User login
For MD-IQ only
Adding apalutamide delays radiographic progression in mCRPC
Key clinical point: Addition of apalutamide to abiraterone and prednisone improves radiographic progression-free survival (rPFS) in chemotherapy-naïve patients with metastatic castration-resistant prostate cancer (mCRPC).
Major finding: At a median follow-up of 54.8 months, apalutamide in combination with abiraterone plus prednisone vs. abiraterone plus prednisone significantly improves rPFS (24.0 months vs 16.6 months; hazard ratio, 0.70; P < .0001).
Study details: A randomized, placebo-controlled, double-blind, phase 3 ACIS study of 982 chemotherapy-naïve patients with mCRPC who were randomly assigned to either apalutamide plus abiraterone and prednisone or abiraterone plus prednisone.
Disclosures: The study received funding from Janssen Research & Development. The authors reported leadership roles and receiving financial support, grants/contracts, consulting fees, and payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing, and/or educational events from various sources, and/or being employed with and holding stocks in pharmaceutical companies.
Source: Saad F et al. Lancet Oncol. 2021 Sep 30. doi: 10.1016/ S1470-2045(21)00402-2.
Key clinical point: Addition of apalutamide to abiraterone and prednisone improves radiographic progression-free survival (rPFS) in chemotherapy-naïve patients with metastatic castration-resistant prostate cancer (mCRPC).
Major finding: At a median follow-up of 54.8 months, apalutamide in combination with abiraterone plus prednisone vs. abiraterone plus prednisone significantly improves rPFS (24.0 months vs 16.6 months; hazard ratio, 0.70; P < .0001).
Study details: A randomized, placebo-controlled, double-blind, phase 3 ACIS study of 982 chemotherapy-naïve patients with mCRPC who were randomly assigned to either apalutamide plus abiraterone and prednisone or abiraterone plus prednisone.
Disclosures: The study received funding from Janssen Research & Development. The authors reported leadership roles and receiving financial support, grants/contracts, consulting fees, and payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing, and/or educational events from various sources, and/or being employed with and holding stocks in pharmaceutical companies.
Source: Saad F et al. Lancet Oncol. 2021 Sep 30. doi: 10.1016/ S1470-2045(21)00402-2.
Key clinical point: Addition of apalutamide to abiraterone and prednisone improves radiographic progression-free survival (rPFS) in chemotherapy-naïve patients with metastatic castration-resistant prostate cancer (mCRPC).
Major finding: At a median follow-up of 54.8 months, apalutamide in combination with abiraterone plus prednisone vs. abiraterone plus prednisone significantly improves rPFS (24.0 months vs 16.6 months; hazard ratio, 0.70; P < .0001).
Study details: A randomized, placebo-controlled, double-blind, phase 3 ACIS study of 982 chemotherapy-naïve patients with mCRPC who were randomly assigned to either apalutamide plus abiraterone and prednisone or abiraterone plus prednisone.
Disclosures: The study received funding from Janssen Research & Development. The authors reported leadership roles and receiving financial support, grants/contracts, consulting fees, and payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing, and/or educational events from various sources, and/or being employed with and holding stocks in pharmaceutical companies.
Source: Saad F et al. Lancet Oncol. 2021 Sep 30. doi: 10.1016/ S1470-2045(21)00402-2.
Prostate cancer: 68Ga-PSMA-11 PET scan may miss pelvic nodal metastasis
Key clinical point: In patients with intermediate-/high-risk prostate cancer undergoing radical prostatectomy and lymph node dissection, Gallium 68 prostate-specific membrane antigen (68Ga-PSMA)-11 positron emission tomographic (PET) imaging shows sensitivity and specificity of 0.40 and 0.95, respectively.
Major finding: Compared with histopathology, 68Ga-PSMA-11 PET imaging showed a sensitivity, specificity, positive predictive value, and negative predictive value for pelvic nodal metastases of 0.40, 0.95, 0.75, and 0.81, respectively.
Study details: A single-arm, open-label, phase 3 imaging trial (NCT03368547, NCT02611882, and NCT02919111) of 764 patients with intermediate- to high-risk prostate cancer considered for prostatectomy who underwent 68Ga-PSMA-11 PET scan.
Disclosures: This work is supported by grants from the National Cancer Institute, Prostate Cancer Foundation, Society of Nuclear Medicine and Molecular Imaging, Philippe Foundation Inc, ARC Foundation, German Research Foundation, Doctor Robert Pfleger Foundation, and Wiedenfeld Foundation. The authors received grants, personal fees, cooperation projects, and speaker/advisory/consulting fees; held a patent; and/or reported being founder/shareholder outside this work.
Source: Hope TA et al. JAMA Oncol. 2021 Sep 16. doi: 10.1001/jamaoncol.2021.3771.
Key clinical point: In patients with intermediate-/high-risk prostate cancer undergoing radical prostatectomy and lymph node dissection, Gallium 68 prostate-specific membrane antigen (68Ga-PSMA)-11 positron emission tomographic (PET) imaging shows sensitivity and specificity of 0.40 and 0.95, respectively.
Major finding: Compared with histopathology, 68Ga-PSMA-11 PET imaging showed a sensitivity, specificity, positive predictive value, and negative predictive value for pelvic nodal metastases of 0.40, 0.95, 0.75, and 0.81, respectively.
Study details: A single-arm, open-label, phase 3 imaging trial (NCT03368547, NCT02611882, and NCT02919111) of 764 patients with intermediate- to high-risk prostate cancer considered for prostatectomy who underwent 68Ga-PSMA-11 PET scan.
Disclosures: This work is supported by grants from the National Cancer Institute, Prostate Cancer Foundation, Society of Nuclear Medicine and Molecular Imaging, Philippe Foundation Inc, ARC Foundation, German Research Foundation, Doctor Robert Pfleger Foundation, and Wiedenfeld Foundation. The authors received grants, personal fees, cooperation projects, and speaker/advisory/consulting fees; held a patent; and/or reported being founder/shareholder outside this work.
Source: Hope TA et al. JAMA Oncol. 2021 Sep 16. doi: 10.1001/jamaoncol.2021.3771.
Key clinical point: In patients with intermediate-/high-risk prostate cancer undergoing radical prostatectomy and lymph node dissection, Gallium 68 prostate-specific membrane antigen (68Ga-PSMA)-11 positron emission tomographic (PET) imaging shows sensitivity and specificity of 0.40 and 0.95, respectively.
Major finding: Compared with histopathology, 68Ga-PSMA-11 PET imaging showed a sensitivity, specificity, positive predictive value, and negative predictive value for pelvic nodal metastases of 0.40, 0.95, 0.75, and 0.81, respectively.
Study details: A single-arm, open-label, phase 3 imaging trial (NCT03368547, NCT02611882, and NCT02919111) of 764 patients with intermediate- to high-risk prostate cancer considered for prostatectomy who underwent 68Ga-PSMA-11 PET scan.
Disclosures: This work is supported by grants from the National Cancer Institute, Prostate Cancer Foundation, Society of Nuclear Medicine and Molecular Imaging, Philippe Foundation Inc, ARC Foundation, German Research Foundation, Doctor Robert Pfleger Foundation, and Wiedenfeld Foundation. The authors received grants, personal fees, cooperation projects, and speaker/advisory/consulting fees; held a patent; and/or reported being founder/shareholder outside this work.
Source: Hope TA et al. JAMA Oncol. 2021 Sep 16. doi: 10.1001/jamaoncol.2021.3771.
Prostate cancer: 68Ga-PSMA-11 PET scan may miss pelvic nodal metastasis
Key clinical point: In patients with intermediate-/high-risk prostate cancer undergoing radical prostatectomy and lymph node dissection, Gallium 68 prostate-specific membrane antigen (68Ga-PSMA)-11 positron emission tomographic (PET) imaging shows sensitivity and specificity of 0.40 and 0.95, respectively.
Major finding: Compared with histopathology, 68Ga-PSMA-11 PET imaging showed a sensitivity, specificity, positive predictive value, and negative predictive value for pelvic nodal metastases of 0.40, 0.95, 0.75, and 0.81, respectively.
Study details: A single-arm, open-label, phase 3 imaging trial (NCT03368547, NCT02611882, and NCT02919111) of 764 patients with intermediate- to high-risk prostate cancer considered for prostatectomy who underwent 68Ga-PSMA-11 PET scan.
Disclosures: This work is supported by grants from the National Cancer Institute, Prostate Cancer Foundation, Society of Nuclear Medicine and Molecular Imaging, Philippe Foundation Inc, ARC Foundation, German Research Foundation, Doctor Robert Pfleger Foundation, and Wiedenfeld Foundation. The authors received grants, personal fees, cooperation projects, and speaker/advisory/consulting fees; held a patent; and/or reported being founder/shareholder outside this work.
Source: Hope TA et al. JAMA Oncol. 2021 Sep 16. doi: 10.1001/jamaoncol.2021.3771.
Key clinical point: In patients with intermediate-/high-risk prostate cancer undergoing radical prostatectomy and lymph node dissection, Gallium 68 prostate-specific membrane antigen (68Ga-PSMA)-11 positron emission tomographic (PET) imaging shows sensitivity and specificity of 0.40 and 0.95, respectively.
Major finding: Compared with histopathology, 68Ga-PSMA-11 PET imaging showed a sensitivity, specificity, positive predictive value, and negative predictive value for pelvic nodal metastases of 0.40, 0.95, 0.75, and 0.81, respectively.
Study details: A single-arm, open-label, phase 3 imaging trial (NCT03368547, NCT02611882, and NCT02919111) of 764 patients with intermediate- to high-risk prostate cancer considered for prostatectomy who underwent 68Ga-PSMA-11 PET scan.
Disclosures: This work is supported by grants from the National Cancer Institute, Prostate Cancer Foundation, Society of Nuclear Medicine and Molecular Imaging, Philippe Foundation Inc, ARC Foundation, German Research Foundation, Doctor Robert Pfleger Foundation, and Wiedenfeld Foundation. The authors received grants, personal fees, cooperation projects, and speaker/advisory/consulting fees; held a patent; and/or reported being founder/shareholder outside this work.
Source: Hope TA et al. JAMA Oncol. 2021 Sep 16. doi: 10.1001/jamaoncol.2021.3771.
Key clinical point: In patients with intermediate-/high-risk prostate cancer undergoing radical prostatectomy and lymph node dissection, Gallium 68 prostate-specific membrane antigen (68Ga-PSMA)-11 positron emission tomographic (PET) imaging shows sensitivity and specificity of 0.40 and 0.95, respectively.
Major finding: Compared with histopathology, 68Ga-PSMA-11 PET imaging showed a sensitivity, specificity, positive predictive value, and negative predictive value for pelvic nodal metastases of 0.40, 0.95, 0.75, and 0.81, respectively.
Study details: A single-arm, open-label, phase 3 imaging trial (NCT03368547, NCT02611882, and NCT02919111) of 764 patients with intermediate- to high-risk prostate cancer considered for prostatectomy who underwent 68Ga-PSMA-11 PET scan.
Disclosures: This work is supported by grants from the National Cancer Institute, Prostate Cancer Foundation, Society of Nuclear Medicine and Molecular Imaging, Philippe Foundation Inc, ARC Foundation, German Research Foundation, Doctor Robert Pfleger Foundation, and Wiedenfeld Foundation. The authors received grants, personal fees, cooperation projects, and speaker/advisory/consulting fees; held a patent; and/or reported being founder/shareholder outside this work.
Source: Hope TA et al. JAMA Oncol. 2021 Sep 16. doi: 10.1001/jamaoncol.2021.3771.
Prostate Cancer: Prostate-Specific Antigen Testing
Weakness in the legs and edema
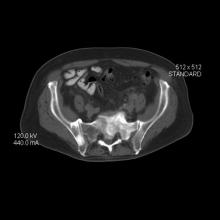
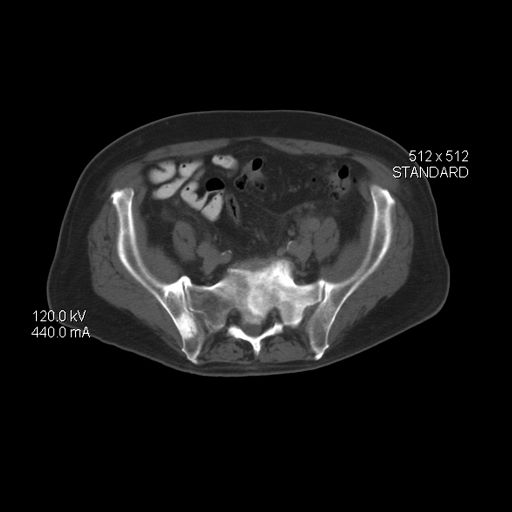
Carcinomas like prostate cancer possess notable bony tropism and can metastasize to the lumbar‐sacral spine and pelvis, draining through the pelvic plexus of the lumbar region. Approximately 90% of patients with advanced prostate cancer develop bone metastasis, the spine being the most common site. Manifestations of metastatic prostate cancer include weight loss and loss of appetite; bone pain, with or without pathologic fracture; and lower-extremity pain and edema. Urinary symptoms are also common. Other physical examination findings are adenopathy, bony tenderness, and lower-extremity edema, as seen in the present case.
Radiologic findings of bone metastases can mimic Paget disease, and even though bone metastases are blastic, lytic lesions may develop and cause pathologic fractures. Such fractures must be distinguished from osteoporotic fractures that can occur after prolonged luteinizing hormone–releasing hormone therapy. Also included in the differential of the present case are lymphomas, which can manifest as pelvic masses and bone lesions and have been reported with prostate cancer. However, considering the patient's history, physical examination, and lab results, bone metastasis is the most likely diagnosis.
Bone imaging should be performed for any patient with suspected bone metastases; specifically, multiparametric MRI outperforms bone scan and targeted x-rays for detection of bone metastases. Because activity in the bone scan may not be observed until 5 years after micrometastasis has occurred, negative bone scan results cannot be used to definitively exclude metastasis.
The alpha emitter radium-223 is a category 1 option to treat symptomatic bone metastases (but should not be used in patients with visceral metastases). It is not recommended for use in combination with docetaxel or any other systemic therapy but may be used with androgen-deprivation therapy (ADT), as studies have suggested that the addition of ADT improves progression-free survival in patients with castrate-resistant prostate cancer with metastasis. Concomitant use of denosumab or zoledronic acid is also recommended.
Kyle A. Richards, MD, Assistant Professor, Department of Urology, University of Wisconsin-Madison; Chief of Urology, William S. Middleton Memorial VA Hospital, Madison, Wisconsin
Kyle A. Richards, MD, has disclosed no relevant financial relationships
Carcinomas like prostate cancer possess notable bony tropism and can metastasize to the lumbar‐sacral spine and pelvis, draining through the pelvic plexus of the lumbar region. Approximately 90% of patients with advanced prostate cancer develop bone metastasis, the spine being the most common site. Manifestations of metastatic prostate cancer include weight loss and loss of appetite; bone pain, with or without pathologic fracture; and lower-extremity pain and edema. Urinary symptoms are also common. Other physical examination findings are adenopathy, bony tenderness, and lower-extremity edema, as seen in the present case.
Radiologic findings of bone metastases can mimic Paget disease, and even though bone metastases are blastic, lytic lesions may develop and cause pathologic fractures. Such fractures must be distinguished from osteoporotic fractures that can occur after prolonged luteinizing hormone–releasing hormone therapy. Also included in the differential of the present case are lymphomas, which can manifest as pelvic masses and bone lesions and have been reported with prostate cancer. However, considering the patient's history, physical examination, and lab results, bone metastasis is the most likely diagnosis.
Bone imaging should be performed for any patient with suspected bone metastases; specifically, multiparametric MRI outperforms bone scan and targeted x-rays for detection of bone metastases. Because activity in the bone scan may not be observed until 5 years after micrometastasis has occurred, negative bone scan results cannot be used to definitively exclude metastasis.
The alpha emitter radium-223 is a category 1 option to treat symptomatic bone metastases (but should not be used in patients with visceral metastases). It is not recommended for use in combination with docetaxel or any other systemic therapy but may be used with androgen-deprivation therapy (ADT), as studies have suggested that the addition of ADT improves progression-free survival in patients with castrate-resistant prostate cancer with metastasis. Concomitant use of denosumab or zoledronic acid is also recommended.
Kyle A. Richards, MD, Assistant Professor, Department of Urology, University of Wisconsin-Madison; Chief of Urology, William S. Middleton Memorial VA Hospital, Madison, Wisconsin
Kyle A. Richards, MD, has disclosed no relevant financial relationships
Carcinomas like prostate cancer possess notable bony tropism and can metastasize to the lumbar‐sacral spine and pelvis, draining through the pelvic plexus of the lumbar region. Approximately 90% of patients with advanced prostate cancer develop bone metastasis, the spine being the most common site. Manifestations of metastatic prostate cancer include weight loss and loss of appetite; bone pain, with or without pathologic fracture; and lower-extremity pain and edema. Urinary symptoms are also common. Other physical examination findings are adenopathy, bony tenderness, and lower-extremity edema, as seen in the present case.
Radiologic findings of bone metastases can mimic Paget disease, and even though bone metastases are blastic, lytic lesions may develop and cause pathologic fractures. Such fractures must be distinguished from osteoporotic fractures that can occur after prolonged luteinizing hormone–releasing hormone therapy. Also included in the differential of the present case are lymphomas, which can manifest as pelvic masses and bone lesions and have been reported with prostate cancer. However, considering the patient's history, physical examination, and lab results, bone metastasis is the most likely diagnosis.
Bone imaging should be performed for any patient with suspected bone metastases; specifically, multiparametric MRI outperforms bone scan and targeted x-rays for detection of bone metastases. Because activity in the bone scan may not be observed until 5 years after micrometastasis has occurred, negative bone scan results cannot be used to definitively exclude metastasis.
The alpha emitter radium-223 is a category 1 option to treat symptomatic bone metastases (but should not be used in patients with visceral metastases). It is not recommended for use in combination with docetaxel or any other systemic therapy but may be used with androgen-deprivation therapy (ADT), as studies have suggested that the addition of ADT improves progression-free survival in patients with castrate-resistant prostate cancer with metastasis. Concomitant use of denosumab or zoledronic acid is also recommended.
Kyle A. Richards, MD, Assistant Professor, Department of Urology, University of Wisconsin-Madison; Chief of Urology, William S. Middleton Memorial VA Hospital, Madison, Wisconsin
Kyle A. Richards, MD, has disclosed no relevant financial relationships
A 66-year-old male patient presents with weakness in the legs and edema. He takes medication to control his hypertension. About 8 years ago, he was diagnosed with prostate cancer during screening. Tumor staging was T3 and Gleason score was 8. The patient underwent successful radiation combined with hormone therapy. While he does not have urologic symptoms at this time, he does report that he is easily fatigued. Serum calcium is 10.6 mg/dL and hemoglobin is 10.5 g/dL. There is no evidence of neurologic deficit.
Clinical Edge Journal Scan Commentary: Prostate Cancer October 2021
Radiation has long been a part of treatment for localized prostate cancer. However, radiation and radiation-delivery modalities continue to evolve to decrease toxicity without sacrificing efficacy, expand the roles of these modalities, and enhance efficacy of other concomitantly delivered treatment modalities. The accompanying studies describe efforts to those effects.
Hypofractionated delivery of radiation is now often considered preferred over conventionally fractionated radiation for localized prostate cancer to maintain efficacy while decreasing toxicity. To determine if the benefit of lower toxicity holds over time, Staffurth et al assessed patient-reported outcomes (PROs) in participants of the CHHiP trial where men were randomized to conventional radiation versus 2 alternative hypofraction schedules. At 5 years post-radiation, there were no significant differences amongst the 3 groups with respect to bowel bother, urinary bother, or sexual bother.
Evidence is emerging that stereotactic body radiation therapy (SBRT) has benefits for oligometastatic disease; however, studies are emerging that support the hypothesis that radiation may enhance immunotherapy in many cancers in the metastatic setting. Kwan et al evaluated the effects of a single fraction of SBRT administered to 1 or 2 disease sites before the first and second doses of avelumab (a PD-L1 antibody) in patients with metastatic castrate-resistant prostate cancer (with prior exposure to at least one second-generation androgen receptor inhibitor). In this single arm phase II study, the disease control rate was 48%, overall response rate was 31%, median radiographic progression-free survival (rPFS) was 8.4 months, and median overall survival (OS) was 14.1 months in this heavily pretreated group of 31 participants. As immunotherapy has been considered efficacious in select groups of prostate cancer (such as tumors with microsatellite instability), this study supports ongoing and new efforts to evaluate how to enhance immunotherapy against prostate cancer.
Radium-223 has been demonstrated to have efficacy in metastatic disease of the bone. Ongoing efforts to determine whether combined modality treatment in this setting may improve outcomes in metastatic disease, Maughn et al evaluated the combination of radium-223 and enzalutamide for safety and efficacy in a small and hypothesis-generating study 47 participants where 35 received enzalutamide plus radium-223 and 12 received enzlutamide alone. Of note, there were no increase in fractures (45 of the 47 participants received bone protective therapy, however), but there were no differences in the secondary endpoints of PSA-PFS, rPFS, or OS (primary endpoints of decline in bone metabolism markers were previously reported). This is a hypothesis generating study that supports the likely safety of this modality if bone protecting agents are utilized.
The 3 studies summarized here represent the ongoing evolution of radiation or radiation-delivery modalities in localized and metastatic prostate cancer. It is encouraging to see the ongoing evaluate of PROs over time, as long-term quality of life in patients potentially cured of disease is of utmost importance. In addition, efforts to evaluate radiation or radium-223 in widely metastatic disease as part of combination therapy may reveal not only situations with modest benefits in efficacy, but more importantly, may reveal molecular insights into newer treatment strategies.
Radiation has long been a part of treatment for localized prostate cancer. However, radiation and radiation-delivery modalities continue to evolve to decrease toxicity without sacrificing efficacy, expand the roles of these modalities, and enhance efficacy of other concomitantly delivered treatment modalities. The accompanying studies describe efforts to those effects.
Hypofractionated delivery of radiation is now often considered preferred over conventionally fractionated radiation for localized prostate cancer to maintain efficacy while decreasing toxicity. To determine if the benefit of lower toxicity holds over time, Staffurth et al assessed patient-reported outcomes (PROs) in participants of the CHHiP trial where men were randomized to conventional radiation versus 2 alternative hypofraction schedules. At 5 years post-radiation, there were no significant differences amongst the 3 groups with respect to bowel bother, urinary bother, or sexual bother.
Evidence is emerging that stereotactic body radiation therapy (SBRT) has benefits for oligometastatic disease; however, studies are emerging that support the hypothesis that radiation may enhance immunotherapy in many cancers in the metastatic setting. Kwan et al evaluated the effects of a single fraction of SBRT administered to 1 or 2 disease sites before the first and second doses of avelumab (a PD-L1 antibody) in patients with metastatic castrate-resistant prostate cancer (with prior exposure to at least one second-generation androgen receptor inhibitor). In this single arm phase II study, the disease control rate was 48%, overall response rate was 31%, median radiographic progression-free survival (rPFS) was 8.4 months, and median overall survival (OS) was 14.1 months in this heavily pretreated group of 31 participants. As immunotherapy has been considered efficacious in select groups of prostate cancer (such as tumors with microsatellite instability), this study supports ongoing and new efforts to evaluate how to enhance immunotherapy against prostate cancer.
Radium-223 has been demonstrated to have efficacy in metastatic disease of the bone. Ongoing efforts to determine whether combined modality treatment in this setting may improve outcomes in metastatic disease, Maughn et al evaluated the combination of radium-223 and enzalutamide for safety and efficacy in a small and hypothesis-generating study 47 participants where 35 received enzalutamide plus radium-223 and 12 received enzlutamide alone. Of note, there were no increase in fractures (45 of the 47 participants received bone protective therapy, however), but there were no differences in the secondary endpoints of PSA-PFS, rPFS, or OS (primary endpoints of decline in bone metabolism markers were previously reported). This is a hypothesis generating study that supports the likely safety of this modality if bone protecting agents are utilized.
The 3 studies summarized here represent the ongoing evolution of radiation or radiation-delivery modalities in localized and metastatic prostate cancer. It is encouraging to see the ongoing evaluate of PROs over time, as long-term quality of life in patients potentially cured of disease is of utmost importance. In addition, efforts to evaluate radiation or radium-223 in widely metastatic disease as part of combination therapy may reveal not only situations with modest benefits in efficacy, but more importantly, may reveal molecular insights into newer treatment strategies.
Radiation has long been a part of treatment for localized prostate cancer. However, radiation and radiation-delivery modalities continue to evolve to decrease toxicity without sacrificing efficacy, expand the roles of these modalities, and enhance efficacy of other concomitantly delivered treatment modalities. The accompanying studies describe efforts to those effects.
Hypofractionated delivery of radiation is now often considered preferred over conventionally fractionated radiation for localized prostate cancer to maintain efficacy while decreasing toxicity. To determine if the benefit of lower toxicity holds over time, Staffurth et al assessed patient-reported outcomes (PROs) in participants of the CHHiP trial where men were randomized to conventional radiation versus 2 alternative hypofraction schedules. At 5 years post-radiation, there were no significant differences amongst the 3 groups with respect to bowel bother, urinary bother, or sexual bother.
Evidence is emerging that stereotactic body radiation therapy (SBRT) has benefits for oligometastatic disease; however, studies are emerging that support the hypothesis that radiation may enhance immunotherapy in many cancers in the metastatic setting. Kwan et al evaluated the effects of a single fraction of SBRT administered to 1 or 2 disease sites before the first and second doses of avelumab (a PD-L1 antibody) in patients with metastatic castrate-resistant prostate cancer (with prior exposure to at least one second-generation androgen receptor inhibitor). In this single arm phase II study, the disease control rate was 48%, overall response rate was 31%, median radiographic progression-free survival (rPFS) was 8.4 months, and median overall survival (OS) was 14.1 months in this heavily pretreated group of 31 participants. As immunotherapy has been considered efficacious in select groups of prostate cancer (such as tumors with microsatellite instability), this study supports ongoing and new efforts to evaluate how to enhance immunotherapy against prostate cancer.
Radium-223 has been demonstrated to have efficacy in metastatic disease of the bone. Ongoing efforts to determine whether combined modality treatment in this setting may improve outcomes in metastatic disease, Maughn et al evaluated the combination of radium-223 and enzalutamide for safety and efficacy in a small and hypothesis-generating study 47 participants where 35 received enzalutamide plus radium-223 and 12 received enzlutamide alone. Of note, there were no increase in fractures (45 of the 47 participants received bone protective therapy, however), but there were no differences in the secondary endpoints of PSA-PFS, rPFS, or OS (primary endpoints of decline in bone metabolism markers were previously reported). This is a hypothesis generating study that supports the likely safety of this modality if bone protecting agents are utilized.
The 3 studies summarized here represent the ongoing evolution of radiation or radiation-delivery modalities in localized and metastatic prostate cancer. It is encouraging to see the ongoing evaluate of PROs over time, as long-term quality of life in patients potentially cured of disease is of utmost importance. In addition, efforts to evaluate radiation or radium-223 in widely metastatic disease as part of combination therapy may reveal not only situations with modest benefits in efficacy, but more importantly, may reveal molecular insights into newer treatment strategies.
Metastatic prostate cancer: Abiraterone tied to worse cardiovascular outcomes
Key clinical point: Compared with enzalutamide, abiraterone was associated with a 31% higher risk for myocardial infarction or stroke in patients with metastatic prostate cancer.
Major finding: Abiraterone vs. enzalutamide was associated with an increased risk for myocardial infarction or stroke (hazard ratio [HR], 1.31; P = .01). A higher risk for stroke was observed in patients who received abiraterone vs enzalutamide (HR, 1.52; P = .008). No difference was seen in the risk for myocardial infarction between the 2 treatments (P = .92).
Study details: A retrospective study of 6,294 patients with metastatic prostate cancer who received androgen deprivation therapy with either abiraterone or enzalutamide.
Disclosures: No funding source was identified for this study. The authors received research funding, advisory/speaker fees, and/or honoraria from various sources.
Source: Kulkarni AA et al. ESMO Open. 2021 Sep 9. doi: 10.1016/j.esmoop.2021.100261.
Key clinical point: Compared with enzalutamide, abiraterone was associated with a 31% higher risk for myocardial infarction or stroke in patients with metastatic prostate cancer.
Major finding: Abiraterone vs. enzalutamide was associated with an increased risk for myocardial infarction or stroke (hazard ratio [HR], 1.31; P = .01). A higher risk for stroke was observed in patients who received abiraterone vs enzalutamide (HR, 1.52; P = .008). No difference was seen in the risk for myocardial infarction between the 2 treatments (P = .92).
Study details: A retrospective study of 6,294 patients with metastatic prostate cancer who received androgen deprivation therapy with either abiraterone or enzalutamide.
Disclosures: No funding source was identified for this study. The authors received research funding, advisory/speaker fees, and/or honoraria from various sources.
Source: Kulkarni AA et al. ESMO Open. 2021 Sep 9. doi: 10.1016/j.esmoop.2021.100261.
Key clinical point: Compared with enzalutamide, abiraterone was associated with a 31% higher risk for myocardial infarction or stroke in patients with metastatic prostate cancer.
Major finding: Abiraterone vs. enzalutamide was associated with an increased risk for myocardial infarction or stroke (hazard ratio [HR], 1.31; P = .01). A higher risk for stroke was observed in patients who received abiraterone vs enzalutamide (HR, 1.52; P = .008). No difference was seen in the risk for myocardial infarction between the 2 treatments (P = .92).
Study details: A retrospective study of 6,294 patients with metastatic prostate cancer who received androgen deprivation therapy with either abiraterone or enzalutamide.
Disclosures: No funding source was identified for this study. The authors received research funding, advisory/speaker fees, and/or honoraria from various sources.
Source: Kulkarni AA et al. ESMO Open. 2021 Sep 9. doi: 10.1016/j.esmoop.2021.100261.
High-risk prostate cancer: Persistent PSA after surgery linked to worse outcomes
Key clinical point: In patients with high-risk prostate cancer undergoing radical prostatectomy, persistent prostate-specific antigen (PSA) is associated with poor oncological outcomes. Early salvage radiotherapy with or without androgen deprivation therapy (ADT) improves survival in patients with persistent PSA.
Major finding: In patients with persistent vs. undetectable PSA, estimated clinical progression-free survival (63.8% vs 93.5%; P < .0001) and overall survival (OS; 54.0% vs 83.2%; P < .0001) were significantly lower at 10 years. In patients with persistent PSA, ADT alone vs salvage radiotherapy was associated with a higher risk for worse OS (hazard ratio, 4.7; P < .0001).
Study details: A retrospective study of 414 consecutive patients with high-risk prostate cancer who underwent radical prostatectomy; 125 patients had persistent PSA.
Disclosures: The study did not receive any funding. The authors declared no competing interests.
Source: Milonas D et al. Clin Transl Oncol. 2021 Aug 28. doi: 10.1007/s12094-021-02700-y.
Key clinical point: In patients with high-risk prostate cancer undergoing radical prostatectomy, persistent prostate-specific antigen (PSA) is associated with poor oncological outcomes. Early salvage radiotherapy with or without androgen deprivation therapy (ADT) improves survival in patients with persistent PSA.
Major finding: In patients with persistent vs. undetectable PSA, estimated clinical progression-free survival (63.8% vs 93.5%; P < .0001) and overall survival (OS; 54.0% vs 83.2%; P < .0001) were significantly lower at 10 years. In patients with persistent PSA, ADT alone vs salvage radiotherapy was associated with a higher risk for worse OS (hazard ratio, 4.7; P < .0001).
Study details: A retrospective study of 414 consecutive patients with high-risk prostate cancer who underwent radical prostatectomy; 125 patients had persistent PSA.
Disclosures: The study did not receive any funding. The authors declared no competing interests.
Source: Milonas D et al. Clin Transl Oncol. 2021 Aug 28. doi: 10.1007/s12094-021-02700-y.
Key clinical point: In patients with high-risk prostate cancer undergoing radical prostatectomy, persistent prostate-specific antigen (PSA) is associated with poor oncological outcomes. Early salvage radiotherapy with or without androgen deprivation therapy (ADT) improves survival in patients with persistent PSA.
Major finding: In patients with persistent vs. undetectable PSA, estimated clinical progression-free survival (63.8% vs 93.5%; P < .0001) and overall survival (OS; 54.0% vs 83.2%; P < .0001) were significantly lower at 10 years. In patients with persistent PSA, ADT alone vs salvage radiotherapy was associated with a higher risk for worse OS (hazard ratio, 4.7; P < .0001).
Study details: A retrospective study of 414 consecutive patients with high-risk prostate cancer who underwent radical prostatectomy; 125 patients had persistent PSA.
Disclosures: The study did not receive any funding. The authors declared no competing interests.
Source: Milonas D et al. Clin Transl Oncol. 2021 Aug 28. doi: 10.1007/s12094-021-02700-y.
Prostate cancer: Exercise during active surveillance improves cardiorespiratory fitness
Key clinical point: High-intensity interval training (HIIT) improves cardiorespiratory fitness levels and lowers prostate-specific antigen (PSA) levels, PSA velocity, and prostate cancer cell growth in patients with localized prostate cancer undergoing active surveillance.
Major finding: The peak oxygen consumption increased by 0.9 mL/kg/minute in the HIIT group and decreased by 0.5 mL/kg/minute in the usual-care group (P = .01). Compared with the usual care, HIIT was associated with a significant decrease in PSA levels (P = .04), PSA velocity (P = .04), and growth of prostate cancer cell line (P = .02).
Study details: Phase 2 ERASE study of 52 men with prostate cancer undergoing active surveillance who were randomly assigned to HIIT or to usual care.
Disclosures: This study was supported by Canadian Institutes of Health Research and Prostate Cancer Canada. The authors reported no conflict of interests.
Source: Kang DW et al. JAMA Oncol. 2021 Aug 19. doi: 10.1001/jamaoncol.2021.3067.
Key clinical point: High-intensity interval training (HIIT) improves cardiorespiratory fitness levels and lowers prostate-specific antigen (PSA) levels, PSA velocity, and prostate cancer cell growth in patients with localized prostate cancer undergoing active surveillance.
Major finding: The peak oxygen consumption increased by 0.9 mL/kg/minute in the HIIT group and decreased by 0.5 mL/kg/minute in the usual-care group (P = .01). Compared with the usual care, HIIT was associated with a significant decrease in PSA levels (P = .04), PSA velocity (P = .04), and growth of prostate cancer cell line (P = .02).
Study details: Phase 2 ERASE study of 52 men with prostate cancer undergoing active surveillance who were randomly assigned to HIIT or to usual care.
Disclosures: This study was supported by Canadian Institutes of Health Research and Prostate Cancer Canada. The authors reported no conflict of interests.
Source: Kang DW et al. JAMA Oncol. 2021 Aug 19. doi: 10.1001/jamaoncol.2021.3067.
Key clinical point: High-intensity interval training (HIIT) improves cardiorespiratory fitness levels and lowers prostate-specific antigen (PSA) levels, PSA velocity, and prostate cancer cell growth in patients with localized prostate cancer undergoing active surveillance.
Major finding: The peak oxygen consumption increased by 0.9 mL/kg/minute in the HIIT group and decreased by 0.5 mL/kg/minute in the usual-care group (P = .01). Compared with the usual care, HIIT was associated with a significant decrease in PSA levels (P = .04), PSA velocity (P = .04), and growth of prostate cancer cell line (P = .02).
Study details: Phase 2 ERASE study of 52 men with prostate cancer undergoing active surveillance who were randomly assigned to HIIT or to usual care.
Disclosures: This study was supported by Canadian Institutes of Health Research and Prostate Cancer Canada. The authors reported no conflict of interests.
Source: Kang DW et al. JAMA Oncol. 2021 Aug 19. doi: 10.1001/jamaoncol.2021.3067.
Radium-223 plus enzalutamide safe in mCRPC
Key clinical point: Radium-223 in combination with enzalutamide is safe in the long term and improves second-line prostate-specific antigen (PSA)-progression-free survival (PFS) in patients with metastatic castration-resistant prostate cancer (mCRPC) vs. enzalutamide alone.
Major finding: Median follow-up was 22 months. Radium-223 plus enzalutamide vs. enzalutamide alone did not improve median overall survival (P = .73), PSA-PFS (P = .97), and radiographic PFS (P= .96). Radium-223 plus enzalutamide vs enzalutamide alone significantly improved second-line PSA-PFS (18.7 months vs 8.41 months; P = .033).
Study details: A phase 2 randomized trial of 47 patients with mCRPC randomly assigned to receive either radium-223 dichloride with enzalutamide or enzalutamide alone.
Disclosures: The study was supported by the University of Utah. The authors did not disclose any conflict of interests.
Source: Maughan BL et al. Oncologist. 2021 Aug 22. doi: 10.1002/onco.13949.
Key clinical point: Radium-223 in combination with enzalutamide is safe in the long term and improves second-line prostate-specific antigen (PSA)-progression-free survival (PFS) in patients with metastatic castration-resistant prostate cancer (mCRPC) vs. enzalutamide alone.
Major finding: Median follow-up was 22 months. Radium-223 plus enzalutamide vs. enzalutamide alone did not improve median overall survival (P = .73), PSA-PFS (P = .97), and radiographic PFS (P= .96). Radium-223 plus enzalutamide vs enzalutamide alone significantly improved second-line PSA-PFS (18.7 months vs 8.41 months; P = .033).
Study details: A phase 2 randomized trial of 47 patients with mCRPC randomly assigned to receive either radium-223 dichloride with enzalutamide or enzalutamide alone.
Disclosures: The study was supported by the University of Utah. The authors did not disclose any conflict of interests.
Source: Maughan BL et al. Oncologist. 2021 Aug 22. doi: 10.1002/onco.13949.
Key clinical point: Radium-223 in combination with enzalutamide is safe in the long term and improves second-line prostate-specific antigen (PSA)-progression-free survival (PFS) in patients with metastatic castration-resistant prostate cancer (mCRPC) vs. enzalutamide alone.
Major finding: Median follow-up was 22 months. Radium-223 plus enzalutamide vs. enzalutamide alone did not improve median overall survival (P = .73), PSA-PFS (P = .97), and radiographic PFS (P= .96). Radium-223 plus enzalutamide vs enzalutamide alone significantly improved second-line PSA-PFS (18.7 months vs 8.41 months; P = .033).
Study details: A phase 2 randomized trial of 47 patients with mCRPC randomly assigned to receive either radium-223 dichloride with enzalutamide or enzalutamide alone.
Disclosures: The study was supported by the University of Utah. The authors did not disclose any conflict of interests.
Source: Maughan BL et al. Oncologist. 2021 Aug 22. doi: 10.1002/onco.13949.