User login
Brentuximab vedotin beat methotrexate, bexarotene in cutaneous T-cell lymphoma
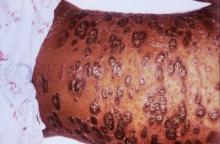
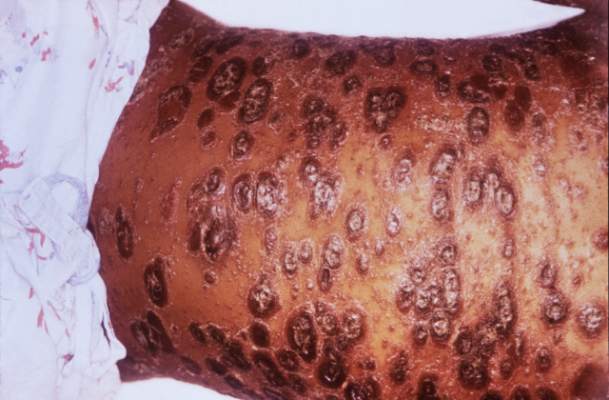
SAN DIEGO – For patients with CD30 expressing cutaneous T-cell lymphoma, antibody-drug conjugate therapy with brentuximab vedotin significantly outperformed two standard regimens in the phase III ALCANZA trial.
After a median of 17.5 months of follow-up, 56% of patients receiving brentuximab vedotin had an objective response lasting at least 4 months, versus 13% of patients treated with physician’s choice of methotrexate or bexarotene (P less than .0001), Youn H. Kim, MD, said during an oral presentation at the annual meeting of the American Society of Hematology.
As in past studies, brentuximab vedotin caused high rates of peripheral neuropathy, but more than 80% of cases improved or resolved over time, she said.
This is the first reported phase III trial to convincingly show that a new systemic agent outperformed standard therapies for cutaneous T-cell lymphoma (CTCL), which tend to have inadequate and short-lived efficacy, stated Dr. Kim, of Stanford (Calif.) University. Brentuximab vedotin not only met the primary endpoint, but all other predefined endpoints, including progression-free survival and a quality-of-life measure, she said.
“These compelling results have potential practice-changing implications,” she concluded.
Brentuximab vedotin (Adcetris) targets CD30, which is expressed in skin lesions of about half of patients with CTCL. A protease-cleavable linker attaches an anti-CD30 monoclonal antibody to monomethyl auristatin E, which disrupts microtubules when released into CD30-positive tumor cells (Blood. 2013;122:367). The agent showed clinical activity against CTCL in two previous phase II trials of CTCL.
Accordingly, the international, open-label phase III ALCANZA study enrolled 128 treatment-experienced patients with CD30-expressing mycosis fungoides or primary cutaneous anaplastic large cell lymphoma. Patients were randomly assigned to receive brentuximab vedotin (1.8 mg/kg once every 3 weeks) or physician’s choice of either methotrexate (5 to 50 mg once weekly) or bexarotene (300 mg/m2 once daily) for up to 16 3-week cycles, or until disease progression or unacceptable toxicity. Methotrexate or bexarotene were designated “physician’s choice” because they are used worldwide for treating CTCL, according to Dr. Kim.
To capture both the rate and duration of response, researchers defined objective response lasting at least 4 months as the primary endpoint. Brentuximab vedotin more than quadrupled the likelihood of this outcome when compared with the standard CTCL regimens, a trend that spanned key demographic and clinical subgroups, Dr. Kim said.
“All endpoints were highly [statistically] significant,” she further reported. For example, the objective response rate with brentuximab vedotin was 67%, versus 20% for methotrexate or bexarotene. Respective rates of complete response were 16% and 2%, and median durations of progression-free survival were 17 and 4 months, translating to a 73% lower risk of progression or death with brentuximab vedotin (95% confidence interval, 57%-83%). Patients who received brentuximab vedotin also reported about a three-fold greater improvement on the Skindex-29 symptom domain, compared with the physician’s choice group (–29 vs. –9 points; P less than .0001).
The safety profile of brentuximab vedotin resembled that seen in previous studies, Dr. Kim said. Most notably, 67% of patients developed peripheral neuropathy, and 9% developed grade 3 peripheral neuropathy. This usually improved or resolved over about the next 22 months. Diarrhea, fatigue, and vomiting affected about a third of patients on brentuximab vedotin, and about one in four stopped treatment because of adverse events, compared with 8% of the physician’s choice arm. Rates of serious adverse events were 41% and 47%, respectively. One brentuximab vedotin recipient died of multiple organ dysfunction syndrome that investigators attributed to treatment-associated necrosis of peripheral tumors. They identified no other treatment-related deaths.
Seattle Genetics and Takeda funded the trial. Dr. Kim disclosed ties to Takeda and Seattle Genetics, as well as several other pharmaceutical companies.
SAN DIEGO – For patients with CD30 expressing cutaneous T-cell lymphoma, antibody-drug conjugate therapy with brentuximab vedotin significantly outperformed two standard regimens in the phase III ALCANZA trial.
After a median of 17.5 months of follow-up, 56% of patients receiving brentuximab vedotin had an objective response lasting at least 4 months, versus 13% of patients treated with physician’s choice of methotrexate or bexarotene (P less than .0001), Youn H. Kim, MD, said during an oral presentation at the annual meeting of the American Society of Hematology.
As in past studies, brentuximab vedotin caused high rates of peripheral neuropathy, but more than 80% of cases improved or resolved over time, she said.
This is the first reported phase III trial to convincingly show that a new systemic agent outperformed standard therapies for cutaneous T-cell lymphoma (CTCL), which tend to have inadequate and short-lived efficacy, stated Dr. Kim, of Stanford (Calif.) University. Brentuximab vedotin not only met the primary endpoint, but all other predefined endpoints, including progression-free survival and a quality-of-life measure, she said.
“These compelling results have potential practice-changing implications,” she concluded.
Brentuximab vedotin (Adcetris) targets CD30, which is expressed in skin lesions of about half of patients with CTCL. A protease-cleavable linker attaches an anti-CD30 monoclonal antibody to monomethyl auristatin E, which disrupts microtubules when released into CD30-positive tumor cells (Blood. 2013;122:367). The agent showed clinical activity against CTCL in two previous phase II trials of CTCL.
Accordingly, the international, open-label phase III ALCANZA study enrolled 128 treatment-experienced patients with CD30-expressing mycosis fungoides or primary cutaneous anaplastic large cell lymphoma. Patients were randomly assigned to receive brentuximab vedotin (1.8 mg/kg once every 3 weeks) or physician’s choice of either methotrexate (5 to 50 mg once weekly) or bexarotene (300 mg/m2 once daily) for up to 16 3-week cycles, or until disease progression or unacceptable toxicity. Methotrexate or bexarotene were designated “physician’s choice” because they are used worldwide for treating CTCL, according to Dr. Kim.
To capture both the rate and duration of response, researchers defined objective response lasting at least 4 months as the primary endpoint. Brentuximab vedotin more than quadrupled the likelihood of this outcome when compared with the standard CTCL regimens, a trend that spanned key demographic and clinical subgroups, Dr. Kim said.
“All endpoints were highly [statistically] significant,” she further reported. For example, the objective response rate with brentuximab vedotin was 67%, versus 20% for methotrexate or bexarotene. Respective rates of complete response were 16% and 2%, and median durations of progression-free survival were 17 and 4 months, translating to a 73% lower risk of progression or death with brentuximab vedotin (95% confidence interval, 57%-83%). Patients who received brentuximab vedotin also reported about a three-fold greater improvement on the Skindex-29 symptom domain, compared with the physician’s choice group (–29 vs. –9 points; P less than .0001).
The safety profile of brentuximab vedotin resembled that seen in previous studies, Dr. Kim said. Most notably, 67% of patients developed peripheral neuropathy, and 9% developed grade 3 peripheral neuropathy. This usually improved or resolved over about the next 22 months. Diarrhea, fatigue, and vomiting affected about a third of patients on brentuximab vedotin, and about one in four stopped treatment because of adverse events, compared with 8% of the physician’s choice arm. Rates of serious adverse events were 41% and 47%, respectively. One brentuximab vedotin recipient died of multiple organ dysfunction syndrome that investigators attributed to treatment-associated necrosis of peripheral tumors. They identified no other treatment-related deaths.
Seattle Genetics and Takeda funded the trial. Dr. Kim disclosed ties to Takeda and Seattle Genetics, as well as several other pharmaceutical companies.
SAN DIEGO – For patients with CD30 expressing cutaneous T-cell lymphoma, antibody-drug conjugate therapy with brentuximab vedotin significantly outperformed two standard regimens in the phase III ALCANZA trial.
After a median of 17.5 months of follow-up, 56% of patients receiving brentuximab vedotin had an objective response lasting at least 4 months, versus 13% of patients treated with physician’s choice of methotrexate or bexarotene (P less than .0001), Youn H. Kim, MD, said during an oral presentation at the annual meeting of the American Society of Hematology.
As in past studies, brentuximab vedotin caused high rates of peripheral neuropathy, but more than 80% of cases improved or resolved over time, she said.
This is the first reported phase III trial to convincingly show that a new systemic agent outperformed standard therapies for cutaneous T-cell lymphoma (CTCL), which tend to have inadequate and short-lived efficacy, stated Dr. Kim, of Stanford (Calif.) University. Brentuximab vedotin not only met the primary endpoint, but all other predefined endpoints, including progression-free survival and a quality-of-life measure, she said.
“These compelling results have potential practice-changing implications,” she concluded.
Brentuximab vedotin (Adcetris) targets CD30, which is expressed in skin lesions of about half of patients with CTCL. A protease-cleavable linker attaches an anti-CD30 monoclonal antibody to monomethyl auristatin E, which disrupts microtubules when released into CD30-positive tumor cells (Blood. 2013;122:367). The agent showed clinical activity against CTCL in two previous phase II trials of CTCL.
Accordingly, the international, open-label phase III ALCANZA study enrolled 128 treatment-experienced patients with CD30-expressing mycosis fungoides or primary cutaneous anaplastic large cell lymphoma. Patients were randomly assigned to receive brentuximab vedotin (1.8 mg/kg once every 3 weeks) or physician’s choice of either methotrexate (5 to 50 mg once weekly) or bexarotene (300 mg/m2 once daily) for up to 16 3-week cycles, or until disease progression or unacceptable toxicity. Methotrexate or bexarotene were designated “physician’s choice” because they are used worldwide for treating CTCL, according to Dr. Kim.
To capture both the rate and duration of response, researchers defined objective response lasting at least 4 months as the primary endpoint. Brentuximab vedotin more than quadrupled the likelihood of this outcome when compared with the standard CTCL regimens, a trend that spanned key demographic and clinical subgroups, Dr. Kim said.
“All endpoints were highly [statistically] significant,” she further reported. For example, the objective response rate with brentuximab vedotin was 67%, versus 20% for methotrexate or bexarotene. Respective rates of complete response were 16% and 2%, and median durations of progression-free survival were 17 and 4 months, translating to a 73% lower risk of progression or death with brentuximab vedotin (95% confidence interval, 57%-83%). Patients who received brentuximab vedotin also reported about a three-fold greater improvement on the Skindex-29 symptom domain, compared with the physician’s choice group (–29 vs. –9 points; P less than .0001).
The safety profile of brentuximab vedotin resembled that seen in previous studies, Dr. Kim said. Most notably, 67% of patients developed peripheral neuropathy, and 9% developed grade 3 peripheral neuropathy. This usually improved or resolved over about the next 22 months. Diarrhea, fatigue, and vomiting affected about a third of patients on brentuximab vedotin, and about one in four stopped treatment because of adverse events, compared with 8% of the physician’s choice arm. Rates of serious adverse events were 41% and 47%, respectively. One brentuximab vedotin recipient died of multiple organ dysfunction syndrome that investigators attributed to treatment-associated necrosis of peripheral tumors. They identified no other treatment-related deaths.
Seattle Genetics and Takeda funded the trial. Dr. Kim disclosed ties to Takeda and Seattle Genetics, as well as several other pharmaceutical companies.
AT ASH 2016
Key clinical point: Brentuximab vedotin met all its endpoints but often caused peripheral neuropathy in a phase III trial of patients with CD30 expressing cutaneous T-cell lymphoma.
Major finding: After a median of 17.5 months of follow-up, 56% of patients receiving brentuximab vedotin had an objective response lasting at least 4 months, versus 13% of those receiving physician’s choice of methotrexate or bexarotene (P less than .0001).
Data source: A multicenter, open-label phase III trial of 128 patients with CD30-expressing mycosis fungoides or primary cutaneous anaplastic large cell lymphoma.
Disclosures: Seattle Genetics and Takeda funded the trial. Dr. Kim disclosed ties to Seattle Genetics and Takeda, as well as several other pharmaceutical companies.
‘Meaningful’ antitumor activity with lenalidomide monotherapy in ATL
Lenalidomide monotherapy demonstrated “meaningful” antitumor activity in patients with relapsed or recurrent aggressive adult T-cell leukemia/lymphoma (ATL), according to new findings.
Among 26 patients enrolled in the study, 11 responses were observed, for an overall response rate of 42% (95% CI, 23%-63%). This included four complete responses and one unconfirmed complete response.
The tumor control rate was 73%, achieved in 19 patients, and the toxicity profile was manageable. Overall, these findings hint at the potential of lenalidomide to “become a treatment option in this patient population,” wrote Takashi Ishida, MD, of Nagoya City University Graduate School of Medical Sciences, Aichi, Japan, and his colleagues (J Clin Oncol. 2016 Sep 12. doi: 10.1200/JCO.2016.67.7732).
ATL is a difficult disease to treat, and it has a poor prognosis, as it is resistant to conventional chemotherapeutic agents and treatment options are currently limited. Lenalidomide, an oral immunomodulatory agent, has demonstrated both antiproliferative and antineoplastic activity in B-cell lymphomas in preclinical studies, and a previous phase I trial established a maximum tolerated dosage (25 mg/d) in a small cohort of Japanese patients with relapsed ATL or other peripheral T-cell lymphomas (PTCL).
Based on these preliminary results, Dr. Ishida and his coauthors designed the current multicenter phase II study, to evaluate the efficacy and safety of lenalidomide monotherapy in 26 patients with relapsed or recurrent ATL.
At a median follow-up of 3.9 months, responses were observed in 33% of patients (5 of 15) with acute disease, 57% (4 of 7) with lymphoma, and 50% (2 of 4) for unfavorable chronic ATL. Patient responses according to disease site were 31% for target (nodal and extranodal) lesions, 75% for cutaneous lesions, and 60% for peripheral blood.
The median time to relapse was 1.9 months (range, 1.8-3.7 months), while the median time to progression was 3.8 months (95% CI, 1.9 to not estimable [NE]). The median and mean duration of response for the entire cohort were NE (95% CI, 0.5 months to NE) and 5.2 months (range, 0 to 16.6 months), respectively.
Progression-free survival was 3.8 months (95% CI, 1.9 months to NE) and for overall survival, it was 20.3 months (95% CI, 9.1 months to NE).
Adverse events occurred in more than 20% of patients and the most common hematologic event was thrombocytopenia (77%). The most common nonhematologic event was increased C-reactive protein (42%), and hypoalbuminemia and hypoproteinemia were observed in about a third of patients, as were constipation, hyponatremia, and hypocalcemia (all 31%).
Dr. Ishida and several coauthors reported multiple relationships with industry, including Celgene K.K. (Tokyo), the study’s sponsor.
Lenalidomide monotherapy demonstrated “meaningful” antitumor activity in patients with relapsed or recurrent aggressive adult T-cell leukemia/lymphoma (ATL), according to new findings.
Among 26 patients enrolled in the study, 11 responses were observed, for an overall response rate of 42% (95% CI, 23%-63%). This included four complete responses and one unconfirmed complete response.
The tumor control rate was 73%, achieved in 19 patients, and the toxicity profile was manageable. Overall, these findings hint at the potential of lenalidomide to “become a treatment option in this patient population,” wrote Takashi Ishida, MD, of Nagoya City University Graduate School of Medical Sciences, Aichi, Japan, and his colleagues (J Clin Oncol. 2016 Sep 12. doi: 10.1200/JCO.2016.67.7732).
ATL is a difficult disease to treat, and it has a poor prognosis, as it is resistant to conventional chemotherapeutic agents and treatment options are currently limited. Lenalidomide, an oral immunomodulatory agent, has demonstrated both antiproliferative and antineoplastic activity in B-cell lymphomas in preclinical studies, and a previous phase I trial established a maximum tolerated dosage (25 mg/d) in a small cohort of Japanese patients with relapsed ATL or other peripheral T-cell lymphomas (PTCL).
Based on these preliminary results, Dr. Ishida and his coauthors designed the current multicenter phase II study, to evaluate the efficacy and safety of lenalidomide monotherapy in 26 patients with relapsed or recurrent ATL.
At a median follow-up of 3.9 months, responses were observed in 33% of patients (5 of 15) with acute disease, 57% (4 of 7) with lymphoma, and 50% (2 of 4) for unfavorable chronic ATL. Patient responses according to disease site were 31% for target (nodal and extranodal) lesions, 75% for cutaneous lesions, and 60% for peripheral blood.
The median time to relapse was 1.9 months (range, 1.8-3.7 months), while the median time to progression was 3.8 months (95% CI, 1.9 to not estimable [NE]). The median and mean duration of response for the entire cohort were NE (95% CI, 0.5 months to NE) and 5.2 months (range, 0 to 16.6 months), respectively.
Progression-free survival was 3.8 months (95% CI, 1.9 months to NE) and for overall survival, it was 20.3 months (95% CI, 9.1 months to NE).
Adverse events occurred in more than 20% of patients and the most common hematologic event was thrombocytopenia (77%). The most common nonhematologic event was increased C-reactive protein (42%), and hypoalbuminemia and hypoproteinemia were observed in about a third of patients, as were constipation, hyponatremia, and hypocalcemia (all 31%).
Dr. Ishida and several coauthors reported multiple relationships with industry, including Celgene K.K. (Tokyo), the study’s sponsor.
Lenalidomide monotherapy demonstrated “meaningful” antitumor activity in patients with relapsed or recurrent aggressive adult T-cell leukemia/lymphoma (ATL), according to new findings.
Among 26 patients enrolled in the study, 11 responses were observed, for an overall response rate of 42% (95% CI, 23%-63%). This included four complete responses and one unconfirmed complete response.
The tumor control rate was 73%, achieved in 19 patients, and the toxicity profile was manageable. Overall, these findings hint at the potential of lenalidomide to “become a treatment option in this patient population,” wrote Takashi Ishida, MD, of Nagoya City University Graduate School of Medical Sciences, Aichi, Japan, and his colleagues (J Clin Oncol. 2016 Sep 12. doi: 10.1200/JCO.2016.67.7732).
ATL is a difficult disease to treat, and it has a poor prognosis, as it is resistant to conventional chemotherapeutic agents and treatment options are currently limited. Lenalidomide, an oral immunomodulatory agent, has demonstrated both antiproliferative and antineoplastic activity in B-cell lymphomas in preclinical studies, and a previous phase I trial established a maximum tolerated dosage (25 mg/d) in a small cohort of Japanese patients with relapsed ATL or other peripheral T-cell lymphomas (PTCL).
Based on these preliminary results, Dr. Ishida and his coauthors designed the current multicenter phase II study, to evaluate the efficacy and safety of lenalidomide monotherapy in 26 patients with relapsed or recurrent ATL.
At a median follow-up of 3.9 months, responses were observed in 33% of patients (5 of 15) with acute disease, 57% (4 of 7) with lymphoma, and 50% (2 of 4) for unfavorable chronic ATL. Patient responses according to disease site were 31% for target (nodal and extranodal) lesions, 75% for cutaneous lesions, and 60% for peripheral blood.
The median time to relapse was 1.9 months (range, 1.8-3.7 months), while the median time to progression was 3.8 months (95% CI, 1.9 to not estimable [NE]). The median and mean duration of response for the entire cohort were NE (95% CI, 0.5 months to NE) and 5.2 months (range, 0 to 16.6 months), respectively.
Progression-free survival was 3.8 months (95% CI, 1.9 months to NE) and for overall survival, it was 20.3 months (95% CI, 9.1 months to NE).
Adverse events occurred in more than 20% of patients and the most common hematologic event was thrombocytopenia (77%). The most common nonhematologic event was increased C-reactive protein (42%), and hypoalbuminemia and hypoproteinemia were observed in about a third of patients, as were constipation, hyponatremia, and hypocalcemia (all 31%).
Dr. Ishida and several coauthors reported multiple relationships with industry, including Celgene K.K. (Tokyo), the study’s sponsor.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Lenalidomide demonstrated clinical activity with acceptable toxicity in recurrent or relapsed ATL.
Major finding: The overall response rate was 42%, and this included four complete responses and one unconfirmed complete response.
Data source: Multicenter phase II trial comprising 26 patients with relapsed or recurrent ATL.
Disclosures: Dr. Ishida and several coauthors reported multiple relationships with industry, including Celgene K.K. (Tokyo), the study’s sponsor.
Pretransplantation mogamulizumab for ATLL raises risk of GVHD
The use of mogamulizumab before allogeneic hematopoietic stem-cell transplantation in aggressive adult T-cell leukemia/lymphoma is associated with an increased risk of acute graft-versus-host disease (GVHD), which leads to an inferior overall survival, investigators report in the Journal of Clinical Oncology.
Mogamulizumab is an anti-CCR4 monoclonal antibody that showed promise in small clinical studies when added to conventional chemotherapy as first-line treatment. It was recently approved for the treatment of adult T-cell leukemia/lymphoma in Japan, and eventually may be approved in the U.S. and other countries, said Shigeo Fuji, MD, of the department of hematopoietic stem-cell transplantation, National Cancer Center Hospital, Tokyo, and his associates.
The agent significantly depleted regulatory T cells for several months in animal models. This prompted concern regarding the possibility of exacerbating GVHD in human patients who don’t respond completely to first-line chemotherapy and then undergo stem-cell transplantation. “However, no direct evidence has demonstrated [regulatory T-cell] depletion in humans,” the investigators noted.
To examine this issue, they assessed clinical outcomes in a cohort of 996 patients across Japan who had aggressive adult T-cell leukemia/lymphoma, were aged 20-69 years, were diagnosed in 2000-2013, and received intensive, multiagent chemotherapy before undergoing allogeneic hematopoietic stem-cell transplantation.
Grade 2-4 acute GVHD developed in 381 of 873 patients who didn’t receive mogamulizumab (43.6%), compared with 47 of 81 patients who did receive the agent (58.0%), for a relative risk of 1.33 (P = .01). Grade 3-4 acute GVHD developed in 150 patients who didn’t receive mogamulizumab (17.2%), compared with 25 who did (30.9%), for an RR of 1.80 (P less than .01) .
The agent not only raised the rate of GVHD, it also increased the severity of the disorder. GVHD was refractory to systemic corticosteroids in 23.5% of patients who didn’t receive mogamulizumab, compared with 48.9% of those who did, for an RR of 2.09 (P less than .01), the investigators reported (J Clin Oncol. 2016. doi: 10.1200/JCO.2016.67.8250).
In addition, 1-year disease-free mortality was 25.1% without mogamulizumab, compared with 43.7% with it. The estimated 1-year overall survival was 49.4% without mogamulizumab, compared with 32.3% with it. And in multivariable analyses, receiving mogamulizumab before undergoing stem-cell transplantation was a significant risk factor for both disease-free mortality (hazard ratio, 1.93) and overall mortality (HR, 1.67).
“All hematologists should take the risks and benefits of mogamulizumab into consideration before they use [it] in transplantation-eligible patients,” Dr. Fuji and his associates said.
The use of mogamulizumab before allogeneic hematopoietic stem-cell transplantation in aggressive adult T-cell leukemia/lymphoma is associated with an increased risk of acute graft-versus-host disease (GVHD), which leads to an inferior overall survival, investigators report in the Journal of Clinical Oncology.
Mogamulizumab is an anti-CCR4 monoclonal antibody that showed promise in small clinical studies when added to conventional chemotherapy as first-line treatment. It was recently approved for the treatment of adult T-cell leukemia/lymphoma in Japan, and eventually may be approved in the U.S. and other countries, said Shigeo Fuji, MD, of the department of hematopoietic stem-cell transplantation, National Cancer Center Hospital, Tokyo, and his associates.
The agent significantly depleted regulatory T cells for several months in animal models. This prompted concern regarding the possibility of exacerbating GVHD in human patients who don’t respond completely to first-line chemotherapy and then undergo stem-cell transplantation. “However, no direct evidence has demonstrated [regulatory T-cell] depletion in humans,” the investigators noted.
To examine this issue, they assessed clinical outcomes in a cohort of 996 patients across Japan who had aggressive adult T-cell leukemia/lymphoma, were aged 20-69 years, were diagnosed in 2000-2013, and received intensive, multiagent chemotherapy before undergoing allogeneic hematopoietic stem-cell transplantation.
Grade 2-4 acute GVHD developed in 381 of 873 patients who didn’t receive mogamulizumab (43.6%), compared with 47 of 81 patients who did receive the agent (58.0%), for a relative risk of 1.33 (P = .01). Grade 3-4 acute GVHD developed in 150 patients who didn’t receive mogamulizumab (17.2%), compared with 25 who did (30.9%), for an RR of 1.80 (P less than .01) .
The agent not only raised the rate of GVHD, it also increased the severity of the disorder. GVHD was refractory to systemic corticosteroids in 23.5% of patients who didn’t receive mogamulizumab, compared with 48.9% of those who did, for an RR of 2.09 (P less than .01), the investigators reported (J Clin Oncol. 2016. doi: 10.1200/JCO.2016.67.8250).
In addition, 1-year disease-free mortality was 25.1% without mogamulizumab, compared with 43.7% with it. The estimated 1-year overall survival was 49.4% without mogamulizumab, compared with 32.3% with it. And in multivariable analyses, receiving mogamulizumab before undergoing stem-cell transplantation was a significant risk factor for both disease-free mortality (hazard ratio, 1.93) and overall mortality (HR, 1.67).
“All hematologists should take the risks and benefits of mogamulizumab into consideration before they use [it] in transplantation-eligible patients,” Dr. Fuji and his associates said.
The use of mogamulizumab before allogeneic hematopoietic stem-cell transplantation in aggressive adult T-cell leukemia/lymphoma is associated with an increased risk of acute graft-versus-host disease (GVHD), which leads to an inferior overall survival, investigators report in the Journal of Clinical Oncology.
Mogamulizumab is an anti-CCR4 monoclonal antibody that showed promise in small clinical studies when added to conventional chemotherapy as first-line treatment. It was recently approved for the treatment of adult T-cell leukemia/lymphoma in Japan, and eventually may be approved in the U.S. and other countries, said Shigeo Fuji, MD, of the department of hematopoietic stem-cell transplantation, National Cancer Center Hospital, Tokyo, and his associates.
The agent significantly depleted regulatory T cells for several months in animal models. This prompted concern regarding the possibility of exacerbating GVHD in human patients who don’t respond completely to first-line chemotherapy and then undergo stem-cell transplantation. “However, no direct evidence has demonstrated [regulatory T-cell] depletion in humans,” the investigators noted.
To examine this issue, they assessed clinical outcomes in a cohort of 996 patients across Japan who had aggressive adult T-cell leukemia/lymphoma, were aged 20-69 years, were diagnosed in 2000-2013, and received intensive, multiagent chemotherapy before undergoing allogeneic hematopoietic stem-cell transplantation.
Grade 2-4 acute GVHD developed in 381 of 873 patients who didn’t receive mogamulizumab (43.6%), compared with 47 of 81 patients who did receive the agent (58.0%), for a relative risk of 1.33 (P = .01). Grade 3-4 acute GVHD developed in 150 patients who didn’t receive mogamulizumab (17.2%), compared with 25 who did (30.9%), for an RR of 1.80 (P less than .01) .
The agent not only raised the rate of GVHD, it also increased the severity of the disorder. GVHD was refractory to systemic corticosteroids in 23.5% of patients who didn’t receive mogamulizumab, compared with 48.9% of those who did, for an RR of 2.09 (P less than .01), the investigators reported (J Clin Oncol. 2016. doi: 10.1200/JCO.2016.67.8250).
In addition, 1-year disease-free mortality was 25.1% without mogamulizumab, compared with 43.7% with it. The estimated 1-year overall survival was 49.4% without mogamulizumab, compared with 32.3% with it. And in multivariable analyses, receiving mogamulizumab before undergoing stem-cell transplantation was a significant risk factor for both disease-free mortality (hazard ratio, 1.93) and overall mortality (HR, 1.67).
“All hematologists should take the risks and benefits of mogamulizumab into consideration before they use [it] in transplantation-eligible patients,” Dr. Fuji and his associates said.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: The use of mogamulizumab before allogeneic hematopoietic stem-cell transplantation in aggressive adult T-cell leukemia/lymphoma was associated with GVHD and increased mortality.
Major finding: Grade 3-4 acute GVHD developed in 17.2% of patients who didn’t receive mogamulizumab, compared with 30.9% who did, for a relative risk of 1.80.
Data source: A retrospective cohort study involving 996 patients with adult T-cell leukemia/lymphoma in Japan.
Disclosures: This study was supported in part by Practical Research for Innovative Cancer Control and the Japan Agency for Medical Research and Development. Dr. Fuji and one associate reported receiving honoraria from Kyowa Hakko Kirin; another associate reported ties to numerous industry sources.
Severe psoriasis upped lymphoma risk in large cohort study
SCOTTSDALE, ARIZ. – Psoriasis of all severities was linked to a 3.5-fold increase in risk of cutaneous T-cell lymphoma, and severe psoriasis upped the associated risk of Hodgkin lymphoma by about 2.5 times, in a large, longitudinal, population-based cohort study.
Psoriasis also was tied to a smaller but statistically significant increase in the risk of non-Hodgkin lymphoma, said Zelma Chiesa Fuxench, MD, of the department of dermatology, the University of Pennsylvania, Philadelphia. Overall, lymphoma risk was highest in people with severe psoriasis, independent of traditional risk factors and exposure to immunosuppressive medications, Dr. Fuxench said at the annual meeting of the Society for Investigative Dermatology.
Psoriasis affects more than 125 million people worldwide, and severe cases are a major cause of cancer-related mortality. “Prior studies have suggested an increased risk of lymphoma in psoriasis patients, but it is unclear if this due to chronic inflammation, exposure to immunosuppressive therapies, or a combination of both factors,” Dr. Fuxench said.
To further explore these links, she and her associates analyzed electronic medical records from THIN (The Health Information Network), which includes about 12 million patients across the United Kingdom. Adults with psoriasis were matched to up to five nonpsoriatic controls based on date and clinic location. Patients who needed systemic medications or phototherapy were categorized as having severe psoriasis. The final dataset included more than 12,000 such patients, as well as 184,000 patients with mild psoriasis and more than 965,000 patients without psoriasis.
Psoriasis patients were younger and more likely to be overweight, male, and smoke and drink alcohol than patients without psoriasis, Dr. Fuxench said. Almost 80% of patients with severe disease had received systemic therapies, most often methotrexate (70% of systemic treatments) or cyclosporine (10%), while only 1% had received biologics.
Patients with severe psoriasis were more likely to be diagnosed with Hodgkin disease, non-Hodgkin lymphoma, and cutaneous T-cell lymphoma than were patients with mild psoriasis or controls. Over a median follow-up of 5.3 years, 34 patients with severe psoriasis were diagnosed with any type of lymphoma, for an incidence of 5.2 cases per 10,000 person-years (95% confidence interval, 3.7-7.3). In contrast, incidence rates for patients with mild psoriasis and controls were 3.3 and 3.2 cases per 10,000 person-years, respectively, Dr. Fuxench said.
In the multivariable analysis, patients with psoriasis were about 18% more likely to develop any type of lymphoma than were controls, an association that reached statistical significance (adjusted hazard ratio, 1.18; 95% CI, 1.06-1.31). Mild psoriasis increased lymphoma risk by 14%, and severe psoriasis upped it by about 83%, and both associations were statistically significant.
The increase in risk of non-Hodgkin lymphoma was 13% greater with mild psoriasis and 56% greater with severe disease, compared with controls, and these associations also reached statistical significance. Mild psoriasis was not linked to Hodgkin lymphoma, but patients with severe psoriasis were about 250% more likely to develop it than controls, with a trend toward statistical significance (aHR, 2.54; 95% CI, 0.94-6.87).
Finally, severe psoriasis was linked to a more than ninefold increase in risk of cutaneous T-cell lymphoma (aHR, 9.3; 95% CI, 4.1-21.4), while mild psoriasis was linked to about a threefold increase in risk.
“These results were robust in multiple sensitivity analyses, including analyses that excluded patients with rheumatoid arthritis, psoriatic arthritis, or a history of exposure to methotrexate, cyclosporine, or biologics,” Dr. Fuxench said. Future studies should explore the effect of treatment timing and selection on cancer risk, she added. “For those of us who care for these patients, we are increasingly using systemic agents that selectively target the immune system, and these questions will arise in clinics.”
The study’s design made it possible to pinpoint dates of diagnosis more effectively than investigators could estimate disease duration or confirm whether patients initially diagnosed with psoriasis actually had cutaneous T-cell lymphoma, Dr. Fuxench noted. “Ideally, we could have another cohort study of incident psoriasis with prospective follow-up, but lymphoma is so rare that there is currently not enough power [in the THIN database] to determine associations.”
The study was funded by grants from the National Institutes of Health and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Fuxench disclosed unrestricted research funding from Pfizer outside the submitted work.
SCOTTSDALE, ARIZ. – Psoriasis of all severities was linked to a 3.5-fold increase in risk of cutaneous T-cell lymphoma, and severe psoriasis upped the associated risk of Hodgkin lymphoma by about 2.5 times, in a large, longitudinal, population-based cohort study.
Psoriasis also was tied to a smaller but statistically significant increase in the risk of non-Hodgkin lymphoma, said Zelma Chiesa Fuxench, MD, of the department of dermatology, the University of Pennsylvania, Philadelphia. Overall, lymphoma risk was highest in people with severe psoriasis, independent of traditional risk factors and exposure to immunosuppressive medications, Dr. Fuxench said at the annual meeting of the Society for Investigative Dermatology.
Psoriasis affects more than 125 million people worldwide, and severe cases are a major cause of cancer-related mortality. “Prior studies have suggested an increased risk of lymphoma in psoriasis patients, but it is unclear if this due to chronic inflammation, exposure to immunosuppressive therapies, or a combination of both factors,” Dr. Fuxench said.
To further explore these links, she and her associates analyzed electronic medical records from THIN (The Health Information Network), which includes about 12 million patients across the United Kingdom. Adults with psoriasis were matched to up to five nonpsoriatic controls based on date and clinic location. Patients who needed systemic medications or phototherapy were categorized as having severe psoriasis. The final dataset included more than 12,000 such patients, as well as 184,000 patients with mild psoriasis and more than 965,000 patients without psoriasis.
Psoriasis patients were younger and more likely to be overweight, male, and smoke and drink alcohol than patients without psoriasis, Dr. Fuxench said. Almost 80% of patients with severe disease had received systemic therapies, most often methotrexate (70% of systemic treatments) or cyclosporine (10%), while only 1% had received biologics.
Patients with severe psoriasis were more likely to be diagnosed with Hodgkin disease, non-Hodgkin lymphoma, and cutaneous T-cell lymphoma than were patients with mild psoriasis or controls. Over a median follow-up of 5.3 years, 34 patients with severe psoriasis were diagnosed with any type of lymphoma, for an incidence of 5.2 cases per 10,000 person-years (95% confidence interval, 3.7-7.3). In contrast, incidence rates for patients with mild psoriasis and controls were 3.3 and 3.2 cases per 10,000 person-years, respectively, Dr. Fuxench said.
In the multivariable analysis, patients with psoriasis were about 18% more likely to develop any type of lymphoma than were controls, an association that reached statistical significance (adjusted hazard ratio, 1.18; 95% CI, 1.06-1.31). Mild psoriasis increased lymphoma risk by 14%, and severe psoriasis upped it by about 83%, and both associations were statistically significant.
The increase in risk of non-Hodgkin lymphoma was 13% greater with mild psoriasis and 56% greater with severe disease, compared with controls, and these associations also reached statistical significance. Mild psoriasis was not linked to Hodgkin lymphoma, but patients with severe psoriasis were about 250% more likely to develop it than controls, with a trend toward statistical significance (aHR, 2.54; 95% CI, 0.94-6.87).
Finally, severe psoriasis was linked to a more than ninefold increase in risk of cutaneous T-cell lymphoma (aHR, 9.3; 95% CI, 4.1-21.4), while mild psoriasis was linked to about a threefold increase in risk.
“These results were robust in multiple sensitivity analyses, including analyses that excluded patients with rheumatoid arthritis, psoriatic arthritis, or a history of exposure to methotrexate, cyclosporine, or biologics,” Dr. Fuxench said. Future studies should explore the effect of treatment timing and selection on cancer risk, she added. “For those of us who care for these patients, we are increasingly using systemic agents that selectively target the immune system, and these questions will arise in clinics.”
The study’s design made it possible to pinpoint dates of diagnosis more effectively than investigators could estimate disease duration or confirm whether patients initially diagnosed with psoriasis actually had cutaneous T-cell lymphoma, Dr. Fuxench noted. “Ideally, we could have another cohort study of incident psoriasis with prospective follow-up, but lymphoma is so rare that there is currently not enough power [in the THIN database] to determine associations.”
The study was funded by grants from the National Institutes of Health and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Fuxench disclosed unrestricted research funding from Pfizer outside the submitted work.
SCOTTSDALE, ARIZ. – Psoriasis of all severities was linked to a 3.5-fold increase in risk of cutaneous T-cell lymphoma, and severe psoriasis upped the associated risk of Hodgkin lymphoma by about 2.5 times, in a large, longitudinal, population-based cohort study.
Psoriasis also was tied to a smaller but statistically significant increase in the risk of non-Hodgkin lymphoma, said Zelma Chiesa Fuxench, MD, of the department of dermatology, the University of Pennsylvania, Philadelphia. Overall, lymphoma risk was highest in people with severe psoriasis, independent of traditional risk factors and exposure to immunosuppressive medications, Dr. Fuxench said at the annual meeting of the Society for Investigative Dermatology.
Psoriasis affects more than 125 million people worldwide, and severe cases are a major cause of cancer-related mortality. “Prior studies have suggested an increased risk of lymphoma in psoriasis patients, but it is unclear if this due to chronic inflammation, exposure to immunosuppressive therapies, or a combination of both factors,” Dr. Fuxench said.
To further explore these links, she and her associates analyzed electronic medical records from THIN (The Health Information Network), which includes about 12 million patients across the United Kingdom. Adults with psoriasis were matched to up to five nonpsoriatic controls based on date and clinic location. Patients who needed systemic medications or phototherapy were categorized as having severe psoriasis. The final dataset included more than 12,000 such patients, as well as 184,000 patients with mild psoriasis and more than 965,000 patients without psoriasis.
Psoriasis patients were younger and more likely to be overweight, male, and smoke and drink alcohol than patients without psoriasis, Dr. Fuxench said. Almost 80% of patients with severe disease had received systemic therapies, most often methotrexate (70% of systemic treatments) or cyclosporine (10%), while only 1% had received biologics.
Patients with severe psoriasis were more likely to be diagnosed with Hodgkin disease, non-Hodgkin lymphoma, and cutaneous T-cell lymphoma than were patients with mild psoriasis or controls. Over a median follow-up of 5.3 years, 34 patients with severe psoriasis were diagnosed with any type of lymphoma, for an incidence of 5.2 cases per 10,000 person-years (95% confidence interval, 3.7-7.3). In contrast, incidence rates for patients with mild psoriasis and controls were 3.3 and 3.2 cases per 10,000 person-years, respectively, Dr. Fuxench said.
In the multivariable analysis, patients with psoriasis were about 18% more likely to develop any type of lymphoma than were controls, an association that reached statistical significance (adjusted hazard ratio, 1.18; 95% CI, 1.06-1.31). Mild psoriasis increased lymphoma risk by 14%, and severe psoriasis upped it by about 83%, and both associations were statistically significant.
The increase in risk of non-Hodgkin lymphoma was 13% greater with mild psoriasis and 56% greater with severe disease, compared with controls, and these associations also reached statistical significance. Mild psoriasis was not linked to Hodgkin lymphoma, but patients with severe psoriasis were about 250% more likely to develop it than controls, with a trend toward statistical significance (aHR, 2.54; 95% CI, 0.94-6.87).
Finally, severe psoriasis was linked to a more than ninefold increase in risk of cutaneous T-cell lymphoma (aHR, 9.3; 95% CI, 4.1-21.4), while mild psoriasis was linked to about a threefold increase in risk.
“These results were robust in multiple sensitivity analyses, including analyses that excluded patients with rheumatoid arthritis, psoriatic arthritis, or a history of exposure to methotrexate, cyclosporine, or biologics,” Dr. Fuxench said. Future studies should explore the effect of treatment timing and selection on cancer risk, she added. “For those of us who care for these patients, we are increasingly using systemic agents that selectively target the immune system, and these questions will arise in clinics.”
The study’s design made it possible to pinpoint dates of diagnosis more effectively than investigators could estimate disease duration or confirm whether patients initially diagnosed with psoriasis actually had cutaneous T-cell lymphoma, Dr. Fuxench noted. “Ideally, we could have another cohort study of incident psoriasis with prospective follow-up, but lymphoma is so rare that there is currently not enough power [in the THIN database] to determine associations.”
The study was funded by grants from the National Institutes of Health and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Fuxench disclosed unrestricted research funding from Pfizer outside the submitted work.
AT THE 2016 SID ANNUAL MEETING
Key clinical point: Psoriasis was identified as an independent risk factor for lymphoma, with the risk of lymphoma increasing with disease severity.
Major finding: The strongest association was between severe psoriasis and cutaneous T-cell lymphoma (aHR, 9.3; 95% CI, 4.1-21.4).
Data source: A population-based longitudinal cohort study of 12,198 patients with severe psoriasis, 184,870 patients with mild psoriasis, and 965,730 nonpsoriatic controls.
Disclosures: The study was funded by grants from the National Institutes of Health and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Fuxench disclosed unrestricted research support from Pfizer outside the submitted work.
Mogamulizumab achieves objective responses in relapsed/refractory adult T-cell leukemia-lymphoma
CHICAGO – The anti-CCR4 monoclonal antibody mogamulizumab was superior to other investigator-selected therapies for the treatment of patients with relapsed/refractory adult T-cell leukemia-lymphoma (ATL), based on results from 71 patients in a prospective, multicenter, randomized study reported at the annual meeting of the American Society of Clinical Oncology.
Commonly used cytotoxic regimens provided limited therapeutic benefit for these patients, but mogamulizumab resulted in an objective response rate that supports its therapeutic potential in this setting, reported Dr. Adrienne Alise Phillips of New York Presbyterian/Weill Cornell Medical College, New York.
A malignancy of T-cells infected with HTLV-1, ATL has a poor prognosis with a median overall survival of less than 3 months in patients with relapsed/refractory disease. CCR4 is expressed in over 90% of ATL patients, and mogamulizumab is approved in Japan for ATL as well as for peripheral T-cell lymphoma and cutaneous T-cell lymphoma.
The 71 patients in the study were from the United States, the European Union and Latin America. The study is the largest randomized clinical trial of relapsed/refractory adult T-cell leukemia-lymphoma thus far conducted. The patients were randomized in 2:1 fashion 47:24 patients) to mogamulizumab, 1.0 mg/kg, given weekly for the first 4-week cycle and then biweekly, or to one of three investigator choice regimens [gemcitabine and oxaliplatin, DHAP (dexamethasone, high-dose cytarabine, and cisplatin), or pralatrexate]. Patients who were in the investigator-choice arm and whose disease progressed were permitted to cross over to mogamulizumab.
The primary endpoint was objective response rate based on modified Tsukasaki criteria and assessed by the treating investigator and in blinded fashion by independent review.
The objective response rate in the mogamulizumab-treated group was 23.4% (11 of 47) by independent review and 34% (16 of 47) by the treating investigator. In the investigator choice group, the overall response rate was 2 of 24 by independent review and 0 of 24 by the treating investigator.
The confirmed objective response rate after 1 month in the mogamulizumab-treated group was 10.6% by independent review and 14.9% by the treating investigator; there were no confirmed responses in the investigator-choice arm. Of 18 patients who crossed over to mogamulizumab, 3 responded. The median duration of response for mogamulizumab was 5 months; one patient had a complete response that lasted over 9 months and the survival data are not yet mature.
Mogamulizumab had few drug-related adverse events, primarily infusion reactions (46.8%), rash/drug eruption (25.5%) and infections (14.9%).
Dr. Phillips disclosed ties to Celgene, Genentech, and Takeda, as well as research funding from Kyowa Hakko Kirin, the sponsor of the study.
On Twitter @maryjodales

|
| Mary Jo Dales/Frontline Medical News Dr. Sonali M. Smith |
Mogamulizumab was superior to investigator’s choice therapy in the largest prospective randomized trial of this very rare disease. Approximately one-third of patients responded, while the response to investigator’s choice therapies was zero. The potential impact of mogamulizumab on T-cell regulation is intriguing. Could it have applications in other T-cell non-Hodgkin’s lymphomas and cutaneous T-cell lymphomas?
Dr. Sonali M. Smith is with the University of Chicago and was the invited discussant of the study.

|
| Mary Jo Dales/Frontline Medical News Dr. Sonali M. Smith |
Mogamulizumab was superior to investigator’s choice therapy in the largest prospective randomized trial of this very rare disease. Approximately one-third of patients responded, while the response to investigator’s choice therapies was zero. The potential impact of mogamulizumab on T-cell regulation is intriguing. Could it have applications in other T-cell non-Hodgkin’s lymphomas and cutaneous T-cell lymphomas?
Dr. Sonali M. Smith is with the University of Chicago and was the invited discussant of the study.

|
| Mary Jo Dales/Frontline Medical News Dr. Sonali M. Smith |
Mogamulizumab was superior to investigator’s choice therapy in the largest prospective randomized trial of this very rare disease. Approximately one-third of patients responded, while the response to investigator’s choice therapies was zero. The potential impact of mogamulizumab on T-cell regulation is intriguing. Could it have applications in other T-cell non-Hodgkin’s lymphomas and cutaneous T-cell lymphomas?
Dr. Sonali M. Smith is with the University of Chicago and was the invited discussant of the study.
CHICAGO – The anti-CCR4 monoclonal antibody mogamulizumab was superior to other investigator-selected therapies for the treatment of patients with relapsed/refractory adult T-cell leukemia-lymphoma (ATL), based on results from 71 patients in a prospective, multicenter, randomized study reported at the annual meeting of the American Society of Clinical Oncology.
Commonly used cytotoxic regimens provided limited therapeutic benefit for these patients, but mogamulizumab resulted in an objective response rate that supports its therapeutic potential in this setting, reported Dr. Adrienne Alise Phillips of New York Presbyterian/Weill Cornell Medical College, New York.
A malignancy of T-cells infected with HTLV-1, ATL has a poor prognosis with a median overall survival of less than 3 months in patients with relapsed/refractory disease. CCR4 is expressed in over 90% of ATL patients, and mogamulizumab is approved in Japan for ATL as well as for peripheral T-cell lymphoma and cutaneous T-cell lymphoma.
The 71 patients in the study were from the United States, the European Union and Latin America. The study is the largest randomized clinical trial of relapsed/refractory adult T-cell leukemia-lymphoma thus far conducted. The patients were randomized in 2:1 fashion 47:24 patients) to mogamulizumab, 1.0 mg/kg, given weekly for the first 4-week cycle and then biweekly, or to one of three investigator choice regimens [gemcitabine and oxaliplatin, DHAP (dexamethasone, high-dose cytarabine, and cisplatin), or pralatrexate]. Patients who were in the investigator-choice arm and whose disease progressed were permitted to cross over to mogamulizumab.
The primary endpoint was objective response rate based on modified Tsukasaki criteria and assessed by the treating investigator and in blinded fashion by independent review.
The objective response rate in the mogamulizumab-treated group was 23.4% (11 of 47) by independent review and 34% (16 of 47) by the treating investigator. In the investigator choice group, the overall response rate was 2 of 24 by independent review and 0 of 24 by the treating investigator.
The confirmed objective response rate after 1 month in the mogamulizumab-treated group was 10.6% by independent review and 14.9% by the treating investigator; there were no confirmed responses in the investigator-choice arm. Of 18 patients who crossed over to mogamulizumab, 3 responded. The median duration of response for mogamulizumab was 5 months; one patient had a complete response that lasted over 9 months and the survival data are not yet mature.
Mogamulizumab had few drug-related adverse events, primarily infusion reactions (46.8%), rash/drug eruption (25.5%) and infections (14.9%).
Dr. Phillips disclosed ties to Celgene, Genentech, and Takeda, as well as research funding from Kyowa Hakko Kirin, the sponsor of the study.
On Twitter @maryjodales
CHICAGO – The anti-CCR4 monoclonal antibody mogamulizumab was superior to other investigator-selected therapies for the treatment of patients with relapsed/refractory adult T-cell leukemia-lymphoma (ATL), based on results from 71 patients in a prospective, multicenter, randomized study reported at the annual meeting of the American Society of Clinical Oncology.
Commonly used cytotoxic regimens provided limited therapeutic benefit for these patients, but mogamulizumab resulted in an objective response rate that supports its therapeutic potential in this setting, reported Dr. Adrienne Alise Phillips of New York Presbyterian/Weill Cornell Medical College, New York.
A malignancy of T-cells infected with HTLV-1, ATL has a poor prognosis with a median overall survival of less than 3 months in patients with relapsed/refractory disease. CCR4 is expressed in over 90% of ATL patients, and mogamulizumab is approved in Japan for ATL as well as for peripheral T-cell lymphoma and cutaneous T-cell lymphoma.
The 71 patients in the study were from the United States, the European Union and Latin America. The study is the largest randomized clinical trial of relapsed/refractory adult T-cell leukemia-lymphoma thus far conducted. The patients were randomized in 2:1 fashion 47:24 patients) to mogamulizumab, 1.0 mg/kg, given weekly for the first 4-week cycle and then biweekly, or to one of three investigator choice regimens [gemcitabine and oxaliplatin, DHAP (dexamethasone, high-dose cytarabine, and cisplatin), or pralatrexate]. Patients who were in the investigator-choice arm and whose disease progressed were permitted to cross over to mogamulizumab.
The primary endpoint was objective response rate based on modified Tsukasaki criteria and assessed by the treating investigator and in blinded fashion by independent review.
The objective response rate in the mogamulizumab-treated group was 23.4% (11 of 47) by independent review and 34% (16 of 47) by the treating investigator. In the investigator choice group, the overall response rate was 2 of 24 by independent review and 0 of 24 by the treating investigator.
The confirmed objective response rate after 1 month in the mogamulizumab-treated group was 10.6% by independent review and 14.9% by the treating investigator; there were no confirmed responses in the investigator-choice arm. Of 18 patients who crossed over to mogamulizumab, 3 responded. The median duration of response for mogamulizumab was 5 months; one patient had a complete response that lasted over 9 months and the survival data are not yet mature.
Mogamulizumab had few drug-related adverse events, primarily infusion reactions (46.8%), rash/drug eruption (25.5%) and infections (14.9%).
Dr. Phillips disclosed ties to Celgene, Genentech, and Takeda, as well as research funding from Kyowa Hakko Kirin, the sponsor of the study.
On Twitter @maryjodales
AT THE 2016 ASCO ANNUAL MEETING
Key clinical point: The anti-CCR4 monoclonal antibody mogamulizumab was superior to other investigator-selected therapies for the treatment of patients with relapsed/refractory adult T-cell leukemia-lymphoma.
Major finding: The confirmed objective response rate after 1 month in the mogamulizumab-treated group was 10.6% by independent review and 14.9% by the treating investigator; there were no confirmed responses in the investigator-choice arm.
Data source: Prospective, multicenter, randomized study of 71 patients from the United States, the European Union, and Latin America.
Disclosures: Dr. Phillips disclosed ties to Celgene, Genentech, and Takeda, as well as research funding from Kyowa Hakko Kirin, the sponsor of the study.
Alemtuzumab plus CHOP didn’t boost survival in elderly patients with peripheral T-cell lymphomas
CHICAGO – Adding the monoclonal anti-CD52 antibody alemtuzumab to CHOP (A-CHOP) increased response rates in elderly patients with peripheral T-cell lymphomas but did not improve their survival, based on the final results from 116 patients treated in the international ACT-2 phase III trial.
Complete responses were seen in 43% of 58 patients given CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisolone) and in 60% of 58 patients given A-CHOP in the trial. However, trial participants did not significantly differ in event-free survival and progression-free survival at 3 years.
Further, overall survival at 3 years was 38% for the patients given A-CHOP and 56% for the patients given CHOP. The poorer overall survival was mainly the result of treatment-related toxicity, Dr. Lorenz H. Trümper reported at the annual meeting of the American Society of Clinical Oncology.
The estimated 3-year disease-free survival is 25% for elderly patients with peripheral T-cell lymphomas. Previous phase II trials had indicated that alemtuzumab was active in primary and relapsed T-cell lymphoma, prompting the study of adjuvant alemtuzumab in combination with dose-dense CHOP-14 in patients with previously untreated peripheral T-cell lymphoma, he said.
Although the treatment protocol demanded stringent monitoring for cytomegalovirus and Epstein-Barr virus and anti-infective prophylaxis, there were more grade 3 or higher infections in the A-CHOP group (40%) than the CHOP group (21%). The higher infection rates were attributed to higher rates of grade 3/4 hematotoxicity in patients given A-CHOP. Grade 4 leukocytopenia was seen in 70% with A-CHOP and 54% with CHOP; grade 3/4 thrombocytopenia was seen in 19% given A-CHOP and 13% given CHOP, according to Dr. Trümper of the University of Göttingen, Germany.
For the study, 116 patients from 52 centers were randomized to receive either six cycles of CHOP or A-CHOP given at 14-day intervals with granulocyte-colony stimulating factor (G-CSF) support. Initially, patients received a total of 360 mg of alemtuzumab (90 mg given at each of the first four cycles of CHOP). After patient 39 was enrolled, the dose was reduced to 120 mg (30 mg given at each of the first four cycles of CHOP). Median patient age was 69 years, and 58% of the trial participants were men.
Treatment was completed as planned in 79% of the CHOP patients and in 57% of the A-CHOP patients.
The study was sponsored by the University of Göttingen. Dr. Trümper is a consultant or adviser to Hexal and Janssen-Ortho, and receives research funding from Genzyme, the maker of alemtuzumab (Lemtrada), as well as other drug companies.
On Twitter @maryjodales
CHICAGO – Adding the monoclonal anti-CD52 antibody alemtuzumab to CHOP (A-CHOP) increased response rates in elderly patients with peripheral T-cell lymphomas but did not improve their survival, based on the final results from 116 patients treated in the international ACT-2 phase III trial.
Complete responses were seen in 43% of 58 patients given CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisolone) and in 60% of 58 patients given A-CHOP in the trial. However, trial participants did not significantly differ in event-free survival and progression-free survival at 3 years.
Further, overall survival at 3 years was 38% for the patients given A-CHOP and 56% for the patients given CHOP. The poorer overall survival was mainly the result of treatment-related toxicity, Dr. Lorenz H. Trümper reported at the annual meeting of the American Society of Clinical Oncology.
The estimated 3-year disease-free survival is 25% for elderly patients with peripheral T-cell lymphomas. Previous phase II trials had indicated that alemtuzumab was active in primary and relapsed T-cell lymphoma, prompting the study of adjuvant alemtuzumab in combination with dose-dense CHOP-14 in patients with previously untreated peripheral T-cell lymphoma, he said.
Although the treatment protocol demanded stringent monitoring for cytomegalovirus and Epstein-Barr virus and anti-infective prophylaxis, there were more grade 3 or higher infections in the A-CHOP group (40%) than the CHOP group (21%). The higher infection rates were attributed to higher rates of grade 3/4 hematotoxicity in patients given A-CHOP. Grade 4 leukocytopenia was seen in 70% with A-CHOP and 54% with CHOP; grade 3/4 thrombocytopenia was seen in 19% given A-CHOP and 13% given CHOP, according to Dr. Trümper of the University of Göttingen, Germany.
For the study, 116 patients from 52 centers were randomized to receive either six cycles of CHOP or A-CHOP given at 14-day intervals with granulocyte-colony stimulating factor (G-CSF) support. Initially, patients received a total of 360 mg of alemtuzumab (90 mg given at each of the first four cycles of CHOP). After patient 39 was enrolled, the dose was reduced to 120 mg (30 mg given at each of the first four cycles of CHOP). Median patient age was 69 years, and 58% of the trial participants were men.
Treatment was completed as planned in 79% of the CHOP patients and in 57% of the A-CHOP patients.
The study was sponsored by the University of Göttingen. Dr. Trümper is a consultant or adviser to Hexal and Janssen-Ortho, and receives research funding from Genzyme, the maker of alemtuzumab (Lemtrada), as well as other drug companies.
On Twitter @maryjodales
CHICAGO – Adding the monoclonal anti-CD52 antibody alemtuzumab to CHOP (A-CHOP) increased response rates in elderly patients with peripheral T-cell lymphomas but did not improve their survival, based on the final results from 116 patients treated in the international ACT-2 phase III trial.
Complete responses were seen in 43% of 58 patients given CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisolone) and in 60% of 58 patients given A-CHOP in the trial. However, trial participants did not significantly differ in event-free survival and progression-free survival at 3 years.
Further, overall survival at 3 years was 38% for the patients given A-CHOP and 56% for the patients given CHOP. The poorer overall survival was mainly the result of treatment-related toxicity, Dr. Lorenz H. Trümper reported at the annual meeting of the American Society of Clinical Oncology.
The estimated 3-year disease-free survival is 25% for elderly patients with peripheral T-cell lymphomas. Previous phase II trials had indicated that alemtuzumab was active in primary and relapsed T-cell lymphoma, prompting the study of adjuvant alemtuzumab in combination with dose-dense CHOP-14 in patients with previously untreated peripheral T-cell lymphoma, he said.
Although the treatment protocol demanded stringent monitoring for cytomegalovirus and Epstein-Barr virus and anti-infective prophylaxis, there were more grade 3 or higher infections in the A-CHOP group (40%) than the CHOP group (21%). The higher infection rates were attributed to higher rates of grade 3/4 hematotoxicity in patients given A-CHOP. Grade 4 leukocytopenia was seen in 70% with A-CHOP and 54% with CHOP; grade 3/4 thrombocytopenia was seen in 19% given A-CHOP and 13% given CHOP, according to Dr. Trümper of the University of Göttingen, Germany.
For the study, 116 patients from 52 centers were randomized to receive either six cycles of CHOP or A-CHOP given at 14-day intervals with granulocyte-colony stimulating factor (G-CSF) support. Initially, patients received a total of 360 mg of alemtuzumab (90 mg given at each of the first four cycles of CHOP). After patient 39 was enrolled, the dose was reduced to 120 mg (30 mg given at each of the first four cycles of CHOP). Median patient age was 69 years, and 58% of the trial participants were men.
Treatment was completed as planned in 79% of the CHOP patients and in 57% of the A-CHOP patients.
The study was sponsored by the University of Göttingen. Dr. Trümper is a consultant or adviser to Hexal and Janssen-Ortho, and receives research funding from Genzyme, the maker of alemtuzumab (Lemtrada), as well as other drug companies.
On Twitter @maryjodales
AT ASCO 16
Key clinical point: Adding the monoclonal anti-CD52 antibody alemtuzumab to CHOP (A-CHOP) increased response rates in elderly patients with peripheral T-cell lymphomas but did not improve their survival.
Major finding: Overall survival at 3 years was 38% for the patients given A-CHOP and 56% for the patients given CHOP.
Data source: Results from 116 patients treated in the international ACT-2 phase III trial.
Disclosures: The study was sponsored by the University of Göttingen, Germany. Dr. Trümper is a consultant or adviser to Hexal and Janssen-Ortho, and receives research funding from Genzyme, the maker of alemtuzumab, as well as other drug companies.
Dr. Matt Kalaycio’s top 10 hematologic oncology abstracts for ASCO 2016
Hematology News’ Editor-in-Chief Matt Kalaycio selected the following as his “top 10” picks for hematologic oncology abstracts at ASCO 2016:
Abstract 7000: Final results of a phase III randomized trial of CPX-351 versus 7+3 in older patients with newly diagnosed high risk (secondary) AML
Comment: When any treatment appears to improve survival, compared with 7+3 for AML, all must take notice.
Abstract 7001: Treatment-free remission (TFR) in patients (pts) with chronic myeloid leukemia in chronic phase (CML-CP) treated with frontline nilotinib: Results from the ENESTFreedom study
Comment: About 50% of the CML patients treated with frontline nilotinib are eventually able to stop the drug and successfully stay off of it. That means more patients in treatment-free remission, compared with those initially treated with imatinib.
Link to abstract 7001
Abstract 7007: Phase Ib/2 study of venetoclax with low-dose cytarabine in treatment-naive patients age ≥ 65 with acute myelogenous leukemia
Abstract 7009: Results of a phase 1b study of venetoclax plus decitabine or azacitidine in untreated acute myeloid leukemia patients ≥ 65 years ineligible for standard induction therapy
Comment: The response rates in these older AML patients are remarkable and challenge results typically seen with 7+3 in a younger population.
Link to abstract 7007 and 7009
Abstract 7501: A prospective, multicenter, randomized study of anti-CCR4 monoclonal antibody mogamulizumab (moga) vs investigator’s choice (IC) in the treatment of patients (pts) with relapsed/refractory (R/R) adult T-cell leukemia-lymphoma (ATL)
Comment: The response rate to mogamulizumab was outstanding in the largest randomized clinical trial thus far conducted for this cancer. Although rare in the USA, ATL is more common in Asia.
Link to abstract 7501
Abstract 7507: Effect of bortezomib on complete remission (CR) rate when added to bendamustine-rituximab (BR) in previously untreated high-risk (HR) follicular lymphoma (FL): A randomized phase II trial of the ECOG-ACRIN Cancer Research Group (E2408)
Comment: This interesting observation of improved complete remission needs longer follow-up.
Link to abstract 7507
Abstract 7519: Venetoclax activity in CLL patients who have relapsed after or are refractory to ibrutinib or idelalisib
Comment: This study has implications for practice. Venetoclax elicits a 50%-60% response rate after patients with CLL progress during treatment with B-cell receptor pathway inhibitors.
Link to abstract 7519
Abstract 7521: Acalabrutinib, a second-generation bruton tyrosine kinase (Btk) inhibitor, in previously untreated chronic lymphocytic leukemia (CLL)
Comment: This next-generation variation on ibrutinib was associated with a 96% overall response rate with fewer adverse effects such as atrial fibrillation.
Link to abstract 7521
Abstract 8000: Upfront autologous stem cell transplantation (ASCT) versus novel agent-based therapy for multiple myeloma (MM): A randomized phase 3 study of the European Myeloma Network (EMN02/HO95 MM trial)
Comment: Other trials are underway to address the role of upfront ASCT for newly diagnosed multiple myeloma. While the last word on this issue has yet to be written, ASCT remains the standard of care for MM patients after induction.
Link to abstract 8000
LBA4: Phase III randomized controlled study of daratumumab, bortezomib, and dexamethasone (DVd) versus bortezomib and dexamethasone (Vd) in patients (pts) with relapsed or refractory multiple myeloma (RRMM): CASTOR study
Comment: As predicted by most, the addition of daratumumab to bortezomib-based therapy increases response rates, compared with bortezomib-based alone. Efficacy is becoming less of a concern with myeloma treatment than is economics..
Look for the full, final text of this abstract to be posted online at 7:30 AM (EDT) on Sunday, June 5.
Hematology News’ Editor-in-Chief Matt Kalaycio selected the following as his “top 10” picks for hematologic oncology abstracts at ASCO 2016:
Abstract 7000: Final results of a phase III randomized trial of CPX-351 versus 7+3 in older patients with newly diagnosed high risk (secondary) AML
Comment: When any treatment appears to improve survival, compared with 7+3 for AML, all must take notice.
Abstract 7001: Treatment-free remission (TFR) in patients (pts) with chronic myeloid leukemia in chronic phase (CML-CP) treated with frontline nilotinib: Results from the ENESTFreedom study
Comment: About 50% of the CML patients treated with frontline nilotinib are eventually able to stop the drug and successfully stay off of it. That means more patients in treatment-free remission, compared with those initially treated with imatinib.
Link to abstract 7001
Abstract 7007: Phase Ib/2 study of venetoclax with low-dose cytarabine in treatment-naive patients age ≥ 65 with acute myelogenous leukemia
Abstract 7009: Results of a phase 1b study of venetoclax plus decitabine or azacitidine in untreated acute myeloid leukemia patients ≥ 65 years ineligible for standard induction therapy
Comment: The response rates in these older AML patients are remarkable and challenge results typically seen with 7+3 in a younger population.
Link to abstract 7007 and 7009
Abstract 7501: A prospective, multicenter, randomized study of anti-CCR4 monoclonal antibody mogamulizumab (moga) vs investigator’s choice (IC) in the treatment of patients (pts) with relapsed/refractory (R/R) adult T-cell leukemia-lymphoma (ATL)
Comment: The response rate to mogamulizumab was outstanding in the largest randomized clinical trial thus far conducted for this cancer. Although rare in the USA, ATL is more common in Asia.
Link to abstract 7501
Abstract 7507: Effect of bortezomib on complete remission (CR) rate when added to bendamustine-rituximab (BR) in previously untreated high-risk (HR) follicular lymphoma (FL): A randomized phase II trial of the ECOG-ACRIN Cancer Research Group (E2408)
Comment: This interesting observation of improved complete remission needs longer follow-up.
Link to abstract 7507
Abstract 7519: Venetoclax activity in CLL patients who have relapsed after or are refractory to ibrutinib or idelalisib
Comment: This study has implications for practice. Venetoclax elicits a 50%-60% response rate after patients with CLL progress during treatment with B-cell receptor pathway inhibitors.
Link to abstract 7519
Abstract 7521: Acalabrutinib, a second-generation bruton tyrosine kinase (Btk) inhibitor, in previously untreated chronic lymphocytic leukemia (CLL)
Comment: This next-generation variation on ibrutinib was associated with a 96% overall response rate with fewer adverse effects such as atrial fibrillation.
Link to abstract 7521
Abstract 8000: Upfront autologous stem cell transplantation (ASCT) versus novel agent-based therapy for multiple myeloma (MM): A randomized phase 3 study of the European Myeloma Network (EMN02/HO95 MM trial)
Comment: Other trials are underway to address the role of upfront ASCT for newly diagnosed multiple myeloma. While the last word on this issue has yet to be written, ASCT remains the standard of care for MM patients after induction.
Link to abstract 8000
LBA4: Phase III randomized controlled study of daratumumab, bortezomib, and dexamethasone (DVd) versus bortezomib and dexamethasone (Vd) in patients (pts) with relapsed or refractory multiple myeloma (RRMM): CASTOR study
Comment: As predicted by most, the addition of daratumumab to bortezomib-based therapy increases response rates, compared with bortezomib-based alone. Efficacy is becoming less of a concern with myeloma treatment than is economics..
Look for the full, final text of this abstract to be posted online at 7:30 AM (EDT) on Sunday, June 5.
Hematology News’ Editor-in-Chief Matt Kalaycio selected the following as his “top 10” picks for hematologic oncology abstracts at ASCO 2016:
Abstract 7000: Final results of a phase III randomized trial of CPX-351 versus 7+3 in older patients with newly diagnosed high risk (secondary) AML
Comment: When any treatment appears to improve survival, compared with 7+3 for AML, all must take notice.
Abstract 7001: Treatment-free remission (TFR) in patients (pts) with chronic myeloid leukemia in chronic phase (CML-CP) treated with frontline nilotinib: Results from the ENESTFreedom study
Comment: About 50% of the CML patients treated with frontline nilotinib are eventually able to stop the drug and successfully stay off of it. That means more patients in treatment-free remission, compared with those initially treated with imatinib.
Link to abstract 7001
Abstract 7007: Phase Ib/2 study of venetoclax with low-dose cytarabine in treatment-naive patients age ≥ 65 with acute myelogenous leukemia
Abstract 7009: Results of a phase 1b study of venetoclax plus decitabine or azacitidine in untreated acute myeloid leukemia patients ≥ 65 years ineligible for standard induction therapy
Comment: The response rates in these older AML patients are remarkable and challenge results typically seen with 7+3 in a younger population.
Link to abstract 7007 and 7009
Abstract 7501: A prospective, multicenter, randomized study of anti-CCR4 monoclonal antibody mogamulizumab (moga) vs investigator’s choice (IC) in the treatment of patients (pts) with relapsed/refractory (R/R) adult T-cell leukemia-lymphoma (ATL)
Comment: The response rate to mogamulizumab was outstanding in the largest randomized clinical trial thus far conducted for this cancer. Although rare in the USA, ATL is more common in Asia.
Link to abstract 7501
Abstract 7507: Effect of bortezomib on complete remission (CR) rate when added to bendamustine-rituximab (BR) in previously untreated high-risk (HR) follicular lymphoma (FL): A randomized phase II trial of the ECOG-ACRIN Cancer Research Group (E2408)
Comment: This interesting observation of improved complete remission needs longer follow-up.
Link to abstract 7507
Abstract 7519: Venetoclax activity in CLL patients who have relapsed after or are refractory to ibrutinib or idelalisib
Comment: This study has implications for practice. Venetoclax elicits a 50%-60% response rate after patients with CLL progress during treatment with B-cell receptor pathway inhibitors.
Link to abstract 7519
Abstract 7521: Acalabrutinib, a second-generation bruton tyrosine kinase (Btk) inhibitor, in previously untreated chronic lymphocytic leukemia (CLL)
Comment: This next-generation variation on ibrutinib was associated with a 96% overall response rate with fewer adverse effects such as atrial fibrillation.
Link to abstract 7521
Abstract 8000: Upfront autologous stem cell transplantation (ASCT) versus novel agent-based therapy for multiple myeloma (MM): A randomized phase 3 study of the European Myeloma Network (EMN02/HO95 MM trial)
Comment: Other trials are underway to address the role of upfront ASCT for newly diagnosed multiple myeloma. While the last word on this issue has yet to be written, ASCT remains the standard of care for MM patients after induction.
Link to abstract 8000
LBA4: Phase III randomized controlled study of daratumumab, bortezomib, and dexamethasone (DVd) versus bortezomib and dexamethasone (Vd) in patients (pts) with relapsed or refractory multiple myeloma (RRMM): CASTOR study
Comment: As predicted by most, the addition of daratumumab to bortezomib-based therapy increases response rates, compared with bortezomib-based alone. Efficacy is becoming less of a concern with myeloma treatment than is economics..
Look for the full, final text of this abstract to be posted online at 7:30 AM (EDT) on Sunday, June 5.
Peripheral T-cell lymphoma incidence and survival varies significantly by race
Peripheral T-cell lymphoma (PTCL) incidence, proportions of subtypes, and survival differed markedly among racial/ethnic subpopulations in the United States, according to analysis of SEER cancer registries.
Compared with non-Hispanic whites, blacks had higher incidence rates overall of PTCL, due to higher rates of PTCL not otherwise specified (PTCL-NOS) (incidence rate ratio [IRR], 1.67; 95% CI, 1.53-1.82) and adult T-cell leukemia/lymphoma (ATLL) (IRR, 4.37; 3.42-5.56). By contrast, blacks had lower incidence rates of angioimmunoblastic T-cell lymphoma (AITL) and extranodal NK/T-cell lymphoma and natural killer–cell leukemia (ENKCL) than non-Hispanic whites. Underlying causes for these differences are not understood.
“Our findings also highlight the need for stronger efforts to increase recruitment of blacks into clinical trials of PTCL therapies,” wrote Dr. Scott Adams of Fred Hutchinson Cancer Research Center, Seattle, and colleagues (J Clin Oncol. 2016 Jan 25. doi: 10.1200/JCO.2015.635540).
Asians/Pacific Islanders and Hispanic whites had higher rates of ENKCL (IRR, 3.61; 2.93-4.42), and ENKCL comprised a larger proportion of PTCL (14%) in these populations than for non-Hispanic whites (3%), findings which reflect global variations. High rates of ENKCL may be influenced by both genetic and environmental factors, as 35% of this subpopulation was born outside the U.S., compared with 16% of all patients with PTCL. Epstein-Barre virus infection is associated with ENKCL.
Similar to global incidence patterns, Asians/Pacific Islanders had lower rates of anaplastic large-cell lymphoma (ALCL).
Higher incidence of ATLL among blacks and Asians/Pacific Islanders corresponded to a higher prevalence of human T-lymphotropic virus type 1 in these populations. Substantial differences in survival were also observed. Compared with non-Hispanic whites, survival with PTCL-NOS was shorter for every minority group, and blacks had worse prognoses for almost every histology. For patients with any PTCL, median survival time varied by race: 49 months for non-Hispanic whites, 24 for blacks, 22 for Asians/Pacific Islanders, 28 for Hispanic whites, and 36 for American Indians/Alaskan natives.
Reasons for survival disparities are not known, but contributing factors may include racial variations in pharmacokinetics or pharmacodynamics of therapeutic agents and genomic variations of the tumors, as well as healthcare disparities and socioeconomic factors.
The analysis used data from SEER cancer registries of 13,107 patients 15 years and older who had PTCL diagnosed in the U.S. between 2000 and 2012.
For non-Hispanic whites, the annual incidence rate of all PTCLs was 1.56 (95% CI; 1.52-1.59) per 100,000. Compared with non-Hispanic whites, incidence rate ratios were 1.32 (1.25-1.39; P less than .001) for blacks, 0.89 (0.83-0.95; P less than .001) for Asians/Pacific Islanders, 0.63 (0.49-0.79; P less than .001) for American Indians/Alaskan natives, and 0.96 (0.90-1.01; P = .13) for Hispanic whites.
Peripheral T-cell lymphoma (PTCL) incidence, proportions of subtypes, and survival differed markedly among racial/ethnic subpopulations in the United States, according to analysis of SEER cancer registries.
Compared with non-Hispanic whites, blacks had higher incidence rates overall of PTCL, due to higher rates of PTCL not otherwise specified (PTCL-NOS) (incidence rate ratio [IRR], 1.67; 95% CI, 1.53-1.82) and adult T-cell leukemia/lymphoma (ATLL) (IRR, 4.37; 3.42-5.56). By contrast, blacks had lower incidence rates of angioimmunoblastic T-cell lymphoma (AITL) and extranodal NK/T-cell lymphoma and natural killer–cell leukemia (ENKCL) than non-Hispanic whites. Underlying causes for these differences are not understood.
“Our findings also highlight the need for stronger efforts to increase recruitment of blacks into clinical trials of PTCL therapies,” wrote Dr. Scott Adams of Fred Hutchinson Cancer Research Center, Seattle, and colleagues (J Clin Oncol. 2016 Jan 25. doi: 10.1200/JCO.2015.635540).
Asians/Pacific Islanders and Hispanic whites had higher rates of ENKCL (IRR, 3.61; 2.93-4.42), and ENKCL comprised a larger proportion of PTCL (14%) in these populations than for non-Hispanic whites (3%), findings which reflect global variations. High rates of ENKCL may be influenced by both genetic and environmental factors, as 35% of this subpopulation was born outside the U.S., compared with 16% of all patients with PTCL. Epstein-Barre virus infection is associated with ENKCL.
Similar to global incidence patterns, Asians/Pacific Islanders had lower rates of anaplastic large-cell lymphoma (ALCL).
Higher incidence of ATLL among blacks and Asians/Pacific Islanders corresponded to a higher prevalence of human T-lymphotropic virus type 1 in these populations. Substantial differences in survival were also observed. Compared with non-Hispanic whites, survival with PTCL-NOS was shorter for every minority group, and blacks had worse prognoses for almost every histology. For patients with any PTCL, median survival time varied by race: 49 months for non-Hispanic whites, 24 for blacks, 22 for Asians/Pacific Islanders, 28 for Hispanic whites, and 36 for American Indians/Alaskan natives.
Reasons for survival disparities are not known, but contributing factors may include racial variations in pharmacokinetics or pharmacodynamics of therapeutic agents and genomic variations of the tumors, as well as healthcare disparities and socioeconomic factors.
The analysis used data from SEER cancer registries of 13,107 patients 15 years and older who had PTCL diagnosed in the U.S. between 2000 and 2012.
For non-Hispanic whites, the annual incidence rate of all PTCLs was 1.56 (95% CI; 1.52-1.59) per 100,000. Compared with non-Hispanic whites, incidence rate ratios were 1.32 (1.25-1.39; P less than .001) for blacks, 0.89 (0.83-0.95; P less than .001) for Asians/Pacific Islanders, 0.63 (0.49-0.79; P less than .001) for American Indians/Alaskan natives, and 0.96 (0.90-1.01; P = .13) for Hispanic whites.
Peripheral T-cell lymphoma (PTCL) incidence, proportions of subtypes, and survival differed markedly among racial/ethnic subpopulations in the United States, according to analysis of SEER cancer registries.
Compared with non-Hispanic whites, blacks had higher incidence rates overall of PTCL, due to higher rates of PTCL not otherwise specified (PTCL-NOS) (incidence rate ratio [IRR], 1.67; 95% CI, 1.53-1.82) and adult T-cell leukemia/lymphoma (ATLL) (IRR, 4.37; 3.42-5.56). By contrast, blacks had lower incidence rates of angioimmunoblastic T-cell lymphoma (AITL) and extranodal NK/T-cell lymphoma and natural killer–cell leukemia (ENKCL) than non-Hispanic whites. Underlying causes for these differences are not understood.
“Our findings also highlight the need for stronger efforts to increase recruitment of blacks into clinical trials of PTCL therapies,” wrote Dr. Scott Adams of Fred Hutchinson Cancer Research Center, Seattle, and colleagues (J Clin Oncol. 2016 Jan 25. doi: 10.1200/JCO.2015.635540).
Asians/Pacific Islanders and Hispanic whites had higher rates of ENKCL (IRR, 3.61; 2.93-4.42), and ENKCL comprised a larger proportion of PTCL (14%) in these populations than for non-Hispanic whites (3%), findings which reflect global variations. High rates of ENKCL may be influenced by both genetic and environmental factors, as 35% of this subpopulation was born outside the U.S., compared with 16% of all patients with PTCL. Epstein-Barre virus infection is associated with ENKCL.
Similar to global incidence patterns, Asians/Pacific Islanders had lower rates of anaplastic large-cell lymphoma (ALCL).
Higher incidence of ATLL among blacks and Asians/Pacific Islanders corresponded to a higher prevalence of human T-lymphotropic virus type 1 in these populations. Substantial differences in survival were also observed. Compared with non-Hispanic whites, survival with PTCL-NOS was shorter for every minority group, and blacks had worse prognoses for almost every histology. For patients with any PTCL, median survival time varied by race: 49 months for non-Hispanic whites, 24 for blacks, 22 for Asians/Pacific Islanders, 28 for Hispanic whites, and 36 for American Indians/Alaskan natives.
Reasons for survival disparities are not known, but contributing factors may include racial variations in pharmacokinetics or pharmacodynamics of therapeutic agents and genomic variations of the tumors, as well as healthcare disparities and socioeconomic factors.
The analysis used data from SEER cancer registries of 13,107 patients 15 years and older who had PTCL diagnosed in the U.S. between 2000 and 2012.
For non-Hispanic whites, the annual incidence rate of all PTCLs was 1.56 (95% CI; 1.52-1.59) per 100,000. Compared with non-Hispanic whites, incidence rate ratios were 1.32 (1.25-1.39; P less than .001) for blacks, 0.89 (0.83-0.95; P less than .001) for Asians/Pacific Islanders, 0.63 (0.49-0.79; P less than .001) for American Indians/Alaskan natives, and 0.96 (0.90-1.01; P = .13) for Hispanic whites.
Key clinical point: Peripheral T-cell lymphoma incidence, proportions of subtypes, and survival differed markedly among racial/ethnic subpopulations in the United States.
Major finding: Compared with non-Hispanic whites, blacks had higher incidence rates of PTCL not otherwise specified (PTCL-NOS) and adult T-cell leukemia/lymphoma (ATLL), and lower incidence rates of angioimmunoblastic T-cell lymphoma (AITL) and extranodal NK/T-cell lymphoma and natural killer–cell leukemia (ENKCL); Asians/Pacific Islanders and Hispanic whites had higher rates of ENKCL; Asians/Pacific Islanders had lower rates of anaplastic large-cell lymphoma (ALCL); survival with PTCL-NOS was shorter for every minority group compared with non-Hispanic whites.
Data source: SEER cancer registries of 13,107 patients 15 years and older who had PTCL diagnosed in the U.S. between 2000 and 2012.
Disclosures: Dr. Adams reported having no disclosures. Dr. Shustov, a coauthor, reported financial ties to Celgene, Spectrum Pharmaceuticals, Bristol-Myers Squibb, Millennium, Gilead Sciences, Seattle Genetics, and Pfizer.
Antilymphocyte globulin curbs chronic graft-versus-host disease
Antilymphocyte globulin (ATG) added to the myeloablative conditioning regimen of patients undergoing allogeneic stem cell transplantation resulted in a lower incidence of chronic graft-versus-host disease (GVHD) compared with conditioning regimens without ATG.
At two years, the cumulative incidence of chronic GVHD was 32.2% (95% CI, 22.1–46.7) for the ATG group vs 68.7% (58.4-80.7) for the non-ATG group (P < .001). At one year, 91% of the ATG group had discontinued cyclosporine, compared with 39% in the non-ATG group. The difference between groups was most pronounced in rates of the clinical extensive form of chronic GVHD: 7.6% (3.0-19.6) with ATG compared with 52.4% (39.3-69.9) without ATG.
T-cell depletion may cause a loss of graft-versus-leukemia effects, which is cause for concern. This study showed similar rates of relapse-free and overall survival for the ATG and non-ATG groups. Two-year relapse-free survival was 59.4% (47.8-69.2) in the ATG group and 64.6% (50.9-75.3) in the non-ATG group. The composite 2-year survival, free from chronic GVHD and relapse, was 36.6% (25.2-48.0) for the ATG group vs 16.8% (9.2-26.4) for the non-ATG group, according to the study reported in the January 7 issue of the New England Journal of Medicine (2016;374:43-53).
Chronic GVHD is the leading cause of later illness and death after allogeneic hematopoietic stem-cell transplantation, according to the authors. “Even if a modest increase in the rate of relapse in the ATG group cannot be ruled out, the significantly lower incidence of chronic GVHD with ATG resulted in a significantly higher rate of 2-year survival free from chronic GVD among patients who received ATG than among those who did not receive ATG (50% vs. 23%),” wrote Dr. Nicolaus Kröger, Professor and Medical Director of the Department of Stem Cell Transplantation at the University Hospital Hamburg-Eppendorf, Germany, and colleagues.
The prospective, open-label, randomized phase 3 trial evaluated 155 patients at 27 centers from 2006 to 2012. Patients with acute leukemia undergoing allogeneic stem cell transplantation with peripheral blood stem cells were randomized 1:1 to receive myeloablative conditioning with or without ATG.
Post-transplantation lymphoproliferative disorder was not observed in either group. The ATG and non-ATG groups had similar rates of infectious complications (57.8% and 54.2%, respectively), and nonrelapse-related death at 2 years (14.0% and 12.0%, respectively).
The study was funded by the Neovii Biotech and the European Society for Blood and Marrow Transplantation; ClinicalTrials.gov number, NCT00678275. Dr. Kröger reported grant support from Neovii Biotech. Several of his coauthors reported ties to industry.
Antilymphocyte globulin (ATG) added to the myeloablative conditioning regimen of patients undergoing allogeneic stem cell transplantation resulted in a lower incidence of chronic graft-versus-host disease (GVHD) compared with conditioning regimens without ATG.
At two years, the cumulative incidence of chronic GVHD was 32.2% (95% CI, 22.1–46.7) for the ATG group vs 68.7% (58.4-80.7) for the non-ATG group (P < .001). At one year, 91% of the ATG group had discontinued cyclosporine, compared with 39% in the non-ATG group. The difference between groups was most pronounced in rates of the clinical extensive form of chronic GVHD: 7.6% (3.0-19.6) with ATG compared with 52.4% (39.3-69.9) without ATG.
T-cell depletion may cause a loss of graft-versus-leukemia effects, which is cause for concern. This study showed similar rates of relapse-free and overall survival for the ATG and non-ATG groups. Two-year relapse-free survival was 59.4% (47.8-69.2) in the ATG group and 64.6% (50.9-75.3) in the non-ATG group. The composite 2-year survival, free from chronic GVHD and relapse, was 36.6% (25.2-48.0) for the ATG group vs 16.8% (9.2-26.4) for the non-ATG group, according to the study reported in the January 7 issue of the New England Journal of Medicine (2016;374:43-53).
Chronic GVHD is the leading cause of later illness and death after allogeneic hematopoietic stem-cell transplantation, according to the authors. “Even if a modest increase in the rate of relapse in the ATG group cannot be ruled out, the significantly lower incidence of chronic GVHD with ATG resulted in a significantly higher rate of 2-year survival free from chronic GVD among patients who received ATG than among those who did not receive ATG (50% vs. 23%),” wrote Dr. Nicolaus Kröger, Professor and Medical Director of the Department of Stem Cell Transplantation at the University Hospital Hamburg-Eppendorf, Germany, and colleagues.
The prospective, open-label, randomized phase 3 trial evaluated 155 patients at 27 centers from 2006 to 2012. Patients with acute leukemia undergoing allogeneic stem cell transplantation with peripheral blood stem cells were randomized 1:1 to receive myeloablative conditioning with or without ATG.
Post-transplantation lymphoproliferative disorder was not observed in either group. The ATG and non-ATG groups had similar rates of infectious complications (57.8% and 54.2%, respectively), and nonrelapse-related death at 2 years (14.0% and 12.0%, respectively).
The study was funded by the Neovii Biotech and the European Society for Blood and Marrow Transplantation; ClinicalTrials.gov number, NCT00678275. Dr. Kröger reported grant support from Neovii Biotech. Several of his coauthors reported ties to industry.
Antilymphocyte globulin (ATG) added to the myeloablative conditioning regimen of patients undergoing allogeneic stem cell transplantation resulted in a lower incidence of chronic graft-versus-host disease (GVHD) compared with conditioning regimens without ATG.
At two years, the cumulative incidence of chronic GVHD was 32.2% (95% CI, 22.1–46.7) for the ATG group vs 68.7% (58.4-80.7) for the non-ATG group (P < .001). At one year, 91% of the ATG group had discontinued cyclosporine, compared with 39% in the non-ATG group. The difference between groups was most pronounced in rates of the clinical extensive form of chronic GVHD: 7.6% (3.0-19.6) with ATG compared with 52.4% (39.3-69.9) without ATG.
T-cell depletion may cause a loss of graft-versus-leukemia effects, which is cause for concern. This study showed similar rates of relapse-free and overall survival for the ATG and non-ATG groups. Two-year relapse-free survival was 59.4% (47.8-69.2) in the ATG group and 64.6% (50.9-75.3) in the non-ATG group. The composite 2-year survival, free from chronic GVHD and relapse, was 36.6% (25.2-48.0) for the ATG group vs 16.8% (9.2-26.4) for the non-ATG group, according to the study reported in the January 7 issue of the New England Journal of Medicine (2016;374:43-53).
Chronic GVHD is the leading cause of later illness and death after allogeneic hematopoietic stem-cell transplantation, according to the authors. “Even if a modest increase in the rate of relapse in the ATG group cannot be ruled out, the significantly lower incidence of chronic GVHD with ATG resulted in a significantly higher rate of 2-year survival free from chronic GVD among patients who received ATG than among those who did not receive ATG (50% vs. 23%),” wrote Dr. Nicolaus Kröger, Professor and Medical Director of the Department of Stem Cell Transplantation at the University Hospital Hamburg-Eppendorf, Germany, and colleagues.
The prospective, open-label, randomized phase 3 trial evaluated 155 patients at 27 centers from 2006 to 2012. Patients with acute leukemia undergoing allogeneic stem cell transplantation with peripheral blood stem cells were randomized 1:1 to receive myeloablative conditioning with or without ATG.
Post-transplantation lymphoproliferative disorder was not observed in either group. The ATG and non-ATG groups had similar rates of infectious complications (57.8% and 54.2%, respectively), and nonrelapse-related death at 2 years (14.0% and 12.0%, respectively).
The study was funded by the Neovii Biotech and the European Society for Blood and Marrow Transplantation; ClinicalTrials.gov number, NCT00678275. Dr. Kröger reported grant support from Neovii Biotech. Several of his coauthors reported ties to industry.
FROM NEJM
Key clinical point: Chronic graft-versus-host disease (GVHD) after allogeneic stem cell transplantation with peripheral blood stem cells was less than half as frequent with antilymphocyte globulin added to the conditioning regimen.
Major finding: Cumulative incidence of chronic GVHD at 2 years was 32.2% with antilymphocyte globulin vs 68.7% without it.
Data source: The prospective, open-label, randomized phase 3 trial evaluated 155 patients at 27 centers from 2006 to 2012.
Disclosures: Research was supported in part by Neovii Biotech. Dr. Kröger reported grant support from Neovii Biotech. Several of his coauthors reported ties to industry.
Medical Roundtable: Peripheral T-Cell Lymphomas: A Practical Approach to Newly Diagnosed and Relapsed Patients
Moderator: Steven M. Horwitz, MD1
Discussants: Alison Moskowitz, MD1; Michelle Fanale, MD2; Andrei Shustov, MD3
From Memorial Sloan Kettering Cancer Center, New York, NY1; MD Anderson Cancer Center, Houston, TX2; University of Washington Medical Center, Seattle, WA3
Address for correspondence: Steven M. Horwitz, MD, Memorial Sloan Kettering Cancer Center, 1275 York Avenue, New York, NY 10065
E-mail: [email protected]
DR. HORWITZ: My name is Dr. Steven Horwitz from Memorial Sloan-Kettering Cancer Center. I’m joined today by Drs. Alison Moskowitz, Memorial Sloan-Kettering, Michelle Fanale of MD Anderson, and Andrei Shustov, University of Washington in Seattle. Thank you all for joining us for this conversation on T-cell lymphoma. My colleagues are all well known as experts in T-cell lymphomas. Those of you who treat these diseases recognize the systemic T-cell lymphomas as one of our greater challenges in hematologic malignancies in terms of the treatment options for patients and the frequent lack of definitive data to guide our decisions.
I thought what we would do today is have a very practical discussion about the way we think about these diseases, the decisions we make, and the way we make those decisions. I'll start off by asking when you get a referral of a patient with a new diagnosis of peripheral T-cell lymphoma (PTCL), what are some of the basic things that you first think about in terms of approaching a new patient?
DR. SHUSTOV: I think one of the biggest challenges in T-cell lymphomas still remains making the proper diagnosis. In general, pathologists in the United States have a pretty good idea when they see T-cell lymphomas, however, subclassification remains a challenge even for expert hematopathologists due to frequent histologic overlap between the subtypes of PTCL, and even with non-malignant autoimmune disorders. I frequently see patients who are diagnosed with or misdiagnosed with a different subtype of T-cell lymphoma. The most challenging is differentiation between angioimmunoblastic T-cell lymphoma (AITL), anaplastic large cell T-cell lymphoma (ALCL), and PTCL not otherwise specified (PTCL-NOS), especially when the latter patients have high expression of CD30 and/or bear features resembling AITL.
Sometimes they are a slam-dunk diagnosis, but frequently our hematopathologists reverse the diagnosis after doing additional studies on the biopsy material. The most recent case I've seen in my clinic for consultation was a patient with a diagnosis of extranodal NK-cell lymphoma that was reclassified as a gamma-delta T-cell lymphoma after additional work-up. I truly believe that it is advisable that majority, if not all PTCL cases are reviewed by expert hematopathology teams at academic centers that see large volumes of these cases.
I think it's very important to educate community physicians and patients about a proper PTCL diagnosis, especially now that more targeted therapies are being developed and the gene expression profiling techniques will probably lead to identification of specific pathways that are amenable to therapy with specific biologic agents.
DR. FANALE: I'd like to expand upon what Andrei just said. For me the next step after confirming the pathology diagnosis is to think about two things. To think about whether or not this patient is a patient who might be eligible for an ongoing front-line trial, typically if the patient meets eligibility criteria for one of our ongoing front-line trials I would really recommend to the patient to consider being enrolled in that trial, and I also think about whether the patient, if he or she enters into remission with front-line therapy, can be considered for a consolidative autologous stem cell transplant.
I think it's very important to educate community physicians and patients about a proper PTCL diagnosis. Right now, our ongoing front-line trial is the ECHELON-2 trial which is evaluating brentuximab vedotin (BV) plus cyclophosphamide, doxorubicin, prednisone (CHP [BV-CHP]) chemotherapy compared to standard of care cyclophosphamide, doxorubicin, vincristine, prednisone (CHOP) chemotherapy, and that trial is based on the promising data that we've seen in the initial phase I trial that combined BV plus CHP chemotherapy followed by maintenance BV which demonstrated both high and durable complete remission (CR) rates.1 BV is an antibody drug conjugate that's targeted at CD30 and carries initial US Food and Drug Administration approval for patients who have relapsed or refractory ALCL which has a 100% level of CD30 positivity and then also has a National Comprehensive Cancer Network listing for treating other types of relapsed or refractory CD30 positive PTCL as well.
Also there is another upcoming front-line trial, which is to combine pralatrexate plus CHOP. If a patient isn't eligible for a clinical trial or it's just not feasible for that patient to enroll in a clinical trial, I will then look further at what would be potentially the best standard of care option for that patient.
I'll look at the patient's age and performance status and if they are generally less than age 65 or so and if otherwise well, I'll preferentially treat that patient with CHOEP which is CHOP plus etoposide. And then, for a patient who's either older than this or has multiple other comorbidities, I would treat that patient typically with CHOP alone.
DR. HORWITZ: Thank you, we'll go further into the selection of initial therapies but first circle back, I was curious as to how often your center comes to a different diagnosis than the referring center, and are there pitfalls you see that alert you to be suspicious of diagnosis.
DR. SHUSTOV: I'd say probably 10% of cases that we see at our center will be reclassified by our hematopathologists. In most cases, they do not necessarily reverse the diagnosis, but provide further clarification. It is occurring to me that in the community, pathologists are less likely to call the subtype of T-cell lymphoma and limit the report to the general description of T-cell lymphoprolipherative disorder, or state something like “consistent with T-cell lymphoma with features of AITL, or with features of anaplastic lymphoma.” I would admit though, that sometimes it's very difficult to identify the specific subtype of PTCL even in the expert hands; but I'd say these cases would constitute no more than 5% of PTCL patients.
DR. HORWITZ: And in your experience is it mostly fine tuning a T-cell lymphoma diagnosis, or do you see totally different diagnoses?
DR. MOSKOWITZ: Usually review by expert hematopathologists simply leads to fine-tuning the T-cell lymphoma diagnosis, however, I occasionally see significant changes in diagnosis. Often, alterations or clarification of a diagnosis are made possible only after we provide the pathologist with clinical history. For example, a lymph node biopsy may be interpreted as ALCL, however the knowledge that the particular patient has a history of mycosis fungoides would lead the pathologist to consider the diagnosis of large cell transformation of mycosis fungoides rather than ALCL. In such a case, molecular studies are helpful in confirming that the lymph node findings originate from the same clone as that in the original mycosis fungoides lesions, rather than representing a second primary.
DR. FANALE: Very occasionally, I've seen patients truly “with a misdiagnosis and a complete revision of diagnosis” and, usually, those pitfalls that I've seen them occasionally have been the young patients—the young patients who generally have disease in the thorax and neck, who are treated as though they have classical Hodgkin lymphoma, who have very significant progression of disease on standard of care treatment. So it's important that not all cases that have CD30 positivity are classical Hodgkin lymphoma even if the patient is young.
DR. HORWITZ: I often think the systemic T-cell lymphomas usually behave in an aggressive fashion so when the clinical picture and the diagnosis don't really fit, I think about getting a second biopsy before we finalize the diagnosis. Of course, when people are ill with obviously progressing disease, you may need to move more quickly.
I often think the systemic T-cell lymphomas usually behave in an aggressive fashion so when the clinical picture and the diagnosis don't really fit, I think about getting a second biopsy before we finalize the diagnosis. To move on, when you first see a patient, what is the decision tree in your mind in terms of picking therapy or planning therapy, or what kind of things do you consider?
DR. MOSKOWITZ: The first thing I think about when deciding upon treatment for a patient with T-cell lymphoma is whether or not I plan to use a curative approach to therapy. As was mentioned by Michelle, this decision is partly based upon the patients’ comorbidities and age. For patients who are eligible for curative therapy, our frontline approach for the most common types of T-cell lymphoma is to use CHOP with or without etoposide followed by consideration for autologous stem cell transplant in first remission. There are certainly individuals for whom such an aggressive strategy would not be appropriate due to age or co-morbidities. In these individuals, we may consider CHOP-based therapy alone or sometimes even milder approaches aimed at disease control.
DR. HORWITZ: Andrei, are you similar in the CHOP/CHOEP paradigm? And if so, what do you think of those data? How do you decide between the two and when do you do that regimen versus something different?
DR. SHUSTOV: I think I will double or triple what Michelle and Alison just said; for me, the two most important decisions that I have to make in the first encounter with patients with newly diagnosed PTCL are: 1) whether we're going to pursue curative approach strategy; and 2) whether the patient can tolerate the intensity of treatments that would provide him/her with the best chance of cure or long term remission. Patients who are elderly and have high risk disease would be very hard to cure, especially considering that the consolidative transplant might carry high rates of morbidity or mortality; more conservative strategies might be appropriate in these cases. On the other hand, younger and more fit patients might benefit more from intensified initial regimens—ie, CHOEP—followed by high-dose therapy and either autologous or allogeneic stem cell transplant (ALLO).
I usually have a long initial discussion with patients and families during which we decide on the intent of treatment and what to expect from certain regimens in terms of toxicities. I typically choose CHOEP regimen (or infusional version, etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin [EPOCH]) for younger patients based on recent German data, even though this was a retrospective study and benefit of adding etoposide only approached statistical significance for the majority of PTCL subtypes.2 In older patients, I try to avoid anthracyclines, especially in the palliative intent setting, based on the retrospective analysis by the International T-Cell Lymphoma Project, and frequently use CEOP regimen (CHOEP minus anthracycline).3 I find that the majority of older patients tolerate this treatment somewhat better than anthracycline-containing combinations like CHOP.
In the very elderly and frail patients, I try to avoid combination chemotherapy all together. It is a somewhat easier decision for patients with AITL. Some of them are more indolent than other sub-types, and I would treat such patients with immunomodulatory approach, ie with a combination of prednisone and cyclosporine; then, I would consider single agent therapy with one of the recently approved agents for relapsed and refractory PTCL. I would also double what Michelle said in regard to making the best attempt to enroll patients into open clinical trials, because the current standards are not really satisfactory for many T-cell lymphoma patients.
DR. HORWITZ: It sounds like we all approach a new patient similarly. However, several of our up front trials are randomized against CHOP as opposed to CHOEP. When you're consenting a patient to those trials, how do you explain it to a patient or how important do you think the etoposide is?
DR. SHUSTOV: I share your frustration with quite a few of the current study designs for that exact reason. Some of these are confirmatory trials after conditional approvals of the new agents and are important. However, as often happens, in the confirmatory trials, we use controls that are a default from a lot of historical data, or the “common” way that the majority of patients are being treated. It is really a challenge when you consent patients to studies where a control arm is something that you think might not be quite adequate.
Having said that, the CHOEP study that was mentioned several times is a retrospective subgroup analysis and the addition of etoposide had marginal benefit that approached significance, but certainly was not a home run. I don't think we are ready to say that etoposide provides survival advantage in T-cell lymphoma patients and dismiss clinical trials in favor of just giving a patient the CHOEP, even though CHOP is admittedly not the best comparator. I discuss this controversy with patients and tell them frankly that the data that we base addition of etoposide on are not the best evidence one may have. Then, I ask patients to decide which approach sounds more reasonable to them and they make their choice.
DR. FANALE: To expand just slightly upon what Andrei said, I do emphasize to the patient and to the referring doctors that if you look at National Comprehensive Cancer Network guidelines, CHOP, CHOEP, EPOCH, all of these are potential options. So there's really not yet one trial that would say that one is clearly superior to the other at this time. I also emphasize to the patient and to the referring clinician that the only way, let's say, the patient could get the targeted agent plus the chemotherapy and have that 50% chance of, potentially, receiving that combination is really through the randomized clinical trial.
DR. HORWITZ: That was excellent, thank you. So you have alluded to this a bit. How much does subtype specific treatment come into play?
DR. MOSKOWITZ: At this point in time, outside of a clinical trial, for PTCL-NOS, AITL, and ALCL, the front-line approach would typically be CHOEP followed by autologous stem cell transplant. As Michelle mentioned, for a patient with ALCL, I would offer enrollment on the ECHELON-2 study in which patients are randomized to CHOP or BV-CHP, in order to give patients the option of potentially receiving BV.
For AITL, we are now obtaining molecular testing for certain mutations, particularly IDH2, because we currently have a study open with an IDH2 inhibitor that specifically enrolls patients with IDH2-mutant AITL. Because the testing takes some time to get back, we typically test patients’ biopsies at the time of diagnosis, so that we know if they're eligible for future clinical trials in case their disease does not respond to upfront therapy.
There are T-cell lymphoma subtypes that we treat quite differently from the entities discussed so far. For human T-lymphotropic virus-1 associated adult T-cell lymphoma, for example, the data with CHOP are really not good and we typically used EPOCH with the aim to consolidate with an allogeneic stem cell transplant.
Likewise, for hepatosplenic T-cell lymphoma, we have not observed good responses with the CHOP-based therapy and therefore we typically use platinum-based or ifosfamide-based therapy upfront with the aim to consolidate with allogeneic stem cell transplant. Extranodal NK/T-cell lymphoma is another entity for which CHOP is typically not effective and we have adopted asparaginase-based regimens, such as dexamethasone, methotrexate, ifosfamide, L-asparaginase, etoposide (SMILE) for this disease.
DR. SHUSTOV: I completely agree with Alison; for more common nodal lymphomas it is really hard at this point to base a treatment decision on histology, at least, in the front-line setting. However, some of the rare and unique subtypes, we generally treat with a completely different approach (ie, extranodal NK-cell lymphomas, enteropathy-associated T-cell lymphomas, adult T-cell leukemia/lymphomas).
DR. HORWITZ: Excellent. I’m curious, in a patient with ALCL who refused randomization of the ECHELON-2 trial, would you give them BV-CHP off study or would you discuss that as an alternative were they not to participate in the trial?
DR. FANALE: No. I don't urge treatment that's not approved for a particular line of therapy off clinical protocol with the exception that, let's say, if a patient is not a candidate for chemotherapy in second line setting for a particular lymphoma such as Hodgkin lymphoma—then, I would reference published data like Alison's data for a second line use of BV say for classical Hodgkin lymphoma and work with the insurance company to get the drug approved for that patient population, but if a patient just doesn't want to go on trial because they don't like the 50/50 chance of receiving BV-CHP compared to CHOP and they say, “I really want the 100% chance. Why don't you just contact my insurance?” I will explain to them the rationale of why the trial is being conducted including the hope that if the endpoints are met and the regimen is approved for front-line therapy then patients in the future can get BV-CHP at any oncology clinic, but I tell them for now it is a protocol based treatment option.
DR. HORWITZ: That's a very good answer. Can we now touch on the idea of transplant vs no transplant in first remission. Always, sometimes, never; how do you all think about that?
DR. SHUSTOV: I think that the idea of consolidative transplant is very heavily debated and discussed at the majority of specialized PTCL meetings. The reason for that is controversial nature of current clinical evidence. In the absence of randomized trials it is very hard to compare prospective phase II trial data to historical controls. You can interpret this in two ways; you might say, well, these are good data, outcome numbers look better than historical controls, and all patients with PTCL should have a consolidative transplant; or you can say, well, I don't have randomized data, and only a randomized study would really tell us whether post-induction consolidative transplant improves survival.
That is why it is such a controversial subject, and we agree that the phase II perspective studies of transplantation are hampered by significant bias; patients who are able and willing to travel to academic centers, were more robust and able to tolerate high dose therapy. They also have chemosensitive disease and that puts them in a completely different category than those you see in populational or retrospective studies, or other historical control trials.
Having said that, when I talk to patients, and I think that this is where I involve patients into treatment decision very heavily, I present them with the data and say that it is not wrong to do or not do the transplant in first CR. I make patients and family a big part of the decision. When they ask me what would give the patient the best chance being in remission five years from today, my answer is transplant, however, the data are not perfect, and the curative potential of autologous transplant in PTCL is not known.
DR. FANALE: So typically here, we would refer most patients on for front-line consolidative autologous stem cell transplant, I think, some exceptions are the ones that Andrei already touched on. If we're seeing a patient who has PTCL-NOS and this patient is the very rare patient who has early stage disease, with really no significant risk factors, that patient, unless there were pathologic features that showed a higher level of aggressiveness as Alison commented on, this patient might be one where we might defer doing the consolidative stem cell transplant for particularly if the patient’s disease entered into remission very quickly, but this still is controversial.
I'm not sure how you practice within each of your centers, but another patient, or where we would potentially defer, is a patient with fairly limited nasal NK/T-cell lymphoma, a patient that we’ve treated, let's say, with the dexamethasone, etoposide, ifosfamide, carboplatin (DeVIC) chemotherapy regimen plus a concomitant high-dose radiation, we typically historically here have deferred doing consolidative stem cell transplant for that patient population.
In terms of what Andrei commented on for when we might consider an ALLO and for a frontline consolidation, here we typically would not. The exception to that rule is that I've had a couple of patients here who have PTCL with a secondary hemophagocytic lymphohistiocytosis syndrome and then, for those patients because the concern is that the autologous stem cell transplant probably wouldn't be enough and for those patients given that they have such a dismal prognosis from the secondary hemophagocytic lymphohistiocytosis, we send those patients on for an ALLO.
DR. MOSKOWITZ: To expand upon early stage extranodal NK/T-cell lymphoma, I agree with Michelle that we wouldn't use an autologous stem cell transplant in first remission. Typically, for patients with localized disease, we recommend 2 cycles of SMILE followed by involved field radiation. Our patients treated with this approach have typically done quite well without needing a transplant.
DR. HORWITZ: That was great. If there are no further comments on upfront therapy, we can move over to relapse setting. When you see a patient at relapse, what are your strategies and how do you approach that patient?
DR. MOSKOWITZ: The first question in my mind when a patient is either not responding to or relapsing following front-line therapy is: what is my ultimate goal? For a patient who is fit and refractory to CHOP or has relapsed after CHOP or autologous stem cell transplant, my ultimate goal would be to try to get them to an ALLO. My choice of treatment following front-line therapy depends upon whether or not we are aiming for an ALLO as well as whether a donor has been identified for the patient.
Usually, if a patient just relapsed following front-line therapy, we are not necessarily ready to proceed quickly to ALLO. This may be because a donor has not yet been identified or because the patients’ eligibility for an ALLO is not clear. In this situation, my choice of treatment would be something that can be continued, potentially for several months, while we get things in place for the transplant. Treatment options in this situation would include the approved single agents for T-cell lymphoma, such as romidepsin, belinostat, pralatrexate, as well as BV (which would be specific for ALCL). In addition, I would consider enrollment on a promising clinical trial. Among these options, my choice of treatment typically depends upon the side effect profile and/or schedule, which is individualized for the patient. The one caveat would be that I typically will aim to use a histone deacetylase inhibitor (HDAC) inhibitor as my first choice for a patient with AITL.
Usually, if a patient just relapsed following front-line therapy, we are not necessarily ready to proceed quickly to ALLO. This may be because a donor has not yet been identified or because the patients’ eligibility for an ALLO is not clear. If my patient has a donor available and we are ready to move quickly to transplant, I would use one of the multi-agent regimens, such as ifosfamide, carboplatin, etoposide (ICE) or cisplatin, cytosine arabinoside, dexamethasone (DHAP), with the aim to try to get them into remission and relatively quickly proceed to transplant. The problem with using one of these types of regimens for everyone in the second-line setting is that the treatment cannot be continued indefinitely due to cumulative toxicity; therefore we need to know that we are ready to proceed to transplant if we are going to use one of the more intense regimens.
DR. HORWITZ: How do the others approach relapse?
DR. FANALE: Generally, a lot of similarities with what Alison discussed. Typically, it's if we're going to be sending the patient on for consideration for ALLO, I think the favor would be to try to use a regimen that has a high CR rate knowing that there is a trend toward improved progression free survival for the patient who enters into CR when compared to those who did not. I think the only slight difference might be just the timing and the selection, so, typically, here, even if the patient doesn't actually have a definite established donor quite yet we'll typically favor a combination type of a treatment approach, so whether or not that would be a platinum-based regimen such as ICE, as Alison mentioned, or gemcitabine-based chemotherapy regimen or similar.
I would generally favor one of those options, usually, over a single-agent therapeutic approach just knowing that the CR rates, generally, with exception of BV for ALCL are, generally, ranging about the 10%–15% range. And then, of course, first priority would be if there is a clinical trial available whether that's with a doublet of targeted agents or with the targeted agent plus chemotherapy in the relapse setting, we would typically favor that approach for a patient being considered for a stem cell transplant, ALLO approach.
DR. SHUSTOV: I'll consolidate what Michelle and Alison just said. I think we're on the same page. To me, the most important decision (even more important in the relapse setting) is to figure out whether we are still going to try to cure the lymphoma or we will pursue a palliative approach for which now we have several treatment options. So again, I’d have a long discussion with the patient, whether we're going to try and cure the disease, or we will pursue a palliative approach.
If this is a curative approach strategy and we're heading toward ALLO, we would start searching for the donor immediately. The choice of salvage therapy depends on duration of first remission. Primary refractory disease patients who failed CHOP, CHOEP, or EPOCH outright or within 3 months, I typically consider using the single agent approach, because even ICE and DHAP type regimens don’t work well for these patients. I put my “money” into HDAC inhibitors or antifolates, or other targeted therapy, if this is anaplastic lymphoma.
For patients who had at least 6 months remission from the initial therapy, younger patients who are still fit, I agree, I would consider standard salvage regimens like DHAP, etoposide, methylprednisolone, high-dosecytarabine, cisplatin (ESHAP) and ICE, and then, follow this with allograft.
DR. HORWITZ: You have all talked about allografting, I have two questions. What is your best guess in terms of percentage of patients you see with relapsed T-cell lymphoma who actually undergo ALLO at some point and do you ever consider autologous transplantation in those who didn't have it as part of their initial therapy?
DR. SHUSTOV: It certainly depends on the type of the academic center that patients are referred to, and we all practice at academic institutions with very strong ALLO programs. In patients who we select for a curative approach (one half to two thirds) we will at least make the best effort to take them to allogeneic grafting. Patients might fall off this track if they don't respond to salvage therapies or develop significant treatment/disease-related complications, ie fungal infections, organ toxicities, poor performance status, etc. Overall, I'd say that we successfully take about half of the patients with relapsed PTCL that are willing to pursue curative approach to allograft.
In addition, I consider autologous stem cell transplant in relapse setting for three situations. One is relapsed ALK-positive ALCL, some patients with ALK-negative ALCL who have had long initial remission and after relapse, and achieved second remission with BV or other salvage therapy. Finally, I’d consider auto-transplant in patients with AITL who achieved second CR with salvage therapy.
DR. HORWITZ: In terms of the relapse setting—some of you talked about angioimmunoblastic and HDAC inhibitors or BV for ALCL—when you have a relapse patient with PTCL-NOS, how do you pick a second line agent if you're not going directly to transplant, and are there new drugs that you're particularly excited about?
DR. FANALE: To answer your questions in terms of how I might choose a targeted agent for a patient who has PTCL-NOS and doesn't have AITL or ALCL, if the patient isn't eligible for trial or trial isn't feasible for that patient, I'll typically look at schedule, and then I'll look at side effect profile. For instance, for comparison, there are two HDAC inhibitor drugs right now approved, romidepsin and belinostat, so I think that potentially in a private practice setting, belinostat might be one when you look at the efficacy, being generally, around equivalent on where a patient might prefer that regimen because of the fact that they come in for five days in a row and then, their treatment is done for that cycle.
At a larger referral center to which patients are often traveling back and forth, it can be quite difficult for a patient to stay in town to do five days of treatment in a row. Often, these patients will prefer the romidepsin schedule because of the fact that they're coming back in once per week for three weeks in a row per cycle. The other way that I'll look at it is side effect profile, so, let's say, pralatrexate compared to the HDAC inhibitors, I will usually favor the HDAC inhibitors because of the potential side effect of mucositis with pralatrexate, although I have been able to manage through the mucositis by giving the patients more of a cutaneous T-cell lymphoma dosing format.
There had been a high level of interest in new emerging agents such as the aurora A-kinase inhibitors, alisertib, and that trial is now complete and the data are to be presented at The American Society of Hematology’s Annual Meeting this year.4 Other new therapies that are emerging are the PI3K inhibitors such as duvelisib and others. One advantage for many of the drugs in current trials is that they are oral agents.
My interest also lies in the combination approach, such as two or three targeted agent combinations, with the goal that if you see favorable CR rates and progression-free survival rates in the relapsed setting that you can then potentially move these treatments to the front-line setting to potentially move beyond the CHOP-based platform of therapies.
DR. SHUSTOV: For patients outside anaplastic lymphoma where, obviously, most of us would likely use BV, given the response rate to this agent, the decision is really based on convenience of the schedule, toxicity profile, and—sometimes—patient’s preference. So, toxicity and quality of life become kind of more decision-driving factors than disease biology. I’d have a discussion with patients about all three drugs, how they are administered, and what toxicities can be expected. Four-hour infusion of romidepsin might not be acceptable for some patients who have to travel long distances to treatment centers. They may prefer more rapid infusion (they would favor a trial of pralatrexate; or, as Alison mentioned, patient prefers a five-day/daily versus weekly administration.
It's kind of a coin toss decision outside clinical trials. Certainly, participation in studies in relapse setting is the high priority. We are all really looking forward to developing doublet combinations of novel agents, ie studies like the one Michelle is running at MD Anderson with a combination of alisertib and romidepsin.
DR. MOSKOWITZ: I'll echo what both Andrei and Michelle said that our choice of treatment is individualized and depends upon side-effect profile and the schedule. With regard to novel agents, I am also very excited about the studies that will be opening with the PI-3 kinase inhibitor, IPI-145. There have been promising results seen with single-agent IPI-1455 and we will be opening a study evaluating it in combination with bortezomib or romidepsin.
In addition, I mentioned earlier the IDH2 inhibitor, which we're studying right now in patients with AITL that is characterized by the presence of the IDH2 mutation. It would be exciting to see if targeting a specific mutation in this disease translates into responses.
DR. HORWITZ: Great, I think that was fantastic. I would like to thank Drs. Moskowitz, Shustov, and Fanale for a very thorough impractical discussion on how they approach and manage patients with T-cell lymphoma. I’m impressed that there is significant consistency among these experts in terms of how they manage these uncommon and often challenging lymphomas in terms of upfront combination chemotherapy approaches, combination considerations for the use of transplantation, as well as their enthusiasm for and dedication to incorporating clinical trials as part of everyday management.
References
1. Fanale MA, Horwitz SM, Forero-Torres A, et al. Brentuximab vedotin in the front-line treatment of patients with CD30+ peripheral T-cell lymphomas: results of a phase I study. J Clin Oncol. 2014;32(28):3137–3143.
2. Schmitz N, Trümper L, Ziepert M, et al. Treatment and prognosis of mature T-cell and NK-cell lymphoma: an analysis of patients with T-cell lymphoma treated in studies of the German High-Grade Non-Hodgkin Lymphoma Study Group. Blood. 2010;116(18):3418–3425. doi: 10.1182/blood-2010-02-270785. Epub 2010 Jul 21.
3. Vose J, Armitage J, Weisenburger D, for the International T-Cell Lymphoma Project. J Clin Oncol. 2008;26(25):4124–4130. doi: 10.1200/JCO.2008.16.4558. Epub 2008 Jul 14.
4. O’Connor OA, Ozcan M, Jacobsen ED, et al. First multicenter, randomized phase 3 study in patients with relapsed/refractory peripheral T-cell lymphoma: alisertib versus investigator’s choice. Paper to be presented at: American Society of Hematology 57th Annual Meeting & Exposition; December 5–8, 2015; Orlando, FL.
5. Horwitz SM, Porcu P, Flinn I, et al. Duvelisib (IPI-145), a phosphoinositide-3-kinsae-γδ inhibitor, shows activity in patients with relapsed/refractory T-cell lymphoma. Paper presented at: American Society of Hematology 56th Annual Meeting & Exposition; December 6–9, 2014; San Francisco, CA.
Moderator: Steven M. Horwitz, MD1
Discussants: Alison Moskowitz, MD1; Michelle Fanale, MD2; Andrei Shustov, MD3
From Memorial Sloan Kettering Cancer Center, New York, NY1; MD Anderson Cancer Center, Houston, TX2; University of Washington Medical Center, Seattle, WA3
Address for correspondence: Steven M. Horwitz, MD, Memorial Sloan Kettering Cancer Center, 1275 York Avenue, New York, NY 10065
E-mail: [email protected]
DR. HORWITZ: My name is Dr. Steven Horwitz from Memorial Sloan-Kettering Cancer Center. I’m joined today by Drs. Alison Moskowitz, Memorial Sloan-Kettering, Michelle Fanale of MD Anderson, and Andrei Shustov, University of Washington in Seattle. Thank you all for joining us for this conversation on T-cell lymphoma. My colleagues are all well known as experts in T-cell lymphomas. Those of you who treat these diseases recognize the systemic T-cell lymphomas as one of our greater challenges in hematologic malignancies in terms of the treatment options for patients and the frequent lack of definitive data to guide our decisions.
I thought what we would do today is have a very practical discussion about the way we think about these diseases, the decisions we make, and the way we make those decisions. I'll start off by asking when you get a referral of a patient with a new diagnosis of peripheral T-cell lymphoma (PTCL), what are some of the basic things that you first think about in terms of approaching a new patient?
DR. SHUSTOV: I think one of the biggest challenges in T-cell lymphomas still remains making the proper diagnosis. In general, pathologists in the United States have a pretty good idea when they see T-cell lymphomas, however, subclassification remains a challenge even for expert hematopathologists due to frequent histologic overlap between the subtypes of PTCL, and even with non-malignant autoimmune disorders. I frequently see patients who are diagnosed with or misdiagnosed with a different subtype of T-cell lymphoma. The most challenging is differentiation between angioimmunoblastic T-cell lymphoma (AITL), anaplastic large cell T-cell lymphoma (ALCL), and PTCL not otherwise specified (PTCL-NOS), especially when the latter patients have high expression of CD30 and/or bear features resembling AITL.
Sometimes they are a slam-dunk diagnosis, but frequently our hematopathologists reverse the diagnosis after doing additional studies on the biopsy material. The most recent case I've seen in my clinic for consultation was a patient with a diagnosis of extranodal NK-cell lymphoma that was reclassified as a gamma-delta T-cell lymphoma after additional work-up. I truly believe that it is advisable that majority, if not all PTCL cases are reviewed by expert hematopathology teams at academic centers that see large volumes of these cases.
I think it's very important to educate community physicians and patients about a proper PTCL diagnosis, especially now that more targeted therapies are being developed and the gene expression profiling techniques will probably lead to identification of specific pathways that are amenable to therapy with specific biologic agents.
DR. FANALE: I'd like to expand upon what Andrei just said. For me the next step after confirming the pathology diagnosis is to think about two things. To think about whether or not this patient is a patient who might be eligible for an ongoing front-line trial, typically if the patient meets eligibility criteria for one of our ongoing front-line trials I would really recommend to the patient to consider being enrolled in that trial, and I also think about whether the patient, if he or she enters into remission with front-line therapy, can be considered for a consolidative autologous stem cell transplant.
I think it's very important to educate community physicians and patients about a proper PTCL diagnosis. Right now, our ongoing front-line trial is the ECHELON-2 trial which is evaluating brentuximab vedotin (BV) plus cyclophosphamide, doxorubicin, prednisone (CHP [BV-CHP]) chemotherapy compared to standard of care cyclophosphamide, doxorubicin, vincristine, prednisone (CHOP) chemotherapy, and that trial is based on the promising data that we've seen in the initial phase I trial that combined BV plus CHP chemotherapy followed by maintenance BV which demonstrated both high and durable complete remission (CR) rates.1 BV is an antibody drug conjugate that's targeted at CD30 and carries initial US Food and Drug Administration approval for patients who have relapsed or refractory ALCL which has a 100% level of CD30 positivity and then also has a National Comprehensive Cancer Network listing for treating other types of relapsed or refractory CD30 positive PTCL as well.
Also there is another upcoming front-line trial, which is to combine pralatrexate plus CHOP. If a patient isn't eligible for a clinical trial or it's just not feasible for that patient to enroll in a clinical trial, I will then look further at what would be potentially the best standard of care option for that patient.
I'll look at the patient's age and performance status and if they are generally less than age 65 or so and if otherwise well, I'll preferentially treat that patient with CHOEP which is CHOP plus etoposide. And then, for a patient who's either older than this or has multiple other comorbidities, I would treat that patient typically with CHOP alone.
DR. HORWITZ: Thank you, we'll go further into the selection of initial therapies but first circle back, I was curious as to how often your center comes to a different diagnosis than the referring center, and are there pitfalls you see that alert you to be suspicious of diagnosis.
DR. SHUSTOV: I'd say probably 10% of cases that we see at our center will be reclassified by our hematopathologists. In most cases, they do not necessarily reverse the diagnosis, but provide further clarification. It is occurring to me that in the community, pathologists are less likely to call the subtype of T-cell lymphoma and limit the report to the general description of T-cell lymphoprolipherative disorder, or state something like “consistent with T-cell lymphoma with features of AITL, or with features of anaplastic lymphoma.” I would admit though, that sometimes it's very difficult to identify the specific subtype of PTCL even in the expert hands; but I'd say these cases would constitute no more than 5% of PTCL patients.
DR. HORWITZ: And in your experience is it mostly fine tuning a T-cell lymphoma diagnosis, or do you see totally different diagnoses?
DR. MOSKOWITZ: Usually review by expert hematopathologists simply leads to fine-tuning the T-cell lymphoma diagnosis, however, I occasionally see significant changes in diagnosis. Often, alterations or clarification of a diagnosis are made possible only after we provide the pathologist with clinical history. For example, a lymph node biopsy may be interpreted as ALCL, however the knowledge that the particular patient has a history of mycosis fungoides would lead the pathologist to consider the diagnosis of large cell transformation of mycosis fungoides rather than ALCL. In such a case, molecular studies are helpful in confirming that the lymph node findings originate from the same clone as that in the original mycosis fungoides lesions, rather than representing a second primary.
DR. FANALE: Very occasionally, I've seen patients truly “with a misdiagnosis and a complete revision of diagnosis” and, usually, those pitfalls that I've seen them occasionally have been the young patients—the young patients who generally have disease in the thorax and neck, who are treated as though they have classical Hodgkin lymphoma, who have very significant progression of disease on standard of care treatment. So it's important that not all cases that have CD30 positivity are classical Hodgkin lymphoma even if the patient is young.
DR. HORWITZ: I often think the systemic T-cell lymphomas usually behave in an aggressive fashion so when the clinical picture and the diagnosis don't really fit, I think about getting a second biopsy before we finalize the diagnosis. Of course, when people are ill with obviously progressing disease, you may need to move more quickly.
I often think the systemic T-cell lymphomas usually behave in an aggressive fashion so when the clinical picture and the diagnosis don't really fit, I think about getting a second biopsy before we finalize the diagnosis. To move on, when you first see a patient, what is the decision tree in your mind in terms of picking therapy or planning therapy, or what kind of things do you consider?
DR. MOSKOWITZ: The first thing I think about when deciding upon treatment for a patient with T-cell lymphoma is whether or not I plan to use a curative approach to therapy. As was mentioned by Michelle, this decision is partly based upon the patients’ comorbidities and age. For patients who are eligible for curative therapy, our frontline approach for the most common types of T-cell lymphoma is to use CHOP with or without etoposide followed by consideration for autologous stem cell transplant in first remission. There are certainly individuals for whom such an aggressive strategy would not be appropriate due to age or co-morbidities. In these individuals, we may consider CHOP-based therapy alone or sometimes even milder approaches aimed at disease control.
DR. HORWITZ: Andrei, are you similar in the CHOP/CHOEP paradigm? And if so, what do you think of those data? How do you decide between the two and when do you do that regimen versus something different?
DR. SHUSTOV: I think I will double or triple what Michelle and Alison just said; for me, the two most important decisions that I have to make in the first encounter with patients with newly diagnosed PTCL are: 1) whether we're going to pursue curative approach strategy; and 2) whether the patient can tolerate the intensity of treatments that would provide him/her with the best chance of cure or long term remission. Patients who are elderly and have high risk disease would be very hard to cure, especially considering that the consolidative transplant might carry high rates of morbidity or mortality; more conservative strategies might be appropriate in these cases. On the other hand, younger and more fit patients might benefit more from intensified initial regimens—ie, CHOEP—followed by high-dose therapy and either autologous or allogeneic stem cell transplant (ALLO).
I usually have a long initial discussion with patients and families during which we decide on the intent of treatment and what to expect from certain regimens in terms of toxicities. I typically choose CHOEP regimen (or infusional version, etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin [EPOCH]) for younger patients based on recent German data, even though this was a retrospective study and benefit of adding etoposide only approached statistical significance for the majority of PTCL subtypes.2 In older patients, I try to avoid anthracyclines, especially in the palliative intent setting, based on the retrospective analysis by the International T-Cell Lymphoma Project, and frequently use CEOP regimen (CHOEP minus anthracycline).3 I find that the majority of older patients tolerate this treatment somewhat better than anthracycline-containing combinations like CHOP.
In the very elderly and frail patients, I try to avoid combination chemotherapy all together. It is a somewhat easier decision for patients with AITL. Some of them are more indolent than other sub-types, and I would treat such patients with immunomodulatory approach, ie with a combination of prednisone and cyclosporine; then, I would consider single agent therapy with one of the recently approved agents for relapsed and refractory PTCL. I would also double what Michelle said in regard to making the best attempt to enroll patients into open clinical trials, because the current standards are not really satisfactory for many T-cell lymphoma patients.
DR. HORWITZ: It sounds like we all approach a new patient similarly. However, several of our up front trials are randomized against CHOP as opposed to CHOEP. When you're consenting a patient to those trials, how do you explain it to a patient or how important do you think the etoposide is?
DR. SHUSTOV: I share your frustration with quite a few of the current study designs for that exact reason. Some of these are confirmatory trials after conditional approvals of the new agents and are important. However, as often happens, in the confirmatory trials, we use controls that are a default from a lot of historical data, or the “common” way that the majority of patients are being treated. It is really a challenge when you consent patients to studies where a control arm is something that you think might not be quite adequate.
Having said that, the CHOEP study that was mentioned several times is a retrospective subgroup analysis and the addition of etoposide had marginal benefit that approached significance, but certainly was not a home run. I don't think we are ready to say that etoposide provides survival advantage in T-cell lymphoma patients and dismiss clinical trials in favor of just giving a patient the CHOEP, even though CHOP is admittedly not the best comparator. I discuss this controversy with patients and tell them frankly that the data that we base addition of etoposide on are not the best evidence one may have. Then, I ask patients to decide which approach sounds more reasonable to them and they make their choice.
DR. FANALE: To expand just slightly upon what Andrei said, I do emphasize to the patient and to the referring doctors that if you look at National Comprehensive Cancer Network guidelines, CHOP, CHOEP, EPOCH, all of these are potential options. So there's really not yet one trial that would say that one is clearly superior to the other at this time. I also emphasize to the patient and to the referring clinician that the only way, let's say, the patient could get the targeted agent plus the chemotherapy and have that 50% chance of, potentially, receiving that combination is really through the randomized clinical trial.
DR. HORWITZ: That was excellent, thank you. So you have alluded to this a bit. How much does subtype specific treatment come into play?
DR. MOSKOWITZ: At this point in time, outside of a clinical trial, for PTCL-NOS, AITL, and ALCL, the front-line approach would typically be CHOEP followed by autologous stem cell transplant. As Michelle mentioned, for a patient with ALCL, I would offer enrollment on the ECHELON-2 study in which patients are randomized to CHOP or BV-CHP, in order to give patients the option of potentially receiving BV.
For AITL, we are now obtaining molecular testing for certain mutations, particularly IDH2, because we currently have a study open with an IDH2 inhibitor that specifically enrolls patients with IDH2-mutant AITL. Because the testing takes some time to get back, we typically test patients’ biopsies at the time of diagnosis, so that we know if they're eligible for future clinical trials in case their disease does not respond to upfront therapy.
There are T-cell lymphoma subtypes that we treat quite differently from the entities discussed so far. For human T-lymphotropic virus-1 associated adult T-cell lymphoma, for example, the data with CHOP are really not good and we typically used EPOCH with the aim to consolidate with an allogeneic stem cell transplant.
Likewise, for hepatosplenic T-cell lymphoma, we have not observed good responses with the CHOP-based therapy and therefore we typically use platinum-based or ifosfamide-based therapy upfront with the aim to consolidate with allogeneic stem cell transplant. Extranodal NK/T-cell lymphoma is another entity for which CHOP is typically not effective and we have adopted asparaginase-based regimens, such as dexamethasone, methotrexate, ifosfamide, L-asparaginase, etoposide (SMILE) for this disease.
DR. SHUSTOV: I completely agree with Alison; for more common nodal lymphomas it is really hard at this point to base a treatment decision on histology, at least, in the front-line setting. However, some of the rare and unique subtypes, we generally treat with a completely different approach (ie, extranodal NK-cell lymphomas, enteropathy-associated T-cell lymphomas, adult T-cell leukemia/lymphomas).
DR. HORWITZ: Excellent. I’m curious, in a patient with ALCL who refused randomization of the ECHELON-2 trial, would you give them BV-CHP off study or would you discuss that as an alternative were they not to participate in the trial?
DR. FANALE: No. I don't urge treatment that's not approved for a particular line of therapy off clinical protocol with the exception that, let's say, if a patient is not a candidate for chemotherapy in second line setting for a particular lymphoma such as Hodgkin lymphoma—then, I would reference published data like Alison's data for a second line use of BV say for classical Hodgkin lymphoma and work with the insurance company to get the drug approved for that patient population, but if a patient just doesn't want to go on trial because they don't like the 50/50 chance of receiving BV-CHP compared to CHOP and they say, “I really want the 100% chance. Why don't you just contact my insurance?” I will explain to them the rationale of why the trial is being conducted including the hope that if the endpoints are met and the regimen is approved for front-line therapy then patients in the future can get BV-CHP at any oncology clinic, but I tell them for now it is a protocol based treatment option.
DR. HORWITZ: That's a very good answer. Can we now touch on the idea of transplant vs no transplant in first remission. Always, sometimes, never; how do you all think about that?
DR. SHUSTOV: I think that the idea of consolidative transplant is very heavily debated and discussed at the majority of specialized PTCL meetings. The reason for that is controversial nature of current clinical evidence. In the absence of randomized trials it is very hard to compare prospective phase II trial data to historical controls. You can interpret this in two ways; you might say, well, these are good data, outcome numbers look better than historical controls, and all patients with PTCL should have a consolidative transplant; or you can say, well, I don't have randomized data, and only a randomized study would really tell us whether post-induction consolidative transplant improves survival.
That is why it is such a controversial subject, and we agree that the phase II perspective studies of transplantation are hampered by significant bias; patients who are able and willing to travel to academic centers, were more robust and able to tolerate high dose therapy. They also have chemosensitive disease and that puts them in a completely different category than those you see in populational or retrospective studies, or other historical control trials.
Having said that, when I talk to patients, and I think that this is where I involve patients into treatment decision very heavily, I present them with the data and say that it is not wrong to do or not do the transplant in first CR. I make patients and family a big part of the decision. When they ask me what would give the patient the best chance being in remission five years from today, my answer is transplant, however, the data are not perfect, and the curative potential of autologous transplant in PTCL is not known.
DR. FANALE: So typically here, we would refer most patients on for front-line consolidative autologous stem cell transplant, I think, some exceptions are the ones that Andrei already touched on. If we're seeing a patient who has PTCL-NOS and this patient is the very rare patient who has early stage disease, with really no significant risk factors, that patient, unless there were pathologic features that showed a higher level of aggressiveness as Alison commented on, this patient might be one where we might defer doing the consolidative stem cell transplant for particularly if the patient’s disease entered into remission very quickly, but this still is controversial.
I'm not sure how you practice within each of your centers, but another patient, or where we would potentially defer, is a patient with fairly limited nasal NK/T-cell lymphoma, a patient that we’ve treated, let's say, with the dexamethasone, etoposide, ifosfamide, carboplatin (DeVIC) chemotherapy regimen plus a concomitant high-dose radiation, we typically historically here have deferred doing consolidative stem cell transplant for that patient population.
In terms of what Andrei commented on for when we might consider an ALLO and for a frontline consolidation, here we typically would not. The exception to that rule is that I've had a couple of patients here who have PTCL with a secondary hemophagocytic lymphohistiocytosis syndrome and then, for those patients because the concern is that the autologous stem cell transplant probably wouldn't be enough and for those patients given that they have such a dismal prognosis from the secondary hemophagocytic lymphohistiocytosis, we send those patients on for an ALLO.
DR. MOSKOWITZ: To expand upon early stage extranodal NK/T-cell lymphoma, I agree with Michelle that we wouldn't use an autologous stem cell transplant in first remission. Typically, for patients with localized disease, we recommend 2 cycles of SMILE followed by involved field radiation. Our patients treated with this approach have typically done quite well without needing a transplant.
DR. HORWITZ: That was great. If there are no further comments on upfront therapy, we can move over to relapse setting. When you see a patient at relapse, what are your strategies and how do you approach that patient?
DR. MOSKOWITZ: The first question in my mind when a patient is either not responding to or relapsing following front-line therapy is: what is my ultimate goal? For a patient who is fit and refractory to CHOP or has relapsed after CHOP or autologous stem cell transplant, my ultimate goal would be to try to get them to an ALLO. My choice of treatment following front-line therapy depends upon whether or not we are aiming for an ALLO as well as whether a donor has been identified for the patient.
Usually, if a patient just relapsed following front-line therapy, we are not necessarily ready to proceed quickly to ALLO. This may be because a donor has not yet been identified or because the patients’ eligibility for an ALLO is not clear. In this situation, my choice of treatment would be something that can be continued, potentially for several months, while we get things in place for the transplant. Treatment options in this situation would include the approved single agents for T-cell lymphoma, such as romidepsin, belinostat, pralatrexate, as well as BV (which would be specific for ALCL). In addition, I would consider enrollment on a promising clinical trial. Among these options, my choice of treatment typically depends upon the side effect profile and/or schedule, which is individualized for the patient. The one caveat would be that I typically will aim to use a histone deacetylase inhibitor (HDAC) inhibitor as my first choice for a patient with AITL.
Usually, if a patient just relapsed following front-line therapy, we are not necessarily ready to proceed quickly to ALLO. This may be because a donor has not yet been identified or because the patients’ eligibility for an ALLO is not clear. If my patient has a donor available and we are ready to move quickly to transplant, I would use one of the multi-agent regimens, such as ifosfamide, carboplatin, etoposide (ICE) or cisplatin, cytosine arabinoside, dexamethasone (DHAP), with the aim to try to get them into remission and relatively quickly proceed to transplant. The problem with using one of these types of regimens for everyone in the second-line setting is that the treatment cannot be continued indefinitely due to cumulative toxicity; therefore we need to know that we are ready to proceed to transplant if we are going to use one of the more intense regimens.
DR. HORWITZ: How do the others approach relapse?
DR. FANALE: Generally, a lot of similarities with what Alison discussed. Typically, it's if we're going to be sending the patient on for consideration for ALLO, I think the favor would be to try to use a regimen that has a high CR rate knowing that there is a trend toward improved progression free survival for the patient who enters into CR when compared to those who did not. I think the only slight difference might be just the timing and the selection, so, typically, here, even if the patient doesn't actually have a definite established donor quite yet we'll typically favor a combination type of a treatment approach, so whether or not that would be a platinum-based regimen such as ICE, as Alison mentioned, or gemcitabine-based chemotherapy regimen or similar.
I would generally favor one of those options, usually, over a single-agent therapeutic approach just knowing that the CR rates, generally, with exception of BV for ALCL are, generally, ranging about the 10%–15% range. And then, of course, first priority would be if there is a clinical trial available whether that's with a doublet of targeted agents or with the targeted agent plus chemotherapy in the relapse setting, we would typically favor that approach for a patient being considered for a stem cell transplant, ALLO approach.
DR. SHUSTOV: I'll consolidate what Michelle and Alison just said. I think we're on the same page. To me, the most important decision (even more important in the relapse setting) is to figure out whether we are still going to try to cure the lymphoma or we will pursue a palliative approach for which now we have several treatment options. So again, I’d have a long discussion with the patient, whether we're going to try and cure the disease, or we will pursue a palliative approach.
If this is a curative approach strategy and we're heading toward ALLO, we would start searching for the donor immediately. The choice of salvage therapy depends on duration of first remission. Primary refractory disease patients who failed CHOP, CHOEP, or EPOCH outright or within 3 months, I typically consider using the single agent approach, because even ICE and DHAP type regimens don’t work well for these patients. I put my “money” into HDAC inhibitors or antifolates, or other targeted therapy, if this is anaplastic lymphoma.
For patients who had at least 6 months remission from the initial therapy, younger patients who are still fit, I agree, I would consider standard salvage regimens like DHAP, etoposide, methylprednisolone, high-dosecytarabine, cisplatin (ESHAP) and ICE, and then, follow this with allograft.
DR. HORWITZ: You have all talked about allografting, I have two questions. What is your best guess in terms of percentage of patients you see with relapsed T-cell lymphoma who actually undergo ALLO at some point and do you ever consider autologous transplantation in those who didn't have it as part of their initial therapy?
DR. SHUSTOV: It certainly depends on the type of the academic center that patients are referred to, and we all practice at academic institutions with very strong ALLO programs. In patients who we select for a curative approach (one half to two thirds) we will at least make the best effort to take them to allogeneic grafting. Patients might fall off this track if they don't respond to salvage therapies or develop significant treatment/disease-related complications, ie fungal infections, organ toxicities, poor performance status, etc. Overall, I'd say that we successfully take about half of the patients with relapsed PTCL that are willing to pursue curative approach to allograft.
In addition, I consider autologous stem cell transplant in relapse setting for three situations. One is relapsed ALK-positive ALCL, some patients with ALK-negative ALCL who have had long initial remission and after relapse, and achieved second remission with BV or other salvage therapy. Finally, I’d consider auto-transplant in patients with AITL who achieved second CR with salvage therapy.
DR. HORWITZ: In terms of the relapse setting—some of you talked about angioimmunoblastic and HDAC inhibitors or BV for ALCL—when you have a relapse patient with PTCL-NOS, how do you pick a second line agent if you're not going directly to transplant, and are there new drugs that you're particularly excited about?
DR. FANALE: To answer your questions in terms of how I might choose a targeted agent for a patient who has PTCL-NOS and doesn't have AITL or ALCL, if the patient isn't eligible for trial or trial isn't feasible for that patient, I'll typically look at schedule, and then I'll look at side effect profile. For instance, for comparison, there are two HDAC inhibitor drugs right now approved, romidepsin and belinostat, so I think that potentially in a private practice setting, belinostat might be one when you look at the efficacy, being generally, around equivalent on where a patient might prefer that regimen because of the fact that they come in for five days in a row and then, their treatment is done for that cycle.
At a larger referral center to which patients are often traveling back and forth, it can be quite difficult for a patient to stay in town to do five days of treatment in a row. Often, these patients will prefer the romidepsin schedule because of the fact that they're coming back in once per week for three weeks in a row per cycle. The other way that I'll look at it is side effect profile, so, let's say, pralatrexate compared to the HDAC inhibitors, I will usually favor the HDAC inhibitors because of the potential side effect of mucositis with pralatrexate, although I have been able to manage through the mucositis by giving the patients more of a cutaneous T-cell lymphoma dosing format.
There had been a high level of interest in new emerging agents such as the aurora A-kinase inhibitors, alisertib, and that trial is now complete and the data are to be presented at The American Society of Hematology’s Annual Meeting this year.4 Other new therapies that are emerging are the PI3K inhibitors such as duvelisib and others. One advantage for many of the drugs in current trials is that they are oral agents.
My interest also lies in the combination approach, such as two or three targeted agent combinations, with the goal that if you see favorable CR rates and progression-free survival rates in the relapsed setting that you can then potentially move these treatments to the front-line setting to potentially move beyond the CHOP-based platform of therapies.
DR. SHUSTOV: For patients outside anaplastic lymphoma where, obviously, most of us would likely use BV, given the response rate to this agent, the decision is really based on convenience of the schedule, toxicity profile, and—sometimes—patient’s preference. So, toxicity and quality of life become kind of more decision-driving factors than disease biology. I’d have a discussion with patients about all three drugs, how they are administered, and what toxicities can be expected. Four-hour infusion of romidepsin might not be acceptable for some patients who have to travel long distances to treatment centers. They may prefer more rapid infusion (they would favor a trial of pralatrexate; or, as Alison mentioned, patient prefers a five-day/daily versus weekly administration.
It's kind of a coin toss decision outside clinical trials. Certainly, participation in studies in relapse setting is the high priority. We are all really looking forward to developing doublet combinations of novel agents, ie studies like the one Michelle is running at MD Anderson with a combination of alisertib and romidepsin.
DR. MOSKOWITZ: I'll echo what both Andrei and Michelle said that our choice of treatment is individualized and depends upon side-effect profile and the schedule. With regard to novel agents, I am also very excited about the studies that will be opening with the PI-3 kinase inhibitor, IPI-145. There have been promising results seen with single-agent IPI-1455 and we will be opening a study evaluating it in combination with bortezomib or romidepsin.
In addition, I mentioned earlier the IDH2 inhibitor, which we're studying right now in patients with AITL that is characterized by the presence of the IDH2 mutation. It would be exciting to see if targeting a specific mutation in this disease translates into responses.
DR. HORWITZ: Great, I think that was fantastic. I would like to thank Drs. Moskowitz, Shustov, and Fanale for a very thorough impractical discussion on how they approach and manage patients with T-cell lymphoma. I’m impressed that there is significant consistency among these experts in terms of how they manage these uncommon and often challenging lymphomas in terms of upfront combination chemotherapy approaches, combination considerations for the use of transplantation, as well as their enthusiasm for and dedication to incorporating clinical trials as part of everyday management.
References
1. Fanale MA, Horwitz SM, Forero-Torres A, et al. Brentuximab vedotin in the front-line treatment of patients with CD30+ peripheral T-cell lymphomas: results of a phase I study. J Clin Oncol. 2014;32(28):3137–3143.
2. Schmitz N, Trümper L, Ziepert M, et al. Treatment and prognosis of mature T-cell and NK-cell lymphoma: an analysis of patients with T-cell lymphoma treated in studies of the German High-Grade Non-Hodgkin Lymphoma Study Group. Blood. 2010;116(18):3418–3425. doi: 10.1182/blood-2010-02-270785. Epub 2010 Jul 21.
3. Vose J, Armitage J, Weisenburger D, for the International T-Cell Lymphoma Project. J Clin Oncol. 2008;26(25):4124–4130. doi: 10.1200/JCO.2008.16.4558. Epub 2008 Jul 14.
4. O’Connor OA, Ozcan M, Jacobsen ED, et al. First multicenter, randomized phase 3 study in patients with relapsed/refractory peripheral T-cell lymphoma: alisertib versus investigator’s choice. Paper to be presented at: American Society of Hematology 57th Annual Meeting & Exposition; December 5–8, 2015; Orlando, FL.
5. Horwitz SM, Porcu P, Flinn I, et al. Duvelisib (IPI-145), a phosphoinositide-3-kinsae-γδ inhibitor, shows activity in patients with relapsed/refractory T-cell lymphoma. Paper presented at: American Society of Hematology 56th Annual Meeting & Exposition; December 6–9, 2014; San Francisco, CA.
Moderator: Steven M. Horwitz, MD1
Discussants: Alison Moskowitz, MD1; Michelle Fanale, MD2; Andrei Shustov, MD3
From Memorial Sloan Kettering Cancer Center, New York, NY1; MD Anderson Cancer Center, Houston, TX2; University of Washington Medical Center, Seattle, WA3
Address for correspondence: Steven M. Horwitz, MD, Memorial Sloan Kettering Cancer Center, 1275 York Avenue, New York, NY 10065
E-mail: [email protected]
DR. HORWITZ: My name is Dr. Steven Horwitz from Memorial Sloan-Kettering Cancer Center. I’m joined today by Drs. Alison Moskowitz, Memorial Sloan-Kettering, Michelle Fanale of MD Anderson, and Andrei Shustov, University of Washington in Seattle. Thank you all for joining us for this conversation on T-cell lymphoma. My colleagues are all well known as experts in T-cell lymphomas. Those of you who treat these diseases recognize the systemic T-cell lymphomas as one of our greater challenges in hematologic malignancies in terms of the treatment options for patients and the frequent lack of definitive data to guide our decisions.
I thought what we would do today is have a very practical discussion about the way we think about these diseases, the decisions we make, and the way we make those decisions. I'll start off by asking when you get a referral of a patient with a new diagnosis of peripheral T-cell lymphoma (PTCL), what are some of the basic things that you first think about in terms of approaching a new patient?
DR. SHUSTOV: I think one of the biggest challenges in T-cell lymphomas still remains making the proper diagnosis. In general, pathologists in the United States have a pretty good idea when they see T-cell lymphomas, however, subclassification remains a challenge even for expert hematopathologists due to frequent histologic overlap between the subtypes of PTCL, and even with non-malignant autoimmune disorders. I frequently see patients who are diagnosed with or misdiagnosed with a different subtype of T-cell lymphoma. The most challenging is differentiation between angioimmunoblastic T-cell lymphoma (AITL), anaplastic large cell T-cell lymphoma (ALCL), and PTCL not otherwise specified (PTCL-NOS), especially when the latter patients have high expression of CD30 and/or bear features resembling AITL.
Sometimes they are a slam-dunk diagnosis, but frequently our hematopathologists reverse the diagnosis after doing additional studies on the biopsy material. The most recent case I've seen in my clinic for consultation was a patient with a diagnosis of extranodal NK-cell lymphoma that was reclassified as a gamma-delta T-cell lymphoma after additional work-up. I truly believe that it is advisable that majority, if not all PTCL cases are reviewed by expert hematopathology teams at academic centers that see large volumes of these cases.
I think it's very important to educate community physicians and patients about a proper PTCL diagnosis, especially now that more targeted therapies are being developed and the gene expression profiling techniques will probably lead to identification of specific pathways that are amenable to therapy with specific biologic agents.
DR. FANALE: I'd like to expand upon what Andrei just said. For me the next step after confirming the pathology diagnosis is to think about two things. To think about whether or not this patient is a patient who might be eligible for an ongoing front-line trial, typically if the patient meets eligibility criteria for one of our ongoing front-line trials I would really recommend to the patient to consider being enrolled in that trial, and I also think about whether the patient, if he or she enters into remission with front-line therapy, can be considered for a consolidative autologous stem cell transplant.
I think it's very important to educate community physicians and patients about a proper PTCL diagnosis. Right now, our ongoing front-line trial is the ECHELON-2 trial which is evaluating brentuximab vedotin (BV) plus cyclophosphamide, doxorubicin, prednisone (CHP [BV-CHP]) chemotherapy compared to standard of care cyclophosphamide, doxorubicin, vincristine, prednisone (CHOP) chemotherapy, and that trial is based on the promising data that we've seen in the initial phase I trial that combined BV plus CHP chemotherapy followed by maintenance BV which demonstrated both high and durable complete remission (CR) rates.1 BV is an antibody drug conjugate that's targeted at CD30 and carries initial US Food and Drug Administration approval for patients who have relapsed or refractory ALCL which has a 100% level of CD30 positivity and then also has a National Comprehensive Cancer Network listing for treating other types of relapsed or refractory CD30 positive PTCL as well.
Also there is another upcoming front-line trial, which is to combine pralatrexate plus CHOP. If a patient isn't eligible for a clinical trial or it's just not feasible for that patient to enroll in a clinical trial, I will then look further at what would be potentially the best standard of care option for that patient.
I'll look at the patient's age and performance status and if they are generally less than age 65 or so and if otherwise well, I'll preferentially treat that patient with CHOEP which is CHOP plus etoposide. And then, for a patient who's either older than this or has multiple other comorbidities, I would treat that patient typically with CHOP alone.
DR. HORWITZ: Thank you, we'll go further into the selection of initial therapies but first circle back, I was curious as to how often your center comes to a different diagnosis than the referring center, and are there pitfalls you see that alert you to be suspicious of diagnosis.
DR. SHUSTOV: I'd say probably 10% of cases that we see at our center will be reclassified by our hematopathologists. In most cases, they do not necessarily reverse the diagnosis, but provide further clarification. It is occurring to me that in the community, pathologists are less likely to call the subtype of T-cell lymphoma and limit the report to the general description of T-cell lymphoprolipherative disorder, or state something like “consistent with T-cell lymphoma with features of AITL, or with features of anaplastic lymphoma.” I would admit though, that sometimes it's very difficult to identify the specific subtype of PTCL even in the expert hands; but I'd say these cases would constitute no more than 5% of PTCL patients.
DR. HORWITZ: And in your experience is it mostly fine tuning a T-cell lymphoma diagnosis, or do you see totally different diagnoses?
DR. MOSKOWITZ: Usually review by expert hematopathologists simply leads to fine-tuning the T-cell lymphoma diagnosis, however, I occasionally see significant changes in diagnosis. Often, alterations or clarification of a diagnosis are made possible only after we provide the pathologist with clinical history. For example, a lymph node biopsy may be interpreted as ALCL, however the knowledge that the particular patient has a history of mycosis fungoides would lead the pathologist to consider the diagnosis of large cell transformation of mycosis fungoides rather than ALCL. In such a case, molecular studies are helpful in confirming that the lymph node findings originate from the same clone as that in the original mycosis fungoides lesions, rather than representing a second primary.
DR. FANALE: Very occasionally, I've seen patients truly “with a misdiagnosis and a complete revision of diagnosis” and, usually, those pitfalls that I've seen them occasionally have been the young patients—the young patients who generally have disease in the thorax and neck, who are treated as though they have classical Hodgkin lymphoma, who have very significant progression of disease on standard of care treatment. So it's important that not all cases that have CD30 positivity are classical Hodgkin lymphoma even if the patient is young.
DR. HORWITZ: I often think the systemic T-cell lymphomas usually behave in an aggressive fashion so when the clinical picture and the diagnosis don't really fit, I think about getting a second biopsy before we finalize the diagnosis. Of course, when people are ill with obviously progressing disease, you may need to move more quickly.
I often think the systemic T-cell lymphomas usually behave in an aggressive fashion so when the clinical picture and the diagnosis don't really fit, I think about getting a second biopsy before we finalize the diagnosis. To move on, when you first see a patient, what is the decision tree in your mind in terms of picking therapy or planning therapy, or what kind of things do you consider?
DR. MOSKOWITZ: The first thing I think about when deciding upon treatment for a patient with T-cell lymphoma is whether or not I plan to use a curative approach to therapy. As was mentioned by Michelle, this decision is partly based upon the patients’ comorbidities and age. For patients who are eligible for curative therapy, our frontline approach for the most common types of T-cell lymphoma is to use CHOP with or without etoposide followed by consideration for autologous stem cell transplant in first remission. There are certainly individuals for whom such an aggressive strategy would not be appropriate due to age or co-morbidities. In these individuals, we may consider CHOP-based therapy alone or sometimes even milder approaches aimed at disease control.
DR. HORWITZ: Andrei, are you similar in the CHOP/CHOEP paradigm? And if so, what do you think of those data? How do you decide between the two and when do you do that regimen versus something different?
DR. SHUSTOV: I think I will double or triple what Michelle and Alison just said; for me, the two most important decisions that I have to make in the first encounter with patients with newly diagnosed PTCL are: 1) whether we're going to pursue curative approach strategy; and 2) whether the patient can tolerate the intensity of treatments that would provide him/her with the best chance of cure or long term remission. Patients who are elderly and have high risk disease would be very hard to cure, especially considering that the consolidative transplant might carry high rates of morbidity or mortality; more conservative strategies might be appropriate in these cases. On the other hand, younger and more fit patients might benefit more from intensified initial regimens—ie, CHOEP—followed by high-dose therapy and either autologous or allogeneic stem cell transplant (ALLO).
I usually have a long initial discussion with patients and families during which we decide on the intent of treatment and what to expect from certain regimens in terms of toxicities. I typically choose CHOEP regimen (or infusional version, etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin [EPOCH]) for younger patients based on recent German data, even though this was a retrospective study and benefit of adding etoposide only approached statistical significance for the majority of PTCL subtypes.2 In older patients, I try to avoid anthracyclines, especially in the palliative intent setting, based on the retrospective analysis by the International T-Cell Lymphoma Project, and frequently use CEOP regimen (CHOEP minus anthracycline).3 I find that the majority of older patients tolerate this treatment somewhat better than anthracycline-containing combinations like CHOP.
In the very elderly and frail patients, I try to avoid combination chemotherapy all together. It is a somewhat easier decision for patients with AITL. Some of them are more indolent than other sub-types, and I would treat such patients with immunomodulatory approach, ie with a combination of prednisone and cyclosporine; then, I would consider single agent therapy with one of the recently approved agents for relapsed and refractory PTCL. I would also double what Michelle said in regard to making the best attempt to enroll patients into open clinical trials, because the current standards are not really satisfactory for many T-cell lymphoma patients.
DR. HORWITZ: It sounds like we all approach a new patient similarly. However, several of our up front trials are randomized against CHOP as opposed to CHOEP. When you're consenting a patient to those trials, how do you explain it to a patient or how important do you think the etoposide is?
DR. SHUSTOV: I share your frustration with quite a few of the current study designs for that exact reason. Some of these are confirmatory trials after conditional approvals of the new agents and are important. However, as often happens, in the confirmatory trials, we use controls that are a default from a lot of historical data, or the “common” way that the majority of patients are being treated. It is really a challenge when you consent patients to studies where a control arm is something that you think might not be quite adequate.
Having said that, the CHOEP study that was mentioned several times is a retrospective subgroup analysis and the addition of etoposide had marginal benefit that approached significance, but certainly was not a home run. I don't think we are ready to say that etoposide provides survival advantage in T-cell lymphoma patients and dismiss clinical trials in favor of just giving a patient the CHOEP, even though CHOP is admittedly not the best comparator. I discuss this controversy with patients and tell them frankly that the data that we base addition of etoposide on are not the best evidence one may have. Then, I ask patients to decide which approach sounds more reasonable to them and they make their choice.
DR. FANALE: To expand just slightly upon what Andrei said, I do emphasize to the patient and to the referring doctors that if you look at National Comprehensive Cancer Network guidelines, CHOP, CHOEP, EPOCH, all of these are potential options. So there's really not yet one trial that would say that one is clearly superior to the other at this time. I also emphasize to the patient and to the referring clinician that the only way, let's say, the patient could get the targeted agent plus the chemotherapy and have that 50% chance of, potentially, receiving that combination is really through the randomized clinical trial.
DR. HORWITZ: That was excellent, thank you. So you have alluded to this a bit. How much does subtype specific treatment come into play?
DR. MOSKOWITZ: At this point in time, outside of a clinical trial, for PTCL-NOS, AITL, and ALCL, the front-line approach would typically be CHOEP followed by autologous stem cell transplant. As Michelle mentioned, for a patient with ALCL, I would offer enrollment on the ECHELON-2 study in which patients are randomized to CHOP or BV-CHP, in order to give patients the option of potentially receiving BV.
For AITL, we are now obtaining molecular testing for certain mutations, particularly IDH2, because we currently have a study open with an IDH2 inhibitor that specifically enrolls patients with IDH2-mutant AITL. Because the testing takes some time to get back, we typically test patients’ biopsies at the time of diagnosis, so that we know if they're eligible for future clinical trials in case their disease does not respond to upfront therapy.
There are T-cell lymphoma subtypes that we treat quite differently from the entities discussed so far. For human T-lymphotropic virus-1 associated adult T-cell lymphoma, for example, the data with CHOP are really not good and we typically used EPOCH with the aim to consolidate with an allogeneic stem cell transplant.
Likewise, for hepatosplenic T-cell lymphoma, we have not observed good responses with the CHOP-based therapy and therefore we typically use platinum-based or ifosfamide-based therapy upfront with the aim to consolidate with allogeneic stem cell transplant. Extranodal NK/T-cell lymphoma is another entity for which CHOP is typically not effective and we have adopted asparaginase-based regimens, such as dexamethasone, methotrexate, ifosfamide, L-asparaginase, etoposide (SMILE) for this disease.
DR. SHUSTOV: I completely agree with Alison; for more common nodal lymphomas it is really hard at this point to base a treatment decision on histology, at least, in the front-line setting. However, some of the rare and unique subtypes, we generally treat with a completely different approach (ie, extranodal NK-cell lymphomas, enteropathy-associated T-cell lymphomas, adult T-cell leukemia/lymphomas).
DR. HORWITZ: Excellent. I’m curious, in a patient with ALCL who refused randomization of the ECHELON-2 trial, would you give them BV-CHP off study or would you discuss that as an alternative were they not to participate in the trial?
DR. FANALE: No. I don't urge treatment that's not approved for a particular line of therapy off clinical protocol with the exception that, let's say, if a patient is not a candidate for chemotherapy in second line setting for a particular lymphoma such as Hodgkin lymphoma—then, I would reference published data like Alison's data for a second line use of BV say for classical Hodgkin lymphoma and work with the insurance company to get the drug approved for that patient population, but if a patient just doesn't want to go on trial because they don't like the 50/50 chance of receiving BV-CHP compared to CHOP and they say, “I really want the 100% chance. Why don't you just contact my insurance?” I will explain to them the rationale of why the trial is being conducted including the hope that if the endpoints are met and the regimen is approved for front-line therapy then patients in the future can get BV-CHP at any oncology clinic, but I tell them for now it is a protocol based treatment option.
DR. HORWITZ: That's a very good answer. Can we now touch on the idea of transplant vs no transplant in first remission. Always, sometimes, never; how do you all think about that?
DR. SHUSTOV: I think that the idea of consolidative transplant is very heavily debated and discussed at the majority of specialized PTCL meetings. The reason for that is controversial nature of current clinical evidence. In the absence of randomized trials it is very hard to compare prospective phase II trial data to historical controls. You can interpret this in two ways; you might say, well, these are good data, outcome numbers look better than historical controls, and all patients with PTCL should have a consolidative transplant; or you can say, well, I don't have randomized data, and only a randomized study would really tell us whether post-induction consolidative transplant improves survival.
That is why it is such a controversial subject, and we agree that the phase II perspective studies of transplantation are hampered by significant bias; patients who are able and willing to travel to academic centers, were more robust and able to tolerate high dose therapy. They also have chemosensitive disease and that puts them in a completely different category than those you see in populational or retrospective studies, or other historical control trials.
Having said that, when I talk to patients, and I think that this is where I involve patients into treatment decision very heavily, I present them with the data and say that it is not wrong to do or not do the transplant in first CR. I make patients and family a big part of the decision. When they ask me what would give the patient the best chance being in remission five years from today, my answer is transplant, however, the data are not perfect, and the curative potential of autologous transplant in PTCL is not known.
DR. FANALE: So typically here, we would refer most patients on for front-line consolidative autologous stem cell transplant, I think, some exceptions are the ones that Andrei already touched on. If we're seeing a patient who has PTCL-NOS and this patient is the very rare patient who has early stage disease, with really no significant risk factors, that patient, unless there were pathologic features that showed a higher level of aggressiveness as Alison commented on, this patient might be one where we might defer doing the consolidative stem cell transplant for particularly if the patient’s disease entered into remission very quickly, but this still is controversial.
I'm not sure how you practice within each of your centers, but another patient, or where we would potentially defer, is a patient with fairly limited nasal NK/T-cell lymphoma, a patient that we’ve treated, let's say, with the dexamethasone, etoposide, ifosfamide, carboplatin (DeVIC) chemotherapy regimen plus a concomitant high-dose radiation, we typically historically here have deferred doing consolidative stem cell transplant for that patient population.
In terms of what Andrei commented on for when we might consider an ALLO and for a frontline consolidation, here we typically would not. The exception to that rule is that I've had a couple of patients here who have PTCL with a secondary hemophagocytic lymphohistiocytosis syndrome and then, for those patients because the concern is that the autologous stem cell transplant probably wouldn't be enough and for those patients given that they have such a dismal prognosis from the secondary hemophagocytic lymphohistiocytosis, we send those patients on for an ALLO.
DR. MOSKOWITZ: To expand upon early stage extranodal NK/T-cell lymphoma, I agree with Michelle that we wouldn't use an autologous stem cell transplant in first remission. Typically, for patients with localized disease, we recommend 2 cycles of SMILE followed by involved field radiation. Our patients treated with this approach have typically done quite well without needing a transplant.
DR. HORWITZ: That was great. If there are no further comments on upfront therapy, we can move over to relapse setting. When you see a patient at relapse, what are your strategies and how do you approach that patient?
DR. MOSKOWITZ: The first question in my mind when a patient is either not responding to or relapsing following front-line therapy is: what is my ultimate goal? For a patient who is fit and refractory to CHOP or has relapsed after CHOP or autologous stem cell transplant, my ultimate goal would be to try to get them to an ALLO. My choice of treatment following front-line therapy depends upon whether or not we are aiming for an ALLO as well as whether a donor has been identified for the patient.
Usually, if a patient just relapsed following front-line therapy, we are not necessarily ready to proceed quickly to ALLO. This may be because a donor has not yet been identified or because the patients’ eligibility for an ALLO is not clear. In this situation, my choice of treatment would be something that can be continued, potentially for several months, while we get things in place for the transplant. Treatment options in this situation would include the approved single agents for T-cell lymphoma, such as romidepsin, belinostat, pralatrexate, as well as BV (which would be specific for ALCL). In addition, I would consider enrollment on a promising clinical trial. Among these options, my choice of treatment typically depends upon the side effect profile and/or schedule, which is individualized for the patient. The one caveat would be that I typically will aim to use a histone deacetylase inhibitor (HDAC) inhibitor as my first choice for a patient with AITL.
Usually, if a patient just relapsed following front-line therapy, we are not necessarily ready to proceed quickly to ALLO. This may be because a donor has not yet been identified or because the patients’ eligibility for an ALLO is not clear. If my patient has a donor available and we are ready to move quickly to transplant, I would use one of the multi-agent regimens, such as ifosfamide, carboplatin, etoposide (ICE) or cisplatin, cytosine arabinoside, dexamethasone (DHAP), with the aim to try to get them into remission and relatively quickly proceed to transplant. The problem with using one of these types of regimens for everyone in the second-line setting is that the treatment cannot be continued indefinitely due to cumulative toxicity; therefore we need to know that we are ready to proceed to transplant if we are going to use one of the more intense regimens.
DR. HORWITZ: How do the others approach relapse?
DR. FANALE: Generally, a lot of similarities with what Alison discussed. Typically, it's if we're going to be sending the patient on for consideration for ALLO, I think the favor would be to try to use a regimen that has a high CR rate knowing that there is a trend toward improved progression free survival for the patient who enters into CR when compared to those who did not. I think the only slight difference might be just the timing and the selection, so, typically, here, even if the patient doesn't actually have a definite established donor quite yet we'll typically favor a combination type of a treatment approach, so whether or not that would be a platinum-based regimen such as ICE, as Alison mentioned, or gemcitabine-based chemotherapy regimen or similar.
I would generally favor one of those options, usually, over a single-agent therapeutic approach just knowing that the CR rates, generally, with exception of BV for ALCL are, generally, ranging about the 10%–15% range. And then, of course, first priority would be if there is a clinical trial available whether that's with a doublet of targeted agents or with the targeted agent plus chemotherapy in the relapse setting, we would typically favor that approach for a patient being considered for a stem cell transplant, ALLO approach.
DR. SHUSTOV: I'll consolidate what Michelle and Alison just said. I think we're on the same page. To me, the most important decision (even more important in the relapse setting) is to figure out whether we are still going to try to cure the lymphoma or we will pursue a palliative approach for which now we have several treatment options. So again, I’d have a long discussion with the patient, whether we're going to try and cure the disease, or we will pursue a palliative approach.
If this is a curative approach strategy and we're heading toward ALLO, we would start searching for the donor immediately. The choice of salvage therapy depends on duration of first remission. Primary refractory disease patients who failed CHOP, CHOEP, or EPOCH outright or within 3 months, I typically consider using the single agent approach, because even ICE and DHAP type regimens don’t work well for these patients. I put my “money” into HDAC inhibitors or antifolates, or other targeted therapy, if this is anaplastic lymphoma.
For patients who had at least 6 months remission from the initial therapy, younger patients who are still fit, I agree, I would consider standard salvage regimens like DHAP, etoposide, methylprednisolone, high-dosecytarabine, cisplatin (ESHAP) and ICE, and then, follow this with allograft.
DR. HORWITZ: You have all talked about allografting, I have two questions. What is your best guess in terms of percentage of patients you see with relapsed T-cell lymphoma who actually undergo ALLO at some point and do you ever consider autologous transplantation in those who didn't have it as part of their initial therapy?
DR. SHUSTOV: It certainly depends on the type of the academic center that patients are referred to, and we all practice at academic institutions with very strong ALLO programs. In patients who we select for a curative approach (one half to two thirds) we will at least make the best effort to take them to allogeneic grafting. Patients might fall off this track if they don't respond to salvage therapies or develop significant treatment/disease-related complications, ie fungal infections, organ toxicities, poor performance status, etc. Overall, I'd say that we successfully take about half of the patients with relapsed PTCL that are willing to pursue curative approach to allograft.
In addition, I consider autologous stem cell transplant in relapse setting for three situations. One is relapsed ALK-positive ALCL, some patients with ALK-negative ALCL who have had long initial remission and after relapse, and achieved second remission with BV or other salvage therapy. Finally, I’d consider auto-transplant in patients with AITL who achieved second CR with salvage therapy.
DR. HORWITZ: In terms of the relapse setting—some of you talked about angioimmunoblastic and HDAC inhibitors or BV for ALCL—when you have a relapse patient with PTCL-NOS, how do you pick a second line agent if you're not going directly to transplant, and are there new drugs that you're particularly excited about?
DR. FANALE: To answer your questions in terms of how I might choose a targeted agent for a patient who has PTCL-NOS and doesn't have AITL or ALCL, if the patient isn't eligible for trial or trial isn't feasible for that patient, I'll typically look at schedule, and then I'll look at side effect profile. For instance, for comparison, there are two HDAC inhibitor drugs right now approved, romidepsin and belinostat, so I think that potentially in a private practice setting, belinostat might be one when you look at the efficacy, being generally, around equivalent on where a patient might prefer that regimen because of the fact that they come in for five days in a row and then, their treatment is done for that cycle.
At a larger referral center to which patients are often traveling back and forth, it can be quite difficult for a patient to stay in town to do five days of treatment in a row. Often, these patients will prefer the romidepsin schedule because of the fact that they're coming back in once per week for three weeks in a row per cycle. The other way that I'll look at it is side effect profile, so, let's say, pralatrexate compared to the HDAC inhibitors, I will usually favor the HDAC inhibitors because of the potential side effect of mucositis with pralatrexate, although I have been able to manage through the mucositis by giving the patients more of a cutaneous T-cell lymphoma dosing format.
There had been a high level of interest in new emerging agents such as the aurora A-kinase inhibitors, alisertib, and that trial is now complete and the data are to be presented at The American Society of Hematology’s Annual Meeting this year.4 Other new therapies that are emerging are the PI3K inhibitors such as duvelisib and others. One advantage for many of the drugs in current trials is that they are oral agents.
My interest also lies in the combination approach, such as two or three targeted agent combinations, with the goal that if you see favorable CR rates and progression-free survival rates in the relapsed setting that you can then potentially move these treatments to the front-line setting to potentially move beyond the CHOP-based platform of therapies.
DR. SHUSTOV: For patients outside anaplastic lymphoma where, obviously, most of us would likely use BV, given the response rate to this agent, the decision is really based on convenience of the schedule, toxicity profile, and—sometimes—patient’s preference. So, toxicity and quality of life become kind of more decision-driving factors than disease biology. I’d have a discussion with patients about all three drugs, how they are administered, and what toxicities can be expected. Four-hour infusion of romidepsin might not be acceptable for some patients who have to travel long distances to treatment centers. They may prefer more rapid infusion (they would favor a trial of pralatrexate; or, as Alison mentioned, patient prefers a five-day/daily versus weekly administration.
It's kind of a coin toss decision outside clinical trials. Certainly, participation in studies in relapse setting is the high priority. We are all really looking forward to developing doublet combinations of novel agents, ie studies like the one Michelle is running at MD Anderson with a combination of alisertib and romidepsin.
DR. MOSKOWITZ: I'll echo what both Andrei and Michelle said that our choice of treatment is individualized and depends upon side-effect profile and the schedule. With regard to novel agents, I am also very excited about the studies that will be opening with the PI-3 kinase inhibitor, IPI-145. There have been promising results seen with single-agent IPI-1455 and we will be opening a study evaluating it in combination with bortezomib or romidepsin.
In addition, I mentioned earlier the IDH2 inhibitor, which we're studying right now in patients with AITL that is characterized by the presence of the IDH2 mutation. It would be exciting to see if targeting a specific mutation in this disease translates into responses.
DR. HORWITZ: Great, I think that was fantastic. I would like to thank Drs. Moskowitz, Shustov, and Fanale for a very thorough impractical discussion on how they approach and manage patients with T-cell lymphoma. I’m impressed that there is significant consistency among these experts in terms of how they manage these uncommon and often challenging lymphomas in terms of upfront combination chemotherapy approaches, combination considerations for the use of transplantation, as well as their enthusiasm for and dedication to incorporating clinical trials as part of everyday management.
References
1. Fanale MA, Horwitz SM, Forero-Torres A, et al. Brentuximab vedotin in the front-line treatment of patients with CD30+ peripheral T-cell lymphomas: results of a phase I study. J Clin Oncol. 2014;32(28):3137–3143.
2. Schmitz N, Trümper L, Ziepert M, et al. Treatment and prognosis of mature T-cell and NK-cell lymphoma: an analysis of patients with T-cell lymphoma treated in studies of the German High-Grade Non-Hodgkin Lymphoma Study Group. Blood. 2010;116(18):3418–3425. doi: 10.1182/blood-2010-02-270785. Epub 2010 Jul 21.
3. Vose J, Armitage J, Weisenburger D, for the International T-Cell Lymphoma Project. J Clin Oncol. 2008;26(25):4124–4130. doi: 10.1200/JCO.2008.16.4558. Epub 2008 Jul 14.
4. O’Connor OA, Ozcan M, Jacobsen ED, et al. First multicenter, randomized phase 3 study in patients with relapsed/refractory peripheral T-cell lymphoma: alisertib versus investigator’s choice. Paper to be presented at: American Society of Hematology 57th Annual Meeting & Exposition; December 5–8, 2015; Orlando, FL.
5. Horwitz SM, Porcu P, Flinn I, et al. Duvelisib (IPI-145), a phosphoinositide-3-kinsae-γδ inhibitor, shows activity in patients with relapsed/refractory T-cell lymphoma. Paper presented at: American Society of Hematology 56th Annual Meeting & Exposition; December 6–9, 2014; San Francisco, CA.