User login
Official Newspaper of the American College of Surgeons
TAVR can be performed safely within 30 days of PCI
WASHINGTON – The risk of adverse events from a transcatheter aortic valve replacement (TAVR) does not appear to be significantly increased in those who have undergone a recent percutaneous intervention (PCI), according to a matched retrospective analysis.
“PCI prior to TAVR in patients with severe aortic stenosis and significant coronary artery disease appears to be feasible and safe,” reported Ashwat S. Dhillon, MD, a cardiology fellow at the University of Southern California, Los Angeles, at CRT 2017 sponsored by the Cardiovascular Research Institute at Washington Hospital Center.
The conclusion that PCI can be performed safely prior to TAVR was drawn from a series of 286 patients treated with TAVR over a nearly 5-year period. Within this group, 29 patients underwent PCI for CAD within 30 days prior to TAVR. They were matched in a 1:1 fashion based on age, sex, history of prior myocardial infarction, and left ventricular ejection fraction to patients undergoing PCI without subsequent TAVR.
The primary endpoint of the analysis was a composite of major in-hospital adverse cardiovascular events (MACE) that included MI and stroke. In addition, the two groups were compared for mortality and readmission rates 30 days after TAVR.
Most of the patients (69%) were male, and the mean age was 77 years. About 20% had a prior MI, roughly 30% had a prior coronary artery bypass graft procedure, and approximately 30% had a prior PCI. There were numerical differences in the rates of hypertension, chronic kidney disease, and diabetes when the two groups were compared, but none were statistically significant.
The procedural details of the PCI were also similar, according to Dr. Dhillon. Although there was a significantly greater proportion of patients treated for lesions in the left circumflex artery in the group that did not undergo TAVR (31.03% vs. 3.45%; P = .02), there were no significant differences in procedures performed in other arteries. There were also no significant differences in the average number of stents and the average total stent length for those who underwent TAVR relative to those who did not.
The rate of in-hospital MI was 14% in both groups. No patient in either group had a stroke. At 30 days, mortality was 3% in each group. Although 30-day readmissions were higher in the group that underwent both PCI and TAVR than those who underwent PCI alone (10% vs. 0%), the difference did not reach significance.
Data evaluating the safety of performing PCI and TAVR procedures in close proximity is needed because “a significant proportion of patients with severe aortic stenosis have coexisting and significant CAD,” Dr. Dhillon explained. Although he suggested that a larger pool of data is needed to confirm the preliminary findings of this study, he suggested that these data are reassuring.
WASHINGTON – The risk of adverse events from a transcatheter aortic valve replacement (TAVR) does not appear to be significantly increased in those who have undergone a recent percutaneous intervention (PCI), according to a matched retrospective analysis.
“PCI prior to TAVR in patients with severe aortic stenosis and significant coronary artery disease appears to be feasible and safe,” reported Ashwat S. Dhillon, MD, a cardiology fellow at the University of Southern California, Los Angeles, at CRT 2017 sponsored by the Cardiovascular Research Institute at Washington Hospital Center.
The conclusion that PCI can be performed safely prior to TAVR was drawn from a series of 286 patients treated with TAVR over a nearly 5-year period. Within this group, 29 patients underwent PCI for CAD within 30 days prior to TAVR. They were matched in a 1:1 fashion based on age, sex, history of prior myocardial infarction, and left ventricular ejection fraction to patients undergoing PCI without subsequent TAVR.
The primary endpoint of the analysis was a composite of major in-hospital adverse cardiovascular events (MACE) that included MI and stroke. In addition, the two groups were compared for mortality and readmission rates 30 days after TAVR.
Most of the patients (69%) were male, and the mean age was 77 years. About 20% had a prior MI, roughly 30% had a prior coronary artery bypass graft procedure, and approximately 30% had a prior PCI. There were numerical differences in the rates of hypertension, chronic kidney disease, and diabetes when the two groups were compared, but none were statistically significant.
The procedural details of the PCI were also similar, according to Dr. Dhillon. Although there was a significantly greater proportion of patients treated for lesions in the left circumflex artery in the group that did not undergo TAVR (31.03% vs. 3.45%; P = .02), there were no significant differences in procedures performed in other arteries. There were also no significant differences in the average number of stents and the average total stent length for those who underwent TAVR relative to those who did not.
The rate of in-hospital MI was 14% in both groups. No patient in either group had a stroke. At 30 days, mortality was 3% in each group. Although 30-day readmissions were higher in the group that underwent both PCI and TAVR than those who underwent PCI alone (10% vs. 0%), the difference did not reach significance.
Data evaluating the safety of performing PCI and TAVR procedures in close proximity is needed because “a significant proportion of patients with severe aortic stenosis have coexisting and significant CAD,” Dr. Dhillon explained. Although he suggested that a larger pool of data is needed to confirm the preliminary findings of this study, he suggested that these data are reassuring.
WASHINGTON – The risk of adverse events from a transcatheter aortic valve replacement (TAVR) does not appear to be significantly increased in those who have undergone a recent percutaneous intervention (PCI), according to a matched retrospective analysis.
“PCI prior to TAVR in patients with severe aortic stenosis and significant coronary artery disease appears to be feasible and safe,” reported Ashwat S. Dhillon, MD, a cardiology fellow at the University of Southern California, Los Angeles, at CRT 2017 sponsored by the Cardiovascular Research Institute at Washington Hospital Center.
The conclusion that PCI can be performed safely prior to TAVR was drawn from a series of 286 patients treated with TAVR over a nearly 5-year period. Within this group, 29 patients underwent PCI for CAD within 30 days prior to TAVR. They were matched in a 1:1 fashion based on age, sex, history of prior myocardial infarction, and left ventricular ejection fraction to patients undergoing PCI without subsequent TAVR.
The primary endpoint of the analysis was a composite of major in-hospital adverse cardiovascular events (MACE) that included MI and stroke. In addition, the two groups were compared for mortality and readmission rates 30 days after TAVR.
Most of the patients (69%) were male, and the mean age was 77 years. About 20% had a prior MI, roughly 30% had a prior coronary artery bypass graft procedure, and approximately 30% had a prior PCI. There were numerical differences in the rates of hypertension, chronic kidney disease, and diabetes when the two groups were compared, but none were statistically significant.
The procedural details of the PCI were also similar, according to Dr. Dhillon. Although there was a significantly greater proportion of patients treated for lesions in the left circumflex artery in the group that did not undergo TAVR (31.03% vs. 3.45%; P = .02), there were no significant differences in procedures performed in other arteries. There were also no significant differences in the average number of stents and the average total stent length for those who underwent TAVR relative to those who did not.
The rate of in-hospital MI was 14% in both groups. No patient in either group had a stroke. At 30 days, mortality was 3% in each group. Although 30-day readmissions were higher in the group that underwent both PCI and TAVR than those who underwent PCI alone (10% vs. 0%), the difference did not reach significance.
Data evaluating the safety of performing PCI and TAVR procedures in close proximity is needed because “a significant proportion of patients with severe aortic stenosis have coexisting and significant CAD,” Dr. Dhillon explained. Although he suggested that a larger pool of data is needed to confirm the preliminary findings of this study, he suggested that these data are reassuring.
AT CRT 2017
Key clinical point: Adverse outcomes from transcatheter aortic valve replacement are not increased in patients who had a recent percutaneous intervention.
Major finding: Rates of in-hospital MI (14%), stroke (0%), and 30-day mortality (3%) were exactly the same for those with or without prior PCI.
Data source: A nonrandomized, retrospective, matched analysis.
Disclosures: Dr. Dhillon reported no financial relationships to disclose.
House Republicans start work on ACA repeal/replace
House Republicans have formally begun their efforts to repeal and replace the Affordable Care Act, but a group of four GOP senators could present a significant hurdle to getting the budget reconciliation package to the president’s desk.
The budget reconciliation language – introduced March 6 – represents the first of three phases in their planned effort to quash the ACA.
“Second are all the regulatory modifications and changes that can be put into place,” Dr. Price said, noting that the Obama administration made 192 specific rules related to the ACA and published more than 5,000 letters of guidance. “We are going to go through every single one of those and ... if they help patients, we need to continue them. If they harm patients or increase costs, they need to be addressed.”
He added that additional legislation could be needed to address changes that cannot be made through the reconciliation process, such as addressing prescription drug costs and allowing health insurance to be sold across state lines.
Sections of the reconciliation package were introduced in the House Energy and Commerce and House Ways and Means Committees, covering areas relative to each committee’s jurisdiction. Taken together, they put forth the plan to repeal and replace certain revenue aspects of the ACA.
Provisions to repeal Medicaid expansion likely will prove the most problematic. The Energy and Commerce language calls for the repeal of Medicaid expansion provisions by 2020. It also repeals a requirement that state Medicaid plans offer the same minimum essential health benefits that are required by plans on the exchanges.
Starting in fiscal 2020, states would begin to receive a per capita allotment to fund their Medicaid programs, a fixed amount per Medicaid enrollee that will adjust over time for inflation using the consumer price index. States spending more than their annual allotment per beneficiary would be penalized with reduced funding in the following year.
That provision has run afoul of certain Republican senators whose votes are needed to gain a simple majority and pass the budget reconciliation package. Because of the slim nature of the Republican majority, any defection from the party line could endanger passage.
“We are concerned that any poorly implemented or poorly timed change in the current funding structure in Medicaid could result in a reduction in access to life-saving health care services,” Sen. Rob Portman (R-Ohio), Sen. Shelley Moore Capito (R-W.Va.), Sen. Cory Gardner (R-Colo.), and Sen. Lisa Murkowski (R-Alaska) said in a letter to Senate Majority Leader Mitch McConnell (R-Ky.). The letter was based on an earlier draft of the legislative language but remains valid as little substantive change has occurred in the formal, introduced language.
“We believe Medicaid needs to be reformed, but reform should not come at the cost of disruption in access to health care for our country’s most vulnerable and sickest individuals,” the senators continued. “Any changes made to how Medicaid is financed through the state and federal governments should be coupled with significant new flexibility so they can efficiently and effectively manage their Medicaid programs to best meet their own needs. ... [The House proposal] does not meet the test of stability for individuals currently enrolled in the program, and we will not support a plan that does not include stability for Medicaid expansion populations or flexibility for states.”
The Energy and Commerce language also includes provisions for block grants to states that would allow them to be used in a manner they see fit within a defined list, including providing financial assistance to high-risk individuals; arrangements that help stabilize premiums in the individual market; reduce the cost of providing insurance in the small group and individual markets; promoting participation in the small group and individual markets; and promoting access to preventive, dental, and vision services as well as treatment for mental and substance abuse disorders. Funds could also be used to provide payments for care or assistance to reduce out-of-pocket costs.
The House proposal also would repeal the premium cost sharing subsidies in the ACA, which would be replaced with refundable tax credits, according to the language released by the Ways and Means Committee.
The credit can be used to purchase a state-approved, major health insurance or unsubsidized COBRA (the Consolidated Omnibus Budget Reconciliation Act) coverage and increases based on age. Individuals younger than age 30 years would get $2,000, and the credit increases in $500 increments per 10-year age block, plateauing at $4,000 for those aged 60 years and older. The caps will adjust over time, based on inflation, and are available to those making up to $75,000 ($150,000 for joint filers); the credit phases out by $100 for every $1,000 over those thresholds.
The Ways and Means language also repeals a number of revenue provisions, including the individual mandate and associated penalties; the employer mandate and associated penalties; taxes on high-cost health plans (Cadillac tax); over-the-counter and prescription medications; health savings accounts; tanning; investment; and on health insurers.
In place of the individual mandate, the language will allow insurers to raise premiums by up to 30%.
The bill does not repeal the provisions regarding young adults being able to stay on their parents’ policies to the age of 26 years, nor does it allow insurers to deny coverage for preexisting conditions.
The House committees will begin consideration of their respective languages on March 8.
House Republicans have formally begun their efforts to repeal and replace the Affordable Care Act, but a group of four GOP senators could present a significant hurdle to getting the budget reconciliation package to the president’s desk.
The budget reconciliation language – introduced March 6 – represents the first of three phases in their planned effort to quash the ACA.
“Second are all the regulatory modifications and changes that can be put into place,” Dr. Price said, noting that the Obama administration made 192 specific rules related to the ACA and published more than 5,000 letters of guidance. “We are going to go through every single one of those and ... if they help patients, we need to continue them. If they harm patients or increase costs, they need to be addressed.”
He added that additional legislation could be needed to address changes that cannot be made through the reconciliation process, such as addressing prescription drug costs and allowing health insurance to be sold across state lines.
Sections of the reconciliation package were introduced in the House Energy and Commerce and House Ways and Means Committees, covering areas relative to each committee’s jurisdiction. Taken together, they put forth the plan to repeal and replace certain revenue aspects of the ACA.
Provisions to repeal Medicaid expansion likely will prove the most problematic. The Energy and Commerce language calls for the repeal of Medicaid expansion provisions by 2020. It also repeals a requirement that state Medicaid plans offer the same minimum essential health benefits that are required by plans on the exchanges.
Starting in fiscal 2020, states would begin to receive a per capita allotment to fund their Medicaid programs, a fixed amount per Medicaid enrollee that will adjust over time for inflation using the consumer price index. States spending more than their annual allotment per beneficiary would be penalized with reduced funding in the following year.
That provision has run afoul of certain Republican senators whose votes are needed to gain a simple majority and pass the budget reconciliation package. Because of the slim nature of the Republican majority, any defection from the party line could endanger passage.
“We are concerned that any poorly implemented or poorly timed change in the current funding structure in Medicaid could result in a reduction in access to life-saving health care services,” Sen. Rob Portman (R-Ohio), Sen. Shelley Moore Capito (R-W.Va.), Sen. Cory Gardner (R-Colo.), and Sen. Lisa Murkowski (R-Alaska) said in a letter to Senate Majority Leader Mitch McConnell (R-Ky.). The letter was based on an earlier draft of the legislative language but remains valid as little substantive change has occurred in the formal, introduced language.
“We believe Medicaid needs to be reformed, but reform should not come at the cost of disruption in access to health care for our country’s most vulnerable and sickest individuals,” the senators continued. “Any changes made to how Medicaid is financed through the state and federal governments should be coupled with significant new flexibility so they can efficiently and effectively manage their Medicaid programs to best meet their own needs. ... [The House proposal] does not meet the test of stability for individuals currently enrolled in the program, and we will not support a plan that does not include stability for Medicaid expansion populations or flexibility for states.”
The Energy and Commerce language also includes provisions for block grants to states that would allow them to be used in a manner they see fit within a defined list, including providing financial assistance to high-risk individuals; arrangements that help stabilize premiums in the individual market; reduce the cost of providing insurance in the small group and individual markets; promoting participation in the small group and individual markets; and promoting access to preventive, dental, and vision services as well as treatment for mental and substance abuse disorders. Funds could also be used to provide payments for care or assistance to reduce out-of-pocket costs.
The House proposal also would repeal the premium cost sharing subsidies in the ACA, which would be replaced with refundable tax credits, according to the language released by the Ways and Means Committee.
The credit can be used to purchase a state-approved, major health insurance or unsubsidized COBRA (the Consolidated Omnibus Budget Reconciliation Act) coverage and increases based on age. Individuals younger than age 30 years would get $2,000, and the credit increases in $500 increments per 10-year age block, plateauing at $4,000 for those aged 60 years and older. The caps will adjust over time, based on inflation, and are available to those making up to $75,000 ($150,000 for joint filers); the credit phases out by $100 for every $1,000 over those thresholds.
The Ways and Means language also repeals a number of revenue provisions, including the individual mandate and associated penalties; the employer mandate and associated penalties; taxes on high-cost health plans (Cadillac tax); over-the-counter and prescription medications; health savings accounts; tanning; investment; and on health insurers.
In place of the individual mandate, the language will allow insurers to raise premiums by up to 30%.
The bill does not repeal the provisions regarding young adults being able to stay on their parents’ policies to the age of 26 years, nor does it allow insurers to deny coverage for preexisting conditions.
The House committees will begin consideration of their respective languages on March 8.
House Republicans have formally begun their efforts to repeal and replace the Affordable Care Act, but a group of four GOP senators could present a significant hurdle to getting the budget reconciliation package to the president’s desk.
The budget reconciliation language – introduced March 6 – represents the first of three phases in their planned effort to quash the ACA.
“Second are all the regulatory modifications and changes that can be put into place,” Dr. Price said, noting that the Obama administration made 192 specific rules related to the ACA and published more than 5,000 letters of guidance. “We are going to go through every single one of those and ... if they help patients, we need to continue them. If they harm patients or increase costs, they need to be addressed.”
He added that additional legislation could be needed to address changes that cannot be made through the reconciliation process, such as addressing prescription drug costs and allowing health insurance to be sold across state lines.
Sections of the reconciliation package were introduced in the House Energy and Commerce and House Ways and Means Committees, covering areas relative to each committee’s jurisdiction. Taken together, they put forth the plan to repeal and replace certain revenue aspects of the ACA.
Provisions to repeal Medicaid expansion likely will prove the most problematic. The Energy and Commerce language calls for the repeal of Medicaid expansion provisions by 2020. It also repeals a requirement that state Medicaid plans offer the same minimum essential health benefits that are required by plans on the exchanges.
Starting in fiscal 2020, states would begin to receive a per capita allotment to fund their Medicaid programs, a fixed amount per Medicaid enrollee that will adjust over time for inflation using the consumer price index. States spending more than their annual allotment per beneficiary would be penalized with reduced funding in the following year.
That provision has run afoul of certain Republican senators whose votes are needed to gain a simple majority and pass the budget reconciliation package. Because of the slim nature of the Republican majority, any defection from the party line could endanger passage.
“We are concerned that any poorly implemented or poorly timed change in the current funding structure in Medicaid could result in a reduction in access to life-saving health care services,” Sen. Rob Portman (R-Ohio), Sen. Shelley Moore Capito (R-W.Va.), Sen. Cory Gardner (R-Colo.), and Sen. Lisa Murkowski (R-Alaska) said in a letter to Senate Majority Leader Mitch McConnell (R-Ky.). The letter was based on an earlier draft of the legislative language but remains valid as little substantive change has occurred in the formal, introduced language.
“We believe Medicaid needs to be reformed, but reform should not come at the cost of disruption in access to health care for our country’s most vulnerable and sickest individuals,” the senators continued. “Any changes made to how Medicaid is financed through the state and federal governments should be coupled with significant new flexibility so they can efficiently and effectively manage their Medicaid programs to best meet their own needs. ... [The House proposal] does not meet the test of stability for individuals currently enrolled in the program, and we will not support a plan that does not include stability for Medicaid expansion populations or flexibility for states.”
The Energy and Commerce language also includes provisions for block grants to states that would allow them to be used in a manner they see fit within a defined list, including providing financial assistance to high-risk individuals; arrangements that help stabilize premiums in the individual market; reduce the cost of providing insurance in the small group and individual markets; promoting participation in the small group and individual markets; and promoting access to preventive, dental, and vision services as well as treatment for mental and substance abuse disorders. Funds could also be used to provide payments for care or assistance to reduce out-of-pocket costs.
The House proposal also would repeal the premium cost sharing subsidies in the ACA, which would be replaced with refundable tax credits, according to the language released by the Ways and Means Committee.
The credit can be used to purchase a state-approved, major health insurance or unsubsidized COBRA (the Consolidated Omnibus Budget Reconciliation Act) coverage and increases based on age. Individuals younger than age 30 years would get $2,000, and the credit increases in $500 increments per 10-year age block, plateauing at $4,000 for those aged 60 years and older. The caps will adjust over time, based on inflation, and are available to those making up to $75,000 ($150,000 for joint filers); the credit phases out by $100 for every $1,000 over those thresholds.
The Ways and Means language also repeals a number of revenue provisions, including the individual mandate and associated penalties; the employer mandate and associated penalties; taxes on high-cost health plans (Cadillac tax); over-the-counter and prescription medications; health savings accounts; tanning; investment; and on health insurers.
In place of the individual mandate, the language will allow insurers to raise premiums by up to 30%.
The bill does not repeal the provisions regarding young adults being able to stay on their parents’ policies to the age of 26 years, nor does it allow insurers to deny coverage for preexisting conditions.
The House committees will begin consideration of their respective languages on March 8.
Arteriovenous coupler for structural hypertension poised for sham-controlled study
WASHINGTON – A new device may be the best opportunity yet to achieve sustained blood pressure control in individuals aged 60 years and older, according to Paul Sobotka, MD, Chief Scientific Officer at ROX Medical, the maker of the device.
The experimental device is an arteriovenous coupler that provides an anastomosis between artery and vein to lower arterial volume and reduce blood pressure due to a structural cause. The device has already performed well in initial clinical studies, including a controlled, open-label trial, he reported at CRT 2017 sponsored by the Cardiovascular Research Institute at Washington Hospital Center.
While neurohormonally driven elevations drive increases in diastolic and systolic blood pressures in younger patients, aortic stiffness and loss of vascular elasticity characterize the structurally driven hypertension of older patients, he said. While diuretics can lower arterial volume to achieve reductions in structurally related systolic hypertension, large doses are typically required to have meaningful results and are likely to be accompanied by unacceptable side effects in a large proportion of patients, he said.
“Somewhere between the age of 50 and 60 years, the majority of hypertensive patients will no longer be principally responsive to drugs acting on neurohormonal pathways or to renal denervation strategies.” In the elderly, cardiovascular risk is largely driven by hypertension principally related to the loss of aortic elasticity, which does not respond to most antihypertensive drugs.
The investigational arteriovenous coupler being developed by ROX Medical is intended to lower systolic blood pressure by reducing vascular resistance and therefore arterial volume. The coupler can be placed during cardiac catheterization, does not require sedation, takes about 1 hour to insert, and can be removed if necessary.
In a randomized, open-label study (Lancet. 2015 Apr;385:1634-41), mean systolic blood pressure was reduced by 26.9 mmHg on average in the 44 patients who received the arteriovenous coupler (P less than .001 vs. baseline) and by 3.7 mmHg (P = .31 vs. baseline) in the 39 patients who were maintained on antihypertensive medication, said Dr. Sobotka, who was a coauthor on this and several other clinical studies testing the efficacy and safety of the coupler. Systolic blood pressure falls almost immediately after the device is positioned, and blood pressure control has been sustained for up to 2 years of follow-up so far.
The procedure has been effective in patients resistant to antihypertensive medications and in those who have failed renal denervation, he said.
“The most significant adverse event observed has been venous stenosis related to turbulence, which occurs within in the first 12 months” after device placement, Dr. Sobotka reported. He said that venous stenting has resolved the problem in all affected patients. “Adverse events beyond that have been trivial.”
WASHINGTON – A new device may be the best opportunity yet to achieve sustained blood pressure control in individuals aged 60 years and older, according to Paul Sobotka, MD, Chief Scientific Officer at ROX Medical, the maker of the device.
The experimental device is an arteriovenous coupler that provides an anastomosis between artery and vein to lower arterial volume and reduce blood pressure due to a structural cause. The device has already performed well in initial clinical studies, including a controlled, open-label trial, he reported at CRT 2017 sponsored by the Cardiovascular Research Institute at Washington Hospital Center.
While neurohormonally driven elevations drive increases in diastolic and systolic blood pressures in younger patients, aortic stiffness and loss of vascular elasticity characterize the structurally driven hypertension of older patients, he said. While diuretics can lower arterial volume to achieve reductions in structurally related systolic hypertension, large doses are typically required to have meaningful results and are likely to be accompanied by unacceptable side effects in a large proportion of patients, he said.
“Somewhere between the age of 50 and 60 years, the majority of hypertensive patients will no longer be principally responsive to drugs acting on neurohormonal pathways or to renal denervation strategies.” In the elderly, cardiovascular risk is largely driven by hypertension principally related to the loss of aortic elasticity, which does not respond to most antihypertensive drugs.
The investigational arteriovenous coupler being developed by ROX Medical is intended to lower systolic blood pressure by reducing vascular resistance and therefore arterial volume. The coupler can be placed during cardiac catheterization, does not require sedation, takes about 1 hour to insert, and can be removed if necessary.
In a randomized, open-label study (Lancet. 2015 Apr;385:1634-41), mean systolic blood pressure was reduced by 26.9 mmHg on average in the 44 patients who received the arteriovenous coupler (P less than .001 vs. baseline) and by 3.7 mmHg (P = .31 vs. baseline) in the 39 patients who were maintained on antihypertensive medication, said Dr. Sobotka, who was a coauthor on this and several other clinical studies testing the efficacy and safety of the coupler. Systolic blood pressure falls almost immediately after the device is positioned, and blood pressure control has been sustained for up to 2 years of follow-up so far.
The procedure has been effective in patients resistant to antihypertensive medications and in those who have failed renal denervation, he said.
“The most significant adverse event observed has been venous stenosis related to turbulence, which occurs within in the first 12 months” after device placement, Dr. Sobotka reported. He said that venous stenting has resolved the problem in all affected patients. “Adverse events beyond that have been trivial.”
WASHINGTON – A new device may be the best opportunity yet to achieve sustained blood pressure control in individuals aged 60 years and older, according to Paul Sobotka, MD, Chief Scientific Officer at ROX Medical, the maker of the device.
The experimental device is an arteriovenous coupler that provides an anastomosis between artery and vein to lower arterial volume and reduce blood pressure due to a structural cause. The device has already performed well in initial clinical studies, including a controlled, open-label trial, he reported at CRT 2017 sponsored by the Cardiovascular Research Institute at Washington Hospital Center.
While neurohormonally driven elevations drive increases in diastolic and systolic blood pressures in younger patients, aortic stiffness and loss of vascular elasticity characterize the structurally driven hypertension of older patients, he said. While diuretics can lower arterial volume to achieve reductions in structurally related systolic hypertension, large doses are typically required to have meaningful results and are likely to be accompanied by unacceptable side effects in a large proportion of patients, he said.
“Somewhere between the age of 50 and 60 years, the majority of hypertensive patients will no longer be principally responsive to drugs acting on neurohormonal pathways or to renal denervation strategies.” In the elderly, cardiovascular risk is largely driven by hypertension principally related to the loss of aortic elasticity, which does not respond to most antihypertensive drugs.
The investigational arteriovenous coupler being developed by ROX Medical is intended to lower systolic blood pressure by reducing vascular resistance and therefore arterial volume. The coupler can be placed during cardiac catheterization, does not require sedation, takes about 1 hour to insert, and can be removed if necessary.
In a randomized, open-label study (Lancet. 2015 Apr;385:1634-41), mean systolic blood pressure was reduced by 26.9 mmHg on average in the 44 patients who received the arteriovenous coupler (P less than .001 vs. baseline) and by 3.7 mmHg (P = .31 vs. baseline) in the 39 patients who were maintained on antihypertensive medication, said Dr. Sobotka, who was a coauthor on this and several other clinical studies testing the efficacy and safety of the coupler. Systolic blood pressure falls almost immediately after the device is positioned, and blood pressure control has been sustained for up to 2 years of follow-up so far.
The procedure has been effective in patients resistant to antihypertensive medications and in those who have failed renal denervation, he said.
“The most significant adverse event observed has been venous stenosis related to turbulence, which occurs within in the first 12 months” after device placement, Dr. Sobotka reported. He said that venous stenting has resolved the problem in all affected patients. “Adverse events beyond that have been trivial.”
EXPERT ANALYSIS AT CRT 2017
When STEMI door-to-balloon times deteriorate
WASHINGTON – The interventional cardiology team at Geisinger Medical Center, Danville, Pa., reorganized their care for ST segment elevation MI (STEMI) in 2004 to improve their door-to-balloon angioplasty times. Their highly successful efforts soon streamlined the process, placing Geisinger in the top 10% nationally for low average door-to-balloon (DTB) times.
Then, Geisinger began to see their DTB times creep up.
After witnessing a 5- to 10-minute increase in DTB times for STEMI patients – whether picked up by ambulance, appearing in the emergency department (ED), or referred from neighboring hospitals – James Blankenship, MD, director of the division of cardiology, and fellow Geisinger researchers took on an institutional analysis, which was presented at CRT 2017 sponsored by the Cardiovascular Research Institute at Washington Hospital Center.
Their analysis failed to identify a single explanation, but it did reinforce the importance of constantly reinvigorating processes, Dr. Blankenship said. And their analysis indicated that delays don’t necessarily translate into poorer outcomes.
One proposed explanation was that delays had been caused by an increased reliance on radial rather than femoral catheter access. Dr. Blankenship cited studies suggesting that radial access increases DTB times by 1 to 12 minutes. The proportion of percutaneous coronary interventions performed radially at Geisinger had risen from 2% to 85% over the time period that DTB times had risen.
“There is evidence of benefit from radial access, so even if it slows you down a few minutes, it may be worth doing,” Dr. Blankenship noted. However, when this variable was evaluated, the DTB times were, if anything, slightly faster with radial relative to femoral access.
Another theory was that the decision to provide fellows with a greater role in STEMI management had produced treatment delays. In the cath lab at Geisinger, the increased fellow participation “correlated perfectly” with the decline in DTB times, according to Dr. Blankenship. However, a close look at this variable failed to show any meaningful impact on DTB times.
Changes in process were also examined. For one example, a form must now be completed documenting airway assessment. However, Dr. Blankenship found that filling out this form only takes about a minute and could account for only a small part of the observed loss.
The most significant cause for the increased DTB times among STEMI patients presenting in the ED may well have been a 2012 change in the configuration of the hospital. Prior to 2012, the distance from the ED to the cath lab was less than 100 yards and a 1-minute walk. After the change in the configuration, the cath lab was approximately 7 minutes away, a change that “correlated somewhat” to a prolongation in DTB times.
Similarly, regional hospital STEMI referral patterns changed when a hospital in relatively close proximity opened a cath lab. Up until that time, most referrals had been a 5-minute helicopter transfer, according to Dr. Blankenship. Afterwards, some helicopter transfer times rose to 25 minutes.
Yet, no explanation seemed to be more important than simply ensuring that new staff understand and adhere to the processes. Recounting his experience with a “secret shopper” approach in which he called Geisinger posing as a referring physician to report a potential STEMI, he was disappointed to reach a staff member uncertain of the meaning of the term STEMI.
“STEMI systems need a lot of maintenance,” Dr. Blankenship said. He cautioned that staff changes are common and frequent, making it important to continually assess whether all the participants in delivering STEMI care understand their role.
Some published studies challenge the importance of small changes in DTB times as a predictor of STEMI survival, according to Dr. Blankenship, citing one national survey unable to link a reduction in DTB times with a change in in-hospital mortality (N Engl J Med. 2013;369:901-09). However, he has been more convinced by those studies that do show a relationship. He cited one large study that found an 8% loss in the mortality benefit for each 10-minute delay in DTB (Lancet. 2015;385:1114-22).
Rapid DTB times cannot be divorced from quality of care, Dr. Blankenship said. He cited an experience at one center in which an aggressive program for reducing DTB times led to an increase in mortality among false-positive patients. “It is okay to sacrifice time for quality.”
It was at the 2016 CRT meeting that Dr. Blankenship first provided data showing the decline in DTB times at his institution. At that time and prior to the more comprehensive evaluation of the reasons for the delay presented at this year’s meeting, he speculated that it may be just a question of fatigue from sustaining a rapid response posture over several years.
“My original observation, that after a while you just get tired, is probably still true,” he said.
WASHINGTON – The interventional cardiology team at Geisinger Medical Center, Danville, Pa., reorganized their care for ST segment elevation MI (STEMI) in 2004 to improve their door-to-balloon angioplasty times. Their highly successful efforts soon streamlined the process, placing Geisinger in the top 10% nationally for low average door-to-balloon (DTB) times.
Then, Geisinger began to see their DTB times creep up.
After witnessing a 5- to 10-minute increase in DTB times for STEMI patients – whether picked up by ambulance, appearing in the emergency department (ED), or referred from neighboring hospitals – James Blankenship, MD, director of the division of cardiology, and fellow Geisinger researchers took on an institutional analysis, which was presented at CRT 2017 sponsored by the Cardiovascular Research Institute at Washington Hospital Center.
Their analysis failed to identify a single explanation, but it did reinforce the importance of constantly reinvigorating processes, Dr. Blankenship said. And their analysis indicated that delays don’t necessarily translate into poorer outcomes.
One proposed explanation was that delays had been caused by an increased reliance on radial rather than femoral catheter access. Dr. Blankenship cited studies suggesting that radial access increases DTB times by 1 to 12 minutes. The proportion of percutaneous coronary interventions performed radially at Geisinger had risen from 2% to 85% over the time period that DTB times had risen.
“There is evidence of benefit from radial access, so even if it slows you down a few minutes, it may be worth doing,” Dr. Blankenship noted. However, when this variable was evaluated, the DTB times were, if anything, slightly faster with radial relative to femoral access.
Another theory was that the decision to provide fellows with a greater role in STEMI management had produced treatment delays. In the cath lab at Geisinger, the increased fellow participation “correlated perfectly” with the decline in DTB times, according to Dr. Blankenship. However, a close look at this variable failed to show any meaningful impact on DTB times.
Changes in process were also examined. For one example, a form must now be completed documenting airway assessment. However, Dr. Blankenship found that filling out this form only takes about a minute and could account for only a small part of the observed loss.
The most significant cause for the increased DTB times among STEMI patients presenting in the ED may well have been a 2012 change in the configuration of the hospital. Prior to 2012, the distance from the ED to the cath lab was less than 100 yards and a 1-minute walk. After the change in the configuration, the cath lab was approximately 7 minutes away, a change that “correlated somewhat” to a prolongation in DTB times.
Similarly, regional hospital STEMI referral patterns changed when a hospital in relatively close proximity opened a cath lab. Up until that time, most referrals had been a 5-minute helicopter transfer, according to Dr. Blankenship. Afterwards, some helicopter transfer times rose to 25 minutes.
Yet, no explanation seemed to be more important than simply ensuring that new staff understand and adhere to the processes. Recounting his experience with a “secret shopper” approach in which he called Geisinger posing as a referring physician to report a potential STEMI, he was disappointed to reach a staff member uncertain of the meaning of the term STEMI.
“STEMI systems need a lot of maintenance,” Dr. Blankenship said. He cautioned that staff changes are common and frequent, making it important to continually assess whether all the participants in delivering STEMI care understand their role.
Some published studies challenge the importance of small changes in DTB times as a predictor of STEMI survival, according to Dr. Blankenship, citing one national survey unable to link a reduction in DTB times with a change in in-hospital mortality (N Engl J Med. 2013;369:901-09). However, he has been more convinced by those studies that do show a relationship. He cited one large study that found an 8% loss in the mortality benefit for each 10-minute delay in DTB (Lancet. 2015;385:1114-22).
Rapid DTB times cannot be divorced from quality of care, Dr. Blankenship said. He cited an experience at one center in which an aggressive program for reducing DTB times led to an increase in mortality among false-positive patients. “It is okay to sacrifice time for quality.”
It was at the 2016 CRT meeting that Dr. Blankenship first provided data showing the decline in DTB times at his institution. At that time and prior to the more comprehensive evaluation of the reasons for the delay presented at this year’s meeting, he speculated that it may be just a question of fatigue from sustaining a rapid response posture over several years.
“My original observation, that after a while you just get tired, is probably still true,” he said.
WASHINGTON – The interventional cardiology team at Geisinger Medical Center, Danville, Pa., reorganized their care for ST segment elevation MI (STEMI) in 2004 to improve their door-to-balloon angioplasty times. Their highly successful efforts soon streamlined the process, placing Geisinger in the top 10% nationally for low average door-to-balloon (DTB) times.
Then, Geisinger began to see their DTB times creep up.
After witnessing a 5- to 10-minute increase in DTB times for STEMI patients – whether picked up by ambulance, appearing in the emergency department (ED), or referred from neighboring hospitals – James Blankenship, MD, director of the division of cardiology, and fellow Geisinger researchers took on an institutional analysis, which was presented at CRT 2017 sponsored by the Cardiovascular Research Institute at Washington Hospital Center.
Their analysis failed to identify a single explanation, but it did reinforce the importance of constantly reinvigorating processes, Dr. Blankenship said. And their analysis indicated that delays don’t necessarily translate into poorer outcomes.
One proposed explanation was that delays had been caused by an increased reliance on radial rather than femoral catheter access. Dr. Blankenship cited studies suggesting that radial access increases DTB times by 1 to 12 minutes. The proportion of percutaneous coronary interventions performed radially at Geisinger had risen from 2% to 85% over the time period that DTB times had risen.
“There is evidence of benefit from radial access, so even if it slows you down a few minutes, it may be worth doing,” Dr. Blankenship noted. However, when this variable was evaluated, the DTB times were, if anything, slightly faster with radial relative to femoral access.
Another theory was that the decision to provide fellows with a greater role in STEMI management had produced treatment delays. In the cath lab at Geisinger, the increased fellow participation “correlated perfectly” with the decline in DTB times, according to Dr. Blankenship. However, a close look at this variable failed to show any meaningful impact on DTB times.
Changes in process were also examined. For one example, a form must now be completed documenting airway assessment. However, Dr. Blankenship found that filling out this form only takes about a minute and could account for only a small part of the observed loss.
The most significant cause for the increased DTB times among STEMI patients presenting in the ED may well have been a 2012 change in the configuration of the hospital. Prior to 2012, the distance from the ED to the cath lab was less than 100 yards and a 1-minute walk. After the change in the configuration, the cath lab was approximately 7 minutes away, a change that “correlated somewhat” to a prolongation in DTB times.
Similarly, regional hospital STEMI referral patterns changed when a hospital in relatively close proximity opened a cath lab. Up until that time, most referrals had been a 5-minute helicopter transfer, according to Dr. Blankenship. Afterwards, some helicopter transfer times rose to 25 minutes.
Yet, no explanation seemed to be more important than simply ensuring that new staff understand and adhere to the processes. Recounting his experience with a “secret shopper” approach in which he called Geisinger posing as a referring physician to report a potential STEMI, he was disappointed to reach a staff member uncertain of the meaning of the term STEMI.
“STEMI systems need a lot of maintenance,” Dr. Blankenship said. He cautioned that staff changes are common and frequent, making it important to continually assess whether all the participants in delivering STEMI care understand their role.
Some published studies challenge the importance of small changes in DTB times as a predictor of STEMI survival, according to Dr. Blankenship, citing one national survey unable to link a reduction in DTB times with a change in in-hospital mortality (N Engl J Med. 2013;369:901-09). However, he has been more convinced by those studies that do show a relationship. He cited one large study that found an 8% loss in the mortality benefit for each 10-minute delay in DTB (Lancet. 2015;385:1114-22).
Rapid DTB times cannot be divorced from quality of care, Dr. Blankenship said. He cited an experience at one center in which an aggressive program for reducing DTB times led to an increase in mortality among false-positive patients. “It is okay to sacrifice time for quality.”
It was at the 2016 CRT meeting that Dr. Blankenship first provided data showing the decline in DTB times at his institution. At that time and prior to the more comprehensive evaluation of the reasons for the delay presented at this year’s meeting, he speculated that it may be just a question of fatigue from sustaining a rapid response posture over several years.
“My original observation, that after a while you just get tired, is probably still true,” he said.
AT CRT 2017
Key clinical point: With small deviations in process over time, delays build in the emergency treatment of ST segment elevation MI (STEMI).
Major finding: Processes must be regularly reinvigorated, a systematic evaluation of deteriorating door-to-balloon times for STEMI care suggests.
Data source: Nonrandomized retrospective analysis.
Disclosures: Dr. Blankenship reported no financial relationships to disclose.
Protocol speeds thrombectomy stroke patients from primary centers
HOUSTON – A novel protocol designed to speed patients with large-vessel occlusion strokes in and out of primary stroke centers and on to centers where they can undergo definitive thrombectomy treatment produced significant improvements in treatment speed and outcomes among 22 Rhode Island patients managed with the full protocol.
Streamlining the path in and out of a primary stroke center is key for delivering mechanical thrombectomy as quickly as possible to patients with an emergent large vessel occlusion, said Ryan A. McTaggart, MD, at the International Stroke Conference sponsored by the American Heart Association. “Door-in door-out time should be the standard metric for all partnerships between primary and comprehensive stroke centers,” said Dr. McTaggart, a neuroradiologist at Rhode Island Hospital in Providence, the state’s only comprehensive stroke center.
• When a patient with a suspected large vessel occlusion with a Los Angeles Motor Score of 4 or 5 arrives soon after onset at the primary stroke center, a call immediately goes out to the EMS transfer center of Rhode Island Hospital to coordinate the transport that will move the patient from the primary center to the comprehensive when needed.
• The initial CT scan at the primary center is run as the definitive scan, including a conventional CT scan to rule out hemorrhage and allow intravenous thrombolytic therapy with tissue plasminogen activator (TPA) and CT angiography to locate the occluding clot.
• The CT images are immediately uploaded to a cloud-based library so that neurologists at Rhode Island Hospital can read the images on their phones and plan the management strategy.
During the 11 months following the start of the protocol, the Rhode Island network identified 70 patients as candidates for thrombectomy, including 22 managed using the complete protocol and 48 managed using only parts of the new protocol.
The median time from onset of stroke symptoms to revascularization with thrombectomy was 184 minutes in the 22 patients managed under the full protocol and 233 minutes among 48 similar patients who were not fully managed with the protocol, Dr. McTaggart reported. This “dramatic” difference in median times was entirely driven by a difference in the door-in door-out time at the primary stroke center, which was a median of 64 minutes for the 22 patients managed with the full protocol and a median of 104 minutes without the full protocol, a 38% relative decrease that was statistically significant.
Time to initiation of intravenous TPA at the primary stroke center also improved, from a median of 65 minutes without the full protocol to a median of 40 minutes with it, a statistically significant difference. “The primary stroke center physicians tell us they have greater confidence to start TPA when they have a consult that can identify the patient’s clot,” he said.
Consistent with the shorter time to revascularization, the prevalence after 90 days of a functionally good outcome – a modified Rankin scale score of 0-2 – occurred in 50% of patients managed with the full protocol and 25% of those managed with a partial protocol, a statistically significant difference.
To put the 184 minutes median time from stroke onset to reperfusion into perspective, Dr. McTaggart noted that it is comparable to the time to reperfusion documented recently in a U.S. registry of thrombectomy patients who had been transported directly to the comprehensive stroke centers where their thrombectomy was done.
He also acknowledged the heavy lifting he and his associates had to do to set up this network. Getting buy-in from all the regional primary strokes centers was “a ton of work,” Dr. McTaggart said in an interview. “We told the primary stroke center staffs that thrombectomy is a powerful treatment, with a number needed to treat of three to get one improved outcome. That’s a convincing argument. The thrombectomy data [that became available in early 2015] made the argument for the protocol and network more compelling.”
Primary stroke centers keep the stroke patients who don’t have a clot occlusion suitable for thrombectomy, which means the comprehensive center thrombectomy team receives fewer false-alarm patients. Dr. McTaggart’s current goal is to have primary stroke centers get incoming patients out and on the road to a thrombectomy center within 45 minutes. In the future, primary stroke centers will perform CT imaging on all patients with suspected strokes, not just the severely affected patients with a Los Angeles Motor Score of 4 or 5. Additional useful steps toward speeding appropriate stroke patients to thrombectomy is direct ambulance transport of selected, high probability patients directly to a comprehensive stroke center and use of mobile stroke units to bring CT imaging and the start of TPA treatment out into the field.
Dr. McTaggart had no disclosures.
[email protected]
On Twitter @mitchelzoler
HOUSTON – A novel protocol designed to speed patients with large-vessel occlusion strokes in and out of primary stroke centers and on to centers where they can undergo definitive thrombectomy treatment produced significant improvements in treatment speed and outcomes among 22 Rhode Island patients managed with the full protocol.
Streamlining the path in and out of a primary stroke center is key for delivering mechanical thrombectomy as quickly as possible to patients with an emergent large vessel occlusion, said Ryan A. McTaggart, MD, at the International Stroke Conference sponsored by the American Heart Association. “Door-in door-out time should be the standard metric for all partnerships between primary and comprehensive stroke centers,” said Dr. McTaggart, a neuroradiologist at Rhode Island Hospital in Providence, the state’s only comprehensive stroke center.
• When a patient with a suspected large vessel occlusion with a Los Angeles Motor Score of 4 or 5 arrives soon after onset at the primary stroke center, a call immediately goes out to the EMS transfer center of Rhode Island Hospital to coordinate the transport that will move the patient from the primary center to the comprehensive when needed.
• The initial CT scan at the primary center is run as the definitive scan, including a conventional CT scan to rule out hemorrhage and allow intravenous thrombolytic therapy with tissue plasminogen activator (TPA) and CT angiography to locate the occluding clot.
• The CT images are immediately uploaded to a cloud-based library so that neurologists at Rhode Island Hospital can read the images on their phones and plan the management strategy.
During the 11 months following the start of the protocol, the Rhode Island network identified 70 patients as candidates for thrombectomy, including 22 managed using the complete protocol and 48 managed using only parts of the new protocol.
The median time from onset of stroke symptoms to revascularization with thrombectomy was 184 minutes in the 22 patients managed under the full protocol and 233 minutes among 48 similar patients who were not fully managed with the protocol, Dr. McTaggart reported. This “dramatic” difference in median times was entirely driven by a difference in the door-in door-out time at the primary stroke center, which was a median of 64 minutes for the 22 patients managed with the full protocol and a median of 104 minutes without the full protocol, a 38% relative decrease that was statistically significant.
Time to initiation of intravenous TPA at the primary stroke center also improved, from a median of 65 minutes without the full protocol to a median of 40 minutes with it, a statistically significant difference. “The primary stroke center physicians tell us they have greater confidence to start TPA when they have a consult that can identify the patient’s clot,” he said.
Consistent with the shorter time to revascularization, the prevalence after 90 days of a functionally good outcome – a modified Rankin scale score of 0-2 – occurred in 50% of patients managed with the full protocol and 25% of those managed with a partial protocol, a statistically significant difference.
To put the 184 minutes median time from stroke onset to reperfusion into perspective, Dr. McTaggart noted that it is comparable to the time to reperfusion documented recently in a U.S. registry of thrombectomy patients who had been transported directly to the comprehensive stroke centers where their thrombectomy was done.
He also acknowledged the heavy lifting he and his associates had to do to set up this network. Getting buy-in from all the regional primary strokes centers was “a ton of work,” Dr. McTaggart said in an interview. “We told the primary stroke center staffs that thrombectomy is a powerful treatment, with a number needed to treat of three to get one improved outcome. That’s a convincing argument. The thrombectomy data [that became available in early 2015] made the argument for the protocol and network more compelling.”
Primary stroke centers keep the stroke patients who don’t have a clot occlusion suitable for thrombectomy, which means the comprehensive center thrombectomy team receives fewer false-alarm patients. Dr. McTaggart’s current goal is to have primary stroke centers get incoming patients out and on the road to a thrombectomy center within 45 minutes. In the future, primary stroke centers will perform CT imaging on all patients with suspected strokes, not just the severely affected patients with a Los Angeles Motor Score of 4 or 5. Additional useful steps toward speeding appropriate stroke patients to thrombectomy is direct ambulance transport of selected, high probability patients directly to a comprehensive stroke center and use of mobile stroke units to bring CT imaging and the start of TPA treatment out into the field.
Dr. McTaggart had no disclosures.
[email protected]
On Twitter @mitchelzoler
HOUSTON – A novel protocol designed to speed patients with large-vessel occlusion strokes in and out of primary stroke centers and on to centers where they can undergo definitive thrombectomy treatment produced significant improvements in treatment speed and outcomes among 22 Rhode Island patients managed with the full protocol.
Streamlining the path in and out of a primary stroke center is key for delivering mechanical thrombectomy as quickly as possible to patients with an emergent large vessel occlusion, said Ryan A. McTaggart, MD, at the International Stroke Conference sponsored by the American Heart Association. “Door-in door-out time should be the standard metric for all partnerships between primary and comprehensive stroke centers,” said Dr. McTaggart, a neuroradiologist at Rhode Island Hospital in Providence, the state’s only comprehensive stroke center.
• When a patient with a suspected large vessel occlusion with a Los Angeles Motor Score of 4 or 5 arrives soon after onset at the primary stroke center, a call immediately goes out to the EMS transfer center of Rhode Island Hospital to coordinate the transport that will move the patient from the primary center to the comprehensive when needed.
• The initial CT scan at the primary center is run as the definitive scan, including a conventional CT scan to rule out hemorrhage and allow intravenous thrombolytic therapy with tissue plasminogen activator (TPA) and CT angiography to locate the occluding clot.
• The CT images are immediately uploaded to a cloud-based library so that neurologists at Rhode Island Hospital can read the images on their phones and plan the management strategy.
During the 11 months following the start of the protocol, the Rhode Island network identified 70 patients as candidates for thrombectomy, including 22 managed using the complete protocol and 48 managed using only parts of the new protocol.
The median time from onset of stroke symptoms to revascularization with thrombectomy was 184 minutes in the 22 patients managed under the full protocol and 233 minutes among 48 similar patients who were not fully managed with the protocol, Dr. McTaggart reported. This “dramatic” difference in median times was entirely driven by a difference in the door-in door-out time at the primary stroke center, which was a median of 64 minutes for the 22 patients managed with the full protocol and a median of 104 minutes without the full protocol, a 38% relative decrease that was statistically significant.
Time to initiation of intravenous TPA at the primary stroke center also improved, from a median of 65 minutes without the full protocol to a median of 40 minutes with it, a statistically significant difference. “The primary stroke center physicians tell us they have greater confidence to start TPA when they have a consult that can identify the patient’s clot,” he said.
Consistent with the shorter time to revascularization, the prevalence after 90 days of a functionally good outcome – a modified Rankin scale score of 0-2 – occurred in 50% of patients managed with the full protocol and 25% of those managed with a partial protocol, a statistically significant difference.
To put the 184 minutes median time from stroke onset to reperfusion into perspective, Dr. McTaggart noted that it is comparable to the time to reperfusion documented recently in a U.S. registry of thrombectomy patients who had been transported directly to the comprehensive stroke centers where their thrombectomy was done.
He also acknowledged the heavy lifting he and his associates had to do to set up this network. Getting buy-in from all the regional primary strokes centers was “a ton of work,” Dr. McTaggart said in an interview. “We told the primary stroke center staffs that thrombectomy is a powerful treatment, with a number needed to treat of three to get one improved outcome. That’s a convincing argument. The thrombectomy data [that became available in early 2015] made the argument for the protocol and network more compelling.”
Primary stroke centers keep the stroke patients who don’t have a clot occlusion suitable for thrombectomy, which means the comprehensive center thrombectomy team receives fewer false-alarm patients. Dr. McTaggart’s current goal is to have primary stroke centers get incoming patients out and on the road to a thrombectomy center within 45 minutes. In the future, primary stroke centers will perform CT imaging on all patients with suspected strokes, not just the severely affected patients with a Los Angeles Motor Score of 4 or 5. Additional useful steps toward speeding appropriate stroke patients to thrombectomy is direct ambulance transport of selected, high probability patients directly to a comprehensive stroke center and use of mobile stroke units to bring CT imaging and the start of TPA treatment out into the field.
Dr. McTaggart had no disclosures.
[email protected]
On Twitter @mitchelzoler
AT THE INTERNATIONAL STROKE CONFERENCE
Key clinical point:
Major finding: Median time from stroke onset to thrombectomy reperfusion was 184 minutes, including transfer between a primary and comprehensive stroke center.
Data source: Review of 70 acute ischemic stroke patients treated at Rhode Island Hospital.
Disclosures: Dr. McTaggart had no disclosures.
Patient transfer before thrombectomy worsens stroke outcomes
HOUSTON – Drip and ship may not be the most time-effective way to treat acute ischemic stroke patients who are candidates for endovascular thrombectomy.
Results from two separate real-world, observational studies showed that acute ischemic stroke patients with large vessel occlusions amenable to mechanical thrombectomy had significantly worse clinical outcomes when their management path included a stop at a primary stroke center followed by transfer to a comprehensive stroke center that had the capacity to perform thrombectomy, compared with going straight to the thrombectomy site.
The findings show “the system of care has room for improvement. Patients with large vessel occlusions clearly do better when we get them to mechanical thrombectomy as quickly as possible,” said Dr. Froehler, a vascular neurologist at Vanderbilt University in Nashville, Tenn. Thrombectomy “has a more powerful treatment effect than TPA [tissue plasminogen activator] and we need to adjust our standard of care to best deliver” thrombectomy, he said in an interview.
The study run by Dr. Froehler used data collected in the Systematic Evaluation of Patients Treated With Stroke Devices for Acute Ischemic Stroke (STRATIS) registry, which began in 2014 and has data for 984 acute ischemic stroke patients with large vessel occlusions treated by mechanical thrombectomy seen at any of 55 U.S. centers. The series included 445 (45%) patients first seen as a primary stroke center and then transferred to a comprehensive center and 539 (55%) who went directly to a comprehensive stroke center (direct patients). Prior to thrombectomy, 628 of all patients (64%) received TPA, with a roughly similar percentage in both the transferred and direct patients.
The data showed that the median time from symptom onset to revascularization was 202 minutes among the direct patients and 312 minutes among those first seen at a primary stroke center and then transferred, a statistically significant difference. The average time difference per patient between the two subgroups was 100 minutes, Dr. Froehler reported.
This difference in time to reperfusion led directly to significant differences in functional outcomes after 90 days measured on the modified Rankin Scale (mRS). The percentage of patients with a mRS score of 0 or 1 (an excellent functional outcome) was 38% for the patients first seen at primary stroke centers and 47% in direct patients, a 47% relative rise in excellent outcomes among the direct patients. The percentage of patients with a mRS score of 0-2, which identifies functional independence post stroke, was 52% among transferred patients and 60% in direct patients, a 38% relative improvement for this outcome among direct patients.
The second study of stroke transfer times and outcomes used data from 562 acute ischemic stroke patients with large vessel occlusions treated in the Providence Health & Services system in five western U.S. states during 2012-2016. Nearly half the patients required a transfer and the other half went directly to a center able to perform thrombectomy. The analysis used clinical outcomes scored on the mRS at the time of hospital discharge.
“Right now, the big delay at primary stroke centers is the door-in door-out time,” commented Ryan A. McTaggart, MD, an interventional neuroradiologist at Rhode Island Hospital in Providence, the only comprehensive stroke center in Rhode Island. He helped organize a partnership with 14 primary stroke centers in Rhode Island that uses a streamlined imaging, treatment (with TPA), and transfer protocol that hacked dozens of minutes off transfer times and produced a median time from onset of symptoms to revascularization by thrombectomy of 184 minutes in patients first seen at a primary stroke center. This clocking blows past the 202 minute median for stroke onset to revascularization in the direct patients from Dr. Froehler’s study.
Stroke transport and treatment networks are now undergoing refinement in Tennessee, said Dr. Froehler, based in part on the data he reported. Considerations in Tennessee include how EMS workers assess possible stroke patients, decisions by EMS on where to take patients, and how quality of care is measured at primary and comprehensive stroke centers.
The STRATIS registry is sponsored by Medtronic. Dr. Froehler is a consultant to Medtronic, Blockade, Stryker, and Control Medical. Dr. Smith, Dr. Tarpley, and Dr. McTaggart had no disclosures.
[email protected]
On Twitter @mitchelzoler
HOUSTON – Drip and ship may not be the most time-effective way to treat acute ischemic stroke patients who are candidates for endovascular thrombectomy.
Results from two separate real-world, observational studies showed that acute ischemic stroke patients with large vessel occlusions amenable to mechanical thrombectomy had significantly worse clinical outcomes when their management path included a stop at a primary stroke center followed by transfer to a comprehensive stroke center that had the capacity to perform thrombectomy, compared with going straight to the thrombectomy site.
The findings show “the system of care has room for improvement. Patients with large vessel occlusions clearly do better when we get them to mechanical thrombectomy as quickly as possible,” said Dr. Froehler, a vascular neurologist at Vanderbilt University in Nashville, Tenn. Thrombectomy “has a more powerful treatment effect than TPA [tissue plasminogen activator] and we need to adjust our standard of care to best deliver” thrombectomy, he said in an interview.
The study run by Dr. Froehler used data collected in the Systematic Evaluation of Patients Treated With Stroke Devices for Acute Ischemic Stroke (STRATIS) registry, which began in 2014 and has data for 984 acute ischemic stroke patients with large vessel occlusions treated by mechanical thrombectomy seen at any of 55 U.S. centers. The series included 445 (45%) patients first seen as a primary stroke center and then transferred to a comprehensive center and 539 (55%) who went directly to a comprehensive stroke center (direct patients). Prior to thrombectomy, 628 of all patients (64%) received TPA, with a roughly similar percentage in both the transferred and direct patients.
The data showed that the median time from symptom onset to revascularization was 202 minutes among the direct patients and 312 minutes among those first seen at a primary stroke center and then transferred, a statistically significant difference. The average time difference per patient between the two subgroups was 100 minutes, Dr. Froehler reported.
This difference in time to reperfusion led directly to significant differences in functional outcomes after 90 days measured on the modified Rankin Scale (mRS). The percentage of patients with a mRS score of 0 or 1 (an excellent functional outcome) was 38% for the patients first seen at primary stroke centers and 47% in direct patients, a 47% relative rise in excellent outcomes among the direct patients. The percentage of patients with a mRS score of 0-2, which identifies functional independence post stroke, was 52% among transferred patients and 60% in direct patients, a 38% relative improvement for this outcome among direct patients.
The second study of stroke transfer times and outcomes used data from 562 acute ischemic stroke patients with large vessel occlusions treated in the Providence Health & Services system in five western U.S. states during 2012-2016. Nearly half the patients required a transfer and the other half went directly to a center able to perform thrombectomy. The analysis used clinical outcomes scored on the mRS at the time of hospital discharge.
“Right now, the big delay at primary stroke centers is the door-in door-out time,” commented Ryan A. McTaggart, MD, an interventional neuroradiologist at Rhode Island Hospital in Providence, the only comprehensive stroke center in Rhode Island. He helped organize a partnership with 14 primary stroke centers in Rhode Island that uses a streamlined imaging, treatment (with TPA), and transfer protocol that hacked dozens of minutes off transfer times and produced a median time from onset of symptoms to revascularization by thrombectomy of 184 minutes in patients first seen at a primary stroke center. This clocking blows past the 202 minute median for stroke onset to revascularization in the direct patients from Dr. Froehler’s study.
Stroke transport and treatment networks are now undergoing refinement in Tennessee, said Dr. Froehler, based in part on the data he reported. Considerations in Tennessee include how EMS workers assess possible stroke patients, decisions by EMS on where to take patients, and how quality of care is measured at primary and comprehensive stroke centers.
The STRATIS registry is sponsored by Medtronic. Dr. Froehler is a consultant to Medtronic, Blockade, Stryker, and Control Medical. Dr. Smith, Dr. Tarpley, and Dr. McTaggart had no disclosures.
[email protected]
On Twitter @mitchelzoler
HOUSTON – Drip and ship may not be the most time-effective way to treat acute ischemic stroke patients who are candidates for endovascular thrombectomy.
Results from two separate real-world, observational studies showed that acute ischemic stroke patients with large vessel occlusions amenable to mechanical thrombectomy had significantly worse clinical outcomes when their management path included a stop at a primary stroke center followed by transfer to a comprehensive stroke center that had the capacity to perform thrombectomy, compared with going straight to the thrombectomy site.
The findings show “the system of care has room for improvement. Patients with large vessel occlusions clearly do better when we get them to mechanical thrombectomy as quickly as possible,” said Dr. Froehler, a vascular neurologist at Vanderbilt University in Nashville, Tenn. Thrombectomy “has a more powerful treatment effect than TPA [tissue plasminogen activator] and we need to adjust our standard of care to best deliver” thrombectomy, he said in an interview.
The study run by Dr. Froehler used data collected in the Systematic Evaluation of Patients Treated With Stroke Devices for Acute Ischemic Stroke (STRATIS) registry, which began in 2014 and has data for 984 acute ischemic stroke patients with large vessel occlusions treated by mechanical thrombectomy seen at any of 55 U.S. centers. The series included 445 (45%) patients first seen as a primary stroke center and then transferred to a comprehensive center and 539 (55%) who went directly to a comprehensive stroke center (direct patients). Prior to thrombectomy, 628 of all patients (64%) received TPA, with a roughly similar percentage in both the transferred and direct patients.
The data showed that the median time from symptom onset to revascularization was 202 minutes among the direct patients and 312 minutes among those first seen at a primary stroke center and then transferred, a statistically significant difference. The average time difference per patient between the two subgroups was 100 minutes, Dr. Froehler reported.
This difference in time to reperfusion led directly to significant differences in functional outcomes after 90 days measured on the modified Rankin Scale (mRS). The percentage of patients with a mRS score of 0 or 1 (an excellent functional outcome) was 38% for the patients first seen at primary stroke centers and 47% in direct patients, a 47% relative rise in excellent outcomes among the direct patients. The percentage of patients with a mRS score of 0-2, which identifies functional independence post stroke, was 52% among transferred patients and 60% in direct patients, a 38% relative improvement for this outcome among direct patients.
The second study of stroke transfer times and outcomes used data from 562 acute ischemic stroke patients with large vessel occlusions treated in the Providence Health & Services system in five western U.S. states during 2012-2016. Nearly half the patients required a transfer and the other half went directly to a center able to perform thrombectomy. The analysis used clinical outcomes scored on the mRS at the time of hospital discharge.
“Right now, the big delay at primary stroke centers is the door-in door-out time,” commented Ryan A. McTaggart, MD, an interventional neuroradiologist at Rhode Island Hospital in Providence, the only comprehensive stroke center in Rhode Island. He helped organize a partnership with 14 primary stroke centers in Rhode Island that uses a streamlined imaging, treatment (with TPA), and transfer protocol that hacked dozens of minutes off transfer times and produced a median time from onset of symptoms to revascularization by thrombectomy of 184 minutes in patients first seen at a primary stroke center. This clocking blows past the 202 minute median for stroke onset to revascularization in the direct patients from Dr. Froehler’s study.
Stroke transport and treatment networks are now undergoing refinement in Tennessee, said Dr. Froehler, based in part on the data he reported. Considerations in Tennessee include how EMS workers assess possible stroke patients, decisions by EMS on where to take patients, and how quality of care is measured at primary and comprehensive stroke centers.
The STRATIS registry is sponsored by Medtronic. Dr. Froehler is a consultant to Medtronic, Blockade, Stryker, and Control Medical. Dr. Smith, Dr. Tarpley, and Dr. McTaggart had no disclosures.
[email protected]
On Twitter @mitchelzoler
AT THE INTERNATIONAL STROKE CONFERENCE
Key clinical point:
Major finding: In STRATIS, excellent outcomes occurred in 47% of patients sent directly to a thrombectomy hospital and in 38% of transferred patients.
Data source: The STRATIS registry, with 984 U.S. acute ischemic stroke patients, and 562 U.S. acute ischemic stroke patients from the Providence Health & Services network.
Disclosures: The STRATIS registry is sponsored by Medtronic. Dr. Froehler is a consultant to Medtronic, Blockade, Stryker, and Control Medical. Dr. Smith, Dr. Tarpley, and Dr. McTaggart had no disclosures.
The percutaneous mitral valve replacement pipe dream
SNOWMASS, COLO. – Percutaneous mitral valve replacement is unlikely to ever catch on in any way remotely approaching that of transcatheter aortic valve replacement for the treatment of aortic stenosis, Blase A. Carabello, MD, predicted at the Annual Cardiovascular Conference at Snowmass.
“We’ve spent $2 billion looking for methods of percutaneous mitral valve replacement, and yet, I have to wonder if that makes any sense,” said Dr. Carabello, professor of medicine and chief of cardiology at East Carolina University in Greenville, N.C.
“If repair is superior to replacement in primary MR [mitral regurgitation], which I think we all agree is true, and you don’t need to get rid of every last molecule of blood going backward across the mitral valve when you’ve got a good left ventricle, then a percutaneous replacement in primary MR would have only the niche of patients who are inoperable and whose leaflets can’t be grabbed by the MitraClip or some new percutaneous device down the road. And, in secondary MR, it doesn’t seem to matter whether you replace or repair the valve, so why not just repair it with a clip?” he argued.
Numerous nonrandomized studies have invariably demonstrated superior survival for surgical repair versus replacement in patients with primary MR.
“There’s never going to be a randomized controlled trial of repair versus replacement; there’s no equipoise there. We all believe that, in primary MR, repair is superior to replacement. There are no data anywhere to suggest the opposite. It’s essentially sacrosanct,” according to the cardiologist.
In contrast, a major randomized trial of surgical repair versus replacement has been conducted in patients with severe secondary MR. This NIH-funded study conducted by the Cardiothoracic Surgical Trials Network found no difference in survival between the two groups (N Engl J Med. 2016 Jan 28; 374[4]:344-53). That’s not a surprising result, Dr. Carabello said, since the underlying cause of this type of valve disease is a sick left ventricle. But, since surgical repair entails less morbidity than replacement – and a percutaneous repair with a leaflet-grasping device such as the MitraClip is simpler and safer than a surgical repair – it seems likely that the future treatment for secondary MR will be a percutaneous device, he said.
That future could depend upon the results of the ongoing COAPT trial (Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy), in which the MitraClip is being studied as an alternative to surgical repair for significant secondary MR. The MitraClip, which doesn’t entail a concomitant annuloplasty, is currently approved by the Food and Drug Administration only for patients with primary, degenerative mitral regurgitation not amenable to surgical repair. But, if COAPT yields positive results, the role of the MitraClip will greatly expand.
An intriguing and poorly understood difference exists in the significance of residual mitral regurgitation following surgical repair as opposed to percutaneous MitraClip repair, Dr. Carabello observed.
“I go to the OR a lot, and I know of no surgeon [who] will leave 2+ MR behind. Most surgeons won’t leave 1+ MR behind. They’ll put the patient back on the pump to repair even mild residual MR, accepting only trace MR or zero before they leave the OR because they know that the best predictor of a failed mitral repair is the presence of residual MR in the OR,” he said.
In contrast, following successful deployment of the MitraClip most patients are left with 1-2+ MR. Yet, as was demonstrated in the 5-year results of the randomized EVEREST II trial (Endovascular Valve Edge-to-Edge Repair Study), this residual MR wasn’t a harbinger of poor outcomes long-term (J Am Coll Cardiol. 2015 Dec 29;66[25]:2844-54).
“You would have expected, with that much residual MR, there would be a perpetually increasing failure rate over time, but that didn’t happen. In Everest II, there was an early failure rate for percutaneous repair, where the MitraClip didn’t work and those patients required surgical mitral valve repair. But, after the first 6 months, the failure rate for the clip was exactly the same as the surgical failure rate, even though, with the clip, you start with more MR to begin with,” the cardiologist noted.
The MitraClip procedure is modeled after the surgical Alfieri double-orifice end-to-end stitch technique, which has been shown to have durable results when performed in conjunction with an annuloplasty ring for primary MR.
“The MitraClip essentially joins the valve in the middle the way the Alfieri stitch does, but it doesn’t appear to behave the same way. Why is that? Maybe the clip does something different than the Alfieri stitch on which it was modeled. Maybe that bar in the middle of the mitral valve does something in terms of scarring or stabilization that we don’t know about yet,” he speculated.
As for the prospects for percutaneous mitral valve replacement, Dr. Carabello said that this type of procedure “is a very difficult thing to do, and so far, has been met with a fair amount of failure. It’ll be very interesting to see what percentage of market share it gets 10 years down the road. My prediction is that, for mitral regurgitation, repair is always going to be it.”
Dr. Carabello reported serving on a data safety monitoring board for Edwards Lifesciences.
The author provides valuable insight into how the definition of “success” of a procedure can change depending on the approach to the problem. While the gold standard of open mitral valve repair is 1+ regurgitation or less, those promoting percutaneous valve replacement are willing to accept long term 1+ to 2+ regurgitation. New technology and innovation is critical in medicine, provided the results are at least equivalent or superior to the standard techniques.
The author provides valuable insight into how the definition of “success” of a procedure can change depending on the approach to the problem. While the gold standard of open mitral valve repair is 1+ regurgitation or less, those promoting percutaneous valve replacement are willing to accept long term 1+ to 2+ regurgitation. New technology and innovation is critical in medicine, provided the results are at least equivalent or superior to the standard techniques.
The author provides valuable insight into how the definition of “success” of a procedure can change depending on the approach to the problem. While the gold standard of open mitral valve repair is 1+ regurgitation or less, those promoting percutaneous valve replacement are willing to accept long term 1+ to 2+ regurgitation. New technology and innovation is critical in medicine, provided the results are at least equivalent or superior to the standard techniques.
SNOWMASS, COLO. – Percutaneous mitral valve replacement is unlikely to ever catch on in any way remotely approaching that of transcatheter aortic valve replacement for the treatment of aortic stenosis, Blase A. Carabello, MD, predicted at the Annual Cardiovascular Conference at Snowmass.
“We’ve spent $2 billion looking for methods of percutaneous mitral valve replacement, and yet, I have to wonder if that makes any sense,” said Dr. Carabello, professor of medicine and chief of cardiology at East Carolina University in Greenville, N.C.
“If repair is superior to replacement in primary MR [mitral regurgitation], which I think we all agree is true, and you don’t need to get rid of every last molecule of blood going backward across the mitral valve when you’ve got a good left ventricle, then a percutaneous replacement in primary MR would have only the niche of patients who are inoperable and whose leaflets can’t be grabbed by the MitraClip or some new percutaneous device down the road. And, in secondary MR, it doesn’t seem to matter whether you replace or repair the valve, so why not just repair it with a clip?” he argued.
Numerous nonrandomized studies have invariably demonstrated superior survival for surgical repair versus replacement in patients with primary MR.
“There’s never going to be a randomized controlled trial of repair versus replacement; there’s no equipoise there. We all believe that, in primary MR, repair is superior to replacement. There are no data anywhere to suggest the opposite. It’s essentially sacrosanct,” according to the cardiologist.
In contrast, a major randomized trial of surgical repair versus replacement has been conducted in patients with severe secondary MR. This NIH-funded study conducted by the Cardiothoracic Surgical Trials Network found no difference in survival between the two groups (N Engl J Med. 2016 Jan 28; 374[4]:344-53). That’s not a surprising result, Dr. Carabello said, since the underlying cause of this type of valve disease is a sick left ventricle. But, since surgical repair entails less morbidity than replacement – and a percutaneous repair with a leaflet-grasping device such as the MitraClip is simpler and safer than a surgical repair – it seems likely that the future treatment for secondary MR will be a percutaneous device, he said.
That future could depend upon the results of the ongoing COAPT trial (Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy), in which the MitraClip is being studied as an alternative to surgical repair for significant secondary MR. The MitraClip, which doesn’t entail a concomitant annuloplasty, is currently approved by the Food and Drug Administration only for patients with primary, degenerative mitral regurgitation not amenable to surgical repair. But, if COAPT yields positive results, the role of the MitraClip will greatly expand.
An intriguing and poorly understood difference exists in the significance of residual mitral regurgitation following surgical repair as opposed to percutaneous MitraClip repair, Dr. Carabello observed.
“I go to the OR a lot, and I know of no surgeon [who] will leave 2+ MR behind. Most surgeons won’t leave 1+ MR behind. They’ll put the patient back on the pump to repair even mild residual MR, accepting only trace MR or zero before they leave the OR because they know that the best predictor of a failed mitral repair is the presence of residual MR in the OR,” he said.
In contrast, following successful deployment of the MitraClip most patients are left with 1-2+ MR. Yet, as was demonstrated in the 5-year results of the randomized EVEREST II trial (Endovascular Valve Edge-to-Edge Repair Study), this residual MR wasn’t a harbinger of poor outcomes long-term (J Am Coll Cardiol. 2015 Dec 29;66[25]:2844-54).
“You would have expected, with that much residual MR, there would be a perpetually increasing failure rate over time, but that didn’t happen. In Everest II, there was an early failure rate for percutaneous repair, where the MitraClip didn’t work and those patients required surgical mitral valve repair. But, after the first 6 months, the failure rate for the clip was exactly the same as the surgical failure rate, even though, with the clip, you start with more MR to begin with,” the cardiologist noted.
The MitraClip procedure is modeled after the surgical Alfieri double-orifice end-to-end stitch technique, which has been shown to have durable results when performed in conjunction with an annuloplasty ring for primary MR.
“The MitraClip essentially joins the valve in the middle the way the Alfieri stitch does, but it doesn’t appear to behave the same way. Why is that? Maybe the clip does something different than the Alfieri stitch on which it was modeled. Maybe that bar in the middle of the mitral valve does something in terms of scarring or stabilization that we don’t know about yet,” he speculated.
As for the prospects for percutaneous mitral valve replacement, Dr. Carabello said that this type of procedure “is a very difficult thing to do, and so far, has been met with a fair amount of failure. It’ll be very interesting to see what percentage of market share it gets 10 years down the road. My prediction is that, for mitral regurgitation, repair is always going to be it.”
Dr. Carabello reported serving on a data safety monitoring board for Edwards Lifesciences.
SNOWMASS, COLO. – Percutaneous mitral valve replacement is unlikely to ever catch on in any way remotely approaching that of transcatheter aortic valve replacement for the treatment of aortic stenosis, Blase A. Carabello, MD, predicted at the Annual Cardiovascular Conference at Snowmass.
“We’ve spent $2 billion looking for methods of percutaneous mitral valve replacement, and yet, I have to wonder if that makes any sense,” said Dr. Carabello, professor of medicine and chief of cardiology at East Carolina University in Greenville, N.C.
“If repair is superior to replacement in primary MR [mitral regurgitation], which I think we all agree is true, and you don’t need to get rid of every last molecule of blood going backward across the mitral valve when you’ve got a good left ventricle, then a percutaneous replacement in primary MR would have only the niche of patients who are inoperable and whose leaflets can’t be grabbed by the MitraClip or some new percutaneous device down the road. And, in secondary MR, it doesn’t seem to matter whether you replace or repair the valve, so why not just repair it with a clip?” he argued.
Numerous nonrandomized studies have invariably demonstrated superior survival for surgical repair versus replacement in patients with primary MR.
“There’s never going to be a randomized controlled trial of repair versus replacement; there’s no equipoise there. We all believe that, in primary MR, repair is superior to replacement. There are no data anywhere to suggest the opposite. It’s essentially sacrosanct,” according to the cardiologist.
In contrast, a major randomized trial of surgical repair versus replacement has been conducted in patients with severe secondary MR. This NIH-funded study conducted by the Cardiothoracic Surgical Trials Network found no difference in survival between the two groups (N Engl J Med. 2016 Jan 28; 374[4]:344-53). That’s not a surprising result, Dr. Carabello said, since the underlying cause of this type of valve disease is a sick left ventricle. But, since surgical repair entails less morbidity than replacement – and a percutaneous repair with a leaflet-grasping device such as the MitraClip is simpler and safer than a surgical repair – it seems likely that the future treatment for secondary MR will be a percutaneous device, he said.
That future could depend upon the results of the ongoing COAPT trial (Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy), in which the MitraClip is being studied as an alternative to surgical repair for significant secondary MR. The MitraClip, which doesn’t entail a concomitant annuloplasty, is currently approved by the Food and Drug Administration only for patients with primary, degenerative mitral regurgitation not amenable to surgical repair. But, if COAPT yields positive results, the role of the MitraClip will greatly expand.
An intriguing and poorly understood difference exists in the significance of residual mitral regurgitation following surgical repair as opposed to percutaneous MitraClip repair, Dr. Carabello observed.
“I go to the OR a lot, and I know of no surgeon [who] will leave 2+ MR behind. Most surgeons won’t leave 1+ MR behind. They’ll put the patient back on the pump to repair even mild residual MR, accepting only trace MR or zero before they leave the OR because they know that the best predictor of a failed mitral repair is the presence of residual MR in the OR,” he said.
In contrast, following successful deployment of the MitraClip most patients are left with 1-2+ MR. Yet, as was demonstrated in the 5-year results of the randomized EVEREST II trial (Endovascular Valve Edge-to-Edge Repair Study), this residual MR wasn’t a harbinger of poor outcomes long-term (J Am Coll Cardiol. 2015 Dec 29;66[25]:2844-54).
“You would have expected, with that much residual MR, there would be a perpetually increasing failure rate over time, but that didn’t happen. In Everest II, there was an early failure rate for percutaneous repair, where the MitraClip didn’t work and those patients required surgical mitral valve repair. But, after the first 6 months, the failure rate for the clip was exactly the same as the surgical failure rate, even though, with the clip, you start with more MR to begin with,” the cardiologist noted.
The MitraClip procedure is modeled after the surgical Alfieri double-orifice end-to-end stitch technique, which has been shown to have durable results when performed in conjunction with an annuloplasty ring for primary MR.
“The MitraClip essentially joins the valve in the middle the way the Alfieri stitch does, but it doesn’t appear to behave the same way. Why is that? Maybe the clip does something different than the Alfieri stitch on which it was modeled. Maybe that bar in the middle of the mitral valve does something in terms of scarring or stabilization that we don’t know about yet,” he speculated.
As for the prospects for percutaneous mitral valve replacement, Dr. Carabello said that this type of procedure “is a very difficult thing to do, and so far, has been met with a fair amount of failure. It’ll be very interesting to see what percentage of market share it gets 10 years down the road. My prediction is that, for mitral regurgitation, repair is always going to be it.”
Dr. Carabello reported serving on a data safety monitoring board for Edwards Lifesciences.
Postoperative pain in women with preexisting chronic pain
Chronic pain disorders have reached epidemic levels in the United States, with the Institute of Medicine reporting more than 100 million Americans affected and health care costs more than $500 billion annually.1 Although many pain disorders are confined to the abdomen or pelvis (chronic pelvic pain, vulvodynia, irritable bowel syndrome, and bladder pain syndrome), others present with global symptoms (fibromyalgia and chronic fatigue syndrome). Women are more likely to be diagnosed with a chronic pain condition and more likely to seek treatment for chronic pain, including undergoing a surgical intervention. In fact, chronic pelvic pain alone affects upward of 20% of women in the United States, and, of the 400,000 hysterectomies performed each year (54.2%, abdominal; 16.7%, vaginal; and 16.8%, laparoscopic/robotic assisted), approximately 15% are for chronic pain.2
Neurobiology of pain
Perioperative pain control, specifically in women with preexisting pain disorders, can provide an additional challenge. Unlike acute pain, chronic pain (lasting more than 6 months) is associated with an amplified pain response of the central nervous system. This abnormal pain processing, known as centralization of pain, may result in a decrease of the inhibitory pain pathways and/or an increase of the amplification pathways, often augmenting the pain response of the original peripheral insult, specifically surgery. Because of these physiologic changes, a multimodal approach to perioperative pain should be offered, especially in women with preexisting pain. The approach ideally ought to target the different mechanisms of actions in both the peripheral and central nervous systems to provide an overall reduction in pain perception.
Preoperative visit
Perhaps the most underutilized opportunity to optimize postoperative pain is a proactive, preoperative approach. Preoperative education, including goal setting of postoperative pain expectations, has been associated with a significant reduction in postoperative opioid use, less preoperative anxiety, and a decreased length of surgical stay.3 While it is unknown exactly when this should be provided to the patient in the treatment course, it should occur prior to the day of surgery to allow for appropriate intervention.
The use of a shared decision-making model between the clinician and the chronic pain patient in the development of a pain management plan has been highly successful in improving pain outcomes in the outpatient setting.4 A similar method can be applied to the preoperative course as well. A detailed history (including the use of an opioid risk assessment tool) allows the clinician to identify patients at risk for opioid misuse and abuse. This is also an opportunity to review a plan for an opioid taper with the patient and the prescriber, if the postoperative plan includes opioid reduction/cessation. The preoperative visit may be an opportunity to adjust centrally acting medications (antidepressants, anticonvulsants) before surgery or to reduce the dose or frequency of high-risk medications, such as benzodiazepines.
Perioperative strategy
One of the most impactful ways for us, as surgeons, to reduce tissue injury and decrease pain from surgery is by offering a minimally invasive approach. The benefits of minimally invasive surgery are well established, resulting in improved perioperative pain control, decreased blood loss, lower infection rates, decreased length of hospital stay, and a faster recovery, compared with laparotomy. Because patients with chronic pain disorders are at increased risk of greater acute postoperative pain and have an elevated risk for the development of chronic postsurgical pain, a minimally invasive surgical approach should be prioritized, when available.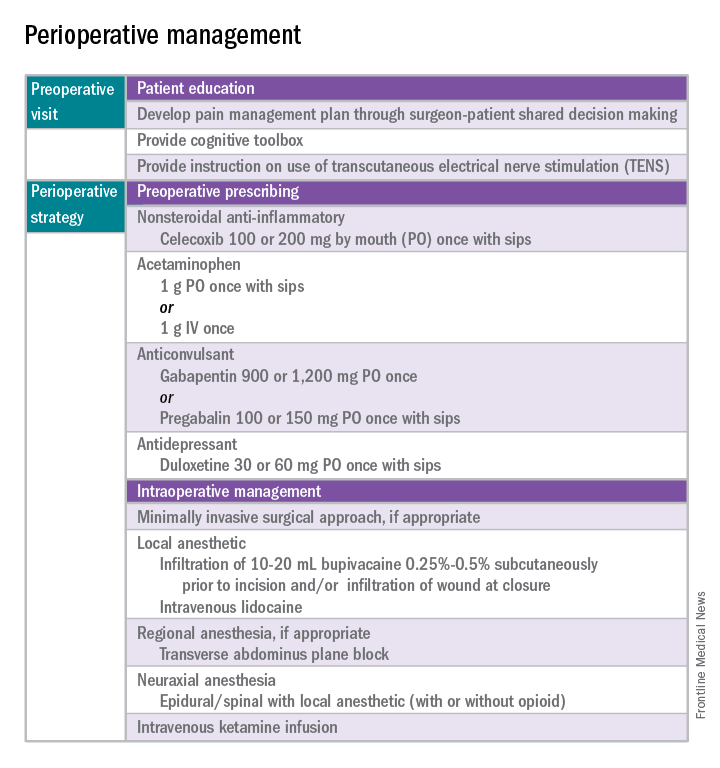
Perioperative multimodal drug therapy is associated with significant decreases in opioid consumption and reductions in acute postoperative pain.6 Recently, a multidisciplinary expert panel from the American Pain Society devised an evidence-based clinical practice guideline for postoperative pain.7 While there is no consensus as to the best regimen specific to gynecologic surgery, the general principles are similar across disciplines.
The postoperative period
Opioid-tolerant patients may experience greater pain during the first 24 hours postoperatively and require an increase in opioids, compared with opioid-naive patients.8 In the event that a postoperative patient does not respond as expected to the usual course, that patient should be evaluated for barriers to routine postoperative care, such as a surgical complication, opioid tolerance, or psychological distress. Surgeons should be aggressive with pain management immediately after surgery, even in the opioid-tolerant patient, and make short-term adjustments as needed based on the pain response. These patients will require pain medications beyond their baseline dose. Additionally, if an opioid taper is not planned in a chronic opioid user, work with the patient and the long-term opioid prescriber in restarting baseline opioid therapy outside of the acute surgical window. 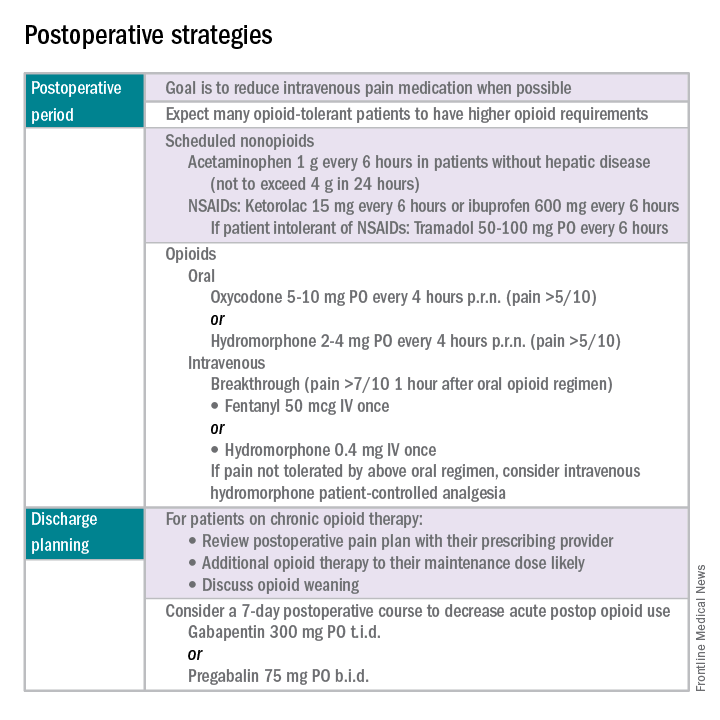
References
1. Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: The National Academies Press, 2011.
2. Obstet Gynecol. 2013 Aug;122(2 Pt 1):233-41.
3. N Engl J Med. 1964 Apr 16;270:825-7.
4. J Pain Symptom Manage. 1999 Jul;18(1):38-48.
5. Pain. 2010 Dec;151(3):694-702.
6. Anesthesiology. 2005 Dec;103(6):1296-304.
7. J Pain. 2016 Feb;17(2):131-57.
8. Pharmacotherapy. 2008 Dec;28(12):1453-60.
Dr. Carey is the director of minimally invasive gynecologic surgery at the University of North Carolina at Chapel Hill, and specializes in the medical and surgical management of pelvic pain disorders. Dr. Rossi is an assistant professor in the division of gynecologic oncology at UNC–Chapel Hill. They reported having no relevant financial disclosures.
Chronic pain disorders have reached epidemic levels in the United States, with the Institute of Medicine reporting more than 100 million Americans affected and health care costs more than $500 billion annually.1 Although many pain disorders are confined to the abdomen or pelvis (chronic pelvic pain, vulvodynia, irritable bowel syndrome, and bladder pain syndrome), others present with global symptoms (fibromyalgia and chronic fatigue syndrome). Women are more likely to be diagnosed with a chronic pain condition and more likely to seek treatment for chronic pain, including undergoing a surgical intervention. In fact, chronic pelvic pain alone affects upward of 20% of women in the United States, and, of the 400,000 hysterectomies performed each year (54.2%, abdominal; 16.7%, vaginal; and 16.8%, laparoscopic/robotic assisted), approximately 15% are for chronic pain.2
Neurobiology of pain
Perioperative pain control, specifically in women with preexisting pain disorders, can provide an additional challenge. Unlike acute pain, chronic pain (lasting more than 6 months) is associated with an amplified pain response of the central nervous system. This abnormal pain processing, known as centralization of pain, may result in a decrease of the inhibitory pain pathways and/or an increase of the amplification pathways, often augmenting the pain response of the original peripheral insult, specifically surgery. Because of these physiologic changes, a multimodal approach to perioperative pain should be offered, especially in women with preexisting pain. The approach ideally ought to target the different mechanisms of actions in both the peripheral and central nervous systems to provide an overall reduction in pain perception.
Preoperative visit
Perhaps the most underutilized opportunity to optimize postoperative pain is a proactive, preoperative approach. Preoperative education, including goal setting of postoperative pain expectations, has been associated with a significant reduction in postoperative opioid use, less preoperative anxiety, and a decreased length of surgical stay.3 While it is unknown exactly when this should be provided to the patient in the treatment course, it should occur prior to the day of surgery to allow for appropriate intervention.
The use of a shared decision-making model between the clinician and the chronic pain patient in the development of a pain management plan has been highly successful in improving pain outcomes in the outpatient setting.4 A similar method can be applied to the preoperative course as well. A detailed history (including the use of an opioid risk assessment tool) allows the clinician to identify patients at risk for opioid misuse and abuse. This is also an opportunity to review a plan for an opioid taper with the patient and the prescriber, if the postoperative plan includes opioid reduction/cessation. The preoperative visit may be an opportunity to adjust centrally acting medications (antidepressants, anticonvulsants) before surgery or to reduce the dose or frequency of high-risk medications, such as benzodiazepines.
Perioperative strategy
One of the most impactful ways for us, as surgeons, to reduce tissue injury and decrease pain from surgery is by offering a minimally invasive approach. The benefits of minimally invasive surgery are well established, resulting in improved perioperative pain control, decreased blood loss, lower infection rates, decreased length of hospital stay, and a faster recovery, compared with laparotomy. Because patients with chronic pain disorders are at increased risk of greater acute postoperative pain and have an elevated risk for the development of chronic postsurgical pain, a minimally invasive surgical approach should be prioritized, when available.
Perioperative multimodal drug therapy is associated with significant decreases in opioid consumption and reductions in acute postoperative pain.6 Recently, a multidisciplinary expert panel from the American Pain Society devised an evidence-based clinical practice guideline for postoperative pain.7 While there is no consensus as to the best regimen specific to gynecologic surgery, the general principles are similar across disciplines.
The postoperative period
Opioid-tolerant patients may experience greater pain during the first 24 hours postoperatively and require an increase in opioids, compared with opioid-naive patients.8 In the event that a postoperative patient does not respond as expected to the usual course, that patient should be evaluated for barriers to routine postoperative care, such as a surgical complication, opioid tolerance, or psychological distress. Surgeons should be aggressive with pain management immediately after surgery, even in the opioid-tolerant patient, and make short-term adjustments as needed based on the pain response. These patients will require pain medications beyond their baseline dose. Additionally, if an opioid taper is not planned in a chronic opioid user, work with the patient and the long-term opioid prescriber in restarting baseline opioid therapy outside of the acute surgical window. 
References
1. Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: The National Academies Press, 2011.
2. Obstet Gynecol. 2013 Aug;122(2 Pt 1):233-41.
3. N Engl J Med. 1964 Apr 16;270:825-7.
4. J Pain Symptom Manage. 1999 Jul;18(1):38-48.
5. Pain. 2010 Dec;151(3):694-702.
6. Anesthesiology. 2005 Dec;103(6):1296-304.
7. J Pain. 2016 Feb;17(2):131-57.
8. Pharmacotherapy. 2008 Dec;28(12):1453-60.
Dr. Carey is the director of minimally invasive gynecologic surgery at the University of North Carolina at Chapel Hill, and specializes in the medical and surgical management of pelvic pain disorders. Dr. Rossi is an assistant professor in the division of gynecologic oncology at UNC–Chapel Hill. They reported having no relevant financial disclosures.
Chronic pain disorders have reached epidemic levels in the United States, with the Institute of Medicine reporting more than 100 million Americans affected and health care costs more than $500 billion annually.1 Although many pain disorders are confined to the abdomen or pelvis (chronic pelvic pain, vulvodynia, irritable bowel syndrome, and bladder pain syndrome), others present with global symptoms (fibromyalgia and chronic fatigue syndrome). Women are more likely to be diagnosed with a chronic pain condition and more likely to seek treatment for chronic pain, including undergoing a surgical intervention. In fact, chronic pelvic pain alone affects upward of 20% of women in the United States, and, of the 400,000 hysterectomies performed each year (54.2%, abdominal; 16.7%, vaginal; and 16.8%, laparoscopic/robotic assisted), approximately 15% are for chronic pain.2
Neurobiology of pain
Perioperative pain control, specifically in women with preexisting pain disorders, can provide an additional challenge. Unlike acute pain, chronic pain (lasting more than 6 months) is associated with an amplified pain response of the central nervous system. This abnormal pain processing, known as centralization of pain, may result in a decrease of the inhibitory pain pathways and/or an increase of the amplification pathways, often augmenting the pain response of the original peripheral insult, specifically surgery. Because of these physiologic changes, a multimodal approach to perioperative pain should be offered, especially in women with preexisting pain. The approach ideally ought to target the different mechanisms of actions in both the peripheral and central nervous systems to provide an overall reduction in pain perception.
Preoperative visit
Perhaps the most underutilized opportunity to optimize postoperative pain is a proactive, preoperative approach. Preoperative education, including goal setting of postoperative pain expectations, has been associated with a significant reduction in postoperative opioid use, less preoperative anxiety, and a decreased length of surgical stay.3 While it is unknown exactly when this should be provided to the patient in the treatment course, it should occur prior to the day of surgery to allow for appropriate intervention.
The use of a shared decision-making model between the clinician and the chronic pain patient in the development of a pain management plan has been highly successful in improving pain outcomes in the outpatient setting.4 A similar method can be applied to the preoperative course as well. A detailed history (including the use of an opioid risk assessment tool) allows the clinician to identify patients at risk for opioid misuse and abuse. This is also an opportunity to review a plan for an opioid taper with the patient and the prescriber, if the postoperative plan includes opioid reduction/cessation. The preoperative visit may be an opportunity to adjust centrally acting medications (antidepressants, anticonvulsants) before surgery or to reduce the dose or frequency of high-risk medications, such as benzodiazepines.
Perioperative strategy
One of the most impactful ways for us, as surgeons, to reduce tissue injury and decrease pain from surgery is by offering a minimally invasive approach. The benefits of minimally invasive surgery are well established, resulting in improved perioperative pain control, decreased blood loss, lower infection rates, decreased length of hospital stay, and a faster recovery, compared with laparotomy. Because patients with chronic pain disorders are at increased risk of greater acute postoperative pain and have an elevated risk for the development of chronic postsurgical pain, a minimally invasive surgical approach should be prioritized, when available.
Perioperative multimodal drug therapy is associated with significant decreases in opioid consumption and reductions in acute postoperative pain.6 Recently, a multidisciplinary expert panel from the American Pain Society devised an evidence-based clinical practice guideline for postoperative pain.7 While there is no consensus as to the best regimen specific to gynecologic surgery, the general principles are similar across disciplines.
The postoperative period
Opioid-tolerant patients may experience greater pain during the first 24 hours postoperatively and require an increase in opioids, compared with opioid-naive patients.8 In the event that a postoperative patient does not respond as expected to the usual course, that patient should be evaluated for barriers to routine postoperative care, such as a surgical complication, opioid tolerance, or psychological distress. Surgeons should be aggressive with pain management immediately after surgery, even in the opioid-tolerant patient, and make short-term adjustments as needed based on the pain response. These patients will require pain medications beyond their baseline dose. Additionally, if an opioid taper is not planned in a chronic opioid user, work with the patient and the long-term opioid prescriber in restarting baseline opioid therapy outside of the acute surgical window. 
References
1. Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: The National Academies Press, 2011.
2. Obstet Gynecol. 2013 Aug;122(2 Pt 1):233-41.
3. N Engl J Med. 1964 Apr 16;270:825-7.
4. J Pain Symptom Manage. 1999 Jul;18(1):38-48.
5. Pain. 2010 Dec;151(3):694-702.
6. Anesthesiology. 2005 Dec;103(6):1296-304.
7. J Pain. 2016 Feb;17(2):131-57.
8. Pharmacotherapy. 2008 Dec;28(12):1453-60.
Dr. Carey is the director of minimally invasive gynecologic surgery at the University of North Carolina at Chapel Hill, and specializes in the medical and surgical management of pelvic pain disorders. Dr. Rossi is an assistant professor in the division of gynecologic oncology at UNC–Chapel Hill. They reported having no relevant financial disclosures.
CMS nominee Verma clears Senate Finance hurdle
Seema Verma has moved one step closer to becoming the administrator of the Centers for Medicare & Medicaid Services.
The Senate Finance Committee voted 13-12 on March 2 to approve Ms. Verma’s nomination after a delayed vote the day before. The vote, conducted during a meeting off the floor, was a straight party-line vote, with 13 Republicans voting for Ms. Verma and 12 Democrats voting against. One proxy vote was not counted into the final tally per Senate rules.
Her nomination will now be considered by the full Senate.
Senate Finance Committee Chairman Orrin Hatch (R-Utah) praised Ms. Verma as a qualified leader who will help improve CMS.
Senate Finance Committee Ranking Member Ron Wyden, (D-Ore.) denounced Ms. Verma, stressing that she failed to adequately answer questions during her nomination hearing and has presented no clear vision of her plans as the next CMS administrator.
During her nomination hearing on Feb. 16, Ms. Verma came under fire for past consulting agreements with states while working for Hewlett Packard, a company that had financial interests in the health programs she designed. The issue was raised again during a preliminary vote by the Finance Committee on March 1.
“Ms. Verma was on both sides of the deal, helping manage the state’s health programs while being paid by vendors to those same programs,” Sen. Wyden said during the hearing. “I am concerned that if Ms. Verma is confirmed to lead CMS, where many of the companies she worked for are major vendors, there will not be adequate scrutiny of her past relationships with them, just as there wasn’t in Indiana.”
Ms. Verma previously said she never negotiated on behalf of Hewlett Packard and that the work she conducted for the states did not overlap with work she completed for HP.
“I hold honesty and integrity and adherence to a high ethical standard as part of my personal philosophy. That’s for me, I demand that from my employees, and I set that example for my own children,” she said during the Feb. 16 hearing. “We were never in a position where we were negotiating on behalf of HP or any other contractor with the state that we had a relationship with. If there was the potential [for a conflict], we would recuse ourselves.”
Ms. Verma’s nomination will now move to the full Senate. No date has yet been set for the vote.
[email protected]
On Twitter @legal_med
Seema Verma has moved one step closer to becoming the administrator of the Centers for Medicare & Medicaid Services.
The Senate Finance Committee voted 13-12 on March 2 to approve Ms. Verma’s nomination after a delayed vote the day before. The vote, conducted during a meeting off the floor, was a straight party-line vote, with 13 Republicans voting for Ms. Verma and 12 Democrats voting against. One proxy vote was not counted into the final tally per Senate rules.
Her nomination will now be considered by the full Senate.
Senate Finance Committee Chairman Orrin Hatch (R-Utah) praised Ms. Verma as a qualified leader who will help improve CMS.
Senate Finance Committee Ranking Member Ron Wyden, (D-Ore.) denounced Ms. Verma, stressing that she failed to adequately answer questions during her nomination hearing and has presented no clear vision of her plans as the next CMS administrator.
During her nomination hearing on Feb. 16, Ms. Verma came under fire for past consulting agreements with states while working for Hewlett Packard, a company that had financial interests in the health programs she designed. The issue was raised again during a preliminary vote by the Finance Committee on March 1.
“Ms. Verma was on both sides of the deal, helping manage the state’s health programs while being paid by vendors to those same programs,” Sen. Wyden said during the hearing. “I am concerned that if Ms. Verma is confirmed to lead CMS, where many of the companies she worked for are major vendors, there will not be adequate scrutiny of her past relationships with them, just as there wasn’t in Indiana.”
Ms. Verma previously said she never negotiated on behalf of Hewlett Packard and that the work she conducted for the states did not overlap with work she completed for HP.
“I hold honesty and integrity and adherence to a high ethical standard as part of my personal philosophy. That’s for me, I demand that from my employees, and I set that example for my own children,” she said during the Feb. 16 hearing. “We were never in a position where we were negotiating on behalf of HP or any other contractor with the state that we had a relationship with. If there was the potential [for a conflict], we would recuse ourselves.”
Ms. Verma’s nomination will now move to the full Senate. No date has yet been set for the vote.
[email protected]
On Twitter @legal_med
Seema Verma has moved one step closer to becoming the administrator of the Centers for Medicare & Medicaid Services.
The Senate Finance Committee voted 13-12 on March 2 to approve Ms. Verma’s nomination after a delayed vote the day before. The vote, conducted during a meeting off the floor, was a straight party-line vote, with 13 Republicans voting for Ms. Verma and 12 Democrats voting against. One proxy vote was not counted into the final tally per Senate rules.
Her nomination will now be considered by the full Senate.
Senate Finance Committee Chairman Orrin Hatch (R-Utah) praised Ms. Verma as a qualified leader who will help improve CMS.
Senate Finance Committee Ranking Member Ron Wyden, (D-Ore.) denounced Ms. Verma, stressing that she failed to adequately answer questions during her nomination hearing and has presented no clear vision of her plans as the next CMS administrator.
During her nomination hearing on Feb. 16, Ms. Verma came under fire for past consulting agreements with states while working for Hewlett Packard, a company that had financial interests in the health programs she designed. The issue was raised again during a preliminary vote by the Finance Committee on March 1.
“Ms. Verma was on both sides of the deal, helping manage the state’s health programs while being paid by vendors to those same programs,” Sen. Wyden said during the hearing. “I am concerned that if Ms. Verma is confirmed to lead CMS, where many of the companies she worked for are major vendors, there will not be adequate scrutiny of her past relationships with them, just as there wasn’t in Indiana.”
Ms. Verma previously said she never negotiated on behalf of Hewlett Packard and that the work she conducted for the states did not overlap with work she completed for HP.
“I hold honesty and integrity and adherence to a high ethical standard as part of my personal philosophy. That’s for me, I demand that from my employees, and I set that example for my own children,” she said during the Feb. 16 hearing. “We were never in a position where we were negotiating on behalf of HP or any other contractor with the state that we had a relationship with. If there was the potential [for a conflict], we would recuse ourselves.”
Ms. Verma’s nomination will now move to the full Senate. No date has yet been set for the vote.
[email protected]
On Twitter @legal_med
Standardize opioid prescribing after endocrine neck surgery, researchers say
Twenty oral morphine equivalents is the best option for pain relief medication with which to discharge outpatients after thyroidectomy or parathyroidectomy surgery, according to researchers. The report was published online in Annals of Surgical Oncology.
Irene Lou, MD, of the department of surgery at the University of Wisconsin–Madison, and her coauthors conducted a prospective observational cohort study collecting data on pain scores and the oral morphine equivalents used by 313 adult patients undergoing thyroidectomy or parathyroidectomy at two large endocrine surgery centers.
While patients were prescribed a median of 30 oral morphine equivalents at discharge – with a range from 0 to 120 – the median number of equivalents taken was 3 (with a range of 0-60).
Overall, 68.4% of patients took at least one oral morphine equivalent. The majority of patients (83%) took 10 or fewer oral morphine equivalents, and only 7% of patients took more than 20 oral morphine equivalents (Ann Surg Oncol. 2017 Feb 3. doi: 10.1245/s10434-017-5781-y).
Among the patients who took more than 10 oral morphine equivalents, 85% said it was for incisional pain, 4% said it was for sore throat, and 11% said it was for some other pain.
While the overall mean pain score after surgery was 2, the study found that mean pain scores in the patients who took more than 10 oral morphine equivalents were significantly higher than in patients who took 10 or fewer. Among patients who used narcotic pain relief, 1% said they did so because they were instructed to despite having reported no pain.
Other factors predicting higher oral morphine equivalent use were age – patients tended to be younger than 45 years – total thyroidectomy, or a history of previous narcotic use.
“Based on our results, we have changed our practices to discharge all patients undergoing parathyroid or thyroid surgery and to request an oral narcotic prescription with no more than 20 equivalents, which translates to 20 tablets of hydrocodone/acetaminophen 5/325” the authors wrote.
Noting that the abuse and misuse of prescription opioids is the leading cause of overdose deaths in the United States, they argued that standardized prescribing practices are a way to not only reduce waste but also to improve patient safety.
“We also discovered that even between our two institutions, there was no standard prescribing pattern, with a wide range of prescriptions and number of equivalents dispensed.”
The authors also examined alternative and adjunctive methods of pain relief, pointing to previous studies suggesting benefits from preoperative gabapentin, postoperative music therapy, postoperative ice packs, and nonopioid analgesics.
They noted that because their study covered the breadth of endocrine neck operations, it did include patients who had minimally invasive surgery through to those who underwent total thyroidectomy with neck dissections. They also pointed out that the data pain scores and oral morphine equivalent use was based on patient recollection.
“Notwithstanding these limitations, our study is the first to examine outpatient narcotic pain medication use after thyroid and parathyroid surgery,” they said. “A standardized practice of prescribing stands to increase patient safety and minimize the risks of dependence and overdose.”
Two authors were supported by National Institutes of Health grants. No other conflicts of interest were declared.
Twenty oral morphine equivalents is the best option for pain relief medication with which to discharge outpatients after thyroidectomy or parathyroidectomy surgery, according to researchers. The report was published online in Annals of Surgical Oncology.
Irene Lou, MD, of the department of surgery at the University of Wisconsin–Madison, and her coauthors conducted a prospective observational cohort study collecting data on pain scores and the oral morphine equivalents used by 313 adult patients undergoing thyroidectomy or parathyroidectomy at two large endocrine surgery centers.
While patients were prescribed a median of 30 oral morphine equivalents at discharge – with a range from 0 to 120 – the median number of equivalents taken was 3 (with a range of 0-60).
Overall, 68.4% of patients took at least one oral morphine equivalent. The majority of patients (83%) took 10 or fewer oral morphine equivalents, and only 7% of patients took more than 20 oral morphine equivalents (Ann Surg Oncol. 2017 Feb 3. doi: 10.1245/s10434-017-5781-y).
Among the patients who took more than 10 oral morphine equivalents, 85% said it was for incisional pain, 4% said it was for sore throat, and 11% said it was for some other pain.
While the overall mean pain score after surgery was 2, the study found that mean pain scores in the patients who took more than 10 oral morphine equivalents were significantly higher than in patients who took 10 or fewer. Among patients who used narcotic pain relief, 1% said they did so because they were instructed to despite having reported no pain.
Other factors predicting higher oral morphine equivalent use were age – patients tended to be younger than 45 years – total thyroidectomy, or a history of previous narcotic use.
“Based on our results, we have changed our practices to discharge all patients undergoing parathyroid or thyroid surgery and to request an oral narcotic prescription with no more than 20 equivalents, which translates to 20 tablets of hydrocodone/acetaminophen 5/325” the authors wrote.
Noting that the abuse and misuse of prescription opioids is the leading cause of overdose deaths in the United States, they argued that standardized prescribing practices are a way to not only reduce waste but also to improve patient safety.
“We also discovered that even between our two institutions, there was no standard prescribing pattern, with a wide range of prescriptions and number of equivalents dispensed.”
The authors also examined alternative and adjunctive methods of pain relief, pointing to previous studies suggesting benefits from preoperative gabapentin, postoperative music therapy, postoperative ice packs, and nonopioid analgesics.
They noted that because their study covered the breadth of endocrine neck operations, it did include patients who had minimally invasive surgery through to those who underwent total thyroidectomy with neck dissections. They also pointed out that the data pain scores and oral morphine equivalent use was based on patient recollection.
“Notwithstanding these limitations, our study is the first to examine outpatient narcotic pain medication use after thyroid and parathyroid surgery,” they said. “A standardized practice of prescribing stands to increase patient safety and minimize the risks of dependence and overdose.”
Two authors were supported by National Institutes of Health grants. No other conflicts of interest were declared.
Twenty oral morphine equivalents is the best option for pain relief medication with which to discharge outpatients after thyroidectomy or parathyroidectomy surgery, according to researchers. The report was published online in Annals of Surgical Oncology.
Irene Lou, MD, of the department of surgery at the University of Wisconsin–Madison, and her coauthors conducted a prospective observational cohort study collecting data on pain scores and the oral morphine equivalents used by 313 adult patients undergoing thyroidectomy or parathyroidectomy at two large endocrine surgery centers.
While patients were prescribed a median of 30 oral morphine equivalents at discharge – with a range from 0 to 120 – the median number of equivalents taken was 3 (with a range of 0-60).
Overall, 68.4% of patients took at least one oral morphine equivalent. The majority of patients (83%) took 10 or fewer oral morphine equivalents, and only 7% of patients took more than 20 oral morphine equivalents (Ann Surg Oncol. 2017 Feb 3. doi: 10.1245/s10434-017-5781-y).
Among the patients who took more than 10 oral morphine equivalents, 85% said it was for incisional pain, 4% said it was for sore throat, and 11% said it was for some other pain.
While the overall mean pain score after surgery was 2, the study found that mean pain scores in the patients who took more than 10 oral morphine equivalents were significantly higher than in patients who took 10 or fewer. Among patients who used narcotic pain relief, 1% said they did so because they were instructed to despite having reported no pain.
Other factors predicting higher oral morphine equivalent use were age – patients tended to be younger than 45 years – total thyroidectomy, or a history of previous narcotic use.
“Based on our results, we have changed our practices to discharge all patients undergoing parathyroid or thyroid surgery and to request an oral narcotic prescription with no more than 20 equivalents, which translates to 20 tablets of hydrocodone/acetaminophen 5/325” the authors wrote.
Noting that the abuse and misuse of prescription opioids is the leading cause of overdose deaths in the United States, they argued that standardized prescribing practices are a way to not only reduce waste but also to improve patient safety.
“We also discovered that even between our two institutions, there was no standard prescribing pattern, with a wide range of prescriptions and number of equivalents dispensed.”
The authors also examined alternative and adjunctive methods of pain relief, pointing to previous studies suggesting benefits from preoperative gabapentin, postoperative music therapy, postoperative ice packs, and nonopioid analgesics.
They noted that because their study covered the breadth of endocrine neck operations, it did include patients who had minimally invasive surgery through to those who underwent total thyroidectomy with neck dissections. They also pointed out that the data pain scores and oral morphine equivalent use was based on patient recollection.
“Notwithstanding these limitations, our study is the first to examine outpatient narcotic pain medication use after thyroid and parathyroid surgery,” they said. “A standardized practice of prescribing stands to increase patient safety and minimize the risks of dependence and overdose.”
Two authors were supported by National Institutes of Health grants. No other conflicts of interest were declared.
FROM ANNALS OF SURGICAL ONCOLOGY
Key clinical point: Twenty oral morphine equivalents is the ideal amount of pain relief medication with which to discharge outpatients after thyroidectomy or parathyroidectomy surgery.
Major finding: Only 7% of patients who undergo thyroidectomy or parathyroidectomy use more than 20 oral morphine equivalents for postoperative pain relief.
Data source: Observational cohort study of 313 adult patients undergoing thyroidectomy or parathyroidectomy.
Disclosures: Two authors were supported by National Institutes of Health grants. No other conflicts of interest were declared.