User login
The American Journal of Orthopedics is an Index Medicus publication that is valued by orthopedic surgeons for its peer-reviewed, practice-oriented clinical information. Most articles are written by specialists at leading teaching institutions and help incorporate the latest technology into everyday practice.
The Female Athlete Triad Can Lead to Bone Health Problems
Sports-related injuries among female athletes have soared and women with symptoms known as the female athlete triad are at greater risk of bone stress injuries and fractures, according to a study published in the July issue of the Journal of the American Academy of Orthopaedic Surgeons.
“The female athlete triad is a spectrum of symptoms that include low energy availability, menstrual cycle abnormalities, and low bone mineral density. Low energy availability can mean taking in inadequate calories or expending more energy than the body is designed to do. It can result from poor nutrition or eating habits or any type of eating disorder. Any combination of these conditions can lead to premature bone loss in females,” explained lead study author Elizabeth Matzkin, MD, Surgical Director of Women’s Musculoskeletal Health at Brigham and Women's Hospital and Assistant Professor at Harvard Medical School in Boston.
Athletes and non-athletes participating in any sports and exercise can develop symptoms of the female athlete triad. However, the likelihood of female athletes sustaining a bone stress injury significantly increases as the number of symptoms they have increases—one symptom conferred a 15% to 21% increase in risk, two symptoms bumped the risk to 21% to 30%, and all three symptoms pushed the increase in risk to 29% to 50%.
The study authors also found that female athletes diagnosed with poor nutrition or low energy availability are 2 to 4 times more likely to sustain a sports-related injury. Female athletes who self-reported menstrual cycle abnormalities had a nearly 3 times greater risk of a bone and joint injury.
“Any athlete who falls under the ‘umbrella’ of the triad should be questioned by their physicians and educated regarding all of the components and potential health risks of this condition. By preventing premature bone loss in young female athletes, we can potentially prevent future fragility fractures,” said Dr. Matzkin.
Suggested Reading
Matzkin E, Curry EJ, Whitlock K. Female athlete triad: past, present, and future. J Am Acad Orthop Surg. 2015;23(7):424-432.
Sports-related injuries among female athletes have soared and women with symptoms known as the female athlete triad are at greater risk of bone stress injuries and fractures, according to a study published in the July issue of the Journal of the American Academy of Orthopaedic Surgeons.
“The female athlete triad is a spectrum of symptoms that include low energy availability, menstrual cycle abnormalities, and low bone mineral density. Low energy availability can mean taking in inadequate calories or expending more energy than the body is designed to do. It can result from poor nutrition or eating habits or any type of eating disorder. Any combination of these conditions can lead to premature bone loss in females,” explained lead study author Elizabeth Matzkin, MD, Surgical Director of Women’s Musculoskeletal Health at Brigham and Women's Hospital and Assistant Professor at Harvard Medical School in Boston.
Athletes and non-athletes participating in any sports and exercise can develop symptoms of the female athlete triad. However, the likelihood of female athletes sustaining a bone stress injury significantly increases as the number of symptoms they have increases—one symptom conferred a 15% to 21% increase in risk, two symptoms bumped the risk to 21% to 30%, and all three symptoms pushed the increase in risk to 29% to 50%.
The study authors also found that female athletes diagnosed with poor nutrition or low energy availability are 2 to 4 times more likely to sustain a sports-related injury. Female athletes who self-reported menstrual cycle abnormalities had a nearly 3 times greater risk of a bone and joint injury.
“Any athlete who falls under the ‘umbrella’ of the triad should be questioned by their physicians and educated regarding all of the components and potential health risks of this condition. By preventing premature bone loss in young female athletes, we can potentially prevent future fragility fractures,” said Dr. Matzkin.
Sports-related injuries among female athletes have soared and women with symptoms known as the female athlete triad are at greater risk of bone stress injuries and fractures, according to a study published in the July issue of the Journal of the American Academy of Orthopaedic Surgeons.
“The female athlete triad is a spectrum of symptoms that include low energy availability, menstrual cycle abnormalities, and low bone mineral density. Low energy availability can mean taking in inadequate calories or expending more energy than the body is designed to do. It can result from poor nutrition or eating habits or any type of eating disorder. Any combination of these conditions can lead to premature bone loss in females,” explained lead study author Elizabeth Matzkin, MD, Surgical Director of Women’s Musculoskeletal Health at Brigham and Women's Hospital and Assistant Professor at Harvard Medical School in Boston.
Athletes and non-athletes participating in any sports and exercise can develop symptoms of the female athlete triad. However, the likelihood of female athletes sustaining a bone stress injury significantly increases as the number of symptoms they have increases—one symptom conferred a 15% to 21% increase in risk, two symptoms bumped the risk to 21% to 30%, and all three symptoms pushed the increase in risk to 29% to 50%.
The study authors also found that female athletes diagnosed with poor nutrition or low energy availability are 2 to 4 times more likely to sustain a sports-related injury. Female athletes who self-reported menstrual cycle abnormalities had a nearly 3 times greater risk of a bone and joint injury.
“Any athlete who falls under the ‘umbrella’ of the triad should be questioned by their physicians and educated regarding all of the components and potential health risks of this condition. By preventing premature bone loss in young female athletes, we can potentially prevent future fragility fractures,” said Dr. Matzkin.
Suggested Reading
Matzkin E, Curry EJ, Whitlock K. Female athlete triad: past, present, and future. J Am Acad Orthop Surg. 2015;23(7):424-432.
Suggested Reading
Matzkin E, Curry EJ, Whitlock K. Female athlete triad: past, present, and future. J Am Acad Orthop Surg. 2015;23(7):424-432.
SSRIs Taken for Menopausal Symptoms Can Increase Bone Fracture Risk
Selective serotonin reuptake inhibitors (SSRIs), taken to curb menopausal symptoms, may boost bone fracture risk, according to a study published online ahead of print June 25 in the journal Injury Prevention. The heightened risk may persist for several years, according to the study findings, prompting the researchers to suggest that shorter treatment length may be preferable.
Lead author Yi-han Sheu, a doctoral student at Harvard University, and research colleagues at Harvard and the University of North Carolina at Chapel Hill used the PharMetrics Claims Database, which contains detailed information about medical and drug treatment claims made by 61 million patients in more than 98 managed care plans in the US.
The researchers focused on 137,031 women with no mental health issues who were between the ages of 40 and 64 and who started treatment with SSRIs between 1998 and 2010. The SSRIs included citalopram, hyrdrobromide, escitalopram oxalate, fluoxetine hyrdrochloride, fluvoxamine maleate, paroxetine hydrochloride, and sertraline hydrochloride.
Participants were compared with more than 236,294 women of the same age who were prescribed H2 antagonists or proton pump inhibitors, typically used to treat indigestion, over the same timeframe. An analysis of the data showed that fracture rates were significantly higher among the women treated with SSRIs.
The fracture rate was 76% higher among those prescribed SSRIs 1 year after starting treatment, 73% higher after 2 years, and 67% higher after 5 years than it was among those treated with indigestion drugs.
While the observational study offered no definitive conclusions on causality, the researchers suggested that antidepressants may alter bone turnover, shifting the balance in favor of bone thinning rather than bone strengthening activities.
“SSRIs appear to increase fracture risk among middle aged women without psychiatric disorders, an effect sustained over time, suggesting that shorter duration of treatment may decrease [this],” stated the researchers.
The study authors pointed out that the number of women prescribed SSRIs for menopausal symptoms is likely to increase in the wake of the FDA’s approval of another SSRI for this treatment indication.
Suggested Reading
Sheu YH, Lanteigne A, Stürmer T, et al. SSRI use and risk of fractures among perimenopausal women without mental disorders. Inj Prev. 2015 June 25 [Epub ahead of print].
Selective serotonin reuptake inhibitors (SSRIs), taken to curb menopausal symptoms, may boost bone fracture risk, according to a study published online ahead of print June 25 in the journal Injury Prevention. The heightened risk may persist for several years, according to the study findings, prompting the researchers to suggest that shorter treatment length may be preferable.
Lead author Yi-han Sheu, a doctoral student at Harvard University, and research colleagues at Harvard and the University of North Carolina at Chapel Hill used the PharMetrics Claims Database, which contains detailed information about medical and drug treatment claims made by 61 million patients in more than 98 managed care plans in the US.
The researchers focused on 137,031 women with no mental health issues who were between the ages of 40 and 64 and who started treatment with SSRIs between 1998 and 2010. The SSRIs included citalopram, hyrdrobromide, escitalopram oxalate, fluoxetine hyrdrochloride, fluvoxamine maleate, paroxetine hydrochloride, and sertraline hydrochloride.
Participants were compared with more than 236,294 women of the same age who were prescribed H2 antagonists or proton pump inhibitors, typically used to treat indigestion, over the same timeframe. An analysis of the data showed that fracture rates were significantly higher among the women treated with SSRIs.
The fracture rate was 76% higher among those prescribed SSRIs 1 year after starting treatment, 73% higher after 2 years, and 67% higher after 5 years than it was among those treated with indigestion drugs.
While the observational study offered no definitive conclusions on causality, the researchers suggested that antidepressants may alter bone turnover, shifting the balance in favor of bone thinning rather than bone strengthening activities.
“SSRIs appear to increase fracture risk among middle aged women without psychiatric disorders, an effect sustained over time, suggesting that shorter duration of treatment may decrease [this],” stated the researchers.
The study authors pointed out that the number of women prescribed SSRIs for menopausal symptoms is likely to increase in the wake of the FDA’s approval of another SSRI for this treatment indication.
Selective serotonin reuptake inhibitors (SSRIs), taken to curb menopausal symptoms, may boost bone fracture risk, according to a study published online ahead of print June 25 in the journal Injury Prevention. The heightened risk may persist for several years, according to the study findings, prompting the researchers to suggest that shorter treatment length may be preferable.
Lead author Yi-han Sheu, a doctoral student at Harvard University, and research colleagues at Harvard and the University of North Carolina at Chapel Hill used the PharMetrics Claims Database, which contains detailed information about medical and drug treatment claims made by 61 million patients in more than 98 managed care plans in the US.
The researchers focused on 137,031 women with no mental health issues who were between the ages of 40 and 64 and who started treatment with SSRIs between 1998 and 2010. The SSRIs included citalopram, hyrdrobromide, escitalopram oxalate, fluoxetine hyrdrochloride, fluvoxamine maleate, paroxetine hydrochloride, and sertraline hydrochloride.
Participants were compared with more than 236,294 women of the same age who were prescribed H2 antagonists or proton pump inhibitors, typically used to treat indigestion, over the same timeframe. An analysis of the data showed that fracture rates were significantly higher among the women treated with SSRIs.
The fracture rate was 76% higher among those prescribed SSRIs 1 year after starting treatment, 73% higher after 2 years, and 67% higher after 5 years than it was among those treated with indigestion drugs.
While the observational study offered no definitive conclusions on causality, the researchers suggested that antidepressants may alter bone turnover, shifting the balance in favor of bone thinning rather than bone strengthening activities.
“SSRIs appear to increase fracture risk among middle aged women without psychiatric disorders, an effect sustained over time, suggesting that shorter duration of treatment may decrease [this],” stated the researchers.
The study authors pointed out that the number of women prescribed SSRIs for menopausal symptoms is likely to increase in the wake of the FDA’s approval of another SSRI for this treatment indication.
Suggested Reading
Sheu YH, Lanteigne A, Stürmer T, et al. SSRI use and risk of fractures among perimenopausal women without mental disorders. Inj Prev. 2015 June 25 [Epub ahead of print].
Suggested Reading
Sheu YH, Lanteigne A, Stürmer T, et al. SSRI use and risk of fractures among perimenopausal women without mental disorders. Inj Prev. 2015 June 25 [Epub ahead of print].
Rates of Joint Replacements Among Military Service Members Is on the Rise
The overall incidence rate for joint replacements among US active component service members increased during an 11-year surveillance period, according to a report published in the May issue of the Medical Surveillance Monthly Report. The report also found that service members in their 30s and early 40s are having the procedures more often and are remaining in the military longer after rehabilitation.
During the surveillance period, overall incidence rates increased 10.5% during 2004 through 2009 and 61.9% during 2009 through 2014. Knee and hip joint replacements accounted for a majority of cases. In 2014, the rates of knee and hip replacements were identical (1.6 per 10,000 person-years) and were the highest for each during the 11-year surveillance period.
The report suggested that service members and their clinicians may be electing to have joint replacements at earlier ages amid improvements in surgical techniques and increased durability and longevity of prosthetic joints.
Service members are at risk for joint replacement for several reasons, the report noted. Military training and operational activities are often physically demanding, and sometimes traumatic. Some occupations are more stressful for bones and joints, and have been associated with higher frequencies of musculoskeletal disorders among service members during wartime and repeated deployments. In addition, recent increases in the incidence of overweight and obesity in service members can contribute to an increase in osteoarthritis and joint and back disorders among service members.
The report documents 3,905 joint replacements among 3,805 active component members of the Army, Navy, Air Force, Marine Corps, and Coast Guard during 2004 through 2014. Knee and hip joint replacements numbered 1,825 and 1,694, respectively.
Among the 2,902 service members who had a joint replaced during 2004 through 2012, 18.2% had retired, 5.2% had been medically disqualified from service, 6.3% had otherwise left service, and 70.3% were still in service 1 year after joint replacement. By 2 years post-joint replacement, 30.2% had retired, 13.0% had been medically disqualified, 10.0% had otherwise left service, and 46.8% were still in service.
The Army and Coast Guard had the highest overall rates of joint replacement (2.89 and 2.88 per 10,000 person-years, respectively). The Coast Guard had the highest rate of hip replacement (1.54 per 10,000 person-years) and the Army had the highest rate of knee replacement (1.46 per 10,000 person-years). The Army and Coast Guard had the highest rates of shoulder replacement (0.16 per 10,000 person-years for each). The study authors noted that these findings may, in part, be accounted for by differences in age and occupational distributions between the services.
Among racial and ethnic groups, black, non-Hispanic service members had the highest rates of joint replacement and in the 2 largest joint replacement categories, hip and knee. Previous surveillance has documented that osteoarthritis is diagnosed at higher rates among active component service members who are black, non-Hispanic. Among black, non-Hispanic service members ages 40 years or older, rates of osteoarthritis were 57% higher than rates among white, non-Hispanic counterparts.
In civilian settings, rates of joint replacement are reportedly higher among white, non-Hispanics than among all other racial and ethnic groups.
Suggested Reading
Daniele DO, Taubman SB, Clark LL. Incidence of joint replacement among active component service members, US Armed Forces, 2004–2014. MSMR. 2015;22(5):2-8.
The overall incidence rate for joint replacements among US active component service members increased during an 11-year surveillance period, according to a report published in the May issue of the Medical Surveillance Monthly Report. The report also found that service members in their 30s and early 40s are having the procedures more often and are remaining in the military longer after rehabilitation.
During the surveillance period, overall incidence rates increased 10.5% during 2004 through 2009 and 61.9% during 2009 through 2014. Knee and hip joint replacements accounted for a majority of cases. In 2014, the rates of knee and hip replacements were identical (1.6 per 10,000 person-years) and were the highest for each during the 11-year surveillance period.
The report suggested that service members and their clinicians may be electing to have joint replacements at earlier ages amid improvements in surgical techniques and increased durability and longevity of prosthetic joints.
Service members are at risk for joint replacement for several reasons, the report noted. Military training and operational activities are often physically demanding, and sometimes traumatic. Some occupations are more stressful for bones and joints, and have been associated with higher frequencies of musculoskeletal disorders among service members during wartime and repeated deployments. In addition, recent increases in the incidence of overweight and obesity in service members can contribute to an increase in osteoarthritis and joint and back disorders among service members.
The report documents 3,905 joint replacements among 3,805 active component members of the Army, Navy, Air Force, Marine Corps, and Coast Guard during 2004 through 2014. Knee and hip joint replacements numbered 1,825 and 1,694, respectively.
Among the 2,902 service members who had a joint replaced during 2004 through 2012, 18.2% had retired, 5.2% had been medically disqualified from service, 6.3% had otherwise left service, and 70.3% were still in service 1 year after joint replacement. By 2 years post-joint replacement, 30.2% had retired, 13.0% had been medically disqualified, 10.0% had otherwise left service, and 46.8% were still in service.
The Army and Coast Guard had the highest overall rates of joint replacement (2.89 and 2.88 per 10,000 person-years, respectively). The Coast Guard had the highest rate of hip replacement (1.54 per 10,000 person-years) and the Army had the highest rate of knee replacement (1.46 per 10,000 person-years). The Army and Coast Guard had the highest rates of shoulder replacement (0.16 per 10,000 person-years for each). The study authors noted that these findings may, in part, be accounted for by differences in age and occupational distributions between the services.
Among racial and ethnic groups, black, non-Hispanic service members had the highest rates of joint replacement and in the 2 largest joint replacement categories, hip and knee. Previous surveillance has documented that osteoarthritis is diagnosed at higher rates among active component service members who are black, non-Hispanic. Among black, non-Hispanic service members ages 40 years or older, rates of osteoarthritis were 57% higher than rates among white, non-Hispanic counterparts.
In civilian settings, rates of joint replacement are reportedly higher among white, non-Hispanics than among all other racial and ethnic groups.
The overall incidence rate for joint replacements among US active component service members increased during an 11-year surveillance period, according to a report published in the May issue of the Medical Surveillance Monthly Report. The report also found that service members in their 30s and early 40s are having the procedures more often and are remaining in the military longer after rehabilitation.
During the surveillance period, overall incidence rates increased 10.5% during 2004 through 2009 and 61.9% during 2009 through 2014. Knee and hip joint replacements accounted for a majority of cases. In 2014, the rates of knee and hip replacements were identical (1.6 per 10,000 person-years) and were the highest for each during the 11-year surveillance period.
The report suggested that service members and their clinicians may be electing to have joint replacements at earlier ages amid improvements in surgical techniques and increased durability and longevity of prosthetic joints.
Service members are at risk for joint replacement for several reasons, the report noted. Military training and operational activities are often physically demanding, and sometimes traumatic. Some occupations are more stressful for bones and joints, and have been associated with higher frequencies of musculoskeletal disorders among service members during wartime and repeated deployments. In addition, recent increases in the incidence of overweight and obesity in service members can contribute to an increase in osteoarthritis and joint and back disorders among service members.
The report documents 3,905 joint replacements among 3,805 active component members of the Army, Navy, Air Force, Marine Corps, and Coast Guard during 2004 through 2014. Knee and hip joint replacements numbered 1,825 and 1,694, respectively.
Among the 2,902 service members who had a joint replaced during 2004 through 2012, 18.2% had retired, 5.2% had been medically disqualified from service, 6.3% had otherwise left service, and 70.3% were still in service 1 year after joint replacement. By 2 years post-joint replacement, 30.2% had retired, 13.0% had been medically disqualified, 10.0% had otherwise left service, and 46.8% were still in service.
The Army and Coast Guard had the highest overall rates of joint replacement (2.89 and 2.88 per 10,000 person-years, respectively). The Coast Guard had the highest rate of hip replacement (1.54 per 10,000 person-years) and the Army had the highest rate of knee replacement (1.46 per 10,000 person-years). The Army and Coast Guard had the highest rates of shoulder replacement (0.16 per 10,000 person-years for each). The study authors noted that these findings may, in part, be accounted for by differences in age and occupational distributions between the services.
Among racial and ethnic groups, black, non-Hispanic service members had the highest rates of joint replacement and in the 2 largest joint replacement categories, hip and knee. Previous surveillance has documented that osteoarthritis is diagnosed at higher rates among active component service members who are black, non-Hispanic. Among black, non-Hispanic service members ages 40 years or older, rates of osteoarthritis were 57% higher than rates among white, non-Hispanic counterparts.
In civilian settings, rates of joint replacement are reportedly higher among white, non-Hispanics than among all other racial and ethnic groups.
Suggested Reading
Daniele DO, Taubman SB, Clark LL. Incidence of joint replacement among active component service members, US Armed Forces, 2004–2014. MSMR. 2015;22(5):2-8.
Suggested Reading
Daniele DO, Taubman SB, Clark LL. Incidence of joint replacement among active component service members, US Armed Forces, 2004–2014. MSMR. 2015;22(5):2-8.
Commentary to "The Burden of Craft in Arthroscopic Rotator Cuff Repair: Where We Have Been and Where We Are Going"
“The Burden of Craft in Arthroscopic Rotator Cuff Repair” is a summary of the annual Neer Lecture that was delivered by Stephen S. Burkhart, MD, at the 2014 annual meeting of American Shoulder and Elbow Surgeons. It is a fascinating personal story of the 35-year evolution of arthroscopic rotator cuff surgery presented by one of the most respected arthroscopic innovators of our times. I especially enjoyed his apt citations of classic leaders—Churchill and Gandhi—but 3 points I believe deserve special comment.
First, Steve describes the challenges he faced bringing new products to market in the 1980s. How do we resolve the inherent conflict between innovation that introduces new technology and the “tried and true” standards of established practice? Do the hard work that Steve has done over the years: pose a hypothesis, design a study to answer the question, publish results in peer-reviewed journals, and embrace the techniques that demonstrate better outcomes for patients.
My second point relates to surgeon–device industry relationships, a subject of great interest to The American Journal of Orthopedics dating back to 2006.1-3 Dr. Burkhart learned early on that he could not fashion new arthroscopic instruments in his garage. Nor could a company develop useful instruments without a knowledgeable surgeon’s input. Hence, a partnership between the innovator-surgeon and the device industry is essential to bring new and effective “tools” to market. Dr. Burkhart’s partnership with Arthrex has benefited many thousands of patients.
The agreements announced in 2007 between the US Department of Justice and 5 orthopedic device manufacturers (interestingly, current presidential candidate and Governor of New Jersey Chris Christie was the lead US Attorney on the case!) dramatically altered the surgeon–industry interaction and established strict guidelines that governed these relationships.4 These were needed reforms. However, the changes did not preclude an entrepreneurial surgeon with great ideas and a device manufacturer from profiting from excellent products that advanced patient care, provided, quoting from my editorial of 2006, “that these partnerships comply with legal and ethical standards” and are transparent as well as fully disclosed.1
Finally, Steve’s last point focuses on the “burden of craft,” a topic dear to all orthopedic surgeons and our professional societies. All of us are committed to improving our surgical skills and, as a profession, we are consistently engaged in learning from our talented colleagues, who are only too willing to share their expertise. The burden of craft requires eager students and dedicated teachers, all committed to the same goal—better outcomes for our patients. We are indeed fortunate that, as orthopedic surgeons, we fundamentally support a culture of continued learning.
I thank Steve for his eloquent paper on this important principle.
1. McCann PD. Are surgeons accepting bribes? Am J Orthop. 2006;35(3):114.
2. Byrd AB, Tearney MB. Are you being bribed? Health care ethics and compliance in the AdvaMed Code era. Part I. Am J Orthop. 2006;35(3):117-120.
3. Byrd AB, Tearney MB. Are you being bribed? Health care ethics and compliance in the AdvaMed Code era. Part II. Am J Orthop. 2006;35(4):166-171.
4 Five companies in hip and knee replacement industry avoid prosecution by agreeing to compliance rules and monitoring [press release]. US Department of Justice website. http://www.justice.gov/usao/nj/Press/files/pdffiles/Older/hips0927.rel.pdf. Published September 27, 2007. Accessed July 14, 2015.
“The Burden of Craft in Arthroscopic Rotator Cuff Repair” is a summary of the annual Neer Lecture that was delivered by Stephen S. Burkhart, MD, at the 2014 annual meeting of American Shoulder and Elbow Surgeons. It is a fascinating personal story of the 35-year evolution of arthroscopic rotator cuff surgery presented by one of the most respected arthroscopic innovators of our times. I especially enjoyed his apt citations of classic leaders—Churchill and Gandhi—but 3 points I believe deserve special comment.
First, Steve describes the challenges he faced bringing new products to market in the 1980s. How do we resolve the inherent conflict between innovation that introduces new technology and the “tried and true” standards of established practice? Do the hard work that Steve has done over the years: pose a hypothesis, design a study to answer the question, publish results in peer-reviewed journals, and embrace the techniques that demonstrate better outcomes for patients.
My second point relates to surgeon–device industry relationships, a subject of great interest to The American Journal of Orthopedics dating back to 2006.1-3 Dr. Burkhart learned early on that he could not fashion new arthroscopic instruments in his garage. Nor could a company develop useful instruments without a knowledgeable surgeon’s input. Hence, a partnership between the innovator-surgeon and the device industry is essential to bring new and effective “tools” to market. Dr. Burkhart’s partnership with Arthrex has benefited many thousands of patients.
The agreements announced in 2007 between the US Department of Justice and 5 orthopedic device manufacturers (interestingly, current presidential candidate and Governor of New Jersey Chris Christie was the lead US Attorney on the case!) dramatically altered the surgeon–industry interaction and established strict guidelines that governed these relationships.4 These were needed reforms. However, the changes did not preclude an entrepreneurial surgeon with great ideas and a device manufacturer from profiting from excellent products that advanced patient care, provided, quoting from my editorial of 2006, “that these partnerships comply with legal and ethical standards” and are transparent as well as fully disclosed.1
Finally, Steve’s last point focuses on the “burden of craft,” a topic dear to all orthopedic surgeons and our professional societies. All of us are committed to improving our surgical skills and, as a profession, we are consistently engaged in learning from our talented colleagues, who are only too willing to share their expertise. The burden of craft requires eager students and dedicated teachers, all committed to the same goal—better outcomes for our patients. We are indeed fortunate that, as orthopedic surgeons, we fundamentally support a culture of continued learning.
I thank Steve for his eloquent paper on this important principle.
“The Burden of Craft in Arthroscopic Rotator Cuff Repair” is a summary of the annual Neer Lecture that was delivered by Stephen S. Burkhart, MD, at the 2014 annual meeting of American Shoulder and Elbow Surgeons. It is a fascinating personal story of the 35-year evolution of arthroscopic rotator cuff surgery presented by one of the most respected arthroscopic innovators of our times. I especially enjoyed his apt citations of classic leaders—Churchill and Gandhi—but 3 points I believe deserve special comment.
First, Steve describes the challenges he faced bringing new products to market in the 1980s. How do we resolve the inherent conflict between innovation that introduces new technology and the “tried and true” standards of established practice? Do the hard work that Steve has done over the years: pose a hypothesis, design a study to answer the question, publish results in peer-reviewed journals, and embrace the techniques that demonstrate better outcomes for patients.
My second point relates to surgeon–device industry relationships, a subject of great interest to The American Journal of Orthopedics dating back to 2006.1-3 Dr. Burkhart learned early on that he could not fashion new arthroscopic instruments in his garage. Nor could a company develop useful instruments without a knowledgeable surgeon’s input. Hence, a partnership between the innovator-surgeon and the device industry is essential to bring new and effective “tools” to market. Dr. Burkhart’s partnership with Arthrex has benefited many thousands of patients.
The agreements announced in 2007 between the US Department of Justice and 5 orthopedic device manufacturers (interestingly, current presidential candidate and Governor of New Jersey Chris Christie was the lead US Attorney on the case!) dramatically altered the surgeon–industry interaction and established strict guidelines that governed these relationships.4 These were needed reforms. However, the changes did not preclude an entrepreneurial surgeon with great ideas and a device manufacturer from profiting from excellent products that advanced patient care, provided, quoting from my editorial of 2006, “that these partnerships comply with legal and ethical standards” and are transparent as well as fully disclosed.1
Finally, Steve’s last point focuses on the “burden of craft,” a topic dear to all orthopedic surgeons and our professional societies. All of us are committed to improving our surgical skills and, as a profession, we are consistently engaged in learning from our talented colleagues, who are only too willing to share their expertise. The burden of craft requires eager students and dedicated teachers, all committed to the same goal—better outcomes for our patients. We are indeed fortunate that, as orthopedic surgeons, we fundamentally support a culture of continued learning.
I thank Steve for his eloquent paper on this important principle.
1. McCann PD. Are surgeons accepting bribes? Am J Orthop. 2006;35(3):114.
2. Byrd AB, Tearney MB. Are you being bribed? Health care ethics and compliance in the AdvaMed Code era. Part I. Am J Orthop. 2006;35(3):117-120.
3. Byrd AB, Tearney MB. Are you being bribed? Health care ethics and compliance in the AdvaMed Code era. Part II. Am J Orthop. 2006;35(4):166-171.
4 Five companies in hip and knee replacement industry avoid prosecution by agreeing to compliance rules and monitoring [press release]. US Department of Justice website. http://www.justice.gov/usao/nj/Press/files/pdffiles/Older/hips0927.rel.pdf. Published September 27, 2007. Accessed July 14, 2015.
1. McCann PD. Are surgeons accepting bribes? Am J Orthop. 2006;35(3):114.
2. Byrd AB, Tearney MB. Are you being bribed? Health care ethics and compliance in the AdvaMed Code era. Part I. Am J Orthop. 2006;35(3):117-120.
3. Byrd AB, Tearney MB. Are you being bribed? Health care ethics and compliance in the AdvaMed Code era. Part II. Am J Orthop. 2006;35(4):166-171.
4 Five companies in hip and knee replacement industry avoid prosecution by agreeing to compliance rules and monitoring [press release]. US Department of Justice website. http://www.justice.gov/usao/nj/Press/files/pdffiles/Older/hips0927.rel.pdf. Published September 27, 2007. Accessed July 14, 2015.
Thoracic Outlet Syndrome: Current Concepts, Imaging Features, and Therapeutic Strategies
Thoracic outlet syndrome (TOS) was first described by Coot in 1861,1,2 and the term was coined by Peet and colleagues3 in 1956 to cover a spectrum of conditions caused by dynamic compression of the brachial plexus (neurogenic), subclavian artery (arterial), or subclavian vein (venous). The estimated incidence of TOS is 10 in 100,000.4 However, cadaveric studies have suggested that up to 90% of the population may have what is considered abnormal anatomy of the thoracic outlet,5 which in turn suggests a multifactorial etiology for symptomatic disease. TOS is most commonly diagnosed in patients 20 to 40 years of age, with females affected in a 4:1 ratio.6 Although historically TOS is a clinical diagnosis, advanced imaging is often helpful in determining the nature and location of the structure undergoing compression and the structure producing compression, which help guide management. Computed tomography angiography (CTA) and magnetic resonance imaging (MRI) performed in association with postural maneuvers aid in the diagnosis in patients with dynamically acquired compression.7
Pathophysiology
The pathophysiology of TOS is attributable to the unique anatomy of the thoracic outlet. Compromise of the neurovascular structures can occur through congenital or acquired narrowing in 3 distinct compartments: the interscalene triangle, the costoclavicular space, and the retropectoralis minor space. The interscalene triangle is the most medial of the compartments. Containing the subclavian artery and the 3 trunks of the brachial plexus, it is bordered anteriorly by the anterior scalene muscle, posteriorly by the middle and posterior scalene muscles, and inferiorly by the first rib. The interscalene triangle is the most frequent site of neurologic compression.8 The middle compartment is the costoclavicular space, which is bordered superiorly by the clavicle, anteriorly by the subclavius muscle, and posteriorly by the first rib and the middle scalene muscle. The costoclavicular space is the most frequent site of arterial compression,8 where the artery lies directly anterior to the subclavian vein and is surrounded by the 3 cords of the brachial plexus. The most lateral compartment is the retropectoralis minor space, which is bordered anteriorly by the pectoralis minor muscle, superiorly by the subscapularis muscle, and inferiorly by the anterior chest wall. Sources of neurovascular compression within any of the spaces include cervical ribs9; elongated C7 transverse processes; hypertrophy of the anterior or middle scalene, subclavius, or pectoralis minor muscles10; anomalous scalenus minimus muscle; repetitive overhead arm movements (pitching, swimming)11; anomalous fascial bands; degenerative spine disease; bone destruction from primary or secondary neoplasms (Pancoast tumor); hyperextension/flexion injury of the neck12; and malunion of clavicle fractures, among others.13
Classification
Three distinct TOSs have been described, individually or combined, depending on the injured component: neurogenic from brachial plexus compression, arterial from subclavian artery compression, and venous from subclavian or axillary vein compression.14,15
Neurogenic TOS has 2 reported types: true (classic) and disputed. True neurogenic TOS is rare, with an estimated incidence of 1 in 1 million.16 First described in 1970 as a lower trunk plexopathy involving slowly progressive unilateral weakness of the intrinsic hand muscles and sensory abnormalities in the ulnar and medial antebrachial cutaneous nerve distributions, true neurogenic TOS was originally called Gilliatt-Sumner hand syndrome.17 A congenital band extending between the first rib and an elongated C7 transverse process was thought to be the location of brachial plexus injury in true neurogenic TOS. Conversely, disputed neurogenic TOS is the most common form of TOS, occurring in 3 to 80 per 100018 and accounting for 90% to 95% of all TOS cases.13,19 In contrast to true neurogenic TOS, in which anatomical and electrodiagnostic evidence supports the diagnosis, objective clinical findings are often lacking in the disputed form.18 Patients with disputed neurogenic TOS present with a diverse array of symptoms, including pain, numbness, and weakness affecting the neck, shoulder, and arm, exacerbated by activities requiring elevation or sustained use of the extremity.20
Arterial TOS accounts for 1% to 5% of all TOS cases.21 Arterial TOS typically affects patients who perform repetitive movements of the upper extremities with their arms above their shoulders, resulting in compression of the subclavian artery. Symptoms of arterial TOS include pain, weakness, coolness, pallor, and paresthesia.18,22 In severe cases of compression, subclavian artery damage can result in thrombosis with distal embolization, poststenotic aneurysm, or even retrograde extension causing stroke.22,23
Last, representing 2% to 3% of all TOS cases, venous TOS results from compression of the subclavian or axillary vein.18,24 Two mechanisms for vascular compromise have been described. The first involves compression of the vein between the clavicle and the first rib with overhead activities.18 Patients often experience intermittent “heaviness” of the extremity with repeated overhead use. The second mechanism involves repeated stress between the clavicle and vein, causing an intravascular thrombosis.18 Patients may experience pain, edema, cyanosis, venous distention, and even spontaneous venous thrombosis, referred to as Paget-Schroetter syndrome, which can lead to pulmonary embolism.6,25,26
Clinical Features
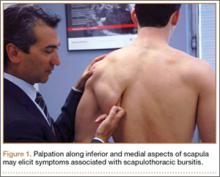
In cases of suspected TOS, clinicians should take a thorough history and perform a thorough physical examination. The differential diagnosis for unilateral, upper limb pain, numbness, tingling, and/or weakness exacerbated by movement includes shoulder and rotator cuff pathology, cervical spine injury, cervical radiculitis, distal compressive neuropathies (carpal or cubital tunnel syndrome), and neuralgic amyotrophy (Parsonage-Turner syndrome/acute brachial radiculitis).27,28 The clinician should pursue a history of trauma to the shoulder or neck as well as any occupational or recreational activities involving elevation of the upper extremity for extended periods.29 Physical examination must include an evaluation of the contralateral side and may begin with visual inspection to assess for muscle asymmetry, atrophy, color changes, edema, or deformities.18 Next, palpation should be used to assess for any tenderness, texture changes, masses, or vascular pulsations. Attention should be directed at examination of the cervical spine as well as neurologic and vascular assessments of the bilateral upper extremities, including range of motion and strength testing,18 to rule out alternative etiologies.
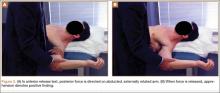
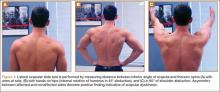
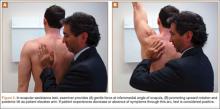
Four basic maneuvers—the Roos test,30 Adson test,31 Wright test,32 and costoclavicular test—traditionally have been used to diagnose TOS. A positive Roos test involves symptom reproduction with the patient slowly opening and closing the hand for 3 minutes with the arm externally rotated and abducted to 90°.33 However, the false-positive rate of the Roos test is as high as 77% in patients with carpal tunnel syndrome and up to 47% in normal subjects.34 The Adson test is performed by having the patient inhale deeply while the arm is kept in the anatomical position with the head extended and turned toward the involved extremity. The examiner monitors the radial pulse; an absent or diminished radial pulse suggests compression of the subclavian artery. The Adson test is not very reliable, however, because the pulse diminishes even in normal subjects,6,26 with a reported false-positive rate of 13.5%.35 A positive costoclavicular compression test occurs when depressing a patient’s shoulder reproduces symptoms. In one study, the false-positive rate of the costoclavicular compression test was 48% in patients with carpal tunnel syndrome and 16% in normal subjects.34 Last, the Wright test is performed by hyperabducting and externally rotating the affected shoulder. It is positive with a diminished pulse or reproduction of symptoms. One study found that the Wright test had 70% to 90% sensitivity and 29% to 53% specificity.36
Clinically distinguishing between the various forms of TOS may be difficult, and occasionally multiple types exist in a single patient, exacerbating one another and adding to the diagnostic difficulty. For example, arterial insufficiency may lead to disruption of the neural microcirculation, leading to concurrent arterial and neurogenic TOS. Because most cases present with nonspecific symptoms, advanced imaging modalities are often required to establish a definitive diagnosis and to target therapy to the appropriate site of compression.
Imaging Features
Plain Radiography
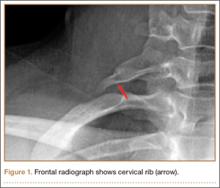
First, cervical spine and chest radiographs should be obtained to assess for bone abnormalities, including cervical ribs, long transverse processes, rib/clavicle fracture callus, rib anomalies, degenerative spine disease, and neoplasm (Pancoast/apical tumor) (Figure 1).18,25
Ultrasonography
Ultrasonography is useful in evaluating arterial or venous TOS because of its low cost, noninvasive nature, and high specificity for vessel occlusion.37,38 In arterial TOS, ultrasound may demonstrate increased flow velocity through a stenosis or an aneurysmal degeneration distal to the stenosis.7 In venous TOS, duplex ultrasound can identify stasis and thrombus.7 Obtaining duplex ultrasound with the upper extremity in multiple positions allows clinicians to correlate dynamically induced symptoms with ultrasonographic findings of altered blood flow.39-41 Despite the purported benefits of ultrasound, its drawback is that it is operator-dependent,42 with some studies reporting a high false-positive rate24 for diagnosis of venous TOS.
Electrodiagnostic Testing
Ruling out etiologies such as cervical radiculitis (Parsonage-Turner syndrome), cervical radiculopathies, brachial plexus lesions, and other distal compressive neuropathies requires nerve conduction studies and electromyography.18,43-46 In true neurogenic TOS, a combination of decreased sensory nerve action potentials in the ulnar and medial antebrachial cutaneous nerves and decreased compound motor action potentials in the median nerve is often found.18 Specifically, an abnormal ulnar sensory nerve action potential suggests the lesion is situated away from the intraspinal canal, which argues against a diagnosis of radiculopathy or myelopathy.43,44 In the disputed form of neurogenic TOS, the role of electrodiagnostic testing is less clear.18
Conventional Arteriography and Venography
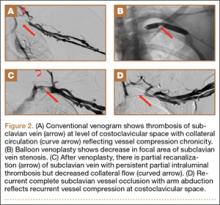
Although CTA has superseded conventional arteriography and venography in most treatment centers, it may still be used in patients with acute symptoms requiring immediate thrombolytic therapy. Catheter angiography and venography with postural maneuvers are often the first invasive treatment modality in cases of thoracic outlet vascular compression.22,24 Presence of intraluminal thrombus, vessel dilatation, and collateral vessels is readily demonstrated (Figure 2A). Recanalization of occluded vessels can be attempted using balloon angioplasty and venoplasty (Figure 2B), but it is usually only temporarily successful if the cause of extrinsic compression is not corrected (Figures 2C, 2D). CTA or conventional angiography, used if sophisticated CTA with 3-dimensional (3-D) reconstruction is unavailable, is the gold standard in diagnosis of TOS.
CTA and Venography
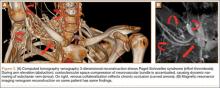
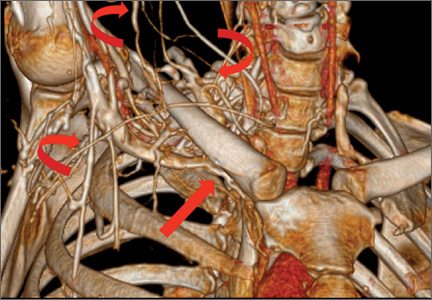
Computed tomography (CT) is a valuable modality because it can be performed rapidly and effectively to depict the relationship of vascular structures to surrounding bone and muscle.47 In addition, CTA and venography provide high-quality representations of the vasculature, and 3-D reconstruction reliably identifies areas of neurovascular compression in patients with TOS.47,48 Furthermore, CT may be performed in a dynamic fashion, with the upper extremity in various positions to reproduce dynamic compression of the neurovascular structures (Figure 3A). Comparison of the images with the upper extremities in the anatomical position and elevated allows the physician to evaluate narrowing of the compartments and dynamic compression of neurovascular structures.8 CT is particularly valuable in arterial and venous TOS. In arterial TOS, the cross-sectional area or diameter of the artery can be measured to calculate the degree of stenosis.8,47 In venous TOS, dynamic narrowing of the vein can be visualized and may be associated with venous thrombosis or collateral circulation (Figure 3B). Although a variety of maneuvers is possible during CTA, the size of the CT tunnel as well as mandatory supine positioning of the patient may limit the series. Drawbacks of CT for diagnosing TOS include difficulties in analyzing the brachial plexus because of limited contrast resolution. In addition, the risks of CT (ionizing radiation, administration of iodinated contrast medium) must be considered before image acquisition.
MRI
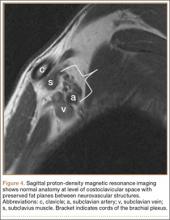
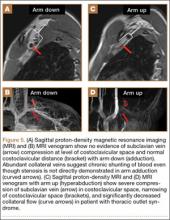
MRI is a noninvasive and nonionizing technique that offers good resolution of the anatomical components of the thoracic outlet8 and that, because of its superior soft-tissue contrast, is the modality of choice for imaging brachial plexus nerve compression in TOS (Figure 4). Neurologic compression is identified with MRI when the fat surrounding the brachial plexus disappears.8 MRI reliably identifies the source of compression, which may include bony structures, muscle hypertrophy (scalenus, scalenus minimus, subclavius, pectoralis minor), and fibrous bands.49 Because of their craniocaudal direction, the sagittal plane is often most useful in demonstrating neurovascular compression.42 Analyzing the caliber of the vessel along its course may evaluate vascular compression, and magnetic resonance (MR) angiography and venography (Figures 5A, 5B) can often complement the findings.50 Specifically, in arterial TOS, poststenotic aneurysmal dilatation may be seen, whereas thrombosis and collateral circulation can be visualized in cases of venous TOS.50 Limitations of MRI in the diagnosis of TOS historically were similar to those of CT, and included supine positioning as well as restricted upper extremity maneuvers because of the size of the tunnel and the presence of surface coils.42 However, newer higher channel surface coils and wider bores allow for imaging in a wider range of motion, including arm hyperabduction (Figures 5C, 5D), which is often necessary to elicit pathology.
Management
Generally, therapeutic options for TOS are aimed at relieving the source of neurovascular compression. It is important that treatment be directed only toward symptomatic patients, as many patients have anatomy consistent with TOS and remain asymptomatic.5 Treatment of TOS is predominately conservative and involves a combination of patient education, activity modification, medication, and rehabilitation to promote appropriate body mechanics and posture.18
Physical Therapy
Physical therapy should be aimed at decreasing pressure on the neurovascular structures of the thoracic outlet by relaxing the scalene muscles, strengthening the shoulder muscles, and working on postural exercises to help the patient sit and stand straighter.51 The scalene muscles are the primary targets for TOS rehabilitation, but focus should also be given to the upper trapezius, levator scapulae, sternocleidomastoid, pectoral, and suboccipital muscles.18 Physical therapy is often combined with hydrotherapy, massage, nonsteroidal anti-inflammatory drugs, and muscle relaxants for maximal symptomatic relief. Some patients have found relief with selective anesthetic or botulinum toxin A injections in the scalene muscles.18 A minimum of 4 to 6 weeks (often 4-6 months) of physical therapy and conservative treatment should be attempted before consideration of any invasive intervention.13,18
Anticoagulation
In venous TOS with evidence of thrombus but no obstructive clot, conservative management is typically sufficient. In rare cases, however, intimal damage secondary to vascular compression in arterial and venous TOS leads to thrombus formation, impairing upper extremity perfusion and producing symptoms. Treatment guidelines for venous TOS involve catheter-directed thrombolysis within 2 weeks of symptom onset.15 Thrombolysis replaced the prior recommendation of systemic anticoagulation combined with extremity rest and elevation because anticoagulation and rest alone result in up to 75% morbidity,52,53 whereas thrombolysis reestablishes vessel patency in nearly all patients.54 After thrombolysis, patients should receive intravenous heparin, and conversion to oral anticoagulation should occur as soon as manageable. In patients with arterial TOS, the goal of treatment is revascularization to prevent or decrease ischemia. In mild arterial ischemia, catheter-directed thrombolysis can be attempted. However, the threshold for surgical thromboembolectomy must remain low, as acute upper extremity ischemia may result in compartment syndrome and permanent loss of function.13 Fixed arterial lesions, whether occlusive or aneurysmal, are an absolute indication for thromboembolectomy with possible thoracic outlet decompression.13
Thoracic Outlet Decompression
Indications for surgical decompression are controversial. They include symptomatic patients who have vascular (arterial or venous) TOS and are not at high risk for surgery, patients with true neurologic TOS and acute progressive neurologic weakness or disabling pain,55 and patients who have disputed neurologic TOS and have failed conservative management—keeping in mind that high recurrence rates and iatrogenic brachial plexopathy have been reported in this population.56 In general, surgical procedures are aimed at reducing soft-tissue compression (scalene release or neurolysis) or bony compression (cervical or first thoracic rib excision). Three surgical approaches (transaxillary, supraclavicular, infraclavicular) are commonly used for decompression, and surgeons choose one over another depending on the anatomical abnormality causing the compression. The transaxillary approach requires limited dissection but still allows for adequate visualization of the rib during resection.57 In this approach, a transverse incision along the inferior border of the axilla extends from the pectoralis major to the latissimus dorsi. After dissection of the axillary vessels and the first thoracic nerve root, the first rib is identified and can be removed, when indicated. In contrast, the supraclavicular approach provides a wide exposure, and the site of compression is directly visualized, allowing for arterial reconstruction.58 Through this approach, the anterior and middle scalene muscles can be resected, and neurolysis of the brachial plexus can be performed. Last, the infraclavicular approach allows for exposure of the central veins through extension of the incision medially, which allows for venous reconstruction. Some patients with neurogenic or arterial TOS present with symptoms of sympathetic overactivity, in which case cervical sympathectomy can be used with decompression.
Outcomes of surgical decompression for TOS depend on the clinical type but are generally good. For instance, in cases of disputed neurogenic TOS, symptom resolution after decompression is reportedly between 80% and 90%.59 However, major depression, work-related injuries,60 and diffuse preoperative arm symptoms61 all influence long-term results. In true neurogenic TOS, postoperative pain relief is often substantial, though recovery of strength can be slow because of the axonal injury.55 In arterial TOS, outcomes are influenced by time to surgical intervention, with early surgery demonstrating better outcomes than later surgery.62 In one study, Cormier and colleagues14 evaluated 47 patients who underwent correction of subclavian-axillary artery compression; 91% were asymptomatic a mean of 5.7 months after decompression. Last, outcomes of successful thrombolysis and decompression for venous TOS demonstrated patency rates higher than 95% at 5-year follow-up.54,63
Conclusions
TOS is a spectrum of disorders caused by compression of the brachial plexus, subclavian artery, or subclavian vein. Early recognition of TOS is imperative, as diagnostic or treatment delays may be associated with significant morbidity. Clinical examination alone is often inadequate for determining the compression site and the structure causing compression. CTA and MRI performed in association with postural maneuvers may demonstrate dynamic compression of the neurovascular structures in the thoracic outlet. These imaging modalities reliably identify the structures causing compression and can be crucial for effective management.
1. Urschel HC Jr. The history of surgery for thoracic outlet syndrome. Chest Surg Clin North Am. 2000;10(1):183-188, x-xi.
2. Atasoy E. History of thoracic outlet syndrome. Hand Clin. 2004;20(1):15-16, v.
3. Peet RM, Henriksen JD, Anderson TP, Martin GM. Thoracic-outlet syndrome: evaluation of a therapeutic exercise program. Proc Staff Meet Mayo Clin. 1956;31(9):281-287.
4. Edwards DP, Mulkern E, Raja AN, Barker P. Trans-axillary first rib excision for thoracic outlet syndrome. J R Coll Surg Edinb. 1999;44(6):362-365.
5. Juvonen T, Satta J, Laitala P, Luukkonen K, Nissinen J. Anomalies at the thoracic outlet are frequent in the general population. Am J Surg. 1995;170(1):33-37.
6. Atasoy E. Thoracic outlet compression syndrome. Orthop Clin North Am. 1996;27(2):265-303.
7. Demondion X, Herbinet P, Van Sint Jan S, Boutry N, Chantelot C, Cotten A. Imaging assessment of thoracic outlet syndrome. Radiographics. 2006;26(6):1735-1750.
8. Demondion X, Bacqueville E, Paul C, Duquesnoy B, Hachulla E, Cotten A. Thoracic outlet: assessment with MR imaging in asymptomatic and symptomatic populations. Radiology. 2003;227(2):461-468.
9. Makhoul RG, Machleder HI. Developmental anomalies at the thoracic outlet: an analysis of 200 consecutive cases. J Vasc Surg. 1992;16(4):534-542.
10. Sanders RJ, Jackson CG, Banchero N, Pearce WH. Scalene muscle abnormalities in traumatic thoracic outlet syndrome. Am J Surg. 1990;159(2):231-236.
11. Katirji B, Hardy RW Jr. Classic neurogenic thoracic outlet syndrome in a competitive swimmer: a true scalenus anticus syndrome. Muscle Nerve. 1995;18(2):229-233.
12. Casbas L, Chauffour X, Cau J, et al. Post-traumatic thoracic outlet syndromes. Ann Vasc Surg. 2005;19(1):25-28.
13. Povlsen B, Belzberg A, Hansson T, Dorsi M. Treatment for thoracic outlet syndrome. Cochrane Database Syst Rev. 2010;(1):CD007218.
14. Cormier JM, Amrane M, Ward A, Laurian C, Gigou F. Arterial complications of the thoracic outlet syndrome: fifty-five operative cases. J Vasc Surg. 1989;9(6):778-787.
15. Hood DB, Kuehne J, Yellin AE, Weaver FA. Vascular complications of thoracic outlet syndrome. Am Surg. 1997;63(10):913-917.
16. Ferrante MA. Brachial plexopathies: classification, causes, and consequences. Muscle Nerve. 2004;30(5):547-568.
17. Gilliatt RW, Le Quesne PM, Logue V, Sumner AJ. Wasting of the hand associated with a cervical rib or band. J Neurol Neurosurg Psychiatry. 1970;33(5):615-624.
18. Ozoa G, Alves D, Fish DE. Thoracic outlet syndrome. Phys Med Rehabil Clin North Am. 2011;22(3):473-483, viii-ix.
19. Schwartzman RJ. Brachial plexus traction injuries. Hand Clin. 1991;7(3):547-556.
20. Christo PJ, McGreevy K. Updated perspectives on neurogenic thoracic outlet syndrome. Curr Pain Headache Rep. 2011;15(1):14-21.
21. Vanti C, Natalini L, Romeo A, Tosarelli D, Pillastrini P. Conservative treatment of thoracic outlet syndrome. A review of the literature. Eura Medicophys. 2007;43(1):55-70.22. Patton GM. Arterial thoracic outlet syndrome. Hand Clin. 2004;20(1):107-111, viii.
23. Lee TS, Hines GL. Cerebral embolic stroke and arm ischemia in a teenager with arterial thoracic outlet syndrome: a case report. Vasc Endovasc Surg. 2007;41(3):254-257.
24. Sanders RJ, Hammond SL. Venous thoracic outlet syndrome. Hand Clin. 2004;20(1):113-118, viii.
25. Sanders RJ, Hammond SL, Rao NM. Diagnosis of thoracic outlet syndrome. J Vasc Surg. 2007;46(3):601-604.
26. Luoma A, Nelems B. Thoracic outlet syndrome. Thoracic surgery perspective. Neurosurg Clin North Am. 1991;2(1):187-226.
27. Cup EH, Ijspeert J, Janssen RJ, et al. Residual complaints after neuralgic amyotrophy. Arch Phys Med Rehabil. 2013;94(1):67-73.
28. van Alfen N, van Engelen BG. The clinical spectrum of neuralgic amyotrophy in 246 cases. Brain. 2006;129(pt 2):438-450.
29. Nichols AW. The thoracic outlet syndrome in athletes. J Am Board Fam Pract. 1996;9(5):346-355.
30. Roos DB, Owens JC. Thoracic outlet syndrome. Arch Surg. 1966;93(1):71-74.
31. Adson AW, Coffey JR. Cervical rib: a method of anterior approach for relief of symptoms by division of the scalenus anticus. Ann Surg. 1927;85(6):839-857.
32. Wright IS. The neurovascular syndrome produced by hyperabduction of the arms. Am Heart J. 1945;29:1-19.
33. Rayan GM, Jensen C. Thoracic outlet syndrome: provocative examination maneuvers in a typical population. J Shoulder Elbow Surg. 1995;4(2):113-117.
34. Nord KM, Kapoor P, Fisher J, et al. False positive rate of thoracic outlet syndrome diagnostic maneuvers. Electromyogr Clin Neurophysiol. 2008;48(2):67-74.
35. Novak CB. Thoracic outlet syndrome. Clin Plast Surg. 2003;30(2):175-188.
36. Gillard J, Pérez-Cousin M, Hachulla E, et al. Diagnosing thoracic outlet syndrome: contribution of provocative tests, ultrasonography, electrophysiology, and helical computed tomography in 48 patients. Joint Bone Spine. 2001;68(5):416-424.
37. Baxter GM, Kincaid W, Jeffrey RF, Millar GM, Porteous C, Morley P. Comparison of colour Doppler ultrasound with venography in the diagnosis of axillary and subclavian vein thrombosis. Br J Radiol. 1991;64(765):777-781.
38. Passman MA, Criado E, Farber MA, et al. Efficacy of color flow duplex imaging for proximal upper extremity venous outflow obstruction in hemodialysis patients. J Vasc Surg. 1998;28(5):869-875.
39. Wadhwani R, Chaubal N, Sukthankar R, Shroff M, Agarwala S. Color Doppler and duplex sonography in 5 patients with thoracic outlet syndrome. J Ultrasound Med. 2001;20(7):795-801.
40. Napoli V, Vignali C, Braccini G, et al. Echography and echo-Doppler in the study of thoracic outlet syndrome. Correlation with angiographic data [in Italian]. Radiol Med. 1993;85(6):733-740.
41. Longley DG, Yedlicka JW, Molina EJ, Schwabacher S, Hunter DW, Letourneau JG. Thoracic outlet syndrome: evaluation of the subclavian vessels by color duplex sonography. AJR Am J Roentgenol. 1992;158(3):623-630.
42. Demondion X, Herbinet P, Boutry N, Fontaine C, Francke JP, Cotten A. Sonographic mapping of the normal brachial plexus. AJNR Am J Neuroradiol. 2003;24(7):1303-1309.
43. Cruz-Martinez A, Arpa J. Electrophysiological assessment in neurogenic thoracic outlet syndrome. Electromyogr Clin Neurophysiol. 2001;41(4):253-256.
44. Ferrante MA, Wilbourn AJ. The utility of various sensory nerve conduction responses in assessing brachial plexopathies. Muscle Nerve. 1995;18(8):879-889.
45. Aminoff MJ, Olney RK, Parry GJ, Raskin NH. Relative utility of different electrophysiologic techniques in the evaluation of brachial plexopathies. Neurology. 1988;38(4):546-550.
46. Komanetsky RM, Novak CB, Mackinnon SE, Russo MH, Padberg AM, Louis S. Somatosensory evoked potentials fail to diagnose thoracic outlet syndrome. J Hand Surg Am. 1996;21(4):662-666.
47. Remy-Jardin M, Remy J, Masson P, et al. Helical CT angiography of thoracic outlet syndrome: functional anatomy. AJR Am J Roentgenol. 2000;174(6):1667-1674.
48. Matsumura JS, Rilling WS, Pearce WH, Nemcek AA Jr, Vogelzang RL, Yao JS. Helical computed tomography of the normal thoracic outlet. J Vasc Surg. 1997;26(5):776-783.
49. Dymarkowski S, Bosmans H, Marchal G, Bogaert J. Three-dimensional MR angiography in the evaluation of thoracic outlet syndrome. AJR Am J Roentgenol. 1999;173(4):1005-1008.
50. Charon JP, Milne W, Sheppard DG, Houston JG. Evaluation of MR angiographic technique in the assessment of thoracic outlet syndrome. Clin Radiol. 2004;59(7):588-595.
51. Cuetter AC, Bartoszek DM. The thoracic outlet syndrome: controversies, overdiagnosis, overtreatment, and recommendations for management. Muscle Nerve. 1989;12(5):410-419.
52. Urschel HC Jr, Razzuk MA. Paget-Schroetter syndrome: what is the best management? Ann Thorac Surg. 2000;69(6):1663-1668.
53. Lee JT, Karwowski JK, Harris EJ, Haukoos JS, Olcott C 4th. Long-term thrombotic recurrence after nonoperative management of Paget-Schroetter syndrome. J Vasc Surg. 2006;43(6):1236-1243.
54. Molina JE, Hunter DW, Dietz CA. Paget-Schroetter syndrome treated with thrombolytics and immediate surgery. J Vasc Surg. 2007;45(2):328-334.
55. Le Forestier N, Mouton P, Maisonobe T, et al. True neurological thoracic outlet syndrome [in French]. Rev Neurol (Paris). 2000;156(1):34-40.
56. Wilbourn AJ. Thoracic outlet syndrome surgery causing severe brachial plexopathy. Muscle Nerve. 1988;11(1):66-74.
57. Likes K, Dapash T, Rochlin DH, Freischlag JA. Remaining or residual first ribs are the cause of recurrent thoracic outlet syndrome. Ann Vasc Surg. 2014;28(4):939-945.
58. Aljabri B, Al-Omran M. Surgical management of vascular thoracic outlet syndrome: a teaching hospital experience. Ann Vasc Dis. 2013;6(1):74-79.
59. Sanders RJ, Pearce WH. The treatment of thoracic outlet syndrome: a comparison of different operations. J Vasc Surg. 1989;10(6):626-634.
60. Franklin GM, Fulton-Kehoe D, Bradley C, Smith-Weller T. Outcome of surgery for thoracic outlet syndrome in Washington state workers’ compensation. Neurology. 2000;54(6):1252-1257.
61. Axelrod DA, Proctor MC, Geisser ME, Roth RS, Greenfield LJ. Outcomes after surgery for thoracic outlet syndrome. J Vasc Surg. 2001;33(6):1220-1225.
62. Taylor JM, Telford RJ, Kinsella DC, Watkinson AF, Thompson JF. Long-term clinical and functional outcome following treatment for Paget-Schroetter syndrome. Br J Surg. 2013;100(11):1459-1464.
63. Schneider DB, Dimuzio PJ, Martin ND, et al. Combination treatment of venous thoracic outlet syndrome: open surgical decompression and intraoperative angioplasty. J Vasc Surg. 2004;40(4):599-603.
Thoracic outlet syndrome (TOS) was first described by Coot in 1861,1,2 and the term was coined by Peet and colleagues3 in 1956 to cover a spectrum of conditions caused by dynamic compression of the brachial plexus (neurogenic), subclavian artery (arterial), or subclavian vein (venous). The estimated incidence of TOS is 10 in 100,000.4 However, cadaveric studies have suggested that up to 90% of the population may have what is considered abnormal anatomy of the thoracic outlet,5 which in turn suggests a multifactorial etiology for symptomatic disease. TOS is most commonly diagnosed in patients 20 to 40 years of age, with females affected in a 4:1 ratio.6 Although historically TOS is a clinical diagnosis, advanced imaging is often helpful in determining the nature and location of the structure undergoing compression and the structure producing compression, which help guide management. Computed tomography angiography (CTA) and magnetic resonance imaging (MRI) performed in association with postural maneuvers aid in the diagnosis in patients with dynamically acquired compression.7
Pathophysiology
The pathophysiology of TOS is attributable to the unique anatomy of the thoracic outlet. Compromise of the neurovascular structures can occur through congenital or acquired narrowing in 3 distinct compartments: the interscalene triangle, the costoclavicular space, and the retropectoralis minor space. The interscalene triangle is the most medial of the compartments. Containing the subclavian artery and the 3 trunks of the brachial plexus, it is bordered anteriorly by the anterior scalene muscle, posteriorly by the middle and posterior scalene muscles, and inferiorly by the first rib. The interscalene triangle is the most frequent site of neurologic compression.8 The middle compartment is the costoclavicular space, which is bordered superiorly by the clavicle, anteriorly by the subclavius muscle, and posteriorly by the first rib and the middle scalene muscle. The costoclavicular space is the most frequent site of arterial compression,8 where the artery lies directly anterior to the subclavian vein and is surrounded by the 3 cords of the brachial plexus. The most lateral compartment is the retropectoralis minor space, which is bordered anteriorly by the pectoralis minor muscle, superiorly by the subscapularis muscle, and inferiorly by the anterior chest wall. Sources of neurovascular compression within any of the spaces include cervical ribs9; elongated C7 transverse processes; hypertrophy of the anterior or middle scalene, subclavius, or pectoralis minor muscles10; anomalous scalenus minimus muscle; repetitive overhead arm movements (pitching, swimming)11; anomalous fascial bands; degenerative spine disease; bone destruction from primary or secondary neoplasms (Pancoast tumor); hyperextension/flexion injury of the neck12; and malunion of clavicle fractures, among others.13
Classification
Three distinct TOSs have been described, individually or combined, depending on the injured component: neurogenic from brachial plexus compression, arterial from subclavian artery compression, and venous from subclavian or axillary vein compression.14,15
Neurogenic TOS has 2 reported types: true (classic) and disputed. True neurogenic TOS is rare, with an estimated incidence of 1 in 1 million.16 First described in 1970 as a lower trunk plexopathy involving slowly progressive unilateral weakness of the intrinsic hand muscles and sensory abnormalities in the ulnar and medial antebrachial cutaneous nerve distributions, true neurogenic TOS was originally called Gilliatt-Sumner hand syndrome.17 A congenital band extending between the first rib and an elongated C7 transverse process was thought to be the location of brachial plexus injury in true neurogenic TOS. Conversely, disputed neurogenic TOS is the most common form of TOS, occurring in 3 to 80 per 100018 and accounting for 90% to 95% of all TOS cases.13,19 In contrast to true neurogenic TOS, in which anatomical and electrodiagnostic evidence supports the diagnosis, objective clinical findings are often lacking in the disputed form.18 Patients with disputed neurogenic TOS present with a diverse array of symptoms, including pain, numbness, and weakness affecting the neck, shoulder, and arm, exacerbated by activities requiring elevation or sustained use of the extremity.20
Arterial TOS accounts for 1% to 5% of all TOS cases.21 Arterial TOS typically affects patients who perform repetitive movements of the upper extremities with their arms above their shoulders, resulting in compression of the subclavian artery. Symptoms of arterial TOS include pain, weakness, coolness, pallor, and paresthesia.18,22 In severe cases of compression, subclavian artery damage can result in thrombosis with distal embolization, poststenotic aneurysm, or even retrograde extension causing stroke.22,23
Last, representing 2% to 3% of all TOS cases, venous TOS results from compression of the subclavian or axillary vein.18,24 Two mechanisms for vascular compromise have been described. The first involves compression of the vein between the clavicle and the first rib with overhead activities.18 Patients often experience intermittent “heaviness” of the extremity with repeated overhead use. The second mechanism involves repeated stress between the clavicle and vein, causing an intravascular thrombosis.18 Patients may experience pain, edema, cyanosis, venous distention, and even spontaneous venous thrombosis, referred to as Paget-Schroetter syndrome, which can lead to pulmonary embolism.6,25,26
Clinical Features
In cases of suspected TOS, clinicians should take a thorough history and perform a thorough physical examination. The differential diagnosis for unilateral, upper limb pain, numbness, tingling, and/or weakness exacerbated by movement includes shoulder and rotator cuff pathology, cervical spine injury, cervical radiculitis, distal compressive neuropathies (carpal or cubital tunnel syndrome), and neuralgic amyotrophy (Parsonage-Turner syndrome/acute brachial radiculitis).27,28 The clinician should pursue a history of trauma to the shoulder or neck as well as any occupational or recreational activities involving elevation of the upper extremity for extended periods.29 Physical examination must include an evaluation of the contralateral side and may begin with visual inspection to assess for muscle asymmetry, atrophy, color changes, edema, or deformities.18 Next, palpation should be used to assess for any tenderness, texture changes, masses, or vascular pulsations. Attention should be directed at examination of the cervical spine as well as neurologic and vascular assessments of the bilateral upper extremities, including range of motion and strength testing,18 to rule out alternative etiologies.
Four basic maneuvers—the Roos test,30 Adson test,31 Wright test,32 and costoclavicular test—traditionally have been used to diagnose TOS. A positive Roos test involves symptom reproduction with the patient slowly opening and closing the hand for 3 minutes with the arm externally rotated and abducted to 90°.33 However, the false-positive rate of the Roos test is as high as 77% in patients with carpal tunnel syndrome and up to 47% in normal subjects.34 The Adson test is performed by having the patient inhale deeply while the arm is kept in the anatomical position with the head extended and turned toward the involved extremity. The examiner monitors the radial pulse; an absent or diminished radial pulse suggests compression of the subclavian artery. The Adson test is not very reliable, however, because the pulse diminishes even in normal subjects,6,26 with a reported false-positive rate of 13.5%.35 A positive costoclavicular compression test occurs when depressing a patient’s shoulder reproduces symptoms. In one study, the false-positive rate of the costoclavicular compression test was 48% in patients with carpal tunnel syndrome and 16% in normal subjects.34 Last, the Wright test is performed by hyperabducting and externally rotating the affected shoulder. It is positive with a diminished pulse or reproduction of symptoms. One study found that the Wright test had 70% to 90% sensitivity and 29% to 53% specificity.36
Clinically distinguishing between the various forms of TOS may be difficult, and occasionally multiple types exist in a single patient, exacerbating one another and adding to the diagnostic difficulty. For example, arterial insufficiency may lead to disruption of the neural microcirculation, leading to concurrent arterial and neurogenic TOS. Because most cases present with nonspecific symptoms, advanced imaging modalities are often required to establish a definitive diagnosis and to target therapy to the appropriate site of compression.
Imaging Features
Plain Radiography
First, cervical spine and chest radiographs should be obtained to assess for bone abnormalities, including cervical ribs, long transverse processes, rib/clavicle fracture callus, rib anomalies, degenerative spine disease, and neoplasm (Pancoast/apical tumor) (Figure 1).18,25
Ultrasonography
Ultrasonography is useful in evaluating arterial or venous TOS because of its low cost, noninvasive nature, and high specificity for vessel occlusion.37,38 In arterial TOS, ultrasound may demonstrate increased flow velocity through a stenosis or an aneurysmal degeneration distal to the stenosis.7 In venous TOS, duplex ultrasound can identify stasis and thrombus.7 Obtaining duplex ultrasound with the upper extremity in multiple positions allows clinicians to correlate dynamically induced symptoms with ultrasonographic findings of altered blood flow.39-41 Despite the purported benefits of ultrasound, its drawback is that it is operator-dependent,42 with some studies reporting a high false-positive rate24 for diagnosis of venous TOS.
Electrodiagnostic Testing
Ruling out etiologies such as cervical radiculitis (Parsonage-Turner syndrome), cervical radiculopathies, brachial plexus lesions, and other distal compressive neuropathies requires nerve conduction studies and electromyography.18,43-46 In true neurogenic TOS, a combination of decreased sensory nerve action potentials in the ulnar and medial antebrachial cutaneous nerves and decreased compound motor action potentials in the median nerve is often found.18 Specifically, an abnormal ulnar sensory nerve action potential suggests the lesion is situated away from the intraspinal canal, which argues against a diagnosis of radiculopathy or myelopathy.43,44 In the disputed form of neurogenic TOS, the role of electrodiagnostic testing is less clear.18
Conventional Arteriography and Venography
Although CTA has superseded conventional arteriography and venography in most treatment centers, it may still be used in patients with acute symptoms requiring immediate thrombolytic therapy. Catheter angiography and venography with postural maneuvers are often the first invasive treatment modality in cases of thoracic outlet vascular compression.22,24 Presence of intraluminal thrombus, vessel dilatation, and collateral vessels is readily demonstrated (Figure 2A). Recanalization of occluded vessels can be attempted using balloon angioplasty and venoplasty (Figure 2B), but it is usually only temporarily successful if the cause of extrinsic compression is not corrected (Figures 2C, 2D). CTA or conventional angiography, used if sophisticated CTA with 3-dimensional (3-D) reconstruction is unavailable, is the gold standard in diagnosis of TOS.
CTA and Venography
Computed tomography (CT) is a valuable modality because it can be performed rapidly and effectively to depict the relationship of vascular structures to surrounding bone and muscle.47 In addition, CTA and venography provide high-quality representations of the vasculature, and 3-D reconstruction reliably identifies areas of neurovascular compression in patients with TOS.47,48 Furthermore, CT may be performed in a dynamic fashion, with the upper extremity in various positions to reproduce dynamic compression of the neurovascular structures (Figure 3A). Comparison of the images with the upper extremities in the anatomical position and elevated allows the physician to evaluate narrowing of the compartments and dynamic compression of neurovascular structures.8 CT is particularly valuable in arterial and venous TOS. In arterial TOS, the cross-sectional area or diameter of the artery can be measured to calculate the degree of stenosis.8,47 In venous TOS, dynamic narrowing of the vein can be visualized and may be associated with venous thrombosis or collateral circulation (Figure 3B). Although a variety of maneuvers is possible during CTA, the size of the CT tunnel as well as mandatory supine positioning of the patient may limit the series. Drawbacks of CT for diagnosing TOS include difficulties in analyzing the brachial plexus because of limited contrast resolution. In addition, the risks of CT (ionizing radiation, administration of iodinated contrast medium) must be considered before image acquisition.
MRI
MRI is a noninvasive and nonionizing technique that offers good resolution of the anatomical components of the thoracic outlet8 and that, because of its superior soft-tissue contrast, is the modality of choice for imaging brachial plexus nerve compression in TOS (Figure 4). Neurologic compression is identified with MRI when the fat surrounding the brachial plexus disappears.8 MRI reliably identifies the source of compression, which may include bony structures, muscle hypertrophy (scalenus, scalenus minimus, subclavius, pectoralis minor), and fibrous bands.49 Because of their craniocaudal direction, the sagittal plane is often most useful in demonstrating neurovascular compression.42 Analyzing the caliber of the vessel along its course may evaluate vascular compression, and magnetic resonance (MR) angiography and venography (Figures 5A, 5B) can often complement the findings.50 Specifically, in arterial TOS, poststenotic aneurysmal dilatation may be seen, whereas thrombosis and collateral circulation can be visualized in cases of venous TOS.50 Limitations of MRI in the diagnosis of TOS historically were similar to those of CT, and included supine positioning as well as restricted upper extremity maneuvers because of the size of the tunnel and the presence of surface coils.42 However, newer higher channel surface coils and wider bores allow for imaging in a wider range of motion, including arm hyperabduction (Figures 5C, 5D), which is often necessary to elicit pathology.
Management
Generally, therapeutic options for TOS are aimed at relieving the source of neurovascular compression. It is important that treatment be directed only toward symptomatic patients, as many patients have anatomy consistent with TOS and remain asymptomatic.5 Treatment of TOS is predominately conservative and involves a combination of patient education, activity modification, medication, and rehabilitation to promote appropriate body mechanics and posture.18
Physical Therapy
Physical therapy should be aimed at decreasing pressure on the neurovascular structures of the thoracic outlet by relaxing the scalene muscles, strengthening the shoulder muscles, and working on postural exercises to help the patient sit and stand straighter.51 The scalene muscles are the primary targets for TOS rehabilitation, but focus should also be given to the upper trapezius, levator scapulae, sternocleidomastoid, pectoral, and suboccipital muscles.18 Physical therapy is often combined with hydrotherapy, massage, nonsteroidal anti-inflammatory drugs, and muscle relaxants for maximal symptomatic relief. Some patients have found relief with selective anesthetic or botulinum toxin A injections in the scalene muscles.18 A minimum of 4 to 6 weeks (often 4-6 months) of physical therapy and conservative treatment should be attempted before consideration of any invasive intervention.13,18
Anticoagulation
In venous TOS with evidence of thrombus but no obstructive clot, conservative management is typically sufficient. In rare cases, however, intimal damage secondary to vascular compression in arterial and venous TOS leads to thrombus formation, impairing upper extremity perfusion and producing symptoms. Treatment guidelines for venous TOS involve catheter-directed thrombolysis within 2 weeks of symptom onset.15 Thrombolysis replaced the prior recommendation of systemic anticoagulation combined with extremity rest and elevation because anticoagulation and rest alone result in up to 75% morbidity,52,53 whereas thrombolysis reestablishes vessel patency in nearly all patients.54 After thrombolysis, patients should receive intravenous heparin, and conversion to oral anticoagulation should occur as soon as manageable. In patients with arterial TOS, the goal of treatment is revascularization to prevent or decrease ischemia. In mild arterial ischemia, catheter-directed thrombolysis can be attempted. However, the threshold for surgical thromboembolectomy must remain low, as acute upper extremity ischemia may result in compartment syndrome and permanent loss of function.13 Fixed arterial lesions, whether occlusive or aneurysmal, are an absolute indication for thromboembolectomy with possible thoracic outlet decompression.13
Thoracic Outlet Decompression
Indications for surgical decompression are controversial. They include symptomatic patients who have vascular (arterial or venous) TOS and are not at high risk for surgery, patients with true neurologic TOS and acute progressive neurologic weakness or disabling pain,55 and patients who have disputed neurologic TOS and have failed conservative management—keeping in mind that high recurrence rates and iatrogenic brachial plexopathy have been reported in this population.56 In general, surgical procedures are aimed at reducing soft-tissue compression (scalene release or neurolysis) or bony compression (cervical or first thoracic rib excision). Three surgical approaches (transaxillary, supraclavicular, infraclavicular) are commonly used for decompression, and surgeons choose one over another depending on the anatomical abnormality causing the compression. The transaxillary approach requires limited dissection but still allows for adequate visualization of the rib during resection.57 In this approach, a transverse incision along the inferior border of the axilla extends from the pectoralis major to the latissimus dorsi. After dissection of the axillary vessels and the first thoracic nerve root, the first rib is identified and can be removed, when indicated. In contrast, the supraclavicular approach provides a wide exposure, and the site of compression is directly visualized, allowing for arterial reconstruction.58 Through this approach, the anterior and middle scalene muscles can be resected, and neurolysis of the brachial plexus can be performed. Last, the infraclavicular approach allows for exposure of the central veins through extension of the incision medially, which allows for venous reconstruction. Some patients with neurogenic or arterial TOS present with symptoms of sympathetic overactivity, in which case cervical sympathectomy can be used with decompression.
Outcomes of surgical decompression for TOS depend on the clinical type but are generally good. For instance, in cases of disputed neurogenic TOS, symptom resolution after decompression is reportedly between 80% and 90%.59 However, major depression, work-related injuries,60 and diffuse preoperative arm symptoms61 all influence long-term results. In true neurogenic TOS, postoperative pain relief is often substantial, though recovery of strength can be slow because of the axonal injury.55 In arterial TOS, outcomes are influenced by time to surgical intervention, with early surgery demonstrating better outcomes than later surgery.62 In one study, Cormier and colleagues14 evaluated 47 patients who underwent correction of subclavian-axillary artery compression; 91% were asymptomatic a mean of 5.7 months after decompression. Last, outcomes of successful thrombolysis and decompression for venous TOS demonstrated patency rates higher than 95% at 5-year follow-up.54,63
Conclusions
TOS is a spectrum of disorders caused by compression of the brachial plexus, subclavian artery, or subclavian vein. Early recognition of TOS is imperative, as diagnostic or treatment delays may be associated with significant morbidity. Clinical examination alone is often inadequate for determining the compression site and the structure causing compression. CTA and MRI performed in association with postural maneuvers may demonstrate dynamic compression of the neurovascular structures in the thoracic outlet. These imaging modalities reliably identify the structures causing compression and can be crucial for effective management.
Thoracic outlet syndrome (TOS) was first described by Coot in 1861,1,2 and the term was coined by Peet and colleagues3 in 1956 to cover a spectrum of conditions caused by dynamic compression of the brachial plexus (neurogenic), subclavian artery (arterial), or subclavian vein (venous). The estimated incidence of TOS is 10 in 100,000.4 However, cadaveric studies have suggested that up to 90% of the population may have what is considered abnormal anatomy of the thoracic outlet,5 which in turn suggests a multifactorial etiology for symptomatic disease. TOS is most commonly diagnosed in patients 20 to 40 years of age, with females affected in a 4:1 ratio.6 Although historically TOS is a clinical diagnosis, advanced imaging is often helpful in determining the nature and location of the structure undergoing compression and the structure producing compression, which help guide management. Computed tomography angiography (CTA) and magnetic resonance imaging (MRI) performed in association with postural maneuvers aid in the diagnosis in patients with dynamically acquired compression.7
Pathophysiology
The pathophysiology of TOS is attributable to the unique anatomy of the thoracic outlet. Compromise of the neurovascular structures can occur through congenital or acquired narrowing in 3 distinct compartments: the interscalene triangle, the costoclavicular space, and the retropectoralis minor space. The interscalene triangle is the most medial of the compartments. Containing the subclavian artery and the 3 trunks of the brachial plexus, it is bordered anteriorly by the anterior scalene muscle, posteriorly by the middle and posterior scalene muscles, and inferiorly by the first rib. The interscalene triangle is the most frequent site of neurologic compression.8 The middle compartment is the costoclavicular space, which is bordered superiorly by the clavicle, anteriorly by the subclavius muscle, and posteriorly by the first rib and the middle scalene muscle. The costoclavicular space is the most frequent site of arterial compression,8 where the artery lies directly anterior to the subclavian vein and is surrounded by the 3 cords of the brachial plexus. The most lateral compartment is the retropectoralis minor space, which is bordered anteriorly by the pectoralis minor muscle, superiorly by the subscapularis muscle, and inferiorly by the anterior chest wall. Sources of neurovascular compression within any of the spaces include cervical ribs9; elongated C7 transverse processes; hypertrophy of the anterior or middle scalene, subclavius, or pectoralis minor muscles10; anomalous scalenus minimus muscle; repetitive overhead arm movements (pitching, swimming)11; anomalous fascial bands; degenerative spine disease; bone destruction from primary or secondary neoplasms (Pancoast tumor); hyperextension/flexion injury of the neck12; and malunion of clavicle fractures, among others.13
Classification
Three distinct TOSs have been described, individually or combined, depending on the injured component: neurogenic from brachial plexus compression, arterial from subclavian artery compression, and venous from subclavian or axillary vein compression.14,15
Neurogenic TOS has 2 reported types: true (classic) and disputed. True neurogenic TOS is rare, with an estimated incidence of 1 in 1 million.16 First described in 1970 as a lower trunk plexopathy involving slowly progressive unilateral weakness of the intrinsic hand muscles and sensory abnormalities in the ulnar and medial antebrachial cutaneous nerve distributions, true neurogenic TOS was originally called Gilliatt-Sumner hand syndrome.17 A congenital band extending between the first rib and an elongated C7 transverse process was thought to be the location of brachial plexus injury in true neurogenic TOS. Conversely, disputed neurogenic TOS is the most common form of TOS, occurring in 3 to 80 per 100018 and accounting for 90% to 95% of all TOS cases.13,19 In contrast to true neurogenic TOS, in which anatomical and electrodiagnostic evidence supports the diagnosis, objective clinical findings are often lacking in the disputed form.18 Patients with disputed neurogenic TOS present with a diverse array of symptoms, including pain, numbness, and weakness affecting the neck, shoulder, and arm, exacerbated by activities requiring elevation or sustained use of the extremity.20
Arterial TOS accounts for 1% to 5% of all TOS cases.21 Arterial TOS typically affects patients who perform repetitive movements of the upper extremities with their arms above their shoulders, resulting in compression of the subclavian artery. Symptoms of arterial TOS include pain, weakness, coolness, pallor, and paresthesia.18,22 In severe cases of compression, subclavian artery damage can result in thrombosis with distal embolization, poststenotic aneurysm, or even retrograde extension causing stroke.22,23
Last, representing 2% to 3% of all TOS cases, venous TOS results from compression of the subclavian or axillary vein.18,24 Two mechanisms for vascular compromise have been described. The first involves compression of the vein between the clavicle and the first rib with overhead activities.18 Patients often experience intermittent “heaviness” of the extremity with repeated overhead use. The second mechanism involves repeated stress between the clavicle and vein, causing an intravascular thrombosis.18 Patients may experience pain, edema, cyanosis, venous distention, and even spontaneous venous thrombosis, referred to as Paget-Schroetter syndrome, which can lead to pulmonary embolism.6,25,26
Clinical Features
In cases of suspected TOS, clinicians should take a thorough history and perform a thorough physical examination. The differential diagnosis for unilateral, upper limb pain, numbness, tingling, and/or weakness exacerbated by movement includes shoulder and rotator cuff pathology, cervical spine injury, cervical radiculitis, distal compressive neuropathies (carpal or cubital tunnel syndrome), and neuralgic amyotrophy (Parsonage-Turner syndrome/acute brachial radiculitis).27,28 The clinician should pursue a history of trauma to the shoulder or neck as well as any occupational or recreational activities involving elevation of the upper extremity for extended periods.29 Physical examination must include an evaluation of the contralateral side and may begin with visual inspection to assess for muscle asymmetry, atrophy, color changes, edema, or deformities.18 Next, palpation should be used to assess for any tenderness, texture changes, masses, or vascular pulsations. Attention should be directed at examination of the cervical spine as well as neurologic and vascular assessments of the bilateral upper extremities, including range of motion and strength testing,18 to rule out alternative etiologies.
Four basic maneuvers—the Roos test,30 Adson test,31 Wright test,32 and costoclavicular test—traditionally have been used to diagnose TOS. A positive Roos test involves symptom reproduction with the patient slowly opening and closing the hand for 3 minutes with the arm externally rotated and abducted to 90°.33 However, the false-positive rate of the Roos test is as high as 77% in patients with carpal tunnel syndrome and up to 47% in normal subjects.34 The Adson test is performed by having the patient inhale deeply while the arm is kept in the anatomical position with the head extended and turned toward the involved extremity. The examiner monitors the radial pulse; an absent or diminished radial pulse suggests compression of the subclavian artery. The Adson test is not very reliable, however, because the pulse diminishes even in normal subjects,6,26 with a reported false-positive rate of 13.5%.35 A positive costoclavicular compression test occurs when depressing a patient’s shoulder reproduces symptoms. In one study, the false-positive rate of the costoclavicular compression test was 48% in patients with carpal tunnel syndrome and 16% in normal subjects.34 Last, the Wright test is performed by hyperabducting and externally rotating the affected shoulder. It is positive with a diminished pulse or reproduction of symptoms. One study found that the Wright test had 70% to 90% sensitivity and 29% to 53% specificity.36
Clinically distinguishing between the various forms of TOS may be difficult, and occasionally multiple types exist in a single patient, exacerbating one another and adding to the diagnostic difficulty. For example, arterial insufficiency may lead to disruption of the neural microcirculation, leading to concurrent arterial and neurogenic TOS. Because most cases present with nonspecific symptoms, advanced imaging modalities are often required to establish a definitive diagnosis and to target therapy to the appropriate site of compression.
Imaging Features
Plain Radiography
First, cervical spine and chest radiographs should be obtained to assess for bone abnormalities, including cervical ribs, long transverse processes, rib/clavicle fracture callus, rib anomalies, degenerative spine disease, and neoplasm (Pancoast/apical tumor) (Figure 1).18,25
Ultrasonography
Ultrasonography is useful in evaluating arterial or venous TOS because of its low cost, noninvasive nature, and high specificity for vessel occlusion.37,38 In arterial TOS, ultrasound may demonstrate increased flow velocity through a stenosis or an aneurysmal degeneration distal to the stenosis.7 In venous TOS, duplex ultrasound can identify stasis and thrombus.7 Obtaining duplex ultrasound with the upper extremity in multiple positions allows clinicians to correlate dynamically induced symptoms with ultrasonographic findings of altered blood flow.39-41 Despite the purported benefits of ultrasound, its drawback is that it is operator-dependent,42 with some studies reporting a high false-positive rate24 for diagnosis of venous TOS.
Electrodiagnostic Testing
Ruling out etiologies such as cervical radiculitis (Parsonage-Turner syndrome), cervical radiculopathies, brachial plexus lesions, and other distal compressive neuropathies requires nerve conduction studies and electromyography.18,43-46 In true neurogenic TOS, a combination of decreased sensory nerve action potentials in the ulnar and medial antebrachial cutaneous nerves and decreased compound motor action potentials in the median nerve is often found.18 Specifically, an abnormal ulnar sensory nerve action potential suggests the lesion is situated away from the intraspinal canal, which argues against a diagnosis of radiculopathy or myelopathy.43,44 In the disputed form of neurogenic TOS, the role of electrodiagnostic testing is less clear.18
Conventional Arteriography and Venography
Although CTA has superseded conventional arteriography and venography in most treatment centers, it may still be used in patients with acute symptoms requiring immediate thrombolytic therapy. Catheter angiography and venography with postural maneuvers are often the first invasive treatment modality in cases of thoracic outlet vascular compression.22,24 Presence of intraluminal thrombus, vessel dilatation, and collateral vessels is readily demonstrated (Figure 2A). Recanalization of occluded vessels can be attempted using balloon angioplasty and venoplasty (Figure 2B), but it is usually only temporarily successful if the cause of extrinsic compression is not corrected (Figures 2C, 2D). CTA or conventional angiography, used if sophisticated CTA with 3-dimensional (3-D) reconstruction is unavailable, is the gold standard in diagnosis of TOS.
CTA and Venography
Computed tomography (CT) is a valuable modality because it can be performed rapidly and effectively to depict the relationship of vascular structures to surrounding bone and muscle.47 In addition, CTA and venography provide high-quality representations of the vasculature, and 3-D reconstruction reliably identifies areas of neurovascular compression in patients with TOS.47,48 Furthermore, CT may be performed in a dynamic fashion, with the upper extremity in various positions to reproduce dynamic compression of the neurovascular structures (Figure 3A). Comparison of the images with the upper extremities in the anatomical position and elevated allows the physician to evaluate narrowing of the compartments and dynamic compression of neurovascular structures.8 CT is particularly valuable in arterial and venous TOS. In arterial TOS, the cross-sectional area or diameter of the artery can be measured to calculate the degree of stenosis.8,47 In venous TOS, dynamic narrowing of the vein can be visualized and may be associated with venous thrombosis or collateral circulation (Figure 3B). Although a variety of maneuvers is possible during CTA, the size of the CT tunnel as well as mandatory supine positioning of the patient may limit the series. Drawbacks of CT for diagnosing TOS include difficulties in analyzing the brachial plexus because of limited contrast resolution. In addition, the risks of CT (ionizing radiation, administration of iodinated contrast medium) must be considered before image acquisition.
MRI
MRI is a noninvasive and nonionizing technique that offers good resolution of the anatomical components of the thoracic outlet8 and that, because of its superior soft-tissue contrast, is the modality of choice for imaging brachial plexus nerve compression in TOS (Figure 4). Neurologic compression is identified with MRI when the fat surrounding the brachial plexus disappears.8 MRI reliably identifies the source of compression, which may include bony structures, muscle hypertrophy (scalenus, scalenus minimus, subclavius, pectoralis minor), and fibrous bands.49 Because of their craniocaudal direction, the sagittal plane is often most useful in demonstrating neurovascular compression.42 Analyzing the caliber of the vessel along its course may evaluate vascular compression, and magnetic resonance (MR) angiography and venography (Figures 5A, 5B) can often complement the findings.50 Specifically, in arterial TOS, poststenotic aneurysmal dilatation may be seen, whereas thrombosis and collateral circulation can be visualized in cases of venous TOS.50 Limitations of MRI in the diagnosis of TOS historically were similar to those of CT, and included supine positioning as well as restricted upper extremity maneuvers because of the size of the tunnel and the presence of surface coils.42 However, newer higher channel surface coils and wider bores allow for imaging in a wider range of motion, including arm hyperabduction (Figures 5C, 5D), which is often necessary to elicit pathology.
Management
Generally, therapeutic options for TOS are aimed at relieving the source of neurovascular compression. It is important that treatment be directed only toward symptomatic patients, as many patients have anatomy consistent with TOS and remain asymptomatic.5 Treatment of TOS is predominately conservative and involves a combination of patient education, activity modification, medication, and rehabilitation to promote appropriate body mechanics and posture.18
Physical Therapy
Physical therapy should be aimed at decreasing pressure on the neurovascular structures of the thoracic outlet by relaxing the scalene muscles, strengthening the shoulder muscles, and working on postural exercises to help the patient sit and stand straighter.51 The scalene muscles are the primary targets for TOS rehabilitation, but focus should also be given to the upper trapezius, levator scapulae, sternocleidomastoid, pectoral, and suboccipital muscles.18 Physical therapy is often combined with hydrotherapy, massage, nonsteroidal anti-inflammatory drugs, and muscle relaxants for maximal symptomatic relief. Some patients have found relief with selective anesthetic or botulinum toxin A injections in the scalene muscles.18 A minimum of 4 to 6 weeks (often 4-6 months) of physical therapy and conservative treatment should be attempted before consideration of any invasive intervention.13,18
Anticoagulation
In venous TOS with evidence of thrombus but no obstructive clot, conservative management is typically sufficient. In rare cases, however, intimal damage secondary to vascular compression in arterial and venous TOS leads to thrombus formation, impairing upper extremity perfusion and producing symptoms. Treatment guidelines for venous TOS involve catheter-directed thrombolysis within 2 weeks of symptom onset.15 Thrombolysis replaced the prior recommendation of systemic anticoagulation combined with extremity rest and elevation because anticoagulation and rest alone result in up to 75% morbidity,52,53 whereas thrombolysis reestablishes vessel patency in nearly all patients.54 After thrombolysis, patients should receive intravenous heparin, and conversion to oral anticoagulation should occur as soon as manageable. In patients with arterial TOS, the goal of treatment is revascularization to prevent or decrease ischemia. In mild arterial ischemia, catheter-directed thrombolysis can be attempted. However, the threshold for surgical thromboembolectomy must remain low, as acute upper extremity ischemia may result in compartment syndrome and permanent loss of function.13 Fixed arterial lesions, whether occlusive or aneurysmal, are an absolute indication for thromboembolectomy with possible thoracic outlet decompression.13
Thoracic Outlet Decompression
Indications for surgical decompression are controversial. They include symptomatic patients who have vascular (arterial or venous) TOS and are not at high risk for surgery, patients with true neurologic TOS and acute progressive neurologic weakness or disabling pain,55 and patients who have disputed neurologic TOS and have failed conservative management—keeping in mind that high recurrence rates and iatrogenic brachial plexopathy have been reported in this population.56 In general, surgical procedures are aimed at reducing soft-tissue compression (scalene release or neurolysis) or bony compression (cervical or first thoracic rib excision). Three surgical approaches (transaxillary, supraclavicular, infraclavicular) are commonly used for decompression, and surgeons choose one over another depending on the anatomical abnormality causing the compression. The transaxillary approach requires limited dissection but still allows for adequate visualization of the rib during resection.57 In this approach, a transverse incision along the inferior border of the axilla extends from the pectoralis major to the latissimus dorsi. After dissection of the axillary vessels and the first thoracic nerve root, the first rib is identified and can be removed, when indicated. In contrast, the supraclavicular approach provides a wide exposure, and the site of compression is directly visualized, allowing for arterial reconstruction.58 Through this approach, the anterior and middle scalene muscles can be resected, and neurolysis of the brachial plexus can be performed. Last, the infraclavicular approach allows for exposure of the central veins through extension of the incision medially, which allows for venous reconstruction. Some patients with neurogenic or arterial TOS present with symptoms of sympathetic overactivity, in which case cervical sympathectomy can be used with decompression.
Outcomes of surgical decompression for TOS depend on the clinical type but are generally good. For instance, in cases of disputed neurogenic TOS, symptom resolution after decompression is reportedly between 80% and 90%.59 However, major depression, work-related injuries,60 and diffuse preoperative arm symptoms61 all influence long-term results. In true neurogenic TOS, postoperative pain relief is often substantial, though recovery of strength can be slow because of the axonal injury.55 In arterial TOS, outcomes are influenced by time to surgical intervention, with early surgery demonstrating better outcomes than later surgery.62 In one study, Cormier and colleagues14 evaluated 47 patients who underwent correction of subclavian-axillary artery compression; 91% were asymptomatic a mean of 5.7 months after decompression. Last, outcomes of successful thrombolysis and decompression for venous TOS demonstrated patency rates higher than 95% at 5-year follow-up.54,63
Conclusions
TOS is a spectrum of disorders caused by compression of the brachial plexus, subclavian artery, or subclavian vein. Early recognition of TOS is imperative, as diagnostic or treatment delays may be associated with significant morbidity. Clinical examination alone is often inadequate for determining the compression site and the structure causing compression. CTA and MRI performed in association with postural maneuvers may demonstrate dynamic compression of the neurovascular structures in the thoracic outlet. These imaging modalities reliably identify the structures causing compression and can be crucial for effective management.
1. Urschel HC Jr. The history of surgery for thoracic outlet syndrome. Chest Surg Clin North Am. 2000;10(1):183-188, x-xi.
2. Atasoy E. History of thoracic outlet syndrome. Hand Clin. 2004;20(1):15-16, v.
3. Peet RM, Henriksen JD, Anderson TP, Martin GM. Thoracic-outlet syndrome: evaluation of a therapeutic exercise program. Proc Staff Meet Mayo Clin. 1956;31(9):281-287.
4. Edwards DP, Mulkern E, Raja AN, Barker P. Trans-axillary first rib excision for thoracic outlet syndrome. J R Coll Surg Edinb. 1999;44(6):362-365.
5. Juvonen T, Satta J, Laitala P, Luukkonen K, Nissinen J. Anomalies at the thoracic outlet are frequent in the general population. Am J Surg. 1995;170(1):33-37.
6. Atasoy E. Thoracic outlet compression syndrome. Orthop Clin North Am. 1996;27(2):265-303.
7. Demondion X, Herbinet P, Van Sint Jan S, Boutry N, Chantelot C, Cotten A. Imaging assessment of thoracic outlet syndrome. Radiographics. 2006;26(6):1735-1750.
8. Demondion X, Bacqueville E, Paul C, Duquesnoy B, Hachulla E, Cotten A. Thoracic outlet: assessment with MR imaging in asymptomatic and symptomatic populations. Radiology. 2003;227(2):461-468.
9. Makhoul RG, Machleder HI. Developmental anomalies at the thoracic outlet: an analysis of 200 consecutive cases. J Vasc Surg. 1992;16(4):534-542.
10. Sanders RJ, Jackson CG, Banchero N, Pearce WH. Scalene muscle abnormalities in traumatic thoracic outlet syndrome. Am J Surg. 1990;159(2):231-236.
11. Katirji B, Hardy RW Jr. Classic neurogenic thoracic outlet syndrome in a competitive swimmer: a true scalenus anticus syndrome. Muscle Nerve. 1995;18(2):229-233.
12. Casbas L, Chauffour X, Cau J, et al. Post-traumatic thoracic outlet syndromes. Ann Vasc Surg. 2005;19(1):25-28.
13. Povlsen B, Belzberg A, Hansson T, Dorsi M. Treatment for thoracic outlet syndrome. Cochrane Database Syst Rev. 2010;(1):CD007218.
14. Cormier JM, Amrane M, Ward A, Laurian C, Gigou F. Arterial complications of the thoracic outlet syndrome: fifty-five operative cases. J Vasc Surg. 1989;9(6):778-787.
15. Hood DB, Kuehne J, Yellin AE, Weaver FA. Vascular complications of thoracic outlet syndrome. Am Surg. 1997;63(10):913-917.
16. Ferrante MA. Brachial plexopathies: classification, causes, and consequences. Muscle Nerve. 2004;30(5):547-568.
17. Gilliatt RW, Le Quesne PM, Logue V, Sumner AJ. Wasting of the hand associated with a cervical rib or band. J Neurol Neurosurg Psychiatry. 1970;33(5):615-624.
18. Ozoa G, Alves D, Fish DE. Thoracic outlet syndrome. Phys Med Rehabil Clin North Am. 2011;22(3):473-483, viii-ix.
19. Schwartzman RJ. Brachial plexus traction injuries. Hand Clin. 1991;7(3):547-556.
20. Christo PJ, McGreevy K. Updated perspectives on neurogenic thoracic outlet syndrome. Curr Pain Headache Rep. 2011;15(1):14-21.
21. Vanti C, Natalini L, Romeo A, Tosarelli D, Pillastrini P. Conservative treatment of thoracic outlet syndrome. A review of the literature. Eura Medicophys. 2007;43(1):55-70.22. Patton GM. Arterial thoracic outlet syndrome. Hand Clin. 2004;20(1):107-111, viii.
23. Lee TS, Hines GL. Cerebral embolic stroke and arm ischemia in a teenager with arterial thoracic outlet syndrome: a case report. Vasc Endovasc Surg. 2007;41(3):254-257.
24. Sanders RJ, Hammond SL. Venous thoracic outlet syndrome. Hand Clin. 2004;20(1):113-118, viii.
25. Sanders RJ, Hammond SL, Rao NM. Diagnosis of thoracic outlet syndrome. J Vasc Surg. 2007;46(3):601-604.
26. Luoma A, Nelems B. Thoracic outlet syndrome. Thoracic surgery perspective. Neurosurg Clin North Am. 1991;2(1):187-226.
27. Cup EH, Ijspeert J, Janssen RJ, et al. Residual complaints after neuralgic amyotrophy. Arch Phys Med Rehabil. 2013;94(1):67-73.
28. van Alfen N, van Engelen BG. The clinical spectrum of neuralgic amyotrophy in 246 cases. Brain. 2006;129(pt 2):438-450.
29. Nichols AW. The thoracic outlet syndrome in athletes. J Am Board Fam Pract. 1996;9(5):346-355.
30. Roos DB, Owens JC. Thoracic outlet syndrome. Arch Surg. 1966;93(1):71-74.
31. Adson AW, Coffey JR. Cervical rib: a method of anterior approach for relief of symptoms by division of the scalenus anticus. Ann Surg. 1927;85(6):839-857.
32. Wright IS. The neurovascular syndrome produced by hyperabduction of the arms. Am Heart J. 1945;29:1-19.
33. Rayan GM, Jensen C. Thoracic outlet syndrome: provocative examination maneuvers in a typical population. J Shoulder Elbow Surg. 1995;4(2):113-117.
34. Nord KM, Kapoor P, Fisher J, et al. False positive rate of thoracic outlet syndrome diagnostic maneuvers. Electromyogr Clin Neurophysiol. 2008;48(2):67-74.
35. Novak CB. Thoracic outlet syndrome. Clin Plast Surg. 2003;30(2):175-188.
36. Gillard J, Pérez-Cousin M, Hachulla E, et al. Diagnosing thoracic outlet syndrome: contribution of provocative tests, ultrasonography, electrophysiology, and helical computed tomography in 48 patients. Joint Bone Spine. 2001;68(5):416-424.
37. Baxter GM, Kincaid W, Jeffrey RF, Millar GM, Porteous C, Morley P. Comparison of colour Doppler ultrasound with venography in the diagnosis of axillary and subclavian vein thrombosis. Br J Radiol. 1991;64(765):777-781.
38. Passman MA, Criado E, Farber MA, et al. Efficacy of color flow duplex imaging for proximal upper extremity venous outflow obstruction in hemodialysis patients. J Vasc Surg. 1998;28(5):869-875.
39. Wadhwani R, Chaubal N, Sukthankar R, Shroff M, Agarwala S. Color Doppler and duplex sonography in 5 patients with thoracic outlet syndrome. J Ultrasound Med. 2001;20(7):795-801.
40. Napoli V, Vignali C, Braccini G, et al. Echography and echo-Doppler in the study of thoracic outlet syndrome. Correlation with angiographic data [in Italian]. Radiol Med. 1993;85(6):733-740.
41. Longley DG, Yedlicka JW, Molina EJ, Schwabacher S, Hunter DW, Letourneau JG. Thoracic outlet syndrome: evaluation of the subclavian vessels by color duplex sonography. AJR Am J Roentgenol. 1992;158(3):623-630.
42. Demondion X, Herbinet P, Boutry N, Fontaine C, Francke JP, Cotten A. Sonographic mapping of the normal brachial plexus. AJNR Am J Neuroradiol. 2003;24(7):1303-1309.
43. Cruz-Martinez A, Arpa J. Electrophysiological assessment in neurogenic thoracic outlet syndrome. Electromyogr Clin Neurophysiol. 2001;41(4):253-256.
44. Ferrante MA, Wilbourn AJ. The utility of various sensory nerve conduction responses in assessing brachial plexopathies. Muscle Nerve. 1995;18(8):879-889.
45. Aminoff MJ, Olney RK, Parry GJ, Raskin NH. Relative utility of different electrophysiologic techniques in the evaluation of brachial plexopathies. Neurology. 1988;38(4):546-550.
46. Komanetsky RM, Novak CB, Mackinnon SE, Russo MH, Padberg AM, Louis S. Somatosensory evoked potentials fail to diagnose thoracic outlet syndrome. J Hand Surg Am. 1996;21(4):662-666.
47. Remy-Jardin M, Remy J, Masson P, et al. Helical CT angiography of thoracic outlet syndrome: functional anatomy. AJR Am J Roentgenol. 2000;174(6):1667-1674.
48. Matsumura JS, Rilling WS, Pearce WH, Nemcek AA Jr, Vogelzang RL, Yao JS. Helical computed tomography of the normal thoracic outlet. J Vasc Surg. 1997;26(5):776-783.
49. Dymarkowski S, Bosmans H, Marchal G, Bogaert J. Three-dimensional MR angiography in the evaluation of thoracic outlet syndrome. AJR Am J Roentgenol. 1999;173(4):1005-1008.
50. Charon JP, Milne W, Sheppard DG, Houston JG. Evaluation of MR angiographic technique in the assessment of thoracic outlet syndrome. Clin Radiol. 2004;59(7):588-595.
51. Cuetter AC, Bartoszek DM. The thoracic outlet syndrome: controversies, overdiagnosis, overtreatment, and recommendations for management. Muscle Nerve. 1989;12(5):410-419.
52. Urschel HC Jr, Razzuk MA. Paget-Schroetter syndrome: what is the best management? Ann Thorac Surg. 2000;69(6):1663-1668.
53. Lee JT, Karwowski JK, Harris EJ, Haukoos JS, Olcott C 4th. Long-term thrombotic recurrence after nonoperative management of Paget-Schroetter syndrome. J Vasc Surg. 2006;43(6):1236-1243.
54. Molina JE, Hunter DW, Dietz CA. Paget-Schroetter syndrome treated with thrombolytics and immediate surgery. J Vasc Surg. 2007;45(2):328-334.
55. Le Forestier N, Mouton P, Maisonobe T, et al. True neurological thoracic outlet syndrome [in French]. Rev Neurol (Paris). 2000;156(1):34-40.
56. Wilbourn AJ. Thoracic outlet syndrome surgery causing severe brachial plexopathy. Muscle Nerve. 1988;11(1):66-74.
57. Likes K, Dapash T, Rochlin DH, Freischlag JA. Remaining or residual first ribs are the cause of recurrent thoracic outlet syndrome. Ann Vasc Surg. 2014;28(4):939-945.
58. Aljabri B, Al-Omran M. Surgical management of vascular thoracic outlet syndrome: a teaching hospital experience. Ann Vasc Dis. 2013;6(1):74-79.
59. Sanders RJ, Pearce WH. The treatment of thoracic outlet syndrome: a comparison of different operations. J Vasc Surg. 1989;10(6):626-634.
60. Franklin GM, Fulton-Kehoe D, Bradley C, Smith-Weller T. Outcome of surgery for thoracic outlet syndrome in Washington state workers’ compensation. Neurology. 2000;54(6):1252-1257.
61. Axelrod DA, Proctor MC, Geisser ME, Roth RS, Greenfield LJ. Outcomes after surgery for thoracic outlet syndrome. J Vasc Surg. 2001;33(6):1220-1225.
62. Taylor JM, Telford RJ, Kinsella DC, Watkinson AF, Thompson JF. Long-term clinical and functional outcome following treatment for Paget-Schroetter syndrome. Br J Surg. 2013;100(11):1459-1464.
63. Schneider DB, Dimuzio PJ, Martin ND, et al. Combination treatment of venous thoracic outlet syndrome: open surgical decompression and intraoperative angioplasty. J Vasc Surg. 2004;40(4):599-603.
1. Urschel HC Jr. The history of surgery for thoracic outlet syndrome. Chest Surg Clin North Am. 2000;10(1):183-188, x-xi.
2. Atasoy E. History of thoracic outlet syndrome. Hand Clin. 2004;20(1):15-16, v.
3. Peet RM, Henriksen JD, Anderson TP, Martin GM. Thoracic-outlet syndrome: evaluation of a therapeutic exercise program. Proc Staff Meet Mayo Clin. 1956;31(9):281-287.
4. Edwards DP, Mulkern E, Raja AN, Barker P. Trans-axillary first rib excision for thoracic outlet syndrome. J R Coll Surg Edinb. 1999;44(6):362-365.
5. Juvonen T, Satta J, Laitala P, Luukkonen K, Nissinen J. Anomalies at the thoracic outlet are frequent in the general population. Am J Surg. 1995;170(1):33-37.
6. Atasoy E. Thoracic outlet compression syndrome. Orthop Clin North Am. 1996;27(2):265-303.
7. Demondion X, Herbinet P, Van Sint Jan S, Boutry N, Chantelot C, Cotten A. Imaging assessment of thoracic outlet syndrome. Radiographics. 2006;26(6):1735-1750.
8. Demondion X, Bacqueville E, Paul C, Duquesnoy B, Hachulla E, Cotten A. Thoracic outlet: assessment with MR imaging in asymptomatic and symptomatic populations. Radiology. 2003;227(2):461-468.
9. Makhoul RG, Machleder HI. Developmental anomalies at the thoracic outlet: an analysis of 200 consecutive cases. J Vasc Surg. 1992;16(4):534-542.
10. Sanders RJ, Jackson CG, Banchero N, Pearce WH. Scalene muscle abnormalities in traumatic thoracic outlet syndrome. Am J Surg. 1990;159(2):231-236.
11. Katirji B, Hardy RW Jr. Classic neurogenic thoracic outlet syndrome in a competitive swimmer: a true scalenus anticus syndrome. Muscle Nerve. 1995;18(2):229-233.
12. Casbas L, Chauffour X, Cau J, et al. Post-traumatic thoracic outlet syndromes. Ann Vasc Surg. 2005;19(1):25-28.
13. Povlsen B, Belzberg A, Hansson T, Dorsi M. Treatment for thoracic outlet syndrome. Cochrane Database Syst Rev. 2010;(1):CD007218.
14. Cormier JM, Amrane M, Ward A, Laurian C, Gigou F. Arterial complications of the thoracic outlet syndrome: fifty-five operative cases. J Vasc Surg. 1989;9(6):778-787.
15. Hood DB, Kuehne J, Yellin AE, Weaver FA. Vascular complications of thoracic outlet syndrome. Am Surg. 1997;63(10):913-917.
16. Ferrante MA. Brachial plexopathies: classification, causes, and consequences. Muscle Nerve. 2004;30(5):547-568.
17. Gilliatt RW, Le Quesne PM, Logue V, Sumner AJ. Wasting of the hand associated with a cervical rib or band. J Neurol Neurosurg Psychiatry. 1970;33(5):615-624.
18. Ozoa G, Alves D, Fish DE. Thoracic outlet syndrome. Phys Med Rehabil Clin North Am. 2011;22(3):473-483, viii-ix.
19. Schwartzman RJ. Brachial plexus traction injuries. Hand Clin. 1991;7(3):547-556.
20. Christo PJ, McGreevy K. Updated perspectives on neurogenic thoracic outlet syndrome. Curr Pain Headache Rep. 2011;15(1):14-21.
21. Vanti C, Natalini L, Romeo A, Tosarelli D, Pillastrini P. Conservative treatment of thoracic outlet syndrome. A review of the literature. Eura Medicophys. 2007;43(1):55-70.22. Patton GM. Arterial thoracic outlet syndrome. Hand Clin. 2004;20(1):107-111, viii.
23. Lee TS, Hines GL. Cerebral embolic stroke and arm ischemia in a teenager with arterial thoracic outlet syndrome: a case report. Vasc Endovasc Surg. 2007;41(3):254-257.
24. Sanders RJ, Hammond SL. Venous thoracic outlet syndrome. Hand Clin. 2004;20(1):113-118, viii.
25. Sanders RJ, Hammond SL, Rao NM. Diagnosis of thoracic outlet syndrome. J Vasc Surg. 2007;46(3):601-604.
26. Luoma A, Nelems B. Thoracic outlet syndrome. Thoracic surgery perspective. Neurosurg Clin North Am. 1991;2(1):187-226.
27. Cup EH, Ijspeert J, Janssen RJ, et al. Residual complaints after neuralgic amyotrophy. Arch Phys Med Rehabil. 2013;94(1):67-73.
28. van Alfen N, van Engelen BG. The clinical spectrum of neuralgic amyotrophy in 246 cases. Brain. 2006;129(pt 2):438-450.
29. Nichols AW. The thoracic outlet syndrome in athletes. J Am Board Fam Pract. 1996;9(5):346-355.
30. Roos DB, Owens JC. Thoracic outlet syndrome. Arch Surg. 1966;93(1):71-74.
31. Adson AW, Coffey JR. Cervical rib: a method of anterior approach for relief of symptoms by division of the scalenus anticus. Ann Surg. 1927;85(6):839-857.
32. Wright IS. The neurovascular syndrome produced by hyperabduction of the arms. Am Heart J. 1945;29:1-19.
33. Rayan GM, Jensen C. Thoracic outlet syndrome: provocative examination maneuvers in a typical population. J Shoulder Elbow Surg. 1995;4(2):113-117.
34. Nord KM, Kapoor P, Fisher J, et al. False positive rate of thoracic outlet syndrome diagnostic maneuvers. Electromyogr Clin Neurophysiol. 2008;48(2):67-74.
35. Novak CB. Thoracic outlet syndrome. Clin Plast Surg. 2003;30(2):175-188.
36. Gillard J, Pérez-Cousin M, Hachulla E, et al. Diagnosing thoracic outlet syndrome: contribution of provocative tests, ultrasonography, electrophysiology, and helical computed tomography in 48 patients. Joint Bone Spine. 2001;68(5):416-424.
37. Baxter GM, Kincaid W, Jeffrey RF, Millar GM, Porteous C, Morley P. Comparison of colour Doppler ultrasound with venography in the diagnosis of axillary and subclavian vein thrombosis. Br J Radiol. 1991;64(765):777-781.
38. Passman MA, Criado E, Farber MA, et al. Efficacy of color flow duplex imaging for proximal upper extremity venous outflow obstruction in hemodialysis patients. J Vasc Surg. 1998;28(5):869-875.
39. Wadhwani R, Chaubal N, Sukthankar R, Shroff M, Agarwala S. Color Doppler and duplex sonography in 5 patients with thoracic outlet syndrome. J Ultrasound Med. 2001;20(7):795-801.
40. Napoli V, Vignali C, Braccini G, et al. Echography and echo-Doppler in the study of thoracic outlet syndrome. Correlation with angiographic data [in Italian]. Radiol Med. 1993;85(6):733-740.
41. Longley DG, Yedlicka JW, Molina EJ, Schwabacher S, Hunter DW, Letourneau JG. Thoracic outlet syndrome: evaluation of the subclavian vessels by color duplex sonography. AJR Am J Roentgenol. 1992;158(3):623-630.
42. Demondion X, Herbinet P, Boutry N, Fontaine C, Francke JP, Cotten A. Sonographic mapping of the normal brachial plexus. AJNR Am J Neuroradiol. 2003;24(7):1303-1309.
43. Cruz-Martinez A, Arpa J. Electrophysiological assessment in neurogenic thoracic outlet syndrome. Electromyogr Clin Neurophysiol. 2001;41(4):253-256.
44. Ferrante MA, Wilbourn AJ. The utility of various sensory nerve conduction responses in assessing brachial plexopathies. Muscle Nerve. 1995;18(8):879-889.
45. Aminoff MJ, Olney RK, Parry GJ, Raskin NH. Relative utility of different electrophysiologic techniques in the evaluation of brachial plexopathies. Neurology. 1988;38(4):546-550.
46. Komanetsky RM, Novak CB, Mackinnon SE, Russo MH, Padberg AM, Louis S. Somatosensory evoked potentials fail to diagnose thoracic outlet syndrome. J Hand Surg Am. 1996;21(4):662-666.
47. Remy-Jardin M, Remy J, Masson P, et al. Helical CT angiography of thoracic outlet syndrome: functional anatomy. AJR Am J Roentgenol. 2000;174(6):1667-1674.
48. Matsumura JS, Rilling WS, Pearce WH, Nemcek AA Jr, Vogelzang RL, Yao JS. Helical computed tomography of the normal thoracic outlet. J Vasc Surg. 1997;26(5):776-783.
49. Dymarkowski S, Bosmans H, Marchal G, Bogaert J. Three-dimensional MR angiography in the evaluation of thoracic outlet syndrome. AJR Am J Roentgenol. 1999;173(4):1005-1008.
50. Charon JP, Milne W, Sheppard DG, Houston JG. Evaluation of MR angiographic technique in the assessment of thoracic outlet syndrome. Clin Radiol. 2004;59(7):588-595.
51. Cuetter AC, Bartoszek DM. The thoracic outlet syndrome: controversies, overdiagnosis, overtreatment, and recommendations for management. Muscle Nerve. 1989;12(5):410-419.
52. Urschel HC Jr, Razzuk MA. Paget-Schroetter syndrome: what is the best management? Ann Thorac Surg. 2000;69(6):1663-1668.
53. Lee JT, Karwowski JK, Harris EJ, Haukoos JS, Olcott C 4th. Long-term thrombotic recurrence after nonoperative management of Paget-Schroetter syndrome. J Vasc Surg. 2006;43(6):1236-1243.
54. Molina JE, Hunter DW, Dietz CA. Paget-Schroetter syndrome treated with thrombolytics and immediate surgery. J Vasc Surg. 2007;45(2):328-334.
55. Le Forestier N, Mouton P, Maisonobe T, et al. True neurological thoracic outlet syndrome [in French]. Rev Neurol (Paris). 2000;156(1):34-40.
56. Wilbourn AJ. Thoracic outlet syndrome surgery causing severe brachial plexopathy. Muscle Nerve. 1988;11(1):66-74.
57. Likes K, Dapash T, Rochlin DH, Freischlag JA. Remaining or residual first ribs are the cause of recurrent thoracic outlet syndrome. Ann Vasc Surg. 2014;28(4):939-945.
58. Aljabri B, Al-Omran M. Surgical management of vascular thoracic outlet syndrome: a teaching hospital experience. Ann Vasc Dis. 2013;6(1):74-79.
59. Sanders RJ, Pearce WH. The treatment of thoracic outlet syndrome: a comparison of different operations. J Vasc Surg. 1989;10(6):626-634.
60. Franklin GM, Fulton-Kehoe D, Bradley C, Smith-Weller T. Outcome of surgery for thoracic outlet syndrome in Washington state workers’ compensation. Neurology. 2000;54(6):1252-1257.
61. Axelrod DA, Proctor MC, Geisser ME, Roth RS, Greenfield LJ. Outcomes after surgery for thoracic outlet syndrome. J Vasc Surg. 2001;33(6):1220-1225.
62. Taylor JM, Telford RJ, Kinsella DC, Watkinson AF, Thompson JF. Long-term clinical and functional outcome following treatment for Paget-Schroetter syndrome. Br J Surg. 2013;100(11):1459-1464.
63. Schneider DB, Dimuzio PJ, Martin ND, et al. Combination treatment of venous thoracic outlet syndrome: open surgical decompression and intraoperative angioplasty. J Vasc Surg. 2004;40(4):599-603.
The Burden of Craft in Arthroscopic Rotator Cuff Repair: Where We Have Been and Where We Are Going
I am very honored that Dr. Rob Bell, past president of the American Shoulder and Elbow Surgeons, invited me to give last year’s Neer Lecture. Dr. Bell asked me to specifically address my role in the development of arthroscopic rotator cuff repair and to recount the significant resistance that the early arthroscopic shoulder surgeons faced from the shoulder establishment as we struggled to achieve mainstream acceptance for this new technology. Tasked with such a personal topic, I find myself in a position analogous to that of Winston Churchill at the end of World War II. When a journalist asked him to speculate on how historians would portray his role in the war, he replied without hesitation, “History will be kind to me because I intend to write it.”
So let’s start at the beginning. And for me it makes the most sense to travel back to the year I started my practice: 1981. The world then was very different from today’s world. On January 20, 1981, Ronald Reagan was inaugurated President of the United States. The same day, 52 US hostages in Iran were released after having been held captive for 442 days. In March 1981, Reagan survived an assassination attempt; 3 months earlier, John Lennon had not been so lucky. Lennon’s hit song “Starting Over” garnered the highest musical awards posthumously.
The world of shoulder surgery was also very different in 1981. The arthroscope was the “instrument of the devil,” according to Dr. Rockwood. And shoulder surgery was ruled by the Charlies—Dr. Charles Neer, Dr. Charlie Rockwood, and any other Charlie who felt compelled to marginalize shoulder arthroscopy.
My personal world in the early 1980s was daunting as well. I had just completed my residency at the Mayo Clinic and my sports medicine fellowship in Eugene, Oregon. I had a young son, a new daughter, and a new job with the San Antonio Orthopaedic Group. I had a new house with a 21% mortgage loan and a “new” used car with a 23% car loan.
I was simultaneously energized and intimidated by my new job, where I was doing general orthopedics with a “special interest” in shoulder surgery and sports medicine. I was initially very proud and humbled by the fact that my senior partners had entrusted me with the care of the most difficult shoulder cases within the practice. But that pride got cut down to its appropriate size the day after I had thanked one of my partners, Dr. Lamar Collie, for his confidence in my potential as a shoulder surgeon. Dr. Collie replied matter-of-factly, “Sure … but you need to understand that we always make the new guy the shoulder expert because shoulders never do worth a damn.”
For shoulder arthroscopy, the early 1980s were exciting. Most of us who were scoping shoulders had already been doing knee arthroscopy and were trying to adapt knee instruments to the shoulder. This worked for some simple excisional cases. For example, I recall excising the bucket-handle portion of a type III SLAP (superior labral tear from anterior to posterior) lesion in 1983. In general, however, shoulder problems were different from knee problems and usually involved repair rather than excision of damaged tissues. Therefore, the technology used in knee arthroscopy was often not directly transferable to the shoulder. Furthermore, treatment of the rotator cuff necessitated development of arthroscopic techniques in a virtual space, the subacromial space, and this was an entirely new arthroscopic concept.
Development of Arthroscopic Rotator Cuff Repair
A major mind-expanding turning point for me occurred in 1984 when I attended one of Dr. Jim Esch’s early San Diego shoulder courses. During that course, Dr. Harvard Ellman of Los Angeles demonstrated to me on a cadaver shoulder how he created a virtual subacromial working space that allowed enough visualization for an arthroscopic acromioplasty. At that moment, I knew that arthroscopic rotator cuff repair was just around the corner. Up until then, I had not been able to envision complex extra-articular reconstructive surgery, as all previous arthroscopic surgery had been intra-articular. But now, having realized a virtual working space could always be created, I knew it would be relatively straightforward to develop the portals to approach the cuff as well as the implants and the instruments to repair it. But I also knew that progression to all-arthroscopic repair techniques would have to be stepwise and that the final repair constructs would need to be at least as strong as those of open repair in order to be acceptable. With an undergraduate degree in mechanical engineering, I had a reasonably clear idea of the concepts I wanted to apply to the instrumentation and techniques, though I could never have envisioned how circuitous the route to the end result would be.
First Steps
I sketched out my ideas for arthroscopic suture passers and knot-tying instruments and presented them to a couple of the major arthroscopy companies in the United States, but the companies were not interested. They did not believe arthroscopy would have any meaningful applications in the shoulder. So, I enlisted the services of a local San Antonio aircraft machinist to fabricate instruments for me. By 1987, I was doing arthroscopic side-to-side margin convergence1 cuff repairs for U-shape tears on a regular basis. And I was doing these at the most hostile point in the universe for arthroscopic shoulder surgery: San Antonio, Texas.
Only a few surgeons were doing arthroscopic shoulder surgery in the 1980s and early 1990s, and without exception these surgeons became the leader-pioneers in the new discipline. In general, these were young surgeons who were in private practice and removed from academia and professional organizations, and thus relatively sheltered from the actions of the shoulder rule-makers of the day. They accepted their status as pariahs as they developed their techniques out of the view of mainstream orthopedics. These leaders included Jim Esch, Steve Snyder, Dick Caspari, Lanny Johnson, Gene Wolf, Gary Gartsman, Rob Bell, and Howard Sweeney. We shared our techniques and our ideas with one another, encouraged one another, and generally became good friends.
Thomas Kuhn, in his classic book The Structure of Scientific Revolutions,2 observed that paradigm shifts within a given field were usually achieved by practitioners who were either very young (naïve) or outside the established hierarchy in the field. The surgeons who contributed most to the shift of shoulder surgery from open to arthroscopic techniques were generally young men who were in private practice and had little to lose by inciting the disdain of the shoulder establishment. Predictably, resistance from the mainstream open shoulder surgeons increased as arthroscopic techniques became more successful and more threatening to the primacy of the open shoulder surgeons. The disdain yielded to disruption and finally to transformation as the paradigm shift occurred. The conflict between the open shoulder surgeons and the arthroscopic shoulder surgeons passed through all the phases that Mahatma Gandhi had described many years before. “First they ignore you; then they laugh at you; then they fight you; then you win.”
Building a Ship in a Bottle
At the start of the 1990s, I recognized that my progress in arthroscopic rotator cuff repair would be extremely slow unless I could find an industry partner who shared my vision for full-scale conversion to arthroscopic means of repair and would be willing to help make it a reality. In 1991, I happened to meet Reinhold Schmieding, the owner of Arthrex, a small arthroscopic device company in Naples, Florida. Reinhold invited me to visit him to discuss the feasibility of developing arthroscopic repair systems for the shoulder. At the time, the world headquarters of Arthrex was a 20×30-ft storage room in an office service center, and there were 2 employees. One employee, Don Grafton, was a talented engineer without medical experience. By the end of my first day there, Reinhold and Don and I had agreed that developing arthroscopic repair systems for shoulder instability and rotator cuff repair would become a top priority for Arthrex.
My initial bias toward arthroscopic cuff repair was that a transosseous bone tunnel technique not only would be possible but would be superior to suture anchor fixation. In fact, my first 2 patents with Arthrex were for instrumentation for an arthroscopic transosseous repair technique. I tested my hypothesis with 2 successive biomechanical studies. The first examined cyclic loading of bone tunnel repairs, and the second examined cyclic loading of anchor-based repairs.3,4 Evaluating the data from these 2 studies, I was surprised to find that anchor-based repairs were significantly stronger than bone tunnel repairs. In addition, anchors shifted the weak link from the bone–suture interface to the tendon–suture interface; in essence, anchors optimized bone fixation by shifting the weak link in the construct to the tendon. I was then completely convinced of the superiority of suture anchors over bone tunnels, and that conviction has become even stronger over the years. After these 2 cyclic loading studies, I shifted my focus, and that of Arthrex, toward arthroscopic suture anchor repair of the rotator cuff.
Reconciling Technique and Instrumentation With Anatomy and Biomechanics
Having recognized the importance of the rotator cable attachments both anatomically5 and biomechanically,6,7 I thought it important to reinforce them as a routine part of performing rotator cuff repairs. Our anatomical and biomechanical studies had had great translational implications in the development of our techniques and instrumentation.
As mentioned earlier, Don Grafton was the chief (and for a long time only) engineer at Arthrex. As he had no medical experience, I invited him to come to San Antonio to observe surgery. During Don’s many visits, I showed him pathology in the operating room and pointed out what I could do with the instruments I had and what I could not do. Then in the evening we went to my house and brainstormed how to perform the “missing” surgical manipulations, how to improve manipulations that were suboptimal, and how to optimize final surgical constructs.
Passing suture through tendon was an early challenge. One must remember that, in the early 1990s, it was not possible for machinists to fabricate complex shapes. Therefore, straight tubular retrograde suture passers were the logical first option. We initially developed spring-loaded retrograde hook retrievers (Figure 1) and then curved suture hooks with shuttling wires (Lasso). To me, the most unappealing feature of retrograde suture passage was the oblique angle of approach through the tendon, which caused a length–tension mismatch between the upper fibers and the lower fibers of the muscle–tendon unit. We recognized we could eliminate the mismatch if we passed the suture antegrade, such that it would pass perpendicular to the tendon fibers. These insights and efforts culminated in development of the Viper suture passer and then the FastPass Scorpion suture passer, which has a spring-loaded trapdoor on the upper jaw for ergonomic self-retrieving of the suture once it is passed through the tendon.
To develop a knot pusher that optimized knot tying (yielding the highest knot security and the tightest loop security), we used prototype instruments to tie and test literally thousands of knots in the laboratory. We were thus able to verify that the Surgeon’s Sixth Finger Knot Pusher (Arthrex) reproducibly tied optimized knots8,9 and also optimized knot fixation and bone fixation. However, our suture was not yet optimized and was prone to breakage, and our suture–tendon interface was not yet optimized. Clearly, improvement was needed in 2 more areas.
Don came up with the idea for a virtually unbreakable suture and developed that idea into FiberWire.10 Shortly thereafter, I contributed the idea and design for FiberTape, which dramatically enhanced suture pullout strength and footprint compression.
Anchor designs improved rapidly and dramatically. We made the second-generation BioCorkscrew fully threaded, which virtually eliminated anchor failure, even in soft bone.
Optimization of the suture–tendon interface took a giant step forward when Park and colleagues11,12 introduced linked double-row rotator cuff repair. Much as with a Chinese finger trap, the harder you pull, the stronger it becomes, with yield load approaching ultimate load.
At this point, it seemed we had optimized virtually every segment of the rotator cuff repair construct. Each component was just about as good as it could be. Or was it?
The Accidental Quest for Knotless Fixation
In November 1998, I made my first trip to China as a guest speaker at the Congress of the Hong Kong Orthopaedic Association. My first view of the magnificent Hong Kong skyline across Victoria Harbour was truly breathtaking. As I admired the gleaming glass towers and the concrete canyons of the city, I had no idea that the very next day these modern skyscrapers would reveal an ancient secret that would change my approach to arthroscopic rotator cuff repair.
The day after my arrival, Dr. James Lam took me to lunch. As we approached the restaurant, he pointed across the street to a tall building that was being renovated and had scaffolding supporting workers alongside the first 9 stories of the exterior wall. Dr. Lam said that, after lunch, he would take me to the construction site for a closer look at the scaffolding.
After lunch, we walked to the base of the scaffolding. Dr. Lam told me it was constructed entirely of bamboo poles held together with lashings but no knots (Figure 2). Lashings were secured by turning them back on themselves and wrapping them in an entirely knotless manner.13 I found it incredible that this knotless fixation was so secure that it could support the weight of workers many stories above the ground. I resolved to determine how this fixation method worked and see if the same mechanism might help us achieve reliable knotless fixation in surgery.
When I returned home, I broke out my college engineering books and reacquainted myself with the concept of cable friction. As has happened so often in the past, however, it took a practical lesson from the ranch to truly illustrate for me how cable friction works.
Every cowboy knows that a spirited horse cannot be restrained with only one lead rope. However, a cowboy can wrap a lead rope around a “snubbing post” and thereby gain complete control over the animal, despite the horse’s superior size and strength. The cable friction between the rope and the post creates such a large restraining force that the cowboy can easily hold the animal without the help of a knotted rope (Figure 3). In similar fashion in the Hong Kong scaffolding, fixation strength results from the significant amount of cable friction produced when the lashings wrap around one another and around the bamboo poles.
The cable friction concept was pivotal in the development of knotless fixation in arthroscopic rotator cuff repair. In lateral row fixation, the eyelet of the PushLock and SwiveLock suture anchors (Arthrex) produces significant cable friction at the eyelet–suture interface, in addition to frictional force wedging the suture between anchor and bone.
As with so many other devices in shoulder arthroscopy, the SwiveLock suture anchor developed in stages. In the first stage, a chainlike suture with consecutive intersecting links was used (FiberChain). The idea for an adjustable fixation construct came to me because I thought that a forked eyelet on a SwiveLock would provide a firm fixation point when inserted into the appropriate suture link, yet would be totally adjustable simply by choosing a tighter or looser link (Figure 4). Although the system worked very well, it was technically challenging. The process was greatly simplified after Don Grafton and I developed FiberTape and recognized that the power of cable friction was dramatically increased by the larger contact area between the eyelet and the braided FiberTape. The SpeedBridge construct (Arthrex), which enhanced cable friction fixation by means of passing FiberTape through the anchor eyelets, also provided a larger compressive interface at the repair site by using FiberTape rather than conventional suture. These incremental improvements led to what I would characterize as today’s gold standard for arthroscopic rotator cuff repair: a largely knotless linked double-row construct using FiberTape, with cinch-loop sutures at the anterior and posterior margins of the tear to reinforce the cable attachments and simultaneously reduce the dog-ears that typically occur in those locations, and a double-pulley medial mattress if tendon quality is poor (Figure 5).
The Burden of Craft
With all the recent enthusiasm for level I studies, I think we need to examine whether they will accelerate technological advancement in rotator cuff repair. The answer, in my opinion, is a resounding no. This answer is based on a major disconnect I have detected in how we evaluate these studies in rotator cuff disease and repair.
An irony related to technological advancement in surgery is that the more technically advanced the surgery becomes, the more skill is required. This fact is completely at odds with the public’s perception that technological advances make procedures easier. In arthroscopic rotator cuff repair, the surgeon must look, feel, and be aware to a greater degree than in open surgery.
Edward Tenner, in his book Why Things Bite Back, described the burden of the practitioner of any advanced technology as the burden of craft.14 The burden of craft is the inherent demand on all craftspeople, but particularly surgeons, to “up our game” if we are to be successful in our craft. For arthroscopic rotator cuff repair, the burden of craft requires patience, attention to detail, and the ability to work in a virtual space. Not everyone has these skills. But anyone who wants to practice in this discipline has an obligation to learn the skills required, and then to teach them to others and assess how well they are being applied.
The problem with relying on level I studies to assess the efficacy of a surgical procedure is that they are inherently biased by the surgeons involved. As results depend on surgeons’ skills, and surgeons’ skill levels are not equal, level I studies cannot prove what is possible, cannot demonstrate the limits of a technique, and cannot demonstrate the equivalence of techniques.
Amazingly enough, there are still rotator cuff repair “deniers” who confidently assert from the podium that a large percentage of massive cuff tears cannot be repaired and that, even if they can be repaired, they do not have the biological potential to heal. Given the disparity in surgeons’ skills and results, however, one must ask whether poor results are a consequence of a biological deficit in the patient, or of a skill deficit in the surgeon.
What I know is that we have techniques for predictable arthroscopic repair and healing of the vast majority of rotator cuff tears, even massive tears,15-17 and patients do very well clinically. Yet, among many orthopedic surgeons, there is a trend to go straight to reverse total shoulder arthroplasty (rTSA) for massive tears—despite the evidence against it. As reported in the literature, rTSA results are not as good as arthroscopic cuff repair results, and the complication rate for rTSA is much higher.
Why has this trend toward rTSA for massive tears gained so much momentum? The only reason I can surmise is that, for the average surgeon, rTSA is easier and quicker than arthroscopic repair for massive tears. But the reason for choosing a specific type of surgery for a given problem should not be that it is easiest for the surgeon; it should be that it is best for the patient.
The surgeon should start by asking what procedure he or she would want if the roles were reversed—if the surgeon were the patient with the massive rotator cuff tear. If a surgeon does not have the skill set for the best procedure for a particular patient, he or she is obligated to send that patient to a surgeon who does have the skills. In addition, given that infection is the most feared complication in most shoulder surgeries, the surgeon should ask which infection rate would be personally acceptable. Arthroscopic rotator cuff repair has a reported infection rate of 1.6 per 1000, or .0016,18 whereas rTSA has an infection rate about 25 times higher, or .04.19 Further, the surgeon must consider the relative severity of the consequences of infection. By any measure, an infected arthroscopy is a straightforward treatable complication, but an infected shoulder replacement is a human tragedy. Patients vastly prefer the minimally invasive arthroscopic approach, and through online searches can easily identify who can offer an arthroscopic solution.
To reproducibly achieve successful arthroscopic repair of massive rotator cuff tears, the surgeon must know advanced techniques, including subscapularis repair techniques,20,21 interval slides,22,23 and self-reinforcing constructs.24,25
“It’s a poor carpenter who blames his tools.” This 18th-century English proverb is as true today as it was 300 years ago. The tools for arthroscopic cuff repair exist, and they are excellent. The burden of craft is the surgeon’s burden and obligation. As surgeons, we must accept that obligation and the responsibility of that burden.
As mentioned earlier, Dr. Rob Bell’s charge to me when he invited me to give the Neer Lecture was to sum up my involvement in the development of arthroscopic shoulder surgery. The short version is that I have been doing shoulder arthroscopy for 31 years; have received 28 US patents related to shoulder instruments and implants and have 12 US patents pending; have published 167 peer-reviewed articles, a couple dozen book chapters, and 2 textbooks on shoulder arthroscopy; have trained 25 fellows; and have hosted approximately 3000 visiting surgeons in my operating room. My greatest professional dream was to see the standard of care for rotator cuff repair and shoulder instability transition from open to arthroscopic techniques, and I have been fortunate enough to have observed that paradigm shift during my career.
What do I envision over the next 31 years? As we all know, history runs in both directions, and some things simply have not happened yet. In terms of rotator cuff treatment, I think over the next few years the guiding principle of treatment will be joint preservation. All rotator cuff tears, even massive tears, will be repaired arthroscopically. Patients and insurers will demand arthroscopic repair, and surgeons without the skill set will migrate to other subspecialties. As for the role of arthroplasty in the treatment of rotator cuff tears, rTSA will be indicated only for pseudoparalysis after failed cuff repair in low-demand elderly patients.
In rotator cuff treatment, I envision a standard of care that is almost entirely arthroscopic. This standard will demand that surgeons who treat rotator cuff tears be proficient in arthroscopic repair of the full range of tears. Acquiring the skills for arthroscopic repair may not be easy, but then “there’s the easy way, and there’s the Cowboy Way.” As my dad used to tell me when I complained about working too hard, “No man ever drowned in his own sweat.” We shoulder surgeons must accept the burden of craft that accompanies the new standard of arthroscopic cuff repair, and we must offer our patients the same level of care we would choose for ourselves.
Happy trails!
1. Burkhart SS, Athanasiou KA, Wirth MA. Margin convergence: a method of reducing strain in massive rotator cuff tears. Arthroscopy. 1996:12(3):335-338.
2. Kuhn TS. The Structure of Scientific Revolutions. Chicago, IL: University of Chicago Press; 1962.
3. Burkhart SS, Johnson TC, Wirth MA, Athanasiou KA. Cyclic loading of transosseous rotator cuff repairs: tension overload as a possible cause of failure. Arthroscopy. 1997;13(2):172-176.
4. Burkhart SS, Diaz Pagàn JL, Wirth MA, Athansiou KA. Cyclic loading of anchor-based rotator cuff repairs: confirmation of the tension overload phenomenon and comparison of suture anchor fixation with transosseous fixation. Arthroscopy. 1997;13(6):720-724.
5. Burkhart SS, Esch JC, Jolson RS. The rotator crescent and rotator cable: an anatomic description of the shoulder’s “suspension bridge.” Arthroscopy. 1993;9(6):611-616.
6. Burkhart SS, Nottage WM, Ogilvie-Harris DJ, Kohn HS, Pachelli A. Partial repair of irreparable rotator cuff tears. Arthroscopy. 1994;10(4):363-370.
7. Halder AM, O’Driscoll SW, Heers G, et al. Biomechanical comparison of effects of supraspinatus tendon detachments, tendon defects, and muscle retractions. J Bone Joint Surg Am. 2002;84(5):780-785.
8. Lo IK, Burkhart SS, Chan KC, Athanasiou K. Arthroscopic knots: determining the optimal balance of loop security and knot security. Arthroscopy. 2004;20(5):489-502.
9. Lo IK, Ochoa E Jr, Burkhart SS. A comparison of knot security and loop security in arthroscopic knots tied with newer high-strength suture materials. Arthroscopy. 2010;26(9 suppl):S120-S126.
10. Lo IK, Burkhart SS, Athanasiou K. Abrasion resistance of two types of nonabsorbable braided suture. Arthroscopy. 2004;20(4):407-413.
11. Park MC, ElAttrache NS, Tibone JE, Ahmad CS, Jun BJ, Lee TQ. Part I: footprint contact characteristics for a “transosseous-equivalent” rotator cuff repair technique compared to a double-row technique. J Shoulder Elbow Surg. 2007;16(4):461-468.
12. Park MC, Tibone JE, ElAttrache NS, Ahmad CS, Jun BJ, Lee TQ. Part II: biomechanical assessment for a footprint-restoring transosseous-equivalent rotator cuff repair technique compared with a double-row repair technique. J Shoulder Elbow Surg. 2007;16(4):469-476.
13. Burkhart SS, Athanasiou KA. The twist-lock concept of tissue transport and suture fixation without knots: observations along the Hong Kong skyline. Arthroscopy. 2003;19(6):613-625.
14. Tenner E. Why Things Bite Back. New York, NY: Random House; 1996.
15. Denard PJ, Jiwani AZ, Lädermann A, Burkhart SS. Long-term outcome of arthroscopic massive rotator cuff repair: the importance of double-row fixation. Arthroscopy. 2012;28(7):909-915.
16. Denard PJ, Lädermann A, Jiwani AZ, Burkhart SS. Functional outcome after arthroscopic repair of massive rotator cuff tears in individuals with pseudoparalysis. Arthroscopy. 2012;28(9):1214-1219.
17. Lädermann A, Denard PJ, Burkhart SS. Revision arthroscopic rotator cuff repair: systematic review and authors’ preferred surgical technique. Arthroscopy. 2012;28(8):1160-1169.
18. Randelli P, Castagna A, Cabitza F, Cabitza P, Arrigoni P, Denti M. Infectious and thromboembolic complications of arthroscopic shoulder surgery. J Shoulder Elbow Surg. 2010;19(1):97-101.
19. Zumstein MA, Pinedo M, Old J, Boileau P. Problems, complications, reoperations, and revisions in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2011;20(1):146-157.
20. Adams CR, Schoolfield JD, Burkhart SS. The results of arthroscopic subscapularis tendon repairs. Arthroscopy. 2008;24(12):1381-1389.
21. Denard PJ, Jiwani AZ, Lädermann A, Burkhart SS. Long-term outcome of a consecutive series of subscapularis tendon tears repaired arthroscopically. Arthroscopy. 2012;28(11):1587-1591.
22. Lo IK, Burkhart SS. Arthroscopic repair of massive, contracted, immobile rotator cuff tears using single and double interval slides: technique and preliminary results. Arthroscopy. 2004;20(1):22-33.
23. Lo IK, Burkhart SS. The interval slide in continuity: a method of mobilizing the anterosuperior rotator cuff without disrupting the tear margins. Arthroscopy. 2004;20(4):435-441.
24. Denard PJ, Burkhart SS. Techniques for managing poor quality tissue and bone during arthroscopic rotator cuff repair. Arthroscopy. 2011;27(10):1409-1421.
25. Burkhart SS, Denard PJ, Konicek J, Hanypsiak BT. Biomechanical validation of load-sharing rip-stop fixation for the repair of tissue-deficient rotator cuff tears. Am J Sports Med. 2014;42(2):457-462.
I am very honored that Dr. Rob Bell, past president of the American Shoulder and Elbow Surgeons, invited me to give last year’s Neer Lecture. Dr. Bell asked me to specifically address my role in the development of arthroscopic rotator cuff repair and to recount the significant resistance that the early arthroscopic shoulder surgeons faced from the shoulder establishment as we struggled to achieve mainstream acceptance for this new technology. Tasked with such a personal topic, I find myself in a position analogous to that of Winston Churchill at the end of World War II. When a journalist asked him to speculate on how historians would portray his role in the war, he replied without hesitation, “History will be kind to me because I intend to write it.”
So let’s start at the beginning. And for me it makes the most sense to travel back to the year I started my practice: 1981. The world then was very different from today’s world. On January 20, 1981, Ronald Reagan was inaugurated President of the United States. The same day, 52 US hostages in Iran were released after having been held captive for 442 days. In March 1981, Reagan survived an assassination attempt; 3 months earlier, John Lennon had not been so lucky. Lennon’s hit song “Starting Over” garnered the highest musical awards posthumously.
The world of shoulder surgery was also very different in 1981. The arthroscope was the “instrument of the devil,” according to Dr. Rockwood. And shoulder surgery was ruled by the Charlies—Dr. Charles Neer, Dr. Charlie Rockwood, and any other Charlie who felt compelled to marginalize shoulder arthroscopy.
My personal world in the early 1980s was daunting as well. I had just completed my residency at the Mayo Clinic and my sports medicine fellowship in Eugene, Oregon. I had a young son, a new daughter, and a new job with the San Antonio Orthopaedic Group. I had a new house with a 21% mortgage loan and a “new” used car with a 23% car loan.
I was simultaneously energized and intimidated by my new job, where I was doing general orthopedics with a “special interest” in shoulder surgery and sports medicine. I was initially very proud and humbled by the fact that my senior partners had entrusted me with the care of the most difficult shoulder cases within the practice. But that pride got cut down to its appropriate size the day after I had thanked one of my partners, Dr. Lamar Collie, for his confidence in my potential as a shoulder surgeon. Dr. Collie replied matter-of-factly, “Sure … but you need to understand that we always make the new guy the shoulder expert because shoulders never do worth a damn.”
For shoulder arthroscopy, the early 1980s were exciting. Most of us who were scoping shoulders had already been doing knee arthroscopy and were trying to adapt knee instruments to the shoulder. This worked for some simple excisional cases. For example, I recall excising the bucket-handle portion of a type III SLAP (superior labral tear from anterior to posterior) lesion in 1983. In general, however, shoulder problems were different from knee problems and usually involved repair rather than excision of damaged tissues. Therefore, the technology used in knee arthroscopy was often not directly transferable to the shoulder. Furthermore, treatment of the rotator cuff necessitated development of arthroscopic techniques in a virtual space, the subacromial space, and this was an entirely new arthroscopic concept.
Development of Arthroscopic Rotator Cuff Repair
A major mind-expanding turning point for me occurred in 1984 when I attended one of Dr. Jim Esch’s early San Diego shoulder courses. During that course, Dr. Harvard Ellman of Los Angeles demonstrated to me on a cadaver shoulder how he created a virtual subacromial working space that allowed enough visualization for an arthroscopic acromioplasty. At that moment, I knew that arthroscopic rotator cuff repair was just around the corner. Up until then, I had not been able to envision complex extra-articular reconstructive surgery, as all previous arthroscopic surgery had been intra-articular. But now, having realized a virtual working space could always be created, I knew it would be relatively straightforward to develop the portals to approach the cuff as well as the implants and the instruments to repair it. But I also knew that progression to all-arthroscopic repair techniques would have to be stepwise and that the final repair constructs would need to be at least as strong as those of open repair in order to be acceptable. With an undergraduate degree in mechanical engineering, I had a reasonably clear idea of the concepts I wanted to apply to the instrumentation and techniques, though I could never have envisioned how circuitous the route to the end result would be.
First Steps
I sketched out my ideas for arthroscopic suture passers and knot-tying instruments and presented them to a couple of the major arthroscopy companies in the United States, but the companies were not interested. They did not believe arthroscopy would have any meaningful applications in the shoulder. So, I enlisted the services of a local San Antonio aircraft machinist to fabricate instruments for me. By 1987, I was doing arthroscopic side-to-side margin convergence1 cuff repairs for U-shape tears on a regular basis. And I was doing these at the most hostile point in the universe for arthroscopic shoulder surgery: San Antonio, Texas.
Only a few surgeons were doing arthroscopic shoulder surgery in the 1980s and early 1990s, and without exception these surgeons became the leader-pioneers in the new discipline. In general, these were young surgeons who were in private practice and removed from academia and professional organizations, and thus relatively sheltered from the actions of the shoulder rule-makers of the day. They accepted their status as pariahs as they developed their techniques out of the view of mainstream orthopedics. These leaders included Jim Esch, Steve Snyder, Dick Caspari, Lanny Johnson, Gene Wolf, Gary Gartsman, Rob Bell, and Howard Sweeney. We shared our techniques and our ideas with one another, encouraged one another, and generally became good friends.
Thomas Kuhn, in his classic book The Structure of Scientific Revolutions,2 observed that paradigm shifts within a given field were usually achieved by practitioners who were either very young (naïve) or outside the established hierarchy in the field. The surgeons who contributed most to the shift of shoulder surgery from open to arthroscopic techniques were generally young men who were in private practice and had little to lose by inciting the disdain of the shoulder establishment. Predictably, resistance from the mainstream open shoulder surgeons increased as arthroscopic techniques became more successful and more threatening to the primacy of the open shoulder surgeons. The disdain yielded to disruption and finally to transformation as the paradigm shift occurred. The conflict between the open shoulder surgeons and the arthroscopic shoulder surgeons passed through all the phases that Mahatma Gandhi had described many years before. “First they ignore you; then they laugh at you; then they fight you; then you win.”
Building a Ship in a Bottle
At the start of the 1990s, I recognized that my progress in arthroscopic rotator cuff repair would be extremely slow unless I could find an industry partner who shared my vision for full-scale conversion to arthroscopic means of repair and would be willing to help make it a reality. In 1991, I happened to meet Reinhold Schmieding, the owner of Arthrex, a small arthroscopic device company in Naples, Florida. Reinhold invited me to visit him to discuss the feasibility of developing arthroscopic repair systems for the shoulder. At the time, the world headquarters of Arthrex was a 20×30-ft storage room in an office service center, and there were 2 employees. One employee, Don Grafton, was a talented engineer without medical experience. By the end of my first day there, Reinhold and Don and I had agreed that developing arthroscopic repair systems for shoulder instability and rotator cuff repair would become a top priority for Arthrex.
My initial bias toward arthroscopic cuff repair was that a transosseous bone tunnel technique not only would be possible but would be superior to suture anchor fixation. In fact, my first 2 patents with Arthrex were for instrumentation for an arthroscopic transosseous repair technique. I tested my hypothesis with 2 successive biomechanical studies. The first examined cyclic loading of bone tunnel repairs, and the second examined cyclic loading of anchor-based repairs.3,4 Evaluating the data from these 2 studies, I was surprised to find that anchor-based repairs were significantly stronger than bone tunnel repairs. In addition, anchors shifted the weak link from the bone–suture interface to the tendon–suture interface; in essence, anchors optimized bone fixation by shifting the weak link in the construct to the tendon. I was then completely convinced of the superiority of suture anchors over bone tunnels, and that conviction has become even stronger over the years. After these 2 cyclic loading studies, I shifted my focus, and that of Arthrex, toward arthroscopic suture anchor repair of the rotator cuff.
Reconciling Technique and Instrumentation With Anatomy and Biomechanics
Having recognized the importance of the rotator cable attachments both anatomically5 and biomechanically,6,7 I thought it important to reinforce them as a routine part of performing rotator cuff repairs. Our anatomical and biomechanical studies had had great translational implications in the development of our techniques and instrumentation.
As mentioned earlier, Don Grafton was the chief (and for a long time only) engineer at Arthrex. As he had no medical experience, I invited him to come to San Antonio to observe surgery. During Don’s many visits, I showed him pathology in the operating room and pointed out what I could do with the instruments I had and what I could not do. Then in the evening we went to my house and brainstormed how to perform the “missing” surgical manipulations, how to improve manipulations that were suboptimal, and how to optimize final surgical constructs.
Passing suture through tendon was an early challenge. One must remember that, in the early 1990s, it was not possible for machinists to fabricate complex shapes. Therefore, straight tubular retrograde suture passers were the logical first option. We initially developed spring-loaded retrograde hook retrievers (Figure 1) and then curved suture hooks with shuttling wires (Lasso). To me, the most unappealing feature of retrograde suture passage was the oblique angle of approach through the tendon, which caused a length–tension mismatch between the upper fibers and the lower fibers of the muscle–tendon unit. We recognized we could eliminate the mismatch if we passed the suture antegrade, such that it would pass perpendicular to the tendon fibers. These insights and efforts culminated in development of the Viper suture passer and then the FastPass Scorpion suture passer, which has a spring-loaded trapdoor on the upper jaw for ergonomic self-retrieving of the suture once it is passed through the tendon.
To develop a knot pusher that optimized knot tying (yielding the highest knot security and the tightest loop security), we used prototype instruments to tie and test literally thousands of knots in the laboratory. We were thus able to verify that the Surgeon’s Sixth Finger Knot Pusher (Arthrex) reproducibly tied optimized knots8,9 and also optimized knot fixation and bone fixation. However, our suture was not yet optimized and was prone to breakage, and our suture–tendon interface was not yet optimized. Clearly, improvement was needed in 2 more areas.
Don came up with the idea for a virtually unbreakable suture and developed that idea into FiberWire.10 Shortly thereafter, I contributed the idea and design for FiberTape, which dramatically enhanced suture pullout strength and footprint compression.
Anchor designs improved rapidly and dramatically. We made the second-generation BioCorkscrew fully threaded, which virtually eliminated anchor failure, even in soft bone.
Optimization of the suture–tendon interface took a giant step forward when Park and colleagues11,12 introduced linked double-row rotator cuff repair. Much as with a Chinese finger trap, the harder you pull, the stronger it becomes, with yield load approaching ultimate load.
At this point, it seemed we had optimized virtually every segment of the rotator cuff repair construct. Each component was just about as good as it could be. Or was it?
The Accidental Quest for Knotless Fixation
In November 1998, I made my first trip to China as a guest speaker at the Congress of the Hong Kong Orthopaedic Association. My first view of the magnificent Hong Kong skyline across Victoria Harbour was truly breathtaking. As I admired the gleaming glass towers and the concrete canyons of the city, I had no idea that the very next day these modern skyscrapers would reveal an ancient secret that would change my approach to arthroscopic rotator cuff repair.
The day after my arrival, Dr. James Lam took me to lunch. As we approached the restaurant, he pointed across the street to a tall building that was being renovated and had scaffolding supporting workers alongside the first 9 stories of the exterior wall. Dr. Lam said that, after lunch, he would take me to the construction site for a closer look at the scaffolding.
After lunch, we walked to the base of the scaffolding. Dr. Lam told me it was constructed entirely of bamboo poles held together with lashings but no knots (Figure 2). Lashings were secured by turning them back on themselves and wrapping them in an entirely knotless manner.13 I found it incredible that this knotless fixation was so secure that it could support the weight of workers many stories above the ground. I resolved to determine how this fixation method worked and see if the same mechanism might help us achieve reliable knotless fixation in surgery.
When I returned home, I broke out my college engineering books and reacquainted myself with the concept of cable friction. As has happened so often in the past, however, it took a practical lesson from the ranch to truly illustrate for me how cable friction works.
Every cowboy knows that a spirited horse cannot be restrained with only one lead rope. However, a cowboy can wrap a lead rope around a “snubbing post” and thereby gain complete control over the animal, despite the horse’s superior size and strength. The cable friction between the rope and the post creates such a large restraining force that the cowboy can easily hold the animal without the help of a knotted rope (Figure 3). In similar fashion in the Hong Kong scaffolding, fixation strength results from the significant amount of cable friction produced when the lashings wrap around one another and around the bamboo poles.
The cable friction concept was pivotal in the development of knotless fixation in arthroscopic rotator cuff repair. In lateral row fixation, the eyelet of the PushLock and SwiveLock suture anchors (Arthrex) produces significant cable friction at the eyelet–suture interface, in addition to frictional force wedging the suture between anchor and bone.
As with so many other devices in shoulder arthroscopy, the SwiveLock suture anchor developed in stages. In the first stage, a chainlike suture with consecutive intersecting links was used (FiberChain). The idea for an adjustable fixation construct came to me because I thought that a forked eyelet on a SwiveLock would provide a firm fixation point when inserted into the appropriate suture link, yet would be totally adjustable simply by choosing a tighter or looser link (Figure 4). Although the system worked very well, it was technically challenging. The process was greatly simplified after Don Grafton and I developed FiberTape and recognized that the power of cable friction was dramatically increased by the larger contact area between the eyelet and the braided FiberTape. The SpeedBridge construct (Arthrex), which enhanced cable friction fixation by means of passing FiberTape through the anchor eyelets, also provided a larger compressive interface at the repair site by using FiberTape rather than conventional suture. These incremental improvements led to what I would characterize as today’s gold standard for arthroscopic rotator cuff repair: a largely knotless linked double-row construct using FiberTape, with cinch-loop sutures at the anterior and posterior margins of the tear to reinforce the cable attachments and simultaneously reduce the dog-ears that typically occur in those locations, and a double-pulley medial mattress if tendon quality is poor (Figure 5).
The Burden of Craft
With all the recent enthusiasm for level I studies, I think we need to examine whether they will accelerate technological advancement in rotator cuff repair. The answer, in my opinion, is a resounding no. This answer is based on a major disconnect I have detected in how we evaluate these studies in rotator cuff disease and repair.
An irony related to technological advancement in surgery is that the more technically advanced the surgery becomes, the more skill is required. This fact is completely at odds with the public’s perception that technological advances make procedures easier. In arthroscopic rotator cuff repair, the surgeon must look, feel, and be aware to a greater degree than in open surgery.
Edward Tenner, in his book Why Things Bite Back, described the burden of the practitioner of any advanced technology as the burden of craft.14 The burden of craft is the inherent demand on all craftspeople, but particularly surgeons, to “up our game” if we are to be successful in our craft. For arthroscopic rotator cuff repair, the burden of craft requires patience, attention to detail, and the ability to work in a virtual space. Not everyone has these skills. But anyone who wants to practice in this discipline has an obligation to learn the skills required, and then to teach them to others and assess how well they are being applied.
The problem with relying on level I studies to assess the efficacy of a surgical procedure is that they are inherently biased by the surgeons involved. As results depend on surgeons’ skills, and surgeons’ skill levels are not equal, level I studies cannot prove what is possible, cannot demonstrate the limits of a technique, and cannot demonstrate the equivalence of techniques.
Amazingly enough, there are still rotator cuff repair “deniers” who confidently assert from the podium that a large percentage of massive cuff tears cannot be repaired and that, even if they can be repaired, they do not have the biological potential to heal. Given the disparity in surgeons’ skills and results, however, one must ask whether poor results are a consequence of a biological deficit in the patient, or of a skill deficit in the surgeon.
What I know is that we have techniques for predictable arthroscopic repair and healing of the vast majority of rotator cuff tears, even massive tears,15-17 and patients do very well clinically. Yet, among many orthopedic surgeons, there is a trend to go straight to reverse total shoulder arthroplasty (rTSA) for massive tears—despite the evidence against it. As reported in the literature, rTSA results are not as good as arthroscopic cuff repair results, and the complication rate for rTSA is much higher.
Why has this trend toward rTSA for massive tears gained so much momentum? The only reason I can surmise is that, for the average surgeon, rTSA is easier and quicker than arthroscopic repair for massive tears. But the reason for choosing a specific type of surgery for a given problem should not be that it is easiest for the surgeon; it should be that it is best for the patient.
The surgeon should start by asking what procedure he or she would want if the roles were reversed—if the surgeon were the patient with the massive rotator cuff tear. If a surgeon does not have the skill set for the best procedure for a particular patient, he or she is obligated to send that patient to a surgeon who does have the skills. In addition, given that infection is the most feared complication in most shoulder surgeries, the surgeon should ask which infection rate would be personally acceptable. Arthroscopic rotator cuff repair has a reported infection rate of 1.6 per 1000, or .0016,18 whereas rTSA has an infection rate about 25 times higher, or .04.19 Further, the surgeon must consider the relative severity of the consequences of infection. By any measure, an infected arthroscopy is a straightforward treatable complication, but an infected shoulder replacement is a human tragedy. Patients vastly prefer the minimally invasive arthroscopic approach, and through online searches can easily identify who can offer an arthroscopic solution.
To reproducibly achieve successful arthroscopic repair of massive rotator cuff tears, the surgeon must know advanced techniques, including subscapularis repair techniques,20,21 interval slides,22,23 and self-reinforcing constructs.24,25
“It’s a poor carpenter who blames his tools.” This 18th-century English proverb is as true today as it was 300 years ago. The tools for arthroscopic cuff repair exist, and they are excellent. The burden of craft is the surgeon’s burden and obligation. As surgeons, we must accept that obligation and the responsibility of that burden.
As mentioned earlier, Dr. Rob Bell’s charge to me when he invited me to give the Neer Lecture was to sum up my involvement in the development of arthroscopic shoulder surgery. The short version is that I have been doing shoulder arthroscopy for 31 years; have received 28 US patents related to shoulder instruments and implants and have 12 US patents pending; have published 167 peer-reviewed articles, a couple dozen book chapters, and 2 textbooks on shoulder arthroscopy; have trained 25 fellows; and have hosted approximately 3000 visiting surgeons in my operating room. My greatest professional dream was to see the standard of care for rotator cuff repair and shoulder instability transition from open to arthroscopic techniques, and I have been fortunate enough to have observed that paradigm shift during my career.
What do I envision over the next 31 years? As we all know, history runs in both directions, and some things simply have not happened yet. In terms of rotator cuff treatment, I think over the next few years the guiding principle of treatment will be joint preservation. All rotator cuff tears, even massive tears, will be repaired arthroscopically. Patients and insurers will demand arthroscopic repair, and surgeons without the skill set will migrate to other subspecialties. As for the role of arthroplasty in the treatment of rotator cuff tears, rTSA will be indicated only for pseudoparalysis after failed cuff repair in low-demand elderly patients.
In rotator cuff treatment, I envision a standard of care that is almost entirely arthroscopic. This standard will demand that surgeons who treat rotator cuff tears be proficient in arthroscopic repair of the full range of tears. Acquiring the skills for arthroscopic repair may not be easy, but then “there’s the easy way, and there’s the Cowboy Way.” As my dad used to tell me when I complained about working too hard, “No man ever drowned in his own sweat.” We shoulder surgeons must accept the burden of craft that accompanies the new standard of arthroscopic cuff repair, and we must offer our patients the same level of care we would choose for ourselves.
Happy trails!
I am very honored that Dr. Rob Bell, past president of the American Shoulder and Elbow Surgeons, invited me to give last year’s Neer Lecture. Dr. Bell asked me to specifically address my role in the development of arthroscopic rotator cuff repair and to recount the significant resistance that the early arthroscopic shoulder surgeons faced from the shoulder establishment as we struggled to achieve mainstream acceptance for this new technology. Tasked with such a personal topic, I find myself in a position analogous to that of Winston Churchill at the end of World War II. When a journalist asked him to speculate on how historians would portray his role in the war, he replied without hesitation, “History will be kind to me because I intend to write it.”
So let’s start at the beginning. And for me it makes the most sense to travel back to the year I started my practice: 1981. The world then was very different from today’s world. On January 20, 1981, Ronald Reagan was inaugurated President of the United States. The same day, 52 US hostages in Iran were released after having been held captive for 442 days. In March 1981, Reagan survived an assassination attempt; 3 months earlier, John Lennon had not been so lucky. Lennon’s hit song “Starting Over” garnered the highest musical awards posthumously.
The world of shoulder surgery was also very different in 1981. The arthroscope was the “instrument of the devil,” according to Dr. Rockwood. And shoulder surgery was ruled by the Charlies—Dr. Charles Neer, Dr. Charlie Rockwood, and any other Charlie who felt compelled to marginalize shoulder arthroscopy.
My personal world in the early 1980s was daunting as well. I had just completed my residency at the Mayo Clinic and my sports medicine fellowship in Eugene, Oregon. I had a young son, a new daughter, and a new job with the San Antonio Orthopaedic Group. I had a new house with a 21% mortgage loan and a “new” used car with a 23% car loan.
I was simultaneously energized and intimidated by my new job, where I was doing general orthopedics with a “special interest” in shoulder surgery and sports medicine. I was initially very proud and humbled by the fact that my senior partners had entrusted me with the care of the most difficult shoulder cases within the practice. But that pride got cut down to its appropriate size the day after I had thanked one of my partners, Dr. Lamar Collie, for his confidence in my potential as a shoulder surgeon. Dr. Collie replied matter-of-factly, “Sure … but you need to understand that we always make the new guy the shoulder expert because shoulders never do worth a damn.”
For shoulder arthroscopy, the early 1980s were exciting. Most of us who were scoping shoulders had already been doing knee arthroscopy and were trying to adapt knee instruments to the shoulder. This worked for some simple excisional cases. For example, I recall excising the bucket-handle portion of a type III SLAP (superior labral tear from anterior to posterior) lesion in 1983. In general, however, shoulder problems were different from knee problems and usually involved repair rather than excision of damaged tissues. Therefore, the technology used in knee arthroscopy was often not directly transferable to the shoulder. Furthermore, treatment of the rotator cuff necessitated development of arthroscopic techniques in a virtual space, the subacromial space, and this was an entirely new arthroscopic concept.
Development of Arthroscopic Rotator Cuff Repair
A major mind-expanding turning point for me occurred in 1984 when I attended one of Dr. Jim Esch’s early San Diego shoulder courses. During that course, Dr. Harvard Ellman of Los Angeles demonstrated to me on a cadaver shoulder how he created a virtual subacromial working space that allowed enough visualization for an arthroscopic acromioplasty. At that moment, I knew that arthroscopic rotator cuff repair was just around the corner. Up until then, I had not been able to envision complex extra-articular reconstructive surgery, as all previous arthroscopic surgery had been intra-articular. But now, having realized a virtual working space could always be created, I knew it would be relatively straightforward to develop the portals to approach the cuff as well as the implants and the instruments to repair it. But I also knew that progression to all-arthroscopic repair techniques would have to be stepwise and that the final repair constructs would need to be at least as strong as those of open repair in order to be acceptable. With an undergraduate degree in mechanical engineering, I had a reasonably clear idea of the concepts I wanted to apply to the instrumentation and techniques, though I could never have envisioned how circuitous the route to the end result would be.
First Steps
I sketched out my ideas for arthroscopic suture passers and knot-tying instruments and presented them to a couple of the major arthroscopy companies in the United States, but the companies were not interested. They did not believe arthroscopy would have any meaningful applications in the shoulder. So, I enlisted the services of a local San Antonio aircraft machinist to fabricate instruments for me. By 1987, I was doing arthroscopic side-to-side margin convergence1 cuff repairs for U-shape tears on a regular basis. And I was doing these at the most hostile point in the universe for arthroscopic shoulder surgery: San Antonio, Texas.
Only a few surgeons were doing arthroscopic shoulder surgery in the 1980s and early 1990s, and without exception these surgeons became the leader-pioneers in the new discipline. In general, these were young surgeons who were in private practice and removed from academia and professional organizations, and thus relatively sheltered from the actions of the shoulder rule-makers of the day. They accepted their status as pariahs as they developed their techniques out of the view of mainstream orthopedics. These leaders included Jim Esch, Steve Snyder, Dick Caspari, Lanny Johnson, Gene Wolf, Gary Gartsman, Rob Bell, and Howard Sweeney. We shared our techniques and our ideas with one another, encouraged one another, and generally became good friends.
Thomas Kuhn, in his classic book The Structure of Scientific Revolutions,2 observed that paradigm shifts within a given field were usually achieved by practitioners who were either very young (naïve) or outside the established hierarchy in the field. The surgeons who contributed most to the shift of shoulder surgery from open to arthroscopic techniques were generally young men who were in private practice and had little to lose by inciting the disdain of the shoulder establishment. Predictably, resistance from the mainstream open shoulder surgeons increased as arthroscopic techniques became more successful and more threatening to the primacy of the open shoulder surgeons. The disdain yielded to disruption and finally to transformation as the paradigm shift occurred. The conflict between the open shoulder surgeons and the arthroscopic shoulder surgeons passed through all the phases that Mahatma Gandhi had described many years before. “First they ignore you; then they laugh at you; then they fight you; then you win.”
Building a Ship in a Bottle
At the start of the 1990s, I recognized that my progress in arthroscopic rotator cuff repair would be extremely slow unless I could find an industry partner who shared my vision for full-scale conversion to arthroscopic means of repair and would be willing to help make it a reality. In 1991, I happened to meet Reinhold Schmieding, the owner of Arthrex, a small arthroscopic device company in Naples, Florida. Reinhold invited me to visit him to discuss the feasibility of developing arthroscopic repair systems for the shoulder. At the time, the world headquarters of Arthrex was a 20×30-ft storage room in an office service center, and there were 2 employees. One employee, Don Grafton, was a talented engineer without medical experience. By the end of my first day there, Reinhold and Don and I had agreed that developing arthroscopic repair systems for shoulder instability and rotator cuff repair would become a top priority for Arthrex.
My initial bias toward arthroscopic cuff repair was that a transosseous bone tunnel technique not only would be possible but would be superior to suture anchor fixation. In fact, my first 2 patents with Arthrex were for instrumentation for an arthroscopic transosseous repair technique. I tested my hypothesis with 2 successive biomechanical studies. The first examined cyclic loading of bone tunnel repairs, and the second examined cyclic loading of anchor-based repairs.3,4 Evaluating the data from these 2 studies, I was surprised to find that anchor-based repairs were significantly stronger than bone tunnel repairs. In addition, anchors shifted the weak link from the bone–suture interface to the tendon–suture interface; in essence, anchors optimized bone fixation by shifting the weak link in the construct to the tendon. I was then completely convinced of the superiority of suture anchors over bone tunnels, and that conviction has become even stronger over the years. After these 2 cyclic loading studies, I shifted my focus, and that of Arthrex, toward arthroscopic suture anchor repair of the rotator cuff.
Reconciling Technique and Instrumentation With Anatomy and Biomechanics
Having recognized the importance of the rotator cable attachments both anatomically5 and biomechanically,6,7 I thought it important to reinforce them as a routine part of performing rotator cuff repairs. Our anatomical and biomechanical studies had had great translational implications in the development of our techniques and instrumentation.
As mentioned earlier, Don Grafton was the chief (and for a long time only) engineer at Arthrex. As he had no medical experience, I invited him to come to San Antonio to observe surgery. During Don’s many visits, I showed him pathology in the operating room and pointed out what I could do with the instruments I had and what I could not do. Then in the evening we went to my house and brainstormed how to perform the “missing” surgical manipulations, how to improve manipulations that were suboptimal, and how to optimize final surgical constructs.
Passing suture through tendon was an early challenge. One must remember that, in the early 1990s, it was not possible for machinists to fabricate complex shapes. Therefore, straight tubular retrograde suture passers were the logical first option. We initially developed spring-loaded retrograde hook retrievers (Figure 1) and then curved suture hooks with shuttling wires (Lasso). To me, the most unappealing feature of retrograde suture passage was the oblique angle of approach through the tendon, which caused a length–tension mismatch between the upper fibers and the lower fibers of the muscle–tendon unit. We recognized we could eliminate the mismatch if we passed the suture antegrade, such that it would pass perpendicular to the tendon fibers. These insights and efforts culminated in development of the Viper suture passer and then the FastPass Scorpion suture passer, which has a spring-loaded trapdoor on the upper jaw for ergonomic self-retrieving of the suture once it is passed through the tendon.
To develop a knot pusher that optimized knot tying (yielding the highest knot security and the tightest loop security), we used prototype instruments to tie and test literally thousands of knots in the laboratory. We were thus able to verify that the Surgeon’s Sixth Finger Knot Pusher (Arthrex) reproducibly tied optimized knots8,9 and also optimized knot fixation and bone fixation. However, our suture was not yet optimized and was prone to breakage, and our suture–tendon interface was not yet optimized. Clearly, improvement was needed in 2 more areas.
Don came up with the idea for a virtually unbreakable suture and developed that idea into FiberWire.10 Shortly thereafter, I contributed the idea and design for FiberTape, which dramatically enhanced suture pullout strength and footprint compression.
Anchor designs improved rapidly and dramatically. We made the second-generation BioCorkscrew fully threaded, which virtually eliminated anchor failure, even in soft bone.
Optimization of the suture–tendon interface took a giant step forward when Park and colleagues11,12 introduced linked double-row rotator cuff repair. Much as with a Chinese finger trap, the harder you pull, the stronger it becomes, with yield load approaching ultimate load.
At this point, it seemed we had optimized virtually every segment of the rotator cuff repair construct. Each component was just about as good as it could be. Or was it?
The Accidental Quest for Knotless Fixation
In November 1998, I made my first trip to China as a guest speaker at the Congress of the Hong Kong Orthopaedic Association. My first view of the magnificent Hong Kong skyline across Victoria Harbour was truly breathtaking. As I admired the gleaming glass towers and the concrete canyons of the city, I had no idea that the very next day these modern skyscrapers would reveal an ancient secret that would change my approach to arthroscopic rotator cuff repair.
The day after my arrival, Dr. James Lam took me to lunch. As we approached the restaurant, he pointed across the street to a tall building that was being renovated and had scaffolding supporting workers alongside the first 9 stories of the exterior wall. Dr. Lam said that, after lunch, he would take me to the construction site for a closer look at the scaffolding.
After lunch, we walked to the base of the scaffolding. Dr. Lam told me it was constructed entirely of bamboo poles held together with lashings but no knots (Figure 2). Lashings were secured by turning them back on themselves and wrapping them in an entirely knotless manner.13 I found it incredible that this knotless fixation was so secure that it could support the weight of workers many stories above the ground. I resolved to determine how this fixation method worked and see if the same mechanism might help us achieve reliable knotless fixation in surgery.
When I returned home, I broke out my college engineering books and reacquainted myself with the concept of cable friction. As has happened so often in the past, however, it took a practical lesson from the ranch to truly illustrate for me how cable friction works.
Every cowboy knows that a spirited horse cannot be restrained with only one lead rope. However, a cowboy can wrap a lead rope around a “snubbing post” and thereby gain complete control over the animal, despite the horse’s superior size and strength. The cable friction between the rope and the post creates such a large restraining force that the cowboy can easily hold the animal without the help of a knotted rope (Figure 3). In similar fashion in the Hong Kong scaffolding, fixation strength results from the significant amount of cable friction produced when the lashings wrap around one another and around the bamboo poles.
The cable friction concept was pivotal in the development of knotless fixation in arthroscopic rotator cuff repair. In lateral row fixation, the eyelet of the PushLock and SwiveLock suture anchors (Arthrex) produces significant cable friction at the eyelet–suture interface, in addition to frictional force wedging the suture between anchor and bone.
As with so many other devices in shoulder arthroscopy, the SwiveLock suture anchor developed in stages. In the first stage, a chainlike suture with consecutive intersecting links was used (FiberChain). The idea for an adjustable fixation construct came to me because I thought that a forked eyelet on a SwiveLock would provide a firm fixation point when inserted into the appropriate suture link, yet would be totally adjustable simply by choosing a tighter or looser link (Figure 4). Although the system worked very well, it was technically challenging. The process was greatly simplified after Don Grafton and I developed FiberTape and recognized that the power of cable friction was dramatically increased by the larger contact area between the eyelet and the braided FiberTape. The SpeedBridge construct (Arthrex), which enhanced cable friction fixation by means of passing FiberTape through the anchor eyelets, also provided a larger compressive interface at the repair site by using FiberTape rather than conventional suture. These incremental improvements led to what I would characterize as today’s gold standard for arthroscopic rotator cuff repair: a largely knotless linked double-row construct using FiberTape, with cinch-loop sutures at the anterior and posterior margins of the tear to reinforce the cable attachments and simultaneously reduce the dog-ears that typically occur in those locations, and a double-pulley medial mattress if tendon quality is poor (Figure 5).
The Burden of Craft
With all the recent enthusiasm for level I studies, I think we need to examine whether they will accelerate technological advancement in rotator cuff repair. The answer, in my opinion, is a resounding no. This answer is based on a major disconnect I have detected in how we evaluate these studies in rotator cuff disease and repair.
An irony related to technological advancement in surgery is that the more technically advanced the surgery becomes, the more skill is required. This fact is completely at odds with the public’s perception that technological advances make procedures easier. In arthroscopic rotator cuff repair, the surgeon must look, feel, and be aware to a greater degree than in open surgery.
Edward Tenner, in his book Why Things Bite Back, described the burden of the practitioner of any advanced technology as the burden of craft.14 The burden of craft is the inherent demand on all craftspeople, but particularly surgeons, to “up our game” if we are to be successful in our craft. For arthroscopic rotator cuff repair, the burden of craft requires patience, attention to detail, and the ability to work in a virtual space. Not everyone has these skills. But anyone who wants to practice in this discipline has an obligation to learn the skills required, and then to teach them to others and assess how well they are being applied.
The problem with relying on level I studies to assess the efficacy of a surgical procedure is that they are inherently biased by the surgeons involved. As results depend on surgeons’ skills, and surgeons’ skill levels are not equal, level I studies cannot prove what is possible, cannot demonstrate the limits of a technique, and cannot demonstrate the equivalence of techniques.
Amazingly enough, there are still rotator cuff repair “deniers” who confidently assert from the podium that a large percentage of massive cuff tears cannot be repaired and that, even if they can be repaired, they do not have the biological potential to heal. Given the disparity in surgeons’ skills and results, however, one must ask whether poor results are a consequence of a biological deficit in the patient, or of a skill deficit in the surgeon.
What I know is that we have techniques for predictable arthroscopic repair and healing of the vast majority of rotator cuff tears, even massive tears,15-17 and patients do very well clinically. Yet, among many orthopedic surgeons, there is a trend to go straight to reverse total shoulder arthroplasty (rTSA) for massive tears—despite the evidence against it. As reported in the literature, rTSA results are not as good as arthroscopic cuff repair results, and the complication rate for rTSA is much higher.
Why has this trend toward rTSA for massive tears gained so much momentum? The only reason I can surmise is that, for the average surgeon, rTSA is easier and quicker than arthroscopic repair for massive tears. But the reason for choosing a specific type of surgery for a given problem should not be that it is easiest for the surgeon; it should be that it is best for the patient.
The surgeon should start by asking what procedure he or she would want if the roles were reversed—if the surgeon were the patient with the massive rotator cuff tear. If a surgeon does not have the skill set for the best procedure for a particular patient, he or she is obligated to send that patient to a surgeon who does have the skills. In addition, given that infection is the most feared complication in most shoulder surgeries, the surgeon should ask which infection rate would be personally acceptable. Arthroscopic rotator cuff repair has a reported infection rate of 1.6 per 1000, or .0016,18 whereas rTSA has an infection rate about 25 times higher, or .04.19 Further, the surgeon must consider the relative severity of the consequences of infection. By any measure, an infected arthroscopy is a straightforward treatable complication, but an infected shoulder replacement is a human tragedy. Patients vastly prefer the minimally invasive arthroscopic approach, and through online searches can easily identify who can offer an arthroscopic solution.
To reproducibly achieve successful arthroscopic repair of massive rotator cuff tears, the surgeon must know advanced techniques, including subscapularis repair techniques,20,21 interval slides,22,23 and self-reinforcing constructs.24,25
“It’s a poor carpenter who blames his tools.” This 18th-century English proverb is as true today as it was 300 years ago. The tools for arthroscopic cuff repair exist, and they are excellent. The burden of craft is the surgeon’s burden and obligation. As surgeons, we must accept that obligation and the responsibility of that burden.
As mentioned earlier, Dr. Rob Bell’s charge to me when he invited me to give the Neer Lecture was to sum up my involvement in the development of arthroscopic shoulder surgery. The short version is that I have been doing shoulder arthroscopy for 31 years; have received 28 US patents related to shoulder instruments and implants and have 12 US patents pending; have published 167 peer-reviewed articles, a couple dozen book chapters, and 2 textbooks on shoulder arthroscopy; have trained 25 fellows; and have hosted approximately 3000 visiting surgeons in my operating room. My greatest professional dream was to see the standard of care for rotator cuff repair and shoulder instability transition from open to arthroscopic techniques, and I have been fortunate enough to have observed that paradigm shift during my career.
What do I envision over the next 31 years? As we all know, history runs in both directions, and some things simply have not happened yet. In terms of rotator cuff treatment, I think over the next few years the guiding principle of treatment will be joint preservation. All rotator cuff tears, even massive tears, will be repaired arthroscopically. Patients and insurers will demand arthroscopic repair, and surgeons without the skill set will migrate to other subspecialties. As for the role of arthroplasty in the treatment of rotator cuff tears, rTSA will be indicated only for pseudoparalysis after failed cuff repair in low-demand elderly patients.
In rotator cuff treatment, I envision a standard of care that is almost entirely arthroscopic. This standard will demand that surgeons who treat rotator cuff tears be proficient in arthroscopic repair of the full range of tears. Acquiring the skills for arthroscopic repair may not be easy, but then “there’s the easy way, and there’s the Cowboy Way.” As my dad used to tell me when I complained about working too hard, “No man ever drowned in his own sweat.” We shoulder surgeons must accept the burden of craft that accompanies the new standard of arthroscopic cuff repair, and we must offer our patients the same level of care we would choose for ourselves.
Happy trails!
1. Burkhart SS, Athanasiou KA, Wirth MA. Margin convergence: a method of reducing strain in massive rotator cuff tears. Arthroscopy. 1996:12(3):335-338.
2. Kuhn TS. The Structure of Scientific Revolutions. Chicago, IL: University of Chicago Press; 1962.
3. Burkhart SS, Johnson TC, Wirth MA, Athanasiou KA. Cyclic loading of transosseous rotator cuff repairs: tension overload as a possible cause of failure. Arthroscopy. 1997;13(2):172-176.
4. Burkhart SS, Diaz Pagàn JL, Wirth MA, Athansiou KA. Cyclic loading of anchor-based rotator cuff repairs: confirmation of the tension overload phenomenon and comparison of suture anchor fixation with transosseous fixation. Arthroscopy. 1997;13(6):720-724.
5. Burkhart SS, Esch JC, Jolson RS. The rotator crescent and rotator cable: an anatomic description of the shoulder’s “suspension bridge.” Arthroscopy. 1993;9(6):611-616.
6. Burkhart SS, Nottage WM, Ogilvie-Harris DJ, Kohn HS, Pachelli A. Partial repair of irreparable rotator cuff tears. Arthroscopy. 1994;10(4):363-370.
7. Halder AM, O’Driscoll SW, Heers G, et al. Biomechanical comparison of effects of supraspinatus tendon detachments, tendon defects, and muscle retractions. J Bone Joint Surg Am. 2002;84(5):780-785.
8. Lo IK, Burkhart SS, Chan KC, Athanasiou K. Arthroscopic knots: determining the optimal balance of loop security and knot security. Arthroscopy. 2004;20(5):489-502.
9. Lo IK, Ochoa E Jr, Burkhart SS. A comparison of knot security and loop security in arthroscopic knots tied with newer high-strength suture materials. Arthroscopy. 2010;26(9 suppl):S120-S126.
10. Lo IK, Burkhart SS, Athanasiou K. Abrasion resistance of two types of nonabsorbable braided suture. Arthroscopy. 2004;20(4):407-413.
11. Park MC, ElAttrache NS, Tibone JE, Ahmad CS, Jun BJ, Lee TQ. Part I: footprint contact characteristics for a “transosseous-equivalent” rotator cuff repair technique compared to a double-row technique. J Shoulder Elbow Surg. 2007;16(4):461-468.
12. Park MC, Tibone JE, ElAttrache NS, Ahmad CS, Jun BJ, Lee TQ. Part II: biomechanical assessment for a footprint-restoring transosseous-equivalent rotator cuff repair technique compared with a double-row repair technique. J Shoulder Elbow Surg. 2007;16(4):469-476.
13. Burkhart SS, Athanasiou KA. The twist-lock concept of tissue transport and suture fixation without knots: observations along the Hong Kong skyline. Arthroscopy. 2003;19(6):613-625.
14. Tenner E. Why Things Bite Back. New York, NY: Random House; 1996.
15. Denard PJ, Jiwani AZ, Lädermann A, Burkhart SS. Long-term outcome of arthroscopic massive rotator cuff repair: the importance of double-row fixation. Arthroscopy. 2012;28(7):909-915.
16. Denard PJ, Lädermann A, Jiwani AZ, Burkhart SS. Functional outcome after arthroscopic repair of massive rotator cuff tears in individuals with pseudoparalysis. Arthroscopy. 2012;28(9):1214-1219.
17. Lädermann A, Denard PJ, Burkhart SS. Revision arthroscopic rotator cuff repair: systematic review and authors’ preferred surgical technique. Arthroscopy. 2012;28(8):1160-1169.
18. Randelli P, Castagna A, Cabitza F, Cabitza P, Arrigoni P, Denti M. Infectious and thromboembolic complications of arthroscopic shoulder surgery. J Shoulder Elbow Surg. 2010;19(1):97-101.
19. Zumstein MA, Pinedo M, Old J, Boileau P. Problems, complications, reoperations, and revisions in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2011;20(1):146-157.
20. Adams CR, Schoolfield JD, Burkhart SS. The results of arthroscopic subscapularis tendon repairs. Arthroscopy. 2008;24(12):1381-1389.
21. Denard PJ, Jiwani AZ, Lädermann A, Burkhart SS. Long-term outcome of a consecutive series of subscapularis tendon tears repaired arthroscopically. Arthroscopy. 2012;28(11):1587-1591.
22. Lo IK, Burkhart SS. Arthroscopic repair of massive, contracted, immobile rotator cuff tears using single and double interval slides: technique and preliminary results. Arthroscopy. 2004;20(1):22-33.
23. Lo IK, Burkhart SS. The interval slide in continuity: a method of mobilizing the anterosuperior rotator cuff without disrupting the tear margins. Arthroscopy. 2004;20(4):435-441.
24. Denard PJ, Burkhart SS. Techniques for managing poor quality tissue and bone during arthroscopic rotator cuff repair. Arthroscopy. 2011;27(10):1409-1421.
25. Burkhart SS, Denard PJ, Konicek J, Hanypsiak BT. Biomechanical validation of load-sharing rip-stop fixation for the repair of tissue-deficient rotator cuff tears. Am J Sports Med. 2014;42(2):457-462.
1. Burkhart SS, Athanasiou KA, Wirth MA. Margin convergence: a method of reducing strain in massive rotator cuff tears. Arthroscopy. 1996:12(3):335-338.
2. Kuhn TS. The Structure of Scientific Revolutions. Chicago, IL: University of Chicago Press; 1962.
3. Burkhart SS, Johnson TC, Wirth MA, Athanasiou KA. Cyclic loading of transosseous rotator cuff repairs: tension overload as a possible cause of failure. Arthroscopy. 1997;13(2):172-176.
4. Burkhart SS, Diaz Pagàn JL, Wirth MA, Athansiou KA. Cyclic loading of anchor-based rotator cuff repairs: confirmation of the tension overload phenomenon and comparison of suture anchor fixation with transosseous fixation. Arthroscopy. 1997;13(6):720-724.
5. Burkhart SS, Esch JC, Jolson RS. The rotator crescent and rotator cable: an anatomic description of the shoulder’s “suspension bridge.” Arthroscopy. 1993;9(6):611-616.
6. Burkhart SS, Nottage WM, Ogilvie-Harris DJ, Kohn HS, Pachelli A. Partial repair of irreparable rotator cuff tears. Arthroscopy. 1994;10(4):363-370.
7. Halder AM, O’Driscoll SW, Heers G, et al. Biomechanical comparison of effects of supraspinatus tendon detachments, tendon defects, and muscle retractions. J Bone Joint Surg Am. 2002;84(5):780-785.
8. Lo IK, Burkhart SS, Chan KC, Athanasiou K. Arthroscopic knots: determining the optimal balance of loop security and knot security. Arthroscopy. 2004;20(5):489-502.
9. Lo IK, Ochoa E Jr, Burkhart SS. A comparison of knot security and loop security in arthroscopic knots tied with newer high-strength suture materials. Arthroscopy. 2010;26(9 suppl):S120-S126.
10. Lo IK, Burkhart SS, Athanasiou K. Abrasion resistance of two types of nonabsorbable braided suture. Arthroscopy. 2004;20(4):407-413.
11. Park MC, ElAttrache NS, Tibone JE, Ahmad CS, Jun BJ, Lee TQ. Part I: footprint contact characteristics for a “transosseous-equivalent” rotator cuff repair technique compared to a double-row technique. J Shoulder Elbow Surg. 2007;16(4):461-468.
12. Park MC, Tibone JE, ElAttrache NS, Ahmad CS, Jun BJ, Lee TQ. Part II: biomechanical assessment for a footprint-restoring transosseous-equivalent rotator cuff repair technique compared with a double-row repair technique. J Shoulder Elbow Surg. 2007;16(4):469-476.
13. Burkhart SS, Athanasiou KA. The twist-lock concept of tissue transport and suture fixation without knots: observations along the Hong Kong skyline. Arthroscopy. 2003;19(6):613-625.
14. Tenner E. Why Things Bite Back. New York, NY: Random House; 1996.
15. Denard PJ, Jiwani AZ, Lädermann A, Burkhart SS. Long-term outcome of arthroscopic massive rotator cuff repair: the importance of double-row fixation. Arthroscopy. 2012;28(7):909-915.
16. Denard PJ, Lädermann A, Jiwani AZ, Burkhart SS. Functional outcome after arthroscopic repair of massive rotator cuff tears in individuals with pseudoparalysis. Arthroscopy. 2012;28(9):1214-1219.
17. Lädermann A, Denard PJ, Burkhart SS. Revision arthroscopic rotator cuff repair: systematic review and authors’ preferred surgical technique. Arthroscopy. 2012;28(8):1160-1169.
18. Randelli P, Castagna A, Cabitza F, Cabitza P, Arrigoni P, Denti M. Infectious and thromboembolic complications of arthroscopic shoulder surgery. J Shoulder Elbow Surg. 2010;19(1):97-101.
19. Zumstein MA, Pinedo M, Old J, Boileau P. Problems, complications, reoperations, and revisions in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2011;20(1):146-157.
20. Adams CR, Schoolfield JD, Burkhart SS. The results of arthroscopic subscapularis tendon repairs. Arthroscopy. 2008;24(12):1381-1389.
21. Denard PJ, Jiwani AZ, Lädermann A, Burkhart SS. Long-term outcome of a consecutive series of subscapularis tendon tears repaired arthroscopically. Arthroscopy. 2012;28(11):1587-1591.
22. Lo IK, Burkhart SS. Arthroscopic repair of massive, contracted, immobile rotator cuff tears using single and double interval slides: technique and preliminary results. Arthroscopy. 2004;20(1):22-33.
23. Lo IK, Burkhart SS. The interval slide in continuity: a method of mobilizing the anterosuperior rotator cuff without disrupting the tear margins. Arthroscopy. 2004;20(4):435-441.
24. Denard PJ, Burkhart SS. Techniques for managing poor quality tissue and bone during arthroscopic rotator cuff repair. Arthroscopy. 2011;27(10):1409-1421.
25. Burkhart SS, Denard PJ, Konicek J, Hanypsiak BT. Biomechanical validation of load-sharing rip-stop fixation for the repair of tissue-deficient rotator cuff tears. Am J Sports Med. 2014;42(2):457-462.
Shoulder Examination of the Overhead Athlete
The overhead athlete’s shoulder is exposed to extremes of stress and range of motion (ROM), predisposing this joint to unique injury patterns. Prompt diagnosis and management begin with a comprehensive history and a physical examination, supplemented by imaging studies as needed. Furthermore, the throwing shoulder undergoes adaptive changes, such as partial undersurface rotator cuff tears and capsular laxity. Imaging studies typically demonstrate abnormalities in asymptomatic throwers. Therefore, clinicians must be skilled in history taking and physical examination in throwing athletes to accurately determine the cause of symptoms and provide optimal treatment. This primer provides orthopedic surgeons with the key points in performing a thorough physical examination of the shoulder in overhead athletes.
When working with overhead athletes, surgeons must elicit the precise nature of symptoms. For example, it is important to distinguish pain from fatigue, as well as complaints related purely to decline in performance. Often, collaboration with the player’s parent or coach may help clarify the chief complaint. In addition, surgeons must have an intricate knowledge of the various stages of the overhead motion, as symptoms in specific stages (late cocking/early acceleration) may raise suspicion for distinctive pathology (labral/biceps complex). Last, it is imperative to understand that the shoulder represents only one part of the kinetic chain in overhead athletes. Successful throwing relies on integrity of the entire kinetic chain, starting with the lower extremity and trunk, extending through the spine, scapula, and shoulder, and terminating with the hand and fingers. Pathology anywhere in the chain must be evaluated and addressed.
When examining the shoulder in overhead athletes, surgeons must address several anatomical structures, both bony and soft tissue. Proper examination begins with comprehensive assessment of the ROM and strength of the various muscles around the shoulder, along with visual inspection to identify any asymmetry of these structures. In addition, the scapulothoracic structures must be examined in detail to rule out underlying dyskinesis. The capsular and ligamentous components of the shoulder joint must be further assessed to note any capsular contracture causing glenohumeral internal rotation deficit (GIRD) or any pathology with the rotator cuff or labral/biceps complex. Last, a comprehensive neurovascular examination should be performed to rule out any compression or neuropathy affecting the shoulder and overhead motion. Findings from the physical examination may then require further imaging to correlate the history and physical examination findings.
1. Inspection, palpation, strength testing
Every examination of the shoulder must begin with visual inspection, along with assessment of basic ROM and strength. The patient must be positioned and exposed adequately to promote visualization of the entire shoulder and scapular girdle, from both anterior and posterior. Visual inspection focuses on identifying any areas of asymmetry, such as position of the bony prominences or bulk of the muscular fossae. Asymmetry of the bony architecture may indicate prior trauma, and atrophy of the muscular fossae may indicate nerve compression. For example, atrophy of the infraspinatus fossa may be caused by compression of the suprascapular nerve at the spinoglenoid notch (likely by a cyst, often associated with labral pathology, but infraspinatus atrophy can result even without the presence of a compressive cyst1). Alternatively, atrophy of both the supraspinatus and infraspinatus fossae may indicate underlying compression of the suprascapular nerve at the suprascapular notch (either by a cyst or by the transverse scapular ligament). Static and dynamic observation of the posterior aspect of the shoulder may help identify gross pathology with scapular positioning or retraction, indicating underlying dyskinesis (discussed later). Deformity of the acromioclavicular joint may indicate prior trauma or separation. Last, all prior surgical scars should be noted.
Selective palpation may help identify pathology in the shoulder of the throwing athlete. Tenderness at the acromioclavicular joint may be especially common in patients who have had prior sprains of this joint or who have degenerative changes. Tenderness along the biceps tendon may be present in those with biceps tendinitis or partial tear. In addition, tenderness at the coracoid may be present in those with scapular dyskinesis. Posteriorly, palpation at the inferomedial aspect of the scapula (Figure 1), as with palpation along the medial border of the scapula, may elicit tenderness in those with scapulothoracic bursitis.
Strength testing in the shoulder is performed to elicit any deficiencies of the rotator cuff/musculature or surrounding structures. Weakness in forward elevation may indicate pathology in the supraspinatus, whereas weakness in external rotation may reflect deficiency in the infraspinatus or teres minor. Teres minor deficiency may be more isolated with weakness in a position of shoulder abduction to 90°. Last, weakness in internal rotation may indicate subscapularis deficiency. Lag signs and other provocative maneuvers are similarly elicited but typically are positive only in the event of large tears of the rotator cuff. These signs and maneuvers include the internal rotation lag sign or belly press test for subscapularis integrity, the drop-arm sign for supraspinatus function, the external rotation lag sign for infraspinatus function, and the hornblower sign for teres minor integrity. Supporting muscles of the shoulder may also be tested. Latissimus strength may be tested with resisted downward rotation of the arm with the shoulder in abduction and the elbow flexed to 90°.
2. ROM and GIRD assessment
After inspection and palpation, the shoulder should be ranged in all relevant planes of motion. Our standard examination includes forward elevation in the frontal and scapular planes, along with external rotation at the side and at 90° of abduction, as well as internal rotation behind the back with documentation of the highest spinal level that the patient can reach. This examination may be performed with the patient upright, but supine positioning can help stabilize the scapula and provide more accurate views of motion. Deficits of internal rotation may be a common finding in overhead athletes, and the degree of this deficit should be quantitatively noted.
Bony and soft-tissue remodeling of the shoulder (and associated structures) in the overhead athlete can lead to contracture of the posterior capsule. This contracture can cause excessive external rotation and subsequent decrease in internal rotation, leading to pain and anterior instability in the throwing shoulder.2 For precise measurements of the internal and external rotation arc, the scapula must be stabilized. This can be done with the patient supine on the examining table or seated upright with manual stabilization of the scapula by the examiner. Once the scapula is stabilized, the arc of internal and external rotation (with the arm in about 90° of abduction) can be measured with a goniometer, with maximum values obtained as the scapula begins to move along the posterior chest wall.2 The difference in internal rotation between the dominant and nondominant arms defines the extent of the athlete’s GIRD. Internal rotation can also be qualitatively assessed by having the athlete internally rotate each arm and reach up the spine while the examiner notes the difference in level achieved. However, this does not provide a quantitative assessment of the patient’s GIRD.
In general, the sum of the internal and external rotation arcs on the 2 sides should be symmetric. Consequently, in GIRD, excessive external rotation is balanced by decreased internal rotation. Symptomatic GIRD may be present when there is more than 25° of discrepancy in internal rotation between the athlete’s dominant and nondominant arms.2 The goal is to reduce this discrepancy to less than 20°.
3. Internal impingement: rotator cuff and labrum
In overhead athletes, an intricate relationship involving rotator cuff, labrum, and biceps tendon allows for efficient, pain-free force delivery at the shoulder. However, because of the significant external rotation and abduction required in the overhead motion, there may be internal impingement of the posterosuperior rotator cuff (infraspinatus and posterior aspect of supraspinatus) between the posterior labrum and the greater tuberosity. Detailed examination of these structures must be performed in any assessment of an overhead athlete. Symptomatic patients may complain of pain during the throwing cycle, particularly in late cocking and early acceleration.
The modified relocation examination is a common maneuver to detect internal impingement.3 In this examination, the patient’s arm is brought into a position of maximal external rotation and abduction mimicking that found in late cocking or early acceleration. In this position, a patient with internal impingement complains of pain in the posterior shoulder. A posteriorly directed force on the humerus relieves this pain.
There are also many examinations for detecting labral pathology, specifically a SLAP (superior labrum, anterior to posterior) lesion, which is commonly found in patients with internal impingement. One commonly tested maneuver is the O’Brien active compression test (Figures 2A, 2B), which has excellent sensitivity and specificity in detecting type II SLAP lesions.4 In this examination, the patient holds the arm in about 15° of adduction and 90° of forward elevation. A downward force is applied with the forearm pronated and subsequently supinated. If pain is noted on the force applied to the pronated arm, and if this pain decreases in the supinated examination, the test is positive for labral pathology.
Anterior instability is routinely found in these patients. Translation is measured with the anterior load and shift test. Anterior translation is tested with the patient supine, with the arm in abduction and external rotation, and with the examiner placing an anteriorly directed force on the humeral head. Translation is compared with the contralateral side and graded on a 3-point scale (1+ is translation to glenoid rim, 2+ is translation over glenoid rim but reduces, 3+ is translation over glenoid and locking). We also use the anterior release test, in which the patient is supine, the arm is brought into abduction and external rotation, and the examiner places a posteriorly directed force on the humeral head. When the examiner removes this force, the patient notices symptoms of instability caused by subluxation (Figures 3A, 3B).
Biceps tendon testing should also be performed to help elicit signs of labral pathology. The Speed test is performed by placing a downward force on the patient’s arm, which is held in 90° forward elevation, and with elbows in extension and forearm in supination. Pain in the long head of the biceps tendon is considered a positive sign and suggestive of SLAP lesion. Although not commonly found in these athletes, external impingement should also be elicited through both the Neer test and the Hawkins test. In the Neer test, the patient’s arm is brought to maximal forward elevation with the forearm supinated and elbow extended, while the scapula is stabilized by the examiner. Pain in the shoulder indicates a positive examination. In the Hawkins test, the patient’s arm is brought into a position of forward elevation, internal rotation, and elbow flexion. The arm is then further internally rotated, and shoulder pain defines a positive examination.
Any of these findings can be concomitant with scapular dyskinesis. Moreover, symptoms related to internal impingement may be exacerbated by concomitant scapular pathology, and therefore proper assessment of scapulothoracic motion must also be performed.
4. Scapulothoracic examination
Motion coupled between the scapula and the rest of the arm (scapular rhythm) allows for efficient use of the shoulder girdle. The scapula helps transfer the force generated by the core so that the hand can efficiently deliver it. Therefore, scapular pathology (or dyskinesis) results in inefficient functioning of the arm, which can be especially debilitating in an overhead athlete.
Scapular assessment begins with visual inspection of the patient, typically from the posterior view, which allows for assessment of the resting position of the scapula. Evidence of prominence of the medial or inferomedial border, coracoid malposition (or pain on palpation), or general scapular malposition should be noted. On active ROM, as the patient forward-elevates the arm, any asymmetric prominence of the inferomedial border of the scapula should be noted. Such asymmetry may indicate underlying scapular dyskinesis. In another important test, the lateral scapular slide test (described by Kibler5), the distance from the inferomedial angle of the scapula to the thoracic spine should be measured for both sides and in 3 difference positions, noting any asymmetry between the affected and nonaffected sides. These 3 positions (Figures 4A–4C) are with arms at side, with hands on hips (internal rotation of humerus in 45° abduction), and in 90° of shoulder abduction. Last, medial and lateral scapular winging—caused by long thoracic nerve and spinal accessory nerve pathology, respectively—can be detected by asking the patient to do a “push-up” against the wall while the examiner views from posterior.
After assessment of scapular position at rest and through motion, a series of provocative maneuvers6 may aid in the diagnosis of scapular dyskinesis. The first maneuver is the scapular assistance test, in which the examiner provides a gentle force at the inferomedial angle of the scapula, promoting upward rotation and posterior tilt as the patient elevates the arm (Figures 5A, 5B). If the patient experiences a decrease or absence of symptoms through this arc, the test is considered positive. The second maneuver is the scapular retraction test, in which strength testing of the supraspinatus is performed before and after retraction stabilization of the scapula. In the baseline state, the strength of the supraspinatus is tested in standard fashion, with resisted elevation of the internally rotated and abducted arm. The strength is then tested with the scapula stabilized in retraction (the examiner medially stabilizes the scapula). With scapular stabilization, an increase in strength or a decrease in symptoms is considered a positive test.
5. Neurovascular examination
It is essential to perform a comprehensive neurovascular examination in all overhead athletes. This includes basic cervical spine testing for any motor or sensory deficits, along with assessment of scapular winging to detect long thoracic or spinal accessory nerve palsy for medial and lateral winging, respectively. Although neurovascular injury may be a rare finding in the overhead athlete, a detailed examination must still be performed to rule it out.
Thoracic outlet syndrome
Thoracic syndrome is a compressive neuropathy of nerves and vasculature exiting the thorax and entering the upper extremity. Common symptoms include pain and tingling (sometimes vague) in the neck and upper extremity. These symptoms may be positional as well.
Diagnosis of thoracic outlet syndrome begins with visual inspection of the involved upper extremity, noting atrophy or asymmetry. Weakness may also be present. Additional provocative maneuvers can be used to detect decrease or loss of pulses, along with reproduction of symptoms, during a provocative maneuver with subsequent return of pulses and resolution of symptoms after the maneuver is completed.
One examination that can be used to detect thoracic outlet syndrome is the Adson test.7 During this maneuver, the radial pulse is palpated with the arm at rest on the patient’s side. The patient then turns to the symptomatic side, hyperextends the arm, and holds inspiration. A positive test coincides with both decreased pulse and reproduction of symptoms, indicating compression within the scalene triangle. In the Wright test,7 the pulse is again palpated at rest with the arm at the side. The patient then holds inspiration and places the arm in a position of abduction and external rotation. If the pulses decrease with this maneuver, the test is considered positive, indicating compression in the sub–pectoralis minor region deep to the coracoid. In a third test, the costoclavicular test, again pulses are measured before and during the provocative maneuver, which is with the shoulders thrust backward and depressed downward. A positive test indicates compression between the clavicle and the first rib. In our practice, we use a modified Wright test in which the arm is held in abduction and external rotation while radial pulses are palpated. The fist is then opened and clenched rapidly, and diminution of radial pulses is considered a positive examination (Figures 6A, 6B).
Effort thrombosis
Overhead athletes are at increased risk for developing effort thrombosis8 (Paget-Schroetter syndrome). This thrombosis, which results from repetitive motion involving the upper extremity, is not limited to overhead sports; it may be caused by underlying compression of or microtrauma to the venous infrastructure. On physical examination, there may be swelling of the affected limb, along with diffuse pain and fatigue, as well as dermatologic changes. Positive findings warrant further testing, such as coagulation profile testing and advanced imaging or venography.
Arterial aneurysm
Although rare, arterial aneurysms, especially of the axillary artery, must be ruled out in the overhead athlete with vague upper extremity pain (especially distally) and without clear diagnosis.9 Aneurysm of the axillary artery can result from repetitive microtrauma related to repetitive overhead motion of the upper extremity. This condition may cause showering of emboli distally to the vasculature of the hand and fingers (Figure 7). Patients may complain of pain in the fingers, difficulty with grip, cyanosis, or cold sensation. On examination, the sufficiency of the radial and ulnar arteries should be assessed, as with detailed sensorimotor examination of the fingers. The fingernails should be examined for splinter hemorrhages.
Conclusion
Overhead athletes place extreme stress on the shoulder during the throwing motion and are at high risk for injury because of repetitive stress on the shoulder girdle. When examining overhead athletes with shoulder pain, surgeons must consider the entire kinetic chain, as inefficiencies anywhere along the chain can lead to altered mechanics and pathology in the shoulder.
1. Cummins CA, Messer TM, Schafer MF. Infraspinatus muscle atrophy in professional baseball players. Am J Sports Med. 2004;32(1):116-120.
2. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology part I: pathoanatomy and biomechanics. Arthroscopy. 2003;19(4):404-420.
3. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology. Part II: evaluation and treatment of SLAP lesions in throwers. Arthroscopy. 2003;19(5):531-539.
4. O’Brien SJ, Pagnani MJ, Fealy S, McGlynn SR, Wilson JB. The active compression test: a new and effective test for diagnosing labral tears and acromioclavicular joint abnormality. Am J Sports Med. 1998;26(5):610-613.
5. Kibler WB. The role of the scapula in athletic shoulder function. Am J Sports Med. 1998;26(2):325-337.
6. Kibler WB, Sciascia A, Wilkes T. Scapular dyskinesis and its relation to shoulder injury. J Am Acad Orthop Surg. 2012;20(6):364-372.
7. Leffert RD. Thoracic outlet syndrome. J Am Acad Orthop Surg. 1994;2(6):317-325.
8. Alla VM, Natarajan N, Kaushik M, Warrier R, Nair CK. Paget-Schroetter syndrome: review of pathogenesis and treatment of effort thrombosis. West J Emerg Med. 2010;11(4):358-362.
9. Baumgarten KM, Dines JS, Winchester PA, et al. Axillary artery aneurysm with distal embolization in a Major League Baseball pitcher. Am J Sports Med. 2007;35(4):650-653.
The overhead athlete’s shoulder is exposed to extremes of stress and range of motion (ROM), predisposing this joint to unique injury patterns. Prompt diagnosis and management begin with a comprehensive history and a physical examination, supplemented by imaging studies as needed. Furthermore, the throwing shoulder undergoes adaptive changes, such as partial undersurface rotator cuff tears and capsular laxity. Imaging studies typically demonstrate abnormalities in asymptomatic throwers. Therefore, clinicians must be skilled in history taking and physical examination in throwing athletes to accurately determine the cause of symptoms and provide optimal treatment. This primer provides orthopedic surgeons with the key points in performing a thorough physical examination of the shoulder in overhead athletes.
When working with overhead athletes, surgeons must elicit the precise nature of symptoms. For example, it is important to distinguish pain from fatigue, as well as complaints related purely to decline in performance. Often, collaboration with the player’s parent or coach may help clarify the chief complaint. In addition, surgeons must have an intricate knowledge of the various stages of the overhead motion, as symptoms in specific stages (late cocking/early acceleration) may raise suspicion for distinctive pathology (labral/biceps complex). Last, it is imperative to understand that the shoulder represents only one part of the kinetic chain in overhead athletes. Successful throwing relies on integrity of the entire kinetic chain, starting with the lower extremity and trunk, extending through the spine, scapula, and shoulder, and terminating with the hand and fingers. Pathology anywhere in the chain must be evaluated and addressed.
When examining the shoulder in overhead athletes, surgeons must address several anatomical structures, both bony and soft tissue. Proper examination begins with comprehensive assessment of the ROM and strength of the various muscles around the shoulder, along with visual inspection to identify any asymmetry of these structures. In addition, the scapulothoracic structures must be examined in detail to rule out underlying dyskinesis. The capsular and ligamentous components of the shoulder joint must be further assessed to note any capsular contracture causing glenohumeral internal rotation deficit (GIRD) or any pathology with the rotator cuff or labral/biceps complex. Last, a comprehensive neurovascular examination should be performed to rule out any compression or neuropathy affecting the shoulder and overhead motion. Findings from the physical examination may then require further imaging to correlate the history and physical examination findings.
1. Inspection, palpation, strength testing
Every examination of the shoulder must begin with visual inspection, along with assessment of basic ROM and strength. The patient must be positioned and exposed adequately to promote visualization of the entire shoulder and scapular girdle, from both anterior and posterior. Visual inspection focuses on identifying any areas of asymmetry, such as position of the bony prominences or bulk of the muscular fossae. Asymmetry of the bony architecture may indicate prior trauma, and atrophy of the muscular fossae may indicate nerve compression. For example, atrophy of the infraspinatus fossa may be caused by compression of the suprascapular nerve at the spinoglenoid notch (likely by a cyst, often associated with labral pathology, but infraspinatus atrophy can result even without the presence of a compressive cyst1). Alternatively, atrophy of both the supraspinatus and infraspinatus fossae may indicate underlying compression of the suprascapular nerve at the suprascapular notch (either by a cyst or by the transverse scapular ligament). Static and dynamic observation of the posterior aspect of the shoulder may help identify gross pathology with scapular positioning or retraction, indicating underlying dyskinesis (discussed later). Deformity of the acromioclavicular joint may indicate prior trauma or separation. Last, all prior surgical scars should be noted.
Selective palpation may help identify pathology in the shoulder of the throwing athlete. Tenderness at the acromioclavicular joint may be especially common in patients who have had prior sprains of this joint or who have degenerative changes. Tenderness along the biceps tendon may be present in those with biceps tendinitis or partial tear. In addition, tenderness at the coracoid may be present in those with scapular dyskinesis. Posteriorly, palpation at the inferomedial aspect of the scapula (Figure 1), as with palpation along the medial border of the scapula, may elicit tenderness in those with scapulothoracic bursitis.
Strength testing in the shoulder is performed to elicit any deficiencies of the rotator cuff/musculature or surrounding structures. Weakness in forward elevation may indicate pathology in the supraspinatus, whereas weakness in external rotation may reflect deficiency in the infraspinatus or teres minor. Teres minor deficiency may be more isolated with weakness in a position of shoulder abduction to 90°. Last, weakness in internal rotation may indicate subscapularis deficiency. Lag signs and other provocative maneuvers are similarly elicited but typically are positive only in the event of large tears of the rotator cuff. These signs and maneuvers include the internal rotation lag sign or belly press test for subscapularis integrity, the drop-arm sign for supraspinatus function, the external rotation lag sign for infraspinatus function, and the hornblower sign for teres minor integrity. Supporting muscles of the shoulder may also be tested. Latissimus strength may be tested with resisted downward rotation of the arm with the shoulder in abduction and the elbow flexed to 90°.
2. ROM and GIRD assessment
After inspection and palpation, the shoulder should be ranged in all relevant planes of motion. Our standard examination includes forward elevation in the frontal and scapular planes, along with external rotation at the side and at 90° of abduction, as well as internal rotation behind the back with documentation of the highest spinal level that the patient can reach. This examination may be performed with the patient upright, but supine positioning can help stabilize the scapula and provide more accurate views of motion. Deficits of internal rotation may be a common finding in overhead athletes, and the degree of this deficit should be quantitatively noted.
Bony and soft-tissue remodeling of the shoulder (and associated structures) in the overhead athlete can lead to contracture of the posterior capsule. This contracture can cause excessive external rotation and subsequent decrease in internal rotation, leading to pain and anterior instability in the throwing shoulder.2 For precise measurements of the internal and external rotation arc, the scapula must be stabilized. This can be done with the patient supine on the examining table or seated upright with manual stabilization of the scapula by the examiner. Once the scapula is stabilized, the arc of internal and external rotation (with the arm in about 90° of abduction) can be measured with a goniometer, with maximum values obtained as the scapula begins to move along the posterior chest wall.2 The difference in internal rotation between the dominant and nondominant arms defines the extent of the athlete’s GIRD. Internal rotation can also be qualitatively assessed by having the athlete internally rotate each arm and reach up the spine while the examiner notes the difference in level achieved. However, this does not provide a quantitative assessment of the patient’s GIRD.
In general, the sum of the internal and external rotation arcs on the 2 sides should be symmetric. Consequently, in GIRD, excessive external rotation is balanced by decreased internal rotation. Symptomatic GIRD may be present when there is more than 25° of discrepancy in internal rotation between the athlete’s dominant and nondominant arms.2 The goal is to reduce this discrepancy to less than 20°.
3. Internal impingement: rotator cuff and labrum
In overhead athletes, an intricate relationship involving rotator cuff, labrum, and biceps tendon allows for efficient, pain-free force delivery at the shoulder. However, because of the significant external rotation and abduction required in the overhead motion, there may be internal impingement of the posterosuperior rotator cuff (infraspinatus and posterior aspect of supraspinatus) between the posterior labrum and the greater tuberosity. Detailed examination of these structures must be performed in any assessment of an overhead athlete. Symptomatic patients may complain of pain during the throwing cycle, particularly in late cocking and early acceleration.
The modified relocation examination is a common maneuver to detect internal impingement.3 In this examination, the patient’s arm is brought into a position of maximal external rotation and abduction mimicking that found in late cocking or early acceleration. In this position, a patient with internal impingement complains of pain in the posterior shoulder. A posteriorly directed force on the humerus relieves this pain.
There are also many examinations for detecting labral pathology, specifically a SLAP (superior labrum, anterior to posterior) lesion, which is commonly found in patients with internal impingement. One commonly tested maneuver is the O’Brien active compression test (Figures 2A, 2B), which has excellent sensitivity and specificity in detecting type II SLAP lesions.4 In this examination, the patient holds the arm in about 15° of adduction and 90° of forward elevation. A downward force is applied with the forearm pronated and subsequently supinated. If pain is noted on the force applied to the pronated arm, and if this pain decreases in the supinated examination, the test is positive for labral pathology.
Anterior instability is routinely found in these patients. Translation is measured with the anterior load and shift test. Anterior translation is tested with the patient supine, with the arm in abduction and external rotation, and with the examiner placing an anteriorly directed force on the humeral head. Translation is compared with the contralateral side and graded on a 3-point scale (1+ is translation to glenoid rim, 2+ is translation over glenoid rim but reduces, 3+ is translation over glenoid and locking). We also use the anterior release test, in which the patient is supine, the arm is brought into abduction and external rotation, and the examiner places a posteriorly directed force on the humeral head. When the examiner removes this force, the patient notices symptoms of instability caused by subluxation (Figures 3A, 3B).
Biceps tendon testing should also be performed to help elicit signs of labral pathology. The Speed test is performed by placing a downward force on the patient’s arm, which is held in 90° forward elevation, and with elbows in extension and forearm in supination. Pain in the long head of the biceps tendon is considered a positive sign and suggestive of SLAP lesion. Although not commonly found in these athletes, external impingement should also be elicited through both the Neer test and the Hawkins test. In the Neer test, the patient’s arm is brought to maximal forward elevation with the forearm supinated and elbow extended, while the scapula is stabilized by the examiner. Pain in the shoulder indicates a positive examination. In the Hawkins test, the patient’s arm is brought into a position of forward elevation, internal rotation, and elbow flexion. The arm is then further internally rotated, and shoulder pain defines a positive examination.
Any of these findings can be concomitant with scapular dyskinesis. Moreover, symptoms related to internal impingement may be exacerbated by concomitant scapular pathology, and therefore proper assessment of scapulothoracic motion must also be performed.
4. Scapulothoracic examination
Motion coupled between the scapula and the rest of the arm (scapular rhythm) allows for efficient use of the shoulder girdle. The scapula helps transfer the force generated by the core so that the hand can efficiently deliver it. Therefore, scapular pathology (or dyskinesis) results in inefficient functioning of the arm, which can be especially debilitating in an overhead athlete.
Scapular assessment begins with visual inspection of the patient, typically from the posterior view, which allows for assessment of the resting position of the scapula. Evidence of prominence of the medial or inferomedial border, coracoid malposition (or pain on palpation), or general scapular malposition should be noted. On active ROM, as the patient forward-elevates the arm, any asymmetric prominence of the inferomedial border of the scapula should be noted. Such asymmetry may indicate underlying scapular dyskinesis. In another important test, the lateral scapular slide test (described by Kibler5), the distance from the inferomedial angle of the scapula to the thoracic spine should be measured for both sides and in 3 difference positions, noting any asymmetry between the affected and nonaffected sides. These 3 positions (Figures 4A–4C) are with arms at side, with hands on hips (internal rotation of humerus in 45° abduction), and in 90° of shoulder abduction. Last, medial and lateral scapular winging—caused by long thoracic nerve and spinal accessory nerve pathology, respectively—can be detected by asking the patient to do a “push-up” against the wall while the examiner views from posterior.
After assessment of scapular position at rest and through motion, a series of provocative maneuvers6 may aid in the diagnosis of scapular dyskinesis. The first maneuver is the scapular assistance test, in which the examiner provides a gentle force at the inferomedial angle of the scapula, promoting upward rotation and posterior tilt as the patient elevates the arm (Figures 5A, 5B). If the patient experiences a decrease or absence of symptoms through this arc, the test is considered positive. The second maneuver is the scapular retraction test, in which strength testing of the supraspinatus is performed before and after retraction stabilization of the scapula. In the baseline state, the strength of the supraspinatus is tested in standard fashion, with resisted elevation of the internally rotated and abducted arm. The strength is then tested with the scapula stabilized in retraction (the examiner medially stabilizes the scapula). With scapular stabilization, an increase in strength or a decrease in symptoms is considered a positive test.
5. Neurovascular examination
It is essential to perform a comprehensive neurovascular examination in all overhead athletes. This includes basic cervical spine testing for any motor or sensory deficits, along with assessment of scapular winging to detect long thoracic or spinal accessory nerve palsy for medial and lateral winging, respectively. Although neurovascular injury may be a rare finding in the overhead athlete, a detailed examination must still be performed to rule it out.
Thoracic outlet syndrome
Thoracic syndrome is a compressive neuropathy of nerves and vasculature exiting the thorax and entering the upper extremity. Common symptoms include pain and tingling (sometimes vague) in the neck and upper extremity. These symptoms may be positional as well.
Diagnosis of thoracic outlet syndrome begins with visual inspection of the involved upper extremity, noting atrophy or asymmetry. Weakness may also be present. Additional provocative maneuvers can be used to detect decrease or loss of pulses, along with reproduction of symptoms, during a provocative maneuver with subsequent return of pulses and resolution of symptoms after the maneuver is completed.
One examination that can be used to detect thoracic outlet syndrome is the Adson test.7 During this maneuver, the radial pulse is palpated with the arm at rest on the patient’s side. The patient then turns to the symptomatic side, hyperextends the arm, and holds inspiration. A positive test coincides with both decreased pulse and reproduction of symptoms, indicating compression within the scalene triangle. In the Wright test,7 the pulse is again palpated at rest with the arm at the side. The patient then holds inspiration and places the arm in a position of abduction and external rotation. If the pulses decrease with this maneuver, the test is considered positive, indicating compression in the sub–pectoralis minor region deep to the coracoid. In a third test, the costoclavicular test, again pulses are measured before and during the provocative maneuver, which is with the shoulders thrust backward and depressed downward. A positive test indicates compression between the clavicle and the first rib. In our practice, we use a modified Wright test in which the arm is held in abduction and external rotation while radial pulses are palpated. The fist is then opened and clenched rapidly, and diminution of radial pulses is considered a positive examination (Figures 6A, 6B).
Effort thrombosis
Overhead athletes are at increased risk for developing effort thrombosis8 (Paget-Schroetter syndrome). This thrombosis, which results from repetitive motion involving the upper extremity, is not limited to overhead sports; it may be caused by underlying compression of or microtrauma to the venous infrastructure. On physical examination, there may be swelling of the affected limb, along with diffuse pain and fatigue, as well as dermatologic changes. Positive findings warrant further testing, such as coagulation profile testing and advanced imaging or venography.
Arterial aneurysm
Although rare, arterial aneurysms, especially of the axillary artery, must be ruled out in the overhead athlete with vague upper extremity pain (especially distally) and without clear diagnosis.9 Aneurysm of the axillary artery can result from repetitive microtrauma related to repetitive overhead motion of the upper extremity. This condition may cause showering of emboli distally to the vasculature of the hand and fingers (Figure 7). Patients may complain of pain in the fingers, difficulty with grip, cyanosis, or cold sensation. On examination, the sufficiency of the radial and ulnar arteries should be assessed, as with detailed sensorimotor examination of the fingers. The fingernails should be examined for splinter hemorrhages.
Conclusion
Overhead athletes place extreme stress on the shoulder during the throwing motion and are at high risk for injury because of repetitive stress on the shoulder girdle. When examining overhead athletes with shoulder pain, surgeons must consider the entire kinetic chain, as inefficiencies anywhere along the chain can lead to altered mechanics and pathology in the shoulder.
The overhead athlete’s shoulder is exposed to extremes of stress and range of motion (ROM), predisposing this joint to unique injury patterns. Prompt diagnosis and management begin with a comprehensive history and a physical examination, supplemented by imaging studies as needed. Furthermore, the throwing shoulder undergoes adaptive changes, such as partial undersurface rotator cuff tears and capsular laxity. Imaging studies typically demonstrate abnormalities in asymptomatic throwers. Therefore, clinicians must be skilled in history taking and physical examination in throwing athletes to accurately determine the cause of symptoms and provide optimal treatment. This primer provides orthopedic surgeons with the key points in performing a thorough physical examination of the shoulder in overhead athletes.
When working with overhead athletes, surgeons must elicit the precise nature of symptoms. For example, it is important to distinguish pain from fatigue, as well as complaints related purely to decline in performance. Often, collaboration with the player’s parent or coach may help clarify the chief complaint. In addition, surgeons must have an intricate knowledge of the various stages of the overhead motion, as symptoms in specific stages (late cocking/early acceleration) may raise suspicion for distinctive pathology (labral/biceps complex). Last, it is imperative to understand that the shoulder represents only one part of the kinetic chain in overhead athletes. Successful throwing relies on integrity of the entire kinetic chain, starting with the lower extremity and trunk, extending through the spine, scapula, and shoulder, and terminating with the hand and fingers. Pathology anywhere in the chain must be evaluated and addressed.
When examining the shoulder in overhead athletes, surgeons must address several anatomical structures, both bony and soft tissue. Proper examination begins with comprehensive assessment of the ROM and strength of the various muscles around the shoulder, along with visual inspection to identify any asymmetry of these structures. In addition, the scapulothoracic structures must be examined in detail to rule out underlying dyskinesis. The capsular and ligamentous components of the shoulder joint must be further assessed to note any capsular contracture causing glenohumeral internal rotation deficit (GIRD) or any pathology with the rotator cuff or labral/biceps complex. Last, a comprehensive neurovascular examination should be performed to rule out any compression or neuropathy affecting the shoulder and overhead motion. Findings from the physical examination may then require further imaging to correlate the history and physical examination findings.
1. Inspection, palpation, strength testing
Every examination of the shoulder must begin with visual inspection, along with assessment of basic ROM and strength. The patient must be positioned and exposed adequately to promote visualization of the entire shoulder and scapular girdle, from both anterior and posterior. Visual inspection focuses on identifying any areas of asymmetry, such as position of the bony prominences or bulk of the muscular fossae. Asymmetry of the bony architecture may indicate prior trauma, and atrophy of the muscular fossae may indicate nerve compression. For example, atrophy of the infraspinatus fossa may be caused by compression of the suprascapular nerve at the spinoglenoid notch (likely by a cyst, often associated with labral pathology, but infraspinatus atrophy can result even without the presence of a compressive cyst1). Alternatively, atrophy of both the supraspinatus and infraspinatus fossae may indicate underlying compression of the suprascapular nerve at the suprascapular notch (either by a cyst or by the transverse scapular ligament). Static and dynamic observation of the posterior aspect of the shoulder may help identify gross pathology with scapular positioning or retraction, indicating underlying dyskinesis (discussed later). Deformity of the acromioclavicular joint may indicate prior trauma or separation. Last, all prior surgical scars should be noted.
Selective palpation may help identify pathology in the shoulder of the throwing athlete. Tenderness at the acromioclavicular joint may be especially common in patients who have had prior sprains of this joint or who have degenerative changes. Tenderness along the biceps tendon may be present in those with biceps tendinitis or partial tear. In addition, tenderness at the coracoid may be present in those with scapular dyskinesis. Posteriorly, palpation at the inferomedial aspect of the scapula (Figure 1), as with palpation along the medial border of the scapula, may elicit tenderness in those with scapulothoracic bursitis.
Strength testing in the shoulder is performed to elicit any deficiencies of the rotator cuff/musculature or surrounding structures. Weakness in forward elevation may indicate pathology in the supraspinatus, whereas weakness in external rotation may reflect deficiency in the infraspinatus or teres minor. Teres minor deficiency may be more isolated with weakness in a position of shoulder abduction to 90°. Last, weakness in internal rotation may indicate subscapularis deficiency. Lag signs and other provocative maneuvers are similarly elicited but typically are positive only in the event of large tears of the rotator cuff. These signs and maneuvers include the internal rotation lag sign or belly press test for subscapularis integrity, the drop-arm sign for supraspinatus function, the external rotation lag sign for infraspinatus function, and the hornblower sign for teres minor integrity. Supporting muscles of the shoulder may also be tested. Latissimus strength may be tested with resisted downward rotation of the arm with the shoulder in abduction and the elbow flexed to 90°.
2. ROM and GIRD assessment
After inspection and palpation, the shoulder should be ranged in all relevant planes of motion. Our standard examination includes forward elevation in the frontal and scapular planes, along with external rotation at the side and at 90° of abduction, as well as internal rotation behind the back with documentation of the highest spinal level that the patient can reach. This examination may be performed with the patient upright, but supine positioning can help stabilize the scapula and provide more accurate views of motion. Deficits of internal rotation may be a common finding in overhead athletes, and the degree of this deficit should be quantitatively noted.
Bony and soft-tissue remodeling of the shoulder (and associated structures) in the overhead athlete can lead to contracture of the posterior capsule. This contracture can cause excessive external rotation and subsequent decrease in internal rotation, leading to pain and anterior instability in the throwing shoulder.2 For precise measurements of the internal and external rotation arc, the scapula must be stabilized. This can be done with the patient supine on the examining table or seated upright with manual stabilization of the scapula by the examiner. Once the scapula is stabilized, the arc of internal and external rotation (with the arm in about 90° of abduction) can be measured with a goniometer, with maximum values obtained as the scapula begins to move along the posterior chest wall.2 The difference in internal rotation between the dominant and nondominant arms defines the extent of the athlete’s GIRD. Internal rotation can also be qualitatively assessed by having the athlete internally rotate each arm and reach up the spine while the examiner notes the difference in level achieved. However, this does not provide a quantitative assessment of the patient’s GIRD.
In general, the sum of the internal and external rotation arcs on the 2 sides should be symmetric. Consequently, in GIRD, excessive external rotation is balanced by decreased internal rotation. Symptomatic GIRD may be present when there is more than 25° of discrepancy in internal rotation between the athlete’s dominant and nondominant arms.2 The goal is to reduce this discrepancy to less than 20°.
3. Internal impingement: rotator cuff and labrum
In overhead athletes, an intricate relationship involving rotator cuff, labrum, and biceps tendon allows for efficient, pain-free force delivery at the shoulder. However, because of the significant external rotation and abduction required in the overhead motion, there may be internal impingement of the posterosuperior rotator cuff (infraspinatus and posterior aspect of supraspinatus) between the posterior labrum and the greater tuberosity. Detailed examination of these structures must be performed in any assessment of an overhead athlete. Symptomatic patients may complain of pain during the throwing cycle, particularly in late cocking and early acceleration.
The modified relocation examination is a common maneuver to detect internal impingement.3 In this examination, the patient’s arm is brought into a position of maximal external rotation and abduction mimicking that found in late cocking or early acceleration. In this position, a patient with internal impingement complains of pain in the posterior shoulder. A posteriorly directed force on the humerus relieves this pain.
There are also many examinations for detecting labral pathology, specifically a SLAP (superior labrum, anterior to posterior) lesion, which is commonly found in patients with internal impingement. One commonly tested maneuver is the O’Brien active compression test (Figures 2A, 2B), which has excellent sensitivity and specificity in detecting type II SLAP lesions.4 In this examination, the patient holds the arm in about 15° of adduction and 90° of forward elevation. A downward force is applied with the forearm pronated and subsequently supinated. If pain is noted on the force applied to the pronated arm, and if this pain decreases in the supinated examination, the test is positive for labral pathology.
Anterior instability is routinely found in these patients. Translation is measured with the anterior load and shift test. Anterior translation is tested with the patient supine, with the arm in abduction and external rotation, and with the examiner placing an anteriorly directed force on the humeral head. Translation is compared with the contralateral side and graded on a 3-point scale (1+ is translation to glenoid rim, 2+ is translation over glenoid rim but reduces, 3+ is translation over glenoid and locking). We also use the anterior release test, in which the patient is supine, the arm is brought into abduction and external rotation, and the examiner places a posteriorly directed force on the humeral head. When the examiner removes this force, the patient notices symptoms of instability caused by subluxation (Figures 3A, 3B).
Biceps tendon testing should also be performed to help elicit signs of labral pathology. The Speed test is performed by placing a downward force on the patient’s arm, which is held in 90° forward elevation, and with elbows in extension and forearm in supination. Pain in the long head of the biceps tendon is considered a positive sign and suggestive of SLAP lesion. Although not commonly found in these athletes, external impingement should also be elicited through both the Neer test and the Hawkins test. In the Neer test, the patient’s arm is brought to maximal forward elevation with the forearm supinated and elbow extended, while the scapula is stabilized by the examiner. Pain in the shoulder indicates a positive examination. In the Hawkins test, the patient’s arm is brought into a position of forward elevation, internal rotation, and elbow flexion. The arm is then further internally rotated, and shoulder pain defines a positive examination.
Any of these findings can be concomitant with scapular dyskinesis. Moreover, symptoms related to internal impingement may be exacerbated by concomitant scapular pathology, and therefore proper assessment of scapulothoracic motion must also be performed.
4. Scapulothoracic examination
Motion coupled between the scapula and the rest of the arm (scapular rhythm) allows for efficient use of the shoulder girdle. The scapula helps transfer the force generated by the core so that the hand can efficiently deliver it. Therefore, scapular pathology (or dyskinesis) results in inefficient functioning of the arm, which can be especially debilitating in an overhead athlete.
Scapular assessment begins with visual inspection of the patient, typically from the posterior view, which allows for assessment of the resting position of the scapula. Evidence of prominence of the medial or inferomedial border, coracoid malposition (or pain on palpation), or general scapular malposition should be noted. On active ROM, as the patient forward-elevates the arm, any asymmetric prominence of the inferomedial border of the scapula should be noted. Such asymmetry may indicate underlying scapular dyskinesis. In another important test, the lateral scapular slide test (described by Kibler5), the distance from the inferomedial angle of the scapula to the thoracic spine should be measured for both sides and in 3 difference positions, noting any asymmetry between the affected and nonaffected sides. These 3 positions (Figures 4A–4C) are with arms at side, with hands on hips (internal rotation of humerus in 45° abduction), and in 90° of shoulder abduction. Last, medial and lateral scapular winging—caused by long thoracic nerve and spinal accessory nerve pathology, respectively—can be detected by asking the patient to do a “push-up” against the wall while the examiner views from posterior.
After assessment of scapular position at rest and through motion, a series of provocative maneuvers6 may aid in the diagnosis of scapular dyskinesis. The first maneuver is the scapular assistance test, in which the examiner provides a gentle force at the inferomedial angle of the scapula, promoting upward rotation and posterior tilt as the patient elevates the arm (Figures 5A, 5B). If the patient experiences a decrease or absence of symptoms through this arc, the test is considered positive. The second maneuver is the scapular retraction test, in which strength testing of the supraspinatus is performed before and after retraction stabilization of the scapula. In the baseline state, the strength of the supraspinatus is tested in standard fashion, with resisted elevation of the internally rotated and abducted arm. The strength is then tested with the scapula stabilized in retraction (the examiner medially stabilizes the scapula). With scapular stabilization, an increase in strength or a decrease in symptoms is considered a positive test.
5. Neurovascular examination
It is essential to perform a comprehensive neurovascular examination in all overhead athletes. This includes basic cervical spine testing for any motor or sensory deficits, along with assessment of scapular winging to detect long thoracic or spinal accessory nerve palsy for medial and lateral winging, respectively. Although neurovascular injury may be a rare finding in the overhead athlete, a detailed examination must still be performed to rule it out.
Thoracic outlet syndrome
Thoracic syndrome is a compressive neuropathy of nerves and vasculature exiting the thorax and entering the upper extremity. Common symptoms include pain and tingling (sometimes vague) in the neck and upper extremity. These symptoms may be positional as well.
Diagnosis of thoracic outlet syndrome begins with visual inspection of the involved upper extremity, noting atrophy or asymmetry. Weakness may also be present. Additional provocative maneuvers can be used to detect decrease or loss of pulses, along with reproduction of symptoms, during a provocative maneuver with subsequent return of pulses and resolution of symptoms after the maneuver is completed.
One examination that can be used to detect thoracic outlet syndrome is the Adson test.7 During this maneuver, the radial pulse is palpated with the arm at rest on the patient’s side. The patient then turns to the symptomatic side, hyperextends the arm, and holds inspiration. A positive test coincides with both decreased pulse and reproduction of symptoms, indicating compression within the scalene triangle. In the Wright test,7 the pulse is again palpated at rest with the arm at the side. The patient then holds inspiration and places the arm in a position of abduction and external rotation. If the pulses decrease with this maneuver, the test is considered positive, indicating compression in the sub–pectoralis minor region deep to the coracoid. In a third test, the costoclavicular test, again pulses are measured before and during the provocative maneuver, which is with the shoulders thrust backward and depressed downward. A positive test indicates compression between the clavicle and the first rib. In our practice, we use a modified Wright test in which the arm is held in abduction and external rotation while radial pulses are palpated. The fist is then opened and clenched rapidly, and diminution of radial pulses is considered a positive examination (Figures 6A, 6B).
Effort thrombosis
Overhead athletes are at increased risk for developing effort thrombosis8 (Paget-Schroetter syndrome). This thrombosis, which results from repetitive motion involving the upper extremity, is not limited to overhead sports; it may be caused by underlying compression of or microtrauma to the venous infrastructure. On physical examination, there may be swelling of the affected limb, along with diffuse pain and fatigue, as well as dermatologic changes. Positive findings warrant further testing, such as coagulation profile testing and advanced imaging or venography.
Arterial aneurysm
Although rare, arterial aneurysms, especially of the axillary artery, must be ruled out in the overhead athlete with vague upper extremity pain (especially distally) and without clear diagnosis.9 Aneurysm of the axillary artery can result from repetitive microtrauma related to repetitive overhead motion of the upper extremity. This condition may cause showering of emboli distally to the vasculature of the hand and fingers (Figure 7). Patients may complain of pain in the fingers, difficulty with grip, cyanosis, or cold sensation. On examination, the sufficiency of the radial and ulnar arteries should be assessed, as with detailed sensorimotor examination of the fingers. The fingernails should be examined for splinter hemorrhages.
Conclusion
Overhead athletes place extreme stress on the shoulder during the throwing motion and are at high risk for injury because of repetitive stress on the shoulder girdle. When examining overhead athletes with shoulder pain, surgeons must consider the entire kinetic chain, as inefficiencies anywhere along the chain can lead to altered mechanics and pathology in the shoulder.
1. Cummins CA, Messer TM, Schafer MF. Infraspinatus muscle atrophy in professional baseball players. Am J Sports Med. 2004;32(1):116-120.
2. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology part I: pathoanatomy and biomechanics. Arthroscopy. 2003;19(4):404-420.
3. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology. Part II: evaluation and treatment of SLAP lesions in throwers. Arthroscopy. 2003;19(5):531-539.
4. O’Brien SJ, Pagnani MJ, Fealy S, McGlynn SR, Wilson JB. The active compression test: a new and effective test for diagnosing labral tears and acromioclavicular joint abnormality. Am J Sports Med. 1998;26(5):610-613.
5. Kibler WB. The role of the scapula in athletic shoulder function. Am J Sports Med. 1998;26(2):325-337.
6. Kibler WB, Sciascia A, Wilkes T. Scapular dyskinesis and its relation to shoulder injury. J Am Acad Orthop Surg. 2012;20(6):364-372.
7. Leffert RD. Thoracic outlet syndrome. J Am Acad Orthop Surg. 1994;2(6):317-325.
8. Alla VM, Natarajan N, Kaushik M, Warrier R, Nair CK. Paget-Schroetter syndrome: review of pathogenesis and treatment of effort thrombosis. West J Emerg Med. 2010;11(4):358-362.
9. Baumgarten KM, Dines JS, Winchester PA, et al. Axillary artery aneurysm with distal embolization in a Major League Baseball pitcher. Am J Sports Med. 2007;35(4):650-653.
1. Cummins CA, Messer TM, Schafer MF. Infraspinatus muscle atrophy in professional baseball players. Am J Sports Med. 2004;32(1):116-120.
2. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology part I: pathoanatomy and biomechanics. Arthroscopy. 2003;19(4):404-420.
3. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology. Part II: evaluation and treatment of SLAP lesions in throwers. Arthroscopy. 2003;19(5):531-539.
4. O’Brien SJ, Pagnani MJ, Fealy S, McGlynn SR, Wilson JB. The active compression test: a new and effective test for diagnosing labral tears and acromioclavicular joint abnormality. Am J Sports Med. 1998;26(5):610-613.
5. Kibler WB. The role of the scapula in athletic shoulder function. Am J Sports Med. 1998;26(2):325-337.
6. Kibler WB, Sciascia A, Wilkes T. Scapular dyskinesis and its relation to shoulder injury. J Am Acad Orthop Surg. 2012;20(6):364-372.
7. Leffert RD. Thoracic outlet syndrome. J Am Acad Orthop Surg. 1994;2(6):317-325.
8. Alla VM, Natarajan N, Kaushik M, Warrier R, Nair CK. Paget-Schroetter syndrome: review of pathogenesis and treatment of effort thrombosis. West J Emerg Med. 2010;11(4):358-362.
9. Baumgarten KM, Dines JS, Winchester PA, et al. Axillary artery aneurysm with distal embolization in a Major League Baseball pitcher. Am J Sports Med. 2007;35(4):650-653.
A Summer Bonanza of Upper Extremity Articles
This issue of The American Journal of Orthopedics has several very interesting articles for the upper extremity surgeon. The first one that I would like to talk about is “Trends in Thumb Carpometacarpal Interposition Arthroplasty in the United States, 2005–2011” by Dr. Werner and colleagues (pages 363-368). This is a condition that has deep penetration in the US population. As a group, surgical treatments have been evolving, with a number of innovations over the last few decades. Like many things in orthopedics, it is not easy to get “head to head” comparisons between different treatment arms. Nonetheless, although there are some studies that have indicated no particular advantage of 1 mechanism to another, it is interesting as a physician to review this data and follow these trends. This article indicates that, despite lack of strong evidence, individual surgeons have the impression that the operative treatments for basal joint or thumb arthritis are functioning better overall. I share that belief.
I also enjoyed the article “5 Points on Shoulder Examination of the Overhead Athlete” by Dr. Makhni and Dr. Ahmad (pages 347-352). I think that the care of the musculoskeletal patient is important both in terms of screening and in terms of establishing reasonable indications and goals for rehabilitation as well as for surgical treatment. In this light, I found a lot of illuminating information in this review of the approach to the overhead athlete by these authors with deep experience in this arena.
The next article that I would like to address is that on thoracic outlet syndrome by Dr. Buller and colleagues (pages 376-382). It has amazed me during my 3 decades in practice how common the condition of thoracic outlet syndrome is and how frequently the diagnosis is made in my own upper extremity practice. Unfortunately, these patients don’t come “labeled,” as this diagnosis remains somewhat mysterious and, certainly, the treatment somewhat controversial. However, identification and recognition of this clinical entity as well as being able to perform an adequate history and do the physical examination maneuvers to elicit the “nerve tension signs” around the thoracic outlet and brachial plexus are important. The descriptions of the history and physical examimation in this article are excellent. Certainly, advanced imaging and diagnostics can be helpful, but I feel that these tests are not adequate as screening tests, and the index of suspicion by you, the clinician, remains paramount in identifying and managing these patients. In my own practice, the vast majority of patients respond to physical therapy and home exercise programs when adequately performed and monitored.
I was fascinated to read Dr. Steve Burkhart’s Neer Guest Lecture, “The Burden of Craft in Arthroscopic Rotator Cuff Repair: Where We Have Been and Where We Are Going” (pages 353-358). He touches on many things in this lecture. Certainly he talks about the innovations that he has been responsible for and how some of these have come about. Interestingly enough, he has views on the role of the private practitioner and those outside of the “shoulder establishment” in contributing to a paradigm shift in treatment from open to arthroscopic techniques, of which he was certainly at the forefront. Additionally, he has some interesting thoughts on the limitations of level I evidence studies. This is a huge issue in orthopedics as it becomes very difficult to try to “randomize” patients into various treatment arms. Most people take their own bodies and the health of their bodies seriously enough to not want to determine treatment with a “flip of the coin.” I think this is quite different than taking a “red pill” or a “blue pill” in a drug study. Dr. Burkhart emphasizes the role of technical expertise as a variable that is not really adequately considered in level I evidence studies, and I wholeheartedly agree with him.
This issue of The American Journal of Orthopedics is rich in terms of its content, and I hope you enjoy reading these articles as much as I have enjoyed commenting on them. ◾
This issue of The American Journal of Orthopedics has several very interesting articles for the upper extremity surgeon. The first one that I would like to talk about is “Trends in Thumb Carpometacarpal Interposition Arthroplasty in the United States, 2005–2011” by Dr. Werner and colleagues (pages 363-368). This is a condition that has deep penetration in the US population. As a group, surgical treatments have been evolving, with a number of innovations over the last few decades. Like many things in orthopedics, it is not easy to get “head to head” comparisons between different treatment arms. Nonetheless, although there are some studies that have indicated no particular advantage of 1 mechanism to another, it is interesting as a physician to review this data and follow these trends. This article indicates that, despite lack of strong evidence, individual surgeons have the impression that the operative treatments for basal joint or thumb arthritis are functioning better overall. I share that belief.
I also enjoyed the article “5 Points on Shoulder Examination of the Overhead Athlete” by Dr. Makhni and Dr. Ahmad (pages 347-352). I think that the care of the musculoskeletal patient is important both in terms of screening and in terms of establishing reasonable indications and goals for rehabilitation as well as for surgical treatment. In this light, I found a lot of illuminating information in this review of the approach to the overhead athlete by these authors with deep experience in this arena.
The next article that I would like to address is that on thoracic outlet syndrome by Dr. Buller and colleagues (pages 376-382). It has amazed me during my 3 decades in practice how common the condition of thoracic outlet syndrome is and how frequently the diagnosis is made in my own upper extremity practice. Unfortunately, these patients don’t come “labeled,” as this diagnosis remains somewhat mysterious and, certainly, the treatment somewhat controversial. However, identification and recognition of this clinical entity as well as being able to perform an adequate history and do the physical examination maneuvers to elicit the “nerve tension signs” around the thoracic outlet and brachial plexus are important. The descriptions of the history and physical examimation in this article are excellent. Certainly, advanced imaging and diagnostics can be helpful, but I feel that these tests are not adequate as screening tests, and the index of suspicion by you, the clinician, remains paramount in identifying and managing these patients. In my own practice, the vast majority of patients respond to physical therapy and home exercise programs when adequately performed and monitored.
I was fascinated to read Dr. Steve Burkhart’s Neer Guest Lecture, “The Burden of Craft in Arthroscopic Rotator Cuff Repair: Where We Have Been and Where We Are Going” (pages 353-358). He touches on many things in this lecture. Certainly he talks about the innovations that he has been responsible for and how some of these have come about. Interestingly enough, he has views on the role of the private practitioner and those outside of the “shoulder establishment” in contributing to a paradigm shift in treatment from open to arthroscopic techniques, of which he was certainly at the forefront. Additionally, he has some interesting thoughts on the limitations of level I evidence studies. This is a huge issue in orthopedics as it becomes very difficult to try to “randomize” patients into various treatment arms. Most people take their own bodies and the health of their bodies seriously enough to not want to determine treatment with a “flip of the coin.” I think this is quite different than taking a “red pill” or a “blue pill” in a drug study. Dr. Burkhart emphasizes the role of technical expertise as a variable that is not really adequately considered in level I evidence studies, and I wholeheartedly agree with him.
This issue of The American Journal of Orthopedics is rich in terms of its content, and I hope you enjoy reading these articles as much as I have enjoyed commenting on them. ◾
This issue of The American Journal of Orthopedics has several very interesting articles for the upper extremity surgeon. The first one that I would like to talk about is “Trends in Thumb Carpometacarpal Interposition Arthroplasty in the United States, 2005–2011” by Dr. Werner and colleagues (pages 363-368). This is a condition that has deep penetration in the US population. As a group, surgical treatments have been evolving, with a number of innovations over the last few decades. Like many things in orthopedics, it is not easy to get “head to head” comparisons between different treatment arms. Nonetheless, although there are some studies that have indicated no particular advantage of 1 mechanism to another, it is interesting as a physician to review this data and follow these trends. This article indicates that, despite lack of strong evidence, individual surgeons have the impression that the operative treatments for basal joint or thumb arthritis are functioning better overall. I share that belief.
I also enjoyed the article “5 Points on Shoulder Examination of the Overhead Athlete” by Dr. Makhni and Dr. Ahmad (pages 347-352). I think that the care of the musculoskeletal patient is important both in terms of screening and in terms of establishing reasonable indications and goals for rehabilitation as well as for surgical treatment. In this light, I found a lot of illuminating information in this review of the approach to the overhead athlete by these authors with deep experience in this arena.
The next article that I would like to address is that on thoracic outlet syndrome by Dr. Buller and colleagues (pages 376-382). It has amazed me during my 3 decades in practice how common the condition of thoracic outlet syndrome is and how frequently the diagnosis is made in my own upper extremity practice. Unfortunately, these patients don’t come “labeled,” as this diagnosis remains somewhat mysterious and, certainly, the treatment somewhat controversial. However, identification and recognition of this clinical entity as well as being able to perform an adequate history and do the physical examination maneuvers to elicit the “nerve tension signs” around the thoracic outlet and brachial plexus are important. The descriptions of the history and physical examimation in this article are excellent. Certainly, advanced imaging and diagnostics can be helpful, but I feel that these tests are not adequate as screening tests, and the index of suspicion by you, the clinician, remains paramount in identifying and managing these patients. In my own practice, the vast majority of patients respond to physical therapy and home exercise programs when adequately performed and monitored.
I was fascinated to read Dr. Steve Burkhart’s Neer Guest Lecture, “The Burden of Craft in Arthroscopic Rotator Cuff Repair: Where We Have Been and Where We Are Going” (pages 353-358). He touches on many things in this lecture. Certainly he talks about the innovations that he has been responsible for and how some of these have come about. Interestingly enough, he has views on the role of the private practitioner and those outside of the “shoulder establishment” in contributing to a paradigm shift in treatment from open to arthroscopic techniques, of which he was certainly at the forefront. Additionally, he has some interesting thoughts on the limitations of level I evidence studies. This is a huge issue in orthopedics as it becomes very difficult to try to “randomize” patients into various treatment arms. Most people take their own bodies and the health of their bodies seriously enough to not want to determine treatment with a “flip of the coin.” I think this is quite different than taking a “red pill” or a “blue pill” in a drug study. Dr. Burkhart emphasizes the role of technical expertise as a variable that is not really adequately considered in level I evidence studies, and I wholeheartedly agree with him.
This issue of The American Journal of Orthopedics is rich in terms of its content, and I hope you enjoy reading these articles as much as I have enjoyed commenting on them. ◾
Closed Rupture of the Flexor Profundus Tendon of Ring Finger: Case Report and Treatment Recommendations
Flexor tendons are considered the strongest component of the musculotendinous unit; they generally do not rupture unless weakened by an underlying pathologic condition.1 According to traditional teaching, when the musculotendinous unit is subjected to excessive forces, failure invariably occurs at the tendon insertion, at the musculotendinous junction, within the muscle substance, or at its origin from the bone before the tendon itself ruptures.1
Midsubstance tears in nonrheumatoid patients are less frequent and are typically attributable to an underlying cause.2 Possible pathologic conditions include, but are not limited to, osteoarthritis of the pisotriquetral joint,3 nonunion fracture of the hook of the hamate,4 lunate dislocation,5 accessory carpal bone,6 gouty infiltration of the flexor tendon,7 and tumor.8 In 1960, Boyes and colleagues9 presented a series of 80 flexor tendon ruptures in 78 patients over a 13-year period. Only 3 cases had no identifiable cause. The authors recommended using the term spontaneous for those ruptures that occur within the tendon substance without underlying or associated pathologic changes.
We describe a patient with spontaneous rupture of the flexor digitorum profundus (FDP) tendon at zone III, satisfying Boyes’ definition of the term spontaneous. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 65-year-old, right-handed manual worker was assessed in our hand clinic 3 days after he felt a cramp in his left palm while lifting a heavy object. Shortly thereafter, he noted he could not flex his ring finger distal interphalangeal (DIP) joint. He could not recall any previous injury to his finger. No predisposing pathologic conditions or bone abnormalities were identified. Clinically, there was no tenderness, swelling, or ecchymosis evident. He had full passive range of motion (ROM) of his ring finger, and proximal interphalangeal (PIP) joint active ROM was 0/110º; however, he had no activity of the FDP of the ring finger. Preoperative radiographs were normal. The hook of the hamate was clinically and radiographically normal.
A preoperative diagnosis of FDP avulsion from the distal phalanx was made, and the operation was carried out 16 days after injury. Surgical exploration started in zone II and extended proximally into the distal palmar crease, but no stump was found in either location. Therefore, exploration was carried out to the midpalmar region, revealing the tendon rupture in zone III, in the region of the origin of the ring finger lumbrical muscle (Figure 1). The flexor digitorum superficialis tendon was intact. Macroscopically, both tendon and carpal tunnel appeared normal, with no evidence of tendon attrition; thus, the tendon was not sent for histologic examination. The ends of the ruptured FDP tendon to the ring finger were at the level of the superficial palmar arch, with the distal end appearing as though it had been cut sharply with a knife. Because of the short period of time from injury to exploration, delayed primary tendon repair was possible, along with side-to-side tenodesis to the intact ring finger flexor superficialis tendon in the palm (Figure 2). Two days after surgery, the patient started a controlled mobilization program using the Duran method.10
At final follow-up of 18 months, total active motion was 126°, which corresponds to a good outcome, according to the Strickland and Glogovac criteria.11 Grip strength was 50 kg, which was 84% of grip strength on the uninjured side. The patient was back to recreational activity but had not returned to work.
Discussion
Most flexor tendon ruptures result from avulsion of the FDP tendon at its distal phalanx insertion, commonly known as Jersey finger. However, true midsubstance spontaneous ruptures are infrequent. Reports of spontaneous tendon ruptures of all types, including those of the hand, have increased in incidence in most countries.12 Bois and colleagues,13 who have reviewed the literature over a 50-year period, found a total of 50 spontaneous ruptures of “normal” flexor tendon in 43 cases. The authors point to unique historical and physical examinaton findings that help differentiate spontaneous tendon ruptures from the more common FDP avulsions. Such findings include the sensation of a pop or snap, or a sudden sharp pain or cramp within the palmar region. In contrast, most avulsion ruptures cause discomfort within the region of the digit. In type I avulsion injuries of the FDP tendon, the proximal tendon stump usually retracts proximal to the digital tendon sheath, causing a tender mass in the palm.14 Flexor digitorum profundus tendon avulsions, however, are not typically associated with a snap or pop in the palm. When spontaneous ruptures of the hand occur, they typically involve the profundus tendon of the small finger, in the area of the lumbrical origin.13
In equivocal cases when the site of rupture is uncertain, ultrasound and magnetic resonance imaging may assist in making the diagnosis and provide important preoperative information for surgical decision-making and planning; this information may decrease postoperative morbidity by minimizing surgical dissection.
The etiology of spontaneous ruptures is incompletely understood. For any rupture of the ulnar flexor tendons, the hook of the hamate should be examined to rule out a previous fracture as a cause of tendon attrition.15 Tendon vascularization may be a cause for tendon rupture in the hand. When the blood supply of the lumbrical muscles was examined in 100 upper extremities from human cadavers using vascular injection studies,16 it was discovered that each lumbrical muscle received its arterial supply from 4 sources: the superficial palmar arch, the common palmar digital artery, the deep palmar arch, and the dorsal digital artery. There were no anastomoses between the networks supplying the lumbrical muscles and the FDP tendons within the palm, suggesting a possible watershed zone between the FDP tendon and lumbrical muscle origin. The patient described in this case had the tendon rupture in the area of potential hypovascularity at the lumbrical origin.
Important factors in the decision-making process for surgical treatment include the length of time between rupture and treatment, the site of rupture, and the condition of the ruptured tendon ends. Patients who present in the first 3 weeks of injury can be treated by primary tendon repair, provided that the ruptured tendon ends are not significantly frayed or attenuated. For patients presenting more than 3 weeks after injury, interposition tendon grafts or tendon transfers are suitable options for ruptures in zone III. Distal interphalangeal joint arthrodesis is another alternative in specific cases where reconstruction is not possible. In this case, direct end-to-end repair was possible, as well as tenodesis to the intact ring finger superficialis in order to prevent stretching of the repair.
Localizing the level of the tendon rupture clinically is difficult. When the site of the profundus tendon rupture is uncertain, and there is no tenderness in zone I or the PIP joint, the first incision should be made at the metacarpophalangeal joint level. This first incision will indicate if the rupture occurred in zone III. If the tendon is intact at that location, then the next incision should be at the level of the PIP joint.
Conclusion
We report a patient treated for spontaneous rupture of the flexor tendon in zone III. He was treated in the acute setting with direct tendon repair. It is important to consider spontaneous rupture of the tendon in patients presenting with a snap/pop and the sudden inability to flex a finger. A tendon rupture can be diagnosed as spontaneous in the absence of an underlying pathologic condition such as rheumatoid arthritis, gout, or occult carpal fractures. In the acute setting, these may be repaired primarily; however, if presenting after a few weeks, alternative surgical options, including interposition tendon grafts, tendon transfer, and DIP joint arthrodesis, should be considered.
1. McMaster PE. Tendon and muscle ruptures, clinical and experimental studies on the causes and location of subcutaneous ruptures. J Bone Joint Surg Am. 1933;15(3):705-722.
2. Folmar RC, Nelson CL, Phalen GS. Ruptures of the flexor tendons in hands of non-rheumatoid patients. J Bone Joint Surg Am. 1972;54(3):579-584.
3. Grant I, Berger AC, Ireland DC. Rupture of the flexor digitorum profundus tendon to the small finger within the carpal tunnel. Hand Surg. 2005;10(1):109-114.
4. Hartford JM, Murphy JM. Flexor digitorum profundus rupture of the small finger secondary to nonunion of the hook of the hamate: a case report. J Hand Surg Am. 1996;21(14):621-623.
5. Johnston GH, Bowen CV. Attritional flexor tendon ruptures by an old lunate dislocation. J Hand Surg Am. 1988;13(5):701-703.
6. Koizumi M, Kanda T, Satoh S, Yoshizu T, Maki Y, Tsubokawa N. Attritional rupture of the flexor digitorum profundus tendon to the index finger caused by accessory carpal bone in the carpal tunnel: a case report. J Hand Surg Am. 2005;30(1):142-146.
7. Wurapa RK, Zelouf DS. Flexor tendon rupture caused by gout: a case report. J Hand Surg Am. 2002;27(4):591-593.
8. Masada K, Kanazawa M, Fuji T. Flexor tendon ruptures caused by an intraosseous ganglion of the hook of the hamate. J Hand Surg Br. 1997;22(3)383-385.
9. Boyes JH, Wilson JN, Smith JW. Flexor-tendon ruptures in the forearm and hand. J Bone Joint Surg Am. 1960;42(4):637-646.
10. Duran R, Houser R, Coleman C, et al. A preliminary report in the use of controlled passive motion following flexor tendon repair in zones II and III [abstract]. J Hand Surg. 1976;1(1):79.
11. Strickland JW, Glogovac SV. Digital function following flexor tendon repair in Zone II: A comparison of immobilization and controlled passive motion techniques. J Hand Surg Am. 1980;5(6):537-543.
12. Kannus P, Jozsa L. Histopathological changes preceding spontaneous rupture of a tendon. A controlled study of 891 patients. J Bone Joint Surg Am. 1991;73(10):1507-1525.
13. Bois AJ, Johnston G, Classen D. Spontaneous flexor tendon ruptures of the hand: case series and review of the literature. J Hand Surg Am. 2007;32(7):1061-1071.
14. Leddy JP, Packer JW. Avulsion of the profundus tendon insertion in athletes. J Hand Surg Am. 1977;2(1):66-69.
15. Jebson PJ, Ferlic RJ, Engber WF. Spontaneous rupture of ulnar-sided digital flexor tendons: don’t forget the hamate. Iowa Orthop J. 1995;15:225-227.
16. Zbrodowski A, Mariéthoz E, Bednarkiewicz M, Gajisin S. The blood supply of the lumbrical muscles. J Hand Surg Br. 1998;23(3):384-388.
Flexor tendons are considered the strongest component of the musculotendinous unit; they generally do not rupture unless weakened by an underlying pathologic condition.1 According to traditional teaching, when the musculotendinous unit is subjected to excessive forces, failure invariably occurs at the tendon insertion, at the musculotendinous junction, within the muscle substance, or at its origin from the bone before the tendon itself ruptures.1
Midsubstance tears in nonrheumatoid patients are less frequent and are typically attributable to an underlying cause.2 Possible pathologic conditions include, but are not limited to, osteoarthritis of the pisotriquetral joint,3 nonunion fracture of the hook of the hamate,4 lunate dislocation,5 accessory carpal bone,6 gouty infiltration of the flexor tendon,7 and tumor.8 In 1960, Boyes and colleagues9 presented a series of 80 flexor tendon ruptures in 78 patients over a 13-year period. Only 3 cases had no identifiable cause. The authors recommended using the term spontaneous for those ruptures that occur within the tendon substance without underlying or associated pathologic changes.
We describe a patient with spontaneous rupture of the flexor digitorum profundus (FDP) tendon at zone III, satisfying Boyes’ definition of the term spontaneous. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 65-year-old, right-handed manual worker was assessed in our hand clinic 3 days after he felt a cramp in his left palm while lifting a heavy object. Shortly thereafter, he noted he could not flex his ring finger distal interphalangeal (DIP) joint. He could not recall any previous injury to his finger. No predisposing pathologic conditions or bone abnormalities were identified. Clinically, there was no tenderness, swelling, or ecchymosis evident. He had full passive range of motion (ROM) of his ring finger, and proximal interphalangeal (PIP) joint active ROM was 0/110º; however, he had no activity of the FDP of the ring finger. Preoperative radiographs were normal. The hook of the hamate was clinically and radiographically normal.
A preoperative diagnosis of FDP avulsion from the distal phalanx was made, and the operation was carried out 16 days after injury. Surgical exploration started in zone II and extended proximally into the distal palmar crease, but no stump was found in either location. Therefore, exploration was carried out to the midpalmar region, revealing the tendon rupture in zone III, in the region of the origin of the ring finger lumbrical muscle (Figure 1). The flexor digitorum superficialis tendon was intact. Macroscopically, both tendon and carpal tunnel appeared normal, with no evidence of tendon attrition; thus, the tendon was not sent for histologic examination. The ends of the ruptured FDP tendon to the ring finger were at the level of the superficial palmar arch, with the distal end appearing as though it had been cut sharply with a knife. Because of the short period of time from injury to exploration, delayed primary tendon repair was possible, along with side-to-side tenodesis to the intact ring finger flexor superficialis tendon in the palm (Figure 2). Two days after surgery, the patient started a controlled mobilization program using the Duran method.10
At final follow-up of 18 months, total active motion was 126°, which corresponds to a good outcome, according to the Strickland and Glogovac criteria.11 Grip strength was 50 kg, which was 84% of grip strength on the uninjured side. The patient was back to recreational activity but had not returned to work.
Discussion
Most flexor tendon ruptures result from avulsion of the FDP tendon at its distal phalanx insertion, commonly known as Jersey finger. However, true midsubstance spontaneous ruptures are infrequent. Reports of spontaneous tendon ruptures of all types, including those of the hand, have increased in incidence in most countries.12 Bois and colleagues,13 who have reviewed the literature over a 50-year period, found a total of 50 spontaneous ruptures of “normal” flexor tendon in 43 cases. The authors point to unique historical and physical examinaton findings that help differentiate spontaneous tendon ruptures from the more common FDP avulsions. Such findings include the sensation of a pop or snap, or a sudden sharp pain or cramp within the palmar region. In contrast, most avulsion ruptures cause discomfort within the region of the digit. In type I avulsion injuries of the FDP tendon, the proximal tendon stump usually retracts proximal to the digital tendon sheath, causing a tender mass in the palm.14 Flexor digitorum profundus tendon avulsions, however, are not typically associated with a snap or pop in the palm. When spontaneous ruptures of the hand occur, they typically involve the profundus tendon of the small finger, in the area of the lumbrical origin.13
In equivocal cases when the site of rupture is uncertain, ultrasound and magnetic resonance imaging may assist in making the diagnosis and provide important preoperative information for surgical decision-making and planning; this information may decrease postoperative morbidity by minimizing surgical dissection.
The etiology of spontaneous ruptures is incompletely understood. For any rupture of the ulnar flexor tendons, the hook of the hamate should be examined to rule out a previous fracture as a cause of tendon attrition.15 Tendon vascularization may be a cause for tendon rupture in the hand. When the blood supply of the lumbrical muscles was examined in 100 upper extremities from human cadavers using vascular injection studies,16 it was discovered that each lumbrical muscle received its arterial supply from 4 sources: the superficial palmar arch, the common palmar digital artery, the deep palmar arch, and the dorsal digital artery. There were no anastomoses between the networks supplying the lumbrical muscles and the FDP tendons within the palm, suggesting a possible watershed zone between the FDP tendon and lumbrical muscle origin. The patient described in this case had the tendon rupture in the area of potential hypovascularity at the lumbrical origin.
Important factors in the decision-making process for surgical treatment include the length of time between rupture and treatment, the site of rupture, and the condition of the ruptured tendon ends. Patients who present in the first 3 weeks of injury can be treated by primary tendon repair, provided that the ruptured tendon ends are not significantly frayed or attenuated. For patients presenting more than 3 weeks after injury, interposition tendon grafts or tendon transfers are suitable options for ruptures in zone III. Distal interphalangeal joint arthrodesis is another alternative in specific cases where reconstruction is not possible. In this case, direct end-to-end repair was possible, as well as tenodesis to the intact ring finger superficialis in order to prevent stretching of the repair.
Localizing the level of the tendon rupture clinically is difficult. When the site of the profundus tendon rupture is uncertain, and there is no tenderness in zone I or the PIP joint, the first incision should be made at the metacarpophalangeal joint level. This first incision will indicate if the rupture occurred in zone III. If the tendon is intact at that location, then the next incision should be at the level of the PIP joint.
Conclusion
We report a patient treated for spontaneous rupture of the flexor tendon in zone III. He was treated in the acute setting with direct tendon repair. It is important to consider spontaneous rupture of the tendon in patients presenting with a snap/pop and the sudden inability to flex a finger. A tendon rupture can be diagnosed as spontaneous in the absence of an underlying pathologic condition such as rheumatoid arthritis, gout, or occult carpal fractures. In the acute setting, these may be repaired primarily; however, if presenting after a few weeks, alternative surgical options, including interposition tendon grafts, tendon transfer, and DIP joint arthrodesis, should be considered.
Flexor tendons are considered the strongest component of the musculotendinous unit; they generally do not rupture unless weakened by an underlying pathologic condition.1 According to traditional teaching, when the musculotendinous unit is subjected to excessive forces, failure invariably occurs at the tendon insertion, at the musculotendinous junction, within the muscle substance, or at its origin from the bone before the tendon itself ruptures.1
Midsubstance tears in nonrheumatoid patients are less frequent and are typically attributable to an underlying cause.2 Possible pathologic conditions include, but are not limited to, osteoarthritis of the pisotriquetral joint,3 nonunion fracture of the hook of the hamate,4 lunate dislocation,5 accessory carpal bone,6 gouty infiltration of the flexor tendon,7 and tumor.8 In 1960, Boyes and colleagues9 presented a series of 80 flexor tendon ruptures in 78 patients over a 13-year period. Only 3 cases had no identifiable cause. The authors recommended using the term spontaneous for those ruptures that occur within the tendon substance without underlying or associated pathologic changes.
We describe a patient with spontaneous rupture of the flexor digitorum profundus (FDP) tendon at zone III, satisfying Boyes’ definition of the term spontaneous. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 65-year-old, right-handed manual worker was assessed in our hand clinic 3 days after he felt a cramp in his left palm while lifting a heavy object. Shortly thereafter, he noted he could not flex his ring finger distal interphalangeal (DIP) joint. He could not recall any previous injury to his finger. No predisposing pathologic conditions or bone abnormalities were identified. Clinically, there was no tenderness, swelling, or ecchymosis evident. He had full passive range of motion (ROM) of his ring finger, and proximal interphalangeal (PIP) joint active ROM was 0/110º; however, he had no activity of the FDP of the ring finger. Preoperative radiographs were normal. The hook of the hamate was clinically and radiographically normal.
A preoperative diagnosis of FDP avulsion from the distal phalanx was made, and the operation was carried out 16 days after injury. Surgical exploration started in zone II and extended proximally into the distal palmar crease, but no stump was found in either location. Therefore, exploration was carried out to the midpalmar region, revealing the tendon rupture in zone III, in the region of the origin of the ring finger lumbrical muscle (Figure 1). The flexor digitorum superficialis tendon was intact. Macroscopically, both tendon and carpal tunnel appeared normal, with no evidence of tendon attrition; thus, the tendon was not sent for histologic examination. The ends of the ruptured FDP tendon to the ring finger were at the level of the superficial palmar arch, with the distal end appearing as though it had been cut sharply with a knife. Because of the short period of time from injury to exploration, delayed primary tendon repair was possible, along with side-to-side tenodesis to the intact ring finger flexor superficialis tendon in the palm (Figure 2). Two days after surgery, the patient started a controlled mobilization program using the Duran method.10
At final follow-up of 18 months, total active motion was 126°, which corresponds to a good outcome, according to the Strickland and Glogovac criteria.11 Grip strength was 50 kg, which was 84% of grip strength on the uninjured side. The patient was back to recreational activity but had not returned to work.
Discussion
Most flexor tendon ruptures result from avulsion of the FDP tendon at its distal phalanx insertion, commonly known as Jersey finger. However, true midsubstance spontaneous ruptures are infrequent. Reports of spontaneous tendon ruptures of all types, including those of the hand, have increased in incidence in most countries.12 Bois and colleagues,13 who have reviewed the literature over a 50-year period, found a total of 50 spontaneous ruptures of “normal” flexor tendon in 43 cases. The authors point to unique historical and physical examinaton findings that help differentiate spontaneous tendon ruptures from the more common FDP avulsions. Such findings include the sensation of a pop or snap, or a sudden sharp pain or cramp within the palmar region. In contrast, most avulsion ruptures cause discomfort within the region of the digit. In type I avulsion injuries of the FDP tendon, the proximal tendon stump usually retracts proximal to the digital tendon sheath, causing a tender mass in the palm.14 Flexor digitorum profundus tendon avulsions, however, are not typically associated with a snap or pop in the palm. When spontaneous ruptures of the hand occur, they typically involve the profundus tendon of the small finger, in the area of the lumbrical origin.13
In equivocal cases when the site of rupture is uncertain, ultrasound and magnetic resonance imaging may assist in making the diagnosis and provide important preoperative information for surgical decision-making and planning; this information may decrease postoperative morbidity by minimizing surgical dissection.
The etiology of spontaneous ruptures is incompletely understood. For any rupture of the ulnar flexor tendons, the hook of the hamate should be examined to rule out a previous fracture as a cause of tendon attrition.15 Tendon vascularization may be a cause for tendon rupture in the hand. When the blood supply of the lumbrical muscles was examined in 100 upper extremities from human cadavers using vascular injection studies,16 it was discovered that each lumbrical muscle received its arterial supply from 4 sources: the superficial palmar arch, the common palmar digital artery, the deep palmar arch, and the dorsal digital artery. There were no anastomoses between the networks supplying the lumbrical muscles and the FDP tendons within the palm, suggesting a possible watershed zone between the FDP tendon and lumbrical muscle origin. The patient described in this case had the tendon rupture in the area of potential hypovascularity at the lumbrical origin.
Important factors in the decision-making process for surgical treatment include the length of time between rupture and treatment, the site of rupture, and the condition of the ruptured tendon ends. Patients who present in the first 3 weeks of injury can be treated by primary tendon repair, provided that the ruptured tendon ends are not significantly frayed or attenuated. For patients presenting more than 3 weeks after injury, interposition tendon grafts or tendon transfers are suitable options for ruptures in zone III. Distal interphalangeal joint arthrodesis is another alternative in specific cases where reconstruction is not possible. In this case, direct end-to-end repair was possible, as well as tenodesis to the intact ring finger superficialis in order to prevent stretching of the repair.
Localizing the level of the tendon rupture clinically is difficult. When the site of the profundus tendon rupture is uncertain, and there is no tenderness in zone I or the PIP joint, the first incision should be made at the metacarpophalangeal joint level. This first incision will indicate if the rupture occurred in zone III. If the tendon is intact at that location, then the next incision should be at the level of the PIP joint.
Conclusion
We report a patient treated for spontaneous rupture of the flexor tendon in zone III. He was treated in the acute setting with direct tendon repair. It is important to consider spontaneous rupture of the tendon in patients presenting with a snap/pop and the sudden inability to flex a finger. A tendon rupture can be diagnosed as spontaneous in the absence of an underlying pathologic condition such as rheumatoid arthritis, gout, or occult carpal fractures. In the acute setting, these may be repaired primarily; however, if presenting after a few weeks, alternative surgical options, including interposition tendon grafts, tendon transfer, and DIP joint arthrodesis, should be considered.
1. McMaster PE. Tendon and muscle ruptures, clinical and experimental studies on the causes and location of subcutaneous ruptures. J Bone Joint Surg Am. 1933;15(3):705-722.
2. Folmar RC, Nelson CL, Phalen GS. Ruptures of the flexor tendons in hands of non-rheumatoid patients. J Bone Joint Surg Am. 1972;54(3):579-584.
3. Grant I, Berger AC, Ireland DC. Rupture of the flexor digitorum profundus tendon to the small finger within the carpal tunnel. Hand Surg. 2005;10(1):109-114.
4. Hartford JM, Murphy JM. Flexor digitorum profundus rupture of the small finger secondary to nonunion of the hook of the hamate: a case report. J Hand Surg Am. 1996;21(14):621-623.
5. Johnston GH, Bowen CV. Attritional flexor tendon ruptures by an old lunate dislocation. J Hand Surg Am. 1988;13(5):701-703.
6. Koizumi M, Kanda T, Satoh S, Yoshizu T, Maki Y, Tsubokawa N. Attritional rupture of the flexor digitorum profundus tendon to the index finger caused by accessory carpal bone in the carpal tunnel: a case report. J Hand Surg Am. 2005;30(1):142-146.
7. Wurapa RK, Zelouf DS. Flexor tendon rupture caused by gout: a case report. J Hand Surg Am. 2002;27(4):591-593.
8. Masada K, Kanazawa M, Fuji T. Flexor tendon ruptures caused by an intraosseous ganglion of the hook of the hamate. J Hand Surg Br. 1997;22(3)383-385.
9. Boyes JH, Wilson JN, Smith JW. Flexor-tendon ruptures in the forearm and hand. J Bone Joint Surg Am. 1960;42(4):637-646.
10. Duran R, Houser R, Coleman C, et al. A preliminary report in the use of controlled passive motion following flexor tendon repair in zones II and III [abstract]. J Hand Surg. 1976;1(1):79.
11. Strickland JW, Glogovac SV. Digital function following flexor tendon repair in Zone II: A comparison of immobilization and controlled passive motion techniques. J Hand Surg Am. 1980;5(6):537-543.
12. Kannus P, Jozsa L. Histopathological changes preceding spontaneous rupture of a tendon. A controlled study of 891 patients. J Bone Joint Surg Am. 1991;73(10):1507-1525.
13. Bois AJ, Johnston G, Classen D. Spontaneous flexor tendon ruptures of the hand: case series and review of the literature. J Hand Surg Am. 2007;32(7):1061-1071.
14. Leddy JP, Packer JW. Avulsion of the profundus tendon insertion in athletes. J Hand Surg Am. 1977;2(1):66-69.
15. Jebson PJ, Ferlic RJ, Engber WF. Spontaneous rupture of ulnar-sided digital flexor tendons: don’t forget the hamate. Iowa Orthop J. 1995;15:225-227.
16. Zbrodowski A, Mariéthoz E, Bednarkiewicz M, Gajisin S. The blood supply of the lumbrical muscles. J Hand Surg Br. 1998;23(3):384-388.
1. McMaster PE. Tendon and muscle ruptures, clinical and experimental studies on the causes and location of subcutaneous ruptures. J Bone Joint Surg Am. 1933;15(3):705-722.
2. Folmar RC, Nelson CL, Phalen GS. Ruptures of the flexor tendons in hands of non-rheumatoid patients. J Bone Joint Surg Am. 1972;54(3):579-584.
3. Grant I, Berger AC, Ireland DC. Rupture of the flexor digitorum profundus tendon to the small finger within the carpal tunnel. Hand Surg. 2005;10(1):109-114.
4. Hartford JM, Murphy JM. Flexor digitorum profundus rupture of the small finger secondary to nonunion of the hook of the hamate: a case report. J Hand Surg Am. 1996;21(14):621-623.
5. Johnston GH, Bowen CV. Attritional flexor tendon ruptures by an old lunate dislocation. J Hand Surg Am. 1988;13(5):701-703.
6. Koizumi M, Kanda T, Satoh S, Yoshizu T, Maki Y, Tsubokawa N. Attritional rupture of the flexor digitorum profundus tendon to the index finger caused by accessory carpal bone in the carpal tunnel: a case report. J Hand Surg Am. 2005;30(1):142-146.
7. Wurapa RK, Zelouf DS. Flexor tendon rupture caused by gout: a case report. J Hand Surg Am. 2002;27(4):591-593.
8. Masada K, Kanazawa M, Fuji T. Flexor tendon ruptures caused by an intraosseous ganglion of the hook of the hamate. J Hand Surg Br. 1997;22(3)383-385.
9. Boyes JH, Wilson JN, Smith JW. Flexor-tendon ruptures in the forearm and hand. J Bone Joint Surg Am. 1960;42(4):637-646.
10. Duran R, Houser R, Coleman C, et al. A preliminary report in the use of controlled passive motion following flexor tendon repair in zones II and III [abstract]. J Hand Surg. 1976;1(1):79.
11. Strickland JW, Glogovac SV. Digital function following flexor tendon repair in Zone II: A comparison of immobilization and controlled passive motion techniques. J Hand Surg Am. 1980;5(6):537-543.
12. Kannus P, Jozsa L. Histopathological changes preceding spontaneous rupture of a tendon. A controlled study of 891 patients. J Bone Joint Surg Am. 1991;73(10):1507-1525.
13. Bois AJ, Johnston G, Classen D. Spontaneous flexor tendon ruptures of the hand: case series and review of the literature. J Hand Surg Am. 2007;32(7):1061-1071.
14. Leddy JP, Packer JW. Avulsion of the profundus tendon insertion in athletes. J Hand Surg Am. 1977;2(1):66-69.
15. Jebson PJ, Ferlic RJ, Engber WF. Spontaneous rupture of ulnar-sided digital flexor tendons: don’t forget the hamate. Iowa Orthop J. 1995;15:225-227.
16. Zbrodowski A, Mariéthoz E, Bednarkiewicz M, Gajisin S. The blood supply of the lumbrical muscles. J Hand Surg Br. 1998;23(3):384-388.
Cadaveric Study of Appropriate Screw Length for Distal Radius Stabilization Using Volar Plate Fixation
Distal radius fractures constitute 15% of all extremity fractures and are the most common upper extremity fractures.1-3 The incidence of distal radius fractures is continuing to escalate because of the expanding elderly population and concurrent increase in osteoporosis.3,4 In addition, open reduction and internal fixation with a volar locking plate for distal radius fractures are more commonly being performed by general orthopedists, who may not perform these surgeries frequently. Surgically treated patients experience less time immobilized and have a higher chance of regaining previous functional status.2 In a commonly used technique, volar fixed-angle plating is used to stabilize the distal radius. With the rising popularity of this method, more patients are having postoperative complications.1,3,5,6 Extensor tendon irritation and attritional rupture constitute up to 50% of all complications stemming from volar plating of the distal radius.1
Volar plate fixation of the distal radius was originally designed to decrease postoperative tendon complications by preventing the flexor and extensor tendons from coming into direct contact with the surgically placed plates and/or screws.1 This technique places the volar plate under the belly of the pronator quadratus muscle. Shielding the flexor tendons, the pronator quadratus can prevent the volar plate from causing flexor tendon attrition. This shielding does not occur on the dorsal side of the wrist because the extensor tendons are in full contact with the dorsal radius. As such, volar fixation gained in popularity on the premise of preventing extensor tendon complications by directly avoiding the dorsal compartment.1,7
The most common complication of volar plating ironically involves the dorsal compartment.1,7 The typical distal radius fracture occurs when a fall on an outstretched hand results in significant dorsal comminution. In these cases, it can be difficult to judge the appropriate screw length, as the depth gauge does not have an intact cortex to hook. There is the temptation to use intraoperative fluoroscopy and the depth gauge to estimate screw lengths at the distal radius, especially in cases in which a surgeon may not perform this type of surgery often. More specifically, use of a lateral image to gauge the appropriate length for screws may be tempting, but a false estimate is possible.
Screw prominence on the dorsal cortex may be caused by the complex geometry of the distal radius. This geometry is produced by the Lister tubercle and its adjacent groove for the extensor pollicis longus.7 The dorsal shape of the distal radius is a dome or dihedral with the thickest part at the Lister tubercle. The dihedral shape may hide possible dorsal screw prominence on a lateral radiograph, but screw prominence can be appreciated with computed tomography (CT) (Figures 1, 2).
We conducted a study to determine if and where screw prominence occurs, and in what amount, to establish general guidelines for screw depth based on lateral radiographs. We also wanted to be able to highlight the potential source of postoperative complications.
Materials and Methods
Twelve preserved cadaveric forearms were used for this study. Two sets of arms were paired, and the other arms came from different cadavers. In total, 5 male arms (3 left, 2 right) and 7 female arms (5 left, 2 right) were used.
The arms were harvested using a bone saw to cut through the humerus just proximal to the epicondyles, keeping the ulna and radius completely intact. Each arm was examined by the naked eye and by fluoroscopy to determine if any significant anatomical or traumatic variations in the distal radius were present. None showed any abnormal variation.
The flexor tendons and volar structures were removed to allow easy visualization and access to the distal radius. The volar locking plates (Precise SD; Small Bone Innovations) were positioned to the best anatomical and radiographic fit and secured with a proximal and distal Kirschner wire (Figure 3). A single cortical screw was placed through the shaft for compression. All 7 distal holes were drilled bicortically using an appropriately sized 2.0-mm drill and the standard block drill guide. A depth gauge was used in concordance with fluoroscopy to estimate the distance between cortices and appropriate screw lengths for each hole. A standard lateral view was used to determine the depth based on aligning the depth gauge at the dorsal cortex. The hook was not used to hook the dorsal cortex, as typically the dorsal cortex is severely comminuted and unavailable for measurement. Next, all 7 locking screws of premeasured length were secured into their respective holes. Anteroposterior, lateral, and oblique (forearm supinated and pronated 45°) radiographs were obtained to visualize screw placement and possible dorsal screw prominence (Figures 4-6).8 The extensor tendons and dorsal structures were then dissected away to expose any violation of the dorsal compartments, and calipers were used to measure absolute dorsal screw prominence and the depth of the Lister tubercle (Figure 7).
Mean (SD) dorsal prominence at each screw position was calculated. The screws were also categorized into radial (1,4), central (2,5), and ulnar (3,6,7) groups based on location within the plate (Figure 3). Equality of means testing was performed using a 1-way analysis of variance followed by a Bonferroni test.
Results
Mean (SD) dorsal prominence in millimeters is listed in Table 1. Positions 1 and 4 had significantly more dorsal prominence than the other 5 screw positions (P < .01 for all comparisons). Mean (SD) dorsal prominence based on grouped screw positions is listed in Table 2. There was significantly more dorsal prominence in the radial group that in the central group (P < .001) and ulnar group (P < .001). Mean depth of the Lister tubercle was 3.25 mm.
All prominent screws in the radial aspect of the radius were detected using a supinated 45° view. A 45° pronated view was not successful in demonstrating screw prominence on the ulnar side of the wrist because of overlap of the ulnar head.
Discussion
Extensor tendon irritation and extensor tendon rupture are frequent yet preventable complications of using volar plating systems to stabilize distal radius fractures. Many recent studies have investigated the intraoperative methodologies in order to identify real-time adjustments the surgeon can make to prevent negative outcomes. The first report of extensor tendon injury caused by volar plate fixation (published in 1989) was attributed to dorsal screw prominence.9,10 Even today, extensor tendon complications remain a challenge, as screw prominence is difficult to ascertain even with multiple intraoperative radiologic views.1,8
This study simulated real-time radiographic views to estimate if screws had extended into the dorsal compartment. These radiographic predictions were then correlated with the absolute dorsal screw prominence seen after dorsal compartment dissection. We determined that the supinated oblique view was the best imaging view for identifying radial styloid screw prominence.
Mean depth of the Lister tubercle was 3.25 mm (similar to previously reported 2 mm11). However, there was no correlation identified between depth of the Lister tubercle and amount of dorsal screw prominence.
We wanted to identify high-risk areas and estimate expected dorsal screw prominence in order to make appropriate intraoperative screw length adjustments. The radius is divided into radial, central, and ulnar columns. The central screw positions had the least dorsal screw prominence (mean, 0.50 mm). This central position was considered low-risk. Both the radial and the ulnar screw positions had more dorsal screw prominence (means, 3.38 mm and 1.03 mm, respectively). Only the radial screws had significantly more prominence. However, this study was not powered to detect a difference as small as that between the central and ulnar screw positions. Despite the lack of statistical significance, it is clear from the data that the ulnar screws trend toward more dorsal prominence, and, therefore, screw measurements at both the radial and ulnar screw locations (using the depth gauge) require adjustments.
Extensor tendon contact was difficult to determine based on any specific screw length, as the extensor tendon had to be dissected to determine prominence. Based on observations, a prominence of 2 mm seemed to present a risk for tendon irritation. The periosteum and the rounded end of the screw may obviate the risk with 1 mm of prominence. However, this observation may not hold true in an in vivo situation.
This study had several limitations. First, only a single brand of plate was used, making these findings specific to this system. However, concepts and conclusions can be extrapolated to all systems. The radial side had the highest risk for prominence, and this factor should be accounted for when selecting screw lengths. In addition, the ulnar column also poses some risk, but not to the degree of the radial column. Another limitation is that fractures were not created in these radii; therefore, dorsal comminution was not recreated. In some cases, the dorsal cortex may be displaced dorsally and be somewhat protective. This study is not meant to be an exhaustive study on all volar plates or provide absolute recommendations. It is meant to suggest caution to surgeons who may not be familiar with the complex anatomy of the dorsal radius and to identify areas where the risk for screw penetration is highest.
Shortening screw lengths at the positions described may trigger surgeons’ concerns about stabilizing distal radius fractures. In a 2012 biomechanical study, Wall and colleagues12 found no difference between unicortical screws (placed at 75% of the distance to the dorsal cortex) and bicortical screws in effectiveness in stabilizing distal radius fractures.12 The proposed reduction will result in the desired bicortical screw lengths but limit prominence. In addition, in the setting of dorsal comminution, the increased stability gained by bicortical fixation is minimal.
In fractures with an intact dorsal cortex, standard depth gauges will likely produce appropriate screw length measurements. However, even in this situation, and based on the results reported by Wall and colleagues,12 subtraction of 1 to 2 mm may prove prudent. In cases in which the dorsal cortex is comminuted and screw estimates based on fluoroscopy are used, the lateral image may provide estimates that lead to screw prominence. A 45° supinated view should be used to check screw length for the radial side, the column most at risk. However, comminution may also obscure this view. We cannot comment on that, as the present study did not create comminuted fractures of the distal radius. In addition, the ulnar column posed a lesser but real risk of screw prominence, which must also be accounted for, and typically is not appreciated with alternate views.
Last, use of live fluoroscopy instead of standard anteroposterior and lateral views may prove valuable in assessing hardware placement and screw length in the setting of a comminuted distal radius fracture. Through use of live fluoroscopy, prominent screws, especially those on the radial side, may be identified, and potential tendon injury may be avoided. Keeping in mind the shape of the dorsal aspect of the distal radius should assist surgeons in preventing screw prominence dorsally and limit complications.
1. Maschke SD, Evans PJ, Schub D, Drake R, Lawton JN. Radiographic evaluation of dorsal screw penetration after volar fixed-angle plating of the distal radius: a cadaveric study. Hand. 2007;2(3):144-150.
2. Nana AD, Joshi A, Lichtman DM. Plating of the distal radius. J Am Acad Orthop Surg. 2005;13(3):159-171.
3. Orbay JL, Fernandez DL. Volar fixed-angle plate fixation for unstable distal radius fractures in the elderly patient. J Hand Surg. 2004;29(1):96-102.
4. Protopsaltis TS, Ruch DS. Volar approach to distal radius fractures. J Hand Surg. 2008;33(6):958-965.
5. Koval KJ, Harrast JJ, Anglen JO, Weinstein JN. Fractures of the distal part of the radius. The evolution of practice over time. Where’s the evidence? J Bone Joint Surg Am. 2008;90(9):1855-1861.
6. Gruber G, Zacherl M, Giessauf C, et al. Quality of life after volar plate fixation of articular fractures of the distal part of the radius. J Bone Joint Surg Am. 2010;92(5):1170-1178.
7. Clement H, Pichler W, Nelson D, Hausleitner L, Tesch NP, Grechenig W. Morphometric analysis of Lister’s tubercle and its consequences on volar plate fixation of distal radius fractures. J Hand Surg. 2008;33(10):1716-1719.
8. Ozer K, Wolf JM, Watkins B, Hak DJ. Comparison of 4 fluoroscopic views for dorsal cortex screw penetration after volar plating of the distal radius. J Hand Surg. 2012;37(5):963-967.
9. Perry DC, Machin DM, Casaletto JA, Brown DJ. Minimising the risk of extensor pollicis longus rupture following volar plate fixation of distal radius fractures: a cadaveric study. Ann R Coll Surg Engl. 2011;93(1):57-60.
10. Wong-Chung J, Quinlan W. Rupture of extensor pollicis longus following fixation of a distal radius fracture. Injury. 1989;20(6):375-376.
11. Park DH, Goldie BS. Volar plating for distal radius fractures—do not trust the image intensifier when judging distal subchondral screw length. Tech Hand Up Extrem Surg. 2012;16(3):169-172.
12. Wall LB, Brodt MD, Silva MJ, Boyer MI, Calfee RP. The effects of screw length on stability of simulated osteoporotic distal radius fractures fixed with volar locking plates. J Hand Surg. 2012;37(3):446-453.
Distal radius fractures constitute 15% of all extremity fractures and are the most common upper extremity fractures.1-3 The incidence of distal radius fractures is continuing to escalate because of the expanding elderly population and concurrent increase in osteoporosis.3,4 In addition, open reduction and internal fixation with a volar locking plate for distal radius fractures are more commonly being performed by general orthopedists, who may not perform these surgeries frequently. Surgically treated patients experience less time immobilized and have a higher chance of regaining previous functional status.2 In a commonly used technique, volar fixed-angle plating is used to stabilize the distal radius. With the rising popularity of this method, more patients are having postoperative complications.1,3,5,6 Extensor tendon irritation and attritional rupture constitute up to 50% of all complications stemming from volar plating of the distal radius.1
Volar plate fixation of the distal radius was originally designed to decrease postoperative tendon complications by preventing the flexor and extensor tendons from coming into direct contact with the surgically placed plates and/or screws.1 This technique places the volar plate under the belly of the pronator quadratus muscle. Shielding the flexor tendons, the pronator quadratus can prevent the volar plate from causing flexor tendon attrition. This shielding does not occur on the dorsal side of the wrist because the extensor tendons are in full contact with the dorsal radius. As such, volar fixation gained in popularity on the premise of preventing extensor tendon complications by directly avoiding the dorsal compartment.1,7
The most common complication of volar plating ironically involves the dorsal compartment.1,7 The typical distal radius fracture occurs when a fall on an outstretched hand results in significant dorsal comminution. In these cases, it can be difficult to judge the appropriate screw length, as the depth gauge does not have an intact cortex to hook. There is the temptation to use intraoperative fluoroscopy and the depth gauge to estimate screw lengths at the distal radius, especially in cases in which a surgeon may not perform this type of surgery often. More specifically, use of a lateral image to gauge the appropriate length for screws may be tempting, but a false estimate is possible.
Screw prominence on the dorsal cortex may be caused by the complex geometry of the distal radius. This geometry is produced by the Lister tubercle and its adjacent groove for the extensor pollicis longus.7 The dorsal shape of the distal radius is a dome or dihedral with the thickest part at the Lister tubercle. The dihedral shape may hide possible dorsal screw prominence on a lateral radiograph, but screw prominence can be appreciated with computed tomography (CT) (Figures 1, 2).
We conducted a study to determine if and where screw prominence occurs, and in what amount, to establish general guidelines for screw depth based on lateral radiographs. We also wanted to be able to highlight the potential source of postoperative complications.
Materials and Methods
Twelve preserved cadaveric forearms were used for this study. Two sets of arms were paired, and the other arms came from different cadavers. In total, 5 male arms (3 left, 2 right) and 7 female arms (5 left, 2 right) were used.
The arms were harvested using a bone saw to cut through the humerus just proximal to the epicondyles, keeping the ulna and radius completely intact. Each arm was examined by the naked eye and by fluoroscopy to determine if any significant anatomical or traumatic variations in the distal radius were present. None showed any abnormal variation.
The flexor tendons and volar structures were removed to allow easy visualization and access to the distal radius. The volar locking plates (Precise SD; Small Bone Innovations) were positioned to the best anatomical and radiographic fit and secured with a proximal and distal Kirschner wire (Figure 3). A single cortical screw was placed through the shaft for compression. All 7 distal holes were drilled bicortically using an appropriately sized 2.0-mm drill and the standard block drill guide. A depth gauge was used in concordance with fluoroscopy to estimate the distance between cortices and appropriate screw lengths for each hole. A standard lateral view was used to determine the depth based on aligning the depth gauge at the dorsal cortex. The hook was not used to hook the dorsal cortex, as typically the dorsal cortex is severely comminuted and unavailable for measurement. Next, all 7 locking screws of premeasured length were secured into their respective holes. Anteroposterior, lateral, and oblique (forearm supinated and pronated 45°) radiographs were obtained to visualize screw placement and possible dorsal screw prominence (Figures 4-6).8 The extensor tendons and dorsal structures were then dissected away to expose any violation of the dorsal compartments, and calipers were used to measure absolute dorsal screw prominence and the depth of the Lister tubercle (Figure 7).
Mean (SD) dorsal prominence at each screw position was calculated. The screws were also categorized into radial (1,4), central (2,5), and ulnar (3,6,7) groups based on location within the plate (Figure 3). Equality of means testing was performed using a 1-way analysis of variance followed by a Bonferroni test.
Results
Mean (SD) dorsal prominence in millimeters is listed in Table 1. Positions 1 and 4 had significantly more dorsal prominence than the other 5 screw positions (P < .01 for all comparisons). Mean (SD) dorsal prominence based on grouped screw positions is listed in Table 2. There was significantly more dorsal prominence in the radial group that in the central group (P < .001) and ulnar group (P < .001). Mean depth of the Lister tubercle was 3.25 mm.
All prominent screws in the radial aspect of the radius were detected using a supinated 45° view. A 45° pronated view was not successful in demonstrating screw prominence on the ulnar side of the wrist because of overlap of the ulnar head.
Discussion
Extensor tendon irritation and extensor tendon rupture are frequent yet preventable complications of using volar plating systems to stabilize distal radius fractures. Many recent studies have investigated the intraoperative methodologies in order to identify real-time adjustments the surgeon can make to prevent negative outcomes. The first report of extensor tendon injury caused by volar plate fixation (published in 1989) was attributed to dorsal screw prominence.9,10 Even today, extensor tendon complications remain a challenge, as screw prominence is difficult to ascertain even with multiple intraoperative radiologic views.1,8
This study simulated real-time radiographic views to estimate if screws had extended into the dorsal compartment. These radiographic predictions were then correlated with the absolute dorsal screw prominence seen after dorsal compartment dissection. We determined that the supinated oblique view was the best imaging view for identifying radial styloid screw prominence.
Mean depth of the Lister tubercle was 3.25 mm (similar to previously reported 2 mm11). However, there was no correlation identified between depth of the Lister tubercle and amount of dorsal screw prominence.
We wanted to identify high-risk areas and estimate expected dorsal screw prominence in order to make appropriate intraoperative screw length adjustments. The radius is divided into radial, central, and ulnar columns. The central screw positions had the least dorsal screw prominence (mean, 0.50 mm). This central position was considered low-risk. Both the radial and the ulnar screw positions had more dorsal screw prominence (means, 3.38 mm and 1.03 mm, respectively). Only the radial screws had significantly more prominence. However, this study was not powered to detect a difference as small as that between the central and ulnar screw positions. Despite the lack of statistical significance, it is clear from the data that the ulnar screws trend toward more dorsal prominence, and, therefore, screw measurements at both the radial and ulnar screw locations (using the depth gauge) require adjustments.
Extensor tendon contact was difficult to determine based on any specific screw length, as the extensor tendon had to be dissected to determine prominence. Based on observations, a prominence of 2 mm seemed to present a risk for tendon irritation. The periosteum and the rounded end of the screw may obviate the risk with 1 mm of prominence. However, this observation may not hold true in an in vivo situation.
This study had several limitations. First, only a single brand of plate was used, making these findings specific to this system. However, concepts and conclusions can be extrapolated to all systems. The radial side had the highest risk for prominence, and this factor should be accounted for when selecting screw lengths. In addition, the ulnar column also poses some risk, but not to the degree of the radial column. Another limitation is that fractures were not created in these radii; therefore, dorsal comminution was not recreated. In some cases, the dorsal cortex may be displaced dorsally and be somewhat protective. This study is not meant to be an exhaustive study on all volar plates or provide absolute recommendations. It is meant to suggest caution to surgeons who may not be familiar with the complex anatomy of the dorsal radius and to identify areas where the risk for screw penetration is highest.
Shortening screw lengths at the positions described may trigger surgeons’ concerns about stabilizing distal radius fractures. In a 2012 biomechanical study, Wall and colleagues12 found no difference between unicortical screws (placed at 75% of the distance to the dorsal cortex) and bicortical screws in effectiveness in stabilizing distal radius fractures.12 The proposed reduction will result in the desired bicortical screw lengths but limit prominence. In addition, in the setting of dorsal comminution, the increased stability gained by bicortical fixation is minimal.
In fractures with an intact dorsal cortex, standard depth gauges will likely produce appropriate screw length measurements. However, even in this situation, and based on the results reported by Wall and colleagues,12 subtraction of 1 to 2 mm may prove prudent. In cases in which the dorsal cortex is comminuted and screw estimates based on fluoroscopy are used, the lateral image may provide estimates that lead to screw prominence. A 45° supinated view should be used to check screw length for the radial side, the column most at risk. However, comminution may also obscure this view. We cannot comment on that, as the present study did not create comminuted fractures of the distal radius. In addition, the ulnar column posed a lesser but real risk of screw prominence, which must also be accounted for, and typically is not appreciated with alternate views.
Last, use of live fluoroscopy instead of standard anteroposterior and lateral views may prove valuable in assessing hardware placement and screw length in the setting of a comminuted distal radius fracture. Through use of live fluoroscopy, prominent screws, especially those on the radial side, may be identified, and potential tendon injury may be avoided. Keeping in mind the shape of the dorsal aspect of the distal radius should assist surgeons in preventing screw prominence dorsally and limit complications.
Distal radius fractures constitute 15% of all extremity fractures and are the most common upper extremity fractures.1-3 The incidence of distal radius fractures is continuing to escalate because of the expanding elderly population and concurrent increase in osteoporosis.3,4 In addition, open reduction and internal fixation with a volar locking plate for distal radius fractures are more commonly being performed by general orthopedists, who may not perform these surgeries frequently. Surgically treated patients experience less time immobilized and have a higher chance of regaining previous functional status.2 In a commonly used technique, volar fixed-angle plating is used to stabilize the distal radius. With the rising popularity of this method, more patients are having postoperative complications.1,3,5,6 Extensor tendon irritation and attritional rupture constitute up to 50% of all complications stemming from volar plating of the distal radius.1
Volar plate fixation of the distal radius was originally designed to decrease postoperative tendon complications by preventing the flexor and extensor tendons from coming into direct contact with the surgically placed plates and/or screws.1 This technique places the volar plate under the belly of the pronator quadratus muscle. Shielding the flexor tendons, the pronator quadratus can prevent the volar plate from causing flexor tendon attrition. This shielding does not occur on the dorsal side of the wrist because the extensor tendons are in full contact with the dorsal radius. As such, volar fixation gained in popularity on the premise of preventing extensor tendon complications by directly avoiding the dorsal compartment.1,7
The most common complication of volar plating ironically involves the dorsal compartment.1,7 The typical distal radius fracture occurs when a fall on an outstretched hand results in significant dorsal comminution. In these cases, it can be difficult to judge the appropriate screw length, as the depth gauge does not have an intact cortex to hook. There is the temptation to use intraoperative fluoroscopy and the depth gauge to estimate screw lengths at the distal radius, especially in cases in which a surgeon may not perform this type of surgery often. More specifically, use of a lateral image to gauge the appropriate length for screws may be tempting, but a false estimate is possible.
Screw prominence on the dorsal cortex may be caused by the complex geometry of the distal radius. This geometry is produced by the Lister tubercle and its adjacent groove for the extensor pollicis longus.7 The dorsal shape of the distal radius is a dome or dihedral with the thickest part at the Lister tubercle. The dihedral shape may hide possible dorsal screw prominence on a lateral radiograph, but screw prominence can be appreciated with computed tomography (CT) (Figures 1, 2).
We conducted a study to determine if and where screw prominence occurs, and in what amount, to establish general guidelines for screw depth based on lateral radiographs. We also wanted to be able to highlight the potential source of postoperative complications.
Materials and Methods
Twelve preserved cadaveric forearms were used for this study. Two sets of arms were paired, and the other arms came from different cadavers. In total, 5 male arms (3 left, 2 right) and 7 female arms (5 left, 2 right) were used.
The arms were harvested using a bone saw to cut through the humerus just proximal to the epicondyles, keeping the ulna and radius completely intact. Each arm was examined by the naked eye and by fluoroscopy to determine if any significant anatomical or traumatic variations in the distal radius were present. None showed any abnormal variation.
The flexor tendons and volar structures were removed to allow easy visualization and access to the distal radius. The volar locking plates (Precise SD; Small Bone Innovations) were positioned to the best anatomical and radiographic fit and secured with a proximal and distal Kirschner wire (Figure 3). A single cortical screw was placed through the shaft for compression. All 7 distal holes were drilled bicortically using an appropriately sized 2.0-mm drill and the standard block drill guide. A depth gauge was used in concordance with fluoroscopy to estimate the distance between cortices and appropriate screw lengths for each hole. A standard lateral view was used to determine the depth based on aligning the depth gauge at the dorsal cortex. The hook was not used to hook the dorsal cortex, as typically the dorsal cortex is severely comminuted and unavailable for measurement. Next, all 7 locking screws of premeasured length were secured into their respective holes. Anteroposterior, lateral, and oblique (forearm supinated and pronated 45°) radiographs were obtained to visualize screw placement and possible dorsal screw prominence (Figures 4-6).8 The extensor tendons and dorsal structures were then dissected away to expose any violation of the dorsal compartments, and calipers were used to measure absolute dorsal screw prominence and the depth of the Lister tubercle (Figure 7).
Mean (SD) dorsal prominence at each screw position was calculated. The screws were also categorized into radial (1,4), central (2,5), and ulnar (3,6,7) groups based on location within the plate (Figure 3). Equality of means testing was performed using a 1-way analysis of variance followed by a Bonferroni test.
Results
Mean (SD) dorsal prominence in millimeters is listed in Table 1. Positions 1 and 4 had significantly more dorsal prominence than the other 5 screw positions (P < .01 for all comparisons). Mean (SD) dorsal prominence based on grouped screw positions is listed in Table 2. There was significantly more dorsal prominence in the radial group that in the central group (P < .001) and ulnar group (P < .001). Mean depth of the Lister tubercle was 3.25 mm.
All prominent screws in the radial aspect of the radius were detected using a supinated 45° view. A 45° pronated view was not successful in demonstrating screw prominence on the ulnar side of the wrist because of overlap of the ulnar head.
Discussion
Extensor tendon irritation and extensor tendon rupture are frequent yet preventable complications of using volar plating systems to stabilize distal radius fractures. Many recent studies have investigated the intraoperative methodologies in order to identify real-time adjustments the surgeon can make to prevent negative outcomes. The first report of extensor tendon injury caused by volar plate fixation (published in 1989) was attributed to dorsal screw prominence.9,10 Even today, extensor tendon complications remain a challenge, as screw prominence is difficult to ascertain even with multiple intraoperative radiologic views.1,8
This study simulated real-time radiographic views to estimate if screws had extended into the dorsal compartment. These radiographic predictions were then correlated with the absolute dorsal screw prominence seen after dorsal compartment dissection. We determined that the supinated oblique view was the best imaging view for identifying radial styloid screw prominence.
Mean depth of the Lister tubercle was 3.25 mm (similar to previously reported 2 mm11). However, there was no correlation identified between depth of the Lister tubercle and amount of dorsal screw prominence.
We wanted to identify high-risk areas and estimate expected dorsal screw prominence in order to make appropriate intraoperative screw length adjustments. The radius is divided into radial, central, and ulnar columns. The central screw positions had the least dorsal screw prominence (mean, 0.50 mm). This central position was considered low-risk. Both the radial and the ulnar screw positions had more dorsal screw prominence (means, 3.38 mm and 1.03 mm, respectively). Only the radial screws had significantly more prominence. However, this study was not powered to detect a difference as small as that between the central and ulnar screw positions. Despite the lack of statistical significance, it is clear from the data that the ulnar screws trend toward more dorsal prominence, and, therefore, screw measurements at both the radial and ulnar screw locations (using the depth gauge) require adjustments.
Extensor tendon contact was difficult to determine based on any specific screw length, as the extensor tendon had to be dissected to determine prominence. Based on observations, a prominence of 2 mm seemed to present a risk for tendon irritation. The periosteum and the rounded end of the screw may obviate the risk with 1 mm of prominence. However, this observation may not hold true in an in vivo situation.
This study had several limitations. First, only a single brand of plate was used, making these findings specific to this system. However, concepts and conclusions can be extrapolated to all systems. The radial side had the highest risk for prominence, and this factor should be accounted for when selecting screw lengths. In addition, the ulnar column also poses some risk, but not to the degree of the radial column. Another limitation is that fractures were not created in these radii; therefore, dorsal comminution was not recreated. In some cases, the dorsal cortex may be displaced dorsally and be somewhat protective. This study is not meant to be an exhaustive study on all volar plates or provide absolute recommendations. It is meant to suggest caution to surgeons who may not be familiar with the complex anatomy of the dorsal radius and to identify areas where the risk for screw penetration is highest.
Shortening screw lengths at the positions described may trigger surgeons’ concerns about stabilizing distal radius fractures. In a 2012 biomechanical study, Wall and colleagues12 found no difference between unicortical screws (placed at 75% of the distance to the dorsal cortex) and bicortical screws in effectiveness in stabilizing distal radius fractures.12 The proposed reduction will result in the desired bicortical screw lengths but limit prominence. In addition, in the setting of dorsal comminution, the increased stability gained by bicortical fixation is minimal.
In fractures with an intact dorsal cortex, standard depth gauges will likely produce appropriate screw length measurements. However, even in this situation, and based on the results reported by Wall and colleagues,12 subtraction of 1 to 2 mm may prove prudent. In cases in which the dorsal cortex is comminuted and screw estimates based on fluoroscopy are used, the lateral image may provide estimates that lead to screw prominence. A 45° supinated view should be used to check screw length for the radial side, the column most at risk. However, comminution may also obscure this view. We cannot comment on that, as the present study did not create comminuted fractures of the distal radius. In addition, the ulnar column posed a lesser but real risk of screw prominence, which must also be accounted for, and typically is not appreciated with alternate views.
Last, use of live fluoroscopy instead of standard anteroposterior and lateral views may prove valuable in assessing hardware placement and screw length in the setting of a comminuted distal radius fracture. Through use of live fluoroscopy, prominent screws, especially those on the radial side, may be identified, and potential tendon injury may be avoided. Keeping in mind the shape of the dorsal aspect of the distal radius should assist surgeons in preventing screw prominence dorsally and limit complications.
1. Maschke SD, Evans PJ, Schub D, Drake R, Lawton JN. Radiographic evaluation of dorsal screw penetration after volar fixed-angle plating of the distal radius: a cadaveric study. Hand. 2007;2(3):144-150.
2. Nana AD, Joshi A, Lichtman DM. Plating of the distal radius. J Am Acad Orthop Surg. 2005;13(3):159-171.
3. Orbay JL, Fernandez DL. Volar fixed-angle plate fixation for unstable distal radius fractures in the elderly patient. J Hand Surg. 2004;29(1):96-102.
4. Protopsaltis TS, Ruch DS. Volar approach to distal radius fractures. J Hand Surg. 2008;33(6):958-965.
5. Koval KJ, Harrast JJ, Anglen JO, Weinstein JN. Fractures of the distal part of the radius. The evolution of practice over time. Where’s the evidence? J Bone Joint Surg Am. 2008;90(9):1855-1861.
6. Gruber G, Zacherl M, Giessauf C, et al. Quality of life after volar plate fixation of articular fractures of the distal part of the radius. J Bone Joint Surg Am. 2010;92(5):1170-1178.
7. Clement H, Pichler W, Nelson D, Hausleitner L, Tesch NP, Grechenig W. Morphometric analysis of Lister’s tubercle and its consequences on volar plate fixation of distal radius fractures. J Hand Surg. 2008;33(10):1716-1719.
8. Ozer K, Wolf JM, Watkins B, Hak DJ. Comparison of 4 fluoroscopic views for dorsal cortex screw penetration after volar plating of the distal radius. J Hand Surg. 2012;37(5):963-967.
9. Perry DC, Machin DM, Casaletto JA, Brown DJ. Minimising the risk of extensor pollicis longus rupture following volar plate fixation of distal radius fractures: a cadaveric study. Ann R Coll Surg Engl. 2011;93(1):57-60.
10. Wong-Chung J, Quinlan W. Rupture of extensor pollicis longus following fixation of a distal radius fracture. Injury. 1989;20(6):375-376.
11. Park DH, Goldie BS. Volar plating for distal radius fractures—do not trust the image intensifier when judging distal subchondral screw length. Tech Hand Up Extrem Surg. 2012;16(3):169-172.
12. Wall LB, Brodt MD, Silva MJ, Boyer MI, Calfee RP. The effects of screw length on stability of simulated osteoporotic distal radius fractures fixed with volar locking plates. J Hand Surg. 2012;37(3):446-453.
1. Maschke SD, Evans PJ, Schub D, Drake R, Lawton JN. Radiographic evaluation of dorsal screw penetration after volar fixed-angle plating of the distal radius: a cadaveric study. Hand. 2007;2(3):144-150.
2. Nana AD, Joshi A, Lichtman DM. Plating of the distal radius. J Am Acad Orthop Surg. 2005;13(3):159-171.
3. Orbay JL, Fernandez DL. Volar fixed-angle plate fixation for unstable distal radius fractures in the elderly patient. J Hand Surg. 2004;29(1):96-102.
4. Protopsaltis TS, Ruch DS. Volar approach to distal radius fractures. J Hand Surg. 2008;33(6):958-965.
5. Koval KJ, Harrast JJ, Anglen JO, Weinstein JN. Fractures of the distal part of the radius. The evolution of practice over time. Where’s the evidence? J Bone Joint Surg Am. 2008;90(9):1855-1861.
6. Gruber G, Zacherl M, Giessauf C, et al. Quality of life after volar plate fixation of articular fractures of the distal part of the radius. J Bone Joint Surg Am. 2010;92(5):1170-1178.
7. Clement H, Pichler W, Nelson D, Hausleitner L, Tesch NP, Grechenig W. Morphometric analysis of Lister’s tubercle and its consequences on volar plate fixation of distal radius fractures. J Hand Surg. 2008;33(10):1716-1719.
8. Ozer K, Wolf JM, Watkins B, Hak DJ. Comparison of 4 fluoroscopic views for dorsal cortex screw penetration after volar plating of the distal radius. J Hand Surg. 2012;37(5):963-967.
9. Perry DC, Machin DM, Casaletto JA, Brown DJ. Minimising the risk of extensor pollicis longus rupture following volar plate fixation of distal radius fractures: a cadaveric study. Ann R Coll Surg Engl. 2011;93(1):57-60.
10. Wong-Chung J, Quinlan W. Rupture of extensor pollicis longus following fixation of a distal radius fracture. Injury. 1989;20(6):375-376.
11. Park DH, Goldie BS. Volar plating for distal radius fractures—do not trust the image intensifier when judging distal subchondral screw length. Tech Hand Up Extrem Surg. 2012;16(3):169-172.
12. Wall LB, Brodt MD, Silva MJ, Boyer MI, Calfee RP. The effects of screw length on stability of simulated osteoporotic distal radius fractures fixed with volar locking plates. J Hand Surg. 2012;37(3):446-453.