User login
Donor’s lack of sleep may impact HSCT

Sleep-deprived mice make poor donors for hematopoietic stem cell transplants (HSCTs), according to a study published in Nature Communications.
The research showed that a sleep deficit of just 4 hours can reduce—by more than 50%—the ability of HSCs to engraft and reconstitute the blood and bone marrow of an irradiated recipient mouse.
Researchers believe these findings may also apply to humans.
“Considering how little attention we typically pay to sleep in the hospital setting, this finding is troubling,” said study author Asya Rolls, PhD, of Technion–Israel Institute of Technology in Haifa.
“We go to all this trouble to find a matching donor, but this research suggests that, if the donor is not well-rested, it can impact the outcome of the transplantation. However, it’s heartening to think that this is not an insurmountable obstacle. A short period of recovery sleep before transplant can restore the donor’s cells’ ability to function normally.”
Dr Rolls and her colleagues studied mice that had been gently handled for 4 hours to prevent them from sleeping while control mice dozed. The team then collected HSCs from the sleep-deprived and well-rested mice and injected the cells into 12 irradiated mice.
The recipient mice also received an injection of their own HSCs collected prior to radiation so the researchers could quantify the relative abilities of the donated HSCs to engraft successfully.
The team then assessed the prevalence of myeloid cells derived from donated HSCs at 8 weeks and 16 weeks after transplant. At 16 weeks, donor myeloid chimerism was about 26% in HSCT recipients with well-rested donors and about 12% in recipients with sleep-deprived donors (P<0.0001).
Dr Rolls and her colleagues also compared the ability of fluorescently labeled HSCs from sleep-deprived mice and rested mice to home to the bone marrow. After 12 hours, 3.3% percent of HSCs from rested mice were found in the bone marrow, compared to 1.7% of HSCs from sleep-deprived mice (P<0.05).
Further investigation revealed that sleep deprivation downregulates the expression of miR-19b, a negative regulator of the suppressor of cytokine signaling (SOCS) genes, which inhibit HSC migration and homing.
Finally, Dr Rolls and her colleagues found the effects of sleep deprivation on HSCs could be reversed by letting mice catch up on sleep. Even 2 hours of recovery sleep restored the HSCs’ ability to function normally in transplantation tests.
“We still don’t know how sleep deprivation affects us all, not just bone marrow donors,” Dr Rolls said. “The fact that recovery sleep is so helpful only emphasizes how important it is to pay attention to sleep.” ![]()

Sleep-deprived mice make poor donors for hematopoietic stem cell transplants (HSCTs), according to a study published in Nature Communications.
The research showed that a sleep deficit of just 4 hours can reduce—by more than 50%—the ability of HSCs to engraft and reconstitute the blood and bone marrow of an irradiated recipient mouse.
Researchers believe these findings may also apply to humans.
“Considering how little attention we typically pay to sleep in the hospital setting, this finding is troubling,” said study author Asya Rolls, PhD, of Technion–Israel Institute of Technology in Haifa.
“We go to all this trouble to find a matching donor, but this research suggests that, if the donor is not well-rested, it can impact the outcome of the transplantation. However, it’s heartening to think that this is not an insurmountable obstacle. A short period of recovery sleep before transplant can restore the donor’s cells’ ability to function normally.”
Dr Rolls and her colleagues studied mice that had been gently handled for 4 hours to prevent them from sleeping while control mice dozed. The team then collected HSCs from the sleep-deprived and well-rested mice and injected the cells into 12 irradiated mice.
The recipient mice also received an injection of their own HSCs collected prior to radiation so the researchers could quantify the relative abilities of the donated HSCs to engraft successfully.
The team then assessed the prevalence of myeloid cells derived from donated HSCs at 8 weeks and 16 weeks after transplant. At 16 weeks, donor myeloid chimerism was about 26% in HSCT recipients with well-rested donors and about 12% in recipients with sleep-deprived donors (P<0.0001).
Dr Rolls and her colleagues also compared the ability of fluorescently labeled HSCs from sleep-deprived mice and rested mice to home to the bone marrow. After 12 hours, 3.3% percent of HSCs from rested mice were found in the bone marrow, compared to 1.7% of HSCs from sleep-deprived mice (P<0.05).
Further investigation revealed that sleep deprivation downregulates the expression of miR-19b, a negative regulator of the suppressor of cytokine signaling (SOCS) genes, which inhibit HSC migration and homing.
Finally, Dr Rolls and her colleagues found the effects of sleep deprivation on HSCs could be reversed by letting mice catch up on sleep. Even 2 hours of recovery sleep restored the HSCs’ ability to function normally in transplantation tests.
“We still don’t know how sleep deprivation affects us all, not just bone marrow donors,” Dr Rolls said. “The fact that recovery sleep is so helpful only emphasizes how important it is to pay attention to sleep.” ![]()

Sleep-deprived mice make poor donors for hematopoietic stem cell transplants (HSCTs), according to a study published in Nature Communications.
The research showed that a sleep deficit of just 4 hours can reduce—by more than 50%—the ability of HSCs to engraft and reconstitute the blood and bone marrow of an irradiated recipient mouse.
Researchers believe these findings may also apply to humans.
“Considering how little attention we typically pay to sleep in the hospital setting, this finding is troubling,” said study author Asya Rolls, PhD, of Technion–Israel Institute of Technology in Haifa.
“We go to all this trouble to find a matching donor, but this research suggests that, if the donor is not well-rested, it can impact the outcome of the transplantation. However, it’s heartening to think that this is not an insurmountable obstacle. A short period of recovery sleep before transplant can restore the donor’s cells’ ability to function normally.”
Dr Rolls and her colleagues studied mice that had been gently handled for 4 hours to prevent them from sleeping while control mice dozed. The team then collected HSCs from the sleep-deprived and well-rested mice and injected the cells into 12 irradiated mice.
The recipient mice also received an injection of their own HSCs collected prior to radiation so the researchers could quantify the relative abilities of the donated HSCs to engraft successfully.
The team then assessed the prevalence of myeloid cells derived from donated HSCs at 8 weeks and 16 weeks after transplant. At 16 weeks, donor myeloid chimerism was about 26% in HSCT recipients with well-rested donors and about 12% in recipients with sleep-deprived donors (P<0.0001).
Dr Rolls and her colleagues also compared the ability of fluorescently labeled HSCs from sleep-deprived mice and rested mice to home to the bone marrow. After 12 hours, 3.3% percent of HSCs from rested mice were found in the bone marrow, compared to 1.7% of HSCs from sleep-deprived mice (P<0.05).
Further investigation revealed that sleep deprivation downregulates the expression of miR-19b, a negative regulator of the suppressor of cytokine signaling (SOCS) genes, which inhibit HSC migration and homing.
Finally, Dr Rolls and her colleagues found the effects of sleep deprivation on HSCs could be reversed by letting mice catch up on sleep. Even 2 hours of recovery sleep restored the HSCs’ ability to function normally in transplantation tests.
“We still don’t know how sleep deprivation affects us all, not just bone marrow donors,” Dr Rolls said. “The fact that recovery sleep is so helpful only emphasizes how important it is to pay attention to sleep.” ![]()
Regimen may lengthen survival in AL amyloidosis
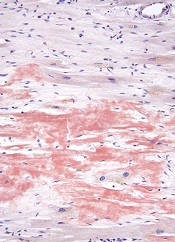
High-dose melphalan and autologous stem cell transplant (HDM/SCT) may enable long-term survival in patients with light-chain (AL) amyloidosis, according to research published in Blood.
The study included more than 500 patients who were followed for a median of 8 years, and the median overall survival (OS) was 7.63 years.
Patients tended to live longer if they had a hematologic complete response (CR) to treatment and if they received the full dose of melphalan as opposed to a modified dose.
“While survival is strongly dependent upon achieving hematologic CR, the survival of patients who did not achieve a CR and of those who relapsed after CR is notable, suggesting a benefit of aggressive treatment,” said Vaishali Sanchorawala, MD, of Boston University School of Medicine in Massachusetts.
Dr Sanchorawala and her colleagues conducted this study by analyzing data from 629 patients with AL amyloidosis who received HDM/SCT between 1994 and 2014. The patients’ median age was 57 (range, 28 to 80).
They received full-dose melphalan at 200 mg/m2 (n=350, 55.6%) or modified-dose melphalan at 100-140 mg/m2 (n=279, 44.3%), based on their age and organ function. All patients received growth factor for stem cell mobilization.
Treatment-related mortality (TRM) was defined as death within 100 days of SCT. The rate of TRM was 7.4% (n=47). Eleven deaths occurred during stem cell mobilization and collection (before melphalan was given).
After 2005, there were no deaths during stem cell mobilization and collection, and TRM improved to 3.4% (n=10).
Overall, 543 patients (86.3%) were evaluable for response at 6 to 12 months after SCT. Of these patients, 40.3% (n=219) achieved a hematologic CR. However, 18.2% (n=40) of these patients later relapsed, at a median of 3.97 years (range, 1.89-12.45).
Hematologic CR was more likely among patients who received full-dose melphalan than those who received the modified dose, occurring in 44.9% and 33.7% of patients, respectively (P=0.0091).
Relapse was more likely among patients who received melphalan at the modified dose than the full dose, occurring in 60% and 40%, respectively.
At a median follow-up of 8 years, the median OS was 7.63 years. The median OS has not been reached among patients achieving a hematologic CR, but it was 6.3 years for patients who did not achieve a hematologic CR (P<0.0001). The median OS for patients who relapsed was 4.3 years.
The median OS was significantly longer for patients who received full-dose melphalan—10.47 years, compared to 5.15 years for patients who received the modified dose (P<0.0001).
Likewise, the estimated OS rates at 1, 5, 10, and 15 years were higher for patients with a hematologic CR than for those without one. The 1-year OS is 100% and 94%, respectively. The OS at 5 years is 88% and 60%, respectively. The 10-year OS is 72% and 34%, respectively. And the OS at 15 years is 57% and 18%, respectively.
Forty patients who achieved a hematologic CR died of a cause other than relapse, including sudden death (n=7), metastatic malignancy (n=6), heart failure (n=5), renal failure (n=5), therapy-related myelodysplastic syndrome/acute myeloid leukemia (n=4), sepsis (n=4), stroke (n=3), bleeding complications (n=2), and unknown cause (n=4).
“Strategies to better understand which patients may benefit the most from this treatment and reducing treatment-related mortality, as well as using combination therapies with novel agents to increase the CR rate, will likely improve outcomes in the future for patients who, just a few years ago, were considered to have a rapidly fatal diagnosis,” Dr Sanchorawala said.
She and her colleagues also noted that this study included a “highly selected” group of new patients. ![]()

High-dose melphalan and autologous stem cell transplant (HDM/SCT) may enable long-term survival in patients with light-chain (AL) amyloidosis, according to research published in Blood.
The study included more than 500 patients who were followed for a median of 8 years, and the median overall survival (OS) was 7.63 years.
Patients tended to live longer if they had a hematologic complete response (CR) to treatment and if they received the full dose of melphalan as opposed to a modified dose.
“While survival is strongly dependent upon achieving hematologic CR, the survival of patients who did not achieve a CR and of those who relapsed after CR is notable, suggesting a benefit of aggressive treatment,” said Vaishali Sanchorawala, MD, of Boston University School of Medicine in Massachusetts.
Dr Sanchorawala and her colleagues conducted this study by analyzing data from 629 patients with AL amyloidosis who received HDM/SCT between 1994 and 2014. The patients’ median age was 57 (range, 28 to 80).
They received full-dose melphalan at 200 mg/m2 (n=350, 55.6%) or modified-dose melphalan at 100-140 mg/m2 (n=279, 44.3%), based on their age and organ function. All patients received growth factor for stem cell mobilization.
Treatment-related mortality (TRM) was defined as death within 100 days of SCT. The rate of TRM was 7.4% (n=47). Eleven deaths occurred during stem cell mobilization and collection (before melphalan was given).
After 2005, there were no deaths during stem cell mobilization and collection, and TRM improved to 3.4% (n=10).
Overall, 543 patients (86.3%) were evaluable for response at 6 to 12 months after SCT. Of these patients, 40.3% (n=219) achieved a hematologic CR. However, 18.2% (n=40) of these patients later relapsed, at a median of 3.97 years (range, 1.89-12.45).
Hematologic CR was more likely among patients who received full-dose melphalan than those who received the modified dose, occurring in 44.9% and 33.7% of patients, respectively (P=0.0091).
Relapse was more likely among patients who received melphalan at the modified dose than the full dose, occurring in 60% and 40%, respectively.
At a median follow-up of 8 years, the median OS was 7.63 years. The median OS has not been reached among patients achieving a hematologic CR, but it was 6.3 years for patients who did not achieve a hematologic CR (P<0.0001). The median OS for patients who relapsed was 4.3 years.
The median OS was significantly longer for patients who received full-dose melphalan—10.47 years, compared to 5.15 years for patients who received the modified dose (P<0.0001).
Likewise, the estimated OS rates at 1, 5, 10, and 15 years were higher for patients with a hematologic CR than for those without one. The 1-year OS is 100% and 94%, respectively. The OS at 5 years is 88% and 60%, respectively. The 10-year OS is 72% and 34%, respectively. And the OS at 15 years is 57% and 18%, respectively.
Forty patients who achieved a hematologic CR died of a cause other than relapse, including sudden death (n=7), metastatic malignancy (n=6), heart failure (n=5), renal failure (n=5), therapy-related myelodysplastic syndrome/acute myeloid leukemia (n=4), sepsis (n=4), stroke (n=3), bleeding complications (n=2), and unknown cause (n=4).
“Strategies to better understand which patients may benefit the most from this treatment and reducing treatment-related mortality, as well as using combination therapies with novel agents to increase the CR rate, will likely improve outcomes in the future for patients who, just a few years ago, were considered to have a rapidly fatal diagnosis,” Dr Sanchorawala said.
She and her colleagues also noted that this study included a “highly selected” group of new patients. ![]()

High-dose melphalan and autologous stem cell transplant (HDM/SCT) may enable long-term survival in patients with light-chain (AL) amyloidosis, according to research published in Blood.
The study included more than 500 patients who were followed for a median of 8 years, and the median overall survival (OS) was 7.63 years.
Patients tended to live longer if they had a hematologic complete response (CR) to treatment and if they received the full dose of melphalan as opposed to a modified dose.
“While survival is strongly dependent upon achieving hematologic CR, the survival of patients who did not achieve a CR and of those who relapsed after CR is notable, suggesting a benefit of aggressive treatment,” said Vaishali Sanchorawala, MD, of Boston University School of Medicine in Massachusetts.
Dr Sanchorawala and her colleagues conducted this study by analyzing data from 629 patients with AL amyloidosis who received HDM/SCT between 1994 and 2014. The patients’ median age was 57 (range, 28 to 80).
They received full-dose melphalan at 200 mg/m2 (n=350, 55.6%) or modified-dose melphalan at 100-140 mg/m2 (n=279, 44.3%), based on their age and organ function. All patients received growth factor for stem cell mobilization.
Treatment-related mortality (TRM) was defined as death within 100 days of SCT. The rate of TRM was 7.4% (n=47). Eleven deaths occurred during stem cell mobilization and collection (before melphalan was given).
After 2005, there were no deaths during stem cell mobilization and collection, and TRM improved to 3.4% (n=10).
Overall, 543 patients (86.3%) were evaluable for response at 6 to 12 months after SCT. Of these patients, 40.3% (n=219) achieved a hematologic CR. However, 18.2% (n=40) of these patients later relapsed, at a median of 3.97 years (range, 1.89-12.45).
Hematologic CR was more likely among patients who received full-dose melphalan than those who received the modified dose, occurring in 44.9% and 33.7% of patients, respectively (P=0.0091).
Relapse was more likely among patients who received melphalan at the modified dose than the full dose, occurring in 60% and 40%, respectively.
At a median follow-up of 8 years, the median OS was 7.63 years. The median OS has not been reached among patients achieving a hematologic CR, but it was 6.3 years for patients who did not achieve a hematologic CR (P<0.0001). The median OS for patients who relapsed was 4.3 years.
The median OS was significantly longer for patients who received full-dose melphalan—10.47 years, compared to 5.15 years for patients who received the modified dose (P<0.0001).
Likewise, the estimated OS rates at 1, 5, 10, and 15 years were higher for patients with a hematologic CR than for those without one. The 1-year OS is 100% and 94%, respectively. The OS at 5 years is 88% and 60%, respectively. The 10-year OS is 72% and 34%, respectively. And the OS at 15 years is 57% and 18%, respectively.
Forty patients who achieved a hematologic CR died of a cause other than relapse, including sudden death (n=7), metastatic malignancy (n=6), heart failure (n=5), renal failure (n=5), therapy-related myelodysplastic syndrome/acute myeloid leukemia (n=4), sepsis (n=4), stroke (n=3), bleeding complications (n=2), and unknown cause (n=4).
“Strategies to better understand which patients may benefit the most from this treatment and reducing treatment-related mortality, as well as using combination therapies with novel agents to increase the CR rate, will likely improve outcomes in the future for patients who, just a few years ago, were considered to have a rapidly fatal diagnosis,” Dr Sanchorawala said.
She and her colleagues also noted that this study included a “highly selected” group of new patients. ![]()
Haplo-HSCT appears comparable to fully matched HSCT

Photo by Chad McNeeley
A retrospective study suggests that, for patients with hematologic disorders, a haploidentical hematopoietic stem cell transplant (HSCT)
can be roughly as safe and effective as a fully matched HSCT.
The study showed that, when patients received an identical conditioning regimen, graft T-cell dose, and graft-vs-host disease (GVHD) prophylaxis, haploidentical and fully matched HSCTs produced comparable results.
Patients had similar rates of overall and progression-free survival, relapse, non-relapse mortality, and chronic GVHD.
However, patients who received haploidentical transplants had higher rates of grade 2-4 acute GVHD and cytomegalovirus reactivation.
Researchers reported these results in Biology of Blood and Marrow Transplantation.
“This is the first study to compare the gold standard to a half-match using an identical protocol,” said Neal Flomenberg, MD, of Thomas Jefferson University in Philadelphia, Pennsylvania.
“The field has debated whether the differences in outcomes between full and partial matches were caused by the quality of the match or by all the procedures the patient goes through before and after the donor cells are administered. We haven’t had a clear answer.”
With that in mind, Dr Flomenberg and his colleagues compared 3-year outcome data from patients who received haploidentical HSCTs (n=50) or fully matched HSCTs (n=27), when both groups of patients were treated with a 2-step protocol.
The patients had acute myeloid leukemia (n=38), acute lymphoblastic leukemia (n=20), myelodysplastic syndromes/myeloproliferative neoplasms (n=7), non-Hodgkin lymphoma (n=11), and aplastic anemia (n=1).
The 2-step protocol
All patients received a myeloablative conditioning regimen consisting of 12 Gy of total body irradiation administered in 8 fractions over 4 days. After the last fraction, they received a fixed T-cell dose (2 x 108 cells/kg), which was followed, 2 days later, by cyclophosphamide at 60 mg/kg/day for 2 days.
Twenty-four hours after they completed cyclophosphamide, patients received CD34-selected peripheral blood stem cells from a half-matched or fully matched donor.
On day -1, patients began taking tacrolimus and mycophenolate mofetil as GVHD prophylaxis. They also received growth factor support (granulocyte-macrophage colony-stimulating factor at 250 μg/m2) starting on day +1.
In the absence of GVHD, mycophenolate mofetil was discontinued on day 28 and tacrolimus was tapered, starting on day +60 after HSCT.
Results
The researchers said that early immune recovery was comparable between the patient groups in nearly all assessed T-cell subsets. The exception was the median CD3/CD8 cell count, which was significantly higher at day 28 in the fully matched group than the haploidentical group (P=0.029).
Survival rates were comparable between the groups. The estimated 3-year overall survival was 70% in the haploidentical group and 71% in the fully matched group (P=0.81). The 3-year progression-free survival was 68% and 70%, respectively (P=0.97).
The 3-year cumulative incidence of non-relapse mortality was 10% in the haploidentical group and 4% in the fully matched group (P=0.34). The 3-year cumulative incidence of relapse was 21% and 27%, respectively (P=0.93).
The 100-day cumulative incidence of grade 2-4 acute GVHD was significantly higher in the haploidentical group than the fully matched group—40% and 8%, respectively (P<0.001). But there was no significant difference in the incidence of grade 3-4 acute GVHD—6% and 4%, respectively (P=0.49).
The cumulative incidence of chronic GVHD at 2 years was not significantly different between the haploidentical and fully matched groups—19% and 12%, respectively (P=0.47). The same was true for severe chronic GVHD—4% and 8%, respectively (P=0.49).
The cumulative incidence of cytomegalovirus reactivation was significantly higher in the haploidentical group than the fully matched group—68% and 19%, respectively (P<0.001).
There were no deaths from infections or GVHD in either group.
“The results of the current study are certainly encouraging and suggest that outcomes from a half-matched, related donor are similar to fully matched donors,” said study author Sameh Gaballa, MD, also of Thomas Jefferson University.
“It might be time to reassess whether half-matched, related transplants can be considered the best alternative donor source for patients lacking a fully matched family member donor. For that, we’ll need more evidence from a randomly controlled, prospective trial, rather than studies that look at patient data retrospectively, to help solidify our findings here.” ![]()

Photo by Chad McNeeley
A retrospective study suggests that, for patients with hematologic disorders, a haploidentical hematopoietic stem cell transplant (HSCT)
can be roughly as safe and effective as a fully matched HSCT.
The study showed that, when patients received an identical conditioning regimen, graft T-cell dose, and graft-vs-host disease (GVHD) prophylaxis, haploidentical and fully matched HSCTs produced comparable results.
Patients had similar rates of overall and progression-free survival, relapse, non-relapse mortality, and chronic GVHD.
However, patients who received haploidentical transplants had higher rates of grade 2-4 acute GVHD and cytomegalovirus reactivation.
Researchers reported these results in Biology of Blood and Marrow Transplantation.
“This is the first study to compare the gold standard to a half-match using an identical protocol,” said Neal Flomenberg, MD, of Thomas Jefferson University in Philadelphia, Pennsylvania.
“The field has debated whether the differences in outcomes between full and partial matches were caused by the quality of the match or by all the procedures the patient goes through before and after the donor cells are administered. We haven’t had a clear answer.”
With that in mind, Dr Flomenberg and his colleagues compared 3-year outcome data from patients who received haploidentical HSCTs (n=50) or fully matched HSCTs (n=27), when both groups of patients were treated with a 2-step protocol.
The patients had acute myeloid leukemia (n=38), acute lymphoblastic leukemia (n=20), myelodysplastic syndromes/myeloproliferative neoplasms (n=7), non-Hodgkin lymphoma (n=11), and aplastic anemia (n=1).
The 2-step protocol
All patients received a myeloablative conditioning regimen consisting of 12 Gy of total body irradiation administered in 8 fractions over 4 days. After the last fraction, they received a fixed T-cell dose (2 x 108 cells/kg), which was followed, 2 days later, by cyclophosphamide at 60 mg/kg/day for 2 days.
Twenty-four hours after they completed cyclophosphamide, patients received CD34-selected peripheral blood stem cells from a half-matched or fully matched donor.
On day -1, patients began taking tacrolimus and mycophenolate mofetil as GVHD prophylaxis. They also received growth factor support (granulocyte-macrophage colony-stimulating factor at 250 μg/m2) starting on day +1.
In the absence of GVHD, mycophenolate mofetil was discontinued on day 28 and tacrolimus was tapered, starting on day +60 after HSCT.
Results
The researchers said that early immune recovery was comparable between the patient groups in nearly all assessed T-cell subsets. The exception was the median CD3/CD8 cell count, which was significantly higher at day 28 in the fully matched group than the haploidentical group (P=0.029).
Survival rates were comparable between the groups. The estimated 3-year overall survival was 70% in the haploidentical group and 71% in the fully matched group (P=0.81). The 3-year progression-free survival was 68% and 70%, respectively (P=0.97).
The 3-year cumulative incidence of non-relapse mortality was 10% in the haploidentical group and 4% in the fully matched group (P=0.34). The 3-year cumulative incidence of relapse was 21% and 27%, respectively (P=0.93).
The 100-day cumulative incidence of grade 2-4 acute GVHD was significantly higher in the haploidentical group than the fully matched group—40% and 8%, respectively (P<0.001). But there was no significant difference in the incidence of grade 3-4 acute GVHD—6% and 4%, respectively (P=0.49).
The cumulative incidence of chronic GVHD at 2 years was not significantly different between the haploidentical and fully matched groups—19% and 12%, respectively (P=0.47). The same was true for severe chronic GVHD—4% and 8%, respectively (P=0.49).
The cumulative incidence of cytomegalovirus reactivation was significantly higher in the haploidentical group than the fully matched group—68% and 19%, respectively (P<0.001).
There were no deaths from infections or GVHD in either group.
“The results of the current study are certainly encouraging and suggest that outcomes from a half-matched, related donor are similar to fully matched donors,” said study author Sameh Gaballa, MD, also of Thomas Jefferson University.
“It might be time to reassess whether half-matched, related transplants can be considered the best alternative donor source for patients lacking a fully matched family member donor. For that, we’ll need more evidence from a randomly controlled, prospective trial, rather than studies that look at patient data retrospectively, to help solidify our findings here.” ![]()

Photo by Chad McNeeley
A retrospective study suggests that, for patients with hematologic disorders, a haploidentical hematopoietic stem cell transplant (HSCT)
can be roughly as safe and effective as a fully matched HSCT.
The study showed that, when patients received an identical conditioning regimen, graft T-cell dose, and graft-vs-host disease (GVHD) prophylaxis, haploidentical and fully matched HSCTs produced comparable results.
Patients had similar rates of overall and progression-free survival, relapse, non-relapse mortality, and chronic GVHD.
However, patients who received haploidentical transplants had higher rates of grade 2-4 acute GVHD and cytomegalovirus reactivation.
Researchers reported these results in Biology of Blood and Marrow Transplantation.
“This is the first study to compare the gold standard to a half-match using an identical protocol,” said Neal Flomenberg, MD, of Thomas Jefferson University in Philadelphia, Pennsylvania.
“The field has debated whether the differences in outcomes between full and partial matches were caused by the quality of the match or by all the procedures the patient goes through before and after the donor cells are administered. We haven’t had a clear answer.”
With that in mind, Dr Flomenberg and his colleagues compared 3-year outcome data from patients who received haploidentical HSCTs (n=50) or fully matched HSCTs (n=27), when both groups of patients were treated with a 2-step protocol.
The patients had acute myeloid leukemia (n=38), acute lymphoblastic leukemia (n=20), myelodysplastic syndromes/myeloproliferative neoplasms (n=7), non-Hodgkin lymphoma (n=11), and aplastic anemia (n=1).
The 2-step protocol
All patients received a myeloablative conditioning regimen consisting of 12 Gy of total body irradiation administered in 8 fractions over 4 days. After the last fraction, they received a fixed T-cell dose (2 x 108 cells/kg), which was followed, 2 days later, by cyclophosphamide at 60 mg/kg/day for 2 days.
Twenty-four hours after they completed cyclophosphamide, patients received CD34-selected peripheral blood stem cells from a half-matched or fully matched donor.
On day -1, patients began taking tacrolimus and mycophenolate mofetil as GVHD prophylaxis. They also received growth factor support (granulocyte-macrophage colony-stimulating factor at 250 μg/m2) starting on day +1.
In the absence of GVHD, mycophenolate mofetil was discontinued on day 28 and tacrolimus was tapered, starting on day +60 after HSCT.
Results
The researchers said that early immune recovery was comparable between the patient groups in nearly all assessed T-cell subsets. The exception was the median CD3/CD8 cell count, which was significantly higher at day 28 in the fully matched group than the haploidentical group (P=0.029).
Survival rates were comparable between the groups. The estimated 3-year overall survival was 70% in the haploidentical group and 71% in the fully matched group (P=0.81). The 3-year progression-free survival was 68% and 70%, respectively (P=0.97).
The 3-year cumulative incidence of non-relapse mortality was 10% in the haploidentical group and 4% in the fully matched group (P=0.34). The 3-year cumulative incidence of relapse was 21% and 27%, respectively (P=0.93).
The 100-day cumulative incidence of grade 2-4 acute GVHD was significantly higher in the haploidentical group than the fully matched group—40% and 8%, respectively (P<0.001). But there was no significant difference in the incidence of grade 3-4 acute GVHD—6% and 4%, respectively (P=0.49).
The cumulative incidence of chronic GVHD at 2 years was not significantly different between the haploidentical and fully matched groups—19% and 12%, respectively (P=0.47). The same was true for severe chronic GVHD—4% and 8%, respectively (P=0.49).
The cumulative incidence of cytomegalovirus reactivation was significantly higher in the haploidentical group than the fully matched group—68% and 19%, respectively (P<0.001).
There were no deaths from infections or GVHD in either group.
“The results of the current study are certainly encouraging and suggest that outcomes from a half-matched, related donor are similar to fully matched donors,” said study author Sameh Gaballa, MD, also of Thomas Jefferson University.
“It might be time to reassess whether half-matched, related transplants can be considered the best alternative donor source for patients lacking a fully matched family member donor. For that, we’ll need more evidence from a randomly controlled, prospective trial, rather than studies that look at patient data retrospectively, to help solidify our findings here.” ![]()
Creating off-the-shelf VSTs

Photo courtesy of Baylor
College of Medicine
NEW YORK—Researchers are creating virus-specific T cells (VST) to treat and prevent viral infections in patients who undergo hematopoietic stem cell transplant.
Thus far, the group has modified T cells with 5 viral vectors—Epstein-Barr virus (EBV), cytomegalovirus (CMV), adenovirus (ADV), BK virus (BKV), and human herpesvirus 6 (HHV6)—and are devising methods whereby these VSTs can be made readily available, off-the-shelf products.
Helen Heslop, MD, of Baylor College of Medicine in Houston, Texas, described the efforts of the Baylor research team at the inaugural CRI-CIMT-EATI-AACR International Cancer Immunotherapy Conference.
The team’s tri-virus and 5-virus VST approaches have been described previously. Here, we focus on the team’s efforts to create an off-the-shelf product.
A companion story describes the team’s VST approach to treating type 2 EBV-associated lymphomas.
Despite promising results with these earlier methodologies both as prophylaxis and treatment after stem cell transplant, problems existed with the approaches that would impede broader implementation of VSTs.
“Most of these initial methodologies were complex,” Dr Heslop said.
And even though the production time of the 5-virus VSTs is only 10 days, it does not allow for urgent use.
To solve this problem, the researchers are developing VSTs as off-the-shelf, third-party banked cells for patients who don’t have the time to wait for donor-specific cells to be made.
“The strategy here is that we make lines that are well-characterized but are HLA-restricted and tied to specific viruses,” Dr Heslop said.
The cells are then cryopreserved so that they’re available, and patients receive VSTs according to their HLA type and the line that has suitable activity for their infections.
“We initially evaluated this approach through a multicenter study sponsored by the NHLBI [National Heart, Lung, and Blood Institute],” Dr Heslop said.
Tri-virus approach
The researchers used the original tri-virus methodology, which was lengthy, “but, in this case, because the cells were already available, the time was not a major issue,” Dr Heslop said.
The team also made some new lines for donors with common alleles. In all, they had 32 lines available while the study was running.
They treated 50 patients: 23 received VSTs for CMV, 18 for ADV, and 9 for EBV.
One patient who had CMV colitis received the VSTs and had a complete response (CR), as evidenced by a normal endoscopy with no viral inclusions.
Another patient had EBV persistent after 6 doses of rituximab. One month after receiving the VST infusion, the patient had a CR, even though the cell line had only 1 class 2 allele. And the patient has sustained the CR for several years.
The overall response rate for the study is 74.0% at day 42 after infusion.
The response rate was not significantly different for each virus, Dr Heslop pointed out. Patients with CMV had a 73.9% response rate, those with EBV had a 66.7% response rate, and those with ADV had a 77.8% response rate.
“This is a little bit lower than with the donor-specific T cells, but I think it’s still a promising approach,” she added.
5-virus peptide mix
The researchers are now evaluating the more rapid, 5-virus peptide mix method for creating off-the-shelf VSTs.
This method replaced live viruses with overlapping peptide pools and added immunogenic antigens for 5 viruses, including BKV and HHV6. The process takes only 10 days to produce VSTs.
The team has identified a line for over 90% of the patients screened.
“That’s because we will accept a line that’s only matched at 1 HLA allele, as long as we have activity against the infecting virus through the shared allele,” Dr Heslop explained.
So they’re conducting a clinical trial using this method, and thus far, they have enrolled 22 patients. Sixteen patients have had 1 infusion, and 6 patients have had multiple infusions.
The 22 patients had 25 infections: 10 CMV, 10 BKV, 2 EBV, 2 ADV, and 1 HHV6.
The overall response rate is 88%. Nine of 10 responses in patients with BKV were partial because BK is difficult to clear from urine. However, the BK patients who had hemorrhagic cystitis all had symptomatic improvement.
Dr Heslop pointed out that these results are similar to those at other centers using EBV third-party T cells.
The initial third-party studies were done in Scotland and had a response rate of about 60%, which increased to around 80% in follow-up studies, where they characterized the T cells more extensively.
Both donor-specific and third-party VSTs have low toxicity, sustained response rates, and activity confirmed by studies in multiple centers.
Dr Heslop believes the rapid manufacturing methodologies will facilitate definitive clinical trials.
She said Cell Medica provided support for some of the trials with EBV tumors. ![]()

Photo courtesy of Baylor
College of Medicine
NEW YORK—Researchers are creating virus-specific T cells (VST) to treat and prevent viral infections in patients who undergo hematopoietic stem cell transplant.
Thus far, the group has modified T cells with 5 viral vectors—Epstein-Barr virus (EBV), cytomegalovirus (CMV), adenovirus (ADV), BK virus (BKV), and human herpesvirus 6 (HHV6)—and are devising methods whereby these VSTs can be made readily available, off-the-shelf products.
Helen Heslop, MD, of Baylor College of Medicine in Houston, Texas, described the efforts of the Baylor research team at the inaugural CRI-CIMT-EATI-AACR International Cancer Immunotherapy Conference.
The team’s tri-virus and 5-virus VST approaches have been described previously. Here, we focus on the team’s efforts to create an off-the-shelf product.
A companion story describes the team’s VST approach to treating type 2 EBV-associated lymphomas.
Despite promising results with these earlier methodologies both as prophylaxis and treatment after stem cell transplant, problems existed with the approaches that would impede broader implementation of VSTs.
“Most of these initial methodologies were complex,” Dr Heslop said.
And even though the production time of the 5-virus VSTs is only 10 days, it does not allow for urgent use.
To solve this problem, the researchers are developing VSTs as off-the-shelf, third-party banked cells for patients who don’t have the time to wait for donor-specific cells to be made.
“The strategy here is that we make lines that are well-characterized but are HLA-restricted and tied to specific viruses,” Dr Heslop said.
The cells are then cryopreserved so that they’re available, and patients receive VSTs according to their HLA type and the line that has suitable activity for their infections.
“We initially evaluated this approach through a multicenter study sponsored by the NHLBI [National Heart, Lung, and Blood Institute],” Dr Heslop said.
Tri-virus approach
The researchers used the original tri-virus methodology, which was lengthy, “but, in this case, because the cells were already available, the time was not a major issue,” Dr Heslop said.
The team also made some new lines for donors with common alleles. In all, they had 32 lines available while the study was running.
They treated 50 patients: 23 received VSTs for CMV, 18 for ADV, and 9 for EBV.
One patient who had CMV colitis received the VSTs and had a complete response (CR), as evidenced by a normal endoscopy with no viral inclusions.
Another patient had EBV persistent after 6 doses of rituximab. One month after receiving the VST infusion, the patient had a CR, even though the cell line had only 1 class 2 allele. And the patient has sustained the CR for several years.
The overall response rate for the study is 74.0% at day 42 after infusion.
The response rate was not significantly different for each virus, Dr Heslop pointed out. Patients with CMV had a 73.9% response rate, those with EBV had a 66.7% response rate, and those with ADV had a 77.8% response rate.
“This is a little bit lower than with the donor-specific T cells, but I think it’s still a promising approach,” she added.
5-virus peptide mix
The researchers are now evaluating the more rapid, 5-virus peptide mix method for creating off-the-shelf VSTs.
This method replaced live viruses with overlapping peptide pools and added immunogenic antigens for 5 viruses, including BKV and HHV6. The process takes only 10 days to produce VSTs.
The team has identified a line for over 90% of the patients screened.
“That’s because we will accept a line that’s only matched at 1 HLA allele, as long as we have activity against the infecting virus through the shared allele,” Dr Heslop explained.
So they’re conducting a clinical trial using this method, and thus far, they have enrolled 22 patients. Sixteen patients have had 1 infusion, and 6 patients have had multiple infusions.
The 22 patients had 25 infections: 10 CMV, 10 BKV, 2 EBV, 2 ADV, and 1 HHV6.
The overall response rate is 88%. Nine of 10 responses in patients with BKV were partial because BK is difficult to clear from urine. However, the BK patients who had hemorrhagic cystitis all had symptomatic improvement.
Dr Heslop pointed out that these results are similar to those at other centers using EBV third-party T cells.
The initial third-party studies were done in Scotland and had a response rate of about 60%, which increased to around 80% in follow-up studies, where they characterized the T cells more extensively.
Both donor-specific and third-party VSTs have low toxicity, sustained response rates, and activity confirmed by studies in multiple centers.
Dr Heslop believes the rapid manufacturing methodologies will facilitate definitive clinical trials.
She said Cell Medica provided support for some of the trials with EBV tumors. ![]()

Photo courtesy of Baylor
College of Medicine
NEW YORK—Researchers are creating virus-specific T cells (VST) to treat and prevent viral infections in patients who undergo hematopoietic stem cell transplant.
Thus far, the group has modified T cells with 5 viral vectors—Epstein-Barr virus (EBV), cytomegalovirus (CMV), adenovirus (ADV), BK virus (BKV), and human herpesvirus 6 (HHV6)—and are devising methods whereby these VSTs can be made readily available, off-the-shelf products.
Helen Heslop, MD, of Baylor College of Medicine in Houston, Texas, described the efforts of the Baylor research team at the inaugural CRI-CIMT-EATI-AACR International Cancer Immunotherapy Conference.
The team’s tri-virus and 5-virus VST approaches have been described previously. Here, we focus on the team’s efforts to create an off-the-shelf product.
A companion story describes the team’s VST approach to treating type 2 EBV-associated lymphomas.
Despite promising results with these earlier methodologies both as prophylaxis and treatment after stem cell transplant, problems existed with the approaches that would impede broader implementation of VSTs.
“Most of these initial methodologies were complex,” Dr Heslop said.
And even though the production time of the 5-virus VSTs is only 10 days, it does not allow for urgent use.
To solve this problem, the researchers are developing VSTs as off-the-shelf, third-party banked cells for patients who don’t have the time to wait for donor-specific cells to be made.
“The strategy here is that we make lines that are well-characterized but are HLA-restricted and tied to specific viruses,” Dr Heslop said.
The cells are then cryopreserved so that they’re available, and patients receive VSTs according to their HLA type and the line that has suitable activity for their infections.
“We initially evaluated this approach through a multicenter study sponsored by the NHLBI [National Heart, Lung, and Blood Institute],” Dr Heslop said.
Tri-virus approach
The researchers used the original tri-virus methodology, which was lengthy, “but, in this case, because the cells were already available, the time was not a major issue,” Dr Heslop said.
The team also made some new lines for donors with common alleles. In all, they had 32 lines available while the study was running.
They treated 50 patients: 23 received VSTs for CMV, 18 for ADV, and 9 for EBV.
One patient who had CMV colitis received the VSTs and had a complete response (CR), as evidenced by a normal endoscopy with no viral inclusions.
Another patient had EBV persistent after 6 doses of rituximab. One month after receiving the VST infusion, the patient had a CR, even though the cell line had only 1 class 2 allele. And the patient has sustained the CR for several years.
The overall response rate for the study is 74.0% at day 42 after infusion.
The response rate was not significantly different for each virus, Dr Heslop pointed out. Patients with CMV had a 73.9% response rate, those with EBV had a 66.7% response rate, and those with ADV had a 77.8% response rate.
“This is a little bit lower than with the donor-specific T cells, but I think it’s still a promising approach,” she added.
5-virus peptide mix
The researchers are now evaluating the more rapid, 5-virus peptide mix method for creating off-the-shelf VSTs.
This method replaced live viruses with overlapping peptide pools and added immunogenic antigens for 5 viruses, including BKV and HHV6. The process takes only 10 days to produce VSTs.
The team has identified a line for over 90% of the patients screened.
“That’s because we will accept a line that’s only matched at 1 HLA allele, as long as we have activity against the infecting virus through the shared allele,” Dr Heslop explained.
So they’re conducting a clinical trial using this method, and thus far, they have enrolled 22 patients. Sixteen patients have had 1 infusion, and 6 patients have had multiple infusions.
The 22 patients had 25 infections: 10 CMV, 10 BKV, 2 EBV, 2 ADV, and 1 HHV6.
The overall response rate is 88%. Nine of 10 responses in patients with BKV were partial because BK is difficult to clear from urine. However, the BK patients who had hemorrhagic cystitis all had symptomatic improvement.
Dr Heslop pointed out that these results are similar to those at other centers using EBV third-party T cells.
The initial third-party studies were done in Scotland and had a response rate of about 60%, which increased to around 80% in follow-up studies, where they characterized the T cells more extensively.
Both donor-specific and third-party VSTs have low toxicity, sustained response rates, and activity confirmed by studies in multiple centers.
Dr Heslop believes the rapid manufacturing methodologies will facilitate definitive clinical trials.
She said Cell Medica provided support for some of the trials with EBV tumors. ![]()
Nonviral gene transfer of CARs tested in humans

Photo courtesy of MDACC
NEW YORK—Researchers have used a nonviral approach to create chimeric antigen receptor (CAR) T cells and tested these cells in safety trials.
Patients with advanced lymphoma or leukemia were infused with the nonvirally modified CD19-directed CAR T cells after autologous or allogeneic hematopoietic stem cell transplant (HSCT).
Eighty-six percent of autologous HSCT recipients were alive 24 months after infusion, and 53% of allogeneic HSCT recipients were alive with a median follow-up of 6.5 months.
“Gratifyingly, the patients have not demonstrated any acute or late toxicity to these CAR T-cell infusions,” said Laurence Cooper, MD, PhD, formerly of MD Anderson Cancer Center (MDACC) in Houston, Texas, and now with Ziopharm Oncology.
Dr Cooper presented these results at the inaugural CRI-CIMT-EATI-AACR International Cancer Immunotherapy Conference.
Some of the technology he described was conducted at MDACC. Dr Cooper is currently a visiting scientist there and will continue to supervise the development of this technology.
Dr Cooper said the appeal of this nonviral approach, which is a modified Sleeping Beauty approach, “is it essentially avoids the complexity of making a virus, a lentivirus or a retrovirus, it can be done at quite low cost, and really allows for a nimbleness to this system.”
Using a simple blood draw of 200 cc of peripheral blood—the process does not require apheresis—the T cells can be expanded on a feeder cell layer and genetically reprogrammed.
Sleeping Beauty system
The researchers reprogrammed the T cells using a 2-plasmid Sleeping Beauty system, which is a transposon/transposase system.
The transposon DNA plasmid codes for the cargo load, which, in this case, is the CAR. At the same time, the transposase DNA plasmid is electroporated, “which is really the secret sauce of the transposition event,” Dr Cooper explained.
After electroporation, the transposon/transposase are co-cultured with K562-derived artificial antigen-presenting cells (aAPC) and expanded with the integrated transposon of K562-aAPC. In this case, CD19 is on the aAPC.
CD19 is co-expressed with other co-stimulatory molecules, CD86 and 4-1BB ligand.
In addition, the researchers added a molecule of interleukin 15 that’s sewn in frame to the Fc region of an immunoglobulin that then activates the T cell in the context of these co-stimulatory molecules.
The T cells that have stable integrants of the CAR grow out over time. And those that have transient expression of the CAR die by neglect.
“By day 14, most of the T cells have the CAR sewn into the genome and are stably expressed,” Dr Cooper said.
The CAR used for these safety trials at MDACC targets CD19 and uses mouse scFv held in frame with an immunoglobulin 4 Fc (IgG4Fc) stalk.
It’s tunneled through the T-cell membrane and has 2 costimulatory molecules, signal 1 delivered by phosphorylation of the immunoreceptor tyrosine-based activation motif in CD3ζ and signal 2 by the costimulatory domain CD28.
The researchers tested the CD19 CARs in 2 clinical settings—one with T cells that were patient-derived and infused after autologous HSCT, and the second with T cells that were derived from a third party and infused after allogeneic HSCT.
Infusion after autologous HSCT
The researchers first tried the CARs in 7 non-Hodgkin lymphoma patients who had an autologous HSCT. Their median age was 52 (range, 36-61).
Five patients received a starting CAR T-cell dose of 5x108 cells/m2, and 2 received 5x109 cells/m2.
Six patients (86%) remain alive and are in complete remission (CR) at a median follow-up of 24 months.
Infusion after allogeneic HSCT
The researchers expanded the investigation to a wider cohort of 19 patients who had undergone allogeneic HSCT.
Seventeen patients had advanced CD19-positive acute lymphoblastic leukemia, and 2 had non-Hodgkin lymphoma. Their median age was 35 (range, 21-56).
All patients were on graft-versus-host disease (GVHD) prophylaxis with tacrolimus at the time of CAR infusion. A subset of these allogeneic transplant patients had haploidentical donors rather than matched sibling donors.
Five patients received a CAR T-cell dose of 106, 6 patients received 107, 5 received 5x107, and 3 received 5x108 cells/m2 based on recipient body surface area.
Fifty-eight percent of patients (11/19) achieved a CR, and 10 remain alive a median of 6.5 months after CAR T-cell infusion.
Three patients developed GVHD, 1 with steroid-refractory acute liver disease, 1 with grade 2 acute skin disease, and 1 with chronic limited skin disease. The incidence of GVHD was lower than historical controls at MDACC, Dr Cooper said.
“[G]ratifyingly, in this clinical setting of minimal disease, patients did not have any acute or late toxicity from these infusions,” he added.
And the rate of cytomegalovirus reactivation after CAR T-cell infusion was 24%, compared with 41% for patients after transplant at MDACC without CAR T-cell infusion.
Eight patients received haploidentical HSCT followed by CAR T-cell infusion, and 75% (6/8) remain in CR.
Persistence of infused T cells
The researchers used 2 forms of PCR—qPCR and droplet PCR—to map the fate of the CARs.
“Roughly speaking, for these patients, and this is in line with the literature, in terms of those T cells that are activated through CD28 in contrast to 4-1BB, these T cells are, on average, living about 28 or so days post-infusion,” Dr Cooper noted.
He said this is similar to results observed with CARs being tested at the National Cancer Institute and Memorial Sloan-Kettering Cancer Center. ![]()

Photo courtesy of MDACC
NEW YORK—Researchers have used a nonviral approach to create chimeric antigen receptor (CAR) T cells and tested these cells in safety trials.
Patients with advanced lymphoma or leukemia were infused with the nonvirally modified CD19-directed CAR T cells after autologous or allogeneic hematopoietic stem cell transplant (HSCT).
Eighty-six percent of autologous HSCT recipients were alive 24 months after infusion, and 53% of allogeneic HSCT recipients were alive with a median follow-up of 6.5 months.
“Gratifyingly, the patients have not demonstrated any acute or late toxicity to these CAR T-cell infusions,” said Laurence Cooper, MD, PhD, formerly of MD Anderson Cancer Center (MDACC) in Houston, Texas, and now with Ziopharm Oncology.
Dr Cooper presented these results at the inaugural CRI-CIMT-EATI-AACR International Cancer Immunotherapy Conference.
Some of the technology he described was conducted at MDACC. Dr Cooper is currently a visiting scientist there and will continue to supervise the development of this technology.
Dr Cooper said the appeal of this nonviral approach, which is a modified Sleeping Beauty approach, “is it essentially avoids the complexity of making a virus, a lentivirus or a retrovirus, it can be done at quite low cost, and really allows for a nimbleness to this system.”
Using a simple blood draw of 200 cc of peripheral blood—the process does not require apheresis—the T cells can be expanded on a feeder cell layer and genetically reprogrammed.
Sleeping Beauty system
The researchers reprogrammed the T cells using a 2-plasmid Sleeping Beauty system, which is a transposon/transposase system.
The transposon DNA plasmid codes for the cargo load, which, in this case, is the CAR. At the same time, the transposase DNA plasmid is electroporated, “which is really the secret sauce of the transposition event,” Dr Cooper explained.
After electroporation, the transposon/transposase are co-cultured with K562-derived artificial antigen-presenting cells (aAPC) and expanded with the integrated transposon of K562-aAPC. In this case, CD19 is on the aAPC.
CD19 is co-expressed with other co-stimulatory molecules, CD86 and 4-1BB ligand.
In addition, the researchers added a molecule of interleukin 15 that’s sewn in frame to the Fc region of an immunoglobulin that then activates the T cell in the context of these co-stimulatory molecules.
The T cells that have stable integrants of the CAR grow out over time. And those that have transient expression of the CAR die by neglect.
“By day 14, most of the T cells have the CAR sewn into the genome and are stably expressed,” Dr Cooper said.
The CAR used for these safety trials at MDACC targets CD19 and uses mouse scFv held in frame with an immunoglobulin 4 Fc (IgG4Fc) stalk.
It’s tunneled through the T-cell membrane and has 2 costimulatory molecules, signal 1 delivered by phosphorylation of the immunoreceptor tyrosine-based activation motif in CD3ζ and signal 2 by the costimulatory domain CD28.
The researchers tested the CD19 CARs in 2 clinical settings—one with T cells that were patient-derived and infused after autologous HSCT, and the second with T cells that were derived from a third party and infused after allogeneic HSCT.
Infusion after autologous HSCT
The researchers first tried the CARs in 7 non-Hodgkin lymphoma patients who had an autologous HSCT. Their median age was 52 (range, 36-61).
Five patients received a starting CAR T-cell dose of 5x108 cells/m2, and 2 received 5x109 cells/m2.
Six patients (86%) remain alive and are in complete remission (CR) at a median follow-up of 24 months.
Infusion after allogeneic HSCT
The researchers expanded the investigation to a wider cohort of 19 patients who had undergone allogeneic HSCT.
Seventeen patients had advanced CD19-positive acute lymphoblastic leukemia, and 2 had non-Hodgkin lymphoma. Their median age was 35 (range, 21-56).
All patients were on graft-versus-host disease (GVHD) prophylaxis with tacrolimus at the time of CAR infusion. A subset of these allogeneic transplant patients had haploidentical donors rather than matched sibling donors.
Five patients received a CAR T-cell dose of 106, 6 patients received 107, 5 received 5x107, and 3 received 5x108 cells/m2 based on recipient body surface area.
Fifty-eight percent of patients (11/19) achieved a CR, and 10 remain alive a median of 6.5 months after CAR T-cell infusion.
Three patients developed GVHD, 1 with steroid-refractory acute liver disease, 1 with grade 2 acute skin disease, and 1 with chronic limited skin disease. The incidence of GVHD was lower than historical controls at MDACC, Dr Cooper said.
“[G]ratifyingly, in this clinical setting of minimal disease, patients did not have any acute or late toxicity from these infusions,” he added.
And the rate of cytomegalovirus reactivation after CAR T-cell infusion was 24%, compared with 41% for patients after transplant at MDACC without CAR T-cell infusion.
Eight patients received haploidentical HSCT followed by CAR T-cell infusion, and 75% (6/8) remain in CR.
Persistence of infused T cells
The researchers used 2 forms of PCR—qPCR and droplet PCR—to map the fate of the CARs.
“Roughly speaking, for these patients, and this is in line with the literature, in terms of those T cells that are activated through CD28 in contrast to 4-1BB, these T cells are, on average, living about 28 or so days post-infusion,” Dr Cooper noted.
He said this is similar to results observed with CARs being tested at the National Cancer Institute and Memorial Sloan-Kettering Cancer Center. ![]()

Photo courtesy of MDACC
NEW YORK—Researchers have used a nonviral approach to create chimeric antigen receptor (CAR) T cells and tested these cells in safety trials.
Patients with advanced lymphoma or leukemia were infused with the nonvirally modified CD19-directed CAR T cells after autologous or allogeneic hematopoietic stem cell transplant (HSCT).
Eighty-six percent of autologous HSCT recipients were alive 24 months after infusion, and 53% of allogeneic HSCT recipients were alive with a median follow-up of 6.5 months.
“Gratifyingly, the patients have not demonstrated any acute or late toxicity to these CAR T-cell infusions,” said Laurence Cooper, MD, PhD, formerly of MD Anderson Cancer Center (MDACC) in Houston, Texas, and now with Ziopharm Oncology.
Dr Cooper presented these results at the inaugural CRI-CIMT-EATI-AACR International Cancer Immunotherapy Conference.
Some of the technology he described was conducted at MDACC. Dr Cooper is currently a visiting scientist there and will continue to supervise the development of this technology.
Dr Cooper said the appeal of this nonviral approach, which is a modified Sleeping Beauty approach, “is it essentially avoids the complexity of making a virus, a lentivirus or a retrovirus, it can be done at quite low cost, and really allows for a nimbleness to this system.”
Using a simple blood draw of 200 cc of peripheral blood—the process does not require apheresis—the T cells can be expanded on a feeder cell layer and genetically reprogrammed.
Sleeping Beauty system
The researchers reprogrammed the T cells using a 2-plasmid Sleeping Beauty system, which is a transposon/transposase system.
The transposon DNA plasmid codes for the cargo load, which, in this case, is the CAR. At the same time, the transposase DNA plasmid is electroporated, “which is really the secret sauce of the transposition event,” Dr Cooper explained.
After electroporation, the transposon/transposase are co-cultured with K562-derived artificial antigen-presenting cells (aAPC) and expanded with the integrated transposon of K562-aAPC. In this case, CD19 is on the aAPC.
CD19 is co-expressed with other co-stimulatory molecules, CD86 and 4-1BB ligand.
In addition, the researchers added a molecule of interleukin 15 that’s sewn in frame to the Fc region of an immunoglobulin that then activates the T cell in the context of these co-stimulatory molecules.
The T cells that have stable integrants of the CAR grow out over time. And those that have transient expression of the CAR die by neglect.
“By day 14, most of the T cells have the CAR sewn into the genome and are stably expressed,” Dr Cooper said.
The CAR used for these safety trials at MDACC targets CD19 and uses mouse scFv held in frame with an immunoglobulin 4 Fc (IgG4Fc) stalk.
It’s tunneled through the T-cell membrane and has 2 costimulatory molecules, signal 1 delivered by phosphorylation of the immunoreceptor tyrosine-based activation motif in CD3ζ and signal 2 by the costimulatory domain CD28.
The researchers tested the CD19 CARs in 2 clinical settings—one with T cells that were patient-derived and infused after autologous HSCT, and the second with T cells that were derived from a third party and infused after allogeneic HSCT.
Infusion after autologous HSCT
The researchers first tried the CARs in 7 non-Hodgkin lymphoma patients who had an autologous HSCT. Their median age was 52 (range, 36-61).
Five patients received a starting CAR T-cell dose of 5x108 cells/m2, and 2 received 5x109 cells/m2.
Six patients (86%) remain alive and are in complete remission (CR) at a median follow-up of 24 months.
Infusion after allogeneic HSCT
The researchers expanded the investigation to a wider cohort of 19 patients who had undergone allogeneic HSCT.
Seventeen patients had advanced CD19-positive acute lymphoblastic leukemia, and 2 had non-Hodgkin lymphoma. Their median age was 35 (range, 21-56).
All patients were on graft-versus-host disease (GVHD) prophylaxis with tacrolimus at the time of CAR infusion. A subset of these allogeneic transplant patients had haploidentical donors rather than matched sibling donors.
Five patients received a CAR T-cell dose of 106, 6 patients received 107, 5 received 5x107, and 3 received 5x108 cells/m2 based on recipient body surface area.
Fifty-eight percent of patients (11/19) achieved a CR, and 10 remain alive a median of 6.5 months after CAR T-cell infusion.
Three patients developed GVHD, 1 with steroid-refractory acute liver disease, 1 with grade 2 acute skin disease, and 1 with chronic limited skin disease. The incidence of GVHD was lower than historical controls at MDACC, Dr Cooper said.
“[G]ratifyingly, in this clinical setting of minimal disease, patients did not have any acute or late toxicity from these infusions,” he added.
And the rate of cytomegalovirus reactivation after CAR T-cell infusion was 24%, compared with 41% for patients after transplant at MDACC without CAR T-cell infusion.
Eight patients received haploidentical HSCT followed by CAR T-cell infusion, and 75% (6/8) remain in CR.
Persistence of infused T cells
The researchers used 2 forms of PCR—qPCR and droplet PCR—to map the fate of the CARs.
“Roughly speaking, for these patients, and this is in line with the literature, in terms of those T cells that are activated through CD28 in contrast to 4-1BB, these T cells are, on average, living about 28 or so days post-infusion,” Dr Cooper noted.
He said this is similar to results observed with CARs being tested at the National Cancer Institute and Memorial Sloan-Kettering Cancer Center. ![]()
Abs from transplanted AML patients enhance GvL effect in vitro

NEW YORK—Investigators have found that B cells may play a role in stimulating graft-versus-leukemia (GvL) responses in patients with acute myeloid leukemia (AML) who have undergone allogeneic hematopoietic stem cell transplant (HSCT).
The team created B cell lines from these patients, isolated AML-specific antibodies, and found that these antibodies can induce the death of AML cells through oncosis.
Oncosis is a non-apoptotic type of cell death that involves swelling and coagulation of the cytoplasm.
Mette Hazenberg, MD, PhD, from the Academic Medical Center in Amsterdam, The Netherlands, reported these findings at the inaugural CRI-CIMT-EATI-AACR International Cancer Immunotherapy Conference as poster B052.
The investigators cloned B cells from 3 high-risk AML patients who had a strong GvL response after HSCT.
The team transduced memory B cells from the patients’ peripheral blood using BCL-6 and BCL-xL. They then screened the B cells for those that produced antibodies that bound specifically to surface antigens on AML cell lines and blasts.
Six of the 15 AML antibodies retrieved from the patients bound specifically to snRNp200. In normal cells, snRNp200 is in the nucleus, but, in AML, it is exposed on the cell membrane.
The investigators then confirmed this by ELISA.
They found 7 of the 15 AML antibodies directly lysed AML blasts without the addition of effector cells or complement. Time-lapse images showed that cell death by the AML antibodies occurred rapidly, within minutes after incubation.
“The leukemia blasts popped like balloons,” Dr Hazenberg said.
The investigators confirmed that the antibodies induced cell death by oncosis and that oncosis occurred independently of temperature. The antibodies were cytotoxic at 4°C and 37°C.
Cytotoxicity of the antibodies could be blocked by the membrane stabilizer cytochalasin D but not by apoptosis inhibitors.
The team concluded that a potent GvL response could be mediated by these antibodies against tumor-associated antigens on AML cells.
Dr Hazenberg’s hope is that, at some point, these antibodies can be combined with chemotherapy—as is rituximab—so patients won’t need to undergo transplant. ![]()

NEW YORK—Investigators have found that B cells may play a role in stimulating graft-versus-leukemia (GvL) responses in patients with acute myeloid leukemia (AML) who have undergone allogeneic hematopoietic stem cell transplant (HSCT).
The team created B cell lines from these patients, isolated AML-specific antibodies, and found that these antibodies can induce the death of AML cells through oncosis.
Oncosis is a non-apoptotic type of cell death that involves swelling and coagulation of the cytoplasm.
Mette Hazenberg, MD, PhD, from the Academic Medical Center in Amsterdam, The Netherlands, reported these findings at the inaugural CRI-CIMT-EATI-AACR International Cancer Immunotherapy Conference as poster B052.
The investigators cloned B cells from 3 high-risk AML patients who had a strong GvL response after HSCT.
The team transduced memory B cells from the patients’ peripheral blood using BCL-6 and BCL-xL. They then screened the B cells for those that produced antibodies that bound specifically to surface antigens on AML cell lines and blasts.
Six of the 15 AML antibodies retrieved from the patients bound specifically to snRNp200. In normal cells, snRNp200 is in the nucleus, but, in AML, it is exposed on the cell membrane.
The investigators then confirmed this by ELISA.
They found 7 of the 15 AML antibodies directly lysed AML blasts without the addition of effector cells or complement. Time-lapse images showed that cell death by the AML antibodies occurred rapidly, within minutes after incubation.
“The leukemia blasts popped like balloons,” Dr Hazenberg said.
The investigators confirmed that the antibodies induced cell death by oncosis and that oncosis occurred independently of temperature. The antibodies were cytotoxic at 4°C and 37°C.
Cytotoxicity of the antibodies could be blocked by the membrane stabilizer cytochalasin D but not by apoptosis inhibitors.
The team concluded that a potent GvL response could be mediated by these antibodies against tumor-associated antigens on AML cells.
Dr Hazenberg’s hope is that, at some point, these antibodies can be combined with chemotherapy—as is rituximab—so patients won’t need to undergo transplant. ![]()

NEW YORK—Investigators have found that B cells may play a role in stimulating graft-versus-leukemia (GvL) responses in patients with acute myeloid leukemia (AML) who have undergone allogeneic hematopoietic stem cell transplant (HSCT).
The team created B cell lines from these patients, isolated AML-specific antibodies, and found that these antibodies can induce the death of AML cells through oncosis.
Oncosis is a non-apoptotic type of cell death that involves swelling and coagulation of the cytoplasm.
Mette Hazenberg, MD, PhD, from the Academic Medical Center in Amsterdam, The Netherlands, reported these findings at the inaugural CRI-CIMT-EATI-AACR International Cancer Immunotherapy Conference as poster B052.
The investigators cloned B cells from 3 high-risk AML patients who had a strong GvL response after HSCT.
The team transduced memory B cells from the patients’ peripheral blood using BCL-6 and BCL-xL. They then screened the B cells for those that produced antibodies that bound specifically to surface antigens on AML cell lines and blasts.
Six of the 15 AML antibodies retrieved from the patients bound specifically to snRNp200. In normal cells, snRNp200 is in the nucleus, but, in AML, it is exposed on the cell membrane.
The investigators then confirmed this by ELISA.
They found 7 of the 15 AML antibodies directly lysed AML blasts without the addition of effector cells or complement. Time-lapse images showed that cell death by the AML antibodies occurred rapidly, within minutes after incubation.
“The leukemia blasts popped like balloons,” Dr Hazenberg said.
The investigators confirmed that the antibodies induced cell death by oncosis and that oncosis occurred independently of temperature. The antibodies were cytotoxic at 4°C and 37°C.
Cytotoxicity of the antibodies could be blocked by the membrane stabilizer cytochalasin D but not by apoptosis inhibitors.
The team concluded that a potent GvL response could be mediated by these antibodies against tumor-associated antigens on AML cells.
Dr Hazenberg’s hope is that, at some point, these antibodies can be combined with chemotherapy—as is rituximab—so patients won’t need to undergo transplant. ![]()
Imaging provides clearer picture of HSCs

in the bone marrow
By imaging the bone marrow of mice, researchers have uncovered new details about hematopoietic stem cells (HSCs).
The team’s deep imaging technique confirmed some previous findings and unearthed new information about where HSCs are located and how they are maintained.
The researchers said these findings, published in Nature, provide a significant advance toward understanding the microenvironment in which HSCs reside.
“The bone marrow and [HSCs] are like a haystack with needles inside,” said study author Sean Morrison, PhD, of the University of Texas Southwestern Medical Center in Dallas.
“Researchers in the past have been able to find a few stem cells, but they’ve only seen a small percentage of the stem cells that are there, so there has been some controversy about where exactly they’re located.”
“We developed a technique that allows us to digitally reconstruct the entire haystack and see all the needles—all the [HSCs] that are present in the bone marrow—and to know exactly where they are and how far they are from every other cell type.”
The team began by identifying a genetic marker that is almost exclusively expressed in HSCs. They then took green fluorescent protein from jellyfish and inserted it into the genetic marker, Ctnnal1, so they could identify the HSCs.
“Using a tissue-clearing technique that makes the bone and bone marrow see-through, and employing a high-resolution, confocal microscope to scan the entire bone marrow compartment, we were able to image large segments of bone marrow to locate every [HSC] and its relation to other cells,” said Melih Acar, PhD, also of the University of Texas Southwestern Medical Center.
The team’s work yielded new findings and confirmed others. They found that HSCs tend to be clustered in the center of the bone marrow, not closer to bone surfaces as some researchers previously thought.
They also found that HSCs are indeed associated with sinusoidal blood vessels, and there are no spatially distinct niches for dividing and non-dividing HSCs.
“With this improved understanding of the microenvironment and mechanisms that maintain [HSCs], we are closer to being able to replicate the environment for [HSCs] in culture,” Dr Morrison said.
“That achievement would significantly improve the safety and effectiveness of bone marrow transplants and potentially save thousands of additional lives each year.” ![]()

in the bone marrow
By imaging the bone marrow of mice, researchers have uncovered new details about hematopoietic stem cells (HSCs).
The team’s deep imaging technique confirmed some previous findings and unearthed new information about where HSCs are located and how they are maintained.
The researchers said these findings, published in Nature, provide a significant advance toward understanding the microenvironment in which HSCs reside.
“The bone marrow and [HSCs] are like a haystack with needles inside,” said study author Sean Morrison, PhD, of the University of Texas Southwestern Medical Center in Dallas.
“Researchers in the past have been able to find a few stem cells, but they’ve only seen a small percentage of the stem cells that are there, so there has been some controversy about where exactly they’re located.”
“We developed a technique that allows us to digitally reconstruct the entire haystack and see all the needles—all the [HSCs] that are present in the bone marrow—and to know exactly where they are and how far they are from every other cell type.”
The team began by identifying a genetic marker that is almost exclusively expressed in HSCs. They then took green fluorescent protein from jellyfish and inserted it into the genetic marker, Ctnnal1, so they could identify the HSCs.
“Using a tissue-clearing technique that makes the bone and bone marrow see-through, and employing a high-resolution, confocal microscope to scan the entire bone marrow compartment, we were able to image large segments of bone marrow to locate every [HSC] and its relation to other cells,” said Melih Acar, PhD, also of the University of Texas Southwestern Medical Center.
The team’s work yielded new findings and confirmed others. They found that HSCs tend to be clustered in the center of the bone marrow, not closer to bone surfaces as some researchers previously thought.
They also found that HSCs are indeed associated with sinusoidal blood vessels, and there are no spatially distinct niches for dividing and non-dividing HSCs.
“With this improved understanding of the microenvironment and mechanisms that maintain [HSCs], we are closer to being able to replicate the environment for [HSCs] in culture,” Dr Morrison said.
“That achievement would significantly improve the safety and effectiveness of bone marrow transplants and potentially save thousands of additional lives each year.” ![]()

in the bone marrow
By imaging the bone marrow of mice, researchers have uncovered new details about hematopoietic stem cells (HSCs).
The team’s deep imaging technique confirmed some previous findings and unearthed new information about where HSCs are located and how they are maintained.
The researchers said these findings, published in Nature, provide a significant advance toward understanding the microenvironment in which HSCs reside.
“The bone marrow and [HSCs] are like a haystack with needles inside,” said study author Sean Morrison, PhD, of the University of Texas Southwestern Medical Center in Dallas.
“Researchers in the past have been able to find a few stem cells, but they’ve only seen a small percentage of the stem cells that are there, so there has been some controversy about where exactly they’re located.”
“We developed a technique that allows us to digitally reconstruct the entire haystack and see all the needles—all the [HSCs] that are present in the bone marrow—and to know exactly where they are and how far they are from every other cell type.”
The team began by identifying a genetic marker that is almost exclusively expressed in HSCs. They then took green fluorescent protein from jellyfish and inserted it into the genetic marker, Ctnnal1, so they could identify the HSCs.
“Using a tissue-clearing technique that makes the bone and bone marrow see-through, and employing a high-resolution, confocal microscope to scan the entire bone marrow compartment, we were able to image large segments of bone marrow to locate every [HSC] and its relation to other cells,” said Melih Acar, PhD, also of the University of Texas Southwestern Medical Center.
The team’s work yielded new findings and confirmed others. They found that HSCs tend to be clustered in the center of the bone marrow, not closer to bone surfaces as some researchers previously thought.
They also found that HSCs are indeed associated with sinusoidal blood vessels, and there are no spatially distinct niches for dividing and non-dividing HSCs.
“With this improved understanding of the microenvironment and mechanisms that maintain [HSCs], we are closer to being able to replicate the environment for [HSCs] in culture,” Dr Morrison said.
“That achievement would significantly improve the safety and effectiveness of bone marrow transplants and potentially save thousands of additional lives each year.”
Readmissions due to infection after HSCT

Photo courtesy of the CDC
SAN DIEGO—A retrospective study has provided insight into hospital readmissions related to opportunistic infection following hematopoietic stem cell transplant (HSCT).
Of the roughly 4200 HSCT recipients studied, 26% were readmitted to the hospital due to opportunistic infection.
About 1 in 3 infection-related readmissions were due to double-stranded DNA (dsDNA) viral infections, and cytomegalovirus (CMV) infections were the most common.
Nearly half of the dsDNA viral infections occurred within the first month of HSCT discharge.
These findings were presented at ICAAC/ICC 2015 (poster T-1360). The study was sponsored by Chimerix, Inc.
Investigators searched the Premier hospital database for patients who underwent HSCT between January 2009 and September 2013. The team identified 4393 patients with a mean age of 50.4 years. Most were adults (91.2%), most were male (57.9%), and most received an autologous HSCT (63.2%).
About 42% (n=1841) of patients had a diagnostic code for opportunistic infection in their HSCT discharge records. Overall, 7.3% (n=319) of patients had dsDNA virus infections, including 13.4% (n=216) of patients who received an allogeneic HSCT.
One hundred and fifty-seven patients died during HSCT hospitalization, leaving 4236 patients evaluable for readmission analysis.
In all, 37.7% (n=1595) of the surviving patients were readmitted to the hospital for any reason during the 12 months after HSCT discharge. And 65.6% of the readmissions occurred within the first 3 months of HSCT discharge.
Readmissions were most frequently related to opportunistic infections (25.8%, n=1091), followed by graft-versus-host disease (13.7%, n=579), renal impairment (11.1%, n=470), and neutropenia (10.0%, n=422).
The investigators noted that patients may have had multiple readmissions or readmission with multiple diagnoses.
Of the hospital readmissions related to opportunistic infections, 32.0% (n=349) were related to dsDNA virus infections. This included CMV (65.9%, n=230), BK virus (13.8%, n=48), adenovirus (5.2%, n=18), and other dsDNA virus infections (32.7%, n=114).
Patients may have experienced more than one viral infection, so the number of hospital readmissions related to each dsDNA virus was not mutually exclusive.
Readmission within the first month of HSCT discharge occurred in 41.8% of patients with any dsDNA virus infection, 49.6% with CMV infection, and 56.3% with BK virus infection. More than half (55.6%) of readmissions related to adenovirus infection occurred within the first 3 months of HSCT discharge.
Taking these results together, the investigators concluded that hospital readmissions related to opportunistic infections were relatively common among HSCT recipients. So strategies that minimize the risks of these infections might have significant clinical and economic advantages.

Photo courtesy of the CDC
SAN DIEGO—A retrospective study has provided insight into hospital readmissions related to opportunistic infection following hematopoietic stem cell transplant (HSCT).
Of the roughly 4200 HSCT recipients studied, 26% were readmitted to the hospital due to opportunistic infection.
About 1 in 3 infection-related readmissions were due to double-stranded DNA (dsDNA) viral infections, and cytomegalovirus (CMV) infections were the most common.
Nearly half of the dsDNA viral infections occurred within the first month of HSCT discharge.
These findings were presented at ICAAC/ICC 2015 (poster T-1360). The study was sponsored by Chimerix, Inc.
Investigators searched the Premier hospital database for patients who underwent HSCT between January 2009 and September 2013. The team identified 4393 patients with a mean age of 50.4 years. Most were adults (91.2%), most were male (57.9%), and most received an autologous HSCT (63.2%).
About 42% (n=1841) of patients had a diagnostic code for opportunistic infection in their HSCT discharge records. Overall, 7.3% (n=319) of patients had dsDNA virus infections, including 13.4% (n=216) of patients who received an allogeneic HSCT.
One hundred and fifty-seven patients died during HSCT hospitalization, leaving 4236 patients evaluable for readmission analysis.
In all, 37.7% (n=1595) of the surviving patients were readmitted to the hospital for any reason during the 12 months after HSCT discharge. And 65.6% of the readmissions occurred within the first 3 months of HSCT discharge.
Readmissions were most frequently related to opportunistic infections (25.8%, n=1091), followed by graft-versus-host disease (13.7%, n=579), renal impairment (11.1%, n=470), and neutropenia (10.0%, n=422).
The investigators noted that patients may have had multiple readmissions or readmission with multiple diagnoses.
Of the hospital readmissions related to opportunistic infections, 32.0% (n=349) were related to dsDNA virus infections. This included CMV (65.9%, n=230), BK virus (13.8%, n=48), adenovirus (5.2%, n=18), and other dsDNA virus infections (32.7%, n=114).
Patients may have experienced more than one viral infection, so the number of hospital readmissions related to each dsDNA virus was not mutually exclusive.
Readmission within the first month of HSCT discharge occurred in 41.8% of patients with any dsDNA virus infection, 49.6% with CMV infection, and 56.3% with BK virus infection. More than half (55.6%) of readmissions related to adenovirus infection occurred within the first 3 months of HSCT discharge.
Taking these results together, the investigators concluded that hospital readmissions related to opportunistic infections were relatively common among HSCT recipients. So strategies that minimize the risks of these infections might have significant clinical and economic advantages.

Photo courtesy of the CDC
SAN DIEGO—A retrospective study has provided insight into hospital readmissions related to opportunistic infection following hematopoietic stem cell transplant (HSCT).
Of the roughly 4200 HSCT recipients studied, 26% were readmitted to the hospital due to opportunistic infection.
About 1 in 3 infection-related readmissions were due to double-stranded DNA (dsDNA) viral infections, and cytomegalovirus (CMV) infections were the most common.
Nearly half of the dsDNA viral infections occurred within the first month of HSCT discharge.
These findings were presented at ICAAC/ICC 2015 (poster T-1360). The study was sponsored by Chimerix, Inc.
Investigators searched the Premier hospital database for patients who underwent HSCT between January 2009 and September 2013. The team identified 4393 patients with a mean age of 50.4 years. Most were adults (91.2%), most were male (57.9%), and most received an autologous HSCT (63.2%).
About 42% (n=1841) of patients had a diagnostic code for opportunistic infection in their HSCT discharge records. Overall, 7.3% (n=319) of patients had dsDNA virus infections, including 13.4% (n=216) of patients who received an allogeneic HSCT.
One hundred and fifty-seven patients died during HSCT hospitalization, leaving 4236 patients evaluable for readmission analysis.
In all, 37.7% (n=1595) of the surviving patients were readmitted to the hospital for any reason during the 12 months after HSCT discharge. And 65.6% of the readmissions occurred within the first 3 months of HSCT discharge.
Readmissions were most frequently related to opportunistic infections (25.8%, n=1091), followed by graft-versus-host disease (13.7%, n=579), renal impairment (11.1%, n=470), and neutropenia (10.0%, n=422).
The investigators noted that patients may have had multiple readmissions or readmission with multiple diagnoses.
Of the hospital readmissions related to opportunistic infections, 32.0% (n=349) were related to dsDNA virus infections. This included CMV (65.9%, n=230), BK virus (13.8%, n=48), adenovirus (5.2%, n=18), and other dsDNA virus infections (32.7%, n=114).
Patients may have experienced more than one viral infection, so the number of hospital readmissions related to each dsDNA virus was not mutually exclusive.
Readmission within the first month of HSCT discharge occurred in 41.8% of patients with any dsDNA virus infection, 49.6% with CMV infection, and 56.3% with BK virus infection. More than half (55.6%) of readmissions related to adenovirus infection occurred within the first 3 months of HSCT discharge.
Taking these results together, the investigators concluded that hospital readmissions related to opportunistic infections were relatively common among HSCT recipients. So strategies that minimize the risks of these infections might have significant clinical and economic advantages.
Chemo-free transplant can cure SCD, team says

Photo by Chad McNeeley
Chemotherapy-free allogeneic transplant can cure sickle cell disease (SCD) in adults, according to researchers.
In a phase 1/2 trial, the treatment normalized hemoglobin concentrations, reduced SCD-related complications, and improved cardiopulmonary function in 12 of 13 patients.
The single graft failure was due to noncompliance with post-transplant treatment.
There were no deaths and no cases of graft-vs-host disease. However, most patients did experience some form of transplant-related toxicity.
These transplants were performed at the University of Illinois Hospital & Health Sciences System in Chicago. But the chemotherapy-free transplant regimen was developed—and initially tested—at the National Institutes of Health in Bethesda, Maryland.
Physicians there have treated 30 patients with the regimen. An account of that work was published in JAMA last year.
The current study has been published in Biology of Blood & Marrow Transplantation.
“Adults with sickle cell disease can be cured without chemotherapy—the main barrier that has stood in the way for them for so long,” said study author Damiano Rondelli, MD, of the University of Illinois Hospital & Health Sciences System.
“Our data provide more support that this therapy is safe and effective and prevents patients from living shortened lives, condemned to pain and progressive complications.”
Treatment and outcome
The study included 13 patients, ages 17 to 40, who were transplanted between November 2011 and June 2014. Prior to transplant, patients received alemtuzumab and total-body irradiation (300 cGy).
They then received peripheral blood stem cells from matched related donors. All donors were a 10/10 human leukocyte antigen match, but 2 donors had different blood types than the recipients. After transplant, the patients received sirolimus.
All 13 patients initially engrafted, but 1 patient experienced secondary graft failure due to noncompliance with sirolimus.
At a median follow-up of 22 months (range, 12-44), all 13 patients are alive, and 12 have maintained a stable mixed donor/recipient chimerism.
At 1 year after transplant, the 12 patients with stable donor chimerism had significant improvements from baseline in hemoglobin, reticulocyte percentage, lactate dehydrogenase concentration, and cardiopulmonary function.
One of these patients required readmission to the hospital for vaso-occlusive crisis. Before transplant, this patient experienced about 12 crises a year.
No other SCD-related complications have occurred. And 4 patients have been able to stop taking sirolimus without transplant rejection or other complications.
Nine of the engrafted patients completed quality of life assessments before transplant and at 1 year after the procedure. They reported improvements in pain, general health, vitality, and social functioning.
“[W]ith this chemotherapy-free transplant, we are curing adults with sickle cell disease, and we see that their quality of life improves vastly within just 1 month of the transplant,” Dr Rondelli said. “They are able to go back to school, go back to work, and can experience life without pain.”
Toxicity
Four patients did not experience any transplant-related toxicity. And there were no cases of acute or chronic graft-vs-host disease.
One patient developed gram-negative rods in her hip prosthesis after transplant, 1 patient experienced delayed hemolytic transfusion reaction after exchange transfusion, 1 patient developed viral pharyngitis, and 1 patient developed Coxsackie B.
One patient developed a urinary tract infection due to extended spectrum beta-lactamase, Clostridium difficile colitis, and Mycoplasma pneumoniae.
Two patients had grade 2 mucositis, one of whom also developed line-associated deep vein thrombosis and cytomegalovirus (CMV) reactivation. Two other patients had CMV reactivation as well, one of whom also had methicillin-resistant Staphylococcus aureus pneumonia.
All 3 patients with CMV reactivation were successfully treated with valgancyclovir and did not develop CMV disease.
Six patients had arthralgias attributed to sirolimus, and 2 of them required dose reductions.
One patient developed chest pain and a decline in carbon monoxide diffusion capacity by 30% that was attributed to sirolimus. The patient was switched to cyclosporine but developed posterior reversible encephalopathy syndrome and was then put on mycophenolate mofetil.
The patient has since maintained stable donor chimerism, and the carbon monoxide diffusing capacity has increased to near-baseline value.

Photo by Chad McNeeley
Chemotherapy-free allogeneic transplant can cure sickle cell disease (SCD) in adults, according to researchers.
In a phase 1/2 trial, the treatment normalized hemoglobin concentrations, reduced SCD-related complications, and improved cardiopulmonary function in 12 of 13 patients.
The single graft failure was due to noncompliance with post-transplant treatment.
There were no deaths and no cases of graft-vs-host disease. However, most patients did experience some form of transplant-related toxicity.
These transplants were performed at the University of Illinois Hospital & Health Sciences System in Chicago. But the chemotherapy-free transplant regimen was developed—and initially tested—at the National Institutes of Health in Bethesda, Maryland.
Physicians there have treated 30 patients with the regimen. An account of that work was published in JAMA last year.
The current study has been published in Biology of Blood & Marrow Transplantation.
“Adults with sickle cell disease can be cured without chemotherapy—the main barrier that has stood in the way for them for so long,” said study author Damiano Rondelli, MD, of the University of Illinois Hospital & Health Sciences System.
“Our data provide more support that this therapy is safe and effective and prevents patients from living shortened lives, condemned to pain and progressive complications.”
Treatment and outcome
The study included 13 patients, ages 17 to 40, who were transplanted between November 2011 and June 2014. Prior to transplant, patients received alemtuzumab and total-body irradiation (300 cGy).
They then received peripheral blood stem cells from matched related donors. All donors were a 10/10 human leukocyte antigen match, but 2 donors had different blood types than the recipients. After transplant, the patients received sirolimus.
All 13 patients initially engrafted, but 1 patient experienced secondary graft failure due to noncompliance with sirolimus.
At a median follow-up of 22 months (range, 12-44), all 13 patients are alive, and 12 have maintained a stable mixed donor/recipient chimerism.
At 1 year after transplant, the 12 patients with stable donor chimerism had significant improvements from baseline in hemoglobin, reticulocyte percentage, lactate dehydrogenase concentration, and cardiopulmonary function.
One of these patients required readmission to the hospital for vaso-occlusive crisis. Before transplant, this patient experienced about 12 crises a year.
No other SCD-related complications have occurred. And 4 patients have been able to stop taking sirolimus without transplant rejection or other complications.
Nine of the engrafted patients completed quality of life assessments before transplant and at 1 year after the procedure. They reported improvements in pain, general health, vitality, and social functioning.
“[W]ith this chemotherapy-free transplant, we are curing adults with sickle cell disease, and we see that their quality of life improves vastly within just 1 month of the transplant,” Dr Rondelli said. “They are able to go back to school, go back to work, and can experience life without pain.”
Toxicity
Four patients did not experience any transplant-related toxicity. And there were no cases of acute or chronic graft-vs-host disease.
One patient developed gram-negative rods in her hip prosthesis after transplant, 1 patient experienced delayed hemolytic transfusion reaction after exchange transfusion, 1 patient developed viral pharyngitis, and 1 patient developed Coxsackie B.
One patient developed a urinary tract infection due to extended spectrum beta-lactamase, Clostridium difficile colitis, and Mycoplasma pneumoniae.
Two patients had grade 2 mucositis, one of whom also developed line-associated deep vein thrombosis and cytomegalovirus (CMV) reactivation. Two other patients had CMV reactivation as well, one of whom also had methicillin-resistant Staphylococcus aureus pneumonia.
All 3 patients with CMV reactivation were successfully treated with valgancyclovir and did not develop CMV disease.
Six patients had arthralgias attributed to sirolimus, and 2 of them required dose reductions.
One patient developed chest pain and a decline in carbon monoxide diffusion capacity by 30% that was attributed to sirolimus. The patient was switched to cyclosporine but developed posterior reversible encephalopathy syndrome and was then put on mycophenolate mofetil.
The patient has since maintained stable donor chimerism, and the carbon monoxide diffusing capacity has increased to near-baseline value.

Photo by Chad McNeeley
Chemotherapy-free allogeneic transplant can cure sickle cell disease (SCD) in adults, according to researchers.
In a phase 1/2 trial, the treatment normalized hemoglobin concentrations, reduced SCD-related complications, and improved cardiopulmonary function in 12 of 13 patients.
The single graft failure was due to noncompliance with post-transplant treatment.
There were no deaths and no cases of graft-vs-host disease. However, most patients did experience some form of transplant-related toxicity.
These transplants were performed at the University of Illinois Hospital & Health Sciences System in Chicago. But the chemotherapy-free transplant regimen was developed—and initially tested—at the National Institutes of Health in Bethesda, Maryland.
Physicians there have treated 30 patients with the regimen. An account of that work was published in JAMA last year.
The current study has been published in Biology of Blood & Marrow Transplantation.
“Adults with sickle cell disease can be cured without chemotherapy—the main barrier that has stood in the way for them for so long,” said study author Damiano Rondelli, MD, of the University of Illinois Hospital & Health Sciences System.
“Our data provide more support that this therapy is safe and effective and prevents patients from living shortened lives, condemned to pain and progressive complications.”
Treatment and outcome
The study included 13 patients, ages 17 to 40, who were transplanted between November 2011 and June 2014. Prior to transplant, patients received alemtuzumab and total-body irradiation (300 cGy).
They then received peripheral blood stem cells from matched related donors. All donors were a 10/10 human leukocyte antigen match, but 2 donors had different blood types than the recipients. After transplant, the patients received sirolimus.
All 13 patients initially engrafted, but 1 patient experienced secondary graft failure due to noncompliance with sirolimus.
At a median follow-up of 22 months (range, 12-44), all 13 patients are alive, and 12 have maintained a stable mixed donor/recipient chimerism.
At 1 year after transplant, the 12 patients with stable donor chimerism had significant improvements from baseline in hemoglobin, reticulocyte percentage, lactate dehydrogenase concentration, and cardiopulmonary function.
One of these patients required readmission to the hospital for vaso-occlusive crisis. Before transplant, this patient experienced about 12 crises a year.
No other SCD-related complications have occurred. And 4 patients have been able to stop taking sirolimus without transplant rejection or other complications.
Nine of the engrafted patients completed quality of life assessments before transplant and at 1 year after the procedure. They reported improvements in pain, general health, vitality, and social functioning.
“[W]ith this chemotherapy-free transplant, we are curing adults with sickle cell disease, and we see that their quality of life improves vastly within just 1 month of the transplant,” Dr Rondelli said. “They are able to go back to school, go back to work, and can experience life without pain.”
Toxicity
Four patients did not experience any transplant-related toxicity. And there were no cases of acute or chronic graft-vs-host disease.
One patient developed gram-negative rods in her hip prosthesis after transplant, 1 patient experienced delayed hemolytic transfusion reaction after exchange transfusion, 1 patient developed viral pharyngitis, and 1 patient developed Coxsackie B.
One patient developed a urinary tract infection due to extended spectrum beta-lactamase, Clostridium difficile colitis, and Mycoplasma pneumoniae.
Two patients had grade 2 mucositis, one of whom also developed line-associated deep vein thrombosis and cytomegalovirus (CMV) reactivation. Two other patients had CMV reactivation as well, one of whom also had methicillin-resistant Staphylococcus aureus pneumonia.
All 3 patients with CMV reactivation were successfully treated with valgancyclovir and did not develop CMV disease.
Six patients had arthralgias attributed to sirolimus, and 2 of them required dose reductions.
One patient developed chest pain and a decline in carbon monoxide diffusion capacity by 30% that was attributed to sirolimus. The patient was switched to cyclosporine but developed posterior reversible encephalopathy syndrome and was then put on mycophenolate mofetil.
The patient has since maintained stable donor chimerism, and the carbon monoxide diffusing capacity has increased to near-baseline value.
First biosimilar launched in US
© Sandoz Inc. 2015
The leukocyte growth factor Zarxio (filgrastim-sndz), the first biosimilar product to gain approval from the US Food and Drug Administration (FDA), is now available in the US.
Zarxio was approved by the FDA on March 6. The product, made by Sandoz, Inc., is biosimilar to Amgen Inc.’s Neupogen, which was originally licensed in 1991.
Zarxio is marketed as Zarzio outside the US. The biosimilar is available in more than 60 countries worldwide.
In the US, Zarxio is approved for the same indications as Neupogen. So Zarxio can be prescribed for the following 5 indications.
Patients with cancer receiving myelosuppressive chemotherapy: to decrease the incidence of infection, as manifested by febrile neutropenia, in patients with nonmyeloid malignancies receiving myelosuppressive anticancer drugs associated with a significant incidence of severe neutropenia with fever.
Patients with acute myeloid leukemia receiving induction or consolidation chemotherapy: to reduce the time to neutrophil recovery and the duration of fever, following induction or consolidation chemotherapy.
Patients with cancer undergoing bone marrow transplant: to reduce the duration of neutropenia and neutropenia-related clinical sequelae—eg, febrile neutropenia—in patients with nonmyeloid malignancies undergoing myeloablative chemotherapy followed by bone marrow transplant.
Patients undergoing autologous peripheral blood progenitor cell collection and therapy: for the mobilization of autologous hematopoietic progenitor cells into the peripheral blood for collection by leukapheresis.
Patients with severe chronic neutropenia: for chronic administration to reduce the incidence and duration of sequelae of neutropenia—eg, fever, infections, oropharyngeal ulcers—in symptomatic patients with congenital neutropenia, cyclic neutropenia, or idiopathic neutropenia.
PIONEER trial
The FDA’s approval of Zarxio was based on data showing that Zarxio is highly similar to Neupogen, with no clinically meaningful differences between the products.
The head-to-head PIONEER study was the final piece of evidence the FDA used to approve Zarxio as biosimilar to Neupogen. Results of the trial were presented at ASH 2014.
Zarxio and Neupogen both produced the expected reduction in the duration of severe neutropenia in breast cancer patients undergoing myelosuppressive chemotherapy—1.17 ± 1.11 and 1.20 ±1.02 days, respectively.
The mean time to absolute neutrophil count recovery in cycle 1 was also similar—1.8 ± 0.97 days in the Zarxio arm and 1.7 ± 0.81 days in the Neupogen arm. No immunogenicity or antibodies against rhG-CSF were detected throughout the study.
The researchers said there were no obvious differences between Zarxio and Neupogen with regard to treatment-emergent adverse events.
The most common side effects observed with Zarxio are aching bones/muscles and redness, swelling, or itching at the injection site. Serious side effects may include spleen rupture; serious allergic reactions that may cause rash, shortness of breath, wheezing and/or swelling around the mouth and eyes; fast pulse and sweating; and acute respiratory distress syndrome.
For more details on Zarxio, see the full prescribing information or visit www.zarxio.com.

© Sandoz Inc. 2015
The leukocyte growth factor Zarxio (filgrastim-sndz), the first biosimilar product to gain approval from the US Food and Drug Administration (FDA), is now available in the US.
Zarxio was approved by the FDA on March 6. The product, made by Sandoz, Inc., is biosimilar to Amgen Inc.’s Neupogen, which was originally licensed in 1991.
Zarxio is marketed as Zarzio outside the US. The biosimilar is available in more than 60 countries worldwide.
In the US, Zarxio is approved for the same indications as Neupogen. So Zarxio can be prescribed for the following 5 indications.
Patients with cancer receiving myelosuppressive chemotherapy: to decrease the incidence of infection, as manifested by febrile neutropenia, in patients with nonmyeloid malignancies receiving myelosuppressive anticancer drugs associated with a significant incidence of severe neutropenia with fever.
Patients with acute myeloid leukemia receiving induction or consolidation chemotherapy: to reduce the time to neutrophil recovery and the duration of fever, following induction or consolidation chemotherapy.
Patients with cancer undergoing bone marrow transplant: to reduce the duration of neutropenia and neutropenia-related clinical sequelae—eg, febrile neutropenia—in patients with nonmyeloid malignancies undergoing myeloablative chemotherapy followed by bone marrow transplant.
Patients undergoing autologous peripheral blood progenitor cell collection and therapy: for the mobilization of autologous hematopoietic progenitor cells into the peripheral blood for collection by leukapheresis.
Patients with severe chronic neutropenia: for chronic administration to reduce the incidence and duration of sequelae of neutropenia—eg, fever, infections, oropharyngeal ulcers—in symptomatic patients with congenital neutropenia, cyclic neutropenia, or idiopathic neutropenia.
PIONEER trial
The FDA’s approval of Zarxio was based on data showing that Zarxio is highly similar to Neupogen, with no clinically meaningful differences between the products.
The head-to-head PIONEER study was the final piece of evidence the FDA used to approve Zarxio as biosimilar to Neupogen. Results of the trial were presented at ASH 2014.
Zarxio and Neupogen both produced the expected reduction in the duration of severe neutropenia in breast cancer patients undergoing myelosuppressive chemotherapy—1.17 ± 1.11 and 1.20 ±1.02 days, respectively.
The mean time to absolute neutrophil count recovery in cycle 1 was also similar—1.8 ± 0.97 days in the Zarxio arm and 1.7 ± 0.81 days in the Neupogen arm. No immunogenicity or antibodies against rhG-CSF were detected throughout the study.
The researchers said there were no obvious differences between Zarxio and Neupogen with regard to treatment-emergent adverse events.
The most common side effects observed with Zarxio are aching bones/muscles and redness, swelling, or itching at the injection site. Serious side effects may include spleen rupture; serious allergic reactions that may cause rash, shortness of breath, wheezing and/or swelling around the mouth and eyes; fast pulse and sweating; and acute respiratory distress syndrome.
For more details on Zarxio, see the full prescribing information or visit www.zarxio.com.

© Sandoz Inc. 2015
The leukocyte growth factor Zarxio (filgrastim-sndz), the first biosimilar product to gain approval from the US Food and Drug Administration (FDA), is now available in the US.
Zarxio was approved by the FDA on March 6. The product, made by Sandoz, Inc., is biosimilar to Amgen Inc.’s Neupogen, which was originally licensed in 1991.
Zarxio is marketed as Zarzio outside the US. The biosimilar is available in more than 60 countries worldwide.
In the US, Zarxio is approved for the same indications as Neupogen. So Zarxio can be prescribed for the following 5 indications.
Patients with cancer receiving myelosuppressive chemotherapy: to decrease the incidence of infection, as manifested by febrile neutropenia, in patients with nonmyeloid malignancies receiving myelosuppressive anticancer drugs associated with a significant incidence of severe neutropenia with fever.
Patients with acute myeloid leukemia receiving induction or consolidation chemotherapy: to reduce the time to neutrophil recovery and the duration of fever, following induction or consolidation chemotherapy.
Patients with cancer undergoing bone marrow transplant: to reduce the duration of neutropenia and neutropenia-related clinical sequelae—eg, febrile neutropenia—in patients with nonmyeloid malignancies undergoing myeloablative chemotherapy followed by bone marrow transplant.
Patients undergoing autologous peripheral blood progenitor cell collection and therapy: for the mobilization of autologous hematopoietic progenitor cells into the peripheral blood for collection by leukapheresis.
Patients with severe chronic neutropenia: for chronic administration to reduce the incidence and duration of sequelae of neutropenia—eg, fever, infections, oropharyngeal ulcers—in symptomatic patients with congenital neutropenia, cyclic neutropenia, or idiopathic neutropenia.
PIONEER trial
The FDA’s approval of Zarxio was based on data showing that Zarxio is highly similar to Neupogen, with no clinically meaningful differences between the products.
The head-to-head PIONEER study was the final piece of evidence the FDA used to approve Zarxio as biosimilar to Neupogen. Results of the trial were presented at ASH 2014.
Zarxio and Neupogen both produced the expected reduction in the duration of severe neutropenia in breast cancer patients undergoing myelosuppressive chemotherapy—1.17 ± 1.11 and 1.20 ±1.02 days, respectively.
The mean time to absolute neutrophil count recovery in cycle 1 was also similar—1.8 ± 0.97 days in the Zarxio arm and 1.7 ± 0.81 days in the Neupogen arm. No immunogenicity or antibodies against rhG-CSF were detected throughout the study.
The researchers said there were no obvious differences between Zarxio and Neupogen with regard to treatment-emergent adverse events.
The most common side effects observed with Zarxio are aching bones/muscles and redness, swelling, or itching at the injection site. Serious side effects may include spleen rupture; serious allergic reactions that may cause rash, shortness of breath, wheezing and/or swelling around the mouth and eyes; fast pulse and sweating; and acute respiratory distress syndrome.
For more details on Zarxio, see the full prescribing information or visit www.zarxio.com.