User login
No rise in complications with concomitant gynecologic cancer, PFD surgery
DENVER – Concomitantly treating pelvic floor disorders during surgery for gynecologic cancer does not increase the risk of postoperative complications, according to an analysis of 4 years of data from the American College of Surgeons’ National Surgical Quality Improvement Program.
Among 23,501 surgical gynecologic cancer patients, 556 (2.4%) underwent concomitant surgery for symptomatic pelvic organ prolapse or urinary incontinence, Katarzyna Bochenska, MD, reported in a poster at Pelvic Floor Disorders Week, sponsored by the American Urogynecologic Society. This subgroup had similar 30-day rates of reoperation, venous thromboembolism, and infectious, pulmonary, and cardiac complications as patients who had surgery only for gynecologic cancer, reported Dr. Bochenska and her associates at Northwestern University in Chicago.
Urinary incontinence and symptomatic pelvic organ prolapse often accompany gynecologic cancer, the researchers noted. In one study, more than half of women with gynecologic cancer reported urinary incontinence, while 11% described feeling a bulge of tissue from their vaginas (Obstet Gynecol. 2013 Nov;122[5]:976-80). But few studies have examined outcomes after concomitant surgery for pelvic floor disorders and gynecologic cancer, according to the researchers.
In the current study, the Northwestern University researchers used postoperative ICD-9 codes to identify patients in the American College of Surgeons’ National Surgical Quality Improvement Program (ACS NSQIP) who underwent surgery for uterine, cervical, ovarian, or vulvar or vaginal cancer between 2010 and 2014. Most patients had uterine or ovarian cancer, while the most common pelvic floor disorder procedures included anterior and/or posterior colporrhaphy, laparoscopic colpopexy, and midurethral slings.
None of the complications studied differed significantly between the groups. Rates of 30-day reoperation were 1.1% among concomitant surgery patients and 2.3% among patients who underwent only cancer surgery (P = .09). Rates of procedure-related infections such as sepsis, deep wound infections, and abscesses also were similar between groups (3.1% and 3.9%, respectively), as were rates of postoperative urinary tract infections (1.8% and 3.2%), pulmonary complications (0.7% and 1.3%), and cardiac complications (0.2% and 0.4%), with all P-values exceeding .05.
Patients who underwent concomitant surgery for pelvic floor disorders were an average of about 3.5 years older than other patients, but otherwise resembled them in term of body mass index and prevalence of comorbidities, such as diabetes, chronic obstructive pulmonary disease, and hypertension.
“Our data suggest that serious postoperative complication rates are not increased in this population,” the researchers concluded. “Therefore, gynecologic surgeons should consider offering concomitant treatment for pelvic floor symptoms at the time of gynecologic cancer surgery.”
Dr. Bochenska and her associates did not report information on funding sources or financial disclosures.
DENVER – Concomitantly treating pelvic floor disorders during surgery for gynecologic cancer does not increase the risk of postoperative complications, according to an analysis of 4 years of data from the American College of Surgeons’ National Surgical Quality Improvement Program.
Among 23,501 surgical gynecologic cancer patients, 556 (2.4%) underwent concomitant surgery for symptomatic pelvic organ prolapse or urinary incontinence, Katarzyna Bochenska, MD, reported in a poster at Pelvic Floor Disorders Week, sponsored by the American Urogynecologic Society. This subgroup had similar 30-day rates of reoperation, venous thromboembolism, and infectious, pulmonary, and cardiac complications as patients who had surgery only for gynecologic cancer, reported Dr. Bochenska and her associates at Northwestern University in Chicago.
Urinary incontinence and symptomatic pelvic organ prolapse often accompany gynecologic cancer, the researchers noted. In one study, more than half of women with gynecologic cancer reported urinary incontinence, while 11% described feeling a bulge of tissue from their vaginas (Obstet Gynecol. 2013 Nov;122[5]:976-80). But few studies have examined outcomes after concomitant surgery for pelvic floor disorders and gynecologic cancer, according to the researchers.
In the current study, the Northwestern University researchers used postoperative ICD-9 codes to identify patients in the American College of Surgeons’ National Surgical Quality Improvement Program (ACS NSQIP) who underwent surgery for uterine, cervical, ovarian, or vulvar or vaginal cancer between 2010 and 2014. Most patients had uterine or ovarian cancer, while the most common pelvic floor disorder procedures included anterior and/or posterior colporrhaphy, laparoscopic colpopexy, and midurethral slings.
None of the complications studied differed significantly between the groups. Rates of 30-day reoperation were 1.1% among concomitant surgery patients and 2.3% among patients who underwent only cancer surgery (P = .09). Rates of procedure-related infections such as sepsis, deep wound infections, and abscesses also were similar between groups (3.1% and 3.9%, respectively), as were rates of postoperative urinary tract infections (1.8% and 3.2%), pulmonary complications (0.7% and 1.3%), and cardiac complications (0.2% and 0.4%), with all P-values exceeding .05.
Patients who underwent concomitant surgery for pelvic floor disorders were an average of about 3.5 years older than other patients, but otherwise resembled them in term of body mass index and prevalence of comorbidities, such as diabetes, chronic obstructive pulmonary disease, and hypertension.
“Our data suggest that serious postoperative complication rates are not increased in this population,” the researchers concluded. “Therefore, gynecologic surgeons should consider offering concomitant treatment for pelvic floor symptoms at the time of gynecologic cancer surgery.”
Dr. Bochenska and her associates did not report information on funding sources or financial disclosures.
DENVER – Concomitantly treating pelvic floor disorders during surgery for gynecologic cancer does not increase the risk of postoperative complications, according to an analysis of 4 years of data from the American College of Surgeons’ National Surgical Quality Improvement Program.
Among 23,501 surgical gynecologic cancer patients, 556 (2.4%) underwent concomitant surgery for symptomatic pelvic organ prolapse or urinary incontinence, Katarzyna Bochenska, MD, reported in a poster at Pelvic Floor Disorders Week, sponsored by the American Urogynecologic Society. This subgroup had similar 30-day rates of reoperation, venous thromboembolism, and infectious, pulmonary, and cardiac complications as patients who had surgery only for gynecologic cancer, reported Dr. Bochenska and her associates at Northwestern University in Chicago.
Urinary incontinence and symptomatic pelvic organ prolapse often accompany gynecologic cancer, the researchers noted. In one study, more than half of women with gynecologic cancer reported urinary incontinence, while 11% described feeling a bulge of tissue from their vaginas (Obstet Gynecol. 2013 Nov;122[5]:976-80). But few studies have examined outcomes after concomitant surgery for pelvic floor disorders and gynecologic cancer, according to the researchers.
In the current study, the Northwestern University researchers used postoperative ICD-9 codes to identify patients in the American College of Surgeons’ National Surgical Quality Improvement Program (ACS NSQIP) who underwent surgery for uterine, cervical, ovarian, or vulvar or vaginal cancer between 2010 and 2014. Most patients had uterine or ovarian cancer, while the most common pelvic floor disorder procedures included anterior and/or posterior colporrhaphy, laparoscopic colpopexy, and midurethral slings.
None of the complications studied differed significantly between the groups. Rates of 30-day reoperation were 1.1% among concomitant surgery patients and 2.3% among patients who underwent only cancer surgery (P = .09). Rates of procedure-related infections such as sepsis, deep wound infections, and abscesses also were similar between groups (3.1% and 3.9%, respectively), as were rates of postoperative urinary tract infections (1.8% and 3.2%), pulmonary complications (0.7% and 1.3%), and cardiac complications (0.2% and 0.4%), with all P-values exceeding .05.
Patients who underwent concomitant surgery for pelvic floor disorders were an average of about 3.5 years older than other patients, but otherwise resembled them in term of body mass index and prevalence of comorbidities, such as diabetes, chronic obstructive pulmonary disease, and hypertension.
“Our data suggest that serious postoperative complication rates are not increased in this population,” the researchers concluded. “Therefore, gynecologic surgeons should consider offering concomitant treatment for pelvic floor symptoms at the time of gynecologic cancer surgery.”
Dr. Bochenska and her associates did not report information on funding sources or financial disclosures.
AT PFD WEEK 2016
Key clinical point:
Major finding: Women who underwent concomitant surgeries had similar rates of infectious, pulmonary, and cardiac complications as those who underwent surgery only for gynecologic cancer, with all P-values exceeding .05.
Data source: A study of 23,501 gynecologic cancer patients in the ACS National Surgical Quality Improvement Program dataset.
Disclosures: Dr. Bochenska and her associates did not report information on funding sources or financial disclosures.
Patients with stage 1 NSCLC more likely to die of other causes in short term
Patients with stage 1 non–small cell lung cancer who underwent resection with intent to cure were more likely, over the short term, to die of other causes, investigators reported.
“Non–cancer-specific mortality represents a significant competing event for lung cancer–specific mortality, with an increasing impact as age increases,” Takashi Eguchi, MD, and his associates at Memorial Sloan Kettering Cancer Center, New York wrote (J Clin Oncol. 2016 Oct 10. doi: 10.1200/JCO.2016.69.0834).
“These findings can provide patients with more accurate information on survivorship on the basis of their individual preoperative status, and help determine patients’ optimal treatment options.”
The study included 2,186 patients who underwent curative-intent resection of stage 1 non-small cell lung cancer at Memorial Sloan Kettering between 2000 and 2011. The cumulative 5-year lung cancer death rate was 10.4%, but rose with age from 7.5% among patients younger than 65 years to 13.2% among patients who were at least 75 years old. Cumulative 5-year rates of mortality not due to cancer were lower, at 5.3% overall, 1.8% in the youngest cohort, and 9% in the oldest cohort. But a competing risk analysis of the entire cohort showed that non–cancer-specific cumulative mortality was higher than mortality from lung cancer for up to 1.5 years after resection, the investigators found. Furthermore, patients who were at least 75 years old were more likely to die of causes other than cancer for up to 2.5 years after surgery.
In the multivariable analysis, low predicted postoperative diffusing capacity of lung for carbon monoxide (DCLO) independently predicted severe morbidity (P less than .001), 1-year mortality (P less than .001), and non–cancer-specific mortality (P less than .001), the researchers said. These findings reflect prior work linking low DCLO with obstructive, restrictive, and pulmonary vascular disease, chronic heart failure, and poor postoperative outcomes, they noted.
Senior author Prasad S. Adusumilli, MD, provided funding. Dr. Eguchi and Dr. Adusumilli had no relevant financial disclosures.
Patients with stage 1 non–small cell lung cancer who underwent resection with intent to cure were more likely, over the short term, to die of other causes, investigators reported.
“Non–cancer-specific mortality represents a significant competing event for lung cancer–specific mortality, with an increasing impact as age increases,” Takashi Eguchi, MD, and his associates at Memorial Sloan Kettering Cancer Center, New York wrote (J Clin Oncol. 2016 Oct 10. doi: 10.1200/JCO.2016.69.0834).
“These findings can provide patients with more accurate information on survivorship on the basis of their individual preoperative status, and help determine patients’ optimal treatment options.”
The study included 2,186 patients who underwent curative-intent resection of stage 1 non-small cell lung cancer at Memorial Sloan Kettering between 2000 and 2011. The cumulative 5-year lung cancer death rate was 10.4%, but rose with age from 7.5% among patients younger than 65 years to 13.2% among patients who were at least 75 years old. Cumulative 5-year rates of mortality not due to cancer were lower, at 5.3% overall, 1.8% in the youngest cohort, and 9% in the oldest cohort. But a competing risk analysis of the entire cohort showed that non–cancer-specific cumulative mortality was higher than mortality from lung cancer for up to 1.5 years after resection, the investigators found. Furthermore, patients who were at least 75 years old were more likely to die of causes other than cancer for up to 2.5 years after surgery.
In the multivariable analysis, low predicted postoperative diffusing capacity of lung for carbon monoxide (DCLO) independently predicted severe morbidity (P less than .001), 1-year mortality (P less than .001), and non–cancer-specific mortality (P less than .001), the researchers said. These findings reflect prior work linking low DCLO with obstructive, restrictive, and pulmonary vascular disease, chronic heart failure, and poor postoperative outcomes, they noted.
Senior author Prasad S. Adusumilli, MD, provided funding. Dr. Eguchi and Dr. Adusumilli had no relevant financial disclosures.
Patients with stage 1 non–small cell lung cancer who underwent resection with intent to cure were more likely, over the short term, to die of other causes, investigators reported.
“Non–cancer-specific mortality represents a significant competing event for lung cancer–specific mortality, with an increasing impact as age increases,” Takashi Eguchi, MD, and his associates at Memorial Sloan Kettering Cancer Center, New York wrote (J Clin Oncol. 2016 Oct 10. doi: 10.1200/JCO.2016.69.0834).
“These findings can provide patients with more accurate information on survivorship on the basis of their individual preoperative status, and help determine patients’ optimal treatment options.”
The study included 2,186 patients who underwent curative-intent resection of stage 1 non-small cell lung cancer at Memorial Sloan Kettering between 2000 and 2011. The cumulative 5-year lung cancer death rate was 10.4%, but rose with age from 7.5% among patients younger than 65 years to 13.2% among patients who were at least 75 years old. Cumulative 5-year rates of mortality not due to cancer were lower, at 5.3% overall, 1.8% in the youngest cohort, and 9% in the oldest cohort. But a competing risk analysis of the entire cohort showed that non–cancer-specific cumulative mortality was higher than mortality from lung cancer for up to 1.5 years after resection, the investigators found. Furthermore, patients who were at least 75 years old were more likely to die of causes other than cancer for up to 2.5 years after surgery.
In the multivariable analysis, low predicted postoperative diffusing capacity of lung for carbon monoxide (DCLO) independently predicted severe morbidity (P less than .001), 1-year mortality (P less than .001), and non–cancer-specific mortality (P less than .001), the researchers said. These findings reflect prior work linking low DCLO with obstructive, restrictive, and pulmonary vascular disease, chronic heart failure, and poor postoperative outcomes, they noted.
Senior author Prasad S. Adusumilli, MD, provided funding. Dr. Eguchi and Dr. Adusumilli had no relevant financial disclosures.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Patients with resected stage 1 non-small cell lung cancer are more likely to die of other causes in the short term. Major finding: The non–cancer-specific cumulative incidence of death was higher than CID from lung cancer for up to 1.5 years after resection.
Data source: A single-center competing risk analysis of 2,186 patients who underwent curative-intent resection for stage 1 non–small cell lung cancer.
Disclosures: Senior author Prasad Adusumilli, MD, provided funding. Dr. Eguchi and Dr. Adusumilli had no relevant financial disclosures.
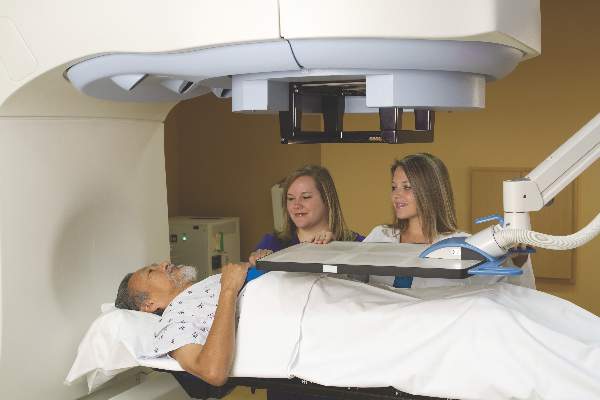
Partial-breast irradiation alternative to mastectomy following recurrence
BOSTON – In women with in-breast failures following breast-conserving surgery and whole-breast irradiation, partial-breast irradiation with 3D conformal radiation appears to be an effective and safe alternative to mastectomy, results of a phase II trial indicate.
At a median follow-up of 3.6 years, there were only two ipsilateral breast recurrences, and four mastectomies required among 58 women treated with partial-breast irradiation after a second lumpectomy, reported Douglas W. Arthur, MD, chair of radiation oncology at Virginia Commonwealth University in Richmond.
“I think this information adds to the growing data supporting this treatment approach as an alternative to mastectomy for those who continue to want to preserve the breast,” he said at a late-breaking abstracts session at the annual meeting of the American Society of Radiation Oncology.
The NRG/RTOG 1014 study is a prospective phase II trial designed to evaluate skin, breast, and chest wall events occurring within 1 year of re-irradiation. One-year toxicity results from the trial were reported at the 2015 ASTRO annual meeting.
Dr. Arthur presented 3-year efficacy results from the trial. The investigators enrolled women with in-breast recurrences more than 1 year after whole-breast irradiation (WBI), with tumors that were smaller than 3 cm, unifocal, and resected with negative margins. Axillary involvement was limited to pathologic NO or N1 without extracapsular extension.
Partial-breast irradiation was targeted to the surgical cavity with a safety margin of plus 1.5 cm for the clinical target volume, and an additional 1-cm expansion. The prescribed dose was 45 Gy in 1.5-Gy fractions delivered via 3D conformal radiotherapy in 30 treatments.
Of 65 patients accrued, 58 completed treatment and were evaluable for efficacy. The median age was 67.5 years. In all, 23 patients had ductal carcinoma in situ, and 35 had invasive cancers. All patients were node negative.
A total of four patients (6.9%) reported late grade 3 toxicities, which included breast infection, fibrous deep connective tissue, skin induration, and breast atrophy, pain, and volume loss. There were no grade 4 toxicities, and no treatment-related deaths.
Two patients had in-breast recurrences, which translated into a year estimate of 3.7%. Four patients underwent mastectomies, for a 3-year estimated mastectomy failure rate of 5.2%. Two of the mastectomies were for the in-breast recurrences, one for a nonhealing wound, and one occurred in a patient who developed cancer in the contralateral breast and opted for a bilateral mastectomy.
BOSTON – In women with in-breast failures following breast-conserving surgery and whole-breast irradiation, partial-breast irradiation with 3D conformal radiation appears to be an effective and safe alternative to mastectomy, results of a phase II trial indicate.
At a median follow-up of 3.6 years, there were only two ipsilateral breast recurrences, and four mastectomies required among 58 women treated with partial-breast irradiation after a second lumpectomy, reported Douglas W. Arthur, MD, chair of radiation oncology at Virginia Commonwealth University in Richmond.
“I think this information adds to the growing data supporting this treatment approach as an alternative to mastectomy for those who continue to want to preserve the breast,” he said at a late-breaking abstracts session at the annual meeting of the American Society of Radiation Oncology.
The NRG/RTOG 1014 study is a prospective phase II trial designed to evaluate skin, breast, and chest wall events occurring within 1 year of re-irradiation. One-year toxicity results from the trial were reported at the 2015 ASTRO annual meeting.
Dr. Arthur presented 3-year efficacy results from the trial. The investigators enrolled women with in-breast recurrences more than 1 year after whole-breast irradiation (WBI), with tumors that were smaller than 3 cm, unifocal, and resected with negative margins. Axillary involvement was limited to pathologic NO or N1 without extracapsular extension.
Partial-breast irradiation was targeted to the surgical cavity with a safety margin of plus 1.5 cm for the clinical target volume, and an additional 1-cm expansion. The prescribed dose was 45 Gy in 1.5-Gy fractions delivered via 3D conformal radiotherapy in 30 treatments.
Of 65 patients accrued, 58 completed treatment and were evaluable for efficacy. The median age was 67.5 years. In all, 23 patients had ductal carcinoma in situ, and 35 had invasive cancers. All patients were node negative.
A total of four patients (6.9%) reported late grade 3 toxicities, which included breast infection, fibrous deep connective tissue, skin induration, and breast atrophy, pain, and volume loss. There were no grade 4 toxicities, and no treatment-related deaths.
Two patients had in-breast recurrences, which translated into a year estimate of 3.7%. Four patients underwent mastectomies, for a 3-year estimated mastectomy failure rate of 5.2%. Two of the mastectomies were for the in-breast recurrences, one for a nonhealing wound, and one occurred in a patient who developed cancer in the contralateral breast and opted for a bilateral mastectomy.
BOSTON – In women with in-breast failures following breast-conserving surgery and whole-breast irradiation, partial-breast irradiation with 3D conformal radiation appears to be an effective and safe alternative to mastectomy, results of a phase II trial indicate.
At a median follow-up of 3.6 years, there were only two ipsilateral breast recurrences, and four mastectomies required among 58 women treated with partial-breast irradiation after a second lumpectomy, reported Douglas W. Arthur, MD, chair of radiation oncology at Virginia Commonwealth University in Richmond.
“I think this information adds to the growing data supporting this treatment approach as an alternative to mastectomy for those who continue to want to preserve the breast,” he said at a late-breaking abstracts session at the annual meeting of the American Society of Radiation Oncology.
The NRG/RTOG 1014 study is a prospective phase II trial designed to evaluate skin, breast, and chest wall events occurring within 1 year of re-irradiation. One-year toxicity results from the trial were reported at the 2015 ASTRO annual meeting.
Dr. Arthur presented 3-year efficacy results from the trial. The investigators enrolled women with in-breast recurrences more than 1 year after whole-breast irradiation (WBI), with tumors that were smaller than 3 cm, unifocal, and resected with negative margins. Axillary involvement was limited to pathologic NO or N1 without extracapsular extension.
Partial-breast irradiation was targeted to the surgical cavity with a safety margin of plus 1.5 cm for the clinical target volume, and an additional 1-cm expansion. The prescribed dose was 45 Gy in 1.5-Gy fractions delivered via 3D conformal radiotherapy in 30 treatments.
Of 65 patients accrued, 58 completed treatment and were evaluable for efficacy. The median age was 67.5 years. In all, 23 patients had ductal carcinoma in situ, and 35 had invasive cancers. All patients were node negative.
A total of four patients (6.9%) reported late grade 3 toxicities, which included breast infection, fibrous deep connective tissue, skin induration, and breast atrophy, pain, and volume loss. There were no grade 4 toxicities, and no treatment-related deaths.
Two patients had in-breast recurrences, which translated into a year estimate of 3.7%. Four patients underwent mastectomies, for a 3-year estimated mastectomy failure rate of 5.2%. Two of the mastectomies were for the in-breast recurrences, one for a nonhealing wound, and one occurred in a patient who developed cancer in the contralateral breast and opted for a bilateral mastectomy.
AT ASTRO 2016
Key clinical point: Re-irradiation following second lumpectomy after in-breast cancer recurrences appears to be a safe and effective alternative to mastectomy.
Major finding: The 3-year estimated in-breast recurrence rate was 3.7%.
Data source: Phase II nonrandomized study in 58 women with in-breast failures following breast-conserving surgery and whole-breast irradiation.
Disclosures: The study was supported by the National Cancer Institute. Dr. Arthur reported formerly serving on the medical advisory board of Impedimed,
Glimmer of promise for nivolumab in neoadjuvant NSCLC therapy
COPENHAGEN – Neoadjuvant therapy with the PD-1 checkpoint inhibitor nivolumab is safe, does not delay surgery, and may offer clinical benefit in some patients with early-stage non–small cell lung cancer, preliminary results of a clinical trial show.
Among 17 patients with previously untreated stage I-IIIA non–small cell lung cancer (NSCLC) who had two courses of nivolumab (Opdivo) followed by surgical resection, 12 had pathologic evidence of tumor regression, including 7 who had what investigators termed a “major pathologic response,” defined as less than 10% residual viable tumor, reported Patrick M. Forde, MBBCh, of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University in Baltimore.
To see whether immunotherapy could be safe and practical in the neoadjuvant setting, the investigators enrolled 18 patients with untreated, resectable disease and performed pretreatment tumor biopsies. Patients then received doses of nivolumab 3 mg/kg delivered at 4 weeks and 2 weeks prior to scheduled surgery. Following surgery, patients received adjuvant chemotherapy at the investigator’s discretion.
Treatment related adverse events included one grade 3 to 4 toxicity and one event leading to discontinuation. There were no adverse events requiring delay of surgery.
The investigators also conducted an exploratory analysis of response to treatment among 17 who had sufficient follow-up data for evaluation of the primary endpoints of safety and feasibility.
As noted, 12 patients had a measurable tumor response, and 7 had a major pathologic response. Of this latter group, three had no radiographic evidence of response, but tumor specimens from all 7 showed evidence of “substantial” T-cell infiltration, indicating an enhanced immune response, Dr. Forde said.
In all, 9 of the 17 patients had tumor regression of more than 50%, and 7 patients had pathologic downstaging from their pretreatment clinical stage.
Four of the seven tumors with the major pathologic response were tested with a programmed death–1 ligand immunohistochemical assay, and three were positive for the ligand.
The investigators also isolated both unique and shared T-cell clones from peripheral blood, and detected new infiltration of T-cell clones that were not seen in the tumor specimens taken prior to nivolumab therapy.
Based on these early results, the investigators plan to expand the study, with one cohort planned to receive a third presurgical dose of nivolumab, and a second cohort scheduled to receive both nivolumab and ipilimumab (Yervoy).
It remains to be seen, he said, whether patients should also receive maintenance therapy, and whether combined checkpoint inhibitors might be more effective.
However, Pieter Postmus, chair of thoracic oncology at the University of Liverpool (England), who was not involved in the study, said that he is reserving judgment on efficacy until further data are available.
“There is a potential for bias when comparing a small biopsy, which might not represent the whole tumor, with the resected tumor,” he said in a statement. “This is not a validated way to measure response to a treatment. It describes a biological effect but whether that has any clinical impact on survival is unproven.”
“Although we do not know for the time being if a major pathological response is correlated with improved survival, this method could first be validated in a cohort of patients with advanced disease by comparing the percentages of viable tumor cells in tumor biopsies taken before and 4 to 8 weeks after immunotherapy,” he added. “If in this way regression – as defined in the preoperative study – correlates with survival in patients with advanced cancer, it is likely to hold true in less advanced or resectable patients. Long-term survival data will be the ultimate test for these neoadjuvant immunotherapy strategies.”
COPENHAGEN – Neoadjuvant therapy with the PD-1 checkpoint inhibitor nivolumab is safe, does not delay surgery, and may offer clinical benefit in some patients with early-stage non–small cell lung cancer, preliminary results of a clinical trial show.
Among 17 patients with previously untreated stage I-IIIA non–small cell lung cancer (NSCLC) who had two courses of nivolumab (Opdivo) followed by surgical resection, 12 had pathologic evidence of tumor regression, including 7 who had what investigators termed a “major pathologic response,” defined as less than 10% residual viable tumor, reported Patrick M. Forde, MBBCh, of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University in Baltimore.
To see whether immunotherapy could be safe and practical in the neoadjuvant setting, the investigators enrolled 18 patients with untreated, resectable disease and performed pretreatment tumor biopsies. Patients then received doses of nivolumab 3 mg/kg delivered at 4 weeks and 2 weeks prior to scheduled surgery. Following surgery, patients received adjuvant chemotherapy at the investigator’s discretion.
Treatment related adverse events included one grade 3 to 4 toxicity and one event leading to discontinuation. There were no adverse events requiring delay of surgery.
The investigators also conducted an exploratory analysis of response to treatment among 17 who had sufficient follow-up data for evaluation of the primary endpoints of safety and feasibility.
As noted, 12 patients had a measurable tumor response, and 7 had a major pathologic response. Of this latter group, three had no radiographic evidence of response, but tumor specimens from all 7 showed evidence of “substantial” T-cell infiltration, indicating an enhanced immune response, Dr. Forde said.
In all, 9 of the 17 patients had tumor regression of more than 50%, and 7 patients had pathologic downstaging from their pretreatment clinical stage.
Four of the seven tumors with the major pathologic response were tested with a programmed death–1 ligand immunohistochemical assay, and three were positive for the ligand.
The investigators also isolated both unique and shared T-cell clones from peripheral blood, and detected new infiltration of T-cell clones that were not seen in the tumor specimens taken prior to nivolumab therapy.
Based on these early results, the investigators plan to expand the study, with one cohort planned to receive a third presurgical dose of nivolumab, and a second cohort scheduled to receive both nivolumab and ipilimumab (Yervoy).
It remains to be seen, he said, whether patients should also receive maintenance therapy, and whether combined checkpoint inhibitors might be more effective.
However, Pieter Postmus, chair of thoracic oncology at the University of Liverpool (England), who was not involved in the study, said that he is reserving judgment on efficacy until further data are available.
“There is a potential for bias when comparing a small biopsy, which might not represent the whole tumor, with the resected tumor,” he said in a statement. “This is not a validated way to measure response to a treatment. It describes a biological effect but whether that has any clinical impact on survival is unproven.”
“Although we do not know for the time being if a major pathological response is correlated with improved survival, this method could first be validated in a cohort of patients with advanced disease by comparing the percentages of viable tumor cells in tumor biopsies taken before and 4 to 8 weeks after immunotherapy,” he added. “If in this way regression – as defined in the preoperative study – correlates with survival in patients with advanced cancer, it is likely to hold true in less advanced or resectable patients. Long-term survival data will be the ultimate test for these neoadjuvant immunotherapy strategies.”
COPENHAGEN – Neoadjuvant therapy with the PD-1 checkpoint inhibitor nivolumab is safe, does not delay surgery, and may offer clinical benefit in some patients with early-stage non–small cell lung cancer, preliminary results of a clinical trial show.
Among 17 patients with previously untreated stage I-IIIA non–small cell lung cancer (NSCLC) who had two courses of nivolumab (Opdivo) followed by surgical resection, 12 had pathologic evidence of tumor regression, including 7 who had what investigators termed a “major pathologic response,” defined as less than 10% residual viable tumor, reported Patrick M. Forde, MBBCh, of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University in Baltimore.
To see whether immunotherapy could be safe and practical in the neoadjuvant setting, the investigators enrolled 18 patients with untreated, resectable disease and performed pretreatment tumor biopsies. Patients then received doses of nivolumab 3 mg/kg delivered at 4 weeks and 2 weeks prior to scheduled surgery. Following surgery, patients received adjuvant chemotherapy at the investigator’s discretion.
Treatment related adverse events included one grade 3 to 4 toxicity and one event leading to discontinuation. There were no adverse events requiring delay of surgery.
The investigators also conducted an exploratory analysis of response to treatment among 17 who had sufficient follow-up data for evaluation of the primary endpoints of safety and feasibility.
As noted, 12 patients had a measurable tumor response, and 7 had a major pathologic response. Of this latter group, three had no radiographic evidence of response, but tumor specimens from all 7 showed evidence of “substantial” T-cell infiltration, indicating an enhanced immune response, Dr. Forde said.
In all, 9 of the 17 patients had tumor regression of more than 50%, and 7 patients had pathologic downstaging from their pretreatment clinical stage.
Four of the seven tumors with the major pathologic response were tested with a programmed death–1 ligand immunohistochemical assay, and three were positive for the ligand.
The investigators also isolated both unique and shared T-cell clones from peripheral blood, and detected new infiltration of T-cell clones that were not seen in the tumor specimens taken prior to nivolumab therapy.
Based on these early results, the investigators plan to expand the study, with one cohort planned to receive a third presurgical dose of nivolumab, and a second cohort scheduled to receive both nivolumab and ipilimumab (Yervoy).
It remains to be seen, he said, whether patients should also receive maintenance therapy, and whether combined checkpoint inhibitors might be more effective.
However, Pieter Postmus, chair of thoracic oncology at the University of Liverpool (England), who was not involved in the study, said that he is reserving judgment on efficacy until further data are available.
“There is a potential for bias when comparing a small biopsy, which might not represent the whole tumor, with the resected tumor,” he said in a statement. “This is not a validated way to measure response to a treatment. It describes a biological effect but whether that has any clinical impact on survival is unproven.”
“Although we do not know for the time being if a major pathological response is correlated with improved survival, this method could first be validated in a cohort of patients with advanced disease by comparing the percentages of viable tumor cells in tumor biopsies taken before and 4 to 8 weeks after immunotherapy,” he added. “If in this way regression – as defined in the preoperative study – correlates with survival in patients with advanced cancer, it is likely to hold true in less advanced or resectable patients. Long-term survival data will be the ultimate test for these neoadjuvant immunotherapy strategies.”
AT ESMO 2016
Key clinical point: Checkpoint inhibitors have shown good efficacy for treatment of advanced NSCLC, but use in the neoadjuvant setting is still investigational.
Major finding: Seven of 17 patients had a major pathologic response (less than 10% viable tumor remaining).
Data source: Safety and feasibility study in 18 patients with stage I-IIIA non–small cell lung cancer.
Disclosures: The study was supported by the American Association for Cancer Research, the Cancer Research Institute, LUNGevity, and Stand Up to Cancer. Bristol-Myers Squibb donated nivolumab and provided funding for PD-L1 testing. Dr. Forde disclosed institution research grants from Bristol-Myers Squibb, Kyowa, Novartis, and uncompensated consulting for AstraZeneca and Celgene. Dr. Baas disclosed grants and research support from Bristol-Myers Squibb. Dr. Postmus had no disclosures.
Boost dose pays off long term after surgery, WBRT for DCIS
BOSTON – Adding a boost dose after surgery and whole-breast radiation therapy (WBRT) for ductal carcinoma in situ offers a small but significant reduction in long-term risk of same-breast recurrence, according to results of a multicenter study.
An analysis of pooled data from 10 institutions showed that after 15 years of follow-up, 91.6% of women who had been treated with breast-conserving surgery, WBRT, and a boost dose remained free of ipsilateral breast tumor recurrence (IBTR), compared with 88% of women who received WBRT alone, reported Meena Savur Moran, MD, director of the breast cancer radiotherapy program at Smilow Cancer Hospital, Yale New Haven, Connecticut.
The absolute differences between the groups – 3% at 10 years, and 4% at 15 years – “don’t seem like a lot, but it is clinically important for patients, because what we’ve learned from the invasive cancer data is that this small, incremental decrease results in about a 40% less mastectomy rate for salvage mastectomies for recurrences,” she said.
It is common practice in many centers to give a radiation boost to the tumor bed of 8-16 Gy in 4-8 additional fractions following breast-conserving surgery and WBRT. This has been shown in invasive cancers to be associated with a statistically significant decrease in risk for IBTR in all age groups of about 4% at 20 years.
Yet because DCIS generally has an excellent prognosis with very few recurrences after whole-breast irradiation, it has been harder to determine whether radiation boosting would offer similar benefit to that seen in invasive cancers,
In an attempt to answer this question, investigators from 10 centers collaborated to create a DCIS database of patients treated with WBRT either with or without boost, and then analyzed the results.
The final cohort included 4,131 patients, far exceeding the minimum sample size the statisticians calculated would be needed to detect a benefit.
The median age of patients at the time of therapy was 56.1 years, median follow-up was 9 years, and the median boost dose was 14 Gy. In all, 4% of patients had positive tumor margins after surgery.
Rates of IBTR-free survival with boost vs. no boost were 97.1% vs. 96.3% at 5 years, 94.1% vs. 92.5% at 10 years, and, as noted before, 91.6% vs. 88% at 15 years (P = .0389).
In both univariate and in multivariate analysis controlling for tumor grade, comedo carcinoma, tamoxifen use, margin status, and age, IBTR was significantly associated with lower risk for recurrence.
The investigators also conducted a boost/no boost analysis stratified by margin status using both the National Surgical Adjuvant Breast and Bowel Project (NSABP) definition of negative margins as no ink on tumor, and the joint Society of Surgical Oncology, ASTRO, and American Society of Clinical Oncology guideline definition as less than 2 mm, and in each case found that among patients with negative margins, boosting offered a significant recurrence-free advantage.
Finally, they found that boosting offered a significant advantage both for patients 50 and older (P = .0073) and those 49 and younger (P = .0166).
“For DCIS, most of who treat breast cancer have used a boost, but we really extrapolated from the treatment from invasive breast cancer. Evidence for this practice until now in specific for DCIS has been lacking,” said Geraldine Jacobson, MD, MPH, professor and chair of radiation oncology at West Virginia University, Morgantown. Dr. Jacobson moderated the briefing.
She noted that the large sample size and long follow-up of the study was necessary for the treatment benefit to emerge.
BOSTON – Adding a boost dose after surgery and whole-breast radiation therapy (WBRT) for ductal carcinoma in situ offers a small but significant reduction in long-term risk of same-breast recurrence, according to results of a multicenter study.
An analysis of pooled data from 10 institutions showed that after 15 years of follow-up, 91.6% of women who had been treated with breast-conserving surgery, WBRT, and a boost dose remained free of ipsilateral breast tumor recurrence (IBTR), compared with 88% of women who received WBRT alone, reported Meena Savur Moran, MD, director of the breast cancer radiotherapy program at Smilow Cancer Hospital, Yale New Haven, Connecticut.
The absolute differences between the groups – 3% at 10 years, and 4% at 15 years – “don’t seem like a lot, but it is clinically important for patients, because what we’ve learned from the invasive cancer data is that this small, incremental decrease results in about a 40% less mastectomy rate for salvage mastectomies for recurrences,” she said.
It is common practice in many centers to give a radiation boost to the tumor bed of 8-16 Gy in 4-8 additional fractions following breast-conserving surgery and WBRT. This has been shown in invasive cancers to be associated with a statistically significant decrease in risk for IBTR in all age groups of about 4% at 20 years.
Yet because DCIS generally has an excellent prognosis with very few recurrences after whole-breast irradiation, it has been harder to determine whether radiation boosting would offer similar benefit to that seen in invasive cancers,
In an attempt to answer this question, investigators from 10 centers collaborated to create a DCIS database of patients treated with WBRT either with or without boost, and then analyzed the results.
The final cohort included 4,131 patients, far exceeding the minimum sample size the statisticians calculated would be needed to detect a benefit.
The median age of patients at the time of therapy was 56.1 years, median follow-up was 9 years, and the median boost dose was 14 Gy. In all, 4% of patients had positive tumor margins after surgery.
Rates of IBTR-free survival with boost vs. no boost were 97.1% vs. 96.3% at 5 years, 94.1% vs. 92.5% at 10 years, and, as noted before, 91.6% vs. 88% at 15 years (P = .0389).
In both univariate and in multivariate analysis controlling for tumor grade, comedo carcinoma, tamoxifen use, margin status, and age, IBTR was significantly associated with lower risk for recurrence.
The investigators also conducted a boost/no boost analysis stratified by margin status using both the National Surgical Adjuvant Breast and Bowel Project (NSABP) definition of negative margins as no ink on tumor, and the joint Society of Surgical Oncology, ASTRO, and American Society of Clinical Oncology guideline definition as less than 2 mm, and in each case found that among patients with negative margins, boosting offered a significant recurrence-free advantage.
Finally, they found that boosting offered a significant advantage both for patients 50 and older (P = .0073) and those 49 and younger (P = .0166).
“For DCIS, most of who treat breast cancer have used a boost, but we really extrapolated from the treatment from invasive breast cancer. Evidence for this practice until now in specific for DCIS has been lacking,” said Geraldine Jacobson, MD, MPH, professor and chair of radiation oncology at West Virginia University, Morgantown. Dr. Jacobson moderated the briefing.
She noted that the large sample size and long follow-up of the study was necessary for the treatment benefit to emerge.
BOSTON – Adding a boost dose after surgery and whole-breast radiation therapy (WBRT) for ductal carcinoma in situ offers a small but significant reduction in long-term risk of same-breast recurrence, according to results of a multicenter study.
An analysis of pooled data from 10 institutions showed that after 15 years of follow-up, 91.6% of women who had been treated with breast-conserving surgery, WBRT, and a boost dose remained free of ipsilateral breast tumor recurrence (IBTR), compared with 88% of women who received WBRT alone, reported Meena Savur Moran, MD, director of the breast cancer radiotherapy program at Smilow Cancer Hospital, Yale New Haven, Connecticut.
The absolute differences between the groups – 3% at 10 years, and 4% at 15 years – “don’t seem like a lot, but it is clinically important for patients, because what we’ve learned from the invasive cancer data is that this small, incremental decrease results in about a 40% less mastectomy rate for salvage mastectomies for recurrences,” she said.
It is common practice in many centers to give a radiation boost to the tumor bed of 8-16 Gy in 4-8 additional fractions following breast-conserving surgery and WBRT. This has been shown in invasive cancers to be associated with a statistically significant decrease in risk for IBTR in all age groups of about 4% at 20 years.
Yet because DCIS generally has an excellent prognosis with very few recurrences after whole-breast irradiation, it has been harder to determine whether radiation boosting would offer similar benefit to that seen in invasive cancers,
In an attempt to answer this question, investigators from 10 centers collaborated to create a DCIS database of patients treated with WBRT either with or without boost, and then analyzed the results.
The final cohort included 4,131 patients, far exceeding the minimum sample size the statisticians calculated would be needed to detect a benefit.
The median age of patients at the time of therapy was 56.1 years, median follow-up was 9 years, and the median boost dose was 14 Gy. In all, 4% of patients had positive tumor margins after surgery.
Rates of IBTR-free survival with boost vs. no boost were 97.1% vs. 96.3% at 5 years, 94.1% vs. 92.5% at 10 years, and, as noted before, 91.6% vs. 88% at 15 years (P = .0389).
In both univariate and in multivariate analysis controlling for tumor grade, comedo carcinoma, tamoxifen use, margin status, and age, IBTR was significantly associated with lower risk for recurrence.
The investigators also conducted a boost/no boost analysis stratified by margin status using both the National Surgical Adjuvant Breast and Bowel Project (NSABP) definition of negative margins as no ink on tumor, and the joint Society of Surgical Oncology, ASTRO, and American Society of Clinical Oncology guideline definition as less than 2 mm, and in each case found that among patients with negative margins, boosting offered a significant recurrence-free advantage.
Finally, they found that boosting offered a significant advantage both for patients 50 and older (P = .0073) and those 49 and younger (P = .0166).
“For DCIS, most of who treat breast cancer have used a boost, but we really extrapolated from the treatment from invasive breast cancer. Evidence for this practice until now in specific for DCIS has been lacking,” said Geraldine Jacobson, MD, MPH, professor and chair of radiation oncology at West Virginia University, Morgantown. Dr. Jacobson moderated the briefing.
She noted that the large sample size and long follow-up of the study was necessary for the treatment benefit to emerge.
AT THE ASTRO ANNUAL MEETING 2016
Key clinical point: This study shows a significant benefit for adding a boost radiation dose following breast-conserving surgery and whole-breast radiation for ductal carcinoma in situ (DCIS).
Major finding: After 15 years of follow-up, 91.6% of women who had received a boost dose were free of ipsilateral breast recurrence, compared with 88% of women who did not have a boost.
Data source: Analysis of pooled data on 4,131 patients with DCIS treated at 10 academic medical centers.
Disclosures: The study was supported by participating institutions. Dr. Moran and Dr. Jacobson reported no conflicts of interest.
ATA’s risk assessment guidelines for thyroid nodules using sonography patterns validated
DENVER – The malignancy risk of thyroid nodules can be assessed with reassuring accuracy using ultrasound and the guidelines developed by the American Thyroid Association.
Ultrasound assessment is the first step of the evaluation of any patient with one or more thyroid nodules. “Maybe it shouldn’t be, but, for now, it is,” noted David L. Steward, MD, at the annual meeting of the American Thyroid Association.
The ATA guidelines categorize thyroid nodules on the basis of their ultrasound patterns, with the high risk of malignancy being in nodules that are taller than they are wide and /or have microcalcifications, irregular margins, hypoechoic areas, extrathyroidal extension, interrupted rim calcification with soft tissue extrusion, and suspicious lymph nodes. Between 70% and 90% of thyroids with such patterns will contain malignancy, according to the ATA guidelines. Lesions with an intermediate risk of malignancy have such sonographic findings as hypoechoic solid tissue and regular margins; between 10% and 20% of these are malignant. The third category in the ATA’s guidelines are those that are of low suspicion, with hyperechoic solid tissue, isoechoic solid tissue, partially cystic with eccentric solid area, and regular margins; 5%-10% of these are malignant. Thyroid nodules with a very-low risk of malignancy (less than 3%) are spongiform or partially cystic with no suspicious findings. Finally, benign nodules, of which less than 1% contain malignancy, are cysts, he said.
“We found that the size of the nodule on ultrasound that underwent fine needle aspiration was inversely correlated with malignancy risk: The lower risk nodules were larger,” he said.
Using the ATA’s system, 9 (4%) of the nodules were high risk, 64 (31%) were intermediate risk, 79 (38%) were low risk, 54 (26%) were very-low risk, and none were benign. Five of the nodules were not included in the results presented.
There was good correlation between the Bethesda and ATA classification systems. Of the lesions that were malignant or suspicious for malignancy in the Bethesda system, 77% were very-high risk for malignancy on ultrasound according to the ATA. Of the lesions that were atypia of undetermined significance (AUS)/follicular lesion of undetermined significance (FLUS), 22% were very high risk according to the ATA. Neither of the systems classified as malignant any of the lesions as follicular/Hurthle cell cancer, benign, or nondiagnostic.
The AUS/FLUS nodules “tend to be all over the map,” he noted. Looking at just the AUS/FLUS nodules, malignancy was found on pathology in 100% classified by the ATA system as being high risk; in 21% of those called intermediate risk; in 17% of those called low risk; and in 12% of the very-low risk group.
The study was funded by the University of Cincinnati. Dr. Steward said his only disclosure is that he was a member of the ATA committee that wrote the guidelines under evaluation in this study.
DENVER – The malignancy risk of thyroid nodules can be assessed with reassuring accuracy using ultrasound and the guidelines developed by the American Thyroid Association.
Ultrasound assessment is the first step of the evaluation of any patient with one or more thyroid nodules. “Maybe it shouldn’t be, but, for now, it is,” noted David L. Steward, MD, at the annual meeting of the American Thyroid Association.
The ATA guidelines categorize thyroid nodules on the basis of their ultrasound patterns, with the high risk of malignancy being in nodules that are taller than they are wide and /or have microcalcifications, irregular margins, hypoechoic areas, extrathyroidal extension, interrupted rim calcification with soft tissue extrusion, and suspicious lymph nodes. Between 70% and 90% of thyroids with such patterns will contain malignancy, according to the ATA guidelines. Lesions with an intermediate risk of malignancy have such sonographic findings as hypoechoic solid tissue and regular margins; between 10% and 20% of these are malignant. The third category in the ATA’s guidelines are those that are of low suspicion, with hyperechoic solid tissue, isoechoic solid tissue, partially cystic with eccentric solid area, and regular margins; 5%-10% of these are malignant. Thyroid nodules with a very-low risk of malignancy (less than 3%) are spongiform or partially cystic with no suspicious findings. Finally, benign nodules, of which less than 1% contain malignancy, are cysts, he said.
“We found that the size of the nodule on ultrasound that underwent fine needle aspiration was inversely correlated with malignancy risk: The lower risk nodules were larger,” he said.
Using the ATA’s system, 9 (4%) of the nodules were high risk, 64 (31%) were intermediate risk, 79 (38%) were low risk, 54 (26%) were very-low risk, and none were benign. Five of the nodules were not included in the results presented.
There was good correlation between the Bethesda and ATA classification systems. Of the lesions that were malignant or suspicious for malignancy in the Bethesda system, 77% were very-high risk for malignancy on ultrasound according to the ATA. Of the lesions that were atypia of undetermined significance (AUS)/follicular lesion of undetermined significance (FLUS), 22% were very high risk according to the ATA. Neither of the systems classified as malignant any of the lesions as follicular/Hurthle cell cancer, benign, or nondiagnostic.
The AUS/FLUS nodules “tend to be all over the map,” he noted. Looking at just the AUS/FLUS nodules, malignancy was found on pathology in 100% classified by the ATA system as being high risk; in 21% of those called intermediate risk; in 17% of those called low risk; and in 12% of the very-low risk group.
The study was funded by the University of Cincinnati. Dr. Steward said his only disclosure is that he was a member of the ATA committee that wrote the guidelines under evaluation in this study.
DENVER – The malignancy risk of thyroid nodules can be assessed with reassuring accuracy using ultrasound and the guidelines developed by the American Thyroid Association.
Ultrasound assessment is the first step of the evaluation of any patient with one or more thyroid nodules. “Maybe it shouldn’t be, but, for now, it is,” noted David L. Steward, MD, at the annual meeting of the American Thyroid Association.
The ATA guidelines categorize thyroid nodules on the basis of their ultrasound patterns, with the high risk of malignancy being in nodules that are taller than they are wide and /or have microcalcifications, irregular margins, hypoechoic areas, extrathyroidal extension, interrupted rim calcification with soft tissue extrusion, and suspicious lymph nodes. Between 70% and 90% of thyroids with such patterns will contain malignancy, according to the ATA guidelines. Lesions with an intermediate risk of malignancy have such sonographic findings as hypoechoic solid tissue and regular margins; between 10% and 20% of these are malignant. The third category in the ATA’s guidelines are those that are of low suspicion, with hyperechoic solid tissue, isoechoic solid tissue, partially cystic with eccentric solid area, and regular margins; 5%-10% of these are malignant. Thyroid nodules with a very-low risk of malignancy (less than 3%) are spongiform or partially cystic with no suspicious findings. Finally, benign nodules, of which less than 1% contain malignancy, are cysts, he said.
“We found that the size of the nodule on ultrasound that underwent fine needle aspiration was inversely correlated with malignancy risk: The lower risk nodules were larger,” he said.
Using the ATA’s system, 9 (4%) of the nodules were high risk, 64 (31%) were intermediate risk, 79 (38%) were low risk, 54 (26%) were very-low risk, and none were benign. Five of the nodules were not included in the results presented.
There was good correlation between the Bethesda and ATA classification systems. Of the lesions that were malignant or suspicious for malignancy in the Bethesda system, 77% were very-high risk for malignancy on ultrasound according to the ATA. Of the lesions that were atypia of undetermined significance (AUS)/follicular lesion of undetermined significance (FLUS), 22% were very high risk according to the ATA. Neither of the systems classified as malignant any of the lesions as follicular/Hurthle cell cancer, benign, or nondiagnostic.
The AUS/FLUS nodules “tend to be all over the map,” he noted. Looking at just the AUS/FLUS nodules, malignancy was found on pathology in 100% classified by the ATA system as being high risk; in 21% of those called intermediate risk; in 17% of those called low risk; and in 12% of the very-low risk group.
The study was funded by the University of Cincinnati. Dr. Steward said his only disclosure is that he was a member of the ATA committee that wrote the guidelines under evaluation in this study.
Key clinical point:
Major finding: Of the lesions that were malignant or suspicious for malignancy in the Bethesda system, 77% were very-high risk for malignancy on ultrasound, according to the ATA.
Data source: Prospective validation of the ATA’s ultrasound risk assessment guidelines on 211 thyroid nodules excised from 199 patients.
Disclosures: The study was funded by the University of Cincinnati. Dr. Steward said his only disclosure is that he was a member of the ATA committee that wrote the guidelines under evaluation in this study.
Less pain, quicker discharge with post-TORS dexamethasone
SEATTLE – A longer course of dexamethasone was a bit better than the usual single intraoperative dose for controlling pain and dysphagia after transoral robotic surgery in a randomized trial from the Oregon Health and Science University, Portland.
Thirty-five subjects were randomized to the standard 10-mg intraoperative dexamethasone dose plus 8 mg every 8 hours for up to 4 days; 33 others were randomized to the intraoperative dose plus placebo. All the subjects had transoral robotic surgery (TORS) resection for T1 or T2 oropharyngeal squamous cell carcinoma, either partial pharyngectomy/radical tonsillectomy, base of tongue resection, or both.
The dexamethasone group had significantly less pain on postop day 3 (about 1.5 points less on the 10-point visual analogue scale) and were discharged, on average, a day earlier. They also advanced more quickly toward solid food at 1- and 3-weeks’ follow-up. “They were much more likely to be on a full-soft diet, while the placebo group was mostly still on purees, and just starting into soft foods,” said lead investigator Daniel Clayburgh, MD, a head and neck cancer specialist at the university.
Otherwise, however, the extra dexamethasone wasn’t much help; pain scores were the same in both groups for the first couple days after surgery and at follow-up, and both groups used the same amount of post-op opioids. Other than food tolerance, dysphagia metrics were pretty much the same.
“I was actually anticipating a little bit more of a benefit, but there are potentially some benefits to extended corticosteroid courses after TORS. It’s safe, and well tolerated so long as you screen out diabetes and other problems with hyperglycemia,” as was done in the study, he said. “It does decrease post-op length of stay and may provide a modest decrease in post-op pain, and may slightly accelerate advancement of dietary consistency,” Dr. Clayburgh said at the International Conference on Head and Neck Cancer, held by the American Head and Neck Society.
Although he and his colleagues are mulling over what to do with the findings in light of other initiatives to reduce post-TORS pain, they are now likely to extend dexamethasone courses when significant post-op pain seems likely, and doing so is not otherwise contraindicated, he said.
Intraoperative corticosteroids are now routine for TORS, based on the strength of benefit in the tonsillectomy literature. The team decided to try an extended course because “being rather simple minded surgeons, we thought that if one dose is good, more should be better,” Dr. Clayburgh said.
The dexamethasone group was slightly younger than the placebo group (56 vs. 61 years) but otherwise similar; most were men. In addition to patients with hyperglycemia issues, those with confounders for post-op speech and swallowing recovery were among those excluded from the trial. Subjects required nasogastric feeding tubes for a median of 6.5 days postoperatively, lost a mean of 10 pounds in the first 2 post-op weeks, and were hospitalized for a mean of about 5 days. Dexamethasone was delivered orally or by nasogastric tube.
There was no external funding for the study, and Dr. Clayburgh had no relevant financial disclosures.
SEATTLE – A longer course of dexamethasone was a bit better than the usual single intraoperative dose for controlling pain and dysphagia after transoral robotic surgery in a randomized trial from the Oregon Health and Science University, Portland.
Thirty-five subjects were randomized to the standard 10-mg intraoperative dexamethasone dose plus 8 mg every 8 hours for up to 4 days; 33 others were randomized to the intraoperative dose plus placebo. All the subjects had transoral robotic surgery (TORS) resection for T1 or T2 oropharyngeal squamous cell carcinoma, either partial pharyngectomy/radical tonsillectomy, base of tongue resection, or both.
The dexamethasone group had significantly less pain on postop day 3 (about 1.5 points less on the 10-point visual analogue scale) and were discharged, on average, a day earlier. They also advanced more quickly toward solid food at 1- and 3-weeks’ follow-up. “They were much more likely to be on a full-soft diet, while the placebo group was mostly still on purees, and just starting into soft foods,” said lead investigator Daniel Clayburgh, MD, a head and neck cancer specialist at the university.
Otherwise, however, the extra dexamethasone wasn’t much help; pain scores were the same in both groups for the first couple days after surgery and at follow-up, and both groups used the same amount of post-op opioids. Other than food tolerance, dysphagia metrics were pretty much the same.
“I was actually anticipating a little bit more of a benefit, but there are potentially some benefits to extended corticosteroid courses after TORS. It’s safe, and well tolerated so long as you screen out diabetes and other problems with hyperglycemia,” as was done in the study, he said. “It does decrease post-op length of stay and may provide a modest decrease in post-op pain, and may slightly accelerate advancement of dietary consistency,” Dr. Clayburgh said at the International Conference on Head and Neck Cancer, held by the American Head and Neck Society.
Although he and his colleagues are mulling over what to do with the findings in light of other initiatives to reduce post-TORS pain, they are now likely to extend dexamethasone courses when significant post-op pain seems likely, and doing so is not otherwise contraindicated, he said.
Intraoperative corticosteroids are now routine for TORS, based on the strength of benefit in the tonsillectomy literature. The team decided to try an extended course because “being rather simple minded surgeons, we thought that if one dose is good, more should be better,” Dr. Clayburgh said.
The dexamethasone group was slightly younger than the placebo group (56 vs. 61 years) but otherwise similar; most were men. In addition to patients with hyperglycemia issues, those with confounders for post-op speech and swallowing recovery were among those excluded from the trial. Subjects required nasogastric feeding tubes for a median of 6.5 days postoperatively, lost a mean of 10 pounds in the first 2 post-op weeks, and were hospitalized for a mean of about 5 days. Dexamethasone was delivered orally or by nasogastric tube.
There was no external funding for the study, and Dr. Clayburgh had no relevant financial disclosures.
SEATTLE – A longer course of dexamethasone was a bit better than the usual single intraoperative dose for controlling pain and dysphagia after transoral robotic surgery in a randomized trial from the Oregon Health and Science University, Portland.
Thirty-five subjects were randomized to the standard 10-mg intraoperative dexamethasone dose plus 8 mg every 8 hours for up to 4 days; 33 others were randomized to the intraoperative dose plus placebo. All the subjects had transoral robotic surgery (TORS) resection for T1 or T2 oropharyngeal squamous cell carcinoma, either partial pharyngectomy/radical tonsillectomy, base of tongue resection, or both.
The dexamethasone group had significantly less pain on postop day 3 (about 1.5 points less on the 10-point visual analogue scale) and were discharged, on average, a day earlier. They also advanced more quickly toward solid food at 1- and 3-weeks’ follow-up. “They were much more likely to be on a full-soft diet, while the placebo group was mostly still on purees, and just starting into soft foods,” said lead investigator Daniel Clayburgh, MD, a head and neck cancer specialist at the university.
Otherwise, however, the extra dexamethasone wasn’t much help; pain scores were the same in both groups for the first couple days after surgery and at follow-up, and both groups used the same amount of post-op opioids. Other than food tolerance, dysphagia metrics were pretty much the same.
“I was actually anticipating a little bit more of a benefit, but there are potentially some benefits to extended corticosteroid courses after TORS. It’s safe, and well tolerated so long as you screen out diabetes and other problems with hyperglycemia,” as was done in the study, he said. “It does decrease post-op length of stay and may provide a modest decrease in post-op pain, and may slightly accelerate advancement of dietary consistency,” Dr. Clayburgh said at the International Conference on Head and Neck Cancer, held by the American Head and Neck Society.
Although he and his colleagues are mulling over what to do with the findings in light of other initiatives to reduce post-TORS pain, they are now likely to extend dexamethasone courses when significant post-op pain seems likely, and doing so is not otherwise contraindicated, he said.
Intraoperative corticosteroids are now routine for TORS, based on the strength of benefit in the tonsillectomy literature. The team decided to try an extended course because “being rather simple minded surgeons, we thought that if one dose is good, more should be better,” Dr. Clayburgh said.
The dexamethasone group was slightly younger than the placebo group (56 vs. 61 years) but otherwise similar; most were men. In addition to patients with hyperglycemia issues, those with confounders for post-op speech and swallowing recovery were among those excluded from the trial. Subjects required nasogastric feeding tubes for a median of 6.5 days postoperatively, lost a mean of 10 pounds in the first 2 post-op weeks, and were hospitalized for a mean of about 5 days. Dexamethasone was delivered orally or by nasogastric tube.
There was no external funding for the study, and Dr. Clayburgh had no relevant financial disclosures.
AT AHNS 2016
Key clinical point: A longer course of dexamethasone is a bit better than the usual single intraoperative dose for controlling pain and dysphagia after transoral robotic surgery.
Major finding: The dexamethasone group had significantly less pain on post-op day 3 (about 1.5 points less on the 10-point visual analogue scale) and were discharged, on average, a day earlier. They also advanced more quickly toward solid food at 1- and 3-weeks’ follow-up.
Data source: A randomized trial of 68 TORS patients.
Disclosures: There was no external funding for the study, and the lead investigator had no relevant financial disclosures.
Palliative cancer surgery: Prioritize patient values
Risk-assessment tools can give surgeons a clinical framework to help inform decisions about palliative care surgery in patients with advanced malignancies, but cannot replace nuanced clinical judgment that incorporates patients’ priorities, according to results of a meta-analysis.
Ian W. Folkert, MD, and Robert E. Roses, MD, of the department of surgery at the Hospital of the University of Pennsylvania, Philadelphia, reviewed the available research on the indications for palliative surgery for patients with advanced disease and risk-assessment tools for patient selection. Emergent and palliative surgery in such situations require a careful consideration of many clinical factors such as overall prognosis and risk of a surgical approach, compared with nonsurgical interventions (J Surg Oncol. 2016;114:[3]311-15). But the investigators concluded that while an evidence-based approach to patient selection for palliative cancer surgery can offer some guidance on the potential for achieving clinical goals, ultimately the decision to proceed must prioritize patient values and orientation to treatment.
Tumor bleeding
Tumor-related complications often initiate the question of palliative surgical and nonsurgical interventions.
Studies of acute hemorrhage from malignancies indicate that bleeding originating from a tumor is rarely massive and usually can be managed endoscopically (Clin Endosc. 2015 Mar;48[2]:121-7; Aliment Pharmacol Ther. 2013;38:144-50; Mayo Clin Proc. 1994;69[8]:736-40). Transcatheter arterial embolization also is used successfully to manage tumor bleeding (J Vasc Interv Radiol. 2015 Sep;26[9]:1297-304; Indian J Cancer. 2014 Feb; 51[6]56-9). The investigators stated, “Although tumor rebleeding may be frequent, repeat endoscopy is often effective and is self-recommending given the high risk of major morbidity after a palliative foregut resection ... [and] all efforts should be made to avoid emergency gastrectomy or esophagectomy.”
Obstructing tumors
Patients with acute colonic obstruction because of colon cancer typically have been treated with a proximal diverting colostomy, but palliative self-expanding metallic stent placement (SEMS) has emerged as an option. Recent studies have shown both short- and long-term clinical success of SEMS, but rates of major morbidity and mortality for emergent surgery and SEMS were similar, as were rates of overall survival (Surg Endosc. 2015;29[6];1580-5; World J Gastroenterol. 2013 Sep 7;19[33]:5565-74). SEMS-related mortality was primarily because of perforation (Endoscopy. 2008 Mar;40[3]:184-91). Stenting for esophageal and gastroesophageal (GE) function obstruction is also emerging as a nonsurgical option. The investigators noted, “There is a very limited role for palliative surgery for esophageal and GE junction tumors.” Gastric outlet obstruction, proximal duodenal obstruction, and biliary tract obstruction are treated palliatively with stents, but “gastrojejunostomy and other bypass operations may provide effective palliation in carefully selected patients.”
Tumor perforation
Few nonsurgical treatment options are available for tumor perforation. Palliative surgical intervention often is undertaken in the context of neutropenia and abdominal pain, the investigators said. These patients are at high risk for morbidity and mortality. One study reviewed found that “prolonged neutropenia and severe sepsis were associated with poor outcomes in all patients, while surgical management was associated with improved survival (Ann Surg. 2008;248[1];104-9), but nonoperative management and comfort care were deemed appropriate for those patients with advanced disease and for those in whom surgery is high risk.
Patient selection for palliative surgery
The studies examined suggest that patient selection for palliative surgical intervention requires the weighing of clinical variables of frailty, morbidity, and mortality. The investigators reviewed a variety of risk-assessment tools developed to help surgeons with that decision (J Am Coll Surg. 2003;197[1];16-21; J Palliat Med. 2014;17:37-42; Ann Surg. 2011;254[2]:333-8). Among the factors considered are the amplified risks of mortality in these patients, the high cost of emergent operations, and most importantly, the chances of extending survival. The complexity of palliative and emergent surgical indications means that risk-assessment studies are “frequently too reductive to provide meaningful guidance” to the surgeon.
Dr. Folkert and Dr. Roses concluded that risk-assessment tools underscore the poor outcomes associated with operations in this setting, and, to some extent, guide decision making, but “they do not supplant clinical judgment, nor do they account for patient values and orientation toward treatment. It remains impossible to place a uniform value on length and quality of life, and patients’ values are paramount in informing treatment decisions.”
The authors had no conflicts to disclose.
Risk-assessment tools can give surgeons a clinical framework to help inform decisions about palliative care surgery in patients with advanced malignancies, but cannot replace nuanced clinical judgment that incorporates patients’ priorities, according to results of a meta-analysis.
Ian W. Folkert, MD, and Robert E. Roses, MD, of the department of surgery at the Hospital of the University of Pennsylvania, Philadelphia, reviewed the available research on the indications for palliative surgery for patients with advanced disease and risk-assessment tools for patient selection. Emergent and palliative surgery in such situations require a careful consideration of many clinical factors such as overall prognosis and risk of a surgical approach, compared with nonsurgical interventions (J Surg Oncol. 2016;114:[3]311-15). But the investigators concluded that while an evidence-based approach to patient selection for palliative cancer surgery can offer some guidance on the potential for achieving clinical goals, ultimately the decision to proceed must prioritize patient values and orientation to treatment.
Tumor bleeding
Tumor-related complications often initiate the question of palliative surgical and nonsurgical interventions.
Studies of acute hemorrhage from malignancies indicate that bleeding originating from a tumor is rarely massive and usually can be managed endoscopically (Clin Endosc. 2015 Mar;48[2]:121-7; Aliment Pharmacol Ther. 2013;38:144-50; Mayo Clin Proc. 1994;69[8]:736-40). Transcatheter arterial embolization also is used successfully to manage tumor bleeding (J Vasc Interv Radiol. 2015 Sep;26[9]:1297-304; Indian J Cancer. 2014 Feb; 51[6]56-9). The investigators stated, “Although tumor rebleeding may be frequent, repeat endoscopy is often effective and is self-recommending given the high risk of major morbidity after a palliative foregut resection ... [and] all efforts should be made to avoid emergency gastrectomy or esophagectomy.”
Obstructing tumors
Patients with acute colonic obstruction because of colon cancer typically have been treated with a proximal diverting colostomy, but palliative self-expanding metallic stent placement (SEMS) has emerged as an option. Recent studies have shown both short- and long-term clinical success of SEMS, but rates of major morbidity and mortality for emergent surgery and SEMS were similar, as were rates of overall survival (Surg Endosc. 2015;29[6];1580-5; World J Gastroenterol. 2013 Sep 7;19[33]:5565-74). SEMS-related mortality was primarily because of perforation (Endoscopy. 2008 Mar;40[3]:184-91). Stenting for esophageal and gastroesophageal (GE) function obstruction is also emerging as a nonsurgical option. The investigators noted, “There is a very limited role for palliative surgery for esophageal and GE junction tumors.” Gastric outlet obstruction, proximal duodenal obstruction, and biliary tract obstruction are treated palliatively with stents, but “gastrojejunostomy and other bypass operations may provide effective palliation in carefully selected patients.”
Tumor perforation
Few nonsurgical treatment options are available for tumor perforation. Palliative surgical intervention often is undertaken in the context of neutropenia and abdominal pain, the investigators said. These patients are at high risk for morbidity and mortality. One study reviewed found that “prolonged neutropenia and severe sepsis were associated with poor outcomes in all patients, while surgical management was associated with improved survival (Ann Surg. 2008;248[1];104-9), but nonoperative management and comfort care were deemed appropriate for those patients with advanced disease and for those in whom surgery is high risk.
Patient selection for palliative surgery
The studies examined suggest that patient selection for palliative surgical intervention requires the weighing of clinical variables of frailty, morbidity, and mortality. The investigators reviewed a variety of risk-assessment tools developed to help surgeons with that decision (J Am Coll Surg. 2003;197[1];16-21; J Palliat Med. 2014;17:37-42; Ann Surg. 2011;254[2]:333-8). Among the factors considered are the amplified risks of mortality in these patients, the high cost of emergent operations, and most importantly, the chances of extending survival. The complexity of palliative and emergent surgical indications means that risk-assessment studies are “frequently too reductive to provide meaningful guidance” to the surgeon.
Dr. Folkert and Dr. Roses concluded that risk-assessment tools underscore the poor outcomes associated with operations in this setting, and, to some extent, guide decision making, but “they do not supplant clinical judgment, nor do they account for patient values and orientation toward treatment. It remains impossible to place a uniform value on length and quality of life, and patients’ values are paramount in informing treatment decisions.”
The authors had no conflicts to disclose.
Risk-assessment tools can give surgeons a clinical framework to help inform decisions about palliative care surgery in patients with advanced malignancies, but cannot replace nuanced clinical judgment that incorporates patients’ priorities, according to results of a meta-analysis.
Ian W. Folkert, MD, and Robert E. Roses, MD, of the department of surgery at the Hospital of the University of Pennsylvania, Philadelphia, reviewed the available research on the indications for palliative surgery for patients with advanced disease and risk-assessment tools for patient selection. Emergent and palliative surgery in such situations require a careful consideration of many clinical factors such as overall prognosis and risk of a surgical approach, compared with nonsurgical interventions (J Surg Oncol. 2016;114:[3]311-15). But the investigators concluded that while an evidence-based approach to patient selection for palliative cancer surgery can offer some guidance on the potential for achieving clinical goals, ultimately the decision to proceed must prioritize patient values and orientation to treatment.
Tumor bleeding
Tumor-related complications often initiate the question of palliative surgical and nonsurgical interventions.
Studies of acute hemorrhage from malignancies indicate that bleeding originating from a tumor is rarely massive and usually can be managed endoscopically (Clin Endosc. 2015 Mar;48[2]:121-7; Aliment Pharmacol Ther. 2013;38:144-50; Mayo Clin Proc. 1994;69[8]:736-40). Transcatheter arterial embolization also is used successfully to manage tumor bleeding (J Vasc Interv Radiol. 2015 Sep;26[9]:1297-304; Indian J Cancer. 2014 Feb; 51[6]56-9). The investigators stated, “Although tumor rebleeding may be frequent, repeat endoscopy is often effective and is self-recommending given the high risk of major morbidity after a palliative foregut resection ... [and] all efforts should be made to avoid emergency gastrectomy or esophagectomy.”
Obstructing tumors
Patients with acute colonic obstruction because of colon cancer typically have been treated with a proximal diverting colostomy, but palliative self-expanding metallic stent placement (SEMS) has emerged as an option. Recent studies have shown both short- and long-term clinical success of SEMS, but rates of major morbidity and mortality for emergent surgery and SEMS were similar, as were rates of overall survival (Surg Endosc. 2015;29[6];1580-5; World J Gastroenterol. 2013 Sep 7;19[33]:5565-74). SEMS-related mortality was primarily because of perforation (Endoscopy. 2008 Mar;40[3]:184-91). Stenting for esophageal and gastroesophageal (GE) function obstruction is also emerging as a nonsurgical option. The investigators noted, “There is a very limited role for palliative surgery for esophageal and GE junction tumors.” Gastric outlet obstruction, proximal duodenal obstruction, and biliary tract obstruction are treated palliatively with stents, but “gastrojejunostomy and other bypass operations may provide effective palliation in carefully selected patients.”
Tumor perforation
Few nonsurgical treatment options are available for tumor perforation. Palliative surgical intervention often is undertaken in the context of neutropenia and abdominal pain, the investigators said. These patients are at high risk for morbidity and mortality. One study reviewed found that “prolonged neutropenia and severe sepsis were associated with poor outcomes in all patients, while surgical management was associated with improved survival (Ann Surg. 2008;248[1];104-9), but nonoperative management and comfort care were deemed appropriate for those patients with advanced disease and for those in whom surgery is high risk.
Patient selection for palliative surgery
The studies examined suggest that patient selection for palliative surgical intervention requires the weighing of clinical variables of frailty, morbidity, and mortality. The investigators reviewed a variety of risk-assessment tools developed to help surgeons with that decision (J Am Coll Surg. 2003;197[1];16-21; J Palliat Med. 2014;17:37-42; Ann Surg. 2011;254[2]:333-8). Among the factors considered are the amplified risks of mortality in these patients, the high cost of emergent operations, and most importantly, the chances of extending survival. The complexity of palliative and emergent surgical indications means that risk-assessment studies are “frequently too reductive to provide meaningful guidance” to the surgeon.
Dr. Folkert and Dr. Roses concluded that risk-assessment tools underscore the poor outcomes associated with operations in this setting, and, to some extent, guide decision making, but “they do not supplant clinical judgment, nor do they account for patient values and orientation toward treatment. It remains impossible to place a uniform value on length and quality of life, and patients’ values are paramount in informing treatment decisions.”
The authors had no conflicts to disclose.
FROM THE JOURNAL OF SURGICAL ONCOLOGY
10-year follow-up: Localized prostate cancer treatments offer similar efficacy
The three main approaches for treating localized prostate cancer – surgery, radiotherapy, or active monitoring – yield similar efficacy outcomes but different quality-of-life outcomes, according to two reports published on the New England Journal of Medicine.
Both reports present the findings of the ongoing Protect (Prostate Testing for Cancer and Treatment) trial, a large prospective, randomized trial in the United Kingdom comparing mortality and other health outcomes in men with PSA-detected localized disease. The trial involved 82,429 men aged 50-69 years who had a PSA test between 1999 and 2009, of whom 2,664 were found to have localized prostate cancer. A total of 1,643 of these participants agreed to be randomly assigned to radical prostatectomy (553 men), radical radiotherapy (545 men), or active monitoring (545 men).
“Active monitoring” involved avoiding any immediate therapy and regularly monitoring disease progression so that radical treatment with curative intent could be given if the need arose. Patients were monitored every 3 months for the first year, then every 6-12 months thereafter. This differs from “watchful waiting,” which doesn’t involve any plan for curative radical treatment if disease progresses.
The first report focused on mortality and disease progression in these 1,643 participants at a median of 10 years of follow-up. The primary outcome measure, prostate cancer–specific survival, was 98.8% or greater across all three study groups, and there was no significant difference among them. Thus, all three approaches yielded the same efficacy: prostate cancer–specific mortality of approximately 1%, said Freddie C. Hamdy, MD, of the Nuffield Department of Surgical Sciences, University of Oxford, and his associates.
However, the rate of disease progression among men assigned to surgery (8.9/1,000 person-years) or to radiotherapy (9.0/1,000 person-years) was less than half the rate among men assigned to active monitoring (22.9/1,000 person-years). The rate of metastasis followed this same pattern (2.4, 3.0, and 6.3 per 1,000 person-years, respectively).
“These differences show the effectiveness of immediate radical therapy over active monitoring, but they have not translated into significant differences – nor have they ruled out equivalence – in disease-specific or all-cause mortality; thus, longer-term follow-up is necessary,” Dr. Hamdy and his associates said (N Engl J Med. 2016 Sep 14. doi: 10.1056/NEJMoa1606220).
The trade-off was that 44% of the men assigned to active monitoring were able to forgo both radical surgery and radical radiotherapy, avoiding the adverse effects of those treatments. These included nine thromboembolic or cardiovascular events, 14 transfusions, one rectal injury, and nine anastomotic problems requiring intervention, they noted.
It is important to remember that approximately one-fourth of the men assigned to active monitoring went on to undergo radical treatment within 3 years, and that more than half did so by the 10-year follow-up date, the investigators added.
The second report focused on patient-reported outcomes concerning urinary, bowel, sexual, and quality-of-life issues in the 1,643 participants at 6 years of follow-up. These differed markedly among the three study groups, said Jenny L. Donovan, PhD, of the School of Social and Community Medicine, University of Bristol, U.K., and her associates.
Prostatectomy had a clear negative effect on urinary continence and sexual function, particularly erectile function, compared with radiotherapy and active monitoring. This peaked at 6 months after surgery, and though some patients recovered some function over time, urinary incontinence remained worse in the prostatectomy group than in the other two groups throughout follow-up. The use of absorbent pads rose from 1% at baseline to 46% at 6 months in the prostatectomy group. In comparison, the 6-month rate in the radiotherapy group rose to only 5% and that in the active-monitoring group to only 4%.
Radiotherapy plus neoadjuvant androgen-deprivation therapy had more of a negative effect on bowel function, urinary voiding, and nocturia than did the other two treatment approaches. However, many patients eventually showed considerable recovery on most of these measures, except that they continued to have bloody stools more frequently than did men who had prostatectomy or active monitoring.
Men in the active-monitoring group had substantially less difficulty with urinary, sexual, and bowel function, as expected. However, this gradually worsened over time as increasing numbers of these men eventually underwent radical treatments, Dr. Donovan and her associates wrote (N Engl J Med. 2016 Sep 14. doi: 10.1056/NEJMoa1606221).
Quality-of-life measures generally reflected these differences among the three study groups, “with some evidence of accommodation to changes over time.” General mental and physical health, cancer-specific quality of life, and anxiety and depression all were similar across the three groups at 6 years.
“Follow-up for an additional 5-10 years is required to fully inform decisions involving the trade-off between the shorter-term effects of the management strategies shown here and the longer course of progression and treatment of prostate cancer in the context of other life-threatening conditions,” they said.
The Protect trial was supported by the U.K. National Institute for Health Research Health Technology Assessment Programme, the University of Oxford, University Hospitals Bristol, the Oxford NIHR Biomedical Research Centre, and the Cancer Research U.K. Oxford Centre. Dr. Hamdy and Dr. Donovan and their associates reported having no relevant financial disclosures.
As both groups of researchers noted, longer follow-up is needed to definitively assess outcomes in the Protect trial. For now, however, we can conclude that active monitoring leads to increased metastasis, compared with either surgery or radiotherapy.
So if a man wants to avoid metastatic prostate cancer and the adverse effects of its treatment, active monitoring should be considered only if he has life-shortening, coexisting disease and his life expectancy is less than the 10-year median follow-up of this study.
Men who have low- or intermediate-risk prostate cancer should feel free to select either surgery or radiotherapy on the basis of the treatments’ QOL profiles, since the mortality profiles are equivalent.
Anthony V. D’Amico, MD, is in the department of radiation oncology at Brigham and Women’s Hospital and the Dana-Farber Cancer Institute, both in Boston. He reported having no relevant financial disclosures. Dr. D’Amico made these remarks in an editorial accompanying the two reports on the Protect trial (N Engl J Med. 2016 Sep 14. doi: 10.1056/NEHMe1610395).
As both groups of researchers noted, longer follow-up is needed to definitively assess outcomes in the Protect trial. For now, however, we can conclude that active monitoring leads to increased metastasis, compared with either surgery or radiotherapy.
So if a man wants to avoid metastatic prostate cancer and the adverse effects of its treatment, active monitoring should be considered only if he has life-shortening, coexisting disease and his life expectancy is less than the 10-year median follow-up of this study.
Men who have low- or intermediate-risk prostate cancer should feel free to select either surgery or radiotherapy on the basis of the treatments’ QOL profiles, since the mortality profiles are equivalent.
Anthony V. D’Amico, MD, is in the department of radiation oncology at Brigham and Women’s Hospital and the Dana-Farber Cancer Institute, both in Boston. He reported having no relevant financial disclosures. Dr. D’Amico made these remarks in an editorial accompanying the two reports on the Protect trial (N Engl J Med. 2016 Sep 14. doi: 10.1056/NEHMe1610395).
As both groups of researchers noted, longer follow-up is needed to definitively assess outcomes in the Protect trial. For now, however, we can conclude that active monitoring leads to increased metastasis, compared with either surgery or radiotherapy.
So if a man wants to avoid metastatic prostate cancer and the adverse effects of its treatment, active monitoring should be considered only if he has life-shortening, coexisting disease and his life expectancy is less than the 10-year median follow-up of this study.
Men who have low- or intermediate-risk prostate cancer should feel free to select either surgery or radiotherapy on the basis of the treatments’ QOL profiles, since the mortality profiles are equivalent.
Anthony V. D’Amico, MD, is in the department of radiation oncology at Brigham and Women’s Hospital and the Dana-Farber Cancer Institute, both in Boston. He reported having no relevant financial disclosures. Dr. D’Amico made these remarks in an editorial accompanying the two reports on the Protect trial (N Engl J Med. 2016 Sep 14. doi: 10.1056/NEHMe1610395).
The three main approaches for treating localized prostate cancer – surgery, radiotherapy, or active monitoring – yield similar efficacy outcomes but different quality-of-life outcomes, according to two reports published on the New England Journal of Medicine.
Both reports present the findings of the ongoing Protect (Prostate Testing for Cancer and Treatment) trial, a large prospective, randomized trial in the United Kingdom comparing mortality and other health outcomes in men with PSA-detected localized disease. The trial involved 82,429 men aged 50-69 years who had a PSA test between 1999 and 2009, of whom 2,664 were found to have localized prostate cancer. A total of 1,643 of these participants agreed to be randomly assigned to radical prostatectomy (553 men), radical radiotherapy (545 men), or active monitoring (545 men).
“Active monitoring” involved avoiding any immediate therapy and regularly monitoring disease progression so that radical treatment with curative intent could be given if the need arose. Patients were monitored every 3 months for the first year, then every 6-12 months thereafter. This differs from “watchful waiting,” which doesn’t involve any plan for curative radical treatment if disease progresses.
The first report focused on mortality and disease progression in these 1,643 participants at a median of 10 years of follow-up. The primary outcome measure, prostate cancer–specific survival, was 98.8% or greater across all three study groups, and there was no significant difference among them. Thus, all three approaches yielded the same efficacy: prostate cancer–specific mortality of approximately 1%, said Freddie C. Hamdy, MD, of the Nuffield Department of Surgical Sciences, University of Oxford, and his associates.
However, the rate of disease progression among men assigned to surgery (8.9/1,000 person-years) or to radiotherapy (9.0/1,000 person-years) was less than half the rate among men assigned to active monitoring (22.9/1,000 person-years). The rate of metastasis followed this same pattern (2.4, 3.0, and 6.3 per 1,000 person-years, respectively).
“These differences show the effectiveness of immediate radical therapy over active monitoring, but they have not translated into significant differences – nor have they ruled out equivalence – in disease-specific or all-cause mortality; thus, longer-term follow-up is necessary,” Dr. Hamdy and his associates said (N Engl J Med. 2016 Sep 14. doi: 10.1056/NEJMoa1606220).
The trade-off was that 44% of the men assigned to active monitoring were able to forgo both radical surgery and radical radiotherapy, avoiding the adverse effects of those treatments. These included nine thromboembolic or cardiovascular events, 14 transfusions, one rectal injury, and nine anastomotic problems requiring intervention, they noted.
It is important to remember that approximately one-fourth of the men assigned to active monitoring went on to undergo radical treatment within 3 years, and that more than half did so by the 10-year follow-up date, the investigators added.
The second report focused on patient-reported outcomes concerning urinary, bowel, sexual, and quality-of-life issues in the 1,643 participants at 6 years of follow-up. These differed markedly among the three study groups, said Jenny L. Donovan, PhD, of the School of Social and Community Medicine, University of Bristol, U.K., and her associates.
Prostatectomy had a clear negative effect on urinary continence and sexual function, particularly erectile function, compared with radiotherapy and active monitoring. This peaked at 6 months after surgery, and though some patients recovered some function over time, urinary incontinence remained worse in the prostatectomy group than in the other two groups throughout follow-up. The use of absorbent pads rose from 1% at baseline to 46% at 6 months in the prostatectomy group. In comparison, the 6-month rate in the radiotherapy group rose to only 5% and that in the active-monitoring group to only 4%.
Radiotherapy plus neoadjuvant androgen-deprivation therapy had more of a negative effect on bowel function, urinary voiding, and nocturia than did the other two treatment approaches. However, many patients eventually showed considerable recovery on most of these measures, except that they continued to have bloody stools more frequently than did men who had prostatectomy or active monitoring.
Men in the active-monitoring group had substantially less difficulty with urinary, sexual, and bowel function, as expected. However, this gradually worsened over time as increasing numbers of these men eventually underwent radical treatments, Dr. Donovan and her associates wrote (N Engl J Med. 2016 Sep 14. doi: 10.1056/NEJMoa1606221).
Quality-of-life measures generally reflected these differences among the three study groups, “with some evidence of accommodation to changes over time.” General mental and physical health, cancer-specific quality of life, and anxiety and depression all were similar across the three groups at 6 years.
“Follow-up for an additional 5-10 years is required to fully inform decisions involving the trade-off between the shorter-term effects of the management strategies shown here and the longer course of progression and treatment of prostate cancer in the context of other life-threatening conditions,” they said.
The Protect trial was supported by the U.K. National Institute for Health Research Health Technology Assessment Programme, the University of Oxford, University Hospitals Bristol, the Oxford NIHR Biomedical Research Centre, and the Cancer Research U.K. Oxford Centre. Dr. Hamdy and Dr. Donovan and their associates reported having no relevant financial disclosures.
The three main approaches for treating localized prostate cancer – surgery, radiotherapy, or active monitoring – yield similar efficacy outcomes but different quality-of-life outcomes, according to two reports published on the New England Journal of Medicine.
Both reports present the findings of the ongoing Protect (Prostate Testing for Cancer and Treatment) trial, a large prospective, randomized trial in the United Kingdom comparing mortality and other health outcomes in men with PSA-detected localized disease. The trial involved 82,429 men aged 50-69 years who had a PSA test between 1999 and 2009, of whom 2,664 were found to have localized prostate cancer. A total of 1,643 of these participants agreed to be randomly assigned to radical prostatectomy (553 men), radical radiotherapy (545 men), or active monitoring (545 men).
“Active monitoring” involved avoiding any immediate therapy and regularly monitoring disease progression so that radical treatment with curative intent could be given if the need arose. Patients were monitored every 3 months for the first year, then every 6-12 months thereafter. This differs from “watchful waiting,” which doesn’t involve any plan for curative radical treatment if disease progresses.
The first report focused on mortality and disease progression in these 1,643 participants at a median of 10 years of follow-up. The primary outcome measure, prostate cancer–specific survival, was 98.8% or greater across all three study groups, and there was no significant difference among them. Thus, all three approaches yielded the same efficacy: prostate cancer–specific mortality of approximately 1%, said Freddie C. Hamdy, MD, of the Nuffield Department of Surgical Sciences, University of Oxford, and his associates.
However, the rate of disease progression among men assigned to surgery (8.9/1,000 person-years) or to radiotherapy (9.0/1,000 person-years) was less than half the rate among men assigned to active monitoring (22.9/1,000 person-years). The rate of metastasis followed this same pattern (2.4, 3.0, and 6.3 per 1,000 person-years, respectively).
“These differences show the effectiveness of immediate radical therapy over active monitoring, but they have not translated into significant differences – nor have they ruled out equivalence – in disease-specific or all-cause mortality; thus, longer-term follow-up is necessary,” Dr. Hamdy and his associates said (N Engl J Med. 2016 Sep 14. doi: 10.1056/NEJMoa1606220).
The trade-off was that 44% of the men assigned to active monitoring were able to forgo both radical surgery and radical radiotherapy, avoiding the adverse effects of those treatments. These included nine thromboembolic or cardiovascular events, 14 transfusions, one rectal injury, and nine anastomotic problems requiring intervention, they noted.
It is important to remember that approximately one-fourth of the men assigned to active monitoring went on to undergo radical treatment within 3 years, and that more than half did so by the 10-year follow-up date, the investigators added.
The second report focused on patient-reported outcomes concerning urinary, bowel, sexual, and quality-of-life issues in the 1,643 participants at 6 years of follow-up. These differed markedly among the three study groups, said Jenny L. Donovan, PhD, of the School of Social and Community Medicine, University of Bristol, U.K., and her associates.
Prostatectomy had a clear negative effect on urinary continence and sexual function, particularly erectile function, compared with radiotherapy and active monitoring. This peaked at 6 months after surgery, and though some patients recovered some function over time, urinary incontinence remained worse in the prostatectomy group than in the other two groups throughout follow-up. The use of absorbent pads rose from 1% at baseline to 46% at 6 months in the prostatectomy group. In comparison, the 6-month rate in the radiotherapy group rose to only 5% and that in the active-monitoring group to only 4%.
Radiotherapy plus neoadjuvant androgen-deprivation therapy had more of a negative effect on bowel function, urinary voiding, and nocturia than did the other two treatment approaches. However, many patients eventually showed considerable recovery on most of these measures, except that they continued to have bloody stools more frequently than did men who had prostatectomy or active monitoring.
Men in the active-monitoring group had substantially less difficulty with urinary, sexual, and bowel function, as expected. However, this gradually worsened over time as increasing numbers of these men eventually underwent radical treatments, Dr. Donovan and her associates wrote (N Engl J Med. 2016 Sep 14. doi: 10.1056/NEJMoa1606221).
Quality-of-life measures generally reflected these differences among the three study groups, “with some evidence of accommodation to changes over time.” General mental and physical health, cancer-specific quality of life, and anxiety and depression all were similar across the three groups at 6 years.
“Follow-up for an additional 5-10 years is required to fully inform decisions involving the trade-off between the shorter-term effects of the management strategies shown here and the longer course of progression and treatment of prostate cancer in the context of other life-threatening conditions,” they said.
The Protect trial was supported by the U.K. National Institute for Health Research Health Technology Assessment Programme, the University of Oxford, University Hospitals Bristol, the Oxford NIHR Biomedical Research Centre, and the Cancer Research U.K. Oxford Centre. Dr. Hamdy and Dr. Donovan and their associates reported having no relevant financial disclosures.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: The three main approaches for treating localized prostate cancer yield similar efficacy outcomes but different quality-of-life outcomes.
Major finding: All three treatment approaches – surgery, radiotherapy, and active monitoring – yielded the same efficacy: 10-year prostate cancer–specific mortality of approximately 1%.
Data source: The Protect trial, a prospective randomized study involving 1,643 prostate cancer patients in the U.K. followed for 10 years.
Disclosures: The Protect trial was supported by the U.K. National Institute for Health Research Health Technology Assessment Programme, the University of Oxford, University Hospitals Bristol, the Oxford NIHR Biomedical Research Centre, and the Cancer Research U.K. Oxford Centre. Dr. Hamdy and Dr. Donovan and their associates reported having no relevant financial disclosures.
ASCO updates postmastectomy radiotherapy guideline
The 2001 American Society of Clinical Oncology clinical practice guideline on postmastectomy radiotherapy has been updated to help clinicians and patients decide which cases of T1-T2 breast cancer with one-three positive axillary lymph nodes will not benefit from the treatment. Recent evidence suggests that some subsets of this patient group are likely to have such a low risk of locoregional recurrence that the potential adverse effects of radiotherapy would outweigh its benefit.
“We still don’t have a single, validated formula that can determine who needs postmastectomy radiotherapy, but we hope that the research evidence summarized in this guideline update will help doctors and patients make more informed decisions,” Abram Recht, MD, cochair of the expert panel that developed the update, said in a press statement.
“Thanks to advances in systemic therapy, fewer women need radiation therapy after a mastectomy. This means we can be more selective when recommending this treatment to our patients,” said Dr. Recht of Beth Israel Deaconess Medical Center, Boston.
ASCO, in collaboration with the American Society for Radiation Oncology and the Society of Surgical Oncology, reviewed the literature since 2001, including a meta-analysis of 22 clinical trials involving 8,135 patients who were randomly assigned to receive or not receive postmastectomy radiotherapy. “Our panel focused on the results for the 3,786 women who underwent axillary dissection.” The 10-year rate of locoregional treatment failure was only 4.3% with irradiation, compared with 21.0% without irradiation. The 10-year rate of local or distant treatment failure was 33.8% with irradiation and 45.5% without it, and the 20-year rates of breast cancer mortality were 41.5% and 49.4%, respectively.
“The panel unanimously agreed that the available evidence shows that postmastectomy radiotherapy reduces the risks of locoregional failure, any recurrence, and breast cancer mortality for patients with T1-T2 breast cancer and one-three positive lymph nodes,” according to the guideline.
However, clinicians and patients “should consider factors that may decrease the risk of locoregional failure, attenuate the benefit of reduced breast cancer–specific mortality, and/or increase the risk of complications.” These include older patient age, limited life expectancy, coexisting conditions that affect the risk of complications, small tumor size, absence of lymphovascular invasion, small nodal metastases, substantial response to neoadjuvant systemic therapy, low tumor grade, or strong tumor sensitivity to hormonal therapy, Dr. Recht and his associates said (J Clin Oncol. 2016 Sep 19. doi: 10.1200/JCO.2016.69.1188).
The guideline also addressed other areas of controversy.
Some clinicians now omit performing axillary lymph node dissection if one-two sentinel nodes are positive, “primarily based on extrapolation of data from randomized trials of patients treated exclusively or predominantly with breast-conserving surgery.” In such cases, the updated guideline “recommends that these patients receive postmastectomy radiotherapy only if there is already sufficient information to justify its use without needing to know that additional axillary nodes are involved.”
Many clinicians are uncertain whether to pursue postmastectomy radiotherapy in patients who have received neoadjuvant systemic therapy, because observational studies suggest the risk of locoregional recurrence is low for those who either have clinically negative nodes before undergoing the treatment or have a complete pathologic response to it in the lymph nodes. The panel decided that current evidence is insufficient to recommend whether radiotherapy should be administered or can be omitted in these groups.
The guideline also recommends that regional nodal irradiation generally should target both the internal mammary and the supraclavicular-axillary apical nodes, in addition to the chest wall or reconstructed breast. “There may be subgroups that will experience limited, if any, benefits from treating both these nodal areas, compared with treating only one or perhaps treating only the chest wall or reconstructed breast. [However,] there is insufficient evidence at this time to define such subgroups in detail.”
Copies of the updated guideline, as well as additional information such as details regarding the panel’s methodology, evidence tables, and clinical tools, are avail at www.asco.org/pmrt-guideline and www.asco.org/guidelineswiki.
This work was supported by the American Society of Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology. Dr. Recht reported serving as a consultant or advisor to CareCore and U.S. Oncology and receiving research funding from Genomic Health; his associates reported ties to numerous industry sources.
The 2001 American Society of Clinical Oncology clinical practice guideline on postmastectomy radiotherapy has been updated to help clinicians and patients decide which cases of T1-T2 breast cancer with one-three positive axillary lymph nodes will not benefit from the treatment. Recent evidence suggests that some subsets of this patient group are likely to have such a low risk of locoregional recurrence that the potential adverse effects of radiotherapy would outweigh its benefit.
“We still don’t have a single, validated formula that can determine who needs postmastectomy radiotherapy, but we hope that the research evidence summarized in this guideline update will help doctors and patients make more informed decisions,” Abram Recht, MD, cochair of the expert panel that developed the update, said in a press statement.
“Thanks to advances in systemic therapy, fewer women need radiation therapy after a mastectomy. This means we can be more selective when recommending this treatment to our patients,” said Dr. Recht of Beth Israel Deaconess Medical Center, Boston.
ASCO, in collaboration with the American Society for Radiation Oncology and the Society of Surgical Oncology, reviewed the literature since 2001, including a meta-analysis of 22 clinical trials involving 8,135 patients who were randomly assigned to receive or not receive postmastectomy radiotherapy. “Our panel focused on the results for the 3,786 women who underwent axillary dissection.” The 10-year rate of locoregional treatment failure was only 4.3% with irradiation, compared with 21.0% without irradiation. The 10-year rate of local or distant treatment failure was 33.8% with irradiation and 45.5% without it, and the 20-year rates of breast cancer mortality were 41.5% and 49.4%, respectively.
“The panel unanimously agreed that the available evidence shows that postmastectomy radiotherapy reduces the risks of locoregional failure, any recurrence, and breast cancer mortality for patients with T1-T2 breast cancer and one-three positive lymph nodes,” according to the guideline.
However, clinicians and patients “should consider factors that may decrease the risk of locoregional failure, attenuate the benefit of reduced breast cancer–specific mortality, and/or increase the risk of complications.” These include older patient age, limited life expectancy, coexisting conditions that affect the risk of complications, small tumor size, absence of lymphovascular invasion, small nodal metastases, substantial response to neoadjuvant systemic therapy, low tumor grade, or strong tumor sensitivity to hormonal therapy, Dr. Recht and his associates said (J Clin Oncol. 2016 Sep 19. doi: 10.1200/JCO.2016.69.1188).
The guideline also addressed other areas of controversy.
Some clinicians now omit performing axillary lymph node dissection if one-two sentinel nodes are positive, “primarily based on extrapolation of data from randomized trials of patients treated exclusively or predominantly with breast-conserving surgery.” In such cases, the updated guideline “recommends that these patients receive postmastectomy radiotherapy only if there is already sufficient information to justify its use without needing to know that additional axillary nodes are involved.”
Many clinicians are uncertain whether to pursue postmastectomy radiotherapy in patients who have received neoadjuvant systemic therapy, because observational studies suggest the risk of locoregional recurrence is low for those who either have clinically negative nodes before undergoing the treatment or have a complete pathologic response to it in the lymph nodes. The panel decided that current evidence is insufficient to recommend whether radiotherapy should be administered or can be omitted in these groups.
The guideline also recommends that regional nodal irradiation generally should target both the internal mammary and the supraclavicular-axillary apical nodes, in addition to the chest wall or reconstructed breast. “There may be subgroups that will experience limited, if any, benefits from treating both these nodal areas, compared with treating only one or perhaps treating only the chest wall or reconstructed breast. [However,] there is insufficient evidence at this time to define such subgroups in detail.”
Copies of the updated guideline, as well as additional information such as details regarding the panel’s methodology, evidence tables, and clinical tools, are avail at www.asco.org/pmrt-guideline and www.asco.org/guidelineswiki.
This work was supported by the American Society of Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology. Dr. Recht reported serving as a consultant or advisor to CareCore and U.S. Oncology and receiving research funding from Genomic Health; his associates reported ties to numerous industry sources.
The 2001 American Society of Clinical Oncology clinical practice guideline on postmastectomy radiotherapy has been updated to help clinicians and patients decide which cases of T1-T2 breast cancer with one-three positive axillary lymph nodes will not benefit from the treatment. Recent evidence suggests that some subsets of this patient group are likely to have such a low risk of locoregional recurrence that the potential adverse effects of radiotherapy would outweigh its benefit.
“We still don’t have a single, validated formula that can determine who needs postmastectomy radiotherapy, but we hope that the research evidence summarized in this guideline update will help doctors and patients make more informed decisions,” Abram Recht, MD, cochair of the expert panel that developed the update, said in a press statement.
“Thanks to advances in systemic therapy, fewer women need radiation therapy after a mastectomy. This means we can be more selective when recommending this treatment to our patients,” said Dr. Recht of Beth Israel Deaconess Medical Center, Boston.
ASCO, in collaboration with the American Society for Radiation Oncology and the Society of Surgical Oncology, reviewed the literature since 2001, including a meta-analysis of 22 clinical trials involving 8,135 patients who were randomly assigned to receive or not receive postmastectomy radiotherapy. “Our panel focused on the results for the 3,786 women who underwent axillary dissection.” The 10-year rate of locoregional treatment failure was only 4.3% with irradiation, compared with 21.0% without irradiation. The 10-year rate of local or distant treatment failure was 33.8% with irradiation and 45.5% without it, and the 20-year rates of breast cancer mortality were 41.5% and 49.4%, respectively.
“The panel unanimously agreed that the available evidence shows that postmastectomy radiotherapy reduces the risks of locoregional failure, any recurrence, and breast cancer mortality for patients with T1-T2 breast cancer and one-three positive lymph nodes,” according to the guideline.
However, clinicians and patients “should consider factors that may decrease the risk of locoregional failure, attenuate the benefit of reduced breast cancer–specific mortality, and/or increase the risk of complications.” These include older patient age, limited life expectancy, coexisting conditions that affect the risk of complications, small tumor size, absence of lymphovascular invasion, small nodal metastases, substantial response to neoadjuvant systemic therapy, low tumor grade, or strong tumor sensitivity to hormonal therapy, Dr. Recht and his associates said (J Clin Oncol. 2016 Sep 19. doi: 10.1200/JCO.2016.69.1188).
The guideline also addressed other areas of controversy.
Some clinicians now omit performing axillary lymph node dissection if one-two sentinel nodes are positive, “primarily based on extrapolation of data from randomized trials of patients treated exclusively or predominantly with breast-conserving surgery.” In such cases, the updated guideline “recommends that these patients receive postmastectomy radiotherapy only if there is already sufficient information to justify its use without needing to know that additional axillary nodes are involved.”
Many clinicians are uncertain whether to pursue postmastectomy radiotherapy in patients who have received neoadjuvant systemic therapy, because observational studies suggest the risk of locoregional recurrence is low for those who either have clinically negative nodes before undergoing the treatment or have a complete pathologic response to it in the lymph nodes. The panel decided that current evidence is insufficient to recommend whether radiotherapy should be administered or can be omitted in these groups.
The guideline also recommends that regional nodal irradiation generally should target both the internal mammary and the supraclavicular-axillary apical nodes, in addition to the chest wall or reconstructed breast. “There may be subgroups that will experience limited, if any, benefits from treating both these nodal areas, compared with treating only one or perhaps treating only the chest wall or reconstructed breast. [However,] there is insufficient evidence at this time to define such subgroups in detail.”
Copies of the updated guideline, as well as additional information such as details regarding the panel’s methodology, evidence tables, and clinical tools, are avail at www.asco.org/pmrt-guideline and www.asco.org/guidelineswiki.
This work was supported by the American Society of Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology. Dr. Recht reported serving as a consultant or advisor to CareCore and U.S. Oncology and receiving research funding from Genomic Health; his associates reported ties to numerous industry sources.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: The ASCO clinical practice guideline on postmastectomy radiotherapy has been updated to help clinicians decide which patients who have T1-T2 breast cancer with one-three positive axillary lymph nodes will not benefit from the treatment.
Major finding: The 10-year rate of locoregional treatment failure was only 4.3% with irradiation, compared with 21.0% without irradiation, and the 10-year rate of local or distant treatment failure was 33.8% with irradiation and 45.5% without it.
Data source: A review of the literature and compilation of several treatment recommendations.
Disclosures: This work was supported by the American Society of Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology. Dr. Recht reported serving as a consultant or advisor to CareCore and U.S. Oncology and receiving research funding from Genomic Health; his associates reported ties to numerous industry sources.