User login
Official Newspaper of the American College of Surgeons
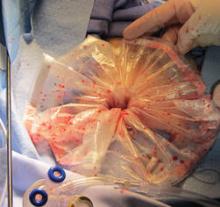
FDA panel addresses risks of power morcellators in fibroid surgery
SILVER SPRING, MD. – Expert advisers expressed little confidence in the Food and Drug Administration estimate that women who undergo a hysterectomy or myomectomy for a presumed fibroid have about a 1 in 350 chance of having an unsuspected sarcoma – the first of several issues the advisers will address at a 2-day meeting on the risks of power morcellation in this setting.
Members of the FDA Obstetrics and Gynecology Devices Advisory Committee said July 10 that they considered the data weak and confusing. Several pointed out that the studies used in the FDA analysis were limited by publication bias, that many patients with fibroids were not included in these estimates, and that better estimates could be obtained with other data sources.
Based on the FDA estimates, clinicians would expect to see about one case of an undiagnosed sarcoma per year, but most do not, one panelist noted.
The estimate is based on an analysis of nine studies of unsuspected uterine sarcomas and leiomyosarcomas (LMSs) treated with a morcellator during a hysterectomy or myomectomy, published between 1990 and 2012. The agency estimated that peritoneal dissemination and/or cancer up-staging to FIGO stage III/IV occurs in about 25%-64% of cases where a uterine sarcoma is morcellated, and that these women have poorer disease-free survival and overall survival than women not treated with morcellation.
The FDA convened the advisory panel meeting to address the benefits, risks, and clinical role of laparoscopic power morcellators (LPMs) in the treatment of women with uterine fibroids, and the use of what the agency described as “potential” mitigation strategies that might reduce or eliminate the risks of disseminating cancerous tissue into the pelvis and abdomen of women with an unsuspected uterine sarcoma or LMS.
In April, the FDA issued a safety communication about the risks of spreading cancerous tissue beyond the uterus when morcellation is used in a patient with an unsuspected uterine sarcoma – and first cited the 1-in-350 risk, notably higher than previously reported rates.The FDA recommended that the use of LPMs during a hysterectomy or myomectomy in women with fibroids should be discouraged and that physicians should discuss alternative treatment options with these patients. But if power morcellation was considered the best option, patients should be informed that their fibroids “may contain unexpected cancerous tissue and that laparoscopic power morcellation may spread the cancer, significantly worsening their prognosis,” the FDA advisory said.
At the meeting, Dr. Ron Yustein, of the FDA Office of Surveillance and Biometrics, said that as of June 22, the voluntary FDA medical device adverse event reporting program had received 21 reports of dissemination of malignancy (mostly LMS) after morcellation of suspected fibroids, including seven reported to be fatal.
Family members of women who had developed disseminated disease after morcellation of an unsuspected sarcoma interrupted the panel discussion to point out they are aware of at least 130 such cases.
LPMs, also cleared for general surgery and urologic indications, are considered class II “moderate risk” devices and were cleared for use based on older devices that were already on the market, with little if any clinical testing in humans. The first LPM for a gynecologic indication was cleared in 1995. LPMs include electromechanical morcellators, which typically have spinning blades to cut up the tissue, and one electrosurgical device that uses radiofrequency energy.
Clinical data are required for class III medical devices to be approved, and the FDA is considering whether to require clinical data for LPMs used in gynecologic indications.
Dr. Craig Sobolewski, who spoke about surgical options for fibroids at the FDA’s invitation, said that the use of specimen collection bags during morcellation dropped because the varied sizes of specimens make it difficult to use bags, and it can be difficult to view a spinning blade through the bag.
The use of collection bags was “minuscule,” but has increased subsequent to the April FDA advisory, said Dr. Sobolewski, chief of the division of minimally invasive gynecologic surgery, Duke University, Durham, N.C.
One “very positive outcome” of this attention will be innovations to address this risk, which will “hopefully enable us to continue to offer this approach ... while simultaneously mitigating the risk,” he said.
The meeting continues July 11, when the panel will address labeling of these devices to reflect the risk of morcellating an unsuspected sarcoma. The panel will not be asked whether these devices should be taken off the market.
Dr. Sobolewski disclosed being a consultant and speaker for Covidien, and is a consultant for and holds stock options in TransEnterix. (Neither company has morcellation products; Covidien manufactures specimen retrieval bags.) None of the panel members has relevant disclosures.
SILVER SPRING, MD. – Expert advisers expressed little confidence in the Food and Drug Administration estimate that women who undergo a hysterectomy or myomectomy for a presumed fibroid have about a 1 in 350 chance of having an unsuspected sarcoma – the first of several issues the advisers will address at a 2-day meeting on the risks of power morcellation in this setting.
Members of the FDA Obstetrics and Gynecology Devices Advisory Committee said July 10 that they considered the data weak and confusing. Several pointed out that the studies used in the FDA analysis were limited by publication bias, that many patients with fibroids were not included in these estimates, and that better estimates could be obtained with other data sources.
Based on the FDA estimates, clinicians would expect to see about one case of an undiagnosed sarcoma per year, but most do not, one panelist noted.
The estimate is based on an analysis of nine studies of unsuspected uterine sarcomas and leiomyosarcomas (LMSs) treated with a morcellator during a hysterectomy or myomectomy, published between 1990 and 2012. The agency estimated that peritoneal dissemination and/or cancer up-staging to FIGO stage III/IV occurs in about 25%-64% of cases where a uterine sarcoma is morcellated, and that these women have poorer disease-free survival and overall survival than women not treated with morcellation.
The FDA convened the advisory panel meeting to address the benefits, risks, and clinical role of laparoscopic power morcellators (LPMs) in the treatment of women with uterine fibroids, and the use of what the agency described as “potential” mitigation strategies that might reduce or eliminate the risks of disseminating cancerous tissue into the pelvis and abdomen of women with an unsuspected uterine sarcoma or LMS.
In April, the FDA issued a safety communication about the risks of spreading cancerous tissue beyond the uterus when morcellation is used in a patient with an unsuspected uterine sarcoma – and first cited the 1-in-350 risk, notably higher than previously reported rates.The FDA recommended that the use of LPMs during a hysterectomy or myomectomy in women with fibroids should be discouraged and that physicians should discuss alternative treatment options with these patients. But if power morcellation was considered the best option, patients should be informed that their fibroids “may contain unexpected cancerous tissue and that laparoscopic power morcellation may spread the cancer, significantly worsening their prognosis,” the FDA advisory said.
At the meeting, Dr. Ron Yustein, of the FDA Office of Surveillance and Biometrics, said that as of June 22, the voluntary FDA medical device adverse event reporting program had received 21 reports of dissemination of malignancy (mostly LMS) after morcellation of suspected fibroids, including seven reported to be fatal.
Family members of women who had developed disseminated disease after morcellation of an unsuspected sarcoma interrupted the panel discussion to point out they are aware of at least 130 such cases.
LPMs, also cleared for general surgery and urologic indications, are considered class II “moderate risk” devices and were cleared for use based on older devices that were already on the market, with little if any clinical testing in humans. The first LPM for a gynecologic indication was cleared in 1995. LPMs include electromechanical morcellators, which typically have spinning blades to cut up the tissue, and one electrosurgical device that uses radiofrequency energy.
Clinical data are required for class III medical devices to be approved, and the FDA is considering whether to require clinical data for LPMs used in gynecologic indications.
Dr. Craig Sobolewski, who spoke about surgical options for fibroids at the FDA’s invitation, said that the use of specimen collection bags during morcellation dropped because the varied sizes of specimens make it difficult to use bags, and it can be difficult to view a spinning blade through the bag.
The use of collection bags was “minuscule,” but has increased subsequent to the April FDA advisory, said Dr. Sobolewski, chief of the division of minimally invasive gynecologic surgery, Duke University, Durham, N.C.
One “very positive outcome” of this attention will be innovations to address this risk, which will “hopefully enable us to continue to offer this approach ... while simultaneously mitigating the risk,” he said.
The meeting continues July 11, when the panel will address labeling of these devices to reflect the risk of morcellating an unsuspected sarcoma. The panel will not be asked whether these devices should be taken off the market.
Dr. Sobolewski disclosed being a consultant and speaker for Covidien, and is a consultant for and holds stock options in TransEnterix. (Neither company has morcellation products; Covidien manufactures specimen retrieval bags.) None of the panel members has relevant disclosures.
SILVER SPRING, MD. – Expert advisers expressed little confidence in the Food and Drug Administration estimate that women who undergo a hysterectomy or myomectomy for a presumed fibroid have about a 1 in 350 chance of having an unsuspected sarcoma – the first of several issues the advisers will address at a 2-day meeting on the risks of power morcellation in this setting.
Members of the FDA Obstetrics and Gynecology Devices Advisory Committee said July 10 that they considered the data weak and confusing. Several pointed out that the studies used in the FDA analysis were limited by publication bias, that many patients with fibroids were not included in these estimates, and that better estimates could be obtained with other data sources.
Based on the FDA estimates, clinicians would expect to see about one case of an undiagnosed sarcoma per year, but most do not, one panelist noted.
The estimate is based on an analysis of nine studies of unsuspected uterine sarcomas and leiomyosarcomas (LMSs) treated with a morcellator during a hysterectomy or myomectomy, published between 1990 and 2012. The agency estimated that peritoneal dissemination and/or cancer up-staging to FIGO stage III/IV occurs in about 25%-64% of cases where a uterine sarcoma is morcellated, and that these women have poorer disease-free survival and overall survival than women not treated with morcellation.
The FDA convened the advisory panel meeting to address the benefits, risks, and clinical role of laparoscopic power morcellators (LPMs) in the treatment of women with uterine fibroids, and the use of what the agency described as “potential” mitigation strategies that might reduce or eliminate the risks of disseminating cancerous tissue into the pelvis and abdomen of women with an unsuspected uterine sarcoma or LMS.
In April, the FDA issued a safety communication about the risks of spreading cancerous tissue beyond the uterus when morcellation is used in a patient with an unsuspected uterine sarcoma – and first cited the 1-in-350 risk, notably higher than previously reported rates.The FDA recommended that the use of LPMs during a hysterectomy or myomectomy in women with fibroids should be discouraged and that physicians should discuss alternative treatment options with these patients. But if power morcellation was considered the best option, patients should be informed that their fibroids “may contain unexpected cancerous tissue and that laparoscopic power morcellation may spread the cancer, significantly worsening their prognosis,” the FDA advisory said.
At the meeting, Dr. Ron Yustein, of the FDA Office of Surveillance and Biometrics, said that as of June 22, the voluntary FDA medical device adverse event reporting program had received 21 reports of dissemination of malignancy (mostly LMS) after morcellation of suspected fibroids, including seven reported to be fatal.
Family members of women who had developed disseminated disease after morcellation of an unsuspected sarcoma interrupted the panel discussion to point out they are aware of at least 130 such cases.
LPMs, also cleared for general surgery and urologic indications, are considered class II “moderate risk” devices and were cleared for use based on older devices that were already on the market, with little if any clinical testing in humans. The first LPM for a gynecologic indication was cleared in 1995. LPMs include electromechanical morcellators, which typically have spinning blades to cut up the tissue, and one electrosurgical device that uses radiofrequency energy.
Clinical data are required for class III medical devices to be approved, and the FDA is considering whether to require clinical data for LPMs used in gynecologic indications.
Dr. Craig Sobolewski, who spoke about surgical options for fibroids at the FDA’s invitation, said that the use of specimen collection bags during morcellation dropped because the varied sizes of specimens make it difficult to use bags, and it can be difficult to view a spinning blade through the bag.
The use of collection bags was “minuscule,” but has increased subsequent to the April FDA advisory, said Dr. Sobolewski, chief of the division of minimally invasive gynecologic surgery, Duke University, Durham, N.C.
One “very positive outcome” of this attention will be innovations to address this risk, which will “hopefully enable us to continue to offer this approach ... while simultaneously mitigating the risk,” he said.
The meeting continues July 11, when the panel will address labeling of these devices to reflect the risk of morcellating an unsuspected sarcoma. The panel will not be asked whether these devices should be taken off the market.
Dr. Sobolewski disclosed being a consultant and speaker for Covidien, and is a consultant for and holds stock options in TransEnterix. (Neither company has morcellation products; Covidien manufactures specimen retrieval bags.) None of the panel members has relevant disclosures.
Obesity malpractice claims up 64%, study shows
Obesity-related lawsuits against health providers are on the rise, according to a claims analysis by national medical liability insurer The Doctors Company. Claims associated with obesity totaled 415 between 2007 and 2012, an increase of 64% in the number of such lawsuits from the period between 1992 and 2002.
The increase "is not at all surprising, as obesity itself has increased a great deal in the population," said Dr. Neil Skolnik, associate director of the family practice residency program at Abington (Pa.) Memorial Hospital. "We’re going to see that reflected in malpractice suits."
The Doctors Company study analyzed 415 obesity-related claims from 2007 through 2012 and compared them with a previous analysis of claims from 1992 to 2002. The most recent suits involved instances such as a 290-pound patient who had complications following a gastric bypass surgery, a 325-pound patient with hypertension and diabetes who died after a physician did not diagnose myocardial infarction and pneumonia, and a 350-pound patient with nerve damage that resulted from improper positioning on a treatment table. Twenty-five percent of the patients involved in the claims died, according to the study, published on The Doctors Company website in May.
Orthopedists were the top specialty sued for obesity-related claims, followed by family physicians, anesthesiologists, plastic surgeons, general surgeons, and internists.
Family physicians may be sued more often than some other specialties because of their interaction with patients who develop conditions associated with obesity over time, said Paul Nagle, director of physician patient safety for The Doctors Company.
"Family medicine physicians can be at risk due to the latent risk of obesity," he said in an interview. "If a patient’s weight gradually increases, what is a family medicine physician doing to monitor the weight and the potentially resulting medical issues, including diabetes, hypertension, cardiac, vascular, joint, or pulmonary health issues?"
Communicating clearly with obese patients early is essential to avoid obesity-related claims, said Liz Brott, regional vice president, risk resource for ProAssurance, a national medical liability insurer.
"It’s extremely important as far as communicating with them about what to expect from procedures or treatment," she said in an interview. "You don’t want to leave this to your staff to communicate. [It should be] the physician sitting down, one-on-one, whether it’s the primary care physician or the surgeon. There should be appropriate communication by the physician."
Ms. Brott said ProAssurance has handled obesity-related claims continually, but their numbers do not show a dramatic rise. In her experience, obesity claims against surgeons related to bariatric procedures are most common, she said.
Along with informed consent, physicians also may want to consider an informed refusal approach for noncompliant patients, Mr. Nagle said. This refers to informing patients about how obesity impacts their overall health and/or a specific health issue and having patients sign a document stating that they were advised but chose not to follow recommendations.
"This may reduce any claims that you did not address and warn of obesity’s impact," he said.
Physicians should also document all activity related to weight interventions and conversations with patients about weight education and guidance, he added
The analysis is a reminder that obesity is a serious disease that has its own direct complications, as well as acting as a catalyst for related conditions, said Dr. Skolnik, who is also a professor of family medicine at Temple University in Philadelphia.
"The overriding theme here is obesity is an area to which patients are paying increasing attention and to which lawyers also must be paying increasing attention," he said. "It’s an area that should be addressed directly and with sensitivity to the often emotional issues that go along with addressing weight issues."
Obesity-related lawsuits against health providers are on the rise, according to a claims analysis by national medical liability insurer The Doctors Company. Claims associated with obesity totaled 415 between 2007 and 2012, an increase of 64% in the number of such lawsuits from the period between 1992 and 2002.
The increase "is not at all surprising, as obesity itself has increased a great deal in the population," said Dr. Neil Skolnik, associate director of the family practice residency program at Abington (Pa.) Memorial Hospital. "We’re going to see that reflected in malpractice suits."
The Doctors Company study analyzed 415 obesity-related claims from 2007 through 2012 and compared them with a previous analysis of claims from 1992 to 2002. The most recent suits involved instances such as a 290-pound patient who had complications following a gastric bypass surgery, a 325-pound patient with hypertension and diabetes who died after a physician did not diagnose myocardial infarction and pneumonia, and a 350-pound patient with nerve damage that resulted from improper positioning on a treatment table. Twenty-five percent of the patients involved in the claims died, according to the study, published on The Doctors Company website in May.
Orthopedists were the top specialty sued for obesity-related claims, followed by family physicians, anesthesiologists, plastic surgeons, general surgeons, and internists.
Family physicians may be sued more often than some other specialties because of their interaction with patients who develop conditions associated with obesity over time, said Paul Nagle, director of physician patient safety for The Doctors Company.
"Family medicine physicians can be at risk due to the latent risk of obesity," he said in an interview. "If a patient’s weight gradually increases, what is a family medicine physician doing to monitor the weight and the potentially resulting medical issues, including diabetes, hypertension, cardiac, vascular, joint, or pulmonary health issues?"
Communicating clearly with obese patients early is essential to avoid obesity-related claims, said Liz Brott, regional vice president, risk resource for ProAssurance, a national medical liability insurer.
"It’s extremely important as far as communicating with them about what to expect from procedures or treatment," she said in an interview. "You don’t want to leave this to your staff to communicate. [It should be] the physician sitting down, one-on-one, whether it’s the primary care physician or the surgeon. There should be appropriate communication by the physician."
Ms. Brott said ProAssurance has handled obesity-related claims continually, but their numbers do not show a dramatic rise. In her experience, obesity claims against surgeons related to bariatric procedures are most common, she said.
Along with informed consent, physicians also may want to consider an informed refusal approach for noncompliant patients, Mr. Nagle said. This refers to informing patients about how obesity impacts their overall health and/or a specific health issue and having patients sign a document stating that they were advised but chose not to follow recommendations.
"This may reduce any claims that you did not address and warn of obesity’s impact," he said.
Physicians should also document all activity related to weight interventions and conversations with patients about weight education and guidance, he added
The analysis is a reminder that obesity is a serious disease that has its own direct complications, as well as acting as a catalyst for related conditions, said Dr. Skolnik, who is also a professor of family medicine at Temple University in Philadelphia.
"The overriding theme here is obesity is an area to which patients are paying increasing attention and to which lawyers also must be paying increasing attention," he said. "It’s an area that should be addressed directly and with sensitivity to the often emotional issues that go along with addressing weight issues."
Obesity-related lawsuits against health providers are on the rise, according to a claims analysis by national medical liability insurer The Doctors Company. Claims associated with obesity totaled 415 between 2007 and 2012, an increase of 64% in the number of such lawsuits from the period between 1992 and 2002.
The increase "is not at all surprising, as obesity itself has increased a great deal in the population," said Dr. Neil Skolnik, associate director of the family practice residency program at Abington (Pa.) Memorial Hospital. "We’re going to see that reflected in malpractice suits."
The Doctors Company study analyzed 415 obesity-related claims from 2007 through 2012 and compared them with a previous analysis of claims from 1992 to 2002. The most recent suits involved instances such as a 290-pound patient who had complications following a gastric bypass surgery, a 325-pound patient with hypertension and diabetes who died after a physician did not diagnose myocardial infarction and pneumonia, and a 350-pound patient with nerve damage that resulted from improper positioning on a treatment table. Twenty-five percent of the patients involved in the claims died, according to the study, published on The Doctors Company website in May.
Orthopedists were the top specialty sued for obesity-related claims, followed by family physicians, anesthesiologists, plastic surgeons, general surgeons, and internists.
Family physicians may be sued more often than some other specialties because of their interaction with patients who develop conditions associated with obesity over time, said Paul Nagle, director of physician patient safety for The Doctors Company.
"Family medicine physicians can be at risk due to the latent risk of obesity," he said in an interview. "If a patient’s weight gradually increases, what is a family medicine physician doing to monitor the weight and the potentially resulting medical issues, including diabetes, hypertension, cardiac, vascular, joint, or pulmonary health issues?"
Communicating clearly with obese patients early is essential to avoid obesity-related claims, said Liz Brott, regional vice president, risk resource for ProAssurance, a national medical liability insurer.
"It’s extremely important as far as communicating with them about what to expect from procedures or treatment," she said in an interview. "You don’t want to leave this to your staff to communicate. [It should be] the physician sitting down, one-on-one, whether it’s the primary care physician or the surgeon. There should be appropriate communication by the physician."
Ms. Brott said ProAssurance has handled obesity-related claims continually, but their numbers do not show a dramatic rise. In her experience, obesity claims against surgeons related to bariatric procedures are most common, she said.
Along with informed consent, physicians also may want to consider an informed refusal approach for noncompliant patients, Mr. Nagle said. This refers to informing patients about how obesity impacts their overall health and/or a specific health issue and having patients sign a document stating that they were advised but chose not to follow recommendations.
"This may reduce any claims that you did not address and warn of obesity’s impact," he said.
Physicians should also document all activity related to weight interventions and conversations with patients about weight education and guidance, he added
The analysis is a reminder that obesity is a serious disease that has its own direct complications, as well as acting as a catalyst for related conditions, said Dr. Skolnik, who is also a professor of family medicine at Temple University in Philadelphia.
"The overriding theme here is obesity is an area to which patients are paying increasing attention and to which lawyers also must be paying increasing attention," he said. "It’s an area that should be addressed directly and with sensitivity to the often emotional issues that go along with addressing weight issues."
Helping breast cancer patients analyze risk
Dr. Sarah Hawley and her coinvestigators are to be applauded for generating insightful data regarding factors and concerns that motivate a woman to undergo contralateral prophylactic mastectomy in the setting of unilateral breast cancer (JAMA Surgery 2014 May 21 [doi:10.1001/jamasurg.2013.5689]).
Hawley et al. found that fear of recurrence was one of the strongest factors leading women to choose contralateral prophylactic mastectomy (CPM). This finding clearly demonstrates that we need to do a better job of explaining and defining the significance of (i) breast cancer local recurrence; (ii) breast cancer distant recurrence; and (iii) the development of a new/second primary breast cancer. Since cross-metastasis of a primary breast cancer to the contralateral breast is an extremely rare event, and since distant metastasis from the initial primary breast cancer tends to determine survival rates, CPM by definition will influence the incidence of only the third pattern. Furthermore, since the risk of experiencing a new contralateral malignancy is less than 1% per year for the general population of breast cancer patients, only a minority of these women will actually become bilateral breast cancer patients. Fear of recurrence is therefore a totally inappropriate reason for patients to pursue CPM, and the reasonableness of CPM to reduce the risk of a contralateral new primary breast cancer is debatable.
It can be reasonably stated that prophylactic surgery by definition is never a medically indicated necessity. Furthermore, despite the fact that a personal history of breast cancer is indeed a risk factor for developing a second primary cancer in the contralateral breast, numerous studies have demonstrated equivalent survival rates for women with unilateral breast cancer, compared with those diagnosed with bilateral/metachronous breast cancer (Cancer 2001;91:1845-53; Am. J. Clin. Oncol. 1997;20:541-5). Survival tends to be driven by the stage and effectiveness of treatment for the first cancer. By virtue of its earlier presentation, it is likely that the initially diagnosed cancer has established itself as the faster-growing malignancy with a lead time advantage in establishing distant organ micrometastatic disease; furthermore, patients with a unilateral breast cancer diagnosis are generally undergoing diligent surveillance and a contralateral malignancy is more often detected at an early stage.
Messages to our patients
It is essential for those of us who manage breast cancer to clearly emphasize several messages to our newly diagnosed breast cancer patients: First, although unilateral breast cancer increases the likelihood of developing a second primary tumor, it is certainly not inevitable, and in fact, the majority of patients are not destined to develop contralateral disease. Second, reducing the risk of being diagnosed with a contralateral breast cancer does not mitigate the mortality risk associated with the first cancer. And, finally, prophylactic mastectomy is the most aggressive and effective strategy for reducing the incidence of primary breast cancer (by approximately 90%), but it does not confer complete protection, as microscopic foci of breast tissue may be left behind in the mastectomy skin flaps, along the pectoralis, or in the axilla.
The messages above are critical: Our patients must understand that the priority is to address the known cancer. In this regard, appropriately selected patients should be encouraged to strongly consider breast-conserving surgery whenever feasible, as this low-morbidity treatment is equivalent to mastectomy from the perspective of overall survival. The question of CPM is most relevant for those patients that are ineligible for breast conservation or patients unwilling to undergo lumpectomy and breast radiation.
If a mastectomy for the cancerous breast is planned, we must then address the questions that routinely arise regarding bilateral surgery. In our efforts to clarify the reality of what CPM can and cannot achieve, we must also avoid being too dogmatic and paternalistic with our patients. There are clearly specific scenarios, as delineated in Dr. Hawley’s work, where the risk of a second primary breast cancer is likely to be considered excessive by most women, and where the decision to pursue CPM may be easier. Examples of such cases would be women known to harbor BRCA mutations or women with suspected hereditary susceptibility based on a strong family history of breast and/or ovarian cancer. The risk of a new contralateral breast cancer can be in the range of 4%-5% per year in cases of hereditary disease, compared with the general population of women with sporadic breast cancer, where the risk ranges from 0.25% to 1% per year.
Conveying an understanding of risk
Patients must understand that the risk to the contralateral breast is predominantly expressed in the future – the likelihood of having a clinically occult, incidentally detected cancer identified in the contralateral mastectomy specimen is only 6%, as demonstrated most recently by King et al. (Ann. Surg. 2011;254:2-7), and with ductal carcinoma in situ accounting for the high majority of these lesions.
Defining the threshold for the amount of risk that an individual woman finds to be acceptable, however, can be a very difficult and personal decision. Even after a patient comes to understand that CPM is unlikely to provide a survival advantage, she may continue to request bilateral surgery purely for the risk-reducing benefits, and out of a desire to minimize her chances of having to repeat the breast cancer diagnosis and treatment experience. In some cases this choice will be influenced by reconstruction factors. A woman may be motivated to pursue bilateral surgery if she has an adequate volume of abdominal tissue because of the fact that the autogenous TRAM (transverse rectus abdominis myocutaneous) flap can be harvested only once. In other cases the decision is influenced by body habitus, for example, a woman with large pendulous breasts who is not interested in breast reconstruction may decide that she is more comfortable with a symmetrically flat chest wall in order to avoid chest wall imbalance and the inconvenience of finding/wearing a prosthesis that matches the remaining breast.
As breast cancer surgeons we should openly discuss these issues with our patients and present viable alternatives when feasible, such as reduction mammoplasty for the large-breasted patient. Ultimately, however, the patient must decide the surgical approach that provides her with the optimal sense of treatment satisfaction, quality of life, and comfort.
Discussion strategies
In my own practice I have found two discussion strategies to be particularly useful in guiding patients through the decision about CPM.
The first approach is relevant for women who are lumpectomy candidates, but who express a "reflex" interest in bilateral mastectomy while they are still in the emotional fog of processing the new cancer diagnosis. For these women it is obviously important to stress the survival equivalence of mastectomy and breast-conserving surgery, and this is also a great opportunity to educate patients about the potential axillary surgery advantages of breast conservation. The American College of Surgeons Oncology Group Z11 trial (JAMA 2011;305:69-75) has provided strong evidence supporting the safety of avoiding an axillary lymph node dissection (ALND) in women with sentinel lymph node (SLN) metastatic disease if the primary breast cancer is managed by lumpectomy and breast radiation.
At this point in time, we do not have comparably strong data to justify avoiding the ALND in the setting of mastectomy patients with SLN metastatic disease. The mastectomy patient with SLN metastasis is usually committed to undergo the completion axillary lymph node dissection specifically so that definitive decisions can be made regarding the need for postmastectomy radiation, and many of these patients become ineligible for immediate reconstruction because of this possible radiation. I therefore accentuate the advantage of at least initiating treatment with lumpectomy and sentinel lymph node biopsy. The patient preserves all of her surgical options with the benefit of having more staging information. If she is found to have SLN metastatic disease then she is in a better position to avoid the ALND with lumpectomy and radiation, and the option of future mastectomy and immediate reconstruction would still be available to her in the future (after completing all of her cancer treatment and healing from her radiation); if the SLN is negative, she can either continue with the breast-conservation treatment plan or she can pursue mastectomy (with or without immediate breast reconstruction, since prophylactic mamillary radiation therapy is not likely to be indicated for node-negative disease).
The second approach is relevant to the patient requiring mastectomy but for whom delayed reconstruction is planned because of medical issues or anticipated postmastectomy radiation. I encourage these patients to at least consider deferring the decision for the CPM until they return for the delayed reconstruction of the cancerous mastectomy, because at that time they can undergo the prophylactic mastectomy with the cosmetic advantages of immediate reconstruction.
Cost considerations
From the public health and population-based breast cancer burden perspectives as well as for individual patients, there are additional issues to be factored into the CPM discussion. It is a basic reality that cost is relevant when it comes to sorting out the net benefit of particular medical interventions, especially those that are prophylactic. Interestingly, a cost analysis study by Zendejas et al. (J. Clin. Oncol. 2011;29:2993-3000) from the Mayo Clinic demonstrated that CPM is actually cost effective, compared with surveillance for patients diagnosed when they are younger than 70 years of age.
The Women’s Health and Cancer Rights Act was implemented in 1999, mandating insurance coverage for breast reconstruction after mastectomy performed for cancer. This legislation promoted more widespread acceptance (and reimbursement) for contralateral mastectomy/reconstruction, but patients should nonetheless be proactive about confirming that their individual policy will indeed cover the expenses of prophylactic surgery. Furthermore, we must continue to monitor outcomes in women who choose to undergo CPM, as advances in breast cancer therapies may influence the survival benefits of this surgical approach. Indeed, selected retrospective studies have recently demonstrated that patients undergoing CPM have an improved survival, compared with those focusing on unilateral breast cancer surgery (Ann. Surg. Oncol. 2010;17:2702-9; J. Natl. Cancer Inst. 2010;102:401-9; J. Clin. Oncol. 2005;23:4275-86; Am. J. Surg. 2000;180:439-45). These results suggest a survival advantage associated with avoidance of a contralateral breast cancer, in contrast to the historical data alluded to above, regarding survival equivalence for patients with unilateral compared to metachronous bilateral breast cancer. As adjuvant systemic therapies for breast cancer continue to improve in effectiveness and ability to completely eliminate distant organ micrometastases, it is likely that we will continue to increase the pool of women who are essentially "cured" of the first cancer. This in turn could potentially increase the longevity threat of a second/metachronous cancer though a renewed metastatic risk. Nonetheless, data on possible survival advantages of CPM have not yet matured to the point where it can be recommended as a medically "indicated" procedure.
Our breast cancer patients face an abundance of very legitimate fears related to the morbidity and mortality risks of the actual cancer as well as the adverse effects and toxicities of treatment for that cancer. Fortunately, we can assure them that for the majority of cases these treatments will be effective and their longevity will be protected. It is therefore understandable that the desire to avoid repeating this particular life experience may be strong. We have an obligation to explain the advantages and disadvantages, as well as the alternatives to CPM, with sensitivity and patience. We must also strive to make sure that our patients do not make premature decisions without understanding the consequences. Last, but certainly not least, we are ethically bound to offer only those treatments that we feel are medically reasonable and safe as well as oncologically sound. But we must also remember that the decision to pursue treatment and the choice between the options that we offer are ultimately rights that belong to the patient.
Dr. Newman in an ACS Fellow, professor of surgery, and director of the Breast Care Center and Multidisciplinary Breast Fellowship Program, University of Michigan Comprehensive Cancer Center, Ann Arbor.
Dr. Sarah Hawley and her coinvestigators are to be applauded for generating insightful data regarding factors and concerns that motivate a woman to undergo contralateral prophylactic mastectomy in the setting of unilateral breast cancer (JAMA Surgery 2014 May 21 [doi:10.1001/jamasurg.2013.5689]).
Hawley et al. found that fear of recurrence was one of the strongest factors leading women to choose contralateral prophylactic mastectomy (CPM). This finding clearly demonstrates that we need to do a better job of explaining and defining the significance of (i) breast cancer local recurrence; (ii) breast cancer distant recurrence; and (iii) the development of a new/second primary breast cancer. Since cross-metastasis of a primary breast cancer to the contralateral breast is an extremely rare event, and since distant metastasis from the initial primary breast cancer tends to determine survival rates, CPM by definition will influence the incidence of only the third pattern. Furthermore, since the risk of experiencing a new contralateral malignancy is less than 1% per year for the general population of breast cancer patients, only a minority of these women will actually become bilateral breast cancer patients. Fear of recurrence is therefore a totally inappropriate reason for patients to pursue CPM, and the reasonableness of CPM to reduce the risk of a contralateral new primary breast cancer is debatable.
It can be reasonably stated that prophylactic surgery by definition is never a medically indicated necessity. Furthermore, despite the fact that a personal history of breast cancer is indeed a risk factor for developing a second primary cancer in the contralateral breast, numerous studies have demonstrated equivalent survival rates for women with unilateral breast cancer, compared with those diagnosed with bilateral/metachronous breast cancer (Cancer 2001;91:1845-53; Am. J. Clin. Oncol. 1997;20:541-5). Survival tends to be driven by the stage and effectiveness of treatment for the first cancer. By virtue of its earlier presentation, it is likely that the initially diagnosed cancer has established itself as the faster-growing malignancy with a lead time advantage in establishing distant organ micrometastatic disease; furthermore, patients with a unilateral breast cancer diagnosis are generally undergoing diligent surveillance and a contralateral malignancy is more often detected at an early stage.
Messages to our patients
It is essential for those of us who manage breast cancer to clearly emphasize several messages to our newly diagnosed breast cancer patients: First, although unilateral breast cancer increases the likelihood of developing a second primary tumor, it is certainly not inevitable, and in fact, the majority of patients are not destined to develop contralateral disease. Second, reducing the risk of being diagnosed with a contralateral breast cancer does not mitigate the mortality risk associated with the first cancer. And, finally, prophylactic mastectomy is the most aggressive and effective strategy for reducing the incidence of primary breast cancer (by approximately 90%), but it does not confer complete protection, as microscopic foci of breast tissue may be left behind in the mastectomy skin flaps, along the pectoralis, or in the axilla.
The messages above are critical: Our patients must understand that the priority is to address the known cancer. In this regard, appropriately selected patients should be encouraged to strongly consider breast-conserving surgery whenever feasible, as this low-morbidity treatment is equivalent to mastectomy from the perspective of overall survival. The question of CPM is most relevant for those patients that are ineligible for breast conservation or patients unwilling to undergo lumpectomy and breast radiation.
If a mastectomy for the cancerous breast is planned, we must then address the questions that routinely arise regarding bilateral surgery. In our efforts to clarify the reality of what CPM can and cannot achieve, we must also avoid being too dogmatic and paternalistic with our patients. There are clearly specific scenarios, as delineated in Dr. Hawley’s work, where the risk of a second primary breast cancer is likely to be considered excessive by most women, and where the decision to pursue CPM may be easier. Examples of such cases would be women known to harbor BRCA mutations or women with suspected hereditary susceptibility based on a strong family history of breast and/or ovarian cancer. The risk of a new contralateral breast cancer can be in the range of 4%-5% per year in cases of hereditary disease, compared with the general population of women with sporadic breast cancer, where the risk ranges from 0.25% to 1% per year.
Conveying an understanding of risk
Patients must understand that the risk to the contralateral breast is predominantly expressed in the future – the likelihood of having a clinically occult, incidentally detected cancer identified in the contralateral mastectomy specimen is only 6%, as demonstrated most recently by King et al. (Ann. Surg. 2011;254:2-7), and with ductal carcinoma in situ accounting for the high majority of these lesions.
Defining the threshold for the amount of risk that an individual woman finds to be acceptable, however, can be a very difficult and personal decision. Even after a patient comes to understand that CPM is unlikely to provide a survival advantage, she may continue to request bilateral surgery purely for the risk-reducing benefits, and out of a desire to minimize her chances of having to repeat the breast cancer diagnosis and treatment experience. In some cases this choice will be influenced by reconstruction factors. A woman may be motivated to pursue bilateral surgery if she has an adequate volume of abdominal tissue because of the fact that the autogenous TRAM (transverse rectus abdominis myocutaneous) flap can be harvested only once. In other cases the decision is influenced by body habitus, for example, a woman with large pendulous breasts who is not interested in breast reconstruction may decide that she is more comfortable with a symmetrically flat chest wall in order to avoid chest wall imbalance and the inconvenience of finding/wearing a prosthesis that matches the remaining breast.
As breast cancer surgeons we should openly discuss these issues with our patients and present viable alternatives when feasible, such as reduction mammoplasty for the large-breasted patient. Ultimately, however, the patient must decide the surgical approach that provides her with the optimal sense of treatment satisfaction, quality of life, and comfort.
Discussion strategies
In my own practice I have found two discussion strategies to be particularly useful in guiding patients through the decision about CPM.
The first approach is relevant for women who are lumpectomy candidates, but who express a "reflex" interest in bilateral mastectomy while they are still in the emotional fog of processing the new cancer diagnosis. For these women it is obviously important to stress the survival equivalence of mastectomy and breast-conserving surgery, and this is also a great opportunity to educate patients about the potential axillary surgery advantages of breast conservation. The American College of Surgeons Oncology Group Z11 trial (JAMA 2011;305:69-75) has provided strong evidence supporting the safety of avoiding an axillary lymph node dissection (ALND) in women with sentinel lymph node (SLN) metastatic disease if the primary breast cancer is managed by lumpectomy and breast radiation.
At this point in time, we do not have comparably strong data to justify avoiding the ALND in the setting of mastectomy patients with SLN metastatic disease. The mastectomy patient with SLN metastasis is usually committed to undergo the completion axillary lymph node dissection specifically so that definitive decisions can be made regarding the need for postmastectomy radiation, and many of these patients become ineligible for immediate reconstruction because of this possible radiation. I therefore accentuate the advantage of at least initiating treatment with lumpectomy and sentinel lymph node biopsy. The patient preserves all of her surgical options with the benefit of having more staging information. If she is found to have SLN metastatic disease then she is in a better position to avoid the ALND with lumpectomy and radiation, and the option of future mastectomy and immediate reconstruction would still be available to her in the future (after completing all of her cancer treatment and healing from her radiation); if the SLN is negative, she can either continue with the breast-conservation treatment plan or she can pursue mastectomy (with or without immediate breast reconstruction, since prophylactic mamillary radiation therapy is not likely to be indicated for node-negative disease).
The second approach is relevant to the patient requiring mastectomy but for whom delayed reconstruction is planned because of medical issues or anticipated postmastectomy radiation. I encourage these patients to at least consider deferring the decision for the CPM until they return for the delayed reconstruction of the cancerous mastectomy, because at that time they can undergo the prophylactic mastectomy with the cosmetic advantages of immediate reconstruction.
Cost considerations
From the public health and population-based breast cancer burden perspectives as well as for individual patients, there are additional issues to be factored into the CPM discussion. It is a basic reality that cost is relevant when it comes to sorting out the net benefit of particular medical interventions, especially those that are prophylactic. Interestingly, a cost analysis study by Zendejas et al. (J. Clin. Oncol. 2011;29:2993-3000) from the Mayo Clinic demonstrated that CPM is actually cost effective, compared with surveillance for patients diagnosed when they are younger than 70 years of age.
The Women’s Health and Cancer Rights Act was implemented in 1999, mandating insurance coverage for breast reconstruction after mastectomy performed for cancer. This legislation promoted more widespread acceptance (and reimbursement) for contralateral mastectomy/reconstruction, but patients should nonetheless be proactive about confirming that their individual policy will indeed cover the expenses of prophylactic surgery. Furthermore, we must continue to monitor outcomes in women who choose to undergo CPM, as advances in breast cancer therapies may influence the survival benefits of this surgical approach. Indeed, selected retrospective studies have recently demonstrated that patients undergoing CPM have an improved survival, compared with those focusing on unilateral breast cancer surgery (Ann. Surg. Oncol. 2010;17:2702-9; J. Natl. Cancer Inst. 2010;102:401-9; J. Clin. Oncol. 2005;23:4275-86; Am. J. Surg. 2000;180:439-45). These results suggest a survival advantage associated with avoidance of a contralateral breast cancer, in contrast to the historical data alluded to above, regarding survival equivalence for patients with unilateral compared to metachronous bilateral breast cancer. As adjuvant systemic therapies for breast cancer continue to improve in effectiveness and ability to completely eliminate distant organ micrometastases, it is likely that we will continue to increase the pool of women who are essentially "cured" of the first cancer. This in turn could potentially increase the longevity threat of a second/metachronous cancer though a renewed metastatic risk. Nonetheless, data on possible survival advantages of CPM have not yet matured to the point where it can be recommended as a medically "indicated" procedure.
Our breast cancer patients face an abundance of very legitimate fears related to the morbidity and mortality risks of the actual cancer as well as the adverse effects and toxicities of treatment for that cancer. Fortunately, we can assure them that for the majority of cases these treatments will be effective and their longevity will be protected. It is therefore understandable that the desire to avoid repeating this particular life experience may be strong. We have an obligation to explain the advantages and disadvantages, as well as the alternatives to CPM, with sensitivity and patience. We must also strive to make sure that our patients do not make premature decisions without understanding the consequences. Last, but certainly not least, we are ethically bound to offer only those treatments that we feel are medically reasonable and safe as well as oncologically sound. But we must also remember that the decision to pursue treatment and the choice between the options that we offer are ultimately rights that belong to the patient.
Dr. Newman in an ACS Fellow, professor of surgery, and director of the Breast Care Center and Multidisciplinary Breast Fellowship Program, University of Michigan Comprehensive Cancer Center, Ann Arbor.
Dr. Sarah Hawley and her coinvestigators are to be applauded for generating insightful data regarding factors and concerns that motivate a woman to undergo contralateral prophylactic mastectomy in the setting of unilateral breast cancer (JAMA Surgery 2014 May 21 [doi:10.1001/jamasurg.2013.5689]).
Hawley et al. found that fear of recurrence was one of the strongest factors leading women to choose contralateral prophylactic mastectomy (CPM). This finding clearly demonstrates that we need to do a better job of explaining and defining the significance of (i) breast cancer local recurrence; (ii) breast cancer distant recurrence; and (iii) the development of a new/second primary breast cancer. Since cross-metastasis of a primary breast cancer to the contralateral breast is an extremely rare event, and since distant metastasis from the initial primary breast cancer tends to determine survival rates, CPM by definition will influence the incidence of only the third pattern. Furthermore, since the risk of experiencing a new contralateral malignancy is less than 1% per year for the general population of breast cancer patients, only a minority of these women will actually become bilateral breast cancer patients. Fear of recurrence is therefore a totally inappropriate reason for patients to pursue CPM, and the reasonableness of CPM to reduce the risk of a contralateral new primary breast cancer is debatable.
It can be reasonably stated that prophylactic surgery by definition is never a medically indicated necessity. Furthermore, despite the fact that a personal history of breast cancer is indeed a risk factor for developing a second primary cancer in the contralateral breast, numerous studies have demonstrated equivalent survival rates for women with unilateral breast cancer, compared with those diagnosed with bilateral/metachronous breast cancer (Cancer 2001;91:1845-53; Am. J. Clin. Oncol. 1997;20:541-5). Survival tends to be driven by the stage and effectiveness of treatment for the first cancer. By virtue of its earlier presentation, it is likely that the initially diagnosed cancer has established itself as the faster-growing malignancy with a lead time advantage in establishing distant organ micrometastatic disease; furthermore, patients with a unilateral breast cancer diagnosis are generally undergoing diligent surveillance and a contralateral malignancy is more often detected at an early stage.
Messages to our patients
It is essential for those of us who manage breast cancer to clearly emphasize several messages to our newly diagnosed breast cancer patients: First, although unilateral breast cancer increases the likelihood of developing a second primary tumor, it is certainly not inevitable, and in fact, the majority of patients are not destined to develop contralateral disease. Second, reducing the risk of being diagnosed with a contralateral breast cancer does not mitigate the mortality risk associated with the first cancer. And, finally, prophylactic mastectomy is the most aggressive and effective strategy for reducing the incidence of primary breast cancer (by approximately 90%), but it does not confer complete protection, as microscopic foci of breast tissue may be left behind in the mastectomy skin flaps, along the pectoralis, or in the axilla.
The messages above are critical: Our patients must understand that the priority is to address the known cancer. In this regard, appropriately selected patients should be encouraged to strongly consider breast-conserving surgery whenever feasible, as this low-morbidity treatment is equivalent to mastectomy from the perspective of overall survival. The question of CPM is most relevant for those patients that are ineligible for breast conservation or patients unwilling to undergo lumpectomy and breast radiation.
If a mastectomy for the cancerous breast is planned, we must then address the questions that routinely arise regarding bilateral surgery. In our efforts to clarify the reality of what CPM can and cannot achieve, we must also avoid being too dogmatic and paternalistic with our patients. There are clearly specific scenarios, as delineated in Dr. Hawley’s work, where the risk of a second primary breast cancer is likely to be considered excessive by most women, and where the decision to pursue CPM may be easier. Examples of such cases would be women known to harbor BRCA mutations or women with suspected hereditary susceptibility based on a strong family history of breast and/or ovarian cancer. The risk of a new contralateral breast cancer can be in the range of 4%-5% per year in cases of hereditary disease, compared with the general population of women with sporadic breast cancer, where the risk ranges from 0.25% to 1% per year.
Conveying an understanding of risk
Patients must understand that the risk to the contralateral breast is predominantly expressed in the future – the likelihood of having a clinically occult, incidentally detected cancer identified in the contralateral mastectomy specimen is only 6%, as demonstrated most recently by King et al. (Ann. Surg. 2011;254:2-7), and with ductal carcinoma in situ accounting for the high majority of these lesions.
Defining the threshold for the amount of risk that an individual woman finds to be acceptable, however, can be a very difficult and personal decision. Even after a patient comes to understand that CPM is unlikely to provide a survival advantage, she may continue to request bilateral surgery purely for the risk-reducing benefits, and out of a desire to minimize her chances of having to repeat the breast cancer diagnosis and treatment experience. In some cases this choice will be influenced by reconstruction factors. A woman may be motivated to pursue bilateral surgery if she has an adequate volume of abdominal tissue because of the fact that the autogenous TRAM (transverse rectus abdominis myocutaneous) flap can be harvested only once. In other cases the decision is influenced by body habitus, for example, a woman with large pendulous breasts who is not interested in breast reconstruction may decide that she is more comfortable with a symmetrically flat chest wall in order to avoid chest wall imbalance and the inconvenience of finding/wearing a prosthesis that matches the remaining breast.
As breast cancer surgeons we should openly discuss these issues with our patients and present viable alternatives when feasible, such as reduction mammoplasty for the large-breasted patient. Ultimately, however, the patient must decide the surgical approach that provides her with the optimal sense of treatment satisfaction, quality of life, and comfort.
Discussion strategies
In my own practice I have found two discussion strategies to be particularly useful in guiding patients through the decision about CPM.
The first approach is relevant for women who are lumpectomy candidates, but who express a "reflex" interest in bilateral mastectomy while they are still in the emotional fog of processing the new cancer diagnosis. For these women it is obviously important to stress the survival equivalence of mastectomy and breast-conserving surgery, and this is also a great opportunity to educate patients about the potential axillary surgery advantages of breast conservation. The American College of Surgeons Oncology Group Z11 trial (JAMA 2011;305:69-75) has provided strong evidence supporting the safety of avoiding an axillary lymph node dissection (ALND) in women with sentinel lymph node (SLN) metastatic disease if the primary breast cancer is managed by lumpectomy and breast radiation.
At this point in time, we do not have comparably strong data to justify avoiding the ALND in the setting of mastectomy patients with SLN metastatic disease. The mastectomy patient with SLN metastasis is usually committed to undergo the completion axillary lymph node dissection specifically so that definitive decisions can be made regarding the need for postmastectomy radiation, and many of these patients become ineligible for immediate reconstruction because of this possible radiation. I therefore accentuate the advantage of at least initiating treatment with lumpectomy and sentinel lymph node biopsy. The patient preserves all of her surgical options with the benefit of having more staging information. If she is found to have SLN metastatic disease then she is in a better position to avoid the ALND with lumpectomy and radiation, and the option of future mastectomy and immediate reconstruction would still be available to her in the future (after completing all of her cancer treatment and healing from her radiation); if the SLN is negative, she can either continue with the breast-conservation treatment plan or she can pursue mastectomy (with or without immediate breast reconstruction, since prophylactic mamillary radiation therapy is not likely to be indicated for node-negative disease).
The second approach is relevant to the patient requiring mastectomy but for whom delayed reconstruction is planned because of medical issues or anticipated postmastectomy radiation. I encourage these patients to at least consider deferring the decision for the CPM until they return for the delayed reconstruction of the cancerous mastectomy, because at that time they can undergo the prophylactic mastectomy with the cosmetic advantages of immediate reconstruction.
Cost considerations
From the public health and population-based breast cancer burden perspectives as well as for individual patients, there are additional issues to be factored into the CPM discussion. It is a basic reality that cost is relevant when it comes to sorting out the net benefit of particular medical interventions, especially those that are prophylactic. Interestingly, a cost analysis study by Zendejas et al. (J. Clin. Oncol. 2011;29:2993-3000) from the Mayo Clinic demonstrated that CPM is actually cost effective, compared with surveillance for patients diagnosed when they are younger than 70 years of age.
The Women’s Health and Cancer Rights Act was implemented in 1999, mandating insurance coverage for breast reconstruction after mastectomy performed for cancer. This legislation promoted more widespread acceptance (and reimbursement) for contralateral mastectomy/reconstruction, but patients should nonetheless be proactive about confirming that their individual policy will indeed cover the expenses of prophylactic surgery. Furthermore, we must continue to monitor outcomes in women who choose to undergo CPM, as advances in breast cancer therapies may influence the survival benefits of this surgical approach. Indeed, selected retrospective studies have recently demonstrated that patients undergoing CPM have an improved survival, compared with those focusing on unilateral breast cancer surgery (Ann. Surg. Oncol. 2010;17:2702-9; J. Natl. Cancer Inst. 2010;102:401-9; J. Clin. Oncol. 2005;23:4275-86; Am. J. Surg. 2000;180:439-45). These results suggest a survival advantage associated with avoidance of a contralateral breast cancer, in contrast to the historical data alluded to above, regarding survival equivalence for patients with unilateral compared to metachronous bilateral breast cancer. As adjuvant systemic therapies for breast cancer continue to improve in effectiveness and ability to completely eliminate distant organ micrometastases, it is likely that we will continue to increase the pool of women who are essentially "cured" of the first cancer. This in turn could potentially increase the longevity threat of a second/metachronous cancer though a renewed metastatic risk. Nonetheless, data on possible survival advantages of CPM have not yet matured to the point where it can be recommended as a medically "indicated" procedure.
Our breast cancer patients face an abundance of very legitimate fears related to the morbidity and mortality risks of the actual cancer as well as the adverse effects and toxicities of treatment for that cancer. Fortunately, we can assure them that for the majority of cases these treatments will be effective and their longevity will be protected. It is therefore understandable that the desire to avoid repeating this particular life experience may be strong. We have an obligation to explain the advantages and disadvantages, as well as the alternatives to CPM, with sensitivity and patience. We must also strive to make sure that our patients do not make premature decisions without understanding the consequences. Last, but certainly not least, we are ethically bound to offer only those treatments that we feel are medically reasonable and safe as well as oncologically sound. But we must also remember that the decision to pursue treatment and the choice between the options that we offer are ultimately rights that belong to the patient.
Dr. Newman in an ACS Fellow, professor of surgery, and director of the Breast Care Center and Multidisciplinary Breast Fellowship Program, University of Michigan Comprehensive Cancer Center, Ann Arbor.
Adaptability is the surgeon’s best friend
At my semiannual palliative medicine fellowship evaluation, I was asked, "What is your best strength?" and after a few seconds I said, "Adaptability."
Often, the most profound thoughts come spontaneously before habit interferes. As a practicing cardiothoracic surgeon for more than 16 years, my transition to palliative medicine and a hospice fellowship required the ability to reinvent myself, to embrace new ideas, to let go of old routines, and to accept new possibilities. In other words, to adapt to change.
During my surgical training I was most impressed with surgeons who could think calmly and rationally on the fly. As surgeons, we all understand the benefit of preparation and how contingency planning optimizes safety. But we also know from experience that no matter how well prepared you think you are, something can and almost invariably does happen that is unintentional, unanticipated, and unplanned that sabotages your preparation. So, too, is it with life.
I would never have predicted at the start of my career as a cardiothoracic and vascular surgeon that I would change, at midcareer, to palliative medicine. I am not going to explain all the events that pushed me to make a change, but suffice it to say I came to the proverbial fork in the road. I could have continued down the same path, frustrated and unhappy, but comfortable with my routines, or I could stop feeling sorry for myself, stop complaining, adapt to the changes, and go in a different direction. I chose the latter. I do miss the exhilaration and teamwork of surgery, but I have replaced it with the more profound collaboration of the palliative inter-disciplinary team that includes nurses, chaplains, social workers, therapists, and patients.
The most frequent question I am asked when people find out that I used to be a heart surgeon is why I changed careers. It’s really not as crazy as it seems. Fundamental to both surgery and palliative medicine are evidence-based options and patient-centered, informed decision making.
Historically, palliative care has had limited acceptance by the surgical community except at the end of life in an actively dying patient, but through the efforts of a few visionaries, palliative surgical care is now valued and deemed worthy of incorporation into general surgical residency training. During the past year I have been introduced to this interdisciplinary approach that improves the quality of life of patients and their families. Because this approach assesses and supports physical, psychological, social, and spiritual needs, it needs to occur with, not after, other appropriate medical treatments.
There is good empiric evidence that the earlier palliative medicine is involved in medical treatment, whether potentially curative or not, the quality of care improves: Caregiver, patient, and family satisfaction increases and resource utilization improves no matter what the delivery setting. These are very compelling data, and they validate the role of palliative medicine in a changing paradigm of health care delivery.
My vision for palliative medicine includes complete integration throughout the trajectory of all chronic illness, especially heart failure, cancer, and dementia, where coordination of care is critical and has been historically fragmented. Palliative medicine is increasing its role in acute care with improved symptom management and early consultation in the emergency department and ICU where better communication with the care team and advance care planning can help define goals of care and limit inappropriate and unwanted treatment.
The adaptability that is so crucial for patients and their families to adjust to progressive and critical illness is the same quality we need as practitioners to accommodate them. I challenge all my surgical colleagues to have the courage and wisdom to change, and to allow the integration of palliative medicine into your practice where and whenever possible.
Dr. Strzalka is a Fellow in Hospice and Palliative Medicine, Section of Palliative Medicine and Supportive Oncology, Taussig Cancer Institute, Cleveland Clinic.
At my semiannual palliative medicine fellowship evaluation, I was asked, "What is your best strength?" and after a few seconds I said, "Adaptability."
Often, the most profound thoughts come spontaneously before habit interferes. As a practicing cardiothoracic surgeon for more than 16 years, my transition to palliative medicine and a hospice fellowship required the ability to reinvent myself, to embrace new ideas, to let go of old routines, and to accept new possibilities. In other words, to adapt to change.
During my surgical training I was most impressed with surgeons who could think calmly and rationally on the fly. As surgeons, we all understand the benefit of preparation and how contingency planning optimizes safety. But we also know from experience that no matter how well prepared you think you are, something can and almost invariably does happen that is unintentional, unanticipated, and unplanned that sabotages your preparation. So, too, is it with life.
I would never have predicted at the start of my career as a cardiothoracic and vascular surgeon that I would change, at midcareer, to palliative medicine. I am not going to explain all the events that pushed me to make a change, but suffice it to say I came to the proverbial fork in the road. I could have continued down the same path, frustrated and unhappy, but comfortable with my routines, or I could stop feeling sorry for myself, stop complaining, adapt to the changes, and go in a different direction. I chose the latter. I do miss the exhilaration and teamwork of surgery, but I have replaced it with the more profound collaboration of the palliative inter-disciplinary team that includes nurses, chaplains, social workers, therapists, and patients.
The most frequent question I am asked when people find out that I used to be a heart surgeon is why I changed careers. It’s really not as crazy as it seems. Fundamental to both surgery and palliative medicine are evidence-based options and patient-centered, informed decision making.
Historically, palliative care has had limited acceptance by the surgical community except at the end of life in an actively dying patient, but through the efforts of a few visionaries, palliative surgical care is now valued and deemed worthy of incorporation into general surgical residency training. During the past year I have been introduced to this interdisciplinary approach that improves the quality of life of patients and their families. Because this approach assesses and supports physical, psychological, social, and spiritual needs, it needs to occur with, not after, other appropriate medical treatments.
There is good empiric evidence that the earlier palliative medicine is involved in medical treatment, whether potentially curative or not, the quality of care improves: Caregiver, patient, and family satisfaction increases and resource utilization improves no matter what the delivery setting. These are very compelling data, and they validate the role of palliative medicine in a changing paradigm of health care delivery.
My vision for palliative medicine includes complete integration throughout the trajectory of all chronic illness, especially heart failure, cancer, and dementia, where coordination of care is critical and has been historically fragmented. Palliative medicine is increasing its role in acute care with improved symptom management and early consultation in the emergency department and ICU where better communication with the care team and advance care planning can help define goals of care and limit inappropriate and unwanted treatment.
The adaptability that is so crucial for patients and their families to adjust to progressive and critical illness is the same quality we need as practitioners to accommodate them. I challenge all my surgical colleagues to have the courage and wisdom to change, and to allow the integration of palliative medicine into your practice where and whenever possible.
Dr. Strzalka is a Fellow in Hospice and Palliative Medicine, Section of Palliative Medicine and Supportive Oncology, Taussig Cancer Institute, Cleveland Clinic.
At my semiannual palliative medicine fellowship evaluation, I was asked, "What is your best strength?" and after a few seconds I said, "Adaptability."
Often, the most profound thoughts come spontaneously before habit interferes. As a practicing cardiothoracic surgeon for more than 16 years, my transition to palliative medicine and a hospice fellowship required the ability to reinvent myself, to embrace new ideas, to let go of old routines, and to accept new possibilities. In other words, to adapt to change.
During my surgical training I was most impressed with surgeons who could think calmly and rationally on the fly. As surgeons, we all understand the benefit of preparation and how contingency planning optimizes safety. But we also know from experience that no matter how well prepared you think you are, something can and almost invariably does happen that is unintentional, unanticipated, and unplanned that sabotages your preparation. So, too, is it with life.
I would never have predicted at the start of my career as a cardiothoracic and vascular surgeon that I would change, at midcareer, to palliative medicine. I am not going to explain all the events that pushed me to make a change, but suffice it to say I came to the proverbial fork in the road. I could have continued down the same path, frustrated and unhappy, but comfortable with my routines, or I could stop feeling sorry for myself, stop complaining, adapt to the changes, and go in a different direction. I chose the latter. I do miss the exhilaration and teamwork of surgery, but I have replaced it with the more profound collaboration of the palliative inter-disciplinary team that includes nurses, chaplains, social workers, therapists, and patients.
The most frequent question I am asked when people find out that I used to be a heart surgeon is why I changed careers. It’s really not as crazy as it seems. Fundamental to both surgery and palliative medicine are evidence-based options and patient-centered, informed decision making.
Historically, palliative care has had limited acceptance by the surgical community except at the end of life in an actively dying patient, but through the efforts of a few visionaries, palliative surgical care is now valued and deemed worthy of incorporation into general surgical residency training. During the past year I have been introduced to this interdisciplinary approach that improves the quality of life of patients and their families. Because this approach assesses and supports physical, psychological, social, and spiritual needs, it needs to occur with, not after, other appropriate medical treatments.
There is good empiric evidence that the earlier palliative medicine is involved in medical treatment, whether potentially curative or not, the quality of care improves: Caregiver, patient, and family satisfaction increases and resource utilization improves no matter what the delivery setting. These are very compelling data, and they validate the role of palliative medicine in a changing paradigm of health care delivery.
My vision for palliative medicine includes complete integration throughout the trajectory of all chronic illness, especially heart failure, cancer, and dementia, where coordination of care is critical and has been historically fragmented. Palliative medicine is increasing its role in acute care with improved symptom management and early consultation in the emergency department and ICU where better communication with the care team and advance care planning can help define goals of care and limit inappropriate and unwanted treatment.
The adaptability that is so crucial for patients and their families to adjust to progressive and critical illness is the same quality we need as practitioners to accommodate them. I challenge all my surgical colleagues to have the courage and wisdom to change, and to allow the integration of palliative medicine into your practice where and whenever possible.
Dr. Strzalka is a Fellow in Hospice and Palliative Medicine, Section of Palliative Medicine and Supportive Oncology, Taussig Cancer Institute, Cleveland Clinic.
Dr. Sachdeva elected VP of SACME
Ajit K. Sachdeva, MD, FACS, FRCSC, Director, Division of Education, American College of Surgeons, was recently elected to the position of vice-president of the Society for Academic Continuing Medical Education (SACME). In this role, Dr. Sachdeva will serve on the Board of SACME and as a member of the joint working group of SACME and the Association of American Medical Colleges.
SACME is the national organization of continuing medical education/continuous professional development professionals, and includes leaders in this field from medical schools, specialty societies, and other stakeholder groups. SACME aims to advance the field of continuing medical education/continuous professional development through research, scholarship, practical application of innovations, and dissemination of best practices.
Ajit K. Sachdeva, MD, FACS, FRCSC, Director, Division of Education, American College of Surgeons, was recently elected to the position of vice-president of the Society for Academic Continuing Medical Education (SACME). In this role, Dr. Sachdeva will serve on the Board of SACME and as a member of the joint working group of SACME and the Association of American Medical Colleges.
SACME is the national organization of continuing medical education/continuous professional development professionals, and includes leaders in this field from medical schools, specialty societies, and other stakeholder groups. SACME aims to advance the field of continuing medical education/continuous professional development through research, scholarship, practical application of innovations, and dissemination of best practices.
Ajit K. Sachdeva, MD, FACS, FRCSC, Director, Division of Education, American College of Surgeons, was recently elected to the position of vice-president of the Society for Academic Continuing Medical Education (SACME). In this role, Dr. Sachdeva will serve on the Board of SACME and as a member of the joint working group of SACME and the Association of American Medical Colleges.
SACME is the national organization of continuing medical education/continuous professional development professionals, and includes leaders in this field from medical schools, specialty societies, and other stakeholder groups. SACME aims to advance the field of continuing medical education/continuous professional development through research, scholarship, practical application of innovations, and dissemination of best practices.
ACS accepting applications for Clowes Award
The American College of Surgeons (ACS) is accepting applications for the George H.A. Clowes, Jr., MD, FACS, Memorial Research Career Development Award. This award, offered through the generosity of The Clowes Fund, of Indianapolis, IN, is intended to provide support for the research of a promising young surgical investigator. The award consists of a stipend of $45,000 for each of five years and is not renewable thereafter. Applications are due August 1, 2014. General requirements General policies concerning the granting of the George H.A. Clowes, Jr., MD, FACS, Memorial Research Career Development Award are as follows:
• The award is restricted to a Fellow or an Associate Fellow of the ACS who has completed an accredited residency in general surgery within the preceding seven years and has received a full-time faculty appointment at a medical school accredited by the Liaison Committee on Medical Education in the U.S. or by the Committee for Accreditation of Canadian Medical Schools. The applicant’s academic appointment may not be above the level of assistant professor. Applicants should provide evidence, by publication or otherwise, of productive initial efforts in laboratory research.
• The award may be used for salary support or other purposes at the discretion of the recipient and the institution. Indirect costs are not paid to the recipient or to the recipient’s institution.
• The ACS Scholarships Committee will look preferentially upon applicants who have received investigator-initiated, peer-reviewed, federally funded research awards (for example, National Institutes of Health [NIH] R01/K08/ K23, Veterans Affairs Merit Review, and Canadian Institutes of Health Research grants). The committee will not consider applicants who have already received research career development awards from professional societies. The recipient must notify the College’s Scholarships Administrator to request approval if another source of scholarship or fellowship funding is received.
• Approval of the application is required from the administration (dean or fiscal officer) and the head of the applicant’s department or administrative unit. This approval includes a commitment to continuation of the academic position and facilities for research throughout the entire period of the award. In addition, assurance must be provided that at least 50 percent of the applicant’s time will be spent conducting the research proposed in the application. This percentage may run concurrently with the time requirements of NIH or other accepted funding.
• The applicant must submit, in addition to the application form, an NIH-style biosketch, a detailed research plan of up to eight pages in length, and propose a budget for the five-year period of the award. The applicant also must to submit a cover letter of no more than one page describing personal career objectives, how these career objectives will be achieved, and how the research protocol furthers the applicant’s career development. The Scholarships Committee requires an annual narrative progress report from the recipient on which annual renewal of the award is based.
While holding the award, the recipient is required to attend the ACS Clinical Congress in 2016, 2018, and 2020 and present reports to the Scholarships Committee and its guests.
• Upon completion of the five-year funding period, the recipient will be required to submit a final report summarizing research progress and providing information regarding current academic rank, sources of research support, and future plans. The recipient also is required to apply to the Surgical Forum at the conclusion of the award period. The closing date for receipt of completed applications and all related documents is August 1, 2014.
The application form may be accessed at http://www.facs.org/memberservices/acsclowes.html.
Additional documents and questions are to be directed to theScholarships Administrator at [email protected].
The American College of Surgeons (ACS) is accepting applications for the George H.A. Clowes, Jr., MD, FACS, Memorial Research Career Development Award. This award, offered through the generosity of The Clowes Fund, of Indianapolis, IN, is intended to provide support for the research of a promising young surgical investigator. The award consists of a stipend of $45,000 for each of five years and is not renewable thereafter. Applications are due August 1, 2014. General requirements General policies concerning the granting of the George H.A. Clowes, Jr., MD, FACS, Memorial Research Career Development Award are as follows:
• The award is restricted to a Fellow or an Associate Fellow of the ACS who has completed an accredited residency in general surgery within the preceding seven years and has received a full-time faculty appointment at a medical school accredited by the Liaison Committee on Medical Education in the U.S. or by the Committee for Accreditation of Canadian Medical Schools. The applicant’s academic appointment may not be above the level of assistant professor. Applicants should provide evidence, by publication or otherwise, of productive initial efforts in laboratory research.
• The award may be used for salary support or other purposes at the discretion of the recipient and the institution. Indirect costs are not paid to the recipient or to the recipient’s institution.
• The ACS Scholarships Committee will look preferentially upon applicants who have received investigator-initiated, peer-reviewed, federally funded research awards (for example, National Institutes of Health [NIH] R01/K08/ K23, Veterans Affairs Merit Review, and Canadian Institutes of Health Research grants). The committee will not consider applicants who have already received research career development awards from professional societies. The recipient must notify the College’s Scholarships Administrator to request approval if another source of scholarship or fellowship funding is received.
• Approval of the application is required from the administration (dean or fiscal officer) and the head of the applicant’s department or administrative unit. This approval includes a commitment to continuation of the academic position and facilities for research throughout the entire period of the award. In addition, assurance must be provided that at least 50 percent of the applicant’s time will be spent conducting the research proposed in the application. This percentage may run concurrently with the time requirements of NIH or other accepted funding.
• The applicant must submit, in addition to the application form, an NIH-style biosketch, a detailed research plan of up to eight pages in length, and propose a budget for the five-year period of the award. The applicant also must to submit a cover letter of no more than one page describing personal career objectives, how these career objectives will be achieved, and how the research protocol furthers the applicant’s career development. The Scholarships Committee requires an annual narrative progress report from the recipient on which annual renewal of the award is based.
While holding the award, the recipient is required to attend the ACS Clinical Congress in 2016, 2018, and 2020 and present reports to the Scholarships Committee and its guests.
• Upon completion of the five-year funding period, the recipient will be required to submit a final report summarizing research progress and providing information regarding current academic rank, sources of research support, and future plans. The recipient also is required to apply to the Surgical Forum at the conclusion of the award period. The closing date for receipt of completed applications and all related documents is August 1, 2014.
The application form may be accessed at http://www.facs.org/memberservices/acsclowes.html.
Additional documents and questions are to be directed to theScholarships Administrator at [email protected].
The American College of Surgeons (ACS) is accepting applications for the George H.A. Clowes, Jr., MD, FACS, Memorial Research Career Development Award. This award, offered through the generosity of The Clowes Fund, of Indianapolis, IN, is intended to provide support for the research of a promising young surgical investigator. The award consists of a stipend of $45,000 for each of five years and is not renewable thereafter. Applications are due August 1, 2014. General requirements General policies concerning the granting of the George H.A. Clowes, Jr., MD, FACS, Memorial Research Career Development Award are as follows:
• The award is restricted to a Fellow or an Associate Fellow of the ACS who has completed an accredited residency in general surgery within the preceding seven years and has received a full-time faculty appointment at a medical school accredited by the Liaison Committee on Medical Education in the U.S. or by the Committee for Accreditation of Canadian Medical Schools. The applicant’s academic appointment may not be above the level of assistant professor. Applicants should provide evidence, by publication or otherwise, of productive initial efforts in laboratory research.
• The award may be used for salary support or other purposes at the discretion of the recipient and the institution. Indirect costs are not paid to the recipient or to the recipient’s institution.
• The ACS Scholarships Committee will look preferentially upon applicants who have received investigator-initiated, peer-reviewed, federally funded research awards (for example, National Institutes of Health [NIH] R01/K08/ K23, Veterans Affairs Merit Review, and Canadian Institutes of Health Research grants). The committee will not consider applicants who have already received research career development awards from professional societies. The recipient must notify the College’s Scholarships Administrator to request approval if another source of scholarship or fellowship funding is received.
• Approval of the application is required from the administration (dean or fiscal officer) and the head of the applicant’s department or administrative unit. This approval includes a commitment to continuation of the academic position and facilities for research throughout the entire period of the award. In addition, assurance must be provided that at least 50 percent of the applicant’s time will be spent conducting the research proposed in the application. This percentage may run concurrently with the time requirements of NIH or other accepted funding.
• The applicant must submit, in addition to the application form, an NIH-style biosketch, a detailed research plan of up to eight pages in length, and propose a budget for the five-year period of the award. The applicant also must to submit a cover letter of no more than one page describing personal career objectives, how these career objectives will be achieved, and how the research protocol furthers the applicant’s career development. The Scholarships Committee requires an annual narrative progress report from the recipient on which annual renewal of the award is based.
While holding the award, the recipient is required to attend the ACS Clinical Congress in 2016, 2018, and 2020 and present reports to the Scholarships Committee and its guests.
• Upon completion of the five-year funding period, the recipient will be required to submit a final report summarizing research progress and providing information regarding current academic rank, sources of research support, and future plans. The recipient also is required to apply to the Surgical Forum at the conclusion of the award period. The closing date for receipt of completed applications and all related documents is August 1, 2014.
The application form may be accessed at http://www.facs.org/memberservices/acsclowes.html.
Additional documents and questions are to be directed to theScholarships Administrator at [email protected].
Program Planner guides you to ACS Clinical Congress 2014
Browse the Program Planner that was sent to all members in early June to get an idea of the vast number of courses and sessions that are available for you to experience at the American College of Surgeons (ACS) Clinical Congress 2014, October 26-30, at the Moscone Center in San Francisco, CA. Portions of the Program Planner for the ACS Clinical Congress—"the Best Surgical Education All in One Place"—were reprinted in the July issue of the Bulletin of the American College of Surgeons.
The Program Committee, chaired by Valerie W. Rusch, MD, FACS, together with the Division of Education, under the leadership of Ajit K. Sachdeva, MD, FACS, hasve organized a Scientific Program that will enlighten and inspire attendees. ACS President Carlos A. Pellegrini, MD, FACS, FRCSI (Hon), has articulated his theme for this year, "The Surgeon of the Future: Anchoring Innovation and Science with Moral Values." A number of sessions will address cutting-edge technology, evidence-based surgery, surgical education, professionalism, ethics, and social responsibility.
The Educational Program will includetimely topics presented iin variousa variety of formats. Diverse Panel Sessionspresented by experts from across surgical specialties and nonsurgical disciplines are included. There is also a compelling series of Named Lectures will to be delivered by recognized surgical leaders recognized in their respective fields. Didactic and Skills Courses will focus on advanced knowledge and skill acquisition.
The Scientific Program will include presentations of innovative research and surgical practices delivered orally as Paper and Surgical Forum Sessions in addition to Poster Presentations. The Video-Based Education presentations will include topic-centered symposiums. These sessions will be complemented by the more intimately set Meet-the Expert Luncheon and Town Hall Meetings.
The Clinical Congress Program has been arranged in key thematic tracks, and that address specialty-based tracks forthat address the learning needs of specialty groups.
The Program Planner isalso available online at http://www.facs.org/clincon2014/index.html.
Browse the Program Planner that was sent to all members in early June to get an idea of the vast number of courses and sessions that are available for you to experience at the American College of Surgeons (ACS) Clinical Congress 2014, October 26-30, at the Moscone Center in San Francisco, CA. Portions of the Program Planner for the ACS Clinical Congress—"the Best Surgical Education All in One Place"—were reprinted in the July issue of the Bulletin of the American College of Surgeons.
The Program Committee, chaired by Valerie W. Rusch, MD, FACS, together with the Division of Education, under the leadership of Ajit K. Sachdeva, MD, FACS, hasve organized a Scientific Program that will enlighten and inspire attendees. ACS President Carlos A. Pellegrini, MD, FACS, FRCSI (Hon), has articulated his theme for this year, "The Surgeon of the Future: Anchoring Innovation and Science with Moral Values." A number of sessions will address cutting-edge technology, evidence-based surgery, surgical education, professionalism, ethics, and social responsibility.
The Educational Program will includetimely topics presented iin variousa variety of formats. Diverse Panel Sessionspresented by experts from across surgical specialties and nonsurgical disciplines are included. There is also a compelling series of Named Lectures will to be delivered by recognized surgical leaders recognized in their respective fields. Didactic and Skills Courses will focus on advanced knowledge and skill acquisition.
The Scientific Program will include presentations of innovative research and surgical practices delivered orally as Paper and Surgical Forum Sessions in addition to Poster Presentations. The Video-Based Education presentations will include topic-centered symposiums. These sessions will be complemented by the more intimately set Meet-the Expert Luncheon and Town Hall Meetings.
The Clinical Congress Program has been arranged in key thematic tracks, and that address specialty-based tracks forthat address the learning needs of specialty groups.
The Program Planner isalso available online at http://www.facs.org/clincon2014/index.html.
Browse the Program Planner that was sent to all members in early June to get an idea of the vast number of courses and sessions that are available for you to experience at the American College of Surgeons (ACS) Clinical Congress 2014, October 26-30, at the Moscone Center in San Francisco, CA. Portions of the Program Planner for the ACS Clinical Congress—"the Best Surgical Education All in One Place"—were reprinted in the July issue of the Bulletin of the American College of Surgeons.
The Program Committee, chaired by Valerie W. Rusch, MD, FACS, together with the Division of Education, under the leadership of Ajit K. Sachdeva, MD, FACS, hasve organized a Scientific Program that will enlighten and inspire attendees. ACS President Carlos A. Pellegrini, MD, FACS, FRCSI (Hon), has articulated his theme for this year, "The Surgeon of the Future: Anchoring Innovation and Science with Moral Values." A number of sessions will address cutting-edge technology, evidence-based surgery, surgical education, professionalism, ethics, and social responsibility.
The Educational Program will includetimely topics presented iin variousa variety of formats. Diverse Panel Sessionspresented by experts from across surgical specialties and nonsurgical disciplines are included. There is also a compelling series of Named Lectures will to be delivered by recognized surgical leaders recognized in their respective fields. Didactic and Skills Courses will focus on advanced knowledge and skill acquisition.
The Scientific Program will include presentations of innovative research and surgical practices delivered orally as Paper and Surgical Forum Sessions in addition to Poster Presentations. The Video-Based Education presentations will include topic-centered symposiums. These sessions will be complemented by the more intimately set Meet-the Expert Luncheon and Town Hall Meetings.
The Clinical Congress Program has been arranged in key thematic tracks, and that address specialty-based tracks forthat address the learning needs of specialty groups.
The Program Planner isalso available online at http://www.facs.org/clincon2014/index.html.
Dr. Robin Cotton receives Jacobson Innovation Award
Robin T. Cotton, MD, FACS,FRCSC, Cincinnati, OH, received the American College of Surgeons (ACS) 2014 Jacobson Innovation Award at a dinner held in his honor June 6 in Chicago, IL. Dr. Cotton is the director of the Aerodigestive Center at Cincinnati Children’s Hospital, and professor, department of pediatrics, at the University of Cincinnati’s department of otolaryngology. The prestigious Jacobson Innovation Award, made possible through a gift from Julius H. Jacobson II, MD, FACS, and his wife Joan, honors living surgeons who have been innovators of new surgical developments or techniques. Dr. Jacobson is a general vascular surgeon known for his pion-eering work in the development of microsurgery.
The 2014 award honors Dr. Cotton’s seminal work in the care and reconstruction of the stenotic pediatric airway. His efforts have led to reconstruction of the larynx and trachea in children with laryngotracheal stenosis, allowing them to live and breathe normally. Dr. Cotton built the world’s first center for the diagnosis and treatment of airway abnormalities. In addition, he developed the anterior cricoid split procedure, a technique to avoid tracheotomy in neonates with acquired subglottic stenosis, and the supraglottoplasty, which he popularized in the U.S.
Dr. Cotton is considered one of the premier pediatric otolaryngologists in the world. View the ACS press release announcing the award at http://www.facs.org/news/2014/cotton-jacobson0614.html.
Robin T. Cotton, MD, FACS,FRCSC, Cincinnati, OH, received the American College of Surgeons (ACS) 2014 Jacobson Innovation Award at a dinner held in his honor June 6 in Chicago, IL. Dr. Cotton is the director of the Aerodigestive Center at Cincinnati Children’s Hospital, and professor, department of pediatrics, at the University of Cincinnati’s department of otolaryngology. The prestigious Jacobson Innovation Award, made possible through a gift from Julius H. Jacobson II, MD, FACS, and his wife Joan, honors living surgeons who have been innovators of new surgical developments or techniques. Dr. Jacobson is a general vascular surgeon known for his pion-eering work in the development of microsurgery.
The 2014 award honors Dr. Cotton’s seminal work in the care and reconstruction of the stenotic pediatric airway. His efforts have led to reconstruction of the larynx and trachea in children with laryngotracheal stenosis, allowing them to live and breathe normally. Dr. Cotton built the world’s first center for the diagnosis and treatment of airway abnormalities. In addition, he developed the anterior cricoid split procedure, a technique to avoid tracheotomy in neonates with acquired subglottic stenosis, and the supraglottoplasty, which he popularized in the U.S.
Dr. Cotton is considered one of the premier pediatric otolaryngologists in the world. View the ACS press release announcing the award at http://www.facs.org/news/2014/cotton-jacobson0614.html.
Robin T. Cotton, MD, FACS,FRCSC, Cincinnati, OH, received the American College of Surgeons (ACS) 2014 Jacobson Innovation Award at a dinner held in his honor June 6 in Chicago, IL. Dr. Cotton is the director of the Aerodigestive Center at Cincinnati Children’s Hospital, and professor, department of pediatrics, at the University of Cincinnati’s department of otolaryngology. The prestigious Jacobson Innovation Award, made possible through a gift from Julius H. Jacobson II, MD, FACS, and his wife Joan, honors living surgeons who have been innovators of new surgical developments or techniques. Dr. Jacobson is a general vascular surgeon known for his pion-eering work in the development of microsurgery.
The 2014 award honors Dr. Cotton’s seminal work in the care and reconstruction of the stenotic pediatric airway. His efforts have led to reconstruction of the larynx and trachea in children with laryngotracheal stenosis, allowing them to live and breathe normally. Dr. Cotton built the world’s first center for the diagnosis and treatment of airway abnormalities. In addition, he developed the anterior cricoid split procedure, a technique to avoid tracheotomy in neonates with acquired subglottic stenosis, and the supraglottoplasty, which he popularized in the U.S.
Dr. Cotton is considered one of the premier pediatric otolaryngologists in the world. View the ACS press release announcing the award at http://www.facs.org/news/2014/cotton-jacobson0614.html.
ACS Women in Surgery Committee issues call for mentees: July 31 deadline
The Women in Surgery Committee of the American College of Surgeons (ACS) is seeking applications for the Mentorship Program for Women Surgeons. Applications for mentees are due July 31. This program is an opportunity for early-career female surgeons to develop a mentoring relationship with established surgeons in all of the specialties represented within the ACS. This year, the program will include up to 12 participants who should plan to attend the ACS Clinical Congress 2014 in San Francisco, CA. Applicants to the program must be either ACS Fellows or Associate Fellows, or currently in the process of applying for Fellowship.
Requirements and responsibilities
Applicants should need mentorship in one or more of the following areas:
• Career development
• Research
• Work-life balance
• Practice development
• Transition to practice
• Leadership development
Mentee responsibilities will include the following:
• Participate in an introductory call with her mentor
• Attend the ACS Clinical Congress in San Francisco to meet her mentor
• Establish a plan to cultivate one or more of the areas in need of mentoring and identify specific goals
• Commit to connecting with the mentor at least on a quarterly basis
• Complete an evaluation form and submit a brief summary on the mentee experience at the conclusion of the program in October 2015.
Interested individuals should contact Connie Bura, Assistant Director, ACS Member Services, at [email protected] to receive an application. In addition to the application, candidates must submit their curriculum vitae by July 31, along with a personal statement discussing the benefits that the program will provide to their career.
Mentors
The Women in Surgery Committee also seeks individuals to serve as mentors for the program. Mentor responsibilities will include the following:
• Participate in an introductory call with the mentee
• Attend the ACS Clinical Congress in San Francisco to meet the mentee
• Provide opportunities for interaction, at least quarterly, with the mentee
• Assist the mentee in her mentorship area(s) of interest and in identifying specific goals
• Complete an evaluation form and submit a brief summary on the mentor experience at the conclusion of the program in October 2015
Individuals interested in serving as mentors should contact Ms. Bura at [email protected] before July 31.
The Women in Surgery Committee of the American College of Surgeons (ACS) is seeking applications for the Mentorship Program for Women Surgeons. Applications for mentees are due July 31. This program is an opportunity for early-career female surgeons to develop a mentoring relationship with established surgeons in all of the specialties represented within the ACS. This year, the program will include up to 12 participants who should plan to attend the ACS Clinical Congress 2014 in San Francisco, CA. Applicants to the program must be either ACS Fellows or Associate Fellows, or currently in the process of applying for Fellowship.
Requirements and responsibilities
Applicants should need mentorship in one or more of the following areas:
• Career development
• Research
• Work-life balance
• Practice development
• Transition to practice
• Leadership development
Mentee responsibilities will include the following:
• Participate in an introductory call with her mentor
• Attend the ACS Clinical Congress in San Francisco to meet her mentor
• Establish a plan to cultivate one or more of the areas in need of mentoring and identify specific goals
• Commit to connecting with the mentor at least on a quarterly basis
• Complete an evaluation form and submit a brief summary on the mentee experience at the conclusion of the program in October 2015.
Interested individuals should contact Connie Bura, Assistant Director, ACS Member Services, at [email protected] to receive an application. In addition to the application, candidates must submit their curriculum vitae by July 31, along with a personal statement discussing the benefits that the program will provide to their career.
Mentors
The Women in Surgery Committee also seeks individuals to serve as mentors for the program. Mentor responsibilities will include the following:
• Participate in an introductory call with the mentee
• Attend the ACS Clinical Congress in San Francisco to meet the mentee
• Provide opportunities for interaction, at least quarterly, with the mentee
• Assist the mentee in her mentorship area(s) of interest and in identifying specific goals
• Complete an evaluation form and submit a brief summary on the mentor experience at the conclusion of the program in October 2015
Individuals interested in serving as mentors should contact Ms. Bura at [email protected] before July 31.
The Women in Surgery Committee of the American College of Surgeons (ACS) is seeking applications for the Mentorship Program for Women Surgeons. Applications for mentees are due July 31. This program is an opportunity for early-career female surgeons to develop a mentoring relationship with established surgeons in all of the specialties represented within the ACS. This year, the program will include up to 12 participants who should plan to attend the ACS Clinical Congress 2014 in San Francisco, CA. Applicants to the program must be either ACS Fellows or Associate Fellows, or currently in the process of applying for Fellowship.
Requirements and responsibilities
Applicants should need mentorship in one or more of the following areas:
• Career development
• Research
• Work-life balance
• Practice development
• Transition to practice
• Leadership development
Mentee responsibilities will include the following:
• Participate in an introductory call with her mentor
• Attend the ACS Clinical Congress in San Francisco to meet her mentor
• Establish a plan to cultivate one or more of the areas in need of mentoring and identify specific goals
• Commit to connecting with the mentor at least on a quarterly basis
• Complete an evaluation form and submit a brief summary on the mentee experience at the conclusion of the program in October 2015.
Interested individuals should contact Connie Bura, Assistant Director, ACS Member Services, at [email protected] to receive an application. In addition to the application, candidates must submit their curriculum vitae by July 31, along with a personal statement discussing the benefits that the program will provide to their career.
Mentors
The Women in Surgery Committee also seeks individuals to serve as mentors for the program. Mentor responsibilities will include the following:
• Participate in an introductory call with the mentee
• Attend the ACS Clinical Congress in San Francisco to meet the mentee
• Provide opportunities for interaction, at least quarterly, with the mentee
• Assist the mentee in her mentorship area(s) of interest and in identifying specific goals
• Complete an evaluation form and submit a brief summary on the mentor experience at the conclusion of the program in October 2015
Individuals interested in serving as mentors should contact Ms. Bura at [email protected] before July 31.
Residents, prepare to take your ACS membershipto the next level
Resident Members of the American College of Surgeons (ACS) who are transitioning from training into practice are encouraged to apply for Associate Fellowship.
To apply, you must provide basic information regarding education and training, licensure, board certification, and current hospital and academic affiliations—some of which already exist in your Resident Member record. If you are current with your Resident Membership dues, the ACS will waive the $75 Associate Fellow application fee.
Associate Fellowship is limited to a period of six years to foster progression to the Fellowship level. The ACS encourages Associate Fellows to begin thinking about applying for full Fellowship once they have met the following requirements:
• Certification by an appropriate American Board of Medical Specialties surgical specialty board, an American osteopathic surgical specialty board, or the Royal College of Surgeons in Canada
• One year of surgical practice after the completion of all formal training (including fellowships)
• A current appointment at a primary hospital
To submit the electronic application for Associate Fellowship, go to the ACS website, http://www.facs.org/memberservices/documents.html#associate.
You will need to have your ACS members-only website log-in information to access the application. If you do not have your log-in information, please contact staff in the Division of Member Services at 800-293-4029 or via e-mail at [email protected] for assistance.
Once the application has beenis completed, you will be asked to verify satisfactory completion of a surgical residency program and, if applicable, continued training in a second surgical residency or research or fellowship program.
When the application has been processed, you will receive an e-mail notification with updated membership information.
Resident Members of the American College of Surgeons (ACS) who are transitioning from training into practice are encouraged to apply for Associate Fellowship.
To apply, you must provide basic information regarding education and training, licensure, board certification, and current hospital and academic affiliations—some of which already exist in your Resident Member record. If you are current with your Resident Membership dues, the ACS will waive the $75 Associate Fellow application fee.
Associate Fellowship is limited to a period of six years to foster progression to the Fellowship level. The ACS encourages Associate Fellows to begin thinking about applying for full Fellowship once they have met the following requirements:
• Certification by an appropriate American Board of Medical Specialties surgical specialty board, an American osteopathic surgical specialty board, or the Royal College of Surgeons in Canada
• One year of surgical practice after the completion of all formal training (including fellowships)
• A current appointment at a primary hospital
To submit the electronic application for Associate Fellowship, go to the ACS website, http://www.facs.org/memberservices/documents.html#associate.
You will need to have your ACS members-only website log-in information to access the application. If you do not have your log-in information, please contact staff in the Division of Member Services at 800-293-4029 or via e-mail at [email protected] for assistance.
Once the application has beenis completed, you will be asked to verify satisfactory completion of a surgical residency program and, if applicable, continued training in a second surgical residency or research or fellowship program.
When the application has been processed, you will receive an e-mail notification with updated membership information.
Resident Members of the American College of Surgeons (ACS) who are transitioning from training into practice are encouraged to apply for Associate Fellowship.
To apply, you must provide basic information regarding education and training, licensure, board certification, and current hospital and academic affiliations—some of which already exist in your Resident Member record. If you are current with your Resident Membership dues, the ACS will waive the $75 Associate Fellow application fee.
Associate Fellowship is limited to a period of six years to foster progression to the Fellowship level. The ACS encourages Associate Fellows to begin thinking about applying for full Fellowship once they have met the following requirements:
• Certification by an appropriate American Board of Medical Specialties surgical specialty board, an American osteopathic surgical specialty board, or the Royal College of Surgeons in Canada
• One year of surgical practice after the completion of all formal training (including fellowships)
• A current appointment at a primary hospital
To submit the electronic application for Associate Fellowship, go to the ACS website, http://www.facs.org/memberservices/documents.html#associate.
You will need to have your ACS members-only website log-in information to access the application. If you do not have your log-in information, please contact staff in the Division of Member Services at 800-293-4029 or via e-mail at [email protected] for assistance.
Once the application has beenis completed, you will be asked to verify satisfactory completion of a surgical residency program and, if applicable, continued training in a second surgical residency or research or fellowship program.
When the application has been processed, you will receive an e-mail notification with updated membership information.