User login
FDA’s arthritis advisory committee unanimously backs biosimilar Humira
In a 26-0 vote, the Food and Drug Administration’s arthritis advisory committee recommended July 12 that the agency license an Amgen product as biosimilar to Humira (adalimumab) for seven distinct indications: rheumatoid arthritis, juvenile idiopathic arthritis (in patients at least 4 years old), psoriatic arthritis, ankylosing spondylitis, plaque psoriasis, adult Crohn’s disease, and adult ulcerative colitis.
Developing Story
In a 26-0 vote, the Food and Drug Administration’s arthritis advisory committee recommended July 12 that the agency license an Amgen product as biosimilar to Humira (adalimumab) for seven distinct indications: rheumatoid arthritis, juvenile idiopathic arthritis (in patients at least 4 years old), psoriatic arthritis, ankylosing spondylitis, plaque psoriasis, adult Crohn’s disease, and adult ulcerative colitis.
Developing Story
In a 26-0 vote, the Food and Drug Administration’s arthritis advisory committee recommended July 12 that the agency license an Amgen product as biosimilar to Humira (adalimumab) for seven distinct indications: rheumatoid arthritis, juvenile idiopathic arthritis (in patients at least 4 years old), psoriatic arthritis, ankylosing spondylitis, plaque psoriasis, adult Crohn’s disease, and adult ulcerative colitis.
Developing Story
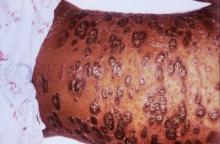
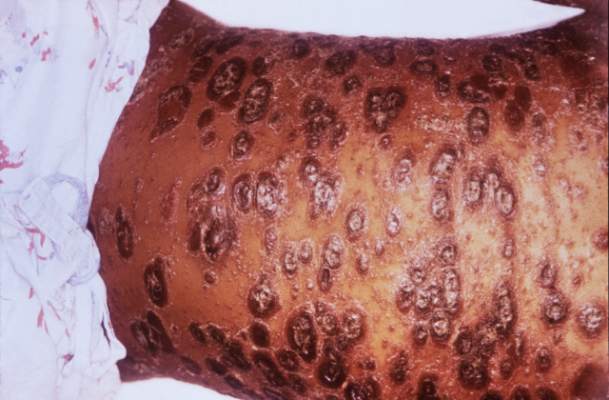
Severe psoriasis upped lymphoma risk in large cohort study
SCOTTSDALE, ARIZ. – Psoriasis of all severities was linked to a 3.5-fold increase in risk of cutaneous T-cell lymphoma, and severe psoriasis upped the associated risk of Hodgkin lymphoma by about 2.5 times, in a large, longitudinal, population-based cohort study.
Psoriasis also was tied to a smaller but statistically significant increase in the risk of non-Hodgkin lymphoma, said Zelma Chiesa Fuxench, MD, of the department of dermatology, the University of Pennsylvania, Philadelphia. Overall, lymphoma risk was highest in people with severe psoriasis, independent of traditional risk factors and exposure to immunosuppressive medications, Dr. Fuxench said at the annual meeting of the Society for Investigative Dermatology.
Psoriasis affects more than 125 million people worldwide, and severe cases are a major cause of cancer-related mortality. “Prior studies have suggested an increased risk of lymphoma in psoriasis patients, but it is unclear if this due to chronic inflammation, exposure to immunosuppressive therapies, or a combination of both factors,” Dr. Fuxench said.
To further explore these links, she and her associates analyzed electronic medical records from THIN (The Health Information Network), which includes about 12 million patients across the United Kingdom. Adults with psoriasis were matched to up to five nonpsoriatic controls based on date and clinic location. Patients who needed systemic medications or phototherapy were categorized as having severe psoriasis. The final dataset included more than 12,000 such patients, as well as 184,000 patients with mild psoriasis and more than 965,000 patients without psoriasis.
Psoriasis patients were younger and more likely to be overweight, male, and smoke and drink alcohol than patients without psoriasis, Dr. Fuxench said. Almost 80% of patients with severe disease had received systemic therapies, most often methotrexate (70% of systemic treatments) or cyclosporine (10%), while only 1% had received biologics.
Patients with severe psoriasis were more likely to be diagnosed with Hodgkin disease, non-Hodgkin lymphoma, and cutaneous T-cell lymphoma than were patients with mild psoriasis or controls. Over a median follow-up of 5.3 years, 34 patients with severe psoriasis were diagnosed with any type of lymphoma, for an incidence of 5.2 cases per 10,000 person-years (95% confidence interval, 3.7-7.3). In contrast, incidence rates for patients with mild psoriasis and controls were 3.3 and 3.2 cases per 10,000 person-years, respectively, Dr. Fuxench said.
In the multivariable analysis, patients with psoriasis were about 18% more likely to develop any type of lymphoma than were controls, an association that reached statistical significance (adjusted hazard ratio, 1.18; 95% CI, 1.06-1.31). Mild psoriasis increased lymphoma risk by 14%, and severe psoriasis upped it by about 83%, and both associations were statistically significant.
The increase in risk of non-Hodgkin lymphoma was 13% greater with mild psoriasis and 56% greater with severe disease, compared with controls, and these associations also reached statistical significance. Mild psoriasis was not linked to Hodgkin lymphoma, but patients with severe psoriasis were about 250% more likely to develop it than controls, with a trend toward statistical significance (aHR, 2.54; 95% CI, 0.94-6.87).
Finally, severe psoriasis was linked to a more than ninefold increase in risk of cutaneous T-cell lymphoma (aHR, 9.3; 95% CI, 4.1-21.4), while mild psoriasis was linked to about a threefold increase in risk.
“These results were robust in multiple sensitivity analyses, including analyses that excluded patients with rheumatoid arthritis, psoriatic arthritis, or a history of exposure to methotrexate, cyclosporine, or biologics,” Dr. Fuxench said. Future studies should explore the effect of treatment timing and selection on cancer risk, she added. “For those of us who care for these patients, we are increasingly using systemic agents that selectively target the immune system, and these questions will arise in clinics.”
The study’s design made it possible to pinpoint dates of diagnosis more effectively than investigators could estimate disease duration or confirm whether patients initially diagnosed with psoriasis actually had cutaneous T-cell lymphoma, Dr. Fuxench noted. “Ideally, we could have another cohort study of incident psoriasis with prospective follow-up, but lymphoma is so rare that there is currently not enough power [in the THIN database] to determine associations.”
The study was funded by grants from the National Institutes of Health and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Fuxench disclosed unrestricted research funding from Pfizer outside the submitted work.
SCOTTSDALE, ARIZ. – Psoriasis of all severities was linked to a 3.5-fold increase in risk of cutaneous T-cell lymphoma, and severe psoriasis upped the associated risk of Hodgkin lymphoma by about 2.5 times, in a large, longitudinal, population-based cohort study.
Psoriasis also was tied to a smaller but statistically significant increase in the risk of non-Hodgkin lymphoma, said Zelma Chiesa Fuxench, MD, of the department of dermatology, the University of Pennsylvania, Philadelphia. Overall, lymphoma risk was highest in people with severe psoriasis, independent of traditional risk factors and exposure to immunosuppressive medications, Dr. Fuxench said at the annual meeting of the Society for Investigative Dermatology.
Psoriasis affects more than 125 million people worldwide, and severe cases are a major cause of cancer-related mortality. “Prior studies have suggested an increased risk of lymphoma in psoriasis patients, but it is unclear if this due to chronic inflammation, exposure to immunosuppressive therapies, or a combination of both factors,” Dr. Fuxench said.
To further explore these links, she and her associates analyzed electronic medical records from THIN (The Health Information Network), which includes about 12 million patients across the United Kingdom. Adults with psoriasis were matched to up to five nonpsoriatic controls based on date and clinic location. Patients who needed systemic medications or phototherapy were categorized as having severe psoriasis. The final dataset included more than 12,000 such patients, as well as 184,000 patients with mild psoriasis and more than 965,000 patients without psoriasis.
Psoriasis patients were younger and more likely to be overweight, male, and smoke and drink alcohol than patients without psoriasis, Dr. Fuxench said. Almost 80% of patients with severe disease had received systemic therapies, most often methotrexate (70% of systemic treatments) or cyclosporine (10%), while only 1% had received biologics.
Patients with severe psoriasis were more likely to be diagnosed with Hodgkin disease, non-Hodgkin lymphoma, and cutaneous T-cell lymphoma than were patients with mild psoriasis or controls. Over a median follow-up of 5.3 years, 34 patients with severe psoriasis were diagnosed with any type of lymphoma, for an incidence of 5.2 cases per 10,000 person-years (95% confidence interval, 3.7-7.3). In contrast, incidence rates for patients with mild psoriasis and controls were 3.3 and 3.2 cases per 10,000 person-years, respectively, Dr. Fuxench said.
In the multivariable analysis, patients with psoriasis were about 18% more likely to develop any type of lymphoma than were controls, an association that reached statistical significance (adjusted hazard ratio, 1.18; 95% CI, 1.06-1.31). Mild psoriasis increased lymphoma risk by 14%, and severe psoriasis upped it by about 83%, and both associations were statistically significant.
The increase in risk of non-Hodgkin lymphoma was 13% greater with mild psoriasis and 56% greater with severe disease, compared with controls, and these associations also reached statistical significance. Mild psoriasis was not linked to Hodgkin lymphoma, but patients with severe psoriasis were about 250% more likely to develop it than controls, with a trend toward statistical significance (aHR, 2.54; 95% CI, 0.94-6.87).
Finally, severe psoriasis was linked to a more than ninefold increase in risk of cutaneous T-cell lymphoma (aHR, 9.3; 95% CI, 4.1-21.4), while mild psoriasis was linked to about a threefold increase in risk.
“These results were robust in multiple sensitivity analyses, including analyses that excluded patients with rheumatoid arthritis, psoriatic arthritis, or a history of exposure to methotrexate, cyclosporine, or biologics,” Dr. Fuxench said. Future studies should explore the effect of treatment timing and selection on cancer risk, she added. “For those of us who care for these patients, we are increasingly using systemic agents that selectively target the immune system, and these questions will arise in clinics.”
The study’s design made it possible to pinpoint dates of diagnosis more effectively than investigators could estimate disease duration or confirm whether patients initially diagnosed with psoriasis actually had cutaneous T-cell lymphoma, Dr. Fuxench noted. “Ideally, we could have another cohort study of incident psoriasis with prospective follow-up, but lymphoma is so rare that there is currently not enough power [in the THIN database] to determine associations.”
The study was funded by grants from the National Institutes of Health and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Fuxench disclosed unrestricted research funding from Pfizer outside the submitted work.
SCOTTSDALE, ARIZ. – Psoriasis of all severities was linked to a 3.5-fold increase in risk of cutaneous T-cell lymphoma, and severe psoriasis upped the associated risk of Hodgkin lymphoma by about 2.5 times, in a large, longitudinal, population-based cohort study.
Psoriasis also was tied to a smaller but statistically significant increase in the risk of non-Hodgkin lymphoma, said Zelma Chiesa Fuxench, MD, of the department of dermatology, the University of Pennsylvania, Philadelphia. Overall, lymphoma risk was highest in people with severe psoriasis, independent of traditional risk factors and exposure to immunosuppressive medications, Dr. Fuxench said at the annual meeting of the Society for Investigative Dermatology.
Psoriasis affects more than 125 million people worldwide, and severe cases are a major cause of cancer-related mortality. “Prior studies have suggested an increased risk of lymphoma in psoriasis patients, but it is unclear if this due to chronic inflammation, exposure to immunosuppressive therapies, or a combination of both factors,” Dr. Fuxench said.
To further explore these links, she and her associates analyzed electronic medical records from THIN (The Health Information Network), which includes about 12 million patients across the United Kingdom. Adults with psoriasis were matched to up to five nonpsoriatic controls based on date and clinic location. Patients who needed systemic medications or phototherapy were categorized as having severe psoriasis. The final dataset included more than 12,000 such patients, as well as 184,000 patients with mild psoriasis and more than 965,000 patients without psoriasis.
Psoriasis patients were younger and more likely to be overweight, male, and smoke and drink alcohol than patients without psoriasis, Dr. Fuxench said. Almost 80% of patients with severe disease had received systemic therapies, most often methotrexate (70% of systemic treatments) or cyclosporine (10%), while only 1% had received biologics.
Patients with severe psoriasis were more likely to be diagnosed with Hodgkin disease, non-Hodgkin lymphoma, and cutaneous T-cell lymphoma than were patients with mild psoriasis or controls. Over a median follow-up of 5.3 years, 34 patients with severe psoriasis were diagnosed with any type of lymphoma, for an incidence of 5.2 cases per 10,000 person-years (95% confidence interval, 3.7-7.3). In contrast, incidence rates for patients with mild psoriasis and controls were 3.3 and 3.2 cases per 10,000 person-years, respectively, Dr. Fuxench said.
In the multivariable analysis, patients with psoriasis were about 18% more likely to develop any type of lymphoma than were controls, an association that reached statistical significance (adjusted hazard ratio, 1.18; 95% CI, 1.06-1.31). Mild psoriasis increased lymphoma risk by 14%, and severe psoriasis upped it by about 83%, and both associations were statistically significant.
The increase in risk of non-Hodgkin lymphoma was 13% greater with mild psoriasis and 56% greater with severe disease, compared with controls, and these associations also reached statistical significance. Mild psoriasis was not linked to Hodgkin lymphoma, but patients with severe psoriasis were about 250% more likely to develop it than controls, with a trend toward statistical significance (aHR, 2.54; 95% CI, 0.94-6.87).
Finally, severe psoriasis was linked to a more than ninefold increase in risk of cutaneous T-cell lymphoma (aHR, 9.3; 95% CI, 4.1-21.4), while mild psoriasis was linked to about a threefold increase in risk.
“These results were robust in multiple sensitivity analyses, including analyses that excluded patients with rheumatoid arthritis, psoriatic arthritis, or a history of exposure to methotrexate, cyclosporine, or biologics,” Dr. Fuxench said. Future studies should explore the effect of treatment timing and selection on cancer risk, she added. “For those of us who care for these patients, we are increasingly using systemic agents that selectively target the immune system, and these questions will arise in clinics.”
The study’s design made it possible to pinpoint dates of diagnosis more effectively than investigators could estimate disease duration or confirm whether patients initially diagnosed with psoriasis actually had cutaneous T-cell lymphoma, Dr. Fuxench noted. “Ideally, we could have another cohort study of incident psoriasis with prospective follow-up, but lymphoma is so rare that there is currently not enough power [in the THIN database] to determine associations.”
The study was funded by grants from the National Institutes of Health and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Fuxench disclosed unrestricted research funding from Pfizer outside the submitted work.
AT THE 2016 SID ANNUAL MEETING
Key clinical point: Psoriasis was identified as an independent risk factor for lymphoma, with the risk of lymphoma increasing with disease severity.
Major finding: The strongest association was between severe psoriasis and cutaneous T-cell lymphoma (aHR, 9.3; 95% CI, 4.1-21.4).
Data source: A population-based longitudinal cohort study of 12,198 patients with severe psoriasis, 184,870 patients with mild psoriasis, and 965,730 nonpsoriatic controls.
Disclosures: The study was funded by grants from the National Institutes of Health and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Fuxench disclosed unrestricted research support from Pfizer outside the submitted work.
Adalimumab approved to treat noninfectious uveitis
The U.S. Food and Drug Administration has approved adalimumab for treatment of noninfectious intermediate, posterior, and panuveitis in adults, making it the only approved drug for the condition that is not a corticosteroid.
The approval marks the 10th approved indication for adalimumab (Humira) in the United States. It was recently approved for this indication in the European Union as well.
Patients on adalimumab had about half the risk of those on placebo to experience treatment failure (a combination of uveitis flare and decrease in visual acuity) in two pivotal phase III studies, VISUAL-I (hazard ratio, 0.5 for VISUAL-I; P less than .001) and VISUAL-II (HR, 0.57; P = .004). In the trials, patients were treated with an 80-mg loading dose of adalimumab at baseline, followed by a 40-mg subcutaneous injection at week 1 and then 40 mg every other week for up to 80 weeks.
“These approvals provide a valuable option for patients experiencing flare and vision impairment associated with this group of inflammatory diseases of the eye,” Glenn J. Jaffe, MD, of Duke University, Durham, N.C., said in an announcement from the manufacturer of adalimumab, AbbVie. “Data from the robust VISUAL clinical trial program demonstrate the value of Humira as a treatment option for patients with these serious diseases.”
The U.S. Food and Drug Administration has approved adalimumab for treatment of noninfectious intermediate, posterior, and panuveitis in adults, making it the only approved drug for the condition that is not a corticosteroid.
The approval marks the 10th approved indication for adalimumab (Humira) in the United States. It was recently approved for this indication in the European Union as well.
Patients on adalimumab had about half the risk of those on placebo to experience treatment failure (a combination of uveitis flare and decrease in visual acuity) in two pivotal phase III studies, VISUAL-I (hazard ratio, 0.5 for VISUAL-I; P less than .001) and VISUAL-II (HR, 0.57; P = .004). In the trials, patients were treated with an 80-mg loading dose of adalimumab at baseline, followed by a 40-mg subcutaneous injection at week 1 and then 40 mg every other week for up to 80 weeks.
“These approvals provide a valuable option for patients experiencing flare and vision impairment associated with this group of inflammatory diseases of the eye,” Glenn J. Jaffe, MD, of Duke University, Durham, N.C., said in an announcement from the manufacturer of adalimumab, AbbVie. “Data from the robust VISUAL clinical trial program demonstrate the value of Humira as a treatment option for patients with these serious diseases.”
The U.S. Food and Drug Administration has approved adalimumab for treatment of noninfectious intermediate, posterior, and panuveitis in adults, making it the only approved drug for the condition that is not a corticosteroid.
The approval marks the 10th approved indication for adalimumab (Humira) in the United States. It was recently approved for this indication in the European Union as well.
Patients on adalimumab had about half the risk of those on placebo to experience treatment failure (a combination of uveitis flare and decrease in visual acuity) in two pivotal phase III studies, VISUAL-I (hazard ratio, 0.5 for VISUAL-I; P less than .001) and VISUAL-II (HR, 0.57; P = .004). In the trials, patients were treated with an 80-mg loading dose of adalimumab at baseline, followed by a 40-mg subcutaneous injection at week 1 and then 40 mg every other week for up to 80 weeks.
“These approvals provide a valuable option for patients experiencing flare and vision impairment associated with this group of inflammatory diseases of the eye,” Glenn J. Jaffe, MD, of Duke University, Durham, N.C., said in an announcement from the manufacturer of adalimumab, AbbVie. “Data from the robust VISUAL clinical trial program demonstrate the value of Humira as a treatment option for patients with these serious diseases.”
Pharma jousts statistically for an ankylosing spondylitis edge
Now that the interleukin-17 inhibitor secukinumab and tumor necrosis factor inhibitors are competing options for treatment of patients with ankylosing spondylitis, the companies that make those drugs must feel pressure to find some sort of advantage for their agents.
How else to explain the remarkable pair of similar post hoc analyses presented in June at the European Congress of Rheumatology in London? One of the analyses was funded by Novartis – the company that markets secukinumab (Cosentyx) – and included several Novartis employees as coauthors. The second study, presented immediately afterward in the main session at the meeting devoted to ankylosing spondylitis (AS) treatments, had backing from AbbVie, which markets adalimumab (Humira), the largest-selling tumor necrosis factor inhibitor worldwide, and had several AbbVie employees as coauthors.
Both analyses used a “matching adjusted indirect comparison,” a fairly new way to compare the performance of interventions studied in two totally independent trials by propensity matching patients from each of the two trials. It’s purportedly a way to make a legitimate comparison in the absence of head-to-head data.
Making the two reports even more surreal was their use of essentially the same data.
The first report came from Walter P. Maksymowych, MD, an AS clinician and researcher from the University of Alberta, who with his coauthors used data collected on secukinumab in the MEASURE 1 pivotal trial and on adalimumab in the ATLAS pivotal trial. He spent much of his presentation describing the methods behind the indirect comparison, and I don’t think I can be blamed for calling the results of this Novartis-sponsored analysis predictable: overall better performance by secukinumab, compared “indirectly” with adalimumab for clinical responses and patient quality of life.
The second report, the one sponsored by AbbVie, came from Keith A. Betts, PhD, a biostatistician who works for the Analysis Group, an international consulting firm. He also used the ATLAS database as the source for adalimumab outcomes, and differed marginally from Dr. Maksymowych by taking data on secukinumab patients from both the MEASURE 1 and MEASURE 2 pivotal trials. Although Dr. Betts also used the matching adjusted indirect comparison approach and broadened his data source modestly, his results showed a distinctly different outcome: similar efficacy for the two drugs. Dr. Betts also included a cost efficacy analysis, and in this part adalimumab showed superior performance after he factored in the cost per responding AS patient.
During the combined discussion period following the two talks, both presenters defended the legitimacy of their approaches, although Dr. Maksymowych conceded that these indirect comparisons are “hypothesis generating rather than producing a definitive answer.” But a couple of active European AS researchers rose to comment from the floor and discredit the whole process.
“These two presentations show why I am not a proponent of indirect comparisons. The statistical models squeeze the data until they confess,” said Robert Landewé, MD, an AS specialist at the University of Amsterdam. “This is now a commercial rather than a scientific clash between two important drugs. I challenge these companies to perform a head-to-head trial. Indirect comparisons are not good,” he concluded, to a round of audience applause.
“There are so many methodological issues,” said Désirée van der Heijde, MD, another Dutch AS clinician and researcher who rose to critique both studies. “The only thing you can rely on is head-to-head trials.”
I later spoke with Dr. Maksymowych, and he expressed some pessimism about the prospects for a fully-powered, head-to-head trial of an interleukin-17 inhibitor and tumor necrosis factor inhibitor because it would need to enroll so many patients. “Randomized studies of active comparators need to be huge because it’s hard to show improvements when the response rates are high,” he said. Plus, he added, it isn’t entirely about a drug’s efficacy against AS spinal symptoms anyway.
“We also have to think about the impact of treatment on other aspects of this disease, such as psoriasis and colitis, as well as radiographic disease progression,” he said. These aspects of the activity of both classes of drugs have not received much study in AS patients until now.
In other words, the battle between treatment options for AS has just begun, and seems likely to be fought on many fronts.
On Twitter @mitchelzoler
Now that the interleukin-17 inhibitor secukinumab and tumor necrosis factor inhibitors are competing options for treatment of patients with ankylosing spondylitis, the companies that make those drugs must feel pressure to find some sort of advantage for their agents.
How else to explain the remarkable pair of similar post hoc analyses presented in June at the European Congress of Rheumatology in London? One of the analyses was funded by Novartis – the company that markets secukinumab (Cosentyx) – and included several Novartis employees as coauthors. The second study, presented immediately afterward in the main session at the meeting devoted to ankylosing spondylitis (AS) treatments, had backing from AbbVie, which markets adalimumab (Humira), the largest-selling tumor necrosis factor inhibitor worldwide, and had several AbbVie employees as coauthors.
Both analyses used a “matching adjusted indirect comparison,” a fairly new way to compare the performance of interventions studied in two totally independent trials by propensity matching patients from each of the two trials. It’s purportedly a way to make a legitimate comparison in the absence of head-to-head data.
Making the two reports even more surreal was their use of essentially the same data.
The first report came from Walter P. Maksymowych, MD, an AS clinician and researcher from the University of Alberta, who with his coauthors used data collected on secukinumab in the MEASURE 1 pivotal trial and on adalimumab in the ATLAS pivotal trial. He spent much of his presentation describing the methods behind the indirect comparison, and I don’t think I can be blamed for calling the results of this Novartis-sponsored analysis predictable: overall better performance by secukinumab, compared “indirectly” with adalimumab for clinical responses and patient quality of life.
The second report, the one sponsored by AbbVie, came from Keith A. Betts, PhD, a biostatistician who works for the Analysis Group, an international consulting firm. He also used the ATLAS database as the source for adalimumab outcomes, and differed marginally from Dr. Maksymowych by taking data on secukinumab patients from both the MEASURE 1 and MEASURE 2 pivotal trials. Although Dr. Betts also used the matching adjusted indirect comparison approach and broadened his data source modestly, his results showed a distinctly different outcome: similar efficacy for the two drugs. Dr. Betts also included a cost efficacy analysis, and in this part adalimumab showed superior performance after he factored in the cost per responding AS patient.
During the combined discussion period following the two talks, both presenters defended the legitimacy of their approaches, although Dr. Maksymowych conceded that these indirect comparisons are “hypothesis generating rather than producing a definitive answer.” But a couple of active European AS researchers rose to comment from the floor and discredit the whole process.
“These two presentations show why I am not a proponent of indirect comparisons. The statistical models squeeze the data until they confess,” said Robert Landewé, MD, an AS specialist at the University of Amsterdam. “This is now a commercial rather than a scientific clash between two important drugs. I challenge these companies to perform a head-to-head trial. Indirect comparisons are not good,” he concluded, to a round of audience applause.
“There are so many methodological issues,” said Désirée van der Heijde, MD, another Dutch AS clinician and researcher who rose to critique both studies. “The only thing you can rely on is head-to-head trials.”
I later spoke with Dr. Maksymowych, and he expressed some pessimism about the prospects for a fully-powered, head-to-head trial of an interleukin-17 inhibitor and tumor necrosis factor inhibitor because it would need to enroll so many patients. “Randomized studies of active comparators need to be huge because it’s hard to show improvements when the response rates are high,” he said. Plus, he added, it isn’t entirely about a drug’s efficacy against AS spinal symptoms anyway.
“We also have to think about the impact of treatment on other aspects of this disease, such as psoriasis and colitis, as well as radiographic disease progression,” he said. These aspects of the activity of both classes of drugs have not received much study in AS patients until now.
In other words, the battle between treatment options for AS has just begun, and seems likely to be fought on many fronts.
On Twitter @mitchelzoler
Now that the interleukin-17 inhibitor secukinumab and tumor necrosis factor inhibitors are competing options for treatment of patients with ankylosing spondylitis, the companies that make those drugs must feel pressure to find some sort of advantage for their agents.
How else to explain the remarkable pair of similar post hoc analyses presented in June at the European Congress of Rheumatology in London? One of the analyses was funded by Novartis – the company that markets secukinumab (Cosentyx) – and included several Novartis employees as coauthors. The second study, presented immediately afterward in the main session at the meeting devoted to ankylosing spondylitis (AS) treatments, had backing from AbbVie, which markets adalimumab (Humira), the largest-selling tumor necrosis factor inhibitor worldwide, and had several AbbVie employees as coauthors.
Both analyses used a “matching adjusted indirect comparison,” a fairly new way to compare the performance of interventions studied in two totally independent trials by propensity matching patients from each of the two trials. It’s purportedly a way to make a legitimate comparison in the absence of head-to-head data.
Making the two reports even more surreal was their use of essentially the same data.
The first report came from Walter P. Maksymowych, MD, an AS clinician and researcher from the University of Alberta, who with his coauthors used data collected on secukinumab in the MEASURE 1 pivotal trial and on adalimumab in the ATLAS pivotal trial. He spent much of his presentation describing the methods behind the indirect comparison, and I don’t think I can be blamed for calling the results of this Novartis-sponsored analysis predictable: overall better performance by secukinumab, compared “indirectly” with adalimumab for clinical responses and patient quality of life.
The second report, the one sponsored by AbbVie, came from Keith A. Betts, PhD, a biostatistician who works for the Analysis Group, an international consulting firm. He also used the ATLAS database as the source for adalimumab outcomes, and differed marginally from Dr. Maksymowych by taking data on secukinumab patients from both the MEASURE 1 and MEASURE 2 pivotal trials. Although Dr. Betts also used the matching adjusted indirect comparison approach and broadened his data source modestly, his results showed a distinctly different outcome: similar efficacy for the two drugs. Dr. Betts also included a cost efficacy analysis, and in this part adalimumab showed superior performance after he factored in the cost per responding AS patient.
During the combined discussion period following the two talks, both presenters defended the legitimacy of their approaches, although Dr. Maksymowych conceded that these indirect comparisons are “hypothesis generating rather than producing a definitive answer.” But a couple of active European AS researchers rose to comment from the floor and discredit the whole process.
“These two presentations show why I am not a proponent of indirect comparisons. The statistical models squeeze the data until they confess,” said Robert Landewé, MD, an AS specialist at the University of Amsterdam. “This is now a commercial rather than a scientific clash between two important drugs. I challenge these companies to perform a head-to-head trial. Indirect comparisons are not good,” he concluded, to a round of audience applause.
“There are so many methodological issues,” said Désirée van der Heijde, MD, another Dutch AS clinician and researcher who rose to critique both studies. “The only thing you can rely on is head-to-head trials.”
I later spoke with Dr. Maksymowych, and he expressed some pessimism about the prospects for a fully-powered, head-to-head trial of an interleukin-17 inhibitor and tumor necrosis factor inhibitor because it would need to enroll so many patients. “Randomized studies of active comparators need to be huge because it’s hard to show improvements when the response rates are high,” he said. Plus, he added, it isn’t entirely about a drug’s efficacy against AS spinal symptoms anyway.
“We also have to think about the impact of treatment on other aspects of this disease, such as psoriasis and colitis, as well as radiographic disease progression,” he said. These aspects of the activity of both classes of drugs have not received much study in AS patients until now.
In other words, the battle between treatment options for AS has just begun, and seems likely to be fought on many fronts.
On Twitter @mitchelzoler
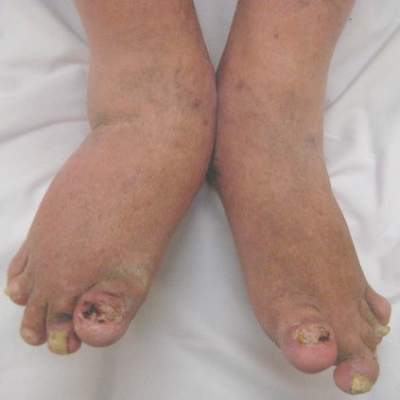
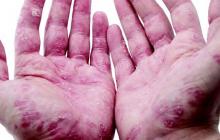
Analysis supports daily folate for children with psoriasis on methotrexate
SCOTTSDALE, ARIZ. – Children and adolescents receiving methotrexate for psoriasis were significantly less likely to experience gastrointestinal side effects when they took a folate supplement every day instead of once weekly or 6 days a week, in a retrospective study of more than 400 pediatric psoriasis patients.
Laboratory abnormalities were significantly more common among children who received a folate supplement 6 days per week rather than daily, noted Inge Bronckers of the department of dermatology, Radboud University, Nijmegen, the Netherlands. “These results support the use of daily folate” in this group of patients,” she said in a poster presentation at the annual meeting of the Society for Investigative Dermatology.
Few studies have examined patterns of use or adverse effects of pediatric psoriasis therapies. Although methotrexate is a folate antagonist with related toxicities, whether folate supplementation counteracts the efficacy of methotrexate is also unclear. Because of these uncertainties, some clinicians recommend a supplement 6 days per week, avoiding the day methotrexate is given, while others recommend it daily or once weekly.
To better understand the effects of these regimens, Ms. Inge and her coinvestigators studied 446 children and adolescents who received phototherapy or systemic treatments for moderate to severe psoriasis at 20 centers in the United States, Canada, and Europe between 1990 and 2014. The patients’ average age was 8 years (standard deviation, 4 years); 238 were female and 208 were male.
Among the 390 patients receiving systemic medications, almost 70% were receiving methotrexate, while 27% were being treated with etanercept or another biologic, 15% were using retinoids, 8% were using cyclosporine, and 5% were using fumaric acid. About 19% of patients were receiving more than one of these medications. Methotrexate most often led to nausea (affecting 18% of patients), elevated hepatic transaminases (13%), dyspepsia (7%), and infections (4%), usually of the skin and upper airways. In contrast, biologics most often caused injection-site reactions (19%) and upper airways infections (10%).
Most (253) of the 270 patients on methotrexate had been prescribed folic acid, typically at a dose of about 8 mg/wk, and nearly always in the form of pure folic acid, rather than a multivitamin. Of the patients taking folic acid, about 34% took it 6 days per week, 34% received it daily and 30% – including most patients in Europe – received it once weekly.
Notably, the odds of gastrointestinal side effects were 75% lower for patients who received folic acid daily or 6 days per week, compared with those who received folic acid once a week (odds ratio, 0.25, in both cases; P less than .001), the investigators found. However, laboratory abnormalities were significantly more likely when folic acid was given 6 days a week, compared with daily (OR, 2.31; P = .03) or weekly (OR, 3.9; P = .002). Patients in Europe, who usually received folic acid weekly, were significantly more likely to have methotrexate-related gastrointestinal side effects than were patients in North America (OR, 3.4; P less than .001), and were less likely to have laboratory abnormalities (OR, 0.32; P = .004).
Patients on biologic therapy were less likely to develop laboratory abnormalities or stop treatment because of side effects than were those on other systemic therapies, Ms. Inge and her associates found. Because methotrexate was associated with elevated liver enzymes, it also was dose adjusted more often than other therapies. No patient on any therapy was diagnosed with tuberculosis or malignancy, but three patients on methotrexate had severe adverse effects, including liver disease, methotrexate hypersensitivity pneumonitis, and severe personality changes. In contrast, fumarate was associated with one case each of pericarditis and bone marrow suppression, while one patient on the biologic adalimumab developed appendicitis.
The study underscores the need to monitor the long-term risks of pediatric psoriasis treatments, the researchers concluded. Data and lessons from the study are being used to develop a prospective pediatric psoriasis registry. “If industry joins forces to use this prospective international registry to capture prospective pediatric data, we will ensure early detection of safety signals and facilitate comparative analyses of efficacy and safety,” Ms. Inge said in the poster.
The International Psoriasis Council funded the study. The investigators did not list disclosures.
SCOTTSDALE, ARIZ. – Children and adolescents receiving methotrexate for psoriasis were significantly less likely to experience gastrointestinal side effects when they took a folate supplement every day instead of once weekly or 6 days a week, in a retrospective study of more than 400 pediatric psoriasis patients.
Laboratory abnormalities were significantly more common among children who received a folate supplement 6 days per week rather than daily, noted Inge Bronckers of the department of dermatology, Radboud University, Nijmegen, the Netherlands. “These results support the use of daily folate” in this group of patients,” she said in a poster presentation at the annual meeting of the Society for Investigative Dermatology.
Few studies have examined patterns of use or adverse effects of pediatric psoriasis therapies. Although methotrexate is a folate antagonist with related toxicities, whether folate supplementation counteracts the efficacy of methotrexate is also unclear. Because of these uncertainties, some clinicians recommend a supplement 6 days per week, avoiding the day methotrexate is given, while others recommend it daily or once weekly.
To better understand the effects of these regimens, Ms. Inge and her coinvestigators studied 446 children and adolescents who received phototherapy or systemic treatments for moderate to severe psoriasis at 20 centers in the United States, Canada, and Europe between 1990 and 2014. The patients’ average age was 8 years (standard deviation, 4 years); 238 were female and 208 were male.
Among the 390 patients receiving systemic medications, almost 70% were receiving methotrexate, while 27% were being treated with etanercept or another biologic, 15% were using retinoids, 8% were using cyclosporine, and 5% were using fumaric acid. About 19% of patients were receiving more than one of these medications. Methotrexate most often led to nausea (affecting 18% of patients), elevated hepatic transaminases (13%), dyspepsia (7%), and infections (4%), usually of the skin and upper airways. In contrast, biologics most often caused injection-site reactions (19%) and upper airways infections (10%).
Most (253) of the 270 patients on methotrexate had been prescribed folic acid, typically at a dose of about 8 mg/wk, and nearly always in the form of pure folic acid, rather than a multivitamin. Of the patients taking folic acid, about 34% took it 6 days per week, 34% received it daily and 30% – including most patients in Europe – received it once weekly.
Notably, the odds of gastrointestinal side effects were 75% lower for patients who received folic acid daily or 6 days per week, compared with those who received folic acid once a week (odds ratio, 0.25, in both cases; P less than .001), the investigators found. However, laboratory abnormalities were significantly more likely when folic acid was given 6 days a week, compared with daily (OR, 2.31; P = .03) or weekly (OR, 3.9; P = .002). Patients in Europe, who usually received folic acid weekly, were significantly more likely to have methotrexate-related gastrointestinal side effects than were patients in North America (OR, 3.4; P less than .001), and were less likely to have laboratory abnormalities (OR, 0.32; P = .004).
Patients on biologic therapy were less likely to develop laboratory abnormalities or stop treatment because of side effects than were those on other systemic therapies, Ms. Inge and her associates found. Because methotrexate was associated with elevated liver enzymes, it also was dose adjusted more often than other therapies. No patient on any therapy was diagnosed with tuberculosis or malignancy, but three patients on methotrexate had severe adverse effects, including liver disease, methotrexate hypersensitivity pneumonitis, and severe personality changes. In contrast, fumarate was associated with one case each of pericarditis and bone marrow suppression, while one patient on the biologic adalimumab developed appendicitis.
The study underscores the need to monitor the long-term risks of pediatric psoriasis treatments, the researchers concluded. Data and lessons from the study are being used to develop a prospective pediatric psoriasis registry. “If industry joins forces to use this prospective international registry to capture prospective pediatric data, we will ensure early detection of safety signals and facilitate comparative analyses of efficacy and safety,” Ms. Inge said in the poster.
The International Psoriasis Council funded the study. The investigators did not list disclosures.
SCOTTSDALE, ARIZ. – Children and adolescents receiving methotrexate for psoriasis were significantly less likely to experience gastrointestinal side effects when they took a folate supplement every day instead of once weekly or 6 days a week, in a retrospective study of more than 400 pediatric psoriasis patients.
Laboratory abnormalities were significantly more common among children who received a folate supplement 6 days per week rather than daily, noted Inge Bronckers of the department of dermatology, Radboud University, Nijmegen, the Netherlands. “These results support the use of daily folate” in this group of patients,” she said in a poster presentation at the annual meeting of the Society for Investigative Dermatology.
Few studies have examined patterns of use or adverse effects of pediatric psoriasis therapies. Although methotrexate is a folate antagonist with related toxicities, whether folate supplementation counteracts the efficacy of methotrexate is also unclear. Because of these uncertainties, some clinicians recommend a supplement 6 days per week, avoiding the day methotrexate is given, while others recommend it daily or once weekly.
To better understand the effects of these regimens, Ms. Inge and her coinvestigators studied 446 children and adolescents who received phototherapy or systemic treatments for moderate to severe psoriasis at 20 centers in the United States, Canada, and Europe between 1990 and 2014. The patients’ average age was 8 years (standard deviation, 4 years); 238 were female and 208 were male.
Among the 390 patients receiving systemic medications, almost 70% were receiving methotrexate, while 27% were being treated with etanercept or another biologic, 15% were using retinoids, 8% were using cyclosporine, and 5% were using fumaric acid. About 19% of patients were receiving more than one of these medications. Methotrexate most often led to nausea (affecting 18% of patients), elevated hepatic transaminases (13%), dyspepsia (7%), and infections (4%), usually of the skin and upper airways. In contrast, biologics most often caused injection-site reactions (19%) and upper airways infections (10%).
Most (253) of the 270 patients on methotrexate had been prescribed folic acid, typically at a dose of about 8 mg/wk, and nearly always in the form of pure folic acid, rather than a multivitamin. Of the patients taking folic acid, about 34% took it 6 days per week, 34% received it daily and 30% – including most patients in Europe – received it once weekly.
Notably, the odds of gastrointestinal side effects were 75% lower for patients who received folic acid daily or 6 days per week, compared with those who received folic acid once a week (odds ratio, 0.25, in both cases; P less than .001), the investigators found. However, laboratory abnormalities were significantly more likely when folic acid was given 6 days a week, compared with daily (OR, 2.31; P = .03) or weekly (OR, 3.9; P = .002). Patients in Europe, who usually received folic acid weekly, were significantly more likely to have methotrexate-related gastrointestinal side effects than were patients in North America (OR, 3.4; P less than .001), and were less likely to have laboratory abnormalities (OR, 0.32; P = .004).
Patients on biologic therapy were less likely to develop laboratory abnormalities or stop treatment because of side effects than were those on other systemic therapies, Ms. Inge and her associates found. Because methotrexate was associated with elevated liver enzymes, it also was dose adjusted more often than other therapies. No patient on any therapy was diagnosed with tuberculosis or malignancy, but three patients on methotrexate had severe adverse effects, including liver disease, methotrexate hypersensitivity pneumonitis, and severe personality changes. In contrast, fumarate was associated with one case each of pericarditis and bone marrow suppression, while one patient on the biologic adalimumab developed appendicitis.
The study underscores the need to monitor the long-term risks of pediatric psoriasis treatments, the researchers concluded. Data and lessons from the study are being used to develop a prospective pediatric psoriasis registry. “If industry joins forces to use this prospective international registry to capture prospective pediatric data, we will ensure early detection of safety signals and facilitate comparative analyses of efficacy and safety,” Ms. Inge said in the poster.
The International Psoriasis Council funded the study. The investigators did not list disclosures.
AT THE 2016 SID ANNUAL MEETING
Key clinical point: Consider daily folate to reduce the likelihood of gastrointestinal side effects of methotrexate in children with psoriasis.
Major finding: The odds of gastrointestinal adverse effects were about 75% lower with daily folate, compared with weekly dosing or 6 days per week dosing that spared the methotrexate day (odds ratio, 0.25; P less than .001).
Data source: An international retrospective study of 446 children receiving phototherapy or systemic therapy for psoriasis.
Disclosures: The International Psoriasis Council funded the study. The investigators did not list disclosures.
NSAID plus TNFi linked to less ankylosing spondylitis progression
LONDON – Patients with ankylosing spondylitis who remained on long-term treatment with a nonsteroidal anti-inflammatory drug and a tumor necrosis factor inhibitor had significantly less new spinal-bone formation in a cross-sectional analysis of a multicenter cohort of 527 U.S. patients.
A related analysis of the same cohort also showed significantly less ankylosing spondylitis (AS) progression as measured by radiographic progression among patients who received treatment with a tumor necrosis factor–alpha (TNF) inhibitor for 2.1-3.5 years regardless of their treatment with a nonsteroidal anti-inflammatory drug (NSAID), although this link trended to a stronger effect among the patients taking both, Lianne S. Gensler, MD, reported in a pair of posters at the European Congress of Rheumatology.
These finding suggest “there may be synergy between NSAIDs and TNF inhibitors [for slowing or preventing progression] in a select group of AS patients at high risk for progression,” said Dr. Gensler, a rheumatologist and director of the Ankylosing Spondylitis Clinic at the University of California, San Francisco.
But Dr. Gensler also cautioned that these findings are merely “hypothesis generating” and should not be used as a rationale to place or maintain AS patients on long-term treatment with an NSAID, a TNF inhibitor, or both drugs.
“You treat the disease burden. The message is absolutely not to always put AS patients on both types of drugs. When an AS patient is well controlled on a TNF inhibitor alone, I would not tell them to also take a NSAID,” she said in an interview. “This is only relevant for patients who require treatment with both drug classes because of their clinical status.”
As the list of treatment options for patients with AS grows – it now includes NSAIDs, TNF inhibitors, and the interleukin-17 inhibitor secukinumab (Cosentyx) – the impact of these agents on disease progression as assessed by radiography and new bone formation becomes a new dimension to start to consider in addition to the standard criterion of immediate clinical response, Dr. Gensler explained. AS progression “is another factor to think about as we decide on treatment strategies. There is growing evidence that long-term treatment with a tumor necrosis factor inhibitor and with a NSAID have potential roles in disease modification.” But the evidence is indirect, from cohort studies that make cause and effect assessments difficult because of possible unidentified confounding factors, she noted.
“There has never been a randomized, controlled trial examining the disease-modifying effects of a TNF inhibitor because you can’t keep patients on placebo for a long enough time to see this benefit,” Dr. Gensler said. It takes a long time to see progression in AS patients, she noted.
A prior cohort analysis run by Dr. Gensler and her associates found evidence for an effect of long-term treatment with a TNF inhibitor and reduced AS progression measured using the modified Stoke AS Spine Score (mSASSS), compared with AS patients not on a TNF inhibitor in a propensity-score matched analysis of 334 patients. A link between TNF inhibitor use and a discernible difference in mSASSS only occurred when patients were on TNF inhibitor treatment for at least 3.9 years (Arthritis Rheum. 2013 Oct;65[10]:2645-54). In addition, a separate report at the EULAR congress on 168 AS patients maintained on treatment with secukinumab for 2 years showed evidence for slowed progression of mSASSS scores, compared with historical controls as well as with similar patients who were not on secukinumab treatment for as long a period of time.
The new analysis reported by Dr. Gensler looked at 527 AS patients in the multicenter Prospective Study of Outcomes in AS cohort followed for a median of 3.7 years. Clinicians participating in this cohort saw patients every 6 months, and radiographic assessments by mSASSS and for new bone formation occurred every 2 years. At entry into the registry, about 57% of patients received a TNF inhibitor and about 63% received an NSAID, with a third on an NSAID only, 27% on a TNF inhibitor only, 30% on both drugs, and 10% receiving neither drug.
The analysis showed that among patients followed for 2.1-3.5 years, the fraction of patients on a TNF inhibitor who showed progression of their mSASSS was 77% lower than patients not on a TNF inhibitor, a statistically significant difference, Dr. Gensler reported. The researchers saw no statistically significant difference in mSASSS progression rates between the patients on a TNF inhibitor at baseline and those not on a TNF inhibitor at baseline among patients followed for 2 years and among those followed for more than 3.5 years, although the analysis did show nominally higher levels of response among TNF-inhibitor users followed for more than 3.5 years. Dr. Gensler speculated that one reason for the loss of a statistically significant difference during longer follow-up could be that fewer patients reached these levels of prolonged follow-up, making statistically significant differences harder to see. This analysis also showed a strong trend for less progression among the patients treated with an NSAID, a 51% relative reduction in mSASSS progression, compared with patients not taking an NSAID, but this relationship just missed statistical significance.
A second analysis by Dr. Gensler and her associates used data from the same cohort but focused on new bone formation during follow-up. This analysis again showed a statistically significant, 72% reduction in this outcome among patients taking a TNF inhibitor at baseline, compared with those not on a TNF inhibitor, when followed for 2.1-3.5 years, with no statistically significant relationship seen among patients followed for less or more time, Dr. Gensler reported. However, the results from this analysis also showed a statistically significant impact from NSAID treatment: Patients on a TNF inhibitor and an NSAID at baseline had 67% less new bone formation, compared with those who received a TNF inhibitor but were not on an NSAID at baseline.
Dr. Gensler has been a consultant to or investigator funded by AbbVie, Amgen, Janssen, Novartis, and UCB. The Prospective Study of Outcomes in Ankylosing Spondylitis receives no commercial support.
On Twitter @mitchelzoler
LONDON – Patients with ankylosing spondylitis who remained on long-term treatment with a nonsteroidal anti-inflammatory drug and a tumor necrosis factor inhibitor had significantly less new spinal-bone formation in a cross-sectional analysis of a multicenter cohort of 527 U.S. patients.
A related analysis of the same cohort also showed significantly less ankylosing spondylitis (AS) progression as measured by radiographic progression among patients who received treatment with a tumor necrosis factor–alpha (TNF) inhibitor for 2.1-3.5 years regardless of their treatment with a nonsteroidal anti-inflammatory drug (NSAID), although this link trended to a stronger effect among the patients taking both, Lianne S. Gensler, MD, reported in a pair of posters at the European Congress of Rheumatology.
These finding suggest “there may be synergy between NSAIDs and TNF inhibitors [for slowing or preventing progression] in a select group of AS patients at high risk for progression,” said Dr. Gensler, a rheumatologist and director of the Ankylosing Spondylitis Clinic at the University of California, San Francisco.
But Dr. Gensler also cautioned that these findings are merely “hypothesis generating” and should not be used as a rationale to place or maintain AS patients on long-term treatment with an NSAID, a TNF inhibitor, or both drugs.
“You treat the disease burden. The message is absolutely not to always put AS patients on both types of drugs. When an AS patient is well controlled on a TNF inhibitor alone, I would not tell them to also take a NSAID,” she said in an interview. “This is only relevant for patients who require treatment with both drug classes because of their clinical status.”
As the list of treatment options for patients with AS grows – it now includes NSAIDs, TNF inhibitors, and the interleukin-17 inhibitor secukinumab (Cosentyx) – the impact of these agents on disease progression as assessed by radiography and new bone formation becomes a new dimension to start to consider in addition to the standard criterion of immediate clinical response, Dr. Gensler explained. AS progression “is another factor to think about as we decide on treatment strategies. There is growing evidence that long-term treatment with a tumor necrosis factor inhibitor and with a NSAID have potential roles in disease modification.” But the evidence is indirect, from cohort studies that make cause and effect assessments difficult because of possible unidentified confounding factors, she noted.
“There has never been a randomized, controlled trial examining the disease-modifying effects of a TNF inhibitor because you can’t keep patients on placebo for a long enough time to see this benefit,” Dr. Gensler said. It takes a long time to see progression in AS patients, she noted.
A prior cohort analysis run by Dr. Gensler and her associates found evidence for an effect of long-term treatment with a TNF inhibitor and reduced AS progression measured using the modified Stoke AS Spine Score (mSASSS), compared with AS patients not on a TNF inhibitor in a propensity-score matched analysis of 334 patients. A link between TNF inhibitor use and a discernible difference in mSASSS only occurred when patients were on TNF inhibitor treatment for at least 3.9 years (Arthritis Rheum. 2013 Oct;65[10]:2645-54). In addition, a separate report at the EULAR congress on 168 AS patients maintained on treatment with secukinumab for 2 years showed evidence for slowed progression of mSASSS scores, compared with historical controls as well as with similar patients who were not on secukinumab treatment for as long a period of time.
The new analysis reported by Dr. Gensler looked at 527 AS patients in the multicenter Prospective Study of Outcomes in AS cohort followed for a median of 3.7 years. Clinicians participating in this cohort saw patients every 6 months, and radiographic assessments by mSASSS and for new bone formation occurred every 2 years. At entry into the registry, about 57% of patients received a TNF inhibitor and about 63% received an NSAID, with a third on an NSAID only, 27% on a TNF inhibitor only, 30% on both drugs, and 10% receiving neither drug.
The analysis showed that among patients followed for 2.1-3.5 years, the fraction of patients on a TNF inhibitor who showed progression of their mSASSS was 77% lower than patients not on a TNF inhibitor, a statistically significant difference, Dr. Gensler reported. The researchers saw no statistically significant difference in mSASSS progression rates between the patients on a TNF inhibitor at baseline and those not on a TNF inhibitor at baseline among patients followed for 2 years and among those followed for more than 3.5 years, although the analysis did show nominally higher levels of response among TNF-inhibitor users followed for more than 3.5 years. Dr. Gensler speculated that one reason for the loss of a statistically significant difference during longer follow-up could be that fewer patients reached these levels of prolonged follow-up, making statistically significant differences harder to see. This analysis also showed a strong trend for less progression among the patients treated with an NSAID, a 51% relative reduction in mSASSS progression, compared with patients not taking an NSAID, but this relationship just missed statistical significance.
A second analysis by Dr. Gensler and her associates used data from the same cohort but focused on new bone formation during follow-up. This analysis again showed a statistically significant, 72% reduction in this outcome among patients taking a TNF inhibitor at baseline, compared with those not on a TNF inhibitor, when followed for 2.1-3.5 years, with no statistically significant relationship seen among patients followed for less or more time, Dr. Gensler reported. However, the results from this analysis also showed a statistically significant impact from NSAID treatment: Patients on a TNF inhibitor and an NSAID at baseline had 67% less new bone formation, compared with those who received a TNF inhibitor but were not on an NSAID at baseline.
Dr. Gensler has been a consultant to or investigator funded by AbbVie, Amgen, Janssen, Novartis, and UCB. The Prospective Study of Outcomes in Ankylosing Spondylitis receives no commercial support.
On Twitter @mitchelzoler
LONDON – Patients with ankylosing spondylitis who remained on long-term treatment with a nonsteroidal anti-inflammatory drug and a tumor necrosis factor inhibitor had significantly less new spinal-bone formation in a cross-sectional analysis of a multicenter cohort of 527 U.S. patients.
A related analysis of the same cohort also showed significantly less ankylosing spondylitis (AS) progression as measured by radiographic progression among patients who received treatment with a tumor necrosis factor–alpha (TNF) inhibitor for 2.1-3.5 years regardless of their treatment with a nonsteroidal anti-inflammatory drug (NSAID), although this link trended to a stronger effect among the patients taking both, Lianne S. Gensler, MD, reported in a pair of posters at the European Congress of Rheumatology.
These finding suggest “there may be synergy between NSAIDs and TNF inhibitors [for slowing or preventing progression] in a select group of AS patients at high risk for progression,” said Dr. Gensler, a rheumatologist and director of the Ankylosing Spondylitis Clinic at the University of California, San Francisco.
But Dr. Gensler also cautioned that these findings are merely “hypothesis generating” and should not be used as a rationale to place or maintain AS patients on long-term treatment with an NSAID, a TNF inhibitor, or both drugs.
“You treat the disease burden. The message is absolutely not to always put AS patients on both types of drugs. When an AS patient is well controlled on a TNF inhibitor alone, I would not tell them to also take a NSAID,” she said in an interview. “This is only relevant for patients who require treatment with both drug classes because of their clinical status.”
As the list of treatment options for patients with AS grows – it now includes NSAIDs, TNF inhibitors, and the interleukin-17 inhibitor secukinumab (Cosentyx) – the impact of these agents on disease progression as assessed by radiography and new bone formation becomes a new dimension to start to consider in addition to the standard criterion of immediate clinical response, Dr. Gensler explained. AS progression “is another factor to think about as we decide on treatment strategies. There is growing evidence that long-term treatment with a tumor necrosis factor inhibitor and with a NSAID have potential roles in disease modification.” But the evidence is indirect, from cohort studies that make cause and effect assessments difficult because of possible unidentified confounding factors, she noted.
“There has never been a randomized, controlled trial examining the disease-modifying effects of a TNF inhibitor because you can’t keep patients on placebo for a long enough time to see this benefit,” Dr. Gensler said. It takes a long time to see progression in AS patients, she noted.
A prior cohort analysis run by Dr. Gensler and her associates found evidence for an effect of long-term treatment with a TNF inhibitor and reduced AS progression measured using the modified Stoke AS Spine Score (mSASSS), compared with AS patients not on a TNF inhibitor in a propensity-score matched analysis of 334 patients. A link between TNF inhibitor use and a discernible difference in mSASSS only occurred when patients were on TNF inhibitor treatment for at least 3.9 years (Arthritis Rheum. 2013 Oct;65[10]:2645-54). In addition, a separate report at the EULAR congress on 168 AS patients maintained on treatment with secukinumab for 2 years showed evidence for slowed progression of mSASSS scores, compared with historical controls as well as with similar patients who were not on secukinumab treatment for as long a period of time.
The new analysis reported by Dr. Gensler looked at 527 AS patients in the multicenter Prospective Study of Outcomes in AS cohort followed for a median of 3.7 years. Clinicians participating in this cohort saw patients every 6 months, and radiographic assessments by mSASSS and for new bone formation occurred every 2 years. At entry into the registry, about 57% of patients received a TNF inhibitor and about 63% received an NSAID, with a third on an NSAID only, 27% on a TNF inhibitor only, 30% on both drugs, and 10% receiving neither drug.
The analysis showed that among patients followed for 2.1-3.5 years, the fraction of patients on a TNF inhibitor who showed progression of their mSASSS was 77% lower than patients not on a TNF inhibitor, a statistically significant difference, Dr. Gensler reported. The researchers saw no statistically significant difference in mSASSS progression rates between the patients on a TNF inhibitor at baseline and those not on a TNF inhibitor at baseline among patients followed for 2 years and among those followed for more than 3.5 years, although the analysis did show nominally higher levels of response among TNF-inhibitor users followed for more than 3.5 years. Dr. Gensler speculated that one reason for the loss of a statistically significant difference during longer follow-up could be that fewer patients reached these levels of prolonged follow-up, making statistically significant differences harder to see. This analysis also showed a strong trend for less progression among the patients treated with an NSAID, a 51% relative reduction in mSASSS progression, compared with patients not taking an NSAID, but this relationship just missed statistical significance.
A second analysis by Dr. Gensler and her associates used data from the same cohort but focused on new bone formation during follow-up. This analysis again showed a statistically significant, 72% reduction in this outcome among patients taking a TNF inhibitor at baseline, compared with those not on a TNF inhibitor, when followed for 2.1-3.5 years, with no statistically significant relationship seen among patients followed for less or more time, Dr. Gensler reported. However, the results from this analysis also showed a statistically significant impact from NSAID treatment: Patients on a TNF inhibitor and an NSAID at baseline had 67% less new bone formation, compared with those who received a TNF inhibitor but were not on an NSAID at baseline.
Dr. Gensler has been a consultant to or investigator funded by AbbVie, Amgen, Janssen, Novartis, and UCB. The Prospective Study of Outcomes in Ankylosing Spondylitis receives no commercial support.
On Twitter @mitchelzoler
AT THE EULAR 2016 CONGRESS
Key clinical point: Ankylosing spondylitis patients treated with a TNF inhibitor plus an NSAID had the lowest level of new bone formation during treatment for more than 2 years.
Major finding: Patients on a TNF inhibitor and an NSAID had 67% less new bone formation, compared with patients not on an NSAID.
Data source: Cross-sectional cohort study of 527 patients with ankylosing spondylitis enrolled in the Prospective Study of Outcomes in Ankylosing Spondylitis.
Disclosures: Dr. Gensler has been a consultant to or investigator funded by AbbVie, Amgen, Janssen, Novartis, and UCB. The Prospective Study of Outcomes in Ankylosing Spondylitis receives no commercial support.
NSAID plus TNFi linked to less ankylosing spondylitis progression
LONDON – Patients with ankylosing spondylitis who remained on long-term treatment with a nonsteroidal anti-inflammatory drug and a tumor necrosis factor inhibitor had significantly less new spinal-bone formation in a cross-sectional analysis of a multicenter cohort of 527 U.S. patients.
A related analysis of the same cohort also showed significantly less ankylosing spondylitis (AS) progression as measured by radiographic progression among patients who received treatment with a tumor necrosis factor–alpha (TNF) inhibitor for 2.1-3.5 years regardless of their treatment with a nonsteroidal anti-inflammatory drug (NSAID), although this link trended to a stronger effect among the patients taking both, Lianne S. Gensler, MD, reported in a pair of posters at the European Congress of Rheumatology.
These finding suggest “there may be synergy between NSAIDs and TNF inhibitors [for slowing or preventing progression] in a select group of AS patients at high risk for progression,” said Dr. Gensler, a rheumatologist and director of the Ankylosing Spondylitis Clinic at the University of California, San Francisco.
But Dr. Gensler also cautioned that these findings are merely “hypothesis generating” and should not be used as a rationale to place or maintain AS patients on long-term treatment with an NSAID, a TNF inhibitor, or both drugs.
“You treat the disease burden. The message is absolutely not to always put AS patients on both types of drugs. When an AS patient is well controlled on a TNF inhibitor alone, I would not tell them to also take a NSAID,” she said in an interview. “This is only relevant for patients who require treatment with both drug classes because of their clinical status.”
As the list of treatment options for patients with AS grows – it now includes NSAIDs, TNF inhibitors, and the interleukin-17 inhibitor secukinumab (Cosentyx) – the impact of these agents on disease progression as assessed by radiography and new bone formation becomes a new dimension to start to consider in addition to the standard criterion of immediate clinical response, Dr. Gensler explained. AS progression “is another factor to think about as we decide on treatment strategies. There is growing evidence that long-term treatment with a tumor necrosis factor inhibitor and with a NSAID have potential roles in disease modification.” But the evidence is indirect, from cohort studies that make cause and effect assessments difficult because of possible unidentified confounding factors, she noted.
“There has never been a randomized, controlled trial examining the disease-modifying effects of a TNF inhibitor because you can’t keep patients on placebo for a long enough time to see this benefit,” Dr. Gensler said. It takes a long time to see progression in AS patients, she noted.
A prior cohort analysis run by Dr. Gensler and her associates found evidence for an effect of long-term treatment with a TNF inhibitor and reduced AS progression measured using the modified Stoke AS Spine Score (mSASSS), compared with AS patients not on a TNF inhibitor in a propensity-score matched analysis of 334 patients. A link between TNF inhibitor use and a discernible difference in mSASSS only occurred when patients were on TNF inhibitor treatment for at least 3.9 years (Arthritis Rheum. 2013 Oct;65[10]:2645-54). In addition, a separate report at the EULAR congress on 168 AS patients maintained on treatment with secukinumab for 2 years showed evidence for slowed progression of mSASSS scores, compared with historical controls as well as with similar patients who were not on secukinumab treatment for as long a period of time.
The new analysis reported by Dr. Gensler looked at 527 AS patients in the multicenter Prospective Study of Outcomes in AS cohort followed for a median of 3.7 years. Clinicians participating in this cohort saw patients every 6 months, and radiographic assessments by mSASSS and for new bone formation occurred every 2 years. At entry into the registry, about 57% of patients received a TNF inhibitor and about 63% received an NSAID, with a third on an NSAID only, 27% on a TNF inhibitor only, 30% on both drugs, and 10% receiving neither drug.
The analysis showed that among patients followed for 2.1-3.5 years, the fraction of patients on a TNF inhibitor who showed progression of their mSASSS was 77% lower than patients not on a TNF inhibitor, a statistically significant difference, Dr. Gensler reported. The researchers saw no statistically significant difference in mSASSS progression rates between the patients on a TNF inhibitor at baseline and those not on a TNF inhibitor at baseline among patients followed for 2 years and among those followed for more than 3.5 years, although the analysis did show nominally higher levels of response among TNF-inhibitor users followed for more than 3.5 years. Dr. Gensler speculated that one reason for the loss of a statistically significant difference during longer follow-up could be that fewer patients reached these levels of prolonged follow-up, making statistically significant differences harder to see. This analysis also showed a strong trend for less progression among the patients treated with an NSAID, a 51% relative reduction in mSASSS progression, compared with patients not taking an NSAID, but this relationship just missed statistical significance.
A second analysis by Dr. Gensler and her associates used data from the same cohort but focused on new bone formation during follow-up. This analysis again showed a statistically significant, 72% reduction in this outcome among patients taking a TNF inhibitor at baseline, compared with those not on a TNF inhibitor, when followed for 2.1-3.5 years, with no statistically significant relationship seen among patients followed for less or more time, Dr. Gensler reported. However, the results from this analysis also showed a statistically significant impact from NSAID treatment: Patients on a TNF inhibitor and an NSAID at baseline had 67% less new bone formation, compared with those who received a TNF inhibitor but were not on an NSAID at baseline.
Dr. Gensler has been a consultant to or investigator funded by AbbVie, Amgen, Janssen, Novartis, and UCB. The Prospective Study of Outcomes in Ankylosing Spondylitis receives no commercial support.
On Twitter @mitchelzoler
LONDON – Patients with ankylosing spondylitis who remained on long-term treatment with a nonsteroidal anti-inflammatory drug and a tumor necrosis factor inhibitor had significantly less new spinal-bone formation in a cross-sectional analysis of a multicenter cohort of 527 U.S. patients.
A related analysis of the same cohort also showed significantly less ankylosing spondylitis (AS) progression as measured by radiographic progression among patients who received treatment with a tumor necrosis factor–alpha (TNF) inhibitor for 2.1-3.5 years regardless of their treatment with a nonsteroidal anti-inflammatory drug (NSAID), although this link trended to a stronger effect among the patients taking both, Lianne S. Gensler, MD, reported in a pair of posters at the European Congress of Rheumatology.
These finding suggest “there may be synergy between NSAIDs and TNF inhibitors [for slowing or preventing progression] in a select group of AS patients at high risk for progression,” said Dr. Gensler, a rheumatologist and director of the Ankylosing Spondylitis Clinic at the University of California, San Francisco.
But Dr. Gensler also cautioned that these findings are merely “hypothesis generating” and should not be used as a rationale to place or maintain AS patients on long-term treatment with an NSAID, a TNF inhibitor, or both drugs.
“You treat the disease burden. The message is absolutely not to always put AS patients on both types of drugs. When an AS patient is well controlled on a TNF inhibitor alone, I would not tell them to also take a NSAID,” she said in an interview. “This is only relevant for patients who require treatment with both drug classes because of their clinical status.”
As the list of treatment options for patients with AS grows – it now includes NSAIDs, TNF inhibitors, and the interleukin-17 inhibitor secukinumab (Cosentyx) – the impact of these agents on disease progression as assessed by radiography and new bone formation becomes a new dimension to start to consider in addition to the standard criterion of immediate clinical response, Dr. Gensler explained. AS progression “is another factor to think about as we decide on treatment strategies. There is growing evidence that long-term treatment with a tumor necrosis factor inhibitor and with a NSAID have potential roles in disease modification.” But the evidence is indirect, from cohort studies that make cause and effect assessments difficult because of possible unidentified confounding factors, she noted.
“There has never been a randomized, controlled trial examining the disease-modifying effects of a TNF inhibitor because you can’t keep patients on placebo for a long enough time to see this benefit,” Dr. Gensler said. It takes a long time to see progression in AS patients, she noted.
A prior cohort analysis run by Dr. Gensler and her associates found evidence for an effect of long-term treatment with a TNF inhibitor and reduced AS progression measured using the modified Stoke AS Spine Score (mSASSS), compared with AS patients not on a TNF inhibitor in a propensity-score matched analysis of 334 patients. A link between TNF inhibitor use and a discernible difference in mSASSS only occurred when patients were on TNF inhibitor treatment for at least 3.9 years (Arthritis Rheum. 2013 Oct;65[10]:2645-54). In addition, a separate report at the EULAR congress on 168 AS patients maintained on treatment with secukinumab for 2 years showed evidence for slowed progression of mSASSS scores, compared with historical controls as well as with similar patients who were not on secukinumab treatment for as long a period of time.
The new analysis reported by Dr. Gensler looked at 527 AS patients in the multicenter Prospective Study of Outcomes in AS cohort followed for a median of 3.7 years. Clinicians participating in this cohort saw patients every 6 months, and radiographic assessments by mSASSS and for new bone formation occurred every 2 years. At entry into the registry, about 57% of patients received a TNF inhibitor and about 63% received an NSAID, with a third on an NSAID only, 27% on a TNF inhibitor only, 30% on both drugs, and 10% receiving neither drug.
The analysis showed that among patients followed for 2.1-3.5 years, the fraction of patients on a TNF inhibitor who showed progression of their mSASSS was 77% lower than patients not on a TNF inhibitor, a statistically significant difference, Dr. Gensler reported. The researchers saw no statistically significant difference in mSASSS progression rates between the patients on a TNF inhibitor at baseline and those not on a TNF inhibitor at baseline among patients followed for 2 years and among those followed for more than 3.5 years, although the analysis did show nominally higher levels of response among TNF-inhibitor users followed for more than 3.5 years. Dr. Gensler speculated that one reason for the loss of a statistically significant difference during longer follow-up could be that fewer patients reached these levels of prolonged follow-up, making statistically significant differences harder to see. This analysis also showed a strong trend for less progression among the patients treated with an NSAID, a 51% relative reduction in mSASSS progression, compared with patients not taking an NSAID, but this relationship just missed statistical significance.
A second analysis by Dr. Gensler and her associates used data from the same cohort but focused on new bone formation during follow-up. This analysis again showed a statistically significant, 72% reduction in this outcome among patients taking a TNF inhibitor at baseline, compared with those not on a TNF inhibitor, when followed for 2.1-3.5 years, with no statistically significant relationship seen among patients followed for less or more time, Dr. Gensler reported. However, the results from this analysis also showed a statistically significant impact from NSAID treatment: Patients on a TNF inhibitor and an NSAID at baseline had 67% less new bone formation, compared with those who received a TNF inhibitor but were not on an NSAID at baseline.
Dr. Gensler has been a consultant to or investigator funded by AbbVie, Amgen, Janssen, Novartis, and UCB. The Prospective Study of Outcomes in Ankylosing Spondylitis receives no commercial support.
On Twitter @mitchelzoler
LONDON – Patients with ankylosing spondylitis who remained on long-term treatment with a nonsteroidal anti-inflammatory drug and a tumor necrosis factor inhibitor had significantly less new spinal-bone formation in a cross-sectional analysis of a multicenter cohort of 527 U.S. patients.
A related analysis of the same cohort also showed significantly less ankylosing spondylitis (AS) progression as measured by radiographic progression among patients who received treatment with a tumor necrosis factor–alpha (TNF) inhibitor for 2.1-3.5 years regardless of their treatment with a nonsteroidal anti-inflammatory drug (NSAID), although this link trended to a stronger effect among the patients taking both, Lianne S. Gensler, MD, reported in a pair of posters at the European Congress of Rheumatology.
These finding suggest “there may be synergy between NSAIDs and TNF inhibitors [for slowing or preventing progression] in a select group of AS patients at high risk for progression,” said Dr. Gensler, a rheumatologist and director of the Ankylosing Spondylitis Clinic at the University of California, San Francisco.
But Dr. Gensler also cautioned that these findings are merely “hypothesis generating” and should not be used as a rationale to place or maintain AS patients on long-term treatment with an NSAID, a TNF inhibitor, or both drugs.
“You treat the disease burden. The message is absolutely not to always put AS patients on both types of drugs. When an AS patient is well controlled on a TNF inhibitor alone, I would not tell them to also take a NSAID,” she said in an interview. “This is only relevant for patients who require treatment with both drug classes because of their clinical status.”
As the list of treatment options for patients with AS grows – it now includes NSAIDs, TNF inhibitors, and the interleukin-17 inhibitor secukinumab (Cosentyx) – the impact of these agents on disease progression as assessed by radiography and new bone formation becomes a new dimension to start to consider in addition to the standard criterion of immediate clinical response, Dr. Gensler explained. AS progression “is another factor to think about as we decide on treatment strategies. There is growing evidence that long-term treatment with a tumor necrosis factor inhibitor and with a NSAID have potential roles in disease modification.” But the evidence is indirect, from cohort studies that make cause and effect assessments difficult because of possible unidentified confounding factors, she noted.
“There has never been a randomized, controlled trial examining the disease-modifying effects of a TNF inhibitor because you can’t keep patients on placebo for a long enough time to see this benefit,” Dr. Gensler said. It takes a long time to see progression in AS patients, she noted.
A prior cohort analysis run by Dr. Gensler and her associates found evidence for an effect of long-term treatment with a TNF inhibitor and reduced AS progression measured using the modified Stoke AS Spine Score (mSASSS), compared with AS patients not on a TNF inhibitor in a propensity-score matched analysis of 334 patients. A link between TNF inhibitor use and a discernible difference in mSASSS only occurred when patients were on TNF inhibitor treatment for at least 3.9 years (Arthritis Rheum. 2013 Oct;65[10]:2645-54). In addition, a separate report at the EULAR congress on 168 AS patients maintained on treatment with secukinumab for 2 years showed evidence for slowed progression of mSASSS scores, compared with historical controls as well as with similar patients who were not on secukinumab treatment for as long a period of time.
The new analysis reported by Dr. Gensler looked at 527 AS patients in the multicenter Prospective Study of Outcomes in AS cohort followed for a median of 3.7 years. Clinicians participating in this cohort saw patients every 6 months, and radiographic assessments by mSASSS and for new bone formation occurred every 2 years. At entry into the registry, about 57% of patients received a TNF inhibitor and about 63% received an NSAID, with a third on an NSAID only, 27% on a TNF inhibitor only, 30% on both drugs, and 10% receiving neither drug.
The analysis showed that among patients followed for 2.1-3.5 years, the fraction of patients on a TNF inhibitor who showed progression of their mSASSS was 77% lower than patients not on a TNF inhibitor, a statistically significant difference, Dr. Gensler reported. The researchers saw no statistically significant difference in mSASSS progression rates between the patients on a TNF inhibitor at baseline and those not on a TNF inhibitor at baseline among patients followed for 2 years and among those followed for more than 3.5 years, although the analysis did show nominally higher levels of response among TNF-inhibitor users followed for more than 3.5 years. Dr. Gensler speculated that one reason for the loss of a statistically significant difference during longer follow-up could be that fewer patients reached these levels of prolonged follow-up, making statistically significant differences harder to see. This analysis also showed a strong trend for less progression among the patients treated with an NSAID, a 51% relative reduction in mSASSS progression, compared with patients not taking an NSAID, but this relationship just missed statistical significance.
A second analysis by Dr. Gensler and her associates used data from the same cohort but focused on new bone formation during follow-up. This analysis again showed a statistically significant, 72% reduction in this outcome among patients taking a TNF inhibitor at baseline, compared with those not on a TNF inhibitor, when followed for 2.1-3.5 years, with no statistically significant relationship seen among patients followed for less or more time, Dr. Gensler reported. However, the results from this analysis also showed a statistically significant impact from NSAID treatment: Patients on a TNF inhibitor and an NSAID at baseline had 67% less new bone formation, compared with those who received a TNF inhibitor but were not on an NSAID at baseline.
Dr. Gensler has been a consultant to or investigator funded by AbbVie, Amgen, Janssen, Novartis, and UCB. The Prospective Study of Outcomes in Ankylosing Spondylitis receives no commercial support.
On Twitter @mitchelzoler
AT THE EULAR 2016 CONGRESS
Key clinical point: Ankylosing spondylitis patients treated with a TNF inhibitor plus an NSAID had the lowest level of new bone formation during treatment for more than 2 years.
Major finding: Patients on a TNF inhibitor and an NSAID had 67% less new bone formation, compared with patients not on an NSAID.
Data source: Cross-sectional cohort study of 527 patients with ankylosing spondylitis enrolled in the Prospective Study of Outcomes in Ankylosing Spondylitis.
Disclosures: Dr. Gensler has been a consultant to or investigator funded by AbbVie, Amgen, Janssen, Novartis, and UCB. The Prospective Study of Outcomes in Ankylosing Spondylitis receives no commercial support.
Obesity may attenuate anti-TNF response in psoriatic arthritis
LONDON – Patients with psoriatic arthritis appear less likely to achieve a good response to their first anti–tumor necrosis factor (anti-TNF) therapy if they are obese, according to data taken from two Nordic registries.
In a large observational cohort study, obese individuals with psoriatic arthritis (PsA) were significantly less likely than their nonobese counterparts to achieve a European League Against Rheumatism (EULAR) good or moderate response at 6 months (55% vs. 65%, P = .02). The overall odds ratio for achieving a good or moderate response was 0.47 when comparing obese with nonobese individuals.
The findings are potentially important because, with the exception of infliximab, anti-TNF therapy is not currently adjusted according to body weight, said presenting study author Pil Højgaard in an interview at the European Congress of Rheumatology.
Ms. Højgaard, who is an M.D. Ph.D. student at the department of rheumatology, Copenhagen University Hospital Gentofte, Rigshospitalet and the Parker Institute in Copenhagen, noted that obesity was a frequent comorbid condition in patients with PsA and that it is a known proinflammatory condition. As such, obesity could potentially affect immunologic processes, the pharmacokinetics of treatments, and ultimately patient outcomes.
Since TNF-alpha inhibitor (TNFi) treatment fails in around half of all patients with PsA treated in routine care, Ms. Højgaard noted that the aim of the cohort study was to investigate whether obesity could be having any influence on this.
Data on baseline characteristics, EULAR response rates, and drug adherence were obtained for 1,943 patients with PsA prescribed their anti-TNF therapy from two nationwide registries of disease-modifying therapies being used to treat rheumatic conditions in Denmark and Iceland, DANBIO (Rheumatology. 2011;50:69–77) and ICEBIO, respectively.
At baseline, body mass index (BMI) data were available for 1,271 patients and 408 (32%) of these had a BMI of 30 kg/m2 or more and were classed as being obese. The majority (39%) had received a first prescription for adalimumab, with around a quarter each prescribed etanercept (26%) or infliximab (24%), and the remainder prescribed golimumab (7%) and certolizumab (4%).
Compared to the 863 (68%) nonobese individuals, the obese patients were older (47 vs. 49 years, P = .01), less likely to smoke (30% vs. 23%, P = .01), and had higher disease activity measured on the Disease Activity Score 28 (DAS28) (4.4 vs. 4.6, P = .01). Health Assessment Questionnaire scores were also higher in obese than in nonobese individuals (1.1 vs. 0.9, P less than .01), and there were higher tender joint counts (6 vs. 5, P = .01), and higher pain levels assessed on a visual analog scale (VAS). Obese patients also had higher scores on a VAS patient global scale. The median follow-up time was 1.5 years.
Patients who were obese were found to adhere to TNFi treatment for shorter periods of time than nonobese patients, with median durations of 1.76 and 3.08 years, respectively (P less than .001). This discrepancy was most pronounced among men, a finding that may account for the fact that they were less likely to achieve a good EULAR response than their nonobese counterparts (OR = 0.5).
Being obese versus not being obese independently predicted TNFi withdrawal overall (hazard ratio, 1.6), especially in men (HR, 1.8; HR, 1.5 in women). TNFi withdrawal was more likely in obese than in nonobese patients even when individual treatments were considered; adalimumab: HR, 1.6; etanercept: HR, 2.0; infliximab: HR, 1.6.
An association between obesity and reduced response to anti-TNF therapy has also been observed in patients with rheumatoid arthritis, Ms. Højgaard acknowledged. There have also been a few studies of PsA and psoriasis “but to my knowledge, I think in the field of psoriatic arthritis, we are one of the few that have been looking at long-time drug survival,” she said. “We also include quite a lot of patients.”
“Of course this is not a randomized clinical study, so there could be residual confounding factors,” Ms. Højgaard cautioned. “It is always a bit difficult to say something about causality when it is a database study,” she added. “I think what we can see here is that there is an association, but in order to recommend weight loss we need some prospective studies.”
She noted that there was one published clinical study (Ann Rheum Dis. 2014;73:1157–62) that had looked at the benefit of a weight reduction program started at the same time as TNFi initiation in patients with PsA. This found there was a benefit of weight loss on response to TNFis, regardless of the type of diet.
DANBIO is supported by unrestricted grants from Abbott, Pfizer, MSD, Bristol-Myers Squibb, Roche, and UCB-Nordic. The sponsors have had no influence on data collection, analysis, or publication. ICEBIO is part of the electronic medical record system held by the University of Reykjavik and receives no industrial funding. Ms. Højgaard has received speaking fees from Celgene and UCB not related to this work.
LONDON – Patients with psoriatic arthritis appear less likely to achieve a good response to their first anti–tumor necrosis factor (anti-TNF) therapy if they are obese, according to data taken from two Nordic registries.
In a large observational cohort study, obese individuals with psoriatic arthritis (PsA) were significantly less likely than their nonobese counterparts to achieve a European League Against Rheumatism (EULAR) good or moderate response at 6 months (55% vs. 65%, P = .02). The overall odds ratio for achieving a good or moderate response was 0.47 when comparing obese with nonobese individuals.
The findings are potentially important because, with the exception of infliximab, anti-TNF therapy is not currently adjusted according to body weight, said presenting study author Pil Højgaard in an interview at the European Congress of Rheumatology.
Ms. Højgaard, who is an M.D. Ph.D. student at the department of rheumatology, Copenhagen University Hospital Gentofte, Rigshospitalet and the Parker Institute in Copenhagen, noted that obesity was a frequent comorbid condition in patients with PsA and that it is a known proinflammatory condition. As such, obesity could potentially affect immunologic processes, the pharmacokinetics of treatments, and ultimately patient outcomes.
Since TNF-alpha inhibitor (TNFi) treatment fails in around half of all patients with PsA treated in routine care, Ms. Højgaard noted that the aim of the cohort study was to investigate whether obesity could be having any influence on this.
Data on baseline characteristics, EULAR response rates, and drug adherence were obtained for 1,943 patients with PsA prescribed their anti-TNF therapy from two nationwide registries of disease-modifying therapies being used to treat rheumatic conditions in Denmark and Iceland, DANBIO (Rheumatology. 2011;50:69–77) and ICEBIO, respectively.
At baseline, body mass index (BMI) data were available for 1,271 patients and 408 (32%) of these had a BMI of 30 kg/m2 or more and were classed as being obese. The majority (39%) had received a first prescription for adalimumab, with around a quarter each prescribed etanercept (26%) or infliximab (24%), and the remainder prescribed golimumab (7%) and certolizumab (4%).
Compared to the 863 (68%) nonobese individuals, the obese patients were older (47 vs. 49 years, P = .01), less likely to smoke (30% vs. 23%, P = .01), and had higher disease activity measured on the Disease Activity Score 28 (DAS28) (4.4 vs. 4.6, P = .01). Health Assessment Questionnaire scores were also higher in obese than in nonobese individuals (1.1 vs. 0.9, P less than .01), and there were higher tender joint counts (6 vs. 5, P = .01), and higher pain levels assessed on a visual analog scale (VAS). Obese patients also had higher scores on a VAS patient global scale. The median follow-up time was 1.5 years.
Patients who were obese were found to adhere to TNFi treatment for shorter periods of time than nonobese patients, with median durations of 1.76 and 3.08 years, respectively (P less than .001). This discrepancy was most pronounced among men, a finding that may account for the fact that they were less likely to achieve a good EULAR response than their nonobese counterparts (OR = 0.5).
Being obese versus not being obese independently predicted TNFi withdrawal overall (hazard ratio, 1.6), especially in men (HR, 1.8; HR, 1.5 in women). TNFi withdrawal was more likely in obese than in nonobese patients even when individual treatments were considered; adalimumab: HR, 1.6; etanercept: HR, 2.0; infliximab: HR, 1.6.
An association between obesity and reduced response to anti-TNF therapy has also been observed in patients with rheumatoid arthritis, Ms. Højgaard acknowledged. There have also been a few studies of PsA and psoriasis “but to my knowledge, I think in the field of psoriatic arthritis, we are one of the few that have been looking at long-time drug survival,” she said. “We also include quite a lot of patients.”
“Of course this is not a randomized clinical study, so there could be residual confounding factors,” Ms. Højgaard cautioned. “It is always a bit difficult to say something about causality when it is a database study,” she added. “I think what we can see here is that there is an association, but in order to recommend weight loss we need some prospective studies.”
She noted that there was one published clinical study (Ann Rheum Dis. 2014;73:1157–62) that had looked at the benefit of a weight reduction program started at the same time as TNFi initiation in patients with PsA. This found there was a benefit of weight loss on response to TNFis, regardless of the type of diet.
DANBIO is supported by unrestricted grants from Abbott, Pfizer, MSD, Bristol-Myers Squibb, Roche, and UCB-Nordic. The sponsors have had no influence on data collection, analysis, or publication. ICEBIO is part of the electronic medical record system held by the University of Reykjavik and receives no industrial funding. Ms. Højgaard has received speaking fees from Celgene and UCB not related to this work.
LONDON – Patients with psoriatic arthritis appear less likely to achieve a good response to their first anti–tumor necrosis factor (anti-TNF) therapy if they are obese, according to data taken from two Nordic registries.
In a large observational cohort study, obese individuals with psoriatic arthritis (PsA) were significantly less likely than their nonobese counterparts to achieve a European League Against Rheumatism (EULAR) good or moderate response at 6 months (55% vs. 65%, P = .02). The overall odds ratio for achieving a good or moderate response was 0.47 when comparing obese with nonobese individuals.
The findings are potentially important because, with the exception of infliximab, anti-TNF therapy is not currently adjusted according to body weight, said presenting study author Pil Højgaard in an interview at the European Congress of Rheumatology.
Ms. Højgaard, who is an M.D. Ph.D. student at the department of rheumatology, Copenhagen University Hospital Gentofte, Rigshospitalet and the Parker Institute in Copenhagen, noted that obesity was a frequent comorbid condition in patients with PsA and that it is a known proinflammatory condition. As such, obesity could potentially affect immunologic processes, the pharmacokinetics of treatments, and ultimately patient outcomes.
Since TNF-alpha inhibitor (TNFi) treatment fails in around half of all patients with PsA treated in routine care, Ms. Højgaard noted that the aim of the cohort study was to investigate whether obesity could be having any influence on this.
Data on baseline characteristics, EULAR response rates, and drug adherence were obtained for 1,943 patients with PsA prescribed their anti-TNF therapy from two nationwide registries of disease-modifying therapies being used to treat rheumatic conditions in Denmark and Iceland, DANBIO (Rheumatology. 2011;50:69–77) and ICEBIO, respectively.
At baseline, body mass index (BMI) data were available for 1,271 patients and 408 (32%) of these had a BMI of 30 kg/m2 or more and were classed as being obese. The majority (39%) had received a first prescription for adalimumab, with around a quarter each prescribed etanercept (26%) or infliximab (24%), and the remainder prescribed golimumab (7%) and certolizumab (4%).
Compared to the 863 (68%) nonobese individuals, the obese patients were older (47 vs. 49 years, P = .01), less likely to smoke (30% vs. 23%, P = .01), and had higher disease activity measured on the Disease Activity Score 28 (DAS28) (4.4 vs. 4.6, P = .01). Health Assessment Questionnaire scores were also higher in obese than in nonobese individuals (1.1 vs. 0.9, P less than .01), and there were higher tender joint counts (6 vs. 5, P = .01), and higher pain levels assessed on a visual analog scale (VAS). Obese patients also had higher scores on a VAS patient global scale. The median follow-up time was 1.5 years.
Patients who were obese were found to adhere to TNFi treatment for shorter periods of time than nonobese patients, with median durations of 1.76 and 3.08 years, respectively (P less than .001). This discrepancy was most pronounced among men, a finding that may account for the fact that they were less likely to achieve a good EULAR response than their nonobese counterparts (OR = 0.5).
Being obese versus not being obese independently predicted TNFi withdrawal overall (hazard ratio, 1.6), especially in men (HR, 1.8; HR, 1.5 in women). TNFi withdrawal was more likely in obese than in nonobese patients even when individual treatments were considered; adalimumab: HR, 1.6; etanercept: HR, 2.0; infliximab: HR, 1.6.
An association between obesity and reduced response to anti-TNF therapy has also been observed in patients with rheumatoid arthritis, Ms. Højgaard acknowledged. There have also been a few studies of PsA and psoriasis “but to my knowledge, I think in the field of psoriatic arthritis, we are one of the few that have been looking at long-time drug survival,” she said. “We also include quite a lot of patients.”
“Of course this is not a randomized clinical study, so there could be residual confounding factors,” Ms. Højgaard cautioned. “It is always a bit difficult to say something about causality when it is a database study,” she added. “I think what we can see here is that there is an association, but in order to recommend weight loss we need some prospective studies.”
She noted that there was one published clinical study (Ann Rheum Dis. 2014;73:1157–62) that had looked at the benefit of a weight reduction program started at the same time as TNFi initiation in patients with PsA. This found there was a benefit of weight loss on response to TNFis, regardless of the type of diet.
DANBIO is supported by unrestricted grants from Abbott, Pfizer, MSD, Bristol-Myers Squibb, Roche, and UCB-Nordic. The sponsors have had no influence on data collection, analysis, or publication. ICEBIO is part of the electronic medical record system held by the University of Reykjavik and receives no industrial funding. Ms. Højgaard has received speaking fees from Celgene and UCB not related to this work.
Key clinical point: Obesity may reduce response and adherence to tumor necrosis factor–inhibitor therapy in patients with psoriatic arthritis.
Major finding: The EULAR good or moderate response rate at 6 months was 55% in obese vs. 65% in nonobese patients (P = .02).
Data source: Observational cohort study based on two Nordic registries of 1,943 patients with PsA prescribed their first TNFi.
Disclosures: DANBIO is supported by unrestricted grants from Abbott, Pfizer, MSD, Bristol-Myers Squibb, Roche, and UCB-Nordic. The sponsors have had no influence on data collection, analysis, or publication. ICEBIO is part of the electronic medical record system held by the University of Reykjavik and receives no industrial funding. Ms. Højgaard has received speaking fees from Celgene and UCB not related to this work.
Genetic therapy lowers joint bleeding in hemophilia B
COPENHAGEN – It’s early days yet, but results look highly promising for the ability of an experimental gene-transfer therapy to improve coagulation parameters in patients with severe hemophilia B.
In a phase I/II dose-escalation study, a single 1-hour infusion of the gene-transfer product, labeled SPK-9001, resulted in factor IX activity levels ranging from 25% to 39% of normal in four men with severe or moderately severe hemophilia B, reported Dr. Katherine A High, president and chief scientific officer of Spark Therapeutics, maker of the product.
“One of the most remarkable features of the data in my mind has been a very consistent performance,” she said at a briefing at the annual congress of the European Hematology Association.
The product consists of a vector containing a novel bio-engineered adeno-associated virus (AAV) capsid with tropism for liver, and a factor IX cassette that carries a strong liver-specific promoter to drive the expression of the factor IX variant, dubbed factor IX Padua.
“The hypothesis of the work was that if we could engineer a vector efficient enough, we would be able to infuse it at a dose low enough that it would drive loads of expression greater than 12% of normal, which in previous work has been shown to be associated with an absence of joint bleeds in natural history studies of people with mild disease, and that infusion at a low dose would eliminate the need for any type of immune suppression with steroids,” Dr. High said.
Other attempts at genetic engineering in patients with hemophilia B have been hampered by the need to use high doses of vector that can induce an immune response, thereby negating the benefit of therapy.
In this ongoing study, conducted in Mississippi, Pennsylvania, and California, males 18 and older with a confirmed diagnosis of hemophilia B (defined as equal to or less than 2 IU/dL or 2% endogenous factor IX) who have received 50 or more days of exposure to factor IX products are enrolled. The patients must have a minimum average of four bleeding events per year requiring episodic treatment of factor IX infusions or prophylactic factor IX infusions, no measurable factor IX inhibitor as assessed by the central laboratory, and no prior history of inhibitors to factor IX protein.
In an oral session at the congress, Dr. Spencer Sullivan, assistant professor of pediatrics and medicine at the University of Mississippi Medical Center in Jackson, presented data on four men whose age ranges from 18 to 47 years.
In the dose-escalation phase of the study, the patients received single infusions of SPK-9001 at an initial starting dose of 5 x 1011 vector genomes of body weight. They were followed for 7-26 weeks after gene transfer for factor IX activity levels, liver enzymes, bleeding episode, and factor usage. As of May 22, 2016, the first four patients showed factor IX activity levels of 32%, 39%, 25%, and 27% of normal, respectively.
All subjects are currently off prophylactic factor IX infusions. During the course of follow-up, one patient infused himself with factor IX once, treating himself 2 days after vector infusion for a suspected ankle bleed.
Asked by a reporter how durable the effect was, Dr. High replied that in dog models of hemophilia B the effect of the gene transfer has been durable.
The best evidence to date of durability in humans, she said, comes from investigators at University College in London (England), who found that if patients can make it past the first 8-10 weeks without developing an immune response to the transfer product, they are likely to do well, and to have a durable effect, she said.
Dr. Anton Hagenbeek, from the Academic Medical Center, University of Amsterdam, who moderated the briefing but was not involved in the study, said that Dr. High was “to be congratulated for these best data ever seen.”
He asked, facetiously, whether she thought that “thousands of patients would buy a ticket to Philadelphia.” The “City of Brotherly Love” is home to one of the trial sites and to Spark headquarters.
The study is sponsored by Spark Therapeutics and Pfizer. Dr. High is president and chief scientific officer of Spark. Dr. Sullivan and Dr. Hagenbeek reported no relevant disclosures.
COPENHAGEN – It’s early days yet, but results look highly promising for the ability of an experimental gene-transfer therapy to improve coagulation parameters in patients with severe hemophilia B.
In a phase I/II dose-escalation study, a single 1-hour infusion of the gene-transfer product, labeled SPK-9001, resulted in factor IX activity levels ranging from 25% to 39% of normal in four men with severe or moderately severe hemophilia B, reported Dr. Katherine A High, president and chief scientific officer of Spark Therapeutics, maker of the product.
“One of the most remarkable features of the data in my mind has been a very consistent performance,” she said at a briefing at the annual congress of the European Hematology Association.
The product consists of a vector containing a novel bio-engineered adeno-associated virus (AAV) capsid with tropism for liver, and a factor IX cassette that carries a strong liver-specific promoter to drive the expression of the factor IX variant, dubbed factor IX Padua.
“The hypothesis of the work was that if we could engineer a vector efficient enough, we would be able to infuse it at a dose low enough that it would drive loads of expression greater than 12% of normal, which in previous work has been shown to be associated with an absence of joint bleeds in natural history studies of people with mild disease, and that infusion at a low dose would eliminate the need for any type of immune suppression with steroids,” Dr. High said.
Other attempts at genetic engineering in patients with hemophilia B have been hampered by the need to use high doses of vector that can induce an immune response, thereby negating the benefit of therapy.
In this ongoing study, conducted in Mississippi, Pennsylvania, and California, males 18 and older with a confirmed diagnosis of hemophilia B (defined as equal to or less than 2 IU/dL or 2% endogenous factor IX) who have received 50 or more days of exposure to factor IX products are enrolled. The patients must have a minimum average of four bleeding events per year requiring episodic treatment of factor IX infusions or prophylactic factor IX infusions, no measurable factor IX inhibitor as assessed by the central laboratory, and no prior history of inhibitors to factor IX protein.
In an oral session at the congress, Dr. Spencer Sullivan, assistant professor of pediatrics and medicine at the University of Mississippi Medical Center in Jackson, presented data on four men whose age ranges from 18 to 47 years.
In the dose-escalation phase of the study, the patients received single infusions of SPK-9001 at an initial starting dose of 5 x 1011 vector genomes of body weight. They were followed for 7-26 weeks after gene transfer for factor IX activity levels, liver enzymes, bleeding episode, and factor usage. As of May 22, 2016, the first four patients showed factor IX activity levels of 32%, 39%, 25%, and 27% of normal, respectively.
All subjects are currently off prophylactic factor IX infusions. During the course of follow-up, one patient infused himself with factor IX once, treating himself 2 days after vector infusion for a suspected ankle bleed.
Asked by a reporter how durable the effect was, Dr. High replied that in dog models of hemophilia B the effect of the gene transfer has been durable.
The best evidence to date of durability in humans, she said, comes from investigators at University College in London (England), who found that if patients can make it past the first 8-10 weeks without developing an immune response to the transfer product, they are likely to do well, and to have a durable effect, she said.
Dr. Anton Hagenbeek, from the Academic Medical Center, University of Amsterdam, who moderated the briefing but was not involved in the study, said that Dr. High was “to be congratulated for these best data ever seen.”
He asked, facetiously, whether she thought that “thousands of patients would buy a ticket to Philadelphia.” The “City of Brotherly Love” is home to one of the trial sites and to Spark headquarters.
The study is sponsored by Spark Therapeutics and Pfizer. Dr. High is president and chief scientific officer of Spark. Dr. Sullivan and Dr. Hagenbeek reported no relevant disclosures.
COPENHAGEN – It’s early days yet, but results look highly promising for the ability of an experimental gene-transfer therapy to improve coagulation parameters in patients with severe hemophilia B.
In a phase I/II dose-escalation study, a single 1-hour infusion of the gene-transfer product, labeled SPK-9001, resulted in factor IX activity levels ranging from 25% to 39% of normal in four men with severe or moderately severe hemophilia B, reported Dr. Katherine A High, president and chief scientific officer of Spark Therapeutics, maker of the product.
“One of the most remarkable features of the data in my mind has been a very consistent performance,” she said at a briefing at the annual congress of the European Hematology Association.
The product consists of a vector containing a novel bio-engineered adeno-associated virus (AAV) capsid with tropism for liver, and a factor IX cassette that carries a strong liver-specific promoter to drive the expression of the factor IX variant, dubbed factor IX Padua.
“The hypothesis of the work was that if we could engineer a vector efficient enough, we would be able to infuse it at a dose low enough that it would drive loads of expression greater than 12% of normal, which in previous work has been shown to be associated with an absence of joint bleeds in natural history studies of people with mild disease, and that infusion at a low dose would eliminate the need for any type of immune suppression with steroids,” Dr. High said.
Other attempts at genetic engineering in patients with hemophilia B have been hampered by the need to use high doses of vector that can induce an immune response, thereby negating the benefit of therapy.
In this ongoing study, conducted in Mississippi, Pennsylvania, and California, males 18 and older with a confirmed diagnosis of hemophilia B (defined as equal to or less than 2 IU/dL or 2% endogenous factor IX) who have received 50 or more days of exposure to factor IX products are enrolled. The patients must have a minimum average of four bleeding events per year requiring episodic treatment of factor IX infusions or prophylactic factor IX infusions, no measurable factor IX inhibitor as assessed by the central laboratory, and no prior history of inhibitors to factor IX protein.
In an oral session at the congress, Dr. Spencer Sullivan, assistant professor of pediatrics and medicine at the University of Mississippi Medical Center in Jackson, presented data on four men whose age ranges from 18 to 47 years.
In the dose-escalation phase of the study, the patients received single infusions of SPK-9001 at an initial starting dose of 5 x 1011 vector genomes of body weight. They were followed for 7-26 weeks after gene transfer for factor IX activity levels, liver enzymes, bleeding episode, and factor usage. As of May 22, 2016, the first four patients showed factor IX activity levels of 32%, 39%, 25%, and 27% of normal, respectively.
All subjects are currently off prophylactic factor IX infusions. During the course of follow-up, one patient infused himself with factor IX once, treating himself 2 days after vector infusion for a suspected ankle bleed.
Asked by a reporter how durable the effect was, Dr. High replied that in dog models of hemophilia B the effect of the gene transfer has been durable.
The best evidence to date of durability in humans, she said, comes from investigators at University College in London (England), who found that if patients can make it past the first 8-10 weeks without developing an immune response to the transfer product, they are likely to do well, and to have a durable effect, she said.
Dr. Anton Hagenbeek, from the Academic Medical Center, University of Amsterdam, who moderated the briefing but was not involved in the study, said that Dr. High was “to be congratulated for these best data ever seen.”
He asked, facetiously, whether she thought that “thousands of patients would buy a ticket to Philadelphia.” The “City of Brotherly Love” is home to one of the trial sites and to Spark headquarters.
The study is sponsored by Spark Therapeutics and Pfizer. Dr. High is president and chief scientific officer of Spark. Dr. Sullivan and Dr. Hagenbeek reported no relevant disclosures.
At THE EHA CONGRESS
Key clinical point:. The experimental SPK-9001 is a gene transfer product carrying a strong factor IX promoter.
Major finding: A single one-hour infusion of SPK-9001 was associated with factor IX activity levels ranging from 25% to 39% of normal.
Data source: Dose-escalation cohort of four adult males in a phase I/II study.
Disclosures: The study is sponsored by Spark Therapeutics and Pfizer. Dr. High is president and chief scientific officer of Spark. Dr. Sullivan and Dr. Hagenbeek reported no relevant disclosures.
Study: TNF inhibitors improve extraintestinal IBD manifestations
SAN DIEGO – Tumor necrosis factor inhibitors improved the extraintestinal manifestations of inflammatory bowel disease (IBD) among more than half of affected patients, according to a national cohort study.
“The best response rates were for psoriasis, aphthous stomatitis, uveitis, and peripheral arthritis,” said Dr. Thomas Greuter of University Hospital in Zürich. Patients responded similarly whether they received oral infliximab or subcutaneous adalimumab or certolizumab, he noted.
IBD often is associated with debilitating disorders of the skin, joints, eyes, and hepatobiliary tract, but “due to the lack of randomized, controlled trials, the therapy of extraintestinal manifestations remains rather empirical,” Dr. Greuter said at the annual Digestive Disease Week.
To study the role of anti-TNF agents in treating these disorders, he and his associates analyzed data for 1,249 patients from the national Swiss IBD Cohort Study between 2006 and 2010. Patients were typically in their mid-30s and had lived with IBD for about 9 years, he said.
A total of 366 patients (29%) had at least one extraintestinal manifestation of IBD – most commonly peripheral arthritis (75%), followed by aphthous stomatitis (24%), and ankylosing spondylitis (22%). In all, 213 (58%) patients received at least one anti-TNF agent, and 40% received the prescription specifically for extraintestinal manifestations. Nearly two-thirds of the patients received infliximab, while 22% received adalimumab and 15% received certolizumab.
About 55% of patients improved on anti-TNF therapy over an average of 7 years of follow-up, Dr. Greuter and his associates reported. Among all three anti-TNF agents, response rates ranged from 100% for psoriasis, to 80% for erythema nodosum and stomatitis, to 73% for arthritis and uveitis, to 50% for pyoderma granulosum. Overall rates of improvement were slightly higher for infliximab than for the other two drugs, but “adalimumab and certolizumab were used mostly as a second or a third-line anti-TNF agent, and the response rate to a second or third-line treatment was lower than for the first one,” Dr. Greuter said. Some patients also received corticosteroids and immunomodulators, but excluding this subgroup had little effect on rates of response to anti-TNF therapy, he added.
Dr. Greuter also reported that 11 patients (about 5% of the cohort) developed 14 new extraintestinal manifestations after starting anti-TNF agents – usually peripheral arthritis, but also pyoderma granulosum, aphthous stomatitis, psoriasis, and uveitis. “We cannot say if this was primary, or a side effect of treatment,” he said. These disorders usually improved if patients stayed on their anti-TNF agent, he added.
About two-thirds of patients in the cohort were female, more than three-quarters had Crohn’s disease, 19% had ulcerative colitis, and 3% had indeterminate colitis, he noted.
A research grant from the Swiss National Science Foundation funded the study. Dr. Greuter had no disclosures.
SAN DIEGO – Tumor necrosis factor inhibitors improved the extraintestinal manifestations of inflammatory bowel disease (IBD) among more than half of affected patients, according to a national cohort study.
“The best response rates were for psoriasis, aphthous stomatitis, uveitis, and peripheral arthritis,” said Dr. Thomas Greuter of University Hospital in Zürich. Patients responded similarly whether they received oral infliximab or subcutaneous adalimumab or certolizumab, he noted.
IBD often is associated with debilitating disorders of the skin, joints, eyes, and hepatobiliary tract, but “due to the lack of randomized, controlled trials, the therapy of extraintestinal manifestations remains rather empirical,” Dr. Greuter said at the annual Digestive Disease Week.
To study the role of anti-TNF agents in treating these disorders, he and his associates analyzed data for 1,249 patients from the national Swiss IBD Cohort Study between 2006 and 2010. Patients were typically in their mid-30s and had lived with IBD for about 9 years, he said.
A total of 366 patients (29%) had at least one extraintestinal manifestation of IBD – most commonly peripheral arthritis (75%), followed by aphthous stomatitis (24%), and ankylosing spondylitis (22%). In all, 213 (58%) patients received at least one anti-TNF agent, and 40% received the prescription specifically for extraintestinal manifestations. Nearly two-thirds of the patients received infliximab, while 22% received adalimumab and 15% received certolizumab.
About 55% of patients improved on anti-TNF therapy over an average of 7 years of follow-up, Dr. Greuter and his associates reported. Among all three anti-TNF agents, response rates ranged from 100% for psoriasis, to 80% for erythema nodosum and stomatitis, to 73% for arthritis and uveitis, to 50% for pyoderma granulosum. Overall rates of improvement were slightly higher for infliximab than for the other two drugs, but “adalimumab and certolizumab were used mostly as a second or a third-line anti-TNF agent, and the response rate to a second or third-line treatment was lower than for the first one,” Dr. Greuter said. Some patients also received corticosteroids and immunomodulators, but excluding this subgroup had little effect on rates of response to anti-TNF therapy, he added.
Dr. Greuter also reported that 11 patients (about 5% of the cohort) developed 14 new extraintestinal manifestations after starting anti-TNF agents – usually peripheral arthritis, but also pyoderma granulosum, aphthous stomatitis, psoriasis, and uveitis. “We cannot say if this was primary, or a side effect of treatment,” he said. These disorders usually improved if patients stayed on their anti-TNF agent, he added.
About two-thirds of patients in the cohort were female, more than three-quarters had Crohn’s disease, 19% had ulcerative colitis, and 3% had indeterminate colitis, he noted.
A research grant from the Swiss National Science Foundation funded the study. Dr. Greuter had no disclosures.
SAN DIEGO – Tumor necrosis factor inhibitors improved the extraintestinal manifestations of inflammatory bowel disease (IBD) among more than half of affected patients, according to a national cohort study.
“The best response rates were for psoriasis, aphthous stomatitis, uveitis, and peripheral arthritis,” said Dr. Thomas Greuter of University Hospital in Zürich. Patients responded similarly whether they received oral infliximab or subcutaneous adalimumab or certolizumab, he noted.
IBD often is associated with debilitating disorders of the skin, joints, eyes, and hepatobiliary tract, but “due to the lack of randomized, controlled trials, the therapy of extraintestinal manifestations remains rather empirical,” Dr. Greuter said at the annual Digestive Disease Week.
To study the role of anti-TNF agents in treating these disorders, he and his associates analyzed data for 1,249 patients from the national Swiss IBD Cohort Study between 2006 and 2010. Patients were typically in their mid-30s and had lived with IBD for about 9 years, he said.
A total of 366 patients (29%) had at least one extraintestinal manifestation of IBD – most commonly peripheral arthritis (75%), followed by aphthous stomatitis (24%), and ankylosing spondylitis (22%). In all, 213 (58%) patients received at least one anti-TNF agent, and 40% received the prescription specifically for extraintestinal manifestations. Nearly two-thirds of the patients received infliximab, while 22% received adalimumab and 15% received certolizumab.
About 55% of patients improved on anti-TNF therapy over an average of 7 years of follow-up, Dr. Greuter and his associates reported. Among all three anti-TNF agents, response rates ranged from 100% for psoriasis, to 80% for erythema nodosum and stomatitis, to 73% for arthritis and uveitis, to 50% for pyoderma granulosum. Overall rates of improvement were slightly higher for infliximab than for the other two drugs, but “adalimumab and certolizumab were used mostly as a second or a third-line anti-TNF agent, and the response rate to a second or third-line treatment was lower than for the first one,” Dr. Greuter said. Some patients also received corticosteroids and immunomodulators, but excluding this subgroup had little effect on rates of response to anti-TNF therapy, he added.
Dr. Greuter also reported that 11 patients (about 5% of the cohort) developed 14 new extraintestinal manifestations after starting anti-TNF agents – usually peripheral arthritis, but also pyoderma granulosum, aphthous stomatitis, psoriasis, and uveitis. “We cannot say if this was primary, or a side effect of treatment,” he said. These disorders usually improved if patients stayed on their anti-TNF agent, he added.
About two-thirds of patients in the cohort were female, more than three-quarters had Crohn’s disease, 19% had ulcerative colitis, and 3% had indeterminate colitis, he noted.
A research grant from the Swiss National Science Foundation funded the study. Dr. Greuter had no disclosures.
AT DDW® 2016
Key clinical point: Tumor necrosis factor inhibitors improved the extraintestinal manifestations of inflammatory bowel disease among more than half of affected patients.
Major finding: About 55% of patients who received infliximab, adalimumab, or certolizumab had a clinical response.
Data source: A study of 1,249 patients with IBD from a national cohort.
Disclosures: A research grant from the Swiss National Science Foundation funded the study. Dr. Greuter had no disclosures.