User login
Obinutuzumab-based regimens yield durable remissions in CLL
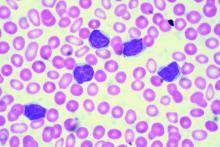
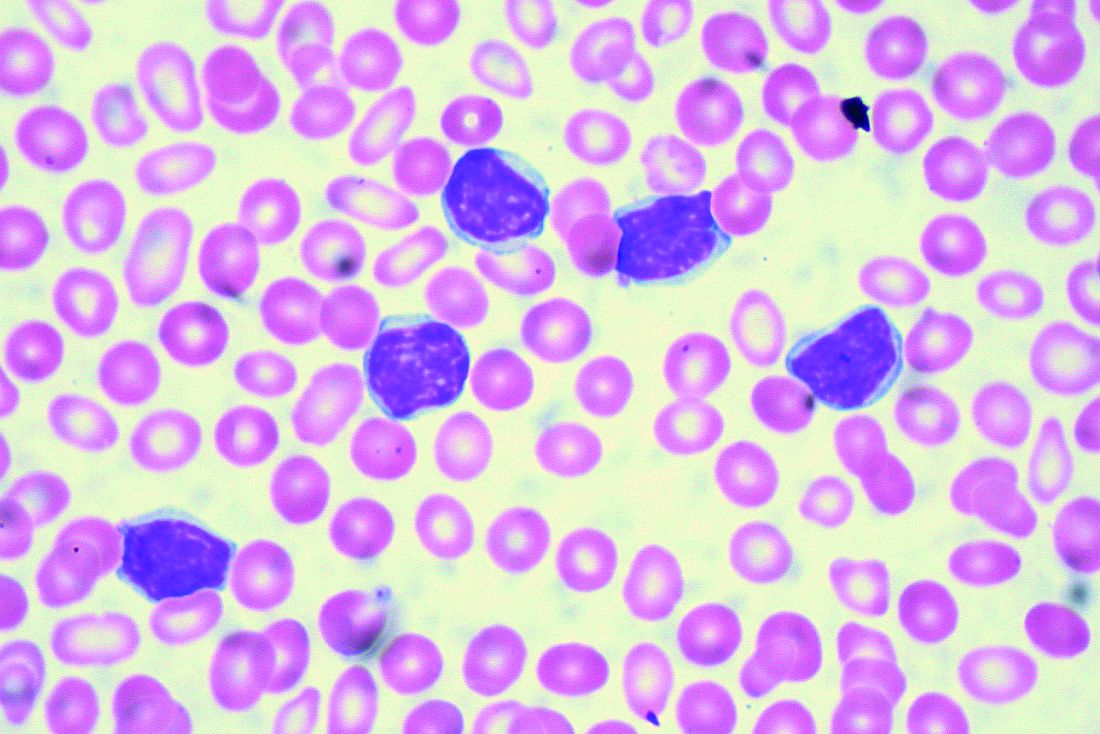
Two different obinutuzumab-based chemoimmunotherapy regimens resulted in excellent long-term disease control as front-line therapy for chronic lymphocytic leukemia (CLL), investigators said in a follow-up report on a phase 1b study.
Both obinutuzumab plus fludarabine/cyclophosphamide (G-FC) and obinutuzumab plus bendamustine (G-B) were well tolerated, with adverse events similar to what has been reported in rituximab-containing immunotherapy regimens, they said in the report of final results from the GALTON trial.
Most evaluable patients had B-cell recovery by 36 months in the study, which included a population of CLL patients largely without 17p deletions, said Jennifer R. Brown, MD, PhD, of Dana-Farber Cancer Institute, Boston, and her coinvestigators.
“These data support moving forward with these regimens in subsequent trials, which are currently ongoing,” they said in their report on the study, which appears in Blood.
The open-label, parallel-arm, multicenter phase 1b GALTON study included 41 patients with CLL, of whom 21 received G-FC and 20 received G-B for up to six cycles of 28 days each. The median age was 60 years, and about one-third of patients had Rai stage III or IV disease. Only one patient had del(17p), and nearly half of patients tested (17 of 38 patients) had unmutated immunoglobulin heavy-chain variable region gene (IGHV). Six patients had del(11q), including four in the G-FC arm and two in the G-B arm.
Both G-FC and G-B had manageable toxicities, with infusion-related reactions being the most common adverse event, occurring in 88% (20% grade 3 or 4), Dr. Brown and her colleagues reported, adding that grade 3 or 4 neutropenia was seen in 48% of the G-FC arm and 55% of the G-B arm.
The objective response rate (ORR) was 62% for G-FC and 90% for GB.
“The ORR in the G-FC arm likely does not reflect the true activity of the regimen, as it is based on an intent-to-treat analysis,” the investigators said.
With a median observation time of 40.4 months, 95% of patients were alive, and 90% had not experienced a progression-free survival event.
Nine patients in the G-FC arm underwent minimal residual disease (MRD) testing in peripheral blood; 100% had undetectable MRD, according to the report.
“With the caveat of small patient numbers and inevitable differences in patient populations across studies, these results suggest that G-FC may clear residual disease more effectively than rituximab plus FC,” the investigators wrote.
Previous studies of R-FC showed an undetectable MRD rate of 45% or less, they said.
The study was sponsored by Genentech. The investigators reported disclosures related to Genentech/Roche and other companies.
SOURCE: Brown JR et al. Blood. 2018 Dec 28. doi: 10.1182/blood-2018-06-857714.
Two different obinutuzumab-based chemoimmunotherapy regimens resulted in excellent long-term disease control as front-line therapy for chronic lymphocytic leukemia (CLL), investigators said in a follow-up report on a phase 1b study.
Both obinutuzumab plus fludarabine/cyclophosphamide (G-FC) and obinutuzumab plus bendamustine (G-B) were well tolerated, with adverse events similar to what has been reported in rituximab-containing immunotherapy regimens, they said in the report of final results from the GALTON trial.
Most evaluable patients had B-cell recovery by 36 months in the study, which included a population of CLL patients largely without 17p deletions, said Jennifer R. Brown, MD, PhD, of Dana-Farber Cancer Institute, Boston, and her coinvestigators.
“These data support moving forward with these regimens in subsequent trials, which are currently ongoing,” they said in their report on the study, which appears in Blood.
The open-label, parallel-arm, multicenter phase 1b GALTON study included 41 patients with CLL, of whom 21 received G-FC and 20 received G-B for up to six cycles of 28 days each. The median age was 60 years, and about one-third of patients had Rai stage III or IV disease. Only one patient had del(17p), and nearly half of patients tested (17 of 38 patients) had unmutated immunoglobulin heavy-chain variable region gene (IGHV). Six patients had del(11q), including four in the G-FC arm and two in the G-B arm.
Both G-FC and G-B had manageable toxicities, with infusion-related reactions being the most common adverse event, occurring in 88% (20% grade 3 or 4), Dr. Brown and her colleagues reported, adding that grade 3 or 4 neutropenia was seen in 48% of the G-FC arm and 55% of the G-B arm.
The objective response rate (ORR) was 62% for G-FC and 90% for GB.
“The ORR in the G-FC arm likely does not reflect the true activity of the regimen, as it is based on an intent-to-treat analysis,” the investigators said.
With a median observation time of 40.4 months, 95% of patients were alive, and 90% had not experienced a progression-free survival event.
Nine patients in the G-FC arm underwent minimal residual disease (MRD) testing in peripheral blood; 100% had undetectable MRD, according to the report.
“With the caveat of small patient numbers and inevitable differences in patient populations across studies, these results suggest that G-FC may clear residual disease more effectively than rituximab plus FC,” the investigators wrote.
Previous studies of R-FC showed an undetectable MRD rate of 45% or less, they said.
The study was sponsored by Genentech. The investigators reported disclosures related to Genentech/Roche and other companies.
SOURCE: Brown JR et al. Blood. 2018 Dec 28. doi: 10.1182/blood-2018-06-857714.
Two different obinutuzumab-based chemoimmunotherapy regimens resulted in excellent long-term disease control as front-line therapy for chronic lymphocytic leukemia (CLL), investigators said in a follow-up report on a phase 1b study.
Both obinutuzumab plus fludarabine/cyclophosphamide (G-FC) and obinutuzumab plus bendamustine (G-B) were well tolerated, with adverse events similar to what has been reported in rituximab-containing immunotherapy regimens, they said in the report of final results from the GALTON trial.
Most evaluable patients had B-cell recovery by 36 months in the study, which included a population of CLL patients largely without 17p deletions, said Jennifer R. Brown, MD, PhD, of Dana-Farber Cancer Institute, Boston, and her coinvestigators.
“These data support moving forward with these regimens in subsequent trials, which are currently ongoing,” they said in their report on the study, which appears in Blood.
The open-label, parallel-arm, multicenter phase 1b GALTON study included 41 patients with CLL, of whom 21 received G-FC and 20 received G-B for up to six cycles of 28 days each. The median age was 60 years, and about one-third of patients had Rai stage III or IV disease. Only one patient had del(17p), and nearly half of patients tested (17 of 38 patients) had unmutated immunoglobulin heavy-chain variable region gene (IGHV). Six patients had del(11q), including four in the G-FC arm and two in the G-B arm.
Both G-FC and G-B had manageable toxicities, with infusion-related reactions being the most common adverse event, occurring in 88% (20% grade 3 or 4), Dr. Brown and her colleagues reported, adding that grade 3 or 4 neutropenia was seen in 48% of the G-FC arm and 55% of the G-B arm.
The objective response rate (ORR) was 62% for G-FC and 90% for GB.
“The ORR in the G-FC arm likely does not reflect the true activity of the regimen, as it is based on an intent-to-treat analysis,” the investigators said.
With a median observation time of 40.4 months, 95% of patients were alive, and 90% had not experienced a progression-free survival event.
Nine patients in the G-FC arm underwent minimal residual disease (MRD) testing in peripheral blood; 100% had undetectable MRD, according to the report.
“With the caveat of small patient numbers and inevitable differences in patient populations across studies, these results suggest that G-FC may clear residual disease more effectively than rituximab plus FC,” the investigators wrote.
Previous studies of R-FC showed an undetectable MRD rate of 45% or less, they said.
The study was sponsored by Genentech. The investigators reported disclosures related to Genentech/Roche and other companies.
SOURCE: Brown JR et al. Blood. 2018 Dec 28. doi: 10.1182/blood-2018-06-857714.
FROM BLOOD
Key clinical point:
Major finding: With a median observation time of 40.4 months, 95% of patients were alive, and 90% had not experienced a progression-free survival event.
Study details: Long-term follow-up of the phase 1b GALTON trial, including 41 patients with CLL.
Disclosures: The study was sponsored by Genentech. The study authors reported disclosures related to Genentech/Roche and other companies.
Source: Brown JR et al. Blood. 2018 Dec 28. doi: 10.1182/blood-2018-06-857714.
FDA approves new ALL treatment for children, young adults
The in pediatric and young adult patients aged 1 month to 21 years.
Calaspargase pegol-mknl is an asparagine-specific enzyme intended to provide a longer interval between doses, compared with other available pegaspargase products. The recommended dosage of calaspargase pegol-mknl is 2,500 units/m2 given no more frequently than every 21 days.
The FDA said it approved calaspargase pegol-mknl because the drug maintained nadir serum asparaginase activity above the level of 0.1 U/mL when given at 2,500 U/m2 every 3 weeks.
Calaspargase pegol-mknl was evaluated in Study DFCI 11-001, a trial of 237 children and adolescents with newly diagnosed ALL or lymphoblastic lymphoma. The patients’ median age was 5 years.
Study participants received calaspargase pegol-mknl at 2,500 U/m2 (n = 118) or pegaspargase at 2,500 U/m2 (n = 119) as part of a Dana-Farber Cancer Institute ALL Consortium backbone therapy. The median duration of exposure was 8 months for both calaspargase pegol-mknl and pegaspargase. Among the patients with B-cell lineage ALL, the complete remission rate was 98% in the calaspargase pegol-mknl arm and 99% in the pegaspargase arm. Estimated overall survival rates were comparable between the arms.
Common grade 3 or higher adverse events in the calaspargase pegol-mknl and pegaspargase arms included elevated transaminase (52% and 66%, respectively), bilirubin increase (20% and 25%), pancreatitis (18% and 24%), and abnormal clotting studies (14% and 21%). There was one fatal adverse event among patients on calaspargase pegol-mknl – multiorgan failure in the setting of chronic pancreatitis associated with a pancreatic pseudocyst.
The safety of calaspargase pegol-mknl was also evaluated in Study AALL07P4, a trial of patients with newly diagnosed, high-risk B-precursor ALL. The patients received calaspargase pegol-mknl at 2,500 U/m2 (n = 43) or 2,100 U/m2 (n = 68) or pegaspargase at 2,500 U/m2 (n = 52) as a component of an augmented Berlin-Frankfurt-Münster regimen. The patients’ median age was 11 years. The median duration of exposure was 7 months for both calaspargase pegol-mknl and pegaspargase. There were 3 induction deaths among the 111 patients who received calaspargase pegol-mknl (2.8%) but no induction deaths among the 52 patients treated with pegaspargase.
Additional details on these studies and calaspargase pegol-mknl can be found in the drug’s prescribing information. Calaspargase pegol-mknl is a product of Servier.
The in pediatric and young adult patients aged 1 month to 21 years.
Calaspargase pegol-mknl is an asparagine-specific enzyme intended to provide a longer interval between doses, compared with other available pegaspargase products. The recommended dosage of calaspargase pegol-mknl is 2,500 units/m2 given no more frequently than every 21 days.
The FDA said it approved calaspargase pegol-mknl because the drug maintained nadir serum asparaginase activity above the level of 0.1 U/mL when given at 2,500 U/m2 every 3 weeks.
Calaspargase pegol-mknl was evaluated in Study DFCI 11-001, a trial of 237 children and adolescents with newly diagnosed ALL or lymphoblastic lymphoma. The patients’ median age was 5 years.
Study participants received calaspargase pegol-mknl at 2,500 U/m2 (n = 118) or pegaspargase at 2,500 U/m2 (n = 119) as part of a Dana-Farber Cancer Institute ALL Consortium backbone therapy. The median duration of exposure was 8 months for both calaspargase pegol-mknl and pegaspargase. Among the patients with B-cell lineage ALL, the complete remission rate was 98% in the calaspargase pegol-mknl arm and 99% in the pegaspargase arm. Estimated overall survival rates were comparable between the arms.
Common grade 3 or higher adverse events in the calaspargase pegol-mknl and pegaspargase arms included elevated transaminase (52% and 66%, respectively), bilirubin increase (20% and 25%), pancreatitis (18% and 24%), and abnormal clotting studies (14% and 21%). There was one fatal adverse event among patients on calaspargase pegol-mknl – multiorgan failure in the setting of chronic pancreatitis associated with a pancreatic pseudocyst.
The safety of calaspargase pegol-mknl was also evaluated in Study AALL07P4, a trial of patients with newly diagnosed, high-risk B-precursor ALL. The patients received calaspargase pegol-mknl at 2,500 U/m2 (n = 43) or 2,100 U/m2 (n = 68) or pegaspargase at 2,500 U/m2 (n = 52) as a component of an augmented Berlin-Frankfurt-Münster regimen. The patients’ median age was 11 years. The median duration of exposure was 7 months for both calaspargase pegol-mknl and pegaspargase. There were 3 induction deaths among the 111 patients who received calaspargase pegol-mknl (2.8%) but no induction deaths among the 52 patients treated with pegaspargase.
Additional details on these studies and calaspargase pegol-mknl can be found in the drug’s prescribing information. Calaspargase pegol-mknl is a product of Servier.
The in pediatric and young adult patients aged 1 month to 21 years.
Calaspargase pegol-mknl is an asparagine-specific enzyme intended to provide a longer interval between doses, compared with other available pegaspargase products. The recommended dosage of calaspargase pegol-mknl is 2,500 units/m2 given no more frequently than every 21 days.
The FDA said it approved calaspargase pegol-mknl because the drug maintained nadir serum asparaginase activity above the level of 0.1 U/mL when given at 2,500 U/m2 every 3 weeks.
Calaspargase pegol-mknl was evaluated in Study DFCI 11-001, a trial of 237 children and adolescents with newly diagnosed ALL or lymphoblastic lymphoma. The patients’ median age was 5 years.
Study participants received calaspargase pegol-mknl at 2,500 U/m2 (n = 118) or pegaspargase at 2,500 U/m2 (n = 119) as part of a Dana-Farber Cancer Institute ALL Consortium backbone therapy. The median duration of exposure was 8 months for both calaspargase pegol-mknl and pegaspargase. Among the patients with B-cell lineage ALL, the complete remission rate was 98% in the calaspargase pegol-mknl arm and 99% in the pegaspargase arm. Estimated overall survival rates were comparable between the arms.
Common grade 3 or higher adverse events in the calaspargase pegol-mknl and pegaspargase arms included elevated transaminase (52% and 66%, respectively), bilirubin increase (20% and 25%), pancreatitis (18% and 24%), and abnormal clotting studies (14% and 21%). There was one fatal adverse event among patients on calaspargase pegol-mknl – multiorgan failure in the setting of chronic pancreatitis associated with a pancreatic pseudocyst.
The safety of calaspargase pegol-mknl was also evaluated in Study AALL07P4, a trial of patients with newly diagnosed, high-risk B-precursor ALL. The patients received calaspargase pegol-mknl at 2,500 U/m2 (n = 43) or 2,100 U/m2 (n = 68) or pegaspargase at 2,500 U/m2 (n = 52) as a component of an augmented Berlin-Frankfurt-Münster regimen. The patients’ median age was 11 years. The median duration of exposure was 7 months for both calaspargase pegol-mknl and pegaspargase. There were 3 induction deaths among the 111 patients who received calaspargase pegol-mknl (2.8%) but no induction deaths among the 52 patients treated with pegaspargase.
Additional details on these studies and calaspargase pegol-mknl can be found in the drug’s prescribing information. Calaspargase pegol-mknl is a product of Servier.
Chemo for solid tumors and risk of tMDS/AML
Chemotherapy for solid tumors is associated with an increased risk of therapy-related myelodysplastic syndromes or acute myeloid leukemia (tMDS/AML), according to a retrospective analysis.
Long-term, population-based cohort data showed the risk of tMDS/AML was significantly elevated after chemotherapy for 22 solid tumor types.
The relative risk of tMDS/AML was 1.5- to 39.0-fold greater among patients treated for these tumors than among the general population.
Lindsay M. Morton, PhD, of the National Institutes of Health in Rockville, Maryland, and her colleagues reported these findings in JAMA Oncology.
“We undertook an investigation to quantify tMDS/AML risks after chemotherapy for solid tumors in the modern treatment era, 2000-2014, using United States cancer registry data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program,” the investigators wrote.
They retrospectively analyzed data from 1619 patients with tMDS/AML who were diagnosed with an initial primary solid tumor from 2000 to 2013.
Patients were given initial chemotherapy and lived for at least 1 year after treatment. Subsequently, Dr. Morton and her colleagues linked patient database records with Medicare insurance claim information to confirm the accuracy of chemotherapy data.
“Because registry data do not include treatment details, we used an alternative database to provide descriptive information on population-based patterns of chemotherapeutic drug use,” the investigators noted.
The team found the risk of developing tMDS/AML was significantly increased following chemotherapy administration for 22 of 23 solid tumor types, excluding colon cancer.
The standardized incidence ratio (SIR) for tMDS/AML ranged from 1.5 to 39.0, and the excess absolute risk (EAR) ranged from 1.4 to 23.6 cases per 10,000 person-years.
SIRs were greatest in patients who received chemotherapy for malignancy of the bone (SIR=39.0, EAR=23.6), testis (SIR, 12.3, EAR=4.4), soft tissue (SIR=10.4, EAR=12.6), fallopian tube (SIR=8.7, EAR=16.0), small cell lung (SIR=8.1, EAR=19.9), peritoneum (SIR=7.5, EAR=15.8), brain or central nervous system (SIR=7.2, EAR=6.0), and ovary (SIR=5.8, EAR=8.2).
The investigators also found that patients who were given chemotherapy at a young age had the highest risk of developing tMDS/AML.
“For patients treated with chemotherapy at the present time, approximately three-quarters of tMDS/AML cases expected to occur within the next 5 years will be attributable to chemotherapy,” the investigators said.
They acknowledged a key limitation of this study was the limited data on patient-specific chemotherapy and dosing information. Given these limitations, Dr. Morton and her colleagues said, “the exact magnitude of our risk estimates, including the proportions of excess cases, should therefore be interpreted cautiously.”
This study was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, and the California Department of Public Health. The authors reported no conflicts of interest.
Chemotherapy for solid tumors is associated with an increased risk of therapy-related myelodysplastic syndromes or acute myeloid leukemia (tMDS/AML), according to a retrospective analysis.
Long-term, population-based cohort data showed the risk of tMDS/AML was significantly elevated after chemotherapy for 22 solid tumor types.
The relative risk of tMDS/AML was 1.5- to 39.0-fold greater among patients treated for these tumors than among the general population.
Lindsay M. Morton, PhD, of the National Institutes of Health in Rockville, Maryland, and her colleagues reported these findings in JAMA Oncology.
“We undertook an investigation to quantify tMDS/AML risks after chemotherapy for solid tumors in the modern treatment era, 2000-2014, using United States cancer registry data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program,” the investigators wrote.
They retrospectively analyzed data from 1619 patients with tMDS/AML who were diagnosed with an initial primary solid tumor from 2000 to 2013.
Patients were given initial chemotherapy and lived for at least 1 year after treatment. Subsequently, Dr. Morton and her colleagues linked patient database records with Medicare insurance claim information to confirm the accuracy of chemotherapy data.
“Because registry data do not include treatment details, we used an alternative database to provide descriptive information on population-based patterns of chemotherapeutic drug use,” the investigators noted.
The team found the risk of developing tMDS/AML was significantly increased following chemotherapy administration for 22 of 23 solid tumor types, excluding colon cancer.
The standardized incidence ratio (SIR) for tMDS/AML ranged from 1.5 to 39.0, and the excess absolute risk (EAR) ranged from 1.4 to 23.6 cases per 10,000 person-years.
SIRs were greatest in patients who received chemotherapy for malignancy of the bone (SIR=39.0, EAR=23.6), testis (SIR, 12.3, EAR=4.4), soft tissue (SIR=10.4, EAR=12.6), fallopian tube (SIR=8.7, EAR=16.0), small cell lung (SIR=8.1, EAR=19.9), peritoneum (SIR=7.5, EAR=15.8), brain or central nervous system (SIR=7.2, EAR=6.0), and ovary (SIR=5.8, EAR=8.2).
The investigators also found that patients who were given chemotherapy at a young age had the highest risk of developing tMDS/AML.
“For patients treated with chemotherapy at the present time, approximately three-quarters of tMDS/AML cases expected to occur within the next 5 years will be attributable to chemotherapy,” the investigators said.
They acknowledged a key limitation of this study was the limited data on patient-specific chemotherapy and dosing information. Given these limitations, Dr. Morton and her colleagues said, “the exact magnitude of our risk estimates, including the proportions of excess cases, should therefore be interpreted cautiously.”
This study was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, and the California Department of Public Health. The authors reported no conflicts of interest.
Chemotherapy for solid tumors is associated with an increased risk of therapy-related myelodysplastic syndromes or acute myeloid leukemia (tMDS/AML), according to a retrospective analysis.
Long-term, population-based cohort data showed the risk of tMDS/AML was significantly elevated after chemotherapy for 22 solid tumor types.
The relative risk of tMDS/AML was 1.5- to 39.0-fold greater among patients treated for these tumors than among the general population.
Lindsay M. Morton, PhD, of the National Institutes of Health in Rockville, Maryland, and her colleagues reported these findings in JAMA Oncology.
“We undertook an investigation to quantify tMDS/AML risks after chemotherapy for solid tumors in the modern treatment era, 2000-2014, using United States cancer registry data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program,” the investigators wrote.
They retrospectively analyzed data from 1619 patients with tMDS/AML who were diagnosed with an initial primary solid tumor from 2000 to 2013.
Patients were given initial chemotherapy and lived for at least 1 year after treatment. Subsequently, Dr. Morton and her colleagues linked patient database records with Medicare insurance claim information to confirm the accuracy of chemotherapy data.
“Because registry data do not include treatment details, we used an alternative database to provide descriptive information on population-based patterns of chemotherapeutic drug use,” the investigators noted.
The team found the risk of developing tMDS/AML was significantly increased following chemotherapy administration for 22 of 23 solid tumor types, excluding colon cancer.
The standardized incidence ratio (SIR) for tMDS/AML ranged from 1.5 to 39.0, and the excess absolute risk (EAR) ranged from 1.4 to 23.6 cases per 10,000 person-years.
SIRs were greatest in patients who received chemotherapy for malignancy of the bone (SIR=39.0, EAR=23.6), testis (SIR, 12.3, EAR=4.4), soft tissue (SIR=10.4, EAR=12.6), fallopian tube (SIR=8.7, EAR=16.0), small cell lung (SIR=8.1, EAR=19.9), peritoneum (SIR=7.5, EAR=15.8), brain or central nervous system (SIR=7.2, EAR=6.0), and ovary (SIR=5.8, EAR=8.2).
The investigators also found that patients who were given chemotherapy at a young age had the highest risk of developing tMDS/AML.
“For patients treated with chemotherapy at the present time, approximately three-quarters of tMDS/AML cases expected to occur within the next 5 years will be attributable to chemotherapy,” the investigators said.
They acknowledged a key limitation of this study was the limited data on patient-specific chemotherapy and dosing information. Given these limitations, Dr. Morton and her colleagues said, “the exact magnitude of our risk estimates, including the proportions of excess cases, should therefore be interpreted cautiously.”
This study was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, and the California Department of Public Health. The authors reported no conflicts of interest.
ALL chemotherapy looks effective in mixed phenotype leukemia
SAN DIEGO – The majority of pediatric patients with mixed phenotype acute leukemia (MPAL) who were treated with acute lymphoblastic leukemia (ALL)–directed chemotherapy achieved a minimum residual disease (MRD)–negative complete response by the end of consolidation, according to findings from a multicenter retrospective cohort study.
The cohort included 94 patients aged 1-21 years who met strict World Health Organization MPAL criteria and were treated between 2008 and 2016 at one of six U.S. institutions. Most had B/myeloid phenotype (89%), and 87 patients were treated with an ALL regimen, Etan Orgel, MD, reported at the annual meeting of the American Society of Hematology.
Of those 87 patients, 81 (93%) experienced an end-of-induction (EOI) complete response. One patient died during induction and six had induction failures, defined as either disease progression before EOI (two patients) or EOI MRD of 5% or greater (three patients), said Dr. Orgel of the University of Southern California, Los Angeles, and Children’s Hospital Los Angeles.
The MRD-negative rates, defined as MRD less than 0.01%, were 70% at EOI and 86% at EOI or end of consolidation (EOC); 12 of 14 patients who were MRD positive at EOI and continued on ALL therapy achieved an EOC MRD-negative complete response, including 8 of 8 with EOI MRD of 0.01%-0.09% and 4 of 6 with EOI MRD of 1% or greater.
Event-free survival at 5 years in the 78 patients without hematopoietic stem cell transplant at first remission was 75%, and 5-year overall survival was 89%, “thus demonstrating that, for a majority of patients, transplant in first remission may not be necessary,” Dr. Orgel said. “This is very different from the approach used at many adult centers and many of the adult recommendations.”
Overall 5-year EOI event-free survival was 80% in the 59 patients who were MRD negative at EOI, and 13% in 25 patients who were MRD-positive at EOI. The corresponding overall survival rates were 91% and 84%.
Overall 5-year EOC event-free survival was 77% in 74 patients who were MRD negative at EOC and was unavailable in 3 patients who were MRD positive at EOC, although all three were salvaged. The corresponding EOC overall survival rates were 89% and “not available,” Dr. Orgel reported.
Multivariable analysis confirmed the predictive value of MRD at EOI (hazard ratio for event-free survival and overall survival, 3.77 and 3.54, respectively).
Of note, there was a possible trend toward earlier failure and a trend toward worse overall survival (HR, 4.49, P = .074) for T-lineage–containing MPAL.
“That indicates that this might be a group that needs careful scrutiny of which form of ALL therapy they receive,” he said.
MRD in pediatric MPAL is rare. Recent studies of MPAL biology show areas of similarity with ALL and AML, and while this could eventually help further subcategorize or classify the disease and lead to biology-driven therapies, it is important to know how to treat the disease today, Dr. Orgel said.
The evolving consensus is that ALL therapy is adequate for most MPAL, but there is no established threshold for MRD to enable a risk-stratified MPAL approach, he added.
The current findings suggest that ALL therapy – without hematopoietic stem cell transplant – may be sufficient to treat most patients with pediatric MPAL, Dr. Orgen reported, noting that clinical trials are necessary to prospectively validate MRD thresholds at EOI and EOC and to establish the threshold for favorable survival.
“Future research should explore either intensification of therapy or different therapies for patients with persistent MRD,” he said.
Dr. Orgel reported having no financial disclosures.
SOURCE: Oberley M et al. ASH 2018, Abstract 558.
SAN DIEGO – The majority of pediatric patients with mixed phenotype acute leukemia (MPAL) who were treated with acute lymphoblastic leukemia (ALL)–directed chemotherapy achieved a minimum residual disease (MRD)–negative complete response by the end of consolidation, according to findings from a multicenter retrospective cohort study.
The cohort included 94 patients aged 1-21 years who met strict World Health Organization MPAL criteria and were treated between 2008 and 2016 at one of six U.S. institutions. Most had B/myeloid phenotype (89%), and 87 patients were treated with an ALL regimen, Etan Orgel, MD, reported at the annual meeting of the American Society of Hematology.
Of those 87 patients, 81 (93%) experienced an end-of-induction (EOI) complete response. One patient died during induction and six had induction failures, defined as either disease progression before EOI (two patients) or EOI MRD of 5% or greater (three patients), said Dr. Orgel of the University of Southern California, Los Angeles, and Children’s Hospital Los Angeles.
The MRD-negative rates, defined as MRD less than 0.01%, were 70% at EOI and 86% at EOI or end of consolidation (EOC); 12 of 14 patients who were MRD positive at EOI and continued on ALL therapy achieved an EOC MRD-negative complete response, including 8 of 8 with EOI MRD of 0.01%-0.09% and 4 of 6 with EOI MRD of 1% or greater.
Event-free survival at 5 years in the 78 patients without hematopoietic stem cell transplant at first remission was 75%, and 5-year overall survival was 89%, “thus demonstrating that, for a majority of patients, transplant in first remission may not be necessary,” Dr. Orgel said. “This is very different from the approach used at many adult centers and many of the adult recommendations.”
Overall 5-year EOI event-free survival was 80% in the 59 patients who were MRD negative at EOI, and 13% in 25 patients who were MRD-positive at EOI. The corresponding overall survival rates were 91% and 84%.
Overall 5-year EOC event-free survival was 77% in 74 patients who were MRD negative at EOC and was unavailable in 3 patients who were MRD positive at EOC, although all three were salvaged. The corresponding EOC overall survival rates were 89% and “not available,” Dr. Orgel reported.
Multivariable analysis confirmed the predictive value of MRD at EOI (hazard ratio for event-free survival and overall survival, 3.77 and 3.54, respectively).
Of note, there was a possible trend toward earlier failure and a trend toward worse overall survival (HR, 4.49, P = .074) for T-lineage–containing MPAL.
“That indicates that this might be a group that needs careful scrutiny of which form of ALL therapy they receive,” he said.
MRD in pediatric MPAL is rare. Recent studies of MPAL biology show areas of similarity with ALL and AML, and while this could eventually help further subcategorize or classify the disease and lead to biology-driven therapies, it is important to know how to treat the disease today, Dr. Orgel said.
The evolving consensus is that ALL therapy is adequate for most MPAL, but there is no established threshold for MRD to enable a risk-stratified MPAL approach, he added.
The current findings suggest that ALL therapy – without hematopoietic stem cell transplant – may be sufficient to treat most patients with pediatric MPAL, Dr. Orgen reported, noting that clinical trials are necessary to prospectively validate MRD thresholds at EOI and EOC and to establish the threshold for favorable survival.
“Future research should explore either intensification of therapy or different therapies for patients with persistent MRD,” he said.
Dr. Orgel reported having no financial disclosures.
SOURCE: Oberley M et al. ASH 2018, Abstract 558.
SAN DIEGO – The majority of pediatric patients with mixed phenotype acute leukemia (MPAL) who were treated with acute lymphoblastic leukemia (ALL)–directed chemotherapy achieved a minimum residual disease (MRD)–negative complete response by the end of consolidation, according to findings from a multicenter retrospective cohort study.
The cohort included 94 patients aged 1-21 years who met strict World Health Organization MPAL criteria and were treated between 2008 and 2016 at one of six U.S. institutions. Most had B/myeloid phenotype (89%), and 87 patients were treated with an ALL regimen, Etan Orgel, MD, reported at the annual meeting of the American Society of Hematology.
Of those 87 patients, 81 (93%) experienced an end-of-induction (EOI) complete response. One patient died during induction and six had induction failures, defined as either disease progression before EOI (two patients) or EOI MRD of 5% or greater (three patients), said Dr. Orgel of the University of Southern California, Los Angeles, and Children’s Hospital Los Angeles.
The MRD-negative rates, defined as MRD less than 0.01%, were 70% at EOI and 86% at EOI or end of consolidation (EOC); 12 of 14 patients who were MRD positive at EOI and continued on ALL therapy achieved an EOC MRD-negative complete response, including 8 of 8 with EOI MRD of 0.01%-0.09% and 4 of 6 with EOI MRD of 1% or greater.
Event-free survival at 5 years in the 78 patients without hematopoietic stem cell transplant at first remission was 75%, and 5-year overall survival was 89%, “thus demonstrating that, for a majority of patients, transplant in first remission may not be necessary,” Dr. Orgel said. “This is very different from the approach used at many adult centers and many of the adult recommendations.”
Overall 5-year EOI event-free survival was 80% in the 59 patients who were MRD negative at EOI, and 13% in 25 patients who were MRD-positive at EOI. The corresponding overall survival rates were 91% and 84%.
Overall 5-year EOC event-free survival was 77% in 74 patients who were MRD negative at EOC and was unavailable in 3 patients who were MRD positive at EOC, although all three were salvaged. The corresponding EOC overall survival rates were 89% and “not available,” Dr. Orgel reported.
Multivariable analysis confirmed the predictive value of MRD at EOI (hazard ratio for event-free survival and overall survival, 3.77 and 3.54, respectively).
Of note, there was a possible trend toward earlier failure and a trend toward worse overall survival (HR, 4.49, P = .074) for T-lineage–containing MPAL.
“That indicates that this might be a group that needs careful scrutiny of which form of ALL therapy they receive,” he said.
MRD in pediatric MPAL is rare. Recent studies of MPAL biology show areas of similarity with ALL and AML, and while this could eventually help further subcategorize or classify the disease and lead to biology-driven therapies, it is important to know how to treat the disease today, Dr. Orgel said.
The evolving consensus is that ALL therapy is adequate for most MPAL, but there is no established threshold for MRD to enable a risk-stratified MPAL approach, he added.
The current findings suggest that ALL therapy – without hematopoietic stem cell transplant – may be sufficient to treat most patients with pediatric MPAL, Dr. Orgen reported, noting that clinical trials are necessary to prospectively validate MRD thresholds at EOI and EOC and to establish the threshold for favorable survival.
“Future research should explore either intensification of therapy or different therapies for patients with persistent MRD,” he said.
Dr. Orgel reported having no financial disclosures.
SOURCE: Oberley M et al. ASH 2018, Abstract 558.
REPORTING FROM ASH 2018
Key clinical point:
Major finding: MRD-negative rates were 70% at end of induction and 86% at end of induction or consolidation.
Study details: A retrospective cohort study of 87 pediatric MPAL patients.
Disclosures: Dr. Orgel reported having no financial disclosures.
Source: Oberley M et al. ASH 2018, Abstract 558.
Higher AML, MDS risk linked to solid tumor chemotherapy
There is an increased risk for therapy-related myelodysplastic syndrome or acute myeloid leukemia (tMDS/AML) following chemotherapy for the majority of solid tumor types, according to an analysis of cancer registry data.
These findings suggest a substantial expansion in the patients at risk for tMDS/AML because, in the past, excess risks were established only after chemotherapy for cancers of the lung, ovary, breast, soft tissue, testis, and brain or central nervous system,” Lindsay M. Morton, PhD, of the National Institutes of Health, and her colleagues wrote in JAMA Oncology.
The researchers retrospectively analyzed data from 1,619 patients with tMDS/AML who were diagnosed with an initial primary solid tumor from 2000 to 2013. Data came from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Program and Medicare claims.
Study participants were given initial chemotherapy and lived for at least 1 year after treatment. Subsequently, Dr. Morton and her colleagues linked patient database records with Medicare insurance claim information to confirm the accuracy of chemotherapy data.
“Because registry data [does] not include treatment details, we used an alternative database to provide descriptive information on population-based patterns of chemotherapeutic drug use,” the researchers wrote in JAMA Oncology.
After statistical analysis, the researchers found that the risk of developing tMDS/AML was significantly elevated following chemotherapy administration for 22 of 23 solid tumor types, excluding colon cancer. They reported a 1.5-fold to more than 10-fold increased relative risk for tMDS/AML in those patients who received chemotherapy for those 22 solid cancer types, compared with the general population.
The relative risks were highest after chemotherapy for bone, soft-tissue, and testis cancers.
The researchers found that the absolute risk of developing tMDS/AML was low. Excess absolute risks ranged from 1.4 to greater than 15 cases per 10,000 person-years, compared with the general population, in those 22 solid cancer types. The greatest absolute risks were for peritoneum, small-cell lung, bone, soft-tissue, and fallopian tube cancers.
“For patients treated with chemotherapy at the present time, approximately three-quarters of tMDS/AML cases expected to occur within the next 5 years will be attributable to chemotherapy,” they added.
The researchers acknowledged a key limitation of the study was the limited data on dosing and patient-specific chemotherapy. As a result, Dr. Morton and her colleagues called for a cautious interpretation of the magnitude of the risk.
The study was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, and the California Department of Public Health. The authors reported having no conflicts of interest.
SOURCE: Morton LM et al. JAMA Oncol. 2018 Dec 20. doi: 10.1001/jamaoncol.2018.5625.
Possibly the most clinical relevant finding of the study by Lindsay M. Morton, PhD, and her colleagues is that patients who received chemotherapy for solid tumor treatment at a younger age were at the highest relative risk for tMDS/AML.
The incidence of tMDS/AML was highest among patients treated with chemotherapy for bone, soft-tissue, and testicular cancers, where the median age of onset is often by 30 years, and the mean onset occurs before age 50.
The researchers also noted an increased risk for tMDS/AML associated with prolonged survival from primary tumors.
Going forward, research should consider those patients at highest risk for tMDS/AML and risk-assessment models for these therapy-related myeloid neoplasms should take into account the clonal evolution of subclinical mutations into overt disease.
The study findings point to the unanswered question of how best to perform risk assessment of chemotherapy in solid tumors. That risk stratification could include the probability of the specific chemotherapy agent initiating disease, the benefit of tumor regression from chemotherapy, and the potential consequences of tumor progression if chemotherapy is not administered.
Shyam A. Patel, MD, PhD, is with the department of medicine at Stanford (Calif.) University. Dr. Patel reported having no financial disclosures. These comments are adapted from his accompanying editorial (JAMA Oncol. 2018 Dec 20. doi: 10.1001/jamaoncol.2018.5617 ).
Possibly the most clinical relevant finding of the study by Lindsay M. Morton, PhD, and her colleagues is that patients who received chemotherapy for solid tumor treatment at a younger age were at the highest relative risk for tMDS/AML.
The incidence of tMDS/AML was highest among patients treated with chemotherapy for bone, soft-tissue, and testicular cancers, where the median age of onset is often by 30 years, and the mean onset occurs before age 50.
The researchers also noted an increased risk for tMDS/AML associated with prolonged survival from primary tumors.
Going forward, research should consider those patients at highest risk for tMDS/AML and risk-assessment models for these therapy-related myeloid neoplasms should take into account the clonal evolution of subclinical mutations into overt disease.
The study findings point to the unanswered question of how best to perform risk assessment of chemotherapy in solid tumors. That risk stratification could include the probability of the specific chemotherapy agent initiating disease, the benefit of tumor regression from chemotherapy, and the potential consequences of tumor progression if chemotherapy is not administered.
Shyam A. Patel, MD, PhD, is with the department of medicine at Stanford (Calif.) University. Dr. Patel reported having no financial disclosures. These comments are adapted from his accompanying editorial (JAMA Oncol. 2018 Dec 20. doi: 10.1001/jamaoncol.2018.5617 ).
Possibly the most clinical relevant finding of the study by Lindsay M. Morton, PhD, and her colleagues is that patients who received chemotherapy for solid tumor treatment at a younger age were at the highest relative risk for tMDS/AML.
The incidence of tMDS/AML was highest among patients treated with chemotherapy for bone, soft-tissue, and testicular cancers, where the median age of onset is often by 30 years, and the mean onset occurs before age 50.
The researchers also noted an increased risk for tMDS/AML associated with prolonged survival from primary tumors.
Going forward, research should consider those patients at highest risk for tMDS/AML and risk-assessment models for these therapy-related myeloid neoplasms should take into account the clonal evolution of subclinical mutations into overt disease.
The study findings point to the unanswered question of how best to perform risk assessment of chemotherapy in solid tumors. That risk stratification could include the probability of the specific chemotherapy agent initiating disease, the benefit of tumor regression from chemotherapy, and the potential consequences of tumor progression if chemotherapy is not administered.
Shyam A. Patel, MD, PhD, is with the department of medicine at Stanford (Calif.) University. Dr. Patel reported having no financial disclosures. These comments are adapted from his accompanying editorial (JAMA Oncol. 2018 Dec 20. doi: 10.1001/jamaoncol.2018.5617 ).
There is an increased risk for therapy-related myelodysplastic syndrome or acute myeloid leukemia (tMDS/AML) following chemotherapy for the majority of solid tumor types, according to an analysis of cancer registry data.
These findings suggest a substantial expansion in the patients at risk for tMDS/AML because, in the past, excess risks were established only after chemotherapy for cancers of the lung, ovary, breast, soft tissue, testis, and brain or central nervous system,” Lindsay M. Morton, PhD, of the National Institutes of Health, and her colleagues wrote in JAMA Oncology.
The researchers retrospectively analyzed data from 1,619 patients with tMDS/AML who were diagnosed with an initial primary solid tumor from 2000 to 2013. Data came from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Program and Medicare claims.
Study participants were given initial chemotherapy and lived for at least 1 year after treatment. Subsequently, Dr. Morton and her colleagues linked patient database records with Medicare insurance claim information to confirm the accuracy of chemotherapy data.
“Because registry data [does] not include treatment details, we used an alternative database to provide descriptive information on population-based patterns of chemotherapeutic drug use,” the researchers wrote in JAMA Oncology.
After statistical analysis, the researchers found that the risk of developing tMDS/AML was significantly elevated following chemotherapy administration for 22 of 23 solid tumor types, excluding colon cancer. They reported a 1.5-fold to more than 10-fold increased relative risk for tMDS/AML in those patients who received chemotherapy for those 22 solid cancer types, compared with the general population.
The relative risks were highest after chemotherapy for bone, soft-tissue, and testis cancers.
The researchers found that the absolute risk of developing tMDS/AML was low. Excess absolute risks ranged from 1.4 to greater than 15 cases per 10,000 person-years, compared with the general population, in those 22 solid cancer types. The greatest absolute risks were for peritoneum, small-cell lung, bone, soft-tissue, and fallopian tube cancers.
“For patients treated with chemotherapy at the present time, approximately three-quarters of tMDS/AML cases expected to occur within the next 5 years will be attributable to chemotherapy,” they added.
The researchers acknowledged a key limitation of the study was the limited data on dosing and patient-specific chemotherapy. As a result, Dr. Morton and her colleagues called for a cautious interpretation of the magnitude of the risk.
The study was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, and the California Department of Public Health. The authors reported having no conflicts of interest.
SOURCE: Morton LM et al. JAMA Oncol. 2018 Dec 20. doi: 10.1001/jamaoncol.2018.5625.
There is an increased risk for therapy-related myelodysplastic syndrome or acute myeloid leukemia (tMDS/AML) following chemotherapy for the majority of solid tumor types, according to an analysis of cancer registry data.
These findings suggest a substantial expansion in the patients at risk for tMDS/AML because, in the past, excess risks were established only after chemotherapy for cancers of the lung, ovary, breast, soft tissue, testis, and brain or central nervous system,” Lindsay M. Morton, PhD, of the National Institutes of Health, and her colleagues wrote in JAMA Oncology.
The researchers retrospectively analyzed data from 1,619 patients with tMDS/AML who were diagnosed with an initial primary solid tumor from 2000 to 2013. Data came from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Program and Medicare claims.
Study participants were given initial chemotherapy and lived for at least 1 year after treatment. Subsequently, Dr. Morton and her colleagues linked patient database records with Medicare insurance claim information to confirm the accuracy of chemotherapy data.
“Because registry data [does] not include treatment details, we used an alternative database to provide descriptive information on population-based patterns of chemotherapeutic drug use,” the researchers wrote in JAMA Oncology.
After statistical analysis, the researchers found that the risk of developing tMDS/AML was significantly elevated following chemotherapy administration for 22 of 23 solid tumor types, excluding colon cancer. They reported a 1.5-fold to more than 10-fold increased relative risk for tMDS/AML in those patients who received chemotherapy for those 22 solid cancer types, compared with the general population.
The relative risks were highest after chemotherapy for bone, soft-tissue, and testis cancers.
The researchers found that the absolute risk of developing tMDS/AML was low. Excess absolute risks ranged from 1.4 to greater than 15 cases per 10,000 person-years, compared with the general population, in those 22 solid cancer types. The greatest absolute risks were for peritoneum, small-cell lung, bone, soft-tissue, and fallopian tube cancers.
“For patients treated with chemotherapy at the present time, approximately three-quarters of tMDS/AML cases expected to occur within the next 5 years will be attributable to chemotherapy,” they added.
The researchers acknowledged a key limitation of the study was the limited data on dosing and patient-specific chemotherapy. As a result, Dr. Morton and her colleagues called for a cautious interpretation of the magnitude of the risk.
The study was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, and the California Department of Public Health. The authors reported having no conflicts of interest.
SOURCE: Morton LM et al. JAMA Oncol. 2018 Dec 20. doi: 10.1001/jamaoncol.2018.5625.
FROM JAMA ONCOLOGY
Key clinical point:
Major finding: Treatment with chemotherapy was linked with a 1.5-fold to more than 10-fold increased risk for tMDS/AML.
Study details: A retrospective analysis of 1,619 patients with tMDS/AML who were diagnosed with an initial primary solid tumor from 2000 to 2013.
Disclosures: The study was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, and the California Department of Public Health. The authors reported having no conflicts of interest.
Source: Morton LM et al. JAMA Oncol. 2018 Dec 20. doi: 10.1001/jamaoncol.2018.5625.
FDA expands dasatinib indication to children with Ph+ ALL
The .
The tyrosine kinase inhibitor is now approved for use in combination with chemotherapy to treat pediatric patients aged 1 year and older who have newly diagnosed, Philadelphia-chromosome-positive (Ph+) acute lymphoblastic leukemia (ALL).
Dasatinib is already approved for use in children aged 1 year and older who have chronic phase, Ph+ chronic myeloid leukemia (CML).
In adults, dasatinib is approved to treat newly diagnosed, Ph+, chronic phase CML; chronic, accelerated, or myeloid/lymphoid blast phase, Ph+ CML with resistance or intolerance to prior therapy including imatinib; and Ph+ ALL with resistance or intolerance to prior therapy. The approval in children with Ph+ ALL is based on data from a phase 2 study (CA180-372, NCT01460160).
In this trial, researchers evaluated dasatinib in combination with the AIEOP-BFM ALL 2000 multi-agent chemotherapy protocol in patients (aged 1-17 years) with newly diagnosed, B-cell precursor, Ph+ ALL.
There were 78 patients evaluated for efficacy in cohort 1. They received dasatinib at a daily dose of 60 mg/m2 for up to 24 months.
Patients with central nervous system 3 disease received cranial irradiation, and patients were assigned to stem cell transplant based on minimal residual disease if they were thought to have a high risk of relapse.
The 3-year event-free survival rate in the 78 patients was 64.1%.
There were 81 patients evaluable for safety who received dasatinib continuously in combination with chemotherapy. Their median duration of treatment was 24 months.
The most common adverse events (AEs) in these patients were mucositis, febrile neutropenia, pyrexia, diarrhea, nausea, vomiting, musculoskeletal pain, abdominal pain, cough, headache, rash, fatigue, and constipation.
Eight patients (10%) had AEs leading to treatment discontinuation. These included fungal sepsis, hepatotoxicity in the setting of graft-versus-host disease, thrombocytopenia, cytomegalovirus infection, pneumonia, nausea, enteritis, and drug hypersensitivity.
Three patients (4%) had fatal AEs, all infections.
This trial was sponsored by Bristol-Myers Squibb. Additional data are available in the prescribing information for dasatinib.
The .
The tyrosine kinase inhibitor is now approved for use in combination with chemotherapy to treat pediatric patients aged 1 year and older who have newly diagnosed, Philadelphia-chromosome-positive (Ph+) acute lymphoblastic leukemia (ALL).
Dasatinib is already approved for use in children aged 1 year and older who have chronic phase, Ph+ chronic myeloid leukemia (CML).
In adults, dasatinib is approved to treat newly diagnosed, Ph+, chronic phase CML; chronic, accelerated, or myeloid/lymphoid blast phase, Ph+ CML with resistance or intolerance to prior therapy including imatinib; and Ph+ ALL with resistance or intolerance to prior therapy. The approval in children with Ph+ ALL is based on data from a phase 2 study (CA180-372, NCT01460160).
In this trial, researchers evaluated dasatinib in combination with the AIEOP-BFM ALL 2000 multi-agent chemotherapy protocol in patients (aged 1-17 years) with newly diagnosed, B-cell precursor, Ph+ ALL.
There were 78 patients evaluated for efficacy in cohort 1. They received dasatinib at a daily dose of 60 mg/m2 for up to 24 months.
Patients with central nervous system 3 disease received cranial irradiation, and patients were assigned to stem cell transplant based on minimal residual disease if they were thought to have a high risk of relapse.
The 3-year event-free survival rate in the 78 patients was 64.1%.
There were 81 patients evaluable for safety who received dasatinib continuously in combination with chemotherapy. Their median duration of treatment was 24 months.
The most common adverse events (AEs) in these patients were mucositis, febrile neutropenia, pyrexia, diarrhea, nausea, vomiting, musculoskeletal pain, abdominal pain, cough, headache, rash, fatigue, and constipation.
Eight patients (10%) had AEs leading to treatment discontinuation. These included fungal sepsis, hepatotoxicity in the setting of graft-versus-host disease, thrombocytopenia, cytomegalovirus infection, pneumonia, nausea, enteritis, and drug hypersensitivity.
Three patients (4%) had fatal AEs, all infections.
This trial was sponsored by Bristol-Myers Squibb. Additional data are available in the prescribing information for dasatinib.
The .
The tyrosine kinase inhibitor is now approved for use in combination with chemotherapy to treat pediatric patients aged 1 year and older who have newly diagnosed, Philadelphia-chromosome-positive (Ph+) acute lymphoblastic leukemia (ALL).
Dasatinib is already approved for use in children aged 1 year and older who have chronic phase, Ph+ chronic myeloid leukemia (CML).
In adults, dasatinib is approved to treat newly diagnosed, Ph+, chronic phase CML; chronic, accelerated, or myeloid/lymphoid blast phase, Ph+ CML with resistance or intolerance to prior therapy including imatinib; and Ph+ ALL with resistance or intolerance to prior therapy. The approval in children with Ph+ ALL is based on data from a phase 2 study (CA180-372, NCT01460160).
In this trial, researchers evaluated dasatinib in combination with the AIEOP-BFM ALL 2000 multi-agent chemotherapy protocol in patients (aged 1-17 years) with newly diagnosed, B-cell precursor, Ph+ ALL.
There were 78 patients evaluated for efficacy in cohort 1. They received dasatinib at a daily dose of 60 mg/m2 for up to 24 months.
Patients with central nervous system 3 disease received cranial irradiation, and patients were assigned to stem cell transplant based on minimal residual disease if they were thought to have a high risk of relapse.
The 3-year event-free survival rate in the 78 patients was 64.1%.
There were 81 patients evaluable for safety who received dasatinib continuously in combination with chemotherapy. Their median duration of treatment was 24 months.
The most common adverse events (AEs) in these patients were mucositis, febrile neutropenia, pyrexia, diarrhea, nausea, vomiting, musculoskeletal pain, abdominal pain, cough, headache, rash, fatigue, and constipation.
Eight patients (10%) had AEs leading to treatment discontinuation. These included fungal sepsis, hepatotoxicity in the setting of graft-versus-host disease, thrombocytopenia, cytomegalovirus infection, pneumonia, nausea, enteritis, and drug hypersensitivity.
Three patients (4%) had fatal AEs, all infections.
This trial was sponsored by Bristol-Myers Squibb. Additional data are available in the prescribing information for dasatinib.
FDA approves dasatinib for kids with Ph+ ALL
The U.S. Food and Drug Administration (FDA) has approved a second pediatric indication for dasatinib (Sprycel®).
The tyrosine kinase inhibitor is now approved for use in combination with chemotherapy to treat pediatric patients age 1 year and older who have newly diagnosed, Philadelphia-chromosome-positive (Ph+) acute lymphoblastic leukemia (ALL).
Dasatinib is also FDA-approved for use in children age 1 year and older who have chronic phase, Ph+ chronic myeloid leukemia (CML).
In adults, dasatinib is FDA-approved to treat:
- Newly diagnosed, Ph+, chronic phase CML
- Chronic, accelerated, or myeloid/lymphoid blast phase, Ph+ CML with resistance or intolerance to prior therapy including imatinib
- Ph+ ALL with resistance or intolerance to prior therapy.
Trial results
The FDA’s approval of dasatinib in children with Ph+ ALL is based on data from a phase 2 study (CA180-372, NCT01460160).
In this trial, researchers evaluated dasatinib in combination with the AIEOP-BFM ALL 2000 chemotherapy protocol in patients (ages 1 to 17) with newly diagnosed, B-cell precursor, Ph+ ALL.
There were 78 patients evaluated for efficacy in cohort 1. They had a median age of 10.4 years (range, 2.6 to 17.9 years). They received dasatinib at a daily dose of 60 mg/m2 for up to 24 months.
Patients with central nervous system 3 disease received cranial irradiation, and patients were assigned to stem cell transplant based on minimal residual disease if they were thought to have a high risk of relapse.
The 3-year event-free survival rate in the 78 patients was 64.1%.
There were 81 patients evaluable for safety who received dasatinib continuously in combination with chemotherapy. Their median duration of treatment was 24 months (range, 2 to 27 months).
The most common adverse events (AEs) in these patients were mucositis (93%), febrile neutropenia (86%), pyrexia (85%), diarrhea (84%), nausea (84%), vomiting (83%), musculoskeletal pain (83%), abdominal pain (78%), cough (78%), headache (77%), rash (68%), fatigue (59%), and constipation (57%).
Eight (10%) patients had AEs leading to treatment discontinuation. These included fungal sepsis, hepatotoxicity in the setting of graft-versus-host disease, thrombocytopenia, cytomegalovirus infection, pneumonia, nausea, enteritis, and drug hypersensitivity.
Three patients (4%) had fatal AEs, all infections.
This trial was sponsored by Bristol-Myers Squibb. Additional data are available in the prescribing information for dasatinib.
The U.S. Food and Drug Administration (FDA) has approved a second pediatric indication for dasatinib (Sprycel®).
The tyrosine kinase inhibitor is now approved for use in combination with chemotherapy to treat pediatric patients age 1 year and older who have newly diagnosed, Philadelphia-chromosome-positive (Ph+) acute lymphoblastic leukemia (ALL).
Dasatinib is also FDA-approved for use in children age 1 year and older who have chronic phase, Ph+ chronic myeloid leukemia (CML).
In adults, dasatinib is FDA-approved to treat:
- Newly diagnosed, Ph+, chronic phase CML
- Chronic, accelerated, or myeloid/lymphoid blast phase, Ph+ CML with resistance or intolerance to prior therapy including imatinib
- Ph+ ALL with resistance or intolerance to prior therapy.
Trial results
The FDA’s approval of dasatinib in children with Ph+ ALL is based on data from a phase 2 study (CA180-372, NCT01460160).
In this trial, researchers evaluated dasatinib in combination with the AIEOP-BFM ALL 2000 chemotherapy protocol in patients (ages 1 to 17) with newly diagnosed, B-cell precursor, Ph+ ALL.
There were 78 patients evaluated for efficacy in cohort 1. They had a median age of 10.4 years (range, 2.6 to 17.9 years). They received dasatinib at a daily dose of 60 mg/m2 for up to 24 months.
Patients with central nervous system 3 disease received cranial irradiation, and patients were assigned to stem cell transplant based on minimal residual disease if they were thought to have a high risk of relapse.
The 3-year event-free survival rate in the 78 patients was 64.1%.
There were 81 patients evaluable for safety who received dasatinib continuously in combination with chemotherapy. Their median duration of treatment was 24 months (range, 2 to 27 months).
The most common adverse events (AEs) in these patients were mucositis (93%), febrile neutropenia (86%), pyrexia (85%), diarrhea (84%), nausea (84%), vomiting (83%), musculoskeletal pain (83%), abdominal pain (78%), cough (78%), headache (77%), rash (68%), fatigue (59%), and constipation (57%).
Eight (10%) patients had AEs leading to treatment discontinuation. These included fungal sepsis, hepatotoxicity in the setting of graft-versus-host disease, thrombocytopenia, cytomegalovirus infection, pneumonia, nausea, enteritis, and drug hypersensitivity.
Three patients (4%) had fatal AEs, all infections.
This trial was sponsored by Bristol-Myers Squibb. Additional data are available in the prescribing information for dasatinib.
The U.S. Food and Drug Administration (FDA) has approved a second pediatric indication for dasatinib (Sprycel®).
The tyrosine kinase inhibitor is now approved for use in combination with chemotherapy to treat pediatric patients age 1 year and older who have newly diagnosed, Philadelphia-chromosome-positive (Ph+) acute lymphoblastic leukemia (ALL).
Dasatinib is also FDA-approved for use in children age 1 year and older who have chronic phase, Ph+ chronic myeloid leukemia (CML).
In adults, dasatinib is FDA-approved to treat:
- Newly diagnosed, Ph+, chronic phase CML
- Chronic, accelerated, or myeloid/lymphoid blast phase, Ph+ CML with resistance or intolerance to prior therapy including imatinib
- Ph+ ALL with resistance or intolerance to prior therapy.
Trial results
The FDA’s approval of dasatinib in children with Ph+ ALL is based on data from a phase 2 study (CA180-372, NCT01460160).
In this trial, researchers evaluated dasatinib in combination with the AIEOP-BFM ALL 2000 chemotherapy protocol in patients (ages 1 to 17) with newly diagnosed, B-cell precursor, Ph+ ALL.
There were 78 patients evaluated for efficacy in cohort 1. They had a median age of 10.4 years (range, 2.6 to 17.9 years). They received dasatinib at a daily dose of 60 mg/m2 for up to 24 months.
Patients with central nervous system 3 disease received cranial irradiation, and patients were assigned to stem cell transplant based on minimal residual disease if they were thought to have a high risk of relapse.
The 3-year event-free survival rate in the 78 patients was 64.1%.
There were 81 patients evaluable for safety who received dasatinib continuously in combination with chemotherapy. Their median duration of treatment was 24 months (range, 2 to 27 months).
The most common adverse events (AEs) in these patients were mucositis (93%), febrile neutropenia (86%), pyrexia (85%), diarrhea (84%), nausea (84%), vomiting (83%), musculoskeletal pain (83%), abdominal pain (78%), cough (78%), headache (77%), rash (68%), fatigue (59%), and constipation (57%).
Eight (10%) patients had AEs leading to treatment discontinuation. These included fungal sepsis, hepatotoxicity in the setting of graft-versus-host disease, thrombocytopenia, cytomegalovirus infection, pneumonia, nausea, enteritis, and drug hypersensitivity.
Three patients (4%) had fatal AEs, all infections.
This trial was sponsored by Bristol-Myers Squibb. Additional data are available in the prescribing information for dasatinib.
Group proposes new grading systems for CRS, neurotoxicity
A group of experts has proposed new consensus definitions and grading systems for cytokine release syndrome (CRS) and neurotoxicity related to immune effector cell therapies.
The group hopes their recommendations will be widely accepted and used in both trials and the clinical setting.
The recommendations were devised by 49 experts at a meeting supported by the American Society for Blood and Marrow Transplantation (ASBMT), compiled by a writing group, and reviewed by stakeholders.
Daniel W. Lee, MD, of the University of Virginia School of Medicine in Charlottesville, and his colleagues described the ASBMT consensus definitions and grading systems in Biology of Blood and Marrow Transplantation.
CRS
The ASBMT consensus definition for CRS is “a supraphysiologic response following any immune therapy that results in the activation or engagement of endogenous or infused T cells and/or other immune effector cells.”
To be diagnosed with CRS, a patient must have a fever and may have the following symptoms:
- Hypotension
- Capillary leak (hypoxia)
- End organ dysfunction.
The ASBMT consensus for grading CRS is as follows:
- Grade 1—Patient has a fever, defined as a temperature of 38.0°C or higher
- Grade 2—Patient has a fever, hypotension that doesn’t require vasopressors, and/or hypoxia that requires oxygen delivered by low-flow nasal cannula (≤6 L/min) or blow-by
- Grade 3—Patient has a fever, hypotension requiring one vasopressor (with or without vasopressin), and/or hypoxia (not attributable to any other cause) that requires high-flow nasal cannula (>6 L/min), facemask, non-rebreather mask, or venturi mask
- Grade 4—Patient has a fever, hypotension requiring multiple vasopressors (excluding vasopressin), and/or hypoxia (not attributable to any other cause) requiring positive-pressure ventilation
- Grade 5—Death due to CRS when there is no other “principle factor” leading to death.
Typically, severe CRS can be considered resolved if “fever, oxygen, and pressor requirements have resolved,” Dr. Lee and his coauthors said.
The authors also stressed that neurotoxicity that occurs with or after CRS “does not inform the grade of CRS but is instead captured separately in the neurotoxicity scale.”
Neurotoxicity
Dr. Lee and his coauthors said neurotoxicity in this setting is called “immune effector cell-associated neurotoxicity syndrome (ICANS).”
The ASBMT consensus definition for ICANs is “a disorder characterized by a pathologic process involving the central nervous system following any immune therapy that results in the activation or engagement of endogenous or infused T cells and/or other immune effector cells.”
Symptoms of ICANS may include:
- Aphasia
- Altered level of consciousness
- Impairment of cognitive skills
- Motor weakness
- Seizures
- Cerebral edema.
The ASBMT consensus for grading ICANS in adults and children age 12 and older is as follows:
- Grade 1—Patient has a score of 7-9 on the 10-point immune effector cell-associated encephalopathy (ICE) assessment and awakens spontaneously
- Grade 2—Patient has a score of 3-6 on the ICE assessment and will awaken to the sound of a voice
- Grade 3—Patient has a score of 0-2 on the ICE assessment, awakens only to tactile stimulus, has any clinical seizure that resolves rapidly or non-convulsive seizures that resolve with intervention, has focal/local edema on neuroimaging
- Grade 4—Patient is unable to perform the ICE assessment, is unarousable or requires “vigorous stimuli” to be aroused, has life-threatening seizure (lasting more than 5 minutes) or repetitive clinical or electrical seizures without return to baseline in between, has deep focal motor weakness, and/or has decerebrate or decorticate posturing, cranial nerve VI palsy, papilledema, Cushing’s triad, or signs of diffuse cerebral edema on neuroimaging
- Grade 5—Death due to ICANS when there is no other “principle factor” leading to death.
Dr. Lee and his coauthors noted that the ICE assessment is not suitable for children younger than 12. For these patients (and older patients with baseline developmental delays), ICANS can be assessed using the Cornell Assessment of Pediatric Delirium (CAPD).
The ASBMT consensus for grading ICANS in children younger than 12 (or older patients with developmental delays) is as follows:
- Grade 1—Patient has a CAPD score lower than 9 and awakens spontaneously
- Grade 2—Patient has a CAPD score lower than 9 and will awaken to the sound of a voice
- Grade 3—Patient has a CAPD score of 9 or higher, awakens only to tactile stimulus, has any clinical seizure that resolves rapidly or non-convulsive seizures that resolve with intervention, and/or has focal/local edema on neuroimaging
- Grade 4—Patient is unable to perform CAPD, is unarousable or requires “vigorous stimuli” to be aroused, has life-threatening seizure (lasting more than 5 minutes) or repetitive clinical or electrical seizures without return to baseline in between, has deep focal motor weakness, and/or has decerebrate or decorticate posturing, cranial nerve VI palsy, papilledema, Cushing’s triad, or signs of diffuse cerebral edema on neuroimaging
- Grade 5—Death due to ICANS when there is no other “principle factor” leading to death.
Dr. Lee and his coauthors reported relationships with a range of companies.
A group of experts has proposed new consensus definitions and grading systems for cytokine release syndrome (CRS) and neurotoxicity related to immune effector cell therapies.
The group hopes their recommendations will be widely accepted and used in both trials and the clinical setting.
The recommendations were devised by 49 experts at a meeting supported by the American Society for Blood and Marrow Transplantation (ASBMT), compiled by a writing group, and reviewed by stakeholders.
Daniel W. Lee, MD, of the University of Virginia School of Medicine in Charlottesville, and his colleagues described the ASBMT consensus definitions and grading systems in Biology of Blood and Marrow Transplantation.
CRS
The ASBMT consensus definition for CRS is “a supraphysiologic response following any immune therapy that results in the activation or engagement of endogenous or infused T cells and/or other immune effector cells.”
To be diagnosed with CRS, a patient must have a fever and may have the following symptoms:
- Hypotension
- Capillary leak (hypoxia)
- End organ dysfunction.
The ASBMT consensus for grading CRS is as follows:
- Grade 1—Patient has a fever, defined as a temperature of 38.0°C or higher
- Grade 2—Patient has a fever, hypotension that doesn’t require vasopressors, and/or hypoxia that requires oxygen delivered by low-flow nasal cannula (≤6 L/min) or blow-by
- Grade 3—Patient has a fever, hypotension requiring one vasopressor (with or without vasopressin), and/or hypoxia (not attributable to any other cause) that requires high-flow nasal cannula (>6 L/min), facemask, non-rebreather mask, or venturi mask
- Grade 4—Patient has a fever, hypotension requiring multiple vasopressors (excluding vasopressin), and/or hypoxia (not attributable to any other cause) requiring positive-pressure ventilation
- Grade 5—Death due to CRS when there is no other “principle factor” leading to death.
Typically, severe CRS can be considered resolved if “fever, oxygen, and pressor requirements have resolved,” Dr. Lee and his coauthors said.
The authors also stressed that neurotoxicity that occurs with or after CRS “does not inform the grade of CRS but is instead captured separately in the neurotoxicity scale.”
Neurotoxicity
Dr. Lee and his coauthors said neurotoxicity in this setting is called “immune effector cell-associated neurotoxicity syndrome (ICANS).”
The ASBMT consensus definition for ICANs is “a disorder characterized by a pathologic process involving the central nervous system following any immune therapy that results in the activation or engagement of endogenous or infused T cells and/or other immune effector cells.”
Symptoms of ICANS may include:
- Aphasia
- Altered level of consciousness
- Impairment of cognitive skills
- Motor weakness
- Seizures
- Cerebral edema.
The ASBMT consensus for grading ICANS in adults and children age 12 and older is as follows:
- Grade 1—Patient has a score of 7-9 on the 10-point immune effector cell-associated encephalopathy (ICE) assessment and awakens spontaneously
- Grade 2—Patient has a score of 3-6 on the ICE assessment and will awaken to the sound of a voice
- Grade 3—Patient has a score of 0-2 on the ICE assessment, awakens only to tactile stimulus, has any clinical seizure that resolves rapidly or non-convulsive seizures that resolve with intervention, has focal/local edema on neuroimaging
- Grade 4—Patient is unable to perform the ICE assessment, is unarousable or requires “vigorous stimuli” to be aroused, has life-threatening seizure (lasting more than 5 minutes) or repetitive clinical or electrical seizures without return to baseline in between, has deep focal motor weakness, and/or has decerebrate or decorticate posturing, cranial nerve VI palsy, papilledema, Cushing’s triad, or signs of diffuse cerebral edema on neuroimaging
- Grade 5—Death due to ICANS when there is no other “principle factor” leading to death.
Dr. Lee and his coauthors noted that the ICE assessment is not suitable for children younger than 12. For these patients (and older patients with baseline developmental delays), ICANS can be assessed using the Cornell Assessment of Pediatric Delirium (CAPD).
The ASBMT consensus for grading ICANS in children younger than 12 (or older patients with developmental delays) is as follows:
- Grade 1—Patient has a CAPD score lower than 9 and awakens spontaneously
- Grade 2—Patient has a CAPD score lower than 9 and will awaken to the sound of a voice
- Grade 3—Patient has a CAPD score of 9 or higher, awakens only to tactile stimulus, has any clinical seizure that resolves rapidly or non-convulsive seizures that resolve with intervention, and/or has focal/local edema on neuroimaging
- Grade 4—Patient is unable to perform CAPD, is unarousable or requires “vigorous stimuli” to be aroused, has life-threatening seizure (lasting more than 5 minutes) or repetitive clinical or electrical seizures without return to baseline in between, has deep focal motor weakness, and/or has decerebrate or decorticate posturing, cranial nerve VI palsy, papilledema, Cushing’s triad, or signs of diffuse cerebral edema on neuroimaging
- Grade 5—Death due to ICANS when there is no other “principle factor” leading to death.
Dr. Lee and his coauthors reported relationships with a range of companies.
A group of experts has proposed new consensus definitions and grading systems for cytokine release syndrome (CRS) and neurotoxicity related to immune effector cell therapies.
The group hopes their recommendations will be widely accepted and used in both trials and the clinical setting.
The recommendations were devised by 49 experts at a meeting supported by the American Society for Blood and Marrow Transplantation (ASBMT), compiled by a writing group, and reviewed by stakeholders.
Daniel W. Lee, MD, of the University of Virginia School of Medicine in Charlottesville, and his colleagues described the ASBMT consensus definitions and grading systems in Biology of Blood and Marrow Transplantation.
CRS
The ASBMT consensus definition for CRS is “a supraphysiologic response following any immune therapy that results in the activation or engagement of endogenous or infused T cells and/or other immune effector cells.”
To be diagnosed with CRS, a patient must have a fever and may have the following symptoms:
- Hypotension
- Capillary leak (hypoxia)
- End organ dysfunction.
The ASBMT consensus for grading CRS is as follows:
- Grade 1—Patient has a fever, defined as a temperature of 38.0°C or higher
- Grade 2—Patient has a fever, hypotension that doesn’t require vasopressors, and/or hypoxia that requires oxygen delivered by low-flow nasal cannula (≤6 L/min) or blow-by
- Grade 3—Patient has a fever, hypotension requiring one vasopressor (with or without vasopressin), and/or hypoxia (not attributable to any other cause) that requires high-flow nasal cannula (>6 L/min), facemask, non-rebreather mask, or venturi mask
- Grade 4—Patient has a fever, hypotension requiring multiple vasopressors (excluding vasopressin), and/or hypoxia (not attributable to any other cause) requiring positive-pressure ventilation
- Grade 5—Death due to CRS when there is no other “principle factor” leading to death.
Typically, severe CRS can be considered resolved if “fever, oxygen, and pressor requirements have resolved,” Dr. Lee and his coauthors said.
The authors also stressed that neurotoxicity that occurs with or after CRS “does not inform the grade of CRS but is instead captured separately in the neurotoxicity scale.”
Neurotoxicity
Dr. Lee and his coauthors said neurotoxicity in this setting is called “immune effector cell-associated neurotoxicity syndrome (ICANS).”
The ASBMT consensus definition for ICANs is “a disorder characterized by a pathologic process involving the central nervous system following any immune therapy that results in the activation or engagement of endogenous or infused T cells and/or other immune effector cells.”
Symptoms of ICANS may include:
- Aphasia
- Altered level of consciousness
- Impairment of cognitive skills
- Motor weakness
- Seizures
- Cerebral edema.
The ASBMT consensus for grading ICANS in adults and children age 12 and older is as follows:
- Grade 1—Patient has a score of 7-9 on the 10-point immune effector cell-associated encephalopathy (ICE) assessment and awakens spontaneously
- Grade 2—Patient has a score of 3-6 on the ICE assessment and will awaken to the sound of a voice
- Grade 3—Patient has a score of 0-2 on the ICE assessment, awakens only to tactile stimulus, has any clinical seizure that resolves rapidly or non-convulsive seizures that resolve with intervention, has focal/local edema on neuroimaging
- Grade 4—Patient is unable to perform the ICE assessment, is unarousable or requires “vigorous stimuli” to be aroused, has life-threatening seizure (lasting more than 5 minutes) or repetitive clinical or electrical seizures without return to baseline in between, has deep focal motor weakness, and/or has decerebrate or decorticate posturing, cranial nerve VI palsy, papilledema, Cushing’s triad, or signs of diffuse cerebral edema on neuroimaging
- Grade 5—Death due to ICANS when there is no other “principle factor” leading to death.
Dr. Lee and his coauthors noted that the ICE assessment is not suitable for children younger than 12. For these patients (and older patients with baseline developmental delays), ICANS can be assessed using the Cornell Assessment of Pediatric Delirium (CAPD).
The ASBMT consensus for grading ICANS in children younger than 12 (or older patients with developmental delays) is as follows:
- Grade 1—Patient has a CAPD score lower than 9 and awakens spontaneously
- Grade 2—Patient has a CAPD score lower than 9 and will awaken to the sound of a voice
- Grade 3—Patient has a CAPD score of 9 or higher, awakens only to tactile stimulus, has any clinical seizure that resolves rapidly or non-convulsive seizures that resolve with intervention, and/or has focal/local edema on neuroimaging
- Grade 4—Patient is unable to perform CAPD, is unarousable or requires “vigorous stimuli” to be aroused, has life-threatening seizure (lasting more than 5 minutes) or repetitive clinical or electrical seizures without return to baseline in between, has deep focal motor weakness, and/or has decerebrate or decorticate posturing, cranial nerve VI palsy, papilledema, Cushing’s triad, or signs of diffuse cerebral edema on neuroimaging
- Grade 5—Death due to ICANS when there is no other “principle factor” leading to death.
Dr. Lee and his coauthors reported relationships with a range of companies.
Quizartinib improves survival of FLT3-mutated AML
Single-agent therapy with quizartinib slightly but significantly prolonged survival – compared with salvage chemotherapy – for patients with relapsed/refractory acute myeloid leukemia (AML) bearing the FLT3-ITD mutation, results of the phase 3 randomized QuANTUM-R trial showed.
Median overall survival (OS), the trial’s primary endpoint, was 6.2 months for 245 patients randomized to quizartinib, compared with 4.7 months for 122 patients assigned to salvage chemotherapy, a difference that translated into a hazard ratio (HR) for death of 0.76 (P = .0177), reported Jorge E. Cortes, MD, of the University of Texas MD Anderson Cancer Center in Houston.
“This study is the first study that demonstrates in a randomized fashion an overall survival benefit in the salvage setting for patients with FLT-3 mutated refractory or relapsed AML,” he said at the annual meeting of the American Society of Hematology. “I will also add that these results you saw here are very consistent with all the trials previously with quizartinib with more than 1,000 patients treated.”
Quizartinib, a tyrosine kinase inhibitor (TKI), has previously been shown to be associated with higher response rates among patients with AML bearing the FLT3-ITD mutation than in patients with AML without the deleterious mutation.
Investigators in the QuANTUM-R trial enrolled 367 adults with FTL3-ITD mutated AML that was refractory to the most recent line of therapy or had relapsed within 6 months of first remission, with or without hematopoietic stem cell transplant (HSCT).
The patients had all received at least one cycle of standard-dose induction therapy containing an anthracycline or mitoxantrone, and had a 3% or greater FLT3-ITD allelic ratio in their AML cells.
The patients were randomly assigned on a 2:1 basis to receive either quizartinib or salvage chemotherapy. Quizartinib was dosed 30 mg per day for 15 days, which could be titrated upward to 60 mg daily if the corrected QT interval by Fredericia (QTcF) was 450 ms or less on day 16.
Chemotherapy was the investigator’s choice of one of three specified regimens: either low-dose cytarabine (LoDAC); mitoxantrone, etoposide, and intermediate-dose cytarabine (MEC); or fludarabine, cytarabine, and granulocyte-colony stimulating factor (G-CSF) with idarubicin (FLAG-IDA). Up to two cycles of MEC or FLAG-IDA were permitted; quizartinib and LoDAC were given until lack of benefit, unacceptable toxicity, or until the patient went on to HSCT.
The analysis was by intention-to-treat. In the quizartinib arm, 241 of the 245 randomized patients (98.4%) received treatment. In the chemotherapy arm, 94 of 122 randomized patients (77%) received chemotherapy. Of this group, 22 received LoDAC, 25 received MEC, and 47 received FLAG-IDA.
The median treatment duration was 97 days in the quizartinib arm versus 28 days (one cycle) in the chemotherapy arm.
The 1-year overall survival rate was 27% for patients assigned to quizartinib, compared with 20% for patients assigned to chemotherapy.
An analysis of OS by subgroup indicated a trend or significant benefit for quizartinib in all categories, including age over or under 65 years, sex, low or high-intensity chemotherapy, response to prior therapy, FLT3 variant allele frequency, prior allogenic HSCT, and AML risk score.
For the secondary endpoint of event-free survival in the ITT population, there was no significant difference between the study arms. In a per-protocol analysis, however, median event-free survival was better with quizartinib, at 1.4 months versus 0.0 months (P = .006).
In all, 32% of patients assigned to quizartinib went on to HSCT, compared with 12% of patients randomized to chemotherapy.
Rates of treatment-emergent adverse events (TEAEs) were similar between the study arms, despite higher total drug exposure in patients randomized to quizartinib. The most frequent grade 3 or greater TEAEs in each arm were infections and cytopenia-related events.
Two patients discontinued quizartinib due to QTcF prolongation. Grade 3 QTcF (greater than 500 ms) occurred in 3% of patients treated with quizartinib, but no grade 4 cases were seen.
The adverse event profile for patients who resumed quizartinib following HSCT was similar to that of patients who received the drug pretransplant.
The combination of standard chemotherapy, with or without quizartinib, is currently being explored in the phase 3 QuANTUM-First trial, Dr. Cortes said.
Daiichi Sankyo sponsored the trial. Dr. Cortes reported financial relationships with Daiichi Sankyo, Pfizer, Arog, Astellas Pharma, and Novartis.
SOURCE: Cortes JE et al. ASH 2018, Abstract 563.
Single-agent therapy with quizartinib slightly but significantly prolonged survival – compared with salvage chemotherapy – for patients with relapsed/refractory acute myeloid leukemia (AML) bearing the FLT3-ITD mutation, results of the phase 3 randomized QuANTUM-R trial showed.
Median overall survival (OS), the trial’s primary endpoint, was 6.2 months for 245 patients randomized to quizartinib, compared with 4.7 months for 122 patients assigned to salvage chemotherapy, a difference that translated into a hazard ratio (HR) for death of 0.76 (P = .0177), reported Jorge E. Cortes, MD, of the University of Texas MD Anderson Cancer Center in Houston.
“This study is the first study that demonstrates in a randomized fashion an overall survival benefit in the salvage setting for patients with FLT-3 mutated refractory or relapsed AML,” he said at the annual meeting of the American Society of Hematology. “I will also add that these results you saw here are very consistent with all the trials previously with quizartinib with more than 1,000 patients treated.”
Quizartinib, a tyrosine kinase inhibitor (TKI), has previously been shown to be associated with higher response rates among patients with AML bearing the FLT3-ITD mutation than in patients with AML without the deleterious mutation.
Investigators in the QuANTUM-R trial enrolled 367 adults with FTL3-ITD mutated AML that was refractory to the most recent line of therapy or had relapsed within 6 months of first remission, with or without hematopoietic stem cell transplant (HSCT).
The patients had all received at least one cycle of standard-dose induction therapy containing an anthracycline or mitoxantrone, and had a 3% or greater FLT3-ITD allelic ratio in their AML cells.
The patients were randomly assigned on a 2:1 basis to receive either quizartinib or salvage chemotherapy. Quizartinib was dosed 30 mg per day for 15 days, which could be titrated upward to 60 mg daily if the corrected QT interval by Fredericia (QTcF) was 450 ms or less on day 16.
Chemotherapy was the investigator’s choice of one of three specified regimens: either low-dose cytarabine (LoDAC); mitoxantrone, etoposide, and intermediate-dose cytarabine (MEC); or fludarabine, cytarabine, and granulocyte-colony stimulating factor (G-CSF) with idarubicin (FLAG-IDA). Up to two cycles of MEC or FLAG-IDA were permitted; quizartinib and LoDAC were given until lack of benefit, unacceptable toxicity, or until the patient went on to HSCT.
The analysis was by intention-to-treat. In the quizartinib arm, 241 of the 245 randomized patients (98.4%) received treatment. In the chemotherapy arm, 94 of 122 randomized patients (77%) received chemotherapy. Of this group, 22 received LoDAC, 25 received MEC, and 47 received FLAG-IDA.
The median treatment duration was 97 days in the quizartinib arm versus 28 days (one cycle) in the chemotherapy arm.
The 1-year overall survival rate was 27% for patients assigned to quizartinib, compared with 20% for patients assigned to chemotherapy.
An analysis of OS by subgroup indicated a trend or significant benefit for quizartinib in all categories, including age over or under 65 years, sex, low or high-intensity chemotherapy, response to prior therapy, FLT3 variant allele frequency, prior allogenic HSCT, and AML risk score.
For the secondary endpoint of event-free survival in the ITT population, there was no significant difference between the study arms. In a per-protocol analysis, however, median event-free survival was better with quizartinib, at 1.4 months versus 0.0 months (P = .006).
In all, 32% of patients assigned to quizartinib went on to HSCT, compared with 12% of patients randomized to chemotherapy.
Rates of treatment-emergent adverse events (TEAEs) were similar between the study arms, despite higher total drug exposure in patients randomized to quizartinib. The most frequent grade 3 or greater TEAEs in each arm were infections and cytopenia-related events.
Two patients discontinued quizartinib due to QTcF prolongation. Grade 3 QTcF (greater than 500 ms) occurred in 3% of patients treated with quizartinib, but no grade 4 cases were seen.
The adverse event profile for patients who resumed quizartinib following HSCT was similar to that of patients who received the drug pretransplant.
The combination of standard chemotherapy, with or without quizartinib, is currently being explored in the phase 3 QuANTUM-First trial, Dr. Cortes said.
Daiichi Sankyo sponsored the trial. Dr. Cortes reported financial relationships with Daiichi Sankyo, Pfizer, Arog, Astellas Pharma, and Novartis.
SOURCE: Cortes JE et al. ASH 2018, Abstract 563.
Single-agent therapy with quizartinib slightly but significantly prolonged survival – compared with salvage chemotherapy – for patients with relapsed/refractory acute myeloid leukemia (AML) bearing the FLT3-ITD mutation, results of the phase 3 randomized QuANTUM-R trial showed.
Median overall survival (OS), the trial’s primary endpoint, was 6.2 months for 245 patients randomized to quizartinib, compared with 4.7 months for 122 patients assigned to salvage chemotherapy, a difference that translated into a hazard ratio (HR) for death of 0.76 (P = .0177), reported Jorge E. Cortes, MD, of the University of Texas MD Anderson Cancer Center in Houston.
“This study is the first study that demonstrates in a randomized fashion an overall survival benefit in the salvage setting for patients with FLT-3 mutated refractory or relapsed AML,” he said at the annual meeting of the American Society of Hematology. “I will also add that these results you saw here are very consistent with all the trials previously with quizartinib with more than 1,000 patients treated.”
Quizartinib, a tyrosine kinase inhibitor (TKI), has previously been shown to be associated with higher response rates among patients with AML bearing the FLT3-ITD mutation than in patients with AML without the deleterious mutation.
Investigators in the QuANTUM-R trial enrolled 367 adults with FTL3-ITD mutated AML that was refractory to the most recent line of therapy or had relapsed within 6 months of first remission, with or without hematopoietic stem cell transplant (HSCT).
The patients had all received at least one cycle of standard-dose induction therapy containing an anthracycline or mitoxantrone, and had a 3% or greater FLT3-ITD allelic ratio in their AML cells.
The patients were randomly assigned on a 2:1 basis to receive either quizartinib or salvage chemotherapy. Quizartinib was dosed 30 mg per day for 15 days, which could be titrated upward to 60 mg daily if the corrected QT interval by Fredericia (QTcF) was 450 ms or less on day 16.
Chemotherapy was the investigator’s choice of one of three specified regimens: either low-dose cytarabine (LoDAC); mitoxantrone, etoposide, and intermediate-dose cytarabine (MEC); or fludarabine, cytarabine, and granulocyte-colony stimulating factor (G-CSF) with idarubicin (FLAG-IDA). Up to two cycles of MEC or FLAG-IDA were permitted; quizartinib and LoDAC were given until lack of benefit, unacceptable toxicity, or until the patient went on to HSCT.
The analysis was by intention-to-treat. In the quizartinib arm, 241 of the 245 randomized patients (98.4%) received treatment. In the chemotherapy arm, 94 of 122 randomized patients (77%) received chemotherapy. Of this group, 22 received LoDAC, 25 received MEC, and 47 received FLAG-IDA.
The median treatment duration was 97 days in the quizartinib arm versus 28 days (one cycle) in the chemotherapy arm.
The 1-year overall survival rate was 27% for patients assigned to quizartinib, compared with 20% for patients assigned to chemotherapy.
An analysis of OS by subgroup indicated a trend or significant benefit for quizartinib in all categories, including age over or under 65 years, sex, low or high-intensity chemotherapy, response to prior therapy, FLT3 variant allele frequency, prior allogenic HSCT, and AML risk score.
For the secondary endpoint of event-free survival in the ITT population, there was no significant difference between the study arms. In a per-protocol analysis, however, median event-free survival was better with quizartinib, at 1.4 months versus 0.0 months (P = .006).
In all, 32% of patients assigned to quizartinib went on to HSCT, compared with 12% of patients randomized to chemotherapy.
Rates of treatment-emergent adverse events (TEAEs) were similar between the study arms, despite higher total drug exposure in patients randomized to quizartinib. The most frequent grade 3 or greater TEAEs in each arm were infections and cytopenia-related events.
Two patients discontinued quizartinib due to QTcF prolongation. Grade 3 QTcF (greater than 500 ms) occurred in 3% of patients treated with quizartinib, but no grade 4 cases were seen.
The adverse event profile for patients who resumed quizartinib following HSCT was similar to that of patients who received the drug pretransplant.
The combination of standard chemotherapy, with or without quizartinib, is currently being explored in the phase 3 QuANTUM-First trial, Dr. Cortes said.
Daiichi Sankyo sponsored the trial. Dr. Cortes reported financial relationships with Daiichi Sankyo, Pfizer, Arog, Astellas Pharma, and Novartis.
SOURCE: Cortes JE et al. ASH 2018, Abstract 563.
REPORTING FROM ASH 2018
Key clinical point:
Major finding: The hazard ratio for death with quizartinib was 0.76 (P = .0177).
Study details: A randomized phase 3 trial comparing quizartinib to salvage chemotherapy on a 2:1 basis in 367 adults with FLT3-ITD mutated AML.
Disclosures: Daiichi Sankyo sponsored the trial. Dr. Cortes reported financial relationships with Daiichi Sankyo, Pfizer, Arog, Astellas Pharma, and Novartis.
Source: Cortes JE et al. ASH 2018, Abstract 563.
FDA approves first treatment for BPDCN
The U.S. Food and Drug Administration (FDA) has approved tagraxofusp-erzs (Elzonris) to treat patients age 2 and older who have blastic plasmacytoid dendritic cell neoplasm (BPDCN).
Tagraxofusp-erzs (formerly SL-401) is a CD123-directed cytotoxin that is the first FDA-approved treatment for BPDCN.
Tagraxofusp-erzs will be commercially available in early 2019, according to Stemline Therapeutics, makers of the drug.
The prescribing information for tagraxofusp-erzs contains a boxed warning noting that the drug is associated with an increased risk of capillary leak syndrome (CLS), which may be life-threatening or fatal.
The FDA previously granted tagraxofusp-erzs breakthrough therapy and orphan drug designations and assessed the drug under priority review.
The FDA’s approval of tagraxofusp-erzs was based on a phase 1 trial (STML-401-0114; NCT02113982).
The trial enrolled 47 patients with BPDCN, including 32 who were treatment-naïve and 15 who were previously treated.
Patients received tagraxofusp-erzs intravenously on days 1-5 of a 21-day cycle for multiple consecutive cycles. The trial had a dose-escalation stage (stage 1), an expansion stage (stage 2), a confirmatory stage (stage 3), and a stage that enabled uninterrupted access to tagraxofusp-erzs (stage 4).
In the confirmatory stage, 13 patients with treatment-naïve BPDCN received tagraxofusp-erzs at the recommended dose and schedule—12 mcg/kg daily for 5 days of a 21-day cycle.
Efficacy was based on the rate of complete response (CR) or clinical complete response (CRc). CRc was defined as CR with residual skin abnormality not indicative of active disease.
The CR/CRc rate was 53.8% (7/13), and the median duration of CR/CRc was not reached (range, 3.9 to 12.2 months).
The safety of tagraxofusp-erzs was assessed in 94 adults with treatment-naïve or previously treated myeloid malignancies, including 58 patients with BPDCN, who were treated at the recommended dose and schedule.
There were two fatal adverse events—both CLS. Eleven percent of patients discontinued treatment with tagraxofusp-erzs due to an adverse event. The most common of these were hepatic toxicities and CLS.
The most common adverse events overall were CLS (55%), nausea (49%), fatigue (45%), peripheral edema (43%), pyrexia (43%), and weight increase (31%).
The most common laboratory abnormalities were decreases in albumin (77%), platelets (67%), hemoglobin (60%), calcium (57%), and sodium (50%), as well as increases in glucose (87%), alanine aminotransferase (82%), and aspartate aminotransferase (79%).
The U.S. Food and Drug Administration (FDA) has approved tagraxofusp-erzs (Elzonris) to treat patients age 2 and older who have blastic plasmacytoid dendritic cell neoplasm (BPDCN).
Tagraxofusp-erzs (formerly SL-401) is a CD123-directed cytotoxin that is the first FDA-approved treatment for BPDCN.
Tagraxofusp-erzs will be commercially available in early 2019, according to Stemline Therapeutics, makers of the drug.
The prescribing information for tagraxofusp-erzs contains a boxed warning noting that the drug is associated with an increased risk of capillary leak syndrome (CLS), which may be life-threatening or fatal.
The FDA previously granted tagraxofusp-erzs breakthrough therapy and orphan drug designations and assessed the drug under priority review.
The FDA’s approval of tagraxofusp-erzs was based on a phase 1 trial (STML-401-0114; NCT02113982).
The trial enrolled 47 patients with BPDCN, including 32 who were treatment-naïve and 15 who were previously treated.
Patients received tagraxofusp-erzs intravenously on days 1-5 of a 21-day cycle for multiple consecutive cycles. The trial had a dose-escalation stage (stage 1), an expansion stage (stage 2), a confirmatory stage (stage 3), and a stage that enabled uninterrupted access to tagraxofusp-erzs (stage 4).
In the confirmatory stage, 13 patients with treatment-naïve BPDCN received tagraxofusp-erzs at the recommended dose and schedule—12 mcg/kg daily for 5 days of a 21-day cycle.
Efficacy was based on the rate of complete response (CR) or clinical complete response (CRc). CRc was defined as CR with residual skin abnormality not indicative of active disease.
The CR/CRc rate was 53.8% (7/13), and the median duration of CR/CRc was not reached (range, 3.9 to 12.2 months).
The safety of tagraxofusp-erzs was assessed in 94 adults with treatment-naïve or previously treated myeloid malignancies, including 58 patients with BPDCN, who were treated at the recommended dose and schedule.
There were two fatal adverse events—both CLS. Eleven percent of patients discontinued treatment with tagraxofusp-erzs due to an adverse event. The most common of these were hepatic toxicities and CLS.
The most common adverse events overall were CLS (55%), nausea (49%), fatigue (45%), peripheral edema (43%), pyrexia (43%), and weight increase (31%).
The most common laboratory abnormalities were decreases in albumin (77%), platelets (67%), hemoglobin (60%), calcium (57%), and sodium (50%), as well as increases in glucose (87%), alanine aminotransferase (82%), and aspartate aminotransferase (79%).
The U.S. Food and Drug Administration (FDA) has approved tagraxofusp-erzs (Elzonris) to treat patients age 2 and older who have blastic plasmacytoid dendritic cell neoplasm (BPDCN).
Tagraxofusp-erzs (formerly SL-401) is a CD123-directed cytotoxin that is the first FDA-approved treatment for BPDCN.
Tagraxofusp-erzs will be commercially available in early 2019, according to Stemline Therapeutics, makers of the drug.
The prescribing information for tagraxofusp-erzs contains a boxed warning noting that the drug is associated with an increased risk of capillary leak syndrome (CLS), which may be life-threatening or fatal.
The FDA previously granted tagraxofusp-erzs breakthrough therapy and orphan drug designations and assessed the drug under priority review.
The FDA’s approval of tagraxofusp-erzs was based on a phase 1 trial (STML-401-0114; NCT02113982).
The trial enrolled 47 patients with BPDCN, including 32 who were treatment-naïve and 15 who were previously treated.
Patients received tagraxofusp-erzs intravenously on days 1-5 of a 21-day cycle for multiple consecutive cycles. The trial had a dose-escalation stage (stage 1), an expansion stage (stage 2), a confirmatory stage (stage 3), and a stage that enabled uninterrupted access to tagraxofusp-erzs (stage 4).
In the confirmatory stage, 13 patients with treatment-naïve BPDCN received tagraxofusp-erzs at the recommended dose and schedule—12 mcg/kg daily for 5 days of a 21-day cycle.
Efficacy was based on the rate of complete response (CR) or clinical complete response (CRc). CRc was defined as CR with residual skin abnormality not indicative of active disease.
The CR/CRc rate was 53.8% (7/13), and the median duration of CR/CRc was not reached (range, 3.9 to 12.2 months).
The safety of tagraxofusp-erzs was assessed in 94 adults with treatment-naïve or previously treated myeloid malignancies, including 58 patients with BPDCN, who were treated at the recommended dose and schedule.
There were two fatal adverse events—both CLS. Eleven percent of patients discontinued treatment with tagraxofusp-erzs due to an adverse event. The most common of these were hepatic toxicities and CLS.
The most common adverse events overall were CLS (55%), nausea (49%), fatigue (45%), peripheral edema (43%), pyrexia (43%), and weight increase (31%).
The most common laboratory abnormalities were decreases in albumin (77%), platelets (67%), hemoglobin (60%), calcium (57%), and sodium (50%), as well as increases in glucose (87%), alanine aminotransferase (82%), and aspartate aminotransferase (79%).