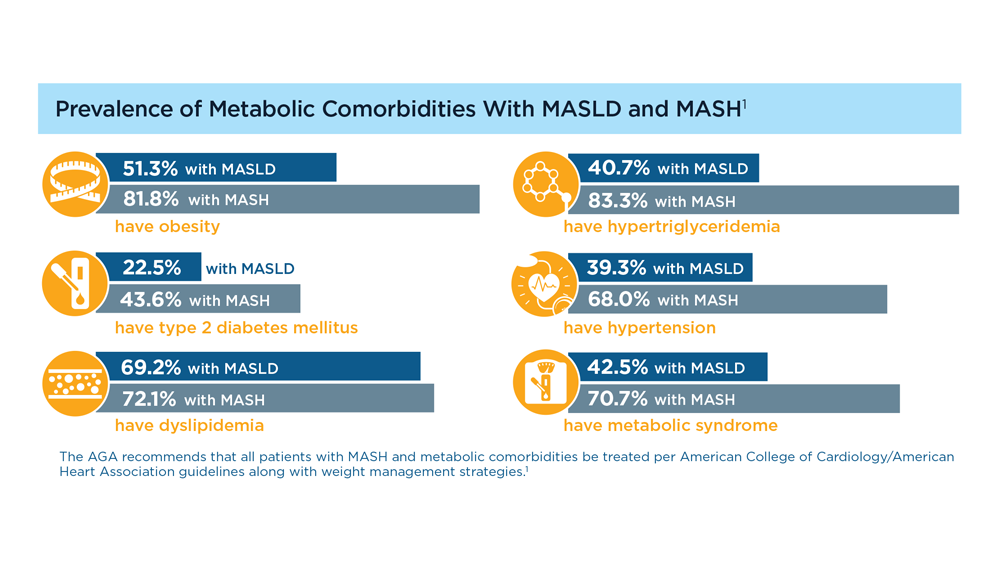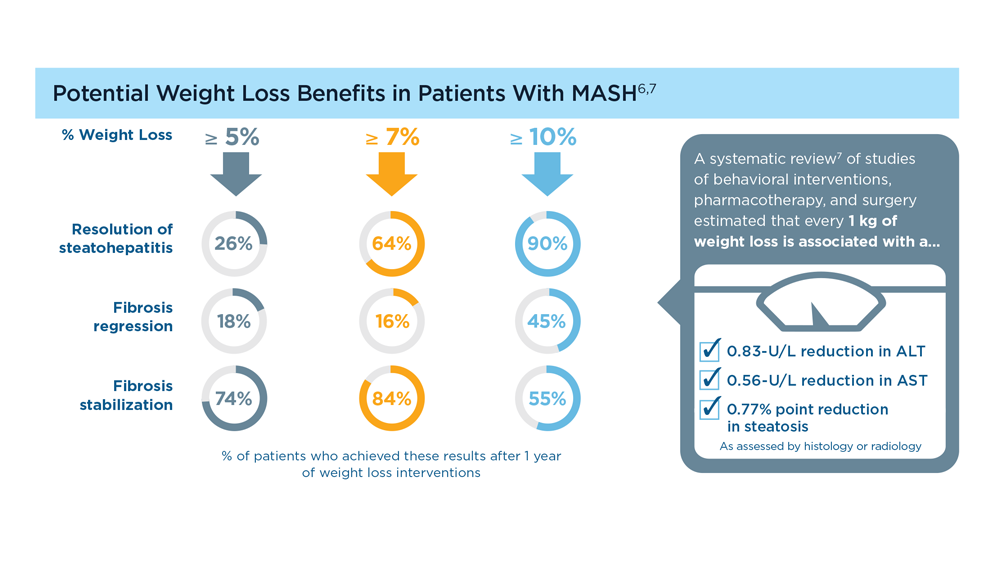User login
Commentary: DMARD types, guselkumab, and interleukin inhibitors in PsA, October 2023
To address this gap in knowledge, Möller and colleagues compared the effectiveness of the first bDMARD in patients with PsA with low vs high joint counts (LJC and HJC, respectively). Using the Swiss Clinical Quality Management registry for rheumatic diseases, they obtained data on 387 patients with PsA who had either LJC (n = 197) or HJC (n = 190) and received bDMARD. As expected, patients with HJC had a higher burden of disease. Despite the higher burden, patients in both groups showed similar treatment efficacy in terms of drug retention. Consistent with previous reports, female sex was associated with lower treatment persistence, whereas concomitant treatment with conventional synthetic DMARD (csDMARD) was associated with longer bDMARD persistence. Thus, baseline joint counts may not be a good criterion for choosing who should be treated with bDMARD. The presence of active disease and lack of response to prior csDMARD is sufficient.
Persistence with therapy is an important indicator of drug effectiveness in the real world. A recent report from the CorEvitas registry by Mease and colleagues demonstrated that nearly 80% of patients with PsA persisted with guselkumab (an interleukin [IL]–23 inhibitor) treatment for 6 months and showed improvements in peripheral joint and skin symptoms. This study evaluated 114 patients with active PsA, > 90% of whom were previously treated with b- and tsDMARD. The mean scores for clinical Disease Activity Index in PsA, overall joint and skin activity, patient-reported pain, and body surface area with psoriasis improved significantly.
Choosing the next therapy after lack of success with treatment with a tumour necrosis factor (TNF) inhibitor and an IL-17A inhibitor is difficult. One question is whether one should try another IL-17A inhibitor or move to another class of therapy. Hansen and colleagues tried to address this question by analyses of data from the Danish Rheumatology Registry. Patients with PsA who underwent prior treatment with one or more TNF inhibitor and switched to either first-line (n = 534) or second-line (n = 102) IL-17A inhibitors (ixekizumab or secukinumab) were included. Similar persistence with therapy was observed between first-line and second-line IL-17A inhibitor switchers and between second-line secukinumab and second-line ixekizumab switchers. Withdrawal reasons were similar for both first-line and second-line switchers when considering adverse events; however, withdrawal due to lack of successful therapy was higher for the first-line vs second-line switchers (34% vs 18%). An important piece of information missing in the report was whether the lack of successful treatment with first-line therapy with an IL-17A inhibitor was primary (no response at all) or secondary (initial response and later failure). One presumes that patients with primary failures are less likely to respond to another IL-17A inhibitor compared with patients with secondary failures. Nevertheless, this large population-based study suggests that the failure of first-line IL-17A inhibitor therapy should not deter treatment with second-line IL-17A inhibitors.
Finally, Schett and colleagues looked at serum cytokine changes after treatment with guselkumab in patients with PsA with inadequate response to TNF inhibitor (TNFI-IR). Using clinical data and biosamples from patients enrolled in the COSMOS study, which included patients with active PsA and TNFI-IR who were randomly assigned to receive either guselkumab (n = 189) or placebo (n = 96), they showed that the serum levels of IL-17A, IL-17F, IL-22, and serum amyloid A were reduced significantly by week 4 and were sustained through week 48 in the guselkumab group vs the placebo group. Patients who achieved a clinical response to guselkumab at week 24 showed higher baseline IL-22 and interferon-γ levels as well as a significant reduction in IL-6 levels at week 4 compared with nonresponders. These markers are candidates for predictors for response to guselkumab in this population.
To address this gap in knowledge, Möller and colleagues compared the effectiveness of the first bDMARD in patients with PsA with low vs high joint counts (LJC and HJC, respectively). Using the Swiss Clinical Quality Management registry for rheumatic diseases, they obtained data on 387 patients with PsA who had either LJC (n = 197) or HJC (n = 190) and received bDMARD. As expected, patients with HJC had a higher burden of disease. Despite the higher burden, patients in both groups showed similar treatment efficacy in terms of drug retention. Consistent with previous reports, female sex was associated with lower treatment persistence, whereas concomitant treatment with conventional synthetic DMARD (csDMARD) was associated with longer bDMARD persistence. Thus, baseline joint counts may not be a good criterion for choosing who should be treated with bDMARD. The presence of active disease and lack of response to prior csDMARD is sufficient.
Persistence with therapy is an important indicator of drug effectiveness in the real world. A recent report from the CorEvitas registry by Mease and colleagues demonstrated that nearly 80% of patients with PsA persisted with guselkumab (an interleukin [IL]–23 inhibitor) treatment for 6 months and showed improvements in peripheral joint and skin symptoms. This study evaluated 114 patients with active PsA, > 90% of whom were previously treated with b- and tsDMARD. The mean scores for clinical Disease Activity Index in PsA, overall joint and skin activity, patient-reported pain, and body surface area with psoriasis improved significantly.
Choosing the next therapy after lack of success with treatment with a tumour necrosis factor (TNF) inhibitor and an IL-17A inhibitor is difficult. One question is whether one should try another IL-17A inhibitor or move to another class of therapy. Hansen and colleagues tried to address this question by analyses of data from the Danish Rheumatology Registry. Patients with PsA who underwent prior treatment with one or more TNF inhibitor and switched to either first-line (n = 534) or second-line (n = 102) IL-17A inhibitors (ixekizumab or secukinumab) were included. Similar persistence with therapy was observed between first-line and second-line IL-17A inhibitor switchers and between second-line secukinumab and second-line ixekizumab switchers. Withdrawal reasons were similar for both first-line and second-line switchers when considering adverse events; however, withdrawal due to lack of successful therapy was higher for the first-line vs second-line switchers (34% vs 18%). An important piece of information missing in the report was whether the lack of successful treatment with first-line therapy with an IL-17A inhibitor was primary (no response at all) or secondary (initial response and later failure). One presumes that patients with primary failures are less likely to respond to another IL-17A inhibitor compared with patients with secondary failures. Nevertheless, this large population-based study suggests that the failure of first-line IL-17A inhibitor therapy should not deter treatment with second-line IL-17A inhibitors.
Finally, Schett and colleagues looked at serum cytokine changes after treatment with guselkumab in patients with PsA with inadequate response to TNF inhibitor (TNFI-IR). Using clinical data and biosamples from patients enrolled in the COSMOS study, which included patients with active PsA and TNFI-IR who were randomly assigned to receive either guselkumab (n = 189) or placebo (n = 96), they showed that the serum levels of IL-17A, IL-17F, IL-22, and serum amyloid A were reduced significantly by week 4 and were sustained through week 48 in the guselkumab group vs the placebo group. Patients who achieved a clinical response to guselkumab at week 24 showed higher baseline IL-22 and interferon-γ levels as well as a significant reduction in IL-6 levels at week 4 compared with nonresponders. These markers are candidates for predictors for response to guselkumab in this population.
To address this gap in knowledge, Möller and colleagues compared the effectiveness of the first bDMARD in patients with PsA with low vs high joint counts (LJC and HJC, respectively). Using the Swiss Clinical Quality Management registry for rheumatic diseases, they obtained data on 387 patients with PsA who had either LJC (n = 197) or HJC (n = 190) and received bDMARD. As expected, patients with HJC had a higher burden of disease. Despite the higher burden, patients in both groups showed similar treatment efficacy in terms of drug retention. Consistent with previous reports, female sex was associated with lower treatment persistence, whereas concomitant treatment with conventional synthetic DMARD (csDMARD) was associated with longer bDMARD persistence. Thus, baseline joint counts may not be a good criterion for choosing who should be treated with bDMARD. The presence of active disease and lack of response to prior csDMARD is sufficient.
Persistence with therapy is an important indicator of drug effectiveness in the real world. A recent report from the CorEvitas registry by Mease and colleagues demonstrated that nearly 80% of patients with PsA persisted with guselkumab (an interleukin [IL]–23 inhibitor) treatment for 6 months and showed improvements in peripheral joint and skin symptoms. This study evaluated 114 patients with active PsA, > 90% of whom were previously treated with b- and tsDMARD. The mean scores for clinical Disease Activity Index in PsA, overall joint and skin activity, patient-reported pain, and body surface area with psoriasis improved significantly.
Choosing the next therapy after lack of success with treatment with a tumour necrosis factor (TNF) inhibitor and an IL-17A inhibitor is difficult. One question is whether one should try another IL-17A inhibitor or move to another class of therapy. Hansen and colleagues tried to address this question by analyses of data from the Danish Rheumatology Registry. Patients with PsA who underwent prior treatment with one or more TNF inhibitor and switched to either first-line (n = 534) or second-line (n = 102) IL-17A inhibitors (ixekizumab or secukinumab) were included. Similar persistence with therapy was observed between first-line and second-line IL-17A inhibitor switchers and between second-line secukinumab and second-line ixekizumab switchers. Withdrawal reasons were similar for both first-line and second-line switchers when considering adverse events; however, withdrawal due to lack of successful therapy was higher for the first-line vs second-line switchers (34% vs 18%). An important piece of information missing in the report was whether the lack of successful treatment with first-line therapy with an IL-17A inhibitor was primary (no response at all) or secondary (initial response and later failure). One presumes that patients with primary failures are less likely to respond to another IL-17A inhibitor compared with patients with secondary failures. Nevertheless, this large population-based study suggests that the failure of first-line IL-17A inhibitor therapy should not deter treatment with second-line IL-17A inhibitors.
Finally, Schett and colleagues looked at serum cytokine changes after treatment with guselkumab in patients with PsA with inadequate response to TNF inhibitor (TNFI-IR). Using clinical data and biosamples from patients enrolled in the COSMOS study, which included patients with active PsA and TNFI-IR who were randomly assigned to receive either guselkumab (n = 189) or placebo (n = 96), they showed that the serum levels of IL-17A, IL-17F, IL-22, and serum amyloid A were reduced significantly by week 4 and were sustained through week 48 in the guselkumab group vs the placebo group. Patients who achieved a clinical response to guselkumab at week 24 showed higher baseline IL-22 and interferon-γ levels as well as a significant reduction in IL-6 levels at week 4 compared with nonresponders. These markers are candidates for predictors for response to guselkumab in this population.
Hypotrichosis and Hair Loss on the Occipital Scalp
The Diagnosis: Monilethrix
A diagnosis of monilethrix was rendered based on the clinical and trichoscopic findings. Simple surveillance of the patient’s condition and prevention of further hair trauma were proposed as management options.
Monilethrix is a hair shaft disorder that is inherited in a predominantly autosomal-dominant pattern with variable expressiveness and penetrance resulting from heterozygous mutations in hair keratin genes KRT81, KRT83, and KRT86 in a region of chromosome 12q13.13.1,2 An autosomalrecessive form has been described with mutation in desmoglein 4, but it differs from the classical form by the variable periodicity of the region between the nodules.3,4
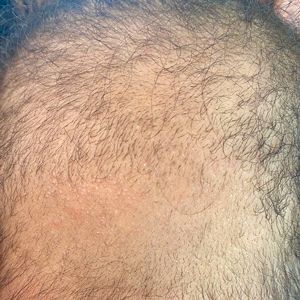
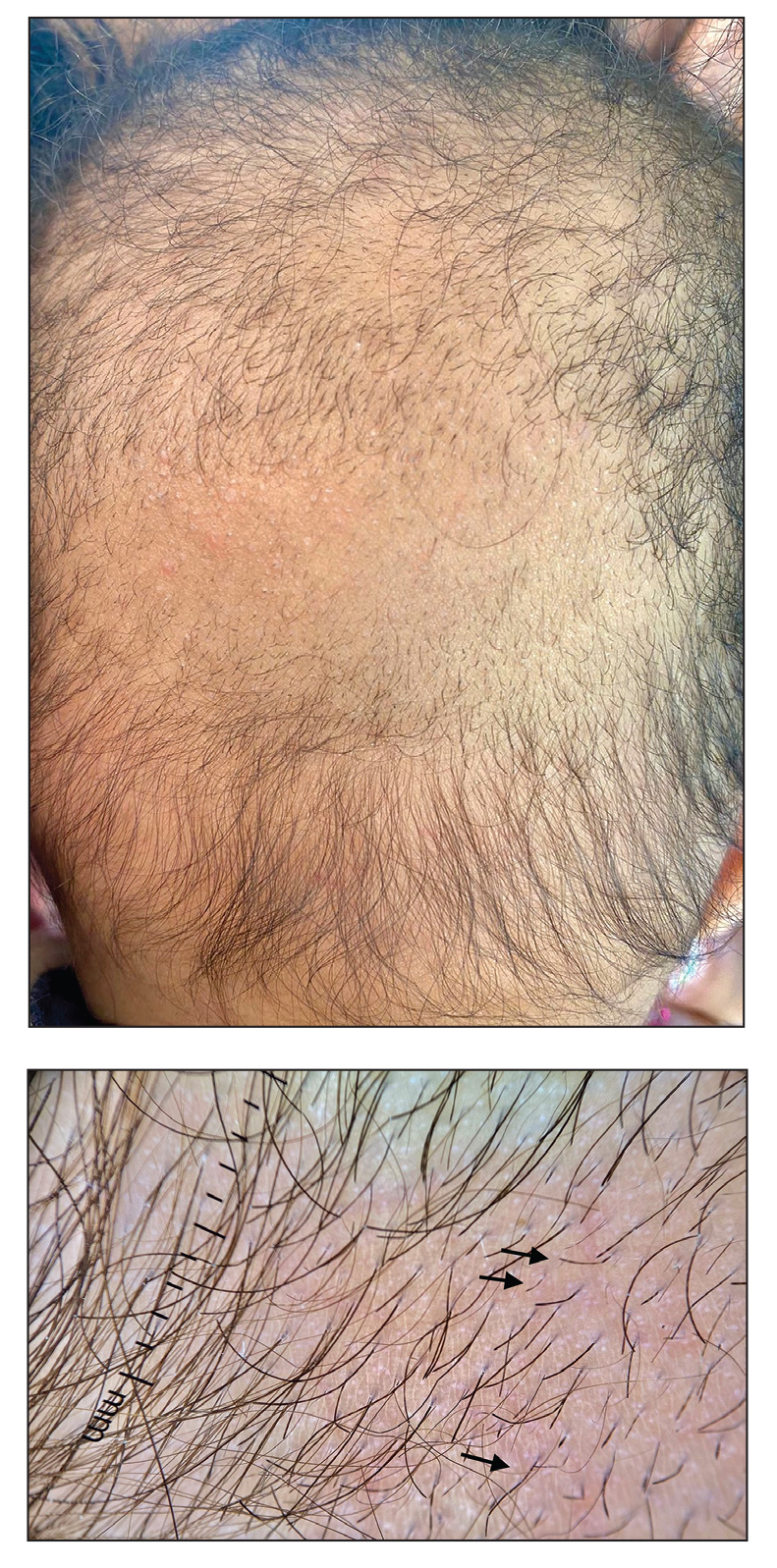
The morphologic alteration consists of the formation of fusiform nodules of normal structure alternated with narrow and dystrophic constrictions (Figure). These internodes are fragile areas that cause breakage at constricted points.5 Clinically, monilethrix presents as areas of focal or diffuse alopecia with frequent involvement of the terminal follicles, mainly in areas of friction. The hair is normal at birth due to the predominance of lanugo in the neonatal period, but it subsequently is replaced by abnormal hairs in the first months of life.6 Initial clinical signs begin to appear when the terminal hairs begin to form.7 Although rarer, the eyebrows and eyelashes, as well as the axillary, pubic, and body hair, may be involved.5
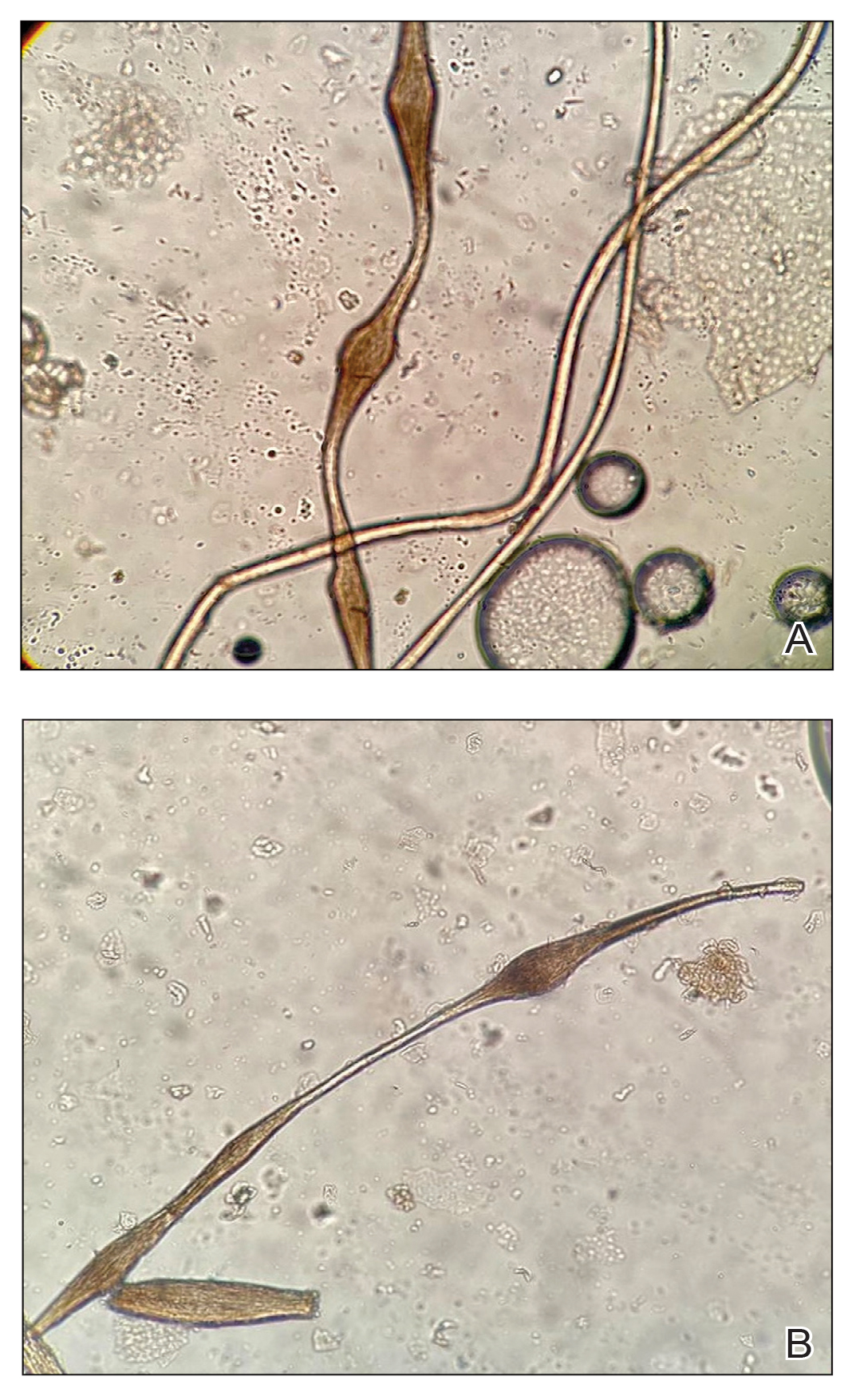
Other hair shaft anomalies merit consideration in the differential diagnosis of monilethrix, including pseudomonilethrix, pressure alopecia, trichorrhexis invaginata, ectodermal dysplasia, tinea capitis, and trichothiodystrophy.6 The diagnosis is reached by clinical history and physical examination. Trichoscopy and light microscopy are used to confirm the diagnosis. Trichoscopic examination shows markedly higher rates of anagen hair. The shafts examined in our patient revealed 0.7- to 1-mm intervals between nodes. Hair can be better visualized under a polarized microscope, and the condition can be distinguished from pseudomonilethrix using this approach.5,6 In our patient, the diagnosis was made based on light microscopy and trichoscopic findings with no genetic testing; however, genetic testing for the classic mutations of the keratin genes would be desirable to confirm the diagnosis but was not done in our patient.6 The prognosis of monilethrix is variable; most cases persist into adulthood, though spontaneous improvement may occur with advancing age, during summer, and during pregnancy.8
There is no definitive therapy for monilethrix. Although there have been reports of cases treated with systemic corticosteroids, oral retinoids, topical minoxidil, vitamins, and peeling ointments (desquamative oil), the cornerstone of management is protecting the hair against traumatic procedures such as excessive combing, brushing, and friction, as well as parent and patient education about the benign nature of the condition.9 Additionally, some cases have shown improvement with minoxidil solution at 2% and 5% concentrations, oral minoxidil, or acitretin.7-9
- Fontenelle de Oliveira E, Cotta de Alencar Araripe AL. Monilethrix: a typical case report with microscopic and dermatoscopic findings. An Bras Dermatol. 2015;90:126-127.
- de Cruz R, Horev L, Green J, et al. A novel monilethrix mutation in coil 2A of KRT86 causing autosomal dominant monilethrix with incomplete penetrance. Br J Dermatol. 2012;166(suppl 2):20-26.
- Baltazard T, Dhaille F, Chaby G, et al. Value of dermoscopy for the diagnosis of monilethrix. Dermatol Online J. 2017;23:13030 /qt9hf1p3xm.
- Kato M, Shimizu A, Yokoyama Y, et al. An autosomal recessive mutation of DSG4 causes monilethrix through the ER stress response. J Invest Dermatol. 2015;135:1253-1260.
- Gummer CL, Dawber RP, Swift JA. Monilethrix: an electron microscopic and electron histochemical study. Br J Dermatol. 1981;105:529-541.
- Sharma VK, Chiramel MJ, Rao A. Dermoscopy: a rapid bedside tool to assess monilethrix. Indian J Dermatol Venereol Leprol. 2016;82:73-74.
- Sinclair R. Treatment of monilethrix with oral minoxidil. JAAD Case Rep. 2016;2:212-215.
- Rakowska A, Slowinska M, Czuwara J, et al. Dermoscopy as a tool for rapid diagnosis of monilethrix. J Drugs Dermatol. 2007;6:222-224.
- Karincaoglu Y, Coskun BK, Seyhan ME, et al. Monilethrix. Am J Clin Dermatol. 2005;6:407-410.
The Diagnosis: Monilethrix
A diagnosis of monilethrix was rendered based on the clinical and trichoscopic findings. Simple surveillance of the patient’s condition and prevention of further hair trauma were proposed as management options.
Monilethrix is a hair shaft disorder that is inherited in a predominantly autosomal-dominant pattern with variable expressiveness and penetrance resulting from heterozygous mutations in hair keratin genes KRT81, KRT83, and KRT86 in a region of chromosome 12q13.13.1,2 An autosomalrecessive form has been described with mutation in desmoglein 4, but it differs from the classical form by the variable periodicity of the region between the nodules.3,4
The morphologic alteration consists of the formation of fusiform nodules of normal structure alternated with narrow and dystrophic constrictions (Figure). These internodes are fragile areas that cause breakage at constricted points.5 Clinically, monilethrix presents as areas of focal or diffuse alopecia with frequent involvement of the terminal follicles, mainly in areas of friction. The hair is normal at birth due to the predominance of lanugo in the neonatal period, but it subsequently is replaced by abnormal hairs in the first months of life.6 Initial clinical signs begin to appear when the terminal hairs begin to form.7 Although rarer, the eyebrows and eyelashes, as well as the axillary, pubic, and body hair, may be involved.5

Other hair shaft anomalies merit consideration in the differential diagnosis of monilethrix, including pseudomonilethrix, pressure alopecia, trichorrhexis invaginata, ectodermal dysplasia, tinea capitis, and trichothiodystrophy.6 The diagnosis is reached by clinical history and physical examination. Trichoscopy and light microscopy are used to confirm the diagnosis. Trichoscopic examination shows markedly higher rates of anagen hair. The shafts examined in our patient revealed 0.7- to 1-mm intervals between nodes. Hair can be better visualized under a polarized microscope, and the condition can be distinguished from pseudomonilethrix using this approach.5,6 In our patient, the diagnosis was made based on light microscopy and trichoscopic findings with no genetic testing; however, genetic testing for the classic mutations of the keratin genes would be desirable to confirm the diagnosis but was not done in our patient.6 The prognosis of monilethrix is variable; most cases persist into adulthood, though spontaneous improvement may occur with advancing age, during summer, and during pregnancy.8
There is no definitive therapy for monilethrix. Although there have been reports of cases treated with systemic corticosteroids, oral retinoids, topical minoxidil, vitamins, and peeling ointments (desquamative oil), the cornerstone of management is protecting the hair against traumatic procedures such as excessive combing, brushing, and friction, as well as parent and patient education about the benign nature of the condition.9 Additionally, some cases have shown improvement with minoxidil solution at 2% and 5% concentrations, oral minoxidil, or acitretin.7-9
The Diagnosis: Monilethrix
A diagnosis of monilethrix was rendered based on the clinical and trichoscopic findings. Simple surveillance of the patient’s condition and prevention of further hair trauma were proposed as management options.
Monilethrix is a hair shaft disorder that is inherited in a predominantly autosomal-dominant pattern with variable expressiveness and penetrance resulting from heterozygous mutations in hair keratin genes KRT81, KRT83, and KRT86 in a region of chromosome 12q13.13.1,2 An autosomalrecessive form has been described with mutation in desmoglein 4, but it differs from the classical form by the variable periodicity of the region between the nodules.3,4
The morphologic alteration consists of the formation of fusiform nodules of normal structure alternated with narrow and dystrophic constrictions (Figure). These internodes are fragile areas that cause breakage at constricted points.5 Clinically, monilethrix presents as areas of focal or diffuse alopecia with frequent involvement of the terminal follicles, mainly in areas of friction. The hair is normal at birth due to the predominance of lanugo in the neonatal period, but it subsequently is replaced by abnormal hairs in the first months of life.6 Initial clinical signs begin to appear when the terminal hairs begin to form.7 Although rarer, the eyebrows and eyelashes, as well as the axillary, pubic, and body hair, may be involved.5

Other hair shaft anomalies merit consideration in the differential diagnosis of monilethrix, including pseudomonilethrix, pressure alopecia, trichorrhexis invaginata, ectodermal dysplasia, tinea capitis, and trichothiodystrophy.6 The diagnosis is reached by clinical history and physical examination. Trichoscopy and light microscopy are used to confirm the diagnosis. Trichoscopic examination shows markedly higher rates of anagen hair. The shafts examined in our patient revealed 0.7- to 1-mm intervals between nodes. Hair can be better visualized under a polarized microscope, and the condition can be distinguished from pseudomonilethrix using this approach.5,6 In our patient, the diagnosis was made based on light microscopy and trichoscopic findings with no genetic testing; however, genetic testing for the classic mutations of the keratin genes would be desirable to confirm the diagnosis but was not done in our patient.6 The prognosis of monilethrix is variable; most cases persist into adulthood, though spontaneous improvement may occur with advancing age, during summer, and during pregnancy.8
There is no definitive therapy for monilethrix. Although there have been reports of cases treated with systemic corticosteroids, oral retinoids, topical minoxidil, vitamins, and peeling ointments (desquamative oil), the cornerstone of management is protecting the hair against traumatic procedures such as excessive combing, brushing, and friction, as well as parent and patient education about the benign nature of the condition.9 Additionally, some cases have shown improvement with minoxidil solution at 2% and 5% concentrations, oral minoxidil, or acitretin.7-9
- Fontenelle de Oliveira E, Cotta de Alencar Araripe AL. Monilethrix: a typical case report with microscopic and dermatoscopic findings. An Bras Dermatol. 2015;90:126-127.
- de Cruz R, Horev L, Green J, et al. A novel monilethrix mutation in coil 2A of KRT86 causing autosomal dominant monilethrix with incomplete penetrance. Br J Dermatol. 2012;166(suppl 2):20-26.
- Baltazard T, Dhaille F, Chaby G, et al. Value of dermoscopy for the diagnosis of monilethrix. Dermatol Online J. 2017;23:13030 /qt9hf1p3xm.
- Kato M, Shimizu A, Yokoyama Y, et al. An autosomal recessive mutation of DSG4 causes monilethrix through the ER stress response. J Invest Dermatol. 2015;135:1253-1260.
- Gummer CL, Dawber RP, Swift JA. Monilethrix: an electron microscopic and electron histochemical study. Br J Dermatol. 1981;105:529-541.
- Sharma VK, Chiramel MJ, Rao A. Dermoscopy: a rapid bedside tool to assess monilethrix. Indian J Dermatol Venereol Leprol. 2016;82:73-74.
- Sinclair R. Treatment of monilethrix with oral minoxidil. JAAD Case Rep. 2016;2:212-215.
- Rakowska A, Slowinska M, Czuwara J, et al. Dermoscopy as a tool for rapid diagnosis of monilethrix. J Drugs Dermatol. 2007;6:222-224.
- Karincaoglu Y, Coskun BK, Seyhan ME, et al. Monilethrix. Am J Clin Dermatol. 2005;6:407-410.
- Fontenelle de Oliveira E, Cotta de Alencar Araripe AL. Monilethrix: a typical case report with microscopic and dermatoscopic findings. An Bras Dermatol. 2015;90:126-127.
- de Cruz R, Horev L, Green J, et al. A novel monilethrix mutation in coil 2A of KRT86 causing autosomal dominant monilethrix with incomplete penetrance. Br J Dermatol. 2012;166(suppl 2):20-26.
- Baltazard T, Dhaille F, Chaby G, et al. Value of dermoscopy for the diagnosis of monilethrix. Dermatol Online J. 2017;23:13030 /qt9hf1p3xm.
- Kato M, Shimizu A, Yokoyama Y, et al. An autosomal recessive mutation of DSG4 causes monilethrix through the ER stress response. J Invest Dermatol. 2015;135:1253-1260.
- Gummer CL, Dawber RP, Swift JA. Monilethrix: an electron microscopic and electron histochemical study. Br J Dermatol. 1981;105:529-541.
- Sharma VK, Chiramel MJ, Rao A. Dermoscopy: a rapid bedside tool to assess monilethrix. Indian J Dermatol Venereol Leprol. 2016;82:73-74.
- Sinclair R. Treatment of monilethrix with oral minoxidil. JAAD Case Rep. 2016;2:212-215.
- Rakowska A, Slowinska M, Czuwara J, et al. Dermoscopy as a tool for rapid diagnosis of monilethrix. J Drugs Dermatol. 2007;6:222-224.
- Karincaoglu Y, Coskun BK, Seyhan ME, et al. Monilethrix. Am J Clin Dermatol. 2005;6:407-410.
A 6-month-old infant girl was referred to the dermatology service with hypotrichosis and hair loss on the occipital region of the scalp of 4 months’ duration (top). The patient was born at full term by cesarean delivery without complications. There were no comorbidities or family history of alopecia. Clinical examination revealed an alopecic plaque in the occipital region with broken hairs and some dystrophic hairs associated with follicular papules and perifollicular hyperkeratosis. A hair pull test was positive for telogen hairs. Trichoscopy revealed black dots and broken hairs resembling Morse code (bottom). Hair microscopy showed regular alternation of constriction zones separated by intervals of normal thickness.
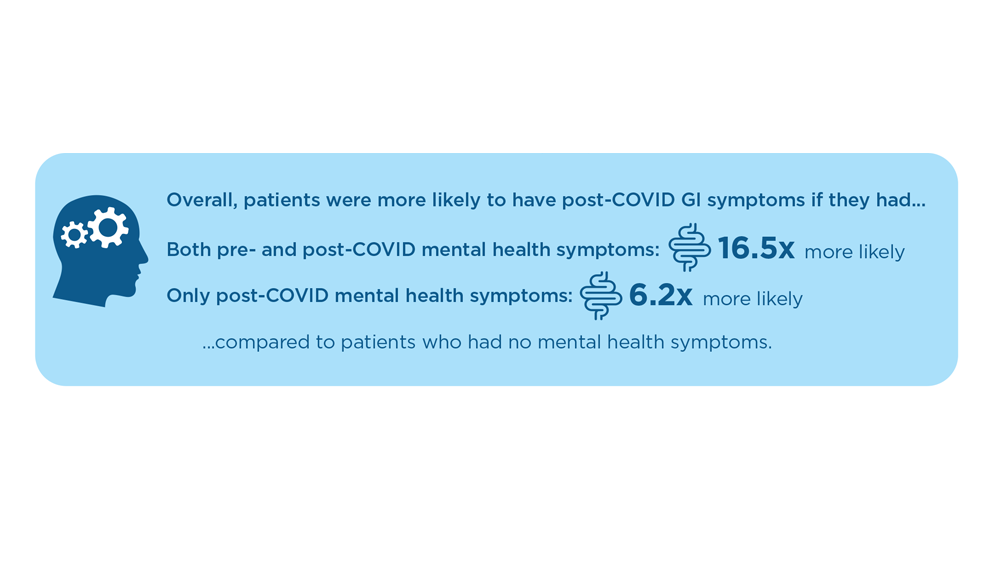
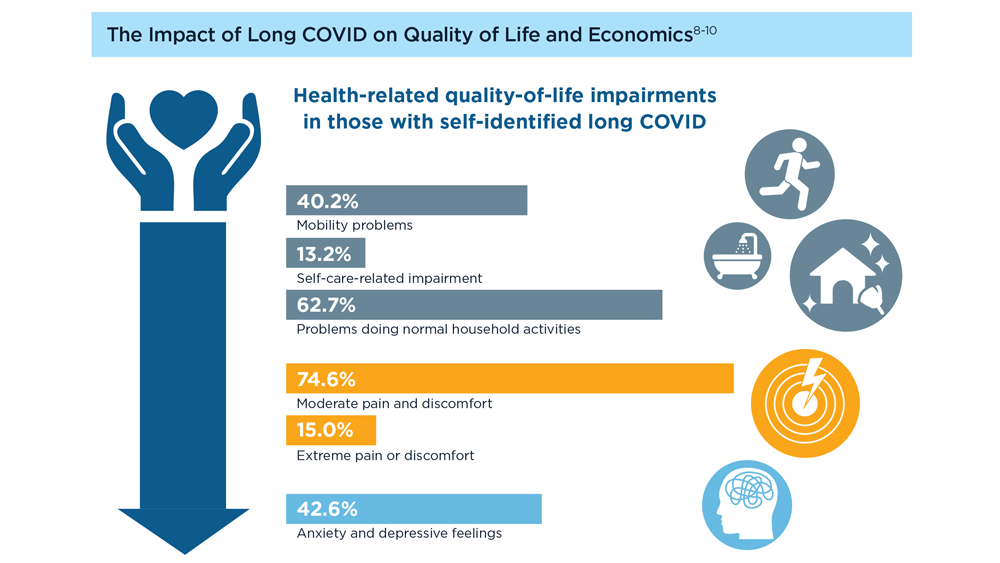
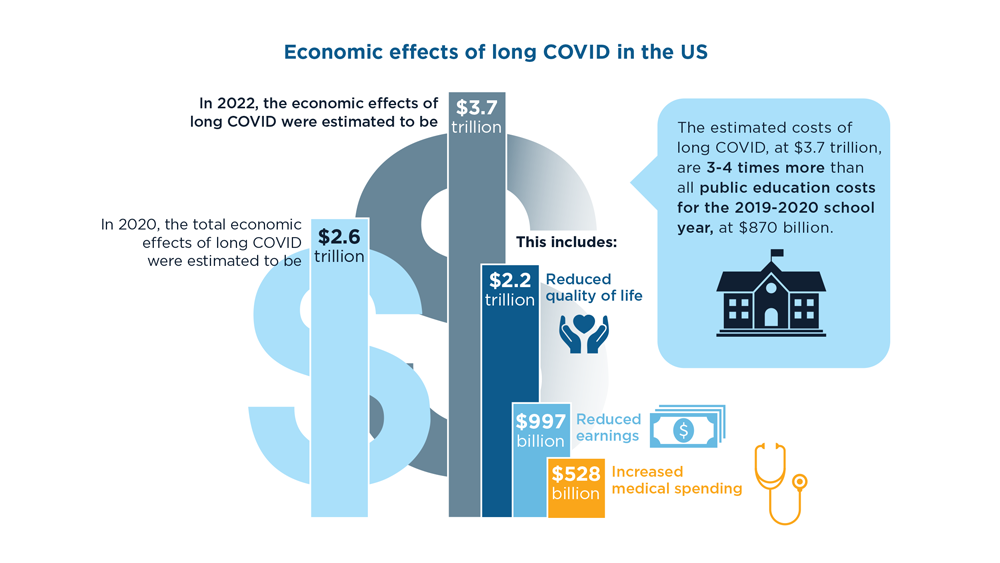
Long COVID and the Gastrointestinal System: Emerging Evidence
- Lutchmansingh DD et al. Semin Respir Crit Care Med. 2023;44(1):130-142. doi:10.1055/s-0042-1759568
- Choudhury A et al. Therap Adv Gastroenterol. 2022;15:17562848221118403. doi:10.1177/17562848221118403
- Xu E et al. Nat Commun. 2023;14(1):983. doi:10.1038/s41467-023-36223-7
- Freedberg DE, Chang L. Curr Opin Gastroenterol. 2022;38(6):555-561. doi:10.1097/MOG.0000000000000876
- Blackett JW et al. Gastroenterology. 2022;162(2):648-650.e2. doi:10.1053/j.gastro.2021.10.040
- Chey WD et al. Gastroenterology. 2021;160(1):47-62. doi:10.1053/j.gastro.2020.06.099
- Líška D et al. Front Public Health. 2022;10:975992. doi:10.3389/fpubh.2022.975992
- Moens M et al. Front Public Health. 2022;10:991572. doi:10.3389/fpubh.2022.991572
- Cutler DM. The economic cost of long COVID: an update. Scholars at Harvard. Published July 2022. Accessed July 20, 2023. https://scholar.harvard.edu/sites/scholar.harvard.edu/files/cutler/files/long_covid_update_7-22.pdf
- National Center for Education Statistics (2023). Public School Expenditures. Condition of Education. US Department of Education, Institute of Education Sciences. Accessed August 4, 2023. https://nces.ed.gov/programs/coe/indicator/cmb
- Lutchmansingh DD et al. Semin Respir Crit Care Med. 2023;44(1):130-142. doi:10.1055/s-0042-1759568
- Choudhury A et al. Therap Adv Gastroenterol. 2022;15:17562848221118403. doi:10.1177/17562848221118403
- Xu E et al. Nat Commun. 2023;14(1):983. doi:10.1038/s41467-023-36223-7
- Freedberg DE, Chang L. Curr Opin Gastroenterol. 2022;38(6):555-561. doi:10.1097/MOG.0000000000000876
- Blackett JW et al. Gastroenterology. 2022;162(2):648-650.e2. doi:10.1053/j.gastro.2021.10.040
- Chey WD et al. Gastroenterology. 2021;160(1):47-62. doi:10.1053/j.gastro.2020.06.099
- Líška D et al. Front Public Health. 2022;10:975992. doi:10.3389/fpubh.2022.975992
- Moens M et al. Front Public Health. 2022;10:991572. doi:10.3389/fpubh.2022.991572
- Cutler DM. The economic cost of long COVID: an update. Scholars at Harvard. Published July 2022. Accessed July 20, 2023. https://scholar.harvard.edu/sites/scholar.harvard.edu/files/cutler/files/long_covid_update_7-22.pdf
- National Center for Education Statistics (2023). Public School Expenditures. Condition of Education. US Department of Education, Institute of Education Sciences. Accessed August 4, 2023. https://nces.ed.gov/programs/coe/indicator/cmb
- Lutchmansingh DD et al. Semin Respir Crit Care Med. 2023;44(1):130-142. doi:10.1055/s-0042-1759568
- Choudhury A et al. Therap Adv Gastroenterol. 2022;15:17562848221118403. doi:10.1177/17562848221118403
- Xu E et al. Nat Commun. 2023;14(1):983. doi:10.1038/s41467-023-36223-7
- Freedberg DE, Chang L. Curr Opin Gastroenterol. 2022;38(6):555-561. doi:10.1097/MOG.0000000000000876
- Blackett JW et al. Gastroenterology. 2022;162(2):648-650.e2. doi:10.1053/j.gastro.2021.10.040
- Chey WD et al. Gastroenterology. 2021;160(1):47-62. doi:10.1053/j.gastro.2020.06.099
- Líška D et al. Front Public Health. 2022;10:975992. doi:10.3389/fpubh.2022.975992
- Moens M et al. Front Public Health. 2022;10:991572. doi:10.3389/fpubh.2022.991572
- Cutler DM. The economic cost of long COVID: an update. Scholars at Harvard. Published July 2022. Accessed July 20, 2023. https://scholar.harvard.edu/sites/scholar.harvard.edu/files/cutler/files/long_covid_update_7-22.pdf
- National Center for Education Statistics (2023). Public School Expenditures. Condition of Education. US Department of Education, Institute of Education Sciences. Accessed August 4, 2023. https://nces.ed.gov/programs/coe/indicator/cmb
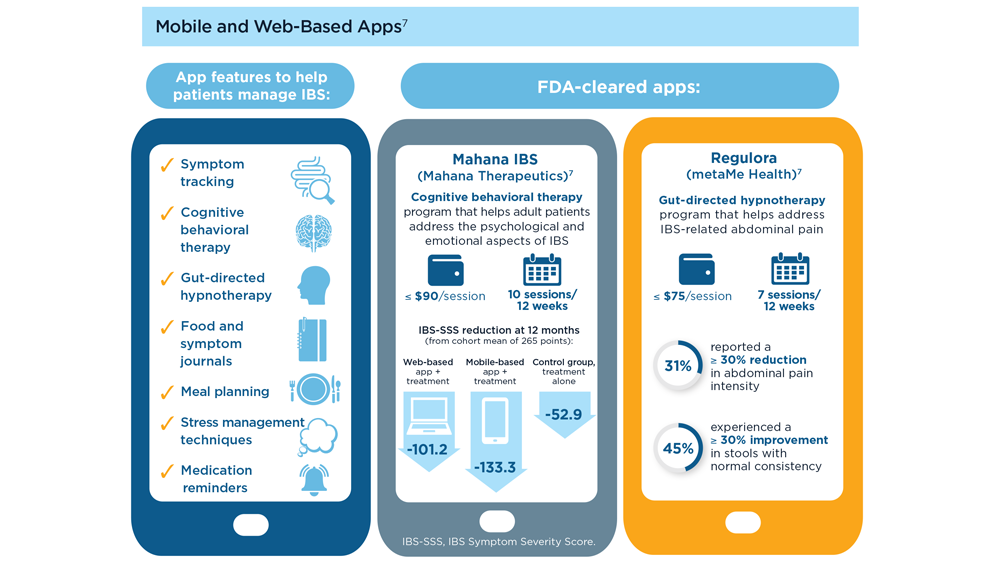
Digital Tools in the Management of IBS/ Functional GI Disorders
- Hasan SS et al. Neurogastroenterol Motil. 2023;35(4):e14554. doi:10.1111/nmo.14554
- Peters SL et al. Neurogastroenterol Motil. 2023;35(4):e14533. doi:10.1111/nmo.14533
- Zhou C et al. Neurogastroenterol Motil. 2019;31(2):e13461. doi:10.1111/nmo.13461
- Staudacher HM et al. Nat Rev Gastroenterol Hepatol. 2023;1-15. doi:10.1038/s41575-023-00794-z
- Qin HY et al. World J Gastroenterol. 2014;20(39):14126-14131. doi:10.3748/wjg.v20.i39.14126
- Varjú P et al. PLoS One. 2017;12(8):e0182942. doi:10.1371/journal.pone.0182942
- Saleh ZM et al. Am J Gastroenterol. 2023. doi:10.14309/ajg.0000000000002220
- Yu C et al. Clin Transl Gastroenterol. 2022;13(9):e00515. doi:10.14309/ctg.0000000000000515
- Jagannath B et al. Inflamm Bowel Dis. 2020;26(10):1533-1542. doi:10.1093/ibd/izaa191
- Zhang H et al. J Nutr. 2023;153(4):924-939. doi:10.1016/j.tjnut.2023.01.026
- Karakan T et al. Gut Microbes. 2022;14(1):2138672. doi:10.1080/19490976.2022.2138672
- Kordi M et al. Inform Med Unlocked. 2022;29:100891. doi:10.1016/j.imu.2022.100891
- Gubatan J et al. World J Gastroenterol. 2021;27(17):1920-1935. doi:10.3748/wjg.v27.i17.1920
- Boucher EM et al. Expert Rev Med Devices. 2021;18(suppl 1):37-49. doi:10.1080/17434440.2021.2013200
- Babel A et al. Front Digit Health. 2021;3:669869. doi:10.3389/fdgth.2021.669869
- Hasan SS et al. Neurogastroenterol Motil. 2023;35(4):e14554. doi:10.1111/nmo.14554
- Peters SL et al. Neurogastroenterol Motil. 2023;35(4):e14533. doi:10.1111/nmo.14533
- Zhou C et al. Neurogastroenterol Motil. 2019;31(2):e13461. doi:10.1111/nmo.13461
- Staudacher HM et al. Nat Rev Gastroenterol Hepatol. 2023;1-15. doi:10.1038/s41575-023-00794-z
- Qin HY et al. World J Gastroenterol. 2014;20(39):14126-14131. doi:10.3748/wjg.v20.i39.14126
- Varjú P et al. PLoS One. 2017;12(8):e0182942. doi:10.1371/journal.pone.0182942
- Saleh ZM et al. Am J Gastroenterol. 2023. doi:10.14309/ajg.0000000000002220
- Yu C et al. Clin Transl Gastroenterol. 2022;13(9):e00515. doi:10.14309/ctg.0000000000000515
- Jagannath B et al. Inflamm Bowel Dis. 2020;26(10):1533-1542. doi:10.1093/ibd/izaa191
- Zhang H et al. J Nutr. 2023;153(4):924-939. doi:10.1016/j.tjnut.2023.01.026
- Karakan T et al. Gut Microbes. 2022;14(1):2138672. doi:10.1080/19490976.2022.2138672
- Kordi M et al. Inform Med Unlocked. 2022;29:100891. doi:10.1016/j.imu.2022.100891
- Gubatan J et al. World J Gastroenterol. 2021;27(17):1920-1935. doi:10.3748/wjg.v27.i17.1920
- Boucher EM et al. Expert Rev Med Devices. 2021;18(suppl 1):37-49. doi:10.1080/17434440.2021.2013200
- Babel A et al. Front Digit Health. 2021;3:669869. doi:10.3389/fdgth.2021.669869
- Hasan SS et al. Neurogastroenterol Motil. 2023;35(4):e14554. doi:10.1111/nmo.14554
- Peters SL et al. Neurogastroenterol Motil. 2023;35(4):e14533. doi:10.1111/nmo.14533
- Zhou C et al. Neurogastroenterol Motil. 2019;31(2):e13461. doi:10.1111/nmo.13461
- Staudacher HM et al. Nat Rev Gastroenterol Hepatol. 2023;1-15. doi:10.1038/s41575-023-00794-z
- Qin HY et al. World J Gastroenterol. 2014;20(39):14126-14131. doi:10.3748/wjg.v20.i39.14126
- Varjú P et al. PLoS One. 2017;12(8):e0182942. doi:10.1371/journal.pone.0182942
- Saleh ZM et al. Am J Gastroenterol. 2023. doi:10.14309/ajg.0000000000002220
- Yu C et al. Clin Transl Gastroenterol. 2022;13(9):e00515. doi:10.14309/ctg.0000000000000515
- Jagannath B et al. Inflamm Bowel Dis. 2020;26(10):1533-1542. doi:10.1093/ibd/izaa191
- Zhang H et al. J Nutr. 2023;153(4):924-939. doi:10.1016/j.tjnut.2023.01.026
- Karakan T et al. Gut Microbes. 2022;14(1):2138672. doi:10.1080/19490976.2022.2138672
- Kordi M et al. Inform Med Unlocked. 2022;29:100891. doi:10.1016/j.imu.2022.100891
- Gubatan J et al. World J Gastroenterol. 2021;27(17):1920-1935. doi:10.3748/wjg.v27.i17.1920
- Boucher EM et al. Expert Rev Med Devices. 2021;18(suppl 1):37-49. doi:10.1080/17434440.2021.2013200
- Babel A et al. Front Digit Health. 2021;3:669869. doi:10.3389/fdgth.2021.669869
Anxiety and panic attacks
Given the patient's insidious cognitive decline, as well as increased agitation, irritability, anxiety, social isolation, inability to fully manage finances, loss of routine hygienic practices, and loss of interest in regular meals, this patient is diagnosed with probable Alzheimer's disease (AD) dementia and is referred to a specialist for further testing.
AD is one of the most common forms of dementia. More than 6 million people in the United States have clinical AD or mild cognitive impairment because of AD. By 2060, the incidence of AD is expected to grow to 15 million people. AD is classified into four stages: preclinical, mild, moderate, and severe. Patients with preclinical AD — a relatively new classification currently only used for research — do not yet show abnormal results on physical exam or mental status testing, but areas of the brain are undergoing pathologic changes. Mild AD signs and symptoms include memory loss, compromised judgment, trouble handling money and paying bills, mood and personality changes, and increased anxiety. People with moderate AD show increasing signs of memory loss and confusion, problems with recognizing family and friends, and difficulty with organizing thoughts and thinking logically, and they repeat themselves in conversation, among other symptoms. Severe AD is generally described as a complete loss of self, with the inability to recognize family and friends, inability to communicate effectively, and complete dependence on others for care.
Diagnosing AD currently relies on a clinical approach. A complete physical examination, with a detailed neurologic examination and a mental status examination, is used to evaluate disease stage and rule out comorbid conditions. Initial mental status testing should evaluate attention and concentration, recent and remote memory, language, praxis, executive function, and visuospatial function. Imaging studies may be performed to rule out other treatable causes of cognitive decline. In addition, volumetric studies of the hippocampus and 2-[18F]fluoro-2-deoxy-D-glucose PET with or without amyloid imaging can be used for early detection and differentiating dementia etiologies. Lumbar puncture as a diagnostic measure for levels of tau (which is often elevated in AD) and amyloid (which is often reduced in AD) is currently reserved for research settings.
Although the cause of AD is unknown, experts believe that environmental and genetic risk factors trigger a pathophysiologic cascade that, over decades, leads to Alzheimer's pathology and dementia. Universally accepted pathologic hallmarks of AD are beta-amyloid plaques and neurofibrillary tangles (NFTs). NFTs result from changes in the tau protein, a key chemical in neuronal support structures, and are associated with malfunctions in communication between neurons as well as cell death. Beta-amyloid plaques are dense, mostly insoluble deposits that develop around neurons in the hippocampus and other regions in the cerebral cortex used for decision-making, disrupting function and leading to brain atrophy. Risk factors for AD include advancing age, family history, APOE e4 genotype, insulin resistance, hypertension, depression, and traumatic brain injury.
After an AD diagnosis, physicians should encourage the involvement of family and friends who agree to become more involved in the patient's care as the disease progresses. These individuals need to understand the patient's wishes around care, especially for the future, when the patient is no longer able to make decisions. The patient may also consider establishing medical advance directives and durable power of attorney for medical and financial decision-making. Caregivers supporting the patient are encouraged to help balance the physical needs of the patient while maintaining respect for them as a competent adult to the extent allowed by the progression of their disease.
Currently, AD treatments are focused on symptomatic therapies that modulate neurotransmitters — either acetylcholine or glutamate. The standard medical treatment includes cholinesterase inhibitors and a partial N-methyl-D-aspartate antagonist. Two amyloid-directed antibodies (aducanumab, lecanemab) are currently available in the US for individuals with AD exhibiting mild cognitive impairment or mild dementia. A third agent currently in clinical trials (donanemab) has shown significantly slowed clinical progression after 1.5 years among clinical trial participants with early symptomatic AD and amyloid and tau pathology.
Jasvinder Chawla, MD, Professor of Neurology, Loyola University Medical Center, Maywood; Director, Clinical Neurophysiology Lab, Department of Neurology, Hines VA Hospital, Hines, IL.
Jasvinder Chawla, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Given the patient's insidious cognitive decline, as well as increased agitation, irritability, anxiety, social isolation, inability to fully manage finances, loss of routine hygienic practices, and loss of interest in regular meals, this patient is diagnosed with probable Alzheimer's disease (AD) dementia and is referred to a specialist for further testing.
AD is one of the most common forms of dementia. More than 6 million people in the United States have clinical AD or mild cognitive impairment because of AD. By 2060, the incidence of AD is expected to grow to 15 million people. AD is classified into four stages: preclinical, mild, moderate, and severe. Patients with preclinical AD — a relatively new classification currently only used for research — do not yet show abnormal results on physical exam or mental status testing, but areas of the brain are undergoing pathologic changes. Mild AD signs and symptoms include memory loss, compromised judgment, trouble handling money and paying bills, mood and personality changes, and increased anxiety. People with moderate AD show increasing signs of memory loss and confusion, problems with recognizing family and friends, and difficulty with organizing thoughts and thinking logically, and they repeat themselves in conversation, among other symptoms. Severe AD is generally described as a complete loss of self, with the inability to recognize family and friends, inability to communicate effectively, and complete dependence on others for care.
Diagnosing AD currently relies on a clinical approach. A complete physical examination, with a detailed neurologic examination and a mental status examination, is used to evaluate disease stage and rule out comorbid conditions. Initial mental status testing should evaluate attention and concentration, recent and remote memory, language, praxis, executive function, and visuospatial function. Imaging studies may be performed to rule out other treatable causes of cognitive decline. In addition, volumetric studies of the hippocampus and 2-[18F]fluoro-2-deoxy-D-glucose PET with or without amyloid imaging can be used for early detection and differentiating dementia etiologies. Lumbar puncture as a diagnostic measure for levels of tau (which is often elevated in AD) and amyloid (which is often reduced in AD) is currently reserved for research settings.
Although the cause of AD is unknown, experts believe that environmental and genetic risk factors trigger a pathophysiologic cascade that, over decades, leads to Alzheimer's pathology and dementia. Universally accepted pathologic hallmarks of AD are beta-amyloid plaques and neurofibrillary tangles (NFTs). NFTs result from changes in the tau protein, a key chemical in neuronal support structures, and are associated with malfunctions in communication between neurons as well as cell death. Beta-amyloid plaques are dense, mostly insoluble deposits that develop around neurons in the hippocampus and other regions in the cerebral cortex used for decision-making, disrupting function and leading to brain atrophy. Risk factors for AD include advancing age, family history, APOE e4 genotype, insulin resistance, hypertension, depression, and traumatic brain injury.
After an AD diagnosis, physicians should encourage the involvement of family and friends who agree to become more involved in the patient's care as the disease progresses. These individuals need to understand the patient's wishes around care, especially for the future, when the patient is no longer able to make decisions. The patient may also consider establishing medical advance directives and durable power of attorney for medical and financial decision-making. Caregivers supporting the patient are encouraged to help balance the physical needs of the patient while maintaining respect for them as a competent adult to the extent allowed by the progression of their disease.
Currently, AD treatments are focused on symptomatic therapies that modulate neurotransmitters — either acetylcholine or glutamate. The standard medical treatment includes cholinesterase inhibitors and a partial N-methyl-D-aspartate antagonist. Two amyloid-directed antibodies (aducanumab, lecanemab) are currently available in the US for individuals with AD exhibiting mild cognitive impairment or mild dementia. A third agent currently in clinical trials (donanemab) has shown significantly slowed clinical progression after 1.5 years among clinical trial participants with early symptomatic AD and amyloid and tau pathology.
Jasvinder Chawla, MD, Professor of Neurology, Loyola University Medical Center, Maywood; Director, Clinical Neurophysiology Lab, Department of Neurology, Hines VA Hospital, Hines, IL.
Jasvinder Chawla, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Given the patient's insidious cognitive decline, as well as increased agitation, irritability, anxiety, social isolation, inability to fully manage finances, loss of routine hygienic practices, and loss of interest in regular meals, this patient is diagnosed with probable Alzheimer's disease (AD) dementia and is referred to a specialist for further testing.
AD is one of the most common forms of dementia. More than 6 million people in the United States have clinical AD or mild cognitive impairment because of AD. By 2060, the incidence of AD is expected to grow to 15 million people. AD is classified into four stages: preclinical, mild, moderate, and severe. Patients with preclinical AD — a relatively new classification currently only used for research — do not yet show abnormal results on physical exam or mental status testing, but areas of the brain are undergoing pathologic changes. Mild AD signs and symptoms include memory loss, compromised judgment, trouble handling money and paying bills, mood and personality changes, and increased anxiety. People with moderate AD show increasing signs of memory loss and confusion, problems with recognizing family and friends, and difficulty with organizing thoughts and thinking logically, and they repeat themselves in conversation, among other symptoms. Severe AD is generally described as a complete loss of self, with the inability to recognize family and friends, inability to communicate effectively, and complete dependence on others for care.
Diagnosing AD currently relies on a clinical approach. A complete physical examination, with a detailed neurologic examination and a mental status examination, is used to evaluate disease stage and rule out comorbid conditions. Initial mental status testing should evaluate attention and concentration, recent and remote memory, language, praxis, executive function, and visuospatial function. Imaging studies may be performed to rule out other treatable causes of cognitive decline. In addition, volumetric studies of the hippocampus and 2-[18F]fluoro-2-deoxy-D-glucose PET with or without amyloid imaging can be used for early detection and differentiating dementia etiologies. Lumbar puncture as a diagnostic measure for levels of tau (which is often elevated in AD) and amyloid (which is often reduced in AD) is currently reserved for research settings.
Although the cause of AD is unknown, experts believe that environmental and genetic risk factors trigger a pathophysiologic cascade that, over decades, leads to Alzheimer's pathology and dementia. Universally accepted pathologic hallmarks of AD are beta-amyloid plaques and neurofibrillary tangles (NFTs). NFTs result from changes in the tau protein, a key chemical in neuronal support structures, and are associated with malfunctions in communication between neurons as well as cell death. Beta-amyloid plaques are dense, mostly insoluble deposits that develop around neurons in the hippocampus and other regions in the cerebral cortex used for decision-making, disrupting function and leading to brain atrophy. Risk factors for AD include advancing age, family history, APOE e4 genotype, insulin resistance, hypertension, depression, and traumatic brain injury.
After an AD diagnosis, physicians should encourage the involvement of family and friends who agree to become more involved in the patient's care as the disease progresses. These individuals need to understand the patient's wishes around care, especially for the future, when the patient is no longer able to make decisions. The patient may also consider establishing medical advance directives and durable power of attorney for medical and financial decision-making. Caregivers supporting the patient are encouraged to help balance the physical needs of the patient while maintaining respect for them as a competent adult to the extent allowed by the progression of their disease.
Currently, AD treatments are focused on symptomatic therapies that modulate neurotransmitters — either acetylcholine or glutamate. The standard medical treatment includes cholinesterase inhibitors and a partial N-methyl-D-aspartate antagonist. Two amyloid-directed antibodies (aducanumab, lecanemab) are currently available in the US for individuals with AD exhibiting mild cognitive impairment or mild dementia. A third agent currently in clinical trials (donanemab) has shown significantly slowed clinical progression after 1.5 years among clinical trial participants with early symptomatic AD and amyloid and tau pathology.
Jasvinder Chawla, MD, Professor of Neurology, Loyola University Medical Center, Maywood; Director, Clinical Neurophysiology Lab, Department of Neurology, Hines VA Hospital, Hines, IL.
Jasvinder Chawla, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 73-year-old man who lives independently presents to his primary care physician (PCP) with irritability, anxiety, and panic attacks. Last year, he saw his PCP at the urging of his brother, who noticed that the patient was becoming more forgetful and agitated. At that time, the brother reported concerns that the patient, who normally enjoyed spending time with his extended family, was beginning to regularly forget to show up at family functions. When asked why he hadn't attended, the patient would become irate, saying it was his family who failed to invite him. The patient wouldn't have agreed to seeing the PCP except he was having issues with insomnia that he wanted to address. During last year's visit, the physician conducted a complete physical examination, as well as detailed neurologic and mental status examinations; all came back normal.
At today's visit, in addition to patient-reported mood fluctuations, the brother tells the physician that the patient has become reclusive, skipping nearly all family functions as well as daily walks with friends. His daily hygiene has suffered, and he has stopped eating regularly. The brother also mentions to the doctor that the patient has received some late-payment notices for utilities that he normally meticulously paid on time. The PCP orders another round of cognitive, behavioral, and functional assessments, which reveal a decline in all areas from last year's results, as well as a complete neurologic examination that reveals mild hyposmia.
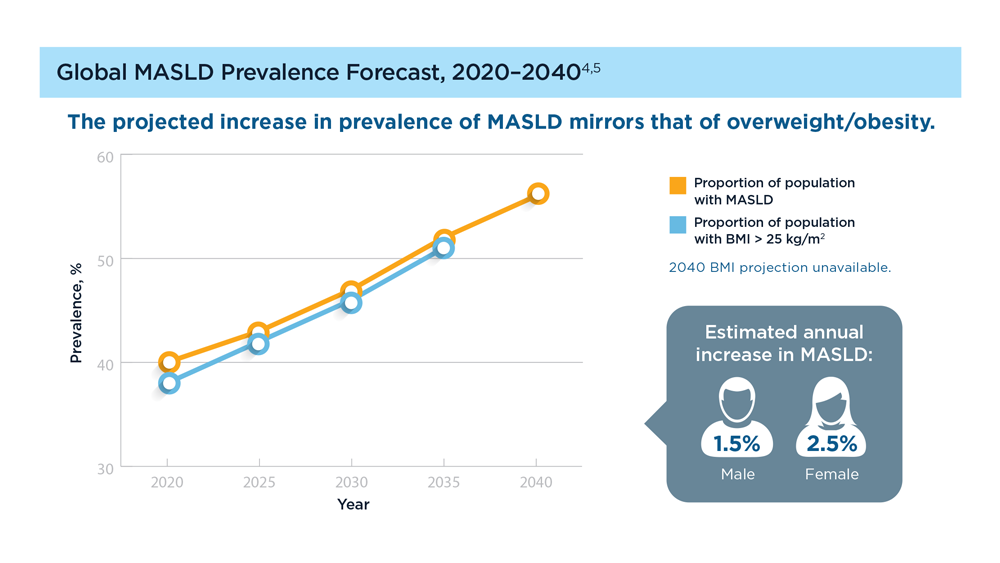
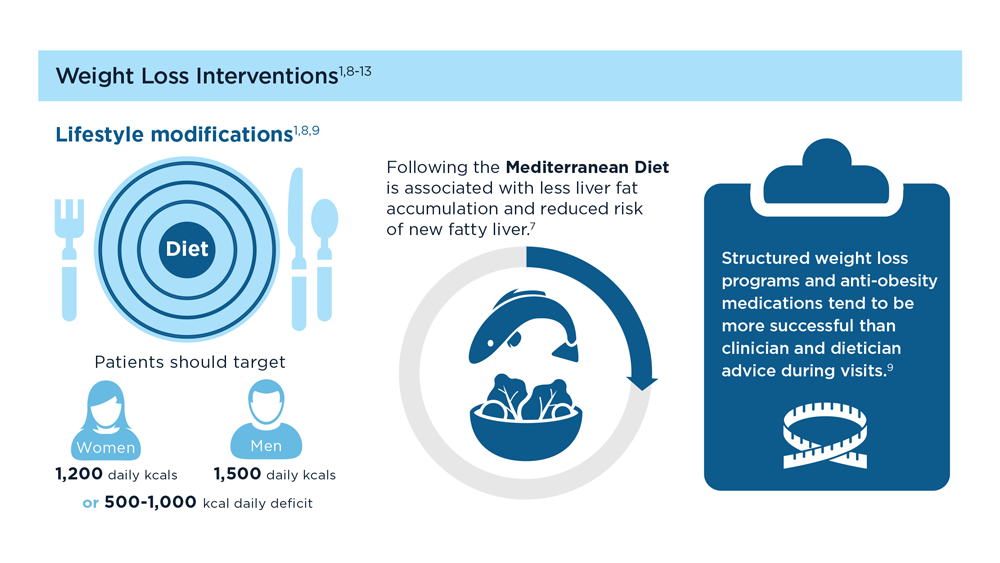
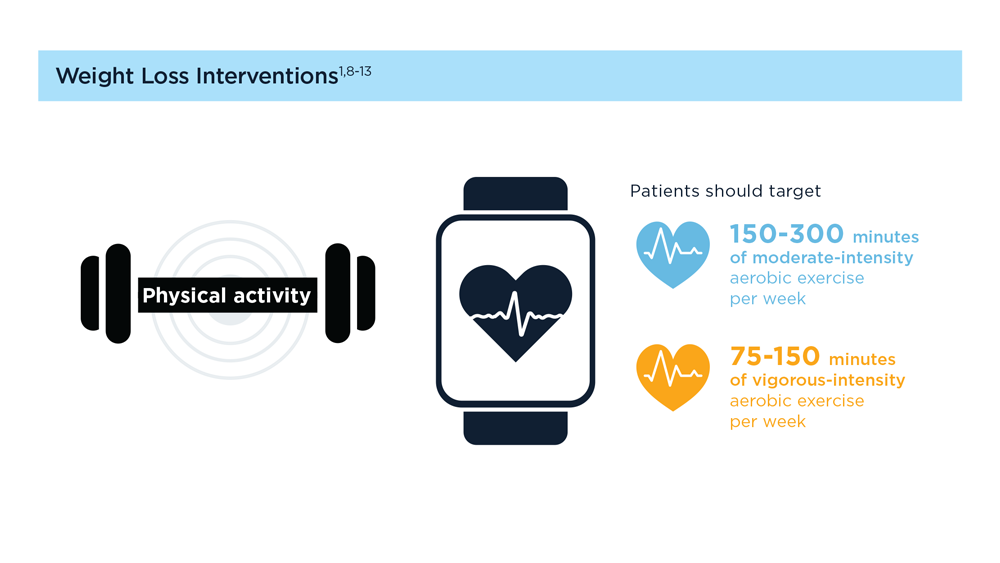
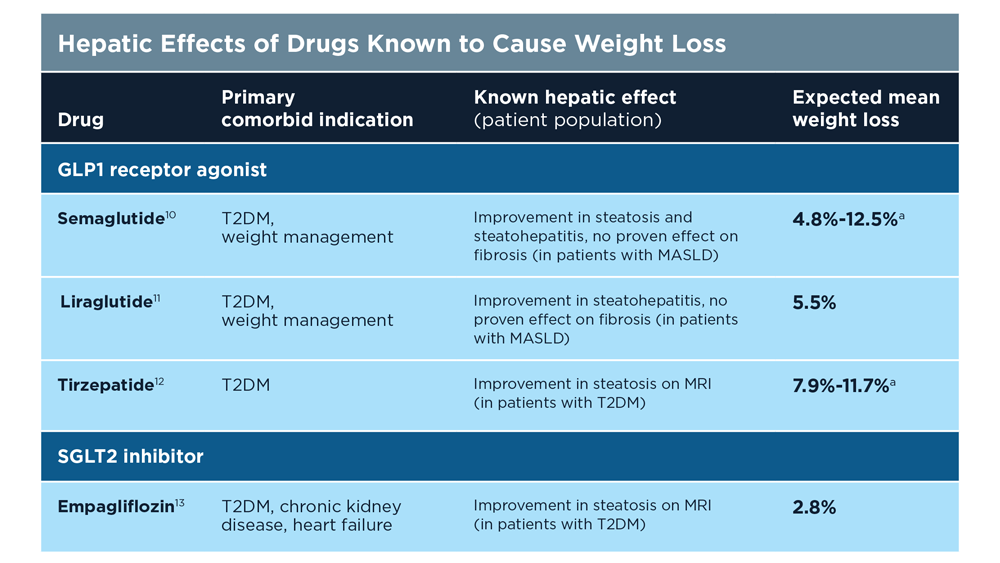
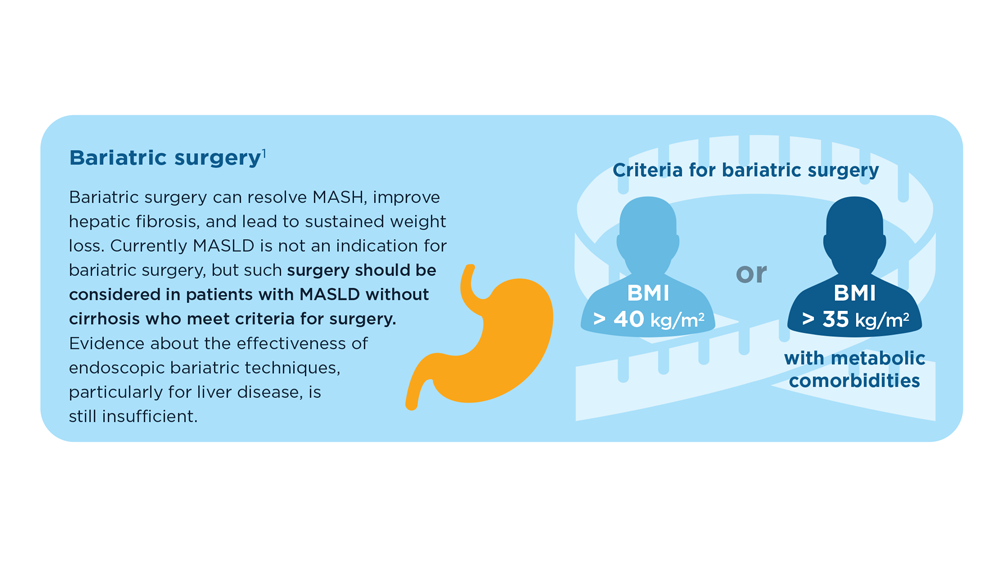
MASLD/MASH and Weight Loss
- Younossi ZM et al. Gastroenterology. 2021;160(3):912-918. doi:10.1053/j.astro.2020.11.051
- Cusi K et al. Endocr Pract. 2022;28(5):528-562. doi:10.1016/j.eprac.2022.03.010
- Rinella ME et al. Hepatology. 2023;77(5):1797-1835. doi:10.1097/HEP.0000000000000323
- World obesity atlas 2023. World Obesity Day. Published March 2023. Accessed July 23, 2023. https://www.worldobesityday.org/assets/downloads/World_Obesity_Atlas_2023_Report.pdf
- Le MH et al. Clin Mol Hepatol. 2022;28(4):841-850. doi:10.3350/cmh.2022.0239
- Vilar-Gomez E et al. Gastroenterology. 2015;149(2):367-78.e5. doi:10.1053/j.gastro.2015.04.005
- Koutoukidis DA et al. Metabolism. 2021;115:154455. doi:10.1016/j.metabol.2020.154455
- Ma J et al. Gastroenterology. 2018;155(1):107-117. doi:10.1053/j.gastro.2018.03.038
- Ahern AL et al. Lancet. 2017;389(10085):2214-2225. doi:10.1016/S0140-6736(17)30647-5
- Newsome PN et al; NN9931-4296 Investigators. N Engl J Med. 2021;384(12):1113-1124. doi:10.1056/NEJMoa2028395
- Armstrong MJ et al. Lancet. 2016;387(10019):679-690. doi:10.1016/S0140-6736(15)00803-X
- Gastaldelli A et al. Lancet Diabetes Endocrinol. 2022;10(6):393-406. doi:10.1016/S2213-8587(22)00070-5
- Kahl S et al. Diabetes Care. 2020;43(2):298-305. doi:10.2337/dc19-0641
- Younossi ZM et al. Gastroenterology. 2021;160(3):912-918. doi:10.1053/j.astro.2020.11.051
- Cusi K et al. Endocr Pract. 2022;28(5):528-562. doi:10.1016/j.eprac.2022.03.010
- Rinella ME et al. Hepatology. 2023;77(5):1797-1835. doi:10.1097/HEP.0000000000000323
- World obesity atlas 2023. World Obesity Day. Published March 2023. Accessed July 23, 2023. https://www.worldobesityday.org/assets/downloads/World_Obesity_Atlas_2023_Report.pdf
- Le MH et al. Clin Mol Hepatol. 2022;28(4):841-850. doi:10.3350/cmh.2022.0239
- Vilar-Gomez E et al. Gastroenterology. 2015;149(2):367-78.e5. doi:10.1053/j.gastro.2015.04.005
- Koutoukidis DA et al. Metabolism. 2021;115:154455. doi:10.1016/j.metabol.2020.154455
- Ma J et al. Gastroenterology. 2018;155(1):107-117. doi:10.1053/j.gastro.2018.03.038
- Ahern AL et al. Lancet. 2017;389(10085):2214-2225. doi:10.1016/S0140-6736(17)30647-5
- Newsome PN et al; NN9931-4296 Investigators. N Engl J Med. 2021;384(12):1113-1124. doi:10.1056/NEJMoa2028395
- Armstrong MJ et al. Lancet. 2016;387(10019):679-690. doi:10.1016/S0140-6736(15)00803-X
- Gastaldelli A et al. Lancet Diabetes Endocrinol. 2022;10(6):393-406. doi:10.1016/S2213-8587(22)00070-5
- Kahl S et al. Diabetes Care. 2020;43(2):298-305. doi:10.2337/dc19-0641
- Younossi ZM et al. Gastroenterology. 2021;160(3):912-918. doi:10.1053/j.astro.2020.11.051
- Cusi K et al. Endocr Pract. 2022;28(5):528-562. doi:10.1016/j.eprac.2022.03.010
- Rinella ME et al. Hepatology. 2023;77(5):1797-1835. doi:10.1097/HEP.0000000000000323
- World obesity atlas 2023. World Obesity Day. Published March 2023. Accessed July 23, 2023. https://www.worldobesityday.org/assets/downloads/World_Obesity_Atlas_2023_Report.pdf
- Le MH et al. Clin Mol Hepatol. 2022;28(4):841-850. doi:10.3350/cmh.2022.0239
- Vilar-Gomez E et al. Gastroenterology. 2015;149(2):367-78.e5. doi:10.1053/j.gastro.2015.04.005
- Koutoukidis DA et al. Metabolism. 2021;115:154455. doi:10.1016/j.metabol.2020.154455
- Ma J et al. Gastroenterology. 2018;155(1):107-117. doi:10.1053/j.gastro.2018.03.038
- Ahern AL et al. Lancet. 2017;389(10085):2214-2225. doi:10.1016/S0140-6736(17)30647-5
- Newsome PN et al; NN9931-4296 Investigators. N Engl J Med. 2021;384(12):1113-1124. doi:10.1056/NEJMoa2028395
- Armstrong MJ et al. Lancet. 2016;387(10019):679-690. doi:10.1016/S0140-6736(15)00803-X
- Gastaldelli A et al. Lancet Diabetes Endocrinol. 2022;10(6):393-406. doi:10.1016/S2213-8587(22)00070-5
- Kahl S et al. Diabetes Care. 2020;43(2):298-305. doi:10.2337/dc19-0641
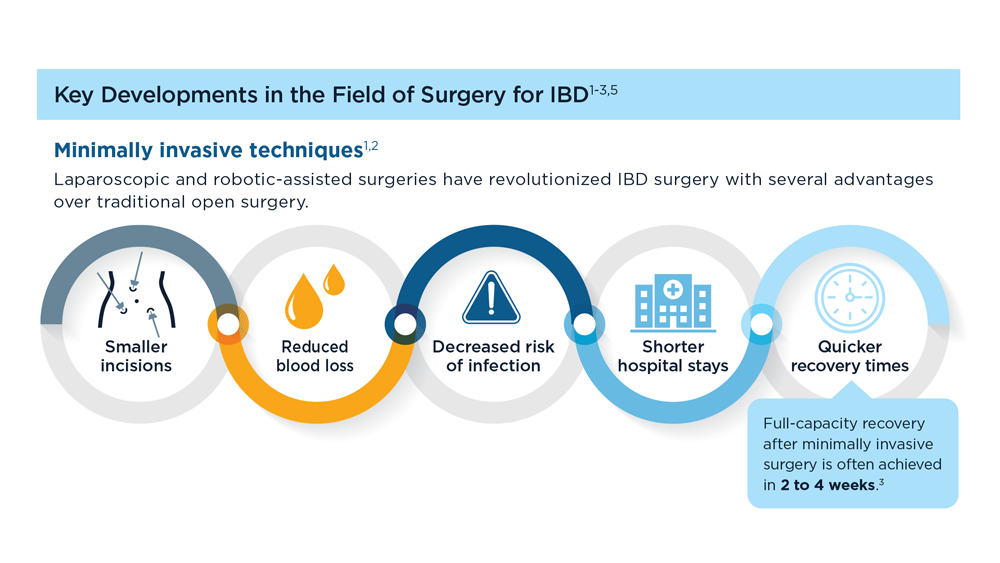
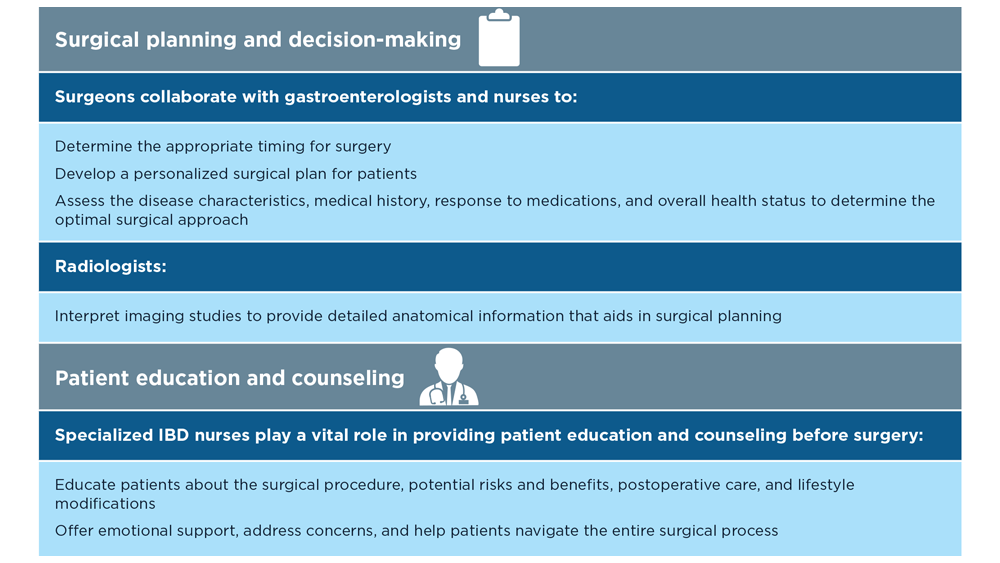
The Evolving Role of Surgery for IBD
- Gul F et al. Ann Med Surg (Lond). 2022;81:104476. doi:10.1016/j.amsu.2022.104476
- Kotze PG et al. Clin Colon Rectal Surg. 2021;34(3):172-180. doi:10.1055/s-0040-1718685
- Bemelman WA; S-ECCO collaborators. J Crohns Colitis. 2018;12(8):1005-1007. doi:10.1093/ecco-jcc/jjy056
- Ricci C et al. Dig Liver Dis. 2008;40(suppl 2):S285-S288. doi:10.1016/S1590-8658(08)60539-3
- Lin X et al. Therap Adv Gastroenterol. 2022;15:17562848221104951. doi:10.1177/17562848221104951
- Parigi TL et al. Dis Colon Rectum. 2022;65(suppl 1):S119-S128. doi:10.1097/DCR.0000000000002548
- Pilonis ND et al. Transl Gastroenterol Hepatol. 2022;7:7. doi:10.21037/tgh.2020.04.02
- Misawa M et al. Clin Endosc. 2021;54(4):455-463. doi:10.5946/ce.2021.165
- de Sousa HT et al. Curr Opin Gastroenterol. 2018;34(4):194-207. doi:10.1097/MOG.0000000000000440
- Whitehead A, Cataldo PA. Clin Colon Rectal Surg. 2017;30(3):162-171. doi:10.1055/s-0037-1598156
- Cannon LM. The use of enhanced recovery pathways in patients undergoing surgery for inflammatory bowel disease. In: Hyman N, Fleshner P, Strong S, eds Mastery of IBD Surgery. Chicago, IL: University of Chicago Press; 2019:29-38. doi:10.1007/978-3-030-16755-4_4
- Ljungqvist O et al. World J Surg. 2020;44(10):3197–3198. doi:10.1007/s00268-020-05734-5
- Gul F et al. Ann Med Surg (Lond). 2022;81:104476. doi:10.1016/j.amsu.2022.104476
- Kotze PG et al. Clin Colon Rectal Surg. 2021;34(3):172-180. doi:10.1055/s-0040-1718685
- Bemelman WA; S-ECCO collaborators. J Crohns Colitis. 2018;12(8):1005-1007. doi:10.1093/ecco-jcc/jjy056
- Ricci C et al. Dig Liver Dis. 2008;40(suppl 2):S285-S288. doi:10.1016/S1590-8658(08)60539-3
- Lin X et al. Therap Adv Gastroenterol. 2022;15:17562848221104951. doi:10.1177/17562848221104951
- Parigi TL et al. Dis Colon Rectum. 2022;65(suppl 1):S119-S128. doi:10.1097/DCR.0000000000002548
- Pilonis ND et al. Transl Gastroenterol Hepatol. 2022;7:7. doi:10.21037/tgh.2020.04.02
- Misawa M et al. Clin Endosc. 2021;54(4):455-463. doi:10.5946/ce.2021.165
- de Sousa HT et al. Curr Opin Gastroenterol. 2018;34(4):194-207. doi:10.1097/MOG.0000000000000440
- Whitehead A, Cataldo PA. Clin Colon Rectal Surg. 2017;30(3):162-171. doi:10.1055/s-0037-1598156
- Cannon LM. The use of enhanced recovery pathways in patients undergoing surgery for inflammatory bowel disease. In: Hyman N, Fleshner P, Strong S, eds Mastery of IBD Surgery. Chicago, IL: University of Chicago Press; 2019:29-38. doi:10.1007/978-3-030-16755-4_4
- Ljungqvist O et al. World J Surg. 2020;44(10):3197–3198. doi:10.1007/s00268-020-05734-5
- Gul F et al. Ann Med Surg (Lond). 2022;81:104476. doi:10.1016/j.amsu.2022.104476
- Kotze PG et al. Clin Colon Rectal Surg. 2021;34(3):172-180. doi:10.1055/s-0040-1718685
- Bemelman WA; S-ECCO collaborators. J Crohns Colitis. 2018;12(8):1005-1007. doi:10.1093/ecco-jcc/jjy056
- Ricci C et al. Dig Liver Dis. 2008;40(suppl 2):S285-S288. doi:10.1016/S1590-8658(08)60539-3
- Lin X et al. Therap Adv Gastroenterol. 2022;15:17562848221104951. doi:10.1177/17562848221104951
- Parigi TL et al. Dis Colon Rectum. 2022;65(suppl 1):S119-S128. doi:10.1097/DCR.0000000000002548
- Pilonis ND et al. Transl Gastroenterol Hepatol. 2022;7:7. doi:10.21037/tgh.2020.04.02
- Misawa M et al. Clin Endosc. 2021;54(4):455-463. doi:10.5946/ce.2021.165
- de Sousa HT et al. Curr Opin Gastroenterol. 2018;34(4):194-207. doi:10.1097/MOG.0000000000000440
- Whitehead A, Cataldo PA. Clin Colon Rectal Surg. 2017;30(3):162-171. doi:10.1055/s-0037-1598156
- Cannon LM. The use of enhanced recovery pathways in patients undergoing surgery for inflammatory bowel disease. In: Hyman N, Fleshner P, Strong S, eds Mastery of IBD Surgery. Chicago, IL: University of Chicago Press; 2019:29-38. doi:10.1007/978-3-030-16755-4_4
- Ljungqvist O et al. World J Surg. 2020;44(10):3197–3198. doi:10.1007/s00268-020-05734-5
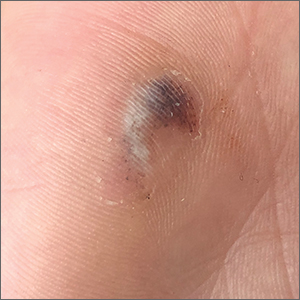
Hyperpigmented lesion on palm
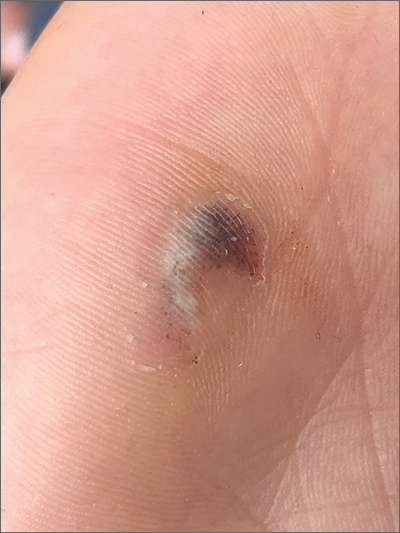
This patient had a posttraumatic tache noir (also known as talon noir on the volar aspect of the feet); it is a subcorneal hematoma. The diagnosis is made clinically. Dermoscopic evaluation of tache/talon noir will reveal “pebbles on a ridge” or “satellite globules.” Confirmation of tache/talon noir can be made by paring the corneum with a #15 blade, which will reveal blood in the shavings and punctate lesions.1
This patient noted that the knob of his baseball bat rubbed the hypothenar eminence of his nondominant hand when he took a swing. The sheer force of the knob led to the subcorneal hematoma. Tache noir was high on the differential due to his physician’s clinical experience with similar cases. Tache noir occurs predominantly in people ages 12 to 24 years, without regard to gender.2 The condition is commonly found in athletes who participate in baseball, cricket, racquet sports, weightlifting, and rock climbing.2-4
Talon noir occurs most commonly in athletes who are frequently jumping, turning, and pivoting, as in football, basketball, tennis, and lacrosse.
Tache noir can be differentiated from other conditions by the presence of preserved architecture of the skin surface and punctate capillaries beneath the stratum corneum. The differential diagnosis includes verruca vulgaris, acral melanoma, and a traumatic tattoo.
Talon/tache noir are benign conditions that do not require treatment and do not affect sports performance. The lesion will usually self-resolve within a matter of weeks from onset or can even be gently scraped with a sterile needle or blade.
This patient was advised that the lesion would resolve on its own. His knee pain was determined to be a simple case of patellofemoral syndrome or “runner’s knee” and he opted to complete a home exercise program to obtain relief.
This case was adapted from: Warden D. Hyperpigmented lesion on left palm. J Fam Pract. 2021;70:459-460. Photos courtesy of Daniel Warden, MD
1. Googe AB, Schulmeier JS, Jackson AR, et al. Talon noir: paring can eliminate the need for biopsy. Postgrad Med J. 2014;90:730-731. doi: 10.1136/postgradmedj-2014-132996
2. Burkhart C, Nguyen N. Talon noire. Dermatology Advisor. Accessed October 19, 2021. www.dermatologyadvisor.com/home/decision-support-in-medicine/dermatology/talon-noire-black-heel-calcaneal-petechiae-runners-heel-basketball-heel-tennis-heel-hyperkeratosis-hemorrhagica-pseudochromhidrosis-plantaris-chromidrose-plantaire-eccrine-intracorne/
3. Talon noir. Primary Care Dermatology Society. Updated August 1, 2021. Accessed October 19, 2021. www.pcds.org.uk/clinical-guidance/talon-noir
4. Birrer RB, Griesemer BA, Cataletto MB, eds. Pediatric Sports Medicine for Primary Care. Lippincott Williams & Wilkins; 2002.

This patient had a posttraumatic tache noir (also known as talon noir on the volar aspect of the feet); it is a subcorneal hematoma. The diagnosis is made clinically. Dermoscopic evaluation of tache/talon noir will reveal “pebbles on a ridge” or “satellite globules.” Confirmation of tache/talon noir can be made by paring the corneum with a #15 blade, which will reveal blood in the shavings and punctate lesions.1
This patient noted that the knob of his baseball bat rubbed the hypothenar eminence of his nondominant hand when he took a swing. The sheer force of the knob led to the subcorneal hematoma. Tache noir was high on the differential due to his physician’s clinical experience with similar cases. Tache noir occurs predominantly in people ages 12 to 24 years, without regard to gender.2 The condition is commonly found in athletes who participate in baseball, cricket, racquet sports, weightlifting, and rock climbing.2-4
Talon noir occurs most commonly in athletes who are frequently jumping, turning, and pivoting, as in football, basketball, tennis, and lacrosse.
Tache noir can be differentiated from other conditions by the presence of preserved architecture of the skin surface and punctate capillaries beneath the stratum corneum. The differential diagnosis includes verruca vulgaris, acral melanoma, and a traumatic tattoo.
Talon/tache noir are benign conditions that do not require treatment and do not affect sports performance. The lesion will usually self-resolve within a matter of weeks from onset or can even be gently scraped with a sterile needle or blade.
This patient was advised that the lesion would resolve on its own. His knee pain was determined to be a simple case of patellofemoral syndrome or “runner’s knee” and he opted to complete a home exercise program to obtain relief.
This case was adapted from: Warden D. Hyperpigmented lesion on left palm. J Fam Pract. 2021;70:459-460. Photos courtesy of Daniel Warden, MD

This patient had a posttraumatic tache noir (also known as talon noir on the volar aspect of the feet); it is a subcorneal hematoma. The diagnosis is made clinically. Dermoscopic evaluation of tache/talon noir will reveal “pebbles on a ridge” or “satellite globules.” Confirmation of tache/talon noir can be made by paring the corneum with a #15 blade, which will reveal blood in the shavings and punctate lesions.1
This patient noted that the knob of his baseball bat rubbed the hypothenar eminence of his nondominant hand when he took a swing. The sheer force of the knob led to the subcorneal hematoma. Tache noir was high on the differential due to his physician’s clinical experience with similar cases. Tache noir occurs predominantly in people ages 12 to 24 years, without regard to gender.2 The condition is commonly found in athletes who participate in baseball, cricket, racquet sports, weightlifting, and rock climbing.2-4
Talon noir occurs most commonly in athletes who are frequently jumping, turning, and pivoting, as in football, basketball, tennis, and lacrosse.
Tache noir can be differentiated from other conditions by the presence of preserved architecture of the skin surface and punctate capillaries beneath the stratum corneum. The differential diagnosis includes verruca vulgaris, acral melanoma, and a traumatic tattoo.
Talon/tache noir are benign conditions that do not require treatment and do not affect sports performance. The lesion will usually self-resolve within a matter of weeks from onset or can even be gently scraped with a sterile needle or blade.
This patient was advised that the lesion would resolve on its own. His knee pain was determined to be a simple case of patellofemoral syndrome or “runner’s knee” and he opted to complete a home exercise program to obtain relief.
This case was adapted from: Warden D. Hyperpigmented lesion on left palm. J Fam Pract. 2021;70:459-460. Photos courtesy of Daniel Warden, MD
1. Googe AB, Schulmeier JS, Jackson AR, et al. Talon noir: paring can eliminate the need for biopsy. Postgrad Med J. 2014;90:730-731. doi: 10.1136/postgradmedj-2014-132996
2. Burkhart C, Nguyen N. Talon noire. Dermatology Advisor. Accessed October 19, 2021. www.dermatologyadvisor.com/home/decision-support-in-medicine/dermatology/talon-noire-black-heel-calcaneal-petechiae-runners-heel-basketball-heel-tennis-heel-hyperkeratosis-hemorrhagica-pseudochromhidrosis-plantaris-chromidrose-plantaire-eccrine-intracorne/
3. Talon noir. Primary Care Dermatology Society. Updated August 1, 2021. Accessed October 19, 2021. www.pcds.org.uk/clinical-guidance/talon-noir
4. Birrer RB, Griesemer BA, Cataletto MB, eds. Pediatric Sports Medicine for Primary Care. Lippincott Williams & Wilkins; 2002.
1. Googe AB, Schulmeier JS, Jackson AR, et al. Talon noir: paring can eliminate the need for biopsy. Postgrad Med J. 2014;90:730-731. doi: 10.1136/postgradmedj-2014-132996
2. Burkhart C, Nguyen N. Talon noire. Dermatology Advisor. Accessed October 19, 2021. www.dermatologyadvisor.com/home/decision-support-in-medicine/dermatology/talon-noire-black-heel-calcaneal-petechiae-runners-heel-basketball-heel-tennis-heel-hyperkeratosis-hemorrhagica-pseudochromhidrosis-plantaris-chromidrose-plantaire-eccrine-intracorne/
3. Talon noir. Primary Care Dermatology Society. Updated August 1, 2021. Accessed October 19, 2021. www.pcds.org.uk/clinical-guidance/talon-noir
4. Birrer RB, Griesemer BA, Cataletto MB, eds. Pediatric Sports Medicine for Primary Care. Lippincott Williams & Wilkins; 2002.
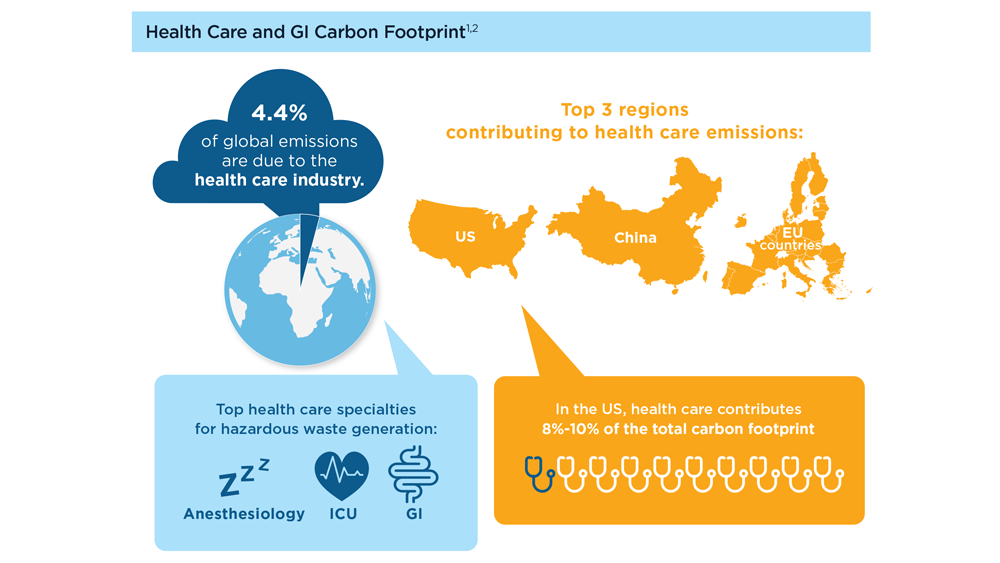
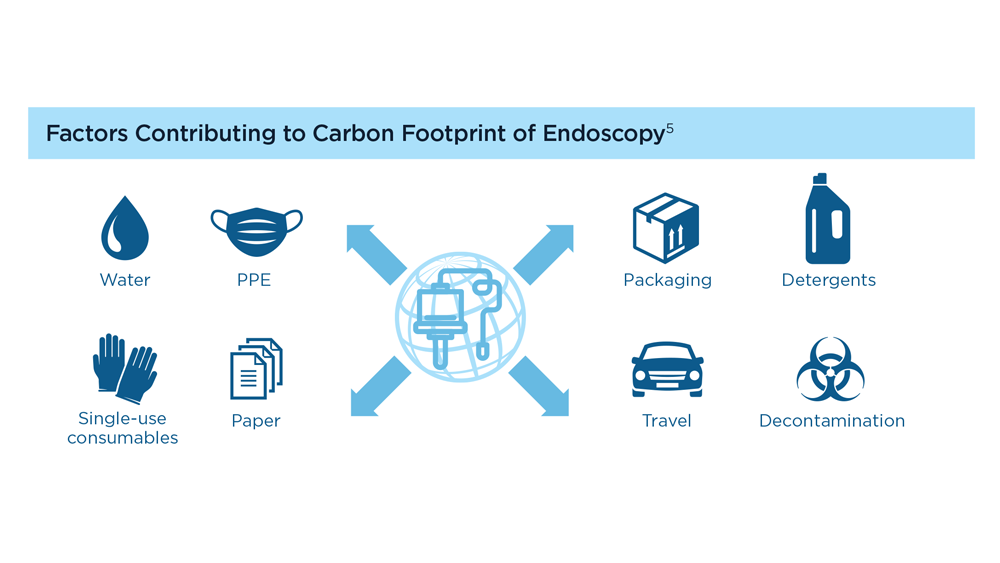
Gastroenterology and Climate Change: Assessing and Mitigating Impacts
- Karliner J et al. Eur J Public Health. 2020;30(suppl 5):v311. doi:10.1093/eurpub/ckaa165.843
- Vaccari M et al. Waste Manag Res. 2018;36(1):39-47. doi:10.1177/0734242X17739968
- Peery AF et al. Gastroenterology. 2019;156(1):254-272.e11. doi:10.1053/j.gastro.2018.08.063
- Sorge A et al. Endoscopy. 2023;55(suppl 2):S72-S73. https://www.esge.com/assets/downloads/pdfs/guidelines/ESGE_Days_2023.pdf
- Maurice JB et al. Lancet Gastroenterol Hepatol. 2020;5(7):636-638. doi:10.1016/S2468-1253(20)30157-6
- Gayam S. Am J Gastroenterol. 2020;115(12):1931-1932. doi:10.14309/ajg.0000000000001005
- Siau K et al. Tech Innov Gastrointest Endosc. 2021;23(4):344-352. doi:10.1016/j.tige.2021.06.005
- Namburar S et al. Gut. 2022;71(7):1326-1331. doi:10.1136/gutjnl-2021-324729
- Haddock R et al. Am J Gastroenterol. 2022;117(3):394-400. doi:10.14309/ajg.0000000000001604
- Donnelly MC et al. J Hepatol. 2022;76(5):995-1000. doi:10.1016/j.jhep.2022.02.01
- Leddin D, Macrae F. J Clin Gastroenterol. 2020;54(5):393-397. doi:10.1097/MCG.0000000000001336
- Pohl H et al. Hepatology. 2022;76(6):1836-1844. doi:10.1002/hep.32810
- Rodríguez de Santiago E et al. Endoscopy. 2022;54(8):797-826. doi:10.1055/a-1859-3726
- Sebastian S et al. Gut. 2023;72(1):12-26. doi:10.1136/gutjnl-2022-328460
- Cunha Neves JA et al. Gut. 2023;72(2):306-313. doi:10.1136/gutjnl-2022-327005
- Kaplan S et al. Issue Brief (Commonw Fund). 2012;29:1-14. PMID:23214181
- López-Muñoz P et al. Gut. 2023;gutjnl-2023-329544. doi:10.1136/gutjnl-2023-329544
- Karliner J et al. Eur J Public Health. 2020;30(suppl 5):v311. doi:10.1093/eurpub/ckaa165.843
- Vaccari M et al. Waste Manag Res. 2018;36(1):39-47. doi:10.1177/0734242X17739968
- Peery AF et al. Gastroenterology. 2019;156(1):254-272.e11. doi:10.1053/j.gastro.2018.08.063
- Sorge A et al. Endoscopy. 2023;55(suppl 2):S72-S73. https://www.esge.com/assets/downloads/pdfs/guidelines/ESGE_Days_2023.pdf
- Maurice JB et al. Lancet Gastroenterol Hepatol. 2020;5(7):636-638. doi:10.1016/S2468-1253(20)30157-6
- Gayam S. Am J Gastroenterol. 2020;115(12):1931-1932. doi:10.14309/ajg.0000000000001005
- Siau K et al. Tech Innov Gastrointest Endosc. 2021;23(4):344-352. doi:10.1016/j.tige.2021.06.005
- Namburar S et al. Gut. 2022;71(7):1326-1331. doi:10.1136/gutjnl-2021-324729
- Haddock R et al. Am J Gastroenterol. 2022;117(3):394-400. doi:10.14309/ajg.0000000000001604
- Donnelly MC et al. J Hepatol. 2022;76(5):995-1000. doi:10.1016/j.jhep.2022.02.01
- Leddin D, Macrae F. J Clin Gastroenterol. 2020;54(5):393-397. doi:10.1097/MCG.0000000000001336
- Pohl H et al. Hepatology. 2022;76(6):1836-1844. doi:10.1002/hep.32810
- Rodríguez de Santiago E et al. Endoscopy. 2022;54(8):797-826. doi:10.1055/a-1859-3726
- Sebastian S et al. Gut. 2023;72(1):12-26. doi:10.1136/gutjnl-2022-328460
- Cunha Neves JA et al. Gut. 2023;72(2):306-313. doi:10.1136/gutjnl-2022-327005
- Kaplan S et al. Issue Brief (Commonw Fund). 2012;29:1-14. PMID:23214181
- López-Muñoz P et al. Gut. 2023;gutjnl-2023-329544. doi:10.1136/gutjnl-2023-329544
- Karliner J et al. Eur J Public Health. 2020;30(suppl 5):v311. doi:10.1093/eurpub/ckaa165.843
- Vaccari M et al. Waste Manag Res. 2018;36(1):39-47. doi:10.1177/0734242X17739968
- Peery AF et al. Gastroenterology. 2019;156(1):254-272.e11. doi:10.1053/j.gastro.2018.08.063
- Sorge A et al. Endoscopy. 2023;55(suppl 2):S72-S73. https://www.esge.com/assets/downloads/pdfs/guidelines/ESGE_Days_2023.pdf
- Maurice JB et al. Lancet Gastroenterol Hepatol. 2020;5(7):636-638. doi:10.1016/S2468-1253(20)30157-6
- Gayam S. Am J Gastroenterol. 2020;115(12):1931-1932. doi:10.14309/ajg.0000000000001005
- Siau K et al. Tech Innov Gastrointest Endosc. 2021;23(4):344-352. doi:10.1016/j.tige.2021.06.005
- Namburar S et al. Gut. 2022;71(7):1326-1331. doi:10.1136/gutjnl-2021-324729
- Haddock R et al. Am J Gastroenterol. 2022;117(3):394-400. doi:10.14309/ajg.0000000000001604
- Donnelly MC et al. J Hepatol. 2022;76(5):995-1000. doi:10.1016/j.jhep.2022.02.01
- Leddin D, Macrae F. J Clin Gastroenterol. 2020;54(5):393-397. doi:10.1097/MCG.0000000000001336
- Pohl H et al. Hepatology. 2022;76(6):1836-1844. doi:10.1002/hep.32810
- Rodríguez de Santiago E et al. Endoscopy. 2022;54(8):797-826. doi:10.1055/a-1859-3726
- Sebastian S et al. Gut. 2023;72(1):12-26. doi:10.1136/gutjnl-2022-328460
- Cunha Neves JA et al. Gut. 2023;72(2):306-313. doi:10.1136/gutjnl-2022-327005
- Kaplan S et al. Issue Brief (Commonw Fund). 2012;29:1-14. PMID:23214181
- López-Muñoz P et al. Gut. 2023;gutjnl-2023-329544. doi:10.1136/gutjnl-2023-329544