User login
Plan a gift that offers a better future for GI
Planned giving provides an opportunity for all who have benefited from digestive disease research to give back to the field in a unique and lasting way.
Your investment in the AGA Research Foundation will enable the foundation to continue our investment in the future of gastroenterological research and innovation. With donations from AGA members, we can provide young researchers with a secure, ongoing stable source of funding that drives advancement in the diagnosis, treatment, and cure of digestive diseases.
If you make a contribution, it will be because you believe in what we do and because you want to help make a difference in the lives of others. But we’d also like to make sure you benefit from making a gift to the AGA Research Foundation.
Your giving options
There are several gift arrangements to choose from. The chart below summarizes the benefits of some of the main types of charitable gifts. Just think of what you want to accomplish with your gift, and there’s probably a way to do it!
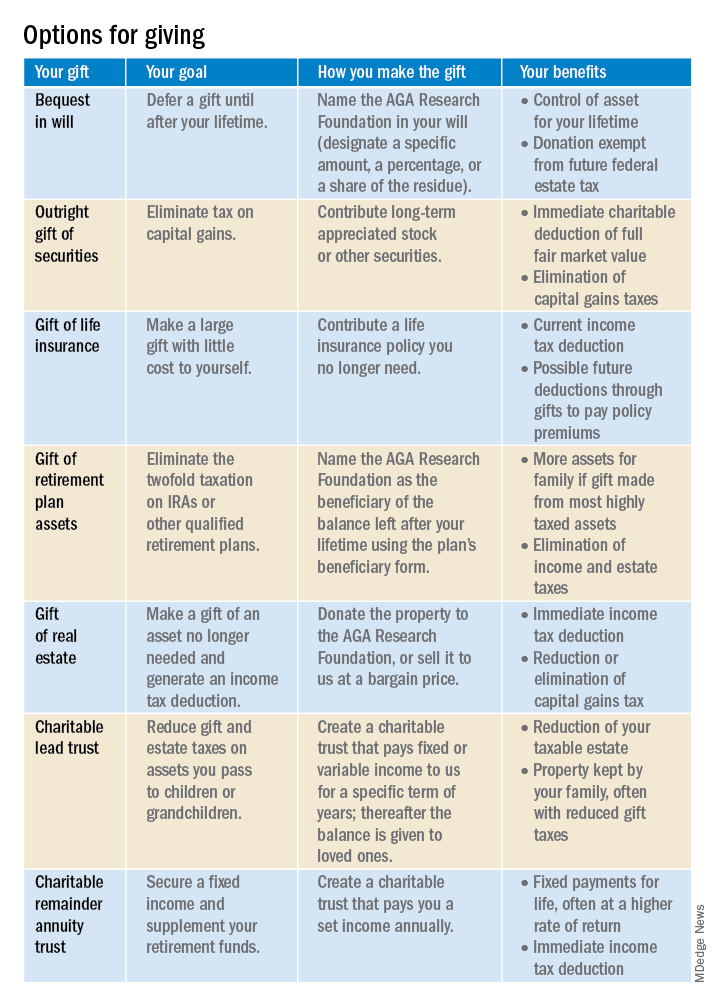
Learn more by visiting http://gastro.planmylegacy.org.
Planned giving provides an opportunity for all who have benefited from digestive disease research to give back to the field in a unique and lasting way.
Your investment in the AGA Research Foundation will enable the foundation to continue our investment in the future of gastroenterological research and innovation. With donations from AGA members, we can provide young researchers with a secure, ongoing stable source of funding that drives advancement in the diagnosis, treatment, and cure of digestive diseases.
If you make a contribution, it will be because you believe in what we do and because you want to help make a difference in the lives of others. But we’d also like to make sure you benefit from making a gift to the AGA Research Foundation.
Your giving options
There are several gift arrangements to choose from. The chart below summarizes the benefits of some of the main types of charitable gifts. Just think of what you want to accomplish with your gift, and there’s probably a way to do it!

Learn more by visiting http://gastro.planmylegacy.org.
Planned giving provides an opportunity for all who have benefited from digestive disease research to give back to the field in a unique and lasting way.
Your investment in the AGA Research Foundation will enable the foundation to continue our investment in the future of gastroenterological research and innovation. With donations from AGA members, we can provide young researchers with a secure, ongoing stable source of funding that drives advancement in the diagnosis, treatment, and cure of digestive diseases.
If you make a contribution, it will be because you believe in what we do and because you want to help make a difference in the lives of others. But we’d also like to make sure you benefit from making a gift to the AGA Research Foundation.
Your giving options
There are several gift arrangements to choose from. The chart below summarizes the benefits of some of the main types of charitable gifts. Just think of what you want to accomplish with your gift, and there’s probably a way to do it!

Learn more by visiting http://gastro.planmylegacy.org.
2022 Billing and coding updates: Critical care services
The principal idea behind this article is to summarize comprehensively yet concisely the 2022 CMS updates regarding the critical care services. I would encourage and urge all the members to read this section attentively to stay abreast with all the recent developments.
As a general reminder the two critical care services billing codes for the evaluation and management of the critically ill injured patients are:
99291: First 30-74 minutes
99292: Each additional 30 minutes
And, the five major changes for 2022 as proposed by the CMS for critical care services are:
1. It is allowed for the physicians and APPs in the same specialty to bill concurrent critical care services.
Previously, same specialty practitioners were required to bill and were paid as “one” when multiple practitioners provided services on the same date. Now, they can bill for critical care services as subsequent care or as aggregate time, and they are highlighted below with examples:
Subsequent care
Initial visit by a provider for 65 minutes (bill as 99291 as the first claim)
Subsequent visit at a later time on the same day for 60 minutes (bill as 99292 x2 as the second claim)
Aggregate time
Time of multiple practitioners in the same specialty can be added to meet 99291 or 99292. If Practitioner A spends 15 minutes of critical care, then 99291 cannot be billed; but, if Practitioner B spends 30 minutes of critical care, they can bill 99291 with a total time of 45 minutes as one claim
The prerequisites are that the visits are medically necessary, and each visit meets the definition of critical care.
2. Modifier FS needs to be used for split sharing of critical care services.
Previously, critical care services could not be split shared, but it can be done in 2022. The practitioner who provides the significant portion of the visit needs to bill. A significant or substantive portion is considered to be more than half the cumulative total time of both providers.
Example: The APP spends 20 minutes in critical care services and the physician spends 30 minutes. Total time spent is 50 minutes, and the physician may bill 99291.
It is crucial to note that each provider needs to document a note for the medically necessary critical care that they personally performed and the time they spent. Additionally, upon review of the medical records, the two providers should be easily identifiable, and the medical record must be signed and dated by the provider who performed the substantive portion and billed.
Lastly, do not forget to submit the modifier FS.
3. Modifier 25 needs to be used to get paid for an ED visit or other E/M service on the same day as critical care.
Previously, hospital ED services were not paid on the same date as critical care by the same provider. But, in 2022, the practitioners may bill for ED visit at the hospital and also for other E/M services on the same day when there is supporting documentation. The practitioners will need to document that the E/M service was provided prior to the time when the patient did not require critical care, that the service was medically necessary, and that the service was separate and distinct with no duplication.
Of note, do not forget to submit the modifier 25.
4. Critical care visits will be separately billable from global surgery when unrelated with the use of modifier FT.
Previously pre- and postoperative critical care was included in the surgical package of many procedures with a global period of 10-90 days, and critical care visits would be paid only if the service was unrelated to the procedure. The concept remains the same in 2022 but, now, new modifier FT will need to be used to report critical care services unrelated to the procedure. Also, the service provided will need to meet the definition of critical care, which is usually above and beyond the procedure performed and should be unrelated to the specific injury or general surgical procedure performed.
5. There will be certain critical care medical record documentation requirements.
It is paramount that each practitioner must document the exact total critical care time and not a range or approximation of time. Additionally, it is equally as important for the documentation to indicate that the services provided were medically reasonable and necessary. In the setting of split/shared billing, the role of each practitioner should be clearly identifiable (the condition for which each practitioner treated the patient, how the care was concurrent either subsequent or aggregate, and the total time of each practitioner).
Hopefully, this review will provide a good perception for our members in regards to major updates for 2022, help them navigate the regulatory rules, and avoid any unnecessary setbacks. In the upcoming months, we will try to cover some more topics on practice management and administration, such as Medicare Physician Fee Schedule Rule, Hospital Outpatient Prospective Payment Rule, and coding/billing for teaching physicians, telehealth, and pulmonary rehabilitation services.
The principal idea behind this article is to summarize comprehensively yet concisely the 2022 CMS updates regarding the critical care services. I would encourage and urge all the members to read this section attentively to stay abreast with all the recent developments.
As a general reminder the two critical care services billing codes for the evaluation and management of the critically ill injured patients are:
99291: First 30-74 minutes
99292: Each additional 30 minutes
And, the five major changes for 2022 as proposed by the CMS for critical care services are:
1. It is allowed for the physicians and APPs in the same specialty to bill concurrent critical care services.
Previously, same specialty practitioners were required to bill and were paid as “one” when multiple practitioners provided services on the same date. Now, they can bill for critical care services as subsequent care or as aggregate time, and they are highlighted below with examples:
Subsequent care
Initial visit by a provider for 65 minutes (bill as 99291 as the first claim)
Subsequent visit at a later time on the same day for 60 minutes (bill as 99292 x2 as the second claim)
Aggregate time
Time of multiple practitioners in the same specialty can be added to meet 99291 or 99292. If Practitioner A spends 15 minutes of critical care, then 99291 cannot be billed; but, if Practitioner B spends 30 minutes of critical care, they can bill 99291 with a total time of 45 minutes as one claim
The prerequisites are that the visits are medically necessary, and each visit meets the definition of critical care.
2. Modifier FS needs to be used for split sharing of critical care services.
Previously, critical care services could not be split shared, but it can be done in 2022. The practitioner who provides the significant portion of the visit needs to bill. A significant or substantive portion is considered to be more than half the cumulative total time of both providers.
Example: The APP spends 20 minutes in critical care services and the physician spends 30 minutes. Total time spent is 50 minutes, and the physician may bill 99291.
It is crucial to note that each provider needs to document a note for the medically necessary critical care that they personally performed and the time they spent. Additionally, upon review of the medical records, the two providers should be easily identifiable, and the medical record must be signed and dated by the provider who performed the substantive portion and billed.
Lastly, do not forget to submit the modifier FS.
3. Modifier 25 needs to be used to get paid for an ED visit or other E/M service on the same day as critical care.
Previously, hospital ED services were not paid on the same date as critical care by the same provider. But, in 2022, the practitioners may bill for ED visit at the hospital and also for other E/M services on the same day when there is supporting documentation. The practitioners will need to document that the E/M service was provided prior to the time when the patient did not require critical care, that the service was medically necessary, and that the service was separate and distinct with no duplication.
Of note, do not forget to submit the modifier 25.
4. Critical care visits will be separately billable from global surgery when unrelated with the use of modifier FT.
Previously pre- and postoperative critical care was included in the surgical package of many procedures with a global period of 10-90 days, and critical care visits would be paid only if the service was unrelated to the procedure. The concept remains the same in 2022 but, now, new modifier FT will need to be used to report critical care services unrelated to the procedure. Also, the service provided will need to meet the definition of critical care, which is usually above and beyond the procedure performed and should be unrelated to the specific injury or general surgical procedure performed.
5. There will be certain critical care medical record documentation requirements.
It is paramount that each practitioner must document the exact total critical care time and not a range or approximation of time. Additionally, it is equally as important for the documentation to indicate that the services provided were medically reasonable and necessary. In the setting of split/shared billing, the role of each practitioner should be clearly identifiable (the condition for which each practitioner treated the patient, how the care was concurrent either subsequent or aggregate, and the total time of each practitioner).
Hopefully, this review will provide a good perception for our members in regards to major updates for 2022, help them navigate the regulatory rules, and avoid any unnecessary setbacks. In the upcoming months, we will try to cover some more topics on practice management and administration, such as Medicare Physician Fee Schedule Rule, Hospital Outpatient Prospective Payment Rule, and coding/billing for teaching physicians, telehealth, and pulmonary rehabilitation services.
The principal idea behind this article is to summarize comprehensively yet concisely the 2022 CMS updates regarding the critical care services. I would encourage and urge all the members to read this section attentively to stay abreast with all the recent developments.
As a general reminder the two critical care services billing codes for the evaluation and management of the critically ill injured patients are:
99291: First 30-74 minutes
99292: Each additional 30 minutes
And, the five major changes for 2022 as proposed by the CMS for critical care services are:
1. It is allowed for the physicians and APPs in the same specialty to bill concurrent critical care services.
Previously, same specialty practitioners were required to bill and were paid as “one” when multiple practitioners provided services on the same date. Now, they can bill for critical care services as subsequent care or as aggregate time, and they are highlighted below with examples:
Subsequent care
Initial visit by a provider for 65 minutes (bill as 99291 as the first claim)
Subsequent visit at a later time on the same day for 60 minutes (bill as 99292 x2 as the second claim)
Aggregate time
Time of multiple practitioners in the same specialty can be added to meet 99291 or 99292. If Practitioner A spends 15 minutes of critical care, then 99291 cannot be billed; but, if Practitioner B spends 30 minutes of critical care, they can bill 99291 with a total time of 45 minutes as one claim
The prerequisites are that the visits are medically necessary, and each visit meets the definition of critical care.
2. Modifier FS needs to be used for split sharing of critical care services.
Previously, critical care services could not be split shared, but it can be done in 2022. The practitioner who provides the significant portion of the visit needs to bill. A significant or substantive portion is considered to be more than half the cumulative total time of both providers.
Example: The APP spends 20 minutes in critical care services and the physician spends 30 minutes. Total time spent is 50 minutes, and the physician may bill 99291.
It is crucial to note that each provider needs to document a note for the medically necessary critical care that they personally performed and the time they spent. Additionally, upon review of the medical records, the two providers should be easily identifiable, and the medical record must be signed and dated by the provider who performed the substantive portion and billed.
Lastly, do not forget to submit the modifier FS.
3. Modifier 25 needs to be used to get paid for an ED visit or other E/M service on the same day as critical care.
Previously, hospital ED services were not paid on the same date as critical care by the same provider. But, in 2022, the practitioners may bill for ED visit at the hospital and also for other E/M services on the same day when there is supporting documentation. The practitioners will need to document that the E/M service was provided prior to the time when the patient did not require critical care, that the service was medically necessary, and that the service was separate and distinct with no duplication.
Of note, do not forget to submit the modifier 25.
4. Critical care visits will be separately billable from global surgery when unrelated with the use of modifier FT.
Previously pre- and postoperative critical care was included in the surgical package of many procedures with a global period of 10-90 days, and critical care visits would be paid only if the service was unrelated to the procedure. The concept remains the same in 2022 but, now, new modifier FT will need to be used to report critical care services unrelated to the procedure. Also, the service provided will need to meet the definition of critical care, which is usually above and beyond the procedure performed and should be unrelated to the specific injury or general surgical procedure performed.
5. There will be certain critical care medical record documentation requirements.
It is paramount that each practitioner must document the exact total critical care time and not a range or approximation of time. Additionally, it is equally as important for the documentation to indicate that the services provided were medically reasonable and necessary. In the setting of split/shared billing, the role of each practitioner should be clearly identifiable (the condition for which each practitioner treated the patient, how the care was concurrent either subsequent or aggregate, and the total time of each practitioner).
Hopefully, this review will provide a good perception for our members in regards to major updates for 2022, help them navigate the regulatory rules, and avoid any unnecessary setbacks. In the upcoming months, we will try to cover some more topics on practice management and administration, such as Medicare Physician Fee Schedule Rule, Hospital Outpatient Prospective Payment Rule, and coding/billing for teaching physicians, telehealth, and pulmonary rehabilitation services.
In memoriam
CHEST has been informed of the following deaths of CHEST members.
We remember our colleagues and extend our sincere condolences.
Edward C. Rosenow III, MD, Master FCCP
Jack Stanko, MD, MS, FCCP
Arthur S. Turetsky, MD, FCCP
CHEST has been informed of the following deaths of CHEST members.
We remember our colleagues and extend our sincere condolences.
Edward C. Rosenow III, MD, Master FCCP
Jack Stanko, MD, MS, FCCP
Arthur S. Turetsky, MD, FCCP
CHEST has been informed of the following deaths of CHEST members.
We remember our colleagues and extend our sincere condolences.
Edward C. Rosenow III, MD, Master FCCP
Jack Stanko, MD, MS, FCCP
Arthur S. Turetsky, MD, FCCP
Living and leading with lung disease
Fred Schick and Betsy Glaeser use their diagnoses to help others
Receiving a chronic disease diagnosis can be paralyzing, with a wide range of associated emotions. A patient’s family, physicians, and other health care professionals can provide a source of support, but, often, the strongest support comes from those who can empathize.
Someone who has lived with a diagnosis can provide guidance and empathy at a more personal level because, to them, it is just that – personal. Fred Schick and Betsy Glaeser have done just that by taking their personal experiences and using them to help others navigate their diagnoses.
Improving patients’ lives is the core focus of the American College of Chest Physicians and the CHEST Foundation. Events like the Belmont Stakes Dinner and Auction provide an opportunity for us to recognize and celebrate powerful stories such as Fred and Betsy’s, while also raising funds to support important initiatives that will improve patient care. Please consider joining the fight against lung disease by making a donation to the CHEST Foundation today at chestfoundation.org/donate.
Patient Advocate – Fred Schick
Increasing awareness of pulmonary fibrosis
Fred Schick of the Chicagoland area was diagnosed with idiopathic pulmonary fibrosis (IPF) in 2017 after years of searching for the root cause of his worsening symptoms.
Fred started experiencing shortness of breath and labored breathing—once to the extent that he needed to be pulled out of the water on vacation despite being an active swimmer. Because Fred was a former cardiac patient, his doctors looked to his heart for a diagnosis.
It wasn’t until his primary care physician retired that he started seeing a new doctor who took a different look at his symptoms. In hearing about the strong changes in his exercise endurance, this particular doctor made the decision to refer Fred to a pulmonologist, which ultimately led Fred on the right path to his IPF diagnosis.
Helping others navigate the path
In his 5 years since being diagnosed with IPF, Fred uses his experience to advocate for others living with this illness. Active in support groups for those with IPF, he is especially focused on helping others navigate the first few months after receiving their diagnosis.
Fred knows from experience that receiving the IPF diagnosis is something to come to terms with but encourages others to look to him for an example of how to live with the illness.
“The first thing I say to someone who has been recently diagnosed with pulmonary fibrosis is, ‘Whatever you’ve read on the Internet, don’t believe it,’ because there are a lot of people who live well beyond the 3- to 5-year expectancy you’ll see in your Google search.”
“I also encourage everyone to be their own health advocate – tell your doctor if anything in your life is abnormal because you know your body better than anyone.”
Like Fred, many living with IPF wait years for a diagnosis because of the commonality in the way the symptoms present, including shortness of breath, fatigue, difficulty breathing, and others. To address this delay, the American College of Chest Physicians, supported by the CHEST Foundation, partnered with the Three Lakes Foundation to create an initiative led by a steering committee of pulmonologists and primary care physicians to join together to shorten the time to diagnosis for interstitial lung diseases like IPF. Among other activities, the steering committee will work to create tools for physicians to use during patient intake that can more quickly bring IPF into the conversation when it is pertinent.
Patient Advocate – Betsy Glaeser
Blazing the trail for NTM
Local to New York, Betsy Glaeser was diagnosed with pulmonary nontuberculous mycobacteria disease (NTM) more than 20 years ago.
Leading up to her diagnosis, Betsy was frequently short of breath with overwhelming fatigue and fevers. She was hospitalized multiple times for pneumonia and treated again and again with short-term standard antibiotics. At the time (1998), there were no clinical programs dedicated to NTM, and when her sputum was tested, it was only for pneumonia.
As a financial consultant required to travel 4 days per week for work, Betsy grew especially concerned about her illness when she developed hemoptysis and began coughing up blood. Lacking local resources, she sought care at the Mayo Clinic in Rochester, Minnesota, where she received her NTM diagnosis.
Based on the severity of her illness and her worsening symptoms, the recommendation of the Mayo Clinic was that she stop working. After 30 years of challenging jobs, quitting was very painful, but a Mayo doctor asked Betsy a very poignant question that resonated with her: “Are you planning to die for your employer?”
With that, she left her job and sought care for her illness. As her NTM developed a second, more resistant strain associated with her disease, requiring daily, constant treatment, Betsy was fortunate to be accepted into the National Institutes of Health NTM protocol, which has directed her care, coordinated with NYU-Langone.
Despite the challenges of having NTM, Betsy maintains an active and enriching life.
Leading with experience
Betsy uses her diagnosis and her experience with NTM to help others who are hearing their diagnoses for the first time. She serves as a charter member and co-leader of a New York NTM patient support group and serves as a member of the NTM Info & Research (NTMir) Board of Directors.
Her goal is to ensure that no one living with NTM feels alone or frightened.
“Not so long ago – and now, too, even – there were doctors who did not know how to treat NTM,” says Betsy. “But, it has really gotten better – as I’ve progressed through all of my medications and lived with this disease, NTM has progressed as well. I hope I helped expand NTM knowledge with my lived experiences, but I’ve been so fortunate to receive medical care from those doctors who knew the most about NTM.”
Fred Schick and Betsy Glaeser use their diagnoses to help others
Fred Schick and Betsy Glaeser use their diagnoses to help others
Receiving a chronic disease diagnosis can be paralyzing, with a wide range of associated emotions. A patient’s family, physicians, and other health care professionals can provide a source of support, but, often, the strongest support comes from those who can empathize.
Someone who has lived with a diagnosis can provide guidance and empathy at a more personal level because, to them, it is just that – personal. Fred Schick and Betsy Glaeser have done just that by taking their personal experiences and using them to help others navigate their diagnoses.
Improving patients’ lives is the core focus of the American College of Chest Physicians and the CHEST Foundation. Events like the Belmont Stakes Dinner and Auction provide an opportunity for us to recognize and celebrate powerful stories such as Fred and Betsy’s, while also raising funds to support important initiatives that will improve patient care. Please consider joining the fight against lung disease by making a donation to the CHEST Foundation today at chestfoundation.org/donate.
Patient Advocate – Fred Schick
Increasing awareness of pulmonary fibrosis
Fred Schick of the Chicagoland area was diagnosed with idiopathic pulmonary fibrosis (IPF) in 2017 after years of searching for the root cause of his worsening symptoms.
Fred started experiencing shortness of breath and labored breathing—once to the extent that he needed to be pulled out of the water on vacation despite being an active swimmer. Because Fred was a former cardiac patient, his doctors looked to his heart for a diagnosis.
It wasn’t until his primary care physician retired that he started seeing a new doctor who took a different look at his symptoms. In hearing about the strong changes in his exercise endurance, this particular doctor made the decision to refer Fred to a pulmonologist, which ultimately led Fred on the right path to his IPF diagnosis.
Helping others navigate the path
In his 5 years since being diagnosed with IPF, Fred uses his experience to advocate for others living with this illness. Active in support groups for those with IPF, he is especially focused on helping others navigate the first few months after receiving their diagnosis.
Fred knows from experience that receiving the IPF diagnosis is something to come to terms with but encourages others to look to him for an example of how to live with the illness.
“The first thing I say to someone who has been recently diagnosed with pulmonary fibrosis is, ‘Whatever you’ve read on the Internet, don’t believe it,’ because there are a lot of people who live well beyond the 3- to 5-year expectancy you’ll see in your Google search.”
“I also encourage everyone to be their own health advocate – tell your doctor if anything in your life is abnormal because you know your body better than anyone.”
Like Fred, many living with IPF wait years for a diagnosis because of the commonality in the way the symptoms present, including shortness of breath, fatigue, difficulty breathing, and others. To address this delay, the American College of Chest Physicians, supported by the CHEST Foundation, partnered with the Three Lakes Foundation to create an initiative led by a steering committee of pulmonologists and primary care physicians to join together to shorten the time to diagnosis for interstitial lung diseases like IPF. Among other activities, the steering committee will work to create tools for physicians to use during patient intake that can more quickly bring IPF into the conversation when it is pertinent.
Patient Advocate – Betsy Glaeser
Blazing the trail for NTM
Local to New York, Betsy Glaeser was diagnosed with pulmonary nontuberculous mycobacteria disease (NTM) more than 20 years ago.
Leading up to her diagnosis, Betsy was frequently short of breath with overwhelming fatigue and fevers. She was hospitalized multiple times for pneumonia and treated again and again with short-term standard antibiotics. At the time (1998), there were no clinical programs dedicated to NTM, and when her sputum was tested, it was only for pneumonia.
As a financial consultant required to travel 4 days per week for work, Betsy grew especially concerned about her illness when she developed hemoptysis and began coughing up blood. Lacking local resources, she sought care at the Mayo Clinic in Rochester, Minnesota, where she received her NTM diagnosis.
Based on the severity of her illness and her worsening symptoms, the recommendation of the Mayo Clinic was that she stop working. After 30 years of challenging jobs, quitting was very painful, but a Mayo doctor asked Betsy a very poignant question that resonated with her: “Are you planning to die for your employer?”
With that, she left her job and sought care for her illness. As her NTM developed a second, more resistant strain associated with her disease, requiring daily, constant treatment, Betsy was fortunate to be accepted into the National Institutes of Health NTM protocol, which has directed her care, coordinated with NYU-Langone.
Despite the challenges of having NTM, Betsy maintains an active and enriching life.
Leading with experience
Betsy uses her diagnosis and her experience with NTM to help others who are hearing their diagnoses for the first time. She serves as a charter member and co-leader of a New York NTM patient support group and serves as a member of the NTM Info & Research (NTMir) Board of Directors.
Her goal is to ensure that no one living with NTM feels alone or frightened.
“Not so long ago – and now, too, even – there were doctors who did not know how to treat NTM,” says Betsy. “But, it has really gotten better – as I’ve progressed through all of my medications and lived with this disease, NTM has progressed as well. I hope I helped expand NTM knowledge with my lived experiences, but I’ve been so fortunate to receive medical care from those doctors who knew the most about NTM.”
Receiving a chronic disease diagnosis can be paralyzing, with a wide range of associated emotions. A patient’s family, physicians, and other health care professionals can provide a source of support, but, often, the strongest support comes from those who can empathize.
Someone who has lived with a diagnosis can provide guidance and empathy at a more personal level because, to them, it is just that – personal. Fred Schick and Betsy Glaeser have done just that by taking their personal experiences and using them to help others navigate their diagnoses.
Improving patients’ lives is the core focus of the American College of Chest Physicians and the CHEST Foundation. Events like the Belmont Stakes Dinner and Auction provide an opportunity for us to recognize and celebrate powerful stories such as Fred and Betsy’s, while also raising funds to support important initiatives that will improve patient care. Please consider joining the fight against lung disease by making a donation to the CHEST Foundation today at chestfoundation.org/donate.
Patient Advocate – Fred Schick
Increasing awareness of pulmonary fibrosis
Fred Schick of the Chicagoland area was diagnosed with idiopathic pulmonary fibrosis (IPF) in 2017 after years of searching for the root cause of his worsening symptoms.
Fred started experiencing shortness of breath and labored breathing—once to the extent that he needed to be pulled out of the water on vacation despite being an active swimmer. Because Fred was a former cardiac patient, his doctors looked to his heart for a diagnosis.
It wasn’t until his primary care physician retired that he started seeing a new doctor who took a different look at his symptoms. In hearing about the strong changes in his exercise endurance, this particular doctor made the decision to refer Fred to a pulmonologist, which ultimately led Fred on the right path to his IPF diagnosis.
Helping others navigate the path
In his 5 years since being diagnosed with IPF, Fred uses his experience to advocate for others living with this illness. Active in support groups for those with IPF, he is especially focused on helping others navigate the first few months after receiving their diagnosis.
Fred knows from experience that receiving the IPF diagnosis is something to come to terms with but encourages others to look to him for an example of how to live with the illness.
“The first thing I say to someone who has been recently diagnosed with pulmonary fibrosis is, ‘Whatever you’ve read on the Internet, don’t believe it,’ because there are a lot of people who live well beyond the 3- to 5-year expectancy you’ll see in your Google search.”
“I also encourage everyone to be their own health advocate – tell your doctor if anything in your life is abnormal because you know your body better than anyone.”
Like Fred, many living with IPF wait years for a diagnosis because of the commonality in the way the symptoms present, including shortness of breath, fatigue, difficulty breathing, and others. To address this delay, the American College of Chest Physicians, supported by the CHEST Foundation, partnered with the Three Lakes Foundation to create an initiative led by a steering committee of pulmonologists and primary care physicians to join together to shorten the time to diagnosis for interstitial lung diseases like IPF. Among other activities, the steering committee will work to create tools for physicians to use during patient intake that can more quickly bring IPF into the conversation when it is pertinent.
Patient Advocate – Betsy Glaeser
Blazing the trail for NTM
Local to New York, Betsy Glaeser was diagnosed with pulmonary nontuberculous mycobacteria disease (NTM) more than 20 years ago.
Leading up to her diagnosis, Betsy was frequently short of breath with overwhelming fatigue and fevers. She was hospitalized multiple times for pneumonia and treated again and again with short-term standard antibiotics. At the time (1998), there were no clinical programs dedicated to NTM, and when her sputum was tested, it was only for pneumonia.
As a financial consultant required to travel 4 days per week for work, Betsy grew especially concerned about her illness when she developed hemoptysis and began coughing up blood. Lacking local resources, she sought care at the Mayo Clinic in Rochester, Minnesota, where she received her NTM diagnosis.
Based on the severity of her illness and her worsening symptoms, the recommendation of the Mayo Clinic was that she stop working. After 30 years of challenging jobs, quitting was very painful, but a Mayo doctor asked Betsy a very poignant question that resonated with her: “Are you planning to die for your employer?”
With that, she left her job and sought care for her illness. As her NTM developed a second, more resistant strain associated with her disease, requiring daily, constant treatment, Betsy was fortunate to be accepted into the National Institutes of Health NTM protocol, which has directed her care, coordinated with NYU-Langone.
Despite the challenges of having NTM, Betsy maintains an active and enriching life.
Leading with experience
Betsy uses her diagnosis and her experience with NTM to help others who are hearing their diagnoses for the first time. She serves as a charter member and co-leader of a New York NTM patient support group and serves as a member of the NTM Info & Research (NTMir) Board of Directors.
Her goal is to ensure that no one living with NTM feels alone or frightened.
“Not so long ago – and now, too, even – there were doctors who did not know how to treat NTM,” says Betsy. “But, it has really gotten better – as I’ve progressed through all of my medications and lived with this disease, NTM has progressed as well. I hope I helped expand NTM knowledge with my lived experiences, but I’ve been so fortunate to receive medical care from those doctors who knew the most about NTM.”
Bridging Specialties™: Timely diagnosis for patients with ILD
Experts in pulmonary and primary care medicine come together to reduce delays in diagnosing complex lung diseases.
Affecting around 400,000 people in the United States, interstitial lung diseases (ILD), like pulmonary fibrosis (PF), present with symptoms that are similar to other more common lung diseases, frequently resulting in misdiagnosis or delayed diagnosis. Some studies show that reaching a proper diagnosis for rarer lung diseases can take upwards of several years.
Despite scientific advancements and increased information available, timely and accurate diagnosis for PF remains a challenge. The course of the disease varies from person to person and can progress rapidly in some cases, increasing the necessity to have the condition diagnosed in its earliest stages. By the time patients learn they have PF, the condition may require reliance on oxygen use and hospitalizations, and it can lead to poor quality of life and a significantly shortened lifespan.
To address this issue, Three Lakes Foundation (TLF) and the American College of Chest Physicians (CHEST) recently announced their collaboration on a multiphase educational initiative led by a steering committee of medical experts aiming to reduce the time it takes to diagnose patients with ILDs like PF. Composed of pulmonologists, primary care physicians, and a nursing professional, the steering committee will work to create materials that will aid in identifying and diagnosing complex lung diseases quicker.
“As a catalyst for change in the PF community, Three Lakes Foundation spoke with patients, health care professionals, physicians, and advocacy groups to advance an understanding of the PF diagnostic experience,” said Dana Ball, executive director for Three Lakes Foundation. “We approached CHEST when it became apparent that primary care physicians could use specific tools to identify high-risk patients with pulmonary conditions. This collaboration is the result of our common need to increase awareness among health care professionals and to improve patient outcomes.”
Members of the expert steering committee include individuals from leading medical institutions, health systems, and organizations across the country:
- Daniel F. Dilling, MD, FCCP, Professor of Medicine, Division of Pulmonary and Critical Care, Loyola University Chicago, Stritch School of Medicine, Maywood, IL.
- Andrew Duggan, MPH, Patient Engagement and Innovation Leader representing Three Lakes Foundation, Boston, MA.
- Jessica Glennie, APRN, MSN, Nurse Practitioner, Interstitial Lung Disease Clinic, Cleveland Clinic, Cleveland, OH.
- Timothy Hernandez, MD, Family Medicine Physician, Chief Executive Officer of Entira Family Clinics, San Antonio, TX.
- Corey D. Kershaw, MD, FCCP, Associate Professor of Medicine, Division of Pulmonary and Critical Care Medicine, University of Texas Southwestern Medical Center, Dallas, TX.
- Tejaswini Kulkarni, MD, MPH, FCCP, Assistant Professor, Director, Interstitial Lung Disease Program, Division of Pulmonary, Allergy and Critical Care Medicine, The University of Alabama at Birmingham, Birmingham, AL.
- William Lago, MD, Family Medicine Physician, Wooster Family Health Center, Cleveland Clinic Foundation, Wooster, OH.
- Andrew H. Limper, MD, FCCP, Annenberg Professor of Pulmonary Medicine, Professor of Biochemistry and Molecular Biology, Director – Thoracic Disease Research Unit, Mayo Clinic College of Medicine, Rochester, MN.
- Anoop M. Nambiar, MD, MS, FCCP, Professor of Medicine, Founding Director of the UT Health San Antonio Center for Interstitial Lung Diseases, Division of Pulmonary and Critical Care Medicine, Department of Medicine, The University of Texas Health Science Center at San Antonio and South Texas Veterans Health Care System, San Antonio, TX.
- Mary Beth Scholand, MD, Associate Professor of Internal Medicine, Division of Pulmonary Diseases, Director, Interstitial Lung Program, University of Utah, Salt Lake City, UT
“While interstitial lung diseases do not affect a substantial amount of the population, those touched by the disease are impacted tremendously,” says steering committee member and pulmonologist, Dr. Andrew H. Limper. “Any delay in receiving a diagnosis is time that could be dedicated to finding a treatment therapy that can improve their quality of life. I look forward to the work of this committee helping to shape how patients with ILDs are diagnosed and treated in the future.”
Starting with data-gathering surveys sent to both primary care physicians and pulmonologists, the committee will evaluate the findings to develop tools that can be used to aid in diagnosing complex lung diseases.
“Having experts from both pulmonary and primary care medicine as members of the steering committee is critical,” says steering committee member and family medicine physician, Dr. William Lago. “Patients first see their family medicine or primary care clinicians and, all too often, the most complex lung diseases present in ways that are indistinguishable from more common conditions like asthma and COPD. Bringing together experts in both fields will yield the best results in creating a path to diagnosis.”
Three Lakes Foundation is providing the initial funding for CHEST to begin designing an educational intervention that addresses the gaps in knowledge and practice and will play an active role in overseeing the development of the program.
For more information on the Bridging Specialties™: Timely Diagnosis for Patients With ILD initiative and to sign up for updates, visit info.chestnet.org/bridging-specialties-timely-diagnosis-for-ild-patients.
Experts in pulmonary and primary care medicine come together to reduce delays in diagnosing complex lung diseases.
Experts in pulmonary and primary care medicine come together to reduce delays in diagnosing complex lung diseases.
Affecting around 400,000 people in the United States, interstitial lung diseases (ILD), like pulmonary fibrosis (PF), present with symptoms that are similar to other more common lung diseases, frequently resulting in misdiagnosis or delayed diagnosis. Some studies show that reaching a proper diagnosis for rarer lung diseases can take upwards of several years.
Despite scientific advancements and increased information available, timely and accurate diagnosis for PF remains a challenge. The course of the disease varies from person to person and can progress rapidly in some cases, increasing the necessity to have the condition diagnosed in its earliest stages. By the time patients learn they have PF, the condition may require reliance on oxygen use and hospitalizations, and it can lead to poor quality of life and a significantly shortened lifespan.
To address this issue, Three Lakes Foundation (TLF) and the American College of Chest Physicians (CHEST) recently announced their collaboration on a multiphase educational initiative led by a steering committee of medical experts aiming to reduce the time it takes to diagnose patients with ILDs like PF. Composed of pulmonologists, primary care physicians, and a nursing professional, the steering committee will work to create materials that will aid in identifying and diagnosing complex lung diseases quicker.
“As a catalyst for change in the PF community, Three Lakes Foundation spoke with patients, health care professionals, physicians, and advocacy groups to advance an understanding of the PF diagnostic experience,” said Dana Ball, executive director for Three Lakes Foundation. “We approached CHEST when it became apparent that primary care physicians could use specific tools to identify high-risk patients with pulmonary conditions. This collaboration is the result of our common need to increase awareness among health care professionals and to improve patient outcomes.”
Members of the expert steering committee include individuals from leading medical institutions, health systems, and organizations across the country:
- Daniel F. Dilling, MD, FCCP, Professor of Medicine, Division of Pulmonary and Critical Care, Loyola University Chicago, Stritch School of Medicine, Maywood, IL.
- Andrew Duggan, MPH, Patient Engagement and Innovation Leader representing Three Lakes Foundation, Boston, MA.
- Jessica Glennie, APRN, MSN, Nurse Practitioner, Interstitial Lung Disease Clinic, Cleveland Clinic, Cleveland, OH.
- Timothy Hernandez, MD, Family Medicine Physician, Chief Executive Officer of Entira Family Clinics, San Antonio, TX.
- Corey D. Kershaw, MD, FCCP, Associate Professor of Medicine, Division of Pulmonary and Critical Care Medicine, University of Texas Southwestern Medical Center, Dallas, TX.
- Tejaswini Kulkarni, MD, MPH, FCCP, Assistant Professor, Director, Interstitial Lung Disease Program, Division of Pulmonary, Allergy and Critical Care Medicine, The University of Alabama at Birmingham, Birmingham, AL.
- William Lago, MD, Family Medicine Physician, Wooster Family Health Center, Cleveland Clinic Foundation, Wooster, OH.
- Andrew H. Limper, MD, FCCP, Annenberg Professor of Pulmonary Medicine, Professor of Biochemistry and Molecular Biology, Director – Thoracic Disease Research Unit, Mayo Clinic College of Medicine, Rochester, MN.
- Anoop M. Nambiar, MD, MS, FCCP, Professor of Medicine, Founding Director of the UT Health San Antonio Center for Interstitial Lung Diseases, Division of Pulmonary and Critical Care Medicine, Department of Medicine, The University of Texas Health Science Center at San Antonio and South Texas Veterans Health Care System, San Antonio, TX.
- Mary Beth Scholand, MD, Associate Professor of Internal Medicine, Division of Pulmonary Diseases, Director, Interstitial Lung Program, University of Utah, Salt Lake City, UT
“While interstitial lung diseases do not affect a substantial amount of the population, those touched by the disease are impacted tremendously,” says steering committee member and pulmonologist, Dr. Andrew H. Limper. “Any delay in receiving a diagnosis is time that could be dedicated to finding a treatment therapy that can improve their quality of life. I look forward to the work of this committee helping to shape how patients with ILDs are diagnosed and treated in the future.”
Starting with data-gathering surveys sent to both primary care physicians and pulmonologists, the committee will evaluate the findings to develop tools that can be used to aid in diagnosing complex lung diseases.
“Having experts from both pulmonary and primary care medicine as members of the steering committee is critical,” says steering committee member and family medicine physician, Dr. William Lago. “Patients first see their family medicine or primary care clinicians and, all too often, the most complex lung diseases present in ways that are indistinguishable from more common conditions like asthma and COPD. Bringing together experts in both fields will yield the best results in creating a path to diagnosis.”
Three Lakes Foundation is providing the initial funding for CHEST to begin designing an educational intervention that addresses the gaps in knowledge and practice and will play an active role in overseeing the development of the program.
For more information on the Bridging Specialties™: Timely Diagnosis for Patients With ILD initiative and to sign up for updates, visit info.chestnet.org/bridging-specialties-timely-diagnosis-for-ild-patients.
Affecting around 400,000 people in the United States, interstitial lung diseases (ILD), like pulmonary fibrosis (PF), present with symptoms that are similar to other more common lung diseases, frequently resulting in misdiagnosis or delayed diagnosis. Some studies show that reaching a proper diagnosis for rarer lung diseases can take upwards of several years.
Despite scientific advancements and increased information available, timely and accurate diagnosis for PF remains a challenge. The course of the disease varies from person to person and can progress rapidly in some cases, increasing the necessity to have the condition diagnosed in its earliest stages. By the time patients learn they have PF, the condition may require reliance on oxygen use and hospitalizations, and it can lead to poor quality of life and a significantly shortened lifespan.
To address this issue, Three Lakes Foundation (TLF) and the American College of Chest Physicians (CHEST) recently announced their collaboration on a multiphase educational initiative led by a steering committee of medical experts aiming to reduce the time it takes to diagnose patients with ILDs like PF. Composed of pulmonologists, primary care physicians, and a nursing professional, the steering committee will work to create materials that will aid in identifying and diagnosing complex lung diseases quicker.
“As a catalyst for change in the PF community, Three Lakes Foundation spoke with patients, health care professionals, physicians, and advocacy groups to advance an understanding of the PF diagnostic experience,” said Dana Ball, executive director for Three Lakes Foundation. “We approached CHEST when it became apparent that primary care physicians could use specific tools to identify high-risk patients with pulmonary conditions. This collaboration is the result of our common need to increase awareness among health care professionals and to improve patient outcomes.”
Members of the expert steering committee include individuals from leading medical institutions, health systems, and organizations across the country:
- Daniel F. Dilling, MD, FCCP, Professor of Medicine, Division of Pulmonary and Critical Care, Loyola University Chicago, Stritch School of Medicine, Maywood, IL.
- Andrew Duggan, MPH, Patient Engagement and Innovation Leader representing Three Lakes Foundation, Boston, MA.
- Jessica Glennie, APRN, MSN, Nurse Practitioner, Interstitial Lung Disease Clinic, Cleveland Clinic, Cleveland, OH.
- Timothy Hernandez, MD, Family Medicine Physician, Chief Executive Officer of Entira Family Clinics, San Antonio, TX.
- Corey D. Kershaw, MD, FCCP, Associate Professor of Medicine, Division of Pulmonary and Critical Care Medicine, University of Texas Southwestern Medical Center, Dallas, TX.
- Tejaswini Kulkarni, MD, MPH, FCCP, Assistant Professor, Director, Interstitial Lung Disease Program, Division of Pulmonary, Allergy and Critical Care Medicine, The University of Alabama at Birmingham, Birmingham, AL.
- William Lago, MD, Family Medicine Physician, Wooster Family Health Center, Cleveland Clinic Foundation, Wooster, OH.
- Andrew H. Limper, MD, FCCP, Annenberg Professor of Pulmonary Medicine, Professor of Biochemistry and Molecular Biology, Director – Thoracic Disease Research Unit, Mayo Clinic College of Medicine, Rochester, MN.
- Anoop M. Nambiar, MD, MS, FCCP, Professor of Medicine, Founding Director of the UT Health San Antonio Center for Interstitial Lung Diseases, Division of Pulmonary and Critical Care Medicine, Department of Medicine, The University of Texas Health Science Center at San Antonio and South Texas Veterans Health Care System, San Antonio, TX.
- Mary Beth Scholand, MD, Associate Professor of Internal Medicine, Division of Pulmonary Diseases, Director, Interstitial Lung Program, University of Utah, Salt Lake City, UT
“While interstitial lung diseases do not affect a substantial amount of the population, those touched by the disease are impacted tremendously,” says steering committee member and pulmonologist, Dr. Andrew H. Limper. “Any delay in receiving a diagnosis is time that could be dedicated to finding a treatment therapy that can improve their quality of life. I look forward to the work of this committee helping to shape how patients with ILDs are diagnosed and treated in the future.”
Starting with data-gathering surveys sent to both primary care physicians and pulmonologists, the committee will evaluate the findings to develop tools that can be used to aid in diagnosing complex lung diseases.
“Having experts from both pulmonary and primary care medicine as members of the steering committee is critical,” says steering committee member and family medicine physician, Dr. William Lago. “Patients first see their family medicine or primary care clinicians and, all too often, the most complex lung diseases present in ways that are indistinguishable from more common conditions like asthma and COPD. Bringing together experts in both fields will yield the best results in creating a path to diagnosis.”
Three Lakes Foundation is providing the initial funding for CHEST to begin designing an educational intervention that addresses the gaps in knowledge and practice and will play an active role in overseeing the development of the program.
For more information on the Bridging Specialties™: Timely Diagnosis for Patients With ILD initiative and to sign up for updates, visit info.chestnet.org/bridging-specialties-timely-diagnosis-for-ild-patients.
Bronchiectasis, microplastics, and end-of-life
Airways disorders network, bronchiectasis section
Phenotyping bronchiectasis: Focus on eosinophilic bronchiectasis
Bronchiectasis has been often linked to neutrophilic inflammation; however, 20% may have a predominantly eosinophilic inflammation.
Eosinophilic bronchiectasis has been associated with a distinct airway microbiome. Shoemark and colleagues showed in an analysis of 1,007 patients from five countries that 22.6% of patients had blood eosinophil counts (BEC) of >300 cells/μL. BEC of <100 cells/μL were associated with higher bronchiectasis severity and increased mortality (Shoemark et al. Am J Respir Crit Care Med. 2022;205[8]:894-902).
BEC of >300 cells/μL were correlated with Streptococcus- and Pseudomonas-dominated microbiome profiles. Compared with patients with BEC of <100 cells/μL, patients with 100-299 cells/μL (hazard ratio [HR], 2.38; 95% confidence interval, 1.33–4.25; P = .003) and those with >300 cells/μL (HR, 3.99; 95% confidence interval, 2.20–7.85; P = .0001) were associated with shorter time to exacerbation.
Eosinophilic inflammation is a risk factor for exacerbations in patients with P. aeruginosa infection and may be considered as a treatable trait. Shoemark and colleagues’ data show that quality of life was improved with inhaled corticosteroid treatment in patients with bronchiectasis who had blood eosinophil counts of >3%, and eosinophils contribute to bronchiectasis exacerbations.
Dharani Narendra, MD
Navitha Ramesh, MD, FCCP
Diego Maselli Caceres, MD, FCCP
Section Members-at-Large
Diffuse lung disease and lung transplant network, occupational and environmental health section
A ubiquitous invasion: The rise of microplastics
About 6.3 billion tons of plastic waste were produced between 1950 and 2015.1 Their degradation into submillimeter fragments of 1 μm to 5 mm, is called microplastics (MP).2 MP are vectors of pollutants, pathologic microorganisms, and chemical additives used in their fabrication.3 Exposure to MP is unavoidable as they are bio-persistent and ubiquitous, even indoors.4 MP have been detected in the snow of large metropolitan areas and in remote locations.5 Humans are exposed to MP via oral ingestion and inhalation. A Brazilian study of human lung autopsy specimens revealed the presence of MP in 13 of 20 subjects.3
In vitro studies have suggested a causal role of polystyrene-MP in the development of chronic pulmonary disease through the formation of reactive oxygen species, inhibition of cell proliferation, and cellular morphology aberration.6 MP can cause local effects due to macrophage-induced inflammation, or alternatively, be transported distantly to the pleura and the systemic circulation.
In addition, MP may disrupt the endocrine pathway due to its estrogenic effects.7 Larger MPs of 8 to 10 µm, like nylon, have been associated with interstitial lung disease.8 Lung biopsies from workers exposed to airborne synthetic fibers (acrylic, polyester, and terylene) have revealed different degrees of inflammation, granulomas, and interstitial fibrosis.9 Factory workers exposed to polyvinyl chloride dust have increased risk of exertional dyspnea and decreased pulmonary function.10 Due to the pervasive nature of MP, it is essential to establish the global burden of airborne MP and to determine its role in lung health.
Bathmapriya Balakrishnan, MD
Member-at-Large
*Tyler Church, DO
Fellow-in-Training Member
*Disclaimer: The views expressed in this article are those of the author(s) and do not reflect the official policy of the Department of Army/Navy/Air Force, Department of Defense, or U.S. Government.
References
1. Rhodes CJ. Plastic pollution and potential solutions. Sci Prog. 2018;101(3):207-60.
2. Danopoulos E et al. Microplastic contamination of drinking water: A systematic review. PLoS One. 2020;15(7):e0236838.
3. Amato-Lourenço LF et al. Presence of airborne microplastics in human lung tissue. J Hazard Mater. 2021;416:126.
4. Al Horr Y et al. Occupant productivity and office indoor environment quality: A review of the literature. Building and Environment. 2016;105:369-89.
5. Bergmann M et al. White and wonderful? Microplastics prevail in snow from the Alps to the Arctic. Sci Adv. 2019;5:eaax1157.
6. Dong CD et al. Polystyrene microplastic particles: In vitro pulmonary toxicity assessment. J Hazard Mater. 2020;385:121575.
7. Amato-Lourenço LF et al. An emerging class of air pollutants: Potential effects of microplastics to respiratory human health. Sci Total Environ. 2020;749:141676.
8. Kern DG et al. Flock worker’s lung: Chronic interstitial lung disease in the nylon flocking industry. Ann Intern Med. 1998;129[4]:261-72. Erratum in: Ann Intern Med. 1999;130[3]:246.
9. Pimentel JC et al. Respiratory disease caused by synthetic fibers: a new occupational disease. Thorax. 1975;30:204-19.
10. Soutar CA et al. Epidemiological study of respiratory disease in workers exposed to polyvinyl chloride dust. Thorax. 1980;35:644-52.
Critical care network, palliative and end-of-life section
Discussing code status with families of critically ill patients
Discussing code status with patients is complex and emotional, especially when critically ill.
The complexity further increases when these conversations have to take place with family members.
Here are some practical tips to help have these conversations in a concise and compassionate manner.
Introduction
- Introduce yourself, and make sure to identify the correct decision-maker.
- Get to know the patient.
–What kind of person are they?
–What brings them joy?
- Find out what the family knows about the current clinical condition of their family member.
–What have you been hearing from the medical team?
–What are you worried about?
Update
- Fill in the gaps – update them on the clinical condition and ongoing management.
- Discuss how you think they will respond to current management and further management options.
- Allow them to process the information.
Provide a medical recommendation
- Example: We are worried he might die, and if his heart stops, interventions like CPR or intubation would not work, and we would not recommend them.
- Do not pressure for a decision right away. (You can say “We do not need a decision today, so please take time to process this information.”)
Respond to emotions
- I can’t image how hard this must be.
- Offer chaplain services if that is important to them.
Things to avoid
- Avoid aggressive language.
–We will have to pound on their chest, break ribs.
–They would be suffering.
- Blaming or judgmental language.
While this complex discussion r equires individualization, these tips will help set a framework for goals of care conversations that lead to high quality care for patients that aligns with their goals.
Reference
Goldfish and Rosielle. Language for Routine Code Status Discussions, Fast Facts and Concepts #365, Palliative Care Network of Wisconsin.
Syed Nazeer Mahmood, MD
Fellow-in-Training Member
Anne Kelemen, LCSW
Member-at-Large
Airways disorders network, bronchiectasis section
Phenotyping bronchiectasis: Focus on eosinophilic bronchiectasis
Bronchiectasis has been often linked to neutrophilic inflammation; however, 20% may have a predominantly eosinophilic inflammation.
Eosinophilic bronchiectasis has been associated with a distinct airway microbiome. Shoemark and colleagues showed in an analysis of 1,007 patients from five countries that 22.6% of patients had blood eosinophil counts (BEC) of >300 cells/μL. BEC of <100 cells/μL were associated with higher bronchiectasis severity and increased mortality (Shoemark et al. Am J Respir Crit Care Med. 2022;205[8]:894-902).
BEC of >300 cells/μL were correlated with Streptococcus- and Pseudomonas-dominated microbiome profiles. Compared with patients with BEC of <100 cells/μL, patients with 100-299 cells/μL (hazard ratio [HR], 2.38; 95% confidence interval, 1.33–4.25; P = .003) and those with >300 cells/μL (HR, 3.99; 95% confidence interval, 2.20–7.85; P = .0001) were associated with shorter time to exacerbation.
Eosinophilic inflammation is a risk factor for exacerbations in patients with P. aeruginosa infection and may be considered as a treatable trait. Shoemark and colleagues’ data show that quality of life was improved with inhaled corticosteroid treatment in patients with bronchiectasis who had blood eosinophil counts of >3%, and eosinophils contribute to bronchiectasis exacerbations.
Dharani Narendra, MD
Navitha Ramesh, MD, FCCP
Diego Maselli Caceres, MD, FCCP
Section Members-at-Large
Diffuse lung disease and lung transplant network, occupational and environmental health section
A ubiquitous invasion: The rise of microplastics
About 6.3 billion tons of plastic waste were produced between 1950 and 2015.1 Their degradation into submillimeter fragments of 1 μm to 5 mm, is called microplastics (MP).2 MP are vectors of pollutants, pathologic microorganisms, and chemical additives used in their fabrication.3 Exposure to MP is unavoidable as they are bio-persistent and ubiquitous, even indoors.4 MP have been detected in the snow of large metropolitan areas and in remote locations.5 Humans are exposed to MP via oral ingestion and inhalation. A Brazilian study of human lung autopsy specimens revealed the presence of MP in 13 of 20 subjects.3
In vitro studies have suggested a causal role of polystyrene-MP in the development of chronic pulmonary disease through the formation of reactive oxygen species, inhibition of cell proliferation, and cellular morphology aberration.6 MP can cause local effects due to macrophage-induced inflammation, or alternatively, be transported distantly to the pleura and the systemic circulation.
In addition, MP may disrupt the endocrine pathway due to its estrogenic effects.7 Larger MPs of 8 to 10 µm, like nylon, have been associated with interstitial lung disease.8 Lung biopsies from workers exposed to airborne synthetic fibers (acrylic, polyester, and terylene) have revealed different degrees of inflammation, granulomas, and interstitial fibrosis.9 Factory workers exposed to polyvinyl chloride dust have increased risk of exertional dyspnea and decreased pulmonary function.10 Due to the pervasive nature of MP, it is essential to establish the global burden of airborne MP and to determine its role in lung health.
Bathmapriya Balakrishnan, MD
Member-at-Large
*Tyler Church, DO
Fellow-in-Training Member
*Disclaimer: The views expressed in this article are those of the author(s) and do not reflect the official policy of the Department of Army/Navy/Air Force, Department of Defense, or U.S. Government.
References
1. Rhodes CJ. Plastic pollution and potential solutions. Sci Prog. 2018;101(3):207-60.
2. Danopoulos E et al. Microplastic contamination of drinking water: A systematic review. PLoS One. 2020;15(7):e0236838.
3. Amato-Lourenço LF et al. Presence of airborne microplastics in human lung tissue. J Hazard Mater. 2021;416:126.
4. Al Horr Y et al. Occupant productivity and office indoor environment quality: A review of the literature. Building and Environment. 2016;105:369-89.
5. Bergmann M et al. White and wonderful? Microplastics prevail in snow from the Alps to the Arctic. Sci Adv. 2019;5:eaax1157.
6. Dong CD et al. Polystyrene microplastic particles: In vitro pulmonary toxicity assessment. J Hazard Mater. 2020;385:121575.
7. Amato-Lourenço LF et al. An emerging class of air pollutants: Potential effects of microplastics to respiratory human health. Sci Total Environ. 2020;749:141676.
8. Kern DG et al. Flock worker’s lung: Chronic interstitial lung disease in the nylon flocking industry. Ann Intern Med. 1998;129[4]:261-72. Erratum in: Ann Intern Med. 1999;130[3]:246.
9. Pimentel JC et al. Respiratory disease caused by synthetic fibers: a new occupational disease. Thorax. 1975;30:204-19.
10. Soutar CA et al. Epidemiological study of respiratory disease in workers exposed to polyvinyl chloride dust. Thorax. 1980;35:644-52.
Critical care network, palliative and end-of-life section
Discussing code status with families of critically ill patients
Discussing code status with patients is complex and emotional, especially when critically ill.
The complexity further increases when these conversations have to take place with family members.
Here are some practical tips to help have these conversations in a concise and compassionate manner.
Introduction
- Introduce yourself, and make sure to identify the correct decision-maker.
- Get to know the patient.
–What kind of person are they?
–What brings them joy?
- Find out what the family knows about the current clinical condition of their family member.
–What have you been hearing from the medical team?
–What are you worried about?
Update
- Fill in the gaps – update them on the clinical condition and ongoing management.
- Discuss how you think they will respond to current management and further management options.
- Allow them to process the information.
Provide a medical recommendation
- Example: We are worried he might die, and if his heart stops, interventions like CPR or intubation would not work, and we would not recommend them.
- Do not pressure for a decision right away. (You can say “We do not need a decision today, so please take time to process this information.”)
Respond to emotions
- I can’t image how hard this must be.
- Offer chaplain services if that is important to them.
Things to avoid
- Avoid aggressive language.
–We will have to pound on their chest, break ribs.
–They would be suffering.
- Blaming or judgmental language.
While this complex discussion r equires individualization, these tips will help set a framework for goals of care conversations that lead to high quality care for patients that aligns with their goals.
Reference
Goldfish and Rosielle. Language for Routine Code Status Discussions, Fast Facts and Concepts #365, Palliative Care Network of Wisconsin.
Syed Nazeer Mahmood, MD
Fellow-in-Training Member
Anne Kelemen, LCSW
Member-at-Large
Airways disorders network, bronchiectasis section
Phenotyping bronchiectasis: Focus on eosinophilic bronchiectasis
Bronchiectasis has been often linked to neutrophilic inflammation; however, 20% may have a predominantly eosinophilic inflammation.
Eosinophilic bronchiectasis has been associated with a distinct airway microbiome. Shoemark and colleagues showed in an analysis of 1,007 patients from five countries that 22.6% of patients had blood eosinophil counts (BEC) of >300 cells/μL. BEC of <100 cells/μL were associated with higher bronchiectasis severity and increased mortality (Shoemark et al. Am J Respir Crit Care Med. 2022;205[8]:894-902).
BEC of >300 cells/μL were correlated with Streptococcus- and Pseudomonas-dominated microbiome profiles. Compared with patients with BEC of <100 cells/μL, patients with 100-299 cells/μL (hazard ratio [HR], 2.38; 95% confidence interval, 1.33–4.25; P = .003) and those with >300 cells/μL (HR, 3.99; 95% confidence interval, 2.20–7.85; P = .0001) were associated with shorter time to exacerbation.
Eosinophilic inflammation is a risk factor for exacerbations in patients with P. aeruginosa infection and may be considered as a treatable trait. Shoemark and colleagues’ data show that quality of life was improved with inhaled corticosteroid treatment in patients with bronchiectasis who had blood eosinophil counts of >3%, and eosinophils contribute to bronchiectasis exacerbations.
Dharani Narendra, MD
Navitha Ramesh, MD, FCCP
Diego Maselli Caceres, MD, FCCP
Section Members-at-Large
Diffuse lung disease and lung transplant network, occupational and environmental health section
A ubiquitous invasion: The rise of microplastics
About 6.3 billion tons of plastic waste were produced between 1950 and 2015.1 Their degradation into submillimeter fragments of 1 μm to 5 mm, is called microplastics (MP).2 MP are vectors of pollutants, pathologic microorganisms, and chemical additives used in their fabrication.3 Exposure to MP is unavoidable as they are bio-persistent and ubiquitous, even indoors.4 MP have been detected in the snow of large metropolitan areas and in remote locations.5 Humans are exposed to MP via oral ingestion and inhalation. A Brazilian study of human lung autopsy specimens revealed the presence of MP in 13 of 20 subjects.3
In vitro studies have suggested a causal role of polystyrene-MP in the development of chronic pulmonary disease through the formation of reactive oxygen species, inhibition of cell proliferation, and cellular morphology aberration.6 MP can cause local effects due to macrophage-induced inflammation, or alternatively, be transported distantly to the pleura and the systemic circulation.
In addition, MP may disrupt the endocrine pathway due to its estrogenic effects.7 Larger MPs of 8 to 10 µm, like nylon, have been associated with interstitial lung disease.8 Lung biopsies from workers exposed to airborne synthetic fibers (acrylic, polyester, and terylene) have revealed different degrees of inflammation, granulomas, and interstitial fibrosis.9 Factory workers exposed to polyvinyl chloride dust have increased risk of exertional dyspnea and decreased pulmonary function.10 Due to the pervasive nature of MP, it is essential to establish the global burden of airborne MP and to determine its role in lung health.
Bathmapriya Balakrishnan, MD
Member-at-Large
*Tyler Church, DO
Fellow-in-Training Member
*Disclaimer: The views expressed in this article are those of the author(s) and do not reflect the official policy of the Department of Army/Navy/Air Force, Department of Defense, or U.S. Government.
References
1. Rhodes CJ. Plastic pollution and potential solutions. Sci Prog. 2018;101(3):207-60.
2. Danopoulos E et al. Microplastic contamination of drinking water: A systematic review. PLoS One. 2020;15(7):e0236838.
3. Amato-Lourenço LF et al. Presence of airborne microplastics in human lung tissue. J Hazard Mater. 2021;416:126.
4. Al Horr Y et al. Occupant productivity and office indoor environment quality: A review of the literature. Building and Environment. 2016;105:369-89.
5. Bergmann M et al. White and wonderful? Microplastics prevail in snow from the Alps to the Arctic. Sci Adv. 2019;5:eaax1157.
6. Dong CD et al. Polystyrene microplastic particles: In vitro pulmonary toxicity assessment. J Hazard Mater. 2020;385:121575.
7. Amato-Lourenço LF et al. An emerging class of air pollutants: Potential effects of microplastics to respiratory human health. Sci Total Environ. 2020;749:141676.
8. Kern DG et al. Flock worker’s lung: Chronic interstitial lung disease in the nylon flocking industry. Ann Intern Med. 1998;129[4]:261-72. Erratum in: Ann Intern Med. 1999;130[3]:246.
9. Pimentel JC et al. Respiratory disease caused by synthetic fibers: a new occupational disease. Thorax. 1975;30:204-19.
10. Soutar CA et al. Epidemiological study of respiratory disease in workers exposed to polyvinyl chloride dust. Thorax. 1980;35:644-52.
Critical care network, palliative and end-of-life section
Discussing code status with families of critically ill patients
Discussing code status with patients is complex and emotional, especially when critically ill.
The complexity further increases when these conversations have to take place with family members.
Here are some practical tips to help have these conversations in a concise and compassionate manner.
Introduction
- Introduce yourself, and make sure to identify the correct decision-maker.
- Get to know the patient.
–What kind of person are they?
–What brings them joy?
- Find out what the family knows about the current clinical condition of their family member.
–What have you been hearing from the medical team?
–What are you worried about?
Update
- Fill in the gaps – update them on the clinical condition and ongoing management.
- Discuss how you think they will respond to current management and further management options.
- Allow them to process the information.
Provide a medical recommendation
- Example: We are worried he might die, and if his heart stops, interventions like CPR or intubation would not work, and we would not recommend them.
- Do not pressure for a decision right away. (You can say “We do not need a decision today, so please take time to process this information.”)
Respond to emotions
- I can’t image how hard this must be.
- Offer chaplain services if that is important to them.
Things to avoid
- Avoid aggressive language.
–We will have to pound on their chest, break ribs.
–They would be suffering.
- Blaming or judgmental language.
While this complex discussion r equires individualization, these tips will help set a framework for goals of care conversations that lead to high quality care for patients that aligns with their goals.
Reference
Goldfish and Rosielle. Language for Routine Code Status Discussions, Fast Facts and Concepts #365, Palliative Care Network of Wisconsin.
Syed Nazeer Mahmood, MD
Fellow-in-Training Member
Anne Kelemen, LCSW
Member-at-Large
This month in the journal CHEST®
Editor’s picks
The Relationship Between Insurance Status and The Affordable Care Act on Asthma Outcomes Among Low-Income Us Adults. By Dr. Rajat Suri et al.
Characteristics and Outcomes of Intensive Care Unit Patients With Respiratory Syncytial Virus Compared to Those With Influenza Infection: A Multicentre Matched Cohort Study. By Dr. Julien Coussement et al
“Can Do, Do Do” Quadrants and 6-Year All-Cause Mortality in Patients with COPD. By Dr. Anouk W. Vaes et al.
Trends in Geriatric Conditions Among Older Adults Admitted to US ICUs Between 1998 and 2015. By Dr. Julien Cobert et al.
Setting and Titrating Positive End-Expiratory Pressure. By Dr. Scott J. Millington et al.
COVID-19 in Lymphangioleiomyomatosis: An International Study of Outcomes and Impact of Mechanistic Target of Rapamycin Inhibition. By Dr. Bruno Guedes Baldi et al.
Perceptions of Life Support and Advance Care Planning During the COVID-19 Pandemic: A Global Study of Twitter Users. By Vishal R. Patel et al.
Framework for Integrating Equity Into Machine Learning Models: A Case Study. By Dr. Juan C. Rojas et al.
Comparison of Guidelines for Evaluation of Suspected Pulmonary Embolism in Pregnancy: A Cost-Effectiveness Analysis. By John Austin McCandlish et al.
Relationship Between CPAP Termination and All-Cause Mortality: A French Nationwide Database Analysis. By Dr. Jean-Louis Pépin et al.
Clinical Outcomes of Immune Checkpoint Inhibitor Therapy in Patients With Advanced Non-small Cell Lung Cancer and Preexisting Interstitial Lung Diseases: A Systematic Review and Meta-Analysis. By Dr. Meng Zhang, et al.
The Impact of Persistent Smoking After Surgery on Long-Term Outcomes After Stage I Non–Small Cell Lung Cancer Resection. By Dr. Brendan T. Heiden et al.
Editor’s picks
Editor’s picks
The Relationship Between Insurance Status and The Affordable Care Act on Asthma Outcomes Among Low-Income Us Adults. By Dr. Rajat Suri et al.
Characteristics and Outcomes of Intensive Care Unit Patients With Respiratory Syncytial Virus Compared to Those With Influenza Infection: A Multicentre Matched Cohort Study. By Dr. Julien Coussement et al
“Can Do, Do Do” Quadrants and 6-Year All-Cause Mortality in Patients with COPD. By Dr. Anouk W. Vaes et al.
Trends in Geriatric Conditions Among Older Adults Admitted to US ICUs Between 1998 and 2015. By Dr. Julien Cobert et al.
Setting and Titrating Positive End-Expiratory Pressure. By Dr. Scott J. Millington et al.
COVID-19 in Lymphangioleiomyomatosis: An International Study of Outcomes and Impact of Mechanistic Target of Rapamycin Inhibition. By Dr. Bruno Guedes Baldi et al.
Perceptions of Life Support and Advance Care Planning During the COVID-19 Pandemic: A Global Study of Twitter Users. By Vishal R. Patel et al.
Framework for Integrating Equity Into Machine Learning Models: A Case Study. By Dr. Juan C. Rojas et al.
Comparison of Guidelines for Evaluation of Suspected Pulmonary Embolism in Pregnancy: A Cost-Effectiveness Analysis. By John Austin McCandlish et al.
Relationship Between CPAP Termination and All-Cause Mortality: A French Nationwide Database Analysis. By Dr. Jean-Louis Pépin et al.
Clinical Outcomes of Immune Checkpoint Inhibitor Therapy in Patients With Advanced Non-small Cell Lung Cancer and Preexisting Interstitial Lung Diseases: A Systematic Review and Meta-Analysis. By Dr. Meng Zhang, et al.
The Impact of Persistent Smoking After Surgery on Long-Term Outcomes After Stage I Non–Small Cell Lung Cancer Resection. By Dr. Brendan T. Heiden et al.
The Relationship Between Insurance Status and The Affordable Care Act on Asthma Outcomes Among Low-Income Us Adults. By Dr. Rajat Suri et al.
Characteristics and Outcomes of Intensive Care Unit Patients With Respiratory Syncytial Virus Compared to Those With Influenza Infection: A Multicentre Matched Cohort Study. By Dr. Julien Coussement et al
“Can Do, Do Do” Quadrants and 6-Year All-Cause Mortality in Patients with COPD. By Dr. Anouk W. Vaes et al.
Trends in Geriatric Conditions Among Older Adults Admitted to US ICUs Between 1998 and 2015. By Dr. Julien Cobert et al.
Setting and Titrating Positive End-Expiratory Pressure. By Dr. Scott J. Millington et al.
COVID-19 in Lymphangioleiomyomatosis: An International Study of Outcomes and Impact of Mechanistic Target of Rapamycin Inhibition. By Dr. Bruno Guedes Baldi et al.
Perceptions of Life Support and Advance Care Planning During the COVID-19 Pandemic: A Global Study of Twitter Users. By Vishal R. Patel et al.
Framework for Integrating Equity Into Machine Learning Models: A Case Study. By Dr. Juan C. Rojas et al.
Comparison of Guidelines for Evaluation of Suspected Pulmonary Embolism in Pregnancy: A Cost-Effectiveness Analysis. By John Austin McCandlish et al.
Relationship Between CPAP Termination and All-Cause Mortality: A French Nationwide Database Analysis. By Dr. Jean-Louis Pépin et al.
Clinical Outcomes of Immune Checkpoint Inhibitor Therapy in Patients With Advanced Non-small Cell Lung Cancer and Preexisting Interstitial Lung Diseases: A Systematic Review and Meta-Analysis. By Dr. Meng Zhang, et al.
The Impact of Persistent Smoking After Surgery on Long-Term Outcomes After Stage I Non–Small Cell Lung Cancer Resection. By Dr. Brendan T. Heiden et al.
The AGA Research Foundation awards $2.56 million in funding
AGA is proud to announce the 61 recipients selected to receive research funding through its annual AGA Research Foundation Awards Program. The program serves as a catalyst for discovery and career growth among the most promising researchers in gastroenterology and hepatology.
“Our award recipients demonstrate an undeniable determination to improve the care of digestive health patients,” said Robert S. Sandler, MD, MPH, AGAF, chair of the AGA Research Foundation. “We are investing in talented early-career investigators knowing that their work will ultimately benefit patients with critical needs.”

“In the past year, we expanded our awards program and elevated the importance of engaging underrepresented groups into the field of GI research,” Dr. Sandler said. “We are encouraged by the range of candidates who applied for funding and look forward to the results of their research.”
The AGA Research Foundation Awards Program is made possible thanks to generous donors and funders.
Here are this year’s award recipients:
Research Scholar Awards
AGA Research Scholar Award
Kathleen Curtius, PhD, MS, University of California, San Diego, La Jolla
Trisha Satya Pasricha, MD, MPH, Massachusetts General Hospital, Boston
Bomi Lee, PhD, MS, Stanford University, Palo Alto, Calif.
Christine E. Eyler, MD, PhD, Duke University, Durham, N.C.
Joel Gabre, MD, Columbia University Irving Medical Center, New York
AGA–Bern Schwartz Family Fund Research Scholar Award in Pancreatic Cancer
Srinivas Gaddam, MD, MPH, Cedars-Sinai Medical Center, Los Angeles
AGA–Takeda Pharmaceuticals Research Scholar Award in Celiac Disease
Claire L. Jansson-Knodell, MD, Cleveland Clinic Foundation, Cleveland
Specialty Awards
AGA–R. Robert & Sally Funderburg Research Award in Gastric Cancer
Eunyoung Choi, PhD, Vanderbilt University Medical Center, Nashville, Tenn.
AGA–Caroline Craig Augustyn & Damian Augustyn Award in Digestive Cancer
Sarah Palmer Short, PhD, Vanderbilt University Medical Center, Nashville, Tenn.
Pilot Awards
AGA–Medtronic Pilot Research Award in Artificial Intelligence
Dennis Shung, MD, MHS, Yale School of Medicine, New Haven, Conn.
AGA–Merck Pilot Research Award in Colorectal Cancer Health Disparities
Sonia Kupfer, MD, The University of Chicago, Chicago
AGA–Bristol Myers Squibb Pilot Research Award in Inflammatory Bowel Disease Health Disparities
Chung Sang Tse, MD, University of California, San Diego
AGA Pilot Research Award in Health Disparities (funded by Janssen Biotech)
Jennifer Flemming, MD, MAS, Queen’s University, Kingston, Ont.
AGA Pilot Research Award in Digestive Disease Health Disparities
Young-Rock Hong, PhD, MPH, University of Florida, Gainesville, Fla.
AGA–Amgen Pilot Research Award in Digestive Disease Health Disparities
Zachary Reichenbach, MD, PhD, Lewis Katz School of Medicine, Temple University, Philadelphia
AGA–Pfizer Pilot Research Award in Inflammatory Bowel Disease
Melinda Engevik, PhD, MS, Medical University of South Carolina, Charleston
Andre Paes Batista da Silva, PhD, MSC, DDS, Case Western Reserve University, Cleveland
Karen Edelblum, PhD, Rutgers New Jersey Medical School, Newark, N.J.
Undergraduate Research Awards
AGA–Aman Armaan Ahmed Family Summer Undergraduate Research Award
Gabriela Ortiz, Washington University School of Medicine, St. Louis
Daniella Montalvo, University of Miami Miller School of Medicine, Miami
Subear Hussein, Children’s Hospital, Boston
Hussein Herz, University of Iowa Carver College of Medicine, Iowa City
Kaleb Tesfai, University of California, San Diego
Varun Ponnusamy, University of Michigan Medical School, Ann Arbor, Mich.
Abstract Awards
AGA Fellow Abstract of the Year Award
Masaru Sasaki, MD, PhD, The Children’s Hospital of Philadelphia
AGA Student Abstract of the Year Award
Anitha Vijay, MS, Penn State University, State College, Pa.
Maafi Rizwana Islam, PhD, Marshall University, Huntington, W.V.
Fellow Abstract Awards
Nicolette Rodriguez, MD, MPH, Brigham and Women’s Hospital, Boston
Hyunseok Kim, MD, PhD, MPH, Baylor College of Medicine, Houston
Margaret Zhou, MD, Stanford University, Palo Alto, Calif.
Steven Steinway, MD, PhD, Johns Hopkins University, Baltimore
Su-Hyung Lee, PhD, DVM, Vanderbilt University Medical Center, Nashville, Tenn.
Ian Greenberg, MD, Dallas Methodist Hospital, Dallas
Jonathan Xia, MD, PhD, Northwestern Memorial Hospital, Chicago
Donevan Westerveld, MD, NewYork-Presbyterian Weill Cornell Medicine, New York
Haley Zylberberg, MD, Columbia University Irving Medical Center, New York
Maria Jesus Villanueva Millan, PhD, Cedars-Sinai Medical Center, Los Angeles
Duke Geem, MD, PhD. Children s Healthcare of Atlanta/Emory University, Atlanta
Fauzi Feris Jassir, MD, Mayo Clinic, Rochester, Minn.
Melissa Musser, MD, PhD, Boston Children’s Hospital, Boston
Student Abstract Awards
Kushal Saha, MS, BS, Penn State College of Medicine, Hershey, Pa.
Winston Liu, BS. Duke University, Durham, N.C.
Yoojin Sohn, BS, Vanderbilt University Medical Center, Nashville, Tenn.
Jamie Yang, BS, David Geffen School of Medicine at University of California, Los Angeles
Rachel Hopton, BS, University of Oregon, Eugene
Alina Li, BS, Columbia University, New York
Eleazar Montalvan Sanchez, MD, Indiana University School of Medicine, Indianapolis
Christina Lin, MD, BA, BS, Kaiser Permanente Northern California, Santa Clara, Calif.
Conrad Fernandes, MD, BA, Hospital of the University of Pennsylvania, Philadelphia
Hajar Hazime, MS, BS, University of Miami
Blaine Prichard, BS, Pennsylvania State University College of Medicine, Hershey, Pa.
Georgetta Skinner, MS, BS, A.T. Still University, Kirksville, Mo.
AGA Abstract Award for Health Disparities Research
Kai Wang, PhD (Fellow), Harvard T.H. Chan School of Public Health, Boston
Alan De La Rosa, MD (Fellow), Mayo Clinic, Rochester, Minn.
Timothy Andrew Zaki, MD, BS (Student), UT Southwestern Medical Center, Dallas
Megan McLeod, MD, MS, BA, University of California, Los Angeles (student)
AGA–APFED Abstract Award in Eosinophilic GI Diseases
Takeo Hara, MD, PhD, Children’s Hospital of Philadelphia
Michael Wang, BS, Duke University School of Medicine, Durham, N.C.
Melissa Nelson, MD, Baylor University Medical Center, Dallas
AGA–Moti L. & Kamla Rustgi International Travel Award
Joost Algera, MD, University of Gothenburg (Sweden)
Ashkan Rezazadeh Ardabili, MD, MS, BS, Maastricht (Netherlands) University Medical Center+
AGA research awards cycle now open
This year the AGA Research Foundation is awarding more than $2.5 million dollars to investigators who are passionate about improving digestive health. Get your piece of the research funding pie with one of our awards!
The AGA Research Foundation Awards Program recruits, retains, and supports the most promising researchers in gastroenterology and hepatology. With funding from the foundation, recipients have protected time to take their research to the next level. View our awards portfolio by career stage below, then mark your calendar for upcoming application deadlines. View additional information about each award.
AGA is proud to announce the 61 recipients selected to receive research funding through its annual AGA Research Foundation Awards Program. The program serves as a catalyst for discovery and career growth among the most promising researchers in gastroenterology and hepatology.
“Our award recipients demonstrate an undeniable determination to improve the care of digestive health patients,” said Robert S. Sandler, MD, MPH, AGAF, chair of the AGA Research Foundation. “We are investing in talented early-career investigators knowing that their work will ultimately benefit patients with critical needs.”

“In the past year, we expanded our awards program and elevated the importance of engaging underrepresented groups into the field of GI research,” Dr. Sandler said. “We are encouraged by the range of candidates who applied for funding and look forward to the results of their research.”
The AGA Research Foundation Awards Program is made possible thanks to generous donors and funders.
Here are this year’s award recipients:
Research Scholar Awards
AGA Research Scholar Award
Kathleen Curtius, PhD, MS, University of California, San Diego, La Jolla
Trisha Satya Pasricha, MD, MPH, Massachusetts General Hospital, Boston
Bomi Lee, PhD, MS, Stanford University, Palo Alto, Calif.
Christine E. Eyler, MD, PhD, Duke University, Durham, N.C.
Joel Gabre, MD, Columbia University Irving Medical Center, New York
AGA–Bern Schwartz Family Fund Research Scholar Award in Pancreatic Cancer
Srinivas Gaddam, MD, MPH, Cedars-Sinai Medical Center, Los Angeles
AGA–Takeda Pharmaceuticals Research Scholar Award in Celiac Disease
Claire L. Jansson-Knodell, MD, Cleveland Clinic Foundation, Cleveland
Specialty Awards
AGA–R. Robert & Sally Funderburg Research Award in Gastric Cancer
Eunyoung Choi, PhD, Vanderbilt University Medical Center, Nashville, Tenn.
AGA–Caroline Craig Augustyn & Damian Augustyn Award in Digestive Cancer
Sarah Palmer Short, PhD, Vanderbilt University Medical Center, Nashville, Tenn.
Pilot Awards
AGA–Medtronic Pilot Research Award in Artificial Intelligence
Dennis Shung, MD, MHS, Yale School of Medicine, New Haven, Conn.
AGA–Merck Pilot Research Award in Colorectal Cancer Health Disparities
Sonia Kupfer, MD, The University of Chicago, Chicago
AGA–Bristol Myers Squibb Pilot Research Award in Inflammatory Bowel Disease Health Disparities
Chung Sang Tse, MD, University of California, San Diego
AGA Pilot Research Award in Health Disparities (funded by Janssen Biotech)
Jennifer Flemming, MD, MAS, Queen’s University, Kingston, Ont.
AGA Pilot Research Award in Digestive Disease Health Disparities
Young-Rock Hong, PhD, MPH, University of Florida, Gainesville, Fla.
AGA–Amgen Pilot Research Award in Digestive Disease Health Disparities
Zachary Reichenbach, MD, PhD, Lewis Katz School of Medicine, Temple University, Philadelphia
AGA–Pfizer Pilot Research Award in Inflammatory Bowel Disease
Melinda Engevik, PhD, MS, Medical University of South Carolina, Charleston
Andre Paes Batista da Silva, PhD, MSC, DDS, Case Western Reserve University, Cleveland
Karen Edelblum, PhD, Rutgers New Jersey Medical School, Newark, N.J.
Undergraduate Research Awards
AGA–Aman Armaan Ahmed Family Summer Undergraduate Research Award
Gabriela Ortiz, Washington University School of Medicine, St. Louis
Daniella Montalvo, University of Miami Miller School of Medicine, Miami
Subear Hussein, Children’s Hospital, Boston
Hussein Herz, University of Iowa Carver College of Medicine, Iowa City
Kaleb Tesfai, University of California, San Diego
Varun Ponnusamy, University of Michigan Medical School, Ann Arbor, Mich.
Abstract Awards
AGA Fellow Abstract of the Year Award
Masaru Sasaki, MD, PhD, The Children’s Hospital of Philadelphia
AGA Student Abstract of the Year Award
Anitha Vijay, MS, Penn State University, State College, Pa.
Maafi Rizwana Islam, PhD, Marshall University, Huntington, W.V.
Fellow Abstract Awards
Nicolette Rodriguez, MD, MPH, Brigham and Women’s Hospital, Boston
Hyunseok Kim, MD, PhD, MPH, Baylor College of Medicine, Houston
Margaret Zhou, MD, Stanford University, Palo Alto, Calif.
Steven Steinway, MD, PhD, Johns Hopkins University, Baltimore
Su-Hyung Lee, PhD, DVM, Vanderbilt University Medical Center, Nashville, Tenn.
Ian Greenberg, MD, Dallas Methodist Hospital, Dallas
Jonathan Xia, MD, PhD, Northwestern Memorial Hospital, Chicago
Donevan Westerveld, MD, NewYork-Presbyterian Weill Cornell Medicine, New York
Haley Zylberberg, MD, Columbia University Irving Medical Center, New York
Maria Jesus Villanueva Millan, PhD, Cedars-Sinai Medical Center, Los Angeles
Duke Geem, MD, PhD. Children s Healthcare of Atlanta/Emory University, Atlanta
Fauzi Feris Jassir, MD, Mayo Clinic, Rochester, Minn.
Melissa Musser, MD, PhD, Boston Children’s Hospital, Boston
Student Abstract Awards
Kushal Saha, MS, BS, Penn State College of Medicine, Hershey, Pa.
Winston Liu, BS. Duke University, Durham, N.C.
Yoojin Sohn, BS, Vanderbilt University Medical Center, Nashville, Tenn.
Jamie Yang, BS, David Geffen School of Medicine at University of California, Los Angeles
Rachel Hopton, BS, University of Oregon, Eugene
Alina Li, BS, Columbia University, New York
Eleazar Montalvan Sanchez, MD, Indiana University School of Medicine, Indianapolis
Christina Lin, MD, BA, BS, Kaiser Permanente Northern California, Santa Clara, Calif.
Conrad Fernandes, MD, BA, Hospital of the University of Pennsylvania, Philadelphia
Hajar Hazime, MS, BS, University of Miami
Blaine Prichard, BS, Pennsylvania State University College of Medicine, Hershey, Pa.
Georgetta Skinner, MS, BS, A.T. Still University, Kirksville, Mo.
AGA Abstract Award for Health Disparities Research
Kai Wang, PhD (Fellow), Harvard T.H. Chan School of Public Health, Boston
Alan De La Rosa, MD (Fellow), Mayo Clinic, Rochester, Minn.
Timothy Andrew Zaki, MD, BS (Student), UT Southwestern Medical Center, Dallas
Megan McLeod, MD, MS, BA, University of California, Los Angeles (student)
AGA–APFED Abstract Award in Eosinophilic GI Diseases
Takeo Hara, MD, PhD, Children’s Hospital of Philadelphia
Michael Wang, BS, Duke University School of Medicine, Durham, N.C.
Melissa Nelson, MD, Baylor University Medical Center, Dallas
AGA–Moti L. & Kamla Rustgi International Travel Award
Joost Algera, MD, University of Gothenburg (Sweden)
Ashkan Rezazadeh Ardabili, MD, MS, BS, Maastricht (Netherlands) University Medical Center+
AGA research awards cycle now open
This year the AGA Research Foundation is awarding more than $2.5 million dollars to investigators who are passionate about improving digestive health. Get your piece of the research funding pie with one of our awards!
The AGA Research Foundation Awards Program recruits, retains, and supports the most promising researchers in gastroenterology and hepatology. With funding from the foundation, recipients have protected time to take their research to the next level. View our awards portfolio by career stage below, then mark your calendar for upcoming application deadlines. View additional information about each award.
AGA is proud to announce the 61 recipients selected to receive research funding through its annual AGA Research Foundation Awards Program. The program serves as a catalyst for discovery and career growth among the most promising researchers in gastroenterology and hepatology.
“Our award recipients demonstrate an undeniable determination to improve the care of digestive health patients,” said Robert S. Sandler, MD, MPH, AGAF, chair of the AGA Research Foundation. “We are investing in talented early-career investigators knowing that their work will ultimately benefit patients with critical needs.”

“In the past year, we expanded our awards program and elevated the importance of engaging underrepresented groups into the field of GI research,” Dr. Sandler said. “We are encouraged by the range of candidates who applied for funding and look forward to the results of their research.”
The AGA Research Foundation Awards Program is made possible thanks to generous donors and funders.
Here are this year’s award recipients:
Research Scholar Awards
AGA Research Scholar Award
Kathleen Curtius, PhD, MS, University of California, San Diego, La Jolla
Trisha Satya Pasricha, MD, MPH, Massachusetts General Hospital, Boston
Bomi Lee, PhD, MS, Stanford University, Palo Alto, Calif.
Christine E. Eyler, MD, PhD, Duke University, Durham, N.C.
Joel Gabre, MD, Columbia University Irving Medical Center, New York
AGA–Bern Schwartz Family Fund Research Scholar Award in Pancreatic Cancer
Srinivas Gaddam, MD, MPH, Cedars-Sinai Medical Center, Los Angeles
AGA–Takeda Pharmaceuticals Research Scholar Award in Celiac Disease
Claire L. Jansson-Knodell, MD, Cleveland Clinic Foundation, Cleveland
Specialty Awards
AGA–R. Robert & Sally Funderburg Research Award in Gastric Cancer
Eunyoung Choi, PhD, Vanderbilt University Medical Center, Nashville, Tenn.
AGA–Caroline Craig Augustyn & Damian Augustyn Award in Digestive Cancer
Sarah Palmer Short, PhD, Vanderbilt University Medical Center, Nashville, Tenn.
Pilot Awards
AGA–Medtronic Pilot Research Award in Artificial Intelligence
Dennis Shung, MD, MHS, Yale School of Medicine, New Haven, Conn.
AGA–Merck Pilot Research Award in Colorectal Cancer Health Disparities
Sonia Kupfer, MD, The University of Chicago, Chicago
AGA–Bristol Myers Squibb Pilot Research Award in Inflammatory Bowel Disease Health Disparities
Chung Sang Tse, MD, University of California, San Diego
AGA Pilot Research Award in Health Disparities (funded by Janssen Biotech)
Jennifer Flemming, MD, MAS, Queen’s University, Kingston, Ont.
AGA Pilot Research Award in Digestive Disease Health Disparities
Young-Rock Hong, PhD, MPH, University of Florida, Gainesville, Fla.
AGA–Amgen Pilot Research Award in Digestive Disease Health Disparities
Zachary Reichenbach, MD, PhD, Lewis Katz School of Medicine, Temple University, Philadelphia
AGA–Pfizer Pilot Research Award in Inflammatory Bowel Disease
Melinda Engevik, PhD, MS, Medical University of South Carolina, Charleston
Andre Paes Batista da Silva, PhD, MSC, DDS, Case Western Reserve University, Cleveland
Karen Edelblum, PhD, Rutgers New Jersey Medical School, Newark, N.J.
Undergraduate Research Awards
AGA–Aman Armaan Ahmed Family Summer Undergraduate Research Award
Gabriela Ortiz, Washington University School of Medicine, St. Louis
Daniella Montalvo, University of Miami Miller School of Medicine, Miami
Subear Hussein, Children’s Hospital, Boston
Hussein Herz, University of Iowa Carver College of Medicine, Iowa City
Kaleb Tesfai, University of California, San Diego
Varun Ponnusamy, University of Michigan Medical School, Ann Arbor, Mich.
Abstract Awards
AGA Fellow Abstract of the Year Award
Masaru Sasaki, MD, PhD, The Children’s Hospital of Philadelphia
AGA Student Abstract of the Year Award
Anitha Vijay, MS, Penn State University, State College, Pa.
Maafi Rizwana Islam, PhD, Marshall University, Huntington, W.V.
Fellow Abstract Awards
Nicolette Rodriguez, MD, MPH, Brigham and Women’s Hospital, Boston
Hyunseok Kim, MD, PhD, MPH, Baylor College of Medicine, Houston
Margaret Zhou, MD, Stanford University, Palo Alto, Calif.
Steven Steinway, MD, PhD, Johns Hopkins University, Baltimore
Su-Hyung Lee, PhD, DVM, Vanderbilt University Medical Center, Nashville, Tenn.
Ian Greenberg, MD, Dallas Methodist Hospital, Dallas
Jonathan Xia, MD, PhD, Northwestern Memorial Hospital, Chicago
Donevan Westerveld, MD, NewYork-Presbyterian Weill Cornell Medicine, New York
Haley Zylberberg, MD, Columbia University Irving Medical Center, New York
Maria Jesus Villanueva Millan, PhD, Cedars-Sinai Medical Center, Los Angeles
Duke Geem, MD, PhD. Children s Healthcare of Atlanta/Emory University, Atlanta
Fauzi Feris Jassir, MD, Mayo Clinic, Rochester, Minn.
Melissa Musser, MD, PhD, Boston Children’s Hospital, Boston
Student Abstract Awards
Kushal Saha, MS, BS, Penn State College of Medicine, Hershey, Pa.
Winston Liu, BS. Duke University, Durham, N.C.
Yoojin Sohn, BS, Vanderbilt University Medical Center, Nashville, Tenn.
Jamie Yang, BS, David Geffen School of Medicine at University of California, Los Angeles
Rachel Hopton, BS, University of Oregon, Eugene
Alina Li, BS, Columbia University, New York
Eleazar Montalvan Sanchez, MD, Indiana University School of Medicine, Indianapolis
Christina Lin, MD, BA, BS, Kaiser Permanente Northern California, Santa Clara, Calif.
Conrad Fernandes, MD, BA, Hospital of the University of Pennsylvania, Philadelphia
Hajar Hazime, MS, BS, University of Miami
Blaine Prichard, BS, Pennsylvania State University College of Medicine, Hershey, Pa.
Georgetta Skinner, MS, BS, A.T. Still University, Kirksville, Mo.
AGA Abstract Award for Health Disparities Research
Kai Wang, PhD (Fellow), Harvard T.H. Chan School of Public Health, Boston
Alan De La Rosa, MD (Fellow), Mayo Clinic, Rochester, Minn.
Timothy Andrew Zaki, MD, BS (Student), UT Southwestern Medical Center, Dallas
Megan McLeod, MD, MS, BA, University of California, Los Angeles (student)
AGA–APFED Abstract Award in Eosinophilic GI Diseases
Takeo Hara, MD, PhD, Children’s Hospital of Philadelphia
Michael Wang, BS, Duke University School of Medicine, Durham, N.C.
Melissa Nelson, MD, Baylor University Medical Center, Dallas
AGA–Moti L. & Kamla Rustgi International Travel Award
Joost Algera, MD, University of Gothenburg (Sweden)
Ashkan Rezazadeh Ardabili, MD, MS, BS, Maastricht (Netherlands) University Medical Center+
AGA research awards cycle now open
This year the AGA Research Foundation is awarding more than $2.5 million dollars to investigators who are passionate about improving digestive health. Get your piece of the research funding pie with one of our awards!
The AGA Research Foundation Awards Program recruits, retains, and supports the most promising researchers in gastroenterology and hepatology. With funding from the foundation, recipients have protected time to take their research to the next level. View our awards portfolio by career stage below, then mark your calendar for upcoming application deadlines. View additional information about each award.
Then and Now: Demographics of the AGA
The demographics of the American Gastroenterological Association have changed markedly since the first issue of GI and Hepatology News (GIHN) was published in 2007. GIHN’s first editorial team published news that was reliable and informative.
The first GIHN editor in chief, Dr. Charles J. Lightdale, described GIHN as “irresistible reading for all those seeking comprehensive, current, authoritative information in the field.” Today, GIHN continues the tradition of publishing news that is “irresistible,” “comprehensive,” and “authoritative.” However, GIHN’s board of editors looks quite different in 2022 than it did in 2007. The current board comprises people from both academic and private practice settings; we are from diverse ethnic and racial backgrounds. Finally, Dr. Megan A. Adams is the first woman to serve as editor in chief of the publication.
The last 15 years have seen an increase in the number of women and underrepresented minorities in gastroenterology. This, in turn, has changed the demographics of the AGA, and more women and underrepresented minorities are assuming leadership roles within the organization. The AGA values diversity and inclusion in its membership, but more importantly in its leadership.
On April 2, 2022, I had the privilege of participating in the AGA Future Leaders Program as a mentor representing Private Practice. The program also included participants of AGA’s FORWARD Program. The meeting started with the usual welcome and introductions. During the morning session, Tom Serena, CEO, spoke about the early history of the AGA. The AGA was an “elite” club 125 years ago, established as a research society with a membership limited to those with investigative achievements.
Next, Dr. John M. Carethers spoke about the importance of diversity and leadership. That afternoon, I sat on a panel with Dr. Anna S. Lok and Dr. Guadalupe Garcia-Tsao. The panel was asked to speak about mentoring across practice settings. As I sat there, I became acutely aware of the diversity represented on the panel and in the audience. The panelists were all women, and women of color. The future leaders of the AGA are from diverse backgrounds.
The physicians participating in that meeting came from academia and private practice – young men and women leaders from various racial and ethnic backgrounds. It was so uplifting to witness how the AGA is evolving. I am proud to be a member of an organization that values having different voices at the table. This diversity will make our organization stronger as we face the challenges in our profession today and in the future.
The traditional 15th-year anniversary gift is crystal, which symbolizes clarity and durability. On GIHN’s 15th-year anniversary, the path before us looks bright. The changing demographics of GI and of our organization brings together unfamiliar faces, fresh perspectives and new ideas that will help the organization build a clear and resilient path forward. Our future is bright!
Kimberly M. Persley, MD, AGAF, is a partner with Texas Digestive Disease Consultants/GI Alliance in Dallas, is on the medical staff of Texas Health Presbyterian Hospital, and is an associate editor of GI & Hepatology News. She has no relevant conflicts of interest.
The demographics of the American Gastroenterological Association have changed markedly since the first issue of GI and Hepatology News (GIHN) was published in 2007. GIHN’s first editorial team published news that was reliable and informative.
The first GIHN editor in chief, Dr. Charles J. Lightdale, described GIHN as “irresistible reading for all those seeking comprehensive, current, authoritative information in the field.” Today, GIHN continues the tradition of publishing news that is “irresistible,” “comprehensive,” and “authoritative.” However, GIHN’s board of editors looks quite different in 2022 than it did in 2007. The current board comprises people from both academic and private practice settings; we are from diverse ethnic and racial backgrounds. Finally, Dr. Megan A. Adams is the first woman to serve as editor in chief of the publication.
The last 15 years have seen an increase in the number of women and underrepresented minorities in gastroenterology. This, in turn, has changed the demographics of the AGA, and more women and underrepresented minorities are assuming leadership roles within the organization. The AGA values diversity and inclusion in its membership, but more importantly in its leadership.
On April 2, 2022, I had the privilege of participating in the AGA Future Leaders Program as a mentor representing Private Practice. The program also included participants of AGA’s FORWARD Program. The meeting started with the usual welcome and introductions. During the morning session, Tom Serena, CEO, spoke about the early history of the AGA. The AGA was an “elite” club 125 years ago, established as a research society with a membership limited to those with investigative achievements.
Next, Dr. John M. Carethers spoke about the importance of diversity and leadership. That afternoon, I sat on a panel with Dr. Anna S. Lok and Dr. Guadalupe Garcia-Tsao. The panel was asked to speak about mentoring across practice settings. As I sat there, I became acutely aware of the diversity represented on the panel and in the audience. The panelists were all women, and women of color. The future leaders of the AGA are from diverse backgrounds.
The physicians participating in that meeting came from academia and private practice – young men and women leaders from various racial and ethnic backgrounds. It was so uplifting to witness how the AGA is evolving. I am proud to be a member of an organization that values having different voices at the table. This diversity will make our organization stronger as we face the challenges in our profession today and in the future.
The traditional 15th-year anniversary gift is crystal, which symbolizes clarity and durability. On GIHN’s 15th-year anniversary, the path before us looks bright. The changing demographics of GI and of our organization brings together unfamiliar faces, fresh perspectives and new ideas that will help the organization build a clear and resilient path forward. Our future is bright!
Kimberly M. Persley, MD, AGAF, is a partner with Texas Digestive Disease Consultants/GI Alliance in Dallas, is on the medical staff of Texas Health Presbyterian Hospital, and is an associate editor of GI & Hepatology News. She has no relevant conflicts of interest.
The demographics of the American Gastroenterological Association have changed markedly since the first issue of GI and Hepatology News (GIHN) was published in 2007. GIHN’s first editorial team published news that was reliable and informative.
The first GIHN editor in chief, Dr. Charles J. Lightdale, described GIHN as “irresistible reading for all those seeking comprehensive, current, authoritative information in the field.” Today, GIHN continues the tradition of publishing news that is “irresistible,” “comprehensive,” and “authoritative.” However, GIHN’s board of editors looks quite different in 2022 than it did in 2007. The current board comprises people from both academic and private practice settings; we are from diverse ethnic and racial backgrounds. Finally, Dr. Megan A. Adams is the first woman to serve as editor in chief of the publication.
The last 15 years have seen an increase in the number of women and underrepresented minorities in gastroenterology. This, in turn, has changed the demographics of the AGA, and more women and underrepresented minorities are assuming leadership roles within the organization. The AGA values diversity and inclusion in its membership, but more importantly in its leadership.
On April 2, 2022, I had the privilege of participating in the AGA Future Leaders Program as a mentor representing Private Practice. The program also included participants of AGA’s FORWARD Program. The meeting started with the usual welcome and introductions. During the morning session, Tom Serena, CEO, spoke about the early history of the AGA. The AGA was an “elite” club 125 years ago, established as a research society with a membership limited to those with investigative achievements.
Next, Dr. John M. Carethers spoke about the importance of diversity and leadership. That afternoon, I sat on a panel with Dr. Anna S. Lok and Dr. Guadalupe Garcia-Tsao. The panel was asked to speak about mentoring across practice settings. As I sat there, I became acutely aware of the diversity represented on the panel and in the audience. The panelists were all women, and women of color. The future leaders of the AGA are from diverse backgrounds.
The physicians participating in that meeting came from academia and private practice – young men and women leaders from various racial and ethnic backgrounds. It was so uplifting to witness how the AGA is evolving. I am proud to be a member of an organization that values having different voices at the table. This diversity will make our organization stronger as we face the challenges in our profession today and in the future.
The traditional 15th-year anniversary gift is crystal, which symbolizes clarity and durability. On GIHN’s 15th-year anniversary, the path before us looks bright. The changing demographics of GI and of our organization brings together unfamiliar faces, fresh perspectives and new ideas that will help the organization build a clear and resilient path forward. Our future is bright!
Kimberly M. Persley, MD, AGAF, is a partner with Texas Digestive Disease Consultants/GI Alliance in Dallas, is on the medical staff of Texas Health Presbyterian Hospital, and is an associate editor of GI & Hepatology News. She has no relevant conflicts of interest.
Journalism or medicine: Why not both?
I had an early attraction to newspapers. As a child growing up in Jersey City, N.J., I delivered them door-to-door. I was editor-in-chief of my high school newspaper and worked as a copy boy and sports reporter on the daily Jersey Journal. At Princeton, I joined the University Press Club, working as a string reporter for the New York Herald Tribune, Philadelphia Inquirer, and Associated Press.
I thought I might become a journalist, but medicine was too strong a calling. During my GI elective as a senior medical resident at New York Hospital, I was able to work with some of the first commercial fiberoptic instruments, which presaged my academic career in endoscopic innovation. I was editor-in-chief of Gastrointestinal Endoscopy from 1988 to 1996, and have been the consulting editor for GI Endoscopy Clinics of North America since 1997.
As the first editor-in-chief of GI & Hepatology News, I had the opportunity to combine a background in peer review with my early newspaper experience. My vision for the new publication was to provide information curated and vetted by experts, in contrast to the torrent pouring down from the Internet that was (pertinent to our specialty) “indigestible.” I put in much effort selecting stories provided by Elsevier Global Medical News, especially in constructing the front page. AGA Institute provided strong support, allowing me to choose an editorial board covering all subspecialties. I wanted to highlight the excitement of researchers balanced by expert review and commentary. The digital version added search features, and I tried to promote the “browse factor” that would also encourage advertising, critical to the success of any newspaper. At the end of my term, I felt I had laid a strong foundation, and have been delighted to see the publication continue to thrive.
Charles Lightdale, MD, is professor of medicine at Columbia University Medical Center in New York. He disclosed having no conflicts of interest.
I had an early attraction to newspapers. As a child growing up in Jersey City, N.J., I delivered them door-to-door. I was editor-in-chief of my high school newspaper and worked as a copy boy and sports reporter on the daily Jersey Journal. At Princeton, I joined the University Press Club, working as a string reporter for the New York Herald Tribune, Philadelphia Inquirer, and Associated Press.
I thought I might become a journalist, but medicine was too strong a calling. During my GI elective as a senior medical resident at New York Hospital, I was able to work with some of the first commercial fiberoptic instruments, which presaged my academic career in endoscopic innovation. I was editor-in-chief of Gastrointestinal Endoscopy from 1988 to 1996, and have been the consulting editor for GI Endoscopy Clinics of North America since 1997.
As the first editor-in-chief of GI & Hepatology News, I had the opportunity to combine a background in peer review with my early newspaper experience. My vision for the new publication was to provide information curated and vetted by experts, in contrast to the torrent pouring down from the Internet that was (pertinent to our specialty) “indigestible.” I put in much effort selecting stories provided by Elsevier Global Medical News, especially in constructing the front page. AGA Institute provided strong support, allowing me to choose an editorial board covering all subspecialties. I wanted to highlight the excitement of researchers balanced by expert review and commentary. The digital version added search features, and I tried to promote the “browse factor” that would also encourage advertising, critical to the success of any newspaper. At the end of my term, I felt I had laid a strong foundation, and have been delighted to see the publication continue to thrive.
Charles Lightdale, MD, is professor of medicine at Columbia University Medical Center in New York. He disclosed having no conflicts of interest.
I had an early attraction to newspapers. As a child growing up in Jersey City, N.J., I delivered them door-to-door. I was editor-in-chief of my high school newspaper and worked as a copy boy and sports reporter on the daily Jersey Journal. At Princeton, I joined the University Press Club, working as a string reporter for the New York Herald Tribune, Philadelphia Inquirer, and Associated Press.
I thought I might become a journalist, but medicine was too strong a calling. During my GI elective as a senior medical resident at New York Hospital, I was able to work with some of the first commercial fiberoptic instruments, which presaged my academic career in endoscopic innovation. I was editor-in-chief of Gastrointestinal Endoscopy from 1988 to 1996, and have been the consulting editor for GI Endoscopy Clinics of North America since 1997.
As the first editor-in-chief of GI & Hepatology News, I had the opportunity to combine a background in peer review with my early newspaper experience. My vision for the new publication was to provide information curated and vetted by experts, in contrast to the torrent pouring down from the Internet that was (pertinent to our specialty) “indigestible.” I put in much effort selecting stories provided by Elsevier Global Medical News, especially in constructing the front page. AGA Institute provided strong support, allowing me to choose an editorial board covering all subspecialties. I wanted to highlight the excitement of researchers balanced by expert review and commentary. The digital version added search features, and I tried to promote the “browse factor” that would also encourage advertising, critical to the success of any newspaper. At the end of my term, I felt I had laid a strong foundation, and have been delighted to see the publication continue to thrive.
Charles Lightdale, MD, is professor of medicine at Columbia University Medical Center in New York. He disclosed having no conflicts of interest.
















