User login
For MD-IQ on Family Practice News, but a regular topic for Rheumatology News
OARSI to FDA: Take osteoarthritis seriously
LAS VEGAS – The Osteoarthritis Research Society International has submitted a 103-page white paper to the Food and Drug Administration, the gist of which is captured in its title: “Osteoarthritis: A Serious Disease.”
The purpose of the voluminous white paper is to persuade FDA officials that osteoarthritis (OA) meets the agency’s formal definition of a serious disease for which there are currently no satisfactory treatments. That recognition would result in removal of current regulatory barriers to development of new structure-modifying treatments for OA, instead allowing such efforts to fall within the agency’s accelerated approval program, Marc C. Hochberg, MD, a coauthor of the white paper, explained at the World Congress on Osteoarthritis.
OARSI would like to see novel investigational therapies be allowed to advance through the developmental pipeline on the basis of favorable changes in clinically relevant biomarkers – be they biochemical or imaging – as intermediate endpoints serving as surrogates for structural change endpoints and meaningful clinical outcomes.
There is an enormous unmet need for effective disease-modifying therapies for OA. Establishing a more flexible regulatory environment for drug development by designating OA as a serious disease is expected to rekindle pharmaceutical industry interest in developing such products, which at present is at an ebb, he continued at the congress sponsored by the Osteoarthritis Research Society International.
The FDA has defined a serious disease as “a disease or condition associated with morbidity that has substantial impact on day-to-day functioning. Short-lived and self-limiting morbidity will usually not be sufficient, but the morbidity need not be irreversible if it is persistent or recurrent. Whether a disease or condition is serious is a matter of clinical judgment, based on its impact on such factors as survival, day-to-day functioning, or the likelihood that the disease, if left untreated, will progress from a less severe condition to a more serious one.”
The white paper makes the case that OA fits that description to a T. Dr. Hochberg said the big picture regarding OA as described in the white paper is this: It’s the most common form of arthritis, affecting more than 250 million people worldwide. And its costs approach 2% of the gross national product in the United States and other developed countries.
“Osteoarthritis accounts for more functional limitation, work loss, and physical disability than any other chronic disease, including cardiovascular disease and chronic obstructive pulmonary disease,” the rheumatologist said.
The white paper cites published data in support of these and other key points. At OARSI 2017, Dr. Hochberg presented highlights from the white paper, submitted to the FDA in December 2016:
• OA prevalence is relentlessly climbing. The Centers for Disease Control and Prevention put the U.S. prevalence of OA at 46 million in 2004 and has projected that it will reach 63 million in 2020 and 78 million Americans by 2040. The rise is being driven by the aging of the baby boomers, the obesity epidemic, predisposing physical injuries, and sedentary behavior.
• OA is expensive for patients and society. The combined direct medical costs and indirect costs stemming from lost earnings from OA amount to an estimated $461 billion annually in the United States.
• OA exacts a steep toll in years lived with disability (YLD). Estimated YLD from OA jumped by 75% during 1990-2013. This increase in YLD was exceeded only by dementia at 84% and diabetes at 135%. OA accounts for 1.6% of overall YLD in the United States, a rate comparable to ischemic heart disease at 1.63% and more than twice that for rheumatoid arthritis at 0.68%.
• Comorbidities are the rule. Various studies have estimated that 59%-87% of adults with OA have at least one additional significant chronic condition. The median number is two. One-third of OA patients have four or more additional comorbid conditions. The most common are cardiovascular disease, diabetes, obesity, metabolic syndrome, depression, anxiety, and falls and fractures.
• No effective treatments exist. There are no approved drugs that can prevent or even slow progression of OA to the point where total joint replacement is needed. Current medications are focused on pain relief and maintenance of functional independence. But these drugs are associated with significant risks of life-threatening side effects. NSAIDs have been linked to increased risk of cardiovascular events, GI bleeding, chronic kidney disease, and heart failure. And while opioids provide a small benefit in terms of pain relief, this is outweighed by the associated risks of falls, fractures, dependence, overdose, and death. All of these risks are accentuated in the presence of the common comorbid conditions associated with OA.
• OA increases the risk of dying prematurely. In a meta-analysis of individual patient data from the Multicenter Osteoarthritis Study and the Johnston County (N.C.) Osteoarthritis Project conducted specifically for the white paper, investigators determined that OA was associated with a 23% increase in the risk of death independent of age, race, and sex. This excess mortality is attributable in part to the presence of the metabolic syndrome and other commonly comorbid conditions, reduced physical activity because of OA disability, and the use of NSAIDs and opioid analgesics for symptomatic control.
The OARSI initiative is supported by EMD Serono, Fidia Pharma, Flexion Therapeutics, Nordic Biosciences, and Spinifex. Dr. Hochberg reported having numerous financial relationships with industry.
LAS VEGAS – The Osteoarthritis Research Society International has submitted a 103-page white paper to the Food and Drug Administration, the gist of which is captured in its title: “Osteoarthritis: A Serious Disease.”
The purpose of the voluminous white paper is to persuade FDA officials that osteoarthritis (OA) meets the agency’s formal definition of a serious disease for which there are currently no satisfactory treatments. That recognition would result in removal of current regulatory barriers to development of new structure-modifying treatments for OA, instead allowing such efforts to fall within the agency’s accelerated approval program, Marc C. Hochberg, MD, a coauthor of the white paper, explained at the World Congress on Osteoarthritis.
OARSI would like to see novel investigational therapies be allowed to advance through the developmental pipeline on the basis of favorable changes in clinically relevant biomarkers – be they biochemical or imaging – as intermediate endpoints serving as surrogates for structural change endpoints and meaningful clinical outcomes.
There is an enormous unmet need for effective disease-modifying therapies for OA. Establishing a more flexible regulatory environment for drug development by designating OA as a serious disease is expected to rekindle pharmaceutical industry interest in developing such products, which at present is at an ebb, he continued at the congress sponsored by the Osteoarthritis Research Society International.
The FDA has defined a serious disease as “a disease or condition associated with morbidity that has substantial impact on day-to-day functioning. Short-lived and self-limiting morbidity will usually not be sufficient, but the morbidity need not be irreversible if it is persistent or recurrent. Whether a disease or condition is serious is a matter of clinical judgment, based on its impact on such factors as survival, day-to-day functioning, or the likelihood that the disease, if left untreated, will progress from a less severe condition to a more serious one.”
The white paper makes the case that OA fits that description to a T. Dr. Hochberg said the big picture regarding OA as described in the white paper is this: It’s the most common form of arthritis, affecting more than 250 million people worldwide. And its costs approach 2% of the gross national product in the United States and other developed countries.
“Osteoarthritis accounts for more functional limitation, work loss, and physical disability than any other chronic disease, including cardiovascular disease and chronic obstructive pulmonary disease,” the rheumatologist said.
The white paper cites published data in support of these and other key points. At OARSI 2017, Dr. Hochberg presented highlights from the white paper, submitted to the FDA in December 2016:
• OA prevalence is relentlessly climbing. The Centers for Disease Control and Prevention put the U.S. prevalence of OA at 46 million in 2004 and has projected that it will reach 63 million in 2020 and 78 million Americans by 2040. The rise is being driven by the aging of the baby boomers, the obesity epidemic, predisposing physical injuries, and sedentary behavior.
• OA is expensive for patients and society. The combined direct medical costs and indirect costs stemming from lost earnings from OA amount to an estimated $461 billion annually in the United States.
• OA exacts a steep toll in years lived with disability (YLD). Estimated YLD from OA jumped by 75% during 1990-2013. This increase in YLD was exceeded only by dementia at 84% and diabetes at 135%. OA accounts for 1.6% of overall YLD in the United States, a rate comparable to ischemic heart disease at 1.63% and more than twice that for rheumatoid arthritis at 0.68%.
• Comorbidities are the rule. Various studies have estimated that 59%-87% of adults with OA have at least one additional significant chronic condition. The median number is two. One-third of OA patients have four or more additional comorbid conditions. The most common are cardiovascular disease, diabetes, obesity, metabolic syndrome, depression, anxiety, and falls and fractures.
• No effective treatments exist. There are no approved drugs that can prevent or even slow progression of OA to the point where total joint replacement is needed. Current medications are focused on pain relief and maintenance of functional independence. But these drugs are associated with significant risks of life-threatening side effects. NSAIDs have been linked to increased risk of cardiovascular events, GI bleeding, chronic kidney disease, and heart failure. And while opioids provide a small benefit in terms of pain relief, this is outweighed by the associated risks of falls, fractures, dependence, overdose, and death. All of these risks are accentuated in the presence of the common comorbid conditions associated with OA.
• OA increases the risk of dying prematurely. In a meta-analysis of individual patient data from the Multicenter Osteoarthritis Study and the Johnston County (N.C.) Osteoarthritis Project conducted specifically for the white paper, investigators determined that OA was associated with a 23% increase in the risk of death independent of age, race, and sex. This excess mortality is attributable in part to the presence of the metabolic syndrome and other commonly comorbid conditions, reduced physical activity because of OA disability, and the use of NSAIDs and opioid analgesics for symptomatic control.
The OARSI initiative is supported by EMD Serono, Fidia Pharma, Flexion Therapeutics, Nordic Biosciences, and Spinifex. Dr. Hochberg reported having numerous financial relationships with industry.
LAS VEGAS – The Osteoarthritis Research Society International has submitted a 103-page white paper to the Food and Drug Administration, the gist of which is captured in its title: “Osteoarthritis: A Serious Disease.”
The purpose of the voluminous white paper is to persuade FDA officials that osteoarthritis (OA) meets the agency’s formal definition of a serious disease for which there are currently no satisfactory treatments. That recognition would result in removal of current regulatory barriers to development of new structure-modifying treatments for OA, instead allowing such efforts to fall within the agency’s accelerated approval program, Marc C. Hochberg, MD, a coauthor of the white paper, explained at the World Congress on Osteoarthritis.
OARSI would like to see novel investigational therapies be allowed to advance through the developmental pipeline on the basis of favorable changes in clinically relevant biomarkers – be they biochemical or imaging – as intermediate endpoints serving as surrogates for structural change endpoints and meaningful clinical outcomes.
There is an enormous unmet need for effective disease-modifying therapies for OA. Establishing a more flexible regulatory environment for drug development by designating OA as a serious disease is expected to rekindle pharmaceutical industry interest in developing such products, which at present is at an ebb, he continued at the congress sponsored by the Osteoarthritis Research Society International.
The FDA has defined a serious disease as “a disease or condition associated with morbidity that has substantial impact on day-to-day functioning. Short-lived and self-limiting morbidity will usually not be sufficient, but the morbidity need not be irreversible if it is persistent or recurrent. Whether a disease or condition is serious is a matter of clinical judgment, based on its impact on such factors as survival, day-to-day functioning, or the likelihood that the disease, if left untreated, will progress from a less severe condition to a more serious one.”
The white paper makes the case that OA fits that description to a T. Dr. Hochberg said the big picture regarding OA as described in the white paper is this: It’s the most common form of arthritis, affecting more than 250 million people worldwide. And its costs approach 2% of the gross national product in the United States and other developed countries.
“Osteoarthritis accounts for more functional limitation, work loss, and physical disability than any other chronic disease, including cardiovascular disease and chronic obstructive pulmonary disease,” the rheumatologist said.
The white paper cites published data in support of these and other key points. At OARSI 2017, Dr. Hochberg presented highlights from the white paper, submitted to the FDA in December 2016:
• OA prevalence is relentlessly climbing. The Centers for Disease Control and Prevention put the U.S. prevalence of OA at 46 million in 2004 and has projected that it will reach 63 million in 2020 and 78 million Americans by 2040. The rise is being driven by the aging of the baby boomers, the obesity epidemic, predisposing physical injuries, and sedentary behavior.
• OA is expensive for patients and society. The combined direct medical costs and indirect costs stemming from lost earnings from OA amount to an estimated $461 billion annually in the United States.
• OA exacts a steep toll in years lived with disability (YLD). Estimated YLD from OA jumped by 75% during 1990-2013. This increase in YLD was exceeded only by dementia at 84% and diabetes at 135%. OA accounts for 1.6% of overall YLD in the United States, a rate comparable to ischemic heart disease at 1.63% and more than twice that for rheumatoid arthritis at 0.68%.
• Comorbidities are the rule. Various studies have estimated that 59%-87% of adults with OA have at least one additional significant chronic condition. The median number is two. One-third of OA patients have four or more additional comorbid conditions. The most common are cardiovascular disease, diabetes, obesity, metabolic syndrome, depression, anxiety, and falls and fractures.
• No effective treatments exist. There are no approved drugs that can prevent or even slow progression of OA to the point where total joint replacement is needed. Current medications are focused on pain relief and maintenance of functional independence. But these drugs are associated with significant risks of life-threatening side effects. NSAIDs have been linked to increased risk of cardiovascular events, GI bleeding, chronic kidney disease, and heart failure. And while opioids provide a small benefit in terms of pain relief, this is outweighed by the associated risks of falls, fractures, dependence, overdose, and death. All of these risks are accentuated in the presence of the common comorbid conditions associated with OA.
• OA increases the risk of dying prematurely. In a meta-analysis of individual patient data from the Multicenter Osteoarthritis Study and the Johnston County (N.C.) Osteoarthritis Project conducted specifically for the white paper, investigators determined that OA was associated with a 23% increase in the risk of death independent of age, race, and sex. This excess mortality is attributable in part to the presence of the metabolic syndrome and other commonly comorbid conditions, reduced physical activity because of OA disability, and the use of NSAIDs and opioid analgesics for symptomatic control.
The OARSI initiative is supported by EMD Serono, Fidia Pharma, Flexion Therapeutics, Nordic Biosciences, and Spinifex. Dr. Hochberg reported having numerous financial relationships with industry.
AT OARSI 2017
Lifetime risk of hand OA comes close to 40%
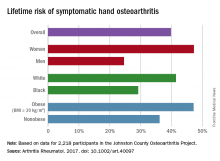
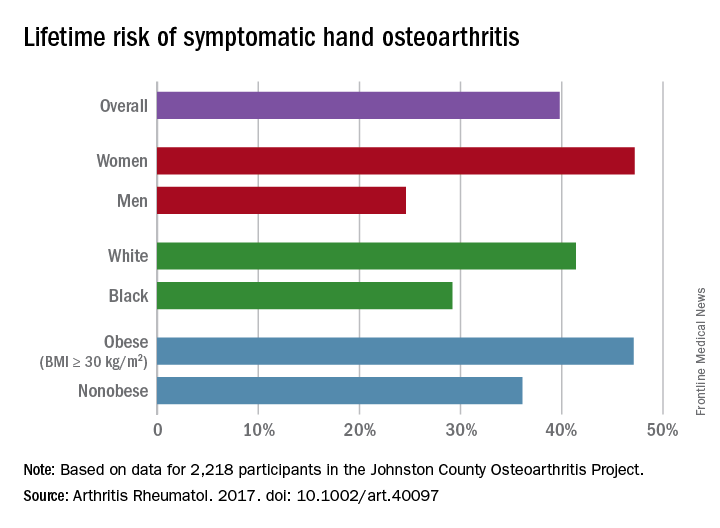
Almost 40% of Americans can expect to develop hand osteoarthritis (OA) in their lifetimes, according to an analysis involving participants from an ongoing population-based, prospective cohort study.
The overall risk, 39.8%, is based on data from 2,218 eligible subjects in the Johnston County Osteoarthritis Project, but there is significant variation among various subgroups, said Jin Qin, ScD, of the Centers for Disease Control and Prevention, and her associates (Arthritis Rheumatol. 2017 May 4. doi: 10.1002/art.40097).
This report is the first to estimate the lifetime risk of symptomatic hand OA, they noted, and “given the aging population and increasing life expectancy in the United States, it is reasonable to expect that more Americans will be affected by this painful and debilitating condition in the years to come.”
The study was funded by the CDC and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. The investigators did not include any disclosures in the report.
Almost 40% of Americans can expect to develop hand osteoarthritis (OA) in their lifetimes, according to an analysis involving participants from an ongoing population-based, prospective cohort study.
The overall risk, 39.8%, is based on data from 2,218 eligible subjects in the Johnston County Osteoarthritis Project, but there is significant variation among various subgroups, said Jin Qin, ScD, of the Centers for Disease Control and Prevention, and her associates (Arthritis Rheumatol. 2017 May 4. doi: 10.1002/art.40097).
This report is the first to estimate the lifetime risk of symptomatic hand OA, they noted, and “given the aging population and increasing life expectancy in the United States, it is reasonable to expect that more Americans will be affected by this painful and debilitating condition in the years to come.”
The study was funded by the CDC and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. The investigators did not include any disclosures in the report.
Almost 40% of Americans can expect to develop hand osteoarthritis (OA) in their lifetimes, according to an analysis involving participants from an ongoing population-based, prospective cohort study.
The overall risk, 39.8%, is based on data from 2,218 eligible subjects in the Johnston County Osteoarthritis Project, but there is significant variation among various subgroups, said Jin Qin, ScD, of the Centers for Disease Control and Prevention, and her associates (Arthritis Rheumatol. 2017 May 4. doi: 10.1002/art.40097).
This report is the first to estimate the lifetime risk of symptomatic hand OA, they noted, and “given the aging population and increasing life expectancy in the United States, it is reasonable to expect that more Americans will be affected by this painful and debilitating condition in the years to come.”
The study was funded by the CDC and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. The investigators did not include any disclosures in the report.
FROM ARTHRITIS & RHEUMATOLOGY
No increase in hand osteoarthritis seen in Sjögren’s syndrome
LAS VEGAS – Patients with Sjögren’s syndrome do not have an increased prevalence of hand osteoarthritis, but they are strongly predisposed to have a history of hypothyroidism, Jeremie Sellam, MD, reported at the World Congress on Osteoarthritis.
Both of these findings in his small case-control study were unexpected, he added at the congress sponsored by the Osteoarthritis Research Society International.
The study included 34 women with primary Sjögren’s syndrome according to the 2002 American-European Consensus Group criteria and 54 female controls with sicca syndrome but no autoantibodies and no Sjögren’s syndrome. All subjects were evaluated at a specialized tertiary Sjögren’s syndrome clinic. The controls were referred there to ascertain whether they had Sjögren’s syndrome.
Among the Sjögren’s syndrome patients, 41% had radiographic evidence of hand osteoarthritis, 12% had symptomatic hand osteoarthritis, and 9% had erosive hand osteoarthritis. In the sicca syndrome–only patients, the rates were similar at 52%, 28%, and 9%, respectively.
Looking for commonalities and differences between the Sjögren’s syndrome patients and controls, Dr. Sellam and his coinvestigators noted that the Sjögren’s syndrome patients were significantly older, with an average age of 64 years, compared with 48.5 years in the controls.
Impressively, two-thirds of the 15 Sjögren’s syndrome patients with hand osteoarthritis had a history of hypothyroidism, compared with just 15% of the 27 non-autoimmune sicca syndrome patients with hand osteoarthritis and one-quarter of the Sjögren’s syndrome patients without hand osteoarthritis. This suggests a possible interaction between Sjögren’s syndrome, hand osteoarthritis, and a history of hypothyroidism which merits further study, according to the rheumatologist.
Because of the relatively small patient numbers in the French study, Dr. Sellam and coworkers ran a crosscheck with data from the Framingham Osteoarthritis Study and found hand osteoarthritis prevalence rates comparable to their own findings. For example, the prevalences of radiographic and erosive hand osteoarthritis in the French Sjögren’s syndrome and non-autoimmune sicca syndrome groups were similar to the 44% and 10% figures, respectively, in the general population of age-matched Framingham women (Ann Rheum Dis. 2011 Sep;70[9]:1581-6).
He reported having no financial conflicts regarding his study, which was conducted free of commercial support.
LAS VEGAS – Patients with Sjögren’s syndrome do not have an increased prevalence of hand osteoarthritis, but they are strongly predisposed to have a history of hypothyroidism, Jeremie Sellam, MD, reported at the World Congress on Osteoarthritis.
Both of these findings in his small case-control study were unexpected, he added at the congress sponsored by the Osteoarthritis Research Society International.
The study included 34 women with primary Sjögren’s syndrome according to the 2002 American-European Consensus Group criteria and 54 female controls with sicca syndrome but no autoantibodies and no Sjögren’s syndrome. All subjects were evaluated at a specialized tertiary Sjögren’s syndrome clinic. The controls were referred there to ascertain whether they had Sjögren’s syndrome.
Among the Sjögren’s syndrome patients, 41% had radiographic evidence of hand osteoarthritis, 12% had symptomatic hand osteoarthritis, and 9% had erosive hand osteoarthritis. In the sicca syndrome–only patients, the rates were similar at 52%, 28%, and 9%, respectively.
Looking for commonalities and differences between the Sjögren’s syndrome patients and controls, Dr. Sellam and his coinvestigators noted that the Sjögren’s syndrome patients were significantly older, with an average age of 64 years, compared with 48.5 years in the controls.
Impressively, two-thirds of the 15 Sjögren’s syndrome patients with hand osteoarthritis had a history of hypothyroidism, compared with just 15% of the 27 non-autoimmune sicca syndrome patients with hand osteoarthritis and one-quarter of the Sjögren’s syndrome patients without hand osteoarthritis. This suggests a possible interaction between Sjögren’s syndrome, hand osteoarthritis, and a history of hypothyroidism which merits further study, according to the rheumatologist.
Because of the relatively small patient numbers in the French study, Dr. Sellam and coworkers ran a crosscheck with data from the Framingham Osteoarthritis Study and found hand osteoarthritis prevalence rates comparable to their own findings. For example, the prevalences of radiographic and erosive hand osteoarthritis in the French Sjögren’s syndrome and non-autoimmune sicca syndrome groups were similar to the 44% and 10% figures, respectively, in the general population of age-matched Framingham women (Ann Rheum Dis. 2011 Sep;70[9]:1581-6).
He reported having no financial conflicts regarding his study, which was conducted free of commercial support.
LAS VEGAS – Patients with Sjögren’s syndrome do not have an increased prevalence of hand osteoarthritis, but they are strongly predisposed to have a history of hypothyroidism, Jeremie Sellam, MD, reported at the World Congress on Osteoarthritis.
Both of these findings in his small case-control study were unexpected, he added at the congress sponsored by the Osteoarthritis Research Society International.
The study included 34 women with primary Sjögren’s syndrome according to the 2002 American-European Consensus Group criteria and 54 female controls with sicca syndrome but no autoantibodies and no Sjögren’s syndrome. All subjects were evaluated at a specialized tertiary Sjögren’s syndrome clinic. The controls were referred there to ascertain whether they had Sjögren’s syndrome.
Among the Sjögren’s syndrome patients, 41% had radiographic evidence of hand osteoarthritis, 12% had symptomatic hand osteoarthritis, and 9% had erosive hand osteoarthritis. In the sicca syndrome–only patients, the rates were similar at 52%, 28%, and 9%, respectively.
Looking for commonalities and differences between the Sjögren’s syndrome patients and controls, Dr. Sellam and his coinvestigators noted that the Sjögren’s syndrome patients were significantly older, with an average age of 64 years, compared with 48.5 years in the controls.
Impressively, two-thirds of the 15 Sjögren’s syndrome patients with hand osteoarthritis had a history of hypothyroidism, compared with just 15% of the 27 non-autoimmune sicca syndrome patients with hand osteoarthritis and one-quarter of the Sjögren’s syndrome patients without hand osteoarthritis. This suggests a possible interaction between Sjögren’s syndrome, hand osteoarthritis, and a history of hypothyroidism which merits further study, according to the rheumatologist.
Because of the relatively small patient numbers in the French study, Dr. Sellam and coworkers ran a crosscheck with data from the Framingham Osteoarthritis Study and found hand osteoarthritis prevalence rates comparable to their own findings. For example, the prevalences of radiographic and erosive hand osteoarthritis in the French Sjögren’s syndrome and non-autoimmune sicca syndrome groups were similar to the 44% and 10% figures, respectively, in the general population of age-matched Framingham women (Ann Rheum Dis. 2011 Sep;70[9]:1581-6).
He reported having no financial conflicts regarding his study, which was conducted free of commercial support.
AT OARSI 2017
Key clinical point:
Major finding: Nine percent of women with Sjögren’s syndrome had erosive hand osteoarthritis, a prevalence identical to that in a group with non-autoimmune sicca syndrome only.
Data source: This case-control study included 34 women with Sjögren’s syndrome and 54 women with non-autoimmune sicca syndrome.
Disclosures: The presenter reported having no financial conflicts regarding the study, which was conducted free of commercial support.
Adalimumab strikes out for hand osteoarthritis
LAS VEGAS – Adalimumab proved no better than placebo for the treatment of erosive hand osteoarthritis in the double-blind, placebo-controlled, randomized HUMOR trial, throwing cold water on hopes that a disease-modifying treatment for osteoarthritis might at long last have been found.
“This randomized controlled trial demonstrated that subcutaneous adalimumab at 40 mg every other week was no different from placebo for alleviation of pain, synovitis, or bone marrow lesions in patients with erosive hand osteoarthritis presenting with MRI-detected synovitis. This suggests that pain and inflammation are not responsive to TNF [tumor necrosis factor] inhibition in this patient population,” Dawn Aitken, PhD, declared at the World Congress on Osteoarthritis.
The primary endpoint was change in the visual analog pain score over the course of 12 weeks of therapy. Scores dropped by an average of 3.0 points from a mean baseline of 63.6 with adalimumab and by 0.7 points with placebo. That between-group difference wasn’t statistically significant, nor were those modest reductions clinically meaningful. By convention, a clinically meaningful treatment result requires at least a 15-point improvement on the self-assessed pain scale, noted Dr. Aitken of the University of Tasmania in Hobart, Australia.
It made no difference which treatment arm came first.
“There was absolutely no placebo effect,” she said.
The study proved negative for all prespecified secondary endpoints, too. These included change in the Australian/Canadian Hand OA Index pain, function, and stiffness subscales, as well as change in bone marrow lesions and synovitis. A mere 12% of patients showed improvement in synovitis scores with adalimumab, as did 10% on placebo. Five percent of the adalimumab group and 7% on placebo showed improvement in bone marrow lesion scores.
The proinflammatory cytokine TNF-alpha has been shown to play a key role in OA development and progression. However, several prior studies failed to show a benefit for anti-TNF therapy in terms of reducing pain in hand OA patients, although in one of those negative studies, a post hoc analysis found that adalimumab halted erosive progression in the subset of interphalangeal joints with palpable soft tissue swelling at baseline (Ann Rheum Dis. 2012 Jun;71[6]:891-8).
However, there was a positive signal: In a subgroup analysis confined to the patients with inflammatory joints, the use of etanercept was associated with less structural damage of those joints over time as assessed using the quantitative Ghent University Scoring System, or GUSS, according to Dr. Kloppenburg, professor of rheumatology at Leiden (the Netherlands) University.
The emphatically negative results of the HUMOR trial were greeted with dismay by several disappointed audience members. They raised questions: Might a study featuring more than 12 weeks of treatment have brought positive results? How about a larger study? Why no placebo effect, given that patients were receiving injections? Is this area of investigation of TNF-inhibitor therapy now a dead end? Or could adalimumab and etanercept have differential efficacy in hand OA, even though they are both TNF inhibitors?
Dr. Aitken had an answer for every question.
“I think our study was big enough based on our power calculations to detect a difference of 15 mm on a visual analog scale, which is a clinically important difference. And that’s what we should be chasing in OA, that big effect. Patients are not going to be interested in a treatment that improves their pain by 5 mm,” she said.
As for the possibility that 12 weeks of adalimumab might have been too short to see a treatment effect, Dr. Aitken noted that anti-TNF therapy in rheumatoid arthritis brings a rapid response.
“Ideally, if you did have a disease-modifying drug for osteoarthritis you would want it to have a relatively quick response for pain; if patients aren’t seeing an effect after 3 months they might be less interested in taking it,” she continued.
Also, studies with a crossover design, like HUMOR, are known to have a much smaller placebo effect because patients know that at some point they’re certain to receive the active agent, Dr. Aitken observed.
As for the possibility that etanercept might be effective for erosive hand OA, while adalimumab is not, one physician commented, “That’s really scraping the bottom of the barrel.”
The HUMOR trial was sponsored by AbbVie. Dr. Aitken reported having no financial conflicts.
LAS VEGAS – Adalimumab proved no better than placebo for the treatment of erosive hand osteoarthritis in the double-blind, placebo-controlled, randomized HUMOR trial, throwing cold water on hopes that a disease-modifying treatment for osteoarthritis might at long last have been found.
“This randomized controlled trial demonstrated that subcutaneous adalimumab at 40 mg every other week was no different from placebo for alleviation of pain, synovitis, or bone marrow lesions in patients with erosive hand osteoarthritis presenting with MRI-detected synovitis. This suggests that pain and inflammation are not responsive to TNF [tumor necrosis factor] inhibition in this patient population,” Dawn Aitken, PhD, declared at the World Congress on Osteoarthritis.
The primary endpoint was change in the visual analog pain score over the course of 12 weeks of therapy. Scores dropped by an average of 3.0 points from a mean baseline of 63.6 with adalimumab and by 0.7 points with placebo. That between-group difference wasn’t statistically significant, nor were those modest reductions clinically meaningful. By convention, a clinically meaningful treatment result requires at least a 15-point improvement on the self-assessed pain scale, noted Dr. Aitken of the University of Tasmania in Hobart, Australia.
It made no difference which treatment arm came first.
“There was absolutely no placebo effect,” she said.
The study proved negative for all prespecified secondary endpoints, too. These included change in the Australian/Canadian Hand OA Index pain, function, and stiffness subscales, as well as change in bone marrow lesions and synovitis. A mere 12% of patients showed improvement in synovitis scores with adalimumab, as did 10% on placebo. Five percent of the adalimumab group and 7% on placebo showed improvement in bone marrow lesion scores.
The proinflammatory cytokine TNF-alpha has been shown to play a key role in OA development and progression. However, several prior studies failed to show a benefit for anti-TNF therapy in terms of reducing pain in hand OA patients, although in one of those negative studies, a post hoc analysis found that adalimumab halted erosive progression in the subset of interphalangeal joints with palpable soft tissue swelling at baseline (Ann Rheum Dis. 2012 Jun;71[6]:891-8).
However, there was a positive signal: In a subgroup analysis confined to the patients with inflammatory joints, the use of etanercept was associated with less structural damage of those joints over time as assessed using the quantitative Ghent University Scoring System, or GUSS, according to Dr. Kloppenburg, professor of rheumatology at Leiden (the Netherlands) University.
The emphatically negative results of the HUMOR trial were greeted with dismay by several disappointed audience members. They raised questions: Might a study featuring more than 12 weeks of treatment have brought positive results? How about a larger study? Why no placebo effect, given that patients were receiving injections? Is this area of investigation of TNF-inhibitor therapy now a dead end? Or could adalimumab and etanercept have differential efficacy in hand OA, even though they are both TNF inhibitors?
Dr. Aitken had an answer for every question.
“I think our study was big enough based on our power calculations to detect a difference of 15 mm on a visual analog scale, which is a clinically important difference. And that’s what we should be chasing in OA, that big effect. Patients are not going to be interested in a treatment that improves their pain by 5 mm,” she said.
As for the possibility that 12 weeks of adalimumab might have been too short to see a treatment effect, Dr. Aitken noted that anti-TNF therapy in rheumatoid arthritis brings a rapid response.
“Ideally, if you did have a disease-modifying drug for osteoarthritis you would want it to have a relatively quick response for pain; if patients aren’t seeing an effect after 3 months they might be less interested in taking it,” she continued.
Also, studies with a crossover design, like HUMOR, are known to have a much smaller placebo effect because patients know that at some point they’re certain to receive the active agent, Dr. Aitken observed.
As for the possibility that etanercept might be effective for erosive hand OA, while adalimumab is not, one physician commented, “That’s really scraping the bottom of the barrel.”
The HUMOR trial was sponsored by AbbVie. Dr. Aitken reported having no financial conflicts.
LAS VEGAS – Adalimumab proved no better than placebo for the treatment of erosive hand osteoarthritis in the double-blind, placebo-controlled, randomized HUMOR trial, throwing cold water on hopes that a disease-modifying treatment for osteoarthritis might at long last have been found.
“This randomized controlled trial demonstrated that subcutaneous adalimumab at 40 mg every other week was no different from placebo for alleviation of pain, synovitis, or bone marrow lesions in patients with erosive hand osteoarthritis presenting with MRI-detected synovitis. This suggests that pain and inflammation are not responsive to TNF [tumor necrosis factor] inhibition in this patient population,” Dawn Aitken, PhD, declared at the World Congress on Osteoarthritis.
The primary endpoint was change in the visual analog pain score over the course of 12 weeks of therapy. Scores dropped by an average of 3.0 points from a mean baseline of 63.6 with adalimumab and by 0.7 points with placebo. That between-group difference wasn’t statistically significant, nor were those modest reductions clinically meaningful. By convention, a clinically meaningful treatment result requires at least a 15-point improvement on the self-assessed pain scale, noted Dr. Aitken of the University of Tasmania in Hobart, Australia.
It made no difference which treatment arm came first.
“There was absolutely no placebo effect,” she said.
The study proved negative for all prespecified secondary endpoints, too. These included change in the Australian/Canadian Hand OA Index pain, function, and stiffness subscales, as well as change in bone marrow lesions and synovitis. A mere 12% of patients showed improvement in synovitis scores with adalimumab, as did 10% on placebo. Five percent of the adalimumab group and 7% on placebo showed improvement in bone marrow lesion scores.
The proinflammatory cytokine TNF-alpha has been shown to play a key role in OA development and progression. However, several prior studies failed to show a benefit for anti-TNF therapy in terms of reducing pain in hand OA patients, although in one of those negative studies, a post hoc analysis found that adalimumab halted erosive progression in the subset of interphalangeal joints with palpable soft tissue swelling at baseline (Ann Rheum Dis. 2012 Jun;71[6]:891-8).
However, there was a positive signal: In a subgroup analysis confined to the patients with inflammatory joints, the use of etanercept was associated with less structural damage of those joints over time as assessed using the quantitative Ghent University Scoring System, or GUSS, according to Dr. Kloppenburg, professor of rheumatology at Leiden (the Netherlands) University.
The emphatically negative results of the HUMOR trial were greeted with dismay by several disappointed audience members. They raised questions: Might a study featuring more than 12 weeks of treatment have brought positive results? How about a larger study? Why no placebo effect, given that patients were receiving injections? Is this area of investigation of TNF-inhibitor therapy now a dead end? Or could adalimumab and etanercept have differential efficacy in hand OA, even though they are both TNF inhibitors?
Dr. Aitken had an answer for every question.
“I think our study was big enough based on our power calculations to detect a difference of 15 mm on a visual analog scale, which is a clinically important difference. And that’s what we should be chasing in OA, that big effect. Patients are not going to be interested in a treatment that improves their pain by 5 mm,” she said.
As for the possibility that 12 weeks of adalimumab might have been too short to see a treatment effect, Dr. Aitken noted that anti-TNF therapy in rheumatoid arthritis brings a rapid response.
“Ideally, if you did have a disease-modifying drug for osteoarthritis you would want it to have a relatively quick response for pain; if patients aren’t seeing an effect after 3 months they might be less interested in taking it,” she continued.
Also, studies with a crossover design, like HUMOR, are known to have a much smaller placebo effect because patients know that at some point they’re certain to receive the active agent, Dr. Aitken observed.
As for the possibility that etanercept might be effective for erosive hand OA, while adalimumab is not, one physician commented, “That’s really scraping the bottom of the barrel.”
The HUMOR trial was sponsored by AbbVie. Dr. Aitken reported having no financial conflicts.
FROM OARSI 2017
Key clinical point:
Major finding: Scores dropped by an average of 3.0 points from a mean baseline of 63.6 with adalimumab and by 0.7 points with placebo on the primary endpoint of change in the visual analog pain score over the course of 12 weeks.
Data source: The HUMOR trial was a double-blind, placebo-controlled, randomized, crossover trial in which 43 participants with erosive hand osteoarthritis received 12 weeks of treatment with adalimumab and 12 weeks of placebo.
Disclosures: The study was sponsored by AbbVie. The presenter reported having no financial conflicts.
Novel agent brings clinically meaningful improvements in knee osteoarthritis
LAS VEGAS – The investigational agent FX006 brought improvements in pain and function in patients with knee osteoarthritis that were not only statistically significant but also clinically meaningful by three different yardsticks, according to Scott Kelley, MD.
FX006 (Zilretta) is an extended-release, microsphere-based formulation of triamcinolone acetonide for intra-articular injection now under review for marketing approval by the Food and Drug Administration, which has said it will render a decision in October, Dr. Kelley reported at the World Congress on Osteoarthritis.
He presented a post hoc pooled analysis of the 798 patients with knee OA who participated in two phase II randomized, double-blind clinical trials and in an identically designed pivotal phase III trial of a single 40-mg intra-articular injection of FX006. The three studies included 324 patients who got FX006, 212 who received a single 40-mg intra-articular injection of standard triamcinolone acetonide crystalline suspension, and 262 who got a placebo injection of 5 mL of saline.
All participants had radiographically documented symptomatic knee OA with baseline daily pain scores of 6-9 on a 0-10 scale, corresponding to pain in the moderate-to-severe range. Patients telephoned in their average daily pain scores every day during the studies, one of which lasted 12 weeks while the other two ran for 24 weeks. Patients also visited their clinician every 4 weeks for Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) assessments of pain, stiffness, and physical function.
This secondary analysis was undertaken to demonstrate that FX006 distinguishes itself from current nonsurgical treatments for knee OA, which typically achieve clinical effects that are small, transient, and not clinically relevant, Dr. Kelley said at the congress sponsored by the Osteoarthritis Research Society International.
“What’s different about this product formulation is it maintains a prolonged release of triamcinolone acetonide inside the synovial fluid. The pharmacology has demonstrated prolonged residence time in synovial fluid out to at least 12 weeks after intra-articular administration. In addition, it mitigates the peak plasma level after intra-articular administration, so it blunts the level of corticosteroid in plasma and has a lower systemic exposure over time,” he said.
The clinical relevance of the outcomes achieved in the pooled studies was assessed using three different metrics: the American Academy of Orthopaedic Surgeons (AAOS) criteria for determining whether a result represents a minimal clinically important improvement; the Outcomes Measures in Rheumatology–Osteoarthritis Research Society International (OMERACT-OARSI) “strict responder” definition; and the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT)–defined proportion of patients achieving substantial improvement in pain.
At the 4- and 8-week follow-up visits post injection, only the FX006 group achieved the AAOS threshold of improvement that’s deemed both statistically and clinically significant. Standard triamcinolone couldn’t reach that bar, even at week 4. This makes FX006 the first intra-articular treatment for knee OA to achieve this stringent standard for a clinically meaningful effect. By week 12, the improvement in pain in the FX006 group had slipped to the level of ‘‘statistically and possibly clinically significant.” At weeks 16-24 following a single injection, the FX006 results were no longer statistically or clinically significant.
A significantly greater percentage of the FX006 group met the OMERACT-OARSI strict responder definition of substantial pain improvement at weeks 4, 8, and 12, compared with the standard triamcinolone and placebo groups. The degree of pain improvement in the FX006 group remained superior to placebo through week 16. The OMERACT-OARSI definition of substantial improvement requires at least a 50% improvement in the WOMAC-A pain score plus an absolute improvement of 20 points or more on the WOMAC-A pain score or WOMAC-C physical function 100-point normalized scale.
Substantial clinical improvement as defined by the IMMPACT criteria requires at least a 50% improvement from baseline in the WOMAC-A pain score. Roughly 60% and 50% of the FX006 group met that standard at weeks 4 and 8, respectively, rates that were significantly higher than for standard triamcinolone. The FX006 group’s IMMPACT substantial improvement rate significantly exceeded that of placebo-treated controls throughout all 24 weeks.
These studies as well as the pooled analysis were funded by Flexion Therapeutics.
FX006 is a type of steroid for joint injection that is formulated to stay in the joint longer than typical steroids through the use of microsphere technology. Typical intra-articular steroid injections tend to provide benefit over the short term, and as described by the Cochrane review, the greatest effect is only moderate at 1-2 weeks and decreases from there, with small effects at 13 weeks and none at 26 weeks (Cochrane Database Syst Rev. 2015;10:CD005328).
As we have previously reported (Semin Arthritis Rheum. 2014;43:701-12), intra-articular corticosteroids are widely recommended by professional societies for the management of knee OA, although a specific formulation is not generally given. This compound may fill an important treatment gap by extending the efficacy of a treatment that is generally accepted as useful and safe for this common painful condition. Also, given the controversy surrounding other intra-articular therapies, it may provide a safe and effective alternative for patients who have not benefited from, or not tolerated, one or more traditional intra-articular steroid injection(s).
Amanda E. Nelson, MD, is with the division of rheumatology, allergy, and immunology and the Thurston Arthritis Research Center at the University of North Carolina, Chapel Hill. She is a consultant to GlaxoSmithKline and has received honoraria for online presentations for MedScape and QuantiaMD regarding OA treatment guidelines.
FX006 is a type of steroid for joint injection that is formulated to stay in the joint longer than typical steroids through the use of microsphere technology. Typical intra-articular steroid injections tend to provide benefit over the short term, and as described by the Cochrane review, the greatest effect is only moderate at 1-2 weeks and decreases from there, with small effects at 13 weeks and none at 26 weeks (Cochrane Database Syst Rev. 2015;10:CD005328).
As we have previously reported (Semin Arthritis Rheum. 2014;43:701-12), intra-articular corticosteroids are widely recommended by professional societies for the management of knee OA, although a specific formulation is not generally given. This compound may fill an important treatment gap by extending the efficacy of a treatment that is generally accepted as useful and safe for this common painful condition. Also, given the controversy surrounding other intra-articular therapies, it may provide a safe and effective alternative for patients who have not benefited from, or not tolerated, one or more traditional intra-articular steroid injection(s).
Amanda E. Nelson, MD, is with the division of rheumatology, allergy, and immunology and the Thurston Arthritis Research Center at the University of North Carolina, Chapel Hill. She is a consultant to GlaxoSmithKline and has received honoraria for online presentations for MedScape and QuantiaMD regarding OA treatment guidelines.
FX006 is a type of steroid for joint injection that is formulated to stay in the joint longer than typical steroids through the use of microsphere technology. Typical intra-articular steroid injections tend to provide benefit over the short term, and as described by the Cochrane review, the greatest effect is only moderate at 1-2 weeks and decreases from there, with small effects at 13 weeks and none at 26 weeks (Cochrane Database Syst Rev. 2015;10:CD005328).
As we have previously reported (Semin Arthritis Rheum. 2014;43:701-12), intra-articular corticosteroids are widely recommended by professional societies for the management of knee OA, although a specific formulation is not generally given. This compound may fill an important treatment gap by extending the efficacy of a treatment that is generally accepted as useful and safe for this common painful condition. Also, given the controversy surrounding other intra-articular therapies, it may provide a safe and effective alternative for patients who have not benefited from, or not tolerated, one or more traditional intra-articular steroid injection(s).
Amanda E. Nelson, MD, is with the division of rheumatology, allergy, and immunology and the Thurston Arthritis Research Center at the University of North Carolina, Chapel Hill. She is a consultant to GlaxoSmithKline and has received honoraria for online presentations for MedScape and QuantiaMD regarding OA treatment guidelines.
LAS VEGAS – The investigational agent FX006 brought improvements in pain and function in patients with knee osteoarthritis that were not only statistically significant but also clinically meaningful by three different yardsticks, according to Scott Kelley, MD.
FX006 (Zilretta) is an extended-release, microsphere-based formulation of triamcinolone acetonide for intra-articular injection now under review for marketing approval by the Food and Drug Administration, which has said it will render a decision in October, Dr. Kelley reported at the World Congress on Osteoarthritis.
He presented a post hoc pooled analysis of the 798 patients with knee OA who participated in two phase II randomized, double-blind clinical trials and in an identically designed pivotal phase III trial of a single 40-mg intra-articular injection of FX006. The three studies included 324 patients who got FX006, 212 who received a single 40-mg intra-articular injection of standard triamcinolone acetonide crystalline suspension, and 262 who got a placebo injection of 5 mL of saline.
All participants had radiographically documented symptomatic knee OA with baseline daily pain scores of 6-9 on a 0-10 scale, corresponding to pain in the moderate-to-severe range. Patients telephoned in their average daily pain scores every day during the studies, one of which lasted 12 weeks while the other two ran for 24 weeks. Patients also visited their clinician every 4 weeks for Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) assessments of pain, stiffness, and physical function.
This secondary analysis was undertaken to demonstrate that FX006 distinguishes itself from current nonsurgical treatments for knee OA, which typically achieve clinical effects that are small, transient, and not clinically relevant, Dr. Kelley said at the congress sponsored by the Osteoarthritis Research Society International.
“What’s different about this product formulation is it maintains a prolonged release of triamcinolone acetonide inside the synovial fluid. The pharmacology has demonstrated prolonged residence time in synovial fluid out to at least 12 weeks after intra-articular administration. In addition, it mitigates the peak plasma level after intra-articular administration, so it blunts the level of corticosteroid in plasma and has a lower systemic exposure over time,” he said.
The clinical relevance of the outcomes achieved in the pooled studies was assessed using three different metrics: the American Academy of Orthopaedic Surgeons (AAOS) criteria for determining whether a result represents a minimal clinically important improvement; the Outcomes Measures in Rheumatology–Osteoarthritis Research Society International (OMERACT-OARSI) “strict responder” definition; and the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT)–defined proportion of patients achieving substantial improvement in pain.
At the 4- and 8-week follow-up visits post injection, only the FX006 group achieved the AAOS threshold of improvement that’s deemed both statistically and clinically significant. Standard triamcinolone couldn’t reach that bar, even at week 4. This makes FX006 the first intra-articular treatment for knee OA to achieve this stringent standard for a clinically meaningful effect. By week 12, the improvement in pain in the FX006 group had slipped to the level of ‘‘statistically and possibly clinically significant.” At weeks 16-24 following a single injection, the FX006 results were no longer statistically or clinically significant.
A significantly greater percentage of the FX006 group met the OMERACT-OARSI strict responder definition of substantial pain improvement at weeks 4, 8, and 12, compared with the standard triamcinolone and placebo groups. The degree of pain improvement in the FX006 group remained superior to placebo through week 16. The OMERACT-OARSI definition of substantial improvement requires at least a 50% improvement in the WOMAC-A pain score plus an absolute improvement of 20 points or more on the WOMAC-A pain score or WOMAC-C physical function 100-point normalized scale.
Substantial clinical improvement as defined by the IMMPACT criteria requires at least a 50% improvement from baseline in the WOMAC-A pain score. Roughly 60% and 50% of the FX006 group met that standard at weeks 4 and 8, respectively, rates that were significantly higher than for standard triamcinolone. The FX006 group’s IMMPACT substantial improvement rate significantly exceeded that of placebo-treated controls throughout all 24 weeks.
These studies as well as the pooled analysis were funded by Flexion Therapeutics.
LAS VEGAS – The investigational agent FX006 brought improvements in pain and function in patients with knee osteoarthritis that were not only statistically significant but also clinically meaningful by three different yardsticks, according to Scott Kelley, MD.
FX006 (Zilretta) is an extended-release, microsphere-based formulation of triamcinolone acetonide for intra-articular injection now under review for marketing approval by the Food and Drug Administration, which has said it will render a decision in October, Dr. Kelley reported at the World Congress on Osteoarthritis.
He presented a post hoc pooled analysis of the 798 patients with knee OA who participated in two phase II randomized, double-blind clinical trials and in an identically designed pivotal phase III trial of a single 40-mg intra-articular injection of FX006. The three studies included 324 patients who got FX006, 212 who received a single 40-mg intra-articular injection of standard triamcinolone acetonide crystalline suspension, and 262 who got a placebo injection of 5 mL of saline.
All participants had radiographically documented symptomatic knee OA with baseline daily pain scores of 6-9 on a 0-10 scale, corresponding to pain in the moderate-to-severe range. Patients telephoned in their average daily pain scores every day during the studies, one of which lasted 12 weeks while the other two ran for 24 weeks. Patients also visited their clinician every 4 weeks for Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) assessments of pain, stiffness, and physical function.
This secondary analysis was undertaken to demonstrate that FX006 distinguishes itself from current nonsurgical treatments for knee OA, which typically achieve clinical effects that are small, transient, and not clinically relevant, Dr. Kelley said at the congress sponsored by the Osteoarthritis Research Society International.
“What’s different about this product formulation is it maintains a prolonged release of triamcinolone acetonide inside the synovial fluid. The pharmacology has demonstrated prolonged residence time in synovial fluid out to at least 12 weeks after intra-articular administration. In addition, it mitigates the peak plasma level after intra-articular administration, so it blunts the level of corticosteroid in plasma and has a lower systemic exposure over time,” he said.
The clinical relevance of the outcomes achieved in the pooled studies was assessed using three different metrics: the American Academy of Orthopaedic Surgeons (AAOS) criteria for determining whether a result represents a minimal clinically important improvement; the Outcomes Measures in Rheumatology–Osteoarthritis Research Society International (OMERACT-OARSI) “strict responder” definition; and the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT)–defined proportion of patients achieving substantial improvement in pain.
At the 4- and 8-week follow-up visits post injection, only the FX006 group achieved the AAOS threshold of improvement that’s deemed both statistically and clinically significant. Standard triamcinolone couldn’t reach that bar, even at week 4. This makes FX006 the first intra-articular treatment for knee OA to achieve this stringent standard for a clinically meaningful effect. By week 12, the improvement in pain in the FX006 group had slipped to the level of ‘‘statistically and possibly clinically significant.” At weeks 16-24 following a single injection, the FX006 results were no longer statistically or clinically significant.
A significantly greater percentage of the FX006 group met the OMERACT-OARSI strict responder definition of substantial pain improvement at weeks 4, 8, and 12, compared with the standard triamcinolone and placebo groups. The degree of pain improvement in the FX006 group remained superior to placebo through week 16. The OMERACT-OARSI definition of substantial improvement requires at least a 50% improvement in the WOMAC-A pain score plus an absolute improvement of 20 points or more on the WOMAC-A pain score or WOMAC-C physical function 100-point normalized scale.
Substantial clinical improvement as defined by the IMMPACT criteria requires at least a 50% improvement from baseline in the WOMAC-A pain score. Roughly 60% and 50% of the FX006 group met that standard at weeks 4 and 8, respectively, rates that were significantly higher than for standard triamcinolone. The FX006 group’s IMMPACT substantial improvement rate significantly exceeded that of placebo-treated controls throughout all 24 weeks.
These studies as well as the pooled analysis were funded by Flexion Therapeutics.
AT OARSI 2017
Key clinical point:
Major finding: The investigational extended-release formulation of triamcinolone, known as FX006 (Zilretta), is the first intra-articular treatment for knee OA to meet the American Academy of Orthopaedic Surgeons’ stringent standard for a clinically meaningful, as opposed to merely statistically significant, effect.
Data source: A post hoc pooled analysis of three randomized, double-blind clinical trials involving nearly 800 patients with knee osteoarthritis.
Disclosures: The pooled analysis was funded by Flexion Therapeutics and presented by a company officer.
Knee osteoarthritis linked to premature mortality
LAS VEGAS – Knee osteoarthritis was independently associated with increased risk of premature mortality in the largest-ever meta-analysis to examine the issue, Kirsten M. Leyland, PhD, reported at the World Congress on Osteoarthritis.
The meta-analysis used individual patient-level data on 11,954 participants in six prospective observational cohort studies conducted in four countries. The key finding: Subjects with symptomatic and radiographically confirmed knee OA were at 17% greater risk of premature mortality, compared with pain-free, radiographically negative participants, independent of age, sex, and race, according to Dr. Leyland, a musculoskeletal epidemiologist at the University of Oxford (England).
The six prospective cohort studies included in the new analysis are well known in the field of osteoarthritis research: the Framingham (Mass.) Osteoarthritis Study, the Rotterdam (the Netherlands) Study, the Multicenter Osteoarthritis Study (MOST), the Johnston County (N.C.) Osteoarthritis Project, the Tasmanian Older Adult Cohort Study, and the Chingford (U.K.) 1000 Women Study. These studies feature 7.4-23.7 years of prospective follow-up.
In an interview, Dr. Leyland said that the current analysis uses all-cause mortality as the outcome because causes of death are recorded differently in the countries where the studies are taking place. It will take several more years for statisticians to harmonize the cause-of-death data and draw definitive conclusions; however, preliminary analysis suggests the causes of premature mortality in the knee OA patients are disproportionately cardiovascular, she added.
Dr. Leyland reported having no financial conflicts regarding the study, which was supported by Arthritis Research UK.
LAS VEGAS – Knee osteoarthritis was independently associated with increased risk of premature mortality in the largest-ever meta-analysis to examine the issue, Kirsten M. Leyland, PhD, reported at the World Congress on Osteoarthritis.
The meta-analysis used individual patient-level data on 11,954 participants in six prospective observational cohort studies conducted in four countries. The key finding: Subjects with symptomatic and radiographically confirmed knee OA were at 17% greater risk of premature mortality, compared with pain-free, radiographically negative participants, independent of age, sex, and race, according to Dr. Leyland, a musculoskeletal epidemiologist at the University of Oxford (England).
The six prospective cohort studies included in the new analysis are well known in the field of osteoarthritis research: the Framingham (Mass.) Osteoarthritis Study, the Rotterdam (the Netherlands) Study, the Multicenter Osteoarthritis Study (MOST), the Johnston County (N.C.) Osteoarthritis Project, the Tasmanian Older Adult Cohort Study, and the Chingford (U.K.) 1000 Women Study. These studies feature 7.4-23.7 years of prospective follow-up.
In an interview, Dr. Leyland said that the current analysis uses all-cause mortality as the outcome because causes of death are recorded differently in the countries where the studies are taking place. It will take several more years for statisticians to harmonize the cause-of-death data and draw definitive conclusions; however, preliminary analysis suggests the causes of premature mortality in the knee OA patients are disproportionately cardiovascular, she added.
Dr. Leyland reported having no financial conflicts regarding the study, which was supported by Arthritis Research UK.
LAS VEGAS – Knee osteoarthritis was independently associated with increased risk of premature mortality in the largest-ever meta-analysis to examine the issue, Kirsten M. Leyland, PhD, reported at the World Congress on Osteoarthritis.
The meta-analysis used individual patient-level data on 11,954 participants in six prospective observational cohort studies conducted in four countries. The key finding: Subjects with symptomatic and radiographically confirmed knee OA were at 17% greater risk of premature mortality, compared with pain-free, radiographically negative participants, independent of age, sex, and race, according to Dr. Leyland, a musculoskeletal epidemiologist at the University of Oxford (England).
The six prospective cohort studies included in the new analysis are well known in the field of osteoarthritis research: the Framingham (Mass.) Osteoarthritis Study, the Rotterdam (the Netherlands) Study, the Multicenter Osteoarthritis Study (MOST), the Johnston County (N.C.) Osteoarthritis Project, the Tasmanian Older Adult Cohort Study, and the Chingford (U.K.) 1000 Women Study. These studies feature 7.4-23.7 years of prospective follow-up.
In an interview, Dr. Leyland said that the current analysis uses all-cause mortality as the outcome because causes of death are recorded differently in the countries where the studies are taking place. It will take several more years for statisticians to harmonize the cause-of-death data and draw definitive conclusions; however, preliminary analysis suggests the causes of premature mortality in the knee OA patients are disproportionately cardiovascular, she added.
Dr. Leyland reported having no financial conflicts regarding the study, which was supported by Arthritis Research UK.
AT OARSI 2017
Key clinical point:
Major finding: Knee osteoarthritis is independently associated with a 17% increased likelihood of premature mortality.
Data source: This meta-analysis harmonized individual patient-level data drawn from six major prospective, population-based cohort studies worldwide.
Disclosures: The presenter reported having no financial conflicts regarding the study, which was supported by Arthritis Research UK.
Short course of prednisolone may help distinguish between RA and hand OA
A short course of prednisolone may help rheumatologists differentiate between patients with rheumatoid arthritis and osteoarthritis, a proof of concept study shows.
However, the investigators caution that a positive response to the 3-day steroid course does not confirm a diagnosis of rheumatoid arthritis (RA).
For many years, rheumatologists have been using short courses of prednisolone in unclear clinical situations to differentiate between inflammatory and non-inflammatory arthritis, according to the investigators led by Uta Kiltz, MD, from Rheumazentrum Ruhrgebiet, Herne, Germany.
The pilot part of the TryCort study involved 15 patients with confirmed osteoarthritis and 15 with rheumatoid arthritis who were given 1 g of paracetamol (acetaminophen) a day for 5 days, and on days 3-5, they were given a 20-mg dose of prednisolone (Arthritis Res Ther. 2017;19:73. doi: 10.1186/s13075-017-1279-z).
Results showed that the patients with RA had greater improvements in their pain scores (0-10 on a numerical rating scale), compared with OA patients. The mean percentage improvement in pain scores at day 5 was 52.3% in the RA group and 22.0% in the OA group.
The research team considered that a 40% improvement in pain scores was the best choice between sensitivity and specificity regarding a diagnosis of RA.
At this 40% improvement cut-off, the “pred-test” was positive in 11 patients with RA and in four patients with OA (P = .012), with a sensitivity and specificity for a diagnosis of RA of 73.3% for both measures.
In order to validate the test, the researchers enrolled 95 patients with pain in their fingers and hands but without a clear diagnosis. These patients completed the 5-day intervention, and then at week 12 a rheumatologist diagnosed 47 as having RA and 48 were thought to not have RA.
The patients with diagnosed RA had a higher reduction in pain scores during the treatment with prednisolone, compared with patients without RA.
The median percentage of improvement at day 5 was higher in patients with RA than in those without RA (50% [interquartile range, 30%-60%] vs. 20% [IQR, 10%-30%]; P = .001). Overall, 40 of the 95 patients had an improvement of more than 40% in pain levels on day 5, fulfilling the criteria of a positive pred-test.
However, the authors noted that 31 patients with RA had a positive pred-test (77.5%), compared with nine (22.5%) patients without RA (P greater than .001).
The sensitivity of the pred-test for a diagnosis of RA was 0.6 (95% confidence interval, 0.5-0.8) and the specificity was 0.8 (95% CI, 0.7-0.9). The positive and negative predictive values were 0.77 and 0.70, respectively.
The authors concluded that the pred-test “performed well” but not “perfectly well.”
“We are aware that the pred-test without confirmation of other surrogate markers is not helpful in clinical decision-making processes,” they said. “We, therefore, recommend use of the test in light of other confirming factors, such as history, physical examination, imaging, and laboratory results.”
The test could be used to triage patients from primary care to rheumatologist care, they suggested.
The study was financially supported by Rheumazentrum Ruhrgebiet. The authors declared no conflicts of interest.
A short course of prednisolone may help rheumatologists differentiate between patients with rheumatoid arthritis and osteoarthritis, a proof of concept study shows.
However, the investigators caution that a positive response to the 3-day steroid course does not confirm a diagnosis of rheumatoid arthritis (RA).
For many years, rheumatologists have been using short courses of prednisolone in unclear clinical situations to differentiate between inflammatory and non-inflammatory arthritis, according to the investigators led by Uta Kiltz, MD, from Rheumazentrum Ruhrgebiet, Herne, Germany.
The pilot part of the TryCort study involved 15 patients with confirmed osteoarthritis and 15 with rheumatoid arthritis who were given 1 g of paracetamol (acetaminophen) a day for 5 days, and on days 3-5, they were given a 20-mg dose of prednisolone (Arthritis Res Ther. 2017;19:73. doi: 10.1186/s13075-017-1279-z).
Results showed that the patients with RA had greater improvements in their pain scores (0-10 on a numerical rating scale), compared with OA patients. The mean percentage improvement in pain scores at day 5 was 52.3% in the RA group and 22.0% in the OA group.
The research team considered that a 40% improvement in pain scores was the best choice between sensitivity and specificity regarding a diagnosis of RA.
At this 40% improvement cut-off, the “pred-test” was positive in 11 patients with RA and in four patients with OA (P = .012), with a sensitivity and specificity for a diagnosis of RA of 73.3% for both measures.
In order to validate the test, the researchers enrolled 95 patients with pain in their fingers and hands but without a clear diagnosis. These patients completed the 5-day intervention, and then at week 12 a rheumatologist diagnosed 47 as having RA and 48 were thought to not have RA.
The patients with diagnosed RA had a higher reduction in pain scores during the treatment with prednisolone, compared with patients without RA.
The median percentage of improvement at day 5 was higher in patients with RA than in those without RA (50% [interquartile range, 30%-60%] vs. 20% [IQR, 10%-30%]; P = .001). Overall, 40 of the 95 patients had an improvement of more than 40% in pain levels on day 5, fulfilling the criteria of a positive pred-test.
However, the authors noted that 31 patients with RA had a positive pred-test (77.5%), compared with nine (22.5%) patients without RA (P greater than .001).
The sensitivity of the pred-test for a diagnosis of RA was 0.6 (95% confidence interval, 0.5-0.8) and the specificity was 0.8 (95% CI, 0.7-0.9). The positive and negative predictive values were 0.77 and 0.70, respectively.
The authors concluded that the pred-test “performed well” but not “perfectly well.”
“We are aware that the pred-test without confirmation of other surrogate markers is not helpful in clinical decision-making processes,” they said. “We, therefore, recommend use of the test in light of other confirming factors, such as history, physical examination, imaging, and laboratory results.”
The test could be used to triage patients from primary care to rheumatologist care, they suggested.
The study was financially supported by Rheumazentrum Ruhrgebiet. The authors declared no conflicts of interest.
A short course of prednisolone may help rheumatologists differentiate between patients with rheumatoid arthritis and osteoarthritis, a proof of concept study shows.
However, the investigators caution that a positive response to the 3-day steroid course does not confirm a diagnosis of rheumatoid arthritis (RA).
For many years, rheumatologists have been using short courses of prednisolone in unclear clinical situations to differentiate between inflammatory and non-inflammatory arthritis, according to the investigators led by Uta Kiltz, MD, from Rheumazentrum Ruhrgebiet, Herne, Germany.
The pilot part of the TryCort study involved 15 patients with confirmed osteoarthritis and 15 with rheumatoid arthritis who were given 1 g of paracetamol (acetaminophen) a day for 5 days, and on days 3-5, they were given a 20-mg dose of prednisolone (Arthritis Res Ther. 2017;19:73. doi: 10.1186/s13075-017-1279-z).
Results showed that the patients with RA had greater improvements in their pain scores (0-10 on a numerical rating scale), compared with OA patients. The mean percentage improvement in pain scores at day 5 was 52.3% in the RA group and 22.0% in the OA group.
The research team considered that a 40% improvement in pain scores was the best choice between sensitivity and specificity regarding a diagnosis of RA.
At this 40% improvement cut-off, the “pred-test” was positive in 11 patients with RA and in four patients with OA (P = .012), with a sensitivity and specificity for a diagnosis of RA of 73.3% for both measures.
In order to validate the test, the researchers enrolled 95 patients with pain in their fingers and hands but without a clear diagnosis. These patients completed the 5-day intervention, and then at week 12 a rheumatologist diagnosed 47 as having RA and 48 were thought to not have RA.
The patients with diagnosed RA had a higher reduction in pain scores during the treatment with prednisolone, compared with patients without RA.
The median percentage of improvement at day 5 was higher in patients with RA than in those without RA (50% [interquartile range, 30%-60%] vs. 20% [IQR, 10%-30%]; P = .001). Overall, 40 of the 95 patients had an improvement of more than 40% in pain levels on day 5, fulfilling the criteria of a positive pred-test.
However, the authors noted that 31 patients with RA had a positive pred-test (77.5%), compared with nine (22.5%) patients without RA (P greater than .001).
The sensitivity of the pred-test for a diagnosis of RA was 0.6 (95% confidence interval, 0.5-0.8) and the specificity was 0.8 (95% CI, 0.7-0.9). The positive and negative predictive values were 0.77 and 0.70, respectively.
The authors concluded that the pred-test “performed well” but not “perfectly well.”
“We are aware that the pred-test without confirmation of other surrogate markers is not helpful in clinical decision-making processes,” they said. “We, therefore, recommend use of the test in light of other confirming factors, such as history, physical examination, imaging, and laboratory results.”
The test could be used to triage patients from primary care to rheumatologist care, they suggested.
The study was financially supported by Rheumazentrum Ruhrgebiet. The authors declared no conflicts of interest.
FROM ARTHRITIS RESEARCH & THERAPY
Key clinical point:
Major finding: The sensitivity of the pred-test for a diagnosis of RA was 0.6 (95% CI, 0.5-0.8) and the specificity was 0.8 (95% CI, 0.7-0.9).
Data source: A pilot study of 20 mg of prednisolone for 3 days in 30 patients with established RA or OA followed by a validation study of the test in 95 patients with pain in their fingers and hands but without a clear diagnosis.
Disclosures: The study was financially supported by Rheumazentrum Ruhrgebiet. The authors declared no conflicts of interest.
Compounding rules challenge practice norms
As new rules about drug compounding get shaped, rheumatologists seek to protect their ability to combine injectable drugs – most commonly a steroid and a local anesthetic – in their own offices.
In a position statement sent to government agencies and members of Congress in February, the American College of Rheumatology voiced concerns that the practice, which it called “critical,” could become a casualty of drug-compounding regulations under revision by the United States Pharmacopeial Convention (USP), a nonprofit group whose standards are enforceable by state and federal regulators.
In the same position statement on compounding, the ACR said it also seeks a change to a Food and Drug Administration rule limiting practitioners’ access to quinacrine, a drug only available through compounding pharmacies that is sometimes used to treat lupus patients. Quinacrine is not on the FDA’s current list of bulk substances approved for compounding, except by special permission. The ACR has asked the agency to add quinacrine to the list, but no one knows when this will happen.
Rheumatologists may also be more restricted than before in terms of which compounding pharmacies they can turn to, as new federal standards divide them into two types – those that can provide medicines in larger quantities and those that can’t.
Steroid fiasco sparked rule revisions
The ACR’s concerns follow a tighter focus by state and federal agencies on drug compounding after a fungal meningitis outbreak in 2012 was traced to contaminated steroids produced in bulk by a compounding pharmacy.
More than 800 infections, 64 of them fatal, occurred after the New England Compounding Center in Framingham, Mass., sold contaminated methylprednisolone acetate that was used in epidural and intra-articular joint injections.
The following year Congress passed the Drug Quality and Security Act, which aims, in part, to prevent compounding pharmacies from engaging in what amounts to unregulated manufacturing.
As part of the law, the FDA created a list of drugs appropriate for compounding and a process by which larger compounding pharmacies must register with the FDA, and agree to inspections. The USP standards, meanwhile, address detailed technical and safety aspects of compounding and are enforceable by the FDA and state agencies.
“USP and FDA have had the ability to regulate compounding for over a decade, but only recently have the rules become actively enforced,” said Donald Miller, PharmD, of North Dakota State University, Fargo, who helped shape the ACR’s position statement on compounding with the help of rheumatologists in private practice.
“When you make guidelines for safety, they make sense, but then you can’t anticipate the way it’s going to affect individuals’ practice. And that’s where rheumatology got caught up,” said Dr. Miller, who was a member of the FDA Arthritis Advisory Committee in 2014-2016.
In-office mixing a top concern
Other specialties, including dermatology and immunology, also stand to be affected by various changes to compounding law and practice – and their societies have been active in voicing concerns.
Though the latest revisions of USP chapter 797, which impacts in-office mixing, are still being sorted out, it’s the No. 1 compounding-related concern for rheumatologists, Dr. Miller said.
Rheumatologists routinely mix an analgesic and a steroid for injection. The analgesic makes the steroids less viscous, and offers patients hours of immediate relief. They also add analgesics to hyaluronic acid injected for viscosupplementation. The mixing is usually conducted bedside, and the injections are administered right away.
Technically, combining these products amounts to sterile compounding, Dr. Miller explained. “And theoretically, under these rules, a physician could still do this, but they’d have to do it under a sterile hood like you find in a pharmacy, and that’s just not practical. It also becomes a matter of interpretation.”
USP chapter 797 sanctions in-office mixing for “immediate use” with individual patients – which is nearly always the case for the steroid injections used in rheumatology. But it’s unclear whether “immediate use” means emergency use only, or allows for routine use, as rheumatologists hope.
“One reason this came to rheumatology’s attention is that some state boards of medicine were inspecting and saying ‘Hey, you can’t do that,’ ” Dr. Miller said.
“There’s that law of unintended consequences where you snare things in a net that you really don’t want to,” Dr. Huffstutter said.
Marcus Snow, MD, a rheumatologist at the University of Nebraska, Omaha, who also worked on the statement, said that most rheumatologists are likely unaware that their ability to mix drugs in-office has been called into question.
“I brought it up at our division meeting with a group of 10 rheumatologists, and no one was aware that this was coming down the pike,” Dr. Snow said in an interview.
Pediatric issues
Pediatric rheumatologists, and adult rheumatologists who see children occasionally, use compounding pharmacies to create palatable oral medicines and adjusted doses of adult treatments.
They also use injections combining steroids with analgesics, and consider the addition of the analgesic a key aid to compliance.
“The biggest barrier we have is patient and parent anxiety about doing the procedure and the associated pain. We always administer our steroids mixed with lidocaine to help with the postprocedural discomfort,” said Adam Reinhardt, MD, chief of pediatric rheumatology at the University of Nebraska and Children’s Hospital and Medical Center in Omaha.
Steroid injections can mean avoiding or delaying systemic treatment in children with oligoarticular arthritis, he said. “Most of us consider them a first-line therapy. The hope is that you can get by without having to use meds like methotrexate if you can get a prolonged response in the one or two joints that are active in that patient.”
But Dr. Reinhardt said that, while he mixed his own injections during his fellowship training, Children’s of Omaha now insists that they be prepared by in-house pharmacists, working under sterile hoods. The delay to receiving them in the clinic or procedure room is 40 minutes to an hour, he said, which the clinicians accommodate through careful scheduling.
The change from mixing in-clinic to relying on the central pharmacy came about in recent years, Dr. Reinhardt said, because of broader concerns related to medication storage in the clinics. While ordering from the central pharmacy works for his practice, he said, “I probably only inject maybe 50-70 joints a year, while adult rheumatologists are injecting far more than that. For a busy private practice, I can see that being a huge time constraint,” he said.
Relevance of rules
None of the rheumatologists interviewed questioned the need for tightened state and federal oversight of compounding practices overall – just the applicability of certain rules to their own practice.
Dr. Snow and Dr. Huffstutter noted that reports of infected joints – a potential result of a contaminated injection – are sporadic and rare. “There’s very little research in this, but [these types of injections] have been standard practice for decades,” Dr. Snow said.
Srikanth Mukkera, MD, a rheumatologist in Tupelo, Miss., agreed that “sporadic cases of joint infection do happen following injection, but it can be hard to show if an injection was the cause.”
Assuring that medicines are mixed only immediately prior to injection, and not stored, reduces the likelihood of contamination, Dr. Mukkera said. Moreover, he noted, epidural injections such as those that resulted in the 2012 meningitis outbreak carry different risks than those seen in intra-articular injections.
Dr. Miller, the lead author of the ACR statement, said that the rheumatologists on our committee “don’t know of anyone that’s had a knee or other joint infection from a contaminated injection. They feel that unless somebody finds some evidence of that, they should be allowed to continue” with their usual practice.
He said that he feels that the USP will ultimately heed the concerns of rheumatologists and hopefully provide a more relaxed interpretation of in-office compounding. “We’re hoping they’ll make some exceptions when they revise 797 standards or at least maybe leave room for organizations to create a best practice statement. We’ll see,” Dr. Miller said.
But this is in no way guaranteed. Dr. Huffstutter said he fears that, if the rules come to be interpreted more narrowly, even standard practices like reconstituting biologic drugs for infusion – something that’s also a routine part of in-office practice – could fall under the rubric of sterile compounding and come into question.
The quinacrine problem
A separate compounding-related issue in rheumatology is clinicians’ access to quinacrine, an antimalarial rheumatology drug that, while infrequently used, represents the only alternative to hydroxychloroquine for some lupus patients.
“There are no alternatives out there for hydroxychloroquine, so we need it as a backup,” Dr. Snow said. “If hydroxychloroquine isn’t an option, there’s nothing out there that we can use. There’s no easy replacement.”
Dr. Huffstutter said he currently had no patients on quinacrine. “It’s not very often that we use it, but in those patients that really need it, it can make a huge difference in how they do.”
Quinacrine is no longer manufactured commercially as a finished drug product but is available in a powder that compounding physicians put into 100-mg capsules. It is not on the FDA’s current list of drugs available for compounding except with special permission.
While the ACR has requested that the FDA add it the list of bulk drug substances that can be used in compounding, quinacrine remains off the list for now – and, providers say, hard to find.
Moreover, while rheumatologists may have previously been able to order and store quantities of quinacrine and other compounded nonsterile medications to dispense to their patients, they can no longer easily do so, as only the FDA-approved compounding “outsourcing facilities” are allowed to process larger orders; the rest can only respond to prescriptions for individual patients.
Dr. Miller said it’s likely that quinacrine will make it onto the FDA’s next list of bulk drugs available for compounding. “The FDA has kind of said, ‘Don’t worry about it,’ ” he said.
As new rules about drug compounding get shaped, rheumatologists seek to protect their ability to combine injectable drugs – most commonly a steroid and a local anesthetic – in their own offices.
In a position statement sent to government agencies and members of Congress in February, the American College of Rheumatology voiced concerns that the practice, which it called “critical,” could become a casualty of drug-compounding regulations under revision by the United States Pharmacopeial Convention (USP), a nonprofit group whose standards are enforceable by state and federal regulators.
In the same position statement on compounding, the ACR said it also seeks a change to a Food and Drug Administration rule limiting practitioners’ access to quinacrine, a drug only available through compounding pharmacies that is sometimes used to treat lupus patients. Quinacrine is not on the FDA’s current list of bulk substances approved for compounding, except by special permission. The ACR has asked the agency to add quinacrine to the list, but no one knows when this will happen.
Rheumatologists may also be more restricted than before in terms of which compounding pharmacies they can turn to, as new federal standards divide them into two types – those that can provide medicines in larger quantities and those that can’t.
Steroid fiasco sparked rule revisions
The ACR’s concerns follow a tighter focus by state and federal agencies on drug compounding after a fungal meningitis outbreak in 2012 was traced to contaminated steroids produced in bulk by a compounding pharmacy.
More than 800 infections, 64 of them fatal, occurred after the New England Compounding Center in Framingham, Mass., sold contaminated methylprednisolone acetate that was used in epidural and intra-articular joint injections.
The following year Congress passed the Drug Quality and Security Act, which aims, in part, to prevent compounding pharmacies from engaging in what amounts to unregulated manufacturing.
As part of the law, the FDA created a list of drugs appropriate for compounding and a process by which larger compounding pharmacies must register with the FDA, and agree to inspections. The USP standards, meanwhile, address detailed technical and safety aspects of compounding and are enforceable by the FDA and state agencies.
“USP and FDA have had the ability to regulate compounding for over a decade, but only recently have the rules become actively enforced,” said Donald Miller, PharmD, of North Dakota State University, Fargo, who helped shape the ACR’s position statement on compounding with the help of rheumatologists in private practice.
“When you make guidelines for safety, they make sense, but then you can’t anticipate the way it’s going to affect individuals’ practice. And that’s where rheumatology got caught up,” said Dr. Miller, who was a member of the FDA Arthritis Advisory Committee in 2014-2016.
In-office mixing a top concern
Other specialties, including dermatology and immunology, also stand to be affected by various changes to compounding law and practice – and their societies have been active in voicing concerns.
Though the latest revisions of USP chapter 797, which impacts in-office mixing, are still being sorted out, it’s the No. 1 compounding-related concern for rheumatologists, Dr. Miller said.
Rheumatologists routinely mix an analgesic and a steroid for injection. The analgesic makes the steroids less viscous, and offers patients hours of immediate relief. They also add analgesics to hyaluronic acid injected for viscosupplementation. The mixing is usually conducted bedside, and the injections are administered right away.
Technically, combining these products amounts to sterile compounding, Dr. Miller explained. “And theoretically, under these rules, a physician could still do this, but they’d have to do it under a sterile hood like you find in a pharmacy, and that’s just not practical. It also becomes a matter of interpretation.”
USP chapter 797 sanctions in-office mixing for “immediate use” with individual patients – which is nearly always the case for the steroid injections used in rheumatology. But it’s unclear whether “immediate use” means emergency use only, or allows for routine use, as rheumatologists hope.
“One reason this came to rheumatology’s attention is that some state boards of medicine were inspecting and saying ‘Hey, you can’t do that,’ ” Dr. Miller said.
“There’s that law of unintended consequences where you snare things in a net that you really don’t want to,” Dr. Huffstutter said.
Marcus Snow, MD, a rheumatologist at the University of Nebraska, Omaha, who also worked on the statement, said that most rheumatologists are likely unaware that their ability to mix drugs in-office has been called into question.
“I brought it up at our division meeting with a group of 10 rheumatologists, and no one was aware that this was coming down the pike,” Dr. Snow said in an interview.
Pediatric issues
Pediatric rheumatologists, and adult rheumatologists who see children occasionally, use compounding pharmacies to create palatable oral medicines and adjusted doses of adult treatments.
They also use injections combining steroids with analgesics, and consider the addition of the analgesic a key aid to compliance.
“The biggest barrier we have is patient and parent anxiety about doing the procedure and the associated pain. We always administer our steroids mixed with lidocaine to help with the postprocedural discomfort,” said Adam Reinhardt, MD, chief of pediatric rheumatology at the University of Nebraska and Children’s Hospital and Medical Center in Omaha.
Steroid injections can mean avoiding or delaying systemic treatment in children with oligoarticular arthritis, he said. “Most of us consider them a first-line therapy. The hope is that you can get by without having to use meds like methotrexate if you can get a prolonged response in the one or two joints that are active in that patient.”
But Dr. Reinhardt said that, while he mixed his own injections during his fellowship training, Children’s of Omaha now insists that they be prepared by in-house pharmacists, working under sterile hoods. The delay to receiving them in the clinic or procedure room is 40 minutes to an hour, he said, which the clinicians accommodate through careful scheduling.
The change from mixing in-clinic to relying on the central pharmacy came about in recent years, Dr. Reinhardt said, because of broader concerns related to medication storage in the clinics. While ordering from the central pharmacy works for his practice, he said, “I probably only inject maybe 50-70 joints a year, while adult rheumatologists are injecting far more than that. For a busy private practice, I can see that being a huge time constraint,” he said.
Relevance of rules
None of the rheumatologists interviewed questioned the need for tightened state and federal oversight of compounding practices overall – just the applicability of certain rules to their own practice.
Dr. Snow and Dr. Huffstutter noted that reports of infected joints – a potential result of a contaminated injection – are sporadic and rare. “There’s very little research in this, but [these types of injections] have been standard practice for decades,” Dr. Snow said.
Srikanth Mukkera, MD, a rheumatologist in Tupelo, Miss., agreed that “sporadic cases of joint infection do happen following injection, but it can be hard to show if an injection was the cause.”
Assuring that medicines are mixed only immediately prior to injection, and not stored, reduces the likelihood of contamination, Dr. Mukkera said. Moreover, he noted, epidural injections such as those that resulted in the 2012 meningitis outbreak carry different risks than those seen in intra-articular injections.
Dr. Miller, the lead author of the ACR statement, said that the rheumatologists on our committee “don’t know of anyone that’s had a knee or other joint infection from a contaminated injection. They feel that unless somebody finds some evidence of that, they should be allowed to continue” with their usual practice.
He said that he feels that the USP will ultimately heed the concerns of rheumatologists and hopefully provide a more relaxed interpretation of in-office compounding. “We’re hoping they’ll make some exceptions when they revise 797 standards or at least maybe leave room for organizations to create a best practice statement. We’ll see,” Dr. Miller said.
But this is in no way guaranteed. Dr. Huffstutter said he fears that, if the rules come to be interpreted more narrowly, even standard practices like reconstituting biologic drugs for infusion – something that’s also a routine part of in-office practice – could fall under the rubric of sterile compounding and come into question.
The quinacrine problem
A separate compounding-related issue in rheumatology is clinicians’ access to quinacrine, an antimalarial rheumatology drug that, while infrequently used, represents the only alternative to hydroxychloroquine for some lupus patients.
“There are no alternatives out there for hydroxychloroquine, so we need it as a backup,” Dr. Snow said. “If hydroxychloroquine isn’t an option, there’s nothing out there that we can use. There’s no easy replacement.”
Dr. Huffstutter said he currently had no patients on quinacrine. “It’s not very often that we use it, but in those patients that really need it, it can make a huge difference in how they do.”
Quinacrine is no longer manufactured commercially as a finished drug product but is available in a powder that compounding physicians put into 100-mg capsules. It is not on the FDA’s current list of drugs available for compounding except with special permission.
While the ACR has requested that the FDA add it the list of bulk drug substances that can be used in compounding, quinacrine remains off the list for now – and, providers say, hard to find.
Moreover, while rheumatologists may have previously been able to order and store quantities of quinacrine and other compounded nonsterile medications to dispense to their patients, they can no longer easily do so, as only the FDA-approved compounding “outsourcing facilities” are allowed to process larger orders; the rest can only respond to prescriptions for individual patients.
Dr. Miller said it’s likely that quinacrine will make it onto the FDA’s next list of bulk drugs available for compounding. “The FDA has kind of said, ‘Don’t worry about it,’ ” he said.
As new rules about drug compounding get shaped, rheumatologists seek to protect their ability to combine injectable drugs – most commonly a steroid and a local anesthetic – in their own offices.
In a position statement sent to government agencies and members of Congress in February, the American College of Rheumatology voiced concerns that the practice, which it called “critical,” could become a casualty of drug-compounding regulations under revision by the United States Pharmacopeial Convention (USP), a nonprofit group whose standards are enforceable by state and federal regulators.
In the same position statement on compounding, the ACR said it also seeks a change to a Food and Drug Administration rule limiting practitioners’ access to quinacrine, a drug only available through compounding pharmacies that is sometimes used to treat lupus patients. Quinacrine is not on the FDA’s current list of bulk substances approved for compounding, except by special permission. The ACR has asked the agency to add quinacrine to the list, but no one knows when this will happen.
Rheumatologists may also be more restricted than before in terms of which compounding pharmacies they can turn to, as new federal standards divide them into two types – those that can provide medicines in larger quantities and those that can’t.
Steroid fiasco sparked rule revisions
The ACR’s concerns follow a tighter focus by state and federal agencies on drug compounding after a fungal meningitis outbreak in 2012 was traced to contaminated steroids produced in bulk by a compounding pharmacy.
More than 800 infections, 64 of them fatal, occurred after the New England Compounding Center in Framingham, Mass., sold contaminated methylprednisolone acetate that was used in epidural and intra-articular joint injections.
The following year Congress passed the Drug Quality and Security Act, which aims, in part, to prevent compounding pharmacies from engaging in what amounts to unregulated manufacturing.
As part of the law, the FDA created a list of drugs appropriate for compounding and a process by which larger compounding pharmacies must register with the FDA, and agree to inspections. The USP standards, meanwhile, address detailed technical and safety aspects of compounding and are enforceable by the FDA and state agencies.
“USP and FDA have had the ability to regulate compounding for over a decade, but only recently have the rules become actively enforced,” said Donald Miller, PharmD, of North Dakota State University, Fargo, who helped shape the ACR’s position statement on compounding with the help of rheumatologists in private practice.
“When you make guidelines for safety, they make sense, but then you can’t anticipate the way it’s going to affect individuals’ practice. And that’s where rheumatology got caught up,” said Dr. Miller, who was a member of the FDA Arthritis Advisory Committee in 2014-2016.
In-office mixing a top concern
Other specialties, including dermatology and immunology, also stand to be affected by various changes to compounding law and practice – and their societies have been active in voicing concerns.
Though the latest revisions of USP chapter 797, which impacts in-office mixing, are still being sorted out, it’s the No. 1 compounding-related concern for rheumatologists, Dr. Miller said.
Rheumatologists routinely mix an analgesic and a steroid for injection. The analgesic makes the steroids less viscous, and offers patients hours of immediate relief. They also add analgesics to hyaluronic acid injected for viscosupplementation. The mixing is usually conducted bedside, and the injections are administered right away.
Technically, combining these products amounts to sterile compounding, Dr. Miller explained. “And theoretically, under these rules, a physician could still do this, but they’d have to do it under a sterile hood like you find in a pharmacy, and that’s just not practical. It also becomes a matter of interpretation.”
USP chapter 797 sanctions in-office mixing for “immediate use” with individual patients – which is nearly always the case for the steroid injections used in rheumatology. But it’s unclear whether “immediate use” means emergency use only, or allows for routine use, as rheumatologists hope.
“One reason this came to rheumatology’s attention is that some state boards of medicine were inspecting and saying ‘Hey, you can’t do that,’ ” Dr. Miller said.
“There’s that law of unintended consequences where you snare things in a net that you really don’t want to,” Dr. Huffstutter said.
Marcus Snow, MD, a rheumatologist at the University of Nebraska, Omaha, who also worked on the statement, said that most rheumatologists are likely unaware that their ability to mix drugs in-office has been called into question.
“I brought it up at our division meeting with a group of 10 rheumatologists, and no one was aware that this was coming down the pike,” Dr. Snow said in an interview.
Pediatric issues
Pediatric rheumatologists, and adult rheumatologists who see children occasionally, use compounding pharmacies to create palatable oral medicines and adjusted doses of adult treatments.
They also use injections combining steroids with analgesics, and consider the addition of the analgesic a key aid to compliance.
“The biggest barrier we have is patient and parent anxiety about doing the procedure and the associated pain. We always administer our steroids mixed with lidocaine to help with the postprocedural discomfort,” said Adam Reinhardt, MD, chief of pediatric rheumatology at the University of Nebraska and Children’s Hospital and Medical Center in Omaha.
Steroid injections can mean avoiding or delaying systemic treatment in children with oligoarticular arthritis, he said. “Most of us consider them a first-line therapy. The hope is that you can get by without having to use meds like methotrexate if you can get a prolonged response in the one or two joints that are active in that patient.”
But Dr. Reinhardt said that, while he mixed his own injections during his fellowship training, Children’s of Omaha now insists that they be prepared by in-house pharmacists, working under sterile hoods. The delay to receiving them in the clinic or procedure room is 40 minutes to an hour, he said, which the clinicians accommodate through careful scheduling.
The change from mixing in-clinic to relying on the central pharmacy came about in recent years, Dr. Reinhardt said, because of broader concerns related to medication storage in the clinics. While ordering from the central pharmacy works for his practice, he said, “I probably only inject maybe 50-70 joints a year, while adult rheumatologists are injecting far more than that. For a busy private practice, I can see that being a huge time constraint,” he said.
Relevance of rules
None of the rheumatologists interviewed questioned the need for tightened state and federal oversight of compounding practices overall – just the applicability of certain rules to their own practice.
Dr. Snow and Dr. Huffstutter noted that reports of infected joints – a potential result of a contaminated injection – are sporadic and rare. “There’s very little research in this, but [these types of injections] have been standard practice for decades,” Dr. Snow said.
Srikanth Mukkera, MD, a rheumatologist in Tupelo, Miss., agreed that “sporadic cases of joint infection do happen following injection, but it can be hard to show if an injection was the cause.”
Assuring that medicines are mixed only immediately prior to injection, and not stored, reduces the likelihood of contamination, Dr. Mukkera said. Moreover, he noted, epidural injections such as those that resulted in the 2012 meningitis outbreak carry different risks than those seen in intra-articular injections.
Dr. Miller, the lead author of the ACR statement, said that the rheumatologists on our committee “don’t know of anyone that’s had a knee or other joint infection from a contaminated injection. They feel that unless somebody finds some evidence of that, they should be allowed to continue” with their usual practice.
He said that he feels that the USP will ultimately heed the concerns of rheumatologists and hopefully provide a more relaxed interpretation of in-office compounding. “We’re hoping they’ll make some exceptions when they revise 797 standards or at least maybe leave room for organizations to create a best practice statement. We’ll see,” Dr. Miller said.
But this is in no way guaranteed. Dr. Huffstutter said he fears that, if the rules come to be interpreted more narrowly, even standard practices like reconstituting biologic drugs for infusion – something that’s also a routine part of in-office practice – could fall under the rubric of sterile compounding and come into question.
The quinacrine problem
A separate compounding-related issue in rheumatology is clinicians’ access to quinacrine, an antimalarial rheumatology drug that, while infrequently used, represents the only alternative to hydroxychloroquine for some lupus patients.
“There are no alternatives out there for hydroxychloroquine, so we need it as a backup,” Dr. Snow said. “If hydroxychloroquine isn’t an option, there’s nothing out there that we can use. There’s no easy replacement.”
Dr. Huffstutter said he currently had no patients on quinacrine. “It’s not very often that we use it, but in those patients that really need it, it can make a huge difference in how they do.”
Quinacrine is no longer manufactured commercially as a finished drug product but is available in a powder that compounding physicians put into 100-mg capsules. It is not on the FDA’s current list of drugs available for compounding except with special permission.
While the ACR has requested that the FDA add it the list of bulk drug substances that can be used in compounding, quinacrine remains off the list for now – and, providers say, hard to find.
Moreover, while rheumatologists may have previously been able to order and store quantities of quinacrine and other compounded nonsterile medications to dispense to their patients, they can no longer easily do so, as only the FDA-approved compounding “outsourcing facilities” are allowed to process larger orders; the rest can only respond to prescriptions for individual patients.
Dr. Miller said it’s likely that quinacrine will make it onto the FDA’s next list of bulk drugs available for compounding. “The FDA has kind of said, ‘Don’t worry about it,’ ” he said.
Osteoarthritis’ link to metabolic syndrome tied to body weight, BMI
The metabolic syndrome’s association with the development of osteoarthritis appears to occur primarily through the influence of factors related to body weight or body mass index, according to an analysis of data from the longitudinal Framingham osteoarthritis study.
Jingbo Niu, DSc, of the Clinical Epidemiology Research and Training Unit at Boston University, and her colleagues found that while the presence of the metabolic syndrome (MetS) and its individual components – central obesity, dyslipidemia, impaired fasting glucose, and hypertension – were associated with a risk of both radiographic osteoarthritis (ROA) and symptomatic OA (SxOA), all associations besides high blood pressure (particularly diastolic blood pressure) disappeared after they adjusted for body mass index (BMI) or body weight (Arthritis Rheumatol. 2017 Mar 3. doi: 10.1002/art.40087).
The research team analyzed 991 participants of the 1992-1995 Framingham Offspring Cohort who did not have existing OA at baseline. Average age was 54.2 years; 55.1% were women. According to Adult Treatment Panel III criteria, 27% of the men and 23% of the women had MetS. Those who had MetS were older, had a higher BMI, and were less likely to drink alcohol or have a college education.
In 2002-2005, the cohort underwent follow-up for the presence of OA. The incidence of ROA in the cohort was 9.8% (78 of 800 knees) in men and 10.5% (105 of 1,003 knees) in women. ROA occurred when a knee without previous radiographic evidence of OA developed a Kellgren and Lawrence score of 2 or higher, and SxOA occurred when a knee developed ROA together with knee pain.
After adjusting for age, education, smoking status, alcohol consumption, and physical activity, the researchers found that MetS, abdominal obesity, and low high-density lipoprotein cholesterol were significantly associated with incident ROA among men. Among women, abdominal obesity and high blood pressure were associated with incident ROA, while MetS was not. However, after adjustment for body weight or BMI, none of the associations remained significant.
The incidence of SxOA was 6.3% (53 of 837 knees) in men and 7.2% (75 of 1,037 knees) in women. Incident SxOA was significantly associated with MetS in women and with the MetS components of abdominal obesity and high blood pressure in both men and women before adjustment for BMI or body weight. However, only an association between diastolic blood pressure and incident SxOA persisted in both sexes after adjustment for body weight, the study authors noted.
“High blood pressure is another MetS component associated with OA in previous studies before adjusting for BMI ... While we found [diastolic blood pressure] was related to incident SxOA even after adjustment for BMI, the relation of [systolic blood pressure] and incident SxOA was nearly significant also, suggesting that both might be related to SxOA,” the study authors wrote.
But a cross-sectional analysis of the relationship would be challenging to interpret, they said, since treatment for SxOA included NSAIDs, which are known to raise blood pressure.
The National Institutes of Health supported the study. The authors declared having no conflicts of interest.
The OA incidence reported in the Framingham study is particularly noteworthy because it shows that preexisting MetS and its components are risk factors for subsequent symptomatic OA and not just radiographic OA, which seems to indicate the existence of common risk factors for both MetS and OA in a manner such that OA could be just a late-occurring component of MetS.
But it may be most appropriate to consider metabolic OA as a complication of MetS, similar to cardiovascular disease.
The fact that any component of MetS remained significantly associated with symptomatic OA after adjustment for BMI and body weight argued strongly for a metabolic driver of OA pathophysiology when considering the likely confounding of body weight between mechanical and metabolic processes.
Nevertheless, carefully designed studies are needed to determine the relative impact of increased body mass in MetS on joint loading versus metabolic derangements, including systemic inflammation, to rule in or out the effects of altered metabolism on incident OA risk independent of biomechanics.
This knowledge would help a great deal toward understanding metabolic OA and other phenotypes as well as for the development of rational treatment approaches.
Thomas Appleton, MD, PhD, of Western University in London, Ont., and his colleagues made these comments in an editorial (Arthritis Rheumatol. 2017 Mar 7. doi: 10.1002/art.40089). They declared having no conflicts of interest to relevant to their editorial.
The OA incidence reported in the Framingham study is particularly noteworthy because it shows that preexisting MetS and its components are risk factors for subsequent symptomatic OA and not just radiographic OA, which seems to indicate the existence of common risk factors for both MetS and OA in a manner such that OA could be just a late-occurring component of MetS.
But it may be most appropriate to consider metabolic OA as a complication of MetS, similar to cardiovascular disease.
The fact that any component of MetS remained significantly associated with symptomatic OA after adjustment for BMI and body weight argued strongly for a metabolic driver of OA pathophysiology when considering the likely confounding of body weight between mechanical and metabolic processes.
Nevertheless, carefully designed studies are needed to determine the relative impact of increased body mass in MetS on joint loading versus metabolic derangements, including systemic inflammation, to rule in or out the effects of altered metabolism on incident OA risk independent of biomechanics.
This knowledge would help a great deal toward understanding metabolic OA and other phenotypes as well as for the development of rational treatment approaches.
Thomas Appleton, MD, PhD, of Western University in London, Ont., and his colleagues made these comments in an editorial (Arthritis Rheumatol. 2017 Mar 7. doi: 10.1002/art.40089). They declared having no conflicts of interest to relevant to their editorial.
The OA incidence reported in the Framingham study is particularly noteworthy because it shows that preexisting MetS and its components are risk factors for subsequent symptomatic OA and not just radiographic OA, which seems to indicate the existence of common risk factors for both MetS and OA in a manner such that OA could be just a late-occurring component of MetS.
But it may be most appropriate to consider metabolic OA as a complication of MetS, similar to cardiovascular disease.
The fact that any component of MetS remained significantly associated with symptomatic OA after adjustment for BMI and body weight argued strongly for a metabolic driver of OA pathophysiology when considering the likely confounding of body weight between mechanical and metabolic processes.
Nevertheless, carefully designed studies are needed to determine the relative impact of increased body mass in MetS on joint loading versus metabolic derangements, including systemic inflammation, to rule in or out the effects of altered metabolism on incident OA risk independent of biomechanics.
This knowledge would help a great deal toward understanding metabolic OA and other phenotypes as well as for the development of rational treatment approaches.
Thomas Appleton, MD, PhD, of Western University in London, Ont., and his colleagues made these comments in an editorial (Arthritis Rheumatol. 2017 Mar 7. doi: 10.1002/art.40089). They declared having no conflicts of interest to relevant to their editorial.
The metabolic syndrome’s association with the development of osteoarthritis appears to occur primarily through the influence of factors related to body weight or body mass index, according to an analysis of data from the longitudinal Framingham osteoarthritis study.
Jingbo Niu, DSc, of the Clinical Epidemiology Research and Training Unit at Boston University, and her colleagues found that while the presence of the metabolic syndrome (MetS) and its individual components – central obesity, dyslipidemia, impaired fasting glucose, and hypertension – were associated with a risk of both radiographic osteoarthritis (ROA) and symptomatic OA (SxOA), all associations besides high blood pressure (particularly diastolic blood pressure) disappeared after they adjusted for body mass index (BMI) or body weight (Arthritis Rheumatol. 2017 Mar 3. doi: 10.1002/art.40087).
The research team analyzed 991 participants of the 1992-1995 Framingham Offspring Cohort who did not have existing OA at baseline. Average age was 54.2 years; 55.1% were women. According to Adult Treatment Panel III criteria, 27% of the men and 23% of the women had MetS. Those who had MetS were older, had a higher BMI, and were less likely to drink alcohol or have a college education.
In 2002-2005, the cohort underwent follow-up for the presence of OA. The incidence of ROA in the cohort was 9.8% (78 of 800 knees) in men and 10.5% (105 of 1,003 knees) in women. ROA occurred when a knee without previous radiographic evidence of OA developed a Kellgren and Lawrence score of 2 or higher, and SxOA occurred when a knee developed ROA together with knee pain.
After adjusting for age, education, smoking status, alcohol consumption, and physical activity, the researchers found that MetS, abdominal obesity, and low high-density lipoprotein cholesterol were significantly associated with incident ROA among men. Among women, abdominal obesity and high blood pressure were associated with incident ROA, while MetS was not. However, after adjustment for body weight or BMI, none of the associations remained significant.
The incidence of SxOA was 6.3% (53 of 837 knees) in men and 7.2% (75 of 1,037 knees) in women. Incident SxOA was significantly associated with MetS in women and with the MetS components of abdominal obesity and high blood pressure in both men and women before adjustment for BMI or body weight. However, only an association between diastolic blood pressure and incident SxOA persisted in both sexes after adjustment for body weight, the study authors noted.
“High blood pressure is another MetS component associated with OA in previous studies before adjusting for BMI ... While we found [diastolic blood pressure] was related to incident SxOA even after adjustment for BMI, the relation of [systolic blood pressure] and incident SxOA was nearly significant also, suggesting that both might be related to SxOA,” the study authors wrote.
But a cross-sectional analysis of the relationship would be challenging to interpret, they said, since treatment for SxOA included NSAIDs, which are known to raise blood pressure.
The National Institutes of Health supported the study. The authors declared having no conflicts of interest.
The metabolic syndrome’s association with the development of osteoarthritis appears to occur primarily through the influence of factors related to body weight or body mass index, according to an analysis of data from the longitudinal Framingham osteoarthritis study.
Jingbo Niu, DSc, of the Clinical Epidemiology Research and Training Unit at Boston University, and her colleagues found that while the presence of the metabolic syndrome (MetS) and its individual components – central obesity, dyslipidemia, impaired fasting glucose, and hypertension – were associated with a risk of both radiographic osteoarthritis (ROA) and symptomatic OA (SxOA), all associations besides high blood pressure (particularly diastolic blood pressure) disappeared after they adjusted for body mass index (BMI) or body weight (Arthritis Rheumatol. 2017 Mar 3. doi: 10.1002/art.40087).
The research team analyzed 991 participants of the 1992-1995 Framingham Offspring Cohort who did not have existing OA at baseline. Average age was 54.2 years; 55.1% were women. According to Adult Treatment Panel III criteria, 27% of the men and 23% of the women had MetS. Those who had MetS were older, had a higher BMI, and were less likely to drink alcohol or have a college education.
In 2002-2005, the cohort underwent follow-up for the presence of OA. The incidence of ROA in the cohort was 9.8% (78 of 800 knees) in men and 10.5% (105 of 1,003 knees) in women. ROA occurred when a knee without previous radiographic evidence of OA developed a Kellgren and Lawrence score of 2 or higher, and SxOA occurred when a knee developed ROA together with knee pain.
After adjusting for age, education, smoking status, alcohol consumption, and physical activity, the researchers found that MetS, abdominal obesity, and low high-density lipoprotein cholesterol were significantly associated with incident ROA among men. Among women, abdominal obesity and high blood pressure were associated with incident ROA, while MetS was not. However, after adjustment for body weight or BMI, none of the associations remained significant.
The incidence of SxOA was 6.3% (53 of 837 knees) in men and 7.2% (75 of 1,037 knees) in women. Incident SxOA was significantly associated with MetS in women and with the MetS components of abdominal obesity and high blood pressure in both men and women before adjustment for BMI or body weight. However, only an association between diastolic blood pressure and incident SxOA persisted in both sexes after adjustment for body weight, the study authors noted.
“High blood pressure is another MetS component associated with OA in previous studies before adjusting for BMI ... While we found [diastolic blood pressure] was related to incident SxOA even after adjustment for BMI, the relation of [systolic blood pressure] and incident SxOA was nearly significant also, suggesting that both might be related to SxOA,” the study authors wrote.
But a cross-sectional analysis of the relationship would be challenging to interpret, they said, since treatment for SxOA included NSAIDs, which are known to raise blood pressure.
The National Institutes of Health supported the study. The authors declared having no conflicts of interest.
FROM ARTHRITIS & RHEUMATOLOGY
Key clinical point: There is no strong association between the metabolic syndrome (MetS) and OA after adjustment for BMI or body weight.
Major finding: Components of MetS were initially associated with incident radiographic and symptomatic OA, but after adjustment for BMI or body weight, most of the associations were weak and insignificant. However, an association between the MetS and high blood pressure persisted in both sexes.
Data source: An analysis of 991 participants of the 1992-1995 Framingham Offspring Cohort who did not have existing OA at baseline and were followed for 10 years.
Disclosures: The National Institutes of Health supported the study. The authors declared having no conflicts of interest.
No benefit found for routine inpatient rehab after knee replacement
A new randomized study from Australia suggests that for many patients, brief inpatient rehabilitation after knee replacement provides no benefits in several measures, compared with rehab at home.
However, “we are not concluding that there is no role for inpatient rehabilitation after knee arthroplasty,” said study coauthor Justine Naylor, PhD, of the University of New South Wales, Liverpool, Australia. “We are simply saying that for people who are otherwise well enough to be discharged directly home, inpatient rehabilitation does not provide more superior recovery across a range of outcomes.”
For the new study, conducted at two Australian hospitals during 2012-2015, researchers recruited patients aged 40 years or older with a primary diagnosis of osteoarthritis who were undergoing a primary unilateral knee arthroplasty and did not have complications such as a need for inpatient care in recovery beyond an initial 5 days after surgery.
The researchers randomly assigned 165 knee arthroplasty patients with uncomplicated cases to undergo either inpatient rehabilitation for 10 days and then recover at home for 6 weeks or a monitored 6-week home rehab program that began 2 weeks after surgery (JAMA. 2017;317[10]:1037-46).
A third group of 112 patients, 87 of whom were included in the primary analysis, were observed as they entered the home rehab protocol. They were in an initial group of 215 who declined to be randomized, mostly because they wanted to get home quickly after surgery.
The average age of all participants was 67 years, and just over two-thirds were women.
At 26 weeks, the researchers found that there was no significant difference in how the patients in the three groups fared on a 6-minute walk test (mean difference, −1.01 meters; 95% confidence interval, −25.56 to 23.55). They also found no significant difference in measurements of patient-reported pain and function (knee score mean difference, 2.06; 95% CI, −0.59 to 4.71), and quality of life (EQ-5D visual analog scale mean difference, 1.41; 95% CI, −6.42 to 3.60).
However, the patients did seem to have a preference. “Satisfaction with rehabilitation was significantly higher with inpatient rehab, average 92% vs. 83%, though both modes were well received overall,” Dr. Naylor said in an interview.
“Many patients who went to inpatient rehabilitation really enjoyed it,” she added. “We have observed that patients and carers like the convenience of inpatient rehab – a one-stop shop where patients get access to multiple clinicians, gyms, and other patients and do not have to prepare meals.”
However, she said, while “we have no doubt the inpatient rehabilitation environment is nurturing, there must also be advantages to being discharged directly home, otherwise we would have seen differences between the groups. It is possible that monitored home programs like the one provided in this study help people gain independence quickly and empower patients in their recovery.”
Dr. Naylor cautioned that inpatient rehabilitation does have potential benefits for certain patients, such as those who are the most impaired and those without someone available to care for them at home. “Future research could focus on what is best in those situations or, at least, design community-based programs [that] offer some of the perceived benefits of inpatient therapy,” she said. “In addition, we also need to know what the best rehabilitation approach is after hip arthroplasty.”
The study was funded by various sources, including a foundation grant. The study authors reported no relevant disclosures.
A new randomized study from Australia suggests that for many patients, brief inpatient rehabilitation after knee replacement provides no benefits in several measures, compared with rehab at home.
However, “we are not concluding that there is no role for inpatient rehabilitation after knee arthroplasty,” said study coauthor Justine Naylor, PhD, of the University of New South Wales, Liverpool, Australia. “We are simply saying that for people who are otherwise well enough to be discharged directly home, inpatient rehabilitation does not provide more superior recovery across a range of outcomes.”
For the new study, conducted at two Australian hospitals during 2012-2015, researchers recruited patients aged 40 years or older with a primary diagnosis of osteoarthritis who were undergoing a primary unilateral knee arthroplasty and did not have complications such as a need for inpatient care in recovery beyond an initial 5 days after surgery.
The researchers randomly assigned 165 knee arthroplasty patients with uncomplicated cases to undergo either inpatient rehabilitation for 10 days and then recover at home for 6 weeks or a monitored 6-week home rehab program that began 2 weeks after surgery (JAMA. 2017;317[10]:1037-46).
A third group of 112 patients, 87 of whom were included in the primary analysis, were observed as they entered the home rehab protocol. They were in an initial group of 215 who declined to be randomized, mostly because they wanted to get home quickly after surgery.
The average age of all participants was 67 years, and just over two-thirds were women.
At 26 weeks, the researchers found that there was no significant difference in how the patients in the three groups fared on a 6-minute walk test (mean difference, −1.01 meters; 95% confidence interval, −25.56 to 23.55). They also found no significant difference in measurements of patient-reported pain and function (knee score mean difference, 2.06; 95% CI, −0.59 to 4.71), and quality of life (EQ-5D visual analog scale mean difference, 1.41; 95% CI, −6.42 to 3.60).
However, the patients did seem to have a preference. “Satisfaction with rehabilitation was significantly higher with inpatient rehab, average 92% vs. 83%, though both modes were well received overall,” Dr. Naylor said in an interview.
“Many patients who went to inpatient rehabilitation really enjoyed it,” she added. “We have observed that patients and carers like the convenience of inpatient rehab – a one-stop shop where patients get access to multiple clinicians, gyms, and other patients and do not have to prepare meals.”
However, she said, while “we have no doubt the inpatient rehabilitation environment is nurturing, there must also be advantages to being discharged directly home, otherwise we would have seen differences between the groups. It is possible that monitored home programs like the one provided in this study help people gain independence quickly and empower patients in their recovery.”
Dr. Naylor cautioned that inpatient rehabilitation does have potential benefits for certain patients, such as those who are the most impaired and those without someone available to care for them at home. “Future research could focus on what is best in those situations or, at least, design community-based programs [that] offer some of the perceived benefits of inpatient therapy,” she said. “In addition, we also need to know what the best rehabilitation approach is after hip arthroplasty.”
The study was funded by various sources, including a foundation grant. The study authors reported no relevant disclosures.
A new randomized study from Australia suggests that for many patients, brief inpatient rehabilitation after knee replacement provides no benefits in several measures, compared with rehab at home.
However, “we are not concluding that there is no role for inpatient rehabilitation after knee arthroplasty,” said study coauthor Justine Naylor, PhD, of the University of New South Wales, Liverpool, Australia. “We are simply saying that for people who are otherwise well enough to be discharged directly home, inpatient rehabilitation does not provide more superior recovery across a range of outcomes.”
For the new study, conducted at two Australian hospitals during 2012-2015, researchers recruited patients aged 40 years or older with a primary diagnosis of osteoarthritis who were undergoing a primary unilateral knee arthroplasty and did not have complications such as a need for inpatient care in recovery beyond an initial 5 days after surgery.
The researchers randomly assigned 165 knee arthroplasty patients with uncomplicated cases to undergo either inpatient rehabilitation for 10 days and then recover at home for 6 weeks or a monitored 6-week home rehab program that began 2 weeks after surgery (JAMA. 2017;317[10]:1037-46).
A third group of 112 patients, 87 of whom were included in the primary analysis, were observed as they entered the home rehab protocol. They were in an initial group of 215 who declined to be randomized, mostly because they wanted to get home quickly after surgery.
The average age of all participants was 67 years, and just over two-thirds were women.
At 26 weeks, the researchers found that there was no significant difference in how the patients in the three groups fared on a 6-minute walk test (mean difference, −1.01 meters; 95% confidence interval, −25.56 to 23.55). They also found no significant difference in measurements of patient-reported pain and function (knee score mean difference, 2.06; 95% CI, −0.59 to 4.71), and quality of life (EQ-5D visual analog scale mean difference, 1.41; 95% CI, −6.42 to 3.60).
However, the patients did seem to have a preference. “Satisfaction with rehabilitation was significantly higher with inpatient rehab, average 92% vs. 83%, though both modes were well received overall,” Dr. Naylor said in an interview.
“Many patients who went to inpatient rehabilitation really enjoyed it,” she added. “We have observed that patients and carers like the convenience of inpatient rehab – a one-stop shop where patients get access to multiple clinicians, gyms, and other patients and do not have to prepare meals.”
However, she said, while “we have no doubt the inpatient rehabilitation environment is nurturing, there must also be advantages to being discharged directly home, otherwise we would have seen differences between the groups. It is possible that monitored home programs like the one provided in this study help people gain independence quickly and empower patients in their recovery.”
Dr. Naylor cautioned that inpatient rehabilitation does have potential benefits for certain patients, such as those who are the most impaired and those without someone available to care for them at home. “Future research could focus on what is best in those situations or, at least, design community-based programs [that] offer some of the perceived benefits of inpatient therapy,” she said. “In addition, we also need to know what the best rehabilitation approach is after hip arthroplasty.”
The study was funded by various sources, including a foundation grant. The study authors reported no relevant disclosures.
FROM JAMA
Key clinical point:
Major finding: There were no significant differences in the primary outcome of a 6-minute walk test at 26 weeks or in pain, function, and quality of life measures between patients randomized to inpatient or at-home rehab protocols.
Data source: A parallel, randomized controlled trial of 165 uncomplicated knee arthroplasty patients assigned to undergo either inpatient rehab for 10 days then in-home monitored rehab for 6 weeks or to a monitored 6-week home rehab 2 weeks after surgery. The study also included a third observational group of 112 patients (87 included in analysis) who underwent at-home rehab.
Disclosures: The study was funded by various sources, including a foundation grant. The study authors reported no relevant disclosures.