User login
Care gaps common after anal sphincter injuries from childbirth
Postpartum complications may go unrecognized in women who incur anal sphincter injuries during childbirth, a review of electronic medical records at one academic health system suggests.
In the first 3 months after delivery, few patients with an obstetric anal sphincter injury (OASI) had documented pelvic floor problems, compared with higher rates documented in medical literature, the researchers found.
“Lack of identified pelvic floor dysfunction in this population differs from the incidence in previously published data and may reflect lack of identification by obstetric providers,” the researchers reported. The findings “highlight a gap in health care that, when addressed, could significantly improve postpartum quality of life.”
The findings are scheduled to be presented at the annual scientific meeting of the American Urogynecologic Society and International Urogynecological Association.
Anal sphincter injuries occur in about 4.4% of vaginal deliveries and are the most common cause of anal incontinence in women of reproductive age.
For the new study, researchers reviewed records of 287 women who underwent a vaginal birth that resulted in an anal sphincter injury at five Ohio hospitals affiliated with Cleveland Clinic from 2013 to 2015.
Of those who met eligibility criteria, 209 (72.8%) were White, 262 (91.3%) were non-Hispanic, and 249 (86.8%) were aged 20-34 years. Most had an epidural (92%), did not require a blood transfusion (97.9%), did not develop a vaginal hematoma (98.9%), and did not have their injury repaired in an operating room (97.2%), the researchers reported.
Among pelvic floor disorders, urinary incontinence was not reported in 96% of patients, fecal incontinence was not reported in 97.1%, and pelvic organ prolapse was not reported in 99.3%. Most had no recorded complications from their lacerations (87.8%) or postpartum depression (92%), the researchers found.
However, a 2015 study found that, 12 weeks after delivery, women with OASIs commonly reported symptoms of incontinence, with 26% reporting urinary stress incontinence, 21.4% urinary urgency incontinence, 59% anal incontinence, and 15% fecal incontinence.
Depression was also seldom identified despite higher risk of mood disorders among women with OASI, the researchers found.
The team also examined interpregnancy intervals, defined as the time between a woman’s first vaginal delivery and conception of a subsequent pregnancy. Of 178 women for whom data were available, the median interval was 26.4 months (95% confidence interval, 23.7-29.9), similar to the median for births nationally.
Lead researcher Alexandra Nutaitis, DO, a resident in obstetrics and gynecology at Cleveland Clinic Akron General, said in an interview that it’s unclear whether physicians did not inquire about symptoms or didn’t record them. She noted that anal sphincter injuries are a “stigmatized topic.”
Not asked, not told
Carolyn Swenson, MD, an associate professor in urogynecology at the University of Utah, Salt Lake City, said physicians in the study may have relied on patients to bring up their symptoms rather than using questionnaires to screen for problems.
“What we know is that if you don’t ask women about pelvic floor disorders, they often don’t tell you that they are experiencing symptoms,” said Dr. Swenson, who was not involved in the new research.
Dr. Swenson called for validated questionnaires to assess pelvic floor symptoms in postpartum patients.
Regarding interpregnancy intervals, Dr. Nutaitis said she would be surprised if women who experienced an OASI didn’t delay having another child longer than women who did not undergo that physical and psychological trauma – but other factors such as societal pressures may override any reluctance to proceed with another pregnancy.
Dr. Swenson said it’s possible that a subgroup of women who have severe complications, such as those with a fourth-degree tear, might put off having another child. However, more research is needed to find out, she said.
Dr. Nutaitis and Dr. Swenson disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Postpartum complications may go unrecognized in women who incur anal sphincter injuries during childbirth, a review of electronic medical records at one academic health system suggests.
In the first 3 months after delivery, few patients with an obstetric anal sphincter injury (OASI) had documented pelvic floor problems, compared with higher rates documented in medical literature, the researchers found.
“Lack of identified pelvic floor dysfunction in this population differs from the incidence in previously published data and may reflect lack of identification by obstetric providers,” the researchers reported. The findings “highlight a gap in health care that, when addressed, could significantly improve postpartum quality of life.”
The findings are scheduled to be presented at the annual scientific meeting of the American Urogynecologic Society and International Urogynecological Association.
Anal sphincter injuries occur in about 4.4% of vaginal deliveries and are the most common cause of anal incontinence in women of reproductive age.
For the new study, researchers reviewed records of 287 women who underwent a vaginal birth that resulted in an anal sphincter injury at five Ohio hospitals affiliated with Cleveland Clinic from 2013 to 2015.
Of those who met eligibility criteria, 209 (72.8%) were White, 262 (91.3%) were non-Hispanic, and 249 (86.8%) were aged 20-34 years. Most had an epidural (92%), did not require a blood transfusion (97.9%), did not develop a vaginal hematoma (98.9%), and did not have their injury repaired in an operating room (97.2%), the researchers reported.
Among pelvic floor disorders, urinary incontinence was not reported in 96% of patients, fecal incontinence was not reported in 97.1%, and pelvic organ prolapse was not reported in 99.3%. Most had no recorded complications from their lacerations (87.8%) or postpartum depression (92%), the researchers found.
However, a 2015 study found that, 12 weeks after delivery, women with OASIs commonly reported symptoms of incontinence, with 26% reporting urinary stress incontinence, 21.4% urinary urgency incontinence, 59% anal incontinence, and 15% fecal incontinence.
Depression was also seldom identified despite higher risk of mood disorders among women with OASI, the researchers found.
The team also examined interpregnancy intervals, defined as the time between a woman’s first vaginal delivery and conception of a subsequent pregnancy. Of 178 women for whom data were available, the median interval was 26.4 months (95% confidence interval, 23.7-29.9), similar to the median for births nationally.
Lead researcher Alexandra Nutaitis, DO, a resident in obstetrics and gynecology at Cleveland Clinic Akron General, said in an interview that it’s unclear whether physicians did not inquire about symptoms or didn’t record them. She noted that anal sphincter injuries are a “stigmatized topic.”
Not asked, not told
Carolyn Swenson, MD, an associate professor in urogynecology at the University of Utah, Salt Lake City, said physicians in the study may have relied on patients to bring up their symptoms rather than using questionnaires to screen for problems.
“What we know is that if you don’t ask women about pelvic floor disorders, they often don’t tell you that they are experiencing symptoms,” said Dr. Swenson, who was not involved in the new research.
Dr. Swenson called for validated questionnaires to assess pelvic floor symptoms in postpartum patients.
Regarding interpregnancy intervals, Dr. Nutaitis said she would be surprised if women who experienced an OASI didn’t delay having another child longer than women who did not undergo that physical and psychological trauma – but other factors such as societal pressures may override any reluctance to proceed with another pregnancy.
Dr. Swenson said it’s possible that a subgroup of women who have severe complications, such as those with a fourth-degree tear, might put off having another child. However, more research is needed to find out, she said.
Dr. Nutaitis and Dr. Swenson disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Postpartum complications may go unrecognized in women who incur anal sphincter injuries during childbirth, a review of electronic medical records at one academic health system suggests.
In the first 3 months after delivery, few patients with an obstetric anal sphincter injury (OASI) had documented pelvic floor problems, compared with higher rates documented in medical literature, the researchers found.
“Lack of identified pelvic floor dysfunction in this population differs from the incidence in previously published data and may reflect lack of identification by obstetric providers,” the researchers reported. The findings “highlight a gap in health care that, when addressed, could significantly improve postpartum quality of life.”
The findings are scheduled to be presented at the annual scientific meeting of the American Urogynecologic Society and International Urogynecological Association.
Anal sphincter injuries occur in about 4.4% of vaginal deliveries and are the most common cause of anal incontinence in women of reproductive age.
For the new study, researchers reviewed records of 287 women who underwent a vaginal birth that resulted in an anal sphincter injury at five Ohio hospitals affiliated with Cleveland Clinic from 2013 to 2015.
Of those who met eligibility criteria, 209 (72.8%) were White, 262 (91.3%) were non-Hispanic, and 249 (86.8%) were aged 20-34 years. Most had an epidural (92%), did not require a blood transfusion (97.9%), did not develop a vaginal hematoma (98.9%), and did not have their injury repaired in an operating room (97.2%), the researchers reported.
Among pelvic floor disorders, urinary incontinence was not reported in 96% of patients, fecal incontinence was not reported in 97.1%, and pelvic organ prolapse was not reported in 99.3%. Most had no recorded complications from their lacerations (87.8%) or postpartum depression (92%), the researchers found.
However, a 2015 study found that, 12 weeks after delivery, women with OASIs commonly reported symptoms of incontinence, with 26% reporting urinary stress incontinence, 21.4% urinary urgency incontinence, 59% anal incontinence, and 15% fecal incontinence.
Depression was also seldom identified despite higher risk of mood disorders among women with OASI, the researchers found.
The team also examined interpregnancy intervals, defined as the time between a woman’s first vaginal delivery and conception of a subsequent pregnancy. Of 178 women for whom data were available, the median interval was 26.4 months (95% confidence interval, 23.7-29.9), similar to the median for births nationally.
Lead researcher Alexandra Nutaitis, DO, a resident in obstetrics and gynecology at Cleveland Clinic Akron General, said in an interview that it’s unclear whether physicians did not inquire about symptoms or didn’t record them. She noted that anal sphincter injuries are a “stigmatized topic.”
Not asked, not told
Carolyn Swenson, MD, an associate professor in urogynecology at the University of Utah, Salt Lake City, said physicians in the study may have relied on patients to bring up their symptoms rather than using questionnaires to screen for problems.
“What we know is that if you don’t ask women about pelvic floor disorders, they often don’t tell you that they are experiencing symptoms,” said Dr. Swenson, who was not involved in the new research.
Dr. Swenson called for validated questionnaires to assess pelvic floor symptoms in postpartum patients.
Regarding interpregnancy intervals, Dr. Nutaitis said she would be surprised if women who experienced an OASI didn’t delay having another child longer than women who did not undergo that physical and psychological trauma – but other factors such as societal pressures may override any reluctance to proceed with another pregnancy.
Dr. Swenson said it’s possible that a subgroup of women who have severe complications, such as those with a fourth-degree tear, might put off having another child. However, more research is needed to find out, she said.
Dr. Nutaitis and Dr. Swenson disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AUGS 2022
Surgeons may underestimate recovery from incontinence operation
Surgeons may significantly underestimate how long it will take women to return to normal activities following sling surgery to correct stress urinary incontinence, a new study has found.
The researchers found that just over 40% of women reported returning to work and other normal activities within 2 weeks of having undergone midurethral sling procedures – a much less optimistic forecast than what surgeons typically provide in these cases.
“This is in contrast to a published survey of physicians that showed the majority of surgeons suggested patients return to work within 2 weeks,” Rui Wang, MD, a fellow in female pelvic medicine and reconstructive surgery at Hartford Hospital, Conn., said in an interview.
Dr. Wang referred to a published survey of 135 physicians that was conducted at a 2018 meeting of the Society of Gynecologic Surgeons. In that survey, 88% of respondents indicated that patients could return to sedentary work within 2 weeks after undergoing sling surgery. Most recommended longer waits before returning to manual labor.
The authors of the survey noted a lack of consensus guidelines and wide variations in recommendations for postoperative restrictions after minimally invasive gynecologic and pelvic reconstructive surgery, which the researchers called a “largely unstudied field.”
Dr. Wang said, “The majority of patients may need more than 2 weeks to return to work and normal activities even following minimally invasive outpatient surgeries such as midurethral sling.”
Dr. Wang is scheduled to present the findings June 18 at the annual meeting of the American Urogynecologic Society.
For the new study, Dr. Wang and a colleague examined how patients answered questions about their activity levels during recovery after sling procedures. The patients were enrolled in the Trial of Mid-Urethral Slings (TOMUS), a randomized controlled trial that compared two types of midurethral slings used for the treatment of stress urinary incontinence: the retropubic midurethral mesh sling and the transobturator midurethral sling. Results of the trial were published in 2010.
Of 597 women enrolled in TOMUS, 441 were included in the new analysis. Patients who underwent another surgery at the same time as their sling procedure were excluded from the analysis.
As part of the trial, patients were asked how many paid workdays they took off after surgery; whether they had returned to full normal activities of daily life, including work, if applicable; and how much time it took for them to fully return to normal activities of daily life, including work.
The researchers found that 183 (41.5%) returned to normal activities within 2 weeks of the procedure. Among those patients, the median recovery time was 6 days. Within 6 weeks of surgery, 308 (70%) had returned to normal activities, including work. After 6 months, 407 (98.3%) were back to their normal routines, the study showed.
Multivariate regression analysis yielded no factor that predicted the timing of returning to normal activity and work. Nor did the researchers observe any significant differences in failure rates and adverse outcomes between patients who returned within 2 weeks or after 2 weeks.
Essential information for patient planning
Dr. Wang said she expects that the findings will help physicians in counseling patients and setting postoperative recovery expectations. “For patients planning elective surgery, one of the most important quality-of-life issues is the time they will need to take off from work and recover,” she said.
Although most patients needed more than 2 weeks to recover, the median paid time off after surgery was 4 days. “Many patients would have taken unpaid days off or used vacation time for their postoperative recovery,” Dr. Wang said.
She added that more research is needed to explore whether that discrepancy disproportionately affects women in jobs with fewer employee benefits. “We did not find that age, race/ethnicity, marital status, occupation, symptom severity, and duration of surgery significantly predicted the timing of return to work or normal activities,” she said. “But are there other factors, such as geographic location, insurance status, [or] income, that may affect this timing?”
Sarah Boyd, MD, an assistant professor in the Division of Female Pelvic Medicine and Reconstructive Surgery at Penn State College of Medicine, Hershey, said the new findings add concrete information that can guide patients in planning their recovery.
“Previously, surgeons could only provide general estimates to these patients based on the experience of their patients,” Dr. Boyd, who was not involved in the study, told this news organization.
The analysis has not been published in a peer-reviewed journal, and Dr. Boyd said that the findings may not pertain to all individuals who undergo midurethral sling procedures, such as people who have had prior surgery for incontinence or those who undergo surgery for other pelvic floor disorders at the same time.
Dr. Wang and Dr. Boyd reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Surgeons may significantly underestimate how long it will take women to return to normal activities following sling surgery to correct stress urinary incontinence, a new study has found.
The researchers found that just over 40% of women reported returning to work and other normal activities within 2 weeks of having undergone midurethral sling procedures – a much less optimistic forecast than what surgeons typically provide in these cases.
“This is in contrast to a published survey of physicians that showed the majority of surgeons suggested patients return to work within 2 weeks,” Rui Wang, MD, a fellow in female pelvic medicine and reconstructive surgery at Hartford Hospital, Conn., said in an interview.
Dr. Wang referred to a published survey of 135 physicians that was conducted at a 2018 meeting of the Society of Gynecologic Surgeons. In that survey, 88% of respondents indicated that patients could return to sedentary work within 2 weeks after undergoing sling surgery. Most recommended longer waits before returning to manual labor.
The authors of the survey noted a lack of consensus guidelines and wide variations in recommendations for postoperative restrictions after minimally invasive gynecologic and pelvic reconstructive surgery, which the researchers called a “largely unstudied field.”
Dr. Wang said, “The majority of patients may need more than 2 weeks to return to work and normal activities even following minimally invasive outpatient surgeries such as midurethral sling.”
Dr. Wang is scheduled to present the findings June 18 at the annual meeting of the American Urogynecologic Society.
For the new study, Dr. Wang and a colleague examined how patients answered questions about their activity levels during recovery after sling procedures. The patients were enrolled in the Trial of Mid-Urethral Slings (TOMUS), a randomized controlled trial that compared two types of midurethral slings used for the treatment of stress urinary incontinence: the retropubic midurethral mesh sling and the transobturator midurethral sling. Results of the trial were published in 2010.
Of 597 women enrolled in TOMUS, 441 were included in the new analysis. Patients who underwent another surgery at the same time as their sling procedure were excluded from the analysis.
As part of the trial, patients were asked how many paid workdays they took off after surgery; whether they had returned to full normal activities of daily life, including work, if applicable; and how much time it took for them to fully return to normal activities of daily life, including work.
The researchers found that 183 (41.5%) returned to normal activities within 2 weeks of the procedure. Among those patients, the median recovery time was 6 days. Within 6 weeks of surgery, 308 (70%) had returned to normal activities, including work. After 6 months, 407 (98.3%) were back to their normal routines, the study showed.
Multivariate regression analysis yielded no factor that predicted the timing of returning to normal activity and work. Nor did the researchers observe any significant differences in failure rates and adverse outcomes between patients who returned within 2 weeks or after 2 weeks.
Essential information for patient planning
Dr. Wang said she expects that the findings will help physicians in counseling patients and setting postoperative recovery expectations. “For patients planning elective surgery, one of the most important quality-of-life issues is the time they will need to take off from work and recover,” she said.
Although most patients needed more than 2 weeks to recover, the median paid time off after surgery was 4 days. “Many patients would have taken unpaid days off or used vacation time for their postoperative recovery,” Dr. Wang said.
She added that more research is needed to explore whether that discrepancy disproportionately affects women in jobs with fewer employee benefits. “We did not find that age, race/ethnicity, marital status, occupation, symptom severity, and duration of surgery significantly predicted the timing of return to work or normal activities,” she said. “But are there other factors, such as geographic location, insurance status, [or] income, that may affect this timing?”
Sarah Boyd, MD, an assistant professor in the Division of Female Pelvic Medicine and Reconstructive Surgery at Penn State College of Medicine, Hershey, said the new findings add concrete information that can guide patients in planning their recovery.
“Previously, surgeons could only provide general estimates to these patients based on the experience of their patients,” Dr. Boyd, who was not involved in the study, told this news organization.
The analysis has not been published in a peer-reviewed journal, and Dr. Boyd said that the findings may not pertain to all individuals who undergo midurethral sling procedures, such as people who have had prior surgery for incontinence or those who undergo surgery for other pelvic floor disorders at the same time.
Dr. Wang and Dr. Boyd reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Surgeons may significantly underestimate how long it will take women to return to normal activities following sling surgery to correct stress urinary incontinence, a new study has found.
The researchers found that just over 40% of women reported returning to work and other normal activities within 2 weeks of having undergone midurethral sling procedures – a much less optimistic forecast than what surgeons typically provide in these cases.
“This is in contrast to a published survey of physicians that showed the majority of surgeons suggested patients return to work within 2 weeks,” Rui Wang, MD, a fellow in female pelvic medicine and reconstructive surgery at Hartford Hospital, Conn., said in an interview.
Dr. Wang referred to a published survey of 135 physicians that was conducted at a 2018 meeting of the Society of Gynecologic Surgeons. In that survey, 88% of respondents indicated that patients could return to sedentary work within 2 weeks after undergoing sling surgery. Most recommended longer waits before returning to manual labor.
The authors of the survey noted a lack of consensus guidelines and wide variations in recommendations for postoperative restrictions after minimally invasive gynecologic and pelvic reconstructive surgery, which the researchers called a “largely unstudied field.”
Dr. Wang said, “The majority of patients may need more than 2 weeks to return to work and normal activities even following minimally invasive outpatient surgeries such as midurethral sling.”
Dr. Wang is scheduled to present the findings June 18 at the annual meeting of the American Urogynecologic Society.
For the new study, Dr. Wang and a colleague examined how patients answered questions about their activity levels during recovery after sling procedures. The patients were enrolled in the Trial of Mid-Urethral Slings (TOMUS), a randomized controlled trial that compared two types of midurethral slings used for the treatment of stress urinary incontinence: the retropubic midurethral mesh sling and the transobturator midurethral sling. Results of the trial were published in 2010.
Of 597 women enrolled in TOMUS, 441 were included in the new analysis. Patients who underwent another surgery at the same time as their sling procedure were excluded from the analysis.
As part of the trial, patients were asked how many paid workdays they took off after surgery; whether they had returned to full normal activities of daily life, including work, if applicable; and how much time it took for them to fully return to normal activities of daily life, including work.
The researchers found that 183 (41.5%) returned to normal activities within 2 weeks of the procedure. Among those patients, the median recovery time was 6 days. Within 6 weeks of surgery, 308 (70%) had returned to normal activities, including work. After 6 months, 407 (98.3%) were back to their normal routines, the study showed.
Multivariate regression analysis yielded no factor that predicted the timing of returning to normal activity and work. Nor did the researchers observe any significant differences in failure rates and adverse outcomes between patients who returned within 2 weeks or after 2 weeks.
Essential information for patient planning
Dr. Wang said she expects that the findings will help physicians in counseling patients and setting postoperative recovery expectations. “For patients planning elective surgery, one of the most important quality-of-life issues is the time they will need to take off from work and recover,” she said.
Although most patients needed more than 2 weeks to recover, the median paid time off after surgery was 4 days. “Many patients would have taken unpaid days off or used vacation time for their postoperative recovery,” Dr. Wang said.
She added that more research is needed to explore whether that discrepancy disproportionately affects women in jobs with fewer employee benefits. “We did not find that age, race/ethnicity, marital status, occupation, symptom severity, and duration of surgery significantly predicted the timing of return to work or normal activities,” she said. “But are there other factors, such as geographic location, insurance status, [or] income, that may affect this timing?”
Sarah Boyd, MD, an assistant professor in the Division of Female Pelvic Medicine and Reconstructive Surgery at Penn State College of Medicine, Hershey, said the new findings add concrete information that can guide patients in planning their recovery.
“Previously, surgeons could only provide general estimates to these patients based on the experience of their patients,” Dr. Boyd, who was not involved in the study, told this news organization.
The analysis has not been published in a peer-reviewed journal, and Dr. Boyd said that the findings may not pertain to all individuals who undergo midurethral sling procedures, such as people who have had prior surgery for incontinence or those who undergo surgery for other pelvic floor disorders at the same time.
Dr. Wang and Dr. Boyd reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AUGS 2022
Challenges and innovations in training gyn surgeons
Obstetrics and gynecology (ObGyn) is a surgical specialty, yet the training of ObGyn residents differs significantly from that of residents in other surgical specialties. In addition to attaining competency in both the distinct but related fields of obstetrics and gynecology, ObGyn residents have their training condensed into 4 years rather than the 5 years’ training of many other surgical specialties. This limits the time dedicated to gynecologic surgery, currently 18 to 20 months in most programs, and has been exacerbated by tighter duty-hour restrictions.1
Additionally, with increasing demand for minimally invasive procedures, residents are expected to attain competency in a growing breadth of gynecologic procedures in a patient population with increasing morbidity, and they may have less autonomy to do so in an increasingly litigious environment.2 Furthermore, annual hysterectomy cases are declining, from about 680,000 in 2002 to 430,000 in 2010,3 and these declining rates are seen in the low case numbers of recent graduates.4
Training time, procedure complexity
With less time to master a growing body of increasingly complex procedures, is the profession adequately training gynecologic surgeons? Many gynecologic surgeons are concerned that the answer is no and that significant shifts in resident training are needed to generate safe and competent gynecologic surgeons. These training deficits represent a deficiency in the quality of care for women specifically, and thus the inattention to training gynecologic surgeons should be considered a health care disparity.
The concern over insufficient attention to gynecologic surgical training is not new, nor are proposed solutions, with many physicians citing the above concerns.5-9 In 2018, the Accreditation Council for Graduate Medical Education (ACGME) case minimums for hysterectomy increased to 85 from 70 hysterectomies, with a shift toward minimally invasive hysterectomy.10 Otherwise, minimal national changes have been made in this century to training gynecologic surgeons.
Tracking as an option
Many critics of current ObGyn training argue that obstetrics and gynecology, while related, have significantly different pathologies, surgical approaches, and skill sets and thus warrant the option to track toward obstetrics or gynecology after attaining limited core skill set in residency. In 2010, the Carnegie Foundation for the Advancement of Teaching called for the need for increased individualization opportunities in graduate medical education, citing that minimal changes have been made to medical education since the Flexner Report a century prior.11
Notably, tracking has been implemented with success at Cleveland Clinic, where residents are given 5 to 10 weeks of time allotted to their specific fields of interest, while still meeting minimum ACGME requirements and, in some cases, exceeding hysterectomy minimums by as much as 500%.12 Tracking is viewed positively by a majority of program directors.13 See the box below for Dr. Ferrando’s experience on tracking at the Cleveland Clinic.
Simulation training
Other educators advocate for maximizing preparedness for the operating room by using high-fidelity simulation.14,15 Simulation allows for the acquisition of basic technical skills needed for surgery as well as for repetition not easily achieved in the current surgical environment. Additionally, it provides lower-level learners the opportunity to acquire basic skills in a safe setting, thereby enhancing the ability to participate meaningfully on arrival in the operating room.16
In 2018, the American Board of Obstetrics and Gynecology added the Fundamentals of Laparoscopic Surgery certification as a new requirement for board certification.17 Laparoscopic and robotic surgery simulators allow trainees to develop coordination and specific skills, like knot tying and suturing. Additionally, models are available with varying levels of fidelity for vaginal and abdominal hysterectomy.18-20 See the box below for Dr. Miyazaki’s experience in developing the Miya Model trainer for vaginal surgery simulation.
Structured feedback
Finally, if a resident has limited exposure to a specific procedure, maximizing the preparation and feedback for each procedure is paramount. However, surgeons receive minimal formal training in teaching trainees, which leads to inconsistent and underutilized feedback.21 Specific structured feedback models have been implemented with success in the general surgery literature, including the SHARP (Set learning objectives, How did it go, Address concerns, Review learning points, Plan ahead) and BID (Briefing, Intraoperative, Debriefing) models.22,23
Reimbursement reform
While surgical reimbursement is not directly tied to resident education, decreased reimbursement to women’s health pathology and procedures has the downstream effect of decreasing the funds available for ObGyn departments to invest in research and education. Additionally, “suboptimal mastery or maintenance of appropriate surgical skills results in procedural inefficiencies that compound surgical cost.”5 Providers and payors alike should therefore be motivated to improve funding in order to improve adequate training of gynecologic surgeons. Payment reform is necessary to equally value women’s health procedures but also can ensure that gynecologic surgeons have the funds needed to train a competent next generation of ObGyn physicians. ●
- Residents and fellows have significant constraints that limit adequate training in gynecologic surgery. In a panel discussion at the 48th annual meeting of the Society of Gynecologic Surgeons, Drs. Zimmerman, Ferrando, and Miyazaki spoke about potential solutions.
- Allowing residents to track toward obstetric or gynecologic subspecialties may improve surgical volume of trainees who aim for a future career in gynecologic surgery.
- Simulation has demonstrated efficacy in enabling residents to prepare and improve their technical skills for specific procedures prior to entering the operating room.
Cecile A. Ferrando, MD, MPH
In his 2013 presidential address at the opening ceremony of the 42nd AAGL Global Congress on Minimally Invasive Gynecology, Javier Magrina, MD, asked the audience, “Isn’t it time to separate the O from the G?”7 Since that address, this catchy question has been posed several times, and it continues to be a topic of interest to many ObGyn educators seeking to innovate the curriculum and to better train our next generation’s gynecologic surgeons.
Several concerns have been raised about the current traditional 4-year residency training program, which has been impacted by the reduction of training hours due to duty-hour rules in the setting of decreased surgical volume and new technologies used to perform surgery. While other surgical specialties have begun to innovate their pathways for trainees, ObGyn has been a little slower to make a significant transition in its approach to training.
In 2012, Cleveland Clinic decided to lead the way in innovation regarding residency training. At its inception, the curriculum was designed to allow “tracking blocks” through each academic year to allow residents to gain additional experience in their specialty of choice. The program was carefully designed to assure that residents would achieve all 28 of the core obstetrics and gynecology milestones while still allowing for curricular flexibility.
Currently, residents are given autonomy to design their own tracking blocks with an assigned mentor for the rotation. Allowing residents to spend more time in their specialty of choice permits them to fine-tune skills that a standard curriculum may not have afforded the opportunity to home in on. It also allows residents to gain exposure to specialties that are not part of the core program, such as vulvar health, breast health and surgery, and gender affirmation surgery.
The Cleveland Clinic experience has been successful thus far. Importantly, preliminary data show that the tracking program does not interfere with the overall case number necessary for graduation. Residents also have succeeded in their postgraduation pursuits, including those who chose to specialize in general obstetrics and gynecology.
Cleveland Clinic is no longer the only program to incorporate tracking into its curriculum. This innovation is likely to become more standard as medical education in ObGyn evolves. We have not yet “separated the O from the G” completely in our specialty. However, thought leaders in our field are recognizing the need to better prepare our trainees, and this flexibility in mindset is bound to lead to a paradigm that may become the new standard for our specialty.
Acknowledgments: John E. Jelovsek, MD, the first Program Director of the Cleveland Clinic Residency in Obstetrics & Gynecology, who was responsible for creating the tracking program; and Vicki Reed, MD, the current Program Director, who has continued to innovate the program.
The Miya Model (Miyazaki Enterprises LLC) is a multiprocedural vaginal surgery simulator born from the need for standardized, scalable training in response to reductions in the average surgical case volume per resident. The Miya Model supports various basic procedures, such as pelvic exams and dilation and curettage, as well as full surgical procedures, including anterior and posterior colporrhaphy, midurethral and retropubic slings, cystoscopy, and vaginal hysterectomy. Training with the Miya Model moves resident surgical education from the operating room to any simulation lab or office-based setting. With rapidly declining resident surgical case volumes, there is an even stronger need to provide additional training outside of the operating room theater. Creation and development of the Miya Model were fueled by a desire to create a safer and more efficient method to educate residents without the risk of patient harm.
Miyazaki Enterprises has taken the Miya Model from a vision on paper to a standardized, commercially available product to help support resident and physician education. The Miya Model has undergone numerous rounds of waterfall and agile development, validity testing, and the creation of internal and external processes to achieve this vision. It serves as an example that ideas originating from significant demonstrated market need can be successfully created and deployed by a physician.
- Espey E, Ogburn T, Puscheck E. Impact of duty hour limitations on resident and student education in obstetrics and gynecology. J Reprod Med. 2007;52:345-348.
- Pulliam SJ, Berkowitz LR. Smaller pieces of the hysterectomy pie: current challenges in resident surgical education. Obstet Gynecol. 2009;113(2 pt 1):395-398. doi: 10.1097/AOG.0b013e3181955011.
- Wright JD, Herzog TJ, Tsui J, et al. Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet Gynecol. 2013;122(2 pt 1):233-241. doi: 10.1097/AOG.0b013e318299a6cf.
- Cadish LA, Kropat G, Muffly TM. Hysterectomy volume among recent obstetrics and gynecology residency graduates. Female Pelvic Med Reconstr Surg. 2021;27:382-387. doi: 10.1097/SPV.0000000000000879.
- Podratz KC. Gynecologic surgery: an imperiled ballet. Presidential address. Am J Obstet Gynecol. 1998;178:1229-1234. doi: 10.1016/ s0002-9378(98)70327-8.
- Bissonnette JM, Gabbe SG, Hammond CB, et al. Restructuring residency training in obstetrics and gynecology. Am J Obstet Gynecol. 1999;180(3 pt 1):516-518. doi: 10.1016/s0002-9378(99)70246-2.
- Magrina JF. Isn’t it time to separate the O from the G? J Minim Invasive Gynecol. 2014;21:501-503. doi: 10.1016/j.jmig.2014.01.022.
- Merrill JA. Needed changes in obstetric-gynecologic training. Obstet Gynecol Surv. 1994;49:1-2.
- Lauer JK, Advincula AP. The future of the gynecologic surgeon: rationale for and steps toward subspecialization of complex gynecologic surgery. J Minim Invasive Gynecol. 2021;28:726-729. doi: 10.1016/j.jmig.2020.12.031.
- Hall EF, Raker CA, Hampton BS. Variability in gynecologic case volume of obstetrician-gynecologist residents graduating from 2009 to 2017. Am J Obstet Gynecol. 2020;222:617.e1-617.e8. doi: 10.1016/j .ajog.2019.11.1258.
- Irby DM, Cooke M, O’Brien BC. Calls for reform of medical education by the Carnegie Foundation for the Advancement of Teaching: 1910 and 2010. Acad Med. 2010;85:220-227. doi: 10.1097 /ACM.0b013e3181c88449.
- Reed VR, Emery J, Farrell RM, et al. Tracking—a flexible obstetrics and gynecology residency curriculum. Obstet Gynecol. 2019;134(suppl 1):29s-33s. doi: 10.1097/AOG.0000000000003464.
- Hariton E, Freret TS, Nitecki R, et al. Program director perceptions of subspecialty tracking in obstetrics and gynecology residency. J Grad Med Educ. 2018;10:665-670. doi: 10.4300/JGME-D-18-00096.1.
- Azadi S, Green IC, Arnold A, et al. Robotic surgery: the impact of simulation and other innovative platforms on performance and training. J Minim Invasive Gynecol. 2021;28:490-495. doi: 10.1016/j .jmig.2020.12.001.
- Wohlrab K, Jelovsek JE, Myers D. Incorporating simulation into gynecologic surgical training. Am J Obstet Gynecol. 2017;217:522-526. doi: 10.1016/j.ajog.2017.05.017.
- Chen CC, Green IC, Colbert-Getz JM, et al. Warm-up on a simulator improves residents’ performance in laparoscopic surgery: a randomized trial. Int Urogynecol J. 2013;24:1615-1622. doi: 10.1007 /s00192-013-2066-2.
- Fundamentals of Laparoscopic Surgery. ABOG announces new eligibility requirement for board certification. January 23, 2018. Accessed May 12, 2022. https://www.flsprogram.org/news/abog -announces-new-eligibility-requirement-board-certification/.
- Zoorob D, Frenn R, Moffitt M, et al. Multi-institutional validation of a vaginal hysterectomy simulation model for resident training. J Minim Invasive Gynecol. 2021;28:1490-1496.e1. doi: 10.1016/j .jmig.2020.12.006.
- Barrier BF, Thompson AB, McCullough MW, et al. A novel and inexpensive vaginal hysterectomy simulator. Simul Healthc. 2012;7:374-379. doi: 10.1097/SIH.0b013e318266d0c6.
- Stickrath E, Alston M. A novel abdominal hysterectomy simulator and its impact on obstetrics and gynecology residents’ surgical confidence. MedEdPORTAL. 2017;13:10636. doi: 10.15766/mep_2374-8265.10636.
- McKendy KM, Watanabe Y, Lee L, et al. Perioperative feedback in surgical training: a systematic review. Am J Surg. 2017;214:117-126. doi: 10.1016/j.amjsurg.2016.12.014.
- Ahmed M, Arora S, Russ S, et al. Operation debrief: a SHARP improvement in performance feedback in the operating room. Ann Surg. 2013;258:958-963. doi: 10.1097/SLA.0b013e31828c88fc.
- Anderson CI, Gupta RN, Larson JR, et al. Impact of objectively assessing surgeons’ teaching on effective perioperative instructional behaviors. JAMA Surg. 2013;148:915-922. doi: 10.1001/jamasurg.2013.2144.
Obstetrics and gynecology (ObGyn) is a surgical specialty, yet the training of ObGyn residents differs significantly from that of residents in other surgical specialties. In addition to attaining competency in both the distinct but related fields of obstetrics and gynecology, ObGyn residents have their training condensed into 4 years rather than the 5 years’ training of many other surgical specialties. This limits the time dedicated to gynecologic surgery, currently 18 to 20 months in most programs, and has been exacerbated by tighter duty-hour restrictions.1
Additionally, with increasing demand for minimally invasive procedures, residents are expected to attain competency in a growing breadth of gynecologic procedures in a patient population with increasing morbidity, and they may have less autonomy to do so in an increasingly litigious environment.2 Furthermore, annual hysterectomy cases are declining, from about 680,000 in 2002 to 430,000 in 2010,3 and these declining rates are seen in the low case numbers of recent graduates.4
Training time, procedure complexity
With less time to master a growing body of increasingly complex procedures, is the profession adequately training gynecologic surgeons? Many gynecologic surgeons are concerned that the answer is no and that significant shifts in resident training are needed to generate safe and competent gynecologic surgeons. These training deficits represent a deficiency in the quality of care for women specifically, and thus the inattention to training gynecologic surgeons should be considered a health care disparity.
The concern over insufficient attention to gynecologic surgical training is not new, nor are proposed solutions, with many physicians citing the above concerns.5-9 In 2018, the Accreditation Council for Graduate Medical Education (ACGME) case minimums for hysterectomy increased to 85 from 70 hysterectomies, with a shift toward minimally invasive hysterectomy.10 Otherwise, minimal national changes have been made in this century to training gynecologic surgeons.
Tracking as an option
Many critics of current ObGyn training argue that obstetrics and gynecology, while related, have significantly different pathologies, surgical approaches, and skill sets and thus warrant the option to track toward obstetrics or gynecology after attaining limited core skill set in residency. In 2010, the Carnegie Foundation for the Advancement of Teaching called for the need for increased individualization opportunities in graduate medical education, citing that minimal changes have been made to medical education since the Flexner Report a century prior.11
Notably, tracking has been implemented with success at Cleveland Clinic, where residents are given 5 to 10 weeks of time allotted to their specific fields of interest, while still meeting minimum ACGME requirements and, in some cases, exceeding hysterectomy minimums by as much as 500%.12 Tracking is viewed positively by a majority of program directors.13 See the box below for Dr. Ferrando’s experience on tracking at the Cleveland Clinic.
Simulation training
Other educators advocate for maximizing preparedness for the operating room by using high-fidelity simulation.14,15 Simulation allows for the acquisition of basic technical skills needed for surgery as well as for repetition not easily achieved in the current surgical environment. Additionally, it provides lower-level learners the opportunity to acquire basic skills in a safe setting, thereby enhancing the ability to participate meaningfully on arrival in the operating room.16
In 2018, the American Board of Obstetrics and Gynecology added the Fundamentals of Laparoscopic Surgery certification as a new requirement for board certification.17 Laparoscopic and robotic surgery simulators allow trainees to develop coordination and specific skills, like knot tying and suturing. Additionally, models are available with varying levels of fidelity for vaginal and abdominal hysterectomy.18-20 See the box below for Dr. Miyazaki’s experience in developing the Miya Model trainer for vaginal surgery simulation.
Structured feedback
Finally, if a resident has limited exposure to a specific procedure, maximizing the preparation and feedback for each procedure is paramount. However, surgeons receive minimal formal training in teaching trainees, which leads to inconsistent and underutilized feedback.21 Specific structured feedback models have been implemented with success in the general surgery literature, including the SHARP (Set learning objectives, How did it go, Address concerns, Review learning points, Plan ahead) and BID (Briefing, Intraoperative, Debriefing) models.22,23
Reimbursement reform
While surgical reimbursement is not directly tied to resident education, decreased reimbursement to women’s health pathology and procedures has the downstream effect of decreasing the funds available for ObGyn departments to invest in research and education. Additionally, “suboptimal mastery or maintenance of appropriate surgical skills results in procedural inefficiencies that compound surgical cost.”5 Providers and payors alike should therefore be motivated to improve funding in order to improve adequate training of gynecologic surgeons. Payment reform is necessary to equally value women’s health procedures but also can ensure that gynecologic surgeons have the funds needed to train a competent next generation of ObGyn physicians. ●
- Residents and fellows have significant constraints that limit adequate training in gynecologic surgery. In a panel discussion at the 48th annual meeting of the Society of Gynecologic Surgeons, Drs. Zimmerman, Ferrando, and Miyazaki spoke about potential solutions.
- Allowing residents to track toward obstetric or gynecologic subspecialties may improve surgical volume of trainees who aim for a future career in gynecologic surgery.
- Simulation has demonstrated efficacy in enabling residents to prepare and improve their technical skills for specific procedures prior to entering the operating room.
Cecile A. Ferrando, MD, MPH
In his 2013 presidential address at the opening ceremony of the 42nd AAGL Global Congress on Minimally Invasive Gynecology, Javier Magrina, MD, asked the audience, “Isn’t it time to separate the O from the G?”7 Since that address, this catchy question has been posed several times, and it continues to be a topic of interest to many ObGyn educators seeking to innovate the curriculum and to better train our next generation’s gynecologic surgeons.
Several concerns have been raised about the current traditional 4-year residency training program, which has been impacted by the reduction of training hours due to duty-hour rules in the setting of decreased surgical volume and new technologies used to perform surgery. While other surgical specialties have begun to innovate their pathways for trainees, ObGyn has been a little slower to make a significant transition in its approach to training.
In 2012, Cleveland Clinic decided to lead the way in innovation regarding residency training. At its inception, the curriculum was designed to allow “tracking blocks” through each academic year to allow residents to gain additional experience in their specialty of choice. The program was carefully designed to assure that residents would achieve all 28 of the core obstetrics and gynecology milestones while still allowing for curricular flexibility.
Currently, residents are given autonomy to design their own tracking blocks with an assigned mentor for the rotation. Allowing residents to spend more time in their specialty of choice permits them to fine-tune skills that a standard curriculum may not have afforded the opportunity to home in on. It also allows residents to gain exposure to specialties that are not part of the core program, such as vulvar health, breast health and surgery, and gender affirmation surgery.
The Cleveland Clinic experience has been successful thus far. Importantly, preliminary data show that the tracking program does not interfere with the overall case number necessary for graduation. Residents also have succeeded in their postgraduation pursuits, including those who chose to specialize in general obstetrics and gynecology.
Cleveland Clinic is no longer the only program to incorporate tracking into its curriculum. This innovation is likely to become more standard as medical education in ObGyn evolves. We have not yet “separated the O from the G” completely in our specialty. However, thought leaders in our field are recognizing the need to better prepare our trainees, and this flexibility in mindset is bound to lead to a paradigm that may become the new standard for our specialty.
Acknowledgments: John E. Jelovsek, MD, the first Program Director of the Cleveland Clinic Residency in Obstetrics & Gynecology, who was responsible for creating the tracking program; and Vicki Reed, MD, the current Program Director, who has continued to innovate the program.
The Miya Model (Miyazaki Enterprises LLC) is a multiprocedural vaginal surgery simulator born from the need for standardized, scalable training in response to reductions in the average surgical case volume per resident. The Miya Model supports various basic procedures, such as pelvic exams and dilation and curettage, as well as full surgical procedures, including anterior and posterior colporrhaphy, midurethral and retropubic slings, cystoscopy, and vaginal hysterectomy. Training with the Miya Model moves resident surgical education from the operating room to any simulation lab or office-based setting. With rapidly declining resident surgical case volumes, there is an even stronger need to provide additional training outside of the operating room theater. Creation and development of the Miya Model were fueled by a desire to create a safer and more efficient method to educate residents without the risk of patient harm.
Miyazaki Enterprises has taken the Miya Model from a vision on paper to a standardized, commercially available product to help support resident and physician education. The Miya Model has undergone numerous rounds of waterfall and agile development, validity testing, and the creation of internal and external processes to achieve this vision. It serves as an example that ideas originating from significant demonstrated market need can be successfully created and deployed by a physician.
Obstetrics and gynecology (ObGyn) is a surgical specialty, yet the training of ObGyn residents differs significantly from that of residents in other surgical specialties. In addition to attaining competency in both the distinct but related fields of obstetrics and gynecology, ObGyn residents have their training condensed into 4 years rather than the 5 years’ training of many other surgical specialties. This limits the time dedicated to gynecologic surgery, currently 18 to 20 months in most programs, and has been exacerbated by tighter duty-hour restrictions.1
Additionally, with increasing demand for minimally invasive procedures, residents are expected to attain competency in a growing breadth of gynecologic procedures in a patient population with increasing morbidity, and they may have less autonomy to do so in an increasingly litigious environment.2 Furthermore, annual hysterectomy cases are declining, from about 680,000 in 2002 to 430,000 in 2010,3 and these declining rates are seen in the low case numbers of recent graduates.4
Training time, procedure complexity
With less time to master a growing body of increasingly complex procedures, is the profession adequately training gynecologic surgeons? Many gynecologic surgeons are concerned that the answer is no and that significant shifts in resident training are needed to generate safe and competent gynecologic surgeons. These training deficits represent a deficiency in the quality of care for women specifically, and thus the inattention to training gynecologic surgeons should be considered a health care disparity.
The concern over insufficient attention to gynecologic surgical training is not new, nor are proposed solutions, with many physicians citing the above concerns.5-9 In 2018, the Accreditation Council for Graduate Medical Education (ACGME) case minimums for hysterectomy increased to 85 from 70 hysterectomies, with a shift toward minimally invasive hysterectomy.10 Otherwise, minimal national changes have been made in this century to training gynecologic surgeons.
Tracking as an option
Many critics of current ObGyn training argue that obstetrics and gynecology, while related, have significantly different pathologies, surgical approaches, and skill sets and thus warrant the option to track toward obstetrics or gynecology after attaining limited core skill set in residency. In 2010, the Carnegie Foundation for the Advancement of Teaching called for the need for increased individualization opportunities in graduate medical education, citing that minimal changes have been made to medical education since the Flexner Report a century prior.11
Notably, tracking has been implemented with success at Cleveland Clinic, where residents are given 5 to 10 weeks of time allotted to their specific fields of interest, while still meeting minimum ACGME requirements and, in some cases, exceeding hysterectomy minimums by as much as 500%.12 Tracking is viewed positively by a majority of program directors.13 See the box below for Dr. Ferrando’s experience on tracking at the Cleveland Clinic.
Simulation training
Other educators advocate for maximizing preparedness for the operating room by using high-fidelity simulation.14,15 Simulation allows for the acquisition of basic technical skills needed for surgery as well as for repetition not easily achieved in the current surgical environment. Additionally, it provides lower-level learners the opportunity to acquire basic skills in a safe setting, thereby enhancing the ability to participate meaningfully on arrival in the operating room.16
In 2018, the American Board of Obstetrics and Gynecology added the Fundamentals of Laparoscopic Surgery certification as a new requirement for board certification.17 Laparoscopic and robotic surgery simulators allow trainees to develop coordination and specific skills, like knot tying and suturing. Additionally, models are available with varying levels of fidelity for vaginal and abdominal hysterectomy.18-20 See the box below for Dr. Miyazaki’s experience in developing the Miya Model trainer for vaginal surgery simulation.
Structured feedback
Finally, if a resident has limited exposure to a specific procedure, maximizing the preparation and feedback for each procedure is paramount. However, surgeons receive minimal formal training in teaching trainees, which leads to inconsistent and underutilized feedback.21 Specific structured feedback models have been implemented with success in the general surgery literature, including the SHARP (Set learning objectives, How did it go, Address concerns, Review learning points, Plan ahead) and BID (Briefing, Intraoperative, Debriefing) models.22,23
Reimbursement reform
While surgical reimbursement is not directly tied to resident education, decreased reimbursement to women’s health pathology and procedures has the downstream effect of decreasing the funds available for ObGyn departments to invest in research and education. Additionally, “suboptimal mastery or maintenance of appropriate surgical skills results in procedural inefficiencies that compound surgical cost.”5 Providers and payors alike should therefore be motivated to improve funding in order to improve adequate training of gynecologic surgeons. Payment reform is necessary to equally value women’s health procedures but also can ensure that gynecologic surgeons have the funds needed to train a competent next generation of ObGyn physicians. ●
- Residents and fellows have significant constraints that limit adequate training in gynecologic surgery. In a panel discussion at the 48th annual meeting of the Society of Gynecologic Surgeons, Drs. Zimmerman, Ferrando, and Miyazaki spoke about potential solutions.
- Allowing residents to track toward obstetric or gynecologic subspecialties may improve surgical volume of trainees who aim for a future career in gynecologic surgery.
- Simulation has demonstrated efficacy in enabling residents to prepare and improve their technical skills for specific procedures prior to entering the operating room.
Cecile A. Ferrando, MD, MPH
In his 2013 presidential address at the opening ceremony of the 42nd AAGL Global Congress on Minimally Invasive Gynecology, Javier Magrina, MD, asked the audience, “Isn’t it time to separate the O from the G?”7 Since that address, this catchy question has been posed several times, and it continues to be a topic of interest to many ObGyn educators seeking to innovate the curriculum and to better train our next generation’s gynecologic surgeons.
Several concerns have been raised about the current traditional 4-year residency training program, which has been impacted by the reduction of training hours due to duty-hour rules in the setting of decreased surgical volume and new technologies used to perform surgery. While other surgical specialties have begun to innovate their pathways for trainees, ObGyn has been a little slower to make a significant transition in its approach to training.
In 2012, Cleveland Clinic decided to lead the way in innovation regarding residency training. At its inception, the curriculum was designed to allow “tracking blocks” through each academic year to allow residents to gain additional experience in their specialty of choice. The program was carefully designed to assure that residents would achieve all 28 of the core obstetrics and gynecology milestones while still allowing for curricular flexibility.
Currently, residents are given autonomy to design their own tracking blocks with an assigned mentor for the rotation. Allowing residents to spend more time in their specialty of choice permits them to fine-tune skills that a standard curriculum may not have afforded the opportunity to home in on. It also allows residents to gain exposure to specialties that are not part of the core program, such as vulvar health, breast health and surgery, and gender affirmation surgery.
The Cleveland Clinic experience has been successful thus far. Importantly, preliminary data show that the tracking program does not interfere with the overall case number necessary for graduation. Residents also have succeeded in their postgraduation pursuits, including those who chose to specialize in general obstetrics and gynecology.
Cleveland Clinic is no longer the only program to incorporate tracking into its curriculum. This innovation is likely to become more standard as medical education in ObGyn evolves. We have not yet “separated the O from the G” completely in our specialty. However, thought leaders in our field are recognizing the need to better prepare our trainees, and this flexibility in mindset is bound to lead to a paradigm that may become the new standard for our specialty.
Acknowledgments: John E. Jelovsek, MD, the first Program Director of the Cleveland Clinic Residency in Obstetrics & Gynecology, who was responsible for creating the tracking program; and Vicki Reed, MD, the current Program Director, who has continued to innovate the program.
The Miya Model (Miyazaki Enterprises LLC) is a multiprocedural vaginal surgery simulator born from the need for standardized, scalable training in response to reductions in the average surgical case volume per resident. The Miya Model supports various basic procedures, such as pelvic exams and dilation and curettage, as well as full surgical procedures, including anterior and posterior colporrhaphy, midurethral and retropubic slings, cystoscopy, and vaginal hysterectomy. Training with the Miya Model moves resident surgical education from the operating room to any simulation lab or office-based setting. With rapidly declining resident surgical case volumes, there is an even stronger need to provide additional training outside of the operating room theater. Creation and development of the Miya Model were fueled by a desire to create a safer and more efficient method to educate residents without the risk of patient harm.
Miyazaki Enterprises has taken the Miya Model from a vision on paper to a standardized, commercially available product to help support resident and physician education. The Miya Model has undergone numerous rounds of waterfall and agile development, validity testing, and the creation of internal and external processes to achieve this vision. It serves as an example that ideas originating from significant demonstrated market need can be successfully created and deployed by a physician.
- Espey E, Ogburn T, Puscheck E. Impact of duty hour limitations on resident and student education in obstetrics and gynecology. J Reprod Med. 2007;52:345-348.
- Pulliam SJ, Berkowitz LR. Smaller pieces of the hysterectomy pie: current challenges in resident surgical education. Obstet Gynecol. 2009;113(2 pt 1):395-398. doi: 10.1097/AOG.0b013e3181955011.
- Wright JD, Herzog TJ, Tsui J, et al. Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet Gynecol. 2013;122(2 pt 1):233-241. doi: 10.1097/AOG.0b013e318299a6cf.
- Cadish LA, Kropat G, Muffly TM. Hysterectomy volume among recent obstetrics and gynecology residency graduates. Female Pelvic Med Reconstr Surg. 2021;27:382-387. doi: 10.1097/SPV.0000000000000879.
- Podratz KC. Gynecologic surgery: an imperiled ballet. Presidential address. Am J Obstet Gynecol. 1998;178:1229-1234. doi: 10.1016/ s0002-9378(98)70327-8.
- Bissonnette JM, Gabbe SG, Hammond CB, et al. Restructuring residency training in obstetrics and gynecology. Am J Obstet Gynecol. 1999;180(3 pt 1):516-518. doi: 10.1016/s0002-9378(99)70246-2.
- Magrina JF. Isn’t it time to separate the O from the G? J Minim Invasive Gynecol. 2014;21:501-503. doi: 10.1016/j.jmig.2014.01.022.
- Merrill JA. Needed changes in obstetric-gynecologic training. Obstet Gynecol Surv. 1994;49:1-2.
- Lauer JK, Advincula AP. The future of the gynecologic surgeon: rationale for and steps toward subspecialization of complex gynecologic surgery. J Minim Invasive Gynecol. 2021;28:726-729. doi: 10.1016/j.jmig.2020.12.031.
- Hall EF, Raker CA, Hampton BS. Variability in gynecologic case volume of obstetrician-gynecologist residents graduating from 2009 to 2017. Am J Obstet Gynecol. 2020;222:617.e1-617.e8. doi: 10.1016/j .ajog.2019.11.1258.
- Irby DM, Cooke M, O’Brien BC. Calls for reform of medical education by the Carnegie Foundation for the Advancement of Teaching: 1910 and 2010. Acad Med. 2010;85:220-227. doi: 10.1097 /ACM.0b013e3181c88449.
- Reed VR, Emery J, Farrell RM, et al. Tracking—a flexible obstetrics and gynecology residency curriculum. Obstet Gynecol. 2019;134(suppl 1):29s-33s. doi: 10.1097/AOG.0000000000003464.
- Hariton E, Freret TS, Nitecki R, et al. Program director perceptions of subspecialty tracking in obstetrics and gynecology residency. J Grad Med Educ. 2018;10:665-670. doi: 10.4300/JGME-D-18-00096.1.
- Azadi S, Green IC, Arnold A, et al. Robotic surgery: the impact of simulation and other innovative platforms on performance and training. J Minim Invasive Gynecol. 2021;28:490-495. doi: 10.1016/j .jmig.2020.12.001.
- Wohlrab K, Jelovsek JE, Myers D. Incorporating simulation into gynecologic surgical training. Am J Obstet Gynecol. 2017;217:522-526. doi: 10.1016/j.ajog.2017.05.017.
- Chen CC, Green IC, Colbert-Getz JM, et al. Warm-up on a simulator improves residents’ performance in laparoscopic surgery: a randomized trial. Int Urogynecol J. 2013;24:1615-1622. doi: 10.1007 /s00192-013-2066-2.
- Fundamentals of Laparoscopic Surgery. ABOG announces new eligibility requirement for board certification. January 23, 2018. Accessed May 12, 2022. https://www.flsprogram.org/news/abog -announces-new-eligibility-requirement-board-certification/.
- Zoorob D, Frenn R, Moffitt M, et al. Multi-institutional validation of a vaginal hysterectomy simulation model for resident training. J Minim Invasive Gynecol. 2021;28:1490-1496.e1. doi: 10.1016/j .jmig.2020.12.006.
- Barrier BF, Thompson AB, McCullough MW, et al. A novel and inexpensive vaginal hysterectomy simulator. Simul Healthc. 2012;7:374-379. doi: 10.1097/SIH.0b013e318266d0c6.
- Stickrath E, Alston M. A novel abdominal hysterectomy simulator and its impact on obstetrics and gynecology residents’ surgical confidence. MedEdPORTAL. 2017;13:10636. doi: 10.15766/mep_2374-8265.10636.
- McKendy KM, Watanabe Y, Lee L, et al. Perioperative feedback in surgical training: a systematic review. Am J Surg. 2017;214:117-126. doi: 10.1016/j.amjsurg.2016.12.014.
- Ahmed M, Arora S, Russ S, et al. Operation debrief: a SHARP improvement in performance feedback in the operating room. Ann Surg. 2013;258:958-963. doi: 10.1097/SLA.0b013e31828c88fc.
- Anderson CI, Gupta RN, Larson JR, et al. Impact of objectively assessing surgeons’ teaching on effective perioperative instructional behaviors. JAMA Surg. 2013;148:915-922. doi: 10.1001/jamasurg.2013.2144.
- Espey E, Ogburn T, Puscheck E. Impact of duty hour limitations on resident and student education in obstetrics and gynecology. J Reprod Med. 2007;52:345-348.
- Pulliam SJ, Berkowitz LR. Smaller pieces of the hysterectomy pie: current challenges in resident surgical education. Obstet Gynecol. 2009;113(2 pt 1):395-398. doi: 10.1097/AOG.0b013e3181955011.
- Wright JD, Herzog TJ, Tsui J, et al. Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet Gynecol. 2013;122(2 pt 1):233-241. doi: 10.1097/AOG.0b013e318299a6cf.
- Cadish LA, Kropat G, Muffly TM. Hysterectomy volume among recent obstetrics and gynecology residency graduates. Female Pelvic Med Reconstr Surg. 2021;27:382-387. doi: 10.1097/SPV.0000000000000879.
- Podratz KC. Gynecologic surgery: an imperiled ballet. Presidential address. Am J Obstet Gynecol. 1998;178:1229-1234. doi: 10.1016/ s0002-9378(98)70327-8.
- Bissonnette JM, Gabbe SG, Hammond CB, et al. Restructuring residency training in obstetrics and gynecology. Am J Obstet Gynecol. 1999;180(3 pt 1):516-518. doi: 10.1016/s0002-9378(99)70246-2.
- Magrina JF. Isn’t it time to separate the O from the G? J Minim Invasive Gynecol. 2014;21:501-503. doi: 10.1016/j.jmig.2014.01.022.
- Merrill JA. Needed changes in obstetric-gynecologic training. Obstet Gynecol Surv. 1994;49:1-2.
- Lauer JK, Advincula AP. The future of the gynecologic surgeon: rationale for and steps toward subspecialization of complex gynecologic surgery. J Minim Invasive Gynecol. 2021;28:726-729. doi: 10.1016/j.jmig.2020.12.031.
- Hall EF, Raker CA, Hampton BS. Variability in gynecologic case volume of obstetrician-gynecologist residents graduating from 2009 to 2017. Am J Obstet Gynecol. 2020;222:617.e1-617.e8. doi: 10.1016/j .ajog.2019.11.1258.
- Irby DM, Cooke M, O’Brien BC. Calls for reform of medical education by the Carnegie Foundation for the Advancement of Teaching: 1910 and 2010. Acad Med. 2010;85:220-227. doi: 10.1097 /ACM.0b013e3181c88449.
- Reed VR, Emery J, Farrell RM, et al. Tracking—a flexible obstetrics and gynecology residency curriculum. Obstet Gynecol. 2019;134(suppl 1):29s-33s. doi: 10.1097/AOG.0000000000003464.
- Hariton E, Freret TS, Nitecki R, et al. Program director perceptions of subspecialty tracking in obstetrics and gynecology residency. J Grad Med Educ. 2018;10:665-670. doi: 10.4300/JGME-D-18-00096.1.
- Azadi S, Green IC, Arnold A, et al. Robotic surgery: the impact of simulation and other innovative platforms on performance and training. J Minim Invasive Gynecol. 2021;28:490-495. doi: 10.1016/j .jmig.2020.12.001.
- Wohlrab K, Jelovsek JE, Myers D. Incorporating simulation into gynecologic surgical training. Am J Obstet Gynecol. 2017;217:522-526. doi: 10.1016/j.ajog.2017.05.017.
- Chen CC, Green IC, Colbert-Getz JM, et al. Warm-up on a simulator improves residents’ performance in laparoscopic surgery: a randomized trial. Int Urogynecol J. 2013;24:1615-1622. doi: 10.1007 /s00192-013-2066-2.
- Fundamentals of Laparoscopic Surgery. ABOG announces new eligibility requirement for board certification. January 23, 2018. Accessed May 12, 2022. https://www.flsprogram.org/news/abog -announces-new-eligibility-requirement-board-certification/.
- Zoorob D, Frenn R, Moffitt M, et al. Multi-institutional validation of a vaginal hysterectomy simulation model for resident training. J Minim Invasive Gynecol. 2021;28:1490-1496.e1. doi: 10.1016/j .jmig.2020.12.006.
- Barrier BF, Thompson AB, McCullough MW, et al. A novel and inexpensive vaginal hysterectomy simulator. Simul Healthc. 2012;7:374-379. doi: 10.1097/SIH.0b013e318266d0c6.
- Stickrath E, Alston M. A novel abdominal hysterectomy simulator and its impact on obstetrics and gynecology residents’ surgical confidence. MedEdPORTAL. 2017;13:10636. doi: 10.15766/mep_2374-8265.10636.
- McKendy KM, Watanabe Y, Lee L, et al. Perioperative feedback in surgical training: a systematic review. Am J Surg. 2017;214:117-126. doi: 10.1016/j.amjsurg.2016.12.014.
- Ahmed M, Arora S, Russ S, et al. Operation debrief: a SHARP improvement in performance feedback in the operating room. Ann Surg. 2013;258:958-963. doi: 10.1097/SLA.0b013e31828c88fc.
- Anderson CI, Gupta RN, Larson JR, et al. Impact of objectively assessing surgeons’ teaching on effective perioperative instructional behaviors. JAMA Surg. 2013;148:915-922. doi: 10.1001/jamasurg.2013.2144.
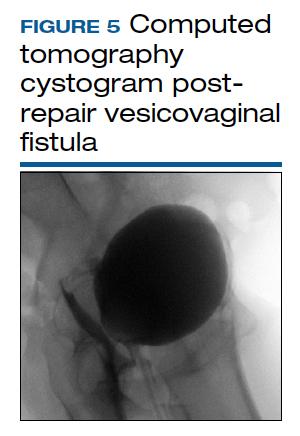
Vesicovaginal and rectovaginal fistulas from obstetric-related causes: Diagnosis and management
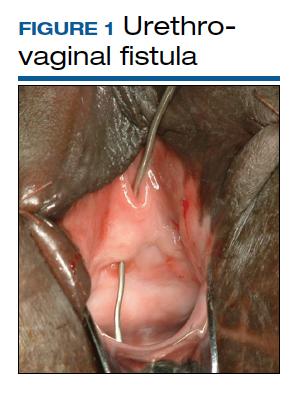
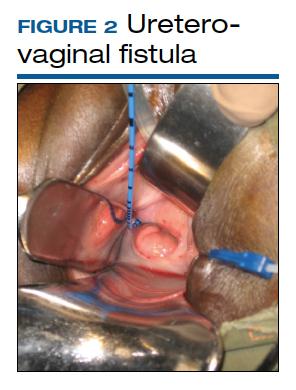
Although rare in the United States and more common in low-resource countries, fistulas due to obstructed labor do occur. In developed countries, other obstetric causes for fistula are usually surgery, trauma, or infection related. An abnormal communication between organs—be it the urethra, bladder, ureter, uterus, cervix, or rectum—can develop1 and lead to vesicovaginal fistula (VVF), urethrovaginal fistula (FIGURE 1), vesicocervical fistula, vesicouterine fistula, ureterovaginal fistula (FIGURE 2), and rectovaginal fistula (RVF). Other nonobstetric causes include gynecologic surgery, radiation, malignancy, and congenital malformations.
During labor, hypoxia, subsequent ischemia, and pressure necrosis contribute to fistula formation. Injury sustained during a cesarean delivery (CD) or cesarean hysterectomy can lead to fistula formation; at times, however, complications are unavoidable given the nature of the pathologic condition that the patient presents with.
VVF and RVF have a devastating impact on a woman’s quality of life as they lead to significant morbidity and short- and long-term psychological distress. The fistula may not be recognized at the time of injury. The presenting signs and symptoms may be intermittent and confusing. Immediate surgical intervention may not be possible due to ongoing inflammation or infection. Recovery often is prolonged. As there is significant concomitant postpartum anxiety and depression, patients with fistula often require psychosocial support and counseling. After repair, there is still a risk for recurrence and voiding dysfunction.
Fistula signs and symptoms and evaluation
In cases of VVF, patients present with continuing large or small volume urinary incontinence. Depending on the time to diagnosis, patients may have calculi formation, prolapse, scarring, external perineal dermatitis, perineal nerve injury, and even motor weakness. Cyclic hematuria may be seen in vesicouterine fistulas.2
Multiple classification systems for diagnosis and staging of VVF have been suggested.3,4 A classification system for RVF was published by Tsang and colleagues.5 All these classification systems have attempted to characterize fistulas in terms of level of surgical complexity for repair, providing a guideline for preoperative assessment. These classification systems do not translate into prediction regarding outcomes.
Evaluation of pelvic fistula from the urinary tract starts with a thorough history that includes onset, duration, and description of leakage (continuous, intermittent, or positional) and whether there is concomitant stress and urge incontinence. A detailed obstetric history, including circumstances around the mode of delivery, underlying risk factors, and psychosocial history, should be obtained.
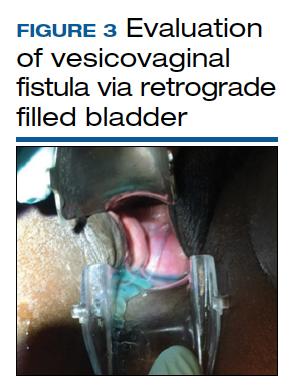
The pelvic examination with a plastic speculum and adequate lighting should assess the external perineum for dermatitis; bulbocavernosus and anal reflexes; and the vagina for length, caliber, level of scarring, and any prolapse. For VVFs, the location, size, and number of the fistula tracts can be visualized and confirmed with a retrograde fill of the bladder via a Foley catheter with saline or water mixed with methylene blue or any other blue dye (FIGURE 3). If a ureterovaginal fistula is suspected, the patient can simultaneously be given oral phenazopyridine and a tampon inserted within the vagina; the patient can then ambulate, and re-examination of the end of the tampon can reveal orange staining. The bladder meanwhile is retrograde filled with blue dye, with no blue staining of the tampon.
For RVF, history taking should include the onset, duration, and description of leakage, and the external anal sphincter should be assessed, with careful examination of the distal vagina at the vestibule as this is the most common location for RVF (fistula in ano). Patients may describe vaginal flatus and sometimes only brownish discharge, which can be intermittent, leading to an incorrect diagnosis of vaginitis that is treated repeatedly without success.
There is no consensus regarding optimal imaging for the assessment of VVF. Imaging used for diagnosis of VVF includes a voiding cystogram with opacification of the vagina after filling the bladder with contrast if there is a fistula. A cystoscopy can evaluate for calculi, retained suture, level of inflammation, and location of the ureters in relation to the fistula. Renal ultrasonography is of limited use. Intravenous pyelography can miss lesions by the trigone. In general, a computed tomography (CT) urogram and magnetic resonance imaging (MRI) with bladder contrast are more sensitive.
In the diagnosis of RVF, contrast vaginoscopy, double contrast barium enema, CT scan with contrast, and MRI can be used. MRI is more sensitive.6 A high index of suspicion is required based on the patient’s history as these imaging modalities do not always confirm RVF despite patient’s clear history of leakage. When the history is convincing, a thorough rectovaginal exam under anesthesia may be imperative. If rectal trauma is present, endoanal ultrasonography can delineate external and internal anal sphincter defects.
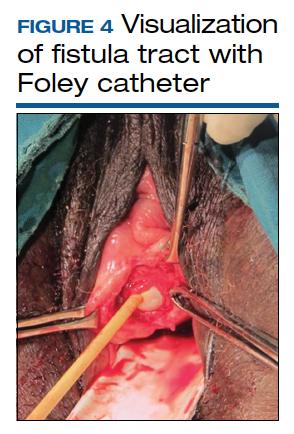
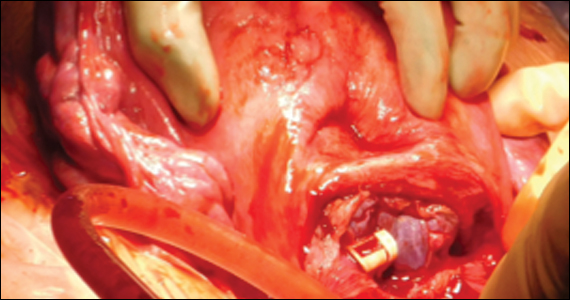
Prolonged Foley catheter placement after obstetric injury can lead to successful closure of a VVF. Prior to surgical intervention, assessing if there is possible ureteral involvement and use of intraoperative ureteral stents is a consideration. The route of surgery can be vaginal, abdominal, combined abdominal-vaginal, laparoscopic, or robotic.7 The robotic approach is increasingly utilized.8,9 However, the general consensus among fistula surgeons is that the vaginal approach should be considered first.
Continue to: Surgical repair...
Surgical repair
VVF repair. Factors that influence successful repair of VVF include the size and number of fistula, location, degree of scarring, bladder capacity, and urethral length.
Surgical technique requires wide mobilization and adequate exposure. The fistula tract can be delineated and manipulated with a pediatric Foley catheter, ureteral stent, or even a ureteral guidewire to aid in dissection (FIGURE 4). Intraoperative visualization of the ureters, including stenting, often is needed. The fistulous track is excised depending on the level of scarring. Closure of the bladder uroepithelium for the first layer is with absorbable interrupted 3-0 or 2-0 sutures in a tension-free closure. The bladder is then evaluated with a retrograde fill with saline and methylene blue to ensure a watertight closure for the first layer. If the first layer is not watertight, the second layer closure will not compensate and the fistula will persist. Particular attention is paid to the angles of the fistula at the first layer closure to prevent recurrence of the fistula at the angles. A running second layer with absorbable 2-0 suture is done. At times, a Martius flap or an omental J flap can be used to provide an additional layer for support and to increase vascularity.10 The patient is sent home with a Foley catheter for drainage for 10 to 14 days.11 Antibiotics are not needed postoperatively for VVF surgery.12
CT cystogram or retrograde cystogram is usually done to evaluate closure of the fistula prior to removal of the Foley catheter; retrograde fill with contrast directly into the bladder with 300 mL is sufficient (FIGURE 5). Patients are advised to refrain from sexual activity for a minimum of 6 weeks, but depending on the level of complexity and scarring, this can be up to 12 weeks.
The success rate in general is in the 95% range. Patients with successful closure of VVF are at risk for urge incontinence due to decreased bladder capacity, stress incontinence especially if the continence mechanism or urethra is involved, vaginal scarring, dyspareunia, and infertility.13 In general, sexual function improves after surgical repair.
RVF repair. Prior to surgical repair of RVF, the integrity of the external anal sphincter must be determined. If it is not involved, a vertical incision is made in the posterior vaginal wall, the vaginal epithelium is separated from the vaginal muscularis, and the fistula tract is identified. After complete wide mobilization of the tissue surrounding the tract, it is excised. The rectal wall is repaired with 3-0 or 4-0 absorbable interrupted sutures; a second layer and if possible even a third layer and finally the vaginal epithelium are all closed with 2-0 absorbable interrupted sutures.
If the sphincter complex is involved, the dissection involves an inverted U incision separating the vaginal wall from the rectum. The fistula tract is excised, the rectal wall is closed, and the internal anal sphincter is identified and reapproximated with interrupted absorbable 2-0 or 0 sutures. The disrupted external sphincter is then reapproximated with 2-0 or 0 sutures, and finally the transverse perineal and bulbocavernosus muscles are brought together with Lembert 0 sutures prior to closure of the external skin. Perioperative antibiotics have been shown to improve success rates in the correction of RVF.5 In patients with sphincter trauma and known RVF, outcomes with a sphincteroplasty are better, compared with endorectal advancement flaps. The patient is discharged with a bowel regimen and dietary precautions that aim for daily soft bowel movements.
After surgical treatment of fistulas, patients benefit from pelvic floor physical therapy that focuses on pelvic floor strengthening. Incorporating the habit of Kegel exercises after every void, timed (scheduled) bladder voiding, and avoidance of straining with urination or defecation should be emphasized.
Continue to: CASE 1 Pregnant woman with rectal bleeding...
CASE 1 Pregnant woman with rectal bleeding
A 37-year-old woman at 36 3/7 weeks’ gestation presented with acute rectal bleeding and pain. This was found to result from a catastrophic rupture of a pelvic arteriovenous malformation that caused an 11 x 7 x 9.5 cm size inferior pelvic hematoma and a full-thickness rectal tear at the dentate line. During examination under anesthesia, the baby was delivered by a stat CD due to breech presentation and a prolonged fetal heart rate deceleration. The patient underwent embolization of the right middle rectal artery and right internal iliac artery by a radiologic intervention. Further bleeding required surgical intervention for evacuation of about 1,000 mL of hematoma, repair of the rectal tear, and laparoscopic diverting loop ileostomy. In total, the patient received 8 U of packed red blood cells, 6 U of fresh frozen plasma, 5 L of crystalloid solution, and 2 g of tranexamic acid. The patient reported increased foul-smelling vaginal discharge, bedside exam suggested possible fistulous tract, and on postoperative day 16, an exam under anesthesia by Urogynecology confirmed a rectovaginal fistula in the right mid vagina. After 2 months of observation to allow resolution of inflammation, successful excision of the fistula tract and repair of RVF using the above-mentioned technique was accomplished.
CASE 2 Patient with VVF after cesarean hysterectomy
A 40-year-old (G6P2222) patient underwent cesarean hysterectomy for placenta percreta and uterine rupture at 24 weeks’ gestation. Intraoperatively, there were right ureteral ligation and posterior bladder wall cystotomies. The right ureter was reimplanted in the right upper posterior wall and the cystostomies were closed. As the patient had continuous urinary leakage postoperatively, a CT urogram was obtained, which showed left ureteral obstruction and VVF. Urinary incontinence persisted despite bilateral robotic ureteral reimplantation with omental flap by the urology team. Percutaneous nephrostomy tubes were placed bilaterally. The patient underwent additional imaging studies, including MRI, with findings of VVF and possible ureterovaginal fistula.
On referral to Urogynecology, the patient underwent cystoscopy with antegrade pyelogram, and the bilateral ureteroneocystostomy orifices had 5 French open-ended ureteral stents placed. A 10 French pediatric Foley catheter was inserted intravaginally into the bladder through the VVF. Via the vaginal approach, cervical remnant and skin bridges overlying the VVF were excised. The scarred fistula tract was excised with a circumferential incision. Horizontal interrupted Lembert sutures with 3-0 absorbable suture were used to reapproximate the first layer, which was confirmed to be watertight on testing with retrograde fill. Second-layer closure was completed with horizontal mattress 2-0 absorbable sutures, followed by a third-layer closure done in similar fashion. Fibrin glue was then placed. The vaginal epithelium was closed with 2-0 absorbable suture. Percutaneous nephrostomy tubes were removed. Postoperatively, the patient had a CT cystogram with no leak and no incontinence, but she developed urgency, which was controlled with timed voids and oxybutynin.
- Adler AJ, Ronsmans C, Calvert C, et al. Estimating the presence of obstetric fistula: a systematic review and meta-analysis BMC Pregnancy Childbirth. 2013;13:246.
- Battacharjee S, Kohli UA, Sood A, et al. Vesicouterine fistula: Youssef’s syndrome. Med J Armed Forces India. 2015;71(suppl 1):S175-S177. doi: 10.1016/j.mjafi.2013.11.006.
- Waaldijk K. Step-by-Step Surgery of Vesicovaginal Fistulas. Campion Press; 1994.
- Goh, JTW. A new classification for female genital tract fistula. Aust N Z J Ob Gynecol. 2004:44:502-504.
- Tsang CB, Rothenberger DA. Rectovaginal fistulas: therapeutic options. Surg Clin North Am. 1997;77:95-114.
- Champagne BJ, McGee MF. Rectovaginal fistula. Surg Clin North Am. 2010;90:69-82.
- Bodner-Adler B, Hanzal E, Pablik E, et al. Management of vesicovaginal fistulas in women following benign gynecologic surgery: a systematic review and meta-analysis. PLoS One. 2017;12:e0171554.
- Randazzo M, Lengauer L, Rochat CH, et al. Best practices in robotic-assisted repair of vesicovaginal fistula: a consensus report from the European Association of Urology Robotic Urology Section Scientific Working Group for Reconstructive Urology. Eur Urol. 2020;78: 432-442.
- Miklos JR, Moore RD, Chinthakanan O. Laparoscopic and robotic assisted vesicovaginal fistula repair: a systematic review of the literature. J Minim Invasive Gynecol. 2015:22:727-736.
- Hancock B. Practical Obstetric Fistula Surgery. Royal Society of Medicine Press; 2009.
- Nardos R, Menber B, Browning A. Outcome of obstetric fistula repair after 10-day versus 14-day Foley catheterization. Int J Gynaecol 0bstet. 2012;118:21-23.
- Tomlinson AJ, Thornton JG. A randomized controlled trial of antibiotic prophylaxis for vesico-vaginal fistula repair. Br J Obstet Gynaecol. 2005;105:397-399.
- Bengtson AM, Kopp D, Tang JH, et al. Identifying patients with vesicovaginal fistula at high risk of urinary incontinence after surgery. Obstet Gynecol. 2016;128:945-953.
Although rare in the United States and more common in low-resource countries, fistulas due to obstructed labor do occur. In developed countries, other obstetric causes for fistula are usually surgery, trauma, or infection related. An abnormal communication between organs—be it the urethra, bladder, ureter, uterus, cervix, or rectum—can develop1 and lead to vesicovaginal fistula (VVF), urethrovaginal fistula (FIGURE 1), vesicocervical fistula, vesicouterine fistula, ureterovaginal fistula (FIGURE 2), and rectovaginal fistula (RVF). Other nonobstetric causes include gynecologic surgery, radiation, malignancy, and congenital malformations.
During labor, hypoxia, subsequent ischemia, and pressure necrosis contribute to fistula formation. Injury sustained during a cesarean delivery (CD) or cesarean hysterectomy can lead to fistula formation; at times, however, complications are unavoidable given the nature of the pathologic condition that the patient presents with.
VVF and RVF have a devastating impact on a woman’s quality of life as they lead to significant morbidity and short- and long-term psychological distress. The fistula may not be recognized at the time of injury. The presenting signs and symptoms may be intermittent and confusing. Immediate surgical intervention may not be possible due to ongoing inflammation or infection. Recovery often is prolonged. As there is significant concomitant postpartum anxiety and depression, patients with fistula often require psychosocial support and counseling. After repair, there is still a risk for recurrence and voiding dysfunction.
Fistula signs and symptoms and evaluation
In cases of VVF, patients present with continuing large or small volume urinary incontinence. Depending on the time to diagnosis, patients may have calculi formation, prolapse, scarring, external perineal dermatitis, perineal nerve injury, and even motor weakness. Cyclic hematuria may be seen in vesicouterine fistulas.2
Multiple classification systems for diagnosis and staging of VVF have been suggested.3,4 A classification system for RVF was published by Tsang and colleagues.5 All these classification systems have attempted to characterize fistulas in terms of level of surgical complexity for repair, providing a guideline for preoperative assessment. These classification systems do not translate into prediction regarding outcomes.
Evaluation of pelvic fistula from the urinary tract starts with a thorough history that includes onset, duration, and description of leakage (continuous, intermittent, or positional) and whether there is concomitant stress and urge incontinence. A detailed obstetric history, including circumstances around the mode of delivery, underlying risk factors, and psychosocial history, should be obtained.
The pelvic examination with a plastic speculum and adequate lighting should assess the external perineum for dermatitis; bulbocavernosus and anal reflexes; and the vagina for length, caliber, level of scarring, and any prolapse. For VVFs, the location, size, and number of the fistula tracts can be visualized and confirmed with a retrograde fill of the bladder via a Foley catheter with saline or water mixed with methylene blue or any other blue dye (FIGURE 3). If a ureterovaginal fistula is suspected, the patient can simultaneously be given oral phenazopyridine and a tampon inserted within the vagina; the patient can then ambulate, and re-examination of the end of the tampon can reveal orange staining. The bladder meanwhile is retrograde filled with blue dye, with no blue staining of the tampon.
For RVF, history taking should include the onset, duration, and description of leakage, and the external anal sphincter should be assessed, with careful examination of the distal vagina at the vestibule as this is the most common location for RVF (fistula in ano). Patients may describe vaginal flatus and sometimes only brownish discharge, which can be intermittent, leading to an incorrect diagnosis of vaginitis that is treated repeatedly without success.
There is no consensus regarding optimal imaging for the assessment of VVF. Imaging used for diagnosis of VVF includes a voiding cystogram with opacification of the vagina after filling the bladder with contrast if there is a fistula. A cystoscopy can evaluate for calculi, retained suture, level of inflammation, and location of the ureters in relation to the fistula. Renal ultrasonography is of limited use. Intravenous pyelography can miss lesions by the trigone. In general, a computed tomography (CT) urogram and magnetic resonance imaging (MRI) with bladder contrast are more sensitive.
In the diagnosis of RVF, contrast vaginoscopy, double contrast barium enema, CT scan with contrast, and MRI can be used. MRI is more sensitive.6 A high index of suspicion is required based on the patient’s history as these imaging modalities do not always confirm RVF despite patient’s clear history of leakage. When the history is convincing, a thorough rectovaginal exam under anesthesia may be imperative. If rectal trauma is present, endoanal ultrasonography can delineate external and internal anal sphincter defects.
Prolonged Foley catheter placement after obstetric injury can lead to successful closure of a VVF. Prior to surgical intervention, assessing if there is possible ureteral involvement and use of intraoperative ureteral stents is a consideration. The route of surgery can be vaginal, abdominal, combined abdominal-vaginal, laparoscopic, or robotic.7 The robotic approach is increasingly utilized.8,9 However, the general consensus among fistula surgeons is that the vaginal approach should be considered first.
Continue to: Surgical repair...
Surgical repair
VVF repair. Factors that influence successful repair of VVF include the size and number of fistula, location, degree of scarring, bladder capacity, and urethral length.
Surgical technique requires wide mobilization and adequate exposure. The fistula tract can be delineated and manipulated with a pediatric Foley catheter, ureteral stent, or even a ureteral guidewire to aid in dissection (FIGURE 4). Intraoperative visualization of the ureters, including stenting, often is needed. The fistulous track is excised depending on the level of scarring. Closure of the bladder uroepithelium for the first layer is with absorbable interrupted 3-0 or 2-0 sutures in a tension-free closure. The bladder is then evaluated with a retrograde fill with saline and methylene blue to ensure a watertight closure for the first layer. If the first layer is not watertight, the second layer closure will not compensate and the fistula will persist. Particular attention is paid to the angles of the fistula at the first layer closure to prevent recurrence of the fistula at the angles. A running second layer with absorbable 2-0 suture is done. At times, a Martius flap or an omental J flap can be used to provide an additional layer for support and to increase vascularity.10 The patient is sent home with a Foley catheter for drainage for 10 to 14 days.11 Antibiotics are not needed postoperatively for VVF surgery.12
CT cystogram or retrograde cystogram is usually done to evaluate closure of the fistula prior to removal of the Foley catheter; retrograde fill with contrast directly into the bladder with 300 mL is sufficient (FIGURE 5). Patients are advised to refrain from sexual activity for a minimum of 6 weeks, but depending on the level of complexity and scarring, this can be up to 12 weeks.
The success rate in general is in the 95% range. Patients with successful closure of VVF are at risk for urge incontinence due to decreased bladder capacity, stress incontinence especially if the continence mechanism or urethra is involved, vaginal scarring, dyspareunia, and infertility.13 In general, sexual function improves after surgical repair.
RVF repair. Prior to surgical repair of RVF, the integrity of the external anal sphincter must be determined. If it is not involved, a vertical incision is made in the posterior vaginal wall, the vaginal epithelium is separated from the vaginal muscularis, and the fistula tract is identified. After complete wide mobilization of the tissue surrounding the tract, it is excised. The rectal wall is repaired with 3-0 or 4-0 absorbable interrupted sutures; a second layer and if possible even a third layer and finally the vaginal epithelium are all closed with 2-0 absorbable interrupted sutures.
If the sphincter complex is involved, the dissection involves an inverted U incision separating the vaginal wall from the rectum. The fistula tract is excised, the rectal wall is closed, and the internal anal sphincter is identified and reapproximated with interrupted absorbable 2-0 or 0 sutures. The disrupted external sphincter is then reapproximated with 2-0 or 0 sutures, and finally the transverse perineal and bulbocavernosus muscles are brought together with Lembert 0 sutures prior to closure of the external skin. Perioperative antibiotics have been shown to improve success rates in the correction of RVF.5 In patients with sphincter trauma and known RVF, outcomes with a sphincteroplasty are better, compared with endorectal advancement flaps. The patient is discharged with a bowel regimen and dietary precautions that aim for daily soft bowel movements.
After surgical treatment of fistulas, patients benefit from pelvic floor physical therapy that focuses on pelvic floor strengthening. Incorporating the habit of Kegel exercises after every void, timed (scheduled) bladder voiding, and avoidance of straining with urination or defecation should be emphasized.
Continue to: CASE 1 Pregnant woman with rectal bleeding...
CASE 1 Pregnant woman with rectal bleeding
A 37-year-old woman at 36 3/7 weeks’ gestation presented with acute rectal bleeding and pain. This was found to result from a catastrophic rupture of a pelvic arteriovenous malformation that caused an 11 x 7 x 9.5 cm size inferior pelvic hematoma and a full-thickness rectal tear at the dentate line. During examination under anesthesia, the baby was delivered by a stat CD due to breech presentation and a prolonged fetal heart rate deceleration. The patient underwent embolization of the right middle rectal artery and right internal iliac artery by a radiologic intervention. Further bleeding required surgical intervention for evacuation of about 1,000 mL of hematoma, repair of the rectal tear, and laparoscopic diverting loop ileostomy. In total, the patient received 8 U of packed red blood cells, 6 U of fresh frozen plasma, 5 L of crystalloid solution, and 2 g of tranexamic acid. The patient reported increased foul-smelling vaginal discharge, bedside exam suggested possible fistulous tract, and on postoperative day 16, an exam under anesthesia by Urogynecology confirmed a rectovaginal fistula in the right mid vagina. After 2 months of observation to allow resolution of inflammation, successful excision of the fistula tract and repair of RVF using the above-mentioned technique was accomplished.
CASE 2 Patient with VVF after cesarean hysterectomy
A 40-year-old (G6P2222) patient underwent cesarean hysterectomy for placenta percreta and uterine rupture at 24 weeks’ gestation. Intraoperatively, there were right ureteral ligation and posterior bladder wall cystotomies. The right ureter was reimplanted in the right upper posterior wall and the cystostomies were closed. As the patient had continuous urinary leakage postoperatively, a CT urogram was obtained, which showed left ureteral obstruction and VVF. Urinary incontinence persisted despite bilateral robotic ureteral reimplantation with omental flap by the urology team. Percutaneous nephrostomy tubes were placed bilaterally. The patient underwent additional imaging studies, including MRI, with findings of VVF and possible ureterovaginal fistula.
On referral to Urogynecology, the patient underwent cystoscopy with antegrade pyelogram, and the bilateral ureteroneocystostomy orifices had 5 French open-ended ureteral stents placed. A 10 French pediatric Foley catheter was inserted intravaginally into the bladder through the VVF. Via the vaginal approach, cervical remnant and skin bridges overlying the VVF were excised. The scarred fistula tract was excised with a circumferential incision. Horizontal interrupted Lembert sutures with 3-0 absorbable suture were used to reapproximate the first layer, which was confirmed to be watertight on testing with retrograde fill. Second-layer closure was completed with horizontal mattress 2-0 absorbable sutures, followed by a third-layer closure done in similar fashion. Fibrin glue was then placed. The vaginal epithelium was closed with 2-0 absorbable suture. Percutaneous nephrostomy tubes were removed. Postoperatively, the patient had a CT cystogram with no leak and no incontinence, but she developed urgency, which was controlled with timed voids and oxybutynin.
Although rare in the United States and more common in low-resource countries, fistulas due to obstructed labor do occur. In developed countries, other obstetric causes for fistula are usually surgery, trauma, or infection related. An abnormal communication between organs—be it the urethra, bladder, ureter, uterus, cervix, or rectum—can develop1 and lead to vesicovaginal fistula (VVF), urethrovaginal fistula (FIGURE 1), vesicocervical fistula, vesicouterine fistula, ureterovaginal fistula (FIGURE 2), and rectovaginal fistula (RVF). Other nonobstetric causes include gynecologic surgery, radiation, malignancy, and congenital malformations.
During labor, hypoxia, subsequent ischemia, and pressure necrosis contribute to fistula formation. Injury sustained during a cesarean delivery (CD) or cesarean hysterectomy can lead to fistula formation; at times, however, complications are unavoidable given the nature of the pathologic condition that the patient presents with.
VVF and RVF have a devastating impact on a woman’s quality of life as they lead to significant morbidity and short- and long-term psychological distress. The fistula may not be recognized at the time of injury. The presenting signs and symptoms may be intermittent and confusing. Immediate surgical intervention may not be possible due to ongoing inflammation or infection. Recovery often is prolonged. As there is significant concomitant postpartum anxiety and depression, patients with fistula often require psychosocial support and counseling. After repair, there is still a risk for recurrence and voiding dysfunction.
Fistula signs and symptoms and evaluation
In cases of VVF, patients present with continuing large or small volume urinary incontinence. Depending on the time to diagnosis, patients may have calculi formation, prolapse, scarring, external perineal dermatitis, perineal nerve injury, and even motor weakness. Cyclic hematuria may be seen in vesicouterine fistulas.2
Multiple classification systems for diagnosis and staging of VVF have been suggested.3,4 A classification system for RVF was published by Tsang and colleagues.5 All these classification systems have attempted to characterize fistulas in terms of level of surgical complexity for repair, providing a guideline for preoperative assessment. These classification systems do not translate into prediction regarding outcomes.
Evaluation of pelvic fistula from the urinary tract starts with a thorough history that includes onset, duration, and description of leakage (continuous, intermittent, or positional) and whether there is concomitant stress and urge incontinence. A detailed obstetric history, including circumstances around the mode of delivery, underlying risk factors, and psychosocial history, should be obtained.
The pelvic examination with a plastic speculum and adequate lighting should assess the external perineum for dermatitis; bulbocavernosus and anal reflexes; and the vagina for length, caliber, level of scarring, and any prolapse. For VVFs, the location, size, and number of the fistula tracts can be visualized and confirmed with a retrograde fill of the bladder via a Foley catheter with saline or water mixed with methylene blue or any other blue dye (FIGURE 3). If a ureterovaginal fistula is suspected, the patient can simultaneously be given oral phenazopyridine and a tampon inserted within the vagina; the patient can then ambulate, and re-examination of the end of the tampon can reveal orange staining. The bladder meanwhile is retrograde filled with blue dye, with no blue staining of the tampon.
For RVF, history taking should include the onset, duration, and description of leakage, and the external anal sphincter should be assessed, with careful examination of the distal vagina at the vestibule as this is the most common location for RVF (fistula in ano). Patients may describe vaginal flatus and sometimes only brownish discharge, which can be intermittent, leading to an incorrect diagnosis of vaginitis that is treated repeatedly without success.
There is no consensus regarding optimal imaging for the assessment of VVF. Imaging used for diagnosis of VVF includes a voiding cystogram with opacification of the vagina after filling the bladder with contrast if there is a fistula. A cystoscopy can evaluate for calculi, retained suture, level of inflammation, and location of the ureters in relation to the fistula. Renal ultrasonography is of limited use. Intravenous pyelography can miss lesions by the trigone. In general, a computed tomography (CT) urogram and magnetic resonance imaging (MRI) with bladder contrast are more sensitive.
In the diagnosis of RVF, contrast vaginoscopy, double contrast barium enema, CT scan with contrast, and MRI can be used. MRI is more sensitive.6 A high index of suspicion is required based on the patient’s history as these imaging modalities do not always confirm RVF despite patient’s clear history of leakage. When the history is convincing, a thorough rectovaginal exam under anesthesia may be imperative. If rectal trauma is present, endoanal ultrasonography can delineate external and internal anal sphincter defects.
Prolonged Foley catheter placement after obstetric injury can lead to successful closure of a VVF. Prior to surgical intervention, assessing if there is possible ureteral involvement and use of intraoperative ureteral stents is a consideration. The route of surgery can be vaginal, abdominal, combined abdominal-vaginal, laparoscopic, or robotic.7 The robotic approach is increasingly utilized.8,9 However, the general consensus among fistula surgeons is that the vaginal approach should be considered first.
Continue to: Surgical repair...
Surgical repair
VVF repair. Factors that influence successful repair of VVF include the size and number of fistula, location, degree of scarring, bladder capacity, and urethral length.
Surgical technique requires wide mobilization and adequate exposure. The fistula tract can be delineated and manipulated with a pediatric Foley catheter, ureteral stent, or even a ureteral guidewire to aid in dissection (FIGURE 4). Intraoperative visualization of the ureters, including stenting, often is needed. The fistulous track is excised depending on the level of scarring. Closure of the bladder uroepithelium for the first layer is with absorbable interrupted 3-0 or 2-0 sutures in a tension-free closure. The bladder is then evaluated with a retrograde fill with saline and methylene blue to ensure a watertight closure for the first layer. If the first layer is not watertight, the second layer closure will not compensate and the fistula will persist. Particular attention is paid to the angles of the fistula at the first layer closure to prevent recurrence of the fistula at the angles. A running second layer with absorbable 2-0 suture is done. At times, a Martius flap or an omental J flap can be used to provide an additional layer for support and to increase vascularity.10 The patient is sent home with a Foley catheter for drainage for 10 to 14 days.11 Antibiotics are not needed postoperatively for VVF surgery.12
CT cystogram or retrograde cystogram is usually done to evaluate closure of the fistula prior to removal of the Foley catheter; retrograde fill with contrast directly into the bladder with 300 mL is sufficient (FIGURE 5). Patients are advised to refrain from sexual activity for a minimum of 6 weeks, but depending on the level of complexity and scarring, this can be up to 12 weeks.
The success rate in general is in the 95% range. Patients with successful closure of VVF are at risk for urge incontinence due to decreased bladder capacity, stress incontinence especially if the continence mechanism or urethra is involved, vaginal scarring, dyspareunia, and infertility.13 In general, sexual function improves after surgical repair.
RVF repair. Prior to surgical repair of RVF, the integrity of the external anal sphincter must be determined. If it is not involved, a vertical incision is made in the posterior vaginal wall, the vaginal epithelium is separated from the vaginal muscularis, and the fistula tract is identified. After complete wide mobilization of the tissue surrounding the tract, it is excised. The rectal wall is repaired with 3-0 or 4-0 absorbable interrupted sutures; a second layer and if possible even a third layer and finally the vaginal epithelium are all closed with 2-0 absorbable interrupted sutures.
If the sphincter complex is involved, the dissection involves an inverted U incision separating the vaginal wall from the rectum. The fistula tract is excised, the rectal wall is closed, and the internal anal sphincter is identified and reapproximated with interrupted absorbable 2-0 or 0 sutures. The disrupted external sphincter is then reapproximated with 2-0 or 0 sutures, and finally the transverse perineal and bulbocavernosus muscles are brought together with Lembert 0 sutures prior to closure of the external skin. Perioperative antibiotics have been shown to improve success rates in the correction of RVF.5 In patients with sphincter trauma and known RVF, outcomes with a sphincteroplasty are better, compared with endorectal advancement flaps. The patient is discharged with a bowel regimen and dietary precautions that aim for daily soft bowel movements.
After surgical treatment of fistulas, patients benefit from pelvic floor physical therapy that focuses on pelvic floor strengthening. Incorporating the habit of Kegel exercises after every void, timed (scheduled) bladder voiding, and avoidance of straining with urination or defecation should be emphasized.
Continue to: CASE 1 Pregnant woman with rectal bleeding...
CASE 1 Pregnant woman with rectal bleeding
A 37-year-old woman at 36 3/7 weeks’ gestation presented with acute rectal bleeding and pain. This was found to result from a catastrophic rupture of a pelvic arteriovenous malformation that caused an 11 x 7 x 9.5 cm size inferior pelvic hematoma and a full-thickness rectal tear at the dentate line. During examination under anesthesia, the baby was delivered by a stat CD due to breech presentation and a prolonged fetal heart rate deceleration. The patient underwent embolization of the right middle rectal artery and right internal iliac artery by a radiologic intervention. Further bleeding required surgical intervention for evacuation of about 1,000 mL of hematoma, repair of the rectal tear, and laparoscopic diverting loop ileostomy. In total, the patient received 8 U of packed red blood cells, 6 U of fresh frozen plasma, 5 L of crystalloid solution, and 2 g of tranexamic acid. The patient reported increased foul-smelling vaginal discharge, bedside exam suggested possible fistulous tract, and on postoperative day 16, an exam under anesthesia by Urogynecology confirmed a rectovaginal fistula in the right mid vagina. After 2 months of observation to allow resolution of inflammation, successful excision of the fistula tract and repair of RVF using the above-mentioned technique was accomplished.
CASE 2 Patient with VVF after cesarean hysterectomy
A 40-year-old (G6P2222) patient underwent cesarean hysterectomy for placenta percreta and uterine rupture at 24 weeks’ gestation. Intraoperatively, there were right ureteral ligation and posterior bladder wall cystotomies. The right ureter was reimplanted in the right upper posterior wall and the cystostomies were closed. As the patient had continuous urinary leakage postoperatively, a CT urogram was obtained, which showed left ureteral obstruction and VVF. Urinary incontinence persisted despite bilateral robotic ureteral reimplantation with omental flap by the urology team. Percutaneous nephrostomy tubes were placed bilaterally. The patient underwent additional imaging studies, including MRI, with findings of VVF and possible ureterovaginal fistula.
On referral to Urogynecology, the patient underwent cystoscopy with antegrade pyelogram, and the bilateral ureteroneocystostomy orifices had 5 French open-ended ureteral stents placed. A 10 French pediatric Foley catheter was inserted intravaginally into the bladder through the VVF. Via the vaginal approach, cervical remnant and skin bridges overlying the VVF were excised. The scarred fistula tract was excised with a circumferential incision. Horizontal interrupted Lembert sutures with 3-0 absorbable suture were used to reapproximate the first layer, which was confirmed to be watertight on testing with retrograde fill. Second-layer closure was completed with horizontal mattress 2-0 absorbable sutures, followed by a third-layer closure done in similar fashion. Fibrin glue was then placed. The vaginal epithelium was closed with 2-0 absorbable suture. Percutaneous nephrostomy tubes were removed. Postoperatively, the patient had a CT cystogram with no leak and no incontinence, but she developed urgency, which was controlled with timed voids and oxybutynin.
- Adler AJ, Ronsmans C, Calvert C, et al. Estimating the presence of obstetric fistula: a systematic review and meta-analysis BMC Pregnancy Childbirth. 2013;13:246.
- Battacharjee S, Kohli UA, Sood A, et al. Vesicouterine fistula: Youssef’s syndrome. Med J Armed Forces India. 2015;71(suppl 1):S175-S177. doi: 10.1016/j.mjafi.2013.11.006.
- Waaldijk K. Step-by-Step Surgery of Vesicovaginal Fistulas. Campion Press; 1994.
- Goh, JTW. A new classification for female genital tract fistula. Aust N Z J Ob Gynecol. 2004:44:502-504.
- Tsang CB, Rothenberger DA. Rectovaginal fistulas: therapeutic options. Surg Clin North Am. 1997;77:95-114.
- Champagne BJ, McGee MF. Rectovaginal fistula. Surg Clin North Am. 2010;90:69-82.
- Bodner-Adler B, Hanzal E, Pablik E, et al. Management of vesicovaginal fistulas in women following benign gynecologic surgery: a systematic review and meta-analysis. PLoS One. 2017;12:e0171554.
- Randazzo M, Lengauer L, Rochat CH, et al. Best practices in robotic-assisted repair of vesicovaginal fistula: a consensus report from the European Association of Urology Robotic Urology Section Scientific Working Group for Reconstructive Urology. Eur Urol. 2020;78: 432-442.
- Miklos JR, Moore RD, Chinthakanan O. Laparoscopic and robotic assisted vesicovaginal fistula repair: a systematic review of the literature. J Minim Invasive Gynecol. 2015:22:727-736.
- Hancock B. Practical Obstetric Fistula Surgery. Royal Society of Medicine Press; 2009.
- Nardos R, Menber B, Browning A. Outcome of obstetric fistula repair after 10-day versus 14-day Foley catheterization. Int J Gynaecol 0bstet. 2012;118:21-23.
- Tomlinson AJ, Thornton JG. A randomized controlled trial of antibiotic prophylaxis for vesico-vaginal fistula repair. Br J Obstet Gynaecol. 2005;105:397-399.
- Bengtson AM, Kopp D, Tang JH, et al. Identifying patients with vesicovaginal fistula at high risk of urinary incontinence after surgery. Obstet Gynecol. 2016;128:945-953.
- Adler AJ, Ronsmans C, Calvert C, et al. Estimating the presence of obstetric fistula: a systematic review and meta-analysis BMC Pregnancy Childbirth. 2013;13:246.
- Battacharjee S, Kohli UA, Sood A, et al. Vesicouterine fistula: Youssef’s syndrome. Med J Armed Forces India. 2015;71(suppl 1):S175-S177. doi: 10.1016/j.mjafi.2013.11.006.
- Waaldijk K. Step-by-Step Surgery of Vesicovaginal Fistulas. Campion Press; 1994.
- Goh, JTW. A new classification for female genital tract fistula. Aust N Z J Ob Gynecol. 2004:44:502-504.
- Tsang CB, Rothenberger DA. Rectovaginal fistulas: therapeutic options. Surg Clin North Am. 1997;77:95-114.
- Champagne BJ, McGee MF. Rectovaginal fistula. Surg Clin North Am. 2010;90:69-82.
- Bodner-Adler B, Hanzal E, Pablik E, et al. Management of vesicovaginal fistulas in women following benign gynecologic surgery: a systematic review and meta-analysis. PLoS One. 2017;12:e0171554.
- Randazzo M, Lengauer L, Rochat CH, et al. Best practices in robotic-assisted repair of vesicovaginal fistula: a consensus report from the European Association of Urology Robotic Urology Section Scientific Working Group for Reconstructive Urology. Eur Urol. 2020;78: 432-442.
- Miklos JR, Moore RD, Chinthakanan O. Laparoscopic and robotic assisted vesicovaginal fistula repair: a systematic review of the literature. J Minim Invasive Gynecol. 2015:22:727-736.
- Hancock B. Practical Obstetric Fistula Surgery. Royal Society of Medicine Press; 2009.
- Nardos R, Menber B, Browning A. Outcome of obstetric fistula repair after 10-day versus 14-day Foley catheterization. Int J Gynaecol 0bstet. 2012;118:21-23.
- Tomlinson AJ, Thornton JG. A randomized controlled trial of antibiotic prophylaxis for vesico-vaginal fistula repair. Br J Obstet Gynaecol. 2005;105:397-399.
- Bengtson AM, Kopp D, Tang JH, et al. Identifying patients with vesicovaginal fistula at high risk of urinary incontinence after surgery. Obstet Gynecol. 2016;128:945-953.
OR safety and efficiency: Measuring and monitoring all factors—including surgical volume
The operating room (OR) is a key contributor to a hospital’s profitability. It is a complex environment with ever-advancing technology. A successful surgery completed without complications within an optimal time depends not only on the surgeon’s experience, skills, and knowledge but also on numerous other structural, human, and nontechnical factors over which the surgeon has limited control.
As in any setting that deals with human life, in the OR, team dynamics, communication, and environment play a major role. Research has indicated the benefits of dedicated teams, reduced handoffs, and innovative modalities that continuously and systematically monitor potential breakdowns and propose solutions for the detected problems.
Finally, who should perform your loved one’s hysterectomy? This article also attempts to address the impact of surgeons’ and hospitals’ volume on operative outcomes with a diminishing number of hysterectomies but an increasing number of approaches.
Human factors in the OR
Human factors research was born as a product of the industrial revolution and mass production. It aims to optimize human experience and improve system performance by studying how humans interact with system. The aviation industry, for example, minimized errors significantly by using methods developed by human factors scientists. As another industry with no tolerance for mistakes, the health care sector followed suit. Ultimately, the goal of human factors research in health care is to improve patient safety, optimize work and environment, reduce costs, and enhance employees’ physical and mental health, engagement, comfort, and quality of life (FIGURE 1).1
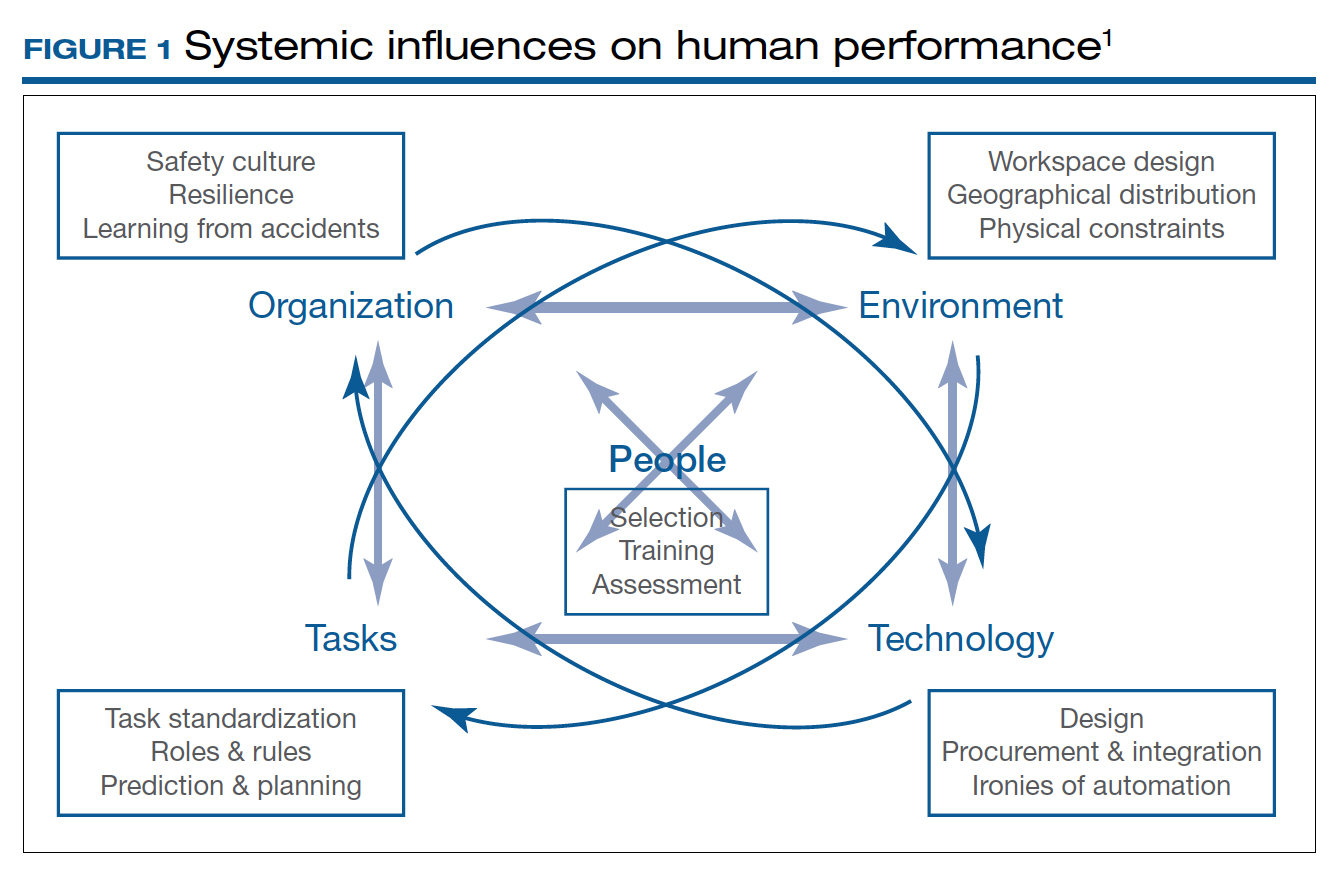
Today’s OR is so complex that it is hard to understand its dynamics without human factors research. Every new OR technology is first tested in controlled and simulated environments to determine “work as imagined.” However, it is necessary to study “work as done” in the real world via direct observation, video recording, questionnaires, and semistructured interviews by an on-site multidisciplinary team. This process not only focuses on surgical skills, process efficiency, and outcomes but also monitors the entire process according to Human Factors and Ergonomics Engineering principles to explore otherwise hidden complexities and latent safety concerns. The Systems Engineering Initiative for Patient Safety (SEIPS) framework is used to study the impact of interactions between people, tasks, technologies, environment, and organization.1
Robot-assisted surgery (RAS), an increasingly popular surgical approach among gynecologic surgeons, recently has been the focus of human factors science. A robotic OR poses unique challenges: the surgeon is not scrubbed, is removed from the operating table, and controls a complex highly technologic device in a crowded and darkened room. These are ideal conditions waiting to be optimized by human factor experts. To demonstrate the importance of human factors in the OR, we review the evidence for RAS.
Continue to: Impact of flow disruptions...
Impact of flow disruptions
Flow disruptions (FDs) were found to be more common in RAS. Catchpole and colleagues identified a mean of 9.62 FDs per hour in 89 robotic procedures, including hysterectomies and sacrocolpopexies, from a variety of fields; FDs occurred more often during the docking stage, followed by the console time, and they mostly were caused by communication breakdown and lack of team familiarity.2
Surgeon experience significantly reduced FDs. Surgeons who had done more than 700 RAS cases experienced 60% fewer FDs than those who had done less than 250 cases (13 vs 8 per hour).2 A study focusing on residents’ impact on RAS outcomes found that each FD increased the total operative time by an average 2.4 minutes, with the number significantly higher when a resident was involved.3 About one-quarter of the training-related FDs were procedure-specific instructions, while one-third were related to instrument and robotic instruction. However, pauses to teach residents did not appear to create significant intraoperative delays. Expectedly, experienced surgeons could anticipate and reduce these disruptions by supporting the whole team.
Human ergonomics, turnover time, and robot-specific skills
In a study of human ergonomics in RAS, Yu and colleagues noted that bedside assistants could experience neck posture problems. Surprisingly, the console could constrain the surgeon’s neck-shoulder region.4 Studies that reported on communication problems in a robotic OR suggest that innovative forms of verbal and nonverbal communication may support successful team communication.5
On the learning curve for RAS, OR turnover time, a key value metric, has been longer. However, turnover time was reduced almost by half from 99.2 to 53.2 minutes over 3 months after concepts from motor racing pit stops were employed, including briefings, leadership, role definition, task allocation, and task sequencing. Average room-ready time also was lowered from 42.2 to 27.2 minutes.6 RAS presents new challenges with sterile instrument processing as well. A successful RAS program, therefore, has organizational needs that include the training of OR and sterile processing staff and appropriate shift management.1
In a robotic OR, not only the surgeon but also the whole team requires robot-specific skills. New training approaches to teamwork, communication, and situation awareness skills are necessary. Robotic equipment, with its data and power cables, 2 consoles, and changing movement paths, necessitate larger rooms with a specific layout.7
In a review of recordings of RAS that used a multidimensional assessment tool to measure team effectiveness and cognitive load, Sexton and colleagues identified anticipation, active team engagement, and higher familiarity scores as the best predictors of team efficiency.8 Several studies emphasized the need for a stable team, especially in the learning phase of robotic surgery.5,9,10 A dedicated robotic team reduced the operative time by 18% during robot-assisted sacrocolpopexy (RASCP).10 RASCP procedures that extended into the afternoon took significantly longer time.9 A dedicated anesthesiologist improved the preoperative time.9 Surgical team handoffs also have reduced OR efficiency.11,12
Studying the impact of human factors is paramount for safe and efficient surgery. It is especially necessary in ORs that are equipped with high technologic instruments such as those used in RAS.
Surgical Black Box: Using data for OR safety and efficiency
Surgical procedures account for more than 50% of medical errors in a hospital setting, many of which are preventable. Postevent analysis with traditional methods, such as “Morbidity and Mortality” meetings held many days later, misses many adverse events in the OR.13 Another challenge with ever-changing and fast-multiplying surgical approaches is the development of effective surgical skill. Reviewing video recording of surgical procedures has been proposed as an instrument for recognizing adverse events and perfecting surgical skills.Recently, an innovative data-capture platform called the OR Black Box, developed by Teodor Grantcharov, MD, PhD, and colleagues, went beyond simple audiovisual recording.14 This high technologic platform not only video records the actual surgical procedure with laparoscopic camera capture (and wearable cameras for open cases) but also monitors the entire OR environment via wide-angle cameras, utilizes sensors, and records both the patient’s and the surgeon’s physiologic parameters.
The OR Black Box generates a holistic view of the OR after synchronization, encryption, and secure storage of all inputs for further analysis by experts and software-based algorithms (FIGURE 2). Computer vision algorithms can recognize improper dissection techniques and complications, such as bleeding. Adverse events are flagged with an automated software on a procedural timeline to facilitate review of procedural steps, disruptive environmental and organizational factors, OR team technical and nontechnical skills, surgeon physiologic stress, and intraoperative errors, events, and rectification processes using validated instruments.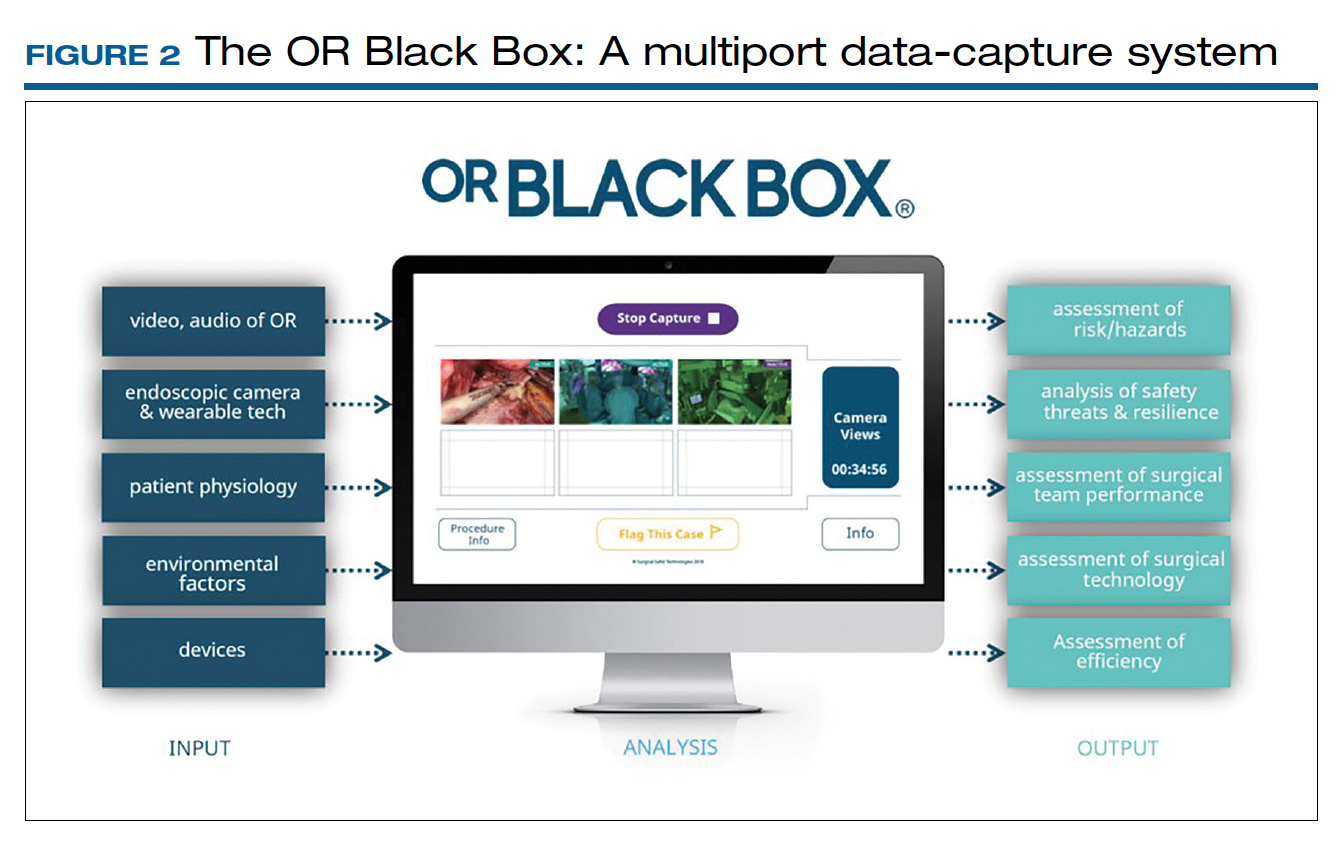
Artificial intelligence built into this platform can automatically extract objective, high-quality, and structured data to generate explainable insights by recognizing adverse events and procedural segments of interest for training and quality improvement and provide a foundation with objective measurements of technical and nontechnical performance for formative and summative assessment. This system, a major step up compared with retrospective review of likely biased medical records and labor-intensive multidisciplinary human observers, has the potential to increase efficiency and reduce costs by studying human factors that include clinical design, technology, and organization. OR efficiency, measured in real time objectively and thoroughly, may save time and resources.
OR Black Box platforms have already started to generate meaningful data. It is not surprising that auditory disruptions—OR doors opening, loud noises, pagers beeping, telephones ringing—were recorded almost every minute during laparoscopic procedures.15 Most technical errors occurred during dissection, resection, and reconstruction and most commonly were associated with improper estimations of force applied to tissue and distance to the target tissue during operative steps of a laparoscopic procedure.16 Another study based on this system showed that technical performance was an independent predictor of postoperative outcomes.17 The OR Black Box identified a device-related interruption in 30% of elective laparoscopic general surgery cases, most commonly in sleeve gastrectomy and oncologic gastrectomy procedures. This sophisticated surgical data recording system also demonstrated a significantly better ability to detect Veress needle injuries (12 vs 3) and near misses (47 vs 0) when compared with traditional chart review.18
Data from the OR Black Box also have been applied to better analyze nontechnical performance, including teamwork and interpersonal dynamics.19 Surgeons most commonly exhibited adept situational awareness and leadership, while the nurse team excelled at task management and situational awareness.19 Of the total care provider team studied, the surgeon and scrub nurse demonstrated the most favorable nontechnical behavior.19 Of note, continuous physiologic monitoring of the surgeon with this system revealed that surgeons under stress had 66% higher adverse events.
The OR Black Box is currently utilized at 20 institutions in North America and Europe. The data compiled from all these institutions revealed that there was a 10% decrease in intraoperative adverse events for each 10-point increase in technical skill score on a scale of 0 to 100 (unpublished data). This centralized data indicated that turnover time ranged widely between 7 and 91 minutes, with variation of cleanup time from 1 to 25 minutes and setup time from 22 to 43 minutes. Institutions can learn from each other using this platform. For example, the information about block time utilization (20%–99%) across institutions provides opportunities for system improvements.
With any revolutionary technology, it is imperative to study its effects on outcomes, training, costs, and privacy before it is widely implemented. We, obstetricians and gynecologists, are very familiar with the impact of electronic fetal monitoring, a great example of a technologic advance that did not improve perinatal outcomes but led to unintended consequences, such as higher rates of cesarean deliveries and lawsuits. Such a tool may lead to potential misrepresentation of intraoperative events unless legal aspects are clearly delineated. As exciting as it is, this disruptive technology requires further exploration with scientific vigor.
Continue to: Surgeon and hospital volume: Surgical outcomes paradigm...
Surgeon and hospital volume: Surgical outcomes paradigm
A landmark study in 1979 that showed decreased mortality in high-volume centers underscored the need for regionalization for certain surgical procedures.20 This association was further substantiated by 2 reports on 2.5 million Medicare beneficiaries that demonstrated significantly lower mortality for all 14 cardiovascular and oncologic procedures for hospitals with larger surgical volume (16% vs 4%) and high-volume surgeons for certain procedures, for example, 15% versus 5% for pancreatic resections for cancer.21,22
A similar association was found for all routes of hysterectomies performed for benign indications. Boyd and colleagues showed that gynecologists who performed fewer than 10 hysterectomies per year had a higher perioperative morbidity rate (16.5%) compared with those who did more (11.7%).23 Specific to vaginal hysterectomy, in a study of more than 6,000 women, surgeons who performed 13 procedures per year had 31% less risk of operative injury than those who did 5.5 procedures per year (2.5% vs 1.7%).24 Overall perioperative complications (5.0% vs 4.0%) and medical complications (5.7% vs 3.9%) were also reduced for higher-volume surgeons. In a cohort of approximately 8,000 women who underwent a laparoscopic hysterectomy, high-volume surgeons had a considerably lower complication rate (4.2% vs 6.2%).25
As expected, lower complication rates of high-volume surgeons led to lower resource utilization, including lower transfusion rates, less intensive care unit utilization, and shorter operative times and, in several studies, length of stay.24,25 Of note, low-volume surgeons were less likely to offer minimally invasive routes and were more likely to convert to laparotomy.26 In addition, significant cost savings have been associated with high surgical volume, which one study showed was 16% ($6,500 vs $5,600) for high-volume surgeons.26 With regard to mortality, a study of 7,800 women found that perioperative mortality increased more than 10-fold for surgeons who performed an average 1 case per year compared with all other surgeons (2.5% vs 0.2%).27
When gynecologic cancers are concerned, arguably, long-term survival outcomes may be more critical than perioperative morbidity and mortality. Higher surgeon and hospital volume are associated with improved perioperative outcomes for endometrial and cervical cancers.28 Importantly, minimally invasive hysterectomy was offered for endometrial cancer significantly more often by surgeons with high volume.28 Survival outcomes were not affected by surgeon or hospital volume, likely due to overall more favorable prognosis for endometrial cancer after treatment.
Although it is intuitive to assume that a surgeon’s skills and experience would make the most impact in procedures for ovarian cancer due to the complexity of ovarian cancer surgery, evidence on short-term outcomes has been mixed. Intriguingly, some studies reported that high-volume institutions had higher complication and readmission rates. However, evidence supports that the surgeon’s volume, and especially hospital volume, improves long-term survival for ovarian cancer, with a negative impact on immediate postoperative morbidity.29 This may suggest that a more aggressive surgical effort improves long-term survival but also can cause more perioperative complications. Further, longer survival may result not only from operative skills but also because of better care by a structured multidisciplinary team at more established high-volume cancer centers.
The association of improved outcomes with higher volume led to public reporting of hospital outcomes. Policy efforts toward regionalization have impacted surgical practice. Based on their analysis of 3.2 million Medicare patients who underwent 1 of 8 different cancer surgeries or cardiovascular operations from 1999 to 2008, Finks and colleagues demonstrated that care was concentrated to fewer hospitals over time for many of these procedures.29 This trend was noted for gynecologic cancer surgery but not for benign gynecologic surgery.
Regionalization of care limits access particularly for minority and underserved communities because of longer travel distances, logistic challenges, and financial strain. An alternative to regionalization of care is targeted quality improvement by rigorous adherence to quality guidelines at low-volume hospitals.
Is there a critical minimum volume that may be used as a requirement for surgeons to maintain their privileges and for hospitals to offer certain procedures? In 2015, minimum volume standards for a number of common procedures were proposed by Johns Hopkins Medicine and Dartmouth-Hitchcock Medical Center, such as 50 hip replacement surgeries per hospital and 25 per physician per year, and 20 pancreatectomies per hospital and 5 per surgeon per year.30 A modeling study for hysterectomy showed that a volume cut point of >1 procedure in the prior year would restrict privileges for a substantial number of surgeons performing abdominal (17.5%), robot-assisted (12.5%), laparoscopic (16.8%), and vaginal (27.6%) hysterectomies.27 This study concluded that minimum-volume standards for hysterectomy for even the lowest volume physicians would restrict a significant number of gynecologic surgeons, including many with outcomes that are better than predicted.
Therefore, while there is good evidence that favors better outcomes in the hands of high-volume surgeons in gynecology, the impact of such policies on gynecologic practice clearly warrants careful monitoring and further study.
- What factors besides the surgeon’s skills influence surgical safety and efficiency?
- Are you ready to have audio, video, and sensor-based recording of everything in the OR?
- Who should perform your loved one’s hysterectomy? Do the surgeon’s and hospital’s volume matter?
- Catchpole K, Bisantz A, Hallbeck MS, et al. Human factors in robotic assisted surgery: lessons from studies ‘in the wild’. Appl Ergon. 2019;78:270-276.
- Catchpole K, Perkins C, Bresee C, et al. Safety, efficiency and learning curves in robotic surgery: a human factors analysis. Surg Endosc. 2016;30:3749-3761.
- Jain M, Fry BT, Hess LW, et al. Barriers to efficiency in robotic surgery: the resident effect. J Surg. Res. 2016;205:296-304.
- Yu D, Dural C, Morrow MM, et al. Intraoperative workload in robotic surgery assessed by wearable motion tracking sensors and questionnaires. Surg Endosc. 2017;31:877-886.
- Randell R, Honey S, Alvarado N, et al. Embedding robotic surgery into routine practice and impacts on communication and decision making: a review of the experience of surgical teams. Cognit Technol Work. 2016;18:423-437.
- Souders CP, Catchpole KR, Wood LN, et al. Reducing operating room turnover time for robotic surgery using a motor racing pit stop model. World J Surg. 2017;4:1943–1949.
- Ahmad N, Hussein AA, Cavuoto L, et al. Ambulatory movements, team dynamics and interactions during robot-assisted surgery. BJU Int. 2016;118:132-139.
- Sexton K, Johnson A, Gotsch A, et al. Anticipation, teamwork, and cognitive load: chasing efficiency during robot-assisted surgery. BMJ Qual Saf. 2018;27:148-154.
- Harmanli O, Solak S, Bayram A, et al. Optimizing the robotic surgery team: an operations management perspective. Int Urogynecol J. 2021;32:1379-1385.
- Carter-Brooks CM, Du AL, Bonidie MJ, et al. The impact of a dedicated robotic team on robotic-assisted sacrocolpopexy outcomes. Female Pelvic Med Reconstr Surg. 2018;24:13-16.
- Giugale LE, Sears S, Lavelle ES, et al. Evaluating the impact of intraoperative surgical team handoffs on patient outcomes. Female Pelvic Med Reconstr Surg. 2017;23:288-292.
- Geynisman-Tan J, Brown O, Mueller M, et al. Operating room efficiency: examining the impact of personnel handoffs. Female Pelvic Med Reconstr Surg. 2018;24:87-89.
- Alsubaie H, Goldenberg M, Grantcharov T. Quantifying recall bias in surgical safety: a need for a modern approach to morbidity and mortality reviews. Can J Surg. 2019;62:39-43.
- Goldenberg MG, Jung J, Grantcharov TP. Using data to enhance performance and improve quality and safety in surgery. JAMA Surg. 2017;152:972-973.
- Jung JJ, Grantcharov TP. The operating room black box: a prospective observational study of the operating room. J Am Coll Surg. 2017;225:S127-S128.
- Jung JJ, Jüni P, Lebovic G, et al. First-year analysis of the operating room black box study. Ann Surg. 2020;271:122-127.
- Jung JJ, Kashfi A, Sharma S, et al. Characterization of device-related interruptions in minimally invasive surgery: need for intraoperative data and effective mitigation strategies. Surg Endosc. 2019;33:717-723.
- Jung JJ, Adams-McGavin RC, Grantcharov TP. Underreporting of Veress needle injuries: comparing direct observation and chart review methods. J Surg Res. 2019;236:266-270.
- Fesco AB, Kuzulugil SS, Babaoglu C, et al. Relationship between intraoperative nontechnical performance and technical events in bariatric surgery. Br J Surg. 2018;105:1044-1050.
- Luft HS, Bunker JP, Enthoven AC. Should operations be regionalized? The empirical relation between surgical volume and mortality. N Engl J Med. 1979;301:1364-1369.
- Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128-1137.
- Birkmeyer JD, Stukel TA, Siewers AE, et al. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349:21172127.
- Boyd LR, Novetsky AP, Curtin JP. Effect of surgical volume on route of hysterectomy and short-term morbidity. Obstet Gynecol. 2010;116:909-915.
- Rogo-Gupta LJ, Lewin SN, Kim JH, et al. The effect of surgeon volume on outcomes and resource use for vaginal hysterectomy. Obstet Gynecol. 2010;116:1341-1347.
- Wallenstein MR, Ananth CV, Kim JH, et al. Effect of surgical volume on outcomes for laparoscopic hysterectomy for benign indications. Obstet Gynecol. 2012;119:709-716.
- Bretschneider CE, Frazzini Padilla P, Das D, et al. The impact of surgeon volume on perioperative adverse events in women undergoing minimally invasive hysterectomy for the large uterus. Am J Obstet Gynecol. 2018;219:490.e1-490.e8.
- Ruiz MP, Chen L, Hou JY, et al. Outcomes of hysterectomy performed by very low-volume surgeons. Obstet Gynecol. 2018;131:981-990.
- Wright JD. The volume-outcome paradigm for gynecologic surgery: clinical and policy implications. Clin Obstet Gynecol. 2020;63:252-265.
- Finks JF, Osborne NH, Birkmeyer JD. Trends in hospital volume and operative mortality for high risk surgery. N Engl J Med. 2011;364:21282137.
- Sternberg S. Hospitals move to limit low-volume surgeries. US News & World Report. May 19, 2015. www.usnews.com/news /articles/2015/05/19/hospitals-move-to-limit-low-volume-surgeries. Accessed April 19, 2022.
The operating room (OR) is a key contributor to a hospital’s profitability. It is a complex environment with ever-advancing technology. A successful surgery completed without complications within an optimal time depends not only on the surgeon’s experience, skills, and knowledge but also on numerous other structural, human, and nontechnical factors over which the surgeon has limited control.
As in any setting that deals with human life, in the OR, team dynamics, communication, and environment play a major role. Research has indicated the benefits of dedicated teams, reduced handoffs, and innovative modalities that continuously and systematically monitor potential breakdowns and propose solutions for the detected problems.
Finally, who should perform your loved one’s hysterectomy? This article also attempts to address the impact of surgeons’ and hospitals’ volume on operative outcomes with a diminishing number of hysterectomies but an increasing number of approaches.
Human factors in the OR
Human factors research was born as a product of the industrial revolution and mass production. It aims to optimize human experience and improve system performance by studying how humans interact with system. The aviation industry, for example, minimized errors significantly by using methods developed by human factors scientists. As another industry with no tolerance for mistakes, the health care sector followed suit. Ultimately, the goal of human factors research in health care is to improve patient safety, optimize work and environment, reduce costs, and enhance employees’ physical and mental health, engagement, comfort, and quality of life (FIGURE 1).1

Today’s OR is so complex that it is hard to understand its dynamics without human factors research. Every new OR technology is first tested in controlled and simulated environments to determine “work as imagined.” However, it is necessary to study “work as done” in the real world via direct observation, video recording, questionnaires, and semistructured interviews by an on-site multidisciplinary team. This process not only focuses on surgical skills, process efficiency, and outcomes but also monitors the entire process according to Human Factors and Ergonomics Engineering principles to explore otherwise hidden complexities and latent safety concerns. The Systems Engineering Initiative for Patient Safety (SEIPS) framework is used to study the impact of interactions between people, tasks, technologies, environment, and organization.1
Robot-assisted surgery (RAS), an increasingly popular surgical approach among gynecologic surgeons, recently has been the focus of human factors science. A robotic OR poses unique challenges: the surgeon is not scrubbed, is removed from the operating table, and controls a complex highly technologic device in a crowded and darkened room. These are ideal conditions waiting to be optimized by human factor experts. To demonstrate the importance of human factors in the OR, we review the evidence for RAS.
Continue to: Impact of flow disruptions...
Impact of flow disruptions
Flow disruptions (FDs) were found to be more common in RAS. Catchpole and colleagues identified a mean of 9.62 FDs per hour in 89 robotic procedures, including hysterectomies and sacrocolpopexies, from a variety of fields; FDs occurred more often during the docking stage, followed by the console time, and they mostly were caused by communication breakdown and lack of team familiarity.2
Surgeon experience significantly reduced FDs. Surgeons who had done more than 700 RAS cases experienced 60% fewer FDs than those who had done less than 250 cases (13 vs 8 per hour).2 A study focusing on residents’ impact on RAS outcomes found that each FD increased the total operative time by an average 2.4 minutes, with the number significantly higher when a resident was involved.3 About one-quarter of the training-related FDs were procedure-specific instructions, while one-third were related to instrument and robotic instruction. However, pauses to teach residents did not appear to create significant intraoperative delays. Expectedly, experienced surgeons could anticipate and reduce these disruptions by supporting the whole team.
Human ergonomics, turnover time, and robot-specific skills
In a study of human ergonomics in RAS, Yu and colleagues noted that bedside assistants could experience neck posture problems. Surprisingly, the console could constrain the surgeon’s neck-shoulder region.4 Studies that reported on communication problems in a robotic OR suggest that innovative forms of verbal and nonverbal communication may support successful team communication.5
On the learning curve for RAS, OR turnover time, a key value metric, has been longer. However, turnover time was reduced almost by half from 99.2 to 53.2 minutes over 3 months after concepts from motor racing pit stops were employed, including briefings, leadership, role definition, task allocation, and task sequencing. Average room-ready time also was lowered from 42.2 to 27.2 minutes.6 RAS presents new challenges with sterile instrument processing as well. A successful RAS program, therefore, has organizational needs that include the training of OR and sterile processing staff and appropriate shift management.1
In a robotic OR, not only the surgeon but also the whole team requires robot-specific skills. New training approaches to teamwork, communication, and situation awareness skills are necessary. Robotic equipment, with its data and power cables, 2 consoles, and changing movement paths, necessitate larger rooms with a specific layout.7
In a review of recordings of RAS that used a multidimensional assessment tool to measure team effectiveness and cognitive load, Sexton and colleagues identified anticipation, active team engagement, and higher familiarity scores as the best predictors of team efficiency.8 Several studies emphasized the need for a stable team, especially in the learning phase of robotic surgery.5,9,10 A dedicated robotic team reduced the operative time by 18% during robot-assisted sacrocolpopexy (RASCP).10 RASCP procedures that extended into the afternoon took significantly longer time.9 A dedicated anesthesiologist improved the preoperative time.9 Surgical team handoffs also have reduced OR efficiency.11,12
Studying the impact of human factors is paramount for safe and efficient surgery. It is especially necessary in ORs that are equipped with high technologic instruments such as those used in RAS.
Surgical Black Box: Using data for OR safety and efficiency
Surgical procedures account for more than 50% of medical errors in a hospital setting, many of which are preventable. Postevent analysis with traditional methods, such as “Morbidity and Mortality” meetings held many days later, misses many adverse events in the OR.13 Another challenge with ever-changing and fast-multiplying surgical approaches is the development of effective surgical skill. Reviewing video recording of surgical procedures has been proposed as an instrument for recognizing adverse events and perfecting surgical skills.Recently, an innovative data-capture platform called the OR Black Box, developed by Teodor Grantcharov, MD, PhD, and colleagues, went beyond simple audiovisual recording.14 This high technologic platform not only video records the actual surgical procedure with laparoscopic camera capture (and wearable cameras for open cases) but also monitors the entire OR environment via wide-angle cameras, utilizes sensors, and records both the patient’s and the surgeon’s physiologic parameters.
The OR Black Box generates a holistic view of the OR after synchronization, encryption, and secure storage of all inputs for further analysis by experts and software-based algorithms (FIGURE 2). Computer vision algorithms can recognize improper dissection techniques and complications, such as bleeding. Adverse events are flagged with an automated software on a procedural timeline to facilitate review of procedural steps, disruptive environmental and organizational factors, OR team technical and nontechnical skills, surgeon physiologic stress, and intraoperative errors, events, and rectification processes using validated instruments.
Artificial intelligence built into this platform can automatically extract objective, high-quality, and structured data to generate explainable insights by recognizing adverse events and procedural segments of interest for training and quality improvement and provide a foundation with objective measurements of technical and nontechnical performance for formative and summative assessment. This system, a major step up compared with retrospective review of likely biased medical records and labor-intensive multidisciplinary human observers, has the potential to increase efficiency and reduce costs by studying human factors that include clinical design, technology, and organization. OR efficiency, measured in real time objectively and thoroughly, may save time and resources.
OR Black Box platforms have already started to generate meaningful data. It is not surprising that auditory disruptions—OR doors opening, loud noises, pagers beeping, telephones ringing—were recorded almost every minute during laparoscopic procedures.15 Most technical errors occurred during dissection, resection, and reconstruction and most commonly were associated with improper estimations of force applied to tissue and distance to the target tissue during operative steps of a laparoscopic procedure.16 Another study based on this system showed that technical performance was an independent predictor of postoperative outcomes.17 The OR Black Box identified a device-related interruption in 30% of elective laparoscopic general surgery cases, most commonly in sleeve gastrectomy and oncologic gastrectomy procedures. This sophisticated surgical data recording system also demonstrated a significantly better ability to detect Veress needle injuries (12 vs 3) and near misses (47 vs 0) when compared with traditional chart review.18
Data from the OR Black Box also have been applied to better analyze nontechnical performance, including teamwork and interpersonal dynamics.19 Surgeons most commonly exhibited adept situational awareness and leadership, while the nurse team excelled at task management and situational awareness.19 Of the total care provider team studied, the surgeon and scrub nurse demonstrated the most favorable nontechnical behavior.19 Of note, continuous physiologic monitoring of the surgeon with this system revealed that surgeons under stress had 66% higher adverse events.
The OR Black Box is currently utilized at 20 institutions in North America and Europe. The data compiled from all these institutions revealed that there was a 10% decrease in intraoperative adverse events for each 10-point increase in technical skill score on a scale of 0 to 100 (unpublished data). This centralized data indicated that turnover time ranged widely between 7 and 91 minutes, with variation of cleanup time from 1 to 25 minutes and setup time from 22 to 43 minutes. Institutions can learn from each other using this platform. For example, the information about block time utilization (20%–99%) across institutions provides opportunities for system improvements.
With any revolutionary technology, it is imperative to study its effects on outcomes, training, costs, and privacy before it is widely implemented. We, obstetricians and gynecologists, are very familiar with the impact of electronic fetal monitoring, a great example of a technologic advance that did not improve perinatal outcomes but led to unintended consequences, such as higher rates of cesarean deliveries and lawsuits. Such a tool may lead to potential misrepresentation of intraoperative events unless legal aspects are clearly delineated. As exciting as it is, this disruptive technology requires further exploration with scientific vigor.
Continue to: Surgeon and hospital volume: Surgical outcomes paradigm...
Surgeon and hospital volume: Surgical outcomes paradigm
A landmark study in 1979 that showed decreased mortality in high-volume centers underscored the need for regionalization for certain surgical procedures.20 This association was further substantiated by 2 reports on 2.5 million Medicare beneficiaries that demonstrated significantly lower mortality for all 14 cardiovascular and oncologic procedures for hospitals with larger surgical volume (16% vs 4%) and high-volume surgeons for certain procedures, for example, 15% versus 5% for pancreatic resections for cancer.21,22
A similar association was found for all routes of hysterectomies performed for benign indications. Boyd and colleagues showed that gynecologists who performed fewer than 10 hysterectomies per year had a higher perioperative morbidity rate (16.5%) compared with those who did more (11.7%).23 Specific to vaginal hysterectomy, in a study of more than 6,000 women, surgeons who performed 13 procedures per year had 31% less risk of operative injury than those who did 5.5 procedures per year (2.5% vs 1.7%).24 Overall perioperative complications (5.0% vs 4.0%) and medical complications (5.7% vs 3.9%) were also reduced for higher-volume surgeons. In a cohort of approximately 8,000 women who underwent a laparoscopic hysterectomy, high-volume surgeons had a considerably lower complication rate (4.2% vs 6.2%).25
As expected, lower complication rates of high-volume surgeons led to lower resource utilization, including lower transfusion rates, less intensive care unit utilization, and shorter operative times and, in several studies, length of stay.24,25 Of note, low-volume surgeons were less likely to offer minimally invasive routes and were more likely to convert to laparotomy.26 In addition, significant cost savings have been associated with high surgical volume, which one study showed was 16% ($6,500 vs $5,600) for high-volume surgeons.26 With regard to mortality, a study of 7,800 women found that perioperative mortality increased more than 10-fold for surgeons who performed an average 1 case per year compared with all other surgeons (2.5% vs 0.2%).27
When gynecologic cancers are concerned, arguably, long-term survival outcomes may be more critical than perioperative morbidity and mortality. Higher surgeon and hospital volume are associated with improved perioperative outcomes for endometrial and cervical cancers.28 Importantly, minimally invasive hysterectomy was offered for endometrial cancer significantly more often by surgeons with high volume.28 Survival outcomes were not affected by surgeon or hospital volume, likely due to overall more favorable prognosis for endometrial cancer after treatment.
Although it is intuitive to assume that a surgeon’s skills and experience would make the most impact in procedures for ovarian cancer due to the complexity of ovarian cancer surgery, evidence on short-term outcomes has been mixed. Intriguingly, some studies reported that high-volume institutions had higher complication and readmission rates. However, evidence supports that the surgeon’s volume, and especially hospital volume, improves long-term survival for ovarian cancer, with a negative impact on immediate postoperative morbidity.29 This may suggest that a more aggressive surgical effort improves long-term survival but also can cause more perioperative complications. Further, longer survival may result not only from operative skills but also because of better care by a structured multidisciplinary team at more established high-volume cancer centers.
The association of improved outcomes with higher volume led to public reporting of hospital outcomes. Policy efforts toward regionalization have impacted surgical practice. Based on their analysis of 3.2 million Medicare patients who underwent 1 of 8 different cancer surgeries or cardiovascular operations from 1999 to 2008, Finks and colleagues demonstrated that care was concentrated to fewer hospitals over time for many of these procedures.29 This trend was noted for gynecologic cancer surgery but not for benign gynecologic surgery.
Regionalization of care limits access particularly for minority and underserved communities because of longer travel distances, logistic challenges, and financial strain. An alternative to regionalization of care is targeted quality improvement by rigorous adherence to quality guidelines at low-volume hospitals.
Is there a critical minimum volume that may be used as a requirement for surgeons to maintain their privileges and for hospitals to offer certain procedures? In 2015, minimum volume standards for a number of common procedures were proposed by Johns Hopkins Medicine and Dartmouth-Hitchcock Medical Center, such as 50 hip replacement surgeries per hospital and 25 per physician per year, and 20 pancreatectomies per hospital and 5 per surgeon per year.30 A modeling study for hysterectomy showed that a volume cut point of >1 procedure in the prior year would restrict privileges for a substantial number of surgeons performing abdominal (17.5%), robot-assisted (12.5%), laparoscopic (16.8%), and vaginal (27.6%) hysterectomies.27 This study concluded that minimum-volume standards for hysterectomy for even the lowest volume physicians would restrict a significant number of gynecologic surgeons, including many with outcomes that are better than predicted.
Therefore, while there is good evidence that favors better outcomes in the hands of high-volume surgeons in gynecology, the impact of such policies on gynecologic practice clearly warrants careful monitoring and further study.
- What factors besides the surgeon’s skills influence surgical safety and efficiency?
- Are you ready to have audio, video, and sensor-based recording of everything in the OR?
- Who should perform your loved one’s hysterectomy? Do the surgeon’s and hospital’s volume matter?
The operating room (OR) is a key contributor to a hospital’s profitability. It is a complex environment with ever-advancing technology. A successful surgery completed without complications within an optimal time depends not only on the surgeon’s experience, skills, and knowledge but also on numerous other structural, human, and nontechnical factors over which the surgeon has limited control.
As in any setting that deals with human life, in the OR, team dynamics, communication, and environment play a major role. Research has indicated the benefits of dedicated teams, reduced handoffs, and innovative modalities that continuously and systematically monitor potential breakdowns and propose solutions for the detected problems.
Finally, who should perform your loved one’s hysterectomy? This article also attempts to address the impact of surgeons’ and hospitals’ volume on operative outcomes with a diminishing number of hysterectomies but an increasing number of approaches.
Human factors in the OR
Human factors research was born as a product of the industrial revolution and mass production. It aims to optimize human experience and improve system performance by studying how humans interact with system. The aviation industry, for example, minimized errors significantly by using methods developed by human factors scientists. As another industry with no tolerance for mistakes, the health care sector followed suit. Ultimately, the goal of human factors research in health care is to improve patient safety, optimize work and environment, reduce costs, and enhance employees’ physical and mental health, engagement, comfort, and quality of life (FIGURE 1).1

Today’s OR is so complex that it is hard to understand its dynamics without human factors research. Every new OR technology is first tested in controlled and simulated environments to determine “work as imagined.” However, it is necessary to study “work as done” in the real world via direct observation, video recording, questionnaires, and semistructured interviews by an on-site multidisciplinary team. This process not only focuses on surgical skills, process efficiency, and outcomes but also monitors the entire process according to Human Factors and Ergonomics Engineering principles to explore otherwise hidden complexities and latent safety concerns. The Systems Engineering Initiative for Patient Safety (SEIPS) framework is used to study the impact of interactions between people, tasks, technologies, environment, and organization.1
Robot-assisted surgery (RAS), an increasingly popular surgical approach among gynecologic surgeons, recently has been the focus of human factors science. A robotic OR poses unique challenges: the surgeon is not scrubbed, is removed from the operating table, and controls a complex highly technologic device in a crowded and darkened room. These are ideal conditions waiting to be optimized by human factor experts. To demonstrate the importance of human factors in the OR, we review the evidence for RAS.
Continue to: Impact of flow disruptions...
Impact of flow disruptions
Flow disruptions (FDs) were found to be more common in RAS. Catchpole and colleagues identified a mean of 9.62 FDs per hour in 89 robotic procedures, including hysterectomies and sacrocolpopexies, from a variety of fields; FDs occurred more often during the docking stage, followed by the console time, and they mostly were caused by communication breakdown and lack of team familiarity.2
Surgeon experience significantly reduced FDs. Surgeons who had done more than 700 RAS cases experienced 60% fewer FDs than those who had done less than 250 cases (13 vs 8 per hour).2 A study focusing on residents’ impact on RAS outcomes found that each FD increased the total operative time by an average 2.4 minutes, with the number significantly higher when a resident was involved.3 About one-quarter of the training-related FDs were procedure-specific instructions, while one-third were related to instrument and robotic instruction. However, pauses to teach residents did not appear to create significant intraoperative delays. Expectedly, experienced surgeons could anticipate and reduce these disruptions by supporting the whole team.
Human ergonomics, turnover time, and robot-specific skills
In a study of human ergonomics in RAS, Yu and colleagues noted that bedside assistants could experience neck posture problems. Surprisingly, the console could constrain the surgeon’s neck-shoulder region.4 Studies that reported on communication problems in a robotic OR suggest that innovative forms of verbal and nonverbal communication may support successful team communication.5
On the learning curve for RAS, OR turnover time, a key value metric, has been longer. However, turnover time was reduced almost by half from 99.2 to 53.2 minutes over 3 months after concepts from motor racing pit stops were employed, including briefings, leadership, role definition, task allocation, and task sequencing. Average room-ready time also was lowered from 42.2 to 27.2 minutes.6 RAS presents new challenges with sterile instrument processing as well. A successful RAS program, therefore, has organizational needs that include the training of OR and sterile processing staff and appropriate shift management.1
In a robotic OR, not only the surgeon but also the whole team requires robot-specific skills. New training approaches to teamwork, communication, and situation awareness skills are necessary. Robotic equipment, with its data and power cables, 2 consoles, and changing movement paths, necessitate larger rooms with a specific layout.7
In a review of recordings of RAS that used a multidimensional assessment tool to measure team effectiveness and cognitive load, Sexton and colleagues identified anticipation, active team engagement, and higher familiarity scores as the best predictors of team efficiency.8 Several studies emphasized the need for a stable team, especially in the learning phase of robotic surgery.5,9,10 A dedicated robotic team reduced the operative time by 18% during robot-assisted sacrocolpopexy (RASCP).10 RASCP procedures that extended into the afternoon took significantly longer time.9 A dedicated anesthesiologist improved the preoperative time.9 Surgical team handoffs also have reduced OR efficiency.11,12
Studying the impact of human factors is paramount for safe and efficient surgery. It is especially necessary in ORs that are equipped with high technologic instruments such as those used in RAS.
Surgical Black Box: Using data for OR safety and efficiency
Surgical procedures account for more than 50% of medical errors in a hospital setting, many of which are preventable. Postevent analysis with traditional methods, such as “Morbidity and Mortality” meetings held many days later, misses many adverse events in the OR.13 Another challenge with ever-changing and fast-multiplying surgical approaches is the development of effective surgical skill. Reviewing video recording of surgical procedures has been proposed as an instrument for recognizing adverse events and perfecting surgical skills.Recently, an innovative data-capture platform called the OR Black Box, developed by Teodor Grantcharov, MD, PhD, and colleagues, went beyond simple audiovisual recording.14 This high technologic platform not only video records the actual surgical procedure with laparoscopic camera capture (and wearable cameras for open cases) but also monitors the entire OR environment via wide-angle cameras, utilizes sensors, and records both the patient’s and the surgeon’s physiologic parameters.
The OR Black Box generates a holistic view of the OR after synchronization, encryption, and secure storage of all inputs for further analysis by experts and software-based algorithms (FIGURE 2). Computer vision algorithms can recognize improper dissection techniques and complications, such as bleeding. Adverse events are flagged with an automated software on a procedural timeline to facilitate review of procedural steps, disruptive environmental and organizational factors, OR team technical and nontechnical skills, surgeon physiologic stress, and intraoperative errors, events, and rectification processes using validated instruments.
Artificial intelligence built into this platform can automatically extract objective, high-quality, and structured data to generate explainable insights by recognizing adverse events and procedural segments of interest for training and quality improvement and provide a foundation with objective measurements of technical and nontechnical performance for formative and summative assessment. This system, a major step up compared with retrospective review of likely biased medical records and labor-intensive multidisciplinary human observers, has the potential to increase efficiency and reduce costs by studying human factors that include clinical design, technology, and organization. OR efficiency, measured in real time objectively and thoroughly, may save time and resources.
OR Black Box platforms have already started to generate meaningful data. It is not surprising that auditory disruptions—OR doors opening, loud noises, pagers beeping, telephones ringing—were recorded almost every minute during laparoscopic procedures.15 Most technical errors occurred during dissection, resection, and reconstruction and most commonly were associated with improper estimations of force applied to tissue and distance to the target tissue during operative steps of a laparoscopic procedure.16 Another study based on this system showed that technical performance was an independent predictor of postoperative outcomes.17 The OR Black Box identified a device-related interruption in 30% of elective laparoscopic general surgery cases, most commonly in sleeve gastrectomy and oncologic gastrectomy procedures. This sophisticated surgical data recording system also demonstrated a significantly better ability to detect Veress needle injuries (12 vs 3) and near misses (47 vs 0) when compared with traditional chart review.18
Data from the OR Black Box also have been applied to better analyze nontechnical performance, including teamwork and interpersonal dynamics.19 Surgeons most commonly exhibited adept situational awareness and leadership, while the nurse team excelled at task management and situational awareness.19 Of the total care provider team studied, the surgeon and scrub nurse demonstrated the most favorable nontechnical behavior.19 Of note, continuous physiologic monitoring of the surgeon with this system revealed that surgeons under stress had 66% higher adverse events.
The OR Black Box is currently utilized at 20 institutions in North America and Europe. The data compiled from all these institutions revealed that there was a 10% decrease in intraoperative adverse events for each 10-point increase in technical skill score on a scale of 0 to 100 (unpublished data). This centralized data indicated that turnover time ranged widely between 7 and 91 minutes, with variation of cleanup time from 1 to 25 minutes and setup time from 22 to 43 minutes. Institutions can learn from each other using this platform. For example, the information about block time utilization (20%–99%) across institutions provides opportunities for system improvements.
With any revolutionary technology, it is imperative to study its effects on outcomes, training, costs, and privacy before it is widely implemented. We, obstetricians and gynecologists, are very familiar with the impact of electronic fetal monitoring, a great example of a technologic advance that did not improve perinatal outcomes but led to unintended consequences, such as higher rates of cesarean deliveries and lawsuits. Such a tool may lead to potential misrepresentation of intraoperative events unless legal aspects are clearly delineated. As exciting as it is, this disruptive technology requires further exploration with scientific vigor.
Continue to: Surgeon and hospital volume: Surgical outcomes paradigm...
Surgeon and hospital volume: Surgical outcomes paradigm
A landmark study in 1979 that showed decreased mortality in high-volume centers underscored the need for regionalization for certain surgical procedures.20 This association was further substantiated by 2 reports on 2.5 million Medicare beneficiaries that demonstrated significantly lower mortality for all 14 cardiovascular and oncologic procedures for hospitals with larger surgical volume (16% vs 4%) and high-volume surgeons for certain procedures, for example, 15% versus 5% for pancreatic resections for cancer.21,22
A similar association was found for all routes of hysterectomies performed for benign indications. Boyd and colleagues showed that gynecologists who performed fewer than 10 hysterectomies per year had a higher perioperative morbidity rate (16.5%) compared with those who did more (11.7%).23 Specific to vaginal hysterectomy, in a study of more than 6,000 women, surgeons who performed 13 procedures per year had 31% less risk of operative injury than those who did 5.5 procedures per year (2.5% vs 1.7%).24 Overall perioperative complications (5.0% vs 4.0%) and medical complications (5.7% vs 3.9%) were also reduced for higher-volume surgeons. In a cohort of approximately 8,000 women who underwent a laparoscopic hysterectomy, high-volume surgeons had a considerably lower complication rate (4.2% vs 6.2%).25
As expected, lower complication rates of high-volume surgeons led to lower resource utilization, including lower transfusion rates, less intensive care unit utilization, and shorter operative times and, in several studies, length of stay.24,25 Of note, low-volume surgeons were less likely to offer minimally invasive routes and were more likely to convert to laparotomy.26 In addition, significant cost savings have been associated with high surgical volume, which one study showed was 16% ($6,500 vs $5,600) for high-volume surgeons.26 With regard to mortality, a study of 7,800 women found that perioperative mortality increased more than 10-fold for surgeons who performed an average 1 case per year compared with all other surgeons (2.5% vs 0.2%).27
When gynecologic cancers are concerned, arguably, long-term survival outcomes may be more critical than perioperative morbidity and mortality. Higher surgeon and hospital volume are associated with improved perioperative outcomes for endometrial and cervical cancers.28 Importantly, minimally invasive hysterectomy was offered for endometrial cancer significantly more often by surgeons with high volume.28 Survival outcomes were not affected by surgeon or hospital volume, likely due to overall more favorable prognosis for endometrial cancer after treatment.
Although it is intuitive to assume that a surgeon’s skills and experience would make the most impact in procedures for ovarian cancer due to the complexity of ovarian cancer surgery, evidence on short-term outcomes has been mixed. Intriguingly, some studies reported that high-volume institutions had higher complication and readmission rates. However, evidence supports that the surgeon’s volume, and especially hospital volume, improves long-term survival for ovarian cancer, with a negative impact on immediate postoperative morbidity.29 This may suggest that a more aggressive surgical effort improves long-term survival but also can cause more perioperative complications. Further, longer survival may result not only from operative skills but also because of better care by a structured multidisciplinary team at more established high-volume cancer centers.
The association of improved outcomes with higher volume led to public reporting of hospital outcomes. Policy efforts toward regionalization have impacted surgical practice. Based on their analysis of 3.2 million Medicare patients who underwent 1 of 8 different cancer surgeries or cardiovascular operations from 1999 to 2008, Finks and colleagues demonstrated that care was concentrated to fewer hospitals over time for many of these procedures.29 This trend was noted for gynecologic cancer surgery but not for benign gynecologic surgery.
Regionalization of care limits access particularly for minority and underserved communities because of longer travel distances, logistic challenges, and financial strain. An alternative to regionalization of care is targeted quality improvement by rigorous adherence to quality guidelines at low-volume hospitals.
Is there a critical minimum volume that may be used as a requirement for surgeons to maintain their privileges and for hospitals to offer certain procedures? In 2015, minimum volume standards for a number of common procedures were proposed by Johns Hopkins Medicine and Dartmouth-Hitchcock Medical Center, such as 50 hip replacement surgeries per hospital and 25 per physician per year, and 20 pancreatectomies per hospital and 5 per surgeon per year.30 A modeling study for hysterectomy showed that a volume cut point of >1 procedure in the prior year would restrict privileges for a substantial number of surgeons performing abdominal (17.5%), robot-assisted (12.5%), laparoscopic (16.8%), and vaginal (27.6%) hysterectomies.27 This study concluded that minimum-volume standards for hysterectomy for even the lowest volume physicians would restrict a significant number of gynecologic surgeons, including many with outcomes that are better than predicted.
Therefore, while there is good evidence that favors better outcomes in the hands of high-volume surgeons in gynecology, the impact of such policies on gynecologic practice clearly warrants careful monitoring and further study.
- What factors besides the surgeon’s skills influence surgical safety and efficiency?
- Are you ready to have audio, video, and sensor-based recording of everything in the OR?
- Who should perform your loved one’s hysterectomy? Do the surgeon’s and hospital’s volume matter?
- Catchpole K, Bisantz A, Hallbeck MS, et al. Human factors in robotic assisted surgery: lessons from studies ‘in the wild’. Appl Ergon. 2019;78:270-276.
- Catchpole K, Perkins C, Bresee C, et al. Safety, efficiency and learning curves in robotic surgery: a human factors analysis. Surg Endosc. 2016;30:3749-3761.
- Jain M, Fry BT, Hess LW, et al. Barriers to efficiency in robotic surgery: the resident effect. J Surg. Res. 2016;205:296-304.
- Yu D, Dural C, Morrow MM, et al. Intraoperative workload in robotic surgery assessed by wearable motion tracking sensors and questionnaires. Surg Endosc. 2017;31:877-886.
- Randell R, Honey S, Alvarado N, et al. Embedding robotic surgery into routine practice and impacts on communication and decision making: a review of the experience of surgical teams. Cognit Technol Work. 2016;18:423-437.
- Souders CP, Catchpole KR, Wood LN, et al. Reducing operating room turnover time for robotic surgery using a motor racing pit stop model. World J Surg. 2017;4:1943–1949.
- Ahmad N, Hussein AA, Cavuoto L, et al. Ambulatory movements, team dynamics and interactions during robot-assisted surgery. BJU Int. 2016;118:132-139.
- Sexton K, Johnson A, Gotsch A, et al. Anticipation, teamwork, and cognitive load: chasing efficiency during robot-assisted surgery. BMJ Qual Saf. 2018;27:148-154.
- Harmanli O, Solak S, Bayram A, et al. Optimizing the robotic surgery team: an operations management perspective. Int Urogynecol J. 2021;32:1379-1385.
- Carter-Brooks CM, Du AL, Bonidie MJ, et al. The impact of a dedicated robotic team on robotic-assisted sacrocolpopexy outcomes. Female Pelvic Med Reconstr Surg. 2018;24:13-16.
- Giugale LE, Sears S, Lavelle ES, et al. Evaluating the impact of intraoperative surgical team handoffs on patient outcomes. Female Pelvic Med Reconstr Surg. 2017;23:288-292.
- Geynisman-Tan J, Brown O, Mueller M, et al. Operating room efficiency: examining the impact of personnel handoffs. Female Pelvic Med Reconstr Surg. 2018;24:87-89.
- Alsubaie H, Goldenberg M, Grantcharov T. Quantifying recall bias in surgical safety: a need for a modern approach to morbidity and mortality reviews. Can J Surg. 2019;62:39-43.
- Goldenberg MG, Jung J, Grantcharov TP. Using data to enhance performance and improve quality and safety in surgery. JAMA Surg. 2017;152:972-973.
- Jung JJ, Grantcharov TP. The operating room black box: a prospective observational study of the operating room. J Am Coll Surg. 2017;225:S127-S128.
- Jung JJ, Jüni P, Lebovic G, et al. First-year analysis of the operating room black box study. Ann Surg. 2020;271:122-127.
- Jung JJ, Kashfi A, Sharma S, et al. Characterization of device-related interruptions in minimally invasive surgery: need for intraoperative data and effective mitigation strategies. Surg Endosc. 2019;33:717-723.
- Jung JJ, Adams-McGavin RC, Grantcharov TP. Underreporting of Veress needle injuries: comparing direct observation and chart review methods. J Surg Res. 2019;236:266-270.
- Fesco AB, Kuzulugil SS, Babaoglu C, et al. Relationship between intraoperative nontechnical performance and technical events in bariatric surgery. Br J Surg. 2018;105:1044-1050.
- Luft HS, Bunker JP, Enthoven AC. Should operations be regionalized? The empirical relation between surgical volume and mortality. N Engl J Med. 1979;301:1364-1369.
- Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128-1137.
- Birkmeyer JD, Stukel TA, Siewers AE, et al. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349:21172127.
- Boyd LR, Novetsky AP, Curtin JP. Effect of surgical volume on route of hysterectomy and short-term morbidity. Obstet Gynecol. 2010;116:909-915.
- Rogo-Gupta LJ, Lewin SN, Kim JH, et al. The effect of surgeon volume on outcomes and resource use for vaginal hysterectomy. Obstet Gynecol. 2010;116:1341-1347.
- Wallenstein MR, Ananth CV, Kim JH, et al. Effect of surgical volume on outcomes for laparoscopic hysterectomy for benign indications. Obstet Gynecol. 2012;119:709-716.
- Bretschneider CE, Frazzini Padilla P, Das D, et al. The impact of surgeon volume on perioperative adverse events in women undergoing minimally invasive hysterectomy for the large uterus. Am J Obstet Gynecol. 2018;219:490.e1-490.e8.
- Ruiz MP, Chen L, Hou JY, et al. Outcomes of hysterectomy performed by very low-volume surgeons. Obstet Gynecol. 2018;131:981-990.
- Wright JD. The volume-outcome paradigm for gynecologic surgery: clinical and policy implications. Clin Obstet Gynecol. 2020;63:252-265.
- Finks JF, Osborne NH, Birkmeyer JD. Trends in hospital volume and operative mortality for high risk surgery. N Engl J Med. 2011;364:21282137.
- Sternberg S. Hospitals move to limit low-volume surgeries. US News & World Report. May 19, 2015. www.usnews.com/news /articles/2015/05/19/hospitals-move-to-limit-low-volume-surgeries. Accessed April 19, 2022.
- Catchpole K, Bisantz A, Hallbeck MS, et al. Human factors in robotic assisted surgery: lessons from studies ‘in the wild’. Appl Ergon. 2019;78:270-276.
- Catchpole K, Perkins C, Bresee C, et al. Safety, efficiency and learning curves in robotic surgery: a human factors analysis. Surg Endosc. 2016;30:3749-3761.
- Jain M, Fry BT, Hess LW, et al. Barriers to efficiency in robotic surgery: the resident effect. J Surg. Res. 2016;205:296-304.
- Yu D, Dural C, Morrow MM, et al. Intraoperative workload in robotic surgery assessed by wearable motion tracking sensors and questionnaires. Surg Endosc. 2017;31:877-886.
- Randell R, Honey S, Alvarado N, et al. Embedding robotic surgery into routine practice and impacts on communication and decision making: a review of the experience of surgical teams. Cognit Technol Work. 2016;18:423-437.
- Souders CP, Catchpole KR, Wood LN, et al. Reducing operating room turnover time for robotic surgery using a motor racing pit stop model. World J Surg. 2017;4:1943–1949.
- Ahmad N, Hussein AA, Cavuoto L, et al. Ambulatory movements, team dynamics and interactions during robot-assisted surgery. BJU Int. 2016;118:132-139.
- Sexton K, Johnson A, Gotsch A, et al. Anticipation, teamwork, and cognitive load: chasing efficiency during robot-assisted surgery. BMJ Qual Saf. 2018;27:148-154.
- Harmanli O, Solak S, Bayram A, et al. Optimizing the robotic surgery team: an operations management perspective. Int Urogynecol J. 2021;32:1379-1385.
- Carter-Brooks CM, Du AL, Bonidie MJ, et al. The impact of a dedicated robotic team on robotic-assisted sacrocolpopexy outcomes. Female Pelvic Med Reconstr Surg. 2018;24:13-16.
- Giugale LE, Sears S, Lavelle ES, et al. Evaluating the impact of intraoperative surgical team handoffs on patient outcomes. Female Pelvic Med Reconstr Surg. 2017;23:288-292.
- Geynisman-Tan J, Brown O, Mueller M, et al. Operating room efficiency: examining the impact of personnel handoffs. Female Pelvic Med Reconstr Surg. 2018;24:87-89.
- Alsubaie H, Goldenberg M, Grantcharov T. Quantifying recall bias in surgical safety: a need for a modern approach to morbidity and mortality reviews. Can J Surg. 2019;62:39-43.
- Goldenberg MG, Jung J, Grantcharov TP. Using data to enhance performance and improve quality and safety in surgery. JAMA Surg. 2017;152:972-973.
- Jung JJ, Grantcharov TP. The operating room black box: a prospective observational study of the operating room. J Am Coll Surg. 2017;225:S127-S128.
- Jung JJ, Jüni P, Lebovic G, et al. First-year analysis of the operating room black box study. Ann Surg. 2020;271:122-127.
- Jung JJ, Kashfi A, Sharma S, et al. Characterization of device-related interruptions in minimally invasive surgery: need for intraoperative data and effective mitigation strategies. Surg Endosc. 2019;33:717-723.
- Jung JJ, Adams-McGavin RC, Grantcharov TP. Underreporting of Veress needle injuries: comparing direct observation and chart review methods. J Surg Res. 2019;236:266-270.
- Fesco AB, Kuzulugil SS, Babaoglu C, et al. Relationship between intraoperative nontechnical performance and technical events in bariatric surgery. Br J Surg. 2018;105:1044-1050.
- Luft HS, Bunker JP, Enthoven AC. Should operations be regionalized? The empirical relation between surgical volume and mortality. N Engl J Med. 1979;301:1364-1369.
- Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128-1137.
- Birkmeyer JD, Stukel TA, Siewers AE, et al. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349:21172127.
- Boyd LR, Novetsky AP, Curtin JP. Effect of surgical volume on route of hysterectomy and short-term morbidity. Obstet Gynecol. 2010;116:909-915.
- Rogo-Gupta LJ, Lewin SN, Kim JH, et al. The effect of surgeon volume on outcomes and resource use for vaginal hysterectomy. Obstet Gynecol. 2010;116:1341-1347.
- Wallenstein MR, Ananth CV, Kim JH, et al. Effect of surgical volume on outcomes for laparoscopic hysterectomy for benign indications. Obstet Gynecol. 2012;119:709-716.
- Bretschneider CE, Frazzini Padilla P, Das D, et al. The impact of surgeon volume on perioperative adverse events in women undergoing minimally invasive hysterectomy for the large uterus. Am J Obstet Gynecol. 2018;219:490.e1-490.e8.
- Ruiz MP, Chen L, Hou JY, et al. Outcomes of hysterectomy performed by very low-volume surgeons. Obstet Gynecol. 2018;131:981-990.
- Wright JD. The volume-outcome paradigm for gynecologic surgery: clinical and policy implications. Clin Obstet Gynecol. 2020;63:252-265.
- Finks JF, Osborne NH, Birkmeyer JD. Trends in hospital volume and operative mortality for high risk surgery. N Engl J Med. 2011;364:21282137.
- Sternberg S. Hospitals move to limit low-volume surgeries. US News & World Report. May 19, 2015. www.usnews.com/news /articles/2015/05/19/hospitals-move-to-limit-low-volume-surgeries. Accessed April 19, 2022.
Steps to minimize morbidity from unanticipated placenta accreta spectrum
CASE Placenta accreta spectrum following uncomplicated vaginal delivery
Imagine you are an obstetric hospitalist taking call at a level II maternal level of care hospital. Your patient is a 35-year-old woman, gravida 2, para 1, with a past history of retained placenta requiring dilation and curettage and intravenous antibiotics for endomyometritis. This is an in vitro fertilization pregnancy that has progressed normally, and the patient labored spontaneously at 38 weeks’ gestation. Following an uncomplicated vaginal delivery, the placenta has not delivered, and you attempt a manual placental extraction after a 40-minute third stage. While there is epidural analgesia and you can reach the uterine fundus, you are unable to create a separation plane between the placenta and uterus.
What do you do next?
Placenta accreta spectrum (PAS) includes a broad range of clinical scenarios with abnormal placental attachment as their common denominator. The condition has classically been defined pathologically, with chorionic villi attaching directly to the myometrium (“accreta”) or extending more deeply into the myometrium (“increta”) or attaching to surrounding tissues and structures (“percreta”).1 It is most commonly encountered in patients with low placental implantation on a prior cesarean section scar; indeed, placenta previa, particularly with a history of cesarean delivery, is the strongest risk factor for the development of PAS.2 In addition to abnormal placental attachment, these placental attachments are often hypervascular and can lead to catastrophic hemorrhage if not managed appropriately. For this reason, patients with sonographic or radiologic signs of PAS should be referred to specialized centers for further workup, counseling, and delivery planning.3
Although delivery at a specialized PAS center has been associated with improved patient outcomes,4 not all patients with PAS will be identified in the antepartum period. Ultrasonography may miss up to 40% to 50% of PAS cases, particularly when the sonologist has not been advised to look for the condition,5 and not all patients with PAS will have a previa implanted in a prior cesarean scar. A recent study found that these patients with nonprevia PAS were identified by imaging less than 40% of the time and were significantly less likely to be managed by a specialized team of clinicians.6 Thus, it falls upon every obstetric care provider to be aware of this diagnosis, promptly recognize its unanticipated presentations, and have a plan to optimize patient safety.
Step 1: Recognition
While PAS is classically defined as a pathologic condition, no clinician has the luxury of histology in the delivery room. Researchers have variously defined PAS clinically, with the common trait of abnormal placental adherence.7-9 The TABLE compares published definitions that have been used in the literature. While some definitions include hemorrhage, no clinician wants to induce significant hemorrhage to confirm their patient’s diagnosis. Thus, practically, the clinical PAS diagnosis comes down to abnormal placental attachment: If it is apparent that some or all of the placenta will not separate from the uterine wall with digital manipulation or careful curettage, then PAS should be suspected, and appropriate steps should be taken before further removal attempts.
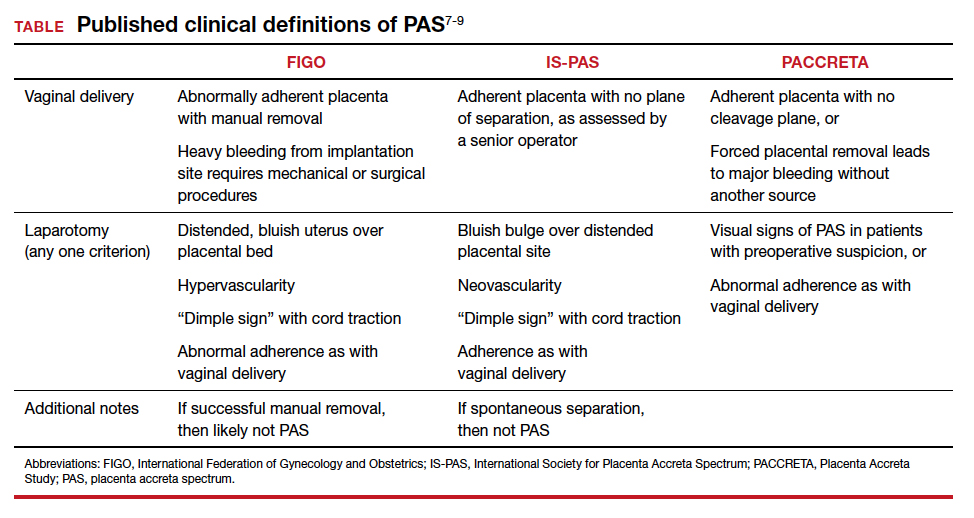
At cesarean delivery, the PAS diagnosis may be aided by visual cues. With placenta previa, the lower uterine segment may bulge and take on a bluish hue, distinctly different from the upper healthy myometrium. PAS may also manifest with neovascularization, particularly behind the bladder. As with vaginal births, the placenta will fail to separate after the delivery, and controlled traction on the umbilical cord can produce a “dimple sign,” or visible myometrial retraction at the site of implantation (FIGURE 1). Finally, if the diagnosis is still in doubt, attempts to gently form a cleavage plane between the placenta and myometrium will be unsuccessful if PAS is present.8
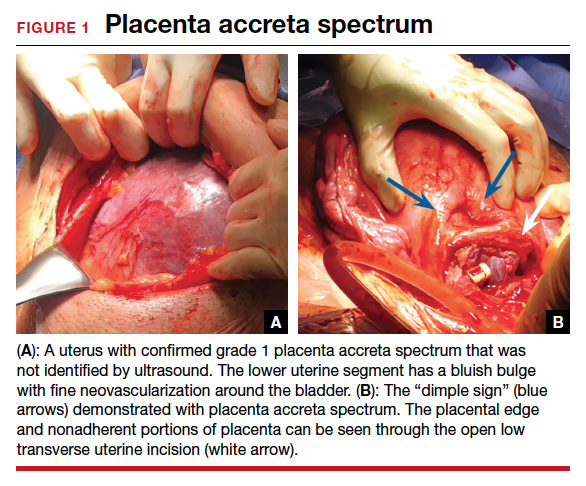
Step 2: Initial management—pause, plan
Most importantly, do not attempt to forcibly remove the placenta. It can be left attached to the uterus until appropriate resources are secured. Efforts to forcibly remove an adherent placenta may well lead to major hemorrhage, and thus it falls on the patient’s care team to pause and plan for PAS care at this point. FIGURE 2 displays an algorithm for patient management. Further steps depend primarily on whether or not the patient is already hemorrhaging. In a stable situation, the patient should be counseled regarding the abnormal findings and the suspected PAS diagnosis. This includes the possibility of further procedures, blood transfusion, and hysterectomy. Local resources, including nursing, anesthesia, and the blood bank, should be notified about the situation and for the potential to call in specialized services. If on-site experienced specialists are not available, then patient transfer to a PAS specialty center should be strongly considered. While awaiting additional help or transport, the patient requires close monitoring for gross and physiologic signs of hemorrhage. If pursued, transport to a PAS specialty center should be expedited.

If the patient is already hemorrhaging or unstable, then appropriate local resources must be activated. At a minimum, this requires an obstetrician and anesthesiologist at the bedside and activation of hemorrhage protocols (eg, a massive transfusion protocol). If blood products are unavailable, consider whether they can be transported from other nearby blood banks, and start that process promptly. Next, contact backup services. Based on local resources and clinical severity, this may include maternal-fetal medicine specialists, pelvic surgeons, general and trauma surgeons, intensivists, interventional radiologists, and transfusion specialists. Even if the patient cannot be safely transferred to another hospital, the obstetrician can call an outside PAS specialist to discuss next steps in care and begin transfer plans, assuming the patient can be stabilized. Based on the Maternal Levels of Care definitions published by the American College of Obstetricians and Gynecologists and the Society of Maternal-Fetal Medicine,10 patients with PAS should be managed at level III or level IV centers. However, delivery units at every level of maternal care should have a protocol for securing local help and reaching an appropriate consultant if a PAS case is encountered. Know which center in your area specializes in PAS so that when an unanticipated case arises, you know who to call.
Continue to: Step 3: Ultimate management—mobilize and prepare for bleeding...
Step 3: Ultimate management—mobilize and prepare for bleeding
If diagnosis occurs intraoperatively at a PAS specialty center, or if safe transport is not possible, then the team should mobilize for the possibility of hysterectomy and prepare for massive bleeding, which can occur regardless of the treatment chosen. Many patients require or will opt for hysterectomy. For example, a patient who has finished childbearing may consent to a hysterectomy upon hearing she likely has PAS. In patients with suspected PAS who are actively hemorrhaging or are unstable, hysterectomy is required.
Uterine conservation may be considered in stable patients who strongly desire future childbearing or uterine retention. This often requires leaving densely adherent placental tissue in situ and thus requires thorough counseling regarding the risks of delayed hemorrhage, infection, and emergent hysterectomy.11 This may not be desirable or safe for some patients, so informed consent is crucial. In such cases, we strongly recommend consultation with a PAS specialist, even if that requires immediate control of the placental blood supply (such as with arterial embolization), and transfer to a PAS specialty center.
Clinical scenarios
Vaginal delivery
The patient in the opening case was never expected to have PAS given her normal placental location and absence of a uterine scar. Even though she had some possible PAS risk factors (past retained placenta with instrumentation and in vitro fertilization), her absolute risk for the condition was low. Nevertheless, inability to create a separation plane should be considered PAS until proven otherwise. Although at this point many obstetricians would move to an operating room for uterine curettage, we recommend that the care team pause and put measures in place for possible PAS and hemorrhage. This involves notification of the blood bank, crossmatching of blood products, alerting the anesthesia team, and having a clear plan in place should a major hemorrhage ensue. This may involve use of balloon tamponade, activation of an interventional radiology team, or possible laparotomy with arterial ligations or hysterectomy. Avoidance of a prolonged third stage should be balanced against the need for preparation with these cases.
It is important for clinicians to bear in mind, and communicate to the patient, that hysterectomy is the standard of care for PAS. Significant delays in performing an indicated hysterectomy can lead to coagulopathy and patient instability. Timeliness is key; we find that delays in the decision to perform an indicated hysterectomy are often at the root of the cause for worsened morbidity in patients with unanticipated PAS. With an unscarred uterus and no placenta previa, a postpartum hysterectomy can be performed by many obstetrician-gynecologists experienced in this abdominal procedure.
Cesarean delivery
Undiagnosed PAS may present at cesarean delivery with or without placenta previa and a prior uterine scar. With this combination, PAS is often visually apparent upon opening the abdominal cavity (TABLE and FIGURE 1). Such surgical findings call for a clinical pause, as further actions at this point can lead to catastrophic hemorrhage. The obstetrician should consider a series of questions:
1. Are appropriate surgical and transfusion resources immediately available? If yes, they should be notified in case they are needed urgently. If not, then the obstetrician should ask whether the delivery must occur now.
2. Is this a scheduled delivery with a stable patient and fetus? If so, then closing the abdominal incision, monitoring the patient and fetus, and either transferring the patient to a PAS center or awaiting appropriate local specialists may be a lifesaving step.
3. Is immediate delivery required? If the fetus must be delivered, then it is imperative to create a hysterotomy out of the way of the placenta. Disrupting the adherent placenta with either an incision or manual manipulation may trigger a massive hemorrhage and should be avoided. This may require rectus muscle transection or creating a “T” incision on the skin to reach the uterine fundus and creating a hysterotomy over the top or even the back of the uterus. Once the fetus is delivered and lack of uterine hemorrhage confirmed (both abdominally and vaginally), the hysterotomy and abdomen can be closed with anticipation of urgent patient transfer to a PAS team or center.
4. Is the patient hemorrhaging? If the patient is hemorrhaging and closure is not an option, then recruitment of local emergent surgical teams is warranted, even if that requires packing the abdomen until an appropriate surgeon can arrive.
Diagnosis at cesarean delivery requires expedited and complex patient counseling. A patient who is unstable or hemorrhaging needs to be told that hysterectomy is lifesaving in this situation. For patients who are stable, it may be appropriate to close the abdomen and leave the placenta in situ, perform comprehensive counseling, and assess the possibility of transfer to a specialty center.
Summary
All obstetric care providers should be familiar with the clinical presentation of undiagnosed accreta spectrum. While hemorrhage is often part of the diagnosis, recognition of abnormal placental adherence and PAS-focused management should ideally be undertaken before this occurs. Once PAS is suspected, avoidance of further placental disruption may save significant morbidity, even if that means leaving the placenta attached until appropriate resources can be obtained. A local protocol for consultation, emergency transfer, and deployment of local resources should be part of every delivery unit’s emergency preparedness plan.
CASE Outcome
This patient is stabilized, with an adherent, retained placenta and no signs of hemorrhage. You administer uterotonics and notify your anesthesiologist and backup obstetrician that you have a likely case of accreta spectrum. A second intravenous line is placed, and blood products are crossmatched. The closest level III hospital is called, and they accept your patient for transfer. There, she is counseled about PAS, and she expresses no desire for future childbearing. After again confirming no placental separation in the operating room, the patient is moved immediately to perform laparotomy and total abdominal hysterectomy through a Pfannenstiel incision. She does not require a blood transfusion, and the pathology returns with grade I placenta accreta spectrum. ●
- American College of Obstetricians and Gynecologists, Society for Maternal-Fetal Medicine. Obstetric Care Consensus No. 7: placenta accreta spectrum. Obstet Gynecol. 2018; 132:e259-e275. doi:10.1097/AOG.0000000000002983.
- Carusi DA. The placenta accreta spectrum: epidemiology and risk factors. Clin Obstet Gynecol. 2018;61:733-742. doi:10.1097/GRF.0000000000000391.
- Silver RM, Fox KA, Barton JR, et al. Center of excellence for placenta accreta. Am J Obstet Gynecol. 2015;212:561-568. doi:10.1016/j.ajog.2014.11.018.
- Shamshirsaz AA, Fox KA, Salmanian B, et al. Maternal morbidity in patients with morbidly adherent placenta treated with and without a standardized multidisciplinary approach. Am J Obstet Gynecol. 2015;212:218.e1-9. doi:10.1016/j.ajog.2014.08.019.
- Bowman ZS, Eller AG, Kennedy AM, et al. Accuracy of ultrasound for the prediction of placenta accreta. Am J Obstet Gynecol. 2014;211:177.e1-7. doi:10.1016/j.ajog.2014.03.029.
- Carusi DA, Fox KA, Lyell DJ, et al. Placenta accreta spectrum without placenta previa. Obstet Gynecol. 2020;136:458-465. doi:10.1097/AOG.0000000000003970.
- Kayem G, Seco A, Beucher G, et al. Clinical profiles of placenta accreta spectrum: the PACCRETA population-based study. BJOG. 2021;128:1646-1655. doi:10.1111/1471-0528.16647.
- Jauniaux E, Ayres-de-Campos D, Langhoff-Roos J, et al. FIGO classification for the clinical diagnosis of placenta accreta spectrum disorders. Int J Gynaecol Obstet. 2019;146:20-24. doi:10.1002/ijgo.12761.
- Collins SL, Alemdar B, van Beekhuizen HJ, et al. Evidence-based guidelines for the management of abnormally invasive placenta: recommendations from the International Society for Abnormally Invasive Placenta. Am J Obstet Gynecol. 2019;220(6):511-526. doi:10.1016/j.ajog.2019.02.054.
- American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine. Obstetric care consensus. No. 7: placenta accreta spectrum. Obstet Gynecol. 2018;132:e259-e275. doi: 10.1097/AOG.0000000000002983.
- Sentilhes L, Kayem G, Silver RM. Conservative management of placenta accreta spectrum. Clin Obstet Gynecol. 2018; 61(4):783-794. doi:10.1097/GRF.0000000000000395.
CASE Placenta accreta spectrum following uncomplicated vaginal delivery
Imagine you are an obstetric hospitalist taking call at a level II maternal level of care hospital. Your patient is a 35-year-old woman, gravida 2, para 1, with a past history of retained placenta requiring dilation and curettage and intravenous antibiotics for endomyometritis. This is an in vitro fertilization pregnancy that has progressed normally, and the patient labored spontaneously at 38 weeks’ gestation. Following an uncomplicated vaginal delivery, the placenta has not delivered, and you attempt a manual placental extraction after a 40-minute third stage. While there is epidural analgesia and you can reach the uterine fundus, you are unable to create a separation plane between the placenta and uterus.
What do you do next?
Placenta accreta spectrum (PAS) includes a broad range of clinical scenarios with abnormal placental attachment as their common denominator. The condition has classically been defined pathologically, with chorionic villi attaching directly to the myometrium (“accreta”) or extending more deeply into the myometrium (“increta”) or attaching to surrounding tissues and structures (“percreta”).1 It is most commonly encountered in patients with low placental implantation on a prior cesarean section scar; indeed, placenta previa, particularly with a history of cesarean delivery, is the strongest risk factor for the development of PAS.2 In addition to abnormal placental attachment, these placental attachments are often hypervascular and can lead to catastrophic hemorrhage if not managed appropriately. For this reason, patients with sonographic or radiologic signs of PAS should be referred to specialized centers for further workup, counseling, and delivery planning.3
Although delivery at a specialized PAS center has been associated with improved patient outcomes,4 not all patients with PAS will be identified in the antepartum period. Ultrasonography may miss up to 40% to 50% of PAS cases, particularly when the sonologist has not been advised to look for the condition,5 and not all patients with PAS will have a previa implanted in a prior cesarean scar. A recent study found that these patients with nonprevia PAS were identified by imaging less than 40% of the time and were significantly less likely to be managed by a specialized team of clinicians.6 Thus, it falls upon every obstetric care provider to be aware of this diagnosis, promptly recognize its unanticipated presentations, and have a plan to optimize patient safety.
Step 1: Recognition
While PAS is classically defined as a pathologic condition, no clinician has the luxury of histology in the delivery room. Researchers have variously defined PAS clinically, with the common trait of abnormal placental adherence.7-9 The TABLE compares published definitions that have been used in the literature. While some definitions include hemorrhage, no clinician wants to induce significant hemorrhage to confirm their patient’s diagnosis. Thus, practically, the clinical PAS diagnosis comes down to abnormal placental attachment: If it is apparent that some or all of the placenta will not separate from the uterine wall with digital manipulation or careful curettage, then PAS should be suspected, and appropriate steps should be taken before further removal attempts.

At cesarean delivery, the PAS diagnosis may be aided by visual cues. With placenta previa, the lower uterine segment may bulge and take on a bluish hue, distinctly different from the upper healthy myometrium. PAS may also manifest with neovascularization, particularly behind the bladder. As with vaginal births, the placenta will fail to separate after the delivery, and controlled traction on the umbilical cord can produce a “dimple sign,” or visible myometrial retraction at the site of implantation (FIGURE 1). Finally, if the diagnosis is still in doubt, attempts to gently form a cleavage plane between the placenta and myometrium will be unsuccessful if PAS is present.8

Step 2: Initial management—pause, plan
Most importantly, do not attempt to forcibly remove the placenta. It can be left attached to the uterus until appropriate resources are secured. Efforts to forcibly remove an adherent placenta may well lead to major hemorrhage, and thus it falls on the patient’s care team to pause and plan for PAS care at this point. FIGURE 2 displays an algorithm for patient management. Further steps depend primarily on whether or not the patient is already hemorrhaging. In a stable situation, the patient should be counseled regarding the abnormal findings and the suspected PAS diagnosis. This includes the possibility of further procedures, blood transfusion, and hysterectomy. Local resources, including nursing, anesthesia, and the blood bank, should be notified about the situation and for the potential to call in specialized services. If on-site experienced specialists are not available, then patient transfer to a PAS specialty center should be strongly considered. While awaiting additional help or transport, the patient requires close monitoring for gross and physiologic signs of hemorrhage. If pursued, transport to a PAS specialty center should be expedited.

If the patient is already hemorrhaging or unstable, then appropriate local resources must be activated. At a minimum, this requires an obstetrician and anesthesiologist at the bedside and activation of hemorrhage protocols (eg, a massive transfusion protocol). If blood products are unavailable, consider whether they can be transported from other nearby blood banks, and start that process promptly. Next, contact backup services. Based on local resources and clinical severity, this may include maternal-fetal medicine specialists, pelvic surgeons, general and trauma surgeons, intensivists, interventional radiologists, and transfusion specialists. Even if the patient cannot be safely transferred to another hospital, the obstetrician can call an outside PAS specialist to discuss next steps in care and begin transfer plans, assuming the patient can be stabilized. Based on the Maternal Levels of Care definitions published by the American College of Obstetricians and Gynecologists and the Society of Maternal-Fetal Medicine,10 patients with PAS should be managed at level III or level IV centers. However, delivery units at every level of maternal care should have a protocol for securing local help and reaching an appropriate consultant if a PAS case is encountered. Know which center in your area specializes in PAS so that when an unanticipated case arises, you know who to call.
Continue to: Step 3: Ultimate management—mobilize and prepare for bleeding...
Step 3: Ultimate management—mobilize and prepare for bleeding
If diagnosis occurs intraoperatively at a PAS specialty center, or if safe transport is not possible, then the team should mobilize for the possibility of hysterectomy and prepare for massive bleeding, which can occur regardless of the treatment chosen. Many patients require or will opt for hysterectomy. For example, a patient who has finished childbearing may consent to a hysterectomy upon hearing she likely has PAS. In patients with suspected PAS who are actively hemorrhaging or are unstable, hysterectomy is required.
Uterine conservation may be considered in stable patients who strongly desire future childbearing or uterine retention. This often requires leaving densely adherent placental tissue in situ and thus requires thorough counseling regarding the risks of delayed hemorrhage, infection, and emergent hysterectomy.11 This may not be desirable or safe for some patients, so informed consent is crucial. In such cases, we strongly recommend consultation with a PAS specialist, even if that requires immediate control of the placental blood supply (such as with arterial embolization), and transfer to a PAS specialty center.
Clinical scenarios
Vaginal delivery
The patient in the opening case was never expected to have PAS given her normal placental location and absence of a uterine scar. Even though she had some possible PAS risk factors (past retained placenta with instrumentation and in vitro fertilization), her absolute risk for the condition was low. Nevertheless, inability to create a separation plane should be considered PAS until proven otherwise. Although at this point many obstetricians would move to an operating room for uterine curettage, we recommend that the care team pause and put measures in place for possible PAS and hemorrhage. This involves notification of the blood bank, crossmatching of blood products, alerting the anesthesia team, and having a clear plan in place should a major hemorrhage ensue. This may involve use of balloon tamponade, activation of an interventional radiology team, or possible laparotomy with arterial ligations or hysterectomy. Avoidance of a prolonged third stage should be balanced against the need for preparation with these cases.
It is important for clinicians to bear in mind, and communicate to the patient, that hysterectomy is the standard of care for PAS. Significant delays in performing an indicated hysterectomy can lead to coagulopathy and patient instability. Timeliness is key; we find that delays in the decision to perform an indicated hysterectomy are often at the root of the cause for worsened morbidity in patients with unanticipated PAS. With an unscarred uterus and no placenta previa, a postpartum hysterectomy can be performed by many obstetrician-gynecologists experienced in this abdominal procedure.
Cesarean delivery
Undiagnosed PAS may present at cesarean delivery with or without placenta previa and a prior uterine scar. With this combination, PAS is often visually apparent upon opening the abdominal cavity (TABLE and FIGURE 1). Such surgical findings call for a clinical pause, as further actions at this point can lead to catastrophic hemorrhage. The obstetrician should consider a series of questions:
1. Are appropriate surgical and transfusion resources immediately available? If yes, they should be notified in case they are needed urgently. If not, then the obstetrician should ask whether the delivery must occur now.
2. Is this a scheduled delivery with a stable patient and fetus? If so, then closing the abdominal incision, monitoring the patient and fetus, and either transferring the patient to a PAS center or awaiting appropriate local specialists may be a lifesaving step.
3. Is immediate delivery required? If the fetus must be delivered, then it is imperative to create a hysterotomy out of the way of the placenta. Disrupting the adherent placenta with either an incision or manual manipulation may trigger a massive hemorrhage and should be avoided. This may require rectus muscle transection or creating a “T” incision on the skin to reach the uterine fundus and creating a hysterotomy over the top or even the back of the uterus. Once the fetus is delivered and lack of uterine hemorrhage confirmed (both abdominally and vaginally), the hysterotomy and abdomen can be closed with anticipation of urgent patient transfer to a PAS team or center.
4. Is the patient hemorrhaging? If the patient is hemorrhaging and closure is not an option, then recruitment of local emergent surgical teams is warranted, even if that requires packing the abdomen until an appropriate surgeon can arrive.
Diagnosis at cesarean delivery requires expedited and complex patient counseling. A patient who is unstable or hemorrhaging needs to be told that hysterectomy is lifesaving in this situation. For patients who are stable, it may be appropriate to close the abdomen and leave the placenta in situ, perform comprehensive counseling, and assess the possibility of transfer to a specialty center.
Summary
All obstetric care providers should be familiar with the clinical presentation of undiagnosed accreta spectrum. While hemorrhage is often part of the diagnosis, recognition of abnormal placental adherence and PAS-focused management should ideally be undertaken before this occurs. Once PAS is suspected, avoidance of further placental disruption may save significant morbidity, even if that means leaving the placenta attached until appropriate resources can be obtained. A local protocol for consultation, emergency transfer, and deployment of local resources should be part of every delivery unit’s emergency preparedness plan.
CASE Outcome
This patient is stabilized, with an adherent, retained placenta and no signs of hemorrhage. You administer uterotonics and notify your anesthesiologist and backup obstetrician that you have a likely case of accreta spectrum. A second intravenous line is placed, and blood products are crossmatched. The closest level III hospital is called, and they accept your patient for transfer. There, she is counseled about PAS, and she expresses no desire for future childbearing. After again confirming no placental separation in the operating room, the patient is moved immediately to perform laparotomy and total abdominal hysterectomy through a Pfannenstiel incision. She does not require a blood transfusion, and the pathology returns with grade I placenta accreta spectrum. ●
CASE Placenta accreta spectrum following uncomplicated vaginal delivery
Imagine you are an obstetric hospitalist taking call at a level II maternal level of care hospital. Your patient is a 35-year-old woman, gravida 2, para 1, with a past history of retained placenta requiring dilation and curettage and intravenous antibiotics for endomyometritis. This is an in vitro fertilization pregnancy that has progressed normally, and the patient labored spontaneously at 38 weeks’ gestation. Following an uncomplicated vaginal delivery, the placenta has not delivered, and you attempt a manual placental extraction after a 40-minute third stage. While there is epidural analgesia and you can reach the uterine fundus, you are unable to create a separation plane between the placenta and uterus.
What do you do next?
Placenta accreta spectrum (PAS) includes a broad range of clinical scenarios with abnormal placental attachment as their common denominator. The condition has classically been defined pathologically, with chorionic villi attaching directly to the myometrium (“accreta”) or extending more deeply into the myometrium (“increta”) or attaching to surrounding tissues and structures (“percreta”).1 It is most commonly encountered in patients with low placental implantation on a prior cesarean section scar; indeed, placenta previa, particularly with a history of cesarean delivery, is the strongest risk factor for the development of PAS.2 In addition to abnormal placental attachment, these placental attachments are often hypervascular and can lead to catastrophic hemorrhage if not managed appropriately. For this reason, patients with sonographic or radiologic signs of PAS should be referred to specialized centers for further workup, counseling, and delivery planning.3
Although delivery at a specialized PAS center has been associated with improved patient outcomes,4 not all patients with PAS will be identified in the antepartum period. Ultrasonography may miss up to 40% to 50% of PAS cases, particularly when the sonologist has not been advised to look for the condition,5 and not all patients with PAS will have a previa implanted in a prior cesarean scar. A recent study found that these patients with nonprevia PAS were identified by imaging less than 40% of the time and were significantly less likely to be managed by a specialized team of clinicians.6 Thus, it falls upon every obstetric care provider to be aware of this diagnosis, promptly recognize its unanticipated presentations, and have a plan to optimize patient safety.
Step 1: Recognition
While PAS is classically defined as a pathologic condition, no clinician has the luxury of histology in the delivery room. Researchers have variously defined PAS clinically, with the common trait of abnormal placental adherence.7-9 The TABLE compares published definitions that have been used in the literature. While some definitions include hemorrhage, no clinician wants to induce significant hemorrhage to confirm their patient’s diagnosis. Thus, practically, the clinical PAS diagnosis comes down to abnormal placental attachment: If it is apparent that some or all of the placenta will not separate from the uterine wall with digital manipulation or careful curettage, then PAS should be suspected, and appropriate steps should be taken before further removal attempts.

At cesarean delivery, the PAS diagnosis may be aided by visual cues. With placenta previa, the lower uterine segment may bulge and take on a bluish hue, distinctly different from the upper healthy myometrium. PAS may also manifest with neovascularization, particularly behind the bladder. As with vaginal births, the placenta will fail to separate after the delivery, and controlled traction on the umbilical cord can produce a “dimple sign,” or visible myometrial retraction at the site of implantation (FIGURE 1). Finally, if the diagnosis is still in doubt, attempts to gently form a cleavage plane between the placenta and myometrium will be unsuccessful if PAS is present.8

Step 2: Initial management—pause, plan
Most importantly, do not attempt to forcibly remove the placenta. It can be left attached to the uterus until appropriate resources are secured. Efforts to forcibly remove an adherent placenta may well lead to major hemorrhage, and thus it falls on the patient’s care team to pause and plan for PAS care at this point. FIGURE 2 displays an algorithm for patient management. Further steps depend primarily on whether or not the patient is already hemorrhaging. In a stable situation, the patient should be counseled regarding the abnormal findings and the suspected PAS diagnosis. This includes the possibility of further procedures, blood transfusion, and hysterectomy. Local resources, including nursing, anesthesia, and the blood bank, should be notified about the situation and for the potential to call in specialized services. If on-site experienced specialists are not available, then patient transfer to a PAS specialty center should be strongly considered. While awaiting additional help or transport, the patient requires close monitoring for gross and physiologic signs of hemorrhage. If pursued, transport to a PAS specialty center should be expedited.

If the patient is already hemorrhaging or unstable, then appropriate local resources must be activated. At a minimum, this requires an obstetrician and anesthesiologist at the bedside and activation of hemorrhage protocols (eg, a massive transfusion protocol). If blood products are unavailable, consider whether they can be transported from other nearby blood banks, and start that process promptly. Next, contact backup services. Based on local resources and clinical severity, this may include maternal-fetal medicine specialists, pelvic surgeons, general and trauma surgeons, intensivists, interventional radiologists, and transfusion specialists. Even if the patient cannot be safely transferred to another hospital, the obstetrician can call an outside PAS specialist to discuss next steps in care and begin transfer plans, assuming the patient can be stabilized. Based on the Maternal Levels of Care definitions published by the American College of Obstetricians and Gynecologists and the Society of Maternal-Fetal Medicine,10 patients with PAS should be managed at level III or level IV centers. However, delivery units at every level of maternal care should have a protocol for securing local help and reaching an appropriate consultant if a PAS case is encountered. Know which center in your area specializes in PAS so that when an unanticipated case arises, you know who to call.
Continue to: Step 3: Ultimate management—mobilize and prepare for bleeding...
Step 3: Ultimate management—mobilize and prepare for bleeding
If diagnosis occurs intraoperatively at a PAS specialty center, or if safe transport is not possible, then the team should mobilize for the possibility of hysterectomy and prepare for massive bleeding, which can occur regardless of the treatment chosen. Many patients require or will opt for hysterectomy. For example, a patient who has finished childbearing may consent to a hysterectomy upon hearing she likely has PAS. In patients with suspected PAS who are actively hemorrhaging or are unstable, hysterectomy is required.
Uterine conservation may be considered in stable patients who strongly desire future childbearing or uterine retention. This often requires leaving densely adherent placental tissue in situ and thus requires thorough counseling regarding the risks of delayed hemorrhage, infection, and emergent hysterectomy.11 This may not be desirable or safe for some patients, so informed consent is crucial. In such cases, we strongly recommend consultation with a PAS specialist, even if that requires immediate control of the placental blood supply (such as with arterial embolization), and transfer to a PAS specialty center.
Clinical scenarios
Vaginal delivery
The patient in the opening case was never expected to have PAS given her normal placental location and absence of a uterine scar. Even though she had some possible PAS risk factors (past retained placenta with instrumentation and in vitro fertilization), her absolute risk for the condition was low. Nevertheless, inability to create a separation plane should be considered PAS until proven otherwise. Although at this point many obstetricians would move to an operating room for uterine curettage, we recommend that the care team pause and put measures in place for possible PAS and hemorrhage. This involves notification of the blood bank, crossmatching of blood products, alerting the anesthesia team, and having a clear plan in place should a major hemorrhage ensue. This may involve use of balloon tamponade, activation of an interventional radiology team, or possible laparotomy with arterial ligations or hysterectomy. Avoidance of a prolonged third stage should be balanced against the need for preparation with these cases.
It is important for clinicians to bear in mind, and communicate to the patient, that hysterectomy is the standard of care for PAS. Significant delays in performing an indicated hysterectomy can lead to coagulopathy and patient instability. Timeliness is key; we find that delays in the decision to perform an indicated hysterectomy are often at the root of the cause for worsened morbidity in patients with unanticipated PAS. With an unscarred uterus and no placenta previa, a postpartum hysterectomy can be performed by many obstetrician-gynecologists experienced in this abdominal procedure.
Cesarean delivery
Undiagnosed PAS may present at cesarean delivery with or without placenta previa and a prior uterine scar. With this combination, PAS is often visually apparent upon opening the abdominal cavity (TABLE and FIGURE 1). Such surgical findings call for a clinical pause, as further actions at this point can lead to catastrophic hemorrhage. The obstetrician should consider a series of questions:
1. Are appropriate surgical and transfusion resources immediately available? If yes, they should be notified in case they are needed urgently. If not, then the obstetrician should ask whether the delivery must occur now.
2. Is this a scheduled delivery with a stable patient and fetus? If so, then closing the abdominal incision, monitoring the patient and fetus, and either transferring the patient to a PAS center or awaiting appropriate local specialists may be a lifesaving step.
3. Is immediate delivery required? If the fetus must be delivered, then it is imperative to create a hysterotomy out of the way of the placenta. Disrupting the adherent placenta with either an incision or manual manipulation may trigger a massive hemorrhage and should be avoided. This may require rectus muscle transection or creating a “T” incision on the skin to reach the uterine fundus and creating a hysterotomy over the top or even the back of the uterus. Once the fetus is delivered and lack of uterine hemorrhage confirmed (both abdominally and vaginally), the hysterotomy and abdomen can be closed with anticipation of urgent patient transfer to a PAS team or center.
4. Is the patient hemorrhaging? If the patient is hemorrhaging and closure is not an option, then recruitment of local emergent surgical teams is warranted, even if that requires packing the abdomen until an appropriate surgeon can arrive.
Diagnosis at cesarean delivery requires expedited and complex patient counseling. A patient who is unstable or hemorrhaging needs to be told that hysterectomy is lifesaving in this situation. For patients who are stable, it may be appropriate to close the abdomen and leave the placenta in situ, perform comprehensive counseling, and assess the possibility of transfer to a specialty center.
Summary
All obstetric care providers should be familiar with the clinical presentation of undiagnosed accreta spectrum. While hemorrhage is often part of the diagnosis, recognition of abnormal placental adherence and PAS-focused management should ideally be undertaken before this occurs. Once PAS is suspected, avoidance of further placental disruption may save significant morbidity, even if that means leaving the placenta attached until appropriate resources can be obtained. A local protocol for consultation, emergency transfer, and deployment of local resources should be part of every delivery unit’s emergency preparedness plan.
CASE Outcome
This patient is stabilized, with an adherent, retained placenta and no signs of hemorrhage. You administer uterotonics and notify your anesthesiologist and backup obstetrician that you have a likely case of accreta spectrum. A second intravenous line is placed, and blood products are crossmatched. The closest level III hospital is called, and they accept your patient for transfer. There, she is counseled about PAS, and she expresses no desire for future childbearing. After again confirming no placental separation in the operating room, the patient is moved immediately to perform laparotomy and total abdominal hysterectomy through a Pfannenstiel incision. She does not require a blood transfusion, and the pathology returns with grade I placenta accreta spectrum. ●
- American College of Obstetricians and Gynecologists, Society for Maternal-Fetal Medicine. Obstetric Care Consensus No. 7: placenta accreta spectrum. Obstet Gynecol. 2018; 132:e259-e275. doi:10.1097/AOG.0000000000002983.
- Carusi DA. The placenta accreta spectrum: epidemiology and risk factors. Clin Obstet Gynecol. 2018;61:733-742. doi:10.1097/GRF.0000000000000391.
- Silver RM, Fox KA, Barton JR, et al. Center of excellence for placenta accreta. Am J Obstet Gynecol. 2015;212:561-568. doi:10.1016/j.ajog.2014.11.018.
- Shamshirsaz AA, Fox KA, Salmanian B, et al. Maternal morbidity in patients with morbidly adherent placenta treated with and without a standardized multidisciplinary approach. Am J Obstet Gynecol. 2015;212:218.e1-9. doi:10.1016/j.ajog.2014.08.019.
- Bowman ZS, Eller AG, Kennedy AM, et al. Accuracy of ultrasound for the prediction of placenta accreta. Am J Obstet Gynecol. 2014;211:177.e1-7. doi:10.1016/j.ajog.2014.03.029.
- Carusi DA, Fox KA, Lyell DJ, et al. Placenta accreta spectrum without placenta previa. Obstet Gynecol. 2020;136:458-465. doi:10.1097/AOG.0000000000003970.
- Kayem G, Seco A, Beucher G, et al. Clinical profiles of placenta accreta spectrum: the PACCRETA population-based study. BJOG. 2021;128:1646-1655. doi:10.1111/1471-0528.16647.
- Jauniaux E, Ayres-de-Campos D, Langhoff-Roos J, et al. FIGO classification for the clinical diagnosis of placenta accreta spectrum disorders. Int J Gynaecol Obstet. 2019;146:20-24. doi:10.1002/ijgo.12761.
- Collins SL, Alemdar B, van Beekhuizen HJ, et al. Evidence-based guidelines for the management of abnormally invasive placenta: recommendations from the International Society for Abnormally Invasive Placenta. Am J Obstet Gynecol. 2019;220(6):511-526. doi:10.1016/j.ajog.2019.02.054.
- American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine. Obstetric care consensus. No. 7: placenta accreta spectrum. Obstet Gynecol. 2018;132:e259-e275. doi: 10.1097/AOG.0000000000002983.
- Sentilhes L, Kayem G, Silver RM. Conservative management of placenta accreta spectrum. Clin Obstet Gynecol. 2018; 61(4):783-794. doi:10.1097/GRF.0000000000000395.
- American College of Obstetricians and Gynecologists, Society for Maternal-Fetal Medicine. Obstetric Care Consensus No. 7: placenta accreta spectrum. Obstet Gynecol. 2018; 132:e259-e275. doi:10.1097/AOG.0000000000002983.
- Carusi DA. The placenta accreta spectrum: epidemiology and risk factors. Clin Obstet Gynecol. 2018;61:733-742. doi:10.1097/GRF.0000000000000391.
- Silver RM, Fox KA, Barton JR, et al. Center of excellence for placenta accreta. Am J Obstet Gynecol. 2015;212:561-568. doi:10.1016/j.ajog.2014.11.018.
- Shamshirsaz AA, Fox KA, Salmanian B, et al. Maternal morbidity in patients with morbidly adherent placenta treated with and without a standardized multidisciplinary approach. Am J Obstet Gynecol. 2015;212:218.e1-9. doi:10.1016/j.ajog.2014.08.019.
- Bowman ZS, Eller AG, Kennedy AM, et al. Accuracy of ultrasound for the prediction of placenta accreta. Am J Obstet Gynecol. 2014;211:177.e1-7. doi:10.1016/j.ajog.2014.03.029.
- Carusi DA, Fox KA, Lyell DJ, et al. Placenta accreta spectrum without placenta previa. Obstet Gynecol. 2020;136:458-465. doi:10.1097/AOG.0000000000003970.
- Kayem G, Seco A, Beucher G, et al. Clinical profiles of placenta accreta spectrum: the PACCRETA population-based study. BJOG. 2021;128:1646-1655. doi:10.1111/1471-0528.16647.
- Jauniaux E, Ayres-de-Campos D, Langhoff-Roos J, et al. FIGO classification for the clinical diagnosis of placenta accreta spectrum disorders. Int J Gynaecol Obstet. 2019;146:20-24. doi:10.1002/ijgo.12761.
- Collins SL, Alemdar B, van Beekhuizen HJ, et al. Evidence-based guidelines for the management of abnormally invasive placenta: recommendations from the International Society for Abnormally Invasive Placenta. Am J Obstet Gynecol. 2019;220(6):511-526. doi:10.1016/j.ajog.2019.02.054.
- American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine. Obstetric care consensus. No. 7: placenta accreta spectrum. Obstet Gynecol. 2018;132:e259-e275. doi: 10.1097/AOG.0000000000002983.
- Sentilhes L, Kayem G, Silver RM. Conservative management of placenta accreta spectrum. Clin Obstet Gynecol. 2018; 61(4):783-794. doi:10.1097/GRF.0000000000000395.
Women are not being warned that anesthetic may reduce birth pill efficacy
The effectiveness of hormonal contraceptives, including the pill and mini-pill, may be compromised by sugammadex, a drug widely used in anesthesia for reversing neuromuscular blockade induced by rocuronium or vecuronium.
Yet women are not routinely informed that the drug may make their contraception less effective, delegates at Euroanaesthesia, the annual meeting of the European Society of Anaesthesiology and Intensive Care in Milan were told.
New research presented at the meeting supports the authors’ experience that “robust methods for identifying at-risk patients and informing them of the associated risk of contraceptive failures is not common practice across anesthetic departments within the United Kingdom, and likely further afield.”
This is according to a survey of almost 150 anesthetic professionals, including consultants, junior doctors, and physician assistants, working at University College London Hospitals NHS Foundation Trust.
Dr. Neha Passi, Dr. Matt Oliver, and colleagues at the trust’s department of anesthesiology sent out a seven-question survey to their 150 colleagues and received 82 responses, 94% of which claimed awareness of the risk of contraceptive failure with sugammadex. However, 70% of the respondents admitted that they do not routinely discuss this with patients who have received the drug.
Risk with all forms of hormonal contraceptive
Yet current guidance is to inform women of child-bearing age that they have received the drug and, because of increased risk of contraceptive failure, advise those taking oral hormonal contraceptives to follow the missed pill advice in the leaflet that comes with their contraceptives. It also counsels that clinicians should advise women using other types of hormonal contraceptive to use an additional nonhormonal means of contraception for 7 days.
The study authors also carried out a retrospective audit of sugammadex use in the trust and reported that during the 6 weeks covered by the audit, 234 patients were administered sugammadex of whom 65 (28%) were women of childbearing age. Of these, 17 had a medical history that meant they weren’t at risk of pregnancy, but the other 48 should have received advice on the risks of contraceptive failure – however there was no record in the medical notes of such advice having been given for any of the at-risk 48 women.
While sugammadex is the only anesthetic drug known to have this effect, it is recognized to interact with progesterone and so may reduce the effectiveness of hormonal contraceptives, including the progesterone-only pill, combined pill, vaginal rings, implants, and intrauterine devices.
Dr. Passi said: “It is concerning that we are so seldom informing patients of the risk of contraceptive failure following sugammadex use.
“Use of sugammadex is expected to rise as it becomes cheaper in the future, and ensuring that women receiving this medicine are aware it may increase their risk of unwanted pregnancy must be a priority.”
She added: “It is important to note, however, that most patients receiving an anesthetic do not need a muscle relaxant and that sugammadex is one of several drugs available to reverse muscle relaxation.”
Dr. Oliver said: “We only studied one hospital trust but we expect the results to be similar in elsewhere in the U.K.”
In response to their findings, the study’s authors have created patient information leaflets and letters and programmed the trust’s electronic patient record system to identify “at-risk” patients and deliver electronic prompts to the anesthetists caring for them in the perioperative period.
A version of this article first appeared on Medscape UK.
The effectiveness of hormonal contraceptives, including the pill and mini-pill, may be compromised by sugammadex, a drug widely used in anesthesia for reversing neuromuscular blockade induced by rocuronium or vecuronium.
Yet women are not routinely informed that the drug may make their contraception less effective, delegates at Euroanaesthesia, the annual meeting of the European Society of Anaesthesiology and Intensive Care in Milan were told.
New research presented at the meeting supports the authors’ experience that “robust methods for identifying at-risk patients and informing them of the associated risk of contraceptive failures is not common practice across anesthetic departments within the United Kingdom, and likely further afield.”
This is according to a survey of almost 150 anesthetic professionals, including consultants, junior doctors, and physician assistants, working at University College London Hospitals NHS Foundation Trust.
Dr. Neha Passi, Dr. Matt Oliver, and colleagues at the trust’s department of anesthesiology sent out a seven-question survey to their 150 colleagues and received 82 responses, 94% of which claimed awareness of the risk of contraceptive failure with sugammadex. However, 70% of the respondents admitted that they do not routinely discuss this with patients who have received the drug.
Risk with all forms of hormonal contraceptive
Yet current guidance is to inform women of child-bearing age that they have received the drug and, because of increased risk of contraceptive failure, advise those taking oral hormonal contraceptives to follow the missed pill advice in the leaflet that comes with their contraceptives. It also counsels that clinicians should advise women using other types of hormonal contraceptive to use an additional nonhormonal means of contraception for 7 days.
The study authors also carried out a retrospective audit of sugammadex use in the trust and reported that during the 6 weeks covered by the audit, 234 patients were administered sugammadex of whom 65 (28%) were women of childbearing age. Of these, 17 had a medical history that meant they weren’t at risk of pregnancy, but the other 48 should have received advice on the risks of contraceptive failure – however there was no record in the medical notes of such advice having been given for any of the at-risk 48 women.
While sugammadex is the only anesthetic drug known to have this effect, it is recognized to interact with progesterone and so may reduce the effectiveness of hormonal contraceptives, including the progesterone-only pill, combined pill, vaginal rings, implants, and intrauterine devices.
Dr. Passi said: “It is concerning that we are so seldom informing patients of the risk of contraceptive failure following sugammadex use.
“Use of sugammadex is expected to rise as it becomes cheaper in the future, and ensuring that women receiving this medicine are aware it may increase their risk of unwanted pregnancy must be a priority.”
She added: “It is important to note, however, that most patients receiving an anesthetic do not need a muscle relaxant and that sugammadex is one of several drugs available to reverse muscle relaxation.”
Dr. Oliver said: “We only studied one hospital trust but we expect the results to be similar in elsewhere in the U.K.”
In response to their findings, the study’s authors have created patient information leaflets and letters and programmed the trust’s electronic patient record system to identify “at-risk” patients and deliver electronic prompts to the anesthetists caring for them in the perioperative period.
A version of this article first appeared on Medscape UK.
The effectiveness of hormonal contraceptives, including the pill and mini-pill, may be compromised by sugammadex, a drug widely used in anesthesia for reversing neuromuscular blockade induced by rocuronium or vecuronium.
Yet women are not routinely informed that the drug may make their contraception less effective, delegates at Euroanaesthesia, the annual meeting of the European Society of Anaesthesiology and Intensive Care in Milan were told.
New research presented at the meeting supports the authors’ experience that “robust methods for identifying at-risk patients and informing them of the associated risk of contraceptive failures is not common practice across anesthetic departments within the United Kingdom, and likely further afield.”
This is according to a survey of almost 150 anesthetic professionals, including consultants, junior doctors, and physician assistants, working at University College London Hospitals NHS Foundation Trust.
Dr. Neha Passi, Dr. Matt Oliver, and colleagues at the trust’s department of anesthesiology sent out a seven-question survey to their 150 colleagues and received 82 responses, 94% of which claimed awareness of the risk of contraceptive failure with sugammadex. However, 70% of the respondents admitted that they do not routinely discuss this with patients who have received the drug.
Risk with all forms of hormonal contraceptive
Yet current guidance is to inform women of child-bearing age that they have received the drug and, because of increased risk of contraceptive failure, advise those taking oral hormonal contraceptives to follow the missed pill advice in the leaflet that comes with their contraceptives. It also counsels that clinicians should advise women using other types of hormonal contraceptive to use an additional nonhormonal means of contraception for 7 days.
The study authors also carried out a retrospective audit of sugammadex use in the trust and reported that during the 6 weeks covered by the audit, 234 patients were administered sugammadex of whom 65 (28%) were women of childbearing age. Of these, 17 had a medical history that meant they weren’t at risk of pregnancy, but the other 48 should have received advice on the risks of contraceptive failure – however there was no record in the medical notes of such advice having been given for any of the at-risk 48 women.
While sugammadex is the only anesthetic drug known to have this effect, it is recognized to interact with progesterone and so may reduce the effectiveness of hormonal contraceptives, including the progesterone-only pill, combined pill, vaginal rings, implants, and intrauterine devices.
Dr. Passi said: “It is concerning that we are so seldom informing patients of the risk of contraceptive failure following sugammadex use.
“Use of sugammadex is expected to rise as it becomes cheaper in the future, and ensuring that women receiving this medicine are aware it may increase their risk of unwanted pregnancy must be a priority.”
She added: “It is important to note, however, that most patients receiving an anesthetic do not need a muscle relaxant and that sugammadex is one of several drugs available to reverse muscle relaxation.”
Dr. Oliver said: “We only studied one hospital trust but we expect the results to be similar in elsewhere in the U.K.”
In response to their findings, the study’s authors have created patient information leaflets and letters and programmed the trust’s electronic patient record system to identify “at-risk” patients and deliver electronic prompts to the anesthetists caring for them in the perioperative period.
A version of this article first appeared on Medscape UK.
FROM EUROANAESTHESIA
Müllerian anomalies: Operative considerations
A Quantification Method to Compare the Value of Surgery and Palliative Care in Patients With Complex Cardiac Disease: A Concept
From the Department of Cardiothoracic Surgery, Stanford University, Stanford, CA.
Abstract
Complex cardiac patients are often referred for surgery or palliative care based on the risk of perioperative mortality. This decision ignores factors such as quality of life or duration of life in either surgery or the palliative path. Here, we propose a model to numerically assess and compare the value of surgery vs palliation. This model includes quality and duration of life, as well as risk of perioperative mortality, and involves a patient’s preferences in the decision-making process.
For each pathway, surgery or palliative care, a value is calculated and compared to a normal life value (no disease symptoms and normal life expectancy). The formula is adjusted for the risk of operative mortality. The model produces a ratio of the value of surgery to the value of palliative care that signifies the superiority of one or another. This model calculation presents an objective estimated numerical value to compare the value of surgery and palliative care. It can be applied to every decision-making process before surgery. In general, if a procedure has the potential to significantly extend life in a patient who otherwise has a very short life expectancy with palliation only, performing high-risk surgery would be a reasonable option. A model that provides a numerical value for surgery vs palliative care and includes quality and duration of life in each pathway could be a useful tool for cardiac surgeons in decision making regarding high-risk surgery.
Keywords: high-risk surgery, palliative care, quality of life, life expectancy.
Patients with complex cardiovascular disease are occasionally considered inoperable due to the high risk of surgical mortality. When the risk of perioperative mortality (POM) is predicted to be too high, surgical intervention is denied, and patients are often referred to palliative care. The risk of POM in cardiac surgery is often calculated using large-scale databases, such as the Society of Thoracic Surgeons (STS) records. The STS risk models, which are regularly updated, are based on large data sets and incorporate precise statistical methods for risk adjustment.1 In general, these calculators provide a percentage value that defines the magnitude of the risk of death, and then an arbitrary range is selected to categorize the procedure as low, medium, or high risk or inoperable status. The STS database does not set a cutoff point or range to define “operability.” Assigning inoperable status to a certain risk rate is problematic, with many ethical, legal, and moral implications, and for this reason, it has mostly remained undefined. In contrast, the low- and medium-risk ranges are easier to define. Another limitation encountered in the STS database is the lack of risk data for less common but very high-risk procedures, such as a triple valve replacement.
A common example where risk classification has been defined is in patients who are candidates for surgical vs transcatheter aortic valve replacement. Some groups have described a risk of <4% as low risk, 4% to 8% as intermediate risk, >8% as high risk, and >15% as inoperable2; for some other groups, a risk of POM >50% is considered extreme risk or inoperable.3,4 This procedure-specific classification is a useful decision-making tool and helps the surgeon perform an initial risk assessment to allocate a specific patient to a group—operable or nonoperable—only by calculating the risk of surgical death. However, this allocation method does not provide any information on how and when death occurs in either group. These 2 parameters of how and when death occurs define the quality of life (QOL) and the duration of life (DOL), respectively, and together could be considered as the value of life in each pathway. A survivor of a high-risk surgery may benefit from good quality and extended life (a high value), or, on the other end of the spectrum, a high-risk patient who does not undergo surgery is spared the mortality risk of the surgery but dies sooner (low value) with symptoms due to the natural course of the untreated disease.
The central question is, if a surgery is high risk but has the potential of providing a good value (for those who survive it), what QOL and DOL values are acceptable to risk or to justify accepting and proceeding with a risky surgery? Or how high a POM risk is justified to proceed with surgery rather than the alternative palliative care with a certain quality and duration? It is obvious that a decision-making process that is based on POM cannot compare the value of surgery (Vs) and the value of palliation (Vp). Furthermore, it ignores patient preferences and their input, as these are excluded from this decision-making process.
To be able to include QOL and DOL in any decision making, one must precisely describe these parameters. Both QOL and DOL are used for estimation of disease burden by health care administrators, public health experts, insurance agencies, and others. Multiple models have been proposed and used to estimate the overall burden of the disease. Most of the models for this purpose are created for large-scale economic purposes and not for decision making in individual cases.
An important measure is the quality-adjusted life year (QALY). This is an important parameter since it includes both measures of quality and quantity of life.5,6 QALY is a simplified measure to assess the value of health outcomes, and it has been used in economic calculations to assess mainly the cost-effectiveness of various interventions. We sought to evaluate the utility of a similar method in adding further insight into the surgical decision-making process. In this article, we propose a simple model to compare the value of surgery vs palliative care, similar to QALY. This model includes and adjusts for the quality and the quantity of life, in addition to the risk of POM, in the decision-making process for high-risk patients.
The Model
The 2 decision pathways, surgery and palliative care, are compared for their value. We define the value as the product of QOL and DOL in each pathway and use the severity of the symptoms as a surrogate for QOL. If duration and quality were depicted on the x and y axes of a graph (Figure 1), then the area under the curve would represent the collective value in each situation. Figure 2 shows the timeline and the different pathways with each decision. The value in each situation is calculated in relation to the full value, which is represented as the value of normal life (Vn), that is, life without disease and with normal life expectancy. The values of each decision pathway, the value of surgery (Vs) and the value of palliation (Vp), are then compared to define the benefit for each decision as follows:
If Vs/Vp > 1, the benefit is toward surgery;
If Vs/Vp < 1, the benefit is for palliative care.
Definitions
Both quality and duration of life are presented on a 1-10 scale, 1 being the lowest and 10 the highest value, to yield a product with a value of 100 in normal, disease-free life. Any lower value is presented as a percentage to represent the comparison to the full value. QOL is determined by degradation of full quality with the average level of symptoms. DOL is calculated as a lost time (
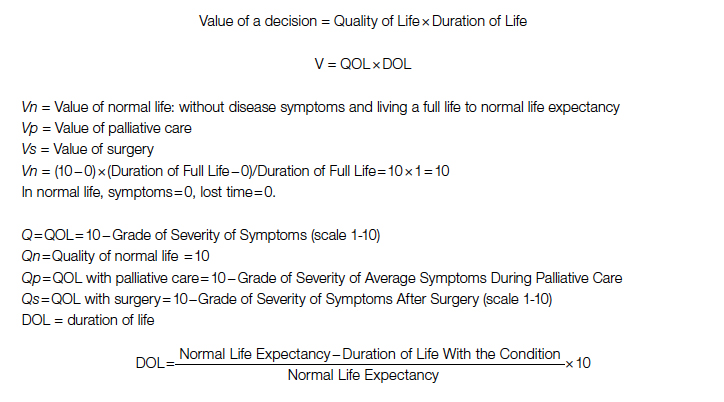
For the DOL under any condition, a 10-year survival rate could be used as a surrogate in this formula. Compared to life expectancy value, using the 10-year survival rate simplifies the calculation since cardiac diseases are more prevalent in older age, close to or beyond the average life expectancy value.
Using the time intervals from the timeline in Figure 2:
dh = time interval from diagnosis to death at life expectancy
dg = time interval from diagnosis to death after successful surgery
df = time interval from diagnosis to death after palliative care
Duration for palliative care:
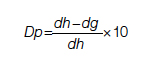
Duration for surgery:
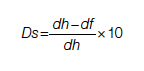
Adjustment: This value is calculated for those who survive the surgery. To adjust for the POM, it is multiplied by the 100 − POM risk.
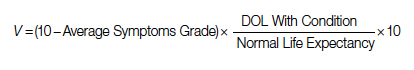
Since value is the base for comparison in this model, and it is the product of 2 equally important factors in the formula (
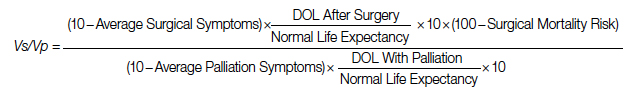
After elimination of normal life expectancy, form the numerator and denominator:
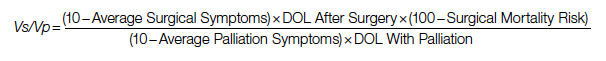
To adjust for surgical outcomes in special circumstances where less than optimal or standard surgical results are expected (eg, in very rare surgeries, limited resource institutions, or suboptimal postoperative surgical care), an optional coefficient R can be added to the numerator (surgical value). This optional coefficient, with values such as 0.8, 0.9 (to degrade the value of surgery) or 1 (standard surgical outcome), adjusts for variability in interinstitutional surgical results or surgeon variability. No coefficient is added to the denominator since palliative care provides minimal differences between clinicians and hospitals. Thus, the final adjusted formula would be as follows:
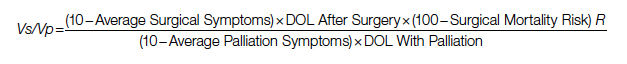
Example
A 60-year-old patient with a 10% POM risk needs to be allocated to surgical or palliative care. With palliative care, if this patient lived 6 years with average symptoms grade 4, the Vp would be 20; that is, 20% of the normal life value (if he lived 18 years instead without the disease).
Using the formula for calculation of value in each pathway:
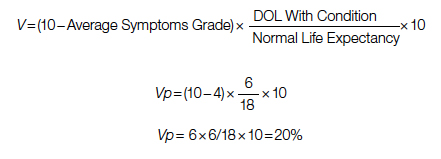
If the same patient undergoes a surgery with a 10% risk of POM, with an average grade 2 related to surgical recovery symptoms for 1 year and then is symptom-free and lives 12 years (instead of 18 years [life expectancy]), his Vs would be 53, or 53% out of the normal life value that is saved if the surgery is 100% successful; adjusted Vs with (chance of survival of 90%) would be 53 × 90% = 48%.
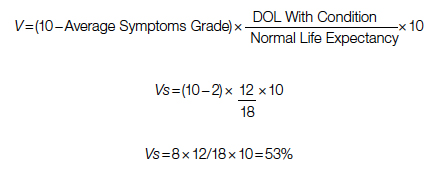
With adjustment of 90% survival chance in surgery, 53 × 90% = 48%. In this example, Vs/Vp = 48/20 = 2.4, showing a significant benefit for surgical care. Notably, the unknown value of normal life expectancy is not needed for the calculation of Vs/Vp, since it is the same in both pathways and it is eliminated by calculation in fraction.
Based on this formula, since the duration of surgical symptoms is short, no matter how severe these are, if the potential duration of life after surgery is high (represented by smaller area under the curve in Figure 1), the numerator becomes larger and the value of the surgery grows. For example, if a patient with a 15% risk of POM, which is generally considered inoperable, lives 5 years, as opposed to 2 years with palliative care with mild symptoms (eg 3/10), Vs/Vp would be 2.7, still showing a significant benefit for surgical care.
Discussion
Any surgical intervention is offered with 2 goals in mind, improving QOL and extending DOL. In a high-risk patient, surgery might be declined due to a high risk of POM, and the patient is offered palliative care, which other than providing symptom relief does not change the course of disease and eventually the patient will die due to the untreated disease. In this decision-making method, mostly completed by a care team only, a potential risk of death due to surgery which possibly could cure the patient is traded for immediate survival; however, the symptomatic course ensues until death. This mostly unilateral decision-making process by a care team, which incorporates minimal input from the patient or ignores patient preferences altogether, is based only on POM risk, and roughly includes a single parameter: years of potential life lost (YPLL). YPLL is a measure of premature mortality, and in the setting of surgical intervention, YPLL is the number of years a patient would lose unless a successful surgery were undertaken. Obviously, patients would live longer if a surgery that was intended to save them failed.
In this article, we proposed a simple method to quantify each decision to decide whether to operate or choose surgical care vs palliative care. Since quality and duration of life are both end factors clinicians and patients aspire to in each decision, they can be considered together as the value of each decision. We believe a numerical framework would provide an objective way to assist both the patient at high risk and the care team in the decision-making process.
The 2 parameters we consider are DOL and QOL. DOL, or survival, can be extracted from large-scale data using statistical methods that have been developed to predict survival under various conditions, such as Kaplan-Meier curves. These methods present the chance of survival in percentages in a defined time frame, such as a 5- or 10-year period.
While the DOL is a numerical parameter and quantifiable, the QOL is a more complex entity. This subjective parameter bears multiple definitions, aspects, and categories, and therefore multiple scales for quantification of QOL have been proposed. These scales have been used extensively for the purpose of health determination in health care policy and economic planning. Most scales acknowledge that QOL is multifactorial and includes interrelated aspects such as mental and socioeconomic factors. We have also noticed that QOL is better determined by the palliative care team than surgeons, so including these care providers in the decision-making process might reduce surgeon bias.
Since our purpose here is only to assist with the decision on medical intervention, we focus on physical QOL. Multiple scales are used to assess health-related QOL, such as the Assessment of Quality of Life (AQoL)-8D,7 EuroQol-5 Dimension (EQ-5D),8 15D,9 and the 36-Item Short Form Survey (SF-36).10 These complex scales are built for systematic reviews, and they are not practical for a clinical user. To simplify and keep this practical, we define QOL by using the severity or grade of symptoms related to the disease the patient has on a scale of 0 to 10. The severity of symptoms can be easily determined using available scales. An applicable scale for this purpose is the Edmonton Symptom Assessment Scale (ESAS), which has been in use for years and has evolved as a useful tool in the medical field.11
Once DOL and QOL are determined on a 1-10 scale, the multiplied value then provides a product that we consider a value. The highest value hoped for in each decision is the achievement of the best QOL and DOL, a value of 100. In Figure 1, a graphic presentation of value in each decision is best seen as the area under the curve. As shown, a successful surgery, even when accompanied by significant symptoms during initial recovery, has a chance (100 – risk of POM%) to gain a larger area under curve (value) by achieving a longer life with no or fewer symptoms. However, in palliative care, progressing disease and even palliated symptoms with a shorter life expectancy impose a large burden on the patient and a much lower value. Note that in this calculation, life expectancy, which is an important but unpredictable factor, is initially included; however, by ratio comparison, it is eliminated, simplifying the calculation further.
Using this formula in different settings reveals that high-risk surgery has a greater potential to reduce YPLL in the general population. Based on this formula, compared to a surgery with potential to significantly extend DOL, a definite shorter and symptomatic life course with palliative care makes it a significantly less favorable option. In fact, in the cardiovascular field, palliative care has minimal or no effect on natural history, as the mechanism of illness is mechanical, such as occlusion of coronary arteries or valve dysfunction, leading eventually to heart failure and death. In a study by Xu et al, although palliative care reduced readmission rates and improved symptoms on a variety of scales, there was no effect on mortality and QOL in patients with heart failure.12
No model in this field has proven to be ideal, and this model bears multiple limitations as well. We have used severity of symptoms as a surrogate for QOL based on the fact that cardiac patients with different pathologies who are untreated will have a common final pathway with development of heart failure symptoms that dictate their QOL. Also, grading QOL is a difficult task at times. Even a model such as QALY, which is one of the most used, is not a perfect model and is not free of problems.6 The difference in surgical results and life expectancy between sexes and ethnic groups might be a source of bias in this formula. Also, multiple factors directly and indirectly affect QOL and DOL and create inaccuracies; therefore, making an exact science from an inexact one naturally relies on multiple assumptions. Although it has previously been shown that most POM occurs in a short period of time after cardiac surgery,13 long-term complications that potentially degrade QOL are not included in this model. By applying this model, one must assume indefinite economic resources. Moreover, applying a single mathematical model in a biologic system and in the general population has intrinsic shortcomings, and it must overlook many other factors (eg, ethical, legal). For example, it will be hard to justify a failed surgery with 15% risk of POM undertaken to eliminate the severe long-lasting symptoms of a disease, while the outcome of a successful surgery with a 20% risk of POM that adds life and quality would be ignored in the current health care system. Thus, regardless of the significant potential, most surgeons would waive a surgery based solely on the percentage rate of POM, perhaps using other terms such as ”peri-nonoperative mortality.”
Conclusion
We have proposed a simple and practical formula for decision making regarding surgical vs palliative care in high-risk patients. By assigning a value that is composed of QOL and DOL in each pathway and including the risk of POM, a ratio of values provides a numerical estimation that can be used to show preference over a specific decision. An advantage of this formula, in addition to presenting an arithmetic value that is easier to understand, is that it can be used in shared decision making with patients. We emphasize that this model is only a preliminary concept at this time and has not been tested or validated for clinical use. Validation of such a model will require extensive work and testing within a large-scale population. We hope that this article will serve as a starting point for the development of other models, and that this formula will become more sophisticated with fewer limitations through larger multidisciplinary efforts in the future.
Corresponding author: Rabin Gerrah, MD, Good Samaritan Regional Medical Center, 3640 NW Samaritan Drive, Suite 100B, Corvallis, OR 97330; [email protected].
Disclosures: None reported.
1. O’Brien SM, Feng L, He X, et al. The Society of Thoracic Surgeons 2018 Adult Cardiac Surgery Risk Models: Part 2-statistical methods and results. Ann Thorac Surg. 2018;105(5):1419-1428. doi: 10.1016/j.athoracsur.2018.03.003
2. Hurtado Rendón IS, Bittenbender P, Dunn JM, Firstenberg MS. Chapter 8: Diagnostic workup and evaluation: eligibility, risk assessment, FDA guidelines. In: Transcatheter Heart Valve Handbook: A Surgeons’ and Interventional Council Review. Akron City Hospital, Summa Health System, Akron, OH.
3. Herrmann HC, Thourani VH, Kodali SK, et al; PARTNER Investigators. One-year clinical outcomes with SAPIEN 3 transcatheter aortic valve replacement in high-risk and inoperable patients with severe aortic stenosis. Circulation. 2016;134:130-140. doi:10.1161/CIRCULATIONAHA
4. Ho C, Argáez C. Transcatheter Aortic Valve Implantation for Patients with Severe Aortic Stenosis at Various Levels of Surgical Risk: A Review of Clinical Effectiveness. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; March 19, 2018.
5. Rios-Diaz AJ, Lam J, Ramos MS, et al. Global patterns of QALY and DALY use in surgical cost-utility analyses: a systematic review. PLoS One. 2016:10;11:e0148304. doi:10.1371/journal.pone.0148304
6. Prieto L, Sacristán JA. Health, Problems and solutions in calculating quality-adjusted life years (QALYs). Qual Life Outcomes. 2003:19;1:80.
7. Centre for Health Economics. Assessment of Quality of Life. 2014. Accessed May 13, 2022. http://www.aqol.com.au/
8. EuroQol Research Foundation. EQ-5D. Accessed May 13, 2022. https://euroqol.org/
9. 15D Instrument. Accessed May 13, 2022. http://www.15d-instrument.net/15d/
10. Rand Corporation. 36-Item Short Form Survey (SF-36).Accessed May 12, 2022. https://www.rand.org/health-care/surveys_tools/mos/36-item-short-form.html
11. Hui D, Bruera E. The Edmonton Symptom Assessment System 25 years later: past, present, and future developments. J Pain Symptom Manage. 2017:53:630-643. doi:10.1016/j.jpainsymman.2016
12. Xu Z, Chen L, Jin S, Yang B, Chen X, Wu Z. Effect of palliative care for patients with heart failure. Int Heart J. 2018:30;59:503-509. doi:10.1536/ihj.17-289
13. Mazzeffi M, Zivot J, Buchman T, Halkos M. In-hospital mortality after cardiac surgery: patient characteristics, timing, and association with postoperative length of intensive care unit and hospital stay. Ann Thorac Surg. 2014;97:1220-1225. doi:10.1016/j.athoracsur.2013.10.040
From the Department of Cardiothoracic Surgery, Stanford University, Stanford, CA.
Abstract
Complex cardiac patients are often referred for surgery or palliative care based on the risk of perioperative mortality. This decision ignores factors such as quality of life or duration of life in either surgery or the palliative path. Here, we propose a model to numerically assess and compare the value of surgery vs palliation. This model includes quality and duration of life, as well as risk of perioperative mortality, and involves a patient’s preferences in the decision-making process.
For each pathway, surgery or palliative care, a value is calculated and compared to a normal life value (no disease symptoms and normal life expectancy). The formula is adjusted for the risk of operative mortality. The model produces a ratio of the value of surgery to the value of palliative care that signifies the superiority of one or another. This model calculation presents an objective estimated numerical value to compare the value of surgery and palliative care. It can be applied to every decision-making process before surgery. In general, if a procedure has the potential to significantly extend life in a patient who otherwise has a very short life expectancy with palliation only, performing high-risk surgery would be a reasonable option. A model that provides a numerical value for surgery vs palliative care and includes quality and duration of life in each pathway could be a useful tool for cardiac surgeons in decision making regarding high-risk surgery.
Keywords: high-risk surgery, palliative care, quality of life, life expectancy.
Patients with complex cardiovascular disease are occasionally considered inoperable due to the high risk of surgical mortality. When the risk of perioperative mortality (POM) is predicted to be too high, surgical intervention is denied, and patients are often referred to palliative care. The risk of POM in cardiac surgery is often calculated using large-scale databases, such as the Society of Thoracic Surgeons (STS) records. The STS risk models, which are regularly updated, are based on large data sets and incorporate precise statistical methods for risk adjustment.1 In general, these calculators provide a percentage value that defines the magnitude of the risk of death, and then an arbitrary range is selected to categorize the procedure as low, medium, or high risk or inoperable status. The STS database does not set a cutoff point or range to define “operability.” Assigning inoperable status to a certain risk rate is problematic, with many ethical, legal, and moral implications, and for this reason, it has mostly remained undefined. In contrast, the low- and medium-risk ranges are easier to define. Another limitation encountered in the STS database is the lack of risk data for less common but very high-risk procedures, such as a triple valve replacement.
A common example where risk classification has been defined is in patients who are candidates for surgical vs transcatheter aortic valve replacement. Some groups have described a risk of <4% as low risk, 4% to 8% as intermediate risk, >8% as high risk, and >15% as inoperable2; for some other groups, a risk of POM >50% is considered extreme risk or inoperable.3,4 This procedure-specific classification is a useful decision-making tool and helps the surgeon perform an initial risk assessment to allocate a specific patient to a group—operable or nonoperable—only by calculating the risk of surgical death. However, this allocation method does not provide any information on how and when death occurs in either group. These 2 parameters of how and when death occurs define the quality of life (QOL) and the duration of life (DOL), respectively, and together could be considered as the value of life in each pathway. A survivor of a high-risk surgery may benefit from good quality and extended life (a high value), or, on the other end of the spectrum, a high-risk patient who does not undergo surgery is spared the mortality risk of the surgery but dies sooner (low value) with symptoms due to the natural course of the untreated disease.
The central question is, if a surgery is high risk but has the potential of providing a good value (for those who survive it), what QOL and DOL values are acceptable to risk or to justify accepting and proceeding with a risky surgery? Or how high a POM risk is justified to proceed with surgery rather than the alternative palliative care with a certain quality and duration? It is obvious that a decision-making process that is based on POM cannot compare the value of surgery (Vs) and the value of palliation (Vp). Furthermore, it ignores patient preferences and their input, as these are excluded from this decision-making process.
To be able to include QOL and DOL in any decision making, one must precisely describe these parameters. Both QOL and DOL are used for estimation of disease burden by health care administrators, public health experts, insurance agencies, and others. Multiple models have been proposed and used to estimate the overall burden of the disease. Most of the models for this purpose are created for large-scale economic purposes and not for decision making in individual cases.
An important measure is the quality-adjusted life year (QALY). This is an important parameter since it includes both measures of quality and quantity of life.5,6 QALY is a simplified measure to assess the value of health outcomes, and it has been used in economic calculations to assess mainly the cost-effectiveness of various interventions. We sought to evaluate the utility of a similar method in adding further insight into the surgical decision-making process. In this article, we propose a simple model to compare the value of surgery vs palliative care, similar to QALY. This model includes and adjusts for the quality and the quantity of life, in addition to the risk of POM, in the decision-making process for high-risk patients.
The Model
The 2 decision pathways, surgery and palliative care, are compared for their value. We define the value as the product of QOL and DOL in each pathway and use the severity of the symptoms as a surrogate for QOL. If duration and quality were depicted on the x and y axes of a graph (Figure 1), then the area under the curve would represent the collective value in each situation. Figure 2 shows the timeline and the different pathways with each decision. The value in each situation is calculated in relation to the full value, which is represented as the value of normal life (Vn), that is, life without disease and with normal life expectancy. The values of each decision pathway, the value of surgery (Vs) and the value of palliation (Vp), are then compared to define the benefit for each decision as follows:
If Vs/Vp > 1, the benefit is toward surgery;
If Vs/Vp < 1, the benefit is for palliative care.


Definitions
Both quality and duration of life are presented on a 1-10 scale, 1 being the lowest and 10 the highest value, to yield a product with a value of 100 in normal, disease-free life. Any lower value is presented as a percentage to represent the comparison to the full value. QOL is determined by degradation of full quality with the average level of symptoms. DOL is calculated as a lost time (

For the DOL under any condition, a 10-year survival rate could be used as a surrogate in this formula. Compared to life expectancy value, using the 10-year survival rate simplifies the calculation since cardiac diseases are more prevalent in older age, close to or beyond the average life expectancy value.
Using the time intervals from the timeline in Figure 2:
dh = time interval from diagnosis to death at life expectancy
dg = time interval from diagnosis to death after successful surgery
df = time interval from diagnosis to death after palliative care
Duration for palliative care:
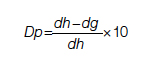
Duration for surgery:
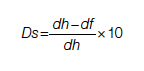
Adjustment: This value is calculated for those who survive the surgery. To adjust for the POM, it is multiplied by the 100 − POM risk.
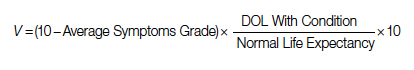
Since value is the base for comparison in this model, and it is the product of 2 equally important factors in the formula (

After elimination of normal life expectancy, form the numerator and denominator:
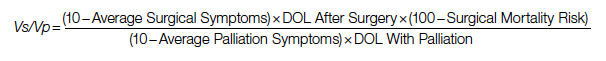
To adjust for surgical outcomes in special circumstances where less than optimal or standard surgical results are expected (eg, in very rare surgeries, limited resource institutions, or suboptimal postoperative surgical care), an optional coefficient R can be added to the numerator (surgical value). This optional coefficient, with values such as 0.8, 0.9 (to degrade the value of surgery) or 1 (standard surgical outcome), adjusts for variability in interinstitutional surgical results or surgeon variability. No coefficient is added to the denominator since palliative care provides minimal differences between clinicians and hospitals. Thus, the final adjusted formula would be as follows:
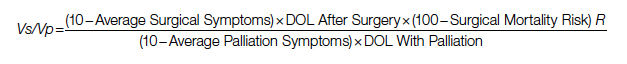
Example
A 60-year-old patient with a 10% POM risk needs to be allocated to surgical or palliative care. With palliative care, if this patient lived 6 years with average symptoms grade 4, the Vp would be 20; that is, 20% of the normal life value (if he lived 18 years instead without the disease).
Using the formula for calculation of value in each pathway:
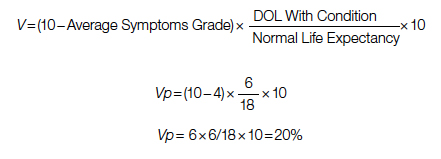
If the same patient undergoes a surgery with a 10% risk of POM, with an average grade 2 related to surgical recovery symptoms for 1 year and then is symptom-free and lives 12 years (instead of 18 years [life expectancy]), his Vs would be 53, or 53% out of the normal life value that is saved if the surgery is 100% successful; adjusted Vs with (chance of survival of 90%) would be 53 × 90% = 48%.
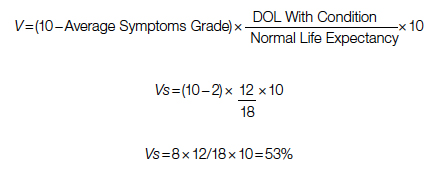
With adjustment of 90% survival chance in surgery, 53 × 90% = 48%. In this example, Vs/Vp = 48/20 = 2.4, showing a significant benefit for surgical care. Notably, the unknown value of normal life expectancy is not needed for the calculation of Vs/Vp, since it is the same in both pathways and it is eliminated by calculation in fraction.
Based on this formula, since the duration of surgical symptoms is short, no matter how severe these are, if the potential duration of life after surgery is high (represented by smaller area under the curve in Figure 1), the numerator becomes larger and the value of the surgery grows. For example, if a patient with a 15% risk of POM, which is generally considered inoperable, lives 5 years, as opposed to 2 years with palliative care with mild symptoms (eg 3/10), Vs/Vp would be 2.7, still showing a significant benefit for surgical care.
Discussion
Any surgical intervention is offered with 2 goals in mind, improving QOL and extending DOL. In a high-risk patient, surgery might be declined due to a high risk of POM, and the patient is offered palliative care, which other than providing symptom relief does not change the course of disease and eventually the patient will die due to the untreated disease. In this decision-making method, mostly completed by a care team only, a potential risk of death due to surgery which possibly could cure the patient is traded for immediate survival; however, the symptomatic course ensues until death. This mostly unilateral decision-making process by a care team, which incorporates minimal input from the patient or ignores patient preferences altogether, is based only on POM risk, and roughly includes a single parameter: years of potential life lost (YPLL). YPLL is a measure of premature mortality, and in the setting of surgical intervention, YPLL is the number of years a patient would lose unless a successful surgery were undertaken. Obviously, patients would live longer if a surgery that was intended to save them failed.
In this article, we proposed a simple method to quantify each decision to decide whether to operate or choose surgical care vs palliative care. Since quality and duration of life are both end factors clinicians and patients aspire to in each decision, they can be considered together as the value of each decision. We believe a numerical framework would provide an objective way to assist both the patient at high risk and the care team in the decision-making process.
The 2 parameters we consider are DOL and QOL. DOL, or survival, can be extracted from large-scale data using statistical methods that have been developed to predict survival under various conditions, such as Kaplan-Meier curves. These methods present the chance of survival in percentages in a defined time frame, such as a 5- or 10-year period.
While the DOL is a numerical parameter and quantifiable, the QOL is a more complex entity. This subjective parameter bears multiple definitions, aspects, and categories, and therefore multiple scales for quantification of QOL have been proposed. These scales have been used extensively for the purpose of health determination in health care policy and economic planning. Most scales acknowledge that QOL is multifactorial and includes interrelated aspects such as mental and socioeconomic factors. We have also noticed that QOL is better determined by the palliative care team than surgeons, so including these care providers in the decision-making process might reduce surgeon bias.
Since our purpose here is only to assist with the decision on medical intervention, we focus on physical QOL. Multiple scales are used to assess health-related QOL, such as the Assessment of Quality of Life (AQoL)-8D,7 EuroQol-5 Dimension (EQ-5D),8 15D,9 and the 36-Item Short Form Survey (SF-36).10 These complex scales are built for systematic reviews, and they are not practical for a clinical user. To simplify and keep this practical, we define QOL by using the severity or grade of symptoms related to the disease the patient has on a scale of 0 to 10. The severity of symptoms can be easily determined using available scales. An applicable scale for this purpose is the Edmonton Symptom Assessment Scale (ESAS), which has been in use for years and has evolved as a useful tool in the medical field.11
Once DOL and QOL are determined on a 1-10 scale, the multiplied value then provides a product that we consider a value. The highest value hoped for in each decision is the achievement of the best QOL and DOL, a value of 100. In Figure 1, a graphic presentation of value in each decision is best seen as the area under the curve. As shown, a successful surgery, even when accompanied by significant symptoms during initial recovery, has a chance (100 – risk of POM%) to gain a larger area under curve (value) by achieving a longer life with no or fewer symptoms. However, in palliative care, progressing disease and even palliated symptoms with a shorter life expectancy impose a large burden on the patient and a much lower value. Note that in this calculation, life expectancy, which is an important but unpredictable factor, is initially included; however, by ratio comparison, it is eliminated, simplifying the calculation further.
Using this formula in different settings reveals that high-risk surgery has a greater potential to reduce YPLL in the general population. Based on this formula, compared to a surgery with potential to significantly extend DOL, a definite shorter and symptomatic life course with palliative care makes it a significantly less favorable option. In fact, in the cardiovascular field, palliative care has minimal or no effect on natural history, as the mechanism of illness is mechanical, such as occlusion of coronary arteries or valve dysfunction, leading eventually to heart failure and death. In a study by Xu et al, although palliative care reduced readmission rates and improved symptoms on a variety of scales, there was no effect on mortality and QOL in patients with heart failure.12
No model in this field has proven to be ideal, and this model bears multiple limitations as well. We have used severity of symptoms as a surrogate for QOL based on the fact that cardiac patients with different pathologies who are untreated will have a common final pathway with development of heart failure symptoms that dictate their QOL. Also, grading QOL is a difficult task at times. Even a model such as QALY, which is one of the most used, is not a perfect model and is not free of problems.6 The difference in surgical results and life expectancy between sexes and ethnic groups might be a source of bias in this formula. Also, multiple factors directly and indirectly affect QOL and DOL and create inaccuracies; therefore, making an exact science from an inexact one naturally relies on multiple assumptions. Although it has previously been shown that most POM occurs in a short period of time after cardiac surgery,13 long-term complications that potentially degrade QOL are not included in this model. By applying this model, one must assume indefinite economic resources. Moreover, applying a single mathematical model in a biologic system and in the general population has intrinsic shortcomings, and it must overlook many other factors (eg, ethical, legal). For example, it will be hard to justify a failed surgery with 15% risk of POM undertaken to eliminate the severe long-lasting symptoms of a disease, while the outcome of a successful surgery with a 20% risk of POM that adds life and quality would be ignored in the current health care system. Thus, regardless of the significant potential, most surgeons would waive a surgery based solely on the percentage rate of POM, perhaps using other terms such as ”peri-nonoperative mortality.”
Conclusion
We have proposed a simple and practical formula for decision making regarding surgical vs palliative care in high-risk patients. By assigning a value that is composed of QOL and DOL in each pathway and including the risk of POM, a ratio of values provides a numerical estimation that can be used to show preference over a specific decision. An advantage of this formula, in addition to presenting an arithmetic value that is easier to understand, is that it can be used in shared decision making with patients. We emphasize that this model is only a preliminary concept at this time and has not been tested or validated for clinical use. Validation of such a model will require extensive work and testing within a large-scale population. We hope that this article will serve as a starting point for the development of other models, and that this formula will become more sophisticated with fewer limitations through larger multidisciplinary efforts in the future.
Corresponding author: Rabin Gerrah, MD, Good Samaritan Regional Medical Center, 3640 NW Samaritan Drive, Suite 100B, Corvallis, OR 97330; [email protected].
Disclosures: None reported.
From the Department of Cardiothoracic Surgery, Stanford University, Stanford, CA.
Abstract
Complex cardiac patients are often referred for surgery or palliative care based on the risk of perioperative mortality. This decision ignores factors such as quality of life or duration of life in either surgery or the palliative path. Here, we propose a model to numerically assess and compare the value of surgery vs palliation. This model includes quality and duration of life, as well as risk of perioperative mortality, and involves a patient’s preferences in the decision-making process.
For each pathway, surgery or palliative care, a value is calculated and compared to a normal life value (no disease symptoms and normal life expectancy). The formula is adjusted for the risk of operative mortality. The model produces a ratio of the value of surgery to the value of palliative care that signifies the superiority of one or another. This model calculation presents an objective estimated numerical value to compare the value of surgery and palliative care. It can be applied to every decision-making process before surgery. In general, if a procedure has the potential to significantly extend life in a patient who otherwise has a very short life expectancy with palliation only, performing high-risk surgery would be a reasonable option. A model that provides a numerical value for surgery vs palliative care and includes quality and duration of life in each pathway could be a useful tool for cardiac surgeons in decision making regarding high-risk surgery.
Keywords: high-risk surgery, palliative care, quality of life, life expectancy.
Patients with complex cardiovascular disease are occasionally considered inoperable due to the high risk of surgical mortality. When the risk of perioperative mortality (POM) is predicted to be too high, surgical intervention is denied, and patients are often referred to palliative care. The risk of POM in cardiac surgery is often calculated using large-scale databases, such as the Society of Thoracic Surgeons (STS) records. The STS risk models, which are regularly updated, are based on large data sets and incorporate precise statistical methods for risk adjustment.1 In general, these calculators provide a percentage value that defines the magnitude of the risk of death, and then an arbitrary range is selected to categorize the procedure as low, medium, or high risk or inoperable status. The STS database does not set a cutoff point or range to define “operability.” Assigning inoperable status to a certain risk rate is problematic, with many ethical, legal, and moral implications, and for this reason, it has mostly remained undefined. In contrast, the low- and medium-risk ranges are easier to define. Another limitation encountered in the STS database is the lack of risk data for less common but very high-risk procedures, such as a triple valve replacement.
A common example where risk classification has been defined is in patients who are candidates for surgical vs transcatheter aortic valve replacement. Some groups have described a risk of <4% as low risk, 4% to 8% as intermediate risk, >8% as high risk, and >15% as inoperable2; for some other groups, a risk of POM >50% is considered extreme risk or inoperable.3,4 This procedure-specific classification is a useful decision-making tool and helps the surgeon perform an initial risk assessment to allocate a specific patient to a group—operable or nonoperable—only by calculating the risk of surgical death. However, this allocation method does not provide any information on how and when death occurs in either group. These 2 parameters of how and when death occurs define the quality of life (QOL) and the duration of life (DOL), respectively, and together could be considered as the value of life in each pathway. A survivor of a high-risk surgery may benefit from good quality and extended life (a high value), or, on the other end of the spectrum, a high-risk patient who does not undergo surgery is spared the mortality risk of the surgery but dies sooner (low value) with symptoms due to the natural course of the untreated disease.
The central question is, if a surgery is high risk but has the potential of providing a good value (for those who survive it), what QOL and DOL values are acceptable to risk or to justify accepting and proceeding with a risky surgery? Or how high a POM risk is justified to proceed with surgery rather than the alternative palliative care with a certain quality and duration? It is obvious that a decision-making process that is based on POM cannot compare the value of surgery (Vs) and the value of palliation (Vp). Furthermore, it ignores patient preferences and their input, as these are excluded from this decision-making process.
To be able to include QOL and DOL in any decision making, one must precisely describe these parameters. Both QOL and DOL are used for estimation of disease burden by health care administrators, public health experts, insurance agencies, and others. Multiple models have been proposed and used to estimate the overall burden of the disease. Most of the models for this purpose are created for large-scale economic purposes and not for decision making in individual cases.
An important measure is the quality-adjusted life year (QALY). This is an important parameter since it includes both measures of quality and quantity of life.5,6 QALY is a simplified measure to assess the value of health outcomes, and it has been used in economic calculations to assess mainly the cost-effectiveness of various interventions. We sought to evaluate the utility of a similar method in adding further insight into the surgical decision-making process. In this article, we propose a simple model to compare the value of surgery vs palliative care, similar to QALY. This model includes and adjusts for the quality and the quantity of life, in addition to the risk of POM, in the decision-making process for high-risk patients.
The Model
The 2 decision pathways, surgery and palliative care, are compared for their value. We define the value as the product of QOL and DOL in each pathway and use the severity of the symptoms as a surrogate for QOL. If duration and quality were depicted on the x and y axes of a graph (Figure 1), then the area under the curve would represent the collective value in each situation. Figure 2 shows the timeline and the different pathways with each decision. The value in each situation is calculated in relation to the full value, which is represented as the value of normal life (Vn), that is, life without disease and with normal life expectancy. The values of each decision pathway, the value of surgery (Vs) and the value of palliation (Vp), are then compared to define the benefit for each decision as follows:
If Vs/Vp > 1, the benefit is toward surgery;
If Vs/Vp < 1, the benefit is for palliative care.


Definitions
Both quality and duration of life are presented on a 1-10 scale, 1 being the lowest and 10 the highest value, to yield a product with a value of 100 in normal, disease-free life. Any lower value is presented as a percentage to represent the comparison to the full value. QOL is determined by degradation of full quality with the average level of symptoms. DOL is calculated as a lost time (

For the DOL under any condition, a 10-year survival rate could be used as a surrogate in this formula. Compared to life expectancy value, using the 10-year survival rate simplifies the calculation since cardiac diseases are more prevalent in older age, close to or beyond the average life expectancy value.
Using the time intervals from the timeline in Figure 2:
dh = time interval from diagnosis to death at life expectancy
dg = time interval from diagnosis to death after successful surgery
df = time interval from diagnosis to death after palliative care
Duration for palliative care:
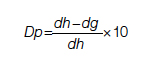
Duration for surgery:
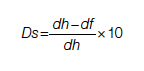
Adjustment: This value is calculated for those who survive the surgery. To adjust for the POM, it is multiplied by the 100 − POM risk.
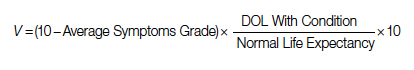
Since value is the base for comparison in this model, and it is the product of 2 equally important factors in the formula (

After elimination of normal life expectancy, form the numerator and denominator:
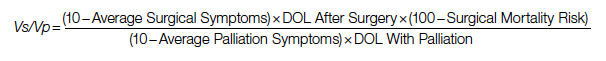
To adjust for surgical outcomes in special circumstances where less than optimal or standard surgical results are expected (eg, in very rare surgeries, limited resource institutions, or suboptimal postoperative surgical care), an optional coefficient R can be added to the numerator (surgical value). This optional coefficient, with values such as 0.8, 0.9 (to degrade the value of surgery) or 1 (standard surgical outcome), adjusts for variability in interinstitutional surgical results or surgeon variability. No coefficient is added to the denominator since palliative care provides minimal differences between clinicians and hospitals. Thus, the final adjusted formula would be as follows:
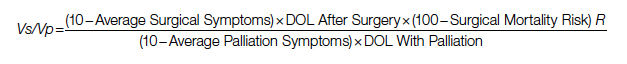
Example
A 60-year-old patient with a 10% POM risk needs to be allocated to surgical or palliative care. With palliative care, if this patient lived 6 years with average symptoms grade 4, the Vp would be 20; that is, 20% of the normal life value (if he lived 18 years instead without the disease).
Using the formula for calculation of value in each pathway:
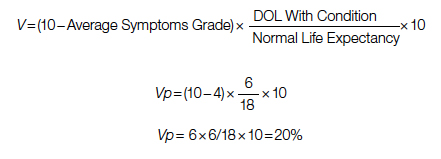
If the same patient undergoes a surgery with a 10% risk of POM, with an average grade 2 related to surgical recovery symptoms for 1 year and then is symptom-free and lives 12 years (instead of 18 years [life expectancy]), his Vs would be 53, or 53% out of the normal life value that is saved if the surgery is 100% successful; adjusted Vs with (chance of survival of 90%) would be 53 × 90% = 48%.
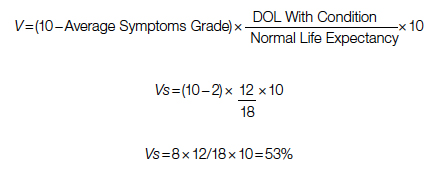
With adjustment of 90% survival chance in surgery, 53 × 90% = 48%. In this example, Vs/Vp = 48/20 = 2.4, showing a significant benefit for surgical care. Notably, the unknown value of normal life expectancy is not needed for the calculation of Vs/Vp, since it is the same in both pathways and it is eliminated by calculation in fraction.
Based on this formula, since the duration of surgical symptoms is short, no matter how severe these are, if the potential duration of life after surgery is high (represented by smaller area under the curve in Figure 1), the numerator becomes larger and the value of the surgery grows. For example, if a patient with a 15% risk of POM, which is generally considered inoperable, lives 5 years, as opposed to 2 years with palliative care with mild symptoms (eg 3/10), Vs/Vp would be 2.7, still showing a significant benefit for surgical care.
Discussion
Any surgical intervention is offered with 2 goals in mind, improving QOL and extending DOL. In a high-risk patient, surgery might be declined due to a high risk of POM, and the patient is offered palliative care, which other than providing symptom relief does not change the course of disease and eventually the patient will die due to the untreated disease. In this decision-making method, mostly completed by a care team only, a potential risk of death due to surgery which possibly could cure the patient is traded for immediate survival; however, the symptomatic course ensues until death. This mostly unilateral decision-making process by a care team, which incorporates minimal input from the patient or ignores patient preferences altogether, is based only on POM risk, and roughly includes a single parameter: years of potential life lost (YPLL). YPLL is a measure of premature mortality, and in the setting of surgical intervention, YPLL is the number of years a patient would lose unless a successful surgery were undertaken. Obviously, patients would live longer if a surgery that was intended to save them failed.
In this article, we proposed a simple method to quantify each decision to decide whether to operate or choose surgical care vs palliative care. Since quality and duration of life are both end factors clinicians and patients aspire to in each decision, they can be considered together as the value of each decision. We believe a numerical framework would provide an objective way to assist both the patient at high risk and the care team in the decision-making process.
The 2 parameters we consider are DOL and QOL. DOL, or survival, can be extracted from large-scale data using statistical methods that have been developed to predict survival under various conditions, such as Kaplan-Meier curves. These methods present the chance of survival in percentages in a defined time frame, such as a 5- or 10-year period.
While the DOL is a numerical parameter and quantifiable, the QOL is a more complex entity. This subjective parameter bears multiple definitions, aspects, and categories, and therefore multiple scales for quantification of QOL have been proposed. These scales have been used extensively for the purpose of health determination in health care policy and economic planning. Most scales acknowledge that QOL is multifactorial and includes interrelated aspects such as mental and socioeconomic factors. We have also noticed that QOL is better determined by the palliative care team than surgeons, so including these care providers in the decision-making process might reduce surgeon bias.
Since our purpose here is only to assist with the decision on medical intervention, we focus on physical QOL. Multiple scales are used to assess health-related QOL, such as the Assessment of Quality of Life (AQoL)-8D,7 EuroQol-5 Dimension (EQ-5D),8 15D,9 and the 36-Item Short Form Survey (SF-36).10 These complex scales are built for systematic reviews, and they are not practical for a clinical user. To simplify and keep this practical, we define QOL by using the severity or grade of symptoms related to the disease the patient has on a scale of 0 to 10. The severity of symptoms can be easily determined using available scales. An applicable scale for this purpose is the Edmonton Symptom Assessment Scale (ESAS), which has been in use for years and has evolved as a useful tool in the medical field.11
Once DOL and QOL are determined on a 1-10 scale, the multiplied value then provides a product that we consider a value. The highest value hoped for in each decision is the achievement of the best QOL and DOL, a value of 100. In Figure 1, a graphic presentation of value in each decision is best seen as the area under the curve. As shown, a successful surgery, even when accompanied by significant symptoms during initial recovery, has a chance (100 – risk of POM%) to gain a larger area under curve (value) by achieving a longer life with no or fewer symptoms. However, in palliative care, progressing disease and even palliated symptoms with a shorter life expectancy impose a large burden on the patient and a much lower value. Note that in this calculation, life expectancy, which is an important but unpredictable factor, is initially included; however, by ratio comparison, it is eliminated, simplifying the calculation further.
Using this formula in different settings reveals that high-risk surgery has a greater potential to reduce YPLL in the general population. Based on this formula, compared to a surgery with potential to significantly extend DOL, a definite shorter and symptomatic life course with palliative care makes it a significantly less favorable option. In fact, in the cardiovascular field, palliative care has minimal or no effect on natural history, as the mechanism of illness is mechanical, such as occlusion of coronary arteries or valve dysfunction, leading eventually to heart failure and death. In a study by Xu et al, although palliative care reduced readmission rates and improved symptoms on a variety of scales, there was no effect on mortality and QOL in patients with heart failure.12
No model in this field has proven to be ideal, and this model bears multiple limitations as well. We have used severity of symptoms as a surrogate for QOL based on the fact that cardiac patients with different pathologies who are untreated will have a common final pathway with development of heart failure symptoms that dictate their QOL. Also, grading QOL is a difficult task at times. Even a model such as QALY, which is one of the most used, is not a perfect model and is not free of problems.6 The difference in surgical results and life expectancy between sexes and ethnic groups might be a source of bias in this formula. Also, multiple factors directly and indirectly affect QOL and DOL and create inaccuracies; therefore, making an exact science from an inexact one naturally relies on multiple assumptions. Although it has previously been shown that most POM occurs in a short period of time after cardiac surgery,13 long-term complications that potentially degrade QOL are not included in this model. By applying this model, one must assume indefinite economic resources. Moreover, applying a single mathematical model in a biologic system and in the general population has intrinsic shortcomings, and it must overlook many other factors (eg, ethical, legal). For example, it will be hard to justify a failed surgery with 15% risk of POM undertaken to eliminate the severe long-lasting symptoms of a disease, while the outcome of a successful surgery with a 20% risk of POM that adds life and quality would be ignored in the current health care system. Thus, regardless of the significant potential, most surgeons would waive a surgery based solely on the percentage rate of POM, perhaps using other terms such as ”peri-nonoperative mortality.”
Conclusion
We have proposed a simple and practical formula for decision making regarding surgical vs palliative care in high-risk patients. By assigning a value that is composed of QOL and DOL in each pathway and including the risk of POM, a ratio of values provides a numerical estimation that can be used to show preference over a specific decision. An advantage of this formula, in addition to presenting an arithmetic value that is easier to understand, is that it can be used in shared decision making with patients. We emphasize that this model is only a preliminary concept at this time and has not been tested or validated for clinical use. Validation of such a model will require extensive work and testing within a large-scale population. We hope that this article will serve as a starting point for the development of other models, and that this formula will become more sophisticated with fewer limitations through larger multidisciplinary efforts in the future.
Corresponding author: Rabin Gerrah, MD, Good Samaritan Regional Medical Center, 3640 NW Samaritan Drive, Suite 100B, Corvallis, OR 97330; [email protected].
Disclosures: None reported.
1. O’Brien SM, Feng L, He X, et al. The Society of Thoracic Surgeons 2018 Adult Cardiac Surgery Risk Models: Part 2-statistical methods and results. Ann Thorac Surg. 2018;105(5):1419-1428. doi: 10.1016/j.athoracsur.2018.03.003
2. Hurtado Rendón IS, Bittenbender P, Dunn JM, Firstenberg MS. Chapter 8: Diagnostic workup and evaluation: eligibility, risk assessment, FDA guidelines. In: Transcatheter Heart Valve Handbook: A Surgeons’ and Interventional Council Review. Akron City Hospital, Summa Health System, Akron, OH.
3. Herrmann HC, Thourani VH, Kodali SK, et al; PARTNER Investigators. One-year clinical outcomes with SAPIEN 3 transcatheter aortic valve replacement in high-risk and inoperable patients with severe aortic stenosis. Circulation. 2016;134:130-140. doi:10.1161/CIRCULATIONAHA
4. Ho C, Argáez C. Transcatheter Aortic Valve Implantation for Patients with Severe Aortic Stenosis at Various Levels of Surgical Risk: A Review of Clinical Effectiveness. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; March 19, 2018.
5. Rios-Diaz AJ, Lam J, Ramos MS, et al. Global patterns of QALY and DALY use in surgical cost-utility analyses: a systematic review. PLoS One. 2016:10;11:e0148304. doi:10.1371/journal.pone.0148304
6. Prieto L, Sacristán JA. Health, Problems and solutions in calculating quality-adjusted life years (QALYs). Qual Life Outcomes. 2003:19;1:80.
7. Centre for Health Economics. Assessment of Quality of Life. 2014. Accessed May 13, 2022. http://www.aqol.com.au/
8. EuroQol Research Foundation. EQ-5D. Accessed May 13, 2022. https://euroqol.org/
9. 15D Instrument. Accessed May 13, 2022. http://www.15d-instrument.net/15d/
10. Rand Corporation. 36-Item Short Form Survey (SF-36).Accessed May 12, 2022. https://www.rand.org/health-care/surveys_tools/mos/36-item-short-form.html
11. Hui D, Bruera E. The Edmonton Symptom Assessment System 25 years later: past, present, and future developments. J Pain Symptom Manage. 2017:53:630-643. doi:10.1016/j.jpainsymman.2016
12. Xu Z, Chen L, Jin S, Yang B, Chen X, Wu Z. Effect of palliative care for patients with heart failure. Int Heart J. 2018:30;59:503-509. doi:10.1536/ihj.17-289
13. Mazzeffi M, Zivot J, Buchman T, Halkos M. In-hospital mortality after cardiac surgery: patient characteristics, timing, and association with postoperative length of intensive care unit and hospital stay. Ann Thorac Surg. 2014;97:1220-1225. doi:10.1016/j.athoracsur.2013.10.040
1. O’Brien SM, Feng L, He X, et al. The Society of Thoracic Surgeons 2018 Adult Cardiac Surgery Risk Models: Part 2-statistical methods and results. Ann Thorac Surg. 2018;105(5):1419-1428. doi: 10.1016/j.athoracsur.2018.03.003
2. Hurtado Rendón IS, Bittenbender P, Dunn JM, Firstenberg MS. Chapter 8: Diagnostic workup and evaluation: eligibility, risk assessment, FDA guidelines. In: Transcatheter Heart Valve Handbook: A Surgeons’ and Interventional Council Review. Akron City Hospital, Summa Health System, Akron, OH.
3. Herrmann HC, Thourani VH, Kodali SK, et al; PARTNER Investigators. One-year clinical outcomes with SAPIEN 3 transcatheter aortic valve replacement in high-risk and inoperable patients with severe aortic stenosis. Circulation. 2016;134:130-140. doi:10.1161/CIRCULATIONAHA
4. Ho C, Argáez C. Transcatheter Aortic Valve Implantation for Patients with Severe Aortic Stenosis at Various Levels of Surgical Risk: A Review of Clinical Effectiveness. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; March 19, 2018.
5. Rios-Diaz AJ, Lam J, Ramos MS, et al. Global patterns of QALY and DALY use in surgical cost-utility analyses: a systematic review. PLoS One. 2016:10;11:e0148304. doi:10.1371/journal.pone.0148304
6. Prieto L, Sacristán JA. Health, Problems and solutions in calculating quality-adjusted life years (QALYs). Qual Life Outcomes. 2003:19;1:80.
7. Centre for Health Economics. Assessment of Quality of Life. 2014. Accessed May 13, 2022. http://www.aqol.com.au/
8. EuroQol Research Foundation. EQ-5D. Accessed May 13, 2022. https://euroqol.org/
9. 15D Instrument. Accessed May 13, 2022. http://www.15d-instrument.net/15d/
10. Rand Corporation. 36-Item Short Form Survey (SF-36).Accessed May 12, 2022. https://www.rand.org/health-care/surveys_tools/mos/36-item-short-form.html
11. Hui D, Bruera E. The Edmonton Symptom Assessment System 25 years later: past, present, and future developments. J Pain Symptom Manage. 2017:53:630-643. doi:10.1016/j.jpainsymman.2016
12. Xu Z, Chen L, Jin S, Yang B, Chen X, Wu Z. Effect of palliative care for patients with heart failure. Int Heart J. 2018:30;59:503-509. doi:10.1536/ihj.17-289
13. Mazzeffi M, Zivot J, Buchman T, Halkos M. In-hospital mortality after cardiac surgery: patient characteristics, timing, and association with postoperative length of intensive care unit and hospital stay. Ann Thorac Surg. 2014;97:1220-1225. doi:10.1016/j.athoracsur.2013.10.040
Coronary CT Angiography Compared to Coronary Angiography or Standard of Care in Patients With Intermediate-Risk Stable Chest Pain
Study 1 Overview (SCOT-HEART Investigators)
Objective: To assess cardiovascular mortality and nonfatal myocardial infarction at 5 years in patients with stable chest pain referred to cardiology clinic for management with either standard care plus computed tomography angiography (CTA) or standard care alone.
Design: Multicenter, randomized, open-label prospective study.
Setting and participants: A total of 4146 patients with stable chest pain were randomized to standard care or standard care plus CTA at 12 centers across Scotland and were followed for 5 years.
Main outcome measures: The primary end point was a composite of death from coronary heart disease or nonfatal myocardial infarction. Main secondary end points were nonfatal myocardial infarction, nonfatal stroke, and frequency of invasive coronary angiography (ICA) and coronary revascularization with percutaneous coronary intervention or coronary artery bypass grafting.
Main results: The primary outcome including the composite of cardiovascular death or nonfatal myocardial infarction was lower in the CTA group than in the standard-care group at 2.3% (48 of 2073 patients) vs 3.9% (81 of 2073 patients), respectively (hazard ratio, 0.59; 95% CI, 0.41-0.84; P = .004). Although there was a higher rate of ICA and coronary revascularization in the CTA group than in the standard-care group in the first few months of follow-up, the overall rates were similar at 5 years, with ICA performed in 491 patients and 502 patients in the CTA vs standard-care groups, respectively (hazard ratio, 1.00; 95% CI, 0.88-1.13). Similarly, coronary revascularization was performed in 279 patients in the CTA group and in 267 patients in the standard-care group (hazard ratio, 1.07; 95% CI, 0.91-1.27). There were, however, more preventive therapies initiated in patients in the CTA group than in the standard-care group (odds ratio, 1.40; 95% CI, 1.19-1.65).
Conclusion: In patients with stable chest pain, the use of CTA in addition to standard care resulted in a significantly lower rate of death from coronary heart disease or nonfatal myocardial infarction at 5 years; the main contributor to this outcome was a reduced nonfatal myocardial infarction rate. There was no difference in the rate of coronary angiography or coronary revascularization between the 2 groups at 5 years.
Study 2 Overview (DISCHARGE Trial Group)
Objective: To compare the effectiveness of computed tomography (CT) with ICA as a diagnostic tool in patients with stable chest pain and intermediate pretest probability of coronary artery disease (CAD).
Design: Multicenter, randomized, assessor-blinded pragmatic prospective study.
Setting and participants: A total of 3667 patients with stable chest pain and intermediate pretest probability of CAD were enrolled at 26 centers and randomized into CT or ICA groups. Only 3561 patients were included in the modified intention-to-treat analysis, with 1808 patients and 1753 patients in the CT and ICA groups, respectively.
Main outcome measures: The primary outcome was a composite of cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke over 3.5 years. The main secondary outcomes were major procedure-related complications and patient-reported angina pectoris during the last 4 weeks of follow up.
Main results: The primary outcome occurred in 38 of 1808 patients (2.1%) in the CT group and in 52 of 1753 patients (3.0%) in the ICA group (hazard ratio, 0.70; 95% CI, 0.46-1.07; P = .10). The secondary outcomes showed that major procedure-related complications occurred in 9 patients (0.5%) in the CT group and in 33 patients (1.9%) in the ICA group (hazard ratio, 0.26; 95% CI, 0.13-0.55). Rates of patient-reported angina in the final 4 weeks of follow-up were 8.8% in the CT group and 7.5% in the ICA group (odds ratio, 1.17; 95% CI, 0.92-1.48).
Conclusion: Risk of major adverse cardiovascular events from the primary outcome were similar in both the CT and ICA groups among patients with stable chest pain and intermediate pretest probability of CAD. Patients referred for CT had a lower rate of coronary angiography leading to fewer major procedure-related complications in these patients than in those referred for ICA.
Commentary
Evaluation and treatment of obstructive atherosclerosis is an important part of clinical care in patients presenting with angina symptoms.1 Thus, the initial investigation for patients with suspected obstructive CAD includes ruling out acute coronary syndrome and assessing quality of life.1 The diagnostic test should be tailored to the pretest probability for the diagnosis of obstructive CAD.2
In the United States, stress testing traditionally has been used for the initial assessment in patients with suspected CAD,3 but recently CTA has been utilized more frequently for this purpose. Compared to a stress test, which often helps identify and assess ischemia, CTA can provide anatomical assessment, with higher sensitivity to identify CAD.4 Furthermore, it can distinguish nonobstructive plaques that can be challenging to identify with stress test alone.
Whether CTA is superior to stress testing as the initial assessment for CAD has been debated. The randomized PROMISE trial compared patients with stable angina who underwent functional stress testing or CTA as an initial strategy.5 They reported a similar outcome between the 2 groups at a median follow-up of 2 years. However, in the original SCOT-HEART trial (CT coronary angiography in patients with suspected angina due to coronary heart disease), which was published in the same year as the PROMISE trial, the patients who underwent initial assessment with CTA had a numerically lower composite end point of cardiac death and myocardial infarction at a median follow-up of 1.7 years (1.3% vs 2.0%, P = .053).6
Given this result, the SCOT-HEART investigators extended the follow-up to evaluate the composite end point of death from coronary heart disease or nonfatal myocardial infarction at 5 years.7 This trial enrolled patients who were initially referred to a cardiology clinic for evaluation of chest pain, and they were randomized to standard care plus CTA or standard care alone. At a median duration of 4.8 years, the primary outcome was lower in the CTA group (2.3%, 48 patients) than in the standard-care group (3.9%, 81 patients) (hazard ratio, 0.58; 95% CI, 0.41-0.84; P = .004). Both groups had similar rates of invasive coronary angiography and had similar coronary revascularization rates.
It is hypothesized that this lower rate of nonfatal myocardial infarction in patients with CTA plus standard care is associated with a higher rate of preventive therapies initiated in patients in the CTA-plus-standard-care group compared to standard care alone. However, the difference in the standard-care group should be noted when compared to the PROMISE trial. In the PROMISE trial, the comparator group had predominantly stress imaging (either nuclear stress test or echocardiography), while in the SCOT-HEART trial, the group had predominantly stress electrocardiogram (ECG), and only 10% of the patients underwent stress imaging. It is possible the difference seen in the rate of nonfatal myocardial infarction was due to suboptimal diagnosis of CAD with stress ECG, which has lower sensitivity compared to stress imaging.
The DISCHARGE trial investigated the effectiveness of CTA vs ICA as the initial diagnostic test in the management of patients with stable chest pain and an intermediate pretest probability of obstructive CAD.8 At 3.5 years of follow-up, the primary composite of cardiovascular death, myocardial infarction, or stroke was similar in both groups (2.1% vs 3.0; hazard ratio, 0.70; 95% CI, 0.46-1.07; P = .10). Importantly, as fewer patients underwent ICA, the risk of procedure-related complication was lower in the CTA group than in the ICA group. However, it is important to note that only 25% of the patients diagnosed with obstructive CAD had greater than 50% vessel stenosis, which raises the question of whether an initial invasive strategy is appropriate for this population.
The strengths of these 2 studies include the large number of patients enrolled along with adequate follow-up, 5 years in the SCOT-HEART trial and 3.5 years in the DISCHARGE trial. The 2 studies overall suggest the usefulness of CTA for assessment of CAD. However, the control groups were very different in these 2 trials. In the SCOT-HEART study, the comparator group was primarily assessed by stress ECG, while in the DISCHARGE study, the comparator group was primary assessed by ICA. In the PROMISE trial, the composite end point of death, myocardial infarction, hospitalization for unstable angina, or major procedural complication was similar when the strategy of initial CTA was compared to functional testing with imaging (exercise ECG, nuclear stress testing, or echocardiography).5 Thus, clinical assessment is still needed when clinicians are selecting the appropriate diagnostic test for patients with suspected CAD. The most recent guidelines give similar recommendations for CTA compared to stress imaging.9 Whether further improvement in CTA acquisition or the addition of CT fractional flow reserve can further improve outcomes requires additional study.
Applications for Clinical Practice and System Implementation
In patients with stable chest pain and intermediate pretest probability of CAD, CTA is useful in diagnosis compared to stress ECG and in reducing utilization of low-yield ICA. Whether CTA is more useful compared to the other noninvasive stress imaging modalities in this population requires further study.
Practice Points
- In patients with stable chest pain and intermediate pretest probability of CAD, CTA is useful compared to stress ECG.
- Use of CTA can potentially reduce the use of low-yield coronary angiography.
–Thai Nguyen, MD, Albert Chan, MD, Taishi Hirai, MD
University of Missouri, Columbia, MO
1. Knuuti J, Wijns W, Saraste A, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41(3):407-477. doi:10.1093/eurheartj/ehz425
2. Nakano S, Kohsaka S, Chikamori T et al. JCS 2022 guideline focused update on diagnosis and treatment in patients with stable coronary artery disease. Circ J. 2022;86(5):882-915. doi:10.1253/circj.CJ-21-1041.
3. Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;60(24):e44-e164. doi:10.1016/j.jacc.2012.07.013
4. Arbab-Zadeh A, Di Carli MF, Cerci R, et al. Accuracy of computed tomographic angiography and single-photon emission computed tomography-acquired myocardial perfusion imaging for the diagnosis of coronary artery disease. Circ Cardiovasc Imaging. 2015;8(10):e003533. doi:10.1161/CIRCIMAGING
5. Douglas PS, Hoffmann U, Patel MR, et al. Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med. 2015;372(14):1291-300. doi:10.1056/NEJMoa1415516
6. SCOT-HEART investigators. CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT-HEART): an open-label, parallel-group, multicentre trial. Lancet. 2015;385:2383-2391. doi:10.1016/S0140-6736(15)60291-4
7. SCOT-HEART Investigators, Newby DE, Adamson PD, et al. Coronary CT angiography and 5-year risk of myocardial infarction. N Engl J Med. 2018;379(10):924-933. doi:10.1056/NEJMoa1805971
8. DISCHARGE Trial Group, Maurovich-Horvat P, Bosserdt M, et al. CT or invasive coronary angiography in stable chest pain. N Engl J Med. 2022;386(17):1591-1602. doi:10.1056/NEJMoa2200963
9. Writing Committee Members, Lawton JS, Tamis-Holland JE, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022;79(2):e21-e129. doi:10.1016/j.jacc.2021.09.006
Study 1 Overview (SCOT-HEART Investigators)
Objective: To assess cardiovascular mortality and nonfatal myocardial infarction at 5 years in patients with stable chest pain referred to cardiology clinic for management with either standard care plus computed tomography angiography (CTA) or standard care alone.
Design: Multicenter, randomized, open-label prospective study.
Setting and participants: A total of 4146 patients with stable chest pain were randomized to standard care or standard care plus CTA at 12 centers across Scotland and were followed for 5 years.
Main outcome measures: The primary end point was a composite of death from coronary heart disease or nonfatal myocardial infarction. Main secondary end points were nonfatal myocardial infarction, nonfatal stroke, and frequency of invasive coronary angiography (ICA) and coronary revascularization with percutaneous coronary intervention or coronary artery bypass grafting.
Main results: The primary outcome including the composite of cardiovascular death or nonfatal myocardial infarction was lower in the CTA group than in the standard-care group at 2.3% (48 of 2073 patients) vs 3.9% (81 of 2073 patients), respectively (hazard ratio, 0.59; 95% CI, 0.41-0.84; P = .004). Although there was a higher rate of ICA and coronary revascularization in the CTA group than in the standard-care group in the first few months of follow-up, the overall rates were similar at 5 years, with ICA performed in 491 patients and 502 patients in the CTA vs standard-care groups, respectively (hazard ratio, 1.00; 95% CI, 0.88-1.13). Similarly, coronary revascularization was performed in 279 patients in the CTA group and in 267 patients in the standard-care group (hazard ratio, 1.07; 95% CI, 0.91-1.27). There were, however, more preventive therapies initiated in patients in the CTA group than in the standard-care group (odds ratio, 1.40; 95% CI, 1.19-1.65).
Conclusion: In patients with stable chest pain, the use of CTA in addition to standard care resulted in a significantly lower rate of death from coronary heart disease or nonfatal myocardial infarction at 5 years; the main contributor to this outcome was a reduced nonfatal myocardial infarction rate. There was no difference in the rate of coronary angiography or coronary revascularization between the 2 groups at 5 years.
Study 2 Overview (DISCHARGE Trial Group)
Objective: To compare the effectiveness of computed tomography (CT) with ICA as a diagnostic tool in patients with stable chest pain and intermediate pretest probability of coronary artery disease (CAD).
Design: Multicenter, randomized, assessor-blinded pragmatic prospective study.
Setting and participants: A total of 3667 patients with stable chest pain and intermediate pretest probability of CAD were enrolled at 26 centers and randomized into CT or ICA groups. Only 3561 patients were included in the modified intention-to-treat analysis, with 1808 patients and 1753 patients in the CT and ICA groups, respectively.
Main outcome measures: The primary outcome was a composite of cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke over 3.5 years. The main secondary outcomes were major procedure-related complications and patient-reported angina pectoris during the last 4 weeks of follow up.
Main results: The primary outcome occurred in 38 of 1808 patients (2.1%) in the CT group and in 52 of 1753 patients (3.0%) in the ICA group (hazard ratio, 0.70; 95% CI, 0.46-1.07; P = .10). The secondary outcomes showed that major procedure-related complications occurred in 9 patients (0.5%) in the CT group and in 33 patients (1.9%) in the ICA group (hazard ratio, 0.26; 95% CI, 0.13-0.55). Rates of patient-reported angina in the final 4 weeks of follow-up were 8.8% in the CT group and 7.5% in the ICA group (odds ratio, 1.17; 95% CI, 0.92-1.48).
Conclusion: Risk of major adverse cardiovascular events from the primary outcome were similar in both the CT and ICA groups among patients with stable chest pain and intermediate pretest probability of CAD. Patients referred for CT had a lower rate of coronary angiography leading to fewer major procedure-related complications in these patients than in those referred for ICA.
Commentary
Evaluation and treatment of obstructive atherosclerosis is an important part of clinical care in patients presenting with angina symptoms.1 Thus, the initial investigation for patients with suspected obstructive CAD includes ruling out acute coronary syndrome and assessing quality of life.1 The diagnostic test should be tailored to the pretest probability for the diagnosis of obstructive CAD.2
In the United States, stress testing traditionally has been used for the initial assessment in patients with suspected CAD,3 but recently CTA has been utilized more frequently for this purpose. Compared to a stress test, which often helps identify and assess ischemia, CTA can provide anatomical assessment, with higher sensitivity to identify CAD.4 Furthermore, it can distinguish nonobstructive plaques that can be challenging to identify with stress test alone.
Whether CTA is superior to stress testing as the initial assessment for CAD has been debated. The randomized PROMISE trial compared patients with stable angina who underwent functional stress testing or CTA as an initial strategy.5 They reported a similar outcome between the 2 groups at a median follow-up of 2 years. However, in the original SCOT-HEART trial (CT coronary angiography in patients with suspected angina due to coronary heart disease), which was published in the same year as the PROMISE trial, the patients who underwent initial assessment with CTA had a numerically lower composite end point of cardiac death and myocardial infarction at a median follow-up of 1.7 years (1.3% vs 2.0%, P = .053).6
Given this result, the SCOT-HEART investigators extended the follow-up to evaluate the composite end point of death from coronary heart disease or nonfatal myocardial infarction at 5 years.7 This trial enrolled patients who were initially referred to a cardiology clinic for evaluation of chest pain, and they were randomized to standard care plus CTA or standard care alone. At a median duration of 4.8 years, the primary outcome was lower in the CTA group (2.3%, 48 patients) than in the standard-care group (3.9%, 81 patients) (hazard ratio, 0.58; 95% CI, 0.41-0.84; P = .004). Both groups had similar rates of invasive coronary angiography and had similar coronary revascularization rates.
It is hypothesized that this lower rate of nonfatal myocardial infarction in patients with CTA plus standard care is associated with a higher rate of preventive therapies initiated in patients in the CTA-plus-standard-care group compared to standard care alone. However, the difference in the standard-care group should be noted when compared to the PROMISE trial. In the PROMISE trial, the comparator group had predominantly stress imaging (either nuclear stress test or echocardiography), while in the SCOT-HEART trial, the group had predominantly stress electrocardiogram (ECG), and only 10% of the patients underwent stress imaging. It is possible the difference seen in the rate of nonfatal myocardial infarction was due to suboptimal diagnosis of CAD with stress ECG, which has lower sensitivity compared to stress imaging.
The DISCHARGE trial investigated the effectiveness of CTA vs ICA as the initial diagnostic test in the management of patients with stable chest pain and an intermediate pretest probability of obstructive CAD.8 At 3.5 years of follow-up, the primary composite of cardiovascular death, myocardial infarction, or stroke was similar in both groups (2.1% vs 3.0; hazard ratio, 0.70; 95% CI, 0.46-1.07; P = .10). Importantly, as fewer patients underwent ICA, the risk of procedure-related complication was lower in the CTA group than in the ICA group. However, it is important to note that only 25% of the patients diagnosed with obstructive CAD had greater than 50% vessel stenosis, which raises the question of whether an initial invasive strategy is appropriate for this population.
The strengths of these 2 studies include the large number of patients enrolled along with adequate follow-up, 5 years in the SCOT-HEART trial and 3.5 years in the DISCHARGE trial. The 2 studies overall suggest the usefulness of CTA for assessment of CAD. However, the control groups were very different in these 2 trials. In the SCOT-HEART study, the comparator group was primarily assessed by stress ECG, while in the DISCHARGE study, the comparator group was primary assessed by ICA. In the PROMISE trial, the composite end point of death, myocardial infarction, hospitalization for unstable angina, or major procedural complication was similar when the strategy of initial CTA was compared to functional testing with imaging (exercise ECG, nuclear stress testing, or echocardiography).5 Thus, clinical assessment is still needed when clinicians are selecting the appropriate diagnostic test for patients with suspected CAD. The most recent guidelines give similar recommendations for CTA compared to stress imaging.9 Whether further improvement in CTA acquisition or the addition of CT fractional flow reserve can further improve outcomes requires additional study.
Applications for Clinical Practice and System Implementation
In patients with stable chest pain and intermediate pretest probability of CAD, CTA is useful in diagnosis compared to stress ECG and in reducing utilization of low-yield ICA. Whether CTA is more useful compared to the other noninvasive stress imaging modalities in this population requires further study.
Practice Points
- In patients with stable chest pain and intermediate pretest probability of CAD, CTA is useful compared to stress ECG.
- Use of CTA can potentially reduce the use of low-yield coronary angiography.
–Thai Nguyen, MD, Albert Chan, MD, Taishi Hirai, MD
University of Missouri, Columbia, MO
Study 1 Overview (SCOT-HEART Investigators)
Objective: To assess cardiovascular mortality and nonfatal myocardial infarction at 5 years in patients with stable chest pain referred to cardiology clinic for management with either standard care plus computed tomography angiography (CTA) or standard care alone.
Design: Multicenter, randomized, open-label prospective study.
Setting and participants: A total of 4146 patients with stable chest pain were randomized to standard care or standard care plus CTA at 12 centers across Scotland and were followed for 5 years.
Main outcome measures: The primary end point was a composite of death from coronary heart disease or nonfatal myocardial infarction. Main secondary end points were nonfatal myocardial infarction, nonfatal stroke, and frequency of invasive coronary angiography (ICA) and coronary revascularization with percutaneous coronary intervention or coronary artery bypass grafting.
Main results: The primary outcome including the composite of cardiovascular death or nonfatal myocardial infarction was lower in the CTA group than in the standard-care group at 2.3% (48 of 2073 patients) vs 3.9% (81 of 2073 patients), respectively (hazard ratio, 0.59; 95% CI, 0.41-0.84; P = .004). Although there was a higher rate of ICA and coronary revascularization in the CTA group than in the standard-care group in the first few months of follow-up, the overall rates were similar at 5 years, with ICA performed in 491 patients and 502 patients in the CTA vs standard-care groups, respectively (hazard ratio, 1.00; 95% CI, 0.88-1.13). Similarly, coronary revascularization was performed in 279 patients in the CTA group and in 267 patients in the standard-care group (hazard ratio, 1.07; 95% CI, 0.91-1.27). There were, however, more preventive therapies initiated in patients in the CTA group than in the standard-care group (odds ratio, 1.40; 95% CI, 1.19-1.65).
Conclusion: In patients with stable chest pain, the use of CTA in addition to standard care resulted in a significantly lower rate of death from coronary heart disease or nonfatal myocardial infarction at 5 years; the main contributor to this outcome was a reduced nonfatal myocardial infarction rate. There was no difference in the rate of coronary angiography or coronary revascularization between the 2 groups at 5 years.
Study 2 Overview (DISCHARGE Trial Group)
Objective: To compare the effectiveness of computed tomography (CT) with ICA as a diagnostic tool in patients with stable chest pain and intermediate pretest probability of coronary artery disease (CAD).
Design: Multicenter, randomized, assessor-blinded pragmatic prospective study.
Setting and participants: A total of 3667 patients with stable chest pain and intermediate pretest probability of CAD were enrolled at 26 centers and randomized into CT or ICA groups. Only 3561 patients were included in the modified intention-to-treat analysis, with 1808 patients and 1753 patients in the CT and ICA groups, respectively.
Main outcome measures: The primary outcome was a composite of cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke over 3.5 years. The main secondary outcomes were major procedure-related complications and patient-reported angina pectoris during the last 4 weeks of follow up.
Main results: The primary outcome occurred in 38 of 1808 patients (2.1%) in the CT group and in 52 of 1753 patients (3.0%) in the ICA group (hazard ratio, 0.70; 95% CI, 0.46-1.07; P = .10). The secondary outcomes showed that major procedure-related complications occurred in 9 patients (0.5%) in the CT group and in 33 patients (1.9%) in the ICA group (hazard ratio, 0.26; 95% CI, 0.13-0.55). Rates of patient-reported angina in the final 4 weeks of follow-up were 8.8% in the CT group and 7.5% in the ICA group (odds ratio, 1.17; 95% CI, 0.92-1.48).
Conclusion: Risk of major adverse cardiovascular events from the primary outcome were similar in both the CT and ICA groups among patients with stable chest pain and intermediate pretest probability of CAD. Patients referred for CT had a lower rate of coronary angiography leading to fewer major procedure-related complications in these patients than in those referred for ICA.
Commentary
Evaluation and treatment of obstructive atherosclerosis is an important part of clinical care in patients presenting with angina symptoms.1 Thus, the initial investigation for patients with suspected obstructive CAD includes ruling out acute coronary syndrome and assessing quality of life.1 The diagnostic test should be tailored to the pretest probability for the diagnosis of obstructive CAD.2
In the United States, stress testing traditionally has been used for the initial assessment in patients with suspected CAD,3 but recently CTA has been utilized more frequently for this purpose. Compared to a stress test, which often helps identify and assess ischemia, CTA can provide anatomical assessment, with higher sensitivity to identify CAD.4 Furthermore, it can distinguish nonobstructive plaques that can be challenging to identify with stress test alone.
Whether CTA is superior to stress testing as the initial assessment for CAD has been debated. The randomized PROMISE trial compared patients with stable angina who underwent functional stress testing or CTA as an initial strategy.5 They reported a similar outcome between the 2 groups at a median follow-up of 2 years. However, in the original SCOT-HEART trial (CT coronary angiography in patients with suspected angina due to coronary heart disease), which was published in the same year as the PROMISE trial, the patients who underwent initial assessment with CTA had a numerically lower composite end point of cardiac death and myocardial infarction at a median follow-up of 1.7 years (1.3% vs 2.0%, P = .053).6
Given this result, the SCOT-HEART investigators extended the follow-up to evaluate the composite end point of death from coronary heart disease or nonfatal myocardial infarction at 5 years.7 This trial enrolled patients who were initially referred to a cardiology clinic for evaluation of chest pain, and they were randomized to standard care plus CTA or standard care alone. At a median duration of 4.8 years, the primary outcome was lower in the CTA group (2.3%, 48 patients) than in the standard-care group (3.9%, 81 patients) (hazard ratio, 0.58; 95% CI, 0.41-0.84; P = .004). Both groups had similar rates of invasive coronary angiography and had similar coronary revascularization rates.
It is hypothesized that this lower rate of nonfatal myocardial infarction in patients with CTA plus standard care is associated with a higher rate of preventive therapies initiated in patients in the CTA-plus-standard-care group compared to standard care alone. However, the difference in the standard-care group should be noted when compared to the PROMISE trial. In the PROMISE trial, the comparator group had predominantly stress imaging (either nuclear stress test or echocardiography), while in the SCOT-HEART trial, the group had predominantly stress electrocardiogram (ECG), and only 10% of the patients underwent stress imaging. It is possible the difference seen in the rate of nonfatal myocardial infarction was due to suboptimal diagnosis of CAD with stress ECG, which has lower sensitivity compared to stress imaging.
The DISCHARGE trial investigated the effectiveness of CTA vs ICA as the initial diagnostic test in the management of patients with stable chest pain and an intermediate pretest probability of obstructive CAD.8 At 3.5 years of follow-up, the primary composite of cardiovascular death, myocardial infarction, or stroke was similar in both groups (2.1% vs 3.0; hazard ratio, 0.70; 95% CI, 0.46-1.07; P = .10). Importantly, as fewer patients underwent ICA, the risk of procedure-related complication was lower in the CTA group than in the ICA group. However, it is important to note that only 25% of the patients diagnosed with obstructive CAD had greater than 50% vessel stenosis, which raises the question of whether an initial invasive strategy is appropriate for this population.
The strengths of these 2 studies include the large number of patients enrolled along with adequate follow-up, 5 years in the SCOT-HEART trial and 3.5 years in the DISCHARGE trial. The 2 studies overall suggest the usefulness of CTA for assessment of CAD. However, the control groups were very different in these 2 trials. In the SCOT-HEART study, the comparator group was primarily assessed by stress ECG, while in the DISCHARGE study, the comparator group was primary assessed by ICA. In the PROMISE trial, the composite end point of death, myocardial infarction, hospitalization for unstable angina, or major procedural complication was similar when the strategy of initial CTA was compared to functional testing with imaging (exercise ECG, nuclear stress testing, or echocardiography).5 Thus, clinical assessment is still needed when clinicians are selecting the appropriate diagnostic test for patients with suspected CAD. The most recent guidelines give similar recommendations for CTA compared to stress imaging.9 Whether further improvement in CTA acquisition or the addition of CT fractional flow reserve can further improve outcomes requires additional study.
Applications for Clinical Practice and System Implementation
In patients with stable chest pain and intermediate pretest probability of CAD, CTA is useful in diagnosis compared to stress ECG and in reducing utilization of low-yield ICA. Whether CTA is more useful compared to the other noninvasive stress imaging modalities in this population requires further study.
Practice Points
- In patients with stable chest pain and intermediate pretest probability of CAD, CTA is useful compared to stress ECG.
- Use of CTA can potentially reduce the use of low-yield coronary angiography.
–Thai Nguyen, MD, Albert Chan, MD, Taishi Hirai, MD
University of Missouri, Columbia, MO
1. Knuuti J, Wijns W, Saraste A, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41(3):407-477. doi:10.1093/eurheartj/ehz425
2. Nakano S, Kohsaka S, Chikamori T et al. JCS 2022 guideline focused update on diagnosis and treatment in patients with stable coronary artery disease. Circ J. 2022;86(5):882-915. doi:10.1253/circj.CJ-21-1041.
3. Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;60(24):e44-e164. doi:10.1016/j.jacc.2012.07.013
4. Arbab-Zadeh A, Di Carli MF, Cerci R, et al. Accuracy of computed tomographic angiography and single-photon emission computed tomography-acquired myocardial perfusion imaging for the diagnosis of coronary artery disease. Circ Cardiovasc Imaging. 2015;8(10):e003533. doi:10.1161/CIRCIMAGING
5. Douglas PS, Hoffmann U, Patel MR, et al. Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med. 2015;372(14):1291-300. doi:10.1056/NEJMoa1415516
6. SCOT-HEART investigators. CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT-HEART): an open-label, parallel-group, multicentre trial. Lancet. 2015;385:2383-2391. doi:10.1016/S0140-6736(15)60291-4
7. SCOT-HEART Investigators, Newby DE, Adamson PD, et al. Coronary CT angiography and 5-year risk of myocardial infarction. N Engl J Med. 2018;379(10):924-933. doi:10.1056/NEJMoa1805971
8. DISCHARGE Trial Group, Maurovich-Horvat P, Bosserdt M, et al. CT or invasive coronary angiography in stable chest pain. N Engl J Med. 2022;386(17):1591-1602. doi:10.1056/NEJMoa2200963
9. Writing Committee Members, Lawton JS, Tamis-Holland JE, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022;79(2):e21-e129. doi:10.1016/j.jacc.2021.09.006
1. Knuuti J, Wijns W, Saraste A, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41(3):407-477. doi:10.1093/eurheartj/ehz425
2. Nakano S, Kohsaka S, Chikamori T et al. JCS 2022 guideline focused update on diagnosis and treatment in patients with stable coronary artery disease. Circ J. 2022;86(5):882-915. doi:10.1253/circj.CJ-21-1041.
3. Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;60(24):e44-e164. doi:10.1016/j.jacc.2012.07.013
4. Arbab-Zadeh A, Di Carli MF, Cerci R, et al. Accuracy of computed tomographic angiography and single-photon emission computed tomography-acquired myocardial perfusion imaging for the diagnosis of coronary artery disease. Circ Cardiovasc Imaging. 2015;8(10):e003533. doi:10.1161/CIRCIMAGING
5. Douglas PS, Hoffmann U, Patel MR, et al. Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med. 2015;372(14):1291-300. doi:10.1056/NEJMoa1415516
6. SCOT-HEART investigators. CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT-HEART): an open-label, parallel-group, multicentre trial. Lancet. 2015;385:2383-2391. doi:10.1016/S0140-6736(15)60291-4
7. SCOT-HEART Investigators, Newby DE, Adamson PD, et al. Coronary CT angiography and 5-year risk of myocardial infarction. N Engl J Med. 2018;379(10):924-933. doi:10.1056/NEJMoa1805971
8. DISCHARGE Trial Group, Maurovich-Horvat P, Bosserdt M, et al. CT or invasive coronary angiography in stable chest pain. N Engl J Med. 2022;386(17):1591-1602. doi:10.1056/NEJMoa2200963
9. Writing Committee Members, Lawton JS, Tamis-Holland JE, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022;79(2):e21-e129. doi:10.1016/j.jacc.2021.09.006