User login
COACT: No benefit of immediate PCI for non–ST-elevation cardiac arrest at 1 year
PHILADELPHIA – Immediate coronary angiography following restoration of spontaneous circulation after out-of-hospital cardiac arrest without ST-elevation MI (STEMI) offered no survival benefit at 1 year, compared with a strategy of delaying angiography until after neurologic recovery, in the landmark COACT trial, Jorrit Lemkes, MD, reported at the American Heart Association scientific sessions.
Nor was immediate coronary angiography advantageous in terms of any of the numerous secondary long-term endpoints, including rates of MI, revascularization, hospitalization for heart failure, implantable cardioverter defibrillator (ICD) shocks, or quality of life measures (see graphic), according to Dr. Lemkes, an interventional cardiologist at Amsterdam University Medical Center.
COACT, a 552-patient randomized trial conducted at 19 Dutch centers, was the first-ever randomized trial to evaluate the role of immediate coronary angiography in patients resuscitated from out-of-hospital cardiac arrest with no signs of ST elevation on ECG. The study hypothesis was that this practice would result in improved survival and other outcomes. But such was not the case in the previously reported 90-day analysis (N Engl J Med. 2019 Apr 11;380[15]:1397-407). Nonetheless, the new 1-year results were eagerly awaited because observational data had suggested that immediate angiography after cardiac arrest without STEMI might provide a survival advantage that manifests late.
It now appears the observational studies were misleading. The 1-year survival rate was 61.4% in the immediate angioplasty group and similar at 64% with delayed angioplasty.
By way of background, Dr. Lemkes noted that both European and American guidelines give a class 1 recommendation to immediate coronary angiography with percutaneous coronary intervention (PCI) in patients who present with STEMI and cardiac arrest. It’s an endorsement grounded in compelling clinical trials evidence demonstrating that this practice reduces mortality and recurrent ischemia, salvages myocardium, and restores left ventricular function. In contrast, current guidelines offer only a tepid recommendation for immediate PCI in patients with cardiac arrest without STEMI because the only supporting evidence has been observational, with its inherent susceptibility to bias.
Discussant Joaquin E. Cigarroa, MD, said that the 1-year outcomes shouldn’t be surprising, since the 90-day results failed to show any between-group differences in myocardial injury or ischemia.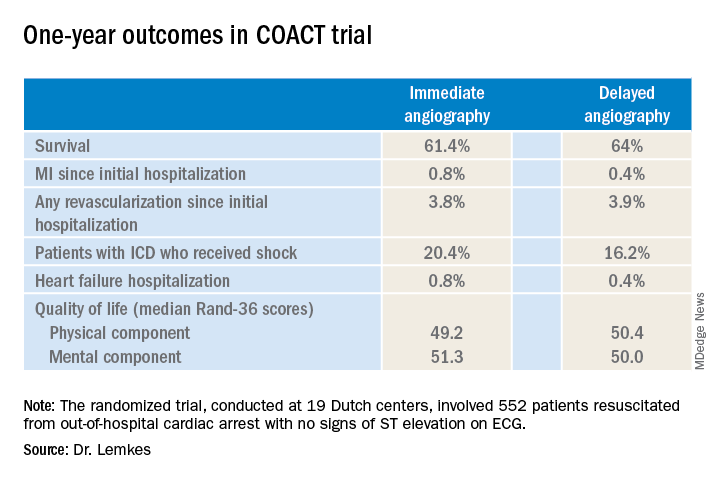
“At present, the results of COACT with regards to primary and secondary outcomes should guide practitioners that angiography remains essential, but that early angiography does not improve outcomes, compared to delayed angiography,” declared Dr. Cigarroa, chief of cardiology and professor of medicine at Oregon Health & Science University, Portland.
His choice of the words “at present” was key, as COACT won’t be the last word on the subject. Dr. Cigarroa believes that it’s critically important to understand the relatively narrow profile of the patients included in the study, because the results may or may not prove to be generalizable to the broader population of out-of-hospital cardiac arrest patients encountered in clinical practice. Nearly 80% of COACT participants had a witnessed arrest, the median time to basic life support was just 2 minutes, and the median time to return of spontaneous circulation was 15 minutes.
He urged his colleagues to stay tuned for reports from ongoing randomized trials examining the potential role of immediate angiography in broader populations of patients with out-of-hospital cardiac arrest without STEMI, including the Swedish DISCO (Direct or Subacute Coronary Angiography in Out-of-Hospital Cardiac Arrest) trial and the smaller University of Minnesota–sponsored ACCESS trial.
Dr. Lemkes reported having no financial conflicts regarding the COACT trial, funded by the Netherlands Heart Institute and unrestricted research grants from Biotronik and AstraZeneca.
PHILADELPHIA – Immediate coronary angiography following restoration of spontaneous circulation after out-of-hospital cardiac arrest without ST-elevation MI (STEMI) offered no survival benefit at 1 year, compared with a strategy of delaying angiography until after neurologic recovery, in the landmark COACT trial, Jorrit Lemkes, MD, reported at the American Heart Association scientific sessions.
Nor was immediate coronary angiography advantageous in terms of any of the numerous secondary long-term endpoints, including rates of MI, revascularization, hospitalization for heart failure, implantable cardioverter defibrillator (ICD) shocks, or quality of life measures (see graphic), according to Dr. Lemkes, an interventional cardiologist at Amsterdam University Medical Center.
COACT, a 552-patient randomized trial conducted at 19 Dutch centers, was the first-ever randomized trial to evaluate the role of immediate coronary angiography in patients resuscitated from out-of-hospital cardiac arrest with no signs of ST elevation on ECG. The study hypothesis was that this practice would result in improved survival and other outcomes. But such was not the case in the previously reported 90-day analysis (N Engl J Med. 2019 Apr 11;380[15]:1397-407). Nonetheless, the new 1-year results were eagerly awaited because observational data had suggested that immediate angiography after cardiac arrest without STEMI might provide a survival advantage that manifests late.
It now appears the observational studies were misleading. The 1-year survival rate was 61.4% in the immediate angioplasty group and similar at 64% with delayed angioplasty.
By way of background, Dr. Lemkes noted that both European and American guidelines give a class 1 recommendation to immediate coronary angiography with percutaneous coronary intervention (PCI) in patients who present with STEMI and cardiac arrest. It’s an endorsement grounded in compelling clinical trials evidence demonstrating that this practice reduces mortality and recurrent ischemia, salvages myocardium, and restores left ventricular function. In contrast, current guidelines offer only a tepid recommendation for immediate PCI in patients with cardiac arrest without STEMI because the only supporting evidence has been observational, with its inherent susceptibility to bias.
Discussant Joaquin E. Cigarroa, MD, said that the 1-year outcomes shouldn’t be surprising, since the 90-day results failed to show any between-group differences in myocardial injury or ischemia.
“At present, the results of COACT with regards to primary and secondary outcomes should guide practitioners that angiography remains essential, but that early angiography does not improve outcomes, compared to delayed angiography,” declared Dr. Cigarroa, chief of cardiology and professor of medicine at Oregon Health & Science University, Portland.
His choice of the words “at present” was key, as COACT won’t be the last word on the subject. Dr. Cigarroa believes that it’s critically important to understand the relatively narrow profile of the patients included in the study, because the results may or may not prove to be generalizable to the broader population of out-of-hospital cardiac arrest patients encountered in clinical practice. Nearly 80% of COACT participants had a witnessed arrest, the median time to basic life support was just 2 minutes, and the median time to return of spontaneous circulation was 15 minutes.
He urged his colleagues to stay tuned for reports from ongoing randomized trials examining the potential role of immediate angiography in broader populations of patients with out-of-hospital cardiac arrest without STEMI, including the Swedish DISCO (Direct or Subacute Coronary Angiography in Out-of-Hospital Cardiac Arrest) trial and the smaller University of Minnesota–sponsored ACCESS trial.
Dr. Lemkes reported having no financial conflicts regarding the COACT trial, funded by the Netherlands Heart Institute and unrestricted research grants from Biotronik and AstraZeneca.
PHILADELPHIA – Immediate coronary angiography following restoration of spontaneous circulation after out-of-hospital cardiac arrest without ST-elevation MI (STEMI) offered no survival benefit at 1 year, compared with a strategy of delaying angiography until after neurologic recovery, in the landmark COACT trial, Jorrit Lemkes, MD, reported at the American Heart Association scientific sessions.
Nor was immediate coronary angiography advantageous in terms of any of the numerous secondary long-term endpoints, including rates of MI, revascularization, hospitalization for heart failure, implantable cardioverter defibrillator (ICD) shocks, or quality of life measures (see graphic), according to Dr. Lemkes, an interventional cardiologist at Amsterdam University Medical Center.
COACT, a 552-patient randomized trial conducted at 19 Dutch centers, was the first-ever randomized trial to evaluate the role of immediate coronary angiography in patients resuscitated from out-of-hospital cardiac arrest with no signs of ST elevation on ECG. The study hypothesis was that this practice would result in improved survival and other outcomes. But such was not the case in the previously reported 90-day analysis (N Engl J Med. 2019 Apr 11;380[15]:1397-407). Nonetheless, the new 1-year results were eagerly awaited because observational data had suggested that immediate angiography after cardiac arrest without STEMI might provide a survival advantage that manifests late.
It now appears the observational studies were misleading. The 1-year survival rate was 61.4% in the immediate angioplasty group and similar at 64% with delayed angioplasty.
By way of background, Dr. Lemkes noted that both European and American guidelines give a class 1 recommendation to immediate coronary angiography with percutaneous coronary intervention (PCI) in patients who present with STEMI and cardiac arrest. It’s an endorsement grounded in compelling clinical trials evidence demonstrating that this practice reduces mortality and recurrent ischemia, salvages myocardium, and restores left ventricular function. In contrast, current guidelines offer only a tepid recommendation for immediate PCI in patients with cardiac arrest without STEMI because the only supporting evidence has been observational, with its inherent susceptibility to bias.
Discussant Joaquin E. Cigarroa, MD, said that the 1-year outcomes shouldn’t be surprising, since the 90-day results failed to show any between-group differences in myocardial injury or ischemia.
“At present, the results of COACT with regards to primary and secondary outcomes should guide practitioners that angiography remains essential, but that early angiography does not improve outcomes, compared to delayed angiography,” declared Dr. Cigarroa, chief of cardiology and professor of medicine at Oregon Health & Science University, Portland.
His choice of the words “at present” was key, as COACT won’t be the last word on the subject. Dr. Cigarroa believes that it’s critically important to understand the relatively narrow profile of the patients included in the study, because the results may or may not prove to be generalizable to the broader population of out-of-hospital cardiac arrest patients encountered in clinical practice. Nearly 80% of COACT participants had a witnessed arrest, the median time to basic life support was just 2 minutes, and the median time to return of spontaneous circulation was 15 minutes.
He urged his colleagues to stay tuned for reports from ongoing randomized trials examining the potential role of immediate angiography in broader populations of patients with out-of-hospital cardiac arrest without STEMI, including the Swedish DISCO (Direct or Subacute Coronary Angiography in Out-of-Hospital Cardiac Arrest) trial and the smaller University of Minnesota–sponsored ACCESS trial.
Dr. Lemkes reported having no financial conflicts regarding the COACT trial, funded by the Netherlands Heart Institute and unrestricted research grants from Biotronik and AstraZeneca.
REPORTING FROM AHA 2019
FUEL trial: Post-Fontan udenafil shows mixed results
PHILADELPHIA – In adolescents who have had a Fontan procedure for congenital heart disease, a randomized trial of the phosphodiesterase type 5 inhibitor udenafil showed that it achieved improved exercise performance but did not lead to significant improvement in oxygen levels or myocardial performance.
That’s according to results of the Pediatric Heart Network’s Fontan Udenafil Exercise Longitudinal Trial (FUEL) presented at the American Heart Association scientific sessions. “Treatment with udenafil was not associated with a statistically significant improvement in oxygen consumption at peak exercise, but it was associated with statistically significant improvements in exercise performance at the ventilatory anaerobic threshold,” said David J. Goldberg, MD, of Children’s Hospital of Philadelphia in reporting the FUEL results. The results were published simultaneously in Circulation (2019 Nov 17. doi: 10.1161/CIRCULATIONAHA.119.044352).
“This is the first large clinical trial to show improvement in measures of clinically relevant exercise performance in those with single-ventricle heart disease after Fontan palliation,” he said.
FUEL enrolled 400 male and female adolescents with a single functional ventricle after Fontan surgical palliation. In these patients, pulmonary vascular resistance (PVR) is critical for the efficient flow of blood through the lungs without the benefit of a ventricular pump. “While this circulation is typically stable through childhood, cardiovascular efficiency deteriorates over time, associated with a decline in exercise performance and the accrual of Fontan-associated morbidities,” Dr. Goldberg said. “Given the importance of pulmonary vascular resistance, modulators of PVR make sense as potential therapies.”
FUEL evaluated the effect of udenafil 87.5 mg twice daily versus placebo in post-Fontan patients who’d been on anticoagulation or antiplatelet therapy. The treatment group had a higher percentage of female patients (44% vs. 36% on placebo), but all other baseline characteristics were similar between the two groups.
While the trial found the drug was well tolerated and safe, with side effects typical of PDE5 inhibitors, it did not lead to changes in myocardial performance index, reactive hyperemia index, or log brain natriuretic peptide, Dr. Goldberg said.
At 6 months, both groups showed a decline in exercise data, “as expected,” Dr. Goldberg said. “But that decline was attenuated in the group receiving udenafil,” he said, with peak oxygen consumption declining an average of 0.23 and 0.89 mL/kg per minute in the treatment and placebo groups, respectively (P = 0.092).
Total oxygen consumption, however, actually improved in the udenafil group and declined in the placebo group, 44 mL/min on average versus –3.7 mL/min (P = 0.071).
“There was no significant difference in the change in peak heart rate or the change in peak oxygen saturation between the groups,” Dr. Goldberg said. But three measures at the ventilatory aerobic threshold (VAT) – oxygen consumption, work rate, and ventilation/carbon dioxide output – all showed statistically significant improvement in exercise performance.
“This has important clinical implications,” Dr. Goldberg said of the study findings. “Our study extends recent findings in highlighting the importance of submaximal exercise in the understanding of Fontan physiology. And unlike peak oxygen consumption, submaximal exercise is not constrained by the physiologic ceiling of central venous pressure inherent in exercise physiology after Fontan palliation.”
Maximum oxygen consumption at VAT is likely a more relevant measure after Fontan palliation than is central venous pressure, discussant Craig A. Sable, MD, a pediatric cardiologist in Potomac, Md., noted in his comments. “This is because VAT occurs at about 70% of maximum VO2 [oxygen consumption] in Fontan as opposed to 55% in two-ventricle physiology,” Dr. Sable said.
In adults with congenital heart disease, maximal VO2 of 45%-50% of predicted levels portends increased risk of heart failure and death. “Therefore, a medication that addresses the central deficiencies of Fontan physiology and results in improved exercise performance may allow for a longer period of symptom-free survival,” he said.
In an invited commentary in Circulation (2019 Nov 17. doi: 10.1161/CIRCULATIONAHA.119.044512), Marc Gewillig, MD, and Alexander van de Bruaene, MD, of University Hospitals Leuven (Belgium) said that the findings of FUEL and other trials of pulmonary vasodilators after Fontan leave “open for debate” whether the treatment effects of a 3%-5% improvement in oxygen consumption is clinically meaningful for adolescents. “For failing Fontan patients (not studied in FUEL), these improvements are minimal but maybe relevant,” the commentators wrote. But the studies do not resolve whether that’s enough to prevent further decline.
Dr. Goldberg disclosed receiving research grants from trial sponsor Mezzion Pharmaceuticals and the National Heart Lung and Blood Institute. Dr. Sable, Dr. Gewillig, and Dr. van de Bruaene have no financial relationships to disclose.
SOURCE: Goldberg D. AHA 2019, Late Breaking Science Session 5.
PHILADELPHIA – In adolescents who have had a Fontan procedure for congenital heart disease, a randomized trial of the phosphodiesterase type 5 inhibitor udenafil showed that it achieved improved exercise performance but did not lead to significant improvement in oxygen levels or myocardial performance.
That’s according to results of the Pediatric Heart Network’s Fontan Udenafil Exercise Longitudinal Trial (FUEL) presented at the American Heart Association scientific sessions. “Treatment with udenafil was not associated with a statistically significant improvement in oxygen consumption at peak exercise, but it was associated with statistically significant improvements in exercise performance at the ventilatory anaerobic threshold,” said David J. Goldberg, MD, of Children’s Hospital of Philadelphia in reporting the FUEL results. The results were published simultaneously in Circulation (2019 Nov 17. doi: 10.1161/CIRCULATIONAHA.119.044352).
“This is the first large clinical trial to show improvement in measures of clinically relevant exercise performance in those with single-ventricle heart disease after Fontan palliation,” he said.
FUEL enrolled 400 male and female adolescents with a single functional ventricle after Fontan surgical palliation. In these patients, pulmonary vascular resistance (PVR) is critical for the efficient flow of blood through the lungs without the benefit of a ventricular pump. “While this circulation is typically stable through childhood, cardiovascular efficiency deteriorates over time, associated with a decline in exercise performance and the accrual of Fontan-associated morbidities,” Dr. Goldberg said. “Given the importance of pulmonary vascular resistance, modulators of PVR make sense as potential therapies.”
FUEL evaluated the effect of udenafil 87.5 mg twice daily versus placebo in post-Fontan patients who’d been on anticoagulation or antiplatelet therapy. The treatment group had a higher percentage of female patients (44% vs. 36% on placebo), but all other baseline characteristics were similar between the two groups.
While the trial found the drug was well tolerated and safe, with side effects typical of PDE5 inhibitors, it did not lead to changes in myocardial performance index, reactive hyperemia index, or log brain natriuretic peptide, Dr. Goldberg said.
At 6 months, both groups showed a decline in exercise data, “as expected,” Dr. Goldberg said. “But that decline was attenuated in the group receiving udenafil,” he said, with peak oxygen consumption declining an average of 0.23 and 0.89 mL/kg per minute in the treatment and placebo groups, respectively (P = 0.092).
Total oxygen consumption, however, actually improved in the udenafil group and declined in the placebo group, 44 mL/min on average versus –3.7 mL/min (P = 0.071).
“There was no significant difference in the change in peak heart rate or the change in peak oxygen saturation between the groups,” Dr. Goldberg said. But three measures at the ventilatory aerobic threshold (VAT) – oxygen consumption, work rate, and ventilation/carbon dioxide output – all showed statistically significant improvement in exercise performance.
“This has important clinical implications,” Dr. Goldberg said of the study findings. “Our study extends recent findings in highlighting the importance of submaximal exercise in the understanding of Fontan physiology. And unlike peak oxygen consumption, submaximal exercise is not constrained by the physiologic ceiling of central venous pressure inherent in exercise physiology after Fontan palliation.”
Maximum oxygen consumption at VAT is likely a more relevant measure after Fontan palliation than is central venous pressure, discussant Craig A. Sable, MD, a pediatric cardiologist in Potomac, Md., noted in his comments. “This is because VAT occurs at about 70% of maximum VO2 [oxygen consumption] in Fontan as opposed to 55% in two-ventricle physiology,” Dr. Sable said.
In adults with congenital heart disease, maximal VO2 of 45%-50% of predicted levels portends increased risk of heart failure and death. “Therefore, a medication that addresses the central deficiencies of Fontan physiology and results in improved exercise performance may allow for a longer period of symptom-free survival,” he said.
In an invited commentary in Circulation (2019 Nov 17. doi: 10.1161/CIRCULATIONAHA.119.044512), Marc Gewillig, MD, and Alexander van de Bruaene, MD, of University Hospitals Leuven (Belgium) said that the findings of FUEL and other trials of pulmonary vasodilators after Fontan leave “open for debate” whether the treatment effects of a 3%-5% improvement in oxygen consumption is clinically meaningful for adolescents. “For failing Fontan patients (not studied in FUEL), these improvements are minimal but maybe relevant,” the commentators wrote. But the studies do not resolve whether that’s enough to prevent further decline.
Dr. Goldberg disclosed receiving research grants from trial sponsor Mezzion Pharmaceuticals and the National Heart Lung and Blood Institute. Dr. Sable, Dr. Gewillig, and Dr. van de Bruaene have no financial relationships to disclose.
SOURCE: Goldberg D. AHA 2019, Late Breaking Science Session 5.
PHILADELPHIA – In adolescents who have had a Fontan procedure for congenital heart disease, a randomized trial of the phosphodiesterase type 5 inhibitor udenafil showed that it achieved improved exercise performance but did not lead to significant improvement in oxygen levels or myocardial performance.
That’s according to results of the Pediatric Heart Network’s Fontan Udenafil Exercise Longitudinal Trial (FUEL) presented at the American Heart Association scientific sessions. “Treatment with udenafil was not associated with a statistically significant improvement in oxygen consumption at peak exercise, but it was associated with statistically significant improvements in exercise performance at the ventilatory anaerobic threshold,” said David J. Goldberg, MD, of Children’s Hospital of Philadelphia in reporting the FUEL results. The results were published simultaneously in Circulation (2019 Nov 17. doi: 10.1161/CIRCULATIONAHA.119.044352).
“This is the first large clinical trial to show improvement in measures of clinically relevant exercise performance in those with single-ventricle heart disease after Fontan palliation,” he said.
FUEL enrolled 400 male and female adolescents with a single functional ventricle after Fontan surgical palliation. In these patients, pulmonary vascular resistance (PVR) is critical for the efficient flow of blood through the lungs without the benefit of a ventricular pump. “While this circulation is typically stable through childhood, cardiovascular efficiency deteriorates over time, associated with a decline in exercise performance and the accrual of Fontan-associated morbidities,” Dr. Goldberg said. “Given the importance of pulmonary vascular resistance, modulators of PVR make sense as potential therapies.”
FUEL evaluated the effect of udenafil 87.5 mg twice daily versus placebo in post-Fontan patients who’d been on anticoagulation or antiplatelet therapy. The treatment group had a higher percentage of female patients (44% vs. 36% on placebo), but all other baseline characteristics were similar between the two groups.
While the trial found the drug was well tolerated and safe, with side effects typical of PDE5 inhibitors, it did not lead to changes in myocardial performance index, reactive hyperemia index, or log brain natriuretic peptide, Dr. Goldberg said.
At 6 months, both groups showed a decline in exercise data, “as expected,” Dr. Goldberg said. “But that decline was attenuated in the group receiving udenafil,” he said, with peak oxygen consumption declining an average of 0.23 and 0.89 mL/kg per minute in the treatment and placebo groups, respectively (P = 0.092).
Total oxygen consumption, however, actually improved in the udenafil group and declined in the placebo group, 44 mL/min on average versus –3.7 mL/min (P = 0.071).
“There was no significant difference in the change in peak heart rate or the change in peak oxygen saturation between the groups,” Dr. Goldberg said. But three measures at the ventilatory aerobic threshold (VAT) – oxygen consumption, work rate, and ventilation/carbon dioxide output – all showed statistically significant improvement in exercise performance.
“This has important clinical implications,” Dr. Goldberg said of the study findings. “Our study extends recent findings in highlighting the importance of submaximal exercise in the understanding of Fontan physiology. And unlike peak oxygen consumption, submaximal exercise is not constrained by the physiologic ceiling of central venous pressure inherent in exercise physiology after Fontan palliation.”
Maximum oxygen consumption at VAT is likely a more relevant measure after Fontan palliation than is central venous pressure, discussant Craig A. Sable, MD, a pediatric cardiologist in Potomac, Md., noted in his comments. “This is because VAT occurs at about 70% of maximum VO2 [oxygen consumption] in Fontan as opposed to 55% in two-ventricle physiology,” Dr. Sable said.
In adults with congenital heart disease, maximal VO2 of 45%-50% of predicted levels portends increased risk of heart failure and death. “Therefore, a medication that addresses the central deficiencies of Fontan physiology and results in improved exercise performance may allow for a longer period of symptom-free survival,” he said.
In an invited commentary in Circulation (2019 Nov 17. doi: 10.1161/CIRCULATIONAHA.119.044512), Marc Gewillig, MD, and Alexander van de Bruaene, MD, of University Hospitals Leuven (Belgium) said that the findings of FUEL and other trials of pulmonary vasodilators after Fontan leave “open for debate” whether the treatment effects of a 3%-5% improvement in oxygen consumption is clinically meaningful for adolescents. “For failing Fontan patients (not studied in FUEL), these improvements are minimal but maybe relevant,” the commentators wrote. But the studies do not resolve whether that’s enough to prevent further decline.
Dr. Goldberg disclosed receiving research grants from trial sponsor Mezzion Pharmaceuticals and the National Heart Lung and Blood Institute. Dr. Sable, Dr. Gewillig, and Dr. van de Bruaene have no financial relationships to disclose.
SOURCE: Goldberg D. AHA 2019, Late Breaking Science Session 5.
REPORTING FROM AHA 2019
Societies dig in to EXCEL trial controversy
Thoracic surgery societies on both sides of the Atlantic have released new statements on a continuing controversy dogging the EXCEL trial, one that has fueled a highly public war of words over how the study was conducted, interpreted, and reported by its investigators.
In a statement dated Dec. 19, 2019, the European Association for Cardio-Thoracic Surgery (EACTS) offered new details on why it withdrew its endorsement of the 2018 EACTS-European Society of Cardiology (ESC) clinical guidelines section covering left-main coronary artery disease.
That part of the guideline had relied in part on 3-year outcomes from EXCEL, which were published in the New England Journal of Medicine in 2016 (2016 Dec 8;375[23]:2223-35) and are central to the ongoing dispute. The trial, in essence, was a comparison of percutaneous coronary intervention (PCI) and coronary bypass surgery (CABG) in left-main disease. In that report, PCI was noninferior to CABG with respect to the composite endpoint of death, stroke, or myocardial infarction at 3 years in patients with left-main disease and low or intermediate anatomical complexity.
The new statement, signed by the society’s secretary general Domenico Pagano, MD, also calls for a new EACTS-ESC evidence review and development of updated recommendations for left-main disease “as a matter of urgency.”
For its part, the ESC had earlier declared its continuing support for the full guideline but hinted that might change pending further details on EXCEL yet to be made public.
The EACTS statement follows the society’s earlier announcement that it would pull support of the guideline section on left-main disease in response to a Dec. 9, 2019, news report from BBC Newsnight that was critical of the EXCEL trial’s methodology and reporting.
The news story made a number of allegations regarding the interpretation and reporting of EXCEL based largely on unpublished data it had obtained through unofficial channels.
Key among them was that reanalysis of myocardial infarction outcomes using the Third Universal Definition of MI, rather than the primarily enzymatic definition on which the reported outcomes were based, substantially raised the MI count in the PCI group, compared with those who had CABG.
The data for that alternative analysis, which had not been publicly reported, seemed to recast the published EXCEL primary outcome from one of parity for PCI and CABG in left-main disease to one that significantly favored CABG, noted the BBC Newsnight story.
Also, the news story claimed that EXCEL investigators had promised to publicly release the trial’s data based on the Third Universal Definition of MI, but had not done so, and had not adequately heeded concerns raised by its Data Safety Monitoring Board (DSMB) over signs of an apparently increased mortality risk from PCI.
Another society weighs in
The unreported data and other issues have led the American Association for Thoracic Surgery (AATS) to issue a statement acknowledging the possibility of misguided treatment recommendations, and therefore patient care, stemming from incomplete reporting of EXCEL.
If there are serious concerns about the “presentation or interpretation” of clinical trials, “then the best way forward is the public release of all trial data for an independent analysis to confirm that the original trial conclusions are valid,” says the statement, signed by AATS president Vaughn A. Starnes, MD, and secretary David R. Jones, MD.
“The AATS agrees with others that all of the data should be made publicly available for analysis and interpretation, as a way to resolve the current controversy around the EXCEL trial, in order to provide patients with the best possible counsel and informed consent,” it states.
The BBC Newsnight story “raised legitimate questions regarding what data was/was not presented to the other EXCEL investigators and to [the] ESC/EACTS guideline committee, and what, when, and to whom were safety warnings raised by the DSMB,” David Taggart, MD, PhD, wrote in an email interview.
“Until these issues are resolved, both EACTS and AATS have expressed concerns about what has happened and, most importantly, the potential implications for patient safety. This stance underpins their sincerity that patient safety, genuine informed consent, and scientific integrity are amongst their highest priorities. Consequently they have my complete support,” said Dr. Taggart, of the University of Oxford (England) a former EXCEL trialist who has been among the most vocal critics of how the EXCEL leadership has interpreted and reported the trial’s outcomes.
“Personally, I do not feel that the current controversy over the EXCEL trial will be resolved until there is full and independent reanalysis of its data. I feel that this would be absolutely crucial in reassuring our patients, the wider medical community, and the general public of the validity of current recommendations.”
An EXCEL principal investigator and prominent public voice for the trial, Gregg W. Stone, MD, of Icahn School of Medicine at Mount Sinai, New York, has not responded to requests for comment on the new society statements.
Point-counterpoint
As previously reported, allegations about EXCEL in news reports and the sometimes fiery public debate led the trialists to release a long and wide-ranging public communique that forcefully disputes the charges. Among them, that they were either remiss or willfully deceptive in not reporting an analysis based on the Third Universal Definition of MI.
In response, Dr. Taggart provided a toughly worded statement that disputes the EXCEL leadership’s missive nearly point by point. It variously describes the assertions as “simplistic,” seemingly “illogical,” “disingenuous,” and “factually completely incorrect,” among other terms.
The document provides Dr. Taggart’s perspective on how MI was defined and interpreted while he was an active member of the EXCEL trial’s leadership, and alleged shortfalls in how outcomes were interpreted and reported.
In it, Taggart also wonders whether or not EXCEL leadership had possibly been aware of a tilt favoring CABG in the analysis based on Third Universal Definition of MI but “decided to suppress it,” and also whether the trial’s sponsor, Abbott Vascular, had influenced the trial’s conduct.
Despite the EXCEL leadership’s communique, “my profound concerns remain the same and, in my opinion, the very long rebuttal response by the EXCEL investigators does not adequately respond to the core issues,” Dr. Taggart writes.
He withdrew his name as an author on the trial’s 5-year outcomes publication, Dr. Taggart says, because “I believed, and still do, that the final interpretation of the actual data in the [New England Journal of Medicine] manuscript did not appropriately reflect its clinical reality, and especially with regards to mortality, and would therefore have potential to do real harm to patients.”
Dr. Taggart has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.
Thoracic surgery societies on both sides of the Atlantic have released new statements on a continuing controversy dogging the EXCEL trial, one that has fueled a highly public war of words over how the study was conducted, interpreted, and reported by its investigators.
In a statement dated Dec. 19, 2019, the European Association for Cardio-Thoracic Surgery (EACTS) offered new details on why it withdrew its endorsement of the 2018 EACTS-European Society of Cardiology (ESC) clinical guidelines section covering left-main coronary artery disease.
That part of the guideline had relied in part on 3-year outcomes from EXCEL, which were published in the New England Journal of Medicine in 2016 (2016 Dec 8;375[23]:2223-35) and are central to the ongoing dispute. The trial, in essence, was a comparison of percutaneous coronary intervention (PCI) and coronary bypass surgery (CABG) in left-main disease. In that report, PCI was noninferior to CABG with respect to the composite endpoint of death, stroke, or myocardial infarction at 3 years in patients with left-main disease and low or intermediate anatomical complexity.
The new statement, signed by the society’s secretary general Domenico Pagano, MD, also calls for a new EACTS-ESC evidence review and development of updated recommendations for left-main disease “as a matter of urgency.”
For its part, the ESC had earlier declared its continuing support for the full guideline but hinted that might change pending further details on EXCEL yet to be made public.
The EACTS statement follows the society’s earlier announcement that it would pull support of the guideline section on left-main disease in response to a Dec. 9, 2019, news report from BBC Newsnight that was critical of the EXCEL trial’s methodology and reporting.
The news story made a number of allegations regarding the interpretation and reporting of EXCEL based largely on unpublished data it had obtained through unofficial channels.
Key among them was that reanalysis of myocardial infarction outcomes using the Third Universal Definition of MI, rather than the primarily enzymatic definition on which the reported outcomes were based, substantially raised the MI count in the PCI group, compared with those who had CABG.
The data for that alternative analysis, which had not been publicly reported, seemed to recast the published EXCEL primary outcome from one of parity for PCI and CABG in left-main disease to one that significantly favored CABG, noted the BBC Newsnight story.
Also, the news story claimed that EXCEL investigators had promised to publicly release the trial’s data based on the Third Universal Definition of MI, but had not done so, and had not adequately heeded concerns raised by its Data Safety Monitoring Board (DSMB) over signs of an apparently increased mortality risk from PCI.
Another society weighs in
The unreported data and other issues have led the American Association for Thoracic Surgery (AATS) to issue a statement acknowledging the possibility of misguided treatment recommendations, and therefore patient care, stemming from incomplete reporting of EXCEL.
If there are serious concerns about the “presentation or interpretation” of clinical trials, “then the best way forward is the public release of all trial data for an independent analysis to confirm that the original trial conclusions are valid,” says the statement, signed by AATS president Vaughn A. Starnes, MD, and secretary David R. Jones, MD.
“The AATS agrees with others that all of the data should be made publicly available for analysis and interpretation, as a way to resolve the current controversy around the EXCEL trial, in order to provide patients with the best possible counsel and informed consent,” it states.
The BBC Newsnight story “raised legitimate questions regarding what data was/was not presented to the other EXCEL investigators and to [the] ESC/EACTS guideline committee, and what, when, and to whom were safety warnings raised by the DSMB,” David Taggart, MD, PhD, wrote in an email interview.
“Until these issues are resolved, both EACTS and AATS have expressed concerns about what has happened and, most importantly, the potential implications for patient safety. This stance underpins their sincerity that patient safety, genuine informed consent, and scientific integrity are amongst their highest priorities. Consequently they have my complete support,” said Dr. Taggart, of the University of Oxford (England) a former EXCEL trialist who has been among the most vocal critics of how the EXCEL leadership has interpreted and reported the trial’s outcomes.
“Personally, I do not feel that the current controversy over the EXCEL trial will be resolved until there is full and independent reanalysis of its data. I feel that this would be absolutely crucial in reassuring our patients, the wider medical community, and the general public of the validity of current recommendations.”
An EXCEL principal investigator and prominent public voice for the trial, Gregg W. Stone, MD, of Icahn School of Medicine at Mount Sinai, New York, has not responded to requests for comment on the new society statements.
Point-counterpoint
As previously reported, allegations about EXCEL in news reports and the sometimes fiery public debate led the trialists to release a long and wide-ranging public communique that forcefully disputes the charges. Among them, that they were either remiss or willfully deceptive in not reporting an analysis based on the Third Universal Definition of MI.
In response, Dr. Taggart provided a toughly worded statement that disputes the EXCEL leadership’s missive nearly point by point. It variously describes the assertions as “simplistic,” seemingly “illogical,” “disingenuous,” and “factually completely incorrect,” among other terms.
The document provides Dr. Taggart’s perspective on how MI was defined and interpreted while he was an active member of the EXCEL trial’s leadership, and alleged shortfalls in how outcomes were interpreted and reported.
In it, Taggart also wonders whether or not EXCEL leadership had possibly been aware of a tilt favoring CABG in the analysis based on Third Universal Definition of MI but “decided to suppress it,” and also whether the trial’s sponsor, Abbott Vascular, had influenced the trial’s conduct.
Despite the EXCEL leadership’s communique, “my profound concerns remain the same and, in my opinion, the very long rebuttal response by the EXCEL investigators does not adequately respond to the core issues,” Dr. Taggart writes.
He withdrew his name as an author on the trial’s 5-year outcomes publication, Dr. Taggart says, because “I believed, and still do, that the final interpretation of the actual data in the [New England Journal of Medicine] manuscript did not appropriately reflect its clinical reality, and especially with regards to mortality, and would therefore have potential to do real harm to patients.”
Dr. Taggart has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.
Thoracic surgery societies on both sides of the Atlantic have released new statements on a continuing controversy dogging the EXCEL trial, one that has fueled a highly public war of words over how the study was conducted, interpreted, and reported by its investigators.
In a statement dated Dec. 19, 2019, the European Association for Cardio-Thoracic Surgery (EACTS) offered new details on why it withdrew its endorsement of the 2018 EACTS-European Society of Cardiology (ESC) clinical guidelines section covering left-main coronary artery disease.
That part of the guideline had relied in part on 3-year outcomes from EXCEL, which were published in the New England Journal of Medicine in 2016 (2016 Dec 8;375[23]:2223-35) and are central to the ongoing dispute. The trial, in essence, was a comparison of percutaneous coronary intervention (PCI) and coronary bypass surgery (CABG) in left-main disease. In that report, PCI was noninferior to CABG with respect to the composite endpoint of death, stroke, or myocardial infarction at 3 years in patients with left-main disease and low or intermediate anatomical complexity.
The new statement, signed by the society’s secretary general Domenico Pagano, MD, also calls for a new EACTS-ESC evidence review and development of updated recommendations for left-main disease “as a matter of urgency.”
For its part, the ESC had earlier declared its continuing support for the full guideline but hinted that might change pending further details on EXCEL yet to be made public.
The EACTS statement follows the society’s earlier announcement that it would pull support of the guideline section on left-main disease in response to a Dec. 9, 2019, news report from BBC Newsnight that was critical of the EXCEL trial’s methodology and reporting.
The news story made a number of allegations regarding the interpretation and reporting of EXCEL based largely on unpublished data it had obtained through unofficial channels.
Key among them was that reanalysis of myocardial infarction outcomes using the Third Universal Definition of MI, rather than the primarily enzymatic definition on which the reported outcomes were based, substantially raised the MI count in the PCI group, compared with those who had CABG.
The data for that alternative analysis, which had not been publicly reported, seemed to recast the published EXCEL primary outcome from one of parity for PCI and CABG in left-main disease to one that significantly favored CABG, noted the BBC Newsnight story.
Also, the news story claimed that EXCEL investigators had promised to publicly release the trial’s data based on the Third Universal Definition of MI, but had not done so, and had not adequately heeded concerns raised by its Data Safety Monitoring Board (DSMB) over signs of an apparently increased mortality risk from PCI.
Another society weighs in
The unreported data and other issues have led the American Association for Thoracic Surgery (AATS) to issue a statement acknowledging the possibility of misguided treatment recommendations, and therefore patient care, stemming from incomplete reporting of EXCEL.
If there are serious concerns about the “presentation or interpretation” of clinical trials, “then the best way forward is the public release of all trial data for an independent analysis to confirm that the original trial conclusions are valid,” says the statement, signed by AATS president Vaughn A. Starnes, MD, and secretary David R. Jones, MD.
“The AATS agrees with others that all of the data should be made publicly available for analysis and interpretation, as a way to resolve the current controversy around the EXCEL trial, in order to provide patients with the best possible counsel and informed consent,” it states.
The BBC Newsnight story “raised legitimate questions regarding what data was/was not presented to the other EXCEL investigators and to [the] ESC/EACTS guideline committee, and what, when, and to whom were safety warnings raised by the DSMB,” David Taggart, MD, PhD, wrote in an email interview.
“Until these issues are resolved, both EACTS and AATS have expressed concerns about what has happened and, most importantly, the potential implications for patient safety. This stance underpins their sincerity that patient safety, genuine informed consent, and scientific integrity are amongst their highest priorities. Consequently they have my complete support,” said Dr. Taggart, of the University of Oxford (England) a former EXCEL trialist who has been among the most vocal critics of how the EXCEL leadership has interpreted and reported the trial’s outcomes.
“Personally, I do not feel that the current controversy over the EXCEL trial will be resolved until there is full and independent reanalysis of its data. I feel that this would be absolutely crucial in reassuring our patients, the wider medical community, and the general public of the validity of current recommendations.”
An EXCEL principal investigator and prominent public voice for the trial, Gregg W. Stone, MD, of Icahn School of Medicine at Mount Sinai, New York, has not responded to requests for comment on the new society statements.
Point-counterpoint
As previously reported, allegations about EXCEL in news reports and the sometimes fiery public debate led the trialists to release a long and wide-ranging public communique that forcefully disputes the charges. Among them, that they were either remiss or willfully deceptive in not reporting an analysis based on the Third Universal Definition of MI.
In response, Dr. Taggart provided a toughly worded statement that disputes the EXCEL leadership’s missive nearly point by point. It variously describes the assertions as “simplistic,” seemingly “illogical,” “disingenuous,” and “factually completely incorrect,” among other terms.
The document provides Dr. Taggart’s perspective on how MI was defined and interpreted while he was an active member of the EXCEL trial’s leadership, and alleged shortfalls in how outcomes were interpreted and reported.
In it, Taggart also wonders whether or not EXCEL leadership had possibly been aware of a tilt favoring CABG in the analysis based on Third Universal Definition of MI but “decided to suppress it,” and also whether the trial’s sponsor, Abbott Vascular, had influenced the trial’s conduct.
Despite the EXCEL leadership’s communique, “my profound concerns remain the same and, in my opinion, the very long rebuttal response by the EXCEL investigators does not adequately respond to the core issues,” Dr. Taggart writes.
He withdrew his name as an author on the trial’s 5-year outcomes publication, Dr. Taggart says, because “I believed, and still do, that the final interpretation of the actual data in the [New England Journal of Medicine] manuscript did not appropriately reflect its clinical reality, and especially with regards to mortality, and would therefore have potential to do real harm to patients.”
Dr. Taggart has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.
Mechanical circulatory support in PCI needs clearer guidance
PHILADELPHIA – Use of the Impella ventricular-assist device in patients with cardiogenic shock having percutaneous coronary interventions (PCI) has increased rapidly since its approval in 2008, but two studies comparing it with intra-aortic balloon pumps in PCI patients have raised questions about the safety, effectiveness, and cost of the ventricular-assist device, according to results of two studies presented at the American Heart Association scientific sessions.
The results of an observational analysis of 48,306 patients and a national real-world study of 28,304 patients may not be telling the complete story of the utility of ventricular assist in patients requiring mechanical circulatory support (MCS), one interventional cardiologist said in an interview. “It’s concerning; it’s sobering,” said Ranya N. Sweis, MD, of Northwestern University, Chicago. However, the data didn’t parse out patients who would have been routed to palliative care and otherwise wouldn’t have been candidates for PCI without MCS.
“What I take from it is that we need to get more randomized data,” she said. “Who are the patients that were doing worse? Who are the patients who really needed the Impella support for the PCI after cardiogenic shock?”
In the observational study, Amit P. Amin, MD, of Washington University, St. Louis, said that the use of MCS devices increased steadily to 32% of all PCI patients receiving MCS from 2008 to 2016 while use of intra-aortic balloon pump (IABP) declined, but that Impella was less likely to be used in critically ill patients. The study analyzed patients in the Premier Healthcare Database who had PCI with MCS at 432 hospitals from 2004 to 2016.
Outcomes in what Dr. Amin called “the Impella era,” showed significantly higher risks for death, acute kidney injury, and stroke, with odds ratios of 1.17, 1.91 and 3.34, respectively (P less than .001 for all). In the patient-level comparison of Impella versus IABP, Impella had a 24% higher risk of death (P less than .0001), 10% for bleeding (P = .0445), 8% for acute kidney injury (P = .0521) and 34% for stroke (P less than .0001). The findings were published simultaneously with the presentation (Circulation. 2019 Nov 17. doi: 10.1161/CIRCULATIONAHA.119.044007)
“The total length of stay, as well as the ICU length of stay, were actually lower with Impella use, by approximately a half day to 1 day,” Dr. Amin said. “Despite that, the total costs were approximately $15,000.”
Yet, the study found wide variation in the use of Impella among hospitals, some doing no cases with the device and others all of them, Dr. Amin said. The risk analysis also found wide variations in outcomes across hospitals using Impella. “We saw a 2.5-fold variation in bleeding across hospitals and a 1.5-fold variation in acute kidney injury, stroke and death,” he noted. The study found less variation in hospital stays and total cost of Impella, “perhaps related to the uniformly high device acquisition costs.”
“These data underscore the need for defining the appropriate use of mechanical circulatory support in patients undergoing PCI,” Dr. Amin said.
Dr. Sweis wasn’t surprised by the cost findings. “New technology is going to cost more,” she said in an interview. “I’m actually surprised that the cost wasn’t more significantly different just knowing the cost of some of these devices.
Patients who require MCS represent a small portion of PCI cases: 2%, according to Dr. Sweis. “It’s not like all PCI has increased because of MCS, and there’s a potential improvement in the length of stay so there are going to be cost savings that way.”
The national real-world study that Sanket S. Dhruva, MD, MHS, of the University of California San Francisco, reported on focused on Impella and IABP in PCI patients with acute MI complicated by cardiogenic shock (CS). The study used outcomes of patients with AMI-CS who had PCI from October 2015 to December 2017 in the National Cardiovascular Data Registry’s CathPCI and Chest Pain–MI registries. An estimated 4%-12% of AMIs present with CS.
Most patients in the study population had medical therapy only, but this study focused on the 1,768 who had Impella only and the 8,471 who had IABP only. The rates of in-hospital death and bleeding were 34.1% 16% in the IABP group, and 45% and 31.3% in the Impella group, Dr. Dhruva said. In this study population, the rate of Impella use increased from 3.5% in 2015 to 8.7% by the end of 2017 (P less than .001).
Dr. Dhruva acknowledged a number of limitations to the study findings, including residual confounding. However, the “robust propensity match” of 95% of the Impella-only patients and the results were consistent across multiple sensitivity analyses. “There may have been questions about the clinical severity of AMI-CS patients in the NCDR Registry,” he said. “However, the registry definition is similar to that used in the trials.”
The trial also failed to distinguish between the different types of Impella devices, but the results mostly pertain to the Impella 2.5 and CP because the 5.0 device requires a surgical cutdown, and the study excluded patients who received multiple devices.
“Better evidence and guidance are needed regarding the optimal management of patients with AMI-CS as well as the role of mechanical circulatory support devices in general and Impella in particular,” he said, adding that Impella has been on the U.S. market since 2008, but with limited randomized clinical trial evidence in cardiogenic shock.
The study population of patient’s with CS is “only a piece of the puzzle,” Dr. Sweis said. “We know that there are sick hearts that aren’t in shock right now, but you’re going to do triple-vessel intervention and use atherectomy. Those patients would not do very well during the procedure itself and it may not even be offered to them if there weren’t support.”
Impella is not going away, Dr. Sweis said. “It provides an option that a patient wouldn’t otherwise have. This is really stressing to me that we need to get rid of that variability in the safety related to these devices.”
Dr. Amin disclosed financial relationships with Terumo and GE Healthcare. Dr. Dhruva had no financial relationships to disclose. The study was supported in part by a Center of Excellence in Regulatory Science and Innovation grant from the Food and Drug Administration and the American College of Cardiology’s National Cardiovascular Data Registry.
PHILADELPHIA – Use of the Impella ventricular-assist device in patients with cardiogenic shock having percutaneous coronary interventions (PCI) has increased rapidly since its approval in 2008, but two studies comparing it with intra-aortic balloon pumps in PCI patients have raised questions about the safety, effectiveness, and cost of the ventricular-assist device, according to results of two studies presented at the American Heart Association scientific sessions.
The results of an observational analysis of 48,306 patients and a national real-world study of 28,304 patients may not be telling the complete story of the utility of ventricular assist in patients requiring mechanical circulatory support (MCS), one interventional cardiologist said in an interview. “It’s concerning; it’s sobering,” said Ranya N. Sweis, MD, of Northwestern University, Chicago. However, the data didn’t parse out patients who would have been routed to palliative care and otherwise wouldn’t have been candidates for PCI without MCS.
“What I take from it is that we need to get more randomized data,” she said. “Who are the patients that were doing worse? Who are the patients who really needed the Impella support for the PCI after cardiogenic shock?”
In the observational study, Amit P. Amin, MD, of Washington University, St. Louis, said that the use of MCS devices increased steadily to 32% of all PCI patients receiving MCS from 2008 to 2016 while use of intra-aortic balloon pump (IABP) declined, but that Impella was less likely to be used in critically ill patients. The study analyzed patients in the Premier Healthcare Database who had PCI with MCS at 432 hospitals from 2004 to 2016.
Outcomes in what Dr. Amin called “the Impella era,” showed significantly higher risks for death, acute kidney injury, and stroke, with odds ratios of 1.17, 1.91 and 3.34, respectively (P less than .001 for all). In the patient-level comparison of Impella versus IABP, Impella had a 24% higher risk of death (P less than .0001), 10% for bleeding (P = .0445), 8% for acute kidney injury (P = .0521) and 34% for stroke (P less than .0001). The findings were published simultaneously with the presentation (Circulation. 2019 Nov 17. doi: 10.1161/CIRCULATIONAHA.119.044007)
“The total length of stay, as well as the ICU length of stay, were actually lower with Impella use, by approximately a half day to 1 day,” Dr. Amin said. “Despite that, the total costs were approximately $15,000.”
Yet, the study found wide variation in the use of Impella among hospitals, some doing no cases with the device and others all of them, Dr. Amin said. The risk analysis also found wide variations in outcomes across hospitals using Impella. “We saw a 2.5-fold variation in bleeding across hospitals and a 1.5-fold variation in acute kidney injury, stroke and death,” he noted. The study found less variation in hospital stays and total cost of Impella, “perhaps related to the uniformly high device acquisition costs.”
“These data underscore the need for defining the appropriate use of mechanical circulatory support in patients undergoing PCI,” Dr. Amin said.
Dr. Sweis wasn’t surprised by the cost findings. “New technology is going to cost more,” she said in an interview. “I’m actually surprised that the cost wasn’t more significantly different just knowing the cost of some of these devices.
Patients who require MCS represent a small portion of PCI cases: 2%, according to Dr. Sweis. “It’s not like all PCI has increased because of MCS, and there’s a potential improvement in the length of stay so there are going to be cost savings that way.”
The national real-world study that Sanket S. Dhruva, MD, MHS, of the University of California San Francisco, reported on focused on Impella and IABP in PCI patients with acute MI complicated by cardiogenic shock (CS). The study used outcomes of patients with AMI-CS who had PCI from October 2015 to December 2017 in the National Cardiovascular Data Registry’s CathPCI and Chest Pain–MI registries. An estimated 4%-12% of AMIs present with CS.
Most patients in the study population had medical therapy only, but this study focused on the 1,768 who had Impella only and the 8,471 who had IABP only. The rates of in-hospital death and bleeding were 34.1% 16% in the IABP group, and 45% and 31.3% in the Impella group, Dr. Dhruva said. In this study population, the rate of Impella use increased from 3.5% in 2015 to 8.7% by the end of 2017 (P less than .001).
Dr. Dhruva acknowledged a number of limitations to the study findings, including residual confounding. However, the “robust propensity match” of 95% of the Impella-only patients and the results were consistent across multiple sensitivity analyses. “There may have been questions about the clinical severity of AMI-CS patients in the NCDR Registry,” he said. “However, the registry definition is similar to that used in the trials.”
The trial also failed to distinguish between the different types of Impella devices, but the results mostly pertain to the Impella 2.5 and CP because the 5.0 device requires a surgical cutdown, and the study excluded patients who received multiple devices.
“Better evidence and guidance are needed regarding the optimal management of patients with AMI-CS as well as the role of mechanical circulatory support devices in general and Impella in particular,” he said, adding that Impella has been on the U.S. market since 2008, but with limited randomized clinical trial evidence in cardiogenic shock.
The study population of patient’s with CS is “only a piece of the puzzle,” Dr. Sweis said. “We know that there are sick hearts that aren’t in shock right now, but you’re going to do triple-vessel intervention and use atherectomy. Those patients would not do very well during the procedure itself and it may not even be offered to them if there weren’t support.”
Impella is not going away, Dr. Sweis said. “It provides an option that a patient wouldn’t otherwise have. This is really stressing to me that we need to get rid of that variability in the safety related to these devices.”
Dr. Amin disclosed financial relationships with Terumo and GE Healthcare. Dr. Dhruva had no financial relationships to disclose. The study was supported in part by a Center of Excellence in Regulatory Science and Innovation grant from the Food and Drug Administration and the American College of Cardiology’s National Cardiovascular Data Registry.
PHILADELPHIA – Use of the Impella ventricular-assist device in patients with cardiogenic shock having percutaneous coronary interventions (PCI) has increased rapidly since its approval in 2008, but two studies comparing it with intra-aortic balloon pumps in PCI patients have raised questions about the safety, effectiveness, and cost of the ventricular-assist device, according to results of two studies presented at the American Heart Association scientific sessions.
The results of an observational analysis of 48,306 patients and a national real-world study of 28,304 patients may not be telling the complete story of the utility of ventricular assist in patients requiring mechanical circulatory support (MCS), one interventional cardiologist said in an interview. “It’s concerning; it’s sobering,” said Ranya N. Sweis, MD, of Northwestern University, Chicago. However, the data didn’t parse out patients who would have been routed to palliative care and otherwise wouldn’t have been candidates for PCI without MCS.
“What I take from it is that we need to get more randomized data,” she said. “Who are the patients that were doing worse? Who are the patients who really needed the Impella support for the PCI after cardiogenic shock?”
In the observational study, Amit P. Amin, MD, of Washington University, St. Louis, said that the use of MCS devices increased steadily to 32% of all PCI patients receiving MCS from 2008 to 2016 while use of intra-aortic balloon pump (IABP) declined, but that Impella was less likely to be used in critically ill patients. The study analyzed patients in the Premier Healthcare Database who had PCI with MCS at 432 hospitals from 2004 to 2016.
Outcomes in what Dr. Amin called “the Impella era,” showed significantly higher risks for death, acute kidney injury, and stroke, with odds ratios of 1.17, 1.91 and 3.34, respectively (P less than .001 for all). In the patient-level comparison of Impella versus IABP, Impella had a 24% higher risk of death (P less than .0001), 10% for bleeding (P = .0445), 8% for acute kidney injury (P = .0521) and 34% for stroke (P less than .0001). The findings were published simultaneously with the presentation (Circulation. 2019 Nov 17. doi: 10.1161/CIRCULATIONAHA.119.044007)
“The total length of stay, as well as the ICU length of stay, were actually lower with Impella use, by approximately a half day to 1 day,” Dr. Amin said. “Despite that, the total costs were approximately $15,000.”
Yet, the study found wide variation in the use of Impella among hospitals, some doing no cases with the device and others all of them, Dr. Amin said. The risk analysis also found wide variations in outcomes across hospitals using Impella. “We saw a 2.5-fold variation in bleeding across hospitals and a 1.5-fold variation in acute kidney injury, stroke and death,” he noted. The study found less variation in hospital stays and total cost of Impella, “perhaps related to the uniformly high device acquisition costs.”
“These data underscore the need for defining the appropriate use of mechanical circulatory support in patients undergoing PCI,” Dr. Amin said.
Dr. Sweis wasn’t surprised by the cost findings. “New technology is going to cost more,” she said in an interview. “I’m actually surprised that the cost wasn’t more significantly different just knowing the cost of some of these devices.
Patients who require MCS represent a small portion of PCI cases: 2%, according to Dr. Sweis. “It’s not like all PCI has increased because of MCS, and there’s a potential improvement in the length of stay so there are going to be cost savings that way.”
The national real-world study that Sanket S. Dhruva, MD, MHS, of the University of California San Francisco, reported on focused on Impella and IABP in PCI patients with acute MI complicated by cardiogenic shock (CS). The study used outcomes of patients with AMI-CS who had PCI from October 2015 to December 2017 in the National Cardiovascular Data Registry’s CathPCI and Chest Pain–MI registries. An estimated 4%-12% of AMIs present with CS.
Most patients in the study population had medical therapy only, but this study focused on the 1,768 who had Impella only and the 8,471 who had IABP only. The rates of in-hospital death and bleeding were 34.1% 16% in the IABP group, and 45% and 31.3% in the Impella group, Dr. Dhruva said. In this study population, the rate of Impella use increased from 3.5% in 2015 to 8.7% by the end of 2017 (P less than .001).
Dr. Dhruva acknowledged a number of limitations to the study findings, including residual confounding. However, the “robust propensity match” of 95% of the Impella-only patients and the results were consistent across multiple sensitivity analyses. “There may have been questions about the clinical severity of AMI-CS patients in the NCDR Registry,” he said. “However, the registry definition is similar to that used in the trials.”
The trial also failed to distinguish between the different types of Impella devices, but the results mostly pertain to the Impella 2.5 and CP because the 5.0 device requires a surgical cutdown, and the study excluded patients who received multiple devices.
“Better evidence and guidance are needed regarding the optimal management of patients with AMI-CS as well as the role of mechanical circulatory support devices in general and Impella in particular,” he said, adding that Impella has been on the U.S. market since 2008, but with limited randomized clinical trial evidence in cardiogenic shock.
The study population of patient’s with CS is “only a piece of the puzzle,” Dr. Sweis said. “We know that there are sick hearts that aren’t in shock right now, but you’re going to do triple-vessel intervention and use atherectomy. Those patients would not do very well during the procedure itself and it may not even be offered to them if there weren’t support.”
Impella is not going away, Dr. Sweis said. “It provides an option that a patient wouldn’t otherwise have. This is really stressing to me that we need to get rid of that variability in the safety related to these devices.”
Dr. Amin disclosed financial relationships with Terumo and GE Healthcare. Dr. Dhruva had no financial relationships to disclose. The study was supported in part by a Center of Excellence in Regulatory Science and Innovation grant from the Food and Drug Administration and the American College of Cardiology’s National Cardiovascular Data Registry.
REPORTING FROM AHA 2019
CvLPRIT: Complete revascularization benefits persist long term
The greater reduction in major adverse cardiovascular events with complete revascularization for ST-segment elevation myocardial infarction, compared with target-lesion only, persists for many years after the procedure, a study has found.
In the Journal of the American College of Cardiology, researchers report the outcomes of long-term follow-up of 272 patients admitted with ST-segment elevation myocardial infarction, who were enrolled in CvLPRIT (Complete Versus Lesion-Only Primary PCI Trial).
The trial randomized patients to complete revascularization or infarct-related artery revascularization only, with a median follow-up of 5.6 years after randomization.
Anthony H. Gershlick, MD, from the University of Leicester (England) and NIHR Leicester Biomedical Research Centre, and coauthors highlighted conflicting evidence on the relative benefit of complete revascularization, compared with revascularization focused on the culprit artery only.
“The aim of this study was, for the first time, to determine if there is a sustained benefit in favor of multivessel percutaneous coronary intervention [PCI] in the longer term,” they wrote.
In the group of patients who underwent complete revascularization, the composite major adverse cardiovascular event rate at 5.6 years was 43% lower than in the infarct-related artery revascularization group (24.0 vs. 37.7%; P = .0079), according to the intention-to-treat analysis.
The complete revascularization group also showed a significantly lower rate of the secondary composite endpoint of death or MI, which was 10% in the complete revascularization group and 18.5% in the target lesion group (hazard ratio, 0.47; P = .0175).
“Our data suggest that total revascularization, known to have benefits in various cohorts with coronary artery disease, should now probably be considered the standard of care in suitable patients with STEMI with multivessel disease,” they wrote.
However they did find that the rates of ischemia-driven revascularization were not significantly different between the two groups at the long-term follow-up.
The authors also did an analysis of outcomes from the end of the original 12-month study to the final follow-up point. This showed a nonsignificant trend toward a lower rate of major adverse cardiovascular events in the group who underwent complete revascularization; 17.1%, compared with 23.3% in the infarct-related artery revascularization group. The rates of the individual components of that primary endpoint also trended toward lower rates in individuals with complete revascularization.
Similarly, the rates of ischemia-driven revascularization were similar in both groups when analyzed after the 12-month mark, and the authors noted that the need for ischemia-driven revascularization was equally spread between infarct-related arteries and non–infarct-related arteries.
The authors commented that the event rate curves for the two groups remained separated even to the median follow-up point of 5.6 years, showing that the highly significant difference in major adverse cardiovascular event rates between the two groups persists.
“All of these data suggest that lower rates of events seen within 12 months do translate into longer-term benefit, predominantly through nonattenuation of benefit,” they wrote.
They speculated that the longer-term benefit of early complete revascularization could be the result of improvement in blood flow to areas around the original site of ischemia, and because it managed lesions in nontarget vessels in patients with disease in multiple arteries.
“Certainly, given that both the MRI and nuclear medicine substudies of CvLPRIT showed no difference between the groups in infarct size (at 1 week) and no difference in ischemic burden at 6 weeks, the benefit we have demonstrated does not appear to be explained simply in terms of ischemic burden being dealt with prophylactically in the complete group,” they wrote.
Commenting on the study’s limitations, the authors noted that the overall numbers of patients were small, and that the use of all-cause mortality rather than cardiovascular mortality may affect the interpretation of results.
The CvLPRIT study was funded by the British Heart Foundation, with support from the National Institute for Health Research Comprehensive Local Research Networks. No conflicts of interest were declared.
SOURCE: Gershlick A et al. J Am Coll Cardiol. 2019 Dec 16;74:3083-9.
Multivessel coronary artery disease is present in around half of all patients presenting with STEMI and is associated with worse outcomes. However the decision about whether to revascularize beyond the culprit lesions – including lesions that may be asymptomatic and cause no ischemia – has been a matter of debate.
This longer-term follow-up from the CvLPRIT trial, along with evidence from other studies, has confirmed that complete revascularization should be considered in STEMI patients with multivessel disease. However, we suggest an individualized approach, rather than one-size-fits-all. This should also take into account factors such as the patient’s age and comorbidities, to avoid futile complex procedures in very old or frail patients. It is also important not to underestimate the importance of revascularization of the target lesion only.
There also remain questions about the best timing for complete revascularization and how to select the nonculprit lesions for revascularization.
Guillaume Cayla, MD, and Benoit Lattuca, MD, are from the Service de cardiologie, CHU de Nimes, ACTION Study Group at the Université de Montpellier, Nimes, France. These comments are adapted from an accompanying editorial (J Am Coll Cardiol. 2019; 74:3095-8. doi. org/10.1016/j.jacc.2019.10.037). Both authors declared research grants and lecture or consultancy fees from the pharmaceutical sector.
Multivessel coronary artery disease is present in around half of all patients presenting with STEMI and is associated with worse outcomes. However the decision about whether to revascularize beyond the culprit lesions – including lesions that may be asymptomatic and cause no ischemia – has been a matter of debate.
This longer-term follow-up from the CvLPRIT trial, along with evidence from other studies, has confirmed that complete revascularization should be considered in STEMI patients with multivessel disease. However, we suggest an individualized approach, rather than one-size-fits-all. This should also take into account factors such as the patient’s age and comorbidities, to avoid futile complex procedures in very old or frail patients. It is also important not to underestimate the importance of revascularization of the target lesion only.
There also remain questions about the best timing for complete revascularization and how to select the nonculprit lesions for revascularization.
Guillaume Cayla, MD, and Benoit Lattuca, MD, are from the Service de cardiologie, CHU de Nimes, ACTION Study Group at the Université de Montpellier, Nimes, France. These comments are adapted from an accompanying editorial (J Am Coll Cardiol. 2019; 74:3095-8. doi. org/10.1016/j.jacc.2019.10.037). Both authors declared research grants and lecture or consultancy fees from the pharmaceutical sector.
Multivessel coronary artery disease is present in around half of all patients presenting with STEMI and is associated with worse outcomes. However the decision about whether to revascularize beyond the culprit lesions – including lesions that may be asymptomatic and cause no ischemia – has been a matter of debate.
This longer-term follow-up from the CvLPRIT trial, along with evidence from other studies, has confirmed that complete revascularization should be considered in STEMI patients with multivessel disease. However, we suggest an individualized approach, rather than one-size-fits-all. This should also take into account factors such as the patient’s age and comorbidities, to avoid futile complex procedures in very old or frail patients. It is also important not to underestimate the importance of revascularization of the target lesion only.
There also remain questions about the best timing for complete revascularization and how to select the nonculprit lesions for revascularization.
Guillaume Cayla, MD, and Benoit Lattuca, MD, are from the Service de cardiologie, CHU de Nimes, ACTION Study Group at the Université de Montpellier, Nimes, France. These comments are adapted from an accompanying editorial (J Am Coll Cardiol. 2019; 74:3095-8. doi. org/10.1016/j.jacc.2019.10.037). Both authors declared research grants and lecture or consultancy fees from the pharmaceutical sector.
The greater reduction in major adverse cardiovascular events with complete revascularization for ST-segment elevation myocardial infarction, compared with target-lesion only, persists for many years after the procedure, a study has found.
In the Journal of the American College of Cardiology, researchers report the outcomes of long-term follow-up of 272 patients admitted with ST-segment elevation myocardial infarction, who were enrolled in CvLPRIT (Complete Versus Lesion-Only Primary PCI Trial).
The trial randomized patients to complete revascularization or infarct-related artery revascularization only, with a median follow-up of 5.6 years after randomization.
Anthony H. Gershlick, MD, from the University of Leicester (England) and NIHR Leicester Biomedical Research Centre, and coauthors highlighted conflicting evidence on the relative benefit of complete revascularization, compared with revascularization focused on the culprit artery only.
“The aim of this study was, for the first time, to determine if there is a sustained benefit in favor of multivessel percutaneous coronary intervention [PCI] in the longer term,” they wrote.
In the group of patients who underwent complete revascularization, the composite major adverse cardiovascular event rate at 5.6 years was 43% lower than in the infarct-related artery revascularization group (24.0 vs. 37.7%; P = .0079), according to the intention-to-treat analysis.
The complete revascularization group also showed a significantly lower rate of the secondary composite endpoint of death or MI, which was 10% in the complete revascularization group and 18.5% in the target lesion group (hazard ratio, 0.47; P = .0175).
“Our data suggest that total revascularization, known to have benefits in various cohorts with coronary artery disease, should now probably be considered the standard of care in suitable patients with STEMI with multivessel disease,” they wrote.
However they did find that the rates of ischemia-driven revascularization were not significantly different between the two groups at the long-term follow-up.
The authors also did an analysis of outcomes from the end of the original 12-month study to the final follow-up point. This showed a nonsignificant trend toward a lower rate of major adverse cardiovascular events in the group who underwent complete revascularization; 17.1%, compared with 23.3% in the infarct-related artery revascularization group. The rates of the individual components of that primary endpoint also trended toward lower rates in individuals with complete revascularization.
Similarly, the rates of ischemia-driven revascularization were similar in both groups when analyzed after the 12-month mark, and the authors noted that the need for ischemia-driven revascularization was equally spread between infarct-related arteries and non–infarct-related arteries.
The authors commented that the event rate curves for the two groups remained separated even to the median follow-up point of 5.6 years, showing that the highly significant difference in major adverse cardiovascular event rates between the two groups persists.
“All of these data suggest that lower rates of events seen within 12 months do translate into longer-term benefit, predominantly through nonattenuation of benefit,” they wrote.
They speculated that the longer-term benefit of early complete revascularization could be the result of improvement in blood flow to areas around the original site of ischemia, and because it managed lesions in nontarget vessels in patients with disease in multiple arteries.
“Certainly, given that both the MRI and nuclear medicine substudies of CvLPRIT showed no difference between the groups in infarct size (at 1 week) and no difference in ischemic burden at 6 weeks, the benefit we have demonstrated does not appear to be explained simply in terms of ischemic burden being dealt with prophylactically in the complete group,” they wrote.
Commenting on the study’s limitations, the authors noted that the overall numbers of patients were small, and that the use of all-cause mortality rather than cardiovascular mortality may affect the interpretation of results.
The CvLPRIT study was funded by the British Heart Foundation, with support from the National Institute for Health Research Comprehensive Local Research Networks. No conflicts of interest were declared.
SOURCE: Gershlick A et al. J Am Coll Cardiol. 2019 Dec 16;74:3083-9.
The greater reduction in major adverse cardiovascular events with complete revascularization for ST-segment elevation myocardial infarction, compared with target-lesion only, persists for many years after the procedure, a study has found.
In the Journal of the American College of Cardiology, researchers report the outcomes of long-term follow-up of 272 patients admitted with ST-segment elevation myocardial infarction, who were enrolled in CvLPRIT (Complete Versus Lesion-Only Primary PCI Trial).
The trial randomized patients to complete revascularization or infarct-related artery revascularization only, with a median follow-up of 5.6 years after randomization.
Anthony H. Gershlick, MD, from the University of Leicester (England) and NIHR Leicester Biomedical Research Centre, and coauthors highlighted conflicting evidence on the relative benefit of complete revascularization, compared with revascularization focused on the culprit artery only.
“The aim of this study was, for the first time, to determine if there is a sustained benefit in favor of multivessel percutaneous coronary intervention [PCI] in the longer term,” they wrote.
In the group of patients who underwent complete revascularization, the composite major adverse cardiovascular event rate at 5.6 years was 43% lower than in the infarct-related artery revascularization group (24.0 vs. 37.7%; P = .0079), according to the intention-to-treat analysis.
The complete revascularization group also showed a significantly lower rate of the secondary composite endpoint of death or MI, which was 10% in the complete revascularization group and 18.5% in the target lesion group (hazard ratio, 0.47; P = .0175).
“Our data suggest that total revascularization, known to have benefits in various cohorts with coronary artery disease, should now probably be considered the standard of care in suitable patients with STEMI with multivessel disease,” they wrote.
However they did find that the rates of ischemia-driven revascularization were not significantly different between the two groups at the long-term follow-up.
The authors also did an analysis of outcomes from the end of the original 12-month study to the final follow-up point. This showed a nonsignificant trend toward a lower rate of major adverse cardiovascular events in the group who underwent complete revascularization; 17.1%, compared with 23.3% in the infarct-related artery revascularization group. The rates of the individual components of that primary endpoint also trended toward lower rates in individuals with complete revascularization.
Similarly, the rates of ischemia-driven revascularization were similar in both groups when analyzed after the 12-month mark, and the authors noted that the need for ischemia-driven revascularization was equally spread between infarct-related arteries and non–infarct-related arteries.
The authors commented that the event rate curves for the two groups remained separated even to the median follow-up point of 5.6 years, showing that the highly significant difference in major adverse cardiovascular event rates between the two groups persists.
“All of these data suggest that lower rates of events seen within 12 months do translate into longer-term benefit, predominantly through nonattenuation of benefit,” they wrote.
They speculated that the longer-term benefit of early complete revascularization could be the result of improvement in blood flow to areas around the original site of ischemia, and because it managed lesions in nontarget vessels in patients with disease in multiple arteries.
“Certainly, given that both the MRI and nuclear medicine substudies of CvLPRIT showed no difference between the groups in infarct size (at 1 week) and no difference in ischemic burden at 6 weeks, the benefit we have demonstrated does not appear to be explained simply in terms of ischemic burden being dealt with prophylactically in the complete group,” they wrote.
Commenting on the study’s limitations, the authors noted that the overall numbers of patients were small, and that the use of all-cause mortality rather than cardiovascular mortality may affect the interpretation of results.
The CvLPRIT study was funded by the British Heart Foundation, with support from the National Institute for Health Research Comprehensive Local Research Networks. No conflicts of interest were declared.
SOURCE: Gershlick A et al. J Am Coll Cardiol. 2019 Dec 16;74:3083-9.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Minimum transcatheter mitral valve intervention case load recommended
U.S. sites that perform transcatheter mitral valve interventions should treat a minimum of 20 such patients each year or at least 40 every 2 years, according to revised recommendations and requirements released on Dec. 16, 2019, by several U.S. cardiology and cardiac surgery societies.
The revised recommendations also for the first time address patient selection for using transcatheter mitral valve (MV) intervention in patients with mitral regurgitation (MR) secondary to heart failure and a left ventricular ejection fraction of 21%-49%, a patient target for transcatheter interventions approved by the Food and Drug Administration in March 2019. The FDA first approved the same transcatheter MV intervention system (MitraClip) in 2013 for patients with primary MR caused by an abnormality of the MV and a prohibitive risk for surgical MV repair or replacement, and the same societies had previously issued their recommendations based on that approval (J Am Coll Cardiol. 2014 Oct; 64[14]:1515-26).
The need for updated recommendations on the delivery of transcatheter MV interventions including the personnel and facilities required for a valid U.S. program was driven by “new transcatheter technology, new trial results, and the new FDA-approved indication” of secondary MR. “We did the update for patients with secondary MR,” said Robert O. Bonow, MD, professor of medicine at Northwestern University, Chicago, and chair of the committee that wrote the revised recommendations.
“Primary and secondary MR are like two different diseases. Primary MR is a disease of the MV leaflets. Secondary MR is a disease of heart failure, left ventricular dysfunction, and left ventricular enlargement.” That makes secondary MR an indication with a potentially huge upside, given how many patients have heart failure today, plus projections for steadily increasing numbers of patients as the geriatric population grows.
“Heart failure is a huge issue, and the numbers are growing, which is why we felt it was important to say which heart failure patients should be candidates. We did not endorse this for all patients, although MR is very common in heart failure,” with estimated prevalence rates as high as two-thirds of all heart failure patients, Dr. Bonow said.
The new recommendations spell out an approach to patient selection that aims to apply transcatheter MV intervention to patients who match those enrolled in the COAPT (Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients With Functional Mitral Regurgitation) trial, which randomized 614 patients with MR secondary to heart failure and found for that MV intervention cut the rate of heart failure hospitalization by about half during 2 years of follow-up, a statistically significant effect for the study’s primary endpoint (N Engl J Med. 2018 Dec 13;379[24]:2307-18). The new recommendations provide “a way to replicate the COAPT trial” in routine practice, Dr. Bonow said in an interview.”We thought it was important to try to replicate the COAPT results,” and a key step toward trying to accomplish that is to apply the multidisciplinary team concept to evaluating patients and determining their management strategy. Dr. Bonow coauthored a commentary that discussed how the COAPT results should influence routine practice (N Engl J Med. 2018 Dec 13;379[24]:2374-6).
“In this case, perhaps the most important member of the multidisciplinary team is the heart failure specialist, because secondary MR is a disease of heart failure and it’s important to also optimize medical therapy to reduce MR and prolong life,” he said. Treatment optimization before undertaking a transcatheter MV intervention involves not only drug treatment but also treatment with indicated devices, such as biventricular pacing, to optimize left ventricular size and function. The 2019 FDA approval of the transcatheter approach for treating MV disease specified that eligible patients had to continue to have significant MR despite receiving optimal drug and device treatment.
Treatment decisions for patients with MR must be highly individualized, and unlike transcatheter aortic valve replacement (TAVR), which occurs against a background of “really good results” for surgical aortic valve replacement, surgical treatment of MR “has never been shown to prolong life, although it can improve function,” Dr. Bonow said. In addition, open surgical repair or replacement of a faulty MV “is much better than the MitraClip for elimination a MV leak; usually with transcatheter intervention there is some residual leak. And other etiologies of MR, such as atrial fibrillation causing atrial enlargement and dilatation of the mitral annulus, generally have much better results with surgery than with a transcatheter intervention. For both primary and secondary MR, deciding when to use surgery and when to do a transcatheter intervention is very individualized,” concluded Dr. Bonow, and a fact that distinguishes transcatheter MV interventions from TAVR, which has been shown to have efficacy that’s comparable with surgical aortic valve replacement. The MitraClip system is currently the only device with U.S. marketing approval for transcatheter MV intervention, although other devices are in development.
Case volume requirements
The designation of a minimum transcatheter MV intervention case load of 20 procedures a year or 40 every 2 years reflected a “consensus among interventional cardiologists and cardiac surgeons of what the experience had to be for MV repair,” Dr. Bonow said. This number contrasts with a minimum case volume of 50 procedures/year or 100 every 2 years to maintain a TAVR program proposed in 2018 by a similar expert panel organized by U.S. cardiology and cardiac surgery societies (J Am Coll of Cardiol. 2019 Jan;73[3]:340-74). The new MV recommendations “follow a similar template [as the TAVR recommendations], but the numbers are what we thought would be best for optimal transcatheter MV expertise. MV interventions will likely increase, and we felt it would be best to define the transcatheter operators and are the right patients; the volume is unclear. There are a lot of heart failure patients, but we know from COAPT that not everyone is a candidate. The existing MV device does not fit all settings. We thought the numbers we selected were most appropriate, at least when we are starting.”
Dr. Bonow and coauthors who wrote the new recommendations will rely on payers, particularly the Centers for Medicare & Medicaid Services, to adopt the societal recommendations as part of their criteria for reimbursement and thereby give them teeth. In June 2019, CMS announced its Medicare coverage determination for TAVR, which included procedure minimums of 20 per year or 40 over 2 years for TAVR programs, a number that fell substantially below the 50 per year or 100 over 2 years that had been proposed by the societies. “We hope CMS will use our MV recommendations as a starting point,” but the final CMS coverage decision for transcatheter MV intervention could again differ from what the societies proposed, Dr. Bonow acknowledged.
In addition to strongly promoting a multidisciplinary team approach (and spelling out the members of the team) and shared decision making involvement of the patient with the team, the new recommendations also endorse participation of MV intervention programs in the Transcatheter Valve Therapy U.S. patient registry that’s maintained by two of the societies that helped organize the writing committee. The recommendations discuss the need to collect 30-day (and longer) outcomes data from transcatheter MV intervention programs through the registry as is now done for TAVR programs (N Engl J Med. 2019 Jun 27;380[26]:2541-50). Dr. Bonow declined to predict when 30-day outcomes data may start appearing for programs performing transcatheter MV interventions.
Dr. Bonow had no disclosures. The COAPT study was funded by Abbott, the company that markets the MitrClip clip delivery system.
SOURCE: Bonow RO et al. J Am Coll Cardiol. 2019 Dec 16. doi: 10.1016/j.jacc.2019.12.002.
U.S. sites that perform transcatheter mitral valve interventions should treat a minimum of 20 such patients each year or at least 40 every 2 years, according to revised recommendations and requirements released on Dec. 16, 2019, by several U.S. cardiology and cardiac surgery societies.
The revised recommendations also for the first time address patient selection for using transcatheter mitral valve (MV) intervention in patients with mitral regurgitation (MR) secondary to heart failure and a left ventricular ejection fraction of 21%-49%, a patient target for transcatheter interventions approved by the Food and Drug Administration in March 2019. The FDA first approved the same transcatheter MV intervention system (MitraClip) in 2013 for patients with primary MR caused by an abnormality of the MV and a prohibitive risk for surgical MV repair or replacement, and the same societies had previously issued their recommendations based on that approval (J Am Coll Cardiol. 2014 Oct; 64[14]:1515-26).
The need for updated recommendations on the delivery of transcatheter MV interventions including the personnel and facilities required for a valid U.S. program was driven by “new transcatheter technology, new trial results, and the new FDA-approved indication” of secondary MR. “We did the update for patients with secondary MR,” said Robert O. Bonow, MD, professor of medicine at Northwestern University, Chicago, and chair of the committee that wrote the revised recommendations.
“Primary and secondary MR are like two different diseases. Primary MR is a disease of the MV leaflets. Secondary MR is a disease of heart failure, left ventricular dysfunction, and left ventricular enlargement.” That makes secondary MR an indication with a potentially huge upside, given how many patients have heart failure today, plus projections for steadily increasing numbers of patients as the geriatric population grows.
“Heart failure is a huge issue, and the numbers are growing, which is why we felt it was important to say which heart failure patients should be candidates. We did not endorse this for all patients, although MR is very common in heart failure,” with estimated prevalence rates as high as two-thirds of all heart failure patients, Dr. Bonow said.
The new recommendations spell out an approach to patient selection that aims to apply transcatheter MV intervention to patients who match those enrolled in the COAPT (Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients With Functional Mitral Regurgitation) trial, which randomized 614 patients with MR secondary to heart failure and found for that MV intervention cut the rate of heart failure hospitalization by about half during 2 years of follow-up, a statistically significant effect for the study’s primary endpoint (N Engl J Med. 2018 Dec 13;379[24]:2307-18). The new recommendations provide “a way to replicate the COAPT trial” in routine practice, Dr. Bonow said in an interview.”We thought it was important to try to replicate the COAPT results,” and a key step toward trying to accomplish that is to apply the multidisciplinary team concept to evaluating patients and determining their management strategy. Dr. Bonow coauthored a commentary that discussed how the COAPT results should influence routine practice (N Engl J Med. 2018 Dec 13;379[24]:2374-6).
“In this case, perhaps the most important member of the multidisciplinary team is the heart failure specialist, because secondary MR is a disease of heart failure and it’s important to also optimize medical therapy to reduce MR and prolong life,” he said. Treatment optimization before undertaking a transcatheter MV intervention involves not only drug treatment but also treatment with indicated devices, such as biventricular pacing, to optimize left ventricular size and function. The 2019 FDA approval of the transcatheter approach for treating MV disease specified that eligible patients had to continue to have significant MR despite receiving optimal drug and device treatment.
Treatment decisions for patients with MR must be highly individualized, and unlike transcatheter aortic valve replacement (TAVR), which occurs against a background of “really good results” for surgical aortic valve replacement, surgical treatment of MR “has never been shown to prolong life, although it can improve function,” Dr. Bonow said. In addition, open surgical repair or replacement of a faulty MV “is much better than the MitraClip for elimination a MV leak; usually with transcatheter intervention there is some residual leak. And other etiologies of MR, such as atrial fibrillation causing atrial enlargement and dilatation of the mitral annulus, generally have much better results with surgery than with a transcatheter intervention. For both primary and secondary MR, deciding when to use surgery and when to do a transcatheter intervention is very individualized,” concluded Dr. Bonow, and a fact that distinguishes transcatheter MV interventions from TAVR, which has been shown to have efficacy that’s comparable with surgical aortic valve replacement. The MitraClip system is currently the only device with U.S. marketing approval for transcatheter MV intervention, although other devices are in development.
Case volume requirements
The designation of a minimum transcatheter MV intervention case load of 20 procedures a year or 40 every 2 years reflected a “consensus among interventional cardiologists and cardiac surgeons of what the experience had to be for MV repair,” Dr. Bonow said. This number contrasts with a minimum case volume of 50 procedures/year or 100 every 2 years to maintain a TAVR program proposed in 2018 by a similar expert panel organized by U.S. cardiology and cardiac surgery societies (J Am Coll of Cardiol. 2019 Jan;73[3]:340-74). The new MV recommendations “follow a similar template [as the TAVR recommendations], but the numbers are what we thought would be best for optimal transcatheter MV expertise. MV interventions will likely increase, and we felt it would be best to define the transcatheter operators and are the right patients; the volume is unclear. There are a lot of heart failure patients, but we know from COAPT that not everyone is a candidate. The existing MV device does not fit all settings. We thought the numbers we selected were most appropriate, at least when we are starting.”
Dr. Bonow and coauthors who wrote the new recommendations will rely on payers, particularly the Centers for Medicare & Medicaid Services, to adopt the societal recommendations as part of their criteria for reimbursement and thereby give them teeth. In June 2019, CMS announced its Medicare coverage determination for TAVR, which included procedure minimums of 20 per year or 40 over 2 years for TAVR programs, a number that fell substantially below the 50 per year or 100 over 2 years that had been proposed by the societies. “We hope CMS will use our MV recommendations as a starting point,” but the final CMS coverage decision for transcatheter MV intervention could again differ from what the societies proposed, Dr. Bonow acknowledged.
In addition to strongly promoting a multidisciplinary team approach (and spelling out the members of the team) and shared decision making involvement of the patient with the team, the new recommendations also endorse participation of MV intervention programs in the Transcatheter Valve Therapy U.S. patient registry that’s maintained by two of the societies that helped organize the writing committee. The recommendations discuss the need to collect 30-day (and longer) outcomes data from transcatheter MV intervention programs through the registry as is now done for TAVR programs (N Engl J Med. 2019 Jun 27;380[26]:2541-50). Dr. Bonow declined to predict when 30-day outcomes data may start appearing for programs performing transcatheter MV interventions.
Dr. Bonow had no disclosures. The COAPT study was funded by Abbott, the company that markets the MitrClip clip delivery system.
SOURCE: Bonow RO et al. J Am Coll Cardiol. 2019 Dec 16. doi: 10.1016/j.jacc.2019.12.002.
U.S. sites that perform transcatheter mitral valve interventions should treat a minimum of 20 such patients each year or at least 40 every 2 years, according to revised recommendations and requirements released on Dec. 16, 2019, by several U.S. cardiology and cardiac surgery societies.
The revised recommendations also for the first time address patient selection for using transcatheter mitral valve (MV) intervention in patients with mitral regurgitation (MR) secondary to heart failure and a left ventricular ejection fraction of 21%-49%, a patient target for transcatheter interventions approved by the Food and Drug Administration in March 2019. The FDA first approved the same transcatheter MV intervention system (MitraClip) in 2013 for patients with primary MR caused by an abnormality of the MV and a prohibitive risk for surgical MV repair or replacement, and the same societies had previously issued their recommendations based on that approval (J Am Coll Cardiol. 2014 Oct; 64[14]:1515-26).
The need for updated recommendations on the delivery of transcatheter MV interventions including the personnel and facilities required for a valid U.S. program was driven by “new transcatheter technology, new trial results, and the new FDA-approved indication” of secondary MR. “We did the update for patients with secondary MR,” said Robert O. Bonow, MD, professor of medicine at Northwestern University, Chicago, and chair of the committee that wrote the revised recommendations.
“Primary and secondary MR are like two different diseases. Primary MR is a disease of the MV leaflets. Secondary MR is a disease of heart failure, left ventricular dysfunction, and left ventricular enlargement.” That makes secondary MR an indication with a potentially huge upside, given how many patients have heart failure today, plus projections for steadily increasing numbers of patients as the geriatric population grows.
“Heart failure is a huge issue, and the numbers are growing, which is why we felt it was important to say which heart failure patients should be candidates. We did not endorse this for all patients, although MR is very common in heart failure,” with estimated prevalence rates as high as two-thirds of all heart failure patients, Dr. Bonow said.
The new recommendations spell out an approach to patient selection that aims to apply transcatheter MV intervention to patients who match those enrolled in the COAPT (Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients With Functional Mitral Regurgitation) trial, which randomized 614 patients with MR secondary to heart failure and found for that MV intervention cut the rate of heart failure hospitalization by about half during 2 years of follow-up, a statistically significant effect for the study’s primary endpoint (N Engl J Med. 2018 Dec 13;379[24]:2307-18). The new recommendations provide “a way to replicate the COAPT trial” in routine practice, Dr. Bonow said in an interview.”We thought it was important to try to replicate the COAPT results,” and a key step toward trying to accomplish that is to apply the multidisciplinary team concept to evaluating patients and determining their management strategy. Dr. Bonow coauthored a commentary that discussed how the COAPT results should influence routine practice (N Engl J Med. 2018 Dec 13;379[24]:2374-6).
“In this case, perhaps the most important member of the multidisciplinary team is the heart failure specialist, because secondary MR is a disease of heart failure and it’s important to also optimize medical therapy to reduce MR and prolong life,” he said. Treatment optimization before undertaking a transcatheter MV intervention involves not only drug treatment but also treatment with indicated devices, such as biventricular pacing, to optimize left ventricular size and function. The 2019 FDA approval of the transcatheter approach for treating MV disease specified that eligible patients had to continue to have significant MR despite receiving optimal drug and device treatment.
Treatment decisions for patients with MR must be highly individualized, and unlike transcatheter aortic valve replacement (TAVR), which occurs against a background of “really good results” for surgical aortic valve replacement, surgical treatment of MR “has never been shown to prolong life, although it can improve function,” Dr. Bonow said. In addition, open surgical repair or replacement of a faulty MV “is much better than the MitraClip for elimination a MV leak; usually with transcatheter intervention there is some residual leak. And other etiologies of MR, such as atrial fibrillation causing atrial enlargement and dilatation of the mitral annulus, generally have much better results with surgery than with a transcatheter intervention. For both primary and secondary MR, deciding when to use surgery and when to do a transcatheter intervention is very individualized,” concluded Dr. Bonow, and a fact that distinguishes transcatheter MV interventions from TAVR, which has been shown to have efficacy that’s comparable with surgical aortic valve replacement. The MitraClip system is currently the only device with U.S. marketing approval for transcatheter MV intervention, although other devices are in development.
Case volume requirements
The designation of a minimum transcatheter MV intervention case load of 20 procedures a year or 40 every 2 years reflected a “consensus among interventional cardiologists and cardiac surgeons of what the experience had to be for MV repair,” Dr. Bonow said. This number contrasts with a minimum case volume of 50 procedures/year or 100 every 2 years to maintain a TAVR program proposed in 2018 by a similar expert panel organized by U.S. cardiology and cardiac surgery societies (J Am Coll of Cardiol. 2019 Jan;73[3]:340-74). The new MV recommendations “follow a similar template [as the TAVR recommendations], but the numbers are what we thought would be best for optimal transcatheter MV expertise. MV interventions will likely increase, and we felt it would be best to define the transcatheter operators and are the right patients; the volume is unclear. There are a lot of heart failure patients, but we know from COAPT that not everyone is a candidate. The existing MV device does not fit all settings. We thought the numbers we selected were most appropriate, at least when we are starting.”
Dr. Bonow and coauthors who wrote the new recommendations will rely on payers, particularly the Centers for Medicare & Medicaid Services, to adopt the societal recommendations as part of their criteria for reimbursement and thereby give them teeth. In June 2019, CMS announced its Medicare coverage determination for TAVR, which included procedure minimums of 20 per year or 40 over 2 years for TAVR programs, a number that fell substantially below the 50 per year or 100 over 2 years that had been proposed by the societies. “We hope CMS will use our MV recommendations as a starting point,” but the final CMS coverage decision for transcatheter MV intervention could again differ from what the societies proposed, Dr. Bonow acknowledged.
In addition to strongly promoting a multidisciplinary team approach (and spelling out the members of the team) and shared decision making involvement of the patient with the team, the new recommendations also endorse participation of MV intervention programs in the Transcatheter Valve Therapy U.S. patient registry that’s maintained by two of the societies that helped organize the writing committee. The recommendations discuss the need to collect 30-day (and longer) outcomes data from transcatheter MV intervention programs through the registry as is now done for TAVR programs (N Engl J Med. 2019 Jun 27;380[26]:2541-50). Dr. Bonow declined to predict when 30-day outcomes data may start appearing for programs performing transcatheter MV interventions.
Dr. Bonow had no disclosures. The COAPT study was funded by Abbott, the company that markets the MitrClip clip delivery system.
SOURCE: Bonow RO et al. J Am Coll Cardiol. 2019 Dec 16. doi: 10.1016/j.jacc.2019.12.002.
ASH releases guidelines on managing cardiopulmonary and kidney disease in SCD
ORLANDO – It is good practice to consult with a pulmonary hypertension (PH) expert before referring a patient with sickle cell disease (SCD) for right-heart catheterization or PH evaluation, according to new American Society of Hematology guidelines for the screening and management of cardiopulmonary and kidney disease in patients with SCD.
That “Good Practice” recommendation is one of several included in the evidence-based guidelines published Dec. 10 in Blood Advances and highlighted during a Special Education Session at the annual ASH meeting.
The guidelines provide 10 main recommendations intended to “support patients, clinicians, and other health care professionals in their decisions about screening, diagnosis, and management of cardiopulmonary and renal complications of SCD,” wrote Robert I. Liem, MD, of Ann & Robert H. Lurie Children’s Hospital of Chicago and colleagues.
The recommendations, agreed upon by a multidisciplinary guideline panel, relate to screening, diagnosis, and management of PH, pulmonary arterial hypertension (PAH), hypertension, proteinuria and chronic kidney disease, and venous thromboembolism (VTE). Most are “conditional,” as opposed to “strong,” because of a paucity of direct, high-quality outcomes data, and they are accompanied by the Good Practice Statements, descriptive remarks and caveats based on the available data, as well as suggestions for future research.
At the special ASH session, Ankit A. Desai, MD, highlighted some of the recommendations and discussed considerations for their practical application.
The Good Practice Statement on consulting a specialist before referring a patient for PH relates specifically to Recommendations 2a and 2b on the management of abnormal echocardiography, explained Dr. Desai of Indiana University, Indianapolis.
For asymptomatic children and adults with SCD and an isolated peak tricuspid regurgitant jet velocity (TRJV) of at least 2.5-2.9 m/s on echocardiography, the panel recommends against right-heart catheterization (Recommendation 2a, conditional), he said.
For children and adults with SCD and a peak TRJV of at least 2.5 m/s who also have a reduced 6-minute walk distance (6MWD) and/or elevated N-terminal proB-type natriuretic peptide (NT-proBNP), the panel supports right-heart catheterization (Recommendation 2b, conditional).
Dr. Desai noted that the 2.5 m/s threshold was found to be suboptimal when used as the sole criteria for right-heart catheterization. Using that threshold alone is associated with “moderate to large” harms, such as starting inappropriate PH-specific therapies and/or performing unnecessary right-heart catheterization. However, when used in combination with 6MWD, the predictive capacity improved significantly, and the risk for potential harm was low, he explained.
Another Good Practice Statement included in the guidelines, and relevant to these recommendations on managing abnormal echocardiography, addresses the importance of basing decisions about the need for right-heart catheterization on echocardiograms obtained at steady state rather than during acute illness, such as during hospitalization for pain or acute chest syndrome.
This is in part because of technical factors, Dr. Desai said.
“We know that repeating [echocardiography] is something that should be considered in patients because ... results vary – sometimes quite a bit – from study to study,” he said.
As for the cutoff values for 6MWD and NT-proBNP, “a decent amount of literature” suggests that less than 333 m and less than 160 pg/ml, respectively, are good thresholds, he said.
“Importantly, this should all be taken in the context of good clinical judgment ... along with discussion with a PH expert,” he added.
The full guidelines are available, along with additional ASH guidelines on immune thrombocytopenia and prevention of venous thromboembolism in surgical hospitalized patients, at the ASH publications website.
Of note, the SCD guidelines on cardiopulmonary disease and kidney disease are one of five sets of SCD guidelines that have been in development; these are the first of those to be published. The remaining four sets of guidelines will address pain, cerebrovascular complications, transfusion, and hematopoietic stem cell transplant. All will be published in Blood Advances, and according to Dr. Liem, the transfusion medicine guidelines have been accepted and should be published in January 2020, followed by those for cerebrovascular complications. Publication of the pain and transplant guidelines are anticipated later in 2020.
Dr. Liem and Dr. Desai reported having no conflicts of interest.
ORLANDO – It is good practice to consult with a pulmonary hypertension (PH) expert before referring a patient with sickle cell disease (SCD) for right-heart catheterization or PH evaluation, according to new American Society of Hematology guidelines for the screening and management of cardiopulmonary and kidney disease in patients with SCD.
That “Good Practice” recommendation is one of several included in the evidence-based guidelines published Dec. 10 in Blood Advances and highlighted during a Special Education Session at the annual ASH meeting.
The guidelines provide 10 main recommendations intended to “support patients, clinicians, and other health care professionals in their decisions about screening, diagnosis, and management of cardiopulmonary and renal complications of SCD,” wrote Robert I. Liem, MD, of Ann & Robert H. Lurie Children’s Hospital of Chicago and colleagues.
The recommendations, agreed upon by a multidisciplinary guideline panel, relate to screening, diagnosis, and management of PH, pulmonary arterial hypertension (PAH), hypertension, proteinuria and chronic kidney disease, and venous thromboembolism (VTE). Most are “conditional,” as opposed to “strong,” because of a paucity of direct, high-quality outcomes data, and they are accompanied by the Good Practice Statements, descriptive remarks and caveats based on the available data, as well as suggestions for future research.
At the special ASH session, Ankit A. Desai, MD, highlighted some of the recommendations and discussed considerations for their practical application.
The Good Practice Statement on consulting a specialist before referring a patient for PH relates specifically to Recommendations 2a and 2b on the management of abnormal echocardiography, explained Dr. Desai of Indiana University, Indianapolis.
For asymptomatic children and adults with SCD and an isolated peak tricuspid regurgitant jet velocity (TRJV) of at least 2.5-2.9 m/s on echocardiography, the panel recommends against right-heart catheterization (Recommendation 2a, conditional), he said.
For children and adults with SCD and a peak TRJV of at least 2.5 m/s who also have a reduced 6-minute walk distance (6MWD) and/or elevated N-terminal proB-type natriuretic peptide (NT-proBNP), the panel supports right-heart catheterization (Recommendation 2b, conditional).
Dr. Desai noted that the 2.5 m/s threshold was found to be suboptimal when used as the sole criteria for right-heart catheterization. Using that threshold alone is associated with “moderate to large” harms, such as starting inappropriate PH-specific therapies and/or performing unnecessary right-heart catheterization. However, when used in combination with 6MWD, the predictive capacity improved significantly, and the risk for potential harm was low, he explained.
Another Good Practice Statement included in the guidelines, and relevant to these recommendations on managing abnormal echocardiography, addresses the importance of basing decisions about the need for right-heart catheterization on echocardiograms obtained at steady state rather than during acute illness, such as during hospitalization for pain or acute chest syndrome.
This is in part because of technical factors, Dr. Desai said.
“We know that repeating [echocardiography] is something that should be considered in patients because ... results vary – sometimes quite a bit – from study to study,” he said.
As for the cutoff values for 6MWD and NT-proBNP, “a decent amount of literature” suggests that less than 333 m and less than 160 pg/ml, respectively, are good thresholds, he said.
“Importantly, this should all be taken in the context of good clinical judgment ... along with discussion with a PH expert,” he added.
The full guidelines are available, along with additional ASH guidelines on immune thrombocytopenia and prevention of venous thromboembolism in surgical hospitalized patients, at the ASH publications website.
Of note, the SCD guidelines on cardiopulmonary disease and kidney disease are one of five sets of SCD guidelines that have been in development; these are the first of those to be published. The remaining four sets of guidelines will address pain, cerebrovascular complications, transfusion, and hematopoietic stem cell transplant. All will be published in Blood Advances, and according to Dr. Liem, the transfusion medicine guidelines have been accepted and should be published in January 2020, followed by those for cerebrovascular complications. Publication of the pain and transplant guidelines are anticipated later in 2020.
Dr. Liem and Dr. Desai reported having no conflicts of interest.
ORLANDO – It is good practice to consult with a pulmonary hypertension (PH) expert before referring a patient with sickle cell disease (SCD) for right-heart catheterization or PH evaluation, according to new American Society of Hematology guidelines for the screening and management of cardiopulmonary and kidney disease in patients with SCD.
That “Good Practice” recommendation is one of several included in the evidence-based guidelines published Dec. 10 in Blood Advances and highlighted during a Special Education Session at the annual ASH meeting.
The guidelines provide 10 main recommendations intended to “support patients, clinicians, and other health care professionals in their decisions about screening, diagnosis, and management of cardiopulmonary and renal complications of SCD,” wrote Robert I. Liem, MD, of Ann & Robert H. Lurie Children’s Hospital of Chicago and colleagues.
The recommendations, agreed upon by a multidisciplinary guideline panel, relate to screening, diagnosis, and management of PH, pulmonary arterial hypertension (PAH), hypertension, proteinuria and chronic kidney disease, and venous thromboembolism (VTE). Most are “conditional,” as opposed to “strong,” because of a paucity of direct, high-quality outcomes data, and they are accompanied by the Good Practice Statements, descriptive remarks and caveats based on the available data, as well as suggestions for future research.
At the special ASH session, Ankit A. Desai, MD, highlighted some of the recommendations and discussed considerations for their practical application.
The Good Practice Statement on consulting a specialist before referring a patient for PH relates specifically to Recommendations 2a and 2b on the management of abnormal echocardiography, explained Dr. Desai of Indiana University, Indianapolis.
For asymptomatic children and adults with SCD and an isolated peak tricuspid regurgitant jet velocity (TRJV) of at least 2.5-2.9 m/s on echocardiography, the panel recommends against right-heart catheterization (Recommendation 2a, conditional), he said.
For children and adults with SCD and a peak TRJV of at least 2.5 m/s who also have a reduced 6-minute walk distance (6MWD) and/or elevated N-terminal proB-type natriuretic peptide (NT-proBNP), the panel supports right-heart catheterization (Recommendation 2b, conditional).
Dr. Desai noted that the 2.5 m/s threshold was found to be suboptimal when used as the sole criteria for right-heart catheterization. Using that threshold alone is associated with “moderate to large” harms, such as starting inappropriate PH-specific therapies and/or performing unnecessary right-heart catheterization. However, when used in combination with 6MWD, the predictive capacity improved significantly, and the risk for potential harm was low, he explained.
Another Good Practice Statement included in the guidelines, and relevant to these recommendations on managing abnormal echocardiography, addresses the importance of basing decisions about the need for right-heart catheterization on echocardiograms obtained at steady state rather than during acute illness, such as during hospitalization for pain or acute chest syndrome.
This is in part because of technical factors, Dr. Desai said.
“We know that repeating [echocardiography] is something that should be considered in patients because ... results vary – sometimes quite a bit – from study to study,” he said.
As for the cutoff values for 6MWD and NT-proBNP, “a decent amount of literature” suggests that less than 333 m and less than 160 pg/ml, respectively, are good thresholds, he said.
“Importantly, this should all be taken in the context of good clinical judgment ... along with discussion with a PH expert,” he added.
The full guidelines are available, along with additional ASH guidelines on immune thrombocytopenia and prevention of venous thromboembolism in surgical hospitalized patients, at the ASH publications website.
Of note, the SCD guidelines on cardiopulmonary disease and kidney disease are one of five sets of SCD guidelines that have been in development; these are the first of those to be published. The remaining four sets of guidelines will address pain, cerebrovascular complications, transfusion, and hematopoietic stem cell transplant. All will be published in Blood Advances, and according to Dr. Liem, the transfusion medicine guidelines have been accepted and should be published in January 2020, followed by those for cerebrovascular complications. Publication of the pain and transplant guidelines are anticipated later in 2020.
Dr. Liem and Dr. Desai reported having no conflicts of interest.
EXPERT ANALYSIS FROM ASH 2019
ACURATE neo falls short against SAPIEN 3 for severe aortic stenosis
SAN FRANCISCO – The ACURATE neo transcatheter aortic valve replacement (TAVR) device failed to meet noninferiority, compared with the SAPIEN 3 device, in terms of the primary composite safety and efficacy endpoint at 30 days, a randomized, comparative trial showed.
“TAVR has become an indispensable treatment option for patients with symptomatic severe aortic stenosis across all risk categories,” Jonas Lanz, MD, MSc, said at the Transcatheter Cardiovascular Therapeutics annual meeting. “The generalizability of outcomes observed in landmark trials comparing TAVR with SAVR [surgical aortic valve replacement] to other commercial TAVR systems is limited by differences in device properties and lack of head-to-head device comparisons. Iterations of the SAPIEN balloon-expandable TAVR system have been extensively investigated in several large-scale, high-quality, randomized trials and registries setting the current benchmark in terms of safety and efficacy.”
The ACURATE neo is a novel self-expanding TAVR system associated with favorable outcomes in nonrandomized studies. It has been available in Europe since 2014 but has not gained Food and Drug Administration approval.
Between February 2017 and February 2019, in a randomized trial known as SCOPE I, Dr. Lanz and colleagues at 20 European sites enrolled 739 patients with severe, symptomatic aortic stenosis at increased surgical risk. Of these, 372 were assigned to transfemoral TAVR with the ACURATE neo and 367 were assigned to the SAPIEN 3 system. The researchers designed the study to investigate noninferiority of the primary endpoint, which was a composite of safety and efficacy derived from the Valve Academic Research Consortium–2 criteria and included all-cause death, any stroke, life-threatening or disabling bleeding, major vascular complications, coronary artery obstruction requiring intervention, acute kidney injury stage 2 or higher, valve-related dysfunction requiring repeat procedure, rehospitalization for valve-related symptoms or heart failure, moderate or severe prosthetic valve regurgitation, or prosthetic valve stenosis at 30 days.
Clinical follow-up information at 30 days was available for 99% and ECG follow-up for about 98% of the total study population. The mean age of the study participants was 82 years, 57% were female, their median STS Risk Score was 3.5, and their mean transvalvular aortic gradient was 42.2 mm Hg. Dr. Lanz, with the department of cardiology at Bern (Switzerland) University Hospital, reported that the primary endpoint rate in the intention-to-treat cohort for ACURATE neo was 23.7%, compared with 16.5% with SAPIEN 3, falling short of the threshold for noninferiority (P for noninferiority = 0.42).
“I think this speaks to the fact of how high the bar is right now – how good the procedure is [and] how good the devices are,” invited discussant Michael J. Mack, MD, a cardiac surgeon at Baylor Scott & White Health, Dallas, said during the media briefing. “It’s an incredibly high bar to meet for any new device.”
Certain single components of the primary endpoint were similar in the ACURATE neo group, compared with the SAPIEN 3 group, including all-cause death (2.5% vs. 0.8%, respectively) and stroke (1.9% vs. 3%), but acute kidney injury was more common in the ACURATE neo group (3% vs. 0.8%, as was paravalvular aortic regurgitation (9.4% vs. 2.8%). “The differences between the two TAVR devices were mainly driven by moderate or severe paravalvular regurgitation and stage 2 or 3 acute kidney injury in favor of the SAPIEN 3 device,” Dr. Lanz said at the meeting sponsored by the Cardiovascular Research Foundation. “An early composite safety and efficacy endpoint proved useful in discriminating the performance of different TAVR systems.”
He pointed out that a trial called SCOPE II is underway in Europe, with a new iteration of the ACURATE neo designed to reduce the risk of paravalvular leak.
The results of SCOPE I were published online at the time of presentation (Lancet. 2019 Sep 27. doi: 10.1016/S0140-6736[19]32220-2). SCOPE I was an investigator-initiated and -conducted study funded by a dedicated research grant from Symetis in Ecublens, Switzerland (part of Boston Scientific). Dr. Lanz had no relevant disclosures.
SAN FRANCISCO – The ACURATE neo transcatheter aortic valve replacement (TAVR) device failed to meet noninferiority, compared with the SAPIEN 3 device, in terms of the primary composite safety and efficacy endpoint at 30 days, a randomized, comparative trial showed.
“TAVR has become an indispensable treatment option for patients with symptomatic severe aortic stenosis across all risk categories,” Jonas Lanz, MD, MSc, said at the Transcatheter Cardiovascular Therapeutics annual meeting. “The generalizability of outcomes observed in landmark trials comparing TAVR with SAVR [surgical aortic valve replacement] to other commercial TAVR systems is limited by differences in device properties and lack of head-to-head device comparisons. Iterations of the SAPIEN balloon-expandable TAVR system have been extensively investigated in several large-scale, high-quality, randomized trials and registries setting the current benchmark in terms of safety and efficacy.”
The ACURATE neo is a novel self-expanding TAVR system associated with favorable outcomes in nonrandomized studies. It has been available in Europe since 2014 but has not gained Food and Drug Administration approval.
Between February 2017 and February 2019, in a randomized trial known as SCOPE I, Dr. Lanz and colleagues at 20 European sites enrolled 739 patients with severe, symptomatic aortic stenosis at increased surgical risk. Of these, 372 were assigned to transfemoral TAVR with the ACURATE neo and 367 were assigned to the SAPIEN 3 system. The researchers designed the study to investigate noninferiority of the primary endpoint, which was a composite of safety and efficacy derived from the Valve Academic Research Consortium–2 criteria and included all-cause death, any stroke, life-threatening or disabling bleeding, major vascular complications, coronary artery obstruction requiring intervention, acute kidney injury stage 2 or higher, valve-related dysfunction requiring repeat procedure, rehospitalization for valve-related symptoms or heart failure, moderate or severe prosthetic valve regurgitation, or prosthetic valve stenosis at 30 days.
Clinical follow-up information at 30 days was available for 99% and ECG follow-up for about 98% of the total study population. The mean age of the study participants was 82 years, 57% were female, their median STS Risk Score was 3.5, and their mean transvalvular aortic gradient was 42.2 mm Hg. Dr. Lanz, with the department of cardiology at Bern (Switzerland) University Hospital, reported that the primary endpoint rate in the intention-to-treat cohort for ACURATE neo was 23.7%, compared with 16.5% with SAPIEN 3, falling short of the threshold for noninferiority (P for noninferiority = 0.42).
“I think this speaks to the fact of how high the bar is right now – how good the procedure is [and] how good the devices are,” invited discussant Michael J. Mack, MD, a cardiac surgeon at Baylor Scott & White Health, Dallas, said during the media briefing. “It’s an incredibly high bar to meet for any new device.”
Certain single components of the primary endpoint were similar in the ACURATE neo group, compared with the SAPIEN 3 group, including all-cause death (2.5% vs. 0.8%, respectively) and stroke (1.9% vs. 3%), but acute kidney injury was more common in the ACURATE neo group (3% vs. 0.8%, as was paravalvular aortic regurgitation (9.4% vs. 2.8%). “The differences between the two TAVR devices were mainly driven by moderate or severe paravalvular regurgitation and stage 2 or 3 acute kidney injury in favor of the SAPIEN 3 device,” Dr. Lanz said at the meeting sponsored by the Cardiovascular Research Foundation. “An early composite safety and efficacy endpoint proved useful in discriminating the performance of different TAVR systems.”
He pointed out that a trial called SCOPE II is underway in Europe, with a new iteration of the ACURATE neo designed to reduce the risk of paravalvular leak.
The results of SCOPE I were published online at the time of presentation (Lancet. 2019 Sep 27. doi: 10.1016/S0140-6736[19]32220-2). SCOPE I was an investigator-initiated and -conducted study funded by a dedicated research grant from Symetis in Ecublens, Switzerland (part of Boston Scientific). Dr. Lanz had no relevant disclosures.
SAN FRANCISCO – The ACURATE neo transcatheter aortic valve replacement (TAVR) device failed to meet noninferiority, compared with the SAPIEN 3 device, in terms of the primary composite safety and efficacy endpoint at 30 days, a randomized, comparative trial showed.
“TAVR has become an indispensable treatment option for patients with symptomatic severe aortic stenosis across all risk categories,” Jonas Lanz, MD, MSc, said at the Transcatheter Cardiovascular Therapeutics annual meeting. “The generalizability of outcomes observed in landmark trials comparing TAVR with SAVR [surgical aortic valve replacement] to other commercial TAVR systems is limited by differences in device properties and lack of head-to-head device comparisons. Iterations of the SAPIEN balloon-expandable TAVR system have been extensively investigated in several large-scale, high-quality, randomized trials and registries setting the current benchmark in terms of safety and efficacy.”
The ACURATE neo is a novel self-expanding TAVR system associated with favorable outcomes in nonrandomized studies. It has been available in Europe since 2014 but has not gained Food and Drug Administration approval.
Between February 2017 and February 2019, in a randomized trial known as SCOPE I, Dr. Lanz and colleagues at 20 European sites enrolled 739 patients with severe, symptomatic aortic stenosis at increased surgical risk. Of these, 372 were assigned to transfemoral TAVR with the ACURATE neo and 367 were assigned to the SAPIEN 3 system. The researchers designed the study to investigate noninferiority of the primary endpoint, which was a composite of safety and efficacy derived from the Valve Academic Research Consortium–2 criteria and included all-cause death, any stroke, life-threatening or disabling bleeding, major vascular complications, coronary artery obstruction requiring intervention, acute kidney injury stage 2 or higher, valve-related dysfunction requiring repeat procedure, rehospitalization for valve-related symptoms or heart failure, moderate or severe prosthetic valve regurgitation, or prosthetic valve stenosis at 30 days.
Clinical follow-up information at 30 days was available for 99% and ECG follow-up for about 98% of the total study population. The mean age of the study participants was 82 years, 57% were female, their median STS Risk Score was 3.5, and their mean transvalvular aortic gradient was 42.2 mm Hg. Dr. Lanz, with the department of cardiology at Bern (Switzerland) University Hospital, reported that the primary endpoint rate in the intention-to-treat cohort for ACURATE neo was 23.7%, compared with 16.5% with SAPIEN 3, falling short of the threshold for noninferiority (P for noninferiority = 0.42).
“I think this speaks to the fact of how high the bar is right now – how good the procedure is [and] how good the devices are,” invited discussant Michael J. Mack, MD, a cardiac surgeon at Baylor Scott & White Health, Dallas, said during the media briefing. “It’s an incredibly high bar to meet for any new device.”
Certain single components of the primary endpoint were similar in the ACURATE neo group, compared with the SAPIEN 3 group, including all-cause death (2.5% vs. 0.8%, respectively) and stroke (1.9% vs. 3%), but acute kidney injury was more common in the ACURATE neo group (3% vs. 0.8%, as was paravalvular aortic regurgitation (9.4% vs. 2.8%). “The differences between the two TAVR devices were mainly driven by moderate or severe paravalvular regurgitation and stage 2 or 3 acute kidney injury in favor of the SAPIEN 3 device,” Dr. Lanz said at the meeting sponsored by the Cardiovascular Research Foundation. “An early composite safety and efficacy endpoint proved useful in discriminating the performance of different TAVR systems.”
He pointed out that a trial called SCOPE II is underway in Europe, with a new iteration of the ACURATE neo designed to reduce the risk of paravalvular leak.
The results of SCOPE I were published online at the time of presentation (Lancet. 2019 Sep 27. doi: 10.1016/S0140-6736[19]32220-2). SCOPE I was an investigator-initiated and -conducted study funded by a dedicated research grant from Symetis in Ecublens, Switzerland (part of Boston Scientific). Dr. Lanz had no relevant disclosures.
REPORTING FROM TCT 2019
Health benefits of TAVR over SAVR sustained at 1 year
SAN FRANCISCO – Among patients with severe aortic stenosis at low surgical risk, both transcatheter and surgical aortic valve replacement resulted in substantial health status benefits at 1 year despite most patients having New York Heart Association class I or II symptoms at baseline.
However, when compared with surgical replacement,
The findings come from an analysis of patients enrolled in the randomized PARTNER 3 trial, which showed that transcatheter aortic valve replacement (TAVR) with the SAPIEN 3 valve. At 1 year post procedure, the rate of the primary composite endpoint comprising death, stroke, or cardiovascular rehospitalization was 8.5% in the TAVR group and 15.1% with surgical aortic valve replacement (SAVR), for a highly significant 46% relative risk reduction (N Engl J Med 2019 May 2;380:1695-705).
“The PARTNER 3 and Evolut Low Risk trials have demonstrated that transfemoral TAVR is both safe and effective when compared with SAVR in patients with severe aortic stenosis at low surgical risk,” Suzanne J. Baron, MD, MSc, said at the Transcatheter Cardiovascular Therapeutics annual meeting. “While prior studies have demonstrated improved early health status with transfemoral TAVR, compared with SAVR in intermediate and high-risk patients, there is little evidence of any late health status benefit with TAVR.”
To address this gap in knowledge, Dr. Baron, director of interventional cardiology research at Lahey Hospital and Medical Center in Burlington, Mass., and associates performed a prospective study alongside the PARTNER 3 randomized trial to understand the impact of valve replacement strategy on early and late health status in aortic stenosis patients at low surgical risk. She reported results from 449 low-risk patients with severe aortic stenosis who were assigned to transfemoral TAVR using a balloon-expandable valve, and 449 who were assigned to surgery in PARTNER 3. At baseline, the mean age of patients was 73 years, 69% were male, and the average STS (Society of Thoracic Surgeons) Risk Score was 1.9%. Rates of other comorbidities were generally low.
Patients in both groups reported a mild baseline impairment in health status. The mean Kansas City Cardiomyopathy Questionnaire–Overall Summary (KCCQ-OS) score was 70, “which corresponds to only New York Heart Association Class II symptoms,” Dr. Baron said. “The SF-36 [Short Form 36] physical summary score was 44 for both groups, which is approximately half of a standard deviation below the population mean.”
As expected, patients who underwent TAVR showed substantially improved health status at 1 month based on the KCCQ-OS (mean difference,16 points; P less than .001). However, in contrast to prior studies, the researchers observed a persistent, although attenuated, benefit of TAVR over SAVR in disease-specific health status at 6 and 12 months (mean difference in KCCQ-OS of 2.6 and 1.8 points respectively; P less than .04 for both).
Dr. Baron said that a sustained benefit of TAVR over SAVR at 6 months and 1 year was observed on several KCCQ subscales, but a similar benefit was not noted on the generic health status measures such as the SF-36 physical summary score. “That’s likely reflective of the fact that, as a disease-specific measure, the KCCQ is much more sensitive in detecting meaningful differences in this population,” she explained. When change in health status was analyzed as an ordinal variable, with death as the worst outcome and large clinical improvement, which was defined as a 20-point or greater increase in the KCCQ-OS score, TAVR showed a significant benefit, compared with surgery at all time points (P less than .05).
In an effort to better understand the mechanism underlying this persistent albeit small late benefit in disease-specific health status with TAVR, the researchers generated cumulative distribution curves to display the proportion of patients who achieved a given change on the KCCQ-OS. A clear separation of the curves emerged, with 5.2% more patients in the TAVR group experiencing a change of at least 20 points, compared with the surgery group. “This suggests that the difference in late health status between the two groups is driven by this 5.2% absolute risk difference in the proportion of patients who experienced a large clinical improvement,” Dr. Baron said at the meeting, which was sponsored by the Cardiovascular Research Foundation.
Next, the researchers performed subgroup analyses to examine the interaction between the 1-year health status benefit of TAVR over surgery and prespecified baseline characteristics including age, gender, STS risk score, ejection fraction, atrial fibrillation, and New York Heart Association (NYHA) class. They observed a significant interaction between NYHA class and treatment effect such that patients who had NYHA class III or IV symptoms at baseline derived greater benefit from TAVR, compared with those who had NYHA class I or II symptoms at baseline.
“This finding suggests that it’s the patients with worse functional impairment at baseline who may be that subset of patients on the cumulative responder curves who gained better health status outcomes with TAVR, compared with surgery in the low-risk population,” Dr. Baron said.
Suzanne V. Arnold, MD, a cardiologist at Saint Luke’s Mid America Heart Institute, Kansas City, Mo., who was an invited discussant, said that it was “remarkable” that patients in the substudy were not particularly symptomatic and yet they still experienced close to a 20-point improvement in the KCCQ-OS score following TAVR, and asked whether frailty may have played a role in the 1.8-point adjusted difference in the KCCQ-OS score between TAVR and surgery at 1 year. Dr. Baron responded that she and her colleagues performed a subgroup analysis of patients who had two or more markers of frailty versus those who had one or less. Noting that there were only 20 patients in that subgroup, she said there was a significant signal that patients who were considered have two or more frail measures were considered to do much better with TAVR.
Dr. Baron concluded that the study’s overall findings, taken together with the clinical outcomes of the PARTNER 3 trial, “further support the use of TAVR in patients with severe [aortic stenosis] at low surgical risk. Longer-term follow up is needed (and ongoing) to determine whether the health status benefits of TAVR at 1 year are durable.”
The content of the study was published online at the time of presentation (J Am Coll Cardiol 2019 Sep 29. doi: 10.1016/j.jacc.2019.09.007). The PARTNER 3 quality of life substudy was funded by Edwards Lifesciences. Dr. Baron disclosed research funding and advisory board compensation from Boston Scientific Corp and consulting fees from Edwards Lifesciences.
SAN FRANCISCO – Among patients with severe aortic stenosis at low surgical risk, both transcatheter and surgical aortic valve replacement resulted in substantial health status benefits at 1 year despite most patients having New York Heart Association class I or II symptoms at baseline.
However, when compared with surgical replacement,
The findings come from an analysis of patients enrolled in the randomized PARTNER 3 trial, which showed that transcatheter aortic valve replacement (TAVR) with the SAPIEN 3 valve. At 1 year post procedure, the rate of the primary composite endpoint comprising death, stroke, or cardiovascular rehospitalization was 8.5% in the TAVR group and 15.1% with surgical aortic valve replacement (SAVR), for a highly significant 46% relative risk reduction (N Engl J Med 2019 May 2;380:1695-705).
“The PARTNER 3 and Evolut Low Risk trials have demonstrated that transfemoral TAVR is both safe and effective when compared with SAVR in patients with severe aortic stenosis at low surgical risk,” Suzanne J. Baron, MD, MSc, said at the Transcatheter Cardiovascular Therapeutics annual meeting. “While prior studies have demonstrated improved early health status with transfemoral TAVR, compared with SAVR in intermediate and high-risk patients, there is little evidence of any late health status benefit with TAVR.”
To address this gap in knowledge, Dr. Baron, director of interventional cardiology research at Lahey Hospital and Medical Center in Burlington, Mass., and associates performed a prospective study alongside the PARTNER 3 randomized trial to understand the impact of valve replacement strategy on early and late health status in aortic stenosis patients at low surgical risk. She reported results from 449 low-risk patients with severe aortic stenosis who were assigned to transfemoral TAVR using a balloon-expandable valve, and 449 who were assigned to surgery in PARTNER 3. At baseline, the mean age of patients was 73 years, 69% were male, and the average STS (Society of Thoracic Surgeons) Risk Score was 1.9%. Rates of other comorbidities were generally low.
Patients in both groups reported a mild baseline impairment in health status. The mean Kansas City Cardiomyopathy Questionnaire–Overall Summary (KCCQ-OS) score was 70, “which corresponds to only New York Heart Association Class II symptoms,” Dr. Baron said. “The SF-36 [Short Form 36] physical summary score was 44 for both groups, which is approximately half of a standard deviation below the population mean.”
As expected, patients who underwent TAVR showed substantially improved health status at 1 month based on the KCCQ-OS (mean difference,16 points; P less than .001). However, in contrast to prior studies, the researchers observed a persistent, although attenuated, benefit of TAVR over SAVR in disease-specific health status at 6 and 12 months (mean difference in KCCQ-OS of 2.6 and 1.8 points respectively; P less than .04 for both).
Dr. Baron said that a sustained benefit of TAVR over SAVR at 6 months and 1 year was observed on several KCCQ subscales, but a similar benefit was not noted on the generic health status measures such as the SF-36 physical summary score. “That’s likely reflective of the fact that, as a disease-specific measure, the KCCQ is much more sensitive in detecting meaningful differences in this population,” she explained. When change in health status was analyzed as an ordinal variable, with death as the worst outcome and large clinical improvement, which was defined as a 20-point or greater increase in the KCCQ-OS score, TAVR showed a significant benefit, compared with surgery at all time points (P less than .05).
In an effort to better understand the mechanism underlying this persistent albeit small late benefit in disease-specific health status with TAVR, the researchers generated cumulative distribution curves to display the proportion of patients who achieved a given change on the KCCQ-OS. A clear separation of the curves emerged, with 5.2% more patients in the TAVR group experiencing a change of at least 20 points, compared with the surgery group. “This suggests that the difference in late health status between the two groups is driven by this 5.2% absolute risk difference in the proportion of patients who experienced a large clinical improvement,” Dr. Baron said at the meeting, which was sponsored by the Cardiovascular Research Foundation.
Next, the researchers performed subgroup analyses to examine the interaction between the 1-year health status benefit of TAVR over surgery and prespecified baseline characteristics including age, gender, STS risk score, ejection fraction, atrial fibrillation, and New York Heart Association (NYHA) class. They observed a significant interaction between NYHA class and treatment effect such that patients who had NYHA class III or IV symptoms at baseline derived greater benefit from TAVR, compared with those who had NYHA class I or II symptoms at baseline.
“This finding suggests that it’s the patients with worse functional impairment at baseline who may be that subset of patients on the cumulative responder curves who gained better health status outcomes with TAVR, compared with surgery in the low-risk population,” Dr. Baron said.
Suzanne V. Arnold, MD, a cardiologist at Saint Luke’s Mid America Heart Institute, Kansas City, Mo., who was an invited discussant, said that it was “remarkable” that patients in the substudy were not particularly symptomatic and yet they still experienced close to a 20-point improvement in the KCCQ-OS score following TAVR, and asked whether frailty may have played a role in the 1.8-point adjusted difference in the KCCQ-OS score between TAVR and surgery at 1 year. Dr. Baron responded that she and her colleagues performed a subgroup analysis of patients who had two or more markers of frailty versus those who had one or less. Noting that there were only 20 patients in that subgroup, she said there was a significant signal that patients who were considered have two or more frail measures were considered to do much better with TAVR.
Dr. Baron concluded that the study’s overall findings, taken together with the clinical outcomes of the PARTNER 3 trial, “further support the use of TAVR in patients with severe [aortic stenosis] at low surgical risk. Longer-term follow up is needed (and ongoing) to determine whether the health status benefits of TAVR at 1 year are durable.”
The content of the study was published online at the time of presentation (J Am Coll Cardiol 2019 Sep 29. doi: 10.1016/j.jacc.2019.09.007). The PARTNER 3 quality of life substudy was funded by Edwards Lifesciences. Dr. Baron disclosed research funding and advisory board compensation from Boston Scientific Corp and consulting fees from Edwards Lifesciences.
SAN FRANCISCO – Among patients with severe aortic stenosis at low surgical risk, both transcatheter and surgical aortic valve replacement resulted in substantial health status benefits at 1 year despite most patients having New York Heart Association class I or II symptoms at baseline.
However, when compared with surgical replacement,
The findings come from an analysis of patients enrolled in the randomized PARTNER 3 trial, which showed that transcatheter aortic valve replacement (TAVR) with the SAPIEN 3 valve. At 1 year post procedure, the rate of the primary composite endpoint comprising death, stroke, or cardiovascular rehospitalization was 8.5% in the TAVR group and 15.1% with surgical aortic valve replacement (SAVR), for a highly significant 46% relative risk reduction (N Engl J Med 2019 May 2;380:1695-705).
“The PARTNER 3 and Evolut Low Risk trials have demonstrated that transfemoral TAVR is both safe and effective when compared with SAVR in patients with severe aortic stenosis at low surgical risk,” Suzanne J. Baron, MD, MSc, said at the Transcatheter Cardiovascular Therapeutics annual meeting. “While prior studies have demonstrated improved early health status with transfemoral TAVR, compared with SAVR in intermediate and high-risk patients, there is little evidence of any late health status benefit with TAVR.”
To address this gap in knowledge, Dr. Baron, director of interventional cardiology research at Lahey Hospital and Medical Center in Burlington, Mass., and associates performed a prospective study alongside the PARTNER 3 randomized trial to understand the impact of valve replacement strategy on early and late health status in aortic stenosis patients at low surgical risk. She reported results from 449 low-risk patients with severe aortic stenosis who were assigned to transfemoral TAVR using a balloon-expandable valve, and 449 who were assigned to surgery in PARTNER 3. At baseline, the mean age of patients was 73 years, 69% were male, and the average STS (Society of Thoracic Surgeons) Risk Score was 1.9%. Rates of other comorbidities were generally low.
Patients in both groups reported a mild baseline impairment in health status. The mean Kansas City Cardiomyopathy Questionnaire–Overall Summary (KCCQ-OS) score was 70, “which corresponds to only New York Heart Association Class II symptoms,” Dr. Baron said. “The SF-36 [Short Form 36] physical summary score was 44 for both groups, which is approximately half of a standard deviation below the population mean.”
As expected, patients who underwent TAVR showed substantially improved health status at 1 month based on the KCCQ-OS (mean difference,16 points; P less than .001). However, in contrast to prior studies, the researchers observed a persistent, although attenuated, benefit of TAVR over SAVR in disease-specific health status at 6 and 12 months (mean difference in KCCQ-OS of 2.6 and 1.8 points respectively; P less than .04 for both).
Dr. Baron said that a sustained benefit of TAVR over SAVR at 6 months and 1 year was observed on several KCCQ subscales, but a similar benefit was not noted on the generic health status measures such as the SF-36 physical summary score. “That’s likely reflective of the fact that, as a disease-specific measure, the KCCQ is much more sensitive in detecting meaningful differences in this population,” she explained. When change in health status was analyzed as an ordinal variable, with death as the worst outcome and large clinical improvement, which was defined as a 20-point or greater increase in the KCCQ-OS score, TAVR showed a significant benefit, compared with surgery at all time points (P less than .05).
In an effort to better understand the mechanism underlying this persistent albeit small late benefit in disease-specific health status with TAVR, the researchers generated cumulative distribution curves to display the proportion of patients who achieved a given change on the KCCQ-OS. A clear separation of the curves emerged, with 5.2% more patients in the TAVR group experiencing a change of at least 20 points, compared with the surgery group. “This suggests that the difference in late health status between the two groups is driven by this 5.2% absolute risk difference in the proportion of patients who experienced a large clinical improvement,” Dr. Baron said at the meeting, which was sponsored by the Cardiovascular Research Foundation.
Next, the researchers performed subgroup analyses to examine the interaction between the 1-year health status benefit of TAVR over surgery and prespecified baseline characteristics including age, gender, STS risk score, ejection fraction, atrial fibrillation, and New York Heart Association (NYHA) class. They observed a significant interaction between NYHA class and treatment effect such that patients who had NYHA class III or IV symptoms at baseline derived greater benefit from TAVR, compared with those who had NYHA class I or II symptoms at baseline.
“This finding suggests that it’s the patients with worse functional impairment at baseline who may be that subset of patients on the cumulative responder curves who gained better health status outcomes with TAVR, compared with surgery in the low-risk population,” Dr. Baron said.
Suzanne V. Arnold, MD, a cardiologist at Saint Luke’s Mid America Heart Institute, Kansas City, Mo., who was an invited discussant, said that it was “remarkable” that patients in the substudy were not particularly symptomatic and yet they still experienced close to a 20-point improvement in the KCCQ-OS score following TAVR, and asked whether frailty may have played a role in the 1.8-point adjusted difference in the KCCQ-OS score between TAVR and surgery at 1 year. Dr. Baron responded that she and her colleagues performed a subgroup analysis of patients who had two or more markers of frailty versus those who had one or less. Noting that there were only 20 patients in that subgroup, she said there was a significant signal that patients who were considered have two or more frail measures were considered to do much better with TAVR.
Dr. Baron concluded that the study’s overall findings, taken together with the clinical outcomes of the PARTNER 3 trial, “further support the use of TAVR in patients with severe [aortic stenosis] at low surgical risk. Longer-term follow up is needed (and ongoing) to determine whether the health status benefits of TAVR at 1 year are durable.”
The content of the study was published online at the time of presentation (J Am Coll Cardiol 2019 Sep 29. doi: 10.1016/j.jacc.2019.09.007). The PARTNER 3 quality of life substudy was funded by Edwards Lifesciences. Dr. Baron disclosed research funding and advisory board compensation from Boston Scientific Corp and consulting fees from Edwards Lifesciences.
REPORTING FROM TCT 2019
Colchicine pre-PCI improves biomarkers, not injury risk
PHILADELPHIA – Giving patients a single shot of colchicine before percutaneous coronary intervention was found to favorably impact inflammatory biomarkers linked to vascular injury, but not to lower the risk of procedure-related myocardial injury, according to results of the COLCHICINE-PCI randomized trial reported at the American Heart Association scientific sessions.
This is the first study to evaluate pre-PCI colchicine versus placebo on markers of myocardial injury and inflammation, said Binita Shah, MD, of Veterans Affairs New York Harbor Healthcare System and New York University.
“More work is needed to determine the optimal dosing and timing regimen of colchicine administration in patients undergoing PCI,” Dr. Shah said in an interview. “In this study, we saw inflammatory markers decrease around the 24-hour time point post PCI, so an earlier start to preprocedural colchicine regimen warrants further investigation.” The study found that pre-PCI colchicine attenuated increases in interleukin-6 and high-sensitivity C-reactive protein (CRP) concentrations at 24 hours post PCI, Dr. Shah said.
The results followed by a day the presentation of results from the COLCOT trial (Colchicine Cardiovascular Outcomes Trial) that showed a 23% reduction in cardiovascular events in patients with coronary disease on colchicine 0.5 mg daily vs. placebo (N Engl J Med. 2019 Nov 16. doi: 10.1056/NEJMoa1912388), as Subodh Verma, MD, PhD, of the University of Toronto noted in his discussant comments. COLCHICINE-PCI “has important implications, since patients with acute coronary syndrome often have variable biomarker responses, as biomarkers often function as acute-phase reactants in that setting.”
The COLCHICINE-PCI study of 400 patients investigated oral 1.8 mg colchicine given 1-2 hours before the patient went to the cath lab. The drug was given in a 1.2-mg dose followed an hour later by an 0.6-mg dose. Patients received placebo at the same intervals. An inflammatory biomarker substudy of 280 patients evaluated differences in plasma interleukin-6 levels at baseline and 1 hour post PCI, as well as other key biomarkers at longer intervals.
The primary outcome of PCI-related myocardial injury showed no statistically significant difference between the two groups, Dr. Shah said: 57.3% for colchicine and 64.2% for placebo (P = 0.19). The same was true of 30-day major adverse cardiovascular events, she said: 11.7% and 12.9% for the treatment and placebo groups, respectively (P = 0.82). Rates of PCI-related MI were also similar between the two groups.
However, the biomarker substudy told a different story. IL-6 levels in the treatment group were stable at 1 and 6-8 hours post PCI. “However, at 22-24 hours we see a significant attenuation in the rise of IL-6 with colchicine,” she said.
While IL-beta levels showed no deviation after PCI, the colchicine group showed a noticeable attenuation in the rise of high-sensitivity CRP levels at 22-24 hours.
“This is the first study to demonstrate that an oral load of colchicine prevents a rise of inflammatory markers in an acute-injury setting,” Dr. Shah said.
While results of the COLCOT trial affirmed a “resounding yes” for the use of colchicine in patients who’ve had a recent MI, Dr. Verma said the COLCHICINE-PCI results did not give as clear an answer.
“What about pre- or peri-PCI?” he said. “I don’t think we’re there yet, but I do think that more studies are needed that target residual inflammatory risk and potentially couple an acute loading dose with a chronic, ongoing treatment.” Results from higher-risk primary prevention studies, such as the CLEAR SYNERGY (OASIS 9) of a colchicine-spironolactone combination in patients with STEMI having PCI, are needed, he said.
Dr. Shah disclosed financial relationships with Phillips Volcano and Radux. The VA Office of Research and Development and AHA provided grant funding and Takeda Pharmaceuticals provided the drug.
SOURCE: Shah B. AHA 2019, Late Breaking Science session IV.
PHILADELPHIA – Giving patients a single shot of colchicine before percutaneous coronary intervention was found to favorably impact inflammatory biomarkers linked to vascular injury, but not to lower the risk of procedure-related myocardial injury, according to results of the COLCHICINE-PCI randomized trial reported at the American Heart Association scientific sessions.
This is the first study to evaluate pre-PCI colchicine versus placebo on markers of myocardial injury and inflammation, said Binita Shah, MD, of Veterans Affairs New York Harbor Healthcare System and New York University.
“More work is needed to determine the optimal dosing and timing regimen of colchicine administration in patients undergoing PCI,” Dr. Shah said in an interview. “In this study, we saw inflammatory markers decrease around the 24-hour time point post PCI, so an earlier start to preprocedural colchicine regimen warrants further investigation.” The study found that pre-PCI colchicine attenuated increases in interleukin-6 and high-sensitivity C-reactive protein (CRP) concentrations at 24 hours post PCI, Dr. Shah said.
The results followed by a day the presentation of results from the COLCOT trial (Colchicine Cardiovascular Outcomes Trial) that showed a 23% reduction in cardiovascular events in patients with coronary disease on colchicine 0.5 mg daily vs. placebo (N Engl J Med. 2019 Nov 16. doi: 10.1056/NEJMoa1912388), as Subodh Verma, MD, PhD, of the University of Toronto noted in his discussant comments. COLCHICINE-PCI “has important implications, since patients with acute coronary syndrome often have variable biomarker responses, as biomarkers often function as acute-phase reactants in that setting.”
The COLCHICINE-PCI study of 400 patients investigated oral 1.8 mg colchicine given 1-2 hours before the patient went to the cath lab. The drug was given in a 1.2-mg dose followed an hour later by an 0.6-mg dose. Patients received placebo at the same intervals. An inflammatory biomarker substudy of 280 patients evaluated differences in plasma interleukin-6 levels at baseline and 1 hour post PCI, as well as other key biomarkers at longer intervals.
The primary outcome of PCI-related myocardial injury showed no statistically significant difference between the two groups, Dr. Shah said: 57.3% for colchicine and 64.2% for placebo (P = 0.19). The same was true of 30-day major adverse cardiovascular events, she said: 11.7% and 12.9% for the treatment and placebo groups, respectively (P = 0.82). Rates of PCI-related MI were also similar between the two groups.
However, the biomarker substudy told a different story. IL-6 levels in the treatment group were stable at 1 and 6-8 hours post PCI. “However, at 22-24 hours we see a significant attenuation in the rise of IL-6 with colchicine,” she said.
While IL-beta levels showed no deviation after PCI, the colchicine group showed a noticeable attenuation in the rise of high-sensitivity CRP levels at 22-24 hours.
“This is the first study to demonstrate that an oral load of colchicine prevents a rise of inflammatory markers in an acute-injury setting,” Dr. Shah said.
While results of the COLCOT trial affirmed a “resounding yes” for the use of colchicine in patients who’ve had a recent MI, Dr. Verma said the COLCHICINE-PCI results did not give as clear an answer.
“What about pre- or peri-PCI?” he said. “I don’t think we’re there yet, but I do think that more studies are needed that target residual inflammatory risk and potentially couple an acute loading dose with a chronic, ongoing treatment.” Results from higher-risk primary prevention studies, such as the CLEAR SYNERGY (OASIS 9) of a colchicine-spironolactone combination in patients with STEMI having PCI, are needed, he said.
Dr. Shah disclosed financial relationships with Phillips Volcano and Radux. The VA Office of Research and Development and AHA provided grant funding and Takeda Pharmaceuticals provided the drug.
SOURCE: Shah B. AHA 2019, Late Breaking Science session IV.
PHILADELPHIA – Giving patients a single shot of colchicine before percutaneous coronary intervention was found to favorably impact inflammatory biomarkers linked to vascular injury, but not to lower the risk of procedure-related myocardial injury, according to results of the COLCHICINE-PCI randomized trial reported at the American Heart Association scientific sessions.
This is the first study to evaluate pre-PCI colchicine versus placebo on markers of myocardial injury and inflammation, said Binita Shah, MD, of Veterans Affairs New York Harbor Healthcare System and New York University.
“More work is needed to determine the optimal dosing and timing regimen of colchicine administration in patients undergoing PCI,” Dr. Shah said in an interview. “In this study, we saw inflammatory markers decrease around the 24-hour time point post PCI, so an earlier start to preprocedural colchicine regimen warrants further investigation.” The study found that pre-PCI colchicine attenuated increases in interleukin-6 and high-sensitivity C-reactive protein (CRP) concentrations at 24 hours post PCI, Dr. Shah said.
The results followed by a day the presentation of results from the COLCOT trial (Colchicine Cardiovascular Outcomes Trial) that showed a 23% reduction in cardiovascular events in patients with coronary disease on colchicine 0.5 mg daily vs. placebo (N Engl J Med. 2019 Nov 16. doi: 10.1056/NEJMoa1912388), as Subodh Verma, MD, PhD, of the University of Toronto noted in his discussant comments. COLCHICINE-PCI “has important implications, since patients with acute coronary syndrome often have variable biomarker responses, as biomarkers often function as acute-phase reactants in that setting.”
The COLCHICINE-PCI study of 400 patients investigated oral 1.8 mg colchicine given 1-2 hours before the patient went to the cath lab. The drug was given in a 1.2-mg dose followed an hour later by an 0.6-mg dose. Patients received placebo at the same intervals. An inflammatory biomarker substudy of 280 patients evaluated differences in plasma interleukin-6 levels at baseline and 1 hour post PCI, as well as other key biomarkers at longer intervals.
The primary outcome of PCI-related myocardial injury showed no statistically significant difference between the two groups, Dr. Shah said: 57.3% for colchicine and 64.2% for placebo (P = 0.19). The same was true of 30-day major adverse cardiovascular events, she said: 11.7% and 12.9% for the treatment and placebo groups, respectively (P = 0.82). Rates of PCI-related MI were also similar between the two groups.
However, the biomarker substudy told a different story. IL-6 levels in the treatment group were stable at 1 and 6-8 hours post PCI. “However, at 22-24 hours we see a significant attenuation in the rise of IL-6 with colchicine,” she said.
While IL-beta levels showed no deviation after PCI, the colchicine group showed a noticeable attenuation in the rise of high-sensitivity CRP levels at 22-24 hours.
“This is the first study to demonstrate that an oral load of colchicine prevents a rise of inflammatory markers in an acute-injury setting,” Dr. Shah said.
While results of the COLCOT trial affirmed a “resounding yes” for the use of colchicine in patients who’ve had a recent MI, Dr. Verma said the COLCHICINE-PCI results did not give as clear an answer.
“What about pre- or peri-PCI?” he said. “I don’t think we’re there yet, but I do think that more studies are needed that target residual inflammatory risk and potentially couple an acute loading dose with a chronic, ongoing treatment.” Results from higher-risk primary prevention studies, such as the CLEAR SYNERGY (OASIS 9) of a colchicine-spironolactone combination in patients with STEMI having PCI, are needed, he said.
Dr. Shah disclosed financial relationships with Phillips Volcano and Radux. The VA Office of Research and Development and AHA provided grant funding and Takeda Pharmaceuticals provided the drug.
SOURCE: Shah B. AHA 2019, Late Breaking Science session IV.
REPORTING FROM AHA 2019