User login
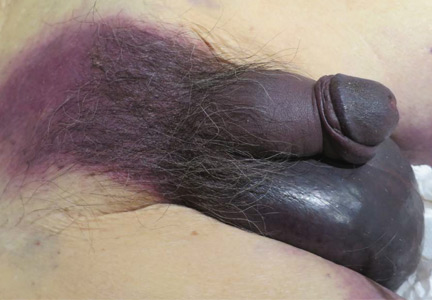
Fournier gangrene
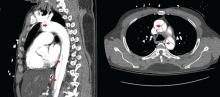
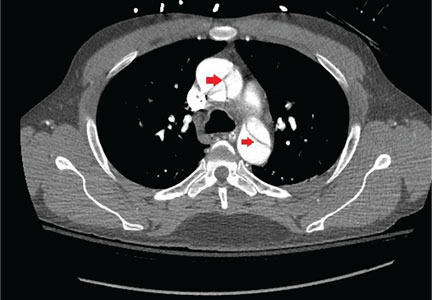
An 88-year-old man with a 1-day history of fever and altered mental status was transferred to the emergency department. He had been receiving conservative management for low-risk localized prostate cancer but had no previous cardiovascular or gastrointestinal problems.
Based on these findings, the diagnosis was Fournier gangrene. Despite aggressive treatment, the patient’s condition deteriorated rapidly, and he died 2 hours after admission.
FOURNIER GANGRENE: NECROTIZING FASCIITIS OF THE PERINEUM
Fournier gangrene is a rare but rapidly progressive necrotizing fasciitis of the perineum with a high death rate.
Predisposing factors for Fournier gangrene include older age, diabetes mellitus, morbid obesity, cardiovascular disorders, chronic alcoholism, long-term corticosteroid treatment, malignancy, and human immunodeficiency virus infection.1,2 Urethral obstruction, instrumentation, urinary extravasation, and trauma have also been associated with this condition.3
In general, organisms from the urinary tract spread along the fascial planes to involve the penis and scrotum.
The differential diagnosis of Fournier gangrene includes scrotal and perineal disorders, as well as intra-abdominal disorders such as cellulitis, abscess, strangulated hernia, pyoderma gangrenosum, allergic vasculitis, vascular occlusion syndromes, and warfarin necrosis.
Delay in the diagnosis of Fournier gangrene leads to an extremely high death rate due to rapid progression of the disease, leading to sepsis, multiple organ failure, and disseminated intravascular coagulation. Immediate diagnosis and appropriate treatment such as broad-spectrum antibiotics and extensive surgical debridement reduce morbidity and control the infection. Antibiotics for methicillin-resistant Staphylococcus aureus should be considered if there is a history of or risk factors for this organism.4
Necrotizing fasciitis, including Fournier gangrene, is a common indication for intravenous immunoglobulin, and this treatment has been reported to be effective in a few cases. However, a double-blind, placebo-controlled trial that evaluated the benefit of this treatment was terminated early due to slow patient recruitment.5
A delay of even a few hours from suspicion of Fournier gangrene to surgical debridement significantly increases the risk of death.6 Thus, when it is suspected, immediate surgical intervention may be necessary to confirm the diagnosis and to treat it. The usual combination of antibiotic therapy for Fournier gangrene includes penicillin for the streptococcal species, a third-generation cephalosporin with or without an aminoglycoside for the gram-negative organisms, and metronidazole for anaerobic bacteria.
- Wang YK, Li YH, Wu ST, Meng E. Fournier’s gangrene. QJM 2017; 110(10):671–672. doi:10.1093/qjmed/hcx124
- Yanar H, Taviloglu K, Ertekin C, et al. Fournier’s gangrene: risk factors and strategies for management. World J Surg 2006; 30(9):1750–1754. doi:10.1007/s00268-005-0777-3
- Paonam SS, Bag S. Fournier gangrene with extensive necrosis of urethra and bladder mucosa: a rare occurrence in a patient with advanced prostate cancer. Urol Ann 2015; 7(4):507–509. doi:10.4103/0974-7796.157975
- Brook I. Microbiology and management of soft tissue and muscle infections. Int J Surg 2008; 6(4):328–338. doi:10.1016/j.ijsu.2007.07.001
- Koch C, Hecker A, Grau V, Padberg W, Wolff M, Henrich M. Intravenous immunoglobulin in necrotizing fasciitis—a case report and review of recent literature. Ann Med Surg (Lond) 2015; 4(3):260–263. doi:10.1016/j.amsu.2015.07.017
- Singh A, Ahmed K, Aydin A, Khan MS, Dasgupta P. Fournier's gangrene. A clinical review. Arch Ital Urol Androl 2016; 88(3):157–164. doi:10.4081/aiua.2016.3.157
An 88-year-old man with a 1-day history of fever and altered mental status was transferred to the emergency department. He had been receiving conservative management for low-risk localized prostate cancer but had no previous cardiovascular or gastrointestinal problems.
Based on these findings, the diagnosis was Fournier gangrene. Despite aggressive treatment, the patient’s condition deteriorated rapidly, and he died 2 hours after admission.
FOURNIER GANGRENE: NECROTIZING FASCIITIS OF THE PERINEUM
Fournier gangrene is a rare but rapidly progressive necrotizing fasciitis of the perineum with a high death rate.
Predisposing factors for Fournier gangrene include older age, diabetes mellitus, morbid obesity, cardiovascular disorders, chronic alcoholism, long-term corticosteroid treatment, malignancy, and human immunodeficiency virus infection.1,2 Urethral obstruction, instrumentation, urinary extravasation, and trauma have also been associated with this condition.3
In general, organisms from the urinary tract spread along the fascial planes to involve the penis and scrotum.
The differential diagnosis of Fournier gangrene includes scrotal and perineal disorders, as well as intra-abdominal disorders such as cellulitis, abscess, strangulated hernia, pyoderma gangrenosum, allergic vasculitis, vascular occlusion syndromes, and warfarin necrosis.
Delay in the diagnosis of Fournier gangrene leads to an extremely high death rate due to rapid progression of the disease, leading to sepsis, multiple organ failure, and disseminated intravascular coagulation. Immediate diagnosis and appropriate treatment such as broad-spectrum antibiotics and extensive surgical debridement reduce morbidity and control the infection. Antibiotics for methicillin-resistant Staphylococcus aureus should be considered if there is a history of or risk factors for this organism.4
Necrotizing fasciitis, including Fournier gangrene, is a common indication for intravenous immunoglobulin, and this treatment has been reported to be effective in a few cases. However, a double-blind, placebo-controlled trial that evaluated the benefit of this treatment was terminated early due to slow patient recruitment.5
A delay of even a few hours from suspicion of Fournier gangrene to surgical debridement significantly increases the risk of death.6 Thus, when it is suspected, immediate surgical intervention may be necessary to confirm the diagnosis and to treat it. The usual combination of antibiotic therapy for Fournier gangrene includes penicillin for the streptococcal species, a third-generation cephalosporin with or without an aminoglycoside for the gram-negative organisms, and metronidazole for anaerobic bacteria.
An 88-year-old man with a 1-day history of fever and altered mental status was transferred to the emergency department. He had been receiving conservative management for low-risk localized prostate cancer but had no previous cardiovascular or gastrointestinal problems.
Based on these findings, the diagnosis was Fournier gangrene. Despite aggressive treatment, the patient’s condition deteriorated rapidly, and he died 2 hours after admission.
FOURNIER GANGRENE: NECROTIZING FASCIITIS OF THE PERINEUM
Fournier gangrene is a rare but rapidly progressive necrotizing fasciitis of the perineum with a high death rate.
Predisposing factors for Fournier gangrene include older age, diabetes mellitus, morbid obesity, cardiovascular disorders, chronic alcoholism, long-term corticosteroid treatment, malignancy, and human immunodeficiency virus infection.1,2 Urethral obstruction, instrumentation, urinary extravasation, and trauma have also been associated with this condition.3
In general, organisms from the urinary tract spread along the fascial planes to involve the penis and scrotum.
The differential diagnosis of Fournier gangrene includes scrotal and perineal disorders, as well as intra-abdominal disorders such as cellulitis, abscess, strangulated hernia, pyoderma gangrenosum, allergic vasculitis, vascular occlusion syndromes, and warfarin necrosis.
Delay in the diagnosis of Fournier gangrene leads to an extremely high death rate due to rapid progression of the disease, leading to sepsis, multiple organ failure, and disseminated intravascular coagulation. Immediate diagnosis and appropriate treatment such as broad-spectrum antibiotics and extensive surgical debridement reduce morbidity and control the infection. Antibiotics for methicillin-resistant Staphylococcus aureus should be considered if there is a history of or risk factors for this organism.4
Necrotizing fasciitis, including Fournier gangrene, is a common indication for intravenous immunoglobulin, and this treatment has been reported to be effective in a few cases. However, a double-blind, placebo-controlled trial that evaluated the benefit of this treatment was terminated early due to slow patient recruitment.5
A delay of even a few hours from suspicion of Fournier gangrene to surgical debridement significantly increases the risk of death.6 Thus, when it is suspected, immediate surgical intervention may be necessary to confirm the diagnosis and to treat it. The usual combination of antibiotic therapy for Fournier gangrene includes penicillin for the streptococcal species, a third-generation cephalosporin with or without an aminoglycoside for the gram-negative organisms, and metronidazole for anaerobic bacteria.
- Wang YK, Li YH, Wu ST, Meng E. Fournier’s gangrene. QJM 2017; 110(10):671–672. doi:10.1093/qjmed/hcx124
- Yanar H, Taviloglu K, Ertekin C, et al. Fournier’s gangrene: risk factors and strategies for management. World J Surg 2006; 30(9):1750–1754. doi:10.1007/s00268-005-0777-3
- Paonam SS, Bag S. Fournier gangrene with extensive necrosis of urethra and bladder mucosa: a rare occurrence in a patient with advanced prostate cancer. Urol Ann 2015; 7(4):507–509. doi:10.4103/0974-7796.157975
- Brook I. Microbiology and management of soft tissue and muscle infections. Int J Surg 2008; 6(4):328–338. doi:10.1016/j.ijsu.2007.07.001
- Koch C, Hecker A, Grau V, Padberg W, Wolff M, Henrich M. Intravenous immunoglobulin in necrotizing fasciitis—a case report and review of recent literature. Ann Med Surg (Lond) 2015; 4(3):260–263. doi:10.1016/j.amsu.2015.07.017
- Singh A, Ahmed K, Aydin A, Khan MS, Dasgupta P. Fournier's gangrene. A clinical review. Arch Ital Urol Androl 2016; 88(3):157–164. doi:10.4081/aiua.2016.3.157
- Wang YK, Li YH, Wu ST, Meng E. Fournier’s gangrene. QJM 2017; 110(10):671–672. doi:10.1093/qjmed/hcx124
- Yanar H, Taviloglu K, Ertekin C, et al. Fournier’s gangrene: risk factors and strategies for management. World J Surg 2006; 30(9):1750–1754. doi:10.1007/s00268-005-0777-3
- Paonam SS, Bag S. Fournier gangrene with extensive necrosis of urethra and bladder mucosa: a rare occurrence in a patient with advanced prostate cancer. Urol Ann 2015; 7(4):507–509. doi:10.4103/0974-7796.157975
- Brook I. Microbiology and management of soft tissue and muscle infections. Int J Surg 2008; 6(4):328–338. doi:10.1016/j.ijsu.2007.07.001
- Koch C, Hecker A, Grau V, Padberg W, Wolff M, Henrich M. Intravenous immunoglobulin in necrotizing fasciitis—a case report and review of recent literature. Ann Med Surg (Lond) 2015; 4(3):260–263. doi:10.1016/j.amsu.2015.07.017
- Singh A, Ahmed K, Aydin A, Khan MS, Dasgupta P. Fournier's gangrene. A clinical review. Arch Ital Urol Androl 2016; 88(3):157–164. doi:10.4081/aiua.2016.3.157
Aortic aneurysm: Fluoroquinolones, genetic counseling
To the Editor: We read with interest the article by Cikach et al on thoracic aortic aneurysm.1 For medical management of this condition, the authors emphasized controlling blood pressure and heart rate and also avoiding isometric exercises and heavy lifting. In addition to their recommendations, we believe there is plausible evidence to advise caution if fluoroquinolone antibiotics are used in this setting.
Three large population-based studies, from Canada,2 Taiwan,3 and Sweden,4 collectively demonstrated a significant 2-fold increase in the incidence of aortic aneurysm and dissection presenting within 60 days of fluoroquinolone use compared with other antibiotic exposure. Moreover, a longer duration of fluoroquinolone use was associated with a significantly higher incidence of aortic aneurysm and dissection.3
Mechanistically, fluoroquinolones have been shown to up-regulate production of several matrix metalloproteinases, including metalloproteinase 2, leading to degradation of type I collagen.2,5 Type I and type III are the dominant collagens in the aortic wall, and collagen degradation is implicated in aortic aneurysm formation and expansion.
Fluoroquinolones are widely prescribed in both outpatient and inpatient settings and are sometimes used for long durations in the geriatric population.2 It is possible that these drugs have a propensity to increase aortic aneurysm expansion and dissection in older patients who already have aortic aneurysm. Accordingly, this might make the risk-benefit ratio unfavorable for using these drugs in these situations, and other antibiotics should be used, if indicated.
Furthermore, if fluoroquinolones are used in patients with aortic aneurysm, perhaps imaging studies of the aneurysm should be done more frequently than once a year to detect accelerated aneurysm growth. Finally, physicians should be aware of the possibility of increased aortic aneurysm expansion and dissection with fluoroquinolone use.
- Cikach F, Desai MY, Roselli EE, Kalahasti V. Thoracic aortic aneurysm: how to counsel, when to refer. Cleve Clin J Med 2018; 85(6):481–492. doi:10.3949/ccjm.85a.17039
- Daneman N, Lu H, Redelmeier DA. Fluoroquinolones and collagen associated severe adverse events: a longitudinal cohort study. BMJ Open 2015; 5:e010077. doi:10.1136/bmjopen-2015-010077
- Lee C-C, Lee MG, Chen Y-S, et al. Risk of aortic dissection and aortic aneurysm in patients taking oral fluoroquinolone. JAMA Intern Med 2015; 175:1839–1847. doi:10.1001/jamainternmed.2015.5389
- Pasternak B, Inghammar M, Svanström H. Fluoroquinolone use and risk of aortic aneurysm and dissection: nationwide cohort study. BMJ Open 2018; 360:k678. doi:10.1136/bmj.k678
- Tsai W-C, Hsu C-C, Chen CPC, et al. Ciprofloxacin up-regulates tendon cells to express matrix metalloproteinase-2 with degradation of type I collagen. J Orthop Res 2011; 29(1):67–73. doi:10.1002/jor.21196
To the Editor: We read with interest the article by Cikach et al on thoracic aortic aneurysm.1 For medical management of this condition, the authors emphasized controlling blood pressure and heart rate and also avoiding isometric exercises and heavy lifting. In addition to their recommendations, we believe there is plausible evidence to advise caution if fluoroquinolone antibiotics are used in this setting.
Three large population-based studies, from Canada,2 Taiwan,3 and Sweden,4 collectively demonstrated a significant 2-fold increase in the incidence of aortic aneurysm and dissection presenting within 60 days of fluoroquinolone use compared with other antibiotic exposure. Moreover, a longer duration of fluoroquinolone use was associated with a significantly higher incidence of aortic aneurysm and dissection.3
Mechanistically, fluoroquinolones have been shown to up-regulate production of several matrix metalloproteinases, including metalloproteinase 2, leading to degradation of type I collagen.2,5 Type I and type III are the dominant collagens in the aortic wall, and collagen degradation is implicated in aortic aneurysm formation and expansion.
Fluoroquinolones are widely prescribed in both outpatient and inpatient settings and are sometimes used for long durations in the geriatric population.2 It is possible that these drugs have a propensity to increase aortic aneurysm expansion and dissection in older patients who already have aortic aneurysm. Accordingly, this might make the risk-benefit ratio unfavorable for using these drugs in these situations, and other antibiotics should be used, if indicated.
Furthermore, if fluoroquinolones are used in patients with aortic aneurysm, perhaps imaging studies of the aneurysm should be done more frequently than once a year to detect accelerated aneurysm growth. Finally, physicians should be aware of the possibility of increased aortic aneurysm expansion and dissection with fluoroquinolone use.
To the Editor: We read with interest the article by Cikach et al on thoracic aortic aneurysm.1 For medical management of this condition, the authors emphasized controlling blood pressure and heart rate and also avoiding isometric exercises and heavy lifting. In addition to their recommendations, we believe there is plausible evidence to advise caution if fluoroquinolone antibiotics are used in this setting.
Three large population-based studies, from Canada,2 Taiwan,3 and Sweden,4 collectively demonstrated a significant 2-fold increase in the incidence of aortic aneurysm and dissection presenting within 60 days of fluoroquinolone use compared with other antibiotic exposure. Moreover, a longer duration of fluoroquinolone use was associated with a significantly higher incidence of aortic aneurysm and dissection.3
Mechanistically, fluoroquinolones have been shown to up-regulate production of several matrix metalloproteinases, including metalloproteinase 2, leading to degradation of type I collagen.2,5 Type I and type III are the dominant collagens in the aortic wall, and collagen degradation is implicated in aortic aneurysm formation and expansion.
Fluoroquinolones are widely prescribed in both outpatient and inpatient settings and are sometimes used for long durations in the geriatric population.2 It is possible that these drugs have a propensity to increase aortic aneurysm expansion and dissection in older patients who already have aortic aneurysm. Accordingly, this might make the risk-benefit ratio unfavorable for using these drugs in these situations, and other antibiotics should be used, if indicated.
Furthermore, if fluoroquinolones are used in patients with aortic aneurysm, perhaps imaging studies of the aneurysm should be done more frequently than once a year to detect accelerated aneurysm growth. Finally, physicians should be aware of the possibility of increased aortic aneurysm expansion and dissection with fluoroquinolone use.
- Cikach F, Desai MY, Roselli EE, Kalahasti V. Thoracic aortic aneurysm: how to counsel, when to refer. Cleve Clin J Med 2018; 85(6):481–492. doi:10.3949/ccjm.85a.17039
- Daneman N, Lu H, Redelmeier DA. Fluoroquinolones and collagen associated severe adverse events: a longitudinal cohort study. BMJ Open 2015; 5:e010077. doi:10.1136/bmjopen-2015-010077
- Lee C-C, Lee MG, Chen Y-S, et al. Risk of aortic dissection and aortic aneurysm in patients taking oral fluoroquinolone. JAMA Intern Med 2015; 175:1839–1847. doi:10.1001/jamainternmed.2015.5389
- Pasternak B, Inghammar M, Svanström H. Fluoroquinolone use and risk of aortic aneurysm and dissection: nationwide cohort study. BMJ Open 2018; 360:k678. doi:10.1136/bmj.k678
- Tsai W-C, Hsu C-C, Chen CPC, et al. Ciprofloxacin up-regulates tendon cells to express matrix metalloproteinase-2 with degradation of type I collagen. J Orthop Res 2011; 29(1):67–73. doi:10.1002/jor.21196
- Cikach F, Desai MY, Roselli EE, Kalahasti V. Thoracic aortic aneurysm: how to counsel, when to refer. Cleve Clin J Med 2018; 85(6):481–492. doi:10.3949/ccjm.85a.17039
- Daneman N, Lu H, Redelmeier DA. Fluoroquinolones and collagen associated severe adverse events: a longitudinal cohort study. BMJ Open 2015; 5:e010077. doi:10.1136/bmjopen-2015-010077
- Lee C-C, Lee MG, Chen Y-S, et al. Risk of aortic dissection and aortic aneurysm in patients taking oral fluoroquinolone. JAMA Intern Med 2015; 175:1839–1847. doi:10.1001/jamainternmed.2015.5389
- Pasternak B, Inghammar M, Svanström H. Fluoroquinolone use and risk of aortic aneurysm and dissection: nationwide cohort study. BMJ Open 2018; 360:k678. doi:10.1136/bmj.k678
- Tsai W-C, Hsu C-C, Chen CPC, et al. Ciprofloxacin up-regulates tendon cells to express matrix metalloproteinase-2 with degradation of type I collagen. J Orthop Res 2011; 29(1):67–73. doi:10.1002/jor.21196
Aortic aneurysm: Fluoroquinolones, genetic counseling
To the Editor: The review of thoracic aortic aneurysm by Cikach et al1 was excellent. However, we noted that referral for clinical genetic counseling and testing is suggested only if 1 or more first-degree relatives have aneurysmal disease.
Absence of a family history does not rule out syndromic aortopathy, which can occur de novo. In addition, a clinical diagnosis of syndromic aortopathy can be made on the basis of physical features that can be very subtle, such as pectus deformities, scoliosis, dolichostenomelia, joint hypermobility or contractures, craniofacial features, or skin fragility.2
Genetic counseling is paramount even if molecular testing is negative or inconclusive, which can occur in more than 50% of patients referred.3 Clinical genetic evaluation would also facilitate testing for other family members who may be affected, and would help to coordinate care for nonvascular conditions that may be associated with the syndrome.
- Cikach F, Desai MY, Roselli EE, Kalahasti V. Thoracic aortic aneurysm: how to counsel, when to refer. Cleve Clin J Med 2018; 85(6):481–492. doi:10.3949/ccjm.85a.17039
- McKusick-Nathans Institute of Genetic Medicine, Johns Hopkins University. OMIM. Online mendelian inheritance in man. https://omim.org. Accessed July 31, 2018.
- Mazine A, Moryousef-Abitbol JH, Faghfoury H, Meza JM, Morel C, Ouzounian M. Yield of genetic testing in patients with thoracic aortic disease. J Am Coll Cardiol 2017; 69(11):2005. doi:10.1016/S0735-1097(17)35394-9
To the Editor: The review of thoracic aortic aneurysm by Cikach et al1 was excellent. However, we noted that referral for clinical genetic counseling and testing is suggested only if 1 or more first-degree relatives have aneurysmal disease.
Absence of a family history does not rule out syndromic aortopathy, which can occur de novo. In addition, a clinical diagnosis of syndromic aortopathy can be made on the basis of physical features that can be very subtle, such as pectus deformities, scoliosis, dolichostenomelia, joint hypermobility or contractures, craniofacial features, or skin fragility.2
Genetic counseling is paramount even if molecular testing is negative or inconclusive, which can occur in more than 50% of patients referred.3 Clinical genetic evaluation would also facilitate testing for other family members who may be affected, and would help to coordinate care for nonvascular conditions that may be associated with the syndrome.
To the Editor: The review of thoracic aortic aneurysm by Cikach et al1 was excellent. However, we noted that referral for clinical genetic counseling and testing is suggested only if 1 or more first-degree relatives have aneurysmal disease.
Absence of a family history does not rule out syndromic aortopathy, which can occur de novo. In addition, a clinical diagnosis of syndromic aortopathy can be made on the basis of physical features that can be very subtle, such as pectus deformities, scoliosis, dolichostenomelia, joint hypermobility or contractures, craniofacial features, or skin fragility.2
Genetic counseling is paramount even if molecular testing is negative or inconclusive, which can occur in more than 50% of patients referred.3 Clinical genetic evaluation would also facilitate testing for other family members who may be affected, and would help to coordinate care for nonvascular conditions that may be associated with the syndrome.
- Cikach F, Desai MY, Roselli EE, Kalahasti V. Thoracic aortic aneurysm: how to counsel, when to refer. Cleve Clin J Med 2018; 85(6):481–492. doi:10.3949/ccjm.85a.17039
- McKusick-Nathans Institute of Genetic Medicine, Johns Hopkins University. OMIM. Online mendelian inheritance in man. https://omim.org. Accessed July 31, 2018.
- Mazine A, Moryousef-Abitbol JH, Faghfoury H, Meza JM, Morel C, Ouzounian M. Yield of genetic testing in patients with thoracic aortic disease. J Am Coll Cardiol 2017; 69(11):2005. doi:10.1016/S0735-1097(17)35394-9
- Cikach F, Desai MY, Roselli EE, Kalahasti V. Thoracic aortic aneurysm: how to counsel, when to refer. Cleve Clin J Med 2018; 85(6):481–492. doi:10.3949/ccjm.85a.17039
- McKusick-Nathans Institute of Genetic Medicine, Johns Hopkins University. OMIM. Online mendelian inheritance in man. https://omim.org. Accessed July 31, 2018.
- Mazine A, Moryousef-Abitbol JH, Faghfoury H, Meza JM, Morel C, Ouzounian M. Yield of genetic testing in patients with thoracic aortic disease. J Am Coll Cardiol 2017; 69(11):2005. doi:10.1016/S0735-1097(17)35394-9
In reply: Aortic aneurysm: Fluoroquinolones, genetic counseling
In Reply: We thank Drs. Goldstein and Mascitelli for their comments regarding fluoroquinolones and thoracic aortic aneurysms. We acknowledge that fluoroquinolones (particularly ciprofloxacin) have been associated with a risk of aortic aneurysm and dissection based on large observational studies from Taiwan, Canada, and Sweden. Although all of the studies have shown an association between ciprofloxacin and aortic aneurysm, the causative role is not well established. In addition, the numbers of events were very small in these large cohorts of patients. In our large tertiary care practice at Cleveland Clinic, we have very few patients with aortic aneurysm or dissection who have used fluoroquinolones.
We recognize the association; however, our paper was intended to emphasize the more common causes and treatment options that primary care physicians are likely to encounter in routine practice.
We also thank Drs. Ayoubieh and MacCarrick for their comments about genetic counseling. We agree that genetic counseling is important, as is a detailed physical examination for subtle features of genetically mediated aortic aneurysm. In fact, we incorporate the physical examination when patients are seen at our aortic center so as to recognize the physical features. We do routinely recommend screening of first-degree relatives even without significant family history on an individual basis and make appropriate referrals for other conditions that can be seen in these patients. Our article, however, is primarily intended to emphasize the importance of referring these patients for more-focused care at a specialized center, where we incorporate all of the suggestions that were made.
In Reply: We thank Drs. Goldstein and Mascitelli for their comments regarding fluoroquinolones and thoracic aortic aneurysms. We acknowledge that fluoroquinolones (particularly ciprofloxacin) have been associated with a risk of aortic aneurysm and dissection based on large observational studies from Taiwan, Canada, and Sweden. Although all of the studies have shown an association between ciprofloxacin and aortic aneurysm, the causative role is not well established. In addition, the numbers of events were very small in these large cohorts of patients. In our large tertiary care practice at Cleveland Clinic, we have very few patients with aortic aneurysm or dissection who have used fluoroquinolones.
We recognize the association; however, our paper was intended to emphasize the more common causes and treatment options that primary care physicians are likely to encounter in routine practice.
We also thank Drs. Ayoubieh and MacCarrick for their comments about genetic counseling. We agree that genetic counseling is important, as is a detailed physical examination for subtle features of genetically mediated aortic aneurysm. In fact, we incorporate the physical examination when patients are seen at our aortic center so as to recognize the physical features. We do routinely recommend screening of first-degree relatives even without significant family history on an individual basis and make appropriate referrals for other conditions that can be seen in these patients. Our article, however, is primarily intended to emphasize the importance of referring these patients for more-focused care at a specialized center, where we incorporate all of the suggestions that were made.
In Reply: We thank Drs. Goldstein and Mascitelli for their comments regarding fluoroquinolones and thoracic aortic aneurysms. We acknowledge that fluoroquinolones (particularly ciprofloxacin) have been associated with a risk of aortic aneurysm and dissection based on large observational studies from Taiwan, Canada, and Sweden. Although all of the studies have shown an association between ciprofloxacin and aortic aneurysm, the causative role is not well established. In addition, the numbers of events were very small in these large cohorts of patients. In our large tertiary care practice at Cleveland Clinic, we have very few patients with aortic aneurysm or dissection who have used fluoroquinolones.
We recognize the association; however, our paper was intended to emphasize the more common causes and treatment options that primary care physicians are likely to encounter in routine practice.
We also thank Drs. Ayoubieh and MacCarrick for their comments about genetic counseling. We agree that genetic counseling is important, as is a detailed physical examination for subtle features of genetically mediated aortic aneurysm. In fact, we incorporate the physical examination when patients are seen at our aortic center so as to recognize the physical features. We do routinely recommend screening of first-degree relatives even without significant family history on an individual basis and make appropriate referrals for other conditions that can be seen in these patients. Our article, however, is primarily intended to emphasize the importance of referring these patients for more-focused care at a specialized center, where we incorporate all of the suggestions that were made.
Open AAA and peripheral bypass surgery patients among the highest users of post-acute care
in Medicare spending, according to the findings of a study that used data from the National Inpatient Sample (NIS) and the Veterans Affairs health system (VA) regarding surgical patients.
PAC, including skilled nursing facilities and inpatient rehabilitation, accounts for 73% of regional variation in Medicare spending, and studies on hospital variation in this area have typically focused on nonsurgical patients or been limited to Medicare data. However, a high degree of variation also appears to hold for surgical patients, according to the authors of this large database study of more than 4 million patients who had aortic aneurysm repair, peripheral vascular bypass, colorectal surgery, hepatectomy, pancreatectomy, or coronary bypass.
“We found that there is significant variation in use of PAC and rates of home discharge following complex cardiac, abdominal, and vascular surgery,” Courtney J. Balentine, MD, of the University of Alabama at Birmingham and his colleagues wrote in their report in the Journal of Surgical Research.
To explore hospital variation in post-surgery PAC, they evaluated 3,487,365 patients from the NIS (39% were aged 70 years or older, and 60% were men) and 60,666 from the VA (32% were aged 70 years or older, and 98% were men) who had surgery during 2008-2011.
Within the NIS, 631,199 patients (18%) were discharged to PAC facilities, and among the 60,666 veterans, 4744 (7.8%) were discharged to PAC facilities. In addition, hospital rates of discharge to PAC facilities varied from 1% to 36% for VA hospitals and from 1% to 59% for non-VA hospitals, according to the researchers. They found that some VA hospitals were four times more likely to discharge patients to PAC facilities than would be expected from their patients’ characteristics, while others were 90% more likely to send patients home than would be expected, according to Dr. Balentine and his colleagues.
Procedure-specific rates of discharge to PAC facilities from VA hospitals ranged from 2% following endovascular aneurysm repair to 10% after pancreatectomy and peripheral vascular bypass. Among the NIS hospitals, in contrast, rates of discharge to PAC facilities ranged from 6% following hepatectomy to as high as 44% following open aneurysm repair.
“These data could be used to characterize practices that promote more effective recovery from surgery and minimize the need for PAC,” the authors wrote. “Given that skilled nursing facilities and inpatient rehabilitation cost [$5,000]-$24,000 more than treatment at home, even minor reductions in the need for PAC facilities could result in substantial cost savings,” they stated.
“Our findings suggest that there is considerable room for improvement in the use of PAC after surgery and that we still have a long way to go in terms of using PAC to help patients recover and regain their independence,” the researchers concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Balentine CJ et al. J Surg Res. 2018 Oct;230:61-70.
in Medicare spending, according to the findings of a study that used data from the National Inpatient Sample (NIS) and the Veterans Affairs health system (VA) regarding surgical patients.
PAC, including skilled nursing facilities and inpatient rehabilitation, accounts for 73% of regional variation in Medicare spending, and studies on hospital variation in this area have typically focused on nonsurgical patients or been limited to Medicare data. However, a high degree of variation also appears to hold for surgical patients, according to the authors of this large database study of more than 4 million patients who had aortic aneurysm repair, peripheral vascular bypass, colorectal surgery, hepatectomy, pancreatectomy, or coronary bypass.
“We found that there is significant variation in use of PAC and rates of home discharge following complex cardiac, abdominal, and vascular surgery,” Courtney J. Balentine, MD, of the University of Alabama at Birmingham and his colleagues wrote in their report in the Journal of Surgical Research.
To explore hospital variation in post-surgery PAC, they evaluated 3,487,365 patients from the NIS (39% were aged 70 years or older, and 60% were men) and 60,666 from the VA (32% were aged 70 years or older, and 98% were men) who had surgery during 2008-2011.
Within the NIS, 631,199 patients (18%) were discharged to PAC facilities, and among the 60,666 veterans, 4744 (7.8%) were discharged to PAC facilities. In addition, hospital rates of discharge to PAC facilities varied from 1% to 36% for VA hospitals and from 1% to 59% for non-VA hospitals, according to the researchers. They found that some VA hospitals were four times more likely to discharge patients to PAC facilities than would be expected from their patients’ characteristics, while others were 90% more likely to send patients home than would be expected, according to Dr. Balentine and his colleagues.
Procedure-specific rates of discharge to PAC facilities from VA hospitals ranged from 2% following endovascular aneurysm repair to 10% after pancreatectomy and peripheral vascular bypass. Among the NIS hospitals, in contrast, rates of discharge to PAC facilities ranged from 6% following hepatectomy to as high as 44% following open aneurysm repair.
“These data could be used to characterize practices that promote more effective recovery from surgery and minimize the need for PAC,” the authors wrote. “Given that skilled nursing facilities and inpatient rehabilitation cost [$5,000]-$24,000 more than treatment at home, even minor reductions in the need for PAC facilities could result in substantial cost savings,” they stated.
“Our findings suggest that there is considerable room for improvement in the use of PAC after surgery and that we still have a long way to go in terms of using PAC to help patients recover and regain their independence,” the researchers concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Balentine CJ et al. J Surg Res. 2018 Oct;230:61-70.
in Medicare spending, according to the findings of a study that used data from the National Inpatient Sample (NIS) and the Veterans Affairs health system (VA) regarding surgical patients.
PAC, including skilled nursing facilities and inpatient rehabilitation, accounts for 73% of regional variation in Medicare spending, and studies on hospital variation in this area have typically focused on nonsurgical patients or been limited to Medicare data. However, a high degree of variation also appears to hold for surgical patients, according to the authors of this large database study of more than 4 million patients who had aortic aneurysm repair, peripheral vascular bypass, colorectal surgery, hepatectomy, pancreatectomy, or coronary bypass.
“We found that there is significant variation in use of PAC and rates of home discharge following complex cardiac, abdominal, and vascular surgery,” Courtney J. Balentine, MD, of the University of Alabama at Birmingham and his colleagues wrote in their report in the Journal of Surgical Research.
To explore hospital variation in post-surgery PAC, they evaluated 3,487,365 patients from the NIS (39% were aged 70 years or older, and 60% were men) and 60,666 from the VA (32% were aged 70 years or older, and 98% were men) who had surgery during 2008-2011.
Within the NIS, 631,199 patients (18%) were discharged to PAC facilities, and among the 60,666 veterans, 4744 (7.8%) were discharged to PAC facilities. In addition, hospital rates of discharge to PAC facilities varied from 1% to 36% for VA hospitals and from 1% to 59% for non-VA hospitals, according to the researchers. They found that some VA hospitals were four times more likely to discharge patients to PAC facilities than would be expected from their patients’ characteristics, while others were 90% more likely to send patients home than would be expected, according to Dr. Balentine and his colleagues.
Procedure-specific rates of discharge to PAC facilities from VA hospitals ranged from 2% following endovascular aneurysm repair to 10% after pancreatectomy and peripheral vascular bypass. Among the NIS hospitals, in contrast, rates of discharge to PAC facilities ranged from 6% following hepatectomy to as high as 44% following open aneurysm repair.
“These data could be used to characterize practices that promote more effective recovery from surgery and minimize the need for PAC,” the authors wrote. “Given that skilled nursing facilities and inpatient rehabilitation cost [$5,000]-$24,000 more than treatment at home, even minor reductions in the need for PAC facilities could result in substantial cost savings,” they stated.
“Our findings suggest that there is considerable room for improvement in the use of PAC after surgery and that we still have a long way to go in terms of using PAC to help patients recover and regain their independence,” the researchers concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Balentine CJ et al. J Surg Res. 2018 Oct;230:61-70.
FROM THE JOURNAL OF SURGICAL RESEARCH
Key clinical point: The wide disparity among hospitals in their rates of postsurgery discharge to post-acute care (PAC) could be an area of focus for cost containment in Medicare spending.
Major finding: Rates of discharge to PAC facilities varied from 1% to 36% for VA hospitals and from 1% to 59% for non-VA hospitals.
Study details: A database analysis of 3,487,365 National Inpatient Sample patients and 60,666 VA patients who had surgery during 2008-2011.
Disclosures: The authors reported that they had no conflicts of interest.
Source: Balentine CJ et al. J Surg Res. 2018 Oct;230:61-70.
Vascular training for general surgery residents continues downward trend
Operative experience in open arterial vascular surgery procedures for general surgery residents has significantly declined, according to the results of a study of the Accreditation Council for Graduate Medical Education (ACGME) national case log reports, which lists the mean numbers of operations performed.
“Because fundamental vascular surgery skills are necessary for operative general surgery, vascular surgery should remain an essential content area. However, programs cannot solely depend on operative experience to teach fundamental vascular surgery skills,” John R. Potts III, MD, FACS, and R. James Valentine, MD, FACS, stated in their report published online in Annals of Surgery.
The number of individuals completing ACGME-accredited general surgery and vascular surgery training each year of the study was obtained from public reports of the ACGME as well as the available summary national data regarding the reported operative experience of residents completing general surgery programs.
The researchers found that over 15 years (academic year 2001-2002 through AY 2016-2017), the total vascular operations performed by general surgery residents significantly declined as did the total open arterial vascular procedures, including those in seven of nine categories (P less than .0001).
The issue of adequate exposure to vascular procedures for general surgery residents is complex. “The number of individuals completing general surgery residency annually has increased by approximately 20% since AY 2001-2. During the same period, the number of open arterial operations reported by general surgery residents decreased by approximately 38%. Thus, the declining experience is clearly not simply a matter of distributing the same number of operations to a larger number of individuals,” the investigators reported.
The ACGME-designated “essential content areas” have increased in recent years for general surgery trainees to now encompass alimentary tract, abdomen, breast, head and neck, endocrine system, the surgical management of trauma, soft tissues, pediatric surgery, surgical critical care, surgical oncology, and vascular surgery. The essential content areas compete to varying degrees for the trainee’s time, potentially cutting into not just vascular cases but other areas as well. It is also the case that general surgery trainees are often in an institutional setting where they are competing with vascular surgery residents for the same pool of vascular surgery patients.
During those last 5 years, significant declines occurred in five categories: aneurysm, cerebrovascular disease, arteriovenous dialysis access, peripheral vascular disease, and extra-anatomic bypass, according to the authors.
“Knowledge of arterial anatomy, approaches, control, and repair are crucial to the practice of operative general surgery. In the face of declining experience for their resident as surgeon in open arterial operations, general surgery programs must augment resident education in the principles of vascular surgery through other means,” the authors concluded.
Portions of this study were presented at the2018 meeting of the American Surgical Association.
Dr. Potts and Dr. Valentine reported that they had no conflicts of interest.
SOURCE: Potts JR et al. Ann Surg. 2018 Jul 24. doi: 10.1097/SLA.0000000000002951.
In the role of discussant at the American Surgical Association meeting where this research was presented, K. Craig Kent, MD, a vascular surgeon and former president of the Society for Vascular Surgery, stated: “We don’t have enough open arterial cases to teach our general surgery residents. There is a problem on the other side, too, which is we don’t have enough open arterial cases to teach our vascular surgeons to be confident, open, vascular surgeons. What’s my solution? ... we need to share. We should take the opportunity whenever there’s a great arterial case to have everybody, vascular and general surgery trainees, participate,” he stated.
In the role of discussant at the American Surgical Association meeting where this research was presented, K. Craig Kent, MD, a vascular surgeon and former president of the Society for Vascular Surgery, stated: “We don’t have enough open arterial cases to teach our general surgery residents. There is a problem on the other side, too, which is we don’t have enough open arterial cases to teach our vascular surgeons to be confident, open, vascular surgeons. What’s my solution? ... we need to share. We should take the opportunity whenever there’s a great arterial case to have everybody, vascular and general surgery trainees, participate,” he stated.
In the role of discussant at the American Surgical Association meeting where this research was presented, K. Craig Kent, MD, a vascular surgeon and former president of the Society for Vascular Surgery, stated: “We don’t have enough open arterial cases to teach our general surgery residents. There is a problem on the other side, too, which is we don’t have enough open arterial cases to teach our vascular surgeons to be confident, open, vascular surgeons. What’s my solution? ... we need to share. We should take the opportunity whenever there’s a great arterial case to have everybody, vascular and general surgery trainees, participate,” he stated.
Operative experience in open arterial vascular surgery procedures for general surgery residents has significantly declined, according to the results of a study of the Accreditation Council for Graduate Medical Education (ACGME) national case log reports, which lists the mean numbers of operations performed.
“Because fundamental vascular surgery skills are necessary for operative general surgery, vascular surgery should remain an essential content area. However, programs cannot solely depend on operative experience to teach fundamental vascular surgery skills,” John R. Potts III, MD, FACS, and R. James Valentine, MD, FACS, stated in their report published online in Annals of Surgery.
The number of individuals completing ACGME-accredited general surgery and vascular surgery training each year of the study was obtained from public reports of the ACGME as well as the available summary national data regarding the reported operative experience of residents completing general surgery programs.
The researchers found that over 15 years (academic year 2001-2002 through AY 2016-2017), the total vascular operations performed by general surgery residents significantly declined as did the total open arterial vascular procedures, including those in seven of nine categories (P less than .0001).
The issue of adequate exposure to vascular procedures for general surgery residents is complex. “The number of individuals completing general surgery residency annually has increased by approximately 20% since AY 2001-2. During the same period, the number of open arterial operations reported by general surgery residents decreased by approximately 38%. Thus, the declining experience is clearly not simply a matter of distributing the same number of operations to a larger number of individuals,” the investigators reported.
The ACGME-designated “essential content areas” have increased in recent years for general surgery trainees to now encompass alimentary tract, abdomen, breast, head and neck, endocrine system, the surgical management of trauma, soft tissues, pediatric surgery, surgical critical care, surgical oncology, and vascular surgery. The essential content areas compete to varying degrees for the trainee’s time, potentially cutting into not just vascular cases but other areas as well. It is also the case that general surgery trainees are often in an institutional setting where they are competing with vascular surgery residents for the same pool of vascular surgery patients.
During those last 5 years, significant declines occurred in five categories: aneurysm, cerebrovascular disease, arteriovenous dialysis access, peripheral vascular disease, and extra-anatomic bypass, according to the authors.
“Knowledge of arterial anatomy, approaches, control, and repair are crucial to the practice of operative general surgery. In the face of declining experience for their resident as surgeon in open arterial operations, general surgery programs must augment resident education in the principles of vascular surgery through other means,” the authors concluded.
Portions of this study were presented at the2018 meeting of the American Surgical Association.
Dr. Potts and Dr. Valentine reported that they had no conflicts of interest.
SOURCE: Potts JR et al. Ann Surg. 2018 Jul 24. doi: 10.1097/SLA.0000000000002951.
Operative experience in open arterial vascular surgery procedures for general surgery residents has significantly declined, according to the results of a study of the Accreditation Council for Graduate Medical Education (ACGME) national case log reports, which lists the mean numbers of operations performed.
“Because fundamental vascular surgery skills are necessary for operative general surgery, vascular surgery should remain an essential content area. However, programs cannot solely depend on operative experience to teach fundamental vascular surgery skills,” John R. Potts III, MD, FACS, and R. James Valentine, MD, FACS, stated in their report published online in Annals of Surgery.
The number of individuals completing ACGME-accredited general surgery and vascular surgery training each year of the study was obtained from public reports of the ACGME as well as the available summary national data regarding the reported operative experience of residents completing general surgery programs.
The researchers found that over 15 years (academic year 2001-2002 through AY 2016-2017), the total vascular operations performed by general surgery residents significantly declined as did the total open arterial vascular procedures, including those in seven of nine categories (P less than .0001).
The issue of adequate exposure to vascular procedures for general surgery residents is complex. “The number of individuals completing general surgery residency annually has increased by approximately 20% since AY 2001-2. During the same period, the number of open arterial operations reported by general surgery residents decreased by approximately 38%. Thus, the declining experience is clearly not simply a matter of distributing the same number of operations to a larger number of individuals,” the investigators reported.
The ACGME-designated “essential content areas” have increased in recent years for general surgery trainees to now encompass alimentary tract, abdomen, breast, head and neck, endocrine system, the surgical management of trauma, soft tissues, pediatric surgery, surgical critical care, surgical oncology, and vascular surgery. The essential content areas compete to varying degrees for the trainee’s time, potentially cutting into not just vascular cases but other areas as well. It is also the case that general surgery trainees are often in an institutional setting where they are competing with vascular surgery residents for the same pool of vascular surgery patients.
During those last 5 years, significant declines occurred in five categories: aneurysm, cerebrovascular disease, arteriovenous dialysis access, peripheral vascular disease, and extra-anatomic bypass, according to the authors.
“Knowledge of arterial anatomy, approaches, control, and repair are crucial to the practice of operative general surgery. In the face of declining experience for their resident as surgeon in open arterial operations, general surgery programs must augment resident education in the principles of vascular surgery through other means,” the authors concluded.
Portions of this study were presented at the2018 meeting of the American Surgical Association.
Dr. Potts and Dr. Valentine reported that they had no conflicts of interest.
SOURCE: Potts JR et al. Ann Surg. 2018 Jul 24. doi: 10.1097/SLA.0000000000002951.
FROM ANNALS OF SURGERY
Key clinical point: General surgery residents may need more open vascular experience.
Major finding: The number of open arterial operations reported by general surgery residents has decreased by approximately 38% since academic year 2001-2002.
Study details: An analysis of 15 years of ACGME resident national case load reports.
Disclosures: Dr. Potts and Dr. Valentine reported that they had no conflicts of interest.
Source: Potts JR III et al. Ann Surg. 2018 Jul 24. doi: 10.1097/SLA.0000000000002951.
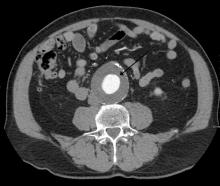
Routine screening for AAA in older men may harm more than help
Deaths from abdominal aortic aneurysm among Swedish men are going down – but not because they’re being screened for the potentially fatal condition.
Although the death rate has decreased by 70% since the early 2000s, screening only saved 2 lives per 10,000 men screened. It did, however, increase by 59% the risk of unnecessary surgery, Minna Johansson, MD, and colleagues wrote in the June 16 issue of the Lancet.
“Screening had only a minor effect on AAA mortality,” wrote Dr. Johansson of the University of Gothenburg (Sweden). “In absolute numbers, only 7% of the benefit estimated in the largest trial of AAA screening was observed. The observed large reductions in AAA mortality were present in both the screened and nonscreened cohorts and were thus mainly caused by other factors – probably reduced smoking. … Our results call the continued justification of AAA screening into question.”
In Sweden, all men aged 65 years are invited to a one-time ultrasound abdominal aorta screening. Most participate. Anyone with an aneurysm is followed up at a vascular surgery clinic, with surgery considered if the aortic diameter is 55 mm or larger.
Dr. Johansson and her colleagues plumbed national health records to estimate the risks and benefits of this routine screening. The study comprised 25,265 men invited to join the AAA screening program in Sweden from 2006 to 2009. Mortality data were compared with those from a contemporaneous cohort of 106,087 men of similar age who were not invited to screen. Finally, the mortality data were compared with national trends in AAA mortality in all Swedish men aged 40-99 years from 1987 to 2015.
A multivariate analysis adjusted for cohort year, marital status, educational level, income, and whether the patient already had an AAA diagnosis at baseline.
From the early 2000s to 2015, AAA mortality among men aged 65-74 years declined from 36 to10 deaths per 100,000. This 70% reduction was similar in both screened and unscreened populations; in fact, the decline began about a decade before population-based screening was introduced and continued to decrease at a steady rate afterward.
After 6 years of screening, there was a 30% reduction of AAA mortality in the screened population, compared with the unscreened, translating to an absolute mortality reduction of two deaths per 10,000 men offered screening.
Screening increased by 52% the number of AAAs detected. The absolute difference in incidence after 6 years of screening translated to an additional 49 overdiagnoses per 10,000 screened men.
Looking back into the mid-1990s, the investigators saw the numbers of elective AAA surgeries rise steadily. In the adjusted model, screened men were 59% more likely to have this procedure than unscreened. The increased risk didn’t come with an equally increased benefit, though. There was a 10% decrease in AAA ruptures, “rendering a risk of overtreatment of 19%, or 19 potentially avoidable elective surgeries per 10,000 men,” the team noted. “Sixty-three percent of all additional elective surgeries for AAA might therefore have constituted overtreat.”
The findings are at odds with large published studies that found a consistent benefit to screening.
“Compared with results at 7-year follow-up of the largest trial of screening for abdominal aortic aneurysm [Multicentre Aneurysm Screening Study (MASS)], we found about half of the benefit in terms of a relative effect and 7% of the estimated benefit in terms of absolute numbers [2 vs. 27 avoided deaths from AAA per 10,000 invited men]. Compared with previous estimates of overdiagnosis and overtreatment, we found a lower absolute number of over-diagnosed cases [49 vs.176 per 10,000 invited men] and fewer overtreated cases [19 vs. 37 per 10,000 invited men]. However, since the harms of screening decreased less than the benefit, the balance between benefits and harms seems much less appealing in today’s setting.”
None of the authors had any financial disclosures.
The study by Johansson et al. indicates a significant risk of overdiagnosis associated with routine screening for abdominal aortic aneurysm: Those risks may not be as clinically harmful as might be assumed, Stefan Acosta, MD, wrote in an accompanying editorial (Lancet 2018; 391: 2394-95).
“Although I agree that having a small AAA that needs long-term follow-up might be associated with negative psychological consequences, there could also be a window of opportunity [eg. with statins, antiplatelet therapy, and blood pressure reduction], for individuals with increased burden of cardiovascular disease. Indeed, screening for AAA, peripheral artery disease, and hypertension, with the initiation of relevant pharmacotherapy, if positive, reduces all-cause mortality and some evidence suggests that this approach of multifaceted vascular screening instead of isolated AAA screening should be considered.”
When performed according to the established criteria for elective AAA surgery, the procedure is associated with less than 1% postoperative mortality, “mainly because of wide implementation of endovascular aneurysm repair, a minimally invasive method.”
The 6-year follow-up time, as the authors noted, is relatively short. A 2016 review of the Swedish Nationwide Abdominal Aortic Aneurysm Screening Program determined that significant mortality benefit could take 10 years to materialize(Circ 2016;134:1141-8).
The full impact of Sweden’s remarkable decrease in smoking is almost certainly making itself known in these outcomes – smoking is implicated in 75% of AAA cases.
“The decreased prevalence of smoking in Sweden, from 44% of the population in 1970 to 15% in 2010, should be viewed as the main cause of the decreasing incidence and mortality of AAA. Every percent drop in the prevalence of smoking will have a huge effect on smoking-related diseases, such as cancer and AAA.”
Dr. Stefan is a vascular disease researcher at Lund (Sweden) University. He had no financial disclosures.
The study by Johansson et al. indicates a significant risk of overdiagnosis associated with routine screening for abdominal aortic aneurysm: Those risks may not be as clinically harmful as might be assumed, Stefan Acosta, MD, wrote in an accompanying editorial (Lancet 2018; 391: 2394-95).
“Although I agree that having a small AAA that needs long-term follow-up might be associated with negative psychological consequences, there could also be a window of opportunity [eg. with statins, antiplatelet therapy, and blood pressure reduction], for individuals with increased burden of cardiovascular disease. Indeed, screening for AAA, peripheral artery disease, and hypertension, with the initiation of relevant pharmacotherapy, if positive, reduces all-cause mortality and some evidence suggests that this approach of multifaceted vascular screening instead of isolated AAA screening should be considered.”
When performed according to the established criteria for elective AAA surgery, the procedure is associated with less than 1% postoperative mortality, “mainly because of wide implementation of endovascular aneurysm repair, a minimally invasive method.”
The 6-year follow-up time, as the authors noted, is relatively short. A 2016 review of the Swedish Nationwide Abdominal Aortic Aneurysm Screening Program determined that significant mortality benefit could take 10 years to materialize(Circ 2016;134:1141-8).
The full impact of Sweden’s remarkable decrease in smoking is almost certainly making itself known in these outcomes – smoking is implicated in 75% of AAA cases.
“The decreased prevalence of smoking in Sweden, from 44% of the population in 1970 to 15% in 2010, should be viewed as the main cause of the decreasing incidence and mortality of AAA. Every percent drop in the prevalence of smoking will have a huge effect on smoking-related diseases, such as cancer and AAA.”
Dr. Stefan is a vascular disease researcher at Lund (Sweden) University. He had no financial disclosures.
The study by Johansson et al. indicates a significant risk of overdiagnosis associated with routine screening for abdominal aortic aneurysm: Those risks may not be as clinically harmful as might be assumed, Stefan Acosta, MD, wrote in an accompanying editorial (Lancet 2018; 391: 2394-95).
“Although I agree that having a small AAA that needs long-term follow-up might be associated with negative psychological consequences, there could also be a window of opportunity [eg. with statins, antiplatelet therapy, and blood pressure reduction], for individuals with increased burden of cardiovascular disease. Indeed, screening for AAA, peripheral artery disease, and hypertension, with the initiation of relevant pharmacotherapy, if positive, reduces all-cause mortality and some evidence suggests that this approach of multifaceted vascular screening instead of isolated AAA screening should be considered.”
When performed according to the established criteria for elective AAA surgery, the procedure is associated with less than 1% postoperative mortality, “mainly because of wide implementation of endovascular aneurysm repair, a minimally invasive method.”
The 6-year follow-up time, as the authors noted, is relatively short. A 2016 review of the Swedish Nationwide Abdominal Aortic Aneurysm Screening Program determined that significant mortality benefit could take 10 years to materialize(Circ 2016;134:1141-8).
The full impact of Sweden’s remarkable decrease in smoking is almost certainly making itself known in these outcomes – smoking is implicated in 75% of AAA cases.
“The decreased prevalence of smoking in Sweden, from 44% of the population in 1970 to 15% in 2010, should be viewed as the main cause of the decreasing incidence and mortality of AAA. Every percent drop in the prevalence of smoking will have a huge effect on smoking-related diseases, such as cancer and AAA.”
Dr. Stefan is a vascular disease researcher at Lund (Sweden) University. He had no financial disclosures.
Deaths from abdominal aortic aneurysm among Swedish men are going down – but not because they’re being screened for the potentially fatal condition.
Although the death rate has decreased by 70% since the early 2000s, screening only saved 2 lives per 10,000 men screened. It did, however, increase by 59% the risk of unnecessary surgery, Minna Johansson, MD, and colleagues wrote in the June 16 issue of the Lancet.
“Screening had only a minor effect on AAA mortality,” wrote Dr. Johansson of the University of Gothenburg (Sweden). “In absolute numbers, only 7% of the benefit estimated in the largest trial of AAA screening was observed. The observed large reductions in AAA mortality were present in both the screened and nonscreened cohorts and were thus mainly caused by other factors – probably reduced smoking. … Our results call the continued justification of AAA screening into question.”
In Sweden, all men aged 65 years are invited to a one-time ultrasound abdominal aorta screening. Most participate. Anyone with an aneurysm is followed up at a vascular surgery clinic, with surgery considered if the aortic diameter is 55 mm or larger.
Dr. Johansson and her colleagues plumbed national health records to estimate the risks and benefits of this routine screening. The study comprised 25,265 men invited to join the AAA screening program in Sweden from 2006 to 2009. Mortality data were compared with those from a contemporaneous cohort of 106,087 men of similar age who were not invited to screen. Finally, the mortality data were compared with national trends in AAA mortality in all Swedish men aged 40-99 years from 1987 to 2015.
A multivariate analysis adjusted for cohort year, marital status, educational level, income, and whether the patient already had an AAA diagnosis at baseline.
From the early 2000s to 2015, AAA mortality among men aged 65-74 years declined from 36 to10 deaths per 100,000. This 70% reduction was similar in both screened and unscreened populations; in fact, the decline began about a decade before population-based screening was introduced and continued to decrease at a steady rate afterward.
After 6 years of screening, there was a 30% reduction of AAA mortality in the screened population, compared with the unscreened, translating to an absolute mortality reduction of two deaths per 10,000 men offered screening.
Screening increased by 52% the number of AAAs detected. The absolute difference in incidence after 6 years of screening translated to an additional 49 overdiagnoses per 10,000 screened men.
Looking back into the mid-1990s, the investigators saw the numbers of elective AAA surgeries rise steadily. In the adjusted model, screened men were 59% more likely to have this procedure than unscreened. The increased risk didn’t come with an equally increased benefit, though. There was a 10% decrease in AAA ruptures, “rendering a risk of overtreatment of 19%, or 19 potentially avoidable elective surgeries per 10,000 men,” the team noted. “Sixty-three percent of all additional elective surgeries for AAA might therefore have constituted overtreat.”
The findings are at odds with large published studies that found a consistent benefit to screening.
“Compared with results at 7-year follow-up of the largest trial of screening for abdominal aortic aneurysm [Multicentre Aneurysm Screening Study (MASS)], we found about half of the benefit in terms of a relative effect and 7% of the estimated benefit in terms of absolute numbers [2 vs. 27 avoided deaths from AAA per 10,000 invited men]. Compared with previous estimates of overdiagnosis and overtreatment, we found a lower absolute number of over-diagnosed cases [49 vs.176 per 10,000 invited men] and fewer overtreated cases [19 vs. 37 per 10,000 invited men]. However, since the harms of screening decreased less than the benefit, the balance between benefits and harms seems much less appealing in today’s setting.”
None of the authors had any financial disclosures.
Deaths from abdominal aortic aneurysm among Swedish men are going down – but not because they’re being screened for the potentially fatal condition.
Although the death rate has decreased by 70% since the early 2000s, screening only saved 2 lives per 10,000 men screened. It did, however, increase by 59% the risk of unnecessary surgery, Minna Johansson, MD, and colleagues wrote in the June 16 issue of the Lancet.
“Screening had only a minor effect on AAA mortality,” wrote Dr. Johansson of the University of Gothenburg (Sweden). “In absolute numbers, only 7% of the benefit estimated in the largest trial of AAA screening was observed. The observed large reductions in AAA mortality were present in both the screened and nonscreened cohorts and were thus mainly caused by other factors – probably reduced smoking. … Our results call the continued justification of AAA screening into question.”
In Sweden, all men aged 65 years are invited to a one-time ultrasound abdominal aorta screening. Most participate. Anyone with an aneurysm is followed up at a vascular surgery clinic, with surgery considered if the aortic diameter is 55 mm or larger.
Dr. Johansson and her colleagues plumbed national health records to estimate the risks and benefits of this routine screening. The study comprised 25,265 men invited to join the AAA screening program in Sweden from 2006 to 2009. Mortality data were compared with those from a contemporaneous cohort of 106,087 men of similar age who were not invited to screen. Finally, the mortality data were compared with national trends in AAA mortality in all Swedish men aged 40-99 years from 1987 to 2015.
A multivariate analysis adjusted for cohort year, marital status, educational level, income, and whether the patient already had an AAA diagnosis at baseline.
From the early 2000s to 2015, AAA mortality among men aged 65-74 years declined from 36 to10 deaths per 100,000. This 70% reduction was similar in both screened and unscreened populations; in fact, the decline began about a decade before population-based screening was introduced and continued to decrease at a steady rate afterward.
After 6 years of screening, there was a 30% reduction of AAA mortality in the screened population, compared with the unscreened, translating to an absolute mortality reduction of two deaths per 10,000 men offered screening.
Screening increased by 52% the number of AAAs detected. The absolute difference in incidence after 6 years of screening translated to an additional 49 overdiagnoses per 10,000 screened men.
Looking back into the mid-1990s, the investigators saw the numbers of elective AAA surgeries rise steadily. In the adjusted model, screened men were 59% more likely to have this procedure than unscreened. The increased risk didn’t come with an equally increased benefit, though. There was a 10% decrease in AAA ruptures, “rendering a risk of overtreatment of 19%, or 19 potentially avoidable elective surgeries per 10,000 men,” the team noted. “Sixty-three percent of all additional elective surgeries for AAA might therefore have constituted overtreat.”
The findings are at odds with large published studies that found a consistent benefit to screening.
“Compared with results at 7-year follow-up of the largest trial of screening for abdominal aortic aneurysm [Multicentre Aneurysm Screening Study (MASS)], we found about half of the benefit in terms of a relative effect and 7% of the estimated benefit in terms of absolute numbers [2 vs. 27 avoided deaths from AAA per 10,000 invited men]. Compared with previous estimates of overdiagnosis and overtreatment, we found a lower absolute number of over-diagnosed cases [49 vs.176 per 10,000 invited men] and fewer overtreated cases [19 vs. 37 per 10,000 invited men]. However, since the harms of screening decreased less than the benefit, the balance between benefits and harms seems much less appealing in today’s setting.”
None of the authors had any financial disclosures.
FROM THE LANCET
Key clinical point: Screening for abdominal aortic aneurysms in men saved few lives, but significantly increased the risk of overdiagnosis and unnecessary surgery.
Major finding: Screening saved two lives per 10,000 men, but showed an increased risk of overtreatment of 19%.
Study details: The population-based longitudinal cohort study comprised 131,352 men.
Disclosures: The authors had no financial disclosures.
Source: Johansson et al. Lancet 2018;391:2441-7.
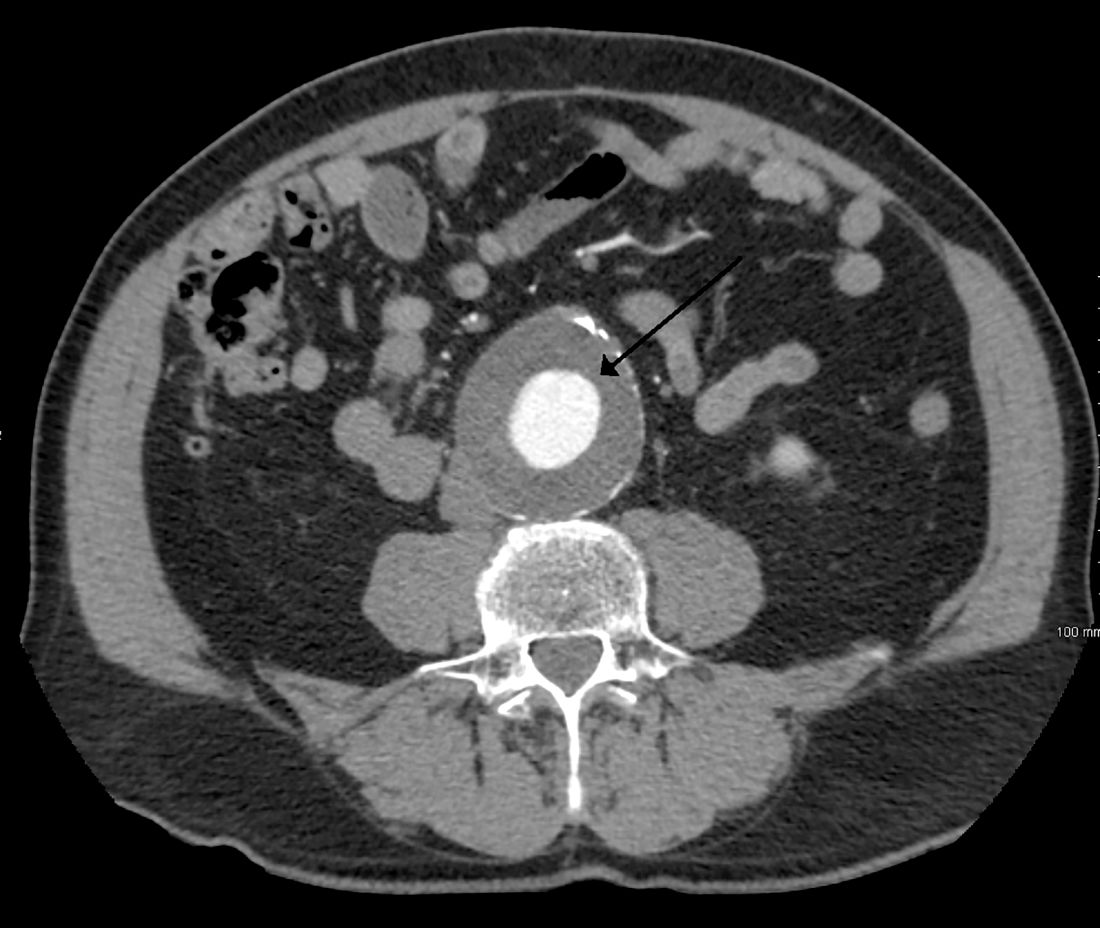
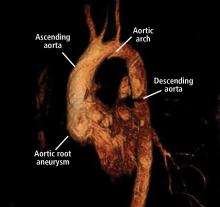
Thoracic aortic aneurysm: How to counsel, when to refer
Thoracic aortic aneurysm (TAA) needs to be detected, monitored, and managed in a timely manner to prevent a serious consequence such as acute dissection or rupture. But only about 5% of patients experience symptoms before an acute event occurs, and for the other 95% the first “symptom” is often death.1 Most cases are detected either incidentally with echocardiography, computed tomography (CT), or magnetic resonance imaging (MRI) during workup for another condition. Patients may also be diagnosed during workup of a murmur or after a family member is found to have an aneurysm. Therefore, its true incidence is difficult to determine.2
With these facts in mind, how would you manage the following 2 cases?
Case 1: Bicuspid aortic valve, ascending aortic aneurysm
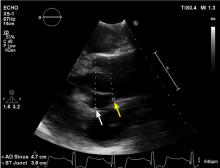
A 45-year-old man with stage 1 hypertension presents for evaluation of a bicuspid aortic valve and ascending aortic aneurysm. He has several first-degree relatives with similar conditions, and his brother recently underwent elective aortic repair. At the urging of his primary care physician, he underwent screening echocardiography, which demonstrated a “dilated root and ascending aorta” 4.6 cm in diameter. He presents today to discuss management options and how the aneurysm could affect his everyday life.
Case 2: Marfan syndrome in a young woman
A 24-year-old woman with Marfan syndrome diagnosed in adolescence presents for annual follow-up. She has many family members with the same condition, and several have undergone prophylactic aortic root repair. Her aortic root has been monitored annually for progression of dilation, and today it is 4.6 cm in diameter, a 3-mm increase from the last measurement. She has grade 2+ aortic insufficiency (on a scale of 1+ to 4+) based on echocardiography, but she has no symptoms. She is curious about what size her aortic root will need to reach for surgery to be considered.
LIKELY UNDERDETECTED
TAA is being detected more often than in the past thanks to better detection methods and heightened awareness among physicians and patients. While an incidence rate of 10.4 per 100,000 patient-years is often cited,3 this figure likely underestimates the true incidence of this clinically silent condition. The most robust data come from studies based on in-hospital diagnostic codes coupled with data from autopsies for out-of-hospital deaths.
Olsson et al,4 in a 2016 study in Sweden, found the incidence of TAA and aortic dissection to be 16.3 per 100,000 per year for men and 9.1 per 100,000 per year for women.
Clouse et al5 reported the incidence of thoracic aortic dissection as 3.5 per 100,000 patient-years, and the same figure for thoracic aortic rupture.
Aneurysmal disease accounts for 52,000 deaths per year in the United States, making it the 19th most common cause of death.6 These figures are likely lower than the true mortality rate for this condition, given that aortic dissection is often mistaken for acute myocardial infarction or other acute event if an autopsy is not done to confirm the cause of death.7
RISK FACTORS FOR THORACIC AORTIC ANEURYSM
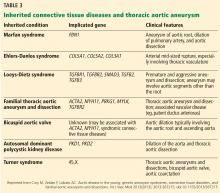
Risk factors for TAA include genetic conditions that lead to aortic medial weakness or destruction such as Loeys-Dietz syndrome and Marfan syndrome.2 In addition, family history is important even in the absence of known genetic mutations. Other risk factors include conditions that increase aortic wall stress, such as hypertension, cocaine abuse, extreme weightlifting, trauma, and aortic coarctation.2
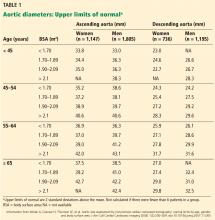
DIAMETER INCREASES WITH AGE, BODY SURFACE AREA
Normal dimensions for the aortic segments differ depending on age, sex, and body surface area.8,44,45 The size of the aortic root may also vary depending on how it is measured, due to the root’s trefoil shape. Measured sinus to sinus, the root is larger than when measured sinus to commissure on CT angiography or cardiac MRI. It is also larger when measured leading edge to leading edge than inner edge to inner edge on echocardiography.10
TAA is defined as an aortic diameter at least 50% greater than the upper limit of normal.8
Geometric changes in the curvature of the ascending aorta, aortic arch, and descending thoracic aorta can occur as the result of hypertension, atherosclerosis, or connective tissue disease.
HOW IS TAA DIAGNOSED?
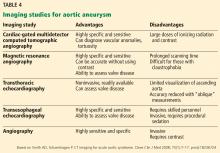
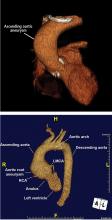
Imaging tests
It is particularly important to obtain a gated CTA image in patients with aortic root aneurysm to avoid motion artifact and possible erroneous measurements. Gated CTA is done with electrocardiographic synchronization and allows for image processing to correct for cardiac motion.
HOW IS TAA CLASSIFIED?
TAA can be caused by a variety of inherited and sporadic conditions. These differences in pathogenesis lend themselves to classification of aneurysms into groups. Table 3 highlights the most common conditions associated with TAA.13
Bicuspid aortic valve aortopathy
From 1% to 2% of people have a bicuspid aortic valve, with a 3-to-1 male predominance.14,15 Aortic dilation occurs in 35% to 80% of people who have a bicuspid aortic valve, conferring a risk of dissection 8 times higher than in the general population.16–18
The pathogenic mechanisms that lead to this condition are widely debated, although a combination of genetic defects leading to intrinsic weakening of the aortic wall and hemodynamic effects likely contribute.19 Evidence of hemodynamic contributions to aortic dilation comes from findings that particular patterns of cusp fusion of the bicuspid aortic valve result in changes in transvalvular flow, placing more stress on specific regions of the ascending aorta.20,21 These hemodynamic alterations result in patterns of aortic dilation that depend on cusp fusion and the presence of valvular disease.
Multiple small studies found that replacing bicuspid aortic valves reduced the rate of aortic dilation, suggesting that hemodynamic factors may play a larger role than intrinsic wall properties in genetically susceptible individuals.22,23 However, larger studies are needed before any definitive conclusions can be made.
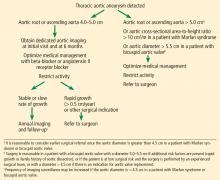
HOW IS ANEURYSM MANAGED ON AN OUTPATIENT BASIS?
Patients with a new diagnosis of TAA should be referred to a cardiologist with expertise in managing aortic disease or to a cardiac surgeon specializing in aortic surgery, depending on the initial size of the aneurysm.
Control blood pressure with beta-blockers
Medical management for patients with TAA has historically been limited to strict blood pressure control aimed at reducing aortic wall stress, mainly with beta-blockers.
Are angiotensin II receptor blockers (ARBs) beneficial? Studies in a mouse model of Marfan syndrome revealed that the ARB losartan attenuated aortic root growth.24 The results of early, small studies in humans were promising,25–27 but larger randomized trials have shown no advantage of losartan over beta-blockers in slowing aortic root growth.28 These negative results led many to question the effectiveness of losartan, although some point out that no studies have shown even beta-blockers to be beneficial in reducing the clinical end points of death or dissection.29 On the other hand, patients with certain FBN1 mutations respond more readily than others to losartan.30 Additional clinical trials of ARBs in Marfan syndrome are ongoing.
Current guidelines recommend stringent blood pressure control and smoking cessation for patients with a small aneurysm not requiring surgery and for those who are considered unsuitable for surgical or percutaneous intervention (level of evidence C, the lowest).2 For patients with TAA, it is considered reasonable to give beta-blockers. Angiotensin-converting enzyme inhibitors or ARBs may be used in combination with beta-blockers, titrated to the lowest tolerable blood pressure without adverse effects (level of evidence B).2
The recommended target blood pressure is less than 140/90 mm Hg, or 130/80 mm Hg in those with diabetes or chronic kidney disease (level of evidence B).2 However, we recommend more stringent blood pressure control: ie, less than 130/80 mm Hg for all patients with aortic aneurysm and a heart rate goal of 70 beats per minute or less, as tolerated.
Activity restriction
Activity restrictions for patients with TAA are largely based on theory, and certain activities may require more modification than others. For example, heavy lifting should be discouraged, as it may increase blood pressure significantly for short periods of time.2,31 The increased wall stress, in theory, could initiate dissection or rupture. However, moderate-intensity aerobic activity is rarely associated with significant elevations in blood pressure and should be encouraged. Stressful emotional states have been anecdotally associated with aortic dissection; thus, measures to reduce stress may offer some benefit.31
Our recommendations. While there are no published guidelines regarding activity restrictions in patients with TAA, we use a graded approach based on aortic diameter:
- 4.0 to 4.4 cm—lift no more than 75 pounds
- 4.5 to 5 cm—lift no more than 50 pounds
- 5 cm—lift no more than 25 pounds.
We also recommend not lifting anything heavier than half of one’s body weight and to avoid breath-holding or performing the Valsalva maneuver while lifting. Although these recommendations are somewhat arbitrary, based on theory and a large clinical experience at our aortic center, they seem reasonable and practical.
Activity restrictions should be stringent and individualized in patients with Marfan, Loeys-Dietz, or Ehlers-Danlos syndrome due to increased risk of dissection or rupture even if the aorta is normal in size.
We sometimes recommend exercise stress testing to assess the heart rate and blood pressure response to exercise, and we are developing research protocols to help tailor activity recommendations.
WHEN SHOULD A PATIENT BE REFERRED?
To a cardiologist at the time of diagnosis
As soon as TAA is diagnosed, the patient should be referred to a cardiologist who has special interest in aortic disease. This will allow for appropriate and timely decisions about medical management, imaging, follow-up, and referral to surgery. Additional recommendations for screening of family members and referral to clinical geneticists can be discussed at this juncture. Activity restrictions should be reviewed at the initial evaluation.
To a surgeon relatively early
Size thresholds for surgical intervention are discussed below, but one should not wait until these thresholds are reached to send the patient for surgical consultation. It is beneficial to the state of mind of a potential surgical candidate to have early discussions pertaining to the types of operations available, their outcomes, and associated risks and benefits. If a patient’s aortic size remains stable over time, he or she may be followed by the cardiologist until significant size or growth has been documented, at which time the patient and surgeon can reconvene to discuss options for definitive treatment.
To a clinical geneticist
If 1 or more first-degree relatives of a patient with TAA or dissection are found to have aneurysmal disease, referral to a clinical geneticist is very important for genetic testing of multiple genes that have been implicated in thoracic aortic aneurysm and dissection.
WHEN SHOULD TAA BE REPAIRED?
Surgery to prevent rupture or dissection remains the definitive treatment of TAA when size thresholds are reached, and symptomatic aneurysm should be operated on regardless of the size. However, rarely are thoracic aneurysms symptomatic unless they rupture or dissect. The size criteria are based on underlying genetic etiology if known and on the behavior and natural course of TAA.
Size and other factors
Treatment should be tailored to the patient’s clinical scenario, family history, and estimated risk of rupture or dissection, balanced against the individual center’s outcomes of elective aortic replacement.32 For example, young and otherwise healthy patients with TAA and a family history of aortic dissection (who may be more likely to have connective tissue disorders such as Marfan syndrome, Loeys-Dietz syndrome, or vascular Ehler-Danlos syndrome) may elect to undergo repair when the aneurysm reaches or nearly reaches the diameter of that of the family member’s aorta when dissection occurred.2 On the other hand, TAA of degenerative etiology (eg, related to smoking or hypertension) measuring less than 5.5 cm in an older patient with comorbidities poses a lower risk of a catastrophic event such as dissection or rupture than the risk of surgery.11
Thresholds for surgery. Once the diameter of the ascending aorta reaches 6 cm, the likelihood of an acute dissection is 31%.11 A similar threshold is reached for the descending aorta at a size of 7 cm.11 Therefore, to avoid high-risk emergency surgery on an acutely dissected aorta, surgery on an ascending aortic aneurysm of degenerative etiology is usually suggested when the aneurysm reaches 5.5 cm or a documented growth rate greater than 0.5 cm/year.2,33
Additionally, in patients already undergoing surgery for valvular or coronary disease, prophylactic aortic replacement is recommended if the ascending aorta is larger than 4.5 cm. The threshold for intervention is lower in patients with connective tissue disease (> 5.0 cm for Marfan syndrome, 4.4–4.6 cm for Loeys-Dietz syndrome).2,33
Observational studies suggest that the risk of aortic complications in patients with bicuspid aortic valve aortopathy is low overall, though significantly greater than in the general population.18,34,35 These findings led to changes in the 2014 American College of Cardiology/American Heart Association guidelines on valvular heart disease,36 suggesting a surgical threshold of 5.5 cm in the absence of significant valve disease or family history of dissection of an aorta of smaller diameter.
A 2015 study of dissection risk in patients with bicuspid aortic valve aortopathy by our group found a dramatic increase in risk of aortic dissection for ascending aortic diameters greater than 5.3 cm, and a gradual increase in risk for aortic root diameters greater than 5.0 cm.37 In addition, a near-constant 3% to 4% risk of dissection was present for aortic diameters ranging from 4.7 cm to 5.0 cm, revealing that watchful waiting carries its own inherent risks.37 In our surgical experience with this population, the hospital mortality rate and risk of stroke from aortic surgery were 0.25% and 0.75%, respectively.37 Thus, the decision to operate for aortic aneurysm in the setting of a bicuspid aortic valve should take into account patient-specific factors and institutional outcomes.
A statement of clarification in the American College of Cardiology/American Heart Association guidelines was published in 2015, recommending surgery for patients with an aortic diameter of 5.0 cm or greater if the patient is at low risk and the surgery is performed by an experienced surgical team at a center with established surgical expertise in this condition.38 However, current recommendations are for surgery at 5.5 cm if the above conditions are not met.
Ratio of aortic cross-sectional area to height
Although size alone has long been used to guide surgical intervention, a recent review from the International Registry of Aortic Dissection revealed that 59% of patients suffered aortic dissection at diameters less than 5.5 cm, and that patients with certain connective tissue diseases such as Loeys-Dietz syndrome or familial thoracic aneurysm and dissection had a documented propensity for dissection at smaller diameters.39–41
Size indices such as the aortic cross-sectional area indexed to height have been implemented in guidelines for certain patient populations (eg, 10 cm2/m in Marfan syndrome) and provide better risk stratification than size cutoffs alone.2,42
The ratio of aortic cross-sectional area to the patient’s height has also been applied to patients with bicuspid aortic valve-associated aortopathy and to those with a dilated aorta and a tricuspid aortic valve.43,44 Notably, a ratio greater than 10 cm2/m has been associated with aortic dissection in these groups, and this cutoff provides better stratification for prediction of death than traditional size metrics.27,28
HOW SHOULD PATIENTS BE SCREENED? WHAT FOLLOW-UP IS NECESSARY?
Initial screening and follow-up
Follow-up of TAA depends on the initial aortic size or rate of growth, or both. For patients presenting for the first time with TAA, it is reasonable to obtain definitive aortic imaging with CT or magnetic resonance angiography (MRA), then to repeat imaging at 6 months to document stability. If the aortic dimensions remain stable, then annual follow-up with CT or MRA is reasonable.2
Our flow chart of initial screening and follow-up is shown in Figure 5.
Screening of family members
In our center, we routinely recommend screening of all first-degree relatives of patients with TAA. Aortic imaging with echocardiography plus CT or MRI should be considered to detect asymptomatic disease.2 In patients with a strong family history (ie, multiple relatives affected with aortic aneurysm, dissection, or sudden cardiac death), genetic screening and testing for known mutations are recommended for the patient as well as for the family members.
If a mutation is identified in a family, then first-degree relatives should undergo genetic screening for the mutation and aortic imaging.2 Imaging in second-degree relatives may also be considered if one or more first-degree relatives are found to have aortic dilation.2
We recommend similar screening of first-degree family members of patients with bicuspid aortic valve aortopathy. In patients with young children, we recommend obtaining an echocardiogram of the child to look for a bicuspid aortic valve or aortic dilation. If an abnormality is detected or suspected, dedicated imaging with MRA to assess aortic dimensions is warranted.
BACK TO OUR PATIENT WITH A BICUSPID AORTIC VALVE
Our patient with a bicuspid aortic valve had a 4.6-cm root, an ascending aortic aneurysm, and several affected family members.
We would obtain dedicated aortic imaging at this patient’s initial visit with either gated CT with contrast or MRA, and we would obtain a cardioaortic surgery consult. We would repeat these studies at a follow-up visit 6 months later to detect any aortic growth compared with initial studies, and follow up annually thereafter. Echocardiography can also be done at the initial visit to determine if valvular disease is present that may influence clinical decisions.
Surgery would likely be recommended once the root reached a maximum area-to-height ratio greater than 10 cm2/m, or if the valve became severely dysfunctional during follow-up.
BACK TO OUR PATIENT WITH MARFAN SYNDROME
The young woman with Marfan syndrome has a 4.6-cm aortic root aneurysm and 2+ aortic insufficiency. Her question pertains to the threshold at which an operation would be considered. This question is complicated and is influenced by several concurrent clinical features in her presentation.
Starting with size criteria, patients with Marfan syndrome should be considered for elective aortic root repair at a diameter greater than 5 cm. However, an aortic cross-sectional area-to-height ratio greater than 10 cm2/m may provide a more robust metric for clinical decision-making than aortic diameter alone. Additional factors such as degree of aortic insufficiency and deleterious left ventricular remodeling may urge one to consider aortic root repair at a diameter of 4.5 cm.
These factors, including rate of growth and the surgeon’s assessment about his or her ability to preserve the aortic valve during repair, should be considered collectively in this scenario.
- Elefteriades JA, Farkas EA. Thoracic aortic aneurysm clinically pertinent controversies and uncertainties. J Am Coll Cardiol 2010; 55(9):841–857. doi:10.1016/j.jacc.2009.08.084
- Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: executive summary. Anesth Analg 2010; 111(2):279–315. doi:10.1213/ANE.0b013e3181dd869b
- Clouse WD, Hallett JW Jr, Schaff HV, Gayari MM, Ilstrup DM, Melton LJ 3rd. Improved prognosis of thoracic aortic aneurysms: a population-based study. JAMA 1998; 280(22):1926–1929. pmid:9851478
- Olsson C, Thelin S, Ståhle E, Ekbom A, Granath F. Thoracic aortic aneurysm and dissection: increasing prevalence and improved outcomes reported in a nationwide population-based study of more than 14,000 cases from 1987 to 2002. Circulation 2006; 114(24):2611–2618. doi:10.1161/CIRCULATIONAHA.106.630400
- Clouse WD, Hallett JW Jr, Schaff HV, et al. Acute aortic dissection: population-based incidence compared with degenerative aortic aneurysm rupture. Mayo Clin Proc 2004; 79(2):176–180. pmid:14959911
- US Centers for Disease Control and Prevention (CDC). National Center for Injury Prevention and Control. WISQARS leading causes of death reports, 1999 – 2007. https://webappa.cdc.gov/sasweb/ncipc/leadcaus10.html. Accessed May 21, 2018.
- Hansen MS, Nogareda GJ, Hutchison SJ. Frequency of and inappropriate treatment of misdiagnosis of acute aortic dissection. Am J Cardiol 2007; 99(6):852–856. doi:10.1016/j.amjcard.2006.10.055
- Goldfinger JZ, Halperin JL, Marin ML, Stewart AS, Eagle KA, Fuster V. Thoracic aortic aneurysm and dissection. J Am Coll Cardiol 2014; 64(16):1725–1739. doi:10.1016/j.jacc.2014.08.025
- Kumar V, Abbas A, Aster J. Robbins and Cotran Pathologic Basis of Disease. 9th ed. Philadelphia, PA: Elsevier/Saunders; 2015.
- Wolak A, Gransar H, Thomson LE, et al. Aortic size assessment by noncontrast cardiac computed tomography: normal limits by age, gender, and body surface area. JACC Cardiovasc Imaging 2008; 1(2):200–209. doi:10.1016/j.jcmg.2007.11.005
- Elefteriades JA. Natural history of thoracic aortic aneurysms: indications for surgery, and surgical versus nonsurgical risks. Ann Thorac Surg 2002; 74(5):S1877–S1880; discussion S1892–S1898. pmid:12440685
- Smith AD, Schoenhagen P. CT imaging for acute aortic syndrome. Cleve Clin J Med 2008; 75(1):7–17. pmid:18236724
- Cury M, Zeidan F, Lobato AC. Aortic disease in the young: genetic aneurysm syndromes, connective tissue disorders, and familial aortic aneurysms and dissections. Int J Vasc Med 2013(2013); 2013:267215. doi:10.1155/2013/267215
- Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol 2002; 39(12):1890–1900. doi:10.1016/S0735-1097(02)01886-7
- Fedak PW, Verma S, David TE, Leask RL, Weisel RD, Butany J. Clinical and pathophysiological implications of a bicuspid aortic valve. Circulation 2002; 106(8):900–904. pmid:12186790
- Della Corte A, Bancone C, Quarto C, et al. Predictors of ascending aortic dilatation with bicuspid aortic valve: a wide spectrum of disease expression. Eur J Cardiothorac Surg 2007; 31(3):397–405. doi:10.1016/j.ejcts.2006.12.006
- Jackson V, Petrini J, Caidahl K, et al. Bicuspid aortic valve leaflet morphology in relation to aortic root morphology: a study of 300 patients undergoing open-heart surgery. Eur J Cardiothorac Surg 2011; 40(3):e118–e124. doi:10.1016/j.ejcts.2011.04.014
- Michelena HI, Khanna AD, Mahoney D, et al. Incidence of aortic complications in patients with bicuspid aortic valves. JAMA 2011; 306(10):1104–1112. doi:10.1001/jama.2011.1286
- Verma S, Siu SC. Aortic dilatation in patients with bicuspid aortic valve. N Engl J Med 2014; 370(20):1920–1929. doi:10.1056/NEJMra1207059
- Barker AJ, Markl M, Bürk J, et al. Bicuspid aortic valve is associated with altered wall shear stress in the ascending aorta. Circ Cardiovasc Imaging 2012; 5(4):457–466. doi:10.1161/CIRCIMAGING.112.973370
- Hope MD, Hope TA, Meadows AK, et al. Bicuspid aortic valve: four-dimensional MR evaluation of ascending aortic systolic flow patterns. Radiology 2010; 255(1):53–61. doi:10.1148/radiol.09091437
- Abdulkareem N, Soppa G, Jones S, Valencia O, Smelt J, Jahangiri M. Dilatation of the remaining aorta after aortic valve or aortic root replacement in patients with bicuspid aortic valve: a 5-year follow-up. Ann Thorac Surg 2013; 96(1):43–49. doi:10.1016/j.athoracsur.2013.03.086
- Regeer MV, Versteegh MI, Klautz RJ, et al. Effect of aortic valve replacement on aortic root dilatation rate in patients with bicuspid and tricuspid aortic valves. Ann Thorac Surg 2016; 102(6):1981–1987. doi:10.1016/j.athoracsur.2016.05.038
- Habashi JP, Judge DP, Holm TM, et al. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science 2006; 312(5770):117–121. doi:10.1126/science.1124287
- Brooke BS, Habashi JP, Judge DP, Patel N, Loeys B, Dietz HC 3rd. Angiotensin II blockade and aortic-root dilation in Marfan’s syndrome. N Engl J Med 2008; 358(26):2787–2795. doi:10.1056/NEJMoa0706585
- Chiu HH, Wu MH, Wang JK, et al. Losartan added to ß-blockade therapy for aortic root dilation in Marfan syndrome: a randomized, open-label pilot study. Mayo Clin Proc 2013; 88(3):271–276. doi:10.1016/j.mayocp.2012.11.005
- Groenink M, den Hartog AW, Franken R, et al. Losartan reduces aortic dilatation rate in adults with Marfan syndrome: a randomized controlled trial. Eur Heart J 2013; 34(45):3491–3500. doi:10.1093/eurheartj/eht334
- Lacro RV, Dietz HC, Sleeper LA, et al; Pediatric Heart Network Investigators. Atenolol versus losartan in children and young adults with Marfan’s syndrome. N Engl J Med 2014; 371(22):2061–2071. doi:10.1056/NEJMoa1404731
- Ziganshin BA, Mukherjee SK, Elefteriades JA, et al. Atenolol versus losartan in Marfan’s syndrome (letters). N Engl J Med 2015; 372(10):977–981. doi:10.1056/NEJMc1500128
- Franken R, den Hartog AW, Radonic T, et al. Beneficial outcome of losartan therapy depends on type of FBN1 mutation in Marfan syndrome. Circ Cardiovasc Genet 2015; 8(2):383–388. doi:10.1161/CIRCGENETICS.114.000950
- Elefteriades JA. Thoracic aortic aneurysm: reading the enemy’s playbook. Curr Probl Cardiol 2008; 33(5):203–277. doi:10.1016/j.cpcardiol.2008.01.004
- Idrees JJ, Roselli EE, Lowry AM, et al. Outcomes after elective proximal aortic replacement: a matched comparison of isolated versus multicomponent operations. Ann Thorac Surg 2016; 101(6):2185–2192. doi:10.1016/j.athoracsur.2015.12.026
- Hiratzka LF, Creager MA, Isselbacher EM, et al. Surgery for aortic dilatation in patients with bicuspid aortic valves: a statement of clarification from the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Thorac Cardiovasc Surg 2016; 151(4):959–966. doi:10.1016/j.jtcvs.2015.12.001
- Tzemos N, Therrien J, Yip J, et al. Outcomes in adults with bicuspid aortic valves. JAMA 2008; 300(11):1317–1325. doi:10.1001/jama.300.11.1317
- Davies RR, Goldstein LJ, Coady MA, et al. Yearly rupture or dissection rates for thoracic aortic aneurysms: simple prediction based on size. Ann Thorac Surg 2002; 73(1):17–28. pmid:11834007
- Nishimura RA, Otto CM, Bono RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American heart Association Task Force on Practice Guidelines. Circulation 2014; 129(23):2440–2492. doi:10.1161/CIR.0000000000000029
- Wojnarski CM, Svensson LG, Roselli EE, et al. Aortic dissection in patients with bicuspid aortic valve–associated aneurysms. Ann Thorac Surg 2015; 100(5):1666–1674. doi:10.1016/j.athoracsur.2015.04.126
- Hiratzka LF, Creager MA, Isselbacher EM, et al. Surgery for aortic dilatation in patients with bicuspid aortic valves: a statement of clarification from the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2016; 133(7):680–686. doi:10.1161/CIR.0000000000000331
- Pape LA, Tsai TT, Isselbacher EM, et al; International Registry of Acute Aortic Dissection (IRAD) Investigators. Aortic diameter > or = 5.5 cm is not a good predictor of type A aortic dissection: observations from the International Registry of Acute Aortic Dissection (IRAD). Circulation 2007; 116(10):1120–1127. doi:10.1161/CIRCULATIONAHA.107.702720
- Loeys BL, Schwarze U, Holm T, et al. Aneurysm syndromes caused by mutations in the TGF-beta receptor. N Engl J Med 2006; 355(8):788–798. doi:10.1056/NEJMoa055695
- Guo DC, Pannu H, Tran-Fadulu V, et al. Mutations in smooth muscle alpha-actin (ACTA2) lead to thoracic aortic aneurysms and dissections. Nat Genet 2007; 39(12):1488–1493. doi:10.1038/ng.2007.6
- Svensson LG, Khitin L. Aortic cross-sectional area/height ratio timing of aortic surgery in asymptomatic patients with Marfan syndrome. J Thorac Cardiovasc Surg 2002; 123(2):360–361. pmid:11828302
- Svensson LG, Kim KH, Lytle BW, Cosgrove DM. Relationship of aortic cross-sectional area to height ratio and the risk of aortic dissection in patients with bicuspid aortic valves. J Thorac Cardiovasc Surg 2003; 126(3):892–893. pmid:14502185
- Masri A, Kalahasti V, Svensson LG, et al. Aortic cross-sectional area/height ratio and outcomes in patients with a trileaflet aortic valve and a dilated aorta. Circulation 2016; 134(22):1724–1737. doi:10.1161/CIRCULATIONAHA.116.022995
Thoracic aortic aneurysm (TAA) needs to be detected, monitored, and managed in a timely manner to prevent a serious consequence such as acute dissection or rupture. But only about 5% of patients experience symptoms before an acute event occurs, and for the other 95% the first “symptom” is often death.1 Most cases are detected either incidentally with echocardiography, computed tomography (CT), or magnetic resonance imaging (MRI) during workup for another condition. Patients may also be diagnosed during workup of a murmur or after a family member is found to have an aneurysm. Therefore, its true incidence is difficult to determine.2
With these facts in mind, how would you manage the following 2 cases?
Case 1: Bicuspid aortic valve, ascending aortic aneurysm
A 45-year-old man with stage 1 hypertension presents for evaluation of a bicuspid aortic valve and ascending aortic aneurysm. He has several first-degree relatives with similar conditions, and his brother recently underwent elective aortic repair. At the urging of his primary care physician, he underwent screening echocardiography, which demonstrated a “dilated root and ascending aorta” 4.6 cm in diameter. He presents today to discuss management options and how the aneurysm could affect his everyday life.
Case 2: Marfan syndrome in a young woman
A 24-year-old woman with Marfan syndrome diagnosed in adolescence presents for annual follow-up. She has many family members with the same condition, and several have undergone prophylactic aortic root repair. Her aortic root has been monitored annually for progression of dilation, and today it is 4.6 cm in diameter, a 3-mm increase from the last measurement. She has grade 2+ aortic insufficiency (on a scale of 1+ to 4+) based on echocardiography, but she has no symptoms. She is curious about what size her aortic root will need to reach for surgery to be considered.
LIKELY UNDERDETECTED
TAA is being detected more often than in the past thanks to better detection methods and heightened awareness among physicians and patients. While an incidence rate of 10.4 per 100,000 patient-years is often cited,3 this figure likely underestimates the true incidence of this clinically silent condition. The most robust data come from studies based on in-hospital diagnostic codes coupled with data from autopsies for out-of-hospital deaths.
Olsson et al,4 in a 2016 study in Sweden, found the incidence of TAA and aortic dissection to be 16.3 per 100,000 per year for men and 9.1 per 100,000 per year for women.
Clouse et al5 reported the incidence of thoracic aortic dissection as 3.5 per 100,000 patient-years, and the same figure for thoracic aortic rupture.
Aneurysmal disease accounts for 52,000 deaths per year in the United States, making it the 19th most common cause of death.6 These figures are likely lower than the true mortality rate for this condition, given that aortic dissection is often mistaken for acute myocardial infarction or other acute event if an autopsy is not done to confirm the cause of death.7
RISK FACTORS FOR THORACIC AORTIC ANEURYSM
Risk factors for TAA include genetic conditions that lead to aortic medial weakness or destruction such as Loeys-Dietz syndrome and Marfan syndrome.2 In addition, family history is important even in the absence of known genetic mutations. Other risk factors include conditions that increase aortic wall stress, such as hypertension, cocaine abuse, extreme weightlifting, trauma, and aortic coarctation.2
DIAMETER INCREASES WITH AGE, BODY SURFACE AREA
Normal dimensions for the aortic segments differ depending on age, sex, and body surface area.8,44,45 The size of the aortic root may also vary depending on how it is measured, due to the root’s trefoil shape. Measured sinus to sinus, the root is larger than when measured sinus to commissure on CT angiography or cardiac MRI. It is also larger when measured leading edge to leading edge than inner edge to inner edge on echocardiography.10
TAA is defined as an aortic diameter at least 50% greater than the upper limit of normal.8
Geometric changes in the curvature of the ascending aorta, aortic arch, and descending thoracic aorta can occur as the result of hypertension, atherosclerosis, or connective tissue disease.
HOW IS TAA DIAGNOSED?
Imaging tests
It is particularly important to obtain a gated CTA image in patients with aortic root aneurysm to avoid motion artifact and possible erroneous measurements. Gated CTA is done with electrocardiographic synchronization and allows for image processing to correct for cardiac motion.
HOW IS TAA CLASSIFIED?
TAA can be caused by a variety of inherited and sporadic conditions. These differences in pathogenesis lend themselves to classification of aneurysms into groups. Table 3 highlights the most common conditions associated with TAA.13
Bicuspid aortic valve aortopathy
From 1% to 2% of people have a bicuspid aortic valve, with a 3-to-1 male predominance.14,15 Aortic dilation occurs in 35% to 80% of people who have a bicuspid aortic valve, conferring a risk of dissection 8 times higher than in the general population.16–18
The pathogenic mechanisms that lead to this condition are widely debated, although a combination of genetic defects leading to intrinsic weakening of the aortic wall and hemodynamic effects likely contribute.19 Evidence of hemodynamic contributions to aortic dilation comes from findings that particular patterns of cusp fusion of the bicuspid aortic valve result in changes in transvalvular flow, placing more stress on specific regions of the ascending aorta.20,21 These hemodynamic alterations result in patterns of aortic dilation that depend on cusp fusion and the presence of valvular disease.
Multiple small studies found that replacing bicuspid aortic valves reduced the rate of aortic dilation, suggesting that hemodynamic factors may play a larger role than intrinsic wall properties in genetically susceptible individuals.22,23 However, larger studies are needed before any definitive conclusions can be made.
HOW IS ANEURYSM MANAGED ON AN OUTPATIENT BASIS?
Patients with a new diagnosis of TAA should be referred to a cardiologist with expertise in managing aortic disease or to a cardiac surgeon specializing in aortic surgery, depending on the initial size of the aneurysm.
Control blood pressure with beta-blockers
Medical management for patients with TAA has historically been limited to strict blood pressure control aimed at reducing aortic wall stress, mainly with beta-blockers.
Are angiotensin II receptor blockers (ARBs) beneficial? Studies in a mouse model of Marfan syndrome revealed that the ARB losartan attenuated aortic root growth.24 The results of early, small studies in humans were promising,25–27 but larger randomized trials have shown no advantage of losartan over beta-blockers in slowing aortic root growth.28 These negative results led many to question the effectiveness of losartan, although some point out that no studies have shown even beta-blockers to be beneficial in reducing the clinical end points of death or dissection.29 On the other hand, patients with certain FBN1 mutations respond more readily than others to losartan.30 Additional clinical trials of ARBs in Marfan syndrome are ongoing.
Current guidelines recommend stringent blood pressure control and smoking cessation for patients with a small aneurysm not requiring surgery and for those who are considered unsuitable for surgical or percutaneous intervention (level of evidence C, the lowest).2 For patients with TAA, it is considered reasonable to give beta-blockers. Angiotensin-converting enzyme inhibitors or ARBs may be used in combination with beta-blockers, titrated to the lowest tolerable blood pressure without adverse effects (level of evidence B).2
The recommended target blood pressure is less than 140/90 mm Hg, or 130/80 mm Hg in those with diabetes or chronic kidney disease (level of evidence B).2 However, we recommend more stringent blood pressure control: ie, less than 130/80 mm Hg for all patients with aortic aneurysm and a heart rate goal of 70 beats per minute or less, as tolerated.
Activity restriction
Activity restrictions for patients with TAA are largely based on theory, and certain activities may require more modification than others. For example, heavy lifting should be discouraged, as it may increase blood pressure significantly for short periods of time.2,31 The increased wall stress, in theory, could initiate dissection or rupture. However, moderate-intensity aerobic activity is rarely associated with significant elevations in blood pressure and should be encouraged. Stressful emotional states have been anecdotally associated with aortic dissection; thus, measures to reduce stress may offer some benefit.31
Our recommendations. While there are no published guidelines regarding activity restrictions in patients with TAA, we use a graded approach based on aortic diameter:
- 4.0 to 4.4 cm—lift no more than 75 pounds
- 4.5 to 5 cm—lift no more than 50 pounds
- 5 cm—lift no more than 25 pounds.
We also recommend not lifting anything heavier than half of one’s body weight and to avoid breath-holding or performing the Valsalva maneuver while lifting. Although these recommendations are somewhat arbitrary, based on theory and a large clinical experience at our aortic center, they seem reasonable and practical.
Activity restrictions should be stringent and individualized in patients with Marfan, Loeys-Dietz, or Ehlers-Danlos syndrome due to increased risk of dissection or rupture even if the aorta is normal in size.
We sometimes recommend exercise stress testing to assess the heart rate and blood pressure response to exercise, and we are developing research protocols to help tailor activity recommendations.
WHEN SHOULD A PATIENT BE REFERRED?
To a cardiologist at the time of diagnosis
As soon as TAA is diagnosed, the patient should be referred to a cardiologist who has special interest in aortic disease. This will allow for appropriate and timely decisions about medical management, imaging, follow-up, and referral to surgery. Additional recommendations for screening of family members and referral to clinical geneticists can be discussed at this juncture. Activity restrictions should be reviewed at the initial evaluation.
To a surgeon relatively early
Size thresholds for surgical intervention are discussed below, but one should not wait until these thresholds are reached to send the patient for surgical consultation. It is beneficial to the state of mind of a potential surgical candidate to have early discussions pertaining to the types of operations available, their outcomes, and associated risks and benefits. If a patient’s aortic size remains stable over time, he or she may be followed by the cardiologist until significant size or growth has been documented, at which time the patient and surgeon can reconvene to discuss options for definitive treatment.
To a clinical geneticist
If 1 or more first-degree relatives of a patient with TAA or dissection are found to have aneurysmal disease, referral to a clinical geneticist is very important for genetic testing of multiple genes that have been implicated in thoracic aortic aneurysm and dissection.
WHEN SHOULD TAA BE REPAIRED?
Surgery to prevent rupture or dissection remains the definitive treatment of TAA when size thresholds are reached, and symptomatic aneurysm should be operated on regardless of the size. However, rarely are thoracic aneurysms symptomatic unless they rupture or dissect. The size criteria are based on underlying genetic etiology if known and on the behavior and natural course of TAA.
Size and other factors
Treatment should be tailored to the patient’s clinical scenario, family history, and estimated risk of rupture or dissection, balanced against the individual center’s outcomes of elective aortic replacement.32 For example, young and otherwise healthy patients with TAA and a family history of aortic dissection (who may be more likely to have connective tissue disorders such as Marfan syndrome, Loeys-Dietz syndrome, or vascular Ehler-Danlos syndrome) may elect to undergo repair when the aneurysm reaches or nearly reaches the diameter of that of the family member’s aorta when dissection occurred.2 On the other hand, TAA of degenerative etiology (eg, related to smoking or hypertension) measuring less than 5.5 cm in an older patient with comorbidities poses a lower risk of a catastrophic event such as dissection or rupture than the risk of surgery.11
Thresholds for surgery. Once the diameter of the ascending aorta reaches 6 cm, the likelihood of an acute dissection is 31%.11 A similar threshold is reached for the descending aorta at a size of 7 cm.11 Therefore, to avoid high-risk emergency surgery on an acutely dissected aorta, surgery on an ascending aortic aneurysm of degenerative etiology is usually suggested when the aneurysm reaches 5.5 cm or a documented growth rate greater than 0.5 cm/year.2,33
Additionally, in patients already undergoing surgery for valvular or coronary disease, prophylactic aortic replacement is recommended if the ascending aorta is larger than 4.5 cm. The threshold for intervention is lower in patients with connective tissue disease (> 5.0 cm for Marfan syndrome, 4.4–4.6 cm for Loeys-Dietz syndrome).2,33
Observational studies suggest that the risk of aortic complications in patients with bicuspid aortic valve aortopathy is low overall, though significantly greater than in the general population.18,34,35 These findings led to changes in the 2014 American College of Cardiology/American Heart Association guidelines on valvular heart disease,36 suggesting a surgical threshold of 5.5 cm in the absence of significant valve disease or family history of dissection of an aorta of smaller diameter.
A 2015 study of dissection risk in patients with bicuspid aortic valve aortopathy by our group found a dramatic increase in risk of aortic dissection for ascending aortic diameters greater than 5.3 cm, and a gradual increase in risk for aortic root diameters greater than 5.0 cm.37 In addition, a near-constant 3% to 4% risk of dissection was present for aortic diameters ranging from 4.7 cm to 5.0 cm, revealing that watchful waiting carries its own inherent risks.37 In our surgical experience with this population, the hospital mortality rate and risk of stroke from aortic surgery were 0.25% and 0.75%, respectively.37 Thus, the decision to operate for aortic aneurysm in the setting of a bicuspid aortic valve should take into account patient-specific factors and institutional outcomes.
A statement of clarification in the American College of Cardiology/American Heart Association guidelines was published in 2015, recommending surgery for patients with an aortic diameter of 5.0 cm or greater if the patient is at low risk and the surgery is performed by an experienced surgical team at a center with established surgical expertise in this condition.38 However, current recommendations are for surgery at 5.5 cm if the above conditions are not met.
Ratio of aortic cross-sectional area to height
Although size alone has long been used to guide surgical intervention, a recent review from the International Registry of Aortic Dissection revealed that 59% of patients suffered aortic dissection at diameters less than 5.5 cm, and that patients with certain connective tissue diseases such as Loeys-Dietz syndrome or familial thoracic aneurysm and dissection had a documented propensity for dissection at smaller diameters.39–41
Size indices such as the aortic cross-sectional area indexed to height have been implemented in guidelines for certain patient populations (eg, 10 cm2/m in Marfan syndrome) and provide better risk stratification than size cutoffs alone.2,42
The ratio of aortic cross-sectional area to the patient’s height has also been applied to patients with bicuspid aortic valve-associated aortopathy and to those with a dilated aorta and a tricuspid aortic valve.43,44 Notably, a ratio greater than 10 cm2/m has been associated with aortic dissection in these groups, and this cutoff provides better stratification for prediction of death than traditional size metrics.27,28
HOW SHOULD PATIENTS BE SCREENED? WHAT FOLLOW-UP IS NECESSARY?
Initial screening and follow-up
Follow-up of TAA depends on the initial aortic size or rate of growth, or both. For patients presenting for the first time with TAA, it is reasonable to obtain definitive aortic imaging with CT or magnetic resonance angiography (MRA), then to repeat imaging at 6 months to document stability. If the aortic dimensions remain stable, then annual follow-up with CT or MRA is reasonable.2
Our flow chart of initial screening and follow-up is shown in Figure 5.
Screening of family members
In our center, we routinely recommend screening of all first-degree relatives of patients with TAA. Aortic imaging with echocardiography plus CT or MRI should be considered to detect asymptomatic disease.2 In patients with a strong family history (ie, multiple relatives affected with aortic aneurysm, dissection, or sudden cardiac death), genetic screening and testing for known mutations are recommended for the patient as well as for the family members.
If a mutation is identified in a family, then first-degree relatives should undergo genetic screening for the mutation and aortic imaging.2 Imaging in second-degree relatives may also be considered if one or more first-degree relatives are found to have aortic dilation.2
We recommend similar screening of first-degree family members of patients with bicuspid aortic valve aortopathy. In patients with young children, we recommend obtaining an echocardiogram of the child to look for a bicuspid aortic valve or aortic dilation. If an abnormality is detected or suspected, dedicated imaging with MRA to assess aortic dimensions is warranted.
BACK TO OUR PATIENT WITH A BICUSPID AORTIC VALVE
Our patient with a bicuspid aortic valve had a 4.6-cm root, an ascending aortic aneurysm, and several affected family members.
We would obtain dedicated aortic imaging at this patient’s initial visit with either gated CT with contrast or MRA, and we would obtain a cardioaortic surgery consult. We would repeat these studies at a follow-up visit 6 months later to detect any aortic growth compared with initial studies, and follow up annually thereafter. Echocardiography can also be done at the initial visit to determine if valvular disease is present that may influence clinical decisions.
Surgery would likely be recommended once the root reached a maximum area-to-height ratio greater than 10 cm2/m, or if the valve became severely dysfunctional during follow-up.
BACK TO OUR PATIENT WITH MARFAN SYNDROME
The young woman with Marfan syndrome has a 4.6-cm aortic root aneurysm and 2+ aortic insufficiency. Her question pertains to the threshold at which an operation would be considered. This question is complicated and is influenced by several concurrent clinical features in her presentation.
Starting with size criteria, patients with Marfan syndrome should be considered for elective aortic root repair at a diameter greater than 5 cm. However, an aortic cross-sectional area-to-height ratio greater than 10 cm2/m may provide a more robust metric for clinical decision-making than aortic diameter alone. Additional factors such as degree of aortic insufficiency and deleterious left ventricular remodeling may urge one to consider aortic root repair at a diameter of 4.5 cm.
These factors, including rate of growth and the surgeon’s assessment about his or her ability to preserve the aortic valve during repair, should be considered collectively in this scenario.
Thoracic aortic aneurysm (TAA) needs to be detected, monitored, and managed in a timely manner to prevent a serious consequence such as acute dissection or rupture. But only about 5% of patients experience symptoms before an acute event occurs, and for the other 95% the first “symptom” is often death.1 Most cases are detected either incidentally with echocardiography, computed tomography (CT), or magnetic resonance imaging (MRI) during workup for another condition. Patients may also be diagnosed during workup of a murmur or after a family member is found to have an aneurysm. Therefore, its true incidence is difficult to determine.2
With these facts in mind, how would you manage the following 2 cases?
Case 1: Bicuspid aortic valve, ascending aortic aneurysm
A 45-year-old man with stage 1 hypertension presents for evaluation of a bicuspid aortic valve and ascending aortic aneurysm. He has several first-degree relatives with similar conditions, and his brother recently underwent elective aortic repair. At the urging of his primary care physician, he underwent screening echocardiography, which demonstrated a “dilated root and ascending aorta” 4.6 cm in diameter. He presents today to discuss management options and how the aneurysm could affect his everyday life.
Case 2: Marfan syndrome in a young woman
A 24-year-old woman with Marfan syndrome diagnosed in adolescence presents for annual follow-up. She has many family members with the same condition, and several have undergone prophylactic aortic root repair. Her aortic root has been monitored annually for progression of dilation, and today it is 4.6 cm in diameter, a 3-mm increase from the last measurement. She has grade 2+ aortic insufficiency (on a scale of 1+ to 4+) based on echocardiography, but she has no symptoms. She is curious about what size her aortic root will need to reach for surgery to be considered.
LIKELY UNDERDETECTED
TAA is being detected more often than in the past thanks to better detection methods and heightened awareness among physicians and patients. While an incidence rate of 10.4 per 100,000 patient-years is often cited,3 this figure likely underestimates the true incidence of this clinically silent condition. The most robust data come from studies based on in-hospital diagnostic codes coupled with data from autopsies for out-of-hospital deaths.
Olsson et al,4 in a 2016 study in Sweden, found the incidence of TAA and aortic dissection to be 16.3 per 100,000 per year for men and 9.1 per 100,000 per year for women.
Clouse et al5 reported the incidence of thoracic aortic dissection as 3.5 per 100,000 patient-years, and the same figure for thoracic aortic rupture.
Aneurysmal disease accounts for 52,000 deaths per year in the United States, making it the 19th most common cause of death.6 These figures are likely lower than the true mortality rate for this condition, given that aortic dissection is often mistaken for acute myocardial infarction or other acute event if an autopsy is not done to confirm the cause of death.7
RISK FACTORS FOR THORACIC AORTIC ANEURYSM
Risk factors for TAA include genetic conditions that lead to aortic medial weakness or destruction such as Loeys-Dietz syndrome and Marfan syndrome.2 In addition, family history is important even in the absence of known genetic mutations. Other risk factors include conditions that increase aortic wall stress, such as hypertension, cocaine abuse, extreme weightlifting, trauma, and aortic coarctation.2
DIAMETER INCREASES WITH AGE, BODY SURFACE AREA
Normal dimensions for the aortic segments differ depending on age, sex, and body surface area.8,44,45 The size of the aortic root may also vary depending on how it is measured, due to the root’s trefoil shape. Measured sinus to sinus, the root is larger than when measured sinus to commissure on CT angiography or cardiac MRI. It is also larger when measured leading edge to leading edge than inner edge to inner edge on echocardiography.10
TAA is defined as an aortic diameter at least 50% greater than the upper limit of normal.8
Geometric changes in the curvature of the ascending aorta, aortic arch, and descending thoracic aorta can occur as the result of hypertension, atherosclerosis, or connective tissue disease.
HOW IS TAA DIAGNOSED?
Imaging tests
It is particularly important to obtain a gated CTA image in patients with aortic root aneurysm to avoid motion artifact and possible erroneous measurements. Gated CTA is done with electrocardiographic synchronization and allows for image processing to correct for cardiac motion.
HOW IS TAA CLASSIFIED?
TAA can be caused by a variety of inherited and sporadic conditions. These differences in pathogenesis lend themselves to classification of aneurysms into groups. Table 3 highlights the most common conditions associated with TAA.13
Bicuspid aortic valve aortopathy
From 1% to 2% of people have a bicuspid aortic valve, with a 3-to-1 male predominance.14,15 Aortic dilation occurs in 35% to 80% of people who have a bicuspid aortic valve, conferring a risk of dissection 8 times higher than in the general population.16–18
The pathogenic mechanisms that lead to this condition are widely debated, although a combination of genetic defects leading to intrinsic weakening of the aortic wall and hemodynamic effects likely contribute.19 Evidence of hemodynamic contributions to aortic dilation comes from findings that particular patterns of cusp fusion of the bicuspid aortic valve result in changes in transvalvular flow, placing more stress on specific regions of the ascending aorta.20,21 These hemodynamic alterations result in patterns of aortic dilation that depend on cusp fusion and the presence of valvular disease.
Multiple small studies found that replacing bicuspid aortic valves reduced the rate of aortic dilation, suggesting that hemodynamic factors may play a larger role than intrinsic wall properties in genetically susceptible individuals.22,23 However, larger studies are needed before any definitive conclusions can be made.
HOW IS ANEURYSM MANAGED ON AN OUTPATIENT BASIS?
Patients with a new diagnosis of TAA should be referred to a cardiologist with expertise in managing aortic disease or to a cardiac surgeon specializing in aortic surgery, depending on the initial size of the aneurysm.
Control blood pressure with beta-blockers
Medical management for patients with TAA has historically been limited to strict blood pressure control aimed at reducing aortic wall stress, mainly with beta-blockers.
Are angiotensin II receptor blockers (ARBs) beneficial? Studies in a mouse model of Marfan syndrome revealed that the ARB losartan attenuated aortic root growth.24 The results of early, small studies in humans were promising,25–27 but larger randomized trials have shown no advantage of losartan over beta-blockers in slowing aortic root growth.28 These negative results led many to question the effectiveness of losartan, although some point out that no studies have shown even beta-blockers to be beneficial in reducing the clinical end points of death or dissection.29 On the other hand, patients with certain FBN1 mutations respond more readily than others to losartan.30 Additional clinical trials of ARBs in Marfan syndrome are ongoing.
Current guidelines recommend stringent blood pressure control and smoking cessation for patients with a small aneurysm not requiring surgery and for those who are considered unsuitable for surgical or percutaneous intervention (level of evidence C, the lowest).2 For patients with TAA, it is considered reasonable to give beta-blockers. Angiotensin-converting enzyme inhibitors or ARBs may be used in combination with beta-blockers, titrated to the lowest tolerable blood pressure without adverse effects (level of evidence B).2
The recommended target blood pressure is less than 140/90 mm Hg, or 130/80 mm Hg in those with diabetes or chronic kidney disease (level of evidence B).2 However, we recommend more stringent blood pressure control: ie, less than 130/80 mm Hg for all patients with aortic aneurysm and a heart rate goal of 70 beats per minute or less, as tolerated.
Activity restriction
Activity restrictions for patients with TAA are largely based on theory, and certain activities may require more modification than others. For example, heavy lifting should be discouraged, as it may increase blood pressure significantly for short periods of time.2,31 The increased wall stress, in theory, could initiate dissection or rupture. However, moderate-intensity aerobic activity is rarely associated with significant elevations in blood pressure and should be encouraged. Stressful emotional states have been anecdotally associated with aortic dissection; thus, measures to reduce stress may offer some benefit.31
Our recommendations. While there are no published guidelines regarding activity restrictions in patients with TAA, we use a graded approach based on aortic diameter:
- 4.0 to 4.4 cm—lift no more than 75 pounds
- 4.5 to 5 cm—lift no more than 50 pounds
- 5 cm—lift no more than 25 pounds.
We also recommend not lifting anything heavier than half of one’s body weight and to avoid breath-holding or performing the Valsalva maneuver while lifting. Although these recommendations are somewhat arbitrary, based on theory and a large clinical experience at our aortic center, they seem reasonable and practical.
Activity restrictions should be stringent and individualized in patients with Marfan, Loeys-Dietz, or Ehlers-Danlos syndrome due to increased risk of dissection or rupture even if the aorta is normal in size.
We sometimes recommend exercise stress testing to assess the heart rate and blood pressure response to exercise, and we are developing research protocols to help tailor activity recommendations.
WHEN SHOULD A PATIENT BE REFERRED?
To a cardiologist at the time of diagnosis
As soon as TAA is diagnosed, the patient should be referred to a cardiologist who has special interest in aortic disease. This will allow for appropriate and timely decisions about medical management, imaging, follow-up, and referral to surgery. Additional recommendations for screening of family members and referral to clinical geneticists can be discussed at this juncture. Activity restrictions should be reviewed at the initial evaluation.
To a surgeon relatively early
Size thresholds for surgical intervention are discussed below, but one should not wait until these thresholds are reached to send the patient for surgical consultation. It is beneficial to the state of mind of a potential surgical candidate to have early discussions pertaining to the types of operations available, their outcomes, and associated risks and benefits. If a patient’s aortic size remains stable over time, he or she may be followed by the cardiologist until significant size or growth has been documented, at which time the patient and surgeon can reconvene to discuss options for definitive treatment.
To a clinical geneticist
If 1 or more first-degree relatives of a patient with TAA or dissection are found to have aneurysmal disease, referral to a clinical geneticist is very important for genetic testing of multiple genes that have been implicated in thoracic aortic aneurysm and dissection.
WHEN SHOULD TAA BE REPAIRED?
Surgery to prevent rupture or dissection remains the definitive treatment of TAA when size thresholds are reached, and symptomatic aneurysm should be operated on regardless of the size. However, rarely are thoracic aneurysms symptomatic unless they rupture or dissect. The size criteria are based on underlying genetic etiology if known and on the behavior and natural course of TAA.
Size and other factors
Treatment should be tailored to the patient’s clinical scenario, family history, and estimated risk of rupture or dissection, balanced against the individual center’s outcomes of elective aortic replacement.32 For example, young and otherwise healthy patients with TAA and a family history of aortic dissection (who may be more likely to have connective tissue disorders such as Marfan syndrome, Loeys-Dietz syndrome, or vascular Ehler-Danlos syndrome) may elect to undergo repair when the aneurysm reaches or nearly reaches the diameter of that of the family member’s aorta when dissection occurred.2 On the other hand, TAA of degenerative etiology (eg, related to smoking or hypertension) measuring less than 5.5 cm in an older patient with comorbidities poses a lower risk of a catastrophic event such as dissection or rupture than the risk of surgery.11
Thresholds for surgery. Once the diameter of the ascending aorta reaches 6 cm, the likelihood of an acute dissection is 31%.11 A similar threshold is reached for the descending aorta at a size of 7 cm.11 Therefore, to avoid high-risk emergency surgery on an acutely dissected aorta, surgery on an ascending aortic aneurysm of degenerative etiology is usually suggested when the aneurysm reaches 5.5 cm or a documented growth rate greater than 0.5 cm/year.2,33
Additionally, in patients already undergoing surgery for valvular or coronary disease, prophylactic aortic replacement is recommended if the ascending aorta is larger than 4.5 cm. The threshold for intervention is lower in patients with connective tissue disease (> 5.0 cm for Marfan syndrome, 4.4–4.6 cm for Loeys-Dietz syndrome).2,33
Observational studies suggest that the risk of aortic complications in patients with bicuspid aortic valve aortopathy is low overall, though significantly greater than in the general population.18,34,35 These findings led to changes in the 2014 American College of Cardiology/American Heart Association guidelines on valvular heart disease,36 suggesting a surgical threshold of 5.5 cm in the absence of significant valve disease or family history of dissection of an aorta of smaller diameter.
A 2015 study of dissection risk in patients with bicuspid aortic valve aortopathy by our group found a dramatic increase in risk of aortic dissection for ascending aortic diameters greater than 5.3 cm, and a gradual increase in risk for aortic root diameters greater than 5.0 cm.37 In addition, a near-constant 3% to 4% risk of dissection was present for aortic diameters ranging from 4.7 cm to 5.0 cm, revealing that watchful waiting carries its own inherent risks.37 In our surgical experience with this population, the hospital mortality rate and risk of stroke from aortic surgery were 0.25% and 0.75%, respectively.37 Thus, the decision to operate for aortic aneurysm in the setting of a bicuspid aortic valve should take into account patient-specific factors and institutional outcomes.
A statement of clarification in the American College of Cardiology/American Heart Association guidelines was published in 2015, recommending surgery for patients with an aortic diameter of 5.0 cm or greater if the patient is at low risk and the surgery is performed by an experienced surgical team at a center with established surgical expertise in this condition.38 However, current recommendations are for surgery at 5.5 cm if the above conditions are not met.
Ratio of aortic cross-sectional area to height
Although size alone has long been used to guide surgical intervention, a recent review from the International Registry of Aortic Dissection revealed that 59% of patients suffered aortic dissection at diameters less than 5.5 cm, and that patients with certain connective tissue diseases such as Loeys-Dietz syndrome or familial thoracic aneurysm and dissection had a documented propensity for dissection at smaller diameters.39–41
Size indices such as the aortic cross-sectional area indexed to height have been implemented in guidelines for certain patient populations (eg, 10 cm2/m in Marfan syndrome) and provide better risk stratification than size cutoffs alone.2,42
The ratio of aortic cross-sectional area to the patient’s height has also been applied to patients with bicuspid aortic valve-associated aortopathy and to those with a dilated aorta and a tricuspid aortic valve.43,44 Notably, a ratio greater than 10 cm2/m has been associated with aortic dissection in these groups, and this cutoff provides better stratification for prediction of death than traditional size metrics.27,28
HOW SHOULD PATIENTS BE SCREENED? WHAT FOLLOW-UP IS NECESSARY?
Initial screening and follow-up
Follow-up of TAA depends on the initial aortic size or rate of growth, or both. For patients presenting for the first time with TAA, it is reasonable to obtain definitive aortic imaging with CT or magnetic resonance angiography (MRA), then to repeat imaging at 6 months to document stability. If the aortic dimensions remain stable, then annual follow-up with CT or MRA is reasonable.2
Our flow chart of initial screening and follow-up is shown in Figure 5.
Screening of family members
In our center, we routinely recommend screening of all first-degree relatives of patients with TAA. Aortic imaging with echocardiography plus CT or MRI should be considered to detect asymptomatic disease.2 In patients with a strong family history (ie, multiple relatives affected with aortic aneurysm, dissection, or sudden cardiac death), genetic screening and testing for known mutations are recommended for the patient as well as for the family members.
If a mutation is identified in a family, then first-degree relatives should undergo genetic screening for the mutation and aortic imaging.2 Imaging in second-degree relatives may also be considered if one or more first-degree relatives are found to have aortic dilation.2
We recommend similar screening of first-degree family members of patients with bicuspid aortic valve aortopathy. In patients with young children, we recommend obtaining an echocardiogram of the child to look for a bicuspid aortic valve or aortic dilation. If an abnormality is detected or suspected, dedicated imaging with MRA to assess aortic dimensions is warranted.
BACK TO OUR PATIENT WITH A BICUSPID AORTIC VALVE
Our patient with a bicuspid aortic valve had a 4.6-cm root, an ascending aortic aneurysm, and several affected family members.
We would obtain dedicated aortic imaging at this patient’s initial visit with either gated CT with contrast or MRA, and we would obtain a cardioaortic surgery consult. We would repeat these studies at a follow-up visit 6 months later to detect any aortic growth compared with initial studies, and follow up annually thereafter. Echocardiography can also be done at the initial visit to determine if valvular disease is present that may influence clinical decisions.
Surgery would likely be recommended once the root reached a maximum area-to-height ratio greater than 10 cm2/m, or if the valve became severely dysfunctional during follow-up.
BACK TO OUR PATIENT WITH MARFAN SYNDROME
The young woman with Marfan syndrome has a 4.6-cm aortic root aneurysm and 2+ aortic insufficiency. Her question pertains to the threshold at which an operation would be considered. This question is complicated and is influenced by several concurrent clinical features in her presentation.
Starting with size criteria, patients with Marfan syndrome should be considered for elective aortic root repair at a diameter greater than 5 cm. However, an aortic cross-sectional area-to-height ratio greater than 10 cm2/m may provide a more robust metric for clinical decision-making than aortic diameter alone. Additional factors such as degree of aortic insufficiency and deleterious left ventricular remodeling may urge one to consider aortic root repair at a diameter of 4.5 cm.
These factors, including rate of growth and the surgeon’s assessment about his or her ability to preserve the aortic valve during repair, should be considered collectively in this scenario.
- Elefteriades JA, Farkas EA. Thoracic aortic aneurysm clinically pertinent controversies and uncertainties. J Am Coll Cardiol 2010; 55(9):841–857. doi:10.1016/j.jacc.2009.08.084
- Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: executive summary. Anesth Analg 2010; 111(2):279–315. doi:10.1213/ANE.0b013e3181dd869b
- Clouse WD, Hallett JW Jr, Schaff HV, Gayari MM, Ilstrup DM, Melton LJ 3rd. Improved prognosis of thoracic aortic aneurysms: a population-based study. JAMA 1998; 280(22):1926–1929. pmid:9851478
- Olsson C, Thelin S, Ståhle E, Ekbom A, Granath F. Thoracic aortic aneurysm and dissection: increasing prevalence and improved outcomes reported in a nationwide population-based study of more than 14,000 cases from 1987 to 2002. Circulation 2006; 114(24):2611–2618. doi:10.1161/CIRCULATIONAHA.106.630400
- Clouse WD, Hallett JW Jr, Schaff HV, et al. Acute aortic dissection: population-based incidence compared with degenerative aortic aneurysm rupture. Mayo Clin Proc 2004; 79(2):176–180. pmid:14959911
- US Centers for Disease Control and Prevention (CDC). National Center for Injury Prevention and Control. WISQARS leading causes of death reports, 1999 – 2007. https://webappa.cdc.gov/sasweb/ncipc/leadcaus10.html. Accessed May 21, 2018.
- Hansen MS, Nogareda GJ, Hutchison SJ. Frequency of and inappropriate treatment of misdiagnosis of acute aortic dissection. Am J Cardiol 2007; 99(6):852–856. doi:10.1016/j.amjcard.2006.10.055
- Goldfinger JZ, Halperin JL, Marin ML, Stewart AS, Eagle KA, Fuster V. Thoracic aortic aneurysm and dissection. J Am Coll Cardiol 2014; 64(16):1725–1739. doi:10.1016/j.jacc.2014.08.025
- Kumar V, Abbas A, Aster J. Robbins and Cotran Pathologic Basis of Disease. 9th ed. Philadelphia, PA: Elsevier/Saunders; 2015.
- Wolak A, Gransar H, Thomson LE, et al. Aortic size assessment by noncontrast cardiac computed tomography: normal limits by age, gender, and body surface area. JACC Cardiovasc Imaging 2008; 1(2):200–209. doi:10.1016/j.jcmg.2007.11.005
- Elefteriades JA. Natural history of thoracic aortic aneurysms: indications for surgery, and surgical versus nonsurgical risks. Ann Thorac Surg 2002; 74(5):S1877–S1880; discussion S1892–S1898. pmid:12440685
- Smith AD, Schoenhagen P. CT imaging for acute aortic syndrome. Cleve Clin J Med 2008; 75(1):7–17. pmid:18236724
- Cury M, Zeidan F, Lobato AC. Aortic disease in the young: genetic aneurysm syndromes, connective tissue disorders, and familial aortic aneurysms and dissections. Int J Vasc Med 2013(2013); 2013:267215. doi:10.1155/2013/267215
- Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol 2002; 39(12):1890–1900. doi:10.1016/S0735-1097(02)01886-7
- Fedak PW, Verma S, David TE, Leask RL, Weisel RD, Butany J. Clinical and pathophysiological implications of a bicuspid aortic valve. Circulation 2002; 106(8):900–904. pmid:12186790
- Della Corte A, Bancone C, Quarto C, et al. Predictors of ascending aortic dilatation with bicuspid aortic valve: a wide spectrum of disease expression. Eur J Cardiothorac Surg 2007; 31(3):397–405. doi:10.1016/j.ejcts.2006.12.006
- Jackson V, Petrini J, Caidahl K, et al. Bicuspid aortic valve leaflet morphology in relation to aortic root morphology: a study of 300 patients undergoing open-heart surgery. Eur J Cardiothorac Surg 2011; 40(3):e118–e124. doi:10.1016/j.ejcts.2011.04.014
- Michelena HI, Khanna AD, Mahoney D, et al. Incidence of aortic complications in patients with bicuspid aortic valves. JAMA 2011; 306(10):1104–1112. doi:10.1001/jama.2011.1286
- Verma S, Siu SC. Aortic dilatation in patients with bicuspid aortic valve. N Engl J Med 2014; 370(20):1920–1929. doi:10.1056/NEJMra1207059
- Barker AJ, Markl M, Bürk J, et al. Bicuspid aortic valve is associated with altered wall shear stress in the ascending aorta. Circ Cardiovasc Imaging 2012; 5(4):457–466. doi:10.1161/CIRCIMAGING.112.973370
- Hope MD, Hope TA, Meadows AK, et al. Bicuspid aortic valve: four-dimensional MR evaluation of ascending aortic systolic flow patterns. Radiology 2010; 255(1):53–61. doi:10.1148/radiol.09091437
- Abdulkareem N, Soppa G, Jones S, Valencia O, Smelt J, Jahangiri M. Dilatation of the remaining aorta after aortic valve or aortic root replacement in patients with bicuspid aortic valve: a 5-year follow-up. Ann Thorac Surg 2013; 96(1):43–49. doi:10.1016/j.athoracsur.2013.03.086
- Regeer MV, Versteegh MI, Klautz RJ, et al. Effect of aortic valve replacement on aortic root dilatation rate in patients with bicuspid and tricuspid aortic valves. Ann Thorac Surg 2016; 102(6):1981–1987. doi:10.1016/j.athoracsur.2016.05.038
- Habashi JP, Judge DP, Holm TM, et al. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science 2006; 312(5770):117–121. doi:10.1126/science.1124287
- Brooke BS, Habashi JP, Judge DP, Patel N, Loeys B, Dietz HC 3rd. Angiotensin II blockade and aortic-root dilation in Marfan’s syndrome. N Engl J Med 2008; 358(26):2787–2795. doi:10.1056/NEJMoa0706585
- Chiu HH, Wu MH, Wang JK, et al. Losartan added to ß-blockade therapy for aortic root dilation in Marfan syndrome: a randomized, open-label pilot study. Mayo Clin Proc 2013; 88(3):271–276. doi:10.1016/j.mayocp.2012.11.005
- Groenink M, den Hartog AW, Franken R, et al. Losartan reduces aortic dilatation rate in adults with Marfan syndrome: a randomized controlled trial. Eur Heart J 2013; 34(45):3491–3500. doi:10.1093/eurheartj/eht334
- Lacro RV, Dietz HC, Sleeper LA, et al; Pediatric Heart Network Investigators. Atenolol versus losartan in children and young adults with Marfan’s syndrome. N Engl J Med 2014; 371(22):2061–2071. doi:10.1056/NEJMoa1404731
- Ziganshin BA, Mukherjee SK, Elefteriades JA, et al. Atenolol versus losartan in Marfan’s syndrome (letters). N Engl J Med 2015; 372(10):977–981. doi:10.1056/NEJMc1500128
- Franken R, den Hartog AW, Radonic T, et al. Beneficial outcome of losartan therapy depends on type of FBN1 mutation in Marfan syndrome. Circ Cardiovasc Genet 2015; 8(2):383–388. doi:10.1161/CIRCGENETICS.114.000950
- Elefteriades JA. Thoracic aortic aneurysm: reading the enemy’s playbook. Curr Probl Cardiol 2008; 33(5):203–277. doi:10.1016/j.cpcardiol.2008.01.004
- Idrees JJ, Roselli EE, Lowry AM, et al. Outcomes after elective proximal aortic replacement: a matched comparison of isolated versus multicomponent operations. Ann Thorac Surg 2016; 101(6):2185–2192. doi:10.1016/j.athoracsur.2015.12.026
- Hiratzka LF, Creager MA, Isselbacher EM, et al. Surgery for aortic dilatation in patients with bicuspid aortic valves: a statement of clarification from the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Thorac Cardiovasc Surg 2016; 151(4):959–966. doi:10.1016/j.jtcvs.2015.12.001
- Tzemos N, Therrien J, Yip J, et al. Outcomes in adults with bicuspid aortic valves. JAMA 2008; 300(11):1317–1325. doi:10.1001/jama.300.11.1317
- Davies RR, Goldstein LJ, Coady MA, et al. Yearly rupture or dissection rates for thoracic aortic aneurysms: simple prediction based on size. Ann Thorac Surg 2002; 73(1):17–28. pmid:11834007
- Nishimura RA, Otto CM, Bono RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American heart Association Task Force on Practice Guidelines. Circulation 2014; 129(23):2440–2492. doi:10.1161/CIR.0000000000000029
- Wojnarski CM, Svensson LG, Roselli EE, et al. Aortic dissection in patients with bicuspid aortic valve–associated aneurysms. Ann Thorac Surg 2015; 100(5):1666–1674. doi:10.1016/j.athoracsur.2015.04.126
- Hiratzka LF, Creager MA, Isselbacher EM, et al. Surgery for aortic dilatation in patients with bicuspid aortic valves: a statement of clarification from the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2016; 133(7):680–686. doi:10.1161/CIR.0000000000000331
- Pape LA, Tsai TT, Isselbacher EM, et al; International Registry of Acute Aortic Dissection (IRAD) Investigators. Aortic diameter > or = 5.5 cm is not a good predictor of type A aortic dissection: observations from the International Registry of Acute Aortic Dissection (IRAD). Circulation 2007; 116(10):1120–1127. doi:10.1161/CIRCULATIONAHA.107.702720
- Loeys BL, Schwarze U, Holm T, et al. Aneurysm syndromes caused by mutations in the TGF-beta receptor. N Engl J Med 2006; 355(8):788–798. doi:10.1056/NEJMoa055695
- Guo DC, Pannu H, Tran-Fadulu V, et al. Mutations in smooth muscle alpha-actin (ACTA2) lead to thoracic aortic aneurysms and dissections. Nat Genet 2007; 39(12):1488–1493. doi:10.1038/ng.2007.6
- Svensson LG, Khitin L. Aortic cross-sectional area/height ratio timing of aortic surgery in asymptomatic patients with Marfan syndrome. J Thorac Cardiovasc Surg 2002; 123(2):360–361. pmid:11828302
- Svensson LG, Kim KH, Lytle BW, Cosgrove DM. Relationship of aortic cross-sectional area to height ratio and the risk of aortic dissection in patients with bicuspid aortic valves. J Thorac Cardiovasc Surg 2003; 126(3):892–893. pmid:14502185
- Masri A, Kalahasti V, Svensson LG, et al. Aortic cross-sectional area/height ratio and outcomes in patients with a trileaflet aortic valve and a dilated aorta. Circulation 2016; 134(22):1724–1737. doi:10.1161/CIRCULATIONAHA.116.022995
- Elefteriades JA, Farkas EA. Thoracic aortic aneurysm clinically pertinent controversies and uncertainties. J Am Coll Cardiol 2010; 55(9):841–857. doi:10.1016/j.jacc.2009.08.084
- Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: executive summary. Anesth Analg 2010; 111(2):279–315. doi:10.1213/ANE.0b013e3181dd869b
- Clouse WD, Hallett JW Jr, Schaff HV, Gayari MM, Ilstrup DM, Melton LJ 3rd. Improved prognosis of thoracic aortic aneurysms: a population-based study. JAMA 1998; 280(22):1926–1929. pmid:9851478
- Olsson C, Thelin S, Ståhle E, Ekbom A, Granath F. Thoracic aortic aneurysm and dissection: increasing prevalence and improved outcomes reported in a nationwide population-based study of more than 14,000 cases from 1987 to 2002. Circulation 2006; 114(24):2611–2618. doi:10.1161/CIRCULATIONAHA.106.630400
- Clouse WD, Hallett JW Jr, Schaff HV, et al. Acute aortic dissection: population-based incidence compared with degenerative aortic aneurysm rupture. Mayo Clin Proc 2004; 79(2):176–180. pmid:14959911
- US Centers for Disease Control and Prevention (CDC). National Center for Injury Prevention and Control. WISQARS leading causes of death reports, 1999 – 2007. https://webappa.cdc.gov/sasweb/ncipc/leadcaus10.html. Accessed May 21, 2018.
- Hansen MS, Nogareda GJ, Hutchison SJ. Frequency of and inappropriate treatment of misdiagnosis of acute aortic dissection. Am J Cardiol 2007; 99(6):852–856. doi:10.1016/j.amjcard.2006.10.055
- Goldfinger JZ, Halperin JL, Marin ML, Stewart AS, Eagle KA, Fuster V. Thoracic aortic aneurysm and dissection. J Am Coll Cardiol 2014; 64(16):1725–1739. doi:10.1016/j.jacc.2014.08.025
- Kumar V, Abbas A, Aster J. Robbins and Cotran Pathologic Basis of Disease. 9th ed. Philadelphia, PA: Elsevier/Saunders; 2015.
- Wolak A, Gransar H, Thomson LE, et al. Aortic size assessment by noncontrast cardiac computed tomography: normal limits by age, gender, and body surface area. JACC Cardiovasc Imaging 2008; 1(2):200–209. doi:10.1016/j.jcmg.2007.11.005
- Elefteriades JA. Natural history of thoracic aortic aneurysms: indications for surgery, and surgical versus nonsurgical risks. Ann Thorac Surg 2002; 74(5):S1877–S1880; discussion S1892–S1898. pmid:12440685
- Smith AD, Schoenhagen P. CT imaging for acute aortic syndrome. Cleve Clin J Med 2008; 75(1):7–17. pmid:18236724
- Cury M, Zeidan F, Lobato AC. Aortic disease in the young: genetic aneurysm syndromes, connective tissue disorders, and familial aortic aneurysms and dissections. Int J Vasc Med 2013(2013); 2013:267215. doi:10.1155/2013/267215
- Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol 2002; 39(12):1890–1900. doi:10.1016/S0735-1097(02)01886-7
- Fedak PW, Verma S, David TE, Leask RL, Weisel RD, Butany J. Clinical and pathophysiological implications of a bicuspid aortic valve. Circulation 2002; 106(8):900–904. pmid:12186790
- Della Corte A, Bancone C, Quarto C, et al. Predictors of ascending aortic dilatation with bicuspid aortic valve: a wide spectrum of disease expression. Eur J Cardiothorac Surg 2007; 31(3):397–405. doi:10.1016/j.ejcts.2006.12.006
- Jackson V, Petrini J, Caidahl K, et al. Bicuspid aortic valve leaflet morphology in relation to aortic root morphology: a study of 300 patients undergoing open-heart surgery. Eur J Cardiothorac Surg 2011; 40(3):e118–e124. doi:10.1016/j.ejcts.2011.04.014
- Michelena HI, Khanna AD, Mahoney D, et al. Incidence of aortic complications in patients with bicuspid aortic valves. JAMA 2011; 306(10):1104–1112. doi:10.1001/jama.2011.1286
- Verma S, Siu SC. Aortic dilatation in patients with bicuspid aortic valve. N Engl J Med 2014; 370(20):1920–1929. doi:10.1056/NEJMra1207059
- Barker AJ, Markl M, Bürk J, et al. Bicuspid aortic valve is associated with altered wall shear stress in the ascending aorta. Circ Cardiovasc Imaging 2012; 5(4):457–466. doi:10.1161/CIRCIMAGING.112.973370
- Hope MD, Hope TA, Meadows AK, et al. Bicuspid aortic valve: four-dimensional MR evaluation of ascending aortic systolic flow patterns. Radiology 2010; 255(1):53–61. doi:10.1148/radiol.09091437
- Abdulkareem N, Soppa G, Jones S, Valencia O, Smelt J, Jahangiri M. Dilatation of the remaining aorta after aortic valve or aortic root replacement in patients with bicuspid aortic valve: a 5-year follow-up. Ann Thorac Surg 2013; 96(1):43–49. doi:10.1016/j.athoracsur.2013.03.086
- Regeer MV, Versteegh MI, Klautz RJ, et al. Effect of aortic valve replacement on aortic root dilatation rate in patients with bicuspid and tricuspid aortic valves. Ann Thorac Surg 2016; 102(6):1981–1987. doi:10.1016/j.athoracsur.2016.05.038
- Habashi JP, Judge DP, Holm TM, et al. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science 2006; 312(5770):117–121. doi:10.1126/science.1124287
- Brooke BS, Habashi JP, Judge DP, Patel N, Loeys B, Dietz HC 3rd. Angiotensin II blockade and aortic-root dilation in Marfan’s syndrome. N Engl J Med 2008; 358(26):2787–2795. doi:10.1056/NEJMoa0706585
- Chiu HH, Wu MH, Wang JK, et al. Losartan added to ß-blockade therapy for aortic root dilation in Marfan syndrome: a randomized, open-label pilot study. Mayo Clin Proc 2013; 88(3):271–276. doi:10.1016/j.mayocp.2012.11.005
- Groenink M, den Hartog AW, Franken R, et al. Losartan reduces aortic dilatation rate in adults with Marfan syndrome: a randomized controlled trial. Eur Heart J 2013; 34(45):3491–3500. doi:10.1093/eurheartj/eht334
- Lacro RV, Dietz HC, Sleeper LA, et al; Pediatric Heart Network Investigators. Atenolol versus losartan in children and young adults with Marfan’s syndrome. N Engl J Med 2014; 371(22):2061–2071. doi:10.1056/NEJMoa1404731
- Ziganshin BA, Mukherjee SK, Elefteriades JA, et al. Atenolol versus losartan in Marfan’s syndrome (letters). N Engl J Med 2015; 372(10):977–981. doi:10.1056/NEJMc1500128
- Franken R, den Hartog AW, Radonic T, et al. Beneficial outcome of losartan therapy depends on type of FBN1 mutation in Marfan syndrome. Circ Cardiovasc Genet 2015; 8(2):383–388. doi:10.1161/CIRCGENETICS.114.000950
- Elefteriades JA. Thoracic aortic aneurysm: reading the enemy’s playbook. Curr Probl Cardiol 2008; 33(5):203–277. doi:10.1016/j.cpcardiol.2008.01.004
- Idrees JJ, Roselli EE, Lowry AM, et al. Outcomes after elective proximal aortic replacement: a matched comparison of isolated versus multicomponent operations. Ann Thorac Surg 2016; 101(6):2185–2192. doi:10.1016/j.athoracsur.2015.12.026
- Hiratzka LF, Creager MA, Isselbacher EM, et al. Surgery for aortic dilatation in patients with bicuspid aortic valves: a statement of clarification from the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Thorac Cardiovasc Surg 2016; 151(4):959–966. doi:10.1016/j.jtcvs.2015.12.001
- Tzemos N, Therrien J, Yip J, et al. Outcomes in adults with bicuspid aortic valves. JAMA 2008; 300(11):1317–1325. doi:10.1001/jama.300.11.1317
- Davies RR, Goldstein LJ, Coady MA, et al. Yearly rupture or dissection rates for thoracic aortic aneurysms: simple prediction based on size. Ann Thorac Surg 2002; 73(1):17–28. pmid:11834007
- Nishimura RA, Otto CM, Bono RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American heart Association Task Force on Practice Guidelines. Circulation 2014; 129(23):2440–2492. doi:10.1161/CIR.0000000000000029
- Wojnarski CM, Svensson LG, Roselli EE, et al. Aortic dissection in patients with bicuspid aortic valve–associated aneurysms. Ann Thorac Surg 2015; 100(5):1666–1674. doi:10.1016/j.athoracsur.2015.04.126
- Hiratzka LF, Creager MA, Isselbacher EM, et al. Surgery for aortic dilatation in patients with bicuspid aortic valves: a statement of clarification from the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2016; 133(7):680–686. doi:10.1161/CIR.0000000000000331
- Pape LA, Tsai TT, Isselbacher EM, et al; International Registry of Acute Aortic Dissection (IRAD) Investigators. Aortic diameter > or = 5.5 cm is not a good predictor of type A aortic dissection: observations from the International Registry of Acute Aortic Dissection (IRAD). Circulation 2007; 116(10):1120–1127. doi:10.1161/CIRCULATIONAHA.107.702720
- Loeys BL, Schwarze U, Holm T, et al. Aneurysm syndromes caused by mutations in the TGF-beta receptor. N Engl J Med 2006; 355(8):788–798. doi:10.1056/NEJMoa055695
- Guo DC, Pannu H, Tran-Fadulu V, et al. Mutations in smooth muscle alpha-actin (ACTA2) lead to thoracic aortic aneurysms and dissections. Nat Genet 2007; 39(12):1488–1493. doi:10.1038/ng.2007.6
- Svensson LG, Khitin L. Aortic cross-sectional area/height ratio timing of aortic surgery in asymptomatic patients with Marfan syndrome. J Thorac Cardiovasc Surg 2002; 123(2):360–361. pmid:11828302
- Svensson LG, Kim KH, Lytle BW, Cosgrove DM. Relationship of aortic cross-sectional area to height ratio and the risk of aortic dissection in patients with bicuspid aortic valves. J Thorac Cardiovasc Surg 2003; 126(3):892–893. pmid:14502185
- Masri A, Kalahasti V, Svensson LG, et al. Aortic cross-sectional area/height ratio and outcomes in patients with a trileaflet aortic valve and a dilated aorta. Circulation 2016; 134(22):1724–1737. doi:10.1161/CIRCULATIONAHA.116.022995
KEY POINTS
- Screening and referral depend on clinical context. A size-based model to determine screening, referral, follow-up, and management serves most cases but should be modified in the context of connective tissue disease or family history of aneurysm and dissection.
- Medical management involves strict blood pressure and heart rate control with beta-blockers and angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers. Activity modifications should be tailored to the individual, although extreme isometric exercises and heavy lifting should be discouraged.
- Patients with TAA should be followed up annually, unless the patient is presenting for initial evaluation or significant changes are seen with dedicated imaging.
Aortic dissection presenting as ischemic limb
A 40-year-old man with a history of hypertension and alcohol abuse presented with acute onset of mild chest tightness, left leg pain, and increasing agitation, which prevented us from obtaining additional meaningful information from him.
On admission, his heart rate was 120 beats per minute, blood pressure 211/122 mm Hg, respiratory rate 18 per minute, and oxygen saturation 92% on room air. Given his history of alcohol abuse, we checked his blood ethanol level, which was less than 0.01%, well below the legal limit for intoxication.
We gave the patient intravenous lorazepam for possible alcohol withdrawal and started labetalol by intravenous infusion to lower his blood pressure.
On physical examination, his left lower extremity was cold and without pulses, including the femoral pulse. Suspecting acute arterial thrombosis, we ordered immediate computed tomographic (CT) angiography of the abdomen and pelvis with left lower extremity runoff. The images showed dissection of the abdominal aorta with extension to both the left and right common iliac arteries and the origin of the right external iliac artery. There was resultant occlusion of the left external iliac artery (Figure 1).
Immediate CT angiography of the chest was then performed, which revealed dissection of the thoracic aorta as well, starting superior to the aortic valve annulus and involving the ascending aorta, aortic arch, and the entire descending thoracic aorta (Figure 2).
The patient underwent emergency surgical repair of the aortic root, ascending aorta, and aortic arch. Residual dissection of the descending aorta was managed conservatively with blood pressure control using intravenous labetalol initially, which was then switched to oral carvedilol, and the pulses returned in his left lower extremity. He had an unremarkable postoperative recovery and was discharged after 1 week.
AORTIC DISSECTION AND MALPERFUSION SYNDROME
Aortic dissection is most often associated with acute onset of sharp chest pain and upper back pain. On rare occasions, it can have an atypical presentation such as stroke, paraplegia, mesenteric ischemia, or lower limb malperfusion.1
Extension of aortic dissection into the iliac and femoral arteries can cause impaired or absent blood flow to the lower extremity. These pulse deficits are a part of limb malperfusion syndrome. Symptoms of malperfusion syndrome vary greatly and depend on the vessels involved. Malperfusion of the branches of the aortic arch can result in stroke or altered sensorium. Compromise of intra-abdominal vessels due to dissection can involve the mesenteric bed, the renal arteries, or both, resulting in laboratory derangements such as lactic acidosis and renal failure.
How aortic dissection and malperfusion syndrome occur
Over time, shear forces on the aortic wall result in degeneration of the tunica intima and media. Dissection occurs when deterioration of the intima causes propagation of blood through a cleavage plane into the outer portion of the diseased media, forming a false lumen.
Anterograde or retrograde progression of dissection depends on the balance of the pressure gradient between true and false lumens.2 With every systolic ventricular contraction, a fluid and pressure wave travels down both lumens (true and false). However, the pressure gradient between the false and true lumens allows the more pliable intimal flap to bulge into the true lumen and ostia of branch vessels, resulting in static or dynamic obstruction.
Static obstruction occurs when the false lumen projects completely into the branch vessel and there is resultant thrombosis. As the name implies, dynamic obstruction is intermittent and is responsible for 80% of the cases of malperfusion syndrome.3 Dynamic obstruction has 2 distinct mechanisms: hypoperfusion through the true lumen due to impaired flow, and prolapse of the false lumen into a branch vessel.
Factors that exacerbate hypoperfusion through the true lumen and make obliteration by the false lumen more likely include large circumference of the dissected aorta, rapid heart rate, and high systolic pressure.4 Therefore, it is important to control the heart rate and blood pressure using beta-blockers in cases of aortic dissection with malperfusion syndrome. This treatment may resolve the dynamic obstruction through expansion and resumption of perfusion through the true lumen.5
MANAGEMENT OF MALPERFUSION SYNDROME
Aortic dissection can be classified as either Stanford type A (involving the ascending aorta) or type B (involving the descending aorta). Type B dissection associated with malperfusion syndrome is termed “complicated” type B aortic dissection. Our patient had both Stanford type A and complicated type B aortic dissection.
Unlike type A aortic dissection, which requires definitive open surgical repair, complicated type B aortic dissection occasionally responds to medical management alone. A plausible explanation for resolution of limb malperfusion with optimal blood pressure control is expansion of the true lumen and obliteration of the false lumen, as was likely the case in our patient.
In most cases, however, limb malperfusion persists despite optimal medical management. In such patients, endovascular graft stenting or open surgical repair may be needed. Open surgical repair procedures like bypass grafting or surgical fenestration are associated with significant rates of mortality and morbidity.5 Therefore, an endovascular approach rather than conventional surgical repair for complicated type B aortic dissection is advocated after optimal medical management.6 Endovascular repair also promotes favorable aortic remodeling without the morbidity associated with open surgical repair.
- Namana V, Balasubramanian R, Kariyanna PT, Sarasam R, Namana S, Shetty V. Aortic dissection with hemopericardium and thrombosed left common iliac artery presenting as acute limb ischemia: a case report and review. Am J Med Case Rep 2015; 3(10):338–343. doi:10.12691/ajmcr-3-10-9
- Crawford TC, Beaulieu RJ, Ehlert BA, Ratchford EV, Black JH 3rd. Malperfusion syndromes in aortic dissections. Vasc Med 2016; 21(3):264–273. doi:10.1177/1358863X15625371
- Williams DM, Lee DY, Hamilton BH, et al. The dissected aorta: percutaneous treatment of ischemic complications—principles and results. J Vasc Interv Radiol 1997; 8(4):605–625. pmid:9232578
- Chung JW, Elkins C, Sakai T, et al. True-lumen collapse in aortic dissection: part II. Evaluation of treatment methods in phantoms with pulsatile flow. Radiology 2000; 214(1):99–106. doi:10.1148/radiology.214.1.r00ja3499
- Gargiulo M, Bianchini Massoni C, Gallitto E, et al. Lower limb malperfusion in type B aortic dissection: a systematic review. Ann Cardiothorac Surg 2014; 3(4):351–367. doi:10.3978/j.issn.2225-319X.2014.07.05
- Dake MD, Kato N, Mitchell RS, et al. Endovascular stent-graft placement for the treatment of acute aortic dissection. N Engl J Med 1999; 340(20):1546–1552. doi:10.1056/NEJM199905203402004
A 40-year-old man with a history of hypertension and alcohol abuse presented with acute onset of mild chest tightness, left leg pain, and increasing agitation, which prevented us from obtaining additional meaningful information from him.
On admission, his heart rate was 120 beats per minute, blood pressure 211/122 mm Hg, respiratory rate 18 per minute, and oxygen saturation 92% on room air. Given his history of alcohol abuse, we checked his blood ethanol level, which was less than 0.01%, well below the legal limit for intoxication.
We gave the patient intravenous lorazepam for possible alcohol withdrawal and started labetalol by intravenous infusion to lower his blood pressure.
On physical examination, his left lower extremity was cold and without pulses, including the femoral pulse. Suspecting acute arterial thrombosis, we ordered immediate computed tomographic (CT) angiography of the abdomen and pelvis with left lower extremity runoff. The images showed dissection of the abdominal aorta with extension to both the left and right common iliac arteries and the origin of the right external iliac artery. There was resultant occlusion of the left external iliac artery (Figure 1).
Immediate CT angiography of the chest was then performed, which revealed dissection of the thoracic aorta as well, starting superior to the aortic valve annulus and involving the ascending aorta, aortic arch, and the entire descending thoracic aorta (Figure 2).
The patient underwent emergency surgical repair of the aortic root, ascending aorta, and aortic arch. Residual dissection of the descending aorta was managed conservatively with blood pressure control using intravenous labetalol initially, which was then switched to oral carvedilol, and the pulses returned in his left lower extremity. He had an unremarkable postoperative recovery and was discharged after 1 week.
AORTIC DISSECTION AND MALPERFUSION SYNDROME
Aortic dissection is most often associated with acute onset of sharp chest pain and upper back pain. On rare occasions, it can have an atypical presentation such as stroke, paraplegia, mesenteric ischemia, or lower limb malperfusion.1
Extension of aortic dissection into the iliac and femoral arteries can cause impaired or absent blood flow to the lower extremity. These pulse deficits are a part of limb malperfusion syndrome. Symptoms of malperfusion syndrome vary greatly and depend on the vessels involved. Malperfusion of the branches of the aortic arch can result in stroke or altered sensorium. Compromise of intra-abdominal vessels due to dissection can involve the mesenteric bed, the renal arteries, or both, resulting in laboratory derangements such as lactic acidosis and renal failure.
How aortic dissection and malperfusion syndrome occur
Over time, shear forces on the aortic wall result in degeneration of the tunica intima and media. Dissection occurs when deterioration of the intima causes propagation of blood through a cleavage plane into the outer portion of the diseased media, forming a false lumen.
Anterograde or retrograde progression of dissection depends on the balance of the pressure gradient between true and false lumens.2 With every systolic ventricular contraction, a fluid and pressure wave travels down both lumens (true and false). However, the pressure gradient between the false and true lumens allows the more pliable intimal flap to bulge into the true lumen and ostia of branch vessels, resulting in static or dynamic obstruction.
Static obstruction occurs when the false lumen projects completely into the branch vessel and there is resultant thrombosis. As the name implies, dynamic obstruction is intermittent and is responsible for 80% of the cases of malperfusion syndrome.3 Dynamic obstruction has 2 distinct mechanisms: hypoperfusion through the true lumen due to impaired flow, and prolapse of the false lumen into a branch vessel.
Factors that exacerbate hypoperfusion through the true lumen and make obliteration by the false lumen more likely include large circumference of the dissected aorta, rapid heart rate, and high systolic pressure.4 Therefore, it is important to control the heart rate and blood pressure using beta-blockers in cases of aortic dissection with malperfusion syndrome. This treatment may resolve the dynamic obstruction through expansion and resumption of perfusion through the true lumen.5
MANAGEMENT OF MALPERFUSION SYNDROME
Aortic dissection can be classified as either Stanford type A (involving the ascending aorta) or type B (involving the descending aorta). Type B dissection associated with malperfusion syndrome is termed “complicated” type B aortic dissection. Our patient had both Stanford type A and complicated type B aortic dissection.
Unlike type A aortic dissection, which requires definitive open surgical repair, complicated type B aortic dissection occasionally responds to medical management alone. A plausible explanation for resolution of limb malperfusion with optimal blood pressure control is expansion of the true lumen and obliteration of the false lumen, as was likely the case in our patient.
In most cases, however, limb malperfusion persists despite optimal medical management. In such patients, endovascular graft stenting or open surgical repair may be needed. Open surgical repair procedures like bypass grafting or surgical fenestration are associated with significant rates of mortality and morbidity.5 Therefore, an endovascular approach rather than conventional surgical repair for complicated type B aortic dissection is advocated after optimal medical management.6 Endovascular repair also promotes favorable aortic remodeling without the morbidity associated with open surgical repair.
A 40-year-old man with a history of hypertension and alcohol abuse presented with acute onset of mild chest tightness, left leg pain, and increasing agitation, which prevented us from obtaining additional meaningful information from him.
On admission, his heart rate was 120 beats per minute, blood pressure 211/122 mm Hg, respiratory rate 18 per minute, and oxygen saturation 92% on room air. Given his history of alcohol abuse, we checked his blood ethanol level, which was less than 0.01%, well below the legal limit for intoxication.
We gave the patient intravenous lorazepam for possible alcohol withdrawal and started labetalol by intravenous infusion to lower his blood pressure.
On physical examination, his left lower extremity was cold and without pulses, including the femoral pulse. Suspecting acute arterial thrombosis, we ordered immediate computed tomographic (CT) angiography of the abdomen and pelvis with left lower extremity runoff. The images showed dissection of the abdominal aorta with extension to both the left and right common iliac arteries and the origin of the right external iliac artery. There was resultant occlusion of the left external iliac artery (Figure 1).
Immediate CT angiography of the chest was then performed, which revealed dissection of the thoracic aorta as well, starting superior to the aortic valve annulus and involving the ascending aorta, aortic arch, and the entire descending thoracic aorta (Figure 2).
The patient underwent emergency surgical repair of the aortic root, ascending aorta, and aortic arch. Residual dissection of the descending aorta was managed conservatively with blood pressure control using intravenous labetalol initially, which was then switched to oral carvedilol, and the pulses returned in his left lower extremity. He had an unremarkable postoperative recovery and was discharged after 1 week.
AORTIC DISSECTION AND MALPERFUSION SYNDROME
Aortic dissection is most often associated with acute onset of sharp chest pain and upper back pain. On rare occasions, it can have an atypical presentation such as stroke, paraplegia, mesenteric ischemia, or lower limb malperfusion.1
Extension of aortic dissection into the iliac and femoral arteries can cause impaired or absent blood flow to the lower extremity. These pulse deficits are a part of limb malperfusion syndrome. Symptoms of malperfusion syndrome vary greatly and depend on the vessels involved. Malperfusion of the branches of the aortic arch can result in stroke or altered sensorium. Compromise of intra-abdominal vessels due to dissection can involve the mesenteric bed, the renal arteries, or both, resulting in laboratory derangements such as lactic acidosis and renal failure.
How aortic dissection and malperfusion syndrome occur
Over time, shear forces on the aortic wall result in degeneration of the tunica intima and media. Dissection occurs when deterioration of the intima causes propagation of blood through a cleavage plane into the outer portion of the diseased media, forming a false lumen.
Anterograde or retrograde progression of dissection depends on the balance of the pressure gradient between true and false lumens.2 With every systolic ventricular contraction, a fluid and pressure wave travels down both lumens (true and false). However, the pressure gradient between the false and true lumens allows the more pliable intimal flap to bulge into the true lumen and ostia of branch vessels, resulting in static or dynamic obstruction.
Static obstruction occurs when the false lumen projects completely into the branch vessel and there is resultant thrombosis. As the name implies, dynamic obstruction is intermittent and is responsible for 80% of the cases of malperfusion syndrome.3 Dynamic obstruction has 2 distinct mechanisms: hypoperfusion through the true lumen due to impaired flow, and prolapse of the false lumen into a branch vessel.
Factors that exacerbate hypoperfusion through the true lumen and make obliteration by the false lumen more likely include large circumference of the dissected aorta, rapid heart rate, and high systolic pressure.4 Therefore, it is important to control the heart rate and blood pressure using beta-blockers in cases of aortic dissection with malperfusion syndrome. This treatment may resolve the dynamic obstruction through expansion and resumption of perfusion through the true lumen.5
MANAGEMENT OF MALPERFUSION SYNDROME
Aortic dissection can be classified as either Stanford type A (involving the ascending aorta) or type B (involving the descending aorta). Type B dissection associated with malperfusion syndrome is termed “complicated” type B aortic dissection. Our patient had both Stanford type A and complicated type B aortic dissection.
Unlike type A aortic dissection, which requires definitive open surgical repair, complicated type B aortic dissection occasionally responds to medical management alone. A plausible explanation for resolution of limb malperfusion with optimal blood pressure control is expansion of the true lumen and obliteration of the false lumen, as was likely the case in our patient.
In most cases, however, limb malperfusion persists despite optimal medical management. In such patients, endovascular graft stenting or open surgical repair may be needed. Open surgical repair procedures like bypass grafting or surgical fenestration are associated with significant rates of mortality and morbidity.5 Therefore, an endovascular approach rather than conventional surgical repair for complicated type B aortic dissection is advocated after optimal medical management.6 Endovascular repair also promotes favorable aortic remodeling without the morbidity associated with open surgical repair.
- Namana V, Balasubramanian R, Kariyanna PT, Sarasam R, Namana S, Shetty V. Aortic dissection with hemopericardium and thrombosed left common iliac artery presenting as acute limb ischemia: a case report and review. Am J Med Case Rep 2015; 3(10):338–343. doi:10.12691/ajmcr-3-10-9
- Crawford TC, Beaulieu RJ, Ehlert BA, Ratchford EV, Black JH 3rd. Malperfusion syndromes in aortic dissections. Vasc Med 2016; 21(3):264–273. doi:10.1177/1358863X15625371
- Williams DM, Lee DY, Hamilton BH, et al. The dissected aorta: percutaneous treatment of ischemic complications—principles and results. J Vasc Interv Radiol 1997; 8(4):605–625. pmid:9232578
- Chung JW, Elkins C, Sakai T, et al. True-lumen collapse in aortic dissection: part II. Evaluation of treatment methods in phantoms with pulsatile flow. Radiology 2000; 214(1):99–106. doi:10.1148/radiology.214.1.r00ja3499
- Gargiulo M, Bianchini Massoni C, Gallitto E, et al. Lower limb malperfusion in type B aortic dissection: a systematic review. Ann Cardiothorac Surg 2014; 3(4):351–367. doi:10.3978/j.issn.2225-319X.2014.07.05
- Dake MD, Kato N, Mitchell RS, et al. Endovascular stent-graft placement for the treatment of acute aortic dissection. N Engl J Med 1999; 340(20):1546–1552. doi:10.1056/NEJM199905203402004
- Namana V, Balasubramanian R, Kariyanna PT, Sarasam R, Namana S, Shetty V. Aortic dissection with hemopericardium and thrombosed left common iliac artery presenting as acute limb ischemia: a case report and review. Am J Med Case Rep 2015; 3(10):338–343. doi:10.12691/ajmcr-3-10-9
- Crawford TC, Beaulieu RJ, Ehlert BA, Ratchford EV, Black JH 3rd. Malperfusion syndromes in aortic dissections. Vasc Med 2016; 21(3):264–273. doi:10.1177/1358863X15625371
- Williams DM, Lee DY, Hamilton BH, et al. The dissected aorta: percutaneous treatment of ischemic complications—principles and results. J Vasc Interv Radiol 1997; 8(4):605–625. pmid:9232578
- Chung JW, Elkins C, Sakai T, et al. True-lumen collapse in aortic dissection: part II. Evaluation of treatment methods in phantoms with pulsatile flow. Radiology 2000; 214(1):99–106. doi:10.1148/radiology.214.1.r00ja3499
- Gargiulo M, Bianchini Massoni C, Gallitto E, et al. Lower limb malperfusion in type B aortic dissection: a systematic review. Ann Cardiothorac Surg 2014; 3(4):351–367. doi:10.3978/j.issn.2225-319X.2014.07.05
- Dake MD, Kato N, Mitchell RS, et al. Endovascular stent-graft placement for the treatment of acute aortic dissection. N Engl J Med 1999; 340(20):1546–1552. doi:10.1056/NEJM199905203402004
‘Non-criteria’ antiphospholipid antibodies and thrombosis
To the Editor: We read with great interest the excellent article on thrombosis secondary to antiphospholipid antibody syndrome.1 We wish to comment on the section “Antiphospholipid antibodies are not all the same,” specifically on question 6: “Which of the following antiphospholipid antibodies have not been associated with an increased thrombotic risk?”
The answer offered was antiphosphatidylserine, and the authors stated, “While lupus anticoagulant, anti-beta-2-glycoprotein I, and anticardiolipin antibodies are associated with thrombosis, antiprothrombin antibodies (including antiprothrombin and antiphosphatidylserine antibodies) are not.”1
Antiphospholipid antibody testing in antiphospholipid antibody syndrome is complicated, but we feel the information provided was inaccurate. It should be noted that 3 antibodies are under discussion: in addition to antiphosphatidylserine (aPS) antibodies, antiprothrombin antibodies are heterogeneous, comprising antibodies to prothrombin alone (aPT-A) and antibodies to the antiphosphatidylserine-prothrombin complex (aPS/PT). While the diagnostic utility of these antibodies is in evolution, there are numerous studies on their association with thrombosis or antiphospholipid antibody syndrome, or both.2,3 Most recently, a systematic review (N = 7,000) concluded that prothrombin antibodies (aPT, aPS/PT) were strong risk factors for thrombosis (odds ratio 2.3, 95% confidence interval 1.72–3.5).4
The revised Sapporo (Sydney) guidelines referenced by the authors addressed these “non-criteria” antiphospholipid antibodies.5 At that time (2006), it was thought premature to include these antibodies as independent criteria for definite antiphospholipid antibody syndrome, even though their association with the syndrome was recognized by the committee. The guidelines considered an interesting scenario: What if a case fulfills the clinical criteria of antiphospholipid antibody syndrome, but serology is positive only for these “non-criteria” antibodies? It was suggested that these cases be classified as “probable” antiphospholipid antibody syndrome. Also, aPS/PT was proposed as a confirmatory assay for lupus anticoagulant testing.
In 2010, the International Congress on Antiphospholipid Antibodies concluded that aPS/PT is truly relevant to thrombosis and antiphospholipid antibody syndrome, with the possibility of aPS/PT becoming a criterion for the syndrome in the future.6 Studies have already started on this.7 Since then, 2 scoring systems to quantify the risk of thrombosis and obstetric events have incorporated aPS/PT—the Antiphospholipid Score (2012) and the Global Anti-Phospholipid Syndrome Score (2013).8.9
In conclusion, these antibodies are associated with thrombosis, can be considered features of antiphospholipid antibody syndrome in the right clinical context, and have a role in contemporary discussion of this disease.
- Serhal M, Evans N, Gornik HL. A 75-year-old with abdominal pain, hypoxia, and weak pulses in the left leg. Cleve Clin J Med 2018; 85(2):145–154. doi:10.3949/ccjm.85a.16069
- Khogeer H, Alfattani A, Al Kaff M, Al Shehri T, Khojah O, Owaidah T. Antiphosphatidylserine antibodies as diagnostic indicators of antiphospholipid syndrome. Lupus 2015; 24(2):186–190. doi:10.1177/0961203314552462
- Sciascia S, Bertolaccini ML. Antibodies to phosphatidylserine/prothrombin complex and the antiphospholipid syndrome. Lupus 2014; 23(12):1309–1312. doi:10.1177/0961203314538332
- Sciascia S, Sanna G, Murru V, Roccatello D, Khamashta MA, Bertolaccini ML. Anti-prothrombin (aPT) and anti-phosphatidylserine/prothrombin (aPS/PT) antibodies and the risk of thrombosis in the antiphospholipid syndrome. A systematic review. Thromb Haemost 2014; 111(2):354–364. doi:10.1160/TH13-06-0509
- Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006; 4(2):295–306. doi:10.1111/j.1538-7836.2006.01753.x
- Bertolaccini ML, Amengual O, Atsumi T, et al. ‘Non-criteria’ aPL tests: report of a task force and preconference workshop at the 13th International Congress on Antiphospholipid Antibodies, Galveston, TX, USA, April 2010. Lupus 2011; 20:191–205. doi:10.1177/0961203310397082
- Fabris M, Giacomello R, Poz A, et al. The introduction of anti-phosphatidylserine/prothrombin autoantibodies in the laboratory diagnostic process of anti-phospholipid antibody syndrome: 6 months of observation. Auto-Immunity Highlights 2014; 5(2):63–67. doi:10.1007/s13317-014-0061-3
- Otomo K, Atsumi T, Amengual O, et al. Efficacy of the antiphospholipid score for the diagnosis of antiphospholipid syndrome and its predictive value for thrombotic events. Arthritis Rheum 2012; 64(2):504–512. doi:10.1002/art.33340
- Sciascia S, Sanna G, Murru V, Roccatello D, Khamashta MA, Bertolaccini ML. GAPSS: the Global Anti-Phospholipid Syndrome Score. Rheumatology (Oxford) 2013; 52(8):1397–1403. doi:10.1093/rheumatology/kes388
To the Editor: We read with great interest the excellent article on thrombosis secondary to antiphospholipid antibody syndrome.1 We wish to comment on the section “Antiphospholipid antibodies are not all the same,” specifically on question 6: “Which of the following antiphospholipid antibodies have not been associated with an increased thrombotic risk?”
The answer offered was antiphosphatidylserine, and the authors stated, “While lupus anticoagulant, anti-beta-2-glycoprotein I, and anticardiolipin antibodies are associated with thrombosis, antiprothrombin antibodies (including antiprothrombin and antiphosphatidylserine antibodies) are not.”1
Antiphospholipid antibody testing in antiphospholipid antibody syndrome is complicated, but we feel the information provided was inaccurate. It should be noted that 3 antibodies are under discussion: in addition to antiphosphatidylserine (aPS) antibodies, antiprothrombin antibodies are heterogeneous, comprising antibodies to prothrombin alone (aPT-A) and antibodies to the antiphosphatidylserine-prothrombin complex (aPS/PT). While the diagnostic utility of these antibodies is in evolution, there are numerous studies on their association with thrombosis or antiphospholipid antibody syndrome, or both.2,3 Most recently, a systematic review (N = 7,000) concluded that prothrombin antibodies (aPT, aPS/PT) were strong risk factors for thrombosis (odds ratio 2.3, 95% confidence interval 1.72–3.5).4
The revised Sapporo (Sydney) guidelines referenced by the authors addressed these “non-criteria” antiphospholipid antibodies.5 At that time (2006), it was thought premature to include these antibodies as independent criteria for definite antiphospholipid antibody syndrome, even though their association with the syndrome was recognized by the committee. The guidelines considered an interesting scenario: What if a case fulfills the clinical criteria of antiphospholipid antibody syndrome, but serology is positive only for these “non-criteria” antibodies? It was suggested that these cases be classified as “probable” antiphospholipid antibody syndrome. Also, aPS/PT was proposed as a confirmatory assay for lupus anticoagulant testing.
In 2010, the International Congress on Antiphospholipid Antibodies concluded that aPS/PT is truly relevant to thrombosis and antiphospholipid antibody syndrome, with the possibility of aPS/PT becoming a criterion for the syndrome in the future.6 Studies have already started on this.7 Since then, 2 scoring systems to quantify the risk of thrombosis and obstetric events have incorporated aPS/PT—the Antiphospholipid Score (2012) and the Global Anti-Phospholipid Syndrome Score (2013).8.9
In conclusion, these antibodies are associated with thrombosis, can be considered features of antiphospholipid antibody syndrome in the right clinical context, and have a role in contemporary discussion of this disease.
To the Editor: We read with great interest the excellent article on thrombosis secondary to antiphospholipid antibody syndrome.1 We wish to comment on the section “Antiphospholipid antibodies are not all the same,” specifically on question 6: “Which of the following antiphospholipid antibodies have not been associated with an increased thrombotic risk?”
The answer offered was antiphosphatidylserine, and the authors stated, “While lupus anticoagulant, anti-beta-2-glycoprotein I, and anticardiolipin antibodies are associated with thrombosis, antiprothrombin antibodies (including antiprothrombin and antiphosphatidylserine antibodies) are not.”1
Antiphospholipid antibody testing in antiphospholipid antibody syndrome is complicated, but we feel the information provided was inaccurate. It should be noted that 3 antibodies are under discussion: in addition to antiphosphatidylserine (aPS) antibodies, antiprothrombin antibodies are heterogeneous, comprising antibodies to prothrombin alone (aPT-A) and antibodies to the antiphosphatidylserine-prothrombin complex (aPS/PT). While the diagnostic utility of these antibodies is in evolution, there are numerous studies on their association with thrombosis or antiphospholipid antibody syndrome, or both.2,3 Most recently, a systematic review (N = 7,000) concluded that prothrombin antibodies (aPT, aPS/PT) were strong risk factors for thrombosis (odds ratio 2.3, 95% confidence interval 1.72–3.5).4
The revised Sapporo (Sydney) guidelines referenced by the authors addressed these “non-criteria” antiphospholipid antibodies.5 At that time (2006), it was thought premature to include these antibodies as independent criteria for definite antiphospholipid antibody syndrome, even though their association with the syndrome was recognized by the committee. The guidelines considered an interesting scenario: What if a case fulfills the clinical criteria of antiphospholipid antibody syndrome, but serology is positive only for these “non-criteria” antibodies? It was suggested that these cases be classified as “probable” antiphospholipid antibody syndrome. Also, aPS/PT was proposed as a confirmatory assay for lupus anticoagulant testing.
In 2010, the International Congress on Antiphospholipid Antibodies concluded that aPS/PT is truly relevant to thrombosis and antiphospholipid antibody syndrome, with the possibility of aPS/PT becoming a criterion for the syndrome in the future.6 Studies have already started on this.7 Since then, 2 scoring systems to quantify the risk of thrombosis and obstetric events have incorporated aPS/PT—the Antiphospholipid Score (2012) and the Global Anti-Phospholipid Syndrome Score (2013).8.9
In conclusion, these antibodies are associated with thrombosis, can be considered features of antiphospholipid antibody syndrome in the right clinical context, and have a role in contemporary discussion of this disease.
- Serhal M, Evans N, Gornik HL. A 75-year-old with abdominal pain, hypoxia, and weak pulses in the left leg. Cleve Clin J Med 2018; 85(2):145–154. doi:10.3949/ccjm.85a.16069
- Khogeer H, Alfattani A, Al Kaff M, Al Shehri T, Khojah O, Owaidah T. Antiphosphatidylserine antibodies as diagnostic indicators of antiphospholipid syndrome. Lupus 2015; 24(2):186–190. doi:10.1177/0961203314552462
- Sciascia S, Bertolaccini ML. Antibodies to phosphatidylserine/prothrombin complex and the antiphospholipid syndrome. Lupus 2014; 23(12):1309–1312. doi:10.1177/0961203314538332
- Sciascia S, Sanna G, Murru V, Roccatello D, Khamashta MA, Bertolaccini ML. Anti-prothrombin (aPT) and anti-phosphatidylserine/prothrombin (aPS/PT) antibodies and the risk of thrombosis in the antiphospholipid syndrome. A systematic review. Thromb Haemost 2014; 111(2):354–364. doi:10.1160/TH13-06-0509
- Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006; 4(2):295–306. doi:10.1111/j.1538-7836.2006.01753.x
- Bertolaccini ML, Amengual O, Atsumi T, et al. ‘Non-criteria’ aPL tests: report of a task force and preconference workshop at the 13th International Congress on Antiphospholipid Antibodies, Galveston, TX, USA, April 2010. Lupus 2011; 20:191–205. doi:10.1177/0961203310397082
- Fabris M, Giacomello R, Poz A, et al. The introduction of anti-phosphatidylserine/prothrombin autoantibodies in the laboratory diagnostic process of anti-phospholipid antibody syndrome: 6 months of observation. Auto-Immunity Highlights 2014; 5(2):63–67. doi:10.1007/s13317-014-0061-3
- Otomo K, Atsumi T, Amengual O, et al. Efficacy of the antiphospholipid score for the diagnosis of antiphospholipid syndrome and its predictive value for thrombotic events. Arthritis Rheum 2012; 64(2):504–512. doi:10.1002/art.33340
- Sciascia S, Sanna G, Murru V, Roccatello D, Khamashta MA, Bertolaccini ML. GAPSS: the Global Anti-Phospholipid Syndrome Score. Rheumatology (Oxford) 2013; 52(8):1397–1403. doi:10.1093/rheumatology/kes388
- Serhal M, Evans N, Gornik HL. A 75-year-old with abdominal pain, hypoxia, and weak pulses in the left leg. Cleve Clin J Med 2018; 85(2):145–154. doi:10.3949/ccjm.85a.16069
- Khogeer H, Alfattani A, Al Kaff M, Al Shehri T, Khojah O, Owaidah T. Antiphosphatidylserine antibodies as diagnostic indicators of antiphospholipid syndrome. Lupus 2015; 24(2):186–190. doi:10.1177/0961203314552462
- Sciascia S, Bertolaccini ML. Antibodies to phosphatidylserine/prothrombin complex and the antiphospholipid syndrome. Lupus 2014; 23(12):1309–1312. doi:10.1177/0961203314538332
- Sciascia S, Sanna G, Murru V, Roccatello D, Khamashta MA, Bertolaccini ML. Anti-prothrombin (aPT) and anti-phosphatidylserine/prothrombin (aPS/PT) antibodies and the risk of thrombosis in the antiphospholipid syndrome. A systematic review. Thromb Haemost 2014; 111(2):354–364. doi:10.1160/TH13-06-0509
- Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006; 4(2):295–306. doi:10.1111/j.1538-7836.2006.01753.x
- Bertolaccini ML, Amengual O, Atsumi T, et al. ‘Non-criteria’ aPL tests: report of a task force and preconference workshop at the 13th International Congress on Antiphospholipid Antibodies, Galveston, TX, USA, April 2010. Lupus 2011; 20:191–205. doi:10.1177/0961203310397082
- Fabris M, Giacomello R, Poz A, et al. The introduction of anti-phosphatidylserine/prothrombin autoantibodies in the laboratory diagnostic process of anti-phospholipid antibody syndrome: 6 months of observation. Auto-Immunity Highlights 2014; 5(2):63–67. doi:10.1007/s13317-014-0061-3
- Otomo K, Atsumi T, Amengual O, et al. Efficacy of the antiphospholipid score for the diagnosis of antiphospholipid syndrome and its predictive value for thrombotic events. Arthritis Rheum 2012; 64(2):504–512. doi:10.1002/art.33340
- Sciascia S, Sanna G, Murru V, Roccatello D, Khamashta MA, Bertolaccini ML. GAPSS: the Global Anti-Phospholipid Syndrome Score. Rheumatology (Oxford) 2013; 52(8):1397–1403. doi:10.1093/rheumatology/kes388