User login
Anticoagulation Hub contains news and clinical review articles for physicians seeking the most up-to-date information on the rapidly evolving treatment options for preventing stroke, acute coronary events, deep vein thrombosis, and pulmonary embolism in at-risk patients. The Anticoagulation Hub is powered by Frontline Medical Communications.
A look at top upcoming clinical trials in electrophysiology
SAN DIEGO – How to prevent sudden arrhythmic death in vulnerable but currently unprotected populations is being addressed by ongoing studies that variously evaluate a pharmacologic, an implanted device-based, or a wearable solution, according to Dr. Bruce D. Lindsay.
Dr. Lindsay, head of the cardiac electrophysiology and pacing section at the Cleveland Clinic, presented an overview of selected major ongoing clinical trials in electrophysiology at the annual meeting of the American College of Cardiology. He focused on five hot topics: prevention of sudden death, atrial fibrillation (AF) ablation, prevention of implantable device-related infections, device-based treatment of heart failure, and leadless pacing systems.
Preventing sudden death
Ongoing phase III trials are evaluating the safety, tolerability, and efficacy of an oral Gilead drug known for now as GS-6615. The drug, a selective late sodium current inhibitor, is designed to shorten the corrected QTc interval in patients with several forms of long QT syndrome.
A different approach is being studied in REFINE-ICD (Risk Estimation Following Infarction Noninvasive Evaluation – ICD Efficacy), a 1,400-subject trial recruiting patients who’ve had an acute MI within the previous year, have abnormal findings on 24-hour Holter monitoring, and have moderate left ventricular dysfunction as defined by an ejection fraction of 35%-50%. The trial will assess whether prophylactic placement of an implantable cardioverter-defibrillator (ICD) guided by noninvasive risk assessment based on heart rate turbulence and T-wave alternans analysis will reduce mortality in MI survivors.
“This is a group that’s at lower risk than current ICD recipients, but because it’s such a large group they account for a lot of sudden deaths,” the cardiologist said.
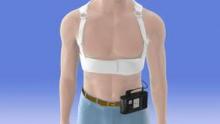
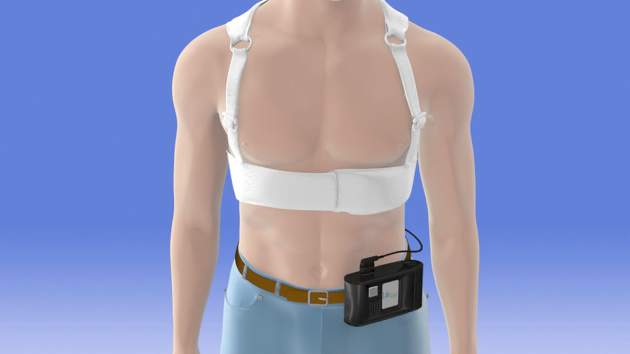
Another phase III trial currently recruiting participants is a 1,900-patient postmarketing study aimed at defining which patients benefit from using the LifeVest wearable defibrillator during the first 3 months following an acute MI resulting in ventricular dysfunction.
AF ablation
The most important ongoing study in this field, in Dr. Lindsay’s view, is CABANA (Catheter Ablation Versus Anti-Arrhythmic Drug Therapy for Atrial Fibrillation Trial). This National Institutes of Health–sponsored study is aimed at showing whether ablation is superior to rate or rhythm control drug therapy in terms of all-cause mortality, disabling stroke, serious bleeding, and/or cardiac arrest. Secondary endpoints include cost, quality of life, hospitalization rates, and the relationship of left atrial size to progression of AF and its contribution to morbidity and mortality.
A promising, innovative ablation strategy known as focal impulse and rotor ablation for paroxysmal AF is under evaluation in the German REAFFIRM (Randomized Evaluation of Atrial Fibrillation Treatment With Focal Impulse and Rotor Modulation Guided Procedures). A similar U.S. study known as FIRMAT-PAF (Focal Impulse and Rotor Modulation Ablation Trial for Treatment of Paroxysmal Atrial Fibrillation) had to be abandoned, however, because of its inability to recruit patients.
New ablation technologies are also being introduced. Three pivotal trials totaling roughly 1,500 patients are evaluating the Biosense Webster nMARQ multielectrode irrigated catheter for paroxysmal AF in the reMARQable trial; a Medtronic phased radiofrequency ablation catheter for persistent AF in the VICTORY AF study; and a CardioFocus endoscopic ablation catheter for paroxysmal AF.
Preventing cardiac implantable device infections
WRAP-IT (the World-Wide Randomized Antibiotic Envelope Infection Trial) is currently enrolling 7,000 patients at 225 sites. This is a Merck-sponsored randomized, prospective, single-blind postmarketing study examining the ability of a proprietary mesh envelope to reduce major infections and costs in the 12 months following device generator replacement, upgrade, or revision, or new implantation of a cardiac resynchronization device. The Tyrx mesh envelope releases minocycline and rifampin for at least 7 days, then eventually becomes fully absorbed.
“Device infection is a huge problem in our field,” Dr. Lindsay noted. “This envelope may have important implications at large, or it may prove to be especially useful in people at high risk for infection.”
Heart failure
Vagal nerve stimulation via an implantable system is one of the hottest areas in the field of heart failure, the electrophysiologist said. Two major trials are ongoing: the Sorin-sponsored VANGUARD (Vagal Nerve Stimulation: Safeguarding Heart Failure Patients) trial, and INNOVATE HF (Increase of Vagal Tone in CHF), sponsored by Biocontrol Medical.
Leadless pacing
St. Jude’s Nanostim and Medtronic’s Micra are very small leadless devices implanted in the right ventricular apex via minimally invasive techniques. Both devices are investigational in the United States, although the Nanostim is approved in Europe. Clinical interest is enormous because lead-related problems have always been the Achilles’ heel of pacemaker therapy. While the Nanostim and Micra can be utilized only for single right ventricular pacing in VVI or VVIR mode, Dr. Lindsay said further advances, including leadless dual-chamber sensing and pacing and biventricular pacing, are likely.
He reported serving as a consultant to Biosense Webster, Boston Scientific, and Medtronic.
SAN DIEGO – How to prevent sudden arrhythmic death in vulnerable but currently unprotected populations is being addressed by ongoing studies that variously evaluate a pharmacologic, an implanted device-based, or a wearable solution, according to Dr. Bruce D. Lindsay.
Dr. Lindsay, head of the cardiac electrophysiology and pacing section at the Cleveland Clinic, presented an overview of selected major ongoing clinical trials in electrophysiology at the annual meeting of the American College of Cardiology. He focused on five hot topics: prevention of sudden death, atrial fibrillation (AF) ablation, prevention of implantable device-related infections, device-based treatment of heart failure, and leadless pacing systems.
Preventing sudden death
Ongoing phase III trials are evaluating the safety, tolerability, and efficacy of an oral Gilead drug known for now as GS-6615. The drug, a selective late sodium current inhibitor, is designed to shorten the corrected QTc interval in patients with several forms of long QT syndrome.
A different approach is being studied in REFINE-ICD (Risk Estimation Following Infarction Noninvasive Evaluation – ICD Efficacy), a 1,400-subject trial recruiting patients who’ve had an acute MI within the previous year, have abnormal findings on 24-hour Holter monitoring, and have moderate left ventricular dysfunction as defined by an ejection fraction of 35%-50%. The trial will assess whether prophylactic placement of an implantable cardioverter-defibrillator (ICD) guided by noninvasive risk assessment based on heart rate turbulence and T-wave alternans analysis will reduce mortality in MI survivors.
“This is a group that’s at lower risk than current ICD recipients, but because it’s such a large group they account for a lot of sudden deaths,” the cardiologist said.
Another phase III trial currently recruiting participants is a 1,900-patient postmarketing study aimed at defining which patients benefit from using the LifeVest wearable defibrillator during the first 3 months following an acute MI resulting in ventricular dysfunction.
AF ablation
The most important ongoing study in this field, in Dr. Lindsay’s view, is CABANA (Catheter Ablation Versus Anti-Arrhythmic Drug Therapy for Atrial Fibrillation Trial). This National Institutes of Health–sponsored study is aimed at showing whether ablation is superior to rate or rhythm control drug therapy in terms of all-cause mortality, disabling stroke, serious bleeding, and/or cardiac arrest. Secondary endpoints include cost, quality of life, hospitalization rates, and the relationship of left atrial size to progression of AF and its contribution to morbidity and mortality.
A promising, innovative ablation strategy known as focal impulse and rotor ablation for paroxysmal AF is under evaluation in the German REAFFIRM (Randomized Evaluation of Atrial Fibrillation Treatment With Focal Impulse and Rotor Modulation Guided Procedures). A similar U.S. study known as FIRMAT-PAF (Focal Impulse and Rotor Modulation Ablation Trial for Treatment of Paroxysmal Atrial Fibrillation) had to be abandoned, however, because of its inability to recruit patients.
New ablation technologies are also being introduced. Three pivotal trials totaling roughly 1,500 patients are evaluating the Biosense Webster nMARQ multielectrode irrigated catheter for paroxysmal AF in the reMARQable trial; a Medtronic phased radiofrequency ablation catheter for persistent AF in the VICTORY AF study; and a CardioFocus endoscopic ablation catheter for paroxysmal AF.
Preventing cardiac implantable device infections
WRAP-IT (the World-Wide Randomized Antibiotic Envelope Infection Trial) is currently enrolling 7,000 patients at 225 sites. This is a Merck-sponsored randomized, prospective, single-blind postmarketing study examining the ability of a proprietary mesh envelope to reduce major infections and costs in the 12 months following device generator replacement, upgrade, or revision, or new implantation of a cardiac resynchronization device. The Tyrx mesh envelope releases minocycline and rifampin for at least 7 days, then eventually becomes fully absorbed.
“Device infection is a huge problem in our field,” Dr. Lindsay noted. “This envelope may have important implications at large, or it may prove to be especially useful in people at high risk for infection.”
Heart failure
Vagal nerve stimulation via an implantable system is one of the hottest areas in the field of heart failure, the electrophysiologist said. Two major trials are ongoing: the Sorin-sponsored VANGUARD (Vagal Nerve Stimulation: Safeguarding Heart Failure Patients) trial, and INNOVATE HF (Increase of Vagal Tone in CHF), sponsored by Biocontrol Medical.
Leadless pacing
St. Jude’s Nanostim and Medtronic’s Micra are very small leadless devices implanted in the right ventricular apex via minimally invasive techniques. Both devices are investigational in the United States, although the Nanostim is approved in Europe. Clinical interest is enormous because lead-related problems have always been the Achilles’ heel of pacemaker therapy. While the Nanostim and Micra can be utilized only for single right ventricular pacing in VVI or VVIR mode, Dr. Lindsay said further advances, including leadless dual-chamber sensing and pacing and biventricular pacing, are likely.
He reported serving as a consultant to Biosense Webster, Boston Scientific, and Medtronic.
SAN DIEGO – How to prevent sudden arrhythmic death in vulnerable but currently unprotected populations is being addressed by ongoing studies that variously evaluate a pharmacologic, an implanted device-based, or a wearable solution, according to Dr. Bruce D. Lindsay.
Dr. Lindsay, head of the cardiac electrophysiology and pacing section at the Cleveland Clinic, presented an overview of selected major ongoing clinical trials in electrophysiology at the annual meeting of the American College of Cardiology. He focused on five hot topics: prevention of sudden death, atrial fibrillation (AF) ablation, prevention of implantable device-related infections, device-based treatment of heart failure, and leadless pacing systems.
Preventing sudden death
Ongoing phase III trials are evaluating the safety, tolerability, and efficacy of an oral Gilead drug known for now as GS-6615. The drug, a selective late sodium current inhibitor, is designed to shorten the corrected QTc interval in patients with several forms of long QT syndrome.
A different approach is being studied in REFINE-ICD (Risk Estimation Following Infarction Noninvasive Evaluation – ICD Efficacy), a 1,400-subject trial recruiting patients who’ve had an acute MI within the previous year, have abnormal findings on 24-hour Holter monitoring, and have moderate left ventricular dysfunction as defined by an ejection fraction of 35%-50%. The trial will assess whether prophylactic placement of an implantable cardioverter-defibrillator (ICD) guided by noninvasive risk assessment based on heart rate turbulence and T-wave alternans analysis will reduce mortality in MI survivors.
“This is a group that’s at lower risk than current ICD recipients, but because it’s such a large group they account for a lot of sudden deaths,” the cardiologist said.
Another phase III trial currently recruiting participants is a 1,900-patient postmarketing study aimed at defining which patients benefit from using the LifeVest wearable defibrillator during the first 3 months following an acute MI resulting in ventricular dysfunction.
AF ablation
The most important ongoing study in this field, in Dr. Lindsay’s view, is CABANA (Catheter Ablation Versus Anti-Arrhythmic Drug Therapy for Atrial Fibrillation Trial). This National Institutes of Health–sponsored study is aimed at showing whether ablation is superior to rate or rhythm control drug therapy in terms of all-cause mortality, disabling stroke, serious bleeding, and/or cardiac arrest. Secondary endpoints include cost, quality of life, hospitalization rates, and the relationship of left atrial size to progression of AF and its contribution to morbidity and mortality.
A promising, innovative ablation strategy known as focal impulse and rotor ablation for paroxysmal AF is under evaluation in the German REAFFIRM (Randomized Evaluation of Atrial Fibrillation Treatment With Focal Impulse and Rotor Modulation Guided Procedures). A similar U.S. study known as FIRMAT-PAF (Focal Impulse and Rotor Modulation Ablation Trial for Treatment of Paroxysmal Atrial Fibrillation) had to be abandoned, however, because of its inability to recruit patients.
New ablation technologies are also being introduced. Three pivotal trials totaling roughly 1,500 patients are evaluating the Biosense Webster nMARQ multielectrode irrigated catheter for paroxysmal AF in the reMARQable trial; a Medtronic phased radiofrequency ablation catheter for persistent AF in the VICTORY AF study; and a CardioFocus endoscopic ablation catheter for paroxysmal AF.
Preventing cardiac implantable device infections
WRAP-IT (the World-Wide Randomized Antibiotic Envelope Infection Trial) is currently enrolling 7,000 patients at 225 sites. This is a Merck-sponsored randomized, prospective, single-blind postmarketing study examining the ability of a proprietary mesh envelope to reduce major infections and costs in the 12 months following device generator replacement, upgrade, or revision, or new implantation of a cardiac resynchronization device. The Tyrx mesh envelope releases minocycline and rifampin for at least 7 days, then eventually becomes fully absorbed.
“Device infection is a huge problem in our field,” Dr. Lindsay noted. “This envelope may have important implications at large, or it may prove to be especially useful in people at high risk for infection.”
Heart failure
Vagal nerve stimulation via an implantable system is one of the hottest areas in the field of heart failure, the electrophysiologist said. Two major trials are ongoing: the Sorin-sponsored VANGUARD (Vagal Nerve Stimulation: Safeguarding Heart Failure Patients) trial, and INNOVATE HF (Increase of Vagal Tone in CHF), sponsored by Biocontrol Medical.
Leadless pacing
St. Jude’s Nanostim and Medtronic’s Micra are very small leadless devices implanted in the right ventricular apex via minimally invasive techniques. Both devices are investigational in the United States, although the Nanostim is approved in Europe. Clinical interest is enormous because lead-related problems have always been the Achilles’ heel of pacemaker therapy. While the Nanostim and Micra can be utilized only for single right ventricular pacing in VVI or VVIR mode, Dr. Lindsay said further advances, including leadless dual-chamber sensing and pacing and biventricular pacing, are likely.
He reported serving as a consultant to Biosense Webster, Boston Scientific, and Medtronic.
EXPERT ANALYSIS FROM ACC 15
Form of catheter-directed thrombolysis cured patients of submassive PEs
Ultrasound-accelerated catheter-directed thrombolysis (USAT) was successful at treating acute submassive pulmonary embolisms, according to a retrospective study.
Acute pulmonary hypertension and right ventricular dysfunction (RVD), on average, became significantly less severe in all of the study’s 45 participants. Specifically, main pulmonary artery pressure decreased to 31.1 mm Hg from 49.8 mm Hg. The improvement in RVD was demonstrated by a decreased right ventricle-to-left-ventricle ratio to 0.93 from 1.59.
Although no complications occurred as a result of catheter placement, six complications resulted from other causes. Those complications included four minor venous access-site hemorrhagic complications and two major bleeding complications: a flank hematoma and an arm hematoma.
“USAT is a safe and efficacious method of treatment of submassive PE to reduce acute pulmonary hypertension and RVD. Future studies should be aimed at examining the long-term effect of USAT on mortality, exercise tolerance, and pulmonary hypertension,” wrote Dr. Sandeep Bagla of the Inova Alexandria (Va.) Hospital and his colleagues.
Find the full study in the Journal of Vascular and Interventional Radiology (doi:10.1016/j.jvir.2014.12.017).
Ultrasound-accelerated catheter-directed thrombolysis (USAT) was successful at treating acute submassive pulmonary embolisms, according to a retrospective study.
Acute pulmonary hypertension and right ventricular dysfunction (RVD), on average, became significantly less severe in all of the study’s 45 participants. Specifically, main pulmonary artery pressure decreased to 31.1 mm Hg from 49.8 mm Hg. The improvement in RVD was demonstrated by a decreased right ventricle-to-left-ventricle ratio to 0.93 from 1.59.
Although no complications occurred as a result of catheter placement, six complications resulted from other causes. Those complications included four minor venous access-site hemorrhagic complications and two major bleeding complications: a flank hematoma and an arm hematoma.
“USAT is a safe and efficacious method of treatment of submassive PE to reduce acute pulmonary hypertension and RVD. Future studies should be aimed at examining the long-term effect of USAT on mortality, exercise tolerance, and pulmonary hypertension,” wrote Dr. Sandeep Bagla of the Inova Alexandria (Va.) Hospital and his colleagues.
Find the full study in the Journal of Vascular and Interventional Radiology (doi:10.1016/j.jvir.2014.12.017).
Ultrasound-accelerated catheter-directed thrombolysis (USAT) was successful at treating acute submassive pulmonary embolisms, according to a retrospective study.
Acute pulmonary hypertension and right ventricular dysfunction (RVD), on average, became significantly less severe in all of the study’s 45 participants. Specifically, main pulmonary artery pressure decreased to 31.1 mm Hg from 49.8 mm Hg. The improvement in RVD was demonstrated by a decreased right ventricle-to-left-ventricle ratio to 0.93 from 1.59.
Although no complications occurred as a result of catheter placement, six complications resulted from other causes. Those complications included four minor venous access-site hemorrhagic complications and two major bleeding complications: a flank hematoma and an arm hematoma.
“USAT is a safe and efficacious method of treatment of submassive PE to reduce acute pulmonary hypertension and RVD. Future studies should be aimed at examining the long-term effect of USAT on mortality, exercise tolerance, and pulmonary hypertension,” wrote Dr. Sandeep Bagla of the Inova Alexandria (Va.) Hospital and his colleagues.
Find the full study in the Journal of Vascular and Interventional Radiology (doi:10.1016/j.jvir.2014.12.017).
Two genes identified as VTE risk loci
A meta-analysis has identified two genes as susceptibility loci for venous thromboembolism, according to Marine Germain of the Institute for Cardiometabolism and Nutrition, Paris, and associates.
The identified risk loci were TSPAN15 and SLC44A2, with the odds ratios for VTE at 1.31 and 1.21, respectively. The most significant single-nucleotide polymorphism for the TSPAN15 loci was the intronic rs78707713; for SLC44A2, the most significant SNP was the nonsynonymous rs2288904, with ORs of 1.42 and 1.28, respectively. Although these associations are not strong, statistical evidence was much more convincing in the discovery and replication stages, the researchers reported.
“The identified VTE-associated SNPs map to genes that are not in conventional pathways to thrombosis that have marked most of the genetic associations to date, suggesting that these genetic variants represent novel biological pathways leading to VTE,” the investigators wrote.
Find the full study in the American Journal of Human Genetics (2015 April 2 [doi:10.1016/j.ajhg.2015.01.019]).
A meta-analysis has identified two genes as susceptibility loci for venous thromboembolism, according to Marine Germain of the Institute for Cardiometabolism and Nutrition, Paris, and associates.
The identified risk loci were TSPAN15 and SLC44A2, with the odds ratios for VTE at 1.31 and 1.21, respectively. The most significant single-nucleotide polymorphism for the TSPAN15 loci was the intronic rs78707713; for SLC44A2, the most significant SNP was the nonsynonymous rs2288904, with ORs of 1.42 and 1.28, respectively. Although these associations are not strong, statistical evidence was much more convincing in the discovery and replication stages, the researchers reported.
“The identified VTE-associated SNPs map to genes that are not in conventional pathways to thrombosis that have marked most of the genetic associations to date, suggesting that these genetic variants represent novel biological pathways leading to VTE,” the investigators wrote.
Find the full study in the American Journal of Human Genetics (2015 April 2 [doi:10.1016/j.ajhg.2015.01.019]).
A meta-analysis has identified two genes as susceptibility loci for venous thromboembolism, according to Marine Germain of the Institute for Cardiometabolism and Nutrition, Paris, and associates.
The identified risk loci were TSPAN15 and SLC44A2, with the odds ratios for VTE at 1.31 and 1.21, respectively. The most significant single-nucleotide polymorphism for the TSPAN15 loci was the intronic rs78707713; for SLC44A2, the most significant SNP was the nonsynonymous rs2288904, with ORs of 1.42 and 1.28, respectively. Although these associations are not strong, statistical evidence was much more convincing in the discovery and replication stages, the researchers reported.
“The identified VTE-associated SNPs map to genes that are not in conventional pathways to thrombosis that have marked most of the genetic associations to date, suggesting that these genetic variants represent novel biological pathways leading to VTE,” the investigators wrote.
Find the full study in the American Journal of Human Genetics (2015 April 2 [doi:10.1016/j.ajhg.2015.01.019]).
Study: More DVTs than expected in patients who had varicose vein surgeries
A retrospective study of patients who underwent varicose vein surgeries with a tourniquet found a greater incidence of deep vein thromboses (DVTs) than previous studies.
Within the first 3 postoperative days, 113 (7.7%) of the 1,461 patients had DVTs. The researchers also found that DVTs occurred significantly more often in patients with gastrocnemius vein dilation (GVD). A total of 410 (28%) of the study’s participants had GVTs, and the incidence of DVTs was significantly greater in individuals with GVD compared to those without such a symptom. GVD had a higher predictive power for postoperative DVT than did all of the other risk factors examined in univariate and multivariate analyses.
The vast majority of the DVTs diagnosed were isolated distal. While 94 patients suffered from this kind of DVT, the remaining 19 DVTs were proximal. According to Dr. Chen Kai of Wenzhou (China) Medical University, and colleagues, proximal DVTs were nearly always asymptomatic and a larger percentage of them took more time to disappear than did the distal DVTs. Within 6 months following anticoagulant therapy, 94.3% of the distal DVTs exhibited thrombus resolution and 55.6% of the proximal DVTs were thrombus free. None of the study’s participants had died because of DVT or pulmonary embolus during the 6 months following their surgeries.
This study’s “present data reflect a higher incidence of postoperative DVT than previous studies, and we also identify GVD as a significant risk factor. Larger prospective studies will be needed to evaluate this issue precisely and to understand the clinical relevance of these results,” wrote the researchers.Find the full study in Thombosis Research (doi: 10.1016/j.thromres.2015.03.008).
A retrospective study of patients who underwent varicose vein surgeries with a tourniquet found a greater incidence of deep vein thromboses (DVTs) than previous studies.
Within the first 3 postoperative days, 113 (7.7%) of the 1,461 patients had DVTs. The researchers also found that DVTs occurred significantly more often in patients with gastrocnemius vein dilation (GVD). A total of 410 (28%) of the study’s participants had GVTs, and the incidence of DVTs was significantly greater in individuals with GVD compared to those without such a symptom. GVD had a higher predictive power for postoperative DVT than did all of the other risk factors examined in univariate and multivariate analyses.
The vast majority of the DVTs diagnosed were isolated distal. While 94 patients suffered from this kind of DVT, the remaining 19 DVTs were proximal. According to Dr. Chen Kai of Wenzhou (China) Medical University, and colleagues, proximal DVTs were nearly always asymptomatic and a larger percentage of them took more time to disappear than did the distal DVTs. Within 6 months following anticoagulant therapy, 94.3% of the distal DVTs exhibited thrombus resolution and 55.6% of the proximal DVTs were thrombus free. None of the study’s participants had died because of DVT or pulmonary embolus during the 6 months following their surgeries.
This study’s “present data reflect a higher incidence of postoperative DVT than previous studies, and we also identify GVD as a significant risk factor. Larger prospective studies will be needed to evaluate this issue precisely and to understand the clinical relevance of these results,” wrote the researchers.Find the full study in Thombosis Research (doi: 10.1016/j.thromres.2015.03.008).
A retrospective study of patients who underwent varicose vein surgeries with a tourniquet found a greater incidence of deep vein thromboses (DVTs) than previous studies.
Within the first 3 postoperative days, 113 (7.7%) of the 1,461 patients had DVTs. The researchers also found that DVTs occurred significantly more often in patients with gastrocnemius vein dilation (GVD). A total of 410 (28%) of the study’s participants had GVTs, and the incidence of DVTs was significantly greater in individuals with GVD compared to those without such a symptom. GVD had a higher predictive power for postoperative DVT than did all of the other risk factors examined in univariate and multivariate analyses.
The vast majority of the DVTs diagnosed were isolated distal. While 94 patients suffered from this kind of DVT, the remaining 19 DVTs were proximal. According to Dr. Chen Kai of Wenzhou (China) Medical University, and colleagues, proximal DVTs were nearly always asymptomatic and a larger percentage of them took more time to disappear than did the distal DVTs. Within 6 months following anticoagulant therapy, 94.3% of the distal DVTs exhibited thrombus resolution and 55.6% of the proximal DVTs were thrombus free. None of the study’s participants had died because of DVT or pulmonary embolus during the 6 months following their surgeries.
This study’s “present data reflect a higher incidence of postoperative DVT than previous studies, and we also identify GVD as a significant risk factor. Larger prospective studies will be needed to evaluate this issue precisely and to understand the clinical relevance of these results,” wrote the researchers.Find the full study in Thombosis Research (doi: 10.1016/j.thromres.2015.03.008).
VTE with transient risk factors is being overtreated
After a first episode of venous thromboembolism, more than 40% of patients with transient risk factors underwent anticoagulation therapy for 12 months or longer – a duration at least four times longer than the period recommended in guidelines, said authors of a large prospective cohort study.
Patients with VTE associated with surgery had about a 0.7% risk/patient-year of recurrence after 3 months of anticoagulation therapy. Patients with transient nonsurgical risk factors had about a 4% risk/patient-year of VTE recurrence.
Further, these patients were more likely to have major bleeding events than recurrent VTEs and were more likely to die of a fatal bleed than from a recurrent pulmonary embolism. Additionally, 38% of major bleeds among patients with transient risk factors occurred during the first 3 months of anticoagulation therapy.
“Our data suggest that in real life, physicians appear to be more concerned about the risk of recurrent VTE after discontinuing therapy than about the risk of bleeding,” said Dr. Walter Ageno at the University of Insubria in Varese, Italy, and his associates. “Clinicians base their treatment decisions on individual risk stratification, taking into account the location of VTE and the presence of additional risk factors for recurrence and bleeding. However, before adequately validated clinical prediction rules become available, this approach may expose a substantial proportion of patients, in particular those with VTE secondary to transient risk factors, to a possibly unnecessary risk of bleeding.”
The American College of Chest Physicians recommends 3 months of anticoagulation therapy for patients with VTE secondary to surgery or a transient, nonsurgical risk factor, and extended (possibly indefinite) anticoagulation for patients with unprovoked VTE or VTE caused by cancer. To look at real-world practice, the researchers carried out a prospective cohort study of 6,944 VTE patients in Italy, Spain, and Belgium. In all, 32% of patients had transient risk factors, 41% had unprovoked VTE, and 27% had cancer (Thrombosis Res. 2015;135:666-72). After excluding patients who died within a year after VTE, 42% of patients with transient risk factors such as recent surgery, pregnancy, or prolonged travel were treated with anticoagulants for more than 12 months, the researchers reported. Significant predictors of extended anticoagulation treatment including being older than 65 years old, having chronic heart failure, pulmonary embolism at presentation, and recurrent VTE during anticoagulation, the researchers also reported. Patients who weighed less than 75 kg, had anemia, or had transient risk factors for VTE were less likely to undergo prolonged treatment than were other patients.
“There is still uncertainty among experts on the optimal duration of secondary prevention of VTE,” concluded the investigators. “This decision should be taken by balancing the risk of recurrence after stopping treatment with the risk of bleeding if treatment is continued.”
Adequately validated clinical prediction rules are needed to make those decisions, the researchers said. Until such tools are validated, a substantial proportion of patients with transiet and secondary risk factors for VTE may be exposed to a possibly unnecessary risk of bleeding, they concluded.
Sanofi Spain and Bayer Pharma AG funded the study. The investigators reported having no relevant conflicts of interest.
After a first episode of venous thromboembolism, more than 40% of patients with transient risk factors underwent anticoagulation therapy for 12 months or longer – a duration at least four times longer than the period recommended in guidelines, said authors of a large prospective cohort study.
Patients with VTE associated with surgery had about a 0.7% risk/patient-year of recurrence after 3 months of anticoagulation therapy. Patients with transient nonsurgical risk factors had about a 4% risk/patient-year of VTE recurrence.
Further, these patients were more likely to have major bleeding events than recurrent VTEs and were more likely to die of a fatal bleed than from a recurrent pulmonary embolism. Additionally, 38% of major bleeds among patients with transient risk factors occurred during the first 3 months of anticoagulation therapy.
“Our data suggest that in real life, physicians appear to be more concerned about the risk of recurrent VTE after discontinuing therapy than about the risk of bleeding,” said Dr. Walter Ageno at the University of Insubria in Varese, Italy, and his associates. “Clinicians base their treatment decisions on individual risk stratification, taking into account the location of VTE and the presence of additional risk factors for recurrence and bleeding. However, before adequately validated clinical prediction rules become available, this approach may expose a substantial proportion of patients, in particular those with VTE secondary to transient risk factors, to a possibly unnecessary risk of bleeding.”
The American College of Chest Physicians recommends 3 months of anticoagulation therapy for patients with VTE secondary to surgery or a transient, nonsurgical risk factor, and extended (possibly indefinite) anticoagulation for patients with unprovoked VTE or VTE caused by cancer. To look at real-world practice, the researchers carried out a prospective cohort study of 6,944 VTE patients in Italy, Spain, and Belgium. In all, 32% of patients had transient risk factors, 41% had unprovoked VTE, and 27% had cancer (Thrombosis Res. 2015;135:666-72). After excluding patients who died within a year after VTE, 42% of patients with transient risk factors such as recent surgery, pregnancy, or prolonged travel were treated with anticoagulants for more than 12 months, the researchers reported. Significant predictors of extended anticoagulation treatment including being older than 65 years old, having chronic heart failure, pulmonary embolism at presentation, and recurrent VTE during anticoagulation, the researchers also reported. Patients who weighed less than 75 kg, had anemia, or had transient risk factors for VTE were less likely to undergo prolonged treatment than were other patients.
“There is still uncertainty among experts on the optimal duration of secondary prevention of VTE,” concluded the investigators. “This decision should be taken by balancing the risk of recurrence after stopping treatment with the risk of bleeding if treatment is continued.”
Adequately validated clinical prediction rules are needed to make those decisions, the researchers said. Until such tools are validated, a substantial proportion of patients with transiet and secondary risk factors for VTE may be exposed to a possibly unnecessary risk of bleeding, they concluded.
Sanofi Spain and Bayer Pharma AG funded the study. The investigators reported having no relevant conflicts of interest.
After a first episode of venous thromboembolism, more than 40% of patients with transient risk factors underwent anticoagulation therapy for 12 months or longer – a duration at least four times longer than the period recommended in guidelines, said authors of a large prospective cohort study.
Patients with VTE associated with surgery had about a 0.7% risk/patient-year of recurrence after 3 months of anticoagulation therapy. Patients with transient nonsurgical risk factors had about a 4% risk/patient-year of VTE recurrence.
Further, these patients were more likely to have major bleeding events than recurrent VTEs and were more likely to die of a fatal bleed than from a recurrent pulmonary embolism. Additionally, 38% of major bleeds among patients with transient risk factors occurred during the first 3 months of anticoagulation therapy.
“Our data suggest that in real life, physicians appear to be more concerned about the risk of recurrent VTE after discontinuing therapy than about the risk of bleeding,” said Dr. Walter Ageno at the University of Insubria in Varese, Italy, and his associates. “Clinicians base their treatment decisions on individual risk stratification, taking into account the location of VTE and the presence of additional risk factors for recurrence and bleeding. However, before adequately validated clinical prediction rules become available, this approach may expose a substantial proportion of patients, in particular those with VTE secondary to transient risk factors, to a possibly unnecessary risk of bleeding.”
The American College of Chest Physicians recommends 3 months of anticoagulation therapy for patients with VTE secondary to surgery or a transient, nonsurgical risk factor, and extended (possibly indefinite) anticoagulation for patients with unprovoked VTE or VTE caused by cancer. To look at real-world practice, the researchers carried out a prospective cohort study of 6,944 VTE patients in Italy, Spain, and Belgium. In all, 32% of patients had transient risk factors, 41% had unprovoked VTE, and 27% had cancer (Thrombosis Res. 2015;135:666-72). After excluding patients who died within a year after VTE, 42% of patients with transient risk factors such as recent surgery, pregnancy, or prolonged travel were treated with anticoagulants for more than 12 months, the researchers reported. Significant predictors of extended anticoagulation treatment including being older than 65 years old, having chronic heart failure, pulmonary embolism at presentation, and recurrent VTE during anticoagulation, the researchers also reported. Patients who weighed less than 75 kg, had anemia, or had transient risk factors for VTE were less likely to undergo prolonged treatment than were other patients.
“There is still uncertainty among experts on the optimal duration of secondary prevention of VTE,” concluded the investigators. “This decision should be taken by balancing the risk of recurrence after stopping treatment with the risk of bleeding if treatment is continued.”
Adequately validated clinical prediction rules are needed to make those decisions, the researchers said. Until such tools are validated, a substantial proportion of patients with transiet and secondary risk factors for VTE may be exposed to a possibly unnecessary risk of bleeding, they concluded.
Sanofi Spain and Bayer Pharma AG funded the study. The investigators reported having no relevant conflicts of interest.
FROM THROMBOSIS RESEARCH
Key clinical point: After venous thromboembolism, patients with transient or removable risk factors for VTE often underwent unneeded, prolonged anticoagulation therapy.
Major finding: Of patients with transient VTE risk factors, 42% underwent anticoagulation therapy for 12 months or longer.
Data source: Prospective cohort study of 6,944 patients with VTE.
Disclosures: Sanofi Spain and Bayer Pharma AG funded the study. The investigators reported having no relevant conflicts of interest.
Few PE patients treated with catheter-directed interventions had complications
A review of research suggests that catheter-directed interventions (CDIs) have fewer complications but are not necessarily better at preventing mortality than are standard treatments for pulmonary embolisms, according to Dr. Efthymios D. Avgerinos and Dr. Rabih A. Chaer of the University of Pittsburgh.
Of 594 patients with massive pulmonary embolisms (PEs) who received various forms of CDI, 86.5% survived (range, 40%-100%), according to a systematic review of 35 noncontrolled studies.
“In 95% of these patients, CDIs were initiated without prior intravenous thrombolysis,” while 60%-67% of the patients also received a thrombolytic agent during the procedure, they wrote. The patient survival rate was 91.2% in studies that provided at least 80% of their patients with local thrombolytic therapy during a CDI, compared with 82.8% in studies in which less than 80% of participants received thrombolytic therapy.
Not all findings, however, suggested that it was more favorable for the patients to receive the thrombolytic therapy, Overall, the pooled rates of major and minor complications were 7.9% and 2.4%, respectively. The 25 major complications reported included bleeding complications requiring transfusion, renal failure requiring hemodialysis, cardiopulmonary events, cerebrovascular events, and death.
Other research on CDIs found that right ventricle dilation was reversed in patients with submassive PEs who received fixed-dose, ultrasound-assisted, catheter-directed thrombosis combined with anticoagulation. According to the recently published randomized controlled trial, which compared the effects of fixed-dose, ultrasound-assisted, catheter-directed thrombosis and anticoagulation to anticoagulation alone, the mean right-to-left-ventricle ratio was reduced for patients in the CDI group after 1 day. Such a change did not occur in the control group, but at 90 days, the average ratio “became comparable between the two groups … with a trend in favor of the [CDI],” according to Dr. Avgerinos and Dr. Chaer. None of this study’s participants suffered from major bleeding complications.
“There is increasing evidence that percutaneous CDIs are an essential, effective and safe alternative to systemic thrombolysis or anticoagulation in the contemporary management of massive and submassive PE,” the reviewers noted. More research is needed to confirm the differences in the outcomes between using systemic thrombolysis and catheter-based techniques for treating PEs, as no clinical trial comparing CDIs with systemic thrombolysis for PE has been done, they added.
Read the full review of research in the Journal of Vascular Surgery (doi:10.1016/j.jvs.2014.10.036).
A review of research suggests that catheter-directed interventions (CDIs) have fewer complications but are not necessarily better at preventing mortality than are standard treatments for pulmonary embolisms, according to Dr. Efthymios D. Avgerinos and Dr. Rabih A. Chaer of the University of Pittsburgh.
Of 594 patients with massive pulmonary embolisms (PEs) who received various forms of CDI, 86.5% survived (range, 40%-100%), according to a systematic review of 35 noncontrolled studies.
“In 95% of these patients, CDIs were initiated without prior intravenous thrombolysis,” while 60%-67% of the patients also received a thrombolytic agent during the procedure, they wrote. The patient survival rate was 91.2% in studies that provided at least 80% of their patients with local thrombolytic therapy during a CDI, compared with 82.8% in studies in which less than 80% of participants received thrombolytic therapy.
Not all findings, however, suggested that it was more favorable for the patients to receive the thrombolytic therapy, Overall, the pooled rates of major and minor complications were 7.9% and 2.4%, respectively. The 25 major complications reported included bleeding complications requiring transfusion, renal failure requiring hemodialysis, cardiopulmonary events, cerebrovascular events, and death.
Other research on CDIs found that right ventricle dilation was reversed in patients with submassive PEs who received fixed-dose, ultrasound-assisted, catheter-directed thrombosis combined with anticoagulation. According to the recently published randomized controlled trial, which compared the effects of fixed-dose, ultrasound-assisted, catheter-directed thrombosis and anticoagulation to anticoagulation alone, the mean right-to-left-ventricle ratio was reduced for patients in the CDI group after 1 day. Such a change did not occur in the control group, but at 90 days, the average ratio “became comparable between the two groups … with a trend in favor of the [CDI],” according to Dr. Avgerinos and Dr. Chaer. None of this study’s participants suffered from major bleeding complications.
“There is increasing evidence that percutaneous CDIs are an essential, effective and safe alternative to systemic thrombolysis or anticoagulation in the contemporary management of massive and submassive PE,” the reviewers noted. More research is needed to confirm the differences in the outcomes between using systemic thrombolysis and catheter-based techniques for treating PEs, as no clinical trial comparing CDIs with systemic thrombolysis for PE has been done, they added.
Read the full review of research in the Journal of Vascular Surgery (doi:10.1016/j.jvs.2014.10.036).
A review of research suggests that catheter-directed interventions (CDIs) have fewer complications but are not necessarily better at preventing mortality than are standard treatments for pulmonary embolisms, according to Dr. Efthymios D. Avgerinos and Dr. Rabih A. Chaer of the University of Pittsburgh.
Of 594 patients with massive pulmonary embolisms (PEs) who received various forms of CDI, 86.5% survived (range, 40%-100%), according to a systematic review of 35 noncontrolled studies.
“In 95% of these patients, CDIs were initiated without prior intravenous thrombolysis,” while 60%-67% of the patients also received a thrombolytic agent during the procedure, they wrote. The patient survival rate was 91.2% in studies that provided at least 80% of their patients with local thrombolytic therapy during a CDI, compared with 82.8% in studies in which less than 80% of participants received thrombolytic therapy.
Not all findings, however, suggested that it was more favorable for the patients to receive the thrombolytic therapy, Overall, the pooled rates of major and minor complications were 7.9% and 2.4%, respectively. The 25 major complications reported included bleeding complications requiring transfusion, renal failure requiring hemodialysis, cardiopulmonary events, cerebrovascular events, and death.
Other research on CDIs found that right ventricle dilation was reversed in patients with submassive PEs who received fixed-dose, ultrasound-assisted, catheter-directed thrombosis combined with anticoagulation. According to the recently published randomized controlled trial, which compared the effects of fixed-dose, ultrasound-assisted, catheter-directed thrombosis and anticoagulation to anticoagulation alone, the mean right-to-left-ventricle ratio was reduced for patients in the CDI group after 1 day. Such a change did not occur in the control group, but at 90 days, the average ratio “became comparable between the two groups … with a trend in favor of the [CDI],” according to Dr. Avgerinos and Dr. Chaer. None of this study’s participants suffered from major bleeding complications.
“There is increasing evidence that percutaneous CDIs are an essential, effective and safe alternative to systemic thrombolysis or anticoagulation in the contemporary management of massive and submassive PE,” the reviewers noted. More research is needed to confirm the differences in the outcomes between using systemic thrombolysis and catheter-based techniques for treating PEs, as no clinical trial comparing CDIs with systemic thrombolysis for PE has been done, they added.
Read the full review of research in the Journal of Vascular Surgery (doi:10.1016/j.jvs.2014.10.036).
Bivalirudin edges unfractionated heparin for PCI in acute coronary syndrome patients
SAN DIEGO– The antithrombin drug bivalirudin received a boost, compared with unfractionated heparin, as the safer drug for preventing ischemic events in patients with acute coronary syndrome undergoing percutaneous coronary intervention in results from a multicenter, randomized trial with more than 7,000 patients.
Although the two primary endpoints from this head-to-head comparison showed no statistically significant differences between the two agents, prespecified secondary endpoints showed that treatment with bivalirudin (Angiomax) during percutaneous coronary intervention (PCI) resulted in significantly fewer deaths after 30 days and significantly fewer major bleeding events, compared with unfractionated heparin (UFH), Dr. Marco Valgimigli said at the annual meeting of the American College of Cardiology. Participating physicians administered the UFH with an antiplatelet glycoprotein IIb/IIIa inhibitor (at the operator’s discretion) 26% of the time.
In the antithrombin-randomization analysis of the MATRIX (Minimizing Adverse Hemorrhagic Events by Transradial Access Site and Systemic Implementation of Angiox) trial, which included 7,213 of the 8,404 acute coronary syndrome patients enrolled in MATRIX, treatment with bivalirudin or UFH led to similar combined rates of all-cause death, myocardial infarction, or stroke, as well as similar rates of death, MI, stroke, and major bleeds, said Dr. Valgimigli, an interventional cardiologist at Erasmus University Medical Center in Rotterdam, the Netherlands. A separate analysis of MATRIX focused on PCI outcomes with transradial vs. transfemoral access (Lancet 2015 [doi:10.1016/S0140-6736(15)60292-6]).
But treatment with bivalirudin cut the 30-day rate of all-cause death by an absolute 0.6%, driven by a concurrent cut in cardiovascular death by 0.7%, which meant that treatment with bivalirudin reduced 30-day cardiovascular deaths by one event for about every 150 patients treated, compared with patients treated with UFH, he reported. Another secondary-endpoint analysis showed that bivalirudin treatment cut the 30-day rate of major bleeding events by an absolute 1.1%, but also increased the rate of definite stent thrombosis by an absolute 0.4%, both statistically significant differences. These important differences got diluted in the primary, combined endpoints by a relatively large number of periprocedural MIs that occurred at an equal rate in both arms of the study, Dr. Valgimigli said.
The antithrombin results from MATRIX followed mixed results in several prior comparisons of bivalirudin and UFH for percutaneous coronary intervention acute coronary syndrome patients (JAMA 2015 [doi:10.1001/jama.2015.2345]). A year ago, results from the HEAT-PCI (Unfractionated Heparin Versus Bivalirudin in Primary Percutaneous Coronary Intervention) trial (Lancet 2014;384:1849-58), which enrolled 1,829 ST-elevation MI patients at one U.K. center, showed a significant reduction in ischemic events and no increased bleeding in patients treated with UFH, compared with those who received bivalirudin, a finding that experts now say seemed to lead to increased use of UFH in both U.S. and global practice relative to the more expensive bivalirudin. But the MATRIX results, as well as results published on the same day as the MATRIX report from the Chinese BRIGHT (Bivalirudin vs. Heparin With or Without Tirofiban During Primary Percutaneous Coronary Intervention in Acute Myocardial Infarction) study (JAMA 2015 [doi:10.1001/jama.2015.232]) that also compared bivalirudin and UFH, seemed to throw the balance of evidence back in bivalirudin’s favor.
MATRIX “was a win for bivalirudin,” commented Dr. Sanjit S. Jolly, an interventional cardiologist at McMaster University in Hamilton, Ont. “Clearly mortality is the most important endpoint, and the totality of data from all the trials suggest that bivalirudin reduced mortality, compared with UFH. I think that physicians who stopped using bivalirudin after HEAT-PCI may go back to bivalirudin,” he said during a press conference at the meeting.
“Following HEAT-PCI there was a shift to greater use of UFH and more selective use of bivalirudin, even in the United States, and especially for patients with ST-elevation MI,” commented Dr. David E. Kandzari , director of interventional cardiology at the Piedmont Heart Institute in Atlanta. The new MATRIX findings “will probably prompt clinicians to revisit this given that MATRIX was the largest study to compare bivalirudin and UFH. That is not to discount the HEAT-PCI findings, but that was a much smaller trial and at a single center.”
Bivalirudin treatment results in additional expense, compared with UFH, and physicians are “under pressure to cut costs, so following the HEAT-PCI results, there was a big reduction in bivalirudin use. But with the subsequent BRIGHT results and now the MATRIX results, I think there will be an uptick again in bivalirudin use,” commented Dr. Cindy L. Grines, an interventional cardiologist at the Detroit Medical Center. The MATRIX results make her “more confident about the benefit from bivalirudin,” she said in an interview.
MATRIX was an investigator-initiated study that received grant support from Terumo and the Medicines Co. Dr. Valgimigli had no disclosures. Dr. Jolly has been a consultant to AstraZeneca, a speaker on behalf of St. Jude, and received research grants from Medtronic. Dr. Kandzari has been a consultant to Medtronic and Boston Scientific and has received research support from Abbott Vascular, Biotronik, Boston Scientific, and Medtronic. Dr. Grines has been a consultant to and received honoraria from Abbott Vascular, the Medicines Co., Merck, and the Volcano Group.
On Twitter @mitchelzoler
SAN DIEGO– The antithrombin drug bivalirudin received a boost, compared with unfractionated heparin, as the safer drug for preventing ischemic events in patients with acute coronary syndrome undergoing percutaneous coronary intervention in results from a multicenter, randomized trial with more than 7,000 patients.
Although the two primary endpoints from this head-to-head comparison showed no statistically significant differences between the two agents, prespecified secondary endpoints showed that treatment with bivalirudin (Angiomax) during percutaneous coronary intervention (PCI) resulted in significantly fewer deaths after 30 days and significantly fewer major bleeding events, compared with unfractionated heparin (UFH), Dr. Marco Valgimigli said at the annual meeting of the American College of Cardiology. Participating physicians administered the UFH with an antiplatelet glycoprotein IIb/IIIa inhibitor (at the operator’s discretion) 26% of the time.
In the antithrombin-randomization analysis of the MATRIX (Minimizing Adverse Hemorrhagic Events by Transradial Access Site and Systemic Implementation of Angiox) trial, which included 7,213 of the 8,404 acute coronary syndrome patients enrolled in MATRIX, treatment with bivalirudin or UFH led to similar combined rates of all-cause death, myocardial infarction, or stroke, as well as similar rates of death, MI, stroke, and major bleeds, said Dr. Valgimigli, an interventional cardiologist at Erasmus University Medical Center in Rotterdam, the Netherlands. A separate analysis of MATRIX focused on PCI outcomes with transradial vs. transfemoral access (Lancet 2015 [doi:10.1016/S0140-6736(15)60292-6]).
But treatment with bivalirudin cut the 30-day rate of all-cause death by an absolute 0.6%, driven by a concurrent cut in cardiovascular death by 0.7%, which meant that treatment with bivalirudin reduced 30-day cardiovascular deaths by one event for about every 150 patients treated, compared with patients treated with UFH, he reported. Another secondary-endpoint analysis showed that bivalirudin treatment cut the 30-day rate of major bleeding events by an absolute 1.1%, but also increased the rate of definite stent thrombosis by an absolute 0.4%, both statistically significant differences. These important differences got diluted in the primary, combined endpoints by a relatively large number of periprocedural MIs that occurred at an equal rate in both arms of the study, Dr. Valgimigli said.
The antithrombin results from MATRIX followed mixed results in several prior comparisons of bivalirudin and UFH for percutaneous coronary intervention acute coronary syndrome patients (JAMA 2015 [doi:10.1001/jama.2015.2345]). A year ago, results from the HEAT-PCI (Unfractionated Heparin Versus Bivalirudin in Primary Percutaneous Coronary Intervention) trial (Lancet 2014;384:1849-58), which enrolled 1,829 ST-elevation MI patients at one U.K. center, showed a significant reduction in ischemic events and no increased bleeding in patients treated with UFH, compared with those who received bivalirudin, a finding that experts now say seemed to lead to increased use of UFH in both U.S. and global practice relative to the more expensive bivalirudin. But the MATRIX results, as well as results published on the same day as the MATRIX report from the Chinese BRIGHT (Bivalirudin vs. Heparin With or Without Tirofiban During Primary Percutaneous Coronary Intervention in Acute Myocardial Infarction) study (JAMA 2015 [doi:10.1001/jama.2015.232]) that also compared bivalirudin and UFH, seemed to throw the balance of evidence back in bivalirudin’s favor.
MATRIX “was a win for bivalirudin,” commented Dr. Sanjit S. Jolly, an interventional cardiologist at McMaster University in Hamilton, Ont. “Clearly mortality is the most important endpoint, and the totality of data from all the trials suggest that bivalirudin reduced mortality, compared with UFH. I think that physicians who stopped using bivalirudin after HEAT-PCI may go back to bivalirudin,” he said during a press conference at the meeting.
“Following HEAT-PCI there was a shift to greater use of UFH and more selective use of bivalirudin, even in the United States, and especially for patients with ST-elevation MI,” commented Dr. David E. Kandzari , director of interventional cardiology at the Piedmont Heart Institute in Atlanta. The new MATRIX findings “will probably prompt clinicians to revisit this given that MATRIX was the largest study to compare bivalirudin and UFH. That is not to discount the HEAT-PCI findings, but that was a much smaller trial and at a single center.”
Bivalirudin treatment results in additional expense, compared with UFH, and physicians are “under pressure to cut costs, so following the HEAT-PCI results, there was a big reduction in bivalirudin use. But with the subsequent BRIGHT results and now the MATRIX results, I think there will be an uptick again in bivalirudin use,” commented Dr. Cindy L. Grines, an interventional cardiologist at the Detroit Medical Center. The MATRIX results make her “more confident about the benefit from bivalirudin,” she said in an interview.
MATRIX was an investigator-initiated study that received grant support from Terumo and the Medicines Co. Dr. Valgimigli had no disclosures. Dr. Jolly has been a consultant to AstraZeneca, a speaker on behalf of St. Jude, and received research grants from Medtronic. Dr. Kandzari has been a consultant to Medtronic and Boston Scientific and has received research support from Abbott Vascular, Biotronik, Boston Scientific, and Medtronic. Dr. Grines has been a consultant to and received honoraria from Abbott Vascular, the Medicines Co., Merck, and the Volcano Group.
On Twitter @mitchelzoler
SAN DIEGO– The antithrombin drug bivalirudin received a boost, compared with unfractionated heparin, as the safer drug for preventing ischemic events in patients with acute coronary syndrome undergoing percutaneous coronary intervention in results from a multicenter, randomized trial with more than 7,000 patients.
Although the two primary endpoints from this head-to-head comparison showed no statistically significant differences between the two agents, prespecified secondary endpoints showed that treatment with bivalirudin (Angiomax) during percutaneous coronary intervention (PCI) resulted in significantly fewer deaths after 30 days and significantly fewer major bleeding events, compared with unfractionated heparin (UFH), Dr. Marco Valgimigli said at the annual meeting of the American College of Cardiology. Participating physicians administered the UFH with an antiplatelet glycoprotein IIb/IIIa inhibitor (at the operator’s discretion) 26% of the time.
In the antithrombin-randomization analysis of the MATRIX (Minimizing Adverse Hemorrhagic Events by Transradial Access Site and Systemic Implementation of Angiox) trial, which included 7,213 of the 8,404 acute coronary syndrome patients enrolled in MATRIX, treatment with bivalirudin or UFH led to similar combined rates of all-cause death, myocardial infarction, or stroke, as well as similar rates of death, MI, stroke, and major bleeds, said Dr. Valgimigli, an interventional cardiologist at Erasmus University Medical Center in Rotterdam, the Netherlands. A separate analysis of MATRIX focused on PCI outcomes with transradial vs. transfemoral access (Lancet 2015 [doi:10.1016/S0140-6736(15)60292-6]).
But treatment with bivalirudin cut the 30-day rate of all-cause death by an absolute 0.6%, driven by a concurrent cut in cardiovascular death by 0.7%, which meant that treatment with bivalirudin reduced 30-day cardiovascular deaths by one event for about every 150 patients treated, compared with patients treated with UFH, he reported. Another secondary-endpoint analysis showed that bivalirudin treatment cut the 30-day rate of major bleeding events by an absolute 1.1%, but also increased the rate of definite stent thrombosis by an absolute 0.4%, both statistically significant differences. These important differences got diluted in the primary, combined endpoints by a relatively large number of periprocedural MIs that occurred at an equal rate in both arms of the study, Dr. Valgimigli said.
The antithrombin results from MATRIX followed mixed results in several prior comparisons of bivalirudin and UFH for percutaneous coronary intervention acute coronary syndrome patients (JAMA 2015 [doi:10.1001/jama.2015.2345]). A year ago, results from the HEAT-PCI (Unfractionated Heparin Versus Bivalirudin in Primary Percutaneous Coronary Intervention) trial (Lancet 2014;384:1849-58), which enrolled 1,829 ST-elevation MI patients at one U.K. center, showed a significant reduction in ischemic events and no increased bleeding in patients treated with UFH, compared with those who received bivalirudin, a finding that experts now say seemed to lead to increased use of UFH in both U.S. and global practice relative to the more expensive bivalirudin. But the MATRIX results, as well as results published on the same day as the MATRIX report from the Chinese BRIGHT (Bivalirudin vs. Heparin With or Without Tirofiban During Primary Percutaneous Coronary Intervention in Acute Myocardial Infarction) study (JAMA 2015 [doi:10.1001/jama.2015.232]) that also compared bivalirudin and UFH, seemed to throw the balance of evidence back in bivalirudin’s favor.
MATRIX “was a win for bivalirudin,” commented Dr. Sanjit S. Jolly, an interventional cardiologist at McMaster University in Hamilton, Ont. “Clearly mortality is the most important endpoint, and the totality of data from all the trials suggest that bivalirudin reduced mortality, compared with UFH. I think that physicians who stopped using bivalirudin after HEAT-PCI may go back to bivalirudin,” he said during a press conference at the meeting.
“Following HEAT-PCI there was a shift to greater use of UFH and more selective use of bivalirudin, even in the United States, and especially for patients with ST-elevation MI,” commented Dr. David E. Kandzari , director of interventional cardiology at the Piedmont Heart Institute in Atlanta. The new MATRIX findings “will probably prompt clinicians to revisit this given that MATRIX was the largest study to compare bivalirudin and UFH. That is not to discount the HEAT-PCI findings, but that was a much smaller trial and at a single center.”
Bivalirudin treatment results in additional expense, compared with UFH, and physicians are “under pressure to cut costs, so following the HEAT-PCI results, there was a big reduction in bivalirudin use. But with the subsequent BRIGHT results and now the MATRIX results, I think there will be an uptick again in bivalirudin use,” commented Dr. Cindy L. Grines, an interventional cardiologist at the Detroit Medical Center. The MATRIX results make her “more confident about the benefit from bivalirudin,” she said in an interview.
MATRIX was an investigator-initiated study that received grant support from Terumo and the Medicines Co. Dr. Valgimigli had no disclosures. Dr. Jolly has been a consultant to AstraZeneca, a speaker on behalf of St. Jude, and received research grants from Medtronic. Dr. Kandzari has been a consultant to Medtronic and Boston Scientific and has received research support from Abbott Vascular, Biotronik, Boston Scientific, and Medtronic. Dr. Grines has been a consultant to and received honoraria from Abbott Vascular, the Medicines Co., Merck, and the Volcano Group.
On Twitter @mitchelzoler
AT ACC 15
Key clinical point: In acute coronary syndrome patients undergoing percutaneous coronary intervention, bivalirudin led to significantly fewer deaths and major bleeds than did unfractionated heparin.
Major finding: Treatment with bivalirudin cut all-cause, 30-day mortality by an absolute 0.6%, compared with unfractionated heparin.
Data source: MATRIX, a multicenter, randomized trial that enrolled 7,213 acute coronary syndrome patients undergoing percutaneous coronary intervention.
Disclosures: MATRIX was an investigator-initiated study that received grant support from Terumo and the Medicines Co. Dr. Valgimigli had no disclosures. Dr. Jolly has been a consultant to AstraZeneca, a speaker on behalf of St. Jude, and received research grants from Medtronic. Dr. Kandzari has been a consultant to Medtronic and Boston Scientific and has received research support from Abbott Vascular, Biotronik, Boston Scientific, and Medtronic. Dr. Grines has been a consultant to and received honoraria from Abbott Vascular, the Medicines Co., Merck, and the Volcano Group.
CoreValve receives first TAVR valve-in-valve indication
The U.S. Food and Drug Administration on March 30 expanded its approved use of the CoreValve transcatheter aortic-valve replacement (TAVR) system to include patients who already have undergone aortic valve replacement and need a second valve replacement done as a valve-in-valve placement.
With this action, CoreValve became the first TAVR system to receive U.S. approval for valve-in-valve use. The CoreValve System received FDA approval for TAVR performed on native aortic valves in January 2014 in patients at “extreme risk,” and in June 2014 for those at “high risk,” for surgical aortic valve replacement.* Valve-in-valve TAVR is only feasible in patients with a failing bioprosthetic aortic valve: It is not an option for patients with a failing mechanical aortic valve.
“The CoreValve System offers a less-invasive treatment option for a significant number of patients with failed tissue aortic valves whose medical teams determine that the risks associated with repeat open-heart surgery are high or extremely high,” Dr. William H. Maisel, deputy center director for science and chief scientist in the FDA’s Center for Devices and Radiological Health, said in a written statement. “The approval is an important expansion of the authorized use of the transcatheter aortic valve replacement technology.”
The CoreValve, which is designed to sit in a supra-annular location 12 mm above the aortic valve annulus, is well suited for valve-in-valve replacement because the only portion of the CoreValve that actually fills the annular space and the ring of the existing valve is the CoreValve’s sealer. This results in a tight seal that produces less paravalvular leak than when the sealer sits in a native annulus that is often deformed with calcium, noted Dr. Michael J. Reardon, professor of cardiothoracic surgery at Methodist Hospital in Houston.
In addition, because the sealer exerts pressure on the old valve ring in the annulus instead of on myocardium, placing the CoreValve as a valve-in-valve produces much less conduction disruption and results in fewer patients who need a pacemaker following TAVR, he said.
The CoreValve as a valve-in-valve “works quite well, and is not hard to position,” said Dr. Reardon, who added that he has now performed several valve-in-valve TAVRs using the CoreValve.
Similar TAVR procedures are usually not possible using the balloon-expandable SAPIEN System because the SAPIEN valve is designed to sit directly in the annulus and, in most patients, the existing valve ring does not provide enough space to accommodate a SAPIEN valve.
Dr. Reardon anticipates that many U.S. patients now in their 80s with a failing bioprosthetic aortic valve will be interested in nonsurgical TAVR replacement. These patients often do not want conventional open-heart surgery, he said in an interview.
To evaluate the safety and efficacy of the CoreValve System for aortic valve-in-valve replacement, the FDA reviewed clinical data collected from a U.S. clinical trial with 143 patients, an agency representative said in the statement. In the clinical trial, the estimated rate of 30-day survival without major stroke was 96%, and 89% after 6 months. “This compares well to the corresponding rates reported previously for trial participants who received the same device to replace their own, native diseased or damaged aortic valve,” the agency’s statement said.
According to the agency, aortic valve-in-valve use of the CoreValve System should be limited to patients who need replacement of a failed tissue aortic valve but are at extreme or high risk of death or serious complications from traditional open-heart surgery. A decision as to whether the product and procedure are appropriate for a patient “should involve careful evaluation by the patient’s heart medical team, including a cardiologist and a cardiac surgeon.”
The FDA said that the CoreValve System should not be used in patients who have any infection, have a mechanical aortic heart valve, cannot tolerate anticoagulant drugs, or have sensitivity to titanium, nickel, or contrast media.
Dr. Maisel had no disclosures. Dr. Reardon has served as an advisor to Medtronic, the company that markets the CoreValve.
On Twitter @mitchelzoler
*Correction, 4/1/2015: An earlier version of this article misstated the device’s approval history.
The U.S. Food and Drug Administration on March 30 expanded its approved use of the CoreValve transcatheter aortic-valve replacement (TAVR) system to include patients who already have undergone aortic valve replacement and need a second valve replacement done as a valve-in-valve placement.
With this action, CoreValve became the first TAVR system to receive U.S. approval for valve-in-valve use. The CoreValve System received FDA approval for TAVR performed on native aortic valves in January 2014 in patients at “extreme risk,” and in June 2014 for those at “high risk,” for surgical aortic valve replacement.* Valve-in-valve TAVR is only feasible in patients with a failing bioprosthetic aortic valve: It is not an option for patients with a failing mechanical aortic valve.
“The CoreValve System offers a less-invasive treatment option for a significant number of patients with failed tissue aortic valves whose medical teams determine that the risks associated with repeat open-heart surgery are high or extremely high,” Dr. William H. Maisel, deputy center director for science and chief scientist in the FDA’s Center for Devices and Radiological Health, said in a written statement. “The approval is an important expansion of the authorized use of the transcatheter aortic valve replacement technology.”
The CoreValve, which is designed to sit in a supra-annular location 12 mm above the aortic valve annulus, is well suited for valve-in-valve replacement because the only portion of the CoreValve that actually fills the annular space and the ring of the existing valve is the CoreValve’s sealer. This results in a tight seal that produces less paravalvular leak than when the sealer sits in a native annulus that is often deformed with calcium, noted Dr. Michael J. Reardon, professor of cardiothoracic surgery at Methodist Hospital in Houston.
In addition, because the sealer exerts pressure on the old valve ring in the annulus instead of on myocardium, placing the CoreValve as a valve-in-valve produces much less conduction disruption and results in fewer patients who need a pacemaker following TAVR, he said.
The CoreValve as a valve-in-valve “works quite well, and is not hard to position,” said Dr. Reardon, who added that he has now performed several valve-in-valve TAVRs using the CoreValve.
Similar TAVR procedures are usually not possible using the balloon-expandable SAPIEN System because the SAPIEN valve is designed to sit directly in the annulus and, in most patients, the existing valve ring does not provide enough space to accommodate a SAPIEN valve.
Dr. Reardon anticipates that many U.S. patients now in their 80s with a failing bioprosthetic aortic valve will be interested in nonsurgical TAVR replacement. These patients often do not want conventional open-heart surgery, he said in an interview.
To evaluate the safety and efficacy of the CoreValve System for aortic valve-in-valve replacement, the FDA reviewed clinical data collected from a U.S. clinical trial with 143 patients, an agency representative said in the statement. In the clinical trial, the estimated rate of 30-day survival without major stroke was 96%, and 89% after 6 months. “This compares well to the corresponding rates reported previously for trial participants who received the same device to replace their own, native diseased or damaged aortic valve,” the agency’s statement said.
According to the agency, aortic valve-in-valve use of the CoreValve System should be limited to patients who need replacement of a failed tissue aortic valve but are at extreme or high risk of death or serious complications from traditional open-heart surgery. A decision as to whether the product and procedure are appropriate for a patient “should involve careful evaluation by the patient’s heart medical team, including a cardiologist and a cardiac surgeon.”
The FDA said that the CoreValve System should not be used in patients who have any infection, have a mechanical aortic heart valve, cannot tolerate anticoagulant drugs, or have sensitivity to titanium, nickel, or contrast media.
Dr. Maisel had no disclosures. Dr. Reardon has served as an advisor to Medtronic, the company that markets the CoreValve.
On Twitter @mitchelzoler
*Correction, 4/1/2015: An earlier version of this article misstated the device’s approval history.
The U.S. Food and Drug Administration on March 30 expanded its approved use of the CoreValve transcatheter aortic-valve replacement (TAVR) system to include patients who already have undergone aortic valve replacement and need a second valve replacement done as a valve-in-valve placement.
With this action, CoreValve became the first TAVR system to receive U.S. approval for valve-in-valve use. The CoreValve System received FDA approval for TAVR performed on native aortic valves in January 2014 in patients at “extreme risk,” and in June 2014 for those at “high risk,” for surgical aortic valve replacement.* Valve-in-valve TAVR is only feasible in patients with a failing bioprosthetic aortic valve: It is not an option for patients with a failing mechanical aortic valve.
“The CoreValve System offers a less-invasive treatment option for a significant number of patients with failed tissue aortic valves whose medical teams determine that the risks associated with repeat open-heart surgery are high or extremely high,” Dr. William H. Maisel, deputy center director for science and chief scientist in the FDA’s Center for Devices and Radiological Health, said in a written statement. “The approval is an important expansion of the authorized use of the transcatheter aortic valve replacement technology.”
The CoreValve, which is designed to sit in a supra-annular location 12 mm above the aortic valve annulus, is well suited for valve-in-valve replacement because the only portion of the CoreValve that actually fills the annular space and the ring of the existing valve is the CoreValve’s sealer. This results in a tight seal that produces less paravalvular leak than when the sealer sits in a native annulus that is often deformed with calcium, noted Dr. Michael J. Reardon, professor of cardiothoracic surgery at Methodist Hospital in Houston.
In addition, because the sealer exerts pressure on the old valve ring in the annulus instead of on myocardium, placing the CoreValve as a valve-in-valve produces much less conduction disruption and results in fewer patients who need a pacemaker following TAVR, he said.
The CoreValve as a valve-in-valve “works quite well, and is not hard to position,” said Dr. Reardon, who added that he has now performed several valve-in-valve TAVRs using the CoreValve.
Similar TAVR procedures are usually not possible using the balloon-expandable SAPIEN System because the SAPIEN valve is designed to sit directly in the annulus and, in most patients, the existing valve ring does not provide enough space to accommodate a SAPIEN valve.
Dr. Reardon anticipates that many U.S. patients now in their 80s with a failing bioprosthetic aortic valve will be interested in nonsurgical TAVR replacement. These patients often do not want conventional open-heart surgery, he said in an interview.
To evaluate the safety and efficacy of the CoreValve System for aortic valve-in-valve replacement, the FDA reviewed clinical data collected from a U.S. clinical trial with 143 patients, an agency representative said in the statement. In the clinical trial, the estimated rate of 30-day survival without major stroke was 96%, and 89% after 6 months. “This compares well to the corresponding rates reported previously for trial participants who received the same device to replace their own, native diseased or damaged aortic valve,” the agency’s statement said.
According to the agency, aortic valve-in-valve use of the CoreValve System should be limited to patients who need replacement of a failed tissue aortic valve but are at extreme or high risk of death or serious complications from traditional open-heart surgery. A decision as to whether the product and procedure are appropriate for a patient “should involve careful evaluation by the patient’s heart medical team, including a cardiologist and a cardiac surgeon.”
The FDA said that the CoreValve System should not be used in patients who have any infection, have a mechanical aortic heart valve, cannot tolerate anticoagulant drugs, or have sensitivity to titanium, nickel, or contrast media.
Dr. Maisel had no disclosures. Dr. Reardon has served as an advisor to Medtronic, the company that markets the CoreValve.
On Twitter @mitchelzoler
*Correction, 4/1/2015: An earlier version of this article misstated the device’s approval history.
Atrial fibrillation patients on dronedarone at greater risk for all-cause hospitalizations
Among nongeriatric atrial fibrillation patients without structural heart disease, those on dronedarone had a greater risk of atrial fibrillation, cardiovascular, and all-cause hospitalizations, compared with patients on amiodarone, sotalol, and class Ic drugs, a study published in Circulation showed. Amiodarone had the lowest risk of atrial fibrillation and cardiovascular hospitalizations, but not overall hospitalizations.
Nancy M. Allen LaPointe, Pharm. D., of the Duke University Medical Center, Durham, N.C., and her associates identified 8,562 atrial fibrillation patients on antiarrhythmic drugs (with a median age of 56 years) from the MarketScan database between 2006 and 2010, and found that the risk of hospitalization for atrial fibrillation was greater with dronedarone than class Ic drugs (hazard ratio, 1.59; 95% confidence interval, 1.13-2.24), amiodarone (HR, 2.63; 1.77-3.89), and sotalol (HR, 1.72; CI, 1.17-2.54), but was lower with amiodarone versus class Ic (HR, 0.68; CI, 0.57-0.80) drugs and sotalol (HR, 0.63; CI, 0.53-0.75).
“There are many potential reasons for these differences in hospitalization rates, including differences in side effects and efficacy of each drug in this patient population. … Additional studies are needed to confirm our findings and focus on potential explanations for differences in hospitalization rates for different AADs [antiarrhythmic drugs],” the investigators wrote.
Read the full article here: Circ. Cardiovasc. Qual. Outcomes 2015 (doi:10.1161/circoutcomes.114.001499).
Among nongeriatric atrial fibrillation patients without structural heart disease, those on dronedarone had a greater risk of atrial fibrillation, cardiovascular, and all-cause hospitalizations, compared with patients on amiodarone, sotalol, and class Ic drugs, a study published in Circulation showed. Amiodarone had the lowest risk of atrial fibrillation and cardiovascular hospitalizations, but not overall hospitalizations.
Nancy M. Allen LaPointe, Pharm. D., of the Duke University Medical Center, Durham, N.C., and her associates identified 8,562 atrial fibrillation patients on antiarrhythmic drugs (with a median age of 56 years) from the MarketScan database between 2006 and 2010, and found that the risk of hospitalization for atrial fibrillation was greater with dronedarone than class Ic drugs (hazard ratio, 1.59; 95% confidence interval, 1.13-2.24), amiodarone (HR, 2.63; 1.77-3.89), and sotalol (HR, 1.72; CI, 1.17-2.54), but was lower with amiodarone versus class Ic (HR, 0.68; CI, 0.57-0.80) drugs and sotalol (HR, 0.63; CI, 0.53-0.75).
“There are many potential reasons for these differences in hospitalization rates, including differences in side effects and efficacy of each drug in this patient population. … Additional studies are needed to confirm our findings and focus on potential explanations for differences in hospitalization rates for different AADs [antiarrhythmic drugs],” the investigators wrote.
Read the full article here: Circ. Cardiovasc. Qual. Outcomes 2015 (doi:10.1161/circoutcomes.114.001499).
Among nongeriatric atrial fibrillation patients without structural heart disease, those on dronedarone had a greater risk of atrial fibrillation, cardiovascular, and all-cause hospitalizations, compared with patients on amiodarone, sotalol, and class Ic drugs, a study published in Circulation showed. Amiodarone had the lowest risk of atrial fibrillation and cardiovascular hospitalizations, but not overall hospitalizations.
Nancy M. Allen LaPointe, Pharm. D., of the Duke University Medical Center, Durham, N.C., and her associates identified 8,562 atrial fibrillation patients on antiarrhythmic drugs (with a median age of 56 years) from the MarketScan database between 2006 and 2010, and found that the risk of hospitalization for atrial fibrillation was greater with dronedarone than class Ic drugs (hazard ratio, 1.59; 95% confidence interval, 1.13-2.24), amiodarone (HR, 2.63; 1.77-3.89), and sotalol (HR, 1.72; CI, 1.17-2.54), but was lower with amiodarone versus class Ic (HR, 0.68; CI, 0.57-0.80) drugs and sotalol (HR, 0.63; CI, 0.53-0.75).
“There are many potential reasons for these differences in hospitalization rates, including differences in side effects and efficacy of each drug in this patient population. … Additional studies are needed to confirm our findings and focus on potential explanations for differences in hospitalization rates for different AADs [antiarrhythmic drugs],” the investigators wrote.
Read the full article here: Circ. Cardiovasc. Qual. Outcomes 2015 (doi:10.1161/circoutcomes.114.001499).
LEGACY: Weight loss markedly improves atrial fibrillation
SAN DIEGO – Maintenance of long-term weight loss in obese or overweight individuals with atrial fibrillation was associated with nearly a sixfold greater likelihood of freedom from recurrent AF during nearly 5 years of active follow-up in the LEGACY study.
“The effect was dose dependent. The most important finding is that 46% of patients with at least a 10% weight loss were free from AF without the use of drugs or ablation procedures through nearly 5 years, versus 22% of those with 3%-9% weight loss, and just 13% with less than a 3% weight loss,” Dr. Rajeev K. Pathak said at the annual meeting of the American College of Cardiology.
LEGACY (Long-Term Effect of Goal-Directed Weight Management in an Atrial Fibrillation Cohort: A Long-Term Follow-Up Study) included 355 overweight or obese participants with paroxsymal or persistent AF who were offered the chance to participate in a dedicated weight-loss clinic. Regular participation in this clinic proved to be a key factor in losing weight, keeping it off, and reducing AF burden; the more frequently patients attended the quarterly sessions the better the outcomes, noted Dr. Pathak, a cardiologist and electrophysiology fellow at the University of Adelaide (Australia).
Of the 355 participants, 135 lost at least 10% of their body weight, 103 had a 3%-9% drop in body weight, and 117 had less than a 3% loss or a weight gain.
Year-by-year weight trends had a significant impact on outcome. The 141 patients with linear weight loss had a 76% AF-free rate with or without the use of drugs or ablation, compared with a 59% in the 179 patients with weight fluctuations and the 38% rate in those with no loss or a weight gain. Atrial fibrillation status was assessed by 7-day Holter monitoring at least annually.
Weight fluctuation – defined as a 1% or greater change in weight between two consecutive annual follow-ups – dampened the benefits conferred by weight loss. Patients who experienced more than a 5% weight fluctuation were 2.2-fold more likely to experience AF recurrence than were those without weight fluctuation.
Patients with a sustained 10% weight loss were 5.6-fold more likely to achieve long-term freedom from AF than were patients with lesser or no weight loss. The goal was 10% weight loss rather than a body mass index of 25 kg/m2 or less because once AF patients get down to a BMI below 27 kg/m2 the incremental benefit of each additional 1 BMI point in terms of freedom from AF becomes much smaller, said Dr. Pathak.
Weight loss also showed a dose-dependent effect on various cardiovascular risk factors. For example, mean systolic blood pressure fell by 18 mm Hg in subjects with at least a 10% weight loss, by 10 mm Hg with a 3%-9% loss, and by 7 mm Hg with a lesser weight loss. Triglycerides, LDL cholesterol, and glycemic control improved in similar fashion. In addition, weight loss showed beneficial effects on cardiac structure, with dose-dependent reductions in left atrial volume indexed for body surface area as well as interventricular septal thickness, Dr. Pathak continued.
He described the weight loss clinic as a “very simple” structured motivational and goal-directed program with face-to-face counseling.
“We have one patient, one physician, no props. We sit with the patient, discuss areas we can improve, then we devise a low-carb, low-fat, high-protein diet in consultation with the patient. Patients maintain a lifestyle journal where they log their meals and exercise. We prescribe at least 200 minutes of moderate-intensity activity per week. Eating is a behavioral pattern, and we have found this approach to be a very effective behavioral tool. Because the plan is developed in consultation with the patient, we’ve found patients tend to adhere to what they have planned,” he explained.
Discussant Dr. Bernard J. Gersh praised LEGACY as “a really wonderful study – very important.
“There are years of epidemiologic evidence suggesting that obesity contributes in a major way to the epidemic of atrial fibrillation, and you’ve taken it a step further,” added Dr. Gersh, professor of medicine at the Mayo Clinic in Rochester, Minn.
Playing devil’s advocate, he asked whether the observed reduction in AF might have nothing to do with weight loss, but could be explained simply by LEGACY perhaps having enrolled a highly compliant group of patients who agreed to attend a clinic and were more willing to take their medications.
Dr. Pathak rejected the compliance factor as an explanation for the results. He noted that while cardiovascular risk factors improved with greater weight loss, the need for antihypertensive, lipid-lowering, and antiarrhythmic medications decreased.
“The effect can’t possibly be due to increased compliance with medications, but rather it’s a true effect of the weight loss itself. So I think this is a true clinical effect and not an epiphenomenon,” he replied.
Dr. Pathak added that he and his coinvestigators are organizing a randomized controlled confirmatory study.
Dr. Prediman K. Shah, who chaired a press conference where the LEGACY study was highlighted, said the study provided him with one of the major take-home lessons from ACC 15.
“We can argue about the mechanism of atrial fibrillation till kingdom come, but the fact is that the association is very strong that weight loss is associated with a reduced burden of atrial fibrillation, and with a very robust magnitude of benefit. That’s one of the messages that I’ll take home with me from this meeting: The next time I see my fat patient with atrial fibrillation, I’m putting him on a weight-reducing diet as the first approach,” declared Dr. Shah, professor of medicine at UCLA and director of the Oppenheimer Atherosclerosis Research Center at Cedars-Sinai Medical Center in Los Angeles.
Dr. Pathak reported having no financial conflicts regarding this study, which was supported by university funds.
Simultaneously with Dr. Pathak’s presentation at ACC 15, the LEGACY study was published online (J. Am. Coll. Cardiol. 2015 [doi: 10.1016/j.jacc.2015.03.002])
SAN DIEGO – Maintenance of long-term weight loss in obese or overweight individuals with atrial fibrillation was associated with nearly a sixfold greater likelihood of freedom from recurrent AF during nearly 5 years of active follow-up in the LEGACY study.
“The effect was dose dependent. The most important finding is that 46% of patients with at least a 10% weight loss were free from AF without the use of drugs or ablation procedures through nearly 5 years, versus 22% of those with 3%-9% weight loss, and just 13% with less than a 3% weight loss,” Dr. Rajeev K. Pathak said at the annual meeting of the American College of Cardiology.
LEGACY (Long-Term Effect of Goal-Directed Weight Management in an Atrial Fibrillation Cohort: A Long-Term Follow-Up Study) included 355 overweight or obese participants with paroxsymal or persistent AF who were offered the chance to participate in a dedicated weight-loss clinic. Regular participation in this clinic proved to be a key factor in losing weight, keeping it off, and reducing AF burden; the more frequently patients attended the quarterly sessions the better the outcomes, noted Dr. Pathak, a cardiologist and electrophysiology fellow at the University of Adelaide (Australia).
Of the 355 participants, 135 lost at least 10% of their body weight, 103 had a 3%-9% drop in body weight, and 117 had less than a 3% loss or a weight gain.
Year-by-year weight trends had a significant impact on outcome. The 141 patients with linear weight loss had a 76% AF-free rate with or without the use of drugs or ablation, compared with a 59% in the 179 patients with weight fluctuations and the 38% rate in those with no loss or a weight gain. Atrial fibrillation status was assessed by 7-day Holter monitoring at least annually.
Weight fluctuation – defined as a 1% or greater change in weight between two consecutive annual follow-ups – dampened the benefits conferred by weight loss. Patients who experienced more than a 5% weight fluctuation were 2.2-fold more likely to experience AF recurrence than were those without weight fluctuation.
Patients with a sustained 10% weight loss were 5.6-fold more likely to achieve long-term freedom from AF than were patients with lesser or no weight loss. The goal was 10% weight loss rather than a body mass index of 25 kg/m2 or less because once AF patients get down to a BMI below 27 kg/m2 the incremental benefit of each additional 1 BMI point in terms of freedom from AF becomes much smaller, said Dr. Pathak.
Weight loss also showed a dose-dependent effect on various cardiovascular risk factors. For example, mean systolic blood pressure fell by 18 mm Hg in subjects with at least a 10% weight loss, by 10 mm Hg with a 3%-9% loss, and by 7 mm Hg with a lesser weight loss. Triglycerides, LDL cholesterol, and glycemic control improved in similar fashion. In addition, weight loss showed beneficial effects on cardiac structure, with dose-dependent reductions in left atrial volume indexed for body surface area as well as interventricular septal thickness, Dr. Pathak continued.
He described the weight loss clinic as a “very simple” structured motivational and goal-directed program with face-to-face counseling.
“We have one patient, one physician, no props. We sit with the patient, discuss areas we can improve, then we devise a low-carb, low-fat, high-protein diet in consultation with the patient. Patients maintain a lifestyle journal where they log their meals and exercise. We prescribe at least 200 minutes of moderate-intensity activity per week. Eating is a behavioral pattern, and we have found this approach to be a very effective behavioral tool. Because the plan is developed in consultation with the patient, we’ve found patients tend to adhere to what they have planned,” he explained.
Discussant Dr. Bernard J. Gersh praised LEGACY as “a really wonderful study – very important.
“There are years of epidemiologic evidence suggesting that obesity contributes in a major way to the epidemic of atrial fibrillation, and you’ve taken it a step further,” added Dr. Gersh, professor of medicine at the Mayo Clinic in Rochester, Minn.
Playing devil’s advocate, he asked whether the observed reduction in AF might have nothing to do with weight loss, but could be explained simply by LEGACY perhaps having enrolled a highly compliant group of patients who agreed to attend a clinic and were more willing to take their medications.
Dr. Pathak rejected the compliance factor as an explanation for the results. He noted that while cardiovascular risk factors improved with greater weight loss, the need for antihypertensive, lipid-lowering, and antiarrhythmic medications decreased.
“The effect can’t possibly be due to increased compliance with medications, but rather it’s a true effect of the weight loss itself. So I think this is a true clinical effect and not an epiphenomenon,” he replied.
Dr. Pathak added that he and his coinvestigators are organizing a randomized controlled confirmatory study.
Dr. Prediman K. Shah, who chaired a press conference where the LEGACY study was highlighted, said the study provided him with one of the major take-home lessons from ACC 15.
“We can argue about the mechanism of atrial fibrillation till kingdom come, but the fact is that the association is very strong that weight loss is associated with a reduced burden of atrial fibrillation, and with a very robust magnitude of benefit. That’s one of the messages that I’ll take home with me from this meeting: The next time I see my fat patient with atrial fibrillation, I’m putting him on a weight-reducing diet as the first approach,” declared Dr. Shah, professor of medicine at UCLA and director of the Oppenheimer Atherosclerosis Research Center at Cedars-Sinai Medical Center in Los Angeles.
Dr. Pathak reported having no financial conflicts regarding this study, which was supported by university funds.
Simultaneously with Dr. Pathak’s presentation at ACC 15, the LEGACY study was published online (J. Am. Coll. Cardiol. 2015 [doi: 10.1016/j.jacc.2015.03.002])
SAN DIEGO – Maintenance of long-term weight loss in obese or overweight individuals with atrial fibrillation was associated with nearly a sixfold greater likelihood of freedom from recurrent AF during nearly 5 years of active follow-up in the LEGACY study.
“The effect was dose dependent. The most important finding is that 46% of patients with at least a 10% weight loss were free from AF without the use of drugs or ablation procedures through nearly 5 years, versus 22% of those with 3%-9% weight loss, and just 13% with less than a 3% weight loss,” Dr. Rajeev K. Pathak said at the annual meeting of the American College of Cardiology.
LEGACY (Long-Term Effect of Goal-Directed Weight Management in an Atrial Fibrillation Cohort: A Long-Term Follow-Up Study) included 355 overweight or obese participants with paroxsymal or persistent AF who were offered the chance to participate in a dedicated weight-loss clinic. Regular participation in this clinic proved to be a key factor in losing weight, keeping it off, and reducing AF burden; the more frequently patients attended the quarterly sessions the better the outcomes, noted Dr. Pathak, a cardiologist and electrophysiology fellow at the University of Adelaide (Australia).
Of the 355 participants, 135 lost at least 10% of their body weight, 103 had a 3%-9% drop in body weight, and 117 had less than a 3% loss or a weight gain.
Year-by-year weight trends had a significant impact on outcome. The 141 patients with linear weight loss had a 76% AF-free rate with or without the use of drugs or ablation, compared with a 59% in the 179 patients with weight fluctuations and the 38% rate in those with no loss or a weight gain. Atrial fibrillation status was assessed by 7-day Holter monitoring at least annually.
Weight fluctuation – defined as a 1% or greater change in weight between two consecutive annual follow-ups – dampened the benefits conferred by weight loss. Patients who experienced more than a 5% weight fluctuation were 2.2-fold more likely to experience AF recurrence than were those without weight fluctuation.
Patients with a sustained 10% weight loss were 5.6-fold more likely to achieve long-term freedom from AF than were patients with lesser or no weight loss. The goal was 10% weight loss rather than a body mass index of 25 kg/m2 or less because once AF patients get down to a BMI below 27 kg/m2 the incremental benefit of each additional 1 BMI point in terms of freedom from AF becomes much smaller, said Dr. Pathak.
Weight loss also showed a dose-dependent effect on various cardiovascular risk factors. For example, mean systolic blood pressure fell by 18 mm Hg in subjects with at least a 10% weight loss, by 10 mm Hg with a 3%-9% loss, and by 7 mm Hg with a lesser weight loss. Triglycerides, LDL cholesterol, and glycemic control improved in similar fashion. In addition, weight loss showed beneficial effects on cardiac structure, with dose-dependent reductions in left atrial volume indexed for body surface area as well as interventricular septal thickness, Dr. Pathak continued.
He described the weight loss clinic as a “very simple” structured motivational and goal-directed program with face-to-face counseling.
“We have one patient, one physician, no props. We sit with the patient, discuss areas we can improve, then we devise a low-carb, low-fat, high-protein diet in consultation with the patient. Patients maintain a lifestyle journal where they log their meals and exercise. We prescribe at least 200 minutes of moderate-intensity activity per week. Eating is a behavioral pattern, and we have found this approach to be a very effective behavioral tool. Because the plan is developed in consultation with the patient, we’ve found patients tend to adhere to what they have planned,” he explained.
Discussant Dr. Bernard J. Gersh praised LEGACY as “a really wonderful study – very important.
“There are years of epidemiologic evidence suggesting that obesity contributes in a major way to the epidemic of atrial fibrillation, and you’ve taken it a step further,” added Dr. Gersh, professor of medicine at the Mayo Clinic in Rochester, Minn.
Playing devil’s advocate, he asked whether the observed reduction in AF might have nothing to do with weight loss, but could be explained simply by LEGACY perhaps having enrolled a highly compliant group of patients who agreed to attend a clinic and were more willing to take their medications.
Dr. Pathak rejected the compliance factor as an explanation for the results. He noted that while cardiovascular risk factors improved with greater weight loss, the need for antihypertensive, lipid-lowering, and antiarrhythmic medications decreased.
“The effect can’t possibly be due to increased compliance with medications, but rather it’s a true effect of the weight loss itself. So I think this is a true clinical effect and not an epiphenomenon,” he replied.
Dr. Pathak added that he and his coinvestigators are organizing a randomized controlled confirmatory study.
Dr. Prediman K. Shah, who chaired a press conference where the LEGACY study was highlighted, said the study provided him with one of the major take-home lessons from ACC 15.
“We can argue about the mechanism of atrial fibrillation till kingdom come, but the fact is that the association is very strong that weight loss is associated with a reduced burden of atrial fibrillation, and with a very robust magnitude of benefit. That’s one of the messages that I’ll take home with me from this meeting: The next time I see my fat patient with atrial fibrillation, I’m putting him on a weight-reducing diet as the first approach,” declared Dr. Shah, professor of medicine at UCLA and director of the Oppenheimer Atherosclerosis Research Center at Cedars-Sinai Medical Center in Los Angeles.
Dr. Pathak reported having no financial conflicts regarding this study, which was supported by university funds.
Simultaneously with Dr. Pathak’s presentation at ACC 15, the LEGACY study was published online (J. Am. Coll. Cardiol. 2015 [doi: 10.1016/j.jacc.2015.03.002])
AT ACC 15
Key clinical point: Nearly half of overweight or obese patients with atrial fibrillation who achieved at least a 10% sustained weight loss remained arrhythmia free without resort to antiarrhythmic drugs or ablation procedures during nearly 5 years of follow-up.
Major finding: The weight-loss-associated freedom from recurrent AF was accompanied by improvements in cardiac structure as well as improved cardiovascular risk factors despite lesser use of risk factor-modifying drugs.
Data source: An observational study of 355 overweight or obese patients with atrial fibrillation who agreed to participate in a weight loss clinic.
Disclosures: The LEGACY study was supported by university funds. The presenter reported no financial conflicts.