User login
Critical Care Network
Sepsis and Shock Section
SEP-1 measure saves lives, let’s not debate!
On December 21, 2021, the National Quality Form (NQF) re-endorsed Measure 0500 Severe Sepsis and Septic Shock: Management Bundle, which CMS adopts as the SEP-1 core measure. The decision was initially met by a request for appeal. On April 29, 2022, the appeals board met to adjudicate the appeal and voted unanimously to uphold the Standards Approval Committee (CSAC) decision to endorse the measure once again (https://tinyurl.com/yc4tjxbz).
The appeals board voted 5-0 on whether procedural errors reasonably affected the outcome of the original endorsement and whether there was new information or evidence unavailable at the time of the CSAC endorsement decision that would reasonably affect the outcome of the original endorsement decision.
even though the results of this bundled approach support an opportunity to save lives. SEP-1 compliance is associated with a lower 30-day mortality, and rendering this care saves lives.
In the Townsend, et al cohort study (Chest. 2022 Feb;161[2]:392) examining patient level Medicare data from October 2015 – March 2017, there was an absolute risk reduction of 5.67% in a standard propensity matched comparison of SEP-1 compliant vs noncompliant care. With a more stringent match, the absolute risk reduction was 4.06%. That’s an outcome that our patients likely appreciate the most…lives saved.
As former CHEST President, Dr. Steven Simpson highlighted in his April 2022 commentary (CHEST Physician. 2022 April;17[4]:15), “Success is not dependent only on what we do but on when we do it.” Let’s not debate any further.
Namita Jayaprakash, MBBCh
Member-at-Large
Sepsis and Shock Section
SEP-1 measure saves lives, let’s not debate!
On December 21, 2021, the National Quality Form (NQF) re-endorsed Measure 0500 Severe Sepsis and Septic Shock: Management Bundle, which CMS adopts as the SEP-1 core measure. The decision was initially met by a request for appeal. On April 29, 2022, the appeals board met to adjudicate the appeal and voted unanimously to uphold the Standards Approval Committee (CSAC) decision to endorse the measure once again (https://tinyurl.com/yc4tjxbz).
The appeals board voted 5-0 on whether procedural errors reasonably affected the outcome of the original endorsement and whether there was new information or evidence unavailable at the time of the CSAC endorsement decision that would reasonably affect the outcome of the original endorsement decision.
even though the results of this bundled approach support an opportunity to save lives. SEP-1 compliance is associated with a lower 30-day mortality, and rendering this care saves lives.
In the Townsend, et al cohort study (Chest. 2022 Feb;161[2]:392) examining patient level Medicare data from October 2015 – March 2017, there was an absolute risk reduction of 5.67% in a standard propensity matched comparison of SEP-1 compliant vs noncompliant care. With a more stringent match, the absolute risk reduction was 4.06%. That’s an outcome that our patients likely appreciate the most…lives saved.
As former CHEST President, Dr. Steven Simpson highlighted in his April 2022 commentary (CHEST Physician. 2022 April;17[4]:15), “Success is not dependent only on what we do but on when we do it.” Let’s not debate any further.
Namita Jayaprakash, MBBCh
Member-at-Large
Sepsis and Shock Section
SEP-1 measure saves lives, let’s not debate!
On December 21, 2021, the National Quality Form (NQF) re-endorsed Measure 0500 Severe Sepsis and Septic Shock: Management Bundle, which CMS adopts as the SEP-1 core measure. The decision was initially met by a request for appeal. On April 29, 2022, the appeals board met to adjudicate the appeal and voted unanimously to uphold the Standards Approval Committee (CSAC) decision to endorse the measure once again (https://tinyurl.com/yc4tjxbz).
The appeals board voted 5-0 on whether procedural errors reasonably affected the outcome of the original endorsement and whether there was new information or evidence unavailable at the time of the CSAC endorsement decision that would reasonably affect the outcome of the original endorsement decision.
even though the results of this bundled approach support an opportunity to save lives. SEP-1 compliance is associated with a lower 30-day mortality, and rendering this care saves lives.
In the Townsend, et al cohort study (Chest. 2022 Feb;161[2]:392) examining patient level Medicare data from October 2015 – March 2017, there was an absolute risk reduction of 5.67% in a standard propensity matched comparison of SEP-1 compliant vs noncompliant care. With a more stringent match, the absolute risk reduction was 4.06%. That’s an outcome that our patients likely appreciate the most…lives saved.
As former CHEST President, Dr. Steven Simpson highlighted in his April 2022 commentary (CHEST Physician. 2022 April;17[4]:15), “Success is not dependent only on what we do but on when we do it.” Let’s not debate any further.
Namita Jayaprakash, MBBCh
Member-at-Large
Sleep Medicine Network
Respiratory-Related Sleep Disorders Section
Reducing racial disparities in sleep apnea
For example, a growing body of research has shown that black race is associated with underdiagnosis of OSA, greater disease severity at time of diagnosis and reduced PAP adherence (Hsu N, et al. J Clin Sleep Med. 2020;16[8]:1249; Thornton JD, et al. Ann Am Thorac Soc. 2022;19[2]:272).
A recent article (Billings ME, et al. Chest. 2021;159[3]:1232) offered potential strategies to mitigate racial disparities in sleep apnea management. To expand access to care, they advocate embracing telemedicine for those who may have difficulty coming to clinic – due to transportation issues, arranging sufficient time off work, or residing in remote locations. On the other hand, an over-reliance on telemedicine has the potential to worsen disparities in populations whose access to technology is limited.
The authors also recommend inpatient screening of high-risk patient populations to detect disease earlier and to help facilitate referrals to a sleep center. They propose the idea of “peer buddies” of similar racial and socioeconomic backgrounds to provide support and counseling, while cautioning against overburden these populations. Finally, they propose broadening the sleep provider workforce by training primary care providers to manage OSA.
The higher proportion of nonwhite providers in these groups as compared with sleep specialists may improve care, since concordant race provision has been associated with better communication. Underlying these interventions is the need to diversify representation within the medical field at large.
Swetha Gogineni, MD, Vice-Chair
Lauren Tobias, MD, Member-at-Large
Respiratory-Related Sleep Disorders Section
Reducing racial disparities in sleep apnea
For example, a growing body of research has shown that black race is associated with underdiagnosis of OSA, greater disease severity at time of diagnosis and reduced PAP adherence (Hsu N, et al. J Clin Sleep Med. 2020;16[8]:1249; Thornton JD, et al. Ann Am Thorac Soc. 2022;19[2]:272).
A recent article (Billings ME, et al. Chest. 2021;159[3]:1232) offered potential strategies to mitigate racial disparities in sleep apnea management. To expand access to care, they advocate embracing telemedicine for those who may have difficulty coming to clinic – due to transportation issues, arranging sufficient time off work, or residing in remote locations. On the other hand, an over-reliance on telemedicine has the potential to worsen disparities in populations whose access to technology is limited.
The authors also recommend inpatient screening of high-risk patient populations to detect disease earlier and to help facilitate referrals to a sleep center. They propose the idea of “peer buddies” of similar racial and socioeconomic backgrounds to provide support and counseling, while cautioning against overburden these populations. Finally, they propose broadening the sleep provider workforce by training primary care providers to manage OSA.
The higher proportion of nonwhite providers in these groups as compared with sleep specialists may improve care, since concordant race provision has been associated with better communication. Underlying these interventions is the need to diversify representation within the medical field at large.
Swetha Gogineni, MD, Vice-Chair
Lauren Tobias, MD, Member-at-Large
Respiratory-Related Sleep Disorders Section
Reducing racial disparities in sleep apnea
For example, a growing body of research has shown that black race is associated with underdiagnosis of OSA, greater disease severity at time of diagnosis and reduced PAP adherence (Hsu N, et al. J Clin Sleep Med. 2020;16[8]:1249; Thornton JD, et al. Ann Am Thorac Soc. 2022;19[2]:272).
A recent article (Billings ME, et al. Chest. 2021;159[3]:1232) offered potential strategies to mitigate racial disparities in sleep apnea management. To expand access to care, they advocate embracing telemedicine for those who may have difficulty coming to clinic – due to transportation issues, arranging sufficient time off work, or residing in remote locations. On the other hand, an over-reliance on telemedicine has the potential to worsen disparities in populations whose access to technology is limited.
The authors also recommend inpatient screening of high-risk patient populations to detect disease earlier and to help facilitate referrals to a sleep center. They propose the idea of “peer buddies” of similar racial and socioeconomic backgrounds to provide support and counseling, while cautioning against overburden these populations. Finally, they propose broadening the sleep provider workforce by training primary care providers to manage OSA.
The higher proportion of nonwhite providers in these groups as compared with sleep specialists may improve care, since concordant race provision has been associated with better communication. Underlying these interventions is the need to diversify representation within the medical field at large.
Swetha Gogineni, MD, Vice-Chair
Lauren Tobias, MD, Member-at-Large
Pulmonary Vascular & Cardiovascular Network
Pulmonary Vascular Disease Section
Restoration of RV function in PAH: Is it the holy grail to improve mortality and long-term outcomes?
Despite several therapeutic advances, PAH continues to be associated with high mortality. Even mild increases in mean pulmonary arterial pressure (mPAP) have been shown to directly impact outcomes (Maron BA, et al. Circulation. 2016 Mar 29;133[13]:1240), leading to a change in the hemodynamic definition of PAH (mPAP > 20 mm Hg) at the 2018 World Symposium on Pulmonary Hypertension (WSPH) (Galiè N, et al. Eur Respir J. 2019;53[1]:1801889). The WSPH also recommended a more aggressive and proactive approach to move patients to “low-risk” status.
Elevated mPAP results in increased RV afterload with subsequent RV dysfunction and consequent abnormal remodeling, which is associated with poor outcomes. Reversal of RV remodeling has been demonstrated in patients after PEA for CTEPH and/or lung transplantation for PAH (D’Armini AM, et al. J Thorac Cardiovasc Surg. 2007;133:162).
Aggressive mPAP reduction facilitates RV recovery, which may alter the course of PAH in the form of improved survival. RV dysfunction is mainly attributed to afterload mismatch and uncoupling of the RV. Although oral therapies have shown significant improvements in symptoms, functional class, and delaying clinical worsening, normalization of RV size and function is often not achieved. More aggressive reduction of mPAP with a combination of parenteral and oral therapies has been shown to be more effective in restoring RV function (Vizza CD, et al. Am J Respir Crit Care Med. 2022;205) with the ultimate goal of improving quality and quantity of life in those affected by PAH.
Vijay Balasubramanian, MD, FCCP, Chair
Jean M. Elwing, MD, FCCP, Ex-Officio
Pulmonary Vascular Disease Section
Restoration of RV function in PAH: Is it the holy grail to improve mortality and long-term outcomes?
Despite several therapeutic advances, PAH continues to be associated with high mortality. Even mild increases in mean pulmonary arterial pressure (mPAP) have been shown to directly impact outcomes (Maron BA, et al. Circulation. 2016 Mar 29;133[13]:1240), leading to a change in the hemodynamic definition of PAH (mPAP > 20 mm Hg) at the 2018 World Symposium on Pulmonary Hypertension (WSPH) (Galiè N, et al. Eur Respir J. 2019;53[1]:1801889). The WSPH also recommended a more aggressive and proactive approach to move patients to “low-risk” status.
Elevated mPAP results in increased RV afterload with subsequent RV dysfunction and consequent abnormal remodeling, which is associated with poor outcomes. Reversal of RV remodeling has been demonstrated in patients after PEA for CTEPH and/or lung transplantation for PAH (D’Armini AM, et al. J Thorac Cardiovasc Surg. 2007;133:162).
Aggressive mPAP reduction facilitates RV recovery, which may alter the course of PAH in the form of improved survival. RV dysfunction is mainly attributed to afterload mismatch and uncoupling of the RV. Although oral therapies have shown significant improvements in symptoms, functional class, and delaying clinical worsening, normalization of RV size and function is often not achieved. More aggressive reduction of mPAP with a combination of parenteral and oral therapies has been shown to be more effective in restoring RV function (Vizza CD, et al. Am J Respir Crit Care Med. 2022;205) with the ultimate goal of improving quality and quantity of life in those affected by PAH.
Vijay Balasubramanian, MD, FCCP, Chair
Jean M. Elwing, MD, FCCP, Ex-Officio
Pulmonary Vascular Disease Section
Restoration of RV function in PAH: Is it the holy grail to improve mortality and long-term outcomes?
Despite several therapeutic advances, PAH continues to be associated with high mortality. Even mild increases in mean pulmonary arterial pressure (mPAP) have been shown to directly impact outcomes (Maron BA, et al. Circulation. 2016 Mar 29;133[13]:1240), leading to a change in the hemodynamic definition of PAH (mPAP > 20 mm Hg) at the 2018 World Symposium on Pulmonary Hypertension (WSPH) (Galiè N, et al. Eur Respir J. 2019;53[1]:1801889). The WSPH also recommended a more aggressive and proactive approach to move patients to “low-risk” status.
Elevated mPAP results in increased RV afterload with subsequent RV dysfunction and consequent abnormal remodeling, which is associated with poor outcomes. Reversal of RV remodeling has been demonstrated in patients after PEA for CTEPH and/or lung transplantation for PAH (D’Armini AM, et al. J Thorac Cardiovasc Surg. 2007;133:162).
Aggressive mPAP reduction facilitates RV recovery, which may alter the course of PAH in the form of improved survival. RV dysfunction is mainly attributed to afterload mismatch and uncoupling of the RV. Although oral therapies have shown significant improvements in symptoms, functional class, and delaying clinical worsening, normalization of RV size and function is often not achieved. More aggressive reduction of mPAP with a combination of parenteral and oral therapies has been shown to be more effective in restoring RV function (Vizza CD, et al. Am J Respir Crit Care Med. 2022;205) with the ultimate goal of improving quality and quantity of life in those affected by PAH.
Vijay Balasubramanian, MD, FCCP, Chair
Jean M. Elwing, MD, FCCP, Ex-Officio
Thoracic Oncology & Chest Procedures Network
Interventional Procedures Section
Lung cancer is the leading cause of cancer-related deaths worldwide and forms of a large burden of cancer-related mortality in the United States. With the rapid advent of new disease-directed therapy, including molecular and targeted therapies, the outlook for management of lung cancer has changed dramatically over the last decade. The choice of therapy, as well as prognosis, is dependent on the stage at diagnosis. It is thus imperative that we accurately differentiate between stages I, II, and III disease by assessment of hilar and mediastinal lymph nodes.
Traditionally, CT and PET/CT scans have been the mainstay to assess stage, with patients with abnormal lymph nodes or high risk of nodal metastasis (≥ T2 disease or “central” location) undergoing invasive mediastinal evaluation (Silvestri, et al. Chest. 2013 May;143(5 Suppl):e211S). The decision to perform invasive mediastinal staging for T1 tumors remains a matter of discussion. DuComb and colleagues, in their study demonstrated a high rate of N2 metastasis (8.1%) even amongst those with T1 tumors, which was independent of tumor location (DuComb, et al. Chest. 2020 Nov;158[5]:2192). This rate is consistent with previous reported rates ranging from 6.9% to 13% of N2 disease in patients with no radiographic evidence of lymph node metastasis (Gonzalez-Stawinski, et al. J Thorac Cardiovasc Surg. 2003 Dec;126[6]:1900; Bao, et al. J Thorac Dis. 2014;6[12]:1697; Shin, et al. Eur Respir J. 2019;53[3]:1801508). The above indicates a possible role of invasive mediastinal staging using EBUS-TBNA in patients with T1 disease to accurately stage the disease prior to curative intent treatment.
While the role of EBUS-TBNA in diagnosis and staging has been a role of ongoing research, data are limited on prognostic implications of EBUS-guided staging in patients with NSCLC. In a recently published paper in Chest, Hwangbo and colleagues assessed the prognostic impact of staging via EBUS in these patients (Hwangbo et al. Chest 2022 May;161[5]:1382). In the 1,089 patients who underwent EBUS-TBNA, they observed a significant difference in survival based on the staging established via EBUS-TBNA, highlighting the importance of EBUS-TBNA in staging for NSCLC. Also of note, patients with false-negative EBUS results had favorable survival that was similar to patients with pathologic N1 disease. While the exact reason for this is unclear and may be related to disease burden, the authors postulated that this may provide a rationale to performing surgery after negative EBUS-TBNA results.
Abhinav Agrawal, MD, FCCP, Member-at-Large
Ellen Volker, MD, MSPH, FCCP, Member-at-Large
Interventional Procedures Section
Lung cancer is the leading cause of cancer-related deaths worldwide and forms of a large burden of cancer-related mortality in the United States. With the rapid advent of new disease-directed therapy, including molecular and targeted therapies, the outlook for management of lung cancer has changed dramatically over the last decade. The choice of therapy, as well as prognosis, is dependent on the stage at diagnosis. It is thus imperative that we accurately differentiate between stages I, II, and III disease by assessment of hilar and mediastinal lymph nodes.
Traditionally, CT and PET/CT scans have been the mainstay to assess stage, with patients with abnormal lymph nodes or high risk of nodal metastasis (≥ T2 disease or “central” location) undergoing invasive mediastinal evaluation (Silvestri, et al. Chest. 2013 May;143(5 Suppl):e211S). The decision to perform invasive mediastinal staging for T1 tumors remains a matter of discussion. DuComb and colleagues, in their study demonstrated a high rate of N2 metastasis (8.1%) even amongst those with T1 tumors, which was independent of tumor location (DuComb, et al. Chest. 2020 Nov;158[5]:2192). This rate is consistent with previous reported rates ranging from 6.9% to 13% of N2 disease in patients with no radiographic evidence of lymph node metastasis (Gonzalez-Stawinski, et al. J Thorac Cardiovasc Surg. 2003 Dec;126[6]:1900; Bao, et al. J Thorac Dis. 2014;6[12]:1697; Shin, et al. Eur Respir J. 2019;53[3]:1801508). The above indicates a possible role of invasive mediastinal staging using EBUS-TBNA in patients with T1 disease to accurately stage the disease prior to curative intent treatment.
While the role of EBUS-TBNA in diagnosis and staging has been a role of ongoing research, data are limited on prognostic implications of EBUS-guided staging in patients with NSCLC. In a recently published paper in Chest, Hwangbo and colleagues assessed the prognostic impact of staging via EBUS in these patients (Hwangbo et al. Chest 2022 May;161[5]:1382). In the 1,089 patients who underwent EBUS-TBNA, they observed a significant difference in survival based on the staging established via EBUS-TBNA, highlighting the importance of EBUS-TBNA in staging for NSCLC. Also of note, patients with false-negative EBUS results had favorable survival that was similar to patients with pathologic N1 disease. While the exact reason for this is unclear and may be related to disease burden, the authors postulated that this may provide a rationale to performing surgery after negative EBUS-TBNA results.
Abhinav Agrawal, MD, FCCP, Member-at-Large
Ellen Volker, MD, MSPH, FCCP, Member-at-Large
Interventional Procedures Section
Lung cancer is the leading cause of cancer-related deaths worldwide and forms of a large burden of cancer-related mortality in the United States. With the rapid advent of new disease-directed therapy, including molecular and targeted therapies, the outlook for management of lung cancer has changed dramatically over the last decade. The choice of therapy, as well as prognosis, is dependent on the stage at diagnosis. It is thus imperative that we accurately differentiate between stages I, II, and III disease by assessment of hilar and mediastinal lymph nodes.
Traditionally, CT and PET/CT scans have been the mainstay to assess stage, with patients with abnormal lymph nodes or high risk of nodal metastasis (≥ T2 disease or “central” location) undergoing invasive mediastinal evaluation (Silvestri, et al. Chest. 2013 May;143(5 Suppl):e211S). The decision to perform invasive mediastinal staging for T1 tumors remains a matter of discussion. DuComb and colleagues, in their study demonstrated a high rate of N2 metastasis (8.1%) even amongst those with T1 tumors, which was independent of tumor location (DuComb, et al. Chest. 2020 Nov;158[5]:2192). This rate is consistent with previous reported rates ranging from 6.9% to 13% of N2 disease in patients with no radiographic evidence of lymph node metastasis (Gonzalez-Stawinski, et al. J Thorac Cardiovasc Surg. 2003 Dec;126[6]:1900; Bao, et al. J Thorac Dis. 2014;6[12]:1697; Shin, et al. Eur Respir J. 2019;53[3]:1801508). The above indicates a possible role of invasive mediastinal staging using EBUS-TBNA in patients with T1 disease to accurately stage the disease prior to curative intent treatment.
While the role of EBUS-TBNA in diagnosis and staging has been a role of ongoing research, data are limited on prognostic implications of EBUS-guided staging in patients with NSCLC. In a recently published paper in Chest, Hwangbo and colleagues assessed the prognostic impact of staging via EBUS in these patients (Hwangbo et al. Chest 2022 May;161[5]:1382). In the 1,089 patients who underwent EBUS-TBNA, they observed a significant difference in survival based on the staging established via EBUS-TBNA, highlighting the importance of EBUS-TBNA in staging for NSCLC. Also of note, patients with false-negative EBUS results had favorable survival that was similar to patients with pathologic N1 disease. While the exact reason for this is unclear and may be related to disease burden, the authors postulated that this may provide a rationale to performing surgery after negative EBUS-TBNA results.
Abhinav Agrawal, MD, FCCP, Member-at-Large
Ellen Volker, MD, MSPH, FCCP, Member-at-Large
Chest Infections & Disaster Response Network
Disaster Response and Global Health Section
Physician response to Ukraine and beyond
Displaced persons, international refugee crises, gun violence, and other disasters remain prevalent in current news. Recent events highlight the need for continued civilian physician leadership and response to disasters.
Before the Ukraine crisis, the United Nations Refugee Agency estimated displaced persons more than doubled to greater than 82 million persons over the last decade (unhcr.org). Since that analysis, there have been over 6.5 million externally displaced persons, 7.5 million internally displaced persons, and significant numbers of injured patients from the Ukraine crisis alone. The Ukraine Ministry of Health has shown preparedness in its ability to handle significant patient surges with minimal assistance.
However, organizations like the Ukraine Medical Association of North America, Razom for Ukraine, Doctors Without Borders (MSF), MedGlobal, Samaritan’s Purse, Global Response Management, and many more have deployed to assist in Ukraine. These NGOs continue to help with medical care, fulfill critical supply needs, and provide training in cutting-edge medicine (POCUS, trauma updates).
Challenges posed by unstable environments, from wars to active shooter situations, further underscore the need for continued education, advances in technology, and preparedness. Providers responding to these events often treat vulnerable populations suffering from physical and mental violence, requiring physicians to step out of their comfort zone.
Physicians should continue to be leaders in the care of vulnerable displaced persons.
Christopher Miller, DO, MPH
Fellow-in-Training Member
Thomas Marston, MD
Member-at-Large
Disaster Response and Global Health Section
Physician response to Ukraine and beyond
Displaced persons, international refugee crises, gun violence, and other disasters remain prevalent in current news. Recent events highlight the need for continued civilian physician leadership and response to disasters.
Before the Ukraine crisis, the United Nations Refugee Agency estimated displaced persons more than doubled to greater than 82 million persons over the last decade (unhcr.org). Since that analysis, there have been over 6.5 million externally displaced persons, 7.5 million internally displaced persons, and significant numbers of injured patients from the Ukraine crisis alone. The Ukraine Ministry of Health has shown preparedness in its ability to handle significant patient surges with minimal assistance.
However, organizations like the Ukraine Medical Association of North America, Razom for Ukraine, Doctors Without Borders (MSF), MedGlobal, Samaritan’s Purse, Global Response Management, and many more have deployed to assist in Ukraine. These NGOs continue to help with medical care, fulfill critical supply needs, and provide training in cutting-edge medicine (POCUS, trauma updates).
Challenges posed by unstable environments, from wars to active shooter situations, further underscore the need for continued education, advances in technology, and preparedness. Providers responding to these events often treat vulnerable populations suffering from physical and mental violence, requiring physicians to step out of their comfort zone.
Physicians should continue to be leaders in the care of vulnerable displaced persons.
Christopher Miller, DO, MPH
Fellow-in-Training Member
Thomas Marston, MD
Member-at-Large
Disaster Response and Global Health Section
Physician response to Ukraine and beyond
Displaced persons, international refugee crises, gun violence, and other disasters remain prevalent in current news. Recent events highlight the need for continued civilian physician leadership and response to disasters.
Before the Ukraine crisis, the United Nations Refugee Agency estimated displaced persons more than doubled to greater than 82 million persons over the last decade (unhcr.org). Since that analysis, there have been over 6.5 million externally displaced persons, 7.5 million internally displaced persons, and significant numbers of injured patients from the Ukraine crisis alone. The Ukraine Ministry of Health has shown preparedness in its ability to handle significant patient surges with minimal assistance.
However, organizations like the Ukraine Medical Association of North America, Razom for Ukraine, Doctors Without Borders (MSF), MedGlobal, Samaritan’s Purse, Global Response Management, and many more have deployed to assist in Ukraine. These NGOs continue to help with medical care, fulfill critical supply needs, and provide training in cutting-edge medicine (POCUS, trauma updates).
Challenges posed by unstable environments, from wars to active shooter situations, further underscore the need for continued education, advances in technology, and preparedness. Providers responding to these events often treat vulnerable populations suffering from physical and mental violence, requiring physicians to step out of their comfort zone.
Physicians should continue to be leaders in the care of vulnerable displaced persons.
Christopher Miller, DO, MPH
Fellow-in-Training Member
Thomas Marston, MD
Member-at-Large
‘The Rock’ assumes the presidency of AGA
We’re honored to announce that John M. Carethers, MD, AGAF, affectionately nicknamed ‘The Rock,’ will begin his term as the 117th president of the AGA Institute on June 1, 2022.
He currently serves as John G. Searle professor of internal medicine and chair of the department of internal medicine at the University of Michigan Health System, a position he has held since 2009.
Dr. Carethers’ research programs focus on familial colon cancer and polyposis syndromes. His research encompasses Lynch syndrome, juvenile polyposis, hyperplastic polyposis, and colorectal cancer. He has published more than 182 articles.
A native of Detroit, Dr. Carethers earned his undergraduate degree in molecular biology and biophysics at Wayne State University. He remained there for medical school, where he graduated at the top of his class. His ability to stay focused on his work earned him the moniker ‘The Rock’. It’s a strength that’s made him an outstanding role model and exemplary leader.
An active member of AGA for more than 20 years, Dr. Carethers received the AGA Gastrointestinal Oncology Section Research Mentor Award as well as the AGA Distinguished Mentor Award in 2017. He has served on several AGA committees, including the AGA Nominating Committee, AGA Underrepresented Minorities Committee, AGA Research Policy Committee, AGA Institute Council and the AGA Trainee & Young GI Committee. He has also served as senior associate editor of Gastroenterology.
His academic career began at the University of California, San Diego, preceded by a gastroenterology fellowship at University of Michigan in Ann Arbor and residency at Massachusetts General Hospital in Boston. From the beginning, he has inspired others with his strong work ethic and intense dedication.
Dr. Carethers joined the AGA Governing Board in June 2020 as vice president and served as president-elect prior to assuming the top leadership role.
We’re honored to announce that John M. Carethers, MD, AGAF, affectionately nicknamed ‘The Rock,’ will begin his term as the 117th president of the AGA Institute on June 1, 2022.
He currently serves as John G. Searle professor of internal medicine and chair of the department of internal medicine at the University of Michigan Health System, a position he has held since 2009.
Dr. Carethers’ research programs focus on familial colon cancer and polyposis syndromes. His research encompasses Lynch syndrome, juvenile polyposis, hyperplastic polyposis, and colorectal cancer. He has published more than 182 articles.
A native of Detroit, Dr. Carethers earned his undergraduate degree in molecular biology and biophysics at Wayne State University. He remained there for medical school, where he graduated at the top of his class. His ability to stay focused on his work earned him the moniker ‘The Rock’. It’s a strength that’s made him an outstanding role model and exemplary leader.
An active member of AGA for more than 20 years, Dr. Carethers received the AGA Gastrointestinal Oncology Section Research Mentor Award as well as the AGA Distinguished Mentor Award in 2017. He has served on several AGA committees, including the AGA Nominating Committee, AGA Underrepresented Minorities Committee, AGA Research Policy Committee, AGA Institute Council and the AGA Trainee & Young GI Committee. He has also served as senior associate editor of Gastroenterology.
His academic career began at the University of California, San Diego, preceded by a gastroenterology fellowship at University of Michigan in Ann Arbor and residency at Massachusetts General Hospital in Boston. From the beginning, he has inspired others with his strong work ethic and intense dedication.
Dr. Carethers joined the AGA Governing Board in June 2020 as vice president and served as president-elect prior to assuming the top leadership role.
We’re honored to announce that John M. Carethers, MD, AGAF, affectionately nicknamed ‘The Rock,’ will begin his term as the 117th president of the AGA Institute on June 1, 2022.
He currently serves as John G. Searle professor of internal medicine and chair of the department of internal medicine at the University of Michigan Health System, a position he has held since 2009.
Dr. Carethers’ research programs focus on familial colon cancer and polyposis syndromes. His research encompasses Lynch syndrome, juvenile polyposis, hyperplastic polyposis, and colorectal cancer. He has published more than 182 articles.
A native of Detroit, Dr. Carethers earned his undergraduate degree in molecular biology and biophysics at Wayne State University. He remained there for medical school, where he graduated at the top of his class. His ability to stay focused on his work earned him the moniker ‘The Rock’. It’s a strength that’s made him an outstanding role model and exemplary leader.
An active member of AGA for more than 20 years, Dr. Carethers received the AGA Gastrointestinal Oncology Section Research Mentor Award as well as the AGA Distinguished Mentor Award in 2017. He has served on several AGA committees, including the AGA Nominating Committee, AGA Underrepresented Minorities Committee, AGA Research Policy Committee, AGA Institute Council and the AGA Trainee & Young GI Committee. He has also served as senior associate editor of Gastroenterology.
His academic career began at the University of California, San Diego, preceded by a gastroenterology fellowship at University of Michigan in Ann Arbor and residency at Massachusetts General Hospital in Boston. From the beginning, he has inspired others with his strong work ethic and intense dedication.
Dr. Carethers joined the AGA Governing Board in June 2020 as vice president and served as president-elect prior to assuming the top leadership role.
New AGA Research Foundation Executive Board members
We’re pleased to share that Michael Camilleri, MD, AGAF, will be taking over the AGA Research Foundation chair role beginning this month. He has recruited five members to be part of the 2022-2024 AGA Research Foundation Executive Board.
Meet the new Foundation Executive Board members
- Michael Camilleri, MD, AGAF, Mayo Clinic, Rochester, MN.
- Aline Charabaty, MD, AGAF, Johns Hopkins School of Medicine, Washington, D.C.
- Eric Esrailian, MD, MPH, AGAF, David Geffen School of Medicine at UCLA, Los Angeles, CA
- Robert A. Ganz, MD, MASGE, MNGI Digestive Health, Minnetonka, MN
- Aja S. McCutchen, MD, Atlanta Gastroenterology Associates, Hoschton, GA
- Michael L. Kochman, MD, AGAF, University of Pennsylvania, Philadelphia, PA
We’re pleased to share that Michael Camilleri, MD, AGAF, will be taking over the AGA Research Foundation chair role beginning this month. He has recruited five members to be part of the 2022-2024 AGA Research Foundation Executive Board.
Meet the new Foundation Executive Board members
- Michael Camilleri, MD, AGAF, Mayo Clinic, Rochester, MN.
- Aline Charabaty, MD, AGAF, Johns Hopkins School of Medicine, Washington, D.C.
- Eric Esrailian, MD, MPH, AGAF, David Geffen School of Medicine at UCLA, Los Angeles, CA
- Robert A. Ganz, MD, MASGE, MNGI Digestive Health, Minnetonka, MN
- Aja S. McCutchen, MD, Atlanta Gastroenterology Associates, Hoschton, GA
- Michael L. Kochman, MD, AGAF, University of Pennsylvania, Philadelphia, PA
We’re pleased to share that Michael Camilleri, MD, AGAF, will be taking over the AGA Research Foundation chair role beginning this month. He has recruited five members to be part of the 2022-2024 AGA Research Foundation Executive Board.
Meet the new Foundation Executive Board members
- Michael Camilleri, MD, AGAF, Mayo Clinic, Rochester, MN.
- Aline Charabaty, MD, AGAF, Johns Hopkins School of Medicine, Washington, D.C.
- Eric Esrailian, MD, MPH, AGAF, David Geffen School of Medicine at UCLA, Los Angeles, CA
- Robert A. Ganz, MD, MASGE, MNGI Digestive Health, Minnetonka, MN
- Aja S. McCutchen, MD, Atlanta Gastroenterology Associates, Hoschton, GA
- Michael L. Kochman, MD, AGAF, University of Pennsylvania, Philadelphia, PA
Plan a gift that offers a better future for GI
Planned giving provides an opportunity for all who have benefited from digestive disease research to give back to the field in a unique and lasting way.
Your investment in the AGA Research Foundation will enable the foundation to continue our investment in the future of gastroenterological research and innovation. With donations from AGA members, we can provide young researchers with a secure, ongoing stable source of funding that drives advancement in the diagnosis, treatment, and cure of digestive diseases.
If you make a contribution, it will be because you believe in what we do and because you want to help make a difference in the lives of others. But we’d also like to make sure you benefit from making a gift to the AGA Research Foundation.
Your giving options
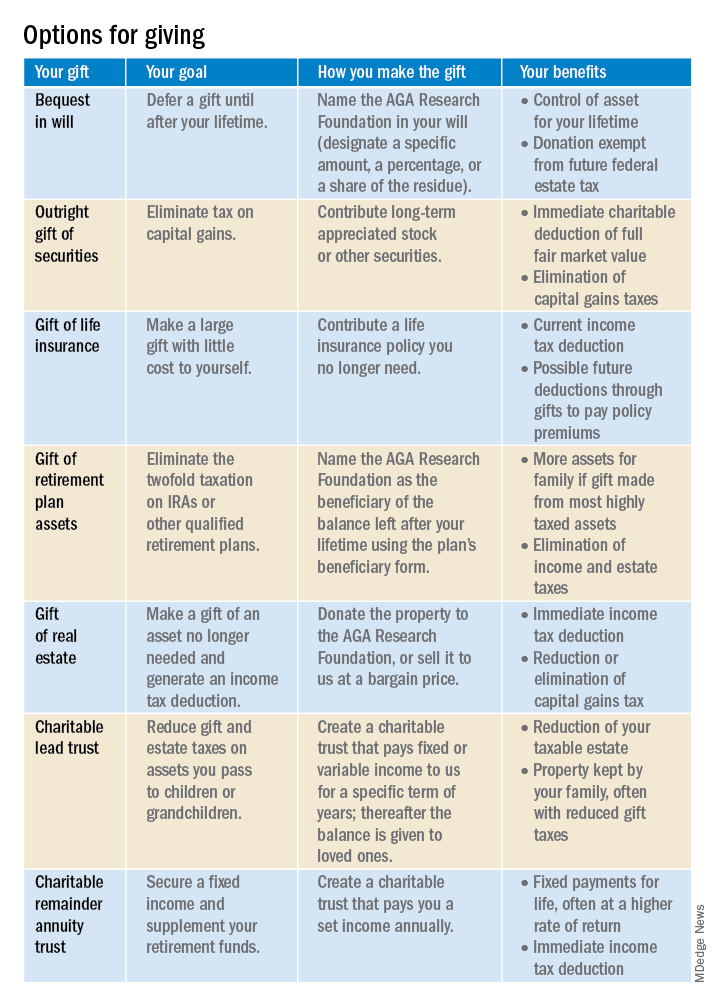
There are several gift arrangements to choose from. The chart below summarizes the benefits of some of the main types of charitable gifts. Just think of what you want to accomplish with your gift, and there’s probably a way to do it!
Learn more by visiting http://gastro.planmylegacy.org.
Planned giving provides an opportunity for all who have benefited from digestive disease research to give back to the field in a unique and lasting way.
Your investment in the AGA Research Foundation will enable the foundation to continue our investment in the future of gastroenterological research and innovation. With donations from AGA members, we can provide young researchers with a secure, ongoing stable source of funding that drives advancement in the diagnosis, treatment, and cure of digestive diseases.
If you make a contribution, it will be because you believe in what we do and because you want to help make a difference in the lives of others. But we’d also like to make sure you benefit from making a gift to the AGA Research Foundation.
Your giving options
There are several gift arrangements to choose from. The chart below summarizes the benefits of some of the main types of charitable gifts. Just think of what you want to accomplish with your gift, and there’s probably a way to do it!

Learn more by visiting http://gastro.planmylegacy.org.
Planned giving provides an opportunity for all who have benefited from digestive disease research to give back to the field in a unique and lasting way.
Your investment in the AGA Research Foundation will enable the foundation to continue our investment in the future of gastroenterological research and innovation. With donations from AGA members, we can provide young researchers with a secure, ongoing stable source of funding that drives advancement in the diagnosis, treatment, and cure of digestive diseases.
If you make a contribution, it will be because you believe in what we do and because you want to help make a difference in the lives of others. But we’d also like to make sure you benefit from making a gift to the AGA Research Foundation.
Your giving options
There are several gift arrangements to choose from. The chart below summarizes the benefits of some of the main types of charitable gifts. Just think of what you want to accomplish with your gift, and there’s probably a way to do it!

Learn more by visiting http://gastro.planmylegacy.org.
2022 Billing and coding updates: Critical care services
The principal idea behind this article is to summarize comprehensively yet concisely the 2022 CMS updates regarding the critical care services. I would encourage and urge all the members to read this section attentively to stay abreast with all the recent developments.
As a general reminder the two critical care services billing codes for the evaluation and management of the critically ill injured patients are:
99291: First 30-74 minutes
99292: Each additional 30 minutes
And, the five major changes for 2022 as proposed by the CMS for critical care services are:
1. It is allowed for the physicians and APPs in the same specialty to bill concurrent critical care services.
Previously, same specialty practitioners were required to bill and were paid as “one” when multiple practitioners provided services on the same date. Now, they can bill for critical care services as subsequent care or as aggregate time, and they are highlighted below with examples:
Subsequent care
Initial visit by a provider for 65 minutes (bill as 99291 as the first claim)
Subsequent visit at a later time on the same day for 60 minutes (bill as 99292 x2 as the second claim)
Aggregate time
Time of multiple practitioners in the same specialty can be added to meet 99291 or 99292. If Practitioner A spends 15 minutes of critical care, then 99291 cannot be billed; but, if Practitioner B spends 30 minutes of critical care, they can bill 99291 with a total time of 45 minutes as one claim
The prerequisites are that the visits are medically necessary, and each visit meets the definition of critical care.
2. Modifier FS needs to be used for split sharing of critical care services.
Previously, critical care services could not be split shared, but it can be done in 2022. The practitioner who provides the significant portion of the visit needs to bill. A significant or substantive portion is considered to be more than half the cumulative total time of both providers.
Example: The APP spends 20 minutes in critical care services and the physician spends 30 minutes. Total time spent is 50 minutes, and the physician may bill 99291.
It is crucial to note that each provider needs to document a note for the medically necessary critical care that they personally performed and the time they spent. Additionally, upon review of the medical records, the two providers should be easily identifiable, and the medical record must be signed and dated by the provider who performed the substantive portion and billed.
Lastly, do not forget to submit the modifier FS.
3. Modifier 25 needs to be used to get paid for an ED visit or other E/M service on the same day as critical care.
Previously, hospital ED services were not paid on the same date as critical care by the same provider. But, in 2022, the practitioners may bill for ED visit at the hospital and also for other E/M services on the same day when there is supporting documentation. The practitioners will need to document that the E/M service was provided prior to the time when the patient did not require critical care, that the service was medically necessary, and that the service was separate and distinct with no duplication.
Of note, do not forget to submit the modifier 25.
4. Critical care visits will be separately billable from global surgery when unrelated with the use of modifier FT.
Previously pre- and postoperative critical care was included in the surgical package of many procedures with a global period of 10-90 days, and critical care visits would be paid only if the service was unrelated to the procedure. The concept remains the same in 2022 but, now, new modifier FT will need to be used to report critical care services unrelated to the procedure. Also, the service provided will need to meet the definition of critical care, which is usually above and beyond the procedure performed and should be unrelated to the specific injury or general surgical procedure performed.
5. There will be certain critical care medical record documentation requirements.
It is paramount that each practitioner must document the exact total critical care time and not a range or approximation of time. Additionally, it is equally as important for the documentation to indicate that the services provided were medically reasonable and necessary. In the setting of split/shared billing, the role of each practitioner should be clearly identifiable (the condition for which each practitioner treated the patient, how the care was concurrent either subsequent or aggregate, and the total time of each practitioner).
Hopefully, this review will provide a good perception for our members in regards to major updates for 2022, help them navigate the regulatory rules, and avoid any unnecessary setbacks. In the upcoming months, we will try to cover some more topics on practice management and administration, such as Medicare Physician Fee Schedule Rule, Hospital Outpatient Prospective Payment Rule, and coding/billing for teaching physicians, telehealth, and pulmonary rehabilitation services.
The principal idea behind this article is to summarize comprehensively yet concisely the 2022 CMS updates regarding the critical care services. I would encourage and urge all the members to read this section attentively to stay abreast with all the recent developments.
As a general reminder the two critical care services billing codes for the evaluation and management of the critically ill injured patients are:
99291: First 30-74 minutes
99292: Each additional 30 minutes
And, the five major changes for 2022 as proposed by the CMS for critical care services are:
1. It is allowed for the physicians and APPs in the same specialty to bill concurrent critical care services.
Previously, same specialty practitioners were required to bill and were paid as “one” when multiple practitioners provided services on the same date. Now, they can bill for critical care services as subsequent care or as aggregate time, and they are highlighted below with examples:
Subsequent care
Initial visit by a provider for 65 minutes (bill as 99291 as the first claim)
Subsequent visit at a later time on the same day for 60 minutes (bill as 99292 x2 as the second claim)
Aggregate time
Time of multiple practitioners in the same specialty can be added to meet 99291 or 99292. If Practitioner A spends 15 minutes of critical care, then 99291 cannot be billed; but, if Practitioner B spends 30 minutes of critical care, they can bill 99291 with a total time of 45 minutes as one claim
The prerequisites are that the visits are medically necessary, and each visit meets the definition of critical care.
2. Modifier FS needs to be used for split sharing of critical care services.
Previously, critical care services could not be split shared, but it can be done in 2022. The practitioner who provides the significant portion of the visit needs to bill. A significant or substantive portion is considered to be more than half the cumulative total time of both providers.
Example: The APP spends 20 minutes in critical care services and the physician spends 30 minutes. Total time spent is 50 minutes, and the physician may bill 99291.
It is crucial to note that each provider needs to document a note for the medically necessary critical care that they personally performed and the time they spent. Additionally, upon review of the medical records, the two providers should be easily identifiable, and the medical record must be signed and dated by the provider who performed the substantive portion and billed.
Lastly, do not forget to submit the modifier FS.
3. Modifier 25 needs to be used to get paid for an ED visit or other E/M service on the same day as critical care.
Previously, hospital ED services were not paid on the same date as critical care by the same provider. But, in 2022, the practitioners may bill for ED visit at the hospital and also for other E/M services on the same day when there is supporting documentation. The practitioners will need to document that the E/M service was provided prior to the time when the patient did not require critical care, that the service was medically necessary, and that the service was separate and distinct with no duplication.
Of note, do not forget to submit the modifier 25.
4. Critical care visits will be separately billable from global surgery when unrelated with the use of modifier FT.
Previously pre- and postoperative critical care was included in the surgical package of many procedures with a global period of 10-90 days, and critical care visits would be paid only if the service was unrelated to the procedure. The concept remains the same in 2022 but, now, new modifier FT will need to be used to report critical care services unrelated to the procedure. Also, the service provided will need to meet the definition of critical care, which is usually above and beyond the procedure performed and should be unrelated to the specific injury or general surgical procedure performed.
5. There will be certain critical care medical record documentation requirements.
It is paramount that each practitioner must document the exact total critical care time and not a range or approximation of time. Additionally, it is equally as important for the documentation to indicate that the services provided were medically reasonable and necessary. In the setting of split/shared billing, the role of each practitioner should be clearly identifiable (the condition for which each practitioner treated the patient, how the care was concurrent either subsequent or aggregate, and the total time of each practitioner).
Hopefully, this review will provide a good perception for our members in regards to major updates for 2022, help them navigate the regulatory rules, and avoid any unnecessary setbacks. In the upcoming months, we will try to cover some more topics on practice management and administration, such as Medicare Physician Fee Schedule Rule, Hospital Outpatient Prospective Payment Rule, and coding/billing for teaching physicians, telehealth, and pulmonary rehabilitation services.
The principal idea behind this article is to summarize comprehensively yet concisely the 2022 CMS updates regarding the critical care services. I would encourage and urge all the members to read this section attentively to stay abreast with all the recent developments.
As a general reminder the two critical care services billing codes for the evaluation and management of the critically ill injured patients are:
99291: First 30-74 minutes
99292: Each additional 30 minutes
And, the five major changes for 2022 as proposed by the CMS for critical care services are:
1. It is allowed for the physicians and APPs in the same specialty to bill concurrent critical care services.
Previously, same specialty practitioners were required to bill and were paid as “one” when multiple practitioners provided services on the same date. Now, they can bill for critical care services as subsequent care or as aggregate time, and they are highlighted below with examples:
Subsequent care
Initial visit by a provider for 65 minutes (bill as 99291 as the first claim)
Subsequent visit at a later time on the same day for 60 minutes (bill as 99292 x2 as the second claim)
Aggregate time
Time of multiple practitioners in the same specialty can be added to meet 99291 or 99292. If Practitioner A spends 15 minutes of critical care, then 99291 cannot be billed; but, if Practitioner B spends 30 minutes of critical care, they can bill 99291 with a total time of 45 minutes as one claim
The prerequisites are that the visits are medically necessary, and each visit meets the definition of critical care.
2. Modifier FS needs to be used for split sharing of critical care services.
Previously, critical care services could not be split shared, but it can be done in 2022. The practitioner who provides the significant portion of the visit needs to bill. A significant or substantive portion is considered to be more than half the cumulative total time of both providers.
Example: The APP spends 20 minutes in critical care services and the physician spends 30 minutes. Total time spent is 50 minutes, and the physician may bill 99291.
It is crucial to note that each provider needs to document a note for the medically necessary critical care that they personally performed and the time they spent. Additionally, upon review of the medical records, the two providers should be easily identifiable, and the medical record must be signed and dated by the provider who performed the substantive portion and billed.
Lastly, do not forget to submit the modifier FS.
3. Modifier 25 needs to be used to get paid for an ED visit or other E/M service on the same day as critical care.
Previously, hospital ED services were not paid on the same date as critical care by the same provider. But, in 2022, the practitioners may bill for ED visit at the hospital and also for other E/M services on the same day when there is supporting documentation. The practitioners will need to document that the E/M service was provided prior to the time when the patient did not require critical care, that the service was medically necessary, and that the service was separate and distinct with no duplication.
Of note, do not forget to submit the modifier 25.
4. Critical care visits will be separately billable from global surgery when unrelated with the use of modifier FT.
Previously pre- and postoperative critical care was included in the surgical package of many procedures with a global period of 10-90 days, and critical care visits would be paid only if the service was unrelated to the procedure. The concept remains the same in 2022 but, now, new modifier FT will need to be used to report critical care services unrelated to the procedure. Also, the service provided will need to meet the definition of critical care, which is usually above and beyond the procedure performed and should be unrelated to the specific injury or general surgical procedure performed.
5. There will be certain critical care medical record documentation requirements.
It is paramount that each practitioner must document the exact total critical care time and not a range or approximation of time. Additionally, it is equally as important for the documentation to indicate that the services provided were medically reasonable and necessary. In the setting of split/shared billing, the role of each practitioner should be clearly identifiable (the condition for which each practitioner treated the patient, how the care was concurrent either subsequent or aggregate, and the total time of each practitioner).
Hopefully, this review will provide a good perception for our members in regards to major updates for 2022, help them navigate the regulatory rules, and avoid any unnecessary setbacks. In the upcoming months, we will try to cover some more topics on practice management and administration, such as Medicare Physician Fee Schedule Rule, Hospital Outpatient Prospective Payment Rule, and coding/billing for teaching physicians, telehealth, and pulmonary rehabilitation services.
In memoriam
CHEST has been informed of the following deaths of CHEST members.
We remember our colleagues and extend our sincere condolences.
Edward C. Rosenow III, MD, Master FCCP
Jack Stanko, MD, MS, FCCP
Arthur S. Turetsky, MD, FCCP
CHEST has been informed of the following deaths of CHEST members.
We remember our colleagues and extend our sincere condolences.
Edward C. Rosenow III, MD, Master FCCP
Jack Stanko, MD, MS, FCCP
Arthur S. Turetsky, MD, FCCP
CHEST has been informed of the following deaths of CHEST members.
We remember our colleagues and extend our sincere condolences.
Edward C. Rosenow III, MD, Master FCCP
Jack Stanko, MD, MS, FCCP
Arthur S. Turetsky, MD, FCCP












