User login
AMA Insights
As many who read CHEST® Physician may know, we have a nucleus of dedicated volunteers who give unselfishly of their time and talent to represent our members in the area of “regulatory advocacy” and “policy advocacy” in the areas of pulmonary, critical care, and sleep medicine. It is our goal to recognize and support this valuable group of individuals who represent us in the space of coding and reimbursement, RUC activities, relationships with organizations like the ACP and the AMA, as well as our sister societies, such as ATS, SCCM, NAMDRC, CCNA, APSR, ALAT, and ERS, among others.
One of our goals, in addition to recognizing this group, is to identify and mentor the next generation of representatives. A great example of this mentorship is reflected in our involvement with the AMA. Dr. Bob McCaffree has represented CHEST for 22 years and is now mentoring Dr. Raj Desai who will be assuming this role of AMA Delegate this year. Special thanks to Dr. McCaffree for his unselfish service in this capacity and for his mentorship of Dr. Desai. I hope that you enjoy this and future CHEST® Physician articles summarizing and reflecting on the activities pertinent to CHEST at the AMA.
John Studdard, MD, FCCP
CHEST President
Collaborating with societies: CHEST and AMA
While the American Medical Association (AMA) is the oldest and largest national medical association, many physicians, both members and nonmembers, have limited understanding of the policies, processes, and strategic foci of the AMA. It is our goal to inform our membership about the workings of the AMA and how those interact with the goals of CHEST and our members. We hope to do this by publishing periodic articles in CHEST® Physician. One of the authors (DRM) has been the CHEST delegate to the AMA for more than 20 years, and the other (NRD) is CHEST’s new delegate.
- Create thriving physician practices.
- Create the medical school of the future.
- Improve health outcomes.
We will expand on these in future articles.
The AMA is both an individual member organization and a federation of geographic, ie, county and state, societies and specialty societies, as well as the uniformed services and the VA. It is this federation that comprises the House of Delegates (HOD or House), which is the principle policy-making body of the AMA. The number of delegates from each member organization (now numbering more than 170 organizations) depends on the number of individual AMA members among that organization’s members. Due to recent bylaws changes, CHEST now has two delegates. The HOD meets twice per year to establish policy on health, medical, professional, and governance matters, as well as the principles within which the AMA’s business activities are conducted.
Most member societies meet in caucuses or Section Councils prior to the voting in the House to discuss the pending business. The Specialty and Service Society (SSS) is the largest caucus in the AMA’s House of Delegates. The SSS meets twice annually in conjunction with the Interim and Annual Meetings of the HOD. There are two categories of groups in the SSS: those societies that have seats in the HOD and those seeking admission to the house.
SSS groups in the HOD include:
- 119 national medical specialties
- 2 professional interest medical associations
- 5 military service groups
An association must first be represented in the SSS for 3 years and meet the required number of AMA members before it is eligible to seek admission to the HOD.
The American College of Chest Physicians (CHEST) is an active member of the SSS but also joins with other societies of similar interests in the Section Council on Chest and Allergic Diseases. This caucus includes the ATS, SCCM, ASSM, and several allergy societies. Through the HOD, the SSS, and the Section Council, CHEST can partner with the AMA and other societies, such as ATS, to support each other’s resolutions or important regulatory issues.
In summary, the AMA plays an important role in many areas of interest to our members. And, it can be a useful forum for connecting with societies with similar interests in directing advocacy and setting policy. We plan to continue this update in future issues of CHEST® Physician.
References
1. https://www.ama-assn.org/content/ama-house-delegates Accessed: January 28, 2018
2. https://www.ama-assn.org/practice-management/ama-steps-forward-practice-improvement-strategies Accessed: January 28, 2018
As many who read CHEST® Physician may know, we have a nucleus of dedicated volunteers who give unselfishly of their time and talent to represent our members in the area of “regulatory advocacy” and “policy advocacy” in the areas of pulmonary, critical care, and sleep medicine. It is our goal to recognize and support this valuable group of individuals who represent us in the space of coding and reimbursement, RUC activities, relationships with organizations like the ACP and the AMA, as well as our sister societies, such as ATS, SCCM, NAMDRC, CCNA, APSR, ALAT, and ERS, among others.
One of our goals, in addition to recognizing this group, is to identify and mentor the next generation of representatives. A great example of this mentorship is reflected in our involvement with the AMA. Dr. Bob McCaffree has represented CHEST for 22 years and is now mentoring Dr. Raj Desai who will be assuming this role of AMA Delegate this year. Special thanks to Dr. McCaffree for his unselfish service in this capacity and for his mentorship of Dr. Desai. I hope that you enjoy this and future CHEST® Physician articles summarizing and reflecting on the activities pertinent to CHEST at the AMA.
John Studdard, MD, FCCP
CHEST President
Collaborating with societies: CHEST and AMA
While the American Medical Association (AMA) is the oldest and largest national medical association, many physicians, both members and nonmembers, have limited understanding of the policies, processes, and strategic foci of the AMA. It is our goal to inform our membership about the workings of the AMA and how those interact with the goals of CHEST and our members. We hope to do this by publishing periodic articles in CHEST® Physician. One of the authors (DRM) has been the CHEST delegate to the AMA for more than 20 years, and the other (NRD) is CHEST’s new delegate.
- Create thriving physician practices.
- Create the medical school of the future.
- Improve health outcomes.
We will expand on these in future articles.
The AMA is both an individual member organization and a federation of geographic, ie, county and state, societies and specialty societies, as well as the uniformed services and the VA. It is this federation that comprises the House of Delegates (HOD or House), which is the principle policy-making body of the AMA. The number of delegates from each member organization (now numbering more than 170 organizations) depends on the number of individual AMA members among that organization’s members. Due to recent bylaws changes, CHEST now has two delegates. The HOD meets twice per year to establish policy on health, medical, professional, and governance matters, as well as the principles within which the AMA’s business activities are conducted.
Most member societies meet in caucuses or Section Councils prior to the voting in the House to discuss the pending business. The Specialty and Service Society (SSS) is the largest caucus in the AMA’s House of Delegates. The SSS meets twice annually in conjunction with the Interim and Annual Meetings of the HOD. There are two categories of groups in the SSS: those societies that have seats in the HOD and those seeking admission to the house.
SSS groups in the HOD include:
- 119 national medical specialties
- 2 professional interest medical associations
- 5 military service groups
An association must first be represented in the SSS for 3 years and meet the required number of AMA members before it is eligible to seek admission to the HOD.
The American College of Chest Physicians (CHEST) is an active member of the SSS but also joins with other societies of similar interests in the Section Council on Chest and Allergic Diseases. This caucus includes the ATS, SCCM, ASSM, and several allergy societies. Through the HOD, the SSS, and the Section Council, CHEST can partner with the AMA and other societies, such as ATS, to support each other’s resolutions or important regulatory issues.
In summary, the AMA plays an important role in many areas of interest to our members. And, it can be a useful forum for connecting with societies with similar interests in directing advocacy and setting policy. We plan to continue this update in future issues of CHEST® Physician.
References
1. https://www.ama-assn.org/content/ama-house-delegates Accessed: January 28, 2018
2. https://www.ama-assn.org/practice-management/ama-steps-forward-practice-improvement-strategies Accessed: January 28, 2018
As many who read CHEST® Physician may know, we have a nucleus of dedicated volunteers who give unselfishly of their time and talent to represent our members in the area of “regulatory advocacy” and “policy advocacy” in the areas of pulmonary, critical care, and sleep medicine. It is our goal to recognize and support this valuable group of individuals who represent us in the space of coding and reimbursement, RUC activities, relationships with organizations like the ACP and the AMA, as well as our sister societies, such as ATS, SCCM, NAMDRC, CCNA, APSR, ALAT, and ERS, among others.
One of our goals, in addition to recognizing this group, is to identify and mentor the next generation of representatives. A great example of this mentorship is reflected in our involvement with the AMA. Dr. Bob McCaffree has represented CHEST for 22 years and is now mentoring Dr. Raj Desai who will be assuming this role of AMA Delegate this year. Special thanks to Dr. McCaffree for his unselfish service in this capacity and for his mentorship of Dr. Desai. I hope that you enjoy this and future CHEST® Physician articles summarizing and reflecting on the activities pertinent to CHEST at the AMA.
John Studdard, MD, FCCP
CHEST President
Collaborating with societies: CHEST and AMA
While the American Medical Association (AMA) is the oldest and largest national medical association, many physicians, both members and nonmembers, have limited understanding of the policies, processes, and strategic foci of the AMA. It is our goal to inform our membership about the workings of the AMA and how those interact with the goals of CHEST and our members. We hope to do this by publishing periodic articles in CHEST® Physician. One of the authors (DRM) has been the CHEST delegate to the AMA for more than 20 years, and the other (NRD) is CHEST’s new delegate.
- Create thriving physician practices.
- Create the medical school of the future.
- Improve health outcomes.
We will expand on these in future articles.
The AMA is both an individual member organization and a federation of geographic, ie, county and state, societies and specialty societies, as well as the uniformed services and the VA. It is this federation that comprises the House of Delegates (HOD or House), which is the principle policy-making body of the AMA. The number of delegates from each member organization (now numbering more than 170 organizations) depends on the number of individual AMA members among that organization’s members. Due to recent bylaws changes, CHEST now has two delegates. The HOD meets twice per year to establish policy on health, medical, professional, and governance matters, as well as the principles within which the AMA’s business activities are conducted.
Most member societies meet in caucuses or Section Councils prior to the voting in the House to discuss the pending business. The Specialty and Service Society (SSS) is the largest caucus in the AMA’s House of Delegates. The SSS meets twice annually in conjunction with the Interim and Annual Meetings of the HOD. There are two categories of groups in the SSS: those societies that have seats in the HOD and those seeking admission to the house.
SSS groups in the HOD include:
- 119 national medical specialties
- 2 professional interest medical associations
- 5 military service groups
An association must first be represented in the SSS for 3 years and meet the required number of AMA members before it is eligible to seek admission to the HOD.
The American College of Chest Physicians (CHEST) is an active member of the SSS but also joins with other societies of similar interests in the Section Council on Chest and Allergic Diseases. This caucus includes the ATS, SCCM, ASSM, and several allergy societies. Through the HOD, the SSS, and the Section Council, CHEST can partner with the AMA and other societies, such as ATS, to support each other’s resolutions or important regulatory issues.
In summary, the AMA plays an important role in many areas of interest to our members. And, it can be a useful forum for connecting with societies with similar interests in directing advocacy and setting policy. We plan to continue this update in future issues of CHEST® Physician.
References
1. https://www.ama-assn.org/content/ama-house-delegates Accessed: January 28, 2018
2. https://www.ama-assn.org/practice-management/ama-steps-forward-practice-improvement-strategies Accessed: January 28, 2018
Palliative care screening, sleep devices, novel biologics
Palliative and end-of-life care
Nurse-driven palliative care screening
Palliative care (PC) aims to improve quality of life for patients with a life-threatening illness, providing holistic patient-centered support along the continuum of the disease process. Although frequently implemented in critical care settings, integrating PC in the neuro ICU has been difficult to adopt in practice due to the uncertainty in prognostication of definitive outcomes and practice culture beliefs such as the self-fulfilling prophecy (Frontera, et al. Crit Care Med. 2015;43[9]:1964; Rubin, et al. Curr Opin Crit Care. 2017;23[2]:134; Knies, et al. Semin Neurol. 2016;36[6]:631).
At our institution, a nursing education project was conducted to pilot nurse-driven PC screenings on admission to the neuro ICU. The project evaluated nurse comfort and knowledge with identifying and recommending PC consults. Pre- and post-intervention surveys revealed that education and introduction of a PC screening tool significantly increased nurse comfort and knowledge of PC eligibility.
PC in the neuro ICU can exist to contribute to successful outcomes in patient and family care. Within neurocritical care, incorporating PC is essential to provide extra support to patients and families (Frontera, et al. 2015).
For these reasons and data from the project, nurse-driven screening may encourage appropriate early PC consults. Patient-centered care is the ultimate goal in the management of our patients. Nurse-driven PC screening can help bring various unmet PC needs to the health-care team for opportunities that might not have been met or otherwise assessed. Consider implementing nurse-driven PC screening protocols at your institution to aid in collaborative and proactive interdisciplinary care.
Danielle McCamey, ACNP
Steering Committee Member
Sleep medicine
Diagnostics, devices, and sleep
The past several months have been busy for the Sleep Medicine NetWork. We have been working to represent the interests of our membership and our patients in many arenas.
Devices coded as E0464, defined as life support mechanical ventilators used with mask-based ventilation in the home are being more frequently used. According to the Office of the Inspector General (OIG), there has been an 89-fold increase in billing for E0464 ventilators for Medicare and its beneficiaries between 2009 and 2015, increasing from $3.8M to $340M. In response, the Agency for Healthcare Research and Quality (AHRQ) requested a response to specific questions related to these devices.
In 2018, the CHEST Sleep Medicine NetWork will be participating in a Federal Drug Association-sponsored workshop entitled “Study Design Considerations for Devices including Digital Health Technologies for Sleep-Disordered Breathing (SDB) in Adults,” along with other national organizations and leaders in our field. This workshop will address available technologies for the diagnosis, monitoring, and treatment of SDB, as well as trends for digital health technologies and clinical trial design considerations.
Finally, the Sleep Medicine NetWork has wasted no time after a successful CHEST 2017 in Toronto in planning for the next annual meeting in San Antonio. We are excited to present an exciting curriculum in Sleep Medicine at CHEST 2018, so stay tuned.
Aneesa M. Das, MD, FCCP
NetWork Chair
Occupational and environmental health
Post-deployment lung disease
Since the early 1990s, ongoing military deployments to Southwest Asia remain a unique challenge from a pulmonary symptomology and diagnostic perspective.
Various airborne hazards in the deployment environment include geologic dusts, burn pit smoke, vehicle emissions, and industrial air pollution. Exposures can give rise to both acute respiratory symptoms and, in some instances, chronic lung disease. Currently, data are limited on whether inhalation of airborne particulate matter by military personnel is linked to increases in pulmonary diseases (Morris MJ, et al. US Army Med Dep J. 2016:173).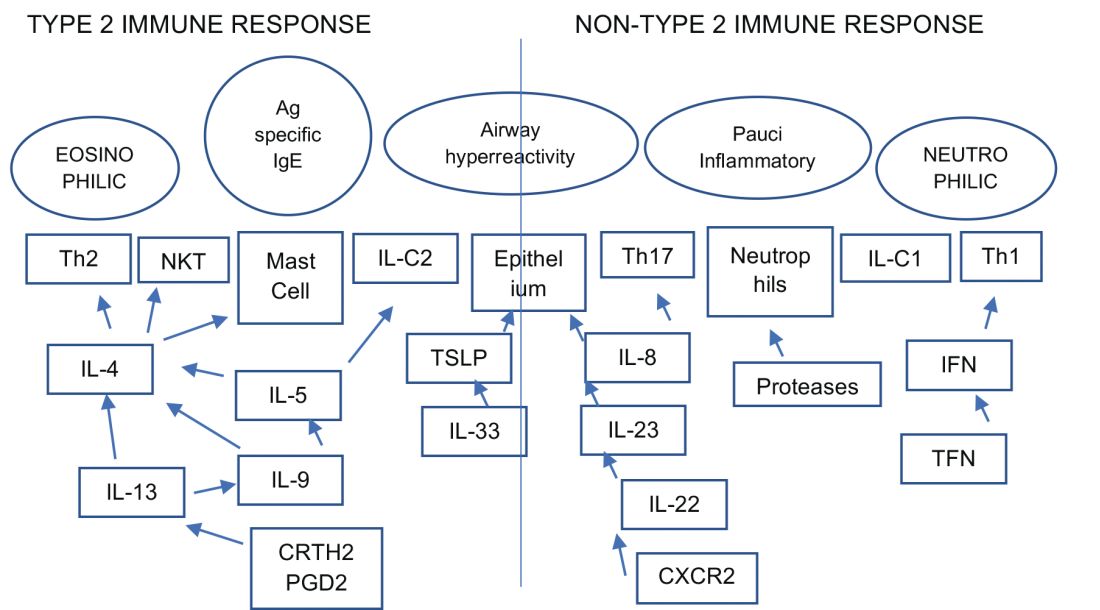
Ongoing research by the Veterans Affairs continues to enroll post-deployed personnel in an Airborne Hazard and Burn Pit Registry. Past approaches in evaluation of deployed individuals ranged from common tests such as spirometry, HRCT scanning, full PFTs, bronchoprovocation challenges, and, in some instances, lung biopsies (Krefft SD, et al. Fed Pract. 2015;32[6]:32). More novel evaluations of postdeployment dyspnea include impulse oscillometry, exhaled nitric oxide, bronchoscopy, and cardiopulmonary exercise testing (Huprikar, et al. Chest. 2016;150[4]:S934A).
Members of the CHEST Occupational and Environmental Health NetWork are currently updating comprehensive approaches to evaluate military personnel with chronic respiratory symptoms from deployments. Continued emphasis, however, should be placed on diagnosing and treating common diseases such as asthma, exercise-induced bronchospasm, GERD, and upper airway disorders.
Pedro F. Lucero, MD, FCCP
Steering Committee Member
Clinical pulmonary medicine
Biologics – Birth of a new era of precision management in asthma
An estimated 10% to 20% of patients with severe uncontrolled asthma do not respond to maximal best standard treatments, leading to substantial health-care costs. A paradigm shift is now underway in our approach to the care of these patients with the emergence of novel biologics targeting the complex and interconnected inflammatory pathways in asthma that result in a diverse profile of asthma endotypes and phenotypes (Fig 1).
Current FDA-approved biologics primarily target patients with a T2 high phenotype (Table1).
Dupilumab binds to the alpha unit of the IL-4 receptor and blocks both IL-4 and IL-13. It shows potential efficacy in patients with T2 high asthma with or without eosinophilia but has not yet received FDA approval.
Multiple newer biologics are currently in development (Table 2).
Pulmonologists need to get familiar with the logistics of administration of these novel agents. The two common methods of administering biologics are (1) buy and bill – where the provider buys the drug directly from the distributor; and (2) assignment of benefits (typically administered by a Pharmacy Benefit Manager) - specific dose of the medication is shipped to the physician’s office and physician only bills for the administration. CPT and J codes are shown in Table 1.
Shyamsunder Subramanian, MD, FCCP
Steering Committee Member
Palliative and end-of-life care
Nurse-driven palliative care screening
Palliative care (PC) aims to improve quality of life for patients with a life-threatening illness, providing holistic patient-centered support along the continuum of the disease process. Although frequently implemented in critical care settings, integrating PC in the neuro ICU has been difficult to adopt in practice due to the uncertainty in prognostication of definitive outcomes and practice culture beliefs such as the self-fulfilling prophecy (Frontera, et al. Crit Care Med. 2015;43[9]:1964; Rubin, et al. Curr Opin Crit Care. 2017;23[2]:134; Knies, et al. Semin Neurol. 2016;36[6]:631).
At our institution, a nursing education project was conducted to pilot nurse-driven PC screenings on admission to the neuro ICU. The project evaluated nurse comfort and knowledge with identifying and recommending PC consults. Pre- and post-intervention surveys revealed that education and introduction of a PC screening tool significantly increased nurse comfort and knowledge of PC eligibility.
PC in the neuro ICU can exist to contribute to successful outcomes in patient and family care. Within neurocritical care, incorporating PC is essential to provide extra support to patients and families (Frontera, et al. 2015).
For these reasons and data from the project, nurse-driven screening may encourage appropriate early PC consults. Patient-centered care is the ultimate goal in the management of our patients. Nurse-driven PC screening can help bring various unmet PC needs to the health-care team for opportunities that might not have been met or otherwise assessed. Consider implementing nurse-driven PC screening protocols at your institution to aid in collaborative and proactive interdisciplinary care.
Danielle McCamey, ACNP
Steering Committee Member
Sleep medicine
Diagnostics, devices, and sleep
The past several months have been busy for the Sleep Medicine NetWork. We have been working to represent the interests of our membership and our patients in many arenas.
Devices coded as E0464, defined as life support mechanical ventilators used with mask-based ventilation in the home are being more frequently used. According to the Office of the Inspector General (OIG), there has been an 89-fold increase in billing for E0464 ventilators for Medicare and its beneficiaries between 2009 and 2015, increasing from $3.8M to $340M. In response, the Agency for Healthcare Research and Quality (AHRQ) requested a response to specific questions related to these devices.
In 2018, the CHEST Sleep Medicine NetWork will be participating in a Federal Drug Association-sponsored workshop entitled “Study Design Considerations for Devices including Digital Health Technologies for Sleep-Disordered Breathing (SDB) in Adults,” along with other national organizations and leaders in our field. This workshop will address available technologies for the diagnosis, monitoring, and treatment of SDB, as well as trends for digital health technologies and clinical trial design considerations.
Finally, the Sleep Medicine NetWork has wasted no time after a successful CHEST 2017 in Toronto in planning for the next annual meeting in San Antonio. We are excited to present an exciting curriculum in Sleep Medicine at CHEST 2018, so stay tuned.
Aneesa M. Das, MD, FCCP
NetWork Chair
Occupational and environmental health
Post-deployment lung disease
Since the early 1990s, ongoing military deployments to Southwest Asia remain a unique challenge from a pulmonary symptomology and diagnostic perspective.
Various airborne hazards in the deployment environment include geologic dusts, burn pit smoke, vehicle emissions, and industrial air pollution. Exposures can give rise to both acute respiratory symptoms and, in some instances, chronic lung disease. Currently, data are limited on whether inhalation of airborne particulate matter by military personnel is linked to increases in pulmonary diseases (Morris MJ, et al. US Army Med Dep J. 2016:173).
Ongoing research by the Veterans Affairs continues to enroll post-deployed personnel in an Airborne Hazard and Burn Pit Registry. Past approaches in evaluation of deployed individuals ranged from common tests such as spirometry, HRCT scanning, full PFTs, bronchoprovocation challenges, and, in some instances, lung biopsies (Krefft SD, et al. Fed Pract. 2015;32[6]:32). More novel evaluations of postdeployment dyspnea include impulse oscillometry, exhaled nitric oxide, bronchoscopy, and cardiopulmonary exercise testing (Huprikar, et al. Chest. 2016;150[4]:S934A).
Members of the CHEST Occupational and Environmental Health NetWork are currently updating comprehensive approaches to evaluate military personnel with chronic respiratory symptoms from deployments. Continued emphasis, however, should be placed on diagnosing and treating common diseases such as asthma, exercise-induced bronchospasm, GERD, and upper airway disorders.
Pedro F. Lucero, MD, FCCP
Steering Committee Member
Clinical pulmonary medicine
Biologics – Birth of a new era of precision management in asthma
An estimated 10% to 20% of patients with severe uncontrolled asthma do not respond to maximal best standard treatments, leading to substantial health-care costs. A paradigm shift is now underway in our approach to the care of these patients with the emergence of novel biologics targeting the complex and interconnected inflammatory pathways in asthma that result in a diverse profile of asthma endotypes and phenotypes (Fig 1).
Current FDA-approved biologics primarily target patients with a T2 high phenotype (Table1).
Dupilumab binds to the alpha unit of the IL-4 receptor and blocks both IL-4 and IL-13. It shows potential efficacy in patients with T2 high asthma with or without eosinophilia but has not yet received FDA approval.
Multiple newer biologics are currently in development (Table 2).
Pulmonologists need to get familiar with the logistics of administration of these novel agents. The two common methods of administering biologics are (1) buy and bill – where the provider buys the drug directly from the distributor; and (2) assignment of benefits (typically administered by a Pharmacy Benefit Manager) - specific dose of the medication is shipped to the physician’s office and physician only bills for the administration. CPT and J codes are shown in Table 1.
Shyamsunder Subramanian, MD, FCCP
Steering Committee Member
Palliative and end-of-life care
Nurse-driven palliative care screening
Palliative care (PC) aims to improve quality of life for patients with a life-threatening illness, providing holistic patient-centered support along the continuum of the disease process. Although frequently implemented in critical care settings, integrating PC in the neuro ICU has been difficult to adopt in practice due to the uncertainty in prognostication of definitive outcomes and practice culture beliefs such as the self-fulfilling prophecy (Frontera, et al. Crit Care Med. 2015;43[9]:1964; Rubin, et al. Curr Opin Crit Care. 2017;23[2]:134; Knies, et al. Semin Neurol. 2016;36[6]:631).
At our institution, a nursing education project was conducted to pilot nurse-driven PC screenings on admission to the neuro ICU. The project evaluated nurse comfort and knowledge with identifying and recommending PC consults. Pre- and post-intervention surveys revealed that education and introduction of a PC screening tool significantly increased nurse comfort and knowledge of PC eligibility.
PC in the neuro ICU can exist to contribute to successful outcomes in patient and family care. Within neurocritical care, incorporating PC is essential to provide extra support to patients and families (Frontera, et al. 2015).
For these reasons and data from the project, nurse-driven screening may encourage appropriate early PC consults. Patient-centered care is the ultimate goal in the management of our patients. Nurse-driven PC screening can help bring various unmet PC needs to the health-care team for opportunities that might not have been met or otherwise assessed. Consider implementing nurse-driven PC screening protocols at your institution to aid in collaborative and proactive interdisciplinary care.
Danielle McCamey, ACNP
Steering Committee Member
Sleep medicine
Diagnostics, devices, and sleep
The past several months have been busy for the Sleep Medicine NetWork. We have been working to represent the interests of our membership and our patients in many arenas.
Devices coded as E0464, defined as life support mechanical ventilators used with mask-based ventilation in the home are being more frequently used. According to the Office of the Inspector General (OIG), there has been an 89-fold increase in billing for E0464 ventilators for Medicare and its beneficiaries between 2009 and 2015, increasing from $3.8M to $340M. In response, the Agency for Healthcare Research and Quality (AHRQ) requested a response to specific questions related to these devices.
In 2018, the CHEST Sleep Medicine NetWork will be participating in a Federal Drug Association-sponsored workshop entitled “Study Design Considerations for Devices including Digital Health Technologies for Sleep-Disordered Breathing (SDB) in Adults,” along with other national organizations and leaders in our field. This workshop will address available technologies for the diagnosis, monitoring, and treatment of SDB, as well as trends for digital health technologies and clinical trial design considerations.
Finally, the Sleep Medicine NetWork has wasted no time after a successful CHEST 2017 in Toronto in planning for the next annual meeting in San Antonio. We are excited to present an exciting curriculum in Sleep Medicine at CHEST 2018, so stay tuned.
Aneesa M. Das, MD, FCCP
NetWork Chair
Occupational and environmental health
Post-deployment lung disease
Since the early 1990s, ongoing military deployments to Southwest Asia remain a unique challenge from a pulmonary symptomology and diagnostic perspective.
Various airborne hazards in the deployment environment include geologic dusts, burn pit smoke, vehicle emissions, and industrial air pollution. Exposures can give rise to both acute respiratory symptoms and, in some instances, chronic lung disease. Currently, data are limited on whether inhalation of airborne particulate matter by military personnel is linked to increases in pulmonary diseases (Morris MJ, et al. US Army Med Dep J. 2016:173).
Ongoing research by the Veterans Affairs continues to enroll post-deployed personnel in an Airborne Hazard and Burn Pit Registry. Past approaches in evaluation of deployed individuals ranged from common tests such as spirometry, HRCT scanning, full PFTs, bronchoprovocation challenges, and, in some instances, lung biopsies (Krefft SD, et al. Fed Pract. 2015;32[6]:32). More novel evaluations of postdeployment dyspnea include impulse oscillometry, exhaled nitric oxide, bronchoscopy, and cardiopulmonary exercise testing (Huprikar, et al. Chest. 2016;150[4]:S934A).
Members of the CHEST Occupational and Environmental Health NetWork are currently updating comprehensive approaches to evaluate military personnel with chronic respiratory symptoms from deployments. Continued emphasis, however, should be placed on diagnosing and treating common diseases such as asthma, exercise-induced bronchospasm, GERD, and upper airway disorders.
Pedro F. Lucero, MD, FCCP
Steering Committee Member
Clinical pulmonary medicine
Biologics – Birth of a new era of precision management in asthma
An estimated 10% to 20% of patients with severe uncontrolled asthma do not respond to maximal best standard treatments, leading to substantial health-care costs. A paradigm shift is now underway in our approach to the care of these patients with the emergence of novel biologics targeting the complex and interconnected inflammatory pathways in asthma that result in a diverse profile of asthma endotypes and phenotypes (Fig 1).
Current FDA-approved biologics primarily target patients with a T2 high phenotype (Table1).
Dupilumab binds to the alpha unit of the IL-4 receptor and blocks both IL-4 and IL-13. It shows potential efficacy in patients with T2 high asthma with or without eosinophilia but has not yet received FDA approval.
Multiple newer biologics are currently in development (Table 2).
Pulmonologists need to get familiar with the logistics of administration of these novel agents. The two common methods of administering biologics are (1) buy and bill – where the provider buys the drug directly from the distributor; and (2) assignment of benefits (typically administered by a Pharmacy Benefit Manager) - specific dose of the medication is shipped to the physician’s office and physician only bills for the administration. CPT and J codes are shown in Table 1.
Shyamsunder Subramanian, MD, FCCP
Steering Committee Member
Bring the Whole Team to VAM
Have you registered yet for the 2018 Vascular Annual Meeting in Boston? This year, bring your vascular team – there will be programming for nurses, technicians, nurse practitioners and PAs. In addition to VAM sessions of interests to nurses, the Society for Vascular Nursing will hold its annual conference in alignment with VAM, on June 20-21. Registration for SVN covers attendance at both meetings. And PAs will their own section of programming from 1 to 5 p.m. Thursday, June 21. Learn more about VAM here. And register today.
Have you registered yet for the 2018 Vascular Annual Meeting in Boston? This year, bring your vascular team – there will be programming for nurses, technicians, nurse practitioners and PAs. In addition to VAM sessions of interests to nurses, the Society for Vascular Nursing will hold its annual conference in alignment with VAM, on June 20-21. Registration for SVN covers attendance at both meetings. And PAs will their own section of programming from 1 to 5 p.m. Thursday, June 21. Learn more about VAM here. And register today.
Have you registered yet for the 2018 Vascular Annual Meeting in Boston? This year, bring your vascular team – there will be programming for nurses, technicians, nurse practitioners and PAs. In addition to VAM sessions of interests to nurses, the Society for Vascular Nursing will hold its annual conference in alignment with VAM, on June 20-21. Registration for SVN covers attendance at both meetings. And PAs will their own section of programming from 1 to 5 p.m. Thursday, June 21. Learn more about VAM here. And register today.
Learn to Negotiate Physician Compensation Agreements
SVS and the SVS Community Practice Committee will hold a webinar for SVS members on April 30 on “Negotiating Physician Employment Agreements.” The 75-minute webinar will begin at 8 p.m. Eastern time. Topics will include current trends, regulatory overview and key contractual provisions. Learn more here. Register here.
SVS and the SVS Community Practice Committee will hold a webinar for SVS members on April 30 on “Negotiating Physician Employment Agreements.” The 75-minute webinar will begin at 8 p.m. Eastern time. Topics will include current trends, regulatory overview and key contractual provisions. Learn more here. Register here.
SVS and the SVS Community Practice Committee will hold a webinar for SVS members on April 30 on “Negotiating Physician Employment Agreements.” The 75-minute webinar will begin at 8 p.m. Eastern time. Topics will include current trends, regulatory overview and key contractual provisions. Learn more here. Register here.
UCLA/SVS Vascular Review Course Set
The Third Annual "UCLA / SVS Symposium: A Comprehensive Review and Update of What's New in Vascular and Endovascular Surgery," is set for Aug. 25 to 27 in California. This course is offered by the Division of Vascular and Endovascular Surgery at UCLA and the Society for Vascular Surgery. It provides an in-depth review of our specialty for those preparing to take the vascular board examinations as well as providing the basic didactic education for vascular residents and fellows in training.
The Third Annual "UCLA / SVS Symposium: A Comprehensive Review and Update of What's New in Vascular and Endovascular Surgery," is set for Aug. 25 to 27 in California. This course is offered by the Division of Vascular and Endovascular Surgery at UCLA and the Society for Vascular Surgery. It provides an in-depth review of our specialty for those preparing to take the vascular board examinations as well as providing the basic didactic education for vascular residents and fellows in training.
The Third Annual "UCLA / SVS Symposium: A Comprehensive Review and Update of What's New in Vascular and Endovascular Surgery," is set for Aug. 25 to 27 in California. This course is offered by the Division of Vascular and Endovascular Surgery at UCLA and the Society for Vascular Surgery. It provides an in-depth review of our specialty for those preparing to take the vascular board examinations as well as providing the basic didactic education for vascular residents and fellows in training.
SVS Seeks Medical Editor for ‘Vascular Specialist’
SVS is accepting applications for Medical Editor of "Vascular Specialist," the monthly news periodical produced by SVS. The editor can expect to spend approximately 10-20 hours each month in the three weeks leading up to publication. A modest annual honorarium is provided. Previous editorial experience will be helpful. Please send letters of interest by April 15 to Kenneth M. Slaw, Ph.D., at [email protected]. He will forward your interest to the SVS Publications Committee for consideration. More information is in the March 29 issue of Pulse.
SVS is accepting applications for Medical Editor of "Vascular Specialist," the monthly news periodical produced by SVS. The editor can expect to spend approximately 10-20 hours each month in the three weeks leading up to publication. A modest annual honorarium is provided. Previous editorial experience will be helpful. Please send letters of interest by April 15 to Kenneth M. Slaw, Ph.D., at [email protected]. He will forward your interest to the SVS Publications Committee for consideration. More information is in the March 29 issue of Pulse.
SVS is accepting applications for Medical Editor of "Vascular Specialist," the monthly news periodical produced by SVS. The editor can expect to spend approximately 10-20 hours each month in the three weeks leading up to publication. A modest annual honorarium is provided. Previous editorial experience will be helpful. Please send letters of interest by April 15 to Kenneth M. Slaw, Ph.D., at [email protected]. He will forward your interest to the SVS Publications Committee for consideration. More information is in the March 29 issue of Pulse.
Better manage acute pancreatitis to improve patient outcomes
AGA has a new clinical guideline on the initial management of acute pancreatitis, published in Gastroenterology. In the U.S., acute pancreatitis (AP) is a leading cause of inpatient care among gastrointestinal conditions with more than 275,000 patients hospitalized annually, at an aggregate cost of over $2.6 billion per year. The guideline focuses on patient care within the first 48-72 hours of admission when management decisions can alter the course of disease and duration of hospitalization.
Guideline recommendations
AGA’s new guideline aims to reduce practice variation and promote high-quality and high-value care for patients suffering from acute pancreatitis. It addresses questions on the benefits of goal-directed fluid resuscitation, early oral feeding, enteral vs. parenteral nutrition, the routine use of prophylactic antibiotics, and routine ERCP in all patients with AP.
The guideline is accompanied by a technical review, a new spotlight (infographic) and a patient companion infographic, which provides key points and important information directly to patients.
AGA has a new clinical guideline on the initial management of acute pancreatitis, published in Gastroenterology. In the U.S., acute pancreatitis (AP) is a leading cause of inpatient care among gastrointestinal conditions with more than 275,000 patients hospitalized annually, at an aggregate cost of over $2.6 billion per year. The guideline focuses on patient care within the first 48-72 hours of admission when management decisions can alter the course of disease and duration of hospitalization.
Guideline recommendations
AGA’s new guideline aims to reduce practice variation and promote high-quality and high-value care for patients suffering from acute pancreatitis. It addresses questions on the benefits of goal-directed fluid resuscitation, early oral feeding, enteral vs. parenteral nutrition, the routine use of prophylactic antibiotics, and routine ERCP in all patients with AP.
The guideline is accompanied by a technical review, a new spotlight (infographic) and a patient companion infographic, which provides key points and important information directly to patients.
AGA has a new clinical guideline on the initial management of acute pancreatitis, published in Gastroenterology. In the U.S., acute pancreatitis (AP) is a leading cause of inpatient care among gastrointestinal conditions with more than 275,000 patients hospitalized annually, at an aggregate cost of over $2.6 billion per year. The guideline focuses on patient care within the first 48-72 hours of admission when management decisions can alter the course of disease and duration of hospitalization.
Guideline recommendations
AGA’s new guideline aims to reduce practice variation and promote high-quality and high-value care for patients suffering from acute pancreatitis. It addresses questions on the benefits of goal-directed fluid resuscitation, early oral feeding, enteral vs. parenteral nutrition, the routine use of prophylactic antibiotics, and routine ERCP in all patients with AP.
The guideline is accompanied by a technical review, a new spotlight (infographic) and a patient companion infographic, which provides key points and important information directly to patients.
How to talk with your patients about PPIs and cognitive decline
A 2018 study published in Clinical Gastroenterology and Hepatology, “Lack of association between proton pump inhibitor use and cognitive decline,” found no association between PPI use and cognitive decline in analyzing data from two large population-based studies in Denmark. While this data is reassuring, clinicians should continue to anticipate questions from their patients about the risks associated with PPI therapy.
- Reassure patients that you prescribed a PPI for a clear-cut indication, in the lowest possible dose, and for an appropriate period of time (lowest dose, shortest time). This advice echoes that offered by AGA and ABIM in the Choosing Wisely campaign.
- Educate patients not to ask “what side effects do PPIs have?” but rather “is it really indicated?” Reassure patients that, when PPIs are indicated, benefits outweigh risks.
- Keep conversation channels open with patients. When patients require long-term use of PPIs, the medication should not be stopped without a discussion with you about the risks and benefits.
- Recommend that patients also consider life-style modifications that may reduce or eliminate the need for PPIs for long-term use.
A 2018 study published in Clinical Gastroenterology and Hepatology, “Lack of association between proton pump inhibitor use and cognitive decline,” found no association between PPI use and cognitive decline in analyzing data from two large population-based studies in Denmark. While this data is reassuring, clinicians should continue to anticipate questions from their patients about the risks associated with PPI therapy.
- Reassure patients that you prescribed a PPI for a clear-cut indication, in the lowest possible dose, and for an appropriate period of time (lowest dose, shortest time). This advice echoes that offered by AGA and ABIM in the Choosing Wisely campaign.
- Educate patients not to ask “what side effects do PPIs have?” but rather “is it really indicated?” Reassure patients that, when PPIs are indicated, benefits outweigh risks.
- Keep conversation channels open with patients. When patients require long-term use of PPIs, the medication should not be stopped without a discussion with you about the risks and benefits.
- Recommend that patients also consider life-style modifications that may reduce or eliminate the need for PPIs for long-term use.
A 2018 study published in Clinical Gastroenterology and Hepatology, “Lack of association between proton pump inhibitor use and cognitive decline,” found no association between PPI use and cognitive decline in analyzing data from two large population-based studies in Denmark. While this data is reassuring, clinicians should continue to anticipate questions from their patients about the risks associated with PPI therapy.
- Reassure patients that you prescribed a PPI for a clear-cut indication, in the lowest possible dose, and for an appropriate period of time (lowest dose, shortest time). This advice echoes that offered by AGA and ABIM in the Choosing Wisely campaign.
- Educate patients not to ask “what side effects do PPIs have?” but rather “is it really indicated?” Reassure patients that, when PPIs are indicated, benefits outweigh risks.
- Keep conversation channels open with patients. When patients require long-term use of PPIs, the medication should not be stopped without a discussion with you about the risks and benefits.
- Recommend that patients also consider life-style modifications that may reduce or eliminate the need for PPIs for long-term use.
Four new and noteworthy IBD drug studies
Inflammatory bowel disease (IBD) is a vibrant area of clinical research. Many of the 250+ abstracts presented at the inaugural Crohn’s & Colitis Congress — a partnership of the Crohn’s & Colitis Foundation and AGA — looked at the efficacy and safety of IBD therapies. You can review all abstracts presented at the Crohn’s & Colitis Congress in Gastroenterology.
Double-blind, randomized, placebo-controlled, crossover trial to evaluate induction of clinical response in patients with moderate-severe Crohn’s disease treated with rifaximin
By Scott D. Lee, University of Washington Medicine, et al.
Significance: It is now known that the intestinal microbiome is integral to the pathogenesis of IBD. However, antibiotic treatments for IBD have previously shown limited effectiveness. In this 8-week clinical trial, there was a fourfold greater response to the antibiotic rifaximin in Crohn’s disease treatment, compared with placebo. The positive impact on clinical disease activity was seen even in patients with a significant disease burden and prior exposure to one or more biologic therapies. Quality of life and laboratory measurements were numerically improved. No new safety concerns were identified. These results offer renewed hope for the use of antibiotics in treating Crohn’s disease.
Post-hoc analysis of tofacitinib Crohn’s disease phase 2 induction efficacy in subgroups with baseline endoscopic or biomarker evidence of inflammation
By Bruce E. Sands, Icahn School of Medicine at Mount Sinai, et al.
Significance: Tofacitinib, a Janus kinase (JAK) inhibitor, is under investigation for treatment of ulcerative colitis and Crohn’s disease. To date, response rates in ulcerative colitis have been higher than for Crohn’s disease. In this report, investigators performed post-hoc analysis studies using objective baseline criteria of disease activity. Their findings showed a greater proportion of patients with moderate to severe Crohn’s disease were in remission with tofacitinib compared to placebo. These results provide evidence of JAK inhibition for the treatment of Crohn’s disease and support further investigation.
Refined population pharmacokinetic model for infliximab precision dosing in pediatric inflammatory bowel disease
By Laura E. Bauman, Cincinnati Children’s Hospital Medical Center, et al.
Significance: Long-term clinical remission from IBD with anti-TNF therapies has generally been limited to less than half of the treated patients. Improved outcomes are seen with optimal pre-infusion trough drug levels, a measurement of the level of drugs in the patient’s bloodstream. However, standard weight-based dosing for pediatric patients has provided widely varying trough drug levels. The investigators report the development of a multifactorial pharmacokinetic model for predicting infliximab trough levels during maintenance therapy for IBD. Such dynamic approaches to treatment address a specific gap in pediatric IBD therapeutic strategies.
Primary nonresponse to tumor necrosis factor antagonists is associated with inferior response to second-line biologics in patients with inflammatory bowel diseases: A systematic review and meta-analysis
By Siddharth Singh, University of California San Diego Health, et al.
Significance: Primary nonresponse to anti-TNF therapy is seen in 35%-65% of IBD patients and another 40%-60% lose responsiveness during the first year of treatment. Physicians struggle with what treatments to recommend for these patients. The investigators in this study performed a literature search and identified eight randomized controlled trials of biologics in patients with prior exposure to anti-TNF and compared outcomes based on their prior responses to anti-TNF. The analysis reveals a 24% decrease in likelihood to achieve remission in patients who changed medications because of immediate nonresponse compared to loss of responsiveness or intolerance during the treatment. These findings raise important questions about the biology of IBD, including the pharmacology of anti-TNF in a subset of patients.
Inflammatory bowel disease (IBD) is a vibrant area of clinical research. Many of the 250+ abstracts presented at the inaugural Crohn’s & Colitis Congress — a partnership of the Crohn’s & Colitis Foundation and AGA — looked at the efficacy and safety of IBD therapies. You can review all abstracts presented at the Crohn’s & Colitis Congress in Gastroenterology.
Double-blind, randomized, placebo-controlled, crossover trial to evaluate induction of clinical response in patients with moderate-severe Crohn’s disease treated with rifaximin
By Scott D. Lee, University of Washington Medicine, et al.
Significance: It is now known that the intestinal microbiome is integral to the pathogenesis of IBD. However, antibiotic treatments for IBD have previously shown limited effectiveness. In this 8-week clinical trial, there was a fourfold greater response to the antibiotic rifaximin in Crohn’s disease treatment, compared with placebo. The positive impact on clinical disease activity was seen even in patients with a significant disease burden and prior exposure to one or more biologic therapies. Quality of life and laboratory measurements were numerically improved. No new safety concerns were identified. These results offer renewed hope for the use of antibiotics in treating Crohn’s disease.
Post-hoc analysis of tofacitinib Crohn’s disease phase 2 induction efficacy in subgroups with baseline endoscopic or biomarker evidence of inflammation
By Bruce E. Sands, Icahn School of Medicine at Mount Sinai, et al.
Significance: Tofacitinib, a Janus kinase (JAK) inhibitor, is under investigation for treatment of ulcerative colitis and Crohn’s disease. To date, response rates in ulcerative colitis have been higher than for Crohn’s disease. In this report, investigators performed post-hoc analysis studies using objective baseline criteria of disease activity. Their findings showed a greater proportion of patients with moderate to severe Crohn’s disease were in remission with tofacitinib compared to placebo. These results provide evidence of JAK inhibition for the treatment of Crohn’s disease and support further investigation.
Refined population pharmacokinetic model for infliximab precision dosing in pediatric inflammatory bowel disease
By Laura E. Bauman, Cincinnati Children’s Hospital Medical Center, et al.
Significance: Long-term clinical remission from IBD with anti-TNF therapies has generally been limited to less than half of the treated patients. Improved outcomes are seen with optimal pre-infusion trough drug levels, a measurement of the level of drugs in the patient’s bloodstream. However, standard weight-based dosing for pediatric patients has provided widely varying trough drug levels. The investigators report the development of a multifactorial pharmacokinetic model for predicting infliximab trough levels during maintenance therapy for IBD. Such dynamic approaches to treatment address a specific gap in pediatric IBD therapeutic strategies.
Primary nonresponse to tumor necrosis factor antagonists is associated with inferior response to second-line biologics in patients with inflammatory bowel diseases: A systematic review and meta-analysis
By Siddharth Singh, University of California San Diego Health, et al.
Significance: Primary nonresponse to anti-TNF therapy is seen in 35%-65% of IBD patients and another 40%-60% lose responsiveness during the first year of treatment. Physicians struggle with what treatments to recommend for these patients. The investigators in this study performed a literature search and identified eight randomized controlled trials of biologics in patients with prior exposure to anti-TNF and compared outcomes based on their prior responses to anti-TNF. The analysis reveals a 24% decrease in likelihood to achieve remission in patients who changed medications because of immediate nonresponse compared to loss of responsiveness or intolerance during the treatment. These findings raise important questions about the biology of IBD, including the pharmacology of anti-TNF in a subset of patients.
Inflammatory bowel disease (IBD) is a vibrant area of clinical research. Many of the 250+ abstracts presented at the inaugural Crohn’s & Colitis Congress — a partnership of the Crohn’s & Colitis Foundation and AGA — looked at the efficacy and safety of IBD therapies. You can review all abstracts presented at the Crohn’s & Colitis Congress in Gastroenterology.
Double-blind, randomized, placebo-controlled, crossover trial to evaluate induction of clinical response in patients with moderate-severe Crohn’s disease treated with rifaximin
By Scott D. Lee, University of Washington Medicine, et al.
Significance: It is now known that the intestinal microbiome is integral to the pathogenesis of IBD. However, antibiotic treatments for IBD have previously shown limited effectiveness. In this 8-week clinical trial, there was a fourfold greater response to the antibiotic rifaximin in Crohn’s disease treatment, compared with placebo. The positive impact on clinical disease activity was seen even in patients with a significant disease burden and prior exposure to one or more biologic therapies. Quality of life and laboratory measurements were numerically improved. No new safety concerns were identified. These results offer renewed hope for the use of antibiotics in treating Crohn’s disease.
Post-hoc analysis of tofacitinib Crohn’s disease phase 2 induction efficacy in subgroups with baseline endoscopic or biomarker evidence of inflammation
By Bruce E. Sands, Icahn School of Medicine at Mount Sinai, et al.
Significance: Tofacitinib, a Janus kinase (JAK) inhibitor, is under investigation for treatment of ulcerative colitis and Crohn’s disease. To date, response rates in ulcerative colitis have been higher than for Crohn’s disease. In this report, investigators performed post-hoc analysis studies using objective baseline criteria of disease activity. Their findings showed a greater proportion of patients with moderate to severe Crohn’s disease were in remission with tofacitinib compared to placebo. These results provide evidence of JAK inhibition for the treatment of Crohn’s disease and support further investigation.
Refined population pharmacokinetic model for infliximab precision dosing in pediatric inflammatory bowel disease
By Laura E. Bauman, Cincinnati Children’s Hospital Medical Center, et al.
Significance: Long-term clinical remission from IBD with anti-TNF therapies has generally been limited to less than half of the treated patients. Improved outcomes are seen with optimal pre-infusion trough drug levels, a measurement of the level of drugs in the patient’s bloodstream. However, standard weight-based dosing for pediatric patients has provided widely varying trough drug levels. The investigators report the development of a multifactorial pharmacokinetic model for predicting infliximab trough levels during maintenance therapy for IBD. Such dynamic approaches to treatment address a specific gap in pediatric IBD therapeutic strategies.
Primary nonresponse to tumor necrosis factor antagonists is associated with inferior response to second-line biologics in patients with inflammatory bowel diseases: A systematic review and meta-analysis
By Siddharth Singh, University of California San Diego Health, et al.
Significance: Primary nonresponse to anti-TNF therapy is seen in 35%-65% of IBD patients and another 40%-60% lose responsiveness during the first year of treatment. Physicians struggle with what treatments to recommend for these patients. The investigators in this study performed a literature search and identified eight randomized controlled trials of biologics in patients with prior exposure to anti-TNF and compared outcomes based on their prior responses to anti-TNF. The analysis reveals a 24% decrease in likelihood to achieve remission in patients who changed medications because of immediate nonresponse compared to loss of responsiveness or intolerance during the treatment. These findings raise important questions about the biology of IBD, including the pharmacology of anti-TNF in a subset of patients.
Remember the AGA Research Foundation in your will or living trust
What if all you had to do to ensure that the AGA Research Foundation can have an impact for years to come is to write a simple sentence? Sound impossible?
The AGA Research Foundation provides a key source of funding at a critical juncture in a young investigators’ career. Securing the future of the talented investigators we serve really is as simple as one sentence.
Including the AGA Research Foundation in your will is a popular gift to give because it is:
- • Affordable. The actual giving of your gift occurs after your lifetime, so your current income is not affected.
- • Flexible. Until your will goes into effect, you are free to alter your plans or change your mind.
- • Versatile. You can give a specific item, a set amount of money, or a percentage of your estate. You can also make your gift contingent upon certain events.
We hope you’ll consider including a gift to the AGA Research Foundation in your will or living trust. It’s simple – just a few sentences in your will or trust are all that is needed. The official bequest language for the AGA Research Foundation is: “I, [name], of [city, state, ZIP], give, devise and bequeath to the AGA Research Foundation [written amount or percentage of the estate or description of property] for its unrestricted use and purpose.”
Join others in donating to the AGA Research Foundation and help fill the funding gap and protect the next generation of investigators.
Please contact us for more information at [email protected] or visit http://gastro.planmylegacy.org/.
What if all you had to do to ensure that the AGA Research Foundation can have an impact for years to come is to write a simple sentence? Sound impossible?
The AGA Research Foundation provides a key source of funding at a critical juncture in a young investigators’ career. Securing the future of the talented investigators we serve really is as simple as one sentence.
Including the AGA Research Foundation in your will is a popular gift to give because it is:
- • Affordable. The actual giving of your gift occurs after your lifetime, so your current income is not affected.
- • Flexible. Until your will goes into effect, you are free to alter your plans or change your mind.
- • Versatile. You can give a specific item, a set amount of money, or a percentage of your estate. You can also make your gift contingent upon certain events.
We hope you’ll consider including a gift to the AGA Research Foundation in your will or living trust. It’s simple – just a few sentences in your will or trust are all that is needed. The official bequest language for the AGA Research Foundation is: “I, [name], of [city, state, ZIP], give, devise and bequeath to the AGA Research Foundation [written amount or percentage of the estate or description of property] for its unrestricted use and purpose.”
Join others in donating to the AGA Research Foundation and help fill the funding gap and protect the next generation of investigators.
Please contact us for more information at [email protected] or visit http://gastro.planmylegacy.org/.
What if all you had to do to ensure that the AGA Research Foundation can have an impact for years to come is to write a simple sentence? Sound impossible?
The AGA Research Foundation provides a key source of funding at a critical juncture in a young investigators’ career. Securing the future of the talented investigators we serve really is as simple as one sentence.
Including the AGA Research Foundation in your will is a popular gift to give because it is:
- • Affordable. The actual giving of your gift occurs after your lifetime, so your current income is not affected.
- • Flexible. Until your will goes into effect, you are free to alter your plans or change your mind.
- • Versatile. You can give a specific item, a set amount of money, or a percentage of your estate. You can also make your gift contingent upon certain events.
We hope you’ll consider including a gift to the AGA Research Foundation in your will or living trust. It’s simple – just a few sentences in your will or trust are all that is needed. The official bequest language for the AGA Research Foundation is: “I, [name], of [city, state, ZIP], give, devise and bequeath to the AGA Research Foundation [written amount or percentage of the estate or description of property] for its unrestricted use and purpose.”
Join others in donating to the AGA Research Foundation and help fill the funding gap and protect the next generation of investigators.
Please contact us for more information at [email protected] or visit http://gastro.planmylegacy.org/.





