User login
Official Newspaper of the American College of Surgeons
Achieving aortic arch replacement without deep hypothermic circulatory arrest
NEW YORK – A novel technique for aortic arch repair, which avoids both circulatory arrest and profound hypothermia, was associated with low mortality and low cerebral morbidity in a group of 62 patients who underwent aortic arch replacement using this procedure.
Advantages of the "branch-first" procedure include visceral organ and cardiac protection and less need for blood/product transfusions, according to Dr. George Matalanis, who presented the findings at the meeting sponsored by the American Association for Thoracic Surgery.
The branch-first technique avoids many of the pitfalls associated with the widely used combination of antegrade perfusion and deep hypothermic circulatory arrest (DHTA). Such pitfalls include a higher incidence of cerebral injury than with proximal aortic surgery, as well as renal failure, ischemic hepatitis, and paraplegia, said Dr. Matalanis, a cardiothoracic surgeon at Austin Hospital, Kew, Australia. He noted that perfusion cannulas used for antegrade perfusion increase the risk of particulate and air emboli and branch injury or dissection, and their insertion extends cumulative cerebral circulatory arrest time.
DHTA also has a number of clinically significant disadvantages, such as prolongation of cardiopulmonary bypass times during cooling and rewarming, increased potential for cerebral ischemic reperfusion injury, and imposition of time constraints.
A feature of the branch-first technique is that it allows a complete and unhurried repair, allowing the surgeon to systematically interrogate each branch anastomosis, resulting in a low incidence of blood product use. By maintaining cardiac and distal body perfusion, even the most complex reconstructions can be carefully accomplished without concern about incomplete organ protection, allowing complete correction of pathology, according to Dr. Matalanis. The technique avoids the need for deep hypothermia, circulatory arrest, extended periods of cardiopulmonary bypass, and cerebral, cardiac and other organ circulatory exclusion.
Dr. Matalanis presented the results of a study involving 62 patients who underwent arch replacement using the branch-first procedure. The mean age was 65 years, and 60% were men. Almost 40% were urgent/emergent, and 32% had acute type A aortic dissection. One quarter had previous cardiac surgery. This work is an extension of an earlier study, which reported outcomes in 42 patients (Ann. Cardiothorac. Surg. 2013;2:194-201).
There were two deaths (3%), both in patients with acute type A aortic dissection with malperfusion. Neurological events occurred in 5%, with one permanent deficit. Six percent required renal support and 2% intra-aortic balloon support. One-quarter did not need red blood cell transfusion, and 15% did not require red blood cells or platelets/factors. Patients awakened as if they had undergone coronary artery bypass grafting (80% within 48 hours and 25% within 11 hours).
"This technique relies on two basic principles," explained Dr. Matalanis "The first is the extensive collateral network that exists between the three major arch branches, in addition to and far more expansive than the circle of Willis, allowing the brief interruption of one branch to be compensated by collateral flow from the other two. The second is the use of a modified trifurcation graft with a perfusion side arm to reconstruct the arch branches, allowing antegrade perfusion to resume as soon as each branch is reconstructed."
The procedure consists of establishing bypass using femoral inflow and moderate hypothermia (28° C), followed by serial disconnection and reconstruction of each arch branch, proceeding from the innominate to left subclavian, using a trifurcation arch graft with a perfusion side arm port. The proximal descending aorta is clamped, the distal arch anastomosis is constructed, and the aortic root reconstruction is completed. Finally, a connection is made between the common stem of the trifurcation graft and the ascending aorta graft.
There are no periods of global circulatory arrest, no interruption to cerebral perfusion during the anastomosis of the two grafts, and cardiac perfusion is maintained during the whole phase of arch branch reconstruction. Distal organ perfusion is also maintained throughout the procedure, says Dr. Matalanis.
Disadvantages of the branch-first technique include loss of the deep hypothermia "security blanket," the possibility of clamp injuries or air/thrombo-embolism, and difficulties with femoral cannulation. Issues may also arise with kinking or stenosis of the trifurcation graft and adhesions.
Dr. Matalanis reported having no relevant financial disclosures.
NEW YORK – A novel technique for aortic arch repair, which avoids both circulatory arrest and profound hypothermia, was associated with low mortality and low cerebral morbidity in a group of 62 patients who underwent aortic arch replacement using this procedure.
Advantages of the "branch-first" procedure include visceral organ and cardiac protection and less need for blood/product transfusions, according to Dr. George Matalanis, who presented the findings at the meeting sponsored by the American Association for Thoracic Surgery.
The branch-first technique avoids many of the pitfalls associated with the widely used combination of antegrade perfusion and deep hypothermic circulatory arrest (DHTA). Such pitfalls include a higher incidence of cerebral injury than with proximal aortic surgery, as well as renal failure, ischemic hepatitis, and paraplegia, said Dr. Matalanis, a cardiothoracic surgeon at Austin Hospital, Kew, Australia. He noted that perfusion cannulas used for antegrade perfusion increase the risk of particulate and air emboli and branch injury or dissection, and their insertion extends cumulative cerebral circulatory arrest time.
DHTA also has a number of clinically significant disadvantages, such as prolongation of cardiopulmonary bypass times during cooling and rewarming, increased potential for cerebral ischemic reperfusion injury, and imposition of time constraints.
A feature of the branch-first technique is that it allows a complete and unhurried repair, allowing the surgeon to systematically interrogate each branch anastomosis, resulting in a low incidence of blood product use. By maintaining cardiac and distal body perfusion, even the most complex reconstructions can be carefully accomplished without concern about incomplete organ protection, allowing complete correction of pathology, according to Dr. Matalanis. The technique avoids the need for deep hypothermia, circulatory arrest, extended periods of cardiopulmonary bypass, and cerebral, cardiac and other organ circulatory exclusion.
Dr. Matalanis presented the results of a study involving 62 patients who underwent arch replacement using the branch-first procedure. The mean age was 65 years, and 60% were men. Almost 40% were urgent/emergent, and 32% had acute type A aortic dissection. One quarter had previous cardiac surgery. This work is an extension of an earlier study, which reported outcomes in 42 patients (Ann. Cardiothorac. Surg. 2013;2:194-201).
There were two deaths (3%), both in patients with acute type A aortic dissection with malperfusion. Neurological events occurred in 5%, with one permanent deficit. Six percent required renal support and 2% intra-aortic balloon support. One-quarter did not need red blood cell transfusion, and 15% did not require red blood cells or platelets/factors. Patients awakened as if they had undergone coronary artery bypass grafting (80% within 48 hours and 25% within 11 hours).
"This technique relies on two basic principles," explained Dr. Matalanis "The first is the extensive collateral network that exists between the three major arch branches, in addition to and far more expansive than the circle of Willis, allowing the brief interruption of one branch to be compensated by collateral flow from the other two. The second is the use of a modified trifurcation graft with a perfusion side arm to reconstruct the arch branches, allowing antegrade perfusion to resume as soon as each branch is reconstructed."
The procedure consists of establishing bypass using femoral inflow and moderate hypothermia (28° C), followed by serial disconnection and reconstruction of each arch branch, proceeding from the innominate to left subclavian, using a trifurcation arch graft with a perfusion side arm port. The proximal descending aorta is clamped, the distal arch anastomosis is constructed, and the aortic root reconstruction is completed. Finally, a connection is made between the common stem of the trifurcation graft and the ascending aorta graft.
There are no periods of global circulatory arrest, no interruption to cerebral perfusion during the anastomosis of the two grafts, and cardiac perfusion is maintained during the whole phase of arch branch reconstruction. Distal organ perfusion is also maintained throughout the procedure, says Dr. Matalanis.
Disadvantages of the branch-first technique include loss of the deep hypothermia "security blanket," the possibility of clamp injuries or air/thrombo-embolism, and difficulties with femoral cannulation. Issues may also arise with kinking or stenosis of the trifurcation graft and adhesions.
Dr. Matalanis reported having no relevant financial disclosures.
NEW YORK – A novel technique for aortic arch repair, which avoids both circulatory arrest and profound hypothermia, was associated with low mortality and low cerebral morbidity in a group of 62 patients who underwent aortic arch replacement using this procedure.
Advantages of the "branch-first" procedure include visceral organ and cardiac protection and less need for blood/product transfusions, according to Dr. George Matalanis, who presented the findings at the meeting sponsored by the American Association for Thoracic Surgery.
The branch-first technique avoids many of the pitfalls associated with the widely used combination of antegrade perfusion and deep hypothermic circulatory arrest (DHTA). Such pitfalls include a higher incidence of cerebral injury than with proximal aortic surgery, as well as renal failure, ischemic hepatitis, and paraplegia, said Dr. Matalanis, a cardiothoracic surgeon at Austin Hospital, Kew, Australia. He noted that perfusion cannulas used for antegrade perfusion increase the risk of particulate and air emboli and branch injury or dissection, and their insertion extends cumulative cerebral circulatory arrest time.
DHTA also has a number of clinically significant disadvantages, such as prolongation of cardiopulmonary bypass times during cooling and rewarming, increased potential for cerebral ischemic reperfusion injury, and imposition of time constraints.
A feature of the branch-first technique is that it allows a complete and unhurried repair, allowing the surgeon to systematically interrogate each branch anastomosis, resulting in a low incidence of blood product use. By maintaining cardiac and distal body perfusion, even the most complex reconstructions can be carefully accomplished without concern about incomplete organ protection, allowing complete correction of pathology, according to Dr. Matalanis. The technique avoids the need for deep hypothermia, circulatory arrest, extended periods of cardiopulmonary bypass, and cerebral, cardiac and other organ circulatory exclusion.
Dr. Matalanis presented the results of a study involving 62 patients who underwent arch replacement using the branch-first procedure. The mean age was 65 years, and 60% were men. Almost 40% were urgent/emergent, and 32% had acute type A aortic dissection. One quarter had previous cardiac surgery. This work is an extension of an earlier study, which reported outcomes in 42 patients (Ann. Cardiothorac. Surg. 2013;2:194-201).
There were two deaths (3%), both in patients with acute type A aortic dissection with malperfusion. Neurological events occurred in 5%, with one permanent deficit. Six percent required renal support and 2% intra-aortic balloon support. One-quarter did not need red blood cell transfusion, and 15% did not require red blood cells or platelets/factors. Patients awakened as if they had undergone coronary artery bypass grafting (80% within 48 hours and 25% within 11 hours).
"This technique relies on two basic principles," explained Dr. Matalanis "The first is the extensive collateral network that exists between the three major arch branches, in addition to and far more expansive than the circle of Willis, allowing the brief interruption of one branch to be compensated by collateral flow from the other two. The second is the use of a modified trifurcation graft with a perfusion side arm to reconstruct the arch branches, allowing antegrade perfusion to resume as soon as each branch is reconstructed."
The procedure consists of establishing bypass using femoral inflow and moderate hypothermia (28° C), followed by serial disconnection and reconstruction of each arch branch, proceeding from the innominate to left subclavian, using a trifurcation arch graft with a perfusion side arm port. The proximal descending aorta is clamped, the distal arch anastomosis is constructed, and the aortic root reconstruction is completed. Finally, a connection is made between the common stem of the trifurcation graft and the ascending aorta graft.
There are no periods of global circulatory arrest, no interruption to cerebral perfusion during the anastomosis of the two grafts, and cardiac perfusion is maintained during the whole phase of arch branch reconstruction. Distal organ perfusion is also maintained throughout the procedure, says Dr. Matalanis.
Disadvantages of the branch-first technique include loss of the deep hypothermia "security blanket," the possibility of clamp injuries or air/thrombo-embolism, and difficulties with femoral cannulation. Issues may also arise with kinking or stenosis of the trifurcation graft and adhesions.
Dr. Matalanis reported having no relevant financial disclosures.
AT AATS AORTIC SYMPOSIUM 2014
Key clinical point: By maintaining cardiac and distal body perfusion, complex reconstructions can be accomplished without concern about organ protection, allowing complete correction of aortic pathology.
Major finding: A method that avoids deep hypothermic circulatory arrest was associated with low mortality (3.2%) and low cerebral morbidity (5%).
Data source: Cohort study of 62 patients.
Disclosures: Dr. Matalanis reported having no relevant financial disclosures.
Mayo Clinic tops hospital rankings for 2014-2015
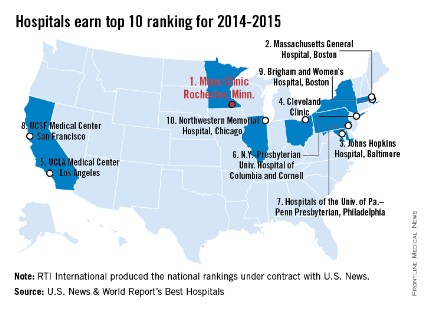
The Mayo Clinic in Rochester, Minn., is the best hospital in the United States, according to the U.S. News & World Report Best Hospitals rankings for 2014-2015.
This is the first time that the Mayo Clinic has taken the top spot in the Best Hospitals Honor Roll. This year’s second-place finisher was Massachusetts General Hospital in Boston, followed by Johns Hopkins Hospital in Baltimore – which won last year – the Cleveland Clinic, and UCLA Medical Center in Los Angeles, U.S. News announced.
For 2014-2015, there were 17 hospitals in the Honor Roll, which is reserved for those institutions that finish at or near the top in 6 or more of the 16 specialties included in the U.S. News rankings. This year, only 144 hospitals did well enough to be nationally ranked in one or more specialties. The 16 ranked specialties are cancer, cardiology and heart surgery; diabetes and endocrinology; otolaryngology; gastroenterology and gastrointestinal surgery; geriatrics; gynecology; nephrology; neurology and neurosurgery; ophthalmology; orthopedics; psychiatry; pulmonology; rehabilitation; rheumatology; and urology.
The ranking process initially comprised 4,743 nonfederal community hospitals, which were rated in the 16 specialties. In the 12 data-driven specialties, scores were based on four elements: reputation with specialists (27.5%), survival (32.5%), patient safety (10%), and other care-related indicators (30%). Four specialties were rated by reputation only. The research organization RTI International conducted the physician survey and produced the Best Hospitals methodology and national rankings under contract with U.S. News.
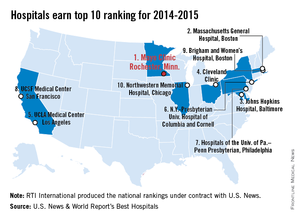
The Mayo Clinic in Rochester, Minn., is the best hospital in the United States, according to the U.S. News & World Report Best Hospitals rankings for 2014-2015.
This is the first time that the Mayo Clinic has taken the top spot in the Best Hospitals Honor Roll. This year’s second-place finisher was Massachusetts General Hospital in Boston, followed by Johns Hopkins Hospital in Baltimore – which won last year – the Cleveland Clinic, and UCLA Medical Center in Los Angeles, U.S. News announced.
For 2014-2015, there were 17 hospitals in the Honor Roll, which is reserved for those institutions that finish at or near the top in 6 or more of the 16 specialties included in the U.S. News rankings. This year, only 144 hospitals did well enough to be nationally ranked in one or more specialties. The 16 ranked specialties are cancer, cardiology and heart surgery; diabetes and endocrinology; otolaryngology; gastroenterology and gastrointestinal surgery; geriatrics; gynecology; nephrology; neurology and neurosurgery; ophthalmology; orthopedics; psychiatry; pulmonology; rehabilitation; rheumatology; and urology.
The ranking process initially comprised 4,743 nonfederal community hospitals, which were rated in the 16 specialties. In the 12 data-driven specialties, scores were based on four elements: reputation with specialists (27.5%), survival (32.5%), patient safety (10%), and other care-related indicators (30%). Four specialties were rated by reputation only. The research organization RTI International conducted the physician survey and produced the Best Hospitals methodology and national rankings under contract with U.S. News.

The Mayo Clinic in Rochester, Minn., is the best hospital in the United States, according to the U.S. News & World Report Best Hospitals rankings for 2014-2015.
This is the first time that the Mayo Clinic has taken the top spot in the Best Hospitals Honor Roll. This year’s second-place finisher was Massachusetts General Hospital in Boston, followed by Johns Hopkins Hospital in Baltimore – which won last year – the Cleveland Clinic, and UCLA Medical Center in Los Angeles, U.S. News announced.
For 2014-2015, there were 17 hospitals in the Honor Roll, which is reserved for those institutions that finish at or near the top in 6 or more of the 16 specialties included in the U.S. News rankings. This year, only 144 hospitals did well enough to be nationally ranked in one or more specialties. The 16 ranked specialties are cancer, cardiology and heart surgery; diabetes and endocrinology; otolaryngology; gastroenterology and gastrointestinal surgery; geriatrics; gynecology; nephrology; neurology and neurosurgery; ophthalmology; orthopedics; psychiatry; pulmonology; rehabilitation; rheumatology; and urology.
The ranking process initially comprised 4,743 nonfederal community hospitals, which were rated in the 16 specialties. In the 12 data-driven specialties, scores were based on four elements: reputation with specialists (27.5%), survival (32.5%), patient safety (10%), and other care-related indicators (30%). Four specialties were rated by reputation only. The research organization RTI International conducted the physician survey and produced the Best Hospitals methodology and national rankings under contract with U.S. News.

Specialists drive overtreatment of low-risk prostate cancer
Low-risk prostate cancer in older men is still being overtreated, according to two separate studies that examined the issue from different perspectives, both of which were published online July 14 in JAMA Internal Medicine.
One group of researchers found that primary androgen deprivation therapy fails to improve either overall or disease-specific survival in this patient population, yet it still is widely used as the initial treatment for localized disease. And another group found that urologists and radiation oncologists are the driving force behind the overly aggressive approach to low-risk prostate cancer in older men.
Both groups of investigators called for efforts to limit these harmful trends.
In the first study, Grace L. Lu-Yao, Ph.D., and her associates analyzed information on 66,717 cases of prostate cancer in the Surveillance, Epidemiology, and End Results (SEER) and Medicaid databases diagnosed in 1992-2009. All the patients were aged 66 years and older, and all had T1/T2 disease. There were 5,275 deaths from prostate cancer and 39,801 deaths from all causes during nearly 20 years of follow-up.
Primary androgen deprivation therapy failed to improve either overall or disease-specific survival at 5 years or 15 years. Further study using instrumental variable analysis to control for an imbalance in risk factors between users and nonusers of androgen deprivation therapy confirmed these results, as did several sensitivity analyses, "suggesting that our conclusions are robust" (JAMA Intern. Med. 2014 July 14 [doi: 10.1001/jamainternmed.2014.3028]).
"For patients with less aggressive cancers, deferred androgen deprivation therapy is safe and reduces the risks of treatment-associated adverse effects, such as osteoporosis, weight gain, decreased libido, decreased muscle tone, diabetes mellitus, and metabolic syndrome," wrote Dr. Lu-Yao of Rutgers Cancer Institute of New Jersey, New Brunswick, and her associates.
"Physicians and patients often believe that treatment is necessary and beneficial. Our data suggest that this may not be the case, at least for primary androgen deprivation therapy," they said.
In the other study, investigators examined physician and patient factors that influence treatment decisions in low-risk prostate cancer. They also analyzed SEER data, this time involving 12,068 men aged 66 years and older (median age, 72 years) who were diagnosed as having low-risk prostate adenocarcinoma in 2006-2009. These men were diagnosed by 2,145 urologists; 68% of them also consulted a radiation oncologist, said Dr. Karen E. Hoffman of the University of Texas M.D. Anderson Cancer Center, Houston, and her associates.
Fully 80% of the low-risk patients diagnosed by a urologist immediately received treatment; only 20% instead underwent observation, as is recommended. The use of observation varied markedly across urologists, with some performing observation for less than 5% of their patients and others performing observation for nearly 65%. Forty urologists (10.2%) had rates of observation that were significantly different from the mean.
In analyses that estimated the relative contributions of numerous factors to treatment decisions, "the diagnosing urologist was the most influential measured factor, responsible for 16.1% of the variance in management choice; just 7.9% of the variance was attributable to patient characteristics," Dr. Hoffman and her associates reported (JAMA Intern. Med. 2014 July 14 [doi: 10.1001/jamainternmed.2014.3021]).
Similarly, of the 7,554 men who consulted 870 radiation oncologists, a remarkable 91.5% underwent immediate treatment (usually radiotherapy), while only 8.5% underwent observation, as is recommended. The use of observation also varied markedly across radiation oncologists, with some advising observation for only 2% of their patients and others advising it for 47%. Again, the variance in treatment decisions attributable to radiation oncologists was at least double that attributable to patient factors.
"In our cohort, 70.0% of men aged 76-80 years and 55.1% of men older than 80 years still received up-front treatment," a striking proportion because the average life expectancy for men 77 years and older in the United States is less than 10 years. "Older men, especially those with multiple medical conditions, are not thought to gain a survival benefit from treatment of low-risk prostate cancer," Dr. Hoffman and her colleagues noted.
Their findings are important because most primary care physicians who refer their patients to specialists for prostate biopsy or consultation probably "assume that patients will receive similar management recommendations regardless of which [specialist] they see." These results demonstrate the opposite: Patients with low-risk prostate cancer could receive widely divergent treatment advice, solely depending on the specialists’ preferences.
Dr. Lu-Yao’s study was supported by the National Cancer Institute and the Cancer Institute of New Jersey. She reported ties to Merck and Schering-Plough, and one of her associates reported receiving research funding from Myriad. Dr. Hoffman’s study was supported by the Cancer Prevention and Research Institute of Texas, the National Cancer Institute, the American Cancer Society, the Duncan Family Institute, the University of Texas M.D. Anderson Cancer Center, and the National Institutes of Health. She reported no potential financial conflicts of interest, and one of her associates reported receiving research support from Varian Medical Systems.
Low-risk prostate cancer in older men is still being overtreated, according to two separate studies that examined the issue from different perspectives, both of which were published online July 14 in JAMA Internal Medicine.
One group of researchers found that primary androgen deprivation therapy fails to improve either overall or disease-specific survival in this patient population, yet it still is widely used as the initial treatment for localized disease. And another group found that urologists and radiation oncologists are the driving force behind the overly aggressive approach to low-risk prostate cancer in older men.
Both groups of investigators called for efforts to limit these harmful trends.
In the first study, Grace L. Lu-Yao, Ph.D., and her associates analyzed information on 66,717 cases of prostate cancer in the Surveillance, Epidemiology, and End Results (SEER) and Medicaid databases diagnosed in 1992-2009. All the patients were aged 66 years and older, and all had T1/T2 disease. There were 5,275 deaths from prostate cancer and 39,801 deaths from all causes during nearly 20 years of follow-up.
Primary androgen deprivation therapy failed to improve either overall or disease-specific survival at 5 years or 15 years. Further study using instrumental variable analysis to control for an imbalance in risk factors between users and nonusers of androgen deprivation therapy confirmed these results, as did several sensitivity analyses, "suggesting that our conclusions are robust" (JAMA Intern. Med. 2014 July 14 [doi: 10.1001/jamainternmed.2014.3028]).
"For patients with less aggressive cancers, deferred androgen deprivation therapy is safe and reduces the risks of treatment-associated adverse effects, such as osteoporosis, weight gain, decreased libido, decreased muscle tone, diabetes mellitus, and metabolic syndrome," wrote Dr. Lu-Yao of Rutgers Cancer Institute of New Jersey, New Brunswick, and her associates.
"Physicians and patients often believe that treatment is necessary and beneficial. Our data suggest that this may not be the case, at least for primary androgen deprivation therapy," they said.
In the other study, investigators examined physician and patient factors that influence treatment decisions in low-risk prostate cancer. They also analyzed SEER data, this time involving 12,068 men aged 66 years and older (median age, 72 years) who were diagnosed as having low-risk prostate adenocarcinoma in 2006-2009. These men were diagnosed by 2,145 urologists; 68% of them also consulted a radiation oncologist, said Dr. Karen E. Hoffman of the University of Texas M.D. Anderson Cancer Center, Houston, and her associates.
Fully 80% of the low-risk patients diagnosed by a urologist immediately received treatment; only 20% instead underwent observation, as is recommended. The use of observation varied markedly across urologists, with some performing observation for less than 5% of their patients and others performing observation for nearly 65%. Forty urologists (10.2%) had rates of observation that were significantly different from the mean.
In analyses that estimated the relative contributions of numerous factors to treatment decisions, "the diagnosing urologist was the most influential measured factor, responsible for 16.1% of the variance in management choice; just 7.9% of the variance was attributable to patient characteristics," Dr. Hoffman and her associates reported (JAMA Intern. Med. 2014 July 14 [doi: 10.1001/jamainternmed.2014.3021]).
Similarly, of the 7,554 men who consulted 870 radiation oncologists, a remarkable 91.5% underwent immediate treatment (usually radiotherapy), while only 8.5% underwent observation, as is recommended. The use of observation also varied markedly across radiation oncologists, with some advising observation for only 2% of their patients and others advising it for 47%. Again, the variance in treatment decisions attributable to radiation oncologists was at least double that attributable to patient factors.
"In our cohort, 70.0% of men aged 76-80 years and 55.1% of men older than 80 years still received up-front treatment," a striking proportion because the average life expectancy for men 77 years and older in the United States is less than 10 years. "Older men, especially those with multiple medical conditions, are not thought to gain a survival benefit from treatment of low-risk prostate cancer," Dr. Hoffman and her colleagues noted.
Their findings are important because most primary care physicians who refer their patients to specialists for prostate biopsy or consultation probably "assume that patients will receive similar management recommendations regardless of which [specialist] they see." These results demonstrate the opposite: Patients with low-risk prostate cancer could receive widely divergent treatment advice, solely depending on the specialists’ preferences.
Dr. Lu-Yao’s study was supported by the National Cancer Institute and the Cancer Institute of New Jersey. She reported ties to Merck and Schering-Plough, and one of her associates reported receiving research funding from Myriad. Dr. Hoffman’s study was supported by the Cancer Prevention and Research Institute of Texas, the National Cancer Institute, the American Cancer Society, the Duncan Family Institute, the University of Texas M.D. Anderson Cancer Center, and the National Institutes of Health. She reported no potential financial conflicts of interest, and one of her associates reported receiving research support from Varian Medical Systems.
Low-risk prostate cancer in older men is still being overtreated, according to two separate studies that examined the issue from different perspectives, both of which were published online July 14 in JAMA Internal Medicine.
One group of researchers found that primary androgen deprivation therapy fails to improve either overall or disease-specific survival in this patient population, yet it still is widely used as the initial treatment for localized disease. And another group found that urologists and radiation oncologists are the driving force behind the overly aggressive approach to low-risk prostate cancer in older men.
Both groups of investigators called for efforts to limit these harmful trends.
In the first study, Grace L. Lu-Yao, Ph.D., and her associates analyzed information on 66,717 cases of prostate cancer in the Surveillance, Epidemiology, and End Results (SEER) and Medicaid databases diagnosed in 1992-2009. All the patients were aged 66 years and older, and all had T1/T2 disease. There were 5,275 deaths from prostate cancer and 39,801 deaths from all causes during nearly 20 years of follow-up.
Primary androgen deprivation therapy failed to improve either overall or disease-specific survival at 5 years or 15 years. Further study using instrumental variable analysis to control for an imbalance in risk factors between users and nonusers of androgen deprivation therapy confirmed these results, as did several sensitivity analyses, "suggesting that our conclusions are robust" (JAMA Intern. Med. 2014 July 14 [doi: 10.1001/jamainternmed.2014.3028]).
"For patients with less aggressive cancers, deferred androgen deprivation therapy is safe and reduces the risks of treatment-associated adverse effects, such as osteoporosis, weight gain, decreased libido, decreased muscle tone, diabetes mellitus, and metabolic syndrome," wrote Dr. Lu-Yao of Rutgers Cancer Institute of New Jersey, New Brunswick, and her associates.
"Physicians and patients often believe that treatment is necessary and beneficial. Our data suggest that this may not be the case, at least for primary androgen deprivation therapy," they said.
In the other study, investigators examined physician and patient factors that influence treatment decisions in low-risk prostate cancer. They also analyzed SEER data, this time involving 12,068 men aged 66 years and older (median age, 72 years) who were diagnosed as having low-risk prostate adenocarcinoma in 2006-2009. These men were diagnosed by 2,145 urologists; 68% of them also consulted a radiation oncologist, said Dr. Karen E. Hoffman of the University of Texas M.D. Anderson Cancer Center, Houston, and her associates.
Fully 80% of the low-risk patients diagnosed by a urologist immediately received treatment; only 20% instead underwent observation, as is recommended. The use of observation varied markedly across urologists, with some performing observation for less than 5% of their patients and others performing observation for nearly 65%. Forty urologists (10.2%) had rates of observation that were significantly different from the mean.
In analyses that estimated the relative contributions of numerous factors to treatment decisions, "the diagnosing urologist was the most influential measured factor, responsible for 16.1% of the variance in management choice; just 7.9% of the variance was attributable to patient characteristics," Dr. Hoffman and her associates reported (JAMA Intern. Med. 2014 July 14 [doi: 10.1001/jamainternmed.2014.3021]).
Similarly, of the 7,554 men who consulted 870 radiation oncologists, a remarkable 91.5% underwent immediate treatment (usually radiotherapy), while only 8.5% underwent observation, as is recommended. The use of observation also varied markedly across radiation oncologists, with some advising observation for only 2% of their patients and others advising it for 47%. Again, the variance in treatment decisions attributable to radiation oncologists was at least double that attributable to patient factors.
"In our cohort, 70.0% of men aged 76-80 years and 55.1% of men older than 80 years still received up-front treatment," a striking proportion because the average life expectancy for men 77 years and older in the United States is less than 10 years. "Older men, especially those with multiple medical conditions, are not thought to gain a survival benefit from treatment of low-risk prostate cancer," Dr. Hoffman and her colleagues noted.
Their findings are important because most primary care physicians who refer their patients to specialists for prostate biopsy or consultation probably "assume that patients will receive similar management recommendations regardless of which [specialist] they see." These results demonstrate the opposite: Patients with low-risk prostate cancer could receive widely divergent treatment advice, solely depending on the specialists’ preferences.
Dr. Lu-Yao’s study was supported by the National Cancer Institute and the Cancer Institute of New Jersey. She reported ties to Merck and Schering-Plough, and one of her associates reported receiving research funding from Myriad. Dr. Hoffman’s study was supported by the Cancer Prevention and Research Institute of Texas, the National Cancer Institute, the American Cancer Society, the Duncan Family Institute, the University of Texas M.D. Anderson Cancer Center, and the National Institutes of Health. She reported no potential financial conflicts of interest, and one of her associates reported receiving research support from Varian Medical Systems.
FROM JAMA INTERNAL MEDICINE
Key clinical point: Patients with low-risk prostate cancer receive widely divergent treatment advice, based on specialists’ preferences above patient characteristics or evidence.
Major finding: A large database analysis showed that primary androgen deprivation therapy failed to improve either overall or disease-specific survival at 5 years or 15 years in men with low-risk prostate cancer. In another study, 80% of low-risk men diagnosed by urologists received immediate treatment rather than undergoing observation as recommended, as did 91.5% of those who consulted radiation oncologists.
Data source: A population-based cohort study involving 66,717 men aged 66 years and older diagnosed as having low-risk prostate cancer in 1992-2009, and a population-based cohort study involving 12,068 men aged 66 and older who were similarly diagnosed in 2006-2009 by 2,145 urologists and who consulted with 870 radiation oncologists.
Disclosures: Dr. Lu-Yao’s study was supported by the National Cancer Institute and the Cancer Institute of New Jersey. She reported ties to Merck and Schering-Plough, and one of her associates reported receiving research funding from Myriad. Dr. Hoffman’s study was supported by the Cancer Prevention and Research Institute of Texas, the National Cancer Institute, the American Cancer Society, the Duncan Family Institute, the University of Texas M.D. Anderson Cancer Center, and the National Institutes of Health. She reported no potential financial conflicts of interest, and one of her associates reported receiving research support from Varian Medical Systems.
J-Tip syringe cuts venipuncture pain in young kids
DALLAS – Jet-injected lidocaine is superior to vapocoolant spray in reducing venipuncture pain in children under 7 years of age, according to a randomized, double-blind clinical trial.
This form of needle-free local anesthesia, administered through what is popularly known as the J-Tip syringe, has been shown previously to decrease venipuncture pain in adults and older children. But data regarding the effectiveness of the J-Tip in young children has been scanty until now, Dr. Maren M. Lunoe noted at the annual meeting of the Society for Academic Emergency Medicine.
She presented the findings of a randomized, sham-controlled, double-blind clinical trial involving 205 children aged 1-6 years who presented for venipuncture. Ninety percent had undergone the procedure before.
The J-Tip device utilizes a cartridge of compressed carbon dioxide to drive buffered lidocaine into the skin. However, it does so with a loud "pop," which is why the study included a control arm featuring a sham J-Tip syringe with the compressed gas but no lidocaine, explained Dr. Lunoe of the Medical College of Wisconsin, Milwaukee.
Participants were randomized 2:1:1 to the J-Tip syringe deployed roughly 30 seconds prior to venipuncture; to a refrigerated vapocoolant spray, also applied immediately prior to venipuncture, which has been usual care for a blood draw at Children’s Hospital of Wisconsin; or to a second control group who received vapocoolant spray and a sham J-Tip.
The primary endpoint was the FLACC score at venipuncture as assessed by two blinded physicians viewing videotapes of every procedure. The FLACC (Face, Legs, Activity, Cry, and Consolability) scale is a validated pain assessment tool scored 0-10.
The median FLACC score while the young children were waiting for the nurse was 2.0. The score jumped by about 2 points in all three groups when the child saw the device. It climbed by another 5.5 points from that point to the actual venipuncture in controls who got vapocoolant spray only, and by 2.5 points in those who got vapocoolant spray plus a sham J-Tip. These increases in FLACC pain score at venipuncture were statistically significant and clinically meaningful. In contrast, patients who received needle-free lidocaine through a loaded J-Tip syringe did not experience a significant increase in FLACC scores at venipuncture.
Forty-five percent of patients in the J-Tip group had no or only mild pain at venipuncture as defined by a FLACC score of 0-3. This was the case in only 23% of the vapocoolant spray–only group and in 30% of controls who got vapocoolant spray and a sham J-Tip.
There were no between-group differences in adverse events, all of which were minor, consisting mostly of mild bruising.
Asked why the study didn’t include a comparison arm pretreated with EMLA cream, Dr. Lunoe said that topical agent takes 30 minutes to take effect, making it unattractive for use in an emergency department or busy clinic.
National Medical Products and Gebauer, which market the J-Tip syringe and the vapocoolant spray, respectively, provided those supplies for the study but had no further involvement. Dr. Lunoe reported no financial conflicts with regard to the investigation.
DALLAS – Jet-injected lidocaine is superior to vapocoolant spray in reducing venipuncture pain in children under 7 years of age, according to a randomized, double-blind clinical trial.
This form of needle-free local anesthesia, administered through what is popularly known as the J-Tip syringe, has been shown previously to decrease venipuncture pain in adults and older children. But data regarding the effectiveness of the J-Tip in young children has been scanty until now, Dr. Maren M. Lunoe noted at the annual meeting of the Society for Academic Emergency Medicine.
She presented the findings of a randomized, sham-controlled, double-blind clinical trial involving 205 children aged 1-6 years who presented for venipuncture. Ninety percent had undergone the procedure before.
The J-Tip device utilizes a cartridge of compressed carbon dioxide to drive buffered lidocaine into the skin. However, it does so with a loud "pop," which is why the study included a control arm featuring a sham J-Tip syringe with the compressed gas but no lidocaine, explained Dr. Lunoe of the Medical College of Wisconsin, Milwaukee.
Participants were randomized 2:1:1 to the J-Tip syringe deployed roughly 30 seconds prior to venipuncture; to a refrigerated vapocoolant spray, also applied immediately prior to venipuncture, which has been usual care for a blood draw at Children’s Hospital of Wisconsin; or to a second control group who received vapocoolant spray and a sham J-Tip.
The primary endpoint was the FLACC score at venipuncture as assessed by two blinded physicians viewing videotapes of every procedure. The FLACC (Face, Legs, Activity, Cry, and Consolability) scale is a validated pain assessment tool scored 0-10.
The median FLACC score while the young children were waiting for the nurse was 2.0. The score jumped by about 2 points in all three groups when the child saw the device. It climbed by another 5.5 points from that point to the actual venipuncture in controls who got vapocoolant spray only, and by 2.5 points in those who got vapocoolant spray plus a sham J-Tip. These increases in FLACC pain score at venipuncture were statistically significant and clinically meaningful. In contrast, patients who received needle-free lidocaine through a loaded J-Tip syringe did not experience a significant increase in FLACC scores at venipuncture.
Forty-five percent of patients in the J-Tip group had no or only mild pain at venipuncture as defined by a FLACC score of 0-3. This was the case in only 23% of the vapocoolant spray–only group and in 30% of controls who got vapocoolant spray and a sham J-Tip.
There were no between-group differences in adverse events, all of which were minor, consisting mostly of mild bruising.
Asked why the study didn’t include a comparison arm pretreated with EMLA cream, Dr. Lunoe said that topical agent takes 30 minutes to take effect, making it unattractive for use in an emergency department or busy clinic.
National Medical Products and Gebauer, which market the J-Tip syringe and the vapocoolant spray, respectively, provided those supplies for the study but had no further involvement. Dr. Lunoe reported no financial conflicts with regard to the investigation.
DALLAS – Jet-injected lidocaine is superior to vapocoolant spray in reducing venipuncture pain in children under 7 years of age, according to a randomized, double-blind clinical trial.
This form of needle-free local anesthesia, administered through what is popularly known as the J-Tip syringe, has been shown previously to decrease venipuncture pain in adults and older children. But data regarding the effectiveness of the J-Tip in young children has been scanty until now, Dr. Maren M. Lunoe noted at the annual meeting of the Society for Academic Emergency Medicine.
She presented the findings of a randomized, sham-controlled, double-blind clinical trial involving 205 children aged 1-6 years who presented for venipuncture. Ninety percent had undergone the procedure before.
The J-Tip device utilizes a cartridge of compressed carbon dioxide to drive buffered lidocaine into the skin. However, it does so with a loud "pop," which is why the study included a control arm featuring a sham J-Tip syringe with the compressed gas but no lidocaine, explained Dr. Lunoe of the Medical College of Wisconsin, Milwaukee.
Participants were randomized 2:1:1 to the J-Tip syringe deployed roughly 30 seconds prior to venipuncture; to a refrigerated vapocoolant spray, also applied immediately prior to venipuncture, which has been usual care for a blood draw at Children’s Hospital of Wisconsin; or to a second control group who received vapocoolant spray and a sham J-Tip.
The primary endpoint was the FLACC score at venipuncture as assessed by two blinded physicians viewing videotapes of every procedure. The FLACC (Face, Legs, Activity, Cry, and Consolability) scale is a validated pain assessment tool scored 0-10.
The median FLACC score while the young children were waiting for the nurse was 2.0. The score jumped by about 2 points in all three groups when the child saw the device. It climbed by another 5.5 points from that point to the actual venipuncture in controls who got vapocoolant spray only, and by 2.5 points in those who got vapocoolant spray plus a sham J-Tip. These increases in FLACC pain score at venipuncture were statistically significant and clinically meaningful. In contrast, patients who received needle-free lidocaine through a loaded J-Tip syringe did not experience a significant increase in FLACC scores at venipuncture.
Forty-five percent of patients in the J-Tip group had no or only mild pain at venipuncture as defined by a FLACC score of 0-3. This was the case in only 23% of the vapocoolant spray–only group and in 30% of controls who got vapocoolant spray and a sham J-Tip.
There were no between-group differences in adverse events, all of which were minor, consisting mostly of mild bruising.
Asked why the study didn’t include a comparison arm pretreated with EMLA cream, Dr. Lunoe said that topical agent takes 30 minutes to take effect, making it unattractive for use in an emergency department or busy clinic.
National Medical Products and Gebauer, which market the J-Tip syringe and the vapocoolant spray, respectively, provided those supplies for the study but had no further involvement. Dr. Lunoe reported no financial conflicts with regard to the investigation.
AT SAEM 2014
Key clinical point: Needle-free local anesthesia via jet-injected lidocaine is a fast and effective means of reducing venipuncture pain for young children.
Major finding: Forty-five percent of young children had no or mild pain at venipuncture if they received jet-injected lidocaine immediately beforehand, a rate twice that seen in controls pretreated with a vapocoolant spray.
Data source: A randomized, prospective, double-blind, sham procedure-controlled study involving 205 children aged 1-6 years undergoing venipuncture.
Disclosures: The presenter reported having no financial conflicts regarding this study, which was carried out with institutional funds.
Hospital use of minimally invasive surgery shows disparity in surgical care nationwide
The use of minimally invasive surgery for appendectomy, colectomy, hysterectomy, and lung lobectomy varies widely in the United States, even though the complication rates were lower from each procedure than with open surgery, results from a large retrospective study demonstrated.
"This study has important implications for quality improvement," researchers led by Dr. Martin A. Makary, professor of surgery at Johns Hopkins University, Baltimore, wrote. "Based on our findings, many hospitals have an opportunity to decrease surgical complications by increasing utilization of minimally invasive surgery."
To investigate the levels of variation in the use of minimally invasive surgery across the United States, Dr. Makary and his associates used the National Inpatient Sample database, which is administered by the Agency for Healthcare Research & Quality, to evaluate hospitalizations at hospitals that performed at least 10 of these procedures in 2010. The sample included 1,051 hospitals in 45 states, and was limited to appendectomy, colectomy, hysterectomy, and lung lobectomy. The researchers used a propensity score model to calculate the predicted proportion of minimally invasive operations for each hospital based on patient characteristics. For each procedure, they categorized hospitals as low, medium, or high based on their actual to predicted proportion of minimally invasive surgery use (BMJ 2014;349:g4198).
On average, the use of minimally invasive surgery by the hospitals sampled was 71% for appendectomy, 28% for colectomy, 13% for hysterectomy, and 32% for lung lobectomy. Overall surgical complications for minimally invasive surgery, compared with open surgery, were, respectively, for appendectomy: 3.94% vs. 7.90% (P less than .001); colectomy: 13.8% vs. 35.8% (P less than .001); hysterectomy: 4.69% vs. 6.64% (P less than .001); and lung lobectomy: 17.1% vs. 25.4% (P less than .05). "In our analysis using Agency for Healthcare Research & Quality patient safety indicators for surgical care, we noted fewer wound, infectious, thrombotic, pulmonary, and mortality complications associated with minimally invasive surgery," the researchers wrote. "Based on our findings, increased hospital utilization of minimally invasive surgery at many U.S. hospitals represents a tremendous opportunity to prevent surgical site infection events."
The use of minimally invasive surgery was highly variable among the sampled hospitals. In fact, some never used minimally invasive surgery for some of the four procedures, while others used minimally invasive surgery for more than 75% of these procedures. Factors associated with the use of minimally invasive surgery were urban location, large hospital size, teaching hospital, and, for certain procedures, the hospital being located in the Midwest, South, or West.
"This [regional] disparity may be due to the broad range of surgical services some surgeons in rural areas are required to provide, and a scarcity of surgical specialists in such areas with advanced skills in minimally invasive surgery. Alternatively, the disparity may be a function of a lack of patient awareness about surgical options, decreased competition for patients, or a lack of minimally invasive surgery equipment, staff, or support in rural areas," the researchers wrote.
The findings of underutilization of minimally invasive surgery may also have something to do with a training gap.
"One reason that hospitals may be underperforming minimally invasive surgery is variability in appropriate training in residency and fellowship," Dr. Makary and his associates wrote. "One strategy that hospitals may consider in managing surgeons who cannot or choose not to acquire skills for performing minimally invasive surgery is to create a division of labor where patients who are not candidates for minimally invasive surgery are cared for by these surgeons. Increased standardization of competencies in minimally invasive surgery in surgical residency is needed to tackle wide variations in training."
The researchers acknowledged certain limitations of the study, including the fact that administrative claims data "can have incomplete coding, particularly of preexisting conditions," they wrote. "Another limitation is the lack of information available in the database for physician factors, such as laparoscopic training and experience that may influence the choice of procedure."
The researchers stated that they had no relevant financial conflicts to disclose.
The use of minimally invasive surgery for appendectomy, colectomy, hysterectomy, and lung lobectomy varies widely in the United States, even though the complication rates were lower from each procedure than with open surgery, results from a large retrospective study demonstrated.
"This study has important implications for quality improvement," researchers led by Dr. Martin A. Makary, professor of surgery at Johns Hopkins University, Baltimore, wrote. "Based on our findings, many hospitals have an opportunity to decrease surgical complications by increasing utilization of minimally invasive surgery."
To investigate the levels of variation in the use of minimally invasive surgery across the United States, Dr. Makary and his associates used the National Inpatient Sample database, which is administered by the Agency for Healthcare Research & Quality, to evaluate hospitalizations at hospitals that performed at least 10 of these procedures in 2010. The sample included 1,051 hospitals in 45 states, and was limited to appendectomy, colectomy, hysterectomy, and lung lobectomy. The researchers used a propensity score model to calculate the predicted proportion of minimally invasive operations for each hospital based on patient characteristics. For each procedure, they categorized hospitals as low, medium, or high based on their actual to predicted proportion of minimally invasive surgery use (BMJ 2014;349:g4198).
On average, the use of minimally invasive surgery by the hospitals sampled was 71% for appendectomy, 28% for colectomy, 13% for hysterectomy, and 32% for lung lobectomy. Overall surgical complications for minimally invasive surgery, compared with open surgery, were, respectively, for appendectomy: 3.94% vs. 7.90% (P less than .001); colectomy: 13.8% vs. 35.8% (P less than .001); hysterectomy: 4.69% vs. 6.64% (P less than .001); and lung lobectomy: 17.1% vs. 25.4% (P less than .05). "In our analysis using Agency for Healthcare Research & Quality patient safety indicators for surgical care, we noted fewer wound, infectious, thrombotic, pulmonary, and mortality complications associated with minimally invasive surgery," the researchers wrote. "Based on our findings, increased hospital utilization of minimally invasive surgery at many U.S. hospitals represents a tremendous opportunity to prevent surgical site infection events."
The use of minimally invasive surgery was highly variable among the sampled hospitals. In fact, some never used minimally invasive surgery for some of the four procedures, while others used minimally invasive surgery for more than 75% of these procedures. Factors associated with the use of minimally invasive surgery were urban location, large hospital size, teaching hospital, and, for certain procedures, the hospital being located in the Midwest, South, or West.
"This [regional] disparity may be due to the broad range of surgical services some surgeons in rural areas are required to provide, and a scarcity of surgical specialists in such areas with advanced skills in minimally invasive surgery. Alternatively, the disparity may be a function of a lack of patient awareness about surgical options, decreased competition for patients, or a lack of minimally invasive surgery equipment, staff, or support in rural areas," the researchers wrote.
The findings of underutilization of minimally invasive surgery may also have something to do with a training gap.
"One reason that hospitals may be underperforming minimally invasive surgery is variability in appropriate training in residency and fellowship," Dr. Makary and his associates wrote. "One strategy that hospitals may consider in managing surgeons who cannot or choose not to acquire skills for performing minimally invasive surgery is to create a division of labor where patients who are not candidates for minimally invasive surgery are cared for by these surgeons. Increased standardization of competencies in minimally invasive surgery in surgical residency is needed to tackle wide variations in training."
The researchers acknowledged certain limitations of the study, including the fact that administrative claims data "can have incomplete coding, particularly of preexisting conditions," they wrote. "Another limitation is the lack of information available in the database for physician factors, such as laparoscopic training and experience that may influence the choice of procedure."
The researchers stated that they had no relevant financial conflicts to disclose.
The use of minimally invasive surgery for appendectomy, colectomy, hysterectomy, and lung lobectomy varies widely in the United States, even though the complication rates were lower from each procedure than with open surgery, results from a large retrospective study demonstrated.
"This study has important implications for quality improvement," researchers led by Dr. Martin A. Makary, professor of surgery at Johns Hopkins University, Baltimore, wrote. "Based on our findings, many hospitals have an opportunity to decrease surgical complications by increasing utilization of minimally invasive surgery."
To investigate the levels of variation in the use of minimally invasive surgery across the United States, Dr. Makary and his associates used the National Inpatient Sample database, which is administered by the Agency for Healthcare Research & Quality, to evaluate hospitalizations at hospitals that performed at least 10 of these procedures in 2010. The sample included 1,051 hospitals in 45 states, and was limited to appendectomy, colectomy, hysterectomy, and lung lobectomy. The researchers used a propensity score model to calculate the predicted proportion of minimally invasive operations for each hospital based on patient characteristics. For each procedure, they categorized hospitals as low, medium, or high based on their actual to predicted proportion of minimally invasive surgery use (BMJ 2014;349:g4198).
On average, the use of minimally invasive surgery by the hospitals sampled was 71% for appendectomy, 28% for colectomy, 13% for hysterectomy, and 32% for lung lobectomy. Overall surgical complications for minimally invasive surgery, compared with open surgery, were, respectively, for appendectomy: 3.94% vs. 7.90% (P less than .001); colectomy: 13.8% vs. 35.8% (P less than .001); hysterectomy: 4.69% vs. 6.64% (P less than .001); and lung lobectomy: 17.1% vs. 25.4% (P less than .05). "In our analysis using Agency for Healthcare Research & Quality patient safety indicators for surgical care, we noted fewer wound, infectious, thrombotic, pulmonary, and mortality complications associated with minimally invasive surgery," the researchers wrote. "Based on our findings, increased hospital utilization of minimally invasive surgery at many U.S. hospitals represents a tremendous opportunity to prevent surgical site infection events."
The use of minimally invasive surgery was highly variable among the sampled hospitals. In fact, some never used minimally invasive surgery for some of the four procedures, while others used minimally invasive surgery for more than 75% of these procedures. Factors associated with the use of minimally invasive surgery were urban location, large hospital size, teaching hospital, and, for certain procedures, the hospital being located in the Midwest, South, or West.
"This [regional] disparity may be due to the broad range of surgical services some surgeons in rural areas are required to provide, and a scarcity of surgical specialists in such areas with advanced skills in minimally invasive surgery. Alternatively, the disparity may be a function of a lack of patient awareness about surgical options, decreased competition for patients, or a lack of minimally invasive surgery equipment, staff, or support in rural areas," the researchers wrote.
The findings of underutilization of minimally invasive surgery may also have something to do with a training gap.
"One reason that hospitals may be underperforming minimally invasive surgery is variability in appropriate training in residency and fellowship," Dr. Makary and his associates wrote. "One strategy that hospitals may consider in managing surgeons who cannot or choose not to acquire skills for performing minimally invasive surgery is to create a division of labor where patients who are not candidates for minimally invasive surgery are cared for by these surgeons. Increased standardization of competencies in minimally invasive surgery in surgical residency is needed to tackle wide variations in training."
The researchers acknowledged certain limitations of the study, including the fact that administrative claims data "can have incomplete coding, particularly of preexisting conditions," they wrote. "Another limitation is the lack of information available in the database for physician factors, such as laparoscopic training and experience that may influence the choice of procedure."
The researchers stated that they had no relevant financial conflicts to disclose.
FROM THE BRITISH MEDICAL JOURNAL
Key clinical point: Hospital use of minimally invasive surgical procedures appears to vary widely in the United States.
Major Finding:. The use of minimally invasive surgery by the hospitals sampled was 71% for appendectomy, 28% for colectomy, 13% for hysterectomy, and 32% for lung lobectomy.
Data Source: An analysis of data from the National Inpatient Sample in 2010 that included 1,051 hospitals in 45 states, and was limited to appendectomy, colectomy, hysterectomy, and lung lobectomy.
Disclosures: The authors stated that they had no relevant financial conflicts to disclose.
Stentless aortic bioprosthesis: Good 1-year outcomes, ‘remarkable’ functional improvement
TORONTO – In a multicenter European study, 30-day mortality and 1-year mortality after implantation of the stentless Freedom Solo aortic bioprosthetic valve were 1.4% and 4.4%, respectively. Patients in the study experienced "remarkable" functional status improvement at 1 year, reported principal investigator Dr. Markus Thalmann at the annual meeting of the American Association for Thoracic Surgery.
"Our trial showed excellent results in terms of morbidity and mortality and low rates of valve-related adverse events in a 12-month follow-up period," said Dr. Thalmann during his late-breaking clinical trials presentation. "It also demonstrated good hemodynamics leading to a remarkable functional status improvement."
The Sorin Freedom Solo aortic bioprosthesis is made of two layers of bovine pericardium, with no synthetic material added. The valve is implanted with a single running suture line technique in a strict supra-annular position and has no contact with the native annulus. The bioprosthesis is not approved in the United States.
Dr. Thalmann, of Krankenhaus Hietzing, Vienna, noted the importance of performing a careful decalcification of the annulus during implantation. "You should do it as properly as you’d do it when implanting a stented valve, even if you don’t put your stitches through the annulus," he said.
In response to a question, he said that the trial was requested by the Food and Drug Administration to provide more information on the single-line suture technique used to implant the valve.
The researchers conducted a prospective, nonrandomized, multicenter trial at 18 clinical centers in eight European countries. All patients with an indication for prosthetic aortic valve replacement were included, except those with a preexisting valve prosthesis in the mitral, pulmonary, or tricuspid positions. Patients needing double or triple valve replacement were also excluded, as were those with active endocarditis and congenital bicuspid valves.
A total of 616 patients received the valve. Patients had a mean age of 74.5 years, 45.9% were female, and the mean logistic EuroSCORE was 10.1%. Concomitant cardiac procedures, including coronary artery bypass grafting, were performed in 43.2% of patients.
Early (30-day) mortality was 1.4%, rising to 4.4% at 1 year. Valve-related mortality at 30 days and 1 year was 0.3% and 1.1%, respectively.
Overall morbidity was low. At 30 days, 4.5% of patients required reintervention for bleeding, 0.3% for perivalvular leakage. No structural valve dysfunction or valve thrombosis was noted at 30 days, with one case of each seen at 1 year (0.2% and 0.2%).
Preoperatively, 49.3% of patients were in New York Heart Association class III or IV heart failure. At 1 year, 97.0% were in NYHA class I or II heart failure.
"We found good hemodynamics after 1 year," said Dr. Thalmann. The overall mean gradient was 7.2 mm Hg, and the effective orifice area was 1.5 cm2.
Patients will be followed for up to 5 years.
The Freedom Solo valve is currently being tested in an investigational device exemption (IDE) study in the United States. The Sorin Solo Smart valve, the evolution of the Freedom Solo valve, received a European CE mark approval in November 2013.
Dr. Thalmann is a consultant for the Sorin Group, which funded the study.
TORONTO – In a multicenter European study, 30-day mortality and 1-year mortality after implantation of the stentless Freedom Solo aortic bioprosthetic valve were 1.4% and 4.4%, respectively. Patients in the study experienced "remarkable" functional status improvement at 1 year, reported principal investigator Dr. Markus Thalmann at the annual meeting of the American Association for Thoracic Surgery.
"Our trial showed excellent results in terms of morbidity and mortality and low rates of valve-related adverse events in a 12-month follow-up period," said Dr. Thalmann during his late-breaking clinical trials presentation. "It also demonstrated good hemodynamics leading to a remarkable functional status improvement."
The Sorin Freedom Solo aortic bioprosthesis is made of two layers of bovine pericardium, with no synthetic material added. The valve is implanted with a single running suture line technique in a strict supra-annular position and has no contact with the native annulus. The bioprosthesis is not approved in the United States.
Dr. Thalmann, of Krankenhaus Hietzing, Vienna, noted the importance of performing a careful decalcification of the annulus during implantation. "You should do it as properly as you’d do it when implanting a stented valve, even if you don’t put your stitches through the annulus," he said.
In response to a question, he said that the trial was requested by the Food and Drug Administration to provide more information on the single-line suture technique used to implant the valve.
The researchers conducted a prospective, nonrandomized, multicenter trial at 18 clinical centers in eight European countries. All patients with an indication for prosthetic aortic valve replacement were included, except those with a preexisting valve prosthesis in the mitral, pulmonary, or tricuspid positions. Patients needing double or triple valve replacement were also excluded, as were those with active endocarditis and congenital bicuspid valves.
A total of 616 patients received the valve. Patients had a mean age of 74.5 years, 45.9% were female, and the mean logistic EuroSCORE was 10.1%. Concomitant cardiac procedures, including coronary artery bypass grafting, were performed in 43.2% of patients.
Early (30-day) mortality was 1.4%, rising to 4.4% at 1 year. Valve-related mortality at 30 days and 1 year was 0.3% and 1.1%, respectively.
Overall morbidity was low. At 30 days, 4.5% of patients required reintervention for bleeding, 0.3% for perivalvular leakage. No structural valve dysfunction or valve thrombosis was noted at 30 days, with one case of each seen at 1 year (0.2% and 0.2%).
Preoperatively, 49.3% of patients were in New York Heart Association class III or IV heart failure. At 1 year, 97.0% were in NYHA class I or II heart failure.
"We found good hemodynamics after 1 year," said Dr. Thalmann. The overall mean gradient was 7.2 mm Hg, and the effective orifice area was 1.5 cm2.
Patients will be followed for up to 5 years.
The Freedom Solo valve is currently being tested in an investigational device exemption (IDE) study in the United States. The Sorin Solo Smart valve, the evolution of the Freedom Solo valve, received a European CE mark approval in November 2013.
Dr. Thalmann is a consultant for the Sorin Group, which funded the study.
TORONTO – In a multicenter European study, 30-day mortality and 1-year mortality after implantation of the stentless Freedom Solo aortic bioprosthetic valve were 1.4% and 4.4%, respectively. Patients in the study experienced "remarkable" functional status improvement at 1 year, reported principal investigator Dr. Markus Thalmann at the annual meeting of the American Association for Thoracic Surgery.
"Our trial showed excellent results in terms of morbidity and mortality and low rates of valve-related adverse events in a 12-month follow-up period," said Dr. Thalmann during his late-breaking clinical trials presentation. "It also demonstrated good hemodynamics leading to a remarkable functional status improvement."
The Sorin Freedom Solo aortic bioprosthesis is made of two layers of bovine pericardium, with no synthetic material added. The valve is implanted with a single running suture line technique in a strict supra-annular position and has no contact with the native annulus. The bioprosthesis is not approved in the United States.
Dr. Thalmann, of Krankenhaus Hietzing, Vienna, noted the importance of performing a careful decalcification of the annulus during implantation. "You should do it as properly as you’d do it when implanting a stented valve, even if you don’t put your stitches through the annulus," he said.
In response to a question, he said that the trial was requested by the Food and Drug Administration to provide more information on the single-line suture technique used to implant the valve.
The researchers conducted a prospective, nonrandomized, multicenter trial at 18 clinical centers in eight European countries. All patients with an indication for prosthetic aortic valve replacement were included, except those with a preexisting valve prosthesis in the mitral, pulmonary, or tricuspid positions. Patients needing double or triple valve replacement were also excluded, as were those with active endocarditis and congenital bicuspid valves.
A total of 616 patients received the valve. Patients had a mean age of 74.5 years, 45.9% were female, and the mean logistic EuroSCORE was 10.1%. Concomitant cardiac procedures, including coronary artery bypass grafting, were performed in 43.2% of patients.
Early (30-day) mortality was 1.4%, rising to 4.4% at 1 year. Valve-related mortality at 30 days and 1 year was 0.3% and 1.1%, respectively.
Overall morbidity was low. At 30 days, 4.5% of patients required reintervention for bleeding, 0.3% for perivalvular leakage. No structural valve dysfunction or valve thrombosis was noted at 30 days, with one case of each seen at 1 year (0.2% and 0.2%).
Preoperatively, 49.3% of patients were in New York Heart Association class III or IV heart failure. At 1 year, 97.0% were in NYHA class I or II heart failure.
"We found good hemodynamics after 1 year," said Dr. Thalmann. The overall mean gradient was 7.2 mm Hg, and the effective orifice area was 1.5 cm2.
Patients will be followed for up to 5 years.
The Freedom Solo valve is currently being tested in an investigational device exemption (IDE) study in the United States. The Sorin Solo Smart valve, the evolution of the Freedom Solo valve, received a European CE mark approval in November 2013.
Dr. Thalmann is a consultant for the Sorin Group, which funded the study.
AT THE AATS ANNUAL MEETING
Key clinical point: The valve is implanted with a single suture line in a supra-annular position, with no contact with the native annulus.
Major finding: Thirty-day mortality and 1-year mortality after implantation of the stentless Freedom Solo bioprosthesis were 1.4% and 4.4%, respectively.
Data source: Nonrandomized, prospective study of 616 patients at 18 centers in eight European countries.
Disclosures: Dr. Thalmann is a consultant for the Sorin Group, which funded the study.
FDA panel recommends informed consent, labeling changes to address morcellator risk
SILVER SPRING, MD. – A requirement for informed consent outlining the risks of morcellation of unsuspected malignancies in women treated with laparoscopic power morcellators for presumably benign fibroids was among the recommendations made by a Food and Drug Administration advisory panel.
On July 11, the second day of a two-day meeting of the FDA’s Obstetrics and Gynecology Devices Advisory Committee, panelists also supported adding a boxed warning to the labels of laparoscopic power morcellators (LPMs). Other suggestions and recommendations included identifying characteristics of patients or fibroids, physical exam findings, and other features that could help determine patients who may have a sarcoma before treatment, as well as strategies to mitigate risks during treatment.
"There should be some labeling or special controls" to ensure that women who are being considered for treatment with this device and their physicians "get the message that we do believe there is an increased risk" and that the device is contraindicated in patients with a known or suspected malignancy, said Dr. Carol Brown, a gynecologic cancer surgeon at Memorial Sloan Kettering Cancer Center, New York.
The advisory panel did not vote on any issues and was not asked whether LPMs should be taken off the market or reclassified as class III medical devices, which require clinical data for approval.
During testimony from women and relatives of those who had been diagnosed with stage 4 LMS after treatment that included morcellation for what was thought to be uterine fibroids, "a recurring theme" was that they had not been told that morcellation could be part of their treatment, and if they had known, they may not have agreed to that treatment and chosen an alternative, she pointed out.
The FDA convened the meeting to discuss the benefits, risks, and clinical role of LPMs in the treatment of women with uterine fibroids. Panelists also were told to discuss strategies that could be used to reduce the risks of morcellation disseminating cancerous tissue into the pelvis and abdomen of women with an unsuspected uterine sarcoma or leiomyosarcoma (LMS).
Concerns about this risk have received widespread attention this year. An FDA safety advisory was issued in April recommending that the use of LPMs during a hysterectomy or myomectomy in women with uterine fibroids be discouraged. The case of Dr. Amy Reed, an anesthesiologist who was diagnosed with stage 4 LMS after undergoing a hysterectomy with morcellation at the age of 40 for what was thought to be benign fibroids, also garnered media attention. She and her husband, Dr. Hooman Noorchashm, a cardiothoracic surgeon, are leading a campaign highlighting these risks, calling for a ban on the use of LPMs. They -- along with women who have had similar experiences, and husbands and sisters of women who died of disseminated LMS after undergoing morcellation for what was thought to be benign fibroids -- also spoke at the meeting. These speakers emphasized that they are aware of at least 130 such cases, despite the small number of cases reported to the FDA (21 as of June 2014).
In April, the FDA recommended that physicians discuss alternative treatment options with women who have symptomatic uterine fibroids. If power morcellation is considered the best option, the agency advised, women should be informed that their fibroids may contain cancerous cells and, if so, morcellation could significantly worsen their prognosis. Among women who undergo a hysterectomy or myomectomy for a presumed fibroid, about one in 350 have a uterine sarcoma, and about one in 500 have a leiomyosarcoma (LMS), the FDA estimates.
The currently available LPMs that are "cleared" for gynecologic indications are regulated as moderate-risk class II devices, which require little or no clinical data. The agency is considering requiring that clinical data be provided for LPMs used for gynecologic indications.
Panelists said that features and tools that could help determine whether a patient could have a sarcoma include an older age, certain symptoms, and genetic susceptibility (a history of retinoblastoma), some imaging techniques, as well as a history of pelvic radiation.
Two panelists – a surgical oncologist and a bioethicist – said that LPMs should not be used for gynecologic indications until better data are available.
"I have not seen anything in isolation or together" that could help predict whether a woman with presumed uterine fibroids has a leiomyosarcoma, said Dr. Craig Shriver, professor of surgery and director of the John P. Murtha Cancer Center at Walter Reed National Navy Military Medical Center, Bethesda, Md.
Referring to the tenets of considering all masses as cancer until proven otherwise, he said, "I have been perplexed over the last two decades watching the introduction of laparoscopic power morcellation techniques that is totally anathema to these and my core principles as a cancer surgeon," he said. Two days of testimony have "only more strongly reaffirmed my commitment and belief that at present, there is no safe way to offer laparoscopic power morcellation as part of any minimally invasive surgery," he added. They should be withdrawn from the market, reclassified as class III devices and studied in clinical trials, he recommended.
But Dr. Brown said that while she agreed with those principles, since fibroids are so common, banning the use of morcellation could result in “hundreds of thousands” of hysterectomies.
Specimen collection bags were used when LPMs were first introduced for gynecologic indications, but dropped over time. Use has increased recently as a result of the increased attention to the risks, but there is no evidence that bags are effective in reducing the risk of disseminating malignant cells in the peritoneal cavity, in the case of an unsuspected malignancy.
“If you are going to morcellate, and it can be done safely in a bag, then that should be encouraged,” said panelist Dr. Keith Isaacson, medical director of the Center for Minimally Invasive Gynecologic Surgery at Newton (Mass.) Wellesley Hospital. More work is needed to evaluate bags and the techniques for their use, and to determine how easy it is train clinicians in how to use them safely, he added. “I still believe that intuitively – and that’s all we have is intuition here – that it more likely mitigates the risk of upstaging a tumor if you morcellate within a containment system, such as a bag.”
The FDA usually follows the recommendations of its advisory panels. Panel members have been cleared of potential conflicts, although occasionally a panelist is given a waiver, but not at this meeting.
Adverse events related to LPMs or other medical devices should be reported to the FDA.
This article has been updated 7/14/2014.
SILVER SPRING, MD. – A requirement for informed consent outlining the risks of morcellation of unsuspected malignancies in women treated with laparoscopic power morcellators for presumably benign fibroids was among the recommendations made by a Food and Drug Administration advisory panel.
On July 11, the second day of a two-day meeting of the FDA’s Obstetrics and Gynecology Devices Advisory Committee, panelists also supported adding a boxed warning to the labels of laparoscopic power morcellators (LPMs). Other suggestions and recommendations included identifying characteristics of patients or fibroids, physical exam findings, and other features that could help determine patients who may have a sarcoma before treatment, as well as strategies to mitigate risks during treatment.
"There should be some labeling or special controls" to ensure that women who are being considered for treatment with this device and their physicians "get the message that we do believe there is an increased risk" and that the device is contraindicated in patients with a known or suspected malignancy, said Dr. Carol Brown, a gynecologic cancer surgeon at Memorial Sloan Kettering Cancer Center, New York.
The advisory panel did not vote on any issues and was not asked whether LPMs should be taken off the market or reclassified as class III medical devices, which require clinical data for approval.
During testimony from women and relatives of those who had been diagnosed with stage 4 LMS after treatment that included morcellation for what was thought to be uterine fibroids, "a recurring theme" was that they had not been told that morcellation could be part of their treatment, and if they had known, they may not have agreed to that treatment and chosen an alternative, she pointed out.
The FDA convened the meeting to discuss the benefits, risks, and clinical role of LPMs in the treatment of women with uterine fibroids. Panelists also were told to discuss strategies that could be used to reduce the risks of morcellation disseminating cancerous tissue into the pelvis and abdomen of women with an unsuspected uterine sarcoma or leiomyosarcoma (LMS).
Concerns about this risk have received widespread attention this year. An FDA safety advisory was issued in April recommending that the use of LPMs during a hysterectomy or myomectomy in women with uterine fibroids be discouraged. The case of Dr. Amy Reed, an anesthesiologist who was diagnosed with stage 4 LMS after undergoing a hysterectomy with morcellation at the age of 40 for what was thought to be benign fibroids, also garnered media attention. She and her husband, Dr. Hooman Noorchashm, a cardiothoracic surgeon, are leading a campaign highlighting these risks, calling for a ban on the use of LPMs. They -- along with women who have had similar experiences, and husbands and sisters of women who died of disseminated LMS after undergoing morcellation for what was thought to be benign fibroids -- also spoke at the meeting. These speakers emphasized that they are aware of at least 130 such cases, despite the small number of cases reported to the FDA (21 as of June 2014).
In April, the FDA recommended that physicians discuss alternative treatment options with women who have symptomatic uterine fibroids. If power morcellation is considered the best option, the agency advised, women should be informed that their fibroids may contain cancerous cells and, if so, morcellation could significantly worsen their prognosis. Among women who undergo a hysterectomy or myomectomy for a presumed fibroid, about one in 350 have a uterine sarcoma, and about one in 500 have a leiomyosarcoma (LMS), the FDA estimates.
The currently available LPMs that are "cleared" for gynecologic indications are regulated as moderate-risk class II devices, which require little or no clinical data. The agency is considering requiring that clinical data be provided for LPMs used for gynecologic indications.
Panelists said that features and tools that could help determine whether a patient could have a sarcoma include an older age, certain symptoms, and genetic susceptibility (a history of retinoblastoma), some imaging techniques, as well as a history of pelvic radiation.
Two panelists – a surgical oncologist and a bioethicist – said that LPMs should not be used for gynecologic indications until better data are available.
"I have not seen anything in isolation or together" that could help predict whether a woman with presumed uterine fibroids has a leiomyosarcoma, said Dr. Craig Shriver, professor of surgery and director of the John P. Murtha Cancer Center at Walter Reed National Navy Military Medical Center, Bethesda, Md.
Referring to the tenets of considering all masses as cancer until proven otherwise, he said, "I have been perplexed over the last two decades watching the introduction of laparoscopic power morcellation techniques that is totally anathema to these and my core principles as a cancer surgeon," he said. Two days of testimony have "only more strongly reaffirmed my commitment and belief that at present, there is no safe way to offer laparoscopic power morcellation as part of any minimally invasive surgery," he added. They should be withdrawn from the market, reclassified as class III devices and studied in clinical trials, he recommended.
But Dr. Brown said that while she agreed with those principles, since fibroids are so common, banning the use of morcellation could result in “hundreds of thousands” of hysterectomies.
Specimen collection bags were used when LPMs were first introduced for gynecologic indications, but dropped over time. Use has increased recently as a result of the increased attention to the risks, but there is no evidence that bags are effective in reducing the risk of disseminating malignant cells in the peritoneal cavity, in the case of an unsuspected malignancy.
“If you are going to morcellate, and it can be done safely in a bag, then that should be encouraged,” said panelist Dr. Keith Isaacson, medical director of the Center for Minimally Invasive Gynecologic Surgery at Newton (Mass.) Wellesley Hospital. More work is needed to evaluate bags and the techniques for their use, and to determine how easy it is train clinicians in how to use them safely, he added. “I still believe that intuitively – and that’s all we have is intuition here – that it more likely mitigates the risk of upstaging a tumor if you morcellate within a containment system, such as a bag.”
The FDA usually follows the recommendations of its advisory panels. Panel members have been cleared of potential conflicts, although occasionally a panelist is given a waiver, but not at this meeting.
Adverse events related to LPMs or other medical devices should be reported to the FDA.
This article has been updated 7/14/2014.
SILVER SPRING, MD. – A requirement for informed consent outlining the risks of morcellation of unsuspected malignancies in women treated with laparoscopic power morcellators for presumably benign fibroids was among the recommendations made by a Food and Drug Administration advisory panel.
On July 11, the second day of a two-day meeting of the FDA’s Obstetrics and Gynecology Devices Advisory Committee, panelists also supported adding a boxed warning to the labels of laparoscopic power morcellators (LPMs). Other suggestions and recommendations included identifying characteristics of patients or fibroids, physical exam findings, and other features that could help determine patients who may have a sarcoma before treatment, as well as strategies to mitigate risks during treatment.
"There should be some labeling or special controls" to ensure that women who are being considered for treatment with this device and their physicians "get the message that we do believe there is an increased risk" and that the device is contraindicated in patients with a known or suspected malignancy, said Dr. Carol Brown, a gynecologic cancer surgeon at Memorial Sloan Kettering Cancer Center, New York.
The advisory panel did not vote on any issues and was not asked whether LPMs should be taken off the market or reclassified as class III medical devices, which require clinical data for approval.
During testimony from women and relatives of those who had been diagnosed with stage 4 LMS after treatment that included morcellation for what was thought to be uterine fibroids, "a recurring theme" was that they had not been told that morcellation could be part of their treatment, and if they had known, they may not have agreed to that treatment and chosen an alternative, she pointed out.
The FDA convened the meeting to discuss the benefits, risks, and clinical role of LPMs in the treatment of women with uterine fibroids. Panelists also were told to discuss strategies that could be used to reduce the risks of morcellation disseminating cancerous tissue into the pelvis and abdomen of women with an unsuspected uterine sarcoma or leiomyosarcoma (LMS).
Concerns about this risk have received widespread attention this year. An FDA safety advisory was issued in April recommending that the use of LPMs during a hysterectomy or myomectomy in women with uterine fibroids be discouraged. The case of Dr. Amy Reed, an anesthesiologist who was diagnosed with stage 4 LMS after undergoing a hysterectomy with morcellation at the age of 40 for what was thought to be benign fibroids, also garnered media attention. She and her husband, Dr. Hooman Noorchashm, a cardiothoracic surgeon, are leading a campaign highlighting these risks, calling for a ban on the use of LPMs. They -- along with women who have had similar experiences, and husbands and sisters of women who died of disseminated LMS after undergoing morcellation for what was thought to be benign fibroids -- also spoke at the meeting. These speakers emphasized that they are aware of at least 130 such cases, despite the small number of cases reported to the FDA (21 as of June 2014).
In April, the FDA recommended that physicians discuss alternative treatment options with women who have symptomatic uterine fibroids. If power morcellation is considered the best option, the agency advised, women should be informed that their fibroids may contain cancerous cells and, if so, morcellation could significantly worsen their prognosis. Among women who undergo a hysterectomy or myomectomy for a presumed fibroid, about one in 350 have a uterine sarcoma, and about one in 500 have a leiomyosarcoma (LMS), the FDA estimates.
The currently available LPMs that are "cleared" for gynecologic indications are regulated as moderate-risk class II devices, which require little or no clinical data. The agency is considering requiring that clinical data be provided for LPMs used for gynecologic indications.
Panelists said that features and tools that could help determine whether a patient could have a sarcoma include an older age, certain symptoms, and genetic susceptibility (a history of retinoblastoma), some imaging techniques, as well as a history of pelvic radiation.
Two panelists – a surgical oncologist and a bioethicist – said that LPMs should not be used for gynecologic indications until better data are available.
"I have not seen anything in isolation or together" that could help predict whether a woman with presumed uterine fibroids has a leiomyosarcoma, said Dr. Craig Shriver, professor of surgery and director of the John P. Murtha Cancer Center at Walter Reed National Navy Military Medical Center, Bethesda, Md.
Referring to the tenets of considering all masses as cancer until proven otherwise, he said, "I have been perplexed over the last two decades watching the introduction of laparoscopic power morcellation techniques that is totally anathema to these and my core principles as a cancer surgeon," he said. Two days of testimony have "only more strongly reaffirmed my commitment and belief that at present, there is no safe way to offer laparoscopic power morcellation as part of any minimally invasive surgery," he added. They should be withdrawn from the market, reclassified as class III devices and studied in clinical trials, he recommended.
But Dr. Brown said that while she agreed with those principles, since fibroids are so common, banning the use of morcellation could result in “hundreds of thousands” of hysterectomies.
Specimen collection bags were used when LPMs were first introduced for gynecologic indications, but dropped over time. Use has increased recently as a result of the increased attention to the risks, but there is no evidence that bags are effective in reducing the risk of disseminating malignant cells in the peritoneal cavity, in the case of an unsuspected malignancy.
“If you are going to morcellate, and it can be done safely in a bag, then that should be encouraged,” said panelist Dr. Keith Isaacson, medical director of the Center for Minimally Invasive Gynecologic Surgery at Newton (Mass.) Wellesley Hospital. More work is needed to evaluate bags and the techniques for their use, and to determine how easy it is train clinicians in how to use them safely, he added. “I still believe that intuitively – and that’s all we have is intuition here – that it more likely mitigates the risk of upstaging a tumor if you morcellate within a containment system, such as a bag.”
The FDA usually follows the recommendations of its advisory panels. Panel members have been cleared of potential conflicts, although occasionally a panelist is given a waiver, but not at this meeting.
Adverse events related to LPMs or other medical devices should be reported to the FDA.
This article has been updated 7/14/2014.
AT AN FDA ADVISORY COMMITTEE MEETING
VIDEO: ‘Improve – but do not abandon – power morcellation’
SILVER SPRING, MD. – Without power morcellation, the number of hysterectomies performed using an open approach would dramatically increase – and the combined mortality from laparoscopic hysterectomy and potential dissemination of leiomyosarcoma would be less than that of open hysterectomy, according to testimony given July 11 at a Food and Drug Administration expert panel meeting.
Dr. Jubilee Brown, director of gynecologic oncology at the Woman's Hospital of Texas, University of Texas M.D. Anderson Cancer Center, Houston, testified at the meeting on behalf of the AAGL, an association that promotes minimally invasive gynecologic surgery. She presented results of a decision analysis suggesting that if all U.S. cases were converted to open hysterectomy from laparoscopic hysterectomy (LH) with morcellation of fibroids, 17 more women each year would die from the open procedure than from the combination LH and morcellation.
"Improve – but do not abandon – power morcellation," Dr. Brown told the FDA Obstetrics and Gynecology Devices Advisory Committee. She discussed her testimony during this video interview.
Dr. Brown said she had no relevant financial conflicts of interest.
SILVER SPRING, MD. – Without power morcellation, the number of hysterectomies performed using an open approach would dramatically increase – and the combined mortality from laparoscopic hysterectomy and potential dissemination of leiomyosarcoma would be less than that of open hysterectomy, according to testimony given July 11 at a Food and Drug Administration expert panel meeting.
Dr. Jubilee Brown, director of gynecologic oncology at the Woman's Hospital of Texas, University of Texas M.D. Anderson Cancer Center, Houston, testified at the meeting on behalf of the AAGL, an association that promotes minimally invasive gynecologic surgery. She presented results of a decision analysis suggesting that if all U.S. cases were converted to open hysterectomy from laparoscopic hysterectomy (LH) with morcellation of fibroids, 17 more women each year would die from the open procedure than from the combination LH and morcellation.
"Improve – but do not abandon – power morcellation," Dr. Brown told the FDA Obstetrics and Gynecology Devices Advisory Committee. She discussed her testimony during this video interview.
Dr. Brown said she had no relevant financial conflicts of interest.
SILVER SPRING, MD. – Without power morcellation, the number of hysterectomies performed using an open approach would dramatically increase – and the combined mortality from laparoscopic hysterectomy and potential dissemination of leiomyosarcoma would be less than that of open hysterectomy, according to testimony given July 11 at a Food and Drug Administration expert panel meeting.
Dr. Jubilee Brown, director of gynecologic oncology at the Woman's Hospital of Texas, University of Texas M.D. Anderson Cancer Center, Houston, testified at the meeting on behalf of the AAGL, an association that promotes minimally invasive gynecologic surgery. She presented results of a decision analysis suggesting that if all U.S. cases were converted to open hysterectomy from laparoscopic hysterectomy (LH) with morcellation of fibroids, 17 more women each year would die from the open procedure than from the combination LH and morcellation.
"Improve – but do not abandon – power morcellation," Dr. Brown told the FDA Obstetrics and Gynecology Devices Advisory Committee. She discussed her testimony during this video interview.
Dr. Brown said she had no relevant financial conflicts of interest.
AT AN FDA ADVISORY COMMITTEE MEETING
ICD-10: Dual coding is only for testing, claims backlog
There’s plenty to be confused about when it comes to the transition to the new ICD-10 coding system in October 2015. But the government has issued some clarification about at least one issue: when to use dual coding.
Dual coding, also called dual processing, generally means using both ICD-9 and ICD-10 at the same time when submitting claims.
In an e-mail July 10, officials at the Centers for Medicare & Medicaid Services (CMS) said physicians and coders may engage in dual coding as a way to test their ICD-10 readiness before the compliance date. They may also code in both systems after the compliance date, if they have a backlog of claims.
Here’s how it works: Before ICD-10 goes into effect on Oct. 1, 2015, physicians and coders can practice by coding current claims in both systems to see if they have the right level of documentation for the new system. ICD-10 codes can also be used to test whether payers and clearinghouses are ready to receive and process the new codes. However, only the ICD-9 code can be sent to payers as part of a "live" transaction before the compliance date.
Physicians may also find that they will use both coding sets for a short period of time after the compliance date, if they need to submit claims with a date of service that occurred before Oct. 1, 2015.
But the physicians and coders don’t get to choose which system to code in. The date of service determines whether ICD-9 or ICD-10 is used, according to CMS, with services performed before Oct. 1 getting an ICD-9 code and services on and after Oct. 1 receiving an ICD-10 code.
CMS is expected to release a regulation soon with more details on the rollout of ICD-10.
On Twitter @maryellenny
There’s plenty to be confused about when it comes to the transition to the new ICD-10 coding system in October 2015. But the government has issued some clarification about at least one issue: when to use dual coding.
Dual coding, also called dual processing, generally means using both ICD-9 and ICD-10 at the same time when submitting claims.
In an e-mail July 10, officials at the Centers for Medicare & Medicaid Services (CMS) said physicians and coders may engage in dual coding as a way to test their ICD-10 readiness before the compliance date. They may also code in both systems after the compliance date, if they have a backlog of claims.
Here’s how it works: Before ICD-10 goes into effect on Oct. 1, 2015, physicians and coders can practice by coding current claims in both systems to see if they have the right level of documentation for the new system. ICD-10 codes can also be used to test whether payers and clearinghouses are ready to receive and process the new codes. However, only the ICD-9 code can be sent to payers as part of a "live" transaction before the compliance date.
Physicians may also find that they will use both coding sets for a short period of time after the compliance date, if they need to submit claims with a date of service that occurred before Oct. 1, 2015.
But the physicians and coders don’t get to choose which system to code in. The date of service determines whether ICD-9 or ICD-10 is used, according to CMS, with services performed before Oct. 1 getting an ICD-9 code and services on and after Oct. 1 receiving an ICD-10 code.
CMS is expected to release a regulation soon with more details on the rollout of ICD-10.
On Twitter @maryellenny
There’s plenty to be confused about when it comes to the transition to the new ICD-10 coding system in October 2015. But the government has issued some clarification about at least one issue: when to use dual coding.
Dual coding, also called dual processing, generally means using both ICD-9 and ICD-10 at the same time when submitting claims.
In an e-mail July 10, officials at the Centers for Medicare & Medicaid Services (CMS) said physicians and coders may engage in dual coding as a way to test their ICD-10 readiness before the compliance date. They may also code in both systems after the compliance date, if they have a backlog of claims.
Here’s how it works: Before ICD-10 goes into effect on Oct. 1, 2015, physicians and coders can practice by coding current claims in both systems to see if they have the right level of documentation for the new system. ICD-10 codes can also be used to test whether payers and clearinghouses are ready to receive and process the new codes. However, only the ICD-9 code can be sent to payers as part of a "live" transaction before the compliance date.
Physicians may also find that they will use both coding sets for a short period of time after the compliance date, if they need to submit claims with a date of service that occurred before Oct. 1, 2015.
But the physicians and coders don’t get to choose which system to code in. The date of service determines whether ICD-9 or ICD-10 is used, according to CMS, with services performed before Oct. 1 getting an ICD-9 code and services on and after Oct. 1 receiving an ICD-10 code.
CMS is expected to release a regulation soon with more details on the rollout of ICD-10.
On Twitter @maryellenny
VIDEO: Public testimony gets heated at FDA panel meeting on morcellation
SILVER SPRING, MD. – Power morcellation devices should be never used for gynecologic procedures, Dr. Hooman Noorchashm testified to a Food and Drug Administration expert panel on July ll.
He called out members of the FDA Obstetrics and Gynecology Devices Panel Advisory Committee by name, seeking to shame them into action to disallow power morcellation for suspected uterine fibroids.
"Is this a safe and logical device?" said Dr. Noorchashm, a cardiothoracic surgeon at Brigham and Women’s Hospital in Boston. "The only classification for this device is banned – unsafe, illogical, incorrect, and deadly."
Dr. Noorchashm’s wife, Dr. Amy Reed, had a hysterectomy with morcellation for suspected fibroids. Biopsy later confirmed sarcoma was present. Use of power morcellation caused tumor cells to spread, upstaging her sarcoma to stage IV.
On Twitter @denisefulton
SILVER SPRING, MD. – Power morcellation devices should be never used for gynecologic procedures, Dr. Hooman Noorchashm testified to a Food and Drug Administration expert panel on July ll.
He called out members of the FDA Obstetrics and Gynecology Devices Panel Advisory Committee by name, seeking to shame them into action to disallow power morcellation for suspected uterine fibroids.
"Is this a safe and logical device?" said Dr. Noorchashm, a cardiothoracic surgeon at Brigham and Women’s Hospital in Boston. "The only classification for this device is banned – unsafe, illogical, incorrect, and deadly."
Dr. Noorchashm’s wife, Dr. Amy Reed, had a hysterectomy with morcellation for suspected fibroids. Biopsy later confirmed sarcoma was present. Use of power morcellation caused tumor cells to spread, upstaging her sarcoma to stage IV.
On Twitter @denisefulton
SILVER SPRING, MD. – Power morcellation devices should be never used for gynecologic procedures, Dr. Hooman Noorchashm testified to a Food and Drug Administration expert panel on July ll.
He called out members of the FDA Obstetrics and Gynecology Devices Panel Advisory Committee by name, seeking to shame them into action to disallow power morcellation for suspected uterine fibroids.
"Is this a safe and logical device?" said Dr. Noorchashm, a cardiothoracic surgeon at Brigham and Women’s Hospital in Boston. "The only classification for this device is banned – unsafe, illogical, incorrect, and deadly."
Dr. Noorchashm’s wife, Dr. Amy Reed, had a hysterectomy with morcellation for suspected fibroids. Biopsy later confirmed sarcoma was present. Use of power morcellation caused tumor cells to spread, upstaging her sarcoma to stage IV.
On Twitter @denisefulton
AT AN FDA ADVISORY COMMITTEE MEETING