User login
Official Newspaper of the American College of Surgeons
Health sector well represented among lobbying top spenders
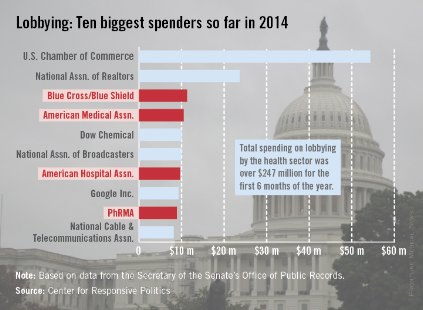
Four of the 10 largest spenders on lobbying for the first 6 months of 2014 are part of the health sector, the Center for Responsive Politics reported.
Blue Cross/Blue Shield was in third place overall, spending almost $11.3 million through the end of June, while the American Medical Association was fourth at $10.5 million, the American Hospital Association was seventh at $9.7 million, and PhRMA (Pharmaceutical Research and Manufacturers of America) was ninth with spending of just under $8.9 million, according to the center.
The U.S. Chamber of Commerce far outdistanced the health sector, however, putting $54.3 million into its lobbying efforts in the first 6 months of 2014 – about $11 million more than the four health-sector representatives combined. The National Association of Realtors was the second-largest lobbyer, spending $23.6 million, the center’s lobbying data showed.
The data on the center’s OpenSecrets website are compiled using lobbying disclosure reports filed with the Secretary of the Senate’s Office of Public Records.

Four of the 10 largest spenders on lobbying for the first 6 months of 2014 are part of the health sector, the Center for Responsive Politics reported.
Blue Cross/Blue Shield was in third place overall, spending almost $11.3 million through the end of June, while the American Medical Association was fourth at $10.5 million, the American Hospital Association was seventh at $9.7 million, and PhRMA (Pharmaceutical Research and Manufacturers of America) was ninth with spending of just under $8.9 million, according to the center.
The U.S. Chamber of Commerce far outdistanced the health sector, however, putting $54.3 million into its lobbying efforts in the first 6 months of 2014 – about $11 million more than the four health-sector representatives combined. The National Association of Realtors was the second-largest lobbyer, spending $23.6 million, the center’s lobbying data showed.
The data on the center’s OpenSecrets website are compiled using lobbying disclosure reports filed with the Secretary of the Senate’s Office of Public Records.

Four of the 10 largest spenders on lobbying for the first 6 months of 2014 are part of the health sector, the Center for Responsive Politics reported.
Blue Cross/Blue Shield was in third place overall, spending almost $11.3 million through the end of June, while the American Medical Association was fourth at $10.5 million, the American Hospital Association was seventh at $9.7 million, and PhRMA (Pharmaceutical Research and Manufacturers of America) was ninth with spending of just under $8.9 million, according to the center.
The U.S. Chamber of Commerce far outdistanced the health sector, however, putting $54.3 million into its lobbying efforts in the first 6 months of 2014 – about $11 million more than the four health-sector representatives combined. The National Association of Realtors was the second-largest lobbyer, spending $23.6 million, the center’s lobbying data showed.
The data on the center’s OpenSecrets website are compiled using lobbying disclosure reports filed with the Secretary of the Senate’s Office of Public Records.

Be alert to less common, but dangerous, thoracic injuries in children
SAN DIEGO – Many thoracic injuries sustained by children and adolescents can be diagnosed with clinical assessment and chest x-ray, and many heal without surgical intervention, according to Dr. Timothy Fairbanks.
"When the pager goes off and says ‘3-year-old involved in a accident,’ that kid may be going home with a physical exam, stickers, and a high five; or he may require a life-saving procedure" in the emergency department. Dr. Fairbanks, of the division of pediatric surgery at Rady Children’s Hospital of San Diego, said at the University of California, San Diego, Critical Care Summer Session. "There’s complete variability in what we see and how serious the injuries can be."
According to data from the National Pediatric Trauma Registry, 86% of injuries are blunt and 14% are penetrating. Chest injuries "are the second leading cause of death to central nervous system injuries," Dr. Fairbanks said. "Mortality rates for blunt and penetrating injuries are similar. In blunt traumas, most kids don’t die from their blunt thoracic injury, but from a CNS injury that they acquired at the same time. Most of the kids who die from penetrating injuries die in the field before reaching the hospital."
Thoracic injuries occur in about 25% of patients who present to a Level I trauma center. While thoracic injuries are less common than abdominal and extremity injuries are, "they are of potentially higher risk and more lethal," he said. "Most can be treated successfully. Male to female injury rate is 2:1 to 3:1 in favor of males having more traumas."
He classifies thoracic trauma injuries by anatomy (rib, pulmonary, bronchial), blunt vs. penetrating, and threat to life (immediate or potentially life threatening). Motor vehicles are the most common mechanism of thoracic injury, and children have an increased risk in all traumas of auto vs. pedestrian, compared with adults. "When children become teenagers, their mechanisms of injuries approach those of the data for adults," Dr. Fairbanks explained. "We see more penetrating knife and gunshot wounds to the chest, although they are rare in our younger patient population. Tracheobronchial lacerations are more common in children, compared with adults. Aortic disruptions are less likely in children."
Common thoracic injuries that he and his associates see at Rady Children’s Hospital are lung contusion, pneumothorax, hemothorax, and fractures to the ribs, sternum, or scapula. Less common "but perhaps more dangerous injuries" are those to the heart, aorta, trachea, bronchi, and diaphragm. "The most common immediately life-threatening thoracic injuries in children are airway obstruction, pneumothorax, hemothorax, and cardiac tamponade," he said. "A large percentage of thoracic injuries have stable vital signs at presentation. Rib fractures are less common in children and are a marker for a significant mechanism of injury or force. Mediastinal structures are more mobile, making tension pneumothorax a bigger problem."
Diagnosis and treatment of thoracic injuries proceeds simultaneously, with an initial goal to rule out life-threatening injuries, which should be identified and treated during the initial resuscitation phase. This includes making sure the airway is clear and secure and providing supplemental oxygen. "If endotracheal intubation is needed, check the position," he advised. "You want to see bilateral chest rise and CO2 waveform on your monitor. It’s very common for a child to have a right mainstem intubation and no breath sounds on the left side." He also makes it a point to assess their ventilation and treat their pneumothorax. "Don’t wait for the chest x-ray if you have a good clinical suspicion," he said.
A chest x-ray can often guide your management decisions in cases of thoracic trauma, while a FAST scan – specifically an ultrasound – is good for seeing a cardiac tamponade.
Sometimes thoracic injuries require an immediate thoracotomy in the emergency department, but its indications "are controversial," Dr. Fairbanks said. "It’s a potentially life-threatening maneuver, especially with penetrating cardiac injuries." The indications for emergency department thoracotomy are post-traumatic arrest or near arrest in all cases of penetrating thoracic injuries; in blunt trauma with loss of vital signs in the ED; or in blunt trauma with loss of vital signs in route to the ED.
"One of the things we struggle with in pediatric trauma is the timing at which they lost the vital signs in the field," he said. "After 20 minutes the chance of survival is almost nil. However, the family is going to want to know that you did everything possible to save their 3- or 4-year-old child."
If the patient is stable, complete the physical exam. "You’re looking for tachypnea, tenderness to the chest wall and abrasions, or signs of thoracic trauma that require further investigation," he said. Important signs include cyanosis, dyspnea, tracheal deviation, hoarseness, jugular engagement, and subcutaneous emphysema.
Chest auscultation is recommended and an anteroposterior (AP) chest x-ray becomes valuable, "because it’s quick, simple, and yields a lot of information," he said. He characterized a thoracic CT scan as "an excellent study" that can help with the diagnosis of aortic and diaphragmatic injuries which can be missed on chest x-ray. "However, there is a lot more radiation with a chest CT," Dr. Fairbanks noted. "Only 1 in 200 chest CTs for trauma yields a new diagnosis." Other studies to consider include EKG, echocardiogram, bronchoscopy, video-assisted thoracic surgery, radionuclide bone scan, and MRI.
A thoracotomy is indicated when the patient is coding or near coding. The other indications are penetrating wound of the heart or great vessels; massive or continuous intrathoracic bleeding; open pneumothorax with major chest wall defect; aortogram indicating injury to the aorta or major branch; massive or continuing air leak, indicating injury to a major airway; cardiac tamponade; esophageal perforation, or diaphragmatic rupture.
Chest wall soft-tissue injuries are usually not clinically significant, "but they’re a marker for a more serious injury under a bruise," he said. "Rib fractures are less common in children. It’s an indicator of significant force. Treatment is pain control and prevention of atelectasis, which is not as big of a problem in kids as it is in adults. They will heal in 6 weeks. Consider child abuse, specifically in cases of multiple rib fractures and those that don’t make sense with the mechanism of reported injury, or rib fractures that are at different stages of healing."
Dr. Fairbanks said that he had no relevant financial conflicts to disclose.
SAN DIEGO – Many thoracic injuries sustained by children and adolescents can be diagnosed with clinical assessment and chest x-ray, and many heal without surgical intervention, according to Dr. Timothy Fairbanks.
"When the pager goes off and says ‘3-year-old involved in a accident,’ that kid may be going home with a physical exam, stickers, and a high five; or he may require a life-saving procedure" in the emergency department. Dr. Fairbanks, of the division of pediatric surgery at Rady Children’s Hospital of San Diego, said at the University of California, San Diego, Critical Care Summer Session. "There’s complete variability in what we see and how serious the injuries can be."
According to data from the National Pediatric Trauma Registry, 86% of injuries are blunt and 14% are penetrating. Chest injuries "are the second leading cause of death to central nervous system injuries," Dr. Fairbanks said. "Mortality rates for blunt and penetrating injuries are similar. In blunt traumas, most kids don’t die from their blunt thoracic injury, but from a CNS injury that they acquired at the same time. Most of the kids who die from penetrating injuries die in the field before reaching the hospital."
Thoracic injuries occur in about 25% of patients who present to a Level I trauma center. While thoracic injuries are less common than abdominal and extremity injuries are, "they are of potentially higher risk and more lethal," he said. "Most can be treated successfully. Male to female injury rate is 2:1 to 3:1 in favor of males having more traumas."
He classifies thoracic trauma injuries by anatomy (rib, pulmonary, bronchial), blunt vs. penetrating, and threat to life (immediate or potentially life threatening). Motor vehicles are the most common mechanism of thoracic injury, and children have an increased risk in all traumas of auto vs. pedestrian, compared with adults. "When children become teenagers, their mechanisms of injuries approach those of the data for adults," Dr. Fairbanks explained. "We see more penetrating knife and gunshot wounds to the chest, although they are rare in our younger patient population. Tracheobronchial lacerations are more common in children, compared with adults. Aortic disruptions are less likely in children."
Common thoracic injuries that he and his associates see at Rady Children’s Hospital are lung contusion, pneumothorax, hemothorax, and fractures to the ribs, sternum, or scapula. Less common "but perhaps more dangerous injuries" are those to the heart, aorta, trachea, bronchi, and diaphragm. "The most common immediately life-threatening thoracic injuries in children are airway obstruction, pneumothorax, hemothorax, and cardiac tamponade," he said. "A large percentage of thoracic injuries have stable vital signs at presentation. Rib fractures are less common in children and are a marker for a significant mechanism of injury or force. Mediastinal structures are more mobile, making tension pneumothorax a bigger problem."
Diagnosis and treatment of thoracic injuries proceeds simultaneously, with an initial goal to rule out life-threatening injuries, which should be identified and treated during the initial resuscitation phase. This includes making sure the airway is clear and secure and providing supplemental oxygen. "If endotracheal intubation is needed, check the position," he advised. "You want to see bilateral chest rise and CO2 waveform on your monitor. It’s very common for a child to have a right mainstem intubation and no breath sounds on the left side." He also makes it a point to assess their ventilation and treat their pneumothorax. "Don’t wait for the chest x-ray if you have a good clinical suspicion," he said.
A chest x-ray can often guide your management decisions in cases of thoracic trauma, while a FAST scan – specifically an ultrasound – is good for seeing a cardiac tamponade.
Sometimes thoracic injuries require an immediate thoracotomy in the emergency department, but its indications "are controversial," Dr. Fairbanks said. "It’s a potentially life-threatening maneuver, especially with penetrating cardiac injuries." The indications for emergency department thoracotomy are post-traumatic arrest or near arrest in all cases of penetrating thoracic injuries; in blunt trauma with loss of vital signs in the ED; or in blunt trauma with loss of vital signs in route to the ED.
"One of the things we struggle with in pediatric trauma is the timing at which they lost the vital signs in the field," he said. "After 20 minutes the chance of survival is almost nil. However, the family is going to want to know that you did everything possible to save their 3- or 4-year-old child."
If the patient is stable, complete the physical exam. "You’re looking for tachypnea, tenderness to the chest wall and abrasions, or signs of thoracic trauma that require further investigation," he said. Important signs include cyanosis, dyspnea, tracheal deviation, hoarseness, jugular engagement, and subcutaneous emphysema.
Chest auscultation is recommended and an anteroposterior (AP) chest x-ray becomes valuable, "because it’s quick, simple, and yields a lot of information," he said. He characterized a thoracic CT scan as "an excellent study" that can help with the diagnosis of aortic and diaphragmatic injuries which can be missed on chest x-ray. "However, there is a lot more radiation with a chest CT," Dr. Fairbanks noted. "Only 1 in 200 chest CTs for trauma yields a new diagnosis." Other studies to consider include EKG, echocardiogram, bronchoscopy, video-assisted thoracic surgery, radionuclide bone scan, and MRI.
A thoracotomy is indicated when the patient is coding or near coding. The other indications are penetrating wound of the heart or great vessels; massive or continuous intrathoracic bleeding; open pneumothorax with major chest wall defect; aortogram indicating injury to the aorta or major branch; massive or continuing air leak, indicating injury to a major airway; cardiac tamponade; esophageal perforation, or diaphragmatic rupture.
Chest wall soft-tissue injuries are usually not clinically significant, "but they’re a marker for a more serious injury under a bruise," he said. "Rib fractures are less common in children. It’s an indicator of significant force. Treatment is pain control and prevention of atelectasis, which is not as big of a problem in kids as it is in adults. They will heal in 6 weeks. Consider child abuse, specifically in cases of multiple rib fractures and those that don’t make sense with the mechanism of reported injury, or rib fractures that are at different stages of healing."
Dr. Fairbanks said that he had no relevant financial conflicts to disclose.
SAN DIEGO – Many thoracic injuries sustained by children and adolescents can be diagnosed with clinical assessment and chest x-ray, and many heal without surgical intervention, according to Dr. Timothy Fairbanks.
"When the pager goes off and says ‘3-year-old involved in a accident,’ that kid may be going home with a physical exam, stickers, and a high five; or he may require a life-saving procedure" in the emergency department. Dr. Fairbanks, of the division of pediatric surgery at Rady Children’s Hospital of San Diego, said at the University of California, San Diego, Critical Care Summer Session. "There’s complete variability in what we see and how serious the injuries can be."
According to data from the National Pediatric Trauma Registry, 86% of injuries are blunt and 14% are penetrating. Chest injuries "are the second leading cause of death to central nervous system injuries," Dr. Fairbanks said. "Mortality rates for blunt and penetrating injuries are similar. In blunt traumas, most kids don’t die from their blunt thoracic injury, but from a CNS injury that they acquired at the same time. Most of the kids who die from penetrating injuries die in the field before reaching the hospital."
Thoracic injuries occur in about 25% of patients who present to a Level I trauma center. While thoracic injuries are less common than abdominal and extremity injuries are, "they are of potentially higher risk and more lethal," he said. "Most can be treated successfully. Male to female injury rate is 2:1 to 3:1 in favor of males having more traumas."
He classifies thoracic trauma injuries by anatomy (rib, pulmonary, bronchial), blunt vs. penetrating, and threat to life (immediate or potentially life threatening). Motor vehicles are the most common mechanism of thoracic injury, and children have an increased risk in all traumas of auto vs. pedestrian, compared with adults. "When children become teenagers, their mechanisms of injuries approach those of the data for adults," Dr. Fairbanks explained. "We see more penetrating knife and gunshot wounds to the chest, although they are rare in our younger patient population. Tracheobronchial lacerations are more common in children, compared with adults. Aortic disruptions are less likely in children."
Common thoracic injuries that he and his associates see at Rady Children’s Hospital are lung contusion, pneumothorax, hemothorax, and fractures to the ribs, sternum, or scapula. Less common "but perhaps more dangerous injuries" are those to the heart, aorta, trachea, bronchi, and diaphragm. "The most common immediately life-threatening thoracic injuries in children are airway obstruction, pneumothorax, hemothorax, and cardiac tamponade," he said. "A large percentage of thoracic injuries have stable vital signs at presentation. Rib fractures are less common in children and are a marker for a significant mechanism of injury or force. Mediastinal structures are more mobile, making tension pneumothorax a bigger problem."
Diagnosis and treatment of thoracic injuries proceeds simultaneously, with an initial goal to rule out life-threatening injuries, which should be identified and treated during the initial resuscitation phase. This includes making sure the airway is clear and secure and providing supplemental oxygen. "If endotracheal intubation is needed, check the position," he advised. "You want to see bilateral chest rise and CO2 waveform on your monitor. It’s very common for a child to have a right mainstem intubation and no breath sounds on the left side." He also makes it a point to assess their ventilation and treat their pneumothorax. "Don’t wait for the chest x-ray if you have a good clinical suspicion," he said.
A chest x-ray can often guide your management decisions in cases of thoracic trauma, while a FAST scan – specifically an ultrasound – is good for seeing a cardiac tamponade.
Sometimes thoracic injuries require an immediate thoracotomy in the emergency department, but its indications "are controversial," Dr. Fairbanks said. "It’s a potentially life-threatening maneuver, especially with penetrating cardiac injuries." The indications for emergency department thoracotomy are post-traumatic arrest or near arrest in all cases of penetrating thoracic injuries; in blunt trauma with loss of vital signs in the ED; or in blunt trauma with loss of vital signs in route to the ED.
"One of the things we struggle with in pediatric trauma is the timing at which they lost the vital signs in the field," he said. "After 20 minutes the chance of survival is almost nil. However, the family is going to want to know that you did everything possible to save their 3- or 4-year-old child."
If the patient is stable, complete the physical exam. "You’re looking for tachypnea, tenderness to the chest wall and abrasions, or signs of thoracic trauma that require further investigation," he said. Important signs include cyanosis, dyspnea, tracheal deviation, hoarseness, jugular engagement, and subcutaneous emphysema.
Chest auscultation is recommended and an anteroposterior (AP) chest x-ray becomes valuable, "because it’s quick, simple, and yields a lot of information," he said. He characterized a thoracic CT scan as "an excellent study" that can help with the diagnosis of aortic and diaphragmatic injuries which can be missed on chest x-ray. "However, there is a lot more radiation with a chest CT," Dr. Fairbanks noted. "Only 1 in 200 chest CTs for trauma yields a new diagnosis." Other studies to consider include EKG, echocardiogram, bronchoscopy, video-assisted thoracic surgery, radionuclide bone scan, and MRI.
A thoracotomy is indicated when the patient is coding or near coding. The other indications are penetrating wound of the heart or great vessels; massive or continuous intrathoracic bleeding; open pneumothorax with major chest wall defect; aortogram indicating injury to the aorta or major branch; massive or continuing air leak, indicating injury to a major airway; cardiac tamponade; esophageal perforation, or diaphragmatic rupture.
Chest wall soft-tissue injuries are usually not clinically significant, "but they’re a marker for a more serious injury under a bruise," he said. "Rib fractures are less common in children. It’s an indicator of significant force. Treatment is pain control and prevention of atelectasis, which is not as big of a problem in kids as it is in adults. They will heal in 6 weeks. Consider child abuse, specifically in cases of multiple rib fractures and those that don’t make sense with the mechanism of reported injury, or rib fractures that are at different stages of healing."
Dr. Fairbanks said that he had no relevant financial conflicts to disclose.
EXPERT ANALYSIS AT THE UCSD CRITICAL CARE SUMMER SESSION
Big data destined for the bedside within 5 years
Big data shows the promise to transform how health care is delivered, utilizing real-time information to assist doctors and patients in making more informed decisions at the point of care, a transformation that could arrive in the next 5 years.
That time prediction was offered by Dr. Harlan Krumholz, a professor at Yale University, New Haven, Conn.
"We are on the cusp of dramatic change in the way in which we are thinking about leveraging data that’s being generated in everyday clinical practice and in everyday daily life," Dr. Krumholz said in an interview. "I think there’s this recognition that the current clinical scientific enterprise is inadequate to keep up with information needs."
But getting to that point is going to require a shift in the research culture and how that information is brought to doctors as they are treating patients.
"One of the biggest problems with our current research approach is it’s very reductionist," Dr. Krumholz observed. "We try to pursue the scientific method with testing hypotheses. We try to pull out all the complexity around the question we are trying to answer and simplify everything so we can cleanly look at a particular effect of one thing on patients. ... By the time [research results] come out, they are very anachronistic because medicine’s progressed and the patients that they’re studying aren’t exactly the people who are being seen in practice, so the studies aren’t necessarily having the effect they could have."
Dr. Krumholz also is a member of the Patient-Centered Outcomes Research Institute board of governors. The organization is building a research network that will facilitate the gathering and usage of big data to help inform patient decisions.
But it’s more than just health-specific data that need to be incorporated into big data analysis.
"It’s not just the pill, but it’s the pill for a specific patient who’s got a specific profile, who is in a certain social situation, who is taking other medication, maybe has certain access to physicians, and a lot of complicated issues coming together," Dr. Krumholz continued. "We need to be able to learn from everyday experience and we need to embrace the complexity, not reject it, so that our studies are taking into account the complex aspects of medicine, not trying to get rid of them so we have clean studies that may not well-relate back to the real-world situations doctors and patients face everyday."
The next step will be to find something that can truly demonstrate the power of big data.
"I think that once we break the dam open and allow the water to flow and turn the turbines of knowledge generation, people are going to demand better and better information in order to be in a stronger position to make decisions," he predicted. "I think the decision support enterprise, that community of people who are working on decision support tools, is going to start competing to develop and create these things that are going to be at the bedside, that is going to make a whole lot of difference from where we are now."
A number of pilots and areas where big data could have an immediate impact were highlighted in a recent article in the July issue of Health Affairs (doi: 10.1377/hlthaff.2014.0041). For example, Kaiser Permanente Northern California; Harvard University, Boston; and the University of California, San Francisco and Santa Cruz, are pilot testing a two-step protocol aimed at reducing antibiotic prescriptions for newborns by using objective maternal data to determine a preliminary probability for early-onset sepsis. The second step uses a set of clinical findings combined with the estimate based on maternal data to yield a new posterior probability for risk of sepsis following birth. Kaiser anticipates that the combination of these two steps could lead to as many as 240,000 fewer U.S. newborns being treated with systemic antibiotics each year.
Lead author Dr. David Bates, chief of the division of general medicine at Brigham and Women’s Hospital, Boston, and others identified several key areas where big data could have an effect at the point of care, including identifying high-cost patients to more efficiently care for them; helping to reduce readmissions; lowering the risk of complications when a patient is first admitted to a hospital; determining the risk that a patient’s condition will worsen; and understanding the risk of adverse events for patients.
"The better we are at getting to good information about options and consequences [regarding] who’s about to get into trouble, the more people are going to demand [the use of big data,] and the race will be on to create the products that are underneath the hood that are leveraging large data," Dr. Krumholz said.
Big data shows the promise to transform how health care is delivered, utilizing real-time information to assist doctors and patients in making more informed decisions at the point of care, a transformation that could arrive in the next 5 years.
That time prediction was offered by Dr. Harlan Krumholz, a professor at Yale University, New Haven, Conn.
"We are on the cusp of dramatic change in the way in which we are thinking about leveraging data that’s being generated in everyday clinical practice and in everyday daily life," Dr. Krumholz said in an interview. "I think there’s this recognition that the current clinical scientific enterprise is inadequate to keep up with information needs."
But getting to that point is going to require a shift in the research culture and how that information is brought to doctors as they are treating patients.
"One of the biggest problems with our current research approach is it’s very reductionist," Dr. Krumholz observed. "We try to pursue the scientific method with testing hypotheses. We try to pull out all the complexity around the question we are trying to answer and simplify everything so we can cleanly look at a particular effect of one thing on patients. ... By the time [research results] come out, they are very anachronistic because medicine’s progressed and the patients that they’re studying aren’t exactly the people who are being seen in practice, so the studies aren’t necessarily having the effect they could have."
Dr. Krumholz also is a member of the Patient-Centered Outcomes Research Institute board of governors. The organization is building a research network that will facilitate the gathering and usage of big data to help inform patient decisions.
But it’s more than just health-specific data that need to be incorporated into big data analysis.
"It’s not just the pill, but it’s the pill for a specific patient who’s got a specific profile, who is in a certain social situation, who is taking other medication, maybe has certain access to physicians, and a lot of complicated issues coming together," Dr. Krumholz continued. "We need to be able to learn from everyday experience and we need to embrace the complexity, not reject it, so that our studies are taking into account the complex aspects of medicine, not trying to get rid of them so we have clean studies that may not well-relate back to the real-world situations doctors and patients face everyday."
The next step will be to find something that can truly demonstrate the power of big data.
"I think that once we break the dam open and allow the water to flow and turn the turbines of knowledge generation, people are going to demand better and better information in order to be in a stronger position to make decisions," he predicted. "I think the decision support enterprise, that community of people who are working on decision support tools, is going to start competing to develop and create these things that are going to be at the bedside, that is going to make a whole lot of difference from where we are now."
A number of pilots and areas where big data could have an immediate impact were highlighted in a recent article in the July issue of Health Affairs (doi: 10.1377/hlthaff.2014.0041). For example, Kaiser Permanente Northern California; Harvard University, Boston; and the University of California, San Francisco and Santa Cruz, are pilot testing a two-step protocol aimed at reducing antibiotic prescriptions for newborns by using objective maternal data to determine a preliminary probability for early-onset sepsis. The second step uses a set of clinical findings combined with the estimate based on maternal data to yield a new posterior probability for risk of sepsis following birth. Kaiser anticipates that the combination of these two steps could lead to as many as 240,000 fewer U.S. newborns being treated with systemic antibiotics each year.
Lead author Dr. David Bates, chief of the division of general medicine at Brigham and Women’s Hospital, Boston, and others identified several key areas where big data could have an effect at the point of care, including identifying high-cost patients to more efficiently care for them; helping to reduce readmissions; lowering the risk of complications when a patient is first admitted to a hospital; determining the risk that a patient’s condition will worsen; and understanding the risk of adverse events for patients.
"The better we are at getting to good information about options and consequences [regarding] who’s about to get into trouble, the more people are going to demand [the use of big data,] and the race will be on to create the products that are underneath the hood that are leveraging large data," Dr. Krumholz said.
Big data shows the promise to transform how health care is delivered, utilizing real-time information to assist doctors and patients in making more informed decisions at the point of care, a transformation that could arrive in the next 5 years.
That time prediction was offered by Dr. Harlan Krumholz, a professor at Yale University, New Haven, Conn.
"We are on the cusp of dramatic change in the way in which we are thinking about leveraging data that’s being generated in everyday clinical practice and in everyday daily life," Dr. Krumholz said in an interview. "I think there’s this recognition that the current clinical scientific enterprise is inadequate to keep up with information needs."
But getting to that point is going to require a shift in the research culture and how that information is brought to doctors as they are treating patients.
"One of the biggest problems with our current research approach is it’s very reductionist," Dr. Krumholz observed. "We try to pursue the scientific method with testing hypotheses. We try to pull out all the complexity around the question we are trying to answer and simplify everything so we can cleanly look at a particular effect of one thing on patients. ... By the time [research results] come out, they are very anachronistic because medicine’s progressed and the patients that they’re studying aren’t exactly the people who are being seen in practice, so the studies aren’t necessarily having the effect they could have."
Dr. Krumholz also is a member of the Patient-Centered Outcomes Research Institute board of governors. The organization is building a research network that will facilitate the gathering and usage of big data to help inform patient decisions.
But it’s more than just health-specific data that need to be incorporated into big data analysis.
"It’s not just the pill, but it’s the pill for a specific patient who’s got a specific profile, who is in a certain social situation, who is taking other medication, maybe has certain access to physicians, and a lot of complicated issues coming together," Dr. Krumholz continued. "We need to be able to learn from everyday experience and we need to embrace the complexity, not reject it, so that our studies are taking into account the complex aspects of medicine, not trying to get rid of them so we have clean studies that may not well-relate back to the real-world situations doctors and patients face everyday."
The next step will be to find something that can truly demonstrate the power of big data.
"I think that once we break the dam open and allow the water to flow and turn the turbines of knowledge generation, people are going to demand better and better information in order to be in a stronger position to make decisions," he predicted. "I think the decision support enterprise, that community of people who are working on decision support tools, is going to start competing to develop and create these things that are going to be at the bedside, that is going to make a whole lot of difference from where we are now."
A number of pilots and areas where big data could have an immediate impact were highlighted in a recent article in the July issue of Health Affairs (doi: 10.1377/hlthaff.2014.0041). For example, Kaiser Permanente Northern California; Harvard University, Boston; and the University of California, San Francisco and Santa Cruz, are pilot testing a two-step protocol aimed at reducing antibiotic prescriptions for newborns by using objective maternal data to determine a preliminary probability for early-onset sepsis. The second step uses a set of clinical findings combined with the estimate based on maternal data to yield a new posterior probability for risk of sepsis following birth. Kaiser anticipates that the combination of these two steps could lead to as many as 240,000 fewer U.S. newborns being treated with systemic antibiotics each year.
Lead author Dr. David Bates, chief of the division of general medicine at Brigham and Women’s Hospital, Boston, and others identified several key areas where big data could have an effect at the point of care, including identifying high-cost patients to more efficiently care for them; helping to reduce readmissions; lowering the risk of complications when a patient is first admitted to a hospital; determining the risk that a patient’s condition will worsen; and understanding the risk of adverse events for patients.
"The better we are at getting to good information about options and consequences [regarding] who’s about to get into trouble, the more people are going to demand [the use of big data,] and the race will be on to create the products that are underneath the hood that are leveraging large data," Dr. Krumholz said.
Questions surround EHR security
Despite government efforts to certify otherwise, questions remain as to whether the Dept. of Health & Human Services Office of the National Coordinator for Health Information Technology is doing enough to ensure that commercially available electronic health record software programs are doing enough to secure patient information.
Concerns were raised by HHS Office of Inspector General (OIG) in a report released Aug. 4. The agency watchdog examined certification work conducted by authorized testing and certification bodies (ATCBs), which early on in the meaningful use program certified that electronic health records (EHRs) met established criteria that would allow doctors and hospitals to obtain Medicare or Medicaid incentive payments.
According to the report, as of Aug. 30, 2013, a total of 3,590 certified EHRs were available to health care providers, 95% of which were certified by ATCBs under a temporary certification program.
In examining the work done by ATCBs, the OIG found that oversight by the HHS Office of the National Coordinator for Health Information Technology (ONC) "did not fully ensure that test procedures and standards could adequately secure and protect patient information contained in EHRs," the report states. OIG claimed that the health IT office did not ensure that ATCBs "developed procedures to periodically evaluate whether certified EHRs continued to meet Federal standards and developed a training program to ensure that their personnel were competent to test and certify EHRs and to secure proprietary or sensitive information."
OIG notes that the ATCB standards and procedures met all National Institute of Standards and Technology (NIST) test procedure requirements that the ONC approved, but those procedures "were not sufficient to ensure that EHRs would adequately secure and protect patient health information; in particular, the procedures allowed ATCBs to certify EHRs that demonstrated the use of a single-character password during testing." NIST procedures also did not address common security issues, including password complexity and logging emergency access or user privilege changes.
In response to the draft, included as part of the report, ONC noted that ATCBs are no longer active in the certification. New certification criteria approved earlier this year have "strengthened test procedures for common security and privacy features for inclusion in EHRs." Additionally, ONC has "substantially revised the ‘auditable events and tamper resistance’ certification criterion, and we adopted a new ‘end-user device encryption’ criterion," as well as other security capabilities, according to a spokesperson. ONC will review the OIG’s comments before determining the appropriate next steps, the spokesperson added.
However, the OIG does not agree that the current certification regulations "sufficiently address our security concerns regarding the Temporary Program," such as multifactor authentication.
OIG also criticized the health IT office for not addressing the authority to remove EHRs from the market that are shown to have privacy and security flaws.
If an EHR "is exploited and used to conduct malicious activities, ONC is not able to remove the EHR, even temporarily, from the product list to prevent further purchases of it."
Despite government efforts to certify otherwise, questions remain as to whether the Dept. of Health & Human Services Office of the National Coordinator for Health Information Technology is doing enough to ensure that commercially available electronic health record software programs are doing enough to secure patient information.
Concerns were raised by HHS Office of Inspector General (OIG) in a report released Aug. 4. The agency watchdog examined certification work conducted by authorized testing and certification bodies (ATCBs), which early on in the meaningful use program certified that electronic health records (EHRs) met established criteria that would allow doctors and hospitals to obtain Medicare or Medicaid incentive payments.
According to the report, as of Aug. 30, 2013, a total of 3,590 certified EHRs were available to health care providers, 95% of which were certified by ATCBs under a temporary certification program.
In examining the work done by ATCBs, the OIG found that oversight by the HHS Office of the National Coordinator for Health Information Technology (ONC) "did not fully ensure that test procedures and standards could adequately secure and protect patient information contained in EHRs," the report states. OIG claimed that the health IT office did not ensure that ATCBs "developed procedures to periodically evaluate whether certified EHRs continued to meet Federal standards and developed a training program to ensure that their personnel were competent to test and certify EHRs and to secure proprietary or sensitive information."
OIG notes that the ATCB standards and procedures met all National Institute of Standards and Technology (NIST) test procedure requirements that the ONC approved, but those procedures "were not sufficient to ensure that EHRs would adequately secure and protect patient health information; in particular, the procedures allowed ATCBs to certify EHRs that demonstrated the use of a single-character password during testing." NIST procedures also did not address common security issues, including password complexity and logging emergency access or user privilege changes.
In response to the draft, included as part of the report, ONC noted that ATCBs are no longer active in the certification. New certification criteria approved earlier this year have "strengthened test procedures for common security and privacy features for inclusion in EHRs." Additionally, ONC has "substantially revised the ‘auditable events and tamper resistance’ certification criterion, and we adopted a new ‘end-user device encryption’ criterion," as well as other security capabilities, according to a spokesperson. ONC will review the OIG’s comments before determining the appropriate next steps, the spokesperson added.
However, the OIG does not agree that the current certification regulations "sufficiently address our security concerns regarding the Temporary Program," such as multifactor authentication.
OIG also criticized the health IT office for not addressing the authority to remove EHRs from the market that are shown to have privacy and security flaws.
If an EHR "is exploited and used to conduct malicious activities, ONC is not able to remove the EHR, even temporarily, from the product list to prevent further purchases of it."
Despite government efforts to certify otherwise, questions remain as to whether the Dept. of Health & Human Services Office of the National Coordinator for Health Information Technology is doing enough to ensure that commercially available electronic health record software programs are doing enough to secure patient information.
Concerns were raised by HHS Office of Inspector General (OIG) in a report released Aug. 4. The agency watchdog examined certification work conducted by authorized testing and certification bodies (ATCBs), which early on in the meaningful use program certified that electronic health records (EHRs) met established criteria that would allow doctors and hospitals to obtain Medicare or Medicaid incentive payments.
According to the report, as of Aug. 30, 2013, a total of 3,590 certified EHRs were available to health care providers, 95% of which were certified by ATCBs under a temporary certification program.
In examining the work done by ATCBs, the OIG found that oversight by the HHS Office of the National Coordinator for Health Information Technology (ONC) "did not fully ensure that test procedures and standards could adequately secure and protect patient information contained in EHRs," the report states. OIG claimed that the health IT office did not ensure that ATCBs "developed procedures to periodically evaluate whether certified EHRs continued to meet Federal standards and developed a training program to ensure that their personnel were competent to test and certify EHRs and to secure proprietary or sensitive information."
OIG notes that the ATCB standards and procedures met all National Institute of Standards and Technology (NIST) test procedure requirements that the ONC approved, but those procedures "were not sufficient to ensure that EHRs would adequately secure and protect patient health information; in particular, the procedures allowed ATCBs to certify EHRs that demonstrated the use of a single-character password during testing." NIST procedures also did not address common security issues, including password complexity and logging emergency access or user privilege changes.
In response to the draft, included as part of the report, ONC noted that ATCBs are no longer active in the certification. New certification criteria approved earlier this year have "strengthened test procedures for common security and privacy features for inclusion in EHRs." Additionally, ONC has "substantially revised the ‘auditable events and tamper resistance’ certification criterion, and we adopted a new ‘end-user device encryption’ criterion," as well as other security capabilities, according to a spokesperson. ONC will review the OIG’s comments before determining the appropriate next steps, the spokesperson added.
However, the OIG does not agree that the current certification regulations "sufficiently address our security concerns regarding the Temporary Program," such as multifactor authentication.
OIG also criticized the health IT office for not addressing the authority to remove EHRs from the market that are shown to have privacy and security flaws.
If an EHR "is exploited and used to conduct malicious activities, ONC is not able to remove the EHR, even temporarily, from the product list to prevent further purchases of it."
Is it time for telemedicine?
In the constantly advancing world of health information technology, the buzzwords are always changing. In the 90s, HIPAA dominated the literature. In the past few years, meaningful use took over. Most recently, a great deal of attention has been paid to telemedicine. For the uninitiated, telemedicine, a.k.a. telehealth, is not simply returning patient phone calls after hours. Telemedicine is the idea of substituting in-person patient encounters with virtual ones, typically using a secure video interface. In this column, we will explore a general overview of telemedicine and consider how this new medium fits into the ever-changing landscape of patient care.
The patient will ‘see’ you now
As evidenced by the feedback we have received on previous columns, many of our readers are still reeling from the arduous task of implementing an EHR and shudder at the thought of introducing even more technology into their office workflow. Others who enjoy being on the cutting edge of medicine are eager to embrace these virtual visits. We’ve spoken to several early adopters and have learned that it can be a rewarding way to handle certain patient interactions, but telemedicine obviously isn’t right for every patient or condition. Some feel that the lack of physical presence is not conducive to handling personal conversations or conveying sensitive information. Others have met with resistance from patients who just can’t get used to the idea of hands-free care. As with anything else, there will be patients who remain skeptical, but there will also be those who prefer it.
One patient we engaged saw his physician’s choice to offer virtual visits as a huge benefit, saying that "the idea of saving the 30 minutes of driving in each direction to visit the doctor, along with avoiding the hassle of taking time off work, makes [telehealth] a perfect way to handle my blood pressure control." In this case, as with others, patients use information from devices such as home blood pressure monitors or glucometers to supplement the visit and provide objective data. As home technology advances, connected devices are becoming more sophisticated and allowing more complicated diagnostics to be done remotely. Smartphone-connected otoscopes and ECG machines are just two examples of what’s to come, and we plan to highlight some of these in an upcoming column.
Getting started: Is it safe?
There are several things to consider when getting started in telemedicine in your practice. First is hardware. In general, this is fairly straightforward as most practices are now set up with broadband Internet and computers or tablets. Obviously, a good quality webcam is essential to ensure quality communication with patients. Beyond this, it’s critical to think about the security of the video interaction.
Standard consumer video-conferencing software such as Skype and Apple’s FaceTime are not HIPAA-compliant and can’t be used for patient communications. An encrypted video portal is essential to maintain patient privacy and make sure the patient interaction remains secure. The good news is that several easy-to-use software packages exist to allow this, if the functionality is not already built in to your EHR. A quick web search for "HIPAA-compliant video conferencing" returned several low-cost examples – some that are even free for a few interactions per month. This may be a good way to see if there is interest from patients prior to a large outlay of money. But what about the question of liability?
Unfortunately, physicians in the 21st century are wired to always be concerned about the threat of litigation, and there is no question that telemedicine offers a new opportunity for scrutiny of our clinical decisions. In addition, many rightfully fear the idea of diagnosis and treatment without the laying on of hands. As of now, there is little-to-no legal precedent for the malpractice implications of telehealth visits, but Teledoc, the country’s largest purveyor of telemedicine, advertises 7.5 million members and zero malpractice claims. Whether or not this is enough to convince the skeptics remains to be seen, but so far telemedicine has seemingly managed to fly below the radar of the courts. Following the pattern of all areas of medicine, this is likely to change, but it may be prevented if physicians limit the kinds of services offered through virtual visits. Many providers, such as Teledoc, for example, do not prescribe Drug Enforcement Administration–controlled or so-called nontherapeutic medications. (If you’re wondering, Viagra and Cialis are both on this list.) In this way, they are able to avoid becoming confused with illegitimate drug fulfillment warehouses and limit encounters that might raise questions of legality.
Can I get paid for this?
Although there are many conceivable benefits to telemedicine, such as access to patients who are homebound or live in isolated rural areas, it is hard to imagine setting off down the virtual path without the promise of reimbursement. According to the American Telemedicine Association, 21 states and Washington, D.C., require coverage of telemedicine services with reimbursement at a rate on par with in-person visits, while providers in the others are left wanting. Currently, 120 members of the U.S. Congress support various bills to expand the reach and acceptance of telemedicine, according to the telemedicine association, but it’s clear there is a long way to go before telemedicine receives the full support of insurers. In the meantime, its only financial benefit may be as a marketing advantage for practices. Those who leverage this may be able to draw new business from patients seeking more options in the delivery of their care.
Are you ready?
As with all burgeoning areas of health IT, telemedicine in its early stages appears at best like science fiction and at worst like just another headache. Unlike the other buzzwords of the past few years, adoption of telemedicine seems to be less of a requirement and more of an option. It may or may not work in your practice or for your patient population. Most importantly, though, it represents one point on the continuum of care that will take on an increasingly prominent role in the consumer-driven health care market, and it may represent a boon to both patients and providers alike.
Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records. Dr. Skolnik is associate director of the family medicine residency program at Abington Memorial Hospital and professor of family and community medicine at Temple University in Philadelphia.
In the constantly advancing world of health information technology, the buzzwords are always changing. In the 90s, HIPAA dominated the literature. In the past few years, meaningful use took over. Most recently, a great deal of attention has been paid to telemedicine. For the uninitiated, telemedicine, a.k.a. telehealth, is not simply returning patient phone calls after hours. Telemedicine is the idea of substituting in-person patient encounters with virtual ones, typically using a secure video interface. In this column, we will explore a general overview of telemedicine and consider how this new medium fits into the ever-changing landscape of patient care.
The patient will ‘see’ you now
As evidenced by the feedback we have received on previous columns, many of our readers are still reeling from the arduous task of implementing an EHR and shudder at the thought of introducing even more technology into their office workflow. Others who enjoy being on the cutting edge of medicine are eager to embrace these virtual visits. We’ve spoken to several early adopters and have learned that it can be a rewarding way to handle certain patient interactions, but telemedicine obviously isn’t right for every patient or condition. Some feel that the lack of physical presence is not conducive to handling personal conversations or conveying sensitive information. Others have met with resistance from patients who just can’t get used to the idea of hands-free care. As with anything else, there will be patients who remain skeptical, but there will also be those who prefer it.
One patient we engaged saw his physician’s choice to offer virtual visits as a huge benefit, saying that "the idea of saving the 30 minutes of driving in each direction to visit the doctor, along with avoiding the hassle of taking time off work, makes [telehealth] a perfect way to handle my blood pressure control." In this case, as with others, patients use information from devices such as home blood pressure monitors or glucometers to supplement the visit and provide objective data. As home technology advances, connected devices are becoming more sophisticated and allowing more complicated diagnostics to be done remotely. Smartphone-connected otoscopes and ECG machines are just two examples of what’s to come, and we plan to highlight some of these in an upcoming column.
Getting started: Is it safe?
There are several things to consider when getting started in telemedicine in your practice. First is hardware. In general, this is fairly straightforward as most practices are now set up with broadband Internet and computers or tablets. Obviously, a good quality webcam is essential to ensure quality communication with patients. Beyond this, it’s critical to think about the security of the video interaction.
Standard consumer video-conferencing software such as Skype and Apple’s FaceTime are not HIPAA-compliant and can’t be used for patient communications. An encrypted video portal is essential to maintain patient privacy and make sure the patient interaction remains secure. The good news is that several easy-to-use software packages exist to allow this, if the functionality is not already built in to your EHR. A quick web search for "HIPAA-compliant video conferencing" returned several low-cost examples – some that are even free for a few interactions per month. This may be a good way to see if there is interest from patients prior to a large outlay of money. But what about the question of liability?
Unfortunately, physicians in the 21st century are wired to always be concerned about the threat of litigation, and there is no question that telemedicine offers a new opportunity for scrutiny of our clinical decisions. In addition, many rightfully fear the idea of diagnosis and treatment without the laying on of hands. As of now, there is little-to-no legal precedent for the malpractice implications of telehealth visits, but Teledoc, the country’s largest purveyor of telemedicine, advertises 7.5 million members and zero malpractice claims. Whether or not this is enough to convince the skeptics remains to be seen, but so far telemedicine has seemingly managed to fly below the radar of the courts. Following the pattern of all areas of medicine, this is likely to change, but it may be prevented if physicians limit the kinds of services offered through virtual visits. Many providers, such as Teledoc, for example, do not prescribe Drug Enforcement Administration–controlled or so-called nontherapeutic medications. (If you’re wondering, Viagra and Cialis are both on this list.) In this way, they are able to avoid becoming confused with illegitimate drug fulfillment warehouses and limit encounters that might raise questions of legality.
Can I get paid for this?
Although there are many conceivable benefits to telemedicine, such as access to patients who are homebound or live in isolated rural areas, it is hard to imagine setting off down the virtual path without the promise of reimbursement. According to the American Telemedicine Association, 21 states and Washington, D.C., require coverage of telemedicine services with reimbursement at a rate on par with in-person visits, while providers in the others are left wanting. Currently, 120 members of the U.S. Congress support various bills to expand the reach and acceptance of telemedicine, according to the telemedicine association, but it’s clear there is a long way to go before telemedicine receives the full support of insurers. In the meantime, its only financial benefit may be as a marketing advantage for practices. Those who leverage this may be able to draw new business from patients seeking more options in the delivery of their care.
Are you ready?
As with all burgeoning areas of health IT, telemedicine in its early stages appears at best like science fiction and at worst like just another headache. Unlike the other buzzwords of the past few years, adoption of telemedicine seems to be less of a requirement and more of an option. It may or may not work in your practice or for your patient population. Most importantly, though, it represents one point on the continuum of care that will take on an increasingly prominent role in the consumer-driven health care market, and it may represent a boon to both patients and providers alike.
Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records. Dr. Skolnik is associate director of the family medicine residency program at Abington Memorial Hospital and professor of family and community medicine at Temple University in Philadelphia.
In the constantly advancing world of health information technology, the buzzwords are always changing. In the 90s, HIPAA dominated the literature. In the past few years, meaningful use took over. Most recently, a great deal of attention has been paid to telemedicine. For the uninitiated, telemedicine, a.k.a. telehealth, is not simply returning patient phone calls after hours. Telemedicine is the idea of substituting in-person patient encounters with virtual ones, typically using a secure video interface. In this column, we will explore a general overview of telemedicine and consider how this new medium fits into the ever-changing landscape of patient care.
The patient will ‘see’ you now
As evidenced by the feedback we have received on previous columns, many of our readers are still reeling from the arduous task of implementing an EHR and shudder at the thought of introducing even more technology into their office workflow. Others who enjoy being on the cutting edge of medicine are eager to embrace these virtual visits. We’ve spoken to several early adopters and have learned that it can be a rewarding way to handle certain patient interactions, but telemedicine obviously isn’t right for every patient or condition. Some feel that the lack of physical presence is not conducive to handling personal conversations or conveying sensitive information. Others have met with resistance from patients who just can’t get used to the idea of hands-free care. As with anything else, there will be patients who remain skeptical, but there will also be those who prefer it.
One patient we engaged saw his physician’s choice to offer virtual visits as a huge benefit, saying that "the idea of saving the 30 minutes of driving in each direction to visit the doctor, along with avoiding the hassle of taking time off work, makes [telehealth] a perfect way to handle my blood pressure control." In this case, as with others, patients use information from devices such as home blood pressure monitors or glucometers to supplement the visit and provide objective data. As home technology advances, connected devices are becoming more sophisticated and allowing more complicated diagnostics to be done remotely. Smartphone-connected otoscopes and ECG machines are just two examples of what’s to come, and we plan to highlight some of these in an upcoming column.
Getting started: Is it safe?
There are several things to consider when getting started in telemedicine in your practice. First is hardware. In general, this is fairly straightforward as most practices are now set up with broadband Internet and computers or tablets. Obviously, a good quality webcam is essential to ensure quality communication with patients. Beyond this, it’s critical to think about the security of the video interaction.
Standard consumer video-conferencing software such as Skype and Apple’s FaceTime are not HIPAA-compliant and can’t be used for patient communications. An encrypted video portal is essential to maintain patient privacy and make sure the patient interaction remains secure. The good news is that several easy-to-use software packages exist to allow this, if the functionality is not already built in to your EHR. A quick web search for "HIPAA-compliant video conferencing" returned several low-cost examples – some that are even free for a few interactions per month. This may be a good way to see if there is interest from patients prior to a large outlay of money. But what about the question of liability?
Unfortunately, physicians in the 21st century are wired to always be concerned about the threat of litigation, and there is no question that telemedicine offers a new opportunity for scrutiny of our clinical decisions. In addition, many rightfully fear the idea of diagnosis and treatment without the laying on of hands. As of now, there is little-to-no legal precedent for the malpractice implications of telehealth visits, but Teledoc, the country’s largest purveyor of telemedicine, advertises 7.5 million members and zero malpractice claims. Whether or not this is enough to convince the skeptics remains to be seen, but so far telemedicine has seemingly managed to fly below the radar of the courts. Following the pattern of all areas of medicine, this is likely to change, but it may be prevented if physicians limit the kinds of services offered through virtual visits. Many providers, such as Teledoc, for example, do not prescribe Drug Enforcement Administration–controlled or so-called nontherapeutic medications. (If you’re wondering, Viagra and Cialis are both on this list.) In this way, they are able to avoid becoming confused with illegitimate drug fulfillment warehouses and limit encounters that might raise questions of legality.
Can I get paid for this?
Although there are many conceivable benefits to telemedicine, such as access to patients who are homebound or live in isolated rural areas, it is hard to imagine setting off down the virtual path without the promise of reimbursement. According to the American Telemedicine Association, 21 states and Washington, D.C., require coverage of telemedicine services with reimbursement at a rate on par with in-person visits, while providers in the others are left wanting. Currently, 120 members of the U.S. Congress support various bills to expand the reach and acceptance of telemedicine, according to the telemedicine association, but it’s clear there is a long way to go before telemedicine receives the full support of insurers. In the meantime, its only financial benefit may be as a marketing advantage for practices. Those who leverage this may be able to draw new business from patients seeking more options in the delivery of their care.
Are you ready?
As with all burgeoning areas of health IT, telemedicine in its early stages appears at best like science fiction and at worst like just another headache. Unlike the other buzzwords of the past few years, adoption of telemedicine seems to be less of a requirement and more of an option. It may or may not work in your practice or for your patient population. Most importantly, though, it represents one point on the continuum of care that will take on an increasingly prominent role in the consumer-driven health care market, and it may represent a boon to both patients and providers alike.
Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records. Dr. Skolnik is associate director of the family medicine residency program at Abington Memorial Hospital and professor of family and community medicine at Temple University in Philadelphia.
Guideline adjusts perioperative cardiac care in noncardiac surgery
A new clinical practice guideline on cardiovascular evaluation and management of patients undergoing noncardiac surgery adds some clarity around the controversial issue of beta-blocker therapy and updates other aspects of care.
If a patient on beta-blocker medication needs noncardiac surgery, continue the beta-blocker, because there is no evidence of harm from doing so; but you risk doing harm if the drug is stopped, according to the new guideline from the American College of Cardiology (ACC) and the American Heart Association (AHA).
Surgeons will be happy to hear that, said Dr. Lee A. Fleisher, the chair of the guideline-writing committee, because that conforms to one of the Surgical Care Improvement Project’s National Measures.
For patients at elevated risk of a cardiovascular event during noncardiac surgery who are not already on beta-blocker therapy, however, the new guideline steps back from the organization’s 2009 position that beta-blockers not be started, and says instead that it’s not unreasonable to start the drug, with a caveat. Be very cautious and start the drug early enough before surgery that you can titrate it to avoid causing hypotension or a low heart rate.
"Make sure that you’re giving the right amount and monitoring their blood pressure and heart rate," Dr. Fleisher, chair of the guideline writing committee, said in an interview. "Really think once, twice, and thrice about starting a protocol," added Dr. Fleisher, the Robert D. Dripps Professor of Anesthesiology and Critical Care at the University of Pennsylvania, Philadelphia.
The ACC and AHA commissioned a committee to review the evidence for and against beta-blockers in patients undergoing noncardiac surgery. A separate writing committee then considered the evidence review committee’s report, reviewed the literature on other aspects of perioperative care for noncardiac surgery, and compiled a 102-page guideline with a 59-page executive summary.
The "2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery" will be published online on the ACC and AHA websites.
Dr. Fleisher described other highlights of the new guideline pertinent to cardiologists, primary care physicians, or surgeons. For the first time, palliative care has been added as an option that may come out of the preoperative evaluation, he said. Patient categories of high risk and intermediate risk have been lumped together as having "elevated" risk for simplicity’s sake because recommendations for the two separate categories were so similar.
The guideline now endorses two tools to choose from for preoperative risk assessments: the Revised Cardiac Risk Index (RCRI), and the National Surgical Quality Improvement Project risk calculator. "There have been a lot of comments that [the NSQIP] is a very useful tool to have shared decision-making conversations with patients," he said.
Another change applies to patients who receive second- or third-generation coronary stents. Instead of a wait of a year after stent implantation to perform noncardiac surgery, a 6-month wait may be reasonable if the risks of delaying noncardiac surgery outweigh the risks of interrupting dual-antiplatelet therapy for the noncardiac surgery.
In addition, the guideline incorporates findings from the recent POISE-2 study to say that aspirin can be stopped and clonidine is not useful in patients without stents undergoing noncardiac surgery (N. Engl. J. Med. 2014;370:1494-503).
A new statement in the guideline about troponin says to check troponin in high-risk patients with signs or symptoms of trouble but not to include troponin in routine screening.
The recommendations on beta-blockers, however, address the most controversial topic in the guideline, Dr. Fleisher said. "There is a lot of confusing evidence" on the use of beta-blockers, "so we’ve tried to clarify as much as we can."
The ACC and AHA funded the work of the committees. Dr. Fleisher reported having no financial disclosures.
On Twitter @sherryboschert
A new clinical practice guideline on cardiovascular evaluation and management of patients undergoing noncardiac surgery adds some clarity around the controversial issue of beta-blocker therapy and updates other aspects of care.
If a patient on beta-blocker medication needs noncardiac surgery, continue the beta-blocker, because there is no evidence of harm from doing so; but you risk doing harm if the drug is stopped, according to the new guideline from the American College of Cardiology (ACC) and the American Heart Association (AHA).
Surgeons will be happy to hear that, said Dr. Lee A. Fleisher, the chair of the guideline-writing committee, because that conforms to one of the Surgical Care Improvement Project’s National Measures.
For patients at elevated risk of a cardiovascular event during noncardiac surgery who are not already on beta-blocker therapy, however, the new guideline steps back from the organization’s 2009 position that beta-blockers not be started, and says instead that it’s not unreasonable to start the drug, with a caveat. Be very cautious and start the drug early enough before surgery that you can titrate it to avoid causing hypotension or a low heart rate.
"Make sure that you’re giving the right amount and monitoring their blood pressure and heart rate," Dr. Fleisher, chair of the guideline writing committee, said in an interview. "Really think once, twice, and thrice about starting a protocol," added Dr. Fleisher, the Robert D. Dripps Professor of Anesthesiology and Critical Care at the University of Pennsylvania, Philadelphia.
The ACC and AHA commissioned a committee to review the evidence for and against beta-blockers in patients undergoing noncardiac surgery. A separate writing committee then considered the evidence review committee’s report, reviewed the literature on other aspects of perioperative care for noncardiac surgery, and compiled a 102-page guideline with a 59-page executive summary.
The "2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery" will be published online on the ACC and AHA websites.
Dr. Fleisher described other highlights of the new guideline pertinent to cardiologists, primary care physicians, or surgeons. For the first time, palliative care has been added as an option that may come out of the preoperative evaluation, he said. Patient categories of high risk and intermediate risk have been lumped together as having "elevated" risk for simplicity’s sake because recommendations for the two separate categories were so similar.
The guideline now endorses two tools to choose from for preoperative risk assessments: the Revised Cardiac Risk Index (RCRI), and the National Surgical Quality Improvement Project risk calculator. "There have been a lot of comments that [the NSQIP] is a very useful tool to have shared decision-making conversations with patients," he said.
Another change applies to patients who receive second- or third-generation coronary stents. Instead of a wait of a year after stent implantation to perform noncardiac surgery, a 6-month wait may be reasonable if the risks of delaying noncardiac surgery outweigh the risks of interrupting dual-antiplatelet therapy for the noncardiac surgery.
In addition, the guideline incorporates findings from the recent POISE-2 study to say that aspirin can be stopped and clonidine is not useful in patients without stents undergoing noncardiac surgery (N. Engl. J. Med. 2014;370:1494-503).
A new statement in the guideline about troponin says to check troponin in high-risk patients with signs or symptoms of trouble but not to include troponin in routine screening.
The recommendations on beta-blockers, however, address the most controversial topic in the guideline, Dr. Fleisher said. "There is a lot of confusing evidence" on the use of beta-blockers, "so we’ve tried to clarify as much as we can."
The ACC and AHA funded the work of the committees. Dr. Fleisher reported having no financial disclosures.
On Twitter @sherryboschert
A new clinical practice guideline on cardiovascular evaluation and management of patients undergoing noncardiac surgery adds some clarity around the controversial issue of beta-blocker therapy and updates other aspects of care.
If a patient on beta-blocker medication needs noncardiac surgery, continue the beta-blocker, because there is no evidence of harm from doing so; but you risk doing harm if the drug is stopped, according to the new guideline from the American College of Cardiology (ACC) and the American Heart Association (AHA).
Surgeons will be happy to hear that, said Dr. Lee A. Fleisher, the chair of the guideline-writing committee, because that conforms to one of the Surgical Care Improvement Project’s National Measures.
For patients at elevated risk of a cardiovascular event during noncardiac surgery who are not already on beta-blocker therapy, however, the new guideline steps back from the organization’s 2009 position that beta-blockers not be started, and says instead that it’s not unreasonable to start the drug, with a caveat. Be very cautious and start the drug early enough before surgery that you can titrate it to avoid causing hypotension or a low heart rate.
"Make sure that you’re giving the right amount and monitoring their blood pressure and heart rate," Dr. Fleisher, chair of the guideline writing committee, said in an interview. "Really think once, twice, and thrice about starting a protocol," added Dr. Fleisher, the Robert D. Dripps Professor of Anesthesiology and Critical Care at the University of Pennsylvania, Philadelphia.
The ACC and AHA commissioned a committee to review the evidence for and against beta-blockers in patients undergoing noncardiac surgery. A separate writing committee then considered the evidence review committee’s report, reviewed the literature on other aspects of perioperative care for noncardiac surgery, and compiled a 102-page guideline with a 59-page executive summary.
The "2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery" will be published online on the ACC and AHA websites.
Dr. Fleisher described other highlights of the new guideline pertinent to cardiologists, primary care physicians, or surgeons. For the first time, palliative care has been added as an option that may come out of the preoperative evaluation, he said. Patient categories of high risk and intermediate risk have been lumped together as having "elevated" risk for simplicity’s sake because recommendations for the two separate categories were so similar.
The guideline now endorses two tools to choose from for preoperative risk assessments: the Revised Cardiac Risk Index (RCRI), and the National Surgical Quality Improvement Project risk calculator. "There have been a lot of comments that [the NSQIP] is a very useful tool to have shared decision-making conversations with patients," he said.
Another change applies to patients who receive second- or third-generation coronary stents. Instead of a wait of a year after stent implantation to perform noncardiac surgery, a 6-month wait may be reasonable if the risks of delaying noncardiac surgery outweigh the risks of interrupting dual-antiplatelet therapy for the noncardiac surgery.
In addition, the guideline incorporates findings from the recent POISE-2 study to say that aspirin can be stopped and clonidine is not useful in patients without stents undergoing noncardiac surgery (N. Engl. J. Med. 2014;370:1494-503).
A new statement in the guideline about troponin says to check troponin in high-risk patients with signs or symptoms of trouble but not to include troponin in routine screening.
The recommendations on beta-blockers, however, address the most controversial topic in the guideline, Dr. Fleisher said. "There is a lot of confusing evidence" on the use of beta-blockers, "so we’ve tried to clarify as much as we can."
The ACC and AHA funded the work of the committees. Dr. Fleisher reported having no financial disclosures.
On Twitter @sherryboschert
Bowel prep before vaginal prolapse surgery offers no postop benefit
WASHINGTON – Bowel preparation for vaginal reconstructive surgery does not affect postoperative bowel function and bowel symptoms one way or the other, according to a secondary analysis of a single-blind randomized trial examining various effects of mechanical bowel preparation.
In a primary analysis published earlier this year, investigators found no benefit to mechanical bowel preparation with regard to the operative field quality. They also found that patients who were randomized not to receive bowel preparation were more satisfied and had fewer abdominal symptoms than those who received the intervention – a clear-liquid diet and enemas (Obstet. Gynecol. 2014;123:232-8).
The new analysis, reported at the scientific meetings of the American Urogynecologic Society and the International Urogynecological Association, looked more closely at postoperative bowel habits after vaginal reconstructive surgery.
"As surgical intervention for pelvic organ prolapse increases, so does our need for knowledge of [best] perioperative management practices," said Dr. Alicia C. Ballard of the division of urogynecology and pelvic reconstructive surgery at the University of Alabama at Birmingham, where the study was conducted.
"Concerns about painful defecation and GI symptoms such as nausea and vomiting are significant concerns for women undergoing surgery," she said.
Women may be predisposed to postsurgical constipation as a result of preoperative bowel preparation (including diet), the lasting effects of anesthesia, the use of narcotics, and the surgery itself. Prior research has shown, moreover, that constipation and incomplete bowel evacuation are not uncommon preoperatively in women with pelvic organ prolapse, she noted.
The study randomized 150 women scheduled to undergo vaginal prolapse surgery with, at a minimum, a planned apical suspension and posterior compartment repair. Surgeries included other prolapse and incontinence procedures.
Women randomized to the bowel preparation group were instructed to have a clear-liquid diet and to self-administer two saline enemas in the late afternoon of the day before surgery.
Those who were randomized to receive no intervention were allowed to have a regular diet. Women in both groups were instructed to eat nothing after midnight on the day of surgery.
All study participants were instructed to complete a bowel diary for 7 days preoperatively and 14 days postoperatively. Those who completed the preoperative diary and 10 of 14 days of the postoperative diary were included in the analysis.
Of the 150 women randomized at the preoperative visit in a 1:1 fashion, 5 withdrew from the study or had surgery canceled, and 121 completed the bowel diary.
The mean time to first bowel movement after surgery was similar in the two groups: 3.3 days in the bowel prep group and 3.2 days in the control group. The groups were also similar with regard to pain, fecal urgency, and stool transit times on the day of the first postoperative bowel movement. The use of antiemetics postoperatively was similar as well (48% and 55% in the bowel prep and no-prep groups, respectively.)
Most of the 121 women – across both groups – used at least one laxative postoperatively, mainly osmotic laxatives. Women who had not received bowel preparation were more likely, however, to report daily fiber use.
"Bowel preparation ... doesn’t appear to affect the return of bowel function and other bowel symptoms postoperatively," Dr. Ballard said. Moreover, "the lack of bowel preparation doesn’t seem to impact painful defecation symptoms, and most women use some type of laxative."
The study’s exclusion criteria included colorectal cancer, inflammatory bowel disease, a history of bowel resection, neurological disorders, and symptoms of colonic inertia (fewer than three bowel movements per week). The decision to exclude patients with significant preoperative constipation was made in order to "decrease the risk of bowel dysfunction and was important for our primary outcomes," Dr. Ballard explained. "But there actually were not that many women excluded."
Dr. Ballard reported that she had no disclosures. Two of her five coinvestigators reported various relationships with St. Jude Medical and Pelvalon.
WASHINGTON – Bowel preparation for vaginal reconstructive surgery does not affect postoperative bowel function and bowel symptoms one way or the other, according to a secondary analysis of a single-blind randomized trial examining various effects of mechanical bowel preparation.
In a primary analysis published earlier this year, investigators found no benefit to mechanical bowel preparation with regard to the operative field quality. They also found that patients who were randomized not to receive bowel preparation were more satisfied and had fewer abdominal symptoms than those who received the intervention – a clear-liquid diet and enemas (Obstet. Gynecol. 2014;123:232-8).
The new analysis, reported at the scientific meetings of the American Urogynecologic Society and the International Urogynecological Association, looked more closely at postoperative bowel habits after vaginal reconstructive surgery.
"As surgical intervention for pelvic organ prolapse increases, so does our need for knowledge of [best] perioperative management practices," said Dr. Alicia C. Ballard of the division of urogynecology and pelvic reconstructive surgery at the University of Alabama at Birmingham, where the study was conducted.
"Concerns about painful defecation and GI symptoms such as nausea and vomiting are significant concerns for women undergoing surgery," she said.
Women may be predisposed to postsurgical constipation as a result of preoperative bowel preparation (including diet), the lasting effects of anesthesia, the use of narcotics, and the surgery itself. Prior research has shown, moreover, that constipation and incomplete bowel evacuation are not uncommon preoperatively in women with pelvic organ prolapse, she noted.
The study randomized 150 women scheduled to undergo vaginal prolapse surgery with, at a minimum, a planned apical suspension and posterior compartment repair. Surgeries included other prolapse and incontinence procedures.
Women randomized to the bowel preparation group were instructed to have a clear-liquid diet and to self-administer two saline enemas in the late afternoon of the day before surgery.
Those who were randomized to receive no intervention were allowed to have a regular diet. Women in both groups were instructed to eat nothing after midnight on the day of surgery.
All study participants were instructed to complete a bowel diary for 7 days preoperatively and 14 days postoperatively. Those who completed the preoperative diary and 10 of 14 days of the postoperative diary were included in the analysis.
Of the 150 women randomized at the preoperative visit in a 1:1 fashion, 5 withdrew from the study or had surgery canceled, and 121 completed the bowel diary.
The mean time to first bowel movement after surgery was similar in the two groups: 3.3 days in the bowel prep group and 3.2 days in the control group. The groups were also similar with regard to pain, fecal urgency, and stool transit times on the day of the first postoperative bowel movement. The use of antiemetics postoperatively was similar as well (48% and 55% in the bowel prep and no-prep groups, respectively.)
Most of the 121 women – across both groups – used at least one laxative postoperatively, mainly osmotic laxatives. Women who had not received bowel preparation were more likely, however, to report daily fiber use.
"Bowel preparation ... doesn’t appear to affect the return of bowel function and other bowel symptoms postoperatively," Dr. Ballard said. Moreover, "the lack of bowel preparation doesn’t seem to impact painful defecation symptoms, and most women use some type of laxative."
The study’s exclusion criteria included colorectal cancer, inflammatory bowel disease, a history of bowel resection, neurological disorders, and symptoms of colonic inertia (fewer than three bowel movements per week). The decision to exclude patients with significant preoperative constipation was made in order to "decrease the risk of bowel dysfunction and was important for our primary outcomes," Dr. Ballard explained. "But there actually were not that many women excluded."
Dr. Ballard reported that she had no disclosures. Two of her five coinvestigators reported various relationships with St. Jude Medical and Pelvalon.
WASHINGTON – Bowel preparation for vaginal reconstructive surgery does not affect postoperative bowel function and bowel symptoms one way or the other, according to a secondary analysis of a single-blind randomized trial examining various effects of mechanical bowel preparation.
In a primary analysis published earlier this year, investigators found no benefit to mechanical bowel preparation with regard to the operative field quality. They also found that patients who were randomized not to receive bowel preparation were more satisfied and had fewer abdominal symptoms than those who received the intervention – a clear-liquid diet and enemas (Obstet. Gynecol. 2014;123:232-8).
The new analysis, reported at the scientific meetings of the American Urogynecologic Society and the International Urogynecological Association, looked more closely at postoperative bowel habits after vaginal reconstructive surgery.
"As surgical intervention for pelvic organ prolapse increases, so does our need for knowledge of [best] perioperative management practices," said Dr. Alicia C. Ballard of the division of urogynecology and pelvic reconstructive surgery at the University of Alabama at Birmingham, where the study was conducted.
"Concerns about painful defecation and GI symptoms such as nausea and vomiting are significant concerns for women undergoing surgery," she said.
Women may be predisposed to postsurgical constipation as a result of preoperative bowel preparation (including diet), the lasting effects of anesthesia, the use of narcotics, and the surgery itself. Prior research has shown, moreover, that constipation and incomplete bowel evacuation are not uncommon preoperatively in women with pelvic organ prolapse, she noted.
The study randomized 150 women scheduled to undergo vaginal prolapse surgery with, at a minimum, a planned apical suspension and posterior compartment repair. Surgeries included other prolapse and incontinence procedures.
Women randomized to the bowel preparation group were instructed to have a clear-liquid diet and to self-administer two saline enemas in the late afternoon of the day before surgery.
Those who were randomized to receive no intervention were allowed to have a regular diet. Women in both groups were instructed to eat nothing after midnight on the day of surgery.
All study participants were instructed to complete a bowel diary for 7 days preoperatively and 14 days postoperatively. Those who completed the preoperative diary and 10 of 14 days of the postoperative diary were included in the analysis.
Of the 150 women randomized at the preoperative visit in a 1:1 fashion, 5 withdrew from the study or had surgery canceled, and 121 completed the bowel diary.
The mean time to first bowel movement after surgery was similar in the two groups: 3.3 days in the bowel prep group and 3.2 days in the control group. The groups were also similar with regard to pain, fecal urgency, and stool transit times on the day of the first postoperative bowel movement. The use of antiemetics postoperatively was similar as well (48% and 55% in the bowel prep and no-prep groups, respectively.)
Most of the 121 women – across both groups – used at least one laxative postoperatively, mainly osmotic laxatives. Women who had not received bowel preparation were more likely, however, to report daily fiber use.
"Bowel preparation ... doesn’t appear to affect the return of bowel function and other bowel symptoms postoperatively," Dr. Ballard said. Moreover, "the lack of bowel preparation doesn’t seem to impact painful defecation symptoms, and most women use some type of laxative."
The study’s exclusion criteria included colorectal cancer, inflammatory bowel disease, a history of bowel resection, neurological disorders, and symptoms of colonic inertia (fewer than three bowel movements per week). The decision to exclude patients with significant preoperative constipation was made in order to "decrease the risk of bowel dysfunction and was important for our primary outcomes," Dr. Ballard explained. "But there actually were not that many women excluded."
Dr. Ballard reported that she had no disclosures. Two of her five coinvestigators reported various relationships with St. Jude Medical and Pelvalon.
AT AUGS/IUGA 2014
Key clinical point: Bowel preparation prior to surgery for vaginal prolapse does not improve postoperative bowel function.
Major finding: The mean time to first bowel movement after surgery was similar in the two groups: 3.3 days in the bowel preparation group and 3.2 days in the control group. The groups also were similar with regard to pain, fecal urgency, and stool transit times on the day of the first postoperative bowel movement.
Data source: A secondary analysis of a single-blind randomized trial of 150 women undergoing vaginal prolapse surgery.
Disclosures: Dr. Ballard reported that she had no disclosures. Two of her five coinvestigators reported various relationships with St. Jude Medical and Pelvalon.
It’s official: Oct. 1, 2015, is the ICD-10 compliance date
Go ahead and mark your calendar: Medicare officials have issued a final regulation setting Oct. 1, 2015, as the official compliance date for switching to the ICD-10 coding system.
Earlier this year, Congress delayed the transition from the ICD-9 to ICD-10 coding systems for at least a year but did not specify the exact compliance date, leaving that to the discretion of officials at the Center for Medicare & Medicaid Services.
CMS first announced the Oct. 1, 2015 compliance date – the earliest start allowed under the law – in May. But the new date wasn’t official until the agency released an interim final rule, which it did July 31.
On Twitter @maryellenny
Go ahead and mark your calendar: Medicare officials have issued a final regulation setting Oct. 1, 2015, as the official compliance date for switching to the ICD-10 coding system.
Earlier this year, Congress delayed the transition from the ICD-9 to ICD-10 coding systems for at least a year but did not specify the exact compliance date, leaving that to the discretion of officials at the Center for Medicare & Medicaid Services.
CMS first announced the Oct. 1, 2015 compliance date – the earliest start allowed under the law – in May. But the new date wasn’t official until the agency released an interim final rule, which it did July 31.
On Twitter @maryellenny
Go ahead and mark your calendar: Medicare officials have issued a final regulation setting Oct. 1, 2015, as the official compliance date for switching to the ICD-10 coding system.
Earlier this year, Congress delayed the transition from the ICD-9 to ICD-10 coding systems for at least a year but did not specify the exact compliance date, leaving that to the discretion of officials at the Center for Medicare & Medicaid Services.
CMS first announced the Oct. 1, 2015 compliance date – the earliest start allowed under the law – in May. But the new date wasn’t official until the agency released an interim final rule, which it did July 31.
On Twitter @maryellenny
House panel looks into rocky rollout of healthcare.gov
WASHINGTON – Despite a scathing report on past and ongoing failures with healthcare.gov, the Affordable Care Act’s website will be ready to go when enrollment begins again in November, Andy Slavitt, principal deputy administrator of the Centers for Medicare & Medicaid Services testified to a House subcommittee July 31.
Mr. Slavitt, who took his post 3 weeks ago, was called to Capitol Hill to respond to a Government Accountability Office report that is critical of both the rollout of healthcare.gov and the ongoing management of the portal. The GAO is a nonpartisan agency that serves as the investigative arm of Congress.
Mr. Slavitt acknowledged problems listed by the GAO. "Fortunately, or unfortunately, the GAO report wasn’t news to the people at CMS," he testified before the House Energy & Commerce Committee Subcommittee on Oversight and Investigations.
But many have been addressed, he said, adding that there is more accountability, better communication with contractors, and clearly defined performance goals.
"This coming year will be one of continuous and visible improvement, but not perfection," Mr. Slavitt testified.
The GAO report chronicled mismanagement, limited oversight, and a chaotic planning process within CMS during the lead-up to the launch of healthcare.gov in October 2013. Officials knew that the site was only 65% ready that spring, but put off a review of performance until September, GAO official William T. Woods testified at the hearing.
Constantly changing requirements led to huge cost increases – which appear to have continued. As of March 2014, the federal government had spent $840 million on the website. The CMS did not properly review or approve work by the contractors and "took only limited steps to hold the contractor accountable," Mr. Woods said in written testimony.
The GAO report notes that the cost of working with Accenture Federal Services – the contractor hired to replace the site’s original builder – had ballooned from $91 million to more than $175 million in the first half of 2014, because of new requirements and other changes ordered by CMS.
Despite rising costs, key parts of the website are still not functional, including the financial management module, which handles financial interactions with insurers. That contract continues, "so costs on that particular contract are almost certainly higher today than they were when we completed our audit work," Mr. Woods said.
Rep. Tim Murphy (R-Pa.), chairman of the oversight subcommittee said, "We still don’t know if the Administration has a system in place capable of handling inconsistencies, inaccurate subsidies, or whether CMS will ever put in place a functioning payments system."
Rep. Murphy and his Republican colleagues also questioned whether top CMS officials, such as Administrator Marilyn Tavenner, were aware that the site was so far behind in its performance goals and deadlines. At one point, Rep. Murphy suggested that, if they had known, they might have perjured themselves in appearances before the Energy & Commerce Committee in 2013.
Democrats on the panel said that it was important to acknowledge the failures of the site’s launch, but only to inform future solutions. "We will stipulate the rollout of the ACA was an unmitigated disaster," said Rep. Diana DeGette (D-Colo.). But she and her colleagues said it was time to move forward.
"My point is not to excuse the healthcare.gov problems but to put them in context," said Rep. Henry Waxman (D-Calif.). He said the problems were fixed, though not quick enough for his liking. But, Rep. Waxman added, "I want to learn what went wrong so CMS can do a better job for the next time."
On Twitter @aliciaault
WASHINGTON – Despite a scathing report on past and ongoing failures with healthcare.gov, the Affordable Care Act’s website will be ready to go when enrollment begins again in November, Andy Slavitt, principal deputy administrator of the Centers for Medicare & Medicaid Services testified to a House subcommittee July 31.
Mr. Slavitt, who took his post 3 weeks ago, was called to Capitol Hill to respond to a Government Accountability Office report that is critical of both the rollout of healthcare.gov and the ongoing management of the portal. The GAO is a nonpartisan agency that serves as the investigative arm of Congress.
Mr. Slavitt acknowledged problems listed by the GAO. "Fortunately, or unfortunately, the GAO report wasn’t news to the people at CMS," he testified before the House Energy & Commerce Committee Subcommittee on Oversight and Investigations.
But many have been addressed, he said, adding that there is more accountability, better communication with contractors, and clearly defined performance goals.
"This coming year will be one of continuous and visible improvement, but not perfection," Mr. Slavitt testified.
The GAO report chronicled mismanagement, limited oversight, and a chaotic planning process within CMS during the lead-up to the launch of healthcare.gov in October 2013. Officials knew that the site was only 65% ready that spring, but put off a review of performance until September, GAO official William T. Woods testified at the hearing.
Constantly changing requirements led to huge cost increases – which appear to have continued. As of March 2014, the federal government had spent $840 million on the website. The CMS did not properly review or approve work by the contractors and "took only limited steps to hold the contractor accountable," Mr. Woods said in written testimony.
The GAO report notes that the cost of working with Accenture Federal Services – the contractor hired to replace the site’s original builder – had ballooned from $91 million to more than $175 million in the first half of 2014, because of new requirements and other changes ordered by CMS.
Despite rising costs, key parts of the website are still not functional, including the financial management module, which handles financial interactions with insurers. That contract continues, "so costs on that particular contract are almost certainly higher today than they were when we completed our audit work," Mr. Woods said.
Rep. Tim Murphy (R-Pa.), chairman of the oversight subcommittee said, "We still don’t know if the Administration has a system in place capable of handling inconsistencies, inaccurate subsidies, or whether CMS will ever put in place a functioning payments system."
Rep. Murphy and his Republican colleagues also questioned whether top CMS officials, such as Administrator Marilyn Tavenner, were aware that the site was so far behind in its performance goals and deadlines. At one point, Rep. Murphy suggested that, if they had known, they might have perjured themselves in appearances before the Energy & Commerce Committee in 2013.
Democrats on the panel said that it was important to acknowledge the failures of the site’s launch, but only to inform future solutions. "We will stipulate the rollout of the ACA was an unmitigated disaster," said Rep. Diana DeGette (D-Colo.). But she and her colleagues said it was time to move forward.
"My point is not to excuse the healthcare.gov problems but to put them in context," said Rep. Henry Waxman (D-Calif.). He said the problems were fixed, though not quick enough for his liking. But, Rep. Waxman added, "I want to learn what went wrong so CMS can do a better job for the next time."
On Twitter @aliciaault
WASHINGTON – Despite a scathing report on past and ongoing failures with healthcare.gov, the Affordable Care Act’s website will be ready to go when enrollment begins again in November, Andy Slavitt, principal deputy administrator of the Centers for Medicare & Medicaid Services testified to a House subcommittee July 31.
Mr. Slavitt, who took his post 3 weeks ago, was called to Capitol Hill to respond to a Government Accountability Office report that is critical of both the rollout of healthcare.gov and the ongoing management of the portal. The GAO is a nonpartisan agency that serves as the investigative arm of Congress.
Mr. Slavitt acknowledged problems listed by the GAO. "Fortunately, or unfortunately, the GAO report wasn’t news to the people at CMS," he testified before the House Energy & Commerce Committee Subcommittee on Oversight and Investigations.
But many have been addressed, he said, adding that there is more accountability, better communication with contractors, and clearly defined performance goals.
"This coming year will be one of continuous and visible improvement, but not perfection," Mr. Slavitt testified.
The GAO report chronicled mismanagement, limited oversight, and a chaotic planning process within CMS during the lead-up to the launch of healthcare.gov in October 2013. Officials knew that the site was only 65% ready that spring, but put off a review of performance until September, GAO official William T. Woods testified at the hearing.
Constantly changing requirements led to huge cost increases – which appear to have continued. As of March 2014, the federal government had spent $840 million on the website. The CMS did not properly review or approve work by the contractors and "took only limited steps to hold the contractor accountable," Mr. Woods said in written testimony.
The GAO report notes that the cost of working with Accenture Federal Services – the contractor hired to replace the site’s original builder – had ballooned from $91 million to more than $175 million in the first half of 2014, because of new requirements and other changes ordered by CMS.
Despite rising costs, key parts of the website are still not functional, including the financial management module, which handles financial interactions with insurers. That contract continues, "so costs on that particular contract are almost certainly higher today than they were when we completed our audit work," Mr. Woods said.
Rep. Tim Murphy (R-Pa.), chairman of the oversight subcommittee said, "We still don’t know if the Administration has a system in place capable of handling inconsistencies, inaccurate subsidies, or whether CMS will ever put in place a functioning payments system."
Rep. Murphy and his Republican colleagues also questioned whether top CMS officials, such as Administrator Marilyn Tavenner, were aware that the site was so far behind in its performance goals and deadlines. At one point, Rep. Murphy suggested that, if they had known, they might have perjured themselves in appearances before the Energy & Commerce Committee in 2013.
Democrats on the panel said that it was important to acknowledge the failures of the site’s launch, but only to inform future solutions. "We will stipulate the rollout of the ACA was an unmitigated disaster," said Rep. Diana DeGette (D-Colo.). But she and her colleagues said it was time to move forward.
"My point is not to excuse the healthcare.gov problems but to put them in context," said Rep. Henry Waxman (D-Calif.). He said the problems were fixed, though not quick enough for his liking. But, Rep. Waxman added, "I want to learn what went wrong so CMS can do a better job for the next time."
On Twitter @aliciaault
FROM A HOUSE ENERGY & COMMERCE COMMITTEE HEARING
Early ID of placenta accreta key to optimal management
SAN DIEGO – An early and accurate diagnosis of placenta accreta is crucial because maternal mortality can be as high as 7% and perinatal mortality can be as high as 10%.
"Historically, the clinical presentation of placenta accreta was a prolonged third-stage or retained placenta after delivery of the baby and subsequent onset of hemorrhage or the onset of hemorrhage at the time of the termination of pregnancy," Dr. Gladys Ramos said at the University of California, San Diego, Critical Care Summer Session. "Now, with predelivery diagnosis, we have changed the management and the clinical presentation."
Placenta accreta is defined as the abnormal presence of villi attached to the myometrium due to a defect in the decidua basalis. In the 1980s, placenta accreta was believed to be rare. However, a study from 2005 showed an increase in the rate of placenta accreta (Am. J. Obstet. Gynecol. 2005;192:1458-61). The condition now occurs in 1 in every 533 pregnancies.
"Mirroring this rise is a rise in cesarean deliveries," said Dr. Ramos, a perinatologist at the UCSD Medical Center. "We think these two conditions are related. At UCSD, we take care of about 3,200 deliveries per year, and from 1990 to 2008 we have seen a linear increase in the rate of placenta accreta. We take care of about two patients per month with this condition."
Risk factors for the placenta accreta are prior uterine surgery, placenta previa, advanced maternal age, parity, and smoking. The published rate of detection by ultrasound ranges from 80% to 100%, "with a low false-positive rate," Dr. Ramos said. Telltale signs on ultrasound include loss of myometrial interface, a heterogeneous "Swiss cheese–looking" appearance to the placenta, an increase in the vascularity of the placenta, and evidence of bladder invasion.
MRI can be used as an adjunct to ultrasound diagnosis. The published detection rate with MRI ranges from 80% to 88%, "with a very low false-positive rate, which is why we use it as an adjunct to an ultrasound," she said. "We see similar findings on MRI that we do on ultrasound: thickened, dark nodular contour to the placenta; extension of the dark bands within the placenta; and mass effect causing a bulge on T1."
Nurses and sonographers at UCSD have been trained to ask patients upon presentation about risk factors for the condition." Then we look for signs of placenta accreta on ultrasound, including endovaginal ultrasound," Dr. Ramos said. "If we are concerned, we plan ahead with a multidisciplinary approach. If we’re still not sure, we proceed with MRI for diagnosis."
In 1995, clinicians at UCSD developed a multidisciplinary approach to treating patients with placenta accreta, mindful that "it took a cast of thousands to be able to make sure our outcomes were optimal for both mom and baby," said Pat Inzano, R.N., an administrative nurse in labor and delivery at UCSD. "Over the years, we’ve recognized the risk factors and the importance of early and accurate diagnosis."
The approach includes detailed maternal counseling and meticulous planning with colleagues at every conceivable step along the way in the care of the mom and baby, from neonatology and gynecologic surgery to surgical ICU staff and social workers. "When a mother gets the news [of placenta accreta], not only does she probably not understand the pathophysiology of what’s going on, but it takes a long time for her to digest this information," Ms. Inzano explained. "One of the things that’s so important at every level is getting her and her family to understand the diagnosis and reminding her that she’s [receiving] the best possible care."
The multidisciplinary team stages a conference to discuss the patient’s hospital delivery and care; availability of blood products; the need for anesthesia, surgical, and radiological expertise in house; and intensive care capability. Proper consents are also required "because the surgery will involve removing the patient’s uterus and rendering her sterile," she said. "We want to get the patient and the family as comfortable as possible with what’s going to happen. We found that providing tours of every single area that she will be ‘parking in’ is stress-relieving for her and her family, including labor and delivery and the neonatal ICU. We also provide consultations with all of the specialties involved in the case. All of this is education and counseling on a lot of different levels."
The team develops a time-line and schedules a planned delivery, including admission to the hospital, cesarean section/hysterectomy in the main operating room (OR), and unit transfers for epidural, central lines, and femoral balloons. In addition, the team coordinates a hospital tour for the patient and family. Most recommended deliveries are at week 34 because "we don’t want her to get near term and go into labor, which would aggravate a bleed of the placenta accreta," Ms. Inzano said.
The team also crafts a "plan B" for emergent delivery, including a detailed list of whom to contact and their pager numbers. "If we need to emergency deliver this patient in the main OR at 3 in the morning, we have our attending physician call the attending trauma physician to put us on trauma bypass in case we need the blood products," she said. "If we don’t have the type and cross-matched blood available, we activate an [obstetric] hemorrhage protocol in order to obtain O-negative blood in massive quantities until she’s cross-matched."
Ms. Inzano said that the multidisciplinary approach to placenta accreta "has become a smooth operation at our institution, but we never drop our awareness of the severity of what can happen. With the multidisciplinary effort, it brings everyone together; everyone’s on the same page, and everyone knows what to anticipate."
Neither Dr. Ramos nor Ms. Inzano had relevant financial conflicts.
On Twitter @dougbrunk
SAN DIEGO – An early and accurate diagnosis of placenta accreta is crucial because maternal mortality can be as high as 7% and perinatal mortality can be as high as 10%.
"Historically, the clinical presentation of placenta accreta was a prolonged third-stage or retained placenta after delivery of the baby and subsequent onset of hemorrhage or the onset of hemorrhage at the time of the termination of pregnancy," Dr. Gladys Ramos said at the University of California, San Diego, Critical Care Summer Session. "Now, with predelivery diagnosis, we have changed the management and the clinical presentation."
Placenta accreta is defined as the abnormal presence of villi attached to the myometrium due to a defect in the decidua basalis. In the 1980s, placenta accreta was believed to be rare. However, a study from 2005 showed an increase in the rate of placenta accreta (Am. J. Obstet. Gynecol. 2005;192:1458-61). The condition now occurs in 1 in every 533 pregnancies.
"Mirroring this rise is a rise in cesarean deliveries," said Dr. Ramos, a perinatologist at the UCSD Medical Center. "We think these two conditions are related. At UCSD, we take care of about 3,200 deliveries per year, and from 1990 to 2008 we have seen a linear increase in the rate of placenta accreta. We take care of about two patients per month with this condition."
Risk factors for the placenta accreta are prior uterine surgery, placenta previa, advanced maternal age, parity, and smoking. The published rate of detection by ultrasound ranges from 80% to 100%, "with a low false-positive rate," Dr. Ramos said. Telltale signs on ultrasound include loss of myometrial interface, a heterogeneous "Swiss cheese–looking" appearance to the placenta, an increase in the vascularity of the placenta, and evidence of bladder invasion.
MRI can be used as an adjunct to ultrasound diagnosis. The published detection rate with MRI ranges from 80% to 88%, "with a very low false-positive rate, which is why we use it as an adjunct to an ultrasound," she said. "We see similar findings on MRI that we do on ultrasound: thickened, dark nodular contour to the placenta; extension of the dark bands within the placenta; and mass effect causing a bulge on T1."
Nurses and sonographers at UCSD have been trained to ask patients upon presentation about risk factors for the condition." Then we look for signs of placenta accreta on ultrasound, including endovaginal ultrasound," Dr. Ramos said. "If we are concerned, we plan ahead with a multidisciplinary approach. If we’re still not sure, we proceed with MRI for diagnosis."
In 1995, clinicians at UCSD developed a multidisciplinary approach to treating patients with placenta accreta, mindful that "it took a cast of thousands to be able to make sure our outcomes were optimal for both mom and baby," said Pat Inzano, R.N., an administrative nurse in labor and delivery at UCSD. "Over the years, we’ve recognized the risk factors and the importance of early and accurate diagnosis."
The approach includes detailed maternal counseling and meticulous planning with colleagues at every conceivable step along the way in the care of the mom and baby, from neonatology and gynecologic surgery to surgical ICU staff and social workers. "When a mother gets the news [of placenta accreta], not only does she probably not understand the pathophysiology of what’s going on, but it takes a long time for her to digest this information," Ms. Inzano explained. "One of the things that’s so important at every level is getting her and her family to understand the diagnosis and reminding her that she’s [receiving] the best possible care."
The multidisciplinary team stages a conference to discuss the patient’s hospital delivery and care; availability of blood products; the need for anesthesia, surgical, and radiological expertise in house; and intensive care capability. Proper consents are also required "because the surgery will involve removing the patient’s uterus and rendering her sterile," she said. "We want to get the patient and the family as comfortable as possible with what’s going to happen. We found that providing tours of every single area that she will be ‘parking in’ is stress-relieving for her and her family, including labor and delivery and the neonatal ICU. We also provide consultations with all of the specialties involved in the case. All of this is education and counseling on a lot of different levels."
The team develops a time-line and schedules a planned delivery, including admission to the hospital, cesarean section/hysterectomy in the main operating room (OR), and unit transfers for epidural, central lines, and femoral balloons. In addition, the team coordinates a hospital tour for the patient and family. Most recommended deliveries are at week 34 because "we don’t want her to get near term and go into labor, which would aggravate a bleed of the placenta accreta," Ms. Inzano said.
The team also crafts a "plan B" for emergent delivery, including a detailed list of whom to contact and their pager numbers. "If we need to emergency deliver this patient in the main OR at 3 in the morning, we have our attending physician call the attending trauma physician to put us on trauma bypass in case we need the blood products," she said. "If we don’t have the type and cross-matched blood available, we activate an [obstetric] hemorrhage protocol in order to obtain O-negative blood in massive quantities until she’s cross-matched."
Ms. Inzano said that the multidisciplinary approach to placenta accreta "has become a smooth operation at our institution, but we never drop our awareness of the severity of what can happen. With the multidisciplinary effort, it brings everyone together; everyone’s on the same page, and everyone knows what to anticipate."
Neither Dr. Ramos nor Ms. Inzano had relevant financial conflicts.
On Twitter @dougbrunk
SAN DIEGO – An early and accurate diagnosis of placenta accreta is crucial because maternal mortality can be as high as 7% and perinatal mortality can be as high as 10%.
"Historically, the clinical presentation of placenta accreta was a prolonged third-stage or retained placenta after delivery of the baby and subsequent onset of hemorrhage or the onset of hemorrhage at the time of the termination of pregnancy," Dr. Gladys Ramos said at the University of California, San Diego, Critical Care Summer Session. "Now, with predelivery diagnosis, we have changed the management and the clinical presentation."
Placenta accreta is defined as the abnormal presence of villi attached to the myometrium due to a defect in the decidua basalis. In the 1980s, placenta accreta was believed to be rare. However, a study from 2005 showed an increase in the rate of placenta accreta (Am. J. Obstet. Gynecol. 2005;192:1458-61). The condition now occurs in 1 in every 533 pregnancies.
"Mirroring this rise is a rise in cesarean deliveries," said Dr. Ramos, a perinatologist at the UCSD Medical Center. "We think these two conditions are related. At UCSD, we take care of about 3,200 deliveries per year, and from 1990 to 2008 we have seen a linear increase in the rate of placenta accreta. We take care of about two patients per month with this condition."
Risk factors for the placenta accreta are prior uterine surgery, placenta previa, advanced maternal age, parity, and smoking. The published rate of detection by ultrasound ranges from 80% to 100%, "with a low false-positive rate," Dr. Ramos said. Telltale signs on ultrasound include loss of myometrial interface, a heterogeneous "Swiss cheese–looking" appearance to the placenta, an increase in the vascularity of the placenta, and evidence of bladder invasion.
MRI can be used as an adjunct to ultrasound diagnosis. The published detection rate with MRI ranges from 80% to 88%, "with a very low false-positive rate, which is why we use it as an adjunct to an ultrasound," she said. "We see similar findings on MRI that we do on ultrasound: thickened, dark nodular contour to the placenta; extension of the dark bands within the placenta; and mass effect causing a bulge on T1."
Nurses and sonographers at UCSD have been trained to ask patients upon presentation about risk factors for the condition." Then we look for signs of placenta accreta on ultrasound, including endovaginal ultrasound," Dr. Ramos said. "If we are concerned, we plan ahead with a multidisciplinary approach. If we’re still not sure, we proceed with MRI for diagnosis."
In 1995, clinicians at UCSD developed a multidisciplinary approach to treating patients with placenta accreta, mindful that "it took a cast of thousands to be able to make sure our outcomes were optimal for both mom and baby," said Pat Inzano, R.N., an administrative nurse in labor and delivery at UCSD. "Over the years, we’ve recognized the risk factors and the importance of early and accurate diagnosis."
The approach includes detailed maternal counseling and meticulous planning with colleagues at every conceivable step along the way in the care of the mom and baby, from neonatology and gynecologic surgery to surgical ICU staff and social workers. "When a mother gets the news [of placenta accreta], not only does she probably not understand the pathophysiology of what’s going on, but it takes a long time for her to digest this information," Ms. Inzano explained. "One of the things that’s so important at every level is getting her and her family to understand the diagnosis and reminding her that she’s [receiving] the best possible care."
The multidisciplinary team stages a conference to discuss the patient’s hospital delivery and care; availability of blood products; the need for anesthesia, surgical, and radiological expertise in house; and intensive care capability. Proper consents are also required "because the surgery will involve removing the patient’s uterus and rendering her sterile," she said. "We want to get the patient and the family as comfortable as possible with what’s going to happen. We found that providing tours of every single area that she will be ‘parking in’ is stress-relieving for her and her family, including labor and delivery and the neonatal ICU. We also provide consultations with all of the specialties involved in the case. All of this is education and counseling on a lot of different levels."
The team develops a time-line and schedules a planned delivery, including admission to the hospital, cesarean section/hysterectomy in the main operating room (OR), and unit transfers for epidural, central lines, and femoral balloons. In addition, the team coordinates a hospital tour for the patient and family. Most recommended deliveries are at week 34 because "we don’t want her to get near term and go into labor, which would aggravate a bleed of the placenta accreta," Ms. Inzano said.
The team also crafts a "plan B" for emergent delivery, including a detailed list of whom to contact and their pager numbers. "If we need to emergency deliver this patient in the main OR at 3 in the morning, we have our attending physician call the attending trauma physician to put us on trauma bypass in case we need the blood products," she said. "If we don’t have the type and cross-matched blood available, we activate an [obstetric] hemorrhage protocol in order to obtain O-negative blood in massive quantities until she’s cross-matched."
Ms. Inzano said that the multidisciplinary approach to placenta accreta "has become a smooth operation at our institution, but we never drop our awareness of the severity of what can happen. With the multidisciplinary effort, it brings everyone together; everyone’s on the same page, and everyone knows what to anticipate."
Neither Dr. Ramos nor Ms. Inzano had relevant financial conflicts.
On Twitter @dougbrunk
EXPERT ANALYSIS AT THE UCSD CRITICAL CARE SUMMER SESSION