User login
For MD-IQ only
Nonmetastatic CRPC: High PSA response with apalutamide
Key clinical point: Apalutamide plus androgen deprivation therapy (ADT) leads to higher PSA responses by 6 months in patients with nonmetastatic castration-resistant prostate cancer (CRPC).
Major finding: At 6 months, a higher proportion of patients in the apalutamide vs placebo group reported a PSA reduction of ≥50% (90% vs 1.5%) and ≥90% (57% vs 0%). Deep PSA responses (PSA reduction of ≥50% or PSA levels of ≤0.2 ng/mL) at 6 months after apalutamide treatment were associated with a significant improvement in metastasis-free survival (P < .001) and overall survival (P < .001).
Study details: A post hoc analysis of phase 3 randomized SPARTAN trial of 1,207 patients with nonmetastatic CRPC randomly assigned to receive apalutamide (n=806) or placebo (n=401) plus ADT.
Disclosures: This study was sponsored by Janssen Research & Development. The authors reported employment, research funding, advisory/consulting roles, travel compensation, honoraria, stock ownership, royalties, or patents outside this work.
Source: Saad F et al. Eur Urol. 2021 Dec 13. doi: 10.1016/j.eururo.2021.11.020.
Key clinical point: Apalutamide plus androgen deprivation therapy (ADT) leads to higher PSA responses by 6 months in patients with nonmetastatic castration-resistant prostate cancer (CRPC).
Major finding: At 6 months, a higher proportion of patients in the apalutamide vs placebo group reported a PSA reduction of ≥50% (90% vs 1.5%) and ≥90% (57% vs 0%). Deep PSA responses (PSA reduction of ≥50% or PSA levels of ≤0.2 ng/mL) at 6 months after apalutamide treatment were associated with a significant improvement in metastasis-free survival (P < .001) and overall survival (P < .001).
Study details: A post hoc analysis of phase 3 randomized SPARTAN trial of 1,207 patients with nonmetastatic CRPC randomly assigned to receive apalutamide (n=806) or placebo (n=401) plus ADT.
Disclosures: This study was sponsored by Janssen Research & Development. The authors reported employment, research funding, advisory/consulting roles, travel compensation, honoraria, stock ownership, royalties, or patents outside this work.
Source: Saad F et al. Eur Urol. 2021 Dec 13. doi: 10.1016/j.eururo.2021.11.020.
Key clinical point: Apalutamide plus androgen deprivation therapy (ADT) leads to higher PSA responses by 6 months in patients with nonmetastatic castration-resistant prostate cancer (CRPC).
Major finding: At 6 months, a higher proportion of patients in the apalutamide vs placebo group reported a PSA reduction of ≥50% (90% vs 1.5%) and ≥90% (57% vs 0%). Deep PSA responses (PSA reduction of ≥50% or PSA levels of ≤0.2 ng/mL) at 6 months after apalutamide treatment were associated with a significant improvement in metastasis-free survival (P < .001) and overall survival (P < .001).
Study details: A post hoc analysis of phase 3 randomized SPARTAN trial of 1,207 patients with nonmetastatic CRPC randomly assigned to receive apalutamide (n=806) or placebo (n=401) plus ADT.
Disclosures: This study was sponsored by Janssen Research & Development. The authors reported employment, research funding, advisory/consulting roles, travel compensation, honoraria, stock ownership, royalties, or patents outside this work.
Source: Saad F et al. Eur Urol. 2021 Dec 13. doi: 10.1016/j.eururo.2021.11.020.
HSPC: Enzalutamide does not worsen overall health and quality of life
Key clinical point: Enzalutamide was associated with worsening of self-reported fatigue, cognitive function, and physical function, but not overall health and quality of life (OHQL) in newly diagnosed patients with metastatic hormone-sensitive prostate cancer (HSPC).
Major finding: Conventional nonsteroidal antiandrogen vs enzalutamide was associated with less worsening of fatigue (mean difference [MD], 5.2; P < .001), cognitive function (MD, 4.0; P < .001), and physical function (MD, 2.6; P < .001), but not OHQL (MD, 1.2; P = .1).
Study details: An open-label, international, randomized, phase 3, cooperative group ENZAMET trial of newly diagnosed patients with metastatic prostate cancer who were randomly assigned to enzalutamide or a conventional nonsteroidal antiandrogen.
Disclosures: This study was funded by Astellas. The authors received speakers/advisory/consulting fees, honoraria, research funding, royalties, and travel/accommodation expenses; were employed by; held stocks/other ownership interests; and had leadership roles outside this work.
Source: Stockler MR et al. J Clin Oncol. 2021 Dec 20. doi: 10.1200/JCO.21.00941.
Key clinical point: Enzalutamide was associated with worsening of self-reported fatigue, cognitive function, and physical function, but not overall health and quality of life (OHQL) in newly diagnosed patients with metastatic hormone-sensitive prostate cancer (HSPC).
Major finding: Conventional nonsteroidal antiandrogen vs enzalutamide was associated with less worsening of fatigue (mean difference [MD], 5.2; P < .001), cognitive function (MD, 4.0; P < .001), and physical function (MD, 2.6; P < .001), but not OHQL (MD, 1.2; P = .1).
Study details: An open-label, international, randomized, phase 3, cooperative group ENZAMET trial of newly diagnosed patients with metastatic prostate cancer who were randomly assigned to enzalutamide or a conventional nonsteroidal antiandrogen.
Disclosures: This study was funded by Astellas. The authors received speakers/advisory/consulting fees, honoraria, research funding, royalties, and travel/accommodation expenses; were employed by; held stocks/other ownership interests; and had leadership roles outside this work.
Source: Stockler MR et al. J Clin Oncol. 2021 Dec 20. doi: 10.1200/JCO.21.00941.
Key clinical point: Enzalutamide was associated with worsening of self-reported fatigue, cognitive function, and physical function, but not overall health and quality of life (OHQL) in newly diagnosed patients with metastatic hormone-sensitive prostate cancer (HSPC).
Major finding: Conventional nonsteroidal antiandrogen vs enzalutamide was associated with less worsening of fatigue (mean difference [MD], 5.2; P < .001), cognitive function (MD, 4.0; P < .001), and physical function (MD, 2.6; P < .001), but not OHQL (MD, 1.2; P = .1).
Study details: An open-label, international, randomized, phase 3, cooperative group ENZAMET trial of newly diagnosed patients with metastatic prostate cancer who were randomly assigned to enzalutamide or a conventional nonsteroidal antiandrogen.
Disclosures: This study was funded by Astellas. The authors received speakers/advisory/consulting fees, honoraria, research funding, royalties, and travel/accommodation expenses; were employed by; held stocks/other ownership interests; and had leadership roles outside this work.
Source: Stockler MR et al. J Clin Oncol. 2021 Dec 20. doi: 10.1200/JCO.21.00941.
mCRPC: Enzalutamide benefit is independent of concurrent corticosteroid use
Key clinical point: In patients with metastatic castration-resistant prostate cancer (mCRPC), enzalutamide improves outcomes independent of concurrent corticosteroid use (CCU).
Major finding: In patients with baseline CCU, enzalutamide vs placebo improved overall survival (OS; hazard ratio [HR], 0.70; P = .012), radiographic progression-free survival (rPFS; HR, 0.59; P < .001), and time to prostate-specific antigen progression (TTPP; HR, 0.36; P < .001). Enzalutamide improved OS (HR, 0.59; P < .001), rPFS (HR, 0.33; P < .001) and TTPP (HR, 0.22; P < .001) in patients with no CCU.
Study details: A post hoc analysis of phase 3, randomized AFFIRM trial of 1,199 patients with mCRPC who were randomly assigned to enzalutamide 160 mg/day or placebo.
Disclosures: This work was supported by Pfizer Inc. and Astellas Pharma, Inc. The authors received honoraria, advisory/consulting fees, nonfinancial support, and/or research funding outside this work. Some authors reported being employed, held patents, and/or were inventors or investigators.
Source: Zhao JL et al. Clin Cancer Res. 2021 Dec 29. doi: 10.1158/1078-0432.CCR-21-1090.
Key clinical point: In patients with metastatic castration-resistant prostate cancer (mCRPC), enzalutamide improves outcomes independent of concurrent corticosteroid use (CCU).
Major finding: In patients with baseline CCU, enzalutamide vs placebo improved overall survival (OS; hazard ratio [HR], 0.70; P = .012), radiographic progression-free survival (rPFS; HR, 0.59; P < .001), and time to prostate-specific antigen progression (TTPP; HR, 0.36; P < .001). Enzalutamide improved OS (HR, 0.59; P < .001), rPFS (HR, 0.33; P < .001) and TTPP (HR, 0.22; P < .001) in patients with no CCU.
Study details: A post hoc analysis of phase 3, randomized AFFIRM trial of 1,199 patients with mCRPC who were randomly assigned to enzalutamide 160 mg/day or placebo.
Disclosures: This work was supported by Pfizer Inc. and Astellas Pharma, Inc. The authors received honoraria, advisory/consulting fees, nonfinancial support, and/or research funding outside this work. Some authors reported being employed, held patents, and/or were inventors or investigators.
Source: Zhao JL et al. Clin Cancer Res. 2021 Dec 29. doi: 10.1158/1078-0432.CCR-21-1090.
Key clinical point: In patients with metastatic castration-resistant prostate cancer (mCRPC), enzalutamide improves outcomes independent of concurrent corticosteroid use (CCU).
Major finding: In patients with baseline CCU, enzalutamide vs placebo improved overall survival (OS; hazard ratio [HR], 0.70; P = .012), radiographic progression-free survival (rPFS; HR, 0.59; P < .001), and time to prostate-specific antigen progression (TTPP; HR, 0.36; P < .001). Enzalutamide improved OS (HR, 0.59; P < .001), rPFS (HR, 0.33; P < .001) and TTPP (HR, 0.22; P < .001) in patients with no CCU.
Study details: A post hoc analysis of phase 3, randomized AFFIRM trial of 1,199 patients with mCRPC who were randomly assigned to enzalutamide 160 mg/day or placebo.
Disclosures: This work was supported by Pfizer Inc. and Astellas Pharma, Inc. The authors received honoraria, advisory/consulting fees, nonfinancial support, and/or research funding outside this work. Some authors reported being employed, held patents, and/or were inventors or investigators.
Source: Zhao JL et al. Clin Cancer Res. 2021 Dec 29. doi: 10.1158/1078-0432.CCR-21-1090.
Abiraterone extends survival in mCSPC with visceral metastases
Key clinical point: Adding abiraterone acetate plus prednisone (AA+P) to androgen deprivation therapy (ADT) extends survival in patients with newly diagnosed metastatic castration-sensitive prostate cancer (mCSPC) and visceral disease.
Major finding: AA+P vs placebo significantly improved overall survival (median, 55.4 months vs 33.0 months; hazard ratio [HR], 0.582; P = .0029) and radiographic progression-free survival (median, 30.7 months vs 18.3 months; HR, 0.527; P = .0005) in patients with vs without visceral metastases.
Study details: A post hoc analysis of LATITUDE study including 1,199 newly diagnosed patients with mCSPC who were randomly assigned to AA+P and ADT or placebo plus ADT.
Disclosures: This work was funded by Janssen Research & Development. The authors reported employment/stock ownership; received personal/advisory fees, funding, honorarium, and travel and accommodation expense outside this work; were employees of Janssen Research & Development; and/or held company stock.
Source: Baciarello G et al. Eur J Cancer. 2021 Dec 22. doi: 10.1016/j.ejca.2021.11.026.
Key clinical point: Adding abiraterone acetate plus prednisone (AA+P) to androgen deprivation therapy (ADT) extends survival in patients with newly diagnosed metastatic castration-sensitive prostate cancer (mCSPC) and visceral disease.
Major finding: AA+P vs placebo significantly improved overall survival (median, 55.4 months vs 33.0 months; hazard ratio [HR], 0.582; P = .0029) and radiographic progression-free survival (median, 30.7 months vs 18.3 months; HR, 0.527; P = .0005) in patients with vs without visceral metastases.
Study details: A post hoc analysis of LATITUDE study including 1,199 newly diagnosed patients with mCSPC who were randomly assigned to AA+P and ADT or placebo plus ADT.
Disclosures: This work was funded by Janssen Research & Development. The authors reported employment/stock ownership; received personal/advisory fees, funding, honorarium, and travel and accommodation expense outside this work; were employees of Janssen Research & Development; and/or held company stock.
Source: Baciarello G et al. Eur J Cancer. 2021 Dec 22. doi: 10.1016/j.ejca.2021.11.026.
Key clinical point: Adding abiraterone acetate plus prednisone (AA+P) to androgen deprivation therapy (ADT) extends survival in patients with newly diagnosed metastatic castration-sensitive prostate cancer (mCSPC) and visceral disease.
Major finding: AA+P vs placebo significantly improved overall survival (median, 55.4 months vs 33.0 months; hazard ratio [HR], 0.582; P = .0029) and radiographic progression-free survival (median, 30.7 months vs 18.3 months; HR, 0.527; P = .0005) in patients with vs without visceral metastases.
Study details: A post hoc analysis of LATITUDE study including 1,199 newly diagnosed patients with mCSPC who were randomly assigned to AA+P and ADT or placebo plus ADT.
Disclosures: This work was funded by Janssen Research & Development. The authors reported employment/stock ownership; received personal/advisory fees, funding, honorarium, and travel and accommodation expense outside this work; were employees of Janssen Research & Development; and/or held company stock.
Source: Baciarello G et al. Eur J Cancer. 2021 Dec 22. doi: 10.1016/j.ejca.2021.11.026.
Prostate cancer: Focal boost to EBRT delays local, regional, and distant failure
Key clinical point: A focal boost to external beam radiotherapy (EBRT) improves local failure-free survival (LFS) and regional/distant metastasis-free survival (rdMFS) in patients with localized high-risk prostate cancer.
Major finding: At a median follow-up of 72 months, focal boost improved LFS (adjusted hazard ratio [aHR], 0.33; P = .01) and rdMFS (aHR, 0.56; P = .02). A higher dose to the tumor resulted in lower LFS and rdMFS rates.
Study details: A phase 3, multicenter, randomized controlled FLAME trial of 571 patients with intermediate- or high-risk localized prostate cancer who were randomly assigned to receive EBRT with or without an additional boost of up to 95 Gy to the macroscopic tumor.
Disclosures: This study was supported by the Dutch Cancer Society. The authors reported no conflict of interests.
Source: Groen VH et al. Eur Urol. 2021 Dec 22. doi: 10.1016/j.eururo.2021.12.012.
Key clinical point: A focal boost to external beam radiotherapy (EBRT) improves local failure-free survival (LFS) and regional/distant metastasis-free survival (rdMFS) in patients with localized high-risk prostate cancer.
Major finding: At a median follow-up of 72 months, focal boost improved LFS (adjusted hazard ratio [aHR], 0.33; P = .01) and rdMFS (aHR, 0.56; P = .02). A higher dose to the tumor resulted in lower LFS and rdMFS rates.
Study details: A phase 3, multicenter, randomized controlled FLAME trial of 571 patients with intermediate- or high-risk localized prostate cancer who were randomly assigned to receive EBRT with or without an additional boost of up to 95 Gy to the macroscopic tumor.
Disclosures: This study was supported by the Dutch Cancer Society. The authors reported no conflict of interests.
Source: Groen VH et al. Eur Urol. 2021 Dec 22. doi: 10.1016/j.eururo.2021.12.012.
Key clinical point: A focal boost to external beam radiotherapy (EBRT) improves local failure-free survival (LFS) and regional/distant metastasis-free survival (rdMFS) in patients with localized high-risk prostate cancer.
Major finding: At a median follow-up of 72 months, focal boost improved LFS (adjusted hazard ratio [aHR], 0.33; P = .01) and rdMFS (aHR, 0.56; P = .02). A higher dose to the tumor resulted in lower LFS and rdMFS rates.
Study details: A phase 3, multicenter, randomized controlled FLAME trial of 571 patients with intermediate- or high-risk localized prostate cancer who were randomly assigned to receive EBRT with or without an additional boost of up to 95 Gy to the macroscopic tumor.
Disclosures: This study was supported by the Dutch Cancer Society. The authors reported no conflict of interests.
Source: Groen VH et al. Eur Urol. 2021 Dec 22. doi: 10.1016/j.eururo.2021.12.012.
High-risk prostate cancer: Add-on abiraterone prolongs survival
Key clinical point: In patients with high-risk nonmetastatic prostate cancer, adding abiraterone acetate and prednisolone to androgen deprivation therapy (ADT) prolongs survival vs ADT alone.
Major finding: Add-on abiraterone acetate plus prednisolone to ADT vs ADT alone significantly improved metastasis-free survival (MFS; hazard ratio [HR], 0.53; P < .0001) and overall survival (HR, 0.60; P < .0001). There was no difference in MFS when enzalutamide and abiraterone acetate were administered concurrently vs abiraterone acetate alone (interaction HR, 1.02; P = .91).
Study details: A meta-analysis of 2 phase 3 STAMPEDE trials including 1,974 patients with high-risk nonmetastatic prostate cancer randomly assigned to ADT alone or ADT plus abiraterone acetate and prednisolone with or without enzalutamide.
Disclosures: This work was supported by Cancer Research UK, UK Medical Research Council, Swiss Group for Clinical Cancer Research, and others. The authors received personal fees, honoraria, grants, travel support, royalty, nonfinancial support, and payments or held patents outside this work.
Source: Attard G et al. Lancet. 2021 Dec 23. doi: 10.1016/S0140-6736(21)02437-5.
Key clinical point: In patients with high-risk nonmetastatic prostate cancer, adding abiraterone acetate and prednisolone to androgen deprivation therapy (ADT) prolongs survival vs ADT alone.
Major finding: Add-on abiraterone acetate plus prednisolone to ADT vs ADT alone significantly improved metastasis-free survival (MFS; hazard ratio [HR], 0.53; P < .0001) and overall survival (HR, 0.60; P < .0001). There was no difference in MFS when enzalutamide and abiraterone acetate were administered concurrently vs abiraterone acetate alone (interaction HR, 1.02; P = .91).
Study details: A meta-analysis of 2 phase 3 STAMPEDE trials including 1,974 patients with high-risk nonmetastatic prostate cancer randomly assigned to ADT alone or ADT plus abiraterone acetate and prednisolone with or without enzalutamide.
Disclosures: This work was supported by Cancer Research UK, UK Medical Research Council, Swiss Group for Clinical Cancer Research, and others. The authors received personal fees, honoraria, grants, travel support, royalty, nonfinancial support, and payments or held patents outside this work.
Source: Attard G et al. Lancet. 2021 Dec 23. doi: 10.1016/S0140-6736(21)02437-5.
Key clinical point: In patients with high-risk nonmetastatic prostate cancer, adding abiraterone acetate and prednisolone to androgen deprivation therapy (ADT) prolongs survival vs ADT alone.
Major finding: Add-on abiraterone acetate plus prednisolone to ADT vs ADT alone significantly improved metastasis-free survival (MFS; hazard ratio [HR], 0.53; P < .0001) and overall survival (HR, 0.60; P < .0001). There was no difference in MFS when enzalutamide and abiraterone acetate were administered concurrently vs abiraterone acetate alone (interaction HR, 1.02; P = .91).
Study details: A meta-analysis of 2 phase 3 STAMPEDE trials including 1,974 patients with high-risk nonmetastatic prostate cancer randomly assigned to ADT alone or ADT plus abiraterone acetate and prednisolone with or without enzalutamide.
Disclosures: This work was supported by Cancer Research UK, UK Medical Research Council, Swiss Group for Clinical Cancer Research, and others. The authors received personal fees, honoraria, grants, travel support, royalty, nonfinancial support, and payments or held patents outside this work.
Source: Attard G et al. Lancet. 2021 Dec 23. doi: 10.1016/S0140-6736(21)02437-5.
Castration Resistant Prostate Cancer
Clinical Edge Journal Scan Commentary: Prostate Cancer January 2022
Extensive efforts have been put forth to evaluate which patients may safely delay, or completely avoid, treatment for prostate cancer. However, identifying who can safely avoid treatment is challenging. Arcot et al. utilized the Michigan Urological Surgery Improvement Collaborative (MUSIC) registry for men to determine whether those with prostate cancer grade group 1 with delayed treatment had increased need for secondary treatments, such as androgen deprivation or radiation therapy, compared with those with upfront surgery. There was a small decrease in the probability of being free from secondary treatment 24 months after diagnosis (96% vs. 93%), suggesting that such an approach is quite reasonable.
These two studies exemplify one of the ongoing points of discussion in treatment of early stage prostate cancer: whether upfront treatments are of net benefit for patients. The study by Wallis et al. further evaluated this point by conducting a prospective cohort study to evaluate the extent of regret of choice of treatment strategy amongst patients with prostate cancer. Out of this cohort of 2,072 men, 16% who underwent surgery, 11% who received radiation, and 7% who chose active surveillance reported regret regarding the treatment choice. However, when controlled for functional outcomes, the differences amongst treatment modalities were not statistically significant. However, perceived treatment efficacy and adverse effects were associated with regret when compared with patient expectations prior to treatment. This suggest that research and focus on shared decision-making in the clinic may be highly beneficial.
Extensive efforts have been put forth to evaluate which patients may safely delay, or completely avoid, treatment for prostate cancer. However, identifying who can safely avoid treatment is challenging. Arcot et al. utilized the Michigan Urological Surgery Improvement Collaborative (MUSIC) registry for men to determine whether those with prostate cancer grade group 1 with delayed treatment had increased need for secondary treatments, such as androgen deprivation or radiation therapy, compared with those with upfront surgery. There was a small decrease in the probability of being free from secondary treatment 24 months after diagnosis (96% vs. 93%), suggesting that such an approach is quite reasonable.
These two studies exemplify one of the ongoing points of discussion in treatment of early stage prostate cancer: whether upfront treatments are of net benefit for patients. The study by Wallis et al. further evaluated this point by conducting a prospective cohort study to evaluate the extent of regret of choice of treatment strategy amongst patients with prostate cancer. Out of this cohort of 2,072 men, 16% who underwent surgery, 11% who received radiation, and 7% who chose active surveillance reported regret regarding the treatment choice. However, when controlled for functional outcomes, the differences amongst treatment modalities were not statistically significant. However, perceived treatment efficacy and adverse effects were associated with regret when compared with patient expectations prior to treatment. This suggest that research and focus on shared decision-making in the clinic may be highly beneficial.
Extensive efforts have been put forth to evaluate which patients may safely delay, or completely avoid, treatment for prostate cancer. However, identifying who can safely avoid treatment is challenging. Arcot et al. utilized the Michigan Urological Surgery Improvement Collaborative (MUSIC) registry for men to determine whether those with prostate cancer grade group 1 with delayed treatment had increased need for secondary treatments, such as androgen deprivation or radiation therapy, compared with those with upfront surgery. There was a small decrease in the probability of being free from secondary treatment 24 months after diagnosis (96% vs. 93%), suggesting that such an approach is quite reasonable.
These two studies exemplify one of the ongoing points of discussion in treatment of early stage prostate cancer: whether upfront treatments are of net benefit for patients. The study by Wallis et al. further evaluated this point by conducting a prospective cohort study to evaluate the extent of regret of choice of treatment strategy amongst patients with prostate cancer. Out of this cohort of 2,072 men, 16% who underwent surgery, 11% who received radiation, and 7% who chose active surveillance reported regret regarding the treatment choice. However, when controlled for functional outcomes, the differences amongst treatment modalities were not statistically significant. However, perceived treatment efficacy and adverse effects were associated with regret when compared with patient expectations prior to treatment. This suggest that research and focus on shared decision-making in the clinic may be highly beneficial.
Complaints of incomplete bladder emptying
Many patients with nonmetastatic prostate cancer are asymptomatic at the time of diagnosis due to widespread routine screening. When localized symptoms do occur, they may include urinary frequency, decreased urine stream, urinary urgency, and hematuria. An increasing proportion of patients with localized disease are asymptomatic, however; such signs and symptoms may well be related to age-associated prostate enlargement or other conditions. Nevertheless, men over the age of 50 years who present with urinary symptoms should be screened for prostate cancer using DRE and PSA. Benign prostatic hyperplasia, for example, can manifest in urinary symptoms and even elevate PSA. Acute prostatitis, on the other hand, presents as a urinary tract infection.
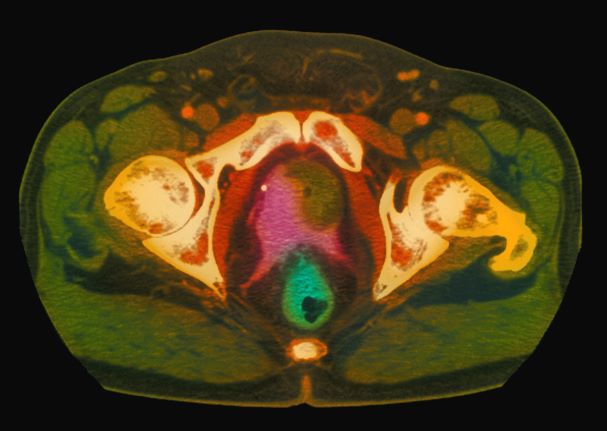
Because this patient showed elevated PSA levels, albeit with normal DRE findings, needle biopsy of the prostate is indicated for tissue diagnosis, usually performed with transrectal ultrasound. A pathologic evaluation of the biopsy specimen will determine the patient's Gleason score. PSA density (amount of PSA per gram of prostate tissue) and PSA doubling time should be collected as well. As seen in the present case, MRI can be used to assess lesions concerning for prostate cancer prior to biopsy. Lesions are then assigned Prostate Imaging–Reporting and Data System (PI-RADS) scores depending on their location within the prostatic zones. Imaging is also useful in staging and active surveillance. Staging is based on the tumor, node, and metastasis (TNM), with clinically localized prostate cancers including any T, N0, M0, NX, or MX cases. The clinician should pursue genetic testing to determine the presence of high-risk germline mutations.
The NCCN Guidelines recommend that for clinically localized prostate cancer, approaches include watchful waiting, active surveillance, radical prostatectomy, and radiation therapy. For asymptomatic patients who are older and/or have other serious comorbidities, active surveillance is often suggested. Radical prostatectomy is typically reserved for patients with a life expectancy of 10 years or more. Pelvic lymph node dissection may be performed on the basis of probability of nodal metastasis. Radiotherapy is also potentially curative in localized prostate cancer and may be delivered via brachytherapy, proton radiation, or external beam radiation therapy (EBRT). EBRT techniques include intensity-modulated radiation therapy (IMRT) and hypofractionated, image-guided stereotactic body radiation therapy (SBRT).
Chad R. Tracy, MD, Professor; Director, Minimally Invasive Surgery, Department of Urology, University of Iowa Hospitals and Clinics, Iowa City, Iowa
Chad R. Tracy, MD, has disclosed the following relevant financial relationships:
Serve(d) as a consultant for: Cvico Medical Solutions
Many patients with nonmetastatic prostate cancer are asymptomatic at the time of diagnosis due to widespread routine screening. When localized symptoms do occur, they may include urinary frequency, decreased urine stream, urinary urgency, and hematuria. An increasing proportion of patients with localized disease are asymptomatic, however; such signs and symptoms may well be related to age-associated prostate enlargement or other conditions. Nevertheless, men over the age of 50 years who present with urinary symptoms should be screened for prostate cancer using DRE and PSA. Benign prostatic hyperplasia, for example, can manifest in urinary symptoms and even elevate PSA. Acute prostatitis, on the other hand, presents as a urinary tract infection.
Because this patient showed elevated PSA levels, albeit with normal DRE findings, needle biopsy of the prostate is indicated for tissue diagnosis, usually performed with transrectal ultrasound. A pathologic evaluation of the biopsy specimen will determine the patient's Gleason score. PSA density (amount of PSA per gram of prostate tissue) and PSA doubling time should be collected as well. As seen in the present case, MRI can be used to assess lesions concerning for prostate cancer prior to biopsy. Lesions are then assigned Prostate Imaging–Reporting and Data System (PI-RADS) scores depending on their location within the prostatic zones. Imaging is also useful in staging and active surveillance. Staging is based on the tumor, node, and metastasis (TNM), with clinically localized prostate cancers including any T, N0, M0, NX, or MX cases. The clinician should pursue genetic testing to determine the presence of high-risk germline mutations.
The NCCN Guidelines recommend that for clinically localized prostate cancer, approaches include watchful waiting, active surveillance, radical prostatectomy, and radiation therapy. For asymptomatic patients who are older and/or have other serious comorbidities, active surveillance is often suggested. Radical prostatectomy is typically reserved for patients with a life expectancy of 10 years or more. Pelvic lymph node dissection may be performed on the basis of probability of nodal metastasis. Radiotherapy is also potentially curative in localized prostate cancer and may be delivered via brachytherapy, proton radiation, or external beam radiation therapy (EBRT). EBRT techniques include intensity-modulated radiation therapy (IMRT) and hypofractionated, image-guided stereotactic body radiation therapy (SBRT).
Chad R. Tracy, MD, Professor; Director, Minimally Invasive Surgery, Department of Urology, University of Iowa Hospitals and Clinics, Iowa City, Iowa
Chad R. Tracy, MD, has disclosed the following relevant financial relationships:
Serve(d) as a consultant for: Cvico Medical Solutions
Many patients with nonmetastatic prostate cancer are asymptomatic at the time of diagnosis due to widespread routine screening. When localized symptoms do occur, they may include urinary frequency, decreased urine stream, urinary urgency, and hematuria. An increasing proportion of patients with localized disease are asymptomatic, however; such signs and symptoms may well be related to age-associated prostate enlargement or other conditions. Nevertheless, men over the age of 50 years who present with urinary symptoms should be screened for prostate cancer using DRE and PSA. Benign prostatic hyperplasia, for example, can manifest in urinary symptoms and even elevate PSA. Acute prostatitis, on the other hand, presents as a urinary tract infection.
Because this patient showed elevated PSA levels, albeit with normal DRE findings, needle biopsy of the prostate is indicated for tissue diagnosis, usually performed with transrectal ultrasound. A pathologic evaluation of the biopsy specimen will determine the patient's Gleason score. PSA density (amount of PSA per gram of prostate tissue) and PSA doubling time should be collected as well. As seen in the present case, MRI can be used to assess lesions concerning for prostate cancer prior to biopsy. Lesions are then assigned Prostate Imaging–Reporting and Data System (PI-RADS) scores depending on their location within the prostatic zones. Imaging is also useful in staging and active surveillance. Staging is based on the tumor, node, and metastasis (TNM), with clinically localized prostate cancers including any T, N0, M0, NX, or MX cases. The clinician should pursue genetic testing to determine the presence of high-risk germline mutations.
The NCCN Guidelines recommend that for clinically localized prostate cancer, approaches include watchful waiting, active surveillance, radical prostatectomy, and radiation therapy. For asymptomatic patients who are older and/or have other serious comorbidities, active surveillance is often suggested. Radical prostatectomy is typically reserved for patients with a life expectancy of 10 years or more. Pelvic lymph node dissection may be performed on the basis of probability of nodal metastasis. Radiotherapy is also potentially curative in localized prostate cancer and may be delivered via brachytherapy, proton radiation, or external beam radiation therapy (EBRT). EBRT techniques include intensity-modulated radiation therapy (IMRT) and hypofractionated, image-guided stereotactic body radiation therapy (SBRT).
Chad R. Tracy, MD, Professor; Director, Minimally Invasive Surgery, Department of Urology, University of Iowa Hospitals and Clinics, Iowa City, Iowa
Chad R. Tracy, MD, has disclosed the following relevant financial relationships:
Serve(d) as a consultant for: Cvico Medical Solutions
A 61-year-old man presents with complaints of frequent urination and incomplete bladder emptying. He also has been feeling fatigued but cannot tell if this is because his symptoms are worse at night and he has not been sleeping well. Despite a family history of atrial fibrillation, he reports no significant medical history beyond appendicitis many years ago. The patient underwent a prostate cancer screening about 18 months ago, which was normal. During a recent office visit, digital rectal examination (DRE) was normal, but prostate-specific antigen (PSA) levels were elevated at 10.2 ng/mL. An MRI is performed as part of the workup.
Prostate cancer: Preoperative mpMRI PI-RAD score is linked to upstaging
Key clinical point: A higher preoperative multiparametric magnetic resonance imaging (mpMRI) Prostate Imaging Reporting and Data System (PI‐RADS) score is associated with upstaging on surgical pathology in patients with prostate cancer.
Major finding: PI-RADS score was greater than 3 in 87% of patients. Upstaging was reported in 47% of patients. PI-RADS was an independent risk predictor for upstaging (adjusted odds ratio, 2.034; P < .001).
Study details: A retrospective study of 294 patients with prostate cancer who underwent a prostate mpMRI and radical prostatectomy between 2016 and 2020.
Disclosures: No funding source was identified for this work. The authors declared no conflict of interests.
Source: Pockros B et al. Prostate. 2021 Dec 8. doi: 10.1002/pros.24280.
Key clinical point: A higher preoperative multiparametric magnetic resonance imaging (mpMRI) Prostate Imaging Reporting and Data System (PI‐RADS) score is associated with upstaging on surgical pathology in patients with prostate cancer.
Major finding: PI-RADS score was greater than 3 in 87% of patients. Upstaging was reported in 47% of patients. PI-RADS was an independent risk predictor for upstaging (adjusted odds ratio, 2.034; P < .001).
Study details: A retrospective study of 294 patients with prostate cancer who underwent a prostate mpMRI and radical prostatectomy between 2016 and 2020.
Disclosures: No funding source was identified for this work. The authors declared no conflict of interests.
Source: Pockros B et al. Prostate. 2021 Dec 8. doi: 10.1002/pros.24280.
Key clinical point: A higher preoperative multiparametric magnetic resonance imaging (mpMRI) Prostate Imaging Reporting and Data System (PI‐RADS) score is associated with upstaging on surgical pathology in patients with prostate cancer.
Major finding: PI-RADS score was greater than 3 in 87% of patients. Upstaging was reported in 47% of patients. PI-RADS was an independent risk predictor for upstaging (adjusted odds ratio, 2.034; P < .001).
Study details: A retrospective study of 294 patients with prostate cancer who underwent a prostate mpMRI and radical prostatectomy between 2016 and 2020.
Disclosures: No funding source was identified for this work. The authors declared no conflict of interests.
Source: Pockros B et al. Prostate. 2021 Dec 8. doi: 10.1002/pros.24280.