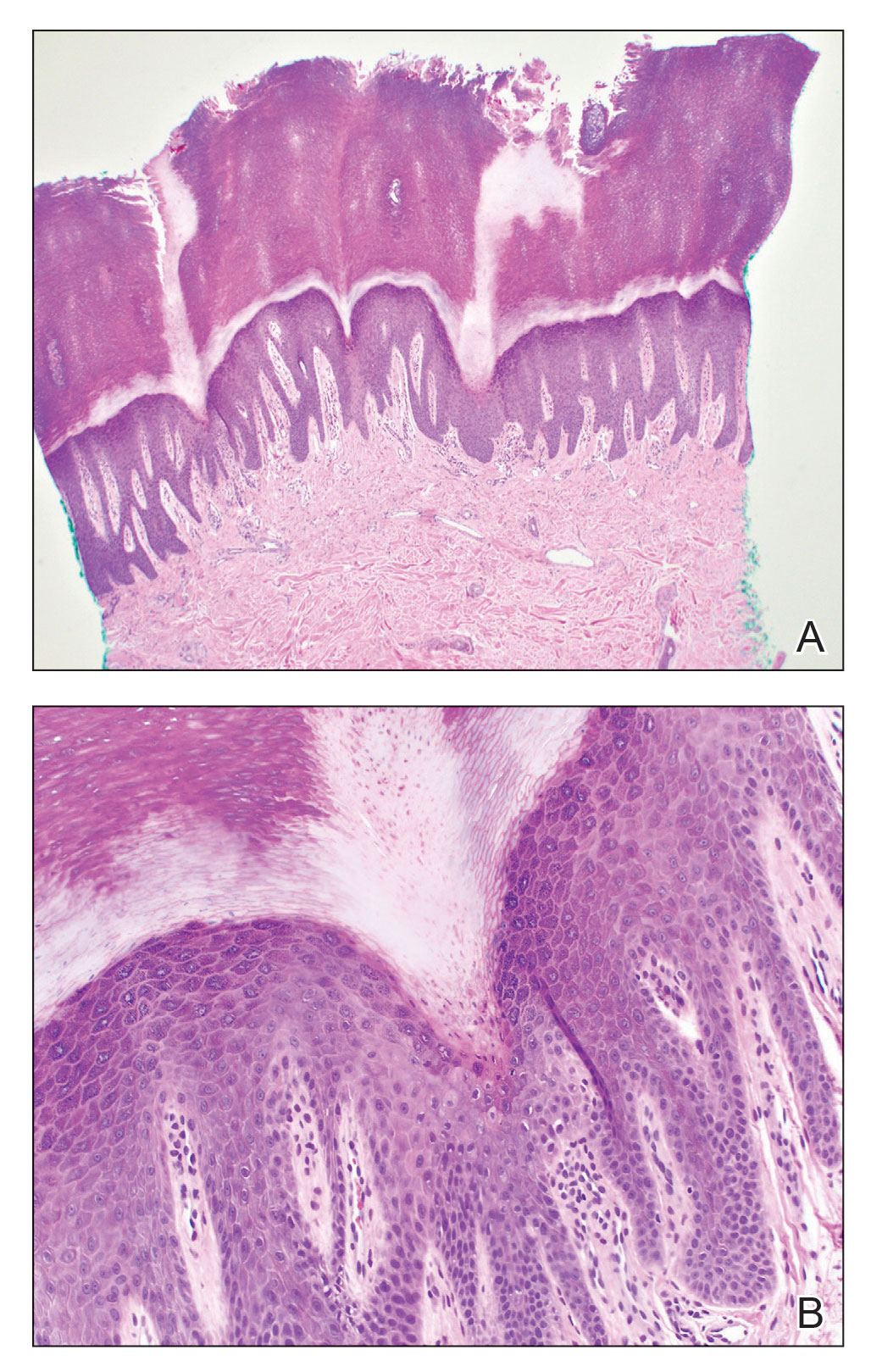
The risks of PPPD as a precancerous entity along with the benefits and side effects of the various management options were discussed with our patient. We recommended that he start low-dose isotretinoin (20 mg/d) due to the large body surface area affected, making focal and field treatments likely insufficient. However, our patient opted not to treat and did not return for follow-up.
Subtypes of porokeratosis, including disseminated superficial actinic porokeratosis (DSAP) and PPPD, are conditions that disrupt the normal maturation of keratin and present clinically with symmetric, crusted, annular papules.1 The signature but nonspecific histopathologic feature shared among the subtypes is the presence of a cornoid lamellae.2 Several triggers of porokeratosis have been proposed, including trauma and exposure to UV and ionizing radiation.2,3 The clinical variants of porokeratosis are important conditions to diagnose correctly because they portend a risk for Bowen disease and invasive squamous cell carcinoma and may indicate the presence of an underlying hematologic and/or solid organ malignancy.4 Management of porokeratosis is difficult, as treatments have shown limited efficacy and variable recurrence rates. Treatment options include focal, field, and systemic options, such as 5-fluorouracil, topical compound of cholesterol and lovastatin, isotretinoin, and acitretin.1,2
Porokeratoses may arise from gene mutations in the mevalonate pathway,5 which is essential for the production of cholesterol.6 Topical cholesterol alone has not been shown to improve porokeratosis, but the combination topical therapy of cholesterol and lovastatin is promising. It is theorized to deliver benefit by both providing the essential end product of the pathway and simultaneously reducing the number of potentially toxic intermediates.6
Porokeratosis plantaris palmaris et disseminata (also known as porokeratosis plantaris) is unique among the subtypes of porokeratosis in that its annular, red-pink, papular rash with scaling and a keratotic border tends to start distally, involving the palms and soles, and progresses proximally to the trunk with smaller lesions.1,7 This centripetal progression can take years, as was seen in our patient.1 The disease is uncommon, with a dearth of published reports on PPPD.2 However, case reports have shown that PPPD is strongly linked to family history and may have an autosomal-dominant inheritance pattern. Penetrance is greater in men than in women, as PPPD is twice as common in men.8 Most cases of PPPD have been diagnosed in patients in their 20s and 30s, but Hartman et al9 reported a case wherein a patient was diagnosed with PPPD after 65 years of age, similar to our patient.
Although the lesions in DSAP can appear similar to those in PPPD, DSAP is more common among the family of porokeratotic conditions, affecting women twice as often as men, with a sporadic pattern of inheritance.2 These same features are present in some other types of porokeratosis but not PPPD. Furthermore, DSAP progresses proximally to distally but often with truncal sparing.2
Akin to PPPD, pityriasis rubra pilaris (PRP) often presents with palmoplantar keratoderma.10 There are at least 6 types of PRP with varying degrees of similarity to PPPD. However, in many cases PRP is associated with a background of diffuse erythema on the body with islands of spared skin. In addition, cases of PRP have been linked to extracutaneous findings such as ectropion and joint pain.11
Darier disease, especially the acrokeratosis verruciformis of Hopf variant, is more common in men and involves younger populations, as in PPPD.11 However, the crusted lesions seen in Darier disease frequently involve the skin folds. These intertriginous lesions may coalesce, mimicking warts in appearance, and are at risk for secondary infection. Nail findings in Darier disease also are distinct and include longitudinal white or red stripes running along the nail bed, in addition to V-shaped nicks at the nail tips.
Psoriasis can occur anywhere on the body and is associated with silver scaling atop a salmon-colored dermatitis.12 It results from aberrant proliferation of keratinocytes. Some distinguishing features of psoriasis include a disease course that waxes and wanes as well as pitting of the nails.
Although PPPD typically affects young adults, we presented a case of PPPD in an older man. Porokeratosis plantaris palmaris et disseminata in older adults may represent a delayed diagnosis, imply a broader range for the age of onset, or suggest its manifestation secondary to radiation treatment or another phenomenon. For example, our patient received 35 radiotherapy cycles for tongue cancer more than 5 years prior to the onset of PPPD.
- Irisawa R, Yamazaki M, Yamamoto T, et al. A case of porokeratosis plantaris palmaris et disseminata and literature review. Dermatol Online J. 2012;18:5.
- Vargas-Mora P, Morgado-Carrasco D, Fusta-Novell X. Porokeratosis: a review of its pathophysiology, clinical manifestations, diagnosis, and treatment. Actas Dermosifiliogr. 2020;111:545-560.
- James AJ, Clarke LE, Elenitsas R, et al. Segmental porokeratosis after radiation therapy for follicular lymphoma. J Am Acad Dermatol. 2008;58(2 suppl):S49-S50.
- Schena D, Papagrigoraki A, Frigo A, et al. Eruptive disseminated porokeratosis associated with internal malignancies: a case report. Cutis. 2010;85:156-159.
- Zhang Z, Li C, Wu F, et al. Genomic variations of the mevalonate pathway in porokeratosis. Elife. 2015;4:E06322. doi:10.7554/eLife.06322
- Atzmony L, Lim YH, Hamilton C, et al. Topical cholesterol/lovastatin for the treatment of porokeratosis: a pathogenesis-directed therapy. J Am Acad Dermatol. 2020;82:123-131. doi:10.1016/j.jaad.2019.08.043
- Guss SB, Osbourn RA, Lutzner MA. Porokeratosis plantaris, palmaris, et disseminata. a third type of porokeratosis. Arch Dermatol. 1971;104:366-373.
- Kanitakis J. Porokeratoses: an update of clinical, aetiopathogenic and therapeutic features. Eur J Dermatol. 2014;24:533-544.
- Hartman R, Mandal R, Sanchez M, et al. Porokeratosis plantaris, palmaris, et disseminata. Dermatol Online J. 2010;16:22.
- Suryawanshi H, Dhobley A, Sharma A, et al. Darier disease: a rare genodermatosis. J Oral Maxillofac Pathol. 2017;21:321. doi:10.4103/jomfp.JOMFP_170_16
- Eastham AB. Pityriasis rubra pilaris. JAMA Dermatol. 2019;155:404. doi:10.1001/jamadermatol.2018.5030
- Nair PA, Badri T. Psoriasis. StatPearls Publishing; 2022. Updated April 6, 2022. Accessed March 13, 2023. https://www.ncbi.nlm.nih.gov/books/NBK448194/
The Diagnosis: Porokeratosis Plantaris Palmaris et Disseminata
A 3-mm punch biopsy of the right upper arm showed incipient cornoid lamellae formation, pigment incontinence, and sparse dermal lymphocytic inflammation (Figure), suggestive of porokeratosis plantaris palmaris et disseminata (PPPD). The dermatopathologist recommended a second biopsy to confirm the diagnosis and to confirm that the lesions on the palms and soles also were suggestive of porokeratosis. A second 4-mm punch biopsy of the left palm was consistent with PPPD.

The risks of PPPD as a precancerous entity along with the benefits and side effects of the various management options were discussed with our patient. We recommended that he start low-dose isotretinoin (20 mg/d) due to the large body surface area affected, making focal and field treatments likely insufficient. However, our patient opted not to treat and did not return for follow-up.
Subtypes of porokeratosis, including disseminated superficial actinic porokeratosis (DSAP) and PPPD, are conditions that disrupt the normal maturation of keratin and present clinically with symmetric, crusted, annular papules.1 The signature but nonspecific histopathologic feature shared among the subtypes is the presence of a cornoid lamellae.2 Several triggers of porokeratosis have been proposed, including trauma and exposure to UV and ionizing radiation.2,3 The clinical variants of porokeratosis are important conditions to diagnose correctly because they portend a risk for Bowen disease and invasive squamous cell carcinoma and may indicate the presence of an underlying hematologic and/or solid organ malignancy.4 Management of porokeratosis is difficult, as treatments have shown limited efficacy and variable recurrence rates. Treatment options include focal, field, and systemic options, such as 5-fluorouracil, topical compound of cholesterol and lovastatin, isotretinoin, and acitretin.1,2
Porokeratoses may arise from gene mutations in the mevalonate pathway,5 which is essential for the production of cholesterol.6 Topical cholesterol alone has not been shown to improve porokeratosis, but the combination topical therapy of cholesterol and lovastatin is promising. It is theorized to deliver benefit by both providing the essential end product of the pathway and simultaneously reducing the number of potentially toxic intermediates.6
Porokeratosis plantaris palmaris et disseminata (also known as porokeratosis plantaris) is unique among the subtypes of porokeratosis in that its annular, red-pink, papular rash with scaling and a keratotic border tends to start distally, involving the palms and soles, and progresses proximally to the trunk with smaller lesions.1,7 This centripetal progression can take years, as was seen in our patient.1 The disease is uncommon, with a dearth of published reports on PPPD.2 However, case reports have shown that PPPD is strongly linked to family history and may have an autosomal-dominant inheritance pattern. Penetrance is greater in men than in women, as PPPD is twice as common in men.8 Most cases of PPPD have been diagnosed in patients in their 20s and 30s, but Hartman et al9 reported a case wherein a patient was diagnosed with PPPD after 65 years of age, similar to our patient.
Although the lesions in DSAP can appear similar to those in PPPD, DSAP is more common among the family of porokeratotic conditions, affecting women twice as often as men, with a sporadic pattern of inheritance.2 These same features are present in some other types of porokeratosis but not PPPD. Furthermore, DSAP progresses proximally to distally but often with truncal sparing.2
Akin to PPPD, pityriasis rubra pilaris (PRP) often presents with palmoplantar keratoderma.10 There are at least 6 types of PRP with varying degrees of similarity to PPPD. However, in many cases PRP is associated with a background of diffuse erythema on the body with islands of spared skin. In addition, cases of PRP have been linked to extracutaneous findings such as ectropion and joint pain.11
Darier disease, especially the acrokeratosis verruciformis of Hopf variant, is more common in men and involves younger populations, as in PPPD.11 However, the crusted lesions seen in Darier disease frequently involve the skin folds. These intertriginous lesions may coalesce, mimicking warts in appearance, and are at risk for secondary infection. Nail findings in Darier disease also are distinct and include longitudinal white or red stripes running along the nail bed, in addition to V-shaped nicks at the nail tips.
Psoriasis can occur anywhere on the body and is associated with silver scaling atop a salmon-colored dermatitis.12 It results from aberrant proliferation of keratinocytes. Some distinguishing features of psoriasis include a disease course that waxes and wanes as well as pitting of the nails.
Although PPPD typically affects young adults, we presented a case of PPPD in an older man. Porokeratosis plantaris palmaris et disseminata in older adults may represent a delayed diagnosis, imply a broader range for the age of onset, or suggest its manifestation secondary to radiation treatment or another phenomenon. For example, our patient received 35 radiotherapy cycles for tongue cancer more than 5 years prior to the onset of PPPD.
The Diagnosis: Porokeratosis Plantaris Palmaris et Disseminata
A 3-mm punch biopsy of the right upper arm showed incipient cornoid lamellae formation, pigment incontinence, and sparse dermal lymphocytic inflammation (Figure), suggestive of porokeratosis plantaris palmaris et disseminata (PPPD). The dermatopathologist recommended a second biopsy to confirm the diagnosis and to confirm that the lesions on the palms and soles also were suggestive of porokeratosis. A second 4-mm punch biopsy of the left palm was consistent with PPPD.

The risks of PPPD as a precancerous entity along with the benefits and side effects of the various management options were discussed with our patient. We recommended that he start low-dose isotretinoin (20 mg/d) due to the large body surface area affected, making focal and field treatments likely insufficient. However, our patient opted not to treat and did not return for follow-up.
Subtypes of porokeratosis, including disseminated superficial actinic porokeratosis (DSAP) and PPPD, are conditions that disrupt the normal maturation of keratin and present clinically with symmetric, crusted, annular papules.1 The signature but nonspecific histopathologic feature shared among the subtypes is the presence of a cornoid lamellae.2 Several triggers of porokeratosis have been proposed, including trauma and exposure to UV and ionizing radiation.2,3 The clinical variants of porokeratosis are important conditions to diagnose correctly because they portend a risk for Bowen disease and invasive squamous cell carcinoma and may indicate the presence of an underlying hematologic and/or solid organ malignancy.4 Management of porokeratosis is difficult, as treatments have shown limited efficacy and variable recurrence rates. Treatment options include focal, field, and systemic options, such as 5-fluorouracil, topical compound of cholesterol and lovastatin, isotretinoin, and acitretin.1,2
Porokeratoses may arise from gene mutations in the mevalonate pathway,5 which is essential for the production of cholesterol.6 Topical cholesterol alone has not been shown to improve porokeratosis, but the combination topical therapy of cholesterol and lovastatin is promising. It is theorized to deliver benefit by both providing the essential end product of the pathway and simultaneously reducing the number of potentially toxic intermediates.6
Porokeratosis plantaris palmaris et disseminata (also known as porokeratosis plantaris) is unique among the subtypes of porokeratosis in that its annular, red-pink, papular rash with scaling and a keratotic border tends to start distally, involving the palms and soles, and progresses proximally to the trunk with smaller lesions.1,7 This centripetal progression can take years, as was seen in our patient.1 The disease is uncommon, with a dearth of published reports on PPPD.2 However, case reports have shown that PPPD is strongly linked to family history and may have an autosomal-dominant inheritance pattern. Penetrance is greater in men than in women, as PPPD is twice as common in men.8 Most cases of PPPD have been diagnosed in patients in their 20s and 30s, but Hartman et al9 reported a case wherein a patient was diagnosed with PPPD after 65 years of age, similar to our patient.
Although the lesions in DSAP can appear similar to those in PPPD, DSAP is more common among the family of porokeratotic conditions, affecting women twice as often as men, with a sporadic pattern of inheritance.2 These same features are present in some other types of porokeratosis but not PPPD. Furthermore, DSAP progresses proximally to distally but often with truncal sparing.2
Akin to PPPD, pityriasis rubra pilaris (PRP) often presents with palmoplantar keratoderma.10 There are at least 6 types of PRP with varying degrees of similarity to PPPD. However, in many cases PRP is associated with a background of diffuse erythema on the body with islands of spared skin. In addition, cases of PRP have been linked to extracutaneous findings such as ectropion and joint pain.11
Darier disease, especially the acrokeratosis verruciformis of Hopf variant, is more common in men and involves younger populations, as in PPPD.11 However, the crusted lesions seen in Darier disease frequently involve the skin folds. These intertriginous lesions may coalesce, mimicking warts in appearance, and are at risk for secondary infection. Nail findings in Darier disease also are distinct and include longitudinal white or red stripes running along the nail bed, in addition to V-shaped nicks at the nail tips.
Psoriasis can occur anywhere on the body and is associated with silver scaling atop a salmon-colored dermatitis.12 It results from aberrant proliferation of keratinocytes. Some distinguishing features of psoriasis include a disease course that waxes and wanes as well as pitting of the nails.
Although PPPD typically affects young adults, we presented a case of PPPD in an older man. Porokeratosis plantaris palmaris et disseminata in older adults may represent a delayed diagnosis, imply a broader range for the age of onset, or suggest its manifestation secondary to radiation treatment or another phenomenon. For example, our patient received 35 radiotherapy cycles for tongue cancer more than 5 years prior to the onset of PPPD.
- Irisawa R, Yamazaki M, Yamamoto T, et al. A case of porokeratosis plantaris palmaris et disseminata and literature review. Dermatol Online J. 2012;18:5.
- Vargas-Mora P, Morgado-Carrasco D, Fusta-Novell X. Porokeratosis: a review of its pathophysiology, clinical manifestations, diagnosis, and treatment. Actas Dermosifiliogr. 2020;111:545-560.
- James AJ, Clarke LE, Elenitsas R, et al. Segmental porokeratosis after radiation therapy for follicular lymphoma. J Am Acad Dermatol. 2008;58(2 suppl):S49-S50.
- Schena D, Papagrigoraki A, Frigo A, et al. Eruptive disseminated porokeratosis associated with internal malignancies: a case report. Cutis. 2010;85:156-159.
- Zhang Z, Li C, Wu F, et al. Genomic variations of the mevalonate pathway in porokeratosis. Elife. 2015;4:E06322. doi:10.7554/eLife.06322
- Atzmony L, Lim YH, Hamilton C, et al. Topical cholesterol/lovastatin for the treatment of porokeratosis: a pathogenesis-directed therapy. J Am Acad Dermatol. 2020;82:123-131. doi:10.1016/j.jaad.2019.08.043
- Guss SB, Osbourn RA, Lutzner MA. Porokeratosis plantaris, palmaris, et disseminata. a third type of porokeratosis. Arch Dermatol. 1971;104:366-373.
- Kanitakis J. Porokeratoses: an update of clinical, aetiopathogenic and therapeutic features. Eur J Dermatol. 2014;24:533-544.
- Hartman R, Mandal R, Sanchez M, et al. Porokeratosis plantaris, palmaris, et disseminata. Dermatol Online J. 2010;16:22.
- Suryawanshi H, Dhobley A, Sharma A, et al. Darier disease: a rare genodermatosis. J Oral Maxillofac Pathol. 2017;21:321. doi:10.4103/jomfp.JOMFP_170_16
- Eastham AB. Pityriasis rubra pilaris. JAMA Dermatol. 2019;155:404. doi:10.1001/jamadermatol.2018.5030
- Nair PA, Badri T. Psoriasis. StatPearls Publishing; 2022. Updated April 6, 2022. Accessed March 13, 2023. https://www.ncbi.nlm.nih.gov/books/NBK448194/
- Irisawa R, Yamazaki M, Yamamoto T, et al. A case of porokeratosis plantaris palmaris et disseminata and literature review. Dermatol Online J. 2012;18:5.
- Vargas-Mora P, Morgado-Carrasco D, Fusta-Novell X. Porokeratosis: a review of its pathophysiology, clinical manifestations, diagnosis, and treatment. Actas Dermosifiliogr. 2020;111:545-560.
- James AJ, Clarke LE, Elenitsas R, et al. Segmental porokeratosis after radiation therapy for follicular lymphoma. J Am Acad Dermatol. 2008;58(2 suppl):S49-S50.
- Schena D, Papagrigoraki A, Frigo A, et al. Eruptive disseminated porokeratosis associated with internal malignancies: a case report. Cutis. 2010;85:156-159.
- Zhang Z, Li C, Wu F, et al. Genomic variations of the mevalonate pathway in porokeratosis. Elife. 2015;4:E06322. doi:10.7554/eLife.06322
- Atzmony L, Lim YH, Hamilton C, et al. Topical cholesterol/lovastatin for the treatment of porokeratosis: a pathogenesis-directed therapy. J Am Acad Dermatol. 2020;82:123-131. doi:10.1016/j.jaad.2019.08.043
- Guss SB, Osbourn RA, Lutzner MA. Porokeratosis plantaris, palmaris, et disseminata. a third type of porokeratosis. Arch Dermatol. 1971;104:366-373.
- Kanitakis J. Porokeratoses: an update of clinical, aetiopathogenic and therapeutic features. Eur J Dermatol. 2014;24:533-544.
- Hartman R, Mandal R, Sanchez M, et al. Porokeratosis plantaris, palmaris, et disseminata. Dermatol Online J. 2010;16:22.
- Suryawanshi H, Dhobley A, Sharma A, et al. Darier disease: a rare genodermatosis. J Oral Maxillofac Pathol. 2017;21:321. doi:10.4103/jomfp.JOMFP_170_16
- Eastham AB. Pityriasis rubra pilaris. JAMA Dermatol. 2019;155:404. doi:10.1001/jamadermatol.2018.5030
- Nair PA, Badri T. Psoriasis. StatPearls Publishing; 2022. Updated April 6, 2022. Accessed March 13, 2023. https://www.ncbi.nlm.nih.gov/books/NBK448194/
A 67-year-old man presented to our office with a rash on the hands, feet, and periungual skin that began with wartlike growths many years prior and recently had started to involve the proximal arms and legs up to the thighs as well as the trunk. He had a medical history of essential hypertension and chronic obstructive pulmonary disease. He had an 18-year smoking history and had quit more than 25 years prior, with tongue cancer diagnosed more than 5 years prior that was treated with surgery, chemotherapy, and radiation. The lesions occasionally were itchy but not painful. He also reported that his nails frequently split down the middle. He denied any oral lesions and was not using any treatments for the rash. He had no history of skin cancer or other skin conditions. His family history was unclear. Physical examination revealed annular red-pink scaling with a keratotic border on the soles of the feet, palms, and periungual skin. There also were small hyperpigmented papules on the arms, legs, thighs, and trunk over a background of dry and discolored skin, as well as dystrophy of all nails.
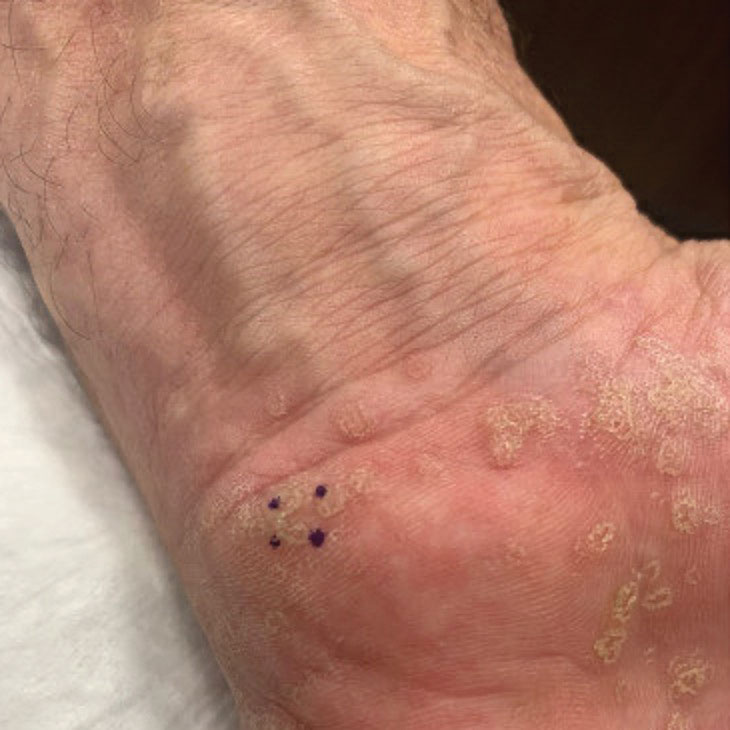
Advanced Breast Cancer Pathophysiology
Kickback Scheme Nets Prison Time for Philadelphia VAMC Service Chief
A former manager at the Philadelphia Veterans Affairs Medical Center (VAMC) has been sentenced to 6 months in federal prison for his part in a bribery scheme.
Ralph Johnson was convicted of accepting $30,000 in kickbacks and bribes for steering contracts to Earron and Carlicha Starks, who ran Ekno Medical Supply and Collondale Medical Supply from 2009 to 2019. Johnson served as chief of environmental services at the medical center. He admitted to receiving cash in binders and packages mailed to his home between 2018 and 2019.
The Starkses pleaded guilty first to paying kickbacks on $7 million worth of contracts to Florida VA facilities, then participated in a sting that implicated Johnson.
The VA Office of Inspector General began investigating Johnson in 2018 after the Starkses, who were indicted for bribing staff at US Department of Veterans Affairs (VA) hospitals in Miami and West Palm Beach, Florida, said they also paid officials in VA facilities on the East Coast.
According to the Philadelphia Inquirer, the judge credited Johnson’s past military service and his “extensive cooperation” with federal authorities investigating fraud within the VA. Johnson apologized to his former employers: “Throughout these 2 and a half years [since the arrest] there’s not a day I don’t think about the wrongness that I did.”
In addition to the prison sentence, Johnson has been ordered to pay back, at $50 a month, the $440,000-plus he cost the Philadelphia VAMC in fraudulent and bloated contracts.
Johnson is at least the third Philadelphia VAMC employee indicted or sentenced for fraud since 2020.
A former manager at the Philadelphia Veterans Affairs Medical Center (VAMC) has been sentenced to 6 months in federal prison for his part in a bribery scheme.
Ralph Johnson was convicted of accepting $30,000 in kickbacks and bribes for steering contracts to Earron and Carlicha Starks, who ran Ekno Medical Supply and Collondale Medical Supply from 2009 to 2019. Johnson served as chief of environmental services at the medical center. He admitted to receiving cash in binders and packages mailed to his home between 2018 and 2019.
The Starkses pleaded guilty first to paying kickbacks on $7 million worth of contracts to Florida VA facilities, then participated in a sting that implicated Johnson.
The VA Office of Inspector General began investigating Johnson in 2018 after the Starkses, who were indicted for bribing staff at US Department of Veterans Affairs (VA) hospitals in Miami and West Palm Beach, Florida, said they also paid officials in VA facilities on the East Coast.
According to the Philadelphia Inquirer, the judge credited Johnson’s past military service and his “extensive cooperation” with federal authorities investigating fraud within the VA. Johnson apologized to his former employers: “Throughout these 2 and a half years [since the arrest] there’s not a day I don’t think about the wrongness that I did.”
In addition to the prison sentence, Johnson has been ordered to pay back, at $50 a month, the $440,000-plus he cost the Philadelphia VAMC in fraudulent and bloated contracts.
Johnson is at least the third Philadelphia VAMC employee indicted or sentenced for fraud since 2020.
A former manager at the Philadelphia Veterans Affairs Medical Center (VAMC) has been sentenced to 6 months in federal prison for his part in a bribery scheme.
Ralph Johnson was convicted of accepting $30,000 in kickbacks and bribes for steering contracts to Earron and Carlicha Starks, who ran Ekno Medical Supply and Collondale Medical Supply from 2009 to 2019. Johnson served as chief of environmental services at the medical center. He admitted to receiving cash in binders and packages mailed to his home between 2018 and 2019.
The Starkses pleaded guilty first to paying kickbacks on $7 million worth of contracts to Florida VA facilities, then participated in a sting that implicated Johnson.
The VA Office of Inspector General began investigating Johnson in 2018 after the Starkses, who were indicted for bribing staff at US Department of Veterans Affairs (VA) hospitals in Miami and West Palm Beach, Florida, said they also paid officials in VA facilities on the East Coast.
According to the Philadelphia Inquirer, the judge credited Johnson’s past military service and his “extensive cooperation” with federal authorities investigating fraud within the VA. Johnson apologized to his former employers: “Throughout these 2 and a half years [since the arrest] there’s not a day I don’t think about the wrongness that I did.”
In addition to the prison sentence, Johnson has been ordered to pay back, at $50 a month, the $440,000-plus he cost the Philadelphia VAMC in fraudulent and bloated contracts.
Johnson is at least the third Philadelphia VAMC employee indicted or sentenced for fraud since 2020.
Targeted Agents Plus Endocrine Therapy in HR+/HER2- Advanced Breast Cancer
The past decade has brought tremendous progress in the treatment of HR+/HER2- advanced breast cancer. The use of newer targeted agents, including PI3K, mTOR, and CDK4/6 inhibitors, in combination with endocrine therapy (ET) may prove a valuable strategy to overcome ET resistance and further extend patient survival.
In this ReCAP, Dr Richard Finn, of the Geffen School of Medicine at UCLA, discusses the growing body of research into CDK4/6 inhibition, PI3K inhibition, and mTOR inhibition, with and without ET. He touches on findings from the EMERALD, MAINTAIN, and monarchE studies to highlight evidence supporting that these targeted agents, in combination with ET, may improve outcomes for patients with advanced disease.
He comments on approaches to sequencing ET and novel agents for patients with recurrence or disease progression, taking into consideration their unique tumor burden, pace of disease, and possible gene mutations. Dr Finn concludes by advising that clinical trial enrollment can provide high-risk patients access to the newest treatments.
--
Professor, Department of Medicine, Division of Hematology/Oncology, Geffen School of Medicine at UCLA, Los Angeles, California
Richard S. Finn, MD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: AstraZeneca; Bayer; CStone; Eisai; Exelixis; Eli Lilly; Pfizer; Merck; Roche; Genentech; Jiangsu Hengrui
Serve(d) as a speaker or a member of a speakers bureau for: Genentech Institution received research grant from: Bayer; Eli Lilly; Eisai; Pfizer; Roche; Genentech
Received income in an amount equal to or greater than $250 from: AstraZeneca; Bayer; CStone; Eisai; Exelixis; Eli Lilly; Pfizer; Merck; Roche; Genentech; Jiangsu Hengrui
The past decade has brought tremendous progress in the treatment of HR+/HER2- advanced breast cancer. The use of newer targeted agents, including PI3K, mTOR, and CDK4/6 inhibitors, in combination with endocrine therapy (ET) may prove a valuable strategy to overcome ET resistance and further extend patient survival.
In this ReCAP, Dr Richard Finn, of the Geffen School of Medicine at UCLA, discusses the growing body of research into CDK4/6 inhibition, PI3K inhibition, and mTOR inhibition, with and without ET. He touches on findings from the EMERALD, MAINTAIN, and monarchE studies to highlight evidence supporting that these targeted agents, in combination with ET, may improve outcomes for patients with advanced disease.
He comments on approaches to sequencing ET and novel agents for patients with recurrence or disease progression, taking into consideration their unique tumor burden, pace of disease, and possible gene mutations. Dr Finn concludes by advising that clinical trial enrollment can provide high-risk patients access to the newest treatments.
--
Professor, Department of Medicine, Division of Hematology/Oncology, Geffen School of Medicine at UCLA, Los Angeles, California
Richard S. Finn, MD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: AstraZeneca; Bayer; CStone; Eisai; Exelixis; Eli Lilly; Pfizer; Merck; Roche; Genentech; Jiangsu Hengrui
Serve(d) as a speaker or a member of a speakers bureau for: Genentech Institution received research grant from: Bayer; Eli Lilly; Eisai; Pfizer; Roche; Genentech
Received income in an amount equal to or greater than $250 from: AstraZeneca; Bayer; CStone; Eisai; Exelixis; Eli Lilly; Pfizer; Merck; Roche; Genentech; Jiangsu Hengrui
The past decade has brought tremendous progress in the treatment of HR+/HER2- advanced breast cancer. The use of newer targeted agents, including PI3K, mTOR, and CDK4/6 inhibitors, in combination with endocrine therapy (ET) may prove a valuable strategy to overcome ET resistance and further extend patient survival.
In this ReCAP, Dr Richard Finn, of the Geffen School of Medicine at UCLA, discusses the growing body of research into CDK4/6 inhibition, PI3K inhibition, and mTOR inhibition, with and without ET. He touches on findings from the EMERALD, MAINTAIN, and monarchE studies to highlight evidence supporting that these targeted agents, in combination with ET, may improve outcomes for patients with advanced disease.
He comments on approaches to sequencing ET and novel agents for patients with recurrence or disease progression, taking into consideration their unique tumor burden, pace of disease, and possible gene mutations. Dr Finn concludes by advising that clinical trial enrollment can provide high-risk patients access to the newest treatments.
--
Professor, Department of Medicine, Division of Hematology/Oncology, Geffen School of Medicine at UCLA, Los Angeles, California
Richard S. Finn, MD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: AstraZeneca; Bayer; CStone; Eisai; Exelixis; Eli Lilly; Pfizer; Merck; Roche; Genentech; Jiangsu Hengrui
Serve(d) as a speaker or a member of a speakers bureau for: Genentech Institution received research grant from: Bayer; Eli Lilly; Eisai; Pfizer; Roche; Genentech
Received income in an amount equal to or greater than $250 from: AstraZeneca; Bayer; CStone; Eisai; Exelixis; Eli Lilly; Pfizer; Merck; Roche; Genentech; Jiangsu Hengrui

Adult ADHD: 6 studies of pharmacologic interventions
Attention-deficit/hyperactivity disorder (ADHD) is a developmental disorder that begins in childhood and continues into adulthood. The clinical presentation is characterized by a persistent pattern of inattention, impulsivity, and/or hyperactivity that causes functional interference.1 ADHD affects patients’ interpersonal and professional lives as well as their daily functioning.2 Adults with ADHD may suffer from excessive self-criticism, low self-esteem, and sensitivity to criticism.3 The overall prevalence of adult ADHD is 4.4%.4 ADHD in adults is frequently associated with comorbid psychiatric disorders.5 The diagnosis of ADHD in adults requires the presence of ≥5 symptoms of inattention and hyperactivity/impulsivity that persist for ≥6 months. Patients must have first had such symptoms before age 12; symptoms need to be present in ≥2 settings and interfere with functioning.1
Treatment of ADHD includes pharmacologic and nonpharmacologic interventions. For most patients, pharmacotherapy—specifically stimulant medications—is advised as first-line treatment,6 with adequate trials of methylphenidate and amphetamines before using second-line agents such as nonstimulants. However, despite these medications’ efficacy in randomized controlled trials (RCTs), adherence is low.7 This could be due to inadequate response or adverse effects.8 Guidelines also recommend the use of nonpharmacologic interventions for adults who cannot adhere to or tolerate medication or have an inadequate response.6 Potential nonpharmacologic interventions include transcranial direct current stimulation, mindfulness, psychoeducation, cognitive-behavioral therapy, and chronotherapy.
In Part 1 of this 2-part article, we review 6 RCTs of pharmacologic interventions for adult ADHD published within the last 5 years (Table9-14). Part 2 will review nonpharmacologic treatments.
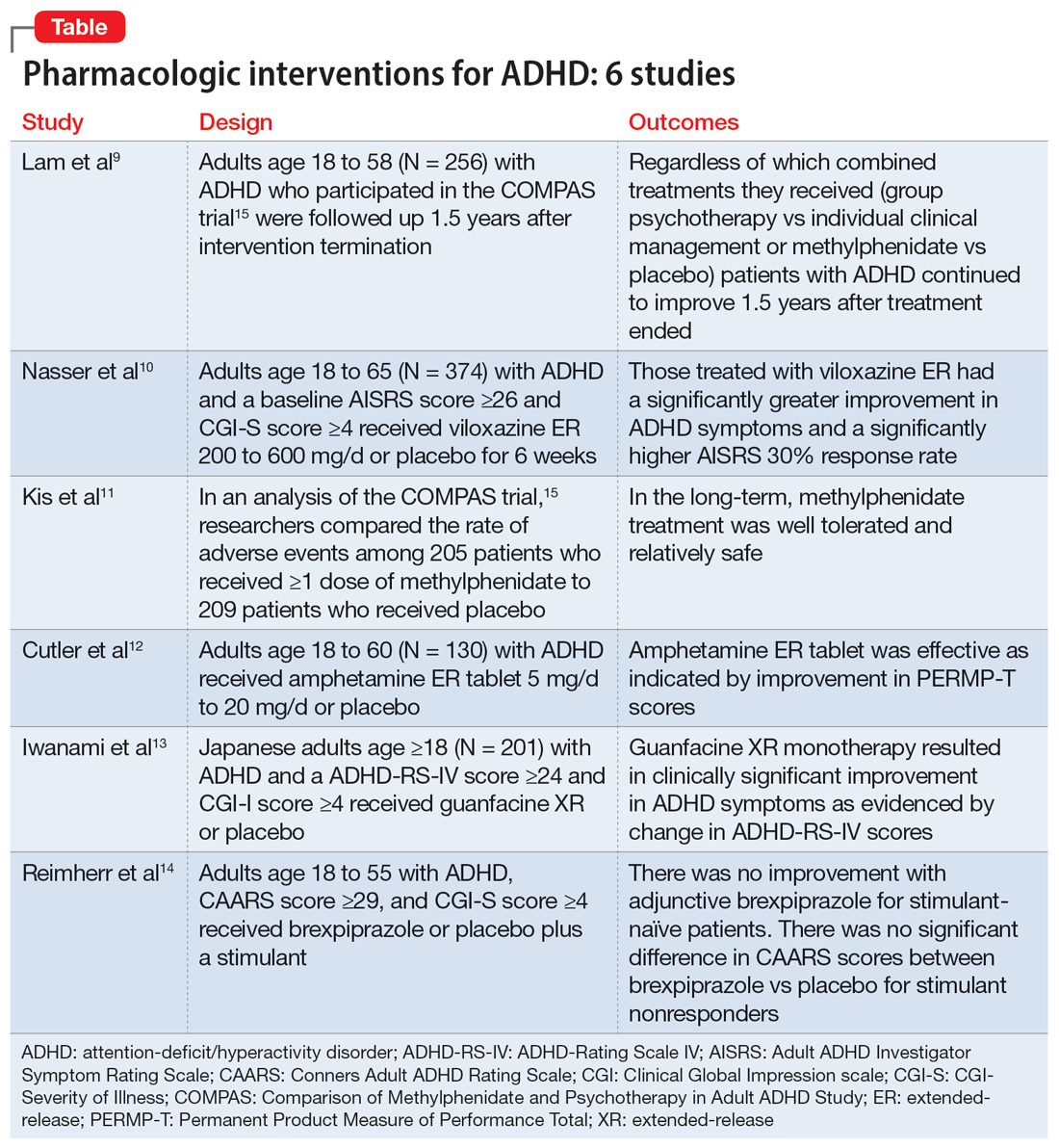
1. Lam AP, Matthies S, Graf E, et al; Comparison of Methylphenidate and Psychotherapy in Adult ADHD Study (COMPAS) Consortium. Long-term effects of multimodal treatment on adult attention-deficit/hyperactivity disorder symptoms: follow-up analysis of the COMPAS Trial. JAMA Netw Open. 2019;2(5):e194980. doi:10.1001/jamanetworkopen.2019.4980
The Comparison of Methylphenidate and Psychotherapy in Adult ADHD Study (COMPAS) was a multicenter prospective, randomized trial of adults age 18 to 58 with ADHD.15 It compared cognitive-behavioral group psychotherapy (GPT) with individual clinical management (CM), and methylphenidate with placebo. When used in conjunction with methylphenidate, psychological treatments produced better results than placebo. However, studies on the long-term effects of multimodal treatment in ADHD are limited. Lam et al9 performed a follow-up analysis of the COMPAS trial.
Study design
- This observer-masked study involved a follow-up of participants in COMPAS 1.5 years after the interventions were terminated. Of the 433 adults with ADHD who participated in COMPAS, 256 participated in this follow-up.
- The inclusion criteria of COMPAS were age 18 to 58; diagnosis of ADHD according to DSM-IV criteria; chronic course of ADHD symptoms from childhood to adulthood; a Wender Utah Rating Scale short version score ≥30; and no pathological abnormality detected on physical examination.
- The exclusion criteria were having an IQ <85; schizophrenia, bipolar disorder (BD), borderline personality disorder, antisocial personality disorder, suicidal or self-injurious behavior, autism, motor tics, or Tourette syndrome; substance abuse/dependence within 6 months prior to screening; positive drug screening; neurologic diseases, seizures, glaucoma, diabetes, hyperlipidemia, uncontrolled arterial hypertension, angina pectoris, tachycardia arrhythmia, or arterial occlusive disease; previous stroke; current bulimia or anorexia; low weight (body mass index [BMI] <20; pregnancy (current or planned) or breastfeeding; treatment with stimulants or ADHD-specific psychotherapy in the past 6 months; methylphenidate intolerance; treatment with antidepressants, norepinephrine reuptake inhibitors, bupropion, antipsychotics, theophylline, amantadine, anticoagulants derived from coumarin, antacids, or alpha-adrenergic agonists in the 2 weeks prior to baseline; and treatment with fluoxetine or monoamine oxidase inhibitors in the 4 weeks prior to baseline.
- The primary outcome was a change from baseline on the ADHD Index of Conners Adult ADHD Rating Scale (CAARS) score. Secondary outcomes were self-ratings on the Beck Depression Inventory (BDI) and observer-masked ratings of the Clinical Global Impression (CGI) scale and other ADHD rating scale scores, such as the Diagnostic Checklist for the diagnosis of ADHD in adults (ADHD-DC) and subscales of the CAARS.
- COMPAS was open regarding patient and therapist assignment to GPT and CM, but double-masked regarding medication. The statistical analysis focused on the 2x2 comparison of GPT vs CM and methylphenidate vs placebo.
Outcomes
- A total of 251 participants had an assessment with the observer-masked CAARS score. The baseline mean (SD) age was 36.3 (10.1), and approximately one-half (49.8%) of participants were male.
- Overall, 9.2% of patients took methylphenidate >31 days from termination of COMPAS before this study but not at the start of this study. Approximately one-third (31.1%) of patients were taking methylphenidate at follow-up. The mean (SD) daily dosage of methylphenidate was 36 (24.77) mg and 0.46 (0.27) mg/kg of body weight.
- The baseline all-group mean ADHD Index of CAARS score was 20.6. At follow-up, it was 14.7 for the CM arm and 14.2 for the GPT arm (difference not significant, P = .48). The mean score decreased to 13.8 for the methylphenidate arm and to 15.2 for the placebo (significant difference, P = .04).
- Overall, methylphenidate was associated with greater improvement in symptoms than placebo. Patients in the GPT arm had fewer severe symptoms as assessed by the self-reported ADHD Symptoms Total Score compared to the CM arm (P = .04).
- There were no significant differences in self-rating CAARS and observer-rated CAARS subscale scores. Compared to CM, GPT significantly decreased pure hyperactive symptoms on the ADHD-DC (P = .08). No significant differences were observed in BDI scores. The difference between GPT and CM remained significant at follow-up in terms of the CGI evaluation of efficacy (P = .04).
Continue to: Conclusions/limitations
Conclusions/limitations
- Regardless of which combined treatments they received, patients with ADHD continued to improve 1.5 years after the 52-week treatment phase ended.
- Patients assigned to methylphenidate performed considerably better on the observer-rated CAARS than patients assigned to placebo.
- Benefits from GPT or CM in addition to methylphenidate therapy lasted 1.5 years. Compared to CM, GPT was not linked to better scores on the CAARS.
- Limitations: Approximately 41% of patients who were recruited did not participate. Daily functioning was measured only by the CGI. There were only marginal differences among the 4 treatments, and the study compared a very regimented approach (GPT) with one that was less focused (CM).
2. Nasser A, Hull JT, Chaturvedi SA, et al. A phase III, randomized, double‐blind, placebo‐controlled trial assessing the efficacy and safety of viloxazine extended‐release capsules in adults with attention‐deficit/hyperactivity disorder. CNS Drugs. 2022;36(8): 897-915. doi:10.1007/s40263-022-00938-w
In 2021, the FDA approved viloxazine extended-release (ER) for treating ADHD in children and adolescents (age 6 to 17). Nasser et al10 reviewed the safety and efficacy of viloxazine ER in adults with ADHD.
Study design
- This phase III, randomized, double-blind, placebo-controlled, multicenter clinical trial included 374 adults with ADHD who received viloxazine ER or placebo.
- Participants were age 18 to 65 and had been given a primary diagnosis of ADHD according to DSM-5 criteria in the last 6 months. Other inclusion criteria were having an Adult ADHD Investigator Symptom Rating Scale (AISRS) total score ≥26 and CGI-Severity of Illness (CGI-S) score ≥4 at baseline, BMI 18 to 35 kg/m2, and being medically healthy.
- Exclusion criteria included having treatment-resistant ADHD, a current diagnosis of any psychiatric disorder other than ADHD, or a history of schizophrenia, schizoaffective disorder, BD, autism, obsessive-compulsive disorder, personality disorder, or posttraumatic stress disorder. Individuals with any significant neurologic disorder, heart condition, arrhythmia, clinically relevant vital sign abnormality, or systemic illness were excluded, as were those with a history (within the past year) or current diagnosis of substance use disorder or a positive drug screen for a drug of abuse. Those with an allergic reaction or intolerance to viloxazine or were breastfeeding, pregnant, or refused to be abstinent or practice birth control were excluded.
- The dosage of viloxazine ER ranged from 200 to 600 mg/d for 6 weeks. This was titrated based on symptom response and adverse effects.
- All individuals received 2 capsules once a day for Week 1 and Week 2. During Week 1 and Week 2, participants in the viloxazine ER group received 200 mg (1 viloxazine ER capsule and 1 placebo capsule) and 400 mg (2 viloxazine ER capsules) of the medication, respectively. Two placebo pills were administered to those in the placebo group. From Week 3 to Week 6, the dose could be titrated or tapered at the investigator’s discretion. Compliance was assessed by comparing the number of pills dispensed vs returned.
- The primary outcome was a change in AISRS score from baselines to Week 6.
- The key secondary outcome was the change in CGI-S score from baseline to Week 6. Scores on the AISRS inattention and hyperactive/impulsivity subscales, Behavioral Regulation Index, Metacognition Index, Behavior Rating Inventory of Executive Function–Adult Version (BRIEF-A), and Generalized Anxiety Disorder-7 item scale (GAD-7) were also evaluated. Also, the rates of 30% and 50% responders on the AISRS (defined as ≥30% or ≥50% reduction from baseline in AISRS total score, respectively), CGI-S scores, and CGI-Improvement (CGI-I) scores were examined.
Outcomes
- Based on change in AISRS total scores, patients who received viloxazine ER had significantly greater improvement in their ADHD symptoms than those taking placebo (P = .0040). Patients in the viloxazine ER group had significantly greater improvement in AISRS hyperactive/impulsive (P = .0380) and inattentive symptoms (P = .0015).
- The decrease in CGI-S score was also significantly greater in the viloxazine ER group than in the placebo group (P = .0023). The viloxazine ER group also had significantly greater improvement in executive function as assessed by the BRIEF-A (P = .0468). The difference in GAD-7 scores between the viloxazine ER group and the placebo group was not significant.
- The viloxazine ER group had a greater AISRS 30% response rate than the placebo group (P = .0395). There were no significant differences between groups in AISRS 50% responder rate or CGI-I responder rate.
- Adverse effects related to viloxazine and occurring in ≥5% of participants included insomnia (14.8%), fatigue (11.6%), nausea, decreased appetite (10.1%), dry mouth (9.0%), and headache (9.0%). The discontinuation rate was 9.0% in the viloxazine ER group vs 4.9% in the placebo group.
Continue to: Conclusions/limitations
Conclusions/limitations
- Compared to placebo, patients treated with viloxazine ER had significantly greater improvements in ADHD symptoms, including both hyperactive/impulsive and inattentive components as well as executive function.
- The viloxazine ER group had a significantly higher AISRS 30% response rate than the placebo group, but there were no significant differences in anxiety symptoms or other measures of response.
- Viloxazine ER was well tolerated and safe.
- Limitations: There was a reduced power to detect differences in treatment due to participants dropping out or discontinuing treatment, a lack of interrater reliability data, and a lack of patient-reported outcome or satisfaction data.
3. Kis B, Lücke C, Abdel-Hamid M, et al. Safety profile of methylphenidate under long-term treatment in adult ADHD patients - results of the COMPAS study. Pharmacopsychiatry. 2020;53(6):263-271. doi:10.1055/a-1207-9851
Kis et al11 analyzed the safety results of COMPAS.15 Details of this trial, including interventions and inclusion/exclusion criteria, are described in the description of Lam et al.9
Study design
- Researchers compared the rate of adverse events (AEs) among 205 patients who received ≥1 dose of methylphenidate with 209 patients who received placebo.
- AEs were documented and analyzed on an “as received” basis during Week 0 to Week 52. Electrocardiogram (ECG) data were recorded at baseline and Week 24. Vital signs were monitored at baseline, every week for the first 12 weeks, then every 4 weeks for the next 52 weeks. Body weight was assessed at Week 6, Week 12, Week 20, Week 28, Week 40, and Week 52. A 12-lead ECG was obtained at baseline and Week 24.
- The sample size was assessed to have 80% power to detect group differences in AEs.
Outcomes
- Overall, 96% of participants in the methylphenidate group and 88% of participants in the placebo group experienced at least 1 AE (difference 8.1%; 95% CI, 2.9% to 13.5%).
- AEs that occurred more frequently with methylphenidate compared to placebo were decreased appetite (22% vs 3.8%); dry mouth (15% vs 4.8%); palpitations (13% vs 3.3%); gastrointestinal (GI) infection (11% vs 4.8%); agitation (11% vs 3.3%); restlessness (10% vs 2.9%); hyperhidrosis, tachycardia, and weight decrease (all 6.3% vs 1.9%); depressive symptoms and influenza (both 4.9% vs 1.0%); and acute tonsillitis (4.4% vs 0.5%). Serious AEs were reported by 7.3% of patients in the methylphenidate group and 4.3% of those in the placebo group, with no difference in frequency (difference 3.0%; 95% CI, 1.6% to 7.9%). The most severe AEs were aggression, depression, somnambulism, and suicidal ideation in the methylphenidate group and car accidents, epicondylitis, and a fall in the placebo group.
- There were no significant differences in AEs between the GPT and CM groups.
- The treatment combinations that included methylphenidate had higher rates of patients experiencing at least 1 AE (CM/methylphenidate 97%, GPT/methylphenidate 96%, CM/placebo 92%, GPT/placebo 84%).
- Overall, 8.8% of patients in the methylphenidate group and 4.8% in the placebo group stopped their medication treatment because of an AE (difference 4.0%; 95% CI, 0.9% to 9.1%). At least 1 dose decrease, increase, or discontinuation was made after an AE in 42% of participants in the placebo group and 69% of those in the methylphenidate group.
- There were no significant differences in clinically pertinent ECG abnormalities between methylphenidate and placebo therapy.
Continue to: Conclusions/limitations
Conclusions/limitations
- AEs were more common in the methylphenidate groups compared to placebo, but there was no significant differences for severe AEs. In the long-term, methylphenidate treatment was well tolerated and relatively safe.
- Limitations: The sample size may have been too small to detect uncommon AEs, all AEs had to be reported and may not have been caused by the treatment, and the original study’s main outcome was efficacy, not safety, which makes this an exploratory analysis of AEs.
4. Cutler AJ, Childress AC, Pardo A, et al. Randomized, double-blind, placebo-controlled, fixed-dose study to evaluate the efficacy and safety of amphetamine extended-release tablets in adults with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2022;83(5):22m14438. doi:10.4088/JCP.22m14438
Once-daily dosing of stimulants, which are commonly used to manage adult ADHD,16 can be beneficial because many patients have schedules that limit taking medication multiple times a day. Cutler et al12 looked at the efficacy and safety of amphetamine extended-release tablet (AMPH ER TAB), which is a 3.2:1 mixture of d- and l-amphetamine released by the LiquiXR drug delivery system. This technology allows for a continuous release following an initial quick onset of action.
Study design
- This parallel-study, double-blind study evaluated adults age 18 to 60 who had a diagnosis of ADHD according to DSM-5 criteria and the Adult ADHD Clinical Diagnostic Scale, normal-range IQ, AISRS score ≥26, and baseline CGI-S score ≥4.
- Women were not lactating or pregnant during the study.
- Exclusion criteria included a history of mental illnesses; chronic medical conditions; clinically significant abnormal ECG or cardiac findings on exam; renal or liver disease; family history of sudden death; significant vital sign findings; uncontrolled hypertension or a resting systolic blood pressure (SBP) >140 mmHg or diastolic blood pressure (DBP) >90 mmHg; recent history of or current alcohol or substance use disorder; use of atomoxetine, monoamine oxidase inhibitors, or tricyclic antidepressants within 14 days of study or the use of other stimulant medications within 1 week of screening; use of GI acidifying agents or urinary acidifying agents within 3 days of screening; answering “yes” to questions 4 or 5 of the Suicidal Ideation section of the Columbia Suicide Severity Rating Scale within 2 years prior to the study; taking another investigational medication within 30 days of screening; allergic to amphetamine or components of the study drug, and a lack of prior response to amphetamine.
- Patients were randomized to receive AMPH ER TAB (n = 65) or placebo (n = 65), taken before 10
am . Participants started at 5 mg/d of the drug/placebo and then entered a 5-week titration period in which the medication was increased by 5 mg/d each week until reaching 20 mg/d, and then continued 20 mg/d for 2 weeks. - The primary outcome was the mean Permanent Product Measure of Performance Total (PERMP-T) score averaged across all time points (0.5-, 1-, 2-, 4-, 8-, 10-, 12-, 13-, and 14-hours postdose) at Visit 5.
- Participants underwent AISRS, CGI-S, and safety evaluations at baseline and at the 5 visits at the end of each treatment week.
Outcomes
- Analyses were completed on participants who received ≥1 dose of the medication and who had ≥1 PERMP-T score at Visit 5.
- Predose PERMP-T scores were similar between the AMPH ER TAB group (259.5) and placebo group (260). The mean postdose PERMP-T score in the AMPH ER TAB group (302.8) was significantly higher (P = .0043) than the placebo group (279.6).
- The PERMP-T scores were significantly different at 0.5-, 1-, 2-, 4-, 8-, and 13-hours postdose but not at 10-, 12-, and 14-hours postdose. The first Visit 5 time point at which the difference between groups was statistically different was at 0.5 hours postdose (P = .01), and the last significant time point was 13 hours (P = .006).
- The improvement in CGI-S scores was significantly greater in the AMPH ER TAB group than the placebo group. The improvement in AISRS scores was significantly greater in the AMPH ER TAB group at Visit 3, Visit 4, and Visit 5. More participants in the AMPH ER TAB group had AEs compared to the placebo group (90% vs 60%). The most common AEs (frequency ≥5% and occurring more in the intervention arm) were decreased appetite, insomnia, dry mouth, irritability, headache, anxiety, nausea, dizziness, and tachycardia.
- The AMPH ER TAB group had nonclinically significant increases in SBP (116.8 to 120.7 mmHg), DBP (74.1 to 77.1 mmHg), and heart rate (73.0 to 81.9 bpm) at Visit 5 compared to baseline.
- No serious AEs occurred. Three participants in the AMPH ER TAB group experienced AEs (increased blood pressure, CNS stimulation, and anxiety) that led them to discontinue the study.
Continue to: Conclusions/limitations
Conclusions/limitations
- AMPH ER TAB reduced symptoms in adults with ADHD as assessed by improvement in PERMP-T scores.
- The safety and tolerability profile of AMPH ER TAB were comparable to other stimulants, with expected rises in blood pressure and heart rate.
- Limitations: Patients were required to be titrated to 20 mg/d of AMPH ER TAB, instead of following a flexible titration based on an individual’s response. Some participants may have had greater improvement at a higher or lower dose. This study did not compare AMPH ER TAB to other stimulants. The 5-week duration of this study limited the ability to evaluate long-term efficacy and tolerability. Patients with a wide range of psychiatric or medical comorbidities were excluded.
5. Iwanami A, Saito K, Fujiwara M, et al. Efficacy and safety of guanfacine extended-release in the treatment of attention-deficit/hyperactivity disorder in adults: results of a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2020;81(3):19m12979. doi:10.4088/JCP.19m12979
Guanfacine extended-release (GXR) is a selective alpha 2A-adrenergic receptor agonist approved for treating ADHD in children and adolescents.17 Iwanami et al13 evaluated the efficacy and safety of GXR for adults.
Study design
- This randomized, double-blinded, placebo-controlled trial enrolled Japanese adults age ≥18 who were diagnosed with ADHD according to DSM-5 criteria and scored ≥24 on the ADHD-Rating Scale IV (ADHD-RS-IV) and ≥4 on CGI- I.
- Exclusion criteria included having anxiety, depression, substance use disorder, tic disorder, BD, personality disorder, schizophrenia, or intellectual disability; a moderate or severe psychiatric disorder requiring treatment other than counseling; seizures; increased risk for suicide; a history of cardiovascular disease, including prolonged QTc/abnormal ECG/abnormal labs, orthostatic hypotension, or continuous bradycardia; or taking medications that affect blood pressure or heart rate.
- Overall, 101 participants were randomized to the GXR group and 100 to the placebo group. Approximately two-thirds of the study population was male. Patients received GXR or placebo once daily at approximately the same time.
- There were 5 phases to the trial. The screening period occurred over 1 to 4 weeks. Part 1 of the treatment period consisted of 5 weeks of medication optimization. Participants were started on GXR 2 mg/d and were required to be receiving a minimum dose of 4 mg/d starting at Week 3. Clinicians were allowed to increase the dose 1 mg/d per week starting at Week 4 based on clinical response to a maximum dosage of 6 mg/d. Part 2 of the treatment period consisted of 5 weeks of maintenance at 4 to 6 mg/d. The tapering period to 2 mg/d occurred over 2 weeks. The follow-up period lasted 1 week.
- Efficacy measurements included the Japanese version of the ADHD-RS-IV and translations of the English-language CAARS, CGI-I, and CGI-S. Participant-reported measures included the Patient Global Impression-Improvement scale (PGI-I), Adult ADHD Quality of Life Questionnaire (AAQoL), and BRIEF-A.
- The primary outcome was the difference in ADHD-RS-IV total score from baseline to the end of the maintenance period (Week 10).
- Safety assessments were completed at Week 5 (end of dose optimization period), Week 10 (end of dose maintenance period), and Week 12 (tapering period).
Outcomes
- The average GXR dose during the maintenance period was 5.07 mg/d.
- Compared to the placebo group, the GXR group had more patients age <30 (47% vs 39%) and fewer patients age ≥40 (17% vs 27%). Baseline ADHD-RS-IV scores in both groups were comparable. At baseline, 51% in the GXR group had a combined inattentive/hyperactive-impulsive presentation and 47% had a predominately inattention presentation, with similar characteristics in the placebo group (49% combined, 49% inattention).
- At Week 10, the least squares mean change from baseline on the ADHD-RS-IV total score was significantly greater in the GXR group than in the placebo group (-11.55 ± 1.10 vs -7.27 ± 1.07; P = .0005), with an effect size of 0.52. There was a greater decrease in the ADHD-RS-IV scores starting at Week 4 and continuing to Week 10 (P < .005).
- There were also significant differences favoring GXR on the ADHD-RS-IV hyperactivity-impulsivity subscale score (P = .0021) and ADHD-RS-IV inattention subscale score (P = .0032).
- There were significant differences in the CAARS total ADHD score (P = .0029) and BRIEF-A scores on the inhibit (P = .0173), initiate (P = .0406), plan/organize (P = .174), and global executive composite index (P = .0404) scales. There was no significant difference in the total AAQoL score (P = .0691), but there was a significant improvement in the AAQoL life productivity subscore (P = .0072).
- At Week 10, there were also significant improvements in the CGI-I scores (P = .0007) and PGI-I scores (P = .0283). The CGI-S scores were similar at all time points.
- Overall, 81.2% of GXR patients reported AEs compared to 62% in the placebo group. There was 1 serious treatment-emergent AE (a suicide attempt) that the authors concluded was unrelated to the study drug. No deaths occurred. The most common AEs (incidence ≥10% in either group) included somnolence, thirst, nasopharyngitis (occurring more in the placebo group), blood pressure decrease, postural dizziness, and constipation. The main AEs leading to discontinuation were somnolence and blood pressure decrease. Overall, 19.8% of patients receiving GXR discontinued treatment due to AEs, compared to 3% in the placebo group.
- Heart rate, blood pressure, and QTc (corrected by the Bazett formula) were decreased in the GXR group at Week 10 while QT and RR intervals increased, and most returned to normal by Week 12.
Continue to: Conclusions/limitations
Conclusions/limitations
- Compared to placebo, GXR monotherapy resulted in clinical improvement in ADHD symptoms, with a moderate effect size.
- The most common AEs were mild to moderate and congruent with known adverse effects of guanfacine. Sedation effects mostly transpired within the first week of medication administration and were transient.
- Limitations: The findings might not be generalizable to non-Japanese patients. The duration of the study was short. Patients with a wide range of psychiatric and medical comorbidities were excluded. Two-thirds of the participants were male, and there was a disparity in participant age in the GXR and placebo groups.
6. Reimherr FW, Gift TE, Steans TA, et al. The use of brexpiprazole combined with a stimulant in adults with treatment-resistant attention-deficit/hyperactivity disorder. J Clin Psychopharmacol. 2022;42(5):445-453. doi:10.1097/JCP.0000000000001592
While stimulants are a mainstay ADHD treatment, some patients have a partial response or do not respond to amphetamines or methylphenidate. Reimherr et el14 assessed the efficacy and safety of adding brexpiprazole (BXP) to a stimulant.
Study design
- This randomized, double-blinded, placebo-controlled trial recruited 559 stimulant-naive patients and 174 patients who had not responded to previous stimulant therapy.
- Participants were adults age 18 to 55 with a primary diagnosis of ADHD according to DSM-IV-TR criteria and the Conners Adult ADHD Diagnostic Interview. Other inclusion criteria were having a CAARS score ≥29 and a CGI-S score ≥4.
- Exclusion criteria included being at risk for suicide; having current substance abuse or positive alcohol/drug screens; a history of good response to prestudy treatment; a clinically significant medical condition; fasting blood glucose >200 mg/dL or hemoglobin A1C >7%; and hospitalization in past 12 months from a diabetic complication, uncontrolled hypertension, ischemic heart disease, or epilepsy. Further exclusion criteria included a history of psychosis, current MDD or BD, current panic disorder, uncontrolled comorbid psychiatric condition, or clinically significant personality disorder. Investigators excluded any patient with severe DSM-IV axis I or II disorders or abnormal/psychopathological behaviors.
- The trial consisted of 3 segments. Part 1 was screening. If the patient was currently receiving a stimulant but not fully responding, the medication was discontinued for at least 5 half-lives.
- Part 2 (5 weeks) involved administering a stimulant plus a single-blind placebo (597 patients completed this phase). The stimulant was chosen by the investigator, who had the option of using 1 of 2 amphetamine derivatives (mixed amphetamine salts capsules or lisdexamfetamine dimesylate capsules) or 1 of 2 methylphenidate derivatives (methylphenidate hydrochloride ER tabs or dexmethylphenidate HCl ER capsules). If a patient did not respond to a particular stimulant prior to the study, they were given a different stimulant from the list. Patients continued the same stimulant throughout the trial. Patients were monitored for a response, defined as a ≥30% decrease in CAARS score or a CAARS score <24, or a CGI-I score of 1 or 2 at Week 5. Patients who did not show this improvement were categorized as open-label nonresponders.
- Part 3 (6 weeks) involved administering a stimulant plus double-blind BXP vs placebo (stimulant-naive n = 167, stimulant nonresponders n = 68). Nonresponders continued the stimulant (at the same dose reached at the end of Part 2) and added either BXP (n = 155) or continued placebo (n = 80). Patients who responded in Part 2 were continued on the stimulant plus placebo and were not randomized. Patients were started on BXP 0.25 mg/d, and the medication could be titrated to 2 mg/d during the following 3 weeks, depending on the benefit vs AE profile. After the third week, the dose could be decreased but not increased.
- The primary outcome was a change in CAARS score. Secondary measurements included the CGI-S, Wender-Reimherr Adult Attention Deficit Disorder Scale (WRAADDS), Montgomery-Åsberg Depression Rating Scale (MADRS), and BDI.
Outcomes
- Stimulant-naive patients were equally divided among the 4 stimulant groups, and previous nonresponders who continued to not respond in Part 2 were more likely to be given methylphenidate HCl or lisdexamfetamine dimesylate.
- Patients with a history of nonresponse had less response to stimulants in Part 2 compared to stimulant-naive patients, as seen by 27% (n = 167) of stimulant-naive patients entering Part 3 compared to 39% of prior nonresponders (n = 68; P = .0249).
- ADHD improvement with BXP appeared to be greater among pretrial nonresponders.
- For stimulant nonresponders before and during the study, at the end of the double-blind endpoint (Part 3; Week 11), WRAADDS total score was significantly improved in the BXP group compared to the placebo group (P = .013; d = 0.74), with most beneficial effects seen in the hyperactivity/restlessness, emotional dysregulation factor, and impulsivity categories.
- For stimulant nonresponders before and during the study, there was no significant difference at the end of Week 11 on the CAARS (P = .64), MADRS (P = .37), or BDI (P = .73). There was a trend toward significance on the CAARS subscale for hyperactive/impulsive (P = .09).
- For prestudy stimulant-naive patients who did not respond to stimulants in Part 2 and were randomized in Part 3, there was not a significant difference between BXP and placebo at Week 11 as assessed on WRAADDS, CAARS, MADRS, or BDI.
- As assessed on WRAADDS, 50% in the BXP group had a response compared to 41% in the placebo group (Fisher exact = 0.334). Under the emotional dysregulation factor category of the WRAADDS, 64% in the BXP group had a response compared to 41% in the placebo group (Fisher exact = 0.064). The attention factor category showed a 40% improvement in the BXP group compared to 32% in the placebo group (Fisher exact = 0.344).
- There were 2 serious AEs in the BXP group (gall bladder inflammation and diarrhea) and 2 in the placebo group (pneumonia and urinary tract infection). There was no statistically significant difference between groups with regards to common AEs (ie, fatigue, heartburn/nausea/stomachache, weight loss), although there was a trend to significant for insomnia in the BXP group (P = .083).
Conclusions/limitations
- Stimulant-naive patients experienced no improvement with adjunctive BXP.
- For prior stimulant nonresponders, there was no significant difference between BXP vs placebo on the primary outcome of the CAARS score, but there was an improvement as observed by assessment with the WRAADDS.
- The largest change in the WRAADDS occurred in the emotional dysregulation factor compared to the attention factor.
- BXP appeared to be well tolerated.
- Limitations: The WRAADDS was administered without the patients’ significant other/collateral. Raters were not trained in the use of the WRAADDS. Patients with a wide range of psychiatric and medical comorbidities were excluded. Fewer patients were recruited in the prior stimulant nonresponder group.
Bottom Line
Recent randomized controlled trials suggest that methylphenidate, amphetamine extended-release, viloxazine extended-release, and guanfacine extended-release improved symptoms of adult attention-deficit/hyperactivity disorder (ADHD). There were no improvements in ADHD symptoms with adjunctive brexpiprazole.
Related Resources
- Parikh AR, Baker SA. Adult ADHD: pharmacologic treatment in the DSM-5 era. Current Psychiatry. 2016;15(10):18-25.
- Akbar HN. Why we should be scrutinizing the rising prevalence of adult ADHD. Current Psychiatry. 2022; 21(7):e1-e2. doi:10.12788/cp.0268
Drug Brand Names
Amantadine • Gocovri
Amphetamine extended-release tablet • Dyanavel XR
Atomoxetine • Strattera
Brexpiprazole • Rexulti
Bupropion • Wellbutrin
Dexmethylphenidate • Focalin
Fluoxetine • Prozac
Guanfacine extended- release • Intuniv
Lisdexamfetamine • Vyvanse
Methylphenidate • Concerta, Methylin
Theophylline • Elixophyllin
Viloxazine • Qelbree
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022.
2. Harpin V, Mazzone L, Raynaud JP, et al. Long-term outcomes of ADHD: a systematic review of self-esteem and social function. J Atten Disord. 2016;20(4):295-305. doi:10.1177/1087054713486516
3. Beaton DM, Sirois F, Milne E. Experiences of criticism in adults with ADHD: a qualitative study. PLoS One. 2022;17(2):e0263366. doi:10.1371/journal.pone.0263366
4. Attention-deficit/hyperactivity disorder (ADHD). National Institute of Mental Health. Accessed February 9, 2023. https://www.nimh.nih.gov/health/statistics/attention-deficit-hyperactivity-disorder-adhd
5. Katzman MA, Bilkey TS, Chokka PR, et al. Adult ADHD and comorbid disorders: clinical implications of a dimensional approach. BMC Psychiatry. 2017;17(1):302. doi:10.1186/s12888-017-1463-3
6. Attention Deficit Hyperactivity Disorder: Diagnosis and Management. NICE Guideline No. 87. National Institute for Health and Care Excellence (NICE); 2019. Accessed February 9, 2023. http://www.ncbi.nlm.nih.gov/books/NBK493361/
7. Adler LD, Nierenberg AA. Review of medication adherence in children and adults with ADHD. Postgrad Med. 2010;122(1):184-191. doi:10.3810/pgm.2010.01.2112
8. Cunill R, Castells X, Tobias A, et al. Efficacy, safety and variability in pharmacotherapy for adults with attention deficit hyperactivity disorder: a meta-analysis and meta-regression in over 9000 patients. Psychopharmacology (Berl). 2016;233(2):187-197. doi:10.1007/s00213-015-4099-3
9. Lam AP, Matthies S, Graf E, et al; Comparison of Methylphenidate and Psychotherapy in Adult ADHD Study (COMPAS) Consortium. Long-term effects of multimodal treatment on adult attention-deficit/hyperactivity disorder symptoms: follow-up analysis of the COMPAS Trial. JAMA Netw Open. 2019;2(5):e194980. doi:10.1001/jamanetworkopen.2019.4980
10. Nasser A, Hull JT, Chaturvedi SA, et al. A phase III, randomized, double-blind, placebo-controlled trial assessing the efficacy and safety of viloxazine extended-release capsules in adults with attention-deficit/hyperactivity disorder. CNS Drugs. 2022;36(8):897-915. doi:10.1007/s40263-022-00938-w
11. Kis B, Lücke C, Abdel-Hamid M, et al. Safety profile of methylphenidate under long-term treatment in adult ADHD patients - results of the COMPAS study. Pharmacopsychiatry. 2020;53(6):263-271. doi:10.1055/a-1207-9851
12. Cutler AJ, Childress AC, Pardo A, et al. Randomized, double-blind, placebo-controlled, fixed-dose study to evaluate the efficacy and safety of amphetamine extended-release tablets in adults with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2022;83(5):22m14438. doi:10.4088/JCP.22m14438
13. Iwanami A, Saito K, Fujiwara M, et al. Efficacy and safety of guanfacine extended-release in the treatment of attention-deficit/hyperactivity disorder in adults: results of a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2020;81(3):19m12979. doi:10.4088/JCP.19m12979
14. Reimherr FW, Gift TE, Steans TA, et al. The use of brexpiprazole combined with a stimulant in adults with treatment-resistant attention-deficit/hyperactivity disorder. J Clin Psychopharmacol. 2022;42(5):445-453. doi:10.1097/JCP.0000000000001592
15. Philipsen A, Jans T, Graf E, et al; Comparison of Methylphenidate and Psychotherapy in Adult ADHD Study (COMPAS) Consortium. Effects of group psychotherapy, individual counseling, methylphenidate, and placebo in the treatment of adult attention-deficit/hyperactivity disorder: a randomized clinical trial. JAMA Psychiatry. 2015;72(12):1199-1210.
16. McGough JJ. Treatment controversies in adult ADHD. Am J Psychiatry. 2016;173(10):960-966. doi:10.1176/appi.ajp.2016.15091207
17. Cruz MP. Guanfacine extended-release tablets (Intuniv), a nonstimulant selective alpha2a-adrenergic receptor agonist for attention-deficit/hyperactivity disorder. P T. 2010;35(8):448-451.
Attention-deficit/hyperactivity disorder (ADHD) is a developmental disorder that begins in childhood and continues into adulthood. The clinical presentation is characterized by a persistent pattern of inattention, impulsivity, and/or hyperactivity that causes functional interference.1 ADHD affects patients’ interpersonal and professional lives as well as their daily functioning.2 Adults with ADHD may suffer from excessive self-criticism, low self-esteem, and sensitivity to criticism.3 The overall prevalence of adult ADHD is 4.4%.4 ADHD in adults is frequently associated with comorbid psychiatric disorders.5 The diagnosis of ADHD in adults requires the presence of ≥5 symptoms of inattention and hyperactivity/impulsivity that persist for ≥6 months. Patients must have first had such symptoms before age 12; symptoms need to be present in ≥2 settings and interfere with functioning.1
Treatment of ADHD includes pharmacologic and nonpharmacologic interventions. For most patients, pharmacotherapy—specifically stimulant medications—is advised as first-line treatment,6 with adequate trials of methylphenidate and amphetamines before using second-line agents such as nonstimulants. However, despite these medications’ efficacy in randomized controlled trials (RCTs), adherence is low.7 This could be due to inadequate response or adverse effects.8 Guidelines also recommend the use of nonpharmacologic interventions for adults who cannot adhere to or tolerate medication or have an inadequate response.6 Potential nonpharmacologic interventions include transcranial direct current stimulation, mindfulness, psychoeducation, cognitive-behavioral therapy, and chronotherapy.
In Part 1 of this 2-part article, we review 6 RCTs of pharmacologic interventions for adult ADHD published within the last 5 years (Table9-14). Part 2 will review nonpharmacologic treatments.

1. Lam AP, Matthies S, Graf E, et al; Comparison of Methylphenidate and Psychotherapy in Adult ADHD Study (COMPAS) Consortium. Long-term effects of multimodal treatment on adult attention-deficit/hyperactivity disorder symptoms: follow-up analysis of the COMPAS Trial. JAMA Netw Open. 2019;2(5):e194980. doi:10.1001/jamanetworkopen.2019.4980
The Comparison of Methylphenidate and Psychotherapy in Adult ADHD Study (COMPAS) was a multicenter prospective, randomized trial of adults age 18 to 58 with ADHD.15 It compared cognitive-behavioral group psychotherapy (GPT) with individual clinical management (CM), and methylphenidate with placebo. When used in conjunction with methylphenidate, psychological treatments produced better results than placebo. However, studies on the long-term effects of multimodal treatment in ADHD are limited. Lam et al9 performed a follow-up analysis of the COMPAS trial.
Study design
- This observer-masked study involved a follow-up of participants in COMPAS 1.5 years after the interventions were terminated. Of the 433 adults with ADHD who participated in COMPAS, 256 participated in this follow-up.
- The inclusion criteria of COMPAS were age 18 to 58; diagnosis of ADHD according to DSM-IV criteria; chronic course of ADHD symptoms from childhood to adulthood; a Wender Utah Rating Scale short version score ≥30; and no pathological abnormality detected on physical examination.
- The exclusion criteria were having an IQ <85; schizophrenia, bipolar disorder (BD), borderline personality disorder, antisocial personality disorder, suicidal or self-injurious behavior, autism, motor tics, or Tourette syndrome; substance abuse/dependence within 6 months prior to screening; positive drug screening; neurologic diseases, seizures, glaucoma, diabetes, hyperlipidemia, uncontrolled arterial hypertension, angina pectoris, tachycardia arrhythmia, or arterial occlusive disease; previous stroke; current bulimia or anorexia; low weight (body mass index [BMI] <20; pregnancy (current or planned) or breastfeeding; treatment with stimulants or ADHD-specific psychotherapy in the past 6 months; methylphenidate intolerance; treatment with antidepressants, norepinephrine reuptake inhibitors, bupropion, antipsychotics, theophylline, amantadine, anticoagulants derived from coumarin, antacids, or alpha-adrenergic agonists in the 2 weeks prior to baseline; and treatment with fluoxetine or monoamine oxidase inhibitors in the 4 weeks prior to baseline.
- The primary outcome was a change from baseline on the ADHD Index of Conners Adult ADHD Rating Scale (CAARS) score. Secondary outcomes were self-ratings on the Beck Depression Inventory (BDI) and observer-masked ratings of the Clinical Global Impression (CGI) scale and other ADHD rating scale scores, such as the Diagnostic Checklist for the diagnosis of ADHD in adults (ADHD-DC) and subscales of the CAARS.
- COMPAS was open regarding patient and therapist assignment to GPT and CM, but double-masked regarding medication. The statistical analysis focused on the 2x2 comparison of GPT vs CM and methylphenidate vs placebo.
Outcomes
- A total of 251 participants had an assessment with the observer-masked CAARS score. The baseline mean (SD) age was 36.3 (10.1), and approximately one-half (49.8%) of participants were male.
- Overall, 9.2% of patients took methylphenidate >31 days from termination of COMPAS before this study but not at the start of this study. Approximately one-third (31.1%) of patients were taking methylphenidate at follow-up. The mean (SD) daily dosage of methylphenidate was 36 (24.77) mg and 0.46 (0.27) mg/kg of body weight.
- The baseline all-group mean ADHD Index of CAARS score was 20.6. At follow-up, it was 14.7 for the CM arm and 14.2 for the GPT arm (difference not significant, P = .48). The mean score decreased to 13.8 for the methylphenidate arm and to 15.2 for the placebo (significant difference, P = .04).
- Overall, methylphenidate was associated with greater improvement in symptoms than placebo. Patients in the GPT arm had fewer severe symptoms as assessed by the self-reported ADHD Symptoms Total Score compared to the CM arm (P = .04).
- There were no significant differences in self-rating CAARS and observer-rated CAARS subscale scores. Compared to CM, GPT significantly decreased pure hyperactive symptoms on the ADHD-DC (P = .08). No significant differences were observed in BDI scores. The difference between GPT and CM remained significant at follow-up in terms of the CGI evaluation of efficacy (P = .04).
Continue to: Conclusions/limitations
Conclusions/limitations
- Regardless of which combined treatments they received, patients with ADHD continued to improve 1.5 years after the 52-week treatment phase ended.
- Patients assigned to methylphenidate performed considerably better on the observer-rated CAARS than patients assigned to placebo.
- Benefits from GPT or CM in addition to methylphenidate therapy lasted 1.5 years. Compared to CM, GPT was not linked to better scores on the CAARS.
- Limitations: Approximately 41% of patients who were recruited did not participate. Daily functioning was measured only by the CGI. There were only marginal differences among the 4 treatments, and the study compared a very regimented approach (GPT) with one that was less focused (CM).
2. Nasser A, Hull JT, Chaturvedi SA, et al. A phase III, randomized, double‐blind, placebo‐controlled trial assessing the efficacy and safety of viloxazine extended‐release capsules in adults with attention‐deficit/hyperactivity disorder. CNS Drugs. 2022;36(8): 897-915. doi:10.1007/s40263-022-00938-w
In 2021, the FDA approved viloxazine extended-release (ER) for treating ADHD in children and adolescents (age 6 to 17). Nasser et al10 reviewed the safety and efficacy of viloxazine ER in adults with ADHD.
Study design
- This phase III, randomized, double-blind, placebo-controlled, multicenter clinical trial included 374 adults with ADHD who received viloxazine ER or placebo.
- Participants were age 18 to 65 and had been given a primary diagnosis of ADHD according to DSM-5 criteria in the last 6 months. Other inclusion criteria were having an Adult ADHD Investigator Symptom Rating Scale (AISRS) total score ≥26 and CGI-Severity of Illness (CGI-S) score ≥4 at baseline, BMI 18 to 35 kg/m2, and being medically healthy.
- Exclusion criteria included having treatment-resistant ADHD, a current diagnosis of any psychiatric disorder other than ADHD, or a history of schizophrenia, schizoaffective disorder, BD, autism, obsessive-compulsive disorder, personality disorder, or posttraumatic stress disorder. Individuals with any significant neurologic disorder, heart condition, arrhythmia, clinically relevant vital sign abnormality, or systemic illness were excluded, as were those with a history (within the past year) or current diagnosis of substance use disorder or a positive drug screen for a drug of abuse. Those with an allergic reaction or intolerance to viloxazine or were breastfeeding, pregnant, or refused to be abstinent or practice birth control were excluded.
- The dosage of viloxazine ER ranged from 200 to 600 mg/d for 6 weeks. This was titrated based on symptom response and adverse effects.
- All individuals received 2 capsules once a day for Week 1 and Week 2. During Week 1 and Week 2, participants in the viloxazine ER group received 200 mg (1 viloxazine ER capsule and 1 placebo capsule) and 400 mg (2 viloxazine ER capsules) of the medication, respectively. Two placebo pills were administered to those in the placebo group. From Week 3 to Week 6, the dose could be titrated or tapered at the investigator’s discretion. Compliance was assessed by comparing the number of pills dispensed vs returned.
- The primary outcome was a change in AISRS score from baselines to Week 6.
- The key secondary outcome was the change in CGI-S score from baseline to Week 6. Scores on the AISRS inattention and hyperactive/impulsivity subscales, Behavioral Regulation Index, Metacognition Index, Behavior Rating Inventory of Executive Function–Adult Version (BRIEF-A), and Generalized Anxiety Disorder-7 item scale (GAD-7) were also evaluated. Also, the rates of 30% and 50% responders on the AISRS (defined as ≥30% or ≥50% reduction from baseline in AISRS total score, respectively), CGI-S scores, and CGI-Improvement (CGI-I) scores were examined.
Outcomes
- Based on change in AISRS total scores, patients who received viloxazine ER had significantly greater improvement in their ADHD symptoms than those taking placebo (P = .0040). Patients in the viloxazine ER group had significantly greater improvement in AISRS hyperactive/impulsive (P = .0380) and inattentive symptoms (P = .0015).
- The decrease in CGI-S score was also significantly greater in the viloxazine ER group than in the placebo group (P = .0023). The viloxazine ER group also had significantly greater improvement in executive function as assessed by the BRIEF-A (P = .0468). The difference in GAD-7 scores between the viloxazine ER group and the placebo group was not significant.
- The viloxazine ER group had a greater AISRS 30% response rate than the placebo group (P = .0395). There were no significant differences between groups in AISRS 50% responder rate or CGI-I responder rate.
- Adverse effects related to viloxazine and occurring in ≥5% of participants included insomnia (14.8%), fatigue (11.6%), nausea, decreased appetite (10.1%), dry mouth (9.0%), and headache (9.0%). The discontinuation rate was 9.0% in the viloxazine ER group vs 4.9% in the placebo group.
Continue to: Conclusions/limitations
Conclusions/limitations
- Compared to placebo, patients treated with viloxazine ER had significantly greater improvements in ADHD symptoms, including both hyperactive/impulsive and inattentive components as well as executive function.
- The viloxazine ER group had a significantly higher AISRS 30% response rate than the placebo group, but there were no significant differences in anxiety symptoms or other measures of response.
- Viloxazine ER was well tolerated and safe.
- Limitations: There was a reduced power to detect differences in treatment due to participants dropping out or discontinuing treatment, a lack of interrater reliability data, and a lack of patient-reported outcome or satisfaction data.
3. Kis B, Lücke C, Abdel-Hamid M, et al. Safety profile of methylphenidate under long-term treatment in adult ADHD patients - results of the COMPAS study. Pharmacopsychiatry. 2020;53(6):263-271. doi:10.1055/a-1207-9851
Kis et al11 analyzed the safety results of COMPAS.15 Details of this trial, including interventions and inclusion/exclusion criteria, are described in the description of Lam et al.9
Study design
- Researchers compared the rate of adverse events (AEs) among 205 patients who received ≥1 dose of methylphenidate with 209 patients who received placebo.
- AEs were documented and analyzed on an “as received” basis during Week 0 to Week 52. Electrocardiogram (ECG) data were recorded at baseline and Week 24. Vital signs were monitored at baseline, every week for the first 12 weeks, then every 4 weeks for the next 52 weeks. Body weight was assessed at Week 6, Week 12, Week 20, Week 28, Week 40, and Week 52. A 12-lead ECG was obtained at baseline and Week 24.
- The sample size was assessed to have 80% power to detect group differences in AEs.
Outcomes
- Overall, 96% of participants in the methylphenidate group and 88% of participants in the placebo group experienced at least 1 AE (difference 8.1%; 95% CI, 2.9% to 13.5%).
- AEs that occurred more frequently with methylphenidate compared to placebo were decreased appetite (22% vs 3.8%); dry mouth (15% vs 4.8%); palpitations (13% vs 3.3%); gastrointestinal (GI) infection (11% vs 4.8%); agitation (11% vs 3.3%); restlessness (10% vs 2.9%); hyperhidrosis, tachycardia, and weight decrease (all 6.3% vs 1.9%); depressive symptoms and influenza (both 4.9% vs 1.0%); and acute tonsillitis (4.4% vs 0.5%). Serious AEs were reported by 7.3% of patients in the methylphenidate group and 4.3% of those in the placebo group, with no difference in frequency (difference 3.0%; 95% CI, 1.6% to 7.9%). The most severe AEs were aggression, depression, somnambulism, and suicidal ideation in the methylphenidate group and car accidents, epicondylitis, and a fall in the placebo group.
- There were no significant differences in AEs between the GPT and CM groups.
- The treatment combinations that included methylphenidate had higher rates of patients experiencing at least 1 AE (CM/methylphenidate 97%, GPT/methylphenidate 96%, CM/placebo 92%, GPT/placebo 84%).
- Overall, 8.8% of patients in the methylphenidate group and 4.8% in the placebo group stopped their medication treatment because of an AE (difference 4.0%; 95% CI, 0.9% to 9.1%). At least 1 dose decrease, increase, or discontinuation was made after an AE in 42% of participants in the placebo group and 69% of those in the methylphenidate group.
- There were no significant differences in clinically pertinent ECG abnormalities between methylphenidate and placebo therapy.
Continue to: Conclusions/limitations
Conclusions/limitations
- AEs were more common in the methylphenidate groups compared to placebo, but there was no significant differences for severe AEs. In the long-term, methylphenidate treatment was well tolerated and relatively safe.
- Limitations: The sample size may have been too small to detect uncommon AEs, all AEs had to be reported and may not have been caused by the treatment, and the original study’s main outcome was efficacy, not safety, which makes this an exploratory analysis of AEs.
4. Cutler AJ, Childress AC, Pardo A, et al. Randomized, double-blind, placebo-controlled, fixed-dose study to evaluate the efficacy and safety of amphetamine extended-release tablets in adults with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2022;83(5):22m14438. doi:10.4088/JCP.22m14438
Once-daily dosing of stimulants, which are commonly used to manage adult ADHD,16 can be beneficial because many patients have schedules that limit taking medication multiple times a day. Cutler et al12 looked at the efficacy and safety of amphetamine extended-release tablet (AMPH ER TAB), which is a 3.2:1 mixture of d- and l-amphetamine released by the LiquiXR drug delivery system. This technology allows for a continuous release following an initial quick onset of action.
Study design
- This parallel-study, double-blind study evaluated adults age 18 to 60 who had a diagnosis of ADHD according to DSM-5 criteria and the Adult ADHD Clinical Diagnostic Scale, normal-range IQ, AISRS score ≥26, and baseline CGI-S score ≥4.
- Women were not lactating or pregnant during the study.
- Exclusion criteria included a history of mental illnesses; chronic medical conditions; clinically significant abnormal ECG or cardiac findings on exam; renal or liver disease; family history of sudden death; significant vital sign findings; uncontrolled hypertension or a resting systolic blood pressure (SBP) >140 mmHg or diastolic blood pressure (DBP) >90 mmHg; recent history of or current alcohol or substance use disorder; use of atomoxetine, monoamine oxidase inhibitors, or tricyclic antidepressants within 14 days of study or the use of other stimulant medications within 1 week of screening; use of GI acidifying agents or urinary acidifying agents within 3 days of screening; answering “yes” to questions 4 or 5 of the Suicidal Ideation section of the Columbia Suicide Severity Rating Scale within 2 years prior to the study; taking another investigational medication within 30 days of screening; allergic to amphetamine or components of the study drug, and a lack of prior response to amphetamine.
- Patients were randomized to receive AMPH ER TAB (n = 65) or placebo (n = 65), taken before 10
am . Participants started at 5 mg/d of the drug/placebo and then entered a 5-week titration period in which the medication was increased by 5 mg/d each week until reaching 20 mg/d, and then continued 20 mg/d for 2 weeks. - The primary outcome was the mean Permanent Product Measure of Performance Total (PERMP-T) score averaged across all time points (0.5-, 1-, 2-, 4-, 8-, 10-, 12-, 13-, and 14-hours postdose) at Visit 5.
- Participants underwent AISRS, CGI-S, and safety evaluations at baseline and at the 5 visits at the end of each treatment week.
Outcomes
- Analyses were completed on participants who received ≥1 dose of the medication and who had ≥1 PERMP-T score at Visit 5.
- Predose PERMP-T scores were similar between the AMPH ER TAB group (259.5) and placebo group (260). The mean postdose PERMP-T score in the AMPH ER TAB group (302.8) was significantly higher (P = .0043) than the placebo group (279.6).
- The PERMP-T scores were significantly different at 0.5-, 1-, 2-, 4-, 8-, and 13-hours postdose but not at 10-, 12-, and 14-hours postdose. The first Visit 5 time point at which the difference between groups was statistically different was at 0.5 hours postdose (P = .01), and the last significant time point was 13 hours (P = .006).
- The improvement in CGI-S scores was significantly greater in the AMPH ER TAB group than the placebo group. The improvement in AISRS scores was significantly greater in the AMPH ER TAB group at Visit 3, Visit 4, and Visit 5. More participants in the AMPH ER TAB group had AEs compared to the placebo group (90% vs 60%). The most common AEs (frequency ≥5% and occurring more in the intervention arm) were decreased appetite, insomnia, dry mouth, irritability, headache, anxiety, nausea, dizziness, and tachycardia.
- The AMPH ER TAB group had nonclinically significant increases in SBP (116.8 to 120.7 mmHg), DBP (74.1 to 77.1 mmHg), and heart rate (73.0 to 81.9 bpm) at Visit 5 compared to baseline.
- No serious AEs occurred. Three participants in the AMPH ER TAB group experienced AEs (increased blood pressure, CNS stimulation, and anxiety) that led them to discontinue the study.
Continue to: Conclusions/limitations
Conclusions/limitations
- AMPH ER TAB reduced symptoms in adults with ADHD as assessed by improvement in PERMP-T scores.
- The safety and tolerability profile of AMPH ER TAB were comparable to other stimulants, with expected rises in blood pressure and heart rate.
- Limitations: Patients were required to be titrated to 20 mg/d of AMPH ER TAB, instead of following a flexible titration based on an individual’s response. Some participants may have had greater improvement at a higher or lower dose. This study did not compare AMPH ER TAB to other stimulants. The 5-week duration of this study limited the ability to evaluate long-term efficacy and tolerability. Patients with a wide range of psychiatric or medical comorbidities were excluded.
5. Iwanami A, Saito K, Fujiwara M, et al. Efficacy and safety of guanfacine extended-release in the treatment of attention-deficit/hyperactivity disorder in adults: results of a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2020;81(3):19m12979. doi:10.4088/JCP.19m12979
Guanfacine extended-release (GXR) is a selective alpha 2A-adrenergic receptor agonist approved for treating ADHD in children and adolescents.17 Iwanami et al13 evaluated the efficacy and safety of GXR for adults.
Study design
- This randomized, double-blinded, placebo-controlled trial enrolled Japanese adults age ≥18 who were diagnosed with ADHD according to DSM-5 criteria and scored ≥24 on the ADHD-Rating Scale IV (ADHD-RS-IV) and ≥4 on CGI- I.
- Exclusion criteria included having anxiety, depression, substance use disorder, tic disorder, BD, personality disorder, schizophrenia, or intellectual disability; a moderate or severe psychiatric disorder requiring treatment other than counseling; seizures; increased risk for suicide; a history of cardiovascular disease, including prolonged QTc/abnormal ECG/abnormal labs, orthostatic hypotension, or continuous bradycardia; or taking medications that affect blood pressure or heart rate.
- Overall, 101 participants were randomized to the GXR group and 100 to the placebo group. Approximately two-thirds of the study population was male. Patients received GXR or placebo once daily at approximately the same time.
- There were 5 phases to the trial. The screening period occurred over 1 to 4 weeks. Part 1 of the treatment period consisted of 5 weeks of medication optimization. Participants were started on GXR 2 mg/d and were required to be receiving a minimum dose of 4 mg/d starting at Week 3. Clinicians were allowed to increase the dose 1 mg/d per week starting at Week 4 based on clinical response to a maximum dosage of 6 mg/d. Part 2 of the treatment period consisted of 5 weeks of maintenance at 4 to 6 mg/d. The tapering period to 2 mg/d occurred over 2 weeks. The follow-up period lasted 1 week.
- Efficacy measurements included the Japanese version of the ADHD-RS-IV and translations of the English-language CAARS, CGI-I, and CGI-S. Participant-reported measures included the Patient Global Impression-Improvement scale (PGI-I), Adult ADHD Quality of Life Questionnaire (AAQoL), and BRIEF-A.
- The primary outcome was the difference in ADHD-RS-IV total score from baseline to the end of the maintenance period (Week 10).
- Safety assessments were completed at Week 5 (end of dose optimization period), Week 10 (end of dose maintenance period), and Week 12 (tapering period).
Outcomes
- The average GXR dose during the maintenance period was 5.07 mg/d.
- Compared to the placebo group, the GXR group had more patients age <30 (47% vs 39%) and fewer patients age ≥40 (17% vs 27%). Baseline ADHD-RS-IV scores in both groups were comparable. At baseline, 51% in the GXR group had a combined inattentive/hyperactive-impulsive presentation and 47% had a predominately inattention presentation, with similar characteristics in the placebo group (49% combined, 49% inattention).
- At Week 10, the least squares mean change from baseline on the ADHD-RS-IV total score was significantly greater in the GXR group than in the placebo group (-11.55 ± 1.10 vs -7.27 ± 1.07; P = .0005), with an effect size of 0.52. There was a greater decrease in the ADHD-RS-IV scores starting at Week 4 and continuing to Week 10 (P < .005).
- There were also significant differences favoring GXR on the ADHD-RS-IV hyperactivity-impulsivity subscale score (P = .0021) and ADHD-RS-IV inattention subscale score (P = .0032).
- There were significant differences in the CAARS total ADHD score (P = .0029) and BRIEF-A scores on the inhibit (P = .0173), initiate (P = .0406), plan/organize (P = .174), and global executive composite index (P = .0404) scales. There was no significant difference in the total AAQoL score (P = .0691), but there was a significant improvement in the AAQoL life productivity subscore (P = .0072).
- At Week 10, there were also significant improvements in the CGI-I scores (P = .0007) and PGI-I scores (P = .0283). The CGI-S scores were similar at all time points.
- Overall, 81.2% of GXR patients reported AEs compared to 62% in the placebo group. There was 1 serious treatment-emergent AE (a suicide attempt) that the authors concluded was unrelated to the study drug. No deaths occurred. The most common AEs (incidence ≥10% in either group) included somnolence, thirst, nasopharyngitis (occurring more in the placebo group), blood pressure decrease, postural dizziness, and constipation. The main AEs leading to discontinuation were somnolence and blood pressure decrease. Overall, 19.8% of patients receiving GXR discontinued treatment due to AEs, compared to 3% in the placebo group.
- Heart rate, blood pressure, and QTc (corrected by the Bazett formula) were decreased in the GXR group at Week 10 while QT and RR intervals increased, and most returned to normal by Week 12.
Continue to: Conclusions/limitations
Conclusions/limitations
- Compared to placebo, GXR monotherapy resulted in clinical improvement in ADHD symptoms, with a moderate effect size.
- The most common AEs were mild to moderate and congruent with known adverse effects of guanfacine. Sedation effects mostly transpired within the first week of medication administration and were transient.
- Limitations: The findings might not be generalizable to non-Japanese patients. The duration of the study was short. Patients with a wide range of psychiatric and medical comorbidities were excluded. Two-thirds of the participants were male, and there was a disparity in participant age in the GXR and placebo groups.
6. Reimherr FW, Gift TE, Steans TA, et al. The use of brexpiprazole combined with a stimulant in adults with treatment-resistant attention-deficit/hyperactivity disorder. J Clin Psychopharmacol. 2022;42(5):445-453. doi:10.1097/JCP.0000000000001592
While stimulants are a mainstay ADHD treatment, some patients have a partial response or do not respond to amphetamines or methylphenidate. Reimherr et el14 assessed the efficacy and safety of adding brexpiprazole (BXP) to a stimulant.
Study design
- This randomized, double-blinded, placebo-controlled trial recruited 559 stimulant-naive patients and 174 patients who had not responded to previous stimulant therapy.
- Participants were adults age 18 to 55 with a primary diagnosis of ADHD according to DSM-IV-TR criteria and the Conners Adult ADHD Diagnostic Interview. Other inclusion criteria were having a CAARS score ≥29 and a CGI-S score ≥4.
- Exclusion criteria included being at risk for suicide; having current substance abuse or positive alcohol/drug screens; a history of good response to prestudy treatment; a clinically significant medical condition; fasting blood glucose >200 mg/dL or hemoglobin A1C >7%; and hospitalization in past 12 months from a diabetic complication, uncontrolled hypertension, ischemic heart disease, or epilepsy. Further exclusion criteria included a history of psychosis, current MDD or BD, current panic disorder, uncontrolled comorbid psychiatric condition, or clinically significant personality disorder. Investigators excluded any patient with severe DSM-IV axis I or II disorders or abnormal/psychopathological behaviors.
- The trial consisted of 3 segments. Part 1 was screening. If the patient was currently receiving a stimulant but not fully responding, the medication was discontinued for at least 5 half-lives.
- Part 2 (5 weeks) involved administering a stimulant plus a single-blind placebo (597 patients completed this phase). The stimulant was chosen by the investigator, who had the option of using 1 of 2 amphetamine derivatives (mixed amphetamine salts capsules or lisdexamfetamine dimesylate capsules) or 1 of 2 methylphenidate derivatives (methylphenidate hydrochloride ER tabs or dexmethylphenidate HCl ER capsules). If a patient did not respond to a particular stimulant prior to the study, they were given a different stimulant from the list. Patients continued the same stimulant throughout the trial. Patients were monitored for a response, defined as a ≥30% decrease in CAARS score or a CAARS score <24, or a CGI-I score of 1 or 2 at Week 5. Patients who did not show this improvement were categorized as open-label nonresponders.
- Part 3 (6 weeks) involved administering a stimulant plus double-blind BXP vs placebo (stimulant-naive n = 167, stimulant nonresponders n = 68). Nonresponders continued the stimulant (at the same dose reached at the end of Part 2) and added either BXP (n = 155) or continued placebo (n = 80). Patients who responded in Part 2 were continued on the stimulant plus placebo and were not randomized. Patients were started on BXP 0.25 mg/d, and the medication could be titrated to 2 mg/d during the following 3 weeks, depending on the benefit vs AE profile. After the third week, the dose could be decreased but not increased.
- The primary outcome was a change in CAARS score. Secondary measurements included the CGI-S, Wender-Reimherr Adult Attention Deficit Disorder Scale (WRAADDS), Montgomery-Åsberg Depression Rating Scale (MADRS), and BDI.
Outcomes
- Stimulant-naive patients were equally divided among the 4 stimulant groups, and previous nonresponders who continued to not respond in Part 2 were more likely to be given methylphenidate HCl or lisdexamfetamine dimesylate.
- Patients with a history of nonresponse had less response to stimulants in Part 2 compared to stimulant-naive patients, as seen by 27% (n = 167) of stimulant-naive patients entering Part 3 compared to 39% of prior nonresponders (n = 68; P = .0249).
- ADHD improvement with BXP appeared to be greater among pretrial nonresponders.
- For stimulant nonresponders before and during the study, at the end of the double-blind endpoint (Part 3; Week 11), WRAADDS total score was significantly improved in the BXP group compared to the placebo group (P = .013; d = 0.74), with most beneficial effects seen in the hyperactivity/restlessness, emotional dysregulation factor, and impulsivity categories.
- For stimulant nonresponders before and during the study, there was no significant difference at the end of Week 11 on the CAARS (P = .64), MADRS (P = .37), or BDI (P = .73). There was a trend toward significance on the CAARS subscale for hyperactive/impulsive (P = .09).
- For prestudy stimulant-naive patients who did not respond to stimulants in Part 2 and were randomized in Part 3, there was not a significant difference between BXP and placebo at Week 11 as assessed on WRAADDS, CAARS, MADRS, or BDI.
- As assessed on WRAADDS, 50% in the BXP group had a response compared to 41% in the placebo group (Fisher exact = 0.334). Under the emotional dysregulation factor category of the WRAADDS, 64% in the BXP group had a response compared to 41% in the placebo group (Fisher exact = 0.064). The attention factor category showed a 40% improvement in the BXP group compared to 32% in the placebo group (Fisher exact = 0.344).
- There were 2 serious AEs in the BXP group (gall bladder inflammation and diarrhea) and 2 in the placebo group (pneumonia and urinary tract infection). There was no statistically significant difference between groups with regards to common AEs (ie, fatigue, heartburn/nausea/stomachache, weight loss), although there was a trend to significant for insomnia in the BXP group (P = .083).
Conclusions/limitations
- Stimulant-naive patients experienced no improvement with adjunctive BXP.
- For prior stimulant nonresponders, there was no significant difference between BXP vs placebo on the primary outcome of the CAARS score, but there was an improvement as observed by assessment with the WRAADDS.
- The largest change in the WRAADDS occurred in the emotional dysregulation factor compared to the attention factor.
- BXP appeared to be well tolerated.
- Limitations: The WRAADDS was administered without the patients’ significant other/collateral. Raters were not trained in the use of the WRAADDS. Patients with a wide range of psychiatric and medical comorbidities were excluded. Fewer patients were recruited in the prior stimulant nonresponder group.
Bottom Line
Recent randomized controlled trials suggest that methylphenidate, amphetamine extended-release, viloxazine extended-release, and guanfacine extended-release improved symptoms of adult attention-deficit/hyperactivity disorder (ADHD). There were no improvements in ADHD symptoms with adjunctive brexpiprazole.
Related Resources
- Parikh AR, Baker SA. Adult ADHD: pharmacologic treatment in the DSM-5 era. Current Psychiatry. 2016;15(10):18-25.
- Akbar HN. Why we should be scrutinizing the rising prevalence of adult ADHD. Current Psychiatry. 2022; 21(7):e1-e2. doi:10.12788/cp.0268
Drug Brand Names
Amantadine • Gocovri
Amphetamine extended-release tablet • Dyanavel XR
Atomoxetine • Strattera
Brexpiprazole • Rexulti
Bupropion • Wellbutrin
Dexmethylphenidate • Focalin
Fluoxetine • Prozac
Guanfacine extended- release • Intuniv
Lisdexamfetamine • Vyvanse
Methylphenidate • Concerta, Methylin
Theophylline • Elixophyllin
Viloxazine • Qelbree
Attention-deficit/hyperactivity disorder (ADHD) is a developmental disorder that begins in childhood and continues into adulthood. The clinical presentation is characterized by a persistent pattern of inattention, impulsivity, and/or hyperactivity that causes functional interference.1 ADHD affects patients’ interpersonal and professional lives as well as their daily functioning.2 Adults with ADHD may suffer from excessive self-criticism, low self-esteem, and sensitivity to criticism.3 The overall prevalence of adult ADHD is 4.4%.4 ADHD in adults is frequently associated with comorbid psychiatric disorders.5 The diagnosis of ADHD in adults requires the presence of ≥5 symptoms of inattention and hyperactivity/impulsivity that persist for ≥6 months. Patients must have first had such symptoms before age 12; symptoms need to be present in ≥2 settings and interfere with functioning.1
Treatment of ADHD includes pharmacologic and nonpharmacologic interventions. For most patients, pharmacotherapy—specifically stimulant medications—is advised as first-line treatment,6 with adequate trials of methylphenidate and amphetamines before using second-line agents such as nonstimulants. However, despite these medications’ efficacy in randomized controlled trials (RCTs), adherence is low.7 This could be due to inadequate response or adverse effects.8 Guidelines also recommend the use of nonpharmacologic interventions for adults who cannot adhere to or tolerate medication or have an inadequate response.6 Potential nonpharmacologic interventions include transcranial direct current stimulation, mindfulness, psychoeducation, cognitive-behavioral therapy, and chronotherapy.
In Part 1 of this 2-part article, we review 6 RCTs of pharmacologic interventions for adult ADHD published within the last 5 years (Table9-14). Part 2 will review nonpharmacologic treatments.

1. Lam AP, Matthies S, Graf E, et al; Comparison of Methylphenidate and Psychotherapy in Adult ADHD Study (COMPAS) Consortium. Long-term effects of multimodal treatment on adult attention-deficit/hyperactivity disorder symptoms: follow-up analysis of the COMPAS Trial. JAMA Netw Open. 2019;2(5):e194980. doi:10.1001/jamanetworkopen.2019.4980
The Comparison of Methylphenidate and Psychotherapy in Adult ADHD Study (COMPAS) was a multicenter prospective, randomized trial of adults age 18 to 58 with ADHD.15 It compared cognitive-behavioral group psychotherapy (GPT) with individual clinical management (CM), and methylphenidate with placebo. When used in conjunction with methylphenidate, psychological treatments produced better results than placebo. However, studies on the long-term effects of multimodal treatment in ADHD are limited. Lam et al9 performed a follow-up analysis of the COMPAS trial.
Study design
- This observer-masked study involved a follow-up of participants in COMPAS 1.5 years after the interventions were terminated. Of the 433 adults with ADHD who participated in COMPAS, 256 participated in this follow-up.
- The inclusion criteria of COMPAS were age 18 to 58; diagnosis of ADHD according to DSM-IV criteria; chronic course of ADHD symptoms from childhood to adulthood; a Wender Utah Rating Scale short version score ≥30; and no pathological abnormality detected on physical examination.
- The exclusion criteria were having an IQ <85; schizophrenia, bipolar disorder (BD), borderline personality disorder, antisocial personality disorder, suicidal or self-injurious behavior, autism, motor tics, or Tourette syndrome; substance abuse/dependence within 6 months prior to screening; positive drug screening; neurologic diseases, seizures, glaucoma, diabetes, hyperlipidemia, uncontrolled arterial hypertension, angina pectoris, tachycardia arrhythmia, or arterial occlusive disease; previous stroke; current bulimia or anorexia; low weight (body mass index [BMI] <20; pregnancy (current or planned) or breastfeeding; treatment with stimulants or ADHD-specific psychotherapy in the past 6 months; methylphenidate intolerance; treatment with antidepressants, norepinephrine reuptake inhibitors, bupropion, antipsychotics, theophylline, amantadine, anticoagulants derived from coumarin, antacids, or alpha-adrenergic agonists in the 2 weeks prior to baseline; and treatment with fluoxetine or monoamine oxidase inhibitors in the 4 weeks prior to baseline.
- The primary outcome was a change from baseline on the ADHD Index of Conners Adult ADHD Rating Scale (CAARS) score. Secondary outcomes were self-ratings on the Beck Depression Inventory (BDI) and observer-masked ratings of the Clinical Global Impression (CGI) scale and other ADHD rating scale scores, such as the Diagnostic Checklist for the diagnosis of ADHD in adults (ADHD-DC) and subscales of the CAARS.
- COMPAS was open regarding patient and therapist assignment to GPT and CM, but double-masked regarding medication. The statistical analysis focused on the 2x2 comparison of GPT vs CM and methylphenidate vs placebo.
Outcomes
- A total of 251 participants had an assessment with the observer-masked CAARS score. The baseline mean (SD) age was 36.3 (10.1), and approximately one-half (49.8%) of participants were male.
- Overall, 9.2% of patients took methylphenidate >31 days from termination of COMPAS before this study but not at the start of this study. Approximately one-third (31.1%) of patients were taking methylphenidate at follow-up. The mean (SD) daily dosage of methylphenidate was 36 (24.77) mg and 0.46 (0.27) mg/kg of body weight.
- The baseline all-group mean ADHD Index of CAARS score was 20.6. At follow-up, it was 14.7 for the CM arm and 14.2 for the GPT arm (difference not significant, P = .48). The mean score decreased to 13.8 for the methylphenidate arm and to 15.2 for the placebo (significant difference, P = .04).
- Overall, methylphenidate was associated with greater improvement in symptoms than placebo. Patients in the GPT arm had fewer severe symptoms as assessed by the self-reported ADHD Symptoms Total Score compared to the CM arm (P = .04).
- There were no significant differences in self-rating CAARS and observer-rated CAARS subscale scores. Compared to CM, GPT significantly decreased pure hyperactive symptoms on the ADHD-DC (P = .08). No significant differences were observed in BDI scores. The difference between GPT and CM remained significant at follow-up in terms of the CGI evaluation of efficacy (P = .04).
Continue to: Conclusions/limitations
Conclusions/limitations
- Regardless of which combined treatments they received, patients with ADHD continued to improve 1.5 years after the 52-week treatment phase ended.
- Patients assigned to methylphenidate performed considerably better on the observer-rated CAARS than patients assigned to placebo.
- Benefits from GPT or CM in addition to methylphenidate therapy lasted 1.5 years. Compared to CM, GPT was not linked to better scores on the CAARS.
- Limitations: Approximately 41% of patients who were recruited did not participate. Daily functioning was measured only by the CGI. There were only marginal differences among the 4 treatments, and the study compared a very regimented approach (GPT) with one that was less focused (CM).
2. Nasser A, Hull JT, Chaturvedi SA, et al. A phase III, randomized, double‐blind, placebo‐controlled trial assessing the efficacy and safety of viloxazine extended‐release capsules in adults with attention‐deficit/hyperactivity disorder. CNS Drugs. 2022;36(8): 897-915. doi:10.1007/s40263-022-00938-w
In 2021, the FDA approved viloxazine extended-release (ER) for treating ADHD in children and adolescents (age 6 to 17). Nasser et al10 reviewed the safety and efficacy of viloxazine ER in adults with ADHD.
Study design
- This phase III, randomized, double-blind, placebo-controlled, multicenter clinical trial included 374 adults with ADHD who received viloxazine ER or placebo.
- Participants were age 18 to 65 and had been given a primary diagnosis of ADHD according to DSM-5 criteria in the last 6 months. Other inclusion criteria were having an Adult ADHD Investigator Symptom Rating Scale (AISRS) total score ≥26 and CGI-Severity of Illness (CGI-S) score ≥4 at baseline, BMI 18 to 35 kg/m2, and being medically healthy.
- Exclusion criteria included having treatment-resistant ADHD, a current diagnosis of any psychiatric disorder other than ADHD, or a history of schizophrenia, schizoaffective disorder, BD, autism, obsessive-compulsive disorder, personality disorder, or posttraumatic stress disorder. Individuals with any significant neurologic disorder, heart condition, arrhythmia, clinically relevant vital sign abnormality, or systemic illness were excluded, as were those with a history (within the past year) or current diagnosis of substance use disorder or a positive drug screen for a drug of abuse. Those with an allergic reaction or intolerance to viloxazine or were breastfeeding, pregnant, or refused to be abstinent or practice birth control were excluded.
- The dosage of viloxazine ER ranged from 200 to 600 mg/d for 6 weeks. This was titrated based on symptom response and adverse effects.
- All individuals received 2 capsules once a day for Week 1 and Week 2. During Week 1 and Week 2, participants in the viloxazine ER group received 200 mg (1 viloxazine ER capsule and 1 placebo capsule) and 400 mg (2 viloxazine ER capsules) of the medication, respectively. Two placebo pills were administered to those in the placebo group. From Week 3 to Week 6, the dose could be titrated or tapered at the investigator’s discretion. Compliance was assessed by comparing the number of pills dispensed vs returned.
- The primary outcome was a change in AISRS score from baselines to Week 6.
- The key secondary outcome was the change in CGI-S score from baseline to Week 6. Scores on the AISRS inattention and hyperactive/impulsivity subscales, Behavioral Regulation Index, Metacognition Index, Behavior Rating Inventory of Executive Function–Adult Version (BRIEF-A), and Generalized Anxiety Disorder-7 item scale (GAD-7) were also evaluated. Also, the rates of 30% and 50% responders on the AISRS (defined as ≥30% or ≥50% reduction from baseline in AISRS total score, respectively), CGI-S scores, and CGI-Improvement (CGI-I) scores were examined.
Outcomes
- Based on change in AISRS total scores, patients who received viloxazine ER had significantly greater improvement in their ADHD symptoms than those taking placebo (P = .0040). Patients in the viloxazine ER group had significantly greater improvement in AISRS hyperactive/impulsive (P = .0380) and inattentive symptoms (P = .0015).
- The decrease in CGI-S score was also significantly greater in the viloxazine ER group than in the placebo group (P = .0023). The viloxazine ER group also had significantly greater improvement in executive function as assessed by the BRIEF-A (P = .0468). The difference in GAD-7 scores between the viloxazine ER group and the placebo group was not significant.
- The viloxazine ER group had a greater AISRS 30% response rate than the placebo group (P = .0395). There were no significant differences between groups in AISRS 50% responder rate or CGI-I responder rate.
- Adverse effects related to viloxazine and occurring in ≥5% of participants included insomnia (14.8%), fatigue (11.6%), nausea, decreased appetite (10.1%), dry mouth (9.0%), and headache (9.0%). The discontinuation rate was 9.0% in the viloxazine ER group vs 4.9% in the placebo group.
Continue to: Conclusions/limitations
Conclusions/limitations
- Compared to placebo, patients treated with viloxazine ER had significantly greater improvements in ADHD symptoms, including both hyperactive/impulsive and inattentive components as well as executive function.
- The viloxazine ER group had a significantly higher AISRS 30% response rate than the placebo group, but there were no significant differences in anxiety symptoms or other measures of response.
- Viloxazine ER was well tolerated and safe.
- Limitations: There was a reduced power to detect differences in treatment due to participants dropping out or discontinuing treatment, a lack of interrater reliability data, and a lack of patient-reported outcome or satisfaction data.
3. Kis B, Lücke C, Abdel-Hamid M, et al. Safety profile of methylphenidate under long-term treatment in adult ADHD patients - results of the COMPAS study. Pharmacopsychiatry. 2020;53(6):263-271. doi:10.1055/a-1207-9851
Kis et al11 analyzed the safety results of COMPAS.15 Details of this trial, including interventions and inclusion/exclusion criteria, are described in the description of Lam et al.9
Study design
- Researchers compared the rate of adverse events (AEs) among 205 patients who received ≥1 dose of methylphenidate with 209 patients who received placebo.
- AEs were documented and analyzed on an “as received” basis during Week 0 to Week 52. Electrocardiogram (ECG) data were recorded at baseline and Week 24. Vital signs were monitored at baseline, every week for the first 12 weeks, then every 4 weeks for the next 52 weeks. Body weight was assessed at Week 6, Week 12, Week 20, Week 28, Week 40, and Week 52. A 12-lead ECG was obtained at baseline and Week 24.
- The sample size was assessed to have 80% power to detect group differences in AEs.
Outcomes
- Overall, 96% of participants in the methylphenidate group and 88% of participants in the placebo group experienced at least 1 AE (difference 8.1%; 95% CI, 2.9% to 13.5%).
- AEs that occurred more frequently with methylphenidate compared to placebo were decreased appetite (22% vs 3.8%); dry mouth (15% vs 4.8%); palpitations (13% vs 3.3%); gastrointestinal (GI) infection (11% vs 4.8%); agitation (11% vs 3.3%); restlessness (10% vs 2.9%); hyperhidrosis, tachycardia, and weight decrease (all 6.3% vs 1.9%); depressive symptoms and influenza (both 4.9% vs 1.0%); and acute tonsillitis (4.4% vs 0.5%). Serious AEs were reported by 7.3% of patients in the methylphenidate group and 4.3% of those in the placebo group, with no difference in frequency (difference 3.0%; 95% CI, 1.6% to 7.9%). The most severe AEs were aggression, depression, somnambulism, and suicidal ideation in the methylphenidate group and car accidents, epicondylitis, and a fall in the placebo group.
- There were no significant differences in AEs between the GPT and CM groups.
- The treatment combinations that included methylphenidate had higher rates of patients experiencing at least 1 AE (CM/methylphenidate 97%, GPT/methylphenidate 96%, CM/placebo 92%, GPT/placebo 84%).
- Overall, 8.8% of patients in the methylphenidate group and 4.8% in the placebo group stopped their medication treatment because of an AE (difference 4.0%; 95% CI, 0.9% to 9.1%). At least 1 dose decrease, increase, or discontinuation was made after an AE in 42% of participants in the placebo group and 69% of those in the methylphenidate group.
- There were no significant differences in clinically pertinent ECG abnormalities between methylphenidate and placebo therapy.
Continue to: Conclusions/limitations
Conclusions/limitations
- AEs were more common in the methylphenidate groups compared to placebo, but there was no significant differences for severe AEs. In the long-term, methylphenidate treatment was well tolerated and relatively safe.
- Limitations: The sample size may have been too small to detect uncommon AEs, all AEs had to be reported and may not have been caused by the treatment, and the original study’s main outcome was efficacy, not safety, which makes this an exploratory analysis of AEs.
4. Cutler AJ, Childress AC, Pardo A, et al. Randomized, double-blind, placebo-controlled, fixed-dose study to evaluate the efficacy and safety of amphetamine extended-release tablets in adults with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2022;83(5):22m14438. doi:10.4088/JCP.22m14438
Once-daily dosing of stimulants, which are commonly used to manage adult ADHD,16 can be beneficial because many patients have schedules that limit taking medication multiple times a day. Cutler et al12 looked at the efficacy and safety of amphetamine extended-release tablet (AMPH ER TAB), which is a 3.2:1 mixture of d- and l-amphetamine released by the LiquiXR drug delivery system. This technology allows for a continuous release following an initial quick onset of action.
Study design
- This parallel-study, double-blind study evaluated adults age 18 to 60 who had a diagnosis of ADHD according to DSM-5 criteria and the Adult ADHD Clinical Diagnostic Scale, normal-range IQ, AISRS score ≥26, and baseline CGI-S score ≥4.
- Women were not lactating or pregnant during the study.
- Exclusion criteria included a history of mental illnesses; chronic medical conditions; clinically significant abnormal ECG or cardiac findings on exam; renal or liver disease; family history of sudden death; significant vital sign findings; uncontrolled hypertension or a resting systolic blood pressure (SBP) >140 mmHg or diastolic blood pressure (DBP) >90 mmHg; recent history of or current alcohol or substance use disorder; use of atomoxetine, monoamine oxidase inhibitors, or tricyclic antidepressants within 14 days of study or the use of other stimulant medications within 1 week of screening; use of GI acidifying agents or urinary acidifying agents within 3 days of screening; answering “yes” to questions 4 or 5 of the Suicidal Ideation section of the Columbia Suicide Severity Rating Scale within 2 years prior to the study; taking another investigational medication within 30 days of screening; allergic to amphetamine or components of the study drug, and a lack of prior response to amphetamine.
- Patients were randomized to receive AMPH ER TAB (n = 65) or placebo (n = 65), taken before 10
am . Participants started at 5 mg/d of the drug/placebo and then entered a 5-week titration period in which the medication was increased by 5 mg/d each week until reaching 20 mg/d, and then continued 20 mg/d for 2 weeks. - The primary outcome was the mean Permanent Product Measure of Performance Total (PERMP-T) score averaged across all time points (0.5-, 1-, 2-, 4-, 8-, 10-, 12-, 13-, and 14-hours postdose) at Visit 5.
- Participants underwent AISRS, CGI-S, and safety evaluations at baseline and at the 5 visits at the end of each treatment week.
Outcomes
- Analyses were completed on participants who received ≥1 dose of the medication and who had ≥1 PERMP-T score at Visit 5.
- Predose PERMP-T scores were similar between the AMPH ER TAB group (259.5) and placebo group (260). The mean postdose PERMP-T score in the AMPH ER TAB group (302.8) was significantly higher (P = .0043) than the placebo group (279.6).
- The PERMP-T scores were significantly different at 0.5-, 1-, 2-, 4-, 8-, and 13-hours postdose but not at 10-, 12-, and 14-hours postdose. The first Visit 5 time point at which the difference between groups was statistically different was at 0.5 hours postdose (P = .01), and the last significant time point was 13 hours (P = .006).
- The improvement in CGI-S scores was significantly greater in the AMPH ER TAB group than the placebo group. The improvement in AISRS scores was significantly greater in the AMPH ER TAB group at Visit 3, Visit 4, and Visit 5. More participants in the AMPH ER TAB group had AEs compared to the placebo group (90% vs 60%). The most common AEs (frequency ≥5% and occurring more in the intervention arm) were decreased appetite, insomnia, dry mouth, irritability, headache, anxiety, nausea, dizziness, and tachycardia.
- The AMPH ER TAB group had nonclinically significant increases in SBP (116.8 to 120.7 mmHg), DBP (74.1 to 77.1 mmHg), and heart rate (73.0 to 81.9 bpm) at Visit 5 compared to baseline.
- No serious AEs occurred. Three participants in the AMPH ER TAB group experienced AEs (increased blood pressure, CNS stimulation, and anxiety) that led them to discontinue the study.
Continue to: Conclusions/limitations
Conclusions/limitations
- AMPH ER TAB reduced symptoms in adults with ADHD as assessed by improvement in PERMP-T scores.
- The safety and tolerability profile of AMPH ER TAB were comparable to other stimulants, with expected rises in blood pressure and heart rate.
- Limitations: Patients were required to be titrated to 20 mg/d of AMPH ER TAB, instead of following a flexible titration based on an individual’s response. Some participants may have had greater improvement at a higher or lower dose. This study did not compare AMPH ER TAB to other stimulants. The 5-week duration of this study limited the ability to evaluate long-term efficacy and tolerability. Patients with a wide range of psychiatric or medical comorbidities were excluded.
5. Iwanami A, Saito K, Fujiwara M, et al. Efficacy and safety of guanfacine extended-release in the treatment of attention-deficit/hyperactivity disorder in adults: results of a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2020;81(3):19m12979. doi:10.4088/JCP.19m12979
Guanfacine extended-release (GXR) is a selective alpha 2A-adrenergic receptor agonist approved for treating ADHD in children and adolescents.17 Iwanami et al13 evaluated the efficacy and safety of GXR for adults.
Study design
- This randomized, double-blinded, placebo-controlled trial enrolled Japanese adults age ≥18 who were diagnosed with ADHD according to DSM-5 criteria and scored ≥24 on the ADHD-Rating Scale IV (ADHD-RS-IV) and ≥4 on CGI- I.
- Exclusion criteria included having anxiety, depression, substance use disorder, tic disorder, BD, personality disorder, schizophrenia, or intellectual disability; a moderate or severe psychiatric disorder requiring treatment other than counseling; seizures; increased risk for suicide; a history of cardiovascular disease, including prolonged QTc/abnormal ECG/abnormal labs, orthostatic hypotension, or continuous bradycardia; or taking medications that affect blood pressure or heart rate.
- Overall, 101 participants were randomized to the GXR group and 100 to the placebo group. Approximately two-thirds of the study population was male. Patients received GXR or placebo once daily at approximately the same time.
- There were 5 phases to the trial. The screening period occurred over 1 to 4 weeks. Part 1 of the treatment period consisted of 5 weeks of medication optimization. Participants were started on GXR 2 mg/d and were required to be receiving a minimum dose of 4 mg/d starting at Week 3. Clinicians were allowed to increase the dose 1 mg/d per week starting at Week 4 based on clinical response to a maximum dosage of 6 mg/d. Part 2 of the treatment period consisted of 5 weeks of maintenance at 4 to 6 mg/d. The tapering period to 2 mg/d occurred over 2 weeks. The follow-up period lasted 1 week.
- Efficacy measurements included the Japanese version of the ADHD-RS-IV and translations of the English-language CAARS, CGI-I, and CGI-S. Participant-reported measures included the Patient Global Impression-Improvement scale (PGI-I), Adult ADHD Quality of Life Questionnaire (AAQoL), and BRIEF-A.
- The primary outcome was the difference in ADHD-RS-IV total score from baseline to the end of the maintenance period (Week 10).
- Safety assessments were completed at Week 5 (end of dose optimization period), Week 10 (end of dose maintenance period), and Week 12 (tapering period).
Outcomes
- The average GXR dose during the maintenance period was 5.07 mg/d.
- Compared to the placebo group, the GXR group had more patients age <30 (47% vs 39%) and fewer patients age ≥40 (17% vs 27%). Baseline ADHD-RS-IV scores in both groups were comparable. At baseline, 51% in the GXR group had a combined inattentive/hyperactive-impulsive presentation and 47% had a predominately inattention presentation, with similar characteristics in the placebo group (49% combined, 49% inattention).
- At Week 10, the least squares mean change from baseline on the ADHD-RS-IV total score was significantly greater in the GXR group than in the placebo group (-11.55 ± 1.10 vs -7.27 ± 1.07; P = .0005), with an effect size of 0.52. There was a greater decrease in the ADHD-RS-IV scores starting at Week 4 and continuing to Week 10 (P < .005).
- There were also significant differences favoring GXR on the ADHD-RS-IV hyperactivity-impulsivity subscale score (P = .0021) and ADHD-RS-IV inattention subscale score (P = .0032).
- There were significant differences in the CAARS total ADHD score (P = .0029) and BRIEF-A scores on the inhibit (P = .0173), initiate (P = .0406), plan/organize (P = .174), and global executive composite index (P = .0404) scales. There was no significant difference in the total AAQoL score (P = .0691), but there was a significant improvement in the AAQoL life productivity subscore (P = .0072).
- At Week 10, there were also significant improvements in the CGI-I scores (P = .0007) and PGI-I scores (P = .0283). The CGI-S scores were similar at all time points.
- Overall, 81.2% of GXR patients reported AEs compared to 62% in the placebo group. There was 1 serious treatment-emergent AE (a suicide attempt) that the authors concluded was unrelated to the study drug. No deaths occurred. The most common AEs (incidence ≥10% in either group) included somnolence, thirst, nasopharyngitis (occurring more in the placebo group), blood pressure decrease, postural dizziness, and constipation. The main AEs leading to discontinuation were somnolence and blood pressure decrease. Overall, 19.8% of patients receiving GXR discontinued treatment due to AEs, compared to 3% in the placebo group.
- Heart rate, blood pressure, and QTc (corrected by the Bazett formula) were decreased in the GXR group at Week 10 while QT and RR intervals increased, and most returned to normal by Week 12.
Continue to: Conclusions/limitations
Conclusions/limitations
- Compared to placebo, GXR monotherapy resulted in clinical improvement in ADHD symptoms, with a moderate effect size.
- The most common AEs were mild to moderate and congruent with known adverse effects of guanfacine. Sedation effects mostly transpired within the first week of medication administration and were transient.
- Limitations: The findings might not be generalizable to non-Japanese patients. The duration of the study was short. Patients with a wide range of psychiatric and medical comorbidities were excluded. Two-thirds of the participants were male, and there was a disparity in participant age in the GXR and placebo groups.
6. Reimherr FW, Gift TE, Steans TA, et al. The use of brexpiprazole combined with a stimulant in adults with treatment-resistant attention-deficit/hyperactivity disorder. J Clin Psychopharmacol. 2022;42(5):445-453. doi:10.1097/JCP.0000000000001592
While stimulants are a mainstay ADHD treatment, some patients have a partial response or do not respond to amphetamines or methylphenidate. Reimherr et el14 assessed the efficacy and safety of adding brexpiprazole (BXP) to a stimulant.
Study design
- This randomized, double-blinded, placebo-controlled trial recruited 559 stimulant-naive patients and 174 patients who had not responded to previous stimulant therapy.
- Participants were adults age 18 to 55 with a primary diagnosis of ADHD according to DSM-IV-TR criteria and the Conners Adult ADHD Diagnostic Interview. Other inclusion criteria were having a CAARS score ≥29 and a CGI-S score ≥4.
- Exclusion criteria included being at risk for suicide; having current substance abuse or positive alcohol/drug screens; a history of good response to prestudy treatment; a clinically significant medical condition; fasting blood glucose >200 mg/dL or hemoglobin A1C >7%; and hospitalization in past 12 months from a diabetic complication, uncontrolled hypertension, ischemic heart disease, or epilepsy. Further exclusion criteria included a history of psychosis, current MDD or BD, current panic disorder, uncontrolled comorbid psychiatric condition, or clinically significant personality disorder. Investigators excluded any patient with severe DSM-IV axis I or II disorders or abnormal/psychopathological behaviors.
- The trial consisted of 3 segments. Part 1 was screening. If the patient was currently receiving a stimulant but not fully responding, the medication was discontinued for at least 5 half-lives.
- Part 2 (5 weeks) involved administering a stimulant plus a single-blind placebo (597 patients completed this phase). The stimulant was chosen by the investigator, who had the option of using 1 of 2 amphetamine derivatives (mixed amphetamine salts capsules or lisdexamfetamine dimesylate capsules) or 1 of 2 methylphenidate derivatives (methylphenidate hydrochloride ER tabs or dexmethylphenidate HCl ER capsules). If a patient did not respond to a particular stimulant prior to the study, they were given a different stimulant from the list. Patients continued the same stimulant throughout the trial. Patients were monitored for a response, defined as a ≥30% decrease in CAARS score or a CAARS score <24, or a CGI-I score of 1 or 2 at Week 5. Patients who did not show this improvement were categorized as open-label nonresponders.
- Part 3 (6 weeks) involved administering a stimulant plus double-blind BXP vs placebo (stimulant-naive n = 167, stimulant nonresponders n = 68). Nonresponders continued the stimulant (at the same dose reached at the end of Part 2) and added either BXP (n = 155) or continued placebo (n = 80). Patients who responded in Part 2 were continued on the stimulant plus placebo and were not randomized. Patients were started on BXP 0.25 mg/d, and the medication could be titrated to 2 mg/d during the following 3 weeks, depending on the benefit vs AE profile. After the third week, the dose could be decreased but not increased.
- The primary outcome was a change in CAARS score. Secondary measurements included the CGI-S, Wender-Reimherr Adult Attention Deficit Disorder Scale (WRAADDS), Montgomery-Åsberg Depression Rating Scale (MADRS), and BDI.
Outcomes
- Stimulant-naive patients were equally divided among the 4 stimulant groups, and previous nonresponders who continued to not respond in Part 2 were more likely to be given methylphenidate HCl or lisdexamfetamine dimesylate.
- Patients with a history of nonresponse had less response to stimulants in Part 2 compared to stimulant-naive patients, as seen by 27% (n = 167) of stimulant-naive patients entering Part 3 compared to 39% of prior nonresponders (n = 68; P = .0249).
- ADHD improvement with BXP appeared to be greater among pretrial nonresponders.
- For stimulant nonresponders before and during the study, at the end of the double-blind endpoint (Part 3; Week 11), WRAADDS total score was significantly improved in the BXP group compared to the placebo group (P = .013; d = 0.74), with most beneficial effects seen in the hyperactivity/restlessness, emotional dysregulation factor, and impulsivity categories.
- For stimulant nonresponders before and during the study, there was no significant difference at the end of Week 11 on the CAARS (P = .64), MADRS (P = .37), or BDI (P = .73). There was a trend toward significance on the CAARS subscale for hyperactive/impulsive (P = .09).
- For prestudy stimulant-naive patients who did not respond to stimulants in Part 2 and were randomized in Part 3, there was not a significant difference between BXP and placebo at Week 11 as assessed on WRAADDS, CAARS, MADRS, or BDI.
- As assessed on WRAADDS, 50% in the BXP group had a response compared to 41% in the placebo group (Fisher exact = 0.334). Under the emotional dysregulation factor category of the WRAADDS, 64% in the BXP group had a response compared to 41% in the placebo group (Fisher exact = 0.064). The attention factor category showed a 40% improvement in the BXP group compared to 32% in the placebo group (Fisher exact = 0.344).
- There were 2 serious AEs in the BXP group (gall bladder inflammation and diarrhea) and 2 in the placebo group (pneumonia and urinary tract infection). There was no statistically significant difference between groups with regards to common AEs (ie, fatigue, heartburn/nausea/stomachache, weight loss), although there was a trend to significant for insomnia in the BXP group (P = .083).
Conclusions/limitations
- Stimulant-naive patients experienced no improvement with adjunctive BXP.
- For prior stimulant nonresponders, there was no significant difference between BXP vs placebo on the primary outcome of the CAARS score, but there was an improvement as observed by assessment with the WRAADDS.
- The largest change in the WRAADDS occurred in the emotional dysregulation factor compared to the attention factor.
- BXP appeared to be well tolerated.
- Limitations: The WRAADDS was administered without the patients’ significant other/collateral. Raters were not trained in the use of the WRAADDS. Patients with a wide range of psychiatric and medical comorbidities were excluded. Fewer patients were recruited in the prior stimulant nonresponder group.
Bottom Line
Recent randomized controlled trials suggest that methylphenidate, amphetamine extended-release, viloxazine extended-release, and guanfacine extended-release improved symptoms of adult attention-deficit/hyperactivity disorder (ADHD). There were no improvements in ADHD symptoms with adjunctive brexpiprazole.
Related Resources
- Parikh AR, Baker SA. Adult ADHD: pharmacologic treatment in the DSM-5 era. Current Psychiatry. 2016;15(10):18-25.
- Akbar HN. Why we should be scrutinizing the rising prevalence of adult ADHD. Current Psychiatry. 2022; 21(7):e1-e2. doi:10.12788/cp.0268
Drug Brand Names
Amantadine • Gocovri
Amphetamine extended-release tablet • Dyanavel XR
Atomoxetine • Strattera
Brexpiprazole • Rexulti
Bupropion • Wellbutrin
Dexmethylphenidate • Focalin
Fluoxetine • Prozac
Guanfacine extended- release • Intuniv
Lisdexamfetamine • Vyvanse
Methylphenidate • Concerta, Methylin
Theophylline • Elixophyllin
Viloxazine • Qelbree
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022.
2. Harpin V, Mazzone L, Raynaud JP, et al. Long-term outcomes of ADHD: a systematic review of self-esteem and social function. J Atten Disord. 2016;20(4):295-305. doi:10.1177/1087054713486516
3. Beaton DM, Sirois F, Milne E. Experiences of criticism in adults with ADHD: a qualitative study. PLoS One. 2022;17(2):e0263366. doi:10.1371/journal.pone.0263366
4. Attention-deficit/hyperactivity disorder (ADHD). National Institute of Mental Health. Accessed February 9, 2023. https://www.nimh.nih.gov/health/statistics/attention-deficit-hyperactivity-disorder-adhd
5. Katzman MA, Bilkey TS, Chokka PR, et al. Adult ADHD and comorbid disorders: clinical implications of a dimensional approach. BMC Psychiatry. 2017;17(1):302. doi:10.1186/s12888-017-1463-3
6. Attention Deficit Hyperactivity Disorder: Diagnosis and Management. NICE Guideline No. 87. National Institute for Health and Care Excellence (NICE); 2019. Accessed February 9, 2023. http://www.ncbi.nlm.nih.gov/books/NBK493361/
7. Adler LD, Nierenberg AA. Review of medication adherence in children and adults with ADHD. Postgrad Med. 2010;122(1):184-191. doi:10.3810/pgm.2010.01.2112
8. Cunill R, Castells X, Tobias A, et al. Efficacy, safety and variability in pharmacotherapy for adults with attention deficit hyperactivity disorder: a meta-analysis and meta-regression in over 9000 patients. Psychopharmacology (Berl). 2016;233(2):187-197. doi:10.1007/s00213-015-4099-3
9. Lam AP, Matthies S, Graf E, et al; Comparison of Methylphenidate and Psychotherapy in Adult ADHD Study (COMPAS) Consortium. Long-term effects of multimodal treatment on adult attention-deficit/hyperactivity disorder symptoms: follow-up analysis of the COMPAS Trial. JAMA Netw Open. 2019;2(5):e194980. doi:10.1001/jamanetworkopen.2019.4980
10. Nasser A, Hull JT, Chaturvedi SA, et al. A phase III, randomized, double-blind, placebo-controlled trial assessing the efficacy and safety of viloxazine extended-release capsules in adults with attention-deficit/hyperactivity disorder. CNS Drugs. 2022;36(8):897-915. doi:10.1007/s40263-022-00938-w
11. Kis B, Lücke C, Abdel-Hamid M, et al. Safety profile of methylphenidate under long-term treatment in adult ADHD patients - results of the COMPAS study. Pharmacopsychiatry. 2020;53(6):263-271. doi:10.1055/a-1207-9851
12. Cutler AJ, Childress AC, Pardo A, et al. Randomized, double-blind, placebo-controlled, fixed-dose study to evaluate the efficacy and safety of amphetamine extended-release tablets in adults with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2022;83(5):22m14438. doi:10.4088/JCP.22m14438
13. Iwanami A, Saito K, Fujiwara M, et al. Efficacy and safety of guanfacine extended-release in the treatment of attention-deficit/hyperactivity disorder in adults: results of a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2020;81(3):19m12979. doi:10.4088/JCP.19m12979
14. Reimherr FW, Gift TE, Steans TA, et al. The use of brexpiprazole combined with a stimulant in adults with treatment-resistant attention-deficit/hyperactivity disorder. J Clin Psychopharmacol. 2022;42(5):445-453. doi:10.1097/JCP.0000000000001592
15. Philipsen A, Jans T, Graf E, et al; Comparison of Methylphenidate and Psychotherapy in Adult ADHD Study (COMPAS) Consortium. Effects of group psychotherapy, individual counseling, methylphenidate, and placebo in the treatment of adult attention-deficit/hyperactivity disorder: a randomized clinical trial. JAMA Psychiatry. 2015;72(12):1199-1210.
16. McGough JJ. Treatment controversies in adult ADHD. Am J Psychiatry. 2016;173(10):960-966. doi:10.1176/appi.ajp.2016.15091207
17. Cruz MP. Guanfacine extended-release tablets (Intuniv), a nonstimulant selective alpha2a-adrenergic receptor agonist for attention-deficit/hyperactivity disorder. P T. 2010;35(8):448-451.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022.
2. Harpin V, Mazzone L, Raynaud JP, et al. Long-term outcomes of ADHD: a systematic review of self-esteem and social function. J Atten Disord. 2016;20(4):295-305. doi:10.1177/1087054713486516
3. Beaton DM, Sirois F, Milne E. Experiences of criticism in adults with ADHD: a qualitative study. PLoS One. 2022;17(2):e0263366. doi:10.1371/journal.pone.0263366
4. Attention-deficit/hyperactivity disorder (ADHD). National Institute of Mental Health. Accessed February 9, 2023. https://www.nimh.nih.gov/health/statistics/attention-deficit-hyperactivity-disorder-adhd
5. Katzman MA, Bilkey TS, Chokka PR, et al. Adult ADHD and comorbid disorders: clinical implications of a dimensional approach. BMC Psychiatry. 2017;17(1):302. doi:10.1186/s12888-017-1463-3
6. Attention Deficit Hyperactivity Disorder: Diagnosis and Management. NICE Guideline No. 87. National Institute for Health and Care Excellence (NICE); 2019. Accessed February 9, 2023. http://www.ncbi.nlm.nih.gov/books/NBK493361/
7. Adler LD, Nierenberg AA. Review of medication adherence in children and adults with ADHD. Postgrad Med. 2010;122(1):184-191. doi:10.3810/pgm.2010.01.2112
8. Cunill R, Castells X, Tobias A, et al. Efficacy, safety and variability in pharmacotherapy for adults with attention deficit hyperactivity disorder: a meta-analysis and meta-regression in over 9000 patients. Psychopharmacology (Berl). 2016;233(2):187-197. doi:10.1007/s00213-015-4099-3
9. Lam AP, Matthies S, Graf E, et al; Comparison of Methylphenidate and Psychotherapy in Adult ADHD Study (COMPAS) Consortium. Long-term effects of multimodal treatment on adult attention-deficit/hyperactivity disorder symptoms: follow-up analysis of the COMPAS Trial. JAMA Netw Open. 2019;2(5):e194980. doi:10.1001/jamanetworkopen.2019.4980
10. Nasser A, Hull JT, Chaturvedi SA, et al. A phase III, randomized, double-blind, placebo-controlled trial assessing the efficacy and safety of viloxazine extended-release capsules in adults with attention-deficit/hyperactivity disorder. CNS Drugs. 2022;36(8):897-915. doi:10.1007/s40263-022-00938-w
11. Kis B, Lücke C, Abdel-Hamid M, et al. Safety profile of methylphenidate under long-term treatment in adult ADHD patients - results of the COMPAS study. Pharmacopsychiatry. 2020;53(6):263-271. doi:10.1055/a-1207-9851
12. Cutler AJ, Childress AC, Pardo A, et al. Randomized, double-blind, placebo-controlled, fixed-dose study to evaluate the efficacy and safety of amphetamine extended-release tablets in adults with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2022;83(5):22m14438. doi:10.4088/JCP.22m14438
13. Iwanami A, Saito K, Fujiwara M, et al. Efficacy and safety of guanfacine extended-release in the treatment of attention-deficit/hyperactivity disorder in adults: results of a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2020;81(3):19m12979. doi:10.4088/JCP.19m12979
14. Reimherr FW, Gift TE, Steans TA, et al. The use of brexpiprazole combined with a stimulant in adults with treatment-resistant attention-deficit/hyperactivity disorder. J Clin Psychopharmacol. 2022;42(5):445-453. doi:10.1097/JCP.0000000000001592
15. Philipsen A, Jans T, Graf E, et al; Comparison of Methylphenidate and Psychotherapy in Adult ADHD Study (COMPAS) Consortium. Effects of group psychotherapy, individual counseling, methylphenidate, and placebo in the treatment of adult attention-deficit/hyperactivity disorder: a randomized clinical trial. JAMA Psychiatry. 2015;72(12):1199-1210.
16. McGough JJ. Treatment controversies in adult ADHD. Am J Psychiatry. 2016;173(10):960-966. doi:10.1176/appi.ajp.2016.15091207
17. Cruz MP. Guanfacine extended-release tablets (Intuniv), a nonstimulant selective alpha2a-adrenergic receptor agonist for attention-deficit/hyperactivity disorder. P T. 2010;35(8):448-451.
Transient global amnesia: Psychiatric precipitants, features, and comorbidities
Ms. A, age 48, is a physician’s assistant with no psychiatric history. She presents to the emergency department (ED) with her partner and daughter due to a 15-minute episode of acute-onset memory loss and concern for stroke. In the ED, Ms. A is confused and repeatedly asks, “Where are we?” “How did we get here?” and “What day is it?” Her partner denies Ms. A has focal neurologic deficits or seizures.
Ms. A had only slept 4 hours the night before she came to the ED because she had just learned that her daughter works in the sex industry. According to her daughter, Ms. A was raped by a soldier many years ago. At that time, her perpetrator told Ms. A that he would kill her entire family if she ever told anyone. As a result, she never pursued any psychological or psychiatric treatment.
During the evaluation, Ms. A shares details regarding her social and medical history; however, she does not recall receiving bad news the night before. She asks the interviewer several times how she got to the hospital, and when a cranial nerve exam is performed, she states, “I am not the patient!”
Ms. A’s vital signs and physical exam are unremarkable. Urinalysis is significant for a ketones level of 20 mmol/L (reference range: negative for ketones), and a urine human chorionic gonadotropin test is negative. A neurologic exam does not identify any focal deficits. No imaging is performed.
Transient global amnesia (TGA) describes an episode of anterograde, and possibly retrograde, amnesia that lasts up to 24 hours. On presentation, patients experiencing TGA repeatedly ask, “Where am I?” “What day is it?” and “How did I get here?” However, semantic memory—such as knowledge of the world and autobiographical information—is preserved.1 The first case of TGA was described in 1956, and its diagnostic criteria were most recently modified in 1990 (Table2).
Though TGA is the most common cause of acute-onset amnesia, it is rare, affecting approximately 3 to 10 individuals per 100,000. The average age of onset is 61 to 63, with most cases occurring after age 50. TGA is generally thought to affect males and females equally, though some studies suggest a female predominance.3 In most cases (approximately 90%), there is a precipitating event such as physical or emotional stress, change in temperature, or sexual intercourse.4
In this article, we provide an overview of the classification, presentation, differential diagnosis, workup, and treatment of TGA. While TGA is a neurologic diagnosis, in a subset of patients it can present with psychiatric features resembling conversion disorder. For such patients, we argue that TGA can be considered a neuropsychiatric condition (Box 15-12). This classification may empower emergency psychiatry clinicians and psychotherapists to identify and treat the condition, which is not described by the current psychiatric diagnostic system.
Box 1
Transient global amnesia (TGA) is a neurologic diagnosis. However, in 1956, Bender8 associated the clinical picture of TGA with psychogenic etiology, 2 years before the term was coined. The same year, Courjon et al9 classified TGA as a functional disorder.
As recent literature on TGA has focused on the neuropsychologic mechanism of memory loss, examination of the condition from a psychodynamic standpoint has fallen out of favor. In fact, the earliest discussions of the condition attributed the absence of TGA from literature prior to the 1950s “to erroneous classification of TGA as psychogenic or hysterical amnesia.”10 However, to refer to this condition as purely neurologic—and without any “psychogenic” or functional features— would be reductive.
In a 2019 case report, Espiridion et al6 considered TGA within the same diagnostic realm as—if not actually a form of—dissociative amnesia (DA). They published the case of a 60-year-old woman with a history of posttraumatic stress disorder (PTSD) who experienced an episode of TGA that had manifested as anterograde and retrograde amnesia for 2 days and was precipitated by a psychotherapy session in which she discussed an individual who had assaulted her 5 years earlier. Much like in the case of Ms. A, the report from Espiridion et al6 clearly exemplifies a psychiatric etiology that shares similar context of a stressor unveiling a past memory too unbearable to maintain in consciousness. They concluded that “this case demonstrates anterograde and identity memory impairments likely induced by her PTSD. It is … possible that this presentation may be labeled PTSD-related dissociative amnesia.”6
Considering TGA as a type of DA within a subset of patients represents progression with regards to considering it as a psychiatric disorder. However, a prominent factor distinguishing TGA from DA is that the latter is more commonly associated with loss of personal identity.5 In TGA, memory of autobiographical information typically is preserved.7
Others have argued for a subtype of “emotional arousal–induced TGA”11 or “emotional TGA.”10 We suggest that this “emotional” subtype of TGA, which clearly was affecting Ms. A, shares similarities with functional neurologic symptom disorder, otherwise known as conversion disorder. The psychoanalytic concept that unconscious psychic distress can be “converted” into a neurologic problem is exemplified by Ms. A. Of note, being female and having an emotional stressor are risk factors for conversion disorder. Additionally, migraine— which was not part of Ms. A’s history—is also a risk factor for both TGA and conversion disorder.12 Despite these similarities, however, TGA’s neurophysiological changes on MRI and self-resolving nature still position the disorder as uniquely neuropsychiatric in the term’s purest sense.
Continue to: Differential diagnosis and workup
Differential diagnosis and workup
The differential diagnosis for acute-onset memory loss in the absence of other neurologic or psychiatric features is broad. It includes:
- dissociative amnesia
- ischemic amnesia
- transient epileptic amnesia
- toxic and metabolic amnesia
- posttraumatic amnesia.
Dissociative amnesia (DA), otherwise known as psychogenic amnesia, is “an inability to recall important autobiographical information, usually of a traumatic or stressful nature, that is inconsistent with ordinary forgetting.”13 According to this definition, DA features only retrograde amnesia, as opposed to TGA, which features anterograde amnesia, with possible retrograde amnesia. A subtype of DA—specifically, “continuous amnesia” or “anterograde dissociative amnesia”— is in DSM-5.13 However, the diagnostic criteria are unclear, and no cases have been identified in the literature since 1903, before TGA became a diagnostic entity.5,14 Moreover, patients with DA cannot recall autobiographical information, which is not a feature of TGA. Within DSM-5, TGA is an exclusion criterion for DA.13 Thus, an episode of anterograde amnesia with acute onset best meets criteria for TGA, even if there are substantial psychiatric risk factors.
Ischemic amnesia—including stroke and transient ischemic attack (TIA)—is often the primary concern of patients with TGA and their families upon initial presentation, as was the case with Ms. A.6,15 TIA presenting with isolated, acute-onset amnesia would be highly unusual, because these attacks usually present with focal symptoms including motor deficits, sensory deficits, visual field deficits, and aphasia or dysarthria. A patient with amnesia experiencing a TIA would likely have symptoms lasting from seconds to minutes, which is much shorter than a typical TGA episode.16
Amnesia secondary to stroke may be transient or permanent.7 Amnesia is present in approximately 1% of all strokes and in approximately 19.3% of posterior cerebral artery strokes.7,17 Unlike TIA and TGA, ischemic amnesia would present with MRI findings detectable at symptom onset. TGA does reveal MRI findings, particularly punctate lesions in the CA1 area of the hippocampus; however, these lesions are typically much smaller than those found in stroke, and are not detectable until 12 to 48 hours after episode onset.1,17 MRI findings in ischemic amnesia are typically associated with extrahippocampal lesions.17 Finally, the presence of vascular risk factors such as hyperlipidemia, smoking, diabetes, and hypertension may also favor a diagnosis of stroke or TIA as opposed to TGA, which is not associated with these risk factors.18 Though ischemic amnesia and TGA usually can be differentiated based on history and presentation, MRI with fluid-attenuated inversion recovery and diffusion-weighted imaging may be performed to definitively distinguish stroke from TGA.7
Transient epileptic amnesia (TEA), a focal form of epilepsy within the temporal lobe, should also be considered in patients who present with acute-onset amnesia. Like TGA, TEA may present with simultaneous anterograde and retrograde amnesia accompanied by repetitive questioning.19 Amnesia can be the sole symptom of TEA in up to 24% of cases. However, several key features distinguish TEA from TGA. TEA most often presents with other clinical signs of seizures, such as oral automatisms and/or olfactory hallucinations.20 There is also a significant difference in episode length; TEA episodes last an average of 30 to 60 minutes and tend to occur upon wakening, whereas TGA episodes last an average of 4 to 6 hours and do not preferentially occur at any particular time.1,21 In the interictal period—between seizures—patients with TEA may also experience accelerated long-term forgetting, autobiographical amnesia, and topographical amnesia.19,20 Finally, a diagnosis of TEA also requires recurrent episodes. Recurrence can happen with TGA, but is less frequent.21 Generally, history and presentation can distinguish TEA from TGA. Though there is no formal protocol for TEA workup, Lanzone et al21 recommend 24-hour EEG or EEG sleep monitoring in patients who present with amnesia as well as other clinical manifestations of epileptic phenomenon.
Continue to: Toxic and metabolic
Toxic and metabolic etiologies of amnesia include opioid and cocaine use, general anesthetics,22 and hypoglycemia.7,23 Toxic and metabolic causes of amnesia may mirror TGA in their acute onset as well as anterograde nature. However, these patients will likely present with fluctuating consciousness and/or other neuropsychiatric features, such as pressured speech, delusions, and/or distractability.23 Obtaining a patient’s medical history, including substance use, medication use, and the presence of diabetes,24 is typically sufficient to rule out toxic and metabolic causes.7
Posttraumatic amnesia (PTA) describes transient memory loss that occurs after a traumatic brain injury. Anterograde amnesia is most common, though approximately 20% of patients may also experience retrograde amnesia pertaining to the events near the date of their injury. Unlike TGA, which typically resolves within 24 hours, the recovery time of amnestic symptoms in PTA ranges from minutes to years.7 A distinguishing feature of PTA is the presence of confusion, which often resembles a state of delirium.25 The presentation of PTA can vary immensely with regards to agitation, psychotic symptoms, and the time to resolution of the amnesia. Though TGA can be distinguished from PTA based on a lack of clouding of consciousness, a case of anterograde amnesia warrants inquiry into a potential history of head injuries to rule out a traumatic cause.26
Box 21,3,23,27-33 outlines current theories of the etiology and pathogenesis of TGA.
Box 2
The etiology and pathogenesis of transient global amnesia (TGA) are poorly understood, and TGA remains one of the most enigmatic syndromes in clinical neurology.27 Theories regarding the pathogenesis of TGA are diverse and include vascular, epileptic, migraine, and stress-related etiologies.1,23
Early theories suggested arterial ischemia28 and epileptic phenomena29 as etiologies of TGA. The venous theory posits that TGA stems from jugular venous incompetency, causing venous flow and subsequent venous congestion in the medial temporal lobe, wherein lies the hippocampus. This theory is supported by several studies showing venous valve insufficiency as detected by ultrasonographic evaluation during the Valsalva maneuver in patients with TGA.30 This pathophysiologic mechanism may explain the occurrence of TGA in a specific cluster of cases, including men whose TGA episodes are precipitated by physical stress or the Valsalva maneuver.3 The migraine theory and stress theory share a similar proposed neurophysiologic mechanism.
The migraine theory stems from migraines being a known risk factor for TGA, particularly in middle-aged women.31 The stress theory is based on the known emotional precipitants and psychiatric comorbidities associated with TGA. Notably, both the migraine theory and stress theory implicate the role of excessive glutamate release as well as CNS depression.31,32 Glutamate targets the CA1 region of the hippocampus, which is involved in TGA and is known to have the highest density of N-methyl-D-aspartate receptors among hippocampal regions.33
Given the heterogeneity of the demographics and stressors associated with TGA, multiple mechanisms for the disease process may coexist, leading to a similar clinical picture. In 2006, Quinette et al3 performed a multivariate analysis of variables associated with TGA, including age, sex, medical history, and presentation. They demonstrated 3 “clusters” of TGA pictures: women with anxiety or a personality disorder; men with physical precipitating events; and younger patients (age <56) with a history of migraine. These findings suggest TGA may have unique precipitants corresponding to multiple neurophysiologic mechanisms.
Transient global ischemia: Psychiatric features
Several studies have demonstrated psychiatric precipitants, features, and comorbidities associated with TGA. Of the TGA cases associated with precipitating events, 29% to 50% are associated with an emotional stressor.3,4 Examples of emotional stressors include a quarrel,4 the announcement of a birth or suicide, and a nightmare.15 For Ms. A, learning her daughter worked in the sex industry was an emotional stressor.2
During its acute phase, TGA has been shown to present with mood and anxiety symptoms.34 Moreover, during episodes, patients often demonstrate features of panic attacks, such as dizziness, fainting, choking, palpitations, and paresthesia.3,35
Continue to: Finally, patients with TGA...
Finally, patients with TGA are more likely to have psychiatric comorbidities than those without the condition. In a study of 25 patients who experienced TGA triggered by a precipitant, Inzitari et al4 found a strong association of TGA with phobic personality traits, including agoraphobia and simple phobic attitudes (ie, fear of traveling far from home or the sight of blood). Pantoni et al35 replicated these results in 2005 and found that in comparison to patients with TIA, patients with TGA are more likely to have personal and family histories of psychiatric disease. A 2014 study by Dohring et al36 found that compared to healthy controls, patients with TGA are more likely to have maladaptive coping strategies and stress responses. Patients with TGA tended to exhibit increased feelings of guilt, take more medication, and exhibit more anxiety compared to healthy controls.36
Treatment: Benzodiazepines
There are no published treatment guidelines for TGA. However, in case reports, benzodiazepines (specifically lorazepam37) have been shown to have utility in diagnosing and treating DA. The success of benzodiazepines is attributed to its gamma-aminobutyric acid mechanism, which involves inhibiting activity of the N-methyl-
However, the benzodiazepine midazolam has been identified as a precipitant of TGA. In a case report, Rinehart et al22 identified flumazenil—a benzodiazepine antagonist used primarily to treat retrograde postoperative amnesia—as an antidote. The potentially paradoxical role of benzodiazepines in both the precipitation and treatment of TGA may relate back to the heterogeneity of the etiologies of TGA. Further research comparing the treatment of TGA in patients with stress-induced TGA vs postoperative TGA is needed to better understand the neurochemical basis of TGA and work toward establishing optimal treatment options for different patient demographics.
A generally favorable prognosis
TGA carries a low risk of recurrence. In studies with 3- to 7-year follow-up periods, the recurrence rates ranged from 1.4% to 23.8%.23,35,38
Memory impairments may be present for 5 to 6 months following a TGA episode. The severity of these impairments may range from clinically unnoticeable to the patient meeting the criteria for mild cognitive impairment.23,39 The risk is higher in patients who have had recurrent TGA compared to those patients who have experienced only a single episode.23
Continue to: TGA does not increase...
TGA does not increase the risk of cerebrovascular events. There is controversy regarding a potentially increased risk for dementia as well as epilepsy, though there is insufficient evidence to support these findings.23,40
CASE CONTINUED
Five hours after the onset of Ms. A’s symptoms, the treatment team initiates oral lorazepam 1 mg. One hour after taking lorazepam, Ms. A’s anterograde and retrograde amnesia improve. She cannot recall why she was brought to the hospital but does remember the date and location, which she was not able to do on initial presentation. She feels safe, states a clear plan for self-care, and is discharged in the care of her partner. Though Ms. A’s memory improved soon after she received lorazepam, this improvement also could be attributed to the natural course of time, as TGA tends to resolve on its own within 24 hours.
Bottom Line
Transient global amnesia (TGA) is an episode of anterograde, and possibly retrograde, amnesia that lasts up to 24 hours. It represents an interesting diagnosis at the intersection of psychiatry and neurology. TGA has many established psychiatric risk factors and features—some of which may resemble conversion disorder—but these may only apply to a particular subset of patients, which reflects the heterogeneity of the condition.
Related Resources
- Sparaco M, Pascarella R, Muccio CF, et al. Forgetting the unforgettable: transient global amnesia part I: pathophysiology and etiology. J Clin Med. 2022;11(12): 3373. doi:10.3390/jcm1112337
- Sparaco M, Pascarella R, Muccio CF, et al. Forgetting the unforgettable: transient global amnesia part II: a clinical road map. J Clin Med. 2022;11(14):3940. doi:10.3390/ jcm11143940
Drug Brand Names
Flumazenil • Romazicon
Lorazepam • Ativan
Midazolam • Versed
1. Miller TD, Butler CR. Acute-onset amnesia: transient global amnesia and other causes. Pract Neurol. 2022;22(3):201-208. doi:10.1136/practneurol-2020-002826
2. Hodges JR, Warlow CP. Syndromes of transient amnesia: towards a classification. A study of 153 cases. J Neurol Neurosurg Psychiatry. 1990;53(10):834-843. doi:10.1136/jnnp.53.10.834
3. Quinette P, Guillery-Girard B, Dayan J, et al. What does transient global amnesia really mean? Review of the literature and thorough study of 142 cases. Brain. 2006;129(Pt 7):1640-1658. doi:10.1093/brain/awl105
4. Inzitari D, Pantoni L, Lamassa M, et al. Emotional arousal and phobia in transient global amnesia. Arch Neurol. 1997;54(7):866-873. doi:10.1001/archneur.1997.00550190056015
5. Staniloiu A, Markowitsch HJ. Dissociative amnesia. Lancet Psychiatry. 2014;1(3):226-241. doi:10.1016/S2215-0366(14)70279-2
6. Espiridion ED, Gupta J, Bshara A, et al. Transient global amnesia in a 60-year-old female with post-traumatic stress disorder. Cureus. 2019;11(9):e5792. doi:10.7759/cureus.5792
7. Alessandro L, Ricciardi M, Chaves H, et al. Acute amnestic syndromes. J Neurol Sci. 2020;413:116781. doi:10.1016/j.jns.2020.116781
8. Bender M. Syndrome of isolated episode of confusion with amnesia. J Hillside Hosp. 1956;5:212-215.
9. Courjon J, Guyotat J. Les ictus amnéstiques [Amnesic strokes]. J Med Lyon. 1956;37(882):697-701.
10. Noel A, Quinette P, Hainselin M, et al. The still enigmatic syndrome of transient global amnesia: interactions between neurological and psychopathological factors. Neuropsychol Rev. 2015;25(2):125-133. doi:10.1007/s11065-015-9284-y
11. Merriam AE, Wyszynski B, Betzler T. Emotional arousal-induced transient global amnesia. A clue to the neural transcription of emotion? Psychosomatics. 1992;33(1):109-113. doi:10.1016/S0033-3182(92)72029-5
12. Hallett M, Aybek S, Dworetzky BA, et al. Functional neurological disorder: new subtypes and shared mechanisms. Lancet Neurol. 2022;21(6):537-550. doi:10.1016/S1474-4422(21)00422-1
13. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
14. Bourdon B, Dide M. A case of continuous amnesia with tactile asymbolia, complicated by other troubles. Ann Psychol. 1903;10:84-115.
15. Marinella MA. Transient global amnesia and a father’s worst nightmare. N Engl J Med. 2004;350(8):843-844. doi:10.1056/NEJM200402193500821
16. Amarenco P. Transient ischemic attack. N Engl J Med. 2020;382(20):1933-1941. doi:10.1056/NEJMcp1908837
17. Szabo K, Forster A, Jager T, et al. Hippocampal lesion patterns in acute posterior cerebral artery stroke: clinical and MRI findings. Stroke. 2009;40(6):2042-2045. doi:10.1161/STROKEAHA.108.536144
18. Liampas I, Raptopoulou M, Siokas V, et al. Conventional cardiovascular risk factors in transient global amnesia: systematic review and proposition of a novel hypothesis. Front Neuroendocrinol. 2021;61:100909. doi:10.1016/j.yfrne.2021.100909
19. Zeman A, Butler C. Transient epileptic amnesia. Curr Opin Neurol. 2010;23(6):610-616. doi:10.1097/WCO.0b013e32834027db
20. Baker J, Savage S, Milton F, et al. The syndrome of transient epileptic amnesia: a combined series of 115 cases and literature review. Brain Commun. 2021;3(2):fcab038. doi:10.1093/braincomms/fcab038
21. Lanzone J, Ricci L, Assenza G, et al Transient epileptic and global amnesia: real-life differential diagnosis. Epilepsy Behav. 2018;88:205-211. doi:10.1016/j.yebeh.2018.07.015
22. Rinehart JB, Baker B, Raphael D. Postoperative global amnesia reversed with flumazenil. Neurologist. 2012;18(4):216-218. doi:10.1097/NRL.0b013e31825bbef4
23. Arena JE, Rabinstein AA. Transient global amnesia. Mayo Clin Proc. 2015;90(2):264-272. doi:10.1016/j.mayocp.2014.12.001
24. Holemans X, Dupuis M, Misson N, et al. Reversible amnesia in a type 1 diabetic patient and bilateral hippocampal lesions on magnetic resonance imaging (MRI). Diabet Med. 2001;18(9):761-763. doi:10.1046/j.1464-5491.2001.00481.x
25. Marshman LAG, Jakabek D, Hennessy M, et al. Post-traumatic amnesia. J Clin Neurosci. 2013;20(11):1475-1481. doi:10.1016/j.jocn.2012.11.022
26. Parker TD, Rees R, Rajagopal S, et al. Post-traumatic amnesia. Pract Neurol. 2022;22(2):129-137. doi:10.1136/practneurol-2021-003056
27. You SH, Kim B, Kim BK. Transient global amnesia: signal alteration in 2D/3D T2-FLAIR sequences. Clin Imaging. 2021;78:154-159. doi:10.1016/j.clinimag.2021.03.029
28. Mathew NT, Meyer JS. Pathogenesis and natural history of transient global amnesia. Stroke. 1974;5(3):303-311. doi:10.1161/01.str.5.3.303
29. Fisher CM, Adams RD. Transient global amnesia. Acta Neurol Scand Suppl. 1964;40(SUPPL 9):1-83.
30. Cejas C, Cisneros LF, Lagos R, et al. Internal jugular vein valve incompetence is highly prevalent in transient global amnesia. Stroke. 2010;41(1):67-71. doi:10.1161/STROKEAHA.109.566315
31. Liampas I, Siouras AS, Siokas V, et al. Migraine in transient global amnesia: a meta-analysis of observational studies. J Neurol. 2022;269(1):184-196. doi:10.1007/s00415-020-10363-y
32. Ding X, Peng D. Transient global amnesia: an electrophysiological disorder based on cortical spreading depression-transient global amnesia model. Front Hum Neurosci. 2020;14:602496. doi:10.3389/fnhum.2020.602496
33. Bartsch T, Dohring J, Reuter S, et al. Selective neuronal vulnerability of human hippocampal CA1 neurons: lesion evolution, temporal course, and pattern of hippocampal damage in diffusion-weighted MR imaging. J Cereb Blood Flow Metab. 2015;35(11):1836-1845. doi:10.1038/jcbfm.2015.137
34. Noel A, Quinette P, Guillery-Girard B, et al. Psychopathological factors, memory disorders and transient global amnesia. Br J Psychiatry. 2008;193(2):145-151. doi:10.1192/bjp.bp.107.045716
35. Pantoni L, Bertini E, Lamassa M, et al. Clinical features, risk factors, and prognosis in transient global amnesia: a follow-up study. Eur J Neurol. 2005;12(5):350-356. doi:10.1111/j.1468-1331.2004.00982.x
36. Dohring J, Schmuck A, Bartsch T. Stress-related factors in the emergence of transient global amnesia with hippocampal lesions. Front Behav Neurosci. 2014;8:287. doi:10.3389/fnbeh.2014.00287
37. Jiang S, Gunther S, Hartney K, et al. An intravenous lorazepam infusion for dissociative amnesia: a case report. Psychosomatics. 2020;61(6):814-818. doi:10.1016/j.psym.2020.01.009
38. He S, Ye Z, Yang Q, et al. Transient global amnesia: risk factors, imaging features, and prognosis. Neuropsychiatr Dis Treat. 2021;17:1611-1619. doi:10.2147/NDT.S299168
39. Borroni B, Agosti C, Brambilla C, et al. Is transient global amnesia a risk factor for amnestic mild cognitive impairment? J Neurol. 2004;251(9):1125-1127. doi:10.1007/s00415-004-0497-x
40. Liampas I, Raptopoulou M, Siokas V, et al. The long-term prognosis of transient global amnesia: a systematic review. Rev Neurosci. 2021;32(5):531-543. doi:10.1515/revneuro-2020-0110
Ms. A, age 48, is a physician’s assistant with no psychiatric history. She presents to the emergency department (ED) with her partner and daughter due to a 15-minute episode of acute-onset memory loss and concern for stroke. In the ED, Ms. A is confused and repeatedly asks, “Where are we?” “How did we get here?” and “What day is it?” Her partner denies Ms. A has focal neurologic deficits or seizures.
Ms. A had only slept 4 hours the night before she came to the ED because she had just learned that her daughter works in the sex industry. According to her daughter, Ms. A was raped by a soldier many years ago. At that time, her perpetrator told Ms. A that he would kill her entire family if she ever told anyone. As a result, she never pursued any psychological or psychiatric treatment.
During the evaluation, Ms. A shares details regarding her social and medical history; however, she does not recall receiving bad news the night before. She asks the interviewer several times how she got to the hospital, and when a cranial nerve exam is performed, she states, “I am not the patient!”
Ms. A’s vital signs and physical exam are unremarkable. Urinalysis is significant for a ketones level of 20 mmol/L (reference range: negative for ketones), and a urine human chorionic gonadotropin test is negative. A neurologic exam does not identify any focal deficits. No imaging is performed.
Transient global amnesia (TGA) describes an episode of anterograde, and possibly retrograde, amnesia that lasts up to 24 hours. On presentation, patients experiencing TGA repeatedly ask, “Where am I?” “What day is it?” and “How did I get here?” However, semantic memory—such as knowledge of the world and autobiographical information—is preserved.1 The first case of TGA was described in 1956, and its diagnostic criteria were most recently modified in 1990 (Table2).
Though TGA is the most common cause of acute-onset amnesia, it is rare, affecting approximately 3 to 10 individuals per 100,000. The average age of onset is 61 to 63, with most cases occurring after age 50. TGA is generally thought to affect males and females equally, though some studies suggest a female predominance.3 In most cases (approximately 90%), there is a precipitating event such as physical or emotional stress, change in temperature, or sexual intercourse.4
In this article, we provide an overview of the classification, presentation, differential diagnosis, workup, and treatment of TGA. While TGA is a neurologic diagnosis, in a subset of patients it can present with psychiatric features resembling conversion disorder. For such patients, we argue that TGA can be considered a neuropsychiatric condition (Box 15-12). This classification may empower emergency psychiatry clinicians and psychotherapists to identify and treat the condition, which is not described by the current psychiatric diagnostic system.
Box 1
Transient global amnesia (TGA) is a neurologic diagnosis. However, in 1956, Bender8 associated the clinical picture of TGA with psychogenic etiology, 2 years before the term was coined. The same year, Courjon et al9 classified TGA as a functional disorder.
As recent literature on TGA has focused on the neuropsychologic mechanism of memory loss, examination of the condition from a psychodynamic standpoint has fallen out of favor. In fact, the earliest discussions of the condition attributed the absence of TGA from literature prior to the 1950s “to erroneous classification of TGA as psychogenic or hysterical amnesia.”10 However, to refer to this condition as purely neurologic—and without any “psychogenic” or functional features— would be reductive.
In a 2019 case report, Espiridion et al6 considered TGA within the same diagnostic realm as—if not actually a form of—dissociative amnesia (DA). They published the case of a 60-year-old woman with a history of posttraumatic stress disorder (PTSD) who experienced an episode of TGA that had manifested as anterograde and retrograde amnesia for 2 days and was precipitated by a psychotherapy session in which she discussed an individual who had assaulted her 5 years earlier. Much like in the case of Ms. A, the report from Espiridion et al6 clearly exemplifies a psychiatric etiology that shares similar context of a stressor unveiling a past memory too unbearable to maintain in consciousness. They concluded that “this case demonstrates anterograde and identity memory impairments likely induced by her PTSD. It is … possible that this presentation may be labeled PTSD-related dissociative amnesia.”6
Considering TGA as a type of DA within a subset of patients represents progression with regards to considering it as a psychiatric disorder. However, a prominent factor distinguishing TGA from DA is that the latter is more commonly associated with loss of personal identity.5 In TGA, memory of autobiographical information typically is preserved.7
Others have argued for a subtype of “emotional arousal–induced TGA”11 or “emotional TGA.”10 We suggest that this “emotional” subtype of TGA, which clearly was affecting Ms. A, shares similarities with functional neurologic symptom disorder, otherwise known as conversion disorder. The psychoanalytic concept that unconscious psychic distress can be “converted” into a neurologic problem is exemplified by Ms. A. Of note, being female and having an emotional stressor are risk factors for conversion disorder. Additionally, migraine— which was not part of Ms. A’s history—is also a risk factor for both TGA and conversion disorder.12 Despite these similarities, however, TGA’s neurophysiological changes on MRI and self-resolving nature still position the disorder as uniquely neuropsychiatric in the term’s purest sense.
Continue to: Differential diagnosis and workup
Differential diagnosis and workup
The differential diagnosis for acute-onset memory loss in the absence of other neurologic or psychiatric features is broad. It includes:
- dissociative amnesia
- ischemic amnesia
- transient epileptic amnesia
- toxic and metabolic amnesia
- posttraumatic amnesia.
Dissociative amnesia (DA), otherwise known as psychogenic amnesia, is “an inability to recall important autobiographical information, usually of a traumatic or stressful nature, that is inconsistent with ordinary forgetting.”13 According to this definition, DA features only retrograde amnesia, as opposed to TGA, which features anterograde amnesia, with possible retrograde amnesia. A subtype of DA—specifically, “continuous amnesia” or “anterograde dissociative amnesia”— is in DSM-5.13 However, the diagnostic criteria are unclear, and no cases have been identified in the literature since 1903, before TGA became a diagnostic entity.5,14 Moreover, patients with DA cannot recall autobiographical information, which is not a feature of TGA. Within DSM-5, TGA is an exclusion criterion for DA.13 Thus, an episode of anterograde amnesia with acute onset best meets criteria for TGA, even if there are substantial psychiatric risk factors.
Ischemic amnesia—including stroke and transient ischemic attack (TIA)—is often the primary concern of patients with TGA and their families upon initial presentation, as was the case with Ms. A.6,15 TIA presenting with isolated, acute-onset amnesia would be highly unusual, because these attacks usually present with focal symptoms including motor deficits, sensory deficits, visual field deficits, and aphasia or dysarthria. A patient with amnesia experiencing a TIA would likely have symptoms lasting from seconds to minutes, which is much shorter than a typical TGA episode.16
Amnesia secondary to stroke may be transient or permanent.7 Amnesia is present in approximately 1% of all strokes and in approximately 19.3% of posterior cerebral artery strokes.7,17 Unlike TIA and TGA, ischemic amnesia would present with MRI findings detectable at symptom onset. TGA does reveal MRI findings, particularly punctate lesions in the CA1 area of the hippocampus; however, these lesions are typically much smaller than those found in stroke, and are not detectable until 12 to 48 hours after episode onset.1,17 MRI findings in ischemic amnesia are typically associated with extrahippocampal lesions.17 Finally, the presence of vascular risk factors such as hyperlipidemia, smoking, diabetes, and hypertension may also favor a diagnosis of stroke or TIA as opposed to TGA, which is not associated with these risk factors.18 Though ischemic amnesia and TGA usually can be differentiated based on history and presentation, MRI with fluid-attenuated inversion recovery and diffusion-weighted imaging may be performed to definitively distinguish stroke from TGA.7
Transient epileptic amnesia (TEA), a focal form of epilepsy within the temporal lobe, should also be considered in patients who present with acute-onset amnesia. Like TGA, TEA may present with simultaneous anterograde and retrograde amnesia accompanied by repetitive questioning.19 Amnesia can be the sole symptom of TEA in up to 24% of cases. However, several key features distinguish TEA from TGA. TEA most often presents with other clinical signs of seizures, such as oral automatisms and/or olfactory hallucinations.20 There is also a significant difference in episode length; TEA episodes last an average of 30 to 60 minutes and tend to occur upon wakening, whereas TGA episodes last an average of 4 to 6 hours and do not preferentially occur at any particular time.1,21 In the interictal period—between seizures—patients with TEA may also experience accelerated long-term forgetting, autobiographical amnesia, and topographical amnesia.19,20 Finally, a diagnosis of TEA also requires recurrent episodes. Recurrence can happen with TGA, but is less frequent.21 Generally, history and presentation can distinguish TEA from TGA. Though there is no formal protocol for TEA workup, Lanzone et al21 recommend 24-hour EEG or EEG sleep monitoring in patients who present with amnesia as well as other clinical manifestations of epileptic phenomenon.
Continue to: Toxic and metabolic
Toxic and metabolic etiologies of amnesia include opioid and cocaine use, general anesthetics,22 and hypoglycemia.7,23 Toxic and metabolic causes of amnesia may mirror TGA in their acute onset as well as anterograde nature. However, these patients will likely present with fluctuating consciousness and/or other neuropsychiatric features, such as pressured speech, delusions, and/or distractability.23 Obtaining a patient’s medical history, including substance use, medication use, and the presence of diabetes,24 is typically sufficient to rule out toxic and metabolic causes.7
Posttraumatic amnesia (PTA) describes transient memory loss that occurs after a traumatic brain injury. Anterograde amnesia is most common, though approximately 20% of patients may also experience retrograde amnesia pertaining to the events near the date of their injury. Unlike TGA, which typically resolves within 24 hours, the recovery time of amnestic symptoms in PTA ranges from minutes to years.7 A distinguishing feature of PTA is the presence of confusion, which often resembles a state of delirium.25 The presentation of PTA can vary immensely with regards to agitation, psychotic symptoms, and the time to resolution of the amnesia. Though TGA can be distinguished from PTA based on a lack of clouding of consciousness, a case of anterograde amnesia warrants inquiry into a potential history of head injuries to rule out a traumatic cause.26
Box 21,3,23,27-33 outlines current theories of the etiology and pathogenesis of TGA.
Box 2
The etiology and pathogenesis of transient global amnesia (TGA) are poorly understood, and TGA remains one of the most enigmatic syndromes in clinical neurology.27 Theories regarding the pathogenesis of TGA are diverse and include vascular, epileptic, migraine, and stress-related etiologies.1,23
Early theories suggested arterial ischemia28 and epileptic phenomena29 as etiologies of TGA. The venous theory posits that TGA stems from jugular venous incompetency, causing venous flow and subsequent venous congestion in the medial temporal lobe, wherein lies the hippocampus. This theory is supported by several studies showing venous valve insufficiency as detected by ultrasonographic evaluation during the Valsalva maneuver in patients with TGA.30 This pathophysiologic mechanism may explain the occurrence of TGA in a specific cluster of cases, including men whose TGA episodes are precipitated by physical stress or the Valsalva maneuver.3 The migraine theory and stress theory share a similar proposed neurophysiologic mechanism.
The migraine theory stems from migraines being a known risk factor for TGA, particularly in middle-aged women.31 The stress theory is based on the known emotional precipitants and psychiatric comorbidities associated with TGA. Notably, both the migraine theory and stress theory implicate the role of excessive glutamate release as well as CNS depression.31,32 Glutamate targets the CA1 region of the hippocampus, which is involved in TGA and is known to have the highest density of N-methyl-D-aspartate receptors among hippocampal regions.33
Given the heterogeneity of the demographics and stressors associated with TGA, multiple mechanisms for the disease process may coexist, leading to a similar clinical picture. In 2006, Quinette et al3 performed a multivariate analysis of variables associated with TGA, including age, sex, medical history, and presentation. They demonstrated 3 “clusters” of TGA pictures: women with anxiety or a personality disorder; men with physical precipitating events; and younger patients (age <56) with a history of migraine. These findings suggest TGA may have unique precipitants corresponding to multiple neurophysiologic mechanisms.
Transient global ischemia: Psychiatric features
Several studies have demonstrated psychiatric precipitants, features, and comorbidities associated with TGA. Of the TGA cases associated with precipitating events, 29% to 50% are associated with an emotional stressor.3,4 Examples of emotional stressors include a quarrel,4 the announcement of a birth or suicide, and a nightmare.15 For Ms. A, learning her daughter worked in the sex industry was an emotional stressor.2
During its acute phase, TGA has been shown to present with mood and anxiety symptoms.34 Moreover, during episodes, patients often demonstrate features of panic attacks, such as dizziness, fainting, choking, palpitations, and paresthesia.3,35
Continue to: Finally, patients with TGA...
Finally, patients with TGA are more likely to have psychiatric comorbidities than those without the condition. In a study of 25 patients who experienced TGA triggered by a precipitant, Inzitari et al4 found a strong association of TGA with phobic personality traits, including agoraphobia and simple phobic attitudes (ie, fear of traveling far from home or the sight of blood). Pantoni et al35 replicated these results in 2005 and found that in comparison to patients with TIA, patients with TGA are more likely to have personal and family histories of psychiatric disease. A 2014 study by Dohring et al36 found that compared to healthy controls, patients with TGA are more likely to have maladaptive coping strategies and stress responses. Patients with TGA tended to exhibit increased feelings of guilt, take more medication, and exhibit more anxiety compared to healthy controls.36
Treatment: Benzodiazepines
There are no published treatment guidelines for TGA. However, in case reports, benzodiazepines (specifically lorazepam37) have been shown to have utility in diagnosing and treating DA. The success of benzodiazepines is attributed to its gamma-aminobutyric acid mechanism, which involves inhibiting activity of the N-methyl-
However, the benzodiazepine midazolam has been identified as a precipitant of TGA. In a case report, Rinehart et al22 identified flumazenil—a benzodiazepine antagonist used primarily to treat retrograde postoperative amnesia—as an antidote. The potentially paradoxical role of benzodiazepines in both the precipitation and treatment of TGA may relate back to the heterogeneity of the etiologies of TGA. Further research comparing the treatment of TGA in patients with stress-induced TGA vs postoperative TGA is needed to better understand the neurochemical basis of TGA and work toward establishing optimal treatment options for different patient demographics.
A generally favorable prognosis
TGA carries a low risk of recurrence. In studies with 3- to 7-year follow-up periods, the recurrence rates ranged from 1.4% to 23.8%.23,35,38
Memory impairments may be present for 5 to 6 months following a TGA episode. The severity of these impairments may range from clinically unnoticeable to the patient meeting the criteria for mild cognitive impairment.23,39 The risk is higher in patients who have had recurrent TGA compared to those patients who have experienced only a single episode.23
Continue to: TGA does not increase...
TGA does not increase the risk of cerebrovascular events. There is controversy regarding a potentially increased risk for dementia as well as epilepsy, though there is insufficient evidence to support these findings.23,40
CASE CONTINUED
Five hours after the onset of Ms. A’s symptoms, the treatment team initiates oral lorazepam 1 mg. One hour after taking lorazepam, Ms. A’s anterograde and retrograde amnesia improve. She cannot recall why she was brought to the hospital but does remember the date and location, which she was not able to do on initial presentation. She feels safe, states a clear plan for self-care, and is discharged in the care of her partner. Though Ms. A’s memory improved soon after she received lorazepam, this improvement also could be attributed to the natural course of time, as TGA tends to resolve on its own within 24 hours.
Bottom Line
Transient global amnesia (TGA) is an episode of anterograde, and possibly retrograde, amnesia that lasts up to 24 hours. It represents an interesting diagnosis at the intersection of psychiatry and neurology. TGA has many established psychiatric risk factors and features—some of which may resemble conversion disorder—but these may only apply to a particular subset of patients, which reflects the heterogeneity of the condition.
Related Resources
- Sparaco M, Pascarella R, Muccio CF, et al. Forgetting the unforgettable: transient global amnesia part I: pathophysiology and etiology. J Clin Med. 2022;11(12): 3373. doi:10.3390/jcm1112337
- Sparaco M, Pascarella R, Muccio CF, et al. Forgetting the unforgettable: transient global amnesia part II: a clinical road map. J Clin Med. 2022;11(14):3940. doi:10.3390/ jcm11143940
Drug Brand Names
Flumazenil • Romazicon
Lorazepam • Ativan
Midazolam • Versed
Ms. A, age 48, is a physician’s assistant with no psychiatric history. She presents to the emergency department (ED) with her partner and daughter due to a 15-minute episode of acute-onset memory loss and concern for stroke. In the ED, Ms. A is confused and repeatedly asks, “Where are we?” “How did we get here?” and “What day is it?” Her partner denies Ms. A has focal neurologic deficits or seizures.
Ms. A had only slept 4 hours the night before she came to the ED because she had just learned that her daughter works in the sex industry. According to her daughter, Ms. A was raped by a soldier many years ago. At that time, her perpetrator told Ms. A that he would kill her entire family if she ever told anyone. As a result, she never pursued any psychological or psychiatric treatment.
During the evaluation, Ms. A shares details regarding her social and medical history; however, she does not recall receiving bad news the night before. She asks the interviewer several times how she got to the hospital, and when a cranial nerve exam is performed, she states, “I am not the patient!”
Ms. A’s vital signs and physical exam are unremarkable. Urinalysis is significant for a ketones level of 20 mmol/L (reference range: negative for ketones), and a urine human chorionic gonadotropin test is negative. A neurologic exam does not identify any focal deficits. No imaging is performed.
Transient global amnesia (TGA) describes an episode of anterograde, and possibly retrograde, amnesia that lasts up to 24 hours. On presentation, patients experiencing TGA repeatedly ask, “Where am I?” “What day is it?” and “How did I get here?” However, semantic memory—such as knowledge of the world and autobiographical information—is preserved.1 The first case of TGA was described in 1956, and its diagnostic criteria were most recently modified in 1990 (Table2).
Though TGA is the most common cause of acute-onset amnesia, it is rare, affecting approximately 3 to 10 individuals per 100,000. The average age of onset is 61 to 63, with most cases occurring after age 50. TGA is generally thought to affect males and females equally, though some studies suggest a female predominance.3 In most cases (approximately 90%), there is a precipitating event such as physical or emotional stress, change in temperature, or sexual intercourse.4
In this article, we provide an overview of the classification, presentation, differential diagnosis, workup, and treatment of TGA. While TGA is a neurologic diagnosis, in a subset of patients it can present with psychiatric features resembling conversion disorder. For such patients, we argue that TGA can be considered a neuropsychiatric condition (Box 15-12). This classification may empower emergency psychiatry clinicians and psychotherapists to identify and treat the condition, which is not described by the current psychiatric diagnostic system.
Box 1
Transient global amnesia (TGA) is a neurologic diagnosis. However, in 1956, Bender8 associated the clinical picture of TGA with psychogenic etiology, 2 years before the term was coined. The same year, Courjon et al9 classified TGA as a functional disorder.
As recent literature on TGA has focused on the neuropsychologic mechanism of memory loss, examination of the condition from a psychodynamic standpoint has fallen out of favor. In fact, the earliest discussions of the condition attributed the absence of TGA from literature prior to the 1950s “to erroneous classification of TGA as psychogenic or hysterical amnesia.”10 However, to refer to this condition as purely neurologic—and without any “psychogenic” or functional features— would be reductive.
In a 2019 case report, Espiridion et al6 considered TGA within the same diagnostic realm as—if not actually a form of—dissociative amnesia (DA). They published the case of a 60-year-old woman with a history of posttraumatic stress disorder (PTSD) who experienced an episode of TGA that had manifested as anterograde and retrograde amnesia for 2 days and was precipitated by a psychotherapy session in which she discussed an individual who had assaulted her 5 years earlier. Much like in the case of Ms. A, the report from Espiridion et al6 clearly exemplifies a psychiatric etiology that shares similar context of a stressor unveiling a past memory too unbearable to maintain in consciousness. They concluded that “this case demonstrates anterograde and identity memory impairments likely induced by her PTSD. It is … possible that this presentation may be labeled PTSD-related dissociative amnesia.”6
Considering TGA as a type of DA within a subset of patients represents progression with regards to considering it as a psychiatric disorder. However, a prominent factor distinguishing TGA from DA is that the latter is more commonly associated with loss of personal identity.5 In TGA, memory of autobiographical information typically is preserved.7
Others have argued for a subtype of “emotional arousal–induced TGA”11 or “emotional TGA.”10 We suggest that this “emotional” subtype of TGA, which clearly was affecting Ms. A, shares similarities with functional neurologic symptom disorder, otherwise known as conversion disorder. The psychoanalytic concept that unconscious psychic distress can be “converted” into a neurologic problem is exemplified by Ms. A. Of note, being female and having an emotional stressor are risk factors for conversion disorder. Additionally, migraine— which was not part of Ms. A’s history—is also a risk factor for both TGA and conversion disorder.12 Despite these similarities, however, TGA’s neurophysiological changes on MRI and self-resolving nature still position the disorder as uniquely neuropsychiatric in the term’s purest sense.
Continue to: Differential diagnosis and workup
Differential diagnosis and workup
The differential diagnosis for acute-onset memory loss in the absence of other neurologic or psychiatric features is broad. It includes:
- dissociative amnesia
- ischemic amnesia
- transient epileptic amnesia
- toxic and metabolic amnesia
- posttraumatic amnesia.
Dissociative amnesia (DA), otherwise known as psychogenic amnesia, is “an inability to recall important autobiographical information, usually of a traumatic or stressful nature, that is inconsistent with ordinary forgetting.”13 According to this definition, DA features only retrograde amnesia, as opposed to TGA, which features anterograde amnesia, with possible retrograde amnesia. A subtype of DA—specifically, “continuous amnesia” or “anterograde dissociative amnesia”— is in DSM-5.13 However, the diagnostic criteria are unclear, and no cases have been identified in the literature since 1903, before TGA became a diagnostic entity.5,14 Moreover, patients with DA cannot recall autobiographical information, which is not a feature of TGA. Within DSM-5, TGA is an exclusion criterion for DA.13 Thus, an episode of anterograde amnesia with acute onset best meets criteria for TGA, even if there are substantial psychiatric risk factors.
Ischemic amnesia—including stroke and transient ischemic attack (TIA)—is often the primary concern of patients with TGA and their families upon initial presentation, as was the case with Ms. A.6,15 TIA presenting with isolated, acute-onset amnesia would be highly unusual, because these attacks usually present with focal symptoms including motor deficits, sensory deficits, visual field deficits, and aphasia or dysarthria. A patient with amnesia experiencing a TIA would likely have symptoms lasting from seconds to minutes, which is much shorter than a typical TGA episode.16
Amnesia secondary to stroke may be transient or permanent.7 Amnesia is present in approximately 1% of all strokes and in approximately 19.3% of posterior cerebral artery strokes.7,17 Unlike TIA and TGA, ischemic amnesia would present with MRI findings detectable at symptom onset. TGA does reveal MRI findings, particularly punctate lesions in the CA1 area of the hippocampus; however, these lesions are typically much smaller than those found in stroke, and are not detectable until 12 to 48 hours after episode onset.1,17 MRI findings in ischemic amnesia are typically associated with extrahippocampal lesions.17 Finally, the presence of vascular risk factors such as hyperlipidemia, smoking, diabetes, and hypertension may also favor a diagnosis of stroke or TIA as opposed to TGA, which is not associated with these risk factors.18 Though ischemic amnesia and TGA usually can be differentiated based on history and presentation, MRI with fluid-attenuated inversion recovery and diffusion-weighted imaging may be performed to definitively distinguish stroke from TGA.7
Transient epileptic amnesia (TEA), a focal form of epilepsy within the temporal lobe, should also be considered in patients who present with acute-onset amnesia. Like TGA, TEA may present with simultaneous anterograde and retrograde amnesia accompanied by repetitive questioning.19 Amnesia can be the sole symptom of TEA in up to 24% of cases. However, several key features distinguish TEA from TGA. TEA most often presents with other clinical signs of seizures, such as oral automatisms and/or olfactory hallucinations.20 There is also a significant difference in episode length; TEA episodes last an average of 30 to 60 minutes and tend to occur upon wakening, whereas TGA episodes last an average of 4 to 6 hours and do not preferentially occur at any particular time.1,21 In the interictal period—between seizures—patients with TEA may also experience accelerated long-term forgetting, autobiographical amnesia, and topographical amnesia.19,20 Finally, a diagnosis of TEA also requires recurrent episodes. Recurrence can happen with TGA, but is less frequent.21 Generally, history and presentation can distinguish TEA from TGA. Though there is no formal protocol for TEA workup, Lanzone et al21 recommend 24-hour EEG or EEG sleep monitoring in patients who present with amnesia as well as other clinical manifestations of epileptic phenomenon.
Continue to: Toxic and metabolic
Toxic and metabolic etiologies of amnesia include opioid and cocaine use, general anesthetics,22 and hypoglycemia.7,23 Toxic and metabolic causes of amnesia may mirror TGA in their acute onset as well as anterograde nature. However, these patients will likely present with fluctuating consciousness and/or other neuropsychiatric features, such as pressured speech, delusions, and/or distractability.23 Obtaining a patient’s medical history, including substance use, medication use, and the presence of diabetes,24 is typically sufficient to rule out toxic and metabolic causes.7
Posttraumatic amnesia (PTA) describes transient memory loss that occurs after a traumatic brain injury. Anterograde amnesia is most common, though approximately 20% of patients may also experience retrograde amnesia pertaining to the events near the date of their injury. Unlike TGA, which typically resolves within 24 hours, the recovery time of amnestic symptoms in PTA ranges from minutes to years.7 A distinguishing feature of PTA is the presence of confusion, which often resembles a state of delirium.25 The presentation of PTA can vary immensely with regards to agitation, psychotic symptoms, and the time to resolution of the amnesia. Though TGA can be distinguished from PTA based on a lack of clouding of consciousness, a case of anterograde amnesia warrants inquiry into a potential history of head injuries to rule out a traumatic cause.26
Box 21,3,23,27-33 outlines current theories of the etiology and pathogenesis of TGA.
Box 2
The etiology and pathogenesis of transient global amnesia (TGA) are poorly understood, and TGA remains one of the most enigmatic syndromes in clinical neurology.27 Theories regarding the pathogenesis of TGA are diverse and include vascular, epileptic, migraine, and stress-related etiologies.1,23
Early theories suggested arterial ischemia28 and epileptic phenomena29 as etiologies of TGA. The venous theory posits that TGA stems from jugular venous incompetency, causing venous flow and subsequent venous congestion in the medial temporal lobe, wherein lies the hippocampus. This theory is supported by several studies showing venous valve insufficiency as detected by ultrasonographic evaluation during the Valsalva maneuver in patients with TGA.30 This pathophysiologic mechanism may explain the occurrence of TGA in a specific cluster of cases, including men whose TGA episodes are precipitated by physical stress or the Valsalva maneuver.3 The migraine theory and stress theory share a similar proposed neurophysiologic mechanism.
The migraine theory stems from migraines being a known risk factor for TGA, particularly in middle-aged women.31 The stress theory is based on the known emotional precipitants and psychiatric comorbidities associated with TGA. Notably, both the migraine theory and stress theory implicate the role of excessive glutamate release as well as CNS depression.31,32 Glutamate targets the CA1 region of the hippocampus, which is involved in TGA and is known to have the highest density of N-methyl-D-aspartate receptors among hippocampal regions.33
Given the heterogeneity of the demographics and stressors associated with TGA, multiple mechanisms for the disease process may coexist, leading to a similar clinical picture. In 2006, Quinette et al3 performed a multivariate analysis of variables associated with TGA, including age, sex, medical history, and presentation. They demonstrated 3 “clusters” of TGA pictures: women with anxiety or a personality disorder; men with physical precipitating events; and younger patients (age <56) with a history of migraine. These findings suggest TGA may have unique precipitants corresponding to multiple neurophysiologic mechanisms.
Transient global ischemia: Psychiatric features
Several studies have demonstrated psychiatric precipitants, features, and comorbidities associated with TGA. Of the TGA cases associated with precipitating events, 29% to 50% are associated with an emotional stressor.3,4 Examples of emotional stressors include a quarrel,4 the announcement of a birth or suicide, and a nightmare.15 For Ms. A, learning her daughter worked in the sex industry was an emotional stressor.2
During its acute phase, TGA has been shown to present with mood and anxiety symptoms.34 Moreover, during episodes, patients often demonstrate features of panic attacks, such as dizziness, fainting, choking, palpitations, and paresthesia.3,35
Continue to: Finally, patients with TGA...
Finally, patients with TGA are more likely to have psychiatric comorbidities than those without the condition. In a study of 25 patients who experienced TGA triggered by a precipitant, Inzitari et al4 found a strong association of TGA with phobic personality traits, including agoraphobia and simple phobic attitudes (ie, fear of traveling far from home or the sight of blood). Pantoni et al35 replicated these results in 2005 and found that in comparison to patients with TIA, patients with TGA are more likely to have personal and family histories of psychiatric disease. A 2014 study by Dohring et al36 found that compared to healthy controls, patients with TGA are more likely to have maladaptive coping strategies and stress responses. Patients with TGA tended to exhibit increased feelings of guilt, take more medication, and exhibit more anxiety compared to healthy controls.36
Treatment: Benzodiazepines
There are no published treatment guidelines for TGA. However, in case reports, benzodiazepines (specifically lorazepam37) have been shown to have utility in diagnosing and treating DA. The success of benzodiazepines is attributed to its gamma-aminobutyric acid mechanism, which involves inhibiting activity of the N-methyl-
However, the benzodiazepine midazolam has been identified as a precipitant of TGA. In a case report, Rinehart et al22 identified flumazenil—a benzodiazepine antagonist used primarily to treat retrograde postoperative amnesia—as an antidote. The potentially paradoxical role of benzodiazepines in both the precipitation and treatment of TGA may relate back to the heterogeneity of the etiologies of TGA. Further research comparing the treatment of TGA in patients with stress-induced TGA vs postoperative TGA is needed to better understand the neurochemical basis of TGA and work toward establishing optimal treatment options for different patient demographics.
A generally favorable prognosis
TGA carries a low risk of recurrence. In studies with 3- to 7-year follow-up periods, the recurrence rates ranged from 1.4% to 23.8%.23,35,38
Memory impairments may be present for 5 to 6 months following a TGA episode. The severity of these impairments may range from clinically unnoticeable to the patient meeting the criteria for mild cognitive impairment.23,39 The risk is higher in patients who have had recurrent TGA compared to those patients who have experienced only a single episode.23
Continue to: TGA does not increase...
TGA does not increase the risk of cerebrovascular events. There is controversy regarding a potentially increased risk for dementia as well as epilepsy, though there is insufficient evidence to support these findings.23,40
CASE CONTINUED
Five hours after the onset of Ms. A’s symptoms, the treatment team initiates oral lorazepam 1 mg. One hour after taking lorazepam, Ms. A’s anterograde and retrograde amnesia improve. She cannot recall why she was brought to the hospital but does remember the date and location, which she was not able to do on initial presentation. She feels safe, states a clear plan for self-care, and is discharged in the care of her partner. Though Ms. A’s memory improved soon after she received lorazepam, this improvement also could be attributed to the natural course of time, as TGA tends to resolve on its own within 24 hours.
Bottom Line
Transient global amnesia (TGA) is an episode of anterograde, and possibly retrograde, amnesia that lasts up to 24 hours. It represents an interesting diagnosis at the intersection of psychiatry and neurology. TGA has many established psychiatric risk factors and features—some of which may resemble conversion disorder—but these may only apply to a particular subset of patients, which reflects the heterogeneity of the condition.
Related Resources
- Sparaco M, Pascarella R, Muccio CF, et al. Forgetting the unforgettable: transient global amnesia part I: pathophysiology and etiology. J Clin Med. 2022;11(12): 3373. doi:10.3390/jcm1112337
- Sparaco M, Pascarella R, Muccio CF, et al. Forgetting the unforgettable: transient global amnesia part II: a clinical road map. J Clin Med. 2022;11(14):3940. doi:10.3390/ jcm11143940
Drug Brand Names
Flumazenil • Romazicon
Lorazepam • Ativan
Midazolam • Versed
1. Miller TD, Butler CR. Acute-onset amnesia: transient global amnesia and other causes. Pract Neurol. 2022;22(3):201-208. doi:10.1136/practneurol-2020-002826
2. Hodges JR, Warlow CP. Syndromes of transient amnesia: towards a classification. A study of 153 cases. J Neurol Neurosurg Psychiatry. 1990;53(10):834-843. doi:10.1136/jnnp.53.10.834
3. Quinette P, Guillery-Girard B, Dayan J, et al. What does transient global amnesia really mean? Review of the literature and thorough study of 142 cases. Brain. 2006;129(Pt 7):1640-1658. doi:10.1093/brain/awl105
4. Inzitari D, Pantoni L, Lamassa M, et al. Emotional arousal and phobia in transient global amnesia. Arch Neurol. 1997;54(7):866-873. doi:10.1001/archneur.1997.00550190056015
5. Staniloiu A, Markowitsch HJ. Dissociative amnesia. Lancet Psychiatry. 2014;1(3):226-241. doi:10.1016/S2215-0366(14)70279-2
6. Espiridion ED, Gupta J, Bshara A, et al. Transient global amnesia in a 60-year-old female with post-traumatic stress disorder. Cureus. 2019;11(9):e5792. doi:10.7759/cureus.5792
7. Alessandro L, Ricciardi M, Chaves H, et al. Acute amnestic syndromes. J Neurol Sci. 2020;413:116781. doi:10.1016/j.jns.2020.116781
8. Bender M. Syndrome of isolated episode of confusion with amnesia. J Hillside Hosp. 1956;5:212-215.
9. Courjon J, Guyotat J. Les ictus amnéstiques [Amnesic strokes]. J Med Lyon. 1956;37(882):697-701.
10. Noel A, Quinette P, Hainselin M, et al. The still enigmatic syndrome of transient global amnesia: interactions between neurological and psychopathological factors. Neuropsychol Rev. 2015;25(2):125-133. doi:10.1007/s11065-015-9284-y
11. Merriam AE, Wyszynski B, Betzler T. Emotional arousal-induced transient global amnesia. A clue to the neural transcription of emotion? Psychosomatics. 1992;33(1):109-113. doi:10.1016/S0033-3182(92)72029-5
12. Hallett M, Aybek S, Dworetzky BA, et al. Functional neurological disorder: new subtypes and shared mechanisms. Lancet Neurol. 2022;21(6):537-550. doi:10.1016/S1474-4422(21)00422-1
13. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
14. Bourdon B, Dide M. A case of continuous amnesia with tactile asymbolia, complicated by other troubles. Ann Psychol. 1903;10:84-115.
15. Marinella MA. Transient global amnesia and a father’s worst nightmare. N Engl J Med. 2004;350(8):843-844. doi:10.1056/NEJM200402193500821
16. Amarenco P. Transient ischemic attack. N Engl J Med. 2020;382(20):1933-1941. doi:10.1056/NEJMcp1908837
17. Szabo K, Forster A, Jager T, et al. Hippocampal lesion patterns in acute posterior cerebral artery stroke: clinical and MRI findings. Stroke. 2009;40(6):2042-2045. doi:10.1161/STROKEAHA.108.536144
18. Liampas I, Raptopoulou M, Siokas V, et al. Conventional cardiovascular risk factors in transient global amnesia: systematic review and proposition of a novel hypothesis. Front Neuroendocrinol. 2021;61:100909. doi:10.1016/j.yfrne.2021.100909
19. Zeman A, Butler C. Transient epileptic amnesia. Curr Opin Neurol. 2010;23(6):610-616. doi:10.1097/WCO.0b013e32834027db
20. Baker J, Savage S, Milton F, et al. The syndrome of transient epileptic amnesia: a combined series of 115 cases and literature review. Brain Commun. 2021;3(2):fcab038. doi:10.1093/braincomms/fcab038
21. Lanzone J, Ricci L, Assenza G, et al Transient epileptic and global amnesia: real-life differential diagnosis. Epilepsy Behav. 2018;88:205-211. doi:10.1016/j.yebeh.2018.07.015
22. Rinehart JB, Baker B, Raphael D. Postoperative global amnesia reversed with flumazenil. Neurologist. 2012;18(4):216-218. doi:10.1097/NRL.0b013e31825bbef4
23. Arena JE, Rabinstein AA. Transient global amnesia. Mayo Clin Proc. 2015;90(2):264-272. doi:10.1016/j.mayocp.2014.12.001
24. Holemans X, Dupuis M, Misson N, et al. Reversible amnesia in a type 1 diabetic patient and bilateral hippocampal lesions on magnetic resonance imaging (MRI). Diabet Med. 2001;18(9):761-763. doi:10.1046/j.1464-5491.2001.00481.x
25. Marshman LAG, Jakabek D, Hennessy M, et al. Post-traumatic amnesia. J Clin Neurosci. 2013;20(11):1475-1481. doi:10.1016/j.jocn.2012.11.022
26. Parker TD, Rees R, Rajagopal S, et al. Post-traumatic amnesia. Pract Neurol. 2022;22(2):129-137. doi:10.1136/practneurol-2021-003056
27. You SH, Kim B, Kim BK. Transient global amnesia: signal alteration in 2D/3D T2-FLAIR sequences. Clin Imaging. 2021;78:154-159. doi:10.1016/j.clinimag.2021.03.029
28. Mathew NT, Meyer JS. Pathogenesis and natural history of transient global amnesia. Stroke. 1974;5(3):303-311. doi:10.1161/01.str.5.3.303
29. Fisher CM, Adams RD. Transient global amnesia. Acta Neurol Scand Suppl. 1964;40(SUPPL 9):1-83.
30. Cejas C, Cisneros LF, Lagos R, et al. Internal jugular vein valve incompetence is highly prevalent in transient global amnesia. Stroke. 2010;41(1):67-71. doi:10.1161/STROKEAHA.109.566315
31. Liampas I, Siouras AS, Siokas V, et al. Migraine in transient global amnesia: a meta-analysis of observational studies. J Neurol. 2022;269(1):184-196. doi:10.1007/s00415-020-10363-y
32. Ding X, Peng D. Transient global amnesia: an electrophysiological disorder based on cortical spreading depression-transient global amnesia model. Front Hum Neurosci. 2020;14:602496. doi:10.3389/fnhum.2020.602496
33. Bartsch T, Dohring J, Reuter S, et al. Selective neuronal vulnerability of human hippocampal CA1 neurons: lesion evolution, temporal course, and pattern of hippocampal damage in diffusion-weighted MR imaging. J Cereb Blood Flow Metab. 2015;35(11):1836-1845. doi:10.1038/jcbfm.2015.137
34. Noel A, Quinette P, Guillery-Girard B, et al. Psychopathological factors, memory disorders and transient global amnesia. Br J Psychiatry. 2008;193(2):145-151. doi:10.1192/bjp.bp.107.045716
35. Pantoni L, Bertini E, Lamassa M, et al. Clinical features, risk factors, and prognosis in transient global amnesia: a follow-up study. Eur J Neurol. 2005;12(5):350-356. doi:10.1111/j.1468-1331.2004.00982.x
36. Dohring J, Schmuck A, Bartsch T. Stress-related factors in the emergence of transient global amnesia with hippocampal lesions. Front Behav Neurosci. 2014;8:287. doi:10.3389/fnbeh.2014.00287
37. Jiang S, Gunther S, Hartney K, et al. An intravenous lorazepam infusion for dissociative amnesia: a case report. Psychosomatics. 2020;61(6):814-818. doi:10.1016/j.psym.2020.01.009
38. He S, Ye Z, Yang Q, et al. Transient global amnesia: risk factors, imaging features, and prognosis. Neuropsychiatr Dis Treat. 2021;17:1611-1619. doi:10.2147/NDT.S299168
39. Borroni B, Agosti C, Brambilla C, et al. Is transient global amnesia a risk factor for amnestic mild cognitive impairment? J Neurol. 2004;251(9):1125-1127. doi:10.1007/s00415-004-0497-x
40. Liampas I, Raptopoulou M, Siokas V, et al. The long-term prognosis of transient global amnesia: a systematic review. Rev Neurosci. 2021;32(5):531-543. doi:10.1515/revneuro-2020-0110
1. Miller TD, Butler CR. Acute-onset amnesia: transient global amnesia and other causes. Pract Neurol. 2022;22(3):201-208. doi:10.1136/practneurol-2020-002826
2. Hodges JR, Warlow CP. Syndromes of transient amnesia: towards a classification. A study of 153 cases. J Neurol Neurosurg Psychiatry. 1990;53(10):834-843. doi:10.1136/jnnp.53.10.834
3. Quinette P, Guillery-Girard B, Dayan J, et al. What does transient global amnesia really mean? Review of the literature and thorough study of 142 cases. Brain. 2006;129(Pt 7):1640-1658. doi:10.1093/brain/awl105
4. Inzitari D, Pantoni L, Lamassa M, et al. Emotional arousal and phobia in transient global amnesia. Arch Neurol. 1997;54(7):866-873. doi:10.1001/archneur.1997.00550190056015
5. Staniloiu A, Markowitsch HJ. Dissociative amnesia. Lancet Psychiatry. 2014;1(3):226-241. doi:10.1016/S2215-0366(14)70279-2
6. Espiridion ED, Gupta J, Bshara A, et al. Transient global amnesia in a 60-year-old female with post-traumatic stress disorder. Cureus. 2019;11(9):e5792. doi:10.7759/cureus.5792
7. Alessandro L, Ricciardi M, Chaves H, et al. Acute amnestic syndromes. J Neurol Sci. 2020;413:116781. doi:10.1016/j.jns.2020.116781
8. Bender M. Syndrome of isolated episode of confusion with amnesia. J Hillside Hosp. 1956;5:212-215.
9. Courjon J, Guyotat J. Les ictus amnéstiques [Amnesic strokes]. J Med Lyon. 1956;37(882):697-701.
10. Noel A, Quinette P, Hainselin M, et al. The still enigmatic syndrome of transient global amnesia: interactions between neurological and psychopathological factors. Neuropsychol Rev. 2015;25(2):125-133. doi:10.1007/s11065-015-9284-y
11. Merriam AE, Wyszynski B, Betzler T. Emotional arousal-induced transient global amnesia. A clue to the neural transcription of emotion? Psychosomatics. 1992;33(1):109-113. doi:10.1016/S0033-3182(92)72029-5
12. Hallett M, Aybek S, Dworetzky BA, et al. Functional neurological disorder: new subtypes and shared mechanisms. Lancet Neurol. 2022;21(6):537-550. doi:10.1016/S1474-4422(21)00422-1
13. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
14. Bourdon B, Dide M. A case of continuous amnesia with tactile asymbolia, complicated by other troubles. Ann Psychol. 1903;10:84-115.
15. Marinella MA. Transient global amnesia and a father’s worst nightmare. N Engl J Med. 2004;350(8):843-844. doi:10.1056/NEJM200402193500821
16. Amarenco P. Transient ischemic attack. N Engl J Med. 2020;382(20):1933-1941. doi:10.1056/NEJMcp1908837
17. Szabo K, Forster A, Jager T, et al. Hippocampal lesion patterns in acute posterior cerebral artery stroke: clinical and MRI findings. Stroke. 2009;40(6):2042-2045. doi:10.1161/STROKEAHA.108.536144
18. Liampas I, Raptopoulou M, Siokas V, et al. Conventional cardiovascular risk factors in transient global amnesia: systematic review and proposition of a novel hypothesis. Front Neuroendocrinol. 2021;61:100909. doi:10.1016/j.yfrne.2021.100909
19. Zeman A, Butler C. Transient epileptic amnesia. Curr Opin Neurol. 2010;23(6):610-616. doi:10.1097/WCO.0b013e32834027db
20. Baker J, Savage S, Milton F, et al. The syndrome of transient epileptic amnesia: a combined series of 115 cases and literature review. Brain Commun. 2021;3(2):fcab038. doi:10.1093/braincomms/fcab038
21. Lanzone J, Ricci L, Assenza G, et al Transient epileptic and global amnesia: real-life differential diagnosis. Epilepsy Behav. 2018;88:205-211. doi:10.1016/j.yebeh.2018.07.015
22. Rinehart JB, Baker B, Raphael D. Postoperative global amnesia reversed with flumazenil. Neurologist. 2012;18(4):216-218. doi:10.1097/NRL.0b013e31825bbef4
23. Arena JE, Rabinstein AA. Transient global amnesia. Mayo Clin Proc. 2015;90(2):264-272. doi:10.1016/j.mayocp.2014.12.001
24. Holemans X, Dupuis M, Misson N, et al. Reversible amnesia in a type 1 diabetic patient and bilateral hippocampal lesions on magnetic resonance imaging (MRI). Diabet Med. 2001;18(9):761-763. doi:10.1046/j.1464-5491.2001.00481.x
25. Marshman LAG, Jakabek D, Hennessy M, et al. Post-traumatic amnesia. J Clin Neurosci. 2013;20(11):1475-1481. doi:10.1016/j.jocn.2012.11.022
26. Parker TD, Rees R, Rajagopal S, et al. Post-traumatic amnesia. Pract Neurol. 2022;22(2):129-137. doi:10.1136/practneurol-2021-003056
27. You SH, Kim B, Kim BK. Transient global amnesia: signal alteration in 2D/3D T2-FLAIR sequences. Clin Imaging. 2021;78:154-159. doi:10.1016/j.clinimag.2021.03.029
28. Mathew NT, Meyer JS. Pathogenesis and natural history of transient global amnesia. Stroke. 1974;5(3):303-311. doi:10.1161/01.str.5.3.303
29. Fisher CM, Adams RD. Transient global amnesia. Acta Neurol Scand Suppl. 1964;40(SUPPL 9):1-83.
30. Cejas C, Cisneros LF, Lagos R, et al. Internal jugular vein valve incompetence is highly prevalent in transient global amnesia. Stroke. 2010;41(1):67-71. doi:10.1161/STROKEAHA.109.566315
31. Liampas I, Siouras AS, Siokas V, et al. Migraine in transient global amnesia: a meta-analysis of observational studies. J Neurol. 2022;269(1):184-196. doi:10.1007/s00415-020-10363-y
32. Ding X, Peng D. Transient global amnesia: an electrophysiological disorder based on cortical spreading depression-transient global amnesia model. Front Hum Neurosci. 2020;14:602496. doi:10.3389/fnhum.2020.602496
33. Bartsch T, Dohring J, Reuter S, et al. Selective neuronal vulnerability of human hippocampal CA1 neurons: lesion evolution, temporal course, and pattern of hippocampal damage in diffusion-weighted MR imaging. J Cereb Blood Flow Metab. 2015;35(11):1836-1845. doi:10.1038/jcbfm.2015.137
34. Noel A, Quinette P, Guillery-Girard B, et al. Psychopathological factors, memory disorders and transient global amnesia. Br J Psychiatry. 2008;193(2):145-151. doi:10.1192/bjp.bp.107.045716
35. Pantoni L, Bertini E, Lamassa M, et al. Clinical features, risk factors, and prognosis in transient global amnesia: a follow-up study. Eur J Neurol. 2005;12(5):350-356. doi:10.1111/j.1468-1331.2004.00982.x
36. Dohring J, Schmuck A, Bartsch T. Stress-related factors in the emergence of transient global amnesia with hippocampal lesions. Front Behav Neurosci. 2014;8:287. doi:10.3389/fnbeh.2014.00287
37. Jiang S, Gunther S, Hartney K, et al. An intravenous lorazepam infusion for dissociative amnesia: a case report. Psychosomatics. 2020;61(6):814-818. doi:10.1016/j.psym.2020.01.009
38. He S, Ye Z, Yang Q, et al. Transient global amnesia: risk factors, imaging features, and prognosis. Neuropsychiatr Dis Treat. 2021;17:1611-1619. doi:10.2147/NDT.S299168
39. Borroni B, Agosti C, Brambilla C, et al. Is transient global amnesia a risk factor for amnestic mild cognitive impairment? J Neurol. 2004;251(9):1125-1127. doi:10.1007/s00415-004-0497-x
40. Liampas I, Raptopoulou M, Siokas V, et al. The long-term prognosis of transient global amnesia: a systematic review. Rev Neurosci. 2021;32(5):531-543. doi:10.1515/revneuro-2020-0110