User login
Improving Care for Sexual and Gender Minority Patients with Disorders of Gut-Brain Interaction
Brief Introduction to the SGM Communities
The sexual and gender minority (SGM) communities (see Table 1), also termed “LGBTQIA+ community” (lesbian, gay, bisexual, transgender, queer, intersex, asexual, plus — including two spirit) are historically minoritized with unique risks for inequities in gastrointestinal health outcomes.1 These potential disparities remain largely uninvestigated because of continued systemic discrimination and inadequate collection of sexual orientation and gender identity (SOGI) data,2 with the National Institutes of Health Sexual & Gender Minority Research Office (SGMRO) having been instructed to address these failures. There is increased SGM self-identification (7.1% of all people in the United States and 20.8% of generation Z).3 Given the high worldwide prevalence of disorders of gut-brain interaction (DGBIs)and the influence of biopsychosocial determinants of health in DGBI incidence,4 it becomes increasingly likely that research in DGBI-related factors in SGM people will be fruitful.
Disorders of Gut-Brain Interaction and the Potential Minority Stress Link in SGM People
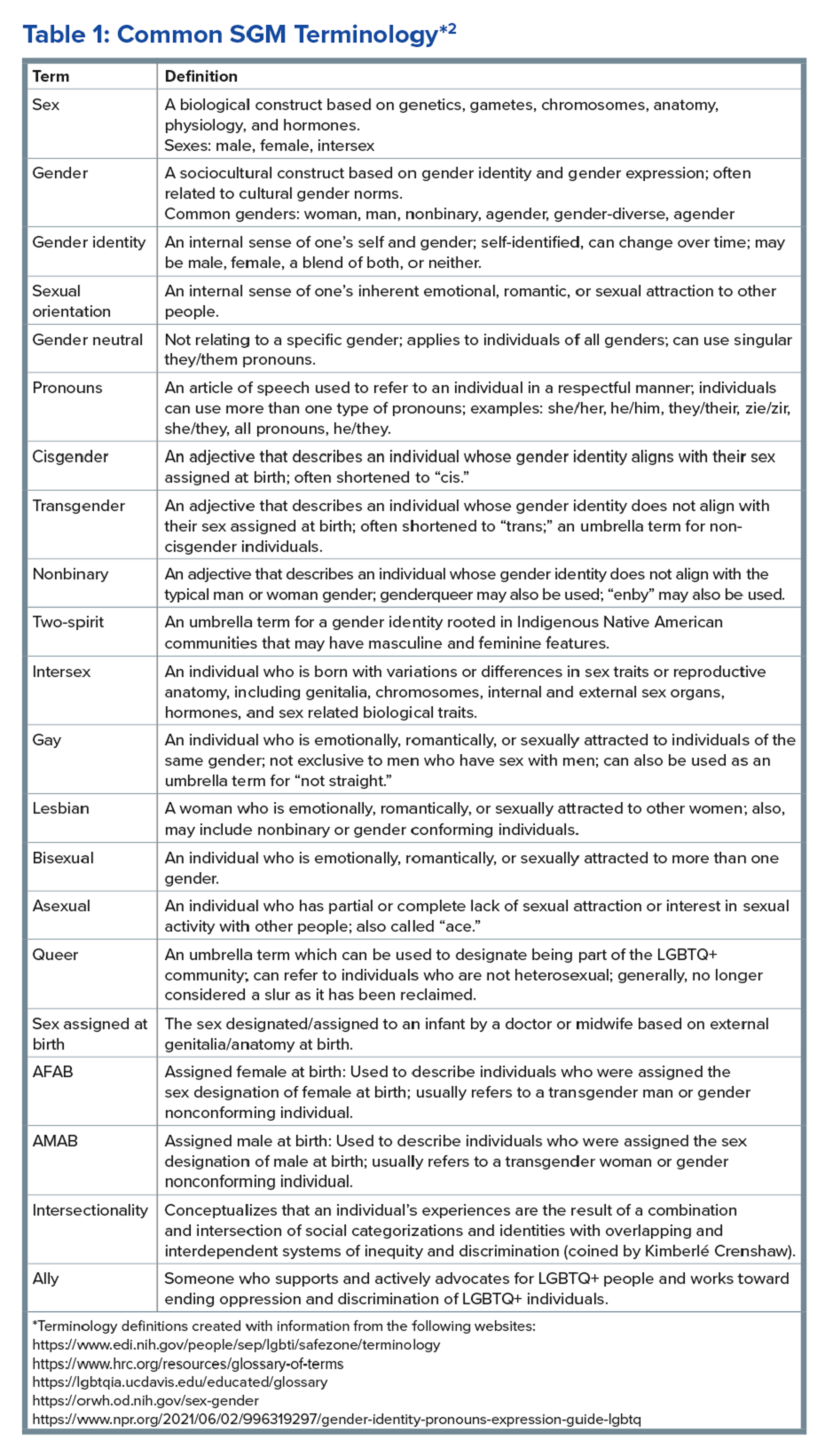
DGBIs are gastrointestinal conditions that occur because of brain-gut axis dysregulation. There is evidence that chronic stress and trauma negatively influence brain-gut interaction, which likely results in minority communities who face increased levels of trauma, stress, discrimination, and social injustice being at higher risk of DGBI development.5-7 Given increased rates of trauma in the SGM community, practicing trauma-informed care is essential to increase patient comfort and decrease the chance of retraumatization in medical settings.8 Trauma-informed care focuses on how trauma influences a patient’s life and response to medical care. To practice trauma-informed care, screening for trauma when appropriate, actively creating a supportive environment with active listening and communication, with informing the patient of planned actions prior to doing them, like physical exams, is key.
Trauma-Informed Care: Examples of Verbiage
Asking about Identity
- Begin by introducing yourself with your pronouns to create a safe environment for patient disclosure. Example: “Hello, I am Dr. Kara Jencks, and my pronouns are she/her. I am one of the gastroenterologists here at XYZ Clinic. How would you prefer to be addressed?”
- You can also wear a pronoun lapel pin or a pronoun button on your ID badge to indicate you are someone who your patient can be themselves around.
- The easiest way to obtain sexual orientation and gender identity is through intake forms. Below are examples of how to ask these questions on intake forms. It is important to offer the option to select more than one option when applicable and to opt out of answering if the patient is not comfortable answering these questions.
Sample Questions for Intake Forms
1. What is your sex assigned at birth? (Select one)
- Female
- Male
- Intersex
- Do not know
- Prefer not to disclose
2. What is your gender identity? (Select all that apply)
- Nonbinary
- Gender queer
- Woman
- Man
- Transwoman
- Transman
- Gender fluid
- Two-spirit
- Agender
- Intersex
- Other: type in response
- Prefer not to disclose
3. What are your pronouns? (Select all that apply)
- They/them/theirs
- She/her/hers
- He/him/his
- Zie/zir/zirs
- Other: type in response
- Prefer not to disclose
4. What is your sexual orientation? (Select all that apply)
- Bisexual
- Pansexual
- Queer
- Lesbian
- Gay
- Asexual
- Demisexual
- Heterosexual or straight
- Other: type in response
- Prefer not to disclose
Screening for Trauma
While there are questionnaires that exist to ask about trauma history, if time allows, it can be helpful to screen verbally with the patient. See reference number 8, for additional prompts and actions to practice trauma-informed care.
- Example: “Many patients with gastrointestinal symptoms and disorders have experienced trauma in the past. We do our best to ensure we are keeping you as comfortable as possible while caring for you. Are you comfortable sharing this information? [if yes->] Do you have a history of trauma, including physical, emotional, or sexual abuse? ... Have these experiences impacted the way in which you navigate your healthcare? ... Is there anything we can do to make you more comfortable today?”
General Physical Examination
Provide details for what you are going to do before you do it. Ask for permission for the examination. Here are two examples:
- “I would like to perform a physical exam to help better understand your symptoms. Is that okay with you?”
- “I would like to examine your abdomen with my stethoscope and my hands. Here is a sheet that we can use to help with your privacy. Please let me know if and when you feel any tenderness or pain.”
Rectal Physical Examination
Let the patient know why it would be helpful to perform a rectal exam, what the rectal exam will entail, and the benefits and risks to doing a rectal exam. An example follows:
- “Based on the symptoms you are describing, I think it would be helpful to perform a rectal exam to make sure you don’t have any fissures or hemorrhoids on the outside around the anus, any blockages or major issues inside the rectum, and to assess the strength and ability of your nerves and muscles or the pelvic floor to coordinate bowel movements. There are no risks aside from discomfort. If it is painful, and you would like me to stop, you tell me to stop, and I will stop right away. What questions do you have? Are we okay to proceed with the rectal exam?”
- “Please pull down your undergarments and your pants to either midthigh, your ankles, or all the way off, whatever your preference is, lie down on the left side on the exam table, and cover yourself with this sheet. In the meantime, I will be getting a chaperone to keep us safe and serve as a patient advocate during the procedure.”
- Upon returning to the exam room: “Here is Sara, who will be chaperoning today. Let myself or Sara know if you are uncomfortable or having pain during this exam. I will be lifting up the sheet to get a good look around the anus. [lifts up sheet] You will feel my hand helping to spread apart the buttocks. I am looking around the anus, and I do not see any fissures, hemorrhoids, or anything else concerning. Please squeeze in like you are trying to hold in gas. Please bear down like you are trying to have a bowel movement or let out gas. Okay, now you may feel some cold gel around the anus, and you will feel my finger go inside. Take a deep breath in. Do you feel any pain as I palpate? Please squeeze in like you are trying to hold in gas. Please bear down like you are trying to have a bowel movement or let out gas. I will be stopping the exam now.”
- You would then wash your hands and allow the patient to get dressed, and then disclose the exam findings and the rest of your visit.
Ilan H. Meyer coined the minority stress model when discussing mental health disorders in SGM patients in the early 2000s.9 With it being well known that DGBIs can overlap with (but are not necessarily caused by) mental health disorders, this model can easily apply to unify multiple individual and societal factors that can combine to result in disorders of brain-gut interaction (see Figure 1) in SGM communities. Let us keep this framework in mind when evaluating the following cases.
Case Presentations
Case 1
A 56-year-old man (pronouns: he/him) assigned male sex at birth, who identifies as gay, presents to your gastroenterology clinic for treatment-refractory constipation-predominant irritable bowel syndrome. It has impacted his sexual function. Outside hospital records report a normal colonoscopy 1 year ago and an unremarkable abdominal computerized tomography 4 months ago, aside from increased stool burden in the entire colon. He has tried to use enemas prior to sex, though these do not always help. Fiber-rich diet and fermentable food avoidance has not been successful. He is currently taking two capfuls of polyethylene glycol 3350 twice per day, as well as senna at night and continues to have a bowel movement every 2-3 days that is Bristol stool form scale type 1-2 unless he uses enemas. How do you counsel this patient about his IBS-C and rectal discomfort?
After assessing for sexual violence or other potential trauma-related factors, your digital rectal examination suggests that an anorectal defecatory disorder is less likely with normal relaxation and perineal movement. You recommend linaclotide. He notices improvement within 1 week, with improved comfort during anoreceptive sex.
Case 2
A 30-year-old woman (pronouns: she/her) assigned male sex at birth who has sex with men underwent vaginoplasty 2 years ago and is referred to the gastroenterology clinic for fecal incontinence and diarrhea. On review of her anatomic inventory, her vaginoplasty was a penile inversion vaginoplasty (no intestinal tissue was used for creation), and her prostate was left intact. The vaginal vault was created in between the urethra and rectum, similar to the pelvic floor anatomy of a woman assigned female sex at birth. Blood, imaging, and endoscopic workup has been negative. She is also not taking any medications associated with diarrhea, only taking estrogen and spironolactone. The diarrhea is not daily, but when present, about once per week, can be up to 10 episodes per day, and she has a sense of incomplete evacuation regularly. She notes having a rectal exam in the past but is not sure if her pelvic floor muscles have ever been assessed. How do you manage this patient?
To complete her evaluation in the office, you perform a trauma-informed rectal exam which reveals a decreased resting anal sphincter tone and paradoxical defecatory maneuvers without tenderness to the puborectalis muscle. Augmentation of the squeeze is also weak. Given her pelvic floor related surgical history, her symptoms, and her rectal exam, you recommend anorectal manometry which is abnormal and send her for anorectal biofeedback pelvic floor physical therapy, which improves her symptoms significantly.
Case 3
A 36-year-old woman (pronouns: she/her) assigned female sex at birth, who identifies as a lesbian, has a history of posttraumatic stress disorder and chronic nausea and vomiting that has begun to affect her quality of life. She notes the nausea and vomiting used to be managed well with evening cannabis gummies, though in the past 3 months, the nausea and vomiting has worsened, and she has lost 20 pounds as a result. As symptom predated cannabis usage, cannabis hyperemesis syndrome (CHS) was less likely (an important point as she has been stigmatized during prior encounters for her cannabis usage). Her primary care physician recommended a gastroscopy which was normal, aside from some residual solid food material in the stomach. Her bowel movements are normal, and she doesn’t have other gastrointestinal symptoms. She and her wife are considering having a third child, so she is worried about medications that may affect pregnancy or breast-feeding. How do you manage her nausea and vomiting?
After validating her concerns and performing a trauma-informed physical exam and encounter, you recommend a 4-hour gastric emptying test with a standard radiolabeled egg meal. Her gastric emptying does reveal significantly delayed gastric emptying at 2 and 4 hours. You discuss the risks and benefits of lifestyle modification (smaller frequent meals), initiating medications (erythromycin and metoclopramide) or cessation of cannabis (despite low likelihood of CHS). Desiring to avoid starting medications around initiation of pregnancy, she opts for the dietary approach and cessation of cannabis. You see her at a follow-up visit in 6 months, and her nausea is now only once a month, and she is excited to begin planning for a pregnancy using assisted reproductive technology.
Case 4
A 20-year-old nonbinary intersex individual (pronouns: he/they) (incorrectly assigned female at birth — is intersex with congenital adrenal hyperplasia) presents to the gastroenterology clinic with 8 years of heartburn, acid reflux, postprandial bloating, alternating diarrhea and constipation, nausea, and vomiting, complicated by avoidant restrictive food intake disorder. They have a history of bipolar II disorder with prior suicidal ideation. He has not yet had diagnostic workup as he previously had a bad encounter with a gastroenterologist where the gastroenterologist blamed his symptoms on his gender-affirming therapy, misgendered the patient, and told the patient their symptoms were “all in her [sic] head.”
You recognize that affirming their gender and using proper pronouns is the best first way to start rapport and help break the cycle of medicalized trauma. You then recommend a holistic work up with interdisciplinary management because of the complexity of his symptoms. For testing, you recommend a colonoscopy, upper endoscopy, a gastric emptying test with a 48-hour transit scintigraphy test, anorectal manometry, a dietitian referral, and a gastrointestinal psychology referral. Their anorectal manometry is consistent with an evacuation disorder. The rest of the work up is unremarkable. You diagnose them with anorectal pelvic floor dysfunction and functional dyspepsia, recommending biofeedback pelvic floor physical therapy, a proton-pump inhibitor, and neuromodulation in coordination with psychiatry and psychology to start with a plan for follow-up. The patient appreciates you for helping them and listening to their symptoms.
Discussion
When approaching DGBIs in the SGM community, it is vital to validate their concerns and be inclusive with diagnostic and treatment modalities. The diagnostic tools and treatments for DGBI are not different for patients in the SGM community. Like with other patients, trauma-informed care should be utilized, particularly given higher rates of trauma and discrimination in this community. Importantly, their DGBI is not a result of their sexual orientation or gender identity, and hormone therapy is not the cause of their DGBI. Recommending cessation of gender-affirming care or recommending lifestyle measures against their identity is generally not appropriate or necessary. among members of the SGM communities.
Dr. Jencks (@karajencks) is based in the division of gastroenterology and hepatology, Mayo Clinic, Rochester, Minnesota. Dr. Vélez (@Chris_Velez_MD) is based in the division of gastroenterology, Massachusetts General Hospital and Harvard Medical School, both in Boston. Both authors do not have any conflicts of interest for this article.
References
1. Duong N et al. 2023 Apr. doi: 10.1016/S2468-1253(23)00005-5.
2. Vélez C et al. Am J Gastroenterol. 2022 Jun. doi: 10.14309/ajg.0000000000001804.
3. Jones JM. Gallup. LGBTQ+ identification in U.S. now at 7.6%. 2024 Mar 13. https://news.gallup.com/poll/611864/lgbtq-identification.aspx
4. Sperber AD et al. Gastroenterology. 2021 Jan. doi: 10.1053/j.gastro.2020.04.014.
5. Wiley JW et al. Neurogastroenterol Motil. 2016 Jan. doi: 10.1111/nmo.12706.
6. Labanski A et al. Psychoneuroendocrinology. 2020 Jan. doi: 10.1016/j.psyneuen.2019.104501.
7. Khlevner J et al. Gastroenterol Clin North Am. 2018 Dec. doi: 10.1016/j.gtc.2018.07.002.
8. Jagielski CH and Harer KN. Gastroenterol Clin North Am. 2022 Dec. doi: 10.1016/j.gtc.2022.07.012.
9. Meyer IH. Psychol Bull. 2003 Sep. doi: 10.1037/0033-2909.129.5.674.
10. Mahurkar-Joshi S and Chang L. Front Psychiatry. 2020 Aug. doi: 10.3389/fpsyt.2020.00805.
Brief Introduction to the SGM Communities
The sexual and gender minority (SGM) communities (see Table 1), also termed “LGBTQIA+ community” (lesbian, gay, bisexual, transgender, queer, intersex, asexual, plus — including two spirit) are historically minoritized with unique risks for inequities in gastrointestinal health outcomes.1 These potential disparities remain largely uninvestigated because of continued systemic discrimination and inadequate collection of sexual orientation and gender identity (SOGI) data,2 with the National Institutes of Health Sexual & Gender Minority Research Office (SGMRO) having been instructed to address these failures. There is increased SGM self-identification (7.1% of all people in the United States and 20.8% of generation Z).3 Given the high worldwide prevalence of disorders of gut-brain interaction (DGBIs)and the influence of biopsychosocial determinants of health in DGBI incidence,4 it becomes increasingly likely that research in DGBI-related factors in SGM people will be fruitful.

Disorders of Gut-Brain Interaction and the Potential Minority Stress Link in SGM People
DGBIs are gastrointestinal conditions that occur because of brain-gut axis dysregulation. There is evidence that chronic stress and trauma negatively influence brain-gut interaction, which likely results in minority communities who face increased levels of trauma, stress, discrimination, and social injustice being at higher risk of DGBI development.5-7 Given increased rates of trauma in the SGM community, practicing trauma-informed care is essential to increase patient comfort and decrease the chance of retraumatization in medical settings.8 Trauma-informed care focuses on how trauma influences a patient’s life and response to medical care. To practice trauma-informed care, screening for trauma when appropriate, actively creating a supportive environment with active listening and communication, with informing the patient of planned actions prior to doing them, like physical exams, is key.
Trauma-Informed Care: Examples of Verbiage
Asking about Identity
- Begin by introducing yourself with your pronouns to create a safe environment for patient disclosure. Example: “Hello, I am Dr. Kara Jencks, and my pronouns are she/her. I am one of the gastroenterologists here at XYZ Clinic. How would you prefer to be addressed?”
- You can also wear a pronoun lapel pin or a pronoun button on your ID badge to indicate you are someone who your patient can be themselves around.
- The easiest way to obtain sexual orientation and gender identity is through intake forms. Below are examples of how to ask these questions on intake forms. It is important to offer the option to select more than one option when applicable and to opt out of answering if the patient is not comfortable answering these questions.
Sample Questions for Intake Forms
1. What is your sex assigned at birth? (Select one)
- Female
- Male
- Intersex
- Do not know
- Prefer not to disclose
2. What is your gender identity? (Select all that apply)
- Nonbinary
- Gender queer
- Woman
- Man
- Transwoman
- Transman
- Gender fluid
- Two-spirit
- Agender
- Intersex
- Other: type in response
- Prefer not to disclose
3. What are your pronouns? (Select all that apply)
- They/them/theirs
- She/her/hers
- He/him/his
- Zie/zir/zirs
- Other: type in response
- Prefer not to disclose
4. What is your sexual orientation? (Select all that apply)
- Bisexual
- Pansexual
- Queer
- Lesbian
- Gay
- Asexual
- Demisexual
- Heterosexual or straight
- Other: type in response
- Prefer not to disclose
Screening for Trauma
While there are questionnaires that exist to ask about trauma history, if time allows, it can be helpful to screen verbally with the patient. See reference number 8, for additional prompts and actions to practice trauma-informed care.
- Example: “Many patients with gastrointestinal symptoms and disorders have experienced trauma in the past. We do our best to ensure we are keeping you as comfortable as possible while caring for you. Are you comfortable sharing this information? [if yes->] Do you have a history of trauma, including physical, emotional, or sexual abuse? ... Have these experiences impacted the way in which you navigate your healthcare? ... Is there anything we can do to make you more comfortable today?”
General Physical Examination
Provide details for what you are going to do before you do it. Ask for permission for the examination. Here are two examples:
- “I would like to perform a physical exam to help better understand your symptoms. Is that okay with you?”
- “I would like to examine your abdomen with my stethoscope and my hands. Here is a sheet that we can use to help with your privacy. Please let me know if and when you feel any tenderness or pain.”
Rectal Physical Examination
Let the patient know why it would be helpful to perform a rectal exam, what the rectal exam will entail, and the benefits and risks to doing a rectal exam. An example follows:
- “Based on the symptoms you are describing, I think it would be helpful to perform a rectal exam to make sure you don’t have any fissures or hemorrhoids on the outside around the anus, any blockages or major issues inside the rectum, and to assess the strength and ability of your nerves and muscles or the pelvic floor to coordinate bowel movements. There are no risks aside from discomfort. If it is painful, and you would like me to stop, you tell me to stop, and I will stop right away. What questions do you have? Are we okay to proceed with the rectal exam?”
- “Please pull down your undergarments and your pants to either midthigh, your ankles, or all the way off, whatever your preference is, lie down on the left side on the exam table, and cover yourself with this sheet. In the meantime, I will be getting a chaperone to keep us safe and serve as a patient advocate during the procedure.”
- Upon returning to the exam room: “Here is Sara, who will be chaperoning today. Let myself or Sara know if you are uncomfortable or having pain during this exam. I will be lifting up the sheet to get a good look around the anus. [lifts up sheet] You will feel my hand helping to spread apart the buttocks. I am looking around the anus, and I do not see any fissures, hemorrhoids, or anything else concerning. Please squeeze in like you are trying to hold in gas. Please bear down like you are trying to have a bowel movement or let out gas. Okay, now you may feel some cold gel around the anus, and you will feel my finger go inside. Take a deep breath in. Do you feel any pain as I palpate? Please squeeze in like you are trying to hold in gas. Please bear down like you are trying to have a bowel movement or let out gas. I will be stopping the exam now.”
- You would then wash your hands and allow the patient to get dressed, and then disclose the exam findings and the rest of your visit.
Ilan H. Meyer coined the minority stress model when discussing mental health disorders in SGM patients in the early 2000s.9 With it being well known that DGBIs can overlap with (but are not necessarily caused by) mental health disorders, this model can easily apply to unify multiple individual and societal factors that can combine to result in disorders of brain-gut interaction (see Figure 1) in SGM communities. Let us keep this framework in mind when evaluating the following cases.

Case Presentations
Case 1
A 56-year-old man (pronouns: he/him) assigned male sex at birth, who identifies as gay, presents to your gastroenterology clinic for treatment-refractory constipation-predominant irritable bowel syndrome. It has impacted his sexual function. Outside hospital records report a normal colonoscopy 1 year ago and an unremarkable abdominal computerized tomography 4 months ago, aside from increased stool burden in the entire colon. He has tried to use enemas prior to sex, though these do not always help. Fiber-rich diet and fermentable food avoidance has not been successful. He is currently taking two capfuls of polyethylene glycol 3350 twice per day, as well as senna at night and continues to have a bowel movement every 2-3 days that is Bristol stool form scale type 1-2 unless he uses enemas. How do you counsel this patient about his IBS-C and rectal discomfort?
After assessing for sexual violence or other potential trauma-related factors, your digital rectal examination suggests that an anorectal defecatory disorder is less likely with normal relaxation and perineal movement. You recommend linaclotide. He notices improvement within 1 week, with improved comfort during anoreceptive sex.
Case 2
A 30-year-old woman (pronouns: she/her) assigned male sex at birth who has sex with men underwent vaginoplasty 2 years ago and is referred to the gastroenterology clinic for fecal incontinence and diarrhea. On review of her anatomic inventory, her vaginoplasty was a penile inversion vaginoplasty (no intestinal tissue was used for creation), and her prostate was left intact. The vaginal vault was created in between the urethra and rectum, similar to the pelvic floor anatomy of a woman assigned female sex at birth. Blood, imaging, and endoscopic workup has been negative. She is also not taking any medications associated with diarrhea, only taking estrogen and spironolactone. The diarrhea is not daily, but when present, about once per week, can be up to 10 episodes per day, and she has a sense of incomplete evacuation regularly. She notes having a rectal exam in the past but is not sure if her pelvic floor muscles have ever been assessed. How do you manage this patient?
To complete her evaluation in the office, you perform a trauma-informed rectal exam which reveals a decreased resting anal sphincter tone and paradoxical defecatory maneuvers without tenderness to the puborectalis muscle. Augmentation of the squeeze is also weak. Given her pelvic floor related surgical history, her symptoms, and her rectal exam, you recommend anorectal manometry which is abnormal and send her for anorectal biofeedback pelvic floor physical therapy, which improves her symptoms significantly.
Case 3
A 36-year-old woman (pronouns: she/her) assigned female sex at birth, who identifies as a lesbian, has a history of posttraumatic stress disorder and chronic nausea and vomiting that has begun to affect her quality of life. She notes the nausea and vomiting used to be managed well with evening cannabis gummies, though in the past 3 months, the nausea and vomiting has worsened, and she has lost 20 pounds as a result. As symptom predated cannabis usage, cannabis hyperemesis syndrome (CHS) was less likely (an important point as she has been stigmatized during prior encounters for her cannabis usage). Her primary care physician recommended a gastroscopy which was normal, aside from some residual solid food material in the stomach. Her bowel movements are normal, and she doesn’t have other gastrointestinal symptoms. She and her wife are considering having a third child, so she is worried about medications that may affect pregnancy or breast-feeding. How do you manage her nausea and vomiting?
After validating her concerns and performing a trauma-informed physical exam and encounter, you recommend a 4-hour gastric emptying test with a standard radiolabeled egg meal. Her gastric emptying does reveal significantly delayed gastric emptying at 2 and 4 hours. You discuss the risks and benefits of lifestyle modification (smaller frequent meals), initiating medications (erythromycin and metoclopramide) or cessation of cannabis (despite low likelihood of CHS). Desiring to avoid starting medications around initiation of pregnancy, she opts for the dietary approach and cessation of cannabis. You see her at a follow-up visit in 6 months, and her nausea is now only once a month, and she is excited to begin planning for a pregnancy using assisted reproductive technology.
Case 4
A 20-year-old nonbinary intersex individual (pronouns: he/they) (incorrectly assigned female at birth — is intersex with congenital adrenal hyperplasia) presents to the gastroenterology clinic with 8 years of heartburn, acid reflux, postprandial bloating, alternating diarrhea and constipation, nausea, and vomiting, complicated by avoidant restrictive food intake disorder. They have a history of bipolar II disorder with prior suicidal ideation. He has not yet had diagnostic workup as he previously had a bad encounter with a gastroenterologist where the gastroenterologist blamed his symptoms on his gender-affirming therapy, misgendered the patient, and told the patient their symptoms were “all in her [sic] head.”
You recognize that affirming their gender and using proper pronouns is the best first way to start rapport and help break the cycle of medicalized trauma. You then recommend a holistic work up with interdisciplinary management because of the complexity of his symptoms. For testing, you recommend a colonoscopy, upper endoscopy, a gastric emptying test with a 48-hour transit scintigraphy test, anorectal manometry, a dietitian referral, and a gastrointestinal psychology referral. Their anorectal manometry is consistent with an evacuation disorder. The rest of the work up is unremarkable. You diagnose them with anorectal pelvic floor dysfunction and functional dyspepsia, recommending biofeedback pelvic floor physical therapy, a proton-pump inhibitor, and neuromodulation in coordination with psychiatry and psychology to start with a plan for follow-up. The patient appreciates you for helping them and listening to their symptoms.
Discussion
When approaching DGBIs in the SGM community, it is vital to validate their concerns and be inclusive with diagnostic and treatment modalities. The diagnostic tools and treatments for DGBI are not different for patients in the SGM community. Like with other patients, trauma-informed care should be utilized, particularly given higher rates of trauma and discrimination in this community. Importantly, their DGBI is not a result of their sexual orientation or gender identity, and hormone therapy is not the cause of their DGBI. Recommending cessation of gender-affirming care or recommending lifestyle measures against their identity is generally not appropriate or necessary. among members of the SGM communities.
Dr. Jencks (@karajencks) is based in the division of gastroenterology and hepatology, Mayo Clinic, Rochester, Minnesota. Dr. Vélez (@Chris_Velez_MD) is based in the division of gastroenterology, Massachusetts General Hospital and Harvard Medical School, both in Boston. Both authors do not have any conflicts of interest for this article.
References
1. Duong N et al. 2023 Apr. doi: 10.1016/S2468-1253(23)00005-5.
2. Vélez C et al. Am J Gastroenterol. 2022 Jun. doi: 10.14309/ajg.0000000000001804.
3. Jones JM. Gallup. LGBTQ+ identification in U.S. now at 7.6%. 2024 Mar 13. https://news.gallup.com/poll/611864/lgbtq-identification.aspx
4. Sperber AD et al. Gastroenterology. 2021 Jan. doi: 10.1053/j.gastro.2020.04.014.
5. Wiley JW et al. Neurogastroenterol Motil. 2016 Jan. doi: 10.1111/nmo.12706.
6. Labanski A et al. Psychoneuroendocrinology. 2020 Jan. doi: 10.1016/j.psyneuen.2019.104501.
7. Khlevner J et al. Gastroenterol Clin North Am. 2018 Dec. doi: 10.1016/j.gtc.2018.07.002.
8. Jagielski CH and Harer KN. Gastroenterol Clin North Am. 2022 Dec. doi: 10.1016/j.gtc.2022.07.012.
9. Meyer IH. Psychol Bull. 2003 Sep. doi: 10.1037/0033-2909.129.5.674.
10. Mahurkar-Joshi S and Chang L. Front Psychiatry. 2020 Aug. doi: 10.3389/fpsyt.2020.00805.
Brief Introduction to the SGM Communities
The sexual and gender minority (SGM) communities (see Table 1), also termed “LGBTQIA+ community” (lesbian, gay, bisexual, transgender, queer, intersex, asexual, plus — including two spirit) are historically minoritized with unique risks for inequities in gastrointestinal health outcomes.1 These potential disparities remain largely uninvestigated because of continued systemic discrimination and inadequate collection of sexual orientation and gender identity (SOGI) data,2 with the National Institutes of Health Sexual & Gender Minority Research Office (SGMRO) having been instructed to address these failures. There is increased SGM self-identification (7.1% of all people in the United States and 20.8% of generation Z).3 Given the high worldwide prevalence of disorders of gut-brain interaction (DGBIs)and the influence of biopsychosocial determinants of health in DGBI incidence,4 it becomes increasingly likely that research in DGBI-related factors in SGM people will be fruitful.

Disorders of Gut-Brain Interaction and the Potential Minority Stress Link in SGM People
DGBIs are gastrointestinal conditions that occur because of brain-gut axis dysregulation. There is evidence that chronic stress and trauma negatively influence brain-gut interaction, which likely results in minority communities who face increased levels of trauma, stress, discrimination, and social injustice being at higher risk of DGBI development.5-7 Given increased rates of trauma in the SGM community, practicing trauma-informed care is essential to increase patient comfort and decrease the chance of retraumatization in medical settings.8 Trauma-informed care focuses on how trauma influences a patient’s life and response to medical care. To practice trauma-informed care, screening for trauma when appropriate, actively creating a supportive environment with active listening and communication, with informing the patient of planned actions prior to doing them, like physical exams, is key.
Trauma-Informed Care: Examples of Verbiage
Asking about Identity
- Begin by introducing yourself with your pronouns to create a safe environment for patient disclosure. Example: “Hello, I am Dr. Kara Jencks, and my pronouns are she/her. I am one of the gastroenterologists here at XYZ Clinic. How would you prefer to be addressed?”
- You can also wear a pronoun lapel pin or a pronoun button on your ID badge to indicate you are someone who your patient can be themselves around.
- The easiest way to obtain sexual orientation and gender identity is through intake forms. Below are examples of how to ask these questions on intake forms. It is important to offer the option to select more than one option when applicable and to opt out of answering if the patient is not comfortable answering these questions.
Sample Questions for Intake Forms
1. What is your sex assigned at birth? (Select one)
- Female
- Male
- Intersex
- Do not know
- Prefer not to disclose
2. What is your gender identity? (Select all that apply)
- Nonbinary
- Gender queer
- Woman
- Man
- Transwoman
- Transman
- Gender fluid
- Two-spirit
- Agender
- Intersex
- Other: type in response
- Prefer not to disclose
3. What are your pronouns? (Select all that apply)
- They/them/theirs
- She/her/hers
- He/him/his
- Zie/zir/zirs
- Other: type in response
- Prefer not to disclose
4. What is your sexual orientation? (Select all that apply)
- Bisexual
- Pansexual
- Queer
- Lesbian
- Gay
- Asexual
- Demisexual
- Heterosexual or straight
- Other: type in response
- Prefer not to disclose
Screening for Trauma
While there are questionnaires that exist to ask about trauma history, if time allows, it can be helpful to screen verbally with the patient. See reference number 8, for additional prompts and actions to practice trauma-informed care.
- Example: “Many patients with gastrointestinal symptoms and disorders have experienced trauma in the past. We do our best to ensure we are keeping you as comfortable as possible while caring for you. Are you comfortable sharing this information? [if yes->] Do you have a history of trauma, including physical, emotional, or sexual abuse? ... Have these experiences impacted the way in which you navigate your healthcare? ... Is there anything we can do to make you more comfortable today?”
General Physical Examination
Provide details for what you are going to do before you do it. Ask for permission for the examination. Here are two examples:
- “I would like to perform a physical exam to help better understand your symptoms. Is that okay with you?”
- “I would like to examine your abdomen with my stethoscope and my hands. Here is a sheet that we can use to help with your privacy. Please let me know if and when you feel any tenderness or pain.”
Rectal Physical Examination
Let the patient know why it would be helpful to perform a rectal exam, what the rectal exam will entail, and the benefits and risks to doing a rectal exam. An example follows:
- “Based on the symptoms you are describing, I think it would be helpful to perform a rectal exam to make sure you don’t have any fissures or hemorrhoids on the outside around the anus, any blockages or major issues inside the rectum, and to assess the strength and ability of your nerves and muscles or the pelvic floor to coordinate bowel movements. There are no risks aside from discomfort. If it is painful, and you would like me to stop, you tell me to stop, and I will stop right away. What questions do you have? Are we okay to proceed with the rectal exam?”
- “Please pull down your undergarments and your pants to either midthigh, your ankles, or all the way off, whatever your preference is, lie down on the left side on the exam table, and cover yourself with this sheet. In the meantime, I will be getting a chaperone to keep us safe and serve as a patient advocate during the procedure.”
- Upon returning to the exam room: “Here is Sara, who will be chaperoning today. Let myself or Sara know if you are uncomfortable or having pain during this exam. I will be lifting up the sheet to get a good look around the anus. [lifts up sheet] You will feel my hand helping to spread apart the buttocks. I am looking around the anus, and I do not see any fissures, hemorrhoids, or anything else concerning. Please squeeze in like you are trying to hold in gas. Please bear down like you are trying to have a bowel movement or let out gas. Okay, now you may feel some cold gel around the anus, and you will feel my finger go inside. Take a deep breath in. Do you feel any pain as I palpate? Please squeeze in like you are trying to hold in gas. Please bear down like you are trying to have a bowel movement or let out gas. I will be stopping the exam now.”
- You would then wash your hands and allow the patient to get dressed, and then disclose the exam findings and the rest of your visit.
Ilan H. Meyer coined the minority stress model when discussing mental health disorders in SGM patients in the early 2000s.9 With it being well known that DGBIs can overlap with (but are not necessarily caused by) mental health disorders, this model can easily apply to unify multiple individual and societal factors that can combine to result in disorders of brain-gut interaction (see Figure 1) in SGM communities. Let us keep this framework in mind when evaluating the following cases.

Case Presentations
Case 1
A 56-year-old man (pronouns: he/him) assigned male sex at birth, who identifies as gay, presents to your gastroenterology clinic for treatment-refractory constipation-predominant irritable bowel syndrome. It has impacted his sexual function. Outside hospital records report a normal colonoscopy 1 year ago and an unremarkable abdominal computerized tomography 4 months ago, aside from increased stool burden in the entire colon. He has tried to use enemas prior to sex, though these do not always help. Fiber-rich diet and fermentable food avoidance has not been successful. He is currently taking two capfuls of polyethylene glycol 3350 twice per day, as well as senna at night and continues to have a bowel movement every 2-3 days that is Bristol stool form scale type 1-2 unless he uses enemas. How do you counsel this patient about his IBS-C and rectal discomfort?
After assessing for sexual violence or other potential trauma-related factors, your digital rectal examination suggests that an anorectal defecatory disorder is less likely with normal relaxation and perineal movement. You recommend linaclotide. He notices improvement within 1 week, with improved comfort during anoreceptive sex.
Case 2
A 30-year-old woman (pronouns: she/her) assigned male sex at birth who has sex with men underwent vaginoplasty 2 years ago and is referred to the gastroenterology clinic for fecal incontinence and diarrhea. On review of her anatomic inventory, her vaginoplasty was a penile inversion vaginoplasty (no intestinal tissue was used for creation), and her prostate was left intact. The vaginal vault was created in between the urethra and rectum, similar to the pelvic floor anatomy of a woman assigned female sex at birth. Blood, imaging, and endoscopic workup has been negative. She is also not taking any medications associated with diarrhea, only taking estrogen and spironolactone. The diarrhea is not daily, but when present, about once per week, can be up to 10 episodes per day, and she has a sense of incomplete evacuation regularly. She notes having a rectal exam in the past but is not sure if her pelvic floor muscles have ever been assessed. How do you manage this patient?
To complete her evaluation in the office, you perform a trauma-informed rectal exam which reveals a decreased resting anal sphincter tone and paradoxical defecatory maneuvers without tenderness to the puborectalis muscle. Augmentation of the squeeze is also weak. Given her pelvic floor related surgical history, her symptoms, and her rectal exam, you recommend anorectal manometry which is abnormal and send her for anorectal biofeedback pelvic floor physical therapy, which improves her symptoms significantly.
Case 3
A 36-year-old woman (pronouns: she/her) assigned female sex at birth, who identifies as a lesbian, has a history of posttraumatic stress disorder and chronic nausea and vomiting that has begun to affect her quality of life. She notes the nausea and vomiting used to be managed well with evening cannabis gummies, though in the past 3 months, the nausea and vomiting has worsened, and she has lost 20 pounds as a result. As symptom predated cannabis usage, cannabis hyperemesis syndrome (CHS) was less likely (an important point as she has been stigmatized during prior encounters for her cannabis usage). Her primary care physician recommended a gastroscopy which was normal, aside from some residual solid food material in the stomach. Her bowel movements are normal, and she doesn’t have other gastrointestinal symptoms. She and her wife are considering having a third child, so she is worried about medications that may affect pregnancy or breast-feeding. How do you manage her nausea and vomiting?
After validating her concerns and performing a trauma-informed physical exam and encounter, you recommend a 4-hour gastric emptying test with a standard radiolabeled egg meal. Her gastric emptying does reveal significantly delayed gastric emptying at 2 and 4 hours. You discuss the risks and benefits of lifestyle modification (smaller frequent meals), initiating medications (erythromycin and metoclopramide) or cessation of cannabis (despite low likelihood of CHS). Desiring to avoid starting medications around initiation of pregnancy, she opts for the dietary approach and cessation of cannabis. You see her at a follow-up visit in 6 months, and her nausea is now only once a month, and she is excited to begin planning for a pregnancy using assisted reproductive technology.
Case 4
A 20-year-old nonbinary intersex individual (pronouns: he/they) (incorrectly assigned female at birth — is intersex with congenital adrenal hyperplasia) presents to the gastroenterology clinic with 8 years of heartburn, acid reflux, postprandial bloating, alternating diarrhea and constipation, nausea, and vomiting, complicated by avoidant restrictive food intake disorder. They have a history of bipolar II disorder with prior suicidal ideation. He has not yet had diagnostic workup as he previously had a bad encounter with a gastroenterologist where the gastroenterologist blamed his symptoms on his gender-affirming therapy, misgendered the patient, and told the patient their symptoms were “all in her [sic] head.”
You recognize that affirming their gender and using proper pronouns is the best first way to start rapport and help break the cycle of medicalized trauma. You then recommend a holistic work up with interdisciplinary management because of the complexity of his symptoms. For testing, you recommend a colonoscopy, upper endoscopy, a gastric emptying test with a 48-hour transit scintigraphy test, anorectal manometry, a dietitian referral, and a gastrointestinal psychology referral. Their anorectal manometry is consistent with an evacuation disorder. The rest of the work up is unremarkable. You diagnose them with anorectal pelvic floor dysfunction and functional dyspepsia, recommending biofeedback pelvic floor physical therapy, a proton-pump inhibitor, and neuromodulation in coordination with psychiatry and psychology to start with a plan for follow-up. The patient appreciates you for helping them and listening to their symptoms.
Discussion
When approaching DGBIs in the SGM community, it is vital to validate their concerns and be inclusive with diagnostic and treatment modalities. The diagnostic tools and treatments for DGBI are not different for patients in the SGM community. Like with other patients, trauma-informed care should be utilized, particularly given higher rates of trauma and discrimination in this community. Importantly, their DGBI is not a result of their sexual orientation or gender identity, and hormone therapy is not the cause of their DGBI. Recommending cessation of gender-affirming care or recommending lifestyle measures against their identity is generally not appropriate or necessary. among members of the SGM communities.
Dr. Jencks (@karajencks) is based in the division of gastroenterology and hepatology, Mayo Clinic, Rochester, Minnesota. Dr. Vélez (@Chris_Velez_MD) is based in the division of gastroenterology, Massachusetts General Hospital and Harvard Medical School, both in Boston. Both authors do not have any conflicts of interest for this article.
References
1. Duong N et al. 2023 Apr. doi: 10.1016/S2468-1253(23)00005-5.
2. Vélez C et al. Am J Gastroenterol. 2022 Jun. doi: 10.14309/ajg.0000000000001804.
3. Jones JM. Gallup. LGBTQ+ identification in U.S. now at 7.6%. 2024 Mar 13. https://news.gallup.com/poll/611864/lgbtq-identification.aspx
4. Sperber AD et al. Gastroenterology. 2021 Jan. doi: 10.1053/j.gastro.2020.04.014.
5. Wiley JW et al. Neurogastroenterol Motil. 2016 Jan. doi: 10.1111/nmo.12706.
6. Labanski A et al. Psychoneuroendocrinology. 2020 Jan. doi: 10.1016/j.psyneuen.2019.104501.
7. Khlevner J et al. Gastroenterol Clin North Am. 2018 Dec. doi: 10.1016/j.gtc.2018.07.002.
8. Jagielski CH and Harer KN. Gastroenterol Clin North Am. 2022 Dec. doi: 10.1016/j.gtc.2022.07.012.
9. Meyer IH. Psychol Bull. 2003 Sep. doi: 10.1037/0033-2909.129.5.674.
10. Mahurkar-Joshi S and Chang L. Front Psychiatry. 2020 Aug. doi: 10.3389/fpsyt.2020.00805.
Avoid Getting Stuck: A Practical Guide to Managing Chronic Constipation
Introduction
Constipation affects one in six people worldwide and accounts for one third of outpatient visits.1 Chronic constipation is defined by difficult, infrequent, and/or incomplete defecation, quantified by less than three spontaneous bowel movements per week, persisting for at least 3 months. Patients may complain of straining during defecation, incomplete evacuation, hard stools (Bristol stool scale [BSS] type 1-2), and fullness or bloating. Chronic constipation can be subclassified as either a primary or secondary disorder.1,2
Primary Constipation Disorders
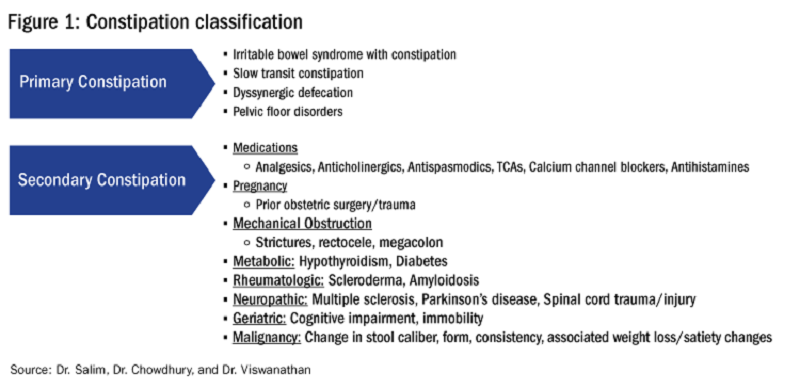
Primary constipation includes disorders of the colon or anorectum. This includes irritable bowel syndrome with constipation (IBS-C), chronic idiopathic constipation (CIC), slow transit constipation (STC), dyssynergic defecation, and pelvic floor disorders (see Figure 1).
IBS-C
IBS-C is a chronic disorder of the gut-brain axis with a worldwide prevalence of 1.3% and a prevalence of 6%-16% in the United States, United Kingdom, and Canada, with females more likely to seek care than males.2 The economic impact of IBS-C is estimated to be $1.5 billion–$10 billion per year in the United States alone.3 The distinguishing characteristic is abdominal pain, however IBS-C can present with a constellation of symptoms. The diagnostic paradigm has shifted from IBS being a diagnosis of exclusion to now using a positive diagnostic strategy.2 Using this Rome IV criteria, one can make the diagnosis with > 95% accuracy.2,4
CIC
CIC, previously defined as functional constipation, is a disorder defined by incomplete defecation and difficult or infrequent stool. CIC is diagnosed in patients without an underlying anatomic or structural abnormality. Rome IV Criteria helps further classify the defining characteristics of chronic idiopathic constipation.2
Slow Transit Constipation
STC is characterized by impaired colonic transit time in the absence of pelvic floor dysfunction. It presents with infrequent bowel movements, diminished urgency, and/or straining with defecation.
Defecatory Disorders: Dyssynergic Defecation and Pelvic Floor Dysfunction
Defecatory disorders (DDs) result from alterations in the colonic-neural pathway with an unclear pathogenesis. A firm understanding of colonic physiology is necessary to identify DDs. The right colon helps to store and mix stool contents, the left colon helps add water to the stool, and the anal canal and rectum enable defecation and maintain continence. Any alteration along this physiologic pathway results in DDs.5
DDs primarily develop via maladaptive pelvic floor contraction during defecation or from muscle or nerve injury and include functional outlet obstruction, anorectal dyssynergia, and pelvic floor dysfunction. Increased resistance to defecation results from anismus, paradoxical anal sphincter contraction, or incomplete relaxation of the pelvic floor and external anal sphincter. This muscle incoordination is described as dyssynergia. DDs can involve either muscle or nerve dysfunction or a combination of the two. Reduced rectal sensation caused by reduced sensory triggers can cause stasis of stool, thus propagating the cycle of constipation. Over time, excessive straining can weaken the pelvic floor, increasing the risk of excessive perineal descent, rectal intussusception, solitary rectal ulcer syndrome, and pudendal neuropathy.5 Thus, identification of DDs is crucial in patients with chronic constipation.
Secondary Constipation Disorders
Secondary constipation disorders are a result of an alternate process and warrant a thorough review of outpatient medications and past medical history. Figure 1 outlines the most common causes of secondary constipation, which span a wide differential.
Clinical Evaluation
The evaluation of constipation begins with a thorough history. Description of bowel habits should include frequency, duration, straining, stool consistency using a Bristol stool chart, complete vs incomplete evacuation, pain, bloating, and use of digital maneuvers (vaginal splinting or digital stool removal). One should inquire about back trauma/surgeries and obstetric history to include vaginal forceps injury or episiotomy.
With increased smartphone use, toilet time on average has increased and can contribute to maladaptive bowel habits.6 Patients may not realize they are constipated, so patient education is critical. A patient with daily bowel movements ranging between BSS type 1-6 with incomplete evacuation might complain of diarrhea but may in fact have constipation with overflow diarrhea, for example. Past medical history is also clinically relevant, as systemic conditions can cause secondary constipation. A constipated patient should also be asked what therapies he/she has tried prior to gastroenterology referral as primary care referrals for constipation account for 8 million visits to gastroenterology per year.7
While a sensitive topic, inquire about abuse history, especially in those with childhood constipation symptoms. There is a positive correlation between childhood constipation and physical, emotional, and sexual abuse and, for any number of reasons, your patient may be reluctant to share this or undergo a digital rectal exam (DRE).8 In such cases, be sensitive in asking for this history in private rather than with other family members around and always perform this exam with a chaperone present.
A detailed physical exam is an indispensable tool all gastroenterologists must master when evaluating a constipated patient. Some key exam findings include abdominal distention, high-pitched bowel sounds, and presence of a succussion splash indicating obstructive pathology. Dry skin and brittle hair indicate hypothyroidism while hypermobile joints and skin laxity suggest connective tissue disease. Finally, a physical examination is incomplete without a DRE.
DRE
DRE is an often-overlooked physical exam component which provides helpful insight that can guide management. An informed DRE can help identify structural disorders such as fissure, hemorrhoids, anorectal mass, fecal impaction, rectal prolapse, and excessive perineal descent syndrome.9 Unless contraindicated, DRE should be a standard part of the workup of a patient with chronic constipation.
Workup
Colonoscopy
The role of colonoscopy in chronic constipation is low yield and only indicated if alarm signs are present.2 When no organic causes can be identified, the patient is deemed to have a functional bowel or motility disorder leading to constipation.
Colonic Transit Time
Colonic transit time (CTT) can be evaluated by assessing the presence of radio-opaque sitz markers in the colon with an abdominal x-ray 5 days after ingestion. The presence of five or more sitz markers may indicate STC. However, this can also signal an obstructive defecatory disorder. Colon scintigraphy can determine whether there is diffuse colonic dysmotility or dysfunction in a specific segment of the colon.10
Anorectal Function Testing (AFT)
AFT can evaluate DDs, such as fecal incontinence, dyssynergic defecation, rectal sensory disorders, anorectal pain, and rectal prolapse. AFT comprises three tests: anorectal manometry (ARM), balloon expulsion test (BET), and rectal sensory testing. These assess the defecation, continence, and sensory mechanisms of the rectum, respectively.
ARM testing employs a thin, flexible probe with an attached sensor that is inserted into the rectum to measure internal and external sphincter pressures while at rest, squeezing, and bearing down to give a functional assessment of sphincter tone.11 Cough or party balloon test assesses continence and sphincter strength. Rectal sensation is assessed by inflating a balloon incrementally and asking the patient to indicate first sensation, urgency to defecate, and discomfort. If both ARM and BET are abnormal, the patient meets diagnostic criteria for dyssynergic defecation.12
Pelvic floor disorders can be further assessed by MR defecography or barium defecography. Barium defecography is the more widely available of the two. MR defecography is a dynamic study that directly assesses pelvic floor muscles and endopelvic fascia during various stages of defecation and considered superior. This testing modality can distinguish between functional causes such as dyssynergia or pelvic floor dysfunction and structural causes of obstruction such as rectocele, rectal prolapse, or rectal intussusception. MR defecography is helpful when dyssynergia is suggested by ARM with a normal BET or if there is an absent recto-anal inhibitory reflex on ARM, which may suggest rectal intussusception.
Management
CIC
Incorporating 20-30 g of total soluble fiber, such as psyllium in individuals with low dietary fiber intake is the first-line recommendation for CIC.13 If response to a trial of fiber supplementation is inadequate, over-the-counter (OTC) osmotic laxatives such as polyethylene glycol and magnesium oxide can be incorporated. In the event of failure of OTC osmotic laxatives, lactulose can be considered. Stimulant laxatives such as senna, bisacodyl, or sodium picosulfate can be added as an adjunctive measure for short periods of time, defined as daily for 4 weeks or less.
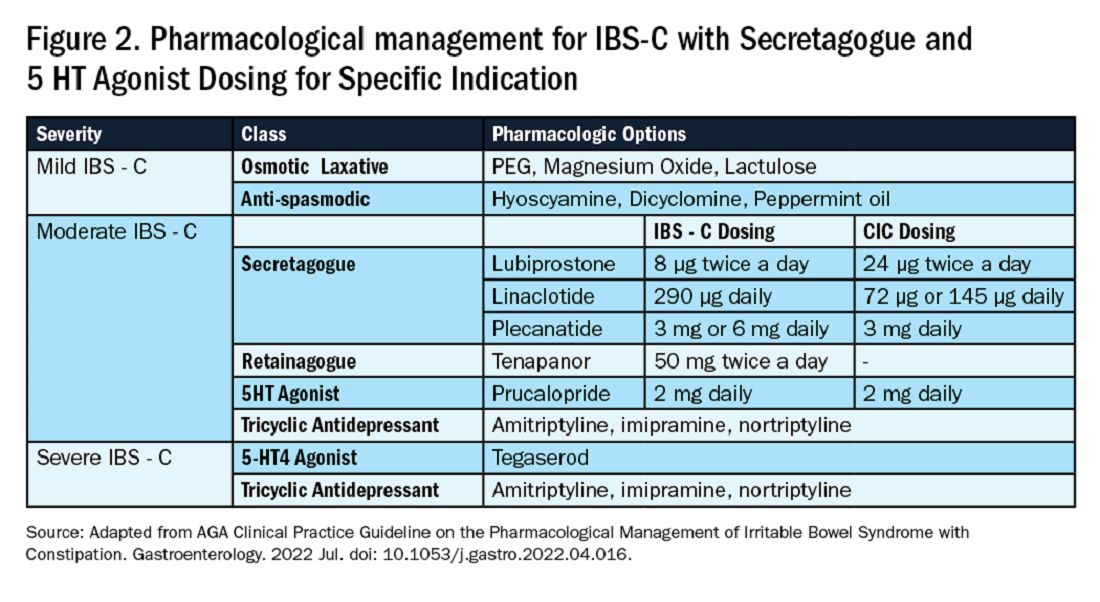
If these measures are inadequate, pharmacological therapy with secretagogues and 5HT agonists can be considered. Prucalopride, a selective agonist of serotonin 5-HT4 receptors, is approved for CIC, prescribed 2 mg daily.14 It can also be used in patients with global motility delays, such as gastroparetics with constipation. The mechanism of action of secretagogues and specific dosing of these medications are discussed in Figure 2.15 Vibrant is a non-pharmacologic, orally ingested, vibrating, and programmable capsule device that has recently received Food and Drug Administration approval for treatment of chronic constipation by stimulating the intestinal wall, thereby promoting colonic contractile activity to achieve more spontaneous bowel movements. Further studies are required to assess its efficacy.16 Additionally, if there is inadequate response to all the above, it would be prudent to evaluate for the presence of pelvic floor dysfunction as well.
IBS-C
Similar to CIC, treatment for mild IBS-C starts with osmotic laxatives with the additional component of pain control. Antispasmodics can be used to manage the abdominal pain, cramping, and spasms associated with IBS-C. Antispasmodics available in the United States include anticholinergic agents that cause smooth muscle relaxation, such as dicyclomine or hyoscyamine or direct smooth muscle relaxants such as peppermint oil.17 IBS-C patients with moderate symptoms may need escalation of therapy to secretagogues or 5HT agonists (see Figure 2). Secretagogues increase fluid retention in the colonic lumen to promote bowel movements and improve visceral hypersensitivity. Lubiprostone is an intestinal chloride channel activator, indicated only for adult women with IBS-C. Linaclotide and plecanatide are guanylate cyclase-C activators which increase intestinal chloride and bicarbonate secretion, and both are indicated in IBS-C and CIC. Tenapanor inhibits the sodium/hydrogen exchanger in the GI tract, leading to increased water secretion, and is recommended for IBS-C in adults who have failed secretagogues.
All four of these drugs can be considered for moderate to severe IBS-C symptoms. In the case of severe IBS-C symptoms, Tegaserod, a 5-HT4 receptor partial agonist has been approved in women under 65 without significant cardiovascular or cerebrovascular disease.18 Regardless of IBS-C symptom severity, persistent visceral hypersensitivity can be treated with low-dose neuromodulators.19 Figure 2 provides treatment recommendations for IBS-C based on symptom severity.
Opioid-Induced Constipation (OIC)
In patients with OIC, peripherally acting mu-opioid receptor antagonists such as methylnaltrexone and naloxegol can be beneficial where stimulant laxatives are insufficient. Additionally, lubiprostone is indicated in OIC in non-cancer patients. At present, there are no head-to-head trials comparing efficacy of these medications.
Defecatory Disorders
Biofeedback therapy is the cornerstone of treatment for dyssynergic defecation, focusing on neuromuscular training to restore a normal pattern of defecation by teaching patients to tense the abdomen and relax the pelvic floor muscles and anal sphincter. It retrains the body to coordinate abdominal, rectal, and anal muscles to achieve synchronous contraction to achieve complete evacuation. It also increases awareness and response to rectal fullness or the need to defecate.
Biofeedback makes patients aware of counterproductive subconscious actions such as contracting of their anal sphincter during defecation followed by simulated defecation training with focus on how to tighten abdominal muscles and relax pelvic floor muscles to initiate and complete defecation.20 This is performed in the office with a physiotherapist or trained nurse for at least six sessions or at home where patients are encouraged to perform the exercises for 20 minutes, twice a day. These sessions utilize tools such as manometry probes, electromyography probes, simulated balloon, or home biofeedback training devices to provide visual feedback while practicing abdominophrenic breathing. Biofeedback is particularly helpful in patients suffering from constipation. Patients with defecatory disorders can also benefit from pelvic floor physical therapy which focuses on strengthening the pelvic and puborectal muscles, external anal sphincter, and pelvic muscles. This is more useful in patients with fecal incontinence. Despite all these treatments, a subset of patients may still not respond and may qualify for surgical evaluation.
Conclusion
While constipation is seldom life-threatening, it has a negative impact on patient quality of life and poses a significant financial burden on our overall healthcare system. The complexity of this condition should be appreciated and understood in order for a complete and thorough evaluation. We trust that our practical guide should serve as a useful tool in the evaluation of a chronically constipated patient.
Dr. Salim (@hamsalim07 on X) is based in the Department of Internal Medicine, University of Texas Medical Branch, Galveston. Dr. Chowdhury (annicho.med on Instagram) is a fellow in the Department of Gastroenterology, Hepatology, and Nutrition, University of Texas MD Anderson Cancer Center, Houston. Dr. Viswanathan (@LavanyaMD on X) is Associate Professor, University of Texas MD Anderson Cancer Center. The authors declare no conflict of interest.
References
1. Mugie S et al. Best Pract Res Clin Gastroenterol. 2011 Feb. doi: 10.1016/j.bpg.2010.12.010.
2. Almario CV et al. Gastroenterology. 2023 Dec. doi: 10.1053/j.gastro.2023.08.010.
3. Canavan C et al. Clin Epidemiol. 2014 Feb. doi: 10.2147/CLEP.S40245.
4. Rao SSC. Gastroenterol Clin North Am. 2007 Sep. doi: 10.1016/j.gtc.2007.07.013.
5. Bharucha AE et al. Gastroenterology. 2020 Apr. doi: 10.1053/j.gastro.2019.12.034.
6. Cinquetti M et al. Clin Exp Pediatr. 2021 Sep. doi: 10.3345/cep.2020.01326.
7. Shah ND et al. Am J Gastroenterol. 2008 Jul. doi: 10.1111/j.1572-0241.2008.01910.x.
8. Rajindrajith S et al. J Pediatr Gastroenterol Nutr. 2014 Apr. doi: 10.1097/MPG.0000000000000249.
9. Talley NJ. Am J Gastroenterol. 2008 Apr. doi: 10.1111/j.1572-0241.2008.01832.x.
10. Maurer AH. J Nucl Med. 2015 Sep. doi: 10.2967/jnumed.113.134551.
11. Frye J et al. Am J Gastroenterol. 2024 Aug. doi: 10.14309/ajg.0000000000002670.
12. Rao SSC et al. J Neurogastroenterol Motil. 2016 Jun. doi: 10.5056/jnm16060.
13. Chang L et al. Gastroenterology. 2023 Jun. doi: 10.1053/j.gastro.2023.03.214.
14. Brenner DM et al. Am J Gastroenterol. 2021 Aug. doi: 10.14309/ajg.0000000000001266.
15. Chang L et al. Gastroenterology. 2022 Jul. doi: 10.1053/j.gastro.2022.04.016.
16. Rao SSC et al. Gastroenterology. 2023 Jun. doi: 10.1053/j.gastro.2023.02.013.
17. Lacy BE et al. Am J Gastroenterol. 2021 Jan. doi: 10.14309/ajg.0000000000001036.
18. Anderson JL et al. J Cardiovasc Pharmacol Ther. 2009 Sep. doi: 10.1177/1074248409340158.
19. Rahimi R et al. World J Gastroenterol. 2009 Apr. doi: 10.3748/wjg.15.1548.
20. Rao SSC. Best Pract Res Clin Gastroenterol. 2011 Feb. doi: 10.1016/j.bpg.2011.01.004.
Introduction
Constipation affects one in six people worldwide and accounts for one third of outpatient visits.1 Chronic constipation is defined by difficult, infrequent, and/or incomplete defecation, quantified by less than three spontaneous bowel movements per week, persisting for at least 3 months. Patients may complain of straining during defecation, incomplete evacuation, hard stools (Bristol stool scale [BSS] type 1-2), and fullness or bloating. Chronic constipation can be subclassified as either a primary or secondary disorder.1,2
Primary Constipation Disorders
Primary constipation includes disorders of the colon or anorectum. This includes irritable bowel syndrome with constipation (IBS-C), chronic idiopathic constipation (CIC), slow transit constipation (STC), dyssynergic defecation, and pelvic floor disorders (see Figure 1).

IBS-C
IBS-C is a chronic disorder of the gut-brain axis with a worldwide prevalence of 1.3% and a prevalence of 6%-16% in the United States, United Kingdom, and Canada, with females more likely to seek care than males.2 The economic impact of IBS-C is estimated to be $1.5 billion–$10 billion per year in the United States alone.3 The distinguishing characteristic is abdominal pain, however IBS-C can present with a constellation of symptoms. The diagnostic paradigm has shifted from IBS being a diagnosis of exclusion to now using a positive diagnostic strategy.2 Using this Rome IV criteria, one can make the diagnosis with > 95% accuracy.2,4
CIC
CIC, previously defined as functional constipation, is a disorder defined by incomplete defecation and difficult or infrequent stool. CIC is diagnosed in patients without an underlying anatomic or structural abnormality. Rome IV Criteria helps further classify the defining characteristics of chronic idiopathic constipation.2
Slow Transit Constipation
STC is characterized by impaired colonic transit time in the absence of pelvic floor dysfunction. It presents with infrequent bowel movements, diminished urgency, and/or straining with defecation.
Defecatory Disorders: Dyssynergic Defecation and Pelvic Floor Dysfunction
Defecatory disorders (DDs) result from alterations in the colonic-neural pathway with an unclear pathogenesis. A firm understanding of colonic physiology is necessary to identify DDs. The right colon helps to store and mix stool contents, the left colon helps add water to the stool, and the anal canal and rectum enable defecation and maintain continence. Any alteration along this physiologic pathway results in DDs.5
DDs primarily develop via maladaptive pelvic floor contraction during defecation or from muscle or nerve injury and include functional outlet obstruction, anorectal dyssynergia, and pelvic floor dysfunction. Increased resistance to defecation results from anismus, paradoxical anal sphincter contraction, or incomplete relaxation of the pelvic floor and external anal sphincter. This muscle incoordination is described as dyssynergia. DDs can involve either muscle or nerve dysfunction or a combination of the two. Reduced rectal sensation caused by reduced sensory triggers can cause stasis of stool, thus propagating the cycle of constipation. Over time, excessive straining can weaken the pelvic floor, increasing the risk of excessive perineal descent, rectal intussusception, solitary rectal ulcer syndrome, and pudendal neuropathy.5 Thus, identification of DDs is crucial in patients with chronic constipation.
Secondary Constipation Disorders
Secondary constipation disorders are a result of an alternate process and warrant a thorough review of outpatient medications and past medical history. Figure 1 outlines the most common causes of secondary constipation, which span a wide differential.
Clinical Evaluation
The evaluation of constipation begins with a thorough history. Description of bowel habits should include frequency, duration, straining, stool consistency using a Bristol stool chart, complete vs incomplete evacuation, pain, bloating, and use of digital maneuvers (vaginal splinting or digital stool removal). One should inquire about back trauma/surgeries and obstetric history to include vaginal forceps injury or episiotomy.
With increased smartphone use, toilet time on average has increased and can contribute to maladaptive bowel habits.6 Patients may not realize they are constipated, so patient education is critical. A patient with daily bowel movements ranging between BSS type 1-6 with incomplete evacuation might complain of diarrhea but may in fact have constipation with overflow diarrhea, for example. Past medical history is also clinically relevant, as systemic conditions can cause secondary constipation. A constipated patient should also be asked what therapies he/she has tried prior to gastroenterology referral as primary care referrals for constipation account for 8 million visits to gastroenterology per year.7
While a sensitive topic, inquire about abuse history, especially in those with childhood constipation symptoms. There is a positive correlation between childhood constipation and physical, emotional, and sexual abuse and, for any number of reasons, your patient may be reluctant to share this or undergo a digital rectal exam (DRE).8 In such cases, be sensitive in asking for this history in private rather than with other family members around and always perform this exam with a chaperone present.
A detailed physical exam is an indispensable tool all gastroenterologists must master when evaluating a constipated patient. Some key exam findings include abdominal distention, high-pitched bowel sounds, and presence of a succussion splash indicating obstructive pathology. Dry skin and brittle hair indicate hypothyroidism while hypermobile joints and skin laxity suggest connective tissue disease. Finally, a physical examination is incomplete without a DRE.
DRE
DRE is an often-overlooked physical exam component which provides helpful insight that can guide management. An informed DRE can help identify structural disorders such as fissure, hemorrhoids, anorectal mass, fecal impaction, rectal prolapse, and excessive perineal descent syndrome.9 Unless contraindicated, DRE should be a standard part of the workup of a patient with chronic constipation.
Workup
Colonoscopy
The role of colonoscopy in chronic constipation is low yield and only indicated if alarm signs are present.2 When no organic causes can be identified, the patient is deemed to have a functional bowel or motility disorder leading to constipation.
Colonic Transit Time
Colonic transit time (CTT) can be evaluated by assessing the presence of radio-opaque sitz markers in the colon with an abdominal x-ray 5 days after ingestion. The presence of five or more sitz markers may indicate STC. However, this can also signal an obstructive defecatory disorder. Colon scintigraphy can determine whether there is diffuse colonic dysmotility or dysfunction in a specific segment of the colon.10
Anorectal Function Testing (AFT)
AFT can evaluate DDs, such as fecal incontinence, dyssynergic defecation, rectal sensory disorders, anorectal pain, and rectal prolapse. AFT comprises three tests: anorectal manometry (ARM), balloon expulsion test (BET), and rectal sensory testing. These assess the defecation, continence, and sensory mechanisms of the rectum, respectively.
ARM testing employs a thin, flexible probe with an attached sensor that is inserted into the rectum to measure internal and external sphincter pressures while at rest, squeezing, and bearing down to give a functional assessment of sphincter tone.11 Cough or party balloon test assesses continence and sphincter strength. Rectal sensation is assessed by inflating a balloon incrementally and asking the patient to indicate first sensation, urgency to defecate, and discomfort. If both ARM and BET are abnormal, the patient meets diagnostic criteria for dyssynergic defecation.12
Pelvic floor disorders can be further assessed by MR defecography or barium defecography. Barium defecography is the more widely available of the two. MR defecography is a dynamic study that directly assesses pelvic floor muscles and endopelvic fascia during various stages of defecation and considered superior. This testing modality can distinguish between functional causes such as dyssynergia or pelvic floor dysfunction and structural causes of obstruction such as rectocele, rectal prolapse, or rectal intussusception. MR defecography is helpful when dyssynergia is suggested by ARM with a normal BET or if there is an absent recto-anal inhibitory reflex on ARM, which may suggest rectal intussusception.
Management
CIC
Incorporating 20-30 g of total soluble fiber, such as psyllium in individuals with low dietary fiber intake is the first-line recommendation for CIC.13 If response to a trial of fiber supplementation is inadequate, over-the-counter (OTC) osmotic laxatives such as polyethylene glycol and magnesium oxide can be incorporated. In the event of failure of OTC osmotic laxatives, lactulose can be considered. Stimulant laxatives such as senna, bisacodyl, or sodium picosulfate can be added as an adjunctive measure for short periods of time, defined as daily for 4 weeks or less.
If these measures are inadequate, pharmacological therapy with secretagogues and 5HT agonists can be considered. Prucalopride, a selective agonist of serotonin 5-HT4 receptors, is approved for CIC, prescribed 2 mg daily.14 It can also be used in patients with global motility delays, such as gastroparetics with constipation. The mechanism of action of secretagogues and specific dosing of these medications are discussed in Figure 2.15 Vibrant is a non-pharmacologic, orally ingested, vibrating, and programmable capsule device that has recently received Food and Drug Administration approval for treatment of chronic constipation by stimulating the intestinal wall, thereby promoting colonic contractile activity to achieve more spontaneous bowel movements. Further studies are required to assess its efficacy.16 Additionally, if there is inadequate response to all the above, it would be prudent to evaluate for the presence of pelvic floor dysfunction as well.

IBS-C
Similar to CIC, treatment for mild IBS-C starts with osmotic laxatives with the additional component of pain control. Antispasmodics can be used to manage the abdominal pain, cramping, and spasms associated with IBS-C. Antispasmodics available in the United States include anticholinergic agents that cause smooth muscle relaxation, such as dicyclomine or hyoscyamine or direct smooth muscle relaxants such as peppermint oil.17 IBS-C patients with moderate symptoms may need escalation of therapy to secretagogues or 5HT agonists (see Figure 2). Secretagogues increase fluid retention in the colonic lumen to promote bowel movements and improve visceral hypersensitivity. Lubiprostone is an intestinal chloride channel activator, indicated only for adult women with IBS-C. Linaclotide and plecanatide are guanylate cyclase-C activators which increase intestinal chloride and bicarbonate secretion, and both are indicated in IBS-C and CIC. Tenapanor inhibits the sodium/hydrogen exchanger in the GI tract, leading to increased water secretion, and is recommended for IBS-C in adults who have failed secretagogues.
All four of these drugs can be considered for moderate to severe IBS-C symptoms. In the case of severe IBS-C symptoms, Tegaserod, a 5-HT4 receptor partial agonist has been approved in women under 65 without significant cardiovascular or cerebrovascular disease.18 Regardless of IBS-C symptom severity, persistent visceral hypersensitivity can be treated with low-dose neuromodulators.19 Figure 2 provides treatment recommendations for IBS-C based on symptom severity.
Opioid-Induced Constipation (OIC)
In patients with OIC, peripherally acting mu-opioid receptor antagonists such as methylnaltrexone and naloxegol can be beneficial where stimulant laxatives are insufficient. Additionally, lubiprostone is indicated in OIC in non-cancer patients. At present, there are no head-to-head trials comparing efficacy of these medications.
Defecatory Disorders
Biofeedback therapy is the cornerstone of treatment for dyssynergic defecation, focusing on neuromuscular training to restore a normal pattern of defecation by teaching patients to tense the abdomen and relax the pelvic floor muscles and anal sphincter. It retrains the body to coordinate abdominal, rectal, and anal muscles to achieve synchronous contraction to achieve complete evacuation. It also increases awareness and response to rectal fullness or the need to defecate.
Biofeedback makes patients aware of counterproductive subconscious actions such as contracting of their anal sphincter during defecation followed by simulated defecation training with focus on how to tighten abdominal muscles and relax pelvic floor muscles to initiate and complete defecation.20 This is performed in the office with a physiotherapist or trained nurse for at least six sessions or at home where patients are encouraged to perform the exercises for 20 minutes, twice a day. These sessions utilize tools such as manometry probes, electromyography probes, simulated balloon, or home biofeedback training devices to provide visual feedback while practicing abdominophrenic breathing. Biofeedback is particularly helpful in patients suffering from constipation. Patients with defecatory disorders can also benefit from pelvic floor physical therapy which focuses on strengthening the pelvic and puborectal muscles, external anal sphincter, and pelvic muscles. This is more useful in patients with fecal incontinence. Despite all these treatments, a subset of patients may still not respond and may qualify for surgical evaluation.
Conclusion
While constipation is seldom life-threatening, it has a negative impact on patient quality of life and poses a significant financial burden on our overall healthcare system. The complexity of this condition should be appreciated and understood in order for a complete and thorough evaluation. We trust that our practical guide should serve as a useful tool in the evaluation of a chronically constipated patient.
Dr. Salim (@hamsalim07 on X) is based in the Department of Internal Medicine, University of Texas Medical Branch, Galveston. Dr. Chowdhury (annicho.med on Instagram) is a fellow in the Department of Gastroenterology, Hepatology, and Nutrition, University of Texas MD Anderson Cancer Center, Houston. Dr. Viswanathan (@LavanyaMD on X) is Associate Professor, University of Texas MD Anderson Cancer Center. The authors declare no conflict of interest.
References
1. Mugie S et al. Best Pract Res Clin Gastroenterol. 2011 Feb. doi: 10.1016/j.bpg.2010.12.010.
2. Almario CV et al. Gastroenterology. 2023 Dec. doi: 10.1053/j.gastro.2023.08.010.
3. Canavan C et al. Clin Epidemiol. 2014 Feb. doi: 10.2147/CLEP.S40245.
4. Rao SSC. Gastroenterol Clin North Am. 2007 Sep. doi: 10.1016/j.gtc.2007.07.013.
5. Bharucha AE et al. Gastroenterology. 2020 Apr. doi: 10.1053/j.gastro.2019.12.034.
6. Cinquetti M et al. Clin Exp Pediatr. 2021 Sep. doi: 10.3345/cep.2020.01326.
7. Shah ND et al. Am J Gastroenterol. 2008 Jul. doi: 10.1111/j.1572-0241.2008.01910.x.
8. Rajindrajith S et al. J Pediatr Gastroenterol Nutr. 2014 Apr. doi: 10.1097/MPG.0000000000000249.
9. Talley NJ. Am J Gastroenterol. 2008 Apr. doi: 10.1111/j.1572-0241.2008.01832.x.
10. Maurer AH. J Nucl Med. 2015 Sep. doi: 10.2967/jnumed.113.134551.
11. Frye J et al. Am J Gastroenterol. 2024 Aug. doi: 10.14309/ajg.0000000000002670.
12. Rao SSC et al. J Neurogastroenterol Motil. 2016 Jun. doi: 10.5056/jnm16060.
13. Chang L et al. Gastroenterology. 2023 Jun. doi: 10.1053/j.gastro.2023.03.214.
14. Brenner DM et al. Am J Gastroenterol. 2021 Aug. doi: 10.14309/ajg.0000000000001266.
15. Chang L et al. Gastroenterology. 2022 Jul. doi: 10.1053/j.gastro.2022.04.016.
16. Rao SSC et al. Gastroenterology. 2023 Jun. doi: 10.1053/j.gastro.2023.02.013.
17. Lacy BE et al. Am J Gastroenterol. 2021 Jan. doi: 10.14309/ajg.0000000000001036.
18. Anderson JL et al. J Cardiovasc Pharmacol Ther. 2009 Sep. doi: 10.1177/1074248409340158.
19. Rahimi R et al. World J Gastroenterol. 2009 Apr. doi: 10.3748/wjg.15.1548.
20. Rao SSC. Best Pract Res Clin Gastroenterol. 2011 Feb. doi: 10.1016/j.bpg.2011.01.004.
Introduction
Constipation affects one in six people worldwide and accounts for one third of outpatient visits.1 Chronic constipation is defined by difficult, infrequent, and/or incomplete defecation, quantified by less than three spontaneous bowel movements per week, persisting for at least 3 months. Patients may complain of straining during defecation, incomplete evacuation, hard stools (Bristol stool scale [BSS] type 1-2), and fullness or bloating. Chronic constipation can be subclassified as either a primary or secondary disorder.1,2
Primary Constipation Disorders
Primary constipation includes disorders of the colon or anorectum. This includes irritable bowel syndrome with constipation (IBS-C), chronic idiopathic constipation (CIC), slow transit constipation (STC), dyssynergic defecation, and pelvic floor disorders (see Figure 1).

IBS-C
IBS-C is a chronic disorder of the gut-brain axis with a worldwide prevalence of 1.3% and a prevalence of 6%-16% in the United States, United Kingdom, and Canada, with females more likely to seek care than males.2 The economic impact of IBS-C is estimated to be $1.5 billion–$10 billion per year in the United States alone.3 The distinguishing characteristic is abdominal pain, however IBS-C can present with a constellation of symptoms. The diagnostic paradigm has shifted from IBS being a diagnosis of exclusion to now using a positive diagnostic strategy.2 Using this Rome IV criteria, one can make the diagnosis with > 95% accuracy.2,4
CIC
CIC, previously defined as functional constipation, is a disorder defined by incomplete defecation and difficult or infrequent stool. CIC is diagnosed in patients without an underlying anatomic or structural abnormality. Rome IV Criteria helps further classify the defining characteristics of chronic idiopathic constipation.2
Slow Transit Constipation
STC is characterized by impaired colonic transit time in the absence of pelvic floor dysfunction. It presents with infrequent bowel movements, diminished urgency, and/or straining with defecation.
Defecatory Disorders: Dyssynergic Defecation and Pelvic Floor Dysfunction
Defecatory disorders (DDs) result from alterations in the colonic-neural pathway with an unclear pathogenesis. A firm understanding of colonic physiology is necessary to identify DDs. The right colon helps to store and mix stool contents, the left colon helps add water to the stool, and the anal canal and rectum enable defecation and maintain continence. Any alteration along this physiologic pathway results in DDs.5
DDs primarily develop via maladaptive pelvic floor contraction during defecation or from muscle or nerve injury and include functional outlet obstruction, anorectal dyssynergia, and pelvic floor dysfunction. Increased resistance to defecation results from anismus, paradoxical anal sphincter contraction, or incomplete relaxation of the pelvic floor and external anal sphincter. This muscle incoordination is described as dyssynergia. DDs can involve either muscle or nerve dysfunction or a combination of the two. Reduced rectal sensation caused by reduced sensory triggers can cause stasis of stool, thus propagating the cycle of constipation. Over time, excessive straining can weaken the pelvic floor, increasing the risk of excessive perineal descent, rectal intussusception, solitary rectal ulcer syndrome, and pudendal neuropathy.5 Thus, identification of DDs is crucial in patients with chronic constipation.
Secondary Constipation Disorders
Secondary constipation disorders are a result of an alternate process and warrant a thorough review of outpatient medications and past medical history. Figure 1 outlines the most common causes of secondary constipation, which span a wide differential.
Clinical Evaluation
The evaluation of constipation begins with a thorough history. Description of bowel habits should include frequency, duration, straining, stool consistency using a Bristol stool chart, complete vs incomplete evacuation, pain, bloating, and use of digital maneuvers (vaginal splinting or digital stool removal). One should inquire about back trauma/surgeries and obstetric history to include vaginal forceps injury or episiotomy.
With increased smartphone use, toilet time on average has increased and can contribute to maladaptive bowel habits.6 Patients may not realize they are constipated, so patient education is critical. A patient with daily bowel movements ranging between BSS type 1-6 with incomplete evacuation might complain of diarrhea but may in fact have constipation with overflow diarrhea, for example. Past medical history is also clinically relevant, as systemic conditions can cause secondary constipation. A constipated patient should also be asked what therapies he/she has tried prior to gastroenterology referral as primary care referrals for constipation account for 8 million visits to gastroenterology per year.7
While a sensitive topic, inquire about abuse history, especially in those with childhood constipation symptoms. There is a positive correlation between childhood constipation and physical, emotional, and sexual abuse and, for any number of reasons, your patient may be reluctant to share this or undergo a digital rectal exam (DRE).8 In such cases, be sensitive in asking for this history in private rather than with other family members around and always perform this exam with a chaperone present.
A detailed physical exam is an indispensable tool all gastroenterologists must master when evaluating a constipated patient. Some key exam findings include abdominal distention, high-pitched bowel sounds, and presence of a succussion splash indicating obstructive pathology. Dry skin and brittle hair indicate hypothyroidism while hypermobile joints and skin laxity suggest connective tissue disease. Finally, a physical examination is incomplete without a DRE.
DRE
DRE is an often-overlooked physical exam component which provides helpful insight that can guide management. An informed DRE can help identify structural disorders such as fissure, hemorrhoids, anorectal mass, fecal impaction, rectal prolapse, and excessive perineal descent syndrome.9 Unless contraindicated, DRE should be a standard part of the workup of a patient with chronic constipation.
Workup
Colonoscopy
The role of colonoscopy in chronic constipation is low yield and only indicated if alarm signs are present.2 When no organic causes can be identified, the patient is deemed to have a functional bowel or motility disorder leading to constipation.
Colonic Transit Time
Colonic transit time (CTT) can be evaluated by assessing the presence of radio-opaque sitz markers in the colon with an abdominal x-ray 5 days after ingestion. The presence of five or more sitz markers may indicate STC. However, this can also signal an obstructive defecatory disorder. Colon scintigraphy can determine whether there is diffuse colonic dysmotility or dysfunction in a specific segment of the colon.10
Anorectal Function Testing (AFT)
AFT can evaluate DDs, such as fecal incontinence, dyssynergic defecation, rectal sensory disorders, anorectal pain, and rectal prolapse. AFT comprises three tests: anorectal manometry (ARM), balloon expulsion test (BET), and rectal sensory testing. These assess the defecation, continence, and sensory mechanisms of the rectum, respectively.
ARM testing employs a thin, flexible probe with an attached sensor that is inserted into the rectum to measure internal and external sphincter pressures while at rest, squeezing, and bearing down to give a functional assessment of sphincter tone.11 Cough or party balloon test assesses continence and sphincter strength. Rectal sensation is assessed by inflating a balloon incrementally and asking the patient to indicate first sensation, urgency to defecate, and discomfort. If both ARM and BET are abnormal, the patient meets diagnostic criteria for dyssynergic defecation.12
Pelvic floor disorders can be further assessed by MR defecography or barium defecography. Barium defecography is the more widely available of the two. MR defecography is a dynamic study that directly assesses pelvic floor muscles and endopelvic fascia during various stages of defecation and considered superior. This testing modality can distinguish between functional causes such as dyssynergia or pelvic floor dysfunction and structural causes of obstruction such as rectocele, rectal prolapse, or rectal intussusception. MR defecography is helpful when dyssynergia is suggested by ARM with a normal BET or if there is an absent recto-anal inhibitory reflex on ARM, which may suggest rectal intussusception.
Management
CIC
Incorporating 20-30 g of total soluble fiber, such as psyllium in individuals with low dietary fiber intake is the first-line recommendation for CIC.13 If response to a trial of fiber supplementation is inadequate, over-the-counter (OTC) osmotic laxatives such as polyethylene glycol and magnesium oxide can be incorporated. In the event of failure of OTC osmotic laxatives, lactulose can be considered. Stimulant laxatives such as senna, bisacodyl, or sodium picosulfate can be added as an adjunctive measure for short periods of time, defined as daily for 4 weeks or less.
If these measures are inadequate, pharmacological therapy with secretagogues and 5HT agonists can be considered. Prucalopride, a selective agonist of serotonin 5-HT4 receptors, is approved for CIC, prescribed 2 mg daily.14 It can also be used in patients with global motility delays, such as gastroparetics with constipation. The mechanism of action of secretagogues and specific dosing of these medications are discussed in Figure 2.15 Vibrant is a non-pharmacologic, orally ingested, vibrating, and programmable capsule device that has recently received Food and Drug Administration approval for treatment of chronic constipation by stimulating the intestinal wall, thereby promoting colonic contractile activity to achieve more spontaneous bowel movements. Further studies are required to assess its efficacy.16 Additionally, if there is inadequate response to all the above, it would be prudent to evaluate for the presence of pelvic floor dysfunction as well.

IBS-C
Similar to CIC, treatment for mild IBS-C starts with osmotic laxatives with the additional component of pain control. Antispasmodics can be used to manage the abdominal pain, cramping, and spasms associated with IBS-C. Antispasmodics available in the United States include anticholinergic agents that cause smooth muscle relaxation, such as dicyclomine or hyoscyamine or direct smooth muscle relaxants such as peppermint oil.17 IBS-C patients with moderate symptoms may need escalation of therapy to secretagogues or 5HT agonists (see Figure 2). Secretagogues increase fluid retention in the colonic lumen to promote bowel movements and improve visceral hypersensitivity. Lubiprostone is an intestinal chloride channel activator, indicated only for adult women with IBS-C. Linaclotide and plecanatide are guanylate cyclase-C activators which increase intestinal chloride and bicarbonate secretion, and both are indicated in IBS-C and CIC. Tenapanor inhibits the sodium/hydrogen exchanger in the GI tract, leading to increased water secretion, and is recommended for IBS-C in adults who have failed secretagogues.
All four of these drugs can be considered for moderate to severe IBS-C symptoms. In the case of severe IBS-C symptoms, Tegaserod, a 5-HT4 receptor partial agonist has been approved in women under 65 without significant cardiovascular or cerebrovascular disease.18 Regardless of IBS-C symptom severity, persistent visceral hypersensitivity can be treated with low-dose neuromodulators.19 Figure 2 provides treatment recommendations for IBS-C based on symptom severity.
Opioid-Induced Constipation (OIC)
In patients with OIC, peripherally acting mu-opioid receptor antagonists such as methylnaltrexone and naloxegol can be beneficial where stimulant laxatives are insufficient. Additionally, lubiprostone is indicated in OIC in non-cancer patients. At present, there are no head-to-head trials comparing efficacy of these medications.
Defecatory Disorders
Biofeedback therapy is the cornerstone of treatment for dyssynergic defecation, focusing on neuromuscular training to restore a normal pattern of defecation by teaching patients to tense the abdomen and relax the pelvic floor muscles and anal sphincter. It retrains the body to coordinate abdominal, rectal, and anal muscles to achieve synchronous contraction to achieve complete evacuation. It also increases awareness and response to rectal fullness or the need to defecate.
Biofeedback makes patients aware of counterproductive subconscious actions such as contracting of their anal sphincter during defecation followed by simulated defecation training with focus on how to tighten abdominal muscles and relax pelvic floor muscles to initiate and complete defecation.20 This is performed in the office with a physiotherapist or trained nurse for at least six sessions or at home where patients are encouraged to perform the exercises for 20 minutes, twice a day. These sessions utilize tools such as manometry probes, electromyography probes, simulated balloon, or home biofeedback training devices to provide visual feedback while practicing abdominophrenic breathing. Biofeedback is particularly helpful in patients suffering from constipation. Patients with defecatory disorders can also benefit from pelvic floor physical therapy which focuses on strengthening the pelvic and puborectal muscles, external anal sphincter, and pelvic muscles. This is more useful in patients with fecal incontinence. Despite all these treatments, a subset of patients may still not respond and may qualify for surgical evaluation.
Conclusion
While constipation is seldom life-threatening, it has a negative impact on patient quality of life and poses a significant financial burden on our overall healthcare system. The complexity of this condition should be appreciated and understood in order for a complete and thorough evaluation. We trust that our practical guide should serve as a useful tool in the evaluation of a chronically constipated patient.
Dr. Salim (@hamsalim07 on X) is based in the Department of Internal Medicine, University of Texas Medical Branch, Galveston. Dr. Chowdhury (annicho.med on Instagram) is a fellow in the Department of Gastroenterology, Hepatology, and Nutrition, University of Texas MD Anderson Cancer Center, Houston. Dr. Viswanathan (@LavanyaMD on X) is Associate Professor, University of Texas MD Anderson Cancer Center. The authors declare no conflict of interest.
References
1. Mugie S et al. Best Pract Res Clin Gastroenterol. 2011 Feb. doi: 10.1016/j.bpg.2010.12.010.
2. Almario CV et al. Gastroenterology. 2023 Dec. doi: 10.1053/j.gastro.2023.08.010.
3. Canavan C et al. Clin Epidemiol. 2014 Feb. doi: 10.2147/CLEP.S40245.
4. Rao SSC. Gastroenterol Clin North Am. 2007 Sep. doi: 10.1016/j.gtc.2007.07.013.
5. Bharucha AE et al. Gastroenterology. 2020 Apr. doi: 10.1053/j.gastro.2019.12.034.
6. Cinquetti M et al. Clin Exp Pediatr. 2021 Sep. doi: 10.3345/cep.2020.01326.
7. Shah ND et al. Am J Gastroenterol. 2008 Jul. doi: 10.1111/j.1572-0241.2008.01910.x.
8. Rajindrajith S et al. J Pediatr Gastroenterol Nutr. 2014 Apr. doi: 10.1097/MPG.0000000000000249.
9. Talley NJ. Am J Gastroenterol. 2008 Apr. doi: 10.1111/j.1572-0241.2008.01832.x.
10. Maurer AH. J Nucl Med. 2015 Sep. doi: 10.2967/jnumed.113.134551.
11. Frye J et al. Am J Gastroenterol. 2024 Aug. doi: 10.14309/ajg.0000000000002670.
12. Rao SSC et al. J Neurogastroenterol Motil. 2016 Jun. doi: 10.5056/jnm16060.
13. Chang L et al. Gastroenterology. 2023 Jun. doi: 10.1053/j.gastro.2023.03.214.
14. Brenner DM et al. Am J Gastroenterol. 2021 Aug. doi: 10.14309/ajg.0000000000001266.
15. Chang L et al. Gastroenterology. 2022 Jul. doi: 10.1053/j.gastro.2022.04.016.
16. Rao SSC et al. Gastroenterology. 2023 Jun. doi: 10.1053/j.gastro.2023.02.013.
17. Lacy BE et al. Am J Gastroenterol. 2021 Jan. doi: 10.14309/ajg.0000000000001036.
18. Anderson JL et al. J Cardiovasc Pharmacol Ther. 2009 Sep. doi: 10.1177/1074248409340158.
19. Rahimi R et al. World J Gastroenterol. 2009 Apr. doi: 10.3748/wjg.15.1548.
20. Rao SSC. Best Pract Res Clin Gastroenterol. 2011 Feb. doi: 10.1016/j.bpg.2011.01.004.
Random Biopsy Improves IBD Dysplasia Detection, With Caveats
according to a recent review and meta-analysis.
Random biopsies collected in studies after 2011 provided limited additional yield, suggesting that high-definition equipment alone may be sufficient to achieve a high detection rate, lead author Li Gao, MD, of Air Force Medical University, Xi’an, China, and colleagues reported. In contrast, patients with primary sclerosing cholangitis (PSC) consistently benefited from random biopsy, offering clearer support for use in this subgroup.
“Random biopsy has been proposed as a strategy that may detect dysplastic lesions that cannot be identified endoscopically, thus minimizing the occurrence of missed colitis-associated dysplasia during colonoscopy,” the investigators wrote in Clinical Gastroenterology and Hepatology. However, the role of random biopsies in colonoscopic surveillance for patients with IBD remains a topic of ongoing debate.”
The SCENIC guidelines remain inconclusive on the role of random biopsy in IBD surveillance, the investigators noted, while other guidelines recommend random biopsy with high-definition white light endoscopy, but not chromoendoscopy.
The present meta-analysis aimed to characterize the impact of random biopsy on dysplasia detection. The investigators aggregated prospective and retrospective studies published in English through September 2023, all of which compared random biopsy with other surveillance techniques and reported the proportion of dysplasia detected exclusively through random biopsy.
“To the best of our knowledge, this systematic review and meta-analysis was the first comprehensive summary of the additional yield of random biopsy during colorectal cancer surveillance in patients with IBD,” Dr. Gao and colleagues noted.
The final dataset comprised 37 studies with 9,051 patients undergoing colorectal cancer surveillance for IBD. Patients had diverse baseline characteristics, including different proportions of ulcerative colitis and Crohn’s disease, as well as varying prevalence of PSC, a known risk factor for colorectal neoplasia.
The pooled additional yield of random biopsy was 10.34% in per-patient analysis and 16.20% in per-lesion analysis, meaning that approximately 1 in 10 patients and 1 in 6 lesions were detected exclusively through random biopsy. Despite these benefits, detection rates were relatively low: 1.31% per patient and 2.82% per lesion.
Subgroup analyses showed a decline in random biopsy additional yield over time. Studies conducted before 2011 reported an additional yield of 14.43% in per-patient analysis, compared to just 0.42% in studies conducted after 2011. This decline coincided with the widespread adoption of high-definition endoscopy.
PSC status strongly influenced detection rates throughout the study period. In patients without PSC (0%-10% PSC prevalence), the additional yield of random biopsy was 4.83% in per-patient analysis and 11.23% in per-lesion analysis. In studies where all patients had PSC, the additional yield increased dramatically to 56.05% and 45.22%, respectively.
“These findings highlight the incremental benefits of random biopsy and provide valuable insights into the management of endoscopic surveillance in patients with IBD,” the investigators wrote. “Considering the decreased additional yields in studies initiated after 2011, and the influence of PSC, endoscopy centers lacking full high-definition equipment should consider incorporating random biopsy in the standard colonoscopy surveillance for IBD patients, especially in those with PSC.”This study was supported by the National Key R&D Program of China, the Key Research and Development Program of Shaanxi Province, and the Nanchang High-Level Scientific and Technological Innovation Talents “Double Hundred Plan” project. The investigators disclosed no conflicts of interest.
Patients with inflammatory bowel diseases (IBD) with colonic involvement are at two- to threefold increased risk of colorectal cancer (CRC), compared with the general population. The development and progression of dysplasia in these patients with IBD does not follow the typical adenoma-carcinoma sequence; rather, patients with IBD at increased risk of colorectal cancer may have field cancerization changes. Historically, these mucosal changes have been difficult to visualize endoscopically, at least with standard definition endoscopes. As a result, systematic, four-quadrant, random biopsies — 8 in each segment of the colon, totaling to 32 biopsies — are recommended for dysplasia detection. The practice has been adopted and accepted widely. Over time, there have been significant advancements in the management of IBD, with improved colonoscopic resolution, adjunct surveillance techniques, focus on quality of colonoscopic exams and evolution of treatments and treatment targets, and these have resulted in a reduction in the risk of CRC in patients with IBD. The value of random biopsies for dysplasia surveillance in patients with colonic IBD has been questioned.
In this context, the systematic review and meta-analysis from Gao and colleagues provides critical insights into the yield of random biopsies for dysplasia surveillance in patients with IBD. Through a detailed analysis of 37 studies published between 2003 to 2023, with 9051 patients who underwent dysplasia surveillance with random biopsies, they ascertained the incremental yield of random biopsies. Overall, 1.3% of patients who underwent random biopsies were detected to have dysplasia. Of these, 1 in 10 patients were detected to have dysplasia only on random biopsies. On per-lesion analysis, one in six dysplastic lesions were only detected on random biopsies. Interestingly, this yield of random biopsies varied markedly depending on the era, as a surrogate for quality of colonoscopies. In studies that fully enrolled and published before 2011 (majority of patients recruited in the 1990s to early 2000s), the per-patient incremental yield of random biopsies was 14%; this dropped precipitously to 0.4% in studies published after 2011 (majority of patients recruited in late 2000s to 2010s). The incremental yield of random biopsies remained markedly high in studies with a high proportion of patients with primary sclerosing cholangitis (PSC), a condition consistently associated with a four- to sixfold higher risk of CRC in patients with IBD.
These findings lend support to the notion that improvements in endoscopy equipment with wide adoption of high-definition white-light colonoscopes and an emphasis on quality of endoscopic examination may be leading to better endoscopic detection of previously “invisible” dysplastic lesions, leading to a markedly lower incremental yield of random biopsies in the current era. This questions the utility of routinely collecting 32 random biopsies during a surveillance exam for a patient with IBD at increased risk of CRC (as long as a thorough high-quality exam is being performed), though there may be subpopulations such as patients with PSC where there may be benefit. Large ongoing trials comparing the yield of targeted biopsies vs random and targeted biopsies in patients with IBD undergoing dysplasia surveillance with high-definition colonoscopes will help to definitively address this question.
Siddharth Singh, MD, MS, is associate professor of medicine and director of the UCSD IBD Center in the division of gastroenterology, University of California, San Diego. He declares no conflicts of interest relative to this article.
Patients with inflammatory bowel diseases (IBD) with colonic involvement are at two- to threefold increased risk of colorectal cancer (CRC), compared with the general population. The development and progression of dysplasia in these patients with IBD does not follow the typical adenoma-carcinoma sequence; rather, patients with IBD at increased risk of colorectal cancer may have field cancerization changes. Historically, these mucosal changes have been difficult to visualize endoscopically, at least with standard definition endoscopes. As a result, systematic, four-quadrant, random biopsies — 8 in each segment of the colon, totaling to 32 biopsies — are recommended for dysplasia detection. The practice has been adopted and accepted widely. Over time, there have been significant advancements in the management of IBD, with improved colonoscopic resolution, adjunct surveillance techniques, focus on quality of colonoscopic exams and evolution of treatments and treatment targets, and these have resulted in a reduction in the risk of CRC in patients with IBD. The value of random biopsies for dysplasia surveillance in patients with colonic IBD has been questioned.
In this context, the systematic review and meta-analysis from Gao and colleagues provides critical insights into the yield of random biopsies for dysplasia surveillance in patients with IBD. Through a detailed analysis of 37 studies published between 2003 to 2023, with 9051 patients who underwent dysplasia surveillance with random biopsies, they ascertained the incremental yield of random biopsies. Overall, 1.3% of patients who underwent random biopsies were detected to have dysplasia. Of these, 1 in 10 patients were detected to have dysplasia only on random biopsies. On per-lesion analysis, one in six dysplastic lesions were only detected on random biopsies. Interestingly, this yield of random biopsies varied markedly depending on the era, as a surrogate for quality of colonoscopies. In studies that fully enrolled and published before 2011 (majority of patients recruited in the 1990s to early 2000s), the per-patient incremental yield of random biopsies was 14%; this dropped precipitously to 0.4% in studies published after 2011 (majority of patients recruited in late 2000s to 2010s). The incremental yield of random biopsies remained markedly high in studies with a high proportion of patients with primary sclerosing cholangitis (PSC), a condition consistently associated with a four- to sixfold higher risk of CRC in patients with IBD.
These findings lend support to the notion that improvements in endoscopy equipment with wide adoption of high-definition white-light colonoscopes and an emphasis on quality of endoscopic examination may be leading to better endoscopic detection of previously “invisible” dysplastic lesions, leading to a markedly lower incremental yield of random biopsies in the current era. This questions the utility of routinely collecting 32 random biopsies during a surveillance exam for a patient with IBD at increased risk of CRC (as long as a thorough high-quality exam is being performed), though there may be subpopulations such as patients with PSC where there may be benefit. Large ongoing trials comparing the yield of targeted biopsies vs random and targeted biopsies in patients with IBD undergoing dysplasia surveillance with high-definition colonoscopes will help to definitively address this question.
Siddharth Singh, MD, MS, is associate professor of medicine and director of the UCSD IBD Center in the division of gastroenterology, University of California, San Diego. He declares no conflicts of interest relative to this article.
Patients with inflammatory bowel diseases (IBD) with colonic involvement are at two- to threefold increased risk of colorectal cancer (CRC), compared with the general population. The development and progression of dysplasia in these patients with IBD does not follow the typical adenoma-carcinoma sequence; rather, patients with IBD at increased risk of colorectal cancer may have field cancerization changes. Historically, these mucosal changes have been difficult to visualize endoscopically, at least with standard definition endoscopes. As a result, systematic, four-quadrant, random biopsies — 8 in each segment of the colon, totaling to 32 biopsies — are recommended for dysplasia detection. The practice has been adopted and accepted widely. Over time, there have been significant advancements in the management of IBD, with improved colonoscopic resolution, adjunct surveillance techniques, focus on quality of colonoscopic exams and evolution of treatments and treatment targets, and these have resulted in a reduction in the risk of CRC in patients with IBD. The value of random biopsies for dysplasia surveillance in patients with colonic IBD has been questioned.
In this context, the systematic review and meta-analysis from Gao and colleagues provides critical insights into the yield of random biopsies for dysplasia surveillance in patients with IBD. Through a detailed analysis of 37 studies published between 2003 to 2023, with 9051 patients who underwent dysplasia surveillance with random biopsies, they ascertained the incremental yield of random biopsies. Overall, 1.3% of patients who underwent random biopsies were detected to have dysplasia. Of these, 1 in 10 patients were detected to have dysplasia only on random biopsies. On per-lesion analysis, one in six dysplastic lesions were only detected on random biopsies. Interestingly, this yield of random biopsies varied markedly depending on the era, as a surrogate for quality of colonoscopies. In studies that fully enrolled and published before 2011 (majority of patients recruited in the 1990s to early 2000s), the per-patient incremental yield of random biopsies was 14%; this dropped precipitously to 0.4% in studies published after 2011 (majority of patients recruited in late 2000s to 2010s). The incremental yield of random biopsies remained markedly high in studies with a high proportion of patients with primary sclerosing cholangitis (PSC), a condition consistently associated with a four- to sixfold higher risk of CRC in patients with IBD.
These findings lend support to the notion that improvements in endoscopy equipment with wide adoption of high-definition white-light colonoscopes and an emphasis on quality of endoscopic examination may be leading to better endoscopic detection of previously “invisible” dysplastic lesions, leading to a markedly lower incremental yield of random biopsies in the current era. This questions the utility of routinely collecting 32 random biopsies during a surveillance exam for a patient with IBD at increased risk of CRC (as long as a thorough high-quality exam is being performed), though there may be subpopulations such as patients with PSC where there may be benefit. Large ongoing trials comparing the yield of targeted biopsies vs random and targeted biopsies in patients with IBD undergoing dysplasia surveillance with high-definition colonoscopes will help to definitively address this question.
Siddharth Singh, MD, MS, is associate professor of medicine and director of the UCSD IBD Center in the division of gastroenterology, University of California, San Diego. He declares no conflicts of interest relative to this article.
according to a recent review and meta-analysis.
Random biopsies collected in studies after 2011 provided limited additional yield, suggesting that high-definition equipment alone may be sufficient to achieve a high detection rate, lead author Li Gao, MD, of Air Force Medical University, Xi’an, China, and colleagues reported. In contrast, patients with primary sclerosing cholangitis (PSC) consistently benefited from random biopsy, offering clearer support for use in this subgroup.
“Random biopsy has been proposed as a strategy that may detect dysplastic lesions that cannot be identified endoscopically, thus minimizing the occurrence of missed colitis-associated dysplasia during colonoscopy,” the investigators wrote in Clinical Gastroenterology and Hepatology. However, the role of random biopsies in colonoscopic surveillance for patients with IBD remains a topic of ongoing debate.”
The SCENIC guidelines remain inconclusive on the role of random biopsy in IBD surveillance, the investigators noted, while other guidelines recommend random biopsy with high-definition white light endoscopy, but not chromoendoscopy.
The present meta-analysis aimed to characterize the impact of random biopsy on dysplasia detection. The investigators aggregated prospective and retrospective studies published in English through September 2023, all of which compared random biopsy with other surveillance techniques and reported the proportion of dysplasia detected exclusively through random biopsy.
“To the best of our knowledge, this systematic review and meta-analysis was the first comprehensive summary of the additional yield of random biopsy during colorectal cancer surveillance in patients with IBD,” Dr. Gao and colleagues noted.
The final dataset comprised 37 studies with 9,051 patients undergoing colorectal cancer surveillance for IBD. Patients had diverse baseline characteristics, including different proportions of ulcerative colitis and Crohn’s disease, as well as varying prevalence of PSC, a known risk factor for colorectal neoplasia.
The pooled additional yield of random biopsy was 10.34% in per-patient analysis and 16.20% in per-lesion analysis, meaning that approximately 1 in 10 patients and 1 in 6 lesions were detected exclusively through random biopsy. Despite these benefits, detection rates were relatively low: 1.31% per patient and 2.82% per lesion.
Subgroup analyses showed a decline in random biopsy additional yield over time. Studies conducted before 2011 reported an additional yield of 14.43% in per-patient analysis, compared to just 0.42% in studies conducted after 2011. This decline coincided with the widespread adoption of high-definition endoscopy.
PSC status strongly influenced detection rates throughout the study period. In patients without PSC (0%-10% PSC prevalence), the additional yield of random biopsy was 4.83% in per-patient analysis and 11.23% in per-lesion analysis. In studies where all patients had PSC, the additional yield increased dramatically to 56.05% and 45.22%, respectively.
“These findings highlight the incremental benefits of random biopsy and provide valuable insights into the management of endoscopic surveillance in patients with IBD,” the investigators wrote. “Considering the decreased additional yields in studies initiated after 2011, and the influence of PSC, endoscopy centers lacking full high-definition equipment should consider incorporating random biopsy in the standard colonoscopy surveillance for IBD patients, especially in those with PSC.”This study was supported by the National Key R&D Program of China, the Key Research and Development Program of Shaanxi Province, and the Nanchang High-Level Scientific and Technological Innovation Talents “Double Hundred Plan” project. The investigators disclosed no conflicts of interest.
according to a recent review and meta-analysis.
Random biopsies collected in studies after 2011 provided limited additional yield, suggesting that high-definition equipment alone may be sufficient to achieve a high detection rate, lead author Li Gao, MD, of Air Force Medical University, Xi’an, China, and colleagues reported. In contrast, patients with primary sclerosing cholangitis (PSC) consistently benefited from random biopsy, offering clearer support for use in this subgroup.
“Random biopsy has been proposed as a strategy that may detect dysplastic lesions that cannot be identified endoscopically, thus minimizing the occurrence of missed colitis-associated dysplasia during colonoscopy,” the investigators wrote in Clinical Gastroenterology and Hepatology. However, the role of random biopsies in colonoscopic surveillance for patients with IBD remains a topic of ongoing debate.”
The SCENIC guidelines remain inconclusive on the role of random biopsy in IBD surveillance, the investigators noted, while other guidelines recommend random biopsy with high-definition white light endoscopy, but not chromoendoscopy.
The present meta-analysis aimed to characterize the impact of random biopsy on dysplasia detection. The investigators aggregated prospective and retrospective studies published in English through September 2023, all of which compared random biopsy with other surveillance techniques and reported the proportion of dysplasia detected exclusively through random biopsy.
“To the best of our knowledge, this systematic review and meta-analysis was the first comprehensive summary of the additional yield of random biopsy during colorectal cancer surveillance in patients with IBD,” Dr. Gao and colleagues noted.
The final dataset comprised 37 studies with 9,051 patients undergoing colorectal cancer surveillance for IBD. Patients had diverse baseline characteristics, including different proportions of ulcerative colitis and Crohn’s disease, as well as varying prevalence of PSC, a known risk factor for colorectal neoplasia.
The pooled additional yield of random biopsy was 10.34% in per-patient analysis and 16.20% in per-lesion analysis, meaning that approximately 1 in 10 patients and 1 in 6 lesions were detected exclusively through random biopsy. Despite these benefits, detection rates were relatively low: 1.31% per patient and 2.82% per lesion.
Subgroup analyses showed a decline in random biopsy additional yield over time. Studies conducted before 2011 reported an additional yield of 14.43% in per-patient analysis, compared to just 0.42% in studies conducted after 2011. This decline coincided with the widespread adoption of high-definition endoscopy.
PSC status strongly influenced detection rates throughout the study period. In patients without PSC (0%-10% PSC prevalence), the additional yield of random biopsy was 4.83% in per-patient analysis and 11.23% in per-lesion analysis. In studies where all patients had PSC, the additional yield increased dramatically to 56.05% and 45.22%, respectively.
“These findings highlight the incremental benefits of random biopsy and provide valuable insights into the management of endoscopic surveillance in patients with IBD,” the investigators wrote. “Considering the decreased additional yields in studies initiated after 2011, and the influence of PSC, endoscopy centers lacking full high-definition equipment should consider incorporating random biopsy in the standard colonoscopy surveillance for IBD patients, especially in those with PSC.”This study was supported by the National Key R&D Program of China, the Key Research and Development Program of Shaanxi Province, and the Nanchang High-Level Scientific and Technological Innovation Talents “Double Hundred Plan” project. The investigators disclosed no conflicts of interest.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Women Researchers Remain Underrepresented in Pharma-Sponsored IBD Presentations
A recent study found that The study was published in Gastroenterology and also appeared concurrently in Clinical Gastroenterology and Hepatology .
Indeed, among gastrointestinal (GI) subspecialties, IBD was selected by 26.5% of all women GI physicians, compared with 18.9% of all their male counterparts, according to a 2021 study.
Thus, conference organizers and pharmaceutical companies should promote speaker diversity by seeking out women presenters, according to a group led by Maria A. Quintero, MD, of the Division of Gastroenterology at the Leonard Miller School of Medicine at the University of Miami, Florida.
“Seeing more women IBD leaders at the podium will inspire other women to engage in IBD clinical research,” Quintero and associates wrote.
In addition, women investigators should be included at every stage of the study process in industry-sponsored research, both as principal investigators and members of steering committees involved in study design, the authors said. Training more women clinical trial investigators in the IBD setting is another way forward.
In another recommendation, pharmaceutical companies need to be more transparent about the way first and senior authors on IBD studies are chosen because in the past the principal investigator who enrolled the most patients became the first author of the study. “That is no longer the case. However, it remains unclear whether all investigators have an equal opportunity to be the first or senior author,” Quintero and associates wrote.
The Study
The investigators analyzed IBD abstracts of presentations at five conferences for two large GI meetings, Digestive Disease Week (DDW) and United European Gastroenterology (UEG) in the period 2021-2023.
They asked whether women investigators were as likely as their male counterparts to present abstracts based on results from industry-supported clinical trials. As a point of comparison, they also looked for possible gender differences in invited-speaker vs investigator-initiated IBD sessions. To do this, they examined all IBD-related abstracts from the two meetings, identified the lead author of each oral presentation, and divided them into women or men. They also assessed whether the presentation was pharma-sponsored, investigator-initiated, or presented by an invited speaker.
Among the study findings:
- Across categories there were 178 invited lectures, 336 investigator-submitted presentations, and 150 industry-supported presentations for UEG (2021, 2022, and 2023) and DDW (2022 and 2023).
- The gender gap for men vs women was significant for industry-supported oral presentations (78.7% vs 21.3%; P < .0001) and for invited lectures (67.4% vs 32.6%; P < .0001) — but not for investigator-submitted abstracts (49.7% vs 50.3%; P = .91).
- The gender gap for industry-supported abstracts, however, was significantly larger than for investigator-submitted abstracts (57.3% vs 0.6%; P < .0001) and larger than for invited lectures (57.3% vs 34.9%; P = .09).
- The gender gap for invited lectures was significantly larger than for investigator-submitted oral presentations (34.9% vs 0.6%; P = .0009).
Why the Discordance?
This disparity may be due to the paucity of women investigators on steering committees for clinical trials. “Although the number of women doing IBD research continues to increase, then number of women senior investigators is still smaller than the number of men senior investigators,” the researchers wrote. “Ideally, there would be transparency in terms of the metrics used by pharma to choose who will be a presenting author and more intentional recruitment of women investigators to steering committees.”
Commenting on the study but not involved in it, internist Shannon M. Ruzycki, MD, MPH, an assistant professor in the Cumming School of Medicine at the University of Calgary Medical Centre in Alberta, Canada, said the findings are not surprising. “In nearly every setting where gender differences are studied in academic medicine, women are found to be disadvantaged compared to men. These differences are not attributable to skill, merit, or career attainment, but rather appear to be arbitrary and due to biases. They add up across time and likely contribute to the larger differences we see between men and women in promotion, compensation, and awards.”
Ruzycki, lead author of a study of women presenters at medical conferences, noted that differences in gender representation in academia, academic medicine, and clinical trials are similar “because the underlying causes are similar.” On the positive side, she added, conference planning committees are using strategies to reduce bias in how presenters are selected by masking the names and/or institutions of those are submitting abstracts and are being more intentional in inviting a diverse panel of qualified speakers.
“However, one strategy alone is unlikely to address such an insidious problem that affects all parts of selection,” she said. “For example, if pharmaceutical companies believe that men presenters are seen as more authoritative or knowledgeable than women presenters, they will select men to be the first author on submitted abstracts which could deprive these opportunities for deserving women candidates.”
Ruzycki attributed the imbalance to systems (academia, medicine, science) designed by men who lack empathy for the experiences of women. “In the same way you can never really understand how exhausting it is to be a parent until you become a parent or how challenging it can be to have a physical disability until you break a leg and have to navigate the world on crutches, it is really challenging for men to understand how cold and hostile these settings can be for women.”
Many of the things that make conferences, academia, and medicine so challenging for women have straightforward solutions, however, Ruzycki added. Onsite childcare, scrubs that fit women, operating room equipment that is ergonomic for women surgeons — even more washroom stalls would help. “If only we listened and cared about things that didn’t directly impact us.”
This study was supported by the 2023 Travel Grant from the International Organization for the Study of Inflammatory Bowel Diseases. One coauthor serves as a consultant or on advisory boards for AbbVie, Amgen, Bristol Myers Squibb, Celsius Therapeutics, Eli Lilly, Gilead Sciences, Janssen Pharmaceuticals, and Pfizer Pharmaceutical. She is a teacher, lecturer, and speaker for Janssen and Takeda Pharmaceuticals. The remaining authors disclosed no conflicts. Ruzycki had no relevant conflicts of interest to declare.
A version of this article appeared on Medscape.com.
A recent study found that The study was published in Gastroenterology and also appeared concurrently in Clinical Gastroenterology and Hepatology .
Indeed, among gastrointestinal (GI) subspecialties, IBD was selected by 26.5% of all women GI physicians, compared with 18.9% of all their male counterparts, according to a 2021 study.
Thus, conference organizers and pharmaceutical companies should promote speaker diversity by seeking out women presenters, according to a group led by Maria A. Quintero, MD, of the Division of Gastroenterology at the Leonard Miller School of Medicine at the University of Miami, Florida.
“Seeing more women IBD leaders at the podium will inspire other women to engage in IBD clinical research,” Quintero and associates wrote.
In addition, women investigators should be included at every stage of the study process in industry-sponsored research, both as principal investigators and members of steering committees involved in study design, the authors said. Training more women clinical trial investigators in the IBD setting is another way forward.
In another recommendation, pharmaceutical companies need to be more transparent about the way first and senior authors on IBD studies are chosen because in the past the principal investigator who enrolled the most patients became the first author of the study. “That is no longer the case. However, it remains unclear whether all investigators have an equal opportunity to be the first or senior author,” Quintero and associates wrote.
The Study
The investigators analyzed IBD abstracts of presentations at five conferences for two large GI meetings, Digestive Disease Week (DDW) and United European Gastroenterology (UEG) in the period 2021-2023.
They asked whether women investigators were as likely as their male counterparts to present abstracts based on results from industry-supported clinical trials. As a point of comparison, they also looked for possible gender differences in invited-speaker vs investigator-initiated IBD sessions. To do this, they examined all IBD-related abstracts from the two meetings, identified the lead author of each oral presentation, and divided them into women or men. They also assessed whether the presentation was pharma-sponsored, investigator-initiated, or presented by an invited speaker.
Among the study findings:
- Across categories there were 178 invited lectures, 336 investigator-submitted presentations, and 150 industry-supported presentations for UEG (2021, 2022, and 2023) and DDW (2022 and 2023).
- The gender gap for men vs women was significant for industry-supported oral presentations (78.7% vs 21.3%; P < .0001) and for invited lectures (67.4% vs 32.6%; P < .0001) — but not for investigator-submitted abstracts (49.7% vs 50.3%; P = .91).
- The gender gap for industry-supported abstracts, however, was significantly larger than for investigator-submitted abstracts (57.3% vs 0.6%; P < .0001) and larger than for invited lectures (57.3% vs 34.9%; P = .09).
- The gender gap for invited lectures was significantly larger than for investigator-submitted oral presentations (34.9% vs 0.6%; P = .0009).
Why the Discordance?
This disparity may be due to the paucity of women investigators on steering committees for clinical trials. “Although the number of women doing IBD research continues to increase, then number of women senior investigators is still smaller than the number of men senior investigators,” the researchers wrote. “Ideally, there would be transparency in terms of the metrics used by pharma to choose who will be a presenting author and more intentional recruitment of women investigators to steering committees.”
Commenting on the study but not involved in it, internist Shannon M. Ruzycki, MD, MPH, an assistant professor in the Cumming School of Medicine at the University of Calgary Medical Centre in Alberta, Canada, said the findings are not surprising. “In nearly every setting where gender differences are studied in academic medicine, women are found to be disadvantaged compared to men. These differences are not attributable to skill, merit, or career attainment, but rather appear to be arbitrary and due to biases. They add up across time and likely contribute to the larger differences we see between men and women in promotion, compensation, and awards.”
Ruzycki, lead author of a study of women presenters at medical conferences, noted that differences in gender representation in academia, academic medicine, and clinical trials are similar “because the underlying causes are similar.” On the positive side, she added, conference planning committees are using strategies to reduce bias in how presenters are selected by masking the names and/or institutions of those are submitting abstracts and are being more intentional in inviting a diverse panel of qualified speakers.
“However, one strategy alone is unlikely to address such an insidious problem that affects all parts of selection,” she said. “For example, if pharmaceutical companies believe that men presenters are seen as more authoritative or knowledgeable than women presenters, they will select men to be the first author on submitted abstracts which could deprive these opportunities for deserving women candidates.”
Ruzycki attributed the imbalance to systems (academia, medicine, science) designed by men who lack empathy for the experiences of women. “In the same way you can never really understand how exhausting it is to be a parent until you become a parent or how challenging it can be to have a physical disability until you break a leg and have to navigate the world on crutches, it is really challenging for men to understand how cold and hostile these settings can be for women.”
Many of the things that make conferences, academia, and medicine so challenging for women have straightforward solutions, however, Ruzycki added. Onsite childcare, scrubs that fit women, operating room equipment that is ergonomic for women surgeons — even more washroom stalls would help. “If only we listened and cared about things that didn’t directly impact us.”
This study was supported by the 2023 Travel Grant from the International Organization for the Study of Inflammatory Bowel Diseases. One coauthor serves as a consultant or on advisory boards for AbbVie, Amgen, Bristol Myers Squibb, Celsius Therapeutics, Eli Lilly, Gilead Sciences, Janssen Pharmaceuticals, and Pfizer Pharmaceutical. She is a teacher, lecturer, and speaker for Janssen and Takeda Pharmaceuticals. The remaining authors disclosed no conflicts. Ruzycki had no relevant conflicts of interest to declare.
A version of this article appeared on Medscape.com.
A recent study found that The study was published in Gastroenterology and also appeared concurrently in Clinical Gastroenterology and Hepatology .
Indeed, among gastrointestinal (GI) subspecialties, IBD was selected by 26.5% of all women GI physicians, compared with 18.9% of all their male counterparts, according to a 2021 study.
Thus, conference organizers and pharmaceutical companies should promote speaker diversity by seeking out women presenters, according to a group led by Maria A. Quintero, MD, of the Division of Gastroenterology at the Leonard Miller School of Medicine at the University of Miami, Florida.
“Seeing more women IBD leaders at the podium will inspire other women to engage in IBD clinical research,” Quintero and associates wrote.
In addition, women investigators should be included at every stage of the study process in industry-sponsored research, both as principal investigators and members of steering committees involved in study design, the authors said. Training more women clinical trial investigators in the IBD setting is another way forward.
In another recommendation, pharmaceutical companies need to be more transparent about the way first and senior authors on IBD studies are chosen because in the past the principal investigator who enrolled the most patients became the first author of the study. “That is no longer the case. However, it remains unclear whether all investigators have an equal opportunity to be the first or senior author,” Quintero and associates wrote.
The Study
The investigators analyzed IBD abstracts of presentations at five conferences for two large GI meetings, Digestive Disease Week (DDW) and United European Gastroenterology (UEG) in the period 2021-2023.
They asked whether women investigators were as likely as their male counterparts to present abstracts based on results from industry-supported clinical trials. As a point of comparison, they also looked for possible gender differences in invited-speaker vs investigator-initiated IBD sessions. To do this, they examined all IBD-related abstracts from the two meetings, identified the lead author of each oral presentation, and divided them into women or men. They also assessed whether the presentation was pharma-sponsored, investigator-initiated, or presented by an invited speaker.
Among the study findings:
- Across categories there were 178 invited lectures, 336 investigator-submitted presentations, and 150 industry-supported presentations for UEG (2021, 2022, and 2023) and DDW (2022 and 2023).
- The gender gap for men vs women was significant for industry-supported oral presentations (78.7% vs 21.3%; P < .0001) and for invited lectures (67.4% vs 32.6%; P < .0001) — but not for investigator-submitted abstracts (49.7% vs 50.3%; P = .91).
- The gender gap for industry-supported abstracts, however, was significantly larger than for investigator-submitted abstracts (57.3% vs 0.6%; P < .0001) and larger than for invited lectures (57.3% vs 34.9%; P = .09).
- The gender gap for invited lectures was significantly larger than for investigator-submitted oral presentations (34.9% vs 0.6%; P = .0009).
Why the Discordance?
This disparity may be due to the paucity of women investigators on steering committees for clinical trials. “Although the number of women doing IBD research continues to increase, then number of women senior investigators is still smaller than the number of men senior investigators,” the researchers wrote. “Ideally, there would be transparency in terms of the metrics used by pharma to choose who will be a presenting author and more intentional recruitment of women investigators to steering committees.”
Commenting on the study but not involved in it, internist Shannon M. Ruzycki, MD, MPH, an assistant professor in the Cumming School of Medicine at the University of Calgary Medical Centre in Alberta, Canada, said the findings are not surprising. “In nearly every setting where gender differences are studied in academic medicine, women are found to be disadvantaged compared to men. These differences are not attributable to skill, merit, or career attainment, but rather appear to be arbitrary and due to biases. They add up across time and likely contribute to the larger differences we see between men and women in promotion, compensation, and awards.”
Ruzycki, lead author of a study of women presenters at medical conferences, noted that differences in gender representation in academia, academic medicine, and clinical trials are similar “because the underlying causes are similar.” On the positive side, she added, conference planning committees are using strategies to reduce bias in how presenters are selected by masking the names and/or institutions of those are submitting abstracts and are being more intentional in inviting a diverse panel of qualified speakers.
“However, one strategy alone is unlikely to address such an insidious problem that affects all parts of selection,” she said. “For example, if pharmaceutical companies believe that men presenters are seen as more authoritative or knowledgeable than women presenters, they will select men to be the first author on submitted abstracts which could deprive these opportunities for deserving women candidates.”
Ruzycki attributed the imbalance to systems (academia, medicine, science) designed by men who lack empathy for the experiences of women. “In the same way you can never really understand how exhausting it is to be a parent until you become a parent or how challenging it can be to have a physical disability until you break a leg and have to navigate the world on crutches, it is really challenging for men to understand how cold and hostile these settings can be for women.”
Many of the things that make conferences, academia, and medicine so challenging for women have straightforward solutions, however, Ruzycki added. Onsite childcare, scrubs that fit women, operating room equipment that is ergonomic for women surgeons — even more washroom stalls would help. “If only we listened and cared about things that didn’t directly impact us.”
This study was supported by the 2023 Travel Grant from the International Organization for the Study of Inflammatory Bowel Diseases. One coauthor serves as a consultant or on advisory boards for AbbVie, Amgen, Bristol Myers Squibb, Celsius Therapeutics, Eli Lilly, Gilead Sciences, Janssen Pharmaceuticals, and Pfizer Pharmaceutical. She is a teacher, lecturer, and speaker for Janssen and Takeda Pharmaceuticals. The remaining authors disclosed no conflicts. Ruzycki had no relevant conflicts of interest to declare.
A version of this article appeared on Medscape.com.
FROM GASTROENTEROLOGY
Quality, Not Type, of Diet Linked to Microbiome Health
new research suggested.
For example, red meat was a strong driver of omnivore microbiomes, with corresponding signature microbes that are negatively correlated with host cardiometabolic health.
In contrast, the signature microbes found in vegans’ gut microbiomes were correlated with favorable cardiometabolic markers and were enriched in omnivores who ate more plant-based foods.
“From the viewpoint of the impact of diet on the gut microbiome, what seems to be more important is the diversity of healthy plant-based foods that are consumed,” principal author Nicola Segata, PhD, University of Trento in Italy, said in an interview. “Whether this comes within a vegan or an omnivore diet is less crucial, as long as there is no specific overconsumption of unhealthy food categories, such as red meat.”
Excluding broad categories of foods also can have consequences, he added. “For example, we saw that the exclusion of dairy fermented foods is associated with decreased presence of potentially probiotic microbes that are constitutive of such foods. Avoiding meat or dairy products does not necessarily have a positive effect if it does not come with a variety of quality plant-based products.”
The study was published online in Nature Microbiology.
Diet Tied to Microbial Signature
The researchers analyzed biological samples from 21,561 individuals across five multi-national cohorts to map how differences in diet patterns (omnivore, vegetarian, and vegan) are reflected in gut microbiomes.
They found that the three diet patterns are highly distinguishable by their microbial profiles and that each diet has corresponding unique signature microbes, including those tied to digestion of specific types of food and sometimes those derived from food itself.
The microbiomes of omnivores had an increased presence of bacteria associated with meat digestion, such as Alistipes putredinis, which is involved in protein fermentation. Omnivores also had more bacteria associated with both inflammatory bowel disease and increased colon cancer risk, such as Ruminococcus torques and Bilophila wadsworthia.
The microbiomes of vegans had an abundance of bacteria involved in fiber fermentation, such as several species of Bacteroides and Firmicutes phyla, which help produce short-chain fatty acids. These compounds have beneficial effects on gut health by reducing inflammation and helping to maintain a better homeostatic balance between an individual’s metabolism and immune system.
The main difference between vegetarians and vegans was the presence in vegetarians’ microbiomes of Streptococcus thermophilus, a bacterium found mainly in dairy products and used in the production of yogurt.
Dietary factors within each diet pattern, such as the amount of plant-based food, shape the microbiome more than the type of diet and are important for gut health, according to the authors. For example, by eating more plant-based foods, people with an omnivorous diet can bring the proportion of beneficial signature microbes in their microbiomes more in line with the levels in people who are vegan or vegetarian.
“Since our data showed that omnivores on average ingest significantly fewer healthy plant-based foods than vegetarians or vegans, optimizing the quality of omnivore diets by increasing dietary plant diversity could lead to better gut health,” they wrote.
The ultimate goal, Segata said, is “a precision nutrition approach that recommends foods based on the configuration of the microbiome of patients and of the aspects of the microbiome one wants to enhance. We are not there yet, but it is nonetheless important to know which foods are usually boosting which types of members of the gut microbiome.”
His team is currently analyzing changes in the gut microbiome induced by diet changes among thousands of participants in various cohorts.
“This is one of the next steps toward unraveling causality along the diet-microbiome-health axis, together with the cultivation of specific microbiome members of interest for potential prebiotic and probiotic strategies,” he said.
Conventional Dietary Advice for Now
The findings are consistent with those of previous studies, Jack Gilbert, MD, director of the Microbiome and Metagenomics Center at the University of California, San Diego, and president of Applied Microbiology International, Cambridge, England, said in an interview.
“Future research needs to focus on whether the gut microbial signature can predict those that develop cardiovascular disease in each cohort — ie, the n-of-1 studies, whereby a vegan develops cardiovascular disease, or a carnivore does not,” said Gilbert, who was not involved in the study.
With more data, he said, “we can also start examining these trends over time to understand what might be going on with these ‘oddballs.’ ”
“There is not much you can do with the ‘eat a healthy balanced diet’ routine,” he noted. “If I got a microbiome signature, I could potentially tell you what to eat to optimize your blood glucose trends and your lipid panels but not to handle long-term disease risk, yet. So sticking with the guideline-recommended dietary advice seems best, until we can provide more nuanced advice for the patient.
“Importantly, I would also like to see time-resolved data,” he added. “Signatures can fluctuate over time, even over days, and so collecting a few weeks of stool samples would help us to better align the microbiome signatures to clinical endpoints.”
Segata is a consultant to and receives options from ZOE. Gilbert is a member of the scientific advisory boards of Holobiome, BiomeSense, EcoBiomics Canadian Research Program, MASTER EU, Sun Genomics, and Oath; the editorial advisory board for The Scientist; and the external advisory board for the Binational Early Asthma & Microbiome Study. He is also an adviser for Bened Life.
A version of this article appeared on Medscape.com.
new research suggested.
For example, red meat was a strong driver of omnivore microbiomes, with corresponding signature microbes that are negatively correlated with host cardiometabolic health.
In contrast, the signature microbes found in vegans’ gut microbiomes were correlated with favorable cardiometabolic markers and were enriched in omnivores who ate more plant-based foods.
“From the viewpoint of the impact of diet on the gut microbiome, what seems to be more important is the diversity of healthy plant-based foods that are consumed,” principal author Nicola Segata, PhD, University of Trento in Italy, said in an interview. “Whether this comes within a vegan or an omnivore diet is less crucial, as long as there is no specific overconsumption of unhealthy food categories, such as red meat.”
Excluding broad categories of foods also can have consequences, he added. “For example, we saw that the exclusion of dairy fermented foods is associated with decreased presence of potentially probiotic microbes that are constitutive of such foods. Avoiding meat or dairy products does not necessarily have a positive effect if it does not come with a variety of quality plant-based products.”
The study was published online in Nature Microbiology.
Diet Tied to Microbial Signature
The researchers analyzed biological samples from 21,561 individuals across five multi-national cohorts to map how differences in diet patterns (omnivore, vegetarian, and vegan) are reflected in gut microbiomes.
They found that the three diet patterns are highly distinguishable by their microbial profiles and that each diet has corresponding unique signature microbes, including those tied to digestion of specific types of food and sometimes those derived from food itself.
The microbiomes of omnivores had an increased presence of bacteria associated with meat digestion, such as Alistipes putredinis, which is involved in protein fermentation. Omnivores also had more bacteria associated with both inflammatory bowel disease and increased colon cancer risk, such as Ruminococcus torques and Bilophila wadsworthia.
The microbiomes of vegans had an abundance of bacteria involved in fiber fermentation, such as several species of Bacteroides and Firmicutes phyla, which help produce short-chain fatty acids. These compounds have beneficial effects on gut health by reducing inflammation and helping to maintain a better homeostatic balance between an individual’s metabolism and immune system.
The main difference between vegetarians and vegans was the presence in vegetarians’ microbiomes of Streptococcus thermophilus, a bacterium found mainly in dairy products and used in the production of yogurt.
Dietary factors within each diet pattern, such as the amount of plant-based food, shape the microbiome more than the type of diet and are important for gut health, according to the authors. For example, by eating more plant-based foods, people with an omnivorous diet can bring the proportion of beneficial signature microbes in their microbiomes more in line with the levels in people who are vegan or vegetarian.
“Since our data showed that omnivores on average ingest significantly fewer healthy plant-based foods than vegetarians or vegans, optimizing the quality of omnivore diets by increasing dietary plant diversity could lead to better gut health,” they wrote.
The ultimate goal, Segata said, is “a precision nutrition approach that recommends foods based on the configuration of the microbiome of patients and of the aspects of the microbiome one wants to enhance. We are not there yet, but it is nonetheless important to know which foods are usually boosting which types of members of the gut microbiome.”
His team is currently analyzing changes in the gut microbiome induced by diet changes among thousands of participants in various cohorts.
“This is one of the next steps toward unraveling causality along the diet-microbiome-health axis, together with the cultivation of specific microbiome members of interest for potential prebiotic and probiotic strategies,” he said.
Conventional Dietary Advice for Now
The findings are consistent with those of previous studies, Jack Gilbert, MD, director of the Microbiome and Metagenomics Center at the University of California, San Diego, and president of Applied Microbiology International, Cambridge, England, said in an interview.
“Future research needs to focus on whether the gut microbial signature can predict those that develop cardiovascular disease in each cohort — ie, the n-of-1 studies, whereby a vegan develops cardiovascular disease, or a carnivore does not,” said Gilbert, who was not involved in the study.
With more data, he said, “we can also start examining these trends over time to understand what might be going on with these ‘oddballs.’ ”
“There is not much you can do with the ‘eat a healthy balanced diet’ routine,” he noted. “If I got a microbiome signature, I could potentially tell you what to eat to optimize your blood glucose trends and your lipid panels but not to handle long-term disease risk, yet. So sticking with the guideline-recommended dietary advice seems best, until we can provide more nuanced advice for the patient.
“Importantly, I would also like to see time-resolved data,” he added. “Signatures can fluctuate over time, even over days, and so collecting a few weeks of stool samples would help us to better align the microbiome signatures to clinical endpoints.”
Segata is a consultant to and receives options from ZOE. Gilbert is a member of the scientific advisory boards of Holobiome, BiomeSense, EcoBiomics Canadian Research Program, MASTER EU, Sun Genomics, and Oath; the editorial advisory board for The Scientist; and the external advisory board for the Binational Early Asthma & Microbiome Study. He is also an adviser for Bened Life.
A version of this article appeared on Medscape.com.
new research suggested.
For example, red meat was a strong driver of omnivore microbiomes, with corresponding signature microbes that are negatively correlated with host cardiometabolic health.
In contrast, the signature microbes found in vegans’ gut microbiomes were correlated with favorable cardiometabolic markers and were enriched in omnivores who ate more plant-based foods.
“From the viewpoint of the impact of diet on the gut microbiome, what seems to be more important is the diversity of healthy plant-based foods that are consumed,” principal author Nicola Segata, PhD, University of Trento in Italy, said in an interview. “Whether this comes within a vegan or an omnivore diet is less crucial, as long as there is no specific overconsumption of unhealthy food categories, such as red meat.”
Excluding broad categories of foods also can have consequences, he added. “For example, we saw that the exclusion of dairy fermented foods is associated with decreased presence of potentially probiotic microbes that are constitutive of such foods. Avoiding meat or dairy products does not necessarily have a positive effect if it does not come with a variety of quality plant-based products.”
The study was published online in Nature Microbiology.
Diet Tied to Microbial Signature
The researchers analyzed biological samples from 21,561 individuals across five multi-national cohorts to map how differences in diet patterns (omnivore, vegetarian, and vegan) are reflected in gut microbiomes.
They found that the three diet patterns are highly distinguishable by their microbial profiles and that each diet has corresponding unique signature microbes, including those tied to digestion of specific types of food and sometimes those derived from food itself.
The microbiomes of omnivores had an increased presence of bacteria associated with meat digestion, such as Alistipes putredinis, which is involved in protein fermentation. Omnivores also had more bacteria associated with both inflammatory bowel disease and increased colon cancer risk, such as Ruminococcus torques and Bilophila wadsworthia.
The microbiomes of vegans had an abundance of bacteria involved in fiber fermentation, such as several species of Bacteroides and Firmicutes phyla, which help produce short-chain fatty acids. These compounds have beneficial effects on gut health by reducing inflammation and helping to maintain a better homeostatic balance between an individual’s metabolism and immune system.
The main difference between vegetarians and vegans was the presence in vegetarians’ microbiomes of Streptococcus thermophilus, a bacterium found mainly in dairy products and used in the production of yogurt.
Dietary factors within each diet pattern, such as the amount of plant-based food, shape the microbiome more than the type of diet and are important for gut health, according to the authors. For example, by eating more plant-based foods, people with an omnivorous diet can bring the proportion of beneficial signature microbes in their microbiomes more in line with the levels in people who are vegan or vegetarian.
“Since our data showed that omnivores on average ingest significantly fewer healthy plant-based foods than vegetarians or vegans, optimizing the quality of omnivore diets by increasing dietary plant diversity could lead to better gut health,” they wrote.
The ultimate goal, Segata said, is “a precision nutrition approach that recommends foods based on the configuration of the microbiome of patients and of the aspects of the microbiome one wants to enhance. We are not there yet, but it is nonetheless important to know which foods are usually boosting which types of members of the gut microbiome.”
His team is currently analyzing changes in the gut microbiome induced by diet changes among thousands of participants in various cohorts.
“This is one of the next steps toward unraveling causality along the diet-microbiome-health axis, together with the cultivation of specific microbiome members of interest for potential prebiotic and probiotic strategies,” he said.
Conventional Dietary Advice for Now
The findings are consistent with those of previous studies, Jack Gilbert, MD, director of the Microbiome and Metagenomics Center at the University of California, San Diego, and president of Applied Microbiology International, Cambridge, England, said in an interview.
“Future research needs to focus on whether the gut microbial signature can predict those that develop cardiovascular disease in each cohort — ie, the n-of-1 studies, whereby a vegan develops cardiovascular disease, or a carnivore does not,” said Gilbert, who was not involved in the study.
With more data, he said, “we can also start examining these trends over time to understand what might be going on with these ‘oddballs.’ ”
“There is not much you can do with the ‘eat a healthy balanced diet’ routine,” he noted. “If I got a microbiome signature, I could potentially tell you what to eat to optimize your blood glucose trends and your lipid panels but not to handle long-term disease risk, yet. So sticking with the guideline-recommended dietary advice seems best, until we can provide more nuanced advice for the patient.
“Importantly, I would also like to see time-resolved data,” he added. “Signatures can fluctuate over time, even over days, and so collecting a few weeks of stool samples would help us to better align the microbiome signatures to clinical endpoints.”
Segata is a consultant to and receives options from ZOE. Gilbert is a member of the scientific advisory boards of Holobiome, BiomeSense, EcoBiomics Canadian Research Program, MASTER EU, Sun Genomics, and Oath; the editorial advisory board for The Scientist; and the external advisory board for the Binational Early Asthma & Microbiome Study. He is also an adviser for Bened Life.
A version of this article appeared on Medscape.com.
FROM NATURE MICROBIOLOGY
Managing GI and Liver Conditions During Pregnancy: New Guidance from AGA
according to a clinical practice update (CPU) from the American Gastroenterological Association.
Notably, procedures, medications, or other interventions intended to improve maternal health shouldn’t be withheld solely because the patient is pregnant, the authors wrote. Instead, treatments should be personalized based on a risk-benefit assessment.
“Pregnancy causes significant physiological changes that can affect the GI tract and liver function. Some common conditions — such as nausea, vomiting, gastroesophageal reflux disease (GERD), and constipation — may be exacerbated, and underlying GI or liver diseases can behave differently during pregnancy,” said lead author Shivangi Kothari, MD, associate professor of medicine and associate director of endoscopy at the University of Rochester Medical Center and Strong Memorial Hospital, both in Rochester, New York.
“These conditions can pose significant risks to both the mother and fetus, and their management requires a specialized, updated approach,” she said. “This clinical practice update stresses the need for coordinated, multidisciplinary care among obstetricians, gastroenterologists, hepatologists, and maternal-and-fetal medicine experts to ensure optimal outcomes, particularly in complex or high-risk cases.”
The update was published online in Gastroenterology.
Pregnancy-Related Concerns
The best path to optimal outcomes is to start early, the authors wrote. Before pregnancy, patients should consider preconception and contraceptive care counseling with a multidisciplinary team that can address GI and liver issues, especially among reproductive-age people who want to become pregnant.
Once pregnant, though, patients shouldn’t be deterred from receiving procedures, medications, or interventions just because they’re pregnant, the authors wrote. Instead, taking an individual approach will help clinicians decide what to do based on the risks and benefits.
At the beginning of pregnancy, early treatment of nausea and vomiting can reduce progression to hyperemesis gravidarum, the authors wrote. Stepwise treatment can include vitamin B6, doxylamine, hydration, and adequate nutrition, followed by ondansetron, metoclopramide, promethazine, and intravenous glucocorticoids in moderate to severe cases.
Constipation may also pose a problem because of hormonal, physiological, and medication-related changes. Treatment options can include dietary fiber, lactulose, and polyethylene glycol-based laxatives.
Patients with certain conditions — such as complex inflammatory bowel disease (IBD), advanced cirrhosis, or liver transplant — should work with a multidisciplinary team to coordinate birth, preferably in a tertiary care center, the authors wrote.
For patients with IBD, clinical remission helps to improve pregnancy outcomes, including before conception, during pregnancy, and throughout the postpartum period. Biologic agents should be used during pregnancy and postpartum, though methotrexate, thalidomide, and ozanimod should be stopped at least 6 months before conception.
For patients with chronic hepatitis B, serum hepatitis B virus DNA and liver biochemical levels should be tested. Patients with a serum level > 200,000 IU/mL during the third trimester should be considered for treatment with tenofovir disoproxil fumarate.
For patients on immunosuppressive therapy for chronic liver diseases or after liver transplantation, therapy should continue at the lowest effective dose. However, mycophenolate mofetil shouldn’t be administered during pregnancy.
Intrahepatic cholestasis of pregnancy may be diagnosed during the second or third trimester based on pruritus and a serum bile acid level > 10 μmol/L. Treatment should include oral ursodeoxycholic acid, with a total daily dose of 10-15 mg/kg.
Other pregnancy-related liver diseases — such as pre-eclampsia; hemolysis, elevated liver enzymes, and low platelets syndrome; and acute fatty liver of pregnancy — require careful birth planning and evaluation for possible liver transplantation. For certain high-risk patients, daily aspirin should start at week 12 of gestation.
In addition, elective endoscopic procedures should wait until after birth, and nonemergent but necessary procedures should be performed during the second trimester. Patients with cirrhosis should undergo evaluation for esophageal varices, and upper endoscopy should happen during the second trimester to guide beta-blocker therapy or endoscopic variceal litigation.
Endoscopic retrograde cholangiopancreatography can be performed for urgent indications, such as choledocholithiasis, cholangitis, and some gallstone pancreatitis cases, ideally during the second trimester.
Cholecystectomy is considered safe during pregnancy, with a laparoscopic approach as the standard of care regardless of trimester, though the second trimester is ideal.

Pregnancy-Related Updates in Practice
Ultimately, clinicians should familiarize themselves with the best practice advice to feel comfortable when counseling and managing pregnancy-related concerns, especially high-risk patients, said Eugenia Shmidt, MD, assistant professor of gastroenterology, hepatology, and nutrition, and founder of the IBD Preconception and Pregnancy Planning Clinic at the University of Minnesota, Minneapolis.
“Half of all patients with GI and liver disease are women, and oftentimes, they don’t have appropriate guidance regarding reproductive health in the context of their disease,” she said. “There exists a very large knowledge gap in this area, particularly because most clinical trials exclude pregnant people.”
Most importantly, the advice statements can guide practitioners on how to help pregnant patients make informed reproductive decisions, she added.
“This CPU makes it clear that preconception counseling and multidisciplinary care are key in optimizing reproductive health, regardless of the underlying GI or liver disease,” Shmidt said. “GI practitioners should be counseling women well in advance of pregnancy and recruiting all relevant stakeholders as early as possible, even prior to conception. This way, pregnancy care is not reactive, but instead proactive.”
The authors received no specific funding for this update. Kothari and Shmidt reported no relevant disclosures.
A version of this article appeared on Medscape.com.
according to a clinical practice update (CPU) from the American Gastroenterological Association.
Notably, procedures, medications, or other interventions intended to improve maternal health shouldn’t be withheld solely because the patient is pregnant, the authors wrote. Instead, treatments should be personalized based on a risk-benefit assessment.
“Pregnancy causes significant physiological changes that can affect the GI tract and liver function. Some common conditions — such as nausea, vomiting, gastroesophageal reflux disease (GERD), and constipation — may be exacerbated, and underlying GI or liver diseases can behave differently during pregnancy,” said lead author Shivangi Kothari, MD, associate professor of medicine and associate director of endoscopy at the University of Rochester Medical Center and Strong Memorial Hospital, both in Rochester, New York.
“These conditions can pose significant risks to both the mother and fetus, and their management requires a specialized, updated approach,” she said. “This clinical practice update stresses the need for coordinated, multidisciplinary care among obstetricians, gastroenterologists, hepatologists, and maternal-and-fetal medicine experts to ensure optimal outcomes, particularly in complex or high-risk cases.”
The update was published online in Gastroenterology.
Pregnancy-Related Concerns
The best path to optimal outcomes is to start early, the authors wrote. Before pregnancy, patients should consider preconception and contraceptive care counseling with a multidisciplinary team that can address GI and liver issues, especially among reproductive-age people who want to become pregnant.
Once pregnant, though, patients shouldn’t be deterred from receiving procedures, medications, or interventions just because they’re pregnant, the authors wrote. Instead, taking an individual approach will help clinicians decide what to do based on the risks and benefits.
At the beginning of pregnancy, early treatment of nausea and vomiting can reduce progression to hyperemesis gravidarum, the authors wrote. Stepwise treatment can include vitamin B6, doxylamine, hydration, and adequate nutrition, followed by ondansetron, metoclopramide, promethazine, and intravenous glucocorticoids in moderate to severe cases.
Constipation may also pose a problem because of hormonal, physiological, and medication-related changes. Treatment options can include dietary fiber, lactulose, and polyethylene glycol-based laxatives.
Patients with certain conditions — such as complex inflammatory bowel disease (IBD), advanced cirrhosis, or liver transplant — should work with a multidisciplinary team to coordinate birth, preferably in a tertiary care center, the authors wrote.
For patients with IBD, clinical remission helps to improve pregnancy outcomes, including before conception, during pregnancy, and throughout the postpartum period. Biologic agents should be used during pregnancy and postpartum, though methotrexate, thalidomide, and ozanimod should be stopped at least 6 months before conception.
For patients with chronic hepatitis B, serum hepatitis B virus DNA and liver biochemical levels should be tested. Patients with a serum level > 200,000 IU/mL during the third trimester should be considered for treatment with tenofovir disoproxil fumarate.
For patients on immunosuppressive therapy for chronic liver diseases or after liver transplantation, therapy should continue at the lowest effective dose. However, mycophenolate mofetil shouldn’t be administered during pregnancy.
Intrahepatic cholestasis of pregnancy may be diagnosed during the second or third trimester based on pruritus and a serum bile acid level > 10 μmol/L. Treatment should include oral ursodeoxycholic acid, with a total daily dose of 10-15 mg/kg.
Other pregnancy-related liver diseases — such as pre-eclampsia; hemolysis, elevated liver enzymes, and low platelets syndrome; and acute fatty liver of pregnancy — require careful birth planning and evaluation for possible liver transplantation. For certain high-risk patients, daily aspirin should start at week 12 of gestation.
In addition, elective endoscopic procedures should wait until after birth, and nonemergent but necessary procedures should be performed during the second trimester. Patients with cirrhosis should undergo evaluation for esophageal varices, and upper endoscopy should happen during the second trimester to guide beta-blocker therapy or endoscopic variceal litigation.
Endoscopic retrograde cholangiopancreatography can be performed for urgent indications, such as choledocholithiasis, cholangitis, and some gallstone pancreatitis cases, ideally during the second trimester.
Cholecystectomy is considered safe during pregnancy, with a laparoscopic approach as the standard of care regardless of trimester, though the second trimester is ideal.

Pregnancy-Related Updates in Practice
Ultimately, clinicians should familiarize themselves with the best practice advice to feel comfortable when counseling and managing pregnancy-related concerns, especially high-risk patients, said Eugenia Shmidt, MD, assistant professor of gastroenterology, hepatology, and nutrition, and founder of the IBD Preconception and Pregnancy Planning Clinic at the University of Minnesota, Minneapolis.
“Half of all patients with GI and liver disease are women, and oftentimes, they don’t have appropriate guidance regarding reproductive health in the context of their disease,” she said. “There exists a very large knowledge gap in this area, particularly because most clinical trials exclude pregnant people.”
Most importantly, the advice statements can guide practitioners on how to help pregnant patients make informed reproductive decisions, she added.
“This CPU makes it clear that preconception counseling and multidisciplinary care are key in optimizing reproductive health, regardless of the underlying GI or liver disease,” Shmidt said. “GI practitioners should be counseling women well in advance of pregnancy and recruiting all relevant stakeholders as early as possible, even prior to conception. This way, pregnancy care is not reactive, but instead proactive.”
The authors received no specific funding for this update. Kothari and Shmidt reported no relevant disclosures.
A version of this article appeared on Medscape.com.
according to a clinical practice update (CPU) from the American Gastroenterological Association.
Notably, procedures, medications, or other interventions intended to improve maternal health shouldn’t be withheld solely because the patient is pregnant, the authors wrote. Instead, treatments should be personalized based on a risk-benefit assessment.
“Pregnancy causes significant physiological changes that can affect the GI tract and liver function. Some common conditions — such as nausea, vomiting, gastroesophageal reflux disease (GERD), and constipation — may be exacerbated, and underlying GI or liver diseases can behave differently during pregnancy,” said lead author Shivangi Kothari, MD, associate professor of medicine and associate director of endoscopy at the University of Rochester Medical Center and Strong Memorial Hospital, both in Rochester, New York.
“These conditions can pose significant risks to both the mother and fetus, and their management requires a specialized, updated approach,” she said. “This clinical practice update stresses the need for coordinated, multidisciplinary care among obstetricians, gastroenterologists, hepatologists, and maternal-and-fetal medicine experts to ensure optimal outcomes, particularly in complex or high-risk cases.”
The update was published online in Gastroenterology.
Pregnancy-Related Concerns
The best path to optimal outcomes is to start early, the authors wrote. Before pregnancy, patients should consider preconception and contraceptive care counseling with a multidisciplinary team that can address GI and liver issues, especially among reproductive-age people who want to become pregnant.
Once pregnant, though, patients shouldn’t be deterred from receiving procedures, medications, or interventions just because they’re pregnant, the authors wrote. Instead, taking an individual approach will help clinicians decide what to do based on the risks and benefits.
At the beginning of pregnancy, early treatment of nausea and vomiting can reduce progression to hyperemesis gravidarum, the authors wrote. Stepwise treatment can include vitamin B6, doxylamine, hydration, and adequate nutrition, followed by ondansetron, metoclopramide, promethazine, and intravenous glucocorticoids in moderate to severe cases.
Constipation may also pose a problem because of hormonal, physiological, and medication-related changes. Treatment options can include dietary fiber, lactulose, and polyethylene glycol-based laxatives.
Patients with certain conditions — such as complex inflammatory bowel disease (IBD), advanced cirrhosis, or liver transplant — should work with a multidisciplinary team to coordinate birth, preferably in a tertiary care center, the authors wrote.
For patients with IBD, clinical remission helps to improve pregnancy outcomes, including before conception, during pregnancy, and throughout the postpartum period. Biologic agents should be used during pregnancy and postpartum, though methotrexate, thalidomide, and ozanimod should be stopped at least 6 months before conception.
For patients with chronic hepatitis B, serum hepatitis B virus DNA and liver biochemical levels should be tested. Patients with a serum level > 200,000 IU/mL during the third trimester should be considered for treatment with tenofovir disoproxil fumarate.
For patients on immunosuppressive therapy for chronic liver diseases or after liver transplantation, therapy should continue at the lowest effective dose. However, mycophenolate mofetil shouldn’t be administered during pregnancy.
Intrahepatic cholestasis of pregnancy may be diagnosed during the second or third trimester based on pruritus and a serum bile acid level > 10 μmol/L. Treatment should include oral ursodeoxycholic acid, with a total daily dose of 10-15 mg/kg.
Other pregnancy-related liver diseases — such as pre-eclampsia; hemolysis, elevated liver enzymes, and low platelets syndrome; and acute fatty liver of pregnancy — require careful birth planning and evaluation for possible liver transplantation. For certain high-risk patients, daily aspirin should start at week 12 of gestation.
In addition, elective endoscopic procedures should wait until after birth, and nonemergent but necessary procedures should be performed during the second trimester. Patients with cirrhosis should undergo evaluation for esophageal varices, and upper endoscopy should happen during the second trimester to guide beta-blocker therapy or endoscopic variceal litigation.
Endoscopic retrograde cholangiopancreatography can be performed for urgent indications, such as choledocholithiasis, cholangitis, and some gallstone pancreatitis cases, ideally during the second trimester.
Cholecystectomy is considered safe during pregnancy, with a laparoscopic approach as the standard of care regardless of trimester, though the second trimester is ideal.

Pregnancy-Related Updates in Practice
Ultimately, clinicians should familiarize themselves with the best practice advice to feel comfortable when counseling and managing pregnancy-related concerns, especially high-risk patients, said Eugenia Shmidt, MD, assistant professor of gastroenterology, hepatology, and nutrition, and founder of the IBD Preconception and Pregnancy Planning Clinic at the University of Minnesota, Minneapolis.
“Half of all patients with GI and liver disease are women, and oftentimes, they don’t have appropriate guidance regarding reproductive health in the context of their disease,” she said. “There exists a very large knowledge gap in this area, particularly because most clinical trials exclude pregnant people.”
Most importantly, the advice statements can guide practitioners on how to help pregnant patients make informed reproductive decisions, she added.
“This CPU makes it clear that preconception counseling and multidisciplinary care are key in optimizing reproductive health, regardless of the underlying GI or liver disease,” Shmidt said. “GI practitioners should be counseling women well in advance of pregnancy and recruiting all relevant stakeholders as early as possible, even prior to conception. This way, pregnancy care is not reactive, but instead proactive.”
The authors received no specific funding for this update. Kothari and Shmidt reported no relevant disclosures.
A version of this article appeared on Medscape.com.
FROM GASTROENTEROLOGY
Noninvasive Microbiome Test May Specifically Identify Crohn’s and Ulcerative Colitis
International researchers have uncovered potentially diagnostic gut microbiome signatures and metabolic pathways associated specifically with ulcerative colitis (UC) and Crohn’s disease (CD).
Targeted droplet digital polymerase chain reaction (ddPCR)‒based quantification of bacterial species led to convenient inflammatory bowel disease (IBD) diagnostic assays that “are sufficiently robust, sensitive and cost-effective for clinical application,” the investigators wrote in a recent study published in Nature Medicine.
“Although traditional modalities used for diagnosis of IBD, including colonoscopy and cross-sectional imaging, are well established, the inconvenience of bowel preparation and radiation represents relevant concerns,” senior author Siew C. Ng, MBBS, PhD, a professor in the Department of Medicine and Therapeutics at the Chinese University of Hong Kong, said in an interview. “Furthermore, existing serological and fecal markers indicate inflammation but lack specificity for IBD.”
Identifying reproducible bacterial biomarkers specific to CD and IBD should enable precise and personalized approaches to detection and management.
As a starting point, the researchers hypothesized that changes in the gut microbiome of IBD patients may reflect underlying functional associations, if not causes, of the disease, said Ng, who is also director of Hong Kong’s Microbiota I-Center (MagIC). “Unlike inflammation, which is a manifestation of the disease, the gut microbiome may serve as a more reliable biomarker less affected by the disease’s fluctuating cycle.”
The study findings showed that bacterial markers remain consistent even during the inactive disease phase. , she added. “With a better performance than the commonly used noninvasive test, fecal calprotectin, we believe the test will be a valuable addition to clinician’s toolbox and a strong option for first-line diagnostics.”
The Study
The group used metagenomic data from 5979 fecal samples from persons with and without IBD from different regions (including the United States) and of different ethnicities. Identifying several microbiota alterations in IBD, they selected bacterial species to construct diagnostic models for UC (n = 10) and CD (n = 9). Some species were deleted and some were enriched in IBD.
Metagenomic findings confirmed, for example, enrichments of Escherichia coli and Bacteroides fragilis in the guts of CD patients, with adherent invasive E coli present in more than half of these. This pathogen has been linked to mucosal dysbiosis and functional alteration, and has been associated with disease activity and endoscopic recurrence following surgery. B fragilis may induce intestinal inflammation through toxin production.
The researchers also identified a new oral bacterium, Actinomyces species oral taxon 181, which was significantly enriched in stool samples with both CD and UC.
The diagnostic models achieved areas under the curve of >.90 for distinguishing IBD patients from controls in the discovery cohort and maintained satisfactory performance in transethnic validation cohorts from eight populations.
Ng’s group further developed a multiplex droplet digital PCR test targeting selected IBD-associated bacterial species. Models based on this test showed numerically higher performance than fecal calprotectin in discriminating UC and CD samples from controls. These universally IBD-associated bacteria suggest the potential applicability of a biomarker panel for noninvasive diagnosis.
Commenting on the paper but not involved in it, Ashwin N. Ananthakrishnan, MBBS, MPH, AGAF, director of the Crohn’s and Colitis Center at Massachusetts General Hospital in Boston and associate professor of medicine at Harvard Medical School, called it “a very important study that highlights the potential role of a microbiome-based diagnostic for screening. It could have application in a wide variety of settings and is very promising.”
More work, however, is necessary to clarify such testing’s role. “The study’s validation in independent cohorts is an important strength, but the sizes of those cohorts are still quite small,” he said in an interview. “It’s important to understand its accuracy across a spectrum of IBD phenotypes and severity.”
Furthermore, endoscopic evaluation at diagnosis is important to establish severity and extent of disease. “It’s not clear this diagnostic biomarker can help supplant that role. But I see potential value to it for patients for whom we may not be considering endoscopy yet but who would like to risk-stratify.”
The Test’s Future
“We expect to see a real shift in clinical practice,” Ng said. “As a cost-effective test, it will help millions of people dealing with nonspecific gastrointestinal symptoms get the diagnoses they need.” Because the bacterial test can identify IBD at an inactive stage, it has the potential for early diagnosis. “This capability allows clinicians to initiate treatment sooner, helping to prevent progression from subclinical to clinical stages of the disease.”
The next research steps involve prospective studies with a larger and more diverse group of patients with various gastrointestinal symptoms. “This will enable a comprehensive evaluation of bacterial biomarkers in real-world populations,” she said. In vivo and in vitro experiments are expected to provide mechanistic insights into the causal role of these bacteria and metabolic dysregulations in the pathogenesis of IBD, as well as their future clinical utility in disease monitoring and predicting treatment response.
Her group plans to work with the biotech industry and regulatory agencies to transform these biomarkers into an approved test kit. “The rollout is likely to be gradual, but we’re optimistic that supportive international and national guidelines will be developed and will pave the way for widespread implementation.”
This study was supported by various academic, charitable, and governmental research-funding bodies, including the governments of Hong Kong and the People’s Republic of China. Ng has served as an advisory board member or speaker for Pfizer, Ferring, Janssen, AbbVie, Tillotts, Menarini, and Takeda. She has received research grants through her institutions from Olympus, Ferring, and AbbVie and is a founding member and shareholder of GenieBiome. She receives patent royalties through her institutions, including MagIC, which holds patents on the therapeutic and diagnostic use of the microbiome in IBD. Several co-authors reported various relationships, including patent holding, with private-sector companies. Ananthakrishnan had no relevant competing interests.
A version of this article first appeared on Medscape.com.
International researchers have uncovered potentially diagnostic gut microbiome signatures and metabolic pathways associated specifically with ulcerative colitis (UC) and Crohn’s disease (CD).
Targeted droplet digital polymerase chain reaction (ddPCR)‒based quantification of bacterial species led to convenient inflammatory bowel disease (IBD) diagnostic assays that “are sufficiently robust, sensitive and cost-effective for clinical application,” the investigators wrote in a recent study published in Nature Medicine.
“Although traditional modalities used for diagnosis of IBD, including colonoscopy and cross-sectional imaging, are well established, the inconvenience of bowel preparation and radiation represents relevant concerns,” senior author Siew C. Ng, MBBS, PhD, a professor in the Department of Medicine and Therapeutics at the Chinese University of Hong Kong, said in an interview. “Furthermore, existing serological and fecal markers indicate inflammation but lack specificity for IBD.”
Identifying reproducible bacterial biomarkers specific to CD and IBD should enable precise and personalized approaches to detection and management.
As a starting point, the researchers hypothesized that changes in the gut microbiome of IBD patients may reflect underlying functional associations, if not causes, of the disease, said Ng, who is also director of Hong Kong’s Microbiota I-Center (MagIC). “Unlike inflammation, which is a manifestation of the disease, the gut microbiome may serve as a more reliable biomarker less affected by the disease’s fluctuating cycle.”
The study findings showed that bacterial markers remain consistent even during the inactive disease phase. , she added. “With a better performance than the commonly used noninvasive test, fecal calprotectin, we believe the test will be a valuable addition to clinician’s toolbox and a strong option for first-line diagnostics.”
The Study
The group used metagenomic data from 5979 fecal samples from persons with and without IBD from different regions (including the United States) and of different ethnicities. Identifying several microbiota alterations in IBD, they selected bacterial species to construct diagnostic models for UC (n = 10) and CD (n = 9). Some species were deleted and some were enriched in IBD.
Metagenomic findings confirmed, for example, enrichments of Escherichia coli and Bacteroides fragilis in the guts of CD patients, with adherent invasive E coli present in more than half of these. This pathogen has been linked to mucosal dysbiosis and functional alteration, and has been associated with disease activity and endoscopic recurrence following surgery. B fragilis may induce intestinal inflammation through toxin production.
The researchers also identified a new oral bacterium, Actinomyces species oral taxon 181, which was significantly enriched in stool samples with both CD and UC.
The diagnostic models achieved areas under the curve of >.90 for distinguishing IBD patients from controls in the discovery cohort and maintained satisfactory performance in transethnic validation cohorts from eight populations.
Ng’s group further developed a multiplex droplet digital PCR test targeting selected IBD-associated bacterial species. Models based on this test showed numerically higher performance than fecal calprotectin in discriminating UC and CD samples from controls. These universally IBD-associated bacteria suggest the potential applicability of a biomarker panel for noninvasive diagnosis.
Commenting on the paper but not involved in it, Ashwin N. Ananthakrishnan, MBBS, MPH, AGAF, director of the Crohn’s and Colitis Center at Massachusetts General Hospital in Boston and associate professor of medicine at Harvard Medical School, called it “a very important study that highlights the potential role of a microbiome-based diagnostic for screening. It could have application in a wide variety of settings and is very promising.”
More work, however, is necessary to clarify such testing’s role. “The study’s validation in independent cohorts is an important strength, but the sizes of those cohorts are still quite small,” he said in an interview. “It’s important to understand its accuracy across a spectrum of IBD phenotypes and severity.”
Furthermore, endoscopic evaluation at diagnosis is important to establish severity and extent of disease. “It’s not clear this diagnostic biomarker can help supplant that role. But I see potential value to it for patients for whom we may not be considering endoscopy yet but who would like to risk-stratify.”
The Test’s Future
“We expect to see a real shift in clinical practice,” Ng said. “As a cost-effective test, it will help millions of people dealing with nonspecific gastrointestinal symptoms get the diagnoses they need.” Because the bacterial test can identify IBD at an inactive stage, it has the potential for early diagnosis. “This capability allows clinicians to initiate treatment sooner, helping to prevent progression from subclinical to clinical stages of the disease.”
The next research steps involve prospective studies with a larger and more diverse group of patients with various gastrointestinal symptoms. “This will enable a comprehensive evaluation of bacterial biomarkers in real-world populations,” she said. In vivo and in vitro experiments are expected to provide mechanistic insights into the causal role of these bacteria and metabolic dysregulations in the pathogenesis of IBD, as well as their future clinical utility in disease monitoring and predicting treatment response.
Her group plans to work with the biotech industry and regulatory agencies to transform these biomarkers into an approved test kit. “The rollout is likely to be gradual, but we’re optimistic that supportive international and national guidelines will be developed and will pave the way for widespread implementation.”
This study was supported by various academic, charitable, and governmental research-funding bodies, including the governments of Hong Kong and the People’s Republic of China. Ng has served as an advisory board member or speaker for Pfizer, Ferring, Janssen, AbbVie, Tillotts, Menarini, and Takeda. She has received research grants through her institutions from Olympus, Ferring, and AbbVie and is a founding member and shareholder of GenieBiome. She receives patent royalties through her institutions, including MagIC, which holds patents on the therapeutic and diagnostic use of the microbiome in IBD. Several co-authors reported various relationships, including patent holding, with private-sector companies. Ananthakrishnan had no relevant competing interests.
A version of this article first appeared on Medscape.com.
International researchers have uncovered potentially diagnostic gut microbiome signatures and metabolic pathways associated specifically with ulcerative colitis (UC) and Crohn’s disease (CD).
Targeted droplet digital polymerase chain reaction (ddPCR)‒based quantification of bacterial species led to convenient inflammatory bowel disease (IBD) diagnostic assays that “are sufficiently robust, sensitive and cost-effective for clinical application,” the investigators wrote in a recent study published in Nature Medicine.
“Although traditional modalities used for diagnosis of IBD, including colonoscopy and cross-sectional imaging, are well established, the inconvenience of bowel preparation and radiation represents relevant concerns,” senior author Siew C. Ng, MBBS, PhD, a professor in the Department of Medicine and Therapeutics at the Chinese University of Hong Kong, said in an interview. “Furthermore, existing serological and fecal markers indicate inflammation but lack specificity for IBD.”
Identifying reproducible bacterial biomarkers specific to CD and IBD should enable precise and personalized approaches to detection and management.
As a starting point, the researchers hypothesized that changes in the gut microbiome of IBD patients may reflect underlying functional associations, if not causes, of the disease, said Ng, who is also director of Hong Kong’s Microbiota I-Center (MagIC). “Unlike inflammation, which is a manifestation of the disease, the gut microbiome may serve as a more reliable biomarker less affected by the disease’s fluctuating cycle.”
The study findings showed that bacterial markers remain consistent even during the inactive disease phase. , she added. “With a better performance than the commonly used noninvasive test, fecal calprotectin, we believe the test will be a valuable addition to clinician’s toolbox and a strong option for first-line diagnostics.”
The Study
The group used metagenomic data from 5979 fecal samples from persons with and without IBD from different regions (including the United States) and of different ethnicities. Identifying several microbiota alterations in IBD, they selected bacterial species to construct diagnostic models for UC (n = 10) and CD (n = 9). Some species were deleted and some were enriched in IBD.
Metagenomic findings confirmed, for example, enrichments of Escherichia coli and Bacteroides fragilis in the guts of CD patients, with adherent invasive E coli present in more than half of these. This pathogen has been linked to mucosal dysbiosis and functional alteration, and has been associated with disease activity and endoscopic recurrence following surgery. B fragilis may induce intestinal inflammation through toxin production.
The researchers also identified a new oral bacterium, Actinomyces species oral taxon 181, which was significantly enriched in stool samples with both CD and UC.
The diagnostic models achieved areas under the curve of >.90 for distinguishing IBD patients from controls in the discovery cohort and maintained satisfactory performance in transethnic validation cohorts from eight populations.
Ng’s group further developed a multiplex droplet digital PCR test targeting selected IBD-associated bacterial species. Models based on this test showed numerically higher performance than fecal calprotectin in discriminating UC and CD samples from controls. These universally IBD-associated bacteria suggest the potential applicability of a biomarker panel for noninvasive diagnosis.
Commenting on the paper but not involved in it, Ashwin N. Ananthakrishnan, MBBS, MPH, AGAF, director of the Crohn’s and Colitis Center at Massachusetts General Hospital in Boston and associate professor of medicine at Harvard Medical School, called it “a very important study that highlights the potential role of a microbiome-based diagnostic for screening. It could have application in a wide variety of settings and is very promising.”
More work, however, is necessary to clarify such testing’s role. “The study’s validation in independent cohorts is an important strength, but the sizes of those cohorts are still quite small,” he said in an interview. “It’s important to understand its accuracy across a spectrum of IBD phenotypes and severity.”
Furthermore, endoscopic evaluation at diagnosis is important to establish severity and extent of disease. “It’s not clear this diagnostic biomarker can help supplant that role. But I see potential value to it for patients for whom we may not be considering endoscopy yet but who would like to risk-stratify.”
The Test’s Future
“We expect to see a real shift in clinical practice,” Ng said. “As a cost-effective test, it will help millions of people dealing with nonspecific gastrointestinal symptoms get the diagnoses they need.” Because the bacterial test can identify IBD at an inactive stage, it has the potential for early diagnosis. “This capability allows clinicians to initiate treatment sooner, helping to prevent progression from subclinical to clinical stages of the disease.”
The next research steps involve prospective studies with a larger and more diverse group of patients with various gastrointestinal symptoms. “This will enable a comprehensive evaluation of bacterial biomarkers in real-world populations,” she said. In vivo and in vitro experiments are expected to provide mechanistic insights into the causal role of these bacteria and metabolic dysregulations in the pathogenesis of IBD, as well as their future clinical utility in disease monitoring and predicting treatment response.
Her group plans to work with the biotech industry and regulatory agencies to transform these biomarkers into an approved test kit. “The rollout is likely to be gradual, but we’re optimistic that supportive international and national guidelines will be developed and will pave the way for widespread implementation.”
This study was supported by various academic, charitable, and governmental research-funding bodies, including the governments of Hong Kong and the People’s Republic of China. Ng has served as an advisory board member or speaker for Pfizer, Ferring, Janssen, AbbVie, Tillotts, Menarini, and Takeda. She has received research grants through her institutions from Olympus, Ferring, and AbbVie and is a founding member and shareholder of GenieBiome. She receives patent royalties through her institutions, including MagIC, which holds patents on the therapeutic and diagnostic use of the microbiome in IBD. Several co-authors reported various relationships, including patent holding, with private-sector companies. Ananthakrishnan had no relevant competing interests.
A version of this article first appeared on Medscape.com.
FROM NATURE MEDICINE
Digestive Disease Mortality Higher for US Indigenous Communities
which experience the highest death rates and ongoing increases, according to a recent study.
Policymakers, healthcare providers, and communities need to respond with targeted interventions and collaborative efforts that address these inequities and advance digestive health equity, lead author Wafa A. Aldhaleei, MD, of Mayo Clinic, Rochester, Minnesota, and colleagues reported.
“Several studies have reported the epidemiological characteristics of certain digestive diseases such as pancreatitis, liver and biliary diseases, and inflammatory bowel disease,” the investigators wrote in Clinical Gastroenterology and Hepatology. “These studies provide insights into the US burden by sex and racial and ethnic disparities of various digestive diseases individually. However, little is known about racial disparities in the United States digestive diseases mortality burden.”
As part of the Global Burden of Disease Study, the investigators analyzed data from the Institute of Health Metrics and Evaluation Global Health Data Exchange, including age-standardized digestive disease mortality rates for five racial and ethnic groups (Black, White, American Indian and Alaska Native, Asian and Pacific Islander, and Latino) between 2000 and 2019, with further subgroups based on sex, state, and county. Joinpoint regression analysis was employed to determine overall temporal trends by demography.
Results showed striking mortality rate differences across racial and ethnic groups. In 2019, digestive disease mortality rates were highest among American Indian and Alaska Native individuals, reaching 86.2 per 100,000 — over twice the rate seen in White (35.5 per 100,000), Black (33.6 per 100,000), and Latino (33.6 per 100,000) populations, and more than five times higher than in Asian and Pacific Islander individuals (15.6 per 100,000). Over the study period, American Indian and Alaska Native individuals experienced a significant 0.87% average annual increase in mortality rates, while White individuals saw a smaller increase of 0.12% annually. In contrast, Latino, Black, and Asian and Pacific Islander individuals had declining average annual rates.
Geographic disparities in digestive disease mortality were significant, with West Virginia recording the highest state-level rate in 2019 at 44.8 deaths per 100,000, well above the national rate of 34.5 per 100,000. Certain regions with high concentrations of American Indian and Alaska Native populations, such as the Southwest Tribes service area (including Arizona and New Mexico) and the Plain Indians service area (spanning Montana, North Dakota, and South Dakota), reported mortality rates exceeding 70 per 100,000, more than double the national average. In Alaska, the American Indian and Alaska Native population’s mortality rate surged with annual increases of up to 3.53% during some periods.
Analyses also revealed some notable sex-based trends. Among American Indian and Alaska Native individuals, males experienced a mortality rate increase of 0.87% annually, reaching 93.5 per 100,000 by 2019, while females saw an even sharper rise at 1.11% per year, with a mortality rate of 79.6 per 100,000 in 2019. For White individuals, the average annual percentage increase was 0.12% for males, bringing their rate to 40.2 per 100,000, and 0.30% for females, with a rate of 31.0 per 100,000 in 2019.
“Our study reveals persistent racial, ethnic, and geographic disparities in digestive diseases mortality in the United States,” the investigators concluded. “Targeted interventions and further research are needed to address these disparities and promote digestive health equity. Collaboration among researchers, policymakers, healthcare providers, and communities is essential to achieve this goal.”This research was conducted as part of Global Burden of Disease, Injuries and Risk Factors Study, coordinated by the Institute of Health Metrics and Evaluation. The investigators disclosed no conflicts of interest.
which experience the highest death rates and ongoing increases, according to a recent study.
Policymakers, healthcare providers, and communities need to respond with targeted interventions and collaborative efforts that address these inequities and advance digestive health equity, lead author Wafa A. Aldhaleei, MD, of Mayo Clinic, Rochester, Minnesota, and colleagues reported.
“Several studies have reported the epidemiological characteristics of certain digestive diseases such as pancreatitis, liver and biliary diseases, and inflammatory bowel disease,” the investigators wrote in Clinical Gastroenterology and Hepatology. “These studies provide insights into the US burden by sex and racial and ethnic disparities of various digestive diseases individually. However, little is known about racial disparities in the United States digestive diseases mortality burden.”
As part of the Global Burden of Disease Study, the investigators analyzed data from the Institute of Health Metrics and Evaluation Global Health Data Exchange, including age-standardized digestive disease mortality rates for five racial and ethnic groups (Black, White, American Indian and Alaska Native, Asian and Pacific Islander, and Latino) between 2000 and 2019, with further subgroups based on sex, state, and county. Joinpoint regression analysis was employed to determine overall temporal trends by demography.
Results showed striking mortality rate differences across racial and ethnic groups. In 2019, digestive disease mortality rates were highest among American Indian and Alaska Native individuals, reaching 86.2 per 100,000 — over twice the rate seen in White (35.5 per 100,000), Black (33.6 per 100,000), and Latino (33.6 per 100,000) populations, and more than five times higher than in Asian and Pacific Islander individuals (15.6 per 100,000). Over the study period, American Indian and Alaska Native individuals experienced a significant 0.87% average annual increase in mortality rates, while White individuals saw a smaller increase of 0.12% annually. In contrast, Latino, Black, and Asian and Pacific Islander individuals had declining average annual rates.
Geographic disparities in digestive disease mortality were significant, with West Virginia recording the highest state-level rate in 2019 at 44.8 deaths per 100,000, well above the national rate of 34.5 per 100,000. Certain regions with high concentrations of American Indian and Alaska Native populations, such as the Southwest Tribes service area (including Arizona and New Mexico) and the Plain Indians service area (spanning Montana, North Dakota, and South Dakota), reported mortality rates exceeding 70 per 100,000, more than double the national average. In Alaska, the American Indian and Alaska Native population’s mortality rate surged with annual increases of up to 3.53% during some periods.
Analyses also revealed some notable sex-based trends. Among American Indian and Alaska Native individuals, males experienced a mortality rate increase of 0.87% annually, reaching 93.5 per 100,000 by 2019, while females saw an even sharper rise at 1.11% per year, with a mortality rate of 79.6 per 100,000 in 2019. For White individuals, the average annual percentage increase was 0.12% for males, bringing their rate to 40.2 per 100,000, and 0.30% for females, with a rate of 31.0 per 100,000 in 2019.
“Our study reveals persistent racial, ethnic, and geographic disparities in digestive diseases mortality in the United States,” the investigators concluded. “Targeted interventions and further research are needed to address these disparities and promote digestive health equity. Collaboration among researchers, policymakers, healthcare providers, and communities is essential to achieve this goal.”This research was conducted as part of Global Burden of Disease, Injuries and Risk Factors Study, coordinated by the Institute of Health Metrics and Evaluation. The investigators disclosed no conflicts of interest.
which experience the highest death rates and ongoing increases, according to a recent study.
Policymakers, healthcare providers, and communities need to respond with targeted interventions and collaborative efforts that address these inequities and advance digestive health equity, lead author Wafa A. Aldhaleei, MD, of Mayo Clinic, Rochester, Minnesota, and colleagues reported.
“Several studies have reported the epidemiological characteristics of certain digestive diseases such as pancreatitis, liver and biliary diseases, and inflammatory bowel disease,” the investigators wrote in Clinical Gastroenterology and Hepatology. “These studies provide insights into the US burden by sex and racial and ethnic disparities of various digestive diseases individually. However, little is known about racial disparities in the United States digestive diseases mortality burden.”
As part of the Global Burden of Disease Study, the investigators analyzed data from the Institute of Health Metrics and Evaluation Global Health Data Exchange, including age-standardized digestive disease mortality rates for five racial and ethnic groups (Black, White, American Indian and Alaska Native, Asian and Pacific Islander, and Latino) between 2000 and 2019, with further subgroups based on sex, state, and county. Joinpoint regression analysis was employed to determine overall temporal trends by demography.
Results showed striking mortality rate differences across racial and ethnic groups. In 2019, digestive disease mortality rates were highest among American Indian and Alaska Native individuals, reaching 86.2 per 100,000 — over twice the rate seen in White (35.5 per 100,000), Black (33.6 per 100,000), and Latino (33.6 per 100,000) populations, and more than five times higher than in Asian and Pacific Islander individuals (15.6 per 100,000). Over the study period, American Indian and Alaska Native individuals experienced a significant 0.87% average annual increase in mortality rates, while White individuals saw a smaller increase of 0.12% annually. In contrast, Latino, Black, and Asian and Pacific Islander individuals had declining average annual rates.
Geographic disparities in digestive disease mortality were significant, with West Virginia recording the highest state-level rate in 2019 at 44.8 deaths per 100,000, well above the national rate of 34.5 per 100,000. Certain regions with high concentrations of American Indian and Alaska Native populations, such as the Southwest Tribes service area (including Arizona and New Mexico) and the Plain Indians service area (spanning Montana, North Dakota, and South Dakota), reported mortality rates exceeding 70 per 100,000, more than double the national average. In Alaska, the American Indian and Alaska Native population’s mortality rate surged with annual increases of up to 3.53% during some periods.
Analyses also revealed some notable sex-based trends. Among American Indian and Alaska Native individuals, males experienced a mortality rate increase of 0.87% annually, reaching 93.5 per 100,000 by 2019, while females saw an even sharper rise at 1.11% per year, with a mortality rate of 79.6 per 100,000 in 2019. For White individuals, the average annual percentage increase was 0.12% for males, bringing their rate to 40.2 per 100,000, and 0.30% for females, with a rate of 31.0 per 100,000 in 2019.
“Our study reveals persistent racial, ethnic, and geographic disparities in digestive diseases mortality in the United States,” the investigators concluded. “Targeted interventions and further research are needed to address these disparities and promote digestive health equity. Collaboration among researchers, policymakers, healthcare providers, and communities is essential to achieve this goal.”This research was conducted as part of Global Burden of Disease, Injuries and Risk Factors Study, coordinated by the Institute of Health Metrics and Evaluation. The investigators disclosed no conflicts of interest.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Human Milk Boosts Intestinal Growth, Immune Health of Fetal Organoids
These findings suggest an important role for human milk in supporting intestinal health, and may inform strategies for reducing the risk of necrotizing enterocolitis (NEC) in preterm infants, lead author Lauren Smith, MD, of Yale School of Medicine, New Haven, Connecticut, and colleagues, reported.
“Compelling evidence has revealed that the largest risk factor for NEC apart from prematurity is formula feeding, while conversely, parental milk (PM) confers protection, with a 6- to 10-fold lower incidence of NEC among PM-fed infants compared to formula,” the investigators wrote in Gastro Hep Advances. “It is unknown whether this is due to the many known protective factors in PM or as a result of an injurious component present in formula or a combination of both.”
To learn more, the investigators studied organoids cultured in a three-dimensional matrix and exposed to one of four dietary conditions: PM, donor human milk (DHM), standard formula (SF), or extensively hydrolyzed formula (HF). Organoids were grown in growth media supplemented with these diets for 5 days, followed by differentiation media for an additional 5 days. Growth, differentiation, and immune-related factors were analyzed using advanced imaging, RNA sequencing, and cytokine profiling.
The results demonstrated that human milk–fed organoids significantly outperformed formula-fed organoids in several measures. By the fifth day of growth media exposure, organoids supplemented with PM or DHM were larger and exhibited higher rates of proliferation, as evidenced by Ki67 staining. Organoids exposed to SF were the smallest and had the lowest proliferation and highest levels of apoptosis, while HF-fed organoids showed intermediate growth performance.
During the differentiation phase, organoids exposed to human milk developed more complex structures, forming buds with greater length and diameter compared to formula-fed organoids. PM was particularly effective, though DHM also promoted substantial differentiation. RNA sequencing revealed that organoids cultured with human milk upregulated genes involved in fatty acid metabolism and Wnt signaling, which are critical for cellular energy production and epithelial proliferation. In contrast, formula-fed organoids exhibited downregulation of cell-cycle-promoting genes and showed an inflammatory gene signature.
Cytokine profiling further underscored the benefits of human milk. Organoids exposed to PM and DHM secreted higher levels of immune-regulating cytokines, such as thymic stromal lymphopoietin (TSLP) and macrophage colony-stimulating factor (M-CSF). In contrast, formula-fed organoids produced lower levels of these beneficial cytokines and higher levels of pro-inflammatory markers, including interleukin-18 (IL-18).
These findings suggest that human milk supports intestinal growth, differentiation, and immune regulation in ways that formula does not, and the investigators emphasized the importance of identifying specific bioactive factors in human milk.
“If the factors responsible for this effect can be identified, there could be significant clinical value in supplementing these components in DHM and formula to help prevent NEC and foster normal intestinal development in preterm infants,” they concluded.
Future research will aim to isolate and supplement key components of human milk to enhance the nutritional and protective value of donor milk and formula. In addition, the investigators noted the need to explore potential sex-based differences in intestinal development, as the current study used only male-derived samples.The research was supported by the Yale School of Medicine Medical Student Research Fellowship. The investigators disclosed no conflicts of interest.
These findings suggest an important role for human milk in supporting intestinal health, and may inform strategies for reducing the risk of necrotizing enterocolitis (NEC) in preterm infants, lead author Lauren Smith, MD, of Yale School of Medicine, New Haven, Connecticut, and colleagues, reported.
“Compelling evidence has revealed that the largest risk factor for NEC apart from prematurity is formula feeding, while conversely, parental milk (PM) confers protection, with a 6- to 10-fold lower incidence of NEC among PM-fed infants compared to formula,” the investigators wrote in Gastro Hep Advances. “It is unknown whether this is due to the many known protective factors in PM or as a result of an injurious component present in formula or a combination of both.”
To learn more, the investigators studied organoids cultured in a three-dimensional matrix and exposed to one of four dietary conditions: PM, donor human milk (DHM), standard formula (SF), or extensively hydrolyzed formula (HF). Organoids were grown in growth media supplemented with these diets for 5 days, followed by differentiation media for an additional 5 days. Growth, differentiation, and immune-related factors were analyzed using advanced imaging, RNA sequencing, and cytokine profiling.
The results demonstrated that human milk–fed organoids significantly outperformed formula-fed organoids in several measures. By the fifth day of growth media exposure, organoids supplemented with PM or DHM were larger and exhibited higher rates of proliferation, as evidenced by Ki67 staining. Organoids exposed to SF were the smallest and had the lowest proliferation and highest levels of apoptosis, while HF-fed organoids showed intermediate growth performance.
During the differentiation phase, organoids exposed to human milk developed more complex structures, forming buds with greater length and diameter compared to formula-fed organoids. PM was particularly effective, though DHM also promoted substantial differentiation. RNA sequencing revealed that organoids cultured with human milk upregulated genes involved in fatty acid metabolism and Wnt signaling, which are critical for cellular energy production and epithelial proliferation. In contrast, formula-fed organoids exhibited downregulation of cell-cycle-promoting genes and showed an inflammatory gene signature.
Cytokine profiling further underscored the benefits of human milk. Organoids exposed to PM and DHM secreted higher levels of immune-regulating cytokines, such as thymic stromal lymphopoietin (TSLP) and macrophage colony-stimulating factor (M-CSF). In contrast, formula-fed organoids produced lower levels of these beneficial cytokines and higher levels of pro-inflammatory markers, including interleukin-18 (IL-18).
These findings suggest that human milk supports intestinal growth, differentiation, and immune regulation in ways that formula does not, and the investigators emphasized the importance of identifying specific bioactive factors in human milk.
“If the factors responsible for this effect can be identified, there could be significant clinical value in supplementing these components in DHM and formula to help prevent NEC and foster normal intestinal development in preterm infants,” they concluded.
Future research will aim to isolate and supplement key components of human milk to enhance the nutritional and protective value of donor milk and formula. In addition, the investigators noted the need to explore potential sex-based differences in intestinal development, as the current study used only male-derived samples.The research was supported by the Yale School of Medicine Medical Student Research Fellowship. The investigators disclosed no conflicts of interest.
These findings suggest an important role for human milk in supporting intestinal health, and may inform strategies for reducing the risk of necrotizing enterocolitis (NEC) in preterm infants, lead author Lauren Smith, MD, of Yale School of Medicine, New Haven, Connecticut, and colleagues, reported.
“Compelling evidence has revealed that the largest risk factor for NEC apart from prematurity is formula feeding, while conversely, parental milk (PM) confers protection, with a 6- to 10-fold lower incidence of NEC among PM-fed infants compared to formula,” the investigators wrote in Gastro Hep Advances. “It is unknown whether this is due to the many known protective factors in PM or as a result of an injurious component present in formula or a combination of both.”
To learn more, the investigators studied organoids cultured in a three-dimensional matrix and exposed to one of four dietary conditions: PM, donor human milk (DHM), standard formula (SF), or extensively hydrolyzed formula (HF). Organoids were grown in growth media supplemented with these diets for 5 days, followed by differentiation media for an additional 5 days. Growth, differentiation, and immune-related factors were analyzed using advanced imaging, RNA sequencing, and cytokine profiling.
The results demonstrated that human milk–fed organoids significantly outperformed formula-fed organoids in several measures. By the fifth day of growth media exposure, organoids supplemented with PM or DHM were larger and exhibited higher rates of proliferation, as evidenced by Ki67 staining. Organoids exposed to SF were the smallest and had the lowest proliferation and highest levels of apoptosis, while HF-fed organoids showed intermediate growth performance.
During the differentiation phase, organoids exposed to human milk developed more complex structures, forming buds with greater length and diameter compared to formula-fed organoids. PM was particularly effective, though DHM also promoted substantial differentiation. RNA sequencing revealed that organoids cultured with human milk upregulated genes involved in fatty acid metabolism and Wnt signaling, which are critical for cellular energy production and epithelial proliferation. In contrast, formula-fed organoids exhibited downregulation of cell-cycle-promoting genes and showed an inflammatory gene signature.
Cytokine profiling further underscored the benefits of human milk. Organoids exposed to PM and DHM secreted higher levels of immune-regulating cytokines, such as thymic stromal lymphopoietin (TSLP) and macrophage colony-stimulating factor (M-CSF). In contrast, formula-fed organoids produced lower levels of these beneficial cytokines and higher levels of pro-inflammatory markers, including interleukin-18 (IL-18).
These findings suggest that human milk supports intestinal growth, differentiation, and immune regulation in ways that formula does not, and the investigators emphasized the importance of identifying specific bioactive factors in human milk.
“If the factors responsible for this effect can be identified, there could be significant clinical value in supplementing these components in DHM and formula to help prevent NEC and foster normal intestinal development in preterm infants,” they concluded.
Future research will aim to isolate and supplement key components of human milk to enhance the nutritional and protective value of donor milk and formula. In addition, the investigators noted the need to explore potential sex-based differences in intestinal development, as the current study used only male-derived samples.The research was supported by the Yale School of Medicine Medical Student Research Fellowship. The investigators disclosed no conflicts of interest.
FROM GASTRO HEP ADVANCES
AGA Guidelines Endorse Earlier Use of High-Efficacy Drugs for Ulcerative Colitis
In a rapidly expanding therapeutic landscape,
“These are the first living guidelines published by a GI society, highlighting the interest and need to provide timely guidance to all stakeholders in a rapidly evolving field,” first author Siddharth Singh, MD, of the Division of Gastroenterology in the Department of Medicine at University of California, San Diego, said in an interview. Living guidance allows for ongoing revision of individual recommendations as new data emerge. Nearly 2 million Americans have UC.
Issued in Gastroenterology and updating the last guidance in 2020, the recommendations suggest more efficacious drugs should be used sooner. “Early use of advanced therapies including biologics and small-molecule drugs are more effective than 5-aminosalicylates [5-ASAs] or thiopurines and methotrexate for most patients with moderate to severe UC and those with poor prognostic factors,” coauthor and gastroenterologist Manasi Agrawal, MD, MS, an assistant professor of medicine at Icahn School of Medicine at Mount Sinai in New York City, said in an interview.
“We provide a practical guidance based on best-available evidence to make it easy for the treating clinician to make informed choices from the multiplicity of available treatments for UC,” added guidelines coauthor Ashwin Ananthakrishnan, MBBS, MPH, AGAF, a gastroenterologist at Massachusetts General Hospital in Boston.
The comprehensive, patient-centered document comes with this caveat from the AGA panel: “These guidelines are meant to be broad recommendations for management of patients with moderate to severe UC and are not intended to address the intricacies of individual patients,” they wrote. “Provider experience and patient values and preferences can inform treating providers and patients to reasonably choose alternative treatment options.”
One gastroenterologist who has been eagerly awaiting these guidelines but not involved in the panel is James D. Lewis, MD, MSCE, AGAF, a professor of medicine and epidemiology at Perelman School of Medicine at the University of Pennsylvania, Philadelphia. “The choice of medications for moderately to severely active UC has expanded tremendously in the past few years,” he said in an interview. “This resulted in the dismantling of the historical therapeutic pyramid.” And while there are many more treatment options, knowing which medication to use for which patient and in which sequence has become much more complicated.
“These guidelines will be extremely helpful for clinicians trying to navigate this new era of UC care,” he said.
The guidelines also outline implementation considerations for optimal use in different scenarios. “Key considerations include patient-related factors such as age, frailty, other health conditions, consideration for pregnancy, patient preferences, and access to healthcare,” Agrawal said.
Specifics
Overall, the guidance recommends advanced or immunomodulatory therapy after failure of 5-ASAs rather than a step-up approach. Moderate to severe disease is defined as a Mayo endoscopic severity subscore of 2 or 3.
The recommendation may also apply to mild disease in the presence of a high burden of inflammation and a poor prognosis or steroid dependence or resistance.
The AGA guideline panelists took account of differences in treatment efficacy between drugs within the same therapeutic class and made their recommendations by specific drugs rather than therapy class.
Based on varying degrees of evidence certainty, the AGA recommends or suggests the following management specifics in adult outpatients with moderate to severe disease:
- Any of the following is recommended over no treatment: infliximab (Remicade), golimumab (Simponi), vedolizumab (Entyvio), tofacitinib (Xeljanz), upadacitinib (Rinvoq), ustekinumab (Stelara), ozanimod (Zeposia), etrasimod (Velsipity), risankizumab (Skyrizi), and guselkumab (Tremfya).
- Adalimumab (Humira), filgotinib (Jyseleca), and mirikizumab (Omvoh) are suggested over no treatment.
- Biosimilars to infliximab, adalimumab, and ustekinumab can be considered of equivalent efficacy to their originator drugs.
- For patients naive to advanced therapies, the AGA panel proposes using a higher-efficacy medication (eg, infliximab, vedolizumab, ozanimod, etrasimod, upadacitinib, risankizumab, and guselkumab) or an intermediate-efficacy medication (golimumab, ustekinumab, tofacitinib, filgotinib, and mirikizumab) rather than a lower-efficacy medication such as adalimumab.
- In patients previously exposed to advanced therapy, particularly tumor necrosis factor (TNF)–alpha antagonists, the panel suggests using a higher-efficacy medication (tofacitinib, upadacitinib, and ustekinumab) or an intermediate-efficacy agent (filgotinib, mirikizumab, risankizumab, and guselkumab) over a lower-efficacy medication (adalimumab, vedolizumab, ozanimod, and etrasimod).
- The panel suggests against the use of thiopurine monotherapy for inducing remission but suggests thiopurine monotherapy over no treatment for maintenance of (typically corticosteroid-induced) remission.
- The panel suggests against the use of methotrexate monotherapy for induction or maintenance of remission.
- Infliximab, adalimumab, and golimumab in combination with an immunomodulator are suggested over monotherapy.
- The panel makes no recommendation for or against non-TNF antagonist biologics in combination with an immunomodulator over non-TNF biologics alone.
- For patients in corticosteroid-free clinical remission for at least 6 months on combination therapy with TNF antagonists and immunomodulators, the panel suggests against withdrawing TNF antagonists but makes no recommendation for or against withdrawing immunomodulators.
- For those who have failed 5-ASAs and have escalated to immunomodulators or advanced therapies, the panel suggests stopping these agents. It suggests the early use of advanced therapies and/or immunomodulator therapy rather than gradual step-up after failure of 5-ASAs.
According to Lewis, the guidance will be useful to both community physicians and highly specialized gastroenterologists. “While few practicing physicians will be able to commit the entirety of the classifications in this guideline to memory, the tool is a quick reference resource to help providers and patients to choose between the many options,” he said.
However, he noted that not all patients and providers may have the same priorities as the guidelines. “There are a few nuances to the methods of the AGA guidelines. For example, the panel prioritized efficacy over safety because the incidence of serious adverse events secondary to medications is relatively rare.”
Lewis also noted that the way the panel classified higher-, intermediate-, and lower-efficacy medications sometimes produced surprising results. “For example, among patients naive to advanced therapies, the IL [interleukin]–23 inhibitors risankizumab and guselkumab were classified as higher efficacy, while the IL-12/23 inhibitor ustekinumab was considered intermediate efficacy,” he said. “These were reversed for patients with prior exposure to advanced therapies, where ustekinumab was considered higher efficacy and all three IL-23 inhibitors were considered intermediate efficacy.”
The Future
The panel identified several knowledge gaps that future studies should address. These include a paucity of head-to-head comparison trials, including active comparators to accurately inform positioning of different treatments and therapeutic mechanisms.
The panelists also noted a literature gap on the efficacy of different therapies in the setting of failure or intolerance to non-TNF antagonist advanced therapy, which could be relevant to drugs that may have a greater overlap in their therapeutic mechanisms — for instance, anti-trafficking agents.
They pointed to a paucity of data on how predictive models can inform future treatment selection in the real-world setting. “There is clearly a need for identifying biomarkers predictive of response to individual therapies, to facilitate optimal choice of therapies,” they wrote.
The panel also recognized that novel therapeutic strategies may soon be in use, including combination advanced therapy or episodic use of nonimmunogenic advanced therapies such as small molecules. “Further primary data are required to accurately inform the positioning of such strategies,” they wrote.
These guidelines were fully funded by the AGA Institute. Singh and Agrawal are supported by the National Institute of Diabetes and Digestive and Kidney Disease (NIDDK), and Ananthakrishnan is supported by the NIDDK, as well as by the Leona M. and Harry B. Helmsley Charitable Trust and the Chleck Family Foundation. Singh disclosed Institutional research grants from Pfizer. Agrawal reported consulting for Douglas Pharmaceuticals. Several coauthors disclosed receiving consulting fees and/or research support from various private companies in the healthcare field. One author reported stock ownership stock in Exact Sciences. Lewis reported consulting, advisory board service, or data monitoring for Amgen, Arena Pharmaceuticals, Bristol Myers Squibb, Celgene, Eli Lilly and Company, Galapagos, Gilead, Janssen Pharmaceuticals, Merck, Pfizer, Protagonist Therapeutics, and Sanofi. He received research funding or in-kind support from Nestle Health Science, Takeda, Janssen Pharmaceuticals, AbbVie, and Eli Lilly and has had educational grants from Janssen.
A version of this article appeared on Medscape.com.
In a rapidly expanding therapeutic landscape,
“These are the first living guidelines published by a GI society, highlighting the interest and need to provide timely guidance to all stakeholders in a rapidly evolving field,” first author Siddharth Singh, MD, of the Division of Gastroenterology in the Department of Medicine at University of California, San Diego, said in an interview. Living guidance allows for ongoing revision of individual recommendations as new data emerge. Nearly 2 million Americans have UC.
Issued in Gastroenterology and updating the last guidance in 2020, the recommendations suggest more efficacious drugs should be used sooner. “Early use of advanced therapies including biologics and small-molecule drugs are more effective than 5-aminosalicylates [5-ASAs] or thiopurines and methotrexate for most patients with moderate to severe UC and those with poor prognostic factors,” coauthor and gastroenterologist Manasi Agrawal, MD, MS, an assistant professor of medicine at Icahn School of Medicine at Mount Sinai in New York City, said in an interview.
“We provide a practical guidance based on best-available evidence to make it easy for the treating clinician to make informed choices from the multiplicity of available treatments for UC,” added guidelines coauthor Ashwin Ananthakrishnan, MBBS, MPH, AGAF, a gastroenterologist at Massachusetts General Hospital in Boston.
The comprehensive, patient-centered document comes with this caveat from the AGA panel: “These guidelines are meant to be broad recommendations for management of patients with moderate to severe UC and are not intended to address the intricacies of individual patients,” they wrote. “Provider experience and patient values and preferences can inform treating providers and patients to reasonably choose alternative treatment options.”
One gastroenterologist who has been eagerly awaiting these guidelines but not involved in the panel is James D. Lewis, MD, MSCE, AGAF, a professor of medicine and epidemiology at Perelman School of Medicine at the University of Pennsylvania, Philadelphia. “The choice of medications for moderately to severely active UC has expanded tremendously in the past few years,” he said in an interview. “This resulted in the dismantling of the historical therapeutic pyramid.” And while there are many more treatment options, knowing which medication to use for which patient and in which sequence has become much more complicated.
“These guidelines will be extremely helpful for clinicians trying to navigate this new era of UC care,” he said.
The guidelines also outline implementation considerations for optimal use in different scenarios. “Key considerations include patient-related factors such as age, frailty, other health conditions, consideration for pregnancy, patient preferences, and access to healthcare,” Agrawal said.
Specifics
Overall, the guidance recommends advanced or immunomodulatory therapy after failure of 5-ASAs rather than a step-up approach. Moderate to severe disease is defined as a Mayo endoscopic severity subscore of 2 or 3.
The recommendation may also apply to mild disease in the presence of a high burden of inflammation and a poor prognosis or steroid dependence or resistance.
The AGA guideline panelists took account of differences in treatment efficacy between drugs within the same therapeutic class and made their recommendations by specific drugs rather than therapy class.
Based on varying degrees of evidence certainty, the AGA recommends or suggests the following management specifics in adult outpatients with moderate to severe disease:
- Any of the following is recommended over no treatment: infliximab (Remicade), golimumab (Simponi), vedolizumab (Entyvio), tofacitinib (Xeljanz), upadacitinib (Rinvoq), ustekinumab (Stelara), ozanimod (Zeposia), etrasimod (Velsipity), risankizumab (Skyrizi), and guselkumab (Tremfya).
- Adalimumab (Humira), filgotinib (Jyseleca), and mirikizumab (Omvoh) are suggested over no treatment.
- Biosimilars to infliximab, adalimumab, and ustekinumab can be considered of equivalent efficacy to their originator drugs.
- For patients naive to advanced therapies, the AGA panel proposes using a higher-efficacy medication (eg, infliximab, vedolizumab, ozanimod, etrasimod, upadacitinib, risankizumab, and guselkumab) or an intermediate-efficacy medication (golimumab, ustekinumab, tofacitinib, filgotinib, and mirikizumab) rather than a lower-efficacy medication such as adalimumab.
- In patients previously exposed to advanced therapy, particularly tumor necrosis factor (TNF)–alpha antagonists, the panel suggests using a higher-efficacy medication (tofacitinib, upadacitinib, and ustekinumab) or an intermediate-efficacy agent (filgotinib, mirikizumab, risankizumab, and guselkumab) over a lower-efficacy medication (adalimumab, vedolizumab, ozanimod, and etrasimod).
- The panel suggests against the use of thiopurine monotherapy for inducing remission but suggests thiopurine monotherapy over no treatment for maintenance of (typically corticosteroid-induced) remission.
- The panel suggests against the use of methotrexate monotherapy for induction or maintenance of remission.
- Infliximab, adalimumab, and golimumab in combination with an immunomodulator are suggested over monotherapy.
- The panel makes no recommendation for or against non-TNF antagonist biologics in combination with an immunomodulator over non-TNF biologics alone.
- For patients in corticosteroid-free clinical remission for at least 6 months on combination therapy with TNF antagonists and immunomodulators, the panel suggests against withdrawing TNF antagonists but makes no recommendation for or against withdrawing immunomodulators.
- For those who have failed 5-ASAs and have escalated to immunomodulators or advanced therapies, the panel suggests stopping these agents. It suggests the early use of advanced therapies and/or immunomodulator therapy rather than gradual step-up after failure of 5-ASAs.
According to Lewis, the guidance will be useful to both community physicians and highly specialized gastroenterologists. “While few practicing physicians will be able to commit the entirety of the classifications in this guideline to memory, the tool is a quick reference resource to help providers and patients to choose between the many options,” he said.
However, he noted that not all patients and providers may have the same priorities as the guidelines. “There are a few nuances to the methods of the AGA guidelines. For example, the panel prioritized efficacy over safety because the incidence of serious adverse events secondary to medications is relatively rare.”
Lewis also noted that the way the panel classified higher-, intermediate-, and lower-efficacy medications sometimes produced surprising results. “For example, among patients naive to advanced therapies, the IL [interleukin]–23 inhibitors risankizumab and guselkumab were classified as higher efficacy, while the IL-12/23 inhibitor ustekinumab was considered intermediate efficacy,” he said. “These were reversed for patients with prior exposure to advanced therapies, where ustekinumab was considered higher efficacy and all three IL-23 inhibitors were considered intermediate efficacy.”
The Future
The panel identified several knowledge gaps that future studies should address. These include a paucity of head-to-head comparison trials, including active comparators to accurately inform positioning of different treatments and therapeutic mechanisms.
The panelists also noted a literature gap on the efficacy of different therapies in the setting of failure or intolerance to non-TNF antagonist advanced therapy, which could be relevant to drugs that may have a greater overlap in their therapeutic mechanisms — for instance, anti-trafficking agents.
They pointed to a paucity of data on how predictive models can inform future treatment selection in the real-world setting. “There is clearly a need for identifying biomarkers predictive of response to individual therapies, to facilitate optimal choice of therapies,” they wrote.
The panel also recognized that novel therapeutic strategies may soon be in use, including combination advanced therapy or episodic use of nonimmunogenic advanced therapies such as small molecules. “Further primary data are required to accurately inform the positioning of such strategies,” they wrote.
These guidelines were fully funded by the AGA Institute. Singh and Agrawal are supported by the National Institute of Diabetes and Digestive and Kidney Disease (NIDDK), and Ananthakrishnan is supported by the NIDDK, as well as by the Leona M. and Harry B. Helmsley Charitable Trust and the Chleck Family Foundation. Singh disclosed Institutional research grants from Pfizer. Agrawal reported consulting for Douglas Pharmaceuticals. Several coauthors disclosed receiving consulting fees and/or research support from various private companies in the healthcare field. One author reported stock ownership stock in Exact Sciences. Lewis reported consulting, advisory board service, or data monitoring for Amgen, Arena Pharmaceuticals, Bristol Myers Squibb, Celgene, Eli Lilly and Company, Galapagos, Gilead, Janssen Pharmaceuticals, Merck, Pfizer, Protagonist Therapeutics, and Sanofi. He received research funding or in-kind support from Nestle Health Science, Takeda, Janssen Pharmaceuticals, AbbVie, and Eli Lilly and has had educational grants from Janssen.
A version of this article appeared on Medscape.com.
In a rapidly expanding therapeutic landscape,
“These are the first living guidelines published by a GI society, highlighting the interest and need to provide timely guidance to all stakeholders in a rapidly evolving field,” first author Siddharth Singh, MD, of the Division of Gastroenterology in the Department of Medicine at University of California, San Diego, said in an interview. Living guidance allows for ongoing revision of individual recommendations as new data emerge. Nearly 2 million Americans have UC.
Issued in Gastroenterology and updating the last guidance in 2020, the recommendations suggest more efficacious drugs should be used sooner. “Early use of advanced therapies including biologics and small-molecule drugs are more effective than 5-aminosalicylates [5-ASAs] or thiopurines and methotrexate for most patients with moderate to severe UC and those with poor prognostic factors,” coauthor and gastroenterologist Manasi Agrawal, MD, MS, an assistant professor of medicine at Icahn School of Medicine at Mount Sinai in New York City, said in an interview.
“We provide a practical guidance based on best-available evidence to make it easy for the treating clinician to make informed choices from the multiplicity of available treatments for UC,” added guidelines coauthor Ashwin Ananthakrishnan, MBBS, MPH, AGAF, a gastroenterologist at Massachusetts General Hospital in Boston.
The comprehensive, patient-centered document comes with this caveat from the AGA panel: “These guidelines are meant to be broad recommendations for management of patients with moderate to severe UC and are not intended to address the intricacies of individual patients,” they wrote. “Provider experience and patient values and preferences can inform treating providers and patients to reasonably choose alternative treatment options.”
One gastroenterologist who has been eagerly awaiting these guidelines but not involved in the panel is James D. Lewis, MD, MSCE, AGAF, a professor of medicine and epidemiology at Perelman School of Medicine at the University of Pennsylvania, Philadelphia. “The choice of medications for moderately to severely active UC has expanded tremendously in the past few years,” he said in an interview. “This resulted in the dismantling of the historical therapeutic pyramid.” And while there are many more treatment options, knowing which medication to use for which patient and in which sequence has become much more complicated.
“These guidelines will be extremely helpful for clinicians trying to navigate this new era of UC care,” he said.
The guidelines also outline implementation considerations for optimal use in different scenarios. “Key considerations include patient-related factors such as age, frailty, other health conditions, consideration for pregnancy, patient preferences, and access to healthcare,” Agrawal said.
Specifics
Overall, the guidance recommends advanced or immunomodulatory therapy after failure of 5-ASAs rather than a step-up approach. Moderate to severe disease is defined as a Mayo endoscopic severity subscore of 2 or 3.
The recommendation may also apply to mild disease in the presence of a high burden of inflammation and a poor prognosis or steroid dependence or resistance.
The AGA guideline panelists took account of differences in treatment efficacy between drugs within the same therapeutic class and made their recommendations by specific drugs rather than therapy class.
Based on varying degrees of evidence certainty, the AGA recommends or suggests the following management specifics in adult outpatients with moderate to severe disease:
- Any of the following is recommended over no treatment: infliximab (Remicade), golimumab (Simponi), vedolizumab (Entyvio), tofacitinib (Xeljanz), upadacitinib (Rinvoq), ustekinumab (Stelara), ozanimod (Zeposia), etrasimod (Velsipity), risankizumab (Skyrizi), and guselkumab (Tremfya).
- Adalimumab (Humira), filgotinib (Jyseleca), and mirikizumab (Omvoh) are suggested over no treatment.
- Biosimilars to infliximab, adalimumab, and ustekinumab can be considered of equivalent efficacy to their originator drugs.
- For patients naive to advanced therapies, the AGA panel proposes using a higher-efficacy medication (eg, infliximab, vedolizumab, ozanimod, etrasimod, upadacitinib, risankizumab, and guselkumab) or an intermediate-efficacy medication (golimumab, ustekinumab, tofacitinib, filgotinib, and mirikizumab) rather than a lower-efficacy medication such as adalimumab.
- In patients previously exposed to advanced therapy, particularly tumor necrosis factor (TNF)–alpha antagonists, the panel suggests using a higher-efficacy medication (tofacitinib, upadacitinib, and ustekinumab) or an intermediate-efficacy agent (filgotinib, mirikizumab, risankizumab, and guselkumab) over a lower-efficacy medication (adalimumab, vedolizumab, ozanimod, and etrasimod).
- The panel suggests against the use of thiopurine monotherapy for inducing remission but suggests thiopurine monotherapy over no treatment for maintenance of (typically corticosteroid-induced) remission.
- The panel suggests against the use of methotrexate monotherapy for induction or maintenance of remission.
- Infliximab, adalimumab, and golimumab in combination with an immunomodulator are suggested over monotherapy.
- The panel makes no recommendation for or against non-TNF antagonist biologics in combination with an immunomodulator over non-TNF biologics alone.
- For patients in corticosteroid-free clinical remission for at least 6 months on combination therapy with TNF antagonists and immunomodulators, the panel suggests against withdrawing TNF antagonists but makes no recommendation for or against withdrawing immunomodulators.
- For those who have failed 5-ASAs and have escalated to immunomodulators or advanced therapies, the panel suggests stopping these agents. It suggests the early use of advanced therapies and/or immunomodulator therapy rather than gradual step-up after failure of 5-ASAs.
According to Lewis, the guidance will be useful to both community physicians and highly specialized gastroenterologists. “While few practicing physicians will be able to commit the entirety of the classifications in this guideline to memory, the tool is a quick reference resource to help providers and patients to choose between the many options,” he said.
However, he noted that not all patients and providers may have the same priorities as the guidelines. “There are a few nuances to the methods of the AGA guidelines. For example, the panel prioritized efficacy over safety because the incidence of serious adverse events secondary to medications is relatively rare.”
Lewis also noted that the way the panel classified higher-, intermediate-, and lower-efficacy medications sometimes produced surprising results. “For example, among patients naive to advanced therapies, the IL [interleukin]–23 inhibitors risankizumab and guselkumab were classified as higher efficacy, while the IL-12/23 inhibitor ustekinumab was considered intermediate efficacy,” he said. “These were reversed for patients with prior exposure to advanced therapies, where ustekinumab was considered higher efficacy and all three IL-23 inhibitors were considered intermediate efficacy.”
The Future
The panel identified several knowledge gaps that future studies should address. These include a paucity of head-to-head comparison trials, including active comparators to accurately inform positioning of different treatments and therapeutic mechanisms.
The panelists also noted a literature gap on the efficacy of different therapies in the setting of failure or intolerance to non-TNF antagonist advanced therapy, which could be relevant to drugs that may have a greater overlap in their therapeutic mechanisms — for instance, anti-trafficking agents.
They pointed to a paucity of data on how predictive models can inform future treatment selection in the real-world setting. “There is clearly a need for identifying biomarkers predictive of response to individual therapies, to facilitate optimal choice of therapies,” they wrote.
The panel also recognized that novel therapeutic strategies may soon be in use, including combination advanced therapy or episodic use of nonimmunogenic advanced therapies such as small molecules. “Further primary data are required to accurately inform the positioning of such strategies,” they wrote.
These guidelines were fully funded by the AGA Institute. Singh and Agrawal are supported by the National Institute of Diabetes and Digestive and Kidney Disease (NIDDK), and Ananthakrishnan is supported by the NIDDK, as well as by the Leona M. and Harry B. Helmsley Charitable Trust and the Chleck Family Foundation. Singh disclosed Institutional research grants from Pfizer. Agrawal reported consulting for Douglas Pharmaceuticals. Several coauthors disclosed receiving consulting fees and/or research support from various private companies in the healthcare field. One author reported stock ownership stock in Exact Sciences. Lewis reported consulting, advisory board service, or data monitoring for Amgen, Arena Pharmaceuticals, Bristol Myers Squibb, Celgene, Eli Lilly and Company, Galapagos, Gilead, Janssen Pharmaceuticals, Merck, Pfizer, Protagonist Therapeutics, and Sanofi. He received research funding or in-kind support from Nestle Health Science, Takeda, Janssen Pharmaceuticals, AbbVie, and Eli Lilly and has had educational grants from Janssen.
A version of this article appeared on Medscape.com.
FROM GASTROENTEROLOGY













