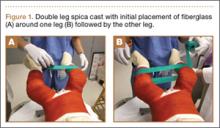
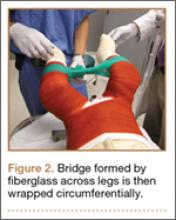
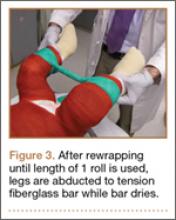
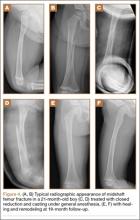
User login
The American Journal of Orthopedics is an Index Medicus publication that is valued by orthopedic surgeons for its peer-reviewed, practice-oriented clinical information. Most articles are written by specialists at leading teaching institutions and help incorporate the latest technology into everyday practice.
Coracoid Fracture After Reverse Total Shoulder Arthroplasty: A Report of 2 Cases
Reverse total shoulder arthroplasty (RTSA) performed in carefully selected patients often leads to satisfactory outcomes.1,2 In recent years, its indications and the number performed per year have expanded. Subsequently, there has been a concomitant rise in reported complications,2,3 with a rate ranging from 19% to 68%.2,3 Some common complications include scapular notching,2-4 fracture,2,3,5-7 dislocation,2,3,7 and infection.2,3,7
In this series, we describe 2 cases of coracoid fracture after RTSA. The patients provided written informed consent for print and electronic publication of these case reports.
Case Series
Case 1
An independently functioning 81-year-old right hand–dominant woman (BMI, 22.1 [height, 160 cm; weight, 56.7 kg]) presented with increasing left shoulder pain and dysfunction after a motor vehicle accident 2 months earlier. She had reported vague chronic left shoulder pain in the past, but after the accident her pain was significantly worse. A subacromial corticosteroid injection by her primary care physician provided temporary symptomatic relief, but her symptoms recurred.
On presentation, there was obvious anterior superior escape of the humeral head, which was accentuated by shoulder shrug. Her deltoid motor function was found to be intact, and her active shoulder range of motion was severely limited (pseudoparesis). There was notable crepitation as well as significant weakness and pain with abduction and external rotation strength testing.
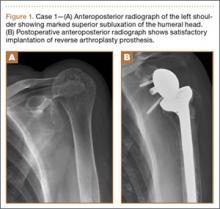
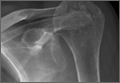
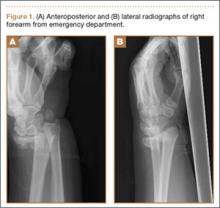
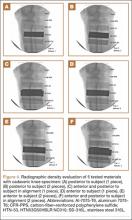
Radiographic imaging showed anterior superior escape of the humeral head with some early degenerative changes (Seebauer type IIB8 [Figure 1A]). Magnetic resonance imaging confirmed a full-thickness retracted massive rotator cuff tear with complete involvement of the supraspinatus, infraspinatus, and most of the subscapularis muscles. Significant glenohumeral degenerative changes consistent with cuff tear arthropathy were also seen without any evidence of fracture.
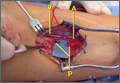
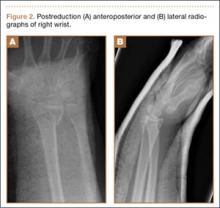
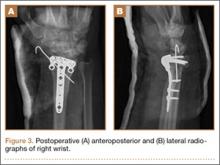
After thorough discussion of options, risks, and benefits, the decision was made to proceed with RTSA. The patient underwent the procedure without complications. A DePuy Delta Xtend prosthesis was used with a cemented humeral stem, polyethylene, and glenosphere, sizes of 12, +3, and 38, respectively. The glenosphere component, positioned inferiorly to avoid scapular notching, was secured with 3 screws, and the stem was placed in neutral version. The patient’s shoulder was reduced, ranged, and noted to be stable, allowing for supple passive range of motion without evidence of excessive tightness. She was placed in a sling with the shoulder positioned in neutral alignment. Her postoperative radiograph (Figure 1B) showed satisfactory implantation of the reverse total shoulder prosthesis. Her postoperative course was uneventful, and rehabilitation consisted of 6 weeks of sling protection, with advancing passive and active range of motion. Strengthening exercises were initiated 6 weeks after surgery.
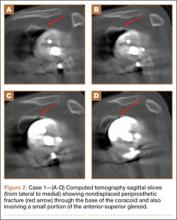
At the patient’s 6-week postoperative visit, she demonstrated pain-free passive elevation to 80° and active forward elevation to 70°. At her 3-month postoperative visit, she reported a 1-week onset of anterior shoulder pain accompanied by a strange noise at the anterior aspect of the operative shoulder. She denied any recent trauma. She continued to have minimal shoulder pain with passive forward flexion of 80°; however, her active forward elevation was very limited because of pain in the anterior aspect of her shoulder. Active external rotation was noted to be 20° and internal rotation was to her buttock. She had pain to palpation of the coracoid process. Radiographs were unchanged from immediate postoperative radiographs. Computed tomography (CT), which was ordered to ensure that the implant was stable with no loosening, showed satisfactory alignment of the prosthesis and no loosening. However, CT was notable for a nondisplaced fracture through the base of the coracoid (Figures 2A-2D). The patient stopped formal physical therapy, and sling immobilization was initiated. After 3 weeks, the sling was discontinued and physical therapy was begun again. She responded satisfactorily to this treatment approach, and, at her 6-month postoperative follow-up, she was without pain, instability, or crepitation. Her range of motion had improved with pain-free active forward flexion, external rotation, and abduction of 100°, 15°, and 90°, respectively. At 28-month postoperative follow-up, her visual analog scale, American Shoulder and Elbow Surgeons score, and Simple Shoulder Test score were 3, 73, and 67, respectively.
Case 2
A 68-year-old, right-handed woman (BMI, 22.5 [height, 160 cm; weight, 57.6 kg]) presented with right shoulder pain and dysfunction of 3 years’ duration. She had undergone an open rotator cuff repair at an outside facility 4 years ago that was unsuccessful. At the time of her presentation to our institution, she had already undergone a failed course of physical therapy. A trial of corticosteroid subacromial injections did not adequately manage her symptoms.
On presentation, her active forward flexion, abduction, and external rotation were 40°, 30°, and 10°, respectively. She had full passive range of motion and pain with active and passive shoulder motion. Radiographic imaging showed superior migration of the humeral head with evidence of glenohumeral arthropathy suggestive of rotator cuff arthropathy (Seebauer type IIA8). After thorough discussion of options, risks, and benefits, the decision was made to proceed with RTSA. She underwent the procedure without complications. A DePuy Delta Xtend prosthesis was used with a cemented humeral stem, polyethylene, and glenosphere, sizes of 8, +3, and 38, respectively. The glenosphere component, positioned inferiorly to avoid scapular notching, was secured with 4 screws, and the stem was placed in neutral version. Her shoulder was reduced, ranged, and noted to be stable, allowing for supple passive range of motion without evidence of excessive tightness. She was placed in a sling with the shoulder positioned in neutral alignment. Her postoperative radiographs revealed satisfactory implantation of the reverse total shoulder prosthesis. Her postoperative course was uneventful. She was taken out of her shoulder immobilizer 4 weeks after surgery and began home-based physical therapy.
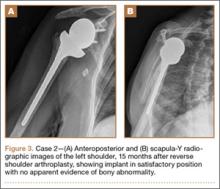
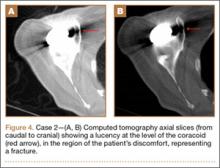
At 1 year after surgery, the patient had minimal shoulder pain with active forward flexion, external rotation, and abduction of 135°, 20°, and 85°, respectively. She presented to our clinic 15 months after RTSA with acute onset of pain about her anterior shoulder. She denied any recent trauma or infectious exposures. On examination, her motion was unchanged from prior examinations. However, she was tender on palpation of the coracoid. Radiographs at that time were unchanged (Figures 3A, 3B). Laboratory tests (erythrocyte sedimentation rate, C-reactive protein, and complete blood count with differential) that were subsequently ordered to rule out an occult infection were within normal limits. Computed tomography, which was ordered for further assessment and to ensure that the implant was stable with no loosening, showed satisfactory alignment of the prosthesis without loosening. However, a lucency was noted in the midportion of the coracoid that was suggestive of a fracture (Figures 4A, 4B). A conservative plan of treatment was advised with sling immobilization for 3 weeks and follow-up visits. The patient responded satisfactorily to this treatment approach, and, at her latest follow-up, 8 months after presenting with a coracoid fracture, she was pain-free. At the 5-year postoperative follow-up, her visual analog scale, American Shoulder and Elbow Surgeons score, and Simple Shoulder Test score were 1-2, 78, and 75, respectively.
Discussion
The reverse prosthesis, a semi-constrained ball-and-socket device, provides satisfactory functional outcomes when used in carefully selected patients with rotator cuff arthropathy and pseudoparalysis, failed shoulder arthroplasty, and fracture sequelae.1,9-11 By the traditional Grammont principles of medializing the center of rotation and lowering the humerus, shear forces about the glenoid are reduced and the deltoid muscle is tensioned, allowing for adequate torque generation, required to facilitate shoulder motion.12,13 While long-term outcomes concerning durability and survivorship are pending, some studies have attempted to improve our understanding of implant and functional longevity. Guery and colleagues14 noted an implant survival of 91% at 120 months. However, increased pain and decreased function were seen at the 6-year mark.14 A more recent study by Cuff and colleagues15 revealed 94% implant survivorship and sustained improvement in range of motion and pain at 5 years.
Despite considerable success, RTSA can be associated with a myriad of complications. The most common complications of RTSA include scapular notching (44%-96%), glenoid side failure (5%-40%), instability (2.4%-31%), and infection (1%-15.3%).2,3 In the setting of inflammatory arthropathy, there is an increased risk for intraoperative and postoperative fractures.16,17 To date, there are only 2 reported cases of coracoid process fractures after RTSA.18,19 In the case by Nolan and colleagues,18 conservative management with a sling for 6 weeks led to successful resolution of symptoms. Although little information is provided on the management of these rare fractures, literature on the slightly more common scapular (0.9%-7.2%) and acromial (0.9%-4.9%) fractures suggest that periscapular fractures are on the rise, may increase the risk for revision surgery, and can lead to inferior outcomes when compared with patients without fractures.5,20,21
Acromial fractures after RTSA have been reported to occur at a rate of 0.9% to 4.9%.5,21 This is a concern because of RTSA reliance on a functional deltoid.5,6 The cause of these fractures remains to be fully elucidated. Wahlquist and colleagues6 in 2011 reported the cases of 5 patients that sustained acromial base fractures after RTSA. All 5 patients were noted to have unsatisfactory functional results despite achieving union (3 were treated with open reduction and internal fixation, and 2 were treated nonoperatively). Acromial fractures tend to present with pain within 6 months of surgery, which may indicate excessive constraint about the scapula, eventually leading to fracture. Furthermore, disruption of this bony structure can lead to devastating results because the acromial base serves as a fulcrum for the deltoid.
Despite a well-placed reverse prosthesis, there is increased reliance on surrounding glenohumeral musculature, resulting from poor rotator cuff function and biomechanical differences compared with a native shoulder. Both our patients were found to have relatively small body habitus. It is possible that, by nature of their smaller statures, they were more susceptible to consequences of excessive joint and soft-tissue tension after RTSA. One explanation for acromial fractures after RTSA is that, by excessively lengthening and/or lateralizing the deltoid, the tension on the acromion in these elderly patients may be sufficient to cause a fracture. A similar mechanism may explain their coracoid fractures. As the arm is lengthened and the prosthesis is tightened, the conjoint tendon is significantly tensioned. We routinely check the tension of these muscles as an extra confirmation of joint stability. However, excessive tension for a significant duration may provide too much stress for bone turnover to match with the inherent repair process, potentially causing a fracture. Recent evidence has also found that bone mineral density of the coracoid diminishes with age, suggesting some predisposition to fracture with lower-energy mechanisms.22
Another possible cause for coracoid fractures may be the orientation of the implants. While we did not have mechanistic evidence, it is possible that, with adduction and internal rotation, prosthetic impingement against the coracoid is feasible, particularly in patients of small stature. Although a glenoid implant placed high can increase the chance for coracoid–implant impingement, the fact that the patients improved without revision makes chronic mechanical impingement less likely. Drill holes, especially multiple ones, placed throughout the base of the coracoid may also predispose to coracoid fractures.
Patients with periscapular fractures (acromion, scapular spine, or coracoid) after RTSA often present with pain and occasional deficits in function. Both patients in this series noted pain out of proportion to examination. The onset of this pain differed, with 1 patient noting pain within the first 3 months and 1 noting discomfort later. Neither patient had any trauma. In the presence of significant symptoms, negative radiographs, and a poor response to conservative treatment, we recommend advanced imaging to rule out fracture. However, prior to obtaining advanced imaging, proper radiographic techniques should be utilized. Eyres and colleagues,23 in a series of 12 fractures of the coracoid process, relied primarily on coracoid views directed 45° in a cephalic direction and thin-slice CT. An isotope bone scan identified 1 case not initially found on radiographs.23
Conservative management with use of a sling until resolution of symptoms was successful in our series. If symptoms persist, a bone stimulator can be used prior to implementing a surgical solution; however, current evidence does not expound on timing and utility of such modalities. Perhaps as important as treatment is education of the patient and the rehabilitation team about the importance of identifying increasing pain as a potential sign of impending fracture in this population. Subsequent activity modification until the pain resolves can help avoid the setback in postoperative recovery that this complication may cause.
Conclusion
We present 2 patients with coracoid fractures encountered at 3 months and 15 months after RTSA. Nonoperative management proved adequate in treating both cases. We suggest a high level of suspicion for possible fracture in the patient who comes in with new-onset pain in a localized region with or without functional deficits.
1. Lawrence TM, Ahmadi S, Sanchez-Sotelo J, Sperling JW, Cofield RH. Patient reported activities after reverse shoulder arthroplasty: part II. J Shoulder Elbow Surg. 2012;21(11):1464-1469.
2. Cheung E, Willis M, Walker M, Clark R, Frankle MA. Complications in reverse total shoulder arthroplasty. J Am Acad Orthop Surg. 2011;19(7):439-449.
3. Affonso J, Nicholson GP, Frankle MA, et al. Complications of the reverse prosthesis: prevention and treatment. Instr Course Lect. 2012;61:157-168.
4. Lévigne C, Garret J, Boileau P, Alami G, Favard L, Walch G. Scapular notching in reverse shoulder arthroplasty: is it important to avoid it and how? Clin Orthop Relat Res. 2011;469(9):2512-2520.
5. Hamid N, Connor PM, Fleischli JF, D’Alessandro DF. Acromial fracture after reverse shoulder arthroplasty. Am J Orthop. 2011;40(7):E125-E129.
6. Wahlquist TC, Hunt AF, Braman JP. Acromial base fractures after reverse total shoulder arthroplasty: report of five cases. J Shoulder Elbow Surg. 2011;20(7):1178-1183.
7. Zumstein MA, Pinedo M, Old J, Boileau P. Problems, complications, reoperations, and revisions in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2011;20(1):146-157.
8. Visotsky JL, Basamania C, Seebauer L, Rockwood CA, Jensen KL. Cuff tear arthropathy: pathogenesis, classification, and algorithm for treatment. J Bone Joint Surg Am. 2004;86(suppl 2):35-40.
9. Gamradt SC, Gelber J, Zhang AL. Shoulder function and pain level after revision of failed reverse shoulder replacement to hemiarthroplasty. Int J Shoulder Surg. 2012;6(2):29-35.
10. Garrigues GE, Johnston PS, Pepe MD, Tucker BS, Ramsey ML, Austin LS. Hemiarthroplasty versus reverse total shoulder arthroplasty for acute proximal humerus fractures in elderly patients. Orthopedics. 2012;35(5):e703-e708.
11. Patel DN, Young B, Onyekwelu I, Zuckerman JD, Kwon YW. Reverse total shoulder arthroplasty for failed shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(11):1473-1483.
12. Grammont PM, Baulot E. The classic: Delta shoulder prosthesis for rotator cuff rupture. 1993. Clin Orthop Relat Res. 2011;469(9):2424.
13. Schwartz DG, Kang SH, Lynch TS, et al. The anterior deltoid’s importance in reverse shoulder arthroplasty: a cadaveric biomechanical study. J Shoulder Elbow Surg. 2013;22(3):357-364.
14. Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty. Survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am. 2006;88(8):1742-1747.
15. Cuff D, Clark R, Pupello D, Frankle M. Reverse shoulder arthroplasty for the treatment of rotator cuff deficiency: a concise follow-up, at a minimum of five years, of a previous report. J Bone Joint Surg Am. 2012;94(21):1996-2000.
16. Young AA, Smith MM, Bacle G, Moraga C, Walch G. Early results of reverse shoulder arthroplasty in patients with rheumatoid arthritis. J Bone Joint Surg. 2011;93(20):1915-1923.
17. Hattrup SJ, Sanchez-Sotelo J, Sperling JW, Cofield RH. Reverse shoulder replacement for patients with inflammatory arthritis. J Hand Surg Am. 2012;37(9):1888-1894.
18. Nolan BM, Ankerson E, Wiater JM. Reverse total shoulder arthroplasty improves function in cuff tear arthropathy. Clin Orthop Relat Res. 2011;469(9):2476-2482.
19. Stechel A, Fuhrmann U, Irlenbusch L, Rott O, Irlenbusch U. Reversed shoulder arthroplasty in cuff tear arthritis, fracture sequelae, and revision arthroplasty. Acta Orthop. 2010;81(3):367-372.
20. Teusink MJ, Otto RJ, Cottrell BJ, Frankle MA. What is the effect of postoperative scapular fracture on outcomes of reverse shoulder arthroplasty? J Shoulder Elbow Surg. 2014;23(6):782-790.
21. Walch G, Bacle G, Lädermann A, Nové-Josserand L, Smithers CJ. Do the indications, results, and complications of reverse shoulder arthroplasty change with surgeon’s experience? J Shoulder Elbow Surg. 2012;21(11):1470-1477.
22. Beranger JS, Maqdes A, Pujol N, Desmoineaux P, Beaufils P. Bone mineral density of the coracoid process decreases with age [published online ahead of print December 17, 2014]. Knee Surg Sports Traumatol Arthrosc.
23. Eyres KS, Brooks A, Stanley D. Fractures of the coracoid process. J Bone Joint Surg Br. 1995;77(3):425-428.
Reverse total shoulder arthroplasty (RTSA) performed in carefully selected patients often leads to satisfactory outcomes.1,2 In recent years, its indications and the number performed per year have expanded. Subsequently, there has been a concomitant rise in reported complications,2,3 with a rate ranging from 19% to 68%.2,3 Some common complications include scapular notching,2-4 fracture,2,3,5-7 dislocation,2,3,7 and infection.2,3,7
In this series, we describe 2 cases of coracoid fracture after RTSA. The patients provided written informed consent for print and electronic publication of these case reports.
Case Series
Case 1
An independently functioning 81-year-old right hand–dominant woman (BMI, 22.1 [height, 160 cm; weight, 56.7 kg]) presented with increasing left shoulder pain and dysfunction after a motor vehicle accident 2 months earlier. She had reported vague chronic left shoulder pain in the past, but after the accident her pain was significantly worse. A subacromial corticosteroid injection by her primary care physician provided temporary symptomatic relief, but her symptoms recurred.
On presentation, there was obvious anterior superior escape of the humeral head, which was accentuated by shoulder shrug. Her deltoid motor function was found to be intact, and her active shoulder range of motion was severely limited (pseudoparesis). There was notable crepitation as well as significant weakness and pain with abduction and external rotation strength testing.
Radiographic imaging showed anterior superior escape of the humeral head with some early degenerative changes (Seebauer type IIB8 [Figure 1A]). Magnetic resonance imaging confirmed a full-thickness retracted massive rotator cuff tear with complete involvement of the supraspinatus, infraspinatus, and most of the subscapularis muscles. Significant glenohumeral degenerative changes consistent with cuff tear arthropathy were also seen without any evidence of fracture.
After thorough discussion of options, risks, and benefits, the decision was made to proceed with RTSA. The patient underwent the procedure without complications. A DePuy Delta Xtend prosthesis was used with a cemented humeral stem, polyethylene, and glenosphere, sizes of 12, +3, and 38, respectively. The glenosphere component, positioned inferiorly to avoid scapular notching, was secured with 3 screws, and the stem was placed in neutral version. The patient’s shoulder was reduced, ranged, and noted to be stable, allowing for supple passive range of motion without evidence of excessive tightness. She was placed in a sling with the shoulder positioned in neutral alignment. Her postoperative radiograph (Figure 1B) showed satisfactory implantation of the reverse total shoulder prosthesis. Her postoperative course was uneventful, and rehabilitation consisted of 6 weeks of sling protection, with advancing passive and active range of motion. Strengthening exercises were initiated 6 weeks after surgery.
At the patient’s 6-week postoperative visit, she demonstrated pain-free passive elevation to 80° and active forward elevation to 70°. At her 3-month postoperative visit, she reported a 1-week onset of anterior shoulder pain accompanied by a strange noise at the anterior aspect of the operative shoulder. She denied any recent trauma. She continued to have minimal shoulder pain with passive forward flexion of 80°; however, her active forward elevation was very limited because of pain in the anterior aspect of her shoulder. Active external rotation was noted to be 20° and internal rotation was to her buttock. She had pain to palpation of the coracoid process. Radiographs were unchanged from immediate postoperative radiographs. Computed tomography (CT), which was ordered to ensure that the implant was stable with no loosening, showed satisfactory alignment of the prosthesis and no loosening. However, CT was notable for a nondisplaced fracture through the base of the coracoid (Figures 2A-2D). The patient stopped formal physical therapy, and sling immobilization was initiated. After 3 weeks, the sling was discontinued and physical therapy was begun again. She responded satisfactorily to this treatment approach, and, at her 6-month postoperative follow-up, she was without pain, instability, or crepitation. Her range of motion had improved with pain-free active forward flexion, external rotation, and abduction of 100°, 15°, and 90°, respectively. At 28-month postoperative follow-up, her visual analog scale, American Shoulder and Elbow Surgeons score, and Simple Shoulder Test score were 3, 73, and 67, respectively.
Case 2
A 68-year-old, right-handed woman (BMI, 22.5 [height, 160 cm; weight, 57.6 kg]) presented with right shoulder pain and dysfunction of 3 years’ duration. She had undergone an open rotator cuff repair at an outside facility 4 years ago that was unsuccessful. At the time of her presentation to our institution, she had already undergone a failed course of physical therapy. A trial of corticosteroid subacromial injections did not adequately manage her symptoms.
On presentation, her active forward flexion, abduction, and external rotation were 40°, 30°, and 10°, respectively. She had full passive range of motion and pain with active and passive shoulder motion. Radiographic imaging showed superior migration of the humeral head with evidence of glenohumeral arthropathy suggestive of rotator cuff arthropathy (Seebauer type IIA8). After thorough discussion of options, risks, and benefits, the decision was made to proceed with RTSA. She underwent the procedure without complications. A DePuy Delta Xtend prosthesis was used with a cemented humeral stem, polyethylene, and glenosphere, sizes of 8, +3, and 38, respectively. The glenosphere component, positioned inferiorly to avoid scapular notching, was secured with 4 screws, and the stem was placed in neutral version. Her shoulder was reduced, ranged, and noted to be stable, allowing for supple passive range of motion without evidence of excessive tightness. She was placed in a sling with the shoulder positioned in neutral alignment. Her postoperative radiographs revealed satisfactory implantation of the reverse total shoulder prosthesis. Her postoperative course was uneventful. She was taken out of her shoulder immobilizer 4 weeks after surgery and began home-based physical therapy.
At 1 year after surgery, the patient had minimal shoulder pain with active forward flexion, external rotation, and abduction of 135°, 20°, and 85°, respectively. She presented to our clinic 15 months after RTSA with acute onset of pain about her anterior shoulder. She denied any recent trauma or infectious exposures. On examination, her motion was unchanged from prior examinations. However, she was tender on palpation of the coracoid. Radiographs at that time were unchanged (Figures 3A, 3B). Laboratory tests (erythrocyte sedimentation rate, C-reactive protein, and complete blood count with differential) that were subsequently ordered to rule out an occult infection were within normal limits. Computed tomography, which was ordered for further assessment and to ensure that the implant was stable with no loosening, showed satisfactory alignment of the prosthesis without loosening. However, a lucency was noted in the midportion of the coracoid that was suggestive of a fracture (Figures 4A, 4B). A conservative plan of treatment was advised with sling immobilization for 3 weeks and follow-up visits. The patient responded satisfactorily to this treatment approach, and, at her latest follow-up, 8 months after presenting with a coracoid fracture, she was pain-free. At the 5-year postoperative follow-up, her visual analog scale, American Shoulder and Elbow Surgeons score, and Simple Shoulder Test score were 1-2, 78, and 75, respectively.
Discussion
The reverse prosthesis, a semi-constrained ball-and-socket device, provides satisfactory functional outcomes when used in carefully selected patients with rotator cuff arthropathy and pseudoparalysis, failed shoulder arthroplasty, and fracture sequelae.1,9-11 By the traditional Grammont principles of medializing the center of rotation and lowering the humerus, shear forces about the glenoid are reduced and the deltoid muscle is tensioned, allowing for adequate torque generation, required to facilitate shoulder motion.12,13 While long-term outcomes concerning durability and survivorship are pending, some studies have attempted to improve our understanding of implant and functional longevity. Guery and colleagues14 noted an implant survival of 91% at 120 months. However, increased pain and decreased function were seen at the 6-year mark.14 A more recent study by Cuff and colleagues15 revealed 94% implant survivorship and sustained improvement in range of motion and pain at 5 years.
Despite considerable success, RTSA can be associated with a myriad of complications. The most common complications of RTSA include scapular notching (44%-96%), glenoid side failure (5%-40%), instability (2.4%-31%), and infection (1%-15.3%).2,3 In the setting of inflammatory arthropathy, there is an increased risk for intraoperative and postoperative fractures.16,17 To date, there are only 2 reported cases of coracoid process fractures after RTSA.18,19 In the case by Nolan and colleagues,18 conservative management with a sling for 6 weeks led to successful resolution of symptoms. Although little information is provided on the management of these rare fractures, literature on the slightly more common scapular (0.9%-7.2%) and acromial (0.9%-4.9%) fractures suggest that periscapular fractures are on the rise, may increase the risk for revision surgery, and can lead to inferior outcomes when compared with patients without fractures.5,20,21
Acromial fractures after RTSA have been reported to occur at a rate of 0.9% to 4.9%.5,21 This is a concern because of RTSA reliance on a functional deltoid.5,6 The cause of these fractures remains to be fully elucidated. Wahlquist and colleagues6 in 2011 reported the cases of 5 patients that sustained acromial base fractures after RTSA. All 5 patients were noted to have unsatisfactory functional results despite achieving union (3 were treated with open reduction and internal fixation, and 2 were treated nonoperatively). Acromial fractures tend to present with pain within 6 months of surgery, which may indicate excessive constraint about the scapula, eventually leading to fracture. Furthermore, disruption of this bony structure can lead to devastating results because the acromial base serves as a fulcrum for the deltoid.
Despite a well-placed reverse prosthesis, there is increased reliance on surrounding glenohumeral musculature, resulting from poor rotator cuff function and biomechanical differences compared with a native shoulder. Both our patients were found to have relatively small body habitus. It is possible that, by nature of their smaller statures, they were more susceptible to consequences of excessive joint and soft-tissue tension after RTSA. One explanation for acromial fractures after RTSA is that, by excessively lengthening and/or lateralizing the deltoid, the tension on the acromion in these elderly patients may be sufficient to cause a fracture. A similar mechanism may explain their coracoid fractures. As the arm is lengthened and the prosthesis is tightened, the conjoint tendon is significantly tensioned. We routinely check the tension of these muscles as an extra confirmation of joint stability. However, excessive tension for a significant duration may provide too much stress for bone turnover to match with the inherent repair process, potentially causing a fracture. Recent evidence has also found that bone mineral density of the coracoid diminishes with age, suggesting some predisposition to fracture with lower-energy mechanisms.22
Another possible cause for coracoid fractures may be the orientation of the implants. While we did not have mechanistic evidence, it is possible that, with adduction and internal rotation, prosthetic impingement against the coracoid is feasible, particularly in patients of small stature. Although a glenoid implant placed high can increase the chance for coracoid–implant impingement, the fact that the patients improved without revision makes chronic mechanical impingement less likely. Drill holes, especially multiple ones, placed throughout the base of the coracoid may also predispose to coracoid fractures.
Patients with periscapular fractures (acromion, scapular spine, or coracoid) after RTSA often present with pain and occasional deficits in function. Both patients in this series noted pain out of proportion to examination. The onset of this pain differed, with 1 patient noting pain within the first 3 months and 1 noting discomfort later. Neither patient had any trauma. In the presence of significant symptoms, negative radiographs, and a poor response to conservative treatment, we recommend advanced imaging to rule out fracture. However, prior to obtaining advanced imaging, proper radiographic techniques should be utilized. Eyres and colleagues,23 in a series of 12 fractures of the coracoid process, relied primarily on coracoid views directed 45° in a cephalic direction and thin-slice CT. An isotope bone scan identified 1 case not initially found on radiographs.23
Conservative management with use of a sling until resolution of symptoms was successful in our series. If symptoms persist, a bone stimulator can be used prior to implementing a surgical solution; however, current evidence does not expound on timing and utility of such modalities. Perhaps as important as treatment is education of the patient and the rehabilitation team about the importance of identifying increasing pain as a potential sign of impending fracture in this population. Subsequent activity modification until the pain resolves can help avoid the setback in postoperative recovery that this complication may cause.
Conclusion
We present 2 patients with coracoid fractures encountered at 3 months and 15 months after RTSA. Nonoperative management proved adequate in treating both cases. We suggest a high level of suspicion for possible fracture in the patient who comes in with new-onset pain in a localized region with or without functional deficits.
Reverse total shoulder arthroplasty (RTSA) performed in carefully selected patients often leads to satisfactory outcomes.1,2 In recent years, its indications and the number performed per year have expanded. Subsequently, there has been a concomitant rise in reported complications,2,3 with a rate ranging from 19% to 68%.2,3 Some common complications include scapular notching,2-4 fracture,2,3,5-7 dislocation,2,3,7 and infection.2,3,7
In this series, we describe 2 cases of coracoid fracture after RTSA. The patients provided written informed consent for print and electronic publication of these case reports.
Case Series
Case 1
An independently functioning 81-year-old right hand–dominant woman (BMI, 22.1 [height, 160 cm; weight, 56.7 kg]) presented with increasing left shoulder pain and dysfunction after a motor vehicle accident 2 months earlier. She had reported vague chronic left shoulder pain in the past, but after the accident her pain was significantly worse. A subacromial corticosteroid injection by her primary care physician provided temporary symptomatic relief, but her symptoms recurred.
On presentation, there was obvious anterior superior escape of the humeral head, which was accentuated by shoulder shrug. Her deltoid motor function was found to be intact, and her active shoulder range of motion was severely limited (pseudoparesis). There was notable crepitation as well as significant weakness and pain with abduction and external rotation strength testing.
Radiographic imaging showed anterior superior escape of the humeral head with some early degenerative changes (Seebauer type IIB8 [Figure 1A]). Magnetic resonance imaging confirmed a full-thickness retracted massive rotator cuff tear with complete involvement of the supraspinatus, infraspinatus, and most of the subscapularis muscles. Significant glenohumeral degenerative changes consistent with cuff tear arthropathy were also seen without any evidence of fracture.
After thorough discussion of options, risks, and benefits, the decision was made to proceed with RTSA. The patient underwent the procedure without complications. A DePuy Delta Xtend prosthesis was used with a cemented humeral stem, polyethylene, and glenosphere, sizes of 12, +3, and 38, respectively. The glenosphere component, positioned inferiorly to avoid scapular notching, was secured with 3 screws, and the stem was placed in neutral version. The patient’s shoulder was reduced, ranged, and noted to be stable, allowing for supple passive range of motion without evidence of excessive tightness. She was placed in a sling with the shoulder positioned in neutral alignment. Her postoperative radiograph (Figure 1B) showed satisfactory implantation of the reverse total shoulder prosthesis. Her postoperative course was uneventful, and rehabilitation consisted of 6 weeks of sling protection, with advancing passive and active range of motion. Strengthening exercises were initiated 6 weeks after surgery.
At the patient’s 6-week postoperative visit, she demonstrated pain-free passive elevation to 80° and active forward elevation to 70°. At her 3-month postoperative visit, she reported a 1-week onset of anterior shoulder pain accompanied by a strange noise at the anterior aspect of the operative shoulder. She denied any recent trauma. She continued to have minimal shoulder pain with passive forward flexion of 80°; however, her active forward elevation was very limited because of pain in the anterior aspect of her shoulder. Active external rotation was noted to be 20° and internal rotation was to her buttock. She had pain to palpation of the coracoid process. Radiographs were unchanged from immediate postoperative radiographs. Computed tomography (CT), which was ordered to ensure that the implant was stable with no loosening, showed satisfactory alignment of the prosthesis and no loosening. However, CT was notable for a nondisplaced fracture through the base of the coracoid (Figures 2A-2D). The patient stopped formal physical therapy, and sling immobilization was initiated. After 3 weeks, the sling was discontinued and physical therapy was begun again. She responded satisfactorily to this treatment approach, and, at her 6-month postoperative follow-up, she was without pain, instability, or crepitation. Her range of motion had improved with pain-free active forward flexion, external rotation, and abduction of 100°, 15°, and 90°, respectively. At 28-month postoperative follow-up, her visual analog scale, American Shoulder and Elbow Surgeons score, and Simple Shoulder Test score were 3, 73, and 67, respectively.
Case 2
A 68-year-old, right-handed woman (BMI, 22.5 [height, 160 cm; weight, 57.6 kg]) presented with right shoulder pain and dysfunction of 3 years’ duration. She had undergone an open rotator cuff repair at an outside facility 4 years ago that was unsuccessful. At the time of her presentation to our institution, she had already undergone a failed course of physical therapy. A trial of corticosteroid subacromial injections did not adequately manage her symptoms.
On presentation, her active forward flexion, abduction, and external rotation were 40°, 30°, and 10°, respectively. She had full passive range of motion and pain with active and passive shoulder motion. Radiographic imaging showed superior migration of the humeral head with evidence of glenohumeral arthropathy suggestive of rotator cuff arthropathy (Seebauer type IIA8). After thorough discussion of options, risks, and benefits, the decision was made to proceed with RTSA. She underwent the procedure without complications. A DePuy Delta Xtend prosthesis was used with a cemented humeral stem, polyethylene, and glenosphere, sizes of 8, +3, and 38, respectively. The glenosphere component, positioned inferiorly to avoid scapular notching, was secured with 4 screws, and the stem was placed in neutral version. Her shoulder was reduced, ranged, and noted to be stable, allowing for supple passive range of motion without evidence of excessive tightness. She was placed in a sling with the shoulder positioned in neutral alignment. Her postoperative radiographs revealed satisfactory implantation of the reverse total shoulder prosthesis. Her postoperative course was uneventful. She was taken out of her shoulder immobilizer 4 weeks after surgery and began home-based physical therapy.
At 1 year after surgery, the patient had minimal shoulder pain with active forward flexion, external rotation, and abduction of 135°, 20°, and 85°, respectively. She presented to our clinic 15 months after RTSA with acute onset of pain about her anterior shoulder. She denied any recent trauma or infectious exposures. On examination, her motion was unchanged from prior examinations. However, she was tender on palpation of the coracoid. Radiographs at that time were unchanged (Figures 3A, 3B). Laboratory tests (erythrocyte sedimentation rate, C-reactive protein, and complete blood count with differential) that were subsequently ordered to rule out an occult infection were within normal limits. Computed tomography, which was ordered for further assessment and to ensure that the implant was stable with no loosening, showed satisfactory alignment of the prosthesis without loosening. However, a lucency was noted in the midportion of the coracoid that was suggestive of a fracture (Figures 4A, 4B). A conservative plan of treatment was advised with sling immobilization for 3 weeks and follow-up visits. The patient responded satisfactorily to this treatment approach, and, at her latest follow-up, 8 months after presenting with a coracoid fracture, she was pain-free. At the 5-year postoperative follow-up, her visual analog scale, American Shoulder and Elbow Surgeons score, and Simple Shoulder Test score were 1-2, 78, and 75, respectively.
Discussion
The reverse prosthesis, a semi-constrained ball-and-socket device, provides satisfactory functional outcomes when used in carefully selected patients with rotator cuff arthropathy and pseudoparalysis, failed shoulder arthroplasty, and fracture sequelae.1,9-11 By the traditional Grammont principles of medializing the center of rotation and lowering the humerus, shear forces about the glenoid are reduced and the deltoid muscle is tensioned, allowing for adequate torque generation, required to facilitate shoulder motion.12,13 While long-term outcomes concerning durability and survivorship are pending, some studies have attempted to improve our understanding of implant and functional longevity. Guery and colleagues14 noted an implant survival of 91% at 120 months. However, increased pain and decreased function were seen at the 6-year mark.14 A more recent study by Cuff and colleagues15 revealed 94% implant survivorship and sustained improvement in range of motion and pain at 5 years.
Despite considerable success, RTSA can be associated with a myriad of complications. The most common complications of RTSA include scapular notching (44%-96%), glenoid side failure (5%-40%), instability (2.4%-31%), and infection (1%-15.3%).2,3 In the setting of inflammatory arthropathy, there is an increased risk for intraoperative and postoperative fractures.16,17 To date, there are only 2 reported cases of coracoid process fractures after RTSA.18,19 In the case by Nolan and colleagues,18 conservative management with a sling for 6 weeks led to successful resolution of symptoms. Although little information is provided on the management of these rare fractures, literature on the slightly more common scapular (0.9%-7.2%) and acromial (0.9%-4.9%) fractures suggest that periscapular fractures are on the rise, may increase the risk for revision surgery, and can lead to inferior outcomes when compared with patients without fractures.5,20,21
Acromial fractures after RTSA have been reported to occur at a rate of 0.9% to 4.9%.5,21 This is a concern because of RTSA reliance on a functional deltoid.5,6 The cause of these fractures remains to be fully elucidated. Wahlquist and colleagues6 in 2011 reported the cases of 5 patients that sustained acromial base fractures after RTSA. All 5 patients were noted to have unsatisfactory functional results despite achieving union (3 were treated with open reduction and internal fixation, and 2 were treated nonoperatively). Acromial fractures tend to present with pain within 6 months of surgery, which may indicate excessive constraint about the scapula, eventually leading to fracture. Furthermore, disruption of this bony structure can lead to devastating results because the acromial base serves as a fulcrum for the deltoid.
Despite a well-placed reverse prosthesis, there is increased reliance on surrounding glenohumeral musculature, resulting from poor rotator cuff function and biomechanical differences compared with a native shoulder. Both our patients were found to have relatively small body habitus. It is possible that, by nature of their smaller statures, they were more susceptible to consequences of excessive joint and soft-tissue tension after RTSA. One explanation for acromial fractures after RTSA is that, by excessively lengthening and/or lateralizing the deltoid, the tension on the acromion in these elderly patients may be sufficient to cause a fracture. A similar mechanism may explain their coracoid fractures. As the arm is lengthened and the prosthesis is tightened, the conjoint tendon is significantly tensioned. We routinely check the tension of these muscles as an extra confirmation of joint stability. However, excessive tension for a significant duration may provide too much stress for bone turnover to match with the inherent repair process, potentially causing a fracture. Recent evidence has also found that bone mineral density of the coracoid diminishes with age, suggesting some predisposition to fracture with lower-energy mechanisms.22
Another possible cause for coracoid fractures may be the orientation of the implants. While we did not have mechanistic evidence, it is possible that, with adduction and internal rotation, prosthetic impingement against the coracoid is feasible, particularly in patients of small stature. Although a glenoid implant placed high can increase the chance for coracoid–implant impingement, the fact that the patients improved without revision makes chronic mechanical impingement less likely. Drill holes, especially multiple ones, placed throughout the base of the coracoid may also predispose to coracoid fractures.
Patients with periscapular fractures (acromion, scapular spine, or coracoid) after RTSA often present with pain and occasional deficits in function. Both patients in this series noted pain out of proportion to examination. The onset of this pain differed, with 1 patient noting pain within the first 3 months and 1 noting discomfort later. Neither patient had any trauma. In the presence of significant symptoms, negative radiographs, and a poor response to conservative treatment, we recommend advanced imaging to rule out fracture. However, prior to obtaining advanced imaging, proper radiographic techniques should be utilized. Eyres and colleagues,23 in a series of 12 fractures of the coracoid process, relied primarily on coracoid views directed 45° in a cephalic direction and thin-slice CT. An isotope bone scan identified 1 case not initially found on radiographs.23
Conservative management with use of a sling until resolution of symptoms was successful in our series. If symptoms persist, a bone stimulator can be used prior to implementing a surgical solution; however, current evidence does not expound on timing and utility of such modalities. Perhaps as important as treatment is education of the patient and the rehabilitation team about the importance of identifying increasing pain as a potential sign of impending fracture in this population. Subsequent activity modification until the pain resolves can help avoid the setback in postoperative recovery that this complication may cause.
Conclusion
We present 2 patients with coracoid fractures encountered at 3 months and 15 months after RTSA. Nonoperative management proved adequate in treating both cases. We suggest a high level of suspicion for possible fracture in the patient who comes in with new-onset pain in a localized region with or without functional deficits.
1. Lawrence TM, Ahmadi S, Sanchez-Sotelo J, Sperling JW, Cofield RH. Patient reported activities after reverse shoulder arthroplasty: part II. J Shoulder Elbow Surg. 2012;21(11):1464-1469.
2. Cheung E, Willis M, Walker M, Clark R, Frankle MA. Complications in reverse total shoulder arthroplasty. J Am Acad Orthop Surg. 2011;19(7):439-449.
3. Affonso J, Nicholson GP, Frankle MA, et al. Complications of the reverse prosthesis: prevention and treatment. Instr Course Lect. 2012;61:157-168.
4. Lévigne C, Garret J, Boileau P, Alami G, Favard L, Walch G. Scapular notching in reverse shoulder arthroplasty: is it important to avoid it and how? Clin Orthop Relat Res. 2011;469(9):2512-2520.
5. Hamid N, Connor PM, Fleischli JF, D’Alessandro DF. Acromial fracture after reverse shoulder arthroplasty. Am J Orthop. 2011;40(7):E125-E129.
6. Wahlquist TC, Hunt AF, Braman JP. Acromial base fractures after reverse total shoulder arthroplasty: report of five cases. J Shoulder Elbow Surg. 2011;20(7):1178-1183.
7. Zumstein MA, Pinedo M, Old J, Boileau P. Problems, complications, reoperations, and revisions in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2011;20(1):146-157.
8. Visotsky JL, Basamania C, Seebauer L, Rockwood CA, Jensen KL. Cuff tear arthropathy: pathogenesis, classification, and algorithm for treatment. J Bone Joint Surg Am. 2004;86(suppl 2):35-40.
9. Gamradt SC, Gelber J, Zhang AL. Shoulder function and pain level after revision of failed reverse shoulder replacement to hemiarthroplasty. Int J Shoulder Surg. 2012;6(2):29-35.
10. Garrigues GE, Johnston PS, Pepe MD, Tucker BS, Ramsey ML, Austin LS. Hemiarthroplasty versus reverse total shoulder arthroplasty for acute proximal humerus fractures in elderly patients. Orthopedics. 2012;35(5):e703-e708.
11. Patel DN, Young B, Onyekwelu I, Zuckerman JD, Kwon YW. Reverse total shoulder arthroplasty for failed shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(11):1473-1483.
12. Grammont PM, Baulot E. The classic: Delta shoulder prosthesis for rotator cuff rupture. 1993. Clin Orthop Relat Res. 2011;469(9):2424.
13. Schwartz DG, Kang SH, Lynch TS, et al. The anterior deltoid’s importance in reverse shoulder arthroplasty: a cadaveric biomechanical study. J Shoulder Elbow Surg. 2013;22(3):357-364.
14. Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty. Survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am. 2006;88(8):1742-1747.
15. Cuff D, Clark R, Pupello D, Frankle M. Reverse shoulder arthroplasty for the treatment of rotator cuff deficiency: a concise follow-up, at a minimum of five years, of a previous report. J Bone Joint Surg Am. 2012;94(21):1996-2000.
16. Young AA, Smith MM, Bacle G, Moraga C, Walch G. Early results of reverse shoulder arthroplasty in patients with rheumatoid arthritis. J Bone Joint Surg. 2011;93(20):1915-1923.
17. Hattrup SJ, Sanchez-Sotelo J, Sperling JW, Cofield RH. Reverse shoulder replacement for patients with inflammatory arthritis. J Hand Surg Am. 2012;37(9):1888-1894.
18. Nolan BM, Ankerson E, Wiater JM. Reverse total shoulder arthroplasty improves function in cuff tear arthropathy. Clin Orthop Relat Res. 2011;469(9):2476-2482.
19. Stechel A, Fuhrmann U, Irlenbusch L, Rott O, Irlenbusch U. Reversed shoulder arthroplasty in cuff tear arthritis, fracture sequelae, and revision arthroplasty. Acta Orthop. 2010;81(3):367-372.
20. Teusink MJ, Otto RJ, Cottrell BJ, Frankle MA. What is the effect of postoperative scapular fracture on outcomes of reverse shoulder arthroplasty? J Shoulder Elbow Surg. 2014;23(6):782-790.
21. Walch G, Bacle G, Lädermann A, Nové-Josserand L, Smithers CJ. Do the indications, results, and complications of reverse shoulder arthroplasty change with surgeon’s experience? J Shoulder Elbow Surg. 2012;21(11):1470-1477.
22. Beranger JS, Maqdes A, Pujol N, Desmoineaux P, Beaufils P. Bone mineral density of the coracoid process decreases with age [published online ahead of print December 17, 2014]. Knee Surg Sports Traumatol Arthrosc.
23. Eyres KS, Brooks A, Stanley D. Fractures of the coracoid process. J Bone Joint Surg Br. 1995;77(3):425-428.
1. Lawrence TM, Ahmadi S, Sanchez-Sotelo J, Sperling JW, Cofield RH. Patient reported activities after reverse shoulder arthroplasty: part II. J Shoulder Elbow Surg. 2012;21(11):1464-1469.
2. Cheung E, Willis M, Walker M, Clark R, Frankle MA. Complications in reverse total shoulder arthroplasty. J Am Acad Orthop Surg. 2011;19(7):439-449.
3. Affonso J, Nicholson GP, Frankle MA, et al. Complications of the reverse prosthesis: prevention and treatment. Instr Course Lect. 2012;61:157-168.
4. Lévigne C, Garret J, Boileau P, Alami G, Favard L, Walch G. Scapular notching in reverse shoulder arthroplasty: is it important to avoid it and how? Clin Orthop Relat Res. 2011;469(9):2512-2520.
5. Hamid N, Connor PM, Fleischli JF, D’Alessandro DF. Acromial fracture after reverse shoulder arthroplasty. Am J Orthop. 2011;40(7):E125-E129.
6. Wahlquist TC, Hunt AF, Braman JP. Acromial base fractures after reverse total shoulder arthroplasty: report of five cases. J Shoulder Elbow Surg. 2011;20(7):1178-1183.
7. Zumstein MA, Pinedo M, Old J, Boileau P. Problems, complications, reoperations, and revisions in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2011;20(1):146-157.
8. Visotsky JL, Basamania C, Seebauer L, Rockwood CA, Jensen KL. Cuff tear arthropathy: pathogenesis, classification, and algorithm for treatment. J Bone Joint Surg Am. 2004;86(suppl 2):35-40.
9. Gamradt SC, Gelber J, Zhang AL. Shoulder function and pain level after revision of failed reverse shoulder replacement to hemiarthroplasty. Int J Shoulder Surg. 2012;6(2):29-35.
10. Garrigues GE, Johnston PS, Pepe MD, Tucker BS, Ramsey ML, Austin LS. Hemiarthroplasty versus reverse total shoulder arthroplasty for acute proximal humerus fractures in elderly patients. Orthopedics. 2012;35(5):e703-e708.
11. Patel DN, Young B, Onyekwelu I, Zuckerman JD, Kwon YW. Reverse total shoulder arthroplasty for failed shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(11):1473-1483.
12. Grammont PM, Baulot E. The classic: Delta shoulder prosthesis for rotator cuff rupture. 1993. Clin Orthop Relat Res. 2011;469(9):2424.
13. Schwartz DG, Kang SH, Lynch TS, et al. The anterior deltoid’s importance in reverse shoulder arthroplasty: a cadaveric biomechanical study. J Shoulder Elbow Surg. 2013;22(3):357-364.
14. Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty. Survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am. 2006;88(8):1742-1747.
15. Cuff D, Clark R, Pupello D, Frankle M. Reverse shoulder arthroplasty for the treatment of rotator cuff deficiency: a concise follow-up, at a minimum of five years, of a previous report. J Bone Joint Surg Am. 2012;94(21):1996-2000.
16. Young AA, Smith MM, Bacle G, Moraga C, Walch G. Early results of reverse shoulder arthroplasty in patients with rheumatoid arthritis. J Bone Joint Surg. 2011;93(20):1915-1923.
17. Hattrup SJ, Sanchez-Sotelo J, Sperling JW, Cofield RH. Reverse shoulder replacement for patients with inflammatory arthritis. J Hand Surg Am. 2012;37(9):1888-1894.
18. Nolan BM, Ankerson E, Wiater JM. Reverse total shoulder arthroplasty improves function in cuff tear arthropathy. Clin Orthop Relat Res. 2011;469(9):2476-2482.
19. Stechel A, Fuhrmann U, Irlenbusch L, Rott O, Irlenbusch U. Reversed shoulder arthroplasty in cuff tear arthritis, fracture sequelae, and revision arthroplasty. Acta Orthop. 2010;81(3):367-372.
20. Teusink MJ, Otto RJ, Cottrell BJ, Frankle MA. What is the effect of postoperative scapular fracture on outcomes of reverse shoulder arthroplasty? J Shoulder Elbow Surg. 2014;23(6):782-790.
21. Walch G, Bacle G, Lädermann A, Nové-Josserand L, Smithers CJ. Do the indications, results, and complications of reverse shoulder arthroplasty change with surgeon’s experience? J Shoulder Elbow Surg. 2012;21(11):1470-1477.
22. Beranger JS, Maqdes A, Pujol N, Desmoineaux P, Beaufils P. Bone mineral density of the coracoid process decreases with age [published online ahead of print December 17, 2014]. Knee Surg Sports Traumatol Arthrosc.
23. Eyres KS, Brooks A, Stanley D. Fractures of the coracoid process. J Bone Joint Surg Br. 1995;77(3):425-428.
Posterior Reversible Encephalopathy Syndrome: Temporary Visual Loss After Spinal Deformity Surgery
First described in 1996, posterior reversible encephalopathy syndrome (PRES) exhibits a wide clinical spectrum and is definitively diagnosed through computed tomography (CT) and/or magnetic resonance imaging (MRI) studies of the brain.1 Clinical presentation may include a spectrum of symptoms, including nausea, emesis, seizures, visual loss, paralysis, and headaches.2,3 The most common imaging finding of PRES is bilateral foci of vasogenic edema located in the parieto-occipital white matter.2-6 Other areas of the brain are frequently affected as well, with the frontal and temporal lobes and the basal or cortical ganglia showing signs of distinctly noncytotoxic edema in 12.5% to 54.2% of all cases.3 With the symptom of visual loss being present in 20% to 62.5% of patients with PRES, the syndrome constitutes a rare potential cause for postoperative visual loss (POVL) after spinal surgery, which has a generally good prognosis because most patients will completely regain their eyesight.2,3
We present a unique account of 2 patients who underwent extensive spinal surgery and received a timely diagnosis and treatment of PRES at a single institution. We aim to elucidate the difference in clinical and radiographic presentation of PRES in relation to other known causes of POVL after spinal surgery. The patients provided written informed consent for print and electronic publication of these case reports.
Case Reports
Case 1
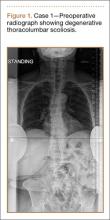
Clinical Presentation. A 78-year-old woman presented to the outpatient clinic with disability due to severe lower back pain. Her surgical history was significant for breast lumpectomy and cataract excision. Her medical history was significant for hypertension, obesity (body mass index, 31.5), hypercholesterolemia, emphysema, and anemia. She had undergone spinal surgery, specifically laminectomies from L2 to S1. The radiographic examination showed degenerative thoracolumbar scoliosis with severe spondylosis, disc space collapse, and ankylosis of L4-L5 (Figure 1).
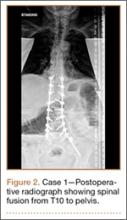
Operative Procedure. The patient underwent transpsoas lumbar interbody fusion (XLIF, NuVasive) from L1 to L4 and posterior spinal fusion from T10 to pelvis (Expedium, Depuy Synthes) (Figure 2). Operative time was 553 minutes; estimated blood loss was 2000 mL due to intraoperative coagulopathy (platelets, 40,000/µL) near the end of the posterior portion of the procedure. Intraoperative hypotension was treated by volume resuscitation and transient use of vasopressor agents. She was transfused with 1700 mL of blood, 150 mL of saline solution, and 420 mL of Lactated Ringer’s solution. No intraoperative complications occurred. The patient was extubated uneventfully on postoperative day 1 and was at baseline neurologically with no visual disturbances.
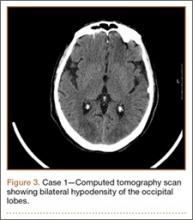
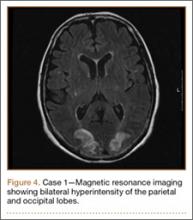
Development and Diagnosis of PRES. The patient made significant progress with physical therapy and developed episodes of hypertension at night on postoperative days 4 to 6. Her mean peak systolic blood pressure was 180 mm Hg. This improved after oral beta-blocker therapy. On postoperative day 6, the patient was ambulating with physical therapy and the aid of a walker. She was found to be neurologically intact, was resting comfortable in a chair reading a book, and was cleared for transfer to a rehabilitation facility the next day. During the morning on postoperative day 7, she developed confusion and visual loss. The patient reported blurry vision followed by complete bilateral painless loss of vision aside from mild light perception. She was unable to identify any objects. She had extinction to double simultaneous stimuli and evidence of agraphesthesia in the left hand. Her neurologic examination was otherwise at baseline. Upon emergent imaging, head CT showed bilateral symmetric areas of hypodensity involving the cortical and subcortical white matter of both occipital lobes (Figure 3). MRI showed extensive bilateral cortical and subcortical signal hyperintensity involving the parietal and occipital lobes (Figure 4). No evidence of petechial or lobar hemorrhage was found.
Treatment and Clinical Course. The patient was transferred to the neurology intensive care unit for neurologic monitoring. She was treated aggressively for recurrent hypertensive episodes. Twenty-four hours after initial blood pressure optimization therapy, she partially recovered her eyesight. She exhibited complete recovery after 48 hours. The patient was discharged to a rehabilitation facility in stable condition on postoperative day 11.
Case 2
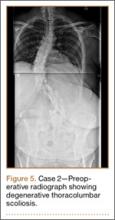
Clinical Presentation. A 51-year-old woman presented to the outpatient clinic with progressive low back pain and decompensation due to degenerative adult scoliosis. Her surgical history was significant for an uneventful Caesarean section. Her medical history was significant for borderline hypertension and obesity (body mass index, 34.4). The radiographic examination showed an S-shaped thoracolumbar curve from T4 to L4 (Figure 5).
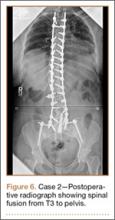
Operative Procedure. After discussions about the risks and benefits of the procedure, the patient underwent posterior spinal fusion from T3 to pelvis (Mesa, K2M) and interbody fusion from L4 to S1 via a presacral approach using the AxiaLIF system (TranS1) (Figure 6). The operation spanned 507 minutes. The patient lost approximately 2200 mL of blood. She was transfused with 1690 mL of blood, 1250 mL of Lactated Ringer’s solution, and 1 unit (50 mL) of albumin. No intraoperative complications occurred.
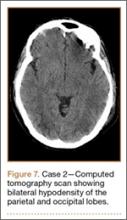
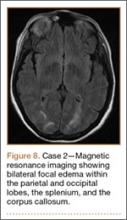
Development and Diagnosis of PRES. The patient was ambulatory with physical therapy and a walker on postoperative day 1. Her albumin levels were noted to be decreased postoperatively (28 mg/mL; normal, >35 mg/mL). She developed intermittent hypertensive episodes and experienced transient peripheral vision loss. After her ophthalmologic symptoms cleared, she was discharged and transferred to a rehabilitation facility on postoperative day 9. Eleven days later, the patient was emergently readmitted for a deep spine wound infection after an onset of wound swelling and fever. She underwent irrigation and débridement of the spine wound with an estimated blood loss of 400 mL. The patient continued to have fevers and was placed on ciprofloxacin and vancomycin, which was changed to levofloxacin on postoperative day 5. Elevated creatinine was noted, and the patient was diagnosed with acute renal failure. On postoperative day 7, oxacillin therapy was commenced. After her cultures grew methicillin-resistant Staphylococcus aureus, a peripherally inserted central catheter line was placed on postoperative day 9. As a result of nausea and constipation, the patient received feeding tubes on postoperative day 11. Additionally, she was diagnosed with a pleural effusion on postoperative day 14. Although her creatinine levels were decreasing, she continued to experience intermittent hypertensive episodes with a mean peak systolic blood pressure of 148 mm Hg. On postoperative day 15, she had a seizure and again developed visual loss. The patient was lethargic and followed only simple commands. She moved all extremities and withdrew symmetrically to noxious stimuli. Upon emergent imaging, head CT showed posterior subcortical white matter hypodensity within the occipital and parietal lobes bilaterally (Figure 7). MRI showed focal regions of symmetric hemispheric edema involving the parietal and occipital lobes in a predominantly subcortical white-matter distribution. Additionally, extensive involvement of the splenium and of the corpus callosum, left greater than right, was observed (Figure 8).
Treatment and Clinical Course. The patient was transferred to the intensive care unit for neuromonitoring. Her hypokalemia and hypertension were treated aggressively to normalize her potassium levels and blood pressure. Her oxacillin therapy was changed to daptomycin. On postoperative day 17, the patient was transferred to another institution for further medical management after achieving full recovery of her eyesight after electrolyte and blood pressure corrections.
Discussion
Posterior reversible encephalopathy syndrome is a rare but frequently devastating complication of spinal surgery, with an estimated incidence of 0.094% to 0.2%.7,8 Pediatric patients, as well as patients undergoing deformity correction surgery and posterior lumbar fusion, which necessitate prone positioning, have a significantly increased risk of POVL after spinal surgery.9 There are several causes of POVL after spinal surgery, each with a unique pathophysiology, clinical presentation, and prognosis.
The most common cause of POVL, accounting for 89% of all cases, is ischemic neuropathy.10 Ischemic neuropathy refers to a hypoperfusion or infarction of the anterior or posterior portion of the optic nerve and presents as painless bilateral vision loss or complete blindness on waking from the surgical procedure.11 Risk factors associated with anterior ischemic neuropathy are primarily diabetes mellitus, prone positioning, nocturnal hypotension, and blood loss.11 Posterior ischemic neuropathy has been most strongly correlated with anemia and hypotension.12 The exact etiology of this complication has not been established, although the prognosis is generally unfavorable, with most vision loss being permanent.10-12
Another potential cause of POVL after spinal surgery is retinal artery occlusion. It is most commonly observed in patients who were improperly positioned, resulting in compression of an orbit on the surface of the headrest or the operating table.13 Retinal artery occlusion characteristically presents as an irreversible unilateral complete loss of vision with a red spot on the macula and an afferent pupillary defect.14
Cortical blindness, another possible common cause of POVL, results from the hypoperfusion of the occipital cortex and has a slightly better prognosis. Cortical blindness generally results from an embolic event that can be visualized through neuroimaging and may be unilateral or bilateral, ranging from mild peripheral vision loss to complete blindness.15
Posterior reversible encephalopathy syndrome, the cause of POVL diagnosed in the 2 patients in this case report, is a neurologic syndrome that differs significantly in its clinical presentation and pathophysiology from the more well-known etiologies. The precise pathophysiologic mechanism of the syndrome is yet to be elucidated. One theory revolves around the failure of cerebral vascular autoregulation. It postulates that intracerebellar hypertension leads to the extravasation of proteins and fluid, resulting in the characteristic vasogenic edema.16,17 The other equally discussed theory postulates that cerebellar vasospasm and subsequent hypoperfusion leading to cellular hypoxemia and ischemia may be responsible.18-20 Posterior reversible encephalopathy syndrome has been reported with increasing frequency, particularly in connection with hypertension, acute renal failure associated with malignancy, cytotoxicity, and corticosteroids, as well as preeclampsia, eclampsia, and autoimmune disorders.1-3,21-23 Traditionally, patients display a combination of different symptoms, including vision changes ranging from slightly decreased perception to complete blindness. Unlike retinal artery occlusion and ischemic optic neuropathy, the onset of vision loss often does not happen immediately after surgery and may occur several hours to days after surgery. Visual disturbance may progressively worsen if the medical cause for the syndrome is not determined and corrected.2,3 In contrast to other known etiologies of POVL, PRES has a relatively favorable prognosis if managed appropriately. Reported case series determined a resolution of the characteristic parieto-occipital vasogenic edema in 83% to 88% of all patients in follow-up neuroimaging after aggressive control of seizures and arterial hypertension.2-3
Both patients undergoing spinal deformity surgery in this report suffered from intermittent hypertensive episodes in the postoperative period. One patient also developed acute renal failure during her hospital stay, and demonstrated low albumin levels postoperatively, which has also been associated with PRES.24 Through the immediate diagnosis and primary control of hypertension, both patients achieved complete neurologic recovery after a mean of 1.5 days (range, 1-2 days); this compares to a recovery period of an average 6.2 days (range, 1-14 days) reported by Ni and colleagues.3 The catastrophic effects of a misdiagnosis and incorrect or untimely treatment were well described in this case report. Several patients who were incorrectly diagnosed with demyelinating disorders or lupus encephalopathy received high doses of immunosuppressants and corticosteroids, known risk factors for the development of PRES.3 The patients subsequently rapidly deteriorated; no patients had a full recovery of their preoperative eyesight, and 1 patient developed complete permanent blindness.3 Optimized multidisciplinary collaboration allowing for a rapid neuro-ophthalmic examination and appropriate neuroimaging will permit an accurate and rapid diagnosis, leading to timely intervention and restoration of vision.
Conclusion
Temporary POVL is a potentially devastating complication of spinal surgery and general anesthesia. The more frequent causes such as ischemic optic neuropathy, retinal artery occlusion, and cortical blindness have very limited effective options for treatment and an overall poor prognosis. The inclusion of PRES in the differential diagnosis of POVL may allow early detection, management, and restoration of vision.
1. Hinchey J, Chaves C, Appignani B, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334(8):494-500.
2. Fugate JE, Claassen DO, Cloft HJ, et al. Posterior reversible encephalopathy syndrome: associated clinical and radiologic findings. Mayo Clin Proc. 2010;85(5):427-432.
3. Ni J, Zhou LX, Hao HL, et al. The clinical and radiological spectrum of posterior reversible encephalopathy syndrome: a retrospective series of 24 patients. J Neuroimaging. 2011;21(3):219-224.
4. Stevens CJ, Heran MK. The many faces of posterior reversible encephalopathy syndrome. Br J Radiol. 2012;85(1020):1566-1575.
5. Bartynski WS. Posterior reversible encephalopathy syndrome, part 1: fundamental imaging and clinical features. AJNR Am J Neuroradiol. 2008;29(6):1036-1042.
6. Yoon SD, Cho BM, Oh SM, et al. Clinical and radiological spectrum of posterior reversible encephalopathy syndrome. J Cerebrovasc Endovasc Neurosurg. 2013;15(3):206-213.
7. Patil CG, Lad EM, Lad SP, Ho C, Boakye M. Visual loss after spine surgery: a population-based study. Spine (Phila Pa 1976). 2008;33(13):1491-1496.
8. Stevens WR, Glazer PA, Kelley SD, Lietman TM, Bradford DS. Ophthalmic complications after spinal surgery. Spine (Phila Pa 1976). 1997;22(12):1319-1324.
9. Shen Y, Drum M, Roth S. The prevalence of perioperative visual loss in the United States: a 10-year study from 1996 to 2005 of spinal, orthopedic, cardiac, and general surgery. Anesth Analg. 2009;109(5):1534-1545.
10. Lee LA, Roth S, Posner KL, et al. The American Society of Anesthesiologists Postoperative Visual Loss Registry: analysis of 93 spine surgery cases with postoperative visual loss. Anesthesiology. 2006;105(4):652-659; quiz 867-868.
11. Hayreh SS. Ischemic optic neuropathies - where are we now? Graefes Arch Clin Exp Ophthalmol. 2013;251(8):1873-1884.
12. Buono LM, Foroozan R. Perioperative posterior ischemic optic neuropathy: review of the literature. Surv Ophthalmol. 2005;50(1):15-26.
13. Katz DA, Karlin LI. Visual field defect after posterior spine fusion. Spine (Phila Pa 1976). 2005;30(3):E83-E85.
14. Hayreh SS, Kolder HE, Weingeist TA. Central retinal artery occlusion and retinal tolerance time. Ophthalmology. 1980;87(1):75-78.
15. Berg KT, Harrison AR, Lee MS. Perioperative visual loss in ocular and nonocular surgery. Clin Ophthalmol. 2010;4:531-546.
16. Primavera A, Audenino D, Mavilio N, Cocito L. Reversible posterior leucoencephalopathy syndrome in systemic lupus and vasculitis. Ann Rheum Dis. 2001;60(5):534-537.
17. Bartynski WS, Boardman JF. Catheter angiography, MR angiography, and MR perfusion in posterior reversible encephalopathy syndrome. AJNR Am J Neuroradiol. 2008;29(3):447-455.
18. Ito T, Sakai T, Inagawa S, Utsu M, Bun T. MR angiography of cerebral vasospasm in preeclampsia. AJNR Am J Neuroradiol. 1995;16(6):1344-1346.
19. Agarwal R, Davis C, Altinok D, Serajee FJ. Posterior reversible encephalopathy and cerebral vasoconstriction in a patient with hemolytic uremic syndrome. Pediatr Neurol. 2014;50(5):518-521.
20. Bartynski WS. Posterior reversible encephalopathy syndrome, part 2: controversies surrounding pathophysiology of vasogenic edema. AJNR Am J Neuroradiol. 2008;29(6):1043-1049.
21. Lee VH, Wijdicks EF, Manno EM, Rabinstein AA. Clinical spectrum of reversible posterior leukoencephalopathy syndrome. Arch Neurol. 2008;65(2):205-210.
22. Ekawa Y, Shiota M, Tobiume T, et al. Reversible posterior leukoencephalopathy syndrome accompanying eclampsia: correct diagnosis using preoperative MRI. Tohoku J Exp Med. 2012;226(1):55-58.
23. Kur JK, Esdaile JM. Posterior reversible encephalopathy syndrome--an underrecognized manifestation of systemic lupus erythematosus. J Rheumatol. 2006;33(11):2178-2183.
24. Pirker A, Kramer L, Voller B, et al. Type of edema in posterior reversible encephalopathy syndrome depends on serum albumin levels: an MR imaging study in 28 patients. AJNR Am J Neuroradiol. 2011;32(3):527-531.
First described in 1996, posterior reversible encephalopathy syndrome (PRES) exhibits a wide clinical spectrum and is definitively diagnosed through computed tomography (CT) and/or magnetic resonance imaging (MRI) studies of the brain.1 Clinical presentation may include a spectrum of symptoms, including nausea, emesis, seizures, visual loss, paralysis, and headaches.2,3 The most common imaging finding of PRES is bilateral foci of vasogenic edema located in the parieto-occipital white matter.2-6 Other areas of the brain are frequently affected as well, with the frontal and temporal lobes and the basal or cortical ganglia showing signs of distinctly noncytotoxic edema in 12.5% to 54.2% of all cases.3 With the symptom of visual loss being present in 20% to 62.5% of patients with PRES, the syndrome constitutes a rare potential cause for postoperative visual loss (POVL) after spinal surgery, which has a generally good prognosis because most patients will completely regain their eyesight.2,3
We present a unique account of 2 patients who underwent extensive spinal surgery and received a timely diagnosis and treatment of PRES at a single institution. We aim to elucidate the difference in clinical and radiographic presentation of PRES in relation to other known causes of POVL after spinal surgery. The patients provided written informed consent for print and electronic publication of these case reports.
Case Reports
Case 1
Clinical Presentation. A 78-year-old woman presented to the outpatient clinic with disability due to severe lower back pain. Her surgical history was significant for breast lumpectomy and cataract excision. Her medical history was significant for hypertension, obesity (body mass index, 31.5), hypercholesterolemia, emphysema, and anemia. She had undergone spinal surgery, specifically laminectomies from L2 to S1. The radiographic examination showed degenerative thoracolumbar scoliosis with severe spondylosis, disc space collapse, and ankylosis of L4-L5 (Figure 1).
Operative Procedure. The patient underwent transpsoas lumbar interbody fusion (XLIF, NuVasive) from L1 to L4 and posterior spinal fusion from T10 to pelvis (Expedium, Depuy Synthes) (Figure 2). Operative time was 553 minutes; estimated blood loss was 2000 mL due to intraoperative coagulopathy (platelets, 40,000/µL) near the end of the posterior portion of the procedure. Intraoperative hypotension was treated by volume resuscitation and transient use of vasopressor agents. She was transfused with 1700 mL of blood, 150 mL of saline solution, and 420 mL of Lactated Ringer’s solution. No intraoperative complications occurred. The patient was extubated uneventfully on postoperative day 1 and was at baseline neurologically with no visual disturbances.
Development and Diagnosis of PRES. The patient made significant progress with physical therapy and developed episodes of hypertension at night on postoperative days 4 to 6. Her mean peak systolic blood pressure was 180 mm Hg. This improved after oral beta-blocker therapy. On postoperative day 6, the patient was ambulating with physical therapy and the aid of a walker. She was found to be neurologically intact, was resting comfortable in a chair reading a book, and was cleared for transfer to a rehabilitation facility the next day. During the morning on postoperative day 7, she developed confusion and visual loss. The patient reported blurry vision followed by complete bilateral painless loss of vision aside from mild light perception. She was unable to identify any objects. She had extinction to double simultaneous stimuli and evidence of agraphesthesia in the left hand. Her neurologic examination was otherwise at baseline. Upon emergent imaging, head CT showed bilateral symmetric areas of hypodensity involving the cortical and subcortical white matter of both occipital lobes (Figure 3). MRI showed extensive bilateral cortical and subcortical signal hyperintensity involving the parietal and occipital lobes (Figure 4). No evidence of petechial or lobar hemorrhage was found.
Treatment and Clinical Course. The patient was transferred to the neurology intensive care unit for neurologic monitoring. She was treated aggressively for recurrent hypertensive episodes. Twenty-four hours after initial blood pressure optimization therapy, she partially recovered her eyesight. She exhibited complete recovery after 48 hours. The patient was discharged to a rehabilitation facility in stable condition on postoperative day 11.
Case 2
Clinical Presentation. A 51-year-old woman presented to the outpatient clinic with progressive low back pain and decompensation due to degenerative adult scoliosis. Her surgical history was significant for an uneventful Caesarean section. Her medical history was significant for borderline hypertension and obesity (body mass index, 34.4). The radiographic examination showed an S-shaped thoracolumbar curve from T4 to L4 (Figure 5).
Operative Procedure. After discussions about the risks and benefits of the procedure, the patient underwent posterior spinal fusion from T3 to pelvis (Mesa, K2M) and interbody fusion from L4 to S1 via a presacral approach using the AxiaLIF system (TranS1) (Figure 6). The operation spanned 507 minutes. The patient lost approximately 2200 mL of blood. She was transfused with 1690 mL of blood, 1250 mL of Lactated Ringer’s solution, and 1 unit (50 mL) of albumin. No intraoperative complications occurred.
Development and Diagnosis of PRES. The patient was ambulatory with physical therapy and a walker on postoperative day 1. Her albumin levels were noted to be decreased postoperatively (28 mg/mL; normal, >35 mg/mL). She developed intermittent hypertensive episodes and experienced transient peripheral vision loss. After her ophthalmologic symptoms cleared, she was discharged and transferred to a rehabilitation facility on postoperative day 9. Eleven days later, the patient was emergently readmitted for a deep spine wound infection after an onset of wound swelling and fever. She underwent irrigation and débridement of the spine wound with an estimated blood loss of 400 mL. The patient continued to have fevers and was placed on ciprofloxacin and vancomycin, which was changed to levofloxacin on postoperative day 5. Elevated creatinine was noted, and the patient was diagnosed with acute renal failure. On postoperative day 7, oxacillin therapy was commenced. After her cultures grew methicillin-resistant Staphylococcus aureus, a peripherally inserted central catheter line was placed on postoperative day 9. As a result of nausea and constipation, the patient received feeding tubes on postoperative day 11. Additionally, she was diagnosed with a pleural effusion on postoperative day 14. Although her creatinine levels were decreasing, she continued to experience intermittent hypertensive episodes with a mean peak systolic blood pressure of 148 mm Hg. On postoperative day 15, she had a seizure and again developed visual loss. The patient was lethargic and followed only simple commands. She moved all extremities and withdrew symmetrically to noxious stimuli. Upon emergent imaging, head CT showed posterior subcortical white matter hypodensity within the occipital and parietal lobes bilaterally (Figure 7). MRI showed focal regions of symmetric hemispheric edema involving the parietal and occipital lobes in a predominantly subcortical white-matter distribution. Additionally, extensive involvement of the splenium and of the corpus callosum, left greater than right, was observed (Figure 8).
Treatment and Clinical Course. The patient was transferred to the intensive care unit for neuromonitoring. Her hypokalemia and hypertension were treated aggressively to normalize her potassium levels and blood pressure. Her oxacillin therapy was changed to daptomycin. On postoperative day 17, the patient was transferred to another institution for further medical management after achieving full recovery of her eyesight after electrolyte and blood pressure corrections.
Discussion
Posterior reversible encephalopathy syndrome is a rare but frequently devastating complication of spinal surgery, with an estimated incidence of 0.094% to 0.2%.7,8 Pediatric patients, as well as patients undergoing deformity correction surgery and posterior lumbar fusion, which necessitate prone positioning, have a significantly increased risk of POVL after spinal surgery.9 There are several causes of POVL after spinal surgery, each with a unique pathophysiology, clinical presentation, and prognosis.
The most common cause of POVL, accounting for 89% of all cases, is ischemic neuropathy.10 Ischemic neuropathy refers to a hypoperfusion or infarction of the anterior or posterior portion of the optic nerve and presents as painless bilateral vision loss or complete blindness on waking from the surgical procedure.11 Risk factors associated with anterior ischemic neuropathy are primarily diabetes mellitus, prone positioning, nocturnal hypotension, and blood loss.11 Posterior ischemic neuropathy has been most strongly correlated with anemia and hypotension.12 The exact etiology of this complication has not been established, although the prognosis is generally unfavorable, with most vision loss being permanent.10-12
Another potential cause of POVL after spinal surgery is retinal artery occlusion. It is most commonly observed in patients who were improperly positioned, resulting in compression of an orbit on the surface of the headrest or the operating table.13 Retinal artery occlusion characteristically presents as an irreversible unilateral complete loss of vision with a red spot on the macula and an afferent pupillary defect.14
Cortical blindness, another possible common cause of POVL, results from the hypoperfusion of the occipital cortex and has a slightly better prognosis. Cortical blindness generally results from an embolic event that can be visualized through neuroimaging and may be unilateral or bilateral, ranging from mild peripheral vision loss to complete blindness.15
Posterior reversible encephalopathy syndrome, the cause of POVL diagnosed in the 2 patients in this case report, is a neurologic syndrome that differs significantly in its clinical presentation and pathophysiology from the more well-known etiologies. The precise pathophysiologic mechanism of the syndrome is yet to be elucidated. One theory revolves around the failure of cerebral vascular autoregulation. It postulates that intracerebellar hypertension leads to the extravasation of proteins and fluid, resulting in the characteristic vasogenic edema.16,17 The other equally discussed theory postulates that cerebellar vasospasm and subsequent hypoperfusion leading to cellular hypoxemia and ischemia may be responsible.18-20 Posterior reversible encephalopathy syndrome has been reported with increasing frequency, particularly in connection with hypertension, acute renal failure associated with malignancy, cytotoxicity, and corticosteroids, as well as preeclampsia, eclampsia, and autoimmune disorders.1-3,21-23 Traditionally, patients display a combination of different symptoms, including vision changes ranging from slightly decreased perception to complete blindness. Unlike retinal artery occlusion and ischemic optic neuropathy, the onset of vision loss often does not happen immediately after surgery and may occur several hours to days after surgery. Visual disturbance may progressively worsen if the medical cause for the syndrome is not determined and corrected.2,3 In contrast to other known etiologies of POVL, PRES has a relatively favorable prognosis if managed appropriately. Reported case series determined a resolution of the characteristic parieto-occipital vasogenic edema in 83% to 88% of all patients in follow-up neuroimaging after aggressive control of seizures and arterial hypertension.2-3
Both patients undergoing spinal deformity surgery in this report suffered from intermittent hypertensive episodes in the postoperative period. One patient also developed acute renal failure during her hospital stay, and demonstrated low albumin levels postoperatively, which has also been associated with PRES.24 Through the immediate diagnosis and primary control of hypertension, both patients achieved complete neurologic recovery after a mean of 1.5 days (range, 1-2 days); this compares to a recovery period of an average 6.2 days (range, 1-14 days) reported by Ni and colleagues.3 The catastrophic effects of a misdiagnosis and incorrect or untimely treatment were well described in this case report. Several patients who were incorrectly diagnosed with demyelinating disorders or lupus encephalopathy received high doses of immunosuppressants and corticosteroids, known risk factors for the development of PRES.3 The patients subsequently rapidly deteriorated; no patients had a full recovery of their preoperative eyesight, and 1 patient developed complete permanent blindness.3 Optimized multidisciplinary collaboration allowing for a rapid neuro-ophthalmic examination and appropriate neuroimaging will permit an accurate and rapid diagnosis, leading to timely intervention and restoration of vision.
Conclusion
Temporary POVL is a potentially devastating complication of spinal surgery and general anesthesia. The more frequent causes such as ischemic optic neuropathy, retinal artery occlusion, and cortical blindness have very limited effective options for treatment and an overall poor prognosis. The inclusion of PRES in the differential diagnosis of POVL may allow early detection, management, and restoration of vision.
First described in 1996, posterior reversible encephalopathy syndrome (PRES) exhibits a wide clinical spectrum and is definitively diagnosed through computed tomography (CT) and/or magnetic resonance imaging (MRI) studies of the brain.1 Clinical presentation may include a spectrum of symptoms, including nausea, emesis, seizures, visual loss, paralysis, and headaches.2,3 The most common imaging finding of PRES is bilateral foci of vasogenic edema located in the parieto-occipital white matter.2-6 Other areas of the brain are frequently affected as well, with the frontal and temporal lobes and the basal or cortical ganglia showing signs of distinctly noncytotoxic edema in 12.5% to 54.2% of all cases.3 With the symptom of visual loss being present in 20% to 62.5% of patients with PRES, the syndrome constitutes a rare potential cause for postoperative visual loss (POVL) after spinal surgery, which has a generally good prognosis because most patients will completely regain their eyesight.2,3
We present a unique account of 2 patients who underwent extensive spinal surgery and received a timely diagnosis and treatment of PRES at a single institution. We aim to elucidate the difference in clinical and radiographic presentation of PRES in relation to other known causes of POVL after spinal surgery. The patients provided written informed consent for print and electronic publication of these case reports.
Case Reports
Case 1
Clinical Presentation. A 78-year-old woman presented to the outpatient clinic with disability due to severe lower back pain. Her surgical history was significant for breast lumpectomy and cataract excision. Her medical history was significant for hypertension, obesity (body mass index, 31.5), hypercholesterolemia, emphysema, and anemia. She had undergone spinal surgery, specifically laminectomies from L2 to S1. The radiographic examination showed degenerative thoracolumbar scoliosis with severe spondylosis, disc space collapse, and ankylosis of L4-L5 (Figure 1).
Operative Procedure. The patient underwent transpsoas lumbar interbody fusion (XLIF, NuVasive) from L1 to L4 and posterior spinal fusion from T10 to pelvis (Expedium, Depuy Synthes) (Figure 2). Operative time was 553 minutes; estimated blood loss was 2000 mL due to intraoperative coagulopathy (platelets, 40,000/µL) near the end of the posterior portion of the procedure. Intraoperative hypotension was treated by volume resuscitation and transient use of vasopressor agents. She was transfused with 1700 mL of blood, 150 mL of saline solution, and 420 mL of Lactated Ringer’s solution. No intraoperative complications occurred. The patient was extubated uneventfully on postoperative day 1 and was at baseline neurologically with no visual disturbances.
Development and Diagnosis of PRES. The patient made significant progress with physical therapy and developed episodes of hypertension at night on postoperative days 4 to 6. Her mean peak systolic blood pressure was 180 mm Hg. This improved after oral beta-blocker therapy. On postoperative day 6, the patient was ambulating with physical therapy and the aid of a walker. She was found to be neurologically intact, was resting comfortable in a chair reading a book, and was cleared for transfer to a rehabilitation facility the next day. During the morning on postoperative day 7, she developed confusion and visual loss. The patient reported blurry vision followed by complete bilateral painless loss of vision aside from mild light perception. She was unable to identify any objects. She had extinction to double simultaneous stimuli and evidence of agraphesthesia in the left hand. Her neurologic examination was otherwise at baseline. Upon emergent imaging, head CT showed bilateral symmetric areas of hypodensity involving the cortical and subcortical white matter of both occipital lobes (Figure 3). MRI showed extensive bilateral cortical and subcortical signal hyperintensity involving the parietal and occipital lobes (Figure 4). No evidence of petechial or lobar hemorrhage was found.
Treatment and Clinical Course. The patient was transferred to the neurology intensive care unit for neurologic monitoring. She was treated aggressively for recurrent hypertensive episodes. Twenty-four hours after initial blood pressure optimization therapy, she partially recovered her eyesight. She exhibited complete recovery after 48 hours. The patient was discharged to a rehabilitation facility in stable condition on postoperative day 11.
Case 2
Clinical Presentation. A 51-year-old woman presented to the outpatient clinic with progressive low back pain and decompensation due to degenerative adult scoliosis. Her surgical history was significant for an uneventful Caesarean section. Her medical history was significant for borderline hypertension and obesity (body mass index, 34.4). The radiographic examination showed an S-shaped thoracolumbar curve from T4 to L4 (Figure 5).
Operative Procedure. After discussions about the risks and benefits of the procedure, the patient underwent posterior spinal fusion from T3 to pelvis (Mesa, K2M) and interbody fusion from L4 to S1 via a presacral approach using the AxiaLIF system (TranS1) (Figure 6). The operation spanned 507 minutes. The patient lost approximately 2200 mL of blood. She was transfused with 1690 mL of blood, 1250 mL of Lactated Ringer’s solution, and 1 unit (50 mL) of albumin. No intraoperative complications occurred.
Development and Diagnosis of PRES. The patient was ambulatory with physical therapy and a walker on postoperative day 1. Her albumin levels were noted to be decreased postoperatively (28 mg/mL; normal, >35 mg/mL). She developed intermittent hypertensive episodes and experienced transient peripheral vision loss. After her ophthalmologic symptoms cleared, she was discharged and transferred to a rehabilitation facility on postoperative day 9. Eleven days later, the patient was emergently readmitted for a deep spine wound infection after an onset of wound swelling and fever. She underwent irrigation and débridement of the spine wound with an estimated blood loss of 400 mL. The patient continued to have fevers and was placed on ciprofloxacin and vancomycin, which was changed to levofloxacin on postoperative day 5. Elevated creatinine was noted, and the patient was diagnosed with acute renal failure. On postoperative day 7, oxacillin therapy was commenced. After her cultures grew methicillin-resistant Staphylococcus aureus, a peripherally inserted central catheter line was placed on postoperative day 9. As a result of nausea and constipation, the patient received feeding tubes on postoperative day 11. Additionally, she was diagnosed with a pleural effusion on postoperative day 14. Although her creatinine levels were decreasing, she continued to experience intermittent hypertensive episodes with a mean peak systolic blood pressure of 148 mm Hg. On postoperative day 15, she had a seizure and again developed visual loss. The patient was lethargic and followed only simple commands. She moved all extremities and withdrew symmetrically to noxious stimuli. Upon emergent imaging, head CT showed posterior subcortical white matter hypodensity within the occipital and parietal lobes bilaterally (Figure 7). MRI showed focal regions of symmetric hemispheric edema involving the parietal and occipital lobes in a predominantly subcortical white-matter distribution. Additionally, extensive involvement of the splenium and of the corpus callosum, left greater than right, was observed (Figure 8).
Treatment and Clinical Course. The patient was transferred to the intensive care unit for neuromonitoring. Her hypokalemia and hypertension were treated aggressively to normalize her potassium levels and blood pressure. Her oxacillin therapy was changed to daptomycin. On postoperative day 17, the patient was transferred to another institution for further medical management after achieving full recovery of her eyesight after electrolyte and blood pressure corrections.
Discussion
Posterior reversible encephalopathy syndrome is a rare but frequently devastating complication of spinal surgery, with an estimated incidence of 0.094% to 0.2%.7,8 Pediatric patients, as well as patients undergoing deformity correction surgery and posterior lumbar fusion, which necessitate prone positioning, have a significantly increased risk of POVL after spinal surgery.9 There are several causes of POVL after spinal surgery, each with a unique pathophysiology, clinical presentation, and prognosis.
The most common cause of POVL, accounting for 89% of all cases, is ischemic neuropathy.10 Ischemic neuropathy refers to a hypoperfusion or infarction of the anterior or posterior portion of the optic nerve and presents as painless bilateral vision loss or complete blindness on waking from the surgical procedure.11 Risk factors associated with anterior ischemic neuropathy are primarily diabetes mellitus, prone positioning, nocturnal hypotension, and blood loss.11 Posterior ischemic neuropathy has been most strongly correlated with anemia and hypotension.12 The exact etiology of this complication has not been established, although the prognosis is generally unfavorable, with most vision loss being permanent.10-12
Another potential cause of POVL after spinal surgery is retinal artery occlusion. It is most commonly observed in patients who were improperly positioned, resulting in compression of an orbit on the surface of the headrest or the operating table.13 Retinal artery occlusion characteristically presents as an irreversible unilateral complete loss of vision with a red spot on the macula and an afferent pupillary defect.14
Cortical blindness, another possible common cause of POVL, results from the hypoperfusion of the occipital cortex and has a slightly better prognosis. Cortical blindness generally results from an embolic event that can be visualized through neuroimaging and may be unilateral or bilateral, ranging from mild peripheral vision loss to complete blindness.15
Posterior reversible encephalopathy syndrome, the cause of POVL diagnosed in the 2 patients in this case report, is a neurologic syndrome that differs significantly in its clinical presentation and pathophysiology from the more well-known etiologies. The precise pathophysiologic mechanism of the syndrome is yet to be elucidated. One theory revolves around the failure of cerebral vascular autoregulation. It postulates that intracerebellar hypertension leads to the extravasation of proteins and fluid, resulting in the characteristic vasogenic edema.16,17 The other equally discussed theory postulates that cerebellar vasospasm and subsequent hypoperfusion leading to cellular hypoxemia and ischemia may be responsible.18-20 Posterior reversible encephalopathy syndrome has been reported with increasing frequency, particularly in connection with hypertension, acute renal failure associated with malignancy, cytotoxicity, and corticosteroids, as well as preeclampsia, eclampsia, and autoimmune disorders.1-3,21-23 Traditionally, patients display a combination of different symptoms, including vision changes ranging from slightly decreased perception to complete blindness. Unlike retinal artery occlusion and ischemic optic neuropathy, the onset of vision loss often does not happen immediately after surgery and may occur several hours to days after surgery. Visual disturbance may progressively worsen if the medical cause for the syndrome is not determined and corrected.2,3 In contrast to other known etiologies of POVL, PRES has a relatively favorable prognosis if managed appropriately. Reported case series determined a resolution of the characteristic parieto-occipital vasogenic edema in 83% to 88% of all patients in follow-up neuroimaging after aggressive control of seizures and arterial hypertension.2-3
Both patients undergoing spinal deformity surgery in this report suffered from intermittent hypertensive episodes in the postoperative period. One patient also developed acute renal failure during her hospital stay, and demonstrated low albumin levels postoperatively, which has also been associated with PRES.24 Through the immediate diagnosis and primary control of hypertension, both patients achieved complete neurologic recovery after a mean of 1.5 days (range, 1-2 days); this compares to a recovery period of an average 6.2 days (range, 1-14 days) reported by Ni and colleagues.3 The catastrophic effects of a misdiagnosis and incorrect or untimely treatment were well described in this case report. Several patients who were incorrectly diagnosed with demyelinating disorders or lupus encephalopathy received high doses of immunosuppressants and corticosteroids, known risk factors for the development of PRES.3 The patients subsequently rapidly deteriorated; no patients had a full recovery of their preoperative eyesight, and 1 patient developed complete permanent blindness.3 Optimized multidisciplinary collaboration allowing for a rapid neuro-ophthalmic examination and appropriate neuroimaging will permit an accurate and rapid diagnosis, leading to timely intervention and restoration of vision.
Conclusion
Temporary POVL is a potentially devastating complication of spinal surgery and general anesthesia. The more frequent causes such as ischemic optic neuropathy, retinal artery occlusion, and cortical blindness have very limited effective options for treatment and an overall poor prognosis. The inclusion of PRES in the differential diagnosis of POVL may allow early detection, management, and restoration of vision.
1. Hinchey J, Chaves C, Appignani B, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334(8):494-500.
2. Fugate JE, Claassen DO, Cloft HJ, et al. Posterior reversible encephalopathy syndrome: associated clinical and radiologic findings. Mayo Clin Proc. 2010;85(5):427-432.
3. Ni J, Zhou LX, Hao HL, et al. The clinical and radiological spectrum of posterior reversible encephalopathy syndrome: a retrospective series of 24 patients. J Neuroimaging. 2011;21(3):219-224.
4. Stevens CJ, Heran MK. The many faces of posterior reversible encephalopathy syndrome. Br J Radiol. 2012;85(1020):1566-1575.
5. Bartynski WS. Posterior reversible encephalopathy syndrome, part 1: fundamental imaging and clinical features. AJNR Am J Neuroradiol. 2008;29(6):1036-1042.
6. Yoon SD, Cho BM, Oh SM, et al. Clinical and radiological spectrum of posterior reversible encephalopathy syndrome. J Cerebrovasc Endovasc Neurosurg. 2013;15(3):206-213.
7. Patil CG, Lad EM, Lad SP, Ho C, Boakye M. Visual loss after spine surgery: a population-based study. Spine (Phila Pa 1976). 2008;33(13):1491-1496.
8. Stevens WR, Glazer PA, Kelley SD, Lietman TM, Bradford DS. Ophthalmic complications after spinal surgery. Spine (Phila Pa 1976). 1997;22(12):1319-1324.
9. Shen Y, Drum M, Roth S. The prevalence of perioperative visual loss in the United States: a 10-year study from 1996 to 2005 of spinal, orthopedic, cardiac, and general surgery. Anesth Analg. 2009;109(5):1534-1545.
10. Lee LA, Roth S, Posner KL, et al. The American Society of Anesthesiologists Postoperative Visual Loss Registry: analysis of 93 spine surgery cases with postoperative visual loss. Anesthesiology. 2006;105(4):652-659; quiz 867-868.
11. Hayreh SS. Ischemic optic neuropathies - where are we now? Graefes Arch Clin Exp Ophthalmol. 2013;251(8):1873-1884.
12. Buono LM, Foroozan R. Perioperative posterior ischemic optic neuropathy: review of the literature. Surv Ophthalmol. 2005;50(1):15-26.
13. Katz DA, Karlin LI. Visual field defect after posterior spine fusion. Spine (Phila Pa 1976). 2005;30(3):E83-E85.
14. Hayreh SS, Kolder HE, Weingeist TA. Central retinal artery occlusion and retinal tolerance time. Ophthalmology. 1980;87(1):75-78.
15. Berg KT, Harrison AR, Lee MS. Perioperative visual loss in ocular and nonocular surgery. Clin Ophthalmol. 2010;4:531-546.
16. Primavera A, Audenino D, Mavilio N, Cocito L. Reversible posterior leucoencephalopathy syndrome in systemic lupus and vasculitis. Ann Rheum Dis. 2001;60(5):534-537.
17. Bartynski WS, Boardman JF. Catheter angiography, MR angiography, and MR perfusion in posterior reversible encephalopathy syndrome. AJNR Am J Neuroradiol. 2008;29(3):447-455.
18. Ito T, Sakai T, Inagawa S, Utsu M, Bun T. MR angiography of cerebral vasospasm in preeclampsia. AJNR Am J Neuroradiol. 1995;16(6):1344-1346.
19. Agarwal R, Davis C, Altinok D, Serajee FJ. Posterior reversible encephalopathy and cerebral vasoconstriction in a patient with hemolytic uremic syndrome. Pediatr Neurol. 2014;50(5):518-521.
20. Bartynski WS. Posterior reversible encephalopathy syndrome, part 2: controversies surrounding pathophysiology of vasogenic edema. AJNR Am J Neuroradiol. 2008;29(6):1043-1049.
21. Lee VH, Wijdicks EF, Manno EM, Rabinstein AA. Clinical spectrum of reversible posterior leukoencephalopathy syndrome. Arch Neurol. 2008;65(2):205-210.
22. Ekawa Y, Shiota M, Tobiume T, et al. Reversible posterior leukoencephalopathy syndrome accompanying eclampsia: correct diagnosis using preoperative MRI. Tohoku J Exp Med. 2012;226(1):55-58.
23. Kur JK, Esdaile JM. Posterior reversible encephalopathy syndrome--an underrecognized manifestation of systemic lupus erythematosus. J Rheumatol. 2006;33(11):2178-2183.
24. Pirker A, Kramer L, Voller B, et al. Type of edema in posterior reversible encephalopathy syndrome depends on serum albumin levels: an MR imaging study in 28 patients. AJNR Am J Neuroradiol. 2011;32(3):527-531.
1. Hinchey J, Chaves C, Appignani B, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334(8):494-500.
2. Fugate JE, Claassen DO, Cloft HJ, et al. Posterior reversible encephalopathy syndrome: associated clinical and radiologic findings. Mayo Clin Proc. 2010;85(5):427-432.
3. Ni J, Zhou LX, Hao HL, et al. The clinical and radiological spectrum of posterior reversible encephalopathy syndrome: a retrospective series of 24 patients. J Neuroimaging. 2011;21(3):219-224.
4. Stevens CJ, Heran MK. The many faces of posterior reversible encephalopathy syndrome. Br J Radiol. 2012;85(1020):1566-1575.
5. Bartynski WS. Posterior reversible encephalopathy syndrome, part 1: fundamental imaging and clinical features. AJNR Am J Neuroradiol. 2008;29(6):1036-1042.
6. Yoon SD, Cho BM, Oh SM, et al. Clinical and radiological spectrum of posterior reversible encephalopathy syndrome. J Cerebrovasc Endovasc Neurosurg. 2013;15(3):206-213.
7. Patil CG, Lad EM, Lad SP, Ho C, Boakye M. Visual loss after spine surgery: a population-based study. Spine (Phila Pa 1976). 2008;33(13):1491-1496.
8. Stevens WR, Glazer PA, Kelley SD, Lietman TM, Bradford DS. Ophthalmic complications after spinal surgery. Spine (Phila Pa 1976). 1997;22(12):1319-1324.
9. Shen Y, Drum M, Roth S. The prevalence of perioperative visual loss in the United States: a 10-year study from 1996 to 2005 of spinal, orthopedic, cardiac, and general surgery. Anesth Analg. 2009;109(5):1534-1545.
10. Lee LA, Roth S, Posner KL, et al. The American Society of Anesthesiologists Postoperative Visual Loss Registry: analysis of 93 spine surgery cases with postoperative visual loss. Anesthesiology. 2006;105(4):652-659; quiz 867-868.
11. Hayreh SS. Ischemic optic neuropathies - where are we now? Graefes Arch Clin Exp Ophthalmol. 2013;251(8):1873-1884.
12. Buono LM, Foroozan R. Perioperative posterior ischemic optic neuropathy: review of the literature. Surv Ophthalmol. 2005;50(1):15-26.
13. Katz DA, Karlin LI. Visual field defect after posterior spine fusion. Spine (Phila Pa 1976). 2005;30(3):E83-E85.
14. Hayreh SS, Kolder HE, Weingeist TA. Central retinal artery occlusion and retinal tolerance time. Ophthalmology. 1980;87(1):75-78.
15. Berg KT, Harrison AR, Lee MS. Perioperative visual loss in ocular and nonocular surgery. Clin Ophthalmol. 2010;4:531-546.
16. Primavera A, Audenino D, Mavilio N, Cocito L. Reversible posterior leucoencephalopathy syndrome in systemic lupus and vasculitis. Ann Rheum Dis. 2001;60(5):534-537.
17. Bartynski WS, Boardman JF. Catheter angiography, MR angiography, and MR perfusion in posterior reversible encephalopathy syndrome. AJNR Am J Neuroradiol. 2008;29(3):447-455.
18. Ito T, Sakai T, Inagawa S, Utsu M, Bun T. MR angiography of cerebral vasospasm in preeclampsia. AJNR Am J Neuroradiol. 1995;16(6):1344-1346.
19. Agarwal R, Davis C, Altinok D, Serajee FJ. Posterior reversible encephalopathy and cerebral vasoconstriction in a patient with hemolytic uremic syndrome. Pediatr Neurol. 2014;50(5):518-521.
20. Bartynski WS. Posterior reversible encephalopathy syndrome, part 2: controversies surrounding pathophysiology of vasogenic edema. AJNR Am J Neuroradiol. 2008;29(6):1043-1049.
21. Lee VH, Wijdicks EF, Manno EM, Rabinstein AA. Clinical spectrum of reversible posterior leukoencephalopathy syndrome. Arch Neurol. 2008;65(2):205-210.
22. Ekawa Y, Shiota M, Tobiume T, et al. Reversible posterior leukoencephalopathy syndrome accompanying eclampsia: correct diagnosis using preoperative MRI. Tohoku J Exp Med. 2012;226(1):55-58.
23. Kur JK, Esdaile JM. Posterior reversible encephalopathy syndrome--an underrecognized manifestation of systemic lupus erythematosus. J Rheumatol. 2006;33(11):2178-2183.
24. Pirker A, Kramer L, Voller B, et al. Type of edema in posterior reversible encephalopathy syndrome depends on serum albumin levels: an MR imaging study in 28 patients. AJNR Am J Neuroradiol. 2011;32(3):527-531.
Posttraumatic Saphenous Neuroma After Open Tibial Fracture
Neuralgia and neuroma secondary to iatrogenic saphenous nerve injury have been described in the setting of orthopedic surgical interventions using a medial parapatellar approach, and in vascular surgery procedures for harvest of the saphenous vein.1-3 However, postoperative neuropathic pain caused by saphenous neuroma in the setting of orthopedic trauma has not been described.
We present a case of symptomatic posttraumatic saphenous neuroma after a displaced and laterally angulated open distal one-third tibial fracture. This unreported cause of postinjury neuralgia is an important complication to address as other similar and more common conditions, such as peripheral neuropathy and complex regional pain syndrome (CRPS), can present in a similar manner. Reaching the correct diagnosis can be challenging for clinicians unfamiliar with this condition or its clinical presentation. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 43-year-old woman presented to our practice 2 years after an open distal one-third metadiaphyseal fracture of the tibia with associated segmental fibular fracture (Gustilo-Anderson type II)4 after an automobile/bicycle accident. At the time of injury, she was noted to have a complex medial wound in the region of an open fracture at the junction of the middle and distal thirds of her tibial shaft. She underwent definitive treatment at an outside facility with initial irrigation and débridement and primary wound closure, followed by staged intramedullary nail fixation. Both soft-tissue and bony injuries healed within the expected time frame, and the patient was discharged from orthopedic care.
Approximately 1 year after her initial injury, the patient began to complain of progressive and persistent anteromedial knee pain as well as gradual-onset, medial-sided leg pain. The leg pain began at the level of her previous fracture site, at the distal one-third metadiaphyseal tibial junction, and radiated from the site of her previous medial open wound distally to the medial aspect of her foot. The pain was burning and tingling in nature, with associated hyperesthesia of the affected area. A diagnosis of CRPS was made, and the patient was prescribed a course of desensitization therapy, oral neuromodulating agents, and physical therapy. After 3 months’ therapy, she remained symptomatic and underwent removal of her proximal tibial interlocking screw fixation (Figure 1). When these measures failed to provide symptomatic relief, and having seen several therapists and physicians, including physiatrists, pain management specialists, and orthopedic surgeons, she presented to our clinic for consultation.
Diagnostic Assessment
On presentation, the patient’s surgical incisions were well healed. At the junction of the middle and distal thirds of the tibia, a 4-cm oblique scar was noted over the anteromedial border of the tibia, the site of her previous open fracture. She demonstrated decreased sensation along the length of this oblique scar, as well as in the distribution of the saphenous nerve distally. Further examination of the previously injured region revealed a positive Tinel sign over the course of the saphenous nerve, with radiating pain down the medial aspect of her leg, recreating her symptoms. She otherwise had full range of motion at the knee with mild tenderness to palpation at the medial joint line and patellar tendon. Her lower extremity motor examination, reflexes, and the remainder of her sensory examination were benign.
These findings were consistent with isolated saphenous neuralgia, and selective injection of the saphenous distribution over the injury site was performed. This injection provided immediate symptomatic relief, with the patient reporting preinjection and postinjection pain scores of 7/10 and 2/10, respectively. Because of the clinical improvement demonstrated with selective injection, surgical intervention with exploration and neurolysis of the saphenous nerve was recommended.
Therapeutic Intervention
The patient underwent surgical exploration of her saphenous nerve at the level of her original open fracture. This was done concurrently with a left-knee diagnostic arthroscopy and removal of her intramedullary tibial implant both to exclude intra-articular pathology (given her medial joint-line tenderness and the limitation of magnetic resonance imaging to diagnose meniscus tear in the presence of her tibial hardware) and to remove any potential hardware irritation in the setting of her anterior knee pain.5,6
Preoperatively, the path of the saphenous nerve was marked using the saphenous vein as a guide. An incision overlying the presumed saphenous nerve course was made at the site of her previous open wound and clinical Tinel sign. The saphenous nerve was carefully dissected with loupe magnification, and the distal divisions of the anterior and posterior branches were identified. The anterior branch was found to be in continuity but encased in fibrotic neuroma. Selective neurolysis of this anterior branch was performed. The posterior branch was found to have been traumatically severed, with both the proximal and distal ends encased in neuromatous scar (Figure 2). Neurectomy of the posterior branch was performed and the severed proximal end of the nerve was buried into the adjacent medial gastrocnemius muscle beneath the fascia of the superficial posterior compartment.7-9
Postoperative Course and Outcome
Upon transport to the postsurgical care unit and emergence from sedation, the patient experienced immediate resolution of her neuralgic symptoms. Pathology of the operative specimen showed a benign, disorganized arrangement of axons, Schwann cells, and perineural fibroblasts amidst a fibrous stroma, consistent with traumatic neuroma. At 1-month and 6-month follow-up visits, the patient remained symptom-free, aside from some continued anterior left knee pain near the site of intramedullary nail entry. Her positive Tinel sign had completely resolved, as did her neuralgic symptoms down the medial aspect of her leg. This proved consistent with a diagnosis of neuroma as the cause of the majority of her symptoms. Subjectively, she reported excellent overall pain relief and satisfaction with her treatment and postoperative course.
Discussion
Postoperative pain after intramedullary fixation of tibial shaft fractures is common and can be caused by several clinical entities.5,10 Anterior knee pain is a well-known complication present in up to 73% of patients treated with tibial nailing.10 Osteoarthritis of the knee or ankle as well as nonarthritic ipsilateral ankle pain are also common complaints, often resulting from tibial malunion or malrotation, leading to altered joint kinematics.11 Additionally, superficial peroneal nerve and tibial neurovascular bundle injuries have been reported as potential complications of distal interlocking screw placement, and should be considered in such patients.12
Another consideration for the development of postoperative pain is CRPS, which is thought to be caused by postinjury sympathetic activation that produces pain out of proportion to clinical examination findings.13 Although no postoperative incidence of CRPS in the setting of tibial nailing has been reported, it is a known contributor to poor functional outcomes after fractures or crush injuries to the lower extremity.9 When attempting to diagnose and treat chronic postoperative pain after tibial nailing, the clinician must keep these common etiologies in mind as well as an understanding of the adjacent anatomy.
The saphenous nerve originates from the third and fourth lumbar nerve roots, coursing beneath the inguinal ligament as part of the femoral nerve. As the terminal branch of the femoral nerve, the saphenous nerve runs in the Hunter canal beneath the fascia of the sartorius muscle. It is bordered laterally by the vastus medialis muscle, and posteriorly and laterally by the adductor longus and magnus muscles. The saphenous nerve then crosses the femoral artery superficially from medial to lateral as it courses distally in the canal. As it emerges from the adductor hiatus, the saphenous nerve runs superficial to the gracilis muscle around the posterior border of the sartorius muscle with the descending genicular artery, and becomes a subcutaneous structure at the level of the knee joint. The infrapatellar branch of the saphenous nerve provides sensation to the medial knee, and continues in a subcutaneous course just medial to the posterior aspect of the tibial shaft with the great saphenous vein. The nerve distally supplies sensory input from the medial foot and ankle.1,3,14
There are several causes of saphenous neuralgia related to surgical and nonsurgical trauma.2,3,15,16 The most common cause of nerve injury is iatrogenic traction or transection causing neuralgic sequelae from subsequent neuroma formation. The anatomy of the saphenous nerve puts it at particular risk when performing saphenectomy for vascular procedures, and its infrapatellar branch is at particular risk when performing a medial parapatellar approach for total knee arthroplasty.2,3 In the case of the surgically naïve patient, saphenous nerve entrapment syndromes have also been described, and occur most frequently at the level of the adductor hiatus or as the saphenous nerve courses between the sartorius and gracilis muscles proximal to the knee joint.16
As is illustrated in the present case, orthopedic trauma may be an additional cause of saphenous neuroma formation, leading to symptomatic neuralgia. This case suggests that symptomatic neuroma should be included in the differential diagnosis of posttraumatic pain in the orthopedic trauma patient. It is important to note that, although this case occurred after a severe injury, the intimate association of the saphenous nerve with the tibia places it in a vulnerable position, and traumatic transection is possible after closed injuries to the tibial metadiaphyseal junction or tibial shaft.
Neuroma formation occurs in response to damage to the endoneurium and axon. For an axon to repair properly, the damaged proximal segment must join with, and reenter, the distal stump. As axons attempt to regenerate, occasionally the proximal stump can escape into the surrounding tissue and form a painful neuroma consisting of a disorganized mass of Schwann cells, fibroblasts, blood vessels, and axons with various degrees of myelination. The subsequent neuralgia associated with neuroma formation is caused by chemical or mechanical stimulation of the damaged axons or by spontaneously evoked potentials in the damaged axons. These signals can manifest as a variety of symptoms, including paresthesia and allodynia.17,18
Making the diagnosis of neuroma-related neuralgia can be challenging and nebulous. A characteristic history and positive Tinel sign over the affected area are helpful clinical indicators. However, the clinical finding most predictive of favorable surgical outcome is symptomatic relief after local injection of 1% lidocaine to the affected area. This is an important diagnostic test, especially when attempting to differentiate painful neuroma from other causes of posttraumatic lower extremity pain (eg, CRPS). Such an injection should be performed in the diagnosis and treatment of symptomatic neuroma, and some authors would suggest that insufficient relief of symptoms with diagnostic nerve block is a contraindication to surgical treatment.19
Several treatments for painful neuromas have been described, with variable results.19,20 The most widely accepted treatment of a complete nerve transection with associated neuroma is neurectomy with reimplantation of the proximal end into adjacent bone, muscle, or vein.14,15 Balcin and colleagues21 suggest that vein transposition produces the most favorable outcomes. Simple neurolysis of in-continuity neuromas has also been described with favorable results.
Conclusion
Neuralgia-producing neuromas of the saphenous nerve are relatively uncommon but can lead to persistent pain and frustrating symptoms for the patient. As noted, the diagnosis may elude clinicians, especially in patients with less obvious clinical presentations. We suggest the following algorithm to help distinguish between painful neuroma and other causes of posttraumatic leg pain: (1) physical examination (including testing for instability, joint line tenderness, patellofemoral pain, Tinel sign, and Semmes-Weinstein testing) should be performed, and plain radiographs taken of the involved bones and joints; (2) if all of the above reveal no abnormality, and there is a positive Tinel sign directly over the course of a nerve, an injection of lidocaine over the region of the potential neuroma can be diagnostic; (3) should several abnormalities be present, further investigation using magnetic resonance imaging, bone scan, and/or electromyography may provide additional information that leads to a diagnosis.
1. Senegor M. Iatrogenic saphenous neuralgia: successful treatment with neuroma resection. J Neurosurg. 1991;28(2):295-298.
2. Mountney J, Wilkinson GA. Saphenous neuralgia after coronary artery bypass grafting. Eur J Cardiothorac Surg. 1999;16(4):440-443.
3. Kachar SM, Williams KM, Finn HA. Neuroma of the infrapatellar branch of the saphenous nerve: a cause of reversible knee stiffness after total knee arthroplasty. J Arthroplasty. 2008;23(6):927-930.
4. Gustilo RB, Anderson AB. Prevention of infection in the treatment of one thousand and twenty-five open fractures of long bones: retrospective and prospective analyses. J Bone Joint Surg Am. 1976;58(4):453-458.
5. Keating JF, Orfaly R, O’Brien PJ. Knee pain after tibial nailing. J Orthop Trauma. 1997;11(1):10-13.
6. Chen CY, Lin KC, Yang SW, Tarng YW, Hsu CJ, Renn JH. Influence of nail prominence and insertion point on anterior knee pain after tibial intramedullary nailing. Orthopedics. 2014;37(3):e221-e225.
7. Lewin-Kowalik J, Marcol W, Kotulska K, Mandera M, Klimczak A. Prevention and management of painful neuroma. Neurol Med Chir (Tokyo). 2006;46(2):62-67.
8. Otfinowski J, Pawelec A, Kaluza J. Implantation of peripheral neural stump into muscle and its effect on the development of posttraumatic neuroma. Pol J Pathol. 1994;45:195-202.
9. Van Beek AL. Management of nerve compression syndromes and painful neuromas. In: McCarthy JG, May JW Jr, Littler JW, eds. Plastic Surgery. Philadelphia, PA: WB Saunders; 1990:4817-4858.
10. Lefaivre KA, Guy P, Chan H, Blachut PA. Long-term follow-up of tibial shaft fractures treated with intramedullary nailing. J Orthop Trauma. 2008;22(8):525-529.
11. Milner SA, Davis TR, Muir KR, Greenwood DC, Doherty M. Long-term outcome after tibial shaft fracture: is malunion important? J Bone Joint Surg Am. 2002;84(6):971-980.
12. Roberts CS, King D, Wang M, Seligson D, Voor MJ. Should distal interlocking of tibial nails be performed from medial or lateral direction? Anatomical and biomechanical considerations. J Orthop Trauma. 1999;13(1):27-32.
13. Hogan CJ, Hurwitz SR. Treatment of complex regional pain syndrome of the lower extremity. J Am Acad Orthop Surg. 2002;10(4):281-289.
14. Gray H, Lewis WH. Anatomy of the Human Body. Philadelphia, PA: Lea & Febiger, 1918. Bartleby.com website. http://www.bartleby.com/br/107.html. Accessed September 29, 2015.
15. Myerson MS, McGarvey WC, Henderson MR, Hakim J. Morbidity after crush injuries to the foot. J Orthop Trauma. 1994;8(4):343-349.
16. Kalenak A. Saphenous nerve entrapment. Op Tech Sports Med. 1996;4(1):40-45.
17. Wolf SW, Hotchkiss RN, Pederson WC, Kozin SH. The peripheral neuroma. In: Green DP, Wolfe SW, eds. Green’s Operative Hand Surgery. 6th ed. Philadelphia, PA: Elsevier Churchill Livingstone, 2011;1063-1071.
18. Thordarson DB, Shean CJ. Nerve and tendon lacerations about the foot and ankle. J Am Acad Orthop Surg. 2005;13(3):186-196.
19. Stokvis A, van der Avoort DJ, van Neck JW, Hovius SE, Coert JH. Surgical management of neuroma pain: a prospective follow-up study. Pain. 2010;151(3):862-869.
20. Burchiel KJ, Johans TJ, Ochoa J. The surgical treatment of painful traumatic neuromas. J Neurosurg. 1993;78(5):714-719.
21. Balcin H, Erba P, Wettstein R, Schaefer DJ, Pierer G, Kalbermatten DF. A comparative study of two methods of surgical treatment for painful neuroma. J Bone Joint Surg Br. 2009;91(6):803-808.
Neuralgia and neuroma secondary to iatrogenic saphenous nerve injury have been described in the setting of orthopedic surgical interventions using a medial parapatellar approach, and in vascular surgery procedures for harvest of the saphenous vein.1-3 However, postoperative neuropathic pain caused by saphenous neuroma in the setting of orthopedic trauma has not been described.
We present a case of symptomatic posttraumatic saphenous neuroma after a displaced and laterally angulated open distal one-third tibial fracture. This unreported cause of postinjury neuralgia is an important complication to address as other similar and more common conditions, such as peripheral neuropathy and complex regional pain syndrome (CRPS), can present in a similar manner. Reaching the correct diagnosis can be challenging for clinicians unfamiliar with this condition or its clinical presentation. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 43-year-old woman presented to our practice 2 years after an open distal one-third metadiaphyseal fracture of the tibia with associated segmental fibular fracture (Gustilo-Anderson type II)4 after an automobile/bicycle accident. At the time of injury, she was noted to have a complex medial wound in the region of an open fracture at the junction of the middle and distal thirds of her tibial shaft. She underwent definitive treatment at an outside facility with initial irrigation and débridement and primary wound closure, followed by staged intramedullary nail fixation. Both soft-tissue and bony injuries healed within the expected time frame, and the patient was discharged from orthopedic care.
Approximately 1 year after her initial injury, the patient began to complain of progressive and persistent anteromedial knee pain as well as gradual-onset, medial-sided leg pain. The leg pain began at the level of her previous fracture site, at the distal one-third metadiaphyseal tibial junction, and radiated from the site of her previous medial open wound distally to the medial aspect of her foot. The pain was burning and tingling in nature, with associated hyperesthesia of the affected area. A diagnosis of CRPS was made, and the patient was prescribed a course of desensitization therapy, oral neuromodulating agents, and physical therapy. After 3 months’ therapy, she remained symptomatic and underwent removal of her proximal tibial interlocking screw fixation (Figure 1). When these measures failed to provide symptomatic relief, and having seen several therapists and physicians, including physiatrists, pain management specialists, and orthopedic surgeons, she presented to our clinic for consultation.
Diagnostic Assessment
On presentation, the patient’s surgical incisions were well healed. At the junction of the middle and distal thirds of the tibia, a 4-cm oblique scar was noted over the anteromedial border of the tibia, the site of her previous open fracture. She demonstrated decreased sensation along the length of this oblique scar, as well as in the distribution of the saphenous nerve distally. Further examination of the previously injured region revealed a positive Tinel sign over the course of the saphenous nerve, with radiating pain down the medial aspect of her leg, recreating her symptoms. She otherwise had full range of motion at the knee with mild tenderness to palpation at the medial joint line and patellar tendon. Her lower extremity motor examination, reflexes, and the remainder of her sensory examination were benign.
These findings were consistent with isolated saphenous neuralgia, and selective injection of the saphenous distribution over the injury site was performed. This injection provided immediate symptomatic relief, with the patient reporting preinjection and postinjection pain scores of 7/10 and 2/10, respectively. Because of the clinical improvement demonstrated with selective injection, surgical intervention with exploration and neurolysis of the saphenous nerve was recommended.
Therapeutic Intervention
The patient underwent surgical exploration of her saphenous nerve at the level of her original open fracture. This was done concurrently with a left-knee diagnostic arthroscopy and removal of her intramedullary tibial implant both to exclude intra-articular pathology (given her medial joint-line tenderness and the limitation of magnetic resonance imaging to diagnose meniscus tear in the presence of her tibial hardware) and to remove any potential hardware irritation in the setting of her anterior knee pain.5,6
Preoperatively, the path of the saphenous nerve was marked using the saphenous vein as a guide. An incision overlying the presumed saphenous nerve course was made at the site of her previous open wound and clinical Tinel sign. The saphenous nerve was carefully dissected with loupe magnification, and the distal divisions of the anterior and posterior branches were identified. The anterior branch was found to be in continuity but encased in fibrotic neuroma. Selective neurolysis of this anterior branch was performed. The posterior branch was found to have been traumatically severed, with both the proximal and distal ends encased in neuromatous scar (Figure 2). Neurectomy of the posterior branch was performed and the severed proximal end of the nerve was buried into the adjacent medial gastrocnemius muscle beneath the fascia of the superficial posterior compartment.7-9
Postoperative Course and Outcome
Upon transport to the postsurgical care unit and emergence from sedation, the patient experienced immediate resolution of her neuralgic symptoms. Pathology of the operative specimen showed a benign, disorganized arrangement of axons, Schwann cells, and perineural fibroblasts amidst a fibrous stroma, consistent with traumatic neuroma. At 1-month and 6-month follow-up visits, the patient remained symptom-free, aside from some continued anterior left knee pain near the site of intramedullary nail entry. Her positive Tinel sign had completely resolved, as did her neuralgic symptoms down the medial aspect of her leg. This proved consistent with a diagnosis of neuroma as the cause of the majority of her symptoms. Subjectively, she reported excellent overall pain relief and satisfaction with her treatment and postoperative course.
Discussion
Postoperative pain after intramedullary fixation of tibial shaft fractures is common and can be caused by several clinical entities.5,10 Anterior knee pain is a well-known complication present in up to 73% of patients treated with tibial nailing.10 Osteoarthritis of the knee or ankle as well as nonarthritic ipsilateral ankle pain are also common complaints, often resulting from tibial malunion or malrotation, leading to altered joint kinematics.11 Additionally, superficial peroneal nerve and tibial neurovascular bundle injuries have been reported as potential complications of distal interlocking screw placement, and should be considered in such patients.12
Another consideration for the development of postoperative pain is CRPS, which is thought to be caused by postinjury sympathetic activation that produces pain out of proportion to clinical examination findings.13 Although no postoperative incidence of CRPS in the setting of tibial nailing has been reported, it is a known contributor to poor functional outcomes after fractures or crush injuries to the lower extremity.9 When attempting to diagnose and treat chronic postoperative pain after tibial nailing, the clinician must keep these common etiologies in mind as well as an understanding of the adjacent anatomy.
The saphenous nerve originates from the third and fourth lumbar nerve roots, coursing beneath the inguinal ligament as part of the femoral nerve. As the terminal branch of the femoral nerve, the saphenous nerve runs in the Hunter canal beneath the fascia of the sartorius muscle. It is bordered laterally by the vastus medialis muscle, and posteriorly and laterally by the adductor longus and magnus muscles. The saphenous nerve then crosses the femoral artery superficially from medial to lateral as it courses distally in the canal. As it emerges from the adductor hiatus, the saphenous nerve runs superficial to the gracilis muscle around the posterior border of the sartorius muscle with the descending genicular artery, and becomes a subcutaneous structure at the level of the knee joint. The infrapatellar branch of the saphenous nerve provides sensation to the medial knee, and continues in a subcutaneous course just medial to the posterior aspect of the tibial shaft with the great saphenous vein. The nerve distally supplies sensory input from the medial foot and ankle.1,3,14
There are several causes of saphenous neuralgia related to surgical and nonsurgical trauma.2,3,15,16 The most common cause of nerve injury is iatrogenic traction or transection causing neuralgic sequelae from subsequent neuroma formation. The anatomy of the saphenous nerve puts it at particular risk when performing saphenectomy for vascular procedures, and its infrapatellar branch is at particular risk when performing a medial parapatellar approach for total knee arthroplasty.2,3 In the case of the surgically naïve patient, saphenous nerve entrapment syndromes have also been described, and occur most frequently at the level of the adductor hiatus or as the saphenous nerve courses between the sartorius and gracilis muscles proximal to the knee joint.16
As is illustrated in the present case, orthopedic trauma may be an additional cause of saphenous neuroma formation, leading to symptomatic neuralgia. This case suggests that symptomatic neuroma should be included in the differential diagnosis of posttraumatic pain in the orthopedic trauma patient. It is important to note that, although this case occurred after a severe injury, the intimate association of the saphenous nerve with the tibia places it in a vulnerable position, and traumatic transection is possible after closed injuries to the tibial metadiaphyseal junction or tibial shaft.
Neuroma formation occurs in response to damage to the endoneurium and axon. For an axon to repair properly, the damaged proximal segment must join with, and reenter, the distal stump. As axons attempt to regenerate, occasionally the proximal stump can escape into the surrounding tissue and form a painful neuroma consisting of a disorganized mass of Schwann cells, fibroblasts, blood vessels, and axons with various degrees of myelination. The subsequent neuralgia associated with neuroma formation is caused by chemical or mechanical stimulation of the damaged axons or by spontaneously evoked potentials in the damaged axons. These signals can manifest as a variety of symptoms, including paresthesia and allodynia.17,18
Making the diagnosis of neuroma-related neuralgia can be challenging and nebulous. A characteristic history and positive Tinel sign over the affected area are helpful clinical indicators. However, the clinical finding most predictive of favorable surgical outcome is symptomatic relief after local injection of 1% lidocaine to the affected area. This is an important diagnostic test, especially when attempting to differentiate painful neuroma from other causes of posttraumatic lower extremity pain (eg, CRPS). Such an injection should be performed in the diagnosis and treatment of symptomatic neuroma, and some authors would suggest that insufficient relief of symptoms with diagnostic nerve block is a contraindication to surgical treatment.19
Several treatments for painful neuromas have been described, with variable results.19,20 The most widely accepted treatment of a complete nerve transection with associated neuroma is neurectomy with reimplantation of the proximal end into adjacent bone, muscle, or vein.14,15 Balcin and colleagues21 suggest that vein transposition produces the most favorable outcomes. Simple neurolysis of in-continuity neuromas has also been described with favorable results.
Conclusion
Neuralgia-producing neuromas of the saphenous nerve are relatively uncommon but can lead to persistent pain and frustrating symptoms for the patient. As noted, the diagnosis may elude clinicians, especially in patients with less obvious clinical presentations. We suggest the following algorithm to help distinguish between painful neuroma and other causes of posttraumatic leg pain: (1) physical examination (including testing for instability, joint line tenderness, patellofemoral pain, Tinel sign, and Semmes-Weinstein testing) should be performed, and plain radiographs taken of the involved bones and joints; (2) if all of the above reveal no abnormality, and there is a positive Tinel sign directly over the course of a nerve, an injection of lidocaine over the region of the potential neuroma can be diagnostic; (3) should several abnormalities be present, further investigation using magnetic resonance imaging, bone scan, and/or electromyography may provide additional information that leads to a diagnosis.
Neuralgia and neuroma secondary to iatrogenic saphenous nerve injury have been described in the setting of orthopedic surgical interventions using a medial parapatellar approach, and in vascular surgery procedures for harvest of the saphenous vein.1-3 However, postoperative neuropathic pain caused by saphenous neuroma in the setting of orthopedic trauma has not been described.
We present a case of symptomatic posttraumatic saphenous neuroma after a displaced and laterally angulated open distal one-third tibial fracture. This unreported cause of postinjury neuralgia is an important complication to address as other similar and more common conditions, such as peripheral neuropathy and complex regional pain syndrome (CRPS), can present in a similar manner. Reaching the correct diagnosis can be challenging for clinicians unfamiliar with this condition or its clinical presentation. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 43-year-old woman presented to our practice 2 years after an open distal one-third metadiaphyseal fracture of the tibia with associated segmental fibular fracture (Gustilo-Anderson type II)4 after an automobile/bicycle accident. At the time of injury, she was noted to have a complex medial wound in the region of an open fracture at the junction of the middle and distal thirds of her tibial shaft. She underwent definitive treatment at an outside facility with initial irrigation and débridement and primary wound closure, followed by staged intramedullary nail fixation. Both soft-tissue and bony injuries healed within the expected time frame, and the patient was discharged from orthopedic care.
Approximately 1 year after her initial injury, the patient began to complain of progressive and persistent anteromedial knee pain as well as gradual-onset, medial-sided leg pain. The leg pain began at the level of her previous fracture site, at the distal one-third metadiaphyseal tibial junction, and radiated from the site of her previous medial open wound distally to the medial aspect of her foot. The pain was burning and tingling in nature, with associated hyperesthesia of the affected area. A diagnosis of CRPS was made, and the patient was prescribed a course of desensitization therapy, oral neuromodulating agents, and physical therapy. After 3 months’ therapy, she remained symptomatic and underwent removal of her proximal tibial interlocking screw fixation (Figure 1). When these measures failed to provide symptomatic relief, and having seen several therapists and physicians, including physiatrists, pain management specialists, and orthopedic surgeons, she presented to our clinic for consultation.
Diagnostic Assessment
On presentation, the patient’s surgical incisions were well healed. At the junction of the middle and distal thirds of the tibia, a 4-cm oblique scar was noted over the anteromedial border of the tibia, the site of her previous open fracture. She demonstrated decreased sensation along the length of this oblique scar, as well as in the distribution of the saphenous nerve distally. Further examination of the previously injured region revealed a positive Tinel sign over the course of the saphenous nerve, with radiating pain down the medial aspect of her leg, recreating her symptoms. She otherwise had full range of motion at the knee with mild tenderness to palpation at the medial joint line and patellar tendon. Her lower extremity motor examination, reflexes, and the remainder of her sensory examination were benign.
These findings were consistent with isolated saphenous neuralgia, and selective injection of the saphenous distribution over the injury site was performed. This injection provided immediate symptomatic relief, with the patient reporting preinjection and postinjection pain scores of 7/10 and 2/10, respectively. Because of the clinical improvement demonstrated with selective injection, surgical intervention with exploration and neurolysis of the saphenous nerve was recommended.
Therapeutic Intervention
The patient underwent surgical exploration of her saphenous nerve at the level of her original open fracture. This was done concurrently with a left-knee diagnostic arthroscopy and removal of her intramedullary tibial implant both to exclude intra-articular pathology (given her medial joint-line tenderness and the limitation of magnetic resonance imaging to diagnose meniscus tear in the presence of her tibial hardware) and to remove any potential hardware irritation in the setting of her anterior knee pain.5,6
Preoperatively, the path of the saphenous nerve was marked using the saphenous vein as a guide. An incision overlying the presumed saphenous nerve course was made at the site of her previous open wound and clinical Tinel sign. The saphenous nerve was carefully dissected with loupe magnification, and the distal divisions of the anterior and posterior branches were identified. The anterior branch was found to be in continuity but encased in fibrotic neuroma. Selective neurolysis of this anterior branch was performed. The posterior branch was found to have been traumatically severed, with both the proximal and distal ends encased in neuromatous scar (Figure 2). Neurectomy of the posterior branch was performed and the severed proximal end of the nerve was buried into the adjacent medial gastrocnemius muscle beneath the fascia of the superficial posterior compartment.7-9
Postoperative Course and Outcome
Upon transport to the postsurgical care unit and emergence from sedation, the patient experienced immediate resolution of her neuralgic symptoms. Pathology of the operative specimen showed a benign, disorganized arrangement of axons, Schwann cells, and perineural fibroblasts amidst a fibrous stroma, consistent with traumatic neuroma. At 1-month and 6-month follow-up visits, the patient remained symptom-free, aside from some continued anterior left knee pain near the site of intramedullary nail entry. Her positive Tinel sign had completely resolved, as did her neuralgic symptoms down the medial aspect of her leg. This proved consistent with a diagnosis of neuroma as the cause of the majority of her symptoms. Subjectively, she reported excellent overall pain relief and satisfaction with her treatment and postoperative course.
Discussion
Postoperative pain after intramedullary fixation of tibial shaft fractures is common and can be caused by several clinical entities.5,10 Anterior knee pain is a well-known complication present in up to 73% of patients treated with tibial nailing.10 Osteoarthritis of the knee or ankle as well as nonarthritic ipsilateral ankle pain are also common complaints, often resulting from tibial malunion or malrotation, leading to altered joint kinematics.11 Additionally, superficial peroneal nerve and tibial neurovascular bundle injuries have been reported as potential complications of distal interlocking screw placement, and should be considered in such patients.12
Another consideration for the development of postoperative pain is CRPS, which is thought to be caused by postinjury sympathetic activation that produces pain out of proportion to clinical examination findings.13 Although no postoperative incidence of CRPS in the setting of tibial nailing has been reported, it is a known contributor to poor functional outcomes after fractures or crush injuries to the lower extremity.9 When attempting to diagnose and treat chronic postoperative pain after tibial nailing, the clinician must keep these common etiologies in mind as well as an understanding of the adjacent anatomy.
The saphenous nerve originates from the third and fourth lumbar nerve roots, coursing beneath the inguinal ligament as part of the femoral nerve. As the terminal branch of the femoral nerve, the saphenous nerve runs in the Hunter canal beneath the fascia of the sartorius muscle. It is bordered laterally by the vastus medialis muscle, and posteriorly and laterally by the adductor longus and magnus muscles. The saphenous nerve then crosses the femoral artery superficially from medial to lateral as it courses distally in the canal. As it emerges from the adductor hiatus, the saphenous nerve runs superficial to the gracilis muscle around the posterior border of the sartorius muscle with the descending genicular artery, and becomes a subcutaneous structure at the level of the knee joint. The infrapatellar branch of the saphenous nerve provides sensation to the medial knee, and continues in a subcutaneous course just medial to the posterior aspect of the tibial shaft with the great saphenous vein. The nerve distally supplies sensory input from the medial foot and ankle.1,3,14
There are several causes of saphenous neuralgia related to surgical and nonsurgical trauma.2,3,15,16 The most common cause of nerve injury is iatrogenic traction or transection causing neuralgic sequelae from subsequent neuroma formation. The anatomy of the saphenous nerve puts it at particular risk when performing saphenectomy for vascular procedures, and its infrapatellar branch is at particular risk when performing a medial parapatellar approach for total knee arthroplasty.2,3 In the case of the surgically naïve patient, saphenous nerve entrapment syndromes have also been described, and occur most frequently at the level of the adductor hiatus or as the saphenous nerve courses between the sartorius and gracilis muscles proximal to the knee joint.16
As is illustrated in the present case, orthopedic trauma may be an additional cause of saphenous neuroma formation, leading to symptomatic neuralgia. This case suggests that symptomatic neuroma should be included in the differential diagnosis of posttraumatic pain in the orthopedic trauma patient. It is important to note that, although this case occurred after a severe injury, the intimate association of the saphenous nerve with the tibia places it in a vulnerable position, and traumatic transection is possible after closed injuries to the tibial metadiaphyseal junction or tibial shaft.
Neuroma formation occurs in response to damage to the endoneurium and axon. For an axon to repair properly, the damaged proximal segment must join with, and reenter, the distal stump. As axons attempt to regenerate, occasionally the proximal stump can escape into the surrounding tissue and form a painful neuroma consisting of a disorganized mass of Schwann cells, fibroblasts, blood vessels, and axons with various degrees of myelination. The subsequent neuralgia associated with neuroma formation is caused by chemical or mechanical stimulation of the damaged axons or by spontaneously evoked potentials in the damaged axons. These signals can manifest as a variety of symptoms, including paresthesia and allodynia.17,18
Making the diagnosis of neuroma-related neuralgia can be challenging and nebulous. A characteristic history and positive Tinel sign over the affected area are helpful clinical indicators. However, the clinical finding most predictive of favorable surgical outcome is symptomatic relief after local injection of 1% lidocaine to the affected area. This is an important diagnostic test, especially when attempting to differentiate painful neuroma from other causes of posttraumatic lower extremity pain (eg, CRPS). Such an injection should be performed in the diagnosis and treatment of symptomatic neuroma, and some authors would suggest that insufficient relief of symptoms with diagnostic nerve block is a contraindication to surgical treatment.19
Several treatments for painful neuromas have been described, with variable results.19,20 The most widely accepted treatment of a complete nerve transection with associated neuroma is neurectomy with reimplantation of the proximal end into adjacent bone, muscle, or vein.14,15 Balcin and colleagues21 suggest that vein transposition produces the most favorable outcomes. Simple neurolysis of in-continuity neuromas has also been described with favorable results.
Conclusion
Neuralgia-producing neuromas of the saphenous nerve are relatively uncommon but can lead to persistent pain and frustrating symptoms for the patient. As noted, the diagnosis may elude clinicians, especially in patients with less obvious clinical presentations. We suggest the following algorithm to help distinguish between painful neuroma and other causes of posttraumatic leg pain: (1) physical examination (including testing for instability, joint line tenderness, patellofemoral pain, Tinel sign, and Semmes-Weinstein testing) should be performed, and plain radiographs taken of the involved bones and joints; (2) if all of the above reveal no abnormality, and there is a positive Tinel sign directly over the course of a nerve, an injection of lidocaine over the region of the potential neuroma can be diagnostic; (3) should several abnormalities be present, further investigation using magnetic resonance imaging, bone scan, and/or electromyography may provide additional information that leads to a diagnosis.
1. Senegor M. Iatrogenic saphenous neuralgia: successful treatment with neuroma resection. J Neurosurg. 1991;28(2):295-298.
2. Mountney J, Wilkinson GA. Saphenous neuralgia after coronary artery bypass grafting. Eur J Cardiothorac Surg. 1999;16(4):440-443.
3. Kachar SM, Williams KM, Finn HA. Neuroma of the infrapatellar branch of the saphenous nerve: a cause of reversible knee stiffness after total knee arthroplasty. J Arthroplasty. 2008;23(6):927-930.
4. Gustilo RB, Anderson AB. Prevention of infection in the treatment of one thousand and twenty-five open fractures of long bones: retrospective and prospective analyses. J Bone Joint Surg Am. 1976;58(4):453-458.
5. Keating JF, Orfaly R, O’Brien PJ. Knee pain after tibial nailing. J Orthop Trauma. 1997;11(1):10-13.
6. Chen CY, Lin KC, Yang SW, Tarng YW, Hsu CJ, Renn JH. Influence of nail prominence and insertion point on anterior knee pain after tibial intramedullary nailing. Orthopedics. 2014;37(3):e221-e225.
7. Lewin-Kowalik J, Marcol W, Kotulska K, Mandera M, Klimczak A. Prevention and management of painful neuroma. Neurol Med Chir (Tokyo). 2006;46(2):62-67.
8. Otfinowski J, Pawelec A, Kaluza J. Implantation of peripheral neural stump into muscle and its effect on the development of posttraumatic neuroma. Pol J Pathol. 1994;45:195-202.
9. Van Beek AL. Management of nerve compression syndromes and painful neuromas. In: McCarthy JG, May JW Jr, Littler JW, eds. Plastic Surgery. Philadelphia, PA: WB Saunders; 1990:4817-4858.
10. Lefaivre KA, Guy P, Chan H, Blachut PA. Long-term follow-up of tibial shaft fractures treated with intramedullary nailing. J Orthop Trauma. 2008;22(8):525-529.
11. Milner SA, Davis TR, Muir KR, Greenwood DC, Doherty M. Long-term outcome after tibial shaft fracture: is malunion important? J Bone Joint Surg Am. 2002;84(6):971-980.
12. Roberts CS, King D, Wang M, Seligson D, Voor MJ. Should distal interlocking of tibial nails be performed from medial or lateral direction? Anatomical and biomechanical considerations. J Orthop Trauma. 1999;13(1):27-32.
13. Hogan CJ, Hurwitz SR. Treatment of complex regional pain syndrome of the lower extremity. J Am Acad Orthop Surg. 2002;10(4):281-289.
14. Gray H, Lewis WH. Anatomy of the Human Body. Philadelphia, PA: Lea & Febiger, 1918. Bartleby.com website. http://www.bartleby.com/br/107.html. Accessed September 29, 2015.
15. Myerson MS, McGarvey WC, Henderson MR, Hakim J. Morbidity after crush injuries to the foot. J Orthop Trauma. 1994;8(4):343-349.
16. Kalenak A. Saphenous nerve entrapment. Op Tech Sports Med. 1996;4(1):40-45.
17. Wolf SW, Hotchkiss RN, Pederson WC, Kozin SH. The peripheral neuroma. In: Green DP, Wolfe SW, eds. Green’s Operative Hand Surgery. 6th ed. Philadelphia, PA: Elsevier Churchill Livingstone, 2011;1063-1071.
18. Thordarson DB, Shean CJ. Nerve and tendon lacerations about the foot and ankle. J Am Acad Orthop Surg. 2005;13(3):186-196.
19. Stokvis A, van der Avoort DJ, van Neck JW, Hovius SE, Coert JH. Surgical management of neuroma pain: a prospective follow-up study. Pain. 2010;151(3):862-869.
20. Burchiel KJ, Johans TJ, Ochoa J. The surgical treatment of painful traumatic neuromas. J Neurosurg. 1993;78(5):714-719.
21. Balcin H, Erba P, Wettstein R, Schaefer DJ, Pierer G, Kalbermatten DF. A comparative study of two methods of surgical treatment for painful neuroma. J Bone Joint Surg Br. 2009;91(6):803-808.
1. Senegor M. Iatrogenic saphenous neuralgia: successful treatment with neuroma resection. J Neurosurg. 1991;28(2):295-298.
2. Mountney J, Wilkinson GA. Saphenous neuralgia after coronary artery bypass grafting. Eur J Cardiothorac Surg. 1999;16(4):440-443.
3. Kachar SM, Williams KM, Finn HA. Neuroma of the infrapatellar branch of the saphenous nerve: a cause of reversible knee stiffness after total knee arthroplasty. J Arthroplasty. 2008;23(6):927-930.
4. Gustilo RB, Anderson AB. Prevention of infection in the treatment of one thousand and twenty-five open fractures of long bones: retrospective and prospective analyses. J Bone Joint Surg Am. 1976;58(4):453-458.
5. Keating JF, Orfaly R, O’Brien PJ. Knee pain after tibial nailing. J Orthop Trauma. 1997;11(1):10-13.
6. Chen CY, Lin KC, Yang SW, Tarng YW, Hsu CJ, Renn JH. Influence of nail prominence and insertion point on anterior knee pain after tibial intramedullary nailing. Orthopedics. 2014;37(3):e221-e225.
7. Lewin-Kowalik J, Marcol W, Kotulska K, Mandera M, Klimczak A. Prevention and management of painful neuroma. Neurol Med Chir (Tokyo). 2006;46(2):62-67.
8. Otfinowski J, Pawelec A, Kaluza J. Implantation of peripheral neural stump into muscle and its effect on the development of posttraumatic neuroma. Pol J Pathol. 1994;45:195-202.
9. Van Beek AL. Management of nerve compression syndromes and painful neuromas. In: McCarthy JG, May JW Jr, Littler JW, eds. Plastic Surgery. Philadelphia, PA: WB Saunders; 1990:4817-4858.
10. Lefaivre KA, Guy P, Chan H, Blachut PA. Long-term follow-up of tibial shaft fractures treated with intramedullary nailing. J Orthop Trauma. 2008;22(8):525-529.
11. Milner SA, Davis TR, Muir KR, Greenwood DC, Doherty M. Long-term outcome after tibial shaft fracture: is malunion important? J Bone Joint Surg Am. 2002;84(6):971-980.
12. Roberts CS, King D, Wang M, Seligson D, Voor MJ. Should distal interlocking of tibial nails be performed from medial or lateral direction? Anatomical and biomechanical considerations. J Orthop Trauma. 1999;13(1):27-32.
13. Hogan CJ, Hurwitz SR. Treatment of complex regional pain syndrome of the lower extremity. J Am Acad Orthop Surg. 2002;10(4):281-289.
14. Gray H, Lewis WH. Anatomy of the Human Body. Philadelphia, PA: Lea & Febiger, 1918. Bartleby.com website. http://www.bartleby.com/br/107.html. Accessed September 29, 2015.
15. Myerson MS, McGarvey WC, Henderson MR, Hakim J. Morbidity after crush injuries to the foot. J Orthop Trauma. 1994;8(4):343-349.
16. Kalenak A. Saphenous nerve entrapment. Op Tech Sports Med. 1996;4(1):40-45.
17. Wolf SW, Hotchkiss RN, Pederson WC, Kozin SH. The peripheral neuroma. In: Green DP, Wolfe SW, eds. Green’s Operative Hand Surgery. 6th ed. Philadelphia, PA: Elsevier Churchill Livingstone, 2011;1063-1071.
18. Thordarson DB, Shean CJ. Nerve and tendon lacerations about the foot and ankle. J Am Acad Orthop Surg. 2005;13(3):186-196.
19. Stokvis A, van der Avoort DJ, van Neck JW, Hovius SE, Coert JH. Surgical management of neuroma pain: a prospective follow-up study. Pain. 2010;151(3):862-869.
20. Burchiel KJ, Johans TJ, Ochoa J. The surgical treatment of painful traumatic neuromas. J Neurosurg. 1993;78(5):714-719.
21. Balcin H, Erba P, Wettstein R, Schaefer DJ, Pierer G, Kalbermatten DF. A comparative study of two methods of surgical treatment for painful neuroma. J Bone Joint Surg Br. 2009;91(6):803-808.
Acute Multiple Flexor Tendon Injury and Carpal Tunnel Syndrome After Open Distal Radius Fracture
The literature on extensor tendon rupture and even chronic flexor tendon rupture after volar plating and distal radius fracture malunion is ubiquitous. However, acute and subacute flexor tendon ruptures caused by distal radius fractures have been reported only in limited case reports. These rare injuries may involve multiple tendons and are associated with high-energy mechanisms. This case report details the involvement of multiple flexor tendon injuries associated with a Gustilo-Anderson type II distal radius fracture and the development of acute carpal tunnel syndrome (CTS) after a motor vehicle collision. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
The patient is a 46-year-old woman who was involved in a motor vehicle collision. She was triaged as a trauma patient via Advanced Trauma Life Support protocol, and treated with antibiotic and tetanus prophylaxis. Radiographs showed an open, comminuted, displaced intra-articular distal radius fracture on the right side (Figures 1A, 1B). The fracture was closed reduced and splinted in the emergency department (Figures 2A, 2B). On initial examination, the patient had diffuse paresthesias in the digits that were most pronounced in the median nerve distribution. Motor examination was limited secondary to pain; however, she demonstrated gentle flexion and extension of the digits. The hand was well perfused, and a palpable radial pulse was present.
After clearance was obtained, she was taken urgently to the operating room. The wound was volar and transverse, approximately 2 cm in length, and approximately 4 cm proximal to the wrist crease. The wound was extended proximally and distally for a standard volar (Henry) approach. The flexor carpi radialis tendon was found to be partially lacerated, comprising 60% of the tendon. The fracture was readily identified because the deep fascia and the pronator quadratus were disrupted. No deep tendon lacerations were identified. The median nerve was found to be in continuity. After satisfactory débridement of the fracture and the wound, reduction and fixation was achieved with a volar locking plate and a single Kirschner wire. The flexor carpi radialis tendon was repaired with a modified Kessler stitch and epitenon repair. The wound was closed primarily in layers (Figures 3A, 3B).
The patient’s immediate postoperative neurologic examination was compromised secondary to the patient having a supraclavicular nerve block for anesthesia. Regional anesthesia was chosen because the patient’s pulmonologist recommended avoiding general anesthesia owing to her history of severe asthma that frequently required corticosteroid treatment. Once the block wore off, she complained of persistent paresthesias in all digits but most pronounced in the median nerve distribution. She was able to flex the interphalangeal joint to the index finger but could not flex the interphalangeal joint to the thumb. Over the course of the night, she was also noted to have worsening pain out of proportion to her injury.
As the paresthesias became denser in the median nerve distribution, she was diagnosed with acute CTS and was taken urgently back to the operating room under general anesthesia. After releasing the carpal tunnel through a separate incision, the original wound was reopened and explored. The median nerve was again visualized and found to be in continuity. All 4 tendons to both the flexor digitorum superficialis and flexor digitorum profundus were identified. The flexor pollicis longus (FPL) was not visualized in the wound. The distal portion of the FPL was retracted in the thumb tendon sheath and retrieved blindly with a tendon passer. The proximal portion was retracted to the mid-forearm. The laceration occurred distal to the musculotendinous junction. The tendon was repaired with a modified Kessler stitch as well as a box suture, resulting in 4 core strands across the tendon. The hand and the wrist were splinted in a thumb spica cast, and the patient was started on a modified Duran protocol 1 week after surgery. Median nerve function improved postoperatively.
Discussion
The rupture of the extensor pollicis longus tendon in nondisplaced distal radius fractures is not uncommon, but occurs in fewer than 5% of nondisplaced distal radius fractures.1 Although less common, chronic complications with flexor tendon rupture after distal radius fracture are well described.1-6 Flexor tendon rupture after distal radius malunion or volar plating is a known complication and is thought to be the result of attritional tendon wear because the flexors rub against protruding bone or plate;3,4,7 however, the initial tendon injury may play a role in those tendons that rupture more quickly.3 When secondary to volar plating, the rupture typically occurs within 1 year of injury,7 but, in both plating and malunion, it has been characterized as a late complication up to 10 years and even 20 years after injury.3,4 Similar to other reports, this rupture was encountered during a volar wrist approach. It has been suggested that, as the incidence of volar plating rises, more acute flexor tendon injuries may be diagnosed because of anatomic exposure,2 but this has not been reported in the literature.
Acute and subacute flexor tendon ruptures are rarely reported in the literature. To our knowledge, there are only 2 other reports of acute flexor tendon rupture2,5 after a distal radius fracture, neither of which involved the FPL. These cases, which involved ruptures of the flexor digitorum superficialis and flexor carpi radialis, were thought to be the result of tendon laceration by a volar bone spike. There is also one report of subacute FPL and flexor digitorum profundus rupture approximately 4 weeks after closed reduction of a distal radius fracture.6 Although sparse, the literature regarding flexor tendon rupture and distal radius fractures suggests that involvement of the flexor digitorum superficialis and the flexor digitorum profundus tendons is most common and that the rupture typically occurs in 1 to 4 months.1
We report a rare case of 2 acute flexor tendon lacerations after a Gustilo-Anderson type II open distal radius fracture, likely caused by the volar spike of bone that created the open injury. This case also was complicated by the development of acute CTS.
To our knowledge, despite a rate of acute CTS reported as high as 5.4% in operatively treated distal radius fractures, there are no established associations between acute CTS and flexor tendon rupture in the setting of distal radius fracture.8,9 In a 2008 retrospective case–control study by Dyer and colleagues,8 fracture translation is the most important risk factor for the development of acute CTS associated with fracture of the distal radius. Although not statistically significant, ipsilateral upper extremity trauma, higher-energy injuries, younger age, and male sex were also associated with the development of acute CTS. Open injuries occurred in only 3 of 50 cases of acute CTS.8
In agreement with published reports, the probability and the timing of tendon rupture are likely related to the severity of the deforming forces applied during the initial insult rather than the resultant stresses.1 Clinicians should have a high suspicion of acute CTS and possible tendon injuries after a high-energy injury with a significantly displaced open distal radius fracture and median nerve paresthesias. A thoughtful and complete preoperative examination of the flexor tendons may prevent the need for reoperation. Concerns for flexor injury and acute CTS should be elevated with the observation of a disrupted pronator. For patients with a volarly displaced fragment after fracture reduction, this concern should be even more elevated.9 Preoperative median nerve symptoms in the setting of the severely displaced fracture should necessitate an acute carpal tunnel release. If 1 flexor tendon is injured, the surgeon should remember that multiple flexor tendons may be involved. We recommend that any injured tendons be repaired primarily, if possible, and the patient started on appropriate rehabilitation.
1. Ashall G. Flexor pollicis longus rupture after fracture of the distal radius. Injury. 1991;22(2):153-155.
2. Dimatteo L, Wolf JM. Flexor carpi radialis tendon rupture as a complication of a closed distal radius fracture: a case report. J Hand Surg Am. 2007;32(6):818-820.
3. Kato N, Nemoto K, Arino H, Ichikawa T, Fujikawa K. Ruptures of flexor tendons at the wrist as a complication of fracture of the distal radius. Scand J Plast Reconstr Surg Hand Surg. 2002;36(4):245-248.
4. Monda MK, Ellis A, Karmani S. Late rupture of flexor pollicis longus tendon 10 years after volar buttress plate fixation of a distal radius fracture: a case report. Acta Orthop Belg. 2010;76(4):549-551.
5. Southmayd WW, Millender LH, Nalebuff EA. Rupture of the flexor tendons of the index finger after Colles’ fracture. Case report. J Bone Joint Surg Am. 1975;57(4):562-563.
6. Wong FY, Pho RW. Median nerve compression, with tendon ruptures, after Colles’ fracture. J Hand Surg Br. 1984;9(2):139-141.
7. Woon CYL, Lee JYL, Ng SW, Teoh LC. Late rupture of flexor pollicis longus tendon after volar distal radius plating: a case report and review of the literature. Inj Extra. 2007;38(7):235-238.
8. Dyer G, Lozano-Calderon S, Gannon C, Baratz M, Ring D. Predictors of acute carpal tunnel syndrome associated with fracture of the distal radius. J Hand Surg Am. 2008;33(8):1309-1313.
9. Paley D, McMurtry RY. Median nerve compression by volarly displaced fragments of the distal radius. Clin Orthop Relat Res. 1987;(215):139-147.
The literature on extensor tendon rupture and even chronic flexor tendon rupture after volar plating and distal radius fracture malunion is ubiquitous. However, acute and subacute flexor tendon ruptures caused by distal radius fractures have been reported only in limited case reports. These rare injuries may involve multiple tendons and are associated with high-energy mechanisms. This case report details the involvement of multiple flexor tendon injuries associated with a Gustilo-Anderson type II distal radius fracture and the development of acute carpal tunnel syndrome (CTS) after a motor vehicle collision. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
The patient is a 46-year-old woman who was involved in a motor vehicle collision. She was triaged as a trauma patient via Advanced Trauma Life Support protocol, and treated with antibiotic and tetanus prophylaxis. Radiographs showed an open, comminuted, displaced intra-articular distal radius fracture on the right side (Figures 1A, 1B). The fracture was closed reduced and splinted in the emergency department (Figures 2A, 2B). On initial examination, the patient had diffuse paresthesias in the digits that were most pronounced in the median nerve distribution. Motor examination was limited secondary to pain; however, she demonstrated gentle flexion and extension of the digits. The hand was well perfused, and a palpable radial pulse was present.
After clearance was obtained, she was taken urgently to the operating room. The wound was volar and transverse, approximately 2 cm in length, and approximately 4 cm proximal to the wrist crease. The wound was extended proximally and distally for a standard volar (Henry) approach. The flexor carpi radialis tendon was found to be partially lacerated, comprising 60% of the tendon. The fracture was readily identified because the deep fascia and the pronator quadratus were disrupted. No deep tendon lacerations were identified. The median nerve was found to be in continuity. After satisfactory débridement of the fracture and the wound, reduction and fixation was achieved with a volar locking plate and a single Kirschner wire. The flexor carpi radialis tendon was repaired with a modified Kessler stitch and epitenon repair. The wound was closed primarily in layers (Figures 3A, 3B).
The patient’s immediate postoperative neurologic examination was compromised secondary to the patient having a supraclavicular nerve block for anesthesia. Regional anesthesia was chosen because the patient’s pulmonologist recommended avoiding general anesthesia owing to her history of severe asthma that frequently required corticosteroid treatment. Once the block wore off, she complained of persistent paresthesias in all digits but most pronounced in the median nerve distribution. She was able to flex the interphalangeal joint to the index finger but could not flex the interphalangeal joint to the thumb. Over the course of the night, she was also noted to have worsening pain out of proportion to her injury.
As the paresthesias became denser in the median nerve distribution, she was diagnosed with acute CTS and was taken urgently back to the operating room under general anesthesia. After releasing the carpal tunnel through a separate incision, the original wound was reopened and explored. The median nerve was again visualized and found to be in continuity. All 4 tendons to both the flexor digitorum superficialis and flexor digitorum profundus were identified. The flexor pollicis longus (FPL) was not visualized in the wound. The distal portion of the FPL was retracted in the thumb tendon sheath and retrieved blindly with a tendon passer. The proximal portion was retracted to the mid-forearm. The laceration occurred distal to the musculotendinous junction. The tendon was repaired with a modified Kessler stitch as well as a box suture, resulting in 4 core strands across the tendon. The hand and the wrist were splinted in a thumb spica cast, and the patient was started on a modified Duran protocol 1 week after surgery. Median nerve function improved postoperatively.
Discussion
The rupture of the extensor pollicis longus tendon in nondisplaced distal radius fractures is not uncommon, but occurs in fewer than 5% of nondisplaced distal radius fractures.1 Although less common, chronic complications with flexor tendon rupture after distal radius fracture are well described.1-6 Flexor tendon rupture after distal radius malunion or volar plating is a known complication and is thought to be the result of attritional tendon wear because the flexors rub against protruding bone or plate;3,4,7 however, the initial tendon injury may play a role in those tendons that rupture more quickly.3 When secondary to volar plating, the rupture typically occurs within 1 year of injury,7 but, in both plating and malunion, it has been characterized as a late complication up to 10 years and even 20 years after injury.3,4 Similar to other reports, this rupture was encountered during a volar wrist approach. It has been suggested that, as the incidence of volar plating rises, more acute flexor tendon injuries may be diagnosed because of anatomic exposure,2 but this has not been reported in the literature.
Acute and subacute flexor tendon ruptures are rarely reported in the literature. To our knowledge, there are only 2 other reports of acute flexor tendon rupture2,5 after a distal radius fracture, neither of which involved the FPL. These cases, which involved ruptures of the flexor digitorum superficialis and flexor carpi radialis, were thought to be the result of tendon laceration by a volar bone spike. There is also one report of subacute FPL and flexor digitorum profundus rupture approximately 4 weeks after closed reduction of a distal radius fracture.6 Although sparse, the literature regarding flexor tendon rupture and distal radius fractures suggests that involvement of the flexor digitorum superficialis and the flexor digitorum profundus tendons is most common and that the rupture typically occurs in 1 to 4 months.1
We report a rare case of 2 acute flexor tendon lacerations after a Gustilo-Anderson type II open distal radius fracture, likely caused by the volar spike of bone that created the open injury. This case also was complicated by the development of acute CTS.
To our knowledge, despite a rate of acute CTS reported as high as 5.4% in operatively treated distal radius fractures, there are no established associations between acute CTS and flexor tendon rupture in the setting of distal radius fracture.8,9 In a 2008 retrospective case–control study by Dyer and colleagues,8 fracture translation is the most important risk factor for the development of acute CTS associated with fracture of the distal radius. Although not statistically significant, ipsilateral upper extremity trauma, higher-energy injuries, younger age, and male sex were also associated with the development of acute CTS. Open injuries occurred in only 3 of 50 cases of acute CTS.8
In agreement with published reports, the probability and the timing of tendon rupture are likely related to the severity of the deforming forces applied during the initial insult rather than the resultant stresses.1 Clinicians should have a high suspicion of acute CTS and possible tendon injuries after a high-energy injury with a significantly displaced open distal radius fracture and median nerve paresthesias. A thoughtful and complete preoperative examination of the flexor tendons may prevent the need for reoperation. Concerns for flexor injury and acute CTS should be elevated with the observation of a disrupted pronator. For patients with a volarly displaced fragment after fracture reduction, this concern should be even more elevated.9 Preoperative median nerve symptoms in the setting of the severely displaced fracture should necessitate an acute carpal tunnel release. If 1 flexor tendon is injured, the surgeon should remember that multiple flexor tendons may be involved. We recommend that any injured tendons be repaired primarily, if possible, and the patient started on appropriate rehabilitation.
The literature on extensor tendon rupture and even chronic flexor tendon rupture after volar plating and distal radius fracture malunion is ubiquitous. However, acute and subacute flexor tendon ruptures caused by distal radius fractures have been reported only in limited case reports. These rare injuries may involve multiple tendons and are associated with high-energy mechanisms. This case report details the involvement of multiple flexor tendon injuries associated with a Gustilo-Anderson type II distal radius fracture and the development of acute carpal tunnel syndrome (CTS) after a motor vehicle collision. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
The patient is a 46-year-old woman who was involved in a motor vehicle collision. She was triaged as a trauma patient via Advanced Trauma Life Support protocol, and treated with antibiotic and tetanus prophylaxis. Radiographs showed an open, comminuted, displaced intra-articular distal radius fracture on the right side (Figures 1A, 1B). The fracture was closed reduced and splinted in the emergency department (Figures 2A, 2B). On initial examination, the patient had diffuse paresthesias in the digits that were most pronounced in the median nerve distribution. Motor examination was limited secondary to pain; however, she demonstrated gentle flexion and extension of the digits. The hand was well perfused, and a palpable radial pulse was present.
After clearance was obtained, she was taken urgently to the operating room. The wound was volar and transverse, approximately 2 cm in length, and approximately 4 cm proximal to the wrist crease. The wound was extended proximally and distally for a standard volar (Henry) approach. The flexor carpi radialis tendon was found to be partially lacerated, comprising 60% of the tendon. The fracture was readily identified because the deep fascia and the pronator quadratus were disrupted. No deep tendon lacerations were identified. The median nerve was found to be in continuity. After satisfactory débridement of the fracture and the wound, reduction and fixation was achieved with a volar locking plate and a single Kirschner wire. The flexor carpi radialis tendon was repaired with a modified Kessler stitch and epitenon repair. The wound was closed primarily in layers (Figures 3A, 3B).
The patient’s immediate postoperative neurologic examination was compromised secondary to the patient having a supraclavicular nerve block for anesthesia. Regional anesthesia was chosen because the patient’s pulmonologist recommended avoiding general anesthesia owing to her history of severe asthma that frequently required corticosteroid treatment. Once the block wore off, she complained of persistent paresthesias in all digits but most pronounced in the median nerve distribution. She was able to flex the interphalangeal joint to the index finger but could not flex the interphalangeal joint to the thumb. Over the course of the night, she was also noted to have worsening pain out of proportion to her injury.
As the paresthesias became denser in the median nerve distribution, she was diagnosed with acute CTS and was taken urgently back to the operating room under general anesthesia. After releasing the carpal tunnel through a separate incision, the original wound was reopened and explored. The median nerve was again visualized and found to be in continuity. All 4 tendons to both the flexor digitorum superficialis and flexor digitorum profundus were identified. The flexor pollicis longus (FPL) was not visualized in the wound. The distal portion of the FPL was retracted in the thumb tendon sheath and retrieved blindly with a tendon passer. The proximal portion was retracted to the mid-forearm. The laceration occurred distal to the musculotendinous junction. The tendon was repaired with a modified Kessler stitch as well as a box suture, resulting in 4 core strands across the tendon. The hand and the wrist were splinted in a thumb spica cast, and the patient was started on a modified Duran protocol 1 week after surgery. Median nerve function improved postoperatively.
Discussion
The rupture of the extensor pollicis longus tendon in nondisplaced distal radius fractures is not uncommon, but occurs in fewer than 5% of nondisplaced distal radius fractures.1 Although less common, chronic complications with flexor tendon rupture after distal radius fracture are well described.1-6 Flexor tendon rupture after distal radius malunion or volar plating is a known complication and is thought to be the result of attritional tendon wear because the flexors rub against protruding bone or plate;3,4,7 however, the initial tendon injury may play a role in those tendons that rupture more quickly.3 When secondary to volar plating, the rupture typically occurs within 1 year of injury,7 but, in both plating and malunion, it has been characterized as a late complication up to 10 years and even 20 years after injury.3,4 Similar to other reports, this rupture was encountered during a volar wrist approach. It has been suggested that, as the incidence of volar plating rises, more acute flexor tendon injuries may be diagnosed because of anatomic exposure,2 but this has not been reported in the literature.
Acute and subacute flexor tendon ruptures are rarely reported in the literature. To our knowledge, there are only 2 other reports of acute flexor tendon rupture2,5 after a distal radius fracture, neither of which involved the FPL. These cases, which involved ruptures of the flexor digitorum superficialis and flexor carpi radialis, were thought to be the result of tendon laceration by a volar bone spike. There is also one report of subacute FPL and flexor digitorum profundus rupture approximately 4 weeks after closed reduction of a distal radius fracture.6 Although sparse, the literature regarding flexor tendon rupture and distal radius fractures suggests that involvement of the flexor digitorum superficialis and the flexor digitorum profundus tendons is most common and that the rupture typically occurs in 1 to 4 months.1
We report a rare case of 2 acute flexor tendon lacerations after a Gustilo-Anderson type II open distal radius fracture, likely caused by the volar spike of bone that created the open injury. This case also was complicated by the development of acute CTS.
To our knowledge, despite a rate of acute CTS reported as high as 5.4% in operatively treated distal radius fractures, there are no established associations between acute CTS and flexor tendon rupture in the setting of distal radius fracture.8,9 In a 2008 retrospective case–control study by Dyer and colleagues,8 fracture translation is the most important risk factor for the development of acute CTS associated with fracture of the distal radius. Although not statistically significant, ipsilateral upper extremity trauma, higher-energy injuries, younger age, and male sex were also associated with the development of acute CTS. Open injuries occurred in only 3 of 50 cases of acute CTS.8
In agreement with published reports, the probability and the timing of tendon rupture are likely related to the severity of the deforming forces applied during the initial insult rather than the resultant stresses.1 Clinicians should have a high suspicion of acute CTS and possible tendon injuries after a high-energy injury with a significantly displaced open distal radius fracture and median nerve paresthesias. A thoughtful and complete preoperative examination of the flexor tendons may prevent the need for reoperation. Concerns for flexor injury and acute CTS should be elevated with the observation of a disrupted pronator. For patients with a volarly displaced fragment after fracture reduction, this concern should be even more elevated.9 Preoperative median nerve symptoms in the setting of the severely displaced fracture should necessitate an acute carpal tunnel release. If 1 flexor tendon is injured, the surgeon should remember that multiple flexor tendons may be involved. We recommend that any injured tendons be repaired primarily, if possible, and the patient started on appropriate rehabilitation.
1. Ashall G. Flexor pollicis longus rupture after fracture of the distal radius. Injury. 1991;22(2):153-155.
2. Dimatteo L, Wolf JM. Flexor carpi radialis tendon rupture as a complication of a closed distal radius fracture: a case report. J Hand Surg Am. 2007;32(6):818-820.
3. Kato N, Nemoto K, Arino H, Ichikawa T, Fujikawa K. Ruptures of flexor tendons at the wrist as a complication of fracture of the distal radius. Scand J Plast Reconstr Surg Hand Surg. 2002;36(4):245-248.
4. Monda MK, Ellis A, Karmani S. Late rupture of flexor pollicis longus tendon 10 years after volar buttress plate fixation of a distal radius fracture: a case report. Acta Orthop Belg. 2010;76(4):549-551.
5. Southmayd WW, Millender LH, Nalebuff EA. Rupture of the flexor tendons of the index finger after Colles’ fracture. Case report. J Bone Joint Surg Am. 1975;57(4):562-563.
6. Wong FY, Pho RW. Median nerve compression, with tendon ruptures, after Colles’ fracture. J Hand Surg Br. 1984;9(2):139-141.
7. Woon CYL, Lee JYL, Ng SW, Teoh LC. Late rupture of flexor pollicis longus tendon after volar distal radius plating: a case report and review of the literature. Inj Extra. 2007;38(7):235-238.
8. Dyer G, Lozano-Calderon S, Gannon C, Baratz M, Ring D. Predictors of acute carpal tunnel syndrome associated with fracture of the distal radius. J Hand Surg Am. 2008;33(8):1309-1313.
9. Paley D, McMurtry RY. Median nerve compression by volarly displaced fragments of the distal radius. Clin Orthop Relat Res. 1987;(215):139-147.
1. Ashall G. Flexor pollicis longus rupture after fracture of the distal radius. Injury. 1991;22(2):153-155.
2. Dimatteo L, Wolf JM. Flexor carpi radialis tendon rupture as a complication of a closed distal radius fracture: a case report. J Hand Surg Am. 2007;32(6):818-820.
3. Kato N, Nemoto K, Arino H, Ichikawa T, Fujikawa K. Ruptures of flexor tendons at the wrist as a complication of fracture of the distal radius. Scand J Plast Reconstr Surg Hand Surg. 2002;36(4):245-248.
4. Monda MK, Ellis A, Karmani S. Late rupture of flexor pollicis longus tendon 10 years after volar buttress plate fixation of a distal radius fracture: a case report. Acta Orthop Belg. 2010;76(4):549-551.
5. Southmayd WW, Millender LH, Nalebuff EA. Rupture of the flexor tendons of the index finger after Colles’ fracture. Case report. J Bone Joint Surg Am. 1975;57(4):562-563.
6. Wong FY, Pho RW. Median nerve compression, with tendon ruptures, after Colles’ fracture. J Hand Surg Br. 1984;9(2):139-141.
7. Woon CYL, Lee JYL, Ng SW, Teoh LC. Late rupture of flexor pollicis longus tendon after volar distal radius plating: a case report and review of the literature. Inj Extra. 2007;38(7):235-238.
8. Dyer G, Lozano-Calderon S, Gannon C, Baratz M, Ring D. Predictors of acute carpal tunnel syndrome associated with fracture of the distal radius. J Hand Surg Am. 2008;33(8):1309-1313.
9. Paley D, McMurtry RY. Median nerve compression by volarly displaced fragments of the distal radius. Clin Orthop Relat Res. 1987;(215):139-147.
Medicaid Insurance Is Associated With Larger Curves in Patients Who Require Scoliosis Surgery
Rising health care costs have led many health insurers to limit benefits, which may be a problem for children in need of specialty care. Uninsured children have poorer access to specialty care than insured children. Children with public health coverage have better access to specialty care than uninsured children but inferior access compared with privately insured children.1,2 It is well documented that children with government insurance have limited access to orthopedic care for fractures, ligamentous knee injuries, and other injuries.1,3-5 Adolescent idiopathic scoliosis (AIS) differs from many other conditions managed by pediatric orthopedists, as it may be progressive, with management becoming increasingly more complex as the curve magnitude increases.6 The ability to access care earlier in the disease process may allow for earlier nonoperative interventions, such as bracing. For patients who require spinal fusion, earlier diagnosis and referral to a specialist could potentially result in shorter fusions and preserve distal motion segments. The ability to access the health care system in a timely fashion would therefore be of utmost importance for patients with scoliosis.
The literature on AIS is lacking in studies focused on care access based on insurance coverage and the potential impact that this may have on curve progression.7-9 We conducted a study to determine whether there is a difference between patients with and without private insurance who present to a busy urban pediatric orthopedic practice for management of scoliosis that eventually resulted in surgical treatment.
Materials and Methods
After obtaining institutional review board approval for this study, we retrospectively reviewed the medical records of patients (age, 10-18 years) who underwent posterior spinal fusion (PSF) for newly diagnosed AIS between 2008 and 2012. We excluded patients treated with growing spine instrumentation (growing rods), patients younger than 10 years or older than 18 years at presentation, and patients without adequate radiographs or clinical data, including insurance status. To focus on newly diagnosed scoliosis, we also excluded patients who had been seen for second opinions or whose scoliosis had been managed elsewhere in the past. Patients with syndromic, neuromuscular, or congenital scoliosis were also excluded.
Medical records were checked to ascertain time from initial evaluation to decision for surgery, time from recommendation for surgery until actual procedure, and insurance status. Distance traveled was figured from patients’ home addresses. Cobb angles were calculated from initial preoperative and final preoperative posteroanterior (PA) radiographs. Curves as seen on PA, lateral, and maximal effort, supine bending thoracic and lumbar radiographs from the initial preoperative visit were classified using the system of Lenke and colleagues.10 Hospital records were queried to determine number of levels fused at surgery, number of implants placed, and length of stay. Patients were evaluated without prior screening of insurance status and without prior consultation with referring physicians. Surgical procedures were scheduled on a first-come, first-served basis without preference for insurance status.
Results
We identified 135 consecutive patients with newly diagnosed AIS treated with PSF by our group between January 2008 and December 2012 (Table 1). Sixty-one percent had private insurance; 39% had Medicaid. There was no difference in age or ASA (American Society of Anesthesiologists) score between groups. Mean (SD) Cobb angle at initial presentation was 47.5° (14.3°) (range, 18.0°-86.0°) for the private insurance group and 57.2° (15.7°) (range, 23.0°-95.0°) for the Medicaid group (P < .0001). At time of surgery, mean (SD) Cobb angles were 54.6° (11.7°) and 60.6° (13.9°) for the private insurance and Medicaid groups, respectively (P = .008). There was no difference in curve types (Lenke and colleagues10 classification) between groups (Table 2, P = .83). Medicaid patients traveled a shorter mean (SD) distance for care, 56.3 (57.0) miles, versus 73.7 (66.7) miles (P = .05). There was no statistical difference (P = .14) in mean (SD) surgical wait time from surgery recommendation to actual surgery, 103.1 (62.4) days and 128.8 (137.5) days for the private insurance and Medicaid groups, respectively. The difference between patient groups in mean (SD) number of levels fused did not reach statistical significance (P = .16), 10.3 (2.2) levels for the Medicaid group and 9.7 (2.3) levels for the private insurance group. Mean (SD) estimated blood loss was higher for Medicaid patients, 445.7 (415.9) mL versus 335.1 (271.5) mL (P = .06), though there was no difference in use of posterior column osteotomies between groups. There was no difference (P = .11) in mean (SD) length of hospital stay between Medicaid patients, 2.6 (0.8) days, and private insurance patients, 2.4 (0.5) days.
Discussion
According to an extensive body of literature, patients with government insurance have limited access to specialty care.1,11,12 Medicaid-insured children in need of orthopedic care are no exception. Sabharwal and colleagues13 examined a database of pediatric fracture cases and found that 52% of the privately insured patients and 22% of the publicly insured patients received orthopedic care (P = .013).13 When Pierce and colleagues14 called 42 orthopedic practices regarding a fictitious 14-year-old patient with an anterior cruciate ligament tear, 38 offered an appointment within 2 weeks to a privately insured patient, and 6 offered such an appointment to a publicly insured patient. Skaggs and colleagues4 surveyed 230 orthopedic practices nationally and found that Medicaid-insured children had limited access to orthopedic care; 41 practices (18%) would not see a child with Medicaid under any circumstances. Using a fictitious case of a 10-year-old boy with a forearm fracture, Iobst and colleagues3 tried making an appointment at 100 orthopedic offices. Eight gave an appointment within 1 week to a Medicaid-insured patient, and 36 gave an appointment to a privately insured patient.3
There are few data regarding insurance status and scoliosis care in children. Spinal deformity differs from simple fractures and ligamentous injuries, as timely care may result in a less invasive treatment (bracing) if the curvature is caught early. Goldstein and colleagues9 recently evaluated 642 patients who presented for scoliosis evaluation over a 10-year period. There was no difference in curve magnitudes between patients with and without Medicaid insurance. Thirty-two percent of these patients were evaluated for a second opinion, and the authors chose not to subdivide patients on the basis of curve severity and treatment needed, noting only no difference between groups. There was no discussion of the potential difference between patients with and without private insurance with respect to surgically versus nonsurgically treated curves. We wanted to focus specifically on patients who required surgical intervention, as our experience has been that many patients with government insurance present with either very mild scoliosis (10°) or very large curves that were not identified because of lack of primary care access or inadequate school screening. Although summing these 2 groups would result in a similar average, they would represent a different cohort than patients with curves along a bell curve. Furthermore, it is the group of patients who would require surgical intervention that is so critical to identify early in order to intervene.
Our data suggest a difference in presenting curves between patients with and without private insurance. The approximately 10° difference between patient groups in this study could potentially represent the difference between bracing and surgery. Furthermore, Miyanji and colleagues6 evaluated the relationship between Cobb angle and health care consumption and correlated larger curve magnitudes with more levels fused, longer surgeries, and higher rates of transfusion. Specifically, every 10° increase in curve magnitude resulted in 7.8 more minutes of operative time, 0.3 extra levels fused, and 1.5 times increased risk for requiring a blood transfusion.
Cho and Egorova15 recently evaluated insurance status with respect to surgical outcomes using a national inpatient database and found that 42.4% of surgeries for AIS in children with Medicaid had fusions involving 9 or more levels, whereas only 33.6% of privately insured patients had fusions of 9 or more levels. There was no difference in osteotomy or reoperation for pseudarthrosis between groups, but there was a slightly higher rate of infectious (1.1% vs 0.6%) and hemorrhagic (2.5% vs 1.7%) complications in the Medicaid group. Hospital stay was longer in patients with Medicaid, though complications were not different between groups.
The mean difference in the magnitude of the curves treated in our study was not more than 10° between patients with and without Medicaid, perhaps explaining the lack of a statistically significant difference in number of levels fused between groups. Although the groups were similar with respect to the percentage requiring posterior column spinal osteotomies, we noted a difference in estimated blood loss between groups, likely explained by the fact that a junior surgeon was added just before initiation of the study period, potentially skewing the estimated blood loss as this surgeon gained experience. Payer status has been correlated to length of hospital stay in children with scoliosis. Vitale and colleagues8 reviewed the effect of payer status on surgical outcomes in 3606 scoliosis patients from a statewide database in California and concluded that, compared with patients having all other payment sources, Medicaid patients had higher odds for complications and longer hospital stay. Our hospital has adopted a highly coordinated care pathway that allows for discharge on postoperative day 2, likely explaining the lack of any difference in postoperative stay.16
The disparity in curve magnitudes among patients with and without private insurance is striking and probably multifactorial. Very likely, the combination of schools with limited screening programs within urban or rural school systems,17 restricted access to pediatricians,18,19 and longer waits to see orthopedic specialists20 all contribute to this disparity. It should be noted that school screening is mandatory in our state. This discrepancy may be related to a previously established tendency in minority populations toward waiting longer to seek care and refusing surgical recommendations, though we were unable to query socioeconomic factors such as race and household income.21,22 It is clearly important to increase access to care for underinsured patients with scoliosis. A comprehensive approach, including providing better education in the schools, establishing communication with referring primary care providers, and increasing access through more physicians or physician extenders, is likely needed. Orthopedists should perhaps treat scoliosis evaluation with the same sense of urgency given to minor fractures, and primary care providers should try to ensure that appropriate referrals for scoliosis are made. Also curious was the shorter travel distance for Medicaid patients versus private insurance patients in this study. We hypothesize this is related to our urban location and its large Medicaid population.
Our study had several limitations. Our electronic medical records (EMR) system does not store data related to the time a patient calls for an initial appointment, limiting our ability to determine how long patients waited for their initial consultation. Furthermore, the decision to undergo surgery is multifactorial and cannot be simplified into time from initial recommendation to surgery, as some patients delay surgery because of school or other obligations. These data should be reasonably consistent over time, as patients seen in the early spring in both groups may delay surgery until the summer, and those diagnosed in June may prefer earlier surgery.
Summary
Children with AIS are at risk for curve progression. Therefore, delays in providing timely care may result in worsening scoliosis. Compared with private insurance patients, Medicaid patients presented with larger curve magnitudes. Further study is needed to better delineate ways to improve care access for patients with scoliosis in communities with larger Medicaid populations.
1. Skaggs DL. Less access to care for children with Medicaid. Orthopedics. 2003;26(12):1184, 1186.
2. Skinner AC, Mayer ML. Effects of insurance status on children’s access to specialty care: a systematic review of the literature. BMC Health Serv Res. 2007;7:194.
3. Iobst C, King W, Baitner A, Tidwell M, Swirsky S, Skaggs DL. Access to care for children with fractures. J Pediatr Orthop. 2010;30(3):244-247.
4. Skaggs DL, Lehmann CL, Rice C, et al. Access to orthopaedic care for children with Medicaid versus private insurance: results of a national survey. J Pediatr Orthop. 2006;26(3):400-404.
5. Skaggs DL, Oda JE, Lerman L, et al. Insurance status and delay in orthotic treatment in children. J Pediatr Orthop. 2007;27(1):94-97.
6. Miyanji F, Slobogean GP, Samdani AF, et al. Is larger scoliosis curve magnitude associated with increased perioperative health-care resource utilization? A multicenter analysis of 325 adolescent idiopathic scoliosis curves. J Bone Joint Surg Am. 2012;94(9):809-813.
7. Nuno M, Drazin DG, Acosta FL Jr. Differences in treatments and outcomes for idiopathic scoliosis patients treated in the United States from 1998 to 2007: impact of socioeconomic variables and ethnicity. Spine J. 2013;13(2):116-123.
8. Vitale MA, Arons RR, Hyman JE, Skaggs DL, Roye DP, Vitale MG. The contribution of hospital volume, payer status, and other factors on the surgical outcomes of scoliosis patients: a review of 3,606 cases in the state of California. J Pediatr Orthop. 2005;25(3):393-399.
9. Goldstein RY, Joiner ER, Skaggs DL. Insurance status does not predict curve magnitude in adolescent idiopathic scoliosis at first presentation to an orthopaedic surgeon. J Pediatr Orthop. 2015;35(1):39-42.
10. Lenke LG, Betz RR, Harms J, et al. Adolescent idiopathic scoliosis: a new classification to determine extent of spinal arthrodesis. J Bone Joint Surg Am. 2001;83(8):1169-1181.
11. Alosh H, Riley LH 3rd, Skolasky RL. Insurance status, geography, race, and ethnicity as predictors of anterior cervical spine surgery rates and in-hospital mortality: an examination of United States trends from 1992 to 2005. Spine. 2009;34(18):1956-1962.
12. Newacheck PW, Hughes DC, Hung YY, Wong S, Stoddard JJ. The unmet health needs of America’s children. Pediatrics. 2000;105(4 pt 2):989-997.
13. Sabharwal S, Zhao C, McClemens E, Kaufmann A. Pediatric orthopaedic patients presenting to a university emergency department after visiting another emergency department: demographics and health insurance status. J Pediatr Orthop. 2007;27(6):690-694.
14. Pierce TR, Mehlman CT, Tamai J, Skaggs DL. Access to care for the adolescent anterior cruciate ligament patient with Medicaid versus private insurance. J Pediatr Orthop. 2012;32(3):245-248.
15. Cho SK, Egorova NN. The association between insurance status and complications, length of stay, and costs for pediatric idiopathic scoliosis. Spine. 2015;40(4):247-256.
16. Fletcher ND, Shourbaji N, Mitchell PM, Oswald TS, Devito DP, Bruce RW Jr. Clinical and economic implications of early discharge following posterior spinal fusion for adolescent idiopathic scoliosis. J Child Orthop. 2014;8(3):257-263.
17. Kasper MJ, Robbins L, Root L, Peterson MG, Allegrante JP. A musculoskeletal outreach screening, treatment, and education program for urban minority children. Arthritis Care Res. 1993;6(3):126-133.
18. Berman S, Dolins J, Tang SF, Yudkowsky B. Factors that influence the willingness of private primary care pediatricians to accept more Medicaid patients. Pediatrics. 2002;110(2 pt 1):239-248.
19. Sommers BD. Protecting low-income children’s access to care: are physician visits associated with reduced patient dropout from Medicaid and the Children’s Health Insurance Program? Pediatrics. 2006;118(1):e36-e42.
20. Bisgaier J, Polsky D, Rhodes KV. Academic medical centers and equity in specialty care access for children. Arch Pediatr Adolesc Med. 2012;166(4):304-310.
21. Sedlis SP, Fisher VJ, Tice D, Esposito R, Madmon L, Steinberg EH. Racial differences in performance of invasive cardiac procedures in a Department of Veterans Affairs medical center. J Clin Epidemiol. 1997;50(8):899-901.
22. Mitchell JB, McCormack LA. Time trends in late-stage diagnosis of cervical cancer. Differences by race/ethnicity and income. Med Care. 1997;35(12):1220-1224.
Rising health care costs have led many health insurers to limit benefits, which may be a problem for children in need of specialty care. Uninsured children have poorer access to specialty care than insured children. Children with public health coverage have better access to specialty care than uninsured children but inferior access compared with privately insured children.1,2 It is well documented that children with government insurance have limited access to orthopedic care for fractures, ligamentous knee injuries, and other injuries.1,3-5 Adolescent idiopathic scoliosis (AIS) differs from many other conditions managed by pediatric orthopedists, as it may be progressive, with management becoming increasingly more complex as the curve magnitude increases.6 The ability to access care earlier in the disease process may allow for earlier nonoperative interventions, such as bracing. For patients who require spinal fusion, earlier diagnosis and referral to a specialist could potentially result in shorter fusions and preserve distal motion segments. The ability to access the health care system in a timely fashion would therefore be of utmost importance for patients with scoliosis.
The literature on AIS is lacking in studies focused on care access based on insurance coverage and the potential impact that this may have on curve progression.7-9 We conducted a study to determine whether there is a difference between patients with and without private insurance who present to a busy urban pediatric orthopedic practice for management of scoliosis that eventually resulted in surgical treatment.
Materials and Methods
After obtaining institutional review board approval for this study, we retrospectively reviewed the medical records of patients (age, 10-18 years) who underwent posterior spinal fusion (PSF) for newly diagnosed AIS between 2008 and 2012. We excluded patients treated with growing spine instrumentation (growing rods), patients younger than 10 years or older than 18 years at presentation, and patients without adequate radiographs or clinical data, including insurance status. To focus on newly diagnosed scoliosis, we also excluded patients who had been seen for second opinions or whose scoliosis had been managed elsewhere in the past. Patients with syndromic, neuromuscular, or congenital scoliosis were also excluded.
Medical records were checked to ascertain time from initial evaluation to decision for surgery, time from recommendation for surgery until actual procedure, and insurance status. Distance traveled was figured from patients’ home addresses. Cobb angles were calculated from initial preoperative and final preoperative posteroanterior (PA) radiographs. Curves as seen on PA, lateral, and maximal effort, supine bending thoracic and lumbar radiographs from the initial preoperative visit were classified using the system of Lenke and colleagues.10 Hospital records were queried to determine number of levels fused at surgery, number of implants placed, and length of stay. Patients were evaluated without prior screening of insurance status and without prior consultation with referring physicians. Surgical procedures were scheduled on a first-come, first-served basis without preference for insurance status.
Results
We identified 135 consecutive patients with newly diagnosed AIS treated with PSF by our group between January 2008 and December 2012 (Table 1). Sixty-one percent had private insurance; 39% had Medicaid. There was no difference in age or ASA (American Society of Anesthesiologists) score between groups. Mean (SD) Cobb angle at initial presentation was 47.5° (14.3°) (range, 18.0°-86.0°) for the private insurance group and 57.2° (15.7°) (range, 23.0°-95.0°) for the Medicaid group (P < .0001). At time of surgery, mean (SD) Cobb angles were 54.6° (11.7°) and 60.6° (13.9°) for the private insurance and Medicaid groups, respectively (P = .008). There was no difference in curve types (Lenke and colleagues10 classification) between groups (Table 2, P = .83). Medicaid patients traveled a shorter mean (SD) distance for care, 56.3 (57.0) miles, versus 73.7 (66.7) miles (P = .05). There was no statistical difference (P = .14) in mean (SD) surgical wait time from surgery recommendation to actual surgery, 103.1 (62.4) days and 128.8 (137.5) days for the private insurance and Medicaid groups, respectively. The difference between patient groups in mean (SD) number of levels fused did not reach statistical significance (P = .16), 10.3 (2.2) levels for the Medicaid group and 9.7 (2.3) levels for the private insurance group. Mean (SD) estimated blood loss was higher for Medicaid patients, 445.7 (415.9) mL versus 335.1 (271.5) mL (P = .06), though there was no difference in use of posterior column osteotomies between groups. There was no difference (P = .11) in mean (SD) length of hospital stay between Medicaid patients, 2.6 (0.8) days, and private insurance patients, 2.4 (0.5) days.
Discussion
According to an extensive body of literature, patients with government insurance have limited access to specialty care.1,11,12 Medicaid-insured children in need of orthopedic care are no exception. Sabharwal and colleagues13 examined a database of pediatric fracture cases and found that 52% of the privately insured patients and 22% of the publicly insured patients received orthopedic care (P = .013).13 When Pierce and colleagues14 called 42 orthopedic practices regarding a fictitious 14-year-old patient with an anterior cruciate ligament tear, 38 offered an appointment within 2 weeks to a privately insured patient, and 6 offered such an appointment to a publicly insured patient. Skaggs and colleagues4 surveyed 230 orthopedic practices nationally and found that Medicaid-insured children had limited access to orthopedic care; 41 practices (18%) would not see a child with Medicaid under any circumstances. Using a fictitious case of a 10-year-old boy with a forearm fracture, Iobst and colleagues3 tried making an appointment at 100 orthopedic offices. Eight gave an appointment within 1 week to a Medicaid-insured patient, and 36 gave an appointment to a privately insured patient.3
There are few data regarding insurance status and scoliosis care in children. Spinal deformity differs from simple fractures and ligamentous injuries, as timely care may result in a less invasive treatment (bracing) if the curvature is caught early. Goldstein and colleagues9 recently evaluated 642 patients who presented for scoliosis evaluation over a 10-year period. There was no difference in curve magnitudes between patients with and without Medicaid insurance. Thirty-two percent of these patients were evaluated for a second opinion, and the authors chose not to subdivide patients on the basis of curve severity and treatment needed, noting only no difference between groups. There was no discussion of the potential difference between patients with and without private insurance with respect to surgically versus nonsurgically treated curves. We wanted to focus specifically on patients who required surgical intervention, as our experience has been that many patients with government insurance present with either very mild scoliosis (10°) or very large curves that were not identified because of lack of primary care access or inadequate school screening. Although summing these 2 groups would result in a similar average, they would represent a different cohort than patients with curves along a bell curve. Furthermore, it is the group of patients who would require surgical intervention that is so critical to identify early in order to intervene.
Our data suggest a difference in presenting curves between patients with and without private insurance. The approximately 10° difference between patient groups in this study could potentially represent the difference between bracing and surgery. Furthermore, Miyanji and colleagues6 evaluated the relationship between Cobb angle and health care consumption and correlated larger curve magnitudes with more levels fused, longer surgeries, and higher rates of transfusion. Specifically, every 10° increase in curve magnitude resulted in 7.8 more minutes of operative time, 0.3 extra levels fused, and 1.5 times increased risk for requiring a blood transfusion.
Cho and Egorova15 recently evaluated insurance status with respect to surgical outcomes using a national inpatient database and found that 42.4% of surgeries for AIS in children with Medicaid had fusions involving 9 or more levels, whereas only 33.6% of privately insured patients had fusions of 9 or more levels. There was no difference in osteotomy or reoperation for pseudarthrosis between groups, but there was a slightly higher rate of infectious (1.1% vs 0.6%) and hemorrhagic (2.5% vs 1.7%) complications in the Medicaid group. Hospital stay was longer in patients with Medicaid, though complications were not different between groups.
The mean difference in the magnitude of the curves treated in our study was not more than 10° between patients with and without Medicaid, perhaps explaining the lack of a statistically significant difference in number of levels fused between groups. Although the groups were similar with respect to the percentage requiring posterior column spinal osteotomies, we noted a difference in estimated blood loss between groups, likely explained by the fact that a junior surgeon was added just before initiation of the study period, potentially skewing the estimated blood loss as this surgeon gained experience. Payer status has been correlated to length of hospital stay in children with scoliosis. Vitale and colleagues8 reviewed the effect of payer status on surgical outcomes in 3606 scoliosis patients from a statewide database in California and concluded that, compared with patients having all other payment sources, Medicaid patients had higher odds for complications and longer hospital stay. Our hospital has adopted a highly coordinated care pathway that allows for discharge on postoperative day 2, likely explaining the lack of any difference in postoperative stay.16
The disparity in curve magnitudes among patients with and without private insurance is striking and probably multifactorial. Very likely, the combination of schools with limited screening programs within urban or rural school systems,17 restricted access to pediatricians,18,19 and longer waits to see orthopedic specialists20 all contribute to this disparity. It should be noted that school screening is mandatory in our state. This discrepancy may be related to a previously established tendency in minority populations toward waiting longer to seek care and refusing surgical recommendations, though we were unable to query socioeconomic factors such as race and household income.21,22 It is clearly important to increase access to care for underinsured patients with scoliosis. A comprehensive approach, including providing better education in the schools, establishing communication with referring primary care providers, and increasing access through more physicians or physician extenders, is likely needed. Orthopedists should perhaps treat scoliosis evaluation with the same sense of urgency given to minor fractures, and primary care providers should try to ensure that appropriate referrals for scoliosis are made. Also curious was the shorter travel distance for Medicaid patients versus private insurance patients in this study. We hypothesize this is related to our urban location and its large Medicaid population.
Our study had several limitations. Our electronic medical records (EMR) system does not store data related to the time a patient calls for an initial appointment, limiting our ability to determine how long patients waited for their initial consultation. Furthermore, the decision to undergo surgery is multifactorial and cannot be simplified into time from initial recommendation to surgery, as some patients delay surgery because of school or other obligations. These data should be reasonably consistent over time, as patients seen in the early spring in both groups may delay surgery until the summer, and those diagnosed in June may prefer earlier surgery.
Summary
Children with AIS are at risk for curve progression. Therefore, delays in providing timely care may result in worsening scoliosis. Compared with private insurance patients, Medicaid patients presented with larger curve magnitudes. Further study is needed to better delineate ways to improve care access for patients with scoliosis in communities with larger Medicaid populations.
Rising health care costs have led many health insurers to limit benefits, which may be a problem for children in need of specialty care. Uninsured children have poorer access to specialty care than insured children. Children with public health coverage have better access to specialty care than uninsured children but inferior access compared with privately insured children.1,2 It is well documented that children with government insurance have limited access to orthopedic care for fractures, ligamentous knee injuries, and other injuries.1,3-5 Adolescent idiopathic scoliosis (AIS) differs from many other conditions managed by pediatric orthopedists, as it may be progressive, with management becoming increasingly more complex as the curve magnitude increases.6 The ability to access care earlier in the disease process may allow for earlier nonoperative interventions, such as bracing. For patients who require spinal fusion, earlier diagnosis and referral to a specialist could potentially result in shorter fusions and preserve distal motion segments. The ability to access the health care system in a timely fashion would therefore be of utmost importance for patients with scoliosis.
The literature on AIS is lacking in studies focused on care access based on insurance coverage and the potential impact that this may have on curve progression.7-9 We conducted a study to determine whether there is a difference between patients with and without private insurance who present to a busy urban pediatric orthopedic practice for management of scoliosis that eventually resulted in surgical treatment.
Materials and Methods
After obtaining institutional review board approval for this study, we retrospectively reviewed the medical records of patients (age, 10-18 years) who underwent posterior spinal fusion (PSF) for newly diagnosed AIS between 2008 and 2012. We excluded patients treated with growing spine instrumentation (growing rods), patients younger than 10 years or older than 18 years at presentation, and patients without adequate radiographs or clinical data, including insurance status. To focus on newly diagnosed scoliosis, we also excluded patients who had been seen for second opinions or whose scoliosis had been managed elsewhere in the past. Patients with syndromic, neuromuscular, or congenital scoliosis were also excluded.
Medical records were checked to ascertain time from initial evaluation to decision for surgery, time from recommendation for surgery until actual procedure, and insurance status. Distance traveled was figured from patients’ home addresses. Cobb angles were calculated from initial preoperative and final preoperative posteroanterior (PA) radiographs. Curves as seen on PA, lateral, and maximal effort, supine bending thoracic and lumbar radiographs from the initial preoperative visit were classified using the system of Lenke and colleagues.10 Hospital records were queried to determine number of levels fused at surgery, number of implants placed, and length of stay. Patients were evaluated without prior screening of insurance status and without prior consultation with referring physicians. Surgical procedures were scheduled on a first-come, first-served basis without preference for insurance status.
Results
We identified 135 consecutive patients with newly diagnosed AIS treated with PSF by our group between January 2008 and December 2012 (Table 1). Sixty-one percent had private insurance; 39% had Medicaid. There was no difference in age or ASA (American Society of Anesthesiologists) score between groups. Mean (SD) Cobb angle at initial presentation was 47.5° (14.3°) (range, 18.0°-86.0°) for the private insurance group and 57.2° (15.7°) (range, 23.0°-95.0°) for the Medicaid group (P < .0001). At time of surgery, mean (SD) Cobb angles were 54.6° (11.7°) and 60.6° (13.9°) for the private insurance and Medicaid groups, respectively (P = .008). There was no difference in curve types (Lenke and colleagues10 classification) between groups (Table 2, P = .83). Medicaid patients traveled a shorter mean (SD) distance for care, 56.3 (57.0) miles, versus 73.7 (66.7) miles (P = .05). There was no statistical difference (P = .14) in mean (SD) surgical wait time from surgery recommendation to actual surgery, 103.1 (62.4) days and 128.8 (137.5) days for the private insurance and Medicaid groups, respectively. The difference between patient groups in mean (SD) number of levels fused did not reach statistical significance (P = .16), 10.3 (2.2) levels for the Medicaid group and 9.7 (2.3) levels for the private insurance group. Mean (SD) estimated blood loss was higher for Medicaid patients, 445.7 (415.9) mL versus 335.1 (271.5) mL (P = .06), though there was no difference in use of posterior column osteotomies between groups. There was no difference (P = .11) in mean (SD) length of hospital stay between Medicaid patients, 2.6 (0.8) days, and private insurance patients, 2.4 (0.5) days.
Discussion
According to an extensive body of literature, patients with government insurance have limited access to specialty care.1,11,12 Medicaid-insured children in need of orthopedic care are no exception. Sabharwal and colleagues13 examined a database of pediatric fracture cases and found that 52% of the privately insured patients and 22% of the publicly insured patients received orthopedic care (P = .013).13 When Pierce and colleagues14 called 42 orthopedic practices regarding a fictitious 14-year-old patient with an anterior cruciate ligament tear, 38 offered an appointment within 2 weeks to a privately insured patient, and 6 offered such an appointment to a publicly insured patient. Skaggs and colleagues4 surveyed 230 orthopedic practices nationally and found that Medicaid-insured children had limited access to orthopedic care; 41 practices (18%) would not see a child with Medicaid under any circumstances. Using a fictitious case of a 10-year-old boy with a forearm fracture, Iobst and colleagues3 tried making an appointment at 100 orthopedic offices. Eight gave an appointment within 1 week to a Medicaid-insured patient, and 36 gave an appointment to a privately insured patient.3
There are few data regarding insurance status and scoliosis care in children. Spinal deformity differs from simple fractures and ligamentous injuries, as timely care may result in a less invasive treatment (bracing) if the curvature is caught early. Goldstein and colleagues9 recently evaluated 642 patients who presented for scoliosis evaluation over a 10-year period. There was no difference in curve magnitudes between patients with and without Medicaid insurance. Thirty-two percent of these patients were evaluated for a second opinion, and the authors chose not to subdivide patients on the basis of curve severity and treatment needed, noting only no difference between groups. There was no discussion of the potential difference between patients with and without private insurance with respect to surgically versus nonsurgically treated curves. We wanted to focus specifically on patients who required surgical intervention, as our experience has been that many patients with government insurance present with either very mild scoliosis (10°) or very large curves that were not identified because of lack of primary care access or inadequate school screening. Although summing these 2 groups would result in a similar average, they would represent a different cohort than patients with curves along a bell curve. Furthermore, it is the group of patients who would require surgical intervention that is so critical to identify early in order to intervene.
Our data suggest a difference in presenting curves between patients with and without private insurance. The approximately 10° difference between patient groups in this study could potentially represent the difference between bracing and surgery. Furthermore, Miyanji and colleagues6 evaluated the relationship between Cobb angle and health care consumption and correlated larger curve magnitudes with more levels fused, longer surgeries, and higher rates of transfusion. Specifically, every 10° increase in curve magnitude resulted in 7.8 more minutes of operative time, 0.3 extra levels fused, and 1.5 times increased risk for requiring a blood transfusion.
Cho and Egorova15 recently evaluated insurance status with respect to surgical outcomes using a national inpatient database and found that 42.4% of surgeries for AIS in children with Medicaid had fusions involving 9 or more levels, whereas only 33.6% of privately insured patients had fusions of 9 or more levels. There was no difference in osteotomy or reoperation for pseudarthrosis between groups, but there was a slightly higher rate of infectious (1.1% vs 0.6%) and hemorrhagic (2.5% vs 1.7%) complications in the Medicaid group. Hospital stay was longer in patients with Medicaid, though complications were not different between groups.
The mean difference in the magnitude of the curves treated in our study was not more than 10° between patients with and without Medicaid, perhaps explaining the lack of a statistically significant difference in number of levels fused between groups. Although the groups were similar with respect to the percentage requiring posterior column spinal osteotomies, we noted a difference in estimated blood loss between groups, likely explained by the fact that a junior surgeon was added just before initiation of the study period, potentially skewing the estimated blood loss as this surgeon gained experience. Payer status has been correlated to length of hospital stay in children with scoliosis. Vitale and colleagues8 reviewed the effect of payer status on surgical outcomes in 3606 scoliosis patients from a statewide database in California and concluded that, compared with patients having all other payment sources, Medicaid patients had higher odds for complications and longer hospital stay. Our hospital has adopted a highly coordinated care pathway that allows for discharge on postoperative day 2, likely explaining the lack of any difference in postoperative stay.16
The disparity in curve magnitudes among patients with and without private insurance is striking and probably multifactorial. Very likely, the combination of schools with limited screening programs within urban or rural school systems,17 restricted access to pediatricians,18,19 and longer waits to see orthopedic specialists20 all contribute to this disparity. It should be noted that school screening is mandatory in our state. This discrepancy may be related to a previously established tendency in minority populations toward waiting longer to seek care and refusing surgical recommendations, though we were unable to query socioeconomic factors such as race and household income.21,22 It is clearly important to increase access to care for underinsured patients with scoliosis. A comprehensive approach, including providing better education in the schools, establishing communication with referring primary care providers, and increasing access through more physicians or physician extenders, is likely needed. Orthopedists should perhaps treat scoliosis evaluation with the same sense of urgency given to minor fractures, and primary care providers should try to ensure that appropriate referrals for scoliosis are made. Also curious was the shorter travel distance for Medicaid patients versus private insurance patients in this study. We hypothesize this is related to our urban location and its large Medicaid population.
Our study had several limitations. Our electronic medical records (EMR) system does not store data related to the time a patient calls for an initial appointment, limiting our ability to determine how long patients waited for their initial consultation. Furthermore, the decision to undergo surgery is multifactorial and cannot be simplified into time from initial recommendation to surgery, as some patients delay surgery because of school or other obligations. These data should be reasonably consistent over time, as patients seen in the early spring in both groups may delay surgery until the summer, and those diagnosed in June may prefer earlier surgery.
Summary
Children with AIS are at risk for curve progression. Therefore, delays in providing timely care may result in worsening scoliosis. Compared with private insurance patients, Medicaid patients presented with larger curve magnitudes. Further study is needed to better delineate ways to improve care access for patients with scoliosis in communities with larger Medicaid populations.
1. Skaggs DL. Less access to care for children with Medicaid. Orthopedics. 2003;26(12):1184, 1186.
2. Skinner AC, Mayer ML. Effects of insurance status on children’s access to specialty care: a systematic review of the literature. BMC Health Serv Res. 2007;7:194.
3. Iobst C, King W, Baitner A, Tidwell M, Swirsky S, Skaggs DL. Access to care for children with fractures. J Pediatr Orthop. 2010;30(3):244-247.
4. Skaggs DL, Lehmann CL, Rice C, et al. Access to orthopaedic care for children with Medicaid versus private insurance: results of a national survey. J Pediatr Orthop. 2006;26(3):400-404.
5. Skaggs DL, Oda JE, Lerman L, et al. Insurance status and delay in orthotic treatment in children. J Pediatr Orthop. 2007;27(1):94-97.
6. Miyanji F, Slobogean GP, Samdani AF, et al. Is larger scoliosis curve magnitude associated with increased perioperative health-care resource utilization? A multicenter analysis of 325 adolescent idiopathic scoliosis curves. J Bone Joint Surg Am. 2012;94(9):809-813.
7. Nuno M, Drazin DG, Acosta FL Jr. Differences in treatments and outcomes for idiopathic scoliosis patients treated in the United States from 1998 to 2007: impact of socioeconomic variables and ethnicity. Spine J. 2013;13(2):116-123.
8. Vitale MA, Arons RR, Hyman JE, Skaggs DL, Roye DP, Vitale MG. The contribution of hospital volume, payer status, and other factors on the surgical outcomes of scoliosis patients: a review of 3,606 cases in the state of California. J Pediatr Orthop. 2005;25(3):393-399.
9. Goldstein RY, Joiner ER, Skaggs DL. Insurance status does not predict curve magnitude in adolescent idiopathic scoliosis at first presentation to an orthopaedic surgeon. J Pediatr Orthop. 2015;35(1):39-42.
10. Lenke LG, Betz RR, Harms J, et al. Adolescent idiopathic scoliosis: a new classification to determine extent of spinal arthrodesis. J Bone Joint Surg Am. 2001;83(8):1169-1181.
11. Alosh H, Riley LH 3rd, Skolasky RL. Insurance status, geography, race, and ethnicity as predictors of anterior cervical spine surgery rates and in-hospital mortality: an examination of United States trends from 1992 to 2005. Spine. 2009;34(18):1956-1962.
12. Newacheck PW, Hughes DC, Hung YY, Wong S, Stoddard JJ. The unmet health needs of America’s children. Pediatrics. 2000;105(4 pt 2):989-997.
13. Sabharwal S, Zhao C, McClemens E, Kaufmann A. Pediatric orthopaedic patients presenting to a university emergency department after visiting another emergency department: demographics and health insurance status. J Pediatr Orthop. 2007;27(6):690-694.
14. Pierce TR, Mehlman CT, Tamai J, Skaggs DL. Access to care for the adolescent anterior cruciate ligament patient with Medicaid versus private insurance. J Pediatr Orthop. 2012;32(3):245-248.
15. Cho SK, Egorova NN. The association between insurance status and complications, length of stay, and costs for pediatric idiopathic scoliosis. Spine. 2015;40(4):247-256.
16. Fletcher ND, Shourbaji N, Mitchell PM, Oswald TS, Devito DP, Bruce RW Jr. Clinical and economic implications of early discharge following posterior spinal fusion for adolescent idiopathic scoliosis. J Child Orthop. 2014;8(3):257-263.
17. Kasper MJ, Robbins L, Root L, Peterson MG, Allegrante JP. A musculoskeletal outreach screening, treatment, and education program for urban minority children. Arthritis Care Res. 1993;6(3):126-133.
18. Berman S, Dolins J, Tang SF, Yudkowsky B. Factors that influence the willingness of private primary care pediatricians to accept more Medicaid patients. Pediatrics. 2002;110(2 pt 1):239-248.
19. Sommers BD. Protecting low-income children’s access to care: are physician visits associated with reduced patient dropout from Medicaid and the Children’s Health Insurance Program? Pediatrics. 2006;118(1):e36-e42.
20. Bisgaier J, Polsky D, Rhodes KV. Academic medical centers and equity in specialty care access for children. Arch Pediatr Adolesc Med. 2012;166(4):304-310.
21. Sedlis SP, Fisher VJ, Tice D, Esposito R, Madmon L, Steinberg EH. Racial differences in performance of invasive cardiac procedures in a Department of Veterans Affairs medical center. J Clin Epidemiol. 1997;50(8):899-901.
22. Mitchell JB, McCormack LA. Time trends in late-stage diagnosis of cervical cancer. Differences by race/ethnicity and income. Med Care. 1997;35(12):1220-1224.
1. Skaggs DL. Less access to care for children with Medicaid. Orthopedics. 2003;26(12):1184, 1186.
2. Skinner AC, Mayer ML. Effects of insurance status on children’s access to specialty care: a systematic review of the literature. BMC Health Serv Res. 2007;7:194.
3. Iobst C, King W, Baitner A, Tidwell M, Swirsky S, Skaggs DL. Access to care for children with fractures. J Pediatr Orthop. 2010;30(3):244-247.
4. Skaggs DL, Lehmann CL, Rice C, et al. Access to orthopaedic care for children with Medicaid versus private insurance: results of a national survey. J Pediatr Orthop. 2006;26(3):400-404.
5. Skaggs DL, Oda JE, Lerman L, et al. Insurance status and delay in orthotic treatment in children. J Pediatr Orthop. 2007;27(1):94-97.
6. Miyanji F, Slobogean GP, Samdani AF, et al. Is larger scoliosis curve magnitude associated with increased perioperative health-care resource utilization? A multicenter analysis of 325 adolescent idiopathic scoliosis curves. J Bone Joint Surg Am. 2012;94(9):809-813.
7. Nuno M, Drazin DG, Acosta FL Jr. Differences in treatments and outcomes for idiopathic scoliosis patients treated in the United States from 1998 to 2007: impact of socioeconomic variables and ethnicity. Spine J. 2013;13(2):116-123.
8. Vitale MA, Arons RR, Hyman JE, Skaggs DL, Roye DP, Vitale MG. The contribution of hospital volume, payer status, and other factors on the surgical outcomes of scoliosis patients: a review of 3,606 cases in the state of California. J Pediatr Orthop. 2005;25(3):393-399.
9. Goldstein RY, Joiner ER, Skaggs DL. Insurance status does not predict curve magnitude in adolescent idiopathic scoliosis at first presentation to an orthopaedic surgeon. J Pediatr Orthop. 2015;35(1):39-42.
10. Lenke LG, Betz RR, Harms J, et al. Adolescent idiopathic scoliosis: a new classification to determine extent of spinal arthrodesis. J Bone Joint Surg Am. 2001;83(8):1169-1181.
11. Alosh H, Riley LH 3rd, Skolasky RL. Insurance status, geography, race, and ethnicity as predictors of anterior cervical spine surgery rates and in-hospital mortality: an examination of United States trends from 1992 to 2005. Spine. 2009;34(18):1956-1962.
12. Newacheck PW, Hughes DC, Hung YY, Wong S, Stoddard JJ. The unmet health needs of America’s children. Pediatrics. 2000;105(4 pt 2):989-997.
13. Sabharwal S, Zhao C, McClemens E, Kaufmann A. Pediatric orthopaedic patients presenting to a university emergency department after visiting another emergency department: demographics and health insurance status. J Pediatr Orthop. 2007;27(6):690-694.
14. Pierce TR, Mehlman CT, Tamai J, Skaggs DL. Access to care for the adolescent anterior cruciate ligament patient with Medicaid versus private insurance. J Pediatr Orthop. 2012;32(3):245-248.
15. Cho SK, Egorova NN. The association between insurance status and complications, length of stay, and costs for pediatric idiopathic scoliosis. Spine. 2015;40(4):247-256.
16. Fletcher ND, Shourbaji N, Mitchell PM, Oswald TS, Devito DP, Bruce RW Jr. Clinical and economic implications of early discharge following posterior spinal fusion for adolescent idiopathic scoliosis. J Child Orthop. 2014;8(3):257-263.
17. Kasper MJ, Robbins L, Root L, Peterson MG, Allegrante JP. A musculoskeletal outreach screening, treatment, and education program for urban minority children. Arthritis Care Res. 1993;6(3):126-133.
18. Berman S, Dolins J, Tang SF, Yudkowsky B. Factors that influence the willingness of private primary care pediatricians to accept more Medicaid patients. Pediatrics. 2002;110(2 pt 1):239-248.
19. Sommers BD. Protecting low-income children’s access to care: are physician visits associated with reduced patient dropout from Medicaid and the Children’s Health Insurance Program? Pediatrics. 2006;118(1):e36-e42.
20. Bisgaier J, Polsky D, Rhodes KV. Academic medical centers and equity in specialty care access for children. Arch Pediatr Adolesc Med. 2012;166(4):304-310.
21. Sedlis SP, Fisher VJ, Tice D, Esposito R, Madmon L, Steinberg EH. Racial differences in performance of invasive cardiac procedures in a Department of Veterans Affairs medical center. J Clin Epidemiol. 1997;50(8):899-901.
22. Mitchell JB, McCormack LA. Time trends in late-stage diagnosis of cervical cancer. Differences by race/ethnicity and income. Med Care. 1997;35(12):1220-1224.
Is the Orthopedic Fellowship Interview Process Broken? A Survey of Program Directors and Residents
Over the past several decades, an increasing number of orthopedic surgery residents have pursued fellowship training.1 This inclination parallels market trends toward subspecialization.2-5 In 1984, 83% of orthopedics job announcements were for general orthopedists. Twenty-five years later, almost 70% of orthopedic opportunities were for fellowship-trained surgeons.6 Further, between 1990 and 2006, the proportion of practicing orthopedic generalists decreased from 44% to 29%.3 In 2007, the American Academy of Orthopaedic Surgery (AAOS) reported 90% of graduating residents were planning to pursue fellowship training.7 Reasons for the explosion in subspecialty training are plentiful and well documented.2-5 Subspecialty positions now dominate the job market, further reinforcing incentives for residents to pursue fellowship training.
The past several decades have seen numerous changes in the orthopedic fellowship interview process. Early on, it was largely unregulated, dependent on personal and professional connections, and flush with the classic “exploding offer” (residents were given a fellowship offer that expired within hours or days). In the 1980s, as the number of fellowship applications surged, the Accreditation Council for Graduate Medical Education (ACGME) pushed for a more regulated process.8 To further standardize the system, the American Orthopaedic Association (AOA), the AAOS, and several other specialty organizations created the Orthopaedic Fellowship Match Program Initiative in 2008.9 Currently, all orthopedic specialties are represented in either the San Francisco Match Program or National Residency Match Program.
As the system currently stands, postgraduate year 4 (PGY-4) residents are required to interview across the country to secure postgraduate training. This process necessitates residents’ absence from their program, reducing educational opportunities and placing potential continuity-of-care constraints on the residency program. Despite the growing competitiveness for fellowship positions, the increasing number of fellowships available, the rising educational debt of residents, and the limitations of the 80-hour work week, the impact of the interview process on both residents and residency programs has received minimal attention.
We conducted a study to elucidate the impact of the fellowship interview process on residents and residency programs. We hypothesized the time and financial costs for fellowship interviews would be substantial.
Materials and Methods
We obtained institutional review board (IRB) approval for this study. Then, in April 2014, we sent 2 mixed-response questionnaires to orthopedic surgery residency directors and residents. There were 8 items on the director questionnaire and 11 on the resident questionnaire. The surveys were designed to determine the impact of the fellowship interview process on residents and residency programs with respect to finances, time, education, and continuity of care. Each survey had at least 1 free-response question, providing the opportunity to recommend changes to the interview process. The surveys were reviewed and approved by our IRB.
An email was sent to 155 orthopedic surgery program directors or their secretaries. The email asked that the director complete the director questionnaire and that the resident questionnaire be forwarded to senior-level residents, PGY-4s and PGY-5s, who had completed the fellowship interview process. Forty-five (29%) of the 155 directors responded, as did 129 (estimated 9.5%) of an estimated 1354 potential PGY-4s and PGY-5s.10
The Survey Monkey surveys could be completed over a 3-week period. All responses were anonymous. Using Survey Monkey, we aggregated individual responses into predefined clusters before performing statistical analysis. Descriptive statistics were generated with Microsoft Excel.
Results
Survey respondents represented all the orthopedic subspecialties (Table). Seventy-eight percent of residents applied to at least 13 programs (average, 19) (Figure 1). Ninety-two percent received at least 8 interview offers (average, 14). Eighty-three percent attended 8 or more interviews (average, 11). Seventy-one percent of all interviews were granted when requested, and 79% of all interviews were attended when offered.
Residents spent an average of $5875 (range, $500-$12,000+) on the fellowship interview process (Figure 2). The highest percentage of respondents, 39.5%, selected an average expense between $4000 and $6000. Forty-nine percent of residents borrowed money (from credit cards, additional loans, family members) to pay their expenses.
Average number of days away from residency programs was 11, with 86% of residents missing more than 8 days (Figure 1). About one-third of residents reported being away from their home program for almost 2 weeks during the interview season. Further, 74% of residents wanted changes made to the fellowship application process.
Thirty-seven (82%) of the 45 program directors were from academic programs, the other 8 from community-based programs. Average number of residents in programs per year was 4 (73% of the programs had 4-6 residents per year). Respondents rated the disruption caused by residents’ interview absences from 1 (least disruptive) to 10 (most disruptive) (Figure 3); the average rating was over 7 (high level of disruption). Although 9% of directors thought the process caused little or no disruption (rating, 1-3), 62% thought it extremely disruptive (rating, 8-10).
Thirty-one (69%) of the 45 directors agreed that the fellowship interview process should undergo fundamental change. Asked about possible solutions to current complaints, 60% of the directors agreed that interviews should be conducted in a central location. Of the directors who thought fundamental change was needed, 59% indicated AAOS and other specialty societies together should lead the change in the fellowship interview process.
Both residents and program directors were given the opportunity to write in suggestions regarding how to improve the fellowship interview process. Suggestions were made by 85 (66%) of the 129 residents and 24 (53%) of the 45 directors (Appendix).
Discussion
Graduating residents are entering a health care environment in which they must be financially conscious because of increasing education debt and decreasing reimbursement prospects.3 Nevertheless, an overwhelming majority of residents delay entering practice to pursue fellowship training—an estimated opportunity cost of $350,000.3 Minimal attention has been given to the potential costs of the fellowship interview process.
Our study results highlight that time away from residency training, financial costs associated with the fellowship interview process, and disruption of the residency program are substantial. On average, residents applied to 19 programs, received 14 interview offers, attended 11 interviews, were away from residency training 11 days, and spent $5875 on travel. The great majority of both residents and program directors wanted changes in the current paradigm governing the orthopedic fellowship interview process.
It is reasonable to think that the number of days residents spend away on interviews would reduce the time available for education and patient care. Although unknown, it is plausible that residents of programs outside major metropolitan centers and residents who apply to more competitive fellowships may be forced to spend even more time away from training. Outside the focus of this study are the impact that residents’ absence might have on their education and the impact of this absence on the people who do the residents’ work while they are away.
Mean fellowship expense was similar to that reported by residents pursuing a pediatric general surgery fellowship ($6974) or a plastic surgery fellowship ($6100).11,12 Unfortunately, we were unable to determine if average cost is influenced by choice of fellowship specialty or location of residency program. Regardless, fellowship cost may impose an additional financial burden on residents. According to the Association of American Medical Colleges (AAMC), the median salary for PGY-4 residents was $56,380 in 2013. Therefore, on average, the fellowship process consumes more than 10% of a resident’s pretax salary. For perspective, this equates to more than $40,000 for a practicing orthopedic surgeon with a median salary of $413,000.13 With an average medical student graduate debt of $175,000 and continuing decreases in reimbursement, further financial hardships to newly graduating residents cannot be understated.5,11,12
Almost 70% of program directors thought the fellowship process significantly disrupted their program. Reasons given for this disruption mainly involved residents’ time away from the program and the resulting strains placed on maintaining adequate coverage for patient care. The overall disruption score of 7.4 out of 10 was consistent with the great majority thinking that the fellowship process negatively affects their residency program. Altering the fellowship interview process may provide unintended benefits to programs and program directors.
Both program directors and residents communicated that change is needed, but there was little consensus regarding how to effect change and who should lead. This lack of consensus highlights how important it is for the various orthopedic leadership committees to actively and collectively participate in discussions about redefining the system. It has been proposed that it would be ideal for the AOA to lead the change, as the AOA consists of a representative cohort of academic orthopedists and leaders across the spectrum of all fellowship specialties.14 Given the abundant concern of both residents and program directors, we find it prudent to issue a call to arms of sorts to the AAOS and the individual orthopedic subspecialty societies to work together on a common goal that would benefit residents, programs, and subspecialties within orthopedics.
In trying to understand the challenges that residents, program directors, and programs face, as well as the inherent complexity of the current system, we incorporated respondents’ write-in comments into suggested ways of improving the fellowship interview process. These comments had broad perspectives but overall were consistent with the survey results (Appendix).
Technology
Health care is continually finding new ways to take advantage of technological advances. This is occurring with the fellowship interview schema. Numerous disciplines are using videoconferencing platforms (eg, Skype) to conduct interviews. This practice is becoming more commonplace in the business sector. In a recent survey, more than 60% of human resource managers reported conducting video interviews.15 Two independent residency programs have used video interviews with mixed success.16,17
Another technological change requested by residents is the creation and updating of fellowship web pages with standardized information. Such a service may prove useful to residents researching a program and may even lead to limiting the number of programs residents apply to, as they may be able to dial in on exactly what distinguishes one program from another before traveling for an interview. A recent study of orthopedic sports medicine fellowship programs found that most of these programs lacked pertinent information on their websites.18 Important information regarding case logs from current and former fellows; number of faculty, residents, and fellows; and schedules and facilities of interview sites are a few of the online data points that may help residents differentiate particular programs.19,20 Questions like these are often asked at interviews and site visits. Having accurate information easily available online may reduce or eliminate the need to travel to a site for such information. Standardizing information would also increase transparency among available fellowships. Although not specifically mentioned, organizational software that improves the productivity of the process may help limit the large number of programs applied to, the interviews offered and attended, the days away, and the financial costs without reducing the match rate.
Timing and Location
The issue of timing—with respect to geographical or meteorological concerns—was another recurring theme among respondents. Numerous respondents indicated that certain programs located in geographic proximity tried to minimize travel by offering interviews around the same time. This coordination potentially minimizes travel expenses and time away from the residency program by allowing residents to interview at multiple locations during a single trip per region. The sports medicine fellowship process was identified as a good example of aligning interviews based on geography. Several respondents suggested an option that also reflects the practice of nonsurgical fellowships—delaying the interview season to bypass potential weather concerns. Winter 2013–2014 saw the most flight delays or cancellations in more than a decade; about 50% of all flights scheduled between December and February were delayed or canceled.21 Beyond the additional factor of more time away or missing an interview because of the weather are safety concerns related to the weather. One resident reported having a motor vehicle accident while traveling to an interview in poor weather conditions (Appendix).
National Meetings
Each orthopedic subspecialty has numerous national meetings. Many programs offer applicants the opportunity to interview at these meetings. One respondent mentioned that the annual meeting of the Orthopaedic Trauma Association offers trauma applicants the opportunity to interview with multiple programs. It might be beneficial to endorse this practice on a larger scale to help reduce travel and time away. We recognize that visiting individual programs is an important aspect of the match process, but doing so on a targeted level may make more sense, increasing financial efficiency and reducing time away from programs.
Proposed Solution
A combined proposed solution that can be implemented without a radical overhaul or significant investments might involve moving the interview season to early spring, switching to a 2-tiered system with a centralized first round of interview screening coinciding with subspecialty national meetings or the AAOS annual meeting, and standardizing online information for all orthopedic fellowship programs. A 2-tiered interview process would allow programs and candidates to obtain exposure to a significant number of programs in the first round without incurring significant costs and then would impose a cap on the number of programs to visit. This would level the playing field between candidates with more time and money and candidates who are more constrained in their training environment and finances. A stopgap or adjunct to residents or fellowship programs unable to attend a centralized meeting would be to combine technological tools, such as Internet-based videoconferencing (Skype), before site visits by residents. After this first round of introductions and interviews, residents could then decide on a limited number of programs to formally visit, attend, and ultimately rank. This proposed system would still be able to function within the confines of the match, and it would benefit from the protections offered to residents and programs. Although capping the number of interviews attended by residents clearly can lower costs across the board, we recognize the difficulty of enforcing such a requirement. These potential changes to the system are not exhaustive, and we hope this work will serve as a springboard to further discussion.
Our study had several inherent weaknesses. Our data came from survey responses, which reflect the perspectives only of the responding residents and program directors. Unfortunately, a small number of orthopedic residents responded to this survey, so there was a potential for bias. However, we think the central themes discovered in this survey are only echoes of the concerns of the larger population of residents and program directors. Our hope in designing such a study was to bring to light some of the discrepancies in the fellowship interview process, the goal being to stimulate interest among the orthopedic leadership representing future orthopedic surgeons. More study is needed to clarify if these issues are reflective of a larger segment of residents and program directors. In addition, action may be needed to fully elucidate the intricate interworking of the fellowship process in order to maximize the interest of the orthopedic surgeons who are seeking fellowship training. Another study limitation was the potential for recall bias in the more senior PGY-5 residents, who were further from the interview process than PGY-4 respondents were. Because of the need for anonymity with the surveys, we could not link some findings (eg, program impact, cost, time away) to individual programs or different specialty fellowships. Although it appears there is a desire for a more cost-effective system, given the financial pressures on medical students and residents, the desire to match increases costs because students are likely to attend more interviews than actually needed. Our proposed solution does not take into account residents’ behavior with respect to the current match system. For example, the prevailing thought is that interviewing at more programs increases the likelihood of matching into a desired subspecialty. Despite these study limitations, we think our results identified important points for discussion, investigation, and potential action by orthopedic leadership.
Conclusion
The challenge of critiquing and improving the orthopedic fellowship process requires the same courageous leadership that was recommended almost a decade ago.14 In this study, we tried to elucidate the impact of the PGY-4 fellowship interview process with respect to residents and residency programs. Our results highlight that time away from residency training, financial costs associated with the fellowship interview process, and disruption of the residency program are substantial and that both residents and program directors want changes made. Leadership needs to further investigate alternatives to the current process to lessen the impact on all parties in this important process.
1. Simon MA. Evolution of the present status of orthopaedic surgery fellowships. J Bone Joint Surg Am. 1998;80(12):1826-1829.
2. Brunworth LS, Chintalapani SR, Gray RR, Cardoso R, Owens PW. Resident selection of hand surgery fellowships: a survey of the 2011, 2012, and 2013 hand fellowship graduates. Hand. 2013;8(2):164-171.
3. Gaskill T, Cook C, Nunley J, Mather RC. The financial impact of orthopaedic fellowship training. J Bone Joint Surg Am. 2009;91(7):1814-1821.
4. Sarmiento A. Additional thoughts on orthopedic residency and fellowships. Orthopedics. 2010;33(10):712-713.
5. Griffin SM, Stoneback JW. Navigating the Orthopaedic Trauma Fellowship Match from a candidate’s perspective. J Orthop Trauma. 2011;25(suppl 3):S101-S103.
6. Morrell NT, Mercer DM, Moneim MS. Trends in the orthopedic job market and the importance of fellowship subspecialty training. Orthopedics. 2012;35(4):e555-e560.
7. Iorio R, Robb WJ, Healy WL, et al. Orthopaedic surgeon workforce and volume assessment for total hip and knee replacement in the United States: preparing for an epidemic. J Bone Joint Surg Am. 2008;90(7):1598-1605.
8. Emery SE, Guss D, Kuremsky MA, Hamlin BR, Herndon JH, Rubash HE. Resident education versus fellowship training—conflict or synergy? AOA critical issues. J Bone Joint Surg Am. 2012;94(21):e159.
9. Harner CD, Ranawat AS, Niederle M, et al. AOA symposium. Current state of fellowship hiring: is a universal match necessary? Is it possible? J Bone Joint Surg Am. 2008;90(6):1375-1384.
10. Ranawat A, Nunley RM, Genuario JW, Sharan AD, Mehta S; Washington Health Policy Fellows. Current state of the fellowship hiring process: Are we in 1957 or 2007? AAOS Now. 2007;1(8).
11. Little DC, Yoder SM, Grikscheit TC, et al. Cost considerations and applicant characteristics for the Pediatric Surgery Match. J Pediatr Surg. 2005;40(1):69-73.
12. Claiborne JR, Crantford JC, Swett KR, David LR. The Plastic Surgery Match: predicting success and improving the process. Ann Plast Surg. 2013;70(6):698-703.
13. Kane L, Peckham C. Medscape Physician Compensation Report 2014. http://www.medscape.com/features/slideshow/compensation/2014/public/overview. Published April 15, 2014. Accessed September 26, 2015.
14. Swiontkowski MF. A simple formula for continued improvement in orthopaedic surgery postgraduate training: courageous leadership. J Bone Joint Surg Am. 2008;90(6):1175.
15. Survey: six in 10 companies conduct video job interviews [news release]. http://www.prnewswire.com/news-releases/survey-six-in-10-companies-conduct-video-job-interviews-167973406.html. Published August 30, 2012. Accessed September 26, 2015.
16. Kerfoot BP, Asher KP, McCullough DL. Financial and educational costs of the residency interview process for urology applicants. Urology. 2008;71(6):990-994.
17. Edje L, Miller C, Kiefer J, Oram D. Using Skype as an alternative for residency selection interviews. J Grad Med Educ. 2013;5(3):503-505.
18. Mulcahey MK, Gosselin MM, Fadale PD. Evaluation of the content and accessibility of web sites for accredited orthopaedic sports medicine fellowships. J Bone Joint Surg Am. 2013;95(12):e85.
19. Gaeta TJ, Birkhahn RH, Lamont D, Banga N, Bove JJ. Aspects of residency programs’ web sites important to student applicants. Acad Emerg Med. 2005;12(1):89-92.
20. Mahler SA, Wagner MJ, Church A, Sokolosky M, Cline DM. Importance of residency program web sites to emergency medicine applicants. J Emerg Med. 2009;36(1):83-88.
21. Davies A. Winter’s toll: 1 million flights cancelled or delayed, costing travelers $5.3 billion. Business Insider. http://www.businessinsider.com/winter-flights-cancelled-delayed-cost-2014-3. Published March 3, 2014. Accessed September 26, 2015.
Over the past several decades, an increasing number of orthopedic surgery residents have pursued fellowship training.1 This inclination parallels market trends toward subspecialization.2-5 In 1984, 83% of orthopedics job announcements were for general orthopedists. Twenty-five years later, almost 70% of orthopedic opportunities were for fellowship-trained surgeons.6 Further, between 1990 and 2006, the proportion of practicing orthopedic generalists decreased from 44% to 29%.3 In 2007, the American Academy of Orthopaedic Surgery (AAOS) reported 90% of graduating residents were planning to pursue fellowship training.7 Reasons for the explosion in subspecialty training are plentiful and well documented.2-5 Subspecialty positions now dominate the job market, further reinforcing incentives for residents to pursue fellowship training.
The past several decades have seen numerous changes in the orthopedic fellowship interview process. Early on, it was largely unregulated, dependent on personal and professional connections, and flush with the classic “exploding offer” (residents were given a fellowship offer that expired within hours or days). In the 1980s, as the number of fellowship applications surged, the Accreditation Council for Graduate Medical Education (ACGME) pushed for a more regulated process.8 To further standardize the system, the American Orthopaedic Association (AOA), the AAOS, and several other specialty organizations created the Orthopaedic Fellowship Match Program Initiative in 2008.9 Currently, all orthopedic specialties are represented in either the San Francisco Match Program or National Residency Match Program.
As the system currently stands, postgraduate year 4 (PGY-4) residents are required to interview across the country to secure postgraduate training. This process necessitates residents’ absence from their program, reducing educational opportunities and placing potential continuity-of-care constraints on the residency program. Despite the growing competitiveness for fellowship positions, the increasing number of fellowships available, the rising educational debt of residents, and the limitations of the 80-hour work week, the impact of the interview process on both residents and residency programs has received minimal attention.
We conducted a study to elucidate the impact of the fellowship interview process on residents and residency programs. We hypothesized the time and financial costs for fellowship interviews would be substantial.
Materials and Methods
We obtained institutional review board (IRB) approval for this study. Then, in April 2014, we sent 2 mixed-response questionnaires to orthopedic surgery residency directors and residents. There were 8 items on the director questionnaire and 11 on the resident questionnaire. The surveys were designed to determine the impact of the fellowship interview process on residents and residency programs with respect to finances, time, education, and continuity of care. Each survey had at least 1 free-response question, providing the opportunity to recommend changes to the interview process. The surveys were reviewed and approved by our IRB.
An email was sent to 155 orthopedic surgery program directors or their secretaries. The email asked that the director complete the director questionnaire and that the resident questionnaire be forwarded to senior-level residents, PGY-4s and PGY-5s, who had completed the fellowship interview process. Forty-five (29%) of the 155 directors responded, as did 129 (estimated 9.5%) of an estimated 1354 potential PGY-4s and PGY-5s.10
The Survey Monkey surveys could be completed over a 3-week period. All responses were anonymous. Using Survey Monkey, we aggregated individual responses into predefined clusters before performing statistical analysis. Descriptive statistics were generated with Microsoft Excel.
Results
Survey respondents represented all the orthopedic subspecialties (Table). Seventy-eight percent of residents applied to at least 13 programs (average, 19) (Figure 1). Ninety-two percent received at least 8 interview offers (average, 14). Eighty-three percent attended 8 or more interviews (average, 11). Seventy-one percent of all interviews were granted when requested, and 79% of all interviews were attended when offered.
Residents spent an average of $5875 (range, $500-$12,000+) on the fellowship interview process (Figure 2). The highest percentage of respondents, 39.5%, selected an average expense between $4000 and $6000. Forty-nine percent of residents borrowed money (from credit cards, additional loans, family members) to pay their expenses.
Average number of days away from residency programs was 11, with 86% of residents missing more than 8 days (Figure 1). About one-third of residents reported being away from their home program for almost 2 weeks during the interview season. Further, 74% of residents wanted changes made to the fellowship application process.
Thirty-seven (82%) of the 45 program directors were from academic programs, the other 8 from community-based programs. Average number of residents in programs per year was 4 (73% of the programs had 4-6 residents per year). Respondents rated the disruption caused by residents’ interview absences from 1 (least disruptive) to 10 (most disruptive) (Figure 3); the average rating was over 7 (high level of disruption). Although 9% of directors thought the process caused little or no disruption (rating, 1-3), 62% thought it extremely disruptive (rating, 8-10).
Thirty-one (69%) of the 45 directors agreed that the fellowship interview process should undergo fundamental change. Asked about possible solutions to current complaints, 60% of the directors agreed that interviews should be conducted in a central location. Of the directors who thought fundamental change was needed, 59% indicated AAOS and other specialty societies together should lead the change in the fellowship interview process.
Both residents and program directors were given the opportunity to write in suggestions regarding how to improve the fellowship interview process. Suggestions were made by 85 (66%) of the 129 residents and 24 (53%) of the 45 directors (Appendix).
Discussion
Graduating residents are entering a health care environment in which they must be financially conscious because of increasing education debt and decreasing reimbursement prospects.3 Nevertheless, an overwhelming majority of residents delay entering practice to pursue fellowship training—an estimated opportunity cost of $350,000.3 Minimal attention has been given to the potential costs of the fellowship interview process.
Our study results highlight that time away from residency training, financial costs associated with the fellowship interview process, and disruption of the residency program are substantial. On average, residents applied to 19 programs, received 14 interview offers, attended 11 interviews, were away from residency training 11 days, and spent $5875 on travel. The great majority of both residents and program directors wanted changes in the current paradigm governing the orthopedic fellowship interview process.
It is reasonable to think that the number of days residents spend away on interviews would reduce the time available for education and patient care. Although unknown, it is plausible that residents of programs outside major metropolitan centers and residents who apply to more competitive fellowships may be forced to spend even more time away from training. Outside the focus of this study are the impact that residents’ absence might have on their education and the impact of this absence on the people who do the residents’ work while they are away.
Mean fellowship expense was similar to that reported by residents pursuing a pediatric general surgery fellowship ($6974) or a plastic surgery fellowship ($6100).11,12 Unfortunately, we were unable to determine if average cost is influenced by choice of fellowship specialty or location of residency program. Regardless, fellowship cost may impose an additional financial burden on residents. According to the Association of American Medical Colleges (AAMC), the median salary for PGY-4 residents was $56,380 in 2013. Therefore, on average, the fellowship process consumes more than 10% of a resident’s pretax salary. For perspective, this equates to more than $40,000 for a practicing orthopedic surgeon with a median salary of $413,000.13 With an average medical student graduate debt of $175,000 and continuing decreases in reimbursement, further financial hardships to newly graduating residents cannot be understated.5,11,12
Almost 70% of program directors thought the fellowship process significantly disrupted their program. Reasons given for this disruption mainly involved residents’ time away from the program and the resulting strains placed on maintaining adequate coverage for patient care. The overall disruption score of 7.4 out of 10 was consistent with the great majority thinking that the fellowship process negatively affects their residency program. Altering the fellowship interview process may provide unintended benefits to programs and program directors.
Both program directors and residents communicated that change is needed, but there was little consensus regarding how to effect change and who should lead. This lack of consensus highlights how important it is for the various orthopedic leadership committees to actively and collectively participate in discussions about redefining the system. It has been proposed that it would be ideal for the AOA to lead the change, as the AOA consists of a representative cohort of academic orthopedists and leaders across the spectrum of all fellowship specialties.14 Given the abundant concern of both residents and program directors, we find it prudent to issue a call to arms of sorts to the AAOS and the individual orthopedic subspecialty societies to work together on a common goal that would benefit residents, programs, and subspecialties within orthopedics.
In trying to understand the challenges that residents, program directors, and programs face, as well as the inherent complexity of the current system, we incorporated respondents’ write-in comments into suggested ways of improving the fellowship interview process. These comments had broad perspectives but overall were consistent with the survey results (Appendix).
Technology
Health care is continually finding new ways to take advantage of technological advances. This is occurring with the fellowship interview schema. Numerous disciplines are using videoconferencing platforms (eg, Skype) to conduct interviews. This practice is becoming more commonplace in the business sector. In a recent survey, more than 60% of human resource managers reported conducting video interviews.15 Two independent residency programs have used video interviews with mixed success.16,17
Another technological change requested by residents is the creation and updating of fellowship web pages with standardized information. Such a service may prove useful to residents researching a program and may even lead to limiting the number of programs residents apply to, as they may be able to dial in on exactly what distinguishes one program from another before traveling for an interview. A recent study of orthopedic sports medicine fellowship programs found that most of these programs lacked pertinent information on their websites.18 Important information regarding case logs from current and former fellows; number of faculty, residents, and fellows; and schedules and facilities of interview sites are a few of the online data points that may help residents differentiate particular programs.19,20 Questions like these are often asked at interviews and site visits. Having accurate information easily available online may reduce or eliminate the need to travel to a site for such information. Standardizing information would also increase transparency among available fellowships. Although not specifically mentioned, organizational software that improves the productivity of the process may help limit the large number of programs applied to, the interviews offered and attended, the days away, and the financial costs without reducing the match rate.
Timing and Location
The issue of timing—with respect to geographical or meteorological concerns—was another recurring theme among respondents. Numerous respondents indicated that certain programs located in geographic proximity tried to minimize travel by offering interviews around the same time. This coordination potentially minimizes travel expenses and time away from the residency program by allowing residents to interview at multiple locations during a single trip per region. The sports medicine fellowship process was identified as a good example of aligning interviews based on geography. Several respondents suggested an option that also reflects the practice of nonsurgical fellowships—delaying the interview season to bypass potential weather concerns. Winter 2013–2014 saw the most flight delays or cancellations in more than a decade; about 50% of all flights scheduled between December and February were delayed or canceled.21 Beyond the additional factor of more time away or missing an interview because of the weather are safety concerns related to the weather. One resident reported having a motor vehicle accident while traveling to an interview in poor weather conditions (Appendix).
National Meetings
Each orthopedic subspecialty has numerous national meetings. Many programs offer applicants the opportunity to interview at these meetings. One respondent mentioned that the annual meeting of the Orthopaedic Trauma Association offers trauma applicants the opportunity to interview with multiple programs. It might be beneficial to endorse this practice on a larger scale to help reduce travel and time away. We recognize that visiting individual programs is an important aspect of the match process, but doing so on a targeted level may make more sense, increasing financial efficiency and reducing time away from programs.
Proposed Solution
A combined proposed solution that can be implemented without a radical overhaul or significant investments might involve moving the interview season to early spring, switching to a 2-tiered system with a centralized first round of interview screening coinciding with subspecialty national meetings or the AAOS annual meeting, and standardizing online information for all orthopedic fellowship programs. A 2-tiered interview process would allow programs and candidates to obtain exposure to a significant number of programs in the first round without incurring significant costs and then would impose a cap on the number of programs to visit. This would level the playing field between candidates with more time and money and candidates who are more constrained in their training environment and finances. A stopgap or adjunct to residents or fellowship programs unable to attend a centralized meeting would be to combine technological tools, such as Internet-based videoconferencing (Skype), before site visits by residents. After this first round of introductions and interviews, residents could then decide on a limited number of programs to formally visit, attend, and ultimately rank. This proposed system would still be able to function within the confines of the match, and it would benefit from the protections offered to residents and programs. Although capping the number of interviews attended by residents clearly can lower costs across the board, we recognize the difficulty of enforcing such a requirement. These potential changes to the system are not exhaustive, and we hope this work will serve as a springboard to further discussion.
Our study had several inherent weaknesses. Our data came from survey responses, which reflect the perspectives only of the responding residents and program directors. Unfortunately, a small number of orthopedic residents responded to this survey, so there was a potential for bias. However, we think the central themes discovered in this survey are only echoes of the concerns of the larger population of residents and program directors. Our hope in designing such a study was to bring to light some of the discrepancies in the fellowship interview process, the goal being to stimulate interest among the orthopedic leadership representing future orthopedic surgeons. More study is needed to clarify if these issues are reflective of a larger segment of residents and program directors. In addition, action may be needed to fully elucidate the intricate interworking of the fellowship process in order to maximize the interest of the orthopedic surgeons who are seeking fellowship training. Another study limitation was the potential for recall bias in the more senior PGY-5 residents, who were further from the interview process than PGY-4 respondents were. Because of the need for anonymity with the surveys, we could not link some findings (eg, program impact, cost, time away) to individual programs or different specialty fellowships. Although it appears there is a desire for a more cost-effective system, given the financial pressures on medical students and residents, the desire to match increases costs because students are likely to attend more interviews than actually needed. Our proposed solution does not take into account residents’ behavior with respect to the current match system. For example, the prevailing thought is that interviewing at more programs increases the likelihood of matching into a desired subspecialty. Despite these study limitations, we think our results identified important points for discussion, investigation, and potential action by orthopedic leadership.
Conclusion
The challenge of critiquing and improving the orthopedic fellowship process requires the same courageous leadership that was recommended almost a decade ago.14 In this study, we tried to elucidate the impact of the PGY-4 fellowship interview process with respect to residents and residency programs. Our results highlight that time away from residency training, financial costs associated with the fellowship interview process, and disruption of the residency program are substantial and that both residents and program directors want changes made. Leadership needs to further investigate alternatives to the current process to lessen the impact on all parties in this important process.
Over the past several decades, an increasing number of orthopedic surgery residents have pursued fellowship training.1 This inclination parallels market trends toward subspecialization.2-5 In 1984, 83% of orthopedics job announcements were for general orthopedists. Twenty-five years later, almost 70% of orthopedic opportunities were for fellowship-trained surgeons.6 Further, between 1990 and 2006, the proportion of practicing orthopedic generalists decreased from 44% to 29%.3 In 2007, the American Academy of Orthopaedic Surgery (AAOS) reported 90% of graduating residents were planning to pursue fellowship training.7 Reasons for the explosion in subspecialty training are plentiful and well documented.2-5 Subspecialty positions now dominate the job market, further reinforcing incentives for residents to pursue fellowship training.
The past several decades have seen numerous changes in the orthopedic fellowship interview process. Early on, it was largely unregulated, dependent on personal and professional connections, and flush with the classic “exploding offer” (residents were given a fellowship offer that expired within hours or days). In the 1980s, as the number of fellowship applications surged, the Accreditation Council for Graduate Medical Education (ACGME) pushed for a more regulated process.8 To further standardize the system, the American Orthopaedic Association (AOA), the AAOS, and several other specialty organizations created the Orthopaedic Fellowship Match Program Initiative in 2008.9 Currently, all orthopedic specialties are represented in either the San Francisco Match Program or National Residency Match Program.
As the system currently stands, postgraduate year 4 (PGY-4) residents are required to interview across the country to secure postgraduate training. This process necessitates residents’ absence from their program, reducing educational opportunities and placing potential continuity-of-care constraints on the residency program. Despite the growing competitiveness for fellowship positions, the increasing number of fellowships available, the rising educational debt of residents, and the limitations of the 80-hour work week, the impact of the interview process on both residents and residency programs has received minimal attention.
We conducted a study to elucidate the impact of the fellowship interview process on residents and residency programs. We hypothesized the time and financial costs for fellowship interviews would be substantial.
Materials and Methods
We obtained institutional review board (IRB) approval for this study. Then, in April 2014, we sent 2 mixed-response questionnaires to orthopedic surgery residency directors and residents. There were 8 items on the director questionnaire and 11 on the resident questionnaire. The surveys were designed to determine the impact of the fellowship interview process on residents and residency programs with respect to finances, time, education, and continuity of care. Each survey had at least 1 free-response question, providing the opportunity to recommend changes to the interview process. The surveys were reviewed and approved by our IRB.
An email was sent to 155 orthopedic surgery program directors or their secretaries. The email asked that the director complete the director questionnaire and that the resident questionnaire be forwarded to senior-level residents, PGY-4s and PGY-5s, who had completed the fellowship interview process. Forty-five (29%) of the 155 directors responded, as did 129 (estimated 9.5%) of an estimated 1354 potential PGY-4s and PGY-5s.10
The Survey Monkey surveys could be completed over a 3-week period. All responses were anonymous. Using Survey Monkey, we aggregated individual responses into predefined clusters before performing statistical analysis. Descriptive statistics were generated with Microsoft Excel.
Results
Survey respondents represented all the orthopedic subspecialties (Table). Seventy-eight percent of residents applied to at least 13 programs (average, 19) (Figure 1). Ninety-two percent received at least 8 interview offers (average, 14). Eighty-three percent attended 8 or more interviews (average, 11). Seventy-one percent of all interviews were granted when requested, and 79% of all interviews were attended when offered.
Residents spent an average of $5875 (range, $500-$12,000+) on the fellowship interview process (Figure 2). The highest percentage of respondents, 39.5%, selected an average expense between $4000 and $6000. Forty-nine percent of residents borrowed money (from credit cards, additional loans, family members) to pay their expenses.
Average number of days away from residency programs was 11, with 86% of residents missing more than 8 days (Figure 1). About one-third of residents reported being away from their home program for almost 2 weeks during the interview season. Further, 74% of residents wanted changes made to the fellowship application process.
Thirty-seven (82%) of the 45 program directors were from academic programs, the other 8 from community-based programs. Average number of residents in programs per year was 4 (73% of the programs had 4-6 residents per year). Respondents rated the disruption caused by residents’ interview absences from 1 (least disruptive) to 10 (most disruptive) (Figure 3); the average rating was over 7 (high level of disruption). Although 9% of directors thought the process caused little or no disruption (rating, 1-3), 62% thought it extremely disruptive (rating, 8-10).
Thirty-one (69%) of the 45 directors agreed that the fellowship interview process should undergo fundamental change. Asked about possible solutions to current complaints, 60% of the directors agreed that interviews should be conducted in a central location. Of the directors who thought fundamental change was needed, 59% indicated AAOS and other specialty societies together should lead the change in the fellowship interview process.
Both residents and program directors were given the opportunity to write in suggestions regarding how to improve the fellowship interview process. Suggestions were made by 85 (66%) of the 129 residents and 24 (53%) of the 45 directors (Appendix).
Discussion
Graduating residents are entering a health care environment in which they must be financially conscious because of increasing education debt and decreasing reimbursement prospects.3 Nevertheless, an overwhelming majority of residents delay entering practice to pursue fellowship training—an estimated opportunity cost of $350,000.3 Minimal attention has been given to the potential costs of the fellowship interview process.
Our study results highlight that time away from residency training, financial costs associated with the fellowship interview process, and disruption of the residency program are substantial. On average, residents applied to 19 programs, received 14 interview offers, attended 11 interviews, were away from residency training 11 days, and spent $5875 on travel. The great majority of both residents and program directors wanted changes in the current paradigm governing the orthopedic fellowship interview process.
It is reasonable to think that the number of days residents spend away on interviews would reduce the time available for education and patient care. Although unknown, it is plausible that residents of programs outside major metropolitan centers and residents who apply to more competitive fellowships may be forced to spend even more time away from training. Outside the focus of this study are the impact that residents’ absence might have on their education and the impact of this absence on the people who do the residents’ work while they are away.
Mean fellowship expense was similar to that reported by residents pursuing a pediatric general surgery fellowship ($6974) or a plastic surgery fellowship ($6100).11,12 Unfortunately, we were unable to determine if average cost is influenced by choice of fellowship specialty or location of residency program. Regardless, fellowship cost may impose an additional financial burden on residents. According to the Association of American Medical Colleges (AAMC), the median salary for PGY-4 residents was $56,380 in 2013. Therefore, on average, the fellowship process consumes more than 10% of a resident’s pretax salary. For perspective, this equates to more than $40,000 for a practicing orthopedic surgeon with a median salary of $413,000.13 With an average medical student graduate debt of $175,000 and continuing decreases in reimbursement, further financial hardships to newly graduating residents cannot be understated.5,11,12
Almost 70% of program directors thought the fellowship process significantly disrupted their program. Reasons given for this disruption mainly involved residents’ time away from the program and the resulting strains placed on maintaining adequate coverage for patient care. The overall disruption score of 7.4 out of 10 was consistent with the great majority thinking that the fellowship process negatively affects their residency program. Altering the fellowship interview process may provide unintended benefits to programs and program directors.
Both program directors and residents communicated that change is needed, but there was little consensus regarding how to effect change and who should lead. This lack of consensus highlights how important it is for the various orthopedic leadership committees to actively and collectively participate in discussions about redefining the system. It has been proposed that it would be ideal for the AOA to lead the change, as the AOA consists of a representative cohort of academic orthopedists and leaders across the spectrum of all fellowship specialties.14 Given the abundant concern of both residents and program directors, we find it prudent to issue a call to arms of sorts to the AAOS and the individual orthopedic subspecialty societies to work together on a common goal that would benefit residents, programs, and subspecialties within orthopedics.
In trying to understand the challenges that residents, program directors, and programs face, as well as the inherent complexity of the current system, we incorporated respondents’ write-in comments into suggested ways of improving the fellowship interview process. These comments had broad perspectives but overall were consistent with the survey results (Appendix).
Technology
Health care is continually finding new ways to take advantage of technological advances. This is occurring with the fellowship interview schema. Numerous disciplines are using videoconferencing platforms (eg, Skype) to conduct interviews. This practice is becoming more commonplace in the business sector. In a recent survey, more than 60% of human resource managers reported conducting video interviews.15 Two independent residency programs have used video interviews with mixed success.16,17
Another technological change requested by residents is the creation and updating of fellowship web pages with standardized information. Such a service may prove useful to residents researching a program and may even lead to limiting the number of programs residents apply to, as they may be able to dial in on exactly what distinguishes one program from another before traveling for an interview. A recent study of orthopedic sports medicine fellowship programs found that most of these programs lacked pertinent information on their websites.18 Important information regarding case logs from current and former fellows; number of faculty, residents, and fellows; and schedules and facilities of interview sites are a few of the online data points that may help residents differentiate particular programs.19,20 Questions like these are often asked at interviews and site visits. Having accurate information easily available online may reduce or eliminate the need to travel to a site for such information. Standardizing information would also increase transparency among available fellowships. Although not specifically mentioned, organizational software that improves the productivity of the process may help limit the large number of programs applied to, the interviews offered and attended, the days away, and the financial costs without reducing the match rate.
Timing and Location
The issue of timing—with respect to geographical or meteorological concerns—was another recurring theme among respondents. Numerous respondents indicated that certain programs located in geographic proximity tried to minimize travel by offering interviews around the same time. This coordination potentially minimizes travel expenses and time away from the residency program by allowing residents to interview at multiple locations during a single trip per region. The sports medicine fellowship process was identified as a good example of aligning interviews based on geography. Several respondents suggested an option that also reflects the practice of nonsurgical fellowships—delaying the interview season to bypass potential weather concerns. Winter 2013–2014 saw the most flight delays or cancellations in more than a decade; about 50% of all flights scheduled between December and February were delayed or canceled.21 Beyond the additional factor of more time away or missing an interview because of the weather are safety concerns related to the weather. One resident reported having a motor vehicle accident while traveling to an interview in poor weather conditions (Appendix).
National Meetings
Each orthopedic subspecialty has numerous national meetings. Many programs offer applicants the opportunity to interview at these meetings. One respondent mentioned that the annual meeting of the Orthopaedic Trauma Association offers trauma applicants the opportunity to interview with multiple programs. It might be beneficial to endorse this practice on a larger scale to help reduce travel and time away. We recognize that visiting individual programs is an important aspect of the match process, but doing so on a targeted level may make more sense, increasing financial efficiency and reducing time away from programs.
Proposed Solution
A combined proposed solution that can be implemented without a radical overhaul or significant investments might involve moving the interview season to early spring, switching to a 2-tiered system with a centralized first round of interview screening coinciding with subspecialty national meetings or the AAOS annual meeting, and standardizing online information for all orthopedic fellowship programs. A 2-tiered interview process would allow programs and candidates to obtain exposure to a significant number of programs in the first round without incurring significant costs and then would impose a cap on the number of programs to visit. This would level the playing field between candidates with more time and money and candidates who are more constrained in their training environment and finances. A stopgap or adjunct to residents or fellowship programs unable to attend a centralized meeting would be to combine technological tools, such as Internet-based videoconferencing (Skype), before site visits by residents. After this first round of introductions and interviews, residents could then decide on a limited number of programs to formally visit, attend, and ultimately rank. This proposed system would still be able to function within the confines of the match, and it would benefit from the protections offered to residents and programs. Although capping the number of interviews attended by residents clearly can lower costs across the board, we recognize the difficulty of enforcing such a requirement. These potential changes to the system are not exhaustive, and we hope this work will serve as a springboard to further discussion.
Our study had several inherent weaknesses. Our data came from survey responses, which reflect the perspectives only of the responding residents and program directors. Unfortunately, a small number of orthopedic residents responded to this survey, so there was a potential for bias. However, we think the central themes discovered in this survey are only echoes of the concerns of the larger population of residents and program directors. Our hope in designing such a study was to bring to light some of the discrepancies in the fellowship interview process, the goal being to stimulate interest among the orthopedic leadership representing future orthopedic surgeons. More study is needed to clarify if these issues are reflective of a larger segment of residents and program directors. In addition, action may be needed to fully elucidate the intricate interworking of the fellowship process in order to maximize the interest of the orthopedic surgeons who are seeking fellowship training. Another study limitation was the potential for recall bias in the more senior PGY-5 residents, who were further from the interview process than PGY-4 respondents were. Because of the need for anonymity with the surveys, we could not link some findings (eg, program impact, cost, time away) to individual programs or different specialty fellowships. Although it appears there is a desire for a more cost-effective system, given the financial pressures on medical students and residents, the desire to match increases costs because students are likely to attend more interviews than actually needed. Our proposed solution does not take into account residents’ behavior with respect to the current match system. For example, the prevailing thought is that interviewing at more programs increases the likelihood of matching into a desired subspecialty. Despite these study limitations, we think our results identified important points for discussion, investigation, and potential action by orthopedic leadership.
Conclusion
The challenge of critiquing and improving the orthopedic fellowship process requires the same courageous leadership that was recommended almost a decade ago.14 In this study, we tried to elucidate the impact of the PGY-4 fellowship interview process with respect to residents and residency programs. Our results highlight that time away from residency training, financial costs associated with the fellowship interview process, and disruption of the residency program are substantial and that both residents and program directors want changes made. Leadership needs to further investigate alternatives to the current process to lessen the impact on all parties in this important process.
1. Simon MA. Evolution of the present status of orthopaedic surgery fellowships. J Bone Joint Surg Am. 1998;80(12):1826-1829.
2. Brunworth LS, Chintalapani SR, Gray RR, Cardoso R, Owens PW. Resident selection of hand surgery fellowships: a survey of the 2011, 2012, and 2013 hand fellowship graduates. Hand. 2013;8(2):164-171.
3. Gaskill T, Cook C, Nunley J, Mather RC. The financial impact of orthopaedic fellowship training. J Bone Joint Surg Am. 2009;91(7):1814-1821.
4. Sarmiento A. Additional thoughts on orthopedic residency and fellowships. Orthopedics. 2010;33(10):712-713.
5. Griffin SM, Stoneback JW. Navigating the Orthopaedic Trauma Fellowship Match from a candidate’s perspective. J Orthop Trauma. 2011;25(suppl 3):S101-S103.
6. Morrell NT, Mercer DM, Moneim MS. Trends in the orthopedic job market and the importance of fellowship subspecialty training. Orthopedics. 2012;35(4):e555-e560.
7. Iorio R, Robb WJ, Healy WL, et al. Orthopaedic surgeon workforce and volume assessment for total hip and knee replacement in the United States: preparing for an epidemic. J Bone Joint Surg Am. 2008;90(7):1598-1605.
8. Emery SE, Guss D, Kuremsky MA, Hamlin BR, Herndon JH, Rubash HE. Resident education versus fellowship training—conflict or synergy? AOA critical issues. J Bone Joint Surg Am. 2012;94(21):e159.
9. Harner CD, Ranawat AS, Niederle M, et al. AOA symposium. Current state of fellowship hiring: is a universal match necessary? Is it possible? J Bone Joint Surg Am. 2008;90(6):1375-1384.
10. Ranawat A, Nunley RM, Genuario JW, Sharan AD, Mehta S; Washington Health Policy Fellows. Current state of the fellowship hiring process: Are we in 1957 or 2007? AAOS Now. 2007;1(8).
11. Little DC, Yoder SM, Grikscheit TC, et al. Cost considerations and applicant characteristics for the Pediatric Surgery Match. J Pediatr Surg. 2005;40(1):69-73.
12. Claiborne JR, Crantford JC, Swett KR, David LR. The Plastic Surgery Match: predicting success and improving the process. Ann Plast Surg. 2013;70(6):698-703.
13. Kane L, Peckham C. Medscape Physician Compensation Report 2014. http://www.medscape.com/features/slideshow/compensation/2014/public/overview. Published April 15, 2014. Accessed September 26, 2015.
14. Swiontkowski MF. A simple formula for continued improvement in orthopaedic surgery postgraduate training: courageous leadership. J Bone Joint Surg Am. 2008;90(6):1175.
15. Survey: six in 10 companies conduct video job interviews [news release]. http://www.prnewswire.com/news-releases/survey-six-in-10-companies-conduct-video-job-interviews-167973406.html. Published August 30, 2012. Accessed September 26, 2015.
16. Kerfoot BP, Asher KP, McCullough DL. Financial and educational costs of the residency interview process for urology applicants. Urology. 2008;71(6):990-994.
17. Edje L, Miller C, Kiefer J, Oram D. Using Skype as an alternative for residency selection interviews. J Grad Med Educ. 2013;5(3):503-505.
18. Mulcahey MK, Gosselin MM, Fadale PD. Evaluation of the content and accessibility of web sites for accredited orthopaedic sports medicine fellowships. J Bone Joint Surg Am. 2013;95(12):e85.
19. Gaeta TJ, Birkhahn RH, Lamont D, Banga N, Bove JJ. Aspects of residency programs’ web sites important to student applicants. Acad Emerg Med. 2005;12(1):89-92.
20. Mahler SA, Wagner MJ, Church A, Sokolosky M, Cline DM. Importance of residency program web sites to emergency medicine applicants. J Emerg Med. 2009;36(1):83-88.
21. Davies A. Winter’s toll: 1 million flights cancelled or delayed, costing travelers $5.3 billion. Business Insider. http://www.businessinsider.com/winter-flights-cancelled-delayed-cost-2014-3. Published March 3, 2014. Accessed September 26, 2015.
1. Simon MA. Evolution of the present status of orthopaedic surgery fellowships. J Bone Joint Surg Am. 1998;80(12):1826-1829.
2. Brunworth LS, Chintalapani SR, Gray RR, Cardoso R, Owens PW. Resident selection of hand surgery fellowships: a survey of the 2011, 2012, and 2013 hand fellowship graduates. Hand. 2013;8(2):164-171.
3. Gaskill T, Cook C, Nunley J, Mather RC. The financial impact of orthopaedic fellowship training. J Bone Joint Surg Am. 2009;91(7):1814-1821.
4. Sarmiento A. Additional thoughts on orthopedic residency and fellowships. Orthopedics. 2010;33(10):712-713.
5. Griffin SM, Stoneback JW. Navigating the Orthopaedic Trauma Fellowship Match from a candidate’s perspective. J Orthop Trauma. 2011;25(suppl 3):S101-S103.
6. Morrell NT, Mercer DM, Moneim MS. Trends in the orthopedic job market and the importance of fellowship subspecialty training. Orthopedics. 2012;35(4):e555-e560.
7. Iorio R, Robb WJ, Healy WL, et al. Orthopaedic surgeon workforce and volume assessment for total hip and knee replacement in the United States: preparing for an epidemic. J Bone Joint Surg Am. 2008;90(7):1598-1605.
8. Emery SE, Guss D, Kuremsky MA, Hamlin BR, Herndon JH, Rubash HE. Resident education versus fellowship training—conflict or synergy? AOA critical issues. J Bone Joint Surg Am. 2012;94(21):e159.
9. Harner CD, Ranawat AS, Niederle M, et al. AOA symposium. Current state of fellowship hiring: is a universal match necessary? Is it possible? J Bone Joint Surg Am. 2008;90(6):1375-1384.
10. Ranawat A, Nunley RM, Genuario JW, Sharan AD, Mehta S; Washington Health Policy Fellows. Current state of the fellowship hiring process: Are we in 1957 or 2007? AAOS Now. 2007;1(8).
11. Little DC, Yoder SM, Grikscheit TC, et al. Cost considerations and applicant characteristics for the Pediatric Surgery Match. J Pediatr Surg. 2005;40(1):69-73.
12. Claiborne JR, Crantford JC, Swett KR, David LR. The Plastic Surgery Match: predicting success and improving the process. Ann Plast Surg. 2013;70(6):698-703.
13. Kane L, Peckham C. Medscape Physician Compensation Report 2014. http://www.medscape.com/features/slideshow/compensation/2014/public/overview. Published April 15, 2014. Accessed September 26, 2015.
14. Swiontkowski MF. A simple formula for continued improvement in orthopaedic surgery postgraduate training: courageous leadership. J Bone Joint Surg Am. 2008;90(6):1175.
15. Survey: six in 10 companies conduct video job interviews [news release]. http://www.prnewswire.com/news-releases/survey-six-in-10-companies-conduct-video-job-interviews-167973406.html. Published August 30, 2012. Accessed September 26, 2015.
16. Kerfoot BP, Asher KP, McCullough DL. Financial and educational costs of the residency interview process for urology applicants. Urology. 2008;71(6):990-994.
17. Edje L, Miller C, Kiefer J, Oram D. Using Skype as an alternative for residency selection interviews. J Grad Med Educ. 2013;5(3):503-505.
18. Mulcahey MK, Gosselin MM, Fadale PD. Evaluation of the content and accessibility of web sites for accredited orthopaedic sports medicine fellowships. J Bone Joint Surg Am. 2013;95(12):e85.
19. Gaeta TJ, Birkhahn RH, Lamont D, Banga N, Bove JJ. Aspects of residency programs’ web sites important to student applicants. Acad Emerg Med. 2005;12(1):89-92.
20. Mahler SA, Wagner MJ, Church A, Sokolosky M, Cline DM. Importance of residency program web sites to emergency medicine applicants. J Emerg Med. 2009;36(1):83-88.
21. Davies A. Winter’s toll: 1 million flights cancelled or delayed, costing travelers $5.3 billion. Business Insider. http://www.businessinsider.com/winter-flights-cancelled-delayed-cost-2014-3. Published March 3, 2014. Accessed September 26, 2015.
Risk Factors for Discharge to Rehabilitation Among Hip Fracture Patients
Length of stay (LOS) is a significant driver of costs after hip fracture surgery.1-3 Multiple studies have identified factors associated with increased LOS in hip fracture patients. These factors include admission time, delay to surgery, presence of comorbidities, and older age.4-9
One significant and potentially modifiable factor affecting LOS is delayed transfer to a rehabilitation center after surgery.8-11 Although patients after orthopedic surgeries require additional rehabilitation services or subacute care directly attributable to their injuries, specialized rehabilitation centers may not always have beds readily available.6-11 Studies have shown that delays in transfer to skilled nursing facilities or rehabilitation centers are highly common among orthopedic patients.8 It is therefore imperative that orthopedists have a mechanism for predicting and identifying which patients require rehabilitation services early in the postoperative period. Identifying risk factors and stratifying patients who are most likely to require rehabilitation would facilitate the early transfer of these patients and thereby directly decrease LOS and hospitalization-related costs.
In this article, we report results from prospective, national, multicenter data to identify commonly measured risk factors for discharge to rehabilitation facilities for hip fracture patients. Through multivariate analysis of ACS-NSQIP (American College of Surgeons National Surgical Quality Improvement Program) data, we determined which risk factors significantly predispose patients to discharge to rehabilitation centers versus discharge home. Knowledge of these risk factors allows the practicing orthopedist to be better equipped to identify patients who require additional rehabilitation early in the postoperative course. By mobilizing case managers and social workers to help avoid delays in the transfers of these identified patients, LOS-associated costs may ultimately decrease.
Materials and Methods
After obtaining institutional review board approval for this study from the Office of Research at Vanderbilt University, we prospectively collected 2011 discharge data from the ACS-NSQIP database (these data are unavailable for earlier years). All patients who underwent hip fracture surgery in 2011 were identified by CPT (Current Procedural Terminology) codes. Cases of patients with unknown discharge information and of those who died during their hospitalizations were excluded from analysis. For the remaining patients, discharge information as categorized by ACS-NSQIP included skilled care (eg, subacute hospital, skilled nursing home), unskilled facility (eg, nursing home, assisted facility), separate acute care, and rehabilitation. All other patients were discharged home without additional assistance or to the previous home where they received chronic care, assisted living, or unskilled aid. Patients were dichotomized according to whether they were discharged home or to one of the rehabilitation facilities mentioned.
To determine which risk factors significantly contributed to a patient’s discharge to rehabilitation, we ran univariate analyses using Fisher exact tests for categorical variables and Student t tests for continuous variables on multiple patient factors, including demographics, preoperative comorbidities, and operative factors. Demographics included age and sex. Preoperative comorbidities included 32 conditions: diabetes mellitus, active smoking status, current alcohol use, dyspnea, history of chronic obstructive pulmonary disease, history of congestive heart failure, hypertension requiring medication, history of esophageal varices, history of myocardial infarction, current renal failure, current dialysis dependence, steroid use, recent weight loss, existing bleeding disorder, transfusion before discharge, presence of central nervous system tumor, recent chemotherapy, recent radiation therapy, previous percutaneous coronary intervention, previous percutaneous coronary stenting, history of angina, peripheral vascular disease, cerebrovascular accidents, recent surgery (within 30 days), rest pain, impaired sensorium, history of transient ischemic attacks, current hemiplegia status, current paraplegia status, current quadriplegia status, current ascites, hypertension, and disseminated cancer. Operative factors included wound infection, DNR (do not resuscitate) status, ventilator support, anesthesia type, wound class, ASA (American Society of Anesthesiologists) class, and operative time.
For the univariate analyses, significance was set at P < .05. Demographics, preoperative comorbidities, and operative factors that were significantly associated with discharge to a rehabilitation facility in the univariate analysis were selected as covariates for a multivariate analysis. We incorporated a binary logistic regression to analyze which of these significant risk factors are correlated with a patient’s discharge to a rehabilitation facility after hip fracture surgery.
Results
A total of 4974 patients undergoing surgery for hip fractures in 2011 were identified. Of these patients, 4815 had complete information on discharge location and were included in the analysis.
Table 1 lists the results of the univariate analysis comparing demographics, preoperative comorbidities, and operative factors between the home and rehabilitation groups. Both age (P < .001) and sex (P = .012) were significantly different between groups; the rehabilitation group was older by about 10 years and included significantly more females. In addition to demographic factors, 16 preoperative comorbidities, and 5 surgical factors were significantly associated with discharge to rehabilitation.
Surgery type significantly affected discharge to rehabilitation (Figure). Patients who were undergoing open plating of a femoral neck fracture or intramedullary nailing of an intertrochanteric, peritrochanteric, or subtrochanteric femoral fracture constituted 30% of all patients discharged to rehabilitation centers. In contrast, patients undergoing percutaneous skeletal fixation of a proximal femoral fracture constituted only 5.5% of all patients discharged to rehabilitation. Based on surgery type, we broke down discharge location further, into categories of skilled nursing facility, unskilled facility (not patient’s previous home), separate acute-care facility, dedicated rehabilitation center, and home. Of all 4815 patients combined, 2102 (43.6%) were discharged to a skilled nursing facility, 31 (0.6%) to an unskilled facility (not home), 106 (2.2%) to separate acute care, 1312 (27.2%) to a dedicated rehabilitation center, and 950 (19.7%) home.
Table 2 lists the significant results from the multivariate logistical analysis comparing discharge to a rehabilitation center and discharge home after controlling for the significant risk factors (Table 1). Current diabetes, history of dyspnea, previous myocardial infarction, history of ischemic attacks, current bleeding disorder, transfusion during hospitalization, previous percutaneous cardiac stenting, chemotherapy, past cerebrovascular accident, presence of cancer, surgery type based on CPT code, history of chronic obstructive pulmonary disease or congestive heart failure, current smoking status, and operative time longer than 90 minutes were not significantly correlated with discharge to rehabilitation in the multivariate analysis. All significant factors were associated with higher odds of discharge to rehabilitation except for DNR status. DNR patients were 2.04 times more likely (95% CI, 1.49-2.78; P < .001) to be discharged home than to rehabilitation centers.
Applying these adjusted odds ratios, we see that an elderly woman (age, >65 years) who underwent general anesthesia with an ASA class higher than 2 was 17.63 times more likely than a patient without these risk factors to be discharged to rehabilitation. If this patient were also dialysis-dependent, she would be 61.52 times more likely than a similar patient without dialysis needs to be discharged to rehabilitation.
Even when controlling for all significant and nonsignificant variables in multivariate logistical analysis, age over 65 years (β = 1.05; P < .001), female sex (β = 1.76; P = .004), dialysis dependence (β = 12.98; P = .036), hypertension requiring medication (β = 1.53; P = .032), and ASA class higher than 2 (β = 1.98; P = .001) were found to be significant risk factors for discharge to rehabilitation.
Discussion
This study was the first to investigate the issue of which patient risk factors allow the practicing orthopedist to identify patients who require rehabilitation after hip fracture surgery. Through our multivariate analysis, which controlled for demographics, comorbidities, and operative factors, we found that older age, female sex, history of percutaneous coronary intervention, dialysis dependence, general anesthesia, and ASA class higher than 2 significantly increased the odds of discharge to a rehabilitation center versus home.
Using our study’s results, we can create a risk stratification model for patients and thereby a means of targeting patients who need rehabilitation and starting the process of finding a rehabilitation bed early in the postoperative course. Our study’s variables are easily measured metrics that may be collected in any hospital setting. Especially for hip fracture patients, early planning and discharge to the appropriate rehabilitation center are important in decreasing LOS and associated hospitalization costs. According to one report,3 about 85% of all hip fracture costs are directly related to LOS, given the unnecessarily long rehabilitation periods in hospitals. Hollingworth and colleagues2 compared costs for patients who remained in the hospital with costs for those discharged with rehabilitation services. Overall costs were significantly lower for patients discharged home with rehabilitation. The authors concluded that 40% of hip fracture patients may be suitable for early discharge.2 In an analysis of Medicare payments for hip fracture treatment, hospital costs including LOS accounted for 60% of all payments.12 The results of these 2 studies suggest that the overall driver of hip fracture costs is prolonged LOS and that, if patients are discharged to rehabilitation, then overall costs may be lowered through a direct reduction in hospital LOS. Given that hip fractures account for almost 350,000 hospital admissions in the United States each year, and using our institution’s average hospital charge per day ($4500), about $1.6 billion may be saved if each patient’s LOS decreased by 1 day.13 Although multiple factors affect LOS, discharge planning is under orthopedists’ direct control. Therefore, early identification of patients who will require rehabilitation may help reduce LOS-associated costs in our health care system.
The patient variables that were significantly associated with discharge to rehabilitation are also associated with increased morbidity and mortality in hip fracture patients, according to the literature,14-20 which provides some external validation of using these risk factors as predictors for rehabilitation. A patient with one of these risk factors may require rehabilitation, given that rehabilitation services are specifically linked to lower morbidity and mortality rates among hip fracture patients. For example, patients with dialysis needs were 3.49 times more likely to be discharged to a rehabilitation center in our study. In a 2000 study by Coco and Rush,16 hip fracture patients on dialysis had a 1-year mortality rate 2.5 times higher than that of patients who were not dialysis-dependent. In 2010, Cameron and colleagues17 found that cardiovascular disease was associated with a 2.68 times higher risk of mortality in hip fracture patients. Similarly in our study, both hypertension and history of percutaneous coronary intervention were associated with discharge to rehabilitation. We found higher odds of discharge to rehabilitation with higher ASA classes, which mirror results from a study by Michel and colleagues,15 who found that higher (vs lower) preoperative ASA classes were associated with higher 1-year mortality in hip fracture patients. Interestingly, DNR status was associated with higher odds of discharge home, which may reflect patients’ desires to forgo noninvasive or lifesaving procedures that may be performed at rehabilitation facilities. Although general anesthesia predisposed patients to discharge to a rehabilitation center, multiple studies have found no association between anesthesia type and postoperative mortality rates for hip fracture patients.18,19 Last, Marcantonio and colleagues20 found delirium specifically had a higher odds ratio for discharge, but our univariate analysis did not find a significant association between impaired sensorium and discharge location. Given the correlation of our risk factors with increased morbidity and mortality in the literature, our study’s results provide the initial groundwork for creating a risk calculator that orthopedists can use to predict discharge to rehabilitation.
Our study had some limitations. Although we analyzed a large number of demographics, preoperative comorbidities, and surgical factors, our univariate analysis was limited to information in the ACS-NSQIP database. We did not incorporate other clinically relevant factors (eg, social factors, including patients’ support networks) that may influence discharge decisions. Furthermore, ACS-NSQIP records patient data only up to 30 days after surgery. Discharge information for the time after that was missing for a subset of hip fracture patients, and these patients had to be excluded, potentially skewing our data. ACS-NSQIP also does not collect cost data for patients based on hospitalization or LOS, so we could not determine whether patients discharged to rehabilitation incurred higher costs because of longer hospitalizations.
Nevertheless, our study identified significant patient and operative variables that are associated with discharge to a rehabilitation center. By identifying hip fracture patients with these risk factors early and mobilizing the appropriate resources, practicing orthopedists should be better equipped to help facilitate the discharge of patients to the appropriate location after surgery. Validation of these risk factors should be prospectively determined with an analysis of LOS and cost implications. Use of a risk calculator may in fact result in decreased LOS and hospital-related costs. Furthermore, using these risk factors in a prospective patient cohort would help validate their use and determine whether there is clinical correlation. The orthopedists in our institution are becoming more aware of these risk factors, but validation is necessary.
1. Garcia AE, Bonnaig JV, Yoneda ZT, et al. Patient variables which may predict length of stay and hospital costs in elderly patients with hip fracture. J Orthop Trauma. 2012;26(11):620-623.
2. Hollingworth W, Todd C, Parker M, Roberts JA, Williams R. Cost analysis of early discharge after hip fracture. BMJ. 1993;307(6909):903-906.
3. Sund R, Riihimäki J, Mäkelä M, et al. Modeling the length of the care episode after hip fracture: does the type of fracture matter? Scand J Surg. 2009;98(3):169-174.
4. Fox KM, Magaziner J, Hebel JR, Kenzora JE, Kashner TM. Intertrochanteric versus femoral neck hip fractures: differential characteristics, treatment, and sequelae. J Gerontol A Biol Sci Med Sci. 1999;54(12):M635-M640.
5. Foss NB, Palm H, Krasheninnikoff M, Kehlet H, Gebuhr P. Impact of surgical complications on length of stay after hip fracture surgery. Injury. 2007;38(7):780-784.
6. Lefaivre KA, Macadam SA, Davidson DJ, Gandhi R, Chan H, Broekhuyse HM. Length of stay, mortality, morbidity and delay to surgery in hip fractures. J Bone Joint Surg Br. 2009;91(7):922-927.
7. Clague JE, Craddock E, Andrew G, Horan MA, Pendleton N. Predictors of outcome following hip fracture. Admission time predicts length of stay and in-hospital mortality. Injury. 2002;33(1):1-6.
8. Parker MJ, Todd CJ, Palmer CR, et al. Inter-hospital variations in length of hospital stay following hip fracture. Age Ageing. 1998;27(31):333-337.
9. Brasel KJ, Rasmussen J, Cauley C, Weigelt JA. Reasons for delayed discharge of trauma patients. J Surg Res. 2002;107(2):223-226.
10. Bonar SK, Tinetti ME, Speechley M, Cooney LM. Factors associated with short- versus long-term skilled nursing facility placement among community-living hip fracture patients. J Am Geriatr Soc. 1990;38(10):1139-1144.
11. Bentler SE, Liu L, Obrizan M, et al. The aftermath of hip fracture: discharge placement, functional status change, and mortality. Am J Epidemiol. 2009;170(10):1290-1299.
12. Birkmeyer JD, Gust C, Baser O, Dimick JB, Sutherland JM, Skinner JS. Medicare payments for common inpatient procedures: implications for episode-based payment bundling. Health Serv Res. 2010;45(6 pt 1):1783-1795.
13. American Academy of Orthopaedic Surgeons. Burden of Musculoskeletal Diseases in the United States: Prevalence, Societal and Economic Cost. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2008.
14. Maciejewski ML, Radcliff A, Henderson WG, et al. Determinants of postsurgical discharge setting for male hip fracture patients. J Rehabil Res Dev. 2013;50(9):1267-1276.
15. Michel JP, Klopfenstein C, Hoffmeyer P, Stern R, Grab B. Hip fracture surgery: is the pre-operative American Society of Anesthesiologists (ASA) score a predictor of functional outcome? Aging Clin Exp Res. 2002;14(5):389-394.
16. Coco M, Rush H. Increased incidence of hip fractures in dialysis patients with low serum parathyroid hormone. Am J Kidney Dis. 2000;36(6):1115-1121.
17. Cameron ID, Chen JS, March LM, et al. Hip fracture causes excess mortality owing to cardiovascular and infectious disease in institutionalized older people: a prospective 5-year study. J Bone Miner Res. 2010;25(4):866-872.
18. White SM, Moppett IK, Griffiths R. Outcome by mode of anaesthesia for hip fracture surgery. An observational audit of 65 535 patients in a national dataset. Anaesthesia. 2014;69(3):224-230.
19. Le-Wendling L, Bihorac A, Baslanti TO, et al. Regional anesthesia as compared with general anesthesia for surgery in geriatric patients with hip fracture: does it decrease morbidity, mortality, and health care costs? Results of a single-centered study. Pain Med. 2012;13(7):948-956.
20. Marcantonio ER, Flacker JM, Michaels M, Resnick NM. Delirium is independently associated with poor functional recovery after hip fracture. J Am Geriatr Soc. 2000;48(6):618-624.
Length of stay (LOS) is a significant driver of costs after hip fracture surgery.1-3 Multiple studies have identified factors associated with increased LOS in hip fracture patients. These factors include admission time, delay to surgery, presence of comorbidities, and older age.4-9
One significant and potentially modifiable factor affecting LOS is delayed transfer to a rehabilitation center after surgery.8-11 Although patients after orthopedic surgeries require additional rehabilitation services or subacute care directly attributable to their injuries, specialized rehabilitation centers may not always have beds readily available.6-11 Studies have shown that delays in transfer to skilled nursing facilities or rehabilitation centers are highly common among orthopedic patients.8 It is therefore imperative that orthopedists have a mechanism for predicting and identifying which patients require rehabilitation services early in the postoperative period. Identifying risk factors and stratifying patients who are most likely to require rehabilitation would facilitate the early transfer of these patients and thereby directly decrease LOS and hospitalization-related costs.
In this article, we report results from prospective, national, multicenter data to identify commonly measured risk factors for discharge to rehabilitation facilities for hip fracture patients. Through multivariate analysis of ACS-NSQIP (American College of Surgeons National Surgical Quality Improvement Program) data, we determined which risk factors significantly predispose patients to discharge to rehabilitation centers versus discharge home. Knowledge of these risk factors allows the practicing orthopedist to be better equipped to identify patients who require additional rehabilitation early in the postoperative course. By mobilizing case managers and social workers to help avoid delays in the transfers of these identified patients, LOS-associated costs may ultimately decrease.
Materials and Methods
After obtaining institutional review board approval for this study from the Office of Research at Vanderbilt University, we prospectively collected 2011 discharge data from the ACS-NSQIP database (these data are unavailable for earlier years). All patients who underwent hip fracture surgery in 2011 were identified by CPT (Current Procedural Terminology) codes. Cases of patients with unknown discharge information and of those who died during their hospitalizations were excluded from analysis. For the remaining patients, discharge information as categorized by ACS-NSQIP included skilled care (eg, subacute hospital, skilled nursing home), unskilled facility (eg, nursing home, assisted facility), separate acute care, and rehabilitation. All other patients were discharged home without additional assistance or to the previous home where they received chronic care, assisted living, or unskilled aid. Patients were dichotomized according to whether they were discharged home or to one of the rehabilitation facilities mentioned.
To determine which risk factors significantly contributed to a patient’s discharge to rehabilitation, we ran univariate analyses using Fisher exact tests for categorical variables and Student t tests for continuous variables on multiple patient factors, including demographics, preoperative comorbidities, and operative factors. Demographics included age and sex. Preoperative comorbidities included 32 conditions: diabetes mellitus, active smoking status, current alcohol use, dyspnea, history of chronic obstructive pulmonary disease, history of congestive heart failure, hypertension requiring medication, history of esophageal varices, history of myocardial infarction, current renal failure, current dialysis dependence, steroid use, recent weight loss, existing bleeding disorder, transfusion before discharge, presence of central nervous system tumor, recent chemotherapy, recent radiation therapy, previous percutaneous coronary intervention, previous percutaneous coronary stenting, history of angina, peripheral vascular disease, cerebrovascular accidents, recent surgery (within 30 days), rest pain, impaired sensorium, history of transient ischemic attacks, current hemiplegia status, current paraplegia status, current quadriplegia status, current ascites, hypertension, and disseminated cancer. Operative factors included wound infection, DNR (do not resuscitate) status, ventilator support, anesthesia type, wound class, ASA (American Society of Anesthesiologists) class, and operative time.
For the univariate analyses, significance was set at P < .05. Demographics, preoperative comorbidities, and operative factors that were significantly associated with discharge to a rehabilitation facility in the univariate analysis were selected as covariates for a multivariate analysis. We incorporated a binary logistic regression to analyze which of these significant risk factors are correlated with a patient’s discharge to a rehabilitation facility after hip fracture surgery.
Results
A total of 4974 patients undergoing surgery for hip fractures in 2011 were identified. Of these patients, 4815 had complete information on discharge location and were included in the analysis.
Table 1 lists the results of the univariate analysis comparing demographics, preoperative comorbidities, and operative factors between the home and rehabilitation groups. Both age (P < .001) and sex (P = .012) were significantly different between groups; the rehabilitation group was older by about 10 years and included significantly more females. In addition to demographic factors, 16 preoperative comorbidities, and 5 surgical factors were significantly associated with discharge to rehabilitation.
Surgery type significantly affected discharge to rehabilitation (Figure). Patients who were undergoing open plating of a femoral neck fracture or intramedullary nailing of an intertrochanteric, peritrochanteric, or subtrochanteric femoral fracture constituted 30% of all patients discharged to rehabilitation centers. In contrast, patients undergoing percutaneous skeletal fixation of a proximal femoral fracture constituted only 5.5% of all patients discharged to rehabilitation. Based on surgery type, we broke down discharge location further, into categories of skilled nursing facility, unskilled facility (not patient’s previous home), separate acute-care facility, dedicated rehabilitation center, and home. Of all 4815 patients combined, 2102 (43.6%) were discharged to a skilled nursing facility, 31 (0.6%) to an unskilled facility (not home), 106 (2.2%) to separate acute care, 1312 (27.2%) to a dedicated rehabilitation center, and 950 (19.7%) home.
Table 2 lists the significant results from the multivariate logistical analysis comparing discharge to a rehabilitation center and discharge home after controlling for the significant risk factors (Table 1). Current diabetes, history of dyspnea, previous myocardial infarction, history of ischemic attacks, current bleeding disorder, transfusion during hospitalization, previous percutaneous cardiac stenting, chemotherapy, past cerebrovascular accident, presence of cancer, surgery type based on CPT code, history of chronic obstructive pulmonary disease or congestive heart failure, current smoking status, and operative time longer than 90 minutes were not significantly correlated with discharge to rehabilitation in the multivariate analysis. All significant factors were associated with higher odds of discharge to rehabilitation except for DNR status. DNR patients were 2.04 times more likely (95% CI, 1.49-2.78; P < .001) to be discharged home than to rehabilitation centers.
Applying these adjusted odds ratios, we see that an elderly woman (age, >65 years) who underwent general anesthesia with an ASA class higher than 2 was 17.63 times more likely than a patient without these risk factors to be discharged to rehabilitation. If this patient were also dialysis-dependent, she would be 61.52 times more likely than a similar patient without dialysis needs to be discharged to rehabilitation.
Even when controlling for all significant and nonsignificant variables in multivariate logistical analysis, age over 65 years (β = 1.05; P < .001), female sex (β = 1.76; P = .004), dialysis dependence (β = 12.98; P = .036), hypertension requiring medication (β = 1.53; P = .032), and ASA class higher than 2 (β = 1.98; P = .001) were found to be significant risk factors for discharge to rehabilitation.
Discussion
This study was the first to investigate the issue of which patient risk factors allow the practicing orthopedist to identify patients who require rehabilitation after hip fracture surgery. Through our multivariate analysis, which controlled for demographics, comorbidities, and operative factors, we found that older age, female sex, history of percutaneous coronary intervention, dialysis dependence, general anesthesia, and ASA class higher than 2 significantly increased the odds of discharge to a rehabilitation center versus home.
Using our study’s results, we can create a risk stratification model for patients and thereby a means of targeting patients who need rehabilitation and starting the process of finding a rehabilitation bed early in the postoperative course. Our study’s variables are easily measured metrics that may be collected in any hospital setting. Especially for hip fracture patients, early planning and discharge to the appropriate rehabilitation center are important in decreasing LOS and associated hospitalization costs. According to one report,3 about 85% of all hip fracture costs are directly related to LOS, given the unnecessarily long rehabilitation periods in hospitals. Hollingworth and colleagues2 compared costs for patients who remained in the hospital with costs for those discharged with rehabilitation services. Overall costs were significantly lower for patients discharged home with rehabilitation. The authors concluded that 40% of hip fracture patients may be suitable for early discharge.2 In an analysis of Medicare payments for hip fracture treatment, hospital costs including LOS accounted for 60% of all payments.12 The results of these 2 studies suggest that the overall driver of hip fracture costs is prolonged LOS and that, if patients are discharged to rehabilitation, then overall costs may be lowered through a direct reduction in hospital LOS. Given that hip fractures account for almost 350,000 hospital admissions in the United States each year, and using our institution’s average hospital charge per day ($4500), about $1.6 billion may be saved if each patient’s LOS decreased by 1 day.13 Although multiple factors affect LOS, discharge planning is under orthopedists’ direct control. Therefore, early identification of patients who will require rehabilitation may help reduce LOS-associated costs in our health care system.
The patient variables that were significantly associated with discharge to rehabilitation are also associated with increased morbidity and mortality in hip fracture patients, according to the literature,14-20 which provides some external validation of using these risk factors as predictors for rehabilitation. A patient with one of these risk factors may require rehabilitation, given that rehabilitation services are specifically linked to lower morbidity and mortality rates among hip fracture patients. For example, patients with dialysis needs were 3.49 times more likely to be discharged to a rehabilitation center in our study. In a 2000 study by Coco and Rush,16 hip fracture patients on dialysis had a 1-year mortality rate 2.5 times higher than that of patients who were not dialysis-dependent. In 2010, Cameron and colleagues17 found that cardiovascular disease was associated with a 2.68 times higher risk of mortality in hip fracture patients. Similarly in our study, both hypertension and history of percutaneous coronary intervention were associated with discharge to rehabilitation. We found higher odds of discharge to rehabilitation with higher ASA classes, which mirror results from a study by Michel and colleagues,15 who found that higher (vs lower) preoperative ASA classes were associated with higher 1-year mortality in hip fracture patients. Interestingly, DNR status was associated with higher odds of discharge home, which may reflect patients’ desires to forgo noninvasive or lifesaving procedures that may be performed at rehabilitation facilities. Although general anesthesia predisposed patients to discharge to a rehabilitation center, multiple studies have found no association between anesthesia type and postoperative mortality rates for hip fracture patients.18,19 Last, Marcantonio and colleagues20 found delirium specifically had a higher odds ratio for discharge, but our univariate analysis did not find a significant association between impaired sensorium and discharge location. Given the correlation of our risk factors with increased morbidity and mortality in the literature, our study’s results provide the initial groundwork for creating a risk calculator that orthopedists can use to predict discharge to rehabilitation.
Our study had some limitations. Although we analyzed a large number of demographics, preoperative comorbidities, and surgical factors, our univariate analysis was limited to information in the ACS-NSQIP database. We did not incorporate other clinically relevant factors (eg, social factors, including patients’ support networks) that may influence discharge decisions. Furthermore, ACS-NSQIP records patient data only up to 30 days after surgery. Discharge information for the time after that was missing for a subset of hip fracture patients, and these patients had to be excluded, potentially skewing our data. ACS-NSQIP also does not collect cost data for patients based on hospitalization or LOS, so we could not determine whether patients discharged to rehabilitation incurred higher costs because of longer hospitalizations.
Nevertheless, our study identified significant patient and operative variables that are associated with discharge to a rehabilitation center. By identifying hip fracture patients with these risk factors early and mobilizing the appropriate resources, practicing orthopedists should be better equipped to help facilitate the discharge of patients to the appropriate location after surgery. Validation of these risk factors should be prospectively determined with an analysis of LOS and cost implications. Use of a risk calculator may in fact result in decreased LOS and hospital-related costs. Furthermore, using these risk factors in a prospective patient cohort would help validate their use and determine whether there is clinical correlation. The orthopedists in our institution are becoming more aware of these risk factors, but validation is necessary.
Length of stay (LOS) is a significant driver of costs after hip fracture surgery.1-3 Multiple studies have identified factors associated with increased LOS in hip fracture patients. These factors include admission time, delay to surgery, presence of comorbidities, and older age.4-9
One significant and potentially modifiable factor affecting LOS is delayed transfer to a rehabilitation center after surgery.8-11 Although patients after orthopedic surgeries require additional rehabilitation services or subacute care directly attributable to their injuries, specialized rehabilitation centers may not always have beds readily available.6-11 Studies have shown that delays in transfer to skilled nursing facilities or rehabilitation centers are highly common among orthopedic patients.8 It is therefore imperative that orthopedists have a mechanism for predicting and identifying which patients require rehabilitation services early in the postoperative period. Identifying risk factors and stratifying patients who are most likely to require rehabilitation would facilitate the early transfer of these patients and thereby directly decrease LOS and hospitalization-related costs.
In this article, we report results from prospective, national, multicenter data to identify commonly measured risk factors for discharge to rehabilitation facilities for hip fracture patients. Through multivariate analysis of ACS-NSQIP (American College of Surgeons National Surgical Quality Improvement Program) data, we determined which risk factors significantly predispose patients to discharge to rehabilitation centers versus discharge home. Knowledge of these risk factors allows the practicing orthopedist to be better equipped to identify patients who require additional rehabilitation early in the postoperative course. By mobilizing case managers and social workers to help avoid delays in the transfers of these identified patients, LOS-associated costs may ultimately decrease.
Materials and Methods
After obtaining institutional review board approval for this study from the Office of Research at Vanderbilt University, we prospectively collected 2011 discharge data from the ACS-NSQIP database (these data are unavailable for earlier years). All patients who underwent hip fracture surgery in 2011 were identified by CPT (Current Procedural Terminology) codes. Cases of patients with unknown discharge information and of those who died during their hospitalizations were excluded from analysis. For the remaining patients, discharge information as categorized by ACS-NSQIP included skilled care (eg, subacute hospital, skilled nursing home), unskilled facility (eg, nursing home, assisted facility), separate acute care, and rehabilitation. All other patients were discharged home without additional assistance or to the previous home where they received chronic care, assisted living, or unskilled aid. Patients were dichotomized according to whether they were discharged home or to one of the rehabilitation facilities mentioned.
To determine which risk factors significantly contributed to a patient’s discharge to rehabilitation, we ran univariate analyses using Fisher exact tests for categorical variables and Student t tests for continuous variables on multiple patient factors, including demographics, preoperative comorbidities, and operative factors. Demographics included age and sex. Preoperative comorbidities included 32 conditions: diabetes mellitus, active smoking status, current alcohol use, dyspnea, history of chronic obstructive pulmonary disease, history of congestive heart failure, hypertension requiring medication, history of esophageal varices, history of myocardial infarction, current renal failure, current dialysis dependence, steroid use, recent weight loss, existing bleeding disorder, transfusion before discharge, presence of central nervous system tumor, recent chemotherapy, recent radiation therapy, previous percutaneous coronary intervention, previous percutaneous coronary stenting, history of angina, peripheral vascular disease, cerebrovascular accidents, recent surgery (within 30 days), rest pain, impaired sensorium, history of transient ischemic attacks, current hemiplegia status, current paraplegia status, current quadriplegia status, current ascites, hypertension, and disseminated cancer. Operative factors included wound infection, DNR (do not resuscitate) status, ventilator support, anesthesia type, wound class, ASA (American Society of Anesthesiologists) class, and operative time.
For the univariate analyses, significance was set at P < .05. Demographics, preoperative comorbidities, and operative factors that were significantly associated with discharge to a rehabilitation facility in the univariate analysis were selected as covariates for a multivariate analysis. We incorporated a binary logistic regression to analyze which of these significant risk factors are correlated with a patient’s discharge to a rehabilitation facility after hip fracture surgery.
Results
A total of 4974 patients undergoing surgery for hip fractures in 2011 were identified. Of these patients, 4815 had complete information on discharge location and were included in the analysis.
Table 1 lists the results of the univariate analysis comparing demographics, preoperative comorbidities, and operative factors between the home and rehabilitation groups. Both age (P < .001) and sex (P = .012) were significantly different between groups; the rehabilitation group was older by about 10 years and included significantly more females. In addition to demographic factors, 16 preoperative comorbidities, and 5 surgical factors were significantly associated with discharge to rehabilitation.
Surgery type significantly affected discharge to rehabilitation (Figure). Patients who were undergoing open plating of a femoral neck fracture or intramedullary nailing of an intertrochanteric, peritrochanteric, or subtrochanteric femoral fracture constituted 30% of all patients discharged to rehabilitation centers. In contrast, patients undergoing percutaneous skeletal fixation of a proximal femoral fracture constituted only 5.5% of all patients discharged to rehabilitation. Based on surgery type, we broke down discharge location further, into categories of skilled nursing facility, unskilled facility (not patient’s previous home), separate acute-care facility, dedicated rehabilitation center, and home. Of all 4815 patients combined, 2102 (43.6%) were discharged to a skilled nursing facility, 31 (0.6%) to an unskilled facility (not home), 106 (2.2%) to separate acute care, 1312 (27.2%) to a dedicated rehabilitation center, and 950 (19.7%) home.
Table 2 lists the significant results from the multivariate logistical analysis comparing discharge to a rehabilitation center and discharge home after controlling for the significant risk factors (Table 1). Current diabetes, history of dyspnea, previous myocardial infarction, history of ischemic attacks, current bleeding disorder, transfusion during hospitalization, previous percutaneous cardiac stenting, chemotherapy, past cerebrovascular accident, presence of cancer, surgery type based on CPT code, history of chronic obstructive pulmonary disease or congestive heart failure, current smoking status, and operative time longer than 90 minutes were not significantly correlated with discharge to rehabilitation in the multivariate analysis. All significant factors were associated with higher odds of discharge to rehabilitation except for DNR status. DNR patients were 2.04 times more likely (95% CI, 1.49-2.78; P < .001) to be discharged home than to rehabilitation centers.
Applying these adjusted odds ratios, we see that an elderly woman (age, >65 years) who underwent general anesthesia with an ASA class higher than 2 was 17.63 times more likely than a patient without these risk factors to be discharged to rehabilitation. If this patient were also dialysis-dependent, she would be 61.52 times more likely than a similar patient without dialysis needs to be discharged to rehabilitation.
Even when controlling for all significant and nonsignificant variables in multivariate logistical analysis, age over 65 years (β = 1.05; P < .001), female sex (β = 1.76; P = .004), dialysis dependence (β = 12.98; P = .036), hypertension requiring medication (β = 1.53; P = .032), and ASA class higher than 2 (β = 1.98; P = .001) were found to be significant risk factors for discharge to rehabilitation.
Discussion
This study was the first to investigate the issue of which patient risk factors allow the practicing orthopedist to identify patients who require rehabilitation after hip fracture surgery. Through our multivariate analysis, which controlled for demographics, comorbidities, and operative factors, we found that older age, female sex, history of percutaneous coronary intervention, dialysis dependence, general anesthesia, and ASA class higher than 2 significantly increased the odds of discharge to a rehabilitation center versus home.
Using our study’s results, we can create a risk stratification model for patients and thereby a means of targeting patients who need rehabilitation and starting the process of finding a rehabilitation bed early in the postoperative course. Our study’s variables are easily measured metrics that may be collected in any hospital setting. Especially for hip fracture patients, early planning and discharge to the appropriate rehabilitation center are important in decreasing LOS and associated hospitalization costs. According to one report,3 about 85% of all hip fracture costs are directly related to LOS, given the unnecessarily long rehabilitation periods in hospitals. Hollingworth and colleagues2 compared costs for patients who remained in the hospital with costs for those discharged with rehabilitation services. Overall costs were significantly lower for patients discharged home with rehabilitation. The authors concluded that 40% of hip fracture patients may be suitable for early discharge.2 In an analysis of Medicare payments for hip fracture treatment, hospital costs including LOS accounted for 60% of all payments.12 The results of these 2 studies suggest that the overall driver of hip fracture costs is prolonged LOS and that, if patients are discharged to rehabilitation, then overall costs may be lowered through a direct reduction in hospital LOS. Given that hip fractures account for almost 350,000 hospital admissions in the United States each year, and using our institution’s average hospital charge per day ($4500), about $1.6 billion may be saved if each patient’s LOS decreased by 1 day.13 Although multiple factors affect LOS, discharge planning is under orthopedists’ direct control. Therefore, early identification of patients who will require rehabilitation may help reduce LOS-associated costs in our health care system.
The patient variables that were significantly associated with discharge to rehabilitation are also associated with increased morbidity and mortality in hip fracture patients, according to the literature,14-20 which provides some external validation of using these risk factors as predictors for rehabilitation. A patient with one of these risk factors may require rehabilitation, given that rehabilitation services are specifically linked to lower morbidity and mortality rates among hip fracture patients. For example, patients with dialysis needs were 3.49 times more likely to be discharged to a rehabilitation center in our study. In a 2000 study by Coco and Rush,16 hip fracture patients on dialysis had a 1-year mortality rate 2.5 times higher than that of patients who were not dialysis-dependent. In 2010, Cameron and colleagues17 found that cardiovascular disease was associated with a 2.68 times higher risk of mortality in hip fracture patients. Similarly in our study, both hypertension and history of percutaneous coronary intervention were associated with discharge to rehabilitation. We found higher odds of discharge to rehabilitation with higher ASA classes, which mirror results from a study by Michel and colleagues,15 who found that higher (vs lower) preoperative ASA classes were associated with higher 1-year mortality in hip fracture patients. Interestingly, DNR status was associated with higher odds of discharge home, which may reflect patients’ desires to forgo noninvasive or lifesaving procedures that may be performed at rehabilitation facilities. Although general anesthesia predisposed patients to discharge to a rehabilitation center, multiple studies have found no association between anesthesia type and postoperative mortality rates for hip fracture patients.18,19 Last, Marcantonio and colleagues20 found delirium specifically had a higher odds ratio for discharge, but our univariate analysis did not find a significant association between impaired sensorium and discharge location. Given the correlation of our risk factors with increased morbidity and mortality in the literature, our study’s results provide the initial groundwork for creating a risk calculator that orthopedists can use to predict discharge to rehabilitation.
Our study had some limitations. Although we analyzed a large number of demographics, preoperative comorbidities, and surgical factors, our univariate analysis was limited to information in the ACS-NSQIP database. We did not incorporate other clinically relevant factors (eg, social factors, including patients’ support networks) that may influence discharge decisions. Furthermore, ACS-NSQIP records patient data only up to 30 days after surgery. Discharge information for the time after that was missing for a subset of hip fracture patients, and these patients had to be excluded, potentially skewing our data. ACS-NSQIP also does not collect cost data for patients based on hospitalization or LOS, so we could not determine whether patients discharged to rehabilitation incurred higher costs because of longer hospitalizations.
Nevertheless, our study identified significant patient and operative variables that are associated with discharge to a rehabilitation center. By identifying hip fracture patients with these risk factors early and mobilizing the appropriate resources, practicing orthopedists should be better equipped to help facilitate the discharge of patients to the appropriate location after surgery. Validation of these risk factors should be prospectively determined with an analysis of LOS and cost implications. Use of a risk calculator may in fact result in decreased LOS and hospital-related costs. Furthermore, using these risk factors in a prospective patient cohort would help validate their use and determine whether there is clinical correlation. The orthopedists in our institution are becoming more aware of these risk factors, but validation is necessary.
1. Garcia AE, Bonnaig JV, Yoneda ZT, et al. Patient variables which may predict length of stay and hospital costs in elderly patients with hip fracture. J Orthop Trauma. 2012;26(11):620-623.
2. Hollingworth W, Todd C, Parker M, Roberts JA, Williams R. Cost analysis of early discharge after hip fracture. BMJ. 1993;307(6909):903-906.
3. Sund R, Riihimäki J, Mäkelä M, et al. Modeling the length of the care episode after hip fracture: does the type of fracture matter? Scand J Surg. 2009;98(3):169-174.
4. Fox KM, Magaziner J, Hebel JR, Kenzora JE, Kashner TM. Intertrochanteric versus femoral neck hip fractures: differential characteristics, treatment, and sequelae. J Gerontol A Biol Sci Med Sci. 1999;54(12):M635-M640.
5. Foss NB, Palm H, Krasheninnikoff M, Kehlet H, Gebuhr P. Impact of surgical complications on length of stay after hip fracture surgery. Injury. 2007;38(7):780-784.
6. Lefaivre KA, Macadam SA, Davidson DJ, Gandhi R, Chan H, Broekhuyse HM. Length of stay, mortality, morbidity and delay to surgery in hip fractures. J Bone Joint Surg Br. 2009;91(7):922-927.
7. Clague JE, Craddock E, Andrew G, Horan MA, Pendleton N. Predictors of outcome following hip fracture. Admission time predicts length of stay and in-hospital mortality. Injury. 2002;33(1):1-6.
8. Parker MJ, Todd CJ, Palmer CR, et al. Inter-hospital variations in length of hospital stay following hip fracture. Age Ageing. 1998;27(31):333-337.
9. Brasel KJ, Rasmussen J, Cauley C, Weigelt JA. Reasons for delayed discharge of trauma patients. J Surg Res. 2002;107(2):223-226.
10. Bonar SK, Tinetti ME, Speechley M, Cooney LM. Factors associated with short- versus long-term skilled nursing facility placement among community-living hip fracture patients. J Am Geriatr Soc. 1990;38(10):1139-1144.
11. Bentler SE, Liu L, Obrizan M, et al. The aftermath of hip fracture: discharge placement, functional status change, and mortality. Am J Epidemiol. 2009;170(10):1290-1299.
12. Birkmeyer JD, Gust C, Baser O, Dimick JB, Sutherland JM, Skinner JS. Medicare payments for common inpatient procedures: implications for episode-based payment bundling. Health Serv Res. 2010;45(6 pt 1):1783-1795.
13. American Academy of Orthopaedic Surgeons. Burden of Musculoskeletal Diseases in the United States: Prevalence, Societal and Economic Cost. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2008.
14. Maciejewski ML, Radcliff A, Henderson WG, et al. Determinants of postsurgical discharge setting for male hip fracture patients. J Rehabil Res Dev. 2013;50(9):1267-1276.
15. Michel JP, Klopfenstein C, Hoffmeyer P, Stern R, Grab B. Hip fracture surgery: is the pre-operative American Society of Anesthesiologists (ASA) score a predictor of functional outcome? Aging Clin Exp Res. 2002;14(5):389-394.
16. Coco M, Rush H. Increased incidence of hip fractures in dialysis patients with low serum parathyroid hormone. Am J Kidney Dis. 2000;36(6):1115-1121.
17. Cameron ID, Chen JS, March LM, et al. Hip fracture causes excess mortality owing to cardiovascular and infectious disease in institutionalized older people: a prospective 5-year study. J Bone Miner Res. 2010;25(4):866-872.
18. White SM, Moppett IK, Griffiths R. Outcome by mode of anaesthesia for hip fracture surgery. An observational audit of 65 535 patients in a national dataset. Anaesthesia. 2014;69(3):224-230.
19. Le-Wendling L, Bihorac A, Baslanti TO, et al. Regional anesthesia as compared with general anesthesia for surgery in geriatric patients with hip fracture: does it decrease morbidity, mortality, and health care costs? Results of a single-centered study. Pain Med. 2012;13(7):948-956.
20. Marcantonio ER, Flacker JM, Michaels M, Resnick NM. Delirium is independently associated with poor functional recovery after hip fracture. J Am Geriatr Soc. 2000;48(6):618-624.
1. Garcia AE, Bonnaig JV, Yoneda ZT, et al. Patient variables which may predict length of stay and hospital costs in elderly patients with hip fracture. J Orthop Trauma. 2012;26(11):620-623.
2. Hollingworth W, Todd C, Parker M, Roberts JA, Williams R. Cost analysis of early discharge after hip fracture. BMJ. 1993;307(6909):903-906.
3. Sund R, Riihimäki J, Mäkelä M, et al. Modeling the length of the care episode after hip fracture: does the type of fracture matter? Scand J Surg. 2009;98(3):169-174.
4. Fox KM, Magaziner J, Hebel JR, Kenzora JE, Kashner TM. Intertrochanteric versus femoral neck hip fractures: differential characteristics, treatment, and sequelae. J Gerontol A Biol Sci Med Sci. 1999;54(12):M635-M640.
5. Foss NB, Palm H, Krasheninnikoff M, Kehlet H, Gebuhr P. Impact of surgical complications on length of stay after hip fracture surgery. Injury. 2007;38(7):780-784.
6. Lefaivre KA, Macadam SA, Davidson DJ, Gandhi R, Chan H, Broekhuyse HM. Length of stay, mortality, morbidity and delay to surgery in hip fractures. J Bone Joint Surg Br. 2009;91(7):922-927.
7. Clague JE, Craddock E, Andrew G, Horan MA, Pendleton N. Predictors of outcome following hip fracture. Admission time predicts length of stay and in-hospital mortality. Injury. 2002;33(1):1-6.
8. Parker MJ, Todd CJ, Palmer CR, et al. Inter-hospital variations in length of hospital stay following hip fracture. Age Ageing. 1998;27(31):333-337.
9. Brasel KJ, Rasmussen J, Cauley C, Weigelt JA. Reasons for delayed discharge of trauma patients. J Surg Res. 2002;107(2):223-226.
10. Bonar SK, Tinetti ME, Speechley M, Cooney LM. Factors associated with short- versus long-term skilled nursing facility placement among community-living hip fracture patients. J Am Geriatr Soc. 1990;38(10):1139-1144.
11. Bentler SE, Liu L, Obrizan M, et al. The aftermath of hip fracture: discharge placement, functional status change, and mortality. Am J Epidemiol. 2009;170(10):1290-1299.
12. Birkmeyer JD, Gust C, Baser O, Dimick JB, Sutherland JM, Skinner JS. Medicare payments for common inpatient procedures: implications for episode-based payment bundling. Health Serv Res. 2010;45(6 pt 1):1783-1795.
13. American Academy of Orthopaedic Surgeons. Burden of Musculoskeletal Diseases in the United States: Prevalence, Societal and Economic Cost. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2008.
14. Maciejewski ML, Radcliff A, Henderson WG, et al. Determinants of postsurgical discharge setting for male hip fracture patients. J Rehabil Res Dev. 2013;50(9):1267-1276.
15. Michel JP, Klopfenstein C, Hoffmeyer P, Stern R, Grab B. Hip fracture surgery: is the pre-operative American Society of Anesthesiologists (ASA) score a predictor of functional outcome? Aging Clin Exp Res. 2002;14(5):389-394.
16. Coco M, Rush H. Increased incidence of hip fractures in dialysis patients with low serum parathyroid hormone. Am J Kidney Dis. 2000;36(6):1115-1121.
17. Cameron ID, Chen JS, March LM, et al. Hip fracture causes excess mortality owing to cardiovascular and infectious disease in institutionalized older people: a prospective 5-year study. J Bone Miner Res. 2010;25(4):866-872.
18. White SM, Moppett IK, Griffiths R. Outcome by mode of anaesthesia for hip fracture surgery. An observational audit of 65 535 patients in a national dataset. Anaesthesia. 2014;69(3):224-230.
19. Le-Wendling L, Bihorac A, Baslanti TO, et al. Regional anesthesia as compared with general anesthesia for surgery in geriatric patients with hip fracture: does it decrease morbidity, mortality, and health care costs? Results of a single-centered study. Pain Med. 2012;13(7):948-956.
20. Marcantonio ER, Flacker JM, Michaels M, Resnick NM. Delirium is independently associated with poor functional recovery after hip fracture. J Am Geriatr Soc. 2000;48(6):618-624.
Incidence and Functional Outcomes of Malunion of Nonoperatively Treated Humeral Shaft Fractures
Humeral shaft fractures account for about 1% of all fractures.1 With the exception of the few absolute indications for surgical intervention, such as the presence of an open fracture, the current teaching on treatment of these fractures is that the majority can be successfully managed nonoperatively.1-3 These conservative measures consist of bandages, abduction splints, U-casts, hanging arm casts, and, most commonly, functional bracing, which is considered the gold standard for treatment of humeral shaft fractures by many authors.1-3 One of the most often cited disadvantages of nonoperative management over surgical treatment is the higher incidence of residual deformity, the most common of which is varus angulation.4
The incidence of malunion (>20° of angulation in any plane or shortening of ≥2.5 cm) after nonoperative treatment varies in the literature from 0% to 13%,2,4-9 with a recent literature review documenting a mean incidence of 4.4% within the frontal plane and 2% within the sagittal plane across all studies.2 As reported initially by Sarmiento and colleagues3,9 and echoed by other authors,2,5,8 angular deformity of less than 20° is thought to be both cosmetically and functionally acceptable. Whether angular deformities or malunion of more than 20° actually leads to functional limitations is unknown. Although some observational reports suggest that the degree of radiographic malalignment does not necessarily correlate with functional outcome,8 no studies have specifically evaluated patient outcomes of humeral shaft fracture malunions.
We conducted a study to determine the overall incidence and long-term clinical and functional outcomes of patients with malunion after nonoperative management of humeral shaft fractures. Long-term outcomes were assessed with current symptoms, physical examination findings, need for subsequent operative intervention, DASH (Disabilities of the Arm, Shoulder, and Hand) scores, and a self-reported questionnaire. We hypothesized that patients who develop a malunion after nonoperative treatment of a closed humeral shaft fracture will have satisfactory functional outcomes based on subjective reports, physical examination findings, and DASH scores.
Methods
After obtaining institutional review board approval for the study, we selected patients from a retrospective medical record review of all those 18 years or older with a humeral shaft fracture managed nonoperatively at our institution between January 1, 2001, and June 30, 2012, with a minimum 1-year follow-up. We identified 156 patients with nonoperatively managed midshaft humerus fractures. Study exclusion criteria included fracture associated with a tumor (3 patients), ipsilateral upper extremity injury (9), open/ballistic injury (18), nonunion (9), underlying cognitive disability or psychiatric illness (4), and insufficient follow-up to clinical or radiographic healing (22). Ninety-one patients were eligible for study inclusion. Radiographs at time of final clinical visit were reviewed to assess for evidence of malunion at the fracture site, as defined by previously reported criteria3 (>20° angulation in anterior/posterior or varus/valgus plane of motion or shortening of ≥2.5 cm). Fifteen patients met all the inclusion criteria for further evaluation.
Medical records were retrospectively reviewed for information on age at injury, sex, comorbidities (eg, diabetes, osteoporosis, smoking), body mass index, type and duration of immobilization, complications, return to work, cosmetic perception, time to final clinical follow-up, and symptoms at final clinical follow-up. Incidence of potential risk factors associated with malunion—obesity, noncompliance, and comorbidities such as smoking and diabetes—was compared between the 15 patients with malunion and the other study patients, who healed without malunion.
For long-term postoperative follow-up, patients were contacted to be seen in clinic to complete an updated physical examination, self-reported questionnaire, and the DASH form. Physical examination included measurements of range of motion (ROM) and strength involving the shoulder, elbow, and forearm, with ROM reported as the difference between the injured and contralateral upper extremities. Neurovascular status and focal tenderness to palpation were also assessed on examination. When in-person examination was not possible, the questionnaire and DASH form were completed over the telephone. The self-reported questionnaire asked for information on smoking status, pain, functional limitations, cosmetic perception, satisfaction, and whether or not the patient would still opt for nonoperative management if presented with the same injury again. Pain and satisfaction were measured on numerical scales: Pain scores ranged from 0 (no pain) to 10 (worst possible pain), and satisfaction scores ranged from 1 (not satisfied) to 5 (very satisfied). Data are presented as mean values.
Results
Of the 91 study-eligible patients, 15 (16%) met the radiographic criteria for the diagnosis of malunion. Retrospective data were available for all 15 patients from time of injury to final clinical follow-up (mean, 19 weeks; range, 7-53 weeks). Mean age at injury was 39 years (range, 20-79 years). Additional demographics are listed in Table 1. Incidence of potential risk factors, such as body mass index (26.5 vs 25.4), smoking (33% vs 33%), and diabetes (0% vs 8%), was not significantly different between the malunion and healed-without-malunion groups, respectively. Furthermore, all malunion patients were compliant with their treatment protocol.
Radiographs were assessed at time of final follow-up to confirm healing and to document malunion. Varus malunion was found in 13 patients (mean, 24°; range, 20.5°-35.5°), and shortening was documented in the other 2 patients (mean, 4 cm; range, 3-5 cm). Patients were immobilized a mean of 10 weeks (range, 6-13 weeks). Initial fracture management consisted of coaptation splinting for 1 to 2 weeks (12 patients), hanging arm cast for 1 week (1 patient), and posterior splint for 1 week (1 patient). Patients were then transitioned to Sarmiento fracture bracing for the duration of their treatment (range, 5-12 months). One patient, followed initially at an outside institution, was managed in a sling throughout the duration of treatment (12 weeks) (Table 1). All 15 patients were neurovascularly intact at time of final clinical examination, with return of full upper extremity ROM in all but 3 patients. Only 1 of these 3 patients reported residual pain and functional limitations 4 months after injury (Table 2). Twelve patients were evaluated for return to work, with all successfully returning to work without restrictions at time of final follow-up. The 1 minor complication noted during the treatment period involved medial-sided elbow skin breakdown from brace wear, which resolved with local wound care. No patient required or requested surgical intervention for their residual malunion.
Of the 15 patients, 8 (53%) were reached for in-person examination (6 patients) or telephone interview (2 patients) for follow-up assessment by means of DASH form and self-reported questionnaire a mean of 47 months (range, 12-99 months) after initial injury. The 6 patients who had a physical examination were neurovascularly intact, lacked focal tenderness to palpation, and demonstrated full (5/5) strength within the deltoid, biceps, triceps, pronator, and supinator musculature. Each patient had equal ROM compared with the contralateral uninjured extremity on shoulder forward flexion and abduction, elbow flexion and extension, and forearm pronation and supination. Three patients (50%) had mild residual loss of ROM, with 2 demonstrating decreased shoulder external rotation of 10° and 15°, respectively, and 1 demonstrating decreased shoulder internal rotation of 10°.
Mean DASH score was 10.4 (range, 0-49.2). Evaluation of the self-reported questionnaire revealed a mean pain score of 1.1 (range, 0-7), with only 2 patients reporting any ongoing pain. In addition, 2 patients reported functional limitations, both related to overhead activities. However, 6 (75%) of the 8 patients reported noticeable cosmetic deformity, most commonly varus angulation (4 patients), as well as palpable bony prominence (2) and muscle atrophy (1). The majority of patients were satisfied with the outcome of their treatment (mean, 4; range, 2-5), with 6 patients reporting being satisfied or very satisfied, and all 6 indicating they would undergo nonoperative management again if presented with the same injury. Two patients reported being dissatisfied with their outcome, 1 because of cosmetic appearance and 1 because of cosmetic appearance and functional limitations. Both patients indicated they would choose operative management if presented with the same injury. There was no apparent relationship between outcome and degree of residual deformity, as both patients with varus angulation of more than 30° reported no residual pain or functional limitation and were very satisfied with the outcome of their treatment (Table 2).
Of the 7 patients who could not be reached for final follow-up, 2 on initial contact expressed overall satisfaction with their outcome and denied functional limitations. However, both asked to complete the study at a later date. Subsequently, these 2 patients could not be reached to complete the formal follow-up.
Discussion
Humeral shaft fractures are usually managed nonoperatively. One of the most commonly cited disadvantages of nonoperative management is its higher incidence of residual angular deformity, up to 13% in previous studies.4 Our study found a slightly higher incidence, 16%, on review of 91 nonoperatively managed humeral shaft fractures treated over an 11.5 year period. Although previous studies have reported acceptable functional and cosmetic outcomes with residual angular deformity of less than 20°,2,3,5,8,9 only observational reports have suggested acceptable function in patients with a documented malunion.8
To our knowledge, ours is the first study to correlate malunion with functional parameters and subjective patient-reported outcomes. We found that malunion was not associated with significant pain or functional limitation after nonoperative management of humeral shaft fractures. Furthermore, 75% of patients were satisfied or very satisfied with the outcome of their treatment and indicated they would undergo nonoperative management if presented with the same injury again. However, 75% of patients reported a noticeable cosmetic deformity, and one-third of these patients cited it as a major reason for dissatisfaction with their overall outcome. Regarding function, all patients returned to full strength and ROM of the affected extremity, aside from small losses of internal or external shoulder rotation on the magnitude of 10° to 15° in 50% of those patients tested. In addition, 75% of patients returned to regular activity without functional limitations; the other 25% reported trouble with overhead activities. There were no significant complications during the treatment or follow-up period, once the fracture had healed.
The major limitation of this study was its small patient population. (Obtaining a larger series of patients with malunion after nonoperative treatment of humeral shaft fractures likely would require a multicenter study.) Some of our study findings, such as lack of correlation between degree of malunion and subsequent functional or subjective outcomes, would require a larger sample size for verification and more definitive conclusions. Another limitation is that the study was not designed to evaluate the cause of malunion. Therefore, we cannot draw any definitive conclusions regarding what may have contributed to the development of malunion in our study population. However, all our malunion patients were compliant with their treatment protocol, and they showed no significant difference in incidence of potential risk factors (eg, obesity, comorbidities) compared with the patients who healed without malunion.
Conclusion
Malunion after nonoperative management of humeral shaft fractures does not appear to result in significant pain, dissatisfaction, or functional limitation as measured on physical examination and with validated objective outcome measures in the majority of patients. Furthermore, no patients in this study required surgical intervention for any residual limitations or complications after malunion. The majority of patients reported a noticeable cosmetic deformity, which left a small subset of patients dissatisfied. Overall, our study findings can be used to help counsel patients before and during nonoperative management—particularly patients who appear to be healing with some malunion. Our findings suggest that operative intervention to prevent malunion is not necessary, as it likely would not result in any overall improvement in patient function or satisfaction, but patients should be counseled regarding the high likelihood of cosmetic deformity, which may or may not be bothersome.
1. Rockwood CA, Green DP, Bucholz RW, eds. Rockwood and Green’s Fractures in Adults. 7th ed. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2010.
2. Papasoulis E, Drosos GI, Ververidis AN, Verettas DA. Functional bracing of humeral shaft fractures. A review of clinical studies. Injury. 2010;41(7):e21-e27.
3. Sarmiento A, Latta LL. Functional fracture bracing. J Am Acad Orthop Surg. 1999;7(1):66-75.
4. Denard A Jr, Richards JE, Obremskey WT, Tucker MC, Floyd M, Herzog GA. Outcome of nonoperative vs operative treatment of humeral shaft fractures: a retrospective study of 213 patients. Orthopedics. 2010;33(8).
5. Fjalestad T, Strømsøe K, Salvesen P, Rostad B. Functional results of braced humeral diaphyseal fractures: why do 38% lose external rotation of the shoulder? Arch Orthop Trauma Surg. 2000;120(5-6):281-285.
6. Koch PP, Gross DF, Gerber C. The results of functional (Sarmiento) bracing of humeral shaft fractures. J Shoulder Elbow Surg. 2002;11(2):143-150.
7. Ozkurt B, Altay M, Aktekin CN, Toprak A, Tabak Y. The role of functional bracing in the treatment of humeral shaft fractures [in Turkish]. Acta Orthop Traumatol Turc. 2007;41(1):15-20.
8. Rutgers M, Ring D. Treatment of diaphyseal fractures of the humerus using a functional brace. J Orthop Trauma. 2006;20(9):597-601.
9. Sarmiento A, Kinman PB, Galvin EG, Schmitt RH, Phillips JG. Functional bracing of fractures of the shaft of the humerus. J Bone Joint Surg Am. 1977;59(5):596-601.
Humeral shaft fractures account for about 1% of all fractures.1 With the exception of the few absolute indications for surgical intervention, such as the presence of an open fracture, the current teaching on treatment of these fractures is that the majority can be successfully managed nonoperatively.1-3 These conservative measures consist of bandages, abduction splints, U-casts, hanging arm casts, and, most commonly, functional bracing, which is considered the gold standard for treatment of humeral shaft fractures by many authors.1-3 One of the most often cited disadvantages of nonoperative management over surgical treatment is the higher incidence of residual deformity, the most common of which is varus angulation.4
The incidence of malunion (>20° of angulation in any plane or shortening of ≥2.5 cm) after nonoperative treatment varies in the literature from 0% to 13%,2,4-9 with a recent literature review documenting a mean incidence of 4.4% within the frontal plane and 2% within the sagittal plane across all studies.2 As reported initially by Sarmiento and colleagues3,9 and echoed by other authors,2,5,8 angular deformity of less than 20° is thought to be both cosmetically and functionally acceptable. Whether angular deformities or malunion of more than 20° actually leads to functional limitations is unknown. Although some observational reports suggest that the degree of radiographic malalignment does not necessarily correlate with functional outcome,8 no studies have specifically evaluated patient outcomes of humeral shaft fracture malunions.
We conducted a study to determine the overall incidence and long-term clinical and functional outcomes of patients with malunion after nonoperative management of humeral shaft fractures. Long-term outcomes were assessed with current symptoms, physical examination findings, need for subsequent operative intervention, DASH (Disabilities of the Arm, Shoulder, and Hand) scores, and a self-reported questionnaire. We hypothesized that patients who develop a malunion after nonoperative treatment of a closed humeral shaft fracture will have satisfactory functional outcomes based on subjective reports, physical examination findings, and DASH scores.
Methods
After obtaining institutional review board approval for the study, we selected patients from a retrospective medical record review of all those 18 years or older with a humeral shaft fracture managed nonoperatively at our institution between January 1, 2001, and June 30, 2012, with a minimum 1-year follow-up. We identified 156 patients with nonoperatively managed midshaft humerus fractures. Study exclusion criteria included fracture associated with a tumor (3 patients), ipsilateral upper extremity injury (9), open/ballistic injury (18), nonunion (9), underlying cognitive disability or psychiatric illness (4), and insufficient follow-up to clinical or radiographic healing (22). Ninety-one patients were eligible for study inclusion. Radiographs at time of final clinical visit were reviewed to assess for evidence of malunion at the fracture site, as defined by previously reported criteria3 (>20° angulation in anterior/posterior or varus/valgus plane of motion or shortening of ≥2.5 cm). Fifteen patients met all the inclusion criteria for further evaluation.
Medical records were retrospectively reviewed for information on age at injury, sex, comorbidities (eg, diabetes, osteoporosis, smoking), body mass index, type and duration of immobilization, complications, return to work, cosmetic perception, time to final clinical follow-up, and symptoms at final clinical follow-up. Incidence of potential risk factors associated with malunion—obesity, noncompliance, and comorbidities such as smoking and diabetes—was compared between the 15 patients with malunion and the other study patients, who healed without malunion.
For long-term postoperative follow-up, patients were contacted to be seen in clinic to complete an updated physical examination, self-reported questionnaire, and the DASH form. Physical examination included measurements of range of motion (ROM) and strength involving the shoulder, elbow, and forearm, with ROM reported as the difference between the injured and contralateral upper extremities. Neurovascular status and focal tenderness to palpation were also assessed on examination. When in-person examination was not possible, the questionnaire and DASH form were completed over the telephone. The self-reported questionnaire asked for information on smoking status, pain, functional limitations, cosmetic perception, satisfaction, and whether or not the patient would still opt for nonoperative management if presented with the same injury again. Pain and satisfaction were measured on numerical scales: Pain scores ranged from 0 (no pain) to 10 (worst possible pain), and satisfaction scores ranged from 1 (not satisfied) to 5 (very satisfied). Data are presented as mean values.
Results
Of the 91 study-eligible patients, 15 (16%) met the radiographic criteria for the diagnosis of malunion. Retrospective data were available for all 15 patients from time of injury to final clinical follow-up (mean, 19 weeks; range, 7-53 weeks). Mean age at injury was 39 years (range, 20-79 years). Additional demographics are listed in Table 1. Incidence of potential risk factors, such as body mass index (26.5 vs 25.4), smoking (33% vs 33%), and diabetes (0% vs 8%), was not significantly different between the malunion and healed-without-malunion groups, respectively. Furthermore, all malunion patients were compliant with their treatment protocol.
Radiographs were assessed at time of final follow-up to confirm healing and to document malunion. Varus malunion was found in 13 patients (mean, 24°; range, 20.5°-35.5°), and shortening was documented in the other 2 patients (mean, 4 cm; range, 3-5 cm). Patients were immobilized a mean of 10 weeks (range, 6-13 weeks). Initial fracture management consisted of coaptation splinting for 1 to 2 weeks (12 patients), hanging arm cast for 1 week (1 patient), and posterior splint for 1 week (1 patient). Patients were then transitioned to Sarmiento fracture bracing for the duration of their treatment (range, 5-12 months). One patient, followed initially at an outside institution, was managed in a sling throughout the duration of treatment (12 weeks) (Table 1). All 15 patients were neurovascularly intact at time of final clinical examination, with return of full upper extremity ROM in all but 3 patients. Only 1 of these 3 patients reported residual pain and functional limitations 4 months after injury (Table 2). Twelve patients were evaluated for return to work, with all successfully returning to work without restrictions at time of final follow-up. The 1 minor complication noted during the treatment period involved medial-sided elbow skin breakdown from brace wear, which resolved with local wound care. No patient required or requested surgical intervention for their residual malunion.
Of the 15 patients, 8 (53%) were reached for in-person examination (6 patients) or telephone interview (2 patients) for follow-up assessment by means of DASH form and self-reported questionnaire a mean of 47 months (range, 12-99 months) after initial injury. The 6 patients who had a physical examination were neurovascularly intact, lacked focal tenderness to palpation, and demonstrated full (5/5) strength within the deltoid, biceps, triceps, pronator, and supinator musculature. Each patient had equal ROM compared with the contralateral uninjured extremity on shoulder forward flexion and abduction, elbow flexion and extension, and forearm pronation and supination. Three patients (50%) had mild residual loss of ROM, with 2 demonstrating decreased shoulder external rotation of 10° and 15°, respectively, and 1 demonstrating decreased shoulder internal rotation of 10°.
Mean DASH score was 10.4 (range, 0-49.2). Evaluation of the self-reported questionnaire revealed a mean pain score of 1.1 (range, 0-7), with only 2 patients reporting any ongoing pain. In addition, 2 patients reported functional limitations, both related to overhead activities. However, 6 (75%) of the 8 patients reported noticeable cosmetic deformity, most commonly varus angulation (4 patients), as well as palpable bony prominence (2) and muscle atrophy (1). The majority of patients were satisfied with the outcome of their treatment (mean, 4; range, 2-5), with 6 patients reporting being satisfied or very satisfied, and all 6 indicating they would undergo nonoperative management again if presented with the same injury. Two patients reported being dissatisfied with their outcome, 1 because of cosmetic appearance and 1 because of cosmetic appearance and functional limitations. Both patients indicated they would choose operative management if presented with the same injury. There was no apparent relationship between outcome and degree of residual deformity, as both patients with varus angulation of more than 30° reported no residual pain or functional limitation and were very satisfied with the outcome of their treatment (Table 2).
Of the 7 patients who could not be reached for final follow-up, 2 on initial contact expressed overall satisfaction with their outcome and denied functional limitations. However, both asked to complete the study at a later date. Subsequently, these 2 patients could not be reached to complete the formal follow-up.
Discussion
Humeral shaft fractures are usually managed nonoperatively. One of the most commonly cited disadvantages of nonoperative management is its higher incidence of residual angular deformity, up to 13% in previous studies.4 Our study found a slightly higher incidence, 16%, on review of 91 nonoperatively managed humeral shaft fractures treated over an 11.5 year period. Although previous studies have reported acceptable functional and cosmetic outcomes with residual angular deformity of less than 20°,2,3,5,8,9 only observational reports have suggested acceptable function in patients with a documented malunion.8
To our knowledge, ours is the first study to correlate malunion with functional parameters and subjective patient-reported outcomes. We found that malunion was not associated with significant pain or functional limitation after nonoperative management of humeral shaft fractures. Furthermore, 75% of patients were satisfied or very satisfied with the outcome of their treatment and indicated they would undergo nonoperative management if presented with the same injury again. However, 75% of patients reported a noticeable cosmetic deformity, and one-third of these patients cited it as a major reason for dissatisfaction with their overall outcome. Regarding function, all patients returned to full strength and ROM of the affected extremity, aside from small losses of internal or external shoulder rotation on the magnitude of 10° to 15° in 50% of those patients tested. In addition, 75% of patients returned to regular activity without functional limitations; the other 25% reported trouble with overhead activities. There were no significant complications during the treatment or follow-up period, once the fracture had healed.
The major limitation of this study was its small patient population. (Obtaining a larger series of patients with malunion after nonoperative treatment of humeral shaft fractures likely would require a multicenter study.) Some of our study findings, such as lack of correlation between degree of malunion and subsequent functional or subjective outcomes, would require a larger sample size for verification and more definitive conclusions. Another limitation is that the study was not designed to evaluate the cause of malunion. Therefore, we cannot draw any definitive conclusions regarding what may have contributed to the development of malunion in our study population. However, all our malunion patients were compliant with their treatment protocol, and they showed no significant difference in incidence of potential risk factors (eg, obesity, comorbidities) compared with the patients who healed without malunion.
Conclusion
Malunion after nonoperative management of humeral shaft fractures does not appear to result in significant pain, dissatisfaction, or functional limitation as measured on physical examination and with validated objective outcome measures in the majority of patients. Furthermore, no patients in this study required surgical intervention for any residual limitations or complications after malunion. The majority of patients reported a noticeable cosmetic deformity, which left a small subset of patients dissatisfied. Overall, our study findings can be used to help counsel patients before and during nonoperative management—particularly patients who appear to be healing with some malunion. Our findings suggest that operative intervention to prevent malunion is not necessary, as it likely would not result in any overall improvement in patient function or satisfaction, but patients should be counseled regarding the high likelihood of cosmetic deformity, which may or may not be bothersome.
Humeral shaft fractures account for about 1% of all fractures.1 With the exception of the few absolute indications for surgical intervention, such as the presence of an open fracture, the current teaching on treatment of these fractures is that the majority can be successfully managed nonoperatively.1-3 These conservative measures consist of bandages, abduction splints, U-casts, hanging arm casts, and, most commonly, functional bracing, which is considered the gold standard for treatment of humeral shaft fractures by many authors.1-3 One of the most often cited disadvantages of nonoperative management over surgical treatment is the higher incidence of residual deformity, the most common of which is varus angulation.4
The incidence of malunion (>20° of angulation in any plane or shortening of ≥2.5 cm) after nonoperative treatment varies in the literature from 0% to 13%,2,4-9 with a recent literature review documenting a mean incidence of 4.4% within the frontal plane and 2% within the sagittal plane across all studies.2 As reported initially by Sarmiento and colleagues3,9 and echoed by other authors,2,5,8 angular deformity of less than 20° is thought to be both cosmetically and functionally acceptable. Whether angular deformities or malunion of more than 20° actually leads to functional limitations is unknown. Although some observational reports suggest that the degree of radiographic malalignment does not necessarily correlate with functional outcome,8 no studies have specifically evaluated patient outcomes of humeral shaft fracture malunions.
We conducted a study to determine the overall incidence and long-term clinical and functional outcomes of patients with malunion after nonoperative management of humeral shaft fractures. Long-term outcomes were assessed with current symptoms, physical examination findings, need for subsequent operative intervention, DASH (Disabilities of the Arm, Shoulder, and Hand) scores, and a self-reported questionnaire. We hypothesized that patients who develop a malunion after nonoperative treatment of a closed humeral shaft fracture will have satisfactory functional outcomes based on subjective reports, physical examination findings, and DASH scores.
Methods
After obtaining institutional review board approval for the study, we selected patients from a retrospective medical record review of all those 18 years or older with a humeral shaft fracture managed nonoperatively at our institution between January 1, 2001, and June 30, 2012, with a minimum 1-year follow-up. We identified 156 patients with nonoperatively managed midshaft humerus fractures. Study exclusion criteria included fracture associated with a tumor (3 patients), ipsilateral upper extremity injury (9), open/ballistic injury (18), nonunion (9), underlying cognitive disability or psychiatric illness (4), and insufficient follow-up to clinical or radiographic healing (22). Ninety-one patients were eligible for study inclusion. Radiographs at time of final clinical visit were reviewed to assess for evidence of malunion at the fracture site, as defined by previously reported criteria3 (>20° angulation in anterior/posterior or varus/valgus plane of motion or shortening of ≥2.5 cm). Fifteen patients met all the inclusion criteria for further evaluation.
Medical records were retrospectively reviewed for information on age at injury, sex, comorbidities (eg, diabetes, osteoporosis, smoking), body mass index, type and duration of immobilization, complications, return to work, cosmetic perception, time to final clinical follow-up, and symptoms at final clinical follow-up. Incidence of potential risk factors associated with malunion—obesity, noncompliance, and comorbidities such as smoking and diabetes—was compared between the 15 patients with malunion and the other study patients, who healed without malunion.
For long-term postoperative follow-up, patients were contacted to be seen in clinic to complete an updated physical examination, self-reported questionnaire, and the DASH form. Physical examination included measurements of range of motion (ROM) and strength involving the shoulder, elbow, and forearm, with ROM reported as the difference between the injured and contralateral upper extremities. Neurovascular status and focal tenderness to palpation were also assessed on examination. When in-person examination was not possible, the questionnaire and DASH form were completed over the telephone. The self-reported questionnaire asked for information on smoking status, pain, functional limitations, cosmetic perception, satisfaction, and whether or not the patient would still opt for nonoperative management if presented with the same injury again. Pain and satisfaction were measured on numerical scales: Pain scores ranged from 0 (no pain) to 10 (worst possible pain), and satisfaction scores ranged from 1 (not satisfied) to 5 (very satisfied). Data are presented as mean values.
Results
Of the 91 study-eligible patients, 15 (16%) met the radiographic criteria for the diagnosis of malunion. Retrospective data were available for all 15 patients from time of injury to final clinical follow-up (mean, 19 weeks; range, 7-53 weeks). Mean age at injury was 39 years (range, 20-79 years). Additional demographics are listed in Table 1. Incidence of potential risk factors, such as body mass index (26.5 vs 25.4), smoking (33% vs 33%), and diabetes (0% vs 8%), was not significantly different between the malunion and healed-without-malunion groups, respectively. Furthermore, all malunion patients were compliant with their treatment protocol.
Radiographs were assessed at time of final follow-up to confirm healing and to document malunion. Varus malunion was found in 13 patients (mean, 24°; range, 20.5°-35.5°), and shortening was documented in the other 2 patients (mean, 4 cm; range, 3-5 cm). Patients were immobilized a mean of 10 weeks (range, 6-13 weeks). Initial fracture management consisted of coaptation splinting for 1 to 2 weeks (12 patients), hanging arm cast for 1 week (1 patient), and posterior splint for 1 week (1 patient). Patients were then transitioned to Sarmiento fracture bracing for the duration of their treatment (range, 5-12 months). One patient, followed initially at an outside institution, was managed in a sling throughout the duration of treatment (12 weeks) (Table 1). All 15 patients were neurovascularly intact at time of final clinical examination, with return of full upper extremity ROM in all but 3 patients. Only 1 of these 3 patients reported residual pain and functional limitations 4 months after injury (Table 2). Twelve patients were evaluated for return to work, with all successfully returning to work without restrictions at time of final follow-up. The 1 minor complication noted during the treatment period involved medial-sided elbow skin breakdown from brace wear, which resolved with local wound care. No patient required or requested surgical intervention for their residual malunion.
Of the 15 patients, 8 (53%) were reached for in-person examination (6 patients) or telephone interview (2 patients) for follow-up assessment by means of DASH form and self-reported questionnaire a mean of 47 months (range, 12-99 months) after initial injury. The 6 patients who had a physical examination were neurovascularly intact, lacked focal tenderness to palpation, and demonstrated full (5/5) strength within the deltoid, biceps, triceps, pronator, and supinator musculature. Each patient had equal ROM compared with the contralateral uninjured extremity on shoulder forward flexion and abduction, elbow flexion and extension, and forearm pronation and supination. Three patients (50%) had mild residual loss of ROM, with 2 demonstrating decreased shoulder external rotation of 10° and 15°, respectively, and 1 demonstrating decreased shoulder internal rotation of 10°.
Mean DASH score was 10.4 (range, 0-49.2). Evaluation of the self-reported questionnaire revealed a mean pain score of 1.1 (range, 0-7), with only 2 patients reporting any ongoing pain. In addition, 2 patients reported functional limitations, both related to overhead activities. However, 6 (75%) of the 8 patients reported noticeable cosmetic deformity, most commonly varus angulation (4 patients), as well as palpable bony prominence (2) and muscle atrophy (1). The majority of patients were satisfied with the outcome of their treatment (mean, 4; range, 2-5), with 6 patients reporting being satisfied or very satisfied, and all 6 indicating they would undergo nonoperative management again if presented with the same injury. Two patients reported being dissatisfied with their outcome, 1 because of cosmetic appearance and 1 because of cosmetic appearance and functional limitations. Both patients indicated they would choose operative management if presented with the same injury. There was no apparent relationship between outcome and degree of residual deformity, as both patients with varus angulation of more than 30° reported no residual pain or functional limitation and were very satisfied with the outcome of their treatment (Table 2).
Of the 7 patients who could not be reached for final follow-up, 2 on initial contact expressed overall satisfaction with their outcome and denied functional limitations. However, both asked to complete the study at a later date. Subsequently, these 2 patients could not be reached to complete the formal follow-up.
Discussion
Humeral shaft fractures are usually managed nonoperatively. One of the most commonly cited disadvantages of nonoperative management is its higher incidence of residual angular deformity, up to 13% in previous studies.4 Our study found a slightly higher incidence, 16%, on review of 91 nonoperatively managed humeral shaft fractures treated over an 11.5 year period. Although previous studies have reported acceptable functional and cosmetic outcomes with residual angular deformity of less than 20°,2,3,5,8,9 only observational reports have suggested acceptable function in patients with a documented malunion.8
To our knowledge, ours is the first study to correlate malunion with functional parameters and subjective patient-reported outcomes. We found that malunion was not associated with significant pain or functional limitation after nonoperative management of humeral shaft fractures. Furthermore, 75% of patients were satisfied or very satisfied with the outcome of their treatment and indicated they would undergo nonoperative management if presented with the same injury again. However, 75% of patients reported a noticeable cosmetic deformity, and one-third of these patients cited it as a major reason for dissatisfaction with their overall outcome. Regarding function, all patients returned to full strength and ROM of the affected extremity, aside from small losses of internal or external shoulder rotation on the magnitude of 10° to 15° in 50% of those patients tested. In addition, 75% of patients returned to regular activity without functional limitations; the other 25% reported trouble with overhead activities. There were no significant complications during the treatment or follow-up period, once the fracture had healed.
The major limitation of this study was its small patient population. (Obtaining a larger series of patients with malunion after nonoperative treatment of humeral shaft fractures likely would require a multicenter study.) Some of our study findings, such as lack of correlation between degree of malunion and subsequent functional or subjective outcomes, would require a larger sample size for verification and more definitive conclusions. Another limitation is that the study was not designed to evaluate the cause of malunion. Therefore, we cannot draw any definitive conclusions regarding what may have contributed to the development of malunion in our study population. However, all our malunion patients were compliant with their treatment protocol, and they showed no significant difference in incidence of potential risk factors (eg, obesity, comorbidities) compared with the patients who healed without malunion.
Conclusion
Malunion after nonoperative management of humeral shaft fractures does not appear to result in significant pain, dissatisfaction, or functional limitation as measured on physical examination and with validated objective outcome measures in the majority of patients. Furthermore, no patients in this study required surgical intervention for any residual limitations or complications after malunion. The majority of patients reported a noticeable cosmetic deformity, which left a small subset of patients dissatisfied. Overall, our study findings can be used to help counsel patients before and during nonoperative management—particularly patients who appear to be healing with some malunion. Our findings suggest that operative intervention to prevent malunion is not necessary, as it likely would not result in any overall improvement in patient function or satisfaction, but patients should be counseled regarding the high likelihood of cosmetic deformity, which may or may not be bothersome.
1. Rockwood CA, Green DP, Bucholz RW, eds. Rockwood and Green’s Fractures in Adults. 7th ed. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2010.
2. Papasoulis E, Drosos GI, Ververidis AN, Verettas DA. Functional bracing of humeral shaft fractures. A review of clinical studies. Injury. 2010;41(7):e21-e27.
3. Sarmiento A, Latta LL. Functional fracture bracing. J Am Acad Orthop Surg. 1999;7(1):66-75.
4. Denard A Jr, Richards JE, Obremskey WT, Tucker MC, Floyd M, Herzog GA. Outcome of nonoperative vs operative treatment of humeral shaft fractures: a retrospective study of 213 patients. Orthopedics. 2010;33(8).
5. Fjalestad T, Strømsøe K, Salvesen P, Rostad B. Functional results of braced humeral diaphyseal fractures: why do 38% lose external rotation of the shoulder? Arch Orthop Trauma Surg. 2000;120(5-6):281-285.
6. Koch PP, Gross DF, Gerber C. The results of functional (Sarmiento) bracing of humeral shaft fractures. J Shoulder Elbow Surg. 2002;11(2):143-150.
7. Ozkurt B, Altay M, Aktekin CN, Toprak A, Tabak Y. The role of functional bracing in the treatment of humeral shaft fractures [in Turkish]. Acta Orthop Traumatol Turc. 2007;41(1):15-20.
8. Rutgers M, Ring D. Treatment of diaphyseal fractures of the humerus using a functional brace. J Orthop Trauma. 2006;20(9):597-601.
9. Sarmiento A, Kinman PB, Galvin EG, Schmitt RH, Phillips JG. Functional bracing of fractures of the shaft of the humerus. J Bone Joint Surg Am. 1977;59(5):596-601.
1. Rockwood CA, Green DP, Bucholz RW, eds. Rockwood and Green’s Fractures in Adults. 7th ed. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2010.
2. Papasoulis E, Drosos GI, Ververidis AN, Verettas DA. Functional bracing of humeral shaft fractures. A review of clinical studies. Injury. 2010;41(7):e21-e27.
3. Sarmiento A, Latta LL. Functional fracture bracing. J Am Acad Orthop Surg. 1999;7(1):66-75.
4. Denard A Jr, Richards JE, Obremskey WT, Tucker MC, Floyd M, Herzog GA. Outcome of nonoperative vs operative treatment of humeral shaft fractures: a retrospective study of 213 patients. Orthopedics. 2010;33(8).
5. Fjalestad T, Strømsøe K, Salvesen P, Rostad B. Functional results of braced humeral diaphyseal fractures: why do 38% lose external rotation of the shoulder? Arch Orthop Trauma Surg. 2000;120(5-6):281-285.
6. Koch PP, Gross DF, Gerber C. The results of functional (Sarmiento) bracing of humeral shaft fractures. J Shoulder Elbow Surg. 2002;11(2):143-150.
7. Ozkurt B, Altay M, Aktekin CN, Toprak A, Tabak Y. The role of functional bracing in the treatment of humeral shaft fractures [in Turkish]. Acta Orthop Traumatol Turc. 2007;41(1):15-20.
8. Rutgers M, Ring D. Treatment of diaphyseal fractures of the humerus using a functional brace. J Orthop Trauma. 2006;20(9):597-601.
9. Sarmiento A, Kinman PB, Galvin EG, Schmitt RH, Phillips JG. Functional bracing of fractures of the shaft of the humerus. J Bone Joint Surg Am. 1977;59(5):596-601.
Long-Term Elastic Durability of Polymer Matrix Composite Materials After Repeated Steam Sterilization
Polymer matrix composite materials have been widely promoted for orthopedic use in a variety of settings, including surgical instruments, medical devices, implants, and bone models.1-13 These types of composites are engineered from 2 or more constituent materials with significantly different physical or chemical properties; these materials remain separate and distinct on a macroscopic level within the finished composite structure. As a result of ongoing biomaterial research, polymer matrix composite materials can be engineered with a wide range of physical, mechanical, and surface properties, tailored to their application. Given their advantages (eg, high strength-to-weight ratio, radiolucency), these polymer matrix composite materials have gained popularity over traditional metallic materials.
Sterilization is an essential day-to-day procedure in the health care sector, both for single- and multiple-use devices or instruments, and thus a composite material used in medical components should remain unaffected by the process. The type of sterilization most commonly performed is steam sterilization, which achieves microbiological death by moist heat and pressure. Steam is created in an autoclave at a temperature of 132°C (270°F) in typical hospital settings. Steam sterilization cycles last 5 to 14 minutes based on specific manufacturer recommendations. Most medical-grade plastics used in health care have been designed and formulated to withstand the required sterilization cycles without sacrificing key properties. The structure integrity and overall performance of polymer matrix composites may be strongly influenced by the stability of the fiber/polymer interfacial region in terms of physical, chemical, and mechanical characteristics of the material at different scales.14 Absorption of moisture causes dilatational expansion and induces stresses, which are associated with the moisture-induced expansion resulting in degradation of structure stability.15 Thus, steam sterilization could affect the properties of the polymer matrix composite materials by excessive absorption of moisture by the polymer.
To our knowledge, no one has studied whether polymer matrix material properties degrade from long-term, repeated steam sterilization followed by mechanical loading. We conducted a study to evaluate the structural properties (short-beam strength, SBS) of several composite materials exposed to repeated sterilization as compared with traditional metal materials: SS-316L (stainless steel 316L) and Al-7075-T6 (aluminum 7075-T6).
Materials and Methods
We evaluated 3 types of composite materials: Tepex (Tepex Dynalite 201; HiPer Technology Inc.), CFR-PPS (carbon-fiber–reinforced polyphenylene sulfide, Cetex PPS; TenCate Advanced Composites USA Inc.), and HTN-53 (Zytel HTN53G50HSLR NC010; HiPer Technology Inc.) (Figure 1). Tepex is being used for orthopedic applications (knee braces, orthoses, insoles) and sporting goods applications. The performance of this material is superior to that of unreinforced thermoplastics. CFR-PPS represented the state of the art in composite materials for aerospace applications (eg, airframe structures, engine nacelles, fan casings, floorboards, interior parts). This is a high-performance material with exceptional high temperature and aggressive chemical resistance characteristics. CFR-PPS is also used to make filter fabric for coal boilers, papermaking felts, electrical insulation, specialty membranes, gaskets, and packing. It is not solubilized by any known solvents, even in long-term exposure, at temperatures up to 200°C. In addition, it exhibits exceptional resistance to organic and inorganic solutions, acids and alkali solutions, and a wide array of miscellaneous chemicals. HTN-53 is a 50% glass-reinforced, lubricated, high-performance polyamide resin with improved flow, developed for applications requiring excellent surface appearance with water-heated molds. This material has specifically shown survivability in hot, cold, chemically aggressive, and load-bearing environments. In addition, it has shown superior moisture and temperature resistance. These 3 composite materials were compared with SS-316L and Al-7075-T6. SS-316L is commonly used for implants in orthopedics, and Al-7075-T6 is a relatively radiolucent alternative for medical applications. Two different tests were performed to evaluate and validate these composite materials: (1) radiographic density evaluation and (2) structural property tests (short-beam load-to-failure [LTF] test, short-beam cyclic compression loading [CCL] test) before and after sterilization cycling.
Radiographic Density Evaluation
The radiographic density of the 5 materials was evaluated with radiographic images of a cadaveric knee specimen (Figure 2). Radiographic image intensification is the gold standard for repeated radiographic imaging in the operating room. Six different radiographic images were obtained for each material superimposed over a cadaveric knee to recreate potential instrument positioning during surgery: posterior to subject (1 piece), posterior to subject (2 pieces), anterior to subject (1 piece), anterior to subject (2 pieces), anterior and posterior to subject in alignment (1 piece), and anterior and posterior to subject in alignment (2 pieces). Image-Pro Plus software (Media Cybernetics) was used to measure the radiographic density of the materials from the grayscale of the images.
Structural Properties Testing Before and After Sterilization Cycling
We used a standard SBS testing method to determine whether any degradation of structural properties resulted from standard repeated sterilization. The material geometries of the test specimens were 18.96×6.50×3.37 mm (length × width × thickness). Standard sterilization procedures were performed with steam sterilization using an autoclave at a temperature of 132°C (270°F) for at least 5 minutes (range, 5-14 minutes). Sample interval testing ran at 0, 200, and 400 sterilization cycles for structural properties in terms of SBS and moisture retention, with the structural properties at the 0th sterilization cycle (material before sterilization was performed) used as a baseline for comparison. Materials were subjected to 400 sterilization cycles, which is representative of the number of sterilization cycles per year an instrument or device would be subjected to.
Three structural tests were performed for each sample interval: moisture retention, LTF, and CCL. Moisture retention was investigated before and after repeated sterilization by measuring the weight of the test materials, as steam sterilization is known to affect the amount of moisture that is absorbed by a material. Twelve specimens of each proposed material were weighed at each sample interval, with the structural weight at the 0th sterilization cycle (material before sterilization is performed) serving as a baseline for comparison.
SBS testing was based on the ASTM (American Society for Testing and Materials) D2344 standard16 for LTF and CCL tests (Figure 3). Six samples of material were used for each test at every sample interval, yielding 180 samples. Seven servohydraulic material testing system instruments (1 MTS 810 and 6 MTS 858 Mini Bionix) were used to test the SBS of each material. For LTF testing, each specimen was loaded in compression from 30 N to complete structural failure at a constant displacement rate of 1.0 mm/min (0.05 in/min). Testing was initiated with 5 preconditioning loading cycles from 30 to 100 N at 1 Hz. The load was then applied continuously until failure occurred; force and displacement data were collected every 0.02 second. This procedure was performed for 6 replicates for each sample interval for each test material.
The calculation for SBS, Fsbs (MPa), for the constant loading rate until structural failure is:
Fsbs = 0.75 × Pm
b × h
where Pm (N) is the maximum applied load observed during the test, b is the measured specimen width (mm), and h is the measured specimen thickness (mm).
CCL testing consisted of each test material axially loaded with 100 to 500 N at a frequency of 1 Hz for 100,000 cycles. The maximum load of 500 N was chosen as a standard based on 80% of the minimum ultimate failure load from previous LTF tests. Displacement and force data were collected every 5 cycles at the maximum compressive load. Degradation of the material was calculated using the difference between the deflection of the initial cycle and the deflection of the final cycle (50th cycle and 100,000th cycle). This procedure was performed for 6 replicates for each sample interval for each test material.
Statistical Analysis
LTF and CCL testing data were analyzed for any differences among the test materials using 1-way analysis of variance with the least significant difference multiple comparisons post hoc test method using SPSS Version 16.0, with P < .05 denoting significance. These analyses were used to determine the statistical relevance of the difference between the SBS (LTF and CCL) of each test material. Means and standard deviations were calculated for all tests.
Results
Radiographic Density Evaluation
Overall, all the tested composite materials were significantly more radiolucent than either SS-316L or Al-7075-T6. Figure 4 shows the 6 different radiographic images obtained for each material superimposed over a cadaveric knee to recreate potential instrument positioning during surgery: posterior to subject (1 piece), posterior to subject (2 pieces), anterior to subject (1 piece), anterior to subject (2 pieces), anterior and posterior to subject in alignment (1 piece), and anterior and posterior to subject in alignment (2 pieces). SS-316L can be considered radiopaque, and Al-7075-T6 has been used as a relatively radiolucent alternative. Tepex was statistically more radiolucent than the other 2 tested composite materials (Table 1). Even with 2 pieces placed anterior to the subject and 2 placed posterior, the radiodensity compared to the cortical bone was still lower than 1 piece of Al-7075-T6 either anterior or posterior to the subject.
Structural Properties Testing
Moisture Retention. Moisture retention was evaluated by weighing the test materials before and after repeated sterilization. There was no significant difference in moisture retention, as weight differences for all the tested materials were less than 0.5 weight percentage compared to the 0th sterilization cycle (Table 2). Therefore, the results of this study showed that all the tested materials exhibited good moisture/temperature resistance after 400 sterilization cycles.
Load to Failure. In the LTF test, significant differences were detected in SBS between all 5 tested materials (P < .05). Figure 5 shows the comparison of the structural properties in terms of SBS between the 5 tested materials, and Figure 6 shows the failure modes for the tested materials. There was no SBS for SS-316L, as the material did not exhibit complete structural failure even after 400 sterilization cycles; however, SS-316L was observed in inelastic deformation failure (Figure 6D). Al-7075-T6 had much higher SBS compared with the other composite materials, and it also resulted in an inelastic deformation failure mode only after 400 sterilization cycles; otherwise, flexure failure modes were observed. Tepex and CFR-PPS exhibited interlaminar shear failure, and HTN-53 exhibited complete structural failure.
Every composite material tested using the short-beam test for LTF showed a decrease in SBS with increased sterilization cycles (Figure 5). This decrease ranged from 17% to 57% compared with the 0th sterilization cycle. SBS was higher for CFR-PPS than for the other 2 composites. No statistically significant difference was found between CFR-PPS and Tepex except at the 200th sterilization cycle. HTN-53 was brittle at the 0th sterilization cycle but performed more like a ductile material at the 200th cycle. In addition, HTN-53 had the lowest SBS in terms of LTF testing when compared with the other 2 composites.
During the complete structural failure test, the failure modes for Tepex and CFR-PPS were visually identified as interlaminar shear failure (Figures 6A, 6B), whereas HTN-53 visually exhibited pure flexure failure (Figure 6C). As for the metals, SS-316L exhibited plastic deformation, and Al-7075-T6 exhibited flexure failure (Figures 6D, 6E).
Cyclic Compression Loading. Tepex was the only material to pass the 100,000 loading cycle without failure (Table 3). HTN-53 had the poorest performance of all: Its failure rates were 33% (2/6 samples) before sterilization (average cycle, 22,213; range, 21,500-22,925), 83% (5/6 samples) at the 200th sterilization cycle (average cycle, 4,210; range, 0-14,360), and 100% after 400 sterilization cycles (average cycle, 12,725; range, 1,190-21,900). CFR-PPS had no failures before the 400th sterilization cycle, and its failure rate after 400 sterilization cycles (average cycle, 50,735; range, 50,270-51,200) was 33% (2/6 samples).
Discussion
The success of a reusable composite material for use in orthopedic surgery depends not only on radiographic density, fabrication methods, and design but also on the ability to withstand repeated sterilization. Over the past 3 decades, investigators have explored several high-performance polymer matrix composite materials for use in orthopedics, especially in trauma, hip stems, and spinal implants.1,3,4,17-34 According to Evans and Gregson,35 composite materials have been widely promoted as possible orthopedic biomaterials but to date have found few successful commercial applications, because of the many challenging problems encountered in fabrication and testing. One of the most important factors in the mechanical properties of many composite materials is the influence of the cooling and loading rates on fiber-matrix interface adhesion.36-38 Our results tended to agree with the findings of Evans and Gregson,35 as some of these composite materials did not withstand repeated sterilizations well.
Guan and colleagues39 evaluated the influence of sterilization treatment on continuous carbon-fiber–reinforced polyolefin composite. Their 3-point bending test results showed that the levels of maximum load of all the specimens undergoing sterilization by autoclave were lower than those of the control group. For these composites, they concluded that autoclave sterilization and Co-60 gamma ray irradiation sterilization should be avoided and that ethylene oxide is the best method. Our results support their findings with a different set of composites.
Although HTN-53 has shown promise in other orthopedic applications because of its superior moisture and temperature resistance, we found that its performance after repeated sterilization was relatively poor. Tepex showed the greatest potential for durability after repeated sterilization; its mechanical properties were stable after 200 steam sterilization cycles.
Clinical Applications
The composite materials investigated in the present study have potential for use in either instrumentation or long-term implantation applications because of their versatility, mechanical strength, fatigue resistance, and biocompatibility. Akay and Aslan40 stated that carbon-fiber–filled composite implants can be designed with more appropriate modulus, strength, toughness, or stiffness by the arrangement of reinforcing fiber volume and orientation, and can provide better fatigue resistance. A notable advantage of using a composite plate with metal screws is that the potential for corrosion of metallic components is eliminated. Another major advantage of composite medical implants (eg, DiPhos-RM) is radiolucency, which allows direct visualization of osseous callus formation as well as monitoring of fracture healing, thereby improving clinical assessment and accuracy.
Numerous studies have documented the successful clinical performance of composite materials in orthopedic, trauma, and spinal surgery applications.41-45 Bagheri and colleagues41 developed a new carbon fiber–flax–epoxy composite plate and biomechanically compared it with a standard clinical metal plate. Their results confirmed that the carbon fiber–flax–epoxy material represents a potential candidate for bone fracture plate applications, as it can simultaneously provide similar mechanical stiffness and lower stress shielding (higher bone stress) compared with a standard clinical metal bone plate. Tarallo and colleagues45 evaluated the clinical results of 40 cases at 12-month follow-up using a new plate made of carbon-fiber–reinforced polyetheretherketon (DiPhos-RM, Lima Corporate) for the treatment of distal radius fractures. They reported good clinical results for this device at early follow-up, and its use allowed maintenance of reduction in complex AO (Arbeitsgemeinschaft für Osteosynthesefragen) fractures.
The main advantage in using composites for surgical instruments is their radiolucency. These materials do not obscure images or radiographs during fluoroscopic visualization. Surgery often requires fluoroscopic visualization of internal organs or bones, which may require temporary removal of radiopaque devices (eg, retractors, clamps, forceps, hooks, distractors). Aside from being inconvenient, removal and subsequent reinsertion consume valuable time and interfere with the smooth flow of an operation.
The shortcomings of using composite materials for surgical instruments involve detectability and sterilization. A significant issue in surgery is the accidental leaving behind of instruments in patients, which can cause serious problems ranging from organ perforation and blood infection to death. Although instrument counting and other safety protocols can reduce the risk of overlooking an instrument, mistakes are bound to happen. The other shortcoming is the influence of repeated sterilization on the mechanical properties of the composite materials, as sterilization is mandatory for surgical instruments used in the operation room. The structural integrity and overall performance of the polymer composite materials—especially the stability of the interface and the interphase zones—are strongly influenced by repeated sterilization.
On the other hand, composite materials have potential advantages that may support their introduction into long-term medical implant applications, as sterilization commonly is performed only once, during packaging. The effects of sterilization by radiation or steam are much less pronounced on composite implants than on composite surgical instruments. However, composite implants require careful consideration with respect to the bioactivity of wear particles that may be produced from articulation. Further, carbon-fiber–reinforced polymer implants are still substantially more difficult to manufacture and more costly than their metallic counterparts.46
Limitations
This study has some limitations. Most important, studies of this nature do not account for biological factors such as corrosion, biological wear, and the soft-tissue attachment effects on structural properties for potential in vivo use. Another limitation was that the study tested only the mechanical properties in terms of SBS and provided no information about other mechanical properties, such as tensile, compression, and torsion strengths. We think SBS testing adequately evaluates challenging scenarios like thin and narrow instruments/devices that are anticipated in application, and information regarding other modes of failure and mechanical properties (compression, tension, torsion) would be a further area of research. An additional limitation was that our model used a relatively small number of samples. A larger study with more samples and varying layout patterns and layers of the carbon fibers may more clearly demonstrate the effect of steam sterilization on composite materials.
Conclusion
This study provided new information on 3 selected composite materials and their structural properties after repeated steam sterilization. We discovered that these composites were similar in radiographic density and water retention but behaved very differently in terms of mechanical durability after repeated steam sterilization. All selected composites demonstrated deterioration of mechanical properties after repeated steam sterilization. Knowing these results could aid in making decisions about the design and manufacturing of operative instruments and orthopedic biomaterials. Although our preliminary findings are intriguing, further study is warranted to seek specific applications for these composite materials in orthopedic surgery.
1. Ali MS, French TA, Hastings GW, et al. Carbon fibre composite bone plates. Development, evaluation and early clinical experience. J Bone Joint Surg Br. 1990;72(4):586-591.
2. Brooks RA, Jones E, Storer A, Rushton N. Biological evaluation of carbon-fibre–reinforced polybutyleneterephthalate (CFRPBT) employed in a novel acetabular cup. Biomaterials. 2004;25(17):3429-3438.
3. Brown SA, Hastings RS, Mason JJ, Moet A. Characterization of short-fibre reinforced thermoplastics for fracture fixation devices. Biomaterials. 1990;11(8):541-547.
4. Skinner HB. Composite technology for total hip arthroplasty. Clin Orthop Relat Res. 1988;(235):224-236.
5. Field RE, Jones E, Nuijten P, Storer A, Cronin M, Rushton N. Pre-clinical evaluation of the Cambridge acetabular cup. J Mater Sci Mater Med. 2008;19(8):2791-2798.
6. Han N, Ahmed I, Parsons AJ, et al. Influence of screw holes and gamma sterilization on properties of phosphate glass fiber–reinforced composite bone plates. J Biomater Appl. 2013;27(8):990-1002.
7. Losi P, Munaò A, Spiller D, et al. Evaluation of a new composite prosthesis for the repair of abdominal wall defects. J Mater Sci Mater Med. 2007;18(10):1939-1944.
8. Pait TG, Kaufman HH, Voelker JL, McAllister HP, Willison C. Use of a carbon composite radiolucent anterior cervical retractor system. Neurosurgery. 1993;33(5):941-942.
9. Elfar J, Menorca RM, Reed JD, Stanbury S. Composite bone models in orthopaedic surgery research and education. J Am Acad Orthop Surg. 2014;22(2):111-120.
10. Gardner MP, Chong AC, Pollock AG, Wooley PH. Mechanical evaluation of large-size fourth-generation composite femur and tibia models. Ann Biomed Eng. 2010;38(3):613-620.
11. Heiner AD. Structural properties of fourth-generation composite femurs and tibias. J Biomech. 2008;41(15):3282-3284.
12. Dunlap JT, Chong AC, Lucas GL, Cooke FW. Structural properties of a novel design of composite analogue humeri models. Ann Biomed Eng. 2008;36(11):1922-1926.
13. Grover P, Albert C, Wang M, Harris GF. Mechanical characterization of fourth generation composite humerus. Proc Inst Mech Eng H. 2011;225(12):1169-1176.
14. Zheng Q, Morgan RJ. Synergistic thermal-moisture damage mechanisms of epoxies and their carbon fiber composites. J Compos Mater. 1993;27(15):1465-1478.
15. Ray BC. Temperature effect during humid ageing on interfaces of glass and carbon fibers reinforced epoxy composites. J Colloid Interface Sci. 2006;298(1):111-117.
16. Standard test method for short-beam strength of polymer matrix composite materials and their laminates [ASTM specification D2344/D2344M-00]. In: Annual Book of ASTM Standards. Vol 15.03. West Conshohocken, PA: American Society for Testing and Materials; 2006.
17. Bradley JS, Hastings GW, Johnson-Nurse C. Carbon fibre reinforced epoxy as a high strength, low modulus material for internal fixation plates. Biomaterials. 1980;1(1):38-40.
18. McKenna GB, Bradley GW, Dunn HK, Statton WO. Mechanical properties of some fibre reinforced polymer composites after implantation as fracture fixation plates. Biomaterials. 1980;1(4):189-192.
19. Tayton K, Johnson-Nurse C, McKibbin B, Bradley J, Hastings G. The use of semi-rigid carbon-fibre–reinforced plastic plates for fixation of human fractures. Results of preliminary trials. J Bone Joint Surg Br. 1982;64(1):105-111.
20. Tayton K, Bradley J. How stiff should semi-rigid fixation of the human tibia be? A clue to the answer. J Bone Joint Surg Br. 1983;65(3):312-315.
21. Tayton K. Corrosive effect of carbon-fibre reinforced plastic on stainless-steel screws during implantation into man. J Med Eng Technol. 1983;7(1):24-26.
22. Howard CB, Tayton KJ, Gibbs A. The response of human tissues to carbon reinforced epoxy resin. J Bone Joint Surg Br. 1985;67(4):656-658.
23. Skirving AP, Day R, Macdonald W, McLaren R. Carbon fiber reinforced plastic (CFRP) plates versus stainless steel dynamic compression plates in the treatment of fractures of the tibiae in dogs. Clin Orthop Relat Res. 1987;(224):117-124.
24. Prakash R, Marwah S, Goel SC, Tuli SM. Carbon fibre reinforced epoxy implants for bridging large osteoperiosteal gaps. Biomaterials. 1988;9(2):198-202.
25. Pemberton DJ, McKibbin B, Savage R, Tayton K, Stuart D. Carbon-fibre reinforced plates for problem fractures. J Bone Joint Surg Br. 1992;74(1):88-92.
26. Pemberton DJ, Evans PD, Grant A, McKibbin B. Fractures of the distal femur in the elderly treated with a carbon fibre supracondylar plate. Injury. 1994;25(5):317-321.
27. Kelsey DJ, Springer GS, Goodman SB. Composite implant for bone replacement. J Compos Mater. 1997;31(16):1593-1632.
28. Corvelli AA, Biermann PJ, Roberts JC. Design, analysis and fabrication of a composite segmental bone replacement implant. J Adv Mater. 1997;28:2-8.
29. Glassman AH, Crowninshield RD, Schenck R, Herberts P. A low stiffness composite biologically fixed prosthesis. Clin Orthop Relat Res. 2001;(393):128-136.
30. Williams D. New horizons for thermoplastic polymers. Med Device Technol. 2001;12(4):8-9.
31. Al-Shawi AK, Smith SP, Anderson GH. The use of a carbon fiber plate for periprosthetic supracondylar femoral fractures. J Arthroplasty. 2002;17(3):320-324.
32. Baker D, Kadambande SS, Alderman PM. Carbon fibre plates in the treatment of femoral periprosthetic fractures. Injury. 2004;35(6):596-598.
33. Akhavan S, Matthiesen MM, Schulte L, et al. Clinical and histologic results related to a low-modulus composite total hip replacement stem. J Bone Joint Surg Am. 2006;88(6):1308-1314.
34. Toth JM, Wang M, Estes BT, Scifert JL, Seim HB 3rd, Turner AS. Polyetheretherketone as a biomaterial for spinal applications. Biomaterials. 2006;27(3):324-334.
35. Evans SL, Gregson PJ. Composite technology in load-bearing orthopaedic implants. Biomaterials. 1998;19(15):1329-1342.
36. Gao SL, Kim JK. Cooling rate influences in carbon fibre/PEEK composites. Part I. Crystallinity and interface adhesion. Composites Part A. 2000;31(6):517-530.
37. Gao SL, Kim JK. Cooling rate influences in carbon fibre/PEEK composites. Part II. Interlaminar fracture toughness. Composites Part A. 2001;32(6):763-774.
38. Gao SL, Kim JK. Cooling rate influences in carbon fibre/PEEK composites. Part III. Impact damage performance. Composites Part A. 2001;32(6):775-785.
39. Guan SB, Hou CL, Chen AM, Zhang W, Wang JE. Influence of sterilization treatments on continuous carbon-fiber reinforced polyolefin composite. Zhonghua Yi Xue Za Zhi. 2007;87(31):2228-2231.
40. Akay M, Aslan N. An estimation of fatigue life for a carbon fibre/poly ether ether ketone hip joint prosthesis. Proc Inst Mech Eng H. 1995;209(2):93-103.
41. Bagheri ZS, Tavakkoli Awal P, Bougherara H, Aziz MS, Schemitsch EH, Zdero R. Biomechanical analysis of a new carbon fiber/flax/epoxy bone fracture plate shows less stress shielding compared to a standard clinical metal plate. J Biomech Eng. 2014;136(9):091002.
42. Rhee PC, Shin AY. The rate of successful four-corner arthrodesis with a locking, dorsal circular polyether-ether-ketone (PEEK-Optima) plate. J Hand Surg Eur Vol. 2013;38(7):767-773.
43. Nakahara I, Takao M, Bandoh S, Bertollo N, Walsh WR, Sugano N. In vivo implant fixation of carbon fiber–reinforced PEEK hip prostheses in an ovine model. J Orthop Res. 2013;31(3):485-492.
44. Kasliwal MK, O’Toole JE. Clinical experience using polyetheretherketone (PEEK) intervertebral structural cage for anterior cervical corpectomy and fusion. J Clin Neurosci. 2014;21(2):217-220.
45. Tarallo L, Mugnai R, Adani R, Zambianchi F, Catani F. A new volar plate made of carbon-fiber–reinforced polyetheretherketon for distal radius fracture: analysis of 40 cases. J Orthop Traumatol. 2014;15(4):277-283.
46. Cordey J, Perren SM, Steinemann SG. Stress protection due to plates: myth or reality? A parametric analysis made using the composite beam theory. Injury. 2000;31(suppl 3):C1-C13.
Polymer matrix composite materials have been widely promoted for orthopedic use in a variety of settings, including surgical instruments, medical devices, implants, and bone models.1-13 These types of composites are engineered from 2 or more constituent materials with significantly different physical or chemical properties; these materials remain separate and distinct on a macroscopic level within the finished composite structure. As a result of ongoing biomaterial research, polymer matrix composite materials can be engineered with a wide range of physical, mechanical, and surface properties, tailored to their application. Given their advantages (eg, high strength-to-weight ratio, radiolucency), these polymer matrix composite materials have gained popularity over traditional metallic materials.
Sterilization is an essential day-to-day procedure in the health care sector, both for single- and multiple-use devices or instruments, and thus a composite material used in medical components should remain unaffected by the process. The type of sterilization most commonly performed is steam sterilization, which achieves microbiological death by moist heat and pressure. Steam is created in an autoclave at a temperature of 132°C (270°F) in typical hospital settings. Steam sterilization cycles last 5 to 14 minutes based on specific manufacturer recommendations. Most medical-grade plastics used in health care have been designed and formulated to withstand the required sterilization cycles without sacrificing key properties. The structure integrity and overall performance of polymer matrix composites may be strongly influenced by the stability of the fiber/polymer interfacial region in terms of physical, chemical, and mechanical characteristics of the material at different scales.14 Absorption of moisture causes dilatational expansion and induces stresses, which are associated with the moisture-induced expansion resulting in degradation of structure stability.15 Thus, steam sterilization could affect the properties of the polymer matrix composite materials by excessive absorption of moisture by the polymer.
To our knowledge, no one has studied whether polymer matrix material properties degrade from long-term, repeated steam sterilization followed by mechanical loading. We conducted a study to evaluate the structural properties (short-beam strength, SBS) of several composite materials exposed to repeated sterilization as compared with traditional metal materials: SS-316L (stainless steel 316L) and Al-7075-T6 (aluminum 7075-T6).
Materials and Methods
We evaluated 3 types of composite materials: Tepex (Tepex Dynalite 201; HiPer Technology Inc.), CFR-PPS (carbon-fiber–reinforced polyphenylene sulfide, Cetex PPS; TenCate Advanced Composites USA Inc.), and HTN-53 (Zytel HTN53G50HSLR NC010; HiPer Technology Inc.) (Figure 1). Tepex is being used for orthopedic applications (knee braces, orthoses, insoles) and sporting goods applications. The performance of this material is superior to that of unreinforced thermoplastics. CFR-PPS represented the state of the art in composite materials for aerospace applications (eg, airframe structures, engine nacelles, fan casings, floorboards, interior parts). This is a high-performance material with exceptional high temperature and aggressive chemical resistance characteristics. CFR-PPS is also used to make filter fabric for coal boilers, papermaking felts, electrical insulation, specialty membranes, gaskets, and packing. It is not solubilized by any known solvents, even in long-term exposure, at temperatures up to 200°C. In addition, it exhibits exceptional resistance to organic and inorganic solutions, acids and alkali solutions, and a wide array of miscellaneous chemicals. HTN-53 is a 50% glass-reinforced, lubricated, high-performance polyamide resin with improved flow, developed for applications requiring excellent surface appearance with water-heated molds. This material has specifically shown survivability in hot, cold, chemically aggressive, and load-bearing environments. In addition, it has shown superior moisture and temperature resistance. These 3 composite materials were compared with SS-316L and Al-7075-T6. SS-316L is commonly used for implants in orthopedics, and Al-7075-T6 is a relatively radiolucent alternative for medical applications. Two different tests were performed to evaluate and validate these composite materials: (1) radiographic density evaluation and (2) structural property tests (short-beam load-to-failure [LTF] test, short-beam cyclic compression loading [CCL] test) before and after sterilization cycling.
Radiographic Density Evaluation
The radiographic density of the 5 materials was evaluated with radiographic images of a cadaveric knee specimen (Figure 2). Radiographic image intensification is the gold standard for repeated radiographic imaging in the operating room. Six different radiographic images were obtained for each material superimposed over a cadaveric knee to recreate potential instrument positioning during surgery: posterior to subject (1 piece), posterior to subject (2 pieces), anterior to subject (1 piece), anterior to subject (2 pieces), anterior and posterior to subject in alignment (1 piece), and anterior and posterior to subject in alignment (2 pieces). Image-Pro Plus software (Media Cybernetics) was used to measure the radiographic density of the materials from the grayscale of the images.
Structural Properties Testing Before and After Sterilization Cycling
We used a standard SBS testing method to determine whether any degradation of structural properties resulted from standard repeated sterilization. The material geometries of the test specimens were 18.96×6.50×3.37 mm (length × width × thickness). Standard sterilization procedures were performed with steam sterilization using an autoclave at a temperature of 132°C (270°F) for at least 5 minutes (range, 5-14 minutes). Sample interval testing ran at 0, 200, and 400 sterilization cycles for structural properties in terms of SBS and moisture retention, with the structural properties at the 0th sterilization cycle (material before sterilization was performed) used as a baseline for comparison. Materials were subjected to 400 sterilization cycles, which is representative of the number of sterilization cycles per year an instrument or device would be subjected to.
Three structural tests were performed for each sample interval: moisture retention, LTF, and CCL. Moisture retention was investigated before and after repeated sterilization by measuring the weight of the test materials, as steam sterilization is known to affect the amount of moisture that is absorbed by a material. Twelve specimens of each proposed material were weighed at each sample interval, with the structural weight at the 0th sterilization cycle (material before sterilization is performed) serving as a baseline for comparison.
SBS testing was based on the ASTM (American Society for Testing and Materials) D2344 standard16 for LTF and CCL tests (Figure 3). Six samples of material were used for each test at every sample interval, yielding 180 samples. Seven servohydraulic material testing system instruments (1 MTS 810 and 6 MTS 858 Mini Bionix) were used to test the SBS of each material. For LTF testing, each specimen was loaded in compression from 30 N to complete structural failure at a constant displacement rate of 1.0 mm/min (0.05 in/min). Testing was initiated with 5 preconditioning loading cycles from 30 to 100 N at 1 Hz. The load was then applied continuously until failure occurred; force and displacement data were collected every 0.02 second. This procedure was performed for 6 replicates for each sample interval for each test material.
The calculation for SBS, Fsbs (MPa), for the constant loading rate until structural failure is:
Fsbs = 0.75 × Pm
b × h
where Pm (N) is the maximum applied load observed during the test, b is the measured specimen width (mm), and h is the measured specimen thickness (mm).
CCL testing consisted of each test material axially loaded with 100 to 500 N at a frequency of 1 Hz for 100,000 cycles. The maximum load of 500 N was chosen as a standard based on 80% of the minimum ultimate failure load from previous LTF tests. Displacement and force data were collected every 5 cycles at the maximum compressive load. Degradation of the material was calculated using the difference between the deflection of the initial cycle and the deflection of the final cycle (50th cycle and 100,000th cycle). This procedure was performed for 6 replicates for each sample interval for each test material.
Statistical Analysis
LTF and CCL testing data were analyzed for any differences among the test materials using 1-way analysis of variance with the least significant difference multiple comparisons post hoc test method using SPSS Version 16.0, with P < .05 denoting significance. These analyses were used to determine the statistical relevance of the difference between the SBS (LTF and CCL) of each test material. Means and standard deviations were calculated for all tests.
Results
Radiographic Density Evaluation
Overall, all the tested composite materials were significantly more radiolucent than either SS-316L or Al-7075-T6. Figure 4 shows the 6 different radiographic images obtained for each material superimposed over a cadaveric knee to recreate potential instrument positioning during surgery: posterior to subject (1 piece), posterior to subject (2 pieces), anterior to subject (1 piece), anterior to subject (2 pieces), anterior and posterior to subject in alignment (1 piece), and anterior and posterior to subject in alignment (2 pieces). SS-316L can be considered radiopaque, and Al-7075-T6 has been used as a relatively radiolucent alternative. Tepex was statistically more radiolucent than the other 2 tested composite materials (Table 1). Even with 2 pieces placed anterior to the subject and 2 placed posterior, the radiodensity compared to the cortical bone was still lower than 1 piece of Al-7075-T6 either anterior or posterior to the subject.
Structural Properties Testing
Moisture Retention. Moisture retention was evaluated by weighing the test materials before and after repeated sterilization. There was no significant difference in moisture retention, as weight differences for all the tested materials were less than 0.5 weight percentage compared to the 0th sterilization cycle (Table 2). Therefore, the results of this study showed that all the tested materials exhibited good moisture/temperature resistance after 400 sterilization cycles.
Load to Failure. In the LTF test, significant differences were detected in SBS between all 5 tested materials (P < .05). Figure 5 shows the comparison of the structural properties in terms of SBS between the 5 tested materials, and Figure 6 shows the failure modes for the tested materials. There was no SBS for SS-316L, as the material did not exhibit complete structural failure even after 400 sterilization cycles; however, SS-316L was observed in inelastic deformation failure (Figure 6D). Al-7075-T6 had much higher SBS compared with the other composite materials, and it also resulted in an inelastic deformation failure mode only after 400 sterilization cycles; otherwise, flexure failure modes were observed. Tepex and CFR-PPS exhibited interlaminar shear failure, and HTN-53 exhibited complete structural failure.
Every composite material tested using the short-beam test for LTF showed a decrease in SBS with increased sterilization cycles (Figure 5). This decrease ranged from 17% to 57% compared with the 0th sterilization cycle. SBS was higher for CFR-PPS than for the other 2 composites. No statistically significant difference was found between CFR-PPS and Tepex except at the 200th sterilization cycle. HTN-53 was brittle at the 0th sterilization cycle but performed more like a ductile material at the 200th cycle. In addition, HTN-53 had the lowest SBS in terms of LTF testing when compared with the other 2 composites.
During the complete structural failure test, the failure modes for Tepex and CFR-PPS were visually identified as interlaminar shear failure (Figures 6A, 6B), whereas HTN-53 visually exhibited pure flexure failure (Figure 6C). As for the metals, SS-316L exhibited plastic deformation, and Al-7075-T6 exhibited flexure failure (Figures 6D, 6E).
Cyclic Compression Loading. Tepex was the only material to pass the 100,000 loading cycle without failure (Table 3). HTN-53 had the poorest performance of all: Its failure rates were 33% (2/6 samples) before sterilization (average cycle, 22,213; range, 21,500-22,925), 83% (5/6 samples) at the 200th sterilization cycle (average cycle, 4,210; range, 0-14,360), and 100% after 400 sterilization cycles (average cycle, 12,725; range, 1,190-21,900). CFR-PPS had no failures before the 400th sterilization cycle, and its failure rate after 400 sterilization cycles (average cycle, 50,735; range, 50,270-51,200) was 33% (2/6 samples).
Discussion
The success of a reusable composite material for use in orthopedic surgery depends not only on radiographic density, fabrication methods, and design but also on the ability to withstand repeated sterilization. Over the past 3 decades, investigators have explored several high-performance polymer matrix composite materials for use in orthopedics, especially in trauma, hip stems, and spinal implants.1,3,4,17-34 According to Evans and Gregson,35 composite materials have been widely promoted as possible orthopedic biomaterials but to date have found few successful commercial applications, because of the many challenging problems encountered in fabrication and testing. One of the most important factors in the mechanical properties of many composite materials is the influence of the cooling and loading rates on fiber-matrix interface adhesion.36-38 Our results tended to agree with the findings of Evans and Gregson,35 as some of these composite materials did not withstand repeated sterilizations well.
Guan and colleagues39 evaluated the influence of sterilization treatment on continuous carbon-fiber–reinforced polyolefin composite. Their 3-point bending test results showed that the levels of maximum load of all the specimens undergoing sterilization by autoclave were lower than those of the control group. For these composites, they concluded that autoclave sterilization and Co-60 gamma ray irradiation sterilization should be avoided and that ethylene oxide is the best method. Our results support their findings with a different set of composites.
Although HTN-53 has shown promise in other orthopedic applications because of its superior moisture and temperature resistance, we found that its performance after repeated sterilization was relatively poor. Tepex showed the greatest potential for durability after repeated sterilization; its mechanical properties were stable after 200 steam sterilization cycles.
Clinical Applications
The composite materials investigated in the present study have potential for use in either instrumentation or long-term implantation applications because of their versatility, mechanical strength, fatigue resistance, and biocompatibility. Akay and Aslan40 stated that carbon-fiber–filled composite implants can be designed with more appropriate modulus, strength, toughness, or stiffness by the arrangement of reinforcing fiber volume and orientation, and can provide better fatigue resistance. A notable advantage of using a composite plate with metal screws is that the potential for corrosion of metallic components is eliminated. Another major advantage of composite medical implants (eg, DiPhos-RM) is radiolucency, which allows direct visualization of osseous callus formation as well as monitoring of fracture healing, thereby improving clinical assessment and accuracy.
Numerous studies have documented the successful clinical performance of composite materials in orthopedic, trauma, and spinal surgery applications.41-45 Bagheri and colleagues41 developed a new carbon fiber–flax–epoxy composite plate and biomechanically compared it with a standard clinical metal plate. Their results confirmed that the carbon fiber–flax–epoxy material represents a potential candidate for bone fracture plate applications, as it can simultaneously provide similar mechanical stiffness and lower stress shielding (higher bone stress) compared with a standard clinical metal bone plate. Tarallo and colleagues45 evaluated the clinical results of 40 cases at 12-month follow-up using a new plate made of carbon-fiber–reinforced polyetheretherketon (DiPhos-RM, Lima Corporate) for the treatment of distal radius fractures. They reported good clinical results for this device at early follow-up, and its use allowed maintenance of reduction in complex AO (Arbeitsgemeinschaft für Osteosynthesefragen) fractures.
The main advantage in using composites for surgical instruments is their radiolucency. These materials do not obscure images or radiographs during fluoroscopic visualization. Surgery often requires fluoroscopic visualization of internal organs or bones, which may require temporary removal of radiopaque devices (eg, retractors, clamps, forceps, hooks, distractors). Aside from being inconvenient, removal and subsequent reinsertion consume valuable time and interfere with the smooth flow of an operation.
The shortcomings of using composite materials for surgical instruments involve detectability and sterilization. A significant issue in surgery is the accidental leaving behind of instruments in patients, which can cause serious problems ranging from organ perforation and blood infection to death. Although instrument counting and other safety protocols can reduce the risk of overlooking an instrument, mistakes are bound to happen. The other shortcoming is the influence of repeated sterilization on the mechanical properties of the composite materials, as sterilization is mandatory for surgical instruments used in the operation room. The structural integrity and overall performance of the polymer composite materials—especially the stability of the interface and the interphase zones—are strongly influenced by repeated sterilization.
On the other hand, composite materials have potential advantages that may support their introduction into long-term medical implant applications, as sterilization commonly is performed only once, during packaging. The effects of sterilization by radiation or steam are much less pronounced on composite implants than on composite surgical instruments. However, composite implants require careful consideration with respect to the bioactivity of wear particles that may be produced from articulation. Further, carbon-fiber–reinforced polymer implants are still substantially more difficult to manufacture and more costly than their metallic counterparts.46
Limitations
This study has some limitations. Most important, studies of this nature do not account for biological factors such as corrosion, biological wear, and the soft-tissue attachment effects on structural properties for potential in vivo use. Another limitation was that the study tested only the mechanical properties in terms of SBS and provided no information about other mechanical properties, such as tensile, compression, and torsion strengths. We think SBS testing adequately evaluates challenging scenarios like thin and narrow instruments/devices that are anticipated in application, and information regarding other modes of failure and mechanical properties (compression, tension, torsion) would be a further area of research. An additional limitation was that our model used a relatively small number of samples. A larger study with more samples and varying layout patterns and layers of the carbon fibers may more clearly demonstrate the effect of steam sterilization on composite materials.
Conclusion
This study provided new information on 3 selected composite materials and their structural properties after repeated steam sterilization. We discovered that these composites were similar in radiographic density and water retention but behaved very differently in terms of mechanical durability after repeated steam sterilization. All selected composites demonstrated deterioration of mechanical properties after repeated steam sterilization. Knowing these results could aid in making decisions about the design and manufacturing of operative instruments and orthopedic biomaterials. Although our preliminary findings are intriguing, further study is warranted to seek specific applications for these composite materials in orthopedic surgery.
Polymer matrix composite materials have been widely promoted for orthopedic use in a variety of settings, including surgical instruments, medical devices, implants, and bone models.1-13 These types of composites are engineered from 2 or more constituent materials with significantly different physical or chemical properties; these materials remain separate and distinct on a macroscopic level within the finished composite structure. As a result of ongoing biomaterial research, polymer matrix composite materials can be engineered with a wide range of physical, mechanical, and surface properties, tailored to their application. Given their advantages (eg, high strength-to-weight ratio, radiolucency), these polymer matrix composite materials have gained popularity over traditional metallic materials.
Sterilization is an essential day-to-day procedure in the health care sector, both for single- and multiple-use devices or instruments, and thus a composite material used in medical components should remain unaffected by the process. The type of sterilization most commonly performed is steam sterilization, which achieves microbiological death by moist heat and pressure. Steam is created in an autoclave at a temperature of 132°C (270°F) in typical hospital settings. Steam sterilization cycles last 5 to 14 minutes based on specific manufacturer recommendations. Most medical-grade plastics used in health care have been designed and formulated to withstand the required sterilization cycles without sacrificing key properties. The structure integrity and overall performance of polymer matrix composites may be strongly influenced by the stability of the fiber/polymer interfacial region in terms of physical, chemical, and mechanical characteristics of the material at different scales.14 Absorption of moisture causes dilatational expansion and induces stresses, which are associated with the moisture-induced expansion resulting in degradation of structure stability.15 Thus, steam sterilization could affect the properties of the polymer matrix composite materials by excessive absorption of moisture by the polymer.
To our knowledge, no one has studied whether polymer matrix material properties degrade from long-term, repeated steam sterilization followed by mechanical loading. We conducted a study to evaluate the structural properties (short-beam strength, SBS) of several composite materials exposed to repeated sterilization as compared with traditional metal materials: SS-316L (stainless steel 316L) and Al-7075-T6 (aluminum 7075-T6).
Materials and Methods
We evaluated 3 types of composite materials: Tepex (Tepex Dynalite 201; HiPer Technology Inc.), CFR-PPS (carbon-fiber–reinforced polyphenylene sulfide, Cetex PPS; TenCate Advanced Composites USA Inc.), and HTN-53 (Zytel HTN53G50HSLR NC010; HiPer Technology Inc.) (Figure 1). Tepex is being used for orthopedic applications (knee braces, orthoses, insoles) and sporting goods applications. The performance of this material is superior to that of unreinforced thermoplastics. CFR-PPS represented the state of the art in composite materials for aerospace applications (eg, airframe structures, engine nacelles, fan casings, floorboards, interior parts). This is a high-performance material with exceptional high temperature and aggressive chemical resistance characteristics. CFR-PPS is also used to make filter fabric for coal boilers, papermaking felts, electrical insulation, specialty membranes, gaskets, and packing. It is not solubilized by any known solvents, even in long-term exposure, at temperatures up to 200°C. In addition, it exhibits exceptional resistance to organic and inorganic solutions, acids and alkali solutions, and a wide array of miscellaneous chemicals. HTN-53 is a 50% glass-reinforced, lubricated, high-performance polyamide resin with improved flow, developed for applications requiring excellent surface appearance with water-heated molds. This material has specifically shown survivability in hot, cold, chemically aggressive, and load-bearing environments. In addition, it has shown superior moisture and temperature resistance. These 3 composite materials were compared with SS-316L and Al-7075-T6. SS-316L is commonly used for implants in orthopedics, and Al-7075-T6 is a relatively radiolucent alternative for medical applications. Two different tests were performed to evaluate and validate these composite materials: (1) radiographic density evaluation and (2) structural property tests (short-beam load-to-failure [LTF] test, short-beam cyclic compression loading [CCL] test) before and after sterilization cycling.
Radiographic Density Evaluation
The radiographic density of the 5 materials was evaluated with radiographic images of a cadaveric knee specimen (Figure 2). Radiographic image intensification is the gold standard for repeated radiographic imaging in the operating room. Six different radiographic images were obtained for each material superimposed over a cadaveric knee to recreate potential instrument positioning during surgery: posterior to subject (1 piece), posterior to subject (2 pieces), anterior to subject (1 piece), anterior to subject (2 pieces), anterior and posterior to subject in alignment (1 piece), and anterior and posterior to subject in alignment (2 pieces). Image-Pro Plus software (Media Cybernetics) was used to measure the radiographic density of the materials from the grayscale of the images.
Structural Properties Testing Before and After Sterilization Cycling
We used a standard SBS testing method to determine whether any degradation of structural properties resulted from standard repeated sterilization. The material geometries of the test specimens were 18.96×6.50×3.37 mm (length × width × thickness). Standard sterilization procedures were performed with steam sterilization using an autoclave at a temperature of 132°C (270°F) for at least 5 minutes (range, 5-14 minutes). Sample interval testing ran at 0, 200, and 400 sterilization cycles for structural properties in terms of SBS and moisture retention, with the structural properties at the 0th sterilization cycle (material before sterilization was performed) used as a baseline for comparison. Materials were subjected to 400 sterilization cycles, which is representative of the number of sterilization cycles per year an instrument or device would be subjected to.
Three structural tests were performed for each sample interval: moisture retention, LTF, and CCL. Moisture retention was investigated before and after repeated sterilization by measuring the weight of the test materials, as steam sterilization is known to affect the amount of moisture that is absorbed by a material. Twelve specimens of each proposed material were weighed at each sample interval, with the structural weight at the 0th sterilization cycle (material before sterilization is performed) serving as a baseline for comparison.
SBS testing was based on the ASTM (American Society for Testing and Materials) D2344 standard16 for LTF and CCL tests (Figure 3). Six samples of material were used for each test at every sample interval, yielding 180 samples. Seven servohydraulic material testing system instruments (1 MTS 810 and 6 MTS 858 Mini Bionix) were used to test the SBS of each material. For LTF testing, each specimen was loaded in compression from 30 N to complete structural failure at a constant displacement rate of 1.0 mm/min (0.05 in/min). Testing was initiated with 5 preconditioning loading cycles from 30 to 100 N at 1 Hz. The load was then applied continuously until failure occurred; force and displacement data were collected every 0.02 second. This procedure was performed for 6 replicates for each sample interval for each test material.
The calculation for SBS, Fsbs (MPa), for the constant loading rate until structural failure is:
Fsbs = 0.75 × Pm
b × h
where Pm (N) is the maximum applied load observed during the test, b is the measured specimen width (mm), and h is the measured specimen thickness (mm).
CCL testing consisted of each test material axially loaded with 100 to 500 N at a frequency of 1 Hz for 100,000 cycles. The maximum load of 500 N was chosen as a standard based on 80% of the minimum ultimate failure load from previous LTF tests. Displacement and force data were collected every 5 cycles at the maximum compressive load. Degradation of the material was calculated using the difference between the deflection of the initial cycle and the deflection of the final cycle (50th cycle and 100,000th cycle). This procedure was performed for 6 replicates for each sample interval for each test material.
Statistical Analysis
LTF and CCL testing data were analyzed for any differences among the test materials using 1-way analysis of variance with the least significant difference multiple comparisons post hoc test method using SPSS Version 16.0, with P < .05 denoting significance. These analyses were used to determine the statistical relevance of the difference between the SBS (LTF and CCL) of each test material. Means and standard deviations were calculated for all tests.
Results
Radiographic Density Evaluation
Overall, all the tested composite materials were significantly more radiolucent than either SS-316L or Al-7075-T6. Figure 4 shows the 6 different radiographic images obtained for each material superimposed over a cadaveric knee to recreate potential instrument positioning during surgery: posterior to subject (1 piece), posterior to subject (2 pieces), anterior to subject (1 piece), anterior to subject (2 pieces), anterior and posterior to subject in alignment (1 piece), and anterior and posterior to subject in alignment (2 pieces). SS-316L can be considered radiopaque, and Al-7075-T6 has been used as a relatively radiolucent alternative. Tepex was statistically more radiolucent than the other 2 tested composite materials (Table 1). Even with 2 pieces placed anterior to the subject and 2 placed posterior, the radiodensity compared to the cortical bone was still lower than 1 piece of Al-7075-T6 either anterior or posterior to the subject.
Structural Properties Testing
Moisture Retention. Moisture retention was evaluated by weighing the test materials before and after repeated sterilization. There was no significant difference in moisture retention, as weight differences for all the tested materials were less than 0.5 weight percentage compared to the 0th sterilization cycle (Table 2). Therefore, the results of this study showed that all the tested materials exhibited good moisture/temperature resistance after 400 sterilization cycles.
Load to Failure. In the LTF test, significant differences were detected in SBS between all 5 tested materials (P < .05). Figure 5 shows the comparison of the structural properties in terms of SBS between the 5 tested materials, and Figure 6 shows the failure modes for the tested materials. There was no SBS for SS-316L, as the material did not exhibit complete structural failure even after 400 sterilization cycles; however, SS-316L was observed in inelastic deformation failure (Figure 6D). Al-7075-T6 had much higher SBS compared with the other composite materials, and it also resulted in an inelastic deformation failure mode only after 400 sterilization cycles; otherwise, flexure failure modes were observed. Tepex and CFR-PPS exhibited interlaminar shear failure, and HTN-53 exhibited complete structural failure.
Every composite material tested using the short-beam test for LTF showed a decrease in SBS with increased sterilization cycles (Figure 5). This decrease ranged from 17% to 57% compared with the 0th sterilization cycle. SBS was higher for CFR-PPS than for the other 2 composites. No statistically significant difference was found between CFR-PPS and Tepex except at the 200th sterilization cycle. HTN-53 was brittle at the 0th sterilization cycle but performed more like a ductile material at the 200th cycle. In addition, HTN-53 had the lowest SBS in terms of LTF testing when compared with the other 2 composites.
During the complete structural failure test, the failure modes for Tepex and CFR-PPS were visually identified as interlaminar shear failure (Figures 6A, 6B), whereas HTN-53 visually exhibited pure flexure failure (Figure 6C). As for the metals, SS-316L exhibited plastic deformation, and Al-7075-T6 exhibited flexure failure (Figures 6D, 6E).
Cyclic Compression Loading. Tepex was the only material to pass the 100,000 loading cycle without failure (Table 3). HTN-53 had the poorest performance of all: Its failure rates were 33% (2/6 samples) before sterilization (average cycle, 22,213; range, 21,500-22,925), 83% (5/6 samples) at the 200th sterilization cycle (average cycle, 4,210; range, 0-14,360), and 100% after 400 sterilization cycles (average cycle, 12,725; range, 1,190-21,900). CFR-PPS had no failures before the 400th sterilization cycle, and its failure rate after 400 sterilization cycles (average cycle, 50,735; range, 50,270-51,200) was 33% (2/6 samples).
Discussion
The success of a reusable composite material for use in orthopedic surgery depends not only on radiographic density, fabrication methods, and design but also on the ability to withstand repeated sterilization. Over the past 3 decades, investigators have explored several high-performance polymer matrix composite materials for use in orthopedics, especially in trauma, hip stems, and spinal implants.1,3,4,17-34 According to Evans and Gregson,35 composite materials have been widely promoted as possible orthopedic biomaterials but to date have found few successful commercial applications, because of the many challenging problems encountered in fabrication and testing. One of the most important factors in the mechanical properties of many composite materials is the influence of the cooling and loading rates on fiber-matrix interface adhesion.36-38 Our results tended to agree with the findings of Evans and Gregson,35 as some of these composite materials did not withstand repeated sterilizations well.
Guan and colleagues39 evaluated the influence of sterilization treatment on continuous carbon-fiber–reinforced polyolefin composite. Their 3-point bending test results showed that the levels of maximum load of all the specimens undergoing sterilization by autoclave were lower than those of the control group. For these composites, they concluded that autoclave sterilization and Co-60 gamma ray irradiation sterilization should be avoided and that ethylene oxide is the best method. Our results support their findings with a different set of composites.
Although HTN-53 has shown promise in other orthopedic applications because of its superior moisture and temperature resistance, we found that its performance after repeated sterilization was relatively poor. Tepex showed the greatest potential for durability after repeated sterilization; its mechanical properties were stable after 200 steam sterilization cycles.
Clinical Applications
The composite materials investigated in the present study have potential for use in either instrumentation or long-term implantation applications because of their versatility, mechanical strength, fatigue resistance, and biocompatibility. Akay and Aslan40 stated that carbon-fiber–filled composite implants can be designed with more appropriate modulus, strength, toughness, or stiffness by the arrangement of reinforcing fiber volume and orientation, and can provide better fatigue resistance. A notable advantage of using a composite plate with metal screws is that the potential for corrosion of metallic components is eliminated. Another major advantage of composite medical implants (eg, DiPhos-RM) is radiolucency, which allows direct visualization of osseous callus formation as well as monitoring of fracture healing, thereby improving clinical assessment and accuracy.
Numerous studies have documented the successful clinical performance of composite materials in orthopedic, trauma, and spinal surgery applications.41-45 Bagheri and colleagues41 developed a new carbon fiber–flax–epoxy composite plate and biomechanically compared it with a standard clinical metal plate. Their results confirmed that the carbon fiber–flax–epoxy material represents a potential candidate for bone fracture plate applications, as it can simultaneously provide similar mechanical stiffness and lower stress shielding (higher bone stress) compared with a standard clinical metal bone plate. Tarallo and colleagues45 evaluated the clinical results of 40 cases at 12-month follow-up using a new plate made of carbon-fiber–reinforced polyetheretherketon (DiPhos-RM, Lima Corporate) for the treatment of distal radius fractures. They reported good clinical results for this device at early follow-up, and its use allowed maintenance of reduction in complex AO (Arbeitsgemeinschaft für Osteosynthesefragen) fractures.
The main advantage in using composites for surgical instruments is their radiolucency. These materials do not obscure images or radiographs during fluoroscopic visualization. Surgery often requires fluoroscopic visualization of internal organs or bones, which may require temporary removal of radiopaque devices (eg, retractors, clamps, forceps, hooks, distractors). Aside from being inconvenient, removal and subsequent reinsertion consume valuable time and interfere with the smooth flow of an operation.
The shortcomings of using composite materials for surgical instruments involve detectability and sterilization. A significant issue in surgery is the accidental leaving behind of instruments in patients, which can cause serious problems ranging from organ perforation and blood infection to death. Although instrument counting and other safety protocols can reduce the risk of overlooking an instrument, mistakes are bound to happen. The other shortcoming is the influence of repeated sterilization on the mechanical properties of the composite materials, as sterilization is mandatory for surgical instruments used in the operation room. The structural integrity and overall performance of the polymer composite materials—especially the stability of the interface and the interphase zones—are strongly influenced by repeated sterilization.
On the other hand, composite materials have potential advantages that may support their introduction into long-term medical implant applications, as sterilization commonly is performed only once, during packaging. The effects of sterilization by radiation or steam are much less pronounced on composite implants than on composite surgical instruments. However, composite implants require careful consideration with respect to the bioactivity of wear particles that may be produced from articulation. Further, carbon-fiber–reinforced polymer implants are still substantially more difficult to manufacture and more costly than their metallic counterparts.46
Limitations
This study has some limitations. Most important, studies of this nature do not account for biological factors such as corrosion, biological wear, and the soft-tissue attachment effects on structural properties for potential in vivo use. Another limitation was that the study tested only the mechanical properties in terms of SBS and provided no information about other mechanical properties, such as tensile, compression, and torsion strengths. We think SBS testing adequately evaluates challenging scenarios like thin and narrow instruments/devices that are anticipated in application, and information regarding other modes of failure and mechanical properties (compression, tension, torsion) would be a further area of research. An additional limitation was that our model used a relatively small number of samples. A larger study with more samples and varying layout patterns and layers of the carbon fibers may more clearly demonstrate the effect of steam sterilization on composite materials.
Conclusion
This study provided new information on 3 selected composite materials and their structural properties after repeated steam sterilization. We discovered that these composites were similar in radiographic density and water retention but behaved very differently in terms of mechanical durability after repeated steam sterilization. All selected composites demonstrated deterioration of mechanical properties after repeated steam sterilization. Knowing these results could aid in making decisions about the design and manufacturing of operative instruments and orthopedic biomaterials. Although our preliminary findings are intriguing, further study is warranted to seek specific applications for these composite materials in orthopedic surgery.
1. Ali MS, French TA, Hastings GW, et al. Carbon fibre composite bone plates. Development, evaluation and early clinical experience. J Bone Joint Surg Br. 1990;72(4):586-591.
2. Brooks RA, Jones E, Storer A, Rushton N. Biological evaluation of carbon-fibre–reinforced polybutyleneterephthalate (CFRPBT) employed in a novel acetabular cup. Biomaterials. 2004;25(17):3429-3438.
3. Brown SA, Hastings RS, Mason JJ, Moet A. Characterization of short-fibre reinforced thermoplastics for fracture fixation devices. Biomaterials. 1990;11(8):541-547.
4. Skinner HB. Composite technology for total hip arthroplasty. Clin Orthop Relat Res. 1988;(235):224-236.
5. Field RE, Jones E, Nuijten P, Storer A, Cronin M, Rushton N. Pre-clinical evaluation of the Cambridge acetabular cup. J Mater Sci Mater Med. 2008;19(8):2791-2798.
6. Han N, Ahmed I, Parsons AJ, et al. Influence of screw holes and gamma sterilization on properties of phosphate glass fiber–reinforced composite bone plates. J Biomater Appl. 2013;27(8):990-1002.
7. Losi P, Munaò A, Spiller D, et al. Evaluation of a new composite prosthesis for the repair of abdominal wall defects. J Mater Sci Mater Med. 2007;18(10):1939-1944.
8. Pait TG, Kaufman HH, Voelker JL, McAllister HP, Willison C. Use of a carbon composite radiolucent anterior cervical retractor system. Neurosurgery. 1993;33(5):941-942.
9. Elfar J, Menorca RM, Reed JD, Stanbury S. Composite bone models in orthopaedic surgery research and education. J Am Acad Orthop Surg. 2014;22(2):111-120.
10. Gardner MP, Chong AC, Pollock AG, Wooley PH. Mechanical evaluation of large-size fourth-generation composite femur and tibia models. Ann Biomed Eng. 2010;38(3):613-620.
11. Heiner AD. Structural properties of fourth-generation composite femurs and tibias. J Biomech. 2008;41(15):3282-3284.
12. Dunlap JT, Chong AC, Lucas GL, Cooke FW. Structural properties of a novel design of composite analogue humeri models. Ann Biomed Eng. 2008;36(11):1922-1926.
13. Grover P, Albert C, Wang M, Harris GF. Mechanical characterization of fourth generation composite humerus. Proc Inst Mech Eng H. 2011;225(12):1169-1176.
14. Zheng Q, Morgan RJ. Synergistic thermal-moisture damage mechanisms of epoxies and their carbon fiber composites. J Compos Mater. 1993;27(15):1465-1478.
15. Ray BC. Temperature effect during humid ageing on interfaces of glass and carbon fibers reinforced epoxy composites. J Colloid Interface Sci. 2006;298(1):111-117.
16. Standard test method for short-beam strength of polymer matrix composite materials and their laminates [ASTM specification D2344/D2344M-00]. In: Annual Book of ASTM Standards. Vol 15.03. West Conshohocken, PA: American Society for Testing and Materials; 2006.
17. Bradley JS, Hastings GW, Johnson-Nurse C. Carbon fibre reinforced epoxy as a high strength, low modulus material for internal fixation plates. Biomaterials. 1980;1(1):38-40.
18. McKenna GB, Bradley GW, Dunn HK, Statton WO. Mechanical properties of some fibre reinforced polymer composites after implantation as fracture fixation plates. Biomaterials. 1980;1(4):189-192.
19. Tayton K, Johnson-Nurse C, McKibbin B, Bradley J, Hastings G. The use of semi-rigid carbon-fibre–reinforced plastic plates for fixation of human fractures. Results of preliminary trials. J Bone Joint Surg Br. 1982;64(1):105-111.
20. Tayton K, Bradley J. How stiff should semi-rigid fixation of the human tibia be? A clue to the answer. J Bone Joint Surg Br. 1983;65(3):312-315.
21. Tayton K. Corrosive effect of carbon-fibre reinforced plastic on stainless-steel screws during implantation into man. J Med Eng Technol. 1983;7(1):24-26.
22. Howard CB, Tayton KJ, Gibbs A. The response of human tissues to carbon reinforced epoxy resin. J Bone Joint Surg Br. 1985;67(4):656-658.
23. Skirving AP, Day R, Macdonald W, McLaren R. Carbon fiber reinforced plastic (CFRP) plates versus stainless steel dynamic compression plates in the treatment of fractures of the tibiae in dogs. Clin Orthop Relat Res. 1987;(224):117-124.
24. Prakash R, Marwah S, Goel SC, Tuli SM. Carbon fibre reinforced epoxy implants for bridging large osteoperiosteal gaps. Biomaterials. 1988;9(2):198-202.
25. Pemberton DJ, McKibbin B, Savage R, Tayton K, Stuart D. Carbon-fibre reinforced plates for problem fractures. J Bone Joint Surg Br. 1992;74(1):88-92.
26. Pemberton DJ, Evans PD, Grant A, McKibbin B. Fractures of the distal femur in the elderly treated with a carbon fibre supracondylar plate. Injury. 1994;25(5):317-321.
27. Kelsey DJ, Springer GS, Goodman SB. Composite implant for bone replacement. J Compos Mater. 1997;31(16):1593-1632.
28. Corvelli AA, Biermann PJ, Roberts JC. Design, analysis and fabrication of a composite segmental bone replacement implant. J Adv Mater. 1997;28:2-8.
29. Glassman AH, Crowninshield RD, Schenck R, Herberts P. A low stiffness composite biologically fixed prosthesis. Clin Orthop Relat Res. 2001;(393):128-136.
30. Williams D. New horizons for thermoplastic polymers. Med Device Technol. 2001;12(4):8-9.
31. Al-Shawi AK, Smith SP, Anderson GH. The use of a carbon fiber plate for periprosthetic supracondylar femoral fractures. J Arthroplasty. 2002;17(3):320-324.
32. Baker D, Kadambande SS, Alderman PM. Carbon fibre plates in the treatment of femoral periprosthetic fractures. Injury. 2004;35(6):596-598.
33. Akhavan S, Matthiesen MM, Schulte L, et al. Clinical and histologic results related to a low-modulus composite total hip replacement stem. J Bone Joint Surg Am. 2006;88(6):1308-1314.
34. Toth JM, Wang M, Estes BT, Scifert JL, Seim HB 3rd, Turner AS. Polyetheretherketone as a biomaterial for spinal applications. Biomaterials. 2006;27(3):324-334.
35. Evans SL, Gregson PJ. Composite technology in load-bearing orthopaedic implants. Biomaterials. 1998;19(15):1329-1342.
36. Gao SL, Kim JK. Cooling rate influences in carbon fibre/PEEK composites. Part I. Crystallinity and interface adhesion. Composites Part A. 2000;31(6):517-530.
37. Gao SL, Kim JK. Cooling rate influences in carbon fibre/PEEK composites. Part II. Interlaminar fracture toughness. Composites Part A. 2001;32(6):763-774.
38. Gao SL, Kim JK. Cooling rate influences in carbon fibre/PEEK composites. Part III. Impact damage performance. Composites Part A. 2001;32(6):775-785.
39. Guan SB, Hou CL, Chen AM, Zhang W, Wang JE. Influence of sterilization treatments on continuous carbon-fiber reinforced polyolefin composite. Zhonghua Yi Xue Za Zhi. 2007;87(31):2228-2231.
40. Akay M, Aslan N. An estimation of fatigue life for a carbon fibre/poly ether ether ketone hip joint prosthesis. Proc Inst Mech Eng H. 1995;209(2):93-103.
41. Bagheri ZS, Tavakkoli Awal P, Bougherara H, Aziz MS, Schemitsch EH, Zdero R. Biomechanical analysis of a new carbon fiber/flax/epoxy bone fracture plate shows less stress shielding compared to a standard clinical metal plate. J Biomech Eng. 2014;136(9):091002.
42. Rhee PC, Shin AY. The rate of successful four-corner arthrodesis with a locking, dorsal circular polyether-ether-ketone (PEEK-Optima) plate. J Hand Surg Eur Vol. 2013;38(7):767-773.
43. Nakahara I, Takao M, Bandoh S, Bertollo N, Walsh WR, Sugano N. In vivo implant fixation of carbon fiber–reinforced PEEK hip prostheses in an ovine model. J Orthop Res. 2013;31(3):485-492.
44. Kasliwal MK, O’Toole JE. Clinical experience using polyetheretherketone (PEEK) intervertebral structural cage for anterior cervical corpectomy and fusion. J Clin Neurosci. 2014;21(2):217-220.
45. Tarallo L, Mugnai R, Adani R, Zambianchi F, Catani F. A new volar plate made of carbon-fiber–reinforced polyetheretherketon for distal radius fracture: analysis of 40 cases. J Orthop Traumatol. 2014;15(4):277-283.
46. Cordey J, Perren SM, Steinemann SG. Stress protection due to plates: myth or reality? A parametric analysis made using the composite beam theory. Injury. 2000;31(suppl 3):C1-C13.
1. Ali MS, French TA, Hastings GW, et al. Carbon fibre composite bone plates. Development, evaluation and early clinical experience. J Bone Joint Surg Br. 1990;72(4):586-591.
2. Brooks RA, Jones E, Storer A, Rushton N. Biological evaluation of carbon-fibre–reinforced polybutyleneterephthalate (CFRPBT) employed in a novel acetabular cup. Biomaterials. 2004;25(17):3429-3438.
3. Brown SA, Hastings RS, Mason JJ, Moet A. Characterization of short-fibre reinforced thermoplastics for fracture fixation devices. Biomaterials. 1990;11(8):541-547.
4. Skinner HB. Composite technology for total hip arthroplasty. Clin Orthop Relat Res. 1988;(235):224-236.
5. Field RE, Jones E, Nuijten P, Storer A, Cronin M, Rushton N. Pre-clinical evaluation of the Cambridge acetabular cup. J Mater Sci Mater Med. 2008;19(8):2791-2798.
6. Han N, Ahmed I, Parsons AJ, et al. Influence of screw holes and gamma sterilization on properties of phosphate glass fiber–reinforced composite bone plates. J Biomater Appl. 2013;27(8):990-1002.
7. Losi P, Munaò A, Spiller D, et al. Evaluation of a new composite prosthesis for the repair of abdominal wall defects. J Mater Sci Mater Med. 2007;18(10):1939-1944.
8. Pait TG, Kaufman HH, Voelker JL, McAllister HP, Willison C. Use of a carbon composite radiolucent anterior cervical retractor system. Neurosurgery. 1993;33(5):941-942.
9. Elfar J, Menorca RM, Reed JD, Stanbury S. Composite bone models in orthopaedic surgery research and education. J Am Acad Orthop Surg. 2014;22(2):111-120.
10. Gardner MP, Chong AC, Pollock AG, Wooley PH. Mechanical evaluation of large-size fourth-generation composite femur and tibia models. Ann Biomed Eng. 2010;38(3):613-620.
11. Heiner AD. Structural properties of fourth-generation composite femurs and tibias. J Biomech. 2008;41(15):3282-3284.
12. Dunlap JT, Chong AC, Lucas GL, Cooke FW. Structural properties of a novel design of composite analogue humeri models. Ann Biomed Eng. 2008;36(11):1922-1926.
13. Grover P, Albert C, Wang M, Harris GF. Mechanical characterization of fourth generation composite humerus. Proc Inst Mech Eng H. 2011;225(12):1169-1176.
14. Zheng Q, Morgan RJ. Synergistic thermal-moisture damage mechanisms of epoxies and their carbon fiber composites. J Compos Mater. 1993;27(15):1465-1478.
15. Ray BC. Temperature effect during humid ageing on interfaces of glass and carbon fibers reinforced epoxy composites. J Colloid Interface Sci. 2006;298(1):111-117.
16. Standard test method for short-beam strength of polymer matrix composite materials and their laminates [ASTM specification D2344/D2344M-00]. In: Annual Book of ASTM Standards. Vol 15.03. West Conshohocken, PA: American Society for Testing and Materials; 2006.
17. Bradley JS, Hastings GW, Johnson-Nurse C. Carbon fibre reinforced epoxy as a high strength, low modulus material for internal fixation plates. Biomaterials. 1980;1(1):38-40.
18. McKenna GB, Bradley GW, Dunn HK, Statton WO. Mechanical properties of some fibre reinforced polymer composites after implantation as fracture fixation plates. Biomaterials. 1980;1(4):189-192.
19. Tayton K, Johnson-Nurse C, McKibbin B, Bradley J, Hastings G. The use of semi-rigid carbon-fibre–reinforced plastic plates for fixation of human fractures. Results of preliminary trials. J Bone Joint Surg Br. 1982;64(1):105-111.
20. Tayton K, Bradley J. How stiff should semi-rigid fixation of the human tibia be? A clue to the answer. J Bone Joint Surg Br. 1983;65(3):312-315.
21. Tayton K. Corrosive effect of carbon-fibre reinforced plastic on stainless-steel screws during implantation into man. J Med Eng Technol. 1983;7(1):24-26.
22. Howard CB, Tayton KJ, Gibbs A. The response of human tissues to carbon reinforced epoxy resin. J Bone Joint Surg Br. 1985;67(4):656-658.
23. Skirving AP, Day R, Macdonald W, McLaren R. Carbon fiber reinforced plastic (CFRP) plates versus stainless steel dynamic compression plates in the treatment of fractures of the tibiae in dogs. Clin Orthop Relat Res. 1987;(224):117-124.
24. Prakash R, Marwah S, Goel SC, Tuli SM. Carbon fibre reinforced epoxy implants for bridging large osteoperiosteal gaps. Biomaterials. 1988;9(2):198-202.
25. Pemberton DJ, McKibbin B, Savage R, Tayton K, Stuart D. Carbon-fibre reinforced plates for problem fractures. J Bone Joint Surg Br. 1992;74(1):88-92.
26. Pemberton DJ, Evans PD, Grant A, McKibbin B. Fractures of the distal femur in the elderly treated with a carbon fibre supracondylar plate. Injury. 1994;25(5):317-321.
27. Kelsey DJ, Springer GS, Goodman SB. Composite implant for bone replacement. J Compos Mater. 1997;31(16):1593-1632.
28. Corvelli AA, Biermann PJ, Roberts JC. Design, analysis and fabrication of a composite segmental bone replacement implant. J Adv Mater. 1997;28:2-8.
29. Glassman AH, Crowninshield RD, Schenck R, Herberts P. A low stiffness composite biologically fixed prosthesis. Clin Orthop Relat Res. 2001;(393):128-136.
30. Williams D. New horizons for thermoplastic polymers. Med Device Technol. 2001;12(4):8-9.
31. Al-Shawi AK, Smith SP, Anderson GH. The use of a carbon fiber plate for periprosthetic supracondylar femoral fractures. J Arthroplasty. 2002;17(3):320-324.
32. Baker D, Kadambande SS, Alderman PM. Carbon fibre plates in the treatment of femoral periprosthetic fractures. Injury. 2004;35(6):596-598.
33. Akhavan S, Matthiesen MM, Schulte L, et al. Clinical and histologic results related to a low-modulus composite total hip replacement stem. J Bone Joint Surg Am. 2006;88(6):1308-1314.
34. Toth JM, Wang M, Estes BT, Scifert JL, Seim HB 3rd, Turner AS. Polyetheretherketone as a biomaterial for spinal applications. Biomaterials. 2006;27(3):324-334.
35. Evans SL, Gregson PJ. Composite technology in load-bearing orthopaedic implants. Biomaterials. 1998;19(15):1329-1342.
36. Gao SL, Kim JK. Cooling rate influences in carbon fibre/PEEK composites. Part I. Crystallinity and interface adhesion. Composites Part A. 2000;31(6):517-530.
37. Gao SL, Kim JK. Cooling rate influences in carbon fibre/PEEK composites. Part II. Interlaminar fracture toughness. Composites Part A. 2001;32(6):763-774.
38. Gao SL, Kim JK. Cooling rate influences in carbon fibre/PEEK composites. Part III. Impact damage performance. Composites Part A. 2001;32(6):775-785.
39. Guan SB, Hou CL, Chen AM, Zhang W, Wang JE. Influence of sterilization treatments on continuous carbon-fiber reinforced polyolefin composite. Zhonghua Yi Xue Za Zhi. 2007;87(31):2228-2231.
40. Akay M, Aslan N. An estimation of fatigue life for a carbon fibre/poly ether ether ketone hip joint prosthesis. Proc Inst Mech Eng H. 1995;209(2):93-103.
41. Bagheri ZS, Tavakkoli Awal P, Bougherara H, Aziz MS, Schemitsch EH, Zdero R. Biomechanical analysis of a new carbon fiber/flax/epoxy bone fracture plate shows less stress shielding compared to a standard clinical metal plate. J Biomech Eng. 2014;136(9):091002.
42. Rhee PC, Shin AY. The rate of successful four-corner arthrodesis with a locking, dorsal circular polyether-ether-ketone (PEEK-Optima) plate. J Hand Surg Eur Vol. 2013;38(7):767-773.
43. Nakahara I, Takao M, Bandoh S, Bertollo N, Walsh WR, Sugano N. In vivo implant fixation of carbon fiber–reinforced PEEK hip prostheses in an ovine model. J Orthop Res. 2013;31(3):485-492.
44. Kasliwal MK, O’Toole JE. Clinical experience using polyetheretherketone (PEEK) intervertebral structural cage for anterior cervical corpectomy and fusion. J Clin Neurosci. 2014;21(2):217-220.
45. Tarallo L, Mugnai R, Adani R, Zambianchi F, Catani F. A new volar plate made of carbon-fiber–reinforced polyetheretherketon for distal radius fracture: analysis of 40 cases. J Orthop Traumatol. 2014;15(4):277-283.
46. Cordey J, Perren SM, Steinemann SG. Stress protection due to plates: myth or reality? A parametric analysis made using the composite beam theory. Injury. 2000;31(suppl 3):C1-C13.
Reinforcing a Spica Cast With a Fiberglass Bar
Femur fractures (Orthopaedic Trauma Association classes 31, 32, 33)1 are common childhood injuries, occurring at a rate of 19 per 100,000 children in the United States.2 Peak occurrence is bimodal at ages 2 and 17 years. The most common mechanism of injury in children under 6 years is a fall, and hip spica casting is the preferred treatment modality in this group.3-5
A bar connecting the legs of the spica cast has been shown to facilitate patient transport5 and significantly decrease mechanical failure of the spica cast.6 This bar often consists of a broom handle or pipe that must be cut to size during the case and subsequently incorporated into the cast—tasks that are often inconvenient and time-consuming for on-call or emergency department staff unfamiliar with orthopedic tools.
In this article, we review a spica cast application that incorporates a low-cost, lightweight technique for fabricating a connecting bar from existing fiberglass casting material. The Institutional Review Board at Connecticut Children’s Medical Center approved this work.
Technique of Double-Leg Spica Casting With Fiberglass Bar
A spica casting table (Orthopedic Systems) with a well-padded post is placed on the operating room table and adjusted to the length of the patient from perineum to just below the shoulders. With the patient under general anesthesia, folded towels are used to provide 2 to 4 cm of padding on the anterior torso, atop which a waterproof pantaloon is applied. The patient is transferred to the spica table, and the patient’s arms are gently secured to the casting table with cast padding or tape in an abducted position at the shoulders. A surgeon controls the legs by holding the feet with the long fingers just above the heels, the index fingers on the anterior ankle, and the thumbs on the soles of the feet. Cast padding is wrapped from the nipple line to the supramalleolar region on each leg. The bony prominences of the malleoli, patella, fibular head, femoral condyles, iliac crests, and coccyx are well padded.
Fiberglass is then rolled without compression onto the patient, beginning with the torso and perineal areas. The injured leg is wrapped to its final length above the malleoli while the uninjured leg is kept free. Maintaining the position of the injured leg with simultaneous molding at the fracture site, typically to promote valgus, allows fracture reduction. The fracture position is then checked under image intensification. For femur fractures, hip abduction and flexion are set to 45° and 90°, respectively, while knee flexion is between 50° and 90°. The uninjured leg is then wrapped with fiberglass. Additional strips of fiberglass can be used to reinforce weak junctional regions between the torso and the legs, posteriorly over the “intern’s triangle” and anteriorly along the hip crease.
A connecting fiberglass bar is then created using a fiberglass roll once the cast is hardened. A 2-inch fiberglass roll is wrapped around one leg to secure its position (Figure 1A) and then rolled around the second limb (Figure 1B). Fiberglass is then pulled taut and rolled around the bridge that has been created in order to thicken the bar (Figure 2). The roll is again brought around the closest limb, wrapped back across the bridge to the other limb, and rolled out to its full length. Last, the legs are abducted 1 to 2 cm to tension the bar (Figure 3). Although this does not produce enough movement to cause a crease and a resultant ulcer, careful inspection of common pressure points (eg, popliteal fossa) should be performed after the cast is complete.
The chest towels are removed, and the final cast is inspected clinically and fluoroscopically at the fracture site before extubation. The cast is trimmed as needed to ensure room for perineal care, as well as full ankle flexion and extension without impingement. Cast edges are further petaled with plastic tape (Hy-Tape International) to provide padding and prevent the waterproof lining from tearing.
Postoperative care involves overnight observation and caregiver practice in perineal care. Frequent rotation from supine to prone is encouraged. Nurses confirm car-seat fit before discharge. If needed, radiographs are obtained 7 to 10 days later to help with wedging adjustment. The cast is removed in the clinic when adequate callus is appreciated on subsequent radiographs.
Case Series
Our experience with this technique in 16 unilateral femur fractures has been favorable (Table). Patient age ranged from 5 months to 3 years. Mean pretreatment angulation was 13° varus and 11° procurvatum. The majority of fractures were femoral shaft fractures; 1 was proximal, 2 distal.
All fractures united without cast revision. Mean cast time was 4.5 weeks (range, 16 days–6 weeks). Immediate postoperative alignment was 2.5° varus (range, 11° valgus to 16° varus) and 7° procurvatum (range, 1° recurvatum to 22° procurvatum). Mean shortening was 1.5 cm (range, 0-2.7 cm). Final alignment was 1° valgus (range, 9° valgus to 12° varus) and 5° procurvatum (range, 0° to 22°). Mean follow-up was 8 months. There were no cases of skin maceration or cast failure. No casts precluded use of a spica car-seat. Figure 4 shows a typical case with a midshaft fracture treated with closed reduction and casting for 4 weeks with good remodeling at final follow-up, 19 months after injury.
Discussion
Although single-leg walking spica casts have been shown to safely treat low-energy femur fractures in children 1 to 6 years old,7 length-unstable femur fractures, bilateral femur fractures, and patients with hip dysplasia continue to be managed with a double-leg hip spica construct. Cast integrity remains fundamental to the control of most fractures and prevention of cast-related complications, such as skin maceration and ulceration. Surgeons typically use spica cast reinforcement schemes—such as cast augments of the torso–limb junction, with multiple layers of casting material or incorporation of a connecting bar between the legs, typically constructed by overwrapping a wooden dowel in casting material—to improve the mechanical stability of casts.6 The present technique of creating a connecting bar from fiberglass casting material significantly simplifies the standard wooden dowel approach and provided excellent results in our treatment group in terms of cast integrity and fracture alignment. In addition, at our institution, a roll of fiberglass costs $2.10, whereas a wooden dowel costs $3 to $10 and can be difficult to locate if not frequently used. Other tube-shaped materials, such as the disposable material used to package implants and tubes, carry an even lower cost. However, we have found that a single fiberglass roll is most readily available and easiest to apply.
Although proper spica cast application remains important in managing pediatric trauma, it lacks a good technical description in the literature. In this technical report, we have presented our standard spica cast application method, which minimizes the range of cast complications that have been reported, from minor skin irritation to superior mesenteric artery syndrome. Two salient technical highlights are use of waterproof pantaloon liners and cast petaling, which we have found almost eliminate the morbidity of potential skin complications, reported to occur at a rate of 28%.8 In addition, we forgo applying the cast on the injured leg in segments. Application of a short-leg cast on the injured leg to allow traction on the leg during cast application is of dubious utility and may be potentially harmful, with described complications of peroneal nerve palsy and compartment syndrome.9-11 Further, it is important to use an abdominal spacer (eg, a stack of towels) under the cast padding to create room for abdominal expansion and minimize pressure thought to induce superior mesenteric artery syndrome. Plastic or rubber abdominal spacers have also been described.12,13 Last, leg position is important for reduction and maintenance of the fracture, as well as patient care. Literature advocates minimizing hip abduction to just that needed for perineal care and maximizing hip flexion and knee extension to optimize car-seat fit and safety.14
Conclusion
Construction of a spica cast lower limb connecting bar from readily available fiberglass casting material allows a facile and rapid addition to the mechanical stability of a spica cast in the treatment of pediatric femur fractures. The technique is low-cost and obviates the need for additional extraneous materials.
1. Slongo TF, Audigé L; AO Pediatric Classification Group. Fracture and dislocation classification compendium for children: the AO Pediatric Comprehensive Classification of Long Bone Fractures (PCCF). J Orthop Trauma. 2007;21(10):S135-S160.
2. Hinton RY, Lincoln A, Crockett MM, Sponseller P, Smith G. Fractures of the femoral shaft in children. Incidence, mechanisms, and sociodemographic risk factors. J Bone Joint Surg Am. 1999;81(4):500-509.
3. Campbell WC, Canale ST, Beaty JH, eds. Campbell’s Operative Orthopaedics. 11th ed. Philadelphia, PA: Mosby Elsevier; 2008.
4. Lovell WW, Winter RB, Morrissy RT, Weinstein SL. Lovell and Winter’s Pediatric Orthopaedics. Philadelphia, PA: Lippincott Williams & Wilkins; 2006.
5. Green NE, Swiontkowski MF, eds. Skeletal Trauma in Children. 4th ed. Philadelphia, PA: Elsevier Health Sciences; 2009.
6. Hosalkar HS, Jones S, Chowdhury M, Chatoo M, Hill RA. Connecting bar for hip spica reinforcement: does it help? J Pediatr Orthop B. 2003;12(2):100-102.
7. Flynn JM, Garner MR, Jones KJ, et al. The treatment of low-energy femoral shaft fractures: a prospective study comparing the “walking spica” with the traditional spica cast. J Bone Joint Surg Am. 2011;93(23):2196-2202.
8. DiFazio R, Vessey J, Zurakowski D, Hresko MT, Matheney T. Incidence of skin complications and associated charges in children treated with hip spica casts for femur fractures. J Pediatr Orthop. 2011;31(1):17-22.
9. Weiss AP, Schenck RC Jr, Sponseller PD, Thompson JD. Peroneal nerve palsy after early cast application for femoral fractures in children. J Pediatr Orthop. 1992;12(1):25-28.
10. Mubarak SJ, Frick S, Sink E, Rathjen K, Noonan KJ. Volkmann contracture and compartment syndromes after femur fractures in children treated with 90/90 spica casts. J Pediatr Orthop. 2006;26(5):567-572.
11. Large TM, Frick SL. Compartment syndrome of the leg after treatment of a femoral fracture with an early sitting spica cast. A report of two cases. J Bone Joint Surg Am. 2003;85(11):2207-2210.
12. Sharma S, Azzopardi T. Reduction of abdominal pressure for prophylaxis of the mesenteric artery syndrome (cast syndrome) in a hip spica—a simple technique. Ann R Coll Surg Engl. 2006;88(3):317.
13. Kiter E, Demirkan F, Kiliç BA, Erkula G. A new technique for creating an abdominal window in a hip spica cast. J Orthop Trauma. 2003;17(6):442-443.
14. Zielinski J, Oliver G, Sybesma J, Walter N, Atkinson P. Casting technique and restraint choice influence child safety during transport of body casted children subjected to a simulated frontal MVA. J Trauma. 2009;66(6):1653-1665.
Femur fractures (Orthopaedic Trauma Association classes 31, 32, 33)1 are common childhood injuries, occurring at a rate of 19 per 100,000 children in the United States.2 Peak occurrence is bimodal at ages 2 and 17 years. The most common mechanism of injury in children under 6 years is a fall, and hip spica casting is the preferred treatment modality in this group.3-5
A bar connecting the legs of the spica cast has been shown to facilitate patient transport5 and significantly decrease mechanical failure of the spica cast.6 This bar often consists of a broom handle or pipe that must be cut to size during the case and subsequently incorporated into the cast—tasks that are often inconvenient and time-consuming for on-call or emergency department staff unfamiliar with orthopedic tools.
In this article, we review a spica cast application that incorporates a low-cost, lightweight technique for fabricating a connecting bar from existing fiberglass casting material. The Institutional Review Board at Connecticut Children’s Medical Center approved this work.
Technique of Double-Leg Spica Casting With Fiberglass Bar
A spica casting table (Orthopedic Systems) with a well-padded post is placed on the operating room table and adjusted to the length of the patient from perineum to just below the shoulders. With the patient under general anesthesia, folded towels are used to provide 2 to 4 cm of padding on the anterior torso, atop which a waterproof pantaloon is applied. The patient is transferred to the spica table, and the patient’s arms are gently secured to the casting table with cast padding or tape in an abducted position at the shoulders. A surgeon controls the legs by holding the feet with the long fingers just above the heels, the index fingers on the anterior ankle, and the thumbs on the soles of the feet. Cast padding is wrapped from the nipple line to the supramalleolar region on each leg. The bony prominences of the malleoli, patella, fibular head, femoral condyles, iliac crests, and coccyx are well padded.
Fiberglass is then rolled without compression onto the patient, beginning with the torso and perineal areas. The injured leg is wrapped to its final length above the malleoli while the uninjured leg is kept free. Maintaining the position of the injured leg with simultaneous molding at the fracture site, typically to promote valgus, allows fracture reduction. The fracture position is then checked under image intensification. For femur fractures, hip abduction and flexion are set to 45° and 90°, respectively, while knee flexion is between 50° and 90°. The uninjured leg is then wrapped with fiberglass. Additional strips of fiberglass can be used to reinforce weak junctional regions between the torso and the legs, posteriorly over the “intern’s triangle” and anteriorly along the hip crease.
A connecting fiberglass bar is then created using a fiberglass roll once the cast is hardened. A 2-inch fiberglass roll is wrapped around one leg to secure its position (Figure 1A) and then rolled around the second limb (Figure 1B). Fiberglass is then pulled taut and rolled around the bridge that has been created in order to thicken the bar (Figure 2). The roll is again brought around the closest limb, wrapped back across the bridge to the other limb, and rolled out to its full length. Last, the legs are abducted 1 to 2 cm to tension the bar (Figure 3). Although this does not produce enough movement to cause a crease and a resultant ulcer, careful inspection of common pressure points (eg, popliteal fossa) should be performed after the cast is complete.
The chest towels are removed, and the final cast is inspected clinically and fluoroscopically at the fracture site before extubation. The cast is trimmed as needed to ensure room for perineal care, as well as full ankle flexion and extension without impingement. Cast edges are further petaled with plastic tape (Hy-Tape International) to provide padding and prevent the waterproof lining from tearing.
Postoperative care involves overnight observation and caregiver practice in perineal care. Frequent rotation from supine to prone is encouraged. Nurses confirm car-seat fit before discharge. If needed, radiographs are obtained 7 to 10 days later to help with wedging adjustment. The cast is removed in the clinic when adequate callus is appreciated on subsequent radiographs.
Case Series
Our experience with this technique in 16 unilateral femur fractures has been favorable (Table). Patient age ranged from 5 months to 3 years. Mean pretreatment angulation was 13° varus and 11° procurvatum. The majority of fractures were femoral shaft fractures; 1 was proximal, 2 distal.
All fractures united without cast revision. Mean cast time was 4.5 weeks (range, 16 days–6 weeks). Immediate postoperative alignment was 2.5° varus (range, 11° valgus to 16° varus) and 7° procurvatum (range, 1° recurvatum to 22° procurvatum). Mean shortening was 1.5 cm (range, 0-2.7 cm). Final alignment was 1° valgus (range, 9° valgus to 12° varus) and 5° procurvatum (range, 0° to 22°). Mean follow-up was 8 months. There were no cases of skin maceration or cast failure. No casts precluded use of a spica car-seat. Figure 4 shows a typical case with a midshaft fracture treated with closed reduction and casting for 4 weeks with good remodeling at final follow-up, 19 months after injury.
Discussion
Although single-leg walking spica casts have been shown to safely treat low-energy femur fractures in children 1 to 6 years old,7 length-unstable femur fractures, bilateral femur fractures, and patients with hip dysplasia continue to be managed with a double-leg hip spica construct. Cast integrity remains fundamental to the control of most fractures and prevention of cast-related complications, such as skin maceration and ulceration. Surgeons typically use spica cast reinforcement schemes—such as cast augments of the torso–limb junction, with multiple layers of casting material or incorporation of a connecting bar between the legs, typically constructed by overwrapping a wooden dowel in casting material—to improve the mechanical stability of casts.6 The present technique of creating a connecting bar from fiberglass casting material significantly simplifies the standard wooden dowel approach and provided excellent results in our treatment group in terms of cast integrity and fracture alignment. In addition, at our institution, a roll of fiberglass costs $2.10, whereas a wooden dowel costs $3 to $10 and can be difficult to locate if not frequently used. Other tube-shaped materials, such as the disposable material used to package implants and tubes, carry an even lower cost. However, we have found that a single fiberglass roll is most readily available and easiest to apply.
Although proper spica cast application remains important in managing pediatric trauma, it lacks a good technical description in the literature. In this technical report, we have presented our standard spica cast application method, which minimizes the range of cast complications that have been reported, from minor skin irritation to superior mesenteric artery syndrome. Two salient technical highlights are use of waterproof pantaloon liners and cast petaling, which we have found almost eliminate the morbidity of potential skin complications, reported to occur at a rate of 28%.8 In addition, we forgo applying the cast on the injured leg in segments. Application of a short-leg cast on the injured leg to allow traction on the leg during cast application is of dubious utility and may be potentially harmful, with described complications of peroneal nerve palsy and compartment syndrome.9-11 Further, it is important to use an abdominal spacer (eg, a stack of towels) under the cast padding to create room for abdominal expansion and minimize pressure thought to induce superior mesenteric artery syndrome. Plastic or rubber abdominal spacers have also been described.12,13 Last, leg position is important for reduction and maintenance of the fracture, as well as patient care. Literature advocates minimizing hip abduction to just that needed for perineal care and maximizing hip flexion and knee extension to optimize car-seat fit and safety.14
Conclusion
Construction of a spica cast lower limb connecting bar from readily available fiberglass casting material allows a facile and rapid addition to the mechanical stability of a spica cast in the treatment of pediatric femur fractures. The technique is low-cost and obviates the need for additional extraneous materials.
Femur fractures (Orthopaedic Trauma Association classes 31, 32, 33)1 are common childhood injuries, occurring at a rate of 19 per 100,000 children in the United States.2 Peak occurrence is bimodal at ages 2 and 17 years. The most common mechanism of injury in children under 6 years is a fall, and hip spica casting is the preferred treatment modality in this group.3-5
A bar connecting the legs of the spica cast has been shown to facilitate patient transport5 and significantly decrease mechanical failure of the spica cast.6 This bar often consists of a broom handle or pipe that must be cut to size during the case and subsequently incorporated into the cast—tasks that are often inconvenient and time-consuming for on-call or emergency department staff unfamiliar with orthopedic tools.
In this article, we review a spica cast application that incorporates a low-cost, lightweight technique for fabricating a connecting bar from existing fiberglass casting material. The Institutional Review Board at Connecticut Children’s Medical Center approved this work.
Technique of Double-Leg Spica Casting With Fiberglass Bar
A spica casting table (Orthopedic Systems) with a well-padded post is placed on the operating room table and adjusted to the length of the patient from perineum to just below the shoulders. With the patient under general anesthesia, folded towels are used to provide 2 to 4 cm of padding on the anterior torso, atop which a waterproof pantaloon is applied. The patient is transferred to the spica table, and the patient’s arms are gently secured to the casting table with cast padding or tape in an abducted position at the shoulders. A surgeon controls the legs by holding the feet with the long fingers just above the heels, the index fingers on the anterior ankle, and the thumbs on the soles of the feet. Cast padding is wrapped from the nipple line to the supramalleolar region on each leg. The bony prominences of the malleoli, patella, fibular head, femoral condyles, iliac crests, and coccyx are well padded.
Fiberglass is then rolled without compression onto the patient, beginning with the torso and perineal areas. The injured leg is wrapped to its final length above the malleoli while the uninjured leg is kept free. Maintaining the position of the injured leg with simultaneous molding at the fracture site, typically to promote valgus, allows fracture reduction. The fracture position is then checked under image intensification. For femur fractures, hip abduction and flexion are set to 45° and 90°, respectively, while knee flexion is between 50° and 90°. The uninjured leg is then wrapped with fiberglass. Additional strips of fiberglass can be used to reinforce weak junctional regions between the torso and the legs, posteriorly over the “intern’s triangle” and anteriorly along the hip crease.
A connecting fiberglass bar is then created using a fiberglass roll once the cast is hardened. A 2-inch fiberglass roll is wrapped around one leg to secure its position (Figure 1A) and then rolled around the second limb (Figure 1B). Fiberglass is then pulled taut and rolled around the bridge that has been created in order to thicken the bar (Figure 2). The roll is again brought around the closest limb, wrapped back across the bridge to the other limb, and rolled out to its full length. Last, the legs are abducted 1 to 2 cm to tension the bar (Figure 3). Although this does not produce enough movement to cause a crease and a resultant ulcer, careful inspection of common pressure points (eg, popliteal fossa) should be performed after the cast is complete.
The chest towels are removed, and the final cast is inspected clinically and fluoroscopically at the fracture site before extubation. The cast is trimmed as needed to ensure room for perineal care, as well as full ankle flexion and extension without impingement. Cast edges are further petaled with plastic tape (Hy-Tape International) to provide padding and prevent the waterproof lining from tearing.
Postoperative care involves overnight observation and caregiver practice in perineal care. Frequent rotation from supine to prone is encouraged. Nurses confirm car-seat fit before discharge. If needed, radiographs are obtained 7 to 10 days later to help with wedging adjustment. The cast is removed in the clinic when adequate callus is appreciated on subsequent radiographs.
Case Series
Our experience with this technique in 16 unilateral femur fractures has been favorable (Table). Patient age ranged from 5 months to 3 years. Mean pretreatment angulation was 13° varus and 11° procurvatum. The majority of fractures were femoral shaft fractures; 1 was proximal, 2 distal.
All fractures united without cast revision. Mean cast time was 4.5 weeks (range, 16 days–6 weeks). Immediate postoperative alignment was 2.5° varus (range, 11° valgus to 16° varus) and 7° procurvatum (range, 1° recurvatum to 22° procurvatum). Mean shortening was 1.5 cm (range, 0-2.7 cm). Final alignment was 1° valgus (range, 9° valgus to 12° varus) and 5° procurvatum (range, 0° to 22°). Mean follow-up was 8 months. There were no cases of skin maceration or cast failure. No casts precluded use of a spica car-seat. Figure 4 shows a typical case with a midshaft fracture treated with closed reduction and casting for 4 weeks with good remodeling at final follow-up, 19 months after injury.
Discussion
Although single-leg walking spica casts have been shown to safely treat low-energy femur fractures in children 1 to 6 years old,7 length-unstable femur fractures, bilateral femur fractures, and patients with hip dysplasia continue to be managed with a double-leg hip spica construct. Cast integrity remains fundamental to the control of most fractures and prevention of cast-related complications, such as skin maceration and ulceration. Surgeons typically use spica cast reinforcement schemes—such as cast augments of the torso–limb junction, with multiple layers of casting material or incorporation of a connecting bar between the legs, typically constructed by overwrapping a wooden dowel in casting material—to improve the mechanical stability of casts.6 The present technique of creating a connecting bar from fiberglass casting material significantly simplifies the standard wooden dowel approach and provided excellent results in our treatment group in terms of cast integrity and fracture alignment. In addition, at our institution, a roll of fiberglass costs $2.10, whereas a wooden dowel costs $3 to $10 and can be difficult to locate if not frequently used. Other tube-shaped materials, such as the disposable material used to package implants and tubes, carry an even lower cost. However, we have found that a single fiberglass roll is most readily available and easiest to apply.
Although proper spica cast application remains important in managing pediatric trauma, it lacks a good technical description in the literature. In this technical report, we have presented our standard spica cast application method, which minimizes the range of cast complications that have been reported, from minor skin irritation to superior mesenteric artery syndrome. Two salient technical highlights are use of waterproof pantaloon liners and cast petaling, which we have found almost eliminate the morbidity of potential skin complications, reported to occur at a rate of 28%.8 In addition, we forgo applying the cast on the injured leg in segments. Application of a short-leg cast on the injured leg to allow traction on the leg during cast application is of dubious utility and may be potentially harmful, with described complications of peroneal nerve palsy and compartment syndrome.9-11 Further, it is important to use an abdominal spacer (eg, a stack of towels) under the cast padding to create room for abdominal expansion and minimize pressure thought to induce superior mesenteric artery syndrome. Plastic or rubber abdominal spacers have also been described.12,13 Last, leg position is important for reduction and maintenance of the fracture, as well as patient care. Literature advocates minimizing hip abduction to just that needed for perineal care and maximizing hip flexion and knee extension to optimize car-seat fit and safety.14
Conclusion
Construction of a spica cast lower limb connecting bar from readily available fiberglass casting material allows a facile and rapid addition to the mechanical stability of a spica cast in the treatment of pediatric femur fractures. The technique is low-cost and obviates the need for additional extraneous materials.
1. Slongo TF, Audigé L; AO Pediatric Classification Group. Fracture and dislocation classification compendium for children: the AO Pediatric Comprehensive Classification of Long Bone Fractures (PCCF). J Orthop Trauma. 2007;21(10):S135-S160.
2. Hinton RY, Lincoln A, Crockett MM, Sponseller P, Smith G. Fractures of the femoral shaft in children. Incidence, mechanisms, and sociodemographic risk factors. J Bone Joint Surg Am. 1999;81(4):500-509.
3. Campbell WC, Canale ST, Beaty JH, eds. Campbell’s Operative Orthopaedics. 11th ed. Philadelphia, PA: Mosby Elsevier; 2008.
4. Lovell WW, Winter RB, Morrissy RT, Weinstein SL. Lovell and Winter’s Pediatric Orthopaedics. Philadelphia, PA: Lippincott Williams & Wilkins; 2006.
5. Green NE, Swiontkowski MF, eds. Skeletal Trauma in Children. 4th ed. Philadelphia, PA: Elsevier Health Sciences; 2009.
6. Hosalkar HS, Jones S, Chowdhury M, Chatoo M, Hill RA. Connecting bar for hip spica reinforcement: does it help? J Pediatr Orthop B. 2003;12(2):100-102.
7. Flynn JM, Garner MR, Jones KJ, et al. The treatment of low-energy femoral shaft fractures: a prospective study comparing the “walking spica” with the traditional spica cast. J Bone Joint Surg Am. 2011;93(23):2196-2202.
8. DiFazio R, Vessey J, Zurakowski D, Hresko MT, Matheney T. Incidence of skin complications and associated charges in children treated with hip spica casts for femur fractures. J Pediatr Orthop. 2011;31(1):17-22.
9. Weiss AP, Schenck RC Jr, Sponseller PD, Thompson JD. Peroneal nerve palsy after early cast application for femoral fractures in children. J Pediatr Orthop. 1992;12(1):25-28.
10. Mubarak SJ, Frick S, Sink E, Rathjen K, Noonan KJ. Volkmann contracture and compartment syndromes after femur fractures in children treated with 90/90 spica casts. J Pediatr Orthop. 2006;26(5):567-572.
11. Large TM, Frick SL. Compartment syndrome of the leg after treatment of a femoral fracture with an early sitting spica cast. A report of two cases. J Bone Joint Surg Am. 2003;85(11):2207-2210.
12. Sharma S, Azzopardi T. Reduction of abdominal pressure for prophylaxis of the mesenteric artery syndrome (cast syndrome) in a hip spica—a simple technique. Ann R Coll Surg Engl. 2006;88(3):317.
13. Kiter E, Demirkan F, Kiliç BA, Erkula G. A new technique for creating an abdominal window in a hip spica cast. J Orthop Trauma. 2003;17(6):442-443.
14. Zielinski J, Oliver G, Sybesma J, Walter N, Atkinson P. Casting technique and restraint choice influence child safety during transport of body casted children subjected to a simulated frontal MVA. J Trauma. 2009;66(6):1653-1665.
1. Slongo TF, Audigé L; AO Pediatric Classification Group. Fracture and dislocation classification compendium for children: the AO Pediatric Comprehensive Classification of Long Bone Fractures (PCCF). J Orthop Trauma. 2007;21(10):S135-S160.
2. Hinton RY, Lincoln A, Crockett MM, Sponseller P, Smith G. Fractures of the femoral shaft in children. Incidence, mechanisms, and sociodemographic risk factors. J Bone Joint Surg Am. 1999;81(4):500-509.
3. Campbell WC, Canale ST, Beaty JH, eds. Campbell’s Operative Orthopaedics. 11th ed. Philadelphia, PA: Mosby Elsevier; 2008.
4. Lovell WW, Winter RB, Morrissy RT, Weinstein SL. Lovell and Winter’s Pediatric Orthopaedics. Philadelphia, PA: Lippincott Williams & Wilkins; 2006.
5. Green NE, Swiontkowski MF, eds. Skeletal Trauma in Children. 4th ed. Philadelphia, PA: Elsevier Health Sciences; 2009.
6. Hosalkar HS, Jones S, Chowdhury M, Chatoo M, Hill RA. Connecting bar for hip spica reinforcement: does it help? J Pediatr Orthop B. 2003;12(2):100-102.
7. Flynn JM, Garner MR, Jones KJ, et al. The treatment of low-energy femoral shaft fractures: a prospective study comparing the “walking spica” with the traditional spica cast. J Bone Joint Surg Am. 2011;93(23):2196-2202.
8. DiFazio R, Vessey J, Zurakowski D, Hresko MT, Matheney T. Incidence of skin complications and associated charges in children treated with hip spica casts for femur fractures. J Pediatr Orthop. 2011;31(1):17-22.
9. Weiss AP, Schenck RC Jr, Sponseller PD, Thompson JD. Peroneal nerve palsy after early cast application for femoral fractures in children. J Pediatr Orthop. 1992;12(1):25-28.
10. Mubarak SJ, Frick S, Sink E, Rathjen K, Noonan KJ. Volkmann contracture and compartment syndromes after femur fractures in children treated with 90/90 spica casts. J Pediatr Orthop. 2006;26(5):567-572.
11. Large TM, Frick SL. Compartment syndrome of the leg after treatment of a femoral fracture with an early sitting spica cast. A report of two cases. J Bone Joint Surg Am. 2003;85(11):2207-2210.
12. Sharma S, Azzopardi T. Reduction of abdominal pressure for prophylaxis of the mesenteric artery syndrome (cast syndrome) in a hip spica—a simple technique. Ann R Coll Surg Engl. 2006;88(3):317.
13. Kiter E, Demirkan F, Kiliç BA, Erkula G. A new technique for creating an abdominal window in a hip spica cast. J Orthop Trauma. 2003;17(6):442-443.
14. Zielinski J, Oliver G, Sybesma J, Walter N, Atkinson P. Casting technique and restraint choice influence child safety during transport of body casted children subjected to a simulated frontal MVA. J Trauma. 2009;66(6):1653-1665.