User login
Game changers in pediatric cancer
Although there have been significant improvements in patient outcomes for some forms of pediatric cancer, progress has been painfully slow for others. An increasing understanding of pediatric cancers is highlighting the unique molecular drivers and challenging the assumption that drugs developed in adults can be applied to children and young adults. Here, we discuss game-changing therapeutic advances and a shifting view of childhood cancers.
Unique genomic background
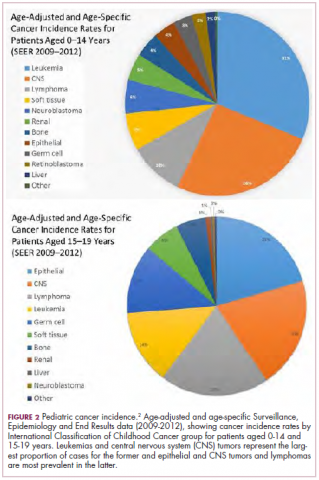
Although pediatric cancers are rare, representing just 1% of all new cancers diagnosed annually in the United States, they are the second leading cause of death in children aged 1 to 14 years. There are many different histological tumor types under the umbrella of childhood cancers, of which the most common are leukemias, central nervous system tumors, and lymphomas (Figure 1).1,2
Significant progress has been made in the treatment of certain pediatric cancers in recent decades, exemplified by pediatric acute lymphoblastic leukemia (ALL), which has been transformed from a virtually incurable cancer to one in which 5-year survival rates now reach up to 90%. In other forms of pediatric cancer, however, survival rates have stagnated and little progress has been made in the development of effective new therapies.3
Because of their rarity, pediatric cancers are difficult to study and adequate enrollment of children in clinical trials can be challenging. Pharmaceutical companies are often hesitant to test drugs in the pediatric population in patients who often cannot advocate for themselves. As a result, the activity of drugs developed in adult patients has often been inferred in pediatric patients with the same tumor type or molecular aberrations. However, as researchers have gathered more information about pediatric cancers, there has been increasing recognition of their unique attributes and the need for dedicated clinical trials in this patient population.
Pediatric cancers tend to be found in the developing mesodermic tissue, whereas adult cancers are more prevalent in the epithelial tissues. Genome sequencing studies have revealed a much lower mutational burden in pediatric cancers and the mechanisms of oncogenesis are also quite different; adult tumors can develop from a series of acquired gene mutations, but pediatric tumors tend to develop from a single catastrophic event.4,5
Even the same type of cancer in a pediatric and adult patient can be quite different, with very different underlying molecular mechanisms. In a recent genomic analysis of different types of pediatric cancer by researchers at St Jude’s Children’s Research Hospital, less than half of the identified mutated genes were found to be similar to those found in adult patients.6
A ‘magic bullet’?
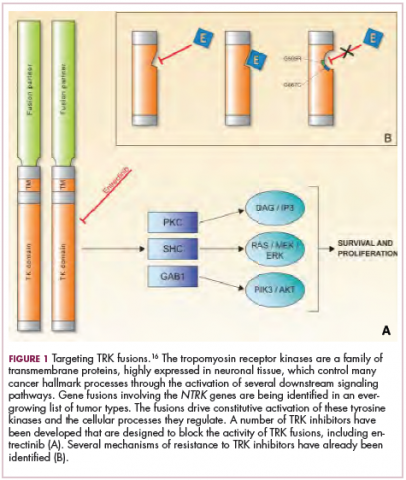
Chromosomal rearrangements are common in pediatric cancers. This type of molecular abnormality can result in a fusion of 2 different genes when the chromosome breaks apart and the pieces join back together in a muddled order. If the genetic code fuses in a manner that is “readable” by the cell, then it can drive aberrant activation of one or both genes.7 Gene fusions often involve kinase enzymes that are essential players in cell signaling pathways regulating hallmark cancer processes, such as unchecked cell proliferation. The fusion drives the constitutive activation of the kinase and, thus, these downstream signaling pathways.
One of the first chromosomal rearrangements linked to cancer, BCR-ABL1 – more commonly known as the Philadelphia chromosome – results in aberrant activation of the ABL1 kinase. It is present in nearly all cases of chronic myeloid leukemia (CML) and 3% to 5% of patients with ALL, and thus became the central focus of targeted drug development. Imatinib was initially approved by the US Food and Drug Administration (FDA) in 2001 for the treatment of adult patients with CML and had such a significant impact on the treatment landscape that it made the cover of Time magazine as a “magic bullet” in the war on cancer.8
Approval was expanded into pediatric patients in 2006 and for pediatric patients with ALL in 2013. However, as with the use of most kinase inhibitors, tumors can evolve under the selective pressure of treatment, developing additional molecular abnormalities that drive resistance.9
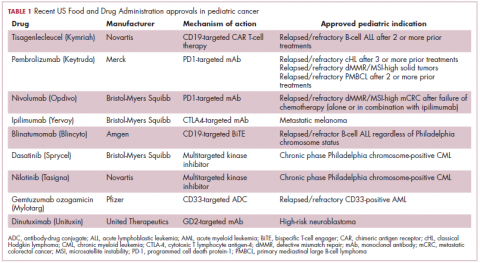
Next-generation multikinase inhibitors that more potently inhibit the BCR-ABL1 fusion protein have been developed to provide additional treatment options for patients who become resistant to imatinib. Dasatinib and nilotinib are among several drugs that have recently been approved for pediatric cancer therapy (Table 1). Both therapies were approved to treat children with Philadelphia chromosome-positive CML in the chronic phase in either the front- or second-line setting after failure of imatinib.
The approval of dasatinib was based on data from 97 patients across 2 trials, 51 of whom were newly diagnosed and 46 previously treated with imatinib. Most of the patients were treated with dasatinib 60 mg/m2 once daily. After 2 years of follow-up, more than 95% of newly diagnosed patients and 82.6% of relapsed/refractory patients had complete cytogenetic response.10
Nilotinib was approved on the basis of findings from 2 clinical trials including 69 patients – 1 trial involving patients who were refractory to or relapsed after dasatinib and imatinib treatment, and 1 that included both relapsed/refractory and newly diagnosed patients. Patients received nilotinib 230 mg/m2 twice daily, rounded to the nearest 50-mg dose, in 28-day cycles. By cycle 12, the cumulative major molecular response rate (MMR) was 47.7% in patients with relapsed/refractory disease, and 64% in newly diagnosed patients.11 Clinical trials of both drugs in the pediatric setting are ongoing.
Other prominent gene fusions
Gene fusions involving the anaplastic lymphoma kinase (ALK) occur in patients with non–small-cell lung cancer and ALK inhibitors have provided an effective new treatment option for patients whose tumors display this abnormality.
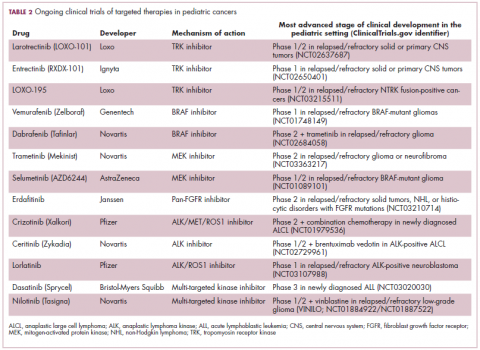
ALK fusions are also a prominent feature of several kinds of pediatric cancers and ALK inhibitors offer promise in this setting.7,12 An NPM-ALK fusion is found in 90% of pediatric anaplastic large cell lymphoma (ALCL) cases,13 whereas a variety of ALK fusions are found in up to half of patients with inflammatory myofibroblastic tumor (IMT), a rare form of soft tissue sarcoma.14 ALK inhibitors are being tested in a variety of clinical trials in pediatric patients (Table 2).
The results of a small phase 1 study of crizotinib in pediatric patients with ALK-positive ALCL (n = 26) or IMT (n = 14) were recently published. ALCL patients received crizotinib at a dose of 165 mg/m2, while IMT patients were given 100, 165, or 280 mg/m2. For the latter, the results were presented as a pooled cohort since safety and efficacy data were similar across dose levels. The overall response rate (ORR) was 83% for patients with ALCL and 86% for those with IMT. Grade 3/4 adverse events occurred in 83% and 71% of patients, respectively, and most commonly involved reduced neutrophil count.15
Most recently and perhaps most promisingly, fusions involving the neurotrophic tropomyosin receptor kinase (NTRK) gene have generated significant buzz. There are 3 NTRK genes, NTRK1, 2, and 3, which encode the TRKA, TRKB, and TRKC proteins, respectively.
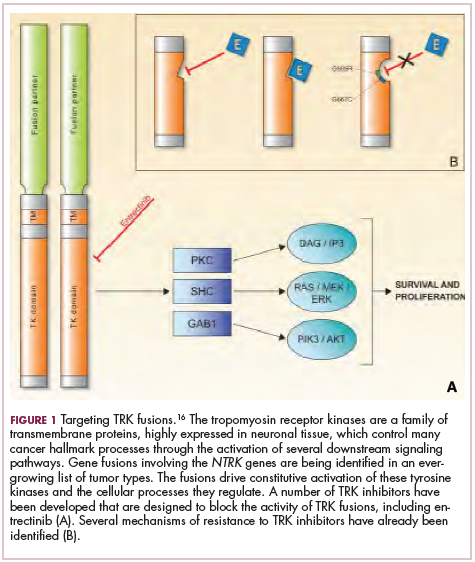
To date, 22 different partner genes have been identified that can fuse with the NTRK genes and, as with other kinase fusions, drive constitutive activation of the receptor proteins and downstream oncogenic signaling pathways, including the mitogen-activated protein kinase (MAPK) pathway (Figure 2).
NTRK fusions are being identified in an ever-growing number of cancer types, but are typically found in a small percentage of patients. However, in certain rare pediatric tumors, including congenital infantile fibrosarcoma and papillary thyroid cancer, they are found at much higher frequencies.
TRK inhibitors have been developed to target the fusion proteins and, given the spread of NTRK fusions across different types of cancers, they offer the most substantial promise as the next tumor agnostic cancer therapy – to treat patients based on the shared presence of a molecular aberration, irrespective of the type of cancer.16
The ongoing SCOUT trial is evaluating larotrectinib (LOXO-101) in pediatric patients. Among 24 patients (17 with NTRK fusions and 7 without) with infantile fibrosarcoma (47%), soft tissue sarcoma (41%) or papillary thyroid cancer (12%), the ORR was 93%, including complete response (CR) in 13% of patients.17
Preliminary results from an ongoing phase 1/2 study of entrectinib in pediatric patients with extracranial solid tumors were also recently presented at the annual meeting of the American Society for Clinical Oncology (ASCO). Among 15 evaluable patients enrolled to date, 3 have NTRK fusions and all experienced an objective response, with 1 (a patient with IMT) ongoing at 10 months.18
CAR T cells transformative in ALL
A variety of different types of immunotherapy have been tested in patients with pediatric cancers. In general, immunotherapy has proved less effective than in adult cancers, possibly because of the lower tumor mutation burden in pediatric cancers, which means there are likely fewer cancer antigens to provoke an anti-tumor immune response.
There are notable exceptions among the disappointments, however, and most exciting is the development of chimeric antigen receptor (CAR) T cells. CAR T cells fall into a category of immunotherapy known as adoptive cell therapy (ACT), in which immune cells are harvested from a patient and grown outside the body to increase their numbers before being reinfused into the patient.
In the case of CAR T-cell therapy, the cells are genetically engineered to express a CAR that endows them with tumor-targeting capabilities. To date, the development of CAR T cells has focused on the use of the CD19 antigen as a target, which is highly expressed on a variety of B-cell malignancies, including several of the most common forms of pediatric cancer. ASCO shined the spotlight on CAR T-cell therapy this year, naming it the Advance of the Year for 2018, saying that the treatment is “poised to transform childhood ALL.”19
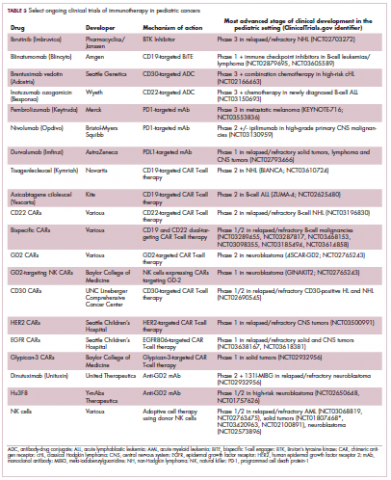
Two CD19-targeted CAR T-cell therapies – tisagenlecleucel and axicabtagene ciloleucel – were brought to market in 2017. Only tisagenlecleucel is approved in the pediatric ALL population, however, having been awarded approval for the treatment of patients aged up to 25 years whose disease is refractory to or relapsed after receiving at least 2 prior therapies. In the pivotal trial, complete responses were observed in more than 60% of patients.20 Clinical trials of both CAR T-cell therapies in pediatric ALL and non-Hodgkin lymphoma are ongoing (Table 3).
CD19 has also proven to be a promising target for other forms of immunotherapy, including a new type of antibody known as a bispecific T-cell engager (BiTE). In 2014, blinatumomab became the first BiTE to receive regulatory approval, for the treatment of adult patients with relapsed/refractory ALL. Blinatumomab also targets the CD3 protein on T cells and helps to bring cancer cells and cytotoxic immune cells into close enough proximity that an immunological synapse can be formed between the two, facilitating tumor cell killing.21
In 2016, the approved indication was expanded into the pediatric population based on the results of a phase 1/2 study in which the safety and efficacy of blinatumomab were evaluated in 93 pediatric patients with relapsed/refractory ALL. Among the 70 patients who received the recommended dose of 5µg/m2 a day for the first 7 days, followed by 15µg/m2 a day thereafter, 51% achieved complete remission within the first 2 cycles, 52% of whom achieved minimal residual disease (MRD).22 Most recently, the FDA expanded the indication for blinatumomab to include patients (both adults and children) who are in remission, but MRD positive.23Despite the dramatic responses, many patients relapse after treatment with CD19-targeted CAR T cells, and researchers have uncovered numerous mechanisms of resistance. Among them is the loss of the CD19 antigen on the surface of target cells, such that a CD19-positive tumor becomes CD19-negative after treatment, driving relapse.24-26Several strategies for overcoming CD19-negative relapse are already being investigated, including the development of CD22-targeted CAR T cells and bispecific CAR T cells that target both CD19 and CD22. The results of a first-in-human trial of anti-CD22 CAR T-cell therapy were recently published. Among 21 pediatric and adult patients with relapsed/refractory B-cell ALL who were treated with either 3 x 105 cells/kg, 1 x 106 cells/kg, or 3 x 106 cells/kg, complete responses were observed in 57%.27
Results from 15 pediatric patients enrolled in a trial evaluating CD22-targeted CAR T cells as salvage therapy for those who relapse after CD19-targeted CAR T cell therapy were presented at the recent Congress of the European Hematology Association in Stockholm, Sweden. Patients who had undergone a stem cell transplant received the CAR T cells at a dose of 0.9 x 105 cell/kg and those who had not undergone a transplant received a dose of 8.2 x 105 cells/kg. At 30 days after CAR T cell infusion, the CR rate was 80% and the treatment was well tolerated.28
More immunotherapy approvals
The immune checkpoint inhibitors, which work by blocking inhibitory receptors on the surface of T cells, have also had recent approvals in pediatric patient populations. Pembrolizumab and nivolumab, inhibitors of the programmed cell death receptor 1 (PD-1) protein, have both been approved for use in adult and pediatric patients (older than 12 years) with relapsed/refractory metastatic colorectal cancer (and other solid tumors in the case of pembrolizumab) that display defects in the mismatch repair pathway that fixes damaged DNA or in patients that have high levels of microsatellite instability. Both deficient mismatch repair and microsatellite instability–high can indicate a high mutation burden in a tumor, which may predict increased sensitivity to immunotherapy.29
The approval in pediatric patients in both of those instances, however, was not based on data in pediatric patient populations but extrapolated from adult patients. Pembrolizumab is also approved for the treatment of adults and pediatric patients with classical Hodgkin lymphoma (cHL) after 3 or more previous treatments, but once again efficacy in the pediatric population was inferred from clinical trials performed in adults. Most recently, pembrolizumab was approved for the treatment of adult and pediatric patients with relapsed or refractory primary mediastinal large B-cell lymphoma.30Ipilimumab, which targets a different T cell receptor – cytotoxic T lymphocyte antigen-4 (CTLA-4) – has been approved for the treatment of pediatric patients aged 12 years and older with metastatic melanoma. This expanded indication, following on from its approval in adult patients in 2011, was based on data from 2 trials in which objective responses were observed in 2 out of 17 patients, including 1 partial response that lasted 16 months.31Finally, antibody-drug conjugates (ADC), in which tumor antigen-targeting monoclonal antibodies are conjugated to cytotoxic payloads to combine the specificity of an antibody with the cell-killing potency of chemotherapy, have also generated some recent successes in pediatric cancers.
Gemtuzumab ozogamicin is an ADC that targets the CD33 protein, which is highly expressed on 85%-90% of cases of acute myeloid leukemia (AML). In 2000, it was the first ADC to be brought to market in the United States, but it was subsequently voluntarily withdrawn by the manufacturer in 2010 after confirmatory trials failed to show a survival benefit.
Recently, a meta-analysis of gemtuzumab ozogamicin trials suggested that the drug likely does improve long-term overall survival (OS) and reduce the risk of relapse and researchers developed an intermittent dosing schedule to help mitigate toxicity.32 This new dosing regimen received FDA approval in 2017 for the treatment of pediatric patients aged 2 years and older on the basis of 2 clinical trials.
In the MyloFrance-1 trial, 57 patients were administered 3 mg/m2 gemtuzumab ozogamicin on days 1, 4, and 7 followed by cytarabine consolidation therapy and demonstrated a 26% CR rate and median recurrence-free survival of 11.6 months. In the phase 3 AML-19 trial, 237 patients received gemtuzumab ozogamicin at a dose of 6 mg/m2 on day 1 and 3 mg/m2 on day 8 or best supportive care. Gemtuzumab ozogamicin improved OS from 3.6 to 4.9 months.33,34
Inotuzumab ozogamicin is a CD22-targeting ADC that has been FDA approved for the treatment of adult patients with relapsed/refractory B-cell precursor ALL since last year. The therapy has been available to pediatric patients through a compassionate access program, but it has not been extensively evaluated in this population. The results of a retrospective analysis of pediatric patients who received at least 1 dose of inotuzumab ozogamicin were presented at ASCO in 2017. Among 29 patients with heavily pretreated disease the CR rate was 62%, 72% of whom achieved MRD negativity.35
1. American Cancer Society. Key statistics for childhood cancers. https://www.cancer.org/cancer/cancer-in-children/key-statistics.html. Last revised September 10, 2018. Accessed September 16, 2018.
2. NHI/National Cancer Institute website. Unusual cancers of childhood treatment (PDQ) - Health Professional Version. https://www.cancer.gov/types/childhood-cancers/hp/unusual-cancers-childhood-pdq. Last updated August 28, 2018. Accessed September 8, 2018.
3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7-30.
4. Marshall GM, Carter DR, Cheung BB, et al. The prenatal origins of cancer. Nat Rev Cancer. 2014;14(4):277-289.
5. Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA, Jr, Kinzler KW. Cancer genome landscapes. Science. 2013;339(6127):1546-1558.
6. Ma X, Liu Y, Liu Y, et al. Pan-cancer genome and transcriptome analyses of 1,699 paediatric leukaemias and solid tumours. Nature. 2018;555:371.
7. Dupain C, Harttrampf AC, Urbinati G, Geoerger B, Massaad-Massade L. Relevance of fusion genes in pediatric cancers: toward precision medicine. Molec Ther Nucleic Acids. 2017;6:315-326.
8. Lemonick MD, Park A. New hope for cancer. http://content.time.com/time/world/article/0,8599,2047900-2,00.html. Published May 28, 2001. Last accessed September 13, 2018.
9. Iqbal N, Iqbal N. Imatinib: a breakthrough of targeted therapy in cancer. https://www.hindawi.com/journals/cherp/2014/357027/. Published May 19, 2014. Accessed September 16, 2018.
10. Gore L, Kearns PR, Martino MLd, et al. Dasatinib in pediatric patients with chronic myeloid leukemia in chronic phase: results from a phase II trial. J Clin Oncol. 2018;36(13):1330-1338.
11. Novartis press release. Novartis drug Tasigna approved by FDA to treat children with rare form of leukemia. 2018; https://www.novartis.com/news/media-releases/novartis-drug-tasignar-approved-fda-treat-children-rare-form-leukemia. Released March 22, 2018. Accessed September 16, 2018.
12. Takita J. The role of anaplastic lymphoma kinase in pediatric cancers. Cancer Sci. 2017;108(10):1913-1920.
13. Turner SD, Lamant L, Kenner L, Brugieres L. Anaplastic large cell lymphoma in paediatric and young adult patients. Br J Haematol. 2016;173(4):560-572.
14. Antonescu CR, Suurmeijer AJH, Zhang L, et al. Molecular characterization of inflammatory myofibroblastic tumors with frequent ALK and ROS1 fusions and rare novel RET gene rearrangement. Am J Surg Pathol. 2015;39(7):957-967.
15. Mosse YP, Voss SD, Lim MS, et al. Targeting ALK with crizotinib in pediatric anaplastic large cell lymphoma and inflammatory myofibroblastic tumor: a children's oncology group study. J Clin Oncol. 2017;35(28):3215-3221.
16. Amatu A, Sartore-Bianchi A, Siena S. NTRK gene fusions as novel targets of cancer therapy across multiple tumour types. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5070277/. Published online March 18, 2016. Accessed September 16, 2018.
17. [Behind paywall.] Laetsch TW, DuBois SG, Mascarenhas L, et al. Larotrectinib for paediatric solid tumours harbouring NTRK gene fusions: phase 1 results from a multicentre, open-label, phase 1/2 study. Lancet Oncol. 2018;19(5):705-714.
18. Desai AV, Brodeur GM, Foster J, et al. Phase 1 study of entrectinib (RXDX-101), a TRK, ROS1, and ALK inhibitor, in children, adolescents, and young adults with recurrent or refractory solid tumors. J Clin Oncol. 2018;36(suppl;):abstr 10536.
19. Heymach J, Krilov L, Alberg A, et al. Clinical cancer advances 2018: annual report on progress against cancer from the American Society of Clinical Oncology. J Clin Oncol. 2018;36(10):1020-1044.
20. Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. NEJM. 2018;378(5):439-448.
21. Wu J, Fu J, Zhang M, Liu D. Blinatumomab: a bispecific T cell engager (BiTE) antibody against CD19/CD3 for refractory acute lymphoid leukemia. J Hematol Oncol. 2015;8:104.
22. Stackelberg Av, Locatelli F, Zugmaier G, et al. Phase I/phase II study of blinatumomab in pediatric patients with relapsed/refractory acute lymphoblastic leukemia. J Clin Oncol. 2016;34(36):4381-4389.
23. Gokbuget N, Dombret H, Bonifacio M, et al. Blinatumomab for minimal residual disease in adults with B-cell precursor acute lymphoblastic leukemia. Blood. 2018;131(14):1522-1531.
24. Fischer J, Paret C, El Malki K, et al. CD19 isoforms enabling resistance to CART-19 immunotherapy are expressed in B-ALL patients at initial diagnosis. J Immunother. 2017;40(5):187-195.
25. Fousek K, Watanabe J, George A, et al. Targeting CD19-negative relapsed B-acute lymphoblastic leukemia using trivalent CAR T cells. J Clin Oncol. 2018;36(5_suppl):121-121.
26. Mejstríková E, Hrusak O, Borowitz MJ, et al. CD19-negative relapse of pediatric B-cell precursor acute lymphoblastic leukemia following blinatumomab treatment. Blood Cancer J. 2017;7(12):659.
27. Fry TJ, Shah NN, Orentas RJ, et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med. 2018;24(1):20-28.
28. Pan J, Deng B, Liu S, et al. Efficacy and safety of CD22-directed CAR T-cell therapy in 15 pediatric refractory or relapsed b acute lymphoblastic leukemia patients. Paper presented at 23rd Congress of the European Hematology Association 2018; Stockholm, Sweden.
29. Boyiadzis MM, Kirkwood JM, Marshall JL, Pritchard CC, Azad NS, Gulley JL. Significance and implications of FDA approval of pembrolizumab for biomarker-defined disease. J Immunother Cancer. 2018;6:35.
30. Drugs.com. Keytruda approval history. 2018; https://www.drugs.com/history/keytruda.html. Last update information not given. Accessed September 16, 2018.
31. Bristol Myers Squibb press release. US Food and Drug Administration expands approval of Yervoy (ipilimumab) to include pediatric patients 12 years and older with unresectable or metastatic melanoma. https://news.bms.com/press-release/corporatefinancial-news/us-food-and-drug-administration-expands-approval-yervoy-ipilim. Released July 24, 2017. Accessed September 16, 2018.
32. Hills RK, Castaigne S, Appelbaum FR, et al. Addition of gemtuzumab ozogamicin to induction chemotherapy in adult patients with acute myeloid leukaemia: a meta-analysis of individual patient data from randomised controlled trials. Lancet Oncol. 2014;15(9):986-996.
33. Amadori S, Suciu S, Selleslag D, et al. Gemtuzumab ozogamicin versus best supportive care in older patients with newly diagnosed acute myeloid leukemia unsuitable for intensive chemotherapy: results of the randomized phase III EORTC-GIMEMA AML-19 trial. J Clin Oncol. 2016;34(9):972-979.
34. Taksin AL, Legrand O, Raffoux E, et al. High efficacy and safety profile of fractionated doses of Mylotarg as induction therapy in patients with relapsed acute myeloblastic leukemia: a prospective study of the alfa group. Leukemia. 2007;21(1):66-71.
35. Bhojwani D, Sposto R, Shah N, et al. Inotuzumab ozogamicin in pediatric patients with relapsed/refractory acute lymphoblastic leukemia (R/R ALL). J Clin Oncol. 2017;35(15_suppl):10512-10512.
Although there have been significant improvements in patient outcomes for some forms of pediatric cancer, progress has been painfully slow for others. An increasing understanding of pediatric cancers is highlighting the unique molecular drivers and challenging the assumption that drugs developed in adults can be applied to children and young adults. Here, we discuss game-changing therapeutic advances and a shifting view of childhood cancers.
Unique genomic background
Although pediatric cancers are rare, representing just 1% of all new cancers diagnosed annually in the United States, they are the second leading cause of death in children aged 1 to 14 years. There are many different histological tumor types under the umbrella of childhood cancers, of which the most common are leukemias, central nervous system tumors, and lymphomas (Figure 1).1,2
Significant progress has been made in the treatment of certain pediatric cancers in recent decades, exemplified by pediatric acute lymphoblastic leukemia (ALL), which has been transformed from a virtually incurable cancer to one in which 5-year survival rates now reach up to 90%. In other forms of pediatric cancer, however, survival rates have stagnated and little progress has been made in the development of effective new therapies.3
Because of their rarity, pediatric cancers are difficult to study and adequate enrollment of children in clinical trials can be challenging. Pharmaceutical companies are often hesitant to test drugs in the pediatric population in patients who often cannot advocate for themselves. As a result, the activity of drugs developed in adult patients has often been inferred in pediatric patients with the same tumor type or molecular aberrations. However, as researchers have gathered more information about pediatric cancers, there has been increasing recognition of their unique attributes and the need for dedicated clinical trials in this patient population.
Pediatric cancers tend to be found in the developing mesodermic tissue, whereas adult cancers are more prevalent in the epithelial tissues. Genome sequencing studies have revealed a much lower mutational burden in pediatric cancers and the mechanisms of oncogenesis are also quite different; adult tumors can develop from a series of acquired gene mutations, but pediatric tumors tend to develop from a single catastrophic event.4,5
Even the same type of cancer in a pediatric and adult patient can be quite different, with very different underlying molecular mechanisms. In a recent genomic analysis of different types of pediatric cancer by researchers at St Jude’s Children’s Research Hospital, less than half of the identified mutated genes were found to be similar to those found in adult patients.6
A ‘magic bullet’?
Chromosomal rearrangements are common in pediatric cancers. This type of molecular abnormality can result in a fusion of 2 different genes when the chromosome breaks apart and the pieces join back together in a muddled order. If the genetic code fuses in a manner that is “readable” by the cell, then it can drive aberrant activation of one or both genes.7 Gene fusions often involve kinase enzymes that are essential players in cell signaling pathways regulating hallmark cancer processes, such as unchecked cell proliferation. The fusion drives the constitutive activation of the kinase and, thus, these downstream signaling pathways.
One of the first chromosomal rearrangements linked to cancer, BCR-ABL1 – more commonly known as the Philadelphia chromosome – results in aberrant activation of the ABL1 kinase. It is present in nearly all cases of chronic myeloid leukemia (CML) and 3% to 5% of patients with ALL, and thus became the central focus of targeted drug development. Imatinib was initially approved by the US Food and Drug Administration (FDA) in 2001 for the treatment of adult patients with CML and had such a significant impact on the treatment landscape that it made the cover of Time magazine as a “magic bullet” in the war on cancer.8
Approval was expanded into pediatric patients in 2006 and for pediatric patients with ALL in 2013. However, as with the use of most kinase inhibitors, tumors can evolve under the selective pressure of treatment, developing additional molecular abnormalities that drive resistance.9
Next-generation multikinase inhibitors that more potently inhibit the BCR-ABL1 fusion protein have been developed to provide additional treatment options for patients who become resistant to imatinib. Dasatinib and nilotinib are among several drugs that have recently been approved for pediatric cancer therapy (Table 1). Both therapies were approved to treat children with Philadelphia chromosome-positive CML in the chronic phase in either the front- or second-line setting after failure of imatinib.
The approval of dasatinib was based on data from 97 patients across 2 trials, 51 of whom were newly diagnosed and 46 previously treated with imatinib. Most of the patients were treated with dasatinib 60 mg/m2 once daily. After 2 years of follow-up, more than 95% of newly diagnosed patients and 82.6% of relapsed/refractory patients had complete cytogenetic response.10
Nilotinib was approved on the basis of findings from 2 clinical trials including 69 patients – 1 trial involving patients who were refractory to or relapsed after dasatinib and imatinib treatment, and 1 that included both relapsed/refractory and newly diagnosed patients. Patients received nilotinib 230 mg/m2 twice daily, rounded to the nearest 50-mg dose, in 28-day cycles. By cycle 12, the cumulative major molecular response rate (MMR) was 47.7% in patients with relapsed/refractory disease, and 64% in newly diagnosed patients.11 Clinical trials of both drugs in the pediatric setting are ongoing.
Other prominent gene fusions
Gene fusions involving the anaplastic lymphoma kinase (ALK) occur in patients with non–small-cell lung cancer and ALK inhibitors have provided an effective new treatment option for patients whose tumors display this abnormality.
ALK fusions are also a prominent feature of several kinds of pediatric cancers and ALK inhibitors offer promise in this setting.7,12 An NPM-ALK fusion is found in 90% of pediatric anaplastic large cell lymphoma (ALCL) cases,13 whereas a variety of ALK fusions are found in up to half of patients with inflammatory myofibroblastic tumor (IMT), a rare form of soft tissue sarcoma.14 ALK inhibitors are being tested in a variety of clinical trials in pediatric patients (Table 2).
The results of a small phase 1 study of crizotinib in pediatric patients with ALK-positive ALCL (n = 26) or IMT (n = 14) were recently published. ALCL patients received crizotinib at a dose of 165 mg/m2, while IMT patients were given 100, 165, or 280 mg/m2. For the latter, the results were presented as a pooled cohort since safety and efficacy data were similar across dose levels. The overall response rate (ORR) was 83% for patients with ALCL and 86% for those with IMT. Grade 3/4 adverse events occurred in 83% and 71% of patients, respectively, and most commonly involved reduced neutrophil count.15
Most recently and perhaps most promisingly, fusions involving the neurotrophic tropomyosin receptor kinase (NTRK) gene have generated significant buzz. There are 3 NTRK genes, NTRK1, 2, and 3, which encode the TRKA, TRKB, and TRKC proteins, respectively.
To date, 22 different partner genes have been identified that can fuse with the NTRK genes and, as with other kinase fusions, drive constitutive activation of the receptor proteins and downstream oncogenic signaling pathways, including the mitogen-activated protein kinase (MAPK) pathway (Figure 2).
NTRK fusions are being identified in an ever-growing number of cancer types, but are typically found in a small percentage of patients. However, in certain rare pediatric tumors, including congenital infantile fibrosarcoma and papillary thyroid cancer, they are found at much higher frequencies.
TRK inhibitors have been developed to target the fusion proteins and, given the spread of NTRK fusions across different types of cancers, they offer the most substantial promise as the next tumor agnostic cancer therapy – to treat patients based on the shared presence of a molecular aberration, irrespective of the type of cancer.16
The ongoing SCOUT trial is evaluating larotrectinib (LOXO-101) in pediatric patients. Among 24 patients (17 with NTRK fusions and 7 without) with infantile fibrosarcoma (47%), soft tissue sarcoma (41%) or papillary thyroid cancer (12%), the ORR was 93%, including complete response (CR) in 13% of patients.17
Preliminary results from an ongoing phase 1/2 study of entrectinib in pediatric patients with extracranial solid tumors were also recently presented at the annual meeting of the American Society for Clinical Oncology (ASCO). Among 15 evaluable patients enrolled to date, 3 have NTRK fusions and all experienced an objective response, with 1 (a patient with IMT) ongoing at 10 months.18
CAR T cells transformative in ALL
A variety of different types of immunotherapy have been tested in patients with pediatric cancers. In general, immunotherapy has proved less effective than in adult cancers, possibly because of the lower tumor mutation burden in pediatric cancers, which means there are likely fewer cancer antigens to provoke an anti-tumor immune response.
There are notable exceptions among the disappointments, however, and most exciting is the development of chimeric antigen receptor (CAR) T cells. CAR T cells fall into a category of immunotherapy known as adoptive cell therapy (ACT), in which immune cells are harvested from a patient and grown outside the body to increase their numbers before being reinfused into the patient.
In the case of CAR T-cell therapy, the cells are genetically engineered to express a CAR that endows them with tumor-targeting capabilities. To date, the development of CAR T cells has focused on the use of the CD19 antigen as a target, which is highly expressed on a variety of B-cell malignancies, including several of the most common forms of pediatric cancer. ASCO shined the spotlight on CAR T-cell therapy this year, naming it the Advance of the Year for 2018, saying that the treatment is “poised to transform childhood ALL.”19
Two CD19-targeted CAR T-cell therapies – tisagenlecleucel and axicabtagene ciloleucel – were brought to market in 2017. Only tisagenlecleucel is approved in the pediatric ALL population, however, having been awarded approval for the treatment of patients aged up to 25 years whose disease is refractory to or relapsed after receiving at least 2 prior therapies. In the pivotal trial, complete responses were observed in more than 60% of patients.20 Clinical trials of both CAR T-cell therapies in pediatric ALL and non-Hodgkin lymphoma are ongoing (Table 3).
CD19 has also proven to be a promising target for other forms of immunotherapy, including a new type of antibody known as a bispecific T-cell engager (BiTE). In 2014, blinatumomab became the first BiTE to receive regulatory approval, for the treatment of adult patients with relapsed/refractory ALL. Blinatumomab also targets the CD3 protein on T cells and helps to bring cancer cells and cytotoxic immune cells into close enough proximity that an immunological synapse can be formed between the two, facilitating tumor cell killing.21
In 2016, the approved indication was expanded into the pediatric population based on the results of a phase 1/2 study in which the safety and efficacy of blinatumomab were evaluated in 93 pediatric patients with relapsed/refractory ALL. Among the 70 patients who received the recommended dose of 5µg/m2 a day for the first 7 days, followed by 15µg/m2 a day thereafter, 51% achieved complete remission within the first 2 cycles, 52% of whom achieved minimal residual disease (MRD).22 Most recently, the FDA expanded the indication for blinatumomab to include patients (both adults and children) who are in remission, but MRD positive.23Despite the dramatic responses, many patients relapse after treatment with CD19-targeted CAR T cells, and researchers have uncovered numerous mechanisms of resistance. Among them is the loss of the CD19 antigen on the surface of target cells, such that a CD19-positive tumor becomes CD19-negative after treatment, driving relapse.24-26Several strategies for overcoming CD19-negative relapse are already being investigated, including the development of CD22-targeted CAR T cells and bispecific CAR T cells that target both CD19 and CD22. The results of a first-in-human trial of anti-CD22 CAR T-cell therapy were recently published. Among 21 pediatric and adult patients with relapsed/refractory B-cell ALL who were treated with either 3 x 105 cells/kg, 1 x 106 cells/kg, or 3 x 106 cells/kg, complete responses were observed in 57%.27
Results from 15 pediatric patients enrolled in a trial evaluating CD22-targeted CAR T cells as salvage therapy for those who relapse after CD19-targeted CAR T cell therapy were presented at the recent Congress of the European Hematology Association in Stockholm, Sweden. Patients who had undergone a stem cell transplant received the CAR T cells at a dose of 0.9 x 105 cell/kg and those who had not undergone a transplant received a dose of 8.2 x 105 cells/kg. At 30 days after CAR T cell infusion, the CR rate was 80% and the treatment was well tolerated.28
More immunotherapy approvals
The immune checkpoint inhibitors, which work by blocking inhibitory receptors on the surface of T cells, have also had recent approvals in pediatric patient populations. Pembrolizumab and nivolumab, inhibitors of the programmed cell death receptor 1 (PD-1) protein, have both been approved for use in adult and pediatric patients (older than 12 years) with relapsed/refractory metastatic colorectal cancer (and other solid tumors in the case of pembrolizumab) that display defects in the mismatch repair pathway that fixes damaged DNA or in patients that have high levels of microsatellite instability. Both deficient mismatch repair and microsatellite instability–high can indicate a high mutation burden in a tumor, which may predict increased sensitivity to immunotherapy.29
The approval in pediatric patients in both of those instances, however, was not based on data in pediatric patient populations but extrapolated from adult patients. Pembrolizumab is also approved for the treatment of adults and pediatric patients with classical Hodgkin lymphoma (cHL) after 3 or more previous treatments, but once again efficacy in the pediatric population was inferred from clinical trials performed in adults. Most recently, pembrolizumab was approved for the treatment of adult and pediatric patients with relapsed or refractory primary mediastinal large B-cell lymphoma.30Ipilimumab, which targets a different T cell receptor – cytotoxic T lymphocyte antigen-4 (CTLA-4) – has been approved for the treatment of pediatric patients aged 12 years and older with metastatic melanoma. This expanded indication, following on from its approval in adult patients in 2011, was based on data from 2 trials in which objective responses were observed in 2 out of 17 patients, including 1 partial response that lasted 16 months.31Finally, antibody-drug conjugates (ADC), in which tumor antigen-targeting monoclonal antibodies are conjugated to cytotoxic payloads to combine the specificity of an antibody with the cell-killing potency of chemotherapy, have also generated some recent successes in pediatric cancers.
Gemtuzumab ozogamicin is an ADC that targets the CD33 protein, which is highly expressed on 85%-90% of cases of acute myeloid leukemia (AML). In 2000, it was the first ADC to be brought to market in the United States, but it was subsequently voluntarily withdrawn by the manufacturer in 2010 after confirmatory trials failed to show a survival benefit.
Recently, a meta-analysis of gemtuzumab ozogamicin trials suggested that the drug likely does improve long-term overall survival (OS) and reduce the risk of relapse and researchers developed an intermittent dosing schedule to help mitigate toxicity.32 This new dosing regimen received FDA approval in 2017 for the treatment of pediatric patients aged 2 years and older on the basis of 2 clinical trials.
In the MyloFrance-1 trial, 57 patients were administered 3 mg/m2 gemtuzumab ozogamicin on days 1, 4, and 7 followed by cytarabine consolidation therapy and demonstrated a 26% CR rate and median recurrence-free survival of 11.6 months. In the phase 3 AML-19 trial, 237 patients received gemtuzumab ozogamicin at a dose of 6 mg/m2 on day 1 and 3 mg/m2 on day 8 or best supportive care. Gemtuzumab ozogamicin improved OS from 3.6 to 4.9 months.33,34
Inotuzumab ozogamicin is a CD22-targeting ADC that has been FDA approved for the treatment of adult patients with relapsed/refractory B-cell precursor ALL since last year. The therapy has been available to pediatric patients through a compassionate access program, but it has not been extensively evaluated in this population. The results of a retrospective analysis of pediatric patients who received at least 1 dose of inotuzumab ozogamicin were presented at ASCO in 2017. Among 29 patients with heavily pretreated disease the CR rate was 62%, 72% of whom achieved MRD negativity.35
Although there have been significant improvements in patient outcomes for some forms of pediatric cancer, progress has been painfully slow for others. An increasing understanding of pediatric cancers is highlighting the unique molecular drivers and challenging the assumption that drugs developed in adults can be applied to children and young adults. Here, we discuss game-changing therapeutic advances and a shifting view of childhood cancers.
Unique genomic background
Although pediatric cancers are rare, representing just 1% of all new cancers diagnosed annually in the United States, they are the second leading cause of death in children aged 1 to 14 years. There are many different histological tumor types under the umbrella of childhood cancers, of which the most common are leukemias, central nervous system tumors, and lymphomas (Figure 1).1,2
Significant progress has been made in the treatment of certain pediatric cancers in recent decades, exemplified by pediatric acute lymphoblastic leukemia (ALL), which has been transformed from a virtually incurable cancer to one in which 5-year survival rates now reach up to 90%. In other forms of pediatric cancer, however, survival rates have stagnated and little progress has been made in the development of effective new therapies.3
Because of their rarity, pediatric cancers are difficult to study and adequate enrollment of children in clinical trials can be challenging. Pharmaceutical companies are often hesitant to test drugs in the pediatric population in patients who often cannot advocate for themselves. As a result, the activity of drugs developed in adult patients has often been inferred in pediatric patients with the same tumor type or molecular aberrations. However, as researchers have gathered more information about pediatric cancers, there has been increasing recognition of their unique attributes and the need for dedicated clinical trials in this patient population.
Pediatric cancers tend to be found in the developing mesodermic tissue, whereas adult cancers are more prevalent in the epithelial tissues. Genome sequencing studies have revealed a much lower mutational burden in pediatric cancers and the mechanisms of oncogenesis are also quite different; adult tumors can develop from a series of acquired gene mutations, but pediatric tumors tend to develop from a single catastrophic event.4,5
Even the same type of cancer in a pediatric and adult patient can be quite different, with very different underlying molecular mechanisms. In a recent genomic analysis of different types of pediatric cancer by researchers at St Jude’s Children’s Research Hospital, less than half of the identified mutated genes were found to be similar to those found in adult patients.6
A ‘magic bullet’?
Chromosomal rearrangements are common in pediatric cancers. This type of molecular abnormality can result in a fusion of 2 different genes when the chromosome breaks apart and the pieces join back together in a muddled order. If the genetic code fuses in a manner that is “readable” by the cell, then it can drive aberrant activation of one or both genes.7 Gene fusions often involve kinase enzymes that are essential players in cell signaling pathways regulating hallmark cancer processes, such as unchecked cell proliferation. The fusion drives the constitutive activation of the kinase and, thus, these downstream signaling pathways.
One of the first chromosomal rearrangements linked to cancer, BCR-ABL1 – more commonly known as the Philadelphia chromosome – results in aberrant activation of the ABL1 kinase. It is present in nearly all cases of chronic myeloid leukemia (CML) and 3% to 5% of patients with ALL, and thus became the central focus of targeted drug development. Imatinib was initially approved by the US Food and Drug Administration (FDA) in 2001 for the treatment of adult patients with CML and had such a significant impact on the treatment landscape that it made the cover of Time magazine as a “magic bullet” in the war on cancer.8
Approval was expanded into pediatric patients in 2006 and for pediatric patients with ALL in 2013. However, as with the use of most kinase inhibitors, tumors can evolve under the selective pressure of treatment, developing additional molecular abnormalities that drive resistance.9
Next-generation multikinase inhibitors that more potently inhibit the BCR-ABL1 fusion protein have been developed to provide additional treatment options for patients who become resistant to imatinib. Dasatinib and nilotinib are among several drugs that have recently been approved for pediatric cancer therapy (Table 1). Both therapies were approved to treat children with Philadelphia chromosome-positive CML in the chronic phase in either the front- or second-line setting after failure of imatinib.
The approval of dasatinib was based on data from 97 patients across 2 trials, 51 of whom were newly diagnosed and 46 previously treated with imatinib. Most of the patients were treated with dasatinib 60 mg/m2 once daily. After 2 years of follow-up, more than 95% of newly diagnosed patients and 82.6% of relapsed/refractory patients had complete cytogenetic response.10
Nilotinib was approved on the basis of findings from 2 clinical trials including 69 patients – 1 trial involving patients who were refractory to or relapsed after dasatinib and imatinib treatment, and 1 that included both relapsed/refractory and newly diagnosed patients. Patients received nilotinib 230 mg/m2 twice daily, rounded to the nearest 50-mg dose, in 28-day cycles. By cycle 12, the cumulative major molecular response rate (MMR) was 47.7% in patients with relapsed/refractory disease, and 64% in newly diagnosed patients.11 Clinical trials of both drugs in the pediatric setting are ongoing.
Other prominent gene fusions
Gene fusions involving the anaplastic lymphoma kinase (ALK) occur in patients with non–small-cell lung cancer and ALK inhibitors have provided an effective new treatment option for patients whose tumors display this abnormality.
ALK fusions are also a prominent feature of several kinds of pediatric cancers and ALK inhibitors offer promise in this setting.7,12 An NPM-ALK fusion is found in 90% of pediatric anaplastic large cell lymphoma (ALCL) cases,13 whereas a variety of ALK fusions are found in up to half of patients with inflammatory myofibroblastic tumor (IMT), a rare form of soft tissue sarcoma.14 ALK inhibitors are being tested in a variety of clinical trials in pediatric patients (Table 2).
The results of a small phase 1 study of crizotinib in pediatric patients with ALK-positive ALCL (n = 26) or IMT (n = 14) were recently published. ALCL patients received crizotinib at a dose of 165 mg/m2, while IMT patients were given 100, 165, or 280 mg/m2. For the latter, the results were presented as a pooled cohort since safety and efficacy data were similar across dose levels. The overall response rate (ORR) was 83% for patients with ALCL and 86% for those with IMT. Grade 3/4 adverse events occurred in 83% and 71% of patients, respectively, and most commonly involved reduced neutrophil count.15
Most recently and perhaps most promisingly, fusions involving the neurotrophic tropomyosin receptor kinase (NTRK) gene have generated significant buzz. There are 3 NTRK genes, NTRK1, 2, and 3, which encode the TRKA, TRKB, and TRKC proteins, respectively.
To date, 22 different partner genes have been identified that can fuse with the NTRK genes and, as with other kinase fusions, drive constitutive activation of the receptor proteins and downstream oncogenic signaling pathways, including the mitogen-activated protein kinase (MAPK) pathway (Figure 2).
NTRK fusions are being identified in an ever-growing number of cancer types, but are typically found in a small percentage of patients. However, in certain rare pediatric tumors, including congenital infantile fibrosarcoma and papillary thyroid cancer, they are found at much higher frequencies.
TRK inhibitors have been developed to target the fusion proteins and, given the spread of NTRK fusions across different types of cancers, they offer the most substantial promise as the next tumor agnostic cancer therapy – to treat patients based on the shared presence of a molecular aberration, irrespective of the type of cancer.16
The ongoing SCOUT trial is evaluating larotrectinib (LOXO-101) in pediatric patients. Among 24 patients (17 with NTRK fusions and 7 without) with infantile fibrosarcoma (47%), soft tissue sarcoma (41%) or papillary thyroid cancer (12%), the ORR was 93%, including complete response (CR) in 13% of patients.17
Preliminary results from an ongoing phase 1/2 study of entrectinib in pediatric patients with extracranial solid tumors were also recently presented at the annual meeting of the American Society for Clinical Oncology (ASCO). Among 15 evaluable patients enrolled to date, 3 have NTRK fusions and all experienced an objective response, with 1 (a patient with IMT) ongoing at 10 months.18
CAR T cells transformative in ALL
A variety of different types of immunotherapy have been tested in patients with pediatric cancers. In general, immunotherapy has proved less effective than in adult cancers, possibly because of the lower tumor mutation burden in pediatric cancers, which means there are likely fewer cancer antigens to provoke an anti-tumor immune response.
There are notable exceptions among the disappointments, however, and most exciting is the development of chimeric antigen receptor (CAR) T cells. CAR T cells fall into a category of immunotherapy known as adoptive cell therapy (ACT), in which immune cells are harvested from a patient and grown outside the body to increase their numbers before being reinfused into the patient.
In the case of CAR T-cell therapy, the cells are genetically engineered to express a CAR that endows them with tumor-targeting capabilities. To date, the development of CAR T cells has focused on the use of the CD19 antigen as a target, which is highly expressed on a variety of B-cell malignancies, including several of the most common forms of pediatric cancer. ASCO shined the spotlight on CAR T-cell therapy this year, naming it the Advance of the Year for 2018, saying that the treatment is “poised to transform childhood ALL.”19
Two CD19-targeted CAR T-cell therapies – tisagenlecleucel and axicabtagene ciloleucel – were brought to market in 2017. Only tisagenlecleucel is approved in the pediatric ALL population, however, having been awarded approval for the treatment of patients aged up to 25 years whose disease is refractory to or relapsed after receiving at least 2 prior therapies. In the pivotal trial, complete responses were observed in more than 60% of patients.20 Clinical trials of both CAR T-cell therapies in pediatric ALL and non-Hodgkin lymphoma are ongoing (Table 3).
CD19 has also proven to be a promising target for other forms of immunotherapy, including a new type of antibody known as a bispecific T-cell engager (BiTE). In 2014, blinatumomab became the first BiTE to receive regulatory approval, for the treatment of adult patients with relapsed/refractory ALL. Blinatumomab also targets the CD3 protein on T cells and helps to bring cancer cells and cytotoxic immune cells into close enough proximity that an immunological synapse can be formed between the two, facilitating tumor cell killing.21
In 2016, the approved indication was expanded into the pediatric population based on the results of a phase 1/2 study in which the safety and efficacy of blinatumomab were evaluated in 93 pediatric patients with relapsed/refractory ALL. Among the 70 patients who received the recommended dose of 5µg/m2 a day for the first 7 days, followed by 15µg/m2 a day thereafter, 51% achieved complete remission within the first 2 cycles, 52% of whom achieved minimal residual disease (MRD).22 Most recently, the FDA expanded the indication for blinatumomab to include patients (both adults and children) who are in remission, but MRD positive.23Despite the dramatic responses, many patients relapse after treatment with CD19-targeted CAR T cells, and researchers have uncovered numerous mechanisms of resistance. Among them is the loss of the CD19 antigen on the surface of target cells, such that a CD19-positive tumor becomes CD19-negative after treatment, driving relapse.24-26Several strategies for overcoming CD19-negative relapse are already being investigated, including the development of CD22-targeted CAR T cells and bispecific CAR T cells that target both CD19 and CD22. The results of a first-in-human trial of anti-CD22 CAR T-cell therapy were recently published. Among 21 pediatric and adult patients with relapsed/refractory B-cell ALL who were treated with either 3 x 105 cells/kg, 1 x 106 cells/kg, or 3 x 106 cells/kg, complete responses were observed in 57%.27
Results from 15 pediatric patients enrolled in a trial evaluating CD22-targeted CAR T cells as salvage therapy for those who relapse after CD19-targeted CAR T cell therapy were presented at the recent Congress of the European Hematology Association in Stockholm, Sweden. Patients who had undergone a stem cell transplant received the CAR T cells at a dose of 0.9 x 105 cell/kg and those who had not undergone a transplant received a dose of 8.2 x 105 cells/kg. At 30 days after CAR T cell infusion, the CR rate was 80% and the treatment was well tolerated.28
More immunotherapy approvals
The immune checkpoint inhibitors, which work by blocking inhibitory receptors on the surface of T cells, have also had recent approvals in pediatric patient populations. Pembrolizumab and nivolumab, inhibitors of the programmed cell death receptor 1 (PD-1) protein, have both been approved for use in adult and pediatric patients (older than 12 years) with relapsed/refractory metastatic colorectal cancer (and other solid tumors in the case of pembrolizumab) that display defects in the mismatch repair pathway that fixes damaged DNA or in patients that have high levels of microsatellite instability. Both deficient mismatch repair and microsatellite instability–high can indicate a high mutation burden in a tumor, which may predict increased sensitivity to immunotherapy.29
The approval in pediatric patients in both of those instances, however, was not based on data in pediatric patient populations but extrapolated from adult patients. Pembrolizumab is also approved for the treatment of adults and pediatric patients with classical Hodgkin lymphoma (cHL) after 3 or more previous treatments, but once again efficacy in the pediatric population was inferred from clinical trials performed in adults. Most recently, pembrolizumab was approved for the treatment of adult and pediatric patients with relapsed or refractory primary mediastinal large B-cell lymphoma.30Ipilimumab, which targets a different T cell receptor – cytotoxic T lymphocyte antigen-4 (CTLA-4) – has been approved for the treatment of pediatric patients aged 12 years and older with metastatic melanoma. This expanded indication, following on from its approval in adult patients in 2011, was based on data from 2 trials in which objective responses were observed in 2 out of 17 patients, including 1 partial response that lasted 16 months.31Finally, antibody-drug conjugates (ADC), in which tumor antigen-targeting monoclonal antibodies are conjugated to cytotoxic payloads to combine the specificity of an antibody with the cell-killing potency of chemotherapy, have also generated some recent successes in pediatric cancers.
Gemtuzumab ozogamicin is an ADC that targets the CD33 protein, which is highly expressed on 85%-90% of cases of acute myeloid leukemia (AML). In 2000, it was the first ADC to be brought to market in the United States, but it was subsequently voluntarily withdrawn by the manufacturer in 2010 after confirmatory trials failed to show a survival benefit.
Recently, a meta-analysis of gemtuzumab ozogamicin trials suggested that the drug likely does improve long-term overall survival (OS) and reduce the risk of relapse and researchers developed an intermittent dosing schedule to help mitigate toxicity.32 This new dosing regimen received FDA approval in 2017 for the treatment of pediatric patients aged 2 years and older on the basis of 2 clinical trials.
In the MyloFrance-1 trial, 57 patients were administered 3 mg/m2 gemtuzumab ozogamicin on days 1, 4, and 7 followed by cytarabine consolidation therapy and demonstrated a 26% CR rate and median recurrence-free survival of 11.6 months. In the phase 3 AML-19 trial, 237 patients received gemtuzumab ozogamicin at a dose of 6 mg/m2 on day 1 and 3 mg/m2 on day 8 or best supportive care. Gemtuzumab ozogamicin improved OS from 3.6 to 4.9 months.33,34
Inotuzumab ozogamicin is a CD22-targeting ADC that has been FDA approved for the treatment of adult patients with relapsed/refractory B-cell precursor ALL since last year. The therapy has been available to pediatric patients through a compassionate access program, but it has not been extensively evaluated in this population. The results of a retrospective analysis of pediatric patients who received at least 1 dose of inotuzumab ozogamicin were presented at ASCO in 2017. Among 29 patients with heavily pretreated disease the CR rate was 62%, 72% of whom achieved MRD negativity.35
1. American Cancer Society. Key statistics for childhood cancers. https://www.cancer.org/cancer/cancer-in-children/key-statistics.html. Last revised September 10, 2018. Accessed September 16, 2018.
2. NHI/National Cancer Institute website. Unusual cancers of childhood treatment (PDQ) - Health Professional Version. https://www.cancer.gov/types/childhood-cancers/hp/unusual-cancers-childhood-pdq. Last updated August 28, 2018. Accessed September 8, 2018.
3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7-30.
4. Marshall GM, Carter DR, Cheung BB, et al. The prenatal origins of cancer. Nat Rev Cancer. 2014;14(4):277-289.
5. Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA, Jr, Kinzler KW. Cancer genome landscapes. Science. 2013;339(6127):1546-1558.
6. Ma X, Liu Y, Liu Y, et al. Pan-cancer genome and transcriptome analyses of 1,699 paediatric leukaemias and solid tumours. Nature. 2018;555:371.
7. Dupain C, Harttrampf AC, Urbinati G, Geoerger B, Massaad-Massade L. Relevance of fusion genes in pediatric cancers: toward precision medicine. Molec Ther Nucleic Acids. 2017;6:315-326.
8. Lemonick MD, Park A. New hope for cancer. http://content.time.com/time/world/article/0,8599,2047900-2,00.html. Published May 28, 2001. Last accessed September 13, 2018.
9. Iqbal N, Iqbal N. Imatinib: a breakthrough of targeted therapy in cancer. https://www.hindawi.com/journals/cherp/2014/357027/. Published May 19, 2014. Accessed September 16, 2018.
10. Gore L, Kearns PR, Martino MLd, et al. Dasatinib in pediatric patients with chronic myeloid leukemia in chronic phase: results from a phase II trial. J Clin Oncol. 2018;36(13):1330-1338.
11. Novartis press release. Novartis drug Tasigna approved by FDA to treat children with rare form of leukemia. 2018; https://www.novartis.com/news/media-releases/novartis-drug-tasignar-approved-fda-treat-children-rare-form-leukemia. Released March 22, 2018. Accessed September 16, 2018.
12. Takita J. The role of anaplastic lymphoma kinase in pediatric cancers. Cancer Sci. 2017;108(10):1913-1920.
13. Turner SD, Lamant L, Kenner L, Brugieres L. Anaplastic large cell lymphoma in paediatric and young adult patients. Br J Haematol. 2016;173(4):560-572.
14. Antonescu CR, Suurmeijer AJH, Zhang L, et al. Molecular characterization of inflammatory myofibroblastic tumors with frequent ALK and ROS1 fusions and rare novel RET gene rearrangement. Am J Surg Pathol. 2015;39(7):957-967.
15. Mosse YP, Voss SD, Lim MS, et al. Targeting ALK with crizotinib in pediatric anaplastic large cell lymphoma and inflammatory myofibroblastic tumor: a children's oncology group study. J Clin Oncol. 2017;35(28):3215-3221.
16. Amatu A, Sartore-Bianchi A, Siena S. NTRK gene fusions as novel targets of cancer therapy across multiple tumour types. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5070277/. Published online March 18, 2016. Accessed September 16, 2018.
17. [Behind paywall.] Laetsch TW, DuBois SG, Mascarenhas L, et al. Larotrectinib for paediatric solid tumours harbouring NTRK gene fusions: phase 1 results from a multicentre, open-label, phase 1/2 study. Lancet Oncol. 2018;19(5):705-714.
18. Desai AV, Brodeur GM, Foster J, et al. Phase 1 study of entrectinib (RXDX-101), a TRK, ROS1, and ALK inhibitor, in children, adolescents, and young adults with recurrent or refractory solid tumors. J Clin Oncol. 2018;36(suppl;):abstr 10536.
19. Heymach J, Krilov L, Alberg A, et al. Clinical cancer advances 2018: annual report on progress against cancer from the American Society of Clinical Oncology. J Clin Oncol. 2018;36(10):1020-1044.
20. Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. NEJM. 2018;378(5):439-448.
21. Wu J, Fu J, Zhang M, Liu D. Blinatumomab: a bispecific T cell engager (BiTE) antibody against CD19/CD3 for refractory acute lymphoid leukemia. J Hematol Oncol. 2015;8:104.
22. Stackelberg Av, Locatelli F, Zugmaier G, et al. Phase I/phase II study of blinatumomab in pediatric patients with relapsed/refractory acute lymphoblastic leukemia. J Clin Oncol. 2016;34(36):4381-4389.
23. Gokbuget N, Dombret H, Bonifacio M, et al. Blinatumomab for minimal residual disease in adults with B-cell precursor acute lymphoblastic leukemia. Blood. 2018;131(14):1522-1531.
24. Fischer J, Paret C, El Malki K, et al. CD19 isoforms enabling resistance to CART-19 immunotherapy are expressed in B-ALL patients at initial diagnosis. J Immunother. 2017;40(5):187-195.
25. Fousek K, Watanabe J, George A, et al. Targeting CD19-negative relapsed B-acute lymphoblastic leukemia using trivalent CAR T cells. J Clin Oncol. 2018;36(5_suppl):121-121.
26. Mejstríková E, Hrusak O, Borowitz MJ, et al. CD19-negative relapse of pediatric B-cell precursor acute lymphoblastic leukemia following blinatumomab treatment. Blood Cancer J. 2017;7(12):659.
27. Fry TJ, Shah NN, Orentas RJ, et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med. 2018;24(1):20-28.
28. Pan J, Deng B, Liu S, et al. Efficacy and safety of CD22-directed CAR T-cell therapy in 15 pediatric refractory or relapsed b acute lymphoblastic leukemia patients. Paper presented at 23rd Congress of the European Hematology Association 2018; Stockholm, Sweden.
29. Boyiadzis MM, Kirkwood JM, Marshall JL, Pritchard CC, Azad NS, Gulley JL. Significance and implications of FDA approval of pembrolizumab for biomarker-defined disease. J Immunother Cancer. 2018;6:35.
30. Drugs.com. Keytruda approval history. 2018; https://www.drugs.com/history/keytruda.html. Last update information not given. Accessed September 16, 2018.
31. Bristol Myers Squibb press release. US Food and Drug Administration expands approval of Yervoy (ipilimumab) to include pediatric patients 12 years and older with unresectable or metastatic melanoma. https://news.bms.com/press-release/corporatefinancial-news/us-food-and-drug-administration-expands-approval-yervoy-ipilim. Released July 24, 2017. Accessed September 16, 2018.
32. Hills RK, Castaigne S, Appelbaum FR, et al. Addition of gemtuzumab ozogamicin to induction chemotherapy in adult patients with acute myeloid leukaemia: a meta-analysis of individual patient data from randomised controlled trials. Lancet Oncol. 2014;15(9):986-996.
33. Amadori S, Suciu S, Selleslag D, et al. Gemtuzumab ozogamicin versus best supportive care in older patients with newly diagnosed acute myeloid leukemia unsuitable for intensive chemotherapy: results of the randomized phase III EORTC-GIMEMA AML-19 trial. J Clin Oncol. 2016;34(9):972-979.
34. Taksin AL, Legrand O, Raffoux E, et al. High efficacy and safety profile of fractionated doses of Mylotarg as induction therapy in patients with relapsed acute myeloblastic leukemia: a prospective study of the alfa group. Leukemia. 2007;21(1):66-71.
35. Bhojwani D, Sposto R, Shah N, et al. Inotuzumab ozogamicin in pediatric patients with relapsed/refractory acute lymphoblastic leukemia (R/R ALL). J Clin Oncol. 2017;35(15_suppl):10512-10512.
1. American Cancer Society. Key statistics for childhood cancers. https://www.cancer.org/cancer/cancer-in-children/key-statistics.html. Last revised September 10, 2018. Accessed September 16, 2018.
2. NHI/National Cancer Institute website. Unusual cancers of childhood treatment (PDQ) - Health Professional Version. https://www.cancer.gov/types/childhood-cancers/hp/unusual-cancers-childhood-pdq. Last updated August 28, 2018. Accessed September 8, 2018.
3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7-30.
4. Marshall GM, Carter DR, Cheung BB, et al. The prenatal origins of cancer. Nat Rev Cancer. 2014;14(4):277-289.
5. Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA, Jr, Kinzler KW. Cancer genome landscapes. Science. 2013;339(6127):1546-1558.
6. Ma X, Liu Y, Liu Y, et al. Pan-cancer genome and transcriptome analyses of 1,699 paediatric leukaemias and solid tumours. Nature. 2018;555:371.
7. Dupain C, Harttrampf AC, Urbinati G, Geoerger B, Massaad-Massade L. Relevance of fusion genes in pediatric cancers: toward precision medicine. Molec Ther Nucleic Acids. 2017;6:315-326.
8. Lemonick MD, Park A. New hope for cancer. http://content.time.com/time/world/article/0,8599,2047900-2,00.html. Published May 28, 2001. Last accessed September 13, 2018.
9. Iqbal N, Iqbal N. Imatinib: a breakthrough of targeted therapy in cancer. https://www.hindawi.com/journals/cherp/2014/357027/. Published May 19, 2014. Accessed September 16, 2018.
10. Gore L, Kearns PR, Martino MLd, et al. Dasatinib in pediatric patients with chronic myeloid leukemia in chronic phase: results from a phase II trial. J Clin Oncol. 2018;36(13):1330-1338.
11. Novartis press release. Novartis drug Tasigna approved by FDA to treat children with rare form of leukemia. 2018; https://www.novartis.com/news/media-releases/novartis-drug-tasignar-approved-fda-treat-children-rare-form-leukemia. Released March 22, 2018. Accessed September 16, 2018.
12. Takita J. The role of anaplastic lymphoma kinase in pediatric cancers. Cancer Sci. 2017;108(10):1913-1920.
13. Turner SD, Lamant L, Kenner L, Brugieres L. Anaplastic large cell lymphoma in paediatric and young adult patients. Br J Haematol. 2016;173(4):560-572.
14. Antonescu CR, Suurmeijer AJH, Zhang L, et al. Molecular characterization of inflammatory myofibroblastic tumors with frequent ALK and ROS1 fusions and rare novel RET gene rearrangement. Am J Surg Pathol. 2015;39(7):957-967.
15. Mosse YP, Voss SD, Lim MS, et al. Targeting ALK with crizotinib in pediatric anaplastic large cell lymphoma and inflammatory myofibroblastic tumor: a children's oncology group study. J Clin Oncol. 2017;35(28):3215-3221.
16. Amatu A, Sartore-Bianchi A, Siena S. NTRK gene fusions as novel targets of cancer therapy across multiple tumour types. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5070277/. Published online March 18, 2016. Accessed September 16, 2018.
17. [Behind paywall.] Laetsch TW, DuBois SG, Mascarenhas L, et al. Larotrectinib for paediatric solid tumours harbouring NTRK gene fusions: phase 1 results from a multicentre, open-label, phase 1/2 study. Lancet Oncol. 2018;19(5):705-714.
18. Desai AV, Brodeur GM, Foster J, et al. Phase 1 study of entrectinib (RXDX-101), a TRK, ROS1, and ALK inhibitor, in children, adolescents, and young adults with recurrent or refractory solid tumors. J Clin Oncol. 2018;36(suppl;):abstr 10536.
19. Heymach J, Krilov L, Alberg A, et al. Clinical cancer advances 2018: annual report on progress against cancer from the American Society of Clinical Oncology. J Clin Oncol. 2018;36(10):1020-1044.
20. Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. NEJM. 2018;378(5):439-448.
21. Wu J, Fu J, Zhang M, Liu D. Blinatumomab: a bispecific T cell engager (BiTE) antibody against CD19/CD3 for refractory acute lymphoid leukemia. J Hematol Oncol. 2015;8:104.
22. Stackelberg Av, Locatelli F, Zugmaier G, et al. Phase I/phase II study of blinatumomab in pediatric patients with relapsed/refractory acute lymphoblastic leukemia. J Clin Oncol. 2016;34(36):4381-4389.
23. Gokbuget N, Dombret H, Bonifacio M, et al. Blinatumomab for minimal residual disease in adults with B-cell precursor acute lymphoblastic leukemia. Blood. 2018;131(14):1522-1531.
24. Fischer J, Paret C, El Malki K, et al. CD19 isoforms enabling resistance to CART-19 immunotherapy are expressed in B-ALL patients at initial diagnosis. J Immunother. 2017;40(5):187-195.
25. Fousek K, Watanabe J, George A, et al. Targeting CD19-negative relapsed B-acute lymphoblastic leukemia using trivalent CAR T cells. J Clin Oncol. 2018;36(5_suppl):121-121.
26. Mejstríková E, Hrusak O, Borowitz MJ, et al. CD19-negative relapse of pediatric B-cell precursor acute lymphoblastic leukemia following blinatumomab treatment. Blood Cancer J. 2017;7(12):659.
27. Fry TJ, Shah NN, Orentas RJ, et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med. 2018;24(1):20-28.
28. Pan J, Deng B, Liu S, et al. Efficacy and safety of CD22-directed CAR T-cell therapy in 15 pediatric refractory or relapsed b acute lymphoblastic leukemia patients. Paper presented at 23rd Congress of the European Hematology Association 2018; Stockholm, Sweden.
29. Boyiadzis MM, Kirkwood JM, Marshall JL, Pritchard CC, Azad NS, Gulley JL. Significance and implications of FDA approval of pembrolizumab for biomarker-defined disease. J Immunother Cancer. 2018;6:35.
30. Drugs.com. Keytruda approval history. 2018; https://www.drugs.com/history/keytruda.html. Last update information not given. Accessed September 16, 2018.
31. Bristol Myers Squibb press release. US Food and Drug Administration expands approval of Yervoy (ipilimumab) to include pediatric patients 12 years and older with unresectable or metastatic melanoma. https://news.bms.com/press-release/corporatefinancial-news/us-food-and-drug-administration-expands-approval-yervoy-ipilim. Released July 24, 2017. Accessed September 16, 2018.
32. Hills RK, Castaigne S, Appelbaum FR, et al. Addition of gemtuzumab ozogamicin to induction chemotherapy in adult patients with acute myeloid leukaemia: a meta-analysis of individual patient data from randomised controlled trials. Lancet Oncol. 2014;15(9):986-996.
33. Amadori S, Suciu S, Selleslag D, et al. Gemtuzumab ozogamicin versus best supportive care in older patients with newly diagnosed acute myeloid leukemia unsuitable for intensive chemotherapy: results of the randomized phase III EORTC-GIMEMA AML-19 trial. J Clin Oncol. 2016;34(9):972-979.
34. Taksin AL, Legrand O, Raffoux E, et al. High efficacy and safety profile of fractionated doses of Mylotarg as induction therapy in patients with relapsed acute myeloblastic leukemia: a prospective study of the alfa group. Leukemia. 2007;21(1):66-71.
35. Bhojwani D, Sposto R, Shah N, et al. Inotuzumab ozogamicin in pediatric patients with relapsed/refractory acute lymphoblastic leukemia (R/R ALL). J Clin Oncol. 2017;35(15_suppl):10512-10512.
Prolonged survival in adenocarcinoma of unknown primary treated with chemoradiotherapy
Cancer of unknown primary (CUP) represents 3% to 5% of all cancer malignancies in the world.1 Since 2003, CUP has been divided into 2 subsets – favorable (20% of the cases) and unfavorable (80% of the cases) – based on histopathologic and clinical manifestations.2 The impact of locoregional therapies, such as surgery and radiation, in addition to systemic chemotherapy in adenocarcinomas of unknown primary is not well described in the literature.
Case presentation and summary
The patient was frustrated by the lack of diagnosis and extensive work-up and decided to travel to Bangladesh for several months. Upon her return in May 2015, the patient underwent dilation and curettage at an outside tertiary care center because of her persistently elevated beta-hCG levels (>500 mIU/mL; reference range for nonpregnant woman, <5 mIU/mL) that found no products of conception and excluded a malignant process. Endoscopy and colonoscopy at that time failed to reveal a primary tumor.
She was then referred to our institution. Her level of beta-hCG remained elevated, and another transvaginal ultrasound was performed but failed to reveal any masses or evidence of pregnancy. Mammogram and a breast ultrasound showed left breast lesions. Biopsy of the breast lesions was performed, and the pathology demonstrated fibrocystic changes.
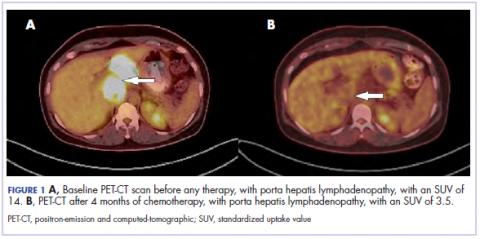
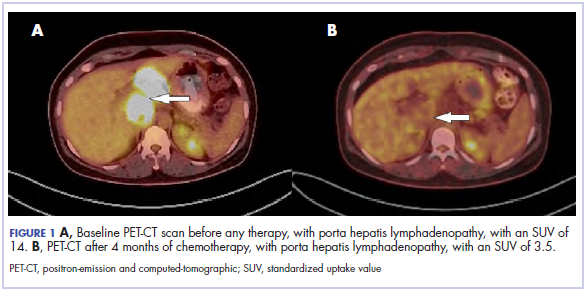
The results of a PET-CT scan in August 2015 showed a lobulated abdominal mass of 5.7 x 3.7 cm, consisting of multiple periportal necrotic lymph nodes with a standardized uptake value (SUV) of 14 (Figure 1A) and a 2.0-cm hypermetabolic retroperitoneal lymph node at the aortic bifurcation level with an SUV of 8.6. The SUV is a ratio of activity per unit volume of a region of interest to the activity per unit whole body volume. An SUV of 2.5 or higher is generally considered to be indicative of malignant tissue. We conducted a detailed review of the lymph node pathologic specimen. Immunohistochemical (IHC) studies were positive for CK7, CDX2, and EMA; focally positive for PR and mammaglobin; and negative for CK20, ER, TTF-1, and WT-1. Nonspecific staining was seen with BRST2, and there was no staining with GATA3. IHC stain for HER2-NEU was equivocal. Molecular analysis did not detect BRAF, KRAS, NRAS, and PIK3CA mutations, but did find a CTNNB1 mutation. The IHC pattern suggested pancreatobiliary origin of the tumor.3
Although serum tumor marker pattern of elevated beta-hCG, AFP, and LDH can be seen in germ cell tumors, the pathology evaluation did not favor a germ cell tumor. No site of origin was evident on radiographic evaluation, and the patient was diagnosed with CUP. Based on tumor metastatic distribution and the elevated beta-hCG level,4 we suspected that an undetected pancreatic primary was possible, and we therefore chose the folinic acid, fluorouracil, irinotecan, oxaliplatin (FOLFIRINOX) chemotherapy regimen for its evidence in prolonging survival in metastatic pancreatic cancer.5 At the initiation of treatment, the patient’s elevated tumor markers were beta-hCG 953.6 mIU/mL (reference for nonpregnant woman, <5 mIU/mL) and AFP 1,800.7 ng/mL (reference range, 0.0-9.0 ng/mL). The patient began FOLFIRINOX chemotherapy in August 2015 and after 1 month of treatment, her beta-hCG and AFP levels declined notably to 1.7 mIU/mL and 11.2 ng/mL, respectively. She completed a total of 8 cycles of FOLFIRINOX in November 2015. After completion of chemotherapy, the PET-CT scan showed a decrease in fluoro-D-glucose (FDG) uptake in the porta hepatis and retroperitoneal lymph nodes (Figure 1B). SUV in the porta hepatis lymph nodes declined from 14 to 3.5. The patient’s case was presented to our institution’s multidisciplinary tumor board, and the members deemed the risk of possible lymph node dissection surgery would outweigh the benefit. It was recommended that we proceed with radiotherapy to the residual lymph node stations.
During December 2015 through February 2016, the patient underwent a course of consolidative chemoradiation therapy to the intra-abdominal lymph nodes to a dose of 5,400 cGy in 30 fractions, with concurrent capecitabine as radiosensitizer, using intensity-modulated radiation therapy. During both chemotherapy and CRT, the patient experienced nausea, vomiting, fatigue, and anorexia, which were treated with antiemetics. She completed therapy without major complications and recovered completely from the adverse effects.
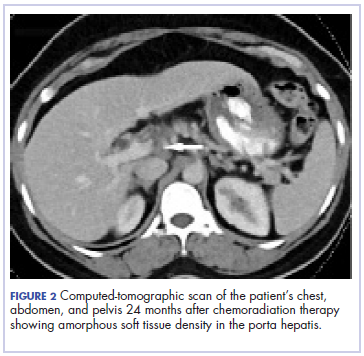
Five weeks after completion of chemoradiation, a restaging PET-CT scan showed a persistent small FDG uptake in the periportal region (SUV, 4.2). After CRT, tumor markers beta-hCG and AFP declined to less than 1.2 mIU/mL and less than 2.0 ng/mL, respectively.
Discussion
CUP is divided into favorable and unfavorable subsets.1 The favorable subset includes women with adenocarcinoma involving axillary lymph nodes, women with papillary adenocarcinoma of peritoneal cavity, and adenocarcinoma with a colon profile. The unfavorable subset includes moderate to poorly differentiated adenocarcinomas (64%) and undifferentiated tumors (36%). It involves the liver in 40% to 50% of the cases, followed by lymph nodes (35%), lungs (31%), bones (28%), and the brain (15%).1,2,6 Although data suggest that CUP with lymph-node–only metastases generally fall into an unfavorable prognosis group, our patient’s survival and progression-free survival have been especially prolonged.
The combined platinum–paclitaxel-based regimens are the treatment of choice in this unfavorable subset of CUP,7,8 with patients showing 16% to 38% response rates and median overall survival times of 6.5 to 13 months.7 Platinum–gemcitabine combinations can also be used as an alternative first-line regimen, with an overall response rate of 55% and a median survival of 8 months.9 The addition of the targeted agents bevacizumab and erlotinib to the carboplatin–paclitaxel combination, followed by bevacizumab and erlotinib maintenance, has been shown to yield a median survival of 12.6 months but was not meaningfully superior to historical studies with chemotherapy alone.10
We chose the FOLFIRINOX regimen for our patient. Conroy and colleagues reported a notably improved survival of 11.1 months with that combination chemotherapy in patients with metastatic pancreatic cancer compared with 6.8 months with gemcitabine alone.5 Given the possible pancreatobiliary site of tumor origin on IHC, the lymph node pattern of spread, and the patient’s young age and robust performance status, we felt that this multiagent systemic therapy would offer the best chance of prolonged survival. FOLFIRINOX includes a platinum agent, oxaliplatin, and platinum agents are recommended to be included in chemotherapy combinations for CUP.9,10 Although there is no data to suggest the superiority of a triplet regimen over a doublet regimen in a CUP, a triplet chemotherapy regimen may be considered in select cases.
There have been only a few reports showing the effectiveness of radiotherapy in the treatment of adenocarcinomas of unknown primary outside of the head and neck. Kubisch and colleagues have reported a case of a woman with hepatic adenocarcinoma of unknown primary that was treated with chemotherapy and surgery. Upon recurrence, the patient was then treated with selective internal radiation therapy (SIRT). She was still alive 3 years after diagnosis, and there had been no tumor relapse 21 months after SIRT.11 Shiota and colleagues have reported a case of a mediastinal lymph node CUP that was treated with docetaxel and cisplatin with concurrent thoracic radiation therapy.12 The patient remained free of symptoms without regrowth of the primary site 22 months after disease onset, and exploration of the body with enhanced and PET-CT scan showed no further abnormalities.
Other reports suggest that locoregional therapy such as surgery and radiation may be of benefit to select patients with CUP. A retrospective study by Löffler and colleagues reported that patients with a limited local involvement who received radical surgery had a median overall survival of 52.7 months compared with those who received radiation (median overall survival, 19.4 months) and those who received chemotherapy alone (median overall survival, 16 months).13 A case of a metastatic undifferentiated CUP also reported a long-term (>5 years), disease-free survivor after pancreaticoduodenectomy and systemic adjuvant chemotherapy.14
Our case further demonstrates that a multidisciplinary approach to CUP may lead to excellent clinical outcomes. Chemotherapy followed by chemoradiation in our patient increased local tumor control and survival.
Adenocarcinomas of unknown primary cases should involve management by a multidisciplinary team. Clinical trials incorporating locoregional therapies for CUP in addition to systemic therapy are warranted.
1. Pavlidis N, Khaled H, Gaafar R. A mini review on cancer of unknown primary site: a clinical puzzle for the oncologists. J Adv Res. 2015;6(3):375-382.
2. Pavlidis N, Briasoulis E, Hainsworth J, Greco FA. Diagnostic and therapeutic management of cancer of an unknown primary. Eur J Cancer. 2003;39(14):1990-2005.
3. Oien KA. Pathologic evaluation of unknown primary cancer. Semin Oncol. 2009;36(1):8-37.
4. Louhimo J, Alfthan H, Stenman UH, Hagland C. Serum HCG beta and CA 72-4 are stronger prognostic factors than CEA, CA 19-9 and CA 242 in pancreatic cancer. Oncology. 2004;66(2):126-131.
5. Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817-1825.
6. Pavlidis N, Pentheroudakis G. Cancer of unknown primary site. Lancet. 2012;379:1428-1435.
7. Bochtler T, Löffler H, Krämer A. Diagnosis and management of metastatic neoplasms with unknown primary. Semin Diagn Pathol. 2017;35(3):199-206.
8. Amela EY, Lauridant-Philippin G, Cousin S, Ryckewaert T, Adenis A, Penel N. Management of 'unfavourable' carcinoma of unknown primary site: synthesis of recent literature. Crit Rev Oncol Hematol. 2012;84(2):213-223.
9. Culine S, Lortholary A, Voigt J-J, et al. Cisplatin in combination with either gemcitabine or irinotecan in carcinomas of unknown primary site: results of a randomized phase II study--trial for the French study group on carcinomas of unknown primary (GEFCAPI 01). J Clin Oncol. 2003;21(18):3479-3482.
10. Hainsworth JD, Spigel DR, Thompson DS, et al. Paclitaxel/carboplatin plus bevacizumab/erlotinib in the first-line treatment of patients with carcinoma of unknown primary site. Oncologist. 2009;14(12):1189-1197.
11. Kubisch CH, Beigel F, Ihrler S, Goke B, Reiser MF, Hoffmann RT. Oesophageal ulceration after selective internal radiation therapy in a patient with carcinoma of unknown primary. Z Gastroenterol. 2010;48(5):546-550.
12. Shiota Y, Imai S, Sasaki N, et al. A case of mediastinal lymph node carcinoma of unknown primary site treated with docetaxel and cisplatin with concurrent thoracic radiation therapy. Acta Med Okayama. 2011;65(6):407-411.
13. Löffler H, Puthenparambil J, Hielscher T, Neben K, Krämer A. Patients with cancer of unknown primary: a retrospective analysis of 223 patients with adenocarcinoma or undifferentiated carcinoma. Dtsch Arztebl Int. 111(27-28):481-487.
14. Nakagawa Y, Todoroki T, Morishita Y, et al. A long-term survivor after pancreaticoduodenectomy for metastatic undifferentiated carcinoma of an unknown primary. Hepatogastroenterology. 2008;55(86-87):1557-1561.
15. Rodríguez-López JL, Toro-Bahamonde AM, Santiago-Méndez RJ, González-Cancel IF, Vélez-Cortés HA. An unusual case of colorectal adenocarcinoma presenting as an anterior mediastinal mass. Clin Colorectal Cancer. 2018;17(1):e115-e119.
Cancer of unknown primary (CUP) represents 3% to 5% of all cancer malignancies in the world.1 Since 2003, CUP has been divided into 2 subsets – favorable (20% of the cases) and unfavorable (80% of the cases) – based on histopathologic and clinical manifestations.2 The impact of locoregional therapies, such as surgery and radiation, in addition to systemic chemotherapy in adenocarcinomas of unknown primary is not well described in the literature.
Case presentation and summary
The patient was frustrated by the lack of diagnosis and extensive work-up and decided to travel to Bangladesh for several months. Upon her return in May 2015, the patient underwent dilation and curettage at an outside tertiary care center because of her persistently elevated beta-hCG levels (>500 mIU/mL; reference range for nonpregnant woman, <5 mIU/mL) that found no products of conception and excluded a malignant process. Endoscopy and colonoscopy at that time failed to reveal a primary tumor.
She was then referred to our institution. Her level of beta-hCG remained elevated, and another transvaginal ultrasound was performed but failed to reveal any masses or evidence of pregnancy. Mammogram and a breast ultrasound showed left breast lesions. Biopsy of the breast lesions was performed, and the pathology demonstrated fibrocystic changes.
The results of a PET-CT scan in August 2015 showed a lobulated abdominal mass of 5.7 x 3.7 cm, consisting of multiple periportal necrotic lymph nodes with a standardized uptake value (SUV) of 14 (Figure 1A) and a 2.0-cm hypermetabolic retroperitoneal lymph node at the aortic bifurcation level with an SUV of 8.6. The SUV is a ratio of activity per unit volume of a region of interest to the activity per unit whole body volume. An SUV of 2.5 or higher is generally considered to be indicative of malignant tissue. We conducted a detailed review of the lymph node pathologic specimen. Immunohistochemical (IHC) studies were positive for CK7, CDX2, and EMA; focally positive for PR and mammaglobin; and negative for CK20, ER, TTF-1, and WT-1. Nonspecific staining was seen with BRST2, and there was no staining with GATA3. IHC stain for HER2-NEU was equivocal. Molecular analysis did not detect BRAF, KRAS, NRAS, and PIK3CA mutations, but did find a CTNNB1 mutation. The IHC pattern suggested pancreatobiliary origin of the tumor.3
Although serum tumor marker pattern of elevated beta-hCG, AFP, and LDH can be seen in germ cell tumors, the pathology evaluation did not favor a germ cell tumor. No site of origin was evident on radiographic evaluation, and the patient was diagnosed with CUP. Based on tumor metastatic distribution and the elevated beta-hCG level,4 we suspected that an undetected pancreatic primary was possible, and we therefore chose the folinic acid, fluorouracil, irinotecan, oxaliplatin (FOLFIRINOX) chemotherapy regimen for its evidence in prolonging survival in metastatic pancreatic cancer.5 At the initiation of treatment, the patient’s elevated tumor markers were beta-hCG 953.6 mIU/mL (reference for nonpregnant woman, <5 mIU/mL) and AFP 1,800.7 ng/mL (reference range, 0.0-9.0 ng/mL). The patient began FOLFIRINOX chemotherapy in August 2015 and after 1 month of treatment, her beta-hCG and AFP levels declined notably to 1.7 mIU/mL and 11.2 ng/mL, respectively. She completed a total of 8 cycles of FOLFIRINOX in November 2015. After completion of chemotherapy, the PET-CT scan showed a decrease in fluoro-D-glucose (FDG) uptake in the porta hepatis and retroperitoneal lymph nodes (Figure 1B). SUV in the porta hepatis lymph nodes declined from 14 to 3.5. The patient’s case was presented to our institution’s multidisciplinary tumor board, and the members deemed the risk of possible lymph node dissection surgery would outweigh the benefit. It was recommended that we proceed with radiotherapy to the residual lymph node stations.
During December 2015 through February 2016, the patient underwent a course of consolidative chemoradiation therapy to the intra-abdominal lymph nodes to a dose of 5,400 cGy in 30 fractions, with concurrent capecitabine as radiosensitizer, using intensity-modulated radiation therapy. During both chemotherapy and CRT, the patient experienced nausea, vomiting, fatigue, and anorexia, which were treated with antiemetics. She completed therapy without major complications and recovered completely from the adverse effects.
Five weeks after completion of chemoradiation, a restaging PET-CT scan showed a persistent small FDG uptake in the periportal region (SUV, 4.2). After CRT, tumor markers beta-hCG and AFP declined to less than 1.2 mIU/mL and less than 2.0 ng/mL, respectively.
Discussion
CUP is divided into favorable and unfavorable subsets.1 The favorable subset includes women with adenocarcinoma involving axillary lymph nodes, women with papillary adenocarcinoma of peritoneal cavity, and adenocarcinoma with a colon profile. The unfavorable subset includes moderate to poorly differentiated adenocarcinomas (64%) and undifferentiated tumors (36%). It involves the liver in 40% to 50% of the cases, followed by lymph nodes (35%), lungs (31%), bones (28%), and the brain (15%).1,2,6 Although data suggest that CUP with lymph-node–only metastases generally fall into an unfavorable prognosis group, our patient’s survival and progression-free survival have been especially prolonged.
The combined platinum–paclitaxel-based regimens are the treatment of choice in this unfavorable subset of CUP,7,8 with patients showing 16% to 38% response rates and median overall survival times of 6.5 to 13 months.7 Platinum–gemcitabine combinations can also be used as an alternative first-line regimen, with an overall response rate of 55% and a median survival of 8 months.9 The addition of the targeted agents bevacizumab and erlotinib to the carboplatin–paclitaxel combination, followed by bevacizumab and erlotinib maintenance, has been shown to yield a median survival of 12.6 months but was not meaningfully superior to historical studies with chemotherapy alone.10
We chose the FOLFIRINOX regimen for our patient. Conroy and colleagues reported a notably improved survival of 11.1 months with that combination chemotherapy in patients with metastatic pancreatic cancer compared with 6.8 months with gemcitabine alone.5 Given the possible pancreatobiliary site of tumor origin on IHC, the lymph node pattern of spread, and the patient’s young age and robust performance status, we felt that this multiagent systemic therapy would offer the best chance of prolonged survival. FOLFIRINOX includes a platinum agent, oxaliplatin, and platinum agents are recommended to be included in chemotherapy combinations for CUP.9,10 Although there is no data to suggest the superiority of a triplet regimen over a doublet regimen in a CUP, a triplet chemotherapy regimen may be considered in select cases.
There have been only a few reports showing the effectiveness of radiotherapy in the treatment of adenocarcinomas of unknown primary outside of the head and neck. Kubisch and colleagues have reported a case of a woman with hepatic adenocarcinoma of unknown primary that was treated with chemotherapy and surgery. Upon recurrence, the patient was then treated with selective internal radiation therapy (SIRT). She was still alive 3 years after diagnosis, and there had been no tumor relapse 21 months after SIRT.11 Shiota and colleagues have reported a case of a mediastinal lymph node CUP that was treated with docetaxel and cisplatin with concurrent thoracic radiation therapy.12 The patient remained free of symptoms without regrowth of the primary site 22 months after disease onset, and exploration of the body with enhanced and PET-CT scan showed no further abnormalities.
Other reports suggest that locoregional therapy such as surgery and radiation may be of benefit to select patients with CUP. A retrospective study by Löffler and colleagues reported that patients with a limited local involvement who received radical surgery had a median overall survival of 52.7 months compared with those who received radiation (median overall survival, 19.4 months) and those who received chemotherapy alone (median overall survival, 16 months).13 A case of a metastatic undifferentiated CUP also reported a long-term (>5 years), disease-free survivor after pancreaticoduodenectomy and systemic adjuvant chemotherapy.14
Our case further demonstrates that a multidisciplinary approach to CUP may lead to excellent clinical outcomes. Chemotherapy followed by chemoradiation in our patient increased local tumor control and survival.
Adenocarcinomas of unknown primary cases should involve management by a multidisciplinary team. Clinical trials incorporating locoregional therapies for CUP in addition to systemic therapy are warranted.
Cancer of unknown primary (CUP) represents 3% to 5% of all cancer malignancies in the world.1 Since 2003, CUP has been divided into 2 subsets – favorable (20% of the cases) and unfavorable (80% of the cases) – based on histopathologic and clinical manifestations.2 The impact of locoregional therapies, such as surgery and radiation, in addition to systemic chemotherapy in adenocarcinomas of unknown primary is not well described in the literature.
Case presentation and summary
The patient was frustrated by the lack of diagnosis and extensive work-up and decided to travel to Bangladesh for several months. Upon her return in May 2015, the patient underwent dilation and curettage at an outside tertiary care center because of her persistently elevated beta-hCG levels (>500 mIU/mL; reference range for nonpregnant woman, <5 mIU/mL) that found no products of conception and excluded a malignant process. Endoscopy and colonoscopy at that time failed to reveal a primary tumor.
She was then referred to our institution. Her level of beta-hCG remained elevated, and another transvaginal ultrasound was performed but failed to reveal any masses or evidence of pregnancy. Mammogram and a breast ultrasound showed left breast lesions. Biopsy of the breast lesions was performed, and the pathology demonstrated fibrocystic changes.
The results of a PET-CT scan in August 2015 showed a lobulated abdominal mass of 5.7 x 3.7 cm, consisting of multiple periportal necrotic lymph nodes with a standardized uptake value (SUV) of 14 (Figure 1A) and a 2.0-cm hypermetabolic retroperitoneal lymph node at the aortic bifurcation level with an SUV of 8.6. The SUV is a ratio of activity per unit volume of a region of interest to the activity per unit whole body volume. An SUV of 2.5 or higher is generally considered to be indicative of malignant tissue. We conducted a detailed review of the lymph node pathologic specimen. Immunohistochemical (IHC) studies were positive for CK7, CDX2, and EMA; focally positive for PR and mammaglobin; and negative for CK20, ER, TTF-1, and WT-1. Nonspecific staining was seen with BRST2, and there was no staining with GATA3. IHC stain for HER2-NEU was equivocal. Molecular analysis did not detect BRAF, KRAS, NRAS, and PIK3CA mutations, but did find a CTNNB1 mutation. The IHC pattern suggested pancreatobiliary origin of the tumor.3
Although serum tumor marker pattern of elevated beta-hCG, AFP, and LDH can be seen in germ cell tumors, the pathology evaluation did not favor a germ cell tumor. No site of origin was evident on radiographic evaluation, and the patient was diagnosed with CUP. Based on tumor metastatic distribution and the elevated beta-hCG level,4 we suspected that an undetected pancreatic primary was possible, and we therefore chose the folinic acid, fluorouracil, irinotecan, oxaliplatin (FOLFIRINOX) chemotherapy regimen for its evidence in prolonging survival in metastatic pancreatic cancer.5 At the initiation of treatment, the patient’s elevated tumor markers were beta-hCG 953.6 mIU/mL (reference for nonpregnant woman, <5 mIU/mL) and AFP 1,800.7 ng/mL (reference range, 0.0-9.0 ng/mL). The patient began FOLFIRINOX chemotherapy in August 2015 and after 1 month of treatment, her beta-hCG and AFP levels declined notably to 1.7 mIU/mL and 11.2 ng/mL, respectively. She completed a total of 8 cycles of FOLFIRINOX in November 2015. After completion of chemotherapy, the PET-CT scan showed a decrease in fluoro-D-glucose (FDG) uptake in the porta hepatis and retroperitoneal lymph nodes (Figure 1B). SUV in the porta hepatis lymph nodes declined from 14 to 3.5. The patient’s case was presented to our institution’s multidisciplinary tumor board, and the members deemed the risk of possible lymph node dissection surgery would outweigh the benefit. It was recommended that we proceed with radiotherapy to the residual lymph node stations.
During December 2015 through February 2016, the patient underwent a course of consolidative chemoradiation therapy to the intra-abdominal lymph nodes to a dose of 5,400 cGy in 30 fractions, with concurrent capecitabine as radiosensitizer, using intensity-modulated radiation therapy. During both chemotherapy and CRT, the patient experienced nausea, vomiting, fatigue, and anorexia, which were treated with antiemetics. She completed therapy without major complications and recovered completely from the adverse effects.
Five weeks after completion of chemoradiation, a restaging PET-CT scan showed a persistent small FDG uptake in the periportal region (SUV, 4.2). After CRT, tumor markers beta-hCG and AFP declined to less than 1.2 mIU/mL and less than 2.0 ng/mL, respectively.
Discussion
CUP is divided into favorable and unfavorable subsets.1 The favorable subset includes women with adenocarcinoma involving axillary lymph nodes, women with papillary adenocarcinoma of peritoneal cavity, and adenocarcinoma with a colon profile. The unfavorable subset includes moderate to poorly differentiated adenocarcinomas (64%) and undifferentiated tumors (36%). It involves the liver in 40% to 50% of the cases, followed by lymph nodes (35%), lungs (31%), bones (28%), and the brain (15%).1,2,6 Although data suggest that CUP with lymph-node–only metastases generally fall into an unfavorable prognosis group, our patient’s survival and progression-free survival have been especially prolonged.
The combined platinum–paclitaxel-based regimens are the treatment of choice in this unfavorable subset of CUP,7,8 with patients showing 16% to 38% response rates and median overall survival times of 6.5 to 13 months.7 Platinum–gemcitabine combinations can also be used as an alternative first-line regimen, with an overall response rate of 55% and a median survival of 8 months.9 The addition of the targeted agents bevacizumab and erlotinib to the carboplatin–paclitaxel combination, followed by bevacizumab and erlotinib maintenance, has been shown to yield a median survival of 12.6 months but was not meaningfully superior to historical studies with chemotherapy alone.10
We chose the FOLFIRINOX regimen for our patient. Conroy and colleagues reported a notably improved survival of 11.1 months with that combination chemotherapy in patients with metastatic pancreatic cancer compared with 6.8 months with gemcitabine alone.5 Given the possible pancreatobiliary site of tumor origin on IHC, the lymph node pattern of spread, and the patient’s young age and robust performance status, we felt that this multiagent systemic therapy would offer the best chance of prolonged survival. FOLFIRINOX includes a platinum agent, oxaliplatin, and platinum agents are recommended to be included in chemotherapy combinations for CUP.9,10 Although there is no data to suggest the superiority of a triplet regimen over a doublet regimen in a CUP, a triplet chemotherapy regimen may be considered in select cases.
There have been only a few reports showing the effectiveness of radiotherapy in the treatment of adenocarcinomas of unknown primary outside of the head and neck. Kubisch and colleagues have reported a case of a woman with hepatic adenocarcinoma of unknown primary that was treated with chemotherapy and surgery. Upon recurrence, the patient was then treated with selective internal radiation therapy (SIRT). She was still alive 3 years after diagnosis, and there had been no tumor relapse 21 months after SIRT.11 Shiota and colleagues have reported a case of a mediastinal lymph node CUP that was treated with docetaxel and cisplatin with concurrent thoracic radiation therapy.12 The patient remained free of symptoms without regrowth of the primary site 22 months after disease onset, and exploration of the body with enhanced and PET-CT scan showed no further abnormalities.
Other reports suggest that locoregional therapy such as surgery and radiation may be of benefit to select patients with CUP. A retrospective study by Löffler and colleagues reported that patients with a limited local involvement who received radical surgery had a median overall survival of 52.7 months compared with those who received radiation (median overall survival, 19.4 months) and those who received chemotherapy alone (median overall survival, 16 months).13 A case of a metastatic undifferentiated CUP also reported a long-term (>5 years), disease-free survivor after pancreaticoduodenectomy and systemic adjuvant chemotherapy.14
Our case further demonstrates that a multidisciplinary approach to CUP may lead to excellent clinical outcomes. Chemotherapy followed by chemoradiation in our patient increased local tumor control and survival.
Adenocarcinomas of unknown primary cases should involve management by a multidisciplinary team. Clinical trials incorporating locoregional therapies for CUP in addition to systemic therapy are warranted.
1. Pavlidis N, Khaled H, Gaafar R. A mini review on cancer of unknown primary site: a clinical puzzle for the oncologists. J Adv Res. 2015;6(3):375-382.
2. Pavlidis N, Briasoulis E, Hainsworth J, Greco FA. Diagnostic and therapeutic management of cancer of an unknown primary. Eur J Cancer. 2003;39(14):1990-2005.
3. Oien KA. Pathologic evaluation of unknown primary cancer. Semin Oncol. 2009;36(1):8-37.
4. Louhimo J, Alfthan H, Stenman UH, Hagland C. Serum HCG beta and CA 72-4 are stronger prognostic factors than CEA, CA 19-9 and CA 242 in pancreatic cancer. Oncology. 2004;66(2):126-131.
5. Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817-1825.
6. Pavlidis N, Pentheroudakis G. Cancer of unknown primary site. Lancet. 2012;379:1428-1435.
7. Bochtler T, Löffler H, Krämer A. Diagnosis and management of metastatic neoplasms with unknown primary. Semin Diagn Pathol. 2017;35(3):199-206.
8. Amela EY, Lauridant-Philippin G, Cousin S, Ryckewaert T, Adenis A, Penel N. Management of 'unfavourable' carcinoma of unknown primary site: synthesis of recent literature. Crit Rev Oncol Hematol. 2012;84(2):213-223.
9. Culine S, Lortholary A, Voigt J-J, et al. Cisplatin in combination with either gemcitabine or irinotecan in carcinomas of unknown primary site: results of a randomized phase II study--trial for the French study group on carcinomas of unknown primary (GEFCAPI 01). J Clin Oncol. 2003;21(18):3479-3482.
10. Hainsworth JD, Spigel DR, Thompson DS, et al. Paclitaxel/carboplatin plus bevacizumab/erlotinib in the first-line treatment of patients with carcinoma of unknown primary site. Oncologist. 2009;14(12):1189-1197.
11. Kubisch CH, Beigel F, Ihrler S, Goke B, Reiser MF, Hoffmann RT. Oesophageal ulceration after selective internal radiation therapy in a patient with carcinoma of unknown primary. Z Gastroenterol. 2010;48(5):546-550.
12. Shiota Y, Imai S, Sasaki N, et al. A case of mediastinal lymph node carcinoma of unknown primary site treated with docetaxel and cisplatin with concurrent thoracic radiation therapy. Acta Med Okayama. 2011;65(6):407-411.
13. Löffler H, Puthenparambil J, Hielscher T, Neben K, Krämer A. Patients with cancer of unknown primary: a retrospective analysis of 223 patients with adenocarcinoma or undifferentiated carcinoma. Dtsch Arztebl Int. 111(27-28):481-487.
14. Nakagawa Y, Todoroki T, Morishita Y, et al. A long-term survivor after pancreaticoduodenectomy for metastatic undifferentiated carcinoma of an unknown primary. Hepatogastroenterology. 2008;55(86-87):1557-1561.
15. Rodríguez-López JL, Toro-Bahamonde AM, Santiago-Méndez RJ, González-Cancel IF, Vélez-Cortés HA. An unusual case of colorectal adenocarcinoma presenting as an anterior mediastinal mass. Clin Colorectal Cancer. 2018;17(1):e115-e119.
1. Pavlidis N, Khaled H, Gaafar R. A mini review on cancer of unknown primary site: a clinical puzzle for the oncologists. J Adv Res. 2015;6(3):375-382.
2. Pavlidis N, Briasoulis E, Hainsworth J, Greco FA. Diagnostic and therapeutic management of cancer of an unknown primary. Eur J Cancer. 2003;39(14):1990-2005.
3. Oien KA. Pathologic evaluation of unknown primary cancer. Semin Oncol. 2009;36(1):8-37.
4. Louhimo J, Alfthan H, Stenman UH, Hagland C. Serum HCG beta and CA 72-4 are stronger prognostic factors than CEA, CA 19-9 and CA 242 in pancreatic cancer. Oncology. 2004;66(2):126-131.
5. Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817-1825.
6. Pavlidis N, Pentheroudakis G. Cancer of unknown primary site. Lancet. 2012;379:1428-1435.
7. Bochtler T, Löffler H, Krämer A. Diagnosis and management of metastatic neoplasms with unknown primary. Semin Diagn Pathol. 2017;35(3):199-206.
8. Amela EY, Lauridant-Philippin G, Cousin S, Ryckewaert T, Adenis A, Penel N. Management of 'unfavourable' carcinoma of unknown primary site: synthesis of recent literature. Crit Rev Oncol Hematol. 2012;84(2):213-223.
9. Culine S, Lortholary A, Voigt J-J, et al. Cisplatin in combination with either gemcitabine or irinotecan in carcinomas of unknown primary site: results of a randomized phase II study--trial for the French study group on carcinomas of unknown primary (GEFCAPI 01). J Clin Oncol. 2003;21(18):3479-3482.
10. Hainsworth JD, Spigel DR, Thompson DS, et al. Paclitaxel/carboplatin plus bevacizumab/erlotinib in the first-line treatment of patients with carcinoma of unknown primary site. Oncologist. 2009;14(12):1189-1197.
11. Kubisch CH, Beigel F, Ihrler S, Goke B, Reiser MF, Hoffmann RT. Oesophageal ulceration after selective internal radiation therapy in a patient with carcinoma of unknown primary. Z Gastroenterol. 2010;48(5):546-550.
12. Shiota Y, Imai S, Sasaki N, et al. A case of mediastinal lymph node carcinoma of unknown primary site treated with docetaxel and cisplatin with concurrent thoracic radiation therapy. Acta Med Okayama. 2011;65(6):407-411.
13. Löffler H, Puthenparambil J, Hielscher T, Neben K, Krämer A. Patients with cancer of unknown primary: a retrospective analysis of 223 patients with adenocarcinoma or undifferentiated carcinoma. Dtsch Arztebl Int. 111(27-28):481-487.
14. Nakagawa Y, Todoroki T, Morishita Y, et al. A long-term survivor after pancreaticoduodenectomy for metastatic undifferentiated carcinoma of an unknown primary. Hepatogastroenterology. 2008;55(86-87):1557-1561.
15. Rodríguez-López JL, Toro-Bahamonde AM, Santiago-Méndez RJ, González-Cancel IF, Vélez-Cortés HA. An unusual case of colorectal adenocarcinoma presenting as an anterior mediastinal mass. Clin Colorectal Cancer. 2018;17(1):e115-e119.
Marriage predicts for survival in patients with stage III non–small-cell lung cancer
Non–small-cell lung cancer (NSCLC) remains the leading cause of cancer death in the United States, where 29% of patients will present with stage III disease.1,2 Ongoing research efforts seek to improve these outcomes using novel systemic therapy options or modern radiation techniques. However, there have also been recent studies showing the importance of marital and/or partner status on clinical outcomes.3-7 For example, in a large Surveillance, Epidemiology, and End Results (SEER) analysis of 734,889 patients diagnosed with several types of cancer (including lung cancer), patients identified as married were less likely to present with metastatic disease, more likely to receive definitive therapy, and had superior cancer-related mortality even after adjusting for other variables such as cancer stage and treatment when compared with single patients.3 Population-based assessments are important in relaying information about trends and general outcomes based on marital status, but because they are large, they often lack patient-specific information such as nutrition, immunologic status, and variability in treatment paradigms, all of which can independently have an impact on overall survival (OS) in stage III NSCLC.8-10 In addition, population analyses have typically included patients of all cancer stages and hence involved a multitude of treatment approaches ranging from curative to palliative. There are limited well-annotated institutional data on the association of marital status on nonmetastatic, locally advanced (LA-NSCLC) in the setting of National Comprehensive Cancer Network-guided, standard-of-care definitive treatment.
The objective of this analysis is to evaluate the effect of marital status on OS and freedom from recurrence (FFR) in patients with stage III NSCLC who were treated at a National Cancer Institute–designated cancer center with curative intent from 2000 through 2013. We performed a detailed multivariate analysis (MVA) of patient-, disease-, and treatment-specific factors, including the interaction with racial, nutritional, and immunologic status, which to our knowledge has not been previously reported, to comprehensively evaluate the benefit of marital status in patients with LA-NSCLC.
Methods
Patient population and treatment
From January 2000 through December 2013, 355 patients diagnosed with clinical stage III NSCLC (American Joint Committee on Cancer 7th edition) were definitively treated at the University of Maryland in Baltimore, Maryland. Their clinical data were retrospectively analyzed under internal review board approval (GCC 1175, Thoracic Oncology Database). All of the patients were evaluated before treatment by a multidisciplinary team consisting of thoracic surgeons and medical and radiation oncologists. Before treatment, the patients underwent standard work-up, which included systemic imaging with positron-emission (PET), computed-tomographic (CT), PET–CT, and/or bone scan, brain imaging consisting of magnetic-resonance imaging or CT with contrast, and routine blood. Patients had documentation of mediastinal disease by either imaging, mediastinoscopy, or endobronchial ultrasound biopsy.
Definitive therapy was administered using the backbone of chemoradiation therapy (CRT) with (trimodality) or without (bimodality) surgical resection. Concurrent CRT was typically administered with weekly carboplatin–paclitaxel (areas under the curve [AUCs], 2 and 50 mg/m2, respectively) and was generally followed with 2 cycles of consolidative treatment with definitive doses of carboplatin–paclitaxel (AUCs, 5-6 and 200-225 mg/m2, respectively) as tolerated. The entire cohort was also assessed for possible trimodality therapy at the time of initial diagnosis, and patients who were potential surgical candidates were reassessed for mediastinal nodal clearance following repeat radiographic staging after full-dose CRT. Patients who experienced pathologic mediastinal clearance of disease underwent resection followed by consolidative chemotherapy. Unless there was evidence of disease progression, patients who did not have mediastinal lymph node clearance or who were found not to be a surgical candidate proceeded directly to consolidative chemotherapy. The details of patient selection for trimodality therapy and the oncological outcomes have been previously reported.10 For follow-up, patients were normally followed with serial CT or PET–CT scans as clinically indicated every 3 months for the first year, 4 to 6 months for the next 2 to 5 years, and then yearly thereafter.
For the analysis, patients were categorized as being either married or single based on self-reporting. As a surrogate for nutrition status, patients were stratified into 4 pretreatment body mass index (BMI) cohorts based on the following World Health Organization criteria: underweight, <18.5 kg/m2; normal weight, 18.5 to <25 kg/m2; overweight, 25 to <30 kg/m2; and obesity, ≥30 kg/m2. Pretreatment albumin was also evaluated as a continuous variable. For assessment of immunological status, neutrophil-to-lymphocyte ratio (NLR) was calculated at the time of diagnosis by dividing the absolute neutrophil count by the absolute lymphocyte count.
Statistics
We used the Pearson chi-square test to compare categorical variables. OS was calculated from the date of diagnosis (by biopsy of either primary tumor or mediastinal nodes) to the time of death or date of last follow-up. Patients were only censored if they were lost to follow-up. FFR was determined by the date of diagnosis to the time of first failure, with either distant or locoregional disease progression. For this analysis, patients were censored at the time of their last follow-up or death. The Kaplan-Meier product limit method was used to estimate OS and FFR, and we applied the log-rank test to compare outcomes between the 2 cohorts.
We conducted the multivariate analyses using Cox regression with forward model selection. Variables analyzed included age (<60 vs ≥60 years), sex, race (black vs nonblack), median household income, insurance status (Yes vs No), Eastern Cooperative Oncology Group Performance Status (ECOG PS) (range: 0-3; 0 = fully active and 3 = capable of limited self-care, confined to bed/chair >50% of day) at time of diagnosis (0 vs ≥1), pre-CRT BMI, smoking (pack-years), chronic obstructive pulmonary disorder (Yes vs No), Charlson Comorbidity Index score (≤6 vs >7; range, 3-15; this score takes into consideration age, cardiovascular disease, malignancy, and other chronic conditions to calculate 1-year mortality), histology, calculated pretreatment NLR (as a continuous variable), pretreatment albumin (as a continuous variable), T stage, N stage, overall stage (IIIA vs IIIB), radiation technique (3D-CRT vs intensity-modulated radiation therapy [IMRT]), date of diagnosis (divided into quartiles based on proportion diagnosed by years: 2000-2002, 2003-2005, 2006-2009, 2010-2013), use of trimodality therapy, and consolidation chemotherapy. SPSS software (version 23.0) was used for statistical analysis (IBM Corp, Armonk, NY).
Results
Treatment cohorts
Table 1 compares and summarizes patient demographics, disease, and treatment characteristics for married (n = 185; 52.1%) and nonmarried (n = 170; 47.9%) patients. Married patients were more likely to self-identify as being white (P < .0001), reside in zip codes with a higher household median income (P < .0001), have an ECOG PS of 0 (P = .001), have a higher distribution of pretreatment albumin levels (P = .009), and undergo trimodality therapy (P = .001), and they were twice as likely to have insurance (P = .029). Both cohorts were evenly distributed in terms of T stage, N stage, and overall staging. There was no difference in pretreatment NLR or pretreatment BMI between married and single patients. Concurrent CRT was used in more than 85% of patients in both groups, with approximately two-thirds also receiving consolidation chemotherapy (Table 1). Median delivered radiation dose was 64.8 Gy (range, 10.8-81.6 Gy). There was no statistically significant difference in radiation dose delivered to either group, with nearly 90% of the cohort receiving ≥60 Gy.
OS and FFR
With a median follow-up of 15 months for all patients and 89 months for surviving patients (range, 1-184 months), married patients had improved OS when compared with the single cohort, with a median survival of 29.6 and 18.4 months, respectively (unadjusted hazard ratio [HR] of married vs nonmarried, .640; 95% confidence interval [CI], 0.502-0.816; P < .0001; Figure 1A). The estimated 2- and 5-year OS for married and single patients were 56% and 31% and 38.6% and 15%, respectively. When stratified by stage, married patients with stage IIIB disease (median survival, 25 months; Figure 1B) had a similar survival to unmarried patients with stage IIIA disease (median survival, 24 months; Figure 1B).
In stage IIIA patients, marital status was associated with an unadjusted HR of .696 (95% CI, 0.497-0.974; P = .035), with a larger OS benefit seen in the IIIB group (unadjusted HR, .601; 95% CI, 0.422-0.856; P = .005).
Survival as it pertains to marital status was further stratified by sex (Figure 2A) and race (Figure 2B). Married men had an improved estimated median survival of 30 months when compared with single men, whose median survival was 16 months (unadjusted HR, .541; 95% CI, 0.392-0.746; P < .0001). On the other hand, marital status had no statistically significant effect on OS when comparing married women with their single counterparts (unadjusted HR, .717; 95% CI, 0.491-1.048; P = .085; Figure 2A), with an overall median survival of approximately 28 months for the entire female cohort. Stratification by race also showed similar results, with married nonblack patients demonstrating better OS when compared with single nonblack patients (HR, .586; 95% CI, 0.420-0.820; P = .002; Figure 2B), with a median survival of 29 and 17 months, respectively. Black patients also had a similar improvement in survival when comparing the married (median survival, 30 months) and nonmarried groups (median survival, 19.6 months; unadjusted HR, .676; 95% CI, 0.457-1.000; P = .050; Figure 2B).
FFR did not differ between the 2 groups, with a median time to failure of 17 and 15 months for married and nonmarried patients, respectively (unadjusted HR, .799; 95% CI, 0.607-1.051; P = .108; Figure 3). Estimated 2- and 5-year FFR for married and nonmarried patients were 39.4% and 27% and 31.5% and 18.5%, respectively (Figure 3).
Clinical predictors of survival
On MVA, factors that were independent predictors for OS are summarized in Table 2. Risk of death was reduced by approximately 65% and 45% in patients who underwent trimodality treatment (P < .0001) or were able to undergo consolidative chemotherapy (P = .004) when compared with those who were treated definitively with bimodality treatment or did not undergo systemic doses of adjuvant chemotherapy, respectively. Having insurance (P = .048) and use of IMRT over 3D-CRT (P = .008) was associated with a reduction of mortality by about half in this cohort. Both gender (improved OS with female sex; P = .004) and marital status (improved OS with marriage; P = .006) were associated with a decreased the risk of death by 40% (Table 2). By contrast, a higher NLR resulted
Discussion
Our study continues to support the notion that marital status is an independent indicator of survival in stage III NSCLC (adjusted HR, .59; 95% CI, 0.404-0.859; P = .006). The benefit of marriage in this population seems to be better than that reported in the SEER analysis for all stages, wherein the HR for death of married patients compared with their single counterparts was .85 (95% CI, 0.83-0.87). In their analysis, the investigators hypothesized that this survival advantage could partially be explained by better access to health care and adherence to therapy, as was supported by the higher likelihood of married patients presenting with localized disease and receiving definitive treatment.3 Another population-based study using the Florida Cancer Data System identified 161,228 lung cancer patients (NSCLC and small-cell lung histology included), and on MVA, marital status remained an important prognostic indicator for OS when compared with never-married patients (HR, .86; P = .001).6 In addition to typically including patients with all stages of diseases, population-based studies often include patients who receive a heterogeneous combination of treatment modalities, possibly confounding the analysis. Furthermore, large population analyses typically do not report on patient-specific variables such as nutrition (ie, BMI and albumin) or immunologic status (ie, NLR), both of which have been shown to be independent predictors of survival in LA-NSCLC.8,9
In contrast, some other studies have failed to demonstrate an OS advantage with marital status in patients with NSCLC. For example, in a meta-analysis that evaluated the influence of race, gender, and marital status on 1,365 nonoperative NSCLC patients who were enrolled in 9 Radiation Therapy Oncology Group (RTOG) trials, the investigators did not find marital status to be independently predictive of survival.11 In addition, for the 5,898 patients who were prospectively enrolled in a Mayo Clinic Lung Cancer Cohort (MCLCC), marital status was also found not to be prognostic for NSCLC outcomes when all stages of the disease were analyzed together.4 There are some possible confounding factors in these studies. Patients recruited for clinical trials tend to be healthier with a better performance status and have a support system (including close monitoring by the study team) when compared with the general population diagnosed with lung cancer. About 70% to 76% of the patients in both the RTOG and MCLCC studies were married, which is significantly higher than both the national average (51%) and our group (52.1%). Like other population-based studies, the MCLCC included patients with all stages getting a variety of treatments. Although no overall impact on survival was noted, the investigators noted that single, divorced, and widowed patients were more likely to not receive cancer therapy(P < .0001). The marital status also influenced the choice of therapy, with subgroup analysis revealing inferior outcomes in widowed and divorced patients with stage IA, IIB, or IIIB disease. The authors also recognized an inherent referral bias from patients, with support system being typically seen at the Mayo clinics, which may have played an additional role. All of the patients in our analysis were appropriately staged and received curative-intent treatment by a team of physicians using essentially identical therapeutic strategies, thus minimizing some of these confounding factors. This allowed us to explore the impact of marital status while a patient was undergoing stage-appropriate treatment. We demonstrated a strong association with marital status and survival that even overcame the effects of stage (IIIA vs IIIB) on clinical outcomes (Figure 1B).
Furthermore, our analysis allowed us to explore the interaction of race and marital status more definitively because the demographics of the patients in the RTOG and MCLCC included 14% and less than 3% of patients identified as being nonwhite, respectively, in contrast to our analysis in which 41% of the patients self-identified as black.12 In our black population, marital status was associated with an observable improvement in OS, similar to our nonblack, predominantly white (97%) cohort (Figure 2B). Also, the results of our analysis may be a more accurate representation of the general population living in large urban or semiurban settings and further implies that an intact social support system could have a greater influence on clinical outcomes.
The current analysis is unique when compared with previous published studies in that beyond conventional demographic and treatment-related factors, we have comprehensively explored potential mechanisms that may explain the survival advantage seen in married patients by evaluating additional factors, such as functional status (ECOG and Charlson’s scores), nutritional status (BMI and albumin), immunologic characteristics (NLR), and other social factors (race, income, insurance status). Although married patients were more likely to have a higher BMI and albumin at diagnosis, when controlling for these factors in the multivariable analysis, marital status remained strongly prognostic (Table 2), suggesting that nutrition alone does not fully account for the observed survival advantage demonstrated. A similar conclusion can be drawn about immunologic status. NLR has previously been shown to be prognostic in a number of cancers,13-16 including in our own cohort.8 Although immune status remains an important predictor for OS in our locally advanced NSCLC population, when we take NLR into consideration in our analysis, marital status continues to be a strong indicator for survival (Table 2). In terms of other variables analyzed, insurance status was a significant predictor of OS in the MVA, though functional status and other social factors including race were not significant.
We also explored cancer control outcomes in the form of FFR. Married patients had an observable, although not statistically significant, improvement in FFR when compared with the single cohort (Figure 2). In our study, married patients were more likely to undergo trimodality therapy (Table 1), which has likely translated to the improvement of FFR seen in our group. In this case, marriage may serve as a surrogate for availability of a support system to undergo aggressive, potentially toxic treatment.3,17,18 Even in the setting of bimodality therapy, the RTOG 0617 study noted about 17.5% treatment interruptions because of adverse effects or illness, with more than 30% of patients experiencing grade 3 or more esophagitis, irrespective of radiation technique.19 In these scenarios, in addition to receiving better attention to nutrition and care, significant others often provide emotional and social support that, in turn, can lead to better compliance. Social supports and socio-demographic factors are especially critical in patient populations in which access to health care is challenging.
Despite the compelling outcomes presented, our study suffers from the common limitations of retrospective analyses. Marital status, in this setting, most likely correlates with improved socioeconomic status and greater support, which have resulted in improved survival. Furthermore, although patients were self-classified as married or single, our data were not able to capture whether patients were single but lived with another adult or had other types of social support. However, even if there was a proportion of the unmarried cohort that had an alternate support system, separating them out is likely to further expand the differences. Quantifying the amount of social, emotional, or even spiritual support was not possible to accomplish in our analysis, though we know that all 3 can play a role in cancer outcomes.20,21 Further prospective studies would have to be done to completely understand how marital status can influence clinical decisions. Understanding whether marital status is a proxy for social provisions may help to identify populations at risk for inferior outcomes. These at-risk patients may benefit from targeted clinical interventions, such as closer physician follow-up, more aggressive supportive care, access to support groups, or nurse navigator visits.
Conclusions
In patients with locally advanced NSCLC treated with curative-intent following uniform treatment algorithms, marital status was linked with improvement in survival even when adjusted for other key variables, with the second highest HR (after insurance status) among pretreatment demographic variables. Although marriage is an unmodifiable factor in itself, it is most likely a surrogate for better psychosocial support. The scale of these positive survival improvements emphasizes the need to institute targeted supportive care strategies to help advance overall outcomes in a tumor for which modern therapeutic approaches (novel systemic therapy and radiation) have yielded only modest improvement in outcomes yet come at the cost of considerable treatment-related toxicity.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7-30.
2. Goldstraw P, Chansky K, Crowley J, et al. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11(1):39-51.
3. Aizer AA, Chen M-H, McCarthy EP, et al. Marital status and survival in patients with cancer. J Clin Oncol. 2013;31(31):3869-3876.
4. Jatoi A, Novotny P, Cassivi S, et al. Does marital status impact survival and quality of life in patients with non-small cell lung cancer? Observations from the mayo clinic lung cancer cohort. Oncologist. 2007;12(12):1456-1463.
5. Kravdal H, Syse A. Changes over time in the effect of marital status on cancer survival. BMC Public Health. 2011;11:804.
6. Tannenbaum SL, Zhao W, Koru-Sengul T, Miao F, Lee D, Byrne MM. Marital status and its effect on lung cancer survival. Springerplus. 2013;2:504.
7. Ellis L, Canchola AJ, Spiegel D, Ladabaum U, Haile R, Gomez SL. Racial and ethnic disparities in cancer survival: the contribution of tumor, sociodemographic, institutional, and neighborhood characteristics [published online October 16, 2017]. J Clin Oncol. 2018;36(1):25-33.
8. Scilla KA, Bentzen SM, Lam VK, et al. Neutrophil-lymphocyte ratio is a prognostic marker in patients with locally advanced (stage IIIA and IIIB) non-small cell lung cancer treated with combined modality therapy. Oncologist. 2017;22(6):737-742.
9. Lam VK, Bentzen SM, Mohindra P, et al. Obesity is associated with long-term improved survival in definitively treated locally advanced non-small cell lung cancer (NSCLC). Lung Cancer. 2017;104:52-57.
10. Vyfhuis MAL, Bhooshan N, Burrows WM, et al. Oncological outcomes from trimodality therapy receiving definitive doses of neoadjuvant chemoradiation (≥60 Gy) and factors influencing consideration for surgery in stage III non-small cell lung cancer. Adv Radiat Oncol. 2017;2(3):259-269.
11. Siddiqui F, Bae K, Langer CJ, et al. The influence of gender, race, and marital status on survival in lung cancer patients: analysis of radiation therapy oncology group trials. J Thorac Oncol. 2010;5(5):631-639.
12. Vyfhuis MAL, Bhooshan N, Molitoris J, et al. Clinical outcomes of black vs. non-black patients with locally advanced non–small cell lung cancer. Lung Cancer. 2017;114:44-49.
13. Beltran BE, Castro D, De La Cruz-Vargas JA, et al. The neutrophil-lymphocyte ratio is prognostic in patients with early stage aggressive peripheral T cell lymphoma [published online February 26, 2018]. Br J Haematol. doi:10.1111/bjh.15141.
14. Lee BM, Chung SY, Chang JS, Lee KJ, Seong J. The neutrophil-lymphocyte ratio and platelet-lymphocyte ratio are prognostic factors in patients with locally advanced pancreatic cancer treated with chemoradiotherapy. Gut Liver. 2018;12(3):342-352.
15. Najjar M, Agrawal S, Emond JC, Halazun KJ. Pretreatment neutrophil-lymphocyte ratio: useful prognostic biomarker in hepatocellular carcinoma. J Hepatocell Carcinoma. 2018;5:17-28.
16. Hu W, Yu J, Huang Y, Hu F, Zhang X, Wang Y. Lymphocyte-related inflammation and immune-based scores predict prognosis of chordoma patients after radical resection. Transl Oncol. 2018;11(2):444-449.
17. Mahal BA, Cooperberg MR, Aizer AA, et al. Who bears the greatest burden of aggressive treatment of indolent prostate cancer? Am J Med. 2015;128(6):609-616.
18. Inverso G, Mahal BA, Aizer AA, Donoff RB, Chau NG, Haddad RI. Marital status and head and neck cancer outcomes. Cancer. 2015;121(8):1273-1278.
19. Chun SG, Hu C, Choy H, et al. Impact of intensity-modulated radiation therapy technique for locally advanced non-small-cell lung cancer: a secondary analysis of the NRG oncology RTOG 0617 randomized clinical trial. J Clin Oncol. 2017;35(1):56-62.
20. Waite LJ, Lehrer EL. The benefits from marriage and religion in the United States: a comparative analysis. Popul Dev Rev. 2003;29(2):255-276.
21. Osborne C, Ostir GV, Du X, Peek MK, Goodwin JS. The influence of marital status on the stage at diagnosis, treatment, and survival of older women with breast cancer. Breast Cancer Res Treat. 2005;93(1):41-47.
Non–small-cell lung cancer (NSCLC) remains the leading cause of cancer death in the United States, where 29% of patients will present with stage III disease.1,2 Ongoing research efforts seek to improve these outcomes using novel systemic therapy options or modern radiation techniques. However, there have also been recent studies showing the importance of marital and/or partner status on clinical outcomes.3-7 For example, in a large Surveillance, Epidemiology, and End Results (SEER) analysis of 734,889 patients diagnosed with several types of cancer (including lung cancer), patients identified as married were less likely to present with metastatic disease, more likely to receive definitive therapy, and had superior cancer-related mortality even after adjusting for other variables such as cancer stage and treatment when compared with single patients.3 Population-based assessments are important in relaying information about trends and general outcomes based on marital status, but because they are large, they often lack patient-specific information such as nutrition, immunologic status, and variability in treatment paradigms, all of which can independently have an impact on overall survival (OS) in stage III NSCLC.8-10 In addition, population analyses have typically included patients of all cancer stages and hence involved a multitude of treatment approaches ranging from curative to palliative. There are limited well-annotated institutional data on the association of marital status on nonmetastatic, locally advanced (LA-NSCLC) in the setting of National Comprehensive Cancer Network-guided, standard-of-care definitive treatment.
The objective of this analysis is to evaluate the effect of marital status on OS and freedom from recurrence (FFR) in patients with stage III NSCLC who were treated at a National Cancer Institute–designated cancer center with curative intent from 2000 through 2013. We performed a detailed multivariate analysis (MVA) of patient-, disease-, and treatment-specific factors, including the interaction with racial, nutritional, and immunologic status, which to our knowledge has not been previously reported, to comprehensively evaluate the benefit of marital status in patients with LA-NSCLC.
Methods
Patient population and treatment
From January 2000 through December 2013, 355 patients diagnosed with clinical stage III NSCLC (American Joint Committee on Cancer 7th edition) were definitively treated at the University of Maryland in Baltimore, Maryland. Their clinical data were retrospectively analyzed under internal review board approval (GCC 1175, Thoracic Oncology Database). All of the patients were evaluated before treatment by a multidisciplinary team consisting of thoracic surgeons and medical and radiation oncologists. Before treatment, the patients underwent standard work-up, which included systemic imaging with positron-emission (PET), computed-tomographic (CT), PET–CT, and/or bone scan, brain imaging consisting of magnetic-resonance imaging or CT with contrast, and routine blood. Patients had documentation of mediastinal disease by either imaging, mediastinoscopy, or endobronchial ultrasound biopsy.
Definitive therapy was administered using the backbone of chemoradiation therapy (CRT) with (trimodality) or without (bimodality) surgical resection. Concurrent CRT was typically administered with weekly carboplatin–paclitaxel (areas under the curve [AUCs], 2 and 50 mg/m2, respectively) and was generally followed with 2 cycles of consolidative treatment with definitive doses of carboplatin–paclitaxel (AUCs, 5-6 and 200-225 mg/m2, respectively) as tolerated. The entire cohort was also assessed for possible trimodality therapy at the time of initial diagnosis, and patients who were potential surgical candidates were reassessed for mediastinal nodal clearance following repeat radiographic staging after full-dose CRT. Patients who experienced pathologic mediastinal clearance of disease underwent resection followed by consolidative chemotherapy. Unless there was evidence of disease progression, patients who did not have mediastinal lymph node clearance or who were found not to be a surgical candidate proceeded directly to consolidative chemotherapy. The details of patient selection for trimodality therapy and the oncological outcomes have been previously reported.10 For follow-up, patients were normally followed with serial CT or PET–CT scans as clinically indicated every 3 months for the first year, 4 to 6 months for the next 2 to 5 years, and then yearly thereafter.
For the analysis, patients were categorized as being either married or single based on self-reporting. As a surrogate for nutrition status, patients were stratified into 4 pretreatment body mass index (BMI) cohorts based on the following World Health Organization criteria: underweight, <18.5 kg/m2; normal weight, 18.5 to <25 kg/m2; overweight, 25 to <30 kg/m2; and obesity, ≥30 kg/m2. Pretreatment albumin was also evaluated as a continuous variable. For assessment of immunological status, neutrophil-to-lymphocyte ratio (NLR) was calculated at the time of diagnosis by dividing the absolute neutrophil count by the absolute lymphocyte count.
Statistics
We used the Pearson chi-square test to compare categorical variables. OS was calculated from the date of diagnosis (by biopsy of either primary tumor or mediastinal nodes) to the time of death or date of last follow-up. Patients were only censored if they were lost to follow-up. FFR was determined by the date of diagnosis to the time of first failure, with either distant or locoregional disease progression. For this analysis, patients were censored at the time of their last follow-up or death. The Kaplan-Meier product limit method was used to estimate OS and FFR, and we applied the log-rank test to compare outcomes between the 2 cohorts.
We conducted the multivariate analyses using Cox regression with forward model selection. Variables analyzed included age (<60 vs ≥60 years), sex, race (black vs nonblack), median household income, insurance status (Yes vs No), Eastern Cooperative Oncology Group Performance Status (ECOG PS) (range: 0-3; 0 = fully active and 3 = capable of limited self-care, confined to bed/chair >50% of day) at time of diagnosis (0 vs ≥1), pre-CRT BMI, smoking (pack-years), chronic obstructive pulmonary disorder (Yes vs No), Charlson Comorbidity Index score (≤6 vs >7; range, 3-15; this score takes into consideration age, cardiovascular disease, malignancy, and other chronic conditions to calculate 1-year mortality), histology, calculated pretreatment NLR (as a continuous variable), pretreatment albumin (as a continuous variable), T stage, N stage, overall stage (IIIA vs IIIB), radiation technique (3D-CRT vs intensity-modulated radiation therapy [IMRT]), date of diagnosis (divided into quartiles based on proportion diagnosed by years: 2000-2002, 2003-2005, 2006-2009, 2010-2013), use of trimodality therapy, and consolidation chemotherapy. SPSS software (version 23.0) was used for statistical analysis (IBM Corp, Armonk, NY).
Results
Treatment cohorts
Table 1 compares and summarizes patient demographics, disease, and treatment characteristics for married (n = 185; 52.1%) and nonmarried (n = 170; 47.9%) patients. Married patients were more likely to self-identify as being white (P < .0001), reside in zip codes with a higher household median income (P < .0001), have an ECOG PS of 0 (P = .001), have a higher distribution of pretreatment albumin levels (P = .009), and undergo trimodality therapy (P = .001), and they were twice as likely to have insurance (P = .029). Both cohorts were evenly distributed in terms of T stage, N stage, and overall staging. There was no difference in pretreatment NLR or pretreatment BMI between married and single patients. Concurrent CRT was used in more than 85% of patients in both groups, with approximately two-thirds also receiving consolidation chemotherapy (Table 1). Median delivered radiation dose was 64.8 Gy (range, 10.8-81.6 Gy). There was no statistically significant difference in radiation dose delivered to either group, with nearly 90% of the cohort receiving ≥60 Gy.
OS and FFR
With a median follow-up of 15 months for all patients and 89 months for surviving patients (range, 1-184 months), married patients had improved OS when compared with the single cohort, with a median survival of 29.6 and 18.4 months, respectively (unadjusted hazard ratio [HR] of married vs nonmarried, .640; 95% confidence interval [CI], 0.502-0.816; P < .0001; Figure 1A). The estimated 2- and 5-year OS for married and single patients were 56% and 31% and 38.6% and 15%, respectively. When stratified by stage, married patients with stage IIIB disease (median survival, 25 months; Figure 1B) had a similar survival to unmarried patients with stage IIIA disease (median survival, 24 months; Figure 1B).
In stage IIIA patients, marital status was associated with an unadjusted HR of .696 (95% CI, 0.497-0.974; P = .035), with a larger OS benefit seen in the IIIB group (unadjusted HR, .601; 95% CI, 0.422-0.856; P = .005).
Survival as it pertains to marital status was further stratified by sex (Figure 2A) and race (Figure 2B). Married men had an improved estimated median survival of 30 months when compared with single men, whose median survival was 16 months (unadjusted HR, .541; 95% CI, 0.392-0.746; P < .0001). On the other hand, marital status had no statistically significant effect on OS when comparing married women with their single counterparts (unadjusted HR, .717; 95% CI, 0.491-1.048; P = .085; Figure 2A), with an overall median survival of approximately 28 months for the entire female cohort. Stratification by race also showed similar results, with married nonblack patients demonstrating better OS when compared with single nonblack patients (HR, .586; 95% CI, 0.420-0.820; P = .002; Figure 2B), with a median survival of 29 and 17 months, respectively. Black patients also had a similar improvement in survival when comparing the married (median survival, 30 months) and nonmarried groups (median survival, 19.6 months; unadjusted HR, .676; 95% CI, 0.457-1.000; P = .050; Figure 2B).
FFR did not differ between the 2 groups, with a median time to failure of 17 and 15 months for married and nonmarried patients, respectively (unadjusted HR, .799; 95% CI, 0.607-1.051; P = .108; Figure 3). Estimated 2- and 5-year FFR for married and nonmarried patients were 39.4% and 27% and 31.5% and 18.5%, respectively (Figure 3).
Clinical predictors of survival
On MVA, factors that were independent predictors for OS are summarized in Table 2. Risk of death was reduced by approximately 65% and 45% in patients who underwent trimodality treatment (P < .0001) or were able to undergo consolidative chemotherapy (P = .004) when compared with those who were treated definitively with bimodality treatment or did not undergo systemic doses of adjuvant chemotherapy, respectively. Having insurance (P = .048) and use of IMRT over 3D-CRT (P = .008) was associated with a reduction of mortality by about half in this cohort. Both gender (improved OS with female sex; P = .004) and marital status (improved OS with marriage; P = .006) were associated with a decreased the risk of death by 40% (Table 2). By contrast, a higher NLR resulted
Discussion
Our study continues to support the notion that marital status is an independent indicator of survival in stage III NSCLC (adjusted HR, .59; 95% CI, 0.404-0.859; P = .006). The benefit of marriage in this population seems to be better than that reported in the SEER analysis for all stages, wherein the HR for death of married patients compared with their single counterparts was .85 (95% CI, 0.83-0.87). In their analysis, the investigators hypothesized that this survival advantage could partially be explained by better access to health care and adherence to therapy, as was supported by the higher likelihood of married patients presenting with localized disease and receiving definitive treatment.3 Another population-based study using the Florida Cancer Data System identified 161,228 lung cancer patients (NSCLC and small-cell lung histology included), and on MVA, marital status remained an important prognostic indicator for OS when compared with never-married patients (HR, .86; P = .001).6 In addition to typically including patients with all stages of diseases, population-based studies often include patients who receive a heterogeneous combination of treatment modalities, possibly confounding the analysis. Furthermore, large population analyses typically do not report on patient-specific variables such as nutrition (ie, BMI and albumin) or immunologic status (ie, NLR), both of which have been shown to be independent predictors of survival in LA-NSCLC.8,9
In contrast, some other studies have failed to demonstrate an OS advantage with marital status in patients with NSCLC. For example, in a meta-analysis that evaluated the influence of race, gender, and marital status on 1,365 nonoperative NSCLC patients who were enrolled in 9 Radiation Therapy Oncology Group (RTOG) trials, the investigators did not find marital status to be independently predictive of survival.11 In addition, for the 5,898 patients who were prospectively enrolled in a Mayo Clinic Lung Cancer Cohort (MCLCC), marital status was also found not to be prognostic for NSCLC outcomes when all stages of the disease were analyzed together.4 There are some possible confounding factors in these studies. Patients recruited for clinical trials tend to be healthier with a better performance status and have a support system (including close monitoring by the study team) when compared with the general population diagnosed with lung cancer. About 70% to 76% of the patients in both the RTOG and MCLCC studies were married, which is significantly higher than both the national average (51%) and our group (52.1%). Like other population-based studies, the MCLCC included patients with all stages getting a variety of treatments. Although no overall impact on survival was noted, the investigators noted that single, divorced, and widowed patients were more likely to not receive cancer therapy(P < .0001). The marital status also influenced the choice of therapy, with subgroup analysis revealing inferior outcomes in widowed and divorced patients with stage IA, IIB, or IIIB disease. The authors also recognized an inherent referral bias from patients, with support system being typically seen at the Mayo clinics, which may have played an additional role. All of the patients in our analysis were appropriately staged and received curative-intent treatment by a team of physicians using essentially identical therapeutic strategies, thus minimizing some of these confounding factors. This allowed us to explore the impact of marital status while a patient was undergoing stage-appropriate treatment. We demonstrated a strong association with marital status and survival that even overcame the effects of stage (IIIA vs IIIB) on clinical outcomes (Figure 1B).
Furthermore, our analysis allowed us to explore the interaction of race and marital status more definitively because the demographics of the patients in the RTOG and MCLCC included 14% and less than 3% of patients identified as being nonwhite, respectively, in contrast to our analysis in which 41% of the patients self-identified as black.12 In our black population, marital status was associated with an observable improvement in OS, similar to our nonblack, predominantly white (97%) cohort (Figure 2B). Also, the results of our analysis may be a more accurate representation of the general population living in large urban or semiurban settings and further implies that an intact social support system could have a greater influence on clinical outcomes.
The current analysis is unique when compared with previous published studies in that beyond conventional demographic and treatment-related factors, we have comprehensively explored potential mechanisms that may explain the survival advantage seen in married patients by evaluating additional factors, such as functional status (ECOG and Charlson’s scores), nutritional status (BMI and albumin), immunologic characteristics (NLR), and other social factors (race, income, insurance status). Although married patients were more likely to have a higher BMI and albumin at diagnosis, when controlling for these factors in the multivariable analysis, marital status remained strongly prognostic (Table 2), suggesting that nutrition alone does not fully account for the observed survival advantage demonstrated. A similar conclusion can be drawn about immunologic status. NLR has previously been shown to be prognostic in a number of cancers,13-16 including in our own cohort.8 Although immune status remains an important predictor for OS in our locally advanced NSCLC population, when we take NLR into consideration in our analysis, marital status continues to be a strong indicator for survival (Table 2). In terms of other variables analyzed, insurance status was a significant predictor of OS in the MVA, though functional status and other social factors including race were not significant.
We also explored cancer control outcomes in the form of FFR. Married patients had an observable, although not statistically significant, improvement in FFR when compared with the single cohort (Figure 2). In our study, married patients were more likely to undergo trimodality therapy (Table 1), which has likely translated to the improvement of FFR seen in our group. In this case, marriage may serve as a surrogate for availability of a support system to undergo aggressive, potentially toxic treatment.3,17,18 Even in the setting of bimodality therapy, the RTOG 0617 study noted about 17.5% treatment interruptions because of adverse effects or illness, with more than 30% of patients experiencing grade 3 or more esophagitis, irrespective of radiation technique.19 In these scenarios, in addition to receiving better attention to nutrition and care, significant others often provide emotional and social support that, in turn, can lead to better compliance. Social supports and socio-demographic factors are especially critical in patient populations in which access to health care is challenging.
Despite the compelling outcomes presented, our study suffers from the common limitations of retrospective analyses. Marital status, in this setting, most likely correlates with improved socioeconomic status and greater support, which have resulted in improved survival. Furthermore, although patients were self-classified as married or single, our data were not able to capture whether patients were single but lived with another adult or had other types of social support. However, even if there was a proportion of the unmarried cohort that had an alternate support system, separating them out is likely to further expand the differences. Quantifying the amount of social, emotional, or even spiritual support was not possible to accomplish in our analysis, though we know that all 3 can play a role in cancer outcomes.20,21 Further prospective studies would have to be done to completely understand how marital status can influence clinical decisions. Understanding whether marital status is a proxy for social provisions may help to identify populations at risk for inferior outcomes. These at-risk patients may benefit from targeted clinical interventions, such as closer physician follow-up, more aggressive supportive care, access to support groups, or nurse navigator visits.
Conclusions
In patients with locally advanced NSCLC treated with curative-intent following uniform treatment algorithms, marital status was linked with improvement in survival even when adjusted for other key variables, with the second highest HR (after insurance status) among pretreatment demographic variables. Although marriage is an unmodifiable factor in itself, it is most likely a surrogate for better psychosocial support. The scale of these positive survival improvements emphasizes the need to institute targeted supportive care strategies to help advance overall outcomes in a tumor for which modern therapeutic approaches (novel systemic therapy and radiation) have yielded only modest improvement in outcomes yet come at the cost of considerable treatment-related toxicity.
Non–small-cell lung cancer (NSCLC) remains the leading cause of cancer death in the United States, where 29% of patients will present with stage III disease.1,2 Ongoing research efforts seek to improve these outcomes using novel systemic therapy options or modern radiation techniques. However, there have also been recent studies showing the importance of marital and/or partner status on clinical outcomes.3-7 For example, in a large Surveillance, Epidemiology, and End Results (SEER) analysis of 734,889 patients diagnosed with several types of cancer (including lung cancer), patients identified as married were less likely to present with metastatic disease, more likely to receive definitive therapy, and had superior cancer-related mortality even after adjusting for other variables such as cancer stage and treatment when compared with single patients.3 Population-based assessments are important in relaying information about trends and general outcomes based on marital status, but because they are large, they often lack patient-specific information such as nutrition, immunologic status, and variability in treatment paradigms, all of which can independently have an impact on overall survival (OS) in stage III NSCLC.8-10 In addition, population analyses have typically included patients of all cancer stages and hence involved a multitude of treatment approaches ranging from curative to palliative. There are limited well-annotated institutional data on the association of marital status on nonmetastatic, locally advanced (LA-NSCLC) in the setting of National Comprehensive Cancer Network-guided, standard-of-care definitive treatment.
The objective of this analysis is to evaluate the effect of marital status on OS and freedom from recurrence (FFR) in patients with stage III NSCLC who were treated at a National Cancer Institute–designated cancer center with curative intent from 2000 through 2013. We performed a detailed multivariate analysis (MVA) of patient-, disease-, and treatment-specific factors, including the interaction with racial, nutritional, and immunologic status, which to our knowledge has not been previously reported, to comprehensively evaluate the benefit of marital status in patients with LA-NSCLC.
Methods
Patient population and treatment
From January 2000 through December 2013, 355 patients diagnosed with clinical stage III NSCLC (American Joint Committee on Cancer 7th edition) were definitively treated at the University of Maryland in Baltimore, Maryland. Their clinical data were retrospectively analyzed under internal review board approval (GCC 1175, Thoracic Oncology Database). All of the patients were evaluated before treatment by a multidisciplinary team consisting of thoracic surgeons and medical and radiation oncologists. Before treatment, the patients underwent standard work-up, which included systemic imaging with positron-emission (PET), computed-tomographic (CT), PET–CT, and/or bone scan, brain imaging consisting of magnetic-resonance imaging or CT with contrast, and routine blood. Patients had documentation of mediastinal disease by either imaging, mediastinoscopy, or endobronchial ultrasound biopsy.
Definitive therapy was administered using the backbone of chemoradiation therapy (CRT) with (trimodality) or without (bimodality) surgical resection. Concurrent CRT was typically administered with weekly carboplatin–paclitaxel (areas under the curve [AUCs], 2 and 50 mg/m2, respectively) and was generally followed with 2 cycles of consolidative treatment with definitive doses of carboplatin–paclitaxel (AUCs, 5-6 and 200-225 mg/m2, respectively) as tolerated. The entire cohort was also assessed for possible trimodality therapy at the time of initial diagnosis, and patients who were potential surgical candidates were reassessed for mediastinal nodal clearance following repeat radiographic staging after full-dose CRT. Patients who experienced pathologic mediastinal clearance of disease underwent resection followed by consolidative chemotherapy. Unless there was evidence of disease progression, patients who did not have mediastinal lymph node clearance or who were found not to be a surgical candidate proceeded directly to consolidative chemotherapy. The details of patient selection for trimodality therapy and the oncological outcomes have been previously reported.10 For follow-up, patients were normally followed with serial CT or PET–CT scans as clinically indicated every 3 months for the first year, 4 to 6 months for the next 2 to 5 years, and then yearly thereafter.
For the analysis, patients were categorized as being either married or single based on self-reporting. As a surrogate for nutrition status, patients were stratified into 4 pretreatment body mass index (BMI) cohorts based on the following World Health Organization criteria: underweight, <18.5 kg/m2; normal weight, 18.5 to <25 kg/m2; overweight, 25 to <30 kg/m2; and obesity, ≥30 kg/m2. Pretreatment albumin was also evaluated as a continuous variable. For assessment of immunological status, neutrophil-to-lymphocyte ratio (NLR) was calculated at the time of diagnosis by dividing the absolute neutrophil count by the absolute lymphocyte count.
Statistics
We used the Pearson chi-square test to compare categorical variables. OS was calculated from the date of diagnosis (by biopsy of either primary tumor or mediastinal nodes) to the time of death or date of last follow-up. Patients were only censored if they were lost to follow-up. FFR was determined by the date of diagnosis to the time of first failure, with either distant or locoregional disease progression. For this analysis, patients were censored at the time of their last follow-up or death. The Kaplan-Meier product limit method was used to estimate OS and FFR, and we applied the log-rank test to compare outcomes between the 2 cohorts.
We conducted the multivariate analyses using Cox regression with forward model selection. Variables analyzed included age (<60 vs ≥60 years), sex, race (black vs nonblack), median household income, insurance status (Yes vs No), Eastern Cooperative Oncology Group Performance Status (ECOG PS) (range: 0-3; 0 = fully active and 3 = capable of limited self-care, confined to bed/chair >50% of day) at time of diagnosis (0 vs ≥1), pre-CRT BMI, smoking (pack-years), chronic obstructive pulmonary disorder (Yes vs No), Charlson Comorbidity Index score (≤6 vs >7; range, 3-15; this score takes into consideration age, cardiovascular disease, malignancy, and other chronic conditions to calculate 1-year mortality), histology, calculated pretreatment NLR (as a continuous variable), pretreatment albumin (as a continuous variable), T stage, N stage, overall stage (IIIA vs IIIB), radiation technique (3D-CRT vs intensity-modulated radiation therapy [IMRT]), date of diagnosis (divided into quartiles based on proportion diagnosed by years: 2000-2002, 2003-2005, 2006-2009, 2010-2013), use of trimodality therapy, and consolidation chemotherapy. SPSS software (version 23.0) was used for statistical analysis (IBM Corp, Armonk, NY).
Results
Treatment cohorts
Table 1 compares and summarizes patient demographics, disease, and treatment characteristics for married (n = 185; 52.1%) and nonmarried (n = 170; 47.9%) patients. Married patients were more likely to self-identify as being white (P < .0001), reside in zip codes with a higher household median income (P < .0001), have an ECOG PS of 0 (P = .001), have a higher distribution of pretreatment albumin levels (P = .009), and undergo trimodality therapy (P = .001), and they were twice as likely to have insurance (P = .029). Both cohorts were evenly distributed in terms of T stage, N stage, and overall staging. There was no difference in pretreatment NLR or pretreatment BMI between married and single patients. Concurrent CRT was used in more than 85% of patients in both groups, with approximately two-thirds also receiving consolidation chemotherapy (Table 1). Median delivered radiation dose was 64.8 Gy (range, 10.8-81.6 Gy). There was no statistically significant difference in radiation dose delivered to either group, with nearly 90% of the cohort receiving ≥60 Gy.
OS and FFR
With a median follow-up of 15 months for all patients and 89 months for surviving patients (range, 1-184 months), married patients had improved OS when compared with the single cohort, with a median survival of 29.6 and 18.4 months, respectively (unadjusted hazard ratio [HR] of married vs nonmarried, .640; 95% confidence interval [CI], 0.502-0.816; P < .0001; Figure 1A). The estimated 2- and 5-year OS for married and single patients were 56% and 31% and 38.6% and 15%, respectively. When stratified by stage, married patients with stage IIIB disease (median survival, 25 months; Figure 1B) had a similar survival to unmarried patients with stage IIIA disease (median survival, 24 months; Figure 1B).
In stage IIIA patients, marital status was associated with an unadjusted HR of .696 (95% CI, 0.497-0.974; P = .035), with a larger OS benefit seen in the IIIB group (unadjusted HR, .601; 95% CI, 0.422-0.856; P = .005).
Survival as it pertains to marital status was further stratified by sex (Figure 2A) and race (Figure 2B). Married men had an improved estimated median survival of 30 months when compared with single men, whose median survival was 16 months (unadjusted HR, .541; 95% CI, 0.392-0.746; P < .0001). On the other hand, marital status had no statistically significant effect on OS when comparing married women with their single counterparts (unadjusted HR, .717; 95% CI, 0.491-1.048; P = .085; Figure 2A), with an overall median survival of approximately 28 months for the entire female cohort. Stratification by race also showed similar results, with married nonblack patients demonstrating better OS when compared with single nonblack patients (HR, .586; 95% CI, 0.420-0.820; P = .002; Figure 2B), with a median survival of 29 and 17 months, respectively. Black patients also had a similar improvement in survival when comparing the married (median survival, 30 months) and nonmarried groups (median survival, 19.6 months; unadjusted HR, .676; 95% CI, 0.457-1.000; P = .050; Figure 2B).
FFR did not differ between the 2 groups, with a median time to failure of 17 and 15 months for married and nonmarried patients, respectively (unadjusted HR, .799; 95% CI, 0.607-1.051; P = .108; Figure 3). Estimated 2- and 5-year FFR for married and nonmarried patients were 39.4% and 27% and 31.5% and 18.5%, respectively (Figure 3).
Clinical predictors of survival
On MVA, factors that were independent predictors for OS are summarized in Table 2. Risk of death was reduced by approximately 65% and 45% in patients who underwent trimodality treatment (P < .0001) or were able to undergo consolidative chemotherapy (P = .004) when compared with those who were treated definitively with bimodality treatment or did not undergo systemic doses of adjuvant chemotherapy, respectively. Having insurance (P = .048) and use of IMRT over 3D-CRT (P = .008) was associated with a reduction of mortality by about half in this cohort. Both gender (improved OS with female sex; P = .004) and marital status (improved OS with marriage; P = .006) were associated with a decreased the risk of death by 40% (Table 2). By contrast, a higher NLR resulted
Discussion
Our study continues to support the notion that marital status is an independent indicator of survival in stage III NSCLC (adjusted HR, .59; 95% CI, 0.404-0.859; P = .006). The benefit of marriage in this population seems to be better than that reported in the SEER analysis for all stages, wherein the HR for death of married patients compared with their single counterparts was .85 (95% CI, 0.83-0.87). In their analysis, the investigators hypothesized that this survival advantage could partially be explained by better access to health care and adherence to therapy, as was supported by the higher likelihood of married patients presenting with localized disease and receiving definitive treatment.3 Another population-based study using the Florida Cancer Data System identified 161,228 lung cancer patients (NSCLC and small-cell lung histology included), and on MVA, marital status remained an important prognostic indicator for OS when compared with never-married patients (HR, .86; P = .001).6 In addition to typically including patients with all stages of diseases, population-based studies often include patients who receive a heterogeneous combination of treatment modalities, possibly confounding the analysis. Furthermore, large population analyses typically do not report on patient-specific variables such as nutrition (ie, BMI and albumin) or immunologic status (ie, NLR), both of which have been shown to be independent predictors of survival in LA-NSCLC.8,9
In contrast, some other studies have failed to demonstrate an OS advantage with marital status in patients with NSCLC. For example, in a meta-analysis that evaluated the influence of race, gender, and marital status on 1,365 nonoperative NSCLC patients who were enrolled in 9 Radiation Therapy Oncology Group (RTOG) trials, the investigators did not find marital status to be independently predictive of survival.11 In addition, for the 5,898 patients who were prospectively enrolled in a Mayo Clinic Lung Cancer Cohort (MCLCC), marital status was also found not to be prognostic for NSCLC outcomes when all stages of the disease were analyzed together.4 There are some possible confounding factors in these studies. Patients recruited for clinical trials tend to be healthier with a better performance status and have a support system (including close monitoring by the study team) when compared with the general population diagnosed with lung cancer. About 70% to 76% of the patients in both the RTOG and MCLCC studies were married, which is significantly higher than both the national average (51%) and our group (52.1%). Like other population-based studies, the MCLCC included patients with all stages getting a variety of treatments. Although no overall impact on survival was noted, the investigators noted that single, divorced, and widowed patients were more likely to not receive cancer therapy(P < .0001). The marital status also influenced the choice of therapy, with subgroup analysis revealing inferior outcomes in widowed and divorced patients with stage IA, IIB, or IIIB disease. The authors also recognized an inherent referral bias from patients, with support system being typically seen at the Mayo clinics, which may have played an additional role. All of the patients in our analysis were appropriately staged and received curative-intent treatment by a team of physicians using essentially identical therapeutic strategies, thus minimizing some of these confounding factors. This allowed us to explore the impact of marital status while a patient was undergoing stage-appropriate treatment. We demonstrated a strong association with marital status and survival that even overcame the effects of stage (IIIA vs IIIB) on clinical outcomes (Figure 1B).
Furthermore, our analysis allowed us to explore the interaction of race and marital status more definitively because the demographics of the patients in the RTOG and MCLCC included 14% and less than 3% of patients identified as being nonwhite, respectively, in contrast to our analysis in which 41% of the patients self-identified as black.12 In our black population, marital status was associated with an observable improvement in OS, similar to our nonblack, predominantly white (97%) cohort (Figure 2B). Also, the results of our analysis may be a more accurate representation of the general population living in large urban or semiurban settings and further implies that an intact social support system could have a greater influence on clinical outcomes.
The current analysis is unique when compared with previous published studies in that beyond conventional demographic and treatment-related factors, we have comprehensively explored potential mechanisms that may explain the survival advantage seen in married patients by evaluating additional factors, such as functional status (ECOG and Charlson’s scores), nutritional status (BMI and albumin), immunologic characteristics (NLR), and other social factors (race, income, insurance status). Although married patients were more likely to have a higher BMI and albumin at diagnosis, when controlling for these factors in the multivariable analysis, marital status remained strongly prognostic (Table 2), suggesting that nutrition alone does not fully account for the observed survival advantage demonstrated. A similar conclusion can be drawn about immunologic status. NLR has previously been shown to be prognostic in a number of cancers,13-16 including in our own cohort.8 Although immune status remains an important predictor for OS in our locally advanced NSCLC population, when we take NLR into consideration in our analysis, marital status continues to be a strong indicator for survival (Table 2). In terms of other variables analyzed, insurance status was a significant predictor of OS in the MVA, though functional status and other social factors including race were not significant.
We also explored cancer control outcomes in the form of FFR. Married patients had an observable, although not statistically significant, improvement in FFR when compared with the single cohort (Figure 2). In our study, married patients were more likely to undergo trimodality therapy (Table 1), which has likely translated to the improvement of FFR seen in our group. In this case, marriage may serve as a surrogate for availability of a support system to undergo aggressive, potentially toxic treatment.3,17,18 Even in the setting of bimodality therapy, the RTOG 0617 study noted about 17.5% treatment interruptions because of adverse effects or illness, with more than 30% of patients experiencing grade 3 or more esophagitis, irrespective of radiation technique.19 In these scenarios, in addition to receiving better attention to nutrition and care, significant others often provide emotional and social support that, in turn, can lead to better compliance. Social supports and socio-demographic factors are especially critical in patient populations in which access to health care is challenging.
Despite the compelling outcomes presented, our study suffers from the common limitations of retrospective analyses. Marital status, in this setting, most likely correlates with improved socioeconomic status and greater support, which have resulted in improved survival. Furthermore, although patients were self-classified as married or single, our data were not able to capture whether patients were single but lived with another adult or had other types of social support. However, even if there was a proportion of the unmarried cohort that had an alternate support system, separating them out is likely to further expand the differences. Quantifying the amount of social, emotional, or even spiritual support was not possible to accomplish in our analysis, though we know that all 3 can play a role in cancer outcomes.20,21 Further prospective studies would have to be done to completely understand how marital status can influence clinical decisions. Understanding whether marital status is a proxy for social provisions may help to identify populations at risk for inferior outcomes. These at-risk patients may benefit from targeted clinical interventions, such as closer physician follow-up, more aggressive supportive care, access to support groups, or nurse navigator visits.
Conclusions
In patients with locally advanced NSCLC treated with curative-intent following uniform treatment algorithms, marital status was linked with improvement in survival even when adjusted for other key variables, with the second highest HR (after insurance status) among pretreatment demographic variables. Although marriage is an unmodifiable factor in itself, it is most likely a surrogate for better psychosocial support. The scale of these positive survival improvements emphasizes the need to institute targeted supportive care strategies to help advance overall outcomes in a tumor for which modern therapeutic approaches (novel systemic therapy and radiation) have yielded only modest improvement in outcomes yet come at the cost of considerable treatment-related toxicity.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7-30.
2. Goldstraw P, Chansky K, Crowley J, et al. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11(1):39-51.
3. Aizer AA, Chen M-H, McCarthy EP, et al. Marital status and survival in patients with cancer. J Clin Oncol. 2013;31(31):3869-3876.
4. Jatoi A, Novotny P, Cassivi S, et al. Does marital status impact survival and quality of life in patients with non-small cell lung cancer? Observations from the mayo clinic lung cancer cohort. Oncologist. 2007;12(12):1456-1463.
5. Kravdal H, Syse A. Changes over time in the effect of marital status on cancer survival. BMC Public Health. 2011;11:804.
6. Tannenbaum SL, Zhao W, Koru-Sengul T, Miao F, Lee D, Byrne MM. Marital status and its effect on lung cancer survival. Springerplus. 2013;2:504.
7. Ellis L, Canchola AJ, Spiegel D, Ladabaum U, Haile R, Gomez SL. Racial and ethnic disparities in cancer survival: the contribution of tumor, sociodemographic, institutional, and neighborhood characteristics [published online October 16, 2017]. J Clin Oncol. 2018;36(1):25-33.
8. Scilla KA, Bentzen SM, Lam VK, et al. Neutrophil-lymphocyte ratio is a prognostic marker in patients with locally advanced (stage IIIA and IIIB) non-small cell lung cancer treated with combined modality therapy. Oncologist. 2017;22(6):737-742.
9. Lam VK, Bentzen SM, Mohindra P, et al. Obesity is associated with long-term improved survival in definitively treated locally advanced non-small cell lung cancer (NSCLC). Lung Cancer. 2017;104:52-57.
10. Vyfhuis MAL, Bhooshan N, Burrows WM, et al. Oncological outcomes from trimodality therapy receiving definitive doses of neoadjuvant chemoradiation (≥60 Gy) and factors influencing consideration for surgery in stage III non-small cell lung cancer. Adv Radiat Oncol. 2017;2(3):259-269.
11. Siddiqui F, Bae K, Langer CJ, et al. The influence of gender, race, and marital status on survival in lung cancer patients: analysis of radiation therapy oncology group trials. J Thorac Oncol. 2010;5(5):631-639.
12. Vyfhuis MAL, Bhooshan N, Molitoris J, et al. Clinical outcomes of black vs. non-black patients with locally advanced non–small cell lung cancer. Lung Cancer. 2017;114:44-49.
13. Beltran BE, Castro D, De La Cruz-Vargas JA, et al. The neutrophil-lymphocyte ratio is prognostic in patients with early stage aggressive peripheral T cell lymphoma [published online February 26, 2018]. Br J Haematol. doi:10.1111/bjh.15141.
14. Lee BM, Chung SY, Chang JS, Lee KJ, Seong J. The neutrophil-lymphocyte ratio and platelet-lymphocyte ratio are prognostic factors in patients with locally advanced pancreatic cancer treated with chemoradiotherapy. Gut Liver. 2018;12(3):342-352.
15. Najjar M, Agrawal S, Emond JC, Halazun KJ. Pretreatment neutrophil-lymphocyte ratio: useful prognostic biomarker in hepatocellular carcinoma. J Hepatocell Carcinoma. 2018;5:17-28.
16. Hu W, Yu J, Huang Y, Hu F, Zhang X, Wang Y. Lymphocyte-related inflammation and immune-based scores predict prognosis of chordoma patients after radical resection. Transl Oncol. 2018;11(2):444-449.
17. Mahal BA, Cooperberg MR, Aizer AA, et al. Who bears the greatest burden of aggressive treatment of indolent prostate cancer? Am J Med. 2015;128(6):609-616.
18. Inverso G, Mahal BA, Aizer AA, Donoff RB, Chau NG, Haddad RI. Marital status and head and neck cancer outcomes. Cancer. 2015;121(8):1273-1278.
19. Chun SG, Hu C, Choy H, et al. Impact of intensity-modulated radiation therapy technique for locally advanced non-small-cell lung cancer: a secondary analysis of the NRG oncology RTOG 0617 randomized clinical trial. J Clin Oncol. 2017;35(1):56-62.
20. Waite LJ, Lehrer EL. The benefits from marriage and religion in the United States: a comparative analysis. Popul Dev Rev. 2003;29(2):255-276.
21. Osborne C, Ostir GV, Du X, Peek MK, Goodwin JS. The influence of marital status on the stage at diagnosis, treatment, and survival of older women with breast cancer. Breast Cancer Res Treat. 2005;93(1):41-47.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7-30.
2. Goldstraw P, Chansky K, Crowley J, et al. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11(1):39-51.
3. Aizer AA, Chen M-H, McCarthy EP, et al. Marital status and survival in patients with cancer. J Clin Oncol. 2013;31(31):3869-3876.
4. Jatoi A, Novotny P, Cassivi S, et al. Does marital status impact survival and quality of life in patients with non-small cell lung cancer? Observations from the mayo clinic lung cancer cohort. Oncologist. 2007;12(12):1456-1463.
5. Kravdal H, Syse A. Changes over time in the effect of marital status on cancer survival. BMC Public Health. 2011;11:804.
6. Tannenbaum SL, Zhao W, Koru-Sengul T, Miao F, Lee D, Byrne MM. Marital status and its effect on lung cancer survival. Springerplus. 2013;2:504.
7. Ellis L, Canchola AJ, Spiegel D, Ladabaum U, Haile R, Gomez SL. Racial and ethnic disparities in cancer survival: the contribution of tumor, sociodemographic, institutional, and neighborhood characteristics [published online October 16, 2017]. J Clin Oncol. 2018;36(1):25-33.
8. Scilla KA, Bentzen SM, Lam VK, et al. Neutrophil-lymphocyte ratio is a prognostic marker in patients with locally advanced (stage IIIA and IIIB) non-small cell lung cancer treated with combined modality therapy. Oncologist. 2017;22(6):737-742.
9. Lam VK, Bentzen SM, Mohindra P, et al. Obesity is associated with long-term improved survival in definitively treated locally advanced non-small cell lung cancer (NSCLC). Lung Cancer. 2017;104:52-57.
10. Vyfhuis MAL, Bhooshan N, Burrows WM, et al. Oncological outcomes from trimodality therapy receiving definitive doses of neoadjuvant chemoradiation (≥60 Gy) and factors influencing consideration for surgery in stage III non-small cell lung cancer. Adv Radiat Oncol. 2017;2(3):259-269.
11. Siddiqui F, Bae K, Langer CJ, et al. The influence of gender, race, and marital status on survival in lung cancer patients: analysis of radiation therapy oncology group trials. J Thorac Oncol. 2010;5(5):631-639.
12. Vyfhuis MAL, Bhooshan N, Molitoris J, et al. Clinical outcomes of black vs. non-black patients with locally advanced non–small cell lung cancer. Lung Cancer. 2017;114:44-49.
13. Beltran BE, Castro D, De La Cruz-Vargas JA, et al. The neutrophil-lymphocyte ratio is prognostic in patients with early stage aggressive peripheral T cell lymphoma [published online February 26, 2018]. Br J Haematol. doi:10.1111/bjh.15141.
14. Lee BM, Chung SY, Chang JS, Lee KJ, Seong J. The neutrophil-lymphocyte ratio and platelet-lymphocyte ratio are prognostic factors in patients with locally advanced pancreatic cancer treated with chemoradiotherapy. Gut Liver. 2018;12(3):342-352.
15. Najjar M, Agrawal S, Emond JC, Halazun KJ. Pretreatment neutrophil-lymphocyte ratio: useful prognostic biomarker in hepatocellular carcinoma. J Hepatocell Carcinoma. 2018;5:17-28.
16. Hu W, Yu J, Huang Y, Hu F, Zhang X, Wang Y. Lymphocyte-related inflammation and immune-based scores predict prognosis of chordoma patients after radical resection. Transl Oncol. 2018;11(2):444-449.
17. Mahal BA, Cooperberg MR, Aizer AA, et al. Who bears the greatest burden of aggressive treatment of indolent prostate cancer? Am J Med. 2015;128(6):609-616.
18. Inverso G, Mahal BA, Aizer AA, Donoff RB, Chau NG, Haddad RI. Marital status and head and neck cancer outcomes. Cancer. 2015;121(8):1273-1278.
19. Chun SG, Hu C, Choy H, et al. Impact of intensity-modulated radiation therapy technique for locally advanced non-small-cell lung cancer: a secondary analysis of the NRG oncology RTOG 0617 randomized clinical trial. J Clin Oncol. 2017;35(1):56-62.
20. Waite LJ, Lehrer EL. The benefits from marriage and religion in the United States: a comparative analysis. Popul Dev Rev. 2003;29(2):255-276.
21. Osborne C, Ostir GV, Du X, Peek MK, Goodwin JS. The influence of marital status on the stage at diagnosis, treatment, and survival of older women with breast cancer. Breast Cancer Res Treat. 2005;93(1):41-47.
Finding that sweet spot where science, practice, and best-possible outcomes come together
The practice of oncology and the science driving it have undergone substantial change in recent years, so it was particularly exciting when this year’s Nobel Prize for Physiology or Medicine was awarded to James Allison and Tasuko Honjo for their discovery that the body’s immune system can be harnessed to fight cancer. The advent of immunotherapy has expanded our therapeutic options, especially for patients whose previous treatments have failed, and in some patients, improvement in overall survival and safety profiles have been encouraging. But we still have a way to go with immunotherapies: not all patients respond to them and they are a costly therapeutic option. In addition, while chemotherapy supresses the immune system, immune-checkpoint inhibitors can hyperactivate it, and patients can experience serious immune-related adverse events that can result in life-threatening toxicities. Among the many things we grapple with in our daily practice is pairing these new and thrilling findings with our patients on a case-by-case basis to ensure the best-possible outcomes at every level – clinical, psychosocial, financial.
In recent years, we have seen an uptick in the number of FDA approvals, and as our therapeutic options have expanded, we have been able to refine and microtarget our treatment approaches, with encouraging clinical and quality-of-life outcomes. Our approach to practice has changed as well – our care is more patient focused, and we work more as part of a team, rather than individually, to ensure that our patients’ clinical and supportive needs are met. We hope our content reflects these shifts. For example, on page e188, Ibrahimi and colleagues looked at the time from admission to treatment initiation (TAT) in patients who were newly diagnosed with acute myeloid leukemia to see if it had an impact on overall survival (OS) and event-free survival. They obtained retrospective data over 5 years, focusing on patients with a TAT of 0-4 days and those with a TAT of >4 days, and found that the median OS in the 0-4 days group was almost double that of the <4 days group (1.3 years and 0.57 years, respectively). Median event-free survival for the groups was 1.21 years and 0.57 years, respectively. Moreover, that association remained significant in a multivariate analysis adjusting for age, white blood cell count, molecular risk group, and undergoing allogeneic stem cell transplant.
Marriage and survival
Does marital status have a prognostic bearing on outcomes in patients with cancer? Vyfhuis and colleagues addressed that question in their study of patients with stage III non–small-cell lung cancer (NSCLC) who had been treated uniformly with curative intent (p. e194). Specifically, they looked at OS and freedom from recurrence and they adjusted for patient-, disease-, and treatment-specific factors, as well as the interaction with racial, nutritional, and immunologic status.
In all, 52% of patients in the study were married, and were more likely to self-identify as white; live in areas with a higher household median income; undergo surgery; and have insurance, an ECOG of 0, and higher pretreatment albumin. The authors report that on multivariate analysis, marital status remained an independent predictor of survival and was associated with a 40% decreased risk of death, further stratifying outcomes beyond gender and stage grouping. Freedom from recurrence was comparable between the married and not-married patients. These findings suggest that in a cancer such as NSCLC, for which survival is modest despite therapeutic advances and which is associated with considerable treatment-related toxicities, marital status might be an independent predictor for survival. The authors suggest that marriage is likely a surrogate for better psychosocial support, and that the survival improvements might justify investment in supportive care interventional strategies to help advance overall outcomes.
Cancer in children and AYAs
Two articles in this issue examine cancers in pediatric patients and in adolescents and young adults (AYAs), and by doing so, demonstrate the importance of having evidence-based research findings to help us refine and deliver better-quality, patient-focused care. On page e217, Sharon Worcester documents the growing efforts by researchers and clinicians to understand and address the disparities in survival outcomes between AYAs with cancer and their pediatric and adult counterparts.
It has been known for a while that some cancers are more common among AYAs compared with the other 2 populations, and others are less common. More recent findings suggest that the biology and molecular make-up of AYA cancers might also be different and therefore necessitate different therapeutic protocols, and that the social and psychological needs unique to this population also require specifically tailored supportive care. What about treatment setting for AYAs with cancer – would outcomes be better in a pediatric or adult care center? There is evidence that the pediatric setting might have some advantage, but a recent study from Canada suggests that the cost of care in that setting might be higher. Despite these encouraging findings, there are very few trials designed specifically for the AYA cancer population, and the “pediatric-versus-adult” question also applies to AYA participation in trials. Worcester’s comprehensive article weaves together these issues and offers insights and useful explanations from a number of experts who study or care for AYAs with cancers.
Pediatric cancers are rare, representing just 1% of all new cancers diagnosed annually in the United States, but they are the second leading cause of death in children aged 1 to 14 years and therefore warrant attention, writes Jane de Lartigue in an article on page e210. She echoes Worcester’s point that better understanding of cancers in this younger population has brought to light their unique molecular drivers and challenged the assumption that drugs developed for adults can be used in children and young adults. Dr de Lartigue drills down into the science behind the unique biology and molecular aberrations in pediatric cancers and provides a useful list of ongoing clinical trials of targeted therapies in this population. She notes that because of their rarity, pediatric cancers are difficult to study and adequate enrollment in trials is challenging, although that is changing with researchers’ greater awareness of the uniqueness of these cancers and need for age-specific trials.
Also included in this issue are Community Translation articles on the approval of an immunotherapy combination – nivolumab plus ipilimumab – for the treatment of advanced RCC (p. e182), and for venetoclax as a therapy for patients with chronic lymphocytic leukemia, regardless of genotype (p. e185); and 2 Case Reports, one describing a diagnostic dilemma relating to a patient eventually diagnosed with primary renal synovial sarcoma (p. e202), and another detailing prolonged survival in a patient with adenocarcinoma of unknown primary who was treated with chemoradiotherapy (p. e206).
The practice of oncology and the science driving it have undergone substantial change in recent years, so it was particularly exciting when this year’s Nobel Prize for Physiology or Medicine was awarded to James Allison and Tasuko Honjo for their discovery that the body’s immune system can be harnessed to fight cancer. The advent of immunotherapy has expanded our therapeutic options, especially for patients whose previous treatments have failed, and in some patients, improvement in overall survival and safety profiles have been encouraging. But we still have a way to go with immunotherapies: not all patients respond to them and they are a costly therapeutic option. In addition, while chemotherapy supresses the immune system, immune-checkpoint inhibitors can hyperactivate it, and patients can experience serious immune-related adverse events that can result in life-threatening toxicities. Among the many things we grapple with in our daily practice is pairing these new and thrilling findings with our patients on a case-by-case basis to ensure the best-possible outcomes at every level – clinical, psychosocial, financial.
In recent years, we have seen an uptick in the number of FDA approvals, and as our therapeutic options have expanded, we have been able to refine and microtarget our treatment approaches, with encouraging clinical and quality-of-life outcomes. Our approach to practice has changed as well – our care is more patient focused, and we work more as part of a team, rather than individually, to ensure that our patients’ clinical and supportive needs are met. We hope our content reflects these shifts. For example, on page e188, Ibrahimi and colleagues looked at the time from admission to treatment initiation (TAT) in patients who were newly diagnosed with acute myeloid leukemia to see if it had an impact on overall survival (OS) and event-free survival. They obtained retrospective data over 5 years, focusing on patients with a TAT of 0-4 days and those with a TAT of >4 days, and found that the median OS in the 0-4 days group was almost double that of the <4 days group (1.3 years and 0.57 years, respectively). Median event-free survival for the groups was 1.21 years and 0.57 years, respectively. Moreover, that association remained significant in a multivariate analysis adjusting for age, white blood cell count, molecular risk group, and undergoing allogeneic stem cell transplant.
Marriage and survival
Does marital status have a prognostic bearing on outcomes in patients with cancer? Vyfhuis and colleagues addressed that question in their study of patients with stage III non–small-cell lung cancer (NSCLC) who had been treated uniformly with curative intent (p. e194). Specifically, they looked at OS and freedom from recurrence and they adjusted for patient-, disease-, and treatment-specific factors, as well as the interaction with racial, nutritional, and immunologic status.
In all, 52% of patients in the study were married, and were more likely to self-identify as white; live in areas with a higher household median income; undergo surgery; and have insurance, an ECOG of 0, and higher pretreatment albumin. The authors report that on multivariate analysis, marital status remained an independent predictor of survival and was associated with a 40% decreased risk of death, further stratifying outcomes beyond gender and stage grouping. Freedom from recurrence was comparable between the married and not-married patients. These findings suggest that in a cancer such as NSCLC, for which survival is modest despite therapeutic advances and which is associated with considerable treatment-related toxicities, marital status might be an independent predictor for survival. The authors suggest that marriage is likely a surrogate for better psychosocial support, and that the survival improvements might justify investment in supportive care interventional strategies to help advance overall outcomes.
Cancer in children and AYAs
Two articles in this issue examine cancers in pediatric patients and in adolescents and young adults (AYAs), and by doing so, demonstrate the importance of having evidence-based research findings to help us refine and deliver better-quality, patient-focused care. On page e217, Sharon Worcester documents the growing efforts by researchers and clinicians to understand and address the disparities in survival outcomes between AYAs with cancer and their pediatric and adult counterparts.
It has been known for a while that some cancers are more common among AYAs compared with the other 2 populations, and others are less common. More recent findings suggest that the biology and molecular make-up of AYA cancers might also be different and therefore necessitate different therapeutic protocols, and that the social and psychological needs unique to this population also require specifically tailored supportive care. What about treatment setting for AYAs with cancer – would outcomes be better in a pediatric or adult care center? There is evidence that the pediatric setting might have some advantage, but a recent study from Canada suggests that the cost of care in that setting might be higher. Despite these encouraging findings, there are very few trials designed specifically for the AYA cancer population, and the “pediatric-versus-adult” question also applies to AYA participation in trials. Worcester’s comprehensive article weaves together these issues and offers insights and useful explanations from a number of experts who study or care for AYAs with cancers.
Pediatric cancers are rare, representing just 1% of all new cancers diagnosed annually in the United States, but they are the second leading cause of death in children aged 1 to 14 years and therefore warrant attention, writes Jane de Lartigue in an article on page e210. She echoes Worcester’s point that better understanding of cancers in this younger population has brought to light their unique molecular drivers and challenged the assumption that drugs developed for adults can be used in children and young adults. Dr de Lartigue drills down into the science behind the unique biology and molecular aberrations in pediatric cancers and provides a useful list of ongoing clinical trials of targeted therapies in this population. She notes that because of their rarity, pediatric cancers are difficult to study and adequate enrollment in trials is challenging, although that is changing with researchers’ greater awareness of the uniqueness of these cancers and need for age-specific trials.
Also included in this issue are Community Translation articles on the approval of an immunotherapy combination – nivolumab plus ipilimumab – for the treatment of advanced RCC (p. e182), and for venetoclax as a therapy for patients with chronic lymphocytic leukemia, regardless of genotype (p. e185); and 2 Case Reports, one describing a diagnostic dilemma relating to a patient eventually diagnosed with primary renal synovial sarcoma (p. e202), and another detailing prolonged survival in a patient with adenocarcinoma of unknown primary who was treated with chemoradiotherapy (p. e206).
The practice of oncology and the science driving it have undergone substantial change in recent years, so it was particularly exciting when this year’s Nobel Prize for Physiology or Medicine was awarded to James Allison and Tasuko Honjo for their discovery that the body’s immune system can be harnessed to fight cancer. The advent of immunotherapy has expanded our therapeutic options, especially for patients whose previous treatments have failed, and in some patients, improvement in overall survival and safety profiles have been encouraging. But we still have a way to go with immunotherapies: not all patients respond to them and they are a costly therapeutic option. In addition, while chemotherapy supresses the immune system, immune-checkpoint inhibitors can hyperactivate it, and patients can experience serious immune-related adverse events that can result in life-threatening toxicities. Among the many things we grapple with in our daily practice is pairing these new and thrilling findings with our patients on a case-by-case basis to ensure the best-possible outcomes at every level – clinical, psychosocial, financial.
In recent years, we have seen an uptick in the number of FDA approvals, and as our therapeutic options have expanded, we have been able to refine and microtarget our treatment approaches, with encouraging clinical and quality-of-life outcomes. Our approach to practice has changed as well – our care is more patient focused, and we work more as part of a team, rather than individually, to ensure that our patients’ clinical and supportive needs are met. We hope our content reflects these shifts. For example, on page e188, Ibrahimi and colleagues looked at the time from admission to treatment initiation (TAT) in patients who were newly diagnosed with acute myeloid leukemia to see if it had an impact on overall survival (OS) and event-free survival. They obtained retrospective data over 5 years, focusing on patients with a TAT of 0-4 days and those with a TAT of >4 days, and found that the median OS in the 0-4 days group was almost double that of the <4 days group (1.3 years and 0.57 years, respectively). Median event-free survival for the groups was 1.21 years and 0.57 years, respectively. Moreover, that association remained significant in a multivariate analysis adjusting for age, white blood cell count, molecular risk group, and undergoing allogeneic stem cell transplant.
Marriage and survival
Does marital status have a prognostic bearing on outcomes in patients with cancer? Vyfhuis and colleagues addressed that question in their study of patients with stage III non–small-cell lung cancer (NSCLC) who had been treated uniformly with curative intent (p. e194). Specifically, they looked at OS and freedom from recurrence and they adjusted for patient-, disease-, and treatment-specific factors, as well as the interaction with racial, nutritional, and immunologic status.
In all, 52% of patients in the study were married, and were more likely to self-identify as white; live in areas with a higher household median income; undergo surgery; and have insurance, an ECOG of 0, and higher pretreatment albumin. The authors report that on multivariate analysis, marital status remained an independent predictor of survival and was associated with a 40% decreased risk of death, further stratifying outcomes beyond gender and stage grouping. Freedom from recurrence was comparable between the married and not-married patients. These findings suggest that in a cancer such as NSCLC, for which survival is modest despite therapeutic advances and which is associated with considerable treatment-related toxicities, marital status might be an independent predictor for survival. The authors suggest that marriage is likely a surrogate for better psychosocial support, and that the survival improvements might justify investment in supportive care interventional strategies to help advance overall outcomes.
Cancer in children and AYAs
Two articles in this issue examine cancers in pediatric patients and in adolescents and young adults (AYAs), and by doing so, demonstrate the importance of having evidence-based research findings to help us refine and deliver better-quality, patient-focused care. On page e217, Sharon Worcester documents the growing efforts by researchers and clinicians to understand and address the disparities in survival outcomes between AYAs with cancer and their pediatric and adult counterparts.
It has been known for a while that some cancers are more common among AYAs compared with the other 2 populations, and others are less common. More recent findings suggest that the biology and molecular make-up of AYA cancers might also be different and therefore necessitate different therapeutic protocols, and that the social and psychological needs unique to this population also require specifically tailored supportive care. What about treatment setting for AYAs with cancer – would outcomes be better in a pediatric or adult care center? There is evidence that the pediatric setting might have some advantage, but a recent study from Canada suggests that the cost of care in that setting might be higher. Despite these encouraging findings, there are very few trials designed specifically for the AYA cancer population, and the “pediatric-versus-adult” question also applies to AYA participation in trials. Worcester’s comprehensive article weaves together these issues and offers insights and useful explanations from a number of experts who study or care for AYAs with cancers.
Pediatric cancers are rare, representing just 1% of all new cancers diagnosed annually in the United States, but they are the second leading cause of death in children aged 1 to 14 years and therefore warrant attention, writes Jane de Lartigue in an article on page e210. She echoes Worcester’s point that better understanding of cancers in this younger population has brought to light their unique molecular drivers and challenged the assumption that drugs developed for adults can be used in children and young adults. Dr de Lartigue drills down into the science behind the unique biology and molecular aberrations in pediatric cancers and provides a useful list of ongoing clinical trials of targeted therapies in this population. She notes that because of their rarity, pediatric cancers are difficult to study and adequate enrollment in trials is challenging, although that is changing with researchers’ greater awareness of the uniqueness of these cancers and need for age-specific trials.
Also included in this issue are Community Translation articles on the approval of an immunotherapy combination – nivolumab plus ipilimumab – for the treatment of advanced RCC (p. e182), and for venetoclax as a therapy for patients with chronic lymphocytic leukemia, regardless of genotype (p. e185); and 2 Case Reports, one describing a diagnostic dilemma relating to a patient eventually diagnosed with primary renal synovial sarcoma (p. e202), and another detailing prolonged survival in a patient with adenocarcinoma of unknown primary who was treated with chemoradiotherapy (p. e206).
No ADT-dementia link in large VA prostate cancer cohort study
In contrast to other recent studies, androgen deprivation therapy (ADT) had no link to dementia in a observational cohort study of more than 45,000 men with prostate cancer who received definitive radiotherapy, investigators have reported.
No significant associations were found between ADT and Alzheimer’s disease or vascular dementia, or between shorter or longer courses of ADT and any dementia studied, according to Rishi Deka, PhD, of Veterans Affairs San Diego Health Care System, La Jolla, Calif., and coinvestigators.
“These results may mitigate concerns regarding the long-term risks of ADT on cognitive health in the treatment of prostate cancer,” Dr. Deka and colleagues wrote in JAMA Oncology.
Two other recent studies showed strong, statistically significant associations between ADT and dementia in prostate cancer. However, those studies combined patients with local and metastatic disease, receiving ADT in the upfront or recurrent settings, while the present study looked specifically at men with nonmetastatic prostate cancer who received radiotherapy.
“Different treatment modalities and disease stages are associated with substantial selection bias that may predispose results to false associations,” noted Dr. Deka and coauthors.
Their observational cohort study comprised 45,218 men diagnosed with nonmetastatic prostate cancer at the U.S. Department of Veterans Affairs who underwent radiotherapy with or without ADT. The investigators excluded men who had a diagnosis of dementia within 1 year of the prostate cancer diagnosis or who had prior diagnoses of dementia, stroke, or cognitive impairment.
A total of 1,497 patients were diagnosed with dementia over a median of 6.8 years of follow-up: 404 with Alzheimer disease, 335 with vascular dementia, and 758 with other types or unclassified dementias.
The investigators found no significant association between use of ADT and development of any dementia, the primary outcome of the analysis (subdistribution hazard ratio [SHR], 1.04; 95% confidence interval, 0.94-1.16; P = .43).
Likewise, there was no association between ADT and vascular dementia, specifically, with an SHR of 1.20 (95% CI, 0.97-1.50; P = .10) or Alzheimer’s disease, with an SHR of 1.11 (95% CI, 0.91-1.36; P = .29).
Duration of ADT longer than 1 year was not significantly associated with dementia, nor was duration shorter than 1 year, with SHRs, of 1.08 and 1.01 respectively, the analysis shows.
The SHRs in these and other analysis reported ranged from 1.00 to 1.21. That is substantially lower than hazard ratios of 1.66 to 2.32 in one previous study linking ADT to dementia, according to the investigators, suggesting that the results of the current analysis were not due to inadequate power to detect differences.
Nevertheless, the findings may not be generalizable to some other populations, they cautioned, since it was focused demographically on veterans, and was limited to radiotherapy-treated patients.
Dr. Deka and coauthors reported no conflict of interest. Their study was funded by grants from the University of California San Diego Center for Precision Radiation Medicine.
SOURCE: Deka R et al. JAMA Oncol. 2018 Oct 11. doi: 10.1001/jamaoncol.2018.4423.
In contrast to other recent studies, androgen deprivation therapy (ADT) had no link to dementia in a observational cohort study of more than 45,000 men with prostate cancer who received definitive radiotherapy, investigators have reported.
No significant associations were found between ADT and Alzheimer’s disease or vascular dementia, or between shorter or longer courses of ADT and any dementia studied, according to Rishi Deka, PhD, of Veterans Affairs San Diego Health Care System, La Jolla, Calif., and coinvestigators.
“These results may mitigate concerns regarding the long-term risks of ADT on cognitive health in the treatment of prostate cancer,” Dr. Deka and colleagues wrote in JAMA Oncology.
Two other recent studies showed strong, statistically significant associations between ADT and dementia in prostate cancer. However, those studies combined patients with local and metastatic disease, receiving ADT in the upfront or recurrent settings, while the present study looked specifically at men with nonmetastatic prostate cancer who received radiotherapy.
“Different treatment modalities and disease stages are associated with substantial selection bias that may predispose results to false associations,” noted Dr. Deka and coauthors.
Their observational cohort study comprised 45,218 men diagnosed with nonmetastatic prostate cancer at the U.S. Department of Veterans Affairs who underwent radiotherapy with or without ADT. The investigators excluded men who had a diagnosis of dementia within 1 year of the prostate cancer diagnosis or who had prior diagnoses of dementia, stroke, or cognitive impairment.
A total of 1,497 patients were diagnosed with dementia over a median of 6.8 years of follow-up: 404 with Alzheimer disease, 335 with vascular dementia, and 758 with other types or unclassified dementias.
The investigators found no significant association between use of ADT and development of any dementia, the primary outcome of the analysis (subdistribution hazard ratio [SHR], 1.04; 95% confidence interval, 0.94-1.16; P = .43).
Likewise, there was no association between ADT and vascular dementia, specifically, with an SHR of 1.20 (95% CI, 0.97-1.50; P = .10) or Alzheimer’s disease, with an SHR of 1.11 (95% CI, 0.91-1.36; P = .29).
Duration of ADT longer than 1 year was not significantly associated with dementia, nor was duration shorter than 1 year, with SHRs, of 1.08 and 1.01 respectively, the analysis shows.
The SHRs in these and other analysis reported ranged from 1.00 to 1.21. That is substantially lower than hazard ratios of 1.66 to 2.32 in one previous study linking ADT to dementia, according to the investigators, suggesting that the results of the current analysis were not due to inadequate power to detect differences.
Nevertheless, the findings may not be generalizable to some other populations, they cautioned, since it was focused demographically on veterans, and was limited to radiotherapy-treated patients.
Dr. Deka and coauthors reported no conflict of interest. Their study was funded by grants from the University of California San Diego Center for Precision Radiation Medicine.
SOURCE: Deka R et al. JAMA Oncol. 2018 Oct 11. doi: 10.1001/jamaoncol.2018.4423.
In contrast to other recent studies, androgen deprivation therapy (ADT) had no link to dementia in a observational cohort study of more than 45,000 men with prostate cancer who received definitive radiotherapy, investigators have reported.
No significant associations were found between ADT and Alzheimer’s disease or vascular dementia, or between shorter or longer courses of ADT and any dementia studied, according to Rishi Deka, PhD, of Veterans Affairs San Diego Health Care System, La Jolla, Calif., and coinvestigators.
“These results may mitigate concerns regarding the long-term risks of ADT on cognitive health in the treatment of prostate cancer,” Dr. Deka and colleagues wrote in JAMA Oncology.
Two other recent studies showed strong, statistically significant associations between ADT and dementia in prostate cancer. However, those studies combined patients with local and metastatic disease, receiving ADT in the upfront or recurrent settings, while the present study looked specifically at men with nonmetastatic prostate cancer who received radiotherapy.
“Different treatment modalities and disease stages are associated with substantial selection bias that may predispose results to false associations,” noted Dr. Deka and coauthors.
Their observational cohort study comprised 45,218 men diagnosed with nonmetastatic prostate cancer at the U.S. Department of Veterans Affairs who underwent radiotherapy with or without ADT. The investigators excluded men who had a diagnosis of dementia within 1 year of the prostate cancer diagnosis or who had prior diagnoses of dementia, stroke, or cognitive impairment.
A total of 1,497 patients were diagnosed with dementia over a median of 6.8 years of follow-up: 404 with Alzheimer disease, 335 with vascular dementia, and 758 with other types or unclassified dementias.
The investigators found no significant association between use of ADT and development of any dementia, the primary outcome of the analysis (subdistribution hazard ratio [SHR], 1.04; 95% confidence interval, 0.94-1.16; P = .43).
Likewise, there was no association between ADT and vascular dementia, specifically, with an SHR of 1.20 (95% CI, 0.97-1.50; P = .10) or Alzheimer’s disease, with an SHR of 1.11 (95% CI, 0.91-1.36; P = .29).
Duration of ADT longer than 1 year was not significantly associated with dementia, nor was duration shorter than 1 year, with SHRs, of 1.08 and 1.01 respectively, the analysis shows.
The SHRs in these and other analysis reported ranged from 1.00 to 1.21. That is substantially lower than hazard ratios of 1.66 to 2.32 in one previous study linking ADT to dementia, according to the investigators, suggesting that the results of the current analysis were not due to inadequate power to detect differences.
Nevertheless, the findings may not be generalizable to some other populations, they cautioned, since it was focused demographically on veterans, and was limited to radiotherapy-treated patients.
Dr. Deka and coauthors reported no conflict of interest. Their study was funded by grants from the University of California San Diego Center for Precision Radiation Medicine.
SOURCE: Deka R et al. JAMA Oncol. 2018 Oct 11. doi: 10.1001/jamaoncol.2018.4423.
FROM JAMA ONCOLOGY
Key clinical point: In contrast with other recent investigations in prostate cancer, researchers found no link between androgen deprivation therapy (ADT) and development of dementia.
Major finding: No significant association was found between use of ADT and development of any dementia (subdistribution hazard ratio [SHR], 1.04; 95% CI, 0.94-1.16; P = .43).
Study details: Observational cohort study of more than 45,000 veterans with nonmetastatic prostate cancer treated with radiotherapy with or without ADT.
Disclosures: This study was funded by grants from the University of California San Diego Center for Precision Radiation Medicine. Dr. Deka and coauthors reported no conflict of interest disclosures related to the work.
Source: Deka R et al. JAMA Oncol. 2018 Oct 11. doi: 10.1001/jamaoncol.2018.4423.
Tech-based cancer company raises access concerns
Oncologists are raising concerns about care access after the launch of a new company that links patients to cancer care options and clinical trials through mobile technology.
Driver, which began in September in the U.S. and China, is a global technology platform that allows patients to access treatment options across a broad network of cancer centers without leaving home. Cancer patients join the platform using a mobile app, through which Driver obtains the required consent to acquire medical records and tumor samples, and the company uses the information to recommend treatment options and clinical trials.
A separate app called Driver for Clinic enables oncologists who belong to Driver’s partner hospitals to manage their institution’s clinical trial information and quickly filter that information based on patients’ medical history to determine the patient’s eligibility for treatments.
Driver’s mission is to connect more patients to the best cancer treatments, regardless of location, said Will Polkinghorn, MD, Driver cofounder and CEO.
“Driver’s cofounders met at Harvard Medical School [in Boston] and saw firsthand the challenges of patients getting access to the latest, cutting-edge treatments available,” Dr. Polkinghorn said in an interview. “As doctors, [we] also witnessed how difficult it was for doctors to manage information in clinic and know about all the treatments that become available all around the world. Driver was created as a platform, with an app for the patient and an app for the doctor, to solve this broken marketplace.”
As part of the model, patients can review their recommended treatment options through video with an expert oncologist and select a hospital within Driver’s network for further evaluation. The company’s global network includes more than 30 leading U.S. cancer centers, including the Cleveland Clinic; multiple locations of the Mayo Clinic; the University of California, San Francisco; and Massachusetts General Hospital, Boston. The U.S. National Cancer Institute (NCI) and the Chinese National Cancer Center are founding members of Driver’s global network, according to the company.
Making more information and treatment options available is a positive for patients, said Walter Stadler, MD, chief of hematology/oncology and director of the genitourinary oncology program at the University of Chicago. However, he noted that the cost for patients to use Driver is prohibitive for many patients. Driver charges patients $3,000 up front and then a $20 monthly fee to use its service. Insurance does not subsidize the cost, nor does Driver help with travel or treatment costs, according to its website.
“It’s inequality of access,” Dr. Stadler said in an interview. “Many of us are very concerned that the clinical trials currently being conducted do not represent the general population well because they don’t represent patients with disparities … Here, we further exacerbate the problem by saying, ‘Okay, we’ll take the 5% of patients who can afford the service and expand their access, and the others, well, that’s not our problem.’ ”
Kashyap Patel, MD, secretary for the Community Oncology Alliance and CEO for the Carolina Blood and Cancer Care in Rock Hill, S.C., also sees positives and negatives about the business model. Using technology to link patients with care and clinical trials can help speed treatment and accelerate drug development, he said. But Driver’s network of large tertiary care centers in metropolitan areas poses challenges for rural cancer patients, he said.
“Access to clinical trials for patients residing in rural areas, as well as those getting their treatment in community based clinics, would not change,” Dr. Patel said in an interview. “Hence, challenges of social and demographic disparities and inequalities in clinical trial access and participation would be altered minimally. There is much greater need for such [platforms to include] community cancer clinics that would be more inclusive and encompass larger geographic areas where the majority of patients receive their care.”
Disadvantaged populations with limited access are not being overlooked by the company, according to Driver leaders. A branch of the company called Driver for All aims to increase access to optimal treatments for free through partnerships with local communities, Dr. Polkinghorn said. Driver for All has thus far partnered with Howard University Hospital in Washington to connect Howard patients to clinical trials at NCI. A partnership with Beijing Children’s Hospital and the Futang Research Center of Pediatric Development, meanwhile, is working to connect patients with rare-disease experts. Driver has funded 100% of the cost of these projects to date, according to its website.
Outside of Driver for All, Dr. Polkinghorn acknowledges that patients must bear the cost of Driver’s consumer products; however, the price should be viewed in context, he said.
“It’s important to remember that today, in order to be evaluated by 30 [plus] centers for treatment options, patients would need to fly to these centers, make appointments, and be seen by a doctor – this would require both time and resources for flights/hotels, which would cost much more than our sticker price,” he said. “So while $3,000 is a lot of money for some patients, Driver’s product is ultimately able to provide more visibility to options that simply would not be realistic today.”
James Gulley, MD, of the National Cancer Institute Center for Cancer Research, said any platform that can efficiently provide access to clinical trial options yields another source of information for patients to utilize in decision making with their health provider. Dr. Gulley, who heads the center’s genitourinary malignancies branch, declined to comment about access-to-care concerns with Driver’s model. He emphasized that patients who participate in NIH research studies are treated without charge.
“The key to finding better [cancer] treatment is to perform science-driven clinical trials,” Dr. Gulley said in an interview. “However, there are many barriers for enrollment in clinical trials. … As a government agency, NCI is open to partnering with any organization that seeks to improve access to clinical trials for cancer patients.”
NCI and Driver recently conducted a study to validate Driver’s platform; it showed that Driver’s technology successfully predicted the eligibility of patients in NCI Center for Cancer Research clinical trials. The study, presented at a recent American Society of Clinical Oncology meeting, evaluated Driver’s processing of 21 metastatic prostate cancer patients enrolled in a therapeutic NCI clinical trial within the last five years. Results showed Driver correctly predicted that 20 of the patients were “potentially eligible” for the trial in which they were enrolled, and that one was ineligible. Based on the study, a protocol is now in development for a new clinical study, which will seek to further determine the efficiency and accuracy of the clinical trial access program created by Driver, according to Dr. Gulley.
Charles Ryan, MD, director of the division of hematology, oncology, and transplantation for the University of Minnesota, Minneapolis, views Driver’s platform as a way to eliminate geographical barriers, which often keep patients from care, while at the same time enabling researchers to find the right patients for clinical trials.
“We need breakthrough technologies and opportunities for patients to be able to access the most successful and promising cancer treatments, regardless of where they live,” Dr. Ryan said in an interview. “Companies like Driver are attempting to bridge that gap by connecting patients to doctors at world class cancer institutes and direct them toward the best care for their particular condition.”
Driver’s model also allows researchers the opportunity to develop specific, unique treatment for less common cancers and remain optimistic that they can attract patients to receive such treatments as they are developed, Dr. Ryan said.
Dr. Stadler, however, worries that Driver may be giving patients the wrong perception that all it takes is a computer and medical records to determine their best treatment route.
“There’s a lot more subtlety to treatment decisions than most people would like to admit,” Dr. Stadler said. “It’s more than just a bunch of data from sophisticated laboratory tests and the written medical record. Obtaining objective information is the first step, but it’s far from the only step.”
Patients may have significant limitations in functional status that is apparent only during an in-person assessment, for example, he said. In other cases, family members may be essential in conveying information about a patient’s cognitive disabilities. Even when such information is documented, it is sometimes difficult to extract the full picture from the record alone, he said. Dr. Stadler is also bothered that the model requires physicians and hospitals to provide their skilled analyses to a for-profit company, which in turn, charges patients to review the information.
“This is our work,” he said. “I agree that patients should have the information, and I don’t mind sharing anything I have with patients, but now I’m going to share it with another business that essentially is competing with me in terms of providing guidance to patients.”
Oncologists are raising concerns about care access after the launch of a new company that links patients to cancer care options and clinical trials through mobile technology.
Driver, which began in September in the U.S. and China, is a global technology platform that allows patients to access treatment options across a broad network of cancer centers without leaving home. Cancer patients join the platform using a mobile app, through which Driver obtains the required consent to acquire medical records and tumor samples, and the company uses the information to recommend treatment options and clinical trials.
A separate app called Driver for Clinic enables oncologists who belong to Driver’s partner hospitals to manage their institution’s clinical trial information and quickly filter that information based on patients’ medical history to determine the patient’s eligibility for treatments.
Driver’s mission is to connect more patients to the best cancer treatments, regardless of location, said Will Polkinghorn, MD, Driver cofounder and CEO.
“Driver’s cofounders met at Harvard Medical School [in Boston] and saw firsthand the challenges of patients getting access to the latest, cutting-edge treatments available,” Dr. Polkinghorn said in an interview. “As doctors, [we] also witnessed how difficult it was for doctors to manage information in clinic and know about all the treatments that become available all around the world. Driver was created as a platform, with an app for the patient and an app for the doctor, to solve this broken marketplace.”
As part of the model, patients can review their recommended treatment options through video with an expert oncologist and select a hospital within Driver’s network for further evaluation. The company’s global network includes more than 30 leading U.S. cancer centers, including the Cleveland Clinic; multiple locations of the Mayo Clinic; the University of California, San Francisco; and Massachusetts General Hospital, Boston. The U.S. National Cancer Institute (NCI) and the Chinese National Cancer Center are founding members of Driver’s global network, according to the company.
Making more information and treatment options available is a positive for patients, said Walter Stadler, MD, chief of hematology/oncology and director of the genitourinary oncology program at the University of Chicago. However, he noted that the cost for patients to use Driver is prohibitive for many patients. Driver charges patients $3,000 up front and then a $20 monthly fee to use its service. Insurance does not subsidize the cost, nor does Driver help with travel or treatment costs, according to its website.
“It’s inequality of access,” Dr. Stadler said in an interview. “Many of us are very concerned that the clinical trials currently being conducted do not represent the general population well because they don’t represent patients with disparities … Here, we further exacerbate the problem by saying, ‘Okay, we’ll take the 5% of patients who can afford the service and expand their access, and the others, well, that’s not our problem.’ ”
Kashyap Patel, MD, secretary for the Community Oncology Alliance and CEO for the Carolina Blood and Cancer Care in Rock Hill, S.C., also sees positives and negatives about the business model. Using technology to link patients with care and clinical trials can help speed treatment and accelerate drug development, he said. But Driver’s network of large tertiary care centers in metropolitan areas poses challenges for rural cancer patients, he said.
“Access to clinical trials for patients residing in rural areas, as well as those getting their treatment in community based clinics, would not change,” Dr. Patel said in an interview. “Hence, challenges of social and demographic disparities and inequalities in clinical trial access and participation would be altered minimally. There is much greater need for such [platforms to include] community cancer clinics that would be more inclusive and encompass larger geographic areas where the majority of patients receive their care.”
Disadvantaged populations with limited access are not being overlooked by the company, according to Driver leaders. A branch of the company called Driver for All aims to increase access to optimal treatments for free through partnerships with local communities, Dr. Polkinghorn said. Driver for All has thus far partnered with Howard University Hospital in Washington to connect Howard patients to clinical trials at NCI. A partnership with Beijing Children’s Hospital and the Futang Research Center of Pediatric Development, meanwhile, is working to connect patients with rare-disease experts. Driver has funded 100% of the cost of these projects to date, according to its website.
Outside of Driver for All, Dr. Polkinghorn acknowledges that patients must bear the cost of Driver’s consumer products; however, the price should be viewed in context, he said.
“It’s important to remember that today, in order to be evaluated by 30 [plus] centers for treatment options, patients would need to fly to these centers, make appointments, and be seen by a doctor – this would require both time and resources for flights/hotels, which would cost much more than our sticker price,” he said. “So while $3,000 is a lot of money for some patients, Driver’s product is ultimately able to provide more visibility to options that simply would not be realistic today.”
James Gulley, MD, of the National Cancer Institute Center for Cancer Research, said any platform that can efficiently provide access to clinical trial options yields another source of information for patients to utilize in decision making with their health provider. Dr. Gulley, who heads the center’s genitourinary malignancies branch, declined to comment about access-to-care concerns with Driver’s model. He emphasized that patients who participate in NIH research studies are treated without charge.
“The key to finding better [cancer] treatment is to perform science-driven clinical trials,” Dr. Gulley said in an interview. “However, there are many barriers for enrollment in clinical trials. … As a government agency, NCI is open to partnering with any organization that seeks to improve access to clinical trials for cancer patients.”
NCI and Driver recently conducted a study to validate Driver’s platform; it showed that Driver’s technology successfully predicted the eligibility of patients in NCI Center for Cancer Research clinical trials. The study, presented at a recent American Society of Clinical Oncology meeting, evaluated Driver’s processing of 21 metastatic prostate cancer patients enrolled in a therapeutic NCI clinical trial within the last five years. Results showed Driver correctly predicted that 20 of the patients were “potentially eligible” for the trial in which they were enrolled, and that one was ineligible. Based on the study, a protocol is now in development for a new clinical study, which will seek to further determine the efficiency and accuracy of the clinical trial access program created by Driver, according to Dr. Gulley.
Charles Ryan, MD, director of the division of hematology, oncology, and transplantation for the University of Minnesota, Minneapolis, views Driver’s platform as a way to eliminate geographical barriers, which often keep patients from care, while at the same time enabling researchers to find the right patients for clinical trials.
“We need breakthrough technologies and opportunities for patients to be able to access the most successful and promising cancer treatments, regardless of where they live,” Dr. Ryan said in an interview. “Companies like Driver are attempting to bridge that gap by connecting patients to doctors at world class cancer institutes and direct them toward the best care for their particular condition.”
Driver’s model also allows researchers the opportunity to develop specific, unique treatment for less common cancers and remain optimistic that they can attract patients to receive such treatments as they are developed, Dr. Ryan said.
Dr. Stadler, however, worries that Driver may be giving patients the wrong perception that all it takes is a computer and medical records to determine their best treatment route.
“There’s a lot more subtlety to treatment decisions than most people would like to admit,” Dr. Stadler said. “It’s more than just a bunch of data from sophisticated laboratory tests and the written medical record. Obtaining objective information is the first step, but it’s far from the only step.”
Patients may have significant limitations in functional status that is apparent only during an in-person assessment, for example, he said. In other cases, family members may be essential in conveying information about a patient’s cognitive disabilities. Even when such information is documented, it is sometimes difficult to extract the full picture from the record alone, he said. Dr. Stadler is also bothered that the model requires physicians and hospitals to provide their skilled analyses to a for-profit company, which in turn, charges patients to review the information.
“This is our work,” he said. “I agree that patients should have the information, and I don’t mind sharing anything I have with patients, but now I’m going to share it with another business that essentially is competing with me in terms of providing guidance to patients.”
Oncologists are raising concerns about care access after the launch of a new company that links patients to cancer care options and clinical trials through mobile technology.
Driver, which began in September in the U.S. and China, is a global technology platform that allows patients to access treatment options across a broad network of cancer centers without leaving home. Cancer patients join the platform using a mobile app, through which Driver obtains the required consent to acquire medical records and tumor samples, and the company uses the information to recommend treatment options and clinical trials.
A separate app called Driver for Clinic enables oncologists who belong to Driver’s partner hospitals to manage their institution’s clinical trial information and quickly filter that information based on patients’ medical history to determine the patient’s eligibility for treatments.
Driver’s mission is to connect more patients to the best cancer treatments, regardless of location, said Will Polkinghorn, MD, Driver cofounder and CEO.
“Driver’s cofounders met at Harvard Medical School [in Boston] and saw firsthand the challenges of patients getting access to the latest, cutting-edge treatments available,” Dr. Polkinghorn said in an interview. “As doctors, [we] also witnessed how difficult it was for doctors to manage information in clinic and know about all the treatments that become available all around the world. Driver was created as a platform, with an app for the patient and an app for the doctor, to solve this broken marketplace.”
As part of the model, patients can review their recommended treatment options through video with an expert oncologist and select a hospital within Driver’s network for further evaluation. The company’s global network includes more than 30 leading U.S. cancer centers, including the Cleveland Clinic; multiple locations of the Mayo Clinic; the University of California, San Francisco; and Massachusetts General Hospital, Boston. The U.S. National Cancer Institute (NCI) and the Chinese National Cancer Center are founding members of Driver’s global network, according to the company.
Making more information and treatment options available is a positive for patients, said Walter Stadler, MD, chief of hematology/oncology and director of the genitourinary oncology program at the University of Chicago. However, he noted that the cost for patients to use Driver is prohibitive for many patients. Driver charges patients $3,000 up front and then a $20 monthly fee to use its service. Insurance does not subsidize the cost, nor does Driver help with travel or treatment costs, according to its website.
“It’s inequality of access,” Dr. Stadler said in an interview. “Many of us are very concerned that the clinical trials currently being conducted do not represent the general population well because they don’t represent patients with disparities … Here, we further exacerbate the problem by saying, ‘Okay, we’ll take the 5% of patients who can afford the service and expand their access, and the others, well, that’s not our problem.’ ”
Kashyap Patel, MD, secretary for the Community Oncology Alliance and CEO for the Carolina Blood and Cancer Care in Rock Hill, S.C., also sees positives and negatives about the business model. Using technology to link patients with care and clinical trials can help speed treatment and accelerate drug development, he said. But Driver’s network of large tertiary care centers in metropolitan areas poses challenges for rural cancer patients, he said.
“Access to clinical trials for patients residing in rural areas, as well as those getting their treatment in community based clinics, would not change,” Dr. Patel said in an interview. “Hence, challenges of social and demographic disparities and inequalities in clinical trial access and participation would be altered minimally. There is much greater need for such [platforms to include] community cancer clinics that would be more inclusive and encompass larger geographic areas where the majority of patients receive their care.”
Disadvantaged populations with limited access are not being overlooked by the company, according to Driver leaders. A branch of the company called Driver for All aims to increase access to optimal treatments for free through partnerships with local communities, Dr. Polkinghorn said. Driver for All has thus far partnered with Howard University Hospital in Washington to connect Howard patients to clinical trials at NCI. A partnership with Beijing Children’s Hospital and the Futang Research Center of Pediatric Development, meanwhile, is working to connect patients with rare-disease experts. Driver has funded 100% of the cost of these projects to date, according to its website.
Outside of Driver for All, Dr. Polkinghorn acknowledges that patients must bear the cost of Driver’s consumer products; however, the price should be viewed in context, he said.
“It’s important to remember that today, in order to be evaluated by 30 [plus] centers for treatment options, patients would need to fly to these centers, make appointments, and be seen by a doctor – this would require both time and resources for flights/hotels, which would cost much more than our sticker price,” he said. “So while $3,000 is a lot of money for some patients, Driver’s product is ultimately able to provide more visibility to options that simply would not be realistic today.”
James Gulley, MD, of the National Cancer Institute Center for Cancer Research, said any platform that can efficiently provide access to clinical trial options yields another source of information for patients to utilize in decision making with their health provider. Dr. Gulley, who heads the center’s genitourinary malignancies branch, declined to comment about access-to-care concerns with Driver’s model. He emphasized that patients who participate in NIH research studies are treated without charge.
“The key to finding better [cancer] treatment is to perform science-driven clinical trials,” Dr. Gulley said in an interview. “However, there are many barriers for enrollment in clinical trials. … As a government agency, NCI is open to partnering with any organization that seeks to improve access to clinical trials for cancer patients.”
NCI and Driver recently conducted a study to validate Driver’s platform; it showed that Driver’s technology successfully predicted the eligibility of patients in NCI Center for Cancer Research clinical trials. The study, presented at a recent American Society of Clinical Oncology meeting, evaluated Driver’s processing of 21 metastatic prostate cancer patients enrolled in a therapeutic NCI clinical trial within the last five years. Results showed Driver correctly predicted that 20 of the patients were “potentially eligible” for the trial in which they were enrolled, and that one was ineligible. Based on the study, a protocol is now in development for a new clinical study, which will seek to further determine the efficiency and accuracy of the clinical trial access program created by Driver, according to Dr. Gulley.
Charles Ryan, MD, director of the division of hematology, oncology, and transplantation for the University of Minnesota, Minneapolis, views Driver’s platform as a way to eliminate geographical barriers, which often keep patients from care, while at the same time enabling researchers to find the right patients for clinical trials.
“We need breakthrough technologies and opportunities for patients to be able to access the most successful and promising cancer treatments, regardless of where they live,” Dr. Ryan said in an interview. “Companies like Driver are attempting to bridge that gap by connecting patients to doctors at world class cancer institutes and direct them toward the best care for their particular condition.”
Driver’s model also allows researchers the opportunity to develop specific, unique treatment for less common cancers and remain optimistic that they can attract patients to receive such treatments as they are developed, Dr. Ryan said.
Dr. Stadler, however, worries that Driver may be giving patients the wrong perception that all it takes is a computer and medical records to determine their best treatment route.
“There’s a lot more subtlety to treatment decisions than most people would like to admit,” Dr. Stadler said. “It’s more than just a bunch of data from sophisticated laboratory tests and the written medical record. Obtaining objective information is the first step, but it’s far from the only step.”
Patients may have significant limitations in functional status that is apparent only during an in-person assessment, for example, he said. In other cases, family members may be essential in conveying information about a patient’s cognitive disabilities. Even when such information is documented, it is sometimes difficult to extract the full picture from the record alone, he said. Dr. Stadler is also bothered that the model requires physicians and hospitals to provide their skilled analyses to a for-profit company, which in turn, charges patients to review the information.
“This is our work,” he said. “I agree that patients should have the information, and I don’t mind sharing anything I have with patients, but now I’m going to share it with another business that essentially is competing with me in terms of providing guidance to patients.”
Beware drug reactions from methotrexate, voriconazole, and BRAF inhibitors
MONTEREY, CALIF. – Cutaneous necrosis. Porphyria cutanea tarda, accelerated photoaging, and actinic keratosis (AK). Cutaneous keratinocytic neoplasias. Two drugs – and a class of drugs commonly used in oncologic dermatology – can produce these skin conditions, a dermatologist cautioned his colleagues.
J. Mark Jackson, MD, of the University of Louisville (Ky.), highlighted these drug reactions in a presentation at the annual Coastal Dermatology Symposium. The
Dr. Jackson referred to reports of cutaneous necrosis associated with methotrexate and highlighted a 2017 case series that compared 24 patients who developed the condition with a control population of patients taking methotrexate who did not develop it. The patients with this reaction were more likely to be older, had a higher starting dose, and had signs of kidney problems. They were also less likely to be taking folic acid supplements (J Am Acad Dermatol. 2017 Aug;77[2]:247-55.e2).
“It’s pretty alarming,” he said. “They look like Stevens-Johnson syndrome/TEN [toxic epidermal necrolysis], but the pathology was differentiated,” he pointed out.
He cautioned, though, that this is not “a typical reaction.”
The oral antifungal drug voriconazole is often used in immunosuppressed patients, such as transplant patients, either as prophylaxis or therapy. It is highly photosensitizing and has been linked to porphyria cutanea tarda, accelerated photoaging, development of AKs, and aggressive cutaneous squamous cell carcinoma (Am J Transplant 2008 Apr;8[4]:877-80; AIDS. 2008 Apr 23;22[7]:905-6; J Am Acad Dermatol. 2010 Jan;62[1]:31-7; Dermatol Surg. 2010 Nov;36[11]:1752-5).
The risk of nonmelanoma skin cancer may be quadrupled in patients who take this medication, Dr. Jackson said.
There also are reports of patients on voriconazole developing tense bullae that are suggestive of porphyria cutanea tarda but with normal porphyrin levels, he said. This resolves over time, once therapy has ceased.
The BRAF inhibitor chemotherapy drugs – vemurafenib (Zelboraf), dabrafenib (Tafinlar), and encorafenib (Braftovi) – are used to treat metastatic melanoma. They’ve been linked to rash and cutaneous keratinocytic neoplasias. Patients on these agents should be “closely monitored” for these conditions (Chem Immunol Allergy. 2012;97:191-202). Dr. Jackson emphasized the importance of photoprotection with these patients and noted that it’s crucial to see these patients every month because neoplasias can develop quickly, even within 4 weeks of starting the medication.
The Coastal Dermatology Symposium is jointly presented by the University of Louisville and Global Academy for Medical Education. This publication and Global Academy for Medical Education are both owned by Frontline Medical Communications.
Dr. Jackson reported relationships with AbbVie, Accuitis, Aclaris, Galderma, Janssen, Lilly, Medimetriks, Novartis, Promius, Ralexar, and TopMD.
MONTEREY, CALIF. – Cutaneous necrosis. Porphyria cutanea tarda, accelerated photoaging, and actinic keratosis (AK). Cutaneous keratinocytic neoplasias. Two drugs – and a class of drugs commonly used in oncologic dermatology – can produce these skin conditions, a dermatologist cautioned his colleagues.
J. Mark Jackson, MD, of the University of Louisville (Ky.), highlighted these drug reactions in a presentation at the annual Coastal Dermatology Symposium. The
Dr. Jackson referred to reports of cutaneous necrosis associated with methotrexate and highlighted a 2017 case series that compared 24 patients who developed the condition with a control population of patients taking methotrexate who did not develop it. The patients with this reaction were more likely to be older, had a higher starting dose, and had signs of kidney problems. They were also less likely to be taking folic acid supplements (J Am Acad Dermatol. 2017 Aug;77[2]:247-55.e2).
“It’s pretty alarming,” he said. “They look like Stevens-Johnson syndrome/TEN [toxic epidermal necrolysis], but the pathology was differentiated,” he pointed out.
He cautioned, though, that this is not “a typical reaction.”
The oral antifungal drug voriconazole is often used in immunosuppressed patients, such as transplant patients, either as prophylaxis or therapy. It is highly photosensitizing and has been linked to porphyria cutanea tarda, accelerated photoaging, development of AKs, and aggressive cutaneous squamous cell carcinoma (Am J Transplant 2008 Apr;8[4]:877-80; AIDS. 2008 Apr 23;22[7]:905-6; J Am Acad Dermatol. 2010 Jan;62[1]:31-7; Dermatol Surg. 2010 Nov;36[11]:1752-5).
The risk of nonmelanoma skin cancer may be quadrupled in patients who take this medication, Dr. Jackson said.
There also are reports of patients on voriconazole developing tense bullae that are suggestive of porphyria cutanea tarda but with normal porphyrin levels, he said. This resolves over time, once therapy has ceased.
The BRAF inhibitor chemotherapy drugs – vemurafenib (Zelboraf), dabrafenib (Tafinlar), and encorafenib (Braftovi) – are used to treat metastatic melanoma. They’ve been linked to rash and cutaneous keratinocytic neoplasias. Patients on these agents should be “closely monitored” for these conditions (Chem Immunol Allergy. 2012;97:191-202). Dr. Jackson emphasized the importance of photoprotection with these patients and noted that it’s crucial to see these patients every month because neoplasias can develop quickly, even within 4 weeks of starting the medication.
The Coastal Dermatology Symposium is jointly presented by the University of Louisville and Global Academy for Medical Education. This publication and Global Academy for Medical Education are both owned by Frontline Medical Communications.
Dr. Jackson reported relationships with AbbVie, Accuitis, Aclaris, Galderma, Janssen, Lilly, Medimetriks, Novartis, Promius, Ralexar, and TopMD.
MONTEREY, CALIF. – Cutaneous necrosis. Porphyria cutanea tarda, accelerated photoaging, and actinic keratosis (AK). Cutaneous keratinocytic neoplasias. Two drugs – and a class of drugs commonly used in oncologic dermatology – can produce these skin conditions, a dermatologist cautioned his colleagues.
J. Mark Jackson, MD, of the University of Louisville (Ky.), highlighted these drug reactions in a presentation at the annual Coastal Dermatology Symposium. The
Dr. Jackson referred to reports of cutaneous necrosis associated with methotrexate and highlighted a 2017 case series that compared 24 patients who developed the condition with a control population of patients taking methotrexate who did not develop it. The patients with this reaction were more likely to be older, had a higher starting dose, and had signs of kidney problems. They were also less likely to be taking folic acid supplements (J Am Acad Dermatol. 2017 Aug;77[2]:247-55.e2).
“It’s pretty alarming,” he said. “They look like Stevens-Johnson syndrome/TEN [toxic epidermal necrolysis], but the pathology was differentiated,” he pointed out.
He cautioned, though, that this is not “a typical reaction.”
The oral antifungal drug voriconazole is often used in immunosuppressed patients, such as transplant patients, either as prophylaxis or therapy. It is highly photosensitizing and has been linked to porphyria cutanea tarda, accelerated photoaging, development of AKs, and aggressive cutaneous squamous cell carcinoma (Am J Transplant 2008 Apr;8[4]:877-80; AIDS. 2008 Apr 23;22[7]:905-6; J Am Acad Dermatol. 2010 Jan;62[1]:31-7; Dermatol Surg. 2010 Nov;36[11]:1752-5).
The risk of nonmelanoma skin cancer may be quadrupled in patients who take this medication, Dr. Jackson said.
There also are reports of patients on voriconazole developing tense bullae that are suggestive of porphyria cutanea tarda but with normal porphyrin levels, he said. This resolves over time, once therapy has ceased.
The BRAF inhibitor chemotherapy drugs – vemurafenib (Zelboraf), dabrafenib (Tafinlar), and encorafenib (Braftovi) – are used to treat metastatic melanoma. They’ve been linked to rash and cutaneous keratinocytic neoplasias. Patients on these agents should be “closely monitored” for these conditions (Chem Immunol Allergy. 2012;97:191-202). Dr. Jackson emphasized the importance of photoprotection with these patients and noted that it’s crucial to see these patients every month because neoplasias can develop quickly, even within 4 weeks of starting the medication.
The Coastal Dermatology Symposium is jointly presented by the University of Louisville and Global Academy for Medical Education. This publication and Global Academy for Medical Education are both owned by Frontline Medical Communications.
Dr. Jackson reported relationships with AbbVie, Accuitis, Aclaris, Galderma, Janssen, Lilly, Medimetriks, Novartis, Promius, Ralexar, and TopMD.
EXPERT ANALYSIS FROM THE COASTAL DERMATOLOGY SYMPOSIUM
Opioid use cut nearly 50% for urologic oncology surgery patients
PHOENIX – Opioid use in urologic oncology patients dropped by 46% after one high-volume surgical center introduced changes to order sets and adopted new patient communication strategies, a researcher has reported.
The changes, which promoted opioid-sparing pain regimens, led to a substantial drop in postoperative opioid use with no compromise in pain control, according to Kerri Stevenson, a nurse practitioner with Stanford Health Care.
“Patients can be successfully managed with minimal opioid medication,” Ms. Stevenson said at a symposium on quality care sponsored by the American Society of Clinical Oncology.
However, “it takes a multidisciplinary team for effective change to occur – this cannot be done in silos,” she told attendees at the meeting.
Seeking to reduce their reliance on opioids to manage postoperative pain, Ms. Stevenson and her colleagues set out to reduce opioid use by 50%, from a baseline morphine equivalent daily dose (MEDD) of 95.1 in June to September 2017 to a target of 47.5 by March 2018.
The actual MEDD at the end of the quality improvement project was 51.5, a 46% reduction that was just shy of that goal, she reported.
Factors fueling opioid use included patient expectations that they would be used and the belief that adjunct medications were not as effective as opioids, Dr. Stevenson found in a team survey.
“We decided to target those,” she said. “Our key drivers were really focused on appropriate prescriptions, increasing patient and provider awareness, standardizing our pathways, and setting expectations.”
To tackle the problem, they revised EMR order sets to default to selection of adjunct medications, educated providers, and introduced new patient communication strategies.
Instead of asking “Would you like me to bring you some oxycodone?” providers would instead start by asking about the patient’s current pain control medications and whether they were working well. When prescribed, opioids should be started at lower doses and escalated only if needed.
“Once we started our interventions, we noticed an immediate effect,” Ms. Stevenson.
The decreases were consistent across a range of surgery types. For example, the MEDD dropped to 55.1 with robotic prostatectomy, a procedure with a 1-day admission and very small incisions, and to 50.6 for open radical cystectomy, which involves a large incision and a stay of approximately 4 days, she said.
To address concerns that they might just be undertreating patients, investigators looked retrospectively at pain scores. They saw no differences pre- and post intervention in pain or anxiety scores within the first 24-48 hours post procedure, Ms. Stevenson reported.
Ms. Stevenson had no disclosures related to the presentation. Coauthor Jay Bakul Shah, MD of Stanford Health Care reported a consulting or advisory role with Pacira Pharmaceuticals.
SOURCE: Stevenson K et al. Quality Care Symposium, Abstract 269.
PHOENIX – Opioid use in urologic oncology patients dropped by 46% after one high-volume surgical center introduced changes to order sets and adopted new patient communication strategies, a researcher has reported.
The changes, which promoted opioid-sparing pain regimens, led to a substantial drop in postoperative opioid use with no compromise in pain control, according to Kerri Stevenson, a nurse practitioner with Stanford Health Care.
“Patients can be successfully managed with minimal opioid medication,” Ms. Stevenson said at a symposium on quality care sponsored by the American Society of Clinical Oncology.
However, “it takes a multidisciplinary team for effective change to occur – this cannot be done in silos,” she told attendees at the meeting.
Seeking to reduce their reliance on opioids to manage postoperative pain, Ms. Stevenson and her colleagues set out to reduce opioid use by 50%, from a baseline morphine equivalent daily dose (MEDD) of 95.1 in June to September 2017 to a target of 47.5 by March 2018.
The actual MEDD at the end of the quality improvement project was 51.5, a 46% reduction that was just shy of that goal, she reported.
Factors fueling opioid use included patient expectations that they would be used and the belief that adjunct medications were not as effective as opioids, Dr. Stevenson found in a team survey.
“We decided to target those,” she said. “Our key drivers were really focused on appropriate prescriptions, increasing patient and provider awareness, standardizing our pathways, and setting expectations.”
To tackle the problem, they revised EMR order sets to default to selection of adjunct medications, educated providers, and introduced new patient communication strategies.
Instead of asking “Would you like me to bring you some oxycodone?” providers would instead start by asking about the patient’s current pain control medications and whether they were working well. When prescribed, opioids should be started at lower doses and escalated only if needed.
“Once we started our interventions, we noticed an immediate effect,” Ms. Stevenson.
The decreases were consistent across a range of surgery types. For example, the MEDD dropped to 55.1 with robotic prostatectomy, a procedure with a 1-day admission and very small incisions, and to 50.6 for open radical cystectomy, which involves a large incision and a stay of approximately 4 days, she said.
To address concerns that they might just be undertreating patients, investigators looked retrospectively at pain scores. They saw no differences pre- and post intervention in pain or anxiety scores within the first 24-48 hours post procedure, Ms. Stevenson reported.
Ms. Stevenson had no disclosures related to the presentation. Coauthor Jay Bakul Shah, MD of Stanford Health Care reported a consulting or advisory role with Pacira Pharmaceuticals.
SOURCE: Stevenson K et al. Quality Care Symposium, Abstract 269.
PHOENIX – Opioid use in urologic oncology patients dropped by 46% after one high-volume surgical center introduced changes to order sets and adopted new patient communication strategies, a researcher has reported.
The changes, which promoted opioid-sparing pain regimens, led to a substantial drop in postoperative opioid use with no compromise in pain control, according to Kerri Stevenson, a nurse practitioner with Stanford Health Care.
“Patients can be successfully managed with minimal opioid medication,” Ms. Stevenson said at a symposium on quality care sponsored by the American Society of Clinical Oncology.
However, “it takes a multidisciplinary team for effective change to occur – this cannot be done in silos,” she told attendees at the meeting.
Seeking to reduce their reliance on opioids to manage postoperative pain, Ms. Stevenson and her colleagues set out to reduce opioid use by 50%, from a baseline morphine equivalent daily dose (MEDD) of 95.1 in June to September 2017 to a target of 47.5 by March 2018.
The actual MEDD at the end of the quality improvement project was 51.5, a 46% reduction that was just shy of that goal, she reported.
Factors fueling opioid use included patient expectations that they would be used and the belief that adjunct medications were not as effective as opioids, Dr. Stevenson found in a team survey.
“We decided to target those,” she said. “Our key drivers were really focused on appropriate prescriptions, increasing patient and provider awareness, standardizing our pathways, and setting expectations.”
To tackle the problem, they revised EMR order sets to default to selection of adjunct medications, educated providers, and introduced new patient communication strategies.
Instead of asking “Would you like me to bring you some oxycodone?” providers would instead start by asking about the patient’s current pain control medications and whether they were working well. When prescribed, opioids should be started at lower doses and escalated only if needed.
“Once we started our interventions, we noticed an immediate effect,” Ms. Stevenson.
The decreases were consistent across a range of surgery types. For example, the MEDD dropped to 55.1 with robotic prostatectomy, a procedure with a 1-day admission and very small incisions, and to 50.6 for open radical cystectomy, which involves a large incision and a stay of approximately 4 days, she said.
To address concerns that they might just be undertreating patients, investigators looked retrospectively at pain scores. They saw no differences pre- and post intervention in pain or anxiety scores within the first 24-48 hours post procedure, Ms. Stevenson reported.
Ms. Stevenson had no disclosures related to the presentation. Coauthor Jay Bakul Shah, MD of Stanford Health Care reported a consulting or advisory role with Pacira Pharmaceuticals.
SOURCE: Stevenson K et al. Quality Care Symposium, Abstract 269.
REPORTING FROM THE QUALITY CARE SYMPOSIUM
Key clinical point: Substantial reductions in postoperative opioid use might be achievable through strategies that promote opioid-sparing pain regimens.
Major finding: Postoperative opioid use dropped 46% for urologic oncology patients after changing default order sets, introducing new patient communication strategies, and educating providers.
Study details: An analysis of opioid prescribing before and after introduction of a quality improvement project at one high-volume surgical center.
Disclosures: One study coauthor reported a consulting or advisory role with Pacira Pharmaceuticals.
Source: Stevenson K et al. Quality Care Symposium, Abstract 269.
MBC care causes more money problems for uninsured – but more financial stress for the insured
PHOENIX – Metastatic breast cancer care may be a bigger financial burden for uninsured patients, but it’s actually causing more financial distress for the insured, results of a recent survey suggest.
The uninsured more often reported material burdens, such as lack of savings or refusing treatment because of cost, according to survey results reported at a symposium on quality care sponsored by the American Society of Clinical Oncology.
By contrast, the insured reported more worry, distress, and frustration related to financial problems, reported Stephanie B. Wheeler, PhD, MPH, of the Gillings School of Global Public Health, University of North Carolina at Chapel Hill.
That divide suggests increased health insurance coverage is not enough to tackle the problem of cancer-related financial harm, said Dr. Wheeler.
“Health insurance expansion is important,” she said, “but it’s going to be ultimately inadequate in solving the problem of financial distress in our cancer patients. We really need to be thinking about other types of interventions that can do a better job of meeting patients where they are.”
Regardless of insurance status, this survey showed an “unprecedented” high level of cancer-related financial harm in metastatic breast cancer patients as compared with previous studies of early-stage cancer patients, Dr. Wheeler said.
The online survey was completed by 1,054 individuals who were members of the Metastatic Breast Cancer Network, a patient advocacy group. Approximately 30% of participants were uninsured, Dr. Wheeler reported.
Overall, 56% of respondents reported not having enough savings to cover costs of care, while 54% stopped or refused treatment because of cost, and 49% said they had been contacted by collection agencies, survey results show.
These material burdens were “perhaps not surprisingly” significantly more often reported by the uninsured respondents, Dr. Wheeler said. What may be surprising, she added, is that psychosocial burdens were more frequently reported by the insured respondents.
The most frequently reported psychosocial burden was worry about cancer-related financial problems, reported by 68% of respondents overall, but nearly 80% of insured and around 45% of uninsured respondents (P less than .001), Dr. Wheeler said.
The least often reported psychosocial issue was worry about the effects of financial stress on the family, at 31% of all respondents. Even so, there was a significant difference in response by insurance status, with the percentage approaching 40% for the insured, but less than 20% for the uninsured (P less than .001).
This high level of worry and distress may indicate that insured cancer patients may be expecting their insurance to cover more that it does, but ultimately, it is inadequate to meet their needs, Dr. Wheeler said.
“It’s also possible that because insured participants are more often affluent – they more often have retirement and other savings to draw down – that they actually have more to lose,” she added, “and when it comes to the legacy that they leave behind for their family, that creates additional stress – not just for them as an individual, but for their entire household.”
Previous research shows that the adverse financial impacts of cancer, also referred to as financial toxicity, affect about 30% of cancer patients, Dr. Wheeler said in her presentation.
Dr. Wheeler had no relationships to disclose. Funding for the project was provided from the National Comprehensive Cancer Network and Pfizer Independent Grants for Learning & Change.
SOURCE: Wheeler SB et al. Quality Care Symposium, Abstract 32.
PHOENIX – Metastatic breast cancer care may be a bigger financial burden for uninsured patients, but it’s actually causing more financial distress for the insured, results of a recent survey suggest.
The uninsured more often reported material burdens, such as lack of savings or refusing treatment because of cost, according to survey results reported at a symposium on quality care sponsored by the American Society of Clinical Oncology.
By contrast, the insured reported more worry, distress, and frustration related to financial problems, reported Stephanie B. Wheeler, PhD, MPH, of the Gillings School of Global Public Health, University of North Carolina at Chapel Hill.
That divide suggests increased health insurance coverage is not enough to tackle the problem of cancer-related financial harm, said Dr. Wheeler.
“Health insurance expansion is important,” she said, “but it’s going to be ultimately inadequate in solving the problem of financial distress in our cancer patients. We really need to be thinking about other types of interventions that can do a better job of meeting patients where they are.”
Regardless of insurance status, this survey showed an “unprecedented” high level of cancer-related financial harm in metastatic breast cancer patients as compared with previous studies of early-stage cancer patients, Dr. Wheeler said.
The online survey was completed by 1,054 individuals who were members of the Metastatic Breast Cancer Network, a patient advocacy group. Approximately 30% of participants were uninsured, Dr. Wheeler reported.
Overall, 56% of respondents reported not having enough savings to cover costs of care, while 54% stopped or refused treatment because of cost, and 49% said they had been contacted by collection agencies, survey results show.
These material burdens were “perhaps not surprisingly” significantly more often reported by the uninsured respondents, Dr. Wheeler said. What may be surprising, she added, is that psychosocial burdens were more frequently reported by the insured respondents.
The most frequently reported psychosocial burden was worry about cancer-related financial problems, reported by 68% of respondents overall, but nearly 80% of insured and around 45% of uninsured respondents (P less than .001), Dr. Wheeler said.
The least often reported psychosocial issue was worry about the effects of financial stress on the family, at 31% of all respondents. Even so, there was a significant difference in response by insurance status, with the percentage approaching 40% for the insured, but less than 20% for the uninsured (P less than .001).
This high level of worry and distress may indicate that insured cancer patients may be expecting their insurance to cover more that it does, but ultimately, it is inadequate to meet their needs, Dr. Wheeler said.
“It’s also possible that because insured participants are more often affluent – they more often have retirement and other savings to draw down – that they actually have more to lose,” she added, “and when it comes to the legacy that they leave behind for their family, that creates additional stress – not just for them as an individual, but for their entire household.”
Previous research shows that the adverse financial impacts of cancer, also referred to as financial toxicity, affect about 30% of cancer patients, Dr. Wheeler said in her presentation.
Dr. Wheeler had no relationships to disclose. Funding for the project was provided from the National Comprehensive Cancer Network and Pfizer Independent Grants for Learning & Change.
SOURCE: Wheeler SB et al. Quality Care Symposium, Abstract 32.
PHOENIX – Metastatic breast cancer care may be a bigger financial burden for uninsured patients, but it’s actually causing more financial distress for the insured, results of a recent survey suggest.
The uninsured more often reported material burdens, such as lack of savings or refusing treatment because of cost, according to survey results reported at a symposium on quality care sponsored by the American Society of Clinical Oncology.
By contrast, the insured reported more worry, distress, and frustration related to financial problems, reported Stephanie B. Wheeler, PhD, MPH, of the Gillings School of Global Public Health, University of North Carolina at Chapel Hill.
That divide suggests increased health insurance coverage is not enough to tackle the problem of cancer-related financial harm, said Dr. Wheeler.
“Health insurance expansion is important,” she said, “but it’s going to be ultimately inadequate in solving the problem of financial distress in our cancer patients. We really need to be thinking about other types of interventions that can do a better job of meeting patients where they are.”
Regardless of insurance status, this survey showed an “unprecedented” high level of cancer-related financial harm in metastatic breast cancer patients as compared with previous studies of early-stage cancer patients, Dr. Wheeler said.
The online survey was completed by 1,054 individuals who were members of the Metastatic Breast Cancer Network, a patient advocacy group. Approximately 30% of participants were uninsured, Dr. Wheeler reported.
Overall, 56% of respondents reported not having enough savings to cover costs of care, while 54% stopped or refused treatment because of cost, and 49% said they had been contacted by collection agencies, survey results show.
These material burdens were “perhaps not surprisingly” significantly more often reported by the uninsured respondents, Dr. Wheeler said. What may be surprising, she added, is that psychosocial burdens were more frequently reported by the insured respondents.
The most frequently reported psychosocial burden was worry about cancer-related financial problems, reported by 68% of respondents overall, but nearly 80% of insured and around 45% of uninsured respondents (P less than .001), Dr. Wheeler said.
The least often reported psychosocial issue was worry about the effects of financial stress on the family, at 31% of all respondents. Even so, there was a significant difference in response by insurance status, with the percentage approaching 40% for the insured, but less than 20% for the uninsured (P less than .001).
This high level of worry and distress may indicate that insured cancer patients may be expecting their insurance to cover more that it does, but ultimately, it is inadequate to meet their needs, Dr. Wheeler said.
“It’s also possible that because insured participants are more often affluent – they more often have retirement and other savings to draw down – that they actually have more to lose,” she added, “and when it comes to the legacy that they leave behind for their family, that creates additional stress – not just for them as an individual, but for their entire household.”
Previous research shows that the adverse financial impacts of cancer, also referred to as financial toxicity, affect about 30% of cancer patients, Dr. Wheeler said in her presentation.
Dr. Wheeler had no relationships to disclose. Funding for the project was provided from the National Comprehensive Cancer Network and Pfizer Independent Grants for Learning & Change.
SOURCE: Wheeler SB et al. Quality Care Symposium, Abstract 32.
REPORTING FROM THE QUALITY CARE SYMPOSIUM
Key clinical point: Survey results suggest that metastatic breast cancer care is a bigger financial burden for uninsured patients vs insured patients, though the insured have more financial distress related to that care.
Major finding: Overall, 68% of respondents said they worried about cancer-related financial problems, and significantly more insured individuals reported this worry (P less than .001).
Study details: Analysis of survey responses from 1,054 members of the Metastatic Breast Cancer Network, of whom about 30% were uninsured.
Disclosures: Funding for the project was provided from the National Comprehensive Cancer Network and Pfizer Independent Grants for Learning & Change.
Source: Wheeler SB et al. Quality Care Symposium, Abstract 32.
Early supportive care cuts costs and admissions in cancer patients undergoing curative treatment
PHOENIX – By starting supportive measures early in the care of cancer patients undergoing curative treatment, a cancer center cut costs, emergency department visits, and admissions, a researcher said at symposium on quality care sponsored by the American Society of Clinical Oncology.
The supportive care pathway resulted in double-digit decreases in admissions and an opportunity cost savings of $1,500 per patient, reported Christopher D. Koprowski, MD, MBA, of Helen F. Graham Cancer Center & Research Institute, Christiana Care Health System, Newark, Del.
Although satisfaction hasn’t been measured yet, anecdotal reports suggest the patient experience has improved because of the multidisciplinary program, which included mandatory supportive care screening and enhancements to computer systems, said Dr. Koprowski, who is director of quality and safety at the cancer center.
“From all outward signs, the patients are extraordinarily grateful in this program,” Dr. Koprowski said in an interview. “I just had one who said that being seen at the same time by all these people just makes things so much easier.”
The Supportive Care of Oncology Patients (SCOOP) clinical pathway, introduced in November 2016, includes palliative and supportive care service screening that occurs during the multidisciplinary visit. The pathway incorporates a checklist integrated into a nurse navigator information system to support care standardization, according to Dr. Koprowski.
Also added were “flags” in the inpatient information system that trigger alerts to navigators, oncologists, and the supportive care service whenever a patient in the SCOOP pathway is admitted, discharged, or seen in the emergency room, he said.
Enrollment in SCOOP was limited to lung, esophageal, head and neck, and colorectal cancer patients receiving concurrent radiation and chemotherapy. Out of approximately 200 eligible patients in the first year, about half entered the clinical pathway, according to Dr. Koprowski.
For that first year, 32% of SCOOP patients had ED visits, compared with 54% of combined modality patients who did not enter the pathway, Dr. Koprowski reported.
Similarly, admissions were 25% for the SCOOP patients and 34% of non-SCOOP patients, and readmissions were seen in 20% versus 32% of those groups, respectively.
These findings are much like what has been seen when early supportive care is introduced in patients with more advanced disease, according to Dr. Koprowski.
He said the SCOOP program was partly inspired by a study in the New England Journal of Medicine showing that patients with advanced non–small cell lung cancer who received early palliative care had longer survival despite less-aggressive care, including reduced use of chemotherapy, at the end of life.
“Early-stage patients aren’t that much different if they are being treated very aggressively with combined modality chemotherapy and radiation,” he said. “The treatment is very, very tough on people.”
Dr. Koprowski and his coinvestigators had no relationships to disclose relevant to the research presented at the ASCO symposium.
SOURCE: Koprowski CD et al. 2018 ASCO Quality Care Symposium, Abstract 142.
PHOENIX – By starting supportive measures early in the care of cancer patients undergoing curative treatment, a cancer center cut costs, emergency department visits, and admissions, a researcher said at symposium on quality care sponsored by the American Society of Clinical Oncology.
The supportive care pathway resulted in double-digit decreases in admissions and an opportunity cost savings of $1,500 per patient, reported Christopher D. Koprowski, MD, MBA, of Helen F. Graham Cancer Center & Research Institute, Christiana Care Health System, Newark, Del.
Although satisfaction hasn’t been measured yet, anecdotal reports suggest the patient experience has improved because of the multidisciplinary program, which included mandatory supportive care screening and enhancements to computer systems, said Dr. Koprowski, who is director of quality and safety at the cancer center.
“From all outward signs, the patients are extraordinarily grateful in this program,” Dr. Koprowski said in an interview. “I just had one who said that being seen at the same time by all these people just makes things so much easier.”
The Supportive Care of Oncology Patients (SCOOP) clinical pathway, introduced in November 2016, includes palliative and supportive care service screening that occurs during the multidisciplinary visit. The pathway incorporates a checklist integrated into a nurse navigator information system to support care standardization, according to Dr. Koprowski.
Also added were “flags” in the inpatient information system that trigger alerts to navigators, oncologists, and the supportive care service whenever a patient in the SCOOP pathway is admitted, discharged, or seen in the emergency room, he said.
Enrollment in SCOOP was limited to lung, esophageal, head and neck, and colorectal cancer patients receiving concurrent radiation and chemotherapy. Out of approximately 200 eligible patients in the first year, about half entered the clinical pathway, according to Dr. Koprowski.
For that first year, 32% of SCOOP patients had ED visits, compared with 54% of combined modality patients who did not enter the pathway, Dr. Koprowski reported.
Similarly, admissions were 25% for the SCOOP patients and 34% of non-SCOOP patients, and readmissions were seen in 20% versus 32% of those groups, respectively.
These findings are much like what has been seen when early supportive care is introduced in patients with more advanced disease, according to Dr. Koprowski.
He said the SCOOP program was partly inspired by a study in the New England Journal of Medicine showing that patients with advanced non–small cell lung cancer who received early palliative care had longer survival despite less-aggressive care, including reduced use of chemotherapy, at the end of life.
“Early-stage patients aren’t that much different if they are being treated very aggressively with combined modality chemotherapy and radiation,” he said. “The treatment is very, very tough on people.”
Dr. Koprowski and his coinvestigators had no relationships to disclose relevant to the research presented at the ASCO symposium.
SOURCE: Koprowski CD et al. 2018 ASCO Quality Care Symposium, Abstract 142.
PHOENIX – By starting supportive measures early in the care of cancer patients undergoing curative treatment, a cancer center cut costs, emergency department visits, and admissions, a researcher said at symposium on quality care sponsored by the American Society of Clinical Oncology.
The supportive care pathway resulted in double-digit decreases in admissions and an opportunity cost savings of $1,500 per patient, reported Christopher D. Koprowski, MD, MBA, of Helen F. Graham Cancer Center & Research Institute, Christiana Care Health System, Newark, Del.
Although satisfaction hasn’t been measured yet, anecdotal reports suggest the patient experience has improved because of the multidisciplinary program, which included mandatory supportive care screening and enhancements to computer systems, said Dr. Koprowski, who is director of quality and safety at the cancer center.
“From all outward signs, the patients are extraordinarily grateful in this program,” Dr. Koprowski said in an interview. “I just had one who said that being seen at the same time by all these people just makes things so much easier.”
The Supportive Care of Oncology Patients (SCOOP) clinical pathway, introduced in November 2016, includes palliative and supportive care service screening that occurs during the multidisciplinary visit. The pathway incorporates a checklist integrated into a nurse navigator information system to support care standardization, according to Dr. Koprowski.
Also added were “flags” in the inpatient information system that trigger alerts to navigators, oncologists, and the supportive care service whenever a patient in the SCOOP pathway is admitted, discharged, or seen in the emergency room, he said.
Enrollment in SCOOP was limited to lung, esophageal, head and neck, and colorectal cancer patients receiving concurrent radiation and chemotherapy. Out of approximately 200 eligible patients in the first year, about half entered the clinical pathway, according to Dr. Koprowski.
For that first year, 32% of SCOOP patients had ED visits, compared with 54% of combined modality patients who did not enter the pathway, Dr. Koprowski reported.
Similarly, admissions were 25% for the SCOOP patients and 34% of non-SCOOP patients, and readmissions were seen in 20% versus 32% of those groups, respectively.
These findings are much like what has been seen when early supportive care is introduced in patients with more advanced disease, according to Dr. Koprowski.
He said the SCOOP program was partly inspired by a study in the New England Journal of Medicine showing that patients with advanced non–small cell lung cancer who received early palliative care had longer survival despite less-aggressive care, including reduced use of chemotherapy, at the end of life.
“Early-stage patients aren’t that much different if they are being treated very aggressively with combined modality chemotherapy and radiation,” he said. “The treatment is very, very tough on people.”
Dr. Koprowski and his coinvestigators had no relationships to disclose relevant to the research presented at the ASCO symposium.
SOURCE: Koprowski CD et al. 2018 ASCO Quality Care Symposium, Abstract 142.
REPORTING FROM THE QUALITY CARE SYMPOSIUM
Key clinical point: Early supportive care measures reduced costs, ED visits, and admissions in cancer patients who underwent combined modality therapy.
Major finding: 32% of patients had ED visits, compared with 54% of patients who did not enter the early supportive care pathway.
Study details: Analysis of cost and health care utilization for approximately 200 patients who underwent concurrent radiation and chemotherapy.
Disclosures: Authors had no relationships to disclose.
Source: Koprowski CD et al. 2018 ASCO Quality Care Symposium, Abstract 142.