User login
The American Journal of Orthopedics is an Index Medicus publication that is valued by orthopedic surgeons for its peer-reviewed, practice-oriented clinical information. Most articles are written by specialists at leading teaching institutions and help incorporate the latest technology into everyday practice.
Isolated Radiopalmar Dislocation of Fifth Carpometacarpal Joint: A Rare Presentation
Isolated dislocation of the carpometacarpal (CMC) joint of the hand is a rare injury. While the dislocation can be dorsal or palmar, dorsal dislocation is more common. Palmar dislocations can be either ulnopalmar or radiopalmar. There are very few reports of isolated radiopalmar dislocations of the fifth CMC joint in the English-language literature.1-3 We present a case of delayed presentation and management of radiopalmar dislocation of the fifth CMC joint. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
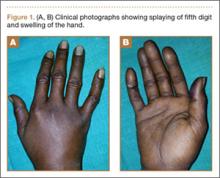
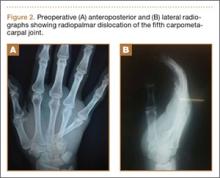
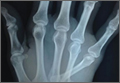
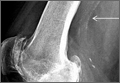
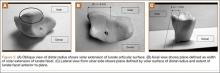
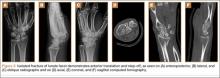
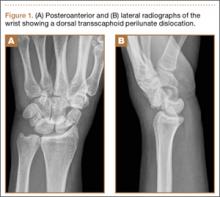
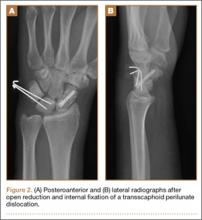
A 42-year-old man presented with polytrauma to our emergency department. He was stabilized initially, and open fractures were treated by débridement and external fixator application. During an examination 3 days after admission, swelling was noted in the right hand. On further study, there was splaying of the fifth digit and tenderness over the fourth and fifth CMC joints (Figure 1). No abnormal mobility or crepitus could be elicited. Plain radiographs of the right hand in anteroposterior and lateral views revealed radiopalmar dislocation of the fifth CMC joint (Figure 2). It was decided to reduce the dislocation immediately after the patient was declared fit for surgery.
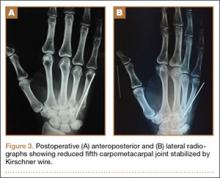
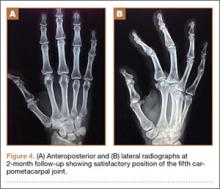
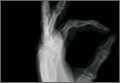
Under axillary block, closed reduction was unsuccessful. Open reduction of the fifth CMC joint was performed through a dorsal incision. The base of the fifth metacarpal bone was found to be stripped of soft-tissue attachments and lying in a radiopalmar location. Reduction, which was checked under image intensifier, was found to be satisfactory (Figure 3). Reduction was stabilized by passing a smooth Kirschner wire (K-wire) from the fifth metacarpal to the hamate bone. After achieving hemostasis, the wound was closed in layers and a below-elbow splint was applied. The perioperative period was uneventful, and sutures were removed on postoperative day 10. The K-wire was removed after 4 weeks, and radiographs showed satisfactory position of the fifth CMC joint. Gentle active and passive mobilization of fingers and wrist were started. The patient had regained good function of the wrist and fingers 2 months after surgery (Figure 4).
Discussion
Carpometacarpal joint dislocations are uncommon injuries and account for less than 1% of hand injuries.4 They are classified as dorsal and volar (palmar) dislocations. Dorsal dislocations of the CMC joints occur more frequently than do volar dislocations, mainly affecting the fourth and fifth digits.5 Isolated volar or palmar dislocation of the fifth CMC joint is an uncommon injury that was first reported in 1918 by McWhorter.6 In 1968, Nalebuff7 classified the volar dislocations into 2 groups according to the direction of the displacement of the fifth metacarpal base: radiopalmar and ulnopalmar. Berg and Murphy8 found the hook of the hamate to deviate the metacarpal bone to either the ulnar or radial side. Tearing of all ligament and tendon attachments of the base of the fifth metacarpal results in radiopalmar dislocation.7 The attachments of ligaments and tendons remain intact in the ulnopalmar dislocation.7
The clinical features of this injury are pain and swelling about the base of the fifth metacarpal and axial deformity of the little finger with apparent shortening. The deep motor branch of the ulnar nerve lies volar to the fifth CMC joint as it courses around the hook of the hamate. It is vulnerable to injury in both dorsal9,10 and volar11 CMC dislocations. For radiologic evaluation, in addition to standard anteroposterior and lateral radiographs, a lateral view in 30º pronation of the hand can provide an improved view of the fifth CMC joint, as suggested by Bora and Didizian.12
The treatment of ulnopalmar dislocation has evolved. Ulnopalmar dislocations have been successfully treated by closed reduction without fixation,8 and by open reduction and K-wire fixation.3,7,13
Radiopalmar dislocations are inherently unstable because of the tearing of all ligament and tendon attachments of the base of the fifth metacarpal.7 In our case of radiopalmar dislocation, diagnosis was delayed and attempts at closed reduction were unsuccessful. Therefore, it was treated by open reduction and K-wire fixation. In our case, open reduction and K-wire fixation for radiopalmar dislocation of the fifth CMC joint provided promising results.
Conclusion
Radiopalmar dislocation of the fifth CMC joint is a rare injury, and very few cases have been reported in the English-language literature. We report one such case, which was successfully treated with open reduction and K-wire fixation.
1. Buzby BF. Palmar carpometacarpal dislocation of the fifth metacarpal. Ann Surg. 1934;100:555-557.
2. Chen VT. Dislocation of carpometacarpal joint of the little finger. J Hand Surg. 1987;12(2):260-263.
3. Dennyson WG, Stother IG. Carpometacarpal dislocation of the little finger. Hand. 1976;8(2):161-164.
4. Domingo A, Font L, Saz L, Arandes JM. Isolated radial palmar dislocation of the fifth carpometacarpal joint with ulnar neuropathy associated: successful treatment with closed reduction and internal fixation. Eur J Orthop Surg Traumatol. 19(2):101-107.
5. Fisher MR, Rogers LF, Hendrix RW. Systematic approach to identifying fourth and fifth carpometacarpal joint dislocations. AJR Am J Roentgenol. 1983;140(2):319-324.
6. McWhorter GL. Isolated and complete dislocation of the fifth carpometacarpal joint: open operation. Surg Clin Chic. 1918;2:793-796.
7. Nalebuff EA. Isolated anterior carpometacarpal dislocation of the fifth finger: classification and case report. J Trauma. 1968;8(6):1119-1123.
8. Berg EE, Murphy DF. Ulnopalmar dislocation of the fifth carpometacarpal joint – successful closed reduction: review of the literature and anatomic reevaluation. J Hand Surg Am. 1986;11(4):521-525.
9. Peterson P, Sacks S. Fracture-dislocation of the base of the fifth metacarpal associated with injury to the deep motor branch of the ulnar nerve: a case report. J Hand Surg Am. 1986;11(4):525-528.
10. Young TB. Dorsal dislocation of the metacarpal base of the little and ring fingers with ulnar nerve branch compression. Injury. 1987;18(1):65-66.
11. O’Rourke PJ, Quinlan W. Fracture dislocation of the fifth metacarpal resulting in compression of the deep branch of the ulnar nerve. J Hand Surg Br. 1993;18(2):190-191.
12. Bora FW Jr, Didizian NH. The treatment of injuries to the carpometacarpal joint of the little finger. J Bone Joint Surg Am. 1974;56(7):1459-1463.
13. Tountas AA, Kwok JM. Isolated volar dislocation of the fifth carpometacarpal joint. Case report. Clin Orthop Relat Res. 1984;187:172-175.
Isolated dislocation of the carpometacarpal (CMC) joint of the hand is a rare injury. While the dislocation can be dorsal or palmar, dorsal dislocation is more common. Palmar dislocations can be either ulnopalmar or radiopalmar. There are very few reports of isolated radiopalmar dislocations of the fifth CMC joint in the English-language literature.1-3 We present a case of delayed presentation and management of radiopalmar dislocation of the fifth CMC joint. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 42-year-old man presented with polytrauma to our emergency department. He was stabilized initially, and open fractures were treated by débridement and external fixator application. During an examination 3 days after admission, swelling was noted in the right hand. On further study, there was splaying of the fifth digit and tenderness over the fourth and fifth CMC joints (Figure 1). No abnormal mobility or crepitus could be elicited. Plain radiographs of the right hand in anteroposterior and lateral views revealed radiopalmar dislocation of the fifth CMC joint (Figure 2). It was decided to reduce the dislocation immediately after the patient was declared fit for surgery.
Under axillary block, closed reduction was unsuccessful. Open reduction of the fifth CMC joint was performed through a dorsal incision. The base of the fifth metacarpal bone was found to be stripped of soft-tissue attachments and lying in a radiopalmar location. Reduction, which was checked under image intensifier, was found to be satisfactory (Figure 3). Reduction was stabilized by passing a smooth Kirschner wire (K-wire) from the fifth metacarpal to the hamate bone. After achieving hemostasis, the wound was closed in layers and a below-elbow splint was applied. The perioperative period was uneventful, and sutures were removed on postoperative day 10. The K-wire was removed after 4 weeks, and radiographs showed satisfactory position of the fifth CMC joint. Gentle active and passive mobilization of fingers and wrist were started. The patient had regained good function of the wrist and fingers 2 months after surgery (Figure 4).
Discussion
Carpometacarpal joint dislocations are uncommon injuries and account for less than 1% of hand injuries.4 They are classified as dorsal and volar (palmar) dislocations. Dorsal dislocations of the CMC joints occur more frequently than do volar dislocations, mainly affecting the fourth and fifth digits.5 Isolated volar or palmar dislocation of the fifth CMC joint is an uncommon injury that was first reported in 1918 by McWhorter.6 In 1968, Nalebuff7 classified the volar dislocations into 2 groups according to the direction of the displacement of the fifth metacarpal base: radiopalmar and ulnopalmar. Berg and Murphy8 found the hook of the hamate to deviate the metacarpal bone to either the ulnar or radial side. Tearing of all ligament and tendon attachments of the base of the fifth metacarpal results in radiopalmar dislocation.7 The attachments of ligaments and tendons remain intact in the ulnopalmar dislocation.7
The clinical features of this injury are pain and swelling about the base of the fifth metacarpal and axial deformity of the little finger with apparent shortening. The deep motor branch of the ulnar nerve lies volar to the fifth CMC joint as it courses around the hook of the hamate. It is vulnerable to injury in both dorsal9,10 and volar11 CMC dislocations. For radiologic evaluation, in addition to standard anteroposterior and lateral radiographs, a lateral view in 30º pronation of the hand can provide an improved view of the fifth CMC joint, as suggested by Bora and Didizian.12
The treatment of ulnopalmar dislocation has evolved. Ulnopalmar dislocations have been successfully treated by closed reduction without fixation,8 and by open reduction and K-wire fixation.3,7,13
Radiopalmar dislocations are inherently unstable because of the tearing of all ligament and tendon attachments of the base of the fifth metacarpal.7 In our case of radiopalmar dislocation, diagnosis was delayed and attempts at closed reduction were unsuccessful. Therefore, it was treated by open reduction and K-wire fixation. In our case, open reduction and K-wire fixation for radiopalmar dislocation of the fifth CMC joint provided promising results.
Conclusion
Radiopalmar dislocation of the fifth CMC joint is a rare injury, and very few cases have been reported in the English-language literature. We report one such case, which was successfully treated with open reduction and K-wire fixation.
Isolated dislocation of the carpometacarpal (CMC) joint of the hand is a rare injury. While the dislocation can be dorsal or palmar, dorsal dislocation is more common. Palmar dislocations can be either ulnopalmar or radiopalmar. There are very few reports of isolated radiopalmar dislocations of the fifth CMC joint in the English-language literature.1-3 We present a case of delayed presentation and management of radiopalmar dislocation of the fifth CMC joint. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 42-year-old man presented with polytrauma to our emergency department. He was stabilized initially, and open fractures were treated by débridement and external fixator application. During an examination 3 days after admission, swelling was noted in the right hand. On further study, there was splaying of the fifth digit and tenderness over the fourth and fifth CMC joints (Figure 1). No abnormal mobility or crepitus could be elicited. Plain radiographs of the right hand in anteroposterior and lateral views revealed radiopalmar dislocation of the fifth CMC joint (Figure 2). It was decided to reduce the dislocation immediately after the patient was declared fit for surgery.
Under axillary block, closed reduction was unsuccessful. Open reduction of the fifth CMC joint was performed through a dorsal incision. The base of the fifth metacarpal bone was found to be stripped of soft-tissue attachments and lying in a radiopalmar location. Reduction, which was checked under image intensifier, was found to be satisfactory (Figure 3). Reduction was stabilized by passing a smooth Kirschner wire (K-wire) from the fifth metacarpal to the hamate bone. After achieving hemostasis, the wound was closed in layers and a below-elbow splint was applied. The perioperative period was uneventful, and sutures were removed on postoperative day 10. The K-wire was removed after 4 weeks, and radiographs showed satisfactory position of the fifth CMC joint. Gentle active and passive mobilization of fingers and wrist were started. The patient had regained good function of the wrist and fingers 2 months after surgery (Figure 4).
Discussion
Carpometacarpal joint dislocations are uncommon injuries and account for less than 1% of hand injuries.4 They are classified as dorsal and volar (palmar) dislocations. Dorsal dislocations of the CMC joints occur more frequently than do volar dislocations, mainly affecting the fourth and fifth digits.5 Isolated volar or palmar dislocation of the fifth CMC joint is an uncommon injury that was first reported in 1918 by McWhorter.6 In 1968, Nalebuff7 classified the volar dislocations into 2 groups according to the direction of the displacement of the fifth metacarpal base: radiopalmar and ulnopalmar. Berg and Murphy8 found the hook of the hamate to deviate the metacarpal bone to either the ulnar or radial side. Tearing of all ligament and tendon attachments of the base of the fifth metacarpal results in radiopalmar dislocation.7 The attachments of ligaments and tendons remain intact in the ulnopalmar dislocation.7
The clinical features of this injury are pain and swelling about the base of the fifth metacarpal and axial deformity of the little finger with apparent shortening. The deep motor branch of the ulnar nerve lies volar to the fifth CMC joint as it courses around the hook of the hamate. It is vulnerable to injury in both dorsal9,10 and volar11 CMC dislocations. For radiologic evaluation, in addition to standard anteroposterior and lateral radiographs, a lateral view in 30º pronation of the hand can provide an improved view of the fifth CMC joint, as suggested by Bora and Didizian.12
The treatment of ulnopalmar dislocation has evolved. Ulnopalmar dislocations have been successfully treated by closed reduction without fixation,8 and by open reduction and K-wire fixation.3,7,13
Radiopalmar dislocations are inherently unstable because of the tearing of all ligament and tendon attachments of the base of the fifth metacarpal.7 In our case of radiopalmar dislocation, diagnosis was delayed and attempts at closed reduction were unsuccessful. Therefore, it was treated by open reduction and K-wire fixation. In our case, open reduction and K-wire fixation for radiopalmar dislocation of the fifth CMC joint provided promising results.
Conclusion
Radiopalmar dislocation of the fifth CMC joint is a rare injury, and very few cases have been reported in the English-language literature. We report one such case, which was successfully treated with open reduction and K-wire fixation.
1. Buzby BF. Palmar carpometacarpal dislocation of the fifth metacarpal. Ann Surg. 1934;100:555-557.
2. Chen VT. Dislocation of carpometacarpal joint of the little finger. J Hand Surg. 1987;12(2):260-263.
3. Dennyson WG, Stother IG. Carpometacarpal dislocation of the little finger. Hand. 1976;8(2):161-164.
4. Domingo A, Font L, Saz L, Arandes JM. Isolated radial palmar dislocation of the fifth carpometacarpal joint with ulnar neuropathy associated: successful treatment with closed reduction and internal fixation. Eur J Orthop Surg Traumatol. 19(2):101-107.
5. Fisher MR, Rogers LF, Hendrix RW. Systematic approach to identifying fourth and fifth carpometacarpal joint dislocations. AJR Am J Roentgenol. 1983;140(2):319-324.
6. McWhorter GL. Isolated and complete dislocation of the fifth carpometacarpal joint: open operation. Surg Clin Chic. 1918;2:793-796.
7. Nalebuff EA. Isolated anterior carpometacarpal dislocation of the fifth finger: classification and case report. J Trauma. 1968;8(6):1119-1123.
8. Berg EE, Murphy DF. Ulnopalmar dislocation of the fifth carpometacarpal joint – successful closed reduction: review of the literature and anatomic reevaluation. J Hand Surg Am. 1986;11(4):521-525.
9. Peterson P, Sacks S. Fracture-dislocation of the base of the fifth metacarpal associated with injury to the deep motor branch of the ulnar nerve: a case report. J Hand Surg Am. 1986;11(4):525-528.
10. Young TB. Dorsal dislocation of the metacarpal base of the little and ring fingers with ulnar nerve branch compression. Injury. 1987;18(1):65-66.
11. O’Rourke PJ, Quinlan W. Fracture dislocation of the fifth metacarpal resulting in compression of the deep branch of the ulnar nerve. J Hand Surg Br. 1993;18(2):190-191.
12. Bora FW Jr, Didizian NH. The treatment of injuries to the carpometacarpal joint of the little finger. J Bone Joint Surg Am. 1974;56(7):1459-1463.
13. Tountas AA, Kwok JM. Isolated volar dislocation of the fifth carpometacarpal joint. Case report. Clin Orthop Relat Res. 1984;187:172-175.
1. Buzby BF. Palmar carpometacarpal dislocation of the fifth metacarpal. Ann Surg. 1934;100:555-557.
2. Chen VT. Dislocation of carpometacarpal joint of the little finger. J Hand Surg. 1987;12(2):260-263.
3. Dennyson WG, Stother IG. Carpometacarpal dislocation of the little finger. Hand. 1976;8(2):161-164.
4. Domingo A, Font L, Saz L, Arandes JM. Isolated radial palmar dislocation of the fifth carpometacarpal joint with ulnar neuropathy associated: successful treatment with closed reduction and internal fixation. Eur J Orthop Surg Traumatol. 19(2):101-107.
5. Fisher MR, Rogers LF, Hendrix RW. Systematic approach to identifying fourth and fifth carpometacarpal joint dislocations. AJR Am J Roentgenol. 1983;140(2):319-324.
6. McWhorter GL. Isolated and complete dislocation of the fifth carpometacarpal joint: open operation. Surg Clin Chic. 1918;2:793-796.
7. Nalebuff EA. Isolated anterior carpometacarpal dislocation of the fifth finger: classification and case report. J Trauma. 1968;8(6):1119-1123.
8. Berg EE, Murphy DF. Ulnopalmar dislocation of the fifth carpometacarpal joint – successful closed reduction: review of the literature and anatomic reevaluation. J Hand Surg Am. 1986;11(4):521-525.
9. Peterson P, Sacks S. Fracture-dislocation of the base of the fifth metacarpal associated with injury to the deep motor branch of the ulnar nerve: a case report. J Hand Surg Am. 1986;11(4):525-528.
10. Young TB. Dorsal dislocation of the metacarpal base of the little and ring fingers with ulnar nerve branch compression. Injury. 1987;18(1):65-66.
11. O’Rourke PJ, Quinlan W. Fracture dislocation of the fifth metacarpal resulting in compression of the deep branch of the ulnar nerve. J Hand Surg Br. 1993;18(2):190-191.
12. Bora FW Jr, Didizian NH. The treatment of injuries to the carpometacarpal joint of the little finger. J Bone Joint Surg Am. 1974;56(7):1459-1463.
13. Tountas AA, Kwok JM. Isolated volar dislocation of the fifth carpometacarpal joint. Case report. Clin Orthop Relat Res. 1984;187:172-175.
Nonoperative Management of Multiple Hand Enchondromas in Ollier Disease With Progressive Ossification
Ollier disease, or multiple enchondromatosis, is a rare nonfamilial condition characterized by multiple cartilaginous tumors often beginning in early childhood. There is significant variation in disease distribution, location, size, number of lesions, and behavior, but the tumors are often located unilaterally.1 Enchondromas are most commonly found in the metacarpals, metatarsals, and phalanges, and develop from metaphyseal bone in close proximity to the physis. They frequently present as painless masses or are incidentally noted during the evaluation of another musculoskeletal condition. Radiographically, enchondromas of the hands and feet appear as oval radiolucencies with thinned, sclerotic rims. The lesions have varying degrees of mineralization and endosteal scalloping, and may expand the bone.2 Enchondromas usually enlarge until skeletal maturity and have been observed to ossify spontaneously.1,3 The clinical course of Ollier disease is variable, and a number of cases of significant hand deformity and malignant transformation have been reported.4-6
In this case report, we present a mild form of Ollier disease isolated to the patient’s left hand, which we followed for 8 years, demonstrating part of the natural history of these lesions. We discuss the patient’s clinical features, radiologic findings, diagnosis, treatment, prognosis, and follow-up, as well as review the literature. The patient and the patient’s family provided written informed consent for print and electronic publication of this case report.
Case Report
A 10-year-old, right-handed girl was referred to our department for the evaluation of left-hand masses. At age 3 years, the patient underwent a chondroma excision from the middle phalanx of her middle finger on her left hand. No operative or pathology report was available from this surgery, and the patient tolerated the procedure well without any complications. At the time of presentation, the masses did not cause any pain, motor or sensory dysfunction, or any systemic symptoms. No history of recent or distant trauma was elicited. The patient’s medical and family history was unremarkable.
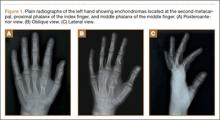
On physical examination, there was a firm, immobile, nontender palpable mass over the dorsal aspect of the distal second metacarpal bone of the left hand. The mass extended medially between the second and third metacarpals. A second small, firm, nontender left-hand mass was palpated over the volar aspect of her proximal phalanx on her index finger. She was neurovascularly intact with full active range of motion of the metacarpophalangeal and proximal and distal interphalangeal joints. There was no angular deformity of the digits. Plain radiographs taken at the time of initial presentation showed a 2.3×1.7-cm radiolucent lesion located in the metaphysis and diaphysis of the second metacarpal of the left hand (Figures 1A-1C). The lesion had varying degrees of mineralization with cortical thinning and expansion in the volar, dorsal, radial, and ulnar directions, consistent with a chondroid lesion. The second and third lesions were oval radiolucencies with sclerotic rims located at the metaphyseal-diaphyseal junction of the proximal phalanx of the index finger and middle phalanx of the middle finger, respectively. No fractures were identified in the radiographs, and the physes were open at this time. The patient was diagnosed with multiple enchondromatosis, or Ollier disease.
Our case showed 1 episode of pain and tenderness to palpation at the second proximal phalanx approximately 6 months after initial presentation. We attributed the pain and tenderness to a small pathologic fracture but did not see radiographic evidence of this. We elected to provide a trial of supportive measures, such as splinting and buddy taping, and to monitor the pain with a tentative plan of open biopsy with curettage and bone grafting if the pain persisted or evidence of fracture was seen on radiographs. The pain and tenderness to palpation resolved at a follow-up visit, and the surgery was deferred.
The patient was treated nonoperatively at initial presentation given the lack of significant cosmetic deformity or functional compromise and was advised close follow-up at 3 and 6 months. Given the absence of disease progression, annual checks (ie, clinical examination and radiographs) in a skeletally immature patient were decided on after consultation with the patient and parent. The family was educated about the possibility of pathologic fracture from minimal trauma to the hand versus the small risk of iatrogenic physeal injury with surgical curettage and bone grafting. No protective splinting was offered. A favorable prognosis and reassurance was provided to the patient and family, given the absence of symptoms, low suspicion and risk of malignant transformation, and stability of the lesion. Serial radiographs showed gradual increases in the lesions’ sizes but were consistent with the stable growth of the metacarpal and phalanges. With the patient nearing skeletal maturity, no pathologic fractures were identified on radiography during follow-up, and the risks of surgery lessened with growth; however, the continued absence of symptoms led to the mutual decision to continue observation.
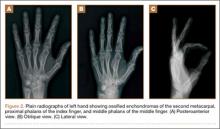
Nearly 8 years after initial presentation, plain radiographs showed closed physes and partially ossified bone masses (Figures 2A-2C). The metacarpal lesion measured 3.2×1.5 cm, and the cortex appeared thickened and regular. The proximal phalanx lesion had a thickened cortex without periosteal reaction, and the middle phalanx lesion appeared to be completely healed. The patient has been asymptomatic for many years, and she has retained complete function of her left hand without any growth retardation, angular deformity, or pathologic fracture. A small but potential risk of malignant transformation was discussed with the patient and her family, as was the need for lifetime follow-up. We intend to follow the enchondromas clinically and radiographically every 2 years and obtain new radiographs if the mass presents with new clinical findings, such as enlargement or pain, for surveillance of tumor transformation. If the patient desired or symptoms developed, curettage and bone grafting would be offered, and the surgical tissue would be sent for pathologic analysis. A bone scan that was obtained at the request of the patient, when she was 21 years old, showed no other sites of disease besides the fingers.
Discussion
Multiple enchondromatosis was first described by Ollier at the turn of the 19th century and has been estimated to affect one in every 100,000 persons.1 The low prevalence and variable manifestations of Ollier disease lead clinicians to handle the disease and its complications, namely skeletal deformity and malignant transformation, on a case-by-case approach. Additionally, the prognosis of Ollier disease with malignant transformation is quite variable, with studies reporting the estimated incidence as 5% to 50%.7 Muramatsu and colleagues6 reported that the occurrence of malignant transformation of multiple enchondromas limited to the bones of the hand was extremely rare, with only 12 cases of malignant transformation. Enchondromas of the pelvis, scapula, and long bones of the extremities have increased risks and rates of secondary transformation to chondrosarcoma.8
A recent large European multicenter retrospective study investigating the clinical characteristics and behavior of enchondromas in 144 patients with Ollier disease has provided new information regarding this rare disease.7 Verdegaal and colleagues7 divided patients into 3 categories depending on their distribution of enchondromas. The development of chondrosarcoma was notably different between individuals with enchondromas limited to the small bones of the hands and feet (15%, group I) versus individuals with enchondromas limited to the long bones and flat bones (43%, group II) or individuals with enchondromas of the short, long, and flat bones (46%, group III).7 The only location found to be statistically significant for the development of chondrosarcoma was the pelvis.
The clinical findings associated with risk of malignant transformation of enchondromas are increasing size of the lesion and onset of pain and tenderness. Dahlin and Salvador9 reported that only 60% of patients with chondrosarcoma of the hand experience pain. The absence of pain may lead to a delay in patient presentation to the clinician.5,6 Radiographic findings of malignant transformation include the classic features of temporal increases in the lesion’s size after skeletal maturity and cortical destruction associated with soft-tissue invasion. However, both findings are nonspecific for differentiating enchondromas from grade 1 chondrosarcomas as described by Geirnaerdt and colleagues.10
Sassoon and colleagues11 reported on a series of hand enchondromas treated operatively. Subgroup analysis between pathologic fractures treated primarily or in delayed fashion showed similar outcomes for achieving full motion and similar number of complications; however, they noted that the delayed group required 7 more weeks of immobilization. Additionally, review of the whole series showed 1 episode of metacarpal shortening and 1 occurrence of angular malalignment. In our patient, we were concerned about introducing an iatrogenic cosmetic deformity, and we believed a pathologic fracture could be managed expectantly. Overall, patients without pathologic fracture treated surgically experienced a complication rate of 12%, whereas patients with a fracture had a complication rate of 20%.11 The majority of patients with multiple enchondromatosis treated with surgical curettage and grafting had successful outcomes, with 86% of patients regaining full motion, but the recurrence rate was 21%.11 Patients with expansile lesions regained less motion than patients with nonexpansile lesions. There was a single lesion believed preoperatively to be an enchondroma, but it underwent malignant transformation, as confirmed on intraoperative pathology. This patient had Maffucci syndrome and was treated with an amputation through the metacarpophalangeal joint.
There are 3 options for treating hand enchondromas: observation, curettage alone, or curettage with bone grafting. There is no consensus about conservative management, timing of intervention, or risk of pathologic fracture. Each patient is treated individually with attention to reason for presentation, number of lesions, associated pain, deformity, or pathologic fracture. Operative criteria include high risk of pathologic fracture based on location of enchondroma, cortical thinning, and previous pathologic fracture with resulting angular deformity. Nonoperative management may increase the risk of pathologic fracture, particularly in patients involved in aggressive contact sports, but the physician may offer protective splinting or counsel the patient on activity modification. Our case provides a study of the natural history of multiple enchondromatosis and shows mild increases in the lesions’ sizes during the 8-year follow-up. This was an expected finding given the patient’s immature skeleton. The lesions’ cortices continued to ossify after the physes closed and now provides an excellent comparison for the identification of future malignant changes.
Histologic analysis of biopsied or surgically treated lesions contributes to the differentiation between benign hand enchondromas and chondrosarcoma. Pathologic findings must be correlated with clinical and radiographic findings because hand enchondromas contain cytologic features of chondrosarcoma.12 In a series of 55 patients with chondrosarcoma, Liu and colleagues8 reported no cases from the hand. Verdegaal and colleagues7 reported a total of 13 chondrosarcomas in the metacarpals and hand phalanges in 97 group I and III patients. Five of these lesions were grade 1, 2 were grade 2, 1 was grade 3, and 5 lesions were unknown.
For patients with multiple enchondromatosis limited to the hands, prognosis is relatively good with respect to risk of secondary chondrosarcoma transformation, metastasis of secondary chondrosarcoma, and death. Verdegaal and colleagues7 reported the rate of secondary transformation in the hand to be 15%. Patil and colleagues13 reported no distant metastases in 23 patients with hand chondrosarcoma at mean follow-up of 8.5 years (range, 2-19 years), although none of their patients had Ollier disease. Verdegaal and colleagues7 reported 7 of the 8 deaths in their study were related to development of pulmonary metastases; however, none originated from chondrosarcomas in the hand. Additionally, there were no disease-related deaths in 29 group I patients. Herget and colleagues,14 in summarizing the literature, postulated that the overall survival rate of patients with secondary chondrosarcoma at 5 years is approximately 90%.
In our case, the patient, who had 3 enchondromas isolated to the left hand, can be categorized in group I. Thus, this case highlights the natural history of a patient with hand enchondromas and demonstrates that enchondromatosis of the short tubular bones of the hands can mature and ossify.
1. Silve C, Jüppner H. Ollier disease. Orphanet J Rare Dis. 2006;1:37-42.
2. Baert A. Encyclopedia of Diagnostic Imaging. Vol. 1. Berlin, Germany: Springer; 2008.
3. Takigawa K. Chondroma of the bones of the hand. A review of 110 cases. J Bone Joint Surg Am. 1971;53(8):1591-1600.
4. Mosher J. Multiple enchondromatosis of the hand. A case report. J Bone Joint Surg Am. 1976;58(5):717-719.
5. Goto T, Motoi T, Komiya K, et al. Chondrosarcoma of the hand secondary to multiple enchondromatosis; report of two cases. Arch Orthop Trauma Surg. 2003;123(1):42-47.
6. Muramatsu K, Kawakami Y, Tani Y, Taguchi T. Malignant transformation of multiple enchondromas in the hand: case report. J Hand Surg Am. 2011;36(2):304-307.
7. Verdegaal SH, Bovee JV, Pansuriya TC, et al. Incidence, predictive factors, and prognosis of chondrosarcoma in patients with Ollier disease and Maffucci syndrome: an international multicenter study of 161 patients. Oncologist. 2011;16(12):1771-1779.
8. Liu J, Hudkins PG, Swee RG, Unni KK. Bone sarcomas associated with Ollier’s disease. Cancer. 1987;59(7):1376-1385.
9. Dahlin D, Salvador AH. Chondrosarcomas of bones of the hands and feet—a study of 30 cases. Cancer. 1974;34(3):755-760.
10. Geirnaerdt MJ, Hermans J, Bloem JL, et al. Usefulness of radiography in differentiating enchondroma from central grade I chondrosarcoma. AJR Am J Roentgenol. 1997;169(4):1097-1104.
11. Sassoon AA, Fitz-Gibbon PD, Harmsen WS, Moran SL. Enchondromas of the hand: factors affecting recurrence, healing, motion, and malignant transformation. J Hand Surg Am. 2012;37(6):1229-1234.
12. Ogose A, Unni KK, Swee R, May GK, Rowland CM, Sim FH. Chondrosarcoma of small bones of the hands and feet. Cancer. 1997;80(1):50-59.
13. Patil S, de Silva MV, Crossan J, Reid R. Chondrosarcoma of small bones of the hand. J Hand Surg Br. 2003;28(6):602-608.
14. Herget GW, Strohm P, Rottenburger C, et al. Insights in Enchondroma, Enchondromatosis and the risk of secondary Chondrosarcoma. Review of the literature with an emphasis on the clinical behaviour, radiology, malignant transformation and the follow up. Neoplasma. 2014;61(4):365-378.
Ollier disease, or multiple enchondromatosis, is a rare nonfamilial condition characterized by multiple cartilaginous tumors often beginning in early childhood. There is significant variation in disease distribution, location, size, number of lesions, and behavior, but the tumors are often located unilaterally.1 Enchondromas are most commonly found in the metacarpals, metatarsals, and phalanges, and develop from metaphyseal bone in close proximity to the physis. They frequently present as painless masses or are incidentally noted during the evaluation of another musculoskeletal condition. Radiographically, enchondromas of the hands and feet appear as oval radiolucencies with thinned, sclerotic rims. The lesions have varying degrees of mineralization and endosteal scalloping, and may expand the bone.2 Enchondromas usually enlarge until skeletal maturity and have been observed to ossify spontaneously.1,3 The clinical course of Ollier disease is variable, and a number of cases of significant hand deformity and malignant transformation have been reported.4-6
In this case report, we present a mild form of Ollier disease isolated to the patient’s left hand, which we followed for 8 years, demonstrating part of the natural history of these lesions. We discuss the patient’s clinical features, radiologic findings, diagnosis, treatment, prognosis, and follow-up, as well as review the literature. The patient and the patient’s family provided written informed consent for print and electronic publication of this case report.
Case Report
A 10-year-old, right-handed girl was referred to our department for the evaluation of left-hand masses. At age 3 years, the patient underwent a chondroma excision from the middle phalanx of her middle finger on her left hand. No operative or pathology report was available from this surgery, and the patient tolerated the procedure well without any complications. At the time of presentation, the masses did not cause any pain, motor or sensory dysfunction, or any systemic symptoms. No history of recent or distant trauma was elicited. The patient’s medical and family history was unremarkable.
On physical examination, there was a firm, immobile, nontender palpable mass over the dorsal aspect of the distal second metacarpal bone of the left hand. The mass extended medially between the second and third metacarpals. A second small, firm, nontender left-hand mass was palpated over the volar aspect of her proximal phalanx on her index finger. She was neurovascularly intact with full active range of motion of the metacarpophalangeal and proximal and distal interphalangeal joints. There was no angular deformity of the digits. Plain radiographs taken at the time of initial presentation showed a 2.3×1.7-cm radiolucent lesion located in the metaphysis and diaphysis of the second metacarpal of the left hand (Figures 1A-1C). The lesion had varying degrees of mineralization with cortical thinning and expansion in the volar, dorsal, radial, and ulnar directions, consistent with a chondroid lesion. The second and third lesions were oval radiolucencies with sclerotic rims located at the metaphyseal-diaphyseal junction of the proximal phalanx of the index finger and middle phalanx of the middle finger, respectively. No fractures were identified in the radiographs, and the physes were open at this time. The patient was diagnosed with multiple enchondromatosis, or Ollier disease.
Our case showed 1 episode of pain and tenderness to palpation at the second proximal phalanx approximately 6 months after initial presentation. We attributed the pain and tenderness to a small pathologic fracture but did not see radiographic evidence of this. We elected to provide a trial of supportive measures, such as splinting and buddy taping, and to monitor the pain with a tentative plan of open biopsy with curettage and bone grafting if the pain persisted or evidence of fracture was seen on radiographs. The pain and tenderness to palpation resolved at a follow-up visit, and the surgery was deferred.
The patient was treated nonoperatively at initial presentation given the lack of significant cosmetic deformity or functional compromise and was advised close follow-up at 3 and 6 months. Given the absence of disease progression, annual checks (ie, clinical examination and radiographs) in a skeletally immature patient were decided on after consultation with the patient and parent. The family was educated about the possibility of pathologic fracture from minimal trauma to the hand versus the small risk of iatrogenic physeal injury with surgical curettage and bone grafting. No protective splinting was offered. A favorable prognosis and reassurance was provided to the patient and family, given the absence of symptoms, low suspicion and risk of malignant transformation, and stability of the lesion. Serial radiographs showed gradual increases in the lesions’ sizes but were consistent with the stable growth of the metacarpal and phalanges. With the patient nearing skeletal maturity, no pathologic fractures were identified on radiography during follow-up, and the risks of surgery lessened with growth; however, the continued absence of symptoms led to the mutual decision to continue observation.
Nearly 8 years after initial presentation, plain radiographs showed closed physes and partially ossified bone masses (Figures 2A-2C). The metacarpal lesion measured 3.2×1.5 cm, and the cortex appeared thickened and regular. The proximal phalanx lesion had a thickened cortex without periosteal reaction, and the middle phalanx lesion appeared to be completely healed. The patient has been asymptomatic for many years, and she has retained complete function of her left hand without any growth retardation, angular deformity, or pathologic fracture. A small but potential risk of malignant transformation was discussed with the patient and her family, as was the need for lifetime follow-up. We intend to follow the enchondromas clinically and radiographically every 2 years and obtain new radiographs if the mass presents with new clinical findings, such as enlargement or pain, for surveillance of tumor transformation. If the patient desired or symptoms developed, curettage and bone grafting would be offered, and the surgical tissue would be sent for pathologic analysis. A bone scan that was obtained at the request of the patient, when she was 21 years old, showed no other sites of disease besides the fingers.
Discussion
Multiple enchondromatosis was first described by Ollier at the turn of the 19th century and has been estimated to affect one in every 100,000 persons.1 The low prevalence and variable manifestations of Ollier disease lead clinicians to handle the disease and its complications, namely skeletal deformity and malignant transformation, on a case-by-case approach. Additionally, the prognosis of Ollier disease with malignant transformation is quite variable, with studies reporting the estimated incidence as 5% to 50%.7 Muramatsu and colleagues6 reported that the occurrence of malignant transformation of multiple enchondromas limited to the bones of the hand was extremely rare, with only 12 cases of malignant transformation. Enchondromas of the pelvis, scapula, and long bones of the extremities have increased risks and rates of secondary transformation to chondrosarcoma.8
A recent large European multicenter retrospective study investigating the clinical characteristics and behavior of enchondromas in 144 patients with Ollier disease has provided new information regarding this rare disease.7 Verdegaal and colleagues7 divided patients into 3 categories depending on their distribution of enchondromas. The development of chondrosarcoma was notably different between individuals with enchondromas limited to the small bones of the hands and feet (15%, group I) versus individuals with enchondromas limited to the long bones and flat bones (43%, group II) or individuals with enchondromas of the short, long, and flat bones (46%, group III).7 The only location found to be statistically significant for the development of chondrosarcoma was the pelvis.
The clinical findings associated with risk of malignant transformation of enchondromas are increasing size of the lesion and onset of pain and tenderness. Dahlin and Salvador9 reported that only 60% of patients with chondrosarcoma of the hand experience pain. The absence of pain may lead to a delay in patient presentation to the clinician.5,6 Radiographic findings of malignant transformation include the classic features of temporal increases in the lesion’s size after skeletal maturity and cortical destruction associated with soft-tissue invasion. However, both findings are nonspecific for differentiating enchondromas from grade 1 chondrosarcomas as described by Geirnaerdt and colleagues.10
Sassoon and colleagues11 reported on a series of hand enchondromas treated operatively. Subgroup analysis between pathologic fractures treated primarily or in delayed fashion showed similar outcomes for achieving full motion and similar number of complications; however, they noted that the delayed group required 7 more weeks of immobilization. Additionally, review of the whole series showed 1 episode of metacarpal shortening and 1 occurrence of angular malalignment. In our patient, we were concerned about introducing an iatrogenic cosmetic deformity, and we believed a pathologic fracture could be managed expectantly. Overall, patients without pathologic fracture treated surgically experienced a complication rate of 12%, whereas patients with a fracture had a complication rate of 20%.11 The majority of patients with multiple enchondromatosis treated with surgical curettage and grafting had successful outcomes, with 86% of patients regaining full motion, but the recurrence rate was 21%.11 Patients with expansile lesions regained less motion than patients with nonexpansile lesions. There was a single lesion believed preoperatively to be an enchondroma, but it underwent malignant transformation, as confirmed on intraoperative pathology. This patient had Maffucci syndrome and was treated with an amputation through the metacarpophalangeal joint.
There are 3 options for treating hand enchondromas: observation, curettage alone, or curettage with bone grafting. There is no consensus about conservative management, timing of intervention, or risk of pathologic fracture. Each patient is treated individually with attention to reason for presentation, number of lesions, associated pain, deformity, or pathologic fracture. Operative criteria include high risk of pathologic fracture based on location of enchondroma, cortical thinning, and previous pathologic fracture with resulting angular deformity. Nonoperative management may increase the risk of pathologic fracture, particularly in patients involved in aggressive contact sports, but the physician may offer protective splinting or counsel the patient on activity modification. Our case provides a study of the natural history of multiple enchondromatosis and shows mild increases in the lesions’ sizes during the 8-year follow-up. This was an expected finding given the patient’s immature skeleton. The lesions’ cortices continued to ossify after the physes closed and now provides an excellent comparison for the identification of future malignant changes.
Histologic analysis of biopsied or surgically treated lesions contributes to the differentiation between benign hand enchondromas and chondrosarcoma. Pathologic findings must be correlated with clinical and radiographic findings because hand enchondromas contain cytologic features of chondrosarcoma.12 In a series of 55 patients with chondrosarcoma, Liu and colleagues8 reported no cases from the hand. Verdegaal and colleagues7 reported a total of 13 chondrosarcomas in the metacarpals and hand phalanges in 97 group I and III patients. Five of these lesions were grade 1, 2 were grade 2, 1 was grade 3, and 5 lesions were unknown.
For patients with multiple enchondromatosis limited to the hands, prognosis is relatively good with respect to risk of secondary chondrosarcoma transformation, metastasis of secondary chondrosarcoma, and death. Verdegaal and colleagues7 reported the rate of secondary transformation in the hand to be 15%. Patil and colleagues13 reported no distant metastases in 23 patients with hand chondrosarcoma at mean follow-up of 8.5 years (range, 2-19 years), although none of their patients had Ollier disease. Verdegaal and colleagues7 reported 7 of the 8 deaths in their study were related to development of pulmonary metastases; however, none originated from chondrosarcomas in the hand. Additionally, there were no disease-related deaths in 29 group I patients. Herget and colleagues,14 in summarizing the literature, postulated that the overall survival rate of patients with secondary chondrosarcoma at 5 years is approximately 90%.
In our case, the patient, who had 3 enchondromas isolated to the left hand, can be categorized in group I. Thus, this case highlights the natural history of a patient with hand enchondromas and demonstrates that enchondromatosis of the short tubular bones of the hands can mature and ossify.
Ollier disease, or multiple enchondromatosis, is a rare nonfamilial condition characterized by multiple cartilaginous tumors often beginning in early childhood. There is significant variation in disease distribution, location, size, number of lesions, and behavior, but the tumors are often located unilaterally.1 Enchondromas are most commonly found in the metacarpals, metatarsals, and phalanges, and develop from metaphyseal bone in close proximity to the physis. They frequently present as painless masses or are incidentally noted during the evaluation of another musculoskeletal condition. Radiographically, enchondromas of the hands and feet appear as oval radiolucencies with thinned, sclerotic rims. The lesions have varying degrees of mineralization and endosteal scalloping, and may expand the bone.2 Enchondromas usually enlarge until skeletal maturity and have been observed to ossify spontaneously.1,3 The clinical course of Ollier disease is variable, and a number of cases of significant hand deformity and malignant transformation have been reported.4-6
In this case report, we present a mild form of Ollier disease isolated to the patient’s left hand, which we followed for 8 years, demonstrating part of the natural history of these lesions. We discuss the patient’s clinical features, radiologic findings, diagnosis, treatment, prognosis, and follow-up, as well as review the literature. The patient and the patient’s family provided written informed consent for print and electronic publication of this case report.
Case Report
A 10-year-old, right-handed girl was referred to our department for the evaluation of left-hand masses. At age 3 years, the patient underwent a chondroma excision from the middle phalanx of her middle finger on her left hand. No operative or pathology report was available from this surgery, and the patient tolerated the procedure well without any complications. At the time of presentation, the masses did not cause any pain, motor or sensory dysfunction, or any systemic symptoms. No history of recent or distant trauma was elicited. The patient’s medical and family history was unremarkable.
On physical examination, there was a firm, immobile, nontender palpable mass over the dorsal aspect of the distal second metacarpal bone of the left hand. The mass extended medially between the second and third metacarpals. A second small, firm, nontender left-hand mass was palpated over the volar aspect of her proximal phalanx on her index finger. She was neurovascularly intact with full active range of motion of the metacarpophalangeal and proximal and distal interphalangeal joints. There was no angular deformity of the digits. Plain radiographs taken at the time of initial presentation showed a 2.3×1.7-cm radiolucent lesion located in the metaphysis and diaphysis of the second metacarpal of the left hand (Figures 1A-1C). The lesion had varying degrees of mineralization with cortical thinning and expansion in the volar, dorsal, radial, and ulnar directions, consistent with a chondroid lesion. The second and third lesions were oval radiolucencies with sclerotic rims located at the metaphyseal-diaphyseal junction of the proximal phalanx of the index finger and middle phalanx of the middle finger, respectively. No fractures were identified in the radiographs, and the physes were open at this time. The patient was diagnosed with multiple enchondromatosis, or Ollier disease.
Our case showed 1 episode of pain and tenderness to palpation at the second proximal phalanx approximately 6 months after initial presentation. We attributed the pain and tenderness to a small pathologic fracture but did not see radiographic evidence of this. We elected to provide a trial of supportive measures, such as splinting and buddy taping, and to monitor the pain with a tentative plan of open biopsy with curettage and bone grafting if the pain persisted or evidence of fracture was seen on radiographs. The pain and tenderness to palpation resolved at a follow-up visit, and the surgery was deferred.
The patient was treated nonoperatively at initial presentation given the lack of significant cosmetic deformity or functional compromise and was advised close follow-up at 3 and 6 months. Given the absence of disease progression, annual checks (ie, clinical examination and radiographs) in a skeletally immature patient were decided on after consultation with the patient and parent. The family was educated about the possibility of pathologic fracture from minimal trauma to the hand versus the small risk of iatrogenic physeal injury with surgical curettage and bone grafting. No protective splinting was offered. A favorable prognosis and reassurance was provided to the patient and family, given the absence of symptoms, low suspicion and risk of malignant transformation, and stability of the lesion. Serial radiographs showed gradual increases in the lesions’ sizes but were consistent with the stable growth of the metacarpal and phalanges. With the patient nearing skeletal maturity, no pathologic fractures were identified on radiography during follow-up, and the risks of surgery lessened with growth; however, the continued absence of symptoms led to the mutual decision to continue observation.
Nearly 8 years after initial presentation, plain radiographs showed closed physes and partially ossified bone masses (Figures 2A-2C). The metacarpal lesion measured 3.2×1.5 cm, and the cortex appeared thickened and regular. The proximal phalanx lesion had a thickened cortex without periosteal reaction, and the middle phalanx lesion appeared to be completely healed. The patient has been asymptomatic for many years, and she has retained complete function of her left hand without any growth retardation, angular deformity, or pathologic fracture. A small but potential risk of malignant transformation was discussed with the patient and her family, as was the need for lifetime follow-up. We intend to follow the enchondromas clinically and radiographically every 2 years and obtain new radiographs if the mass presents with new clinical findings, such as enlargement or pain, for surveillance of tumor transformation. If the patient desired or symptoms developed, curettage and bone grafting would be offered, and the surgical tissue would be sent for pathologic analysis. A bone scan that was obtained at the request of the patient, when she was 21 years old, showed no other sites of disease besides the fingers.
Discussion
Multiple enchondromatosis was first described by Ollier at the turn of the 19th century and has been estimated to affect one in every 100,000 persons.1 The low prevalence and variable manifestations of Ollier disease lead clinicians to handle the disease and its complications, namely skeletal deformity and malignant transformation, on a case-by-case approach. Additionally, the prognosis of Ollier disease with malignant transformation is quite variable, with studies reporting the estimated incidence as 5% to 50%.7 Muramatsu and colleagues6 reported that the occurrence of malignant transformation of multiple enchondromas limited to the bones of the hand was extremely rare, with only 12 cases of malignant transformation. Enchondromas of the pelvis, scapula, and long bones of the extremities have increased risks and rates of secondary transformation to chondrosarcoma.8
A recent large European multicenter retrospective study investigating the clinical characteristics and behavior of enchondromas in 144 patients with Ollier disease has provided new information regarding this rare disease.7 Verdegaal and colleagues7 divided patients into 3 categories depending on their distribution of enchondromas. The development of chondrosarcoma was notably different between individuals with enchondromas limited to the small bones of the hands and feet (15%, group I) versus individuals with enchondromas limited to the long bones and flat bones (43%, group II) or individuals with enchondromas of the short, long, and flat bones (46%, group III).7 The only location found to be statistically significant for the development of chondrosarcoma was the pelvis.
The clinical findings associated with risk of malignant transformation of enchondromas are increasing size of the lesion and onset of pain and tenderness. Dahlin and Salvador9 reported that only 60% of patients with chondrosarcoma of the hand experience pain. The absence of pain may lead to a delay in patient presentation to the clinician.5,6 Radiographic findings of malignant transformation include the classic features of temporal increases in the lesion’s size after skeletal maturity and cortical destruction associated with soft-tissue invasion. However, both findings are nonspecific for differentiating enchondromas from grade 1 chondrosarcomas as described by Geirnaerdt and colleagues.10
Sassoon and colleagues11 reported on a series of hand enchondromas treated operatively. Subgroup analysis between pathologic fractures treated primarily or in delayed fashion showed similar outcomes for achieving full motion and similar number of complications; however, they noted that the delayed group required 7 more weeks of immobilization. Additionally, review of the whole series showed 1 episode of metacarpal shortening and 1 occurrence of angular malalignment. In our patient, we were concerned about introducing an iatrogenic cosmetic deformity, and we believed a pathologic fracture could be managed expectantly. Overall, patients without pathologic fracture treated surgically experienced a complication rate of 12%, whereas patients with a fracture had a complication rate of 20%.11 The majority of patients with multiple enchondromatosis treated with surgical curettage and grafting had successful outcomes, with 86% of patients regaining full motion, but the recurrence rate was 21%.11 Patients with expansile lesions regained less motion than patients with nonexpansile lesions. There was a single lesion believed preoperatively to be an enchondroma, but it underwent malignant transformation, as confirmed on intraoperative pathology. This patient had Maffucci syndrome and was treated with an amputation through the metacarpophalangeal joint.
There are 3 options for treating hand enchondromas: observation, curettage alone, or curettage with bone grafting. There is no consensus about conservative management, timing of intervention, or risk of pathologic fracture. Each patient is treated individually with attention to reason for presentation, number of lesions, associated pain, deformity, or pathologic fracture. Operative criteria include high risk of pathologic fracture based on location of enchondroma, cortical thinning, and previous pathologic fracture with resulting angular deformity. Nonoperative management may increase the risk of pathologic fracture, particularly in patients involved in aggressive contact sports, but the physician may offer protective splinting or counsel the patient on activity modification. Our case provides a study of the natural history of multiple enchondromatosis and shows mild increases in the lesions’ sizes during the 8-year follow-up. This was an expected finding given the patient’s immature skeleton. The lesions’ cortices continued to ossify after the physes closed and now provides an excellent comparison for the identification of future malignant changes.
Histologic analysis of biopsied or surgically treated lesions contributes to the differentiation between benign hand enchondromas and chondrosarcoma. Pathologic findings must be correlated with clinical and radiographic findings because hand enchondromas contain cytologic features of chondrosarcoma.12 In a series of 55 patients with chondrosarcoma, Liu and colleagues8 reported no cases from the hand. Verdegaal and colleagues7 reported a total of 13 chondrosarcomas in the metacarpals and hand phalanges in 97 group I and III patients. Five of these lesions were grade 1, 2 were grade 2, 1 was grade 3, and 5 lesions were unknown.
For patients with multiple enchondromatosis limited to the hands, prognosis is relatively good with respect to risk of secondary chondrosarcoma transformation, metastasis of secondary chondrosarcoma, and death. Verdegaal and colleagues7 reported the rate of secondary transformation in the hand to be 15%. Patil and colleagues13 reported no distant metastases in 23 patients with hand chondrosarcoma at mean follow-up of 8.5 years (range, 2-19 years), although none of their patients had Ollier disease. Verdegaal and colleagues7 reported 7 of the 8 deaths in their study were related to development of pulmonary metastases; however, none originated from chondrosarcomas in the hand. Additionally, there were no disease-related deaths in 29 group I patients. Herget and colleagues,14 in summarizing the literature, postulated that the overall survival rate of patients with secondary chondrosarcoma at 5 years is approximately 90%.
In our case, the patient, who had 3 enchondromas isolated to the left hand, can be categorized in group I. Thus, this case highlights the natural history of a patient with hand enchondromas and demonstrates that enchondromatosis of the short tubular bones of the hands can mature and ossify.
1. Silve C, Jüppner H. Ollier disease. Orphanet J Rare Dis. 2006;1:37-42.
2. Baert A. Encyclopedia of Diagnostic Imaging. Vol. 1. Berlin, Germany: Springer; 2008.
3. Takigawa K. Chondroma of the bones of the hand. A review of 110 cases. J Bone Joint Surg Am. 1971;53(8):1591-1600.
4. Mosher J. Multiple enchondromatosis of the hand. A case report. J Bone Joint Surg Am. 1976;58(5):717-719.
5. Goto T, Motoi T, Komiya K, et al. Chondrosarcoma of the hand secondary to multiple enchondromatosis; report of two cases. Arch Orthop Trauma Surg. 2003;123(1):42-47.
6. Muramatsu K, Kawakami Y, Tani Y, Taguchi T. Malignant transformation of multiple enchondromas in the hand: case report. J Hand Surg Am. 2011;36(2):304-307.
7. Verdegaal SH, Bovee JV, Pansuriya TC, et al. Incidence, predictive factors, and prognosis of chondrosarcoma in patients with Ollier disease and Maffucci syndrome: an international multicenter study of 161 patients. Oncologist. 2011;16(12):1771-1779.
8. Liu J, Hudkins PG, Swee RG, Unni KK. Bone sarcomas associated with Ollier’s disease. Cancer. 1987;59(7):1376-1385.
9. Dahlin D, Salvador AH. Chondrosarcomas of bones of the hands and feet—a study of 30 cases. Cancer. 1974;34(3):755-760.
10. Geirnaerdt MJ, Hermans J, Bloem JL, et al. Usefulness of radiography in differentiating enchondroma from central grade I chondrosarcoma. AJR Am J Roentgenol. 1997;169(4):1097-1104.
11. Sassoon AA, Fitz-Gibbon PD, Harmsen WS, Moran SL. Enchondromas of the hand: factors affecting recurrence, healing, motion, and malignant transformation. J Hand Surg Am. 2012;37(6):1229-1234.
12. Ogose A, Unni KK, Swee R, May GK, Rowland CM, Sim FH. Chondrosarcoma of small bones of the hands and feet. Cancer. 1997;80(1):50-59.
13. Patil S, de Silva MV, Crossan J, Reid R. Chondrosarcoma of small bones of the hand. J Hand Surg Br. 2003;28(6):602-608.
14. Herget GW, Strohm P, Rottenburger C, et al. Insights in Enchondroma, Enchondromatosis and the risk of secondary Chondrosarcoma. Review of the literature with an emphasis on the clinical behaviour, radiology, malignant transformation and the follow up. Neoplasma. 2014;61(4):365-378.
1. Silve C, Jüppner H. Ollier disease. Orphanet J Rare Dis. 2006;1:37-42.
2. Baert A. Encyclopedia of Diagnostic Imaging. Vol. 1. Berlin, Germany: Springer; 2008.
3. Takigawa K. Chondroma of the bones of the hand. A review of 110 cases. J Bone Joint Surg Am. 1971;53(8):1591-1600.
4. Mosher J. Multiple enchondromatosis of the hand. A case report. J Bone Joint Surg Am. 1976;58(5):717-719.
5. Goto T, Motoi T, Komiya K, et al. Chondrosarcoma of the hand secondary to multiple enchondromatosis; report of two cases. Arch Orthop Trauma Surg. 2003;123(1):42-47.
6. Muramatsu K, Kawakami Y, Tani Y, Taguchi T. Malignant transformation of multiple enchondromas in the hand: case report. J Hand Surg Am. 2011;36(2):304-307.
7. Verdegaal SH, Bovee JV, Pansuriya TC, et al. Incidence, predictive factors, and prognosis of chondrosarcoma in patients with Ollier disease and Maffucci syndrome: an international multicenter study of 161 patients. Oncologist. 2011;16(12):1771-1779.
8. Liu J, Hudkins PG, Swee RG, Unni KK. Bone sarcomas associated with Ollier’s disease. Cancer. 1987;59(7):1376-1385.
9. Dahlin D, Salvador AH. Chondrosarcomas of bones of the hands and feet—a study of 30 cases. Cancer. 1974;34(3):755-760.
10. Geirnaerdt MJ, Hermans J, Bloem JL, et al. Usefulness of radiography in differentiating enchondroma from central grade I chondrosarcoma. AJR Am J Roentgenol. 1997;169(4):1097-1104.
11. Sassoon AA, Fitz-Gibbon PD, Harmsen WS, Moran SL. Enchondromas of the hand: factors affecting recurrence, healing, motion, and malignant transformation. J Hand Surg Am. 2012;37(6):1229-1234.
12. Ogose A, Unni KK, Swee R, May GK, Rowland CM, Sim FH. Chondrosarcoma of small bones of the hands and feet. Cancer. 1997;80(1):50-59.
13. Patil S, de Silva MV, Crossan J, Reid R. Chondrosarcoma of small bones of the hand. J Hand Surg Br. 2003;28(6):602-608.
14. Herget GW, Strohm P, Rottenburger C, et al. Insights in Enchondroma, Enchondromatosis and the risk of secondary Chondrosarcoma. Review of the literature with an emphasis on the clinical behaviour, radiology, malignant transformation and the follow up. Neoplasma. 2014;61(4):365-378.
Midterm Follow-Up of Metal-Backed Glenoid Components in Anatomical Total Shoulder Arthroplasties
Total shoulder arthroplasty (TSA) is being performed with increasing frequency. According to recent data, the number of TSAs performed annually increased 2.5-fold from 2000 to 2008.1 As more are performed, the need for improved implant survival will increase as well. In particular, advances in glenoid survivorship will be a primary focus. Previous experience has demonstrated that the glenoid component is the most common source of loosening and failure, and glenoid loosening has been documented in 33% to 44% of arthroplasties, with the rate of radiographically lucent lines even higher.2-5 Thus, a correlation between increasing incidence of procedures and high rates of glenoid loosening represents the potential for a significant increase in the number of future revisions. A recent report from Germany indicated that TSA had a 3-fold higher relative burden of revision than hemiarthroplasty.6
Ingrowth metal-backed glenoid components offer the theoretical advantage of bone growth directly into the prosthesis with a single host–prosthesis interface. Use of a novel tantalum glenoid may avoid the stress-shielding, component-stiffness, dissociation, and backside-wear issues that have produced the high failure rates of conventional metal-backed glenoids. According to the literature, the multiple different-style cementless glenoids being used have had unpredictable outcomes and demonstrated an increased need for revisions.7-11
In this article, we present a case series of midterm radiographic and clinical outcomes for TSAs using porous tantalum glenoid components. Our goals were to further understanding of survivorship and complications associated with ingrowth glenoid components and to demonstrate the differences that may occur with use of tantalum.
Materials and Methods
Data were examined for all TSAs performed at a single institution between 2004 and 2013. Before reviewing the data, we obtained approval from the hospital institutional review board. Our retrospective chart review identified all patients who underwent TSA using a tantalum ingrowth glenoid component. Exclusion criteria included revision arthroplasty, use of a non-tantalum glenoid, reverse shoulder arthroplasty, and conversion from hemiarthroplasty to TSA. Twelve shoulders (11 patients) were identified. We obtained patient consent to examine the data collected, and patients were reexamined if they had not been seen within the past 12 months. Figures 1 and 2 show the preoperative radiographs.
The TSAs were performed by 2 fellowship-trained shoulder surgeons using glenoid components with porous tantalum anchors (Zimmer). Indications for this procedure were age under 60 years, no prior surgery, and glenoid morphology allowing for version correction without bone grafting. Patients with severe posterior erosion that required bone graft or with a dysplastic glenoid were not indicated for this glenoid implant.
In each case, the anesthesia team placed an indwelling interscalene catheter, and then the surgery was performed with the patient under deep sedation. The beach-chair position and a deltopectoral approach were used, and biceps tendon tenodesis was performed. The subscapularis was elevated with a lesser tuberosity osteotomy and was repaired with nonabsorbable braided suture at the end of the case. During glenoid implantation, the periphery of the polyethylene was cemented. This is consistent with the approved method of implantation for this device. Closed suction drainage was used. After surgery, the patient was restricted to no weight-bearing. During the first 6 weeks, passive forward elevation was allowed to 130° and external rotation to 30°. Active and active-assisted range of motion was started at 6 weeks, and muscular strengthening was allowed 12 weeks after surgery.
We analyzed standard radiographs at yearly intervals for trabecular bony architecture and lucency surrounding the tantalum anchor of the glenoid. Before and after surgery, American Shoulder and Elbow Surgeons (ASES) scores and active forward elevation (AFE) and active external rotation (AER) measurements were recorded. These measurements served as endpoints of analysis.
Results
Twelve shoulders (11 patients) were identified and examined. Mean follow-up was 20 months (range, 6-84 months). In all cases, annual standard radiographs showed bony trabeculae adjacent to the tantalum anchor without lucency. There was no sign of glenoid loosening in any patient.
ASES scores and AFE and AER measurements were obtained with physical examinations and compared with t tests. ASES scores, available for 8 patients, increased from 32 before surgery to 85 after surgery (P < .01). Mean AFE increased from 117° to 159° (P < .01), and mean AER increased from 23° to 53° (P < .01). Figures 3 and 4 show the postoperative radiographs, and the Table highlights the ASES and range-of-motion data.
Discussion
Data for the 12 TSAs followed in this series showed promising outcomes for cementless ingrowth glenoid components. Much as with other data in the literature, there were significant improvements in ASES scores, AFE, and AER. What differs from the majority of available data is the survivorship and lack of radiolucent lines on follow-up radiographs.
Boileau and colleagues7 randomized 39 patients (40 shoulders) to either a cemented all-polyethylene glenoid or a cementless metal-backed glenoid component. Although the metal-backed glenoid components had a significantly lower rate of radiolucent lines, the metal-backed glenoids had a significantly higher rate of loosening. The authors subsequently abandoned use of uncemented metal-backed glenoid components. Taunton and colleagues8 reviewed 83 TSAs with a metal-backed bone ingrowth glenoid component. In 74 cases, the preoperative diagnosis was primary osteoarthritis. Mean clinical follow-up was 9.5 years. During follow-up, there were improvements in pain, forward elevation, and external rotation. Radiographic glenoid loosening was noted in 33 shoulders; 9 required revision for glenoid loosening. Both series demonstrated a high rate of revisions for cementless glenoid components.
Similar revision difficulties were noted by Montoya and colleagues.9 In their series of 65 TSAs performed for primary osteoarthritis, a cementless glenoid component was implanted. There were significant improvements in Constant scores, forward flexion, external rotation, and abduction but also an 11.3% revision rate noted at 68 months (mean follow-up). Glenoid revisions were required predominantly in patients with eccentric preoperative glenoid morphology. Lawrence and colleagues10 used a cementless ingrowth glenoid component in 21 shoulder arthroplasties performed for glenoid bone loss (13) or revision (8). They noted a high rate of revisions but good outcomes for the cases not revised. In both studies, there was a high rate of revision for glenoid loosening but also a tendency for revisions to be correlated with more challenging clinical applications.
Wirth and colleagues11 followed 44 TSAs using a minimally cemented ingrowth glenoid component. There were significant improvements in ASES scores, Simple Shoulder Test scores, and visual analog scale pain ratings. No revisions for glenoid loosening were noted. The implants were thought to provide durable outcomes at a mean follow-up of 4 years. These results were similar to those appreciated in the present study. In both series, the revision rate was much lower than described in the literature, and there were predictable improvements in pain and active motion.
Our study had several limitations: small number of patients, no comparison group, and relatively short follow-up. More long-term data are needed to appropriately compare cemented and uncemented glenoid components. In addition, it is difficult to compare our group of patients with those described in the literature, as the implants used differ. Despite these limitations, our data suggest that tantalum ingrowth glenoid components provide predictable and sustainable outcomes in TSA. With longer-term follow-up, tantalum ingrowth glenoids may demonstrate a durable and reliable alternative to cemented glenoid components.
1. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254.
2. Torchia ME, Cofield RH, Settergren CR. Total shoulder arthroplasty with the Neer prosthesis: long-term results. J Shoulder Elbow Surg. 1997;6(6):495-505.
3. Kasten P, Pape G, Raiss P, et al. Mid-term survivorship analysis of a shoulder replacement with a keeled glenoid and a modern cementing technique. J Bone Joint Surg Br. 2010;92(3):387-392.
4. Bohsali KI, Wirth MA, Rockwood CA Jr. Complications of total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(10):2279-2292.
5. Neer CS 2nd, Watson KC, Stanton FJ. Recent experience in total shoulder replacement. J Bone Joint Surg Am. 1982;64(3):319-337.
6. Hollatz MF, Stang A. Nationwide shoulder arthroplasty rates and revision burden in Germany: analysis of the national hospitalization data 2005 to 2006. J Shoulder Elbow Surg. 2014;23(11):e267-e274.
7. Boileau P, Avidor C, Krishnan SG, Walch G, Kempf JF, Molé D. Cemented polyethylene versus uncemented metal-backed glenoid components in total shoulder arthroplasty: a prospective, double-blind, randomized study. J Shoulder Elbow Surg. 2002;11(4):351-359.
8. Taunton MJ, McIntosh AL, Sperling JW, Cofield RH. Total shoulder arthroplasty with a metal-backed, bone-ingrowth glenoid component. Medium to long-term results. J Bone Joint Surg Am. 2008;90(10):2180-2188.
9. Montoya F, Magosch P, Scheiderer B, Lichtenberg S, Melean P, Habermeyer P. Midterm results of a total shoulder prosthesis fixed with a cementless glenoid component. J Shoulder Elbow Surg. 2013;22(5):628-635.
10. Lawrence TM, Ahmadi S, Sperling JW, Cofield RH. Fixation and durability of a bone-ingrowth component for glenoid bone loss. J Shoulder Elbow Surg. 2012;21(12):1764-1769.
11. Wirth MA, Loredo R, Garcia G, Rockwood CA Jr, Southworth C, Iannotti JP. Total shoulder arthroplasty with an all-polyethylene pegged bone-ingrowth glenoid component: a clinical and radiographic outcome study. J Bone Joint Surg Am. 2012;94(3):260-267.
Total shoulder arthroplasty (TSA) is being performed with increasing frequency. According to recent data, the number of TSAs performed annually increased 2.5-fold from 2000 to 2008.1 As more are performed, the need for improved implant survival will increase as well. In particular, advances in glenoid survivorship will be a primary focus. Previous experience has demonstrated that the glenoid component is the most common source of loosening and failure, and glenoid loosening has been documented in 33% to 44% of arthroplasties, with the rate of radiographically lucent lines even higher.2-5 Thus, a correlation between increasing incidence of procedures and high rates of glenoid loosening represents the potential for a significant increase in the number of future revisions. A recent report from Germany indicated that TSA had a 3-fold higher relative burden of revision than hemiarthroplasty.6
Ingrowth metal-backed glenoid components offer the theoretical advantage of bone growth directly into the prosthesis with a single host–prosthesis interface. Use of a novel tantalum glenoid may avoid the stress-shielding, component-stiffness, dissociation, and backside-wear issues that have produced the high failure rates of conventional metal-backed glenoids. According to the literature, the multiple different-style cementless glenoids being used have had unpredictable outcomes and demonstrated an increased need for revisions.7-11
In this article, we present a case series of midterm radiographic and clinical outcomes for TSAs using porous tantalum glenoid components. Our goals were to further understanding of survivorship and complications associated with ingrowth glenoid components and to demonstrate the differences that may occur with use of tantalum.
Materials and Methods
Data were examined for all TSAs performed at a single institution between 2004 and 2013. Before reviewing the data, we obtained approval from the hospital institutional review board. Our retrospective chart review identified all patients who underwent TSA using a tantalum ingrowth glenoid component. Exclusion criteria included revision arthroplasty, use of a non-tantalum glenoid, reverse shoulder arthroplasty, and conversion from hemiarthroplasty to TSA. Twelve shoulders (11 patients) were identified. We obtained patient consent to examine the data collected, and patients were reexamined if they had not been seen within the past 12 months. Figures 1 and 2 show the preoperative radiographs.
The TSAs were performed by 2 fellowship-trained shoulder surgeons using glenoid components with porous tantalum anchors (Zimmer). Indications for this procedure were age under 60 years, no prior surgery, and glenoid morphology allowing for version correction without bone grafting. Patients with severe posterior erosion that required bone graft or with a dysplastic glenoid were not indicated for this glenoid implant.
In each case, the anesthesia team placed an indwelling interscalene catheter, and then the surgery was performed with the patient under deep sedation. The beach-chair position and a deltopectoral approach were used, and biceps tendon tenodesis was performed. The subscapularis was elevated with a lesser tuberosity osteotomy and was repaired with nonabsorbable braided suture at the end of the case. During glenoid implantation, the periphery of the polyethylene was cemented. This is consistent with the approved method of implantation for this device. Closed suction drainage was used. After surgery, the patient was restricted to no weight-bearing. During the first 6 weeks, passive forward elevation was allowed to 130° and external rotation to 30°. Active and active-assisted range of motion was started at 6 weeks, and muscular strengthening was allowed 12 weeks after surgery.
We analyzed standard radiographs at yearly intervals for trabecular bony architecture and lucency surrounding the tantalum anchor of the glenoid. Before and after surgery, American Shoulder and Elbow Surgeons (ASES) scores and active forward elevation (AFE) and active external rotation (AER) measurements were recorded. These measurements served as endpoints of analysis.
Results
Twelve shoulders (11 patients) were identified and examined. Mean follow-up was 20 months (range, 6-84 months). In all cases, annual standard radiographs showed bony trabeculae adjacent to the tantalum anchor without lucency. There was no sign of glenoid loosening in any patient.
ASES scores and AFE and AER measurements were obtained with physical examinations and compared with t tests. ASES scores, available for 8 patients, increased from 32 before surgery to 85 after surgery (P < .01). Mean AFE increased from 117° to 159° (P < .01), and mean AER increased from 23° to 53° (P < .01). Figures 3 and 4 show the postoperative radiographs, and the Table highlights the ASES and range-of-motion data.
Discussion
Data for the 12 TSAs followed in this series showed promising outcomes for cementless ingrowth glenoid components. Much as with other data in the literature, there were significant improvements in ASES scores, AFE, and AER. What differs from the majority of available data is the survivorship and lack of radiolucent lines on follow-up radiographs.
Boileau and colleagues7 randomized 39 patients (40 shoulders) to either a cemented all-polyethylene glenoid or a cementless metal-backed glenoid component. Although the metal-backed glenoid components had a significantly lower rate of radiolucent lines, the metal-backed glenoids had a significantly higher rate of loosening. The authors subsequently abandoned use of uncemented metal-backed glenoid components. Taunton and colleagues8 reviewed 83 TSAs with a metal-backed bone ingrowth glenoid component. In 74 cases, the preoperative diagnosis was primary osteoarthritis. Mean clinical follow-up was 9.5 years. During follow-up, there were improvements in pain, forward elevation, and external rotation. Radiographic glenoid loosening was noted in 33 shoulders; 9 required revision for glenoid loosening. Both series demonstrated a high rate of revisions for cementless glenoid components.
Similar revision difficulties were noted by Montoya and colleagues.9 In their series of 65 TSAs performed for primary osteoarthritis, a cementless glenoid component was implanted. There were significant improvements in Constant scores, forward flexion, external rotation, and abduction but also an 11.3% revision rate noted at 68 months (mean follow-up). Glenoid revisions were required predominantly in patients with eccentric preoperative glenoid morphology. Lawrence and colleagues10 used a cementless ingrowth glenoid component in 21 shoulder arthroplasties performed for glenoid bone loss (13) or revision (8). They noted a high rate of revisions but good outcomes for the cases not revised. In both studies, there was a high rate of revision for glenoid loosening but also a tendency for revisions to be correlated with more challenging clinical applications.
Wirth and colleagues11 followed 44 TSAs using a minimally cemented ingrowth glenoid component. There were significant improvements in ASES scores, Simple Shoulder Test scores, and visual analog scale pain ratings. No revisions for glenoid loosening were noted. The implants were thought to provide durable outcomes at a mean follow-up of 4 years. These results were similar to those appreciated in the present study. In both series, the revision rate was much lower than described in the literature, and there were predictable improvements in pain and active motion.
Our study had several limitations: small number of patients, no comparison group, and relatively short follow-up. More long-term data are needed to appropriately compare cemented and uncemented glenoid components. In addition, it is difficult to compare our group of patients with those described in the literature, as the implants used differ. Despite these limitations, our data suggest that tantalum ingrowth glenoid components provide predictable and sustainable outcomes in TSA. With longer-term follow-up, tantalum ingrowth glenoids may demonstrate a durable and reliable alternative to cemented glenoid components.
Total shoulder arthroplasty (TSA) is being performed with increasing frequency. According to recent data, the number of TSAs performed annually increased 2.5-fold from 2000 to 2008.1 As more are performed, the need for improved implant survival will increase as well. In particular, advances in glenoid survivorship will be a primary focus. Previous experience has demonstrated that the glenoid component is the most common source of loosening and failure, and glenoid loosening has been documented in 33% to 44% of arthroplasties, with the rate of radiographically lucent lines even higher.2-5 Thus, a correlation between increasing incidence of procedures and high rates of glenoid loosening represents the potential for a significant increase in the number of future revisions. A recent report from Germany indicated that TSA had a 3-fold higher relative burden of revision than hemiarthroplasty.6
Ingrowth metal-backed glenoid components offer the theoretical advantage of bone growth directly into the prosthesis with a single host–prosthesis interface. Use of a novel tantalum glenoid may avoid the stress-shielding, component-stiffness, dissociation, and backside-wear issues that have produced the high failure rates of conventional metal-backed glenoids. According to the literature, the multiple different-style cementless glenoids being used have had unpredictable outcomes and demonstrated an increased need for revisions.7-11
In this article, we present a case series of midterm radiographic and clinical outcomes for TSAs using porous tantalum glenoid components. Our goals were to further understanding of survivorship and complications associated with ingrowth glenoid components and to demonstrate the differences that may occur with use of tantalum.
Materials and Methods
Data were examined for all TSAs performed at a single institution between 2004 and 2013. Before reviewing the data, we obtained approval from the hospital institutional review board. Our retrospective chart review identified all patients who underwent TSA using a tantalum ingrowth glenoid component. Exclusion criteria included revision arthroplasty, use of a non-tantalum glenoid, reverse shoulder arthroplasty, and conversion from hemiarthroplasty to TSA. Twelve shoulders (11 patients) were identified. We obtained patient consent to examine the data collected, and patients were reexamined if they had not been seen within the past 12 months. Figures 1 and 2 show the preoperative radiographs.
The TSAs were performed by 2 fellowship-trained shoulder surgeons using glenoid components with porous tantalum anchors (Zimmer). Indications for this procedure were age under 60 years, no prior surgery, and glenoid morphology allowing for version correction without bone grafting. Patients with severe posterior erosion that required bone graft or with a dysplastic glenoid were not indicated for this glenoid implant.
In each case, the anesthesia team placed an indwelling interscalene catheter, and then the surgery was performed with the patient under deep sedation. The beach-chair position and a deltopectoral approach were used, and biceps tendon tenodesis was performed. The subscapularis was elevated with a lesser tuberosity osteotomy and was repaired with nonabsorbable braided suture at the end of the case. During glenoid implantation, the periphery of the polyethylene was cemented. This is consistent with the approved method of implantation for this device. Closed suction drainage was used. After surgery, the patient was restricted to no weight-bearing. During the first 6 weeks, passive forward elevation was allowed to 130° and external rotation to 30°. Active and active-assisted range of motion was started at 6 weeks, and muscular strengthening was allowed 12 weeks after surgery.
We analyzed standard radiographs at yearly intervals for trabecular bony architecture and lucency surrounding the tantalum anchor of the glenoid. Before and after surgery, American Shoulder and Elbow Surgeons (ASES) scores and active forward elevation (AFE) and active external rotation (AER) measurements were recorded. These measurements served as endpoints of analysis.
Results
Twelve shoulders (11 patients) were identified and examined. Mean follow-up was 20 months (range, 6-84 months). In all cases, annual standard radiographs showed bony trabeculae adjacent to the tantalum anchor without lucency. There was no sign of glenoid loosening in any patient.
ASES scores and AFE and AER measurements were obtained with physical examinations and compared with t tests. ASES scores, available for 8 patients, increased from 32 before surgery to 85 after surgery (P < .01). Mean AFE increased from 117° to 159° (P < .01), and mean AER increased from 23° to 53° (P < .01). Figures 3 and 4 show the postoperative radiographs, and the Table highlights the ASES and range-of-motion data.
Discussion
Data for the 12 TSAs followed in this series showed promising outcomes for cementless ingrowth glenoid components. Much as with other data in the literature, there were significant improvements in ASES scores, AFE, and AER. What differs from the majority of available data is the survivorship and lack of radiolucent lines on follow-up radiographs.
Boileau and colleagues7 randomized 39 patients (40 shoulders) to either a cemented all-polyethylene glenoid or a cementless metal-backed glenoid component. Although the metal-backed glenoid components had a significantly lower rate of radiolucent lines, the metal-backed glenoids had a significantly higher rate of loosening. The authors subsequently abandoned use of uncemented metal-backed glenoid components. Taunton and colleagues8 reviewed 83 TSAs with a metal-backed bone ingrowth glenoid component. In 74 cases, the preoperative diagnosis was primary osteoarthritis. Mean clinical follow-up was 9.5 years. During follow-up, there were improvements in pain, forward elevation, and external rotation. Radiographic glenoid loosening was noted in 33 shoulders; 9 required revision for glenoid loosening. Both series demonstrated a high rate of revisions for cementless glenoid components.
Similar revision difficulties were noted by Montoya and colleagues.9 In their series of 65 TSAs performed for primary osteoarthritis, a cementless glenoid component was implanted. There were significant improvements in Constant scores, forward flexion, external rotation, and abduction but also an 11.3% revision rate noted at 68 months (mean follow-up). Glenoid revisions were required predominantly in patients with eccentric preoperative glenoid morphology. Lawrence and colleagues10 used a cementless ingrowth glenoid component in 21 shoulder arthroplasties performed for glenoid bone loss (13) or revision (8). They noted a high rate of revisions but good outcomes for the cases not revised. In both studies, there was a high rate of revision for glenoid loosening but also a tendency for revisions to be correlated with more challenging clinical applications.
Wirth and colleagues11 followed 44 TSAs using a minimally cemented ingrowth glenoid component. There were significant improvements in ASES scores, Simple Shoulder Test scores, and visual analog scale pain ratings. No revisions for glenoid loosening were noted. The implants were thought to provide durable outcomes at a mean follow-up of 4 years. These results were similar to those appreciated in the present study. In both series, the revision rate was much lower than described in the literature, and there were predictable improvements in pain and active motion.
Our study had several limitations: small number of patients, no comparison group, and relatively short follow-up. More long-term data are needed to appropriately compare cemented and uncemented glenoid components. In addition, it is difficult to compare our group of patients with those described in the literature, as the implants used differ. Despite these limitations, our data suggest that tantalum ingrowth glenoid components provide predictable and sustainable outcomes in TSA. With longer-term follow-up, tantalum ingrowth glenoids may demonstrate a durable and reliable alternative to cemented glenoid components.
1. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254.
2. Torchia ME, Cofield RH, Settergren CR. Total shoulder arthroplasty with the Neer prosthesis: long-term results. J Shoulder Elbow Surg. 1997;6(6):495-505.
3. Kasten P, Pape G, Raiss P, et al. Mid-term survivorship analysis of a shoulder replacement with a keeled glenoid and a modern cementing technique. J Bone Joint Surg Br. 2010;92(3):387-392.
4. Bohsali KI, Wirth MA, Rockwood CA Jr. Complications of total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(10):2279-2292.
5. Neer CS 2nd, Watson KC, Stanton FJ. Recent experience in total shoulder replacement. J Bone Joint Surg Am. 1982;64(3):319-337.
6. Hollatz MF, Stang A. Nationwide shoulder arthroplasty rates and revision burden in Germany: analysis of the national hospitalization data 2005 to 2006. J Shoulder Elbow Surg. 2014;23(11):e267-e274.
7. Boileau P, Avidor C, Krishnan SG, Walch G, Kempf JF, Molé D. Cemented polyethylene versus uncemented metal-backed glenoid components in total shoulder arthroplasty: a prospective, double-blind, randomized study. J Shoulder Elbow Surg. 2002;11(4):351-359.
8. Taunton MJ, McIntosh AL, Sperling JW, Cofield RH. Total shoulder arthroplasty with a metal-backed, bone-ingrowth glenoid component. Medium to long-term results. J Bone Joint Surg Am. 2008;90(10):2180-2188.
9. Montoya F, Magosch P, Scheiderer B, Lichtenberg S, Melean P, Habermeyer P. Midterm results of a total shoulder prosthesis fixed with a cementless glenoid component. J Shoulder Elbow Surg. 2013;22(5):628-635.
10. Lawrence TM, Ahmadi S, Sperling JW, Cofield RH. Fixation and durability of a bone-ingrowth component for glenoid bone loss. J Shoulder Elbow Surg. 2012;21(12):1764-1769.
11. Wirth MA, Loredo R, Garcia G, Rockwood CA Jr, Southworth C, Iannotti JP. Total shoulder arthroplasty with an all-polyethylene pegged bone-ingrowth glenoid component: a clinical and radiographic outcome study. J Bone Joint Surg Am. 2012;94(3):260-267.
1. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254.
2. Torchia ME, Cofield RH, Settergren CR. Total shoulder arthroplasty with the Neer prosthesis: long-term results. J Shoulder Elbow Surg. 1997;6(6):495-505.
3. Kasten P, Pape G, Raiss P, et al. Mid-term survivorship analysis of a shoulder replacement with a keeled glenoid and a modern cementing technique. J Bone Joint Surg Br. 2010;92(3):387-392.
4. Bohsali KI, Wirth MA, Rockwood CA Jr. Complications of total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(10):2279-2292.
5. Neer CS 2nd, Watson KC, Stanton FJ. Recent experience in total shoulder replacement. J Bone Joint Surg Am. 1982;64(3):319-337.
6. Hollatz MF, Stang A. Nationwide shoulder arthroplasty rates and revision burden in Germany: analysis of the national hospitalization data 2005 to 2006. J Shoulder Elbow Surg. 2014;23(11):e267-e274.
7. Boileau P, Avidor C, Krishnan SG, Walch G, Kempf JF, Molé D. Cemented polyethylene versus uncemented metal-backed glenoid components in total shoulder arthroplasty: a prospective, double-blind, randomized study. J Shoulder Elbow Surg. 2002;11(4):351-359.
8. Taunton MJ, McIntosh AL, Sperling JW, Cofield RH. Total shoulder arthroplasty with a metal-backed, bone-ingrowth glenoid component. Medium to long-term results. J Bone Joint Surg Am. 2008;90(10):2180-2188.
9. Montoya F, Magosch P, Scheiderer B, Lichtenberg S, Melean P, Habermeyer P. Midterm results of a total shoulder prosthesis fixed with a cementless glenoid component. J Shoulder Elbow Surg. 2013;22(5):628-635.
10. Lawrence TM, Ahmadi S, Sperling JW, Cofield RH. Fixation and durability of a bone-ingrowth component for glenoid bone loss. J Shoulder Elbow Surg. 2012;21(12):1764-1769.
11. Wirth MA, Loredo R, Garcia G, Rockwood CA Jr, Southworth C, Iannotti JP. Total shoulder arthroplasty with an all-polyethylene pegged bone-ingrowth glenoid component: a clinical and radiographic outcome study. J Bone Joint Surg Am. 2012;94(3):260-267.
Fingertip Amputation Treatment: A Survey Study
Finger injuries are common, representing an estimated 3 million emergency department visits per year in the United States, with 44% of these diagnosed as lacerations.1 Amputations of the finger (partial and complete) in non-work-related accidents alone are estimated at 30,000 per year.1 The fingertip is a highly specialized structure that contributes to precision function of the hand through tactile feedback and fine motor control as well as hand aesthetics. An injury can compromise a variety of fingertip structures, including the distal phalanx, which provides length and structural support; the fingernail, germinal matrix, and sterile matrix, which protect the fingertip and function as tools; and the volar skin pad, which is important for sensation and fine motor activity.
There is considerable debate regarding optimal management of fingertip amputations, and to date there have been no prospective, randomly controlled trials to guide treatment.2 Injury characteristics, amputation levels, and patient priorities all contribute to management decisions. Treatment goals are to maintain length when possible; to provide stable, supple, and sensate skin coverage; to ensure the nail plate regrows without complication; and to maintain normal overall finger shape and cosmesis. In addition, a simple, cost-effective treatment with short recovery time and no donor-site morbidity is desired.
Treatment recommendations are wide-ranging, and evidence-based literature is sparse. About 30 years ago, 2 retrospective comparative studies found no difference in outcomes between simpler treatments (primary closure, secondary wound healing) and various operative strategies.3,4 Since then, most of the scientific studies have been retrospective noncomparative case series, all reporting good to excellent results.5-17 Investigators generally implied superior results of a studied procedure over those of more conservative treatments. Recommended treatments include secondary wound healing, simple flaps, staged flaps, pedicle flaps, allograft and autograft coverage, composite grafting, and replantation, for all levels of fingertip injury.
Given our surgical advances, improved techniques, and accumulating experience, we may have expected better outcomes with newer and more complex reconstructive efforts. Unfortunately, in a recent review of 53 fingertip injuries treated with a reconstructive procedure, bone shortening with closure, or secondary healing, Wang and colleagues18 found no discernible differences in outcomes at 4.5-year follow-up. They questioned whether complex reconstructive procedures are worth the time, expense, and risk. In the absence of prospective, comparative studies, surgeons must rely on anecdotal evidence (including predominantly level IV evidence), training bias, previous experience, and the prevailing common wisdom.
Toward that end, we became interested in identifying treatment preferences for fingertip amputations. We conducted a study to better understand how surgeon and patient factors influence the treatment preferences for distal fingertip amputations among a cross section of US and international hand surgeons. We hypothesized that hand surgeons’ treatment preferences would be varied and influenced by surgeon and patient demographics.
Materials and Methods
An online multiple-choice survey was created and powered by Constant Contact. The survey consisted of 6 surgeon demographic questions; 5 treatment preference questions regarding patient age, sex, occupation, and germinal matrix management; and 5 clinical scenarios based on Allen levels 2, 3 (with and without exposed distal phalanx), and 4 and volar oblique middle-finger amputations. The Allen classification designates level 2 injuries as those involving only the distal pulp and nail.19 Level 3 injuries also involve the terminal distal phalanx, and level 4 injuries extend to the lunula. The survey questions are listed in the Appendix. For the clinical scenario questions, treatment choices included wound care, skeletal shortening and closure, composite graft, autograft, allograft, V-Y/Kutler flap, advancement flap, thenar flap, cross-finger flap, pedicle and homodigital flap, replantation, and other.
An email invitation was sent to members of the American Association for Hand Surgery (AAHS). The survey was also submitted to personal contacts of international hand societies named on the AAHS website to expand the international response. A reminder email was sent 1 week after the original invitation. The survey was closed 5 weeks later, and the responses were analyzed with all non-US hand surgeons grouped collectively as an international group, compared with the US group. Institutional review board approval was not needed for this survey study.
Statistics
A generalized linear regression model was used to implement logistic regression with random effects for question and respondent. This approach accounts for multiple observations from the same respondent, assuming that both respondent and question are random samples from a larger population. The model estimated the probability that a given surgical approach (eg, skeletal shortening, wound care) would be selected, based on the predictors of the US versus international respondent, time in practice, practice type, and whether the fingertip was available. The model returned adjusted odds ratios (ORs) for each predictor, controlling for all the others. By convention, P < .05 was considered significant. No attempt was made to prune the model of nonsignificant factors. Analyses were performed using the lme4 package on the R statistical platform (R Foundation for Statistical Computing).
Results
One hundred ninety-eight responses were recorded. Of the 1054 AAHS members invited to take the survey, 174 (US, international) responded (17% response rate). One hundred twenty-three responses and 62% of the total were generated from US hand surgeons. Fifty-eight percent of US responses were from the Mid-South, Midwest, or Mid-Atlantic region. Fifty-seven percent of international responses were from Brazil and Europe. Respondents’ demographic data are listed in Tables 1 and 2.
Responses to the 5 clinical scenarios showed a wide variation in treatment preferences. The top 6 preferred treatment selections for an acute, clean long-finger amputation in a healthy 40-year-old office worker are shown in Figures 1 to 5. When surgeons who preferred replant were asked what they would do if the amputated part was not available, they indicated flap coverage more often than less complex treatments, such as skeletal shortening/primary closure or wound care.
There were statistically significant differences in treatment preferences between US and international hand surgeons when controlling for all other demographic variables. Adjusted ORs and their confidence intervals (CIs) for the aggregate clinical scenarios are presented in a forest plot in Figure 6. Figure 4 shows that US surgeons were more likely to choose wound care (OR, 3.6; P < .0004) and less likely to attempt a replant (OR, 0.01; P < .0001). US surgeons were also less likely to use a pedicle or homodigital island flap when the amputated fingertip was both available (OR, 0.04; P = .039) and unavailable (OR, 0.47; Ps = .029).
Among all respondents and across all clinical scenarios, skeletal shortening with closure was favored among hand surgeons in practice less than 5 years compared with those in practice longer (OR, 2.11; 95% CI, 1.36-3.25; P = .0008). Similarly, surgeons with more than 30 years of experience were the least likely to favor wound care (OR, 0.2; 95% CI, 0.09-0.93; P = .037). Compared with orthopedic surgeons, plastic surgeons opted for wound care less often (OR, 0.44; 95% CI, 0.23-0.98; P = .018) and appeared to prefer replantation, but the difference was not statistically significant (OR, 8.86; 95% CI, 0.99-79.61; P = .054).
Replantation was less often chosen by private practice versus full-time academic surgeons (OR, 0.09; 95% CI, 0.01-0.91; P = .041.) Part-time academics were no more or less likely to perform replantation than full-time academics were (OR, 0.52; 95% CI, 0.05-5.41; P = .58). Of the 59 respondents who performed more than 10 microvascular cases a year, 18 (31%) chose replant for Allen level 4 amputations. In comparison, 9 (20%) of the 45 respondents who performed fewer than 3 microvascular cases a year chose replant for amputations at this level. Amount of time working with fellows did not affect treatment preferences.
Patient demographics (age, sex, occupation) also played a role in treatment decisions (Table 3). The most significant factors appeared to be age and occupation. Regarding age, 41% of respondents chose more complex procedures for patients younger than 15, and 62% chose less complex procedures for patients older than 70 years. Regarding occupation, 61% chose more complex procedures for professional musicians, and 60% chose less complex procedures for manual laborers. Sex did not influence clinical decisions for 78% of respondents. There was also substantial variation in both the indications for germinal matrix ablation and the frequency of sterile matrix transplant (Table 3).
Discussion
Although there is a variety of treatment options and published treatment guidelines for distal fingertip amputations, few comparative studies support use of one treatment over another. In our experience, treatment decisions are based mainly on injury parameters, but surgeon preference and patient factors (age, sex, occupation) can also influence care. Our goal in this study was to better understand how surgeon and patient factors influence treatment preferences for distal fingertip amputations among a cross section of US and international hand surgeons. Our survey results showed lack of consensus among hand surgeons and highlighted several trends.
As expected, we found a wide range of treatment preferences for each clinical scenario queried, ranging from more simple treatments (eg, wound care) to more complex ones (eg, replantation). With patient parameters (age, profession, finger, acuity, injury type, tissue preservation, smoking status) standardized in the clinical scenarios, the treatment differences noted should reflect surgeon preference. However, other patient factors (eg, cultural differences, religious beliefs, surgeon setting, practice pattern, resource availability) that were not included in the clinical scenarios could also affect treatment preferences.
One particularly interesting finding was that international hand surgeons were 6.8 times more likely to replant a distal fingertip amputation. One possible explanation for this variation is the influence of cultural differences. For example, in East Asian countries, there can be a cultural stigma associated with loss of a fingertip, and therefore more of a desire on the part of the patient to restore the original finger.20,21 In addition, the international respondents were biased toward academic practices—which could skew the treatment preference toward replantation, as we found that academic surgeons were more inclined to replantation.
Our finding that replantation was more commonly preferred by academic versus private practice surgeons may suggest a training bias, an affinity for more complex or interesting procedures, or access to hospital equipment and staff, including residents and fellows, not usually found at smaller community hospitals, where private practice surgeons are more commonly based. Jazayeri and colleagues22 found that institutions specializing in microsurgery often produced better outcomes than nonspecializing institutions. Therefore, it is not surprising that private practice hand surgeons may less often opt to replant a distal fingertip amputation. It is also not surprising that plastic surgeons are more inclined to perform a replantation or flap coverage, as their training is more microsurgery-intensive and their practice more focused on aesthetics compared with the other specialists.
Distal fingertip replantation is accepted by most as technically demanding, but it seems that the additional effort and resources would be justified if the procedure provided a superior outcome. However, other factors, such as cost of treatment and length of recovery, should also be considered. Average replantation cost has been estimated to range from $7500 to $14,000, compared with $2800 for non-replantation-related care, and median stay is about 4 days longer for replantation-related care.23,24 These estimates do not include indirect costs, such as for postoperative rehabilitation, which is likely longer and more expensive, even in distal fingertip replantation. These disparities may not justify the outcome (of having a complete fingertip) if more conservative treatments yield similar results.17,18 In addition, there is the expected failure rate of limb replantation surgery. In analysis of the overall societal costs and benefits of larger upper extremity limb replantation, the loss of invested resources sustained with failed limb replantation may be outweighed by the benefit of another patient having a successful outcome. In the case of fingertip replantation, however, does the undefined benefit of the successful patient outcome outweigh the investment of resources lost in cases of replantation failure? Understandably, there is a need for more robust clinical outcome and cost-comparative evidence to better inform decisions regarding distal fingertip amputation.
We found that wound care and skeletal shortening with primary closure (particularly with Allen level 3 injuries) were preferred more by surgeons within the first 5 years of practice. This finding seems to imply a lack of experience or confidence on the part of younger surgeons performing more complex procedures, such as flap coverage. Conversely, this finding may indicate a shift in treatment principle based on recent literature suggesting equivalent outcomes with simpler procedures.17,18 Although our survey study did not provide an option for treatment combinations or staged procedures, several respondents wrote in that skeletal shortening supplemented with various types of autografts and allografts would be their preferred treatment.
Patient factors also play a significant role in clinical decisions. Age and profession seem to be important determinants, with more than 50% of respondents, on average, changing their treatment recommendation based on these 2 factors. A majority of respondents would perform a less involved procedure for a manual laborer, suggesting a quicker return to work is prioritized over a perceived improved clinical outcome. Interestingly, for patients younger than 15 years, the preference was divided, with 41% of surgeons opting for a more complex procedure. This suggests the importance of restoring anatomy in a younger patient, or the perceived decreased risk or failure rate with more involved treatment. Twenty percent preferred a less complex procedure in a younger patient, perhaps relying on the patient’s developmental potential for a good outcome or suggesting a concern for patient intolerance or compliance with complex surgery.
Nail plate regrowth can be a problem with fingertip amputations. Nail deformity is highly correlated with injury level, with amputations proximal to the lunula more likely to cause nail plate deformity.25,26 Jebson and colleagues27 recommended germinal matrix ablation for amputations proximal to the lunula. We found respondents often performed ablations for other indications, including injured or minimal remaining sterile matrix and lack of bony support for the sterile matrix. Forty-six percent of respondents had never performed sterile matrix transplant, which could indicate that they were unfamiliar with the technique or had donor-site concerns, or that postinjury nail deformities are uncommon, well tolerated, or treated along with other procedures, such as germinal matrix ablation.
Several weaknesses of this study must be highlighted. First, our response rate was smaller than desired. Although this work incorporated a large number of surgeon responses, nearly 200, the response rate was only 17%. In addition, although number of responses was likely adequate to show the diversity of opinion, the preferences and trends reported might not be representative of all hand surgeons. We could not perform a nonresponder analysis because of a lack of specific demographic data for the AAHS and international hand society members. However, AAHS has an approximate 50/50 mix of plastic and orthopedic surgeons, similar to our responder demographic, suggesting our smaller subset of responses might be representative of the whole. According to AAHS, a majority of its members are “academic” hand surgeons, so our results might not adequately reflect the preferences of community hand surgeons and ultimately might overstate the frequency of more complex treatments. Last, our international response was limited to a few countries. A larger, more broadly distributed response would provide a better understanding of regional preferences, which could shed light on the importance of cultural differences.
Variations in patient insurance status were not queried in this survey but might also affect treatment decisions. More involved, costly, and highly reimbursing procedures might be deemed reasonable options for a small perceived clinical benefit for insured patients.
When multiple digits or the thumb is injured, or there are other concomitant injuries, surgeons may alter their choice of intervention. In mangled extremities, preservation of salvageable functional units takes precedence over aesthetics and likely affects choice of treatment for the amputated fingertips. Similarly, multiple fingertip amputations, even if all at the same level, may be differently regarded than a solitary injury.
Conclusion
For distal fingertip amputations, there is little evidence supporting one approach over another. Without level I comparative data guiding treatment, anecdotal evidence and surgeon personal preferences likely contribute to the large variation noted in this survey. Our study results showed the disparity of fingertip treatment preferences among a cross section of US and international hand surgeons. More important, results underscored the need for a well-designed comparative study to determine the most effective treatments for distal fingertip amputations.
1. Conn JM, Annest JL, Ryan GW, Budnitz DS. Non-work-related finger amputations in the United States, 2001-2002. Ann Emerg Med. 2005;45(6):630-635.
2. Bickel KD, Dosanjh A. Fingertip reconstruction. J Hand Surg Am. 2008;33(8):1417-1419.
3. Söderberg T, Nyström Å, Hallmans G, Hultén J. Treatment of fingertip amputations with bone exposure. A comparative study between surgical and conservative treatment methods. Scand J Plast Reconstr Surg. 1983;17(2):147-152.
4. Braun M, Horton RC, Snelling CF. Fingertip amputation: review of 100 digits. Can J Surg. 1985;28(1):72-75.
5. Sammut D. Fingertip injuries. A review of indications and methods of management. Curr Orthop. 2002;16:271-285.
6. Mennen U, Wiese A. Fingertip injuries management with semi-occlusive dressing. J Hand Surg Br. 1993;18(4):416-422.
7. Atasoy E, Ioakimidis E, Kasdan ML, Kutz JE, Kleinert HE. Reconstruction of the amputated fingertip with a triangular volar flap. A new surgical procedure. J Bone Joint Surg Am. 1970;52(5):921-926.
8. Kutler W. A new method for finger tip amputation. J Am Med Assoc. 1947;133(1):29-30.
9. Takeishi M, Shinoda A, Sugiyama A, Ui K. Innervated reverse dorsal digital island flap for fingertip reconstruction. J Hand Surg Am. 2006;31(7):1094-1099.
10. Tuncali D, Barutcu AY, Gokrem S, Terzioglu A, Aslan G. The hatchet flap for reconstruction of fingertip amputations. Plast Reconstr Surg. 2006;117(6):1933-1939.
11. Teoh LC, Tay SC, Yong FC, Tan SH, Khoo DB. Heterodigital arterialized flaps for large finger wounds: results and indications. Plast Reconstr Surg. 2003;111(6):1905-1913.
12. Nishikawa H, Smith PJ. The recovery of sensation and function after cross-finger flaps for fingertip injury. J Hand Surg Br. 1992;17(1):102-107.
13. Rinker B. Fingertip reconstruction with the laterally based thenar flap: indications and long-term functional results. Hand. 2006;1(1):2-8.
14. Jung MS, Lim YK, Hong YT, Kim HN. Treatment of fingertip amputation in adults by palmar pocketing of the amputated part. Arch Plast Surg. 2012;39(4):404-410.
15. Venkatramani H, Sabapathy SR. Fingertip replantation: technical considerations and outcome analysis of 24 consecutive fingertip replantations. Indian J Plast Surg. 2011;44(2):237-245.
16. Chen SY, Wang CH, Fu JP, Chang SC, Chen SG. Composite grafting for traumatic fingertip amputation in adults: technique reinforcement and experience in 31 digits. J Trauma. 2011;70(1):148-153.
17. van den Berg WB, Vergeer RA, van der Sluis CK, Ten Duis HJ, Werker PM. Comparison of three types of treatment modalities on the outcome of fingertip injuries. J Trauma Acute Care Surg. 2012;72(6):1681-1687.
18. Wang K, Sears ED, Shauver MJ, Chung KC. A systematic review of outcomes of revision amputation treatment for fingertip amputations. Hand. 2013;8(2):139-145.
19. Allen MJ. Conservative management of finger tip injuries in adults. Hand. 1980;12(3):257-265.
20. Chen CT, Wei FC, Chen HC, Chuang CC, Chen HT, Hsu WM. Distal phalanx replantation. Microsurgery. 1994;15(1):77-82.
21. Kim WK, Lim JH, Han SK. Fingertip replantations: clinical evaluation of 135 digits. Plast Reconstr Surg. 1996;98(3):470-476.
22. Jazayeri L, Klausner JQ, Chang J. Distal digital replantation. Plast Reconstr Surg. 2013;132(5):1207-1217.
23. Hattori Y, Doi K, Sakamoto S, Yamasaki H, Wahegaonkar A, Addosooki A. Fingertip replantation. J Hand Surg Am. 2007;32(4):548-555.
24. Goldner RD, Stevanovic MV, Nunley JA, Urbaniak JR. Digital replantation at the level of the distal interphalangeal joint and the distal phalanx. J Hand Surg Am. 1989;14(2 pt 1):214-220.
25. Nishi G, Shibata Y, Tago K, Kubota M, Suzuki M. Nail regeneration in digits replanted after amputation through the distal phalanx. J Hand Surg Am. 1996;21(2):229-233.
26. Yamano Y. Replantation of the amputated distal part of the fingers. J Hand Surg Am. 1985;10(2):211-218.
27. Jebson PJ, Louis DS, Bagg M. Amputations. In: Wolfe SW, Pederson WC, Hotchkiss RN, Kozin SH, eds. Green’s Operative Hand Surgery. 6th ed. Philadelphia, PA: Churchill Livingstone; 2010:1885-1927.
Finger injuries are common, representing an estimated 3 million emergency department visits per year in the United States, with 44% of these diagnosed as lacerations.1 Amputations of the finger (partial and complete) in non-work-related accidents alone are estimated at 30,000 per year.1 The fingertip is a highly specialized structure that contributes to precision function of the hand through tactile feedback and fine motor control as well as hand aesthetics. An injury can compromise a variety of fingertip structures, including the distal phalanx, which provides length and structural support; the fingernail, germinal matrix, and sterile matrix, which protect the fingertip and function as tools; and the volar skin pad, which is important for sensation and fine motor activity.
There is considerable debate regarding optimal management of fingertip amputations, and to date there have been no prospective, randomly controlled trials to guide treatment.2 Injury characteristics, amputation levels, and patient priorities all contribute to management decisions. Treatment goals are to maintain length when possible; to provide stable, supple, and sensate skin coverage; to ensure the nail plate regrows without complication; and to maintain normal overall finger shape and cosmesis. In addition, a simple, cost-effective treatment with short recovery time and no donor-site morbidity is desired.
Treatment recommendations are wide-ranging, and evidence-based literature is sparse. About 30 years ago, 2 retrospective comparative studies found no difference in outcomes between simpler treatments (primary closure, secondary wound healing) and various operative strategies.3,4 Since then, most of the scientific studies have been retrospective noncomparative case series, all reporting good to excellent results.5-17 Investigators generally implied superior results of a studied procedure over those of more conservative treatments. Recommended treatments include secondary wound healing, simple flaps, staged flaps, pedicle flaps, allograft and autograft coverage, composite grafting, and replantation, for all levels of fingertip injury.
Given our surgical advances, improved techniques, and accumulating experience, we may have expected better outcomes with newer and more complex reconstructive efforts. Unfortunately, in a recent review of 53 fingertip injuries treated with a reconstructive procedure, bone shortening with closure, or secondary healing, Wang and colleagues18 found no discernible differences in outcomes at 4.5-year follow-up. They questioned whether complex reconstructive procedures are worth the time, expense, and risk. In the absence of prospective, comparative studies, surgeons must rely on anecdotal evidence (including predominantly level IV evidence), training bias, previous experience, and the prevailing common wisdom.
Toward that end, we became interested in identifying treatment preferences for fingertip amputations. We conducted a study to better understand how surgeon and patient factors influence the treatment preferences for distal fingertip amputations among a cross section of US and international hand surgeons. We hypothesized that hand surgeons’ treatment preferences would be varied and influenced by surgeon and patient demographics.
Materials and Methods
An online multiple-choice survey was created and powered by Constant Contact. The survey consisted of 6 surgeon demographic questions; 5 treatment preference questions regarding patient age, sex, occupation, and germinal matrix management; and 5 clinical scenarios based on Allen levels 2, 3 (with and without exposed distal phalanx), and 4 and volar oblique middle-finger amputations. The Allen classification designates level 2 injuries as those involving only the distal pulp and nail.19 Level 3 injuries also involve the terminal distal phalanx, and level 4 injuries extend to the lunula. The survey questions are listed in the Appendix. For the clinical scenario questions, treatment choices included wound care, skeletal shortening and closure, composite graft, autograft, allograft, V-Y/Kutler flap, advancement flap, thenar flap, cross-finger flap, pedicle and homodigital flap, replantation, and other.
An email invitation was sent to members of the American Association for Hand Surgery (AAHS). The survey was also submitted to personal contacts of international hand societies named on the AAHS website to expand the international response. A reminder email was sent 1 week after the original invitation. The survey was closed 5 weeks later, and the responses were analyzed with all non-US hand surgeons grouped collectively as an international group, compared with the US group. Institutional review board approval was not needed for this survey study.
Statistics
A generalized linear regression model was used to implement logistic regression with random effects for question and respondent. This approach accounts for multiple observations from the same respondent, assuming that both respondent and question are random samples from a larger population. The model estimated the probability that a given surgical approach (eg, skeletal shortening, wound care) would be selected, based on the predictors of the US versus international respondent, time in practice, practice type, and whether the fingertip was available. The model returned adjusted odds ratios (ORs) for each predictor, controlling for all the others. By convention, P < .05 was considered significant. No attempt was made to prune the model of nonsignificant factors. Analyses were performed using the lme4 package on the R statistical platform (R Foundation for Statistical Computing).
Results
One hundred ninety-eight responses were recorded. Of the 1054 AAHS members invited to take the survey, 174 (US, international) responded (17% response rate). One hundred twenty-three responses and 62% of the total were generated from US hand surgeons. Fifty-eight percent of US responses were from the Mid-South, Midwest, or Mid-Atlantic region. Fifty-seven percent of international responses were from Brazil and Europe. Respondents’ demographic data are listed in Tables 1 and 2.
Responses to the 5 clinical scenarios showed a wide variation in treatment preferences. The top 6 preferred treatment selections for an acute, clean long-finger amputation in a healthy 40-year-old office worker are shown in Figures 1 to 5. When surgeons who preferred replant were asked what they would do if the amputated part was not available, they indicated flap coverage more often than less complex treatments, such as skeletal shortening/primary closure or wound care.
There were statistically significant differences in treatment preferences between US and international hand surgeons when controlling for all other demographic variables. Adjusted ORs and their confidence intervals (CIs) for the aggregate clinical scenarios are presented in a forest plot in Figure 6. Figure 4 shows that US surgeons were more likely to choose wound care (OR, 3.6; P < .0004) and less likely to attempt a replant (OR, 0.01; P < .0001). US surgeons were also less likely to use a pedicle or homodigital island flap when the amputated fingertip was both available (OR, 0.04; P = .039) and unavailable (OR, 0.47; Ps = .029).
Among all respondents and across all clinical scenarios, skeletal shortening with closure was favored among hand surgeons in practice less than 5 years compared with those in practice longer (OR, 2.11; 95% CI, 1.36-3.25; P = .0008). Similarly, surgeons with more than 30 years of experience were the least likely to favor wound care (OR, 0.2; 95% CI, 0.09-0.93; P = .037). Compared with orthopedic surgeons, plastic surgeons opted for wound care less often (OR, 0.44; 95% CI, 0.23-0.98; P = .018) and appeared to prefer replantation, but the difference was not statistically significant (OR, 8.86; 95% CI, 0.99-79.61; P = .054).
Replantation was less often chosen by private practice versus full-time academic surgeons (OR, 0.09; 95% CI, 0.01-0.91; P = .041.) Part-time academics were no more or less likely to perform replantation than full-time academics were (OR, 0.52; 95% CI, 0.05-5.41; P = .58). Of the 59 respondents who performed more than 10 microvascular cases a year, 18 (31%) chose replant for Allen level 4 amputations. In comparison, 9 (20%) of the 45 respondents who performed fewer than 3 microvascular cases a year chose replant for amputations at this level. Amount of time working with fellows did not affect treatment preferences.
Patient demographics (age, sex, occupation) also played a role in treatment decisions (Table 3). The most significant factors appeared to be age and occupation. Regarding age, 41% of respondents chose more complex procedures for patients younger than 15, and 62% chose less complex procedures for patients older than 70 years. Regarding occupation, 61% chose more complex procedures for professional musicians, and 60% chose less complex procedures for manual laborers. Sex did not influence clinical decisions for 78% of respondents. There was also substantial variation in both the indications for germinal matrix ablation and the frequency of sterile matrix transplant (Table 3).
Discussion
Although there is a variety of treatment options and published treatment guidelines for distal fingertip amputations, few comparative studies support use of one treatment over another. In our experience, treatment decisions are based mainly on injury parameters, but surgeon preference and patient factors (age, sex, occupation) can also influence care. Our goal in this study was to better understand how surgeon and patient factors influence treatment preferences for distal fingertip amputations among a cross section of US and international hand surgeons. Our survey results showed lack of consensus among hand surgeons and highlighted several trends.
As expected, we found a wide range of treatment preferences for each clinical scenario queried, ranging from more simple treatments (eg, wound care) to more complex ones (eg, replantation). With patient parameters (age, profession, finger, acuity, injury type, tissue preservation, smoking status) standardized in the clinical scenarios, the treatment differences noted should reflect surgeon preference. However, other patient factors (eg, cultural differences, religious beliefs, surgeon setting, practice pattern, resource availability) that were not included in the clinical scenarios could also affect treatment preferences.
One particularly interesting finding was that international hand surgeons were 6.8 times more likely to replant a distal fingertip amputation. One possible explanation for this variation is the influence of cultural differences. For example, in East Asian countries, there can be a cultural stigma associated with loss of a fingertip, and therefore more of a desire on the part of the patient to restore the original finger.20,21 In addition, the international respondents were biased toward academic practices—which could skew the treatment preference toward replantation, as we found that academic surgeons were more inclined to replantation.
Our finding that replantation was more commonly preferred by academic versus private practice surgeons may suggest a training bias, an affinity for more complex or interesting procedures, or access to hospital equipment and staff, including residents and fellows, not usually found at smaller community hospitals, where private practice surgeons are more commonly based. Jazayeri and colleagues22 found that institutions specializing in microsurgery often produced better outcomes than nonspecializing institutions. Therefore, it is not surprising that private practice hand surgeons may less often opt to replant a distal fingertip amputation. It is also not surprising that plastic surgeons are more inclined to perform a replantation or flap coverage, as their training is more microsurgery-intensive and their practice more focused on aesthetics compared with the other specialists.
Distal fingertip replantation is accepted by most as technically demanding, but it seems that the additional effort and resources would be justified if the procedure provided a superior outcome. However, other factors, such as cost of treatment and length of recovery, should also be considered. Average replantation cost has been estimated to range from $7500 to $14,000, compared with $2800 for non-replantation-related care, and median stay is about 4 days longer for replantation-related care.23,24 These estimates do not include indirect costs, such as for postoperative rehabilitation, which is likely longer and more expensive, even in distal fingertip replantation. These disparities may not justify the outcome (of having a complete fingertip) if more conservative treatments yield similar results.17,18 In addition, there is the expected failure rate of limb replantation surgery. In analysis of the overall societal costs and benefits of larger upper extremity limb replantation, the loss of invested resources sustained with failed limb replantation may be outweighed by the benefit of another patient having a successful outcome. In the case of fingertip replantation, however, does the undefined benefit of the successful patient outcome outweigh the investment of resources lost in cases of replantation failure? Understandably, there is a need for more robust clinical outcome and cost-comparative evidence to better inform decisions regarding distal fingertip amputation.
We found that wound care and skeletal shortening with primary closure (particularly with Allen level 3 injuries) were preferred more by surgeons within the first 5 years of practice. This finding seems to imply a lack of experience or confidence on the part of younger surgeons performing more complex procedures, such as flap coverage. Conversely, this finding may indicate a shift in treatment principle based on recent literature suggesting equivalent outcomes with simpler procedures.17,18 Although our survey study did not provide an option for treatment combinations or staged procedures, several respondents wrote in that skeletal shortening supplemented with various types of autografts and allografts would be their preferred treatment.
Patient factors also play a significant role in clinical decisions. Age and profession seem to be important determinants, with more than 50% of respondents, on average, changing their treatment recommendation based on these 2 factors. A majority of respondents would perform a less involved procedure for a manual laborer, suggesting a quicker return to work is prioritized over a perceived improved clinical outcome. Interestingly, for patients younger than 15 years, the preference was divided, with 41% of surgeons opting for a more complex procedure. This suggests the importance of restoring anatomy in a younger patient, or the perceived decreased risk or failure rate with more involved treatment. Twenty percent preferred a less complex procedure in a younger patient, perhaps relying on the patient’s developmental potential for a good outcome or suggesting a concern for patient intolerance or compliance with complex surgery.
Nail plate regrowth can be a problem with fingertip amputations. Nail deformity is highly correlated with injury level, with amputations proximal to the lunula more likely to cause nail plate deformity.25,26 Jebson and colleagues27 recommended germinal matrix ablation for amputations proximal to the lunula. We found respondents often performed ablations for other indications, including injured or minimal remaining sterile matrix and lack of bony support for the sterile matrix. Forty-six percent of respondents had never performed sterile matrix transplant, which could indicate that they were unfamiliar with the technique or had donor-site concerns, or that postinjury nail deformities are uncommon, well tolerated, or treated along with other procedures, such as germinal matrix ablation.
Several weaknesses of this study must be highlighted. First, our response rate was smaller than desired. Although this work incorporated a large number of surgeon responses, nearly 200, the response rate was only 17%. In addition, although number of responses was likely adequate to show the diversity of opinion, the preferences and trends reported might not be representative of all hand surgeons. We could not perform a nonresponder analysis because of a lack of specific demographic data for the AAHS and international hand society members. However, AAHS has an approximate 50/50 mix of plastic and orthopedic surgeons, similar to our responder demographic, suggesting our smaller subset of responses might be representative of the whole. According to AAHS, a majority of its members are “academic” hand surgeons, so our results might not adequately reflect the preferences of community hand surgeons and ultimately might overstate the frequency of more complex treatments. Last, our international response was limited to a few countries. A larger, more broadly distributed response would provide a better understanding of regional preferences, which could shed light on the importance of cultural differences.
Variations in patient insurance status were not queried in this survey but might also affect treatment decisions. More involved, costly, and highly reimbursing procedures might be deemed reasonable options for a small perceived clinical benefit for insured patients.
When multiple digits or the thumb is injured, or there are other concomitant injuries, surgeons may alter their choice of intervention. In mangled extremities, preservation of salvageable functional units takes precedence over aesthetics and likely affects choice of treatment for the amputated fingertips. Similarly, multiple fingertip amputations, even if all at the same level, may be differently regarded than a solitary injury.
Conclusion
For distal fingertip amputations, there is little evidence supporting one approach over another. Without level I comparative data guiding treatment, anecdotal evidence and surgeon personal preferences likely contribute to the large variation noted in this survey. Our study results showed the disparity of fingertip treatment preferences among a cross section of US and international hand surgeons. More important, results underscored the need for a well-designed comparative study to determine the most effective treatments for distal fingertip amputations.
Finger injuries are common, representing an estimated 3 million emergency department visits per year in the United States, with 44% of these diagnosed as lacerations.1 Amputations of the finger (partial and complete) in non-work-related accidents alone are estimated at 30,000 per year.1 The fingertip is a highly specialized structure that contributes to precision function of the hand through tactile feedback and fine motor control as well as hand aesthetics. An injury can compromise a variety of fingertip structures, including the distal phalanx, which provides length and structural support; the fingernail, germinal matrix, and sterile matrix, which protect the fingertip and function as tools; and the volar skin pad, which is important for sensation and fine motor activity.
There is considerable debate regarding optimal management of fingertip amputations, and to date there have been no prospective, randomly controlled trials to guide treatment.2 Injury characteristics, amputation levels, and patient priorities all contribute to management decisions. Treatment goals are to maintain length when possible; to provide stable, supple, and sensate skin coverage; to ensure the nail plate regrows without complication; and to maintain normal overall finger shape and cosmesis. In addition, a simple, cost-effective treatment with short recovery time and no donor-site morbidity is desired.
Treatment recommendations are wide-ranging, and evidence-based literature is sparse. About 30 years ago, 2 retrospective comparative studies found no difference in outcomes between simpler treatments (primary closure, secondary wound healing) and various operative strategies.3,4 Since then, most of the scientific studies have been retrospective noncomparative case series, all reporting good to excellent results.5-17 Investigators generally implied superior results of a studied procedure over those of more conservative treatments. Recommended treatments include secondary wound healing, simple flaps, staged flaps, pedicle flaps, allograft and autograft coverage, composite grafting, and replantation, for all levels of fingertip injury.
Given our surgical advances, improved techniques, and accumulating experience, we may have expected better outcomes with newer and more complex reconstructive efforts. Unfortunately, in a recent review of 53 fingertip injuries treated with a reconstructive procedure, bone shortening with closure, or secondary healing, Wang and colleagues18 found no discernible differences in outcomes at 4.5-year follow-up. They questioned whether complex reconstructive procedures are worth the time, expense, and risk. In the absence of prospective, comparative studies, surgeons must rely on anecdotal evidence (including predominantly level IV evidence), training bias, previous experience, and the prevailing common wisdom.
Toward that end, we became interested in identifying treatment preferences for fingertip amputations. We conducted a study to better understand how surgeon and patient factors influence the treatment preferences for distal fingertip amputations among a cross section of US and international hand surgeons. We hypothesized that hand surgeons’ treatment preferences would be varied and influenced by surgeon and patient demographics.
Materials and Methods
An online multiple-choice survey was created and powered by Constant Contact. The survey consisted of 6 surgeon demographic questions; 5 treatment preference questions regarding patient age, sex, occupation, and germinal matrix management; and 5 clinical scenarios based on Allen levels 2, 3 (with and without exposed distal phalanx), and 4 and volar oblique middle-finger amputations. The Allen classification designates level 2 injuries as those involving only the distal pulp and nail.19 Level 3 injuries also involve the terminal distal phalanx, and level 4 injuries extend to the lunula. The survey questions are listed in the Appendix. For the clinical scenario questions, treatment choices included wound care, skeletal shortening and closure, composite graft, autograft, allograft, V-Y/Kutler flap, advancement flap, thenar flap, cross-finger flap, pedicle and homodigital flap, replantation, and other.
An email invitation was sent to members of the American Association for Hand Surgery (AAHS). The survey was also submitted to personal contacts of international hand societies named on the AAHS website to expand the international response. A reminder email was sent 1 week after the original invitation. The survey was closed 5 weeks later, and the responses were analyzed with all non-US hand surgeons grouped collectively as an international group, compared with the US group. Institutional review board approval was not needed for this survey study.
Statistics
A generalized linear regression model was used to implement logistic regression with random effects for question and respondent. This approach accounts for multiple observations from the same respondent, assuming that both respondent and question are random samples from a larger population. The model estimated the probability that a given surgical approach (eg, skeletal shortening, wound care) would be selected, based on the predictors of the US versus international respondent, time in practice, practice type, and whether the fingertip was available. The model returned adjusted odds ratios (ORs) for each predictor, controlling for all the others. By convention, P < .05 was considered significant. No attempt was made to prune the model of nonsignificant factors. Analyses were performed using the lme4 package on the R statistical platform (R Foundation for Statistical Computing).
Results
One hundred ninety-eight responses were recorded. Of the 1054 AAHS members invited to take the survey, 174 (US, international) responded (17% response rate). One hundred twenty-three responses and 62% of the total were generated from US hand surgeons. Fifty-eight percent of US responses were from the Mid-South, Midwest, or Mid-Atlantic region. Fifty-seven percent of international responses were from Brazil and Europe. Respondents’ demographic data are listed in Tables 1 and 2.
Responses to the 5 clinical scenarios showed a wide variation in treatment preferences. The top 6 preferred treatment selections for an acute, clean long-finger amputation in a healthy 40-year-old office worker are shown in Figures 1 to 5. When surgeons who preferred replant were asked what they would do if the amputated part was not available, they indicated flap coverage more often than less complex treatments, such as skeletal shortening/primary closure or wound care.
There were statistically significant differences in treatment preferences between US and international hand surgeons when controlling for all other demographic variables. Adjusted ORs and their confidence intervals (CIs) for the aggregate clinical scenarios are presented in a forest plot in Figure 6. Figure 4 shows that US surgeons were more likely to choose wound care (OR, 3.6; P < .0004) and less likely to attempt a replant (OR, 0.01; P < .0001). US surgeons were also less likely to use a pedicle or homodigital island flap when the amputated fingertip was both available (OR, 0.04; P = .039) and unavailable (OR, 0.47; Ps = .029).
Among all respondents and across all clinical scenarios, skeletal shortening with closure was favored among hand surgeons in practice less than 5 years compared with those in practice longer (OR, 2.11; 95% CI, 1.36-3.25; P = .0008). Similarly, surgeons with more than 30 years of experience were the least likely to favor wound care (OR, 0.2; 95% CI, 0.09-0.93; P = .037). Compared with orthopedic surgeons, plastic surgeons opted for wound care less often (OR, 0.44; 95% CI, 0.23-0.98; P = .018) and appeared to prefer replantation, but the difference was not statistically significant (OR, 8.86; 95% CI, 0.99-79.61; P = .054).
Replantation was less often chosen by private practice versus full-time academic surgeons (OR, 0.09; 95% CI, 0.01-0.91; P = .041.) Part-time academics were no more or less likely to perform replantation than full-time academics were (OR, 0.52; 95% CI, 0.05-5.41; P = .58). Of the 59 respondents who performed more than 10 microvascular cases a year, 18 (31%) chose replant for Allen level 4 amputations. In comparison, 9 (20%) of the 45 respondents who performed fewer than 3 microvascular cases a year chose replant for amputations at this level. Amount of time working with fellows did not affect treatment preferences.
Patient demographics (age, sex, occupation) also played a role in treatment decisions (Table 3). The most significant factors appeared to be age and occupation. Regarding age, 41% of respondents chose more complex procedures for patients younger than 15, and 62% chose less complex procedures for patients older than 70 years. Regarding occupation, 61% chose more complex procedures for professional musicians, and 60% chose less complex procedures for manual laborers. Sex did not influence clinical decisions for 78% of respondents. There was also substantial variation in both the indications for germinal matrix ablation and the frequency of sterile matrix transplant (Table 3).
Discussion
Although there is a variety of treatment options and published treatment guidelines for distal fingertip amputations, few comparative studies support use of one treatment over another. In our experience, treatment decisions are based mainly on injury parameters, but surgeon preference and patient factors (age, sex, occupation) can also influence care. Our goal in this study was to better understand how surgeon and patient factors influence treatment preferences for distal fingertip amputations among a cross section of US and international hand surgeons. Our survey results showed lack of consensus among hand surgeons and highlighted several trends.
As expected, we found a wide range of treatment preferences for each clinical scenario queried, ranging from more simple treatments (eg, wound care) to more complex ones (eg, replantation). With patient parameters (age, profession, finger, acuity, injury type, tissue preservation, smoking status) standardized in the clinical scenarios, the treatment differences noted should reflect surgeon preference. However, other patient factors (eg, cultural differences, religious beliefs, surgeon setting, practice pattern, resource availability) that were not included in the clinical scenarios could also affect treatment preferences.
One particularly interesting finding was that international hand surgeons were 6.8 times more likely to replant a distal fingertip amputation. One possible explanation for this variation is the influence of cultural differences. For example, in East Asian countries, there can be a cultural stigma associated with loss of a fingertip, and therefore more of a desire on the part of the patient to restore the original finger.20,21 In addition, the international respondents were biased toward academic practices—which could skew the treatment preference toward replantation, as we found that academic surgeons were more inclined to replantation.
Our finding that replantation was more commonly preferred by academic versus private practice surgeons may suggest a training bias, an affinity for more complex or interesting procedures, or access to hospital equipment and staff, including residents and fellows, not usually found at smaller community hospitals, where private practice surgeons are more commonly based. Jazayeri and colleagues22 found that institutions specializing in microsurgery often produced better outcomes than nonspecializing institutions. Therefore, it is not surprising that private practice hand surgeons may less often opt to replant a distal fingertip amputation. It is also not surprising that plastic surgeons are more inclined to perform a replantation or flap coverage, as their training is more microsurgery-intensive and their practice more focused on aesthetics compared with the other specialists.
Distal fingertip replantation is accepted by most as technically demanding, but it seems that the additional effort and resources would be justified if the procedure provided a superior outcome. However, other factors, such as cost of treatment and length of recovery, should also be considered. Average replantation cost has been estimated to range from $7500 to $14,000, compared with $2800 for non-replantation-related care, and median stay is about 4 days longer for replantation-related care.23,24 These estimates do not include indirect costs, such as for postoperative rehabilitation, which is likely longer and more expensive, even in distal fingertip replantation. These disparities may not justify the outcome (of having a complete fingertip) if more conservative treatments yield similar results.17,18 In addition, there is the expected failure rate of limb replantation surgery. In analysis of the overall societal costs and benefits of larger upper extremity limb replantation, the loss of invested resources sustained with failed limb replantation may be outweighed by the benefit of another patient having a successful outcome. In the case of fingertip replantation, however, does the undefined benefit of the successful patient outcome outweigh the investment of resources lost in cases of replantation failure? Understandably, there is a need for more robust clinical outcome and cost-comparative evidence to better inform decisions regarding distal fingertip amputation.
We found that wound care and skeletal shortening with primary closure (particularly with Allen level 3 injuries) were preferred more by surgeons within the first 5 years of practice. This finding seems to imply a lack of experience or confidence on the part of younger surgeons performing more complex procedures, such as flap coverage. Conversely, this finding may indicate a shift in treatment principle based on recent literature suggesting equivalent outcomes with simpler procedures.17,18 Although our survey study did not provide an option for treatment combinations or staged procedures, several respondents wrote in that skeletal shortening supplemented with various types of autografts and allografts would be their preferred treatment.
Patient factors also play a significant role in clinical decisions. Age and profession seem to be important determinants, with more than 50% of respondents, on average, changing their treatment recommendation based on these 2 factors. A majority of respondents would perform a less involved procedure for a manual laborer, suggesting a quicker return to work is prioritized over a perceived improved clinical outcome. Interestingly, for patients younger than 15 years, the preference was divided, with 41% of surgeons opting for a more complex procedure. This suggests the importance of restoring anatomy in a younger patient, or the perceived decreased risk or failure rate with more involved treatment. Twenty percent preferred a less complex procedure in a younger patient, perhaps relying on the patient’s developmental potential for a good outcome or suggesting a concern for patient intolerance or compliance with complex surgery.
Nail plate regrowth can be a problem with fingertip amputations. Nail deformity is highly correlated with injury level, with amputations proximal to the lunula more likely to cause nail plate deformity.25,26 Jebson and colleagues27 recommended germinal matrix ablation for amputations proximal to the lunula. We found respondents often performed ablations for other indications, including injured or minimal remaining sterile matrix and lack of bony support for the sterile matrix. Forty-six percent of respondents had never performed sterile matrix transplant, which could indicate that they were unfamiliar with the technique or had donor-site concerns, or that postinjury nail deformities are uncommon, well tolerated, or treated along with other procedures, such as germinal matrix ablation.
Several weaknesses of this study must be highlighted. First, our response rate was smaller than desired. Although this work incorporated a large number of surgeon responses, nearly 200, the response rate was only 17%. In addition, although number of responses was likely adequate to show the diversity of opinion, the preferences and trends reported might not be representative of all hand surgeons. We could not perform a nonresponder analysis because of a lack of specific demographic data for the AAHS and international hand society members. However, AAHS has an approximate 50/50 mix of plastic and orthopedic surgeons, similar to our responder demographic, suggesting our smaller subset of responses might be representative of the whole. According to AAHS, a majority of its members are “academic” hand surgeons, so our results might not adequately reflect the preferences of community hand surgeons and ultimately might overstate the frequency of more complex treatments. Last, our international response was limited to a few countries. A larger, more broadly distributed response would provide a better understanding of regional preferences, which could shed light on the importance of cultural differences.
Variations in patient insurance status were not queried in this survey but might also affect treatment decisions. More involved, costly, and highly reimbursing procedures might be deemed reasonable options for a small perceived clinical benefit for insured patients.
When multiple digits or the thumb is injured, or there are other concomitant injuries, surgeons may alter their choice of intervention. In mangled extremities, preservation of salvageable functional units takes precedence over aesthetics and likely affects choice of treatment for the amputated fingertips. Similarly, multiple fingertip amputations, even if all at the same level, may be differently regarded than a solitary injury.
Conclusion
For distal fingertip amputations, there is little evidence supporting one approach over another. Without level I comparative data guiding treatment, anecdotal evidence and surgeon personal preferences likely contribute to the large variation noted in this survey. Our study results showed the disparity of fingertip treatment preferences among a cross section of US and international hand surgeons. More important, results underscored the need for a well-designed comparative study to determine the most effective treatments for distal fingertip amputations.
1. Conn JM, Annest JL, Ryan GW, Budnitz DS. Non-work-related finger amputations in the United States, 2001-2002. Ann Emerg Med. 2005;45(6):630-635.
2. Bickel KD, Dosanjh A. Fingertip reconstruction. J Hand Surg Am. 2008;33(8):1417-1419.
3. Söderberg T, Nyström Å, Hallmans G, Hultén J. Treatment of fingertip amputations with bone exposure. A comparative study between surgical and conservative treatment methods. Scand J Plast Reconstr Surg. 1983;17(2):147-152.
4. Braun M, Horton RC, Snelling CF. Fingertip amputation: review of 100 digits. Can J Surg. 1985;28(1):72-75.
5. Sammut D. Fingertip injuries. A review of indications and methods of management. Curr Orthop. 2002;16:271-285.
6. Mennen U, Wiese A. Fingertip injuries management with semi-occlusive dressing. J Hand Surg Br. 1993;18(4):416-422.
7. Atasoy E, Ioakimidis E, Kasdan ML, Kutz JE, Kleinert HE. Reconstruction of the amputated fingertip with a triangular volar flap. A new surgical procedure. J Bone Joint Surg Am. 1970;52(5):921-926.
8. Kutler W. A new method for finger tip amputation. J Am Med Assoc. 1947;133(1):29-30.
9. Takeishi M, Shinoda A, Sugiyama A, Ui K. Innervated reverse dorsal digital island flap for fingertip reconstruction. J Hand Surg Am. 2006;31(7):1094-1099.
10. Tuncali D, Barutcu AY, Gokrem S, Terzioglu A, Aslan G. The hatchet flap for reconstruction of fingertip amputations. Plast Reconstr Surg. 2006;117(6):1933-1939.
11. Teoh LC, Tay SC, Yong FC, Tan SH, Khoo DB. Heterodigital arterialized flaps for large finger wounds: results and indications. Plast Reconstr Surg. 2003;111(6):1905-1913.
12. Nishikawa H, Smith PJ. The recovery of sensation and function after cross-finger flaps for fingertip injury. J Hand Surg Br. 1992;17(1):102-107.
13. Rinker B. Fingertip reconstruction with the laterally based thenar flap: indications and long-term functional results. Hand. 2006;1(1):2-8.
14. Jung MS, Lim YK, Hong YT, Kim HN. Treatment of fingertip amputation in adults by palmar pocketing of the amputated part. Arch Plast Surg. 2012;39(4):404-410.
15. Venkatramani H, Sabapathy SR. Fingertip replantation: technical considerations and outcome analysis of 24 consecutive fingertip replantations. Indian J Plast Surg. 2011;44(2):237-245.
16. Chen SY, Wang CH, Fu JP, Chang SC, Chen SG. Composite grafting for traumatic fingertip amputation in adults: technique reinforcement and experience in 31 digits. J Trauma. 2011;70(1):148-153.
17. van den Berg WB, Vergeer RA, van der Sluis CK, Ten Duis HJ, Werker PM. Comparison of three types of treatment modalities on the outcome of fingertip injuries. J Trauma Acute Care Surg. 2012;72(6):1681-1687.
18. Wang K, Sears ED, Shauver MJ, Chung KC. A systematic review of outcomes of revision amputation treatment for fingertip amputations. Hand. 2013;8(2):139-145.
19. Allen MJ. Conservative management of finger tip injuries in adults. Hand. 1980;12(3):257-265.
20. Chen CT, Wei FC, Chen HC, Chuang CC, Chen HT, Hsu WM. Distal phalanx replantation. Microsurgery. 1994;15(1):77-82.
21. Kim WK, Lim JH, Han SK. Fingertip replantations: clinical evaluation of 135 digits. Plast Reconstr Surg. 1996;98(3):470-476.
22. Jazayeri L, Klausner JQ, Chang J. Distal digital replantation. Plast Reconstr Surg. 2013;132(5):1207-1217.
23. Hattori Y, Doi K, Sakamoto S, Yamasaki H, Wahegaonkar A, Addosooki A. Fingertip replantation. J Hand Surg Am. 2007;32(4):548-555.
24. Goldner RD, Stevanovic MV, Nunley JA, Urbaniak JR. Digital replantation at the level of the distal interphalangeal joint and the distal phalanx. J Hand Surg Am. 1989;14(2 pt 1):214-220.
25. Nishi G, Shibata Y, Tago K, Kubota M, Suzuki M. Nail regeneration in digits replanted after amputation through the distal phalanx. J Hand Surg Am. 1996;21(2):229-233.
26. Yamano Y. Replantation of the amputated distal part of the fingers. J Hand Surg Am. 1985;10(2):211-218.
27. Jebson PJ, Louis DS, Bagg M. Amputations. In: Wolfe SW, Pederson WC, Hotchkiss RN, Kozin SH, eds. Green’s Operative Hand Surgery. 6th ed. Philadelphia, PA: Churchill Livingstone; 2010:1885-1927.
1. Conn JM, Annest JL, Ryan GW, Budnitz DS. Non-work-related finger amputations in the United States, 2001-2002. Ann Emerg Med. 2005;45(6):630-635.
2. Bickel KD, Dosanjh A. Fingertip reconstruction. J Hand Surg Am. 2008;33(8):1417-1419.
3. Söderberg T, Nyström Å, Hallmans G, Hultén J. Treatment of fingertip amputations with bone exposure. A comparative study between surgical and conservative treatment methods. Scand J Plast Reconstr Surg. 1983;17(2):147-152.
4. Braun M, Horton RC, Snelling CF. Fingertip amputation: review of 100 digits. Can J Surg. 1985;28(1):72-75.
5. Sammut D. Fingertip injuries. A review of indications and methods of management. Curr Orthop. 2002;16:271-285.
6. Mennen U, Wiese A. Fingertip injuries management with semi-occlusive dressing. J Hand Surg Br. 1993;18(4):416-422.
7. Atasoy E, Ioakimidis E, Kasdan ML, Kutz JE, Kleinert HE. Reconstruction of the amputated fingertip with a triangular volar flap. A new surgical procedure. J Bone Joint Surg Am. 1970;52(5):921-926.
8. Kutler W. A new method for finger tip amputation. J Am Med Assoc. 1947;133(1):29-30.
9. Takeishi M, Shinoda A, Sugiyama A, Ui K. Innervated reverse dorsal digital island flap for fingertip reconstruction. J Hand Surg Am. 2006;31(7):1094-1099.
10. Tuncali D, Barutcu AY, Gokrem S, Terzioglu A, Aslan G. The hatchet flap for reconstruction of fingertip amputations. Plast Reconstr Surg. 2006;117(6):1933-1939.
11. Teoh LC, Tay SC, Yong FC, Tan SH, Khoo DB. Heterodigital arterialized flaps for large finger wounds: results and indications. Plast Reconstr Surg. 2003;111(6):1905-1913.
12. Nishikawa H, Smith PJ. The recovery of sensation and function after cross-finger flaps for fingertip injury. J Hand Surg Br. 1992;17(1):102-107.
13. Rinker B. Fingertip reconstruction with the laterally based thenar flap: indications and long-term functional results. Hand. 2006;1(1):2-8.
14. Jung MS, Lim YK, Hong YT, Kim HN. Treatment of fingertip amputation in adults by palmar pocketing of the amputated part. Arch Plast Surg. 2012;39(4):404-410.
15. Venkatramani H, Sabapathy SR. Fingertip replantation: technical considerations and outcome analysis of 24 consecutive fingertip replantations. Indian J Plast Surg. 2011;44(2):237-245.
16. Chen SY, Wang CH, Fu JP, Chang SC, Chen SG. Composite grafting for traumatic fingertip amputation in adults: technique reinforcement and experience in 31 digits. J Trauma. 2011;70(1):148-153.
17. van den Berg WB, Vergeer RA, van der Sluis CK, Ten Duis HJ, Werker PM. Comparison of three types of treatment modalities on the outcome of fingertip injuries. J Trauma Acute Care Surg. 2012;72(6):1681-1687.
18. Wang K, Sears ED, Shauver MJ, Chung KC. A systematic review of outcomes of revision amputation treatment for fingertip amputations. Hand. 2013;8(2):139-145.
19. Allen MJ. Conservative management of finger tip injuries in adults. Hand. 1980;12(3):257-265.
20. Chen CT, Wei FC, Chen HC, Chuang CC, Chen HT, Hsu WM. Distal phalanx replantation. Microsurgery. 1994;15(1):77-82.
21. Kim WK, Lim JH, Han SK. Fingertip replantations: clinical evaluation of 135 digits. Plast Reconstr Surg. 1996;98(3):470-476.
22. Jazayeri L, Klausner JQ, Chang J. Distal digital replantation. Plast Reconstr Surg. 2013;132(5):1207-1217.
23. Hattori Y, Doi K, Sakamoto S, Yamasaki H, Wahegaonkar A, Addosooki A. Fingertip replantation. J Hand Surg Am. 2007;32(4):548-555.
24. Goldner RD, Stevanovic MV, Nunley JA, Urbaniak JR. Digital replantation at the level of the distal interphalangeal joint and the distal phalanx. J Hand Surg Am. 1989;14(2 pt 1):214-220.
25. Nishi G, Shibata Y, Tago K, Kubota M, Suzuki M. Nail regeneration in digits replanted after amputation through the distal phalanx. J Hand Surg Am. 1996;21(2):229-233.
26. Yamano Y. Replantation of the amputated distal part of the fingers. J Hand Surg Am. 1985;10(2):211-218.
27. Jebson PJ, Louis DS, Bagg M. Amputations. In: Wolfe SW, Pederson WC, Hotchkiss RN, Kozin SH, eds. Green’s Operative Hand Surgery. 6th ed. Philadelphia, PA: Churchill Livingstone; 2010:1885-1927.
The Role of Computed Tomography in Evaluating Intra-Articular Distal Humerus Fractures
Elbow fractures constitute 7% of all adult fractures, and 30% of these fractures are distal humerus fractures.1,2 Of these, 96% involve disruption of the articular surface.3 Intra-articular distal humerus fracture patterns can be difficult to characterize on plain radiographs, and therefore computed tomography (CT) is often used. The surgeon’s understanding of the fracture pattern and the deforming forces affects choice of surgical approach. In particular, multiplanar fracture patterns, including coronal shear fractures of the capitellum or trochlea, are often difficult to recognize on plain radiographs. Identification of a multiplanar fracture pattern may require a change in approach or fixation. CT is useful for other intra-articular fractures, such as those of the proximal humerus,3-6 but involves increased radiation and cost.
We conducted a study to determine the effect of adding CT evaluation to plain radiographic evaluation on the classification of, and treatment plans for, intra-articular distal humerus fractures. We hypothesized that adding CT images to plain radiographs would change the classification and treatment of these fractures and would improve interobserver agreement on classification and treatment.
Materials and Methods
After obtaining University of Southern California Institutional Review Board approval, we retrospectively studied 30 consecutive cases of adult intra-articular distal humerus fractures treated by Dr. Itamura at a level I trauma center between 1995 and 2008. In each case, the injured elbow was imaged with plain radiography and CT. Multiple machines were used for CT, but all according to the radiology department’s standard protocol. The images were evaluated by 9 independent observers from the same institution: 3 orthopedic surgeons (1 fellowship-trained shoulder/elbow subspecialist, 1 fellowship-trained upper extremity subspecialist, 1 fellowship-trained orthopedic trauma surgeon), 3 shoulder/elbow fellows, and 3 senior residents pursuing upper extremity fellowships on graduation. No observer was involved in the care of any of the patients. All identifying details were removed from the patient information presented to the observers. For each set of images, the observer was asked to classify the fractures according to the Mehne and Matta classification system,7,8 which is the predominant system used at our institution.
Diagrams of this classification system were provided, but there was no formal observer training or calibration. Seven treatment options were presented: (1) open reduction and internal fixation (ORIF) using a posterior approach with olecranon osteotomy, (2) ORIF using a posterior approach, (3) ORIF using a lateral approach, (4) ORIF using a medial approach, (5) ORIF using an anterior/anterolateral approach, (6) total elbow arthroplasty, and (7) nonoperative management. The only clinical data provided were patient age and sex.
Images were evaluated in blinded fashion. Two rounds of evaluation were compared. In round 1, plain radiographs were evaluated; in round 2, the same radiographs plus corresponding 2-dimensional (2-D) CT images. A minimum of 1 month was required between viewing rounds.
Statistical Analysis
Statistical analysis was performed by the Statistical Consultation and Research Center at our institution. Cohen κ was calculated to estimate the reliability of the fracture classification and treatment plan made by different observers on the same occasion (interobserver reliability). Cramer V9 was calculated to estimate the reliability of the fracture classification and treatment plan made by the same observer on separate occasions (intraobserver reliability). It measures the association between the 2 ratings as a percentage of their total variation. The κ value and Cramer V value were also used to evaluate results based on the observers’ training levels. Both κ and Cramer V values are interpreted as follows: .00 to .20 indicates slight agreement; .21 to .40, fair agreement; .41-.60, moderate agreement; .61 to .80, substantial agreement; and ≥.81, almost perfect agreement. Zero represents no agreement, and 1.00 represents perfect agreement.
Results
Overall intraobserver reliability for classification was fair (.393). It was moderate for the treatment plan (.426) between viewing rounds. Residents had the highest Cramer V value at .60 (moderate) for classification reliability, and attending surgeons had the highest value at .52 (moderate) for treatment plan. All 3 groups (residents, fellows, attending surgeons) showed moderate intraobserver agreement for treatment plan (Table 1).
Interobserver reliability did not improve with the addition of CT in round 2. Reliability was fair at both viewing rounds for classification and for treatment. For classification, the overall κ value was .21 for the first round and .20 for the second round. For treatment plan, the overall κ value was .28 for the first round and .27 for the second round. Attending surgeons decreased in agreement with regard to treatment plan with the addition of CT (.46, moderate, to .32, fair). Fellows had only slight agreement for both rounds with regard to classification as well as treatment (Table 2).
ORIF using a posterior approach with an olecranon osteotomy was the most common choice of treatment method overall at both time points (58.1% and 63.7%) and was still the most common choice when each group of observers (residents, fellows, faculty) was considered separately (Figure 1).
When classifying the fractures, attending surgeons chose the multiplanar fracture pattern 25.6% of the time when viewing radiographs only, and remained consistent in choosing this pattern 23.3% of the time when CT was added to radiographs. Fellows and residents chose this fracture pattern much less often (8.9% and 7.8%, respectively) when viewing radiographs only. Both fellows and residents increased their choice of the multiplanar fracture pattern by 10% (18.9% for fellows, 17.8% for residents) when CT was added (Figure 2).
Overall, the recognition of a multiplanar fracture pattern increased when CT was added. On 30 occasions, an answer was changed from another classification pattern to the multiplanar pattern when CT was added. Only 6 times did an observer change a multiplanar pattern selection at round 1 to another choice at round 2.
Adding CT in round 2 changed the treatment plan for multiplanar fractures. At round 1, 73.7% chose ORIF using a lateral approach for treatment of the multiplanar fracture versus 10.5% who chose ORIF using a posterior approach with an olecranon osteotomy. The choice of the posterior approach with olecranon osteotomy increased to 51.9% at round 2, using the technique we have previously described.5,10
Overall intraobserver reliability for classification was fair (.393). It was moderate for the treatment plan (.426) between viewing rounds. Residents had the highest Cramer V value at .60 (moderate) for classification reliability, and faculty had the highest value at .52 (moderate) for treatment plan. All 3 groups (residents, fellows, attending surgeons) showed moderate intraobserver agreement for treatment plan (Table 1).
Interobserver reliability did not improve with the addition of CT in round 2. Reliability for classification was fair for round 1 and slight for round 2. Reliability was fair at both viewing rounds for treatment. For classification, the overall κ value was .21 for round 1 and .20 for round 2. For treatment plan, the overall κ value was .28 for round 1 and .27 for round 2. Attending surgeons decreased in agreement with regard to treatment plan with the addition of CT (.46, moderate, to .32, fair). Fellows had only slight agreement for both rounds with regard to classification as well as treatment (Table 2).
Discussion
In this study, CT changed both classification and treatment when added to plain radiographs. Interestingly, interobserver reliability did not improve for classification or treatment with the addition of CT. This finding suggests substantial disagreement among qualified observers that is not resolved with more sophisticated imaging. We propose this disagreement is caused by differences in training and experience with specific fracture patterns and surgical approaches.
Our fair to moderate interobserver reliability using radiographs only is consistent with a study by Wainwright and colleagues,11 who demonstrated fair to moderate interobserver reliability with radiographs only using 3 different classification systems. CT did not improve interobserver reliability in the present study.
To our knowledge, the effect of adding CT to plain radiographs on classification and treatment plan has not been evaluated. Doornberg and colleagues2 evaluated the effect of adding 3-dimensional (3-D) CT to a combination of radiographs and 2-D CT. Using the AO (Arbeitsgemeinschaft für Osteosynthesefragen) classification12 and the classification system of Mehne and Matta, they found that 3-D CT improved intraobserver and interobserver reliability for classification but improved only intraobserver agreement for treatment. Interobserver agreement for treatment plan remained fair. In parallel with their study, fracture classification in our study was more often changed with CT than the treatment plan was. Training level appeared not to affect this finding. We found fair interobserver agreement for treatment choice as well, which was not improved by adding CT. Doornberg and colleagues2 concluded that the “relatively small added expense of three-dimensional computed tomography scans seems worthwhile.”
When evaluating specific fracture patterns in the Mehne and Matta classification system, we observed that less experienced surgeons (residents, fellows) were much more likely to identify multiplanar fracture patterns with the aid of CT. Use of CT did not change attending surgeons’ recognition of these multiplanar fractures, suggesting that the faculty were more capable of appreciating these fracture patterns with radiographs only (Figure 3). We also observed that adding CT changed the predominant treatment plan for multiplanar fractures from a lateral approach to a posterior approach with an olecranon osteotomy. Failure to appreciate this component of the fracture before surgery could lead to an increased intraoperative difficulty level. Failure to appreciate it during surgery could lead to unexpected postoperative displacement and ultimately poorer outcome.
There are limitations to our study. There is no gold standard for assessing the accuracy of classification decisions. Intraoperative classification could have served as a gold standard, but the fractures were not routinely assigned a classification during surgery. Brouwer and colleagues13 evaluated the diagnostic accuracy of CT (including 3-D CT) with intraoperative AO classification as a reference point and found improvement in intraobserver agreement but not interobserver agreement when describing fracture characteristics—and no significant effect on classification.
We used a single classification system, the one primarily used at our institution and by Dr. Itamura. There are many systems,7,12,14 all with their strengths and weaknesses, and no one system is used universally. Adding a system would have allowed us to compare results of more than one system. Our aim, however, was to keep our form simple for the sake of participation and completion of the viewings by each volunteer.
Only 2-D CT was used for this study, as 3-D images were not available for all patients. Although this is a potential weakness, it appears that, based on the study by Doornberg and colleagues,2 adding 3-D imaging resulted in only modest improvement in the reliability of classification and no significant improvement in agreement on treatment recommendation.
In addition, our results were likely biased by the fact that 8 of the 9 evaluators were trained by Dr. Itamura, who very often uses a posterior approach with an olecranon osteotomy for internal fixation of distal humerus intra-articular fractures, as previously described.8,10 Therefore, selection of this treatment option may have been overestimated in this study. Nevertheless, after reviewing the literature, Ljungquist and colleagues15 wrote, “There do not seem to be superior functional results associated with any one surgical approach to the distal humerus.”
We did not give the evaluators an indication of patients’ activity demands (only age and sex), which may have been relevant when considering total elbow arthroplasty.
Last, performing another round of evaluations with only plain radiographs, before introducing CT, would have provided intraobserver reliability results on plain radiograph evaluation, which could have been compared with intraobserver reliability when CT was added. Again, this was excluded to encourage participation and create the least cumbersome evaluation experience possible, which was thought appropriate, as this information is already in the literature.
Conclusion
Adding CT changed classifications and treatment plans. Raters were more likely to change their classifications than their treatment plans. The addition of CT did not increase agreement between observers. Despite the added radiation and cost, we recommend performing CT for all intra-articular distal humerus fractures because it improves understanding of the fracture pattern and affects treatment planning, especially for fractures with a coronal shear component, which is often not appreciated on plain radiographs.
1. Anglen J. Distal humerus. J Am Acad Orthop Surg. 2005;13(5):291-297.
2. Doornberg J, Lindenhovius A, Kloen P, van Dijk CN, Zurakowski D, Ring D. Two and three-dimensional computed tomography for the classification and management of distal humerus fractures. Evaluation of reliability and diagnostic accuracy. J Bone Joint Surg Am. 2006;88(8):1795-1801.
3. Pollock JW, Faber KJ, Athwal GS. Distal humerus fractures. Orthop Clin North Am. 2008;39(2):187-200.
4. Castagno AA, Shuman WP, Kilcoyne RF, Haynor DR, Morris ME, Matsen FA. Complex fractures of the proximal humerus: role of CT in treatment. Radiology. 1987;165(3):759-762.
5. Palvanen M, Kannus P, Niemi S, Parkkari J. Secular trends in the osteoporotic fractures of the distal humerus in elderly women. Eur J Epidemiol. 1998;14(2):159-164.
6. Siebenrock KA, Gerber C. The reproducibility of classification of fractures of the proximal end of the humerus. J Bone Joint Surg Am. 1993;75(12):1751-1755.
7. Jupiter JB, Mehne DK. Fractures of the distal humerus. Orthopedics. 1992;15(7):825-833.
8. Zalavras CG, McAllister ET, Singh A, Itamura JM. Operative treatment of intra-articular distal humerus fractures. Am J Orthop. 2007;36(12 suppl):8-12.
9. Cramer H. Mathematical Methods of Statistics. Princeton, NJ: Princeton University Press; 1946.
10. Panossian V, Zalavras C, Mirzayan R, Itamura JM. Intra-articular distal humerus fractures. In: Mirzayan R, Itamura JM, eds. Shoulder and Elbow Trauma. New York, NY: Thieme; 2004:67-78.
11. Wainwright AM, Williams JR, Carr AJ. Interobserver and intraobserver variation in classification systems for fractures of the distal humerus. J Bone Joint Surg Br. 2000;82(5):636-642.
12. Müller ME, Nazarian S, Koch P, Schatzker J. The Comprehensive Classification of Fractures in Long Bones. Berlin, Germany: Springer-Verlag; 1990.
13. Brouwer KM, Lindenhovius AL, Dyer GS, Zurakowski D, Mudgal C, Ring D. Diagnostic accuracy of 2- and 3-dimensional imaging and modeling of distal humerus fractures. J Shoulder Elbow Surg. 2012;21(6):772-776.
14. Riseborough EJ, Radin EL. Intercondylar T fractures of the humerus in the adult. A comparison of operative and non-operative treatment in 29 cases. J Bone Joint Surg Am. 1969;51(1):130-141.
15. Ljungquist KL, Beran MC, Awan H. Effects of surgical approach on functional outcomes of open reduction and internal fixation of intra-articular distal humeral fractures: a systematic review. J Shoulder Elbow Surg. 2012;21(1):126-135.
Elbow fractures constitute 7% of all adult fractures, and 30% of these fractures are distal humerus fractures.1,2 Of these, 96% involve disruption of the articular surface.3 Intra-articular distal humerus fracture patterns can be difficult to characterize on plain radiographs, and therefore computed tomography (CT) is often used. The surgeon’s understanding of the fracture pattern and the deforming forces affects choice of surgical approach. In particular, multiplanar fracture patterns, including coronal shear fractures of the capitellum or trochlea, are often difficult to recognize on plain radiographs. Identification of a multiplanar fracture pattern may require a change in approach or fixation. CT is useful for other intra-articular fractures, such as those of the proximal humerus,3-6 but involves increased radiation and cost.
We conducted a study to determine the effect of adding CT evaluation to plain radiographic evaluation on the classification of, and treatment plans for, intra-articular distal humerus fractures. We hypothesized that adding CT images to plain radiographs would change the classification and treatment of these fractures and would improve interobserver agreement on classification and treatment.
Materials and Methods
After obtaining University of Southern California Institutional Review Board approval, we retrospectively studied 30 consecutive cases of adult intra-articular distal humerus fractures treated by Dr. Itamura at a level I trauma center between 1995 and 2008. In each case, the injured elbow was imaged with plain radiography and CT. Multiple machines were used for CT, but all according to the radiology department’s standard protocol. The images were evaluated by 9 independent observers from the same institution: 3 orthopedic surgeons (1 fellowship-trained shoulder/elbow subspecialist, 1 fellowship-trained upper extremity subspecialist, 1 fellowship-trained orthopedic trauma surgeon), 3 shoulder/elbow fellows, and 3 senior residents pursuing upper extremity fellowships on graduation. No observer was involved in the care of any of the patients. All identifying details were removed from the patient information presented to the observers. For each set of images, the observer was asked to classify the fractures according to the Mehne and Matta classification system,7,8 which is the predominant system used at our institution.
Diagrams of this classification system were provided, but there was no formal observer training or calibration. Seven treatment options were presented: (1) open reduction and internal fixation (ORIF) using a posterior approach with olecranon osteotomy, (2) ORIF using a posterior approach, (3) ORIF using a lateral approach, (4) ORIF using a medial approach, (5) ORIF using an anterior/anterolateral approach, (6) total elbow arthroplasty, and (7) nonoperative management. The only clinical data provided were patient age and sex.
Images were evaluated in blinded fashion. Two rounds of evaluation were compared. In round 1, plain radiographs were evaluated; in round 2, the same radiographs plus corresponding 2-dimensional (2-D) CT images. A minimum of 1 month was required between viewing rounds.
Statistical Analysis
Statistical analysis was performed by the Statistical Consultation and Research Center at our institution. Cohen κ was calculated to estimate the reliability of the fracture classification and treatment plan made by different observers on the same occasion (interobserver reliability). Cramer V9 was calculated to estimate the reliability of the fracture classification and treatment plan made by the same observer on separate occasions (intraobserver reliability). It measures the association between the 2 ratings as a percentage of their total variation. The κ value and Cramer V value were also used to evaluate results based on the observers’ training levels. Both κ and Cramer V values are interpreted as follows: .00 to .20 indicates slight agreement; .21 to .40, fair agreement; .41-.60, moderate agreement; .61 to .80, substantial agreement; and ≥.81, almost perfect agreement. Zero represents no agreement, and 1.00 represents perfect agreement.
Results
Overall intraobserver reliability for classification was fair (.393). It was moderate for the treatment plan (.426) between viewing rounds. Residents had the highest Cramer V value at .60 (moderate) for classification reliability, and attending surgeons had the highest value at .52 (moderate) for treatment plan. All 3 groups (residents, fellows, attending surgeons) showed moderate intraobserver agreement for treatment plan (Table 1).
Interobserver reliability did not improve with the addition of CT in round 2. Reliability was fair at both viewing rounds for classification and for treatment. For classification, the overall κ value was .21 for the first round and .20 for the second round. For treatment plan, the overall κ value was .28 for the first round and .27 for the second round. Attending surgeons decreased in agreement with regard to treatment plan with the addition of CT (.46, moderate, to .32, fair). Fellows had only slight agreement for both rounds with regard to classification as well as treatment (Table 2).
ORIF using a posterior approach with an olecranon osteotomy was the most common choice of treatment method overall at both time points (58.1% and 63.7%) and was still the most common choice when each group of observers (residents, fellows, faculty) was considered separately (Figure 1).
When classifying the fractures, attending surgeons chose the multiplanar fracture pattern 25.6% of the time when viewing radiographs only, and remained consistent in choosing this pattern 23.3% of the time when CT was added to radiographs. Fellows and residents chose this fracture pattern much less often (8.9% and 7.8%, respectively) when viewing radiographs only. Both fellows and residents increased their choice of the multiplanar fracture pattern by 10% (18.9% for fellows, 17.8% for residents) when CT was added (Figure 2).
Overall, the recognition of a multiplanar fracture pattern increased when CT was added. On 30 occasions, an answer was changed from another classification pattern to the multiplanar pattern when CT was added. Only 6 times did an observer change a multiplanar pattern selection at round 1 to another choice at round 2.
Adding CT in round 2 changed the treatment plan for multiplanar fractures. At round 1, 73.7% chose ORIF using a lateral approach for treatment of the multiplanar fracture versus 10.5% who chose ORIF using a posterior approach with an olecranon osteotomy. The choice of the posterior approach with olecranon osteotomy increased to 51.9% at round 2, using the technique we have previously described.5,10
Overall intraobserver reliability for classification was fair (.393). It was moderate for the treatment plan (.426) between viewing rounds. Residents had the highest Cramer V value at .60 (moderate) for classification reliability, and faculty had the highest value at .52 (moderate) for treatment plan. All 3 groups (residents, fellows, attending surgeons) showed moderate intraobserver agreement for treatment plan (Table 1).
Interobserver reliability did not improve with the addition of CT in round 2. Reliability for classification was fair for round 1 and slight for round 2. Reliability was fair at both viewing rounds for treatment. For classification, the overall κ value was .21 for round 1 and .20 for round 2. For treatment plan, the overall κ value was .28 for round 1 and .27 for round 2. Attending surgeons decreased in agreement with regard to treatment plan with the addition of CT (.46, moderate, to .32, fair). Fellows had only slight agreement for both rounds with regard to classification as well as treatment (Table 2).
Discussion
In this study, CT changed both classification and treatment when added to plain radiographs. Interestingly, interobserver reliability did not improve for classification or treatment with the addition of CT. This finding suggests substantial disagreement among qualified observers that is not resolved with more sophisticated imaging. We propose this disagreement is caused by differences in training and experience with specific fracture patterns and surgical approaches.
Our fair to moderate interobserver reliability using radiographs only is consistent with a study by Wainwright and colleagues,11 who demonstrated fair to moderate interobserver reliability with radiographs only using 3 different classification systems. CT did not improve interobserver reliability in the present study.
To our knowledge, the effect of adding CT to plain radiographs on classification and treatment plan has not been evaluated. Doornberg and colleagues2 evaluated the effect of adding 3-dimensional (3-D) CT to a combination of radiographs and 2-D CT. Using the AO (Arbeitsgemeinschaft für Osteosynthesefragen) classification12 and the classification system of Mehne and Matta, they found that 3-D CT improved intraobserver and interobserver reliability for classification but improved only intraobserver agreement for treatment. Interobserver agreement for treatment plan remained fair. In parallel with their study, fracture classification in our study was more often changed with CT than the treatment plan was. Training level appeared not to affect this finding. We found fair interobserver agreement for treatment choice as well, which was not improved by adding CT. Doornberg and colleagues2 concluded that the “relatively small added expense of three-dimensional computed tomography scans seems worthwhile.”
When evaluating specific fracture patterns in the Mehne and Matta classification system, we observed that less experienced surgeons (residents, fellows) were much more likely to identify multiplanar fracture patterns with the aid of CT. Use of CT did not change attending surgeons’ recognition of these multiplanar fractures, suggesting that the faculty were more capable of appreciating these fracture patterns with radiographs only (Figure 3). We also observed that adding CT changed the predominant treatment plan for multiplanar fractures from a lateral approach to a posterior approach with an olecranon osteotomy. Failure to appreciate this component of the fracture before surgery could lead to an increased intraoperative difficulty level. Failure to appreciate it during surgery could lead to unexpected postoperative displacement and ultimately poorer outcome.
There are limitations to our study. There is no gold standard for assessing the accuracy of classification decisions. Intraoperative classification could have served as a gold standard, but the fractures were not routinely assigned a classification during surgery. Brouwer and colleagues13 evaluated the diagnostic accuracy of CT (including 3-D CT) with intraoperative AO classification as a reference point and found improvement in intraobserver agreement but not interobserver agreement when describing fracture characteristics—and no significant effect on classification.
We used a single classification system, the one primarily used at our institution and by Dr. Itamura. There are many systems,7,12,14 all with their strengths and weaknesses, and no one system is used universally. Adding a system would have allowed us to compare results of more than one system. Our aim, however, was to keep our form simple for the sake of participation and completion of the viewings by each volunteer.
Only 2-D CT was used for this study, as 3-D images were not available for all patients. Although this is a potential weakness, it appears that, based on the study by Doornberg and colleagues,2 adding 3-D imaging resulted in only modest improvement in the reliability of classification and no significant improvement in agreement on treatment recommendation.
In addition, our results were likely biased by the fact that 8 of the 9 evaluators were trained by Dr. Itamura, who very often uses a posterior approach with an olecranon osteotomy for internal fixation of distal humerus intra-articular fractures, as previously described.8,10 Therefore, selection of this treatment option may have been overestimated in this study. Nevertheless, after reviewing the literature, Ljungquist and colleagues15 wrote, “There do not seem to be superior functional results associated with any one surgical approach to the distal humerus.”
We did not give the evaluators an indication of patients’ activity demands (only age and sex), which may have been relevant when considering total elbow arthroplasty.
Last, performing another round of evaluations with only plain radiographs, before introducing CT, would have provided intraobserver reliability results on plain radiograph evaluation, which could have been compared with intraobserver reliability when CT was added. Again, this was excluded to encourage participation and create the least cumbersome evaluation experience possible, which was thought appropriate, as this information is already in the literature.
Conclusion
Adding CT changed classifications and treatment plans. Raters were more likely to change their classifications than their treatment plans. The addition of CT did not increase agreement between observers. Despite the added radiation and cost, we recommend performing CT for all intra-articular distal humerus fractures because it improves understanding of the fracture pattern and affects treatment planning, especially for fractures with a coronal shear component, which is often not appreciated on plain radiographs.
Elbow fractures constitute 7% of all adult fractures, and 30% of these fractures are distal humerus fractures.1,2 Of these, 96% involve disruption of the articular surface.3 Intra-articular distal humerus fracture patterns can be difficult to characterize on plain radiographs, and therefore computed tomography (CT) is often used. The surgeon’s understanding of the fracture pattern and the deforming forces affects choice of surgical approach. In particular, multiplanar fracture patterns, including coronal shear fractures of the capitellum or trochlea, are often difficult to recognize on plain radiographs. Identification of a multiplanar fracture pattern may require a change in approach or fixation. CT is useful for other intra-articular fractures, such as those of the proximal humerus,3-6 but involves increased radiation and cost.
We conducted a study to determine the effect of adding CT evaluation to plain radiographic evaluation on the classification of, and treatment plans for, intra-articular distal humerus fractures. We hypothesized that adding CT images to plain radiographs would change the classification and treatment of these fractures and would improve interobserver agreement on classification and treatment.
Materials and Methods
After obtaining University of Southern California Institutional Review Board approval, we retrospectively studied 30 consecutive cases of adult intra-articular distal humerus fractures treated by Dr. Itamura at a level I trauma center between 1995 and 2008. In each case, the injured elbow was imaged with plain radiography and CT. Multiple machines were used for CT, but all according to the radiology department’s standard protocol. The images were evaluated by 9 independent observers from the same institution: 3 orthopedic surgeons (1 fellowship-trained shoulder/elbow subspecialist, 1 fellowship-trained upper extremity subspecialist, 1 fellowship-trained orthopedic trauma surgeon), 3 shoulder/elbow fellows, and 3 senior residents pursuing upper extremity fellowships on graduation. No observer was involved in the care of any of the patients. All identifying details were removed from the patient information presented to the observers. For each set of images, the observer was asked to classify the fractures according to the Mehne and Matta classification system,7,8 which is the predominant system used at our institution.
Diagrams of this classification system were provided, but there was no formal observer training or calibration. Seven treatment options were presented: (1) open reduction and internal fixation (ORIF) using a posterior approach with olecranon osteotomy, (2) ORIF using a posterior approach, (3) ORIF using a lateral approach, (4) ORIF using a medial approach, (5) ORIF using an anterior/anterolateral approach, (6) total elbow arthroplasty, and (7) nonoperative management. The only clinical data provided were patient age and sex.
Images were evaluated in blinded fashion. Two rounds of evaluation were compared. In round 1, plain radiographs were evaluated; in round 2, the same radiographs plus corresponding 2-dimensional (2-D) CT images. A minimum of 1 month was required between viewing rounds.
Statistical Analysis
Statistical analysis was performed by the Statistical Consultation and Research Center at our institution. Cohen κ was calculated to estimate the reliability of the fracture classification and treatment plan made by different observers on the same occasion (interobserver reliability). Cramer V9 was calculated to estimate the reliability of the fracture classification and treatment plan made by the same observer on separate occasions (intraobserver reliability). It measures the association between the 2 ratings as a percentage of their total variation. The κ value and Cramer V value were also used to evaluate results based on the observers’ training levels. Both κ and Cramer V values are interpreted as follows: .00 to .20 indicates slight agreement; .21 to .40, fair agreement; .41-.60, moderate agreement; .61 to .80, substantial agreement; and ≥.81, almost perfect agreement. Zero represents no agreement, and 1.00 represents perfect agreement.
Results
Overall intraobserver reliability for classification was fair (.393). It was moderate for the treatment plan (.426) between viewing rounds. Residents had the highest Cramer V value at .60 (moderate) for classification reliability, and attending surgeons had the highest value at .52 (moderate) for treatment plan. All 3 groups (residents, fellows, attending surgeons) showed moderate intraobserver agreement for treatment plan (Table 1).
Interobserver reliability did not improve with the addition of CT in round 2. Reliability was fair at both viewing rounds for classification and for treatment. For classification, the overall κ value was .21 for the first round and .20 for the second round. For treatment plan, the overall κ value was .28 for the first round and .27 for the second round. Attending surgeons decreased in agreement with regard to treatment plan with the addition of CT (.46, moderate, to .32, fair). Fellows had only slight agreement for both rounds with regard to classification as well as treatment (Table 2).
ORIF using a posterior approach with an olecranon osteotomy was the most common choice of treatment method overall at both time points (58.1% and 63.7%) and was still the most common choice when each group of observers (residents, fellows, faculty) was considered separately (Figure 1).
When classifying the fractures, attending surgeons chose the multiplanar fracture pattern 25.6% of the time when viewing radiographs only, and remained consistent in choosing this pattern 23.3% of the time when CT was added to radiographs. Fellows and residents chose this fracture pattern much less often (8.9% and 7.8%, respectively) when viewing radiographs only. Both fellows and residents increased their choice of the multiplanar fracture pattern by 10% (18.9% for fellows, 17.8% for residents) when CT was added (Figure 2).
Overall, the recognition of a multiplanar fracture pattern increased when CT was added. On 30 occasions, an answer was changed from another classification pattern to the multiplanar pattern when CT was added. Only 6 times did an observer change a multiplanar pattern selection at round 1 to another choice at round 2.
Adding CT in round 2 changed the treatment plan for multiplanar fractures. At round 1, 73.7% chose ORIF using a lateral approach for treatment of the multiplanar fracture versus 10.5% who chose ORIF using a posterior approach with an olecranon osteotomy. The choice of the posterior approach with olecranon osteotomy increased to 51.9% at round 2, using the technique we have previously described.5,10
Overall intraobserver reliability for classification was fair (.393). It was moderate for the treatment plan (.426) between viewing rounds. Residents had the highest Cramer V value at .60 (moderate) for classification reliability, and faculty had the highest value at .52 (moderate) for treatment plan. All 3 groups (residents, fellows, attending surgeons) showed moderate intraobserver agreement for treatment plan (Table 1).
Interobserver reliability did not improve with the addition of CT in round 2. Reliability for classification was fair for round 1 and slight for round 2. Reliability was fair at both viewing rounds for treatment. For classification, the overall κ value was .21 for round 1 and .20 for round 2. For treatment plan, the overall κ value was .28 for round 1 and .27 for round 2. Attending surgeons decreased in agreement with regard to treatment plan with the addition of CT (.46, moderate, to .32, fair). Fellows had only slight agreement for both rounds with regard to classification as well as treatment (Table 2).
Discussion
In this study, CT changed both classification and treatment when added to plain radiographs. Interestingly, interobserver reliability did not improve for classification or treatment with the addition of CT. This finding suggests substantial disagreement among qualified observers that is not resolved with more sophisticated imaging. We propose this disagreement is caused by differences in training and experience with specific fracture patterns and surgical approaches.
Our fair to moderate interobserver reliability using radiographs only is consistent with a study by Wainwright and colleagues,11 who demonstrated fair to moderate interobserver reliability with radiographs only using 3 different classification systems. CT did not improve interobserver reliability in the present study.
To our knowledge, the effect of adding CT to plain radiographs on classification and treatment plan has not been evaluated. Doornberg and colleagues2 evaluated the effect of adding 3-dimensional (3-D) CT to a combination of radiographs and 2-D CT. Using the AO (Arbeitsgemeinschaft für Osteosynthesefragen) classification12 and the classification system of Mehne and Matta, they found that 3-D CT improved intraobserver and interobserver reliability for classification but improved only intraobserver agreement for treatment. Interobserver agreement for treatment plan remained fair. In parallel with their study, fracture classification in our study was more often changed with CT than the treatment plan was. Training level appeared not to affect this finding. We found fair interobserver agreement for treatment choice as well, which was not improved by adding CT. Doornberg and colleagues2 concluded that the “relatively small added expense of three-dimensional computed tomography scans seems worthwhile.”
When evaluating specific fracture patterns in the Mehne and Matta classification system, we observed that less experienced surgeons (residents, fellows) were much more likely to identify multiplanar fracture patterns with the aid of CT. Use of CT did not change attending surgeons’ recognition of these multiplanar fractures, suggesting that the faculty were more capable of appreciating these fracture patterns with radiographs only (Figure 3). We also observed that adding CT changed the predominant treatment plan for multiplanar fractures from a lateral approach to a posterior approach with an olecranon osteotomy. Failure to appreciate this component of the fracture before surgery could lead to an increased intraoperative difficulty level. Failure to appreciate it during surgery could lead to unexpected postoperative displacement and ultimately poorer outcome.
There are limitations to our study. There is no gold standard for assessing the accuracy of classification decisions. Intraoperative classification could have served as a gold standard, but the fractures were not routinely assigned a classification during surgery. Brouwer and colleagues13 evaluated the diagnostic accuracy of CT (including 3-D CT) with intraoperative AO classification as a reference point and found improvement in intraobserver agreement but not interobserver agreement when describing fracture characteristics—and no significant effect on classification.
We used a single classification system, the one primarily used at our institution and by Dr. Itamura. There are many systems,7,12,14 all with their strengths and weaknesses, and no one system is used universally. Adding a system would have allowed us to compare results of more than one system. Our aim, however, was to keep our form simple for the sake of participation and completion of the viewings by each volunteer.
Only 2-D CT was used for this study, as 3-D images were not available for all patients. Although this is a potential weakness, it appears that, based on the study by Doornberg and colleagues,2 adding 3-D imaging resulted in only modest improvement in the reliability of classification and no significant improvement in agreement on treatment recommendation.
In addition, our results were likely biased by the fact that 8 of the 9 evaluators were trained by Dr. Itamura, who very often uses a posterior approach with an olecranon osteotomy for internal fixation of distal humerus intra-articular fractures, as previously described.8,10 Therefore, selection of this treatment option may have been overestimated in this study. Nevertheless, after reviewing the literature, Ljungquist and colleagues15 wrote, “There do not seem to be superior functional results associated with any one surgical approach to the distal humerus.”
We did not give the evaluators an indication of patients’ activity demands (only age and sex), which may have been relevant when considering total elbow arthroplasty.
Last, performing another round of evaluations with only plain radiographs, before introducing CT, would have provided intraobserver reliability results on plain radiograph evaluation, which could have been compared with intraobserver reliability when CT was added. Again, this was excluded to encourage participation and create the least cumbersome evaluation experience possible, which was thought appropriate, as this information is already in the literature.
Conclusion
Adding CT changed classifications and treatment plans. Raters were more likely to change their classifications than their treatment plans. The addition of CT did not increase agreement between observers. Despite the added radiation and cost, we recommend performing CT for all intra-articular distal humerus fractures because it improves understanding of the fracture pattern and affects treatment planning, especially for fractures with a coronal shear component, which is often not appreciated on plain radiographs.
1. Anglen J. Distal humerus. J Am Acad Orthop Surg. 2005;13(5):291-297.
2. Doornberg J, Lindenhovius A, Kloen P, van Dijk CN, Zurakowski D, Ring D. Two and three-dimensional computed tomography for the classification and management of distal humerus fractures. Evaluation of reliability and diagnostic accuracy. J Bone Joint Surg Am. 2006;88(8):1795-1801.
3. Pollock JW, Faber KJ, Athwal GS. Distal humerus fractures. Orthop Clin North Am. 2008;39(2):187-200.
4. Castagno AA, Shuman WP, Kilcoyne RF, Haynor DR, Morris ME, Matsen FA. Complex fractures of the proximal humerus: role of CT in treatment. Radiology. 1987;165(3):759-762.
5. Palvanen M, Kannus P, Niemi S, Parkkari J. Secular trends in the osteoporotic fractures of the distal humerus in elderly women. Eur J Epidemiol. 1998;14(2):159-164.
6. Siebenrock KA, Gerber C. The reproducibility of classification of fractures of the proximal end of the humerus. J Bone Joint Surg Am. 1993;75(12):1751-1755.
7. Jupiter JB, Mehne DK. Fractures of the distal humerus. Orthopedics. 1992;15(7):825-833.
8. Zalavras CG, McAllister ET, Singh A, Itamura JM. Operative treatment of intra-articular distal humerus fractures. Am J Orthop. 2007;36(12 suppl):8-12.
9. Cramer H. Mathematical Methods of Statistics. Princeton, NJ: Princeton University Press; 1946.
10. Panossian V, Zalavras C, Mirzayan R, Itamura JM. Intra-articular distal humerus fractures. In: Mirzayan R, Itamura JM, eds. Shoulder and Elbow Trauma. New York, NY: Thieme; 2004:67-78.
11. Wainwright AM, Williams JR, Carr AJ. Interobserver and intraobserver variation in classification systems for fractures of the distal humerus. J Bone Joint Surg Br. 2000;82(5):636-642.
12. Müller ME, Nazarian S, Koch P, Schatzker J. The Comprehensive Classification of Fractures in Long Bones. Berlin, Germany: Springer-Verlag; 1990.
13. Brouwer KM, Lindenhovius AL, Dyer GS, Zurakowski D, Mudgal C, Ring D. Diagnostic accuracy of 2- and 3-dimensional imaging and modeling of distal humerus fractures. J Shoulder Elbow Surg. 2012;21(6):772-776.
14. Riseborough EJ, Radin EL. Intercondylar T fractures of the humerus in the adult. A comparison of operative and non-operative treatment in 29 cases. J Bone Joint Surg Am. 1969;51(1):130-141.
15. Ljungquist KL, Beran MC, Awan H. Effects of surgical approach on functional outcomes of open reduction and internal fixation of intra-articular distal humeral fractures: a systematic review. J Shoulder Elbow Surg. 2012;21(1):126-135.
1. Anglen J. Distal humerus. J Am Acad Orthop Surg. 2005;13(5):291-297.
2. Doornberg J, Lindenhovius A, Kloen P, van Dijk CN, Zurakowski D, Ring D. Two and three-dimensional computed tomography for the classification and management of distal humerus fractures. Evaluation of reliability and diagnostic accuracy. J Bone Joint Surg Am. 2006;88(8):1795-1801.
3. Pollock JW, Faber KJ, Athwal GS. Distal humerus fractures. Orthop Clin North Am. 2008;39(2):187-200.
4. Castagno AA, Shuman WP, Kilcoyne RF, Haynor DR, Morris ME, Matsen FA. Complex fractures of the proximal humerus: role of CT in treatment. Radiology. 1987;165(3):759-762.
5. Palvanen M, Kannus P, Niemi S, Parkkari J. Secular trends in the osteoporotic fractures of the distal humerus in elderly women. Eur J Epidemiol. 1998;14(2):159-164.
6. Siebenrock KA, Gerber C. The reproducibility of classification of fractures of the proximal end of the humerus. J Bone Joint Surg Am. 1993;75(12):1751-1755.
7. Jupiter JB, Mehne DK. Fractures of the distal humerus. Orthopedics. 1992;15(7):825-833.
8. Zalavras CG, McAllister ET, Singh A, Itamura JM. Operative treatment of intra-articular distal humerus fractures. Am J Orthop. 2007;36(12 suppl):8-12.
9. Cramer H. Mathematical Methods of Statistics. Princeton, NJ: Princeton University Press; 1946.
10. Panossian V, Zalavras C, Mirzayan R, Itamura JM. Intra-articular distal humerus fractures. In: Mirzayan R, Itamura JM, eds. Shoulder and Elbow Trauma. New York, NY: Thieme; 2004:67-78.
11. Wainwright AM, Williams JR, Carr AJ. Interobserver and intraobserver variation in classification systems for fractures of the distal humerus. J Bone Joint Surg Br. 2000;82(5):636-642.
12. Müller ME, Nazarian S, Koch P, Schatzker J. The Comprehensive Classification of Fractures in Long Bones. Berlin, Germany: Springer-Verlag; 1990.
13. Brouwer KM, Lindenhovius AL, Dyer GS, Zurakowski D, Mudgal C, Ring D. Diagnostic accuracy of 2- and 3-dimensional imaging and modeling of distal humerus fractures. J Shoulder Elbow Surg. 2012;21(6):772-776.
14. Riseborough EJ, Radin EL. Intercondylar T fractures of the humerus in the adult. A comparison of operative and non-operative treatment in 29 cases. J Bone Joint Surg Am. 1969;51(1):130-141.
15. Ljungquist KL, Beran MC, Awan H. Effects of surgical approach on functional outcomes of open reduction and internal fixation of intra-articular distal humeral fractures: a systematic review. J Shoulder Elbow Surg. 2012;21(1):126-135.
Factors Affecting Perceptions of Open, Mini-Open, and Arthroscopic Rotator Cuff Repair Techniques Among Medical Professionals
Rotator cuff tears are a common condition affecting the shoulder joint. Initial open repair techniques were associated with several complications, including severe early postoperative pain, deltoid detachment and/or weakness, risk for infection, and arthrofibrosis.1-3 In addition, open procedures cannot address other possible diagnoses, such as labral tears and loose bodies. These disadvantages promoted the development of an arthroscopically assisted mini-open technique.4 Superior long-term results, with more than 90% of patients achieving good to excellent results,5-13 established the mini-open rotator cuff repair (RCR) as the gold standard.3,6,10,12,14-16
Recently, as instrumentation for arthroscopy has improved, enthusiasm for all-arthroscopic techniques (hereafter referred to as arthroscopic repair) has grown. The appeal of arthroscopic repair includes potentially less initial pain, ability to treat intra-articular lesions concurrently, smaller skin incisions with better cosmesis, less soft-tissue dissection, and low risk for deltoid detachment.3,17 The potential advantages of arthroscopic repair can lead to perceptions of quicker healing and shorter recovery, which are not supported by the literature. However, arthroscopic repair is technically more challenging, time-consuming, and expensive than open or mini-open repairs,18,19 and though some investigators have reported a trend toward fewer complications,3 the long-term outcome of arthroscopic RCRs has not been shown to be better than that of other techniques.
Given that no differences have been shown between the emerging arthroscopic repair technique and mini-open repair with respect to range of motion or clinical scores in the short term,3 it is unclear what perceptions influence choice of technique for one’s own personal RCR.
We conducted a study to determine which RCR technique medical professionals (orthopedic attendings and residents, anesthesiologists, internal medicine attendings, main operating room nurses, and physical therapists) preferred for their own surgery and to analyze perceptions shaping those opinions. Orthopedic surgeons have the best concept of rotator cuff surgery, but anesthesiologists and nurses have a “front row seat” and opinions on types of rotator cuff surgery. Physical therapists, who treat patients with rotator cuff tears, also have a working knowledge of rotator cuff surgery. Finally, internists represent a rotator cuff injury referral service and may have patients who have undergone rotator cuff surgery. We hypothesized that most medical professionals, irrespective of specialty or career length, would prefer arthroscopic RCR because of its perceived superior outcome and fast recovery.
Materials and Methods
This cross-sectional, descriptive, survey-based study was approved by our institutional review board (IRB) and offered via 3 emails between April 2011 and June 2011 to attendings (orthopedists, internists, anesthesiologists), residents, and allied health professionals (AHPs; operating room nurses, physical therapists) involved in orthopedic care at our institution. Each email contained a hyperlink to the online survey (Appendix), which took about 10 minutes to complete and explored respondent demographics, exposure to the different techniques, and opinions regarding different aspects of RCR surgery and recovery.
There were 84 respondents. The sexes were equally represented, and age ranged from 25 to 78 years (Table 1). Of the respondents, 41 (49%) were attendings, 20 (24%) were residents, and 23 (27%) were AHPs. Of the attendings, 13 (32%) were orthopedic surgeons, 26 (63%) were primary care physicians, and 2 (5%) did not specify their specialty. Four orthopedic surgeons had fellowship training in sports medicine or shoulder and elbow surgery. The attendings were overall more experienced in their profession than the other groups were, with 68% reporting more than 5 years of experience.
Descriptive statistics, including means and standard errors, were calculated. Fisher exact test was used to compare preferences of RCR type according to type of training and years of experience. Significance was set at P ≤ .05.
Results
Overall Responses (Table 2)
Of the 84 respondents, almost half (46%) preferred deferring their choice of RCR to their surgeon. Most of the other respondents preferred the arthroscopic technique (26%) or the mini-open repair (23%). There was no association between technique preference and medical professional type. Most respondents (63%) had never assisted in or performed rotator cuff surgery.
Seventy-four percent of all respondents indicated they thought arthroscopic, mini-open, and open RCRs are safe, and about half thought these procedures are fast. About half expressed no opinion about the cost-effectiveness of arthroscopic, mini-open, or open RCRs (54%, 52%, and 48%, respectively), and slightly more than half expressed no opinion about whether arthroscopic, mini-open, or open RCR provide the best outcome (58%, 60%, and 62%, respectively). Significantly (P < .05) more respondents thought arthroscopic and mini-open repairs, rather than open repairs, promote quick healing (64% and 45%, respectively, vs 15%), good cosmetic results (81% and 51%, respectively, vs 10%), and patient satisfaction (50% and 48%, respectively, vs 30%). However, a significant (P < .05) number also thought arthroscopic and mini-open repairs are harder to learn/more challenging to perform than open repairs (52% and 38%, respectively, vs 17%).
Of all factors considered, safety of arthroscopic repair garnered the highest consensus: 82%. Respondents were least opinionated about the outcome of the open repair technique, with more than 62% expressing no opinion about the outcome. The responses to the questions on the learning curves for the 3 techniques varied the most.
Responses by Group (Table 2)
Attendings. Of the 41 attendings, 24 (59%) responded they would defer to their surgeon’s technique preference for RCR. Of the other 17 who expressed a preference, most indicated arthroscopic or mini-open repair (17% each). There was a difference (P < .05) between years of experience and RCR preference: of the 13 attendings with less than 5 years of experience, arthroscopic repair was preferred by 31%; in contrast, of the 28 attendings with more than 5 years of experience, only 11% preferred arthroscopic repair.
Of the 11 attendings who performed rotator cuff surgery, 55% used the open technique, but most (8) preferred to have their own rotator cuff fixed arthroscopically or according to their surgeon’s preference. Only 1 surgeon preferred open repair for his own rotator cuff. Of the 4 surgeons who performed arthroscopic RCRs, 3 had less than 5 years of experience. Conversely, all 7 surgeons who performed mini-open or open repairs had more than 5 years of experience.
Of the 30 attendings who did not perform rotator cuff surgery, most (20) responded they would defer to their surgeon’s technique preference for RCR.
The attendings’ opinions on factors affecting rotator cuff surgery were similar to those of the other respondents with respect to safety, cost-effectiveness, recovery, cosmesis, patient satisfaction, outcome, and technical difficulty. Unlike the others, however, attendings considered all 3 repair techniques fast.
Residents. Of the 20 residents, 7 preferred arthroscopic, 5 preferred mini-open, and 1 preferred open repair; the other 7 responded they would defer to their surgeon’s preference. Residents’ opinions on each factor were more polarized and consistent across categories than those of the other groups. Residents overwhelmingly thought all 3 techniques (arthroscopic, mini-open, open) are safe (19, 19, and 18, respectively) and cost-effective (12, 14, and 14, respectively). Although most residents considered the open and mini-open repair techniques fast (19 and 15, respectively), only 8 considered arthroscopic RCR fast, and 4 considered it slow. Residents’ opinions about the technique that produces the best outcome were mixed. As with the other respondents, residents thought arthroscopic RCRs heal fast and produce great cosmetic results, but are challenging to perform and have a steep learning curve. Unlike the other respondents, most residents (12) considered open RCR easy to learn (P = .006), with a learning curve of fewer than 20 procedures.
AHPs. No AHP expressed a preference for open RCR. This group was evenly divided among 3 choices: deferring to their surgeon’s preference, arthroscopic repair, and mini-open repair. The 23 AHPs thought arthroscopic, mini-open, and open repairs are safe (17, 15, and 12, respectively), but most indicated they were “equivocal” about which techniques are cost-effective, challenging to perform, and produce the best outcomes. A significantly (P = .014) larger number of AHPs (7) considered open rotator cuff surgery slow compared with arthroscopic (0) and mini-open (2) repair techniques. As with the overall cohort, AHPs reported arthroscopic and mini-open repairs promote quick healing and good cosmetic results, but are challenging to perform.
Discussion
As our population ages and continues to remain active, the demand for RCR has accelerated. National data show that 272,148 ambulatory RCRs and 20,433 inpatient RCRs were performed in 2006—an overall 141% increase in RCR since 1996.20 In 1996, 41 per 100,000 population underwent RCR.20 By 2006, this number ballooned to 98 per 100,000 population.20 There are 3 predominant techniques for repairing the rotator cuff: open, mini-open, and arthroscopic. As RCR use increases, we should consider the factors that medical professionals consider important when choosing a method for their own RCR.
Of the 84 medical professionals in our cohort, 39 (46%) indicated they would defer to their surgeon’s technique preference for RCR. Of the other 45, about equal numbers preferred arthroscopic and mini-open RCRs; only 2 preferred open RCRs. This finding suggests that the individual opinions of surgeons who perform RCRs have a substantial influence on a large proportion of medical professionals’ ultimate choice of RCR method. Interestingly, of the attendings who performed open RCR, only 1 expressed a preference for the open technique for his own RCR. This finding might suggest a shift in opinion and an emerging perception among surgeons performing RCR about the value of this technique.
Several factors may account for these evolving beliefs. We hypothesized that a biased favorable view of arthroscopic repair outcome might influence opinions. However, our results did not support the hypothesis. Medical professionals in our cohort were equivocal about the best RCR technique. No consensus was evident among attendings, residents, or AHPs. This lack of clinical agreement about rotator cuff surgery has been observed elsewhere—for example, among members of the American Academy of Orthopaedic Surgeons (AAOS)21 and the European Society of Sports Traumatology, Knee Surgery, and Arthroscopy.22 Despite theoretical advantages of arthroscopic repair, there has been no documented significant difference in patient outcomes when compared with other techniques.23 To our knowledge, there have been only a few clinical studies comparing the different RCR techniques. A meta-analysis of 5 clinical studies comparing arthroscopic and mini-open RCR techniques showed no difference in clinical outcomes or complication rates.8 The 2012 AAOS clinical practice guidelines for RCR reflect these observations.24 That consortium of leading shoulder surgeons could not recommend a modality of surgical rotator cuff tear repair given the lack of conclusive evidence.24
At our institution, arthroscopic, mini-open, and open RCRs were performed by 36%, 9%, and 55% of our surgeons, respectively. A survey of AAOS surgeons showed that, of those who perform RCRs, 14.5%, 46.2%, and 36.6% used arthroscopic, mini-open, and open techniques, respectively.21 The greater use of open repairs at our institution might reflect the seniority of our faculty. Dunn and colleagues21 found that surgeons who preferred open RCR had been in practice longer than those who preferred the arthroscopic or mini-open technique. Of our 4 faculty who performed arthroscopic repairs, 3 were less than 5 years from completing their training. In contrast, all faculty who performed mini-open or open repairs were more than 5 years from completing their training. Furthermore, mean age of the surgeons who performed arthroscopic repair was 39.8 years (range, 32-51 years), and these surgeons were significantly younger than those who performed mini-open or open repair (mean age, 56.3 years; range, 41-78 years). Younger surgeon age has been associated with higher rates of arthroscopic repair.25
Attendings unaccustomed to arthroscopy may find it more challenging than the younger generation of surgeons, who are exposed to it early in training. Dunn and colleagues21 noted that the likelihood of performing an arthroscopic repair was influenced by the surgeon’s experience level. Fellowship-trained shoulder and sports medicine surgeons are also more likely to perform arthroscopic repairs than those with training limited to orthopedic residency.25 Arthroscopic RCR demands a high level of technical skill that many acquire in fellowship training.26 Mauro and colleagues26 found that surgeons trained in a sports medicine fellowship performed 82.6% of subacromial decompression and/or RCR procedures arthroscopically, compared with 54.5% to 70.1% for surgeons trained in other fellowships. In our cohort, with the exception of 1 surgeon, all fellowship-trained shoulder and sports medicine surgeons performed arthroscopic RCRs.
Although no conclusive evidence in the literature supports arthroscopic over the other repair types, the demand for arthroscopic RCR has rapidly increased relative to that for the others. Between 1996 and 2006, use of arthroscopic RCR increased 600%, from 8 to 58 per 100,000 population.20 In that same period, use of open RCR increased by only 34%.20 Similarly, Mauro and colleagues26 found that the proportion of subacromial decompression and RCRs performed arthroscopically rose from 58.3% in 2004 to 83.7% in 2009. Using the 2006 New York State Ambulatory Surgery Database, Churchill and Ghorai27 found that 74.5% of RCRs with acromioplasty were performed arthroscopically.
Respondent-indicated factors that may have contributed to the more favorable opinion of arthroscopic and mini-open repair include quick healing, good cosmetic results, and better perceived patient satisfaction. The literature supports these perceptions. Baker and Liu14 found shorter hospital stays and quicker return to activity with arthroscopic repair compared with open repair. Vitale and colleagues25 also noted that, compared with open or mini-open repair techniques, arthroscopic repair resulted in shorter hospitalization and quicker overall recovery.
If these selected health care professionals with some inside information on rotator cuff surgery have biases that affect their selection of rotator cuff procedures, we should acknowledge that nonmedical personnel, in particular our patients, also have biases. The knowledge base of patients may be further influenced by friends or family members who have had rotator cuff surgery, by lay publications, and by the Internet. Satisfaction with any surgical procedure depends not only on the success of the surgery and the rehabilitation but also on patient and provider expectations. Such expectations are influenced, in part, by biases.
Our medical professionals had similar opinions on safety, recovery, cosmesis, and overall outcome of the RCR techniques, but different opinions on procedure durations and associated training requirements. All residents except one indicated open repair was a quick procedure. In contrast, a significant number of AHPs thought open repair was time-consuming. The attendings considered all the methods fast. The residents’ opinions were the most consistent with the true operating times reported. According to the literature, total operating time for mini-open repair ranges from 10 to 16 minutes faster than that for arthroscopic repair.18,20,27 Ultimately, procedure duration did not affect the respondents’ technique preference for RCR.
There was substantial disagreement about the number of procedures needed to become proficient in the different repair techniques. Overall, however, there was consensus that arthroscopic and mini-open repairs had longer learning curves than open repair. Given the lack of agreement among orthopedic department chairmen and sports medicine fellowship directors regarding the minimum exposure needed (during residency) to become proficient in diagnostic shoulder arthroscopy,28 this finding is not surprising. Guttmann and colleagues29 attempted to quantify the learning curve for arthroscopic RCR by tracking operating time as a surrogate measure. They found that RCR operative time decreased rapidly during the initial block of 10 cases to the second block of 10 cases, but thereafter improvement continued at a much lower rate.29 None of our respondents thought the learning curve for arthroscopic RCR was under 10 cases, but no group, not even the attendings who performed RCRs, could agree on the minimum number of cases needed for proficiency. The longer learning curve for arthroscopic RCR did not discourage the respondents who preferred arthroscopic or mini-open RCR.
Cost was not an influential factor in opinions about which RCR method is optimal. Medical professionals were ambivalent about the cost-effectiveness of the different procedures, with most expressing no opinion on cost. Multiple investigators have shown that arthroscopic RCR costs as much as $1144 more than mini-open RCR,18,27 which has many of the advantages of arthroscopic repair but not the costly implants and instruments. As our medical community becomes more cost-conscious, concern about this factor may increase among medical professionals.
Our study had several limitations. Its results must be interpreted carefully, given they represent the viewpoints of a nonrandomized sample of motivated respondents at one institution. A selection bias excluded surgeons who were uncomfortable with RCR and unwilling to report any shortcomings. The conclusions cannot be generalized to other medical professionals or to other institutions. Furthermore, to develop a simple, straightforward survey focused on a specific type of rotator cuff tear, and to avoid confusion, we assumed that the treatment preference for the described tear was generalizable to all encountered tears. However, some surgeons have reported different repair techniques for different types and sizes of rotator cuff tears.25
Conclusion
Most of our surveyed medical professionals were willing to defer to their surgeon’s decision about which technique would be appropriate for their own personal RCR. There is a trend nationally, and at our institution, for increased use of arthroscopic RCR. Although medical professionals readily acknowledge it is unclear which repair method provides the best ultimate outcome, many perceive fast recovery and good cosmetic results with arthroscopic and mini-open repairs. When medical professionals are counseling patients, we need to recognize these personal biases because many patients defer to their surgeon’s counsel. For some medical professionals, cosmesis can be an important factor, but cost, procedure duration, potential technical challenges of arthroscopic repair, and other considerations may make other techniques more desirable for others.
1. Bennett WF. Arthroscopic repair of massive rotator cuff tears: a prospective cohort with 2- to 4-year follow-up. Arthroscopy. 2003;19(4):380-390.
2. Bennett WF. Arthroscopic repair of full-thickness supraspinatus tears (small-to-medium): a prospective study with 2- to 4-year follow-up. Arthroscopy. 2003;19(3):249-256.
3. Nho SJ, Shindle MK, Sherman SL, Freedman KB, Lyman S, MacGillivray JD. Systematic review of arthroscopic rotator cuff repair and mini-open rotator cuff repair. J Bone Joint Surg Am. 2007;89(suppl 3):127-136.
4. Duralde XA, Greene RT. Mini-open rotator cuff repair via an anterosuperior approach. J Shoulder Elbow Surg. 2008;17(5):715-721.
5. Blevins FT, Warren RF, Cavo C, et al. Arthroscopic assisted rotator cuff repair: results using a mini-open deltoid splitting approach. Arthroscopy. 1996;12(1):50-59.
6. Levy HJ, Uribe JW, Delaney LG. Arthroscopic assisted rotator cuff repair: preliminary results. Arthroscopy. 1990;6(1):55-60.
7. Liu SH. Arthroscopically-assisted rotator-cuff repair. J Bone Joint Surg Br. 1994;76(4):592-595.
8. Morse K, Davis AD, Afra R, Kaye EK, Schepsis A, Voloshin I. Arthroscopic versus mini-open rotator cuff repair: a comprehensive review and meta-analysis. Am J Sports Med. 2008;36(9):1824-1828.
9. Park JY, Levine WN, Marra G, Pollock RG, Flatow EL, Bigliani LU. Portal-extension approach for the repair of small and medium rotator cuff tears. Am J Sports Med. 2000;28(3):312-316.
10. Paulos LE, Kody MH. Arthroscopically enhanced “miniapproach” to rotator cuff repair. Am J Sports Med. 1994;22(1):19-25.
11. Posada A, Uribe JW, Hechtman KS, Tjin-A-Tsoi EW, Zvijac JE. Mini-deltoid splitting rotator cuff repair: do results deteriorate with time? Arthroscopy. 2000;16(2):137-141.
12. Shinners TJ, Noordsij PG, Orwin JF. Arthroscopically assisted mini-open rotator cuff repair. Arthroscopy. 2002;18(1):21-26.
13. Weber SC. Arthroscopic debridement and acromioplasty versus mini-open repair in the treatment of significant partial-thickness rotator cuff tears. Arthroscopy. 1999;15(2):126-131.
14. Baker CL, Liu SH. Comparison of open and arthroscopically assisted rotator cuff repairs. Am J Sports Med. 1995;23(1):99-104.
15. Liu SH, Baker CL. Arthroscopically assisted rotator cuff repair: correlation of functional results with integrity of the cuff. Arthroscopy. 1994;10(1):54-60.
16. Pollock RG, Flatow EL. The rotator cuff, part II. Full-thickness tears. Mini-open repair. Orthop Clin North Am. 1997;28(2):169-177.
17. Yamaguchi K, Levine WN, Marra G, Galatz LM, Klepps S, Flatow EL. Transitioning to arthroscopic rotator cuff repair: the pros and cons. Instr Course Lect. 2003;52:81-92.
18. Adla DN, Rowsell M, Pandey R. Cost-effectiveness of open versus arthroscopic rotator cuff repair. J Shoulder Elbow Surg. 2010;19(2):258-261.
19. Kose KC, Tezen E, Cebesoy O, et al. Mini-open versus all-arthroscopic rotator cuff repair: comparison of the operative costs and the clinical outcomes. Adv Ther. 2008;25(3):249-259.
20. Colvin AC, Egorova N, Harrison AK, Moskowitz A, Flatow EL. National trends in rotator cuff repair. J Bone Joint Surg Am. 2012;94(3):227-233.
21. Dunn WR, Schackman BR, Walsh C, et al. Variation in orthopaedic surgeons’ perceptions about the indications for rotator cuff surgery. J Bone Joint Surg Am. 2005;87(9):1978-1984.
22. Randelli P, Arrigoni P, Cabitza F, Ragone V, Cabitza P. Current practice in shoulder pathology: results of a web-based survey among a community of 1,084 orthopedic surgeons. Knee Surg Sports Traumatol Arthrosc. 2012;20(5):803-815.
23. Aleem AW, Brophy RH. Outcomes of rotator cuff surgery: what does the evidence tell us? Clin Sports Med. 2012;31(4):665-674.
24. Pedowitz RA, Yamaguchi K, Ahmad CS, et al. American Academy of Orthopaedic Surgeons clinical practice guideline on: optimizing the management of rotator cuff problems. J Bone Joint Surg Am. 2012;94(2):163-167.
25. Vitale MA, Kleweno CP, Jacir AM, Levine WN, Bigliani LU, Ahmad CS. Training resources in arthroscopic rotator cuff repair. J Bone Joint Surg Am. 2007;89(6):1393-1398.
26. Mauro CS, Jordan SS, Irrgang JJ, Harner CD. Practice patterns for subacromial decompression and rotator cuff repair: an analysis of the American Board of Orthopaedic Surgery database. J Bone Joint Surg Am. 2012;94(16):1492-1499.
27. Churchill RS, Ghorai JK. Total cost and operating room time comparison of rotator cuff repair techniques at low, intermediate, and high volume centers: mini-open versus all-arthroscopic. J Shoulder Elbow Surg. 2010;19(5):716-721.
28. O’Neill PJ, Cosgarea AJ, Freedman JA, Queale WS, McFarland EG. Arthroscopic proficiency: a survey of orthopaedic sports medicine fellowship directors and orthopaedic surgery department chairs. Arthroscopy. 2002;18(7):795-800.
29. Guttmann D, Graham RD, MacLennan MJ, Lubowitz JH. Arthroscopic rotator cuff repair: the learning curve. Arthroscopy. 2005;21(4):394-400.
Rotator cuff tears are a common condition affecting the shoulder joint. Initial open repair techniques were associated with several complications, including severe early postoperative pain, deltoid detachment and/or weakness, risk for infection, and arthrofibrosis.1-3 In addition, open procedures cannot address other possible diagnoses, such as labral tears and loose bodies. These disadvantages promoted the development of an arthroscopically assisted mini-open technique.4 Superior long-term results, with more than 90% of patients achieving good to excellent results,5-13 established the mini-open rotator cuff repair (RCR) as the gold standard.3,6,10,12,14-16
Recently, as instrumentation for arthroscopy has improved, enthusiasm for all-arthroscopic techniques (hereafter referred to as arthroscopic repair) has grown. The appeal of arthroscopic repair includes potentially less initial pain, ability to treat intra-articular lesions concurrently, smaller skin incisions with better cosmesis, less soft-tissue dissection, and low risk for deltoid detachment.3,17 The potential advantages of arthroscopic repair can lead to perceptions of quicker healing and shorter recovery, which are not supported by the literature. However, arthroscopic repair is technically more challenging, time-consuming, and expensive than open or mini-open repairs,18,19 and though some investigators have reported a trend toward fewer complications,3 the long-term outcome of arthroscopic RCRs has not been shown to be better than that of other techniques.
Given that no differences have been shown between the emerging arthroscopic repair technique and mini-open repair with respect to range of motion or clinical scores in the short term,3 it is unclear what perceptions influence choice of technique for one’s own personal RCR.
We conducted a study to determine which RCR technique medical professionals (orthopedic attendings and residents, anesthesiologists, internal medicine attendings, main operating room nurses, and physical therapists) preferred for their own surgery and to analyze perceptions shaping those opinions. Orthopedic surgeons have the best concept of rotator cuff surgery, but anesthesiologists and nurses have a “front row seat” and opinions on types of rotator cuff surgery. Physical therapists, who treat patients with rotator cuff tears, also have a working knowledge of rotator cuff surgery. Finally, internists represent a rotator cuff injury referral service and may have patients who have undergone rotator cuff surgery. We hypothesized that most medical professionals, irrespective of specialty or career length, would prefer arthroscopic RCR because of its perceived superior outcome and fast recovery.
Materials and Methods
This cross-sectional, descriptive, survey-based study was approved by our institutional review board (IRB) and offered via 3 emails between April 2011 and June 2011 to attendings (orthopedists, internists, anesthesiologists), residents, and allied health professionals (AHPs; operating room nurses, physical therapists) involved in orthopedic care at our institution. Each email contained a hyperlink to the online survey (Appendix), which took about 10 minutes to complete and explored respondent demographics, exposure to the different techniques, and opinions regarding different aspects of RCR surgery and recovery.
There were 84 respondents. The sexes were equally represented, and age ranged from 25 to 78 years (Table 1). Of the respondents, 41 (49%) were attendings, 20 (24%) were residents, and 23 (27%) were AHPs. Of the attendings, 13 (32%) were orthopedic surgeons, 26 (63%) were primary care physicians, and 2 (5%) did not specify their specialty. Four orthopedic surgeons had fellowship training in sports medicine or shoulder and elbow surgery. The attendings were overall more experienced in their profession than the other groups were, with 68% reporting more than 5 years of experience.
Descriptive statistics, including means and standard errors, were calculated. Fisher exact test was used to compare preferences of RCR type according to type of training and years of experience. Significance was set at P ≤ .05.
Results
Overall Responses (Table 2)
Of the 84 respondents, almost half (46%) preferred deferring their choice of RCR to their surgeon. Most of the other respondents preferred the arthroscopic technique (26%) or the mini-open repair (23%). There was no association between technique preference and medical professional type. Most respondents (63%) had never assisted in or performed rotator cuff surgery.
Seventy-four percent of all respondents indicated they thought arthroscopic, mini-open, and open RCRs are safe, and about half thought these procedures are fast. About half expressed no opinion about the cost-effectiveness of arthroscopic, mini-open, or open RCRs (54%, 52%, and 48%, respectively), and slightly more than half expressed no opinion about whether arthroscopic, mini-open, or open RCR provide the best outcome (58%, 60%, and 62%, respectively). Significantly (P < .05) more respondents thought arthroscopic and mini-open repairs, rather than open repairs, promote quick healing (64% and 45%, respectively, vs 15%), good cosmetic results (81% and 51%, respectively, vs 10%), and patient satisfaction (50% and 48%, respectively, vs 30%). However, a significant (P < .05) number also thought arthroscopic and mini-open repairs are harder to learn/more challenging to perform than open repairs (52% and 38%, respectively, vs 17%).
Of all factors considered, safety of arthroscopic repair garnered the highest consensus: 82%. Respondents were least opinionated about the outcome of the open repair technique, with more than 62% expressing no opinion about the outcome. The responses to the questions on the learning curves for the 3 techniques varied the most.
Responses by Group (Table 2)
Attendings. Of the 41 attendings, 24 (59%) responded they would defer to their surgeon’s technique preference for RCR. Of the other 17 who expressed a preference, most indicated arthroscopic or mini-open repair (17% each). There was a difference (P < .05) between years of experience and RCR preference: of the 13 attendings with less than 5 years of experience, arthroscopic repair was preferred by 31%; in contrast, of the 28 attendings with more than 5 years of experience, only 11% preferred arthroscopic repair.
Of the 11 attendings who performed rotator cuff surgery, 55% used the open technique, but most (8) preferred to have their own rotator cuff fixed arthroscopically or according to their surgeon’s preference. Only 1 surgeon preferred open repair for his own rotator cuff. Of the 4 surgeons who performed arthroscopic RCRs, 3 had less than 5 years of experience. Conversely, all 7 surgeons who performed mini-open or open repairs had more than 5 years of experience.
Of the 30 attendings who did not perform rotator cuff surgery, most (20) responded they would defer to their surgeon’s technique preference for RCR.
The attendings’ opinions on factors affecting rotator cuff surgery were similar to those of the other respondents with respect to safety, cost-effectiveness, recovery, cosmesis, patient satisfaction, outcome, and technical difficulty. Unlike the others, however, attendings considered all 3 repair techniques fast.
Residents. Of the 20 residents, 7 preferred arthroscopic, 5 preferred mini-open, and 1 preferred open repair; the other 7 responded they would defer to their surgeon’s preference. Residents’ opinions on each factor were more polarized and consistent across categories than those of the other groups. Residents overwhelmingly thought all 3 techniques (arthroscopic, mini-open, open) are safe (19, 19, and 18, respectively) and cost-effective (12, 14, and 14, respectively). Although most residents considered the open and mini-open repair techniques fast (19 and 15, respectively), only 8 considered arthroscopic RCR fast, and 4 considered it slow. Residents’ opinions about the technique that produces the best outcome were mixed. As with the other respondents, residents thought arthroscopic RCRs heal fast and produce great cosmetic results, but are challenging to perform and have a steep learning curve. Unlike the other respondents, most residents (12) considered open RCR easy to learn (P = .006), with a learning curve of fewer than 20 procedures.
AHPs. No AHP expressed a preference for open RCR. This group was evenly divided among 3 choices: deferring to their surgeon’s preference, arthroscopic repair, and mini-open repair. The 23 AHPs thought arthroscopic, mini-open, and open repairs are safe (17, 15, and 12, respectively), but most indicated they were “equivocal” about which techniques are cost-effective, challenging to perform, and produce the best outcomes. A significantly (P = .014) larger number of AHPs (7) considered open rotator cuff surgery slow compared with arthroscopic (0) and mini-open (2) repair techniques. As with the overall cohort, AHPs reported arthroscopic and mini-open repairs promote quick healing and good cosmetic results, but are challenging to perform.
Discussion
As our population ages and continues to remain active, the demand for RCR has accelerated. National data show that 272,148 ambulatory RCRs and 20,433 inpatient RCRs were performed in 2006—an overall 141% increase in RCR since 1996.20 In 1996, 41 per 100,000 population underwent RCR.20 By 2006, this number ballooned to 98 per 100,000 population.20 There are 3 predominant techniques for repairing the rotator cuff: open, mini-open, and arthroscopic. As RCR use increases, we should consider the factors that medical professionals consider important when choosing a method for their own RCR.
Of the 84 medical professionals in our cohort, 39 (46%) indicated they would defer to their surgeon’s technique preference for RCR. Of the other 45, about equal numbers preferred arthroscopic and mini-open RCRs; only 2 preferred open RCRs. This finding suggests that the individual opinions of surgeons who perform RCRs have a substantial influence on a large proportion of medical professionals’ ultimate choice of RCR method. Interestingly, of the attendings who performed open RCR, only 1 expressed a preference for the open technique for his own RCR. This finding might suggest a shift in opinion and an emerging perception among surgeons performing RCR about the value of this technique.
Several factors may account for these evolving beliefs. We hypothesized that a biased favorable view of arthroscopic repair outcome might influence opinions. However, our results did not support the hypothesis. Medical professionals in our cohort were equivocal about the best RCR technique. No consensus was evident among attendings, residents, or AHPs. This lack of clinical agreement about rotator cuff surgery has been observed elsewhere—for example, among members of the American Academy of Orthopaedic Surgeons (AAOS)21 and the European Society of Sports Traumatology, Knee Surgery, and Arthroscopy.22 Despite theoretical advantages of arthroscopic repair, there has been no documented significant difference in patient outcomes when compared with other techniques.23 To our knowledge, there have been only a few clinical studies comparing the different RCR techniques. A meta-analysis of 5 clinical studies comparing arthroscopic and mini-open RCR techniques showed no difference in clinical outcomes or complication rates.8 The 2012 AAOS clinical practice guidelines for RCR reflect these observations.24 That consortium of leading shoulder surgeons could not recommend a modality of surgical rotator cuff tear repair given the lack of conclusive evidence.24
At our institution, arthroscopic, mini-open, and open RCRs were performed by 36%, 9%, and 55% of our surgeons, respectively. A survey of AAOS surgeons showed that, of those who perform RCRs, 14.5%, 46.2%, and 36.6% used arthroscopic, mini-open, and open techniques, respectively.21 The greater use of open repairs at our institution might reflect the seniority of our faculty. Dunn and colleagues21 found that surgeons who preferred open RCR had been in practice longer than those who preferred the arthroscopic or mini-open technique. Of our 4 faculty who performed arthroscopic repairs, 3 were less than 5 years from completing their training. In contrast, all faculty who performed mini-open or open repairs were more than 5 years from completing their training. Furthermore, mean age of the surgeons who performed arthroscopic repair was 39.8 years (range, 32-51 years), and these surgeons were significantly younger than those who performed mini-open or open repair (mean age, 56.3 years; range, 41-78 years). Younger surgeon age has been associated with higher rates of arthroscopic repair.25
Attendings unaccustomed to arthroscopy may find it more challenging than the younger generation of surgeons, who are exposed to it early in training. Dunn and colleagues21 noted that the likelihood of performing an arthroscopic repair was influenced by the surgeon’s experience level. Fellowship-trained shoulder and sports medicine surgeons are also more likely to perform arthroscopic repairs than those with training limited to orthopedic residency.25 Arthroscopic RCR demands a high level of technical skill that many acquire in fellowship training.26 Mauro and colleagues26 found that surgeons trained in a sports medicine fellowship performed 82.6% of subacromial decompression and/or RCR procedures arthroscopically, compared with 54.5% to 70.1% for surgeons trained in other fellowships. In our cohort, with the exception of 1 surgeon, all fellowship-trained shoulder and sports medicine surgeons performed arthroscopic RCRs.
Although no conclusive evidence in the literature supports arthroscopic over the other repair types, the demand for arthroscopic RCR has rapidly increased relative to that for the others. Between 1996 and 2006, use of arthroscopic RCR increased 600%, from 8 to 58 per 100,000 population.20 In that same period, use of open RCR increased by only 34%.20 Similarly, Mauro and colleagues26 found that the proportion of subacromial decompression and RCRs performed arthroscopically rose from 58.3% in 2004 to 83.7% in 2009. Using the 2006 New York State Ambulatory Surgery Database, Churchill and Ghorai27 found that 74.5% of RCRs with acromioplasty were performed arthroscopically.
Respondent-indicated factors that may have contributed to the more favorable opinion of arthroscopic and mini-open repair include quick healing, good cosmetic results, and better perceived patient satisfaction. The literature supports these perceptions. Baker and Liu14 found shorter hospital stays and quicker return to activity with arthroscopic repair compared with open repair. Vitale and colleagues25 also noted that, compared with open or mini-open repair techniques, arthroscopic repair resulted in shorter hospitalization and quicker overall recovery.
If these selected health care professionals with some inside information on rotator cuff surgery have biases that affect their selection of rotator cuff procedures, we should acknowledge that nonmedical personnel, in particular our patients, also have biases. The knowledge base of patients may be further influenced by friends or family members who have had rotator cuff surgery, by lay publications, and by the Internet. Satisfaction with any surgical procedure depends not only on the success of the surgery and the rehabilitation but also on patient and provider expectations. Such expectations are influenced, in part, by biases.
Our medical professionals had similar opinions on safety, recovery, cosmesis, and overall outcome of the RCR techniques, but different opinions on procedure durations and associated training requirements. All residents except one indicated open repair was a quick procedure. In contrast, a significant number of AHPs thought open repair was time-consuming. The attendings considered all the methods fast. The residents’ opinions were the most consistent with the true operating times reported. According to the literature, total operating time for mini-open repair ranges from 10 to 16 minutes faster than that for arthroscopic repair.18,20,27 Ultimately, procedure duration did not affect the respondents’ technique preference for RCR.
There was substantial disagreement about the number of procedures needed to become proficient in the different repair techniques. Overall, however, there was consensus that arthroscopic and mini-open repairs had longer learning curves than open repair. Given the lack of agreement among orthopedic department chairmen and sports medicine fellowship directors regarding the minimum exposure needed (during residency) to become proficient in diagnostic shoulder arthroscopy,28 this finding is not surprising. Guttmann and colleagues29 attempted to quantify the learning curve for arthroscopic RCR by tracking operating time as a surrogate measure. They found that RCR operative time decreased rapidly during the initial block of 10 cases to the second block of 10 cases, but thereafter improvement continued at a much lower rate.29 None of our respondents thought the learning curve for arthroscopic RCR was under 10 cases, but no group, not even the attendings who performed RCRs, could agree on the minimum number of cases needed for proficiency. The longer learning curve for arthroscopic RCR did not discourage the respondents who preferred arthroscopic or mini-open RCR.
Cost was not an influential factor in opinions about which RCR method is optimal. Medical professionals were ambivalent about the cost-effectiveness of the different procedures, with most expressing no opinion on cost. Multiple investigators have shown that arthroscopic RCR costs as much as $1144 more than mini-open RCR,18,27 which has many of the advantages of arthroscopic repair but not the costly implants and instruments. As our medical community becomes more cost-conscious, concern about this factor may increase among medical professionals.
Our study had several limitations. Its results must be interpreted carefully, given they represent the viewpoints of a nonrandomized sample of motivated respondents at one institution. A selection bias excluded surgeons who were uncomfortable with RCR and unwilling to report any shortcomings. The conclusions cannot be generalized to other medical professionals or to other institutions. Furthermore, to develop a simple, straightforward survey focused on a specific type of rotator cuff tear, and to avoid confusion, we assumed that the treatment preference for the described tear was generalizable to all encountered tears. However, some surgeons have reported different repair techniques for different types and sizes of rotator cuff tears.25
Conclusion
Most of our surveyed medical professionals were willing to defer to their surgeon’s decision about which technique would be appropriate for their own personal RCR. There is a trend nationally, and at our institution, for increased use of arthroscopic RCR. Although medical professionals readily acknowledge it is unclear which repair method provides the best ultimate outcome, many perceive fast recovery and good cosmetic results with arthroscopic and mini-open repairs. When medical professionals are counseling patients, we need to recognize these personal biases because many patients defer to their surgeon’s counsel. For some medical professionals, cosmesis can be an important factor, but cost, procedure duration, potential technical challenges of arthroscopic repair, and other considerations may make other techniques more desirable for others.
Rotator cuff tears are a common condition affecting the shoulder joint. Initial open repair techniques were associated with several complications, including severe early postoperative pain, deltoid detachment and/or weakness, risk for infection, and arthrofibrosis.1-3 In addition, open procedures cannot address other possible diagnoses, such as labral tears and loose bodies. These disadvantages promoted the development of an arthroscopically assisted mini-open technique.4 Superior long-term results, with more than 90% of patients achieving good to excellent results,5-13 established the mini-open rotator cuff repair (RCR) as the gold standard.3,6,10,12,14-16
Recently, as instrumentation for arthroscopy has improved, enthusiasm for all-arthroscopic techniques (hereafter referred to as arthroscopic repair) has grown. The appeal of arthroscopic repair includes potentially less initial pain, ability to treat intra-articular lesions concurrently, smaller skin incisions with better cosmesis, less soft-tissue dissection, and low risk for deltoid detachment.3,17 The potential advantages of arthroscopic repair can lead to perceptions of quicker healing and shorter recovery, which are not supported by the literature. However, arthroscopic repair is technically more challenging, time-consuming, and expensive than open or mini-open repairs,18,19 and though some investigators have reported a trend toward fewer complications,3 the long-term outcome of arthroscopic RCRs has not been shown to be better than that of other techniques.
Given that no differences have been shown between the emerging arthroscopic repair technique and mini-open repair with respect to range of motion or clinical scores in the short term,3 it is unclear what perceptions influence choice of technique for one’s own personal RCR.
We conducted a study to determine which RCR technique medical professionals (orthopedic attendings and residents, anesthesiologists, internal medicine attendings, main operating room nurses, and physical therapists) preferred for their own surgery and to analyze perceptions shaping those opinions. Orthopedic surgeons have the best concept of rotator cuff surgery, but anesthesiologists and nurses have a “front row seat” and opinions on types of rotator cuff surgery. Physical therapists, who treat patients with rotator cuff tears, also have a working knowledge of rotator cuff surgery. Finally, internists represent a rotator cuff injury referral service and may have patients who have undergone rotator cuff surgery. We hypothesized that most medical professionals, irrespective of specialty or career length, would prefer arthroscopic RCR because of its perceived superior outcome and fast recovery.
Materials and Methods
This cross-sectional, descriptive, survey-based study was approved by our institutional review board (IRB) and offered via 3 emails between April 2011 and June 2011 to attendings (orthopedists, internists, anesthesiologists), residents, and allied health professionals (AHPs; operating room nurses, physical therapists) involved in orthopedic care at our institution. Each email contained a hyperlink to the online survey (Appendix), which took about 10 minutes to complete and explored respondent demographics, exposure to the different techniques, and opinions regarding different aspects of RCR surgery and recovery.
There were 84 respondents. The sexes were equally represented, and age ranged from 25 to 78 years (Table 1). Of the respondents, 41 (49%) were attendings, 20 (24%) were residents, and 23 (27%) were AHPs. Of the attendings, 13 (32%) were orthopedic surgeons, 26 (63%) were primary care physicians, and 2 (5%) did not specify their specialty. Four orthopedic surgeons had fellowship training in sports medicine or shoulder and elbow surgery. The attendings were overall more experienced in their profession than the other groups were, with 68% reporting more than 5 years of experience.
Descriptive statistics, including means and standard errors, were calculated. Fisher exact test was used to compare preferences of RCR type according to type of training and years of experience. Significance was set at P ≤ .05.
Results
Overall Responses (Table 2)
Of the 84 respondents, almost half (46%) preferred deferring their choice of RCR to their surgeon. Most of the other respondents preferred the arthroscopic technique (26%) or the mini-open repair (23%). There was no association between technique preference and medical professional type. Most respondents (63%) had never assisted in or performed rotator cuff surgery.
Seventy-four percent of all respondents indicated they thought arthroscopic, mini-open, and open RCRs are safe, and about half thought these procedures are fast. About half expressed no opinion about the cost-effectiveness of arthroscopic, mini-open, or open RCRs (54%, 52%, and 48%, respectively), and slightly more than half expressed no opinion about whether arthroscopic, mini-open, or open RCR provide the best outcome (58%, 60%, and 62%, respectively). Significantly (P < .05) more respondents thought arthroscopic and mini-open repairs, rather than open repairs, promote quick healing (64% and 45%, respectively, vs 15%), good cosmetic results (81% and 51%, respectively, vs 10%), and patient satisfaction (50% and 48%, respectively, vs 30%). However, a significant (P < .05) number also thought arthroscopic and mini-open repairs are harder to learn/more challenging to perform than open repairs (52% and 38%, respectively, vs 17%).
Of all factors considered, safety of arthroscopic repair garnered the highest consensus: 82%. Respondents were least opinionated about the outcome of the open repair technique, with more than 62% expressing no opinion about the outcome. The responses to the questions on the learning curves for the 3 techniques varied the most.
Responses by Group (Table 2)
Attendings. Of the 41 attendings, 24 (59%) responded they would defer to their surgeon’s technique preference for RCR. Of the other 17 who expressed a preference, most indicated arthroscopic or mini-open repair (17% each). There was a difference (P < .05) between years of experience and RCR preference: of the 13 attendings with less than 5 years of experience, arthroscopic repair was preferred by 31%; in contrast, of the 28 attendings with more than 5 years of experience, only 11% preferred arthroscopic repair.
Of the 11 attendings who performed rotator cuff surgery, 55% used the open technique, but most (8) preferred to have their own rotator cuff fixed arthroscopically or according to their surgeon’s preference. Only 1 surgeon preferred open repair for his own rotator cuff. Of the 4 surgeons who performed arthroscopic RCRs, 3 had less than 5 years of experience. Conversely, all 7 surgeons who performed mini-open or open repairs had more than 5 years of experience.
Of the 30 attendings who did not perform rotator cuff surgery, most (20) responded they would defer to their surgeon’s technique preference for RCR.
The attendings’ opinions on factors affecting rotator cuff surgery were similar to those of the other respondents with respect to safety, cost-effectiveness, recovery, cosmesis, patient satisfaction, outcome, and technical difficulty. Unlike the others, however, attendings considered all 3 repair techniques fast.
Residents. Of the 20 residents, 7 preferred arthroscopic, 5 preferred mini-open, and 1 preferred open repair; the other 7 responded they would defer to their surgeon’s preference. Residents’ opinions on each factor were more polarized and consistent across categories than those of the other groups. Residents overwhelmingly thought all 3 techniques (arthroscopic, mini-open, open) are safe (19, 19, and 18, respectively) and cost-effective (12, 14, and 14, respectively). Although most residents considered the open and mini-open repair techniques fast (19 and 15, respectively), only 8 considered arthroscopic RCR fast, and 4 considered it slow. Residents’ opinions about the technique that produces the best outcome were mixed. As with the other respondents, residents thought arthroscopic RCRs heal fast and produce great cosmetic results, but are challenging to perform and have a steep learning curve. Unlike the other respondents, most residents (12) considered open RCR easy to learn (P = .006), with a learning curve of fewer than 20 procedures.
AHPs. No AHP expressed a preference for open RCR. This group was evenly divided among 3 choices: deferring to their surgeon’s preference, arthroscopic repair, and mini-open repair. The 23 AHPs thought arthroscopic, mini-open, and open repairs are safe (17, 15, and 12, respectively), but most indicated they were “equivocal” about which techniques are cost-effective, challenging to perform, and produce the best outcomes. A significantly (P = .014) larger number of AHPs (7) considered open rotator cuff surgery slow compared with arthroscopic (0) and mini-open (2) repair techniques. As with the overall cohort, AHPs reported arthroscopic and mini-open repairs promote quick healing and good cosmetic results, but are challenging to perform.
Discussion
As our population ages and continues to remain active, the demand for RCR has accelerated. National data show that 272,148 ambulatory RCRs and 20,433 inpatient RCRs were performed in 2006—an overall 141% increase in RCR since 1996.20 In 1996, 41 per 100,000 population underwent RCR.20 By 2006, this number ballooned to 98 per 100,000 population.20 There are 3 predominant techniques for repairing the rotator cuff: open, mini-open, and arthroscopic. As RCR use increases, we should consider the factors that medical professionals consider important when choosing a method for their own RCR.
Of the 84 medical professionals in our cohort, 39 (46%) indicated they would defer to their surgeon’s technique preference for RCR. Of the other 45, about equal numbers preferred arthroscopic and mini-open RCRs; only 2 preferred open RCRs. This finding suggests that the individual opinions of surgeons who perform RCRs have a substantial influence on a large proportion of medical professionals’ ultimate choice of RCR method. Interestingly, of the attendings who performed open RCR, only 1 expressed a preference for the open technique for his own RCR. This finding might suggest a shift in opinion and an emerging perception among surgeons performing RCR about the value of this technique.
Several factors may account for these evolving beliefs. We hypothesized that a biased favorable view of arthroscopic repair outcome might influence opinions. However, our results did not support the hypothesis. Medical professionals in our cohort were equivocal about the best RCR technique. No consensus was evident among attendings, residents, or AHPs. This lack of clinical agreement about rotator cuff surgery has been observed elsewhere—for example, among members of the American Academy of Orthopaedic Surgeons (AAOS)21 and the European Society of Sports Traumatology, Knee Surgery, and Arthroscopy.22 Despite theoretical advantages of arthroscopic repair, there has been no documented significant difference in patient outcomes when compared with other techniques.23 To our knowledge, there have been only a few clinical studies comparing the different RCR techniques. A meta-analysis of 5 clinical studies comparing arthroscopic and mini-open RCR techniques showed no difference in clinical outcomes or complication rates.8 The 2012 AAOS clinical practice guidelines for RCR reflect these observations.24 That consortium of leading shoulder surgeons could not recommend a modality of surgical rotator cuff tear repair given the lack of conclusive evidence.24
At our institution, arthroscopic, mini-open, and open RCRs were performed by 36%, 9%, and 55% of our surgeons, respectively. A survey of AAOS surgeons showed that, of those who perform RCRs, 14.5%, 46.2%, and 36.6% used arthroscopic, mini-open, and open techniques, respectively.21 The greater use of open repairs at our institution might reflect the seniority of our faculty. Dunn and colleagues21 found that surgeons who preferred open RCR had been in practice longer than those who preferred the arthroscopic or mini-open technique. Of our 4 faculty who performed arthroscopic repairs, 3 were less than 5 years from completing their training. In contrast, all faculty who performed mini-open or open repairs were more than 5 years from completing their training. Furthermore, mean age of the surgeons who performed arthroscopic repair was 39.8 years (range, 32-51 years), and these surgeons were significantly younger than those who performed mini-open or open repair (mean age, 56.3 years; range, 41-78 years). Younger surgeon age has been associated with higher rates of arthroscopic repair.25
Attendings unaccustomed to arthroscopy may find it more challenging than the younger generation of surgeons, who are exposed to it early in training. Dunn and colleagues21 noted that the likelihood of performing an arthroscopic repair was influenced by the surgeon’s experience level. Fellowship-trained shoulder and sports medicine surgeons are also more likely to perform arthroscopic repairs than those with training limited to orthopedic residency.25 Arthroscopic RCR demands a high level of technical skill that many acquire in fellowship training.26 Mauro and colleagues26 found that surgeons trained in a sports medicine fellowship performed 82.6% of subacromial decompression and/or RCR procedures arthroscopically, compared with 54.5% to 70.1% for surgeons trained in other fellowships. In our cohort, with the exception of 1 surgeon, all fellowship-trained shoulder and sports medicine surgeons performed arthroscopic RCRs.
Although no conclusive evidence in the literature supports arthroscopic over the other repair types, the demand for arthroscopic RCR has rapidly increased relative to that for the others. Between 1996 and 2006, use of arthroscopic RCR increased 600%, from 8 to 58 per 100,000 population.20 In that same period, use of open RCR increased by only 34%.20 Similarly, Mauro and colleagues26 found that the proportion of subacromial decompression and RCRs performed arthroscopically rose from 58.3% in 2004 to 83.7% in 2009. Using the 2006 New York State Ambulatory Surgery Database, Churchill and Ghorai27 found that 74.5% of RCRs with acromioplasty were performed arthroscopically.
Respondent-indicated factors that may have contributed to the more favorable opinion of arthroscopic and mini-open repair include quick healing, good cosmetic results, and better perceived patient satisfaction. The literature supports these perceptions. Baker and Liu14 found shorter hospital stays and quicker return to activity with arthroscopic repair compared with open repair. Vitale and colleagues25 also noted that, compared with open or mini-open repair techniques, arthroscopic repair resulted in shorter hospitalization and quicker overall recovery.
If these selected health care professionals with some inside information on rotator cuff surgery have biases that affect their selection of rotator cuff procedures, we should acknowledge that nonmedical personnel, in particular our patients, also have biases. The knowledge base of patients may be further influenced by friends or family members who have had rotator cuff surgery, by lay publications, and by the Internet. Satisfaction with any surgical procedure depends not only on the success of the surgery and the rehabilitation but also on patient and provider expectations. Such expectations are influenced, in part, by biases.
Our medical professionals had similar opinions on safety, recovery, cosmesis, and overall outcome of the RCR techniques, but different opinions on procedure durations and associated training requirements. All residents except one indicated open repair was a quick procedure. In contrast, a significant number of AHPs thought open repair was time-consuming. The attendings considered all the methods fast. The residents’ opinions were the most consistent with the true operating times reported. According to the literature, total operating time for mini-open repair ranges from 10 to 16 minutes faster than that for arthroscopic repair.18,20,27 Ultimately, procedure duration did not affect the respondents’ technique preference for RCR.
There was substantial disagreement about the number of procedures needed to become proficient in the different repair techniques. Overall, however, there was consensus that arthroscopic and mini-open repairs had longer learning curves than open repair. Given the lack of agreement among orthopedic department chairmen and sports medicine fellowship directors regarding the minimum exposure needed (during residency) to become proficient in diagnostic shoulder arthroscopy,28 this finding is not surprising. Guttmann and colleagues29 attempted to quantify the learning curve for arthroscopic RCR by tracking operating time as a surrogate measure. They found that RCR operative time decreased rapidly during the initial block of 10 cases to the second block of 10 cases, but thereafter improvement continued at a much lower rate.29 None of our respondents thought the learning curve for arthroscopic RCR was under 10 cases, but no group, not even the attendings who performed RCRs, could agree on the minimum number of cases needed for proficiency. The longer learning curve for arthroscopic RCR did not discourage the respondents who preferred arthroscopic or mini-open RCR.
Cost was not an influential factor in opinions about which RCR method is optimal. Medical professionals were ambivalent about the cost-effectiveness of the different procedures, with most expressing no opinion on cost. Multiple investigators have shown that arthroscopic RCR costs as much as $1144 more than mini-open RCR,18,27 which has many of the advantages of arthroscopic repair but not the costly implants and instruments. As our medical community becomes more cost-conscious, concern about this factor may increase among medical professionals.
Our study had several limitations. Its results must be interpreted carefully, given they represent the viewpoints of a nonrandomized sample of motivated respondents at one institution. A selection bias excluded surgeons who were uncomfortable with RCR and unwilling to report any shortcomings. The conclusions cannot be generalized to other medical professionals or to other institutions. Furthermore, to develop a simple, straightforward survey focused on a specific type of rotator cuff tear, and to avoid confusion, we assumed that the treatment preference for the described tear was generalizable to all encountered tears. However, some surgeons have reported different repair techniques for different types and sizes of rotator cuff tears.25
Conclusion
Most of our surveyed medical professionals were willing to defer to their surgeon’s decision about which technique would be appropriate for their own personal RCR. There is a trend nationally, and at our institution, for increased use of arthroscopic RCR. Although medical professionals readily acknowledge it is unclear which repair method provides the best ultimate outcome, many perceive fast recovery and good cosmetic results with arthroscopic and mini-open repairs. When medical professionals are counseling patients, we need to recognize these personal biases because many patients defer to their surgeon’s counsel. For some medical professionals, cosmesis can be an important factor, but cost, procedure duration, potential technical challenges of arthroscopic repair, and other considerations may make other techniques more desirable for others.
1. Bennett WF. Arthroscopic repair of massive rotator cuff tears: a prospective cohort with 2- to 4-year follow-up. Arthroscopy. 2003;19(4):380-390.
2. Bennett WF. Arthroscopic repair of full-thickness supraspinatus tears (small-to-medium): a prospective study with 2- to 4-year follow-up. Arthroscopy. 2003;19(3):249-256.
3. Nho SJ, Shindle MK, Sherman SL, Freedman KB, Lyman S, MacGillivray JD. Systematic review of arthroscopic rotator cuff repair and mini-open rotator cuff repair. J Bone Joint Surg Am. 2007;89(suppl 3):127-136.
4. Duralde XA, Greene RT. Mini-open rotator cuff repair via an anterosuperior approach. J Shoulder Elbow Surg. 2008;17(5):715-721.
5. Blevins FT, Warren RF, Cavo C, et al. Arthroscopic assisted rotator cuff repair: results using a mini-open deltoid splitting approach. Arthroscopy. 1996;12(1):50-59.
6. Levy HJ, Uribe JW, Delaney LG. Arthroscopic assisted rotator cuff repair: preliminary results. Arthroscopy. 1990;6(1):55-60.
7. Liu SH. Arthroscopically-assisted rotator-cuff repair. J Bone Joint Surg Br. 1994;76(4):592-595.
8. Morse K, Davis AD, Afra R, Kaye EK, Schepsis A, Voloshin I. Arthroscopic versus mini-open rotator cuff repair: a comprehensive review and meta-analysis. Am J Sports Med. 2008;36(9):1824-1828.
9. Park JY, Levine WN, Marra G, Pollock RG, Flatow EL, Bigliani LU. Portal-extension approach for the repair of small and medium rotator cuff tears. Am J Sports Med. 2000;28(3):312-316.
10. Paulos LE, Kody MH. Arthroscopically enhanced “miniapproach” to rotator cuff repair. Am J Sports Med. 1994;22(1):19-25.
11. Posada A, Uribe JW, Hechtman KS, Tjin-A-Tsoi EW, Zvijac JE. Mini-deltoid splitting rotator cuff repair: do results deteriorate with time? Arthroscopy. 2000;16(2):137-141.
12. Shinners TJ, Noordsij PG, Orwin JF. Arthroscopically assisted mini-open rotator cuff repair. Arthroscopy. 2002;18(1):21-26.
13. Weber SC. Arthroscopic debridement and acromioplasty versus mini-open repair in the treatment of significant partial-thickness rotator cuff tears. Arthroscopy. 1999;15(2):126-131.
14. Baker CL, Liu SH. Comparison of open and arthroscopically assisted rotator cuff repairs. Am J Sports Med. 1995;23(1):99-104.
15. Liu SH, Baker CL. Arthroscopically assisted rotator cuff repair: correlation of functional results with integrity of the cuff. Arthroscopy. 1994;10(1):54-60.
16. Pollock RG, Flatow EL. The rotator cuff, part II. Full-thickness tears. Mini-open repair. Orthop Clin North Am. 1997;28(2):169-177.
17. Yamaguchi K, Levine WN, Marra G, Galatz LM, Klepps S, Flatow EL. Transitioning to arthroscopic rotator cuff repair: the pros and cons. Instr Course Lect. 2003;52:81-92.
18. Adla DN, Rowsell M, Pandey R. Cost-effectiveness of open versus arthroscopic rotator cuff repair. J Shoulder Elbow Surg. 2010;19(2):258-261.
19. Kose KC, Tezen E, Cebesoy O, et al. Mini-open versus all-arthroscopic rotator cuff repair: comparison of the operative costs and the clinical outcomes. Adv Ther. 2008;25(3):249-259.
20. Colvin AC, Egorova N, Harrison AK, Moskowitz A, Flatow EL. National trends in rotator cuff repair. J Bone Joint Surg Am. 2012;94(3):227-233.
21. Dunn WR, Schackman BR, Walsh C, et al. Variation in orthopaedic surgeons’ perceptions about the indications for rotator cuff surgery. J Bone Joint Surg Am. 2005;87(9):1978-1984.
22. Randelli P, Arrigoni P, Cabitza F, Ragone V, Cabitza P. Current practice in shoulder pathology: results of a web-based survey among a community of 1,084 orthopedic surgeons. Knee Surg Sports Traumatol Arthrosc. 2012;20(5):803-815.
23. Aleem AW, Brophy RH. Outcomes of rotator cuff surgery: what does the evidence tell us? Clin Sports Med. 2012;31(4):665-674.
24. Pedowitz RA, Yamaguchi K, Ahmad CS, et al. American Academy of Orthopaedic Surgeons clinical practice guideline on: optimizing the management of rotator cuff problems. J Bone Joint Surg Am. 2012;94(2):163-167.
25. Vitale MA, Kleweno CP, Jacir AM, Levine WN, Bigliani LU, Ahmad CS. Training resources in arthroscopic rotator cuff repair. J Bone Joint Surg Am. 2007;89(6):1393-1398.
26. Mauro CS, Jordan SS, Irrgang JJ, Harner CD. Practice patterns for subacromial decompression and rotator cuff repair: an analysis of the American Board of Orthopaedic Surgery database. J Bone Joint Surg Am. 2012;94(16):1492-1499.
27. Churchill RS, Ghorai JK. Total cost and operating room time comparison of rotator cuff repair techniques at low, intermediate, and high volume centers: mini-open versus all-arthroscopic. J Shoulder Elbow Surg. 2010;19(5):716-721.
28. O’Neill PJ, Cosgarea AJ, Freedman JA, Queale WS, McFarland EG. Arthroscopic proficiency: a survey of orthopaedic sports medicine fellowship directors and orthopaedic surgery department chairs. Arthroscopy. 2002;18(7):795-800.
29. Guttmann D, Graham RD, MacLennan MJ, Lubowitz JH. Arthroscopic rotator cuff repair: the learning curve. Arthroscopy. 2005;21(4):394-400.
1. Bennett WF. Arthroscopic repair of massive rotator cuff tears: a prospective cohort with 2- to 4-year follow-up. Arthroscopy. 2003;19(4):380-390.
2. Bennett WF. Arthroscopic repair of full-thickness supraspinatus tears (small-to-medium): a prospective study with 2- to 4-year follow-up. Arthroscopy. 2003;19(3):249-256.
3. Nho SJ, Shindle MK, Sherman SL, Freedman KB, Lyman S, MacGillivray JD. Systematic review of arthroscopic rotator cuff repair and mini-open rotator cuff repair. J Bone Joint Surg Am. 2007;89(suppl 3):127-136.
4. Duralde XA, Greene RT. Mini-open rotator cuff repair via an anterosuperior approach. J Shoulder Elbow Surg. 2008;17(5):715-721.
5. Blevins FT, Warren RF, Cavo C, et al. Arthroscopic assisted rotator cuff repair: results using a mini-open deltoid splitting approach. Arthroscopy. 1996;12(1):50-59.
6. Levy HJ, Uribe JW, Delaney LG. Arthroscopic assisted rotator cuff repair: preliminary results. Arthroscopy. 1990;6(1):55-60.
7. Liu SH. Arthroscopically-assisted rotator-cuff repair. J Bone Joint Surg Br. 1994;76(4):592-595.
8. Morse K, Davis AD, Afra R, Kaye EK, Schepsis A, Voloshin I. Arthroscopic versus mini-open rotator cuff repair: a comprehensive review and meta-analysis. Am J Sports Med. 2008;36(9):1824-1828.
9. Park JY, Levine WN, Marra G, Pollock RG, Flatow EL, Bigliani LU. Portal-extension approach for the repair of small and medium rotator cuff tears. Am J Sports Med. 2000;28(3):312-316.
10. Paulos LE, Kody MH. Arthroscopically enhanced “miniapproach” to rotator cuff repair. Am J Sports Med. 1994;22(1):19-25.
11. Posada A, Uribe JW, Hechtman KS, Tjin-A-Tsoi EW, Zvijac JE. Mini-deltoid splitting rotator cuff repair: do results deteriorate with time? Arthroscopy. 2000;16(2):137-141.
12. Shinners TJ, Noordsij PG, Orwin JF. Arthroscopically assisted mini-open rotator cuff repair. Arthroscopy. 2002;18(1):21-26.
13. Weber SC. Arthroscopic debridement and acromioplasty versus mini-open repair in the treatment of significant partial-thickness rotator cuff tears. Arthroscopy. 1999;15(2):126-131.
14. Baker CL, Liu SH. Comparison of open and arthroscopically assisted rotator cuff repairs. Am J Sports Med. 1995;23(1):99-104.
15. Liu SH, Baker CL. Arthroscopically assisted rotator cuff repair: correlation of functional results with integrity of the cuff. Arthroscopy. 1994;10(1):54-60.
16. Pollock RG, Flatow EL. The rotator cuff, part II. Full-thickness tears. Mini-open repair. Orthop Clin North Am. 1997;28(2):169-177.
17. Yamaguchi K, Levine WN, Marra G, Galatz LM, Klepps S, Flatow EL. Transitioning to arthroscopic rotator cuff repair: the pros and cons. Instr Course Lect. 2003;52:81-92.
18. Adla DN, Rowsell M, Pandey R. Cost-effectiveness of open versus arthroscopic rotator cuff repair. J Shoulder Elbow Surg. 2010;19(2):258-261.
19. Kose KC, Tezen E, Cebesoy O, et al. Mini-open versus all-arthroscopic rotator cuff repair: comparison of the operative costs and the clinical outcomes. Adv Ther. 2008;25(3):249-259.
20. Colvin AC, Egorova N, Harrison AK, Moskowitz A, Flatow EL. National trends in rotator cuff repair. J Bone Joint Surg Am. 2012;94(3):227-233.
21. Dunn WR, Schackman BR, Walsh C, et al. Variation in orthopaedic surgeons’ perceptions about the indications for rotator cuff surgery. J Bone Joint Surg Am. 2005;87(9):1978-1984.
22. Randelli P, Arrigoni P, Cabitza F, Ragone V, Cabitza P. Current practice in shoulder pathology: results of a web-based survey among a community of 1,084 orthopedic surgeons. Knee Surg Sports Traumatol Arthrosc. 2012;20(5):803-815.
23. Aleem AW, Brophy RH. Outcomes of rotator cuff surgery: what does the evidence tell us? Clin Sports Med. 2012;31(4):665-674.
24. Pedowitz RA, Yamaguchi K, Ahmad CS, et al. American Academy of Orthopaedic Surgeons clinical practice guideline on: optimizing the management of rotator cuff problems. J Bone Joint Surg Am. 2012;94(2):163-167.
25. Vitale MA, Kleweno CP, Jacir AM, Levine WN, Bigliani LU, Ahmad CS. Training resources in arthroscopic rotator cuff repair. J Bone Joint Surg Am. 2007;89(6):1393-1398.
26. Mauro CS, Jordan SS, Irrgang JJ, Harner CD. Practice patterns for subacromial decompression and rotator cuff repair: an analysis of the American Board of Orthopaedic Surgery database. J Bone Joint Surg Am. 2012;94(16):1492-1499.
27. Churchill RS, Ghorai JK. Total cost and operating room time comparison of rotator cuff repair techniques at low, intermediate, and high volume centers: mini-open versus all-arthroscopic. J Shoulder Elbow Surg. 2010;19(5):716-721.
28. O’Neill PJ, Cosgarea AJ, Freedman JA, Queale WS, McFarland EG. Arthroscopic proficiency: a survey of orthopaedic sports medicine fellowship directors and orthopaedic surgery department chairs. Arthroscopy. 2002;18(7):795-800.
29. Guttmann D, Graham RD, MacLennan MJ, Lubowitz JH. Arthroscopic rotator cuff repair: the learning curve. Arthroscopy. 2005;21(4):394-400.
Safety of Tourniquet Use in Total Knee Arthroplasty in Patients With Radiographic Evidence of Vascular Calcifications
Tourniquets are often used in total knee arthroplasty (TKA) to improve visualization of structures, shorten operative time, reduce intraoperative bleeding, and improve cementing technique. Despite these advantages, controversy remains regarding the safety of tourniquet use. Tourniquets have been associated with nerve palsies, vascular injury, and muscle damage.1-5 Some have hypothesized they may cause venous stasis or direct endothelial damage that may develop into deep vein thrombosis (DVT). Abdel-Salam and Eyres6 found an increased incidence of postoperative wound complications and DVTs associated with tourniquet use.
Moreover, investigators have analyzed the role of tourniquets in populations at high risk for wound complications. DeLaurentis and colleagues7 performed a prospective and retrospective analysis of 1182 TKA patients, 24 (2%) of whom had preexisting peripheral vascular disease (PVD), defined as a history of arterial insufficiency, absent dorsalis pedis and/or absent posterior tibial pulsations, and arterial calcifications. A tourniquet was used in each case. Arterial complications occurred in 6 of the 24 patients with PVD. As expected, the authors found that a history of intermittent claudication, pain at rest, and arterial ulcers resulted in a high risk for vascular complications. Further studies have supported this finding and expanded the list of predisposing factors to include previous vascular surgery and absent and asymmetric pedal pulsations.7-11 Of particular concern to total joint arthroplasty surgeons was the finding by DeLaurentis and colleagues7 that patients with radiographic evidence of calcification of the distal superficial femoral artery and/or popliteal artery were at risk for arterial complications. This finding is also supported by other studies.8,11 In TKA, damage to arterial structures proximal to the surgical field could manifest as impaired postoperative wound healing or an ischemic limb. Wound healing depends on adequate blood flow to the healing tissue, and any damage to arterial or venous structures can theoretically compromise this process.
Added to vascular/wound complications as concerning complications in orthopedic surgery is venous thromboembolism (VTE). The role of tourniquets in the formation of VTEs is controversial. A tourniquet has the potential to increase the risk for DVT because of the stasis of venous blood in the lower limb or possible damage to calcified blood vessels. Callam and colleagues12 studied the connection between artery disease and chronic leg ulcers and found that half the patients diagnosed with peripheral artery disease also had stigmata of chronic venous insufficiency. Therefore, the entities can occur in tandem, and surgeons should keep this in mind.
Here we report on a study we conducted to determine whether tourniquet use in TKA in patients with preexisting radiographic evidence of vascular disease increases the risk for wound complications or VTE.
Patients and Methods
We retrospectively reviewed 461 consecutive primary TKAs (373 patients) performed between January 2007 and June 2012 by 2 attending orthopedic surgeons specializing in adult reconstruction. Medical records and operative reports of 583 patients were examined after receiving institutional review board approval. Of these patients, 373 (64%) had a minimum of 12-month follow-up data available. Twelve months was deemed long enough to discover wound complications or DVTs secondary to the index procedure. Most of these outcomes manifest within the first 3 months after surgery and certainly by 12 months. Follow-up longer than 12 months may become a confounder, as wound complications outside the acute to subacute postoperative window could be related to patients’ underlying PVD and not directly to tourniquet use during surgery. Patient demographics and comorbidities were recorded. Comorbidities were obtained from preoperative medical evaluations and surgeons’ preoperative evaluations. All patients had preoperative palpable dorsalis pedis and posterior tibialis arterial pulses. No patient required preoperative vascular studies based on preoperative examination or comorbidities. No patient had prior vascular bypass surgery or stenting.
TKA was performed in a nonlaminar flow, positive-pressure, high-efficiency particulate air-filtered room with sterile toga/surgical helmet systems. For all patients, a pneumatic thigh tourniquet was applied, and the patient was prepared and draped. After limb exsanguination using a rubber bandage, the limb was elevated and the tourniquet inflated to a pressure of 250 to 300 mm Hg. The tourniquet was released either just before closure or immediately after closure in all cases; it was always let down before placement of final bandages.
Prophylactic chemical anticoagulation consisting of warfarin, aspirin, or enoxaparin was used in all patients and continued for 4 to 6 weeks after surgery. All patients received mechanical DVT prophylaxis with sequential compression devices, and all were mobilized out of bed beginning either the day of surgery or the next day. All patients received perioperative intravenous antibiotics, with the preoperative dose given before tourniquet inflation and the last postoperative dose stopped within 24 hours of surgery.
All patients who had primary TKA underwent preoperative medical evaluation and optimization. The patient’s hospital course was monitored closely, and complications noted by the orthopedic team were documented. Follow-up documentation was retrospectively reviewed for evidence of wound complications or VTE. Wound complications were defined as cellulitis, delayed wound healing, wound dehiscence, and/or periprosthetic joint infection. In the case of VTE, physical examination findings were not sufficient for inclusion. Venous duplex ultrasonography demonstrating the clot was reviewed before inclusion.
Preoperative radiographs were examined for arterial calcification (Figure). We refer to calcification seen above the knee joint as proximal calcification and to calcification observed below the joint as distal calcification. Patients exhibited calcification proximally only, distally only, or both proximally and distally. The 373 patients were placed into 2 groups based on whether they had preoperative arterial calcification on plain radiography of the knee. One group (285 patients with no radiographic evidence of preoperative knee arterial calcification) underwent 365 TKAs, and the other group (88 patients with radiographic evidence of preoperative knee arterial calcification) underwent 96 TKAs.
A sample size calculation was performed to determine how many patients were needed in each group with 80% power and an α of 0.05. With an estimated difference in VTE/wound complication rate between the calcification and no-calcification groups of 12%, we needed to review 316 TKAs total. This 12% difference was based on study findings of a 25% complication rate in PVD patients who underwent tourniquet-assisted TKA, and the rate of VTE/wound complication after TKA in patients overall, which can be up to 12%.7,13,14 We exceeded minimal enrollment and had 461 TKAs. Descriptive statistics were reported, with means and ranges provided where appropriate. Independent t test was used to evaluate the differences in continuous data (age) between the groups. Univariate analysis (using Pearson χ2 and Fisher exact tests) and multivariate logistic regression analysis were used to evaluate the effects of categorical variables (sex, comorbidity, calcification [presence, absence], and location of calcification [proximal only, distal only, both]) on wound complication and VTE rates. All tests were 2-tailed and performed with a type I error rate of 0.05. Data analysis was performed with SPSS Version 19.0 (SPSS).
Results
Patient characteristics are summarized in Table 1. Of the 373 patients, 285 lacked calcification, and 88 had calcification. Mean age was 67.73 years (range, 24-92 years) for all patients, 65.99 years (range, 24-89 years) for the no-calcification group, and 74.32 years (range, 54-92 years) for the calcification group; the calcification group demonstrated a trend toward older age, but the difference was not significantly different (P = .07). Of the 373 patients, 156 (41.82%) were male: 110 in the no-calcification group (38.60%) and 46 in the calcification group (52.27%); sex was significantly (P = .002) different between groups, with more males in the calcification group.
Data on total preoperative comorbidities are summarized in Table 2. Hypertension, hyperlipidemia, diabetes, and coronary artery disease (CAD) were the most common comorbidities, and they were all significantly (P ≤ .05) increased in the calcification group.
No patients had reported arterial complications, such as arterial bleeding, aneurysm, intimal tears, or loss of distal pulses. Wound complication after TKA was detected in 3.04% of all cases (Table 3). Rate of DVT after TKA was 2.60% of all cases, and rate of pulmonary embolism after TKA was 2.17% of all cases. Of the 96 TKAs with preoperative radiographic evidence of calcification, 47 (48.96%) had proximal calcification only, 11 (11.46%) had distal calcification only, and 38 (39.58%) had both proximal and distal calcification (Table 4). There was no significant difference between the rate of wound complication or VTE based on location of vascular calcification.
Univariate analysis demonstrated that presence of arterial knee calcification did not increase the risk for postoperative wound complication (odds ratio [OR], 1.04; 95% confidence interval [CI], 0.28-3.80; P > .05) (Table 5). Location of arterial knee calcification also did not increase the risk for postoperative wound complication. In addition, univariate analysis demonstrated that presence of arterial knee calcification did not increase the risk for postoperative VTE (OR, 1.20; 95% CI, 0.43-3.36; P > .05 (Table 6).
Of the 14 wound complications, the most common infections were cellulitis (5/14 cases; 35.71%) and infected hardware that required component revision (5/14 cases; 35.71%). Mean time from TKA to infection was 137.93 days (range, 5-783 days). The most common organism grown in culture from the wound was Staphylococcus (5/14 cases; 35.71%).
Additional univariate statistical analysis revealed that presence of diabetes, hypertension, prior VTE, CAD, and male sex was linked to higher incidence of wound complication (P < .05) (Table 5). When multivariate analysis was performed, hypertension, prior VTE, and male sex remained significant (P < .05) (Table 5).
Discussion
TKA is a safe and effective procedure used to treat osteoarthritis of the knee and improve patients’ quality of life.15 About 700,000 TKAs are performed annually in the United States.16 Because of improvements in preventive medicine and medical technology, life expectancy is increasing, and TKAs are now being performed in higher numbers and in an older patient population. Over the next few decades, these developments will lead to more postoperative complications. It is projected that, by 2030, the need for TKAs in the United States will increase by 673% to 3.48 million.17 Postoperative complications are rare but unfortunately often lead to poor outcomes or even mortality.18 To help minimize the number of postoperative complications, we must understand the safety of tourniquet use in TKA. Other investigators have concluded that tourniquet use is unsafe in patients with preoperative vascular calcifications on plain radiographs.7,8,11 The present study, designed to elucidate whether preoperative evidence of knee arterial calcification may predispose TKA patients to postoperative wound complication or VTE, had some important findings.
In our study, wound complication and VTE occurred in a considerable number of patients after TKA. Despite exceeding the number of patients calculated by the power analysis, our population may have been inadequate to fully detect statistical significance. Thus, our conclusion of failing to reject the null hypothesis may have been because of sample size, a type II error. We found that, after primary TKA, 3.04% of patients developed wound complications and 4.77% VTE. According to the literature, the incidence of infection after primary TKA is between 0.5% and 12%, and that of VTE reported within 3 months after TKA is 1.3% to 10%.13,14 Although we had 100% VTE prophylaxis, meeting the standard of care, VTE after TKA remains a postoperative complication.19 This study also found that a considerable percentage of primary TKA patients (23.59%) had preoperative calcification of the knee arteries. To our knowledge, this study was the first to quantify the incidence of knee arterial calcification in patients who underwent TKA.
Preoperative calcification of the knee arteries in patients who underwent TKA did not increase the risk for wound complication, VTE, or arterial damage. These calcifications, however, do pose an increased systemic vascular risk.20 Calcification of the vascular wall predicts increased cardiovascular risk, independent of classical cardiovascular risk factors.3,18,21-24 Clinically, patients who have both diabetes and calcifications are at significant excess risk for total mortality, stroke mortality, and cardiovascular mortality, compared with patients with diabetes but without such calcifications. They also had a significantly higher incidence of coronary heart disease events, stroke events, and lower extremity amputations.25,26
All our patients underwent tourniquet-assisted TKA. Although previous studies have indicated that tourniquet use may increase arterial complications and wound complications or even limb loss in patients with calcified arteries, we did not find this link.7,27 Our population had no reported arterial complications related to tourniquet use. Other, smaller studies have had similar findings. Vandenbussche and colleagues28 prospectively studied 80 TKA cases randomized to tourniquet use or no tourniquet use and found no postoperative nerve palsies, wound infections, wound healing problems, or hematomas. Our study is also in accord with studies that have reported tourniquet use did not increase risk for DVT.29 Therefore, unlike earlier data, our data demonstrated that tourniquet use in patients with knee arterial calcification was safe.7,27,30,31
Patients with calcification were more likely to have the medical comorbidities of hypertension, diabetes, hyperlipidemia, and CAD. All these comorbidities are linked to the development of arterial calcification, or atherosclerotic occlusive disease.32,33 As life expectancy and the need for TKA increase, it is likely that a larger percentage of TKA patients will have preoperative radiographic evidence of knee arterial calcification. Although current dogma is that tourniquet-assisted TKA is contraindicated for patients with preoperative radiographic evidence of femoral-popliteal calcification, our study results showed that this calcification should not affect preoperative TKA planning for these patients.
We divided our patients into 3 categories: those with proximal calcification (above the joint line), those with distal calcification (below the joint line), and those with both proximal and distal calcification. Location of arterial calcification did not have an effect on their rates of postoperative wound complication or VTE. We hypothesized that patients with proximal calcification would be at increased risk for direct arterial injury and subsequent wound complication because the tourniquet is placed proximally. Previous research has indicated that arterial occlusion and subsequent wound complication can occur because of low blood flow stemming from tourniquet use.7 Further, intraoperative manipulation (flexing) of a knee with calcified vessels causes arterial complications after TKA because these vessels are less elastic than nonatheromatous vessels.31 However, we found no such effect. At the same time, having arterial calcification might also be an indication of venous disease in this location,12 which may be especially important for proximal calcifications. Proximal DVT more likely is a precursor to pulmonary embolic events than distal DVT is.31,34 However, we found no difference in VTE rates among the 3 arterial location groups, which is supported by studies that have found that tourniquet use does not increase DVT incidence.29,35-40
Risk for wound complications was higher in male patients and in patients with diabetes, prior VTE, hypertension, or CAD. This finding is important because, with the increasing age of patients who undergo TKA, those with serious medical comorbidities will continue to need and have this surgery.17 Diabetes may increase the rate of wound complication because patients with diabetes have poor microcirculation, poor collagen synthesis, and reduced wound strength.41 Malinzak and colleagues42 demonstrated that, compared with patients without diabetes, those with diabetes had a significantly higher risk for infection after TKA. Prior VTE, specifically DVT, may increase the rate of wound complication because after DVT the deep veins may be damaged and exhibit valvular dysfunction. Labropoulos and colleagues43 showed that DVT history was strongly associated with ulcer nonhealing. Perhaps hypertension has been overlooked as a risk factor for wound complication in TKA. No previous studies have assessed the link between hypertension and wound complications after TKA. However, a study of wound healing after total hip arthroplasty found that, compared with normotensive patients, hypertensive patients had delayed wound healing, putting them at higher risk for infection.44 In addition, we found that patients with CAD were at increased risk for wound complications—an unexpected finding, as CAD traditionally is not a risk factor for infection or poor wound healing. Recently, however, CAD was identified as an independent risk factor for surgical site infections in posterior lumbar–instrumented arthrodesis.45 The etiology of this association is unknown. Also, male patients were at increased risk for wound complication. Male sex has been implicated as an independent risk factor for development of surgical site infections and has been established as an important predisposing factor for periprosthetic joint infections.46
It is possible that patients who present with diabetes, VTE, hypertension, or CAD before TKA should have a consultation with a vascular surgeon or should have TKA performed without a tourniquet, but this conclusion cannot be considered definitive without a large prospective randomized trial or possibly registry data. Our data indicate that patients with these comorbidities have higher rates of wound complications irrespective of preoperative radiographic calcifications. On the basis of our study results, however, we certainly recommend that patients with these risk factors have preoperative medical optimization. Orthopedic surgeons should take a thorough history and perform a meticulous physical examination on these patients to look for evidence of PVD. We recommend that, if vascular claudication is elicited in the history, or if there is evidence of peripheral arterial disease—such as hair loss, skin discoloration, dystrophic nail changes, or absent or unequal peripheral pulses—the ankle-brachial index test should be performed. If the index value is less than 0.9, then a preoperative vascular surgery consultation should be obtained.
This study had some weaknesses. First, it was retrospective, so it is possible that some wound or VTE complications were not reported and thus not found in the paper charts or electronic medical records. Some patients may have had VTE diagnostic scans at other hospitals, and their results may not have been recorded across databases. Moreover, some patients may have seen wound specialists for wound infections or wound healing problems, and these may not have been reported to the orthopedic surgeons. Second, though our patient population was not small, it may not have been of adequate size to fully detect statistical significance. We met our enrollment numbers based on our sample size calculations from an a priori power analysis; however, we still draw conclusions with the possibility of committing a type II error in mind by failing to reject the null hypothesis when in reality a statistically significant difference does exist. Third, none of our consecutive patients carried the preoperative diagnosis of PVD, and none had preoperative vascular surgery. Therefore, though calcifications were noted on radiographs, clinically our patients were asymptomatic with respect to vascular health. Last, the 2 groups were not randomized. All patients underwent tourniquet-assisted TKA.
Conclusion
To our knowledge, this is the largest study to examine the effect of preoperative knee arterial calcification on wound complication and VTE after tourniquet-assisted TKA. Contrary to previously published recommendations, we conclude that TKA can be safely performed with a tourniquet in the presence of preoperative radiographic evidence of such calcification. However, we recommend that patients with diabetes, hypertension, CAD, or prior VTE undergo an appropriate physical examination to elicit any signs or symptoms of vascular disease. If before surgery there is any question of vascular competence, a vascular surgeon should be consulted.
1. Guanche CA. Tourniquet-induced tibial nerve palsy complicating anterior cruciate ligament reconstruction. Arthroscopy. 1995;11(5):620-622.
2. Irvine GB, Chan RN. Arterial calcification and tourniquets. Lancet. 1986;2(8517):1217.
3. Patterson S, Klenerman L. The effect of pneumatic tourniquets on the ultrastructure of skeletal muscle. J Bone Joint Surg Br. 1979;61(2):178-183.
4. Rorabeck CH, Kennedy JC. Tourniquet-induced nerve ischemia complicating knee ligament surgery. Am J Sports Med. 1980;8(2):98-102.
5. Shenton DW, Spitzer SA, Mulrennan BM. Tourniquet-induced rhabdomyolysis. A case report. J Bone Joint Surg Am. 1990;72(9):1405-1406.
6. Abdel-Salam A, Eyres KS. Effects of tourniquet during total knee arthroplasty. A prospective randomised study. J Bone Joint Surg Br. 1995;77(2):250-253.
7. DeLaurentis DA, Levitsky KA, Booth RE, et al. Arterial and ischemic aspects of total knee arthroplasty. Am J Surg. 1992;164(3):237-240.
8. Holmberg A, Milbrink J, Bergqvist D. Arterial complications and knee arthroplasty. Acta Orthop Scand. 1996;67(1):75-8.
9. Hozack WJ, Cole PA, Gardner R, Corces A. Popliteal aneurysm after total knee arthroplasty. Case reports and review of the literature. J Arthroplasty. 1990;5(4):301-305.
10. Kumar SN, Chapman JA, Rawlins I. Vascular injuries after total knee arthroplasty: a review of the problem with special reference to the possible effects of the tourniquet. J Arthroplasty. 1998;13(2):211-216.
11. Rush JH, Vidovich JD, Johanson MA. Arterial complications and total knee arthroplasty. The Australian experience. J Bone Joint Surg Br. 1987;69(3):400-402.
12. Callam MJ, Harper DR, Dale JJ, Ruckley CV. Arterial disease in chronic leg ulceration: an underestimated hazard? Lothian and Forth Valley Leg Ulcer Study. Br Med J (Clin Res Ed). 1987;294(6577):929-931.
13. Blom AW, Brown J, Taylor AH, Pattison G, Whitehouse S, Bannister GC. Infection after total knee arthroplasty. J Bone Joint Surg Br. 2004;86(5):688-691.
14. Geerts WH, Bergqvist D, Pinco G, et al. Prevention of venous thromboembolism. Chest. 2008;133(6 suppl):381S-453S.
15. Pulido L, Parvizi J, Macgibeny M, et al. In hospital complications after total joint arthroplasty. J Arthroplasty. 2008;23(6 Suppl 1):139-145.
16. Arthritis: data and statistics. Centers for Disease Control and Prevention website. http://www.cdc.gov/arthritis/data_statistics.htm. Updated March 11, 2015. Accessed July 27, 2015.
17. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
18. Pulido L, Ghanem E, Joshi A, Purtill JJ, Parvizi J. Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clin Orthop Relat Res. 2008;466(7):1710-1715.
19. Warwick D. Prevention of venous thromboembolism in total knee and hip replacement. Circulation. 2012;125(17):2151-2155.
20. Rennenberg RJ, Kessels AG, Schurgers LJ, van Engelshoven JM, de Leeuw PW, Kroon AA. Vascular calcifications as a marker of increased cardiovascular risk: a meta-analysis. Vasc Health Risk Manag. 2009;5(1):185-197.
21. Arad Y, Goodman KJ, Roth M, Newstein D, Guerci AD. Coronary calcification, coronary disease risk factors, C-reactive protein, and atherosclerotic cardiovascular disease events: the St. Francis Heart Study. J Am Coll Cardiol. 2005;46(1):158-165.
22. Iribarren C, Sidney S, Sternfeld B, Browner WS. Calcification of the aortic arch: risk factors and association with coronary heart disease, stroke, and peripheral vascular disease. JAMA. 2000;283(21):2810-2815.
23. Shaw LJ, Raggi P, Schisterman E, Berman DS, Callister TQ. Prognostic value of cardiac risk factors and coronary artery calcium screening for all-cause mortality. Radiology. 2003;228(3):826-833.
24. Taylor AJ, Bindeman J, Feuerstein I, Cao F, Brazaitis M, O’Malley PG. Coronary calcium independently predicts incident premature coronary heart disease over measured cardiovascular risk factors: mean three-year outcomes in the Prospective Army Coronary Calcium (PACC) project. J Am Coll Cardiol. 2005;46(5):807-814.
25. Lehto S, Niskanen L, Suhonen M, Rönnemaa T, Laakso M. Medial artery calcification. A neglected harbinger of cardiovascular complications in non-insulin-dependent diabetes mellitus. Arterioscler Thromb Vasc Biol. 1996;16(8):978-983.
26. Niskanen L, Siitonen O, Suhonen M, Uusitupa MI. Medial artery calcification predicts cardiovascular mortality in patients with NIDDM. Diabetes Care. 1994;17(11):1252-1256.
27. Smith DE, McGraw RW, Taylor DC, et al. Arterial complications and total knee arthroplasty. J Am Acad Orthop Surg. 2001;9(4):253-257.
28. Vandenbussche E, Duranthon L, Couturier M, Pidhorz L, Augereau B. The effect of tourniquet use in total knee arthroplasty. Int Orthop. 2002;26(5):306-309.
29. Fukunda A, Hasegawa M, Kato K, Shi D, Sudo A, Uchida A. Effect of tourniquet application on deep vein thrombosis after total knee thrombosis. Arch Orthop Trauma Surg. 2007;127(8):671-675.
30. Butt U, Samuel R, Sahu A, Butt IS, Johnson DS, Turner PG. Arterial injury in total knee arthroplasty. J Arthroplasty. 2010;25(8):1311-1318.
31. Langkamer VG. Local vascular complications after knee replacement: a review with illustrative case reports. Knee. 2001;8(4):259-264.
32. Hussein A, Uno K, Wolski K, et al. Peripheral arterial disease and progression of coronary atherosclerosis. J Am Coll Cardiol. 2011;57(10):1220-1225.
33. Ouriel K. Peripheral arterial disease. Lancet. 2001;358(9289):1257-1264.
34. Monreal M, Rufz J, Olazabal A, Arias A, Roca J. Deep venous thrombosis and the risk of pulmonary embolism. Chest. 1992;102(3):677-681.
35. Angus PD, Nakielny R, Goodrum DT. The pneumatic tourniquet and deep venous thrombosis. J Bone Joint Surg Br. 1983;65(3):336-339.
36. Fahmy NR, Patel DG. Hemostatic changes and postoperative deep-vein thrombosis associated with use of a pneumatic tourniquet. J Bone Joint Surg Am. 1981;63(3):461-465.
37. Harvey EJ, Leclerc J, Brooks CE, Burke DL. Effect of tourniquet use on blood loss and incidence of deep vein thrombosis in total knee arthroplasty. J Arthroplasty. 1997;12(3):291-296.
38. Simon MA, Mass DP, Zarins CK, Bidani N, Gudas CJ, Metz CE. The effect of a thigh tourniquet on the incidence of deep venous thrombosis after operations on the fore part of the foot. J Bone Joint Surg Am. 1982;64(2):188-191.
39. Stulberg BN, Insall JN, Williams GW, Ghelman B. Deep-vein thrombosis following total knee replacement. An analysis of six hundred and thirty-eight arthroplasties. J Bone Joint Surg Am. 1984;66(2):194-201.
40. Wakankar HM, Nicholl JE, Koka R, D’Arcy JC. The tourniquet in total knee arthroplasty. A prospective, randomized study. J Bone Joint Surg Br. 1999;81(1):30-33.
41. Vince K, Chivas D, Droll K. Wound complications after total knee arthroplasty. J Arthroplasty. 2007;22(4 Suppl 1):39-44.
42. Malinzak RA, Ritter MA, Berend ME, Meding JB, Olberding EM, Davis KE. Morbidly obese, diabetic, younger, and unilateral joint arthroplasty patients have elevated total joint arthroplasty infection rates. J Arthroplasty. 2009;24(6 Suppl):84-88.
43. Labropoulos N, Wang E, Lanier S, Khan SU. Factors associated with poor healing and recurrence of venous ulceration. Plast Reconstr Surg. 2011;129(1):179-186.
44. Ahmed AA, Mooar PA, Kleiner M, Torg JS, Miyamoto CT. Hypertensive patients show delayed wound healing following total hip arthroplasty. PLoS One. 2011;6(8):e23224.
45. Koutsoumbelis S, Hughes AP, Girardi FP, et al. Risk factors for postoperative infection following posterior lumbar instrumented arthrodesis. J Bone Joint Surg Am. 2001;93(17):1627-1633.
46. Poultsides LA, Ma Y, Della Valle AG, Chiu YL, Sculco TP, Memtsoudis SG. In-hospital surgical site infections after primary hip and knee arthroplasty—incidence and risk factors. J Arthroplasty. 2013;28(3):385-389.
Tourniquets are often used in total knee arthroplasty (TKA) to improve visualization of structures, shorten operative time, reduce intraoperative bleeding, and improve cementing technique. Despite these advantages, controversy remains regarding the safety of tourniquet use. Tourniquets have been associated with nerve palsies, vascular injury, and muscle damage.1-5 Some have hypothesized they may cause venous stasis or direct endothelial damage that may develop into deep vein thrombosis (DVT). Abdel-Salam and Eyres6 found an increased incidence of postoperative wound complications and DVTs associated with tourniquet use.
Moreover, investigators have analyzed the role of tourniquets in populations at high risk for wound complications. DeLaurentis and colleagues7 performed a prospective and retrospective analysis of 1182 TKA patients, 24 (2%) of whom had preexisting peripheral vascular disease (PVD), defined as a history of arterial insufficiency, absent dorsalis pedis and/or absent posterior tibial pulsations, and arterial calcifications. A tourniquet was used in each case. Arterial complications occurred in 6 of the 24 patients with PVD. As expected, the authors found that a history of intermittent claudication, pain at rest, and arterial ulcers resulted in a high risk for vascular complications. Further studies have supported this finding and expanded the list of predisposing factors to include previous vascular surgery and absent and asymmetric pedal pulsations.7-11 Of particular concern to total joint arthroplasty surgeons was the finding by DeLaurentis and colleagues7 that patients with radiographic evidence of calcification of the distal superficial femoral artery and/or popliteal artery were at risk for arterial complications. This finding is also supported by other studies.8,11 In TKA, damage to arterial structures proximal to the surgical field could manifest as impaired postoperative wound healing or an ischemic limb. Wound healing depends on adequate blood flow to the healing tissue, and any damage to arterial or venous structures can theoretically compromise this process.
Added to vascular/wound complications as concerning complications in orthopedic surgery is venous thromboembolism (VTE). The role of tourniquets in the formation of VTEs is controversial. A tourniquet has the potential to increase the risk for DVT because of the stasis of venous blood in the lower limb or possible damage to calcified blood vessels. Callam and colleagues12 studied the connection between artery disease and chronic leg ulcers and found that half the patients diagnosed with peripheral artery disease also had stigmata of chronic venous insufficiency. Therefore, the entities can occur in tandem, and surgeons should keep this in mind.
Here we report on a study we conducted to determine whether tourniquet use in TKA in patients with preexisting radiographic evidence of vascular disease increases the risk for wound complications or VTE.
Patients and Methods
We retrospectively reviewed 461 consecutive primary TKAs (373 patients) performed between January 2007 and June 2012 by 2 attending orthopedic surgeons specializing in adult reconstruction. Medical records and operative reports of 583 patients were examined after receiving institutional review board approval. Of these patients, 373 (64%) had a minimum of 12-month follow-up data available. Twelve months was deemed long enough to discover wound complications or DVTs secondary to the index procedure. Most of these outcomes manifest within the first 3 months after surgery and certainly by 12 months. Follow-up longer than 12 months may become a confounder, as wound complications outside the acute to subacute postoperative window could be related to patients’ underlying PVD and not directly to tourniquet use during surgery. Patient demographics and comorbidities were recorded. Comorbidities were obtained from preoperative medical evaluations and surgeons’ preoperative evaluations. All patients had preoperative palpable dorsalis pedis and posterior tibialis arterial pulses. No patient required preoperative vascular studies based on preoperative examination or comorbidities. No patient had prior vascular bypass surgery or stenting.
TKA was performed in a nonlaminar flow, positive-pressure, high-efficiency particulate air-filtered room with sterile toga/surgical helmet systems. For all patients, a pneumatic thigh tourniquet was applied, and the patient was prepared and draped. After limb exsanguination using a rubber bandage, the limb was elevated and the tourniquet inflated to a pressure of 250 to 300 mm Hg. The tourniquet was released either just before closure or immediately after closure in all cases; it was always let down before placement of final bandages.
Prophylactic chemical anticoagulation consisting of warfarin, aspirin, or enoxaparin was used in all patients and continued for 4 to 6 weeks after surgery. All patients received mechanical DVT prophylaxis with sequential compression devices, and all were mobilized out of bed beginning either the day of surgery or the next day. All patients received perioperative intravenous antibiotics, with the preoperative dose given before tourniquet inflation and the last postoperative dose stopped within 24 hours of surgery.
All patients who had primary TKA underwent preoperative medical evaluation and optimization. The patient’s hospital course was monitored closely, and complications noted by the orthopedic team were documented. Follow-up documentation was retrospectively reviewed for evidence of wound complications or VTE. Wound complications were defined as cellulitis, delayed wound healing, wound dehiscence, and/or periprosthetic joint infection. In the case of VTE, physical examination findings were not sufficient for inclusion. Venous duplex ultrasonography demonstrating the clot was reviewed before inclusion.
Preoperative radiographs were examined for arterial calcification (Figure). We refer to calcification seen above the knee joint as proximal calcification and to calcification observed below the joint as distal calcification. Patients exhibited calcification proximally only, distally only, or both proximally and distally. The 373 patients were placed into 2 groups based on whether they had preoperative arterial calcification on plain radiography of the knee. One group (285 patients with no radiographic evidence of preoperative knee arterial calcification) underwent 365 TKAs, and the other group (88 patients with radiographic evidence of preoperative knee arterial calcification) underwent 96 TKAs.
A sample size calculation was performed to determine how many patients were needed in each group with 80% power and an α of 0.05. With an estimated difference in VTE/wound complication rate between the calcification and no-calcification groups of 12%, we needed to review 316 TKAs total. This 12% difference was based on study findings of a 25% complication rate in PVD patients who underwent tourniquet-assisted TKA, and the rate of VTE/wound complication after TKA in patients overall, which can be up to 12%.7,13,14 We exceeded minimal enrollment and had 461 TKAs. Descriptive statistics were reported, with means and ranges provided where appropriate. Independent t test was used to evaluate the differences in continuous data (age) between the groups. Univariate analysis (using Pearson χ2 and Fisher exact tests) and multivariate logistic regression analysis were used to evaluate the effects of categorical variables (sex, comorbidity, calcification [presence, absence], and location of calcification [proximal only, distal only, both]) on wound complication and VTE rates. All tests were 2-tailed and performed with a type I error rate of 0.05. Data analysis was performed with SPSS Version 19.0 (SPSS).
Results
Patient characteristics are summarized in Table 1. Of the 373 patients, 285 lacked calcification, and 88 had calcification. Mean age was 67.73 years (range, 24-92 years) for all patients, 65.99 years (range, 24-89 years) for the no-calcification group, and 74.32 years (range, 54-92 years) for the calcification group; the calcification group demonstrated a trend toward older age, but the difference was not significantly different (P = .07). Of the 373 patients, 156 (41.82%) were male: 110 in the no-calcification group (38.60%) and 46 in the calcification group (52.27%); sex was significantly (P = .002) different between groups, with more males in the calcification group.
Data on total preoperative comorbidities are summarized in Table 2. Hypertension, hyperlipidemia, diabetes, and coronary artery disease (CAD) were the most common comorbidities, and they were all significantly (P ≤ .05) increased in the calcification group.
No patients had reported arterial complications, such as arterial bleeding, aneurysm, intimal tears, or loss of distal pulses. Wound complication after TKA was detected in 3.04% of all cases (Table 3). Rate of DVT after TKA was 2.60% of all cases, and rate of pulmonary embolism after TKA was 2.17% of all cases. Of the 96 TKAs with preoperative radiographic evidence of calcification, 47 (48.96%) had proximal calcification only, 11 (11.46%) had distal calcification only, and 38 (39.58%) had both proximal and distal calcification (Table 4). There was no significant difference between the rate of wound complication or VTE based on location of vascular calcification.
Univariate analysis demonstrated that presence of arterial knee calcification did not increase the risk for postoperative wound complication (odds ratio [OR], 1.04; 95% confidence interval [CI], 0.28-3.80; P > .05) (Table 5). Location of arterial knee calcification also did not increase the risk for postoperative wound complication. In addition, univariate analysis demonstrated that presence of arterial knee calcification did not increase the risk for postoperative VTE (OR, 1.20; 95% CI, 0.43-3.36; P > .05 (Table 6).
Of the 14 wound complications, the most common infections were cellulitis (5/14 cases; 35.71%) and infected hardware that required component revision (5/14 cases; 35.71%). Mean time from TKA to infection was 137.93 days (range, 5-783 days). The most common organism grown in culture from the wound was Staphylococcus (5/14 cases; 35.71%).
Additional univariate statistical analysis revealed that presence of diabetes, hypertension, prior VTE, CAD, and male sex was linked to higher incidence of wound complication (P < .05) (Table 5). When multivariate analysis was performed, hypertension, prior VTE, and male sex remained significant (P < .05) (Table 5).
Discussion
TKA is a safe and effective procedure used to treat osteoarthritis of the knee and improve patients’ quality of life.15 About 700,000 TKAs are performed annually in the United States.16 Because of improvements in preventive medicine and medical technology, life expectancy is increasing, and TKAs are now being performed in higher numbers and in an older patient population. Over the next few decades, these developments will lead to more postoperative complications. It is projected that, by 2030, the need for TKAs in the United States will increase by 673% to 3.48 million.17 Postoperative complications are rare but unfortunately often lead to poor outcomes or even mortality.18 To help minimize the number of postoperative complications, we must understand the safety of tourniquet use in TKA. Other investigators have concluded that tourniquet use is unsafe in patients with preoperative vascular calcifications on plain radiographs.7,8,11 The present study, designed to elucidate whether preoperative evidence of knee arterial calcification may predispose TKA patients to postoperative wound complication or VTE, had some important findings.
In our study, wound complication and VTE occurred in a considerable number of patients after TKA. Despite exceeding the number of patients calculated by the power analysis, our population may have been inadequate to fully detect statistical significance. Thus, our conclusion of failing to reject the null hypothesis may have been because of sample size, a type II error. We found that, after primary TKA, 3.04% of patients developed wound complications and 4.77% VTE. According to the literature, the incidence of infection after primary TKA is between 0.5% and 12%, and that of VTE reported within 3 months after TKA is 1.3% to 10%.13,14 Although we had 100% VTE prophylaxis, meeting the standard of care, VTE after TKA remains a postoperative complication.19 This study also found that a considerable percentage of primary TKA patients (23.59%) had preoperative calcification of the knee arteries. To our knowledge, this study was the first to quantify the incidence of knee arterial calcification in patients who underwent TKA.
Preoperative calcification of the knee arteries in patients who underwent TKA did not increase the risk for wound complication, VTE, or arterial damage. These calcifications, however, do pose an increased systemic vascular risk.20 Calcification of the vascular wall predicts increased cardiovascular risk, independent of classical cardiovascular risk factors.3,18,21-24 Clinically, patients who have both diabetes and calcifications are at significant excess risk for total mortality, stroke mortality, and cardiovascular mortality, compared with patients with diabetes but without such calcifications. They also had a significantly higher incidence of coronary heart disease events, stroke events, and lower extremity amputations.25,26
All our patients underwent tourniquet-assisted TKA. Although previous studies have indicated that tourniquet use may increase arterial complications and wound complications or even limb loss in patients with calcified arteries, we did not find this link.7,27 Our population had no reported arterial complications related to tourniquet use. Other, smaller studies have had similar findings. Vandenbussche and colleagues28 prospectively studied 80 TKA cases randomized to tourniquet use or no tourniquet use and found no postoperative nerve palsies, wound infections, wound healing problems, or hematomas. Our study is also in accord with studies that have reported tourniquet use did not increase risk for DVT.29 Therefore, unlike earlier data, our data demonstrated that tourniquet use in patients with knee arterial calcification was safe.7,27,30,31
Patients with calcification were more likely to have the medical comorbidities of hypertension, diabetes, hyperlipidemia, and CAD. All these comorbidities are linked to the development of arterial calcification, or atherosclerotic occlusive disease.32,33 As life expectancy and the need for TKA increase, it is likely that a larger percentage of TKA patients will have preoperative radiographic evidence of knee arterial calcification. Although current dogma is that tourniquet-assisted TKA is contraindicated for patients with preoperative radiographic evidence of femoral-popliteal calcification, our study results showed that this calcification should not affect preoperative TKA planning for these patients.
We divided our patients into 3 categories: those with proximal calcification (above the joint line), those with distal calcification (below the joint line), and those with both proximal and distal calcification. Location of arterial calcification did not have an effect on their rates of postoperative wound complication or VTE. We hypothesized that patients with proximal calcification would be at increased risk for direct arterial injury and subsequent wound complication because the tourniquet is placed proximally. Previous research has indicated that arterial occlusion and subsequent wound complication can occur because of low blood flow stemming from tourniquet use.7 Further, intraoperative manipulation (flexing) of a knee with calcified vessels causes arterial complications after TKA because these vessels are less elastic than nonatheromatous vessels.31 However, we found no such effect. At the same time, having arterial calcification might also be an indication of venous disease in this location,12 which may be especially important for proximal calcifications. Proximal DVT more likely is a precursor to pulmonary embolic events than distal DVT is.31,34 However, we found no difference in VTE rates among the 3 arterial location groups, which is supported by studies that have found that tourniquet use does not increase DVT incidence.29,35-40
Risk for wound complications was higher in male patients and in patients with diabetes, prior VTE, hypertension, or CAD. This finding is important because, with the increasing age of patients who undergo TKA, those with serious medical comorbidities will continue to need and have this surgery.17 Diabetes may increase the rate of wound complication because patients with diabetes have poor microcirculation, poor collagen synthesis, and reduced wound strength.41 Malinzak and colleagues42 demonstrated that, compared with patients without diabetes, those with diabetes had a significantly higher risk for infection after TKA. Prior VTE, specifically DVT, may increase the rate of wound complication because after DVT the deep veins may be damaged and exhibit valvular dysfunction. Labropoulos and colleagues43 showed that DVT history was strongly associated with ulcer nonhealing. Perhaps hypertension has been overlooked as a risk factor for wound complication in TKA. No previous studies have assessed the link between hypertension and wound complications after TKA. However, a study of wound healing after total hip arthroplasty found that, compared with normotensive patients, hypertensive patients had delayed wound healing, putting them at higher risk for infection.44 In addition, we found that patients with CAD were at increased risk for wound complications—an unexpected finding, as CAD traditionally is not a risk factor for infection or poor wound healing. Recently, however, CAD was identified as an independent risk factor for surgical site infections in posterior lumbar–instrumented arthrodesis.45 The etiology of this association is unknown. Also, male patients were at increased risk for wound complication. Male sex has been implicated as an independent risk factor for development of surgical site infections and has been established as an important predisposing factor for periprosthetic joint infections.46
It is possible that patients who present with diabetes, VTE, hypertension, or CAD before TKA should have a consultation with a vascular surgeon or should have TKA performed without a tourniquet, but this conclusion cannot be considered definitive without a large prospective randomized trial or possibly registry data. Our data indicate that patients with these comorbidities have higher rates of wound complications irrespective of preoperative radiographic calcifications. On the basis of our study results, however, we certainly recommend that patients with these risk factors have preoperative medical optimization. Orthopedic surgeons should take a thorough history and perform a meticulous physical examination on these patients to look for evidence of PVD. We recommend that, if vascular claudication is elicited in the history, or if there is evidence of peripheral arterial disease—such as hair loss, skin discoloration, dystrophic nail changes, or absent or unequal peripheral pulses—the ankle-brachial index test should be performed. If the index value is less than 0.9, then a preoperative vascular surgery consultation should be obtained.
This study had some weaknesses. First, it was retrospective, so it is possible that some wound or VTE complications were not reported and thus not found in the paper charts or electronic medical records. Some patients may have had VTE diagnostic scans at other hospitals, and their results may not have been recorded across databases. Moreover, some patients may have seen wound specialists for wound infections or wound healing problems, and these may not have been reported to the orthopedic surgeons. Second, though our patient population was not small, it may not have been of adequate size to fully detect statistical significance. We met our enrollment numbers based on our sample size calculations from an a priori power analysis; however, we still draw conclusions with the possibility of committing a type II error in mind by failing to reject the null hypothesis when in reality a statistically significant difference does exist. Third, none of our consecutive patients carried the preoperative diagnosis of PVD, and none had preoperative vascular surgery. Therefore, though calcifications were noted on radiographs, clinically our patients were asymptomatic with respect to vascular health. Last, the 2 groups were not randomized. All patients underwent tourniquet-assisted TKA.
Conclusion
To our knowledge, this is the largest study to examine the effect of preoperative knee arterial calcification on wound complication and VTE after tourniquet-assisted TKA. Contrary to previously published recommendations, we conclude that TKA can be safely performed with a tourniquet in the presence of preoperative radiographic evidence of such calcification. However, we recommend that patients with diabetes, hypertension, CAD, or prior VTE undergo an appropriate physical examination to elicit any signs or symptoms of vascular disease. If before surgery there is any question of vascular competence, a vascular surgeon should be consulted.
Tourniquets are often used in total knee arthroplasty (TKA) to improve visualization of structures, shorten operative time, reduce intraoperative bleeding, and improve cementing technique. Despite these advantages, controversy remains regarding the safety of tourniquet use. Tourniquets have been associated with nerve palsies, vascular injury, and muscle damage.1-5 Some have hypothesized they may cause venous stasis or direct endothelial damage that may develop into deep vein thrombosis (DVT). Abdel-Salam and Eyres6 found an increased incidence of postoperative wound complications and DVTs associated with tourniquet use.
Moreover, investigators have analyzed the role of tourniquets in populations at high risk for wound complications. DeLaurentis and colleagues7 performed a prospective and retrospective analysis of 1182 TKA patients, 24 (2%) of whom had preexisting peripheral vascular disease (PVD), defined as a history of arterial insufficiency, absent dorsalis pedis and/or absent posterior tibial pulsations, and arterial calcifications. A tourniquet was used in each case. Arterial complications occurred in 6 of the 24 patients with PVD. As expected, the authors found that a history of intermittent claudication, pain at rest, and arterial ulcers resulted in a high risk for vascular complications. Further studies have supported this finding and expanded the list of predisposing factors to include previous vascular surgery and absent and asymmetric pedal pulsations.7-11 Of particular concern to total joint arthroplasty surgeons was the finding by DeLaurentis and colleagues7 that patients with radiographic evidence of calcification of the distal superficial femoral artery and/or popliteal artery were at risk for arterial complications. This finding is also supported by other studies.8,11 In TKA, damage to arterial structures proximal to the surgical field could manifest as impaired postoperative wound healing or an ischemic limb. Wound healing depends on adequate blood flow to the healing tissue, and any damage to arterial or venous structures can theoretically compromise this process.
Added to vascular/wound complications as concerning complications in orthopedic surgery is venous thromboembolism (VTE). The role of tourniquets in the formation of VTEs is controversial. A tourniquet has the potential to increase the risk for DVT because of the stasis of venous blood in the lower limb or possible damage to calcified blood vessels. Callam and colleagues12 studied the connection between artery disease and chronic leg ulcers and found that half the patients diagnosed with peripheral artery disease also had stigmata of chronic venous insufficiency. Therefore, the entities can occur in tandem, and surgeons should keep this in mind.
Here we report on a study we conducted to determine whether tourniquet use in TKA in patients with preexisting radiographic evidence of vascular disease increases the risk for wound complications or VTE.
Patients and Methods
We retrospectively reviewed 461 consecutive primary TKAs (373 patients) performed between January 2007 and June 2012 by 2 attending orthopedic surgeons specializing in adult reconstruction. Medical records and operative reports of 583 patients were examined after receiving institutional review board approval. Of these patients, 373 (64%) had a minimum of 12-month follow-up data available. Twelve months was deemed long enough to discover wound complications or DVTs secondary to the index procedure. Most of these outcomes manifest within the first 3 months after surgery and certainly by 12 months. Follow-up longer than 12 months may become a confounder, as wound complications outside the acute to subacute postoperative window could be related to patients’ underlying PVD and not directly to tourniquet use during surgery. Patient demographics and comorbidities were recorded. Comorbidities were obtained from preoperative medical evaluations and surgeons’ preoperative evaluations. All patients had preoperative palpable dorsalis pedis and posterior tibialis arterial pulses. No patient required preoperative vascular studies based on preoperative examination or comorbidities. No patient had prior vascular bypass surgery or stenting.
TKA was performed in a nonlaminar flow, positive-pressure, high-efficiency particulate air-filtered room with sterile toga/surgical helmet systems. For all patients, a pneumatic thigh tourniquet was applied, and the patient was prepared and draped. After limb exsanguination using a rubber bandage, the limb was elevated and the tourniquet inflated to a pressure of 250 to 300 mm Hg. The tourniquet was released either just before closure or immediately after closure in all cases; it was always let down before placement of final bandages.
Prophylactic chemical anticoagulation consisting of warfarin, aspirin, or enoxaparin was used in all patients and continued for 4 to 6 weeks after surgery. All patients received mechanical DVT prophylaxis with sequential compression devices, and all were mobilized out of bed beginning either the day of surgery or the next day. All patients received perioperative intravenous antibiotics, with the preoperative dose given before tourniquet inflation and the last postoperative dose stopped within 24 hours of surgery.
All patients who had primary TKA underwent preoperative medical evaluation and optimization. The patient’s hospital course was monitored closely, and complications noted by the orthopedic team were documented. Follow-up documentation was retrospectively reviewed for evidence of wound complications or VTE. Wound complications were defined as cellulitis, delayed wound healing, wound dehiscence, and/or periprosthetic joint infection. In the case of VTE, physical examination findings were not sufficient for inclusion. Venous duplex ultrasonography demonstrating the clot was reviewed before inclusion.
Preoperative radiographs were examined for arterial calcification (Figure). We refer to calcification seen above the knee joint as proximal calcification and to calcification observed below the joint as distal calcification. Patients exhibited calcification proximally only, distally only, or both proximally and distally. The 373 patients were placed into 2 groups based on whether they had preoperative arterial calcification on plain radiography of the knee. One group (285 patients with no radiographic evidence of preoperative knee arterial calcification) underwent 365 TKAs, and the other group (88 patients with radiographic evidence of preoperative knee arterial calcification) underwent 96 TKAs.
A sample size calculation was performed to determine how many patients were needed in each group with 80% power and an α of 0.05. With an estimated difference in VTE/wound complication rate between the calcification and no-calcification groups of 12%, we needed to review 316 TKAs total. This 12% difference was based on study findings of a 25% complication rate in PVD patients who underwent tourniquet-assisted TKA, and the rate of VTE/wound complication after TKA in patients overall, which can be up to 12%.7,13,14 We exceeded minimal enrollment and had 461 TKAs. Descriptive statistics were reported, with means and ranges provided where appropriate. Independent t test was used to evaluate the differences in continuous data (age) between the groups. Univariate analysis (using Pearson χ2 and Fisher exact tests) and multivariate logistic regression analysis were used to evaluate the effects of categorical variables (sex, comorbidity, calcification [presence, absence], and location of calcification [proximal only, distal only, both]) on wound complication and VTE rates. All tests were 2-tailed and performed with a type I error rate of 0.05. Data analysis was performed with SPSS Version 19.0 (SPSS).
Results
Patient characteristics are summarized in Table 1. Of the 373 patients, 285 lacked calcification, and 88 had calcification. Mean age was 67.73 years (range, 24-92 years) for all patients, 65.99 years (range, 24-89 years) for the no-calcification group, and 74.32 years (range, 54-92 years) for the calcification group; the calcification group demonstrated a trend toward older age, but the difference was not significantly different (P = .07). Of the 373 patients, 156 (41.82%) were male: 110 in the no-calcification group (38.60%) and 46 in the calcification group (52.27%); sex was significantly (P = .002) different between groups, with more males in the calcification group.
Data on total preoperative comorbidities are summarized in Table 2. Hypertension, hyperlipidemia, diabetes, and coronary artery disease (CAD) were the most common comorbidities, and they were all significantly (P ≤ .05) increased in the calcification group.
No patients had reported arterial complications, such as arterial bleeding, aneurysm, intimal tears, or loss of distal pulses. Wound complication after TKA was detected in 3.04% of all cases (Table 3). Rate of DVT after TKA was 2.60% of all cases, and rate of pulmonary embolism after TKA was 2.17% of all cases. Of the 96 TKAs with preoperative radiographic evidence of calcification, 47 (48.96%) had proximal calcification only, 11 (11.46%) had distal calcification only, and 38 (39.58%) had both proximal and distal calcification (Table 4). There was no significant difference between the rate of wound complication or VTE based on location of vascular calcification.
Univariate analysis demonstrated that presence of arterial knee calcification did not increase the risk for postoperative wound complication (odds ratio [OR], 1.04; 95% confidence interval [CI], 0.28-3.80; P > .05) (Table 5). Location of arterial knee calcification also did not increase the risk for postoperative wound complication. In addition, univariate analysis demonstrated that presence of arterial knee calcification did not increase the risk for postoperative VTE (OR, 1.20; 95% CI, 0.43-3.36; P > .05 (Table 6).
Of the 14 wound complications, the most common infections were cellulitis (5/14 cases; 35.71%) and infected hardware that required component revision (5/14 cases; 35.71%). Mean time from TKA to infection was 137.93 days (range, 5-783 days). The most common organism grown in culture from the wound was Staphylococcus (5/14 cases; 35.71%).
Additional univariate statistical analysis revealed that presence of diabetes, hypertension, prior VTE, CAD, and male sex was linked to higher incidence of wound complication (P < .05) (Table 5). When multivariate analysis was performed, hypertension, prior VTE, and male sex remained significant (P < .05) (Table 5).
Discussion
TKA is a safe and effective procedure used to treat osteoarthritis of the knee and improve patients’ quality of life.15 About 700,000 TKAs are performed annually in the United States.16 Because of improvements in preventive medicine and medical technology, life expectancy is increasing, and TKAs are now being performed in higher numbers and in an older patient population. Over the next few decades, these developments will lead to more postoperative complications. It is projected that, by 2030, the need for TKAs in the United States will increase by 673% to 3.48 million.17 Postoperative complications are rare but unfortunately often lead to poor outcomes or even mortality.18 To help minimize the number of postoperative complications, we must understand the safety of tourniquet use in TKA. Other investigators have concluded that tourniquet use is unsafe in patients with preoperative vascular calcifications on plain radiographs.7,8,11 The present study, designed to elucidate whether preoperative evidence of knee arterial calcification may predispose TKA patients to postoperative wound complication or VTE, had some important findings.
In our study, wound complication and VTE occurred in a considerable number of patients after TKA. Despite exceeding the number of patients calculated by the power analysis, our population may have been inadequate to fully detect statistical significance. Thus, our conclusion of failing to reject the null hypothesis may have been because of sample size, a type II error. We found that, after primary TKA, 3.04% of patients developed wound complications and 4.77% VTE. According to the literature, the incidence of infection after primary TKA is between 0.5% and 12%, and that of VTE reported within 3 months after TKA is 1.3% to 10%.13,14 Although we had 100% VTE prophylaxis, meeting the standard of care, VTE after TKA remains a postoperative complication.19 This study also found that a considerable percentage of primary TKA patients (23.59%) had preoperative calcification of the knee arteries. To our knowledge, this study was the first to quantify the incidence of knee arterial calcification in patients who underwent TKA.
Preoperative calcification of the knee arteries in patients who underwent TKA did not increase the risk for wound complication, VTE, or arterial damage. These calcifications, however, do pose an increased systemic vascular risk.20 Calcification of the vascular wall predicts increased cardiovascular risk, independent of classical cardiovascular risk factors.3,18,21-24 Clinically, patients who have both diabetes and calcifications are at significant excess risk for total mortality, stroke mortality, and cardiovascular mortality, compared with patients with diabetes but without such calcifications. They also had a significantly higher incidence of coronary heart disease events, stroke events, and lower extremity amputations.25,26
All our patients underwent tourniquet-assisted TKA. Although previous studies have indicated that tourniquet use may increase arterial complications and wound complications or even limb loss in patients with calcified arteries, we did not find this link.7,27 Our population had no reported arterial complications related to tourniquet use. Other, smaller studies have had similar findings. Vandenbussche and colleagues28 prospectively studied 80 TKA cases randomized to tourniquet use or no tourniquet use and found no postoperative nerve palsies, wound infections, wound healing problems, or hematomas. Our study is also in accord with studies that have reported tourniquet use did not increase risk for DVT.29 Therefore, unlike earlier data, our data demonstrated that tourniquet use in patients with knee arterial calcification was safe.7,27,30,31
Patients with calcification were more likely to have the medical comorbidities of hypertension, diabetes, hyperlipidemia, and CAD. All these comorbidities are linked to the development of arterial calcification, or atherosclerotic occlusive disease.32,33 As life expectancy and the need for TKA increase, it is likely that a larger percentage of TKA patients will have preoperative radiographic evidence of knee arterial calcification. Although current dogma is that tourniquet-assisted TKA is contraindicated for patients with preoperative radiographic evidence of femoral-popliteal calcification, our study results showed that this calcification should not affect preoperative TKA planning for these patients.
We divided our patients into 3 categories: those with proximal calcification (above the joint line), those with distal calcification (below the joint line), and those with both proximal and distal calcification. Location of arterial calcification did not have an effect on their rates of postoperative wound complication or VTE. We hypothesized that patients with proximal calcification would be at increased risk for direct arterial injury and subsequent wound complication because the tourniquet is placed proximally. Previous research has indicated that arterial occlusion and subsequent wound complication can occur because of low blood flow stemming from tourniquet use.7 Further, intraoperative manipulation (flexing) of a knee with calcified vessels causes arterial complications after TKA because these vessels are less elastic than nonatheromatous vessels.31 However, we found no such effect. At the same time, having arterial calcification might also be an indication of venous disease in this location,12 which may be especially important for proximal calcifications. Proximal DVT more likely is a precursor to pulmonary embolic events than distal DVT is.31,34 However, we found no difference in VTE rates among the 3 arterial location groups, which is supported by studies that have found that tourniquet use does not increase DVT incidence.29,35-40
Risk for wound complications was higher in male patients and in patients with diabetes, prior VTE, hypertension, or CAD. This finding is important because, with the increasing age of patients who undergo TKA, those with serious medical comorbidities will continue to need and have this surgery.17 Diabetes may increase the rate of wound complication because patients with diabetes have poor microcirculation, poor collagen synthesis, and reduced wound strength.41 Malinzak and colleagues42 demonstrated that, compared with patients without diabetes, those with diabetes had a significantly higher risk for infection after TKA. Prior VTE, specifically DVT, may increase the rate of wound complication because after DVT the deep veins may be damaged and exhibit valvular dysfunction. Labropoulos and colleagues43 showed that DVT history was strongly associated with ulcer nonhealing. Perhaps hypertension has been overlooked as a risk factor for wound complication in TKA. No previous studies have assessed the link between hypertension and wound complications after TKA. However, a study of wound healing after total hip arthroplasty found that, compared with normotensive patients, hypertensive patients had delayed wound healing, putting them at higher risk for infection.44 In addition, we found that patients with CAD were at increased risk for wound complications—an unexpected finding, as CAD traditionally is not a risk factor for infection or poor wound healing. Recently, however, CAD was identified as an independent risk factor for surgical site infections in posterior lumbar–instrumented arthrodesis.45 The etiology of this association is unknown. Also, male patients were at increased risk for wound complication. Male sex has been implicated as an independent risk factor for development of surgical site infections and has been established as an important predisposing factor for periprosthetic joint infections.46
It is possible that patients who present with diabetes, VTE, hypertension, or CAD before TKA should have a consultation with a vascular surgeon or should have TKA performed without a tourniquet, but this conclusion cannot be considered definitive without a large prospective randomized trial or possibly registry data. Our data indicate that patients with these comorbidities have higher rates of wound complications irrespective of preoperative radiographic calcifications. On the basis of our study results, however, we certainly recommend that patients with these risk factors have preoperative medical optimization. Orthopedic surgeons should take a thorough history and perform a meticulous physical examination on these patients to look for evidence of PVD. We recommend that, if vascular claudication is elicited in the history, or if there is evidence of peripheral arterial disease—such as hair loss, skin discoloration, dystrophic nail changes, or absent or unequal peripheral pulses—the ankle-brachial index test should be performed. If the index value is less than 0.9, then a preoperative vascular surgery consultation should be obtained.
This study had some weaknesses. First, it was retrospective, so it is possible that some wound or VTE complications were not reported and thus not found in the paper charts or electronic medical records. Some patients may have had VTE diagnostic scans at other hospitals, and their results may not have been recorded across databases. Moreover, some patients may have seen wound specialists for wound infections or wound healing problems, and these may not have been reported to the orthopedic surgeons. Second, though our patient population was not small, it may not have been of adequate size to fully detect statistical significance. We met our enrollment numbers based on our sample size calculations from an a priori power analysis; however, we still draw conclusions with the possibility of committing a type II error in mind by failing to reject the null hypothesis when in reality a statistically significant difference does exist. Third, none of our consecutive patients carried the preoperative diagnosis of PVD, and none had preoperative vascular surgery. Therefore, though calcifications were noted on radiographs, clinically our patients were asymptomatic with respect to vascular health. Last, the 2 groups were not randomized. All patients underwent tourniquet-assisted TKA.
Conclusion
To our knowledge, this is the largest study to examine the effect of preoperative knee arterial calcification on wound complication and VTE after tourniquet-assisted TKA. Contrary to previously published recommendations, we conclude that TKA can be safely performed with a tourniquet in the presence of preoperative radiographic evidence of such calcification. However, we recommend that patients with diabetes, hypertension, CAD, or prior VTE undergo an appropriate physical examination to elicit any signs or symptoms of vascular disease. If before surgery there is any question of vascular competence, a vascular surgeon should be consulted.
1. Guanche CA. Tourniquet-induced tibial nerve palsy complicating anterior cruciate ligament reconstruction. Arthroscopy. 1995;11(5):620-622.
2. Irvine GB, Chan RN. Arterial calcification and tourniquets. Lancet. 1986;2(8517):1217.
3. Patterson S, Klenerman L. The effect of pneumatic tourniquets on the ultrastructure of skeletal muscle. J Bone Joint Surg Br. 1979;61(2):178-183.
4. Rorabeck CH, Kennedy JC. Tourniquet-induced nerve ischemia complicating knee ligament surgery. Am J Sports Med. 1980;8(2):98-102.
5. Shenton DW, Spitzer SA, Mulrennan BM. Tourniquet-induced rhabdomyolysis. A case report. J Bone Joint Surg Am. 1990;72(9):1405-1406.
6. Abdel-Salam A, Eyres KS. Effects of tourniquet during total knee arthroplasty. A prospective randomised study. J Bone Joint Surg Br. 1995;77(2):250-253.
7. DeLaurentis DA, Levitsky KA, Booth RE, et al. Arterial and ischemic aspects of total knee arthroplasty. Am J Surg. 1992;164(3):237-240.
8. Holmberg A, Milbrink J, Bergqvist D. Arterial complications and knee arthroplasty. Acta Orthop Scand. 1996;67(1):75-8.
9. Hozack WJ, Cole PA, Gardner R, Corces A. Popliteal aneurysm after total knee arthroplasty. Case reports and review of the literature. J Arthroplasty. 1990;5(4):301-305.
10. Kumar SN, Chapman JA, Rawlins I. Vascular injuries after total knee arthroplasty: a review of the problem with special reference to the possible effects of the tourniquet. J Arthroplasty. 1998;13(2):211-216.
11. Rush JH, Vidovich JD, Johanson MA. Arterial complications and total knee arthroplasty. The Australian experience. J Bone Joint Surg Br. 1987;69(3):400-402.
12. Callam MJ, Harper DR, Dale JJ, Ruckley CV. Arterial disease in chronic leg ulceration: an underestimated hazard? Lothian and Forth Valley Leg Ulcer Study. Br Med J (Clin Res Ed). 1987;294(6577):929-931.
13. Blom AW, Brown J, Taylor AH, Pattison G, Whitehouse S, Bannister GC. Infection after total knee arthroplasty. J Bone Joint Surg Br. 2004;86(5):688-691.
14. Geerts WH, Bergqvist D, Pinco G, et al. Prevention of venous thromboembolism. Chest. 2008;133(6 suppl):381S-453S.
15. Pulido L, Parvizi J, Macgibeny M, et al. In hospital complications after total joint arthroplasty. J Arthroplasty. 2008;23(6 Suppl 1):139-145.
16. Arthritis: data and statistics. Centers for Disease Control and Prevention website. http://www.cdc.gov/arthritis/data_statistics.htm. Updated March 11, 2015. Accessed July 27, 2015.
17. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
18. Pulido L, Ghanem E, Joshi A, Purtill JJ, Parvizi J. Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clin Orthop Relat Res. 2008;466(7):1710-1715.
19. Warwick D. Prevention of venous thromboembolism in total knee and hip replacement. Circulation. 2012;125(17):2151-2155.
20. Rennenberg RJ, Kessels AG, Schurgers LJ, van Engelshoven JM, de Leeuw PW, Kroon AA. Vascular calcifications as a marker of increased cardiovascular risk: a meta-analysis. Vasc Health Risk Manag. 2009;5(1):185-197.
21. Arad Y, Goodman KJ, Roth M, Newstein D, Guerci AD. Coronary calcification, coronary disease risk factors, C-reactive protein, and atherosclerotic cardiovascular disease events: the St. Francis Heart Study. J Am Coll Cardiol. 2005;46(1):158-165.
22. Iribarren C, Sidney S, Sternfeld B, Browner WS. Calcification of the aortic arch: risk factors and association with coronary heart disease, stroke, and peripheral vascular disease. JAMA. 2000;283(21):2810-2815.
23. Shaw LJ, Raggi P, Schisterman E, Berman DS, Callister TQ. Prognostic value of cardiac risk factors and coronary artery calcium screening for all-cause mortality. Radiology. 2003;228(3):826-833.
24. Taylor AJ, Bindeman J, Feuerstein I, Cao F, Brazaitis M, O’Malley PG. Coronary calcium independently predicts incident premature coronary heart disease over measured cardiovascular risk factors: mean three-year outcomes in the Prospective Army Coronary Calcium (PACC) project. J Am Coll Cardiol. 2005;46(5):807-814.
25. Lehto S, Niskanen L, Suhonen M, Rönnemaa T, Laakso M. Medial artery calcification. A neglected harbinger of cardiovascular complications in non-insulin-dependent diabetes mellitus. Arterioscler Thromb Vasc Biol. 1996;16(8):978-983.
26. Niskanen L, Siitonen O, Suhonen M, Uusitupa MI. Medial artery calcification predicts cardiovascular mortality in patients with NIDDM. Diabetes Care. 1994;17(11):1252-1256.
27. Smith DE, McGraw RW, Taylor DC, et al. Arterial complications and total knee arthroplasty. J Am Acad Orthop Surg. 2001;9(4):253-257.
28. Vandenbussche E, Duranthon L, Couturier M, Pidhorz L, Augereau B. The effect of tourniquet use in total knee arthroplasty. Int Orthop. 2002;26(5):306-309.
29. Fukunda A, Hasegawa M, Kato K, Shi D, Sudo A, Uchida A. Effect of tourniquet application on deep vein thrombosis after total knee thrombosis. Arch Orthop Trauma Surg. 2007;127(8):671-675.
30. Butt U, Samuel R, Sahu A, Butt IS, Johnson DS, Turner PG. Arterial injury in total knee arthroplasty. J Arthroplasty. 2010;25(8):1311-1318.
31. Langkamer VG. Local vascular complications after knee replacement: a review with illustrative case reports. Knee. 2001;8(4):259-264.
32. Hussein A, Uno K, Wolski K, et al. Peripheral arterial disease and progression of coronary atherosclerosis. J Am Coll Cardiol. 2011;57(10):1220-1225.
33. Ouriel K. Peripheral arterial disease. Lancet. 2001;358(9289):1257-1264.
34. Monreal M, Rufz J, Olazabal A, Arias A, Roca J. Deep venous thrombosis and the risk of pulmonary embolism. Chest. 1992;102(3):677-681.
35. Angus PD, Nakielny R, Goodrum DT. The pneumatic tourniquet and deep venous thrombosis. J Bone Joint Surg Br. 1983;65(3):336-339.
36. Fahmy NR, Patel DG. Hemostatic changes and postoperative deep-vein thrombosis associated with use of a pneumatic tourniquet. J Bone Joint Surg Am. 1981;63(3):461-465.
37. Harvey EJ, Leclerc J, Brooks CE, Burke DL. Effect of tourniquet use on blood loss and incidence of deep vein thrombosis in total knee arthroplasty. J Arthroplasty. 1997;12(3):291-296.
38. Simon MA, Mass DP, Zarins CK, Bidani N, Gudas CJ, Metz CE. The effect of a thigh tourniquet on the incidence of deep venous thrombosis after operations on the fore part of the foot. J Bone Joint Surg Am. 1982;64(2):188-191.
39. Stulberg BN, Insall JN, Williams GW, Ghelman B. Deep-vein thrombosis following total knee replacement. An analysis of six hundred and thirty-eight arthroplasties. J Bone Joint Surg Am. 1984;66(2):194-201.
40. Wakankar HM, Nicholl JE, Koka R, D’Arcy JC. The tourniquet in total knee arthroplasty. A prospective, randomized study. J Bone Joint Surg Br. 1999;81(1):30-33.
41. Vince K, Chivas D, Droll K. Wound complications after total knee arthroplasty. J Arthroplasty. 2007;22(4 Suppl 1):39-44.
42. Malinzak RA, Ritter MA, Berend ME, Meding JB, Olberding EM, Davis KE. Morbidly obese, diabetic, younger, and unilateral joint arthroplasty patients have elevated total joint arthroplasty infection rates. J Arthroplasty. 2009;24(6 Suppl):84-88.
43. Labropoulos N, Wang E, Lanier S, Khan SU. Factors associated with poor healing and recurrence of venous ulceration. Plast Reconstr Surg. 2011;129(1):179-186.
44. Ahmed AA, Mooar PA, Kleiner M, Torg JS, Miyamoto CT. Hypertensive patients show delayed wound healing following total hip arthroplasty. PLoS One. 2011;6(8):e23224.
45. Koutsoumbelis S, Hughes AP, Girardi FP, et al. Risk factors for postoperative infection following posterior lumbar instrumented arthrodesis. J Bone Joint Surg Am. 2001;93(17):1627-1633.
46. Poultsides LA, Ma Y, Della Valle AG, Chiu YL, Sculco TP, Memtsoudis SG. In-hospital surgical site infections after primary hip and knee arthroplasty—incidence and risk factors. J Arthroplasty. 2013;28(3):385-389.
1. Guanche CA. Tourniquet-induced tibial nerve palsy complicating anterior cruciate ligament reconstruction. Arthroscopy. 1995;11(5):620-622.
2. Irvine GB, Chan RN. Arterial calcification and tourniquets. Lancet. 1986;2(8517):1217.
3. Patterson S, Klenerman L. The effect of pneumatic tourniquets on the ultrastructure of skeletal muscle. J Bone Joint Surg Br. 1979;61(2):178-183.
4. Rorabeck CH, Kennedy JC. Tourniquet-induced nerve ischemia complicating knee ligament surgery. Am J Sports Med. 1980;8(2):98-102.
5. Shenton DW, Spitzer SA, Mulrennan BM. Tourniquet-induced rhabdomyolysis. A case report. J Bone Joint Surg Am. 1990;72(9):1405-1406.
6. Abdel-Salam A, Eyres KS. Effects of tourniquet during total knee arthroplasty. A prospective randomised study. J Bone Joint Surg Br. 1995;77(2):250-253.
7. DeLaurentis DA, Levitsky KA, Booth RE, et al. Arterial and ischemic aspects of total knee arthroplasty. Am J Surg. 1992;164(3):237-240.
8. Holmberg A, Milbrink J, Bergqvist D. Arterial complications and knee arthroplasty. Acta Orthop Scand. 1996;67(1):75-8.
9. Hozack WJ, Cole PA, Gardner R, Corces A. Popliteal aneurysm after total knee arthroplasty. Case reports and review of the literature. J Arthroplasty. 1990;5(4):301-305.
10. Kumar SN, Chapman JA, Rawlins I. Vascular injuries after total knee arthroplasty: a review of the problem with special reference to the possible effects of the tourniquet. J Arthroplasty. 1998;13(2):211-216.
11. Rush JH, Vidovich JD, Johanson MA. Arterial complications and total knee arthroplasty. The Australian experience. J Bone Joint Surg Br. 1987;69(3):400-402.
12. Callam MJ, Harper DR, Dale JJ, Ruckley CV. Arterial disease in chronic leg ulceration: an underestimated hazard? Lothian and Forth Valley Leg Ulcer Study. Br Med J (Clin Res Ed). 1987;294(6577):929-931.
13. Blom AW, Brown J, Taylor AH, Pattison G, Whitehouse S, Bannister GC. Infection after total knee arthroplasty. J Bone Joint Surg Br. 2004;86(5):688-691.
14. Geerts WH, Bergqvist D, Pinco G, et al. Prevention of venous thromboembolism. Chest. 2008;133(6 suppl):381S-453S.
15. Pulido L, Parvizi J, Macgibeny M, et al. In hospital complications after total joint arthroplasty. J Arthroplasty. 2008;23(6 Suppl 1):139-145.
16. Arthritis: data and statistics. Centers for Disease Control and Prevention website. http://www.cdc.gov/arthritis/data_statistics.htm. Updated March 11, 2015. Accessed July 27, 2015.
17. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
18. Pulido L, Ghanem E, Joshi A, Purtill JJ, Parvizi J. Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clin Orthop Relat Res. 2008;466(7):1710-1715.
19. Warwick D. Prevention of venous thromboembolism in total knee and hip replacement. Circulation. 2012;125(17):2151-2155.
20. Rennenberg RJ, Kessels AG, Schurgers LJ, van Engelshoven JM, de Leeuw PW, Kroon AA. Vascular calcifications as a marker of increased cardiovascular risk: a meta-analysis. Vasc Health Risk Manag. 2009;5(1):185-197.
21. Arad Y, Goodman KJ, Roth M, Newstein D, Guerci AD. Coronary calcification, coronary disease risk factors, C-reactive protein, and atherosclerotic cardiovascular disease events: the St. Francis Heart Study. J Am Coll Cardiol. 2005;46(1):158-165.
22. Iribarren C, Sidney S, Sternfeld B, Browner WS. Calcification of the aortic arch: risk factors and association with coronary heart disease, stroke, and peripheral vascular disease. JAMA. 2000;283(21):2810-2815.
23. Shaw LJ, Raggi P, Schisterman E, Berman DS, Callister TQ. Prognostic value of cardiac risk factors and coronary artery calcium screening for all-cause mortality. Radiology. 2003;228(3):826-833.
24. Taylor AJ, Bindeman J, Feuerstein I, Cao F, Brazaitis M, O’Malley PG. Coronary calcium independently predicts incident premature coronary heart disease over measured cardiovascular risk factors: mean three-year outcomes in the Prospective Army Coronary Calcium (PACC) project. J Am Coll Cardiol. 2005;46(5):807-814.
25. Lehto S, Niskanen L, Suhonen M, Rönnemaa T, Laakso M. Medial artery calcification. A neglected harbinger of cardiovascular complications in non-insulin-dependent diabetes mellitus. Arterioscler Thromb Vasc Biol. 1996;16(8):978-983.
26. Niskanen L, Siitonen O, Suhonen M, Uusitupa MI. Medial artery calcification predicts cardiovascular mortality in patients with NIDDM. Diabetes Care. 1994;17(11):1252-1256.
27. Smith DE, McGraw RW, Taylor DC, et al. Arterial complications and total knee arthroplasty. J Am Acad Orthop Surg. 2001;9(4):253-257.
28. Vandenbussche E, Duranthon L, Couturier M, Pidhorz L, Augereau B. The effect of tourniquet use in total knee arthroplasty. Int Orthop. 2002;26(5):306-309.
29. Fukunda A, Hasegawa M, Kato K, Shi D, Sudo A, Uchida A. Effect of tourniquet application on deep vein thrombosis after total knee thrombosis. Arch Orthop Trauma Surg. 2007;127(8):671-675.
30. Butt U, Samuel R, Sahu A, Butt IS, Johnson DS, Turner PG. Arterial injury in total knee arthroplasty. J Arthroplasty. 2010;25(8):1311-1318.
31. Langkamer VG. Local vascular complications after knee replacement: a review with illustrative case reports. Knee. 2001;8(4):259-264.
32. Hussein A, Uno K, Wolski K, et al. Peripheral arterial disease and progression of coronary atherosclerosis. J Am Coll Cardiol. 2011;57(10):1220-1225.
33. Ouriel K. Peripheral arterial disease. Lancet. 2001;358(9289):1257-1264.
34. Monreal M, Rufz J, Olazabal A, Arias A, Roca J. Deep venous thrombosis and the risk of pulmonary embolism. Chest. 1992;102(3):677-681.
35. Angus PD, Nakielny R, Goodrum DT. The pneumatic tourniquet and deep venous thrombosis. J Bone Joint Surg Br. 1983;65(3):336-339.
36. Fahmy NR, Patel DG. Hemostatic changes and postoperative deep-vein thrombosis associated with use of a pneumatic tourniquet. J Bone Joint Surg Am. 1981;63(3):461-465.
37. Harvey EJ, Leclerc J, Brooks CE, Burke DL. Effect of tourniquet use on blood loss and incidence of deep vein thrombosis in total knee arthroplasty. J Arthroplasty. 1997;12(3):291-296.
38. Simon MA, Mass DP, Zarins CK, Bidani N, Gudas CJ, Metz CE. The effect of a thigh tourniquet on the incidence of deep venous thrombosis after operations on the fore part of the foot. J Bone Joint Surg Am. 1982;64(2):188-191.
39. Stulberg BN, Insall JN, Williams GW, Ghelman B. Deep-vein thrombosis following total knee replacement. An analysis of six hundred and thirty-eight arthroplasties. J Bone Joint Surg Am. 1984;66(2):194-201.
40. Wakankar HM, Nicholl JE, Koka R, D’Arcy JC. The tourniquet in total knee arthroplasty. A prospective, randomized study. J Bone Joint Surg Br. 1999;81(1):30-33.
41. Vince K, Chivas D, Droll K. Wound complications after total knee arthroplasty. J Arthroplasty. 2007;22(4 Suppl 1):39-44.
42. Malinzak RA, Ritter MA, Berend ME, Meding JB, Olberding EM, Davis KE. Morbidly obese, diabetic, younger, and unilateral joint arthroplasty patients have elevated total joint arthroplasty infection rates. J Arthroplasty. 2009;24(6 Suppl):84-88.
43. Labropoulos N, Wang E, Lanier S, Khan SU. Factors associated with poor healing and recurrence of venous ulceration. Plast Reconstr Surg. 2011;129(1):179-186.
44. Ahmed AA, Mooar PA, Kleiner M, Torg JS, Miyamoto CT. Hypertensive patients show delayed wound healing following total hip arthroplasty. PLoS One. 2011;6(8):e23224.
45. Koutsoumbelis S, Hughes AP, Girardi FP, et al. Risk factors for postoperative infection following posterior lumbar instrumented arthrodesis. J Bone Joint Surg Am. 2001;93(17):1627-1633.
46. Poultsides LA, Ma Y, Della Valle AG, Chiu YL, Sculco TP, Memtsoudis SG. In-hospital surgical site infections after primary hip and knee arthroplasty—incidence and risk factors. J Arthroplasty. 2013;28(3):385-389.
Sustentaculum Lunatum: Appreciation of the Palmar Lunate Facet in Management of Complex Intra-Articular Fractures of the Distal Radius
Fracture of the distal radius is the wrist injury most often encountered by orthopedic and hand surgeons.1 The number of fractures of the distal radius in the United States was estimated to be 640,000 in 2001, and the incidence is increasing.2,3 Recent evidence has shown a substantial increase in treating these fractures with internal rather than closed fixation, even in the elderly.4
Treatment of complex intra-articular fractures of the distal radius requires an accurate diagnosis of the fracture pattern and a thoughtful approach to fixation. Although a majority of the fractures that meet the operative criteria are now treated with various anterior locked-plating techniques with good results, a subset requires more technically demanding fixation approaches, including fragment-specific approaches, dorsal and palmar plating, and combined internal and external fixation.
The sustentaculum lunatum, as we have named the palmar lunate facet, deserves specific attention because of its importance in load transmission across the radiocarpal joint and its key role in restoring the anatomy of the palmar distal radial metaphysis during internal fixation. This fragment in comminuted fractures was first ascribed special importance by Melone5 in his description of common fracture patterns. In the present article, we describe the anatomical characteristics of the sustentaculum lunatum and the clinical relevance of this fragment to management of fractures of the distal radius.
Classification
A variety of classification systems have been proposed to characterize and guide treatment of fractures of the distal radius. The earliest descriptions of fracture patterns were presented by Castaing6 and Frykman7 in the 1960s. The Frykman classification historically has been popular but is limited in accuracy in its characterization of fragments and their displacement and is limited in its ability to guide treatment. The classification system proposed by Melone and colleagues5,8-10 was the first to truly describe fracture of the distal radius fragments in a relevant manner, including their characteristic “4 parts” (Figure 1). The authors emphasized the importance of the “medial complex” as the cornerstone of the radiocarpal and radioulnar joints.
The classification system developed by Müller and colleagues,11 which was adopted by the AO (Arbeitsgemeinschaft für Osteosynthesefragen), might be the most descriptive and informative system, and it is widely used to conduct research and direct treatment. This system classifies fractures into A (extra-articular), B (partial articular), and C (complete articular) types and subclassifies them according to fracture location and comminution. These classifications, along with a conceptualization of the distal forearm as a 3-column structure involving the radial, ulnar, and intermediate columns (including the lunate facet), as proposed by Peine and colleagues,12 gave us a framework for approaching fixation of fractures of the distal radius.
Etymology and Definition
Sustentaculum, from the Latin sustinere, “to support, check, or put off,” and taculum, “receptacle or holding space,” is a fitting description of the most distal portion of the palmar lunate facet, as it supports and holds the carpus, and specifically the lunate, on the radial articular surface. This portion is analogous to the sustentaculum tali, the named portion of the calcaneus that supports and articulates with the middle calcaneal articular surface of the talus13 and provides a reliable fragment for internal fixation of the calcaneus.
Anatomical and Biomechanical Considerations
The distal radial articular surface is composed of distinct scaphoid and lunate facets that articulate with their respective carpal bones. Several studies have characterized the anatomy of the distal radius.14-17 Linscheid14 found that the lunate and scaphoid facets account for 46% and 43% of the contact area across the radiocarpal joint, respectively; this has been corroborated by others.15 A biomechanical study by Genda and Horii18 showed that the majority of stress across the wrist joint was concentrated at the palmar side of the distal radius in the neutral position. Although it is recognized that the scaphoid facet bears most of the load across the wrist in the neutral wrist position, most activities of daily living place the wrist in a slightly extended and ulnarly deviated position. This position results in a shift of the majority of load to the radiolunar articulation, constituting 53% of total force transmission.18 Subchondral bone density analyses have supported this lunate-predominant loading pattern across the radiocarpal articulation in most people.19 This loading pattern is also supported by the observation that failure of fixation and carpal subluxation generally occurs at the radiolunate articulation.
The palmar lip of the distal radius traditionally has been depicted and conceptualized as a flat extension of the metaphysis, leading to the development of implants that are not ideally designed for capturing this area in the fracture setting. A 3-dimensional (3-D) computed tomography (CT) study of the distal radii of healthy volunteers, conducted by Andermahr and colleagues,20 showed that the contour of the palmar lunate facet projects from the palmar cortex of the radius by 3 mm on average and is about 19 mm in width (radial to ulnar dimension) (Figures 2A-2C). In the axial plane, the anterior cortex of the distal radius slopes in a palmar direction, from radial to ulnar. This presents a challenge in attempts to support the entire surface (scaphoid and lunate facets) with a single palmar implant.20-25
A study conducted by Harness and colleagues24 showed that the majority of palmar shear fractures are composed of multiple fragments of the lunar articular facet. Anatomical studies of the distal radiocarpal articulation have also described the ligamentous attachments to the sustentaculum lunatum.26 The short radiolunate ligament, which originates from this fragment and inserts onto the lunate, provides stability to the carpus and, if not adequately fixed, leads to an incompetent restraint to palmar carpal translation. Isolated injuries of the short radiolunate ligament or fractures of the palmar lunate facet have been shown to result in palmar carpal translation.27,28 In addition, attachments of the palmar radioulnar ligament and other more ulnar radiocarpal ligaments act as deforming forces on the palmar lunate facet.24,26
Fracture Pattern Recognition
Although the AO type B palmar shear fracture pattern, also known as the Barton fracture, has classically been recognized as the fracture involving the palmar lunate facet and requiring special attention, many complete articular fractures feature involvement and fragmentation of this portion of the distal radius (Figures 3A-3F).29 In highly comminuted complete articular and palmar shear fracture patterns, the morphology of the sustentaculum lunatum should be appreciated, and its adequate fixation to the radial metaphysis ensured, to prevent loss of reduction.
Visualization of the palmar lunate facet as a distinct fragment might be difficult in cases of highly comminuted fracture patterns. Standard CT or more recently described 3-D CT techniques with subtraction of the carpus might facilitate appreciation of this fragment for preoperative planning of approach and fixation.29,30 Our institutional protocol involves obtaining preoperative traction radiographs of every fracture of the distal radius. These radiographs have reduced the need for CT in understanding the fracture pattern and aid in decision making.31
Besides appreciating the existence of the sustentaculum lunatum fragment, we should recognize that some injury patterns that split the lunate facet into unstable dorsal and palmar fragments might necessitate a separate dorsal approach to reduce and fix the dorsal lunate fragment. Traction radiographs can be especially useful in recognizing these patterns (a V sign is present) (Figures 4A, 4B).
Open Fractures
Highly comminuted fractures of the distal radius presenting with displaced lunate facet fragments can have high-energy mechanisms of injury. Although open fractures of the distal radius are associated with lower risk for infection (compared with open fractures of other long bones), they deserve special attention because of associated tendon and neurovascular injuries. Few studies have specifically assessed open fractures of the distal radius.32-35 Only the study by Rozental and Blazar34 listed associated injuries at the wrist level. The authors identified 4 patients (out of 18) with concomitant flexor tendon or neurovascular injuries that included radial or ulnar artery injury. In our experience, many open fractures of the distal radius are caused by an inside-out mechanism and present with an open wound either over the ulnar styloid or in the area of the ulnar side of the palmar radial metaphysis corresponding to the metaphyseal spike that mates with the sustentaculum lunatum (Figures 5A, 5B). Given these findings, we approach this intermediate column with particular care in cases of open fracture, paying attention to important structures (flexors, neurovascular) and looking for contamination from the environment into the fracture.
Fixation Techniques
The approach to fixation of partial articular palmar shear fractures is fairly straightforward. Buttress plate fixation has been well described and has had reliably good results.36 However, in very distal fracture patterns and in cases in which the palmar lunate facet is fragmented as part of a complete articular fracture, a fragment-specific approach to fixation with or without spanning external fixation often is necessary.37 The unrecognized sustentaculum lunatum fragment in comminuted complete articular fractures can lead to inadequate fixation constructs, resulting in loss of reduction and carpal subluxation in a palmar direction.24,34,38
Our surgical approach uses the standard anterior interval between the radial artery and the flexor carpi radialis, as described by Henry.39 The flexor pollicis longus is retracted ulnarly, revealing the pronator quadratus. We then reflect the pronator quadratus from the distal radial metaphysis until the most proximal and ulnar extent of the fracture is easily visualized. The palmar ulnar metaphyseal cortex that mates with the displaced sustentaculum lunatum is, in our experience, often the least comminuted portion of the metaphysis, thus providing a cortical key for restoration of height and alignment (Figures 5A, 5B). At our institution, fixation typically is achieved by contouring miniplates (1.3 or 1.5 mm) to capture and buttress the sustentaculum lunatum (Figures 6A, 6B). In our experience, the screw lengths in the most distal fixed-angle constructs at the palmar lip are limited to 6 mm or less to avoid penetration of the articular surface, though this has not been previously reported in the literature. After restoring the length and tilt of this intermediate column of the distal radius, we proceed with “rebuilding” the remainder of the fragments to our stabilized initial construct.
Various authors40-43 have described alternative fixation methods for the palmar lunate facet fragment. Jupiter and Marent-Huber42 described 2.4-mm locked-plate fixation with either a standard palmar plate or T- or L-plates for cases in which the palmar lip fragment is very distal and small. In fact, some newer anatomical distal radius implants include features designed to target these fragments (Figures 7A, 7B). An alternative fixation method involves use of a 26-gauge stainless steel wire passed through drill holes in the metaphysis 1 cm proximal to the fracture and then passed through the palmar capsule just distal to the fragment and secured in figure-8 fashion while the fragment is manually held reduced.41 Still others have recommended limited internal fixation of the sustentaculum lunatum through an ulna-sided palmar approach to the distal radius (between the ulnar neurovascular bundle and the flexor tendons) combined with external fixation to restore length and palmar tilt in highly comminuted fractures.40,43
A method involving arthroscopically assisted reduction and fixation of the lunate facet has also been described, though this procedure is technically demanding and has limited indications.44 It uses a Freer elevator passed through the standard 3-4 portal after initial visualization and evacuation of hematoma. The Freer elevator is used to disimpact the sustentaculum lunatum and to elevate it from its depressed position. With the dorsal lunate facet left displaced to facilitate access to the palmar fragment, a nerve hook retractor is used to reduce the palmar facet to the radial styloid, and Kirschner wires are used to achieve interfragmentary fixation. The dorsal lunate fragment is then pieced back to the articular segment, and the entire construct is fixed to the radial metaphysis with additional Kirschner wires.
Discussion
Given the increasing incidence of fractures of the distal radius, internal fixation of these injuries will continue to be relevant. American Academy of Orthopaedic Surgeons guidelines recommend operative fixation for fractures with postreduction radial shortening of more than 3 mm, dorsal tilt of more than 10°, or intra-articular displacement or step-off of more than 2 mm.45 Dr. Eglseder and Dr. Pensy indicate operative treatment of any incongruity of more than 2 mm in a young, active adult with a fracture of the distal radius. For the multifragmentary distal radius being treated operatively, attempts are made to achieve reduction more accurate than this, but formal dorsal exposure or direct visualization of the joint surface via dorsal capsulotomy is carefully chosen based on age, activity level, and bone quality. Recent high-level evidence46 showed that closed treatment of unstable fractures of the distal radius results in good outcomes in the elderly. However, it is important to note that fractures displaced in a palmar direction and palmar shear patterns were excluded from that work. It is widely accepted that palmar carpal translation should be addressed with internal fixation, and specific attention must therefore be paid to the lunate facet as the cornerstone of the distal radius. Furthermore, high-energy comminuted fractures in young patients still necessitate internal fixation of fragments to restore alignment and articular congruity.
Conclusion
The importance of the palmar lunate facet in providing support and restraint to palmar carpal translation and the key role of this facet in restoring the anatomy of the distal radius have been known. This fragment deserves special attention because failure to adequately stabilize it results in loss of fixation and carpal subluxation. Various approaches and fixation techniques have been recommended, including the method we prefer and have described here. Our newly proposed term, sustentaculum lunatum, our review of its structure and function, and our descriptions of fixation techniques are intended to promote awareness of this fragment in the treatment of fractures of the distal radius.
1. Jupiter JB. Fractures of the distal end of the radius. J Bone Joint Surg Am. 1991;73(3):461-469.
2. Chung KC, Spilson SV. The frequency and epidemiology of hand and forearm fractures in the United States. J Hand Surg Am. 2001;26(5):908-915.
3. Nellans KW, Kowalski E, Chung KC. The epidemiology of distal radius fractures. Hand Clin. 2012;28(2):113-125.
4. Chung KC, Shauver MJ, Birkmeyer JD. Trends in the United States in the treatment of distal radial fractures in the elderly. J Bone Joint Surg Am. 2009;91(8):1868-1873.
5. Melone CP Jr. Articular fractures of the distal radius. Orthop Clin North Am. 1984;15(2):217-236.
6. Castaing J. Recent fractures of the lower extremity of the radius in adults [in French]. Rev Chir Orthop Reparatrice Appar Mot. 1964;50:581-696.
7. Frykman G. Fracture of the distal radius including sequelae—shoulder-hand-finger syndrome, disturbance in the distal radio-ulnar joint and impairment of nerve function. A clinical and experimental study. Acta Orthop Scand. 1967;(suppl 108):3+.
8. Isani A, Melone CP Jr. Classification and management of intra-articular fractures of the distal radius. Hand Clin. 1988;4(3):349-360.
9. Melone CP Jr. Distal radius fractures: patterns of articular fragmentation. Orthop Clin North Am. 1993;24(2):239-253.
10. Rettig ME, Dassa GL, Raskin KB, Melone CP Jr. Wrist fractures in the athlete: distal radius and carpal fractures. Clin Sports Med. 1998;17(3):469-489.
11. Müller ME, Koch P, Nazarian S, Schatzker J. The Comprehensive Classification of Fractures of Long Bones. Berlin, Germany: Springer-Verlag; 1990.
12. Peine R, Rikli DA, Hoffmann R, Duda G, Regazzoni P. Comparison of three different plating techniques for the dorsum of the distal radius: a biomechanical study. J Hand Surg Am. 2000;25(1):29-33.
13. Williams PL, Warwick R, Dyson M, Bannister LH, eds. Gray’s Anatomy. 37th ed. New York, NY: Churchill Livingstone; 1989.
14. Linscheid RL. Kinematic considerations of the wrist. Clin Orthop Relat Res. 1986;(202):27-39.
15. Mekhail AO, Ebraheim NA, McCreath WA, Jackson WT, Yeasting RA. Anatomic and x-ray film studies of the distal articular surface of the radius. J Hand Surg Am. 1996;21(4):567-573.
16. Schuind FA, Linscheid RL, An KN, Chao EY. A normal data base of posteroanterior roentgenographic measurements of the wrist. J Bone Joint Surg Am. 1992;74(9):1418-1429.
17. Schuind F, Alemzadeh S, Stallenberg B, Burny F. Does the normal contralateral wrist provide the best reference for x-ray film measurements of the pathologic wrist? J Hand Surg Am. 1996;21(1):24-30.
18. Genda E, Horii E. Theoretical stress analysis in wrist joint: neutral position and functional position. J Hand Surg Br. 2000;25(3):292-295.
19. Giunta R, Löwer N, Wilhelm K, Keirse R, Rock C, Müller-Gerbl M. Altered patterns of subchondral bone mineralization in Kienböck’s disease. J Hand Surg Br. 1997;22(1):16-20.
20. Andermahr J, Lozano-Calderon S, Trafton T, Crisco JJ, Ring D. The volar extension of the lunate facet of the distal radius: a quantitative anatomic study. J Hand Surg Am. 2006;31(6):892-895.
21. Bo WJ, Meschan I, Krueger WA. Basic Atlas of Cross-Sectional Anatomy. Philadelphia, PA: Saunders; 1980.
22. Cahill DR, Orland MJ, Miller GM. Atlas of Human Cross-Sectional Anatomy: With CT and MR Images. 3rd ed. New York, NY: Wiley; 1995.
23. El-Khoury GY, Bergman RA, Montgomery WJ. Sectional Anatomy by MRI. 2nd ed. New York, NY: Churchill Livingstone; 1995.
24. Harness NG, Jupiter JB, Orbay JL, Raskin KB, Fernandez DL. Loss of fixation of the volar lunate facet fragment in fractures of the distal part of the radius. J Bone Joint Surg Am. 2004;86(9):1900-1908.
25. Lewis OJ, Hamshere RJ, Bucknill TM. The anatomy of the wrist joint. J Anat. 1970;106(Pt 3):539-552.
26. Berger RA, Landsmeer JM. The palmar radiocarpal ligaments: a study of adult and fetal human wrist joints. J Hand Surg Am. 1990;15(6):847-854.
27. Apergis E, Darmanis S, Theodoratos G, Maris J. Beware of the ulno-palmar distal radial fragment. J Hand Surg Br. 2002;27(2):139-145.
28. Chang EY, Chen KC, Meunier MJ, Chung CB. Acute short radiolunate ligament rupture in a rock climber. Skeletal Radiol. 2014;43(2):235-238.
29. Souer JS, Wiggers J, Ring D. Quantitative 3-dimensional computed tomography measurement of volar shearing fractures of the distal radius. J Hand Surg Am. 2011;36(4):599-603.
30. Pruitt DL, Gilula LA, Manske PR, Vannier MW. Computed tomography scanning with image reconstruction in evaluation of distal radius fractures. J Hand Surg Am. 1994(5);19:720-727.
31. Goldwyn E, Pensy R, O’Toole RV, et al. Do traction radiographs of distal radial fractures influence fracture characterization and treatment? J Bone Joint Surg Am. 2012;94(22):2055-2062.
32. Glueck DA, Charoglu CP, Lawton JN. Factors associated with infection following open distal radius fractures. Hand. 2009;4(3):330-334.
33. Kurylo JC, Axelrad TW, Tornetta P 3rd, Jawa A. Open fractures of the distal radius: the effects of delayed debridement and immediate internal fixation on infection rates and the need for secondary procedures. J Hand Surg Am. 2011;36(7):1131-1134.
34. Rozental TD, Blazar PE. Functional outcome and complications after volar plating for dorsally displaced, unstable fractures of the distal radius. J Hand Surg Am. 2006;31(3):359-365.
35. Rozental TD, Beredjiklian PK, Steinberg DR, Bozentka DJ. Open fractures of the distal radius. J Hand Surg Am. 2002;27(1):77-85.
36. Nana AD, Joshi A, Lichtman DM. Plating of the distal radius. J Am Acad Orthop Surg. 2005;13(3):159-171.
37. Bae DS, Koris MJ. Fragment-specific internal fixation of distal radius fractures. Hand Clin. 2005;21(3):355-362.
38. Berglund LM, Messer TM. Complications of volar plate fixation for managing distal radius fractures. J Am Acad Orthop Surg. 2009;17(6):369-377.
39. Henry AK. Extensile Exposure. 2nd ed. New York, NY: Churchill Livingstone; 1973.
40. Axelrod T, Paley D, Green J, McMurtry RY. Limited open reduction of the lunate facet in comminuted intra-articular fractures of the distal radius. J Hand Surg Am. 1988;13(3):372-377.
41. Chin KR, Jupiter JB. Wire-loop fixation of volar displaced osteochondral fractures of the distal radius. J Hand Surg Am. 1999;24(3):525-533.
42. Jupiter JB, Marent-Huber M; LCP Study Group. Operative management of distal radial fractures with 2.4-millimeter locking plates: a multicenter prospective case series. Surgical technique. J Bone Joint Surg Am. 2010;92(suppl 1, pt 1):96-106.
43. Ruch DS, Yang C, Smith BP. Results of palmar plating of the lunate facet combined with external fixation for the treatment of high-energy compression fractures of the distal radius. J Orthop Trauma. 2004;18(1):28-33.
44. Wiesler ER, Chloros GD, Lucas RM, Kuzma GR. Arthroscopic management of volar lunate facet fractures of the distal radius. Tech Hand Up Extrem Surg. 2006;10(3):139-144.
45. American Academy of Orthopaedic Surgeons. The Treatment of Distal Radius Fractures: Guideline and Evidence Report. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2009. http://www.aaos.org/research/guidelines/drfguideline.pdf. Accessed August 4, 2015.
46. Arora R, Lutz M, Deml C, Krappinger D, Haug L, Gabl M. A prospective randomized trial comparing nonoperative treatment with volar locking plate fixation for displaced and unstable distal radial fractures in patients sixty-five years of age and older. J Bone Joint Surg Am. 2011;93(23):2146-2153.
Fracture of the distal radius is the wrist injury most often encountered by orthopedic and hand surgeons.1 The number of fractures of the distal radius in the United States was estimated to be 640,000 in 2001, and the incidence is increasing.2,3 Recent evidence has shown a substantial increase in treating these fractures with internal rather than closed fixation, even in the elderly.4
Treatment of complex intra-articular fractures of the distal radius requires an accurate diagnosis of the fracture pattern and a thoughtful approach to fixation. Although a majority of the fractures that meet the operative criteria are now treated with various anterior locked-plating techniques with good results, a subset requires more technically demanding fixation approaches, including fragment-specific approaches, dorsal and palmar plating, and combined internal and external fixation.
The sustentaculum lunatum, as we have named the palmar lunate facet, deserves specific attention because of its importance in load transmission across the radiocarpal joint and its key role in restoring the anatomy of the palmar distal radial metaphysis during internal fixation. This fragment in comminuted fractures was first ascribed special importance by Melone5 in his description of common fracture patterns. In the present article, we describe the anatomical characteristics of the sustentaculum lunatum and the clinical relevance of this fragment to management of fractures of the distal radius.
Classification
A variety of classification systems have been proposed to characterize and guide treatment of fractures of the distal radius. The earliest descriptions of fracture patterns were presented by Castaing6 and Frykman7 in the 1960s. The Frykman classification historically has been popular but is limited in accuracy in its characterization of fragments and their displacement and is limited in its ability to guide treatment. The classification system proposed by Melone and colleagues5,8-10 was the first to truly describe fracture of the distal radius fragments in a relevant manner, including their characteristic “4 parts” (Figure 1). The authors emphasized the importance of the “medial complex” as the cornerstone of the radiocarpal and radioulnar joints.
The classification system developed by Müller and colleagues,11 which was adopted by the AO (Arbeitsgemeinschaft für Osteosynthesefragen), might be the most descriptive and informative system, and it is widely used to conduct research and direct treatment. This system classifies fractures into A (extra-articular), B (partial articular), and C (complete articular) types and subclassifies them according to fracture location and comminution. These classifications, along with a conceptualization of the distal forearm as a 3-column structure involving the radial, ulnar, and intermediate columns (including the lunate facet), as proposed by Peine and colleagues,12 gave us a framework for approaching fixation of fractures of the distal radius.
Etymology and Definition
Sustentaculum, from the Latin sustinere, “to support, check, or put off,” and taculum, “receptacle or holding space,” is a fitting description of the most distal portion of the palmar lunate facet, as it supports and holds the carpus, and specifically the lunate, on the radial articular surface. This portion is analogous to the sustentaculum tali, the named portion of the calcaneus that supports and articulates with the middle calcaneal articular surface of the talus13 and provides a reliable fragment for internal fixation of the calcaneus.
Anatomical and Biomechanical Considerations
The distal radial articular surface is composed of distinct scaphoid and lunate facets that articulate with their respective carpal bones. Several studies have characterized the anatomy of the distal radius.14-17 Linscheid14 found that the lunate and scaphoid facets account for 46% and 43% of the contact area across the radiocarpal joint, respectively; this has been corroborated by others.15 A biomechanical study by Genda and Horii18 showed that the majority of stress across the wrist joint was concentrated at the palmar side of the distal radius in the neutral position. Although it is recognized that the scaphoid facet bears most of the load across the wrist in the neutral wrist position, most activities of daily living place the wrist in a slightly extended and ulnarly deviated position. This position results in a shift of the majority of load to the radiolunar articulation, constituting 53% of total force transmission.18 Subchondral bone density analyses have supported this lunate-predominant loading pattern across the radiocarpal articulation in most people.19 This loading pattern is also supported by the observation that failure of fixation and carpal subluxation generally occurs at the radiolunate articulation.
The palmar lip of the distal radius traditionally has been depicted and conceptualized as a flat extension of the metaphysis, leading to the development of implants that are not ideally designed for capturing this area in the fracture setting. A 3-dimensional (3-D) computed tomography (CT) study of the distal radii of healthy volunteers, conducted by Andermahr and colleagues,20 showed that the contour of the palmar lunate facet projects from the palmar cortex of the radius by 3 mm on average and is about 19 mm in width (radial to ulnar dimension) (Figures 2A-2C). In the axial plane, the anterior cortex of the distal radius slopes in a palmar direction, from radial to ulnar. This presents a challenge in attempts to support the entire surface (scaphoid and lunate facets) with a single palmar implant.20-25
A study conducted by Harness and colleagues24 showed that the majority of palmar shear fractures are composed of multiple fragments of the lunar articular facet. Anatomical studies of the distal radiocarpal articulation have also described the ligamentous attachments to the sustentaculum lunatum.26 The short radiolunate ligament, which originates from this fragment and inserts onto the lunate, provides stability to the carpus and, if not adequately fixed, leads to an incompetent restraint to palmar carpal translation. Isolated injuries of the short radiolunate ligament or fractures of the palmar lunate facet have been shown to result in palmar carpal translation.27,28 In addition, attachments of the palmar radioulnar ligament and other more ulnar radiocarpal ligaments act as deforming forces on the palmar lunate facet.24,26
Fracture Pattern Recognition
Although the AO type B palmar shear fracture pattern, also known as the Barton fracture, has classically been recognized as the fracture involving the palmar lunate facet and requiring special attention, many complete articular fractures feature involvement and fragmentation of this portion of the distal radius (Figures 3A-3F).29 In highly comminuted complete articular and palmar shear fracture patterns, the morphology of the sustentaculum lunatum should be appreciated, and its adequate fixation to the radial metaphysis ensured, to prevent loss of reduction.
Visualization of the palmar lunate facet as a distinct fragment might be difficult in cases of highly comminuted fracture patterns. Standard CT or more recently described 3-D CT techniques with subtraction of the carpus might facilitate appreciation of this fragment for preoperative planning of approach and fixation.29,30 Our institutional protocol involves obtaining preoperative traction radiographs of every fracture of the distal radius. These radiographs have reduced the need for CT in understanding the fracture pattern and aid in decision making.31
Besides appreciating the existence of the sustentaculum lunatum fragment, we should recognize that some injury patterns that split the lunate facet into unstable dorsal and palmar fragments might necessitate a separate dorsal approach to reduce and fix the dorsal lunate fragment. Traction radiographs can be especially useful in recognizing these patterns (a V sign is present) (Figures 4A, 4B).
Open Fractures
Highly comminuted fractures of the distal radius presenting with displaced lunate facet fragments can have high-energy mechanisms of injury. Although open fractures of the distal radius are associated with lower risk for infection (compared with open fractures of other long bones), they deserve special attention because of associated tendon and neurovascular injuries. Few studies have specifically assessed open fractures of the distal radius.32-35 Only the study by Rozental and Blazar34 listed associated injuries at the wrist level. The authors identified 4 patients (out of 18) with concomitant flexor tendon or neurovascular injuries that included radial or ulnar artery injury. In our experience, many open fractures of the distal radius are caused by an inside-out mechanism and present with an open wound either over the ulnar styloid or in the area of the ulnar side of the palmar radial metaphysis corresponding to the metaphyseal spike that mates with the sustentaculum lunatum (Figures 5A, 5B). Given these findings, we approach this intermediate column with particular care in cases of open fracture, paying attention to important structures (flexors, neurovascular) and looking for contamination from the environment into the fracture.
Fixation Techniques
The approach to fixation of partial articular palmar shear fractures is fairly straightforward. Buttress plate fixation has been well described and has had reliably good results.36 However, in very distal fracture patterns and in cases in which the palmar lunate facet is fragmented as part of a complete articular fracture, a fragment-specific approach to fixation with or without spanning external fixation often is necessary.37 The unrecognized sustentaculum lunatum fragment in comminuted complete articular fractures can lead to inadequate fixation constructs, resulting in loss of reduction and carpal subluxation in a palmar direction.24,34,38
Our surgical approach uses the standard anterior interval between the radial artery and the flexor carpi radialis, as described by Henry.39 The flexor pollicis longus is retracted ulnarly, revealing the pronator quadratus. We then reflect the pronator quadratus from the distal radial metaphysis until the most proximal and ulnar extent of the fracture is easily visualized. The palmar ulnar metaphyseal cortex that mates with the displaced sustentaculum lunatum is, in our experience, often the least comminuted portion of the metaphysis, thus providing a cortical key for restoration of height and alignment (Figures 5A, 5B). At our institution, fixation typically is achieved by contouring miniplates (1.3 or 1.5 mm) to capture and buttress the sustentaculum lunatum (Figures 6A, 6B). In our experience, the screw lengths in the most distal fixed-angle constructs at the palmar lip are limited to 6 mm or less to avoid penetration of the articular surface, though this has not been previously reported in the literature. After restoring the length and tilt of this intermediate column of the distal radius, we proceed with “rebuilding” the remainder of the fragments to our stabilized initial construct.
Various authors40-43 have described alternative fixation methods for the palmar lunate facet fragment. Jupiter and Marent-Huber42 described 2.4-mm locked-plate fixation with either a standard palmar plate or T- or L-plates for cases in which the palmar lip fragment is very distal and small. In fact, some newer anatomical distal radius implants include features designed to target these fragments (Figures 7A, 7B). An alternative fixation method involves use of a 26-gauge stainless steel wire passed through drill holes in the metaphysis 1 cm proximal to the fracture and then passed through the palmar capsule just distal to the fragment and secured in figure-8 fashion while the fragment is manually held reduced.41 Still others have recommended limited internal fixation of the sustentaculum lunatum through an ulna-sided palmar approach to the distal radius (between the ulnar neurovascular bundle and the flexor tendons) combined with external fixation to restore length and palmar tilt in highly comminuted fractures.40,43
A method involving arthroscopically assisted reduction and fixation of the lunate facet has also been described, though this procedure is technically demanding and has limited indications.44 It uses a Freer elevator passed through the standard 3-4 portal after initial visualization and evacuation of hematoma. The Freer elevator is used to disimpact the sustentaculum lunatum and to elevate it from its depressed position. With the dorsal lunate facet left displaced to facilitate access to the palmar fragment, a nerve hook retractor is used to reduce the palmar facet to the radial styloid, and Kirschner wires are used to achieve interfragmentary fixation. The dorsal lunate fragment is then pieced back to the articular segment, and the entire construct is fixed to the radial metaphysis with additional Kirschner wires.
Discussion
Given the increasing incidence of fractures of the distal radius, internal fixation of these injuries will continue to be relevant. American Academy of Orthopaedic Surgeons guidelines recommend operative fixation for fractures with postreduction radial shortening of more than 3 mm, dorsal tilt of more than 10°, or intra-articular displacement or step-off of more than 2 mm.45 Dr. Eglseder and Dr. Pensy indicate operative treatment of any incongruity of more than 2 mm in a young, active adult with a fracture of the distal radius. For the multifragmentary distal radius being treated operatively, attempts are made to achieve reduction more accurate than this, but formal dorsal exposure or direct visualization of the joint surface via dorsal capsulotomy is carefully chosen based on age, activity level, and bone quality. Recent high-level evidence46 showed that closed treatment of unstable fractures of the distal radius results in good outcomes in the elderly. However, it is important to note that fractures displaced in a palmar direction and palmar shear patterns were excluded from that work. It is widely accepted that palmar carpal translation should be addressed with internal fixation, and specific attention must therefore be paid to the lunate facet as the cornerstone of the distal radius. Furthermore, high-energy comminuted fractures in young patients still necessitate internal fixation of fragments to restore alignment and articular congruity.
Conclusion
The importance of the palmar lunate facet in providing support and restraint to palmar carpal translation and the key role of this facet in restoring the anatomy of the distal radius have been known. This fragment deserves special attention because failure to adequately stabilize it results in loss of fixation and carpal subluxation. Various approaches and fixation techniques have been recommended, including the method we prefer and have described here. Our newly proposed term, sustentaculum lunatum, our review of its structure and function, and our descriptions of fixation techniques are intended to promote awareness of this fragment in the treatment of fractures of the distal radius.
Fracture of the distal radius is the wrist injury most often encountered by orthopedic and hand surgeons.1 The number of fractures of the distal radius in the United States was estimated to be 640,000 in 2001, and the incidence is increasing.2,3 Recent evidence has shown a substantial increase in treating these fractures with internal rather than closed fixation, even in the elderly.4
Treatment of complex intra-articular fractures of the distal radius requires an accurate diagnosis of the fracture pattern and a thoughtful approach to fixation. Although a majority of the fractures that meet the operative criteria are now treated with various anterior locked-plating techniques with good results, a subset requires more technically demanding fixation approaches, including fragment-specific approaches, dorsal and palmar plating, and combined internal and external fixation.
The sustentaculum lunatum, as we have named the palmar lunate facet, deserves specific attention because of its importance in load transmission across the radiocarpal joint and its key role in restoring the anatomy of the palmar distal radial metaphysis during internal fixation. This fragment in comminuted fractures was first ascribed special importance by Melone5 in his description of common fracture patterns. In the present article, we describe the anatomical characteristics of the sustentaculum lunatum and the clinical relevance of this fragment to management of fractures of the distal radius.
Classification
A variety of classification systems have been proposed to characterize and guide treatment of fractures of the distal radius. The earliest descriptions of fracture patterns were presented by Castaing6 and Frykman7 in the 1960s. The Frykman classification historically has been popular but is limited in accuracy in its characterization of fragments and their displacement and is limited in its ability to guide treatment. The classification system proposed by Melone and colleagues5,8-10 was the first to truly describe fracture of the distal radius fragments in a relevant manner, including their characteristic “4 parts” (Figure 1). The authors emphasized the importance of the “medial complex” as the cornerstone of the radiocarpal and radioulnar joints.
The classification system developed by Müller and colleagues,11 which was adopted by the AO (Arbeitsgemeinschaft für Osteosynthesefragen), might be the most descriptive and informative system, and it is widely used to conduct research and direct treatment. This system classifies fractures into A (extra-articular), B (partial articular), and C (complete articular) types and subclassifies them according to fracture location and comminution. These classifications, along with a conceptualization of the distal forearm as a 3-column structure involving the radial, ulnar, and intermediate columns (including the lunate facet), as proposed by Peine and colleagues,12 gave us a framework for approaching fixation of fractures of the distal radius.
Etymology and Definition
Sustentaculum, from the Latin sustinere, “to support, check, or put off,” and taculum, “receptacle or holding space,” is a fitting description of the most distal portion of the palmar lunate facet, as it supports and holds the carpus, and specifically the lunate, on the radial articular surface. This portion is analogous to the sustentaculum tali, the named portion of the calcaneus that supports and articulates with the middle calcaneal articular surface of the talus13 and provides a reliable fragment for internal fixation of the calcaneus.
Anatomical and Biomechanical Considerations
The distal radial articular surface is composed of distinct scaphoid and lunate facets that articulate with their respective carpal bones. Several studies have characterized the anatomy of the distal radius.14-17 Linscheid14 found that the lunate and scaphoid facets account for 46% and 43% of the contact area across the radiocarpal joint, respectively; this has been corroborated by others.15 A biomechanical study by Genda and Horii18 showed that the majority of stress across the wrist joint was concentrated at the palmar side of the distal radius in the neutral position. Although it is recognized that the scaphoid facet bears most of the load across the wrist in the neutral wrist position, most activities of daily living place the wrist in a slightly extended and ulnarly deviated position. This position results in a shift of the majority of load to the radiolunar articulation, constituting 53% of total force transmission.18 Subchondral bone density analyses have supported this lunate-predominant loading pattern across the radiocarpal articulation in most people.19 This loading pattern is also supported by the observation that failure of fixation and carpal subluxation generally occurs at the radiolunate articulation.
The palmar lip of the distal radius traditionally has been depicted and conceptualized as a flat extension of the metaphysis, leading to the development of implants that are not ideally designed for capturing this area in the fracture setting. A 3-dimensional (3-D) computed tomography (CT) study of the distal radii of healthy volunteers, conducted by Andermahr and colleagues,20 showed that the contour of the palmar lunate facet projects from the palmar cortex of the radius by 3 mm on average and is about 19 mm in width (radial to ulnar dimension) (Figures 2A-2C). In the axial plane, the anterior cortex of the distal radius slopes in a palmar direction, from radial to ulnar. This presents a challenge in attempts to support the entire surface (scaphoid and lunate facets) with a single palmar implant.20-25
A study conducted by Harness and colleagues24 showed that the majority of palmar shear fractures are composed of multiple fragments of the lunar articular facet. Anatomical studies of the distal radiocarpal articulation have also described the ligamentous attachments to the sustentaculum lunatum.26 The short radiolunate ligament, which originates from this fragment and inserts onto the lunate, provides stability to the carpus and, if not adequately fixed, leads to an incompetent restraint to palmar carpal translation. Isolated injuries of the short radiolunate ligament or fractures of the palmar lunate facet have been shown to result in palmar carpal translation.27,28 In addition, attachments of the palmar radioulnar ligament and other more ulnar radiocarpal ligaments act as deforming forces on the palmar lunate facet.24,26
Fracture Pattern Recognition
Although the AO type B palmar shear fracture pattern, also known as the Barton fracture, has classically been recognized as the fracture involving the palmar lunate facet and requiring special attention, many complete articular fractures feature involvement and fragmentation of this portion of the distal radius (Figures 3A-3F).29 In highly comminuted complete articular and palmar shear fracture patterns, the morphology of the sustentaculum lunatum should be appreciated, and its adequate fixation to the radial metaphysis ensured, to prevent loss of reduction.
Visualization of the palmar lunate facet as a distinct fragment might be difficult in cases of highly comminuted fracture patterns. Standard CT or more recently described 3-D CT techniques with subtraction of the carpus might facilitate appreciation of this fragment for preoperative planning of approach and fixation.29,30 Our institutional protocol involves obtaining preoperative traction radiographs of every fracture of the distal radius. These radiographs have reduced the need for CT in understanding the fracture pattern and aid in decision making.31
Besides appreciating the existence of the sustentaculum lunatum fragment, we should recognize that some injury patterns that split the lunate facet into unstable dorsal and palmar fragments might necessitate a separate dorsal approach to reduce and fix the dorsal lunate fragment. Traction radiographs can be especially useful in recognizing these patterns (a V sign is present) (Figures 4A, 4B).
Open Fractures
Highly comminuted fractures of the distal radius presenting with displaced lunate facet fragments can have high-energy mechanisms of injury. Although open fractures of the distal radius are associated with lower risk for infection (compared with open fractures of other long bones), they deserve special attention because of associated tendon and neurovascular injuries. Few studies have specifically assessed open fractures of the distal radius.32-35 Only the study by Rozental and Blazar34 listed associated injuries at the wrist level. The authors identified 4 patients (out of 18) with concomitant flexor tendon or neurovascular injuries that included radial or ulnar artery injury. In our experience, many open fractures of the distal radius are caused by an inside-out mechanism and present with an open wound either over the ulnar styloid or in the area of the ulnar side of the palmar radial metaphysis corresponding to the metaphyseal spike that mates with the sustentaculum lunatum (Figures 5A, 5B). Given these findings, we approach this intermediate column with particular care in cases of open fracture, paying attention to important structures (flexors, neurovascular) and looking for contamination from the environment into the fracture.
Fixation Techniques
The approach to fixation of partial articular palmar shear fractures is fairly straightforward. Buttress plate fixation has been well described and has had reliably good results.36 However, in very distal fracture patterns and in cases in which the palmar lunate facet is fragmented as part of a complete articular fracture, a fragment-specific approach to fixation with or without spanning external fixation often is necessary.37 The unrecognized sustentaculum lunatum fragment in comminuted complete articular fractures can lead to inadequate fixation constructs, resulting in loss of reduction and carpal subluxation in a palmar direction.24,34,38
Our surgical approach uses the standard anterior interval between the radial artery and the flexor carpi radialis, as described by Henry.39 The flexor pollicis longus is retracted ulnarly, revealing the pronator quadratus. We then reflect the pronator quadratus from the distal radial metaphysis until the most proximal and ulnar extent of the fracture is easily visualized. The palmar ulnar metaphyseal cortex that mates with the displaced sustentaculum lunatum is, in our experience, often the least comminuted portion of the metaphysis, thus providing a cortical key for restoration of height and alignment (Figures 5A, 5B). At our institution, fixation typically is achieved by contouring miniplates (1.3 or 1.5 mm) to capture and buttress the sustentaculum lunatum (Figures 6A, 6B). In our experience, the screw lengths in the most distal fixed-angle constructs at the palmar lip are limited to 6 mm or less to avoid penetration of the articular surface, though this has not been previously reported in the literature. After restoring the length and tilt of this intermediate column of the distal radius, we proceed with “rebuilding” the remainder of the fragments to our stabilized initial construct.
Various authors40-43 have described alternative fixation methods for the palmar lunate facet fragment. Jupiter and Marent-Huber42 described 2.4-mm locked-plate fixation with either a standard palmar plate or T- or L-plates for cases in which the palmar lip fragment is very distal and small. In fact, some newer anatomical distal radius implants include features designed to target these fragments (Figures 7A, 7B). An alternative fixation method involves use of a 26-gauge stainless steel wire passed through drill holes in the metaphysis 1 cm proximal to the fracture and then passed through the palmar capsule just distal to the fragment and secured in figure-8 fashion while the fragment is manually held reduced.41 Still others have recommended limited internal fixation of the sustentaculum lunatum through an ulna-sided palmar approach to the distal radius (between the ulnar neurovascular bundle and the flexor tendons) combined with external fixation to restore length and palmar tilt in highly comminuted fractures.40,43
A method involving arthroscopically assisted reduction and fixation of the lunate facet has also been described, though this procedure is technically demanding and has limited indications.44 It uses a Freer elevator passed through the standard 3-4 portal after initial visualization and evacuation of hematoma. The Freer elevator is used to disimpact the sustentaculum lunatum and to elevate it from its depressed position. With the dorsal lunate facet left displaced to facilitate access to the palmar fragment, a nerve hook retractor is used to reduce the palmar facet to the radial styloid, and Kirschner wires are used to achieve interfragmentary fixation. The dorsal lunate fragment is then pieced back to the articular segment, and the entire construct is fixed to the radial metaphysis with additional Kirschner wires.
Discussion
Given the increasing incidence of fractures of the distal radius, internal fixation of these injuries will continue to be relevant. American Academy of Orthopaedic Surgeons guidelines recommend operative fixation for fractures with postreduction radial shortening of more than 3 mm, dorsal tilt of more than 10°, or intra-articular displacement or step-off of more than 2 mm.45 Dr. Eglseder and Dr. Pensy indicate operative treatment of any incongruity of more than 2 mm in a young, active adult with a fracture of the distal radius. For the multifragmentary distal radius being treated operatively, attempts are made to achieve reduction more accurate than this, but formal dorsal exposure or direct visualization of the joint surface via dorsal capsulotomy is carefully chosen based on age, activity level, and bone quality. Recent high-level evidence46 showed that closed treatment of unstable fractures of the distal radius results in good outcomes in the elderly. However, it is important to note that fractures displaced in a palmar direction and palmar shear patterns were excluded from that work. It is widely accepted that palmar carpal translation should be addressed with internal fixation, and specific attention must therefore be paid to the lunate facet as the cornerstone of the distal radius. Furthermore, high-energy comminuted fractures in young patients still necessitate internal fixation of fragments to restore alignment and articular congruity.
Conclusion
The importance of the palmar lunate facet in providing support and restraint to palmar carpal translation and the key role of this facet in restoring the anatomy of the distal radius have been known. This fragment deserves special attention because failure to adequately stabilize it results in loss of fixation and carpal subluxation. Various approaches and fixation techniques have been recommended, including the method we prefer and have described here. Our newly proposed term, sustentaculum lunatum, our review of its structure and function, and our descriptions of fixation techniques are intended to promote awareness of this fragment in the treatment of fractures of the distal radius.
1. Jupiter JB. Fractures of the distal end of the radius. J Bone Joint Surg Am. 1991;73(3):461-469.
2. Chung KC, Spilson SV. The frequency and epidemiology of hand and forearm fractures in the United States. J Hand Surg Am. 2001;26(5):908-915.
3. Nellans KW, Kowalski E, Chung KC. The epidemiology of distal radius fractures. Hand Clin. 2012;28(2):113-125.
4. Chung KC, Shauver MJ, Birkmeyer JD. Trends in the United States in the treatment of distal radial fractures in the elderly. J Bone Joint Surg Am. 2009;91(8):1868-1873.
5. Melone CP Jr. Articular fractures of the distal radius. Orthop Clin North Am. 1984;15(2):217-236.
6. Castaing J. Recent fractures of the lower extremity of the radius in adults [in French]. Rev Chir Orthop Reparatrice Appar Mot. 1964;50:581-696.
7. Frykman G. Fracture of the distal radius including sequelae—shoulder-hand-finger syndrome, disturbance in the distal radio-ulnar joint and impairment of nerve function. A clinical and experimental study. Acta Orthop Scand. 1967;(suppl 108):3+.
8. Isani A, Melone CP Jr. Classification and management of intra-articular fractures of the distal radius. Hand Clin. 1988;4(3):349-360.
9. Melone CP Jr. Distal radius fractures: patterns of articular fragmentation. Orthop Clin North Am. 1993;24(2):239-253.
10. Rettig ME, Dassa GL, Raskin KB, Melone CP Jr. Wrist fractures in the athlete: distal radius and carpal fractures. Clin Sports Med. 1998;17(3):469-489.
11. Müller ME, Koch P, Nazarian S, Schatzker J. The Comprehensive Classification of Fractures of Long Bones. Berlin, Germany: Springer-Verlag; 1990.
12. Peine R, Rikli DA, Hoffmann R, Duda G, Regazzoni P. Comparison of three different plating techniques for the dorsum of the distal radius: a biomechanical study. J Hand Surg Am. 2000;25(1):29-33.
13. Williams PL, Warwick R, Dyson M, Bannister LH, eds. Gray’s Anatomy. 37th ed. New York, NY: Churchill Livingstone; 1989.
14. Linscheid RL. Kinematic considerations of the wrist. Clin Orthop Relat Res. 1986;(202):27-39.
15. Mekhail AO, Ebraheim NA, McCreath WA, Jackson WT, Yeasting RA. Anatomic and x-ray film studies of the distal articular surface of the radius. J Hand Surg Am. 1996;21(4):567-573.
16. Schuind FA, Linscheid RL, An KN, Chao EY. A normal data base of posteroanterior roentgenographic measurements of the wrist. J Bone Joint Surg Am. 1992;74(9):1418-1429.
17. Schuind F, Alemzadeh S, Stallenberg B, Burny F. Does the normal contralateral wrist provide the best reference for x-ray film measurements of the pathologic wrist? J Hand Surg Am. 1996;21(1):24-30.
18. Genda E, Horii E. Theoretical stress analysis in wrist joint: neutral position and functional position. J Hand Surg Br. 2000;25(3):292-295.
19. Giunta R, Löwer N, Wilhelm K, Keirse R, Rock C, Müller-Gerbl M. Altered patterns of subchondral bone mineralization in Kienböck’s disease. J Hand Surg Br. 1997;22(1):16-20.
20. Andermahr J, Lozano-Calderon S, Trafton T, Crisco JJ, Ring D. The volar extension of the lunate facet of the distal radius: a quantitative anatomic study. J Hand Surg Am. 2006;31(6):892-895.
21. Bo WJ, Meschan I, Krueger WA. Basic Atlas of Cross-Sectional Anatomy. Philadelphia, PA: Saunders; 1980.
22. Cahill DR, Orland MJ, Miller GM. Atlas of Human Cross-Sectional Anatomy: With CT and MR Images. 3rd ed. New York, NY: Wiley; 1995.
23. El-Khoury GY, Bergman RA, Montgomery WJ. Sectional Anatomy by MRI. 2nd ed. New York, NY: Churchill Livingstone; 1995.
24. Harness NG, Jupiter JB, Orbay JL, Raskin KB, Fernandez DL. Loss of fixation of the volar lunate facet fragment in fractures of the distal part of the radius. J Bone Joint Surg Am. 2004;86(9):1900-1908.
25. Lewis OJ, Hamshere RJ, Bucknill TM. The anatomy of the wrist joint. J Anat. 1970;106(Pt 3):539-552.
26. Berger RA, Landsmeer JM. The palmar radiocarpal ligaments: a study of adult and fetal human wrist joints. J Hand Surg Am. 1990;15(6):847-854.
27. Apergis E, Darmanis S, Theodoratos G, Maris J. Beware of the ulno-palmar distal radial fragment. J Hand Surg Br. 2002;27(2):139-145.
28. Chang EY, Chen KC, Meunier MJ, Chung CB. Acute short radiolunate ligament rupture in a rock climber. Skeletal Radiol. 2014;43(2):235-238.
29. Souer JS, Wiggers J, Ring D. Quantitative 3-dimensional computed tomography measurement of volar shearing fractures of the distal radius. J Hand Surg Am. 2011;36(4):599-603.
30. Pruitt DL, Gilula LA, Manske PR, Vannier MW. Computed tomography scanning with image reconstruction in evaluation of distal radius fractures. J Hand Surg Am. 1994(5);19:720-727.
31. Goldwyn E, Pensy R, O’Toole RV, et al. Do traction radiographs of distal radial fractures influence fracture characterization and treatment? J Bone Joint Surg Am. 2012;94(22):2055-2062.
32. Glueck DA, Charoglu CP, Lawton JN. Factors associated with infection following open distal radius fractures. Hand. 2009;4(3):330-334.
33. Kurylo JC, Axelrad TW, Tornetta P 3rd, Jawa A. Open fractures of the distal radius: the effects of delayed debridement and immediate internal fixation on infection rates and the need for secondary procedures. J Hand Surg Am. 2011;36(7):1131-1134.
34. Rozental TD, Blazar PE. Functional outcome and complications after volar plating for dorsally displaced, unstable fractures of the distal radius. J Hand Surg Am. 2006;31(3):359-365.
35. Rozental TD, Beredjiklian PK, Steinberg DR, Bozentka DJ. Open fractures of the distal radius. J Hand Surg Am. 2002;27(1):77-85.
36. Nana AD, Joshi A, Lichtman DM. Plating of the distal radius. J Am Acad Orthop Surg. 2005;13(3):159-171.
37. Bae DS, Koris MJ. Fragment-specific internal fixation of distal radius fractures. Hand Clin. 2005;21(3):355-362.
38. Berglund LM, Messer TM. Complications of volar plate fixation for managing distal radius fractures. J Am Acad Orthop Surg. 2009;17(6):369-377.
39. Henry AK. Extensile Exposure. 2nd ed. New York, NY: Churchill Livingstone; 1973.
40. Axelrod T, Paley D, Green J, McMurtry RY. Limited open reduction of the lunate facet in comminuted intra-articular fractures of the distal radius. J Hand Surg Am. 1988;13(3):372-377.
41. Chin KR, Jupiter JB. Wire-loop fixation of volar displaced osteochondral fractures of the distal radius. J Hand Surg Am. 1999;24(3):525-533.
42. Jupiter JB, Marent-Huber M; LCP Study Group. Operative management of distal radial fractures with 2.4-millimeter locking plates: a multicenter prospective case series. Surgical technique. J Bone Joint Surg Am. 2010;92(suppl 1, pt 1):96-106.
43. Ruch DS, Yang C, Smith BP. Results of palmar plating of the lunate facet combined with external fixation for the treatment of high-energy compression fractures of the distal radius. J Orthop Trauma. 2004;18(1):28-33.
44. Wiesler ER, Chloros GD, Lucas RM, Kuzma GR. Arthroscopic management of volar lunate facet fractures of the distal radius. Tech Hand Up Extrem Surg. 2006;10(3):139-144.
45. American Academy of Orthopaedic Surgeons. The Treatment of Distal Radius Fractures: Guideline and Evidence Report. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2009. http://www.aaos.org/research/guidelines/drfguideline.pdf. Accessed August 4, 2015.
46. Arora R, Lutz M, Deml C, Krappinger D, Haug L, Gabl M. A prospective randomized trial comparing nonoperative treatment with volar locking plate fixation for displaced and unstable distal radial fractures in patients sixty-five years of age and older. J Bone Joint Surg Am. 2011;93(23):2146-2153.
1. Jupiter JB. Fractures of the distal end of the radius. J Bone Joint Surg Am. 1991;73(3):461-469.
2. Chung KC, Spilson SV. The frequency and epidemiology of hand and forearm fractures in the United States. J Hand Surg Am. 2001;26(5):908-915.
3. Nellans KW, Kowalski E, Chung KC. The epidemiology of distal radius fractures. Hand Clin. 2012;28(2):113-125.
4. Chung KC, Shauver MJ, Birkmeyer JD. Trends in the United States in the treatment of distal radial fractures in the elderly. J Bone Joint Surg Am. 2009;91(8):1868-1873.
5. Melone CP Jr. Articular fractures of the distal radius. Orthop Clin North Am. 1984;15(2):217-236.
6. Castaing J. Recent fractures of the lower extremity of the radius in adults [in French]. Rev Chir Orthop Reparatrice Appar Mot. 1964;50:581-696.
7. Frykman G. Fracture of the distal radius including sequelae—shoulder-hand-finger syndrome, disturbance in the distal radio-ulnar joint and impairment of nerve function. A clinical and experimental study. Acta Orthop Scand. 1967;(suppl 108):3+.
8. Isani A, Melone CP Jr. Classification and management of intra-articular fractures of the distal radius. Hand Clin. 1988;4(3):349-360.
9. Melone CP Jr. Distal radius fractures: patterns of articular fragmentation. Orthop Clin North Am. 1993;24(2):239-253.
10. Rettig ME, Dassa GL, Raskin KB, Melone CP Jr. Wrist fractures in the athlete: distal radius and carpal fractures. Clin Sports Med. 1998;17(3):469-489.
11. Müller ME, Koch P, Nazarian S, Schatzker J. The Comprehensive Classification of Fractures of Long Bones. Berlin, Germany: Springer-Verlag; 1990.
12. Peine R, Rikli DA, Hoffmann R, Duda G, Regazzoni P. Comparison of three different plating techniques for the dorsum of the distal radius: a biomechanical study. J Hand Surg Am. 2000;25(1):29-33.
13. Williams PL, Warwick R, Dyson M, Bannister LH, eds. Gray’s Anatomy. 37th ed. New York, NY: Churchill Livingstone; 1989.
14. Linscheid RL. Kinematic considerations of the wrist. Clin Orthop Relat Res. 1986;(202):27-39.
15. Mekhail AO, Ebraheim NA, McCreath WA, Jackson WT, Yeasting RA. Anatomic and x-ray film studies of the distal articular surface of the radius. J Hand Surg Am. 1996;21(4):567-573.
16. Schuind FA, Linscheid RL, An KN, Chao EY. A normal data base of posteroanterior roentgenographic measurements of the wrist. J Bone Joint Surg Am. 1992;74(9):1418-1429.
17. Schuind F, Alemzadeh S, Stallenberg B, Burny F. Does the normal contralateral wrist provide the best reference for x-ray film measurements of the pathologic wrist? J Hand Surg Am. 1996;21(1):24-30.
18. Genda E, Horii E. Theoretical stress analysis in wrist joint: neutral position and functional position. J Hand Surg Br. 2000;25(3):292-295.
19. Giunta R, Löwer N, Wilhelm K, Keirse R, Rock C, Müller-Gerbl M. Altered patterns of subchondral bone mineralization in Kienböck’s disease. J Hand Surg Br. 1997;22(1):16-20.
20. Andermahr J, Lozano-Calderon S, Trafton T, Crisco JJ, Ring D. The volar extension of the lunate facet of the distal radius: a quantitative anatomic study. J Hand Surg Am. 2006;31(6):892-895.
21. Bo WJ, Meschan I, Krueger WA. Basic Atlas of Cross-Sectional Anatomy. Philadelphia, PA: Saunders; 1980.
22. Cahill DR, Orland MJ, Miller GM. Atlas of Human Cross-Sectional Anatomy: With CT and MR Images. 3rd ed. New York, NY: Wiley; 1995.
23. El-Khoury GY, Bergman RA, Montgomery WJ. Sectional Anatomy by MRI. 2nd ed. New York, NY: Churchill Livingstone; 1995.
24. Harness NG, Jupiter JB, Orbay JL, Raskin KB, Fernandez DL. Loss of fixation of the volar lunate facet fragment in fractures of the distal part of the radius. J Bone Joint Surg Am. 2004;86(9):1900-1908.
25. Lewis OJ, Hamshere RJ, Bucknill TM. The anatomy of the wrist joint. J Anat. 1970;106(Pt 3):539-552.
26. Berger RA, Landsmeer JM. The palmar radiocarpal ligaments: a study of adult and fetal human wrist joints. J Hand Surg Am. 1990;15(6):847-854.
27. Apergis E, Darmanis S, Theodoratos G, Maris J. Beware of the ulno-palmar distal radial fragment. J Hand Surg Br. 2002;27(2):139-145.
28. Chang EY, Chen KC, Meunier MJ, Chung CB. Acute short radiolunate ligament rupture in a rock climber. Skeletal Radiol. 2014;43(2):235-238.
29. Souer JS, Wiggers J, Ring D. Quantitative 3-dimensional computed tomography measurement of volar shearing fractures of the distal radius. J Hand Surg Am. 2011;36(4):599-603.
30. Pruitt DL, Gilula LA, Manske PR, Vannier MW. Computed tomography scanning with image reconstruction in evaluation of distal radius fractures. J Hand Surg Am. 1994(5);19:720-727.
31. Goldwyn E, Pensy R, O’Toole RV, et al. Do traction radiographs of distal radial fractures influence fracture characterization and treatment? J Bone Joint Surg Am. 2012;94(22):2055-2062.
32. Glueck DA, Charoglu CP, Lawton JN. Factors associated with infection following open distal radius fractures. Hand. 2009;4(3):330-334.
33. Kurylo JC, Axelrad TW, Tornetta P 3rd, Jawa A. Open fractures of the distal radius: the effects of delayed debridement and immediate internal fixation on infection rates and the need for secondary procedures. J Hand Surg Am. 2011;36(7):1131-1134.
34. Rozental TD, Blazar PE. Functional outcome and complications after volar plating for dorsally displaced, unstable fractures of the distal radius. J Hand Surg Am. 2006;31(3):359-365.
35. Rozental TD, Beredjiklian PK, Steinberg DR, Bozentka DJ. Open fractures of the distal radius. J Hand Surg Am. 2002;27(1):77-85.
36. Nana AD, Joshi A, Lichtman DM. Plating of the distal radius. J Am Acad Orthop Surg. 2005;13(3):159-171.
37. Bae DS, Koris MJ. Fragment-specific internal fixation of distal radius fractures. Hand Clin. 2005;21(3):355-362.
38. Berglund LM, Messer TM. Complications of volar plate fixation for managing distal radius fractures. J Am Acad Orthop Surg. 2009;17(6):369-377.
39. Henry AK. Extensile Exposure. 2nd ed. New York, NY: Churchill Livingstone; 1973.
40. Axelrod T, Paley D, Green J, McMurtry RY. Limited open reduction of the lunate facet in comminuted intra-articular fractures of the distal radius. J Hand Surg Am. 1988;13(3):372-377.
41. Chin KR, Jupiter JB. Wire-loop fixation of volar displaced osteochondral fractures of the distal radius. J Hand Surg Am. 1999;24(3):525-533.
42. Jupiter JB, Marent-Huber M; LCP Study Group. Operative management of distal radial fractures with 2.4-millimeter locking plates: a multicenter prospective case series. Surgical technique. J Bone Joint Surg Am. 2010;92(suppl 1, pt 1):96-106.
43. Ruch DS, Yang C, Smith BP. Results of palmar plating of the lunate facet combined with external fixation for the treatment of high-energy compression fractures of the distal radius. J Orthop Trauma. 2004;18(1):28-33.
44. Wiesler ER, Chloros GD, Lucas RM, Kuzma GR. Arthroscopic management of volar lunate facet fractures of the distal radius. Tech Hand Up Extrem Surg. 2006;10(3):139-144.
45. American Academy of Orthopaedic Surgeons. The Treatment of Distal Radius Fractures: Guideline and Evidence Report. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2009. http://www.aaos.org/research/guidelines/drfguideline.pdf. Accessed August 4, 2015.
46. Arora R, Lutz M, Deml C, Krappinger D, Haug L, Gabl M. A prospective randomized trial comparing nonoperative treatment with volar locking plate fixation for displaced and unstable distal radial fractures in patients sixty-five years of age and older. J Bone Joint Surg Am. 2011;93(23):2146-2153.
Perilunate Injuries
Perilunate injuries typically stem from a high-energy insult to the carpus. Because of their relative infrequency and often subtle radiographic and physical examination findings, these injuries are often undetected in the emergency department setting.1 Early anatomic reduction of any carpal malalignment is essential. Even with optimal treatment, complications such as generalized wrist stiffness, diminished grip strength, and posttraumatic arthritis, commonly develop; however, recent studies suggest these issues are often well tolerated.1-5 In this article, the diagnosis, treatment, and outcomes after perilunate injuries are examined.
History and Physical Examination
Perilunate injuries result from high-energy trauma to the carpus. Patients with these injuries often present with vague wrist pain and loss of wrist motion. Their fingers are frequently held in slight flexion. The patient may complain of numbness and tingling in the median nerve distribution. An acute carpal tunnel syndrome can rapidly develop. The general belief is that acute carpal tunnel syndrome occurs more commonly in pure volar lunate dislocations than in dorsal perilunate dislocations. However, no studies compare the incidence of acute carpal tunnel syndrome in lunate versus perilunate dislocations.
Radiographic Evaluation
Standard radiographic evaluation of a potential perilunate injury includes posteroanterior (PA), lateral, and oblique views of the wrist (Figure 1). A scaphoid view (ie, PA view with the wrist in ulnar deviation) may also be helpful. The PA view is particularly helpful because it enables assessment of Gilula lines, which are imaginary lines drawn across the proximal and distal aspects of the proximal carpal row and the proximal aspect of the distal carpal row. These lines should appear as 3 smooth arcs running nearly parallel to each other.6 Any disruption in these lines suggests carpal incongruity. It may be possible to note a triangular-shaped lunate on the PA view, which is a sign of lunate dislocation.7
While the PA view is certainly useful, the lateral view is the most important in diagnosing a perilunate injury. The lateral view allows assessment of the collinearity of radius, lunate, and capitate. Any disruption in this collinearity strongly suggests a perilunate dislocation.7,8
Classification
Mayfield and colleagues9,10 described 4 stages of perilunate instability proceeding from a radial to an ulnar direction around the lunate. Stage I involves disruption of the scapholunate joint, while stage II involves both the scapholunate and capitolunate joints. In stage III, the scapholunate, capitolunate, and lunotriquetral ligaments are disrupted, and the result is a perilunate dislocation, usually dorsal. Finally, in stage IV, all the ligaments surrounding the lunate are disrupted and the lunate dislocates, most often volarly.
Lastly, perilunate injuries can be classified as greater-arc injuries if concomitant fracture of the carpus occurs, lesser-arc injuries if the injury is purely ligamentous, or inferior-arc injuries if there is an associated fracture of the volar rim of the distal radius.8
Treatment
Closed Reduction
All acute perilunate dislocations should be managed initially with an attempted closed reduction.11 If the injury is older than 72 hours, such an attempt may be futile. For any closed reduction performed in the emergency department setting, intravenous sedation is generally advised for muscle relaxation. Gentle traction with finger traps can also be used prior to the reduction attempt. For a dorsal perilunate dislocation, longitudinal traction followed by volar flexion of the wrist with volar pressure on the lunate and dorsal pressure on the capitate (ie, Tavernier’s maneuver) is required. Once reduction is complete, PA and lateral views of the wrist should be obtained to assess carpal alignment. If closed reduction is unsuccessful, an open reduction is required, although the timing of said procedure is an area of debate, which we will discuss later.1,3 Restoration of anatomic carpal alignment is essential to optimizing outcome, although it does not guarantee a good overall result.
Open Reduction
If successful closed reduction is achieved, the patient can be immobilized temporarily in a short-arm plaster splint. However, open reduction and either pinning or internal fixation will be required to maintain this alignment. The exact timing of open reduction and fixation is debatable and often dictated by the absence or presence of median nerve symptoms.1,3 If a patient with no median nerve symptoms undergoes a successful closed reduction, he or she may be stabilized surgically within 3 to 5 days (or longer) with either pins or headless screws. If closed reduction is unsuccessful, an open reduction should be done within 2 to 3 days. However, if the patient has progressive numbness in the median nerve distribution upon presentation that fails to improve or worsens despite a successful closed reduction, an urgent open reduction (within 24 hours) and carpal tunnel release should be performed to prevent permanent damage to the median nerve.
Once open reduction is undertaken, a dorsal, volar, and combined approach can be used.2-4 In most cases the dorsal approach is selected first. A longitudinal incision is made over the dorsum of the wrist, centered on the Lister tubercle. Dissection occurs between the third and fourth dorsal compartments. After the capsule is exposed, reduction of the lunate to the capitate is confirmed. If any fractures are present in the carpus (eg, scaphoid), they are internally fixed. The scapholunate articulation is then addressed. In general, the scapholunate ligament is not disrupted with a transscaphoid perilunate dislocation. However, if the scapholunate ligament is disrupted, the joint should be reduced and pinned. Repair or reconstruction of the scapholunate ligament is performed. Finally, the lunotriquetral articulation is reduced and stabilized with pins. There are no studies that specifically suggest direct repair of the lunotriquetral ligament versus pinning of the lunotriquetral articulation, but the lunotriquetral ligament could be repaired in similar fashion to the scapholunate ligament at the surgeon’s discretion.
As an alternative to percutaneous pinning, intercarpal screw fixation can be used to stabilize the carpus. A 2007 study by Souer and colleagues12 showed no substantial difference in outcome between the 2 methods of fixation. However, a second procedure is required to remove the screws.
The volar approach, if selected, is typically done second and performed via an extended carpal tunnel incision. It allows decompression of the carpal tunnel and enables repair of volar capsular ligaments (ie, long and short radiolunate ligaments, volar scapholunate ligament, and volar lunotriquetral ligament), which increases overall carpal stability. Currently, many surgeons favor a combined dorsal-volar approach for its efficacy.2,3 Some use a dorsal approach in all patients and perform a volar procedure only if the patient has median nerve symptoms.4 However, Başar and colleagues13 report use of only the volar approach for treatment of perilunate injuries. The authors repaired the long and short radiolunate ligaments, volar scapholunate ligament, and volar lunotriquetral ligament. They reported reasonably good outcomes, which are equivalent to those reported in similar studies using dorsal or combined dorsal-volar approaches. However, no studies in the literature directly compare any of the different approaches with each other.
Postoperatively, patients are placed in a long-arm thumb-spica cast for 4 weeks, and then in a short-arm cast for 4 to 8 weeks (Figure 2). If present, pins are removed in 3 to 12 weeks, with most authors recommending removal at 8 weeks.2,14
Lastly, carpal tunnel symptoms can develop late and even after a successful reduction and surgical stabilization. One theory is that a significant perilunate injury can create slightly higher baseline carpal tunnel pressures, which can compromise the blood flow to the median nerve and cause carpal tunnel symptoms. Additionally, it is possible that direct median nerve contusion and/or traction injury via a displaced lunate can also cause these symptoms. Whatever the inciting cause of median-nerve irritation, a delayed carpal tunnel release is sometimes required.
Conclusion
Outcomes after either perilunate or lunate dislocation are fair to good at best but can be optimized with prompt, appropriate treatment. Closed reduction and casting as definitive treatment has been abandoned because of frequent loss of reduction.12 Early open reduction (ie, less than 3 days after injury) has been shown to be beneficial.1,2 However, even those treated early and with anatomic restoration of carpal alignment can expect a loss of grip strength and a range of motion of approximately 70% compared with the contralateral side.2-5 A recent study has suggested that lesser-arc injures generally have a poorer overall outcome than their greater-arc counterparts.15
More than half of all patients with perilunate injuries will develop radiographic signs of osteoarthritis, and some will require additional salvage procedures.3-5 Kremer and colleagues4 showed that overall results after perilunate injuries deteriorate with time. However, according to a paper by Forli and colleagues5 in which patients were followed a minimum of 10 years after their injuries, the authors found that, despite radiographic progression of arthritis, most patients maintained reasonable hand function.
1. Herzberg G, Comtet JJ, Linscheid RL, Amadio PC, Cooney WP, Stalder J. Perilunate dislocations and fracture-dislocations: a multicenter study. J Hand Surg Am. 1993;18(5):768-779.
2. Sotereanos DG, Mitsionis GJ, Giannakopoulos PN, Tomaino MM, Herndon JH. Perilunate dislocation and fracture dislocation: a critical analysis of the volar-dorsal approach. J Hand Surg Am. 1997;22(1):49-56.
3. Hildebrand KA, Ross DC, Patterson SD, Roth JH, MacDermid JC, King GJ. Dorsal perilunate dislocations and fracture-dislocations: questionnaire, clinical, and radiographic evaluation. J Hand Surg Am. 2000;25(6):1069-1079.
4. Kremer T, Wendt M, Riedel K, Sauerbier M, Germann G, Bickert B. Open reduction for perilunate injuries--clinical outcome and patient satisfaction. J Hand Surg Am. 2010;35(10):1599-1606.
5. Forli A, Courvoisier A, Wimsey S, Corcella D, Moutet F. Perilunate dislocations and transscaphoid perilunate fracture-dislocations: a retrospective study with minimum ten-year follow-up. J Hand Surg Am. 2010;35(1):62-68.
6. Gilula LA. Carpal injuries: analytic approach and case exercises. AJR Am J Roentgenol. 1979;133(3):503-517.
7. Kozin SH. Perilunate injuries: diagnosis and treatment. J Am Acad Orthop Surg. 1998;6(2):114-120.
8. Graham TJ. The inferior arc injury: an addition to the family of complex carpal fracture-dislocation patterns. Am J Orthop. 2003;32(9 suppl):10-19.
9. Mayfield JK, Johnson RP, Kilcoyne RK. Carpal dislocations: pathomechanics and progressive perilunar instability. J Hand Surg Am. 1980;5(3):226-241.
10. Mayfield JK. Mechanism of carpal injuries. Clin Orthop Relat Res. 1980;149:45-54.
11. Adkison JW, Chapman MW. Treatment of acute lunate and perilunate dislocations. Clin Orthop Relat Res. 1982;164:199-207.
12. Souer JS, Rutgers M, Andermahr J, Jupiter JB, Ring D. Perilunate fracture-dislocations of the wrist: comparison of temporary screw versus K-wire fixation. J Hand Surg Am. 2007;32(3):318-325.
13. Başar H, Başar B, Erol B, Tetik C. Isolated volar surgical approach for the treatment of perilunate and lunate dislocations. Indian J Orthop. 2014;48(3):301-315.
14. Komurcu M, Kürklü M, Ozturan KE, Mahirogullari M, Basbozkurt M. Early and delayed treatment of dorsal transscaphoid perilunate fracture-dislocations. J Orthop Trauma. 2008;22:535-540.
15. Massoud AH, Naam NH. Functional outcome of open reduction of chronic perilunate injuries. J Hand Surg Am. 2012;37(9):1852-1860.
Perilunate injuries typically stem from a high-energy insult to the carpus. Because of their relative infrequency and often subtle radiographic and physical examination findings, these injuries are often undetected in the emergency department setting.1 Early anatomic reduction of any carpal malalignment is essential. Even with optimal treatment, complications such as generalized wrist stiffness, diminished grip strength, and posttraumatic arthritis, commonly develop; however, recent studies suggest these issues are often well tolerated.1-5 In this article, the diagnosis, treatment, and outcomes after perilunate injuries are examined.
History and Physical Examination
Perilunate injuries result from high-energy trauma to the carpus. Patients with these injuries often present with vague wrist pain and loss of wrist motion. Their fingers are frequently held in slight flexion. The patient may complain of numbness and tingling in the median nerve distribution. An acute carpal tunnel syndrome can rapidly develop. The general belief is that acute carpal tunnel syndrome occurs more commonly in pure volar lunate dislocations than in dorsal perilunate dislocations. However, no studies compare the incidence of acute carpal tunnel syndrome in lunate versus perilunate dislocations.
Radiographic Evaluation
Standard radiographic evaluation of a potential perilunate injury includes posteroanterior (PA), lateral, and oblique views of the wrist (Figure 1). A scaphoid view (ie, PA view with the wrist in ulnar deviation) may also be helpful. The PA view is particularly helpful because it enables assessment of Gilula lines, which are imaginary lines drawn across the proximal and distal aspects of the proximal carpal row and the proximal aspect of the distal carpal row. These lines should appear as 3 smooth arcs running nearly parallel to each other.6 Any disruption in these lines suggests carpal incongruity. It may be possible to note a triangular-shaped lunate on the PA view, which is a sign of lunate dislocation.7
While the PA view is certainly useful, the lateral view is the most important in diagnosing a perilunate injury. The lateral view allows assessment of the collinearity of radius, lunate, and capitate. Any disruption in this collinearity strongly suggests a perilunate dislocation.7,8
Classification
Mayfield and colleagues9,10 described 4 stages of perilunate instability proceeding from a radial to an ulnar direction around the lunate. Stage I involves disruption of the scapholunate joint, while stage II involves both the scapholunate and capitolunate joints. In stage III, the scapholunate, capitolunate, and lunotriquetral ligaments are disrupted, and the result is a perilunate dislocation, usually dorsal. Finally, in stage IV, all the ligaments surrounding the lunate are disrupted and the lunate dislocates, most often volarly.
Lastly, perilunate injuries can be classified as greater-arc injuries if concomitant fracture of the carpus occurs, lesser-arc injuries if the injury is purely ligamentous, or inferior-arc injuries if there is an associated fracture of the volar rim of the distal radius.8
Treatment
Closed Reduction
All acute perilunate dislocations should be managed initially with an attempted closed reduction.11 If the injury is older than 72 hours, such an attempt may be futile. For any closed reduction performed in the emergency department setting, intravenous sedation is generally advised for muscle relaxation. Gentle traction with finger traps can also be used prior to the reduction attempt. For a dorsal perilunate dislocation, longitudinal traction followed by volar flexion of the wrist with volar pressure on the lunate and dorsal pressure on the capitate (ie, Tavernier’s maneuver) is required. Once reduction is complete, PA and lateral views of the wrist should be obtained to assess carpal alignment. If closed reduction is unsuccessful, an open reduction is required, although the timing of said procedure is an area of debate, which we will discuss later.1,3 Restoration of anatomic carpal alignment is essential to optimizing outcome, although it does not guarantee a good overall result.
Open Reduction
If successful closed reduction is achieved, the patient can be immobilized temporarily in a short-arm plaster splint. However, open reduction and either pinning or internal fixation will be required to maintain this alignment. The exact timing of open reduction and fixation is debatable and often dictated by the absence or presence of median nerve symptoms.1,3 If a patient with no median nerve symptoms undergoes a successful closed reduction, he or she may be stabilized surgically within 3 to 5 days (or longer) with either pins or headless screws. If closed reduction is unsuccessful, an open reduction should be done within 2 to 3 days. However, if the patient has progressive numbness in the median nerve distribution upon presentation that fails to improve or worsens despite a successful closed reduction, an urgent open reduction (within 24 hours) and carpal tunnel release should be performed to prevent permanent damage to the median nerve.
Once open reduction is undertaken, a dorsal, volar, and combined approach can be used.2-4 In most cases the dorsal approach is selected first. A longitudinal incision is made over the dorsum of the wrist, centered on the Lister tubercle. Dissection occurs between the third and fourth dorsal compartments. After the capsule is exposed, reduction of the lunate to the capitate is confirmed. If any fractures are present in the carpus (eg, scaphoid), they are internally fixed. The scapholunate articulation is then addressed. In general, the scapholunate ligament is not disrupted with a transscaphoid perilunate dislocation. However, if the scapholunate ligament is disrupted, the joint should be reduced and pinned. Repair or reconstruction of the scapholunate ligament is performed. Finally, the lunotriquetral articulation is reduced and stabilized with pins. There are no studies that specifically suggest direct repair of the lunotriquetral ligament versus pinning of the lunotriquetral articulation, but the lunotriquetral ligament could be repaired in similar fashion to the scapholunate ligament at the surgeon’s discretion.
As an alternative to percutaneous pinning, intercarpal screw fixation can be used to stabilize the carpus. A 2007 study by Souer and colleagues12 showed no substantial difference in outcome between the 2 methods of fixation. However, a second procedure is required to remove the screws.
The volar approach, if selected, is typically done second and performed via an extended carpal tunnel incision. It allows decompression of the carpal tunnel and enables repair of volar capsular ligaments (ie, long and short radiolunate ligaments, volar scapholunate ligament, and volar lunotriquetral ligament), which increases overall carpal stability. Currently, many surgeons favor a combined dorsal-volar approach for its efficacy.2,3 Some use a dorsal approach in all patients and perform a volar procedure only if the patient has median nerve symptoms.4 However, Başar and colleagues13 report use of only the volar approach for treatment of perilunate injuries. The authors repaired the long and short radiolunate ligaments, volar scapholunate ligament, and volar lunotriquetral ligament. They reported reasonably good outcomes, which are equivalent to those reported in similar studies using dorsal or combined dorsal-volar approaches. However, no studies in the literature directly compare any of the different approaches with each other.
Postoperatively, patients are placed in a long-arm thumb-spica cast for 4 weeks, and then in a short-arm cast for 4 to 8 weeks (Figure 2). If present, pins are removed in 3 to 12 weeks, with most authors recommending removal at 8 weeks.2,14
Lastly, carpal tunnel symptoms can develop late and even after a successful reduction and surgical stabilization. One theory is that a significant perilunate injury can create slightly higher baseline carpal tunnel pressures, which can compromise the blood flow to the median nerve and cause carpal tunnel symptoms. Additionally, it is possible that direct median nerve contusion and/or traction injury via a displaced lunate can also cause these symptoms. Whatever the inciting cause of median-nerve irritation, a delayed carpal tunnel release is sometimes required.
Conclusion
Outcomes after either perilunate or lunate dislocation are fair to good at best but can be optimized with prompt, appropriate treatment. Closed reduction and casting as definitive treatment has been abandoned because of frequent loss of reduction.12 Early open reduction (ie, less than 3 days after injury) has been shown to be beneficial.1,2 However, even those treated early and with anatomic restoration of carpal alignment can expect a loss of grip strength and a range of motion of approximately 70% compared with the contralateral side.2-5 A recent study has suggested that lesser-arc injures generally have a poorer overall outcome than their greater-arc counterparts.15
More than half of all patients with perilunate injuries will develop radiographic signs of osteoarthritis, and some will require additional salvage procedures.3-5 Kremer and colleagues4 showed that overall results after perilunate injuries deteriorate with time. However, according to a paper by Forli and colleagues5 in which patients were followed a minimum of 10 years after their injuries, the authors found that, despite radiographic progression of arthritis, most patients maintained reasonable hand function.
Perilunate injuries typically stem from a high-energy insult to the carpus. Because of their relative infrequency and often subtle radiographic and physical examination findings, these injuries are often undetected in the emergency department setting.1 Early anatomic reduction of any carpal malalignment is essential. Even with optimal treatment, complications such as generalized wrist stiffness, diminished grip strength, and posttraumatic arthritis, commonly develop; however, recent studies suggest these issues are often well tolerated.1-5 In this article, the diagnosis, treatment, and outcomes after perilunate injuries are examined.
History and Physical Examination
Perilunate injuries result from high-energy trauma to the carpus. Patients with these injuries often present with vague wrist pain and loss of wrist motion. Their fingers are frequently held in slight flexion. The patient may complain of numbness and tingling in the median nerve distribution. An acute carpal tunnel syndrome can rapidly develop. The general belief is that acute carpal tunnel syndrome occurs more commonly in pure volar lunate dislocations than in dorsal perilunate dislocations. However, no studies compare the incidence of acute carpal tunnel syndrome in lunate versus perilunate dislocations.
Radiographic Evaluation
Standard radiographic evaluation of a potential perilunate injury includes posteroanterior (PA), lateral, and oblique views of the wrist (Figure 1). A scaphoid view (ie, PA view with the wrist in ulnar deviation) may also be helpful. The PA view is particularly helpful because it enables assessment of Gilula lines, which are imaginary lines drawn across the proximal and distal aspects of the proximal carpal row and the proximal aspect of the distal carpal row. These lines should appear as 3 smooth arcs running nearly parallel to each other.6 Any disruption in these lines suggests carpal incongruity. It may be possible to note a triangular-shaped lunate on the PA view, which is a sign of lunate dislocation.7
While the PA view is certainly useful, the lateral view is the most important in diagnosing a perilunate injury. The lateral view allows assessment of the collinearity of radius, lunate, and capitate. Any disruption in this collinearity strongly suggests a perilunate dislocation.7,8
Classification
Mayfield and colleagues9,10 described 4 stages of perilunate instability proceeding from a radial to an ulnar direction around the lunate. Stage I involves disruption of the scapholunate joint, while stage II involves both the scapholunate and capitolunate joints. In stage III, the scapholunate, capitolunate, and lunotriquetral ligaments are disrupted, and the result is a perilunate dislocation, usually dorsal. Finally, in stage IV, all the ligaments surrounding the lunate are disrupted and the lunate dislocates, most often volarly.
Lastly, perilunate injuries can be classified as greater-arc injuries if concomitant fracture of the carpus occurs, lesser-arc injuries if the injury is purely ligamentous, or inferior-arc injuries if there is an associated fracture of the volar rim of the distal radius.8
Treatment
Closed Reduction
All acute perilunate dislocations should be managed initially with an attempted closed reduction.11 If the injury is older than 72 hours, such an attempt may be futile. For any closed reduction performed in the emergency department setting, intravenous sedation is generally advised for muscle relaxation. Gentle traction with finger traps can also be used prior to the reduction attempt. For a dorsal perilunate dislocation, longitudinal traction followed by volar flexion of the wrist with volar pressure on the lunate and dorsal pressure on the capitate (ie, Tavernier’s maneuver) is required. Once reduction is complete, PA and lateral views of the wrist should be obtained to assess carpal alignment. If closed reduction is unsuccessful, an open reduction is required, although the timing of said procedure is an area of debate, which we will discuss later.1,3 Restoration of anatomic carpal alignment is essential to optimizing outcome, although it does not guarantee a good overall result.
Open Reduction
If successful closed reduction is achieved, the patient can be immobilized temporarily in a short-arm plaster splint. However, open reduction and either pinning or internal fixation will be required to maintain this alignment. The exact timing of open reduction and fixation is debatable and often dictated by the absence or presence of median nerve symptoms.1,3 If a patient with no median nerve symptoms undergoes a successful closed reduction, he or she may be stabilized surgically within 3 to 5 days (or longer) with either pins or headless screws. If closed reduction is unsuccessful, an open reduction should be done within 2 to 3 days. However, if the patient has progressive numbness in the median nerve distribution upon presentation that fails to improve or worsens despite a successful closed reduction, an urgent open reduction (within 24 hours) and carpal tunnel release should be performed to prevent permanent damage to the median nerve.
Once open reduction is undertaken, a dorsal, volar, and combined approach can be used.2-4 In most cases the dorsal approach is selected first. A longitudinal incision is made over the dorsum of the wrist, centered on the Lister tubercle. Dissection occurs between the third and fourth dorsal compartments. After the capsule is exposed, reduction of the lunate to the capitate is confirmed. If any fractures are present in the carpus (eg, scaphoid), they are internally fixed. The scapholunate articulation is then addressed. In general, the scapholunate ligament is not disrupted with a transscaphoid perilunate dislocation. However, if the scapholunate ligament is disrupted, the joint should be reduced and pinned. Repair or reconstruction of the scapholunate ligament is performed. Finally, the lunotriquetral articulation is reduced and stabilized with pins. There are no studies that specifically suggest direct repair of the lunotriquetral ligament versus pinning of the lunotriquetral articulation, but the lunotriquetral ligament could be repaired in similar fashion to the scapholunate ligament at the surgeon’s discretion.
As an alternative to percutaneous pinning, intercarpal screw fixation can be used to stabilize the carpus. A 2007 study by Souer and colleagues12 showed no substantial difference in outcome between the 2 methods of fixation. However, a second procedure is required to remove the screws.
The volar approach, if selected, is typically done second and performed via an extended carpal tunnel incision. It allows decompression of the carpal tunnel and enables repair of volar capsular ligaments (ie, long and short radiolunate ligaments, volar scapholunate ligament, and volar lunotriquetral ligament), which increases overall carpal stability. Currently, many surgeons favor a combined dorsal-volar approach for its efficacy.2,3 Some use a dorsal approach in all patients and perform a volar procedure only if the patient has median nerve symptoms.4 However, Başar and colleagues13 report use of only the volar approach for treatment of perilunate injuries. The authors repaired the long and short radiolunate ligaments, volar scapholunate ligament, and volar lunotriquetral ligament. They reported reasonably good outcomes, which are equivalent to those reported in similar studies using dorsal or combined dorsal-volar approaches. However, no studies in the literature directly compare any of the different approaches with each other.
Postoperatively, patients are placed in a long-arm thumb-spica cast for 4 weeks, and then in a short-arm cast for 4 to 8 weeks (Figure 2). If present, pins are removed in 3 to 12 weeks, with most authors recommending removal at 8 weeks.2,14
Lastly, carpal tunnel symptoms can develop late and even after a successful reduction and surgical stabilization. One theory is that a significant perilunate injury can create slightly higher baseline carpal tunnel pressures, which can compromise the blood flow to the median nerve and cause carpal tunnel symptoms. Additionally, it is possible that direct median nerve contusion and/or traction injury via a displaced lunate can also cause these symptoms. Whatever the inciting cause of median-nerve irritation, a delayed carpal tunnel release is sometimes required.
Conclusion
Outcomes after either perilunate or lunate dislocation are fair to good at best but can be optimized with prompt, appropriate treatment. Closed reduction and casting as definitive treatment has been abandoned because of frequent loss of reduction.12 Early open reduction (ie, less than 3 days after injury) has been shown to be beneficial.1,2 However, even those treated early and with anatomic restoration of carpal alignment can expect a loss of grip strength and a range of motion of approximately 70% compared with the contralateral side.2-5 A recent study has suggested that lesser-arc injures generally have a poorer overall outcome than their greater-arc counterparts.15
More than half of all patients with perilunate injuries will develop radiographic signs of osteoarthritis, and some will require additional salvage procedures.3-5 Kremer and colleagues4 showed that overall results after perilunate injuries deteriorate with time. However, according to a paper by Forli and colleagues5 in which patients were followed a minimum of 10 years after their injuries, the authors found that, despite radiographic progression of arthritis, most patients maintained reasonable hand function.
1. Herzberg G, Comtet JJ, Linscheid RL, Amadio PC, Cooney WP, Stalder J. Perilunate dislocations and fracture-dislocations: a multicenter study. J Hand Surg Am. 1993;18(5):768-779.
2. Sotereanos DG, Mitsionis GJ, Giannakopoulos PN, Tomaino MM, Herndon JH. Perilunate dislocation and fracture dislocation: a critical analysis of the volar-dorsal approach. J Hand Surg Am. 1997;22(1):49-56.
3. Hildebrand KA, Ross DC, Patterson SD, Roth JH, MacDermid JC, King GJ. Dorsal perilunate dislocations and fracture-dislocations: questionnaire, clinical, and radiographic evaluation. J Hand Surg Am. 2000;25(6):1069-1079.
4. Kremer T, Wendt M, Riedel K, Sauerbier M, Germann G, Bickert B. Open reduction for perilunate injuries--clinical outcome and patient satisfaction. J Hand Surg Am. 2010;35(10):1599-1606.
5. Forli A, Courvoisier A, Wimsey S, Corcella D, Moutet F. Perilunate dislocations and transscaphoid perilunate fracture-dislocations: a retrospective study with minimum ten-year follow-up. J Hand Surg Am. 2010;35(1):62-68.
6. Gilula LA. Carpal injuries: analytic approach and case exercises. AJR Am J Roentgenol. 1979;133(3):503-517.
7. Kozin SH. Perilunate injuries: diagnosis and treatment. J Am Acad Orthop Surg. 1998;6(2):114-120.
8. Graham TJ. The inferior arc injury: an addition to the family of complex carpal fracture-dislocation patterns. Am J Orthop. 2003;32(9 suppl):10-19.
9. Mayfield JK, Johnson RP, Kilcoyne RK. Carpal dislocations: pathomechanics and progressive perilunar instability. J Hand Surg Am. 1980;5(3):226-241.
10. Mayfield JK. Mechanism of carpal injuries. Clin Orthop Relat Res. 1980;149:45-54.
11. Adkison JW, Chapman MW. Treatment of acute lunate and perilunate dislocations. Clin Orthop Relat Res. 1982;164:199-207.
12. Souer JS, Rutgers M, Andermahr J, Jupiter JB, Ring D. Perilunate fracture-dislocations of the wrist: comparison of temporary screw versus K-wire fixation. J Hand Surg Am. 2007;32(3):318-325.
13. Başar H, Başar B, Erol B, Tetik C. Isolated volar surgical approach for the treatment of perilunate and lunate dislocations. Indian J Orthop. 2014;48(3):301-315.
14. Komurcu M, Kürklü M, Ozturan KE, Mahirogullari M, Basbozkurt M. Early and delayed treatment of dorsal transscaphoid perilunate fracture-dislocations. J Orthop Trauma. 2008;22:535-540.
15. Massoud AH, Naam NH. Functional outcome of open reduction of chronic perilunate injuries. J Hand Surg Am. 2012;37(9):1852-1860.
1. Herzberg G, Comtet JJ, Linscheid RL, Amadio PC, Cooney WP, Stalder J. Perilunate dislocations and fracture-dislocations: a multicenter study. J Hand Surg Am. 1993;18(5):768-779.
2. Sotereanos DG, Mitsionis GJ, Giannakopoulos PN, Tomaino MM, Herndon JH. Perilunate dislocation and fracture dislocation: a critical analysis of the volar-dorsal approach. J Hand Surg Am. 1997;22(1):49-56.
3. Hildebrand KA, Ross DC, Patterson SD, Roth JH, MacDermid JC, King GJ. Dorsal perilunate dislocations and fracture-dislocations: questionnaire, clinical, and radiographic evaluation. J Hand Surg Am. 2000;25(6):1069-1079.
4. Kremer T, Wendt M, Riedel K, Sauerbier M, Germann G, Bickert B. Open reduction for perilunate injuries--clinical outcome and patient satisfaction. J Hand Surg Am. 2010;35(10):1599-1606.
5. Forli A, Courvoisier A, Wimsey S, Corcella D, Moutet F. Perilunate dislocations and transscaphoid perilunate fracture-dislocations: a retrospective study with minimum ten-year follow-up. J Hand Surg Am. 2010;35(1):62-68.
6. Gilula LA. Carpal injuries: analytic approach and case exercises. AJR Am J Roentgenol. 1979;133(3):503-517.
7. Kozin SH. Perilunate injuries: diagnosis and treatment. J Am Acad Orthop Surg. 1998;6(2):114-120.
8. Graham TJ. The inferior arc injury: an addition to the family of complex carpal fracture-dislocation patterns. Am J Orthop. 2003;32(9 suppl):10-19.
9. Mayfield JK, Johnson RP, Kilcoyne RK. Carpal dislocations: pathomechanics and progressive perilunar instability. J Hand Surg Am. 1980;5(3):226-241.
10. Mayfield JK. Mechanism of carpal injuries. Clin Orthop Relat Res. 1980;149:45-54.
11. Adkison JW, Chapman MW. Treatment of acute lunate and perilunate dislocations. Clin Orthop Relat Res. 1982;164:199-207.
12. Souer JS, Rutgers M, Andermahr J, Jupiter JB, Ring D. Perilunate fracture-dislocations of the wrist: comparison of temporary screw versus K-wire fixation. J Hand Surg Am. 2007;32(3):318-325.
13. Başar H, Başar B, Erol B, Tetik C. Isolated volar surgical approach for the treatment of perilunate and lunate dislocations. Indian J Orthop. 2014;48(3):301-315.
14. Komurcu M, Kürklü M, Ozturan KE, Mahirogullari M, Basbozkurt M. Early and delayed treatment of dorsal transscaphoid perilunate fracture-dislocations. J Orthop Trauma. 2008;22:535-540.
15. Massoud AH, Naam NH. Functional outcome of open reduction of chronic perilunate injuries. J Hand Surg Am. 2012;37(9):1852-1860.
Is Your Electronic Health Record Putting You at Risk for a Documentation Audit?
A group of 3 busy orthopedists attended coding education each year and did their best to accurately code and document their services. As a risk-reduction strategy, the group engaged our firm to conduct an audit to determine whether they were documenting their services properly and to provide feedback about how they could improve.
What we found was shocking to the surgeons, but all too common, as we review thousands of orthopedic visit notes every year: The same examination had been documented for all visits, with physicians stating in their notes that the examination was medically necessary. In addition, their documentation supported Current Procedural Terminology (CPT) code 99214 at every visit, with visit frequencies of 2 weeks to 4 months.
The culprit of all this sameness? The practice’s electronic health record (EHR).
“Practices with EHRs often have a large volume of visit notes that look almost identical for a patient who is seen for multiple visits,” explains Mary LeGrand, RN, MA, CCS-P, CPC, KarenZupko & Associates consultant and coding educator. “And that is putting physicians at higher risk of being audited or of not passing an audit.”
According to LeGrand, this is because physicians are using the practice’s EHR to “pull forward” the patient’s previous visit note for the current visit, but failing to customize it for the current visit. The unintended consequence of this workflow efficiency is twofold:
1. It creates documentation that looks strikingly similar to, if not exactly like, the patient’s last billed visit note. This is often referred to as note “cloning.”
2. It creates documentation that includes a lot of unnecessary detail that, even if delivered and documented, doesn’t match the medical necessity of the visit, based on the history of present illness statements.
Both of these things can come back to bite you.
Zero in on the Risk
If your practice has an EHR, it is important that you evaluate whether certain workflow efficiency features are putting the practice at risk. You do not necessarily need to dump the EHR, but you may need to take action to reduce the risk of using these features.
In a pre-EHR practice, physicians began each visit with a blank piece of paper or dictated the entire visit. Then along came EHR vendors who, in an effort to make things easier and more efficient, created visit templates and the ability to “pull forward” the last visit note and use it as a basis for the current visit. The intention was always that physicians would modify it based on the current visit. But the reality is that physicians are busy, editing is time-consuming, and the unintended consequence is cloning.
“If you pull in unnecessary history or exam information from a previous visit that’s not relevant to the current visit, you can get dinged in an audit for not customizing the note to the patient’s specific presenting complaint,” LeGrand explains, “or, for attempting to bill a higher-level code by unintentionally padding the note with irrelevant information. What is documented for ‘reference’ has to be separated from what can be used to select the level of service.”
Your first documentation risk-reduction strategy is to review notes and look for signs of cloning.
LeGrand explains that a practice may be predisposed to cloning simply because of the way the EHR templates and workflow were set up when the system was implemented. “But,” she says, “‘the EHR made me do it’ defense won’t hold water, because it’s still the physician’s responsibility to customize or remove the information from templates and make the note unique to the visit.”
Yes, physician time is precious. But the reality is that the onus is on the physician to integrate EHR features with clinic workflow and to follow documentation rules.
The second documentation risk-reduction strategy is to make sure the level of evaluation and management (E/M) service billed is supported by medical necessity, not only by documentation artifacts that were relevant to the patient in the past but irrelevant to his or her current presenting complaint or condition.
“Medicare won’t pay for services that aren’t supported by medical necessity,” says LeGrand, “and you can’t achieve medical necessity by simply documenting additional E/M elements.”
This has always been the rule, LeGrand says. “But with the increased use of EHRs, and templates that automatically document visit elements and drive visits to a higher level of service, the Centers for Medicare & Medicaid Services [CMS] and private payers have added scrutiny to medical necessity reviews. They want to validate that higher-level visits billed indeed required a higher level of history and/or exam.”
To do this, the Office of the Inspector General (OIG) has supplemented its audit team with registered nurses. “The nurses assist certified coders by determining whether medical necessity has been met,” explains LeGrand.
Look at a patient who presents with toe pain. You take a detailed family history, conduct a review of systems (ROS), bill a high-level code, and document all the elements to support it. LeGrand explains, “There is no medical necessity to support doing an eye exam for a patient with toe pain in the absence of any other medical history, or performing a ROS to correlate an eye exam with toe pain. So, even if you do it and document it, the higher-level code won’t pass muster in an audit because the information documented is not medically necessary.”
According to LeGrand, the extent of the history and examination should be based on the presenting problem and the patient’s condition. “If an ankle sprain patient returns 2 weeks after the initial evaluation of the injury with a negative medical or surgical history, and the patient has been treated conservatively, it’s probably not necessary to conduct a ROS that includes 10 organ systems,” she says. “If your standard of care is to perform this level of service, no one will fault you for your care delivery; however, if you also choose a level of service based on this system review, without relevance to the presenting problem, and you bill a higher level of service than is supported by the nature of the presenting problem or the plan of care, the documentation probably won’t hold up in an audit where medical necessity is valued into the equation.”
On the other hand, LeGrand adds, if a patient presents to the emergency department after an automobile accident with an open fracture and other injuries, and the surgeon performs a complete ROS, the medical necessity would most likely be supported as the surgeon is preparing the patient for surgery.
Based on LeGrand’s work with practices, this distinction about medical necessity is news to many nonclinical billing staff. “They confuse medical necessity with medical decision-making, an E/M code documentation component, and incorrectly bill for a high-level visit because medical decision-making elements meet the documentation requirements—yet the code is not supported by medical necessity of the presenting problem.”
Talk with your billing team to make sure all staff members understand this critical difference. They must comprehend that the medically necessary level of service is determined by a number of clinical factors, not medical decision-making. Describe some of these clinical factors, which include, but are not limited to, chief complaint, clinical judgment, standards of practice, acute exacerbations/onsets of medical conditions or injuries, and comorbidities.
EHR Dos and Don’ts
LeGrand recommends the following best practices for using EHR documentation features:
1. DON’T simply cut and paste from a previous note. “This is what leads to verbose notes that have little to do with the patient you are documenting,” she says. “If you don’t cut and paste, you’ll avoid the root cause of this risk.”
2. DON’T pull forward information from previous visit notes that have nothing to do with the nature of the patient’s problem. “We understand that this takes extra time because physicians must review the previous note,” LeGrand says. “So if you don’t have time to review the past note, just don’t pull it forward. Start fresh with a new drop-down menu and select elements pertinent to the current visit. Or, dictate or type a note relevant to the current condition and presenting problems.”
How you choose to work this into your process will vary depending on which EHR system you use. “One surgeon I work with dictates everything because the drop-down menus and templates are cumbersome,” LeGrand says. “Some groups find it faster to use the EHR templates that they have customized. Others find their EHR’s point-and-click features most efficient for customizing quickly.”
3. DO customize your EHR visit templates if the use of templates is critical to your efficiency. “This is the most overlooked step in the EHR implementation process because it takes a fair amount of time to do,” LeGrand says. She suggests avoiding the use of multisystem examination templates created for medicine specialties altogether, and insists, “Don’t assume ‘that is how the vendor built it so we have to use it.’ Customize a template for each of your visit types so you can document in the EHR in the same fashion as when you used a paper system. Doing so will save you loads of documentation time.”
4. DO review your E/M code distribution. Generate a CPT frequency report for each physician and for the practice as a whole. Compare the data with state and national usage in orthopedics as a baseline. The American Academy of Orthopaedic Surgeon’s Code-X tool enables easy comparison of your practice’s E/M code usage with state and national data for orthopedics. Simply generate a CPT frequency report from your practice management system and enter the E/M data. Line graphs are automatically generated, making trends and patterns easy to see (Figure).
“Identify your outliers, pull charts randomly, and review the notes,” recommends LeGrand. “Make sure there is medical necessity for the level of code that’s been billed and that documentation supports it.”
You may be surprised to find you are an outlier on inpatient hospital codes, or your distribution of level-2 or -3 codes varies from your practice, state, or national data. Orthopedic surgeons don’t typically report high volumes of CPT codes 99204, 99205 or 99215, but if your practice does and you are an outlier, best to pay attention before someone else does.
5. DO select auditors with the right skill sets. Evaluating medical necessity in the note requires a clinical background. “If internal documentation reviews are conducted by the billing team, that’s fine,” LeGrand advises. “Just add a physician assistant or nurse to your internal review team. They can provide clinical oversight and review the note when necessary for medical necessity.”
If you are contracting with external auditors or consultants, verify auditor credentials and skill sets to ensure they can abstract and incorporate medical necessity into the review. “Auditors must be able to do more than count elements,” LeGrand says. “They must have clinical knowledge, and expertise in orthopedics is critical. This knowledge should be used to verify that medical necessity is present in every note.” LeGrand is quick to point out that not every note will be at risk, based on the amount of work performed and documented and the level of service billed. “But medical necessity must always be present.”
The addition of nurses to the OIG’s audit team is a big change and will refine the auditing process by adding more clinical scrutiny. The EHR documentation features are intended to improve efficiency, but only a clinician can determine and document unique visit elements and medical necessity.
Address these intersections of risk by ensuring your documentation meets medical necessity as well as E/M documentation elements. Conduct internal audits bi-annually to verify that E/M usage patterns align with peers and physician documentation is appropriate. And be sure there is clinical expertise on your audit team, whether it is internal or external. CMS now has it, and your practice should too. ◾
A group of 3 busy orthopedists attended coding education each year and did their best to accurately code and document their services. As a risk-reduction strategy, the group engaged our firm to conduct an audit to determine whether they were documenting their services properly and to provide feedback about how they could improve.
What we found was shocking to the surgeons, but all too common, as we review thousands of orthopedic visit notes every year: The same examination had been documented for all visits, with physicians stating in their notes that the examination was medically necessary. In addition, their documentation supported Current Procedural Terminology (CPT) code 99214 at every visit, with visit frequencies of 2 weeks to 4 months.
The culprit of all this sameness? The practice’s electronic health record (EHR).
“Practices with EHRs often have a large volume of visit notes that look almost identical for a patient who is seen for multiple visits,” explains Mary LeGrand, RN, MA, CCS-P, CPC, KarenZupko & Associates consultant and coding educator. “And that is putting physicians at higher risk of being audited or of not passing an audit.”
According to LeGrand, this is because physicians are using the practice’s EHR to “pull forward” the patient’s previous visit note for the current visit, but failing to customize it for the current visit. The unintended consequence of this workflow efficiency is twofold:
1. It creates documentation that looks strikingly similar to, if not exactly like, the patient’s last billed visit note. This is often referred to as note “cloning.”
2. It creates documentation that includes a lot of unnecessary detail that, even if delivered and documented, doesn’t match the medical necessity of the visit, based on the history of present illness statements.
Both of these things can come back to bite you.
Zero in on the Risk
If your practice has an EHR, it is important that you evaluate whether certain workflow efficiency features are putting the practice at risk. You do not necessarily need to dump the EHR, but you may need to take action to reduce the risk of using these features.
In a pre-EHR practice, physicians began each visit with a blank piece of paper or dictated the entire visit. Then along came EHR vendors who, in an effort to make things easier and more efficient, created visit templates and the ability to “pull forward” the last visit note and use it as a basis for the current visit. The intention was always that physicians would modify it based on the current visit. But the reality is that physicians are busy, editing is time-consuming, and the unintended consequence is cloning.
“If you pull in unnecessary history or exam information from a previous visit that’s not relevant to the current visit, you can get dinged in an audit for not customizing the note to the patient’s specific presenting complaint,” LeGrand explains, “or, for attempting to bill a higher-level code by unintentionally padding the note with irrelevant information. What is documented for ‘reference’ has to be separated from what can be used to select the level of service.”
Your first documentation risk-reduction strategy is to review notes and look for signs of cloning.
LeGrand explains that a practice may be predisposed to cloning simply because of the way the EHR templates and workflow were set up when the system was implemented. “But,” she says, “‘the EHR made me do it’ defense won’t hold water, because it’s still the physician’s responsibility to customize or remove the information from templates and make the note unique to the visit.”
Yes, physician time is precious. But the reality is that the onus is on the physician to integrate EHR features with clinic workflow and to follow documentation rules.
The second documentation risk-reduction strategy is to make sure the level of evaluation and management (E/M) service billed is supported by medical necessity, not only by documentation artifacts that were relevant to the patient in the past but irrelevant to his or her current presenting complaint or condition.
“Medicare won’t pay for services that aren’t supported by medical necessity,” says LeGrand, “and you can’t achieve medical necessity by simply documenting additional E/M elements.”
This has always been the rule, LeGrand says. “But with the increased use of EHRs, and templates that automatically document visit elements and drive visits to a higher level of service, the Centers for Medicare & Medicaid Services [CMS] and private payers have added scrutiny to medical necessity reviews. They want to validate that higher-level visits billed indeed required a higher level of history and/or exam.”
To do this, the Office of the Inspector General (OIG) has supplemented its audit team with registered nurses. “The nurses assist certified coders by determining whether medical necessity has been met,” explains LeGrand.
Look at a patient who presents with toe pain. You take a detailed family history, conduct a review of systems (ROS), bill a high-level code, and document all the elements to support it. LeGrand explains, “There is no medical necessity to support doing an eye exam for a patient with toe pain in the absence of any other medical history, or performing a ROS to correlate an eye exam with toe pain. So, even if you do it and document it, the higher-level code won’t pass muster in an audit because the information documented is not medically necessary.”
According to LeGrand, the extent of the history and examination should be based on the presenting problem and the patient’s condition. “If an ankle sprain patient returns 2 weeks after the initial evaluation of the injury with a negative medical or surgical history, and the patient has been treated conservatively, it’s probably not necessary to conduct a ROS that includes 10 organ systems,” she says. “If your standard of care is to perform this level of service, no one will fault you for your care delivery; however, if you also choose a level of service based on this system review, without relevance to the presenting problem, and you bill a higher level of service than is supported by the nature of the presenting problem or the plan of care, the documentation probably won’t hold up in an audit where medical necessity is valued into the equation.”
On the other hand, LeGrand adds, if a patient presents to the emergency department after an automobile accident with an open fracture and other injuries, and the surgeon performs a complete ROS, the medical necessity would most likely be supported as the surgeon is preparing the patient for surgery.
Based on LeGrand’s work with practices, this distinction about medical necessity is news to many nonclinical billing staff. “They confuse medical necessity with medical decision-making, an E/M code documentation component, and incorrectly bill for a high-level visit because medical decision-making elements meet the documentation requirements—yet the code is not supported by medical necessity of the presenting problem.”
Talk with your billing team to make sure all staff members understand this critical difference. They must comprehend that the medically necessary level of service is determined by a number of clinical factors, not medical decision-making. Describe some of these clinical factors, which include, but are not limited to, chief complaint, clinical judgment, standards of practice, acute exacerbations/onsets of medical conditions or injuries, and comorbidities.
EHR Dos and Don’ts
LeGrand recommends the following best practices for using EHR documentation features:
1. DON’T simply cut and paste from a previous note. “This is what leads to verbose notes that have little to do with the patient you are documenting,” she says. “If you don’t cut and paste, you’ll avoid the root cause of this risk.”
2. DON’T pull forward information from previous visit notes that have nothing to do with the nature of the patient’s problem. “We understand that this takes extra time because physicians must review the previous note,” LeGrand says. “So if you don’t have time to review the past note, just don’t pull it forward. Start fresh with a new drop-down menu and select elements pertinent to the current visit. Or, dictate or type a note relevant to the current condition and presenting problems.”
How you choose to work this into your process will vary depending on which EHR system you use. “One surgeon I work with dictates everything because the drop-down menus and templates are cumbersome,” LeGrand says. “Some groups find it faster to use the EHR templates that they have customized. Others find their EHR’s point-and-click features most efficient for customizing quickly.”
3. DO customize your EHR visit templates if the use of templates is critical to your efficiency. “This is the most overlooked step in the EHR implementation process because it takes a fair amount of time to do,” LeGrand says. She suggests avoiding the use of multisystem examination templates created for medicine specialties altogether, and insists, “Don’t assume ‘that is how the vendor built it so we have to use it.’ Customize a template for each of your visit types so you can document in the EHR in the same fashion as when you used a paper system. Doing so will save you loads of documentation time.”
4. DO review your E/M code distribution. Generate a CPT frequency report for each physician and for the practice as a whole. Compare the data with state and national usage in orthopedics as a baseline. The American Academy of Orthopaedic Surgeon’s Code-X tool enables easy comparison of your practice’s E/M code usage with state and national data for orthopedics. Simply generate a CPT frequency report from your practice management system and enter the E/M data. Line graphs are automatically generated, making trends and patterns easy to see (Figure).
“Identify your outliers, pull charts randomly, and review the notes,” recommends LeGrand. “Make sure there is medical necessity for the level of code that’s been billed and that documentation supports it.”
You may be surprised to find you are an outlier on inpatient hospital codes, or your distribution of level-2 or -3 codes varies from your practice, state, or national data. Orthopedic surgeons don’t typically report high volumes of CPT codes 99204, 99205 or 99215, but if your practice does and you are an outlier, best to pay attention before someone else does.
5. DO select auditors with the right skill sets. Evaluating medical necessity in the note requires a clinical background. “If internal documentation reviews are conducted by the billing team, that’s fine,” LeGrand advises. “Just add a physician assistant or nurse to your internal review team. They can provide clinical oversight and review the note when necessary for medical necessity.”
If you are contracting with external auditors or consultants, verify auditor credentials and skill sets to ensure they can abstract and incorporate medical necessity into the review. “Auditors must be able to do more than count elements,” LeGrand says. “They must have clinical knowledge, and expertise in orthopedics is critical. This knowledge should be used to verify that medical necessity is present in every note.” LeGrand is quick to point out that not every note will be at risk, based on the amount of work performed and documented and the level of service billed. “But medical necessity must always be present.”
The addition of nurses to the OIG’s audit team is a big change and will refine the auditing process by adding more clinical scrutiny. The EHR documentation features are intended to improve efficiency, but only a clinician can determine and document unique visit elements and medical necessity.
Address these intersections of risk by ensuring your documentation meets medical necessity as well as E/M documentation elements. Conduct internal audits bi-annually to verify that E/M usage patterns align with peers and physician documentation is appropriate. And be sure there is clinical expertise on your audit team, whether it is internal or external. CMS now has it, and your practice should too. ◾
A group of 3 busy orthopedists attended coding education each year and did their best to accurately code and document their services. As a risk-reduction strategy, the group engaged our firm to conduct an audit to determine whether they were documenting their services properly and to provide feedback about how they could improve.
What we found was shocking to the surgeons, but all too common, as we review thousands of orthopedic visit notes every year: The same examination had been documented for all visits, with physicians stating in their notes that the examination was medically necessary. In addition, their documentation supported Current Procedural Terminology (CPT) code 99214 at every visit, with visit frequencies of 2 weeks to 4 months.
The culprit of all this sameness? The practice’s electronic health record (EHR).
“Practices with EHRs often have a large volume of visit notes that look almost identical for a patient who is seen for multiple visits,” explains Mary LeGrand, RN, MA, CCS-P, CPC, KarenZupko & Associates consultant and coding educator. “And that is putting physicians at higher risk of being audited or of not passing an audit.”
According to LeGrand, this is because physicians are using the practice’s EHR to “pull forward” the patient’s previous visit note for the current visit, but failing to customize it for the current visit. The unintended consequence of this workflow efficiency is twofold:
1. It creates documentation that looks strikingly similar to, if not exactly like, the patient’s last billed visit note. This is often referred to as note “cloning.”
2. It creates documentation that includes a lot of unnecessary detail that, even if delivered and documented, doesn’t match the medical necessity of the visit, based on the history of present illness statements.
Both of these things can come back to bite you.
Zero in on the Risk
If your practice has an EHR, it is important that you evaluate whether certain workflow efficiency features are putting the practice at risk. You do not necessarily need to dump the EHR, but you may need to take action to reduce the risk of using these features.
In a pre-EHR practice, physicians began each visit with a blank piece of paper or dictated the entire visit. Then along came EHR vendors who, in an effort to make things easier and more efficient, created visit templates and the ability to “pull forward” the last visit note and use it as a basis for the current visit. The intention was always that physicians would modify it based on the current visit. But the reality is that physicians are busy, editing is time-consuming, and the unintended consequence is cloning.
“If you pull in unnecessary history or exam information from a previous visit that’s not relevant to the current visit, you can get dinged in an audit for not customizing the note to the patient’s specific presenting complaint,” LeGrand explains, “or, for attempting to bill a higher-level code by unintentionally padding the note with irrelevant information. What is documented for ‘reference’ has to be separated from what can be used to select the level of service.”
Your first documentation risk-reduction strategy is to review notes and look for signs of cloning.
LeGrand explains that a practice may be predisposed to cloning simply because of the way the EHR templates and workflow were set up when the system was implemented. “But,” she says, “‘the EHR made me do it’ defense won’t hold water, because it’s still the physician’s responsibility to customize or remove the information from templates and make the note unique to the visit.”
Yes, physician time is precious. But the reality is that the onus is on the physician to integrate EHR features with clinic workflow and to follow documentation rules.
The second documentation risk-reduction strategy is to make sure the level of evaluation and management (E/M) service billed is supported by medical necessity, not only by documentation artifacts that were relevant to the patient in the past but irrelevant to his or her current presenting complaint or condition.
“Medicare won’t pay for services that aren’t supported by medical necessity,” says LeGrand, “and you can’t achieve medical necessity by simply documenting additional E/M elements.”
This has always been the rule, LeGrand says. “But with the increased use of EHRs, and templates that automatically document visit elements and drive visits to a higher level of service, the Centers for Medicare & Medicaid Services [CMS] and private payers have added scrutiny to medical necessity reviews. They want to validate that higher-level visits billed indeed required a higher level of history and/or exam.”
To do this, the Office of the Inspector General (OIG) has supplemented its audit team with registered nurses. “The nurses assist certified coders by determining whether medical necessity has been met,” explains LeGrand.
Look at a patient who presents with toe pain. You take a detailed family history, conduct a review of systems (ROS), bill a high-level code, and document all the elements to support it. LeGrand explains, “There is no medical necessity to support doing an eye exam for a patient with toe pain in the absence of any other medical history, or performing a ROS to correlate an eye exam with toe pain. So, even if you do it and document it, the higher-level code won’t pass muster in an audit because the information documented is not medically necessary.”
According to LeGrand, the extent of the history and examination should be based on the presenting problem and the patient’s condition. “If an ankle sprain patient returns 2 weeks after the initial evaluation of the injury with a negative medical or surgical history, and the patient has been treated conservatively, it’s probably not necessary to conduct a ROS that includes 10 organ systems,” she says. “If your standard of care is to perform this level of service, no one will fault you for your care delivery; however, if you also choose a level of service based on this system review, without relevance to the presenting problem, and you bill a higher level of service than is supported by the nature of the presenting problem or the plan of care, the documentation probably won’t hold up in an audit where medical necessity is valued into the equation.”
On the other hand, LeGrand adds, if a patient presents to the emergency department after an automobile accident with an open fracture and other injuries, and the surgeon performs a complete ROS, the medical necessity would most likely be supported as the surgeon is preparing the patient for surgery.
Based on LeGrand’s work with practices, this distinction about medical necessity is news to many nonclinical billing staff. “They confuse medical necessity with medical decision-making, an E/M code documentation component, and incorrectly bill for a high-level visit because medical decision-making elements meet the documentation requirements—yet the code is not supported by medical necessity of the presenting problem.”
Talk with your billing team to make sure all staff members understand this critical difference. They must comprehend that the medically necessary level of service is determined by a number of clinical factors, not medical decision-making. Describe some of these clinical factors, which include, but are not limited to, chief complaint, clinical judgment, standards of practice, acute exacerbations/onsets of medical conditions or injuries, and comorbidities.
EHR Dos and Don’ts
LeGrand recommends the following best practices for using EHR documentation features:
1. DON’T simply cut and paste from a previous note. “This is what leads to verbose notes that have little to do with the patient you are documenting,” she says. “If you don’t cut and paste, you’ll avoid the root cause of this risk.”
2. DON’T pull forward information from previous visit notes that have nothing to do with the nature of the patient’s problem. “We understand that this takes extra time because physicians must review the previous note,” LeGrand says. “So if you don’t have time to review the past note, just don’t pull it forward. Start fresh with a new drop-down menu and select elements pertinent to the current visit. Or, dictate or type a note relevant to the current condition and presenting problems.”
How you choose to work this into your process will vary depending on which EHR system you use. “One surgeon I work with dictates everything because the drop-down menus and templates are cumbersome,” LeGrand says. “Some groups find it faster to use the EHR templates that they have customized. Others find their EHR’s point-and-click features most efficient for customizing quickly.”
3. DO customize your EHR visit templates if the use of templates is critical to your efficiency. “This is the most overlooked step in the EHR implementation process because it takes a fair amount of time to do,” LeGrand says. She suggests avoiding the use of multisystem examination templates created for medicine specialties altogether, and insists, “Don’t assume ‘that is how the vendor built it so we have to use it.’ Customize a template for each of your visit types so you can document in the EHR in the same fashion as when you used a paper system. Doing so will save you loads of documentation time.”
4. DO review your E/M code distribution. Generate a CPT frequency report for each physician and for the practice as a whole. Compare the data with state and national usage in orthopedics as a baseline. The American Academy of Orthopaedic Surgeon’s Code-X tool enables easy comparison of your practice’s E/M code usage with state and national data for orthopedics. Simply generate a CPT frequency report from your practice management system and enter the E/M data. Line graphs are automatically generated, making trends and patterns easy to see (Figure).
“Identify your outliers, pull charts randomly, and review the notes,” recommends LeGrand. “Make sure there is medical necessity for the level of code that’s been billed and that documentation supports it.”
You may be surprised to find you are an outlier on inpatient hospital codes, or your distribution of level-2 or -3 codes varies from your practice, state, or national data. Orthopedic surgeons don’t typically report high volumes of CPT codes 99204, 99205 or 99215, but if your practice does and you are an outlier, best to pay attention before someone else does.
5. DO select auditors with the right skill sets. Evaluating medical necessity in the note requires a clinical background. “If internal documentation reviews are conducted by the billing team, that’s fine,” LeGrand advises. “Just add a physician assistant or nurse to your internal review team. They can provide clinical oversight and review the note when necessary for medical necessity.”
If you are contracting with external auditors or consultants, verify auditor credentials and skill sets to ensure they can abstract and incorporate medical necessity into the review. “Auditors must be able to do more than count elements,” LeGrand says. “They must have clinical knowledge, and expertise in orthopedics is critical. This knowledge should be used to verify that medical necessity is present in every note.” LeGrand is quick to point out that not every note will be at risk, based on the amount of work performed and documented and the level of service billed. “But medical necessity must always be present.”
The addition of nurses to the OIG’s audit team is a big change and will refine the auditing process by adding more clinical scrutiny. The EHR documentation features are intended to improve efficiency, but only a clinician can determine and document unique visit elements and medical necessity.
Address these intersections of risk by ensuring your documentation meets medical necessity as well as E/M documentation elements. Conduct internal audits bi-annually to verify that E/M usage patterns align with peers and physician documentation is appropriate. And be sure there is clinical expertise on your audit team, whether it is internal or external. CMS now has it, and your practice should too. ◾