User login
Sleep-deprived physicians less empathetic to patient pain?
new research suggests.
In the first of two studies, resident physicians were presented with two hypothetical scenarios involving a patient who complains of pain. They were asked about their likelihood of prescribing pain medication. The test was given to one group of residents who were just starting their day and to another group who were at the end of their night shift after being on call for 26 hours.
Results showed that the night shift residents were less likely than their daytime counterparts to say they would prescribe pain medication to the patients.
In further analysis of discharge notes from more than 13,000 electronic records of patients presenting with pain complaints at hospitals in Israel and the United States, the likelihood of an analgesic being prescribed during the night shift was 11% lower in Israel and 9% lower in the United States, compared with the day shift.
“Pain management is a major challenge, and a doctor’s perception of a patient’s subjective pain is susceptible to bias,” coinvestigator David Gozal, MD, the Marie M. and Harry L. Smith Endowed Chair of Child Health, University of Missouri–Columbia, said in a press release.
“This study demonstrated that night shift work is an important and previously unrecognized source of bias in pain management, likely stemming from impaired perception of pain,” Dr. Gozal added.
The findings were published online in the Proceedings of the National Academy of Sciences.
‘Directional’ differences
Senior investigator Alex Gileles-Hillel, MD, senior pediatric pulmonologist and sleep researcher at Hadassah University Medical Center, Jerusalem, said in an interview that physicians must make “complex assessments of patients’ subjective pain experience” – and the “subjective nature of pain management decisions can give rise to various biases.”
Dr. Gileles-Hillel has previously researched the cognitive toll of night shift work on physicians.
“It’s pretty established, for example, not to drive when sleep deprived because cognition is impaired,” he said. The current study explored whether sleep deprivation could affect areas other than cognition, including emotions and empathy.
The researchers used “two complementary approaches.” First, they administered tests to measure empathy and pain management decisions in 67 resident physicians at Hadassah Medical Centers either following a 26-hour night shift that began at 8:00 a.m. the day before (n = 36) or immediately before starting the workday (n = 31).
There were no significant differences in demographic, sleep, or burnout measures between the two groups, except that night shift physicians had slept less than those in the daytime group (2.93 vs. 5.96 hours).
Participants completed two tasks. In the empathy-for-pain task, they rated their emotional reactions to pictures of individuals in pain. In the empathy accuracy task, they were asked to assess the feelings of videotaped individuals telling emotional stories.
They were then presented with two clinical scenarios: a female patient with a headache and a male patient with a backache. Following that, they were asked to assess the magnitude of the patients’ pain and how likely they would be to prescribe pain medication.
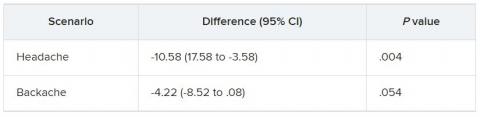
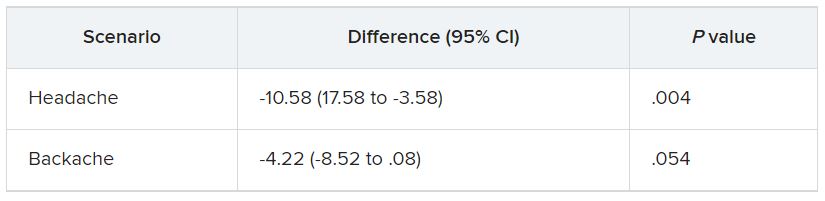
In the empathy-for-pain task, physicians’ empathy scores were significantly lower in the night shift group than in the day group (difference, –0.83; 95% CI, –1.55 to –0.10; P = .026). There were no significant differences between the groups in the empathy accuracy task.
In both scenarios, physicians in the night shift group assessed the patient’s pain as weaker in comparison with physicians in the day group. There was a statistically significant difference in the headache scenario but not the backache scenario.
In the headache scenario, the propensity of the physicians to prescribe analgesics was “directionally lower” but did not reach statistical significance. In the backache scenario, there was no significant difference between the groups’ prescribing propensities.
In both scenarios, pain assessment was positively correlated with the propensity to prescribe analgesics.
Despite the lack of statistical significance, the findings “documented a negative effect of night shift work on physician empathy for pain and a positive association between physician assessment of patient pain and the propensity to prescribe analgesics,” the investigators wrote.
Need for naps?
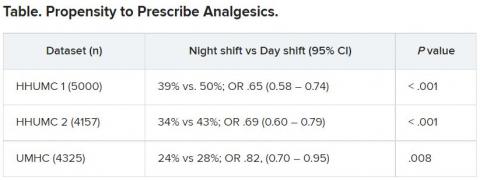
The researchers then analyzed analgesic prescription patterns drawn from three datasets of discharge notes of patients presenting to the emergency department with pain complaints (n = 13,482) at two branches of Hadassah-Hebrew University Medical Center and the University of Missouri Health Center.
The researchers collected data, including discharge time, medications patients were prescribed upon discharge, and patients’ subjective pain rating on a scale of 0-10 on a visual analogue scale (VAS).
Although patients’ VAS scores did not differ with respect to time or shift, patients were discharged with significantly less prescribed analgesics during the night shift in comparison with the day shift.
No similar differences in prescriptions between night shifts and day shifts were found for nonanalgesic medications, such as for diabetes or blood pressure. This suggests “the effect was specific to pain,” Dr. Gileles-Hillel said.
The pattern remained significant after controlling for potential confounders, including patient and physician variables and emergency department characteristics.
In addition, patients seen during night shifts received fewer analgesics, particularly opioids, than recommended by the World Health Organization for pain management.
“The first study enabled us to measure empathy for pain directly and examine our hypothesis in a controlled environment, while the second enabled us to test the implications by examining real-life pain management decisions,” Dr. Gileles-Hillel said.
“Physicians need to be aware of this,” he noted. “I try to be aware when I’m taking calls [at night] that I’m less empathetic to others and I might be more brief or angry with others.”
On a “house management level, perhaps institutions should try to schedule naps either before or during overnight call. A nap might give a boost and reboot not only to cognitive but also to emotional resources,” Dr. Gileles-Hillel added.
Compromised safety
In a comment, Eti Ben Simon, PhD, a postdoctoral fellow at the Center for Human Sleep Science, University of California, Berkeley, called the study “an important contribution to a growing list of studies that reveal how long night shifts reduce overall safety” for both patients and clinicians.
“It’s time to abandon the notion that the human brain can function as normal after being deprived of sleep for 24 hours,” said Dr. Ben Simon, who was not involved with the research.
“This is especially true in medicine, where we trust others to take care of us and feel our pain. These functions are simply not possible without adequate sleep,” she added.
Also commenting, Kannan Ramar, MD, president of the American Academy of Sleep Medicine, suggested that being cognizant of these findings “may help providers to mitigate this bias” of underprescribing pain medications when treating their patients.
Dr. Ramar, who is also a critical care specialist, pulmonologist, and sleep medicine specialist at Mayo Clinic, Rochester, Minn., was not involved with the research.
He noted that “further studies that systematically evaluate this further in a prospective and blinded way will be important.”
The research was supported in part by grants from the Israel Science Foundation, Joy Ventures, the Recanati Fund at the Jerusalem School of Business at the Hebrew University, and a fellowship from the Azrieli Foundation and received grant support to various investigators from the NIH, the Leda J. Sears Foundation, and the University of Missouri. The investigators, Ramar, and Ben Simon have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests.
In the first of two studies, resident physicians were presented with two hypothetical scenarios involving a patient who complains of pain. They were asked about their likelihood of prescribing pain medication. The test was given to one group of residents who were just starting their day and to another group who were at the end of their night shift after being on call for 26 hours.
Results showed that the night shift residents were less likely than their daytime counterparts to say they would prescribe pain medication to the patients.
In further analysis of discharge notes from more than 13,000 electronic records of patients presenting with pain complaints at hospitals in Israel and the United States, the likelihood of an analgesic being prescribed during the night shift was 11% lower in Israel and 9% lower in the United States, compared with the day shift.
“Pain management is a major challenge, and a doctor’s perception of a patient’s subjective pain is susceptible to bias,” coinvestigator David Gozal, MD, the Marie M. and Harry L. Smith Endowed Chair of Child Health, University of Missouri–Columbia, said in a press release.
“This study demonstrated that night shift work is an important and previously unrecognized source of bias in pain management, likely stemming from impaired perception of pain,” Dr. Gozal added.
The findings were published online in the Proceedings of the National Academy of Sciences.
‘Directional’ differences
Senior investigator Alex Gileles-Hillel, MD, senior pediatric pulmonologist and sleep researcher at Hadassah University Medical Center, Jerusalem, said in an interview that physicians must make “complex assessments of patients’ subjective pain experience” – and the “subjective nature of pain management decisions can give rise to various biases.”
Dr. Gileles-Hillel has previously researched the cognitive toll of night shift work on physicians.
“It’s pretty established, for example, not to drive when sleep deprived because cognition is impaired,” he said. The current study explored whether sleep deprivation could affect areas other than cognition, including emotions and empathy.
The researchers used “two complementary approaches.” First, they administered tests to measure empathy and pain management decisions in 67 resident physicians at Hadassah Medical Centers either following a 26-hour night shift that began at 8:00 a.m. the day before (n = 36) or immediately before starting the workday (n = 31).
There were no significant differences in demographic, sleep, or burnout measures between the two groups, except that night shift physicians had slept less than those in the daytime group (2.93 vs. 5.96 hours).
Participants completed two tasks. In the empathy-for-pain task, they rated their emotional reactions to pictures of individuals in pain. In the empathy accuracy task, they were asked to assess the feelings of videotaped individuals telling emotional stories.
They were then presented with two clinical scenarios: a female patient with a headache and a male patient with a backache. Following that, they were asked to assess the magnitude of the patients’ pain and how likely they would be to prescribe pain medication.
In the empathy-for-pain task, physicians’ empathy scores were significantly lower in the night shift group than in the day group (difference, –0.83; 95% CI, –1.55 to –0.10; P = .026). There were no significant differences between the groups in the empathy accuracy task.
In both scenarios, physicians in the night shift group assessed the patient’s pain as weaker in comparison with physicians in the day group. There was a statistically significant difference in the headache scenario but not the backache scenario.
In the headache scenario, the propensity of the physicians to prescribe analgesics was “directionally lower” but did not reach statistical significance. In the backache scenario, there was no significant difference between the groups’ prescribing propensities.
In both scenarios, pain assessment was positively correlated with the propensity to prescribe analgesics.
Despite the lack of statistical significance, the findings “documented a negative effect of night shift work on physician empathy for pain and a positive association between physician assessment of patient pain and the propensity to prescribe analgesics,” the investigators wrote.
Need for naps?
The researchers then analyzed analgesic prescription patterns drawn from three datasets of discharge notes of patients presenting to the emergency department with pain complaints (n = 13,482) at two branches of Hadassah-Hebrew University Medical Center and the University of Missouri Health Center.
The researchers collected data, including discharge time, medications patients were prescribed upon discharge, and patients’ subjective pain rating on a scale of 0-10 on a visual analogue scale (VAS).
Although patients’ VAS scores did not differ with respect to time or shift, patients were discharged with significantly less prescribed analgesics during the night shift in comparison with the day shift.
No similar differences in prescriptions between night shifts and day shifts were found for nonanalgesic medications, such as for diabetes or blood pressure. This suggests “the effect was specific to pain,” Dr. Gileles-Hillel said.
The pattern remained significant after controlling for potential confounders, including patient and physician variables and emergency department characteristics.
In addition, patients seen during night shifts received fewer analgesics, particularly opioids, than recommended by the World Health Organization for pain management.
“The first study enabled us to measure empathy for pain directly and examine our hypothesis in a controlled environment, while the second enabled us to test the implications by examining real-life pain management decisions,” Dr. Gileles-Hillel said.
“Physicians need to be aware of this,” he noted. “I try to be aware when I’m taking calls [at night] that I’m less empathetic to others and I might be more brief or angry with others.”
On a “house management level, perhaps institutions should try to schedule naps either before or during overnight call. A nap might give a boost and reboot not only to cognitive but also to emotional resources,” Dr. Gileles-Hillel added.
Compromised safety
In a comment, Eti Ben Simon, PhD, a postdoctoral fellow at the Center for Human Sleep Science, University of California, Berkeley, called the study “an important contribution to a growing list of studies that reveal how long night shifts reduce overall safety” for both patients and clinicians.
“It’s time to abandon the notion that the human brain can function as normal after being deprived of sleep for 24 hours,” said Dr. Ben Simon, who was not involved with the research.
“This is especially true in medicine, where we trust others to take care of us and feel our pain. These functions are simply not possible without adequate sleep,” she added.
Also commenting, Kannan Ramar, MD, president of the American Academy of Sleep Medicine, suggested that being cognizant of these findings “may help providers to mitigate this bias” of underprescribing pain medications when treating their patients.
Dr. Ramar, who is also a critical care specialist, pulmonologist, and sleep medicine specialist at Mayo Clinic, Rochester, Minn., was not involved with the research.
He noted that “further studies that systematically evaluate this further in a prospective and blinded way will be important.”
The research was supported in part by grants from the Israel Science Foundation, Joy Ventures, the Recanati Fund at the Jerusalem School of Business at the Hebrew University, and a fellowship from the Azrieli Foundation and received grant support to various investigators from the NIH, the Leda J. Sears Foundation, and the University of Missouri. The investigators, Ramar, and Ben Simon have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests.
In the first of two studies, resident physicians were presented with two hypothetical scenarios involving a patient who complains of pain. They were asked about their likelihood of prescribing pain medication. The test was given to one group of residents who were just starting their day and to another group who were at the end of their night shift after being on call for 26 hours.
Results showed that the night shift residents were less likely than their daytime counterparts to say they would prescribe pain medication to the patients.
In further analysis of discharge notes from more than 13,000 electronic records of patients presenting with pain complaints at hospitals in Israel and the United States, the likelihood of an analgesic being prescribed during the night shift was 11% lower in Israel and 9% lower in the United States, compared with the day shift.
“Pain management is a major challenge, and a doctor’s perception of a patient’s subjective pain is susceptible to bias,” coinvestigator David Gozal, MD, the Marie M. and Harry L. Smith Endowed Chair of Child Health, University of Missouri–Columbia, said in a press release.
“This study demonstrated that night shift work is an important and previously unrecognized source of bias in pain management, likely stemming from impaired perception of pain,” Dr. Gozal added.
The findings were published online in the Proceedings of the National Academy of Sciences.
‘Directional’ differences
Senior investigator Alex Gileles-Hillel, MD, senior pediatric pulmonologist and sleep researcher at Hadassah University Medical Center, Jerusalem, said in an interview that physicians must make “complex assessments of patients’ subjective pain experience” – and the “subjective nature of pain management decisions can give rise to various biases.”
Dr. Gileles-Hillel has previously researched the cognitive toll of night shift work on physicians.
“It’s pretty established, for example, not to drive when sleep deprived because cognition is impaired,” he said. The current study explored whether sleep deprivation could affect areas other than cognition, including emotions and empathy.
The researchers used “two complementary approaches.” First, they administered tests to measure empathy and pain management decisions in 67 resident physicians at Hadassah Medical Centers either following a 26-hour night shift that began at 8:00 a.m. the day before (n = 36) or immediately before starting the workday (n = 31).
There were no significant differences in demographic, sleep, or burnout measures between the two groups, except that night shift physicians had slept less than those in the daytime group (2.93 vs. 5.96 hours).
Participants completed two tasks. In the empathy-for-pain task, they rated their emotional reactions to pictures of individuals in pain. In the empathy accuracy task, they were asked to assess the feelings of videotaped individuals telling emotional stories.
They were then presented with two clinical scenarios: a female patient with a headache and a male patient with a backache. Following that, they were asked to assess the magnitude of the patients’ pain and how likely they would be to prescribe pain medication.
In the empathy-for-pain task, physicians’ empathy scores were significantly lower in the night shift group than in the day group (difference, –0.83; 95% CI, –1.55 to –0.10; P = .026). There were no significant differences between the groups in the empathy accuracy task.
In both scenarios, physicians in the night shift group assessed the patient’s pain as weaker in comparison with physicians in the day group. There was a statistically significant difference in the headache scenario but not the backache scenario.
In the headache scenario, the propensity of the physicians to prescribe analgesics was “directionally lower” but did not reach statistical significance. In the backache scenario, there was no significant difference between the groups’ prescribing propensities.
In both scenarios, pain assessment was positively correlated with the propensity to prescribe analgesics.
Despite the lack of statistical significance, the findings “documented a negative effect of night shift work on physician empathy for pain and a positive association between physician assessment of patient pain and the propensity to prescribe analgesics,” the investigators wrote.
Need for naps?
The researchers then analyzed analgesic prescription patterns drawn from three datasets of discharge notes of patients presenting to the emergency department with pain complaints (n = 13,482) at two branches of Hadassah-Hebrew University Medical Center and the University of Missouri Health Center.
The researchers collected data, including discharge time, medications patients were prescribed upon discharge, and patients’ subjective pain rating on a scale of 0-10 on a visual analogue scale (VAS).
Although patients’ VAS scores did not differ with respect to time or shift, patients were discharged with significantly less prescribed analgesics during the night shift in comparison with the day shift.
No similar differences in prescriptions between night shifts and day shifts were found for nonanalgesic medications, such as for diabetes or blood pressure. This suggests “the effect was specific to pain,” Dr. Gileles-Hillel said.
The pattern remained significant after controlling for potential confounders, including patient and physician variables and emergency department characteristics.
In addition, patients seen during night shifts received fewer analgesics, particularly opioids, than recommended by the World Health Organization for pain management.
“The first study enabled us to measure empathy for pain directly and examine our hypothesis in a controlled environment, while the second enabled us to test the implications by examining real-life pain management decisions,” Dr. Gileles-Hillel said.
“Physicians need to be aware of this,” he noted. “I try to be aware when I’m taking calls [at night] that I’m less empathetic to others and I might be more brief or angry with others.”
On a “house management level, perhaps institutions should try to schedule naps either before or during overnight call. A nap might give a boost and reboot not only to cognitive but also to emotional resources,” Dr. Gileles-Hillel added.
Compromised safety
In a comment, Eti Ben Simon, PhD, a postdoctoral fellow at the Center for Human Sleep Science, University of California, Berkeley, called the study “an important contribution to a growing list of studies that reveal how long night shifts reduce overall safety” for both patients and clinicians.
“It’s time to abandon the notion that the human brain can function as normal after being deprived of sleep for 24 hours,” said Dr. Ben Simon, who was not involved with the research.
“This is especially true in medicine, where we trust others to take care of us and feel our pain. These functions are simply not possible without adequate sleep,” she added.
Also commenting, Kannan Ramar, MD, president of the American Academy of Sleep Medicine, suggested that being cognizant of these findings “may help providers to mitigate this bias” of underprescribing pain medications when treating their patients.
Dr. Ramar, who is also a critical care specialist, pulmonologist, and sleep medicine specialist at Mayo Clinic, Rochester, Minn., was not involved with the research.
He noted that “further studies that systematically evaluate this further in a prospective and blinded way will be important.”
The research was supported in part by grants from the Israel Science Foundation, Joy Ventures, the Recanati Fund at the Jerusalem School of Business at the Hebrew University, and a fellowship from the Azrieli Foundation and received grant support to various investigators from the NIH, the Leda J. Sears Foundation, and the University of Missouri. The investigators, Ramar, and Ben Simon have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE PROCEEDINGS OF THE NATIONAL ACADEMY OF SCIENCES
Water birth may have benefits for healthy women: Meta-analysis suggests
Water immersion during labor and birth significantly reduced use of medications, maternal pain, and postpartum hemorrhage, compared with standard care with no water immersion, based on data from 36 studies including more than 150,000 women.
“Resting and laboring in water can reduce fear, anxiety, and pain perception; it helps optimize the physiology of childbirth through the release of endogenous endorphins and oxytocin,” and data from randomized, controlled trials have shown a reduced need for epidural analgesia with water immersion, Ethel Burns, PhD, of Oxford (England) Brookes University Faculty of Health and Life Sciences, and colleagues wrote.
Although previous studies have not shown an increased risk for adverse events for newborns following water birth, “There is a need to understand which clinical practices, when performed as part of water immersion care, result in the optimum outcomes for mother and newborn,” the researchers said.
In a systematic review and meta-analysis published in BMJ Open, the researchers identified studies published since 2000 that examined maternal or neonatal interventions and/or outcomes when birthing pools were used for labor and/or birth.
The primary objective was to compare intrapartum interventions and outcomes for water immersion during labor with standard care with no water immersion.
Water immersion generally involves the use of a birth pool for relaxation and pain relief in early labor, and some women proceed with immersion through the second stage of labor and delivery. Of the 36 included studies, 31 took place in a hospital setting, 4 in a midwife-led setting, and 1 in a mixed setting. Most of the studies (25) involved women who planned to have/had a water birth, and these studies included 151,742 women. Another seven studies including 1,901 women involved in water immersion for labor only, three studies including 3,688 women involved in water immersion during labor and water birth; the timing of water immersion was unclear in the remaining study of 215 women.
Overall, water immersion significantly reduced the use of epidurals (odds ratio, 0.17), injected opioids (OR, 0.22), and episiotomy (OR, 0.16). Maternal pain and postpartum hemorrhage also were significantly reduced with water immersion (OR, 0.24 and OR, 0.69, respectively).
Maternal satisfaction was significantly increased with water immersion, and the odds of an intact perineum increased as well (OR, 1.95 and OR, 1.48).
The overall odds of cord avulsion increased with water immersion (OR, 1.94), but the absolute risk was low, compared with births without water immersion (4.3 vs. 1.3 per 1,000). No significant differences in other identified neonatal outcomes were observed across the studies.
The study findings were limited by several factors including the inconsistency of reporting on birth setting, care practices, interventions, and outcomes, and the inclusion of only three outcomes for meta-regression analysis, the researchers noted. In addition, only four studies were conducted in midwifery-led settings.
“This is important because birth pool use is most prevalent in midwifery-led settings,” the researchers wrote.” Evidence-based practice of water immersion requires research that reflects the context of care provision.
“We suggest that studies incorporate the following fundamentals to advance the evidence: birth pool description, clearly described maternal and obstetric characteristics, the birth setting, the care model and use of standardized definitions.”
Despite the limitations and need for additional research, the data overall support the potential benefits from water immersion births for healthy women and newborns, the researchers concluded.
A Clinical Report issued by the American Academy of Pediatrics in January 2022 advised against water immersion during the second stage of labor and delivery. According to the report, the potential for neonatal infections from organisms such as Legionella and Pseudomonas species, is low, but does exist, and could result in serious complications.
Education is essential
Increasing numbers of women are seeking home births and water births, Marissa Platner, MD, a maternal-fetal medicine specialist at Emory University, Atlanta, said in an interview.
“Given the conflicting data and lack of data, it is important to be able to educate birthing mothers based on best available evidence,” said Dr. Platner, who was not involved in the study.
“I was not surprised by the findings, because the adverse outcomes that are of concern, such as neonatal sepsis, were not clearly addressed,” Dr. Platner said. Given that sepsis “is a rare outcome in the population of low-risk individuals, the study may not have been powered to assess for this. The findings of maternal pain and satisfaction being improved with water immersion are well known. ACOG [American College of Obstetricians and Gynecologists] has also stated that water immersion during the first stage of labor is safe and can help with pain control.”
On a practical level, “I think clinicians can use this guidance to discuss the potential benefits of water immersion in the first stages of labor, but would caution women regarding the unknown but possible risks of the water birth, given these findings are less clear,” Dr. Platner said.
“I think the findings regarding maternal outcomes are valid and consistent with the AAP/ACOG recommendations in terms of improving maternal pain control; however, more research is needed to determine the safety of the second stage of labor occurring in the water, given the potential for neonatal infection and respiratory distress, which could not be adequately addressed in this study,” Dr. Platner emphasized.
The study was supported by Oxford Brookes University. The researchers had no financial conflicts to disclose. Dr. Platner had no financial conflicts to disclose.
Water immersion during labor and birth significantly reduced use of medications, maternal pain, and postpartum hemorrhage, compared with standard care with no water immersion, based on data from 36 studies including more than 150,000 women.
“Resting and laboring in water can reduce fear, anxiety, and pain perception; it helps optimize the physiology of childbirth through the release of endogenous endorphins and oxytocin,” and data from randomized, controlled trials have shown a reduced need for epidural analgesia with water immersion, Ethel Burns, PhD, of Oxford (England) Brookes University Faculty of Health and Life Sciences, and colleagues wrote.
Although previous studies have not shown an increased risk for adverse events for newborns following water birth, “There is a need to understand which clinical practices, when performed as part of water immersion care, result in the optimum outcomes for mother and newborn,” the researchers said.
In a systematic review and meta-analysis published in BMJ Open, the researchers identified studies published since 2000 that examined maternal or neonatal interventions and/or outcomes when birthing pools were used for labor and/or birth.
The primary objective was to compare intrapartum interventions and outcomes for water immersion during labor with standard care with no water immersion.
Water immersion generally involves the use of a birth pool for relaxation and pain relief in early labor, and some women proceed with immersion through the second stage of labor and delivery. Of the 36 included studies, 31 took place in a hospital setting, 4 in a midwife-led setting, and 1 in a mixed setting. Most of the studies (25) involved women who planned to have/had a water birth, and these studies included 151,742 women. Another seven studies including 1,901 women involved in water immersion for labor only, three studies including 3,688 women involved in water immersion during labor and water birth; the timing of water immersion was unclear in the remaining study of 215 women.
Overall, water immersion significantly reduced the use of epidurals (odds ratio, 0.17), injected opioids (OR, 0.22), and episiotomy (OR, 0.16). Maternal pain and postpartum hemorrhage also were significantly reduced with water immersion (OR, 0.24 and OR, 0.69, respectively).
Maternal satisfaction was significantly increased with water immersion, and the odds of an intact perineum increased as well (OR, 1.95 and OR, 1.48).
The overall odds of cord avulsion increased with water immersion (OR, 1.94), but the absolute risk was low, compared with births without water immersion (4.3 vs. 1.3 per 1,000). No significant differences in other identified neonatal outcomes were observed across the studies.
The study findings were limited by several factors including the inconsistency of reporting on birth setting, care practices, interventions, and outcomes, and the inclusion of only three outcomes for meta-regression analysis, the researchers noted. In addition, only four studies were conducted in midwifery-led settings.
“This is important because birth pool use is most prevalent in midwifery-led settings,” the researchers wrote.” Evidence-based practice of water immersion requires research that reflects the context of care provision.
“We suggest that studies incorporate the following fundamentals to advance the evidence: birth pool description, clearly described maternal and obstetric characteristics, the birth setting, the care model and use of standardized definitions.”
Despite the limitations and need for additional research, the data overall support the potential benefits from water immersion births for healthy women and newborns, the researchers concluded.
A Clinical Report issued by the American Academy of Pediatrics in January 2022 advised against water immersion during the second stage of labor and delivery. According to the report, the potential for neonatal infections from organisms such as Legionella and Pseudomonas species, is low, but does exist, and could result in serious complications.
Education is essential
Increasing numbers of women are seeking home births and water births, Marissa Platner, MD, a maternal-fetal medicine specialist at Emory University, Atlanta, said in an interview.
“Given the conflicting data and lack of data, it is important to be able to educate birthing mothers based on best available evidence,” said Dr. Platner, who was not involved in the study.
“I was not surprised by the findings, because the adverse outcomes that are of concern, such as neonatal sepsis, were not clearly addressed,” Dr. Platner said. Given that sepsis “is a rare outcome in the population of low-risk individuals, the study may not have been powered to assess for this. The findings of maternal pain and satisfaction being improved with water immersion are well known. ACOG [American College of Obstetricians and Gynecologists] has also stated that water immersion during the first stage of labor is safe and can help with pain control.”
On a practical level, “I think clinicians can use this guidance to discuss the potential benefits of water immersion in the first stages of labor, but would caution women regarding the unknown but possible risks of the water birth, given these findings are less clear,” Dr. Platner said.
“I think the findings regarding maternal outcomes are valid and consistent with the AAP/ACOG recommendations in terms of improving maternal pain control; however, more research is needed to determine the safety of the second stage of labor occurring in the water, given the potential for neonatal infection and respiratory distress, which could not be adequately addressed in this study,” Dr. Platner emphasized.
The study was supported by Oxford Brookes University. The researchers had no financial conflicts to disclose. Dr. Platner had no financial conflicts to disclose.
Water immersion during labor and birth significantly reduced use of medications, maternal pain, and postpartum hemorrhage, compared with standard care with no water immersion, based on data from 36 studies including more than 150,000 women.
“Resting and laboring in water can reduce fear, anxiety, and pain perception; it helps optimize the physiology of childbirth through the release of endogenous endorphins and oxytocin,” and data from randomized, controlled trials have shown a reduced need for epidural analgesia with water immersion, Ethel Burns, PhD, of Oxford (England) Brookes University Faculty of Health and Life Sciences, and colleagues wrote.
Although previous studies have not shown an increased risk for adverse events for newborns following water birth, “There is a need to understand which clinical practices, when performed as part of water immersion care, result in the optimum outcomes for mother and newborn,” the researchers said.
In a systematic review and meta-analysis published in BMJ Open, the researchers identified studies published since 2000 that examined maternal or neonatal interventions and/or outcomes when birthing pools were used for labor and/or birth.
The primary objective was to compare intrapartum interventions and outcomes for water immersion during labor with standard care with no water immersion.
Water immersion generally involves the use of a birth pool for relaxation and pain relief in early labor, and some women proceed with immersion through the second stage of labor and delivery. Of the 36 included studies, 31 took place in a hospital setting, 4 in a midwife-led setting, and 1 in a mixed setting. Most of the studies (25) involved women who planned to have/had a water birth, and these studies included 151,742 women. Another seven studies including 1,901 women involved in water immersion for labor only, three studies including 3,688 women involved in water immersion during labor and water birth; the timing of water immersion was unclear in the remaining study of 215 women.
Overall, water immersion significantly reduced the use of epidurals (odds ratio, 0.17), injected opioids (OR, 0.22), and episiotomy (OR, 0.16). Maternal pain and postpartum hemorrhage also were significantly reduced with water immersion (OR, 0.24 and OR, 0.69, respectively).
Maternal satisfaction was significantly increased with water immersion, and the odds of an intact perineum increased as well (OR, 1.95 and OR, 1.48).
The overall odds of cord avulsion increased with water immersion (OR, 1.94), but the absolute risk was low, compared with births without water immersion (4.3 vs. 1.3 per 1,000). No significant differences in other identified neonatal outcomes were observed across the studies.
The study findings were limited by several factors including the inconsistency of reporting on birth setting, care practices, interventions, and outcomes, and the inclusion of only three outcomes for meta-regression analysis, the researchers noted. In addition, only four studies were conducted in midwifery-led settings.
“This is important because birth pool use is most prevalent in midwifery-led settings,” the researchers wrote.” Evidence-based practice of water immersion requires research that reflects the context of care provision.
“We suggest that studies incorporate the following fundamentals to advance the evidence: birth pool description, clearly described maternal and obstetric characteristics, the birth setting, the care model and use of standardized definitions.”
Despite the limitations and need for additional research, the data overall support the potential benefits from water immersion births for healthy women and newborns, the researchers concluded.
A Clinical Report issued by the American Academy of Pediatrics in January 2022 advised against water immersion during the second stage of labor and delivery. According to the report, the potential for neonatal infections from organisms such as Legionella and Pseudomonas species, is low, but does exist, and could result in serious complications.
Education is essential
Increasing numbers of women are seeking home births and water births, Marissa Platner, MD, a maternal-fetal medicine specialist at Emory University, Atlanta, said in an interview.
“Given the conflicting data and lack of data, it is important to be able to educate birthing mothers based on best available evidence,” said Dr. Platner, who was not involved in the study.
“I was not surprised by the findings, because the adverse outcomes that are of concern, such as neonatal sepsis, were not clearly addressed,” Dr. Platner said. Given that sepsis “is a rare outcome in the population of low-risk individuals, the study may not have been powered to assess for this. The findings of maternal pain and satisfaction being improved with water immersion are well known. ACOG [American College of Obstetricians and Gynecologists] has also stated that water immersion during the first stage of labor is safe and can help with pain control.”
On a practical level, “I think clinicians can use this guidance to discuss the potential benefits of water immersion in the first stages of labor, but would caution women regarding the unknown but possible risks of the water birth, given these findings are less clear,” Dr. Platner said.
“I think the findings regarding maternal outcomes are valid and consistent with the AAP/ACOG recommendations in terms of improving maternal pain control; however, more research is needed to determine the safety of the second stage of labor occurring in the water, given the potential for neonatal infection and respiratory distress, which could not be adequately addressed in this study,” Dr. Platner emphasized.
The study was supported by Oxford Brookes University. The researchers had no financial conflicts to disclose. Dr. Platner had no financial conflicts to disclose.
FROM BMJ OPEN
How to manage cancer pain when patients misuse opioids
Opioids remain a staple in pain management for cancer, but there is little guidance around how to treat patients who have a history of opioid misuse.
Recently,
“There is a tendency to ignore treatment of opioid use disorder in advanced cancer patients because people think: ‘Oh, this person has bigger fish to fry,’ but that’s not a very patient-centric way of looking at things,” senior author Jessica Merlin, MD, PhD, with the University of Pittsburgh, said in a news release.
“We know that opioid use disorder is a really important factor in quality of life, so addressing opioid addiction and prescription opioid misuse in people with advanced cancer is really critical,” Dr. Merlin added.
The study was published online in JAMA Oncology.
To improve care for people with advanced cancer and cancer-related pain, the researchers first assessed how clinicians currently treat patients with opioid complexity.
Using an online Delphi platform, the team invited 120 clinicians with expertise in palliative care, pain management, and addiction medicine to weigh in on three common clinical scenarios – a patient with a recent history of untreated opioid use disorder, a patient taking more opioids than prescribed, and a patient using nonprescribed benzodiazepines.
For a patient with cancer and a recent history of untreated opioid use disorder, regardless of prognosis, the panel deemed it appropriate to begin treatment with buprenorphine/naloxone for pain but inappropriate to refer the patient to a methadone clinic. The panel felt that going to a methadone clinic would be too burdensome for a patient with advanced cancer and not possible for those with limited prognoses.
“This underscores the importance of access to [opioid use disorder] treatment in cancer treatment settings, including non–addiction specialists waivered to prescribe buprenorphine/naloxone and addiction specialists for more complex cases,” the authors wrote.
For a patient with untreated opioid use disorder, the panel deemed split-dose methadone (two to three times daily) appropriate in those with limited prognosis of weeks to months but was uncertain about the suitability of this approach for patients with longer prognoses of a year or longer.
The appropriateness of initiating treatment with a full-agonist opioid was considered uncertain for a patient with limited prognosis and inappropriate for a patient with longer prognosis.
For a patient with cancer pain and no medical history of opioid use disorder but taking more opioids than prescribed, regardless of prognosis, the panel felt it was appropriate to increase monitoring and inappropriate to taper opioids. The panel was not certain about whether to increase opioids based on the patient’s account of what they need or transition to buprenorphine/naloxone.
For a patient with no history of opioid use disorder who was prescribed traditional opioids for pain and had a positive urine drug test for nonprescribed benzodiazepines, regardless of prognosis, the panel felt it was appropriate to continue opioids with close monitoring and inappropriate to taper opioids or transition to buprenorphine/naloxone.
The researchers said that improving education around buprenorphine and cancer pain management in the context of opioid use disorder or misuse is needed.
In a related editorial, two experts noted that the patients considered in this “important article” require considerable time and expertise from an interdisciplinary team.
“It is important that cancer centers establish and fund such teams mainly as a safety measure for these patients and also as a major contribution to the care of all patients with cancer,” wrote Joseph Arthur, MD, and Eduardo Bruera, MD, with the University of Texas MD Anderson Cancer Center, Houston.
In the wider context, Dr. Arthur and Dr. Bruera highlighted how treatments for patients with advanced cancer have evolved over the past 3 decades, yet patients have continued to be given opioids to address cancer-related pain. Developing more sophisticated drugs that relieve pain without significant side effects or addictive properties is imperative.
Dr. Arthur and Dr. Bruera said the study authors “appropriately emphasize the value of delivering compassionate and expert care for these particularly complex cases and the importance of conducting research on the best ways to alleviate the suffering in this rapidly growing patient population.”
This research was supported by Cambia Health Foundation and the National Institute of Nursing Research. Dr. Merlin, Dr. Arthur, and Dr. Bruera reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
Opioids remain a staple in pain management for cancer, but there is little guidance around how to treat patients who have a history of opioid misuse.
Recently,
“There is a tendency to ignore treatment of opioid use disorder in advanced cancer patients because people think: ‘Oh, this person has bigger fish to fry,’ but that’s not a very patient-centric way of looking at things,” senior author Jessica Merlin, MD, PhD, with the University of Pittsburgh, said in a news release.
“We know that opioid use disorder is a really important factor in quality of life, so addressing opioid addiction and prescription opioid misuse in people with advanced cancer is really critical,” Dr. Merlin added.
The study was published online in JAMA Oncology.
To improve care for people with advanced cancer and cancer-related pain, the researchers first assessed how clinicians currently treat patients with opioid complexity.
Using an online Delphi platform, the team invited 120 clinicians with expertise in palliative care, pain management, and addiction medicine to weigh in on three common clinical scenarios – a patient with a recent history of untreated opioid use disorder, a patient taking more opioids than prescribed, and a patient using nonprescribed benzodiazepines.
For a patient with cancer and a recent history of untreated opioid use disorder, regardless of prognosis, the panel deemed it appropriate to begin treatment with buprenorphine/naloxone for pain but inappropriate to refer the patient to a methadone clinic. The panel felt that going to a methadone clinic would be too burdensome for a patient with advanced cancer and not possible for those with limited prognoses.
“This underscores the importance of access to [opioid use disorder] treatment in cancer treatment settings, including non–addiction specialists waivered to prescribe buprenorphine/naloxone and addiction specialists for more complex cases,” the authors wrote.
For a patient with untreated opioid use disorder, the panel deemed split-dose methadone (two to three times daily) appropriate in those with limited prognosis of weeks to months but was uncertain about the suitability of this approach for patients with longer prognoses of a year or longer.
The appropriateness of initiating treatment with a full-agonist opioid was considered uncertain for a patient with limited prognosis and inappropriate for a patient with longer prognosis.
For a patient with cancer pain and no medical history of opioid use disorder but taking more opioids than prescribed, regardless of prognosis, the panel felt it was appropriate to increase monitoring and inappropriate to taper opioids. The panel was not certain about whether to increase opioids based on the patient’s account of what they need or transition to buprenorphine/naloxone.
For a patient with no history of opioid use disorder who was prescribed traditional opioids for pain and had a positive urine drug test for nonprescribed benzodiazepines, regardless of prognosis, the panel felt it was appropriate to continue opioids with close monitoring and inappropriate to taper opioids or transition to buprenorphine/naloxone.
The researchers said that improving education around buprenorphine and cancer pain management in the context of opioid use disorder or misuse is needed.
In a related editorial, two experts noted that the patients considered in this “important article” require considerable time and expertise from an interdisciplinary team.
“It is important that cancer centers establish and fund such teams mainly as a safety measure for these patients and also as a major contribution to the care of all patients with cancer,” wrote Joseph Arthur, MD, and Eduardo Bruera, MD, with the University of Texas MD Anderson Cancer Center, Houston.
In the wider context, Dr. Arthur and Dr. Bruera highlighted how treatments for patients with advanced cancer have evolved over the past 3 decades, yet patients have continued to be given opioids to address cancer-related pain. Developing more sophisticated drugs that relieve pain without significant side effects or addictive properties is imperative.
Dr. Arthur and Dr. Bruera said the study authors “appropriately emphasize the value of delivering compassionate and expert care for these particularly complex cases and the importance of conducting research on the best ways to alleviate the suffering in this rapidly growing patient population.”
This research was supported by Cambia Health Foundation and the National Institute of Nursing Research. Dr. Merlin, Dr. Arthur, and Dr. Bruera reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
Opioids remain a staple in pain management for cancer, but there is little guidance around how to treat patients who have a history of opioid misuse.
Recently,
“There is a tendency to ignore treatment of opioid use disorder in advanced cancer patients because people think: ‘Oh, this person has bigger fish to fry,’ but that’s not a very patient-centric way of looking at things,” senior author Jessica Merlin, MD, PhD, with the University of Pittsburgh, said in a news release.
“We know that opioid use disorder is a really important factor in quality of life, so addressing opioid addiction and prescription opioid misuse in people with advanced cancer is really critical,” Dr. Merlin added.
The study was published online in JAMA Oncology.
To improve care for people with advanced cancer and cancer-related pain, the researchers first assessed how clinicians currently treat patients with opioid complexity.
Using an online Delphi platform, the team invited 120 clinicians with expertise in palliative care, pain management, and addiction medicine to weigh in on three common clinical scenarios – a patient with a recent history of untreated opioid use disorder, a patient taking more opioids than prescribed, and a patient using nonprescribed benzodiazepines.
For a patient with cancer and a recent history of untreated opioid use disorder, regardless of prognosis, the panel deemed it appropriate to begin treatment with buprenorphine/naloxone for pain but inappropriate to refer the patient to a methadone clinic. The panel felt that going to a methadone clinic would be too burdensome for a patient with advanced cancer and not possible for those with limited prognoses.
“This underscores the importance of access to [opioid use disorder] treatment in cancer treatment settings, including non–addiction specialists waivered to prescribe buprenorphine/naloxone and addiction specialists for more complex cases,” the authors wrote.
For a patient with untreated opioid use disorder, the panel deemed split-dose methadone (two to three times daily) appropriate in those with limited prognosis of weeks to months but was uncertain about the suitability of this approach for patients with longer prognoses of a year or longer.
The appropriateness of initiating treatment with a full-agonist opioid was considered uncertain for a patient with limited prognosis and inappropriate for a patient with longer prognosis.
For a patient with cancer pain and no medical history of opioid use disorder but taking more opioids than prescribed, regardless of prognosis, the panel felt it was appropriate to increase monitoring and inappropriate to taper opioids. The panel was not certain about whether to increase opioids based on the patient’s account of what they need or transition to buprenorphine/naloxone.
For a patient with no history of opioid use disorder who was prescribed traditional opioids for pain and had a positive urine drug test for nonprescribed benzodiazepines, regardless of prognosis, the panel felt it was appropriate to continue opioids with close monitoring and inappropriate to taper opioids or transition to buprenorphine/naloxone.
The researchers said that improving education around buprenorphine and cancer pain management in the context of opioid use disorder or misuse is needed.
In a related editorial, two experts noted that the patients considered in this “important article” require considerable time and expertise from an interdisciplinary team.
“It is important that cancer centers establish and fund such teams mainly as a safety measure for these patients and also as a major contribution to the care of all patients with cancer,” wrote Joseph Arthur, MD, and Eduardo Bruera, MD, with the University of Texas MD Anderson Cancer Center, Houston.
In the wider context, Dr. Arthur and Dr. Bruera highlighted how treatments for patients with advanced cancer have evolved over the past 3 decades, yet patients have continued to be given opioids to address cancer-related pain. Developing more sophisticated drugs that relieve pain without significant side effects or addictive properties is imperative.
Dr. Arthur and Dr. Bruera said the study authors “appropriately emphasize the value of delivering compassionate and expert care for these particularly complex cases and the importance of conducting research on the best ways to alleviate the suffering in this rapidly growing patient population.”
This research was supported by Cambia Health Foundation and the National Institute of Nursing Research. Dr. Merlin, Dr. Arthur, and Dr. Bruera reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM JAMA ONCOLOGY
Will the headache field embrace rofecoxib?
In June, the Concord, Mass.–based company Tremeau Pharmaceuticals announced that the Food and Drug Administration was letting it proceed with a phase 3 clinical trial to test rofecoxib, the once-bestselling painkiller known as Vioxx, in patients with migraine.
The anti-inflammatory drug, a cyclooxygenase-2 (COX-2) inhibitor, received its first FDA approval in 1999 and became widely prescribed for arthritis and acute pain. In 2004 it was withdrawn by its manufacturer, Merck, after being shown to raise the risk of cardiovascular events.
In clinical trials and in real-world epidemiological studies, rofecoxib was associated with elevated heart attack, stroke, and related deaths; one 2005 study estimated that it had been responsible for some 38,000 excess deaths in the United States before being withdrawn. In 2007 Merck, beset with allegations that it had suppressed and mischaracterized rofecoxib’s safety data, paid out nearly $5 billion to settle thousands of lawsuits filed by patients and their families.
, an indication for which it received an orphan drug designation in 2017 and the agency’s green light for trials in 2020.
Brad Sippy, Tremeau’s chief executive officer, said that his company chose the two indications in part because both patient populations have low cardiovascular risk. Migraine patients are generally younger than the arthritis populations formerly treated with rofecoxib and are unlikely to take the drug for more than a day or 2 at time, avoiding the risks associated with extended exposure.
A crowded market
The past several years have seen the emergence of a cornucopia of new migraine treatments, including monoclonal antibodies such as erenumab (Aimovig, Amgen), which help prevent attacks by blocking the vasodilator calcitonin gene-related peptide, or CGRP. In addition to the standard arsenal of triptans and nonsteroidal anti-inflammatory drugs for acute pain relief, migraine patients can now choose among serotonin-blocking agents such as lasmiditan (Reyvow, Eli Lilly), known as “ditans,” and small-molecule CGRP antagonists such as ubrogepant (Ubrelvy, Abbie), known as “gepants.” Some NSAIDs, including one COX inhibitor, have been formulated into rapidly absorbed powders or liquids for migraine.
Mr. Sippy said he sees a role for rofecoxib even in this crowded space. “Migraine as you know is a multimodal situation – few people say that only one drug works for them,” he said. “We think this is an option that would basically be like a high dose of ibuprofen,” but with less frequent dosing and lower gastrointestinal and platelet effects compared with ibuprofen and other NSAIDs.
An improved formulation
Rofecoxib “crosses the blood brain barrier very readily – better than other COX inhibitors on the market,” Mr. Sippy added. “It was well absorbed in its original formulation, and our product is even better absorbed than the original – we estimate it’s probably an hour quicker to [peak concentration].” In addition, he said, “our formulation is more efficient at delivering the drug so we don’t need as much active ingredient – our 17.5 milligrams gets you the same systemic exposure as 25 milligrams of the old product.”
A different mechanism of action
Neurologist Alan M. Rapoport, MD, editor-in-chief of Neurology Reviews and professor of neurology at the University of California, Los Angeles, said that he was “cautiously optimistic” that “if used correctly and not too frequently, [rofecoxib] will find its niche in migraine treatment.”
“Patients liked Vioxx,” said Dr. Rapoport, past president of the International Headache Society. Even people currently on prevention “need to have an acute care drug handy.” While some patients on monoclonal antibodies have had success with gepants for acute care, “these both target the same pathway. It’s always nice to have options with a different mechanism of action.”
One of the arguments Tremeau has cited for reintroducing rofecoxib has been an urgent need for alternatives to opioid painkillers. Indeed some analysts have linked the demise of Vioxx with a subsequent increase in opioid prescribing.
Dr. Rapoport noted that he never prescribes opioids or butalbital, a barbiturate, for migraine, and that most headache specialists avoid them in clinical practice. But in the emergency setting, he said, patients receive them all too frequently.
Mr. Sippy said that opioid prescribing, while not unknown in migraine, was a bigger problem in hemophilic arthropathy, the first indication his company has pursued for rofecoxib. People with hemophilia “have a kind of arthritis that would respond well to an anti-inflammatory drug but they can’t take NSAIDs due to bleeding risk. This is why so many end up on opioids. Rofecoxib, as a COX-2 inhibitor, doesn’t have any effect on platelet aggregation, which would make it another option.”
No unique risks at prescribed doses
The migraine indication originally started out narrower: Patients with both migraine and bleeding disorders. “But in talking with the FDA, they encouraged us to develop it for migraine,” Mr. Sippy said. The company is considering pursuing a third indication: menstrual pain co-occurring with migraine. Tremeau has not ruled out seeking an indication in patients with arthritis who cannot take other painkillers, whether opioids or NSAIDs.
Five years ago, when Tremeau first announced its plans to bring rofecoxib back – indeed the company was set up for that purpose and has only this and another COX-2 inhibitor in development – some experts warned that there is little to prevent the drug from being used off-label, whether in higher doses or for other diseases.
“That’s something else we’re seeking to solve in addition to going for younger populations,” said Mr. Sippy, who worked at Merck during the Vioxx crisis and later headed neurology at Sunovion before starting his own company.
“We’re going for the former middle dose as our high dose and now we know that you don’t want to take more than the prescribed amount. If it doesn’t work you get off it; you don’t want to dose-creep on it. That’s been a key insight: At the appropriate dose, this product has no unique risk relative to the drug class and potentially some unique benefits,” he said.
Risk versus benefit
Joseph Ross, MD, a health policy researcher at Yale University in New Haven, Conn., who in a 2018 editorial expressed concerns about rofecoxib’s revival, said in an email that he felt its use in migraine could be justified, with caveats.
During Vioxx’s original approval and time on the market, “there was a cardiovascular risk associated with use that was not being transparently and clearly reported to patients and clinicians,” Dr. Ross said.
“In terms of testing the product for use in patients with migraine – a population of generally younger patients at lower risk of cardiovascular disease – my only concern is that the risk is clearly communicated and that there is adequate postmarket safety surveillance,” he said. “If patients are making fully informed decisions, the potential benefit of the drug with respect to pain control may be worth the risks.”
Dr. Rapoport serves as an adviser for AbbVie, Amgen, Biohaven, Cala Health, Collegium Pharmaceutical, Satsuma, Teva, Theranica and Xoc; he is on the speakers bureau of AbbVie, Amgen, Biohaven, Impel, Lundbeck, and Teva. Dr. Ross disclosed research support from Johnson and Johnson, the Medical Device Innovation Consortium, and the Laura and John Arnold Foundation, along with government grants; he is also an expert witness in a lawsuit against Biogen.
In June, the Concord, Mass.–based company Tremeau Pharmaceuticals announced that the Food and Drug Administration was letting it proceed with a phase 3 clinical trial to test rofecoxib, the once-bestselling painkiller known as Vioxx, in patients with migraine.
The anti-inflammatory drug, a cyclooxygenase-2 (COX-2) inhibitor, received its first FDA approval in 1999 and became widely prescribed for arthritis and acute pain. In 2004 it was withdrawn by its manufacturer, Merck, after being shown to raise the risk of cardiovascular events.
In clinical trials and in real-world epidemiological studies, rofecoxib was associated with elevated heart attack, stroke, and related deaths; one 2005 study estimated that it had been responsible for some 38,000 excess deaths in the United States before being withdrawn. In 2007 Merck, beset with allegations that it had suppressed and mischaracterized rofecoxib’s safety data, paid out nearly $5 billion to settle thousands of lawsuits filed by patients and their families.
, an indication for which it received an orphan drug designation in 2017 and the agency’s green light for trials in 2020.
Brad Sippy, Tremeau’s chief executive officer, said that his company chose the two indications in part because both patient populations have low cardiovascular risk. Migraine patients are generally younger than the arthritis populations formerly treated with rofecoxib and are unlikely to take the drug for more than a day or 2 at time, avoiding the risks associated with extended exposure.
A crowded market
The past several years have seen the emergence of a cornucopia of new migraine treatments, including monoclonal antibodies such as erenumab (Aimovig, Amgen), which help prevent attacks by blocking the vasodilator calcitonin gene-related peptide, or CGRP. In addition to the standard arsenal of triptans and nonsteroidal anti-inflammatory drugs for acute pain relief, migraine patients can now choose among serotonin-blocking agents such as lasmiditan (Reyvow, Eli Lilly), known as “ditans,” and small-molecule CGRP antagonists such as ubrogepant (Ubrelvy, Abbie), known as “gepants.” Some NSAIDs, including one COX inhibitor, have been formulated into rapidly absorbed powders or liquids for migraine.
Mr. Sippy said he sees a role for rofecoxib even in this crowded space. “Migraine as you know is a multimodal situation – few people say that only one drug works for them,” he said. “We think this is an option that would basically be like a high dose of ibuprofen,” but with less frequent dosing and lower gastrointestinal and platelet effects compared with ibuprofen and other NSAIDs.
An improved formulation
Rofecoxib “crosses the blood brain barrier very readily – better than other COX inhibitors on the market,” Mr. Sippy added. “It was well absorbed in its original formulation, and our product is even better absorbed than the original – we estimate it’s probably an hour quicker to [peak concentration].” In addition, he said, “our formulation is more efficient at delivering the drug so we don’t need as much active ingredient – our 17.5 milligrams gets you the same systemic exposure as 25 milligrams of the old product.”
A different mechanism of action
Neurologist Alan M. Rapoport, MD, editor-in-chief of Neurology Reviews and professor of neurology at the University of California, Los Angeles, said that he was “cautiously optimistic” that “if used correctly and not too frequently, [rofecoxib] will find its niche in migraine treatment.”
“Patients liked Vioxx,” said Dr. Rapoport, past president of the International Headache Society. Even people currently on prevention “need to have an acute care drug handy.” While some patients on monoclonal antibodies have had success with gepants for acute care, “these both target the same pathway. It’s always nice to have options with a different mechanism of action.”
One of the arguments Tremeau has cited for reintroducing rofecoxib has been an urgent need for alternatives to opioid painkillers. Indeed some analysts have linked the demise of Vioxx with a subsequent increase in opioid prescribing.
Dr. Rapoport noted that he never prescribes opioids or butalbital, a barbiturate, for migraine, and that most headache specialists avoid them in clinical practice. But in the emergency setting, he said, patients receive them all too frequently.
Mr. Sippy said that opioid prescribing, while not unknown in migraine, was a bigger problem in hemophilic arthropathy, the first indication his company has pursued for rofecoxib. People with hemophilia “have a kind of arthritis that would respond well to an anti-inflammatory drug but they can’t take NSAIDs due to bleeding risk. This is why so many end up on opioids. Rofecoxib, as a COX-2 inhibitor, doesn’t have any effect on platelet aggregation, which would make it another option.”
No unique risks at prescribed doses
The migraine indication originally started out narrower: Patients with both migraine and bleeding disorders. “But in talking with the FDA, they encouraged us to develop it for migraine,” Mr. Sippy said. The company is considering pursuing a third indication: menstrual pain co-occurring with migraine. Tremeau has not ruled out seeking an indication in patients with arthritis who cannot take other painkillers, whether opioids or NSAIDs.
Five years ago, when Tremeau first announced its plans to bring rofecoxib back – indeed the company was set up for that purpose and has only this and another COX-2 inhibitor in development – some experts warned that there is little to prevent the drug from being used off-label, whether in higher doses or for other diseases.
“That’s something else we’re seeking to solve in addition to going for younger populations,” said Mr. Sippy, who worked at Merck during the Vioxx crisis and later headed neurology at Sunovion before starting his own company.
“We’re going for the former middle dose as our high dose and now we know that you don’t want to take more than the prescribed amount. If it doesn’t work you get off it; you don’t want to dose-creep on it. That’s been a key insight: At the appropriate dose, this product has no unique risk relative to the drug class and potentially some unique benefits,” he said.
Risk versus benefit
Joseph Ross, MD, a health policy researcher at Yale University in New Haven, Conn., who in a 2018 editorial expressed concerns about rofecoxib’s revival, said in an email that he felt its use in migraine could be justified, with caveats.
During Vioxx’s original approval and time on the market, “there was a cardiovascular risk associated with use that was not being transparently and clearly reported to patients and clinicians,” Dr. Ross said.
“In terms of testing the product for use in patients with migraine – a population of generally younger patients at lower risk of cardiovascular disease – my only concern is that the risk is clearly communicated and that there is adequate postmarket safety surveillance,” he said. “If patients are making fully informed decisions, the potential benefit of the drug with respect to pain control may be worth the risks.”
Dr. Rapoport serves as an adviser for AbbVie, Amgen, Biohaven, Cala Health, Collegium Pharmaceutical, Satsuma, Teva, Theranica and Xoc; he is on the speakers bureau of AbbVie, Amgen, Biohaven, Impel, Lundbeck, and Teva. Dr. Ross disclosed research support from Johnson and Johnson, the Medical Device Innovation Consortium, and the Laura and John Arnold Foundation, along with government grants; he is also an expert witness in a lawsuit against Biogen.
In June, the Concord, Mass.–based company Tremeau Pharmaceuticals announced that the Food and Drug Administration was letting it proceed with a phase 3 clinical trial to test rofecoxib, the once-bestselling painkiller known as Vioxx, in patients with migraine.
The anti-inflammatory drug, a cyclooxygenase-2 (COX-2) inhibitor, received its first FDA approval in 1999 and became widely prescribed for arthritis and acute pain. In 2004 it was withdrawn by its manufacturer, Merck, after being shown to raise the risk of cardiovascular events.
In clinical trials and in real-world epidemiological studies, rofecoxib was associated with elevated heart attack, stroke, and related deaths; one 2005 study estimated that it had been responsible for some 38,000 excess deaths in the United States before being withdrawn. In 2007 Merck, beset with allegations that it had suppressed and mischaracterized rofecoxib’s safety data, paid out nearly $5 billion to settle thousands of lawsuits filed by patients and their families.
, an indication for which it received an orphan drug designation in 2017 and the agency’s green light for trials in 2020.
Brad Sippy, Tremeau’s chief executive officer, said that his company chose the two indications in part because both patient populations have low cardiovascular risk. Migraine patients are generally younger than the arthritis populations formerly treated with rofecoxib and are unlikely to take the drug for more than a day or 2 at time, avoiding the risks associated with extended exposure.
A crowded market
The past several years have seen the emergence of a cornucopia of new migraine treatments, including monoclonal antibodies such as erenumab (Aimovig, Amgen), which help prevent attacks by blocking the vasodilator calcitonin gene-related peptide, or CGRP. In addition to the standard arsenal of triptans and nonsteroidal anti-inflammatory drugs for acute pain relief, migraine patients can now choose among serotonin-blocking agents such as lasmiditan (Reyvow, Eli Lilly), known as “ditans,” and small-molecule CGRP antagonists such as ubrogepant (Ubrelvy, Abbie), known as “gepants.” Some NSAIDs, including one COX inhibitor, have been formulated into rapidly absorbed powders or liquids for migraine.
Mr. Sippy said he sees a role for rofecoxib even in this crowded space. “Migraine as you know is a multimodal situation – few people say that only one drug works for them,” he said. “We think this is an option that would basically be like a high dose of ibuprofen,” but with less frequent dosing and lower gastrointestinal and platelet effects compared with ibuprofen and other NSAIDs.
An improved formulation
Rofecoxib “crosses the blood brain barrier very readily – better than other COX inhibitors on the market,” Mr. Sippy added. “It was well absorbed in its original formulation, and our product is even better absorbed than the original – we estimate it’s probably an hour quicker to [peak concentration].” In addition, he said, “our formulation is more efficient at delivering the drug so we don’t need as much active ingredient – our 17.5 milligrams gets you the same systemic exposure as 25 milligrams of the old product.”
A different mechanism of action
Neurologist Alan M. Rapoport, MD, editor-in-chief of Neurology Reviews and professor of neurology at the University of California, Los Angeles, said that he was “cautiously optimistic” that “if used correctly and not too frequently, [rofecoxib] will find its niche in migraine treatment.”
“Patients liked Vioxx,” said Dr. Rapoport, past president of the International Headache Society. Even people currently on prevention “need to have an acute care drug handy.” While some patients on monoclonal antibodies have had success with gepants for acute care, “these both target the same pathway. It’s always nice to have options with a different mechanism of action.”
One of the arguments Tremeau has cited for reintroducing rofecoxib has been an urgent need for alternatives to opioid painkillers. Indeed some analysts have linked the demise of Vioxx with a subsequent increase in opioid prescribing.
Dr. Rapoport noted that he never prescribes opioids or butalbital, a barbiturate, for migraine, and that most headache specialists avoid them in clinical practice. But in the emergency setting, he said, patients receive them all too frequently.
Mr. Sippy said that opioid prescribing, while not unknown in migraine, was a bigger problem in hemophilic arthropathy, the first indication his company has pursued for rofecoxib. People with hemophilia “have a kind of arthritis that would respond well to an anti-inflammatory drug but they can’t take NSAIDs due to bleeding risk. This is why so many end up on opioids. Rofecoxib, as a COX-2 inhibitor, doesn’t have any effect on platelet aggregation, which would make it another option.”
No unique risks at prescribed doses
The migraine indication originally started out narrower: Patients with both migraine and bleeding disorders. “But in talking with the FDA, they encouraged us to develop it for migraine,” Mr. Sippy said. The company is considering pursuing a third indication: menstrual pain co-occurring with migraine. Tremeau has not ruled out seeking an indication in patients with arthritis who cannot take other painkillers, whether opioids or NSAIDs.
Five years ago, when Tremeau first announced its plans to bring rofecoxib back – indeed the company was set up for that purpose and has only this and another COX-2 inhibitor in development – some experts warned that there is little to prevent the drug from being used off-label, whether in higher doses or for other diseases.
“That’s something else we’re seeking to solve in addition to going for younger populations,” said Mr. Sippy, who worked at Merck during the Vioxx crisis and later headed neurology at Sunovion before starting his own company.
“We’re going for the former middle dose as our high dose and now we know that you don’t want to take more than the prescribed amount. If it doesn’t work you get off it; you don’t want to dose-creep on it. That’s been a key insight: At the appropriate dose, this product has no unique risk relative to the drug class and potentially some unique benefits,” he said.
Risk versus benefit
Joseph Ross, MD, a health policy researcher at Yale University in New Haven, Conn., who in a 2018 editorial expressed concerns about rofecoxib’s revival, said in an email that he felt its use in migraine could be justified, with caveats.
During Vioxx’s original approval and time on the market, “there was a cardiovascular risk associated with use that was not being transparently and clearly reported to patients and clinicians,” Dr. Ross said.
“In terms of testing the product for use in patients with migraine – a population of generally younger patients at lower risk of cardiovascular disease – my only concern is that the risk is clearly communicated and that there is adequate postmarket safety surveillance,” he said. “If patients are making fully informed decisions, the potential benefit of the drug with respect to pain control may be worth the risks.”
Dr. Rapoport serves as an adviser for AbbVie, Amgen, Biohaven, Cala Health, Collegium Pharmaceutical, Satsuma, Teva, Theranica and Xoc; he is on the speakers bureau of AbbVie, Amgen, Biohaven, Impel, Lundbeck, and Teva. Dr. Ross disclosed research support from Johnson and Johnson, the Medical Device Innovation Consortium, and the Laura and John Arnold Foundation, along with government grants; he is also an expert witness in a lawsuit against Biogen.
What is palliative care and what’s new in practicing this type of medicine?
The World Health Organization defines palliative care as “an approach that improves the quality of life of patients (adults and children) and their families who are facing problems associated with life-threatening illness. It prevents and relieves suffering through the early identification, correct assessment, and treatment of pain and other problems, whether physical, psychosocial or spiritual.”1
The common misperception is that palliative care is only for those at end of life or only in the advanced stages of their illness. However, palliative care is ideally most helpful following individuals from diagnosis through their illness trajectory. Another misperception is that palliative care and hospice are the same thing. Though all hospice is palliative care, all palliative care is not hospice. Both palliative care and hospice provide care for individuals facing a serious illness and focus on the same philosophy of care, but palliative care can be initiated at any stage of illness, even if the goal is to pursue curative and life-prolonging therapies/interventions.
In contrast, hospice is considered for those who are at the end of life and are usually not pursuing life-prolonging therapies or interventions, instead focusing on comfort, symptom management, and optimization of quality of life.
Though there is a growing need for palliative care, there is a shortage of specialist palliative care providers. Much of the palliative care needs can be met by all providers who can offer basic symptom management, identification surrounding goals of care and discussions of advance care planning, and understanding of illness/prognosis and treatment options, which is called primary palliative care.2 In fact, two-thirds of patients with a serious illness other than cancer prefer discussion of end-of-life care or advance care planning with their primary care providers.3
Referral to specialty palliative care should be considered when there are more complexities to symptom/pain management and goals of care/end of life, transition to hospice, or complex communication dynamics.4
Though specialty palliative care was shown to be more comprehensive, both primary palliative care and specialty palliative care have led to improvements in the quality of life in individuals living with serious illness.5 Early integration of palliative care into routine care has been shown to improve symptom burden, mood, quality of life, survival, and health care costs.6
Updates in alternative and complementary therapies to palliative care
There are several alternative and complementary therapies to palliative care, including cannabis and psychedelics. These therapies are becoming or may become a familiar part of medical therapies that are listed in a patient’s history as part of their medical regimen, especially as more states continue to legalize and/or decriminalize the use of these alternative therapies for recreational or medicinal use.
Both cannabis and psychedelics have a longstanding history of therapeutic and holistic use. Cannabis has been used to manage symptoms such as pain since the 16th and 17th century.7 In palliative care, more patients may turn to various forms of cannabis as a source of relief from symptoms and suffering as their focus shifts more to quality of life.
Even with the increasing popularity of the use of cannabis among seriously ill patients, there is still a lack of evidence of the benefits of medical cannabis use in palliative care, and there is a lack of standardization of type of cannabis used and state regulations regarding their use.7
A recent systematic review found that despite the reported positive treatment effects of cannabis in palliative care, the results of the studies were conflicting. This highlights the need for further high-quality research to determine whether cannabis products are an effective treatment in palliative care patients.8
One limitation to note is that the majority of the included studies focused on cannabis use in patients with cancer for cancer-related symptoms. Few studies included patients with other serious conditions.
Psychedelics
There is evidence that psychedelic assisted therapy (PAT) is a safe and effective treatment for individuals with refractory depression, posttraumatic stress disorder, and substance use disorder.9 Plus, there have been ample studies providing support that PAT improves symptoms such as refractory anxiety/depression, demoralization, and existential distress in seriously ill patients, thus improving their quality of life and overall well-being.9
Nine U.S. cities and the State of Oregon have decriminalized or legalized the psychedelic psilocybin, based on the medical benefits patients have experienced evidenced from using it.10
In light of the increasing interest in PAT, Dr. Ira Byock provided the following points on what “all clinicians should know as they enter this uncharted territory”:
- Psychedelics have been around for a long time.
- Psychedelic-assisted therapies’ therapeutic effects are experiential.
- There are a variety of terms for specific categories of psychedelic compounds.
- Some palliative care teams are already caring for patients who undergo psychedelic experiences.
- Use of psychedelics should be well-observed by a skilled clinician with expertise.
I am hoping this provides a general refresher on palliative care and an overview of updates to alternative and complementary therapies for patients living with serious illness.9
Dr. Kang is a geriatrician and palliative care provider at the University of Washington, Seattle in the division of geriatrics and gerontology. She has no conflicts related to the content of this piece.
References
1. World Health Organization. Palliative care. 2020 Aug 5..
2. Weissman DE and Meier DE. Identifying patients in need of a palliative care assessment in the hospital setting a consensus report from the center to advance palliative care. J Palliat Med. 2011;14(1):17-23.
3. Sherry D et al. Is primary care physician involvement associated with earlier advance care planning? A study of patients in an academic primary care setting. J Palliat Med. 2022;25(1):75-80.
4. Quill TE and Abernethy AP. Generalist plus specialist palliative care-creating a more sustainable model. N Engl J Med. 2013;368:1173-75.
5. Ernecoff NC et al. Comparing specialty and primary palliative care interventions: Analysis of a systematic review. J Palliat Med. 2020;23(3):389-96.
6. Temmel JS et al. Early palliative care for patients with metastatic non–small-cell lung cancer. N Engl J Med. 2011;363:733-42.
7. Kogan M and Sexton M. Medical cannabis: A new old tool for palliative care. J Altern Complement Med . 2020 Sep;26(9):776-8.
8. Doppen M et al. Cannabis in palliative care: A systematic review of the current evidence. J Pain Symptom Manage. 2022 Jun 12;S0885-3924(22)00760-6.
9. Byock I. Psychedelics for serious illness: Five things clinicians need to know. The Center to Advance Palliative Care. Psychedelics for Serious Illness, Palliative in Practice, Center to Advance Palliative Care (capc.org). June 13, 2022.
10. Marks M. A strategy for rescheduling psilocybin. Scientific American. Oct. 11, 2021.
The World Health Organization defines palliative care as “an approach that improves the quality of life of patients (adults and children) and their families who are facing problems associated with life-threatening illness. It prevents and relieves suffering through the early identification, correct assessment, and treatment of pain and other problems, whether physical, psychosocial or spiritual.”1
The common misperception is that palliative care is only for those at end of life or only in the advanced stages of their illness. However, palliative care is ideally most helpful following individuals from diagnosis through their illness trajectory. Another misperception is that palliative care and hospice are the same thing. Though all hospice is palliative care, all palliative care is not hospice. Both palliative care and hospice provide care for individuals facing a serious illness and focus on the same philosophy of care, but palliative care can be initiated at any stage of illness, even if the goal is to pursue curative and life-prolonging therapies/interventions.
In contrast, hospice is considered for those who are at the end of life and are usually not pursuing life-prolonging therapies or interventions, instead focusing on comfort, symptom management, and optimization of quality of life.
Though there is a growing need for palliative care, there is a shortage of specialist palliative care providers. Much of the palliative care needs can be met by all providers who can offer basic symptom management, identification surrounding goals of care and discussions of advance care planning, and understanding of illness/prognosis and treatment options, which is called primary palliative care.2 In fact, two-thirds of patients with a serious illness other than cancer prefer discussion of end-of-life care or advance care planning with their primary care providers.3
Referral to specialty palliative care should be considered when there are more complexities to symptom/pain management and goals of care/end of life, transition to hospice, or complex communication dynamics.4
Though specialty palliative care was shown to be more comprehensive, both primary palliative care and specialty palliative care have led to improvements in the quality of life in individuals living with serious illness.5 Early integration of palliative care into routine care has been shown to improve symptom burden, mood, quality of life, survival, and health care costs.6
Updates in alternative and complementary therapies to palliative care
There are several alternative and complementary therapies to palliative care, including cannabis and psychedelics. These therapies are becoming or may become a familiar part of medical therapies that are listed in a patient’s history as part of their medical regimen, especially as more states continue to legalize and/or decriminalize the use of these alternative therapies for recreational or medicinal use.
Both cannabis and psychedelics have a longstanding history of therapeutic and holistic use. Cannabis has been used to manage symptoms such as pain since the 16th and 17th century.7 In palliative care, more patients may turn to various forms of cannabis as a source of relief from symptoms and suffering as their focus shifts more to quality of life.
Even with the increasing popularity of the use of cannabis among seriously ill patients, there is still a lack of evidence of the benefits of medical cannabis use in palliative care, and there is a lack of standardization of type of cannabis used and state regulations regarding their use.7
A recent systematic review found that despite the reported positive treatment effects of cannabis in palliative care, the results of the studies were conflicting. This highlights the need for further high-quality research to determine whether cannabis products are an effective treatment in palliative care patients.8
One limitation to note is that the majority of the included studies focused on cannabis use in patients with cancer for cancer-related symptoms. Few studies included patients with other serious conditions.
Psychedelics
There is evidence that psychedelic assisted therapy (PAT) is a safe and effective treatment for individuals with refractory depression, posttraumatic stress disorder, and substance use disorder.9 Plus, there have been ample studies providing support that PAT improves symptoms such as refractory anxiety/depression, demoralization, and existential distress in seriously ill patients, thus improving their quality of life and overall well-being.9
Nine U.S. cities and the State of Oregon have decriminalized or legalized the psychedelic psilocybin, based on the medical benefits patients have experienced evidenced from using it.10
In light of the increasing interest in PAT, Dr. Ira Byock provided the following points on what “all clinicians should know as they enter this uncharted territory”:
- Psychedelics have been around for a long time.
- Psychedelic-assisted therapies’ therapeutic effects are experiential.
- There are a variety of terms for specific categories of psychedelic compounds.
- Some palliative care teams are already caring for patients who undergo psychedelic experiences.
- Use of psychedelics should be well-observed by a skilled clinician with expertise.
I am hoping this provides a general refresher on palliative care and an overview of updates to alternative and complementary therapies for patients living with serious illness.9
Dr. Kang is a geriatrician and palliative care provider at the University of Washington, Seattle in the division of geriatrics and gerontology. She has no conflicts related to the content of this piece.
References
1. World Health Organization. Palliative care. 2020 Aug 5..
2. Weissman DE and Meier DE. Identifying patients in need of a palliative care assessment in the hospital setting a consensus report from the center to advance palliative care. J Palliat Med. 2011;14(1):17-23.
3. Sherry D et al. Is primary care physician involvement associated with earlier advance care planning? A study of patients in an academic primary care setting. J Palliat Med. 2022;25(1):75-80.
4. Quill TE and Abernethy AP. Generalist plus specialist palliative care-creating a more sustainable model. N Engl J Med. 2013;368:1173-75.
5. Ernecoff NC et al. Comparing specialty and primary palliative care interventions: Analysis of a systematic review. J Palliat Med. 2020;23(3):389-96.
6. Temmel JS et al. Early palliative care for patients with metastatic non–small-cell lung cancer. N Engl J Med. 2011;363:733-42.
7. Kogan M and Sexton M. Medical cannabis: A new old tool for palliative care. J Altern Complement Med . 2020 Sep;26(9):776-8.
8. Doppen M et al. Cannabis in palliative care: A systematic review of the current evidence. J Pain Symptom Manage. 2022 Jun 12;S0885-3924(22)00760-6.
9. Byock I. Psychedelics for serious illness: Five things clinicians need to know. The Center to Advance Palliative Care. Psychedelics for Serious Illness, Palliative in Practice, Center to Advance Palliative Care (capc.org). June 13, 2022.
10. Marks M. A strategy for rescheduling psilocybin. Scientific American. Oct. 11, 2021.
The World Health Organization defines palliative care as “an approach that improves the quality of life of patients (adults and children) and their families who are facing problems associated with life-threatening illness. It prevents and relieves suffering through the early identification, correct assessment, and treatment of pain and other problems, whether physical, psychosocial or spiritual.”1
The common misperception is that palliative care is only for those at end of life or only in the advanced stages of their illness. However, palliative care is ideally most helpful following individuals from diagnosis through their illness trajectory. Another misperception is that palliative care and hospice are the same thing. Though all hospice is palliative care, all palliative care is not hospice. Both palliative care and hospice provide care for individuals facing a serious illness and focus on the same philosophy of care, but palliative care can be initiated at any stage of illness, even if the goal is to pursue curative and life-prolonging therapies/interventions.
In contrast, hospice is considered for those who are at the end of life and are usually not pursuing life-prolonging therapies or interventions, instead focusing on comfort, symptom management, and optimization of quality of life.
Though there is a growing need for palliative care, there is a shortage of specialist palliative care providers. Much of the palliative care needs can be met by all providers who can offer basic symptom management, identification surrounding goals of care and discussions of advance care planning, and understanding of illness/prognosis and treatment options, which is called primary palliative care.2 In fact, two-thirds of patients with a serious illness other than cancer prefer discussion of end-of-life care or advance care planning with their primary care providers.3
Referral to specialty palliative care should be considered when there are more complexities to symptom/pain management and goals of care/end of life, transition to hospice, or complex communication dynamics.4
Though specialty palliative care was shown to be more comprehensive, both primary palliative care and specialty palliative care have led to improvements in the quality of life in individuals living with serious illness.5 Early integration of palliative care into routine care has been shown to improve symptom burden, mood, quality of life, survival, and health care costs.6
Updates in alternative and complementary therapies to palliative care
There are several alternative and complementary therapies to palliative care, including cannabis and psychedelics. These therapies are becoming or may become a familiar part of medical therapies that are listed in a patient’s history as part of their medical regimen, especially as more states continue to legalize and/or decriminalize the use of these alternative therapies for recreational or medicinal use.
Both cannabis and psychedelics have a longstanding history of therapeutic and holistic use. Cannabis has been used to manage symptoms such as pain since the 16th and 17th century.7 In palliative care, more patients may turn to various forms of cannabis as a source of relief from symptoms and suffering as their focus shifts more to quality of life.
Even with the increasing popularity of the use of cannabis among seriously ill patients, there is still a lack of evidence of the benefits of medical cannabis use in palliative care, and there is a lack of standardization of type of cannabis used and state regulations regarding their use.7
A recent systematic review found that despite the reported positive treatment effects of cannabis in palliative care, the results of the studies were conflicting. This highlights the need for further high-quality research to determine whether cannabis products are an effective treatment in palliative care patients.8
One limitation to note is that the majority of the included studies focused on cannabis use in patients with cancer for cancer-related symptoms. Few studies included patients with other serious conditions.
Psychedelics
There is evidence that psychedelic assisted therapy (PAT) is a safe and effective treatment for individuals with refractory depression, posttraumatic stress disorder, and substance use disorder.9 Plus, there have been ample studies providing support that PAT improves symptoms such as refractory anxiety/depression, demoralization, and existential distress in seriously ill patients, thus improving their quality of life and overall well-being.9
Nine U.S. cities and the State of Oregon have decriminalized or legalized the psychedelic psilocybin, based on the medical benefits patients have experienced evidenced from using it.10
In light of the increasing interest in PAT, Dr. Ira Byock provided the following points on what “all clinicians should know as they enter this uncharted territory”:
- Psychedelics have been around for a long time.
- Psychedelic-assisted therapies’ therapeutic effects are experiential.
- There are a variety of terms for specific categories of psychedelic compounds.
- Some palliative care teams are already caring for patients who undergo psychedelic experiences.
- Use of psychedelics should be well-observed by a skilled clinician with expertise.
I am hoping this provides a general refresher on palliative care and an overview of updates to alternative and complementary therapies for patients living with serious illness.9
Dr. Kang is a geriatrician and palliative care provider at the University of Washington, Seattle in the division of geriatrics and gerontology. She has no conflicts related to the content of this piece.
References
1. World Health Organization. Palliative care. 2020 Aug 5..
2. Weissman DE and Meier DE. Identifying patients in need of a palliative care assessment in the hospital setting a consensus report from the center to advance palliative care. J Palliat Med. 2011;14(1):17-23.
3. Sherry D et al. Is primary care physician involvement associated with earlier advance care planning? A study of patients in an academic primary care setting. J Palliat Med. 2022;25(1):75-80.
4. Quill TE and Abernethy AP. Generalist plus specialist palliative care-creating a more sustainable model. N Engl J Med. 2013;368:1173-75.
5. Ernecoff NC et al. Comparing specialty and primary palliative care interventions: Analysis of a systematic review. J Palliat Med. 2020;23(3):389-96.
6. Temmel JS et al. Early palliative care for patients with metastatic non–small-cell lung cancer. N Engl J Med. 2011;363:733-42.
7. Kogan M and Sexton M. Medical cannabis: A new old tool for palliative care. J Altern Complement Med . 2020 Sep;26(9):776-8.
8. Doppen M et al. Cannabis in palliative care: A systematic review of the current evidence. J Pain Symptom Manage. 2022 Jun 12;S0885-3924(22)00760-6.
9. Byock I. Psychedelics for serious illness: Five things clinicians need to know. The Center to Advance Palliative Care. Psychedelics for Serious Illness, Palliative in Practice, Center to Advance Palliative Care (capc.org). June 13, 2022.
10. Marks M. A strategy for rescheduling psilocybin. Scientific American. Oct. 11, 2021.
CBT may improve comorbid posttraumatic headache, PTSD
Results from a randomized clinical trial of almost 200 military veterans showed that, compared with usual care, CBT for headache led to significant improvement in both headache disability and PTSD symptoms. Cognitive-processing therapy (CPT) also led to significant improvement in PTSD symptoms, but it did not improve headache disability.
Lead author Donald McGeary, PhD, department of rehabilitation medicine, the University of Texas Health Science Center,San Antonio, noted the improvements shown in headache disability after CBT were likely caused by its building of patients’ confidence that they could control or manage their headaches themselves.
That sense of control was key to helping patients “get their lives back. If you can improve a person’s belief that they can control their headache, they function better,” Dr. McGeary said in a news release.
The findings were published online in JAMA Neurology.
Signature wounds
Both mild traumatic brain injury (TBI) and PTSD are signature wounds of post-9/11 military conflicts. The two conditions commonly occur together and can harm quality of life and functioning, the investigators noted. Following mild TBI, many veterans experience persistent posttraumatic headache, which often co-occurs with PTSD.
To gauge the impact of CBTs for this patient population, researchers recruited 193 post-9/11 combat veterans (mean age, 39.7 years) with clinically significant PTSD symptoms and posttraumatic headache that had persisted more than 3 months after TBI. Of these, 167 were men.
All participants were receiving care at the Polytrauma Rehabilitation Center of the South Texas Veterans Health Care System in Houston.
They were randomly allocated to undergo 8 sessions of manualized CBT for headache, 12 sessions of manualized CPT for PTSD, or usual headache treatment.
CBT for headache uses CBT concepts to reduce headache disability and improve mood – and includes key components, such as relaxation, setting goals for activities patients want to resume, and planning for those situations.
CPT is a leading psychotherapy for PTSD. It teaches patients how to evaluate and change upsetting and maladaptive thoughts related to their trauma. The idea is that, by changing thoughts, patients can change the way they feel.
Treatment as usual was consistent with multidisciplinary treatment in a large Veterans Affairs multiple-trauma center and could include pharmacotherapies, physical and occupational therapies, pain medications, acupuncture, and massage.
The coprimary outcomes were headache-related disability on the six-item Headache Impact Test (HIT-6) and PTSD symptom severity on the PTSD Checklist for Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (PCL-5), assessed from end of treatment to 6 months post treatment.
At baseline, all participants reported severe headache-related disability (mean HIT-6 score, 65.8 points) and severe PTSD symptoms (mean PCL-5 score, 48.4 points).
Significant improvement
Compared with usual care, CBT for headache led to significant improvement in headache disability (posttreatment mean change in HIT-6 score, –3.4 points; P < .01) and PTSD symptoms (posttreatment change in PCL-5, –6.5 points; P = .04).
CPT also led to significant improvement in PTSD symptoms (8.9 points lower on the PCL-5 after treatment; P = .01), but it had only a modest effect on headache disability (1.4 points lower after treatment; P = .21).
“This was a surprise,” Dr. McGeary said. “If theories about PTSD driving posttraumatic headache are correct, you’d expect CPT to help both PTSD and headache. Our findings call that into question.”
Despite improvements in headache disability, CBT for headache did not significantly reduce headache frequency or intensity.
The researchers are now hoping to replicate their findings in a larger trial at multiple military and VA sites around the United States.
“We need more women, more racial and ethnic diversity, veterans as well as active military of different branches with varying comorbidities in different geographic regions attached to different hospitals and medical systems, because we’re comparing to usual care,” Dr. McGeary said.
A step forward
Commenting on the study, retired Col. Elspeth Cameron Ritchie, MD, chair of psychiatry, MedStar Washington Hospital Center, Washington, said she was “pleased” to see that this study was conducted and that she was pleased with the results.
“It’s been 20 years since 9/11, and wars are pretty much forgotten, but people are still suffering from the effects of traumatic brain injury and posttraumatic stress disorder. These are not conditions that go away quickly or lightly. They do take work,” said Dr. Ritchie, who was not involved with the research.
Finding therapies besides medication that are helpful is “good and is a step forward. The more alternatives we have, the better,” she concluded.
The study was supported in part by the Department of Defense and the Department of Veterans Affairs. Dr. McGeary and Dr. Ritchie have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Results from a randomized clinical trial of almost 200 military veterans showed that, compared with usual care, CBT for headache led to significant improvement in both headache disability and PTSD symptoms. Cognitive-processing therapy (CPT) also led to significant improvement in PTSD symptoms, but it did not improve headache disability.
Lead author Donald McGeary, PhD, department of rehabilitation medicine, the University of Texas Health Science Center,San Antonio, noted the improvements shown in headache disability after CBT were likely caused by its building of patients’ confidence that they could control or manage their headaches themselves.
That sense of control was key to helping patients “get their lives back. If you can improve a person’s belief that they can control their headache, they function better,” Dr. McGeary said in a news release.
The findings were published online in JAMA Neurology.
Signature wounds
Both mild traumatic brain injury (TBI) and PTSD are signature wounds of post-9/11 military conflicts. The two conditions commonly occur together and can harm quality of life and functioning, the investigators noted. Following mild TBI, many veterans experience persistent posttraumatic headache, which often co-occurs with PTSD.
To gauge the impact of CBTs for this patient population, researchers recruited 193 post-9/11 combat veterans (mean age, 39.7 years) with clinically significant PTSD symptoms and posttraumatic headache that had persisted more than 3 months after TBI. Of these, 167 were men.
All participants were receiving care at the Polytrauma Rehabilitation Center of the South Texas Veterans Health Care System in Houston.
They were randomly allocated to undergo 8 sessions of manualized CBT for headache, 12 sessions of manualized CPT for PTSD, or usual headache treatment.
CBT for headache uses CBT concepts to reduce headache disability and improve mood – and includes key components, such as relaxation, setting goals for activities patients want to resume, and planning for those situations.
CPT is a leading psychotherapy for PTSD. It teaches patients how to evaluate and change upsetting and maladaptive thoughts related to their trauma. The idea is that, by changing thoughts, patients can change the way they feel.
Treatment as usual was consistent with multidisciplinary treatment in a large Veterans Affairs multiple-trauma center and could include pharmacotherapies, physical and occupational therapies, pain medications, acupuncture, and massage.
The coprimary outcomes were headache-related disability on the six-item Headache Impact Test (HIT-6) and PTSD symptom severity on the PTSD Checklist for Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (PCL-5), assessed from end of treatment to 6 months post treatment.
At baseline, all participants reported severe headache-related disability (mean HIT-6 score, 65.8 points) and severe PTSD symptoms (mean PCL-5 score, 48.4 points).
Significant improvement
Compared with usual care, CBT for headache led to significant improvement in headache disability (posttreatment mean change in HIT-6 score, –3.4 points; P < .01) and PTSD symptoms (posttreatment change in PCL-5, –6.5 points; P = .04).
CPT also led to significant improvement in PTSD symptoms (8.9 points lower on the PCL-5 after treatment; P = .01), but it had only a modest effect on headache disability (1.4 points lower after treatment; P = .21).
“This was a surprise,” Dr. McGeary said. “If theories about PTSD driving posttraumatic headache are correct, you’d expect CPT to help both PTSD and headache. Our findings call that into question.”
Despite improvements in headache disability, CBT for headache did not significantly reduce headache frequency or intensity.
The researchers are now hoping to replicate their findings in a larger trial at multiple military and VA sites around the United States.
“We need more women, more racial and ethnic diversity, veterans as well as active military of different branches with varying comorbidities in different geographic regions attached to different hospitals and medical systems, because we’re comparing to usual care,” Dr. McGeary said.
A step forward
Commenting on the study, retired Col. Elspeth Cameron Ritchie, MD, chair of psychiatry, MedStar Washington Hospital Center, Washington, said she was “pleased” to see that this study was conducted and that she was pleased with the results.
“It’s been 20 years since 9/11, and wars are pretty much forgotten, but people are still suffering from the effects of traumatic brain injury and posttraumatic stress disorder. These are not conditions that go away quickly or lightly. They do take work,” said Dr. Ritchie, who was not involved with the research.
Finding therapies besides medication that are helpful is “good and is a step forward. The more alternatives we have, the better,” she concluded.
The study was supported in part by the Department of Defense and the Department of Veterans Affairs. Dr. McGeary and Dr. Ritchie have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Results from a randomized clinical trial of almost 200 military veterans showed that, compared with usual care, CBT for headache led to significant improvement in both headache disability and PTSD symptoms. Cognitive-processing therapy (CPT) also led to significant improvement in PTSD symptoms, but it did not improve headache disability.
Lead author Donald McGeary, PhD, department of rehabilitation medicine, the University of Texas Health Science Center,San Antonio, noted the improvements shown in headache disability after CBT were likely caused by its building of patients’ confidence that they could control or manage their headaches themselves.
That sense of control was key to helping patients “get their lives back. If you can improve a person’s belief that they can control their headache, they function better,” Dr. McGeary said in a news release.
The findings were published online in JAMA Neurology.
Signature wounds
Both mild traumatic brain injury (TBI) and PTSD are signature wounds of post-9/11 military conflicts. The two conditions commonly occur together and can harm quality of life and functioning, the investigators noted. Following mild TBI, many veterans experience persistent posttraumatic headache, which often co-occurs with PTSD.
To gauge the impact of CBTs for this patient population, researchers recruited 193 post-9/11 combat veterans (mean age, 39.7 years) with clinically significant PTSD symptoms and posttraumatic headache that had persisted more than 3 months after TBI. Of these, 167 were men.
All participants were receiving care at the Polytrauma Rehabilitation Center of the South Texas Veterans Health Care System in Houston.
They were randomly allocated to undergo 8 sessions of manualized CBT for headache, 12 sessions of manualized CPT for PTSD, or usual headache treatment.
CBT for headache uses CBT concepts to reduce headache disability and improve mood – and includes key components, such as relaxation, setting goals for activities patients want to resume, and planning for those situations.
CPT is a leading psychotherapy for PTSD. It teaches patients how to evaluate and change upsetting and maladaptive thoughts related to their trauma. The idea is that, by changing thoughts, patients can change the way they feel.
Treatment as usual was consistent with multidisciplinary treatment in a large Veterans Affairs multiple-trauma center and could include pharmacotherapies, physical and occupational therapies, pain medications, acupuncture, and massage.
The coprimary outcomes were headache-related disability on the six-item Headache Impact Test (HIT-6) and PTSD symptom severity on the PTSD Checklist for Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (PCL-5), assessed from end of treatment to 6 months post treatment.
At baseline, all participants reported severe headache-related disability (mean HIT-6 score, 65.8 points) and severe PTSD symptoms (mean PCL-5 score, 48.4 points).
Significant improvement
Compared with usual care, CBT for headache led to significant improvement in headache disability (posttreatment mean change in HIT-6 score, –3.4 points; P < .01) and PTSD symptoms (posttreatment change in PCL-5, –6.5 points; P = .04).
CPT also led to significant improvement in PTSD symptoms (8.9 points lower on the PCL-5 after treatment; P = .01), but it had only a modest effect on headache disability (1.4 points lower after treatment; P = .21).
“This was a surprise,” Dr. McGeary said. “If theories about PTSD driving posttraumatic headache are correct, you’d expect CPT to help both PTSD and headache. Our findings call that into question.”
Despite improvements in headache disability, CBT for headache did not significantly reduce headache frequency or intensity.
The researchers are now hoping to replicate their findings in a larger trial at multiple military and VA sites around the United States.
“We need more women, more racial and ethnic diversity, veterans as well as active military of different branches with varying comorbidities in different geographic regions attached to different hospitals and medical systems, because we’re comparing to usual care,” Dr. McGeary said.
A step forward
Commenting on the study, retired Col. Elspeth Cameron Ritchie, MD, chair of psychiatry, MedStar Washington Hospital Center, Washington, said she was “pleased” to see that this study was conducted and that she was pleased with the results.
“It’s been 20 years since 9/11, and wars are pretty much forgotten, but people are still suffering from the effects of traumatic brain injury and posttraumatic stress disorder. These are not conditions that go away quickly or lightly. They do take work,” said Dr. Ritchie, who was not involved with the research.
Finding therapies besides medication that are helpful is “good and is a step forward. The more alternatives we have, the better,” she concluded.
The study was supported in part by the Department of Defense and the Department of Veterans Affairs. Dr. McGeary and Dr. Ritchie have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NEUROLOGY
A Veteran With Recurrent, Painful Knee Effusion
Case Presentation: A 39-year-old Air Force veteran was admitted to the US Department of Veterans Affairs Boston Healthcare System (VABHS) for evaluation of recurrent, painful right knee effusions. On presentation, his vital signs were stable, and the examination was significant for a right knee with a large effusion and tenderness to palpation without erythema or warmth. His white blood cell count was 12.0 cells/L with an erythrocyte sedimentation rate of 23 mm/h and C-reactive protein of 11.87 mg/L. He was in remission from alcohol use but had relapsed on alcohol in the past day to treat the pain. He had a history of IV drug use but was in remission. He was previously active and enjoyed long hikes. Nine months prior to presentation, he developed his first large right knee effusion associated with pain. He reported no antecedent trauma. At that time, he presented to another hospital and underwent arthrocentesis with orthopedic surgery, but this did not lead to a diagnosis, and the effusion reaccumulated within 24 hours. Four months later, he received a corticosteroid injection that provided only minor, temporary relief. He received 5 additional arthrocenteses over 9 months, all without definitive diagnosis and with rapid reaccumulation of the fluid. His most recent arthrocentesis was 3 weeks before admission.
►Lauren E. Merz, MD, MSc, Chief Medical Resident, VABHS: Dr. Jindal, what is your approach and differential diagnosis for joint effusions in hospitalized patients?
►Shivani Jindal, MD, MPH, Hospitalist, VABHS, Instructor in Medicine, Boston University School of Medicine (BUSM): A thorough history and physical examination are important. I specifically ask about chronicity, pain, and trauma. A medical history of potential infectious exposures and the history of the present illness are also important, such as the risk of sexually transmitted infections, exposure to Lyme disease or other viral illnesses. Gonococcal arthritis is one of the most common causes of nontraumatic monoarthritis in young adults but can also present as a migratory polyarthritis.1
It sounds like he was quite active and liked to hike so a history of tick exposure is important to ascertain. I would also ask about eye inflammation and back pain to assess possible ankylosing spondyarthritis. Other inflammatory etiologies, such as gout are common, but it would be surprising to miss this diagnosis on repeated arthocenteses. A physical examination can confirm monoarthritis over polyarthritis and assess for signs of inflammatory arthritis (eg, warmth and erythema). The most important etiology to assess for and rule out in a person admitted to the hospital is septic arthritis. The severe pain, mild leukocytosis, and mildly elevated inflammatory markers could be consistent with this diagnosis but are nonspecific. However, the chronicity of this patient’s presentation and hemodynamic stability make septic arthritis less likely overall and a more indolent infection or other inflammatory process more likely.
►Dr. Merz: The patient’s medical history included posttraumatic stress disorder (PTSD) and antisocial personality disorder with multiple prior suicide attempts. He also had a history of opioid use disorder (OUD) with prior overdose and alcohol use disorder (AUD). Given his stated preference to avoid opioids and normal liver function and liver chemistry testing, the initial treatment was with acetaminophen. After this failed to provide satisfactory pain control, IV hydromorphone was added.
Dr. Jindal, how do you approach pain control in the hospital for musculoskeletal issues like this?
►Dr. Jindal: Typically, nonsteroidal anti-inflammatory medications (NSAIDs) are most effective for musculoskeletal pain, often in the form of ketorolac or ibuprofen. However, we are often limited in our NSAID use by kidney disease, gastritis, or cardiovascular disease.
►Dr. Merz: On hospital day 1, the patient asked to leave to consume alcohol to ease unremitting pain. He also expressed suicidal ideation and discharge was therefore felt to be unsafe. He was reluctant to engage with psychiatry and became physically combative while attempting to leave the hospital, necessitating the use of sedating medications and physical restraints.
Dr. Shahal, what factors led to the decision to place an involuntary hold, and how do you balance patient autonomy and patient safety?
►Dr. Talya Shahal, MD, Consult-Liaison Psychiatry Service, VABHS, Instructor in Psychiatry, Harvard Medical School: This is a delicate balance that requires constant reassessment. The patient initially presented to the emergency department with suicidal ideation, stating he was not able to tolerate the pain and thus resumed alcohol consumption after a period of nonuse. He had multiple risk factors for suicide, including 9 prior suicide attempts with the latest less than a year before presentation, active substance use with alcohol and other recreational drugs, PTSD, pain, veteran status, male sex, single status, and a history of trauma.3,4 He was also displaying impulsivity and limited insight, did not engage in his psychiatric assessment, and attempted to assault staff. As such, his suicide risk was assessed to be high at the time of the evaluation, which led to the decision to place an involuntary hold. However, we reevaluate this decision at least daily in order to reassess the risk and ensure that the balance between patient safety and autonomy are maintained.
►Dr. Merz: The involuntary hold was removed within 48 hours as the patient remained calm and engaged with the primary and consulting teams. He requested escalating doses of opioids as he felt the short-acting IV medications were not providing sustained relief. However, he was also noted to be walking outside of the hospital without assistance, and he repeatedly declined nonopioid pain modalities as well as buprenorphine/naloxone. The chronic pain service was consulted but was unable to see the patient as he was frequently outside of his room.
Dr. Shahal, how do you address OUD, pain, and stigma in the hospital?
►Dr. Shahal: It is important to remember that patients with substance use disorder (SUD) and mental illness frequently have physical causes for pain and are often undertreated.5 Patients with SUD may also have higher tolerance for opioids and may need higher doses to treat the pain.5 Modalities like buprenorphine/naloxone can be effective to treat OUD and pain, but these usually cannot be initiated while the patient is on short-acting opioids as this would precipitate withdrawal.6 However, withdrawal can be managed while inpatient, and this can be a good time to start these medications as practitioners can aggressively help with symptom control. Proactively addressing mental health concerns, particularly anxiety, AUD, insomnia, PTSD, and depression, can also have a direct impact on the perception of pain and assist with better control.2 In addition, nonpharmacologic options, such as meditation, deep breathing, and even acupuncture and Reiki can be helpful and of course less harmful to treat pain.2
► Dr. Merz: An X-ray of the knee showed no acute fracture or joint space narrowing. Magnetic resonance imaging confirmed a large knee effusion with no evidence of ligament injury. Synovial fluid showed turbid, yellow fluid with 14,110 nucleated cells (84% segmented cells and 4000 RBCs). Gram stain was negative, culture had no growth, and there were no crystals. Anticyclic citrullinated peptide (anti-CCP), rheumatoid factor, HIV testing, and HLA-B27 were negative.
Dr. Serrao, what do these studies tell us about the joint effusion and the possible diagnoses?
► Dr. Richard Serrao, MD, Infectious Disease, VABHS, Clinical Associate Professor in Medicine, BUSM: I would expect the white blood cell (WBC) count to be > 50,000 cells with > 75% polymorphonuclear cells and a positive Gram stain if this was a bacterial infection resulting in septic arthritis.7 This patient’s studies are not consistent with this diagnosis nor is the chronicity of his presentation. There are 2 important bacteria that can present with inflammatory arthritis and less pronounced findings on arthrocentesis: Borrelia burgdorferi (the bacteria causing Lyme arthritis) and Neisseria gonorrhea. Lyme arthritis could be consistent with this relapsing remitting presentation as you expect a WBC count between 3000 and 100,000 cells with a mean value between 10,000 and 25,000 cells, > 50% polymorphonuclear leukocytes, and negative Gram stains.8 Gonococcal infections often do not have marked elevations in the WBC count and the Gram stain can be variable, but you still expect the WBC count to be > 30,000 cells.7 Inflammatory causes such as gout or autoimmune conditions such as lupus often have a WBC count between 2000 and 100,000 with a negative Gram stain, which could be consistent with this patient’s presentation.7 However, the lack of crystals rules out gout and the negative anti-CCP, rheumatoid factor, and HLA-B27 make rheumatologic diseases less likely.
►Dr. Merz: The patient received a phone call from another hospital where an arthrocentesis had been performed 3 weeks before. The results included a positive polymerase chain reaction (PCR) test for Lyme disease in the synovial fluid. A subsequent serum Lyme screen was positive for 1 of 3 immunoglobulin (Ig) M bands and 10 of 10 IgG bands.
Dr. Serrao, how does Lyme arthritis typically present, and are there aspects of this case that make you suspect the diagnosis? Does the serum Lyme test give us any additional information?
►Dr. Serrao: Lyme arthritis is a late manifestation of Lyme disease. Patients typically have persistent or intermittent arthritis, and large joints are more commonly impacted than small joints. Monoarthritis of the knee is the most common, but oligoarthritis is possible as well. The swelling usually begins abruptly, lasts for weeks to months, and effusions typically recur quickly after aspiration. These findings are consistent with the patient’s clinical history.
For diagnostics, the IgG Western blot is positive if 5 of the 10 bands are positive.9 This patient far exceeds the IgG band number to diagnose Lyme disease. All patients with Lyme arthritis will have positive IgG serologies since Lyme arthritis is a late manifestation of the infection. IgM reactivity may be present, but are not necessary to diagnose Lyme arthritis.10 Synovial fluid is often not analyzed for antibody responses as they are susceptible to false positive results, but synovial PCR testing like this patient had detects approximately 70% of patients with untreated Lyme arthritis.11 However, PCR positivity does not necessarily equate with active infection. Serologic testing for Lyme disease by enzyme-linked immunosorbent assay and Western blot as well as careful history and the exclusion of other diagnoses are usually sufficient to make the diagnosis.
► Dr. Merz: On further history the patient reported that 5 years prior he found a tick on his skin with a bull’s-eye rash. He was treated with 28 days of doxycycline at that time. He did not recall any tick bites or rashes in the years since.
Dr. Serrao, is it surprising that he developed Lyme arthritis 5 years after exposure and after being treated appropriately? What is the typical treatment approach for a patient like this?
►Dr. Serrao: It is atypical to develop Lyme arthritis 5 years after reported treatment of what appeared to be early localized disease, namely, erythema migrans. This stage is usually cured with 10 days of treatment alone (he received 28 days) and is generally abortive of subsequent stages, including Lyme arthritis. Furthermore, the patient reported no symptoms of arthritis until recently since that time. Therefore, one can argue that the excessively long span of time from treatment to these first episodes of arthritis suggests the patient could have been reinfected. When available, comparing the types and number of Western blot bands (eg, new and/or more bands on subsequent serologic testing) can support a reinfection diagnosis. A delayed postinfectious inflammatory process from excessive proinflammatory immune responses that block wound repair resulting in proliferative synovitis is also possible.12 This is defined as the postinfectious, postantibiotic, or antibiotic-refractory Lyme arthritis, a diagnosis of exclusion more apparent only after patients receive appropriate antibiotic courses for the possibility of untreated Lyme as an active infection.12
Given the inherent diagnostic uncertainty between an active infection and posttreatment Lyme arthritis syndromes, it is best to approach most cases of Lyme arthritis as an active infection first especially if not yet treated with antibiotics. Diagnosis of postinflammatory processes should be considered if symptoms persist after appropriate antibiotics, and then short-term use of disease-modifying antirheumatic drugs, rather than further courses of antibiotics, is recommended.
► Dr. Merz: The patient was initiated on doxycycline with the plan to transition to ceftriaxone if there was no response. One day after diagnosis and treatment initiation and in the setting of continued pain, the patient again asked to leave the hospital to drink alcohol. After eloping and becoming intoxicated with alcohol, he returned to his room. He remained concerned about his continued pain and lack of adequate pain control. At the time, he was receiving hydromorphone, ketorolac, lorazepam, gabapentin, and quetiapine.
Dr. Serrao, do you expect this degree of pain from Lyme arthritis?
► Dr. Serrao: Lyme arthritis is typically less painful than other forms of infectious or inflammatory arthritis. Pain is usually caused by the pressure from the acute accumulation and reaccumulation of fluid. In this case, the rapid accumulation of fluid that this patient experienced as well as relief with arthrocentesis suggests that the size and acuity of the effusion was causing great discomfort. Repeated arthrocentesis can prove to be a preventative strategy to minimize synovial herniation.
►Dr. Merz: Dr. Shahal, how do you balance the patient subjectively telling you that they are in pain with objective signs that they may be tolerating the pain like walking around unassisted? Is there anything else that could have been done to prevent this adverse outcome?
►Dr. Shahal: This is one of the hardest pieces of pain management. We want to practice beneficence by believing our patients and addressing their discomfort, but we also want to practice nonmaleficence by avoiding inappropriate long-term pain treatments like opioids that have significant harm as well as avoiding exacerbating this patient’s underlying SUD. An agent like buprenorphine/naloxone could have been an excellent fit to treat pain and SUD, but the patient’s lack of interest and the frequent use of short-acting opioids were major barriers. A chronic pain consult early on is helpful in cases like this as well, but they were unable to see him since he was often out of his room. Repeated arthrocentesis may also have helped the pain. Treatment of anxiety and insomnia with medications like hydroxyzine, trazodone, melatonin, gabapentin, or buspirone as well as interventions like sleep hygiene protocols or spiritual care may have helped somewhat as well.
We know that there is a vicious cycle between pain and poorly controlled mood symptoms. Many of our veterans have PTSD, anxiety, and SUD that are exacerbated by hospitalization and pain. Maintaining optimal communication between the patient and the practitioners, using trauma-informed care, understanding the patient’s goals of care, setting expectations and limits, and attempting to address the patient’s needs while attempting to minimize stigma might be helpful. However, despite optimal care, sometimes these events cannot be avoided.
►Dr. Merz: The patient was ultimately transferred to an inpatient psychiatric unit where a taper plan for the short-acting opioids was implemented. He was psychiatrically stabilized and discharged a few days later off opioids and on doxycycline. On follow-up a few weeks later, his pain had markedly improved, and the effusion was significantly reduced in size. His mood and impulsivity had stabilized. He continues to follow-up in the infectious disease clinic.

1. Siva C, Velazquez C, Mody A, Brasington R. Diagnosing acute monoarthritis in adults: a practical approach for the family physician. Am Fam Physician. 2003;68(1):83-90.
2. Qaseem A, McLean RM, O’Gurek D, et al. Nonpharmacologic and pharmacologic management of acute pain from non-low back, musculoskeletal injuries in adults: a clinical guideline from the American College of Physicians and American Academy of Family Physicians. Ann Intern Med. 2020;173(9):739-748. doi:10.7326/M19-3602
3. Silverman MM, Berman AL. Suicide risk assessment and risk formulation part I: a focus on suicide ideation in assessing suicide risk. Suicide Life Threat Behav. 2014;44(4):420-431. doi:10.1111/sltb.12065
4. Berman AL, Silverman MM. Suicide risk assessment and risk formulation part II: Suicide risk formulation and the determination of levels of risk. Suicide Life Threat Behav. 2014;44(4):432-443. doi:10.1111/sltb.12067
5. Quinlan J, Cox F. Acute pain management in patients with drug dependence syndrome. Pain Rep. 2017;2(4):e611. Published 2017 Jul 27. doi:10.1097/PR9.0000000000000611
6. Chou R, Wagner J, Ahmed AY, et al. Treatments for Acute Pain: A Systematic Review. Agency for Healthcare Research and Quality; 2020. https://www.ncbi.nlm.nih.gov/books/NBK566506/
7. Seidman AJ, Limaiem F. Synovial fluid analysis. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022. Updated May 8, 2022. https://www.ncbi.nlm.nih.gov/books/NBK537114
8. Arvikar SL, Steere AC. Diagnosis and treatment of Lyme arthritis. Infect Dis Clin North Am. 2015;29(2):269-280. doi:10.1016/j.idc.2015.02.004
9. Centers for Disease Control and Prevention. Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. JAMA. 1995;274(12):937.
10. Craft JE, Grodzicki RL, Steere AC. Antibody response in Lyme disease: evaluation of diagnostic tests. J Infect Dis. 1984;149(5):789-795. doi:10.1093/infdis/149.5.789
11. Nocton JJ, Dressler F, Rutledge BJ, Rys PN, Persing DH, Steere AC. Detection of Borrelia burgdorferi DNA by polymerase chain reaction in synovial fluid from patients with Lyme arthritis. N Engl J Med. 1994;330(4):229-234. doi:10.1056/NEJM199401273300401
12. Steere AC. Posttreatment Lyme disease syndromes: distinct pathogenesis caused by maladaptive host responses. J Clin Invest. 2020;130(5):2148-2151. doi:10.1172/JCI138062
Case Presentation: A 39-year-old Air Force veteran was admitted to the US Department of Veterans Affairs Boston Healthcare System (VABHS) for evaluation of recurrent, painful right knee effusions. On presentation, his vital signs were stable, and the examination was significant for a right knee with a large effusion and tenderness to palpation without erythema or warmth. His white blood cell count was 12.0 cells/L with an erythrocyte sedimentation rate of 23 mm/h and C-reactive protein of 11.87 mg/L. He was in remission from alcohol use but had relapsed on alcohol in the past day to treat the pain. He had a history of IV drug use but was in remission. He was previously active and enjoyed long hikes. Nine months prior to presentation, he developed his first large right knee effusion associated with pain. He reported no antecedent trauma. At that time, he presented to another hospital and underwent arthrocentesis with orthopedic surgery, but this did not lead to a diagnosis, and the effusion reaccumulated within 24 hours. Four months later, he received a corticosteroid injection that provided only minor, temporary relief. He received 5 additional arthrocenteses over 9 months, all without definitive diagnosis and with rapid reaccumulation of the fluid. His most recent arthrocentesis was 3 weeks before admission.
►Lauren E. Merz, MD, MSc, Chief Medical Resident, VABHS: Dr. Jindal, what is your approach and differential diagnosis for joint effusions in hospitalized patients?
►Shivani Jindal, MD, MPH, Hospitalist, VABHS, Instructor in Medicine, Boston University School of Medicine (BUSM): A thorough history and physical examination are important. I specifically ask about chronicity, pain, and trauma. A medical history of potential infectious exposures and the history of the present illness are also important, such as the risk of sexually transmitted infections, exposure to Lyme disease or other viral illnesses. Gonococcal arthritis is one of the most common causes of nontraumatic monoarthritis in young adults but can also present as a migratory polyarthritis.1
It sounds like he was quite active and liked to hike so a history of tick exposure is important to ascertain. I would also ask about eye inflammation and back pain to assess possible ankylosing spondyarthritis. Other inflammatory etiologies, such as gout are common, but it would be surprising to miss this diagnosis on repeated arthocenteses. A physical examination can confirm monoarthritis over polyarthritis and assess for signs of inflammatory arthritis (eg, warmth and erythema). The most important etiology to assess for and rule out in a person admitted to the hospital is septic arthritis. The severe pain, mild leukocytosis, and mildly elevated inflammatory markers could be consistent with this diagnosis but are nonspecific. However, the chronicity of this patient’s presentation and hemodynamic stability make septic arthritis less likely overall and a more indolent infection or other inflammatory process more likely.
►Dr. Merz: The patient’s medical history included posttraumatic stress disorder (PTSD) and antisocial personality disorder with multiple prior suicide attempts. He also had a history of opioid use disorder (OUD) with prior overdose and alcohol use disorder (AUD). Given his stated preference to avoid opioids and normal liver function and liver chemistry testing, the initial treatment was with acetaminophen. After this failed to provide satisfactory pain control, IV hydromorphone was added.
Dr. Jindal, how do you approach pain control in the hospital for musculoskeletal issues like this?
►Dr. Jindal: Typically, nonsteroidal anti-inflammatory medications (NSAIDs) are most effective for musculoskeletal pain, often in the form of ketorolac or ibuprofen. However, we are often limited in our NSAID use by kidney disease, gastritis, or cardiovascular disease.
►Dr. Merz: On hospital day 1, the patient asked to leave to consume alcohol to ease unremitting pain. He also expressed suicidal ideation and discharge was therefore felt to be unsafe. He was reluctant to engage with psychiatry and became physically combative while attempting to leave the hospital, necessitating the use of sedating medications and physical restraints.
Dr. Shahal, what factors led to the decision to place an involuntary hold, and how do you balance patient autonomy and patient safety?
►Dr. Talya Shahal, MD, Consult-Liaison Psychiatry Service, VABHS, Instructor in Psychiatry, Harvard Medical School: This is a delicate balance that requires constant reassessment. The patient initially presented to the emergency department with suicidal ideation, stating he was not able to tolerate the pain and thus resumed alcohol consumption after a period of nonuse. He had multiple risk factors for suicide, including 9 prior suicide attempts with the latest less than a year before presentation, active substance use with alcohol and other recreational drugs, PTSD, pain, veteran status, male sex, single status, and a history of trauma.3,4 He was also displaying impulsivity and limited insight, did not engage in his psychiatric assessment, and attempted to assault staff. As such, his suicide risk was assessed to be high at the time of the evaluation, which led to the decision to place an involuntary hold. However, we reevaluate this decision at least daily in order to reassess the risk and ensure that the balance between patient safety and autonomy are maintained.
►Dr. Merz: The involuntary hold was removed within 48 hours as the patient remained calm and engaged with the primary and consulting teams. He requested escalating doses of opioids as he felt the short-acting IV medications were not providing sustained relief. However, he was also noted to be walking outside of the hospital without assistance, and he repeatedly declined nonopioid pain modalities as well as buprenorphine/naloxone. The chronic pain service was consulted but was unable to see the patient as he was frequently outside of his room.
Dr. Shahal, how do you address OUD, pain, and stigma in the hospital?
►Dr. Shahal: It is important to remember that patients with substance use disorder (SUD) and mental illness frequently have physical causes for pain and are often undertreated.5 Patients with SUD may also have higher tolerance for opioids and may need higher doses to treat the pain.5 Modalities like buprenorphine/naloxone can be effective to treat OUD and pain, but these usually cannot be initiated while the patient is on short-acting opioids as this would precipitate withdrawal.6 However, withdrawal can be managed while inpatient, and this can be a good time to start these medications as practitioners can aggressively help with symptom control. Proactively addressing mental health concerns, particularly anxiety, AUD, insomnia, PTSD, and depression, can also have a direct impact on the perception of pain and assist with better control.2 In addition, nonpharmacologic options, such as meditation, deep breathing, and even acupuncture and Reiki can be helpful and of course less harmful to treat pain.2
► Dr. Merz: An X-ray of the knee showed no acute fracture or joint space narrowing. Magnetic resonance imaging confirmed a large knee effusion with no evidence of ligament injury. Synovial fluid showed turbid, yellow fluid with 14,110 nucleated cells (84% segmented cells and 4000 RBCs). Gram stain was negative, culture had no growth, and there were no crystals. Anticyclic citrullinated peptide (anti-CCP), rheumatoid factor, HIV testing, and HLA-B27 were negative.
Dr. Serrao, what do these studies tell us about the joint effusion and the possible diagnoses?
► Dr. Richard Serrao, MD, Infectious Disease, VABHS, Clinical Associate Professor in Medicine, BUSM: I would expect the white blood cell (WBC) count to be > 50,000 cells with > 75% polymorphonuclear cells and a positive Gram stain if this was a bacterial infection resulting in septic arthritis.7 This patient’s studies are not consistent with this diagnosis nor is the chronicity of his presentation. There are 2 important bacteria that can present with inflammatory arthritis and less pronounced findings on arthrocentesis: Borrelia burgdorferi (the bacteria causing Lyme arthritis) and Neisseria gonorrhea. Lyme arthritis could be consistent with this relapsing remitting presentation as you expect a WBC count between 3000 and 100,000 cells with a mean value between 10,000 and 25,000 cells, > 50% polymorphonuclear leukocytes, and negative Gram stains.8 Gonococcal infections often do not have marked elevations in the WBC count and the Gram stain can be variable, but you still expect the WBC count to be > 30,000 cells.7 Inflammatory causes such as gout or autoimmune conditions such as lupus often have a WBC count between 2000 and 100,000 with a negative Gram stain, which could be consistent with this patient’s presentation.7 However, the lack of crystals rules out gout and the negative anti-CCP, rheumatoid factor, and HLA-B27 make rheumatologic diseases less likely.
►Dr. Merz: The patient received a phone call from another hospital where an arthrocentesis had been performed 3 weeks before. The results included a positive polymerase chain reaction (PCR) test for Lyme disease in the synovial fluid. A subsequent serum Lyme screen was positive for 1 of 3 immunoglobulin (Ig) M bands and 10 of 10 IgG bands.
Dr. Serrao, how does Lyme arthritis typically present, and are there aspects of this case that make you suspect the diagnosis? Does the serum Lyme test give us any additional information?
►Dr. Serrao: Lyme arthritis is a late manifestation of Lyme disease. Patients typically have persistent or intermittent arthritis, and large joints are more commonly impacted than small joints. Monoarthritis of the knee is the most common, but oligoarthritis is possible as well. The swelling usually begins abruptly, lasts for weeks to months, and effusions typically recur quickly after aspiration. These findings are consistent with the patient’s clinical history.
For diagnostics, the IgG Western blot is positive if 5 of the 10 bands are positive.9 This patient far exceeds the IgG band number to diagnose Lyme disease. All patients with Lyme arthritis will have positive IgG serologies since Lyme arthritis is a late manifestation of the infection. IgM reactivity may be present, but are not necessary to diagnose Lyme arthritis.10 Synovial fluid is often not analyzed for antibody responses as they are susceptible to false positive results, but synovial PCR testing like this patient had detects approximately 70% of patients with untreated Lyme arthritis.11 However, PCR positivity does not necessarily equate with active infection. Serologic testing for Lyme disease by enzyme-linked immunosorbent assay and Western blot as well as careful history and the exclusion of other diagnoses are usually sufficient to make the diagnosis.
► Dr. Merz: On further history the patient reported that 5 years prior he found a tick on his skin with a bull’s-eye rash. He was treated with 28 days of doxycycline at that time. He did not recall any tick bites or rashes in the years since.
Dr. Serrao, is it surprising that he developed Lyme arthritis 5 years after exposure and after being treated appropriately? What is the typical treatment approach for a patient like this?
►Dr. Serrao: It is atypical to develop Lyme arthritis 5 years after reported treatment of what appeared to be early localized disease, namely, erythema migrans. This stage is usually cured with 10 days of treatment alone (he received 28 days) and is generally abortive of subsequent stages, including Lyme arthritis. Furthermore, the patient reported no symptoms of arthritis until recently since that time. Therefore, one can argue that the excessively long span of time from treatment to these first episodes of arthritis suggests the patient could have been reinfected. When available, comparing the types and number of Western blot bands (eg, new and/or more bands on subsequent serologic testing) can support a reinfection diagnosis. A delayed postinfectious inflammatory process from excessive proinflammatory immune responses that block wound repair resulting in proliferative synovitis is also possible.12 This is defined as the postinfectious, postantibiotic, or antibiotic-refractory Lyme arthritis, a diagnosis of exclusion more apparent only after patients receive appropriate antibiotic courses for the possibility of untreated Lyme as an active infection.12
Given the inherent diagnostic uncertainty between an active infection and posttreatment Lyme arthritis syndromes, it is best to approach most cases of Lyme arthritis as an active infection first especially if not yet treated with antibiotics. Diagnosis of postinflammatory processes should be considered if symptoms persist after appropriate antibiotics, and then short-term use of disease-modifying antirheumatic drugs, rather than further courses of antibiotics, is recommended.
► Dr. Merz: The patient was initiated on doxycycline with the plan to transition to ceftriaxone if there was no response. One day after diagnosis and treatment initiation and in the setting of continued pain, the patient again asked to leave the hospital to drink alcohol. After eloping and becoming intoxicated with alcohol, he returned to his room. He remained concerned about his continued pain and lack of adequate pain control. At the time, he was receiving hydromorphone, ketorolac, lorazepam, gabapentin, and quetiapine.
Dr. Serrao, do you expect this degree of pain from Lyme arthritis?
► Dr. Serrao: Lyme arthritis is typically less painful than other forms of infectious or inflammatory arthritis. Pain is usually caused by the pressure from the acute accumulation and reaccumulation of fluid. In this case, the rapid accumulation of fluid that this patient experienced as well as relief with arthrocentesis suggests that the size and acuity of the effusion was causing great discomfort. Repeated arthrocentesis can prove to be a preventative strategy to minimize synovial herniation.
►Dr. Merz: Dr. Shahal, how do you balance the patient subjectively telling you that they are in pain with objective signs that they may be tolerating the pain like walking around unassisted? Is there anything else that could have been done to prevent this adverse outcome?
►Dr. Shahal: This is one of the hardest pieces of pain management. We want to practice beneficence by believing our patients and addressing their discomfort, but we also want to practice nonmaleficence by avoiding inappropriate long-term pain treatments like opioids that have significant harm as well as avoiding exacerbating this patient’s underlying SUD. An agent like buprenorphine/naloxone could have been an excellent fit to treat pain and SUD, but the patient’s lack of interest and the frequent use of short-acting opioids were major barriers. A chronic pain consult early on is helpful in cases like this as well, but they were unable to see him since he was often out of his room. Repeated arthrocentesis may also have helped the pain. Treatment of anxiety and insomnia with medications like hydroxyzine, trazodone, melatonin, gabapentin, or buspirone as well as interventions like sleep hygiene protocols or spiritual care may have helped somewhat as well.
We know that there is a vicious cycle between pain and poorly controlled mood symptoms. Many of our veterans have PTSD, anxiety, and SUD that are exacerbated by hospitalization and pain. Maintaining optimal communication between the patient and the practitioners, using trauma-informed care, understanding the patient’s goals of care, setting expectations and limits, and attempting to address the patient’s needs while attempting to minimize stigma might be helpful. However, despite optimal care, sometimes these events cannot be avoided.
►Dr. Merz: The patient was ultimately transferred to an inpatient psychiatric unit where a taper plan for the short-acting opioids was implemented. He was psychiatrically stabilized and discharged a few days later off opioids and on doxycycline. On follow-up a few weeks later, his pain had markedly improved, and the effusion was significantly reduced in size. His mood and impulsivity had stabilized. He continues to follow-up in the infectious disease clinic.

Case Presentation: A 39-year-old Air Force veteran was admitted to the US Department of Veterans Affairs Boston Healthcare System (VABHS) for evaluation of recurrent, painful right knee effusions. On presentation, his vital signs were stable, and the examination was significant for a right knee with a large effusion and tenderness to palpation without erythema or warmth. His white blood cell count was 12.0 cells/L with an erythrocyte sedimentation rate of 23 mm/h and C-reactive protein of 11.87 mg/L. He was in remission from alcohol use but had relapsed on alcohol in the past day to treat the pain. He had a history of IV drug use but was in remission. He was previously active and enjoyed long hikes. Nine months prior to presentation, he developed his first large right knee effusion associated with pain. He reported no antecedent trauma. At that time, he presented to another hospital and underwent arthrocentesis with orthopedic surgery, but this did not lead to a diagnosis, and the effusion reaccumulated within 24 hours. Four months later, he received a corticosteroid injection that provided only minor, temporary relief. He received 5 additional arthrocenteses over 9 months, all without definitive diagnosis and with rapid reaccumulation of the fluid. His most recent arthrocentesis was 3 weeks before admission.
►Lauren E. Merz, MD, MSc, Chief Medical Resident, VABHS: Dr. Jindal, what is your approach and differential diagnosis for joint effusions in hospitalized patients?
►Shivani Jindal, MD, MPH, Hospitalist, VABHS, Instructor in Medicine, Boston University School of Medicine (BUSM): A thorough history and physical examination are important. I specifically ask about chronicity, pain, and trauma. A medical history of potential infectious exposures and the history of the present illness are also important, such as the risk of sexually transmitted infections, exposure to Lyme disease or other viral illnesses. Gonococcal arthritis is one of the most common causes of nontraumatic monoarthritis in young adults but can also present as a migratory polyarthritis.1
It sounds like he was quite active and liked to hike so a history of tick exposure is important to ascertain. I would also ask about eye inflammation and back pain to assess possible ankylosing spondyarthritis. Other inflammatory etiologies, such as gout are common, but it would be surprising to miss this diagnosis on repeated arthocenteses. A physical examination can confirm monoarthritis over polyarthritis and assess for signs of inflammatory arthritis (eg, warmth and erythema). The most important etiology to assess for and rule out in a person admitted to the hospital is septic arthritis. The severe pain, mild leukocytosis, and mildly elevated inflammatory markers could be consistent with this diagnosis but are nonspecific. However, the chronicity of this patient’s presentation and hemodynamic stability make septic arthritis less likely overall and a more indolent infection or other inflammatory process more likely.
►Dr. Merz: The patient’s medical history included posttraumatic stress disorder (PTSD) and antisocial personality disorder with multiple prior suicide attempts. He also had a history of opioid use disorder (OUD) with prior overdose and alcohol use disorder (AUD). Given his stated preference to avoid opioids and normal liver function and liver chemistry testing, the initial treatment was with acetaminophen. After this failed to provide satisfactory pain control, IV hydromorphone was added.
Dr. Jindal, how do you approach pain control in the hospital for musculoskeletal issues like this?
►Dr. Jindal: Typically, nonsteroidal anti-inflammatory medications (NSAIDs) are most effective for musculoskeletal pain, often in the form of ketorolac or ibuprofen. However, we are often limited in our NSAID use by kidney disease, gastritis, or cardiovascular disease.
►Dr. Merz: On hospital day 1, the patient asked to leave to consume alcohol to ease unremitting pain. He also expressed suicidal ideation and discharge was therefore felt to be unsafe. He was reluctant to engage with psychiatry and became physically combative while attempting to leave the hospital, necessitating the use of sedating medications and physical restraints.
Dr. Shahal, what factors led to the decision to place an involuntary hold, and how do you balance patient autonomy and patient safety?
►Dr. Talya Shahal, MD, Consult-Liaison Psychiatry Service, VABHS, Instructor in Psychiatry, Harvard Medical School: This is a delicate balance that requires constant reassessment. The patient initially presented to the emergency department with suicidal ideation, stating he was not able to tolerate the pain and thus resumed alcohol consumption after a period of nonuse. He had multiple risk factors for suicide, including 9 prior suicide attempts with the latest less than a year before presentation, active substance use with alcohol and other recreational drugs, PTSD, pain, veteran status, male sex, single status, and a history of trauma.3,4 He was also displaying impulsivity and limited insight, did not engage in his psychiatric assessment, and attempted to assault staff. As such, his suicide risk was assessed to be high at the time of the evaluation, which led to the decision to place an involuntary hold. However, we reevaluate this decision at least daily in order to reassess the risk and ensure that the balance between patient safety and autonomy are maintained.
►Dr. Merz: The involuntary hold was removed within 48 hours as the patient remained calm and engaged with the primary and consulting teams. He requested escalating doses of opioids as he felt the short-acting IV medications were not providing sustained relief. However, he was also noted to be walking outside of the hospital without assistance, and he repeatedly declined nonopioid pain modalities as well as buprenorphine/naloxone. The chronic pain service was consulted but was unable to see the patient as he was frequently outside of his room.
Dr. Shahal, how do you address OUD, pain, and stigma in the hospital?
►Dr. Shahal: It is important to remember that patients with substance use disorder (SUD) and mental illness frequently have physical causes for pain and are often undertreated.5 Patients with SUD may also have higher tolerance for opioids and may need higher doses to treat the pain.5 Modalities like buprenorphine/naloxone can be effective to treat OUD and pain, but these usually cannot be initiated while the patient is on short-acting opioids as this would precipitate withdrawal.6 However, withdrawal can be managed while inpatient, and this can be a good time to start these medications as practitioners can aggressively help with symptom control. Proactively addressing mental health concerns, particularly anxiety, AUD, insomnia, PTSD, and depression, can also have a direct impact on the perception of pain and assist with better control.2 In addition, nonpharmacologic options, such as meditation, deep breathing, and even acupuncture and Reiki can be helpful and of course less harmful to treat pain.2
► Dr. Merz: An X-ray of the knee showed no acute fracture or joint space narrowing. Magnetic resonance imaging confirmed a large knee effusion with no evidence of ligament injury. Synovial fluid showed turbid, yellow fluid with 14,110 nucleated cells (84% segmented cells and 4000 RBCs). Gram stain was negative, culture had no growth, and there were no crystals. Anticyclic citrullinated peptide (anti-CCP), rheumatoid factor, HIV testing, and HLA-B27 were negative.
Dr. Serrao, what do these studies tell us about the joint effusion and the possible diagnoses?
► Dr. Richard Serrao, MD, Infectious Disease, VABHS, Clinical Associate Professor in Medicine, BUSM: I would expect the white blood cell (WBC) count to be > 50,000 cells with > 75% polymorphonuclear cells and a positive Gram stain if this was a bacterial infection resulting in septic arthritis.7 This patient’s studies are not consistent with this diagnosis nor is the chronicity of his presentation. There are 2 important bacteria that can present with inflammatory arthritis and less pronounced findings on arthrocentesis: Borrelia burgdorferi (the bacteria causing Lyme arthritis) and Neisseria gonorrhea. Lyme arthritis could be consistent with this relapsing remitting presentation as you expect a WBC count between 3000 and 100,000 cells with a mean value between 10,000 and 25,000 cells, > 50% polymorphonuclear leukocytes, and negative Gram stains.8 Gonococcal infections often do not have marked elevations in the WBC count and the Gram stain can be variable, but you still expect the WBC count to be > 30,000 cells.7 Inflammatory causes such as gout or autoimmune conditions such as lupus often have a WBC count between 2000 and 100,000 with a negative Gram stain, which could be consistent with this patient’s presentation.7 However, the lack of crystals rules out gout and the negative anti-CCP, rheumatoid factor, and HLA-B27 make rheumatologic diseases less likely.
►Dr. Merz: The patient received a phone call from another hospital where an arthrocentesis had been performed 3 weeks before. The results included a positive polymerase chain reaction (PCR) test for Lyme disease in the synovial fluid. A subsequent serum Lyme screen was positive for 1 of 3 immunoglobulin (Ig) M bands and 10 of 10 IgG bands.
Dr. Serrao, how does Lyme arthritis typically present, and are there aspects of this case that make you suspect the diagnosis? Does the serum Lyme test give us any additional information?
►Dr. Serrao: Lyme arthritis is a late manifestation of Lyme disease. Patients typically have persistent or intermittent arthritis, and large joints are more commonly impacted than small joints. Monoarthritis of the knee is the most common, but oligoarthritis is possible as well. The swelling usually begins abruptly, lasts for weeks to months, and effusions typically recur quickly after aspiration. These findings are consistent with the patient’s clinical history.
For diagnostics, the IgG Western blot is positive if 5 of the 10 bands are positive.9 This patient far exceeds the IgG band number to diagnose Lyme disease. All patients with Lyme arthritis will have positive IgG serologies since Lyme arthritis is a late manifestation of the infection. IgM reactivity may be present, but are not necessary to diagnose Lyme arthritis.10 Synovial fluid is often not analyzed for antibody responses as they are susceptible to false positive results, but synovial PCR testing like this patient had detects approximately 70% of patients with untreated Lyme arthritis.11 However, PCR positivity does not necessarily equate with active infection. Serologic testing for Lyme disease by enzyme-linked immunosorbent assay and Western blot as well as careful history and the exclusion of other diagnoses are usually sufficient to make the diagnosis.
► Dr. Merz: On further history the patient reported that 5 years prior he found a tick on his skin with a bull’s-eye rash. He was treated with 28 days of doxycycline at that time. He did not recall any tick bites or rashes in the years since.
Dr. Serrao, is it surprising that he developed Lyme arthritis 5 years after exposure and after being treated appropriately? What is the typical treatment approach for a patient like this?
►Dr. Serrao: It is atypical to develop Lyme arthritis 5 years after reported treatment of what appeared to be early localized disease, namely, erythema migrans. This stage is usually cured with 10 days of treatment alone (he received 28 days) and is generally abortive of subsequent stages, including Lyme arthritis. Furthermore, the patient reported no symptoms of arthritis until recently since that time. Therefore, one can argue that the excessively long span of time from treatment to these first episodes of arthritis suggests the patient could have been reinfected. When available, comparing the types and number of Western blot bands (eg, new and/or more bands on subsequent serologic testing) can support a reinfection diagnosis. A delayed postinfectious inflammatory process from excessive proinflammatory immune responses that block wound repair resulting in proliferative synovitis is also possible.12 This is defined as the postinfectious, postantibiotic, or antibiotic-refractory Lyme arthritis, a diagnosis of exclusion more apparent only after patients receive appropriate antibiotic courses for the possibility of untreated Lyme as an active infection.12
Given the inherent diagnostic uncertainty between an active infection and posttreatment Lyme arthritis syndromes, it is best to approach most cases of Lyme arthritis as an active infection first especially if not yet treated with antibiotics. Diagnosis of postinflammatory processes should be considered if symptoms persist after appropriate antibiotics, and then short-term use of disease-modifying antirheumatic drugs, rather than further courses of antibiotics, is recommended.
► Dr. Merz: The patient was initiated on doxycycline with the plan to transition to ceftriaxone if there was no response. One day after diagnosis and treatment initiation and in the setting of continued pain, the patient again asked to leave the hospital to drink alcohol. After eloping and becoming intoxicated with alcohol, he returned to his room. He remained concerned about his continued pain and lack of adequate pain control. At the time, he was receiving hydromorphone, ketorolac, lorazepam, gabapentin, and quetiapine.
Dr. Serrao, do you expect this degree of pain from Lyme arthritis?
► Dr. Serrao: Lyme arthritis is typically less painful than other forms of infectious or inflammatory arthritis. Pain is usually caused by the pressure from the acute accumulation and reaccumulation of fluid. In this case, the rapid accumulation of fluid that this patient experienced as well as relief with arthrocentesis suggests that the size and acuity of the effusion was causing great discomfort. Repeated arthrocentesis can prove to be a preventative strategy to minimize synovial herniation.
►Dr. Merz: Dr. Shahal, how do you balance the patient subjectively telling you that they are in pain with objective signs that they may be tolerating the pain like walking around unassisted? Is there anything else that could have been done to prevent this adverse outcome?
►Dr. Shahal: This is one of the hardest pieces of pain management. We want to practice beneficence by believing our patients and addressing their discomfort, but we also want to practice nonmaleficence by avoiding inappropriate long-term pain treatments like opioids that have significant harm as well as avoiding exacerbating this patient’s underlying SUD. An agent like buprenorphine/naloxone could have been an excellent fit to treat pain and SUD, but the patient’s lack of interest and the frequent use of short-acting opioids were major barriers. A chronic pain consult early on is helpful in cases like this as well, but they were unable to see him since he was often out of his room. Repeated arthrocentesis may also have helped the pain. Treatment of anxiety and insomnia with medications like hydroxyzine, trazodone, melatonin, gabapentin, or buspirone as well as interventions like sleep hygiene protocols or spiritual care may have helped somewhat as well.
We know that there is a vicious cycle between pain and poorly controlled mood symptoms. Many of our veterans have PTSD, anxiety, and SUD that are exacerbated by hospitalization and pain. Maintaining optimal communication between the patient and the practitioners, using trauma-informed care, understanding the patient’s goals of care, setting expectations and limits, and attempting to address the patient’s needs while attempting to minimize stigma might be helpful. However, despite optimal care, sometimes these events cannot be avoided.
►Dr. Merz: The patient was ultimately transferred to an inpatient psychiatric unit where a taper plan for the short-acting opioids was implemented. He was psychiatrically stabilized and discharged a few days later off opioids and on doxycycline. On follow-up a few weeks later, his pain had markedly improved, and the effusion was significantly reduced in size. His mood and impulsivity had stabilized. He continues to follow-up in the infectious disease clinic.

1. Siva C, Velazquez C, Mody A, Brasington R. Diagnosing acute monoarthritis in adults: a practical approach for the family physician. Am Fam Physician. 2003;68(1):83-90.
2. Qaseem A, McLean RM, O’Gurek D, et al. Nonpharmacologic and pharmacologic management of acute pain from non-low back, musculoskeletal injuries in adults: a clinical guideline from the American College of Physicians and American Academy of Family Physicians. Ann Intern Med. 2020;173(9):739-748. doi:10.7326/M19-3602
3. Silverman MM, Berman AL. Suicide risk assessment and risk formulation part I: a focus on suicide ideation in assessing suicide risk. Suicide Life Threat Behav. 2014;44(4):420-431. doi:10.1111/sltb.12065
4. Berman AL, Silverman MM. Suicide risk assessment and risk formulation part II: Suicide risk formulation and the determination of levels of risk. Suicide Life Threat Behav. 2014;44(4):432-443. doi:10.1111/sltb.12067
5. Quinlan J, Cox F. Acute pain management in patients with drug dependence syndrome. Pain Rep. 2017;2(4):e611. Published 2017 Jul 27. doi:10.1097/PR9.0000000000000611
6. Chou R, Wagner J, Ahmed AY, et al. Treatments for Acute Pain: A Systematic Review. Agency for Healthcare Research and Quality; 2020. https://www.ncbi.nlm.nih.gov/books/NBK566506/
7. Seidman AJ, Limaiem F. Synovial fluid analysis. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022. Updated May 8, 2022. https://www.ncbi.nlm.nih.gov/books/NBK537114
8. Arvikar SL, Steere AC. Diagnosis and treatment of Lyme arthritis. Infect Dis Clin North Am. 2015;29(2):269-280. doi:10.1016/j.idc.2015.02.004
9. Centers for Disease Control and Prevention. Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. JAMA. 1995;274(12):937.
10. Craft JE, Grodzicki RL, Steere AC. Antibody response in Lyme disease: evaluation of diagnostic tests. J Infect Dis. 1984;149(5):789-795. doi:10.1093/infdis/149.5.789
11. Nocton JJ, Dressler F, Rutledge BJ, Rys PN, Persing DH, Steere AC. Detection of Borrelia burgdorferi DNA by polymerase chain reaction in synovial fluid from patients with Lyme arthritis. N Engl J Med. 1994;330(4):229-234. doi:10.1056/NEJM199401273300401
12. Steere AC. Posttreatment Lyme disease syndromes: distinct pathogenesis caused by maladaptive host responses. J Clin Invest. 2020;130(5):2148-2151. doi:10.1172/JCI138062
1. Siva C, Velazquez C, Mody A, Brasington R. Diagnosing acute monoarthritis in adults: a practical approach for the family physician. Am Fam Physician. 2003;68(1):83-90.
2. Qaseem A, McLean RM, O’Gurek D, et al. Nonpharmacologic and pharmacologic management of acute pain from non-low back, musculoskeletal injuries in adults: a clinical guideline from the American College of Physicians and American Academy of Family Physicians. Ann Intern Med. 2020;173(9):739-748. doi:10.7326/M19-3602
3. Silverman MM, Berman AL. Suicide risk assessment and risk formulation part I: a focus on suicide ideation in assessing suicide risk. Suicide Life Threat Behav. 2014;44(4):420-431. doi:10.1111/sltb.12065
4. Berman AL, Silverman MM. Suicide risk assessment and risk formulation part II: Suicide risk formulation and the determination of levels of risk. Suicide Life Threat Behav. 2014;44(4):432-443. doi:10.1111/sltb.12067
5. Quinlan J, Cox F. Acute pain management in patients with drug dependence syndrome. Pain Rep. 2017;2(4):e611. Published 2017 Jul 27. doi:10.1097/PR9.0000000000000611
6. Chou R, Wagner J, Ahmed AY, et al. Treatments for Acute Pain: A Systematic Review. Agency for Healthcare Research and Quality; 2020. https://www.ncbi.nlm.nih.gov/books/NBK566506/
7. Seidman AJ, Limaiem F. Synovial fluid analysis. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022. Updated May 8, 2022. https://www.ncbi.nlm.nih.gov/books/NBK537114
8. Arvikar SL, Steere AC. Diagnosis and treatment of Lyme arthritis. Infect Dis Clin North Am. 2015;29(2):269-280. doi:10.1016/j.idc.2015.02.004
9. Centers for Disease Control and Prevention. Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. JAMA. 1995;274(12):937.
10. Craft JE, Grodzicki RL, Steere AC. Antibody response in Lyme disease: evaluation of diagnostic tests. J Infect Dis. 1984;149(5):789-795. doi:10.1093/infdis/149.5.789
11. Nocton JJ, Dressler F, Rutledge BJ, Rys PN, Persing DH, Steere AC. Detection of Borrelia burgdorferi DNA by polymerase chain reaction in synovial fluid from patients with Lyme arthritis. N Engl J Med. 1994;330(4):229-234. doi:10.1056/NEJM199401273300401
12. Steere AC. Posttreatment Lyme disease syndromes: distinct pathogenesis caused by maladaptive host responses. J Clin Invest. 2020;130(5):2148-2151. doi:10.1172/JCI138062
Children with migraine at high risk of comorbid anxiety, depression
Children and adolescents with migraine are about twice as likely to have an anxiety or depressive disorder as those without migraine, results from a new review and meta-analysis suggest.
“This is compelling, high-level evidence showing there’s this established comorbidity between migraine and anxiety and depressive symptoms and disorders in this age group,” co-investigator Serena L. Orr, MD, a pediatric neurologist and headache specialist at Alberta Children’s Hospital and assistant professor in the department of pediatrics, University of Calgary (Alta.), told this news organization.
The results “should compel every clinician who is seeing a child or adolescent with migraine to screen for anxiety and depression and to manage that if it’s present. That should be the standard of care with this level of evidence,” Dr. Orr said.
The findings were presented at the American Headache Society (AHS) Annual Meeting 2022.
Incidence divergence
Previous studies have suggested that 10%-20% of children and adolescents will experience migraine at some point before adulthood, with the prevalence increasing after puberty.
While the female-to-male ratio is about 1:1 before puberty, there is a “big divergence in incidence curves” afterward – with the female-to-male ratio reaching 2-3:1 in adulthood, Dr. Orr noted. Experts believe hormones drive this divergence, she said, noting that male adults with migraine have lower testosterone levels than male adults without migraine.
Dr. Orr and her colleagues were keen to investigate the relationship between child migraine and anxiety symptoms and disorders, as well as between child migraine and depression symptoms and disorders. They searched the literature for related case-control, cross-sectional, and cohort studies with participants of ages up to 18 years.
The researchers selected 80 studies to include in the review. Most of the studies were carried out in the past 30 to 40 years and were in English and other languages. Both community-based and clinical studies were included.
Of the total, 73 studies reported on the association between the exposures and migraine, and 51 were amenable to quantitative pooling.
Results from a meta-analysis that included 16 studies that compared children and adolescents who had migraine with their healthy peers showed a significant association between migraine and anxiety symptoms (standardized mean difference, 1.13; 95% confidence interval, 0.64-1.63; P < .0001).
Compared with children who did not have migraine, those with migraine had almost twice the odds of an anxiety disorder in 15 studies (odds ratio, 1.93; 95% CI, 1.49-2.50; P < .0001).
In addition, there was an association between migraine and depressive symptoms in 17 relevant studies (SMD, 0.67; 95% CI, 0.46-0.87; P < .0001). Participants with versus without migraine also had higher odds of depressive disorders in 18 studies (OR, 2.01; 95% CI, 1.46-2.78; P < .0001).
Effect sizes were similar between community-based and clinic studies. Dr. Orr said it is important to note that the analysis wasn’t restricted to studies with “just kids with really high disease burden who are going to naturally be more predisposed to psychiatric comorbidity.”
‘Shocking’ lack of research
The researchers were also interested in determining whether having migraine along with anxiety or depression symptoms or disorders could affect headache-specific outcomes and whether such patients’ conditions would be more refractory to treatment. However, these outcomes were “all over the place” in the 18 relevant studies, Dr. Orr reported.
“Some looked at headache frequency, some at disability, some at school functioning; so, we were not able to put them into a meta-analysis,” she said.
Only two studies examined whether anxiety or depression earlier in childhood predisposes to subsequent migraine, so that issue is still unresolved, Dr. Orr added.
The investigators also assessed whether outcomes with migraine are similar to those with other headache types, such as tension-type headaches. “We did not find a difference at the symptom or disorder level, but there were fewer of those studies” – and these, too, were heterogeneous, said Dr. Orr.
The researchers did not find any studies of the association between migraine and trauma, which Dr. Orr said was “shocking.”
“In the broader pediatric chronic-pain literature, there’s research showing that having a trauma or stress-related disorder is associated with more chronic pain and worse chronic pain outcomes, but we could not find a study that specifically looked at that question in migraine,” she added.
Emerging evidence suggests there may be a bidirectional relationship between migraine and anxiety/depression, at least in adults. Dr. Orr said having these symptoms appears to raise the risk for migraine, but whether that’s environmental or driven by shared genetics isn’t clear.
Experiencing chronic pain may also predispose individuals to anxiety and depression, “but we need more studies on this.”
In addition to screening children with migraine for anxiety and depression, clinicians should advocate for better access to mental health resources for patients with these comorbidities, Dr. Orr noted.
She added that a limitation of the review was that 82.5% of the studies reported unadjusted associations and that 26.3% of the studies were of low quality.
High-level evidence
Sara Pavitt, MD, chief of the Pediatric Headache Program and assistant professor in the department of neurology, the University of Texas at Austin, said the investigators “should be applauded” for providing “high-level evidence” to better understand the relationship between migraine and anxiety and depression in pediatric patients.
Such information has been “lacking” for this patient population, said Dr. Pavitt, who was not involved with the research.
She noted that screening kids for mood disorders is challenging, given the relatively few pediatric mental health care providers. A referral for a psychiatric follow-up can mean a 9- to 12-month wait – or even longer for children who do not have insurance or use Medicare.
“Providers need to have more incentives to care for patients with Medicare or lack of insurance – these patients are often excluded from practices because reimbursement is so poor,” Dr. Pavitt said.
Additional pediatric studies are needed to understand how other mental health disorders, such as panic disorder, phobias, and posttraumatic stress disorder, may be related to migraine, she added.
The study received no outside funding. Dr. Orr has received grants from the Canadian Institutes of Health Research and royalties from Cambridge University Press for book publication, and she is on editorial boards of Headache, Neurology, and the American Migraine Foundation. Dr. Pavitt serves on an advisory board for Theranica, which produces a neuromodulation device for acute migraine treatment, although this is not directly relevant to this review.
A version of this article first appeared on Medscape.com.
Children and adolescents with migraine are about twice as likely to have an anxiety or depressive disorder as those without migraine, results from a new review and meta-analysis suggest.
“This is compelling, high-level evidence showing there’s this established comorbidity between migraine and anxiety and depressive symptoms and disorders in this age group,” co-investigator Serena L. Orr, MD, a pediatric neurologist and headache specialist at Alberta Children’s Hospital and assistant professor in the department of pediatrics, University of Calgary (Alta.), told this news organization.
The results “should compel every clinician who is seeing a child or adolescent with migraine to screen for anxiety and depression and to manage that if it’s present. That should be the standard of care with this level of evidence,” Dr. Orr said.
The findings were presented at the American Headache Society (AHS) Annual Meeting 2022.
Incidence divergence
Previous studies have suggested that 10%-20% of children and adolescents will experience migraine at some point before adulthood, with the prevalence increasing after puberty.
While the female-to-male ratio is about 1:1 before puberty, there is a “big divergence in incidence curves” afterward – with the female-to-male ratio reaching 2-3:1 in adulthood, Dr. Orr noted. Experts believe hormones drive this divergence, she said, noting that male adults with migraine have lower testosterone levels than male adults without migraine.
Dr. Orr and her colleagues were keen to investigate the relationship between child migraine and anxiety symptoms and disorders, as well as between child migraine and depression symptoms and disorders. They searched the literature for related case-control, cross-sectional, and cohort studies with participants of ages up to 18 years.
The researchers selected 80 studies to include in the review. Most of the studies were carried out in the past 30 to 40 years and were in English and other languages. Both community-based and clinical studies were included.
Of the total, 73 studies reported on the association between the exposures and migraine, and 51 were amenable to quantitative pooling.
Results from a meta-analysis that included 16 studies that compared children and adolescents who had migraine with their healthy peers showed a significant association between migraine and anxiety symptoms (standardized mean difference, 1.13; 95% confidence interval, 0.64-1.63; P < .0001).
Compared with children who did not have migraine, those with migraine had almost twice the odds of an anxiety disorder in 15 studies (odds ratio, 1.93; 95% CI, 1.49-2.50; P < .0001).
In addition, there was an association between migraine and depressive symptoms in 17 relevant studies (SMD, 0.67; 95% CI, 0.46-0.87; P < .0001). Participants with versus without migraine also had higher odds of depressive disorders in 18 studies (OR, 2.01; 95% CI, 1.46-2.78; P < .0001).
Effect sizes were similar between community-based and clinic studies. Dr. Orr said it is important to note that the analysis wasn’t restricted to studies with “just kids with really high disease burden who are going to naturally be more predisposed to psychiatric comorbidity.”
‘Shocking’ lack of research
The researchers were also interested in determining whether having migraine along with anxiety or depression symptoms or disorders could affect headache-specific outcomes and whether such patients’ conditions would be more refractory to treatment. However, these outcomes were “all over the place” in the 18 relevant studies, Dr. Orr reported.
“Some looked at headache frequency, some at disability, some at school functioning; so, we were not able to put them into a meta-analysis,” she said.
Only two studies examined whether anxiety or depression earlier in childhood predisposes to subsequent migraine, so that issue is still unresolved, Dr. Orr added.
The investigators also assessed whether outcomes with migraine are similar to those with other headache types, such as tension-type headaches. “We did not find a difference at the symptom or disorder level, but there were fewer of those studies” – and these, too, were heterogeneous, said Dr. Orr.
The researchers did not find any studies of the association between migraine and trauma, which Dr. Orr said was “shocking.”
“In the broader pediatric chronic-pain literature, there’s research showing that having a trauma or stress-related disorder is associated with more chronic pain and worse chronic pain outcomes, but we could not find a study that specifically looked at that question in migraine,” she added.
Emerging evidence suggests there may be a bidirectional relationship between migraine and anxiety/depression, at least in adults. Dr. Orr said having these symptoms appears to raise the risk for migraine, but whether that’s environmental or driven by shared genetics isn’t clear.
Experiencing chronic pain may also predispose individuals to anxiety and depression, “but we need more studies on this.”
In addition to screening children with migraine for anxiety and depression, clinicians should advocate for better access to mental health resources for patients with these comorbidities, Dr. Orr noted.
She added that a limitation of the review was that 82.5% of the studies reported unadjusted associations and that 26.3% of the studies were of low quality.
High-level evidence
Sara Pavitt, MD, chief of the Pediatric Headache Program and assistant professor in the department of neurology, the University of Texas at Austin, said the investigators “should be applauded” for providing “high-level evidence” to better understand the relationship between migraine and anxiety and depression in pediatric patients.
Such information has been “lacking” for this patient population, said Dr. Pavitt, who was not involved with the research.
She noted that screening kids for mood disorders is challenging, given the relatively few pediatric mental health care providers. A referral for a psychiatric follow-up can mean a 9- to 12-month wait – or even longer for children who do not have insurance or use Medicare.
“Providers need to have more incentives to care for patients with Medicare or lack of insurance – these patients are often excluded from practices because reimbursement is so poor,” Dr. Pavitt said.
Additional pediatric studies are needed to understand how other mental health disorders, such as panic disorder, phobias, and posttraumatic stress disorder, may be related to migraine, she added.
The study received no outside funding. Dr. Orr has received grants from the Canadian Institutes of Health Research and royalties from Cambridge University Press for book publication, and she is on editorial boards of Headache, Neurology, and the American Migraine Foundation. Dr. Pavitt serves on an advisory board for Theranica, which produces a neuromodulation device for acute migraine treatment, although this is not directly relevant to this review.
A version of this article first appeared on Medscape.com.
Children and adolescents with migraine are about twice as likely to have an anxiety or depressive disorder as those without migraine, results from a new review and meta-analysis suggest.
“This is compelling, high-level evidence showing there’s this established comorbidity between migraine and anxiety and depressive symptoms and disorders in this age group,” co-investigator Serena L. Orr, MD, a pediatric neurologist and headache specialist at Alberta Children’s Hospital and assistant professor in the department of pediatrics, University of Calgary (Alta.), told this news organization.
The results “should compel every clinician who is seeing a child or adolescent with migraine to screen for anxiety and depression and to manage that if it’s present. That should be the standard of care with this level of evidence,” Dr. Orr said.
The findings were presented at the American Headache Society (AHS) Annual Meeting 2022.
Incidence divergence
Previous studies have suggested that 10%-20% of children and adolescents will experience migraine at some point before adulthood, with the prevalence increasing after puberty.
While the female-to-male ratio is about 1:1 before puberty, there is a “big divergence in incidence curves” afterward – with the female-to-male ratio reaching 2-3:1 in adulthood, Dr. Orr noted. Experts believe hormones drive this divergence, she said, noting that male adults with migraine have lower testosterone levels than male adults without migraine.
Dr. Orr and her colleagues were keen to investigate the relationship between child migraine and anxiety symptoms and disorders, as well as between child migraine and depression symptoms and disorders. They searched the literature for related case-control, cross-sectional, and cohort studies with participants of ages up to 18 years.
The researchers selected 80 studies to include in the review. Most of the studies were carried out in the past 30 to 40 years and were in English and other languages. Both community-based and clinical studies were included.
Of the total, 73 studies reported on the association between the exposures and migraine, and 51 were amenable to quantitative pooling.
Results from a meta-analysis that included 16 studies that compared children and adolescents who had migraine with their healthy peers showed a significant association between migraine and anxiety symptoms (standardized mean difference, 1.13; 95% confidence interval, 0.64-1.63; P < .0001).
Compared with children who did not have migraine, those with migraine had almost twice the odds of an anxiety disorder in 15 studies (odds ratio, 1.93; 95% CI, 1.49-2.50; P < .0001).
In addition, there was an association between migraine and depressive symptoms in 17 relevant studies (SMD, 0.67; 95% CI, 0.46-0.87; P < .0001). Participants with versus without migraine also had higher odds of depressive disorders in 18 studies (OR, 2.01; 95% CI, 1.46-2.78; P < .0001).
Effect sizes were similar between community-based and clinic studies. Dr. Orr said it is important to note that the analysis wasn’t restricted to studies with “just kids with really high disease burden who are going to naturally be more predisposed to psychiatric comorbidity.”
‘Shocking’ lack of research
The researchers were also interested in determining whether having migraine along with anxiety or depression symptoms or disorders could affect headache-specific outcomes and whether such patients’ conditions would be more refractory to treatment. However, these outcomes were “all over the place” in the 18 relevant studies, Dr. Orr reported.
“Some looked at headache frequency, some at disability, some at school functioning; so, we were not able to put them into a meta-analysis,” she said.
Only two studies examined whether anxiety or depression earlier in childhood predisposes to subsequent migraine, so that issue is still unresolved, Dr. Orr added.
The investigators also assessed whether outcomes with migraine are similar to those with other headache types, such as tension-type headaches. “We did not find a difference at the symptom or disorder level, but there were fewer of those studies” – and these, too, were heterogeneous, said Dr. Orr.
The researchers did not find any studies of the association between migraine and trauma, which Dr. Orr said was “shocking.”
“In the broader pediatric chronic-pain literature, there’s research showing that having a trauma or stress-related disorder is associated with more chronic pain and worse chronic pain outcomes, but we could not find a study that specifically looked at that question in migraine,” she added.
Emerging evidence suggests there may be a bidirectional relationship between migraine and anxiety/depression, at least in adults. Dr. Orr said having these symptoms appears to raise the risk for migraine, but whether that’s environmental or driven by shared genetics isn’t clear.
Experiencing chronic pain may also predispose individuals to anxiety and depression, “but we need more studies on this.”
In addition to screening children with migraine for anxiety and depression, clinicians should advocate for better access to mental health resources for patients with these comorbidities, Dr. Orr noted.
She added that a limitation of the review was that 82.5% of the studies reported unadjusted associations and that 26.3% of the studies were of low quality.
High-level evidence
Sara Pavitt, MD, chief of the Pediatric Headache Program and assistant professor in the department of neurology, the University of Texas at Austin, said the investigators “should be applauded” for providing “high-level evidence” to better understand the relationship between migraine and anxiety and depression in pediatric patients.
Such information has been “lacking” for this patient population, said Dr. Pavitt, who was not involved with the research.
She noted that screening kids for mood disorders is challenging, given the relatively few pediatric mental health care providers. A referral for a psychiatric follow-up can mean a 9- to 12-month wait – or even longer for children who do not have insurance or use Medicare.
“Providers need to have more incentives to care for patients with Medicare or lack of insurance – these patients are often excluded from practices because reimbursement is so poor,” Dr. Pavitt said.
Additional pediatric studies are needed to understand how other mental health disorders, such as panic disorder, phobias, and posttraumatic stress disorder, may be related to migraine, she added.
The study received no outside funding. Dr. Orr has received grants from the Canadian Institutes of Health Research and royalties from Cambridge University Press for book publication, and she is on editorial boards of Headache, Neurology, and the American Migraine Foundation. Dr. Pavitt serves on an advisory board for Theranica, which produces a neuromodulation device for acute migraine treatment, although this is not directly relevant to this review.
A version of this article first appeared on Medscape.com.
Persistent abdominal pain: Not always IBS
Persistent abdominal pain may be caused by a whole range of different conditions, say French experts who call for more physician awareness to achieve early diagnosis and treatment so as to improve patient outcomes.
Benoit Coffin, MD, PhD, and Henri Duboc, MD, PhD, from Hôpital Louis Mourier, Colombes, France, conducted a literature review to identify rare and less well-known causes of persistent abdominal pain, identifying almost 50 across several categories.
“Some causes of persistent abdominal pain can be effectively treated using established approaches after a definitive diagnosis has been reached,” they wrote.
“Other causes are more complex and may benefit from a multidisciplinary approach involving gastroenterologists, pain specialists, allergists, immunologists, rheumatologists, psychologists, physiotherapists, dietitians, and primary care clinicians,” they wrote.
The research was published online in Alimentary Pharmacology and Therapeutics.
Frequent and frustrating symptoms
Although there is “no commonly accepted definition” for persistent abdominal pain, the authors said it may be defined as “continuous or intermittent abdominal discomfort that persists for at least 6 months and fails to respond to conventional therapeutic approaches.”
They highlight that it is “frequently encountered” by physicians and has a prevalence of 22.9 per 1,000 person-years, regardless of age group, ethnicity, or geographical region, with many patients experiencing pain for more than 5 years.
The cause of persistent abdominal pain can be organic with a clear cause or functional, making diagnosis and management “challenging and frustrating for patients and physicians.”
“Clinicians not only need to recognize somatic abnormalities, but they must also perceive the patient’s cognitions and emotions related to the pain,” they added, suggesting that clinicians take time to “listen to the patient and perceive psychological factors.”
Dr. Coffin and Dr. Duboc write that the most common conditions associated with persistent abdominal pain are irritable bowel syndrome and functional dyspepsia, as well as inflammatory bowel disease, chronic pancreatitis, and gallstones.
To examine the diagnosis and management of its less well-known causes, the authors conducted a literature review, beginning with the diagnosis of persistent abdominal pain.
Diagnostic workup
“Given its chronicity, many patients will have already undergone extensive and redundant medical testing,” they wrote, emphasizing that clinicians should be on the lookout for any change in the description of persistent abdominal pain or new symptoms.
“Other ‘red-flag’ symptoms include fever, vomiting, diarrhea, acute change in bowel habit, obstipation, syncope, tachycardia, hypotension, concomitant chest or back pain, unintentional weight loss, night sweats, and acute gastrointestinal bleeding,” the authors said.
They stressed the need to determine whether the origin of the pain is organic or functional, as well as the importance of identifying a “triggering event, such as an adverse life event, infection, initiating a new medication, or surgical procedure.” They also recommend discussing the patient’s diet.
There are currently no specific algorithms for diagnostic workup of persistent abdominal pain, the authors said. Patients will have undergone repeated laboratory tests, “upper and lower endoscopic examinations, abdominal ultrasounds, and computed tomography scans of the abdominal/pelvic area.”
Consequently, “in the absence of alarm features, any additional tests should be ordered in a conservative and cost-effective manner,” they advised.
They suggested that, at a tertiary center, patients should be assessed in three steps:
- In-depth questioning of the symptoms and medical history
- Summary of all previous investigations and treatments and their effectiveness
- Determination of the complementary explorations to be performed
The authors went on to list 49 rare or less well-known potential causes of persistent abdominal pain, some linked to digestive disorders, such as eosinophilic gastroenteritis, mesenteric panniculitis, and chronic mesenteric ischemia, as well as endometriosis, chronic abdominal wall pain, and referred osteoarticular pain.
Systemic causes of persistent abdominal pain may include adrenal insufficiency and mast cell activation syndrome, while acute hepatic porphyrias and Ehlers-Danlos syndrome may be genetic causes.
There are also centrally mediated disorders that lead to persistent abdominal pain, the authors noted, including postural orthostatic tachycardia syndrome and narcotic bowel syndrome caused by opioid therapy, among others.
Writing support for the manuscript was funded by Alnylam Switzerland. Dr. Coffin has served as a speaker for Kyowa Kyrin and Mayoly Spindler and as an advisory board member for Sanofi and Alnylam. Dr. Duboc reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Persistent abdominal pain may be caused by a whole range of different conditions, say French experts who call for more physician awareness to achieve early diagnosis and treatment so as to improve patient outcomes.
Benoit Coffin, MD, PhD, and Henri Duboc, MD, PhD, from Hôpital Louis Mourier, Colombes, France, conducted a literature review to identify rare and less well-known causes of persistent abdominal pain, identifying almost 50 across several categories.
“Some causes of persistent abdominal pain can be effectively treated using established approaches after a definitive diagnosis has been reached,” they wrote.
“Other causes are more complex and may benefit from a multidisciplinary approach involving gastroenterologists, pain specialists, allergists, immunologists, rheumatologists, psychologists, physiotherapists, dietitians, and primary care clinicians,” they wrote.
The research was published online in Alimentary Pharmacology and Therapeutics.
Frequent and frustrating symptoms
Although there is “no commonly accepted definition” for persistent abdominal pain, the authors said it may be defined as “continuous or intermittent abdominal discomfort that persists for at least 6 months and fails to respond to conventional therapeutic approaches.”
They highlight that it is “frequently encountered” by physicians and has a prevalence of 22.9 per 1,000 person-years, regardless of age group, ethnicity, or geographical region, with many patients experiencing pain for more than 5 years.
The cause of persistent abdominal pain can be organic with a clear cause or functional, making diagnosis and management “challenging and frustrating for patients and physicians.”
“Clinicians not only need to recognize somatic abnormalities, but they must also perceive the patient’s cognitions and emotions related to the pain,” they added, suggesting that clinicians take time to “listen to the patient and perceive psychological factors.”
Dr. Coffin and Dr. Duboc write that the most common conditions associated with persistent abdominal pain are irritable bowel syndrome and functional dyspepsia, as well as inflammatory bowel disease, chronic pancreatitis, and gallstones.
To examine the diagnosis and management of its less well-known causes, the authors conducted a literature review, beginning with the diagnosis of persistent abdominal pain.
Diagnostic workup
“Given its chronicity, many patients will have already undergone extensive and redundant medical testing,” they wrote, emphasizing that clinicians should be on the lookout for any change in the description of persistent abdominal pain or new symptoms.
“Other ‘red-flag’ symptoms include fever, vomiting, diarrhea, acute change in bowel habit, obstipation, syncope, tachycardia, hypotension, concomitant chest or back pain, unintentional weight loss, night sweats, and acute gastrointestinal bleeding,” the authors said.
They stressed the need to determine whether the origin of the pain is organic or functional, as well as the importance of identifying a “triggering event, such as an adverse life event, infection, initiating a new medication, or surgical procedure.” They also recommend discussing the patient’s diet.
There are currently no specific algorithms for diagnostic workup of persistent abdominal pain, the authors said. Patients will have undergone repeated laboratory tests, “upper and lower endoscopic examinations, abdominal ultrasounds, and computed tomography scans of the abdominal/pelvic area.”
Consequently, “in the absence of alarm features, any additional tests should be ordered in a conservative and cost-effective manner,” they advised.
They suggested that, at a tertiary center, patients should be assessed in three steps:
- In-depth questioning of the symptoms and medical history
- Summary of all previous investigations and treatments and their effectiveness
- Determination of the complementary explorations to be performed
The authors went on to list 49 rare or less well-known potential causes of persistent abdominal pain, some linked to digestive disorders, such as eosinophilic gastroenteritis, mesenteric panniculitis, and chronic mesenteric ischemia, as well as endometriosis, chronic abdominal wall pain, and referred osteoarticular pain.
Systemic causes of persistent abdominal pain may include adrenal insufficiency and mast cell activation syndrome, while acute hepatic porphyrias and Ehlers-Danlos syndrome may be genetic causes.
There are also centrally mediated disorders that lead to persistent abdominal pain, the authors noted, including postural orthostatic tachycardia syndrome and narcotic bowel syndrome caused by opioid therapy, among others.
Writing support for the manuscript was funded by Alnylam Switzerland. Dr. Coffin has served as a speaker for Kyowa Kyrin and Mayoly Spindler and as an advisory board member for Sanofi and Alnylam. Dr. Duboc reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Persistent abdominal pain may be caused by a whole range of different conditions, say French experts who call for more physician awareness to achieve early diagnosis and treatment so as to improve patient outcomes.
Benoit Coffin, MD, PhD, and Henri Duboc, MD, PhD, from Hôpital Louis Mourier, Colombes, France, conducted a literature review to identify rare and less well-known causes of persistent abdominal pain, identifying almost 50 across several categories.
“Some causes of persistent abdominal pain can be effectively treated using established approaches after a definitive diagnosis has been reached,” they wrote.
“Other causes are more complex and may benefit from a multidisciplinary approach involving gastroenterologists, pain specialists, allergists, immunologists, rheumatologists, psychologists, physiotherapists, dietitians, and primary care clinicians,” they wrote.
The research was published online in Alimentary Pharmacology and Therapeutics.
Frequent and frustrating symptoms
Although there is “no commonly accepted definition” for persistent abdominal pain, the authors said it may be defined as “continuous or intermittent abdominal discomfort that persists for at least 6 months and fails to respond to conventional therapeutic approaches.”
They highlight that it is “frequently encountered” by physicians and has a prevalence of 22.9 per 1,000 person-years, regardless of age group, ethnicity, or geographical region, with many patients experiencing pain for more than 5 years.
The cause of persistent abdominal pain can be organic with a clear cause or functional, making diagnosis and management “challenging and frustrating for patients and physicians.”
“Clinicians not only need to recognize somatic abnormalities, but they must also perceive the patient’s cognitions and emotions related to the pain,” they added, suggesting that clinicians take time to “listen to the patient and perceive psychological factors.”
Dr. Coffin and Dr. Duboc write that the most common conditions associated with persistent abdominal pain are irritable bowel syndrome and functional dyspepsia, as well as inflammatory bowel disease, chronic pancreatitis, and gallstones.
To examine the diagnosis and management of its less well-known causes, the authors conducted a literature review, beginning with the diagnosis of persistent abdominal pain.
Diagnostic workup
“Given its chronicity, many patients will have already undergone extensive and redundant medical testing,” they wrote, emphasizing that clinicians should be on the lookout for any change in the description of persistent abdominal pain or new symptoms.
“Other ‘red-flag’ symptoms include fever, vomiting, diarrhea, acute change in bowel habit, obstipation, syncope, tachycardia, hypotension, concomitant chest or back pain, unintentional weight loss, night sweats, and acute gastrointestinal bleeding,” the authors said.
They stressed the need to determine whether the origin of the pain is organic or functional, as well as the importance of identifying a “triggering event, such as an adverse life event, infection, initiating a new medication, or surgical procedure.” They also recommend discussing the patient’s diet.
There are currently no specific algorithms for diagnostic workup of persistent abdominal pain, the authors said. Patients will have undergone repeated laboratory tests, “upper and lower endoscopic examinations, abdominal ultrasounds, and computed tomography scans of the abdominal/pelvic area.”
Consequently, “in the absence of alarm features, any additional tests should be ordered in a conservative and cost-effective manner,” they advised.
They suggested that, at a tertiary center, patients should be assessed in three steps:
- In-depth questioning of the symptoms and medical history
- Summary of all previous investigations and treatments and their effectiveness
- Determination of the complementary explorations to be performed
The authors went on to list 49 rare or less well-known potential causes of persistent abdominal pain, some linked to digestive disorders, such as eosinophilic gastroenteritis, mesenteric panniculitis, and chronic mesenteric ischemia, as well as endometriosis, chronic abdominal wall pain, and referred osteoarticular pain.
Systemic causes of persistent abdominal pain may include adrenal insufficiency and mast cell activation syndrome, while acute hepatic porphyrias and Ehlers-Danlos syndrome may be genetic causes.
There are also centrally mediated disorders that lead to persistent abdominal pain, the authors noted, including postural orthostatic tachycardia syndrome and narcotic bowel syndrome caused by opioid therapy, among others.
Writing support for the manuscript was funded by Alnylam Switzerland. Dr. Coffin has served as a speaker for Kyowa Kyrin and Mayoly Spindler and as an advisory board member for Sanofi and Alnylam. Dr. Duboc reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ALIMENTARY PHARMACOLOGY AND THERAPEUTICS
Acupuncture deep needling technique points to greater tension headache relief
(TTH), new research suggests. Result of a randomized trial showed that though the majority of participants reported some relief from TTH after 8 weeks of acupuncture treatment, those who received needling at a depth of 12.5-20.0 mm reported the greatest reduction in headache frequency and severity.
At this depth, acupuncture promotes deqi sensation, a feeling of numbness, soreness, heaviness, or irritating pain in the needling site that is considered key to successful acupuncture treatment in traditional Chinese acupuncture theory.
“Our study showed that deqi sensation could enhance the effect of acupuncture in the treatment of chronic TTH, and the effect of acupuncture lasted at least 6 months when the treatment was stopped,” said co-investigator Ying Li, MD, PhD, The Third Hospital/Acupuncture and Tuina School, Chengdu University of Traditional Chinese Medicine, China.
The findings were published online in Neurology.
Deqi sensation key
TTH is the most common type of headache, with a lifetime prevalence of up to 78% in some studies. The pain is often described as throbbing or a vice-like tightness on both sides of the head. TTH is considered chronic when it occurs at least 15 days a month.
Previous studies have suggested that acupuncture can offer relief from headache pain, but specific information on TTH, especially chronic TTH, has been lacking.
To address the issue, researchers designed a parallel-design, patient-and-assessor blinded randomized controlled trial with 218 individuals with a history of chronic TTH. All were untreated with prophylactic treatment in the previous 3 months.
The treatment group (n = 110) received 20 sessions of true acupuncture (TA) over 8 weeks. This included three sessions per week in the first 4 weeks and two sessions per week in the last 4 weeks. The depth of needling at each point ranged from 12.5 to 20 mm, which is needed to achieve deqi sensation.
The control group (n = 108) received superficial acupuncture (SA) on the same schedule as the TA group and at traditional acupuncture points. However, this was done at a maximum depth of 2 mm, which is not deep enough for deqi sensation.
At week 16, 68.2% of the participants receiving TA reported a greater than 50% reduction in monthly headache days, compared with 48.1% of those receiving SA (odds ratio, 2.65; P < .001).
Mean monthly headache days decreased from 20.38 days at baseline to 7.48 days at week 32 in the TA group versus 22.6 days at baseline to 11.94 days in the SA group.
Headache intensity and severity decreased in both groups, although those who achieved deqi sensation reported the most improvement.
Only four patients reported adverse effects, all of which were mild and none requiring treatment.
Patients in both groups reported some pain relief, suggesting that those who are not comfortable with deqi sensation may still benefit from superficial acupuncture, although to a lesser extent, Dr. Li said.
“We assume that the point-specific effect and placebo effect were combined to give the patients relief of headaches,” Dr. Li added. “Further, the effect of deqi sensation added more treatment effect. This might be explained by gate-control theory or other unknown mechanisms.”
Deeper understanding?
Commenting on the research, Jennifer Bickel, MD, a senior member of neurology at Moffit Cancer Center and professor of oncologic sciences at University of South Florida, Tampa, said that the study provides a deeper understanding of acupuncture’s efficacy for chronic TTH, which could aid clinicians who are unfamiliar with the therapy or when and how to refer treatment.
“This study provides a more descriptive outline for what type of acupuncture treatment and duration can be effective for patients so doctors can prep patients on what to expect and so doctors can better assess if patients received appropriate acupuncture for their headaches,” said Dr. Bickel, who was not involved with the research.
However, she noted that the acupuncture sites and techniques did not vary during the trial. Although that makes sense for a controlled study, it may not reflect real-world clinical practice, she added.
“The downside is that the study didn’t fully reflect that most acupuncturists in clinical practice would alter treatments during the 20 sessions based on the patient’s response and accompanying symptoms or comorbidities,” Dr. Bickel said.
The study also lacked information on medication overuse headache or patients’ prior history of TTH treatments.
“This could be helpful to understand which patients in clinical practice are most likely to benefit from treatment,” Dr. Bickel said.
Study authors received funding from the Department of Science and Technology of Sichuan Province and the National Natural Science Foundation of China. Dr. Li, Dr. Bickel, and Dr. Vickers report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
(TTH), new research suggests. Result of a randomized trial showed that though the majority of participants reported some relief from TTH after 8 weeks of acupuncture treatment, those who received needling at a depth of 12.5-20.0 mm reported the greatest reduction in headache frequency and severity.
At this depth, acupuncture promotes deqi sensation, a feeling of numbness, soreness, heaviness, or irritating pain in the needling site that is considered key to successful acupuncture treatment in traditional Chinese acupuncture theory.
“Our study showed that deqi sensation could enhance the effect of acupuncture in the treatment of chronic TTH, and the effect of acupuncture lasted at least 6 months when the treatment was stopped,” said co-investigator Ying Li, MD, PhD, The Third Hospital/Acupuncture and Tuina School, Chengdu University of Traditional Chinese Medicine, China.
The findings were published online in Neurology.
Deqi sensation key
TTH is the most common type of headache, with a lifetime prevalence of up to 78% in some studies. The pain is often described as throbbing or a vice-like tightness on both sides of the head. TTH is considered chronic when it occurs at least 15 days a month.
Previous studies have suggested that acupuncture can offer relief from headache pain, but specific information on TTH, especially chronic TTH, has been lacking.
To address the issue, researchers designed a parallel-design, patient-and-assessor blinded randomized controlled trial with 218 individuals with a history of chronic TTH. All were untreated with prophylactic treatment in the previous 3 months.
The treatment group (n = 110) received 20 sessions of true acupuncture (TA) over 8 weeks. This included three sessions per week in the first 4 weeks and two sessions per week in the last 4 weeks. The depth of needling at each point ranged from 12.5 to 20 mm, which is needed to achieve deqi sensation.
The control group (n = 108) received superficial acupuncture (SA) on the same schedule as the TA group and at traditional acupuncture points. However, this was done at a maximum depth of 2 mm, which is not deep enough for deqi sensation.
At week 16, 68.2% of the participants receiving TA reported a greater than 50% reduction in monthly headache days, compared with 48.1% of those receiving SA (odds ratio, 2.65; P < .001).
Mean monthly headache days decreased from 20.38 days at baseline to 7.48 days at week 32 in the TA group versus 22.6 days at baseline to 11.94 days in the SA group.
Headache intensity and severity decreased in both groups, although those who achieved deqi sensation reported the most improvement.
Only four patients reported adverse effects, all of which were mild and none requiring treatment.
Patients in both groups reported some pain relief, suggesting that those who are not comfortable with deqi sensation may still benefit from superficial acupuncture, although to a lesser extent, Dr. Li said.
“We assume that the point-specific effect and placebo effect were combined to give the patients relief of headaches,” Dr. Li added. “Further, the effect of deqi sensation added more treatment effect. This might be explained by gate-control theory or other unknown mechanisms.”
Deeper understanding?
Commenting on the research, Jennifer Bickel, MD, a senior member of neurology at Moffit Cancer Center and professor of oncologic sciences at University of South Florida, Tampa, said that the study provides a deeper understanding of acupuncture’s efficacy for chronic TTH, which could aid clinicians who are unfamiliar with the therapy or when and how to refer treatment.
“This study provides a more descriptive outline for what type of acupuncture treatment and duration can be effective for patients so doctors can prep patients on what to expect and so doctors can better assess if patients received appropriate acupuncture for their headaches,” said Dr. Bickel, who was not involved with the research.
However, she noted that the acupuncture sites and techniques did not vary during the trial. Although that makes sense for a controlled study, it may not reflect real-world clinical practice, she added.
“The downside is that the study didn’t fully reflect that most acupuncturists in clinical practice would alter treatments during the 20 sessions based on the patient’s response and accompanying symptoms or comorbidities,” Dr. Bickel said.
The study also lacked information on medication overuse headache or patients’ prior history of TTH treatments.
“This could be helpful to understand which patients in clinical practice are most likely to benefit from treatment,” Dr. Bickel said.
Study authors received funding from the Department of Science and Technology of Sichuan Province and the National Natural Science Foundation of China. Dr. Li, Dr. Bickel, and Dr. Vickers report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
(TTH), new research suggests. Result of a randomized trial showed that though the majority of participants reported some relief from TTH after 8 weeks of acupuncture treatment, those who received needling at a depth of 12.5-20.0 mm reported the greatest reduction in headache frequency and severity.
At this depth, acupuncture promotes deqi sensation, a feeling of numbness, soreness, heaviness, or irritating pain in the needling site that is considered key to successful acupuncture treatment in traditional Chinese acupuncture theory.
“Our study showed that deqi sensation could enhance the effect of acupuncture in the treatment of chronic TTH, and the effect of acupuncture lasted at least 6 months when the treatment was stopped,” said co-investigator Ying Li, MD, PhD, The Third Hospital/Acupuncture and Tuina School, Chengdu University of Traditional Chinese Medicine, China.
The findings were published online in Neurology.
Deqi sensation key
TTH is the most common type of headache, with a lifetime prevalence of up to 78% in some studies. The pain is often described as throbbing or a vice-like tightness on both sides of the head. TTH is considered chronic when it occurs at least 15 days a month.
Previous studies have suggested that acupuncture can offer relief from headache pain, but specific information on TTH, especially chronic TTH, has been lacking.
To address the issue, researchers designed a parallel-design, patient-and-assessor blinded randomized controlled trial with 218 individuals with a history of chronic TTH. All were untreated with prophylactic treatment in the previous 3 months.
The treatment group (n = 110) received 20 sessions of true acupuncture (TA) over 8 weeks. This included three sessions per week in the first 4 weeks and two sessions per week in the last 4 weeks. The depth of needling at each point ranged from 12.5 to 20 mm, which is needed to achieve deqi sensation.
The control group (n = 108) received superficial acupuncture (SA) on the same schedule as the TA group and at traditional acupuncture points. However, this was done at a maximum depth of 2 mm, which is not deep enough for deqi sensation.
At week 16, 68.2% of the participants receiving TA reported a greater than 50% reduction in monthly headache days, compared with 48.1% of those receiving SA (odds ratio, 2.65; P < .001).
Mean monthly headache days decreased from 20.38 days at baseline to 7.48 days at week 32 in the TA group versus 22.6 days at baseline to 11.94 days in the SA group.
Headache intensity and severity decreased in both groups, although those who achieved deqi sensation reported the most improvement.
Only four patients reported adverse effects, all of which were mild and none requiring treatment.
Patients in both groups reported some pain relief, suggesting that those who are not comfortable with deqi sensation may still benefit from superficial acupuncture, although to a lesser extent, Dr. Li said.
“We assume that the point-specific effect and placebo effect were combined to give the patients relief of headaches,” Dr. Li added. “Further, the effect of deqi sensation added more treatment effect. This might be explained by gate-control theory or other unknown mechanisms.”
Deeper understanding?
Commenting on the research, Jennifer Bickel, MD, a senior member of neurology at Moffit Cancer Center and professor of oncologic sciences at University of South Florida, Tampa, said that the study provides a deeper understanding of acupuncture’s efficacy for chronic TTH, which could aid clinicians who are unfamiliar with the therapy or when and how to refer treatment.
“This study provides a more descriptive outline for what type of acupuncture treatment and duration can be effective for patients so doctors can prep patients on what to expect and so doctors can better assess if patients received appropriate acupuncture for their headaches,” said Dr. Bickel, who was not involved with the research.
However, she noted that the acupuncture sites and techniques did not vary during the trial. Although that makes sense for a controlled study, it may not reflect real-world clinical practice, she added.
“The downside is that the study didn’t fully reflect that most acupuncturists in clinical practice would alter treatments during the 20 sessions based on the patient’s response and accompanying symptoms or comorbidities,” Dr. Bickel said.
The study also lacked information on medication overuse headache or patients’ prior history of TTH treatments.
“This could be helpful to understand which patients in clinical practice are most likely to benefit from treatment,” Dr. Bickel said.
Study authors received funding from the Department of Science and Technology of Sichuan Province and the National Natural Science Foundation of China. Dr. Li, Dr. Bickel, and Dr. Vickers report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NEUROLOGY