User login
A new and completely different pain medicine
This transcript has been edited for clarity.
When you stub your toe or get a paper cut on your finger, you feel the pain in that part of your body. It feels like the pain is coming from that place. But, of course, that’s not really what is happening. Pain doesn’t really happen in your toe or your finger. It happens in your brain.
It’s a game of telephone, really. The afferent nerve fiber detects the noxious stimulus, passing that signal to the second-order neuron in the dorsal root ganglia of the spinal cord, which runs it up to the thalamus to be passed to the third-order neuron which brings it to the cortex for localization and conscious perception. It’s not even a very good game of telephone. It takes about 100 ms for a pain signal to get from the hand to the brain – longer from the feet, given the greater distance. You see your foot hit the corner of the coffee table and have just enough time to think: “Oh no!” before the pain hits.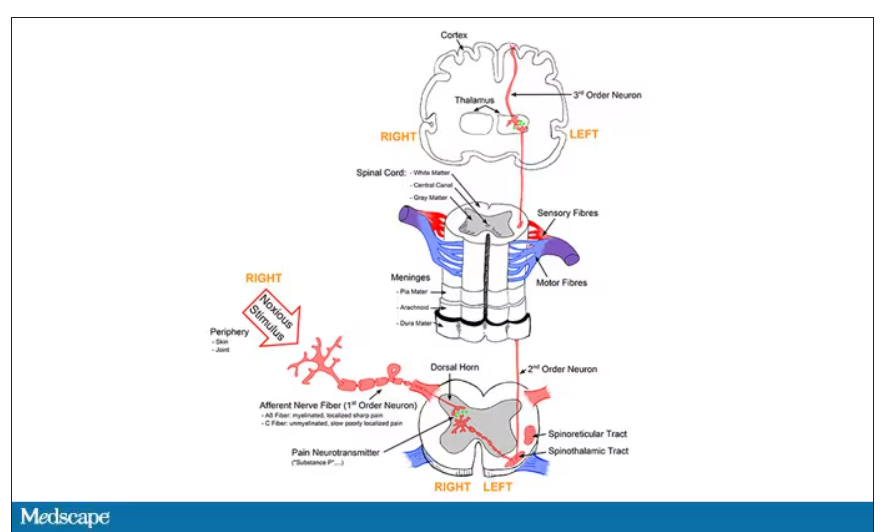
Given the Rube Goldberg nature of the process, it would seem like there are any number of places we could stop pain sensation. And sure, local anesthetics at the site of injury, or even spinal anesthetics, are powerful – if temporary and hard to administer – solutions to acute pain.
But in our everyday armamentarium, let’s be honest – we essentially have three options: opiates and opioids, which activate the mu-receptors in the brain to dull pain (and cause a host of other nasty side effects); NSAIDs, which block prostaglandin synthesis and thus limit the ability for pain-conducting neurons to get excited; and acetaminophen, which, despite being used for a century, is poorly understood.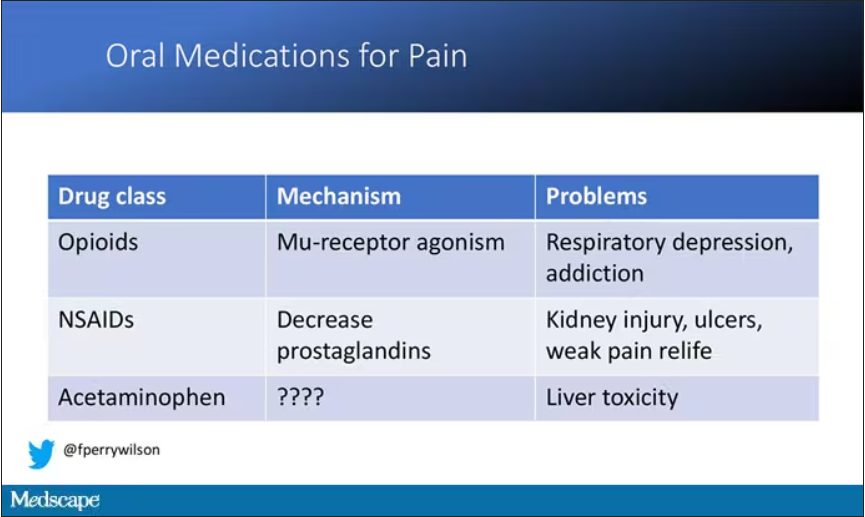
But
If you were to zoom in on the connection between that first afferent pain fiber and the secondary nerve in the spinal cord dorsal root ganglion, you would see a receptor called Nav1.8, a voltage-gated sodium channel.
This receptor is a key part of the apparatus that passes information from nerve 1 to nerve 2, but only for fibers that transmit pain signals. In fact, humans with mutations in this receptor that leave it always in the “open” state have a severe pain syndrome. Blocking the receptor, therefore, might reduce pain.
In preclinical work, researchers identified VX-548, which doesn’t have a brand name yet, as a potent blocker of that channel even in nanomolar concentrations. Importantly, the compound was highly selective for that particular channel – about 30,000 times more selective than it was for the other sodium channels in that family.
Of course, a highly selective and specific drug does not a blockbuster analgesic make. To determine how this drug would work on humans in pain, they turned to two populations: 303 individuals undergoing abdominoplasty and 274 undergoing bunionectomy, as reported in a new paper in the New England Journal of Medicine.
I know this seems a bit random, but abdominoplasty is quite painful and a good model for soft-tissue pain. Bunionectomy is also quite a painful procedure and a useful model of bone pain. After the surgeries, patients were randomized to several different doses of VX-548, hydrocodone plus acetaminophen, or placebo for 48 hours.
At 19 time points over that 48-hour period, participants were asked to rate their pain on a scale from 0 to 10. The primary outcome was the cumulative pain experienced over the 48 hours. So, higher pain would be worse here, but longer duration of pain would also be worse.
The story of the study is really told in this chart.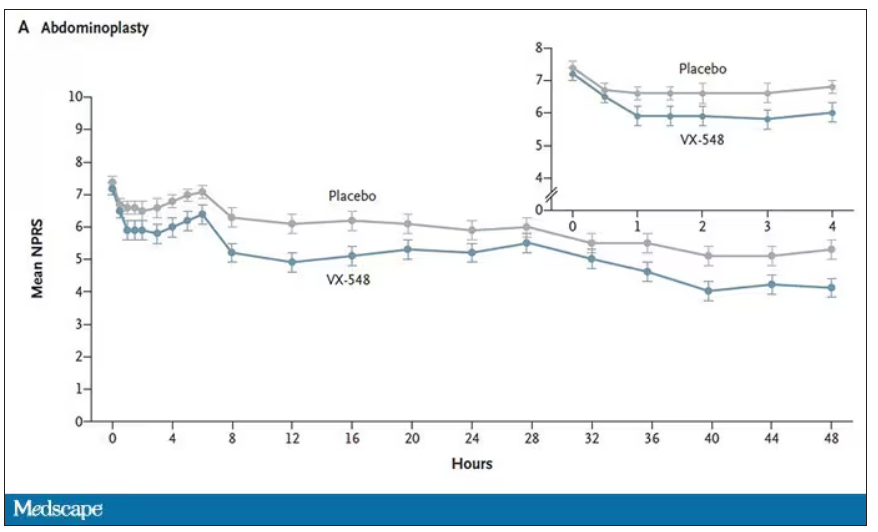
Yes, those assigned to the highest dose of VX-548 had a statistically significant lower cumulative amount of pain in the 48 hours after surgery. But the picture is really worth more than the stats here. You can see that the onset of pain relief was fairly quick, and that pain relief was sustained over time. You can also see that this is not a miracle drug. Pain scores were a bit better 48 hours out, but only by about a point and a half.
Placebo isn’t really the fair comparison here; few of us treat our postabdominoplasty patients with placebo, after all. The authors do not formally compare the effect of VX-548 with that of the opioid hydrocodone, for instance. But that doesn’t stop us.
This graph, which I put together from data in the paper, shows pain control across the four randomization categories, with higher numbers indicating more (cumulative) control. While all the active agents do a bit better than placebo, VX-548 at the higher dose appears to do the best. But I should note that 5 mg of hydrocodone may not be an adequate dose for most people.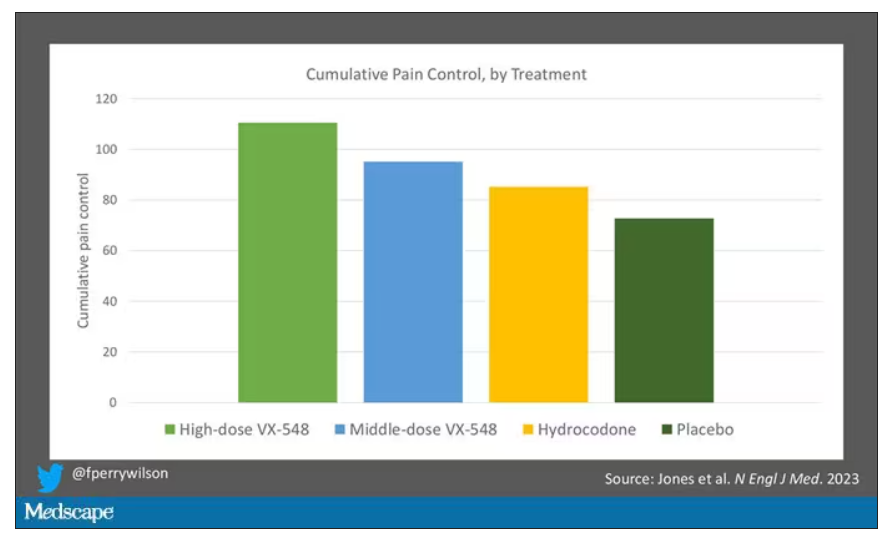
Yes, I would really have killed for an NSAID arm in this trial. Its absence, given that NSAIDs are a staple of postoperative care, is ... well, let’s just say, notable.
Although not a pain-destroying machine, VX-548 has some other things to recommend it. The receptor is really not found in the brain at all, which suggests that the drug should not carry much risk for dependency, though that has not been formally studied.
The side effects were generally mild – headache was the most common – and less prevalent than what you see even in the placebo arm.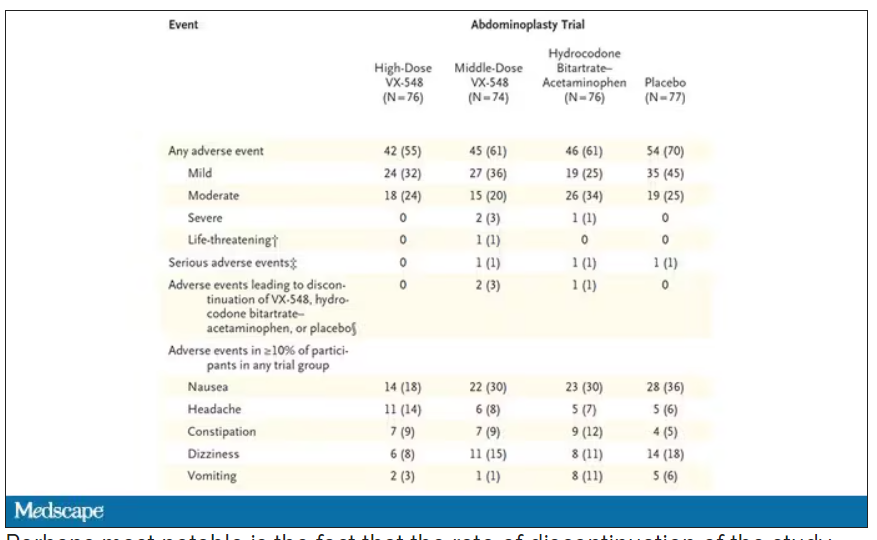
Perhaps most notable is the fact that the rate of discontinuation of the study drug was lowest in the VX-548 arm. Patients could stop taking the pill they were assigned for any reason, ranging from perceived lack of efficacy to side effects. A low discontinuation rate indicates to me a sort of “voting with your feet” that suggests this might be a well-tolerated and reasonably effective drug.
VX-548 isn’t on the market yet; phase 3 trials are ongoing. But whether it is this particular drug or another in this class, I’m happy to see researchers trying to find new ways to target that most primeval form of suffering: pain.
Dr. Wilson is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator, New Haven, Conn. He disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
When you stub your toe or get a paper cut on your finger, you feel the pain in that part of your body. It feels like the pain is coming from that place. But, of course, that’s not really what is happening. Pain doesn’t really happen in your toe or your finger. It happens in your brain.
It’s a game of telephone, really. The afferent nerve fiber detects the noxious stimulus, passing that signal to the second-order neuron in the dorsal root ganglia of the spinal cord, which runs it up to the thalamus to be passed to the third-order neuron which brings it to the cortex for localization and conscious perception. It’s not even a very good game of telephone. It takes about 100 ms for a pain signal to get from the hand to the brain – longer from the feet, given the greater distance. You see your foot hit the corner of the coffee table and have just enough time to think: “Oh no!” before the pain hits.
Given the Rube Goldberg nature of the process, it would seem like there are any number of places we could stop pain sensation. And sure, local anesthetics at the site of injury, or even spinal anesthetics, are powerful – if temporary and hard to administer – solutions to acute pain.
But in our everyday armamentarium, let’s be honest – we essentially have three options: opiates and opioids, which activate the mu-receptors in the brain to dull pain (and cause a host of other nasty side effects); NSAIDs, which block prostaglandin synthesis and thus limit the ability for pain-conducting neurons to get excited; and acetaminophen, which, despite being used for a century, is poorly understood.
But
If you were to zoom in on the connection between that first afferent pain fiber and the secondary nerve in the spinal cord dorsal root ganglion, you would see a receptor called Nav1.8, a voltage-gated sodium channel.
This receptor is a key part of the apparatus that passes information from nerve 1 to nerve 2, but only for fibers that transmit pain signals. In fact, humans with mutations in this receptor that leave it always in the “open” state have a severe pain syndrome. Blocking the receptor, therefore, might reduce pain.
In preclinical work, researchers identified VX-548, which doesn’t have a brand name yet, as a potent blocker of that channel even in nanomolar concentrations. Importantly, the compound was highly selective for that particular channel – about 30,000 times more selective than it was for the other sodium channels in that family.
Of course, a highly selective and specific drug does not a blockbuster analgesic make. To determine how this drug would work on humans in pain, they turned to two populations: 303 individuals undergoing abdominoplasty and 274 undergoing bunionectomy, as reported in a new paper in the New England Journal of Medicine.
I know this seems a bit random, but abdominoplasty is quite painful and a good model for soft-tissue pain. Bunionectomy is also quite a painful procedure and a useful model of bone pain. After the surgeries, patients were randomized to several different doses of VX-548, hydrocodone plus acetaminophen, or placebo for 48 hours.
At 19 time points over that 48-hour period, participants were asked to rate their pain on a scale from 0 to 10. The primary outcome was the cumulative pain experienced over the 48 hours. So, higher pain would be worse here, but longer duration of pain would also be worse.
The story of the study is really told in this chart.
Yes, those assigned to the highest dose of VX-548 had a statistically significant lower cumulative amount of pain in the 48 hours after surgery. But the picture is really worth more than the stats here. You can see that the onset of pain relief was fairly quick, and that pain relief was sustained over time. You can also see that this is not a miracle drug. Pain scores were a bit better 48 hours out, but only by about a point and a half.
Placebo isn’t really the fair comparison here; few of us treat our postabdominoplasty patients with placebo, after all. The authors do not formally compare the effect of VX-548 with that of the opioid hydrocodone, for instance. But that doesn’t stop us.
This graph, which I put together from data in the paper, shows pain control across the four randomization categories, with higher numbers indicating more (cumulative) control. While all the active agents do a bit better than placebo, VX-548 at the higher dose appears to do the best. But I should note that 5 mg of hydrocodone may not be an adequate dose for most people.
Yes, I would really have killed for an NSAID arm in this trial. Its absence, given that NSAIDs are a staple of postoperative care, is ... well, let’s just say, notable.
Although not a pain-destroying machine, VX-548 has some other things to recommend it. The receptor is really not found in the brain at all, which suggests that the drug should not carry much risk for dependency, though that has not been formally studied.
The side effects were generally mild – headache was the most common – and less prevalent than what you see even in the placebo arm.
Perhaps most notable is the fact that the rate of discontinuation of the study drug was lowest in the VX-548 arm. Patients could stop taking the pill they were assigned for any reason, ranging from perceived lack of efficacy to side effects. A low discontinuation rate indicates to me a sort of “voting with your feet” that suggests this might be a well-tolerated and reasonably effective drug.
VX-548 isn’t on the market yet; phase 3 trials are ongoing. But whether it is this particular drug or another in this class, I’m happy to see researchers trying to find new ways to target that most primeval form of suffering: pain.
Dr. Wilson is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator, New Haven, Conn. He disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
When you stub your toe or get a paper cut on your finger, you feel the pain in that part of your body. It feels like the pain is coming from that place. But, of course, that’s not really what is happening. Pain doesn’t really happen in your toe or your finger. It happens in your brain.
It’s a game of telephone, really. The afferent nerve fiber detects the noxious stimulus, passing that signal to the second-order neuron in the dorsal root ganglia of the spinal cord, which runs it up to the thalamus to be passed to the third-order neuron which brings it to the cortex for localization and conscious perception. It’s not even a very good game of telephone. It takes about 100 ms for a pain signal to get from the hand to the brain – longer from the feet, given the greater distance. You see your foot hit the corner of the coffee table and have just enough time to think: “Oh no!” before the pain hits.
Given the Rube Goldberg nature of the process, it would seem like there are any number of places we could stop pain sensation. And sure, local anesthetics at the site of injury, or even spinal anesthetics, are powerful – if temporary and hard to administer – solutions to acute pain.
But in our everyday armamentarium, let’s be honest – we essentially have three options: opiates and opioids, which activate the mu-receptors in the brain to dull pain (and cause a host of other nasty side effects); NSAIDs, which block prostaglandin synthesis and thus limit the ability for pain-conducting neurons to get excited; and acetaminophen, which, despite being used for a century, is poorly understood.
But
If you were to zoom in on the connection between that first afferent pain fiber and the secondary nerve in the spinal cord dorsal root ganglion, you would see a receptor called Nav1.8, a voltage-gated sodium channel.
This receptor is a key part of the apparatus that passes information from nerve 1 to nerve 2, but only for fibers that transmit pain signals. In fact, humans with mutations in this receptor that leave it always in the “open” state have a severe pain syndrome. Blocking the receptor, therefore, might reduce pain.
In preclinical work, researchers identified VX-548, which doesn’t have a brand name yet, as a potent blocker of that channel even in nanomolar concentrations. Importantly, the compound was highly selective for that particular channel – about 30,000 times more selective than it was for the other sodium channels in that family.
Of course, a highly selective and specific drug does not a blockbuster analgesic make. To determine how this drug would work on humans in pain, they turned to two populations: 303 individuals undergoing abdominoplasty and 274 undergoing bunionectomy, as reported in a new paper in the New England Journal of Medicine.
I know this seems a bit random, but abdominoplasty is quite painful and a good model for soft-tissue pain. Bunionectomy is also quite a painful procedure and a useful model of bone pain. After the surgeries, patients were randomized to several different doses of VX-548, hydrocodone plus acetaminophen, or placebo for 48 hours.
At 19 time points over that 48-hour period, participants were asked to rate their pain on a scale from 0 to 10. The primary outcome was the cumulative pain experienced over the 48 hours. So, higher pain would be worse here, but longer duration of pain would also be worse.
The story of the study is really told in this chart.
Yes, those assigned to the highest dose of VX-548 had a statistically significant lower cumulative amount of pain in the 48 hours after surgery. But the picture is really worth more than the stats here. You can see that the onset of pain relief was fairly quick, and that pain relief was sustained over time. You can also see that this is not a miracle drug. Pain scores were a bit better 48 hours out, but only by about a point and a half.
Placebo isn’t really the fair comparison here; few of us treat our postabdominoplasty patients with placebo, after all. The authors do not formally compare the effect of VX-548 with that of the opioid hydrocodone, for instance. But that doesn’t stop us.
This graph, which I put together from data in the paper, shows pain control across the four randomization categories, with higher numbers indicating more (cumulative) control. While all the active agents do a bit better than placebo, VX-548 at the higher dose appears to do the best. But I should note that 5 mg of hydrocodone may not be an adequate dose for most people.
Yes, I would really have killed for an NSAID arm in this trial. Its absence, given that NSAIDs are a staple of postoperative care, is ... well, let’s just say, notable.
Although not a pain-destroying machine, VX-548 has some other things to recommend it. The receptor is really not found in the brain at all, which suggests that the drug should not carry much risk for dependency, though that has not been formally studied.
The side effects were generally mild – headache was the most common – and less prevalent than what you see even in the placebo arm.
Perhaps most notable is the fact that the rate of discontinuation of the study drug was lowest in the VX-548 arm. Patients could stop taking the pill they were assigned for any reason, ranging from perceived lack of efficacy to side effects. A low discontinuation rate indicates to me a sort of “voting with your feet” that suggests this might be a well-tolerated and reasonably effective drug.
VX-548 isn’t on the market yet; phase 3 trials are ongoing. But whether it is this particular drug or another in this class, I’m happy to see researchers trying to find new ways to target that most primeval form of suffering: pain.
Dr. Wilson is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator, New Haven, Conn. He disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Will this trial help solve chronic back pain?
Chronic pain, and back pain in particular, is among the most frequent concerns for patients in the primary care setting. Roughly 8% of adults in the United States say they suffer from chronic low back pain, and many of them say the pain is significant enough to impair their ability to move, work, and otherwise enjoy life. All this, despite decades of research and countless millions in funding to find the optimal approach to treating chronic pain.
As the United States crawls out of the opioid epidemic, a group of pain specialists is hoping to identify effective, personalized approaches to managing back pain. Daniel Clauw, MD, professor of anesthesiology, internal medicine, and psychiatry at the University of Michigan, Ann Arbor, is helping lead the BEST trial. With projected enrollment of nearly 800 patients, BEST will be the largest federally funded clinical trial of interventions to treat chronic low back pain.
In an interview, The interview has been edited for length and clarity.
What are your thoughts on the current state of primary care physicians’ understanding and management of pain?
Primary care physicians need a lot of help in demystifying the diagnosis and treatment of any kind of pain, but back pain is a really good place to start. When it comes to back pain, most primary care physicians are not any more knowledgeable than a layperson.
What has the opioid debacle-cum-tragedy taught you about pain management, particular as regards people with chronic pain?
I don’t feel opioids should ever be used to treat chronic low back pain. The few long-term studies that have been performed using opioids for longer than 3 months suggest that they often make pain worse rather than just failing to make pain better – and we know they are associated with a significantly increased all-cause mortality with increased deaths from myocardial infarction, accidents, and suicides, in addition to overdose.
Given how many patients experience back pain, how did we come to the point at which primary care physicians are so ill equipped?
We’ve had terrible pain curricula in medical schools. To give you an example: I’m one of the leading pain experts in the world and I’m not allowed to teach our medical students their pain curriculum. The students learn about neurophysiology and the anatomy of the nerves, not what’s relevant in pain.
This is notorious in medical school: Curricula are almost impossible to modify and change. So it starts with poor training in medical school. And then, regardless of what education they do or don’t get in medical school, a lot of their education about pain management is through our residencies – mainly in inpatient settings, where you’re really seeing the management of acute pain and not the management of chronic pain.
People get more accustomed to managing acute pain, where opioids are a reasonable option. It’s just that when you start managing subacute or chronic pain, opioids don’t work as well.
The other big problem is that historically, most people trained in medicine think that if you have pain in your elbow, there’s got to be something wrong in your elbow. This third mechanism of pain, central sensitization – or nociplastic pain – the kind of pain that we see in fibromyalgia, headache, and low back pain, where the pain is coming from the brain – that’s confusing to people. People can have pain without any damage or inflammation to that region of the body.
Physicians are trained that if there’s pain, there’s something wrong and we have to do surgery or there’s been some trauma. Most chronic pain is none of that. There’s a big disconnect between how people are trained, and then when they go out and are seeing a tremendous number of people with chronic pain.
What are the different types of pain, and how should they inform clinicians’ understanding about what approaches might work for managing their patients in pain?
The way the central nervous system responds to pain is analogous to the loudness of an electric guitar. You can make an electric guitar louder either by strumming the strings harder or by turning up the amplifier. For many people with fibromyalgia, low back pain, and endometriosis, for example, the problem is really more that the amplifier is turned up too high rather than its being that the guitar is strummed too strongly. That kind of pain where the pain is not due to anatomic damage or inflammation is particularly flummoxing for providers.
Can you explain the design of the new study?
It’s a 13-site study looking at four treatments: enhanced self-care, cognitive-behavioral therapy, physical therapy, and duloxetine. It’s a big precision medicine trial, trying to take everything we’ve learned and putting it all into one big study.
We’re using a SMART design, which randomizes people to two of those treatments, unless they are very much improved from the first treatment. To be eligible for the trial, you have to be able to be randomized to three of the four treatments, and people can’t choose which of the four they get.
We give them one of those treatments for 12 weeks, and at the end of 12 weeks we make the call – “Did you respond or not respond?” – and then we go back to the phenotypic data we collected at the beginning of that trial and say, “What information at baseline that we collected predicts that someone is going to respond better to duloxetine or worse to duloxetine?” And then we create the phenotype that responds best to each of those four treatments.
None of our treatments works so well that someone doesn’t end up getting randomized to a second treatment. About 85% of people so far need a second treatment because they still have enough pain that they want more relief. But the nice thing about that is we’ve already done all the functional brain imaging and all these really expensive and time-consuming things.
We’re hoping to have around 700-800 people total in this trial, which means that around 170 people will get randomized to each of the four initial treatments. No one’s ever done a study that has functional brain imaging and all these other things in it with more than 80 or 100 people. The scale of this is totally unprecedented.
Given that the individual therapies don’t appear to be all that successful on their own, what is your goal?
The primary aim is to match the phenotypic characteristics of a patient with chronic low back pain with treatment response to each of these four treatments. So at the end, we can give clinicians information on which of the patients is going to respond to physical therapy, for instance.
Right now, about one out of three people respond to most treatments for pain. We think by doing a trial like this, we can take treatments that work in one out of three people and make them work in one out of two or two out of three people just by using them in the right people.
How do you differentiate between these types of pain in your study?
We phenotype people by asking them a number of questions. We also do brain imaging, look at their back with MRI, test biomechanics, and then give them four different treatments that we know work in groups of people with low back pain.
We think one of the first parts of the phenotype is, do they have pain just in their back? Or do they have pain in their back plus a lot of other body regions? Because the more body regions that people have pain in, the more likely it is that this is an amplifier problem rather than a guitar problem.
Treatments like physical therapy, surgery, and injections are going to work better for people in whom the pain is a guitar problem rather than an amplifier problem. And drugs like duloxetine, which works in the brain, and cognitive-behavioral therapy are going to work a lot better in the people with pain in multiple sites besides the back.
To pick up on your metaphor, do any symptoms help clinicians differentiate between the guitar and the amplifier?
Sleep problems, fatigue, memory problems, and mood problems are common in patients with chronic pain and are more common with amplifier pain. Because again, those are all central nervous system problems. And so we see that the people that have anxiety, depression, and a lot of distress are more likely to have this kind of pain.
Does medical imaging help?
There’s a terrible relationship between what you see on an MRI of the back and whether someone has pain or how severe the pain is going to be. There’s always going to be individuals that have a lot of anatomic damage who don’t have any pain because they happen to be on the other end of the continuum from fibromyalgia; they’re actually pain-insensitive people.
What are your thoughts about ketamine as a possible treatment for chronic pain?
I have a mentee who’s doing a ketamine trial. We’re doing psilocybin trials in patients with fibromyalgia. Ketamine is such a dirty drug; it has so many different mechanisms of action. It does have some psychedelic effects, but it also is an NMDA blocker. It really has so many different effects.
I think it’s being thrown around like water in settings where we don’t yet know it to be efficacious. Even the data in treatment-refractory depression are pretty weak, but we’re so desperate to do something for those patients. If you’re trying to harness the psychedelic properties of ketamine, I think there’s other psychedelics that are a lot more interesting, which is why we’re using psilocybin for a subset of patients. Most of us in the pain field think that the psychedelics will work best for the people with chronic pain who have a lot of comorbid psychiatric illness, especially the ones with a lot of trauma. These drugs will allow us therapeutically to get at a lot of these patients with the side-by-side psychotherapy that’s being done as people are getting care in the medicalized setting.
Dr. Clauw reported conflicts of interest with Pfizer, Tonix, Theravance, Zynerba, Samumed, Aptinyx, Daiichi Sankyo, Intec, Regeneron, Teva, Lundbeck, Virios, and Cerephex.
A version of this article first appeared on Medscape.com.
Chronic pain, and back pain in particular, is among the most frequent concerns for patients in the primary care setting. Roughly 8% of adults in the United States say they suffer from chronic low back pain, and many of them say the pain is significant enough to impair their ability to move, work, and otherwise enjoy life. All this, despite decades of research and countless millions in funding to find the optimal approach to treating chronic pain.
As the United States crawls out of the opioid epidemic, a group of pain specialists is hoping to identify effective, personalized approaches to managing back pain. Daniel Clauw, MD, professor of anesthesiology, internal medicine, and psychiatry at the University of Michigan, Ann Arbor, is helping lead the BEST trial. With projected enrollment of nearly 800 patients, BEST will be the largest federally funded clinical trial of interventions to treat chronic low back pain.
In an interview, The interview has been edited for length and clarity.
What are your thoughts on the current state of primary care physicians’ understanding and management of pain?
Primary care physicians need a lot of help in demystifying the diagnosis and treatment of any kind of pain, but back pain is a really good place to start. When it comes to back pain, most primary care physicians are not any more knowledgeable than a layperson.
What has the opioid debacle-cum-tragedy taught you about pain management, particular as regards people with chronic pain?
I don’t feel opioids should ever be used to treat chronic low back pain. The few long-term studies that have been performed using opioids for longer than 3 months suggest that they often make pain worse rather than just failing to make pain better – and we know they are associated with a significantly increased all-cause mortality with increased deaths from myocardial infarction, accidents, and suicides, in addition to overdose.
Given how many patients experience back pain, how did we come to the point at which primary care physicians are so ill equipped?
We’ve had terrible pain curricula in medical schools. To give you an example: I’m one of the leading pain experts in the world and I’m not allowed to teach our medical students their pain curriculum. The students learn about neurophysiology and the anatomy of the nerves, not what’s relevant in pain.
This is notorious in medical school: Curricula are almost impossible to modify and change. So it starts with poor training in medical school. And then, regardless of what education they do or don’t get in medical school, a lot of their education about pain management is through our residencies – mainly in inpatient settings, where you’re really seeing the management of acute pain and not the management of chronic pain.
People get more accustomed to managing acute pain, where opioids are a reasonable option. It’s just that when you start managing subacute or chronic pain, opioids don’t work as well.
The other big problem is that historically, most people trained in medicine think that if you have pain in your elbow, there’s got to be something wrong in your elbow. This third mechanism of pain, central sensitization – or nociplastic pain – the kind of pain that we see in fibromyalgia, headache, and low back pain, where the pain is coming from the brain – that’s confusing to people. People can have pain without any damage or inflammation to that region of the body.
Physicians are trained that if there’s pain, there’s something wrong and we have to do surgery or there’s been some trauma. Most chronic pain is none of that. There’s a big disconnect between how people are trained, and then when they go out and are seeing a tremendous number of people with chronic pain.
What are the different types of pain, and how should they inform clinicians’ understanding about what approaches might work for managing their patients in pain?
The way the central nervous system responds to pain is analogous to the loudness of an electric guitar. You can make an electric guitar louder either by strumming the strings harder or by turning up the amplifier. For many people with fibromyalgia, low back pain, and endometriosis, for example, the problem is really more that the amplifier is turned up too high rather than its being that the guitar is strummed too strongly. That kind of pain where the pain is not due to anatomic damage or inflammation is particularly flummoxing for providers.
Can you explain the design of the new study?
It’s a 13-site study looking at four treatments: enhanced self-care, cognitive-behavioral therapy, physical therapy, and duloxetine. It’s a big precision medicine trial, trying to take everything we’ve learned and putting it all into one big study.
We’re using a SMART design, which randomizes people to two of those treatments, unless they are very much improved from the first treatment. To be eligible for the trial, you have to be able to be randomized to three of the four treatments, and people can’t choose which of the four they get.
We give them one of those treatments for 12 weeks, and at the end of 12 weeks we make the call – “Did you respond or not respond?” – and then we go back to the phenotypic data we collected at the beginning of that trial and say, “What information at baseline that we collected predicts that someone is going to respond better to duloxetine or worse to duloxetine?” And then we create the phenotype that responds best to each of those four treatments.
None of our treatments works so well that someone doesn’t end up getting randomized to a second treatment. About 85% of people so far need a second treatment because they still have enough pain that they want more relief. But the nice thing about that is we’ve already done all the functional brain imaging and all these really expensive and time-consuming things.
We’re hoping to have around 700-800 people total in this trial, which means that around 170 people will get randomized to each of the four initial treatments. No one’s ever done a study that has functional brain imaging and all these other things in it with more than 80 or 100 people. The scale of this is totally unprecedented.
Given that the individual therapies don’t appear to be all that successful on their own, what is your goal?
The primary aim is to match the phenotypic characteristics of a patient with chronic low back pain with treatment response to each of these four treatments. So at the end, we can give clinicians information on which of the patients is going to respond to physical therapy, for instance.
Right now, about one out of three people respond to most treatments for pain. We think by doing a trial like this, we can take treatments that work in one out of three people and make them work in one out of two or two out of three people just by using them in the right people.
How do you differentiate between these types of pain in your study?
We phenotype people by asking them a number of questions. We also do brain imaging, look at their back with MRI, test biomechanics, and then give them four different treatments that we know work in groups of people with low back pain.
We think one of the first parts of the phenotype is, do they have pain just in their back? Or do they have pain in their back plus a lot of other body regions? Because the more body regions that people have pain in, the more likely it is that this is an amplifier problem rather than a guitar problem.
Treatments like physical therapy, surgery, and injections are going to work better for people in whom the pain is a guitar problem rather than an amplifier problem. And drugs like duloxetine, which works in the brain, and cognitive-behavioral therapy are going to work a lot better in the people with pain in multiple sites besides the back.
To pick up on your metaphor, do any symptoms help clinicians differentiate between the guitar and the amplifier?
Sleep problems, fatigue, memory problems, and mood problems are common in patients with chronic pain and are more common with amplifier pain. Because again, those are all central nervous system problems. And so we see that the people that have anxiety, depression, and a lot of distress are more likely to have this kind of pain.
Does medical imaging help?
There’s a terrible relationship between what you see on an MRI of the back and whether someone has pain or how severe the pain is going to be. There’s always going to be individuals that have a lot of anatomic damage who don’t have any pain because they happen to be on the other end of the continuum from fibromyalgia; they’re actually pain-insensitive people.
What are your thoughts about ketamine as a possible treatment for chronic pain?
I have a mentee who’s doing a ketamine trial. We’re doing psilocybin trials in patients with fibromyalgia. Ketamine is such a dirty drug; it has so many different mechanisms of action. It does have some psychedelic effects, but it also is an NMDA blocker. It really has so many different effects.
I think it’s being thrown around like water in settings where we don’t yet know it to be efficacious. Even the data in treatment-refractory depression are pretty weak, but we’re so desperate to do something for those patients. If you’re trying to harness the psychedelic properties of ketamine, I think there’s other psychedelics that are a lot more interesting, which is why we’re using psilocybin for a subset of patients. Most of us in the pain field think that the psychedelics will work best for the people with chronic pain who have a lot of comorbid psychiatric illness, especially the ones with a lot of trauma. These drugs will allow us therapeutically to get at a lot of these patients with the side-by-side psychotherapy that’s being done as people are getting care in the medicalized setting.
Dr. Clauw reported conflicts of interest with Pfizer, Tonix, Theravance, Zynerba, Samumed, Aptinyx, Daiichi Sankyo, Intec, Regeneron, Teva, Lundbeck, Virios, and Cerephex.
A version of this article first appeared on Medscape.com.
Chronic pain, and back pain in particular, is among the most frequent concerns for patients in the primary care setting. Roughly 8% of adults in the United States say they suffer from chronic low back pain, and many of them say the pain is significant enough to impair their ability to move, work, and otherwise enjoy life. All this, despite decades of research and countless millions in funding to find the optimal approach to treating chronic pain.
As the United States crawls out of the opioid epidemic, a group of pain specialists is hoping to identify effective, personalized approaches to managing back pain. Daniel Clauw, MD, professor of anesthesiology, internal medicine, and psychiatry at the University of Michigan, Ann Arbor, is helping lead the BEST trial. With projected enrollment of nearly 800 patients, BEST will be the largest federally funded clinical trial of interventions to treat chronic low back pain.
In an interview, The interview has been edited for length and clarity.
What are your thoughts on the current state of primary care physicians’ understanding and management of pain?
Primary care physicians need a lot of help in demystifying the diagnosis and treatment of any kind of pain, but back pain is a really good place to start. When it comes to back pain, most primary care physicians are not any more knowledgeable than a layperson.
What has the opioid debacle-cum-tragedy taught you about pain management, particular as regards people with chronic pain?
I don’t feel opioids should ever be used to treat chronic low back pain. The few long-term studies that have been performed using opioids for longer than 3 months suggest that they often make pain worse rather than just failing to make pain better – and we know they are associated with a significantly increased all-cause mortality with increased deaths from myocardial infarction, accidents, and suicides, in addition to overdose.
Given how many patients experience back pain, how did we come to the point at which primary care physicians are so ill equipped?
We’ve had terrible pain curricula in medical schools. To give you an example: I’m one of the leading pain experts in the world and I’m not allowed to teach our medical students their pain curriculum. The students learn about neurophysiology and the anatomy of the nerves, not what’s relevant in pain.
This is notorious in medical school: Curricula are almost impossible to modify and change. So it starts with poor training in medical school. And then, regardless of what education they do or don’t get in medical school, a lot of their education about pain management is through our residencies – mainly in inpatient settings, where you’re really seeing the management of acute pain and not the management of chronic pain.
People get more accustomed to managing acute pain, where opioids are a reasonable option. It’s just that when you start managing subacute or chronic pain, opioids don’t work as well.
The other big problem is that historically, most people trained in medicine think that if you have pain in your elbow, there’s got to be something wrong in your elbow. This third mechanism of pain, central sensitization – or nociplastic pain – the kind of pain that we see in fibromyalgia, headache, and low back pain, where the pain is coming from the brain – that’s confusing to people. People can have pain without any damage or inflammation to that region of the body.
Physicians are trained that if there’s pain, there’s something wrong and we have to do surgery or there’s been some trauma. Most chronic pain is none of that. There’s a big disconnect between how people are trained, and then when they go out and are seeing a tremendous number of people with chronic pain.
What are the different types of pain, and how should they inform clinicians’ understanding about what approaches might work for managing their patients in pain?
The way the central nervous system responds to pain is analogous to the loudness of an electric guitar. You can make an electric guitar louder either by strumming the strings harder or by turning up the amplifier. For many people with fibromyalgia, low back pain, and endometriosis, for example, the problem is really more that the amplifier is turned up too high rather than its being that the guitar is strummed too strongly. That kind of pain where the pain is not due to anatomic damage or inflammation is particularly flummoxing for providers.
Can you explain the design of the new study?
It’s a 13-site study looking at four treatments: enhanced self-care, cognitive-behavioral therapy, physical therapy, and duloxetine. It’s a big precision medicine trial, trying to take everything we’ve learned and putting it all into one big study.
We’re using a SMART design, which randomizes people to two of those treatments, unless they are very much improved from the first treatment. To be eligible for the trial, you have to be able to be randomized to three of the four treatments, and people can’t choose which of the four they get.
We give them one of those treatments for 12 weeks, and at the end of 12 weeks we make the call – “Did you respond or not respond?” – and then we go back to the phenotypic data we collected at the beginning of that trial and say, “What information at baseline that we collected predicts that someone is going to respond better to duloxetine or worse to duloxetine?” And then we create the phenotype that responds best to each of those four treatments.
None of our treatments works so well that someone doesn’t end up getting randomized to a second treatment. About 85% of people so far need a second treatment because they still have enough pain that they want more relief. But the nice thing about that is we’ve already done all the functional brain imaging and all these really expensive and time-consuming things.
We’re hoping to have around 700-800 people total in this trial, which means that around 170 people will get randomized to each of the four initial treatments. No one’s ever done a study that has functional brain imaging and all these other things in it with more than 80 or 100 people. The scale of this is totally unprecedented.
Given that the individual therapies don’t appear to be all that successful on their own, what is your goal?
The primary aim is to match the phenotypic characteristics of a patient with chronic low back pain with treatment response to each of these four treatments. So at the end, we can give clinicians information on which of the patients is going to respond to physical therapy, for instance.
Right now, about one out of three people respond to most treatments for pain. We think by doing a trial like this, we can take treatments that work in one out of three people and make them work in one out of two or two out of three people just by using them in the right people.
How do you differentiate between these types of pain in your study?
We phenotype people by asking them a number of questions. We also do brain imaging, look at their back with MRI, test biomechanics, and then give them four different treatments that we know work in groups of people with low back pain.
We think one of the first parts of the phenotype is, do they have pain just in their back? Or do they have pain in their back plus a lot of other body regions? Because the more body regions that people have pain in, the more likely it is that this is an amplifier problem rather than a guitar problem.
Treatments like physical therapy, surgery, and injections are going to work better for people in whom the pain is a guitar problem rather than an amplifier problem. And drugs like duloxetine, which works in the brain, and cognitive-behavioral therapy are going to work a lot better in the people with pain in multiple sites besides the back.
To pick up on your metaphor, do any symptoms help clinicians differentiate between the guitar and the amplifier?
Sleep problems, fatigue, memory problems, and mood problems are common in patients with chronic pain and are more common with amplifier pain. Because again, those are all central nervous system problems. And so we see that the people that have anxiety, depression, and a lot of distress are more likely to have this kind of pain.
Does medical imaging help?
There’s a terrible relationship between what you see on an MRI of the back and whether someone has pain or how severe the pain is going to be. There’s always going to be individuals that have a lot of anatomic damage who don’t have any pain because they happen to be on the other end of the continuum from fibromyalgia; they’re actually pain-insensitive people.
What are your thoughts about ketamine as a possible treatment for chronic pain?
I have a mentee who’s doing a ketamine trial. We’re doing psilocybin trials in patients with fibromyalgia. Ketamine is such a dirty drug; it has so many different mechanisms of action. It does have some psychedelic effects, but it also is an NMDA blocker. It really has so many different effects.
I think it’s being thrown around like water in settings where we don’t yet know it to be efficacious. Even the data in treatment-refractory depression are pretty weak, but we’re so desperate to do something for those patients. If you’re trying to harness the psychedelic properties of ketamine, I think there’s other psychedelics that are a lot more interesting, which is why we’re using psilocybin for a subset of patients. Most of us in the pain field think that the psychedelics will work best for the people with chronic pain who have a lot of comorbid psychiatric illness, especially the ones with a lot of trauma. These drugs will allow us therapeutically to get at a lot of these patients with the side-by-side psychotherapy that’s being done as people are getting care in the medicalized setting.
Dr. Clauw reported conflicts of interest with Pfizer, Tonix, Theravance, Zynerba, Samumed, Aptinyx, Daiichi Sankyo, Intec, Regeneron, Teva, Lundbeck, Virios, and Cerephex.
A version of this article first appeared on Medscape.com.
Oxycodone tied to persistent use only after vaginal delivery
“In the last decade in Ontario, oxycodone surpassed codeine as the most commonly prescribed opioid postpartum for pain control,” Jonathan Zipursky, MD, PhD, of Sunnybrook Health Sciences Centre, ICES, Toronto, and the University of Toronto, said in an interview. “This likely had to do with concerns with codeine use during breastfeeding, many of which are unsubstantiated.
“We hypothesized that use of oxycodone would be associated with an increased risk of persistent postpartum opioid use,” he said. “However, we did not find this.”
Instead, other factors, such as the quantity of opioids initially prescribed, were probably more important risks, he said.
The team also was “a bit surprised” that oxycodone was associated with an increased risk of persistent use only among those who had a vaginal delivery, Dr. Zipursky added.
“Receipt of an opioid prescription after vaginal delivery is uncommon in Ontario. People who fill prescriptions for potent opioids, such as oxycodone, after vaginal delivery may have underlying characteristics that predispose them to chronic opioid use,” he suggested. “Some of these factors we were unable to assess using our data.”
The study was published online in the Canadian Medical Association Journal.
Oxycodone okay
The investigators analyzed data from 70,607 people (median age, 32) who filled an opioid prescription within 7 days of discharge from the hospital between 2012 and 2020. Two-thirds (69.8%) received oxycodone and one-third received (30.2%) codeine.
The median gestational age at delivery was 39 weeks, and 80% of participants had a cesarean delivery. The median opioid prescription duration was 3 days. The median opioid content per prescription was 150 morphine milligram equivalents (MMEs) among those prescribed oxycodone and 135 MMEs for codeine.
The main outcome was persistent opioid use. This was defined as one or more additional prescriptions for an opioid within 90 days of the first postpartum prescription and one or more additional prescriptions in the 91-365 days after.
Oxycodone receipt was not associated with persistent opioid use, compared with codeine (relative risk, 1.04).
However, in a secondary analysis by mode of delivery, an association was seen between a prescription for oxycodone and persistent use after vaginal (RR, 1.63), but not after cesarean (RR, 0.85), delivery.
Dr. Zipursky noted that the quantity of opioids prescribed in the initial postpartum prescription “is likely a more important modifiable risk factor for new persistent opioid use, rather than the type of opioid prescribed.”
For example, a prescription containing more than 225 MMEs (equivalent to about 30 tablets of 5 mg oxycodone and to 50 tablets of 30 mg codeine) was associated with a roughly twofold increased risk of persistent use, compared with less than 112.5 MMEs after both vaginal (odds ratio, 2.51) and cesarean (OR, 1.78) delivery.
Furthermore, a prescription duration of more than 7 days was also associated with a roughly twofold increased risk of persistent use, compared with a duration of 1-3 days after both vaginal (OR, 2.43) and cesarean (OR, 1.52) delivery.
Most risk factors for persistent opioid use – a history of mental illness, substance use disorder, and more maternal comorbidities (aggregated diagnosis groups > 10) – were consistent across modes of delivery.
“Awareness of modifiable factors associated with new, persistent opioid use may help clinicians tailor opioid prescribing while ensuring adequate analgesia after delivery,” Dr. Zipursky suggested.
Less is more
In a comment, Elaine Duryea, MD, assistant professor in the department of obstetrics and gynecology at UT Southwestern Medical Center and medical director of the Maternal-Fetal Medicine Clinic at Parkland Health and Hospital System, both in Dallas, said, “It is likely exposure to any opioid, rather than a specific opioid, that can promote continued use – that is, past the medically indicated period.”
Dr. Duryea was principal investigator of a study, published in the American Journal of Obstetrics and Gynecology, that showed a multimodal regimen that included scheduled nonsteroidal anti-inflammatory drugs and acetaminophen, with opioids used as needed, resulted in a decrease in opioid use while adequately controlling pain after cesarean delivery.
“It is important to understand how to appropriately tailor the amount of opioid given to patients at the time of hospital discharge after cesarean in order to treat pain effectively but not send patients home with more opioids than [are] really needed,” she said.
It is also important to “individualize prescribing practices and maximize the use of non-opioid medication to treat postpartum and postoperative pain. Opioids should be a last resort for breakthrough pain, not first-line therapy,” Dr. Duryea concluded.
The study was funded by a Canadian Institutes of Health Research project grant. Dr. Zipursky has received payments from private law firms for medicolegal opinions on the safety and effectiveness of analgesics, including opioids.
A version of this article first appeared on Medscape.com.
“In the last decade in Ontario, oxycodone surpassed codeine as the most commonly prescribed opioid postpartum for pain control,” Jonathan Zipursky, MD, PhD, of Sunnybrook Health Sciences Centre, ICES, Toronto, and the University of Toronto, said in an interview. “This likely had to do with concerns with codeine use during breastfeeding, many of which are unsubstantiated.
“We hypothesized that use of oxycodone would be associated with an increased risk of persistent postpartum opioid use,” he said. “However, we did not find this.”
Instead, other factors, such as the quantity of opioids initially prescribed, were probably more important risks, he said.
The team also was “a bit surprised” that oxycodone was associated with an increased risk of persistent use only among those who had a vaginal delivery, Dr. Zipursky added.
“Receipt of an opioid prescription after vaginal delivery is uncommon in Ontario. People who fill prescriptions for potent opioids, such as oxycodone, after vaginal delivery may have underlying characteristics that predispose them to chronic opioid use,” he suggested. “Some of these factors we were unable to assess using our data.”
The study was published online in the Canadian Medical Association Journal.
Oxycodone okay
The investigators analyzed data from 70,607 people (median age, 32) who filled an opioid prescription within 7 days of discharge from the hospital between 2012 and 2020. Two-thirds (69.8%) received oxycodone and one-third received (30.2%) codeine.
The median gestational age at delivery was 39 weeks, and 80% of participants had a cesarean delivery. The median opioid prescription duration was 3 days. The median opioid content per prescription was 150 morphine milligram equivalents (MMEs) among those prescribed oxycodone and 135 MMEs for codeine.
The main outcome was persistent opioid use. This was defined as one or more additional prescriptions for an opioid within 90 days of the first postpartum prescription and one or more additional prescriptions in the 91-365 days after.
Oxycodone receipt was not associated with persistent opioid use, compared with codeine (relative risk, 1.04).
However, in a secondary analysis by mode of delivery, an association was seen between a prescription for oxycodone and persistent use after vaginal (RR, 1.63), but not after cesarean (RR, 0.85), delivery.
Dr. Zipursky noted that the quantity of opioids prescribed in the initial postpartum prescription “is likely a more important modifiable risk factor for new persistent opioid use, rather than the type of opioid prescribed.”
For example, a prescription containing more than 225 MMEs (equivalent to about 30 tablets of 5 mg oxycodone and to 50 tablets of 30 mg codeine) was associated with a roughly twofold increased risk of persistent use, compared with less than 112.5 MMEs after both vaginal (odds ratio, 2.51) and cesarean (OR, 1.78) delivery.
Furthermore, a prescription duration of more than 7 days was also associated with a roughly twofold increased risk of persistent use, compared with a duration of 1-3 days after both vaginal (OR, 2.43) and cesarean (OR, 1.52) delivery.
Most risk factors for persistent opioid use – a history of mental illness, substance use disorder, and more maternal comorbidities (aggregated diagnosis groups > 10) – were consistent across modes of delivery.
“Awareness of modifiable factors associated with new, persistent opioid use may help clinicians tailor opioid prescribing while ensuring adequate analgesia after delivery,” Dr. Zipursky suggested.
Less is more
In a comment, Elaine Duryea, MD, assistant professor in the department of obstetrics and gynecology at UT Southwestern Medical Center and medical director of the Maternal-Fetal Medicine Clinic at Parkland Health and Hospital System, both in Dallas, said, “It is likely exposure to any opioid, rather than a specific opioid, that can promote continued use – that is, past the medically indicated period.”
Dr. Duryea was principal investigator of a study, published in the American Journal of Obstetrics and Gynecology, that showed a multimodal regimen that included scheduled nonsteroidal anti-inflammatory drugs and acetaminophen, with opioids used as needed, resulted in a decrease in opioid use while adequately controlling pain after cesarean delivery.
“It is important to understand how to appropriately tailor the amount of opioid given to patients at the time of hospital discharge after cesarean in order to treat pain effectively but not send patients home with more opioids than [are] really needed,” she said.
It is also important to “individualize prescribing practices and maximize the use of non-opioid medication to treat postpartum and postoperative pain. Opioids should be a last resort for breakthrough pain, not first-line therapy,” Dr. Duryea concluded.
The study was funded by a Canadian Institutes of Health Research project grant. Dr. Zipursky has received payments from private law firms for medicolegal opinions on the safety and effectiveness of analgesics, including opioids.
A version of this article first appeared on Medscape.com.
“In the last decade in Ontario, oxycodone surpassed codeine as the most commonly prescribed opioid postpartum for pain control,” Jonathan Zipursky, MD, PhD, of Sunnybrook Health Sciences Centre, ICES, Toronto, and the University of Toronto, said in an interview. “This likely had to do with concerns with codeine use during breastfeeding, many of which are unsubstantiated.
“We hypothesized that use of oxycodone would be associated with an increased risk of persistent postpartum opioid use,” he said. “However, we did not find this.”
Instead, other factors, such as the quantity of opioids initially prescribed, were probably more important risks, he said.
The team also was “a bit surprised” that oxycodone was associated with an increased risk of persistent use only among those who had a vaginal delivery, Dr. Zipursky added.
“Receipt of an opioid prescription after vaginal delivery is uncommon in Ontario. People who fill prescriptions for potent opioids, such as oxycodone, after vaginal delivery may have underlying characteristics that predispose them to chronic opioid use,” he suggested. “Some of these factors we were unable to assess using our data.”
The study was published online in the Canadian Medical Association Journal.
Oxycodone okay
The investigators analyzed data from 70,607 people (median age, 32) who filled an opioid prescription within 7 days of discharge from the hospital between 2012 and 2020. Two-thirds (69.8%) received oxycodone and one-third received (30.2%) codeine.
The median gestational age at delivery was 39 weeks, and 80% of participants had a cesarean delivery. The median opioid prescription duration was 3 days. The median opioid content per prescription was 150 morphine milligram equivalents (MMEs) among those prescribed oxycodone and 135 MMEs for codeine.
The main outcome was persistent opioid use. This was defined as one or more additional prescriptions for an opioid within 90 days of the first postpartum prescription and one or more additional prescriptions in the 91-365 days after.
Oxycodone receipt was not associated with persistent opioid use, compared with codeine (relative risk, 1.04).
However, in a secondary analysis by mode of delivery, an association was seen between a prescription for oxycodone and persistent use after vaginal (RR, 1.63), but not after cesarean (RR, 0.85), delivery.
Dr. Zipursky noted that the quantity of opioids prescribed in the initial postpartum prescription “is likely a more important modifiable risk factor for new persistent opioid use, rather than the type of opioid prescribed.”
For example, a prescription containing more than 225 MMEs (equivalent to about 30 tablets of 5 mg oxycodone and to 50 tablets of 30 mg codeine) was associated with a roughly twofold increased risk of persistent use, compared with less than 112.5 MMEs after both vaginal (odds ratio, 2.51) and cesarean (OR, 1.78) delivery.
Furthermore, a prescription duration of more than 7 days was also associated with a roughly twofold increased risk of persistent use, compared with a duration of 1-3 days after both vaginal (OR, 2.43) and cesarean (OR, 1.52) delivery.
Most risk factors for persistent opioid use – a history of mental illness, substance use disorder, and more maternal comorbidities (aggregated diagnosis groups > 10) – were consistent across modes of delivery.
“Awareness of modifiable factors associated with new, persistent opioid use may help clinicians tailor opioid prescribing while ensuring adequate analgesia after delivery,” Dr. Zipursky suggested.
Less is more
In a comment, Elaine Duryea, MD, assistant professor in the department of obstetrics and gynecology at UT Southwestern Medical Center and medical director of the Maternal-Fetal Medicine Clinic at Parkland Health and Hospital System, both in Dallas, said, “It is likely exposure to any opioid, rather than a specific opioid, that can promote continued use – that is, past the medically indicated period.”
Dr. Duryea was principal investigator of a study, published in the American Journal of Obstetrics and Gynecology, that showed a multimodal regimen that included scheduled nonsteroidal anti-inflammatory drugs and acetaminophen, with opioids used as needed, resulted in a decrease in opioid use while adequately controlling pain after cesarean delivery.
“It is important to understand how to appropriately tailor the amount of opioid given to patients at the time of hospital discharge after cesarean in order to treat pain effectively but not send patients home with more opioids than [are] really needed,” she said.
It is also important to “individualize prescribing practices and maximize the use of non-opioid medication to treat postpartum and postoperative pain. Opioids should be a last resort for breakthrough pain, not first-line therapy,” Dr. Duryea concluded.
The study was funded by a Canadian Institutes of Health Research project grant. Dr. Zipursky has received payments from private law firms for medicolegal opinions on the safety and effectiveness of analgesics, including opioids.
A version of this article first appeared on Medscape.com.
FROM THE CANADIAN MEDICAL ASSOCIATION JOURNAL
Innovations in pediatric chronic pain management
At the new Walnut Creek Clinic in the East Bay of the San Francisco Bay area, kids get a “Comfort Promise.”
The clinic extends the work of the Stad Center for Pediatric Pain, Palliative & Integrative Medicine beyond the locations in University of California San Francisco Benioff Children’s Hospitals in San Francisco and Oakland.
At Walnut Creek, clinical acupuncturists, massage therapists, and specialists in hypnosis complement advanced medical care with integrative techniques.
The “Comfort Promise” program, which is being rolled out at that clinic and other UCSF pediatric clinics through the end of 2024, is the clinicians’ pledge to do everything in their power to make tests, infusions, and vaccinations “practically pain free.”
Needle sticks, for example, can be a common source of pain and anxiety for kids. Techniques to minimize pain vary by age. Among the ways the clinicians minimize needle pain for a child 6- to 12-years-old are:
- Giving the child control options to pick which arm; and watch the injection, pause it, or stop it with a communication sign.
- Introducing memory shaping by asking the child about the experience afterward and presenting it in a positive way by praising the acts of sitting still, breathing deeply, or being brave.
- Using distractors such as asking the child to hold a favorite item from home, storytelling, coloring, singing, or using breathing exercises.
Stefan Friedrichsdorf, MD, chief of the UCSF division of pediatric pain, palliative & integrative medicine, said in a statement: “For kids with chronic pain, complex pain medications can cause more harm than benefit. Our goal is to combine exercise and physical therapy with integrative medicine and skills-based psychotherapy to help them become pain free in their everyday life.”
Bundling appointments for early impact
At Lurie Children’s Hospital of Chicago, the chronic pain treatment program bundles visits with experts in several disciplines, include social workers, psychologists, and physical therapists, in addition to the medical team, so that patients can complete a first round of visits with multiple specialists in a short period, as opposed to several months.
Natalie Weatherred, APRN-NP, CPNP-PC, a pediatric nurse practitioner in anesthesiology and the pain clinic coordinator, said in an interview that the up-front visits involve between four and eight follow-up sessions in a short period with everybody in the multidisciplinary team “to really help jump-start their pain treatment.”
She pointed out that many families come from distant parts of the state or beyond so the bundled appointments are also important for easing burden on families.
Sarah Duggan, APRN-NP, CPNP-PC, also a pediatric nurse practitioner in anesthesiology at Lurie’s, pointed out that patients at their clinic often have other chronic conditions as well, such as such as postural orthostatic tachycardia syndrome so the care integration is particularly important.
“We can get them the appropriate care that they need and the resources they need, much sooner than we would have been able to do 5 or 10 years ago,” Ms. Duggan said.
Virtual reality distraction instead of sedation
Henry Huang, MD, anesthesiologist and pain physician at Texas Children’s Hospital, Houston, said a special team there collaborates with the Chariot Program at Stanford (Calif.) University and incorporates virtual reality to distract children from pain and anxiety and harness their imaginations during induction for anesthesia, intravenous placement, and vaccinations.
“At our institution we’ve been recruiting patients to do a proof of concept to do virtual reality distraction for pain procedures, such as nerve blocks or steroid injections,” Dr. Huang said.
Traditionally, kids would have received oral or intravenous sedation to help them cope with the fear and pain.
“We’ve been successful in several cases without relying on any sedation,” he said. “The next target is to expand that to the chronic pain population.”
The distraction techniques are promising for a wide range of ages, he said, and the programming is tailored to the child’s ability to interact with the technology.
He said he is also part of a group promoting use of ultrasound instead of x-rays to guide injections to the spine and chest to reduce children’s exposure to radiation. His group is helping teach these methods to other clinicians nationally.
Dr. Huang said the most important development in chronic pediatric pain has been the growth of rehab centers that include the medical team, and practitioners from psychology as well as occupational and physical therapy.
“More and more hospitals are recognizing the importance of these pain rehab centers,” he said.
The problem, Dr. Huang said, is that these programs have always been resource intensive and involve highly specialized clinicians. The cost and the limited number of specialists make it difficult for widespread rollout.
“That’s always been the challenge from the pediatric pain world,” he said.
Recognizing the complexity of kids’ chronic pain
Angela Garcia, MD, a consulting physician for pediatric rehabilitation medicine at UPMC Children’s Hospital of Pittsburgh said
Techniques such as biofeedback and acupuncture are becoming more mainstream in pediatric chronic care, she said.
At the UPMC clinic, children and their families talk with a care team about their values and what they want to accomplish in managing the child’s pain. They ask what the pain is preventing the child from doing.
“Their goals really are our goals,” she said.
She said she also refers almost all patients to one of the center’s pain psychologists.
“Pain is biopsychosocial,” she said. “We want to make sure we’re addressing how to cope with pain.”
Dr. Garcia said she hopes nutritional therapy is one of the next approaches the clinic will incorporate, particularly surrounding how dietary changes can reduce inflammation “and heal the body from the inside out.”
She said the hospital is also looking at developing an inpatient pain program for kids whose functioning has changed so drastically that they need more intensive therapies.
Whatever the treatment approach, she said, addressing the pain early is critical.
“There is an increased risk of a child with chronic pain becoming an adult with chronic pain,” Dr. Garcia pointed out, “and that can lead to a decrease in the ability to participate in society.”
Ms. Weatherred, Ms. Duggan, Dr. Huang, and Dr. Garcia reported no relevant financial relationships.
At the new Walnut Creek Clinic in the East Bay of the San Francisco Bay area, kids get a “Comfort Promise.”
The clinic extends the work of the Stad Center for Pediatric Pain, Palliative & Integrative Medicine beyond the locations in University of California San Francisco Benioff Children’s Hospitals in San Francisco and Oakland.
At Walnut Creek, clinical acupuncturists, massage therapists, and specialists in hypnosis complement advanced medical care with integrative techniques.
The “Comfort Promise” program, which is being rolled out at that clinic and other UCSF pediatric clinics through the end of 2024, is the clinicians’ pledge to do everything in their power to make tests, infusions, and vaccinations “practically pain free.”
Needle sticks, for example, can be a common source of pain and anxiety for kids. Techniques to minimize pain vary by age. Among the ways the clinicians minimize needle pain for a child 6- to 12-years-old are:
- Giving the child control options to pick which arm; and watch the injection, pause it, or stop it with a communication sign.
- Introducing memory shaping by asking the child about the experience afterward and presenting it in a positive way by praising the acts of sitting still, breathing deeply, or being brave.
- Using distractors such as asking the child to hold a favorite item from home, storytelling, coloring, singing, or using breathing exercises.
Stefan Friedrichsdorf, MD, chief of the UCSF division of pediatric pain, palliative & integrative medicine, said in a statement: “For kids with chronic pain, complex pain medications can cause more harm than benefit. Our goal is to combine exercise and physical therapy with integrative medicine and skills-based psychotherapy to help them become pain free in their everyday life.”
Bundling appointments for early impact
At Lurie Children’s Hospital of Chicago, the chronic pain treatment program bundles visits with experts in several disciplines, include social workers, psychologists, and physical therapists, in addition to the medical team, so that patients can complete a first round of visits with multiple specialists in a short period, as opposed to several months.
Natalie Weatherred, APRN-NP, CPNP-PC, a pediatric nurse practitioner in anesthesiology and the pain clinic coordinator, said in an interview that the up-front visits involve between four and eight follow-up sessions in a short period with everybody in the multidisciplinary team “to really help jump-start their pain treatment.”
She pointed out that many families come from distant parts of the state or beyond so the bundled appointments are also important for easing burden on families.
Sarah Duggan, APRN-NP, CPNP-PC, also a pediatric nurse practitioner in anesthesiology at Lurie’s, pointed out that patients at their clinic often have other chronic conditions as well, such as such as postural orthostatic tachycardia syndrome so the care integration is particularly important.
“We can get them the appropriate care that they need and the resources they need, much sooner than we would have been able to do 5 or 10 years ago,” Ms. Duggan said.
Virtual reality distraction instead of sedation
Henry Huang, MD, anesthesiologist and pain physician at Texas Children’s Hospital, Houston, said a special team there collaborates with the Chariot Program at Stanford (Calif.) University and incorporates virtual reality to distract children from pain and anxiety and harness their imaginations during induction for anesthesia, intravenous placement, and vaccinations.
“At our institution we’ve been recruiting patients to do a proof of concept to do virtual reality distraction for pain procedures, such as nerve blocks or steroid injections,” Dr. Huang said.
Traditionally, kids would have received oral or intravenous sedation to help them cope with the fear and pain.
“We’ve been successful in several cases without relying on any sedation,” he said. “The next target is to expand that to the chronic pain population.”
The distraction techniques are promising for a wide range of ages, he said, and the programming is tailored to the child’s ability to interact with the technology.
He said he is also part of a group promoting use of ultrasound instead of x-rays to guide injections to the spine and chest to reduce children’s exposure to radiation. His group is helping teach these methods to other clinicians nationally.
Dr. Huang said the most important development in chronic pediatric pain has been the growth of rehab centers that include the medical team, and practitioners from psychology as well as occupational and physical therapy.
“More and more hospitals are recognizing the importance of these pain rehab centers,” he said.
The problem, Dr. Huang said, is that these programs have always been resource intensive and involve highly specialized clinicians. The cost and the limited number of specialists make it difficult for widespread rollout.
“That’s always been the challenge from the pediatric pain world,” he said.
Recognizing the complexity of kids’ chronic pain
Angela Garcia, MD, a consulting physician for pediatric rehabilitation medicine at UPMC Children’s Hospital of Pittsburgh said
Techniques such as biofeedback and acupuncture are becoming more mainstream in pediatric chronic care, she said.
At the UPMC clinic, children and their families talk with a care team about their values and what they want to accomplish in managing the child’s pain. They ask what the pain is preventing the child from doing.
“Their goals really are our goals,” she said.
She said she also refers almost all patients to one of the center’s pain psychologists.
“Pain is biopsychosocial,” she said. “We want to make sure we’re addressing how to cope with pain.”
Dr. Garcia said she hopes nutritional therapy is one of the next approaches the clinic will incorporate, particularly surrounding how dietary changes can reduce inflammation “and heal the body from the inside out.”
She said the hospital is also looking at developing an inpatient pain program for kids whose functioning has changed so drastically that they need more intensive therapies.
Whatever the treatment approach, she said, addressing the pain early is critical.
“There is an increased risk of a child with chronic pain becoming an adult with chronic pain,” Dr. Garcia pointed out, “and that can lead to a decrease in the ability to participate in society.”
Ms. Weatherred, Ms. Duggan, Dr. Huang, and Dr. Garcia reported no relevant financial relationships.
At the new Walnut Creek Clinic in the East Bay of the San Francisco Bay area, kids get a “Comfort Promise.”
The clinic extends the work of the Stad Center for Pediatric Pain, Palliative & Integrative Medicine beyond the locations in University of California San Francisco Benioff Children’s Hospitals in San Francisco and Oakland.
At Walnut Creek, clinical acupuncturists, massage therapists, and specialists in hypnosis complement advanced medical care with integrative techniques.
The “Comfort Promise” program, which is being rolled out at that clinic and other UCSF pediatric clinics through the end of 2024, is the clinicians’ pledge to do everything in their power to make tests, infusions, and vaccinations “practically pain free.”
Needle sticks, for example, can be a common source of pain and anxiety for kids. Techniques to minimize pain vary by age. Among the ways the clinicians minimize needle pain for a child 6- to 12-years-old are:
- Giving the child control options to pick which arm; and watch the injection, pause it, or stop it with a communication sign.
- Introducing memory shaping by asking the child about the experience afterward and presenting it in a positive way by praising the acts of sitting still, breathing deeply, or being brave.
- Using distractors such as asking the child to hold a favorite item from home, storytelling, coloring, singing, or using breathing exercises.
Stefan Friedrichsdorf, MD, chief of the UCSF division of pediatric pain, palliative & integrative medicine, said in a statement: “For kids with chronic pain, complex pain medications can cause more harm than benefit. Our goal is to combine exercise and physical therapy with integrative medicine and skills-based psychotherapy to help them become pain free in their everyday life.”
Bundling appointments for early impact
At Lurie Children’s Hospital of Chicago, the chronic pain treatment program bundles visits with experts in several disciplines, include social workers, psychologists, and physical therapists, in addition to the medical team, so that patients can complete a first round of visits with multiple specialists in a short period, as opposed to several months.
Natalie Weatherred, APRN-NP, CPNP-PC, a pediatric nurse practitioner in anesthesiology and the pain clinic coordinator, said in an interview that the up-front visits involve between four and eight follow-up sessions in a short period with everybody in the multidisciplinary team “to really help jump-start their pain treatment.”
She pointed out that many families come from distant parts of the state or beyond so the bundled appointments are also important for easing burden on families.
Sarah Duggan, APRN-NP, CPNP-PC, also a pediatric nurse practitioner in anesthesiology at Lurie’s, pointed out that patients at their clinic often have other chronic conditions as well, such as such as postural orthostatic tachycardia syndrome so the care integration is particularly important.
“We can get them the appropriate care that they need and the resources they need, much sooner than we would have been able to do 5 or 10 years ago,” Ms. Duggan said.
Virtual reality distraction instead of sedation
Henry Huang, MD, anesthesiologist and pain physician at Texas Children’s Hospital, Houston, said a special team there collaborates with the Chariot Program at Stanford (Calif.) University and incorporates virtual reality to distract children from pain and anxiety and harness their imaginations during induction for anesthesia, intravenous placement, and vaccinations.
“At our institution we’ve been recruiting patients to do a proof of concept to do virtual reality distraction for pain procedures, such as nerve blocks or steroid injections,” Dr. Huang said.
Traditionally, kids would have received oral or intravenous sedation to help them cope with the fear and pain.
“We’ve been successful in several cases without relying on any sedation,” he said. “The next target is to expand that to the chronic pain population.”
The distraction techniques are promising for a wide range of ages, he said, and the programming is tailored to the child’s ability to interact with the technology.
He said he is also part of a group promoting use of ultrasound instead of x-rays to guide injections to the spine and chest to reduce children’s exposure to radiation. His group is helping teach these methods to other clinicians nationally.
Dr. Huang said the most important development in chronic pediatric pain has been the growth of rehab centers that include the medical team, and practitioners from psychology as well as occupational and physical therapy.
“More and more hospitals are recognizing the importance of these pain rehab centers,” he said.
The problem, Dr. Huang said, is that these programs have always been resource intensive and involve highly specialized clinicians. The cost and the limited number of specialists make it difficult for widespread rollout.
“That’s always been the challenge from the pediatric pain world,” he said.
Recognizing the complexity of kids’ chronic pain
Angela Garcia, MD, a consulting physician for pediatric rehabilitation medicine at UPMC Children’s Hospital of Pittsburgh said
Techniques such as biofeedback and acupuncture are becoming more mainstream in pediatric chronic care, she said.
At the UPMC clinic, children and their families talk with a care team about their values and what they want to accomplish in managing the child’s pain. They ask what the pain is preventing the child from doing.
“Their goals really are our goals,” she said.
She said she also refers almost all patients to one of the center’s pain psychologists.
“Pain is biopsychosocial,” she said. “We want to make sure we’re addressing how to cope with pain.”
Dr. Garcia said she hopes nutritional therapy is one of the next approaches the clinic will incorporate, particularly surrounding how dietary changes can reduce inflammation “and heal the body from the inside out.”
She said the hospital is also looking at developing an inpatient pain program for kids whose functioning has changed so drastically that they need more intensive therapies.
Whatever the treatment approach, she said, addressing the pain early is critical.
“There is an increased risk of a child with chronic pain becoming an adult with chronic pain,” Dr. Garcia pointed out, “and that can lead to a decrease in the ability to participate in society.”
Ms. Weatherred, Ms. Duggan, Dr. Huang, and Dr. Garcia reported no relevant financial relationships.
Functional MRI shows that empathetic remarks reduce pain
These are the results of a study, recently published in the Proceedings of the National Academy of Sciences, that was conducted by a team led by neuroscientist Dan-Mikael Ellingsen, PhD, from Oslo University Hospital.
The researchers used functional MRI to scan the brains of 20 patients with chronic pain to investigate how a physician’s demeanor may affect patients’ sensitivity to pain, including effects in the central nervous system. During the scans, which were conducted in two sessions, the patients’ legs were exposed to stimuli that ranged from painless to moderately painful. The patients recorded perceived pain intensity using a scale. The physicians also underwent fMRI.
Half of the patients were subjected to the pain stimuli while alone; the other half were subjected to pain while in the presence of a physician. The latter group of patients was divided into two subgroups. Half of the patients had spoken to the accompanying physician before the examination. They discussed the history of the patient’s condition to date, among other things. The other half underwent the brain scans without any prior interaction with a physician.
Worse when alone
Dr. Ellingsen and his colleagues found that patients who were alone during the examination reported greater pain than those who were in the presence of a physician, even though they were subjected to stimuli of the same intensity. In instances in which the physician and patient had already spoken before the brain scan, patients additionally felt that the physician was empathetic and understood their pain. Furthermore, the physicians were better able to estimate the pain that their patients experienced.
The patients who had a physician by their side consistently experienced pain that was milder than the pain experienced by those who were alone. For pairs that had spoken beforehand, the patients considered their physician to be better able to understand their pain, and the physicians estimated the perceived pain intensity of their patients more accurately.
Evidence of trust
There was greater activity in the dorsolateral and ventrolateral prefrontal cortex, as well as in the primary and secondary somatosensory areas, in patients in the subgroup that had spoken to a physician. For the physicians, compared with the comparison group, there was an increase in correspondence between activity in the dorsolateral prefrontal cortex and activity in the secondary somatosensory areas of patients, which is a brain region that is known to react to pain. The brain activity correlation increased in line with the self-reported mutual trust between the physician and patient.
“These results prove that empathy and support can decrease pain intensity,” the investigators write. The data shed light on the brain processes behind the social modulation of pain during the interaction between the physician and the patient. Concordances in the brain are increased by greater therapeutic alliance.
Beyond medication
Winfried Meissner, MD, head of the pain clinic at the department of anesthesiology and intensive care medicine at Jena University Hospital, Germany, and former president of the German Pain Society, said in an interview: “I view this as a vital study that impressively demonstrates that effective, intensive pain therapy is not just a case of administering the correct analgesic.”
“Instead, a focus should be placed on what common sense tells us, which is just how crucial an empathetic attitude from physicians and good communication with patients are when it comes to the success of any therapy,” Dr. Meissner added. Unfortunately, such an attitude and such communication often are not provided in clinical practice because of limitations on time.
“Now, with objectively collected data from patients and physicians, [Dr.] Ellingsen’s team has been able to demonstrate that human interaction has a decisive impact on the treatment of patients experiencing pain,” said Dr. Meissner. “The study should encourage practitioners to treat communication just as seriously as the pharmacology of analgesics.”
Perception and attitude
“The study shows remarkably well that empathetic conversation between the physician and patient represents a valuable therapeutic method and should be recognized as such,” emphasized Dr. Meissner. Of course, conversation cannot replace pharmacologic treatment, but it can supplement and reinforce it. Furthermore, a physician’s empathy presumably has an effect that is at least as great as a suitable analgesic.
“Pain is more than just sensory perception,” explained Dr. Meissner. “We all know that it has a strong affective component, and perception is greatly determined by context.” This can be seen, for example, in athletes, who often attribute less importance to their pain and can successfully perform competitively despite a painful injury.
Positive expectations
Dr. Meissner advised all physicians to treat patients with pain empathetically. He encourages them to ask patients about their pain, accompanying symptoms, possible fears, and other mental stress and to take these factors seriously.
Moreover, the findings accentuate the effect of prescribed analgesics. “Numerous studies have meanwhile shown that the more positive a patient’s expectations, the better the effect of a medication,” said Dr. Meissner. “We physicians must exploit this effect, too.”
This article was translated from the Medscape German Edition and a version appeared on Medscape.com.
These are the results of a study, recently published in the Proceedings of the National Academy of Sciences, that was conducted by a team led by neuroscientist Dan-Mikael Ellingsen, PhD, from Oslo University Hospital.
The researchers used functional MRI to scan the brains of 20 patients with chronic pain to investigate how a physician’s demeanor may affect patients’ sensitivity to pain, including effects in the central nervous system. During the scans, which were conducted in two sessions, the patients’ legs were exposed to stimuli that ranged from painless to moderately painful. The patients recorded perceived pain intensity using a scale. The physicians also underwent fMRI.
Half of the patients were subjected to the pain stimuli while alone; the other half were subjected to pain while in the presence of a physician. The latter group of patients was divided into two subgroups. Half of the patients had spoken to the accompanying physician before the examination. They discussed the history of the patient’s condition to date, among other things. The other half underwent the brain scans without any prior interaction with a physician.
Worse when alone
Dr. Ellingsen and his colleagues found that patients who were alone during the examination reported greater pain than those who were in the presence of a physician, even though they were subjected to stimuli of the same intensity. In instances in which the physician and patient had already spoken before the brain scan, patients additionally felt that the physician was empathetic and understood their pain. Furthermore, the physicians were better able to estimate the pain that their patients experienced.
The patients who had a physician by their side consistently experienced pain that was milder than the pain experienced by those who were alone. For pairs that had spoken beforehand, the patients considered their physician to be better able to understand their pain, and the physicians estimated the perceived pain intensity of their patients more accurately.
Evidence of trust
There was greater activity in the dorsolateral and ventrolateral prefrontal cortex, as well as in the primary and secondary somatosensory areas, in patients in the subgroup that had spoken to a physician. For the physicians, compared with the comparison group, there was an increase in correspondence between activity in the dorsolateral prefrontal cortex and activity in the secondary somatosensory areas of patients, which is a brain region that is known to react to pain. The brain activity correlation increased in line with the self-reported mutual trust between the physician and patient.
“These results prove that empathy and support can decrease pain intensity,” the investigators write. The data shed light on the brain processes behind the social modulation of pain during the interaction between the physician and the patient. Concordances in the brain are increased by greater therapeutic alliance.
Beyond medication
Winfried Meissner, MD, head of the pain clinic at the department of anesthesiology and intensive care medicine at Jena University Hospital, Germany, and former president of the German Pain Society, said in an interview: “I view this as a vital study that impressively demonstrates that effective, intensive pain therapy is not just a case of administering the correct analgesic.”
“Instead, a focus should be placed on what common sense tells us, which is just how crucial an empathetic attitude from physicians and good communication with patients are when it comes to the success of any therapy,” Dr. Meissner added. Unfortunately, such an attitude and such communication often are not provided in clinical practice because of limitations on time.
“Now, with objectively collected data from patients and physicians, [Dr.] Ellingsen’s team has been able to demonstrate that human interaction has a decisive impact on the treatment of patients experiencing pain,” said Dr. Meissner. “The study should encourage practitioners to treat communication just as seriously as the pharmacology of analgesics.”
Perception and attitude
“The study shows remarkably well that empathetic conversation between the physician and patient represents a valuable therapeutic method and should be recognized as such,” emphasized Dr. Meissner. Of course, conversation cannot replace pharmacologic treatment, but it can supplement and reinforce it. Furthermore, a physician’s empathy presumably has an effect that is at least as great as a suitable analgesic.
“Pain is more than just sensory perception,” explained Dr. Meissner. “We all know that it has a strong affective component, and perception is greatly determined by context.” This can be seen, for example, in athletes, who often attribute less importance to their pain and can successfully perform competitively despite a painful injury.
Positive expectations
Dr. Meissner advised all physicians to treat patients with pain empathetically. He encourages them to ask patients about their pain, accompanying symptoms, possible fears, and other mental stress and to take these factors seriously.
Moreover, the findings accentuate the effect of prescribed analgesics. “Numerous studies have meanwhile shown that the more positive a patient’s expectations, the better the effect of a medication,” said Dr. Meissner. “We physicians must exploit this effect, too.”
This article was translated from the Medscape German Edition and a version appeared on Medscape.com.
These are the results of a study, recently published in the Proceedings of the National Academy of Sciences, that was conducted by a team led by neuroscientist Dan-Mikael Ellingsen, PhD, from Oslo University Hospital.
The researchers used functional MRI to scan the brains of 20 patients with chronic pain to investigate how a physician’s demeanor may affect patients’ sensitivity to pain, including effects in the central nervous system. During the scans, which were conducted in two sessions, the patients’ legs were exposed to stimuli that ranged from painless to moderately painful. The patients recorded perceived pain intensity using a scale. The physicians also underwent fMRI.
Half of the patients were subjected to the pain stimuli while alone; the other half were subjected to pain while in the presence of a physician. The latter group of patients was divided into two subgroups. Half of the patients had spoken to the accompanying physician before the examination. They discussed the history of the patient’s condition to date, among other things. The other half underwent the brain scans without any prior interaction with a physician.
Worse when alone
Dr. Ellingsen and his colleagues found that patients who were alone during the examination reported greater pain than those who were in the presence of a physician, even though they were subjected to stimuli of the same intensity. In instances in which the physician and patient had already spoken before the brain scan, patients additionally felt that the physician was empathetic and understood their pain. Furthermore, the physicians were better able to estimate the pain that their patients experienced.
The patients who had a physician by their side consistently experienced pain that was milder than the pain experienced by those who were alone. For pairs that had spoken beforehand, the patients considered their physician to be better able to understand their pain, and the physicians estimated the perceived pain intensity of their patients more accurately.
Evidence of trust
There was greater activity in the dorsolateral and ventrolateral prefrontal cortex, as well as in the primary and secondary somatosensory areas, in patients in the subgroup that had spoken to a physician. For the physicians, compared with the comparison group, there was an increase in correspondence between activity in the dorsolateral prefrontal cortex and activity in the secondary somatosensory areas of patients, which is a brain region that is known to react to pain. The brain activity correlation increased in line with the self-reported mutual trust between the physician and patient.
“These results prove that empathy and support can decrease pain intensity,” the investigators write. The data shed light on the brain processes behind the social modulation of pain during the interaction between the physician and the patient. Concordances in the brain are increased by greater therapeutic alliance.
Beyond medication
Winfried Meissner, MD, head of the pain clinic at the department of anesthesiology and intensive care medicine at Jena University Hospital, Germany, and former president of the German Pain Society, said in an interview: “I view this as a vital study that impressively demonstrates that effective, intensive pain therapy is not just a case of administering the correct analgesic.”
“Instead, a focus should be placed on what common sense tells us, which is just how crucial an empathetic attitude from physicians and good communication with patients are when it comes to the success of any therapy,” Dr. Meissner added. Unfortunately, such an attitude and such communication often are not provided in clinical practice because of limitations on time.
“Now, with objectively collected data from patients and physicians, [Dr.] Ellingsen’s team has been able to demonstrate that human interaction has a decisive impact on the treatment of patients experiencing pain,” said Dr. Meissner. “The study should encourage practitioners to treat communication just as seriously as the pharmacology of analgesics.”
Perception and attitude
“The study shows remarkably well that empathetic conversation between the physician and patient represents a valuable therapeutic method and should be recognized as such,” emphasized Dr. Meissner. Of course, conversation cannot replace pharmacologic treatment, but it can supplement and reinforce it. Furthermore, a physician’s empathy presumably has an effect that is at least as great as a suitable analgesic.
“Pain is more than just sensory perception,” explained Dr. Meissner. “We all know that it has a strong affective component, and perception is greatly determined by context.” This can be seen, for example, in athletes, who often attribute less importance to their pain and can successfully perform competitively despite a painful injury.
Positive expectations
Dr. Meissner advised all physicians to treat patients with pain empathetically. He encourages them to ask patients about their pain, accompanying symptoms, possible fears, and other mental stress and to take these factors seriously.
Moreover, the findings accentuate the effect of prescribed analgesics. “Numerous studies have meanwhile shown that the more positive a patient’s expectations, the better the effect of a medication,” said Dr. Meissner. “We physicians must exploit this effect, too.”
This article was translated from the Medscape German Edition and a version appeared on Medscape.com.
FROM THE PROCEEDINGS OF THE NATIONAL ACADEMY OF SCIENCES
Opioid initiation in dementia tied to an 11-fold increased risk of death
Opioid initiation for older adults with dementia is linked to a significantly increased risk of death, especially in the first 2 weeks, when the risk is elevated 11-fold, new research shows.
“We expected that opioids would be associated with an increased risk of death, but we are surprised by the magnitude,” study investigator Christina Jensen-Dahm, MD, PhD, with the Danish Dementia Research Centre, Copenhagen University Hospital, Rigshospitalet, Denmark, told this news organization.
“It’s important that physicians carefully evaluate the risk and benefits if considering initiating an opioid, and this is particularly important in elderly with dementia,” Dr. Jensen-Dahm added.
The findings were presented at the Alzheimer’s Association International Conference.
Risky business
Using Danish nationwide registries, the researchers analyzed data on all 75,471 adults in Denmark who were aged 65 and older and had been diagnosed with dementia between 2008 and 2018. A total of 31,619 individuals (42%) filled a prescription for an opioid. These “exposed” individuals were matched to 63,235 unexposed individuals.
Among the exposed group, 10,474 (33%) died within 180 days after starting opioid therapy, compared with 3,980 (6.4%) in the unexposed group.
After adjusting for potential differences between groups, new use of an opioid was associated with a greater than fourfold excess mortality risk (adjusted hazard ratio, 4.16; 95% confidence interval, 4.00-4.33).
New use of a strong opioid – defined as morphine, oxycodone, ketobemidone, hydromorphone, pethidine, buprenorphine, and fentanyl – was associated with a greater than sixfold increase in mortality risk (aHR, 6.42; 95% CI, 6.08-6.79).
Among those who used fentanyl patches as their first opioid, 65% died within the first 180 days, compared with 6.7% in the unexposed – an eightfold increased mortality risk (aHR, 8.04; 95% CI, 7.01-9.22).
For all opioids, the risk was greatest in the first 14 days, with a nearly 11-fold increased risk of mortality (aHR, 10.8; 95% CI, 9.74-11.99). However, there remained a twofold increase in risk after taking opioids for 90 days (aHR, 2.32; 95% CI, 2.17-2.48).
“Opioids are associated with severe and well-known side effects, such as sedation, confusion, respiratory depression, falls, and in the most severe cases, death. In the general population, opioids have been associated with an increased risk of death, and similar to ours, greatest in the first 14 days,” said Dr. Jensen-Dahm.
Need to weigh risks, benefits
Commenting on the study, Percy Griffin, PhD, director of scientific engagement at the Alzheimer’s Association, told this news organization that the use of strong opioids has “increased considerably over the past decade among older people with dementia. Opioid therapy should only be considered for pain if the benefits are anticipated to outweigh the risks in individuals who are living with dementia.”
“Opioids are very powerful drugs, and while we need to see additional research in more diverse populations, these initial findings indicate they may put older adults with dementia at much higher risk of death,” Nicole Purcell, DO, neurologist and senior director of clinical practice at the Alzheimer’s Association, added in a conference statement.
“Pain should not go undiagnosed or untreated, in particular in people living with dementia, who may not be able to effectively articulate the location and severity of the pain,” Dr. Purcell added.
These new findings further emphasize the need for discussion between patient, family, and physician. Decisions about prescribing pain medication should be thought through carefully, and if used, there needs to be careful monitoring of the patient, said Dr. Purcell.
The study was supported by a grant from the Capital Region of Denmark. Dr. Jensen-Dahm, Dr. Griffin, and Dr. Purcell have no relevant disclosures.
A version of this article first appeared on Medscape.com.
Opioid initiation for older adults with dementia is linked to a significantly increased risk of death, especially in the first 2 weeks, when the risk is elevated 11-fold, new research shows.
“We expected that opioids would be associated with an increased risk of death, but we are surprised by the magnitude,” study investigator Christina Jensen-Dahm, MD, PhD, with the Danish Dementia Research Centre, Copenhagen University Hospital, Rigshospitalet, Denmark, told this news organization.
“It’s important that physicians carefully evaluate the risk and benefits if considering initiating an opioid, and this is particularly important in elderly with dementia,” Dr. Jensen-Dahm added.
The findings were presented at the Alzheimer’s Association International Conference.
Risky business
Using Danish nationwide registries, the researchers analyzed data on all 75,471 adults in Denmark who were aged 65 and older and had been diagnosed with dementia between 2008 and 2018. A total of 31,619 individuals (42%) filled a prescription for an opioid. These “exposed” individuals were matched to 63,235 unexposed individuals.
Among the exposed group, 10,474 (33%) died within 180 days after starting opioid therapy, compared with 3,980 (6.4%) in the unexposed group.
After adjusting for potential differences between groups, new use of an opioid was associated with a greater than fourfold excess mortality risk (adjusted hazard ratio, 4.16; 95% confidence interval, 4.00-4.33).
New use of a strong opioid – defined as morphine, oxycodone, ketobemidone, hydromorphone, pethidine, buprenorphine, and fentanyl – was associated with a greater than sixfold increase in mortality risk (aHR, 6.42; 95% CI, 6.08-6.79).
Among those who used fentanyl patches as their first opioid, 65% died within the first 180 days, compared with 6.7% in the unexposed – an eightfold increased mortality risk (aHR, 8.04; 95% CI, 7.01-9.22).
For all opioids, the risk was greatest in the first 14 days, with a nearly 11-fold increased risk of mortality (aHR, 10.8; 95% CI, 9.74-11.99). However, there remained a twofold increase in risk after taking opioids for 90 days (aHR, 2.32; 95% CI, 2.17-2.48).
“Opioids are associated with severe and well-known side effects, such as sedation, confusion, respiratory depression, falls, and in the most severe cases, death. In the general population, opioids have been associated with an increased risk of death, and similar to ours, greatest in the first 14 days,” said Dr. Jensen-Dahm.
Need to weigh risks, benefits
Commenting on the study, Percy Griffin, PhD, director of scientific engagement at the Alzheimer’s Association, told this news organization that the use of strong opioids has “increased considerably over the past decade among older people with dementia. Opioid therapy should only be considered for pain if the benefits are anticipated to outweigh the risks in individuals who are living with dementia.”
“Opioids are very powerful drugs, and while we need to see additional research in more diverse populations, these initial findings indicate they may put older adults with dementia at much higher risk of death,” Nicole Purcell, DO, neurologist and senior director of clinical practice at the Alzheimer’s Association, added in a conference statement.
“Pain should not go undiagnosed or untreated, in particular in people living with dementia, who may not be able to effectively articulate the location and severity of the pain,” Dr. Purcell added.
These new findings further emphasize the need for discussion between patient, family, and physician. Decisions about prescribing pain medication should be thought through carefully, and if used, there needs to be careful monitoring of the patient, said Dr. Purcell.
The study was supported by a grant from the Capital Region of Denmark. Dr. Jensen-Dahm, Dr. Griffin, and Dr. Purcell have no relevant disclosures.
A version of this article first appeared on Medscape.com.
Opioid initiation for older adults with dementia is linked to a significantly increased risk of death, especially in the first 2 weeks, when the risk is elevated 11-fold, new research shows.
“We expected that opioids would be associated with an increased risk of death, but we are surprised by the magnitude,” study investigator Christina Jensen-Dahm, MD, PhD, with the Danish Dementia Research Centre, Copenhagen University Hospital, Rigshospitalet, Denmark, told this news organization.
“It’s important that physicians carefully evaluate the risk and benefits if considering initiating an opioid, and this is particularly important in elderly with dementia,” Dr. Jensen-Dahm added.
The findings were presented at the Alzheimer’s Association International Conference.
Risky business
Using Danish nationwide registries, the researchers analyzed data on all 75,471 adults in Denmark who were aged 65 and older and had been diagnosed with dementia between 2008 and 2018. A total of 31,619 individuals (42%) filled a prescription for an opioid. These “exposed” individuals were matched to 63,235 unexposed individuals.
Among the exposed group, 10,474 (33%) died within 180 days after starting opioid therapy, compared with 3,980 (6.4%) in the unexposed group.
After adjusting for potential differences between groups, new use of an opioid was associated with a greater than fourfold excess mortality risk (adjusted hazard ratio, 4.16; 95% confidence interval, 4.00-4.33).
New use of a strong opioid – defined as morphine, oxycodone, ketobemidone, hydromorphone, pethidine, buprenorphine, and fentanyl – was associated with a greater than sixfold increase in mortality risk (aHR, 6.42; 95% CI, 6.08-6.79).
Among those who used fentanyl patches as their first opioid, 65% died within the first 180 days, compared with 6.7% in the unexposed – an eightfold increased mortality risk (aHR, 8.04; 95% CI, 7.01-9.22).
For all opioids, the risk was greatest in the first 14 days, with a nearly 11-fold increased risk of mortality (aHR, 10.8; 95% CI, 9.74-11.99). However, there remained a twofold increase in risk after taking opioids for 90 days (aHR, 2.32; 95% CI, 2.17-2.48).
“Opioids are associated with severe and well-known side effects, such as sedation, confusion, respiratory depression, falls, and in the most severe cases, death. In the general population, opioids have been associated with an increased risk of death, and similar to ours, greatest in the first 14 days,” said Dr. Jensen-Dahm.
Need to weigh risks, benefits
Commenting on the study, Percy Griffin, PhD, director of scientific engagement at the Alzheimer’s Association, told this news organization that the use of strong opioids has “increased considerably over the past decade among older people with dementia. Opioid therapy should only be considered for pain if the benefits are anticipated to outweigh the risks in individuals who are living with dementia.”
“Opioids are very powerful drugs, and while we need to see additional research in more diverse populations, these initial findings indicate they may put older adults with dementia at much higher risk of death,” Nicole Purcell, DO, neurologist and senior director of clinical practice at the Alzheimer’s Association, added in a conference statement.
“Pain should not go undiagnosed or untreated, in particular in people living with dementia, who may not be able to effectively articulate the location and severity of the pain,” Dr. Purcell added.
These new findings further emphasize the need for discussion between patient, family, and physician. Decisions about prescribing pain medication should be thought through carefully, and if used, there needs to be careful monitoring of the patient, said Dr. Purcell.
The study was supported by a grant from the Capital Region of Denmark. Dr. Jensen-Dahm, Dr. Griffin, and Dr. Purcell have no relevant disclosures.
A version of this article first appeared on Medscape.com.
From AAIC 2023
Fibromyalgia linked to higher mortality risk
People who experience chronic pain and tiredness from fibromyalgia have an increased risk for all-cause mortality, a new analysis of evidence says.
The condition can lead people to be vulnerable to accidents, infections, and even suicide, according to the report published in RMD Open.
The researchers suggest that care providers monitor physical and mental health to lower the dangers.
People with fibromyalgia often have other health issues, including rheumatic, gut, neurological, and mental health disorders, according to The BMJ. More and more people are being diagnosed with fibromyalgia. The cause of the illness remains unclear.
The researchers looked at eight studies published between 1999 and 2020 and pooled results from six of them. The studies involved a total of 188,000 adults.
The analysis of the data revealed that fibromyalgia was linked to a 27% greater risk of death from all causes.
Those with fibromyalgia were at a 44% greater risk of infections, including pneumonia. Their suicide risk was more than three times higher.
The greater risk of all-cause death could result from fatigue, poor sleep, and concentration problems, The BMJ said.
The patients had a 12% lower risk of dying from cancer, the analysis found. This could be because they tend to make more visits to health care professionals, the authors suggest.
“Fibromyalgia is often called an ‘imaginary condition,’ with ongoing debates on the legitimacy and clinical usefulness of this diagnosis. Our review provides further proof that fibromyalgia patients should be taken seriously, with particular focus on screening for suicidal ideation, prevention of accidents, and prevention and treatment of infections,” the researchers say.
A version of this article appeared on WebMD.com.
People who experience chronic pain and tiredness from fibromyalgia have an increased risk for all-cause mortality, a new analysis of evidence says.
The condition can lead people to be vulnerable to accidents, infections, and even suicide, according to the report published in RMD Open.
The researchers suggest that care providers monitor physical and mental health to lower the dangers.
People with fibromyalgia often have other health issues, including rheumatic, gut, neurological, and mental health disorders, according to The BMJ. More and more people are being diagnosed with fibromyalgia. The cause of the illness remains unclear.
The researchers looked at eight studies published between 1999 and 2020 and pooled results from six of them. The studies involved a total of 188,000 adults.
The analysis of the data revealed that fibromyalgia was linked to a 27% greater risk of death from all causes.
Those with fibromyalgia were at a 44% greater risk of infections, including pneumonia. Their suicide risk was more than three times higher.
The greater risk of all-cause death could result from fatigue, poor sleep, and concentration problems, The BMJ said.
The patients had a 12% lower risk of dying from cancer, the analysis found. This could be because they tend to make more visits to health care professionals, the authors suggest.
“Fibromyalgia is often called an ‘imaginary condition,’ with ongoing debates on the legitimacy and clinical usefulness of this diagnosis. Our review provides further proof that fibromyalgia patients should be taken seriously, with particular focus on screening for suicidal ideation, prevention of accidents, and prevention and treatment of infections,” the researchers say.
A version of this article appeared on WebMD.com.
People who experience chronic pain and tiredness from fibromyalgia have an increased risk for all-cause mortality, a new analysis of evidence says.
The condition can lead people to be vulnerable to accidents, infections, and even suicide, according to the report published in RMD Open.
The researchers suggest that care providers monitor physical and mental health to lower the dangers.
People with fibromyalgia often have other health issues, including rheumatic, gut, neurological, and mental health disorders, according to The BMJ. More and more people are being diagnosed with fibromyalgia. The cause of the illness remains unclear.
The researchers looked at eight studies published between 1999 and 2020 and pooled results from six of them. The studies involved a total of 188,000 adults.
The analysis of the data revealed that fibromyalgia was linked to a 27% greater risk of death from all causes.
Those with fibromyalgia were at a 44% greater risk of infections, including pneumonia. Their suicide risk was more than three times higher.
The greater risk of all-cause death could result from fatigue, poor sleep, and concentration problems, The BMJ said.
The patients had a 12% lower risk of dying from cancer, the analysis found. This could be because they tend to make more visits to health care professionals, the authors suggest.
“Fibromyalgia is often called an ‘imaginary condition,’ with ongoing debates on the legitimacy and clinical usefulness of this diagnosis. Our review provides further proof that fibromyalgia patients should be taken seriously, with particular focus on screening for suicidal ideation, prevention of accidents, and prevention and treatment of infections,” the researchers say.
A version of this article appeared on WebMD.com.
Medical cannabis does not reduce use of prescription meds
TOPLINE:
, according to a new study published in Annals of Internal Medicine.
METHODOLOGY:
- Cannabis advocates suggest that legal medical cannabis can be a partial solution to the opioid overdose crisis in the United States, which claimed more than 80,000 lives in 2021.
- Current research on how legalized cannabis reduces dependence on prescription pain medication is inconclusive.
- Researchers examined insurance data for the period 2010-2022 from 583,820 adults with chronic noncancer pain.
- They drew from 12 states in which medical cannabis is legal and from 17 in which it is not legal to create a hypothetical randomized trial. The control group simulated prescription rates where medical cannabis was not available.
- Authors evaluated prescription rates for opioids, nonopioid painkillers, and pain interventions, such as physical therapy.
TAKEAWAY:
In a given month during the first 3 years after legalization, for states with medical cannabis, the investigators found the following:
- There was an average decrease of 1.07 percentage points in the proportion of patients who received any opioid prescription, compared to a 1.12 percentage point decrease in the control group.
- There was an average increase of 1.14 percentage points in the proportion of patients who received any nonopioid prescription painkiller, compared to a 1.19 percentage point increase in the control group.
- There was a 0.17 percentage point decrease in the proportion of patients who received any pain procedure, compared to a 0.001 percentage point decrease in the control group.
IN PRACTICE:
“This study did not identify important effects of medical cannabis laws on receipt of opioid or nonopioid pain treatment among patients with chronic noncancer pain,” according to the researchers.
SOURCE:
The study was led by Emma E. McGinty, PhD, of Weill Cornell Medicine, New York, and was funded by the National Institute on Drug Abuse.
LIMITATIONS:
The investigators used a simulated, hypothetical control group that was based on untestable assumptions. They also drew data solely from insured individuals, so the study does not necessarily represent uninsured populations.
DISCLOSURES:
Dr. McGinty reports receiving a grant from NIDA. Her coauthors reported receiving support from NIDA and the National Institutes of Health.
A version of this article first appeared on Medscape.com.
TOPLINE:
, according to a new study published in Annals of Internal Medicine.
METHODOLOGY:
- Cannabis advocates suggest that legal medical cannabis can be a partial solution to the opioid overdose crisis in the United States, which claimed more than 80,000 lives in 2021.
- Current research on how legalized cannabis reduces dependence on prescription pain medication is inconclusive.
- Researchers examined insurance data for the period 2010-2022 from 583,820 adults with chronic noncancer pain.
- They drew from 12 states in which medical cannabis is legal and from 17 in which it is not legal to create a hypothetical randomized trial. The control group simulated prescription rates where medical cannabis was not available.
- Authors evaluated prescription rates for opioids, nonopioid painkillers, and pain interventions, such as physical therapy.
TAKEAWAY:
In a given month during the first 3 years after legalization, for states with medical cannabis, the investigators found the following:
- There was an average decrease of 1.07 percentage points in the proportion of patients who received any opioid prescription, compared to a 1.12 percentage point decrease in the control group.
- There was an average increase of 1.14 percentage points in the proportion of patients who received any nonopioid prescription painkiller, compared to a 1.19 percentage point increase in the control group.
- There was a 0.17 percentage point decrease in the proportion of patients who received any pain procedure, compared to a 0.001 percentage point decrease in the control group.
IN PRACTICE:
“This study did not identify important effects of medical cannabis laws on receipt of opioid or nonopioid pain treatment among patients with chronic noncancer pain,” according to the researchers.
SOURCE:
The study was led by Emma E. McGinty, PhD, of Weill Cornell Medicine, New York, and was funded by the National Institute on Drug Abuse.
LIMITATIONS:
The investigators used a simulated, hypothetical control group that was based on untestable assumptions. They also drew data solely from insured individuals, so the study does not necessarily represent uninsured populations.
DISCLOSURES:
Dr. McGinty reports receiving a grant from NIDA. Her coauthors reported receiving support from NIDA and the National Institutes of Health.
A version of this article first appeared on Medscape.com.
TOPLINE:
, according to a new study published in Annals of Internal Medicine.
METHODOLOGY:
- Cannabis advocates suggest that legal medical cannabis can be a partial solution to the opioid overdose crisis in the United States, which claimed more than 80,000 lives in 2021.
- Current research on how legalized cannabis reduces dependence on prescription pain medication is inconclusive.
- Researchers examined insurance data for the period 2010-2022 from 583,820 adults with chronic noncancer pain.
- They drew from 12 states in which medical cannabis is legal and from 17 in which it is not legal to create a hypothetical randomized trial. The control group simulated prescription rates where medical cannabis was not available.
- Authors evaluated prescription rates for opioids, nonopioid painkillers, and pain interventions, such as physical therapy.
TAKEAWAY:
In a given month during the first 3 years after legalization, for states with medical cannabis, the investigators found the following:
- There was an average decrease of 1.07 percentage points in the proportion of patients who received any opioid prescription, compared to a 1.12 percentage point decrease in the control group.
- There was an average increase of 1.14 percentage points in the proportion of patients who received any nonopioid prescription painkiller, compared to a 1.19 percentage point increase in the control group.
- There was a 0.17 percentage point decrease in the proportion of patients who received any pain procedure, compared to a 0.001 percentage point decrease in the control group.
IN PRACTICE:
“This study did not identify important effects of medical cannabis laws on receipt of opioid or nonopioid pain treatment among patients with chronic noncancer pain,” according to the researchers.
SOURCE:
The study was led by Emma E. McGinty, PhD, of Weill Cornell Medicine, New York, and was funded by the National Institute on Drug Abuse.
LIMITATIONS:
The investigators used a simulated, hypothetical control group that was based on untestable assumptions. They also drew data solely from insured individuals, so the study does not necessarily represent uninsured populations.
DISCLOSURES:
Dr. McGinty reports receiving a grant from NIDA. Her coauthors reported receiving support from NIDA and the National Institutes of Health.
A version of this article first appeared on Medscape.com.
‘Landmark’ trial shows opioids for back, neck pain no better than placebo
Opioids do not relieve acute low back or neck pain in the short term and lead to worse outcomes in the long term, results of the first randomized controlled trial testing the efficacy and safety of a short course of opioids for acute nonspecific low back/neck pain suggest.
After 6 weeks, there was no significant difference in pain scores of patients who took opioids, compared with those who took placebo. After 1 year, patients given the placebo had slightly lower pain scores. Also, patients using opioids were at greater risk of opioid misuse after 1 year.
This is a “landmark” trial with “practice-changing” results, senior author Christine Lin, PhD, with the University of Sydney, told this news organization.
“Before this trial, we did not have good evidence on whether opioids were effective for acute low back pain or neck pain, yet opioids were one of the most commonly used medicines for these conditions,” Dr. Lin explained.
On the basis of these results, “opioids should not be recommended at all for acute low back pain and neck pain,” Dr. Lin said.
Results of the OPAL study were published online in The Lancet.
Rigorous trial
The trial was conducted in 157 primary care or emergency department sites in Australia and involved 347 adults who had been experiencing low back pain, neck pain, or both for 12 weeks or less.
They were randomly allocated (1:1) to receive guideline-recommended care (reassurance and advice to stay active) plus an opioid (oxycodone up to 20 mg daily) or identical placebo for up to 6 weeks. Naloxone was provided to help prevent opioid-induced constipation and improve blinding.
The primary outcome was pain severity at 6 weeks, measured with the pain severity subscale of the Brief Pain Inventory (10-point scale).
After 6 weeks, opioid therapy offered no more relief for acute back/neck pain or functional improvement than placebo.
The mean pain score at 6 weeks was 2.78 in the opioid group, versus 2.25 in the placebo group (adjusted mean difference, 0.53; 95% confidence interval, –0.00 to 1.07; P = .051). At 1 year, mean pain scores in the placebo group were slightly lower than in the opioid group (1.8 vs. 2.4).
In addition, there was a doubling of the risk of opioid misuse at 1 year among patients randomly allocated to receive opioid therapy for 6 weeks, compared with those allocated to receive placebo for 6 weeks.
At 1 year, 24 (20%) of 123 of the patients who received opioids were at risk of misuse, as indicated by the Current Opioid Misuse Measure scale, compared with 13 (10%) of 128 patients in the placebo group (P = .049). The COMM is a widely used measure of current aberrant drug-related behavior among patients with chronic pain who are being prescribed opioid therapy.
Results raise ‘serious questions’
“I believe the findings of the study will need to be disseminated to the doctors and patients, so they receive this latest evidence on opioids,” Dr. Lin said in an interview.
“We need to reassure doctors and patients that most people with acute low back pain and neck pain recover well with time (usually by 6 weeks), so management is simple – staying active, avoiding bed rest, and, if necessary, using a heat pack for short term pain relief. If drugs are required, consider anti-inflammatory drugs,” Dr. Lin added.
The authors of a linked comment say the OPAL trial “raises serious questions about the use of opioid therapy for acute low back and neck pain.”
Mark Sullivan, MD, PhD, and Jane Ballantyne, MD, with the University of Washington, Seattle, note that current clinical guidelines recommend opioids for patients with acute back and neck pain when other drug treatments fail or are contraindicated.
“As many as two-thirds of patients might receive an opioid when presenting for care of back or neck pain. It is time to re-examine these guidelines and these practices,” Dr. Sullivan and Dr. Ballantyne conclude.
Funding for the OPAL study was provided by the National Health and Medical Research Council, the University of Sydney Faculty of Medicine and Health, and SafeWork SA. The study authors have disclosed no relevant financial relationships. Dr. Sullivan and Dr. Ballantyne are board members (unpaid) of Physicians for Responsible Opioid Prescribing and have been paid consultants in opioid litigation.
A version of this article originally appeared on Medscape.com.
Opioids do not relieve acute low back or neck pain in the short term and lead to worse outcomes in the long term, results of the first randomized controlled trial testing the efficacy and safety of a short course of opioids for acute nonspecific low back/neck pain suggest.
After 6 weeks, there was no significant difference in pain scores of patients who took opioids, compared with those who took placebo. After 1 year, patients given the placebo had slightly lower pain scores. Also, patients using opioids were at greater risk of opioid misuse after 1 year.
This is a “landmark” trial with “practice-changing” results, senior author Christine Lin, PhD, with the University of Sydney, told this news organization.
“Before this trial, we did not have good evidence on whether opioids were effective for acute low back pain or neck pain, yet opioids were one of the most commonly used medicines for these conditions,” Dr. Lin explained.
On the basis of these results, “opioids should not be recommended at all for acute low back pain and neck pain,” Dr. Lin said.
Results of the OPAL study were published online in The Lancet.
Rigorous trial
The trial was conducted in 157 primary care or emergency department sites in Australia and involved 347 adults who had been experiencing low back pain, neck pain, or both for 12 weeks or less.
They were randomly allocated (1:1) to receive guideline-recommended care (reassurance and advice to stay active) plus an opioid (oxycodone up to 20 mg daily) or identical placebo for up to 6 weeks. Naloxone was provided to help prevent opioid-induced constipation and improve blinding.
The primary outcome was pain severity at 6 weeks, measured with the pain severity subscale of the Brief Pain Inventory (10-point scale).
After 6 weeks, opioid therapy offered no more relief for acute back/neck pain or functional improvement than placebo.
The mean pain score at 6 weeks was 2.78 in the opioid group, versus 2.25 in the placebo group (adjusted mean difference, 0.53; 95% confidence interval, –0.00 to 1.07; P = .051). At 1 year, mean pain scores in the placebo group were slightly lower than in the opioid group (1.8 vs. 2.4).
In addition, there was a doubling of the risk of opioid misuse at 1 year among patients randomly allocated to receive opioid therapy for 6 weeks, compared with those allocated to receive placebo for 6 weeks.
At 1 year, 24 (20%) of 123 of the patients who received opioids were at risk of misuse, as indicated by the Current Opioid Misuse Measure scale, compared with 13 (10%) of 128 patients in the placebo group (P = .049). The COMM is a widely used measure of current aberrant drug-related behavior among patients with chronic pain who are being prescribed opioid therapy.
Results raise ‘serious questions’
“I believe the findings of the study will need to be disseminated to the doctors and patients, so they receive this latest evidence on opioids,” Dr. Lin said in an interview.
“We need to reassure doctors and patients that most people with acute low back pain and neck pain recover well with time (usually by 6 weeks), so management is simple – staying active, avoiding bed rest, and, if necessary, using a heat pack for short term pain relief. If drugs are required, consider anti-inflammatory drugs,” Dr. Lin added.
The authors of a linked comment say the OPAL trial “raises serious questions about the use of opioid therapy for acute low back and neck pain.”
Mark Sullivan, MD, PhD, and Jane Ballantyne, MD, with the University of Washington, Seattle, note that current clinical guidelines recommend opioids for patients with acute back and neck pain when other drug treatments fail or are contraindicated.
“As many as two-thirds of patients might receive an opioid when presenting for care of back or neck pain. It is time to re-examine these guidelines and these practices,” Dr. Sullivan and Dr. Ballantyne conclude.
Funding for the OPAL study was provided by the National Health and Medical Research Council, the University of Sydney Faculty of Medicine and Health, and SafeWork SA. The study authors have disclosed no relevant financial relationships. Dr. Sullivan and Dr. Ballantyne are board members (unpaid) of Physicians for Responsible Opioid Prescribing and have been paid consultants in opioid litigation.
A version of this article originally appeared on Medscape.com.
Opioids do not relieve acute low back or neck pain in the short term and lead to worse outcomes in the long term, results of the first randomized controlled trial testing the efficacy and safety of a short course of opioids for acute nonspecific low back/neck pain suggest.
After 6 weeks, there was no significant difference in pain scores of patients who took opioids, compared with those who took placebo. After 1 year, patients given the placebo had slightly lower pain scores. Also, patients using opioids were at greater risk of opioid misuse after 1 year.
This is a “landmark” trial with “practice-changing” results, senior author Christine Lin, PhD, with the University of Sydney, told this news organization.
“Before this trial, we did not have good evidence on whether opioids were effective for acute low back pain or neck pain, yet opioids were one of the most commonly used medicines for these conditions,” Dr. Lin explained.
On the basis of these results, “opioids should not be recommended at all for acute low back pain and neck pain,” Dr. Lin said.
Results of the OPAL study were published online in The Lancet.
Rigorous trial
The trial was conducted in 157 primary care or emergency department sites in Australia and involved 347 adults who had been experiencing low back pain, neck pain, or both for 12 weeks or less.
They were randomly allocated (1:1) to receive guideline-recommended care (reassurance and advice to stay active) plus an opioid (oxycodone up to 20 mg daily) or identical placebo for up to 6 weeks. Naloxone was provided to help prevent opioid-induced constipation and improve blinding.
The primary outcome was pain severity at 6 weeks, measured with the pain severity subscale of the Brief Pain Inventory (10-point scale).
After 6 weeks, opioid therapy offered no more relief for acute back/neck pain or functional improvement than placebo.
The mean pain score at 6 weeks was 2.78 in the opioid group, versus 2.25 in the placebo group (adjusted mean difference, 0.53; 95% confidence interval, –0.00 to 1.07; P = .051). At 1 year, mean pain scores in the placebo group were slightly lower than in the opioid group (1.8 vs. 2.4).
In addition, there was a doubling of the risk of opioid misuse at 1 year among patients randomly allocated to receive opioid therapy for 6 weeks, compared with those allocated to receive placebo for 6 weeks.
At 1 year, 24 (20%) of 123 of the patients who received opioids were at risk of misuse, as indicated by the Current Opioid Misuse Measure scale, compared with 13 (10%) of 128 patients in the placebo group (P = .049). The COMM is a widely used measure of current aberrant drug-related behavior among patients with chronic pain who are being prescribed opioid therapy.
Results raise ‘serious questions’
“I believe the findings of the study will need to be disseminated to the doctors and patients, so they receive this latest evidence on opioids,” Dr. Lin said in an interview.
“We need to reassure doctors and patients that most people with acute low back pain and neck pain recover well with time (usually by 6 weeks), so management is simple – staying active, avoiding bed rest, and, if necessary, using a heat pack for short term pain relief. If drugs are required, consider anti-inflammatory drugs,” Dr. Lin added.
The authors of a linked comment say the OPAL trial “raises serious questions about the use of opioid therapy for acute low back and neck pain.”
Mark Sullivan, MD, PhD, and Jane Ballantyne, MD, with the University of Washington, Seattle, note that current clinical guidelines recommend opioids for patients with acute back and neck pain when other drug treatments fail or are contraindicated.
“As many as two-thirds of patients might receive an opioid when presenting for care of back or neck pain. It is time to re-examine these guidelines and these practices,” Dr. Sullivan and Dr. Ballantyne conclude.
Funding for the OPAL study was provided by the National Health and Medical Research Council, the University of Sydney Faculty of Medicine and Health, and SafeWork SA. The study authors have disclosed no relevant financial relationships. Dr. Sullivan and Dr. Ballantyne are board members (unpaid) of Physicians for Responsible Opioid Prescribing and have been paid consultants in opioid litigation.
A version of this article originally appeared on Medscape.com.
New international guidelines on opioid deprescribing
An expert panel of pain management clinicians has released what they say are the first international guidelines for general practitioners on opioid analgesic deprescribing in adults.
The recommendations describe best practices for stopping opioid therapy and emphasize slow tapering and individualized deprescribing plans tailored to each patient.
Developed by general practitioners, pain specialists, addiction specialists, pharmacists, registered nurses, consumers, and physiotherapists, the guidelines note that deprescribing may not be appropriate for every patient and that stopping abruptly can be associated with an increased risk of overdose.
“Internationally, we were seeing significant harms from opioids but also significant harms from unsolicited and abrupt opioid cessation,” said lead author Aili Langford, PhD, who conducted the study as a doctoral student at the University of Sydney. “It was clear that recommendations to support safe and person-centered opioid deprescribing were required.”
The findings were published online in the Medical Journal of Australia.
Deprescribing plan
The consensus guidelines include 11 recommendations for deprescribing in adult patients who take at least one opioid for any type of pain.
Recommendations include implementing a deprescribing plan when opioids are first prescribed and gradual and individualized deprescribing, with regular monitoring and review.
Clinicians should consider opioid deprescribing in patients who experience no clinically meaningful improvement in function, quality of life, or pain at high risk with opioid therapy, they note. Patients who are at high risk for opioid-related harm are also good candidates for deprescribing.
Stopping opioid therapy is not recommended for patients with severe opioid use disorder (OUD). In those patients, medication-assisted OUD treatment and other evidence-based interventions are recommended.
“Opioids can be effective in pain management,” co-author Carl Schneider, PhD, an associate professor of pharmacy at the University of Sydney, said in a press release. “However, over the longer term, the risk of harms may outweigh the benefits.”
A ‘global problem’
Commenting on the guidelines, Orman Trent Hall, DO, assistant professor of addiction medicine, department of psychiatry and behavioral health at the Ohio State University Wexner Medical Center, Columbus, said they are similar to recommendations published by the U.S. Centers for Disease Control and Prevention in 2016 and 2022 but offer additional information that could be helpful.
“This new guideline provides more explicit advice about tapering and withdrawal management, which may be useful to practitioners. The opioid crisis is a global problem, and while individual countries may require local solutions, the new international guideline may offer a framework for approaching this issue,” he said.
The guideline’s emphasis on the potential risks of deprescribing in some patients is also key, Dr. Hall added. Patients who are tapering off opioid therapy may have worsening pain and loss of function that can affect their quality of life.
“Patients may also experience psychological harm and increased risk of opioid use disorder and death by suicide following opioid deprescribing,” Dr. Hall said. “Therefore, it is important for providers to carefully weigh the risks of prescribing and deprescribing and engage patients with person-centered communication and shared decision-making.”
The work was funded by grants from the University of Sydney and the National Health and Medical Research Council. Full disclosures are available in the original article. Dr. Hall has provided expert opinion to the health care consultancy firm Lumanity and Emergent BioSolutions regarding the overdose crisis.
A version of this article originally appeared on Medscape.com.
An expert panel of pain management clinicians has released what they say are the first international guidelines for general practitioners on opioid analgesic deprescribing in adults.
The recommendations describe best practices for stopping opioid therapy and emphasize slow tapering and individualized deprescribing plans tailored to each patient.
Developed by general practitioners, pain specialists, addiction specialists, pharmacists, registered nurses, consumers, and physiotherapists, the guidelines note that deprescribing may not be appropriate for every patient and that stopping abruptly can be associated with an increased risk of overdose.
“Internationally, we were seeing significant harms from opioids but also significant harms from unsolicited and abrupt opioid cessation,” said lead author Aili Langford, PhD, who conducted the study as a doctoral student at the University of Sydney. “It was clear that recommendations to support safe and person-centered opioid deprescribing were required.”
The findings were published online in the Medical Journal of Australia.
Deprescribing plan
The consensus guidelines include 11 recommendations for deprescribing in adult patients who take at least one opioid for any type of pain.
Recommendations include implementing a deprescribing plan when opioids are first prescribed and gradual and individualized deprescribing, with regular monitoring and review.
Clinicians should consider opioid deprescribing in patients who experience no clinically meaningful improvement in function, quality of life, or pain at high risk with opioid therapy, they note. Patients who are at high risk for opioid-related harm are also good candidates for deprescribing.
Stopping opioid therapy is not recommended for patients with severe opioid use disorder (OUD). In those patients, medication-assisted OUD treatment and other evidence-based interventions are recommended.
“Opioids can be effective in pain management,” co-author Carl Schneider, PhD, an associate professor of pharmacy at the University of Sydney, said in a press release. “However, over the longer term, the risk of harms may outweigh the benefits.”
A ‘global problem’
Commenting on the guidelines, Orman Trent Hall, DO, assistant professor of addiction medicine, department of psychiatry and behavioral health at the Ohio State University Wexner Medical Center, Columbus, said they are similar to recommendations published by the U.S. Centers for Disease Control and Prevention in 2016 and 2022 but offer additional information that could be helpful.
“This new guideline provides more explicit advice about tapering and withdrawal management, which may be useful to practitioners. The opioid crisis is a global problem, and while individual countries may require local solutions, the new international guideline may offer a framework for approaching this issue,” he said.
The guideline’s emphasis on the potential risks of deprescribing in some patients is also key, Dr. Hall added. Patients who are tapering off opioid therapy may have worsening pain and loss of function that can affect their quality of life.
“Patients may also experience psychological harm and increased risk of opioid use disorder and death by suicide following opioid deprescribing,” Dr. Hall said. “Therefore, it is important for providers to carefully weigh the risks of prescribing and deprescribing and engage patients with person-centered communication and shared decision-making.”
The work was funded by grants from the University of Sydney and the National Health and Medical Research Council. Full disclosures are available in the original article. Dr. Hall has provided expert opinion to the health care consultancy firm Lumanity and Emergent BioSolutions regarding the overdose crisis.
A version of this article originally appeared on Medscape.com.
An expert panel of pain management clinicians has released what they say are the first international guidelines for general practitioners on opioid analgesic deprescribing in adults.
The recommendations describe best practices for stopping opioid therapy and emphasize slow tapering and individualized deprescribing plans tailored to each patient.
Developed by general practitioners, pain specialists, addiction specialists, pharmacists, registered nurses, consumers, and physiotherapists, the guidelines note that deprescribing may not be appropriate for every patient and that stopping abruptly can be associated with an increased risk of overdose.
“Internationally, we were seeing significant harms from opioids but also significant harms from unsolicited and abrupt opioid cessation,” said lead author Aili Langford, PhD, who conducted the study as a doctoral student at the University of Sydney. “It was clear that recommendations to support safe and person-centered opioid deprescribing were required.”
The findings were published online in the Medical Journal of Australia.
Deprescribing plan
The consensus guidelines include 11 recommendations for deprescribing in adult patients who take at least one opioid for any type of pain.
Recommendations include implementing a deprescribing plan when opioids are first prescribed and gradual and individualized deprescribing, with regular monitoring and review.
Clinicians should consider opioid deprescribing in patients who experience no clinically meaningful improvement in function, quality of life, or pain at high risk with opioid therapy, they note. Patients who are at high risk for opioid-related harm are also good candidates for deprescribing.
Stopping opioid therapy is not recommended for patients with severe opioid use disorder (OUD). In those patients, medication-assisted OUD treatment and other evidence-based interventions are recommended.
“Opioids can be effective in pain management,” co-author Carl Schneider, PhD, an associate professor of pharmacy at the University of Sydney, said in a press release. “However, over the longer term, the risk of harms may outweigh the benefits.”
A ‘global problem’
Commenting on the guidelines, Orman Trent Hall, DO, assistant professor of addiction medicine, department of psychiatry and behavioral health at the Ohio State University Wexner Medical Center, Columbus, said they are similar to recommendations published by the U.S. Centers for Disease Control and Prevention in 2016 and 2022 but offer additional information that could be helpful.
“This new guideline provides more explicit advice about tapering and withdrawal management, which may be useful to practitioners. The opioid crisis is a global problem, and while individual countries may require local solutions, the new international guideline may offer a framework for approaching this issue,” he said.
The guideline’s emphasis on the potential risks of deprescribing in some patients is also key, Dr. Hall added. Patients who are tapering off opioid therapy may have worsening pain and loss of function that can affect their quality of life.
“Patients may also experience psychological harm and increased risk of opioid use disorder and death by suicide following opioid deprescribing,” Dr. Hall said. “Therefore, it is important for providers to carefully weigh the risks of prescribing and deprescribing and engage patients with person-centered communication and shared decision-making.”
The work was funded by grants from the University of Sydney and the National Health and Medical Research Council. Full disclosures are available in the original article. Dr. Hall has provided expert opinion to the health care consultancy firm Lumanity and Emergent BioSolutions regarding the overdose crisis.
A version of this article originally appeared on Medscape.com.


