User login
A nurse’s view: Blood test for severe preeclampsia will save lives
There is amazing news for the world of obstetrics and for all pregnant women. Severe preeclampsia is a critical obstetrical condition that can have serious outcomes for a mother and baby. It can lead to eclampsia, an obstetrical emergency, which often results in death of the mother and/or baby.
Based on research published in the Journal of the American Heart Association, the incidence of new‐onset hypertensive disorders of pregnancy (gestational hypertension and preeclampsia/eclampsia) have nearly doubled in the United States from 2007 to 2019. And they continue to climb.
According to the Preeclampsia Foundation, 5%-8% of all pregnancies in the United States will result in preeclampsia. Black women are at a 60% higher risk than white women, and according to various sources, other risk groups include those who became pregnant via in vitro fertilization, mothers of multiples (twins and triplets), women with gestational diabetes, women over age 35, women with chronic hypertension, obesity, polycystic ovary syndrome, sickle cell disease, rheumatoid arthritis, lupus, migraines, antiphospholipid syndrome, previous pregnancy with preeclampsia, family history, and scleroderma.
Screening and treatment
Preeclampsia is a multiorgan disease of pregnancy, and can be mild, but may quickly progress to severe, which can be life-threatening for mother and baby. It was previously referred to as toxemia or the high blood pressure disease of pregnancy. It primarily involves the cardiovascular, neurologic and renal systems, and the liver. Patients typically present with elevated blood pressures, but other symptoms may include headache, swelling of hands and feet, blurry/double vision or seeing spots, nausea/vomiting, and epigastric pain. It is diagnosed with elevated blood pressures, blood work, and protein in the urine.
Early screening for preeclampsia is done in the first trimester. Presently, a combination of prenatal blood work, blood pressure monitoring, and recognition of high-risk groups is used to determine a treatment plan going forward. The American Congress of Obstetricians and Gynecologists recommends women that fall into this group for potentially developing preeclampsia take daily aspirin as a preventative measure.
In its milder form, a pregnant woman can be observed as an outpatient – monitored with antepartum testing, lab work, and patient education to report significant symptoms as listed above. Teaching patients about fetal kick counts to monitor their baby’s movements is equally important. Women with mild preeclampsia usually can safely deliver at term, being induced between 37-39 weeks’ gestation.
On the other hand, if mild preeclampsia progresses to severe preeclampsia, delivery may be preterm for the safety of mother and baby. Severe preeclampsia can lead to maternal organ damage, seizures, and even death of mother and/or baby.
About 20% of women with severe preeclampsia will develop HELLP (Hemolysis, Elevated Liver enzymes, and Low Platelets) syndrome, a life-threatening disease that often warrants immediate delivery. According to the National Library of Medicine, the mortality rate of women with HELLP syndrome is up to 24% and the perinatal death rate is up as high as 37%. These serious conditions can cause ineffective maternal clotting, liver rupture, placental abruption, and postpartum hemorrhage. It is most prevalent in the third trimester but can occur within 48 hours of delivery.
The only cure for preeclampsia in any form is delivery.
Patients with severe preeclampsia are hospitalized until delivery – sometimes a few days to a couple of weeks. Mother and baby are closely watched for further progression, including signs of organ damage in the mother and changes to the well-being of the baby. If the mother’s health is severely compromised, then the baby will be compromised as well. A preterm delivery may be necessary.
Impact of the new test
The National Institute of Health states that preterm babies born from preeclamptic mothers can suffer many health problems including cerebral palsy, deafness, blindness, epilepsy, and a host of other respiratory, cardiovascular, and endocrine issues. But the biggest issue is preterm birth, defined as birth before 37 weeks gestation. Being born preterm can require a long stay in the intensive care nursery.
This is where the first-of-its-kind prognostic blood test comes into play. The test’s ability to predict severe preeclampsia within 2 weeks can help save lives. The test can offer health care providers the ability to administer steroids for fetal lung maturity before delivery and be more prepared to care for what could be a very compromised newborn.
The blood test, which is recommended between 23-35 weeks gestation, involves analyzing a ratio between two proteins from the placenta, sFlt1 and PIGF. The higher the ratio, the higher the risk that severe preeclampsia will develop. Results can be available within 30 minutes, which is critical when contemplating treatment.
An example of the use of this ratio is illustrated with chronic hypertension in pregnancy, which is defined as elevated blood pressure before 20 weeks or even before conception. Since chronic hypertension can be a primary precursor to preeclampsia, patients with this condition are at higher risk. The FDA-approved blood test would be helpful in determining the plan of care; that is, delivery versus hospitalization versus monitor as an outpatient.
With a positive test result, a pregnant woman can be immediately hospitalized where she can get the care she and baby need as they await delivery. Since health care providers already know the high-risk groups, surveillance can begin early, utilizing this blood test to predict the progression to severe preeclampsia. Conversely, if the test is negative, a treatment plan can be made as an outpatient and the pregnancy continues.
Not all hospitals are equipped to care for premature babies. If delivery is not imminent, providers can use this blood test to identify those that should be transferred to a tertiary center for observation and monitoring. Mother and baby would then not be separated after birth.
We really don’t know who will develop severe preeclampsia and who won’t. This new blood test will be a critical tool as pregnant patients go through their second and third trimesters. It will be especially pivotal for these women, but important for all pregnant women in reducing maternal and fetal mortality and morbidity.
Ms. Barnett is a registered nurse in the department of obstetrics, Mills-Peninsula Medical Center, Burlingame, Calif. She has disclosed no relevant financial relationships.
There is amazing news for the world of obstetrics and for all pregnant women. Severe preeclampsia is a critical obstetrical condition that can have serious outcomes for a mother and baby. It can lead to eclampsia, an obstetrical emergency, which often results in death of the mother and/or baby.
Based on research published in the Journal of the American Heart Association, the incidence of new‐onset hypertensive disorders of pregnancy (gestational hypertension and preeclampsia/eclampsia) have nearly doubled in the United States from 2007 to 2019. And they continue to climb.
According to the Preeclampsia Foundation, 5%-8% of all pregnancies in the United States will result in preeclampsia. Black women are at a 60% higher risk than white women, and according to various sources, other risk groups include those who became pregnant via in vitro fertilization, mothers of multiples (twins and triplets), women with gestational diabetes, women over age 35, women with chronic hypertension, obesity, polycystic ovary syndrome, sickle cell disease, rheumatoid arthritis, lupus, migraines, antiphospholipid syndrome, previous pregnancy with preeclampsia, family history, and scleroderma.
Screening and treatment
Preeclampsia is a multiorgan disease of pregnancy, and can be mild, but may quickly progress to severe, which can be life-threatening for mother and baby. It was previously referred to as toxemia or the high blood pressure disease of pregnancy. It primarily involves the cardiovascular, neurologic and renal systems, and the liver. Patients typically present with elevated blood pressures, but other symptoms may include headache, swelling of hands and feet, blurry/double vision or seeing spots, nausea/vomiting, and epigastric pain. It is diagnosed with elevated blood pressures, blood work, and protein in the urine.
Early screening for preeclampsia is done in the first trimester. Presently, a combination of prenatal blood work, blood pressure monitoring, and recognition of high-risk groups is used to determine a treatment plan going forward. The American Congress of Obstetricians and Gynecologists recommends women that fall into this group for potentially developing preeclampsia take daily aspirin as a preventative measure.
In its milder form, a pregnant woman can be observed as an outpatient – monitored with antepartum testing, lab work, and patient education to report significant symptoms as listed above. Teaching patients about fetal kick counts to monitor their baby’s movements is equally important. Women with mild preeclampsia usually can safely deliver at term, being induced between 37-39 weeks’ gestation.
On the other hand, if mild preeclampsia progresses to severe preeclampsia, delivery may be preterm for the safety of mother and baby. Severe preeclampsia can lead to maternal organ damage, seizures, and even death of mother and/or baby.
About 20% of women with severe preeclampsia will develop HELLP (Hemolysis, Elevated Liver enzymes, and Low Platelets) syndrome, a life-threatening disease that often warrants immediate delivery. According to the National Library of Medicine, the mortality rate of women with HELLP syndrome is up to 24% and the perinatal death rate is up as high as 37%. These serious conditions can cause ineffective maternal clotting, liver rupture, placental abruption, and postpartum hemorrhage. It is most prevalent in the third trimester but can occur within 48 hours of delivery.
The only cure for preeclampsia in any form is delivery.
Patients with severe preeclampsia are hospitalized until delivery – sometimes a few days to a couple of weeks. Mother and baby are closely watched for further progression, including signs of organ damage in the mother and changes to the well-being of the baby. If the mother’s health is severely compromised, then the baby will be compromised as well. A preterm delivery may be necessary.
Impact of the new test
The National Institute of Health states that preterm babies born from preeclamptic mothers can suffer many health problems including cerebral palsy, deafness, blindness, epilepsy, and a host of other respiratory, cardiovascular, and endocrine issues. But the biggest issue is preterm birth, defined as birth before 37 weeks gestation. Being born preterm can require a long stay in the intensive care nursery.
This is where the first-of-its-kind prognostic blood test comes into play. The test’s ability to predict severe preeclampsia within 2 weeks can help save lives. The test can offer health care providers the ability to administer steroids for fetal lung maturity before delivery and be more prepared to care for what could be a very compromised newborn.
The blood test, which is recommended between 23-35 weeks gestation, involves analyzing a ratio between two proteins from the placenta, sFlt1 and PIGF. The higher the ratio, the higher the risk that severe preeclampsia will develop. Results can be available within 30 minutes, which is critical when contemplating treatment.
An example of the use of this ratio is illustrated with chronic hypertension in pregnancy, which is defined as elevated blood pressure before 20 weeks or even before conception. Since chronic hypertension can be a primary precursor to preeclampsia, patients with this condition are at higher risk. The FDA-approved blood test would be helpful in determining the plan of care; that is, delivery versus hospitalization versus monitor as an outpatient.
With a positive test result, a pregnant woman can be immediately hospitalized where she can get the care she and baby need as they await delivery. Since health care providers already know the high-risk groups, surveillance can begin early, utilizing this blood test to predict the progression to severe preeclampsia. Conversely, if the test is negative, a treatment plan can be made as an outpatient and the pregnancy continues.
Not all hospitals are equipped to care for premature babies. If delivery is not imminent, providers can use this blood test to identify those that should be transferred to a tertiary center for observation and monitoring. Mother and baby would then not be separated after birth.
We really don’t know who will develop severe preeclampsia and who won’t. This new blood test will be a critical tool as pregnant patients go through their second and third trimesters. It will be especially pivotal for these women, but important for all pregnant women in reducing maternal and fetal mortality and morbidity.
Ms. Barnett is a registered nurse in the department of obstetrics, Mills-Peninsula Medical Center, Burlingame, Calif. She has disclosed no relevant financial relationships.
There is amazing news for the world of obstetrics and for all pregnant women. Severe preeclampsia is a critical obstetrical condition that can have serious outcomes for a mother and baby. It can lead to eclampsia, an obstetrical emergency, which often results in death of the mother and/or baby.
Based on research published in the Journal of the American Heart Association, the incidence of new‐onset hypertensive disorders of pregnancy (gestational hypertension and preeclampsia/eclampsia) have nearly doubled in the United States from 2007 to 2019. And they continue to climb.
According to the Preeclampsia Foundation, 5%-8% of all pregnancies in the United States will result in preeclampsia. Black women are at a 60% higher risk than white women, and according to various sources, other risk groups include those who became pregnant via in vitro fertilization, mothers of multiples (twins and triplets), women with gestational diabetes, women over age 35, women with chronic hypertension, obesity, polycystic ovary syndrome, sickle cell disease, rheumatoid arthritis, lupus, migraines, antiphospholipid syndrome, previous pregnancy with preeclampsia, family history, and scleroderma.
Screening and treatment
Preeclampsia is a multiorgan disease of pregnancy, and can be mild, but may quickly progress to severe, which can be life-threatening for mother and baby. It was previously referred to as toxemia or the high blood pressure disease of pregnancy. It primarily involves the cardiovascular, neurologic and renal systems, and the liver. Patients typically present with elevated blood pressures, but other symptoms may include headache, swelling of hands and feet, blurry/double vision or seeing spots, nausea/vomiting, and epigastric pain. It is diagnosed with elevated blood pressures, blood work, and protein in the urine.
Early screening for preeclampsia is done in the first trimester. Presently, a combination of prenatal blood work, blood pressure monitoring, and recognition of high-risk groups is used to determine a treatment plan going forward. The American Congress of Obstetricians and Gynecologists recommends women that fall into this group for potentially developing preeclampsia take daily aspirin as a preventative measure.
In its milder form, a pregnant woman can be observed as an outpatient – monitored with antepartum testing, lab work, and patient education to report significant symptoms as listed above. Teaching patients about fetal kick counts to monitor their baby’s movements is equally important. Women with mild preeclampsia usually can safely deliver at term, being induced between 37-39 weeks’ gestation.
On the other hand, if mild preeclampsia progresses to severe preeclampsia, delivery may be preterm for the safety of mother and baby. Severe preeclampsia can lead to maternal organ damage, seizures, and even death of mother and/or baby.
About 20% of women with severe preeclampsia will develop HELLP (Hemolysis, Elevated Liver enzymes, and Low Platelets) syndrome, a life-threatening disease that often warrants immediate delivery. According to the National Library of Medicine, the mortality rate of women with HELLP syndrome is up to 24% and the perinatal death rate is up as high as 37%. These serious conditions can cause ineffective maternal clotting, liver rupture, placental abruption, and postpartum hemorrhage. It is most prevalent in the third trimester but can occur within 48 hours of delivery.
The only cure for preeclampsia in any form is delivery.
Patients with severe preeclampsia are hospitalized until delivery – sometimes a few days to a couple of weeks. Mother and baby are closely watched for further progression, including signs of organ damage in the mother and changes to the well-being of the baby. If the mother’s health is severely compromised, then the baby will be compromised as well. A preterm delivery may be necessary.
Impact of the new test
The National Institute of Health states that preterm babies born from preeclamptic mothers can suffer many health problems including cerebral palsy, deafness, blindness, epilepsy, and a host of other respiratory, cardiovascular, and endocrine issues. But the biggest issue is preterm birth, defined as birth before 37 weeks gestation. Being born preterm can require a long stay in the intensive care nursery.
This is where the first-of-its-kind prognostic blood test comes into play. The test’s ability to predict severe preeclampsia within 2 weeks can help save lives. The test can offer health care providers the ability to administer steroids for fetal lung maturity before delivery and be more prepared to care for what could be a very compromised newborn.
The blood test, which is recommended between 23-35 weeks gestation, involves analyzing a ratio between two proteins from the placenta, sFlt1 and PIGF. The higher the ratio, the higher the risk that severe preeclampsia will develop. Results can be available within 30 minutes, which is critical when contemplating treatment.
An example of the use of this ratio is illustrated with chronic hypertension in pregnancy, which is defined as elevated blood pressure before 20 weeks or even before conception. Since chronic hypertension can be a primary precursor to preeclampsia, patients with this condition are at higher risk. The FDA-approved blood test would be helpful in determining the plan of care; that is, delivery versus hospitalization versus monitor as an outpatient.
With a positive test result, a pregnant woman can be immediately hospitalized where she can get the care she and baby need as they await delivery. Since health care providers already know the high-risk groups, surveillance can begin early, utilizing this blood test to predict the progression to severe preeclampsia. Conversely, if the test is negative, a treatment plan can be made as an outpatient and the pregnancy continues.
Not all hospitals are equipped to care for premature babies. If delivery is not imminent, providers can use this blood test to identify those that should be transferred to a tertiary center for observation and monitoring. Mother and baby would then not be separated after birth.
We really don’t know who will develop severe preeclampsia and who won’t. This new blood test will be a critical tool as pregnant patients go through their second and third trimesters. It will be especially pivotal for these women, but important for all pregnant women in reducing maternal and fetal mortality and morbidity.
Ms. Barnett is a registered nurse in the department of obstetrics, Mills-Peninsula Medical Center, Burlingame, Calif. She has disclosed no relevant financial relationships.
Continuous glucose monitors for pregnant patients?
Patients with pregestational diabetes may benefit from use of a continuous subcutaneous insulin infusion pump paired with a continuous glucose monitor. Use of the tools has been associated with a reduction in maternal and neonatal morbidity, a recent study found.
“We were seeing an unacceptable burden of both maternal and fetal disease in our diabetic population,” said Neil Hamill, MD, a maternal-fetal medicine specialist at Methodist Women’s Hospital, Omaha, Neb., and an author of the study. “We thought the success with this technology in the nonpregnant population would and should translate into the pregnant population.”
Dr. Hamill and his colleagues analyzed data from 55 pregnant patients who received care at the Women’s Hospital Perinatal Center at the Nebraska Methodist Health System between October 2019 and October 2022. Everyone in the cohort had pregestational diabetes and required insulin prior to week 20 of pregnancy. They used CGMs for more than 2 weeks. The study set blood glucose levels of less than 140 mg/dL as a healthy benchmark.
Participants who had severe preeclampsia, who had delivered preterm, who had delivered a neonate with respiratory distress syndrome, and/or who had given birth to a larger-than-expected infant spent less time in the safe zone – having a blood glucose level below 140 mg/dL – than women who did not have those risk factors.
“When blood sugar control is better, maternal and fetal outcomes are improved,” Dr. Hamill said.
Neetu Sodhi, MD, an ob.gyn. at Providence Cedars-Sinai Tarzana Medical Center, Los Angeles, expressed optimism that use of blood glucose monitors and insulin pumps can improve outcomes for pregnant patients with pregestational diabetes.
“This is just another case for why it’s so important for patients to have access to these types of devices that really, really improve their outcomes and their health, and now it’s proven in the case of pregnancy outcomes too – or at least suggested strongly with this data,” Dr. Sodhi said.
Mark Ebell, MD, a professor of epidemiology at the University of Georgia, Athens, was more skeptical, pointing out that study participants might have used other methods in addition to the technology to lower their blood sugar levels.
The findings suggest that insulin pumps are more manageable than multiple, daily self-injections. About 1 in 9 women have diabetes in the United States, and 35% of people newly diagnosed with the condition are women of reproductive age.
Dr. Hamill said that in future research, use of a stricter criterion for baseline blood sugar levels (< 140 mg/dL) would be helpful, as would exploring how much time patients need to spend below that level for optimal outcomes.
“Those questions are really absent in the literature,” Dr. Hamill said. “Most of our obstetrical literature is comparing treatment types. All those things are secondary. It’s the blood sugar that confers the risk, and if we get the blood sugar better, risk is reduced.”
Dr. Hamill added that the benefits of these technologies for patients with gestational diabetes are unclear in consideration of the limited duration of the disease and the time required to implant or install a monitor and pump, as well as associated risks and the cost of the devices.
Dr. Sodhi said clinicians who see patients during family planning visits should review morbidities and medical problems related to diabetes.
“I think this is a study that’s maybe too early,” Dr. Sodhi said. “They did ‘guesstimates’ on what target blood glucose ranges to be looking at, but I think over time, we might, with more studies like this, be building a case to try to put these types of monitors in for patients who are young for the purpose of optimizing pregnancy outcomes.”
The authors have disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Patients with pregestational diabetes may benefit from use of a continuous subcutaneous insulin infusion pump paired with a continuous glucose monitor. Use of the tools has been associated with a reduction in maternal and neonatal morbidity, a recent study found.
“We were seeing an unacceptable burden of both maternal and fetal disease in our diabetic population,” said Neil Hamill, MD, a maternal-fetal medicine specialist at Methodist Women’s Hospital, Omaha, Neb., and an author of the study. “We thought the success with this technology in the nonpregnant population would and should translate into the pregnant population.”
Dr. Hamill and his colleagues analyzed data from 55 pregnant patients who received care at the Women’s Hospital Perinatal Center at the Nebraska Methodist Health System between October 2019 and October 2022. Everyone in the cohort had pregestational diabetes and required insulin prior to week 20 of pregnancy. They used CGMs for more than 2 weeks. The study set blood glucose levels of less than 140 mg/dL as a healthy benchmark.
Participants who had severe preeclampsia, who had delivered preterm, who had delivered a neonate with respiratory distress syndrome, and/or who had given birth to a larger-than-expected infant spent less time in the safe zone – having a blood glucose level below 140 mg/dL – than women who did not have those risk factors.
“When blood sugar control is better, maternal and fetal outcomes are improved,” Dr. Hamill said.
Neetu Sodhi, MD, an ob.gyn. at Providence Cedars-Sinai Tarzana Medical Center, Los Angeles, expressed optimism that use of blood glucose monitors and insulin pumps can improve outcomes for pregnant patients with pregestational diabetes.
“This is just another case for why it’s so important for patients to have access to these types of devices that really, really improve their outcomes and their health, and now it’s proven in the case of pregnancy outcomes too – or at least suggested strongly with this data,” Dr. Sodhi said.
Mark Ebell, MD, a professor of epidemiology at the University of Georgia, Athens, was more skeptical, pointing out that study participants might have used other methods in addition to the technology to lower their blood sugar levels.
The findings suggest that insulin pumps are more manageable than multiple, daily self-injections. About 1 in 9 women have diabetes in the United States, and 35% of people newly diagnosed with the condition are women of reproductive age.
Dr. Hamill said that in future research, use of a stricter criterion for baseline blood sugar levels (< 140 mg/dL) would be helpful, as would exploring how much time patients need to spend below that level for optimal outcomes.
“Those questions are really absent in the literature,” Dr. Hamill said. “Most of our obstetrical literature is comparing treatment types. All those things are secondary. It’s the blood sugar that confers the risk, and if we get the blood sugar better, risk is reduced.”
Dr. Hamill added that the benefits of these technologies for patients with gestational diabetes are unclear in consideration of the limited duration of the disease and the time required to implant or install a monitor and pump, as well as associated risks and the cost of the devices.
Dr. Sodhi said clinicians who see patients during family planning visits should review morbidities and medical problems related to diabetes.
“I think this is a study that’s maybe too early,” Dr. Sodhi said. “They did ‘guesstimates’ on what target blood glucose ranges to be looking at, but I think over time, we might, with more studies like this, be building a case to try to put these types of monitors in for patients who are young for the purpose of optimizing pregnancy outcomes.”
The authors have disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Patients with pregestational diabetes may benefit from use of a continuous subcutaneous insulin infusion pump paired with a continuous glucose monitor. Use of the tools has been associated with a reduction in maternal and neonatal morbidity, a recent study found.
“We were seeing an unacceptable burden of both maternal and fetal disease in our diabetic population,” said Neil Hamill, MD, a maternal-fetal medicine specialist at Methodist Women’s Hospital, Omaha, Neb., and an author of the study. “We thought the success with this technology in the nonpregnant population would and should translate into the pregnant population.”
Dr. Hamill and his colleagues analyzed data from 55 pregnant patients who received care at the Women’s Hospital Perinatal Center at the Nebraska Methodist Health System between October 2019 and October 2022. Everyone in the cohort had pregestational diabetes and required insulin prior to week 20 of pregnancy. They used CGMs for more than 2 weeks. The study set blood glucose levels of less than 140 mg/dL as a healthy benchmark.
Participants who had severe preeclampsia, who had delivered preterm, who had delivered a neonate with respiratory distress syndrome, and/or who had given birth to a larger-than-expected infant spent less time in the safe zone – having a blood glucose level below 140 mg/dL – than women who did not have those risk factors.
“When blood sugar control is better, maternal and fetal outcomes are improved,” Dr. Hamill said.
Neetu Sodhi, MD, an ob.gyn. at Providence Cedars-Sinai Tarzana Medical Center, Los Angeles, expressed optimism that use of blood glucose monitors and insulin pumps can improve outcomes for pregnant patients with pregestational diabetes.
“This is just another case for why it’s so important for patients to have access to these types of devices that really, really improve their outcomes and their health, and now it’s proven in the case of pregnancy outcomes too – or at least suggested strongly with this data,” Dr. Sodhi said.
Mark Ebell, MD, a professor of epidemiology at the University of Georgia, Athens, was more skeptical, pointing out that study participants might have used other methods in addition to the technology to lower their blood sugar levels.
The findings suggest that insulin pumps are more manageable than multiple, daily self-injections. About 1 in 9 women have diabetes in the United States, and 35% of people newly diagnosed with the condition are women of reproductive age.
Dr. Hamill said that in future research, use of a stricter criterion for baseline blood sugar levels (< 140 mg/dL) would be helpful, as would exploring how much time patients need to spend below that level for optimal outcomes.
“Those questions are really absent in the literature,” Dr. Hamill said. “Most of our obstetrical literature is comparing treatment types. All those things are secondary. It’s the blood sugar that confers the risk, and if we get the blood sugar better, risk is reduced.”
Dr. Hamill added that the benefits of these technologies for patients with gestational diabetes are unclear in consideration of the limited duration of the disease and the time required to implant or install a monitor and pump, as well as associated risks and the cost of the devices.
Dr. Sodhi said clinicians who see patients during family planning visits should review morbidities and medical problems related to diabetes.
“I think this is a study that’s maybe too early,” Dr. Sodhi said. “They did ‘guesstimates’ on what target blood glucose ranges to be looking at, but I think over time, we might, with more studies like this, be building a case to try to put these types of monitors in for patients who are young for the purpose of optimizing pregnancy outcomes.”
The authors have disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Atopic dermatitis may be a risk factor for GBS colonization in pregnancy
suggest.
“The rate of GBS colonization among pregnant females with a history of AD has not been previously reported, but AD could be a risk factor for maternal carriage of GBS,” corresponding author David J. Margolis, MD, PhD, of the department of dermatology at the University of Pennsylvania, Philadelphia, and colleagues wrote in the study, which was published as a letter to the editor online in the Journal of Investigative Dermatology. “GBS reporting in a large administrative database represents a unique opportunity to conduct a population-based evaluation of GBS carriage with AD. Understanding this association could expand our understanding of microbial changes associated with AD,” they noted.
To determine if an association between GBS and AD in pregnant women exists, the researchers performed a cross-sectional study using a random sample from an Optum administrative database of pregnant women who had vaginal deliveries between May of 2007 and September 2021. The primary outcome of interest was the presence of GBS based on American College of Obstetricians and Gynecologists–recommended codes for GBS during 36 0/7 to 37 6/7 weeks of pregnancy. They used descriptive statistics to summarize categorical and continuous variables as proportions and means, and logistic regression to examine the association between AD and GBS status.
The cohort included 566,467 pregnant women with an average age of 38.8 years. Of these, 2.9% had a diagnosis of AD or a history of AD, and 24.9% had diagnoses of asthma, seasonal allergies, or both. Women with AD had an increased odds ratio of asthma (OR, 2.55), seasonal allergies (OR, 3.39), or both (OR, 5.35), compared with those without AD.
GBS was reported in 20.6% of the cohort. The median time of follow-up for those with and without GBS was 494 days and 468 days, respectively (P = .134). Among the women with AD, 24.1% had GBS, compared with 20.51% of the women without AD (P <.0001), which translated into an OR of 1.23 (95% confidence interval, 1.18-1.27).
Among the women with GBS, the OR of asthma was 1.08 (95% CI, 1.06-1.10) and was 1.07 (95% CI, 1.05-1.09) among those with seasonal allergies. When adjusted for potential confounders, these findings did not change substantively.
“It is not apparent why pregnant females with AD are more likely to specifically carry GBS,” the authors wrote. “However, several studies have shown that individuals with AD are more likely to carry [Staphylococcus] aureus and that individuals with AD might be deficient in host defenses against S. aureus and other pathogens,” they added.
“Individuals with AD frequently receive antibiotics as part of their AD treatment and this might alter their resident microbiome. Carriage rates may be enhanced by the inhibition of an important barrier protein called filaggrin (FLG) and FLG loss of function genetic variation is known to decrease barrier proteins thought to inhibit the colonization of S. aureus and other pathogens,” the researchers wrote.
They acknowledged certain limitations of their study, including its reliance on an administrative database that does not contain information on past disease.
Asked to comment on the results, Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was not involved with the study, characterized AD as “the poster child for cutaneous dysbiosis – an altered petri dish, so to speak, [that] facilitates survival of the few, leading to decreased microbial diversity that can both enable potential pathogen invasion and immune dysregulation.”
Though it’s not surprising that pregnant AD patients have dysbiosis, the focus on GBS, “which can be a bad actor in the perinatal period, is an interesting connection,” he said. “Will this change practices? Pregnant women should be screened for GBS regardless, but maybe more attention or counseling can be offered to AD patients about the importance of screening. Would decolonization regimens be employed early in pregnancy? This study can’t answer that but certainly raises good questions.”
Dr. Margolis disclosed that he is or recently has been a consultant for Pfizer, Leo, and Sanofi with respect to studies of atopic dermatitis and served on an advisory board for the National Eczema Association. Another author disclosed receiving grants from companies related to work with AD; other authors had no disclosures. Dr. Friedman reported having no relevant disclosures.
suggest.
“The rate of GBS colonization among pregnant females with a history of AD has not been previously reported, but AD could be a risk factor for maternal carriage of GBS,” corresponding author David J. Margolis, MD, PhD, of the department of dermatology at the University of Pennsylvania, Philadelphia, and colleagues wrote in the study, which was published as a letter to the editor online in the Journal of Investigative Dermatology. “GBS reporting in a large administrative database represents a unique opportunity to conduct a population-based evaluation of GBS carriage with AD. Understanding this association could expand our understanding of microbial changes associated with AD,” they noted.
To determine if an association between GBS and AD in pregnant women exists, the researchers performed a cross-sectional study using a random sample from an Optum administrative database of pregnant women who had vaginal deliveries between May of 2007 and September 2021. The primary outcome of interest was the presence of GBS based on American College of Obstetricians and Gynecologists–recommended codes for GBS during 36 0/7 to 37 6/7 weeks of pregnancy. They used descriptive statistics to summarize categorical and continuous variables as proportions and means, and logistic regression to examine the association between AD and GBS status.
The cohort included 566,467 pregnant women with an average age of 38.8 years. Of these, 2.9% had a diagnosis of AD or a history of AD, and 24.9% had diagnoses of asthma, seasonal allergies, or both. Women with AD had an increased odds ratio of asthma (OR, 2.55), seasonal allergies (OR, 3.39), or both (OR, 5.35), compared with those without AD.
GBS was reported in 20.6% of the cohort. The median time of follow-up for those with and without GBS was 494 days and 468 days, respectively (P = .134). Among the women with AD, 24.1% had GBS, compared with 20.51% of the women without AD (P <.0001), which translated into an OR of 1.23 (95% confidence interval, 1.18-1.27).
Among the women with GBS, the OR of asthma was 1.08 (95% CI, 1.06-1.10) and was 1.07 (95% CI, 1.05-1.09) among those with seasonal allergies. When adjusted for potential confounders, these findings did not change substantively.
“It is not apparent why pregnant females with AD are more likely to specifically carry GBS,” the authors wrote. “However, several studies have shown that individuals with AD are more likely to carry [Staphylococcus] aureus and that individuals with AD might be deficient in host defenses against S. aureus and other pathogens,” they added.
“Individuals with AD frequently receive antibiotics as part of their AD treatment and this might alter their resident microbiome. Carriage rates may be enhanced by the inhibition of an important barrier protein called filaggrin (FLG) and FLG loss of function genetic variation is known to decrease barrier proteins thought to inhibit the colonization of S. aureus and other pathogens,” the researchers wrote.
They acknowledged certain limitations of their study, including its reliance on an administrative database that does not contain information on past disease.
Asked to comment on the results, Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was not involved with the study, characterized AD as “the poster child for cutaneous dysbiosis – an altered petri dish, so to speak, [that] facilitates survival of the few, leading to decreased microbial diversity that can both enable potential pathogen invasion and immune dysregulation.”
Though it’s not surprising that pregnant AD patients have dysbiosis, the focus on GBS, “which can be a bad actor in the perinatal period, is an interesting connection,” he said. “Will this change practices? Pregnant women should be screened for GBS regardless, but maybe more attention or counseling can be offered to AD patients about the importance of screening. Would decolonization regimens be employed early in pregnancy? This study can’t answer that but certainly raises good questions.”
Dr. Margolis disclosed that he is or recently has been a consultant for Pfizer, Leo, and Sanofi with respect to studies of atopic dermatitis and served on an advisory board for the National Eczema Association. Another author disclosed receiving grants from companies related to work with AD; other authors had no disclosures. Dr. Friedman reported having no relevant disclosures.
suggest.
“The rate of GBS colonization among pregnant females with a history of AD has not been previously reported, but AD could be a risk factor for maternal carriage of GBS,” corresponding author David J. Margolis, MD, PhD, of the department of dermatology at the University of Pennsylvania, Philadelphia, and colleagues wrote in the study, which was published as a letter to the editor online in the Journal of Investigative Dermatology. “GBS reporting in a large administrative database represents a unique opportunity to conduct a population-based evaluation of GBS carriage with AD. Understanding this association could expand our understanding of microbial changes associated with AD,” they noted.
To determine if an association between GBS and AD in pregnant women exists, the researchers performed a cross-sectional study using a random sample from an Optum administrative database of pregnant women who had vaginal deliveries between May of 2007 and September 2021. The primary outcome of interest was the presence of GBS based on American College of Obstetricians and Gynecologists–recommended codes for GBS during 36 0/7 to 37 6/7 weeks of pregnancy. They used descriptive statistics to summarize categorical and continuous variables as proportions and means, and logistic regression to examine the association between AD and GBS status.
The cohort included 566,467 pregnant women with an average age of 38.8 years. Of these, 2.9% had a diagnosis of AD or a history of AD, and 24.9% had diagnoses of asthma, seasonal allergies, or both. Women with AD had an increased odds ratio of asthma (OR, 2.55), seasonal allergies (OR, 3.39), or both (OR, 5.35), compared with those without AD.
GBS was reported in 20.6% of the cohort. The median time of follow-up for those with and without GBS was 494 days and 468 days, respectively (P = .134). Among the women with AD, 24.1% had GBS, compared with 20.51% of the women without AD (P <.0001), which translated into an OR of 1.23 (95% confidence interval, 1.18-1.27).
Among the women with GBS, the OR of asthma was 1.08 (95% CI, 1.06-1.10) and was 1.07 (95% CI, 1.05-1.09) among those with seasonal allergies. When adjusted for potential confounders, these findings did not change substantively.
“It is not apparent why pregnant females with AD are more likely to specifically carry GBS,” the authors wrote. “However, several studies have shown that individuals with AD are more likely to carry [Staphylococcus] aureus and that individuals with AD might be deficient in host defenses against S. aureus and other pathogens,” they added.
“Individuals with AD frequently receive antibiotics as part of their AD treatment and this might alter their resident microbiome. Carriage rates may be enhanced by the inhibition of an important barrier protein called filaggrin (FLG) and FLG loss of function genetic variation is known to decrease barrier proteins thought to inhibit the colonization of S. aureus and other pathogens,” the researchers wrote.
They acknowledged certain limitations of their study, including its reliance on an administrative database that does not contain information on past disease.
Asked to comment on the results, Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was not involved with the study, characterized AD as “the poster child for cutaneous dysbiosis – an altered petri dish, so to speak, [that] facilitates survival of the few, leading to decreased microbial diversity that can both enable potential pathogen invasion and immune dysregulation.”
Though it’s not surprising that pregnant AD patients have dysbiosis, the focus on GBS, “which can be a bad actor in the perinatal period, is an interesting connection,” he said. “Will this change practices? Pregnant women should be screened for GBS regardless, but maybe more attention or counseling can be offered to AD patients about the importance of screening. Would decolonization regimens be employed early in pregnancy? This study can’t answer that but certainly raises good questions.”
Dr. Margolis disclosed that he is or recently has been a consultant for Pfizer, Leo, and Sanofi with respect to studies of atopic dermatitis and served on an advisory board for the National Eczema Association. Another author disclosed receiving grants from companies related to work with AD; other authors had no disclosures. Dr. Friedman reported having no relevant disclosures.
FROM THE JOURNAL OF INVESTIGATIVE DERMATOLOGY
One in five women report mistreatment during maternity care
“We have to do better at providing respectful and unbiased care to all mothers,” Debra E. Houry, MD, chief medical officer of the Centers for Disease Control and Prevention, said in a press briefing announcing the findings, which were published as a Vital Signs report in the CDC’s Morbidity and Mortality Weekly Report.
Previous research showed an increase in maternal deaths in the United States from 17.4 to 32.9 per 100,000 live births between 2018 and 2021, but approximately 80% of these deaths are preventable, wrote Yousra A. Mohamoud, PhD, of the CDC’s division of reproductive health, and colleagues.
“Maternal mortality review committees have identified discrimination as one factor contributing to pregnancy-related deaths,” the researchers wrote. Respectful care must be part of a larger strategy to prevent these deaths, they emphasized.
In the report, researchers reviewed data from 2,402 women who responded to an opt-in survey. The survey was conducted for the CDC through Porter Novelli, and no personally identifying information was included. Nearly 70% of the participants were White, 10.7% were Black, 10.2% were Hispanic, 4.8% were Asian, 1.5% were American Indian, Alaska Native, Pacific Islander, or Native Hawaiian, 2.8% were multiracial, and 0.5% were another race.
The survey included questions about maternity care experiences during pregnancy and delivery of the youngest child. For 65.5% of respondents, their youngest child was 5 years or older at the time of the survey.
Mistreatment during maternity care was defined using seven validated questions, including questions about violations of physical privacy, verbal abuse, and inattention to requests for help. Satisfaction with maternity care was defined as “very satisfied” or “somewhat satisfied.”
Participants also responded to questions about discrimination during maternity care based on factors such as race, ethnicity, skin color, age, and weight. Finally, participants were asked whether they refrained from asking questions about their health or raising concerns with health care providers.
Overall, 20.4% of respondents reported experiencing one of the defined forms of mistreatment during maternity care. The most common mistreatment reported by the women was being ignored by providers when they requested help (9.7%), followed by being shouted at or scolded (6.7%), having physical privacy violated (5.1%), and being forced to accept unwanted treatment or threatened with withholding of treatment (4.6%).
However, approximately 90% of women overall and 75% of those who reported any mistreatment were very or somewhat satisfied with their maternity care.
When stratified by race, mistreatment was reported most frequently by Black, Hispanic, and multiracial women (30%, 29%, and 27%, respectively).
Overall, 29% of women reported experiencing some type of discrimination; the most frequently reported reasons were age, weight, and income. Black women reported the highest rates of discrimination (40%) followed by multiracial women (39%) and Hispanic women (37%).
With regard to self-advocacy, 45% of women reported holding back from asking questions of health care providers; the most common reasons were thinking their health concerns were normal for pregnancy, being embarrassed, and being concerned that health care providers would consider them difficult.
In addition, more women with no insurance or public insurance at the time of delivery reported mistreatment during their maternity care than did women with private insurance (28%, 26%, and 16%, respectively).
The findings were limited by several factors, including the opt-in nature of the survey, which means that the data are likely not representative of the birthing population in the United States, the researchers noted. Other limitations included the reliance on self-reports, potential recall bias, use of English language only, and use of a combined category for respondents of American Indian, Alaska Native, Native Hawaiian, and Pacific Islander ethnicity.
However, the results highlight the need for improving respectful care as part of a larger strategy to reduce pregnancy-related deaths, the researchers said. At the system level, quality improvement programs are needed to standardize care and support providers in recognizing and reducing biases and increasing cultural awareness and communication. At the provider level, clinicians at all points in the maternity care process can improve patient experiences by providing equitable and respectful care, and by listening to and addressing patients’ concerns.
In addition, communication campaigns and community engagement can include perspectives of patients, families, and communities to support women and encourage them to ask questions and express concerns, the researchers said.
Improving respectful care can be part of actions to reduce mortality at all levels, the researchers noted. The Hear Her campaign, developed by the CDC Foundation with funding from Merck, provides resources for pregnant and postpartum women and their support networks to help reduce pregnancy-related deaths and complications by encouraging women to share concerns with providers and to recognize urgent maternal warning signs.
The study received no outside funding. The researchers had no financial conflicts to disclose.
“We have to do better at providing respectful and unbiased care to all mothers,” Debra E. Houry, MD, chief medical officer of the Centers for Disease Control and Prevention, said in a press briefing announcing the findings, which were published as a Vital Signs report in the CDC’s Morbidity and Mortality Weekly Report.
Previous research showed an increase in maternal deaths in the United States from 17.4 to 32.9 per 100,000 live births between 2018 and 2021, but approximately 80% of these deaths are preventable, wrote Yousra A. Mohamoud, PhD, of the CDC’s division of reproductive health, and colleagues.
“Maternal mortality review committees have identified discrimination as one factor contributing to pregnancy-related deaths,” the researchers wrote. Respectful care must be part of a larger strategy to prevent these deaths, they emphasized.
In the report, researchers reviewed data from 2,402 women who responded to an opt-in survey. The survey was conducted for the CDC through Porter Novelli, and no personally identifying information was included. Nearly 70% of the participants were White, 10.7% were Black, 10.2% were Hispanic, 4.8% were Asian, 1.5% were American Indian, Alaska Native, Pacific Islander, or Native Hawaiian, 2.8% were multiracial, and 0.5% were another race.
The survey included questions about maternity care experiences during pregnancy and delivery of the youngest child. For 65.5% of respondents, their youngest child was 5 years or older at the time of the survey.
Mistreatment during maternity care was defined using seven validated questions, including questions about violations of physical privacy, verbal abuse, and inattention to requests for help. Satisfaction with maternity care was defined as “very satisfied” or “somewhat satisfied.”
Participants also responded to questions about discrimination during maternity care based on factors such as race, ethnicity, skin color, age, and weight. Finally, participants were asked whether they refrained from asking questions about their health or raising concerns with health care providers.
Overall, 20.4% of respondents reported experiencing one of the defined forms of mistreatment during maternity care. The most common mistreatment reported by the women was being ignored by providers when they requested help (9.7%), followed by being shouted at or scolded (6.7%), having physical privacy violated (5.1%), and being forced to accept unwanted treatment or threatened with withholding of treatment (4.6%).
However, approximately 90% of women overall and 75% of those who reported any mistreatment were very or somewhat satisfied with their maternity care.
When stratified by race, mistreatment was reported most frequently by Black, Hispanic, and multiracial women (30%, 29%, and 27%, respectively).
Overall, 29% of women reported experiencing some type of discrimination; the most frequently reported reasons were age, weight, and income. Black women reported the highest rates of discrimination (40%) followed by multiracial women (39%) and Hispanic women (37%).
With regard to self-advocacy, 45% of women reported holding back from asking questions of health care providers; the most common reasons were thinking their health concerns were normal for pregnancy, being embarrassed, and being concerned that health care providers would consider them difficult.
In addition, more women with no insurance or public insurance at the time of delivery reported mistreatment during their maternity care than did women with private insurance (28%, 26%, and 16%, respectively).
The findings were limited by several factors, including the opt-in nature of the survey, which means that the data are likely not representative of the birthing population in the United States, the researchers noted. Other limitations included the reliance on self-reports, potential recall bias, use of English language only, and use of a combined category for respondents of American Indian, Alaska Native, Native Hawaiian, and Pacific Islander ethnicity.
However, the results highlight the need for improving respectful care as part of a larger strategy to reduce pregnancy-related deaths, the researchers said. At the system level, quality improvement programs are needed to standardize care and support providers in recognizing and reducing biases and increasing cultural awareness and communication. At the provider level, clinicians at all points in the maternity care process can improve patient experiences by providing equitable and respectful care, and by listening to and addressing patients’ concerns.
In addition, communication campaigns and community engagement can include perspectives of patients, families, and communities to support women and encourage them to ask questions and express concerns, the researchers said.
Improving respectful care can be part of actions to reduce mortality at all levels, the researchers noted. The Hear Her campaign, developed by the CDC Foundation with funding from Merck, provides resources for pregnant and postpartum women and their support networks to help reduce pregnancy-related deaths and complications by encouraging women to share concerns with providers and to recognize urgent maternal warning signs.
The study received no outside funding. The researchers had no financial conflicts to disclose.
“We have to do better at providing respectful and unbiased care to all mothers,” Debra E. Houry, MD, chief medical officer of the Centers for Disease Control and Prevention, said in a press briefing announcing the findings, which were published as a Vital Signs report in the CDC’s Morbidity and Mortality Weekly Report.
Previous research showed an increase in maternal deaths in the United States from 17.4 to 32.9 per 100,000 live births between 2018 and 2021, but approximately 80% of these deaths are preventable, wrote Yousra A. Mohamoud, PhD, of the CDC’s division of reproductive health, and colleagues.
“Maternal mortality review committees have identified discrimination as one factor contributing to pregnancy-related deaths,” the researchers wrote. Respectful care must be part of a larger strategy to prevent these deaths, they emphasized.
In the report, researchers reviewed data from 2,402 women who responded to an opt-in survey. The survey was conducted for the CDC through Porter Novelli, and no personally identifying information was included. Nearly 70% of the participants were White, 10.7% were Black, 10.2% were Hispanic, 4.8% were Asian, 1.5% were American Indian, Alaska Native, Pacific Islander, or Native Hawaiian, 2.8% were multiracial, and 0.5% were another race.
The survey included questions about maternity care experiences during pregnancy and delivery of the youngest child. For 65.5% of respondents, their youngest child was 5 years or older at the time of the survey.
Mistreatment during maternity care was defined using seven validated questions, including questions about violations of physical privacy, verbal abuse, and inattention to requests for help. Satisfaction with maternity care was defined as “very satisfied” or “somewhat satisfied.”
Participants also responded to questions about discrimination during maternity care based on factors such as race, ethnicity, skin color, age, and weight. Finally, participants were asked whether they refrained from asking questions about their health or raising concerns with health care providers.
Overall, 20.4% of respondents reported experiencing one of the defined forms of mistreatment during maternity care. The most common mistreatment reported by the women was being ignored by providers when they requested help (9.7%), followed by being shouted at or scolded (6.7%), having physical privacy violated (5.1%), and being forced to accept unwanted treatment or threatened with withholding of treatment (4.6%).
However, approximately 90% of women overall and 75% of those who reported any mistreatment were very or somewhat satisfied with their maternity care.
When stratified by race, mistreatment was reported most frequently by Black, Hispanic, and multiracial women (30%, 29%, and 27%, respectively).
Overall, 29% of women reported experiencing some type of discrimination; the most frequently reported reasons were age, weight, and income. Black women reported the highest rates of discrimination (40%) followed by multiracial women (39%) and Hispanic women (37%).
With regard to self-advocacy, 45% of women reported holding back from asking questions of health care providers; the most common reasons were thinking their health concerns were normal for pregnancy, being embarrassed, and being concerned that health care providers would consider them difficult.
In addition, more women with no insurance or public insurance at the time of delivery reported mistreatment during their maternity care than did women with private insurance (28%, 26%, and 16%, respectively).
The findings were limited by several factors, including the opt-in nature of the survey, which means that the data are likely not representative of the birthing population in the United States, the researchers noted. Other limitations included the reliance on self-reports, potential recall bias, use of English language only, and use of a combined category for respondents of American Indian, Alaska Native, Native Hawaiian, and Pacific Islander ethnicity.
However, the results highlight the need for improving respectful care as part of a larger strategy to reduce pregnancy-related deaths, the researchers said. At the system level, quality improvement programs are needed to standardize care and support providers in recognizing and reducing biases and increasing cultural awareness and communication. At the provider level, clinicians at all points in the maternity care process can improve patient experiences by providing equitable and respectful care, and by listening to and addressing patients’ concerns.
In addition, communication campaigns and community engagement can include perspectives of patients, families, and communities to support women and encourage them to ask questions and express concerns, the researchers said.
Improving respectful care can be part of actions to reduce mortality at all levels, the researchers noted. The Hear Her campaign, developed by the CDC Foundation with funding from Merck, provides resources for pregnant and postpartum women and their support networks to help reduce pregnancy-related deaths and complications by encouraging women to share concerns with providers and to recognize urgent maternal warning signs.
The study received no outside funding. The researchers had no financial conflicts to disclose.
FROM MMWR
Self-managed medication abortion shows success at 9-16 weeks’ gestation
Although most abortions happen within the first 9 weeks of pregnancy, it is important to understand the effectiveness of different models of care in a wider gestational range, corresponding author Heidi Moseson, PhD, of Ibis Reproductive Health in Oakland, Calif., said in an interview.
“There will always be people who need abortions after 9 weeks of pregnancy,” she said, whether because of delayed recognition of the pregnancy, changes in the pregnant person’s health, a fetal diagnosis, changes in life circumstances, time required to gather money, transportation to care, or other reasons.
“This study builds on prior research from the same SAFE study cohort that established self-managed medication abortion in the first 9 weeks of pregnancy as safe and effective, and noninferior to clinician-managed abortion,” Dr. Moseson said. “With this analysis, we wanted to explore whether self-managed medication abortion remained effective after 9 weeks of pregnancy, too.”
In the study, published in Obstetrics & Gynecology, Dr. Moseson and colleagues recruited 1,352 women who were initiating self-managed medication abortion through one of three abortion-accompaniment groups in Argentina, Nigeria, and Southeast Asia between 2019 and 2020. Of these, 264 were self-managing a medication abortion at 9 or more weeks’ gestation.
Participants completed a baseline phone survey before beginning the pill regimen, and follow-up surveys at 1 week and 3 weeks after taking the pills. The average age of the participants was 26 years; 75% were at 9-11 weeks’ gestation, 19.3% were at 12-14 weeks’ gestation, and 5.7% were at 15-22 weeks’ gestation. Slightly more than half of the participants (56.4%) used a combination of mifepristone plus misoprostol, and 43.6% used misoprostol only.
The primary outcome was abortion completion. Secondary outcomes included health care seeking and treatment as well as physical experiences.
A total of 89.4% of participants had an abortion completion without the need for procedural intervention. Another 5.3% had a complete abortion with manual vacuum aspiration or dilation and curettage, 4.9% had an incomplete abortion, and one patient reported no abortion outcome.
Of the participants who sought health care during or after the self-managed abortion, 15.9% sought to confirm abortion completion, and 9.1% needed additional medical intervention, including procedural evacuation, antibiotics, additional misoprostol, intravenous fluids, blood transfusion, or an overnight stay in the health care facility.
Overall, women who were at least 12 weeks pregnant were more likely to seek care at a clinic or hospital than those who were 9-11 weeks pregnant (adjusted relative risk, 1.62).
“Particularly in the United States, the [Food and Drug Administration] label only endorsed medication abortion use through 10 weeks of pregnancy; as a result, many people in the U.S. have the incorrect assumption that the pills are not effective after 10 weeks of pregnancy,” Dr. Moseson said. “This isn’t true. There is no magic line at 10 or 12 weeks after which the pills stop working – in fact, the uterus becomes more sensitive, not less, to misoprostol as a pregnancy progresses. This is why the misoprostol dose is reduced by half for abortions after 12-14 weeks or so.”
The findings were limited by several factors including the use of self-reports for gestational age and abortion outcome, without confirmation by ultrasonogram, the researchers noted. Other limitations included the inability to randomize participants to medication regimens because of legal restrictions on abortion access within the study sites, and the small number of participants (three) who underwent self-managed medication abortion at 17-22 weeks’ gestation.
Data support self-management medication abortion later in pregnancy
“Many people are not aware that there is a robust randomized clinical trial literature that demonstrates that both medication abortion regimens remain highly effective up to 24-28 weeks of pregnancy,” as well as a Cochrane review, Dr. Moseson said. “We know that when these pills are administered in a clinical setting well beyond 9 weeks of pregnancy, that they are highly effective and safe.
“We did not expect that the pills would work differently just because someone takes all doses at home, rather than just the second or third dose at home, as happens in most clinician-managed medication abortions,” she noted. However, “we were interested to see differences in likelihood of health care seeking during or after the abortion by country, but in some ways, also not surprised by these differences given that the risks of seeking care and the expectations around care varied significantly across the study sites.”
Looking ahead, “as we think about the United States and we see more and more bans and restrictions on abortion care going into effect, we will see people seeking abortion later into their pregnancies due to these additional barriers people have to overcome to get care,” said Dr. Moseson. “This need for abortion care later in pregnancy extends to self-managed medication abortion, and in that light, I find the results from this study to be reassuring.
“For people who for some reason or another can’t obtain pills until they are 12 or 13 or more weeks’ pregnant, these findings suggest that people can still safely use the pills on their own to end their pregnancy,” she said. Notably, “the participants in this study had high-quality information on how to take the pills, and phone-based counseling and support available to them throughout their abortion via the accompaniment groups, so ensuring that people who self-manage with pills have accurate, accessible information on how to use the pills and monitor for warning signs is also key.
“Additional research is needed to understand the unique informational and support needs of people who are self-managing their abortions beyond 10 weeks of pregnancy,” Dr. Moseson said. “What information do they need and want to feel secure and safe, what resources do they need to protect themselves from legal risk, where and how can they safely access clinical care if needed? These sorts of practical questions feel urgent, and there is much that can be learned from the activist abortion accompaniment groups around the world that have been providing this sort of informational, emotional, and physical support to aborting people for decades.”
Rising rate of self-managed abortions highlights need for more data
“As abortion restrictions increase in the United States, more people may choose to self-manage their abortions,” Lauren Owens, MD, of the University of Washington, Seattle, said in an interview. “Worldwide, self-managed abortion with accompaniment has been shown to be noninferior to medication abortion involving clinical settings at gestational ages less than 9 weeks, as shown in the SAFE study. However, legal and other logistical barriers to care may mean that people can’t access abortion care until after 9 weeks, and we need more data about the effectiveness of these medications when used outside clinical settings.”
Dr. Owens was not surprised by the effectiveness of the medications to end pregnancies between 9 and 16 weeks’ gestation, with few needing follow-up care. However, “it makes sense that as gestational age increases, the percent of people seeking follow-up care also increases, even as it remains a minority of people,” she said.
The World Health Organization’s guidance on self-managed abortion, issued in 2022, was similar to the regimen in the current study, she added.“Self-managed abortion at home can be very safe and effective from 9-16 weeks’ gestation,” said Dr. Owens. “Having access to accompaniment or support, such as the Medication and Abortion Hotline in the United States, can help people through the process.”
According to a recent report, “more than half the abortions in the U.S. were done using medication in 2020, and protocols developed during the pandemic helped us see how safe medication abortion can be without in-person clinic visits,” Dr. Owens said. “I would encourage clinicians who view the 9.1% rate of need for further interventions (such as intravenous fluids, suction, transfusion) in this study as high to compare this to the rate of interventions and morbidity in ongoing pregnancy.”
According to data from the Centers for Disease Control and Prevention, the cesarean rate in the United States varies by state, but ranges from 21% to 35% of pregnancies; “some of the states with the highest cesarean rates are also those with the most abortion restrictions,” Dr. Owens said. “Abortion is generally safer than continuing pregnancy, and patients deserve access to safe options for abortion care and pregnancy care. Clinicians should know that patients can access these medications through Aid Access, accompaniment through the Miscarriage and Abortion Hotline, and legal advice through If/When/How.”
“We still need more data on self-managed abortion at higher gestational ages,” said Dr. Owens. “Few participants in the study were 14 or more weeks’ pregnant; also, despite the WHO recommendation against criminalization of self-managed abortion, we have seen criminalization for adverse pregnancy outcomes in the United States. As self-managed abortion may carry more legal than medical risks for people, creating and evaluating patient and clinician education to minimize that risk is important.”
The study was supported by the David and Lucile Packard Foundation; the researchers also received support for their time from a National Institutes of Health grant. The researchers had no financial conflicts to disclose. Dr. Owens had no financial conflicts to disclose.
Although most abortions happen within the first 9 weeks of pregnancy, it is important to understand the effectiveness of different models of care in a wider gestational range, corresponding author Heidi Moseson, PhD, of Ibis Reproductive Health in Oakland, Calif., said in an interview.
“There will always be people who need abortions after 9 weeks of pregnancy,” she said, whether because of delayed recognition of the pregnancy, changes in the pregnant person’s health, a fetal diagnosis, changes in life circumstances, time required to gather money, transportation to care, or other reasons.
“This study builds on prior research from the same SAFE study cohort that established self-managed medication abortion in the first 9 weeks of pregnancy as safe and effective, and noninferior to clinician-managed abortion,” Dr. Moseson said. “With this analysis, we wanted to explore whether self-managed medication abortion remained effective after 9 weeks of pregnancy, too.”
In the study, published in Obstetrics & Gynecology, Dr. Moseson and colleagues recruited 1,352 women who were initiating self-managed medication abortion through one of three abortion-accompaniment groups in Argentina, Nigeria, and Southeast Asia between 2019 and 2020. Of these, 264 were self-managing a medication abortion at 9 or more weeks’ gestation.
Participants completed a baseline phone survey before beginning the pill regimen, and follow-up surveys at 1 week and 3 weeks after taking the pills. The average age of the participants was 26 years; 75% were at 9-11 weeks’ gestation, 19.3% were at 12-14 weeks’ gestation, and 5.7% were at 15-22 weeks’ gestation. Slightly more than half of the participants (56.4%) used a combination of mifepristone plus misoprostol, and 43.6% used misoprostol only.
The primary outcome was abortion completion. Secondary outcomes included health care seeking and treatment as well as physical experiences.
A total of 89.4% of participants had an abortion completion without the need for procedural intervention. Another 5.3% had a complete abortion with manual vacuum aspiration or dilation and curettage, 4.9% had an incomplete abortion, and one patient reported no abortion outcome.
Of the participants who sought health care during or after the self-managed abortion, 15.9% sought to confirm abortion completion, and 9.1% needed additional medical intervention, including procedural evacuation, antibiotics, additional misoprostol, intravenous fluids, blood transfusion, or an overnight stay in the health care facility.
Overall, women who were at least 12 weeks pregnant were more likely to seek care at a clinic or hospital than those who were 9-11 weeks pregnant (adjusted relative risk, 1.62).
“Particularly in the United States, the [Food and Drug Administration] label only endorsed medication abortion use through 10 weeks of pregnancy; as a result, many people in the U.S. have the incorrect assumption that the pills are not effective after 10 weeks of pregnancy,” Dr. Moseson said. “This isn’t true. There is no magic line at 10 or 12 weeks after which the pills stop working – in fact, the uterus becomes more sensitive, not less, to misoprostol as a pregnancy progresses. This is why the misoprostol dose is reduced by half for abortions after 12-14 weeks or so.”
The findings were limited by several factors including the use of self-reports for gestational age and abortion outcome, without confirmation by ultrasonogram, the researchers noted. Other limitations included the inability to randomize participants to medication regimens because of legal restrictions on abortion access within the study sites, and the small number of participants (three) who underwent self-managed medication abortion at 17-22 weeks’ gestation.
Data support self-management medication abortion later in pregnancy
“Many people are not aware that there is a robust randomized clinical trial literature that demonstrates that both medication abortion regimens remain highly effective up to 24-28 weeks of pregnancy,” as well as a Cochrane review, Dr. Moseson said. “We know that when these pills are administered in a clinical setting well beyond 9 weeks of pregnancy, that they are highly effective and safe.
“We did not expect that the pills would work differently just because someone takes all doses at home, rather than just the second or third dose at home, as happens in most clinician-managed medication abortions,” she noted. However, “we were interested to see differences in likelihood of health care seeking during or after the abortion by country, but in some ways, also not surprised by these differences given that the risks of seeking care and the expectations around care varied significantly across the study sites.”
Looking ahead, “as we think about the United States and we see more and more bans and restrictions on abortion care going into effect, we will see people seeking abortion later into their pregnancies due to these additional barriers people have to overcome to get care,” said Dr. Moseson. “This need for abortion care later in pregnancy extends to self-managed medication abortion, and in that light, I find the results from this study to be reassuring.
“For people who for some reason or another can’t obtain pills until they are 12 or 13 or more weeks’ pregnant, these findings suggest that people can still safely use the pills on their own to end their pregnancy,” she said. Notably, “the participants in this study had high-quality information on how to take the pills, and phone-based counseling and support available to them throughout their abortion via the accompaniment groups, so ensuring that people who self-manage with pills have accurate, accessible information on how to use the pills and monitor for warning signs is also key.
“Additional research is needed to understand the unique informational and support needs of people who are self-managing their abortions beyond 10 weeks of pregnancy,” Dr. Moseson said. “What information do they need and want to feel secure and safe, what resources do they need to protect themselves from legal risk, where and how can they safely access clinical care if needed? These sorts of practical questions feel urgent, and there is much that can be learned from the activist abortion accompaniment groups around the world that have been providing this sort of informational, emotional, and physical support to aborting people for decades.”
Rising rate of self-managed abortions highlights need for more data
“As abortion restrictions increase in the United States, more people may choose to self-manage their abortions,” Lauren Owens, MD, of the University of Washington, Seattle, said in an interview. “Worldwide, self-managed abortion with accompaniment has been shown to be noninferior to medication abortion involving clinical settings at gestational ages less than 9 weeks, as shown in the SAFE study. However, legal and other logistical barriers to care may mean that people can’t access abortion care until after 9 weeks, and we need more data about the effectiveness of these medications when used outside clinical settings.”
Dr. Owens was not surprised by the effectiveness of the medications to end pregnancies between 9 and 16 weeks’ gestation, with few needing follow-up care. However, “it makes sense that as gestational age increases, the percent of people seeking follow-up care also increases, even as it remains a minority of people,” she said.
The World Health Organization’s guidance on self-managed abortion, issued in 2022, was similar to the regimen in the current study, she added.“Self-managed abortion at home can be very safe and effective from 9-16 weeks’ gestation,” said Dr. Owens. “Having access to accompaniment or support, such as the Medication and Abortion Hotline in the United States, can help people through the process.”
According to a recent report, “more than half the abortions in the U.S. were done using medication in 2020, and protocols developed during the pandemic helped us see how safe medication abortion can be without in-person clinic visits,” Dr. Owens said. “I would encourage clinicians who view the 9.1% rate of need for further interventions (such as intravenous fluids, suction, transfusion) in this study as high to compare this to the rate of interventions and morbidity in ongoing pregnancy.”
According to data from the Centers for Disease Control and Prevention, the cesarean rate in the United States varies by state, but ranges from 21% to 35% of pregnancies; “some of the states with the highest cesarean rates are also those with the most abortion restrictions,” Dr. Owens said. “Abortion is generally safer than continuing pregnancy, and patients deserve access to safe options for abortion care and pregnancy care. Clinicians should know that patients can access these medications through Aid Access, accompaniment through the Miscarriage and Abortion Hotline, and legal advice through If/When/How.”
“We still need more data on self-managed abortion at higher gestational ages,” said Dr. Owens. “Few participants in the study were 14 or more weeks’ pregnant; also, despite the WHO recommendation against criminalization of self-managed abortion, we have seen criminalization for adverse pregnancy outcomes in the United States. As self-managed abortion may carry more legal than medical risks for people, creating and evaluating patient and clinician education to minimize that risk is important.”
The study was supported by the David and Lucile Packard Foundation; the researchers also received support for their time from a National Institutes of Health grant. The researchers had no financial conflicts to disclose. Dr. Owens had no financial conflicts to disclose.
Although most abortions happen within the first 9 weeks of pregnancy, it is important to understand the effectiveness of different models of care in a wider gestational range, corresponding author Heidi Moseson, PhD, of Ibis Reproductive Health in Oakland, Calif., said in an interview.
“There will always be people who need abortions after 9 weeks of pregnancy,” she said, whether because of delayed recognition of the pregnancy, changes in the pregnant person’s health, a fetal diagnosis, changes in life circumstances, time required to gather money, transportation to care, or other reasons.
“This study builds on prior research from the same SAFE study cohort that established self-managed medication abortion in the first 9 weeks of pregnancy as safe and effective, and noninferior to clinician-managed abortion,” Dr. Moseson said. “With this analysis, we wanted to explore whether self-managed medication abortion remained effective after 9 weeks of pregnancy, too.”
In the study, published in Obstetrics & Gynecology, Dr. Moseson and colleagues recruited 1,352 women who were initiating self-managed medication abortion through one of three abortion-accompaniment groups in Argentina, Nigeria, and Southeast Asia between 2019 and 2020. Of these, 264 were self-managing a medication abortion at 9 or more weeks’ gestation.
Participants completed a baseline phone survey before beginning the pill regimen, and follow-up surveys at 1 week and 3 weeks after taking the pills. The average age of the participants was 26 years; 75% were at 9-11 weeks’ gestation, 19.3% were at 12-14 weeks’ gestation, and 5.7% were at 15-22 weeks’ gestation. Slightly more than half of the participants (56.4%) used a combination of mifepristone plus misoprostol, and 43.6% used misoprostol only.
The primary outcome was abortion completion. Secondary outcomes included health care seeking and treatment as well as physical experiences.
A total of 89.4% of participants had an abortion completion without the need for procedural intervention. Another 5.3% had a complete abortion with manual vacuum aspiration or dilation and curettage, 4.9% had an incomplete abortion, and one patient reported no abortion outcome.
Of the participants who sought health care during or after the self-managed abortion, 15.9% sought to confirm abortion completion, and 9.1% needed additional medical intervention, including procedural evacuation, antibiotics, additional misoprostol, intravenous fluids, blood transfusion, or an overnight stay in the health care facility.
Overall, women who were at least 12 weeks pregnant were more likely to seek care at a clinic or hospital than those who were 9-11 weeks pregnant (adjusted relative risk, 1.62).
“Particularly in the United States, the [Food and Drug Administration] label only endorsed medication abortion use through 10 weeks of pregnancy; as a result, many people in the U.S. have the incorrect assumption that the pills are not effective after 10 weeks of pregnancy,” Dr. Moseson said. “This isn’t true. There is no magic line at 10 or 12 weeks after which the pills stop working – in fact, the uterus becomes more sensitive, not less, to misoprostol as a pregnancy progresses. This is why the misoprostol dose is reduced by half for abortions after 12-14 weeks or so.”
The findings were limited by several factors including the use of self-reports for gestational age and abortion outcome, without confirmation by ultrasonogram, the researchers noted. Other limitations included the inability to randomize participants to medication regimens because of legal restrictions on abortion access within the study sites, and the small number of participants (three) who underwent self-managed medication abortion at 17-22 weeks’ gestation.
Data support self-management medication abortion later in pregnancy
“Many people are not aware that there is a robust randomized clinical trial literature that demonstrates that both medication abortion regimens remain highly effective up to 24-28 weeks of pregnancy,” as well as a Cochrane review, Dr. Moseson said. “We know that when these pills are administered in a clinical setting well beyond 9 weeks of pregnancy, that they are highly effective and safe.
“We did not expect that the pills would work differently just because someone takes all doses at home, rather than just the second or third dose at home, as happens in most clinician-managed medication abortions,” she noted. However, “we were interested to see differences in likelihood of health care seeking during or after the abortion by country, but in some ways, also not surprised by these differences given that the risks of seeking care and the expectations around care varied significantly across the study sites.”
Looking ahead, “as we think about the United States and we see more and more bans and restrictions on abortion care going into effect, we will see people seeking abortion later into their pregnancies due to these additional barriers people have to overcome to get care,” said Dr. Moseson. “This need for abortion care later in pregnancy extends to self-managed medication abortion, and in that light, I find the results from this study to be reassuring.
“For people who for some reason or another can’t obtain pills until they are 12 or 13 or more weeks’ pregnant, these findings suggest that people can still safely use the pills on their own to end their pregnancy,” she said. Notably, “the participants in this study had high-quality information on how to take the pills, and phone-based counseling and support available to them throughout their abortion via the accompaniment groups, so ensuring that people who self-manage with pills have accurate, accessible information on how to use the pills and monitor for warning signs is also key.
“Additional research is needed to understand the unique informational and support needs of people who are self-managing their abortions beyond 10 weeks of pregnancy,” Dr. Moseson said. “What information do they need and want to feel secure and safe, what resources do they need to protect themselves from legal risk, where and how can they safely access clinical care if needed? These sorts of practical questions feel urgent, and there is much that can be learned from the activist abortion accompaniment groups around the world that have been providing this sort of informational, emotional, and physical support to aborting people for decades.”
Rising rate of self-managed abortions highlights need for more data
“As abortion restrictions increase in the United States, more people may choose to self-manage their abortions,” Lauren Owens, MD, of the University of Washington, Seattle, said in an interview. “Worldwide, self-managed abortion with accompaniment has been shown to be noninferior to medication abortion involving clinical settings at gestational ages less than 9 weeks, as shown in the SAFE study. However, legal and other logistical barriers to care may mean that people can’t access abortion care until after 9 weeks, and we need more data about the effectiveness of these medications when used outside clinical settings.”
Dr. Owens was not surprised by the effectiveness of the medications to end pregnancies between 9 and 16 weeks’ gestation, with few needing follow-up care. However, “it makes sense that as gestational age increases, the percent of people seeking follow-up care also increases, even as it remains a minority of people,” she said.
The World Health Organization’s guidance on self-managed abortion, issued in 2022, was similar to the regimen in the current study, she added.“Self-managed abortion at home can be very safe and effective from 9-16 weeks’ gestation,” said Dr. Owens. “Having access to accompaniment or support, such as the Medication and Abortion Hotline in the United States, can help people through the process.”
According to a recent report, “more than half the abortions in the U.S. were done using medication in 2020, and protocols developed during the pandemic helped us see how safe medication abortion can be without in-person clinic visits,” Dr. Owens said. “I would encourage clinicians who view the 9.1% rate of need for further interventions (such as intravenous fluids, suction, transfusion) in this study as high to compare this to the rate of interventions and morbidity in ongoing pregnancy.”
According to data from the Centers for Disease Control and Prevention, the cesarean rate in the United States varies by state, but ranges from 21% to 35% of pregnancies; “some of the states with the highest cesarean rates are also those with the most abortion restrictions,” Dr. Owens said. “Abortion is generally safer than continuing pregnancy, and patients deserve access to safe options for abortion care and pregnancy care. Clinicians should know that patients can access these medications through Aid Access, accompaniment through the Miscarriage and Abortion Hotline, and legal advice through If/When/How.”
“We still need more data on self-managed abortion at higher gestational ages,” said Dr. Owens. “Few participants in the study were 14 or more weeks’ pregnant; also, despite the WHO recommendation against criminalization of self-managed abortion, we have seen criminalization for adverse pregnancy outcomes in the United States. As self-managed abortion may carry more legal than medical risks for people, creating and evaluating patient and clinician education to minimize that risk is important.”
The study was supported by the David and Lucile Packard Foundation; the researchers also received support for their time from a National Institutes of Health grant. The researchers had no financial conflicts to disclose. Dr. Owens had no financial conflicts to disclose.
FDA approves first RSV vaccine for pregnancy
The vaccine, known as Abrysvo, can be given between weeks 32 and 36 of pregnancy and is designed to protect infants from the virus from birth to 6 months of age.
Administered as a single-dose, intramuscular injection, the FDA approved Abrysvo at the end of May for the prevention of lower respiratory tract illness caused by RSV in people aged 60 years and older.
However, “RSV is a common cause of illness in children, and infants are among those at highest risk for severe disease, which can lead to hospitalization,” Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, pointed out in a news release. “This approval provides an option for health care providers and pregnant individuals to protect infants from this potentially life-threatening disease.”
Most children are infected with the contagious virus at least once by the time they reach age 2 years. Very young children are at particular risk of severe complications, such as pneumonia or bronchitis, and in clinical trials, the new vaccine reduced that risk by up to 82%.
Before the vaccine became available, up to 3% of infants infected with RSV needed to be hospitalized, according to the Centers for Disease Control and Prevention. In the hospital, treatment typically includes oxygen, intravenous fluids, and mechanical ventilation.
RSV often causes common cold symptoms, but the virus poses the risk of severe complications that can lead to death among young children and older people. The CDC estimates 100-300 deaths of children younger than 5 years and 6,000-10,000 deaths of people aged 65 years and older are linked to RSV annually.
This is also the first year that an antibody shot is available to be given after birth to prevent severe RSV in infants younger than 1 year.
In its approval announcement, the FDA pointed out that preeclampsia occurred in 1.8% of pregnancies after Abrysvo, compared with 1.4% of those who received placebo. The FDA also reported that, in infants, low birth weight and jaundice occurred at a higher rate among the pregnant Abrysvo recipients, compared with the placebo group.
Studies have also shown that pregnant vaccine recipients experienced preterm birth at a rate of 5.7%, compared with a rate of 4.7% among those who received placebo. The FDA called the difference “a numerical imbalance” but said in the approval announcement that a “causal relationship” could not be established.
The FDA also noted that people already at high risk of preterm birth were excluded from clinical trials and that Pfizer must conduct ongoing studies to monitor the risk of preeclampsia as well as preterm birth.
A version of this article first appeared on Medscape.com.
The vaccine, known as Abrysvo, can be given between weeks 32 and 36 of pregnancy and is designed to protect infants from the virus from birth to 6 months of age.
Administered as a single-dose, intramuscular injection, the FDA approved Abrysvo at the end of May for the prevention of lower respiratory tract illness caused by RSV in people aged 60 years and older.
However, “RSV is a common cause of illness in children, and infants are among those at highest risk for severe disease, which can lead to hospitalization,” Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, pointed out in a news release. “This approval provides an option for health care providers and pregnant individuals to protect infants from this potentially life-threatening disease.”
Most children are infected with the contagious virus at least once by the time they reach age 2 years. Very young children are at particular risk of severe complications, such as pneumonia or bronchitis, and in clinical trials, the new vaccine reduced that risk by up to 82%.
Before the vaccine became available, up to 3% of infants infected with RSV needed to be hospitalized, according to the Centers for Disease Control and Prevention. In the hospital, treatment typically includes oxygen, intravenous fluids, and mechanical ventilation.
RSV often causes common cold symptoms, but the virus poses the risk of severe complications that can lead to death among young children and older people. The CDC estimates 100-300 deaths of children younger than 5 years and 6,000-10,000 deaths of people aged 65 years and older are linked to RSV annually.
This is also the first year that an antibody shot is available to be given after birth to prevent severe RSV in infants younger than 1 year.
In its approval announcement, the FDA pointed out that preeclampsia occurred in 1.8% of pregnancies after Abrysvo, compared with 1.4% of those who received placebo. The FDA also reported that, in infants, low birth weight and jaundice occurred at a higher rate among the pregnant Abrysvo recipients, compared with the placebo group.
Studies have also shown that pregnant vaccine recipients experienced preterm birth at a rate of 5.7%, compared with a rate of 4.7% among those who received placebo. The FDA called the difference “a numerical imbalance” but said in the approval announcement that a “causal relationship” could not be established.
The FDA also noted that people already at high risk of preterm birth were excluded from clinical trials and that Pfizer must conduct ongoing studies to monitor the risk of preeclampsia as well as preterm birth.
A version of this article first appeared on Medscape.com.
The vaccine, known as Abrysvo, can be given between weeks 32 and 36 of pregnancy and is designed to protect infants from the virus from birth to 6 months of age.
Administered as a single-dose, intramuscular injection, the FDA approved Abrysvo at the end of May for the prevention of lower respiratory tract illness caused by RSV in people aged 60 years and older.
However, “RSV is a common cause of illness in children, and infants are among those at highest risk for severe disease, which can lead to hospitalization,” Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, pointed out in a news release. “This approval provides an option for health care providers and pregnant individuals to protect infants from this potentially life-threatening disease.”
Most children are infected with the contagious virus at least once by the time they reach age 2 years. Very young children are at particular risk of severe complications, such as pneumonia or bronchitis, and in clinical trials, the new vaccine reduced that risk by up to 82%.
Before the vaccine became available, up to 3% of infants infected with RSV needed to be hospitalized, according to the Centers for Disease Control and Prevention. In the hospital, treatment typically includes oxygen, intravenous fluids, and mechanical ventilation.
RSV often causes common cold symptoms, but the virus poses the risk of severe complications that can lead to death among young children and older people. The CDC estimates 100-300 deaths of children younger than 5 years and 6,000-10,000 deaths of people aged 65 years and older are linked to RSV annually.
This is also the first year that an antibody shot is available to be given after birth to prevent severe RSV in infants younger than 1 year.
In its approval announcement, the FDA pointed out that preeclampsia occurred in 1.8% of pregnancies after Abrysvo, compared with 1.4% of those who received placebo. The FDA also reported that, in infants, low birth weight and jaundice occurred at a higher rate among the pregnant Abrysvo recipients, compared with the placebo group.
Studies have also shown that pregnant vaccine recipients experienced preterm birth at a rate of 5.7%, compared with a rate of 4.7% among those who received placebo. The FDA called the difference “a numerical imbalance” but said in the approval announcement that a “causal relationship” could not be established.
The FDA also noted that people already at high risk of preterm birth were excluded from clinical trials and that Pfizer must conduct ongoing studies to monitor the risk of preeclampsia as well as preterm birth.
A version of this article first appeared on Medscape.com.
Big geographic access gaps for oncofertility services in U.S.
TOPLINE:
, a study shows.
METHODOLOGY:
- In this cross-sectional analysis, researchers identified 370 fertility centers in the United States (361 in the continental U.S.) that provide oncofertility services and that met criteria for an oncofertility center.
- Clinics were considered oncofertility centers if they offered oocyte cryopreservation, had performed at least one fertility preservation cycle in 2018, reported serving people without partners, and had an embryology laboratory that was accredited per Centers for Disease Control and Prevention guidelines.
- Researchers then quantified the number of young women potentially eligible for oncofertility services who lived farther than 2 hours from an oncofertility center.
- In a secondary analysis, the team assessed the association between geographic access and state fertility preservation insurance mandates.
TAKEAWAY:
- Overall, 3.63 million (5.7%) young women of reproductive age in the United States (aged 15-4) live more than 2 hours from an oncofertility center.
- The greatest gaps in access are in the Mountain West, West North Central, and Southwest regions; for instance, in Montana, North Dakota, and Wyoming fewer than half of the at-risk population has geographic access to an oncofertility center.
- Among the 11 states with fertility preservation insurance mandates, 98.5% of at-risk women have geographic access to an oncofertility center; in the 17 states without fertility preservation legislation, 79.6% of at-risk women have access to an oncofertility center.
IN PRACTICE:
Just over 3.6 million “reproductive-age female individuals lack geographic access to oncofertility services, especially in the Mountain West and West North Central regions,” the authors concluded. “Significant geographic disparities in access to fertility preservation in the U.S. require strategic expansion of care, especially given the growing demand for oncofertility services.”
SOURCE:
The study, led by Benjamin Peipert, MD, with Duke University, Durham, N.C., was published online in JAMA Oncology.
LIMITATIONS:
The authors relied on clinic data reported to the CDC, made assumptions about reasonable travel time, and may have underestimated access in some areas.
DISCLOSURES:
The study had no commercial funding. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
, a study shows.
METHODOLOGY:
- In this cross-sectional analysis, researchers identified 370 fertility centers in the United States (361 in the continental U.S.) that provide oncofertility services and that met criteria for an oncofertility center.
- Clinics were considered oncofertility centers if they offered oocyte cryopreservation, had performed at least one fertility preservation cycle in 2018, reported serving people without partners, and had an embryology laboratory that was accredited per Centers for Disease Control and Prevention guidelines.
- Researchers then quantified the number of young women potentially eligible for oncofertility services who lived farther than 2 hours from an oncofertility center.
- In a secondary analysis, the team assessed the association between geographic access and state fertility preservation insurance mandates.
TAKEAWAY:
- Overall, 3.63 million (5.7%) young women of reproductive age in the United States (aged 15-4) live more than 2 hours from an oncofertility center.
- The greatest gaps in access are in the Mountain West, West North Central, and Southwest regions; for instance, in Montana, North Dakota, and Wyoming fewer than half of the at-risk population has geographic access to an oncofertility center.
- Among the 11 states with fertility preservation insurance mandates, 98.5% of at-risk women have geographic access to an oncofertility center; in the 17 states without fertility preservation legislation, 79.6% of at-risk women have access to an oncofertility center.
IN PRACTICE:
Just over 3.6 million “reproductive-age female individuals lack geographic access to oncofertility services, especially in the Mountain West and West North Central regions,” the authors concluded. “Significant geographic disparities in access to fertility preservation in the U.S. require strategic expansion of care, especially given the growing demand for oncofertility services.”
SOURCE:
The study, led by Benjamin Peipert, MD, with Duke University, Durham, N.C., was published online in JAMA Oncology.
LIMITATIONS:
The authors relied on clinic data reported to the CDC, made assumptions about reasonable travel time, and may have underestimated access in some areas.
DISCLOSURES:
The study had no commercial funding. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
, a study shows.
METHODOLOGY:
- In this cross-sectional analysis, researchers identified 370 fertility centers in the United States (361 in the continental U.S.) that provide oncofertility services and that met criteria for an oncofertility center.
- Clinics were considered oncofertility centers if they offered oocyte cryopreservation, had performed at least one fertility preservation cycle in 2018, reported serving people without partners, and had an embryology laboratory that was accredited per Centers for Disease Control and Prevention guidelines.
- Researchers then quantified the number of young women potentially eligible for oncofertility services who lived farther than 2 hours from an oncofertility center.
- In a secondary analysis, the team assessed the association between geographic access and state fertility preservation insurance mandates.
TAKEAWAY:
- Overall, 3.63 million (5.7%) young women of reproductive age in the United States (aged 15-4) live more than 2 hours from an oncofertility center.
- The greatest gaps in access are in the Mountain West, West North Central, and Southwest regions; for instance, in Montana, North Dakota, and Wyoming fewer than half of the at-risk population has geographic access to an oncofertility center.
- Among the 11 states with fertility preservation insurance mandates, 98.5% of at-risk women have geographic access to an oncofertility center; in the 17 states without fertility preservation legislation, 79.6% of at-risk women have access to an oncofertility center.
IN PRACTICE:
Just over 3.6 million “reproductive-age female individuals lack geographic access to oncofertility services, especially in the Mountain West and West North Central regions,” the authors concluded. “Significant geographic disparities in access to fertility preservation in the U.S. require strategic expansion of care, especially given the growing demand for oncofertility services.”
SOURCE:
The study, led by Benjamin Peipert, MD, with Duke University, Durham, N.C., was published online in JAMA Oncology.
LIMITATIONS:
The authors relied on clinic data reported to the CDC, made assumptions about reasonable travel time, and may have underestimated access in some areas.
DISCLOSURES:
The study had no commercial funding. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA ONCOLOGY
The three pillars of perinatal care: Babies, parents, dyadic relationships
Perinatal depression (PND) is the most common obstetric complication in the United States. Even when screening results are positive, mothers often do not receive further evaluation, and even when PND is diagnosed, mothers do not receive evidence-based treatments.
Meta-analytic estimates show that pregnant women suffer from PND at rates from 6.5% to 12.9% across pregnancy to 3-months post partum.1 Women from low-income families and adolescent mothers are at highest risk, where rates are double and triple respectively.
Fathers also suffer from PND, with a prevalence rate from 2% to 25%, increasing to 50% when the mother experiences PND.
The American Academy of Pediatrics issued a Policy Statement (January 2019) about the need to recognize and manage PND. They recommended that pediatric medical homes establish a system to implement the screening of mothers at the 1-, 2-, 4-, and 6-month well-child visits, to use community resources for the treatment and referral of the mother with depression, and to provide support for the maternal-child relationship.2
The American Academy of Pediatrics also recommends advocacy for workforce development for mental health professionals who care for young children and mother-infant dyads, and for promotion of evidence-based interventions focused on healthy attachment and parent-child relationships.
Family research
There is a bidirectional association between family relational stress and PND. Lack of family support is both a predictor and a consequence of perinatal depression. Frequent arguments, conflict because one or both partners did not want the pregnancy, division of labor, poor support following stressful life events, lack of partner availability, and low intimacy are associated with increased perinatal depressive symptoms.
Gender role stress is also included as a risk factor. For example, men may fear performance failure related to work and sex, and women may fear disruption in the couple relationship due to the introduction of a child.
When depressed and nondepressed women at 2 months post delivery were compared, the women with depressive symptoms perceived that their partners did not share similar interests, provided little companionship, expressed disinterest in infant care, did not provide a feeling of connection, did not encourage them to get assistance to cope with difficulties, and expressed disagreement in infant care.3
A high-quality intimate relationship is protective for many illnesses and PND is no exception.4
Assessment
Despite the availability of effective treatments, perinatal mental health utilization rates are strikingly low. There are limited providers and a general lack of awareness of the need for this care. The stigma for assessing and treating PND is high because the perception is that pregnancy is supposed to be a joyous time and with time, PND will pass.
The first step is a timely and accurate assessment of the mother, which should, if possible, include the father and other family support people. The preferred standard for women is the Edinburgh Postnatal Depression Scale (EPDS), a checklist of 10 items (listed below) with a maximum score of 30, and any score over 10 warrants further assessment.5 This scale is used worldwide in obstetric clinics and has been used to identify PND in fathers.
- I have been able to laugh and see the funny side of things.
- I have looked forward with enjoyment to things.
- I have blamed myself unnecessarily when things went wrong.
- I have been anxious or worried for no good reason.
- I have felt scared or panicky for no good reason.
- Things have been getting to me.
- I have been so unhappy that I have had difficulty sleeping.
- I have felt sad or miserable.
- I have been so unhappy that I have been crying.
- The thought of harming myself has occurred to me.
A new ultrabrief tool with only four questions is the Brief Multidimensional Assessment Scale (BMAS), which measures the ability to get things done, emotional support in important relationships, quality of life, and sense of purpose in life. It demonstrates concurrent validity with other measures and discriminates between nonclinical participants and participants from most clinical contexts.6
For those interested in assessing family health, an easy-to-use assessment tool is the 12-item Family Assessment Device (FAD).7
Family therapy interventions
A systematic review and meta-analysis of the current evidence on the usefulness of family therapy interventions in the prevention and treatment of PND identified seven studies.
In these studies, there were statistically significant reductions in depressive symptoms at postintervention in intervention group mothers. Intervention intensity and level of family involvement moderated the impacts of intervention on maternal depression, and there was a trend in improved family functioning in intervention group couples.8
Evidence-based interventions are usually psychoeducational or cognitive-behavioral family therapy models where focused interventions target the following three areas:
- Communication skills related to expectations (including those that pertain to gender roles and the transition to parenthood) and emotional support.
- Conflict management.
- Problem-solving skills related to shared responsibility in infant care and household activities.
Intensive day program for mothers and babies
There is a growing awareness of the effectiveness of specialized mother-baby day hospital programs for women with psychiatric distress during the peripartum period.9
The Women & Infants’ Hospital (WIH) in Providence, R.I., established a mother-baby postpartum depression day program in 2000, adjacent to the obstetrical hospital, the ninth largest obstetrical service in the United States. The day program is integrated with the hospital’s obstetric medicine team and referrals are also accepted from the perinatal practices in the surrounding community. The treatment day includes group, individual, and milieu treatment, as well as consultation with psychiatrists, nutritionists, social workers, lactation specialists and others.
The primary theoretical model utilized by the program is interpersonal psychotherapy (IPT), with essential elements of the program incorporating cognitive behavioral therapy (CBT), and experiential strategies (for instance, mindfulness, breathing, progressive muscle relaxation) to improve self-care and relaxation skills. Patient satisfaction surveys collected from 800 women, (54% identified as White) treated at the program between 2007 and 2012 found that women were highly satisfied with the treatment received, noting that the inclusion of the baby in their treatment is a highly valued aspect of care.
A similar program in Minnesota reported that 328 women who consented to participation in research had significant improvements (P < .001) in self-report scales assessing depression, anxiety, and maternal functioning, improving mental health and parenting functioning.10
Lastly, a recent study out of Brussels, on the benefit of a mother-baby day program analyzed patient data from 2015 and 2020. This clinical population of 92 patients (43% identifying as North African) was comparable to the population of the inpatient mother-baby units in terms of psychosocial fragility except that the parents entering the day program had less severe illnesses, more anxiety disorder, and less postpartum psychosis. In the day program, all the babies improved in terms of symptoms and relationships, except for those with significant developmental difficulties.
The dyadic relationship was measured using “levels of adaptation of the parent–child relationship” scale which has four general levels of adjustment, from well-adjusted to troubled or dangerous relationship. Unlike programs in the United States, this program takes children up to 2.5 years old and the assessment period is up to 8 weeks.11
Prevention of mental illness is best achieved by reducing the known determinants of illness. For PND, the research is clear, so why not start at the earliest possible stage, when we know that change is possible? Pushing health care systems to change is not easy, but as the research accumulates and the positive results grow, our arguments become stronger.
Dr. Heru is a psychiatrist in Aurora, Colo. She is editor of “Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals” (New York: Routledge, 2013). She has no conflicts of interest to disclose. Contact Dr. Heru at [email protected].
References
1. Gavin NI et al. Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol. 2005 Nov;106(5 Pt 1):1071-83. doi: 10.1097/01.AOG.0000183597.31630.db.
2. Rafferty J et al. Incorporating recognition and management of perinatal depression into pediatric practice. Pediatrics. 2019 Jan;143(1):e20183260. doi: 10.1542/peds.2018-3260.
3. Cluxton-Keller F, Bruce ML. Clinical effectiveness of family therapeutic interventions in the prevention and treatment of perinatal depression: A systematic review and meta-analysis. PLoS One. 2018 Jun 14;13(6):e0198730. doi: 10.1371/journal.pone.0198730.
4. Kumar SA et al. Promoting resilience to depression among couples during pregnancy: The protective functions of intimate relationship satisfaction and self-compassion. Family Process. 2022 May;62(1):387-405. doi: 10.1111/famp.12788.
5. Cox JL et al. Detection of postnatal depression: Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987 Jun;150:782-6. doi: 10.1192/bjp.150.6.782.
6. Keitner GI et al. The Brief Multidimensional Assessment Scale (BMAS): A broad measure of patient well-being. Am J Psychother. 2023 Feb 1;76(2):75-81. doi: 10.1176/appi.psychotherapy.20220032.
7. Boterhoven de Haan KL et al. Reliability and validity of a short version of the general functioning subscale of the McMaster Family Assessment Device. Fam Process. 2015 Mar;54(1):116-23. doi: 10.1111/famp.12113.
8. Cluxton-Keller F, Bruce ML. Clinical effectiveness of family therapeutic interventions in the prevention and treatment of perinatal depression: A systematic review and meta-analysis. PLoS One. 2018 Jun 14;13(6):e0198730. doi: 10.1371/journal.pone.0198730.
9. Battle CL, Howard MM. A mother-baby psychiatric day hospital: History, rationale, and why perinatal mental health is important for obstetric medicine. Obstet Med. 2014 Jun;7(2):66-70. doi: 10.1177/1753495X13514402.
10. Kim HG et al. Keeping Parent, Child, and Relationship in Mind: Clinical Effectiveness of a Trauma-informed, Multigenerational, Attachment-Based, Mother-Baby Partial Hospital Program in an Urban Safety Net Hospital. Matern Child Health J. 2021 Nov;25(11):1776-86. doi: 10.1007/s10995-021-03221-4.
11. Moureau A et al. A 5 years’ experience of a parent-baby day unit: impact on baby’s development. Front Psychiatry. 2023 June 15;14. doi: 10.3389/fpsyt.2023.1121894.
Perinatal depression (PND) is the most common obstetric complication in the United States. Even when screening results are positive, mothers often do not receive further evaluation, and even when PND is diagnosed, mothers do not receive evidence-based treatments.
Meta-analytic estimates show that pregnant women suffer from PND at rates from 6.5% to 12.9% across pregnancy to 3-months post partum.1 Women from low-income families and adolescent mothers are at highest risk, where rates are double and triple respectively.
Fathers also suffer from PND, with a prevalence rate from 2% to 25%, increasing to 50% when the mother experiences PND.
The American Academy of Pediatrics issued a Policy Statement (January 2019) about the need to recognize and manage PND. They recommended that pediatric medical homes establish a system to implement the screening of mothers at the 1-, 2-, 4-, and 6-month well-child visits, to use community resources for the treatment and referral of the mother with depression, and to provide support for the maternal-child relationship.2
The American Academy of Pediatrics also recommends advocacy for workforce development for mental health professionals who care for young children and mother-infant dyads, and for promotion of evidence-based interventions focused on healthy attachment and parent-child relationships.
Family research
There is a bidirectional association between family relational stress and PND. Lack of family support is both a predictor and a consequence of perinatal depression. Frequent arguments, conflict because one or both partners did not want the pregnancy, division of labor, poor support following stressful life events, lack of partner availability, and low intimacy are associated with increased perinatal depressive symptoms.
Gender role stress is also included as a risk factor. For example, men may fear performance failure related to work and sex, and women may fear disruption in the couple relationship due to the introduction of a child.
When depressed and nondepressed women at 2 months post delivery were compared, the women with depressive symptoms perceived that their partners did not share similar interests, provided little companionship, expressed disinterest in infant care, did not provide a feeling of connection, did not encourage them to get assistance to cope with difficulties, and expressed disagreement in infant care.3
A high-quality intimate relationship is protective for many illnesses and PND is no exception.4
Assessment
Despite the availability of effective treatments, perinatal mental health utilization rates are strikingly low. There are limited providers and a general lack of awareness of the need for this care. The stigma for assessing and treating PND is high because the perception is that pregnancy is supposed to be a joyous time and with time, PND will pass.
The first step is a timely and accurate assessment of the mother, which should, if possible, include the father and other family support people. The preferred standard for women is the Edinburgh Postnatal Depression Scale (EPDS), a checklist of 10 items (listed below) with a maximum score of 30, and any score over 10 warrants further assessment.5 This scale is used worldwide in obstetric clinics and has been used to identify PND in fathers.
- I have been able to laugh and see the funny side of things.
- I have looked forward with enjoyment to things.
- I have blamed myself unnecessarily when things went wrong.
- I have been anxious or worried for no good reason.
- I have felt scared or panicky for no good reason.
- Things have been getting to me.
- I have been so unhappy that I have had difficulty sleeping.
- I have felt sad or miserable.
- I have been so unhappy that I have been crying.
- The thought of harming myself has occurred to me.
A new ultrabrief tool with only four questions is the Brief Multidimensional Assessment Scale (BMAS), which measures the ability to get things done, emotional support in important relationships, quality of life, and sense of purpose in life. It demonstrates concurrent validity with other measures and discriminates between nonclinical participants and participants from most clinical contexts.6
For those interested in assessing family health, an easy-to-use assessment tool is the 12-item Family Assessment Device (FAD).7
Family therapy interventions
A systematic review and meta-analysis of the current evidence on the usefulness of family therapy interventions in the prevention and treatment of PND identified seven studies.
In these studies, there were statistically significant reductions in depressive symptoms at postintervention in intervention group mothers. Intervention intensity and level of family involvement moderated the impacts of intervention on maternal depression, and there was a trend in improved family functioning in intervention group couples.8
Evidence-based interventions are usually psychoeducational or cognitive-behavioral family therapy models where focused interventions target the following three areas:
- Communication skills related to expectations (including those that pertain to gender roles and the transition to parenthood) and emotional support.
- Conflict management.
- Problem-solving skills related to shared responsibility in infant care and household activities.
Intensive day program for mothers and babies
There is a growing awareness of the effectiveness of specialized mother-baby day hospital programs for women with psychiatric distress during the peripartum period.9
The Women & Infants’ Hospital (WIH) in Providence, R.I., established a mother-baby postpartum depression day program in 2000, adjacent to the obstetrical hospital, the ninth largest obstetrical service in the United States. The day program is integrated with the hospital’s obstetric medicine team and referrals are also accepted from the perinatal practices in the surrounding community. The treatment day includes group, individual, and milieu treatment, as well as consultation with psychiatrists, nutritionists, social workers, lactation specialists and others.
The primary theoretical model utilized by the program is interpersonal psychotherapy (IPT), with essential elements of the program incorporating cognitive behavioral therapy (CBT), and experiential strategies (for instance, mindfulness, breathing, progressive muscle relaxation) to improve self-care and relaxation skills. Patient satisfaction surveys collected from 800 women, (54% identified as White) treated at the program between 2007 and 2012 found that women were highly satisfied with the treatment received, noting that the inclusion of the baby in their treatment is a highly valued aspect of care.
A similar program in Minnesota reported that 328 women who consented to participation in research had significant improvements (P < .001) in self-report scales assessing depression, anxiety, and maternal functioning, improving mental health and parenting functioning.10
Lastly, a recent study out of Brussels, on the benefit of a mother-baby day program analyzed patient data from 2015 and 2020. This clinical population of 92 patients (43% identifying as North African) was comparable to the population of the inpatient mother-baby units in terms of psychosocial fragility except that the parents entering the day program had less severe illnesses, more anxiety disorder, and less postpartum psychosis. In the day program, all the babies improved in terms of symptoms and relationships, except for those with significant developmental difficulties.
The dyadic relationship was measured using “levels of adaptation of the parent–child relationship” scale which has four general levels of adjustment, from well-adjusted to troubled or dangerous relationship. Unlike programs in the United States, this program takes children up to 2.5 years old and the assessment period is up to 8 weeks.11
Prevention of mental illness is best achieved by reducing the known determinants of illness. For PND, the research is clear, so why not start at the earliest possible stage, when we know that change is possible? Pushing health care systems to change is not easy, but as the research accumulates and the positive results grow, our arguments become stronger.
Dr. Heru is a psychiatrist in Aurora, Colo. She is editor of “Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals” (New York: Routledge, 2013). She has no conflicts of interest to disclose. Contact Dr. Heru at [email protected].
References
1. Gavin NI et al. Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol. 2005 Nov;106(5 Pt 1):1071-83. doi: 10.1097/01.AOG.0000183597.31630.db.
2. Rafferty J et al. Incorporating recognition and management of perinatal depression into pediatric practice. Pediatrics. 2019 Jan;143(1):e20183260. doi: 10.1542/peds.2018-3260.
3. Cluxton-Keller F, Bruce ML. Clinical effectiveness of family therapeutic interventions in the prevention and treatment of perinatal depression: A systematic review and meta-analysis. PLoS One. 2018 Jun 14;13(6):e0198730. doi: 10.1371/journal.pone.0198730.
4. Kumar SA et al. Promoting resilience to depression among couples during pregnancy: The protective functions of intimate relationship satisfaction and self-compassion. Family Process. 2022 May;62(1):387-405. doi: 10.1111/famp.12788.
5. Cox JL et al. Detection of postnatal depression: Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987 Jun;150:782-6. doi: 10.1192/bjp.150.6.782.
6. Keitner GI et al. The Brief Multidimensional Assessment Scale (BMAS): A broad measure of patient well-being. Am J Psychother. 2023 Feb 1;76(2):75-81. doi: 10.1176/appi.psychotherapy.20220032.
7. Boterhoven de Haan KL et al. Reliability and validity of a short version of the general functioning subscale of the McMaster Family Assessment Device. Fam Process. 2015 Mar;54(1):116-23. doi: 10.1111/famp.12113.
8. Cluxton-Keller F, Bruce ML. Clinical effectiveness of family therapeutic interventions in the prevention and treatment of perinatal depression: A systematic review and meta-analysis. PLoS One. 2018 Jun 14;13(6):e0198730. doi: 10.1371/journal.pone.0198730.
9. Battle CL, Howard MM. A mother-baby psychiatric day hospital: History, rationale, and why perinatal mental health is important for obstetric medicine. Obstet Med. 2014 Jun;7(2):66-70. doi: 10.1177/1753495X13514402.
10. Kim HG et al. Keeping Parent, Child, and Relationship in Mind: Clinical Effectiveness of a Trauma-informed, Multigenerational, Attachment-Based, Mother-Baby Partial Hospital Program in an Urban Safety Net Hospital. Matern Child Health J. 2021 Nov;25(11):1776-86. doi: 10.1007/s10995-021-03221-4.
11. Moureau A et al. A 5 years’ experience of a parent-baby day unit: impact on baby’s development. Front Psychiatry. 2023 June 15;14. doi: 10.3389/fpsyt.2023.1121894.
Perinatal depression (PND) is the most common obstetric complication in the United States. Even when screening results are positive, mothers often do not receive further evaluation, and even when PND is diagnosed, mothers do not receive evidence-based treatments.
Meta-analytic estimates show that pregnant women suffer from PND at rates from 6.5% to 12.9% across pregnancy to 3-months post partum.1 Women from low-income families and adolescent mothers are at highest risk, where rates are double and triple respectively.
Fathers also suffer from PND, with a prevalence rate from 2% to 25%, increasing to 50% when the mother experiences PND.
The American Academy of Pediatrics issued a Policy Statement (January 2019) about the need to recognize and manage PND. They recommended that pediatric medical homes establish a system to implement the screening of mothers at the 1-, 2-, 4-, and 6-month well-child visits, to use community resources for the treatment and referral of the mother with depression, and to provide support for the maternal-child relationship.2
The American Academy of Pediatrics also recommends advocacy for workforce development for mental health professionals who care for young children and mother-infant dyads, and for promotion of evidence-based interventions focused on healthy attachment and parent-child relationships.
Family research
There is a bidirectional association between family relational stress and PND. Lack of family support is both a predictor and a consequence of perinatal depression. Frequent arguments, conflict because one or both partners did not want the pregnancy, division of labor, poor support following stressful life events, lack of partner availability, and low intimacy are associated with increased perinatal depressive symptoms.
Gender role stress is also included as a risk factor. For example, men may fear performance failure related to work and sex, and women may fear disruption in the couple relationship due to the introduction of a child.
When depressed and nondepressed women at 2 months post delivery were compared, the women with depressive symptoms perceived that their partners did not share similar interests, provided little companionship, expressed disinterest in infant care, did not provide a feeling of connection, did not encourage them to get assistance to cope with difficulties, and expressed disagreement in infant care.3
A high-quality intimate relationship is protective for many illnesses and PND is no exception.4
Assessment
Despite the availability of effective treatments, perinatal mental health utilization rates are strikingly low. There are limited providers and a general lack of awareness of the need for this care. The stigma for assessing and treating PND is high because the perception is that pregnancy is supposed to be a joyous time and with time, PND will pass.
The first step is a timely and accurate assessment of the mother, which should, if possible, include the father and other family support people. The preferred standard for women is the Edinburgh Postnatal Depression Scale (EPDS), a checklist of 10 items (listed below) with a maximum score of 30, and any score over 10 warrants further assessment.5 This scale is used worldwide in obstetric clinics and has been used to identify PND in fathers.
- I have been able to laugh and see the funny side of things.
- I have looked forward with enjoyment to things.
- I have blamed myself unnecessarily when things went wrong.
- I have been anxious or worried for no good reason.
- I have felt scared or panicky for no good reason.
- Things have been getting to me.
- I have been so unhappy that I have had difficulty sleeping.
- I have felt sad or miserable.
- I have been so unhappy that I have been crying.
- The thought of harming myself has occurred to me.
A new ultrabrief tool with only four questions is the Brief Multidimensional Assessment Scale (BMAS), which measures the ability to get things done, emotional support in important relationships, quality of life, and sense of purpose in life. It demonstrates concurrent validity with other measures and discriminates between nonclinical participants and participants from most clinical contexts.6
For those interested in assessing family health, an easy-to-use assessment tool is the 12-item Family Assessment Device (FAD).7
Family therapy interventions
A systematic review and meta-analysis of the current evidence on the usefulness of family therapy interventions in the prevention and treatment of PND identified seven studies.
In these studies, there were statistically significant reductions in depressive symptoms at postintervention in intervention group mothers. Intervention intensity and level of family involvement moderated the impacts of intervention on maternal depression, and there was a trend in improved family functioning in intervention group couples.8
Evidence-based interventions are usually psychoeducational or cognitive-behavioral family therapy models where focused interventions target the following three areas:
- Communication skills related to expectations (including those that pertain to gender roles and the transition to parenthood) and emotional support.
- Conflict management.
- Problem-solving skills related to shared responsibility in infant care and household activities.
Intensive day program for mothers and babies
There is a growing awareness of the effectiveness of specialized mother-baby day hospital programs for women with psychiatric distress during the peripartum period.9
The Women & Infants’ Hospital (WIH) in Providence, R.I., established a mother-baby postpartum depression day program in 2000, adjacent to the obstetrical hospital, the ninth largest obstetrical service in the United States. The day program is integrated with the hospital’s obstetric medicine team and referrals are also accepted from the perinatal practices in the surrounding community. The treatment day includes group, individual, and milieu treatment, as well as consultation with psychiatrists, nutritionists, social workers, lactation specialists and others.
The primary theoretical model utilized by the program is interpersonal psychotherapy (IPT), with essential elements of the program incorporating cognitive behavioral therapy (CBT), and experiential strategies (for instance, mindfulness, breathing, progressive muscle relaxation) to improve self-care and relaxation skills. Patient satisfaction surveys collected from 800 women, (54% identified as White) treated at the program between 2007 and 2012 found that women were highly satisfied with the treatment received, noting that the inclusion of the baby in their treatment is a highly valued aspect of care.
A similar program in Minnesota reported that 328 women who consented to participation in research had significant improvements (P < .001) in self-report scales assessing depression, anxiety, and maternal functioning, improving mental health and parenting functioning.10
Lastly, a recent study out of Brussels, on the benefit of a mother-baby day program analyzed patient data from 2015 and 2020. This clinical population of 92 patients (43% identifying as North African) was comparable to the population of the inpatient mother-baby units in terms of psychosocial fragility except that the parents entering the day program had less severe illnesses, more anxiety disorder, and less postpartum psychosis. In the day program, all the babies improved in terms of symptoms and relationships, except for those with significant developmental difficulties.
The dyadic relationship was measured using “levels of adaptation of the parent–child relationship” scale which has four general levels of adjustment, from well-adjusted to troubled or dangerous relationship. Unlike programs in the United States, this program takes children up to 2.5 years old and the assessment period is up to 8 weeks.11
Prevention of mental illness is best achieved by reducing the known determinants of illness. For PND, the research is clear, so why not start at the earliest possible stage, when we know that change is possible? Pushing health care systems to change is not easy, but as the research accumulates and the positive results grow, our arguments become stronger.
Dr. Heru is a psychiatrist in Aurora, Colo. She is editor of “Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals” (New York: Routledge, 2013). She has no conflicts of interest to disclose. Contact Dr. Heru at [email protected].
References
1. Gavin NI et al. Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol. 2005 Nov;106(5 Pt 1):1071-83. doi: 10.1097/01.AOG.0000183597.31630.db.
2. Rafferty J et al. Incorporating recognition and management of perinatal depression into pediatric practice. Pediatrics. 2019 Jan;143(1):e20183260. doi: 10.1542/peds.2018-3260.
3. Cluxton-Keller F, Bruce ML. Clinical effectiveness of family therapeutic interventions in the prevention and treatment of perinatal depression: A systematic review and meta-analysis. PLoS One. 2018 Jun 14;13(6):e0198730. doi: 10.1371/journal.pone.0198730.
4. Kumar SA et al. Promoting resilience to depression among couples during pregnancy: The protective functions of intimate relationship satisfaction and self-compassion. Family Process. 2022 May;62(1):387-405. doi: 10.1111/famp.12788.
5. Cox JL et al. Detection of postnatal depression: Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987 Jun;150:782-6. doi: 10.1192/bjp.150.6.782.
6. Keitner GI et al. The Brief Multidimensional Assessment Scale (BMAS): A broad measure of patient well-being. Am J Psychother. 2023 Feb 1;76(2):75-81. doi: 10.1176/appi.psychotherapy.20220032.
7. Boterhoven de Haan KL et al. Reliability and validity of a short version of the general functioning subscale of the McMaster Family Assessment Device. Fam Process. 2015 Mar;54(1):116-23. doi: 10.1111/famp.12113.
8. Cluxton-Keller F, Bruce ML. Clinical effectiveness of family therapeutic interventions in the prevention and treatment of perinatal depression: A systematic review and meta-analysis. PLoS One. 2018 Jun 14;13(6):e0198730. doi: 10.1371/journal.pone.0198730.
9. Battle CL, Howard MM. A mother-baby psychiatric day hospital: History, rationale, and why perinatal mental health is important for obstetric medicine. Obstet Med. 2014 Jun;7(2):66-70. doi: 10.1177/1753495X13514402.
10. Kim HG et al. Keeping Parent, Child, and Relationship in Mind: Clinical Effectiveness of a Trauma-informed, Multigenerational, Attachment-Based, Mother-Baby Partial Hospital Program in an Urban Safety Net Hospital. Matern Child Health J. 2021 Nov;25(11):1776-86. doi: 10.1007/s10995-021-03221-4.
11. Moureau A et al. A 5 years’ experience of a parent-baby day unit: impact on baby’s development. Front Psychiatry. 2023 June 15;14. doi: 10.3389/fpsyt.2023.1121894.
Ob.gyn. organizations opt for new residency application platform
In a recent joint announcement, the American College of Obstetricians and Gynecologists and the Association of Professors of Gynecology and Obstetrics said the new system, ResidencyCAS, offered by Liaison Centralized Application Service, will replace the Electronic Residency Application Service (ERAS). ERAS was implemented some 25 years ago by the Association of American Medical Colleges.
Efficiencies and lower costs
Potential startup glitches aside, the transition will allegedly lower skyrocketing application fees and provide enhanced efficiencies and a better user experience than ERAS. So far, ob.gyn. is first and the only specialty to jump ship from the established platform. But if other specialties follow suit making the new software the norm, that will have a serious impact on ERAS’s revenues, said J. Bryan Carmody, MD, MPH, a pediatric nephrologist at the Children’s Hospital of the King’s Daughters, Norfolk, Va., who closely monitors and writes about residency selection and discussed the coming transition in a recent blog posting.
“My feeling is that the average program director thinks that ERAS is functional but there are not many, if any, who are in love with ERAS,” Dr. Carmody said in an interview. “I think ERAS will benefit from having a competitor.”
A major drawback for applicants with the removal of ob.gyn. from ERAS, which handles almost all medical specialties, is that those seeking acceptance in more than one specialty will now need to apply twice and incur two sets of costs. “A substantial fraction of applicants do that and now they’ll have to navigate two different systems and collect and format all their documents for both, which will be burdensome,” he said.
Holistic review
According to the ACOG announcement, the new technology promises to manage the deluge of applications more efficiently and, most important, to allow program directors to evaluate candidates holistically in order to better meet the specific needs of different communities.
“The platform makes it much easier to review applicants for important characteristics other than academic, and It will cost applicants about 20% less,” said Maya M. Hammoud, MD, MBA, professor and association chair for education, obstetrics, and gynecology at the University of Michigan, Ann Arbor, and past president of APGO.
So far the announced switch has been positively received. “People are very excited about the change, especially when they see the video,” Dr. Hammoud said.
For Adi Katz, MD, director of gynecology and director of the obstetrics and gynecology residency program at Lenox Hill Hospital, New York, the change signals a step in the right direction, especially when it comes to application reviewing. “The number of applications has been increasing tremendously in the past few years. We have four residency spots and we get almost 900 applications for them, ” she said. “Under the present system it’s hard to give a fair review to all the applicants, and we hope that with change we’ll be able to give each one the attention they deserve.”
An important feature, added Dr. Katz, is that the new software will allow directors to do intuitive, “gut-level” screenings with the help of AI. In this approach, large numbers of candidates can be screened based on intuition in relation to their formal criteria.
Residency program administrators have long sought more holistic ways of screening applicants, and AI has the potential to provide insights into who’s a good fit by finding patterns in very complex data.
“Of course, we won’t know for sure if it’s the right move until we start using the platform,” Dr. Katz said.
“There are many factors beyond academic standing that can help determine which individual applicants would be the best fit for each unique program,” AnnaMarie Connolly, MD, chief of education and academic affairs at ACOG, said in an interview. ”In particular, improved holistic review will allow programs and applicants to better ensure alignment that, for example, considers factors such as applicants’ clinical interests, academic interests, and past life experiences.”
Updated data science is expected better align ob.gyn. programs and applicants, and improve staff efficiency at no cost to programs, Dr. Connolly added. Good alignment of residents with programs is especially important in a patient-interactive specialty such as ob.gyn. Webinars will prepare users to apply the new system.
According to the promotional video, ResidencyCAS integrates all components of application from candidates’ letters and credentials to lists of program directors, applicant reviews, and specialty data analytics. Collecting recommendations and credentials is expected to be streamlined. The software is currently used by 31 U.S. health care professions and across 31,000 programs.
“It’s clear that ob.gyn. residency applicants and ob.gyn. programs have been frustrated by certain aspects of the former application system, one of which being high costs,” Dr. Connolly added. “The feedback we’ve received indicates that programs are excited about a more streamlined process.”
AAMC strikes back
Not all groups are so enthusiastic, however, including, understandably, the AAMC, which expressed “surprise and dismay” at the switch.
“While it is too early to fully understand the consequences of this development – intended and unintended – the AAMC remains committed to creating a fair and equitable process for learners, medical schools, and programs,” wrote AAMC spokespersons David J. Skorton, MD, AAMC’s CEO, and Alison J. Whelan, MD, chief academic officer in a statement. “We are concerned that ob.gyn. program data will no longer be part of the numerous and longstanding AAMC data and research efforts.”
Those efforts include the Residency Readiness Survey, multidecade institution-level data and analytics, and future cross-specialty innovations. Lost with the changeover, the AAMC warned, may be the cross-specialty data it has collected, analyzed, and shared since ERAS’s inception, in particular its advocacy, research, and data support for the ob.gyn. community following the 2022 Supreme Court ruling in Dobbs v. Jackson.
Evolution of specialty application
In a blog posting, Dr. Carmody outlined the evolution of the specialty residency application process. Pre-ERAS application was slow, cumbersome, and done by mail. With the introduction of ERAS, applicants were able to put their information on floppy discs and submit them to the dean’s office, hopefully triggering interview offers via email. The new approach was originally piloted in partnership with ob.gyn. program directors and now ERAS finds itself in a first-in, first-out situation.
Over the years, program directors suffocating under the weight of applications have periodically asked the AAMC to share data or make changes to ERAS protocols or policies, including those on the sharing of collected information. “Its my perception that frustration about the AAMC’s data sharing was one of the things that led to the change,” Dr. Carmody said. While acknowledging that data sharing must be carefully done, he noted that, when program directors asked to see ERAS data to answer important questions, they were often refused.
While it appears that AAMC’s improvement efforts have not gone far or fast enough, the association pointed to significant efforts to streamline applications. It stressed its ongoing commitment to cooperation “with learners, medical schools, and the ERAS program community to further consider the implications of ACOG’s announcement.” It recently announced a collaboration with Thalamus-connecting the docs, a new interview-management software system the AAMC expects will accelerate innovation across the transition-to-residency process.
“We have many questions and few answers at this time,” Dr. Skorton and Dr. Whelan wrote, “and we will work diligently to fully understand the consequences and keep open communication with all of our constituents.”
Financial impact
Ob.gyn., an important but relatively small specialty, represented only 2.8% of the 2022 residency applications on ERAS and $3,362,760 of its $120 million in revenue that year, Dr. Carmody noted. That’s with 2,613 ob.gyn. applicants submitting an average of 63-83 applications depending on their background.
But if the defection of ob.gyn. starts a stampede among program directors in other branches of medicine to ResidencyCAS or some other new platform, that would cost ERAS substantially more.
“The next few years are going to be very telling,” said Dr. Carmody. Although competition may act as a catalyst for needed improvements to ERAS, if momentum grows, the comfortable inertia of staying with a known system may soon be overcome. “And the more specialties that switch, the more that will deprive the AAMC of the revenue it needs to improve the product.”
Dr. Carmody and Dr. Katz disclosed no relevant conflicts of interest with regard to their comments.
In a recent joint announcement, the American College of Obstetricians and Gynecologists and the Association of Professors of Gynecology and Obstetrics said the new system, ResidencyCAS, offered by Liaison Centralized Application Service, will replace the Electronic Residency Application Service (ERAS). ERAS was implemented some 25 years ago by the Association of American Medical Colleges.
Efficiencies and lower costs
Potential startup glitches aside, the transition will allegedly lower skyrocketing application fees and provide enhanced efficiencies and a better user experience than ERAS. So far, ob.gyn. is first and the only specialty to jump ship from the established platform. But if other specialties follow suit making the new software the norm, that will have a serious impact on ERAS’s revenues, said J. Bryan Carmody, MD, MPH, a pediatric nephrologist at the Children’s Hospital of the King’s Daughters, Norfolk, Va., who closely monitors and writes about residency selection and discussed the coming transition in a recent blog posting.
“My feeling is that the average program director thinks that ERAS is functional but there are not many, if any, who are in love with ERAS,” Dr. Carmody said in an interview. “I think ERAS will benefit from having a competitor.”
A major drawback for applicants with the removal of ob.gyn. from ERAS, which handles almost all medical specialties, is that those seeking acceptance in more than one specialty will now need to apply twice and incur two sets of costs. “A substantial fraction of applicants do that and now they’ll have to navigate two different systems and collect and format all their documents for both, which will be burdensome,” he said.
Holistic review
According to the ACOG announcement, the new technology promises to manage the deluge of applications more efficiently and, most important, to allow program directors to evaluate candidates holistically in order to better meet the specific needs of different communities.
“The platform makes it much easier to review applicants for important characteristics other than academic, and It will cost applicants about 20% less,” said Maya M. Hammoud, MD, MBA, professor and association chair for education, obstetrics, and gynecology at the University of Michigan, Ann Arbor, and past president of APGO.
So far the announced switch has been positively received. “People are very excited about the change, especially when they see the video,” Dr. Hammoud said.
For Adi Katz, MD, director of gynecology and director of the obstetrics and gynecology residency program at Lenox Hill Hospital, New York, the change signals a step in the right direction, especially when it comes to application reviewing. “The number of applications has been increasing tremendously in the past few years. We have four residency spots and we get almost 900 applications for them, ” she said. “Under the present system it’s hard to give a fair review to all the applicants, and we hope that with change we’ll be able to give each one the attention they deserve.”
An important feature, added Dr. Katz, is that the new software will allow directors to do intuitive, “gut-level” screenings with the help of AI. In this approach, large numbers of candidates can be screened based on intuition in relation to their formal criteria.
Residency program administrators have long sought more holistic ways of screening applicants, and AI has the potential to provide insights into who’s a good fit by finding patterns in very complex data.
“Of course, we won’t know for sure if it’s the right move until we start using the platform,” Dr. Katz said.
“There are many factors beyond academic standing that can help determine which individual applicants would be the best fit for each unique program,” AnnaMarie Connolly, MD, chief of education and academic affairs at ACOG, said in an interview. ”In particular, improved holistic review will allow programs and applicants to better ensure alignment that, for example, considers factors such as applicants’ clinical interests, academic interests, and past life experiences.”
Updated data science is expected better align ob.gyn. programs and applicants, and improve staff efficiency at no cost to programs, Dr. Connolly added. Good alignment of residents with programs is especially important in a patient-interactive specialty such as ob.gyn. Webinars will prepare users to apply the new system.
According to the promotional video, ResidencyCAS integrates all components of application from candidates’ letters and credentials to lists of program directors, applicant reviews, and specialty data analytics. Collecting recommendations and credentials is expected to be streamlined. The software is currently used by 31 U.S. health care professions and across 31,000 programs.
“It’s clear that ob.gyn. residency applicants and ob.gyn. programs have been frustrated by certain aspects of the former application system, one of which being high costs,” Dr. Connolly added. “The feedback we’ve received indicates that programs are excited about a more streamlined process.”
AAMC strikes back
Not all groups are so enthusiastic, however, including, understandably, the AAMC, which expressed “surprise and dismay” at the switch.
“While it is too early to fully understand the consequences of this development – intended and unintended – the AAMC remains committed to creating a fair and equitable process for learners, medical schools, and programs,” wrote AAMC spokespersons David J. Skorton, MD, AAMC’s CEO, and Alison J. Whelan, MD, chief academic officer in a statement. “We are concerned that ob.gyn. program data will no longer be part of the numerous and longstanding AAMC data and research efforts.”
Those efforts include the Residency Readiness Survey, multidecade institution-level data and analytics, and future cross-specialty innovations. Lost with the changeover, the AAMC warned, may be the cross-specialty data it has collected, analyzed, and shared since ERAS’s inception, in particular its advocacy, research, and data support for the ob.gyn. community following the 2022 Supreme Court ruling in Dobbs v. Jackson.
Evolution of specialty application
In a blog posting, Dr. Carmody outlined the evolution of the specialty residency application process. Pre-ERAS application was slow, cumbersome, and done by mail. With the introduction of ERAS, applicants were able to put their information on floppy discs and submit them to the dean’s office, hopefully triggering interview offers via email. The new approach was originally piloted in partnership with ob.gyn. program directors and now ERAS finds itself in a first-in, first-out situation.
Over the years, program directors suffocating under the weight of applications have periodically asked the AAMC to share data or make changes to ERAS protocols or policies, including those on the sharing of collected information. “Its my perception that frustration about the AAMC’s data sharing was one of the things that led to the change,” Dr. Carmody said. While acknowledging that data sharing must be carefully done, he noted that, when program directors asked to see ERAS data to answer important questions, they were often refused.
While it appears that AAMC’s improvement efforts have not gone far or fast enough, the association pointed to significant efforts to streamline applications. It stressed its ongoing commitment to cooperation “with learners, medical schools, and the ERAS program community to further consider the implications of ACOG’s announcement.” It recently announced a collaboration with Thalamus-connecting the docs, a new interview-management software system the AAMC expects will accelerate innovation across the transition-to-residency process.
“We have many questions and few answers at this time,” Dr. Skorton and Dr. Whelan wrote, “and we will work diligently to fully understand the consequences and keep open communication with all of our constituents.”
Financial impact
Ob.gyn., an important but relatively small specialty, represented only 2.8% of the 2022 residency applications on ERAS and $3,362,760 of its $120 million in revenue that year, Dr. Carmody noted. That’s with 2,613 ob.gyn. applicants submitting an average of 63-83 applications depending on their background.
But if the defection of ob.gyn. starts a stampede among program directors in other branches of medicine to ResidencyCAS or some other new platform, that would cost ERAS substantially more.
“The next few years are going to be very telling,” said Dr. Carmody. Although competition may act as a catalyst for needed improvements to ERAS, if momentum grows, the comfortable inertia of staying with a known system may soon be overcome. “And the more specialties that switch, the more that will deprive the AAMC of the revenue it needs to improve the product.”
Dr. Carmody and Dr. Katz disclosed no relevant conflicts of interest with regard to their comments.
In a recent joint announcement, the American College of Obstetricians and Gynecologists and the Association of Professors of Gynecology and Obstetrics said the new system, ResidencyCAS, offered by Liaison Centralized Application Service, will replace the Electronic Residency Application Service (ERAS). ERAS was implemented some 25 years ago by the Association of American Medical Colleges.
Efficiencies and lower costs
Potential startup glitches aside, the transition will allegedly lower skyrocketing application fees and provide enhanced efficiencies and a better user experience than ERAS. So far, ob.gyn. is first and the only specialty to jump ship from the established platform. But if other specialties follow suit making the new software the norm, that will have a serious impact on ERAS’s revenues, said J. Bryan Carmody, MD, MPH, a pediatric nephrologist at the Children’s Hospital of the King’s Daughters, Norfolk, Va., who closely monitors and writes about residency selection and discussed the coming transition in a recent blog posting.
“My feeling is that the average program director thinks that ERAS is functional but there are not many, if any, who are in love with ERAS,” Dr. Carmody said in an interview. “I think ERAS will benefit from having a competitor.”
A major drawback for applicants with the removal of ob.gyn. from ERAS, which handles almost all medical specialties, is that those seeking acceptance in more than one specialty will now need to apply twice and incur two sets of costs. “A substantial fraction of applicants do that and now they’ll have to navigate two different systems and collect and format all their documents for both, which will be burdensome,” he said.
Holistic review
According to the ACOG announcement, the new technology promises to manage the deluge of applications more efficiently and, most important, to allow program directors to evaluate candidates holistically in order to better meet the specific needs of different communities.
“The platform makes it much easier to review applicants for important characteristics other than academic, and It will cost applicants about 20% less,” said Maya M. Hammoud, MD, MBA, professor and association chair for education, obstetrics, and gynecology at the University of Michigan, Ann Arbor, and past president of APGO.
So far the announced switch has been positively received. “People are very excited about the change, especially when they see the video,” Dr. Hammoud said.
For Adi Katz, MD, director of gynecology and director of the obstetrics and gynecology residency program at Lenox Hill Hospital, New York, the change signals a step in the right direction, especially when it comes to application reviewing. “The number of applications has been increasing tremendously in the past few years. We have four residency spots and we get almost 900 applications for them, ” she said. “Under the present system it’s hard to give a fair review to all the applicants, and we hope that with change we’ll be able to give each one the attention they deserve.”
An important feature, added Dr. Katz, is that the new software will allow directors to do intuitive, “gut-level” screenings with the help of AI. In this approach, large numbers of candidates can be screened based on intuition in relation to their formal criteria.
Residency program administrators have long sought more holistic ways of screening applicants, and AI has the potential to provide insights into who’s a good fit by finding patterns in very complex data.
“Of course, we won’t know for sure if it’s the right move until we start using the platform,” Dr. Katz said.
“There are many factors beyond academic standing that can help determine which individual applicants would be the best fit for each unique program,” AnnaMarie Connolly, MD, chief of education and academic affairs at ACOG, said in an interview. ”In particular, improved holistic review will allow programs and applicants to better ensure alignment that, for example, considers factors such as applicants’ clinical interests, academic interests, and past life experiences.”
Updated data science is expected better align ob.gyn. programs and applicants, and improve staff efficiency at no cost to programs, Dr. Connolly added. Good alignment of residents with programs is especially important in a patient-interactive specialty such as ob.gyn. Webinars will prepare users to apply the new system.
According to the promotional video, ResidencyCAS integrates all components of application from candidates’ letters and credentials to lists of program directors, applicant reviews, and specialty data analytics. Collecting recommendations and credentials is expected to be streamlined. The software is currently used by 31 U.S. health care professions and across 31,000 programs.
“It’s clear that ob.gyn. residency applicants and ob.gyn. programs have been frustrated by certain aspects of the former application system, one of which being high costs,” Dr. Connolly added. “The feedback we’ve received indicates that programs are excited about a more streamlined process.”
AAMC strikes back
Not all groups are so enthusiastic, however, including, understandably, the AAMC, which expressed “surprise and dismay” at the switch.
“While it is too early to fully understand the consequences of this development – intended and unintended – the AAMC remains committed to creating a fair and equitable process for learners, medical schools, and programs,” wrote AAMC spokespersons David J. Skorton, MD, AAMC’s CEO, and Alison J. Whelan, MD, chief academic officer in a statement. “We are concerned that ob.gyn. program data will no longer be part of the numerous and longstanding AAMC data and research efforts.”
Those efforts include the Residency Readiness Survey, multidecade institution-level data and analytics, and future cross-specialty innovations. Lost with the changeover, the AAMC warned, may be the cross-specialty data it has collected, analyzed, and shared since ERAS’s inception, in particular its advocacy, research, and data support for the ob.gyn. community following the 2022 Supreme Court ruling in Dobbs v. Jackson.
Evolution of specialty application
In a blog posting, Dr. Carmody outlined the evolution of the specialty residency application process. Pre-ERAS application was slow, cumbersome, and done by mail. With the introduction of ERAS, applicants were able to put their information on floppy discs and submit them to the dean’s office, hopefully triggering interview offers via email. The new approach was originally piloted in partnership with ob.gyn. program directors and now ERAS finds itself in a first-in, first-out situation.
Over the years, program directors suffocating under the weight of applications have periodically asked the AAMC to share data or make changes to ERAS protocols or policies, including those on the sharing of collected information. “Its my perception that frustration about the AAMC’s data sharing was one of the things that led to the change,” Dr. Carmody said. While acknowledging that data sharing must be carefully done, he noted that, when program directors asked to see ERAS data to answer important questions, they were often refused.
While it appears that AAMC’s improvement efforts have not gone far or fast enough, the association pointed to significant efforts to streamline applications. It stressed its ongoing commitment to cooperation “with learners, medical schools, and the ERAS program community to further consider the implications of ACOG’s announcement.” It recently announced a collaboration with Thalamus-connecting the docs, a new interview-management software system the AAMC expects will accelerate innovation across the transition-to-residency process.
“We have many questions and few answers at this time,” Dr. Skorton and Dr. Whelan wrote, “and we will work diligently to fully understand the consequences and keep open communication with all of our constituents.”
Financial impact
Ob.gyn., an important but relatively small specialty, represented only 2.8% of the 2022 residency applications on ERAS and $3,362,760 of its $120 million in revenue that year, Dr. Carmody noted. That’s with 2,613 ob.gyn. applicants submitting an average of 63-83 applications depending on their background.
But if the defection of ob.gyn. starts a stampede among program directors in other branches of medicine to ResidencyCAS or some other new platform, that would cost ERAS substantially more.
“The next few years are going to be very telling,” said Dr. Carmody. Although competition may act as a catalyst for needed improvements to ERAS, if momentum grows, the comfortable inertia of staying with a known system may soon be overcome. “And the more specialties that switch, the more that will deprive the AAMC of the revenue it needs to improve the product.”
Dr. Carmody and Dr. Katz disclosed no relevant conflicts of interest with regard to their comments.
Adenomyosis: Why we need to reassess our understanding of this condition
CASE Painful, heavy menstruation and recurrent pregnancy loss
A 37-year-old woman (G3P0030) with a history of recurrent pregnancy loss presents for evaluation. She had 3 losses—most recently a miscarriage at 22 weeks with a cerclage in place. She did not undergo any surgical procedures for these losses. Hormonal and thrombophilia workup is negative and semen analysis is normal. She reports a history of painful, heavy periods for many years, as well as dyspareunia and occasional post-coital bleeding. Past medical history was otherwise unremarkable. Pelvic magnetic resonance imaging (MRI) revealed focal thickening of the junctional zone up to 15 mm with 2 foci of T2 hyperintensities suggesting adenomyosis (FIGURE 1).
How do you counsel this patient regarding the MRI findings and their impact on her fertility?
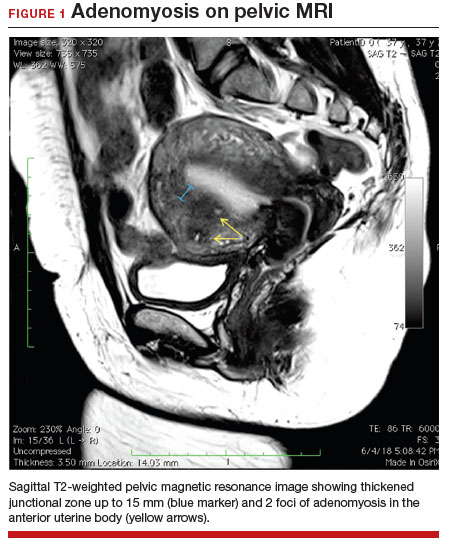
Adenomyosis is a condition in which endometrial glands and stroma are abnormally present in the uterine myometrium, resulting in smooth muscle hypertrophy and abnormal uterine contractility. Traditional teaching describes a woman in her 40s with heavy and painful menses, a “boggy uterus” on examination, who has completed childbearing and desires definitive treatment. Histologic diagnosis of adenomyosis is made from the uterine specimen at the time of hysterectomy, invariably confounding our understanding of the epidemiology of adenomyosis.
More recently, however, we are beginning to learn that this narrative is misguided. Imaging changes of adenomyosis can be seen in women who desire future fertility and in adolescents with severe dysmenorrhea, suggesting an earlier age of incidence.1 In a recent systematic review, prevalence estimates ranged from 15% to 67%, owing to varying diagnostic methods and patient inclusion criteria.2 It is increasingly being recognized as a primary contributor to infertility, with one study estimating a 30% prevalence of infertility in women with adenomyosis.3 Moreover, treatment with gonadotropin-releasing hormone agonists and/or surgical excision may improve fertility outcomes.4
As we learn more about this prevalent and life-altering condition, we owe it to our patients to consider this diagnosis when counseling on dysmenorrhea, heavy menstrual bleeding, or infertility.
Anatomy of the myometrium
The myometrium is composed of the inner and outer myometrium: the inner myometrium (IM) and endometrium are of Müllerian origin, and the outer myometrium (OM) is of mesenchymal origin. The IM thickens in response to steroid hormones during the menstrual cycle with metaplasia of endometrial stromal cells into myocytes and back again, whereas the OM is not responsive to hormones.5 Emerging literature suggests the OM is further divided into a middle and outer section based on different histologic morphologies, though the clinical implications of this are not understood.6 The term “junctional zone” (JZ) refers to the imaging appearance of what is thought to be the IM. Interestingly it cannot be identified on traditional hematoxylin and eosin staining. When the JZ is thickened or demonstrates irregular borders, it is used as a diagnostic marker for adenomyosis and is postulated to play an important role in adenomyosis pathophysiology, particularly heavy menstrual bleeding and infertility.7
Continue to: Subtypes of adenomyosis...
Subtypes of adenomyosis
While various disease classifications have been suggested for adenomyosis, to date there is no international consensus. Adenomyosis is typically described in 3 forms: diffuse, focal, or adenomyoma.8 As implied, the term focal adenomyosis refers to discrete lesions surrounded by normal myometrium, whereas abnormal glandular changes are pervasive throughout the myometrium in diffuse disease. Adenomyomas are a subgroup of focal adenomyosis that are thought to be surrounded by leiomyomatous smooth muscle and may be well demarcated on imaging.9
Recent research uses novel histologic imaging techniques to explore adenomyotic growth patterns in 3-dimensional (3D) reconstructions. Combining tissue-clearing methods with light-sheet fluorescence microscopy enables highly detailed 3D representations of the protein and nucleic acid structure of organs.10 For example, Yamaguchi and colleagues used this technology to explore the 3D morphological features of adenomyotic tissue and observed direct invasion of the endometrial glands into the myometrium and an “ant colony ̶ like network” of ectopic endometrial glands in the myometrium (FIGURE 2).11 These abnormal glandular networks have been visualized beyond the IM, which may not be captured on ultrasonography or MRI. While this work is still in its infancy, it has the potential to provide important insight into disease pathogenesis and to inform future therapy.
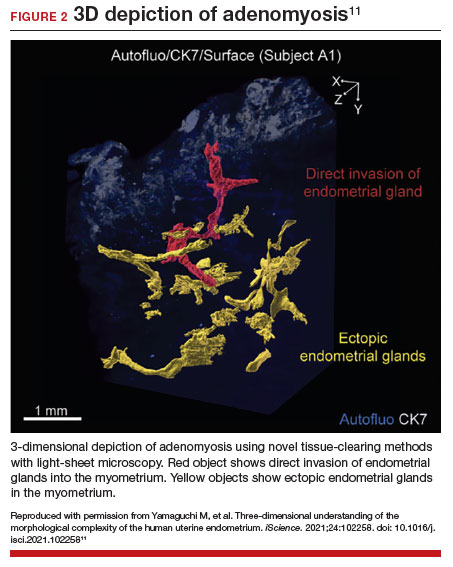
Pathogenesis
Proposed mechanisms for the development of adenomyosis include endometrial invasion, tissue injury and repair (TIAR) mechanisms, and the stem cell theory.12 According to the endometrial invasion theory, glandular epithelial cells from the basalis layer invaginate through an altered IM, slipping through weak muscle fibers and attracted by certain growth factors. In the TIAR mechanism theory, micro- or macro-trauma to the IM (whether from pregnancy, surgery, or infection) results in chronic proliferation and inflammation leading to the development of adenomyosis. Finally, the stem cell theory proposes that adenomyosis might develop from de novo ectopic endometrial tissue.
While the exact pathogenesis of adenomyosis is largely unknown, it has been associated with predictable molecular changes in the endometrium and surrounding myometrium.12 Myometrial hypercontractility is seen in patients with adenomyosis and dysmenorrhea, whereas neovascularization, high microvessel density, and abnormal uterine contractility are seen in those with abnormal uterine bleeding.13 In patients with infertility, increased inflammation, abnormal endometrial receptivity, and alterations in the myometrial architecture have been suggested to impair contractility and sperm transport.12,14
Differential growth factor expression and abnormal estrogen and progesterone signaling pathways have been observed in the IM in patients with adenomyosis, along with dysregulation of immune factors and increased inflammatory oxidative stress.12 This in turn results in myometrial hypertrophy and fibrosis, impairing normal uterine contractility patterns. This abnormal contractility may alter sperm transport and embryo implantation, and animal models that target pathways leading to fibrosis may improve endometrial receptivity.14,15 Further research is needed to elucidate specific molecular pathways and their complex interplay in this disease.
Continue to: Diagnosis...
Diagnosis
The gold standard for diagnosis of adenomyosis is histopathology from hysterectomy specimens, but specific definitions vary. Published criteria include endometrial glands within the myometrial layer greater than 0.5 to 1 low power field from the basal layer of the endometrium, endometrial glands extending deeper than 25% of the myometrial thickness, or endometrial glands a certain distance (ranging from 1-3 mm) from the basalis layer of the endometrium.16 Various methods of non-hysterectomy tissue sampling have been proposed for diagnosis, including needle, hysteroscopic, or laparoscopic sampling, but the sensitivity of these methods is poor.17 Limiting the diagnosis of adenomyosis to specimen pathology relies on invasive methods and clearly we cannot confirm the diagnosis by hysterectomy in patients with a desire for future fertility. It is for this reason that the prevalence of the disease is widely unknown.
The alternative to pathologic diagnosis is to identify radiologic changes that are associated with adenomyosis via either transvaginal ultrasound (TVUS) or MRI. Features suggestive of adenomyosis on MRI overlap with TVUS features, including uterine enlargement, anteroposterior myometrial asymmetry, T1- or T2-intense myometrial cysts or foci, and a thickened JZ.18 A JZ thicker than 12 mm has been thought to be predictive of adenomyosis, whereas a thickness of less than 8 mm is predictive of its absence, although the JZ may vary in thickness with the menstrual cycle.19,20 A 2021 systematic review and meta-analysis comparing MRI diagnosis with histopathologic findings reported a pooled sensitivity and specificity of 60% and 96%, respectively.21 The reported range for sensitivity and specificity is wide: 70% to 93% for sensitivity and 67% to 93% for specificity.22-24
Key TVUS features associated with adenomyosis were defined in 2015 in a consensus statement released by the Morphological Uterus Sonographic Assessment (MUSA) group.25 These include a globally enlarged uterus, anteroposterior myometrial asymmetry, myometrial cysts, fan-shaped shadowing, mixed myometrial echogenicity, translesional vascularity, echogenic subendometrial lines and buds, and a thickened, irregular or discontinuous JZ (FIGURES 3 and 4).25 The accuracy of ultrasonographic diagnosis of adenomyosis using these features has been investigated in multiple systematic reviews and meta-analyses, most recently by Liu and colleagues who found a pooled sensitivity of TVUS of 81% and pooled specificity of 87%.23 The range for ultrasonographic sensitivity and specificity is wide, however, ranging from 33% to 84% for sensitivity and 64% to 100% for specificity.22 Consensus is lacking as to which TVUS features are most predictive of adenomyosis, but in general, the combination of multiple MUSA criteria (particularly myometrial cysts and irregular JZ on 3D imaging) appears to be more accurate than any one feature alone.23 The presence of fibroids may decrease the sensitivity of TVUS, and one study suggested elastography may increase the accuracy of TVUS.24,26 Moreover, given that most radiologists receive limited training on the MUSA criteria, it behooves gynecologists to become familiar with these sonographic features to be able to identify adenomyosis in our patients.
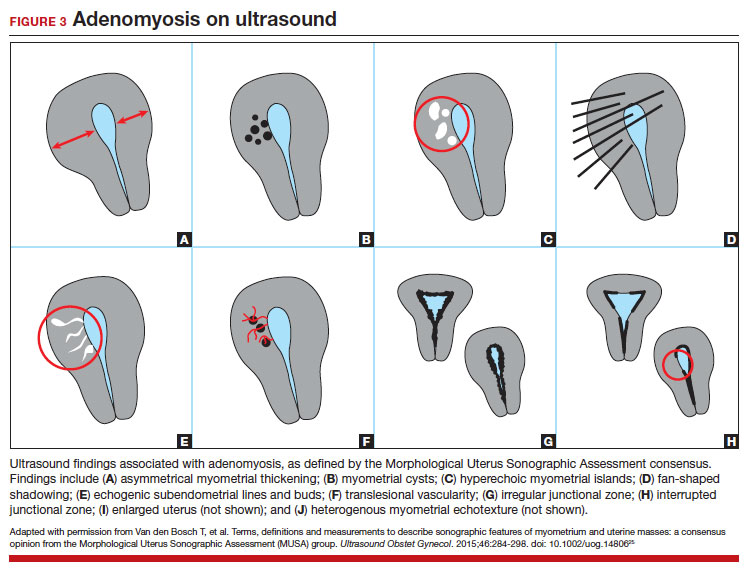

Adenomyosis also may be suspected based on hysteroscopic findings, although a normal hysteroscopy cannot rule out the disease and data are lacking to support these markers as diagnostic. Visual findings can include a “strawberry” pattern, mucosal elevation, cystic hemorrhagic lesions, localized vascularity, or endometrial defects.27 Hysteroscopy may be effective in the treatment of localized lesions, although that discussion is beyond the scope of this review.
Clinical presentation
While many women who are later diagnosed with adenomyosis are asymptomatic, the disease can present with heavy menstrual bleeding and dysmenorrhea, which occur in 50% and 30% of patients, respectively.28 Other symptoms include dyspareunia and infertility. Symptoms were previously reported to develop between the ages of 40 and 50 years; however, this is biased by diagnosis at the time of hysterectomy and the fact that younger patients are less likely to undergo definitive surgery. When using imaging criteria for diagnosis, adenomyosis might be more responsible for dysmenorrhea and chronic pelvic pain in younger patients than previously appreciated.1,29 In a recent study reviewing TVUS in 270 adolescents for any reason, adenomyosis was present in 5% of cases and this increased up to 44% in the presence of endometriosis.30
Adenomyosis often co-exists and shares similar clinical presentations with other gynecologic pathologies such as endometriosis and fibroids, making diagnosis on symptomatology alone challenging. Concurrent adenomyosis has been found in up to 73% and 57% of patients with suspected or diagnosed endometriosis and fibroids, respectively.31,32 Accumulating evidence suggests that pelvic pain previously attributed to endometriosis may in fact be a result of adenomyosis; for example, persistent pelvic pain after optimal resection of endometriosis may be confounded by the presence of adenomyosis.29 In one study of 155 patients with complete resection of deep infiltrating endometriosis, persistent pelvic pain was significantly associated with the presence of adenomyosis on imaging.33
Adenomyosis is increasingly being recognized at the time of infertility evaluation with an estimated prevalence of 30% in women with infertility.3 Among women with infertility, adenomyosis has been associated with a lower clinical pregnancy rate, higher miscarriage rate, and lower live birth rate, as well as obstetric complications such as abnormal placentation.34-36 A study of 37 baboons found the histologic diagnosis of adenomyosis alone at necropsy was associated with a 20-fold increased risk of lifelong infertility (odds ratio [OR], 20.1; 95% CI, 2.1-921), whereas presence of endometriosis was associated with a nonsignificant 3-fold risk of lifelong infertility (OR, 3.6; 95% CI, 0.9-15.8).37
In women with endometriosis and infertility, co-existing adenomyosis portends worse fertility outcomes. In a retrospective study of 244 women who underwent endometriosis surgery, more than five features of adenomyosis on imaging was associated with higher rates of infertility, in vitro fertilization treatments, and a higher number of in vitro fertilization cycles.31 Moreover, in women who underwent surgery for deep infiltrating endometriosis, the presence of adenomyosis on imaging was associated with a 68% reduction in likelihood of pregnancy after surgery.38
Conclusion
As we begin to learn about adenomyosis, our misconceptions become more evident. The notion that it largely affects women at the end of their reproductive lives is biased by using histopathology at hysterectomy as the gold standard for diagnosis. Lack of definitive histologic or imaging criteria and biopsy techniques add to the diagnostic challenge. This in turn leads to inaccurate estimates of incidence and prevalence, as we assume patients’ symptoms must be attributable to what we can see at the time of surgery (for example, Stage I or II endometriosis), rather than what we cannot see. We now know that adenomyosis is present in women of all ages, including adolescents, and can significantly contribute to reduced fertility and quality of life. We owe it to our patients to consider this condition in the differential diagnosis of dysmenorrhea, heavy menstrual bleeding, dyspareunia, and infertility.
CASE Resolved
The patient underwent targeted hysteroscopic resection of adenomyosis (FIGURE 5) and conceived spontaneously the following year. ●
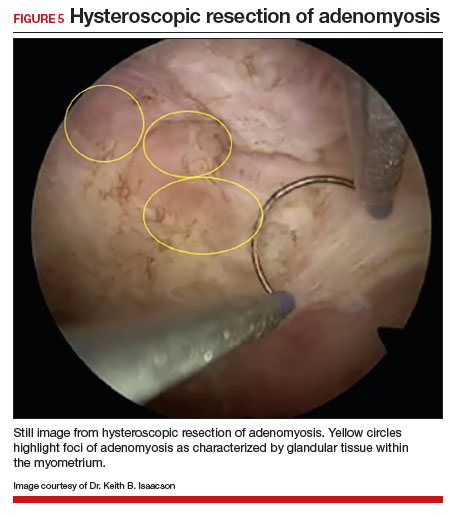
- Exacoustos C, Lazzeri L, Martire FG, et al. Ultrasound findings of adenomyosis in adolescents: type and grade of the disease. J Minim Invasive Gynecol. 2021;29:291.e1-299.e1. doi: 10.1016/j.jmig.2021.08.023
- Loring M, Chen TY, Isaacson KB. A systematic review of adenomyosis: it is time to reassess what we thought we knew about the disease. J Minim Invasive Gynecol. 2021;28:644655. doi: 10.1016/j.jmig.2020.10.012
- Bourdon M, Santulli P, Oliveira J, et al. Focal adenomyosis is associated with primary infertility. Fertil Steril. 2020;114:1271-1277. doi: 10.1016/j.fertnstert.2020.06.018
- Lan J, Wu Y, Wu Z, et al. Ultra-long GnRH agonist protocol during IVF/ICSI improves pregnancy outcomes in women with adenomyosis: a retrospective cohort study. Front Endocrinol (Lausanne). 2021;12:609771. doi: 10.3389 /fendo.2021.609771
- Gnecco JS, Brown AT, Kan EL, et al. Physiomimetic models of adenomyosis. Semin Reprod Med. 2020;38:179-196. doi: 10.1055/s-0040-1719084
- Harmsen MJ, Trommelen LM, de Leeuw RA, et al. Uterine junctional zone and adenomyosis: comparison of MRI, transvaginal ultrasound and histology. Ultrasound Obstet Gynecol. 2023;62:42-60. doi: 10.1002/uog.26117
- Xie T, Xu X, Yang Y, et al. The role of abnormal uterine junction zone in the occurrence and development of adenomyosis. Reprod Sci. 2022;29:2719-2730. doi: 10.1007/s43032-021 -00684-2
- Lazzeri L, Morosetti G, Centini G, et al. A sonographic classification of adenomyosis: interobserver reproducibility in the evaluation of type and degree of the myometrial involvement. Fertil Steril. 2018;110:1154-1161.e3. doi: 10.1016 /j.fertnstert.2018.06.031
- Tahlan A, Nanda A, Mohan H. Uterine adenomyoma: a clinicopathologic review of 26 cases and a review of the literature. Int J Gynecol Pathol. 2006;25:361-365. doi: 10.1097/01.pgp.0000209570.08716.b3
- Chung K, Wallace J, Kim S-Y, et al. Structural and molecular interrogation of intact biological systems. Nature. 2013;497:332-337. doi: 10.1038/nature12107
- Yamaguchi M, Yoshihara K, Suda K, et al. Three-dimensional understanding of the morphological complexity of the human uterine endometrium. iScience. 2021;24:102258. doi: 10.1016/j.isci.2021.102258
- Vannuccini S, Tosti C, Carmona F, et al. Pathogenesis of adenomyosis: an update on molecular mechanisms. Reprod Biomed Online. 2017;35:592-601. doi: 10.1016 /j.rbmo.2017.06.016
- Zhai J, Vannuccini S, Petraglia F, et al. Adenomyosis: mechanisms and pathogenesis. Semin Reprod Med. 2020;38:129-143. doi: 10.1055/s-0040-1716687
- Munro MG. Uterine polyps, adenomyosis, leiomyomas, and endometrial receptivity. Fertil Steril. 2019;111:629-640. doi: 10.1016/j.fertnstert.2019.02.008
- Kay N, Huang C-Y, Shiu L-Y, et al. TGF-β1 neutralization improves pregnancy outcomes by restoring endometrial receptivity in mice with adenomyosis. Reprod Sci. 2021;28:877-887. doi: 10.1007/s43032-020-00308-1
- Habiba M, Benagiano G. Classifying adenomyosis: progress and challenges. Int J Environ Res Public Health. 2021;18:12386. doi: 10.3390/ijerph182312386
- Movilla P, Morris S, Isaacson K. A systematic review of tissue sampling techniques for the diagnosis of adenomyosis. J Minim Invasive Gynecol. 2020;27:344-351. doi: 10.1016 /j.jmig.2019.09.001
- Agostinho L, Cruz R, Osório F, et al. MRI for adenomyosis: a pictorial review. Insights Imaging. 2017;8:549-556. doi: 10.1007/s13244-017-0576-z
- Bazot M, Cortez A, Darai E, et al. Ultrasonography compared with magnetic resonance imaging for the diagnosis of adenomyosis: correlation with histopathology. Hum Reprod. 2001;16:2427-2433. doi: 10.1093/humrep/16.11.2427
- Reinhold C, Tafazoli F, Mehio A, et al. Uterine adenomyosis: endovaginal US and MR imaging features with histopathologic correlation. Radiographics. 1999;19:S147-S160. doi: 10.1148 /radiographics.19.suppl_1.g99oc13s147
- Rees CO, Nederend J, Mischi M, et al. Objective measures of adenomyosis on MRI and their diagnostic accuracy—a systematic review & meta-analysis. Acta Obstet Gynecol Scand. 2021;100:1377-1391.
- Chapron C, Vannuccini S, Santulli P, et al. Diagnosing adenomyosis: an integrated clinical and imaging approach. Hum Reprod Update. 2020;26:392-411. doi: 10.1093 /humupd/dmz049
- Liu L, Li W, Leonardi M, et al. Diagnostic accuracy of transvaginal ultrasound and magnetic resonance imaging for adenomyosis: systematic review and meta-analysis and review of sonographic diagnostic criteria. J Ultrasound Med. 2021;40:2289-2306. doi: 10.1002/jum.15635
- Bazot M, Daraï E. Role of transvaginal sonography and magnetic resonance imaging in the diagnosis of uterine adenomyosis. Fertil Steril. 2018;109:389-397. doi: 10.1016 /j.fertnstert.2018.01.024
- Van den Bosch T, Dueholm M, Leone FPG, et al. Terms, definitions and measurements to describe sonographic features of myometrium and uterine masses: a consensus opinion from the Morphological Uterus Sonographic Assessment (MUSA) group. Ultrasound Obstet Gynecol. 2015;46:284-298. doi: 10.1002/uog.14806
- Săsăran V, Turdean S, Gliga M, et al. Value of strainratio elastography in the diagnosis and differentiation of uterine fibroids and adenomyosis. J Pers Med. 2021;11:824. doi: 10.3390/jpm11080824
- Di Spiezio Sardo A, Calagna G, Santangelo F, et al. The role of hysteroscopy in the diagnosis and treatment of adenomyosis. Biomed Res Int. 2017;2017:2518396. doi: 10.1155/2017/2518396
- Azzi R. Adenomyosis: current perspectives. Obstet Gynecol Clin North Am. 1989;16:221-235.
- Parker JD, Leondires M, Sinaii N, et al. Persistence of dysmenorrhea and nonmenstrual pain after optimal endometriosis surgery may indicate adenomyosis. Fertil Steril. 2006;86:711-715. doi: 10.1016/j.fertnstert.2006.01.030
- Martire FG, Lazzeri L, Conway F, et al. Adolescence and endometriosis: symptoms, ultrasound signs and early diagnosis. Fertil Steril. 2020;114:1049-1057. doi: 10.1016 /j.fertnstert.2020.06.012
- Decter D, Arbib N, Markovitz H, et al. Sonographic signs of adenomyosis in women with endometriosis are associated with infertility. J Clin Med. 2021;10:2355. doi: 10.3390 /jcm10112355
- Brucker SY, Huebner M, Wallwiener M, et al. Clinical characteristics indicating adenomyosis coexisting with leiomyomas: a retrospective, questionnaire-based study. Fertil Steril. 2014;101:237-241.e1. doi: 10.1016 /j.fertnstert.2013.09.038
- Perelló MF, Martínez-Zamora MÁ, Torres X, et al. Endometriotic pain is associated with adenomyosis but not with the compartments affected by deep infiltrating endometriosis. Gynecol Obstet Invest. 2017;82:240-246. doi: 10.1159/000447633
- Younes G, Tulandi T. Effects of adenomyosis on in vitro fertilization treatment outcomes: a metaanalysis. Fertil Steril. 2017;108:483-490.e3. doi: 10.1016 /j.fertnstert.2017.06.025
- Nirgianakis K, Kalaitzopoulos DR, Schwartz ASK, et al. Fertility, pregnancy and neonatal outcomes of patients with adenomyosis: a systematic review and meta-analysis. Reprod BioMed Online. 2021;42:185-206. doi: 10.1016 /j.rbmo.2020.09.023
- Ono Y, Ota H, Takimoto K, et al. Perinatal outcomes associated with the positional relationship between the placenta and the adenomyosis lesion. J Gynecol Obstet Hum Reprod. 2021;50:102114. doi: 10.1016/j.jogoh.2021.102114
- Barrier BF, Malinowski MJ, Dick EJ Jr, et al. Adenomyosis in the baboon is associated with primary infertility. Fertil Steril. 2004;82(suppl 3):1091-1094. doi: 10.1016 /j.fertnstert.2003.11.065
- Vercellini P, Consonni D, Barbara G, et al. Adenomyosis and reproductive performance after surgery for rectovaginal and colorectal endometriosis: a systematic review and meta-analysis. Reprod Biomed Online. 2014;28:704-713. doi: 10.1016/j.rbmo.2014.02.006

CASE Painful, heavy menstruation and recurrent pregnancy loss
A 37-year-old woman (G3P0030) with a history of recurrent pregnancy loss presents for evaluation. She had 3 losses—most recently a miscarriage at 22 weeks with a cerclage in place. She did not undergo any surgical procedures for these losses. Hormonal and thrombophilia workup is negative and semen analysis is normal. She reports a history of painful, heavy periods for many years, as well as dyspareunia and occasional post-coital bleeding. Past medical history was otherwise unremarkable. Pelvic magnetic resonance imaging (MRI) revealed focal thickening of the junctional zone up to 15 mm with 2 foci of T2 hyperintensities suggesting adenomyosis (FIGURE 1).
How do you counsel this patient regarding the MRI findings and their impact on her fertility?

Adenomyosis is a condition in which endometrial glands and stroma are abnormally present in the uterine myometrium, resulting in smooth muscle hypertrophy and abnormal uterine contractility. Traditional teaching describes a woman in her 40s with heavy and painful menses, a “boggy uterus” on examination, who has completed childbearing and desires definitive treatment. Histologic diagnosis of adenomyosis is made from the uterine specimen at the time of hysterectomy, invariably confounding our understanding of the epidemiology of adenomyosis.
More recently, however, we are beginning to learn that this narrative is misguided. Imaging changes of adenomyosis can be seen in women who desire future fertility and in adolescents with severe dysmenorrhea, suggesting an earlier age of incidence.1 In a recent systematic review, prevalence estimates ranged from 15% to 67%, owing to varying diagnostic methods and patient inclusion criteria.2 It is increasingly being recognized as a primary contributor to infertility, with one study estimating a 30% prevalence of infertility in women with adenomyosis.3 Moreover, treatment with gonadotropin-releasing hormone agonists and/or surgical excision may improve fertility outcomes.4
As we learn more about this prevalent and life-altering condition, we owe it to our patients to consider this diagnosis when counseling on dysmenorrhea, heavy menstrual bleeding, or infertility.
Anatomy of the myometrium
The myometrium is composed of the inner and outer myometrium: the inner myometrium (IM) and endometrium are of Müllerian origin, and the outer myometrium (OM) is of mesenchymal origin. The IM thickens in response to steroid hormones during the menstrual cycle with metaplasia of endometrial stromal cells into myocytes and back again, whereas the OM is not responsive to hormones.5 Emerging literature suggests the OM is further divided into a middle and outer section based on different histologic morphologies, though the clinical implications of this are not understood.6 The term “junctional zone” (JZ) refers to the imaging appearance of what is thought to be the IM. Interestingly it cannot be identified on traditional hematoxylin and eosin staining. When the JZ is thickened or demonstrates irregular borders, it is used as a diagnostic marker for adenomyosis and is postulated to play an important role in adenomyosis pathophysiology, particularly heavy menstrual bleeding and infertility.7
Continue to: Subtypes of adenomyosis...
Subtypes of adenomyosis
While various disease classifications have been suggested for adenomyosis, to date there is no international consensus. Adenomyosis is typically described in 3 forms: diffuse, focal, or adenomyoma.8 As implied, the term focal adenomyosis refers to discrete lesions surrounded by normal myometrium, whereas abnormal glandular changes are pervasive throughout the myometrium in diffuse disease. Adenomyomas are a subgroup of focal adenomyosis that are thought to be surrounded by leiomyomatous smooth muscle and may be well demarcated on imaging.9
Recent research uses novel histologic imaging techniques to explore adenomyotic growth patterns in 3-dimensional (3D) reconstructions. Combining tissue-clearing methods with light-sheet fluorescence microscopy enables highly detailed 3D representations of the protein and nucleic acid structure of organs.10 For example, Yamaguchi and colleagues used this technology to explore the 3D morphological features of adenomyotic tissue and observed direct invasion of the endometrial glands into the myometrium and an “ant colony ̶ like network” of ectopic endometrial glands in the myometrium (FIGURE 2).11 These abnormal glandular networks have been visualized beyond the IM, which may not be captured on ultrasonography or MRI. While this work is still in its infancy, it has the potential to provide important insight into disease pathogenesis and to inform future therapy.

Pathogenesis
Proposed mechanisms for the development of adenomyosis include endometrial invasion, tissue injury and repair (TIAR) mechanisms, and the stem cell theory.12 According to the endometrial invasion theory, glandular epithelial cells from the basalis layer invaginate through an altered IM, slipping through weak muscle fibers and attracted by certain growth factors. In the TIAR mechanism theory, micro- or macro-trauma to the IM (whether from pregnancy, surgery, or infection) results in chronic proliferation and inflammation leading to the development of adenomyosis. Finally, the stem cell theory proposes that adenomyosis might develop from de novo ectopic endometrial tissue.
While the exact pathogenesis of adenomyosis is largely unknown, it has been associated with predictable molecular changes in the endometrium and surrounding myometrium.12 Myometrial hypercontractility is seen in patients with adenomyosis and dysmenorrhea, whereas neovascularization, high microvessel density, and abnormal uterine contractility are seen in those with abnormal uterine bleeding.13 In patients with infertility, increased inflammation, abnormal endometrial receptivity, and alterations in the myometrial architecture have been suggested to impair contractility and sperm transport.12,14
Differential growth factor expression and abnormal estrogen and progesterone signaling pathways have been observed in the IM in patients with adenomyosis, along with dysregulation of immune factors and increased inflammatory oxidative stress.12 This in turn results in myometrial hypertrophy and fibrosis, impairing normal uterine contractility patterns. This abnormal contractility may alter sperm transport and embryo implantation, and animal models that target pathways leading to fibrosis may improve endometrial receptivity.14,15 Further research is needed to elucidate specific molecular pathways and their complex interplay in this disease.
Continue to: Diagnosis...
Diagnosis
The gold standard for diagnosis of adenomyosis is histopathology from hysterectomy specimens, but specific definitions vary. Published criteria include endometrial glands within the myometrial layer greater than 0.5 to 1 low power field from the basal layer of the endometrium, endometrial glands extending deeper than 25% of the myometrial thickness, or endometrial glands a certain distance (ranging from 1-3 mm) from the basalis layer of the endometrium.16 Various methods of non-hysterectomy tissue sampling have been proposed for diagnosis, including needle, hysteroscopic, or laparoscopic sampling, but the sensitivity of these methods is poor.17 Limiting the diagnosis of adenomyosis to specimen pathology relies on invasive methods and clearly we cannot confirm the diagnosis by hysterectomy in patients with a desire for future fertility. It is for this reason that the prevalence of the disease is widely unknown.
The alternative to pathologic diagnosis is to identify radiologic changes that are associated with adenomyosis via either transvaginal ultrasound (TVUS) or MRI. Features suggestive of adenomyosis on MRI overlap with TVUS features, including uterine enlargement, anteroposterior myometrial asymmetry, T1- or T2-intense myometrial cysts or foci, and a thickened JZ.18 A JZ thicker than 12 mm has been thought to be predictive of adenomyosis, whereas a thickness of less than 8 mm is predictive of its absence, although the JZ may vary in thickness with the menstrual cycle.19,20 A 2021 systematic review and meta-analysis comparing MRI diagnosis with histopathologic findings reported a pooled sensitivity and specificity of 60% and 96%, respectively.21 The reported range for sensitivity and specificity is wide: 70% to 93% for sensitivity and 67% to 93% for specificity.22-24
Key TVUS features associated with adenomyosis were defined in 2015 in a consensus statement released by the Morphological Uterus Sonographic Assessment (MUSA) group.25 These include a globally enlarged uterus, anteroposterior myometrial asymmetry, myometrial cysts, fan-shaped shadowing, mixed myometrial echogenicity, translesional vascularity, echogenic subendometrial lines and buds, and a thickened, irregular or discontinuous JZ (FIGURES 3 and 4).25 The accuracy of ultrasonographic diagnosis of adenomyosis using these features has been investigated in multiple systematic reviews and meta-analyses, most recently by Liu and colleagues who found a pooled sensitivity of TVUS of 81% and pooled specificity of 87%.23 The range for ultrasonographic sensitivity and specificity is wide, however, ranging from 33% to 84% for sensitivity and 64% to 100% for specificity.22 Consensus is lacking as to which TVUS features are most predictive of adenomyosis, but in general, the combination of multiple MUSA criteria (particularly myometrial cysts and irregular JZ on 3D imaging) appears to be more accurate than any one feature alone.23 The presence of fibroids may decrease the sensitivity of TVUS, and one study suggested elastography may increase the accuracy of TVUS.24,26 Moreover, given that most radiologists receive limited training on the MUSA criteria, it behooves gynecologists to become familiar with these sonographic features to be able to identify adenomyosis in our patients.


Adenomyosis also may be suspected based on hysteroscopic findings, although a normal hysteroscopy cannot rule out the disease and data are lacking to support these markers as diagnostic. Visual findings can include a “strawberry” pattern, mucosal elevation, cystic hemorrhagic lesions, localized vascularity, or endometrial defects.27 Hysteroscopy may be effective in the treatment of localized lesions, although that discussion is beyond the scope of this review.
Clinical presentation
While many women who are later diagnosed with adenomyosis are asymptomatic, the disease can present with heavy menstrual bleeding and dysmenorrhea, which occur in 50% and 30% of patients, respectively.28 Other symptoms include dyspareunia and infertility. Symptoms were previously reported to develop between the ages of 40 and 50 years; however, this is biased by diagnosis at the time of hysterectomy and the fact that younger patients are less likely to undergo definitive surgery. When using imaging criteria for diagnosis, adenomyosis might be more responsible for dysmenorrhea and chronic pelvic pain in younger patients than previously appreciated.1,29 In a recent study reviewing TVUS in 270 adolescents for any reason, adenomyosis was present in 5% of cases and this increased up to 44% in the presence of endometriosis.30
Adenomyosis often co-exists and shares similar clinical presentations with other gynecologic pathologies such as endometriosis and fibroids, making diagnosis on symptomatology alone challenging. Concurrent adenomyosis has been found in up to 73% and 57% of patients with suspected or diagnosed endometriosis and fibroids, respectively.31,32 Accumulating evidence suggests that pelvic pain previously attributed to endometriosis may in fact be a result of adenomyosis; for example, persistent pelvic pain after optimal resection of endometriosis may be confounded by the presence of adenomyosis.29 In one study of 155 patients with complete resection of deep infiltrating endometriosis, persistent pelvic pain was significantly associated with the presence of adenomyosis on imaging.33
Adenomyosis is increasingly being recognized at the time of infertility evaluation with an estimated prevalence of 30% in women with infertility.3 Among women with infertility, adenomyosis has been associated with a lower clinical pregnancy rate, higher miscarriage rate, and lower live birth rate, as well as obstetric complications such as abnormal placentation.34-36 A study of 37 baboons found the histologic diagnosis of adenomyosis alone at necropsy was associated with a 20-fold increased risk of lifelong infertility (odds ratio [OR], 20.1; 95% CI, 2.1-921), whereas presence of endometriosis was associated with a nonsignificant 3-fold risk of lifelong infertility (OR, 3.6; 95% CI, 0.9-15.8).37
In women with endometriosis and infertility, co-existing adenomyosis portends worse fertility outcomes. In a retrospective study of 244 women who underwent endometriosis surgery, more than five features of adenomyosis on imaging was associated with higher rates of infertility, in vitro fertilization treatments, and a higher number of in vitro fertilization cycles.31 Moreover, in women who underwent surgery for deep infiltrating endometriosis, the presence of adenomyosis on imaging was associated with a 68% reduction in likelihood of pregnancy after surgery.38
Conclusion
As we begin to learn about adenomyosis, our misconceptions become more evident. The notion that it largely affects women at the end of their reproductive lives is biased by using histopathology at hysterectomy as the gold standard for diagnosis. Lack of definitive histologic or imaging criteria and biopsy techniques add to the diagnostic challenge. This in turn leads to inaccurate estimates of incidence and prevalence, as we assume patients’ symptoms must be attributable to what we can see at the time of surgery (for example, Stage I or II endometriosis), rather than what we cannot see. We now know that adenomyosis is present in women of all ages, including adolescents, and can significantly contribute to reduced fertility and quality of life. We owe it to our patients to consider this condition in the differential diagnosis of dysmenorrhea, heavy menstrual bleeding, dyspareunia, and infertility.
CASE Resolved
The patient underwent targeted hysteroscopic resection of adenomyosis (FIGURE 5) and conceived spontaneously the following year. ●


CASE Painful, heavy menstruation and recurrent pregnancy loss
A 37-year-old woman (G3P0030) with a history of recurrent pregnancy loss presents for evaluation. She had 3 losses—most recently a miscarriage at 22 weeks with a cerclage in place. She did not undergo any surgical procedures for these losses. Hormonal and thrombophilia workup is negative and semen analysis is normal. She reports a history of painful, heavy periods for many years, as well as dyspareunia and occasional post-coital bleeding. Past medical history was otherwise unremarkable. Pelvic magnetic resonance imaging (MRI) revealed focal thickening of the junctional zone up to 15 mm with 2 foci of T2 hyperintensities suggesting adenomyosis (FIGURE 1).
How do you counsel this patient regarding the MRI findings and their impact on her fertility?

Adenomyosis is a condition in which endometrial glands and stroma are abnormally present in the uterine myometrium, resulting in smooth muscle hypertrophy and abnormal uterine contractility. Traditional teaching describes a woman in her 40s with heavy and painful menses, a “boggy uterus” on examination, who has completed childbearing and desires definitive treatment. Histologic diagnosis of adenomyosis is made from the uterine specimen at the time of hysterectomy, invariably confounding our understanding of the epidemiology of adenomyosis.
More recently, however, we are beginning to learn that this narrative is misguided. Imaging changes of adenomyosis can be seen in women who desire future fertility and in adolescents with severe dysmenorrhea, suggesting an earlier age of incidence.1 In a recent systematic review, prevalence estimates ranged from 15% to 67%, owing to varying diagnostic methods and patient inclusion criteria.2 It is increasingly being recognized as a primary contributor to infertility, with one study estimating a 30% prevalence of infertility in women with adenomyosis.3 Moreover, treatment with gonadotropin-releasing hormone agonists and/or surgical excision may improve fertility outcomes.4
As we learn more about this prevalent and life-altering condition, we owe it to our patients to consider this diagnosis when counseling on dysmenorrhea, heavy menstrual bleeding, or infertility.
Anatomy of the myometrium
The myometrium is composed of the inner and outer myometrium: the inner myometrium (IM) and endometrium are of Müllerian origin, and the outer myometrium (OM) is of mesenchymal origin. The IM thickens in response to steroid hormones during the menstrual cycle with metaplasia of endometrial stromal cells into myocytes and back again, whereas the OM is not responsive to hormones.5 Emerging literature suggests the OM is further divided into a middle and outer section based on different histologic morphologies, though the clinical implications of this are not understood.6 The term “junctional zone” (JZ) refers to the imaging appearance of what is thought to be the IM. Interestingly it cannot be identified on traditional hematoxylin and eosin staining. When the JZ is thickened or demonstrates irregular borders, it is used as a diagnostic marker for adenomyosis and is postulated to play an important role in adenomyosis pathophysiology, particularly heavy menstrual bleeding and infertility.7
Continue to: Subtypes of adenomyosis...
Subtypes of adenomyosis
While various disease classifications have been suggested for adenomyosis, to date there is no international consensus. Adenomyosis is typically described in 3 forms: diffuse, focal, or adenomyoma.8 As implied, the term focal adenomyosis refers to discrete lesions surrounded by normal myometrium, whereas abnormal glandular changes are pervasive throughout the myometrium in diffuse disease. Adenomyomas are a subgroup of focal adenomyosis that are thought to be surrounded by leiomyomatous smooth muscle and may be well demarcated on imaging.9
Recent research uses novel histologic imaging techniques to explore adenomyotic growth patterns in 3-dimensional (3D) reconstructions. Combining tissue-clearing methods with light-sheet fluorescence microscopy enables highly detailed 3D representations of the protein and nucleic acid structure of organs.10 For example, Yamaguchi and colleagues used this technology to explore the 3D morphological features of adenomyotic tissue and observed direct invasion of the endometrial glands into the myometrium and an “ant colony ̶ like network” of ectopic endometrial glands in the myometrium (FIGURE 2).11 These abnormal glandular networks have been visualized beyond the IM, which may not be captured on ultrasonography or MRI. While this work is still in its infancy, it has the potential to provide important insight into disease pathogenesis and to inform future therapy.

Pathogenesis
Proposed mechanisms for the development of adenomyosis include endometrial invasion, tissue injury and repair (TIAR) mechanisms, and the stem cell theory.12 According to the endometrial invasion theory, glandular epithelial cells from the basalis layer invaginate through an altered IM, slipping through weak muscle fibers and attracted by certain growth factors. In the TIAR mechanism theory, micro- or macro-trauma to the IM (whether from pregnancy, surgery, or infection) results in chronic proliferation and inflammation leading to the development of adenomyosis. Finally, the stem cell theory proposes that adenomyosis might develop from de novo ectopic endometrial tissue.
While the exact pathogenesis of adenomyosis is largely unknown, it has been associated with predictable molecular changes in the endometrium and surrounding myometrium.12 Myometrial hypercontractility is seen in patients with adenomyosis and dysmenorrhea, whereas neovascularization, high microvessel density, and abnormal uterine contractility are seen in those with abnormal uterine bleeding.13 In patients with infertility, increased inflammation, abnormal endometrial receptivity, and alterations in the myometrial architecture have been suggested to impair contractility and sperm transport.12,14
Differential growth factor expression and abnormal estrogen and progesterone signaling pathways have been observed in the IM in patients with adenomyosis, along with dysregulation of immune factors and increased inflammatory oxidative stress.12 This in turn results in myometrial hypertrophy and fibrosis, impairing normal uterine contractility patterns. This abnormal contractility may alter sperm transport and embryo implantation, and animal models that target pathways leading to fibrosis may improve endometrial receptivity.14,15 Further research is needed to elucidate specific molecular pathways and their complex interplay in this disease.
Continue to: Diagnosis...
Diagnosis
The gold standard for diagnosis of adenomyosis is histopathology from hysterectomy specimens, but specific definitions vary. Published criteria include endometrial glands within the myometrial layer greater than 0.5 to 1 low power field from the basal layer of the endometrium, endometrial glands extending deeper than 25% of the myometrial thickness, or endometrial glands a certain distance (ranging from 1-3 mm) from the basalis layer of the endometrium.16 Various methods of non-hysterectomy tissue sampling have been proposed for diagnosis, including needle, hysteroscopic, or laparoscopic sampling, but the sensitivity of these methods is poor.17 Limiting the diagnosis of adenomyosis to specimen pathology relies on invasive methods and clearly we cannot confirm the diagnosis by hysterectomy in patients with a desire for future fertility. It is for this reason that the prevalence of the disease is widely unknown.
The alternative to pathologic diagnosis is to identify radiologic changes that are associated with adenomyosis via either transvaginal ultrasound (TVUS) or MRI. Features suggestive of adenomyosis on MRI overlap with TVUS features, including uterine enlargement, anteroposterior myometrial asymmetry, T1- or T2-intense myometrial cysts or foci, and a thickened JZ.18 A JZ thicker than 12 mm has been thought to be predictive of adenomyosis, whereas a thickness of less than 8 mm is predictive of its absence, although the JZ may vary in thickness with the menstrual cycle.19,20 A 2021 systematic review and meta-analysis comparing MRI diagnosis with histopathologic findings reported a pooled sensitivity and specificity of 60% and 96%, respectively.21 The reported range for sensitivity and specificity is wide: 70% to 93% for sensitivity and 67% to 93% for specificity.22-24
Key TVUS features associated with adenomyosis were defined in 2015 in a consensus statement released by the Morphological Uterus Sonographic Assessment (MUSA) group.25 These include a globally enlarged uterus, anteroposterior myometrial asymmetry, myometrial cysts, fan-shaped shadowing, mixed myometrial echogenicity, translesional vascularity, echogenic subendometrial lines and buds, and a thickened, irregular or discontinuous JZ (FIGURES 3 and 4).25 The accuracy of ultrasonographic diagnosis of adenomyosis using these features has been investigated in multiple systematic reviews and meta-analyses, most recently by Liu and colleagues who found a pooled sensitivity of TVUS of 81% and pooled specificity of 87%.23 The range for ultrasonographic sensitivity and specificity is wide, however, ranging from 33% to 84% for sensitivity and 64% to 100% for specificity.22 Consensus is lacking as to which TVUS features are most predictive of adenomyosis, but in general, the combination of multiple MUSA criteria (particularly myometrial cysts and irregular JZ on 3D imaging) appears to be more accurate than any one feature alone.23 The presence of fibroids may decrease the sensitivity of TVUS, and one study suggested elastography may increase the accuracy of TVUS.24,26 Moreover, given that most radiologists receive limited training on the MUSA criteria, it behooves gynecologists to become familiar with these sonographic features to be able to identify adenomyosis in our patients.


Adenomyosis also may be suspected based on hysteroscopic findings, although a normal hysteroscopy cannot rule out the disease and data are lacking to support these markers as diagnostic. Visual findings can include a “strawberry” pattern, mucosal elevation, cystic hemorrhagic lesions, localized vascularity, or endometrial defects.27 Hysteroscopy may be effective in the treatment of localized lesions, although that discussion is beyond the scope of this review.
Clinical presentation
While many women who are later diagnosed with adenomyosis are asymptomatic, the disease can present with heavy menstrual bleeding and dysmenorrhea, which occur in 50% and 30% of patients, respectively.28 Other symptoms include dyspareunia and infertility. Symptoms were previously reported to develop between the ages of 40 and 50 years; however, this is biased by diagnosis at the time of hysterectomy and the fact that younger patients are less likely to undergo definitive surgery. When using imaging criteria for diagnosis, adenomyosis might be more responsible for dysmenorrhea and chronic pelvic pain in younger patients than previously appreciated.1,29 In a recent study reviewing TVUS in 270 adolescents for any reason, adenomyosis was present in 5% of cases and this increased up to 44% in the presence of endometriosis.30
Adenomyosis often co-exists and shares similar clinical presentations with other gynecologic pathologies such as endometriosis and fibroids, making diagnosis on symptomatology alone challenging. Concurrent adenomyosis has been found in up to 73% and 57% of patients with suspected or diagnosed endometriosis and fibroids, respectively.31,32 Accumulating evidence suggests that pelvic pain previously attributed to endometriosis may in fact be a result of adenomyosis; for example, persistent pelvic pain after optimal resection of endometriosis may be confounded by the presence of adenomyosis.29 In one study of 155 patients with complete resection of deep infiltrating endometriosis, persistent pelvic pain was significantly associated with the presence of adenomyosis on imaging.33
Adenomyosis is increasingly being recognized at the time of infertility evaluation with an estimated prevalence of 30% in women with infertility.3 Among women with infertility, adenomyosis has been associated with a lower clinical pregnancy rate, higher miscarriage rate, and lower live birth rate, as well as obstetric complications such as abnormal placentation.34-36 A study of 37 baboons found the histologic diagnosis of adenomyosis alone at necropsy was associated with a 20-fold increased risk of lifelong infertility (odds ratio [OR], 20.1; 95% CI, 2.1-921), whereas presence of endometriosis was associated with a nonsignificant 3-fold risk of lifelong infertility (OR, 3.6; 95% CI, 0.9-15.8).37
In women with endometriosis and infertility, co-existing adenomyosis portends worse fertility outcomes. In a retrospective study of 244 women who underwent endometriosis surgery, more than five features of adenomyosis on imaging was associated with higher rates of infertility, in vitro fertilization treatments, and a higher number of in vitro fertilization cycles.31 Moreover, in women who underwent surgery for deep infiltrating endometriosis, the presence of adenomyosis on imaging was associated with a 68% reduction in likelihood of pregnancy after surgery.38
Conclusion
As we begin to learn about adenomyosis, our misconceptions become more evident. The notion that it largely affects women at the end of their reproductive lives is biased by using histopathology at hysterectomy as the gold standard for diagnosis. Lack of definitive histologic or imaging criteria and biopsy techniques add to the diagnostic challenge. This in turn leads to inaccurate estimates of incidence and prevalence, as we assume patients’ symptoms must be attributable to what we can see at the time of surgery (for example, Stage I or II endometriosis), rather than what we cannot see. We now know that adenomyosis is present in women of all ages, including adolescents, and can significantly contribute to reduced fertility and quality of life. We owe it to our patients to consider this condition in the differential diagnosis of dysmenorrhea, heavy menstrual bleeding, dyspareunia, and infertility.
CASE Resolved
The patient underwent targeted hysteroscopic resection of adenomyosis (FIGURE 5) and conceived spontaneously the following year. ●

- Exacoustos C, Lazzeri L, Martire FG, et al. Ultrasound findings of adenomyosis in adolescents: type and grade of the disease. J Minim Invasive Gynecol. 2021;29:291.e1-299.e1. doi: 10.1016/j.jmig.2021.08.023
- Loring M, Chen TY, Isaacson KB. A systematic review of adenomyosis: it is time to reassess what we thought we knew about the disease. J Minim Invasive Gynecol. 2021;28:644655. doi: 10.1016/j.jmig.2020.10.012
- Bourdon M, Santulli P, Oliveira J, et al. Focal adenomyosis is associated with primary infertility. Fertil Steril. 2020;114:1271-1277. doi: 10.1016/j.fertnstert.2020.06.018
- Lan J, Wu Y, Wu Z, et al. Ultra-long GnRH agonist protocol during IVF/ICSI improves pregnancy outcomes in women with adenomyosis: a retrospective cohort study. Front Endocrinol (Lausanne). 2021;12:609771. doi: 10.3389 /fendo.2021.609771
- Gnecco JS, Brown AT, Kan EL, et al. Physiomimetic models of adenomyosis. Semin Reprod Med. 2020;38:179-196. doi: 10.1055/s-0040-1719084
- Harmsen MJ, Trommelen LM, de Leeuw RA, et al. Uterine junctional zone and adenomyosis: comparison of MRI, transvaginal ultrasound and histology. Ultrasound Obstet Gynecol. 2023;62:42-60. doi: 10.1002/uog.26117
- Xie T, Xu X, Yang Y, et al. The role of abnormal uterine junction zone in the occurrence and development of adenomyosis. Reprod Sci. 2022;29:2719-2730. doi: 10.1007/s43032-021 -00684-2
- Lazzeri L, Morosetti G, Centini G, et al. A sonographic classification of adenomyosis: interobserver reproducibility in the evaluation of type and degree of the myometrial involvement. Fertil Steril. 2018;110:1154-1161.e3. doi: 10.1016 /j.fertnstert.2018.06.031
- Tahlan A, Nanda A, Mohan H. Uterine adenomyoma: a clinicopathologic review of 26 cases and a review of the literature. Int J Gynecol Pathol. 2006;25:361-365. doi: 10.1097/01.pgp.0000209570.08716.b3
- Chung K, Wallace J, Kim S-Y, et al. Structural and molecular interrogation of intact biological systems. Nature. 2013;497:332-337. doi: 10.1038/nature12107
- Yamaguchi M, Yoshihara K, Suda K, et al. Three-dimensional understanding of the morphological complexity of the human uterine endometrium. iScience. 2021;24:102258. doi: 10.1016/j.isci.2021.102258
- Vannuccini S, Tosti C, Carmona F, et al. Pathogenesis of adenomyosis: an update on molecular mechanisms. Reprod Biomed Online. 2017;35:592-601. doi: 10.1016 /j.rbmo.2017.06.016
- Zhai J, Vannuccini S, Petraglia F, et al. Adenomyosis: mechanisms and pathogenesis. Semin Reprod Med. 2020;38:129-143. doi: 10.1055/s-0040-1716687
- Munro MG. Uterine polyps, adenomyosis, leiomyomas, and endometrial receptivity. Fertil Steril. 2019;111:629-640. doi: 10.1016/j.fertnstert.2019.02.008
- Kay N, Huang C-Y, Shiu L-Y, et al. TGF-β1 neutralization improves pregnancy outcomes by restoring endometrial receptivity in mice with adenomyosis. Reprod Sci. 2021;28:877-887. doi: 10.1007/s43032-020-00308-1
- Habiba M, Benagiano G. Classifying adenomyosis: progress and challenges. Int J Environ Res Public Health. 2021;18:12386. doi: 10.3390/ijerph182312386
- Movilla P, Morris S, Isaacson K. A systematic review of tissue sampling techniques for the diagnosis of adenomyosis. J Minim Invasive Gynecol. 2020;27:344-351. doi: 10.1016 /j.jmig.2019.09.001
- Agostinho L, Cruz R, Osório F, et al. MRI for adenomyosis: a pictorial review. Insights Imaging. 2017;8:549-556. doi: 10.1007/s13244-017-0576-z
- Bazot M, Cortez A, Darai E, et al. Ultrasonography compared with magnetic resonance imaging for the diagnosis of adenomyosis: correlation with histopathology. Hum Reprod. 2001;16:2427-2433. doi: 10.1093/humrep/16.11.2427
- Reinhold C, Tafazoli F, Mehio A, et al. Uterine adenomyosis: endovaginal US and MR imaging features with histopathologic correlation. Radiographics. 1999;19:S147-S160. doi: 10.1148 /radiographics.19.suppl_1.g99oc13s147
- Rees CO, Nederend J, Mischi M, et al. Objective measures of adenomyosis on MRI and their diagnostic accuracy—a systematic review & meta-analysis. Acta Obstet Gynecol Scand. 2021;100:1377-1391.
- Chapron C, Vannuccini S, Santulli P, et al. Diagnosing adenomyosis: an integrated clinical and imaging approach. Hum Reprod Update. 2020;26:392-411. doi: 10.1093 /humupd/dmz049
- Liu L, Li W, Leonardi M, et al. Diagnostic accuracy of transvaginal ultrasound and magnetic resonance imaging for adenomyosis: systematic review and meta-analysis and review of sonographic diagnostic criteria. J Ultrasound Med. 2021;40:2289-2306. doi: 10.1002/jum.15635
- Bazot M, Daraï E. Role of transvaginal sonography and magnetic resonance imaging in the diagnosis of uterine adenomyosis. Fertil Steril. 2018;109:389-397. doi: 10.1016 /j.fertnstert.2018.01.024
- Van den Bosch T, Dueholm M, Leone FPG, et al. Terms, definitions and measurements to describe sonographic features of myometrium and uterine masses: a consensus opinion from the Morphological Uterus Sonographic Assessment (MUSA) group. Ultrasound Obstet Gynecol. 2015;46:284-298. doi: 10.1002/uog.14806
- Săsăran V, Turdean S, Gliga M, et al. Value of strainratio elastography in the diagnosis and differentiation of uterine fibroids and adenomyosis. J Pers Med. 2021;11:824. doi: 10.3390/jpm11080824
- Di Spiezio Sardo A, Calagna G, Santangelo F, et al. The role of hysteroscopy in the diagnosis and treatment of adenomyosis. Biomed Res Int. 2017;2017:2518396. doi: 10.1155/2017/2518396
- Azzi R. Adenomyosis: current perspectives. Obstet Gynecol Clin North Am. 1989;16:221-235.
- Parker JD, Leondires M, Sinaii N, et al. Persistence of dysmenorrhea and nonmenstrual pain after optimal endometriosis surgery may indicate adenomyosis. Fertil Steril. 2006;86:711-715. doi: 10.1016/j.fertnstert.2006.01.030
- Martire FG, Lazzeri L, Conway F, et al. Adolescence and endometriosis: symptoms, ultrasound signs and early diagnosis. Fertil Steril. 2020;114:1049-1057. doi: 10.1016 /j.fertnstert.2020.06.012
- Decter D, Arbib N, Markovitz H, et al. Sonographic signs of adenomyosis in women with endometriosis are associated with infertility. J Clin Med. 2021;10:2355. doi: 10.3390 /jcm10112355
- Brucker SY, Huebner M, Wallwiener M, et al. Clinical characteristics indicating adenomyosis coexisting with leiomyomas: a retrospective, questionnaire-based study. Fertil Steril. 2014;101:237-241.e1. doi: 10.1016 /j.fertnstert.2013.09.038
- Perelló MF, Martínez-Zamora MÁ, Torres X, et al. Endometriotic pain is associated with adenomyosis but not with the compartments affected by deep infiltrating endometriosis. Gynecol Obstet Invest. 2017;82:240-246. doi: 10.1159/000447633
- Younes G, Tulandi T. Effects of adenomyosis on in vitro fertilization treatment outcomes: a metaanalysis. Fertil Steril. 2017;108:483-490.e3. doi: 10.1016 /j.fertnstert.2017.06.025
- Nirgianakis K, Kalaitzopoulos DR, Schwartz ASK, et al. Fertility, pregnancy and neonatal outcomes of patients with adenomyosis: a systematic review and meta-analysis. Reprod BioMed Online. 2021;42:185-206. doi: 10.1016 /j.rbmo.2020.09.023
- Ono Y, Ota H, Takimoto K, et al. Perinatal outcomes associated with the positional relationship between the placenta and the adenomyosis lesion. J Gynecol Obstet Hum Reprod. 2021;50:102114. doi: 10.1016/j.jogoh.2021.102114
- Barrier BF, Malinowski MJ, Dick EJ Jr, et al. Adenomyosis in the baboon is associated with primary infertility. Fertil Steril. 2004;82(suppl 3):1091-1094. doi: 10.1016 /j.fertnstert.2003.11.065
- Vercellini P, Consonni D, Barbara G, et al. Adenomyosis and reproductive performance after surgery for rectovaginal and colorectal endometriosis: a systematic review and meta-analysis. Reprod Biomed Online. 2014;28:704-713. doi: 10.1016/j.rbmo.2014.02.006
- Exacoustos C, Lazzeri L, Martire FG, et al. Ultrasound findings of adenomyosis in adolescents: type and grade of the disease. J Minim Invasive Gynecol. 2021;29:291.e1-299.e1. doi: 10.1016/j.jmig.2021.08.023
- Loring M, Chen TY, Isaacson KB. A systematic review of adenomyosis: it is time to reassess what we thought we knew about the disease. J Minim Invasive Gynecol. 2021;28:644655. doi: 10.1016/j.jmig.2020.10.012
- Bourdon M, Santulli P, Oliveira J, et al. Focal adenomyosis is associated with primary infertility. Fertil Steril. 2020;114:1271-1277. doi: 10.1016/j.fertnstert.2020.06.018
- Lan J, Wu Y, Wu Z, et al. Ultra-long GnRH agonist protocol during IVF/ICSI improves pregnancy outcomes in women with adenomyosis: a retrospective cohort study. Front Endocrinol (Lausanne). 2021;12:609771. doi: 10.3389 /fendo.2021.609771
- Gnecco JS, Brown AT, Kan EL, et al. Physiomimetic models of adenomyosis. Semin Reprod Med. 2020;38:179-196. doi: 10.1055/s-0040-1719084
- Harmsen MJ, Trommelen LM, de Leeuw RA, et al. Uterine junctional zone and adenomyosis: comparison of MRI, transvaginal ultrasound and histology. Ultrasound Obstet Gynecol. 2023;62:42-60. doi: 10.1002/uog.26117
- Xie T, Xu X, Yang Y, et al. The role of abnormal uterine junction zone in the occurrence and development of adenomyosis. Reprod Sci. 2022;29:2719-2730. doi: 10.1007/s43032-021 -00684-2
- Lazzeri L, Morosetti G, Centini G, et al. A sonographic classification of adenomyosis: interobserver reproducibility in the evaluation of type and degree of the myometrial involvement. Fertil Steril. 2018;110:1154-1161.e3. doi: 10.1016 /j.fertnstert.2018.06.031
- Tahlan A, Nanda A, Mohan H. Uterine adenomyoma: a clinicopathologic review of 26 cases and a review of the literature. Int J Gynecol Pathol. 2006;25:361-365. doi: 10.1097/01.pgp.0000209570.08716.b3
- Chung K, Wallace J, Kim S-Y, et al. Structural and molecular interrogation of intact biological systems. Nature. 2013;497:332-337. doi: 10.1038/nature12107
- Yamaguchi M, Yoshihara K, Suda K, et al. Three-dimensional understanding of the morphological complexity of the human uterine endometrium. iScience. 2021;24:102258. doi: 10.1016/j.isci.2021.102258
- Vannuccini S, Tosti C, Carmona F, et al. Pathogenesis of adenomyosis: an update on molecular mechanisms. Reprod Biomed Online. 2017;35:592-601. doi: 10.1016 /j.rbmo.2017.06.016
- Zhai J, Vannuccini S, Petraglia F, et al. Adenomyosis: mechanisms and pathogenesis. Semin Reprod Med. 2020;38:129-143. doi: 10.1055/s-0040-1716687
- Munro MG. Uterine polyps, adenomyosis, leiomyomas, and endometrial receptivity. Fertil Steril. 2019;111:629-640. doi: 10.1016/j.fertnstert.2019.02.008
- Kay N, Huang C-Y, Shiu L-Y, et al. TGF-β1 neutralization improves pregnancy outcomes by restoring endometrial receptivity in mice with adenomyosis. Reprod Sci. 2021;28:877-887. doi: 10.1007/s43032-020-00308-1
- Habiba M, Benagiano G. Classifying adenomyosis: progress and challenges. Int J Environ Res Public Health. 2021;18:12386. doi: 10.3390/ijerph182312386
- Movilla P, Morris S, Isaacson K. A systematic review of tissue sampling techniques for the diagnosis of adenomyosis. J Minim Invasive Gynecol. 2020;27:344-351. doi: 10.1016 /j.jmig.2019.09.001
- Agostinho L, Cruz R, Osório F, et al. MRI for adenomyosis: a pictorial review. Insights Imaging. 2017;8:549-556. doi: 10.1007/s13244-017-0576-z
- Bazot M, Cortez A, Darai E, et al. Ultrasonography compared with magnetic resonance imaging for the diagnosis of adenomyosis: correlation with histopathology. Hum Reprod. 2001;16:2427-2433. doi: 10.1093/humrep/16.11.2427
- Reinhold C, Tafazoli F, Mehio A, et al. Uterine adenomyosis: endovaginal US and MR imaging features with histopathologic correlation. Radiographics. 1999;19:S147-S160. doi: 10.1148 /radiographics.19.suppl_1.g99oc13s147
- Rees CO, Nederend J, Mischi M, et al. Objective measures of adenomyosis on MRI and their diagnostic accuracy—a systematic review & meta-analysis. Acta Obstet Gynecol Scand. 2021;100:1377-1391.
- Chapron C, Vannuccini S, Santulli P, et al. Diagnosing adenomyosis: an integrated clinical and imaging approach. Hum Reprod Update. 2020;26:392-411. doi: 10.1093 /humupd/dmz049
- Liu L, Li W, Leonardi M, et al. Diagnostic accuracy of transvaginal ultrasound and magnetic resonance imaging for adenomyosis: systematic review and meta-analysis and review of sonographic diagnostic criteria. J Ultrasound Med. 2021;40:2289-2306. doi: 10.1002/jum.15635
- Bazot M, Daraï E. Role of transvaginal sonography and magnetic resonance imaging in the diagnosis of uterine adenomyosis. Fertil Steril. 2018;109:389-397. doi: 10.1016 /j.fertnstert.2018.01.024
- Van den Bosch T, Dueholm M, Leone FPG, et al. Terms, definitions and measurements to describe sonographic features of myometrium and uterine masses: a consensus opinion from the Morphological Uterus Sonographic Assessment (MUSA) group. Ultrasound Obstet Gynecol. 2015;46:284-298. doi: 10.1002/uog.14806
- Săsăran V, Turdean S, Gliga M, et al. Value of strainratio elastography in the diagnosis and differentiation of uterine fibroids and adenomyosis. J Pers Med. 2021;11:824. doi: 10.3390/jpm11080824
- Di Spiezio Sardo A, Calagna G, Santangelo F, et al. The role of hysteroscopy in the diagnosis and treatment of adenomyosis. Biomed Res Int. 2017;2017:2518396. doi: 10.1155/2017/2518396
- Azzi R. Adenomyosis: current perspectives. Obstet Gynecol Clin North Am. 1989;16:221-235.
- Parker JD, Leondires M, Sinaii N, et al. Persistence of dysmenorrhea and nonmenstrual pain after optimal endometriosis surgery may indicate adenomyosis. Fertil Steril. 2006;86:711-715. doi: 10.1016/j.fertnstert.2006.01.030
- Martire FG, Lazzeri L, Conway F, et al. Adolescence and endometriosis: symptoms, ultrasound signs and early diagnosis. Fertil Steril. 2020;114:1049-1057. doi: 10.1016 /j.fertnstert.2020.06.012
- Decter D, Arbib N, Markovitz H, et al. Sonographic signs of adenomyosis in women with endometriosis are associated with infertility. J Clin Med. 2021;10:2355. doi: 10.3390 /jcm10112355
- Brucker SY, Huebner M, Wallwiener M, et al. Clinical characteristics indicating adenomyosis coexisting with leiomyomas: a retrospective, questionnaire-based study. Fertil Steril. 2014;101:237-241.e1. doi: 10.1016 /j.fertnstert.2013.09.038
- Perelló MF, Martínez-Zamora MÁ, Torres X, et al. Endometriotic pain is associated with adenomyosis but not with the compartments affected by deep infiltrating endometriosis. Gynecol Obstet Invest. 2017;82:240-246. doi: 10.1159/000447633
- Younes G, Tulandi T. Effects of adenomyosis on in vitro fertilization treatment outcomes: a metaanalysis. Fertil Steril. 2017;108:483-490.e3. doi: 10.1016 /j.fertnstert.2017.06.025
- Nirgianakis K, Kalaitzopoulos DR, Schwartz ASK, et al. Fertility, pregnancy and neonatal outcomes of patients with adenomyosis: a systematic review and meta-analysis. Reprod BioMed Online. 2021;42:185-206. doi: 10.1016 /j.rbmo.2020.09.023
- Ono Y, Ota H, Takimoto K, et al. Perinatal outcomes associated with the positional relationship between the placenta and the adenomyosis lesion. J Gynecol Obstet Hum Reprod. 2021;50:102114. doi: 10.1016/j.jogoh.2021.102114
- Barrier BF, Malinowski MJ, Dick EJ Jr, et al. Adenomyosis in the baboon is associated with primary infertility. Fertil Steril. 2004;82(suppl 3):1091-1094. doi: 10.1016 /j.fertnstert.2003.11.065
- Vercellini P, Consonni D, Barbara G, et al. Adenomyosis and reproductive performance after surgery for rectovaginal and colorectal endometriosis: a systematic review and meta-analysis. Reprod Biomed Online. 2014;28:704-713. doi: 10.1016/j.rbmo.2014.02.006