User login
Effect of Hospital Readmission Reduction Program on Hospital Readmissions and Mortality Rates
Chronic obstructive pulmonary disease (COPD) is recognized as the third leading cause of death nationally. Globally, it has been estimated that 10% of the population has COPD; in the United States, approximately 15 million people are affected.1,2 The annual estimated cost of COPD management in the United States is approximately $50 billion, one-third of which is directly related to inpatient hospitalization for COPD exacerbation.3,4,5 The 30-day readmission rate after hospitalization for acute exacerbation of COPD (AECOPD) is approximately 21% with an approximate cost of $13 billion per year.6,7 To reduce the cost and to improve patient outcomes, the Centers for Medicare and Medicaid Services (CMS) has designed several interventions with little effect.8
In October 2012, the Affordable Care Act added section 1886(q) to the Social Security Act and established the Hospital Readmission Reduction Program (HRRP), an initiative to decrease hospitalization costs by penalizing hospitals with high 30-day readmission rates. Under this program, hospitals received up to 3% penalty for excess readmissions after the index hospitalization with acute myocardial infarction (AMI), heart failure (HF), and pneumonia.9-11 Hospitals are penalized if their annual readmission rates are significantly above the average national readmission rate. In 2014, the HRRP was extended to include AECOPD for the FY 2015.
Since the implementation of readmission penalties, data have shown a significant decrease in the 30-day readmission rates for all conditions.12,13 On the other hand, studies have suggested that, at least for some conditions, the decrease in the 30-day readmission rate is associated with higher adverse patients outcomes, including higher mortality.14,15 However, whether a decrease in readmission rates after an AECOPD hospitalization is associated with a concomitant increase in mortality has not been examined. Therefore, our objective was to examine the association of the 30-day risk-adjusted hospital readmission rate with the 30-day risk-adjusted hospital mortality rate for patients discharged with a diagnosis of AECOPD.
METHOD
Data Sources
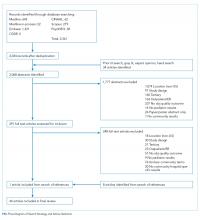
Publicly available data from three sources were used. The all-cause 30-day risk-standardized readmission rate (RSRR) and the 30-day risk-standardized mortality rate (RSMR) of each hospital for patients with AECOPD were obtained from the Hospital Compare database; a database maintained by the CMS.16,17 In 2014, the CMS started reporting three-year running average of 30-day mortality and readmission rate data on hospitals for AECOPD hospitalizations; the data start date was July 2010.18-22 We examined data from the FY 2010-2013 to 2014-2017 cycles on readmission and mortality reported by the CMS; this included data before and after the implementation of penalties.
Hospital characteristics were also obtained from the CMS website. Hospital ownership was defined as government (owned by Federal or state), for-profit (owned by physicians or another proprietary), or nonprofit (owned by a nonprofit organization such as a church). A hospital was considered as a teaching hospital if it obtained graduate medical education funding from the CMS.
Data on local population characteristics according to ZIP codes were obtained from the 2010 decennial census and the American Community Survey five-year (2009-2013) data files available at the United States Census Bureau website.23 For each ZIP code, we obtained data on the total population, percentage of African Americans in the population, median income, poverty level, and insurance status.
We used Hospital service area (HSA) information obtained from the Dartmouth Atlas of Health Care crosswalk files to link local population characteristics to hospitals. The Dartmouth Atlas defined 3,436 HSAs by assigning the ZIP codes to the hospital area where the greatest proportion of their Medicare residents was hospitalized.24,25
Hospital Compare data and Census Bureau population data were matched to the HSAs from the Dartmouth Atlas of Healthcare data at the ZIP code level. First, the ZIP code-level data from the Census Bureau were pooled by the HSAs obtained from the Dartmouth Atlas of Healthcare, followed by matching these data by the HSAs to the Hospital Compare data. Merging data from these three sources generated a dataset that contained information about readmission and mortality rates from a particular hospital and the population characteristics of the local healthcare market or neighborhood. Our final dataset included hospitals that had readmission and mortality information available at the Hospital Compare website and were included in the crosswalk files of the Dartmouth Atlas of Healthcare.
Statistical Analysis
Data are summarized as mean and standard deviation (SD), median with interquartile range, or frequencies as appropriate. To model the dependence of observations from the same hospital over time, we used mixed linear models with random intercept and slope. A strength of this modeling approach is that it incorporates information from all hospitals even when some hospitals are missing data for some time periods. We reached our final model through stages with increasing model complexity at each stage. In the first stage, we developed an empty model without any covariates to determine the unconditional variance components so that we can partition mortality variance into between- and within-hospital components. In the second stage, we developed an unconditional growth curve model to determine the shape of time trend in mortality over time using linear and quadratic (by including squared time in the model) growth curves. In the third stage, we added baseline readmission rates (from 2010 to 2013) to the model to determine the effect of baseline readmission rate on mortality trends and also examined its interaction with time and squared time. We generated a change in the readmission rate variable by subtracting the last readmission rate from the baseline readmission rate (readmission rate in 2010-2013 − readmission rate in 2014-2017). In the fourth stage, we included this change in readmission rate into the third-stage model to examine how changes in the readmission rate affected the time trends of mortality and also examined its interaction with time and squared time. In the final model, we included the following potential confounding variables to the fourth stage model: African American percentage in the HSA, HSA median income, percentage of people living in poverty in the HSA, median age, ownership of hospital (government, for profit), teaching status (teaching vs nonteaching), and acute care hospital beds in the HSA. Within each stage, the models were compared using the Akaike information criterion (AIC) and the Bayesian information criterion (BIC), and the model with the lowest value of each was moved to the next stage of model development. All analyses were performed in Stata 14.1 for Windows (College Station, Texas).
RESULTS
Of the 3,685 acute care hospitals analyzed in the 2010-2013 data cycle for COPD, the 30-day RSRR was 20.7% (1.28), which decreased to 19.6% (1.11) in 2014-2017 (Table 1). During the same period, the 30-day all-cause RSMR increased from 7.8% (1.03) in 2010-2013 to 8.4% (1.11) in 2014-2017. The partitioning of variance showed that 57% of variation in the mortality rate over the study period was due to between-hospital differences.
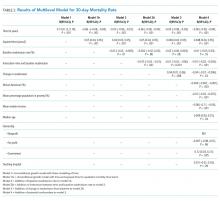
The unconditional growth model examining the linear time trend revealed a 0.13% per year (95% CI = 0.12 to 0.14; P < .0001) increase in mortality rate over the five data cycles. When the squared time variable was added to the model to examine a quadratic trend, both time and squared trend were statistically significant (Table 2) and the AIC and BIC were lower for the quadratic model. Thus, the unconditional growth curve model suggested that the mortality trend was nonlinear and the coefficients demonstrated that not only the mortality rate increased, but the rate of change in the mortality rate was also increasing during the study period.
When we added the baseline readmission rate to the abovementioned quadratic growth model, we found an inverse association; each 1% increase in baseline readmission rate was associated with 0.03% (95% CI = −0.05 to −0.005; P = .02) decrease in mortality rate. These findings suggest that hospitals with higher baseline readmission rates also had lower mortality rates. To examine whether the effect of baseline readmission rate on mortality varied over time, we included the interaction term with time in the model and then added the interaction term with squared time. As the AIC and BIC were the lowest for the model with interaction between time and baseline readmission (and not when interaction between squared time and baseline readmission were included), we accepted this model. In this model, although there was no difference in mortality according to readmissions at baseline, each 1% increase in baseline readmission rate was associated with a smaller increase in mortality rate by 0.015% (95% CI = −0.02 to −0.01; P < .0001; Table 2 and Figure 1). These findings suggest that hospitals with higher readmission rates at baseline had a smaller increase in mortality rate during the study period than those with lower readmission rates.
Inclusion of change in the readmissions variable in the model showed that each 1% decrease in readmission rate during the study period was associated with 0.04% (95% CI = 0.01 to 0.06; P = .008) increase in mortality. However, the interaction between change in readmission and time was not significant and the AIC and BIC of the model were higher than the model without interaction. Therefore, we retained the model without the interaction term and included other potential confounding variables to build our final model. Thus, although hospitals with different baseline readmission rates had different rates of change in mortality rate, the change in readmission rate had a consistent effect on the mortality rate. Including potential confounders in the model did not change the results; the mortality rate and the change in the mortality rate increased during the study period, a high baseline readmission rate was associated with a lower yearly increase in mortality, and a larger decrease in readmission rate was associated with a higher mortality rate (Table 2).
DISCUSSION
As efforts to decrease readmission rates continue as a part of the HRRP implementation by the CMS, our study shows that among hospitals that discharged patients with AECOPD during 2010-2017, the all-cause 30-day RSRR was decreased, whereas the all-cause 30-day RSMR was increased. Of particular concern is that the rate of increase in mortality also increased. We also found that hospitals with higher readmission rates in 2010-2013 had a lower rate of increase in mortality than hospitals with lower readmission rates. In addition, hospitals that had a larger decrease in readmission rates during the study period had a larger rate of increase in mortality than hospitals with a smaller decrease in readmission rates. Our findings were robust to potential confounders such as hospital characteristics and local population characteristics in which hospitals operate.
Our study findings raise the question whether the implementation of the HRRP resulted in unintentional patient harm by forcing hospitals to make changes that may affect overall patient care. This question is particularly important as other studies on hospitalized patients with HF have found similar results.13,14 On the other hand, a similar association between readmission and mortality rates has not been observed in patients with pneumonia or AMI.14 Several possible explanations can be given for the observed discrepancy between the diseases and their effect on the relationship between readmission rate and mortality rate. Both COPD and HF are chronic diseases and characterized by exacerbations, whereas AMI and pneumonia are episodic diseases that are treatable. As the number of patients hospitalized with AECOPD and HF is much larger, hospitals may have a greater focus on reducing the 30-day readmission rates and may attempt to game the process, such as by delaying admissions through the emergency department within the 30-day period or by admitting patients for observation. In fact, a study found a 3% reduction in the within-hospital readmission rate with a concurrent 0.8% increase in observation unit use since the implementation of the HRRP.26 Such approaches to patient care may lead to adverse outcomes.
It is possible that readmissions and mortality act as competing risks and hence hospitals with higher mortality rates are left with fewer patients and thus have fewer readmissions, whereas those with lower mortality rates have more patients and a higher readmission rate.27 Such studies are not possible with hospital-level data, and patient-level studies will be required to examine this competing risk hypothesis. Our study results provide some support to the competing risk hypothesis (hospitals with lower baseline readmission rates had a steeper increase in mortality); however, it is not possible to draw any conclusions due to the high risk of ecological fallacy bias.
This study has important potential implications for healthcare policy, public health, and research. We found that an important national intervention aimed at decreasing readmission rates and improving the quality of care for patients with AECOPD may be associated with higher mortality rates in these patients. There may be a need to redefine measures for determining the performance of an institution. Our study supports research into the underlying mechanisms resulting in an inverse association between readmissions and mortality. In particular, health policy researchers may need to examine how incentives and penalties affect the allocation of resources within hospitals.
This study has several strengths and some potential weaknesses. We used a national dataset to examine readmission and mortality rates that include the majority of hospitals in the United States. We also included data from the local population for each hospital, thus allowing us to examine hospital performance within the context of its target population. One potential limitation is that we used hospital-level data and not patient-level data; however, the readmission penalties are designed for hospitals, which justifies our use of hospital-level data. Furthermore, data were not available for shorter time intervals; data from shorter time intervals may be associated with greater variability. Being an observational study, it is difficult to establish a causal relationship; the longitudinal nature of the study does establish temporality, an important factor in establishing causality.
In conclusion, we found that although the readmission rates decreased, there was an increase in the mortality rate within the 30 days of discharge from the hospital in patients with AECOPD. The rate of increase in mortality was higher in hospitals with lower readmission rates than in hospitals with higher readmission rates. Further research for determining the mechanism responsible for this association is needed. Future health policy interventions may need to consider the potential for adverse outcomes.
1. Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2010. Natl Vital Stat Rep. 2013;61(4):1-117.
2. Halbert RJ, Natoli JL, Gano A, et al. Global burden of copd: systematic review and meta-analysis. Eur Respir J. 2006;28(3):523-532. https://doi.org/10.1183/09031936.06.00124605.
3. Toy EL, Gallagher KF, Stanley EL, Swensen AR, Duh MS. The economic impact of exacerbations of chronic obstructive pulmonary disease and exacerbation definition: a review. COPD. 2010;7(3):214-228. https://doi.org/10.3109/15412555.2010.481697.
4. Shah T, Churpek MM, Coca Perraillon M, Konetzka RT. Understanding why patients with COPD get readmitted: a large national study to delineate the Medicare population for the readmissions penalty expansion. Chest. 2015;147(5):1219-1226. https://doi.org/10.1378/chest.14-2181.
5. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428. https://doi.org/10.1056/NEJMsa0803563.
6. Stein BD, Charbeneau JT, Lee TA, et al. Hospitalizations for acute exacerbations of chronic obstructive pulmonary disease: how you count matters. COPD. 2010;7(3):164-171. https://doi.org/10.3109/15412555.2010.481696.
7. Stein, B. D., Charbeneau, J. T., Lee, T. A., Schumock, G. T., Lindenauer, P. K., Bautista, A., . . . Krishnan, J. A. (2010). Hospitalizations for acute exacerbations of chronic obstructive pulmonary disease: how you count matters. COPD, 7(3), 164-171. doi:10.3109/15412555.2010.481696
8. McIlvennan CK, Eapen ZJ, Allen LA. Hospital readmissions reduction program. Circulation. 2015;131(20):1796-1803. https://doi.org/10.1161/CIRCULATIONAHA.114.010270.
9. McIlvennan CK, Eapen ZJ, Allen LA. Hospital readmissions reduction program. Circulation. 2015;131(20):179-1803. https://doi.org/10.1161/CIRCULATIONAHA.114.010270.
10. Centers for Medicare and Medicaid Services (CMS), HHS. Medicare Program; hospital inpatient prospective payment systems for acute care hospitals and the long-term care hospital prospective payment system and FY 2012 rates; Hospitals’ FTE Resident Caps for Graduate Medical Education Payment. Final Rules. Fed Regist. 2011;76(160):51476-51846.
11. Centers for Medicare and Medicaid Services (CMS). Medicare program; hospital inpatient prospective payment systems for acute care hospitals and the long-term care hospital prospective payment system and fiscal year 2014 rates; quality reporting requirements for specific providers; hospital conditions of participation; payment policies related to patient status. Final rules. Fed Regist. 2013;78(160):50495-51040.
12. Casillas G. Published: Mar 10 and 2017, “aiming for fewer hospital U-turns: the Medicare Hospital readmission reduction program,” [blog]. https://www.kff.org/medicare/issue-brief/aiming-for-fewer-hospital-u-turns-the-medicare-hospital-readmission-reduction-program/; Accessed March 10, 2017. The Henry J. Kaiser Family Foundation.
13. Desai NR, Ross JS, Kwon JY, et al. Association Between hospital penalty status Under the hospital readmission reduction program and readmission rates for target and nontarget conditions. JAMA. 2016;316(24): 2647-2656. https://doi.org/10.1001/jama.2016.18533.
14. Gupta A, Allen LA, Bhatt DL, et al. Association of the hospital readmissions reduction program implementation with readmission and mortality outcomes in heart failure. JAMA Cardiol. 2018;3(1):44-53. https://doi.org/10.1001/jamacardio.2017.4265.
15. Krumholz HM, Lin Z, Keenan PS, et al. Relationship between hospital readmission and mortality rates for patients hospitalized with acute myocardial infarction, heart failure, or pneumonia. JAMA. 2013;309(6):587-593. https://doi.org/10.1001/jama.2013.333.
16. Medicare Hospital compare overview,” https://www.medicare.gov/hospitalcompare/About/What-Is-HOS.html; Accessed April 17, 2019.
17. Archived datasets. Data.Medicare.Gov. Data.Medicare.Gov. Accessed April 17, 2019. https://data.medicare.gov/data/archives/hospital-compare.
18. Krumholz HM, Lin Z, Drye EE, et al. An administrative claims measure suitable for profiling hospital performance based on 30-day all-cause readmission rates among patients with acute myocardial infarction. Circ Cardiovasc Qual Outcomes. 2011;4(2):243-252. https://doi.org/10.1161/CIRCOUTCOMES.110.957498.
19. Bratzler DW, Normand SL, Wang Y, et al. An administrative claims model for profiling hospital 30-day mortality rates for pneumonia patients. PLOS ONE. 2011;6(4):e17401. https://doi.org/10.1371/journal.pone.0017401.
20. Centers for Medicare, Medicaid Services. Security Boulevard Baltimore, and Md21244 USA, “OutcomeMeasures,”. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/OutcomeMeasures.html 7500; Accessed October 13, 2017.
21. Centers for Medicare and Medicaid Services (CMS), HHS. Medicare Program; Hospital Inpatient Prospective Payment Systems for Acute Care Hospitals and the Long-Term Care Hospital Prospective Payment System and Fiscal Year 2014 Rates; Quality Reporting Requirements for Specific Providers; Hospital Conditions of Participation; Payment Policies Related to Patient Status. Final Rules.”
22. Feemster LC, Au DH. Penalizing hospitals for chronic obstructive pulmonary disease readmissions. Am J Respir Crit Care Med. 2014;189(6):634-639. https://doi.org/10.1164/rccm.201308-1541PP.
23. United States Census Bureau. Census.Gov. Accessed April 17, 2019. https://www.census.gov/en.html.
24. Dartmouth atlas data,”. https://atlasdata.dartmouth.edu/. Aaccessed April 17, 2019.
25. Home. Dartmouth Atlas Healthc. https://www.dartmouthatlas.org/. Accessed April 17, 2019.
26. Zuckerman RB, Sheingold SH, Orav EJ, Ruhter J, Epstein AM. Readmissions, observation, and the hospital readmissions reduction program. N Engl J Med. 2016;374(16):1543-1551. https://doi.org/10.1056/NEJMsa1513024.
27. Gorodeski EZ, Starling RC, Blackstone EH. Are All Readmissions Bad Readmissions?, letter. World. 2010. https://doi.org/10.1056/NEJMc1001882.
Chronic obstructive pulmonary disease (COPD) is recognized as the third leading cause of death nationally. Globally, it has been estimated that 10% of the population has COPD; in the United States, approximately 15 million people are affected.1,2 The annual estimated cost of COPD management in the United States is approximately $50 billion, one-third of which is directly related to inpatient hospitalization for COPD exacerbation.3,4,5 The 30-day readmission rate after hospitalization for acute exacerbation of COPD (AECOPD) is approximately 21% with an approximate cost of $13 billion per year.6,7 To reduce the cost and to improve patient outcomes, the Centers for Medicare and Medicaid Services (CMS) has designed several interventions with little effect.8
In October 2012, the Affordable Care Act added section 1886(q) to the Social Security Act and established the Hospital Readmission Reduction Program (HRRP), an initiative to decrease hospitalization costs by penalizing hospitals with high 30-day readmission rates. Under this program, hospitals received up to 3% penalty for excess readmissions after the index hospitalization with acute myocardial infarction (AMI), heart failure (HF), and pneumonia.9-11 Hospitals are penalized if their annual readmission rates are significantly above the average national readmission rate. In 2014, the HRRP was extended to include AECOPD for the FY 2015.
Since the implementation of readmission penalties, data have shown a significant decrease in the 30-day readmission rates for all conditions.12,13 On the other hand, studies have suggested that, at least for some conditions, the decrease in the 30-day readmission rate is associated with higher adverse patients outcomes, including higher mortality.14,15 However, whether a decrease in readmission rates after an AECOPD hospitalization is associated with a concomitant increase in mortality has not been examined. Therefore, our objective was to examine the association of the 30-day risk-adjusted hospital readmission rate with the 30-day risk-adjusted hospital mortality rate for patients discharged with a diagnosis of AECOPD.
METHOD
Data Sources
Publicly available data from three sources were used. The all-cause 30-day risk-standardized readmission rate (RSRR) and the 30-day risk-standardized mortality rate (RSMR) of each hospital for patients with AECOPD were obtained from the Hospital Compare database; a database maintained by the CMS.16,17 In 2014, the CMS started reporting three-year running average of 30-day mortality and readmission rate data on hospitals for AECOPD hospitalizations; the data start date was July 2010.18-22 We examined data from the FY 2010-2013 to 2014-2017 cycles on readmission and mortality reported by the CMS; this included data before and after the implementation of penalties.
Hospital characteristics were also obtained from the CMS website. Hospital ownership was defined as government (owned by Federal or state), for-profit (owned by physicians or another proprietary), or nonprofit (owned by a nonprofit organization such as a church). A hospital was considered as a teaching hospital if it obtained graduate medical education funding from the CMS.
Data on local population characteristics according to ZIP codes were obtained from the 2010 decennial census and the American Community Survey five-year (2009-2013) data files available at the United States Census Bureau website.23 For each ZIP code, we obtained data on the total population, percentage of African Americans in the population, median income, poverty level, and insurance status.
We used Hospital service area (HSA) information obtained from the Dartmouth Atlas of Health Care crosswalk files to link local population characteristics to hospitals. The Dartmouth Atlas defined 3,436 HSAs by assigning the ZIP codes to the hospital area where the greatest proportion of their Medicare residents was hospitalized.24,25
Hospital Compare data and Census Bureau population data were matched to the HSAs from the Dartmouth Atlas of Healthcare data at the ZIP code level. First, the ZIP code-level data from the Census Bureau were pooled by the HSAs obtained from the Dartmouth Atlas of Healthcare, followed by matching these data by the HSAs to the Hospital Compare data. Merging data from these three sources generated a dataset that contained information about readmission and mortality rates from a particular hospital and the population characteristics of the local healthcare market or neighborhood. Our final dataset included hospitals that had readmission and mortality information available at the Hospital Compare website and were included in the crosswalk files of the Dartmouth Atlas of Healthcare.
Statistical Analysis
Data are summarized as mean and standard deviation (SD), median with interquartile range, or frequencies as appropriate. To model the dependence of observations from the same hospital over time, we used mixed linear models with random intercept and slope. A strength of this modeling approach is that it incorporates information from all hospitals even when some hospitals are missing data for some time periods. We reached our final model through stages with increasing model complexity at each stage. In the first stage, we developed an empty model without any covariates to determine the unconditional variance components so that we can partition mortality variance into between- and within-hospital components. In the second stage, we developed an unconditional growth curve model to determine the shape of time trend in mortality over time using linear and quadratic (by including squared time in the model) growth curves. In the third stage, we added baseline readmission rates (from 2010 to 2013) to the model to determine the effect of baseline readmission rate on mortality trends and also examined its interaction with time and squared time. We generated a change in the readmission rate variable by subtracting the last readmission rate from the baseline readmission rate (readmission rate in 2010-2013 − readmission rate in 2014-2017). In the fourth stage, we included this change in readmission rate into the third-stage model to examine how changes in the readmission rate affected the time trends of mortality and also examined its interaction with time and squared time. In the final model, we included the following potential confounding variables to the fourth stage model: African American percentage in the HSA, HSA median income, percentage of people living in poverty in the HSA, median age, ownership of hospital (government, for profit), teaching status (teaching vs nonteaching), and acute care hospital beds in the HSA. Within each stage, the models were compared using the Akaike information criterion (AIC) and the Bayesian information criterion (BIC), and the model with the lowest value of each was moved to the next stage of model development. All analyses were performed in Stata 14.1 for Windows (College Station, Texas).
RESULTS
Of the 3,685 acute care hospitals analyzed in the 2010-2013 data cycle for COPD, the 30-day RSRR was 20.7% (1.28), which decreased to 19.6% (1.11) in 2014-2017 (Table 1). During the same period, the 30-day all-cause RSMR increased from 7.8% (1.03) in 2010-2013 to 8.4% (1.11) in 2014-2017. The partitioning of variance showed that 57% of variation in the mortality rate over the study period was due to between-hospital differences.
The unconditional growth model examining the linear time trend revealed a 0.13% per year (95% CI = 0.12 to 0.14; P < .0001) increase in mortality rate over the five data cycles. When the squared time variable was added to the model to examine a quadratic trend, both time and squared trend were statistically significant (Table 2) and the AIC and BIC were lower for the quadratic model. Thus, the unconditional growth curve model suggested that the mortality trend was nonlinear and the coefficients demonstrated that not only the mortality rate increased, but the rate of change in the mortality rate was also increasing during the study period.
When we added the baseline readmission rate to the abovementioned quadratic growth model, we found an inverse association; each 1% increase in baseline readmission rate was associated with 0.03% (95% CI = −0.05 to −0.005; P = .02) decrease in mortality rate. These findings suggest that hospitals with higher baseline readmission rates also had lower mortality rates. To examine whether the effect of baseline readmission rate on mortality varied over time, we included the interaction term with time in the model and then added the interaction term with squared time. As the AIC and BIC were the lowest for the model with interaction between time and baseline readmission (and not when interaction between squared time and baseline readmission were included), we accepted this model. In this model, although there was no difference in mortality according to readmissions at baseline, each 1% increase in baseline readmission rate was associated with a smaller increase in mortality rate by 0.015% (95% CI = −0.02 to −0.01; P < .0001; Table 2 and Figure 1). These findings suggest that hospitals with higher readmission rates at baseline had a smaller increase in mortality rate during the study period than those with lower readmission rates.
Inclusion of change in the readmissions variable in the model showed that each 1% decrease in readmission rate during the study period was associated with 0.04% (95% CI = 0.01 to 0.06; P = .008) increase in mortality. However, the interaction between change in readmission and time was not significant and the AIC and BIC of the model were higher than the model without interaction. Therefore, we retained the model without the interaction term and included other potential confounding variables to build our final model. Thus, although hospitals with different baseline readmission rates had different rates of change in mortality rate, the change in readmission rate had a consistent effect on the mortality rate. Including potential confounders in the model did not change the results; the mortality rate and the change in the mortality rate increased during the study period, a high baseline readmission rate was associated with a lower yearly increase in mortality, and a larger decrease in readmission rate was associated with a higher mortality rate (Table 2).
DISCUSSION
As efforts to decrease readmission rates continue as a part of the HRRP implementation by the CMS, our study shows that among hospitals that discharged patients with AECOPD during 2010-2017, the all-cause 30-day RSRR was decreased, whereas the all-cause 30-day RSMR was increased. Of particular concern is that the rate of increase in mortality also increased. We also found that hospitals with higher readmission rates in 2010-2013 had a lower rate of increase in mortality than hospitals with lower readmission rates. In addition, hospitals that had a larger decrease in readmission rates during the study period had a larger rate of increase in mortality than hospitals with a smaller decrease in readmission rates. Our findings were robust to potential confounders such as hospital characteristics and local population characteristics in which hospitals operate.
Our study findings raise the question whether the implementation of the HRRP resulted in unintentional patient harm by forcing hospitals to make changes that may affect overall patient care. This question is particularly important as other studies on hospitalized patients with HF have found similar results.13,14 On the other hand, a similar association between readmission and mortality rates has not been observed in patients with pneumonia or AMI.14 Several possible explanations can be given for the observed discrepancy between the diseases and their effect on the relationship between readmission rate and mortality rate. Both COPD and HF are chronic diseases and characterized by exacerbations, whereas AMI and pneumonia are episodic diseases that are treatable. As the number of patients hospitalized with AECOPD and HF is much larger, hospitals may have a greater focus on reducing the 30-day readmission rates and may attempt to game the process, such as by delaying admissions through the emergency department within the 30-day period or by admitting patients for observation. In fact, a study found a 3% reduction in the within-hospital readmission rate with a concurrent 0.8% increase in observation unit use since the implementation of the HRRP.26 Such approaches to patient care may lead to adverse outcomes.
It is possible that readmissions and mortality act as competing risks and hence hospitals with higher mortality rates are left with fewer patients and thus have fewer readmissions, whereas those with lower mortality rates have more patients and a higher readmission rate.27 Such studies are not possible with hospital-level data, and patient-level studies will be required to examine this competing risk hypothesis. Our study results provide some support to the competing risk hypothesis (hospitals with lower baseline readmission rates had a steeper increase in mortality); however, it is not possible to draw any conclusions due to the high risk of ecological fallacy bias.
This study has important potential implications for healthcare policy, public health, and research. We found that an important national intervention aimed at decreasing readmission rates and improving the quality of care for patients with AECOPD may be associated with higher mortality rates in these patients. There may be a need to redefine measures for determining the performance of an institution. Our study supports research into the underlying mechanisms resulting in an inverse association between readmissions and mortality. In particular, health policy researchers may need to examine how incentives and penalties affect the allocation of resources within hospitals.
This study has several strengths and some potential weaknesses. We used a national dataset to examine readmission and mortality rates that include the majority of hospitals in the United States. We also included data from the local population for each hospital, thus allowing us to examine hospital performance within the context of its target population. One potential limitation is that we used hospital-level data and not patient-level data; however, the readmission penalties are designed for hospitals, which justifies our use of hospital-level data. Furthermore, data were not available for shorter time intervals; data from shorter time intervals may be associated with greater variability. Being an observational study, it is difficult to establish a causal relationship; the longitudinal nature of the study does establish temporality, an important factor in establishing causality.
In conclusion, we found that although the readmission rates decreased, there was an increase in the mortality rate within the 30 days of discharge from the hospital in patients with AECOPD. The rate of increase in mortality was higher in hospitals with lower readmission rates than in hospitals with higher readmission rates. Further research for determining the mechanism responsible for this association is needed. Future health policy interventions may need to consider the potential for adverse outcomes.
Chronic obstructive pulmonary disease (COPD) is recognized as the third leading cause of death nationally. Globally, it has been estimated that 10% of the population has COPD; in the United States, approximately 15 million people are affected.1,2 The annual estimated cost of COPD management in the United States is approximately $50 billion, one-third of which is directly related to inpatient hospitalization for COPD exacerbation.3,4,5 The 30-day readmission rate after hospitalization for acute exacerbation of COPD (AECOPD) is approximately 21% with an approximate cost of $13 billion per year.6,7 To reduce the cost and to improve patient outcomes, the Centers for Medicare and Medicaid Services (CMS) has designed several interventions with little effect.8
In October 2012, the Affordable Care Act added section 1886(q) to the Social Security Act and established the Hospital Readmission Reduction Program (HRRP), an initiative to decrease hospitalization costs by penalizing hospitals with high 30-day readmission rates. Under this program, hospitals received up to 3% penalty for excess readmissions after the index hospitalization with acute myocardial infarction (AMI), heart failure (HF), and pneumonia.9-11 Hospitals are penalized if their annual readmission rates are significantly above the average national readmission rate. In 2014, the HRRP was extended to include AECOPD for the FY 2015.
Since the implementation of readmission penalties, data have shown a significant decrease in the 30-day readmission rates for all conditions.12,13 On the other hand, studies have suggested that, at least for some conditions, the decrease in the 30-day readmission rate is associated with higher adverse patients outcomes, including higher mortality.14,15 However, whether a decrease in readmission rates after an AECOPD hospitalization is associated with a concomitant increase in mortality has not been examined. Therefore, our objective was to examine the association of the 30-day risk-adjusted hospital readmission rate with the 30-day risk-adjusted hospital mortality rate for patients discharged with a diagnosis of AECOPD.
METHOD
Data Sources
Publicly available data from three sources were used. The all-cause 30-day risk-standardized readmission rate (RSRR) and the 30-day risk-standardized mortality rate (RSMR) of each hospital for patients with AECOPD were obtained from the Hospital Compare database; a database maintained by the CMS.16,17 In 2014, the CMS started reporting three-year running average of 30-day mortality and readmission rate data on hospitals for AECOPD hospitalizations; the data start date was July 2010.18-22 We examined data from the FY 2010-2013 to 2014-2017 cycles on readmission and mortality reported by the CMS; this included data before and after the implementation of penalties.
Hospital characteristics were also obtained from the CMS website. Hospital ownership was defined as government (owned by Federal or state), for-profit (owned by physicians or another proprietary), or nonprofit (owned by a nonprofit organization such as a church). A hospital was considered as a teaching hospital if it obtained graduate medical education funding from the CMS.
Data on local population characteristics according to ZIP codes were obtained from the 2010 decennial census and the American Community Survey five-year (2009-2013) data files available at the United States Census Bureau website.23 For each ZIP code, we obtained data on the total population, percentage of African Americans in the population, median income, poverty level, and insurance status.
We used Hospital service area (HSA) information obtained from the Dartmouth Atlas of Health Care crosswalk files to link local population characteristics to hospitals. The Dartmouth Atlas defined 3,436 HSAs by assigning the ZIP codes to the hospital area where the greatest proportion of their Medicare residents was hospitalized.24,25
Hospital Compare data and Census Bureau population data were matched to the HSAs from the Dartmouth Atlas of Healthcare data at the ZIP code level. First, the ZIP code-level data from the Census Bureau were pooled by the HSAs obtained from the Dartmouth Atlas of Healthcare, followed by matching these data by the HSAs to the Hospital Compare data. Merging data from these three sources generated a dataset that contained information about readmission and mortality rates from a particular hospital and the population characteristics of the local healthcare market or neighborhood. Our final dataset included hospitals that had readmission and mortality information available at the Hospital Compare website and were included in the crosswalk files of the Dartmouth Atlas of Healthcare.
Statistical Analysis
Data are summarized as mean and standard deviation (SD), median with interquartile range, or frequencies as appropriate. To model the dependence of observations from the same hospital over time, we used mixed linear models with random intercept and slope. A strength of this modeling approach is that it incorporates information from all hospitals even when some hospitals are missing data for some time periods. We reached our final model through stages with increasing model complexity at each stage. In the first stage, we developed an empty model without any covariates to determine the unconditional variance components so that we can partition mortality variance into between- and within-hospital components. In the second stage, we developed an unconditional growth curve model to determine the shape of time trend in mortality over time using linear and quadratic (by including squared time in the model) growth curves. In the third stage, we added baseline readmission rates (from 2010 to 2013) to the model to determine the effect of baseline readmission rate on mortality trends and also examined its interaction with time and squared time. We generated a change in the readmission rate variable by subtracting the last readmission rate from the baseline readmission rate (readmission rate in 2010-2013 − readmission rate in 2014-2017). In the fourth stage, we included this change in readmission rate into the third-stage model to examine how changes in the readmission rate affected the time trends of mortality and also examined its interaction with time and squared time. In the final model, we included the following potential confounding variables to the fourth stage model: African American percentage in the HSA, HSA median income, percentage of people living in poverty in the HSA, median age, ownership of hospital (government, for profit), teaching status (teaching vs nonteaching), and acute care hospital beds in the HSA. Within each stage, the models were compared using the Akaike information criterion (AIC) and the Bayesian information criterion (BIC), and the model with the lowest value of each was moved to the next stage of model development. All analyses were performed in Stata 14.1 for Windows (College Station, Texas).
RESULTS
Of the 3,685 acute care hospitals analyzed in the 2010-2013 data cycle for COPD, the 30-day RSRR was 20.7% (1.28), which decreased to 19.6% (1.11) in 2014-2017 (Table 1). During the same period, the 30-day all-cause RSMR increased from 7.8% (1.03) in 2010-2013 to 8.4% (1.11) in 2014-2017. The partitioning of variance showed that 57% of variation in the mortality rate over the study period was due to between-hospital differences.
The unconditional growth model examining the linear time trend revealed a 0.13% per year (95% CI = 0.12 to 0.14; P < .0001) increase in mortality rate over the five data cycles. When the squared time variable was added to the model to examine a quadratic trend, both time and squared trend were statistically significant (Table 2) and the AIC and BIC were lower for the quadratic model. Thus, the unconditional growth curve model suggested that the mortality trend was nonlinear and the coefficients demonstrated that not only the mortality rate increased, but the rate of change in the mortality rate was also increasing during the study period.
When we added the baseline readmission rate to the abovementioned quadratic growth model, we found an inverse association; each 1% increase in baseline readmission rate was associated with 0.03% (95% CI = −0.05 to −0.005; P = .02) decrease in mortality rate. These findings suggest that hospitals with higher baseline readmission rates also had lower mortality rates. To examine whether the effect of baseline readmission rate on mortality varied over time, we included the interaction term with time in the model and then added the interaction term with squared time. As the AIC and BIC were the lowest for the model with interaction between time and baseline readmission (and not when interaction between squared time and baseline readmission were included), we accepted this model. In this model, although there was no difference in mortality according to readmissions at baseline, each 1% increase in baseline readmission rate was associated with a smaller increase in mortality rate by 0.015% (95% CI = −0.02 to −0.01; P < .0001; Table 2 and Figure 1). These findings suggest that hospitals with higher readmission rates at baseline had a smaller increase in mortality rate during the study period than those with lower readmission rates.
Inclusion of change in the readmissions variable in the model showed that each 1% decrease in readmission rate during the study period was associated with 0.04% (95% CI = 0.01 to 0.06; P = .008) increase in mortality. However, the interaction between change in readmission and time was not significant and the AIC and BIC of the model were higher than the model without interaction. Therefore, we retained the model without the interaction term and included other potential confounding variables to build our final model. Thus, although hospitals with different baseline readmission rates had different rates of change in mortality rate, the change in readmission rate had a consistent effect on the mortality rate. Including potential confounders in the model did not change the results; the mortality rate and the change in the mortality rate increased during the study period, a high baseline readmission rate was associated with a lower yearly increase in mortality, and a larger decrease in readmission rate was associated with a higher mortality rate (Table 2).
DISCUSSION
As efforts to decrease readmission rates continue as a part of the HRRP implementation by the CMS, our study shows that among hospitals that discharged patients with AECOPD during 2010-2017, the all-cause 30-day RSRR was decreased, whereas the all-cause 30-day RSMR was increased. Of particular concern is that the rate of increase in mortality also increased. We also found that hospitals with higher readmission rates in 2010-2013 had a lower rate of increase in mortality than hospitals with lower readmission rates. In addition, hospitals that had a larger decrease in readmission rates during the study period had a larger rate of increase in mortality than hospitals with a smaller decrease in readmission rates. Our findings were robust to potential confounders such as hospital characteristics and local population characteristics in which hospitals operate.
Our study findings raise the question whether the implementation of the HRRP resulted in unintentional patient harm by forcing hospitals to make changes that may affect overall patient care. This question is particularly important as other studies on hospitalized patients with HF have found similar results.13,14 On the other hand, a similar association between readmission and mortality rates has not been observed in patients with pneumonia or AMI.14 Several possible explanations can be given for the observed discrepancy between the diseases and their effect on the relationship between readmission rate and mortality rate. Both COPD and HF are chronic diseases and characterized by exacerbations, whereas AMI and pneumonia are episodic diseases that are treatable. As the number of patients hospitalized with AECOPD and HF is much larger, hospitals may have a greater focus on reducing the 30-day readmission rates and may attempt to game the process, such as by delaying admissions through the emergency department within the 30-day period or by admitting patients for observation. In fact, a study found a 3% reduction in the within-hospital readmission rate with a concurrent 0.8% increase in observation unit use since the implementation of the HRRP.26 Such approaches to patient care may lead to adverse outcomes.
It is possible that readmissions and mortality act as competing risks and hence hospitals with higher mortality rates are left with fewer patients and thus have fewer readmissions, whereas those with lower mortality rates have more patients and a higher readmission rate.27 Such studies are not possible with hospital-level data, and patient-level studies will be required to examine this competing risk hypothesis. Our study results provide some support to the competing risk hypothesis (hospitals with lower baseline readmission rates had a steeper increase in mortality); however, it is not possible to draw any conclusions due to the high risk of ecological fallacy bias.
This study has important potential implications for healthcare policy, public health, and research. We found that an important national intervention aimed at decreasing readmission rates and improving the quality of care for patients with AECOPD may be associated with higher mortality rates in these patients. There may be a need to redefine measures for determining the performance of an institution. Our study supports research into the underlying mechanisms resulting in an inverse association between readmissions and mortality. In particular, health policy researchers may need to examine how incentives and penalties affect the allocation of resources within hospitals.
This study has several strengths and some potential weaknesses. We used a national dataset to examine readmission and mortality rates that include the majority of hospitals in the United States. We also included data from the local population for each hospital, thus allowing us to examine hospital performance within the context of its target population. One potential limitation is that we used hospital-level data and not patient-level data; however, the readmission penalties are designed for hospitals, which justifies our use of hospital-level data. Furthermore, data were not available for shorter time intervals; data from shorter time intervals may be associated with greater variability. Being an observational study, it is difficult to establish a causal relationship; the longitudinal nature of the study does establish temporality, an important factor in establishing causality.
In conclusion, we found that although the readmission rates decreased, there was an increase in the mortality rate within the 30 days of discharge from the hospital in patients with AECOPD. The rate of increase in mortality was higher in hospitals with lower readmission rates than in hospitals with higher readmission rates. Further research for determining the mechanism responsible for this association is needed. Future health policy interventions may need to consider the potential for adverse outcomes.
1. Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2010. Natl Vital Stat Rep. 2013;61(4):1-117.
2. Halbert RJ, Natoli JL, Gano A, et al. Global burden of copd: systematic review and meta-analysis. Eur Respir J. 2006;28(3):523-532. https://doi.org/10.1183/09031936.06.00124605.
3. Toy EL, Gallagher KF, Stanley EL, Swensen AR, Duh MS. The economic impact of exacerbations of chronic obstructive pulmonary disease and exacerbation definition: a review. COPD. 2010;7(3):214-228. https://doi.org/10.3109/15412555.2010.481697.
4. Shah T, Churpek MM, Coca Perraillon M, Konetzka RT. Understanding why patients with COPD get readmitted: a large national study to delineate the Medicare population for the readmissions penalty expansion. Chest. 2015;147(5):1219-1226. https://doi.org/10.1378/chest.14-2181.
5. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428. https://doi.org/10.1056/NEJMsa0803563.
6. Stein BD, Charbeneau JT, Lee TA, et al. Hospitalizations for acute exacerbations of chronic obstructive pulmonary disease: how you count matters. COPD. 2010;7(3):164-171. https://doi.org/10.3109/15412555.2010.481696.
7. Stein, B. D., Charbeneau, J. T., Lee, T. A., Schumock, G. T., Lindenauer, P. K., Bautista, A., . . . Krishnan, J. A. (2010). Hospitalizations for acute exacerbations of chronic obstructive pulmonary disease: how you count matters. COPD, 7(3), 164-171. doi:10.3109/15412555.2010.481696
8. McIlvennan CK, Eapen ZJ, Allen LA. Hospital readmissions reduction program. Circulation. 2015;131(20):1796-1803. https://doi.org/10.1161/CIRCULATIONAHA.114.010270.
9. McIlvennan CK, Eapen ZJ, Allen LA. Hospital readmissions reduction program. Circulation. 2015;131(20):179-1803. https://doi.org/10.1161/CIRCULATIONAHA.114.010270.
10. Centers for Medicare and Medicaid Services (CMS), HHS. Medicare Program; hospital inpatient prospective payment systems for acute care hospitals and the long-term care hospital prospective payment system and FY 2012 rates; Hospitals’ FTE Resident Caps for Graduate Medical Education Payment. Final Rules. Fed Regist. 2011;76(160):51476-51846.
11. Centers for Medicare and Medicaid Services (CMS). Medicare program; hospital inpatient prospective payment systems for acute care hospitals and the long-term care hospital prospective payment system and fiscal year 2014 rates; quality reporting requirements for specific providers; hospital conditions of participation; payment policies related to patient status. Final rules. Fed Regist. 2013;78(160):50495-51040.
12. Casillas G. Published: Mar 10 and 2017, “aiming for fewer hospital U-turns: the Medicare Hospital readmission reduction program,” [blog]. https://www.kff.org/medicare/issue-brief/aiming-for-fewer-hospital-u-turns-the-medicare-hospital-readmission-reduction-program/; Accessed March 10, 2017. The Henry J. Kaiser Family Foundation.
13. Desai NR, Ross JS, Kwon JY, et al. Association Between hospital penalty status Under the hospital readmission reduction program and readmission rates for target and nontarget conditions. JAMA. 2016;316(24): 2647-2656. https://doi.org/10.1001/jama.2016.18533.
14. Gupta A, Allen LA, Bhatt DL, et al. Association of the hospital readmissions reduction program implementation with readmission and mortality outcomes in heart failure. JAMA Cardiol. 2018;3(1):44-53. https://doi.org/10.1001/jamacardio.2017.4265.
15. Krumholz HM, Lin Z, Keenan PS, et al. Relationship between hospital readmission and mortality rates for patients hospitalized with acute myocardial infarction, heart failure, or pneumonia. JAMA. 2013;309(6):587-593. https://doi.org/10.1001/jama.2013.333.
16. Medicare Hospital compare overview,” https://www.medicare.gov/hospitalcompare/About/What-Is-HOS.html; Accessed April 17, 2019.
17. Archived datasets. Data.Medicare.Gov. Data.Medicare.Gov. Accessed April 17, 2019. https://data.medicare.gov/data/archives/hospital-compare.
18. Krumholz HM, Lin Z, Drye EE, et al. An administrative claims measure suitable for profiling hospital performance based on 30-day all-cause readmission rates among patients with acute myocardial infarction. Circ Cardiovasc Qual Outcomes. 2011;4(2):243-252. https://doi.org/10.1161/CIRCOUTCOMES.110.957498.
19. Bratzler DW, Normand SL, Wang Y, et al. An administrative claims model for profiling hospital 30-day mortality rates for pneumonia patients. PLOS ONE. 2011;6(4):e17401. https://doi.org/10.1371/journal.pone.0017401.
20. Centers for Medicare, Medicaid Services. Security Boulevard Baltimore, and Md21244 USA, “OutcomeMeasures,”. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/OutcomeMeasures.html 7500; Accessed October 13, 2017.
21. Centers for Medicare and Medicaid Services (CMS), HHS. Medicare Program; Hospital Inpatient Prospective Payment Systems for Acute Care Hospitals and the Long-Term Care Hospital Prospective Payment System and Fiscal Year 2014 Rates; Quality Reporting Requirements for Specific Providers; Hospital Conditions of Participation; Payment Policies Related to Patient Status. Final Rules.”
22. Feemster LC, Au DH. Penalizing hospitals for chronic obstructive pulmonary disease readmissions. Am J Respir Crit Care Med. 2014;189(6):634-639. https://doi.org/10.1164/rccm.201308-1541PP.
23. United States Census Bureau. Census.Gov. Accessed April 17, 2019. https://www.census.gov/en.html.
24. Dartmouth atlas data,”. https://atlasdata.dartmouth.edu/. Aaccessed April 17, 2019.
25. Home. Dartmouth Atlas Healthc. https://www.dartmouthatlas.org/. Accessed April 17, 2019.
26. Zuckerman RB, Sheingold SH, Orav EJ, Ruhter J, Epstein AM. Readmissions, observation, and the hospital readmissions reduction program. N Engl J Med. 2016;374(16):1543-1551. https://doi.org/10.1056/NEJMsa1513024.
27. Gorodeski EZ, Starling RC, Blackstone EH. Are All Readmissions Bad Readmissions?, letter. World. 2010. https://doi.org/10.1056/NEJMc1001882.
1. Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2010. Natl Vital Stat Rep. 2013;61(4):1-117.
2. Halbert RJ, Natoli JL, Gano A, et al. Global burden of copd: systematic review and meta-analysis. Eur Respir J. 2006;28(3):523-532. https://doi.org/10.1183/09031936.06.00124605.
3. Toy EL, Gallagher KF, Stanley EL, Swensen AR, Duh MS. The economic impact of exacerbations of chronic obstructive pulmonary disease and exacerbation definition: a review. COPD. 2010;7(3):214-228. https://doi.org/10.3109/15412555.2010.481697.
4. Shah T, Churpek MM, Coca Perraillon M, Konetzka RT. Understanding why patients with COPD get readmitted: a large national study to delineate the Medicare population for the readmissions penalty expansion. Chest. 2015;147(5):1219-1226. https://doi.org/10.1378/chest.14-2181.
5. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428. https://doi.org/10.1056/NEJMsa0803563.
6. Stein BD, Charbeneau JT, Lee TA, et al. Hospitalizations for acute exacerbations of chronic obstructive pulmonary disease: how you count matters. COPD. 2010;7(3):164-171. https://doi.org/10.3109/15412555.2010.481696.
7. Stein, B. D., Charbeneau, J. T., Lee, T. A., Schumock, G. T., Lindenauer, P. K., Bautista, A., . . . Krishnan, J. A. (2010). Hospitalizations for acute exacerbations of chronic obstructive pulmonary disease: how you count matters. COPD, 7(3), 164-171. doi:10.3109/15412555.2010.481696
8. McIlvennan CK, Eapen ZJ, Allen LA. Hospital readmissions reduction program. Circulation. 2015;131(20):1796-1803. https://doi.org/10.1161/CIRCULATIONAHA.114.010270.
9. McIlvennan CK, Eapen ZJ, Allen LA. Hospital readmissions reduction program. Circulation. 2015;131(20):179-1803. https://doi.org/10.1161/CIRCULATIONAHA.114.010270.
10. Centers for Medicare and Medicaid Services (CMS), HHS. Medicare Program; hospital inpatient prospective payment systems for acute care hospitals and the long-term care hospital prospective payment system and FY 2012 rates; Hospitals’ FTE Resident Caps for Graduate Medical Education Payment. Final Rules. Fed Regist. 2011;76(160):51476-51846.
11. Centers for Medicare and Medicaid Services (CMS). Medicare program; hospital inpatient prospective payment systems for acute care hospitals and the long-term care hospital prospective payment system and fiscal year 2014 rates; quality reporting requirements for specific providers; hospital conditions of participation; payment policies related to patient status. Final rules. Fed Regist. 2013;78(160):50495-51040.
12. Casillas G. Published: Mar 10 and 2017, “aiming for fewer hospital U-turns: the Medicare Hospital readmission reduction program,” [blog]. https://www.kff.org/medicare/issue-brief/aiming-for-fewer-hospital-u-turns-the-medicare-hospital-readmission-reduction-program/; Accessed March 10, 2017. The Henry J. Kaiser Family Foundation.
13. Desai NR, Ross JS, Kwon JY, et al. Association Between hospital penalty status Under the hospital readmission reduction program and readmission rates for target and nontarget conditions. JAMA. 2016;316(24): 2647-2656. https://doi.org/10.1001/jama.2016.18533.
14. Gupta A, Allen LA, Bhatt DL, et al. Association of the hospital readmissions reduction program implementation with readmission and mortality outcomes in heart failure. JAMA Cardiol. 2018;3(1):44-53. https://doi.org/10.1001/jamacardio.2017.4265.
15. Krumholz HM, Lin Z, Keenan PS, et al. Relationship between hospital readmission and mortality rates for patients hospitalized with acute myocardial infarction, heart failure, or pneumonia. JAMA. 2013;309(6):587-593. https://doi.org/10.1001/jama.2013.333.
16. Medicare Hospital compare overview,” https://www.medicare.gov/hospitalcompare/About/What-Is-HOS.html; Accessed April 17, 2019.
17. Archived datasets. Data.Medicare.Gov. Data.Medicare.Gov. Accessed April 17, 2019. https://data.medicare.gov/data/archives/hospital-compare.
18. Krumholz HM, Lin Z, Drye EE, et al. An administrative claims measure suitable for profiling hospital performance based on 30-day all-cause readmission rates among patients with acute myocardial infarction. Circ Cardiovasc Qual Outcomes. 2011;4(2):243-252. https://doi.org/10.1161/CIRCOUTCOMES.110.957498.
19. Bratzler DW, Normand SL, Wang Y, et al. An administrative claims model for profiling hospital 30-day mortality rates for pneumonia patients. PLOS ONE. 2011;6(4):e17401. https://doi.org/10.1371/journal.pone.0017401.
20. Centers for Medicare, Medicaid Services. Security Boulevard Baltimore, and Md21244 USA, “OutcomeMeasures,”. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/OutcomeMeasures.html 7500; Accessed October 13, 2017.
21. Centers for Medicare and Medicaid Services (CMS), HHS. Medicare Program; Hospital Inpatient Prospective Payment Systems for Acute Care Hospitals and the Long-Term Care Hospital Prospective Payment System and Fiscal Year 2014 Rates; Quality Reporting Requirements for Specific Providers; Hospital Conditions of Participation; Payment Policies Related to Patient Status. Final Rules.”
22. Feemster LC, Au DH. Penalizing hospitals for chronic obstructive pulmonary disease readmissions. Am J Respir Crit Care Med. 2014;189(6):634-639. https://doi.org/10.1164/rccm.201308-1541PP.
23. United States Census Bureau. Census.Gov. Accessed April 17, 2019. https://www.census.gov/en.html.
24. Dartmouth atlas data,”. https://atlasdata.dartmouth.edu/. Aaccessed April 17, 2019.
25. Home. Dartmouth Atlas Healthc. https://www.dartmouthatlas.org/. Accessed April 17, 2019.
26. Zuckerman RB, Sheingold SH, Orav EJ, Ruhter J, Epstein AM. Readmissions, observation, and the hospital readmissions reduction program. N Engl J Med. 2016;374(16):1543-1551. https://doi.org/10.1056/NEJMsa1513024.
27. Gorodeski EZ, Starling RC, Blackstone EH. Are All Readmissions Bad Readmissions?, letter. World. 2010. https://doi.org/10.1056/NEJMc1001882.
© 2019 Society of Hospital Medicine
Choosing Wisely® in Pediatric Hospital Medicine: Time to Celebrate?
The Choosing Wisely® campaign, launched in 2012 by the American Board of Internal Medicine, aims to reduce overuse of tests and treatments that do not add value for patients. The campaign has caught the attention of the medical profession and spread internationally. Over the last seven years, most specialty societies have published specific recommendations on what tests and treatments clinicians should stop doing. However, has this campaign actually had an impact on the testing and treating behaviors of clinicians?
In this issue of the Journal of Hospital Medicine, Reyes and colleagues examine changes in five overuse metrics linked with the 2013 Choosing Wisely® Pediatric Hospital Medicine recommendations at 37 children’s hospitals from 2008 to 2017, five years before and after the recommendations were published.1,2 The tests and treatments targeted by these recommendations are not individually costly, but given the high prevalence of the conditions, the cumulative cost is not insignificant. More importantly, reducing the potentially harmful long-term effects of unnecessary radiation and adverse effects from exposure to inappropriate systemic steroids and antacids is a laudable goal. Results from unnecessary tests may also lead to a further cascade of unnecessary testing and/or treatment.3
The authors used an administrative data source, the Pediatric Health Information System (PHIS), to measure billing charges for the tests and medications linked with the overuse measures in over 278,000 hospitalizations. The good news is that overuse declined over the 10-year study period. After adjusting for differences in patient characteristics over time, they observed a substantial absolute reduction in bronchiolitis bronchodilator use (36.6%, from 64% in 2008 to 27.4% in 2017) and chest x-ray (CXR) use (31.5%, from 58.4% to 26.9%). There were also reductions for the other metrics: acid-suppressing medications for gastroesophageal reflux (24.1%, from 63% to 48.9%), asthma CXR use (20.8%, from 52.8% to 32%), and steroids for lower respiratory tract infections (2.9%, from 15.1% to 12.2%). We would not expect the goal for these overuse metrics to be zero percent given the diagnostic uncertainties in real-world clinical decision-making.
The Choosing Wisely® Pediatric Hospital Medicine recommendations, however, were associated with only a modest impact on the overuse decline. A before-and-after interrupted time series analysis showed that the overuse measures were on the downturn prior to the recommendations being published. Then after publication, only the rate of CXR use in asthma decreased immediately. The rate of bronchodilator use for bronchiolitis declined in the following five-year period. There were no changes in the rate of decline in overuse for the other tests and treatments associated with the recommendations.
With such a widespread national campaign, a control group of hospitals to better understand the specific influence of the Choosing Wisely® recommendations was not possible. The decline in overuse over the 10-year period reported by Reyes et al. is likely due to a combination of efforts at multiple levels—including national society guidelines, local hospital guidelines and pathways, increased awareness by clinicians of the problem of overuse, and focused quality improvement efforts.
The use of the PHIS database provided Reyes et al. a powerful data source to evaluate overuse across a large number of patients and hospitals efficiently. However, there are limitations with administrative data that are important to consider. Detailed clinical data, such as patient disease characteristics and test and treatment indications, are not available, which limits the specificity of these measures. For example, one of the recommendations suggests that gastroesophageal reflux should not be routinely treated with acid suppression therapy. Using administrative data, it is impossible to know whether the use of antacids in hospitalized children with a primary discharge diagnosis code of gastroesophageal reflux was inappropriate or because they failed other treatments in the outpatient setting and/or had complicated disease appropriately warranting treatment. This misclassification would result in an overestimation of overuse. The authors did attempt to minimize the possibility of misclassification by excluding children with comorbidities, those who had longer hospital stays, those admitted to the intensive care unit, and those with greater severity of illness where some of these tests and treatments would be indicated.
While the report by Reyes et al. focuses on Pediatric Hospital Medicine Choosing Wisely®recommendations, it is important to recognize that tests and treatments for conditions like asthma, bronchiolitis, and lower respiratory tract infections are initially performed in the emergency department (ED). Collaboration between the ED and the Hospital Medicine Unit is essential to tackle the issue of overuse.4
The study by Reyes et al. provides a nice description of the trends in the Choosing Wisely®overuse metrics at a group of children’s hospitals and is one of few such reports. The NIH funded, Eliminating Monitor Overuse: pulse oximetry (EMO: SpO2) study is focusing on the 5th Choosing Wisely® Pediatric Hospital Medicine recommendation that was not studied by Reyes.5
So then, with the decline in overuse reported in this study over 10 years, is it time to celebrate? Not yet. There is much work to do in the pursuit of Choosing Wisely®: developing a host of valid measures of overuse in pediatric hospital care, expanding the examination of overuse to community hospitals where the majority of children are hospitalized, and using implementation science theory to de-implement the ingrained practices.
1. Reyes M, Etigner B, Hall M, et al. Impact of the choosing wisely campaign recommendations for hospitalized children on clinical practice: trends from 2008 to 2017 [published online ahead of print September 18, 2019]. J Hosp Medicine. 2020;15(2):124-125. https://doi.org/10.12788/jhm.3291
2. Quinonez RA, Garber MD, Schroeder AR, et al. Choosing wisely in pediatric hospital medicine: five opportunities from improved healthcare value. J Hosp Med. 2013;8(9):479-495. https://doi.org/10.1002/jhm.2064.
3. Schuh S, Lalani A, Allen U, et al. Evaluation of the utility of radiography in acute bronchiolitis. J Pediatr. 2007;150(4):429-433. https://doi.org/16/j.jpeds.2007.01.005.
4. Mussman GM, Lossius M, Wasif F, et al. Multisite emergency department inpatient collaborative to reduce unnecessary bronchiolitis care. Pediatrics. 2018;141(2):e20170830. https://doi.org/10.1542/peds.2017-0830.
5. Rasooly IR, Beidas RS, Wolk CB, Barg F, et al. Measuring overuse of continuous pulse oximetry in bronchiolitis and developing strategies for large-scale deimplementation: study protocol for a feasibility trial. Pilot Feasibility Stud. 2019;5(1):68. https://doi.org/10.1186/s40814-019-0453-2.
The Choosing Wisely® campaign, launched in 2012 by the American Board of Internal Medicine, aims to reduce overuse of tests and treatments that do not add value for patients. The campaign has caught the attention of the medical profession and spread internationally. Over the last seven years, most specialty societies have published specific recommendations on what tests and treatments clinicians should stop doing. However, has this campaign actually had an impact on the testing and treating behaviors of clinicians?
In this issue of the Journal of Hospital Medicine, Reyes and colleagues examine changes in five overuse metrics linked with the 2013 Choosing Wisely® Pediatric Hospital Medicine recommendations at 37 children’s hospitals from 2008 to 2017, five years before and after the recommendations were published.1,2 The tests and treatments targeted by these recommendations are not individually costly, but given the high prevalence of the conditions, the cumulative cost is not insignificant. More importantly, reducing the potentially harmful long-term effects of unnecessary radiation and adverse effects from exposure to inappropriate systemic steroids and antacids is a laudable goal. Results from unnecessary tests may also lead to a further cascade of unnecessary testing and/or treatment.3
The authors used an administrative data source, the Pediatric Health Information System (PHIS), to measure billing charges for the tests and medications linked with the overuse measures in over 278,000 hospitalizations. The good news is that overuse declined over the 10-year study period. After adjusting for differences in patient characteristics over time, they observed a substantial absolute reduction in bronchiolitis bronchodilator use (36.6%, from 64% in 2008 to 27.4% in 2017) and chest x-ray (CXR) use (31.5%, from 58.4% to 26.9%). There were also reductions for the other metrics: acid-suppressing medications for gastroesophageal reflux (24.1%, from 63% to 48.9%), asthma CXR use (20.8%, from 52.8% to 32%), and steroids for lower respiratory tract infections (2.9%, from 15.1% to 12.2%). We would not expect the goal for these overuse metrics to be zero percent given the diagnostic uncertainties in real-world clinical decision-making.
The Choosing Wisely® Pediatric Hospital Medicine recommendations, however, were associated with only a modest impact on the overuse decline. A before-and-after interrupted time series analysis showed that the overuse measures were on the downturn prior to the recommendations being published. Then after publication, only the rate of CXR use in asthma decreased immediately. The rate of bronchodilator use for bronchiolitis declined in the following five-year period. There were no changes in the rate of decline in overuse for the other tests and treatments associated with the recommendations.
With such a widespread national campaign, a control group of hospitals to better understand the specific influence of the Choosing Wisely® recommendations was not possible. The decline in overuse over the 10-year period reported by Reyes et al. is likely due to a combination of efforts at multiple levels—including national society guidelines, local hospital guidelines and pathways, increased awareness by clinicians of the problem of overuse, and focused quality improvement efforts.
The use of the PHIS database provided Reyes et al. a powerful data source to evaluate overuse across a large number of patients and hospitals efficiently. However, there are limitations with administrative data that are important to consider. Detailed clinical data, such as patient disease characteristics and test and treatment indications, are not available, which limits the specificity of these measures. For example, one of the recommendations suggests that gastroesophageal reflux should not be routinely treated with acid suppression therapy. Using administrative data, it is impossible to know whether the use of antacids in hospitalized children with a primary discharge diagnosis code of gastroesophageal reflux was inappropriate or because they failed other treatments in the outpatient setting and/or had complicated disease appropriately warranting treatment. This misclassification would result in an overestimation of overuse. The authors did attempt to minimize the possibility of misclassification by excluding children with comorbidities, those who had longer hospital stays, those admitted to the intensive care unit, and those with greater severity of illness where some of these tests and treatments would be indicated.
While the report by Reyes et al. focuses on Pediatric Hospital Medicine Choosing Wisely®recommendations, it is important to recognize that tests and treatments for conditions like asthma, bronchiolitis, and lower respiratory tract infections are initially performed in the emergency department (ED). Collaboration between the ED and the Hospital Medicine Unit is essential to tackle the issue of overuse.4
The study by Reyes et al. provides a nice description of the trends in the Choosing Wisely®overuse metrics at a group of children’s hospitals and is one of few such reports. The NIH funded, Eliminating Monitor Overuse: pulse oximetry (EMO: SpO2) study is focusing on the 5th Choosing Wisely® Pediatric Hospital Medicine recommendation that was not studied by Reyes.5
So then, with the decline in overuse reported in this study over 10 years, is it time to celebrate? Not yet. There is much work to do in the pursuit of Choosing Wisely®: developing a host of valid measures of overuse in pediatric hospital care, expanding the examination of overuse to community hospitals where the majority of children are hospitalized, and using implementation science theory to de-implement the ingrained practices.
The Choosing Wisely® campaign, launched in 2012 by the American Board of Internal Medicine, aims to reduce overuse of tests and treatments that do not add value for patients. The campaign has caught the attention of the medical profession and spread internationally. Over the last seven years, most specialty societies have published specific recommendations on what tests and treatments clinicians should stop doing. However, has this campaign actually had an impact on the testing and treating behaviors of clinicians?
In this issue of the Journal of Hospital Medicine, Reyes and colleagues examine changes in five overuse metrics linked with the 2013 Choosing Wisely® Pediatric Hospital Medicine recommendations at 37 children’s hospitals from 2008 to 2017, five years before and after the recommendations were published.1,2 The tests and treatments targeted by these recommendations are not individually costly, but given the high prevalence of the conditions, the cumulative cost is not insignificant. More importantly, reducing the potentially harmful long-term effects of unnecessary radiation and adverse effects from exposure to inappropriate systemic steroids and antacids is a laudable goal. Results from unnecessary tests may also lead to a further cascade of unnecessary testing and/or treatment.3
The authors used an administrative data source, the Pediatric Health Information System (PHIS), to measure billing charges for the tests and medications linked with the overuse measures in over 278,000 hospitalizations. The good news is that overuse declined over the 10-year study period. After adjusting for differences in patient characteristics over time, they observed a substantial absolute reduction in bronchiolitis bronchodilator use (36.6%, from 64% in 2008 to 27.4% in 2017) and chest x-ray (CXR) use (31.5%, from 58.4% to 26.9%). There were also reductions for the other metrics: acid-suppressing medications for gastroesophageal reflux (24.1%, from 63% to 48.9%), asthma CXR use (20.8%, from 52.8% to 32%), and steroids for lower respiratory tract infections (2.9%, from 15.1% to 12.2%). We would not expect the goal for these overuse metrics to be zero percent given the diagnostic uncertainties in real-world clinical decision-making.
The Choosing Wisely® Pediatric Hospital Medicine recommendations, however, were associated with only a modest impact on the overuse decline. A before-and-after interrupted time series analysis showed that the overuse measures were on the downturn prior to the recommendations being published. Then after publication, only the rate of CXR use in asthma decreased immediately. The rate of bronchodilator use for bronchiolitis declined in the following five-year period. There were no changes in the rate of decline in overuse for the other tests and treatments associated with the recommendations.
With such a widespread national campaign, a control group of hospitals to better understand the specific influence of the Choosing Wisely® recommendations was not possible. The decline in overuse over the 10-year period reported by Reyes et al. is likely due to a combination of efforts at multiple levels—including national society guidelines, local hospital guidelines and pathways, increased awareness by clinicians of the problem of overuse, and focused quality improvement efforts.
The use of the PHIS database provided Reyes et al. a powerful data source to evaluate overuse across a large number of patients and hospitals efficiently. However, there are limitations with administrative data that are important to consider. Detailed clinical data, such as patient disease characteristics and test and treatment indications, are not available, which limits the specificity of these measures. For example, one of the recommendations suggests that gastroesophageal reflux should not be routinely treated with acid suppression therapy. Using administrative data, it is impossible to know whether the use of antacids in hospitalized children with a primary discharge diagnosis code of gastroesophageal reflux was inappropriate or because they failed other treatments in the outpatient setting and/or had complicated disease appropriately warranting treatment. This misclassification would result in an overestimation of overuse. The authors did attempt to minimize the possibility of misclassification by excluding children with comorbidities, those who had longer hospital stays, those admitted to the intensive care unit, and those with greater severity of illness where some of these tests and treatments would be indicated.
While the report by Reyes et al. focuses on Pediatric Hospital Medicine Choosing Wisely®recommendations, it is important to recognize that tests and treatments for conditions like asthma, bronchiolitis, and lower respiratory tract infections are initially performed in the emergency department (ED). Collaboration between the ED and the Hospital Medicine Unit is essential to tackle the issue of overuse.4
The study by Reyes et al. provides a nice description of the trends in the Choosing Wisely®overuse metrics at a group of children’s hospitals and is one of few such reports. The NIH funded, Eliminating Monitor Overuse: pulse oximetry (EMO: SpO2) study is focusing on the 5th Choosing Wisely® Pediatric Hospital Medicine recommendation that was not studied by Reyes.5
So then, with the decline in overuse reported in this study over 10 years, is it time to celebrate? Not yet. There is much work to do in the pursuit of Choosing Wisely®: developing a host of valid measures of overuse in pediatric hospital care, expanding the examination of overuse to community hospitals where the majority of children are hospitalized, and using implementation science theory to de-implement the ingrained practices.
1. Reyes M, Etigner B, Hall M, et al. Impact of the choosing wisely campaign recommendations for hospitalized children on clinical practice: trends from 2008 to 2017 [published online ahead of print September 18, 2019]. J Hosp Medicine. 2020;15(2):124-125. https://doi.org/10.12788/jhm.3291
2. Quinonez RA, Garber MD, Schroeder AR, et al. Choosing wisely in pediatric hospital medicine: five opportunities from improved healthcare value. J Hosp Med. 2013;8(9):479-495. https://doi.org/10.1002/jhm.2064.
3. Schuh S, Lalani A, Allen U, et al. Evaluation of the utility of radiography in acute bronchiolitis. J Pediatr. 2007;150(4):429-433. https://doi.org/16/j.jpeds.2007.01.005.
4. Mussman GM, Lossius M, Wasif F, et al. Multisite emergency department inpatient collaborative to reduce unnecessary bronchiolitis care. Pediatrics. 2018;141(2):e20170830. https://doi.org/10.1542/peds.2017-0830.
5. Rasooly IR, Beidas RS, Wolk CB, Barg F, et al. Measuring overuse of continuous pulse oximetry in bronchiolitis and developing strategies for large-scale deimplementation: study protocol for a feasibility trial. Pilot Feasibility Stud. 2019;5(1):68. https://doi.org/10.1186/s40814-019-0453-2.
1. Reyes M, Etigner B, Hall M, et al. Impact of the choosing wisely campaign recommendations for hospitalized children on clinical practice: trends from 2008 to 2017 [published online ahead of print September 18, 2019]. J Hosp Medicine. 2020;15(2):124-125. https://doi.org/10.12788/jhm.3291
2. Quinonez RA, Garber MD, Schroeder AR, et al. Choosing wisely in pediatric hospital medicine: five opportunities from improved healthcare value. J Hosp Med. 2013;8(9):479-495. https://doi.org/10.1002/jhm.2064.
3. Schuh S, Lalani A, Allen U, et al. Evaluation of the utility of radiography in acute bronchiolitis. J Pediatr. 2007;150(4):429-433. https://doi.org/16/j.jpeds.2007.01.005.
4. Mussman GM, Lossius M, Wasif F, et al. Multisite emergency department inpatient collaborative to reduce unnecessary bronchiolitis care. Pediatrics. 2018;141(2):e20170830. https://doi.org/10.1542/peds.2017-0830.
5. Rasooly IR, Beidas RS, Wolk CB, Barg F, et al. Measuring overuse of continuous pulse oximetry in bronchiolitis and developing strategies for large-scale deimplementation: study protocol for a feasibility trial. Pilot Feasibility Stud. 2019;5(1):68. https://doi.org/10.1186/s40814-019-0453-2.
© 2019 Society of Hospital Medicine
Quality and Safety of Pediatric Inpatient Care in Community Hospitals: A Scoping Review
Despite efforts to provide high-quality healthcare, Americans die from medical errors each year and many patients do not receive recommended medical care. Risk is particularly acute during times of hospitalization.1-4 In response, the Institute of Medicine (IOM, now the Academy of Medicine) has released “Crossing the Quality Chasm: A New Health System for the 21st Century,” providing a framework to guide delivery and measurement of high-quality healthcare.5
Although the IOM framework has motivated the development of quality improvement (QI) and quality measurement initiatives, relatively few resources have been allocated to improving the quality of pediatric inpatient care.6,7 The resultant gap in our knowledge of quality and safety of pediatric hospital-based care is further widened by the variability of settings in which children are hospitalized. These settings include freestanding children’s hospitals, children’s hospitals nested within larger hospitals, and community hospitals, defined as general, nonuniversity, and nonchildren’s hospitals.8
Although almost three-quarters of children needing hospitalization are cared for outside of freestanding children’s hospitals, we know particularly little about the quality and safety of pediatric hospital-based care outside of these settings.6,9 Therefore, our scoping review aims to summarize literature regarding the quality and safety of pediatric inpatient care within community hospitals.
METHODS
We used a scoping review approach because this methodology, by design, is utilized to synthesize evidence and map existing literature and is particularly useful when a body of literature is heterogeneous, rendering a more targeted systematic review approach infeasible.10 This methodology thereby provided an organized approach to answer our broad research question, “What evidence exists regarding the quality and safety of pediatric inpatient care in United States community hospitals?“ We followed the scoping review guidelines put forth by the Joanna Briggs Institute and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guideline Extension for Scoping Reviews.10,11
Data Sources and Search Strategies
We searched Medline, Medline-In-Process, Embase, the Cochrane Database of Systematic Reviews, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), PsycINFO, and Scopus for studies that reported at least one outcome related to healthcare quality or patient safety and involved pediatric patients (aged <18 years) receiving inpatient care at a community hospital. Outcomes included measures from the IOM-defined aims of quality healthcare: (1) safety, (2) effectiveness, (3) efficiency, (4) timeliness, (5) patient-centeredness, and (6) equity (Appendix Table 1). Terms were searched as controlled vocabulary in applicable databases (Medline, Embase, CINAHL, PsycINFO) and as keywords in all databases. Search strategies tailored to each database were developed, tested, and refined in collaboration with a reference librarian. Date parameters for retrieval were set from 1989 to the search date, with the start date chosen to correspond with the establishment of the Agency for Health Care Policy and Research, currently known as the Agency for Healthcare Research and Quality (AHRQ), targeting literature produced in the wake of the AHRQ emphasis on quality in healthcare. Results were limited to articles published in English. Searches were conducted on October 24 and 25, 2016. Complete search strategies can be found in Appendix Methods.
Independent authors performed handsearches of Academic Pediatrics, BMJ Quality & Safety, Hospital Pediatrics, JAMA Pediatrics, and Pediatrics Quality Reports for the five years preceding the search date (July 2011-October 2016).
Study Selection and Definitions
To identify studies conducted in community hospitals, we operationalized the definition of community hospital proposed by Percelay.8 We used “community”, “general”, “nonuniversity”, and “nonchildren’s” as search terms and operationalized these through the addition of qualifying descriptors (Table 1).
Studies were excluded if they (1) were performed outside of the United States, as community hospital definitions may differ by country; (2) included participants aged >18 years and did not report any pediatric-specific results; (3) were performed exclusively in tertiary hospitals; (4) evaluated only outpatient or emergency department care; (5) did not report any results specific to community hospitals; (6) did not report any data-driven or parent/patient-reported measures of safety, effectiveness, efficiency, timeliness, patient-centeredness, or equity in their results; and (7) were case reports, case series, editorials, or abstracts without an associated full-text article. We read commentaries and literature reviews related to our objectives and reviewed their references to identify additional articles, but did not include these manuscripts.
Two authors independently reviewed each abstract, and full-text articles were reviewed if one or more authors determined that the abstract met the inclusion criteria. Two authors then independently reviewed each full-text article to determine whether the article met the criteria for the final review. Disagreements were resolved through consensus after discussion and review with the entire research team, and reasons for exclusion were recorded.
Charting the Results and Data Synthesis
We used a standardized charting form to collect information regarding study design, community hospital terms, population descriptors, IOM aims of quality healthcare, and outcome measures. To minimize bias in data collection, information from each full-text article was extracted independently by two authors, and differences in extraction were resolved through discussion and re-review among the same two authors. To evaluate the quality of included evidence, two authors (JCL and JKL) independently assessed the risk of bias for each study using modified Newcastle-Ottawa Quality Assessment Scales (NOS), with disagreements resolved through discussion and re-review among the same two authors. For cohort studies, five of the eight NOS domains were relevant and applied to all included studies (maximum score 6). Using the NOS adapted for cross-sectional studies, six of the seven domains were relevant and applied (maximum score 9). For cross-sectional studies, we defined the risk of bias to be low for scores ≥8, moderate for scores 5-7, and high for scores ≤4, consistent with previous work.12 For cohort studies, we similarly defined these strata by scores ≥5, 3-4, and ≤2, given the lower maximum score for this study type.
We categorized studies as either observational, defined as cohort or cross-sectional studies of usual healthcare delivery, or interventional, defined as studies evaluating the development and/or implementation of an intervention designed to improve healthcare quality. We further categorized the studies into the following four overarching medical domains: (1) neonatal, (2) pediatric medicine, (3) surgery, or (4) radiology.
RESULTS
After removal of duplicates, our search identified 2,068 abstracts for screening (Figure). Of these, 1,777 did not meet the inclusion criteria, leaving 291 articles for full-text review. Of these, 43 articles met all the inclusion criteria, and one additional article was included from search of references, resulting in a total of 44 articles.
Study designs, patient populations, and quality outcome measures were heterogeneous. We identified only one randomized controlled trial (RCT). A total of 30 articles were observational studies, 27 of which used retrospective cohort or cross-sectional designs; the remaining three used prospective cohort designs (Table 2). Of these studies, 20 involved multiple hospitals, whereas 10 were conducted at a single community hospital. Sample sizes ranged from 29 (single-site) to 107,727 (multisite) patients. Twenty-two studies aimed to compare quality outcomes at community hospitals with other hospital types, of which 16 performed risk-adjusted analyses (detailed findings of observational studies are summarized in Appendix Table 2). The remaining 14 articles were interventional studies (Table 2). Of these, 12 (86%) reported improvement in quality outcomes after implementation (detailed findings of interventional studies are summarized in Appendix Table 3).
The included studies evaluated quality outcomes addressing all six of the IOM aims of quality healthcare, with safety, effectiveness, and efficiency being the most predominant (Table 3). Patient-centeredness and timeliness were infrequently addressed, and only one study assessed equity.
Risk of bias was moderate or high for 27 (69%) of the observational and interventional cohort studies and four (100%) of the included cross-sectional studies (Table 2), with the median NOS score being 4 (range: 0-6) for cohort studies (Appendix Table 4) and 4 (range: 3-6) for cross-sectional studies (Appendix Table 5). The higher risk of bias was largely driven by low comparability scores due to inadequate risk adjustment or statistical reporting. Of the 12 studies with low risk of bias, 11 (92%) were multisite, 9 (75%) used large regional or national databases, and half reported quality outcomes limited to hospital charges and/or mortality.
Observational Studies
Neonatal Medicine
Five multisite studies focused on neonatal care,13,14,16,19,20 of which four examined outcomes associated with the transfer of neonates to or from community hospitals to tertiary care hospitals, such as neonatal morbidity, readmission, completion of preventative health measures and screening, parent satisfaction, and hospital charges.14,16,19,20 For example, in a study of extremely premature very low birth weight infants born in Hawaii, Kuo et al. demonstrated that the odds of retinopathy of prematurity was 2.9 times higher for infants born at a community hospital and transported to their tertiary center compared with those inborn at the tertiary center (P = .02).16 The fifth study examined neonatal mortality by site of birth, demonstrating that, among infants with birth weights less than 2,000 g, birth at a hospital with a community neonatal intensive care unit (NICU) was associated with 1.4 times higher odds of risk-adjusted mortality compared with birth at a regional NICU (P < .001).13
Three studies evaluated the quality of neonatal care at a single community hospital.15,17,18 Quality outcomes were heterogeneous, including utility of rebound bilirubin levels for infants with jaundice, morbidity of neonates requiring mechanical ventilation, and provision of breastfeeding advice to mothers of breastfeeding infants. For instance, Meadow et al. attempted to determine the quality of care for ventilated neonates at one community NICU compared with a tertiary hospital.18 They found no difference in days on ventilation or need for home oxygen therapy between the community hospital and the tertiary center, although P values and effect sizes were not reported for these outcomes and analyses were not adjusted beyond matching on birthdate and birth weight.
Pediatric Medicine
Nine multisite studies explored the quality and safety of pediatric medical care across hospital types.21-23,25-30 Of these, four were conducted using the Kids’ Inpatient Database (KID)28-30 or the National Inpatient Sample (NIS),27 two were conducted using other national databases,21,25 and two were conducted using electronic medical record data.22,26 All of the KID and NIS studies examined hospital charges, either alone or in conjunction with mortality. Two of these studies found no differences in risk-adjusted hospital charges between children’s hospitals and community hospitals (for burn injuries),28,29 whereas two found that hospitalization at community hospitals was associated with lower risk-adjusted charges (for asthma and sepsis).27,30 The remaining five studies evaluated diverse quality outcomes such as medication errors, therapeutic drug monitoring, practice guideline compliance, antibiotic prescribing, or hospital-to-home transition summary scores.21-23,25,26
Three single-site studies examined quality outcomes for pediatric medical patients, including mortality, outcome ratings for dehydration, and measures of peripherally inserted central catheter (PICC) safety and effectiveness.24,31,32 For example, Frank et al. evaluated safety of care in one community hospital pediatric intensive care unit (PICU) compared with a tertiary hospital using the Pediatric Risk of Mortality (PRISM) score.24 They reported that the observed number of deaths in their community PICU did not differ significantly from the number of deaths predicted in a tertiary center (23 vs 33, respectively, P > .2).
Surgery
Three multisite studies examined quality outcomes among children with surgical conditions, including surgical complications, readmission, and hospital charges.34,35,37 For example, Kelley-Quon et al. examined outcomes following surgery in infants with hypertrophic pyloric stenosis and found that infants who received their surgery at community hospitals had twice the odds of a surgical complication compared with those at children’s hospitals (P = .027).34 They also examined how the risk of appendiceal perforation differed by hospital type, finding that black children who received their surgery at children’s hospitals had twice the odds of appendiceal perforation compared with those who received care at community hospitals.35
In addition, two studies evaluated quality and safety outcomes for pediatric surgical care in a single community hospital.33,36 For example, Beaty et al. prospectively evaluated the incidence of missed injuries in hospitalized pediatric trauma patients, reporting that the incidence of missed injury was 33% when admission evaluation was performed by a trauma surgeon alone compared to 11% when performed by a pediatric doctor or a trauma surgeon and a pediatric doctor together (P < .001).33
Radiology
Three multisite studies examined quality and safety outcomes associated with radiographic imaging in community hospitals, including radiation dosing and frequency of preoperative imaging modalities and accuracy.38,39,41 For instance, Marin et al. found substantial variation in radiation dose across hospital types, with children’s hospitals delivering lower median radiation doses than academic and community hospitals.39 Similarly, Saito et al. demonstrated increased use of radiating modalities when evaluation was performed at community hospitals, with four times higher odds of computed tomography (CT) and five times lower odds of ultrasound use compared to a children’s hospital.41
Two single-site studies also evaluated quality and safety outcomes associated with the use of radiographic imaging for pediatric appendicitis in a community hospital.40,42 For example, York et al. demonstrated that, compared with nonimaged patients, patients who underwent diagnostic imaging for appendicitis experienced a significant time delay from initial evaluation to surgery and incurred significantly higher hospital charges, whereas there were no significant differences in intraoperative findings, antibiotic requirements, and surgical complications between the groups.42
Interventional Studies
Neonatal Medicine
We identified six studies that evaluated interventions to improve healthcare quality for neonates in community hospitals; two involved telemedicine.43-48 Hall et al. described “Telenursery,” a program linking regional perinatal centers with a large academic neonatal practice through real-time teleconferencing, in addition to providing weekly educational conferences.45 After its implementation, there was an increase in the state-recommended delivery of very low birth weight infants at the regional perinatal center from 24% to 33% (P < .05); clinical outcomes were not discussed. In a similar study, Sable et al. found that a videoconferencing system for cardiologists from an academic center to guide care in a community setting provided diagnostic services more quickly (28 minutes vs 12 hours) and had high diagnostic accuracy.47 The remaining four studies evaluated heterogeneous interventions such as a maternal education program to reduce shaking injuries to infants or implementation of evidence-based order sets to reduce early onset group B streptococcal disease in neonates.43,44,46,48 Five of the six neonatal studies demonstrated improved quality outcomes after intervention.43-47
Pediatric Medicine
Seven studies evaluated QI interventions for children at community hospitals,49-55 three of which described interventions to improve quality of management of respiratory diseases.49,51,53 Dayal et al. evaluated the implementation of respiratory illness order sets and an asthma pathway, demonstrating a 41% reduction in asthma hospitalization cost per patient (P < .05), reduced bronchodilator use for all respiratory illnesses, and no change in readmission rates.49 Similarly, Nkoy et al. evaluated an asthma “Evidence-Based Care Practice Model” implemented at seven community hospitals and demonstrated a nonsignificant reduction in readmissions (P = .12), as well as lower hospitalization costs (P = .05).53 Using QI methods, Kuhlmann et al. demonstrated improved compliance from 43% to 97% with the Asthma Home Management Plan of Care measure.51 The remaining four studies evaluated heterogeneous interventions, including telemedicine critical care consultations, use of I-PASS, and consolidation of pediatric care onto one hospital unit.50,52,54,55 Six of the seven pediatric studies (86%) demonstrated improved inpatient quality outcomes after intervention,49,51-55 but only one study was multisite.53
Surgery
We identified only one study evaluating a surgical intervention aimed at improving the quality of pediatric care.56 Kelley-Quon evaluated the impact of a community hospital partnering with an Academic Medical Pediatric Trauma Center to become a Level II Pediatric Trauma Center (PTC). After achieving Level II PTC designation, they reported that children treated at the community hospital had reduced rates of CT use, transfers, and in-hospital mortality (from 81% to 51%, 8.5% to 2.5%, and 2% to 0.4%, respectively, P < .05 for all) compared to those treated predesignation.
Radiology
Within the domain of radiology, our review identified no interventional studies.
DISCUSSION
In this scoping review of the quality and safety of pediatric inpatient care in community hospitals, we identified 44 studies applying heterogeneous study designs and evaluating diverse patient populations and quality outcomes. We identified only one RCT in our search; all the remaining studies applied observational designs.
We found only three clinical areas that were explored in multiple studies, with consistent directionality of results: (1) perinatal regionalization, (2) telemedicine, and (3) imaging radiation. The limited evidence identified in our review suggests that delivery of early premature infants at community hospitals, rather than at tertiary hospitals, may increase risk of neonatal morbidity and/or mortality,13,16,46 that use of telemedicine may improve the effectiveness and efficiency of intensive or specialized care in community settings,45,47,52,55 and that CT use and radiation doses may be higher in community hospitals compared with other settings.38,39,41 However, even within these clinical domains, the literature was limited in amount and heterogeneous; additional research is needed to systematically review the effect of individual interventions or particular community hospital quality outcomes compared with other hospital types.
Our search identified only 14 studies evaluating QI interventions within community hospitals over the almost 30-year review period. Although limited in number, 86% of these demonstrated improvements in healthcare quality and safety, providing a positive “proof of concept” that pediatric care in community hospitals can be improved by multidisciplinary efforts and be sustained over time.43-47,49,51-56 As pediatric departments within community hospitals may have limited resources, aligning pediatric QI efforts with adult initiatives within the same hospitals could prove advantageous. However, we did not identify any studies meeting our inclusion criteria that took this approach. Alternatively, QI collaboratives across structurally diverse hospitals may provide valuable infrastructure for QI in community hospitals. For example, the Value in Inpatient Pediatrics Network has conducted several multisite QI initiatives that have engaged both children’s and community hospitals.57-60 However, none of these studies have reported community hospital-specific quality outcomes, resulting in their exclusion from this review. In future study, researchers may consider separating community hospital results from those of pediatric hospitals to highlight the effect of community hospital-specific QI efforts and to allow valuable direct comparisons between hospital types.
Many studies identified in our scoping review were conducted at single hospitals. Sample sizes were often very small and power calculations were rarely reported, raising questions about the validity of the “nonsignificant” differences reported by some. Although such single-center studies provide valuable information for local improvement of inpatient care, by design the findings from these studies are unlikely generalizable to other community hospital systems. Without inclusion of another hospital type and/or quality measures with clear national benchmark for comparison, the additional conclusions that can be drawn from these studies are limited. In comparison, many of the multicenter studies had large study samples, but more than half used data registries with limited data to evaluate outcomes, clinical context, or important covariates.13,20,21,25,27-30,34,35,37,39 The most frequently reported outcomes in these data sets—hospital charges and lengths of stay—are of limited utility in understanding healthcare quality without clear benchmarks. As a result, the evidence-base from which to draw conclusions about quality and safety in community hospitals is very limited.
Therefore, our review highlights a great need for additional research in community hospital medicine and the need for high-quality evidence generation. Risk of bias was moderate or high for the majority of included studies because of inadequate risk adjustment or statistical analysis. In the future, multicenter collaborations may help to connect research methodologists with community hospital teams to aid in the application of robust study designs and analytic techniques. Multisite collaboration may also overcome the limitation of small sample sizes that are a reality at many community hospitals.
Our study findings must be considered in the setting of several methodological limitations. The lack of a standard definition for a community hospital has led to inconsistent terms and hospital definitions used in the literature. It is possible that, while following our systematic approach to define a community hospital, we inadvertently missed relevant studies that used different terms. We also excluded unpublished articles. Given that publication bias tends to favor studies with significant associations, it is possible that some studies with insignificant changes in quality outcomes were missed. Finally, in our exclusion of all non-US studies, we may have unknowingly missed literature from countries with community hospital definitions similar to those in the United States.
CONCLUSIONS
Recognizing that more than half of all children admitted to hospitals in the US receive their care at community hospitals, understanding healthcare quality in community hospitals is important. This scoping review underscores the need for additional research and higher quality evidence to determine the quality of pediatric inpatient care in these settings and identifies some particularly wide gaps that could be targeted in future research. Acknowledging that further research is necessary to address all aims of quality healthcare, markedly few studies have examined timeliness, equity, or patient-centeredness. Collaborations between academic medical centers and community hospitals may be an effective means to connect researchers with community hospital clinical teams to facilitate the application of robust study designs and analytic approaches and to facilitate multisite investigations. Research in this field would benefit from a standardized definition of a community hospital that could be consistently applied in research and QI endeavors.
Disclosures
The authors have no potential conflicts of interest to disclose.
Funding
Jana Leary was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, Grant Number 5TL1TR0001062-03. JoAnna Leyenaar was supported by the Agency for Healthcare Research and Quality (K08HS024133).
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the AHRQ.
1. McGlynn EA, Asch SM, Adams J, et al. The quality of health care delivered to adults in the United States. N Engl J Med. 2003;348(26):2635-2645. https://doi.org/10.1056/NEJMsa022615.
2. Richardson WC, Berwick DM, Bisgard C, et al. To err is human: building a safer health system-Institute of Medicine. Medscape. http://www.iom.edu/Reports/1999/To-Err-is-Human-Building-A-Safer-Health-System.aspx; 2000. Accessed October 7, 2016.
3. Classen DC, Resar R, Griffin F, et al. ‘Global Trigger Tool’ shows that adverse events in hospitals may be ten times greater than previously measured. Health Aff. 2011;30(4):581-589. https://doi.org/10.1377/hlthaff.2011.0190.
4. Stockwell DC, Landrigan CP, Schuster MA, et al. Using a pediatric trigger tool to estimate total harm burden hospital-acquired conditions represent. Pediatr Qual Saf. 2018;3(3):e081. https://doi.org/10.1097/pq9.0000000000000081.
5. Institute of Medicine (US) Committee on Quality of Health Care in America. Crossing the Quality Chasm. Washington (DC): National Academies Press (US); 2001. https://doi.org/10.17226/10027.
6. Rauch DA, Lye PS, Carlson D, et al. Pediatric hospital medicine: a strategic planning roundtable to chart the future. J Hosp Med. 2012;7(4):329-334. https://doi.org/10.1002/jhm.950.
7. Simpson L, Fairbrother G, Hale S et al. Reauthorizing SCHIP: Opportunities for Promoting Effective Health Coverage and HighQuality Care for Children and Adolescents. (Publication 1051). Commonw Fund; 2007.
8. Percelay JM. Pediatric hospitalists working in community hospitals. Pediatr Clin North Am. 2014;61(4):681-691. https://doi.org/10.1016/j.pcl.2014.04.005.
9. Leyenaar JK, Ralston SL, Shieh MS et al. Epidemiology of pediatric hospitalizations at general hospitals and freestanding children’s hospitals in the United States. J Hosp Med. 2016;11(11):743-749. https://doi.org/10.1002/jhm.2624.
10. Peters MDJ, Godfrey CM, Khalil H et al. Guidance for conducting systematic scoping reviews. Int J Evid Based Healthc. 2015;13(3):141-146. https://doi.org/10.1097/XEB.0000000000000050.
11. Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467-473. https://doi.org/10.7326/M18-0850.
12. Alobaidi R, Morgan C, Basu RK, et al. Association between fluid balance and outcomes in critically ill children: a systematic review and meta-analysis. JAMA Pediatr. 2018;172(3):257-268. https://doi.org/10.1001/jamapediatrics.2017.4540.
13. Cifuentes J, Bronstein J, Phibbs CS et al. Mortality in low birth weight infants according to level of neonatal care at hospital of birth. Pediatrics. 2002;109(5):745-751. https://doi.org/10.1542/peds.109.5.745.
14. Donohue PK, Hussey-Gardner B, Sulpar LJ, Fox R, Aucott SW. Convalescent care of infants in the neonatal intensive care unit in community hospitals: risk or benefit? Pediatrics. 2009;124(1):105-111. https://doi.org/10.1542/peds.2008-0880.
15. Izatt SD. Breastfeeding counseling by health care providers. J Hum Lact. 1997;13(2):109-113. https://doi.org/10.1177/089033449701300210.
16. Kuo S, Kimata C, Akamine K, Young B, Balaraman V. Outcomes of inborn and transported extremely premature very-low-birthweight infants in Hawai’i. Pediatr Int. 2012;54(3):365-369. https://doi.org/10.1111/j.1442-200X.2012.03561.x.
17. Maisels MJ, Kring E. Rebound in serum bilirubin level following intensive phototherapy. Arch Pediatr Adolesc Med. 2002;156(7):669-672. https://doi.org/10.1001/archpedi.156.7.669.
18. Meadow W, Mendez D, Makela J et al. Can and should level II nurseries care for newborns who require mechanical ventilation? Clin Perinatol. 1996;23(3):551-561. https://doi.org/10.1016/S0095-5108(18)30227-6.
19. Phibbs CS, Mortensen L. Back transporting infants from neonatal intensive care units to community hospitals for recovery care: effect on total hospital charges. Pediatrics. 1992;90(1):22-26.
20. Wall SN, Handler AS, Park CG. Hospital factors and nontransfer of small babies: A marker of deregionalized perinatal care? J Perinatol. 2004;24(6):351-359. https://doi.org/10.1038/sj.jp.7211101.
21. Alexander DC, Bundy DG, Shore AD et al. Cardiovascular medication errors in children. Pediatrics. 2009;124(1):324-332. https://doi.org/10.1542/peds.2008-2073.
22. Balch AH, Constance JE, Thorell EA, et al. Pediatric vancomycin dosing: trends over time and the impact of therapeutic drug monitoring. J Clin Pharmacol. 2015;55(2):212-220. https://doi.org/10.1002/jcph.402.
23. Conway PH, Edwards S, Stucky ER et al. Variations in management of common inpatient pediatric illnesses: hospitalists and community pediatricians. Pediatrics. 2006;118(2):441-447. https://doi.org/10.1542/peds.2006-0484.
24. Frank BS, Pollack MM. Quantitative quality assurance in a community hospital pediatric intensive care unit. West J Med. 1992;157(2):149-151.
25. Leyenaar JK, Lagu T, Shieh MS, Pekow PS, Lindenauer PK. Variation in resource utilization for the management of uncomplicated community-acquired pneumonia across community and children’s hospitals. J Pediatr. 2014;165(3):585-591. https://doi.org/10.1016/j.jpeds.2014.04.062.
26. Leyenaar JK, Desai AD, Burkhart Q, et al. Quality measures to assess care transitions for hospitalized children. Pediatrics. 2016;138(2):e20160906. https://doi.org/10.1542/peds.2016-0906.
27. Meurer JR, Kuhn EM, George V, Yauck JS, Layde PM. Charges for childhood asthma by hospital characteristics. Pediatrics. 1998;102(6):E70. https://doi.org/10.1542/peds.102.6.e70.
28. Myers J, Lehna C. Where are lengths of stay longer and total charges higher for pediatric burn patients? J Burn Care Res. 2014;35(5):382-387. https://doi.org/10.1097/BCR.0000000000000012.
29. Myers J, Smith M, Woods C, Espinosa C, Lehna C. The effect of transfers between health care facilities on costs and length of stay for pediatric burn patients. J Burn Care Res. 2015;36(1):178-183. https://doi.org/10.1097/BCR.0000000000000206.
30. Odetola FO, Gebremariam A, Freed GL. Patient and hospital correlates of clinical outcomes and resource utilization in severe pediatric sepsis. Pediatrics. 2007;119(3):487-494. https://doi.org/10.1542/peds.2006-2353.
31. Scherb CA, Stevens MS, Busman C. Outcomes related to dehydration in the pediatric population. J Pediatr Nurs. 2007;22(5):376-382. https://doi.org/10.1016/j.pedn.2006.10.004.
32. Van Winkle P, Whiffen T, Liu IL. Experience using peripherally inserted central venous catheters for outpatient parenteral antibiotic therapy in children at a community hospital. ediatr Infect Dis J. 2008;27(12):1069-1072. https://doi.org/10.1097/INF.0b013e31817d32f2.
33. Beaty JS, Chendrasekhar A, Hopkins J, Gruelke L. Missed injuries in pediatric trauma patients. J Appl Res. 2003;3(1):84-88. https://jrnlappliedresearch.com/articles/Vol3Iss1/CHENDRASEKHAR.htm. Accessed July 8, 2019.
34. Kelley-Quon LI, Tseng CH, Jen HC, Shew SB. Hospital type predicts surgical complications for infants with hypertrophic pyloric stenosis. Am Surg. 2012;78(10):1079-1082.
35. Kelley-Quon LI, Tseng CH, Jen HC, Lee SL, Shew SB. Hospital type as a metric for racial disparities in pediatric appendicitis. J Am Coll Surg. 2013;216(1):74-82. https://doi.org/10.1016/j.jamcollsurg.2012.09.018.
36. Pokala N, Sadhasivam S, Kiran RP, Parithivel V. Complicated appendicitis—is the laparoscopic approach appropriate? A comparative study with the open approach: outcome in a community hospital setting. Am Surg. 2007;73(8):732-737.
37. Smith JT, Price C, Stevens PM, Masters KS, Young M. Does pediatric orthopedic subspecialization affect hospital utilization and charges? J Pediatr Orthop. 1999;19(4):553-555. https://doi.org/10.1097/01241398-199907000-00027.
38. Calvert C, Strauss KJ, Mooney DP. Variation in computed tomography radiation dose in community hospitals. J Pediatr Surg. 2012;47(6):1167-1169. https://doi.org/10.1016/j.jpedsurg.2012.03.021.
39. Marin JR, Sengupta D, Bhargavan-Chatfield M et al. Variation in pediatric cervical spine computed tomography radiation dose index. Acad Emerg Med. 2015;22(12):1499-1505. https://doi.org/10.1111/acem.12822.
40. Reich JD, Brogdon B, Ray WE, Eckert J, Gorell H. Use of CT scan in the diagnosis of pediatric acute appendicitis. Pediatr Emerg Care. 2000;16(4):241-243. https://doi.org/10.1097/00006565-200008000-00006.
41. Saito JM, Yan Y, Evashwick TW, Warner BW, Tarr PI. Use and accuracy of diagnostic imaging by hospital type in pediatric appendicitis. Pediatrics. 2013;131(1):e37-e44. https://doi.org/10.1542/peds.2012-1665.
42. York D, Smith A, Phillips JD, Von Allmen D. The influence of advanced radiographic imaging on the treatment of pediatric appendicitis. J Pediatr Surg. 2005;40(12):1908-1911. https://doi.org/10.1016/j.jpedsurg.2005.08.004.
43. Altman RL, Canter J, Patrick PA et al. Parent education by maternity nurses and prevention of abusive head trauma. Pediatrics. 2011;128(5):e1164-e1172. https://doi.org/10.1542/peds.2010-3260.
44. Clemens CJ, Gable EK. The development of a group B streptococcus prevention policy at a community hospital. J Perinatol. 2002;22(7):523-525. https://doi.org/10.1038/sj.jp.7210794.
45. Hall RW, Hall-Barrow J, Garcia-Rill E. Neonatal regionalization through telemedicine using a community-based research and education core facility. Ethn Dis. 2010;20(1 Suppl 1):S136-S140.
46. Hulsey TC, Pittard WB 3rd, Ebeling M. Regionalized perinatal transport systems: association with changes in location of birth, neonatal transport, and survival of very low birth weight deliveries. J S C Med Assoc. 1991;87(12):581-584.
47. Sable CA, Cummings SD, Pearson GD, et al. Impact of telemedicine on the practice of pediatric cardiology in community hospitals. Pediatrics. 2002;109(1):E3. https://doi.org/10.1542/peds.109.1.e3.
48. Wexelblatt SL, Ward LP, Torok K et al. Universal maternal drug testing in a high-prevalence region of prescription opiate abuse. J Pediatr. 2015;166(3):582-586. https://doi.org/10.1016/j.jpeds.2014.10.004.
49. Dayal A, Alvarez F. The effect of implementation of standardized, evidence-based order sets on efficiency and quality measures for pediatric respiratory illnesses in a community hospital. Hosp Pediatr. 2015;5(12):624-629. https://doi.org/10.1542/hpeds.2015-0140.
50. Krugman SD, Suggs A, Photowala HY, Beck A. Redefining the community pediatric hospitalist: the combined Pediatric ED/Inpatient Unit. Pediatr Emerg Care. 2007;23(1):33-37. https://doi.org/10.1097/01.pec.0000248685.94647.01.
51. Kuhlmann S, Mason B, Ahlers-Schmidt CR. A quality improvement project to improve compliance with the joint commission children’s asthma care-3 measure. Hosp Pediatr. 2013;3(1):45-51. https://doi.org/10.1542/hpeds.2012-0015.
52. Labarbera JM, Ellenby MS, Bouressa P et al. The impact of telemedicine intensivist support and a pediatric hospitalist program on a community hospital. Telemed J E Health. 2013;19(10):760-766. https://doi.org/10.1089/tmj.2012.0303.
53. Nkoy F, Fassl B, Stone B, et al. Improving pediatric asthma care and outcomes Across multiple hospitals. Pediatrics. 2015;136(6):e1602-e1610. https://doi.org/10.1542/peds.2015-0285.
54. Walia J, Qayumi Z, Khawar N, et al. Physician transition of care: benefits of I-PASS and an electronic handoff system in a community pediatric residency program. Acad Pediatr. 2016;16(6):519-523. https://doi.org/10.1016/j.acap.2016.04.001.
55. Yang CP, Hunt EA, Shilkofski N et al. Can telemedicine improve adherence to resuscitation guidelines for critically ill children at community hospitals? A randomized controlled trial using high-fidelity simulation. Pediatr Emerg Care. 2017;33(7):474-479. https://doi.org/10.1097/PEC.0000000000000653.
56. Kelley-Quon LI, Crowley MA, Applebaum H, et al. Academic-community partnerships improve outcomes in pediatric trauma care. J Pediatr Surg. 2015;50(6):1032-1036. https://doi.org/10.1016/j.jpedsurg.2015.03.033.
57. Ralston S, Garber M, Narang S, et al. Decreasing unnecessary utilization in acute bronchiolitis care: results from the value in inpatient pediatrics network. J Hosp Med. 2013;8(1):25-30. https://doi.org/10.1002/jhm.1982.
58. Ralston SL, Garber MD, Rice-Conboy E, et al. A multicenter collaborative to reduce unnecessary care in inpatient bronchiolitis. Pediatrics. 2016;137(1):e20150851. https://doi.org/10.1542/peds.2015-0851.
59. Parikh K, Biondi E, Nazif J, et al. A multicenter collaborative to improve care of community acquired pneumonia in hospitalized children. Pediatrics. 2017;139(3):e20161411. https://doi.org/10.1542/peds.2016-1411.
60. Leyenaar JK, Bergert L, Mallory LA, et al. Pediatric primary care providers’ perspectives regarding hospital discharge communication: a mixed methods analysis. Acad Pediatr. 2015;15(1):61-68. https://doi.org/10.1016/j.acap.2014.07.004.
Despite efforts to provide high-quality healthcare, Americans die from medical errors each year and many patients do not receive recommended medical care. Risk is particularly acute during times of hospitalization.1-4 In response, the Institute of Medicine (IOM, now the Academy of Medicine) has released “Crossing the Quality Chasm: A New Health System for the 21st Century,” providing a framework to guide delivery and measurement of high-quality healthcare.5
Although the IOM framework has motivated the development of quality improvement (QI) and quality measurement initiatives, relatively few resources have been allocated to improving the quality of pediatric inpatient care.6,7 The resultant gap in our knowledge of quality and safety of pediatric hospital-based care is further widened by the variability of settings in which children are hospitalized. These settings include freestanding children’s hospitals, children’s hospitals nested within larger hospitals, and community hospitals, defined as general, nonuniversity, and nonchildren’s hospitals.8
Although almost three-quarters of children needing hospitalization are cared for outside of freestanding children’s hospitals, we know particularly little about the quality and safety of pediatric hospital-based care outside of these settings.6,9 Therefore, our scoping review aims to summarize literature regarding the quality and safety of pediatric inpatient care within community hospitals.
METHODS
We used a scoping review approach because this methodology, by design, is utilized to synthesize evidence and map existing literature and is particularly useful when a body of literature is heterogeneous, rendering a more targeted systematic review approach infeasible.10 This methodology thereby provided an organized approach to answer our broad research question, “What evidence exists regarding the quality and safety of pediatric inpatient care in United States community hospitals?“ We followed the scoping review guidelines put forth by the Joanna Briggs Institute and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guideline Extension for Scoping Reviews.10,11
Data Sources and Search Strategies
We searched Medline, Medline-In-Process, Embase, the Cochrane Database of Systematic Reviews, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), PsycINFO, and Scopus for studies that reported at least one outcome related to healthcare quality or patient safety and involved pediatric patients (aged <18 years) receiving inpatient care at a community hospital. Outcomes included measures from the IOM-defined aims of quality healthcare: (1) safety, (2) effectiveness, (3) efficiency, (4) timeliness, (5) patient-centeredness, and (6) equity (Appendix Table 1). Terms were searched as controlled vocabulary in applicable databases (Medline, Embase, CINAHL, PsycINFO) and as keywords in all databases. Search strategies tailored to each database were developed, tested, and refined in collaboration with a reference librarian. Date parameters for retrieval were set from 1989 to the search date, with the start date chosen to correspond with the establishment of the Agency for Health Care Policy and Research, currently known as the Agency for Healthcare Research and Quality (AHRQ), targeting literature produced in the wake of the AHRQ emphasis on quality in healthcare. Results were limited to articles published in English. Searches were conducted on October 24 and 25, 2016. Complete search strategies can be found in Appendix Methods.
Independent authors performed handsearches of Academic Pediatrics, BMJ Quality & Safety, Hospital Pediatrics, JAMA Pediatrics, and Pediatrics Quality Reports for the five years preceding the search date (July 2011-October 2016).
Study Selection and Definitions
To identify studies conducted in community hospitals, we operationalized the definition of community hospital proposed by Percelay.8 We used “community”, “general”, “nonuniversity”, and “nonchildren’s” as search terms and operationalized these through the addition of qualifying descriptors (Table 1).
Studies were excluded if they (1) were performed outside of the United States, as community hospital definitions may differ by country; (2) included participants aged >18 years and did not report any pediatric-specific results; (3) were performed exclusively in tertiary hospitals; (4) evaluated only outpatient or emergency department care; (5) did not report any results specific to community hospitals; (6) did not report any data-driven or parent/patient-reported measures of safety, effectiveness, efficiency, timeliness, patient-centeredness, or equity in their results; and (7) were case reports, case series, editorials, or abstracts without an associated full-text article. We read commentaries and literature reviews related to our objectives and reviewed their references to identify additional articles, but did not include these manuscripts.
Two authors independently reviewed each abstract, and full-text articles were reviewed if one or more authors determined that the abstract met the inclusion criteria. Two authors then independently reviewed each full-text article to determine whether the article met the criteria for the final review. Disagreements were resolved through consensus after discussion and review with the entire research team, and reasons for exclusion were recorded.
Charting the Results and Data Synthesis
We used a standardized charting form to collect information regarding study design, community hospital terms, population descriptors, IOM aims of quality healthcare, and outcome measures. To minimize bias in data collection, information from each full-text article was extracted independently by two authors, and differences in extraction were resolved through discussion and re-review among the same two authors. To evaluate the quality of included evidence, two authors (JCL and JKL) independently assessed the risk of bias for each study using modified Newcastle-Ottawa Quality Assessment Scales (NOS), with disagreements resolved through discussion and re-review among the same two authors. For cohort studies, five of the eight NOS domains were relevant and applied to all included studies (maximum score 6). Using the NOS adapted for cross-sectional studies, six of the seven domains were relevant and applied (maximum score 9). For cross-sectional studies, we defined the risk of bias to be low for scores ≥8, moderate for scores 5-7, and high for scores ≤4, consistent with previous work.12 For cohort studies, we similarly defined these strata by scores ≥5, 3-4, and ≤2, given the lower maximum score for this study type.
We categorized studies as either observational, defined as cohort or cross-sectional studies of usual healthcare delivery, or interventional, defined as studies evaluating the development and/or implementation of an intervention designed to improve healthcare quality. We further categorized the studies into the following four overarching medical domains: (1) neonatal, (2) pediatric medicine, (3) surgery, or (4) radiology.
RESULTS
After removal of duplicates, our search identified 2,068 abstracts for screening (Figure). Of these, 1,777 did not meet the inclusion criteria, leaving 291 articles for full-text review. Of these, 43 articles met all the inclusion criteria, and one additional article was included from search of references, resulting in a total of 44 articles.
Study designs, patient populations, and quality outcome measures were heterogeneous. We identified only one randomized controlled trial (RCT). A total of 30 articles were observational studies, 27 of which used retrospective cohort or cross-sectional designs; the remaining three used prospective cohort designs (Table 2). Of these studies, 20 involved multiple hospitals, whereas 10 were conducted at a single community hospital. Sample sizes ranged from 29 (single-site) to 107,727 (multisite) patients. Twenty-two studies aimed to compare quality outcomes at community hospitals with other hospital types, of which 16 performed risk-adjusted analyses (detailed findings of observational studies are summarized in Appendix Table 2). The remaining 14 articles were interventional studies (Table 2). Of these, 12 (86%) reported improvement in quality outcomes after implementation (detailed findings of interventional studies are summarized in Appendix Table 3).
The included studies evaluated quality outcomes addressing all six of the IOM aims of quality healthcare, with safety, effectiveness, and efficiency being the most predominant (Table 3). Patient-centeredness and timeliness were infrequently addressed, and only one study assessed equity.
Risk of bias was moderate or high for 27 (69%) of the observational and interventional cohort studies and four (100%) of the included cross-sectional studies (Table 2), with the median NOS score being 4 (range: 0-6) for cohort studies (Appendix Table 4) and 4 (range: 3-6) for cross-sectional studies (Appendix Table 5). The higher risk of bias was largely driven by low comparability scores due to inadequate risk adjustment or statistical reporting. Of the 12 studies with low risk of bias, 11 (92%) were multisite, 9 (75%) used large regional or national databases, and half reported quality outcomes limited to hospital charges and/or mortality.
Observational Studies
Neonatal Medicine
Five multisite studies focused on neonatal care,13,14,16,19,20 of which four examined outcomes associated with the transfer of neonates to or from community hospitals to tertiary care hospitals, such as neonatal morbidity, readmission, completion of preventative health measures and screening, parent satisfaction, and hospital charges.14,16,19,20 For example, in a study of extremely premature very low birth weight infants born in Hawaii, Kuo et al. demonstrated that the odds of retinopathy of prematurity was 2.9 times higher for infants born at a community hospital and transported to their tertiary center compared with those inborn at the tertiary center (P = .02).16 The fifth study examined neonatal mortality by site of birth, demonstrating that, among infants with birth weights less than 2,000 g, birth at a hospital with a community neonatal intensive care unit (NICU) was associated with 1.4 times higher odds of risk-adjusted mortality compared with birth at a regional NICU (P < .001).13
Three studies evaluated the quality of neonatal care at a single community hospital.15,17,18 Quality outcomes were heterogeneous, including utility of rebound bilirubin levels for infants with jaundice, morbidity of neonates requiring mechanical ventilation, and provision of breastfeeding advice to mothers of breastfeeding infants. For instance, Meadow et al. attempted to determine the quality of care for ventilated neonates at one community NICU compared with a tertiary hospital.18 They found no difference in days on ventilation or need for home oxygen therapy between the community hospital and the tertiary center, although P values and effect sizes were not reported for these outcomes and analyses were not adjusted beyond matching on birthdate and birth weight.
Pediatric Medicine
Nine multisite studies explored the quality and safety of pediatric medical care across hospital types.21-23,25-30 Of these, four were conducted using the Kids’ Inpatient Database (KID)28-30 or the National Inpatient Sample (NIS),27 two were conducted using other national databases,21,25 and two were conducted using electronic medical record data.22,26 All of the KID and NIS studies examined hospital charges, either alone or in conjunction with mortality. Two of these studies found no differences in risk-adjusted hospital charges between children’s hospitals and community hospitals (for burn injuries),28,29 whereas two found that hospitalization at community hospitals was associated with lower risk-adjusted charges (for asthma and sepsis).27,30 The remaining five studies evaluated diverse quality outcomes such as medication errors, therapeutic drug monitoring, practice guideline compliance, antibiotic prescribing, or hospital-to-home transition summary scores.21-23,25,26
Three single-site studies examined quality outcomes for pediatric medical patients, including mortality, outcome ratings for dehydration, and measures of peripherally inserted central catheter (PICC) safety and effectiveness.24,31,32 For example, Frank et al. evaluated safety of care in one community hospital pediatric intensive care unit (PICU) compared with a tertiary hospital using the Pediatric Risk of Mortality (PRISM) score.24 They reported that the observed number of deaths in their community PICU did not differ significantly from the number of deaths predicted in a tertiary center (23 vs 33, respectively, P > .2).
Surgery
Three multisite studies examined quality outcomes among children with surgical conditions, including surgical complications, readmission, and hospital charges.34,35,37 For example, Kelley-Quon et al. examined outcomes following surgery in infants with hypertrophic pyloric stenosis and found that infants who received their surgery at community hospitals had twice the odds of a surgical complication compared with those at children’s hospitals (P = .027).34 They also examined how the risk of appendiceal perforation differed by hospital type, finding that black children who received their surgery at children’s hospitals had twice the odds of appendiceal perforation compared with those who received care at community hospitals.35
In addition, two studies evaluated quality and safety outcomes for pediatric surgical care in a single community hospital.33,36 For example, Beaty et al. prospectively evaluated the incidence of missed injuries in hospitalized pediatric trauma patients, reporting that the incidence of missed injury was 33% when admission evaluation was performed by a trauma surgeon alone compared to 11% when performed by a pediatric doctor or a trauma surgeon and a pediatric doctor together (P < .001).33
Radiology
Three multisite studies examined quality and safety outcomes associated with radiographic imaging in community hospitals, including radiation dosing and frequency of preoperative imaging modalities and accuracy.38,39,41 For instance, Marin et al. found substantial variation in radiation dose across hospital types, with children’s hospitals delivering lower median radiation doses than academic and community hospitals.39 Similarly, Saito et al. demonstrated increased use of radiating modalities when evaluation was performed at community hospitals, with four times higher odds of computed tomography (CT) and five times lower odds of ultrasound use compared to a children’s hospital.41
Two single-site studies also evaluated quality and safety outcomes associated with the use of radiographic imaging for pediatric appendicitis in a community hospital.40,42 For example, York et al. demonstrated that, compared with nonimaged patients, patients who underwent diagnostic imaging for appendicitis experienced a significant time delay from initial evaluation to surgery and incurred significantly higher hospital charges, whereas there were no significant differences in intraoperative findings, antibiotic requirements, and surgical complications between the groups.42
Interventional Studies
Neonatal Medicine
We identified six studies that evaluated interventions to improve healthcare quality for neonates in community hospitals; two involved telemedicine.43-48 Hall et al. described “Telenursery,” a program linking regional perinatal centers with a large academic neonatal practice through real-time teleconferencing, in addition to providing weekly educational conferences.45 After its implementation, there was an increase in the state-recommended delivery of very low birth weight infants at the regional perinatal center from 24% to 33% (P < .05); clinical outcomes were not discussed. In a similar study, Sable et al. found that a videoconferencing system for cardiologists from an academic center to guide care in a community setting provided diagnostic services more quickly (28 minutes vs 12 hours) and had high diagnostic accuracy.47 The remaining four studies evaluated heterogeneous interventions such as a maternal education program to reduce shaking injuries to infants or implementation of evidence-based order sets to reduce early onset group B streptococcal disease in neonates.43,44,46,48 Five of the six neonatal studies demonstrated improved quality outcomes after intervention.43-47
Pediatric Medicine
Seven studies evaluated QI interventions for children at community hospitals,49-55 three of which described interventions to improve quality of management of respiratory diseases.49,51,53 Dayal et al. evaluated the implementation of respiratory illness order sets and an asthma pathway, demonstrating a 41% reduction in asthma hospitalization cost per patient (P < .05), reduced bronchodilator use for all respiratory illnesses, and no change in readmission rates.49 Similarly, Nkoy et al. evaluated an asthma “Evidence-Based Care Practice Model” implemented at seven community hospitals and demonstrated a nonsignificant reduction in readmissions (P = .12), as well as lower hospitalization costs (P = .05).53 Using QI methods, Kuhlmann et al. demonstrated improved compliance from 43% to 97% with the Asthma Home Management Plan of Care measure.51 The remaining four studies evaluated heterogeneous interventions, including telemedicine critical care consultations, use of I-PASS, and consolidation of pediatric care onto one hospital unit.50,52,54,55 Six of the seven pediatric studies (86%) demonstrated improved inpatient quality outcomes after intervention,49,51-55 but only one study was multisite.53
Surgery
We identified only one study evaluating a surgical intervention aimed at improving the quality of pediatric care.56 Kelley-Quon evaluated the impact of a community hospital partnering with an Academic Medical Pediatric Trauma Center to become a Level II Pediatric Trauma Center (PTC). After achieving Level II PTC designation, they reported that children treated at the community hospital had reduced rates of CT use, transfers, and in-hospital mortality (from 81% to 51%, 8.5% to 2.5%, and 2% to 0.4%, respectively, P < .05 for all) compared to those treated predesignation.
Radiology
Within the domain of radiology, our review identified no interventional studies.
DISCUSSION
In this scoping review of the quality and safety of pediatric inpatient care in community hospitals, we identified 44 studies applying heterogeneous study designs and evaluating diverse patient populations and quality outcomes. We identified only one RCT in our search; all the remaining studies applied observational designs.
We found only three clinical areas that were explored in multiple studies, with consistent directionality of results: (1) perinatal regionalization, (2) telemedicine, and (3) imaging radiation. The limited evidence identified in our review suggests that delivery of early premature infants at community hospitals, rather than at tertiary hospitals, may increase risk of neonatal morbidity and/or mortality,13,16,46 that use of telemedicine may improve the effectiveness and efficiency of intensive or specialized care in community settings,45,47,52,55 and that CT use and radiation doses may be higher in community hospitals compared with other settings.38,39,41 However, even within these clinical domains, the literature was limited in amount and heterogeneous; additional research is needed to systematically review the effect of individual interventions or particular community hospital quality outcomes compared with other hospital types.
Our search identified only 14 studies evaluating QI interventions within community hospitals over the almost 30-year review period. Although limited in number, 86% of these demonstrated improvements in healthcare quality and safety, providing a positive “proof of concept” that pediatric care in community hospitals can be improved by multidisciplinary efforts and be sustained over time.43-47,49,51-56 As pediatric departments within community hospitals may have limited resources, aligning pediatric QI efforts with adult initiatives within the same hospitals could prove advantageous. However, we did not identify any studies meeting our inclusion criteria that took this approach. Alternatively, QI collaboratives across structurally diverse hospitals may provide valuable infrastructure for QI in community hospitals. For example, the Value in Inpatient Pediatrics Network has conducted several multisite QI initiatives that have engaged both children’s and community hospitals.57-60 However, none of these studies have reported community hospital-specific quality outcomes, resulting in their exclusion from this review. In future study, researchers may consider separating community hospital results from those of pediatric hospitals to highlight the effect of community hospital-specific QI efforts and to allow valuable direct comparisons between hospital types.
Many studies identified in our scoping review were conducted at single hospitals. Sample sizes were often very small and power calculations were rarely reported, raising questions about the validity of the “nonsignificant” differences reported by some. Although such single-center studies provide valuable information for local improvement of inpatient care, by design the findings from these studies are unlikely generalizable to other community hospital systems. Without inclusion of another hospital type and/or quality measures with clear national benchmark for comparison, the additional conclusions that can be drawn from these studies are limited. In comparison, many of the multicenter studies had large study samples, but more than half used data registries with limited data to evaluate outcomes, clinical context, or important covariates.13,20,21,25,27-30,34,35,37,39 The most frequently reported outcomes in these data sets—hospital charges and lengths of stay—are of limited utility in understanding healthcare quality without clear benchmarks. As a result, the evidence-base from which to draw conclusions about quality and safety in community hospitals is very limited.
Therefore, our review highlights a great need for additional research in community hospital medicine and the need for high-quality evidence generation. Risk of bias was moderate or high for the majority of included studies because of inadequate risk adjustment or statistical analysis. In the future, multicenter collaborations may help to connect research methodologists with community hospital teams to aid in the application of robust study designs and analytic techniques. Multisite collaboration may also overcome the limitation of small sample sizes that are a reality at many community hospitals.
Our study findings must be considered in the setting of several methodological limitations. The lack of a standard definition for a community hospital has led to inconsistent terms and hospital definitions used in the literature. It is possible that, while following our systematic approach to define a community hospital, we inadvertently missed relevant studies that used different terms. We also excluded unpublished articles. Given that publication bias tends to favor studies with significant associations, it is possible that some studies with insignificant changes in quality outcomes were missed. Finally, in our exclusion of all non-US studies, we may have unknowingly missed literature from countries with community hospital definitions similar to those in the United States.
CONCLUSIONS
Recognizing that more than half of all children admitted to hospitals in the US receive their care at community hospitals, understanding healthcare quality in community hospitals is important. This scoping review underscores the need for additional research and higher quality evidence to determine the quality of pediatric inpatient care in these settings and identifies some particularly wide gaps that could be targeted in future research. Acknowledging that further research is necessary to address all aims of quality healthcare, markedly few studies have examined timeliness, equity, or patient-centeredness. Collaborations between academic medical centers and community hospitals may be an effective means to connect researchers with community hospital clinical teams to facilitate the application of robust study designs and analytic approaches and to facilitate multisite investigations. Research in this field would benefit from a standardized definition of a community hospital that could be consistently applied in research and QI endeavors.
Disclosures
The authors have no potential conflicts of interest to disclose.
Funding
Jana Leary was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, Grant Number 5TL1TR0001062-03. JoAnna Leyenaar was supported by the Agency for Healthcare Research and Quality (K08HS024133).
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the AHRQ.
Despite efforts to provide high-quality healthcare, Americans die from medical errors each year and many patients do not receive recommended medical care. Risk is particularly acute during times of hospitalization.1-4 In response, the Institute of Medicine (IOM, now the Academy of Medicine) has released “Crossing the Quality Chasm: A New Health System for the 21st Century,” providing a framework to guide delivery and measurement of high-quality healthcare.5
Although the IOM framework has motivated the development of quality improvement (QI) and quality measurement initiatives, relatively few resources have been allocated to improving the quality of pediatric inpatient care.6,7 The resultant gap in our knowledge of quality and safety of pediatric hospital-based care is further widened by the variability of settings in which children are hospitalized. These settings include freestanding children’s hospitals, children’s hospitals nested within larger hospitals, and community hospitals, defined as general, nonuniversity, and nonchildren’s hospitals.8
Although almost three-quarters of children needing hospitalization are cared for outside of freestanding children’s hospitals, we know particularly little about the quality and safety of pediatric hospital-based care outside of these settings.6,9 Therefore, our scoping review aims to summarize literature regarding the quality and safety of pediatric inpatient care within community hospitals.
METHODS
We used a scoping review approach because this methodology, by design, is utilized to synthesize evidence and map existing literature and is particularly useful when a body of literature is heterogeneous, rendering a more targeted systematic review approach infeasible.10 This methodology thereby provided an organized approach to answer our broad research question, “What evidence exists regarding the quality and safety of pediatric inpatient care in United States community hospitals?“ We followed the scoping review guidelines put forth by the Joanna Briggs Institute and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guideline Extension for Scoping Reviews.10,11
Data Sources and Search Strategies
We searched Medline, Medline-In-Process, Embase, the Cochrane Database of Systematic Reviews, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), PsycINFO, and Scopus for studies that reported at least one outcome related to healthcare quality or patient safety and involved pediatric patients (aged <18 years) receiving inpatient care at a community hospital. Outcomes included measures from the IOM-defined aims of quality healthcare: (1) safety, (2) effectiveness, (3) efficiency, (4) timeliness, (5) patient-centeredness, and (6) equity (Appendix Table 1). Terms were searched as controlled vocabulary in applicable databases (Medline, Embase, CINAHL, PsycINFO) and as keywords in all databases. Search strategies tailored to each database were developed, tested, and refined in collaboration with a reference librarian. Date parameters for retrieval were set from 1989 to the search date, with the start date chosen to correspond with the establishment of the Agency for Health Care Policy and Research, currently known as the Agency for Healthcare Research and Quality (AHRQ), targeting literature produced in the wake of the AHRQ emphasis on quality in healthcare. Results were limited to articles published in English. Searches were conducted on October 24 and 25, 2016. Complete search strategies can be found in Appendix Methods.
Independent authors performed handsearches of Academic Pediatrics, BMJ Quality & Safety, Hospital Pediatrics, JAMA Pediatrics, and Pediatrics Quality Reports for the five years preceding the search date (July 2011-October 2016).
Study Selection and Definitions
To identify studies conducted in community hospitals, we operationalized the definition of community hospital proposed by Percelay.8 We used “community”, “general”, “nonuniversity”, and “nonchildren’s” as search terms and operationalized these through the addition of qualifying descriptors (Table 1).
Studies were excluded if they (1) were performed outside of the United States, as community hospital definitions may differ by country; (2) included participants aged >18 years and did not report any pediatric-specific results; (3) were performed exclusively in tertiary hospitals; (4) evaluated only outpatient or emergency department care; (5) did not report any results specific to community hospitals; (6) did not report any data-driven or parent/patient-reported measures of safety, effectiveness, efficiency, timeliness, patient-centeredness, or equity in their results; and (7) were case reports, case series, editorials, or abstracts without an associated full-text article. We read commentaries and literature reviews related to our objectives and reviewed their references to identify additional articles, but did not include these manuscripts.
Two authors independently reviewed each abstract, and full-text articles were reviewed if one or more authors determined that the abstract met the inclusion criteria. Two authors then independently reviewed each full-text article to determine whether the article met the criteria for the final review. Disagreements were resolved through consensus after discussion and review with the entire research team, and reasons for exclusion were recorded.
Charting the Results and Data Synthesis
We used a standardized charting form to collect information regarding study design, community hospital terms, population descriptors, IOM aims of quality healthcare, and outcome measures. To minimize bias in data collection, information from each full-text article was extracted independently by two authors, and differences in extraction were resolved through discussion and re-review among the same two authors. To evaluate the quality of included evidence, two authors (JCL and JKL) independently assessed the risk of bias for each study using modified Newcastle-Ottawa Quality Assessment Scales (NOS), with disagreements resolved through discussion and re-review among the same two authors. For cohort studies, five of the eight NOS domains were relevant and applied to all included studies (maximum score 6). Using the NOS adapted for cross-sectional studies, six of the seven domains were relevant and applied (maximum score 9). For cross-sectional studies, we defined the risk of bias to be low for scores ≥8, moderate for scores 5-7, and high for scores ≤4, consistent with previous work.12 For cohort studies, we similarly defined these strata by scores ≥5, 3-4, and ≤2, given the lower maximum score for this study type.
We categorized studies as either observational, defined as cohort or cross-sectional studies of usual healthcare delivery, or interventional, defined as studies evaluating the development and/or implementation of an intervention designed to improve healthcare quality. We further categorized the studies into the following four overarching medical domains: (1) neonatal, (2) pediatric medicine, (3) surgery, or (4) radiology.
RESULTS
After removal of duplicates, our search identified 2,068 abstracts for screening (Figure). Of these, 1,777 did not meet the inclusion criteria, leaving 291 articles for full-text review. Of these, 43 articles met all the inclusion criteria, and one additional article was included from search of references, resulting in a total of 44 articles.
Study designs, patient populations, and quality outcome measures were heterogeneous. We identified only one randomized controlled trial (RCT). A total of 30 articles were observational studies, 27 of which used retrospective cohort or cross-sectional designs; the remaining three used prospective cohort designs (Table 2). Of these studies, 20 involved multiple hospitals, whereas 10 were conducted at a single community hospital. Sample sizes ranged from 29 (single-site) to 107,727 (multisite) patients. Twenty-two studies aimed to compare quality outcomes at community hospitals with other hospital types, of which 16 performed risk-adjusted analyses (detailed findings of observational studies are summarized in Appendix Table 2). The remaining 14 articles were interventional studies (Table 2). Of these, 12 (86%) reported improvement in quality outcomes after implementation (detailed findings of interventional studies are summarized in Appendix Table 3).
The included studies evaluated quality outcomes addressing all six of the IOM aims of quality healthcare, with safety, effectiveness, and efficiency being the most predominant (Table 3). Patient-centeredness and timeliness were infrequently addressed, and only one study assessed equity.
Risk of bias was moderate or high for 27 (69%) of the observational and interventional cohort studies and four (100%) of the included cross-sectional studies (Table 2), with the median NOS score being 4 (range: 0-6) for cohort studies (Appendix Table 4) and 4 (range: 3-6) for cross-sectional studies (Appendix Table 5). The higher risk of bias was largely driven by low comparability scores due to inadequate risk adjustment or statistical reporting. Of the 12 studies with low risk of bias, 11 (92%) were multisite, 9 (75%) used large regional or national databases, and half reported quality outcomes limited to hospital charges and/or mortality.
Observational Studies
Neonatal Medicine
Five multisite studies focused on neonatal care,13,14,16,19,20 of which four examined outcomes associated with the transfer of neonates to or from community hospitals to tertiary care hospitals, such as neonatal morbidity, readmission, completion of preventative health measures and screening, parent satisfaction, and hospital charges.14,16,19,20 For example, in a study of extremely premature very low birth weight infants born in Hawaii, Kuo et al. demonstrated that the odds of retinopathy of prematurity was 2.9 times higher for infants born at a community hospital and transported to their tertiary center compared with those inborn at the tertiary center (P = .02).16 The fifth study examined neonatal mortality by site of birth, demonstrating that, among infants with birth weights less than 2,000 g, birth at a hospital with a community neonatal intensive care unit (NICU) was associated with 1.4 times higher odds of risk-adjusted mortality compared with birth at a regional NICU (P < .001).13
Three studies evaluated the quality of neonatal care at a single community hospital.15,17,18 Quality outcomes were heterogeneous, including utility of rebound bilirubin levels for infants with jaundice, morbidity of neonates requiring mechanical ventilation, and provision of breastfeeding advice to mothers of breastfeeding infants. For instance, Meadow et al. attempted to determine the quality of care for ventilated neonates at one community NICU compared with a tertiary hospital.18 They found no difference in days on ventilation or need for home oxygen therapy between the community hospital and the tertiary center, although P values and effect sizes were not reported for these outcomes and analyses were not adjusted beyond matching on birthdate and birth weight.
Pediatric Medicine
Nine multisite studies explored the quality and safety of pediatric medical care across hospital types.21-23,25-30 Of these, four were conducted using the Kids’ Inpatient Database (KID)28-30 or the National Inpatient Sample (NIS),27 two were conducted using other national databases,21,25 and two were conducted using electronic medical record data.22,26 All of the KID and NIS studies examined hospital charges, either alone or in conjunction with mortality. Two of these studies found no differences in risk-adjusted hospital charges between children’s hospitals and community hospitals (for burn injuries),28,29 whereas two found that hospitalization at community hospitals was associated with lower risk-adjusted charges (for asthma and sepsis).27,30 The remaining five studies evaluated diverse quality outcomes such as medication errors, therapeutic drug monitoring, practice guideline compliance, antibiotic prescribing, or hospital-to-home transition summary scores.21-23,25,26
Three single-site studies examined quality outcomes for pediatric medical patients, including mortality, outcome ratings for dehydration, and measures of peripherally inserted central catheter (PICC) safety and effectiveness.24,31,32 For example, Frank et al. evaluated safety of care in one community hospital pediatric intensive care unit (PICU) compared with a tertiary hospital using the Pediatric Risk of Mortality (PRISM) score.24 They reported that the observed number of deaths in their community PICU did not differ significantly from the number of deaths predicted in a tertiary center (23 vs 33, respectively, P > .2).
Surgery
Three multisite studies examined quality outcomes among children with surgical conditions, including surgical complications, readmission, and hospital charges.34,35,37 For example, Kelley-Quon et al. examined outcomes following surgery in infants with hypertrophic pyloric stenosis and found that infants who received their surgery at community hospitals had twice the odds of a surgical complication compared with those at children’s hospitals (P = .027).34 They also examined how the risk of appendiceal perforation differed by hospital type, finding that black children who received their surgery at children’s hospitals had twice the odds of appendiceal perforation compared with those who received care at community hospitals.35
In addition, two studies evaluated quality and safety outcomes for pediatric surgical care in a single community hospital.33,36 For example, Beaty et al. prospectively evaluated the incidence of missed injuries in hospitalized pediatric trauma patients, reporting that the incidence of missed injury was 33% when admission evaluation was performed by a trauma surgeon alone compared to 11% when performed by a pediatric doctor or a trauma surgeon and a pediatric doctor together (P < .001).33
Radiology
Three multisite studies examined quality and safety outcomes associated with radiographic imaging in community hospitals, including radiation dosing and frequency of preoperative imaging modalities and accuracy.38,39,41 For instance, Marin et al. found substantial variation in radiation dose across hospital types, with children’s hospitals delivering lower median radiation doses than academic and community hospitals.39 Similarly, Saito et al. demonstrated increased use of radiating modalities when evaluation was performed at community hospitals, with four times higher odds of computed tomography (CT) and five times lower odds of ultrasound use compared to a children’s hospital.41
Two single-site studies also evaluated quality and safety outcomes associated with the use of radiographic imaging for pediatric appendicitis in a community hospital.40,42 For example, York et al. demonstrated that, compared with nonimaged patients, patients who underwent diagnostic imaging for appendicitis experienced a significant time delay from initial evaluation to surgery and incurred significantly higher hospital charges, whereas there were no significant differences in intraoperative findings, antibiotic requirements, and surgical complications between the groups.42
Interventional Studies
Neonatal Medicine
We identified six studies that evaluated interventions to improve healthcare quality for neonates in community hospitals; two involved telemedicine.43-48 Hall et al. described “Telenursery,” a program linking regional perinatal centers with a large academic neonatal practice through real-time teleconferencing, in addition to providing weekly educational conferences.45 After its implementation, there was an increase in the state-recommended delivery of very low birth weight infants at the regional perinatal center from 24% to 33% (P < .05); clinical outcomes were not discussed. In a similar study, Sable et al. found that a videoconferencing system for cardiologists from an academic center to guide care in a community setting provided diagnostic services more quickly (28 minutes vs 12 hours) and had high diagnostic accuracy.47 The remaining four studies evaluated heterogeneous interventions such as a maternal education program to reduce shaking injuries to infants or implementation of evidence-based order sets to reduce early onset group B streptococcal disease in neonates.43,44,46,48 Five of the six neonatal studies demonstrated improved quality outcomes after intervention.43-47
Pediatric Medicine
Seven studies evaluated QI interventions for children at community hospitals,49-55 three of which described interventions to improve quality of management of respiratory diseases.49,51,53 Dayal et al. evaluated the implementation of respiratory illness order sets and an asthma pathway, demonstrating a 41% reduction in asthma hospitalization cost per patient (P < .05), reduced bronchodilator use for all respiratory illnesses, and no change in readmission rates.49 Similarly, Nkoy et al. evaluated an asthma “Evidence-Based Care Practice Model” implemented at seven community hospitals and demonstrated a nonsignificant reduction in readmissions (P = .12), as well as lower hospitalization costs (P = .05).53 Using QI methods, Kuhlmann et al. demonstrated improved compliance from 43% to 97% with the Asthma Home Management Plan of Care measure.51 The remaining four studies evaluated heterogeneous interventions, including telemedicine critical care consultations, use of I-PASS, and consolidation of pediatric care onto one hospital unit.50,52,54,55 Six of the seven pediatric studies (86%) demonstrated improved inpatient quality outcomes after intervention,49,51-55 but only one study was multisite.53
Surgery
We identified only one study evaluating a surgical intervention aimed at improving the quality of pediatric care.56 Kelley-Quon evaluated the impact of a community hospital partnering with an Academic Medical Pediatric Trauma Center to become a Level II Pediatric Trauma Center (PTC). After achieving Level II PTC designation, they reported that children treated at the community hospital had reduced rates of CT use, transfers, and in-hospital mortality (from 81% to 51%, 8.5% to 2.5%, and 2% to 0.4%, respectively, P < .05 for all) compared to those treated predesignation.
Radiology
Within the domain of radiology, our review identified no interventional studies.
DISCUSSION
In this scoping review of the quality and safety of pediatric inpatient care in community hospitals, we identified 44 studies applying heterogeneous study designs and evaluating diverse patient populations and quality outcomes. We identified only one RCT in our search; all the remaining studies applied observational designs.
We found only three clinical areas that were explored in multiple studies, with consistent directionality of results: (1) perinatal regionalization, (2) telemedicine, and (3) imaging radiation. The limited evidence identified in our review suggests that delivery of early premature infants at community hospitals, rather than at tertiary hospitals, may increase risk of neonatal morbidity and/or mortality,13,16,46 that use of telemedicine may improve the effectiveness and efficiency of intensive or specialized care in community settings,45,47,52,55 and that CT use and radiation doses may be higher in community hospitals compared with other settings.38,39,41 However, even within these clinical domains, the literature was limited in amount and heterogeneous; additional research is needed to systematically review the effect of individual interventions or particular community hospital quality outcomes compared with other hospital types.
Our search identified only 14 studies evaluating QI interventions within community hospitals over the almost 30-year review period. Although limited in number, 86% of these demonstrated improvements in healthcare quality and safety, providing a positive “proof of concept” that pediatric care in community hospitals can be improved by multidisciplinary efforts and be sustained over time.43-47,49,51-56 As pediatric departments within community hospitals may have limited resources, aligning pediatric QI efforts with adult initiatives within the same hospitals could prove advantageous. However, we did not identify any studies meeting our inclusion criteria that took this approach. Alternatively, QI collaboratives across structurally diverse hospitals may provide valuable infrastructure for QI in community hospitals. For example, the Value in Inpatient Pediatrics Network has conducted several multisite QI initiatives that have engaged both children’s and community hospitals.57-60 However, none of these studies have reported community hospital-specific quality outcomes, resulting in their exclusion from this review. In future study, researchers may consider separating community hospital results from those of pediatric hospitals to highlight the effect of community hospital-specific QI efforts and to allow valuable direct comparisons between hospital types.
Many studies identified in our scoping review were conducted at single hospitals. Sample sizes were often very small and power calculations were rarely reported, raising questions about the validity of the “nonsignificant” differences reported by some. Although such single-center studies provide valuable information for local improvement of inpatient care, by design the findings from these studies are unlikely generalizable to other community hospital systems. Without inclusion of another hospital type and/or quality measures with clear national benchmark for comparison, the additional conclusions that can be drawn from these studies are limited. In comparison, many of the multicenter studies had large study samples, but more than half used data registries with limited data to evaluate outcomes, clinical context, or important covariates.13,20,21,25,27-30,34,35,37,39 The most frequently reported outcomes in these data sets—hospital charges and lengths of stay—are of limited utility in understanding healthcare quality without clear benchmarks. As a result, the evidence-base from which to draw conclusions about quality and safety in community hospitals is very limited.
Therefore, our review highlights a great need for additional research in community hospital medicine and the need for high-quality evidence generation. Risk of bias was moderate or high for the majority of included studies because of inadequate risk adjustment or statistical analysis. In the future, multicenter collaborations may help to connect research methodologists with community hospital teams to aid in the application of robust study designs and analytic techniques. Multisite collaboration may also overcome the limitation of small sample sizes that are a reality at many community hospitals.
Our study findings must be considered in the setting of several methodological limitations. The lack of a standard definition for a community hospital has led to inconsistent terms and hospital definitions used in the literature. It is possible that, while following our systematic approach to define a community hospital, we inadvertently missed relevant studies that used different terms. We also excluded unpublished articles. Given that publication bias tends to favor studies with significant associations, it is possible that some studies with insignificant changes in quality outcomes were missed. Finally, in our exclusion of all non-US studies, we may have unknowingly missed literature from countries with community hospital definitions similar to those in the United States.
CONCLUSIONS
Recognizing that more than half of all children admitted to hospitals in the US receive their care at community hospitals, understanding healthcare quality in community hospitals is important. This scoping review underscores the need for additional research and higher quality evidence to determine the quality of pediatric inpatient care in these settings and identifies some particularly wide gaps that could be targeted in future research. Acknowledging that further research is necessary to address all aims of quality healthcare, markedly few studies have examined timeliness, equity, or patient-centeredness. Collaborations between academic medical centers and community hospitals may be an effective means to connect researchers with community hospital clinical teams to facilitate the application of robust study designs and analytic approaches and to facilitate multisite investigations. Research in this field would benefit from a standardized definition of a community hospital that could be consistently applied in research and QI endeavors.
Disclosures
The authors have no potential conflicts of interest to disclose.
Funding
Jana Leary was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, Grant Number 5TL1TR0001062-03. JoAnna Leyenaar was supported by the Agency for Healthcare Research and Quality (K08HS024133).
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the AHRQ.
1. McGlynn EA, Asch SM, Adams J, et al. The quality of health care delivered to adults in the United States. N Engl J Med. 2003;348(26):2635-2645. https://doi.org/10.1056/NEJMsa022615.
2. Richardson WC, Berwick DM, Bisgard C, et al. To err is human: building a safer health system-Institute of Medicine. Medscape. http://www.iom.edu/Reports/1999/To-Err-is-Human-Building-A-Safer-Health-System.aspx; 2000. Accessed October 7, 2016.
3. Classen DC, Resar R, Griffin F, et al. ‘Global Trigger Tool’ shows that adverse events in hospitals may be ten times greater than previously measured. Health Aff. 2011;30(4):581-589. https://doi.org/10.1377/hlthaff.2011.0190.
4. Stockwell DC, Landrigan CP, Schuster MA, et al. Using a pediatric trigger tool to estimate total harm burden hospital-acquired conditions represent. Pediatr Qual Saf. 2018;3(3):e081. https://doi.org/10.1097/pq9.0000000000000081.
5. Institute of Medicine (US) Committee on Quality of Health Care in America. Crossing the Quality Chasm. Washington (DC): National Academies Press (US); 2001. https://doi.org/10.17226/10027.
6. Rauch DA, Lye PS, Carlson D, et al. Pediatric hospital medicine: a strategic planning roundtable to chart the future. J Hosp Med. 2012;7(4):329-334. https://doi.org/10.1002/jhm.950.
7. Simpson L, Fairbrother G, Hale S et al. Reauthorizing SCHIP: Opportunities for Promoting Effective Health Coverage and HighQuality Care for Children and Adolescents. (Publication 1051). Commonw Fund; 2007.
8. Percelay JM. Pediatric hospitalists working in community hospitals. Pediatr Clin North Am. 2014;61(4):681-691. https://doi.org/10.1016/j.pcl.2014.04.005.
9. Leyenaar JK, Ralston SL, Shieh MS et al. Epidemiology of pediatric hospitalizations at general hospitals and freestanding children’s hospitals in the United States. J Hosp Med. 2016;11(11):743-749. https://doi.org/10.1002/jhm.2624.
10. Peters MDJ, Godfrey CM, Khalil H et al. Guidance for conducting systematic scoping reviews. Int J Evid Based Healthc. 2015;13(3):141-146. https://doi.org/10.1097/XEB.0000000000000050.
11. Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467-473. https://doi.org/10.7326/M18-0850.
12. Alobaidi R, Morgan C, Basu RK, et al. Association between fluid balance and outcomes in critically ill children: a systematic review and meta-analysis. JAMA Pediatr. 2018;172(3):257-268. https://doi.org/10.1001/jamapediatrics.2017.4540.
13. Cifuentes J, Bronstein J, Phibbs CS et al. Mortality in low birth weight infants according to level of neonatal care at hospital of birth. Pediatrics. 2002;109(5):745-751. https://doi.org/10.1542/peds.109.5.745.
14. Donohue PK, Hussey-Gardner B, Sulpar LJ, Fox R, Aucott SW. Convalescent care of infants in the neonatal intensive care unit in community hospitals: risk or benefit? Pediatrics. 2009;124(1):105-111. https://doi.org/10.1542/peds.2008-0880.
15. Izatt SD. Breastfeeding counseling by health care providers. J Hum Lact. 1997;13(2):109-113. https://doi.org/10.1177/089033449701300210.
16. Kuo S, Kimata C, Akamine K, Young B, Balaraman V. Outcomes of inborn and transported extremely premature very-low-birthweight infants in Hawai’i. Pediatr Int. 2012;54(3):365-369. https://doi.org/10.1111/j.1442-200X.2012.03561.x.
17. Maisels MJ, Kring E. Rebound in serum bilirubin level following intensive phototherapy. Arch Pediatr Adolesc Med. 2002;156(7):669-672. https://doi.org/10.1001/archpedi.156.7.669.
18. Meadow W, Mendez D, Makela J et al. Can and should level II nurseries care for newborns who require mechanical ventilation? Clin Perinatol. 1996;23(3):551-561. https://doi.org/10.1016/S0095-5108(18)30227-6.
19. Phibbs CS, Mortensen L. Back transporting infants from neonatal intensive care units to community hospitals for recovery care: effect on total hospital charges. Pediatrics. 1992;90(1):22-26.
20. Wall SN, Handler AS, Park CG. Hospital factors and nontransfer of small babies: A marker of deregionalized perinatal care? J Perinatol. 2004;24(6):351-359. https://doi.org/10.1038/sj.jp.7211101.
21. Alexander DC, Bundy DG, Shore AD et al. Cardiovascular medication errors in children. Pediatrics. 2009;124(1):324-332. https://doi.org/10.1542/peds.2008-2073.
22. Balch AH, Constance JE, Thorell EA, et al. Pediatric vancomycin dosing: trends over time and the impact of therapeutic drug monitoring. J Clin Pharmacol. 2015;55(2):212-220. https://doi.org/10.1002/jcph.402.
23. Conway PH, Edwards S, Stucky ER et al. Variations in management of common inpatient pediatric illnesses: hospitalists and community pediatricians. Pediatrics. 2006;118(2):441-447. https://doi.org/10.1542/peds.2006-0484.
24. Frank BS, Pollack MM. Quantitative quality assurance in a community hospital pediatric intensive care unit. West J Med. 1992;157(2):149-151.
25. Leyenaar JK, Lagu T, Shieh MS, Pekow PS, Lindenauer PK. Variation in resource utilization for the management of uncomplicated community-acquired pneumonia across community and children’s hospitals. J Pediatr. 2014;165(3):585-591. https://doi.org/10.1016/j.jpeds.2014.04.062.
26. Leyenaar JK, Desai AD, Burkhart Q, et al. Quality measures to assess care transitions for hospitalized children. Pediatrics. 2016;138(2):e20160906. https://doi.org/10.1542/peds.2016-0906.
27. Meurer JR, Kuhn EM, George V, Yauck JS, Layde PM. Charges for childhood asthma by hospital characteristics. Pediatrics. 1998;102(6):E70. https://doi.org/10.1542/peds.102.6.e70.
28. Myers J, Lehna C. Where are lengths of stay longer and total charges higher for pediatric burn patients? J Burn Care Res. 2014;35(5):382-387. https://doi.org/10.1097/BCR.0000000000000012.
29. Myers J, Smith M, Woods C, Espinosa C, Lehna C. The effect of transfers between health care facilities on costs and length of stay for pediatric burn patients. J Burn Care Res. 2015;36(1):178-183. https://doi.org/10.1097/BCR.0000000000000206.
30. Odetola FO, Gebremariam A, Freed GL. Patient and hospital correlates of clinical outcomes and resource utilization in severe pediatric sepsis. Pediatrics. 2007;119(3):487-494. https://doi.org/10.1542/peds.2006-2353.
31. Scherb CA, Stevens MS, Busman C. Outcomes related to dehydration in the pediatric population. J Pediatr Nurs. 2007;22(5):376-382. https://doi.org/10.1016/j.pedn.2006.10.004.
32. Van Winkle P, Whiffen T, Liu IL. Experience using peripherally inserted central venous catheters for outpatient parenteral antibiotic therapy in children at a community hospital. ediatr Infect Dis J. 2008;27(12):1069-1072. https://doi.org/10.1097/INF.0b013e31817d32f2.
33. Beaty JS, Chendrasekhar A, Hopkins J, Gruelke L. Missed injuries in pediatric trauma patients. J Appl Res. 2003;3(1):84-88. https://jrnlappliedresearch.com/articles/Vol3Iss1/CHENDRASEKHAR.htm. Accessed July 8, 2019.
34. Kelley-Quon LI, Tseng CH, Jen HC, Shew SB. Hospital type predicts surgical complications for infants with hypertrophic pyloric stenosis. Am Surg. 2012;78(10):1079-1082.
35. Kelley-Quon LI, Tseng CH, Jen HC, Lee SL, Shew SB. Hospital type as a metric for racial disparities in pediatric appendicitis. J Am Coll Surg. 2013;216(1):74-82. https://doi.org/10.1016/j.jamcollsurg.2012.09.018.
36. Pokala N, Sadhasivam S, Kiran RP, Parithivel V. Complicated appendicitis—is the laparoscopic approach appropriate? A comparative study with the open approach: outcome in a community hospital setting. Am Surg. 2007;73(8):732-737.
37. Smith JT, Price C, Stevens PM, Masters KS, Young M. Does pediatric orthopedic subspecialization affect hospital utilization and charges? J Pediatr Orthop. 1999;19(4):553-555. https://doi.org/10.1097/01241398-199907000-00027.
38. Calvert C, Strauss KJ, Mooney DP. Variation in computed tomography radiation dose in community hospitals. J Pediatr Surg. 2012;47(6):1167-1169. https://doi.org/10.1016/j.jpedsurg.2012.03.021.
39. Marin JR, Sengupta D, Bhargavan-Chatfield M et al. Variation in pediatric cervical spine computed tomography radiation dose index. Acad Emerg Med. 2015;22(12):1499-1505. https://doi.org/10.1111/acem.12822.
40. Reich JD, Brogdon B, Ray WE, Eckert J, Gorell H. Use of CT scan in the diagnosis of pediatric acute appendicitis. Pediatr Emerg Care. 2000;16(4):241-243. https://doi.org/10.1097/00006565-200008000-00006.
41. Saito JM, Yan Y, Evashwick TW, Warner BW, Tarr PI. Use and accuracy of diagnostic imaging by hospital type in pediatric appendicitis. Pediatrics. 2013;131(1):e37-e44. https://doi.org/10.1542/peds.2012-1665.
42. York D, Smith A, Phillips JD, Von Allmen D. The influence of advanced radiographic imaging on the treatment of pediatric appendicitis. J Pediatr Surg. 2005;40(12):1908-1911. https://doi.org/10.1016/j.jpedsurg.2005.08.004.
43. Altman RL, Canter J, Patrick PA et al. Parent education by maternity nurses and prevention of abusive head trauma. Pediatrics. 2011;128(5):e1164-e1172. https://doi.org/10.1542/peds.2010-3260.
44. Clemens CJ, Gable EK. The development of a group B streptococcus prevention policy at a community hospital. J Perinatol. 2002;22(7):523-525. https://doi.org/10.1038/sj.jp.7210794.
45. Hall RW, Hall-Barrow J, Garcia-Rill E. Neonatal regionalization through telemedicine using a community-based research and education core facility. Ethn Dis. 2010;20(1 Suppl 1):S136-S140.
46. Hulsey TC, Pittard WB 3rd, Ebeling M. Regionalized perinatal transport systems: association with changes in location of birth, neonatal transport, and survival of very low birth weight deliveries. J S C Med Assoc. 1991;87(12):581-584.
47. Sable CA, Cummings SD, Pearson GD, et al. Impact of telemedicine on the practice of pediatric cardiology in community hospitals. Pediatrics. 2002;109(1):E3. https://doi.org/10.1542/peds.109.1.e3.
48. Wexelblatt SL, Ward LP, Torok K et al. Universal maternal drug testing in a high-prevalence region of prescription opiate abuse. J Pediatr. 2015;166(3):582-586. https://doi.org/10.1016/j.jpeds.2014.10.004.
49. Dayal A, Alvarez F. The effect of implementation of standardized, evidence-based order sets on efficiency and quality measures for pediatric respiratory illnesses in a community hospital. Hosp Pediatr. 2015;5(12):624-629. https://doi.org/10.1542/hpeds.2015-0140.
50. Krugman SD, Suggs A, Photowala HY, Beck A. Redefining the community pediatric hospitalist: the combined Pediatric ED/Inpatient Unit. Pediatr Emerg Care. 2007;23(1):33-37. https://doi.org/10.1097/01.pec.0000248685.94647.01.
51. Kuhlmann S, Mason B, Ahlers-Schmidt CR. A quality improvement project to improve compliance with the joint commission children’s asthma care-3 measure. Hosp Pediatr. 2013;3(1):45-51. https://doi.org/10.1542/hpeds.2012-0015.
52. Labarbera JM, Ellenby MS, Bouressa P et al. The impact of telemedicine intensivist support and a pediatric hospitalist program on a community hospital. Telemed J E Health. 2013;19(10):760-766. https://doi.org/10.1089/tmj.2012.0303.
53. Nkoy F, Fassl B, Stone B, et al. Improving pediatric asthma care and outcomes Across multiple hospitals. Pediatrics. 2015;136(6):e1602-e1610. https://doi.org/10.1542/peds.2015-0285.
54. Walia J, Qayumi Z, Khawar N, et al. Physician transition of care: benefits of I-PASS and an electronic handoff system in a community pediatric residency program. Acad Pediatr. 2016;16(6):519-523. https://doi.org/10.1016/j.acap.2016.04.001.
55. Yang CP, Hunt EA, Shilkofski N et al. Can telemedicine improve adherence to resuscitation guidelines for critically ill children at community hospitals? A randomized controlled trial using high-fidelity simulation. Pediatr Emerg Care. 2017;33(7):474-479. https://doi.org/10.1097/PEC.0000000000000653.
56. Kelley-Quon LI, Crowley MA, Applebaum H, et al. Academic-community partnerships improve outcomes in pediatric trauma care. J Pediatr Surg. 2015;50(6):1032-1036. https://doi.org/10.1016/j.jpedsurg.2015.03.033.
57. Ralston S, Garber M, Narang S, et al. Decreasing unnecessary utilization in acute bronchiolitis care: results from the value in inpatient pediatrics network. J Hosp Med. 2013;8(1):25-30. https://doi.org/10.1002/jhm.1982.
58. Ralston SL, Garber MD, Rice-Conboy E, et al. A multicenter collaborative to reduce unnecessary care in inpatient bronchiolitis. Pediatrics. 2016;137(1):e20150851. https://doi.org/10.1542/peds.2015-0851.
59. Parikh K, Biondi E, Nazif J, et al. A multicenter collaborative to improve care of community acquired pneumonia in hospitalized children. Pediatrics. 2017;139(3):e20161411. https://doi.org/10.1542/peds.2016-1411.
60. Leyenaar JK, Bergert L, Mallory LA, et al. Pediatric primary care providers’ perspectives regarding hospital discharge communication: a mixed methods analysis. Acad Pediatr. 2015;15(1):61-68. https://doi.org/10.1016/j.acap.2014.07.004.
1. McGlynn EA, Asch SM, Adams J, et al. The quality of health care delivered to adults in the United States. N Engl J Med. 2003;348(26):2635-2645. https://doi.org/10.1056/NEJMsa022615.
2. Richardson WC, Berwick DM, Bisgard C, et al. To err is human: building a safer health system-Institute of Medicine. Medscape. http://www.iom.edu/Reports/1999/To-Err-is-Human-Building-A-Safer-Health-System.aspx; 2000. Accessed October 7, 2016.
3. Classen DC, Resar R, Griffin F, et al. ‘Global Trigger Tool’ shows that adverse events in hospitals may be ten times greater than previously measured. Health Aff. 2011;30(4):581-589. https://doi.org/10.1377/hlthaff.2011.0190.
4. Stockwell DC, Landrigan CP, Schuster MA, et al. Using a pediatric trigger tool to estimate total harm burden hospital-acquired conditions represent. Pediatr Qual Saf. 2018;3(3):e081. https://doi.org/10.1097/pq9.0000000000000081.
5. Institute of Medicine (US) Committee on Quality of Health Care in America. Crossing the Quality Chasm. Washington (DC): National Academies Press (US); 2001. https://doi.org/10.17226/10027.
6. Rauch DA, Lye PS, Carlson D, et al. Pediatric hospital medicine: a strategic planning roundtable to chart the future. J Hosp Med. 2012;7(4):329-334. https://doi.org/10.1002/jhm.950.
7. Simpson L, Fairbrother G, Hale S et al. Reauthorizing SCHIP: Opportunities for Promoting Effective Health Coverage and HighQuality Care for Children and Adolescents. (Publication 1051). Commonw Fund; 2007.
8. Percelay JM. Pediatric hospitalists working in community hospitals. Pediatr Clin North Am. 2014;61(4):681-691. https://doi.org/10.1016/j.pcl.2014.04.005.
9. Leyenaar JK, Ralston SL, Shieh MS et al. Epidemiology of pediatric hospitalizations at general hospitals and freestanding children’s hospitals in the United States. J Hosp Med. 2016;11(11):743-749. https://doi.org/10.1002/jhm.2624.
10. Peters MDJ, Godfrey CM, Khalil H et al. Guidance for conducting systematic scoping reviews. Int J Evid Based Healthc. 2015;13(3):141-146. https://doi.org/10.1097/XEB.0000000000000050.
11. Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467-473. https://doi.org/10.7326/M18-0850.
12. Alobaidi R, Morgan C, Basu RK, et al. Association between fluid balance and outcomes in critically ill children: a systematic review and meta-analysis. JAMA Pediatr. 2018;172(3):257-268. https://doi.org/10.1001/jamapediatrics.2017.4540.
13. Cifuentes J, Bronstein J, Phibbs CS et al. Mortality in low birth weight infants according to level of neonatal care at hospital of birth. Pediatrics. 2002;109(5):745-751. https://doi.org/10.1542/peds.109.5.745.
14. Donohue PK, Hussey-Gardner B, Sulpar LJ, Fox R, Aucott SW. Convalescent care of infants in the neonatal intensive care unit in community hospitals: risk or benefit? Pediatrics. 2009;124(1):105-111. https://doi.org/10.1542/peds.2008-0880.
15. Izatt SD. Breastfeeding counseling by health care providers. J Hum Lact. 1997;13(2):109-113. https://doi.org/10.1177/089033449701300210.
16. Kuo S, Kimata C, Akamine K, Young B, Balaraman V. Outcomes of inborn and transported extremely premature very-low-birthweight infants in Hawai’i. Pediatr Int. 2012;54(3):365-369. https://doi.org/10.1111/j.1442-200X.2012.03561.x.
17. Maisels MJ, Kring E. Rebound in serum bilirubin level following intensive phototherapy. Arch Pediatr Adolesc Med. 2002;156(7):669-672. https://doi.org/10.1001/archpedi.156.7.669.
18. Meadow W, Mendez D, Makela J et al. Can and should level II nurseries care for newborns who require mechanical ventilation? Clin Perinatol. 1996;23(3):551-561. https://doi.org/10.1016/S0095-5108(18)30227-6.
19. Phibbs CS, Mortensen L. Back transporting infants from neonatal intensive care units to community hospitals for recovery care: effect on total hospital charges. Pediatrics. 1992;90(1):22-26.
20. Wall SN, Handler AS, Park CG. Hospital factors and nontransfer of small babies: A marker of deregionalized perinatal care? J Perinatol. 2004;24(6):351-359. https://doi.org/10.1038/sj.jp.7211101.
21. Alexander DC, Bundy DG, Shore AD et al. Cardiovascular medication errors in children. Pediatrics. 2009;124(1):324-332. https://doi.org/10.1542/peds.2008-2073.
22. Balch AH, Constance JE, Thorell EA, et al. Pediatric vancomycin dosing: trends over time and the impact of therapeutic drug monitoring. J Clin Pharmacol. 2015;55(2):212-220. https://doi.org/10.1002/jcph.402.
23. Conway PH, Edwards S, Stucky ER et al. Variations in management of common inpatient pediatric illnesses: hospitalists and community pediatricians. Pediatrics. 2006;118(2):441-447. https://doi.org/10.1542/peds.2006-0484.
24. Frank BS, Pollack MM. Quantitative quality assurance in a community hospital pediatric intensive care unit. West J Med. 1992;157(2):149-151.
25. Leyenaar JK, Lagu T, Shieh MS, Pekow PS, Lindenauer PK. Variation in resource utilization for the management of uncomplicated community-acquired pneumonia across community and children’s hospitals. J Pediatr. 2014;165(3):585-591. https://doi.org/10.1016/j.jpeds.2014.04.062.
26. Leyenaar JK, Desai AD, Burkhart Q, et al. Quality measures to assess care transitions for hospitalized children. Pediatrics. 2016;138(2):e20160906. https://doi.org/10.1542/peds.2016-0906.
27. Meurer JR, Kuhn EM, George V, Yauck JS, Layde PM. Charges for childhood asthma by hospital characteristics. Pediatrics. 1998;102(6):E70. https://doi.org/10.1542/peds.102.6.e70.
28. Myers J, Lehna C. Where are lengths of stay longer and total charges higher for pediatric burn patients? J Burn Care Res. 2014;35(5):382-387. https://doi.org/10.1097/BCR.0000000000000012.
29. Myers J, Smith M, Woods C, Espinosa C, Lehna C. The effect of transfers between health care facilities on costs and length of stay for pediatric burn patients. J Burn Care Res. 2015;36(1):178-183. https://doi.org/10.1097/BCR.0000000000000206.
30. Odetola FO, Gebremariam A, Freed GL. Patient and hospital correlates of clinical outcomes and resource utilization in severe pediatric sepsis. Pediatrics. 2007;119(3):487-494. https://doi.org/10.1542/peds.2006-2353.
31. Scherb CA, Stevens MS, Busman C. Outcomes related to dehydration in the pediatric population. J Pediatr Nurs. 2007;22(5):376-382. https://doi.org/10.1016/j.pedn.2006.10.004.
32. Van Winkle P, Whiffen T, Liu IL. Experience using peripherally inserted central venous catheters for outpatient parenteral antibiotic therapy in children at a community hospital. ediatr Infect Dis J. 2008;27(12):1069-1072. https://doi.org/10.1097/INF.0b013e31817d32f2.
33. Beaty JS, Chendrasekhar A, Hopkins J, Gruelke L. Missed injuries in pediatric trauma patients. J Appl Res. 2003;3(1):84-88. https://jrnlappliedresearch.com/articles/Vol3Iss1/CHENDRASEKHAR.htm. Accessed July 8, 2019.
34. Kelley-Quon LI, Tseng CH, Jen HC, Shew SB. Hospital type predicts surgical complications for infants with hypertrophic pyloric stenosis. Am Surg. 2012;78(10):1079-1082.
35. Kelley-Quon LI, Tseng CH, Jen HC, Lee SL, Shew SB. Hospital type as a metric for racial disparities in pediatric appendicitis. J Am Coll Surg. 2013;216(1):74-82. https://doi.org/10.1016/j.jamcollsurg.2012.09.018.
36. Pokala N, Sadhasivam S, Kiran RP, Parithivel V. Complicated appendicitis—is the laparoscopic approach appropriate? A comparative study with the open approach: outcome in a community hospital setting. Am Surg. 2007;73(8):732-737.
37. Smith JT, Price C, Stevens PM, Masters KS, Young M. Does pediatric orthopedic subspecialization affect hospital utilization and charges? J Pediatr Orthop. 1999;19(4):553-555. https://doi.org/10.1097/01241398-199907000-00027.
38. Calvert C, Strauss KJ, Mooney DP. Variation in computed tomography radiation dose in community hospitals. J Pediatr Surg. 2012;47(6):1167-1169. https://doi.org/10.1016/j.jpedsurg.2012.03.021.
39. Marin JR, Sengupta D, Bhargavan-Chatfield M et al. Variation in pediatric cervical spine computed tomography radiation dose index. Acad Emerg Med. 2015;22(12):1499-1505. https://doi.org/10.1111/acem.12822.
40. Reich JD, Brogdon B, Ray WE, Eckert J, Gorell H. Use of CT scan in the diagnosis of pediatric acute appendicitis. Pediatr Emerg Care. 2000;16(4):241-243. https://doi.org/10.1097/00006565-200008000-00006.
41. Saito JM, Yan Y, Evashwick TW, Warner BW, Tarr PI. Use and accuracy of diagnostic imaging by hospital type in pediatric appendicitis. Pediatrics. 2013;131(1):e37-e44. https://doi.org/10.1542/peds.2012-1665.
42. York D, Smith A, Phillips JD, Von Allmen D. The influence of advanced radiographic imaging on the treatment of pediatric appendicitis. J Pediatr Surg. 2005;40(12):1908-1911. https://doi.org/10.1016/j.jpedsurg.2005.08.004.
43. Altman RL, Canter J, Patrick PA et al. Parent education by maternity nurses and prevention of abusive head trauma. Pediatrics. 2011;128(5):e1164-e1172. https://doi.org/10.1542/peds.2010-3260.
44. Clemens CJ, Gable EK. The development of a group B streptococcus prevention policy at a community hospital. J Perinatol. 2002;22(7):523-525. https://doi.org/10.1038/sj.jp.7210794.
45. Hall RW, Hall-Barrow J, Garcia-Rill E. Neonatal regionalization through telemedicine using a community-based research and education core facility. Ethn Dis. 2010;20(1 Suppl 1):S136-S140.
46. Hulsey TC, Pittard WB 3rd, Ebeling M. Regionalized perinatal transport systems: association with changes in location of birth, neonatal transport, and survival of very low birth weight deliveries. J S C Med Assoc. 1991;87(12):581-584.
47. Sable CA, Cummings SD, Pearson GD, et al. Impact of telemedicine on the practice of pediatric cardiology in community hospitals. Pediatrics. 2002;109(1):E3. https://doi.org/10.1542/peds.109.1.e3.
48. Wexelblatt SL, Ward LP, Torok K et al. Universal maternal drug testing in a high-prevalence region of prescription opiate abuse. J Pediatr. 2015;166(3):582-586. https://doi.org/10.1016/j.jpeds.2014.10.004.
49. Dayal A, Alvarez F. The effect of implementation of standardized, evidence-based order sets on efficiency and quality measures for pediatric respiratory illnesses in a community hospital. Hosp Pediatr. 2015;5(12):624-629. https://doi.org/10.1542/hpeds.2015-0140.
50. Krugman SD, Suggs A, Photowala HY, Beck A. Redefining the community pediatric hospitalist: the combined Pediatric ED/Inpatient Unit. Pediatr Emerg Care. 2007;23(1):33-37. https://doi.org/10.1097/01.pec.0000248685.94647.01.
51. Kuhlmann S, Mason B, Ahlers-Schmidt CR. A quality improvement project to improve compliance with the joint commission children’s asthma care-3 measure. Hosp Pediatr. 2013;3(1):45-51. https://doi.org/10.1542/hpeds.2012-0015.
52. Labarbera JM, Ellenby MS, Bouressa P et al. The impact of telemedicine intensivist support and a pediatric hospitalist program on a community hospital. Telemed J E Health. 2013;19(10):760-766. https://doi.org/10.1089/tmj.2012.0303.
53. Nkoy F, Fassl B, Stone B, et al. Improving pediatric asthma care and outcomes Across multiple hospitals. Pediatrics. 2015;136(6):e1602-e1610. https://doi.org/10.1542/peds.2015-0285.
54. Walia J, Qayumi Z, Khawar N, et al. Physician transition of care: benefits of I-PASS and an electronic handoff system in a community pediatric residency program. Acad Pediatr. 2016;16(6):519-523. https://doi.org/10.1016/j.acap.2016.04.001.
55. Yang CP, Hunt EA, Shilkofski N et al. Can telemedicine improve adherence to resuscitation guidelines for critically ill children at community hospitals? A randomized controlled trial using high-fidelity simulation. Pediatr Emerg Care. 2017;33(7):474-479. https://doi.org/10.1097/PEC.0000000000000653.
56. Kelley-Quon LI, Crowley MA, Applebaum H, et al. Academic-community partnerships improve outcomes in pediatric trauma care. J Pediatr Surg. 2015;50(6):1032-1036. https://doi.org/10.1016/j.jpedsurg.2015.03.033.
57. Ralston S, Garber M, Narang S, et al. Decreasing unnecessary utilization in acute bronchiolitis care: results from the value in inpatient pediatrics network. J Hosp Med. 2013;8(1):25-30. https://doi.org/10.1002/jhm.1982.
58. Ralston SL, Garber MD, Rice-Conboy E, et al. A multicenter collaborative to reduce unnecessary care in inpatient bronchiolitis. Pediatrics. 2016;137(1):e20150851. https://doi.org/10.1542/peds.2015-0851.
59. Parikh K, Biondi E, Nazif J, et al. A multicenter collaborative to improve care of community acquired pneumonia in hospitalized children. Pediatrics. 2017;139(3):e20161411. https://doi.org/10.1542/peds.2016-1411.
60. Leyenaar JK, Bergert L, Mallory LA, et al. Pediatric primary care providers’ perspectives regarding hospital discharge communication: a mixed methods analysis. Acad Pediatr. 2015;15(1):61-68. https://doi.org/10.1016/j.acap.2014.07.004.
© 2019 Society of Hospital Medicine
Catheter-Associated Urinary Tract Infections in Adults: Diagnosis, Treatment, and Prevention
Every day in the United States, approximately 4% of patients in acute care hospitals have at least one hospital-acquired infection (HAI).1,2 Among the top 10 causes of death in the United States, HAIs are associated with increased morbidity, mortality, and hospital length of stay (LOS).2 The direct medical cost of treating HAIs is substantial for both hospitals and patients.3,4 Urinary tract infections (UTIs) are a leading cause of HAI, and 70%-80% of these are catheter-associated urinary tract infections (CAUTIs).5,6 In 2016, 26,983 CAUTIs occurred in acute care hospitals.7 The high incidence of CAUTI can substantially contribute to morbidity, length of stay, and mortality.8-11
The recognition that a substantial proportion of HAIs may be preventable, including 55%-70% of CAUTIs,12 has resulted in implementing multiple strategies to reduce CAUTI rates.13-17 These include simple prevention interventions such as avoiding placement of unnecessary indwelling urinary catheters and early removal of urinary catheters when they are no longer clinically indicated. Hospitalists are responsible for the care of many, if not most, inpatients with indwelling urinary catheters and are integral in antimicrobial stewardship efforts surrounding CAUTIs.18 Diagnostic stewardship, including appropriate urine specimen ordering, collection, processing, and reporting, works synergistically with antimicrobial stewardship and allows for appropriate antibiotic prescribing in symptomatic patients.19
DEFINITIONS
CAUTIs can be defined using either clinical or surveillance definitions. Clinical definitions are used at the bedside and take individual clinical characteristics into consideration, but vary among clinicians since there is no gold standard. Abnormal laboratory urinary findings in the absence of symptoms are not sufficient for the diagnosis of UTI, including CAUTI. Surveillance definitions, such as those used by the Centers for Disease Control and Prevention,20 are designed to be simple, easily applicable in any healthcare setting, and standardized to all patients. Surveillance definitions generally include at least one systemic or local symptom (such as fever or dysuria) and positive urine culture in a patient with an indwelling urinary catheter (or within 48 hours after its removal).
Pyuria is leukocytes or white blood cells (WBCs) in a urine specimen, with a threshold of >10 WBCs/high-power field using urine microscopy. The predictive value of different thresholds of pyuria for UTI is unclear.
Bacteriuria denotes the presence (on microscopy or culture) of bacteria in the urine. In a patient without signs or symptoms of a UTI, this is termed asymptomatic bacteriuria (ASB). A full discussion of bacteriuria, a major reason for inappropriate antibiotic use, is beyond the scope of this article but is discussed in a recent guideline.21
Urinary tract infections are usually characterized by a clinical syndrome along with evidence of pyuria and/or bacteriuria. The two major clinical syndromes that are observed are lower UTI (cystitis or bladder infections) and upper UTI (pyelonephritis or kidney infections). Rarely, patients may develop asymptomatic bacteremic UTI, where blood and urine cultures grow the same pathogen in the absence of clinical symptoms. (Table 1 summarizes the key points for these definitions).
CAUSES AND RISK FACTORS FOR CAUTI
Bacterial biofilm can form on the inner and outer surfaces of an indwelling urinary catheter following its insertion and can be associated with bacteriuria and CAUTI.22,23 The biofilm comprises bacteria from the periurethral area that migrate upwards from a colonized drainage system. Bacteria present in the biofilm tend to exhibit slow growth, are protected from antibiotic exposure, and have less susceptibility to these agents.22-24 When a mature biofilm has formed, catheter removal may be necessary for source control and to facilitate effective antimicrobial treatment. The pathogens that most commonly cause CAUTIs are Escherichia coli (23.9%), Pseudomonas aeruginosa (10.3%), and Klebsiella pneumoniae/oxytoca (10.1%).25 Although urine cultures often grow yeast, particularly Candida spp., nonbacterial pathogens rarely cause UTI.
The risk of developing a CAUTI is directly related to catheter dwell time.26,27 For catheterized patients, the rate of development of catheter-associated bacteriuria is approximately 3% to 7% per day14,28 and is more common in the elderly and females. The likelihood of bacteriuria approaches 100% if a patient has an indwelling urinary catheter for ≥30 days,27,29,30 which is part of the rationale for why a urine culture alone is not sufficient to diagnose a CAUTI. While bacteriuria is a risk factor for UTI, the frequency of progression from bacteriuria to CAUTI is low and treating ASB does not decrease the risk of future CAUTI. Other risk factors for the development of CAUTI include urinary tract instrumentation, diabetes mellitus, and malnutrition.31,32
The two most important factors that lead to the development of CAUTIs and have been the main focus of quality improvement areas are unnecessary urinary catheter placement and inappropriate delay in removing a catheter when it is no longer needed.26,33 Unfortunately, 38% of attending physicians are unaware that their patients have a urinary catheter in place.34 Furthermore, in 20% to 50% of cases, there is no clear indication for catheter placement.2,34
DIAGNOSIS OF CAUTI
A CAUTI diagnosis is typically one of exclusion, as most patients present with fever and no apparent alternative source.14,29 Since catheterized patients may not exhibit common cystitis symptoms,29 most who develop CAUTI present with fever alone. However, most fevers in patients with bacteriuria and catheters are not CAUTIs and can be attributed to other sources. If a patient with an indwelling urinary catheter develops a fever and there is a suspicion of a CAUTI, careful evaluation is warranted for alternative sources of infection. This particularly applies to patients with severe systemic illness, such as hypotension or systemic inflammatory response syndrome, since these are unusual manifestations of CAUTI. The presence of either cloudy or malodorous urine does not indicate a UTI, and should not be the sole rationale for obtaining a urine culture.
Diagnostic workup of fever should include a clinical assessment of the patient. Indeed, professional guidelines recommend against obtaining a urine culture routinely for fever, unless invasive UTI risk is elevated, such as in patients with neutropenia, history of renal transplantation, or recent genitourinary surgery.35 Diagnostic stewardship, focusing on the appropriate use of urine cultures, can reduce CAUTI rates.36 For catheterized patients, hospitals are increasingly adopting reflex urine culture, where urine is simultaneously collected for a urinalysis and urine culture, but a urine culture is performed only if the urinalysis is positive for a predetermined threshold for pyuria, leukocyte esterase, or both. However, the use of reflex urine cultures remains an area of debate.37 In addition, the Infectious Diseases Society of America recommends against screening for or treating ASB in patients with either short-term (<30 days) or long-term indwelling urethral catheters.21
Ideally, a urine culture should be obtained by collecting a midstream sample. In catheterized patients, a sample should be obtained after removal of the catheter; or, in patients with a clinical indication for ongoing catheterization, a sample should be obtained after a new catheter has been placed.14 If an indwelling urinary catheter must be continued, the recommendation is to disinfect the drainage system’s aspiration port and then obtain a urine culture. Urine should never be obtained from a catheter collection bag. (Table 2 summarizes best practices for diagnosis of CAUTI).
TREATMENT OF CAUTI
For all CAUTIs, an indwelling urinary catheter should be removed as soon as possible. If an indwelling urinary catheter remains necessary, but the existing catheter has been in place longer than two weeks, a new catheter should be placed before initiating antibiotic therapy14 to accelerate symptom resolution and reduce the likelihood of relapse or recurrence.32
Urinary tract agents such as fosfomycin and nitrofurantoin are recommended as first-line agents for simple cystitis in women and can be used in patients with lower UTI and sufficient renal function to achieve adequate drug concentration in urine. Upper UTIs require antibiotics with good penetration into renal parenchyma such as ceftriaxone. If empiric antimicrobial therapy is needed before culture results are available, then previous urine culture results, local antibiograms, or practice guidelines can guide selection. Definitive antimicrobial therapy should be based on urine culture results. It is important to narrow empiric therapy14 to reduce risk of Clostridioides difficile infection and emergence of other resistant bacteria. Fluoroquinolones should be avoided for lower UTIs because of these risks and multiple United States Food and Drug Administration warnings.38-40
The optimal duration of antimicrobial therapy for a CAUTI is unclear;14 however, most patients can be treated with a relatively short duration of therapy (≤7 days) if they respond promptly to therapy. Patients with a slow response to therapy may require 10-14 days of treatment.14 (Table 2 summarizes best practices for the treatment of CAUTI).
STRATEGIES FOR CAUTI PREVENTION
Since CAUTI is predicated on the presence of an indwelling urinary catheter, the simplest way to reduce CAUTI is to avoid placing or retaining unnecessary catheters. Some examples of appropriate indications9 for placement and maintenance of an indwelling urinary catheter are listed below:
1. Accurate measurement of urinary output in severely ill patients;
2. Improved comfort for patients receiving end-of-life care;
3. Acute urinary retention or bladder outlet obstruction;
4. Need for a period of prolonged immobilization (eg, potentially unstable lumbar or thoracic spine, or has multiple traumatic injuries);
5. Selected surgical procedures, such as urologic procedures and those that are expected to have a prolonged duration, require intraoperative monitoring of urine output, require the administration of either large volumes of intravenous infusions or diuretics;
6. To promote healing of open perineal or sacral wounds in patients with incontinence;
7. Neurogenic bladder; and
8. Hematuria with clots.
To increase the timely removal of urinary catheters that are no longer indicated, daily assessment of catheter necessity must be an integral part of clinicians’ workflow.32,33 Alternatives, such as external catheters or intermittent catheterization, should be considered before indwelling urinary catheter placement since both options are associated with a reduced CAUTI risk.41-44 Although indwelling urinary catheters can be seen as being more convenient for both patients and healthcare providers, many patients have expressed a preference for the use of intermittent catheterization compared with indwelling urinary catheterization.43
For urinary retention, bladder scanning can noninvasively assess the amount of residual urine in a patient’s bladder and can avoid unnecessary insertion of an indwelling urinary catheter. However, if indwelling urinary catheters are ultimately needed, they must be inserted and maintained appropriately. Of note, the use of antibiotic-impregnated catheters has not been shown to reduce CAUTI rates significantly.45
CAUTI prevention requires a multidisciplinary collaborative approach. Nurse-driven protocols and checklists to remove indwelling urinary catheters that are no longer indicated can be very effective.46,47 Automatic stop orders and catheter removal reminders are useful for reducing the duration of catheter placement.26,48 Both of these approaches require appropriate, consistent documentation with input from bedside nurses, physicians, advanced practice providers, and information technology. (Table 3 summarizes best practices for the prevention of CAUTI).
Importantly, CAUTI prevention supports broader antimicrobial stewardship. Over 55% of inpatients receive at least one dose of an antibiotic during their hospital stay.48,49 In 2015, the White House released the National Action Plan for Combating Antibiotic-Resistant Bacteria with the goals of slowing the emergence of resistant bacteria, preventing the spread of antibiotic-resistant infections, and setting a target of a 20% reduction in the inappropriate use of antibiotics for hospitalized patients.50 Hospitalists care for a substantial number of inpatients and, in turn, can drive actions to decrease CAUTIs, and promote stewardship efforts. Through actions to decrease CAUTIs, hospitalists can promote these stewardship efforts.
CONCLUSIONS
CAUTI is one of the most common types of HAI and is associated with increased morbidity, hospital length of stay, and patient costs. Most CAUTIs are preventable by limiting the placement of unnecessary catheters to instances of true necessity and removing catheters when they are no longer clinically indicated. Proper technique for the insertion and maintenance of catheters is also important for reducing CAUTI rates. Hospitalists care for a substantial number of inpatients and can make major contributions to the appropriate diagnosis, treatment, and prevention of CAUTIs.
1. Centers for Disease Control and Prevention. Estimates of healthcare-associated infections. http://www.cdc.gov/ncidod/dhqp/hai.html. Accessed September 27, 2018.
2. Calfee D. Crisis in hospital-acquired, healthcare-associated infections. Annu Rev Med. 2012;63(1):359-371. https://doi.org/10.1146/annurev-med-081210-144458.
3. Scott R. 2009. The direct medical costs of healthcare-associated infections in U.S. hospitals and the benefits of prevention. Centers for Disease Control and Prevention, Atlanta. http://www.cdc.gov/ncidod/dhqp/pdf/Scott_CostPaper.pdf. Accessed September 27, 2018.
4. Zimlachman E, Henderson D, Tamir O, et al. Health care associated infections. A meta-analysis of costs and financial impact on the US health care system. JAMA Intern Med. 2013;173(22):2039-2046. https://doi.org/10.1001/jamainternmed.2013.9763.
5. Nicolle LE. Catheter-acquired urinary tract infection: the once and future guidelines. Infect Control Hosp Epidemiol. 2010;31(4):327-329. https://doi.org/10.1086/651092.
6. Weber DJ, Sickbert-Bennett EE, Gould CV, et al. Incidence of catheter-associated and noncatheter-associated urinary tract infections in a healthcare system. Infect Control Hosp Epidemiol. 2011;32(8):822-823. https://doi.org/10.1086/661107.
7. Healthcare-associated infections. National and state HAI progress reports or SIR reports. https://www.cdc.gov/hai/data/archive/archive.html. Accessed May 6, 2019.
8. Saint S. Clinical and economic consequences of nosocomial catheter-related bacteriuria. Am J Infect Control. 2000;28(1):68-75. https://doi.org/10.1016/S0196-6553(00)90015-4.
9. Platt R, Polk BF, Murdock B, Rosner B. Mortality associated with nosocomial urinary-tract infection. N Engl J Med. 1982;307(11):637-642. https://doi.org/10.1056/NEJM198209093071101.
10. Tambyah PA, Knasinski V, Maki DG. The direct costs of nosocomial catheter-associated urinary tract infection in the era of managed care. Infect Control Hosp Epidemiol. 2002;23(1):27-31.
11. Chant C, Smith OM, Marshall JC, Friedrich JO. Relationship of catheter-associated urinary tract infection to mortality and length of stay in critically ill patients: a systematic review and meta-analysis of observational studies. Crit Care Med. 2011;39(5):1167-1173. https://doi.org/10.1097/CCM.0b013e31820a8581.
12. Umscheid CA, Mitchell MD, Doshi JA, et al. Estimating the proportion of healthcare-associated infections that are reasonably preventable and the related mortality and costs. Infect Control Hosp Epidemiol. 2011;32(2):101-114. https://doi.org/10.1086/657912.
13. Gould C, Umscheid C, Agarwal R, et al. Healthcare Infection Control Practices Advisory Committee (HICPAC): guideline for the prevention of catheter-associated urinary tract infections, 2009. http://www.cdc.gov/hicpac/pdf/CAUTI/CAUTIguideline2009final.pdf. Accessed November 1, 2018.
14. Hooton TM, Bradley SF, Cardenas DD, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(5):625-663. https://doi.org/10.1086/650482.
15. Conway LJ, Larson EL. Guidelines to prevent catheter-associated urinary tract infection: 1980 to 2010. Heart Lung. 2011;41(3):271-283. https://doi.org/10.1016/j.hrtlng.2011.08.001.
16. Greene L, Marx J, Oriola S. APIC elimination guide: guide to the elimination of catheter-associated urinary tract infections (CAUTIs). http://www.apic.org/Content/NavigationMenu/PracticeGuidance/APICEliminationGuides/CAUTI_Guide_0609.pdf. Accessed October 15, 2018.
17. Yokoe DS, Mermel LA, Anderson DJ, et al. A compendium of strategies to prevent healthcare-associated infections in acute care hospitals. Infect Control Hosp Epidemiol. 2008;29(S1):S12-S21.
18. Wiley Z, Kobaidze K, Sexton ME, Jacob JT. Hospitalists as integral stakeholders in antimicrobial stewardship. Curr Treat Options Infect Dis. 2018;10(2):240-248. https://doi.org/10.1007/s40506-018-0162-z.
19. Claeys KC, Blanco N, Morgan DJ, Leekha S, Sullivan KV. Advances and challenges in the diagnosis and treatment of urinary tract infections: the need for diagnostic stewardship. Curr Infect Dis Rep. 2019;21(4):11. https://doi.org/10.1007/s11908-019-0668-7.
20. Horan T, Andrus M, Dudeck M. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36(5):309-332. https://doi.org/10.1016/j.ajic.2008.03.002.
21. Nicolle LE, Gupta K, Bradley S, et al. Clinical practice guideline for the management of asymptomatic bacteriuria: 2019 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2019;68(10):e83-e110.
22. Donlan R. Biofilms and device-associated infections. Emerg Infect Dis. 2001;7(2):277-281. https://doi.org/10.3201/eid0702.010226.
23. Choe HS, Son SW, Choi HA, et al. Analysis of the distribution of bacteria within urinary catheter biofilms using four different molecular techniques. Am J Infect Control. 2012;40(9):e249-e254. https://doi.org/10.1016/j.ajic.2012.05.010.
24. Mohajer M, Darouiche R. Prevention and treatment of urinary catheter-associated infections. Curr Infect Dis Rep. 2013;15(2):116-123. https://doi.org/10.1007/s11908-013-0316-6.
25. Weiner LM, Webb AK, Limbago B, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011-2014. Infect Control Hosp Epidemiol. 2016;37(11):1288-1301. https://doi.org/10.1017/ice.2016.174.
26. Tambyah PA, Oon J. Catheter-associated urinary tract infection. Curr Opin Infect Dis. 2012;25(4):365-370. https://doi.org/10.1097/QCO.0b013e32835565cc.
27. Warren JW, Tenney JH, Hoopes JM, Muncie HL, Anthony WC. A prospective microbiologic study of bacteriuria in patients with chronic indwelling urethral catheters. J Infect Dis. 1982;146(6):719-723. https://doi.org/10.1093/infdis/146.6.719.
28. Lo E, Nicolle L, Coffin S, et al. Strategies to prevent catheter-associated urinary tract infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(5):464-479. https://doi.org/10.1086/675718.
29. Nicolle LE. Urinary catheter-associated infections. Infect Dis Clin N Am. 2012;26(1):13-27. https://doi.org/10.1016/j.idc.2011.09.009.
30. Titsworth WL, Hester J, Correia T, et al. Reduction of catheter-associated urinary tract infections among patients in a neurological intensive care unit: a single institution’s success. J Neurosurg. 2012;116(4):911-920. https://doi.org/10.3171/2011.11.JNS11974.
31. Lobdell KW, Stamou S, Sanchez JA. Hospital-acquired infections. Surg Clin N Am. 2012;92(1):65-77. https://doi.org/10.1016/j.suc.2011.11.003.
32. Gray M. Reducing catheter-associated urinary tract infection in the critical care unit. AACN Adv Crit Care. 2010;21(3):247-257. 33. Fakih MG, Watson SR, Greene T, et al. Reducing inappropriate urinary catheter use. Arch Intern Med. 2012;172(3):255-260. https://doi.org/10.1001/archinternmed.2011.627.
33. Fakih MG, Watson SR, Greene T, et al. Reducing inappropriate urinary catheter use. Arch Intern Med. 2012;172(3):255-260. https://doi.org/10.1001/archinternmed.2011.627.
34. Saint S, Wiese J, Amory JK, Bernstein ML, et al. Are physicians aware of which of their patients have an indwelling urinary catheters? Am J Med. 2000;109(6):476-480. https://doi.org/10.1016/s0002-9343(00)00531-3.
35. O’Grady NP, Barie PS, Bartlett JG, et al. Guidelines for evaluation of new fever in critically ill adult patients: 2008 update from the American College of Critical Care Medicine and the Infectious Diseases Society of America. Crit Care Med. 2008;36(4):1330-1349. https://doi.org/10.1097/CCM.0b013e318169eda9.
36. Mullin K, Kovacs C, Fatica C, et al. A multifaceted approach to reduction of catheter-associated urinary tract infections in the intensive care unit with an emphasis on “stewardship of culturing.” Infect Control Hosp Epidemiol. 2017;38(2):186-188. https://doi.org/10.1017/ice.2016.266.
37. Humphries R, Bard J. Point-counterpoint: reflex cultures reduce laboratory workload and improve antimicrobial stewardship in patients suspected of having urinary tract infections. J Clin Microbiol. 2016;54(2):254-258. https://doi.org/10.1128/JCM.03021-15.
38. Voelker R. New fluoroquinolone warning. JAMA. 2016;315(23):2514. https://doi.org/10.1001/jama.2016.7290.
39. Tillotson S. FDA and the safe and appropriate antibiotic use of fluoroquinolones. Lancet Infect Dis. 2016;16(3):PE11-E12.
40. Tanne J. FDA adds “black box” warning label to fluoroquinolone antibiotics. BMJ 2008;337:a816. https://doi.org/10.1136/bmj.a816.
41. Wyndaele JJ, Brauner A, Geerlings SE, et al. Clean intermittent catheterization and urinary tract infection: review and guide for future research. BJU Intern. 2012;110(11c):E910-E917. https://doi.org/10.1111/j.1464-410X.2012.11549.x.
42. Hakwoort RA, Thijs SD, Bouwmeester FW, et al. Comparing clean intermittent catheterization and transurethral indwelling catheterization for incomplete voiding after vaginal prolapse surgery: a multicenter randomized trial. BJOG. 2011;118(9):1055-1060. https://doi.org/10.1111/j.1471-0528.2011.02935.x.
43. Hakvoort RA, Nieuwkerk PT, Burger MP, et al. Patient preferences for clean intermittent catheterization and transurethral indwelling catheterization for treatment of abnormal post-void residual bladder volume after vaginal prolapsed surgery. BJOG. 2011;118(11):1324-1328. https://doi.org/10.1111/j.1471-0528.2011.03056.x.
44. Saint S, Kaufman SR, Roger M, et al. Condom versus indwelling urinary catheters: a randomized trial. JAGS. 2006;54(7):1055-1061. https://doi.org/10.1111/j.1532-5415.2006.00785.x.
45. Pickard R, Lam T, MacLennan G, et al. Antimicrobial catheters for reduction of symptomatic urinary tract infection in adults requiring short-term catheterization in hospital: a multicenter randomized controlled trial. Lancet. 2012;380(9857):1927-1935. https://doi.org/10.1016/S0140-6736(12)61380-4.
46. Parry M, Grant B, Sestovic M. Successful reduction in catheter-associated urinary tract infections: focus on nurse-directed catheter removal. Am J Infect Control. 2013;41(12):1178-1181. https://doi.org/10.1016/j.ajic.2013.03.296.
47. Fuchs MA, Sexton DJ, Thornlow DK, Champagne MT. Evaluation of an evidence-based, nurse-driven checklist to prevent hospital-acquired catheter-associated urinary tract infections in intensive care units. J Nurs Care Qual. 2011;26(2):101-109. https://doi.org/10.1097/NCQ.0b013e3181fb7847.
48. Meddings J, Rogers MA, Macy M, Saint S. Systematic review and meta-analysis: reminder systems to reduce catheter-associated urinary tract infections and urinary catheter use in hospitalized patients. Clin Infect Dis. 2010;51(5):550-560. https://doi.org/10.1086/655133.
49. Baggs J, Fridkin SK, Pollack LA, Srinivasan A, Jernigan JA. Estimating national trends in inpatient antibiotic use among US hospitals from 2006 to 2012. JAMA Intern Med. 2016;176(11):1639-1648. https://doi.org/10.1001/jamainternmed.2016.5651.
50. National Action Plan for Combating Antibiotic-resistant Bacteria. 2015. https://obamawhitehouse.archives.gov/sites/default/files/docs/national_action_plan_for_combatin
Every day in the United States, approximately 4% of patients in acute care hospitals have at least one hospital-acquired infection (HAI).1,2 Among the top 10 causes of death in the United States, HAIs are associated with increased morbidity, mortality, and hospital length of stay (LOS).2 The direct medical cost of treating HAIs is substantial for both hospitals and patients.3,4 Urinary tract infections (UTIs) are a leading cause of HAI, and 70%-80% of these are catheter-associated urinary tract infections (CAUTIs).5,6 In 2016, 26,983 CAUTIs occurred in acute care hospitals.7 The high incidence of CAUTI can substantially contribute to morbidity, length of stay, and mortality.8-11
The recognition that a substantial proportion of HAIs may be preventable, including 55%-70% of CAUTIs,12 has resulted in implementing multiple strategies to reduce CAUTI rates.13-17 These include simple prevention interventions such as avoiding placement of unnecessary indwelling urinary catheters and early removal of urinary catheters when they are no longer clinically indicated. Hospitalists are responsible for the care of many, if not most, inpatients with indwelling urinary catheters and are integral in antimicrobial stewardship efforts surrounding CAUTIs.18 Diagnostic stewardship, including appropriate urine specimen ordering, collection, processing, and reporting, works synergistically with antimicrobial stewardship and allows for appropriate antibiotic prescribing in symptomatic patients.19
DEFINITIONS
CAUTIs can be defined using either clinical or surveillance definitions. Clinical definitions are used at the bedside and take individual clinical characteristics into consideration, but vary among clinicians since there is no gold standard. Abnormal laboratory urinary findings in the absence of symptoms are not sufficient for the diagnosis of UTI, including CAUTI. Surveillance definitions, such as those used by the Centers for Disease Control and Prevention,20 are designed to be simple, easily applicable in any healthcare setting, and standardized to all patients. Surveillance definitions generally include at least one systemic or local symptom (such as fever or dysuria) and positive urine culture in a patient with an indwelling urinary catheter (or within 48 hours after its removal).
Pyuria is leukocytes or white blood cells (WBCs) in a urine specimen, with a threshold of >10 WBCs/high-power field using urine microscopy. The predictive value of different thresholds of pyuria for UTI is unclear.
Bacteriuria denotes the presence (on microscopy or culture) of bacteria in the urine. In a patient without signs or symptoms of a UTI, this is termed asymptomatic bacteriuria (ASB). A full discussion of bacteriuria, a major reason for inappropriate antibiotic use, is beyond the scope of this article but is discussed in a recent guideline.21
Urinary tract infections are usually characterized by a clinical syndrome along with evidence of pyuria and/or bacteriuria. The two major clinical syndromes that are observed are lower UTI (cystitis or bladder infections) and upper UTI (pyelonephritis or kidney infections). Rarely, patients may develop asymptomatic bacteremic UTI, where blood and urine cultures grow the same pathogen in the absence of clinical symptoms. (Table 1 summarizes the key points for these definitions).
CAUSES AND RISK FACTORS FOR CAUTI
Bacterial biofilm can form on the inner and outer surfaces of an indwelling urinary catheter following its insertion and can be associated with bacteriuria and CAUTI.22,23 The biofilm comprises bacteria from the periurethral area that migrate upwards from a colonized drainage system. Bacteria present in the biofilm tend to exhibit slow growth, are protected from antibiotic exposure, and have less susceptibility to these agents.22-24 When a mature biofilm has formed, catheter removal may be necessary for source control and to facilitate effective antimicrobial treatment. The pathogens that most commonly cause CAUTIs are Escherichia coli (23.9%), Pseudomonas aeruginosa (10.3%), and Klebsiella pneumoniae/oxytoca (10.1%).25 Although urine cultures often grow yeast, particularly Candida spp., nonbacterial pathogens rarely cause UTI.
The risk of developing a CAUTI is directly related to catheter dwell time.26,27 For catheterized patients, the rate of development of catheter-associated bacteriuria is approximately 3% to 7% per day14,28 and is more common in the elderly and females. The likelihood of bacteriuria approaches 100% if a patient has an indwelling urinary catheter for ≥30 days,27,29,30 which is part of the rationale for why a urine culture alone is not sufficient to diagnose a CAUTI. While bacteriuria is a risk factor for UTI, the frequency of progression from bacteriuria to CAUTI is low and treating ASB does not decrease the risk of future CAUTI. Other risk factors for the development of CAUTI include urinary tract instrumentation, diabetes mellitus, and malnutrition.31,32
The two most important factors that lead to the development of CAUTIs and have been the main focus of quality improvement areas are unnecessary urinary catheter placement and inappropriate delay in removing a catheter when it is no longer needed.26,33 Unfortunately, 38% of attending physicians are unaware that their patients have a urinary catheter in place.34 Furthermore, in 20% to 50% of cases, there is no clear indication for catheter placement.2,34
DIAGNOSIS OF CAUTI
A CAUTI diagnosis is typically one of exclusion, as most patients present with fever and no apparent alternative source.14,29 Since catheterized patients may not exhibit common cystitis symptoms,29 most who develop CAUTI present with fever alone. However, most fevers in patients with bacteriuria and catheters are not CAUTIs and can be attributed to other sources. If a patient with an indwelling urinary catheter develops a fever and there is a suspicion of a CAUTI, careful evaluation is warranted for alternative sources of infection. This particularly applies to patients with severe systemic illness, such as hypotension or systemic inflammatory response syndrome, since these are unusual manifestations of CAUTI. The presence of either cloudy or malodorous urine does not indicate a UTI, and should not be the sole rationale for obtaining a urine culture.
Diagnostic workup of fever should include a clinical assessment of the patient. Indeed, professional guidelines recommend against obtaining a urine culture routinely for fever, unless invasive UTI risk is elevated, such as in patients with neutropenia, history of renal transplantation, or recent genitourinary surgery.35 Diagnostic stewardship, focusing on the appropriate use of urine cultures, can reduce CAUTI rates.36 For catheterized patients, hospitals are increasingly adopting reflex urine culture, where urine is simultaneously collected for a urinalysis and urine culture, but a urine culture is performed only if the urinalysis is positive for a predetermined threshold for pyuria, leukocyte esterase, or both. However, the use of reflex urine cultures remains an area of debate.37 In addition, the Infectious Diseases Society of America recommends against screening for or treating ASB in patients with either short-term (<30 days) or long-term indwelling urethral catheters.21
Ideally, a urine culture should be obtained by collecting a midstream sample. In catheterized patients, a sample should be obtained after removal of the catheter; or, in patients with a clinical indication for ongoing catheterization, a sample should be obtained after a new catheter has been placed.14 If an indwelling urinary catheter must be continued, the recommendation is to disinfect the drainage system’s aspiration port and then obtain a urine culture. Urine should never be obtained from a catheter collection bag. (Table 2 summarizes best practices for diagnosis of CAUTI).
TREATMENT OF CAUTI
For all CAUTIs, an indwelling urinary catheter should be removed as soon as possible. If an indwelling urinary catheter remains necessary, but the existing catheter has been in place longer than two weeks, a new catheter should be placed before initiating antibiotic therapy14 to accelerate symptom resolution and reduce the likelihood of relapse or recurrence.32
Urinary tract agents such as fosfomycin and nitrofurantoin are recommended as first-line agents for simple cystitis in women and can be used in patients with lower UTI and sufficient renal function to achieve adequate drug concentration in urine. Upper UTIs require antibiotics with good penetration into renal parenchyma such as ceftriaxone. If empiric antimicrobial therapy is needed before culture results are available, then previous urine culture results, local antibiograms, or practice guidelines can guide selection. Definitive antimicrobial therapy should be based on urine culture results. It is important to narrow empiric therapy14 to reduce risk of Clostridioides difficile infection and emergence of other resistant bacteria. Fluoroquinolones should be avoided for lower UTIs because of these risks and multiple United States Food and Drug Administration warnings.38-40
The optimal duration of antimicrobial therapy for a CAUTI is unclear;14 however, most patients can be treated with a relatively short duration of therapy (≤7 days) if they respond promptly to therapy. Patients with a slow response to therapy may require 10-14 days of treatment.14 (Table 2 summarizes best practices for the treatment of CAUTI).
STRATEGIES FOR CAUTI PREVENTION
Since CAUTI is predicated on the presence of an indwelling urinary catheter, the simplest way to reduce CAUTI is to avoid placing or retaining unnecessary catheters. Some examples of appropriate indications9 for placement and maintenance of an indwelling urinary catheter are listed below:
1. Accurate measurement of urinary output in severely ill patients;
2. Improved comfort for patients receiving end-of-life care;
3. Acute urinary retention or bladder outlet obstruction;
4. Need for a period of prolonged immobilization (eg, potentially unstable lumbar or thoracic spine, or has multiple traumatic injuries);
5. Selected surgical procedures, such as urologic procedures and those that are expected to have a prolonged duration, require intraoperative monitoring of urine output, require the administration of either large volumes of intravenous infusions or diuretics;
6. To promote healing of open perineal or sacral wounds in patients with incontinence;
7. Neurogenic bladder; and
8. Hematuria with clots.
To increase the timely removal of urinary catheters that are no longer indicated, daily assessment of catheter necessity must be an integral part of clinicians’ workflow.32,33 Alternatives, such as external catheters or intermittent catheterization, should be considered before indwelling urinary catheter placement since both options are associated with a reduced CAUTI risk.41-44 Although indwelling urinary catheters can be seen as being more convenient for both patients and healthcare providers, many patients have expressed a preference for the use of intermittent catheterization compared with indwelling urinary catheterization.43
For urinary retention, bladder scanning can noninvasively assess the amount of residual urine in a patient’s bladder and can avoid unnecessary insertion of an indwelling urinary catheter. However, if indwelling urinary catheters are ultimately needed, they must be inserted and maintained appropriately. Of note, the use of antibiotic-impregnated catheters has not been shown to reduce CAUTI rates significantly.45
CAUTI prevention requires a multidisciplinary collaborative approach. Nurse-driven protocols and checklists to remove indwelling urinary catheters that are no longer indicated can be very effective.46,47 Automatic stop orders and catheter removal reminders are useful for reducing the duration of catheter placement.26,48 Both of these approaches require appropriate, consistent documentation with input from bedside nurses, physicians, advanced practice providers, and information technology. (Table 3 summarizes best practices for the prevention of CAUTI).
Importantly, CAUTI prevention supports broader antimicrobial stewardship. Over 55% of inpatients receive at least one dose of an antibiotic during their hospital stay.48,49 In 2015, the White House released the National Action Plan for Combating Antibiotic-Resistant Bacteria with the goals of slowing the emergence of resistant bacteria, preventing the spread of antibiotic-resistant infections, and setting a target of a 20% reduction in the inappropriate use of antibiotics for hospitalized patients.50 Hospitalists care for a substantial number of inpatients and, in turn, can drive actions to decrease CAUTIs, and promote stewardship efforts. Through actions to decrease CAUTIs, hospitalists can promote these stewardship efforts.
CONCLUSIONS
CAUTI is one of the most common types of HAI and is associated with increased morbidity, hospital length of stay, and patient costs. Most CAUTIs are preventable by limiting the placement of unnecessary catheters to instances of true necessity and removing catheters when they are no longer clinically indicated. Proper technique for the insertion and maintenance of catheters is also important for reducing CAUTI rates. Hospitalists care for a substantial number of inpatients and can make major contributions to the appropriate diagnosis, treatment, and prevention of CAUTIs.
Every day in the United States, approximately 4% of patients in acute care hospitals have at least one hospital-acquired infection (HAI).1,2 Among the top 10 causes of death in the United States, HAIs are associated with increased morbidity, mortality, and hospital length of stay (LOS).2 The direct medical cost of treating HAIs is substantial for both hospitals and patients.3,4 Urinary tract infections (UTIs) are a leading cause of HAI, and 70%-80% of these are catheter-associated urinary tract infections (CAUTIs).5,6 In 2016, 26,983 CAUTIs occurred in acute care hospitals.7 The high incidence of CAUTI can substantially contribute to morbidity, length of stay, and mortality.8-11
The recognition that a substantial proportion of HAIs may be preventable, including 55%-70% of CAUTIs,12 has resulted in implementing multiple strategies to reduce CAUTI rates.13-17 These include simple prevention interventions such as avoiding placement of unnecessary indwelling urinary catheters and early removal of urinary catheters when they are no longer clinically indicated. Hospitalists are responsible for the care of many, if not most, inpatients with indwelling urinary catheters and are integral in antimicrobial stewardship efforts surrounding CAUTIs.18 Diagnostic stewardship, including appropriate urine specimen ordering, collection, processing, and reporting, works synergistically with antimicrobial stewardship and allows for appropriate antibiotic prescribing in symptomatic patients.19
DEFINITIONS
CAUTIs can be defined using either clinical or surveillance definitions. Clinical definitions are used at the bedside and take individual clinical characteristics into consideration, but vary among clinicians since there is no gold standard. Abnormal laboratory urinary findings in the absence of symptoms are not sufficient for the diagnosis of UTI, including CAUTI. Surveillance definitions, such as those used by the Centers for Disease Control and Prevention,20 are designed to be simple, easily applicable in any healthcare setting, and standardized to all patients. Surveillance definitions generally include at least one systemic or local symptom (such as fever or dysuria) and positive urine culture in a patient with an indwelling urinary catheter (or within 48 hours after its removal).
Pyuria is leukocytes or white blood cells (WBCs) in a urine specimen, with a threshold of >10 WBCs/high-power field using urine microscopy. The predictive value of different thresholds of pyuria for UTI is unclear.
Bacteriuria denotes the presence (on microscopy or culture) of bacteria in the urine. In a patient without signs or symptoms of a UTI, this is termed asymptomatic bacteriuria (ASB). A full discussion of bacteriuria, a major reason for inappropriate antibiotic use, is beyond the scope of this article but is discussed in a recent guideline.21
Urinary tract infections are usually characterized by a clinical syndrome along with evidence of pyuria and/or bacteriuria. The two major clinical syndromes that are observed are lower UTI (cystitis or bladder infections) and upper UTI (pyelonephritis or kidney infections). Rarely, patients may develop asymptomatic bacteremic UTI, where blood and urine cultures grow the same pathogen in the absence of clinical symptoms. (Table 1 summarizes the key points for these definitions).
CAUSES AND RISK FACTORS FOR CAUTI
Bacterial biofilm can form on the inner and outer surfaces of an indwelling urinary catheter following its insertion and can be associated with bacteriuria and CAUTI.22,23 The biofilm comprises bacteria from the periurethral area that migrate upwards from a colonized drainage system. Bacteria present in the biofilm tend to exhibit slow growth, are protected from antibiotic exposure, and have less susceptibility to these agents.22-24 When a mature biofilm has formed, catheter removal may be necessary for source control and to facilitate effective antimicrobial treatment. The pathogens that most commonly cause CAUTIs are Escherichia coli (23.9%), Pseudomonas aeruginosa (10.3%), and Klebsiella pneumoniae/oxytoca (10.1%).25 Although urine cultures often grow yeast, particularly Candida spp., nonbacterial pathogens rarely cause UTI.
The risk of developing a CAUTI is directly related to catheter dwell time.26,27 For catheterized patients, the rate of development of catheter-associated bacteriuria is approximately 3% to 7% per day14,28 and is more common in the elderly and females. The likelihood of bacteriuria approaches 100% if a patient has an indwelling urinary catheter for ≥30 days,27,29,30 which is part of the rationale for why a urine culture alone is not sufficient to diagnose a CAUTI. While bacteriuria is a risk factor for UTI, the frequency of progression from bacteriuria to CAUTI is low and treating ASB does not decrease the risk of future CAUTI. Other risk factors for the development of CAUTI include urinary tract instrumentation, diabetes mellitus, and malnutrition.31,32
The two most important factors that lead to the development of CAUTIs and have been the main focus of quality improvement areas are unnecessary urinary catheter placement and inappropriate delay in removing a catheter when it is no longer needed.26,33 Unfortunately, 38% of attending physicians are unaware that their patients have a urinary catheter in place.34 Furthermore, in 20% to 50% of cases, there is no clear indication for catheter placement.2,34
DIAGNOSIS OF CAUTI
A CAUTI diagnosis is typically one of exclusion, as most patients present with fever and no apparent alternative source.14,29 Since catheterized patients may not exhibit common cystitis symptoms,29 most who develop CAUTI present with fever alone. However, most fevers in patients with bacteriuria and catheters are not CAUTIs and can be attributed to other sources. If a patient with an indwelling urinary catheter develops a fever and there is a suspicion of a CAUTI, careful evaluation is warranted for alternative sources of infection. This particularly applies to patients with severe systemic illness, such as hypotension or systemic inflammatory response syndrome, since these are unusual manifestations of CAUTI. The presence of either cloudy or malodorous urine does not indicate a UTI, and should not be the sole rationale for obtaining a urine culture.
Diagnostic workup of fever should include a clinical assessment of the patient. Indeed, professional guidelines recommend against obtaining a urine culture routinely for fever, unless invasive UTI risk is elevated, such as in patients with neutropenia, history of renal transplantation, or recent genitourinary surgery.35 Diagnostic stewardship, focusing on the appropriate use of urine cultures, can reduce CAUTI rates.36 For catheterized patients, hospitals are increasingly adopting reflex urine culture, where urine is simultaneously collected for a urinalysis and urine culture, but a urine culture is performed only if the urinalysis is positive for a predetermined threshold for pyuria, leukocyte esterase, or both. However, the use of reflex urine cultures remains an area of debate.37 In addition, the Infectious Diseases Society of America recommends against screening for or treating ASB in patients with either short-term (<30 days) or long-term indwelling urethral catheters.21
Ideally, a urine culture should be obtained by collecting a midstream sample. In catheterized patients, a sample should be obtained after removal of the catheter; or, in patients with a clinical indication for ongoing catheterization, a sample should be obtained after a new catheter has been placed.14 If an indwelling urinary catheter must be continued, the recommendation is to disinfect the drainage system’s aspiration port and then obtain a urine culture. Urine should never be obtained from a catheter collection bag. (Table 2 summarizes best practices for diagnosis of CAUTI).
TREATMENT OF CAUTI
For all CAUTIs, an indwelling urinary catheter should be removed as soon as possible. If an indwelling urinary catheter remains necessary, but the existing catheter has been in place longer than two weeks, a new catheter should be placed before initiating antibiotic therapy14 to accelerate symptom resolution and reduce the likelihood of relapse or recurrence.32
Urinary tract agents such as fosfomycin and nitrofurantoin are recommended as first-line agents for simple cystitis in women and can be used in patients with lower UTI and sufficient renal function to achieve adequate drug concentration in urine. Upper UTIs require antibiotics with good penetration into renal parenchyma such as ceftriaxone. If empiric antimicrobial therapy is needed before culture results are available, then previous urine culture results, local antibiograms, or practice guidelines can guide selection. Definitive antimicrobial therapy should be based on urine culture results. It is important to narrow empiric therapy14 to reduce risk of Clostridioides difficile infection and emergence of other resistant bacteria. Fluoroquinolones should be avoided for lower UTIs because of these risks and multiple United States Food and Drug Administration warnings.38-40
The optimal duration of antimicrobial therapy for a CAUTI is unclear;14 however, most patients can be treated with a relatively short duration of therapy (≤7 days) if they respond promptly to therapy. Patients with a slow response to therapy may require 10-14 days of treatment.14 (Table 2 summarizes best practices for the treatment of CAUTI).
STRATEGIES FOR CAUTI PREVENTION
Since CAUTI is predicated on the presence of an indwelling urinary catheter, the simplest way to reduce CAUTI is to avoid placing or retaining unnecessary catheters. Some examples of appropriate indications9 for placement and maintenance of an indwelling urinary catheter are listed below:
1. Accurate measurement of urinary output in severely ill patients;
2. Improved comfort for patients receiving end-of-life care;
3. Acute urinary retention or bladder outlet obstruction;
4. Need for a period of prolonged immobilization (eg, potentially unstable lumbar or thoracic spine, or has multiple traumatic injuries);
5. Selected surgical procedures, such as urologic procedures and those that are expected to have a prolonged duration, require intraoperative monitoring of urine output, require the administration of either large volumes of intravenous infusions or diuretics;
6. To promote healing of open perineal or sacral wounds in patients with incontinence;
7. Neurogenic bladder; and
8. Hematuria with clots.
To increase the timely removal of urinary catheters that are no longer indicated, daily assessment of catheter necessity must be an integral part of clinicians’ workflow.32,33 Alternatives, such as external catheters or intermittent catheterization, should be considered before indwelling urinary catheter placement since both options are associated with a reduced CAUTI risk.41-44 Although indwelling urinary catheters can be seen as being more convenient for both patients and healthcare providers, many patients have expressed a preference for the use of intermittent catheterization compared with indwelling urinary catheterization.43
For urinary retention, bladder scanning can noninvasively assess the amount of residual urine in a patient’s bladder and can avoid unnecessary insertion of an indwelling urinary catheter. However, if indwelling urinary catheters are ultimately needed, they must be inserted and maintained appropriately. Of note, the use of antibiotic-impregnated catheters has not been shown to reduce CAUTI rates significantly.45
CAUTI prevention requires a multidisciplinary collaborative approach. Nurse-driven protocols and checklists to remove indwelling urinary catheters that are no longer indicated can be very effective.46,47 Automatic stop orders and catheter removal reminders are useful for reducing the duration of catheter placement.26,48 Both of these approaches require appropriate, consistent documentation with input from bedside nurses, physicians, advanced practice providers, and information technology. (Table 3 summarizes best practices for the prevention of CAUTI).
Importantly, CAUTI prevention supports broader antimicrobial stewardship. Over 55% of inpatients receive at least one dose of an antibiotic during their hospital stay.48,49 In 2015, the White House released the National Action Plan for Combating Antibiotic-Resistant Bacteria with the goals of slowing the emergence of resistant bacteria, preventing the spread of antibiotic-resistant infections, and setting a target of a 20% reduction in the inappropriate use of antibiotics for hospitalized patients.50 Hospitalists care for a substantial number of inpatients and, in turn, can drive actions to decrease CAUTIs, and promote stewardship efforts. Through actions to decrease CAUTIs, hospitalists can promote these stewardship efforts.
CONCLUSIONS
CAUTI is one of the most common types of HAI and is associated with increased morbidity, hospital length of stay, and patient costs. Most CAUTIs are preventable by limiting the placement of unnecessary catheters to instances of true necessity and removing catheters when they are no longer clinically indicated. Proper technique for the insertion and maintenance of catheters is also important for reducing CAUTI rates. Hospitalists care for a substantial number of inpatients and can make major contributions to the appropriate diagnosis, treatment, and prevention of CAUTIs.
1. Centers for Disease Control and Prevention. Estimates of healthcare-associated infections. http://www.cdc.gov/ncidod/dhqp/hai.html. Accessed September 27, 2018.
2. Calfee D. Crisis in hospital-acquired, healthcare-associated infections. Annu Rev Med. 2012;63(1):359-371. https://doi.org/10.1146/annurev-med-081210-144458.
3. Scott R. 2009. The direct medical costs of healthcare-associated infections in U.S. hospitals and the benefits of prevention. Centers for Disease Control and Prevention, Atlanta. http://www.cdc.gov/ncidod/dhqp/pdf/Scott_CostPaper.pdf. Accessed September 27, 2018.
4. Zimlachman E, Henderson D, Tamir O, et al. Health care associated infections. A meta-analysis of costs and financial impact on the US health care system. JAMA Intern Med. 2013;173(22):2039-2046. https://doi.org/10.1001/jamainternmed.2013.9763.
5. Nicolle LE. Catheter-acquired urinary tract infection: the once and future guidelines. Infect Control Hosp Epidemiol. 2010;31(4):327-329. https://doi.org/10.1086/651092.
6. Weber DJ, Sickbert-Bennett EE, Gould CV, et al. Incidence of catheter-associated and noncatheter-associated urinary tract infections in a healthcare system. Infect Control Hosp Epidemiol. 2011;32(8):822-823. https://doi.org/10.1086/661107.
7. Healthcare-associated infections. National and state HAI progress reports or SIR reports. https://www.cdc.gov/hai/data/archive/archive.html. Accessed May 6, 2019.
8. Saint S. Clinical and economic consequences of nosocomial catheter-related bacteriuria. Am J Infect Control. 2000;28(1):68-75. https://doi.org/10.1016/S0196-6553(00)90015-4.
9. Platt R, Polk BF, Murdock B, Rosner B. Mortality associated with nosocomial urinary-tract infection. N Engl J Med. 1982;307(11):637-642. https://doi.org/10.1056/NEJM198209093071101.
10. Tambyah PA, Knasinski V, Maki DG. The direct costs of nosocomial catheter-associated urinary tract infection in the era of managed care. Infect Control Hosp Epidemiol. 2002;23(1):27-31.
11. Chant C, Smith OM, Marshall JC, Friedrich JO. Relationship of catheter-associated urinary tract infection to mortality and length of stay in critically ill patients: a systematic review and meta-analysis of observational studies. Crit Care Med. 2011;39(5):1167-1173. https://doi.org/10.1097/CCM.0b013e31820a8581.
12. Umscheid CA, Mitchell MD, Doshi JA, et al. Estimating the proportion of healthcare-associated infections that are reasonably preventable and the related mortality and costs. Infect Control Hosp Epidemiol. 2011;32(2):101-114. https://doi.org/10.1086/657912.
13. Gould C, Umscheid C, Agarwal R, et al. Healthcare Infection Control Practices Advisory Committee (HICPAC): guideline for the prevention of catheter-associated urinary tract infections, 2009. http://www.cdc.gov/hicpac/pdf/CAUTI/CAUTIguideline2009final.pdf. Accessed November 1, 2018.
14. Hooton TM, Bradley SF, Cardenas DD, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(5):625-663. https://doi.org/10.1086/650482.
15. Conway LJ, Larson EL. Guidelines to prevent catheter-associated urinary tract infection: 1980 to 2010. Heart Lung. 2011;41(3):271-283. https://doi.org/10.1016/j.hrtlng.2011.08.001.
16. Greene L, Marx J, Oriola S. APIC elimination guide: guide to the elimination of catheter-associated urinary tract infections (CAUTIs). http://www.apic.org/Content/NavigationMenu/PracticeGuidance/APICEliminationGuides/CAUTI_Guide_0609.pdf. Accessed October 15, 2018.
17. Yokoe DS, Mermel LA, Anderson DJ, et al. A compendium of strategies to prevent healthcare-associated infections in acute care hospitals. Infect Control Hosp Epidemiol. 2008;29(S1):S12-S21.
18. Wiley Z, Kobaidze K, Sexton ME, Jacob JT. Hospitalists as integral stakeholders in antimicrobial stewardship. Curr Treat Options Infect Dis. 2018;10(2):240-248. https://doi.org/10.1007/s40506-018-0162-z.
19. Claeys KC, Blanco N, Morgan DJ, Leekha S, Sullivan KV. Advances and challenges in the diagnosis and treatment of urinary tract infections: the need for diagnostic stewardship. Curr Infect Dis Rep. 2019;21(4):11. https://doi.org/10.1007/s11908-019-0668-7.
20. Horan T, Andrus M, Dudeck M. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36(5):309-332. https://doi.org/10.1016/j.ajic.2008.03.002.
21. Nicolle LE, Gupta K, Bradley S, et al. Clinical practice guideline for the management of asymptomatic bacteriuria: 2019 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2019;68(10):e83-e110.
22. Donlan R. Biofilms and device-associated infections. Emerg Infect Dis. 2001;7(2):277-281. https://doi.org/10.3201/eid0702.010226.
23. Choe HS, Son SW, Choi HA, et al. Analysis of the distribution of bacteria within urinary catheter biofilms using four different molecular techniques. Am J Infect Control. 2012;40(9):e249-e254. https://doi.org/10.1016/j.ajic.2012.05.010.
24. Mohajer M, Darouiche R. Prevention and treatment of urinary catheter-associated infections. Curr Infect Dis Rep. 2013;15(2):116-123. https://doi.org/10.1007/s11908-013-0316-6.
25. Weiner LM, Webb AK, Limbago B, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011-2014. Infect Control Hosp Epidemiol. 2016;37(11):1288-1301. https://doi.org/10.1017/ice.2016.174.
26. Tambyah PA, Oon J. Catheter-associated urinary tract infection. Curr Opin Infect Dis. 2012;25(4):365-370. https://doi.org/10.1097/QCO.0b013e32835565cc.
27. Warren JW, Tenney JH, Hoopes JM, Muncie HL, Anthony WC. A prospective microbiologic study of bacteriuria in patients with chronic indwelling urethral catheters. J Infect Dis. 1982;146(6):719-723. https://doi.org/10.1093/infdis/146.6.719.
28. Lo E, Nicolle L, Coffin S, et al. Strategies to prevent catheter-associated urinary tract infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(5):464-479. https://doi.org/10.1086/675718.
29. Nicolle LE. Urinary catheter-associated infections. Infect Dis Clin N Am. 2012;26(1):13-27. https://doi.org/10.1016/j.idc.2011.09.009.
30. Titsworth WL, Hester J, Correia T, et al. Reduction of catheter-associated urinary tract infections among patients in a neurological intensive care unit: a single institution’s success. J Neurosurg. 2012;116(4):911-920. https://doi.org/10.3171/2011.11.JNS11974.
31. Lobdell KW, Stamou S, Sanchez JA. Hospital-acquired infections. Surg Clin N Am. 2012;92(1):65-77. https://doi.org/10.1016/j.suc.2011.11.003.
32. Gray M. Reducing catheter-associated urinary tract infection in the critical care unit. AACN Adv Crit Care. 2010;21(3):247-257. 33. Fakih MG, Watson SR, Greene T, et al. Reducing inappropriate urinary catheter use. Arch Intern Med. 2012;172(3):255-260. https://doi.org/10.1001/archinternmed.2011.627.
33. Fakih MG, Watson SR, Greene T, et al. Reducing inappropriate urinary catheter use. Arch Intern Med. 2012;172(3):255-260. https://doi.org/10.1001/archinternmed.2011.627.
34. Saint S, Wiese J, Amory JK, Bernstein ML, et al. Are physicians aware of which of their patients have an indwelling urinary catheters? Am J Med. 2000;109(6):476-480. https://doi.org/10.1016/s0002-9343(00)00531-3.
35. O’Grady NP, Barie PS, Bartlett JG, et al. Guidelines for evaluation of new fever in critically ill adult patients: 2008 update from the American College of Critical Care Medicine and the Infectious Diseases Society of America. Crit Care Med. 2008;36(4):1330-1349. https://doi.org/10.1097/CCM.0b013e318169eda9.
36. Mullin K, Kovacs C, Fatica C, et al. A multifaceted approach to reduction of catheter-associated urinary tract infections in the intensive care unit with an emphasis on “stewardship of culturing.” Infect Control Hosp Epidemiol. 2017;38(2):186-188. https://doi.org/10.1017/ice.2016.266.
37. Humphries R, Bard J. Point-counterpoint: reflex cultures reduce laboratory workload and improve antimicrobial stewardship in patients suspected of having urinary tract infections. J Clin Microbiol. 2016;54(2):254-258. https://doi.org/10.1128/JCM.03021-15.
38. Voelker R. New fluoroquinolone warning. JAMA. 2016;315(23):2514. https://doi.org/10.1001/jama.2016.7290.
39. Tillotson S. FDA and the safe and appropriate antibiotic use of fluoroquinolones. Lancet Infect Dis. 2016;16(3):PE11-E12.
40. Tanne J. FDA adds “black box” warning label to fluoroquinolone antibiotics. BMJ 2008;337:a816. https://doi.org/10.1136/bmj.a816.
41. Wyndaele JJ, Brauner A, Geerlings SE, et al. Clean intermittent catheterization and urinary tract infection: review and guide for future research. BJU Intern. 2012;110(11c):E910-E917. https://doi.org/10.1111/j.1464-410X.2012.11549.x.
42. Hakwoort RA, Thijs SD, Bouwmeester FW, et al. Comparing clean intermittent catheterization and transurethral indwelling catheterization for incomplete voiding after vaginal prolapse surgery: a multicenter randomized trial. BJOG. 2011;118(9):1055-1060. https://doi.org/10.1111/j.1471-0528.2011.02935.x.
43. Hakvoort RA, Nieuwkerk PT, Burger MP, et al. Patient preferences for clean intermittent catheterization and transurethral indwelling catheterization for treatment of abnormal post-void residual bladder volume after vaginal prolapsed surgery. BJOG. 2011;118(11):1324-1328. https://doi.org/10.1111/j.1471-0528.2011.03056.x.
44. Saint S, Kaufman SR, Roger M, et al. Condom versus indwelling urinary catheters: a randomized trial. JAGS. 2006;54(7):1055-1061. https://doi.org/10.1111/j.1532-5415.2006.00785.x.
45. Pickard R, Lam T, MacLennan G, et al. Antimicrobial catheters for reduction of symptomatic urinary tract infection in adults requiring short-term catheterization in hospital: a multicenter randomized controlled trial. Lancet. 2012;380(9857):1927-1935. https://doi.org/10.1016/S0140-6736(12)61380-4.
46. Parry M, Grant B, Sestovic M. Successful reduction in catheter-associated urinary tract infections: focus on nurse-directed catheter removal. Am J Infect Control. 2013;41(12):1178-1181. https://doi.org/10.1016/j.ajic.2013.03.296.
47. Fuchs MA, Sexton DJ, Thornlow DK, Champagne MT. Evaluation of an evidence-based, nurse-driven checklist to prevent hospital-acquired catheter-associated urinary tract infections in intensive care units. J Nurs Care Qual. 2011;26(2):101-109. https://doi.org/10.1097/NCQ.0b013e3181fb7847.
48. Meddings J, Rogers MA, Macy M, Saint S. Systematic review and meta-analysis: reminder systems to reduce catheter-associated urinary tract infections and urinary catheter use in hospitalized patients. Clin Infect Dis. 2010;51(5):550-560. https://doi.org/10.1086/655133.
49. Baggs J, Fridkin SK, Pollack LA, Srinivasan A, Jernigan JA. Estimating national trends in inpatient antibiotic use among US hospitals from 2006 to 2012. JAMA Intern Med. 2016;176(11):1639-1648. https://doi.org/10.1001/jamainternmed.2016.5651.
50. National Action Plan for Combating Antibiotic-resistant Bacteria. 2015. https://obamawhitehouse.archives.gov/sites/default/files/docs/national_action_plan_for_combatin
1. Centers for Disease Control and Prevention. Estimates of healthcare-associated infections. http://www.cdc.gov/ncidod/dhqp/hai.html. Accessed September 27, 2018.
2. Calfee D. Crisis in hospital-acquired, healthcare-associated infections. Annu Rev Med. 2012;63(1):359-371. https://doi.org/10.1146/annurev-med-081210-144458.
3. Scott R. 2009. The direct medical costs of healthcare-associated infections in U.S. hospitals and the benefits of prevention. Centers for Disease Control and Prevention, Atlanta. http://www.cdc.gov/ncidod/dhqp/pdf/Scott_CostPaper.pdf. Accessed September 27, 2018.
4. Zimlachman E, Henderson D, Tamir O, et al. Health care associated infections. A meta-analysis of costs and financial impact on the US health care system. JAMA Intern Med. 2013;173(22):2039-2046. https://doi.org/10.1001/jamainternmed.2013.9763.
5. Nicolle LE. Catheter-acquired urinary tract infection: the once and future guidelines. Infect Control Hosp Epidemiol. 2010;31(4):327-329. https://doi.org/10.1086/651092.
6. Weber DJ, Sickbert-Bennett EE, Gould CV, et al. Incidence of catheter-associated and noncatheter-associated urinary tract infections in a healthcare system. Infect Control Hosp Epidemiol. 2011;32(8):822-823. https://doi.org/10.1086/661107.
7. Healthcare-associated infections. National and state HAI progress reports or SIR reports. https://www.cdc.gov/hai/data/archive/archive.html. Accessed May 6, 2019.
8. Saint S. Clinical and economic consequences of nosocomial catheter-related bacteriuria. Am J Infect Control. 2000;28(1):68-75. https://doi.org/10.1016/S0196-6553(00)90015-4.
9. Platt R, Polk BF, Murdock B, Rosner B. Mortality associated with nosocomial urinary-tract infection. N Engl J Med. 1982;307(11):637-642. https://doi.org/10.1056/NEJM198209093071101.
10. Tambyah PA, Knasinski V, Maki DG. The direct costs of nosocomial catheter-associated urinary tract infection in the era of managed care. Infect Control Hosp Epidemiol. 2002;23(1):27-31.
11. Chant C, Smith OM, Marshall JC, Friedrich JO. Relationship of catheter-associated urinary tract infection to mortality and length of stay in critically ill patients: a systematic review and meta-analysis of observational studies. Crit Care Med. 2011;39(5):1167-1173. https://doi.org/10.1097/CCM.0b013e31820a8581.
12. Umscheid CA, Mitchell MD, Doshi JA, et al. Estimating the proportion of healthcare-associated infections that are reasonably preventable and the related mortality and costs. Infect Control Hosp Epidemiol. 2011;32(2):101-114. https://doi.org/10.1086/657912.
13. Gould C, Umscheid C, Agarwal R, et al. Healthcare Infection Control Practices Advisory Committee (HICPAC): guideline for the prevention of catheter-associated urinary tract infections, 2009. http://www.cdc.gov/hicpac/pdf/CAUTI/CAUTIguideline2009final.pdf. Accessed November 1, 2018.
14. Hooton TM, Bradley SF, Cardenas DD, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(5):625-663. https://doi.org/10.1086/650482.
15. Conway LJ, Larson EL. Guidelines to prevent catheter-associated urinary tract infection: 1980 to 2010. Heart Lung. 2011;41(3):271-283. https://doi.org/10.1016/j.hrtlng.2011.08.001.
16. Greene L, Marx J, Oriola S. APIC elimination guide: guide to the elimination of catheter-associated urinary tract infections (CAUTIs). http://www.apic.org/Content/NavigationMenu/PracticeGuidance/APICEliminationGuides/CAUTI_Guide_0609.pdf. Accessed October 15, 2018.
17. Yokoe DS, Mermel LA, Anderson DJ, et al. A compendium of strategies to prevent healthcare-associated infections in acute care hospitals. Infect Control Hosp Epidemiol. 2008;29(S1):S12-S21.
18. Wiley Z, Kobaidze K, Sexton ME, Jacob JT. Hospitalists as integral stakeholders in antimicrobial stewardship. Curr Treat Options Infect Dis. 2018;10(2):240-248. https://doi.org/10.1007/s40506-018-0162-z.
19. Claeys KC, Blanco N, Morgan DJ, Leekha S, Sullivan KV. Advances and challenges in the diagnosis and treatment of urinary tract infections: the need for diagnostic stewardship. Curr Infect Dis Rep. 2019;21(4):11. https://doi.org/10.1007/s11908-019-0668-7.
20. Horan T, Andrus M, Dudeck M. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36(5):309-332. https://doi.org/10.1016/j.ajic.2008.03.002.
21. Nicolle LE, Gupta K, Bradley S, et al. Clinical practice guideline for the management of asymptomatic bacteriuria: 2019 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2019;68(10):e83-e110.
22. Donlan R. Biofilms and device-associated infections. Emerg Infect Dis. 2001;7(2):277-281. https://doi.org/10.3201/eid0702.010226.
23. Choe HS, Son SW, Choi HA, et al. Analysis of the distribution of bacteria within urinary catheter biofilms using four different molecular techniques. Am J Infect Control. 2012;40(9):e249-e254. https://doi.org/10.1016/j.ajic.2012.05.010.
24. Mohajer M, Darouiche R. Prevention and treatment of urinary catheter-associated infections. Curr Infect Dis Rep. 2013;15(2):116-123. https://doi.org/10.1007/s11908-013-0316-6.
25. Weiner LM, Webb AK, Limbago B, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011-2014. Infect Control Hosp Epidemiol. 2016;37(11):1288-1301. https://doi.org/10.1017/ice.2016.174.
26. Tambyah PA, Oon J. Catheter-associated urinary tract infection. Curr Opin Infect Dis. 2012;25(4):365-370. https://doi.org/10.1097/QCO.0b013e32835565cc.
27. Warren JW, Tenney JH, Hoopes JM, Muncie HL, Anthony WC. A prospective microbiologic study of bacteriuria in patients with chronic indwelling urethral catheters. J Infect Dis. 1982;146(6):719-723. https://doi.org/10.1093/infdis/146.6.719.
28. Lo E, Nicolle L, Coffin S, et al. Strategies to prevent catheter-associated urinary tract infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(5):464-479. https://doi.org/10.1086/675718.
29. Nicolle LE. Urinary catheter-associated infections. Infect Dis Clin N Am. 2012;26(1):13-27. https://doi.org/10.1016/j.idc.2011.09.009.
30. Titsworth WL, Hester J, Correia T, et al. Reduction of catheter-associated urinary tract infections among patients in a neurological intensive care unit: a single institution’s success. J Neurosurg. 2012;116(4):911-920. https://doi.org/10.3171/2011.11.JNS11974.
31. Lobdell KW, Stamou S, Sanchez JA. Hospital-acquired infections. Surg Clin N Am. 2012;92(1):65-77. https://doi.org/10.1016/j.suc.2011.11.003.
32. Gray M. Reducing catheter-associated urinary tract infection in the critical care unit. AACN Adv Crit Care. 2010;21(3):247-257. 33. Fakih MG, Watson SR, Greene T, et al. Reducing inappropriate urinary catheter use. Arch Intern Med. 2012;172(3):255-260. https://doi.org/10.1001/archinternmed.2011.627.
33. Fakih MG, Watson SR, Greene T, et al. Reducing inappropriate urinary catheter use. Arch Intern Med. 2012;172(3):255-260. https://doi.org/10.1001/archinternmed.2011.627.
34. Saint S, Wiese J, Amory JK, Bernstein ML, et al. Are physicians aware of which of their patients have an indwelling urinary catheters? Am J Med. 2000;109(6):476-480. https://doi.org/10.1016/s0002-9343(00)00531-3.
35. O’Grady NP, Barie PS, Bartlett JG, et al. Guidelines for evaluation of new fever in critically ill adult patients: 2008 update from the American College of Critical Care Medicine and the Infectious Diseases Society of America. Crit Care Med. 2008;36(4):1330-1349. https://doi.org/10.1097/CCM.0b013e318169eda9.
36. Mullin K, Kovacs C, Fatica C, et al. A multifaceted approach to reduction of catheter-associated urinary tract infections in the intensive care unit with an emphasis on “stewardship of culturing.” Infect Control Hosp Epidemiol. 2017;38(2):186-188. https://doi.org/10.1017/ice.2016.266.
37. Humphries R, Bard J. Point-counterpoint: reflex cultures reduce laboratory workload and improve antimicrobial stewardship in patients suspected of having urinary tract infections. J Clin Microbiol. 2016;54(2):254-258. https://doi.org/10.1128/JCM.03021-15.
38. Voelker R. New fluoroquinolone warning. JAMA. 2016;315(23):2514. https://doi.org/10.1001/jama.2016.7290.
39. Tillotson S. FDA and the safe and appropriate antibiotic use of fluoroquinolones. Lancet Infect Dis. 2016;16(3):PE11-E12.
40. Tanne J. FDA adds “black box” warning label to fluoroquinolone antibiotics. BMJ 2008;337:a816. https://doi.org/10.1136/bmj.a816.
41. Wyndaele JJ, Brauner A, Geerlings SE, et al. Clean intermittent catheterization and urinary tract infection: review and guide for future research. BJU Intern. 2012;110(11c):E910-E917. https://doi.org/10.1111/j.1464-410X.2012.11549.x.
42. Hakwoort RA, Thijs SD, Bouwmeester FW, et al. Comparing clean intermittent catheterization and transurethral indwelling catheterization for incomplete voiding after vaginal prolapse surgery: a multicenter randomized trial. BJOG. 2011;118(9):1055-1060. https://doi.org/10.1111/j.1471-0528.2011.02935.x.
43. Hakvoort RA, Nieuwkerk PT, Burger MP, et al. Patient preferences for clean intermittent catheterization and transurethral indwelling catheterization for treatment of abnormal post-void residual bladder volume after vaginal prolapsed surgery. BJOG. 2011;118(11):1324-1328. https://doi.org/10.1111/j.1471-0528.2011.03056.x.
44. Saint S, Kaufman SR, Roger M, et al. Condom versus indwelling urinary catheters: a randomized trial. JAGS. 2006;54(7):1055-1061. https://doi.org/10.1111/j.1532-5415.2006.00785.x.
45. Pickard R, Lam T, MacLennan G, et al. Antimicrobial catheters for reduction of symptomatic urinary tract infection in adults requiring short-term catheterization in hospital: a multicenter randomized controlled trial. Lancet. 2012;380(9857):1927-1935. https://doi.org/10.1016/S0140-6736(12)61380-4.
46. Parry M, Grant B, Sestovic M. Successful reduction in catheter-associated urinary tract infections: focus on nurse-directed catheter removal. Am J Infect Control. 2013;41(12):1178-1181. https://doi.org/10.1016/j.ajic.2013.03.296.
47. Fuchs MA, Sexton DJ, Thornlow DK, Champagne MT. Evaluation of an evidence-based, nurse-driven checklist to prevent hospital-acquired catheter-associated urinary tract infections in intensive care units. J Nurs Care Qual. 2011;26(2):101-109. https://doi.org/10.1097/NCQ.0b013e3181fb7847.
48. Meddings J, Rogers MA, Macy M, Saint S. Systematic review and meta-analysis: reminder systems to reduce catheter-associated urinary tract infections and urinary catheter use in hospitalized patients. Clin Infect Dis. 2010;51(5):550-560. https://doi.org/10.1086/655133.
49. Baggs J, Fridkin SK, Pollack LA, Srinivasan A, Jernigan JA. Estimating national trends in inpatient antibiotic use among US hospitals from 2006 to 2012. JAMA Intern Med. 2016;176(11):1639-1648. https://doi.org/10.1001/jamainternmed.2016.5651.
50. National Action Plan for Combating Antibiotic-resistant Bacteria. 2015. https://obamawhitehouse.archives.gov/sites/default/files/docs/national_action_plan_for_combatin
© 2019 Society of Hospital Medicine
Clinical Progress Note: Procalcitonin in the Management of Pediatric Lower Respiratory Tract Infection
Procalcitonin (PCT) is a biomarker that has shown promise to identify bacterial etiology in acute infections, including bacterial lower respiratory tract infection (LRTI). In 2017, the United States Food and Drug Administration (FDA) approved the use of PCT as a diagnostic aid to guide the decisions around antibiotic therapy in acute LRTI.1 Although most of the data supporting the use of PCT for LRTI stems from adult studies, the high disease burden, predominance of viral etiologies, and frequent diagnostic uncertainty resulting in antibiotic overuse make pediatric LRTI an ideal target for the use of PCT as a diagnostic aid. This review evaluates and summarizes the current evidence regarding the role of PCT in the clinical care of pediatric LRTI, including its use in guiding antibiotic use and prognosticating disease severity.
THE ROLE OF PROCALCITONIN IN GUIDING INITIATION OF ANTIBIOTICS
The commonly used PCT cut points for withholding or stopping antibiotics in adults and children are 0.1 µg/L (very low risk of bacterial etiology) or 0.25 µg/L (low risk of bacterial etiology).2-4 Among the 532 children enrolled in the multicenter study of Etiology of Pneumonia in the Community (EPIC), a PCT threshold of 0.25 µg/L demonstrated an approximate sensitivity of 85%, specificity of 45%, positive likelihood ratio of 1.55, and negative likelihood ratio of 0.33 for community acquired pneumonia (CAP) caused by typical bacterial pathogens.5 Lowering the cutoff to <0.1 µg/L increased PCT sensitivity to 100%, decreased specificity, positive likelihood ratio, and negative likelihood ratio to 20%, 1.26, and 0, respectively. Although the EPIC study obtained culture and performed PCR testing on any blood sample, pleural fluid specimen, endotracheal aspirate, or bronchoalveolar–lavage specimens obtained during the study period, currently available laboratory methods show poor sensitivity for defining bacterial LRTI. Thus, bacterial etiologies may have been underestimated. The highly negative predictive value demonstrated in this study highlights the potential of PCT as a biomarker for ruling out bacterial diseases, including LRTI.
Multiple studies have evaluated the potential utility of PCT in guiding antibiotic initiation in adults with LRTI, but data on pediatric patients are sparse.4 In a randomized, single-center Italian study comparing a PCT-guided algorithm (withholding antibiotics when PCT < 0.25 µg/L) versus usual care among 319 hospitalized children with pneumonia, the PCT group experienced fewer antibiotic initiations (15.5% vs 100%, P < .05) without significant differences in recurrence of respiratory symptoms or new antibiotic prescriptions in the month following enrollment.2
A similar randomized trial using a PCT-guided algorithm for the initiation of antibiotics conducted among 337 Swiss children presented to the emergency department (ED) with pneumonia and other LRTIs failed to demonstrate decreases in antibiotic initiation.3 This study used an algorithm that categorized the likelihood of requiring antibiotic treatment for bacterial LRTI as “definitely” if PCT was >0.5 µg/L, “probably” if PCT was 0.26–0.5 µg/L, “probably not” if PCT was 0.1–0.25 µg/L, and “definitely not” if PCT was <0.1 µg/L. In the PCT group, 104 out of 168 (62%) patients received antibiotics within 14 days compared with 93 out of 165 (56%) patients in the control group (odds ratio [OR]: 1.26, 95% CI: 0.81, 1.95). In the subgroup analyses, the odds of administering antibiotics to those with nonpneumonia LRTI was significantly higher than those of the PCT group and control group (OR: 4.09, 95% CI: 1.8, 9.93); the odds of receiving antibiotics also showed no difference in the subgroup of children with pneumonia (OR: 0.66, 95% CI: 0.35, 1.23).
The benefit of PCT for informing decisions around the initiation of antibiotics likely varies based on perceived risk of bacterial diseases. When the pretest probability of bacterial disease is extremely high, the use of PCT is unlikely to alter treatment decisions. Similarly, PCT should not be used in situations where the pretest probability for bacterial pneumonia is very low—in these instances, an elevated PCT may lead to unnecessary antibiotic use among children presenting to the ED. However, the risk of bacterial pneumonia is often equivocal, and in these situations, PCT may provide clinicians with useful insights, primarily for ruling out bacterial disease.
THE ROLE OF PROCALCITONIN IN GUIDING DISCONTINUATION OF ANTIBIOTICS
In the study by Esposito et al., the PCT levels were additionally measured every two days until discharge and during two scheduled follow-up visits; the antibiotics were discontinued when PCT < 0.25 µg/L.2 The PCT-guided group experienced shorter antibiotic duration (mean 5.4 vs 11.0 days, P < .05), shorter length of hospital stay (mean 4.7 vs 5.61 days for mild LRTI and 5.01 vs 5.93 for severe LRTI), and fewer antibiotic-related adverse events (3.9% vs 25.2%, P < .05). Similarly, in the study by Baer et al., the PCT-guided group had PCT levels repeated on days three and five after enrollment, and the antibiotics were discontinued when PCT was less than 0.25 µg/L. The duration of antibiotic administration was significantly lower in the PCT-guided group (mean difference: 1.8 days, 95% CI: −3.1, −0.).3 The rates of hospitalization, duration of hospital stay, and mean impairment of daily activities attributable to LRTI were similar between groups.
Considering the adult studies and the small number of pediatric LRTI research published to date, the use of PCT to safely reduce antibiotic treatment duration is encouraging.4 Although the studies on the kinetics of PCT are limited, the biomarker has been shown to rise two to four hours after a bacterial stimulus, peak in 24-48 hours and achieve a half-life of 24-36 hours.6,7 As such, serial PCT measurements at 24-hour intervals for three to five days may be more beneficial than stand-alone PCT tests. Nonetheless, additional studies are needed to better define groups of patients who will most likely benefit from PCT testing and to understand how to best integrate testing into clinical practice.
PROCALCITONIN FOR SEVERITY PREDICTION OF LRTI
PCT has also been explored as a marker of LRTI disease severity. In a 2008 multicenter cohort encompassing 1,651 adults with pneumonia, PCT < 0.1 µg/L was associated with a decreased 30-day mortality, shorter length of stay, and decreased admission to the intensive care unit (ICU) compared with those with PCT>0.1 µg/L.8 In a 2017 study of 317 adults hospitalized with pneumonia, the PCT level was significantly higher in those with bacteremia and in those admitted to intensive care.9 When used in combination with the pneumonia severity index (PSI), the addition of PCT resulted in improved prognostic performance compared with the PSI alone for both outcomes, increasing the area under the receiver operating characteristic curve from 0.67 to 0.85 for bacteremia and from 0.58 to 0.64 for intensive care. Similarly, in the adult EPIC cohort, the addition of PCT contributed significant prognostic information beyond existing severity scores for predicting the need for invasive respiratory or vasopressor support; each 1 µg/L increase in PCT was associated with a 1% to 2% absolute increase in the need for this outcome.10
A European study of 100 children with pneumonia also demonstrated higher PCT values among hospitalized children (n = 26, median PCT 17.8 µg/L) compared with outpatient children (n = 73, median PCT 0.72 µg/L, P < .01).11 Among the 532 children from the EPIC study, a PCT < 0.25 µg/L was associated with the reduced odds of ICU admission (adjusted OR: 0.48; 95% CI: 0.30, 0.78) and a 2.3-day (95% CI: 1.4, 3.2) decrease in the average length of stay compared with those with higher PCT concentrations.5 Of the 34 children with empyema requiring drainage, 28 (82%) showed a PCT concentration ≥0.5 µg/L. Additional pediatric studies are needed, but the limited data to date suggest that PCT may play a role in predicting pediatric LRTI disease severity, including the need for mechanical ventilatory support and ICU-level care.
LIMITATIONS TO CLINICAL APPLICATION
Although PCT shows promise as a biomarker to reliably rule out bacterial infection, several potential limitations exist in assessing its role in pediatric LRTI. Atypical bacterial infections (ie, Mycoplasma pneumoniae) and localized bacterial infection may not induce significant PCT production, as has been shown in adults and children with tonsillitis, localized skin infections, endocarditis, or empyema (Table).12 The majority of clinical trials in LRTI have been conducted in the adult population,4 with the number of pediatric trials remaining small.2,3 Given the predominance of viral LRTI in children compared with adults, the utility of PCT may differ in these populations.13,14 Furthermore, existing studies demonstrate mixed results regarding the magnitude of benefits that PCT may provide in terms of limiting antibiotic use. Another concern is the potential of PCT to increase unnecessary antibiotic use in those with viral LRTI,3 as PCT may also be increased in populations with systemic inflammation from nonbacterial causes.12,15
CONCLUSIONS AND CLINICAL APPLICATION
The misuse of antibiotics is a public health crisis resulting in the emergence of antibiotic-resistant pathogens and adverse outcomes, including Clostridioides difficile infection, drug toxicities, and increased healthcare costs.16 Pneumonia is responsible for more days of antibiotics than any other disease in children’s hospitals and is an important target for stewardship efforts.17 PCT is a promising biomarker for distinguishing bacterial from viral infection, and its use may help in making informed antibiotic decisions and predicting disease outcomes in pediatric LRTI. Although PCT has been cleared by the FDA for assisting with antibiotic decisions in pediatric LRTI, the majority of evidence supporting this indication is drawn from adults. Additional studies are needed prior to the widespread implementation in the pediatric population, but the results of available pediatric studies show promise. The clinical context and severity of patient presentation are important when considering whether or not to use PCT and how to best interpret PCT levels when making clinical management decisions. The utility of PCT for antibiotic initiation in the pediatric population is encouraging given the predominance of viral etiologies in pediatric LRTI. Currently available data demonstrate the value of serial PCT measurements in antibiotic de-escalation and promoting antibiotic stewardship for children and adults.2-4 As with all new diagnostic modalities, provider education is paramount to ensure a safe and value-driven implementation.
Disclosures
Dr. Katz received investigator-initiated grant funding from Roche and bioMérieux to conduct research involving procalcitonin in the past three years. Dr. Sartori has nothing to disclose. Dr. Williams received investigator-initiated grant funding from bioMérieux to conduct research involving procalcitonin in the past three years.
Funding
This work was supported by the National Institute of Health (1T32AI095202-07).
Disclaimer
This content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, Roche, or bioMérieux.
1. FDA clears test to help manage antibiotic treatment for lower respiratory tract infections and sepsis. US Food and Drug Administration. [Press Release]. Silver Spring, MD, February 23 2017.
2. Esposito S, Tagliabue C, Picciolli I, et al. Procalcitonin measurements for guiding antibiotic treatment in pediatric pneumonia. Respir Med. 2011;105(12):1939-1945. https://doi.org/10.1016/j.rmed.2011.09.003.
3. Baer G, Baumann P, Buettcher M, et al. Procalcitonin guidance to reduce antibiotic treatment of lower respiratory tract infection in children and adolescents (ProPAED): a randomized controlled trial. PLoS One. 2013;8(8):e68419. https://doi.org/10.1371/journal.pone.0068419.
4. Choi JJ MM, Simon MS, Evans AT, Self WH, Glesby MJ. Procalcitonin in the diagnosis and management of community-acquired pneumonia in hospitalized adults. J Hosp Med. 2019;18(X);XXX-XXX. https://doi.org/10.12788/jhm.3272.
5. Stockmann C, Ampofo K, Killpack J, et al. Procalcitonin accurately identifies hospitalized children with low risk of bacterial community-acquired pneumonia. J Pediatric Infect Dis Soc. 2017;7(1): 46-53. https://doi.org/10.1093/jpids/piw091.
6. Dandona P, Nix D, Wilson MF, et al. Procalcitonin increase after endotoxin injection in normal subjects. J Clin Endocrinol Metab. 1994;79(6):1605-1608. https://doi.org/10.1210/jcem.79.6.7989463.
7. Brunkhorst FM, Heinz U, Forycki ZF. Kinetics of procalcitonin in iatrogenic sepsis. Intensive Care Med. 1998;24(8):888-889.
8. Huang DT, Weissfeld LA, Kellum JA, et al; GenIMS Investigators. Risk prediction with procalcitonin and clinical rules in community-acquired pneumonia. Ann Emerg Med. 2008;52(1):48-58 e42. https://doi.org/10.1016/j.annemergmed.2008.01.003.
9. McCluskey SM, Schuetz P, Abers MS, et al. Serial procalcitonin as a predictor of pacteremia and peed for intensive care unit care in adults with pneumonia, including those with highest severity: A Prospective Cohort Study. Open Forum Infect Dis. 2017;4(1):ofw238. https://doi.org/10.1093/ofid/ofw238.
10. Self WH, Grijalva CG, Williams DJ, et al. Procalcitonin as an early marker of the need for invasive respiratory or vasopressor support in adults with community-acquired pneumonia. Chest. 2016;150(4):819-828. https://doi.org/10.1016/j.chest.2016.04.010.
11. Don M, Valent F, Korppi M, et al. Efficacy of serum procalcitonin in evaluating severity of community-acquired pneumonia in childhood. Scand J Infect Dis. 2007;39(2):129-137. https://doi.org/10.1080/00365540600951283.
12. Meisner M. Update on procalcitonin measurements. Ann Lab Med. 2014;34(4):263-273. https://doi.org/10.3343/alm.2014.34.4.263.
13. Jain S, Williams DJ, Arnold SR, et al; CDC EPIC Study Team. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015;372(9):835-845. https://doi.org/10.1056/NEJMoa1405870.
14. Jain S, Self WH, Wunderink RG, et al; CDC EPIC Study Team. Community-Acquired Pneumonia Requiring Hospitalization among U.S. adults. N Engl J Med. 2015;373(5):415-427. https://doi.org/10.1056/NEJMoa1500245.
15. Aloisio E, Dolci A, Panteghini M. Procalcitonin: Between evidence and critical issues. Clin Chim Acta. 2019;496:7-12. https://doi.org/10.1016/j.cca.2019.06.010.
16. Society for Healthcare Epidemiology of A, Infectious Diseases Society of A, Pediatric Infectious Diseases S. Policy statement on antimicrobial stewardship by the Society for Healthcare Epidemiology of America (SHEA), the Infectious Diseases Society of America (IDSA), and the Pediatric Infectious Diseases Society (PIDS). Infect Control Hosp Epidemiol. 2012;33(4):322-327. https://doi.org/10.1086/665010.
17. Gerber JS, Kronman MP, Ross RK, et al. Identifying targets for antimicrobial stewardship in children’s hospitals. Infect Control Hosp Epidemiol. 2013;34(12):1252-1258. https://doi.org/10.1086/673982.
Procalcitonin (PCT) is a biomarker that has shown promise to identify bacterial etiology in acute infections, including bacterial lower respiratory tract infection (LRTI). In 2017, the United States Food and Drug Administration (FDA) approved the use of PCT as a diagnostic aid to guide the decisions around antibiotic therapy in acute LRTI.1 Although most of the data supporting the use of PCT for LRTI stems from adult studies, the high disease burden, predominance of viral etiologies, and frequent diagnostic uncertainty resulting in antibiotic overuse make pediatric LRTI an ideal target for the use of PCT as a diagnostic aid. This review evaluates and summarizes the current evidence regarding the role of PCT in the clinical care of pediatric LRTI, including its use in guiding antibiotic use and prognosticating disease severity.
THE ROLE OF PROCALCITONIN IN GUIDING INITIATION OF ANTIBIOTICS
The commonly used PCT cut points for withholding or stopping antibiotics in adults and children are 0.1 µg/L (very low risk of bacterial etiology) or 0.25 µg/L (low risk of bacterial etiology).2-4 Among the 532 children enrolled in the multicenter study of Etiology of Pneumonia in the Community (EPIC), a PCT threshold of 0.25 µg/L demonstrated an approximate sensitivity of 85%, specificity of 45%, positive likelihood ratio of 1.55, and negative likelihood ratio of 0.33 for community acquired pneumonia (CAP) caused by typical bacterial pathogens.5 Lowering the cutoff to <0.1 µg/L increased PCT sensitivity to 100%, decreased specificity, positive likelihood ratio, and negative likelihood ratio to 20%, 1.26, and 0, respectively. Although the EPIC study obtained culture and performed PCR testing on any blood sample, pleural fluid specimen, endotracheal aspirate, or bronchoalveolar–lavage specimens obtained during the study period, currently available laboratory methods show poor sensitivity for defining bacterial LRTI. Thus, bacterial etiologies may have been underestimated. The highly negative predictive value demonstrated in this study highlights the potential of PCT as a biomarker for ruling out bacterial diseases, including LRTI.
Multiple studies have evaluated the potential utility of PCT in guiding antibiotic initiation in adults with LRTI, but data on pediatric patients are sparse.4 In a randomized, single-center Italian study comparing a PCT-guided algorithm (withholding antibiotics when PCT < 0.25 µg/L) versus usual care among 319 hospitalized children with pneumonia, the PCT group experienced fewer antibiotic initiations (15.5% vs 100%, P < .05) without significant differences in recurrence of respiratory symptoms or new antibiotic prescriptions in the month following enrollment.2
A similar randomized trial using a PCT-guided algorithm for the initiation of antibiotics conducted among 337 Swiss children presented to the emergency department (ED) with pneumonia and other LRTIs failed to demonstrate decreases in antibiotic initiation.3 This study used an algorithm that categorized the likelihood of requiring antibiotic treatment for bacterial LRTI as “definitely” if PCT was >0.5 µg/L, “probably” if PCT was 0.26–0.5 µg/L, “probably not” if PCT was 0.1–0.25 µg/L, and “definitely not” if PCT was <0.1 µg/L. In the PCT group, 104 out of 168 (62%) patients received antibiotics within 14 days compared with 93 out of 165 (56%) patients in the control group (odds ratio [OR]: 1.26, 95% CI: 0.81, 1.95). In the subgroup analyses, the odds of administering antibiotics to those with nonpneumonia LRTI was significantly higher than those of the PCT group and control group (OR: 4.09, 95% CI: 1.8, 9.93); the odds of receiving antibiotics also showed no difference in the subgroup of children with pneumonia (OR: 0.66, 95% CI: 0.35, 1.23).
The benefit of PCT for informing decisions around the initiation of antibiotics likely varies based on perceived risk of bacterial diseases. When the pretest probability of bacterial disease is extremely high, the use of PCT is unlikely to alter treatment decisions. Similarly, PCT should not be used in situations where the pretest probability for bacterial pneumonia is very low—in these instances, an elevated PCT may lead to unnecessary antibiotic use among children presenting to the ED. However, the risk of bacterial pneumonia is often equivocal, and in these situations, PCT may provide clinicians with useful insights, primarily for ruling out bacterial disease.
THE ROLE OF PROCALCITONIN IN GUIDING DISCONTINUATION OF ANTIBIOTICS
In the study by Esposito et al., the PCT levels were additionally measured every two days until discharge and during two scheduled follow-up visits; the antibiotics were discontinued when PCT < 0.25 µg/L.2 The PCT-guided group experienced shorter antibiotic duration (mean 5.4 vs 11.0 days, P < .05), shorter length of hospital stay (mean 4.7 vs 5.61 days for mild LRTI and 5.01 vs 5.93 for severe LRTI), and fewer antibiotic-related adverse events (3.9% vs 25.2%, P < .05). Similarly, in the study by Baer et al., the PCT-guided group had PCT levels repeated on days three and five after enrollment, and the antibiotics were discontinued when PCT was less than 0.25 µg/L. The duration of antibiotic administration was significantly lower in the PCT-guided group (mean difference: 1.8 days, 95% CI: −3.1, −0.).3 The rates of hospitalization, duration of hospital stay, and mean impairment of daily activities attributable to LRTI were similar between groups.
Considering the adult studies and the small number of pediatric LRTI research published to date, the use of PCT to safely reduce antibiotic treatment duration is encouraging.4 Although the studies on the kinetics of PCT are limited, the biomarker has been shown to rise two to four hours after a bacterial stimulus, peak in 24-48 hours and achieve a half-life of 24-36 hours.6,7 As such, serial PCT measurements at 24-hour intervals for three to five days may be more beneficial than stand-alone PCT tests. Nonetheless, additional studies are needed to better define groups of patients who will most likely benefit from PCT testing and to understand how to best integrate testing into clinical practice.
PROCALCITONIN FOR SEVERITY PREDICTION OF LRTI
PCT has also been explored as a marker of LRTI disease severity. In a 2008 multicenter cohort encompassing 1,651 adults with pneumonia, PCT < 0.1 µg/L was associated with a decreased 30-day mortality, shorter length of stay, and decreased admission to the intensive care unit (ICU) compared with those with PCT>0.1 µg/L.8 In a 2017 study of 317 adults hospitalized with pneumonia, the PCT level was significantly higher in those with bacteremia and in those admitted to intensive care.9 When used in combination with the pneumonia severity index (PSI), the addition of PCT resulted in improved prognostic performance compared with the PSI alone for both outcomes, increasing the area under the receiver operating characteristic curve from 0.67 to 0.85 for bacteremia and from 0.58 to 0.64 for intensive care. Similarly, in the adult EPIC cohort, the addition of PCT contributed significant prognostic information beyond existing severity scores for predicting the need for invasive respiratory or vasopressor support; each 1 µg/L increase in PCT was associated with a 1% to 2% absolute increase in the need for this outcome.10
A European study of 100 children with pneumonia also demonstrated higher PCT values among hospitalized children (n = 26, median PCT 17.8 µg/L) compared with outpatient children (n = 73, median PCT 0.72 µg/L, P < .01).11 Among the 532 children from the EPIC study, a PCT < 0.25 µg/L was associated with the reduced odds of ICU admission (adjusted OR: 0.48; 95% CI: 0.30, 0.78) and a 2.3-day (95% CI: 1.4, 3.2) decrease in the average length of stay compared with those with higher PCT concentrations.5 Of the 34 children with empyema requiring drainage, 28 (82%) showed a PCT concentration ≥0.5 µg/L. Additional pediatric studies are needed, but the limited data to date suggest that PCT may play a role in predicting pediatric LRTI disease severity, including the need for mechanical ventilatory support and ICU-level care.
LIMITATIONS TO CLINICAL APPLICATION
Although PCT shows promise as a biomarker to reliably rule out bacterial infection, several potential limitations exist in assessing its role in pediatric LRTI. Atypical bacterial infections (ie, Mycoplasma pneumoniae) and localized bacterial infection may not induce significant PCT production, as has been shown in adults and children with tonsillitis, localized skin infections, endocarditis, or empyema (Table).12 The majority of clinical trials in LRTI have been conducted in the adult population,4 with the number of pediatric trials remaining small.2,3 Given the predominance of viral LRTI in children compared with adults, the utility of PCT may differ in these populations.13,14 Furthermore, existing studies demonstrate mixed results regarding the magnitude of benefits that PCT may provide in terms of limiting antibiotic use. Another concern is the potential of PCT to increase unnecessary antibiotic use in those with viral LRTI,3 as PCT may also be increased in populations with systemic inflammation from nonbacterial causes.12,15
CONCLUSIONS AND CLINICAL APPLICATION
The misuse of antibiotics is a public health crisis resulting in the emergence of antibiotic-resistant pathogens and adverse outcomes, including Clostridioides difficile infection, drug toxicities, and increased healthcare costs.16 Pneumonia is responsible for more days of antibiotics than any other disease in children’s hospitals and is an important target for stewardship efforts.17 PCT is a promising biomarker for distinguishing bacterial from viral infection, and its use may help in making informed antibiotic decisions and predicting disease outcomes in pediatric LRTI. Although PCT has been cleared by the FDA for assisting with antibiotic decisions in pediatric LRTI, the majority of evidence supporting this indication is drawn from adults. Additional studies are needed prior to the widespread implementation in the pediatric population, but the results of available pediatric studies show promise. The clinical context and severity of patient presentation are important when considering whether or not to use PCT and how to best interpret PCT levels when making clinical management decisions. The utility of PCT for antibiotic initiation in the pediatric population is encouraging given the predominance of viral etiologies in pediatric LRTI. Currently available data demonstrate the value of serial PCT measurements in antibiotic de-escalation and promoting antibiotic stewardship for children and adults.2-4 As with all new diagnostic modalities, provider education is paramount to ensure a safe and value-driven implementation.
Disclosures
Dr. Katz received investigator-initiated grant funding from Roche and bioMérieux to conduct research involving procalcitonin in the past three years. Dr. Sartori has nothing to disclose. Dr. Williams received investigator-initiated grant funding from bioMérieux to conduct research involving procalcitonin in the past three years.
Funding
This work was supported by the National Institute of Health (1T32AI095202-07).
Disclaimer
This content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, Roche, or bioMérieux.
Procalcitonin (PCT) is a biomarker that has shown promise to identify bacterial etiology in acute infections, including bacterial lower respiratory tract infection (LRTI). In 2017, the United States Food and Drug Administration (FDA) approved the use of PCT as a diagnostic aid to guide the decisions around antibiotic therapy in acute LRTI.1 Although most of the data supporting the use of PCT for LRTI stems from adult studies, the high disease burden, predominance of viral etiologies, and frequent diagnostic uncertainty resulting in antibiotic overuse make pediatric LRTI an ideal target for the use of PCT as a diagnostic aid. This review evaluates and summarizes the current evidence regarding the role of PCT in the clinical care of pediatric LRTI, including its use in guiding antibiotic use and prognosticating disease severity.
THE ROLE OF PROCALCITONIN IN GUIDING INITIATION OF ANTIBIOTICS
The commonly used PCT cut points for withholding or stopping antibiotics in adults and children are 0.1 µg/L (very low risk of bacterial etiology) or 0.25 µg/L (low risk of bacterial etiology).2-4 Among the 532 children enrolled in the multicenter study of Etiology of Pneumonia in the Community (EPIC), a PCT threshold of 0.25 µg/L demonstrated an approximate sensitivity of 85%, specificity of 45%, positive likelihood ratio of 1.55, and negative likelihood ratio of 0.33 for community acquired pneumonia (CAP) caused by typical bacterial pathogens.5 Lowering the cutoff to <0.1 µg/L increased PCT sensitivity to 100%, decreased specificity, positive likelihood ratio, and negative likelihood ratio to 20%, 1.26, and 0, respectively. Although the EPIC study obtained culture and performed PCR testing on any blood sample, pleural fluid specimen, endotracheal aspirate, or bronchoalveolar–lavage specimens obtained during the study period, currently available laboratory methods show poor sensitivity for defining bacterial LRTI. Thus, bacterial etiologies may have been underestimated. The highly negative predictive value demonstrated in this study highlights the potential of PCT as a biomarker for ruling out bacterial diseases, including LRTI.
Multiple studies have evaluated the potential utility of PCT in guiding antibiotic initiation in adults with LRTI, but data on pediatric patients are sparse.4 In a randomized, single-center Italian study comparing a PCT-guided algorithm (withholding antibiotics when PCT < 0.25 µg/L) versus usual care among 319 hospitalized children with pneumonia, the PCT group experienced fewer antibiotic initiations (15.5% vs 100%, P < .05) without significant differences in recurrence of respiratory symptoms or new antibiotic prescriptions in the month following enrollment.2
A similar randomized trial using a PCT-guided algorithm for the initiation of antibiotics conducted among 337 Swiss children presented to the emergency department (ED) with pneumonia and other LRTIs failed to demonstrate decreases in antibiotic initiation.3 This study used an algorithm that categorized the likelihood of requiring antibiotic treatment for bacterial LRTI as “definitely” if PCT was >0.5 µg/L, “probably” if PCT was 0.26–0.5 µg/L, “probably not” if PCT was 0.1–0.25 µg/L, and “definitely not” if PCT was <0.1 µg/L. In the PCT group, 104 out of 168 (62%) patients received antibiotics within 14 days compared with 93 out of 165 (56%) patients in the control group (odds ratio [OR]: 1.26, 95% CI: 0.81, 1.95). In the subgroup analyses, the odds of administering antibiotics to those with nonpneumonia LRTI was significantly higher than those of the PCT group and control group (OR: 4.09, 95% CI: 1.8, 9.93); the odds of receiving antibiotics also showed no difference in the subgroup of children with pneumonia (OR: 0.66, 95% CI: 0.35, 1.23).
The benefit of PCT for informing decisions around the initiation of antibiotics likely varies based on perceived risk of bacterial diseases. When the pretest probability of bacterial disease is extremely high, the use of PCT is unlikely to alter treatment decisions. Similarly, PCT should not be used in situations where the pretest probability for bacterial pneumonia is very low—in these instances, an elevated PCT may lead to unnecessary antibiotic use among children presenting to the ED. However, the risk of bacterial pneumonia is often equivocal, and in these situations, PCT may provide clinicians with useful insights, primarily for ruling out bacterial disease.
THE ROLE OF PROCALCITONIN IN GUIDING DISCONTINUATION OF ANTIBIOTICS
In the study by Esposito et al., the PCT levels were additionally measured every two days until discharge and during two scheduled follow-up visits; the antibiotics were discontinued when PCT < 0.25 µg/L.2 The PCT-guided group experienced shorter antibiotic duration (mean 5.4 vs 11.0 days, P < .05), shorter length of hospital stay (mean 4.7 vs 5.61 days for mild LRTI and 5.01 vs 5.93 for severe LRTI), and fewer antibiotic-related adverse events (3.9% vs 25.2%, P < .05). Similarly, in the study by Baer et al., the PCT-guided group had PCT levels repeated on days three and five after enrollment, and the antibiotics were discontinued when PCT was less than 0.25 µg/L. The duration of antibiotic administration was significantly lower in the PCT-guided group (mean difference: 1.8 days, 95% CI: −3.1, −0.).3 The rates of hospitalization, duration of hospital stay, and mean impairment of daily activities attributable to LRTI were similar between groups.
Considering the adult studies and the small number of pediatric LRTI research published to date, the use of PCT to safely reduce antibiotic treatment duration is encouraging.4 Although the studies on the kinetics of PCT are limited, the biomarker has been shown to rise two to four hours after a bacterial stimulus, peak in 24-48 hours and achieve a half-life of 24-36 hours.6,7 As such, serial PCT measurements at 24-hour intervals for three to five days may be more beneficial than stand-alone PCT tests. Nonetheless, additional studies are needed to better define groups of patients who will most likely benefit from PCT testing and to understand how to best integrate testing into clinical practice.
PROCALCITONIN FOR SEVERITY PREDICTION OF LRTI
PCT has also been explored as a marker of LRTI disease severity. In a 2008 multicenter cohort encompassing 1,651 adults with pneumonia, PCT < 0.1 µg/L was associated with a decreased 30-day mortality, shorter length of stay, and decreased admission to the intensive care unit (ICU) compared with those with PCT>0.1 µg/L.8 In a 2017 study of 317 adults hospitalized with pneumonia, the PCT level was significantly higher in those with bacteremia and in those admitted to intensive care.9 When used in combination with the pneumonia severity index (PSI), the addition of PCT resulted in improved prognostic performance compared with the PSI alone for both outcomes, increasing the area under the receiver operating characteristic curve from 0.67 to 0.85 for bacteremia and from 0.58 to 0.64 for intensive care. Similarly, in the adult EPIC cohort, the addition of PCT contributed significant prognostic information beyond existing severity scores for predicting the need for invasive respiratory or vasopressor support; each 1 µg/L increase in PCT was associated with a 1% to 2% absolute increase in the need for this outcome.10
A European study of 100 children with pneumonia also demonstrated higher PCT values among hospitalized children (n = 26, median PCT 17.8 µg/L) compared with outpatient children (n = 73, median PCT 0.72 µg/L, P < .01).11 Among the 532 children from the EPIC study, a PCT < 0.25 µg/L was associated with the reduced odds of ICU admission (adjusted OR: 0.48; 95% CI: 0.30, 0.78) and a 2.3-day (95% CI: 1.4, 3.2) decrease in the average length of stay compared with those with higher PCT concentrations.5 Of the 34 children with empyema requiring drainage, 28 (82%) showed a PCT concentration ≥0.5 µg/L. Additional pediatric studies are needed, but the limited data to date suggest that PCT may play a role in predicting pediatric LRTI disease severity, including the need for mechanical ventilatory support and ICU-level care.
LIMITATIONS TO CLINICAL APPLICATION
Although PCT shows promise as a biomarker to reliably rule out bacterial infection, several potential limitations exist in assessing its role in pediatric LRTI. Atypical bacterial infections (ie, Mycoplasma pneumoniae) and localized bacterial infection may not induce significant PCT production, as has been shown in adults and children with tonsillitis, localized skin infections, endocarditis, or empyema (Table).12 The majority of clinical trials in LRTI have been conducted in the adult population,4 with the number of pediatric trials remaining small.2,3 Given the predominance of viral LRTI in children compared with adults, the utility of PCT may differ in these populations.13,14 Furthermore, existing studies demonstrate mixed results regarding the magnitude of benefits that PCT may provide in terms of limiting antibiotic use. Another concern is the potential of PCT to increase unnecessary antibiotic use in those with viral LRTI,3 as PCT may also be increased in populations with systemic inflammation from nonbacterial causes.12,15
CONCLUSIONS AND CLINICAL APPLICATION
The misuse of antibiotics is a public health crisis resulting in the emergence of antibiotic-resistant pathogens and adverse outcomes, including Clostridioides difficile infection, drug toxicities, and increased healthcare costs.16 Pneumonia is responsible for more days of antibiotics than any other disease in children’s hospitals and is an important target for stewardship efforts.17 PCT is a promising biomarker for distinguishing bacterial from viral infection, and its use may help in making informed antibiotic decisions and predicting disease outcomes in pediatric LRTI. Although PCT has been cleared by the FDA for assisting with antibiotic decisions in pediatric LRTI, the majority of evidence supporting this indication is drawn from adults. Additional studies are needed prior to the widespread implementation in the pediatric population, but the results of available pediatric studies show promise. The clinical context and severity of patient presentation are important when considering whether or not to use PCT and how to best interpret PCT levels when making clinical management decisions. The utility of PCT for antibiotic initiation in the pediatric population is encouraging given the predominance of viral etiologies in pediatric LRTI. Currently available data demonstrate the value of serial PCT measurements in antibiotic de-escalation and promoting antibiotic stewardship for children and adults.2-4 As with all new diagnostic modalities, provider education is paramount to ensure a safe and value-driven implementation.
Disclosures
Dr. Katz received investigator-initiated grant funding from Roche and bioMérieux to conduct research involving procalcitonin in the past three years. Dr. Sartori has nothing to disclose. Dr. Williams received investigator-initiated grant funding from bioMérieux to conduct research involving procalcitonin in the past three years.
Funding
This work was supported by the National Institute of Health (1T32AI095202-07).
Disclaimer
This content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, Roche, or bioMérieux.
1. FDA clears test to help manage antibiotic treatment for lower respiratory tract infections and sepsis. US Food and Drug Administration. [Press Release]. Silver Spring, MD, February 23 2017.
2. Esposito S, Tagliabue C, Picciolli I, et al. Procalcitonin measurements for guiding antibiotic treatment in pediatric pneumonia. Respir Med. 2011;105(12):1939-1945. https://doi.org/10.1016/j.rmed.2011.09.003.
3. Baer G, Baumann P, Buettcher M, et al. Procalcitonin guidance to reduce antibiotic treatment of lower respiratory tract infection in children and adolescents (ProPAED): a randomized controlled trial. PLoS One. 2013;8(8):e68419. https://doi.org/10.1371/journal.pone.0068419.
4. Choi JJ MM, Simon MS, Evans AT, Self WH, Glesby MJ. Procalcitonin in the diagnosis and management of community-acquired pneumonia in hospitalized adults. J Hosp Med. 2019;18(X);XXX-XXX. https://doi.org/10.12788/jhm.3272.
5. Stockmann C, Ampofo K, Killpack J, et al. Procalcitonin accurately identifies hospitalized children with low risk of bacterial community-acquired pneumonia. J Pediatric Infect Dis Soc. 2017;7(1): 46-53. https://doi.org/10.1093/jpids/piw091.
6. Dandona P, Nix D, Wilson MF, et al. Procalcitonin increase after endotoxin injection in normal subjects. J Clin Endocrinol Metab. 1994;79(6):1605-1608. https://doi.org/10.1210/jcem.79.6.7989463.
7. Brunkhorst FM, Heinz U, Forycki ZF. Kinetics of procalcitonin in iatrogenic sepsis. Intensive Care Med. 1998;24(8):888-889.
8. Huang DT, Weissfeld LA, Kellum JA, et al; GenIMS Investigators. Risk prediction with procalcitonin and clinical rules in community-acquired pneumonia. Ann Emerg Med. 2008;52(1):48-58 e42. https://doi.org/10.1016/j.annemergmed.2008.01.003.
9. McCluskey SM, Schuetz P, Abers MS, et al. Serial procalcitonin as a predictor of pacteremia and peed for intensive care unit care in adults with pneumonia, including those with highest severity: A Prospective Cohort Study. Open Forum Infect Dis. 2017;4(1):ofw238. https://doi.org/10.1093/ofid/ofw238.
10. Self WH, Grijalva CG, Williams DJ, et al. Procalcitonin as an early marker of the need for invasive respiratory or vasopressor support in adults with community-acquired pneumonia. Chest. 2016;150(4):819-828. https://doi.org/10.1016/j.chest.2016.04.010.
11. Don M, Valent F, Korppi M, et al. Efficacy of serum procalcitonin in evaluating severity of community-acquired pneumonia in childhood. Scand J Infect Dis. 2007;39(2):129-137. https://doi.org/10.1080/00365540600951283.
12. Meisner M. Update on procalcitonin measurements. Ann Lab Med. 2014;34(4):263-273. https://doi.org/10.3343/alm.2014.34.4.263.
13. Jain S, Williams DJ, Arnold SR, et al; CDC EPIC Study Team. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015;372(9):835-845. https://doi.org/10.1056/NEJMoa1405870.
14. Jain S, Self WH, Wunderink RG, et al; CDC EPIC Study Team. Community-Acquired Pneumonia Requiring Hospitalization among U.S. adults. N Engl J Med. 2015;373(5):415-427. https://doi.org/10.1056/NEJMoa1500245.
15. Aloisio E, Dolci A, Panteghini M. Procalcitonin: Between evidence and critical issues. Clin Chim Acta. 2019;496:7-12. https://doi.org/10.1016/j.cca.2019.06.010.
16. Society for Healthcare Epidemiology of A, Infectious Diseases Society of A, Pediatric Infectious Diseases S. Policy statement on antimicrobial stewardship by the Society for Healthcare Epidemiology of America (SHEA), the Infectious Diseases Society of America (IDSA), and the Pediatric Infectious Diseases Society (PIDS). Infect Control Hosp Epidemiol. 2012;33(4):322-327. https://doi.org/10.1086/665010.
17. Gerber JS, Kronman MP, Ross RK, et al. Identifying targets for antimicrobial stewardship in children’s hospitals. Infect Control Hosp Epidemiol. 2013;34(12):1252-1258. https://doi.org/10.1086/673982.
1. FDA clears test to help manage antibiotic treatment for lower respiratory tract infections and sepsis. US Food and Drug Administration. [Press Release]. Silver Spring, MD, February 23 2017.
2. Esposito S, Tagliabue C, Picciolli I, et al. Procalcitonin measurements for guiding antibiotic treatment in pediatric pneumonia. Respir Med. 2011;105(12):1939-1945. https://doi.org/10.1016/j.rmed.2011.09.003.
3. Baer G, Baumann P, Buettcher M, et al. Procalcitonin guidance to reduce antibiotic treatment of lower respiratory tract infection in children and adolescents (ProPAED): a randomized controlled trial. PLoS One. 2013;8(8):e68419. https://doi.org/10.1371/journal.pone.0068419.
4. Choi JJ MM, Simon MS, Evans AT, Self WH, Glesby MJ. Procalcitonin in the diagnosis and management of community-acquired pneumonia in hospitalized adults. J Hosp Med. 2019;18(X);XXX-XXX. https://doi.org/10.12788/jhm.3272.
5. Stockmann C, Ampofo K, Killpack J, et al. Procalcitonin accurately identifies hospitalized children with low risk of bacterial community-acquired pneumonia. J Pediatric Infect Dis Soc. 2017;7(1): 46-53. https://doi.org/10.1093/jpids/piw091.
6. Dandona P, Nix D, Wilson MF, et al. Procalcitonin increase after endotoxin injection in normal subjects. J Clin Endocrinol Metab. 1994;79(6):1605-1608. https://doi.org/10.1210/jcem.79.6.7989463.
7. Brunkhorst FM, Heinz U, Forycki ZF. Kinetics of procalcitonin in iatrogenic sepsis. Intensive Care Med. 1998;24(8):888-889.
8. Huang DT, Weissfeld LA, Kellum JA, et al; GenIMS Investigators. Risk prediction with procalcitonin and clinical rules in community-acquired pneumonia. Ann Emerg Med. 2008;52(1):48-58 e42. https://doi.org/10.1016/j.annemergmed.2008.01.003.
9. McCluskey SM, Schuetz P, Abers MS, et al. Serial procalcitonin as a predictor of pacteremia and peed for intensive care unit care in adults with pneumonia, including those with highest severity: A Prospective Cohort Study. Open Forum Infect Dis. 2017;4(1):ofw238. https://doi.org/10.1093/ofid/ofw238.
10. Self WH, Grijalva CG, Williams DJ, et al. Procalcitonin as an early marker of the need for invasive respiratory or vasopressor support in adults with community-acquired pneumonia. Chest. 2016;150(4):819-828. https://doi.org/10.1016/j.chest.2016.04.010.
11. Don M, Valent F, Korppi M, et al. Efficacy of serum procalcitonin in evaluating severity of community-acquired pneumonia in childhood. Scand J Infect Dis. 2007;39(2):129-137. https://doi.org/10.1080/00365540600951283.
12. Meisner M. Update on procalcitonin measurements. Ann Lab Med. 2014;34(4):263-273. https://doi.org/10.3343/alm.2014.34.4.263.
13. Jain S, Williams DJ, Arnold SR, et al; CDC EPIC Study Team. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015;372(9):835-845. https://doi.org/10.1056/NEJMoa1405870.
14. Jain S, Self WH, Wunderink RG, et al; CDC EPIC Study Team. Community-Acquired Pneumonia Requiring Hospitalization among U.S. adults. N Engl J Med. 2015;373(5):415-427. https://doi.org/10.1056/NEJMoa1500245.
15. Aloisio E, Dolci A, Panteghini M. Procalcitonin: Between evidence and critical issues. Clin Chim Acta. 2019;496:7-12. https://doi.org/10.1016/j.cca.2019.06.010.
16. Society for Healthcare Epidemiology of A, Infectious Diseases Society of A, Pediatric Infectious Diseases S. Policy statement on antimicrobial stewardship by the Society for Healthcare Epidemiology of America (SHEA), the Infectious Diseases Society of America (IDSA), and the Pediatric Infectious Diseases Society (PIDS). Infect Control Hosp Epidemiol. 2012;33(4):322-327. https://doi.org/10.1086/665010.
17. Gerber JS, Kronman MP, Ross RK, et al. Identifying targets for antimicrobial stewardship in children’s hospitals. Infect Control Hosp Epidemiol. 2013;34(12):1252-1258. https://doi.org/10.1086/673982.
© 2019 Society of Hospital Medicine
Impact of the Choosing Wisely® Campaign Recommendations for Hospitalized Children on Clinical Practice: Trends from 2008 to 2017
The Choosing Wisely® Campaign (CWC) was launched in 2012. This ongoing national initiative encourages conversations among patients and clinicians about the need —or the lack thereof—for frequent tests, treatments, and procedures in healthcare. More than 80 professional societies have developed short lists of evidence-based recommendations aimed at avoiding unnecessary, “low-value” care. More than 550 recommendations are currently available.1 The Society of Hospital Medicine (SHM) Pediatric Committee published a list of five recommendations for the CWC in 2013.2
After seven years, the campaign has posted several success stories highlighting the increase in clinicians’ awareness about the recommendations. Several local, regional, and national initiatives and quality improvement (QI) projects have been inspired by the CWC and its tenants.1,3 However, limited research has been performed on the true impact of these recommendations on avoiding “low-value” services. A more comprehensive approach is required to “measure wisely” the impact of the campaign on bedside clinical practice.4 Stakeholders in healthcare value have been challenged to collaborate in creating high-impact lists of “low-value” interventions and designing effective tools to measure their impact on clinical practice and costs.5
We initially developed a report card with five metrics derived from the CWC-SHM pediatric recommendations to help individual institutions and group practices to measure their performance and benchmark their results with peers.6 The report card is available for hospital members of the Children’s Hospital Association (CHA).7
The current study analyzes the frequency of utilization and trends of five metrics included in the CHA/Pediatric Health Information System® (PHIS) CWC report card in tertiary children’s hospitals in the United States. We analyzed data from five years before and five years after the CWC-PHM recommendations were published in 2013. We hypothesize that the publication and dissemination of the CWC-PHM recommendations—the intervention—will result in either an immediate decrease in the use of the “low-value” services studied and/or a change in the trend of utilization over time.
METHODS
Study Design
We conducted an observational, longitudinal retrospective study aimed at evaluating the impact of the CWC-PHM recommendations on clinical practice in tertiary children’s hospitals in the US.
Study Population
The population included inpatient and observation stays for children aged 0-18 years admitted to the 36 children’s hospitals consistently providing data from 2008 to 2017 to the PHIS administrative database (CHA, Lenexa, Kansas). This database contains inpatient, emergency department, ambulatory, and observation encounter–level data from more than 50 not-for-profit, tertiary care pediatric hospitals and accounts for ~20% of all pediatric hospitalizations in the US every year.
A joint effort between the CHA and the participating hospitals ensures the quality of the data submitted, as previously described.8 These data are subjected to a routine quality check with each submission and within each report. Data were fully deidentified for this study. In total, 36 PHIS hospitals met the strict quality standards for inclusion of submitted data. The remaining hospitals were excluded because they did not have complete data or had incomplete billing information.
For external benchmarking purposes, PHIS participating hospitals provide encounter data, including demographics, diagnoses, and procedures (International Classification of Diseases versions 9 and 10).9,10 The transition from ICD-9 to ICD-10 in the US took place during the study period. However, the CHA completed a process of translating and mapping all ICD-9 codes to every possible equivalent ICD-10 code in the PHIS database. Thus, the change from ICD-9 to ICD-10 should not have had any significant effect on population definition and data analytics, including trend analysis.
For each condition, the study population was divided into the following two cohorts for comparison of the trends: all admissions from January 1, 2008 to December 31, 2012 (before) and all admissions from January 1, 2013 to December 31, 2017 (after) the CWC-PHM recommendations were published.
This study was determined to be nonhuman subject research and was therefore exempted by Nicklaus Children’s Hospital Human Research Protection Program.
Outcomes
The outcomes for this study were the percentages of patients receiving the not-recommended “low-value” services targeted by the CWC-PHM recommendations. For this purpose, four of the five recommendations were translated into the following five metrics, operationalized in the PHIS database and displayed in the “Choosing Wisely” report card:6
1. Percentage of patients with uncomplicated asthma receiving chest radiograph (CXR).
2. Percentage of patients with uncomplicated bronchiolitis receiving CXR.
3. Percentage of patients with uncomplicated bronchiolitis receiving bronchodilators.
4. Percentage of patients with lower respiratory tract infection (LRTI) receiving systemic corticosteroids (relievers).
5. Percentage of patients with uncomplicated gastroesophageal reflux (GER) receiving acid suppressor therapy.
The fifth recommendation—limiting the use of continuous pulse oximetry unless the patient is receiving supplemental oxygen—could not be operationalized in the PHIS database because of inconsistent reporting of these resources.6
The resulting percentages represent nonadherence to the recommendations, suggesting overuse of the specific “low-value” intervention. As such, a decreasing trend over time is the desired direction of improvement.
The definition of “uncomplicated” conditions and the metrics are presented in Table 1. A complete list of the inclusion and exclusion criteria to define “uncomplicated” conditions and the complete list of the clinical translation codes used in PHIS to identify the “low-value” services are presented as an electronic supplement.
Statistical Analyses
We compared the demographic and clinical characteristics of the various cohorts before and after the release of the CWC-PHM recommendations—the intervention—using chi-square statistics. To assess the individual hospital-level trends over time for each measure, we modeled the patient-level data of each hospital using generalized linear mixed effects models with a binomial distribution. These models were adjusted for patient demographic and clinical factors that were found to be significantly different (P < .01) before and after the intervention on bivariate analyses. From these models, we generated adjusted estimates for the quarterly percentages for each hospital. We then conducted an interrupted time series (ITS) using these estimates to compare trends in the five years before (2008-2012) and five years after (2013-2017) the publication of the CWC-PHM recommendations. For the ITS analysis, we used a generalized linear mixed effects model with the quarterly adjusted hospital-level utilization rates of “low-value” services for each cohort as the unit of analysis and a random intercept for each hospital. The model used an autoregressive(1) covariance structure to account for autocorrelation. The ITS allowed us to test our hypothesis by assessing the following two important features: (a) if a significant decrease occurred right after the CWC-PHM recommendations were published (level-change) and/or (b) if the intervention altered the secular trend (slope-change). All statistical analyses were performed using SAS v. 9.4 (SAS Institute, Cary, North Carolina), and P values <.01 were considered to be statistically significant.
RESULTS
Table 2 presents the demographic characteristics of the cohorts before (2008-2012) and after (2013-2017) the publication of the CWC-PHM recommendations. Hospitalizations due to asthma represented the largest cohort with 142,067 cases, followed by hospitalizations due to bronchiolitis with 94,253 cases. Hospitalizations due to GER comprised the smallest cohort with 13,635 cases. Most of the children had government insurance and had “minor” severity according to the All Patient Revised Diagnosis Related Group (APR-DRG) system.
We found statistically significant differences in most of the demographic characteristics for the cohorts when comparing cases before and after the introduction of the CWC-PHM recommendations.
After adjusting for demographic characteristics, we estimated the percentages of the utilization of the “low-value” services from 2008 to 2017. We observed a steady decrease in overutilization of all services over time. The absolute percentage decrease was more evident in the reduction of the utilization of relievers by 36.6% and that of CXR by 31.5% for bronchiolitis. We also observed a 20.8% absolute reduction in the use of CXR for asthma.
The use of systemic steroids in LRTI revealed the lowest utilization among the “low-value” services studied, with 15.1% in 2008 and 12.2% in 2017, a 2.9% absolute reduction. However, the prescription of acid suppressors for GER showed the highest utilization among all the overuse metrics studied, ie, 63% in 2008 and 48.9% in 2017, with an absolute decrease of 24.1%. The yearly adjusted estimated percentages of utilization for each “low-value” service are presented in Appendix Table A.
Table 3 and the Figure (attached as supplemental online graphic) respectively present the risk-adjusted ITS parameter estimates and the graphic representation before and after the inception of the CWC-PHM recommendations for the trend analysis.
During the five years preceding the intervention (2008-2012), a statistically significant decrease (P < .01) was already noted in the trend of utilization of relievers and CXR in bronchiolitis and CXR in asthma. However, we found no significant change in the trend of the use of systemic corticosteroids in cases with LRTI or the use of acid suppression therapy for GER.
The immediate effect of the intervention is represented by the level change. We found a statistically significant (P < .01) reduction according to the CWC-PHM recommendations only for the use of CXR in hospitalized children with uncomplicated asthma.
During the five years after the CWC-PHM recommendations were published (2013-2017), a sustained, significant decrease in the trend of the use of CXR in asthma and bronchiolitis and the use of relievers in bronchiolitis (P < .01) was observed. However, there was no significant change in the trend of the use of systemic corticosteroids in cases with LRTI or in the use of acid suppression therapy for GER during this period.
Comparison of the trends before and after the publication of the CWC-PHM recommendations revealed that only the decreasing trend in the use of relievers for bronchiolitis over time significantly correlated with the campaign (P < .01).
DISCUSSION
We found a steady reduction in the frequency of overutilization of five “low-value” services described in the CWC-PHM recommendations from 2008 to 2017 in 36 tertiary children’s hospitals in the US. This trend was more evident in the utilization of relievers and CXR for bronchiolitis. The ITS analysis demonstrated that immediately after the publication of the CWC-PHM recommendations, only the use of CXR for asthma decreased significantly. Then, only the use of relievers for bronchiolitis decreased significantly over time in comparison with the secular trend.
These results support our hypothesis for two of the five metrics studied, suggesting that the publication of the CWC-PHM recommendations had a modest impact in clinical practices related to those services in tertiary children’s hospitals.
These findings align with a limited number of published studies that have consistently found a modest decrease in the use of “low-value” services before 201211-13 and a limited impact of the CWC in clinical practices on the use of “low-value” services after the inception of the campaign.14-17
For instance, in a cross-sectional analysis of the 1999 and 2009 samples of ambulatory care practices in the US, only two of 11 overuse quality indicators showed improvement.11 The authors recognized that reducing inappropriate care will require the same attention to guideline development and performance measurement that was directed at reducing the underuse of needed therapies. However, determining whether a patient received inappropriate care generally requires a much more detailed analysis of clinical information than what is required for assessments of underuse.11
Another study designed claims-based algorithms to measure the prevalence of 11 Choosing Wisely-identified “low-value” services in fee-for-service Medicare patients aged >65 years from 2006 to 2011.12 The annual prevalence of selected CWC “low-value” services ranged from 1.2% (upper urinary tract imaging in men with benign prostatic hyperplasia) to 46.5% (preoperative cardiac testing for low-risk, noncardiac procedures). The study concluded that identifying and measuring “low-value” health services is a prerequisite for improving quality and eliminating waste.12
In pediatric medicine, the authors investigated a large cohort of infants aged one to 24 months hospitalized with bronchiolitis to 41 tertiary children’s hospitals reporting data to the PHIS database from 2004 to 2012.13 The trend analysis revealed a decrease in the utilization of diagnostics and treatment interventions before the publication of the American Academy of Pediatrics 2006 Bronchiolitis Guidelines.18 There was an additional reduction in the use of CXR, steroids, and bronchodilators after the publication of the guidelines.13
After the CWC was launched in 2012, several surveys have demonstrated a tangible increase in awareness of the CWC and its goals, mostly among primary care physicians and subspecialists. Clinicians who were aware of the campaign found the recommendations to be useful as a legitimate source of guidance and were more likely to reduce the indication of unnecessary care and “low-value” clinical services included in the CWC.1,3,19,20
Few studies in adults have focused on measuring the trends in overuse metrics derived from the CWC recommendations.14-16 The initial studies have found limited reduction on the use of “low-value” care after the inception of the CWC. They suggest that clinician education, awareness, and public promotion alone do not appear to be sufficient to achieve widespread changes in clinical practice. Additional interventions are necessary for the wider implementation and success of the CWC recommendations.11,14,15,19,21,22
However, a more recent study was conducted in 91 academic centers from 2013 through 2016, before and after the publication of a CWC recommendation on the use of troponin-only testing for the diagnosis of acute myocardial infarction. Hospitals with low rates of troponin-only testing before the publication of the recommendation demonstrated a statistically significant increase over time in the rate of adherence. The authors postulated that the impact of the CWC might have been significant because of the increase in the institutional and provider attention to “high-value” care as a result of the campaign.16
In pediatrics, a cross-sectional study defined 20 “low-value” services from a list of more than 400 items from the CWC and other sources of highly regarded, evidence-based pediatrics healthcare recommendations. The list included six diagnostic tests, five imaging tests, and nine prescription drugs ordered in a robust cohort of 4.4 million children nationwide in 2014. The study concluded that approximately one in 10 children received a “low-value” service. The majority (59.4%) were related to prescription drugs, specifically the inappropriate use of antibiotics for a variety of conditions. The estimated combined cost of these unnecessary services was approximately $27 million, with one-third of the cost being paid out of pocket, arguing for significant financial harm. However, this study did not perform a trend analysis.17
Our results are comparable with these studies, reporting an initial increase in awareness and beliefs, followed by progressive changes in clinical practice among pediatric hospital-based clinicians in delivering evidence-based, high-value care after the CWC.
The attribution of the steady reduction in the absolute percentages of overuse/waste in the five metrics related to the CWC observed in this study, including the significant changes noted in two of the overuse indicators after the publication of the CWC-PHM recommendations, should be interpreted with caution. For example, the significant decrease in the use of “low-value” services in bronchiolitis could be attributed to multiple factors such as national guidelines released in 2014 after the campaign,23 national multicenter QI collaborative projects,24,25 and multiple local QI efforts.26,27 The increase in the awareness and impact of the CWC recommendations among pediatric providers could also be a contributing factor, but this association cannot be established in the light of our findings.
On the other hand, despite extensive evidence for the lack of efficacy and the potential harm associated with the use of acid suppressors for uncomplicated GER in infants,28-30 the frequency of this “low-value” therapeutic intervention remains high (~50%). The trend in utilization was not impacted by the CWC-PHM recommendations. This finding could be explained by several factors, including the possibility that several hospitalized patients may suffer from GER disease requiring acid suppressors. Another possibility is that acid suppressors are generally prescribed as an outpatient medication, and physicians treating inpatients may be reluctant to discontinue it during hospitalization. Nevertheless, this recommendation represents a target for review, update, and QI interventions in the near future.
The delivery of inappropriate “low-value” care represents the most significant dimension of waste in healthcare.31 The development of quality measures of “low-value” services representing overuse and waste is the most needed step toward assessing the magnitude of the problem. Overuse metrics could be incorporated into QI interventions to decrease the provision of such services. However, systematic efforts aimed at developing quality indicators of overuse based on the CWC recommendations have been limited. To our knowledge, this is the first study on the trends of metrics derived from the CWC recommendations in pediatric medicine.
Future research is needed to develop overuse metrics further to assess the specific outcomes related to the implementation of the CWC. How much has clinical practice changed as a result of the campaign? What are the outcomes and savings attributable to these efforts? These are critical questions for the immediate future that should be answered to sustain the ongoing efforts and results and to validate that the efforts are worthwhile.
This study has several limitations. First, this is a retrospective and observational study. It cannot prove a direct causal relationship between the publication of the CWC-PHM and the observed trends, as other potential factors may have contributed to the outcomes. Second, in administrative databases, the data quality is dependent on proper documentation and coding that may vary among reporting institutions. These data lack clinical information, and a fair assessment of “appropriateness” could be questioned. In addition, the study included only 36 academic, tertiary children’s hospitals. Because approximately two-thirds of all pediatric hospitalizations in the US occur in community settings,32 this study may not fully represent clinical practice in the majority of pediatric hospitalizations in the US. Finally, the validity of the ITS analysis has inherent limitations due to the variability of the data in some metrics that may affect the power of the analysis. This fact could lead to inaccurate conclusions regarding intervention effectiveness due to the data-driven model applied, as well as the lack of control for other time-varying confounders.33
CONCLUSIONS
After seven years, the CWC faces important challenges. Critical to the success of the campaign is to “measure wisely” by developing quality indicators of overuse and operationalizing them into administrative and clinical data sources to assess the impact on clinical practice. Our study highlights some limited but steady reduction in the use of some “low-value” services before the campaign. It also demonstrates a modest impact of the campaign on clinical practices in tertiary care children’s hospitals in the US. Clinicians and institutions still have a long way to go in reducing the use of “low-value” interventions in pediatric medicine. These observations challenge us to step up our efforts to implement QI interventions aimed at incorporating these professional, society-endorsed recommendations into our clinical practice.
Acknowledgments
The authors thank Dr. Kristine De La Torre and Dr. Jennifer McCafferty-Fernandez and the Research Institute of Nicklaus Children’s Hospital for medical writing assistance. They also acknowledge Tatiana Consuegra, library technician, for her clerical assistance in the preparation and submission of this article.
1. Choosing Wisely. Choosing Wisely Campaign Official Site. http://www.choosingwisely.org/. Accessed May 2019.
2. Quinonez RA, Garber MD, Schroeder AR, et al. Choosing wisely in pediatric hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):479-485. https://doi.org/10.1002/jhm.2064.
3. ABIM Foundation CR. Choosing Wisely: A Special Report on the First Five Years. http://www.choosingwisely.org/choosing-wisely-a-special-report-on-the-first-five-years/. Updated 2017. Accessed May 2019.
4. Wolfson D, Santa J, Slass L. Engaging physicians and consumers in conversations about treatment overuse and waste: a short history of the choosing wisely campaign. Acad Med. 2014;89(7):990-995. https://doi.org/10.1097/ACM.0000000000000270.
5. Morden NE, Colla CH, Sequist TD, Rosenthal MB. Choosing wisely—the politics and economics of labeling low-value services. N Engl J Med. 2014;370(7):589-592. https://doi.org/10.1056/NEJMp1314965.
6. Reyes M, Paulus E, Hronek C, et al. Choosing wisely campaign: Report card and achievable benchmarks of care for children’s hospitals. Hosp Pediatr. 2017;7(11):633-641. https://doi.org/10.1542/hpeds.2017-0029.
7. Report Cards. Choosing Wisely Measures - Pediatric Hospital Medicine Detail Reports. Children’s Hospital Association Web site. https://www.childrenshospitals.org/. Accessed May 2019.
8. Mongelluzzo J, Mohamad Z, Ten Have TR, Shah SS. Corticosteroids and mortality in children with bacterial meningitis. JAMA. 2008;299(17):2048-2055. https://doi.org/10.1001/jama.299.17.2048.
9. Buck CJ. 2013 ICD 9 CM for Physicians, Volumes 1 & 2. Chicago, IL: American Medical Association; 2013.
10. Buck CJ. 2018 ICD-10-CM for Physicians. Chicago, IL: American Medical Association; 2018.
11. Kale MS, Bishop TF, Federman AD, Keyhani S. Trends in the overuse of ambulatory health care services in the United States. JAMA Inter Med. 2013;173(2):142-148. https://doi.org/10.1001/2013.jamainternmed.1022.
12. Colla CH, Morden NE, Sequist TD, Schpero WL, Rosenthal MB. Choosing wisely: Prevalence and correlates of low-value health care services in the United States. J Gen Intern Med. 2015;30(2):221-228. https://doi.org/10.1007/s11606-014-3070-z
13. Parikh K, Hall M, Teach SJ. Bronchiolitis management before and after the AAP guidelines. Pediatrics. 2014;133(1): e1-7. https://doi.org/10.1542/peds.2013-2005.
14. Rosenberg A, Agiro A, Gottlieb M, et al. Early trends among seven recommendations from the Choosing Wisely campaign. JAMA Inter Med. 2015;175(12):1913-1920. https://doi.org/10.1001/jamainternmed.2015.5441.
15. Reid RO, Rabideau B, Sood N. Low-value health care services in a commercially insured population. JAMA Inter Med. 2016;176(10):1567-1571. https://doi.org/10.1001/jamainternmed.2016.5031.
16. Prochaska MT, Hohmann SF, Modes M, Arora VM. Trends in troponin-only testing for AMI in academic teaching hospitals and the impact of choosing wisely(R). J Hosp Med. 2017;12(12):957-962. https://doi.org/10.12788/jhm.2846.
17. Chua KP, Schwartz AL, Volerman A, Conti RM, Huang ES. Use of low-value pediatric services among the commercially insured. Pediatrics. 2016;138(6):e20161809. https://doi.org/10.1542/peds.2016-1809.
18. American Academy of Pediatrics Subcommittee on Diagnosis and Management of Bronchiolitis. Diagnosis and management of bronchiolitis. Pediatrics. 2006;118(4):1774-1793.
19. Colla CH, Kinsella EA, Morden NE, Meyers DJ, Rosenthal MB, Sequist TD. Physician perceptions of Choosing Wisely and drivers of overuse. Am J Manag Care. 2016;22(5):337-343.
20. PerryUndem Research/Communication AF. DataBrief: Findings from a National Survey of Physicians. http://www.choosingwisely.org/wp-content/uploads/2017/10/Summary-Research-Report-Survey-2017.pdf. Updated 2017.
21. Wolfson D. Choosing wisely recommendations using administrative claims data. JAMA Inter Med. 2016;176(4):565. https://doi.org/10.1001/jamainternmed.2016.0357.
22. Heekin AM, Kontor J, Sax HC, Keller M, Wellington A, Weingarten S. Choosing wisely clinical decision support adherence and associated patient outcomes. Am J Manag Care. 2018;24(8):361-366.
23. Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134(5):e1474-e502. https://doi.org/10.1542/peds.2014-2742.
24. Ralston SL, Garber MD, Rice-Conboy E, et al. A multicenter collaborative to reduce unnecessary care in inpatient bronchiolitis. Pediatrics. 2016;137(1):e20150851. https://doi.org/10.1542/peds.2015-0851.
25. Mussman GM, Lossius M, Wasif F, et al. Multisite emergency department inpatient collaborative to reduce unnecessary bronchiolitis care. Pediatrics. 2018;141(2):e20170830. https://doi.org/10.1542/peds.2017-0830.
26. Mittal V, Hall M, Morse R, et al. Impact of inpatient bronchiolitis clinical practice guideline implementation on testing and treatment. J Pediatr. 2014;165(3):570-576. https://doi.org/10.1016/j.jpeds.2014.05.021.
27. Tyler A, Krack P, Bakel LA, et al. Interventions to reduce over-utilized tests and treatments in bronchiolitis. Pediatrics. 2018;141(6):e20170485. https://doi.org/10.1542/peds.2017-0485.
28. Rosen R, Vandenplas Y, Singendonk M, et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2018;66(3):516-554. https://doi.org/10.1097/MPG.0b013e3181b7f563.
29. Eichenwald EC, COMMITTEE ON FETUS AND NEWBORN. Diagnosis and management of gastroesophageal reflux in preterm infants. Pediatrics. 2018;142(1):e20181061. https://doi.org/10.1542/peds.2018-1061
30. van der Pol RJ, Smits MJ, van Wijk MP, Omari TI, Tabbers MM, Benninga MA. Efficacy of proton-pump inhibitors in children with gastroesophageal reflux disease: a systematic review. Pediatrics. 2011;127(5):925-935. https://doi.org/10.1542/peds.2010-2719.
31. IOM Report: Estimated $750B Wasted Annually In Health Care System. Kaiser Health News Web site. https://khn.org/morning-breakout/iom-report/. Updated 2012. Accessed May 2019.
32. Leyenaar JK, Ralston SL, Shieh M, Pekow PS, Mangione‐Smith R, Lindenauer PK. Epidemiology of pediatric hospitalizations at general hospitals and freestanding children’s hospitals in the United States. J Hosp Med. 2016;11(11):743-749. https://doi.org/10.1002/jhm.2624.
33. Bernal JL, Cummins S, Gasparrini A. Interrupted time series regression for the evaluation of public health interventions: a tutorial. Int J Epidemiol. 2017;46(1):348-355. https://doi.org/10.1093/ije/dyw098.
The Choosing Wisely® Campaign (CWC) was launched in 2012. This ongoing national initiative encourages conversations among patients and clinicians about the need —or the lack thereof—for frequent tests, treatments, and procedures in healthcare. More than 80 professional societies have developed short lists of evidence-based recommendations aimed at avoiding unnecessary, “low-value” care. More than 550 recommendations are currently available.1 The Society of Hospital Medicine (SHM) Pediatric Committee published a list of five recommendations for the CWC in 2013.2
After seven years, the campaign has posted several success stories highlighting the increase in clinicians’ awareness about the recommendations. Several local, regional, and national initiatives and quality improvement (QI) projects have been inspired by the CWC and its tenants.1,3 However, limited research has been performed on the true impact of these recommendations on avoiding “low-value” services. A more comprehensive approach is required to “measure wisely” the impact of the campaign on bedside clinical practice.4 Stakeholders in healthcare value have been challenged to collaborate in creating high-impact lists of “low-value” interventions and designing effective tools to measure their impact on clinical practice and costs.5
We initially developed a report card with five metrics derived from the CWC-SHM pediatric recommendations to help individual institutions and group practices to measure their performance and benchmark their results with peers.6 The report card is available for hospital members of the Children’s Hospital Association (CHA).7
The current study analyzes the frequency of utilization and trends of five metrics included in the CHA/Pediatric Health Information System® (PHIS) CWC report card in tertiary children’s hospitals in the United States. We analyzed data from five years before and five years after the CWC-PHM recommendations were published in 2013. We hypothesize that the publication and dissemination of the CWC-PHM recommendations—the intervention—will result in either an immediate decrease in the use of the “low-value” services studied and/or a change in the trend of utilization over time.
METHODS
Study Design
We conducted an observational, longitudinal retrospective study aimed at evaluating the impact of the CWC-PHM recommendations on clinical practice in tertiary children’s hospitals in the US.
Study Population
The population included inpatient and observation stays for children aged 0-18 years admitted to the 36 children’s hospitals consistently providing data from 2008 to 2017 to the PHIS administrative database (CHA, Lenexa, Kansas). This database contains inpatient, emergency department, ambulatory, and observation encounter–level data from more than 50 not-for-profit, tertiary care pediatric hospitals and accounts for ~20% of all pediatric hospitalizations in the US every year.
A joint effort between the CHA and the participating hospitals ensures the quality of the data submitted, as previously described.8 These data are subjected to a routine quality check with each submission and within each report. Data were fully deidentified for this study. In total, 36 PHIS hospitals met the strict quality standards for inclusion of submitted data. The remaining hospitals were excluded because they did not have complete data or had incomplete billing information.
For external benchmarking purposes, PHIS participating hospitals provide encounter data, including demographics, diagnoses, and procedures (International Classification of Diseases versions 9 and 10).9,10 The transition from ICD-9 to ICD-10 in the US took place during the study period. However, the CHA completed a process of translating and mapping all ICD-9 codes to every possible equivalent ICD-10 code in the PHIS database. Thus, the change from ICD-9 to ICD-10 should not have had any significant effect on population definition and data analytics, including trend analysis.
For each condition, the study population was divided into the following two cohorts for comparison of the trends: all admissions from January 1, 2008 to December 31, 2012 (before) and all admissions from January 1, 2013 to December 31, 2017 (after) the CWC-PHM recommendations were published.
This study was determined to be nonhuman subject research and was therefore exempted by Nicklaus Children’s Hospital Human Research Protection Program.
Outcomes
The outcomes for this study were the percentages of patients receiving the not-recommended “low-value” services targeted by the CWC-PHM recommendations. For this purpose, four of the five recommendations were translated into the following five metrics, operationalized in the PHIS database and displayed in the “Choosing Wisely” report card:6
1. Percentage of patients with uncomplicated asthma receiving chest radiograph (CXR).
2. Percentage of patients with uncomplicated bronchiolitis receiving CXR.
3. Percentage of patients with uncomplicated bronchiolitis receiving bronchodilators.
4. Percentage of patients with lower respiratory tract infection (LRTI) receiving systemic corticosteroids (relievers).
5. Percentage of patients with uncomplicated gastroesophageal reflux (GER) receiving acid suppressor therapy.
The fifth recommendation—limiting the use of continuous pulse oximetry unless the patient is receiving supplemental oxygen—could not be operationalized in the PHIS database because of inconsistent reporting of these resources.6
The resulting percentages represent nonadherence to the recommendations, suggesting overuse of the specific “low-value” intervention. As such, a decreasing trend over time is the desired direction of improvement.
The definition of “uncomplicated” conditions and the metrics are presented in Table 1. A complete list of the inclusion and exclusion criteria to define “uncomplicated” conditions and the complete list of the clinical translation codes used in PHIS to identify the “low-value” services are presented as an electronic supplement.
Statistical Analyses
We compared the demographic and clinical characteristics of the various cohorts before and after the release of the CWC-PHM recommendations—the intervention—using chi-square statistics. To assess the individual hospital-level trends over time for each measure, we modeled the patient-level data of each hospital using generalized linear mixed effects models with a binomial distribution. These models were adjusted for patient demographic and clinical factors that were found to be significantly different (P < .01) before and after the intervention on bivariate analyses. From these models, we generated adjusted estimates for the quarterly percentages for each hospital. We then conducted an interrupted time series (ITS) using these estimates to compare trends in the five years before (2008-2012) and five years after (2013-2017) the publication of the CWC-PHM recommendations. For the ITS analysis, we used a generalized linear mixed effects model with the quarterly adjusted hospital-level utilization rates of “low-value” services for each cohort as the unit of analysis and a random intercept for each hospital. The model used an autoregressive(1) covariance structure to account for autocorrelation. The ITS allowed us to test our hypothesis by assessing the following two important features: (a) if a significant decrease occurred right after the CWC-PHM recommendations were published (level-change) and/or (b) if the intervention altered the secular trend (slope-change). All statistical analyses were performed using SAS v. 9.4 (SAS Institute, Cary, North Carolina), and P values <.01 were considered to be statistically significant.
RESULTS
Table 2 presents the demographic characteristics of the cohorts before (2008-2012) and after (2013-2017) the publication of the CWC-PHM recommendations. Hospitalizations due to asthma represented the largest cohort with 142,067 cases, followed by hospitalizations due to bronchiolitis with 94,253 cases. Hospitalizations due to GER comprised the smallest cohort with 13,635 cases. Most of the children had government insurance and had “minor” severity according to the All Patient Revised Diagnosis Related Group (APR-DRG) system.
We found statistically significant differences in most of the demographic characteristics for the cohorts when comparing cases before and after the introduction of the CWC-PHM recommendations.
After adjusting for demographic characteristics, we estimated the percentages of the utilization of the “low-value” services from 2008 to 2017. We observed a steady decrease in overutilization of all services over time. The absolute percentage decrease was more evident in the reduction of the utilization of relievers by 36.6% and that of CXR by 31.5% for bronchiolitis. We also observed a 20.8% absolute reduction in the use of CXR for asthma.
The use of systemic steroids in LRTI revealed the lowest utilization among the “low-value” services studied, with 15.1% in 2008 and 12.2% in 2017, a 2.9% absolute reduction. However, the prescription of acid suppressors for GER showed the highest utilization among all the overuse metrics studied, ie, 63% in 2008 and 48.9% in 2017, with an absolute decrease of 24.1%. The yearly adjusted estimated percentages of utilization for each “low-value” service are presented in Appendix Table A.
Table 3 and the Figure (attached as supplemental online graphic) respectively present the risk-adjusted ITS parameter estimates and the graphic representation before and after the inception of the CWC-PHM recommendations for the trend analysis.
During the five years preceding the intervention (2008-2012), a statistically significant decrease (P < .01) was already noted in the trend of utilization of relievers and CXR in bronchiolitis and CXR in asthma. However, we found no significant change in the trend of the use of systemic corticosteroids in cases with LRTI or the use of acid suppression therapy for GER.
The immediate effect of the intervention is represented by the level change. We found a statistically significant (P < .01) reduction according to the CWC-PHM recommendations only for the use of CXR in hospitalized children with uncomplicated asthma.
During the five years after the CWC-PHM recommendations were published (2013-2017), a sustained, significant decrease in the trend of the use of CXR in asthma and bronchiolitis and the use of relievers in bronchiolitis (P < .01) was observed. However, there was no significant change in the trend of the use of systemic corticosteroids in cases with LRTI or in the use of acid suppression therapy for GER during this period.
Comparison of the trends before and after the publication of the CWC-PHM recommendations revealed that only the decreasing trend in the use of relievers for bronchiolitis over time significantly correlated with the campaign (P < .01).
DISCUSSION
We found a steady reduction in the frequency of overutilization of five “low-value” services described in the CWC-PHM recommendations from 2008 to 2017 in 36 tertiary children’s hospitals in the US. This trend was more evident in the utilization of relievers and CXR for bronchiolitis. The ITS analysis demonstrated that immediately after the publication of the CWC-PHM recommendations, only the use of CXR for asthma decreased significantly. Then, only the use of relievers for bronchiolitis decreased significantly over time in comparison with the secular trend.
These results support our hypothesis for two of the five metrics studied, suggesting that the publication of the CWC-PHM recommendations had a modest impact in clinical practices related to those services in tertiary children’s hospitals.
These findings align with a limited number of published studies that have consistently found a modest decrease in the use of “low-value” services before 201211-13 and a limited impact of the CWC in clinical practices on the use of “low-value” services after the inception of the campaign.14-17
For instance, in a cross-sectional analysis of the 1999 and 2009 samples of ambulatory care practices in the US, only two of 11 overuse quality indicators showed improvement.11 The authors recognized that reducing inappropriate care will require the same attention to guideline development and performance measurement that was directed at reducing the underuse of needed therapies. However, determining whether a patient received inappropriate care generally requires a much more detailed analysis of clinical information than what is required for assessments of underuse.11
Another study designed claims-based algorithms to measure the prevalence of 11 Choosing Wisely-identified “low-value” services in fee-for-service Medicare patients aged >65 years from 2006 to 2011.12 The annual prevalence of selected CWC “low-value” services ranged from 1.2% (upper urinary tract imaging in men with benign prostatic hyperplasia) to 46.5% (preoperative cardiac testing for low-risk, noncardiac procedures). The study concluded that identifying and measuring “low-value” health services is a prerequisite for improving quality and eliminating waste.12
In pediatric medicine, the authors investigated a large cohort of infants aged one to 24 months hospitalized with bronchiolitis to 41 tertiary children’s hospitals reporting data to the PHIS database from 2004 to 2012.13 The trend analysis revealed a decrease in the utilization of diagnostics and treatment interventions before the publication of the American Academy of Pediatrics 2006 Bronchiolitis Guidelines.18 There was an additional reduction in the use of CXR, steroids, and bronchodilators after the publication of the guidelines.13
After the CWC was launched in 2012, several surveys have demonstrated a tangible increase in awareness of the CWC and its goals, mostly among primary care physicians and subspecialists. Clinicians who were aware of the campaign found the recommendations to be useful as a legitimate source of guidance and were more likely to reduce the indication of unnecessary care and “low-value” clinical services included in the CWC.1,3,19,20
Few studies in adults have focused on measuring the trends in overuse metrics derived from the CWC recommendations.14-16 The initial studies have found limited reduction on the use of “low-value” care after the inception of the CWC. They suggest that clinician education, awareness, and public promotion alone do not appear to be sufficient to achieve widespread changes in clinical practice. Additional interventions are necessary for the wider implementation and success of the CWC recommendations.11,14,15,19,21,22
However, a more recent study was conducted in 91 academic centers from 2013 through 2016, before and after the publication of a CWC recommendation on the use of troponin-only testing for the diagnosis of acute myocardial infarction. Hospitals with low rates of troponin-only testing before the publication of the recommendation demonstrated a statistically significant increase over time in the rate of adherence. The authors postulated that the impact of the CWC might have been significant because of the increase in the institutional and provider attention to “high-value” care as a result of the campaign.16
In pediatrics, a cross-sectional study defined 20 “low-value” services from a list of more than 400 items from the CWC and other sources of highly regarded, evidence-based pediatrics healthcare recommendations. The list included six diagnostic tests, five imaging tests, and nine prescription drugs ordered in a robust cohort of 4.4 million children nationwide in 2014. The study concluded that approximately one in 10 children received a “low-value” service. The majority (59.4%) were related to prescription drugs, specifically the inappropriate use of antibiotics for a variety of conditions. The estimated combined cost of these unnecessary services was approximately $27 million, with one-third of the cost being paid out of pocket, arguing for significant financial harm. However, this study did not perform a trend analysis.17
Our results are comparable with these studies, reporting an initial increase in awareness and beliefs, followed by progressive changes in clinical practice among pediatric hospital-based clinicians in delivering evidence-based, high-value care after the CWC.
The attribution of the steady reduction in the absolute percentages of overuse/waste in the five metrics related to the CWC observed in this study, including the significant changes noted in two of the overuse indicators after the publication of the CWC-PHM recommendations, should be interpreted with caution. For example, the significant decrease in the use of “low-value” services in bronchiolitis could be attributed to multiple factors such as national guidelines released in 2014 after the campaign,23 national multicenter QI collaborative projects,24,25 and multiple local QI efforts.26,27 The increase in the awareness and impact of the CWC recommendations among pediatric providers could also be a contributing factor, but this association cannot be established in the light of our findings.
On the other hand, despite extensive evidence for the lack of efficacy and the potential harm associated with the use of acid suppressors for uncomplicated GER in infants,28-30 the frequency of this “low-value” therapeutic intervention remains high (~50%). The trend in utilization was not impacted by the CWC-PHM recommendations. This finding could be explained by several factors, including the possibility that several hospitalized patients may suffer from GER disease requiring acid suppressors. Another possibility is that acid suppressors are generally prescribed as an outpatient medication, and physicians treating inpatients may be reluctant to discontinue it during hospitalization. Nevertheless, this recommendation represents a target for review, update, and QI interventions in the near future.
The delivery of inappropriate “low-value” care represents the most significant dimension of waste in healthcare.31 The development of quality measures of “low-value” services representing overuse and waste is the most needed step toward assessing the magnitude of the problem. Overuse metrics could be incorporated into QI interventions to decrease the provision of such services. However, systematic efforts aimed at developing quality indicators of overuse based on the CWC recommendations have been limited. To our knowledge, this is the first study on the trends of metrics derived from the CWC recommendations in pediatric medicine.
Future research is needed to develop overuse metrics further to assess the specific outcomes related to the implementation of the CWC. How much has clinical practice changed as a result of the campaign? What are the outcomes and savings attributable to these efforts? These are critical questions for the immediate future that should be answered to sustain the ongoing efforts and results and to validate that the efforts are worthwhile.
This study has several limitations. First, this is a retrospective and observational study. It cannot prove a direct causal relationship between the publication of the CWC-PHM and the observed trends, as other potential factors may have contributed to the outcomes. Second, in administrative databases, the data quality is dependent on proper documentation and coding that may vary among reporting institutions. These data lack clinical information, and a fair assessment of “appropriateness” could be questioned. In addition, the study included only 36 academic, tertiary children’s hospitals. Because approximately two-thirds of all pediatric hospitalizations in the US occur in community settings,32 this study may not fully represent clinical practice in the majority of pediatric hospitalizations in the US. Finally, the validity of the ITS analysis has inherent limitations due to the variability of the data in some metrics that may affect the power of the analysis. This fact could lead to inaccurate conclusions regarding intervention effectiveness due to the data-driven model applied, as well as the lack of control for other time-varying confounders.33
CONCLUSIONS
After seven years, the CWC faces important challenges. Critical to the success of the campaign is to “measure wisely” by developing quality indicators of overuse and operationalizing them into administrative and clinical data sources to assess the impact on clinical practice. Our study highlights some limited but steady reduction in the use of some “low-value” services before the campaign. It also demonstrates a modest impact of the campaign on clinical practices in tertiary care children’s hospitals in the US. Clinicians and institutions still have a long way to go in reducing the use of “low-value” interventions in pediatric medicine. These observations challenge us to step up our efforts to implement QI interventions aimed at incorporating these professional, society-endorsed recommendations into our clinical practice.
Acknowledgments
The authors thank Dr. Kristine De La Torre and Dr. Jennifer McCafferty-Fernandez and the Research Institute of Nicklaus Children’s Hospital for medical writing assistance. They also acknowledge Tatiana Consuegra, library technician, for her clerical assistance in the preparation and submission of this article.
The Choosing Wisely® Campaign (CWC) was launched in 2012. This ongoing national initiative encourages conversations among patients and clinicians about the need —or the lack thereof—for frequent tests, treatments, and procedures in healthcare. More than 80 professional societies have developed short lists of evidence-based recommendations aimed at avoiding unnecessary, “low-value” care. More than 550 recommendations are currently available.1 The Society of Hospital Medicine (SHM) Pediatric Committee published a list of five recommendations for the CWC in 2013.2
After seven years, the campaign has posted several success stories highlighting the increase in clinicians’ awareness about the recommendations. Several local, regional, and national initiatives and quality improvement (QI) projects have been inspired by the CWC and its tenants.1,3 However, limited research has been performed on the true impact of these recommendations on avoiding “low-value” services. A more comprehensive approach is required to “measure wisely” the impact of the campaign on bedside clinical practice.4 Stakeholders in healthcare value have been challenged to collaborate in creating high-impact lists of “low-value” interventions and designing effective tools to measure their impact on clinical practice and costs.5
We initially developed a report card with five metrics derived from the CWC-SHM pediatric recommendations to help individual institutions and group practices to measure their performance and benchmark their results with peers.6 The report card is available for hospital members of the Children’s Hospital Association (CHA).7
The current study analyzes the frequency of utilization and trends of five metrics included in the CHA/Pediatric Health Information System® (PHIS) CWC report card in tertiary children’s hospitals in the United States. We analyzed data from five years before and five years after the CWC-PHM recommendations were published in 2013. We hypothesize that the publication and dissemination of the CWC-PHM recommendations—the intervention—will result in either an immediate decrease in the use of the “low-value” services studied and/or a change in the trend of utilization over time.
METHODS
Study Design
We conducted an observational, longitudinal retrospective study aimed at evaluating the impact of the CWC-PHM recommendations on clinical practice in tertiary children’s hospitals in the US.
Study Population
The population included inpatient and observation stays for children aged 0-18 years admitted to the 36 children’s hospitals consistently providing data from 2008 to 2017 to the PHIS administrative database (CHA, Lenexa, Kansas). This database contains inpatient, emergency department, ambulatory, and observation encounter–level data from more than 50 not-for-profit, tertiary care pediatric hospitals and accounts for ~20% of all pediatric hospitalizations in the US every year.
A joint effort between the CHA and the participating hospitals ensures the quality of the data submitted, as previously described.8 These data are subjected to a routine quality check with each submission and within each report. Data were fully deidentified for this study. In total, 36 PHIS hospitals met the strict quality standards for inclusion of submitted data. The remaining hospitals were excluded because they did not have complete data or had incomplete billing information.
For external benchmarking purposes, PHIS participating hospitals provide encounter data, including demographics, diagnoses, and procedures (International Classification of Diseases versions 9 and 10).9,10 The transition from ICD-9 to ICD-10 in the US took place during the study period. However, the CHA completed a process of translating and mapping all ICD-9 codes to every possible equivalent ICD-10 code in the PHIS database. Thus, the change from ICD-9 to ICD-10 should not have had any significant effect on population definition and data analytics, including trend analysis.
For each condition, the study population was divided into the following two cohorts for comparison of the trends: all admissions from January 1, 2008 to December 31, 2012 (before) and all admissions from January 1, 2013 to December 31, 2017 (after) the CWC-PHM recommendations were published.
This study was determined to be nonhuman subject research and was therefore exempted by Nicklaus Children’s Hospital Human Research Protection Program.
Outcomes
The outcomes for this study were the percentages of patients receiving the not-recommended “low-value” services targeted by the CWC-PHM recommendations. For this purpose, four of the five recommendations were translated into the following five metrics, operationalized in the PHIS database and displayed in the “Choosing Wisely” report card:6
1. Percentage of patients with uncomplicated asthma receiving chest radiograph (CXR).
2. Percentage of patients with uncomplicated bronchiolitis receiving CXR.
3. Percentage of patients with uncomplicated bronchiolitis receiving bronchodilators.
4. Percentage of patients with lower respiratory tract infection (LRTI) receiving systemic corticosteroids (relievers).
5. Percentage of patients with uncomplicated gastroesophageal reflux (GER) receiving acid suppressor therapy.
The fifth recommendation—limiting the use of continuous pulse oximetry unless the patient is receiving supplemental oxygen—could not be operationalized in the PHIS database because of inconsistent reporting of these resources.6
The resulting percentages represent nonadherence to the recommendations, suggesting overuse of the specific “low-value” intervention. As such, a decreasing trend over time is the desired direction of improvement.
The definition of “uncomplicated” conditions and the metrics are presented in Table 1. A complete list of the inclusion and exclusion criteria to define “uncomplicated” conditions and the complete list of the clinical translation codes used in PHIS to identify the “low-value” services are presented as an electronic supplement.
Statistical Analyses
We compared the demographic and clinical characteristics of the various cohorts before and after the release of the CWC-PHM recommendations—the intervention—using chi-square statistics. To assess the individual hospital-level trends over time for each measure, we modeled the patient-level data of each hospital using generalized linear mixed effects models with a binomial distribution. These models were adjusted for patient demographic and clinical factors that were found to be significantly different (P < .01) before and after the intervention on bivariate analyses. From these models, we generated adjusted estimates for the quarterly percentages for each hospital. We then conducted an interrupted time series (ITS) using these estimates to compare trends in the five years before (2008-2012) and five years after (2013-2017) the publication of the CWC-PHM recommendations. For the ITS analysis, we used a generalized linear mixed effects model with the quarterly adjusted hospital-level utilization rates of “low-value” services for each cohort as the unit of analysis and a random intercept for each hospital. The model used an autoregressive(1) covariance structure to account for autocorrelation. The ITS allowed us to test our hypothesis by assessing the following two important features: (a) if a significant decrease occurred right after the CWC-PHM recommendations were published (level-change) and/or (b) if the intervention altered the secular trend (slope-change). All statistical analyses were performed using SAS v. 9.4 (SAS Institute, Cary, North Carolina), and P values <.01 were considered to be statistically significant.
RESULTS
Table 2 presents the demographic characteristics of the cohorts before (2008-2012) and after (2013-2017) the publication of the CWC-PHM recommendations. Hospitalizations due to asthma represented the largest cohort with 142,067 cases, followed by hospitalizations due to bronchiolitis with 94,253 cases. Hospitalizations due to GER comprised the smallest cohort with 13,635 cases. Most of the children had government insurance and had “minor” severity according to the All Patient Revised Diagnosis Related Group (APR-DRG) system.
We found statistically significant differences in most of the demographic characteristics for the cohorts when comparing cases before and after the introduction of the CWC-PHM recommendations.
After adjusting for demographic characteristics, we estimated the percentages of the utilization of the “low-value” services from 2008 to 2017. We observed a steady decrease in overutilization of all services over time. The absolute percentage decrease was more evident in the reduction of the utilization of relievers by 36.6% and that of CXR by 31.5% for bronchiolitis. We also observed a 20.8% absolute reduction in the use of CXR for asthma.
The use of systemic steroids in LRTI revealed the lowest utilization among the “low-value” services studied, with 15.1% in 2008 and 12.2% in 2017, a 2.9% absolute reduction. However, the prescription of acid suppressors for GER showed the highest utilization among all the overuse metrics studied, ie, 63% in 2008 and 48.9% in 2017, with an absolute decrease of 24.1%. The yearly adjusted estimated percentages of utilization for each “low-value” service are presented in Appendix Table A.
Table 3 and the Figure (attached as supplemental online graphic) respectively present the risk-adjusted ITS parameter estimates and the graphic representation before and after the inception of the CWC-PHM recommendations for the trend analysis.
During the five years preceding the intervention (2008-2012), a statistically significant decrease (P < .01) was already noted in the trend of utilization of relievers and CXR in bronchiolitis and CXR in asthma. However, we found no significant change in the trend of the use of systemic corticosteroids in cases with LRTI or the use of acid suppression therapy for GER.
The immediate effect of the intervention is represented by the level change. We found a statistically significant (P < .01) reduction according to the CWC-PHM recommendations only for the use of CXR in hospitalized children with uncomplicated asthma.
During the five years after the CWC-PHM recommendations were published (2013-2017), a sustained, significant decrease in the trend of the use of CXR in asthma and bronchiolitis and the use of relievers in bronchiolitis (P < .01) was observed. However, there was no significant change in the trend of the use of systemic corticosteroids in cases with LRTI or in the use of acid suppression therapy for GER during this period.
Comparison of the trends before and after the publication of the CWC-PHM recommendations revealed that only the decreasing trend in the use of relievers for bronchiolitis over time significantly correlated with the campaign (P < .01).
DISCUSSION
We found a steady reduction in the frequency of overutilization of five “low-value” services described in the CWC-PHM recommendations from 2008 to 2017 in 36 tertiary children’s hospitals in the US. This trend was more evident in the utilization of relievers and CXR for bronchiolitis. The ITS analysis demonstrated that immediately after the publication of the CWC-PHM recommendations, only the use of CXR for asthma decreased significantly. Then, only the use of relievers for bronchiolitis decreased significantly over time in comparison with the secular trend.
These results support our hypothesis for two of the five metrics studied, suggesting that the publication of the CWC-PHM recommendations had a modest impact in clinical practices related to those services in tertiary children’s hospitals.
These findings align with a limited number of published studies that have consistently found a modest decrease in the use of “low-value” services before 201211-13 and a limited impact of the CWC in clinical practices on the use of “low-value” services after the inception of the campaign.14-17
For instance, in a cross-sectional analysis of the 1999 and 2009 samples of ambulatory care practices in the US, only two of 11 overuse quality indicators showed improvement.11 The authors recognized that reducing inappropriate care will require the same attention to guideline development and performance measurement that was directed at reducing the underuse of needed therapies. However, determining whether a patient received inappropriate care generally requires a much more detailed analysis of clinical information than what is required for assessments of underuse.11
Another study designed claims-based algorithms to measure the prevalence of 11 Choosing Wisely-identified “low-value” services in fee-for-service Medicare patients aged >65 years from 2006 to 2011.12 The annual prevalence of selected CWC “low-value” services ranged from 1.2% (upper urinary tract imaging in men with benign prostatic hyperplasia) to 46.5% (preoperative cardiac testing for low-risk, noncardiac procedures). The study concluded that identifying and measuring “low-value” health services is a prerequisite for improving quality and eliminating waste.12
In pediatric medicine, the authors investigated a large cohort of infants aged one to 24 months hospitalized with bronchiolitis to 41 tertiary children’s hospitals reporting data to the PHIS database from 2004 to 2012.13 The trend analysis revealed a decrease in the utilization of diagnostics and treatment interventions before the publication of the American Academy of Pediatrics 2006 Bronchiolitis Guidelines.18 There was an additional reduction in the use of CXR, steroids, and bronchodilators after the publication of the guidelines.13
After the CWC was launched in 2012, several surveys have demonstrated a tangible increase in awareness of the CWC and its goals, mostly among primary care physicians and subspecialists. Clinicians who were aware of the campaign found the recommendations to be useful as a legitimate source of guidance and were more likely to reduce the indication of unnecessary care and “low-value” clinical services included in the CWC.1,3,19,20
Few studies in adults have focused on measuring the trends in overuse metrics derived from the CWC recommendations.14-16 The initial studies have found limited reduction on the use of “low-value” care after the inception of the CWC. They suggest that clinician education, awareness, and public promotion alone do not appear to be sufficient to achieve widespread changes in clinical practice. Additional interventions are necessary for the wider implementation and success of the CWC recommendations.11,14,15,19,21,22
However, a more recent study was conducted in 91 academic centers from 2013 through 2016, before and after the publication of a CWC recommendation on the use of troponin-only testing for the diagnosis of acute myocardial infarction. Hospitals with low rates of troponin-only testing before the publication of the recommendation demonstrated a statistically significant increase over time in the rate of adherence. The authors postulated that the impact of the CWC might have been significant because of the increase in the institutional and provider attention to “high-value” care as a result of the campaign.16
In pediatrics, a cross-sectional study defined 20 “low-value” services from a list of more than 400 items from the CWC and other sources of highly regarded, evidence-based pediatrics healthcare recommendations. The list included six diagnostic tests, five imaging tests, and nine prescription drugs ordered in a robust cohort of 4.4 million children nationwide in 2014. The study concluded that approximately one in 10 children received a “low-value” service. The majority (59.4%) were related to prescription drugs, specifically the inappropriate use of antibiotics for a variety of conditions. The estimated combined cost of these unnecessary services was approximately $27 million, with one-third of the cost being paid out of pocket, arguing for significant financial harm. However, this study did not perform a trend analysis.17
Our results are comparable with these studies, reporting an initial increase in awareness and beliefs, followed by progressive changes in clinical practice among pediatric hospital-based clinicians in delivering evidence-based, high-value care after the CWC.
The attribution of the steady reduction in the absolute percentages of overuse/waste in the five metrics related to the CWC observed in this study, including the significant changes noted in two of the overuse indicators after the publication of the CWC-PHM recommendations, should be interpreted with caution. For example, the significant decrease in the use of “low-value” services in bronchiolitis could be attributed to multiple factors such as national guidelines released in 2014 after the campaign,23 national multicenter QI collaborative projects,24,25 and multiple local QI efforts.26,27 The increase in the awareness and impact of the CWC recommendations among pediatric providers could also be a contributing factor, but this association cannot be established in the light of our findings.
On the other hand, despite extensive evidence for the lack of efficacy and the potential harm associated with the use of acid suppressors for uncomplicated GER in infants,28-30 the frequency of this “low-value” therapeutic intervention remains high (~50%). The trend in utilization was not impacted by the CWC-PHM recommendations. This finding could be explained by several factors, including the possibility that several hospitalized patients may suffer from GER disease requiring acid suppressors. Another possibility is that acid suppressors are generally prescribed as an outpatient medication, and physicians treating inpatients may be reluctant to discontinue it during hospitalization. Nevertheless, this recommendation represents a target for review, update, and QI interventions in the near future.
The delivery of inappropriate “low-value” care represents the most significant dimension of waste in healthcare.31 The development of quality measures of “low-value” services representing overuse and waste is the most needed step toward assessing the magnitude of the problem. Overuse metrics could be incorporated into QI interventions to decrease the provision of such services. However, systematic efforts aimed at developing quality indicators of overuse based on the CWC recommendations have been limited. To our knowledge, this is the first study on the trends of metrics derived from the CWC recommendations in pediatric medicine.
Future research is needed to develop overuse metrics further to assess the specific outcomes related to the implementation of the CWC. How much has clinical practice changed as a result of the campaign? What are the outcomes and savings attributable to these efforts? These are critical questions for the immediate future that should be answered to sustain the ongoing efforts and results and to validate that the efforts are worthwhile.
This study has several limitations. First, this is a retrospective and observational study. It cannot prove a direct causal relationship between the publication of the CWC-PHM and the observed trends, as other potential factors may have contributed to the outcomes. Second, in administrative databases, the data quality is dependent on proper documentation and coding that may vary among reporting institutions. These data lack clinical information, and a fair assessment of “appropriateness” could be questioned. In addition, the study included only 36 academic, tertiary children’s hospitals. Because approximately two-thirds of all pediatric hospitalizations in the US occur in community settings,32 this study may not fully represent clinical practice in the majority of pediatric hospitalizations in the US. Finally, the validity of the ITS analysis has inherent limitations due to the variability of the data in some metrics that may affect the power of the analysis. This fact could lead to inaccurate conclusions regarding intervention effectiveness due to the data-driven model applied, as well as the lack of control for other time-varying confounders.33
CONCLUSIONS
After seven years, the CWC faces important challenges. Critical to the success of the campaign is to “measure wisely” by developing quality indicators of overuse and operationalizing them into administrative and clinical data sources to assess the impact on clinical practice. Our study highlights some limited but steady reduction in the use of some “low-value” services before the campaign. It also demonstrates a modest impact of the campaign on clinical practices in tertiary care children’s hospitals in the US. Clinicians and institutions still have a long way to go in reducing the use of “low-value” interventions in pediatric medicine. These observations challenge us to step up our efforts to implement QI interventions aimed at incorporating these professional, society-endorsed recommendations into our clinical practice.
Acknowledgments
The authors thank Dr. Kristine De La Torre and Dr. Jennifer McCafferty-Fernandez and the Research Institute of Nicklaus Children’s Hospital for medical writing assistance. They also acknowledge Tatiana Consuegra, library technician, for her clerical assistance in the preparation and submission of this article.
1. Choosing Wisely. Choosing Wisely Campaign Official Site. http://www.choosingwisely.org/. Accessed May 2019.
2. Quinonez RA, Garber MD, Schroeder AR, et al. Choosing wisely in pediatric hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):479-485. https://doi.org/10.1002/jhm.2064.
3. ABIM Foundation CR. Choosing Wisely: A Special Report on the First Five Years. http://www.choosingwisely.org/choosing-wisely-a-special-report-on-the-first-five-years/. Updated 2017. Accessed May 2019.
4. Wolfson D, Santa J, Slass L. Engaging physicians and consumers in conversations about treatment overuse and waste: a short history of the choosing wisely campaign. Acad Med. 2014;89(7):990-995. https://doi.org/10.1097/ACM.0000000000000270.
5. Morden NE, Colla CH, Sequist TD, Rosenthal MB. Choosing wisely—the politics and economics of labeling low-value services. N Engl J Med. 2014;370(7):589-592. https://doi.org/10.1056/NEJMp1314965.
6. Reyes M, Paulus E, Hronek C, et al. Choosing wisely campaign: Report card and achievable benchmarks of care for children’s hospitals. Hosp Pediatr. 2017;7(11):633-641. https://doi.org/10.1542/hpeds.2017-0029.
7. Report Cards. Choosing Wisely Measures - Pediatric Hospital Medicine Detail Reports. Children’s Hospital Association Web site. https://www.childrenshospitals.org/. Accessed May 2019.
8. Mongelluzzo J, Mohamad Z, Ten Have TR, Shah SS. Corticosteroids and mortality in children with bacterial meningitis. JAMA. 2008;299(17):2048-2055. https://doi.org/10.1001/jama.299.17.2048.
9. Buck CJ. 2013 ICD 9 CM for Physicians, Volumes 1 & 2. Chicago, IL: American Medical Association; 2013.
10. Buck CJ. 2018 ICD-10-CM for Physicians. Chicago, IL: American Medical Association; 2018.
11. Kale MS, Bishop TF, Federman AD, Keyhani S. Trends in the overuse of ambulatory health care services in the United States. JAMA Inter Med. 2013;173(2):142-148. https://doi.org/10.1001/2013.jamainternmed.1022.
12. Colla CH, Morden NE, Sequist TD, Schpero WL, Rosenthal MB. Choosing wisely: Prevalence and correlates of low-value health care services in the United States. J Gen Intern Med. 2015;30(2):221-228. https://doi.org/10.1007/s11606-014-3070-z
13. Parikh K, Hall M, Teach SJ. Bronchiolitis management before and after the AAP guidelines. Pediatrics. 2014;133(1): e1-7. https://doi.org/10.1542/peds.2013-2005.
14. Rosenberg A, Agiro A, Gottlieb M, et al. Early trends among seven recommendations from the Choosing Wisely campaign. JAMA Inter Med. 2015;175(12):1913-1920. https://doi.org/10.1001/jamainternmed.2015.5441.
15. Reid RO, Rabideau B, Sood N. Low-value health care services in a commercially insured population. JAMA Inter Med. 2016;176(10):1567-1571. https://doi.org/10.1001/jamainternmed.2016.5031.
16. Prochaska MT, Hohmann SF, Modes M, Arora VM. Trends in troponin-only testing for AMI in academic teaching hospitals and the impact of choosing wisely(R). J Hosp Med. 2017;12(12):957-962. https://doi.org/10.12788/jhm.2846.
17. Chua KP, Schwartz AL, Volerman A, Conti RM, Huang ES. Use of low-value pediatric services among the commercially insured. Pediatrics. 2016;138(6):e20161809. https://doi.org/10.1542/peds.2016-1809.
18. American Academy of Pediatrics Subcommittee on Diagnosis and Management of Bronchiolitis. Diagnosis and management of bronchiolitis. Pediatrics. 2006;118(4):1774-1793.
19. Colla CH, Kinsella EA, Morden NE, Meyers DJ, Rosenthal MB, Sequist TD. Physician perceptions of Choosing Wisely and drivers of overuse. Am J Manag Care. 2016;22(5):337-343.
20. PerryUndem Research/Communication AF. DataBrief: Findings from a National Survey of Physicians. http://www.choosingwisely.org/wp-content/uploads/2017/10/Summary-Research-Report-Survey-2017.pdf. Updated 2017.
21. Wolfson D. Choosing wisely recommendations using administrative claims data. JAMA Inter Med. 2016;176(4):565. https://doi.org/10.1001/jamainternmed.2016.0357.
22. Heekin AM, Kontor J, Sax HC, Keller M, Wellington A, Weingarten S. Choosing wisely clinical decision support adherence and associated patient outcomes. Am J Manag Care. 2018;24(8):361-366.
23. Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134(5):e1474-e502. https://doi.org/10.1542/peds.2014-2742.
24. Ralston SL, Garber MD, Rice-Conboy E, et al. A multicenter collaborative to reduce unnecessary care in inpatient bronchiolitis. Pediatrics. 2016;137(1):e20150851. https://doi.org/10.1542/peds.2015-0851.
25. Mussman GM, Lossius M, Wasif F, et al. Multisite emergency department inpatient collaborative to reduce unnecessary bronchiolitis care. Pediatrics. 2018;141(2):e20170830. https://doi.org/10.1542/peds.2017-0830.
26. Mittal V, Hall M, Morse R, et al. Impact of inpatient bronchiolitis clinical practice guideline implementation on testing and treatment. J Pediatr. 2014;165(3):570-576. https://doi.org/10.1016/j.jpeds.2014.05.021.
27. Tyler A, Krack P, Bakel LA, et al. Interventions to reduce over-utilized tests and treatments in bronchiolitis. Pediatrics. 2018;141(6):e20170485. https://doi.org/10.1542/peds.2017-0485.
28. Rosen R, Vandenplas Y, Singendonk M, et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2018;66(3):516-554. https://doi.org/10.1097/MPG.0b013e3181b7f563.
29. Eichenwald EC, COMMITTEE ON FETUS AND NEWBORN. Diagnosis and management of gastroesophageal reflux in preterm infants. Pediatrics. 2018;142(1):e20181061. https://doi.org/10.1542/peds.2018-1061
30. van der Pol RJ, Smits MJ, van Wijk MP, Omari TI, Tabbers MM, Benninga MA. Efficacy of proton-pump inhibitors in children with gastroesophageal reflux disease: a systematic review. Pediatrics. 2011;127(5):925-935. https://doi.org/10.1542/peds.2010-2719.
31. IOM Report: Estimated $750B Wasted Annually In Health Care System. Kaiser Health News Web site. https://khn.org/morning-breakout/iom-report/. Updated 2012. Accessed May 2019.
32. Leyenaar JK, Ralston SL, Shieh M, Pekow PS, Mangione‐Smith R, Lindenauer PK. Epidemiology of pediatric hospitalizations at general hospitals and freestanding children’s hospitals in the United States. J Hosp Med. 2016;11(11):743-749. https://doi.org/10.1002/jhm.2624.
33. Bernal JL, Cummins S, Gasparrini A. Interrupted time series regression for the evaluation of public health interventions: a tutorial. Int J Epidemiol. 2017;46(1):348-355. https://doi.org/10.1093/ije/dyw098.
1. Choosing Wisely. Choosing Wisely Campaign Official Site. http://www.choosingwisely.org/. Accessed May 2019.
2. Quinonez RA, Garber MD, Schroeder AR, et al. Choosing wisely in pediatric hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):479-485. https://doi.org/10.1002/jhm.2064.
3. ABIM Foundation CR. Choosing Wisely: A Special Report on the First Five Years. http://www.choosingwisely.org/choosing-wisely-a-special-report-on-the-first-five-years/. Updated 2017. Accessed May 2019.
4. Wolfson D, Santa J, Slass L. Engaging physicians and consumers in conversations about treatment overuse and waste: a short history of the choosing wisely campaign. Acad Med. 2014;89(7):990-995. https://doi.org/10.1097/ACM.0000000000000270.
5. Morden NE, Colla CH, Sequist TD, Rosenthal MB. Choosing wisely—the politics and economics of labeling low-value services. N Engl J Med. 2014;370(7):589-592. https://doi.org/10.1056/NEJMp1314965.
6. Reyes M, Paulus E, Hronek C, et al. Choosing wisely campaign: Report card and achievable benchmarks of care for children’s hospitals. Hosp Pediatr. 2017;7(11):633-641. https://doi.org/10.1542/hpeds.2017-0029.
7. Report Cards. Choosing Wisely Measures - Pediatric Hospital Medicine Detail Reports. Children’s Hospital Association Web site. https://www.childrenshospitals.org/. Accessed May 2019.
8. Mongelluzzo J, Mohamad Z, Ten Have TR, Shah SS. Corticosteroids and mortality in children with bacterial meningitis. JAMA. 2008;299(17):2048-2055. https://doi.org/10.1001/jama.299.17.2048.
9. Buck CJ. 2013 ICD 9 CM for Physicians, Volumes 1 & 2. Chicago, IL: American Medical Association; 2013.
10. Buck CJ. 2018 ICD-10-CM for Physicians. Chicago, IL: American Medical Association; 2018.
11. Kale MS, Bishop TF, Federman AD, Keyhani S. Trends in the overuse of ambulatory health care services in the United States. JAMA Inter Med. 2013;173(2):142-148. https://doi.org/10.1001/2013.jamainternmed.1022.
12. Colla CH, Morden NE, Sequist TD, Schpero WL, Rosenthal MB. Choosing wisely: Prevalence and correlates of low-value health care services in the United States. J Gen Intern Med. 2015;30(2):221-228. https://doi.org/10.1007/s11606-014-3070-z
13. Parikh K, Hall M, Teach SJ. Bronchiolitis management before and after the AAP guidelines. Pediatrics. 2014;133(1): e1-7. https://doi.org/10.1542/peds.2013-2005.
14. Rosenberg A, Agiro A, Gottlieb M, et al. Early trends among seven recommendations from the Choosing Wisely campaign. JAMA Inter Med. 2015;175(12):1913-1920. https://doi.org/10.1001/jamainternmed.2015.5441.
15. Reid RO, Rabideau B, Sood N. Low-value health care services in a commercially insured population. JAMA Inter Med. 2016;176(10):1567-1571. https://doi.org/10.1001/jamainternmed.2016.5031.
16. Prochaska MT, Hohmann SF, Modes M, Arora VM. Trends in troponin-only testing for AMI in academic teaching hospitals and the impact of choosing wisely(R). J Hosp Med. 2017;12(12):957-962. https://doi.org/10.12788/jhm.2846.
17. Chua KP, Schwartz AL, Volerman A, Conti RM, Huang ES. Use of low-value pediatric services among the commercially insured. Pediatrics. 2016;138(6):e20161809. https://doi.org/10.1542/peds.2016-1809.
18. American Academy of Pediatrics Subcommittee on Diagnosis and Management of Bronchiolitis. Diagnosis and management of bronchiolitis. Pediatrics. 2006;118(4):1774-1793.
19. Colla CH, Kinsella EA, Morden NE, Meyers DJ, Rosenthal MB, Sequist TD. Physician perceptions of Choosing Wisely and drivers of overuse. Am J Manag Care. 2016;22(5):337-343.
20. PerryUndem Research/Communication AF. DataBrief: Findings from a National Survey of Physicians. http://www.choosingwisely.org/wp-content/uploads/2017/10/Summary-Research-Report-Survey-2017.pdf. Updated 2017.
21. Wolfson D. Choosing wisely recommendations using administrative claims data. JAMA Inter Med. 2016;176(4):565. https://doi.org/10.1001/jamainternmed.2016.0357.
22. Heekin AM, Kontor J, Sax HC, Keller M, Wellington A, Weingarten S. Choosing wisely clinical decision support adherence and associated patient outcomes. Am J Manag Care. 2018;24(8):361-366.
23. Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134(5):e1474-e502. https://doi.org/10.1542/peds.2014-2742.
24. Ralston SL, Garber MD, Rice-Conboy E, et al. A multicenter collaborative to reduce unnecessary care in inpatient bronchiolitis. Pediatrics. 2016;137(1):e20150851. https://doi.org/10.1542/peds.2015-0851.
25. Mussman GM, Lossius M, Wasif F, et al. Multisite emergency department inpatient collaborative to reduce unnecessary bronchiolitis care. Pediatrics. 2018;141(2):e20170830. https://doi.org/10.1542/peds.2017-0830.
26. Mittal V, Hall M, Morse R, et al. Impact of inpatient bronchiolitis clinical practice guideline implementation on testing and treatment. J Pediatr. 2014;165(3):570-576. https://doi.org/10.1016/j.jpeds.2014.05.021.
27. Tyler A, Krack P, Bakel LA, et al. Interventions to reduce over-utilized tests and treatments in bronchiolitis. Pediatrics. 2018;141(6):e20170485. https://doi.org/10.1542/peds.2017-0485.
28. Rosen R, Vandenplas Y, Singendonk M, et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2018;66(3):516-554. https://doi.org/10.1097/MPG.0b013e3181b7f563.
29. Eichenwald EC, COMMITTEE ON FETUS AND NEWBORN. Diagnosis and management of gastroesophageal reflux in preterm infants. Pediatrics. 2018;142(1):e20181061. https://doi.org/10.1542/peds.2018-1061
30. van der Pol RJ, Smits MJ, van Wijk MP, Omari TI, Tabbers MM, Benninga MA. Efficacy of proton-pump inhibitors in children with gastroesophageal reflux disease: a systematic review. Pediatrics. 2011;127(5):925-935. https://doi.org/10.1542/peds.2010-2719.
31. IOM Report: Estimated $750B Wasted Annually In Health Care System. Kaiser Health News Web site. https://khn.org/morning-breakout/iom-report/. Updated 2012. Accessed May 2019.
32. Leyenaar JK, Ralston SL, Shieh M, Pekow PS, Mangione‐Smith R, Lindenauer PK. Epidemiology of pediatric hospitalizations at general hospitals and freestanding children’s hospitals in the United States. J Hosp Med. 2016;11(11):743-749. https://doi.org/10.1002/jhm.2624.
33. Bernal JL, Cummins S, Gasparrini A. Interrupted time series regression for the evaluation of public health interventions: a tutorial. Int J Epidemiol. 2017;46(1):348-355. https://doi.org/10.1093/ije/dyw098.
© 2019 Society of Hospital Medicine
Implementing Pediatric Asthma Pathways in Community Hospitals: A National Qualitative Study
Despite the widespread availability of evidence-based guidelines,1 there is inappropriate variation in the care and outcomes for children with asthma in both the emergency department (ED) and the inpatient setting.2-6 Operational versions of evidence-based guidelines known as “pathways” have been shown to improve adoption of evidence-based guidelines, quality of care, and health outcomes for children with asthma.7-14 However, little is known about how to successfully implement pathways outside of free-standing children’s hospitals.15-19
The majority of children with asthma in the United States are cared for in community hospitals, which provide services for both adults and children.20 However, prior studies of pediatric asthma pathways have largely excluded community hospitals. These studies primarily focused on determining clinical effectiveness, rather than detailing the implementation process. These approaches have left critical gaps that hinder our ability to implement pathways and improve care in community hospitals, which have unique barriers and less resources.21,22 Therefore, understanding the process of pathway implementation in community hospitals is critical to improving care for children.22 Our objective was to identify the key determinants of successful pediatric asthma pathway implementation using a national sample of community hospitals. This knowledge can guide hospital leaders and healthcare providers in efforts to improve pediatric care and outcomes in these settings.
METHODS
Study Setting, Design, and Population
In Fall 2017, the Value in Inpatient Pediatrics (VIP) network launched PIPA, Pathways to Improving Pediatric Asthma care.23 The VIP network, a part of the American Academy of Pediatrics (AAP), aims to improve the value of care delivered to any pediatric patient in a hospital bed, from rural to free-standing children’s hospitals.24 PIPA used a learning collaborative model25 and recruited local project leaders (physicians, nurses, respiratory therapists (RT), and pharmacists) from 89 hospitals around the country. PIPA provided these hospital teams with asthma pathways and several resources for implementation support, including educational meetings, quality improvement (QI) training, audit and feedback, and facilitation. Facilitation is a process of interactive problem-solving and support that occurs in the context of a supportive interpersonal relationship and a recognized need for improvement.26 A facilitator, or a “coach”, is an external expert who provides project mentorship and assists the process of making meaningful changes to improve patient care.26 Facilitation was provided by external consultants with QI expertise.
For this qualitative study, facilitators conducted semi-structured interviews with a convenience sample of project leaders from community hospitals participating in PIPA, with some interviews including multiple project leaders (eg, nursing, inpatient, and Emergency Department [ED] leaders). Verbal consent was obtained from all participants. No incentives were provided. This study was approved by the AAP institutional review board.
Data Collection
We used the constructs described in the Consolidated Framework for Implementation Research (CFIR)27 and adapted those salient to pediatric asthma pathways to develop an interview guide that was used with all participants (Appendix 1). The CFIR offers an overarching typology to understand what works where and why across five major domains that influence implementation: intervention characteristics, inner setting (hospital), outer setting (economic, political, and social context of the hospital), characteristics of the individuals involved, and the process of implementation. Data were collected across these domains to inform our analysis of the key determinants of pediatric asthma pathway implementation in community hospitals.
Interviews were conducted by phone from December 2017 to April 2018 (first four months of pathway implementation). Interviews lasted 30-60 minutes and were recorded and transcribed verbatim. Transcripts were edited for accuracy using the audio recordings. As data collection occurred concurrently with analysis, the interview guide was iteratively revised to reflect new insights and patterns that emerged from our analysis. All sites were anonymized in the data analysis. New interviews were coded until thematic saturation was reached.
Analysis
We conducted an inductive thematic analysis using the CFIR as our conceptual framework.28,29 Four investigators (CM, MJ, ES, and SK) performed the initial open coding of the data. Investigators met twice during the open coding process to develop and then finalize a codebook of standard definitions for codes. This codebook facilitated coding consistency through the remainder of the analytic process. Two investigators (CM and MJ) then independently read and coded all data to ensure intercoder reliability. During this process, CM and MJ met every two weeks to compare coding consistency, resolve discrepancies, and discuss preliminary findings. When the coding was complete, all investigators met to explore and develop themes that encompassed related common codes.
The CFIR was used at two stages of the study: (1) developing the interview guide and (2) cross-checking for any potentially important codes that were missing/needed to be explored further. Thus, the investigators maintained an inductive approach grounded in the data. To assure study rigor, we employed investigator triangulation (use of multiple investigators and participants from multiple clinical roles) and reflexivity (ongoing critique and critical reflection of the individual biases of the investigators).30 Coding was performed using Dedoose (version 7.0.23; Los Angeles, California).
RESULTS
A total of 34 community hospitals completed the PIPA project, of which the project leaders of 25 hospitals connected with the facilitators and were approached to participate; 18 (72%) hospitals’ project leaders participated in the study. We analyzed 18 interviews conducted between facilitators and project leaders, which included a total of 32 project leaders (one to five leaders per interview). The hospitals represented were diverse in geographic location and size (range 4-50 pediatric beds per hospital), and the majority of sites (78%) supported the trainees (Table 1).
We identified four overarching themes that described the key determinants of pathway implementation in community hospitals. These themes are presented in order of their frequency of occurrence in the data. They included (1) building an implementation infrastructure, (2) engaging and motivating providers, (3) addressing organizational and resource limitations, and (4) devising implementation solutions with facilitators. Descriptions and exemplary quotations for each theme are provided in Table 2 and Appendix Figure 1.
Building an Implementation Infrastructure
“So, I’m going to sit down with the primary nursing staff and the other four physicians in the group to go over the expectations…We’re not going to have the actual EMR [changes] and we’re not going to have the nursing documentation field built right away but [we want to] make sure that people are documenting the respiratory score in their generic nursing note so that the information is easily accessible.” (Physician leader, Hospital G)
Participants also described the need to deliver education on the evidence supporting changes in practice and skills training specific to pediatric asthma care:
“Once we realized that we were going to be doing this pathway, we started training our nurses on the inpatient side on [pediatric respiratory scoring].” (Nursing leader, Hospital P)
In addition, pathway implementation required modification of clinical workflows via changes to hospital policies or guidelines, electronic medical records (EMR), and/or the physical environment (eg, placing supplies in proximity to care delivery):
“I think it can help if we could get an order set or a nursing protocol where asthmatics over a certain severity can just get steroids in triage.” (Physician leader, Hospital A)
Engaging and Motivating Providers
Another crucial step in pathway implementation was engaging and motivating providers. This included overcoming inertia to practice change, facilitating multidisciplinary collaboration, and handling conflicts regarding practice changes. Participants discussed the excitement of participating in a national collaborative as especially motivating to help drive engagement and overcome barriers to change, particularly the ability to compare local hospital performance to national peers.
“I think everyone is a little competitive. So I think that when we see how we compare to other institutions—both our group and the ER—I think it also adds a little oomph…I think for our nurses too; we’re able to say, ‘[look how we compare to] most of the other hospitals.’ I think that helps.” (Physician leader, Hospital B)
Multidisciplinary collaboration across a wide variety of frontline pediatric and nonpediatric providers was key to understanding current workflows and identifying needed modifications for pathway implementation:
“I do think clearly our biggest obstacles are the fact that we have adult ED providers. We have the opportunity on the inpatient side [with nursing and respiratory therapy], who really do awesome with pediatric changes, to take our wins where we can and make the changes with the ED. In the ED we have an RN educator. She’s very on board with doing the respiratory scoring and getting this whole thing started.” (Physician leader, Hospital L)
Intentional communication and leadership skills also played key roles in engaging hesitant providers and handling conflict:
“Just sitting and talking with our respiratory therapist about the ability to provide this type of service or support and seeing what their reservations have been, at least it’s open to conversation so that we could provide these types of therapies in the future and we’re able to see like what people’s concerns are. I think just basically increasing familiarity with not only these processes, but different types of therapy will hopefully in the future help us provide better care to our patients.” (Physician leader, Hospital Q)
Addressing Organizational and Resource Limitations
Participants recognized organizational and resource limitations, some of which may be unique to community hospitals that prioritize resources for adult care. The limitations described included EMR staff support, healthcare provider staffing/capacity, navigating IRBs, and addressing administrative processes. Competing demands for information technology staff support and lack of prioritization of pediatric-specific initiatives often hindered efforts to modify the EMR.
“Resource wise, we are hoping to implement an order set in our Epic EMR, [but] finding the availability from the Epic team may be a challenge.” (Physician Leader, Hospital A)
Participants also reported that limited staff capacity (eg, nursing, RT) hindered pathway implementation efforts. This limited capacity hindered workflow changes and limited the time available for education and training on pathways:
“[Respiratory scoring for asthma is] an added responsibility for the [nursing] staff and we don’t have patient technicians. So they’re doing everything from changing the sheets to bringing water to all of the medical patients. So, that I think may be a barrier.” (Physician leader, Hospital B)
Across sites, navigating the IRB posed various challenges. Some sites were required to obtain approval from regional IRBs, which did not have resources to devote to pediatric projects. Other sites did not have IRBs at all, but instead required separate approvals for the project from hospital leadership or other entities:
“On the IRB, I contacted the manager of the IRB and she’s said, ‘No, it’s not an IRB project,’ but she sent it to another director for review, and it took forever to be able to get a data agreement with [the local university hospital] so that we can pull the data. I just couldn’t believe it took months to get done.” (Physician Leader, Hospital K)
Finally, administrative barriers such as addressing formulary changes in the context of adult-focused settings were challenging. For example, at one hospital, metered dose inhalers (MDIs) were not used for adult patients, and the hospital administration was resistant to incorporate their use into practice for pediatric patients due to the cost of such changes.
“The [general hospital] didn’t have MDI’s anymore because of cost reasons, and when we started the pediatric work, we really made it a point to get the MDI’s for pediatric patients back in the formulary.” (Physician leader, Hospital A)
Devising Implementation Solutions with Practice Facilitators
Participants often devised pathway implementation solutions with facilitators in-the-moment during meetings. This problem-solving included figuring out work-arounds, proactive coaching by external facilitators, and just-in-time solution building. Furthermore, in meetings that included more than one project leader, leaders would often work with each other to devise solutions. Meetings provided forums that stimulated identification of implementation barriers, brainstorming, and subsequently solution building.
Physician leader: I’m wondering if we could, as an interim solution, try out an algorithm on paper, I don’t know if that’s allowed, until we get Epic approval. Do you know?
Nurse Leader: You mean having an algorithm posted in triage? Yeah, I don’t see why not. (Hospital A)
Next, problem solving was often driven by the facilitator’s experience and knowledge, drawn from their interactions with other collaborative sites or their own prior experiences with asthma, QI, or pathway implementation. The facilitators brought an outside perspective, not bound by that particular hospital’s local culture or structural intricacies. This proactive coaching spurred the identification of creative, yet practical solutions:
Project Leader: We’re still trying to get all our templates [for the EMR]…because [currently they are] all adult templates.
Facilitator: If you’re making templates right now, could you also add the three asterisks? Like smoking or exposure to second hand tobacco smoke or marijuana…then have the three asterisks there and then “Referral made?***”. That would force people to document in a certain place in the template as well.Project Leader: That’s definitely something we could add right now. (Hospital O)
Check-in meetings with facilitators offered an opportunity to trouble shoot, brainstorm work-arounds, devise in-the-moment site-specific solutions to enable successful pathway implementation, and provide ongoing support throughout implementation.
DISCUSSION
Pathways can improve the quality of care for children with asthma.31 However, there is little evidence-based guidance on how to implement pathways and improve pediatric care in community hospitals,17-20 where the majority of children are cared for nationally. This is the first study to our knowledge that details the key determinants of pediatric asthma pathway implementation in community hospital settings. We identified four key determinants of implementation that can help guide others in similar settings. These include building an implementation infrastructure, engaging and motivating multidisciplinary providers, addressing organizational and resource limitations, and using external facilitators to devise implementation solutions.
Existing frameworks such as the CFIR outline the potential determinants of implementation success but do not provide population- or setting-specific guidance.27 There have been prior studies detailing pathway implementation for pediatric populations, but these studies did not focus on community hospitals.32,33 Our findings align with these prior studies, which highlight the importance of identifying implementation champions, engaging and motivating multidisciplinary providers, establishing a QI infrastructure, and addressing organizational and resource limitations, such as EMR integration.32,33 However, our study provides unique insights into issues that are important to successful pathway implementation in community hospitals, including engagement of adult-focused healthcare providers, reprioritization of resources toward the care of children, and the potentially critical role of external facilitators.
Our findings indicate that community hospitals seeking to improve care for children may particularly benefit from using external facilitators and/or partnering with external organizations. We found that external facilitators played a significant and proactive role in community hospitals’ efforts to improve care for children. Facilitators helped devise work-arounds and engaged in just-in-time solution building with local project leaders. For instance, facilitators helped develop strategies for training healthcare providers in performing new clinical tasks, building reminders of pathway recommendations into clinical workflows, and overcoming resource barriers. Thus, community hospitals may uniquely benefit from participation in national learning collaboratives, which often provide avenues for external facilitation.25,34,35 National networks, such as the VIP network, lead national learning collaboratives that provide external facilitation as well as other resources (eg, educational materials, data analysis support) to community hospitals seeking to improve pediatric care.24 Previous work by McDaniel et al. identified that intentional partnerships between children’s and community hospitals can also potentially provide access to resources for education and training in pediatric care and support in navigating organizational and resource challenges.22
Our results characterize the key determinants of pediatric asthma pathway implementation using a national sample of community hospitals that were diverse in geography, size, and structure. This imparts greater transferability of our findings. We also used strategies to promote the rigor of our findings, including triangulation and reflexivity. However, our study has several limitations. First, we analyzed only the meetings that occurred during the early months of pathway implementation. As such, we did not capture any key determinants that may have arisen later in implementation. However, process analyses of implementation indicate that the majority of implementation efforts occurred within these first three to four months.36 Second, we did not elicit input from hospital administration or leadership. The lack of administrative/leadership input probably affected the CFIR themes we found, as no themes from the outer setting were elicited. However, the goal of our study was to characterize the experiences of those leading implementation efforts, and focusing on these leaders allows our work to better guide those doing similar work in the future. Third, we used CFIR to guide the development of our interview guide and as a reference during analysis, which may have skewed our findings to preferentially reflect CFIR constructs. However, our overall analysis was grounded in the primary data and we employed reflexivity during all stages of our analysis. In addition, having the facilitators conduct the qualitative interviews may have biased our findings toward the perspectives of the facilitators; however, the facilitators represented quite diverse clinical and QI backgrounds. Finally, our findings do not necessarily correlate with improvements in clinical outcomes. As such, they are not meant to serve as explicit recommendations for improving patient outcomes, but rather as a characterization of the context, processes, and experiences of implementing pathways in the community setting to inform others doing this important work.
CONCLUSIONS
We identified the key determinants of pediatric asthma pathway implementation in community hospitals, which may help inform QI efforts in these settings. We also identified organizational and resource limitations that are probably unique to these adult-focused hospitals. Participating in national learning collaboratives and/or working with facilitators may support pathway implementation and improved quality of care for children with asthma in community hospitals.
Future work should seek to correlate these and other determinants of pathway implementation with health outcomes for hospitalized children, as well as integrate broader and more diverse samples of community hospitals.
1. National Asthma E, Prevention P. Expert Panel Report 3 (EPR-3): Guidelines for the diagnosis and management of asthma-summary report 2007. J Allergy Clin Immunol. 2007;120(5):S94-S138. https://doi.org/10.1016/j.jaci.2007.09.043.
2. Bekmezian A, Hersh AL, Maselli JH, Cabana MD. Pediatric emergency departments are more likely than general emergency departments to treat asthma exacerbation with systemic corticosteroids. J Asthma. 2011;48(1):69-74. https://doi.org/10.3109/02770903.2010.535884.
3. Biagini Myers JM, Simmons JM, Kercsmar CM, et al. Heterogeneity in asthma care in a statewide collaborative: the Ohio Pediatric Asthma Repository. Pediatrics. 2015;135(2):271-279. https://doi.org/10.1542/peds.2014-2230.
4. Kharbanda AB, Hall M, Shah SS, et al. Variation in resource utilization across a national sample of pediatric emergency departments. J Pediatr. 2013;163(1):230-236. https://doi.org/10.1016/j.jpeds.2012.12.013.
5. O’Con
6. Lougheed MD, Garvey N, Chapman KR, et al. Variations and gaps in management of acute asthma in Ontario emergency departments. Chest. 2009;135(3):724-736. https://doi.org/10.1378/chest.08-0371.
7. Bekmezian A, Fee C, Weber E. Clinical pathway improves pediatrics asthma management in the emergency department and reduces admissions. J Asthma. 2015;52(8):806-814. https://doi.org/10.3109/02770903.2015.1019086.
8. Chen KH, Chen CC, Liu HE, Tzeng PC, Glasziou PP. Effectiveness of paediatric asthma clinical pathways: a narrative systematic review. J Asthma. 2014;51(5):480-492. https://doi.org/10.3109/02770903.2014.887728.
9. Johnson KB, Blaisdell CJ, Walker A, Eggleston P. Effectiveness of a clinical pathway for inpatient asthma management. Pediatrics. 2000;106(5):1006-1012. https://doi.org/10.1542/peds.106.5.1006.
10. Kelly CS, Andersen CL, Pestian JP, et al. Improved outcomes for hospitalized asthmatic children using a clinical pathway. Ann Allergy Asthma Immunol. 2000;84(5):509-516. https://doi.org/10.1016/S1081-1206(10)62514-8.
11. McDowell KM, Chatburn RL, Myers TR, O’Riordan MA, Kercsmar CM. A cost-saving algorithm for children hospitalized for status asthmaticus. Arch Pediatr Adolesc Med. 1998;152(10):977-984. https://doi.org/10.1001/archpedi.152.10.977.
12. Miller AG, Breslin ME, Pineda LC, Fox JW. An asthma protocol improved adherence to evidence-based guidelines for pediatric subjects with status asthmaticus in the emergency department. Respir Care. 2015;60(12):1759-1764. https://doi.org/10.4187/respcare.04011.
13. Nkoy F, Fassl B, Stone B, et al. Improving pediatric asthma care and outcomes across multiple hospitals. Pediatrics. 2015;136(6):e1602-e1610. https://doi.org/10.1542/peds.2015-0285.
14. Rutman L, Atkins RC, Migita R, et al. Modification of an established pediatric asthma pathway improves evidence-based, efficient care. Pediatrics. 2016;138(6). https://doi.org/10.1542/peds.2016-1248.
15. Glauber JH, Farber HJ, Homer CJ. Asthma clinical pathways: toward what end? Pediatrics. 2001;107(3):590-592. https://doi.org/10.1542/peds.107.3.590.
16. Grimshaw J, Eccles M, Thomas R, et al. Toward evidence-based quality improvement. Evidence (and its limitations) of the effectiveness of guideline dissemination and implementation strategies 1966-1998. J Gen Intern Med. 2006;21(2):S14-S20. https://doi.org/10.1111/j.1525-1497.2006.00357.x.
17. Scott SD, Grimshaw J, Klassen TP, Nettel-Aguirre A, Johnson DW. Understanding implementation processes of clinical pathways and clinical practice guidelines in pediatric contexts: a study protocol. Implement Sci. 2011;6:133. https://doi.org/10.1186/1748-5908-6-133.
18. Walls TA, Hughes NT, Mullan PC, Chamberlain JM, Brown K. Improving pediatric asthma outcomes in a community emergency department. Pediatrics. 2017;139(1). https://doi.org/10.1542/peds.2016-0088.
19. Kaiser SV, Lam R, Cabana MD, et al. Best practices in implementing inpatient pediatric asthma pathways: a qualitative study. J Asthma. 2019:1-11. https://doi.org/10.1080/02770903.2019.1606237.
20. Leyenaar JK, Ralston SL, Shieh MS, Pekow PS, Mangione-Smith R, Lindenauer PK. Epidemiology of pediatric hospitalizations at general hospitals and freestanding children’s hospitals in the United States. J Hosp Med. 2016;11(11):743-749. https://doi.org/10.1002/jhm.2624.
21. Franca UL, McManus ML. Availability of definitive hospital care for children. JAMA Pediatr. 2017;171(9):e171096. https://doi.org/10.1001/jamapediatrics.2017.1096.
22. McDaniel CE, Jennings R, Schroeder AR, Paciorkowski N, Hofmann M, Leyenaar J. Aligning inpatient pediatric research with settings of care: a call to action. Pediatrics. 2019;143(5). https://doi.org/10.1542/peds.2018-2648.
23. Kaiser SV JB. Value in inpatient pediatrics network launches National Asthma Project. In: AAP Quality Connections 2018; 26:8-9. Retrieved from: https://www.aap.org/en-us/Documents/coqips_newsletter_2018_winter_26.pdf
24. Value in Inpatient Pediatrics. https://www.aap.org/en-us/professional-resources/quality-improvement/Pages/Value-in-Inpatient-Pediatrics.aspx. Accessed December 1, 2017.
25. The Breakthrough Series: IHI’s Collaborative Model for Achieving Breakthrough Improvement. IHI Innovation Series white paper. Boston: Institute for Healthcare Improvement; 2003. Retrieved from: www.IHI.org
26. Powell BJ, Waltz TJ, Chinman MJ, et al. A refined compilation of implementation strategies: results from the Expert Recommendations for Implementing Change (ERIC) project. Implement Sci. 2015;10:21. https://doi.org/10.1186/s13012-015-0209-1.
27. Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4:50. https://doi.org/10.1186/1748-5908-4-50.
28. Braun VaC, V. Thematic analysis. In: H. Cooper PC, Long DL, Panter AT, Rindskopf E, Sher KJ, eds. APA handbook of research methods in psychology, Vol 2. Research designs: Quantitative, qualitative, neuropsychologial, and biological. Washington, DC, US: American Psychological Association; 2012. https://doi.org/10.1037/13620-000.
29. Charmaz K. Grounded Theory. 2nd ed. Thousand Oaks, CA: SAGE Publications; 2014.
30. Creswell JW, Poth CNCN CJaP. Qualitative Inquiry and Research Design: Choosing Among Five Approaches. Thousand Oaks, CA: Sage; 2017.
31. Kaiser SV, Rodean J, Bekmezian A, et al. Effectiveness of pediatric asthma pathways for hospitalized children: a multicenter, national analysis. J. Pediatr. 2018;197:165-171. https://doi.org/10.1016/j.jpeds.2018.01.084.
32. Leyenaar JK, Andrews CB, Tyksinski ER, Biondi E, Parikh K, Ralston S. Facilitators of interdepartmental quality improvement: a mixed-methods analysis of a collaborative to improve pediatric community-acquired pneumonia management. BMJ Qual Saf. 2019;28(3):215-222. https://doi.org/10.1136/bmjqs-2018-008065. 33. Ralston SL, Atwood EC, Garber MD, Holmes AV. What works to reduce unnecessary care for bronchiolitis? A qualitative analysis of a national collaborative. Acad Pediatr. 2017;17(2):198-204. https://doi.org/10.1016/j.acap.2016.07.001.
33. Ralston SL, Atwood EC, Garber MD, Holmes AV. What works to reduce unnecessary care for bronchiolitis? A qualitative analysis of a national collaborative. Acad Pediatr. 2017;17(2):198-204. https://doi.org/10.1016/j.acap.2016.07.001.
34. Parikh K, Biondi E, Nazif J, et al. A multicenter collaborative to improve care of community acquired pneumonia in hospitalized children. Pediatrics. 2017;139(2). https://doi.org/10.1542/peds.2016-1411.
35. Ralston S, Garber M, Narang S, et al. Decreasing unnecessary utilization in acute bronchiolitis care: results from the value in inpatient pediatrics network. J Hosp Med. 2013;8(1):25-30. https://doi.org/10.1002/jhm.1982.
36. Gupta N CA, Cabana MD, Jennings B, Parikh K, Kaiser SV. PIPA (Pathways for Improving Pediatric Asthma Care): Process Evaluation of a National Collaborative to Implement Pathways. Platform presented at Pediatric Academic Society National Meeting. Baltimore, Maryland; 2019.
Despite the widespread availability of evidence-based guidelines,1 there is inappropriate variation in the care and outcomes for children with asthma in both the emergency department (ED) and the inpatient setting.2-6 Operational versions of evidence-based guidelines known as “pathways” have been shown to improve adoption of evidence-based guidelines, quality of care, and health outcomes for children with asthma.7-14 However, little is known about how to successfully implement pathways outside of free-standing children’s hospitals.15-19
The majority of children with asthma in the United States are cared for in community hospitals, which provide services for both adults and children.20 However, prior studies of pediatric asthma pathways have largely excluded community hospitals. These studies primarily focused on determining clinical effectiveness, rather than detailing the implementation process. These approaches have left critical gaps that hinder our ability to implement pathways and improve care in community hospitals, which have unique barriers and less resources.21,22 Therefore, understanding the process of pathway implementation in community hospitals is critical to improving care for children.22 Our objective was to identify the key determinants of successful pediatric asthma pathway implementation using a national sample of community hospitals. This knowledge can guide hospital leaders and healthcare providers in efforts to improve pediatric care and outcomes in these settings.
METHODS
Study Setting, Design, and Population
In Fall 2017, the Value in Inpatient Pediatrics (VIP) network launched PIPA, Pathways to Improving Pediatric Asthma care.23 The VIP network, a part of the American Academy of Pediatrics (AAP), aims to improve the value of care delivered to any pediatric patient in a hospital bed, from rural to free-standing children’s hospitals.24 PIPA used a learning collaborative model25 and recruited local project leaders (physicians, nurses, respiratory therapists (RT), and pharmacists) from 89 hospitals around the country. PIPA provided these hospital teams with asthma pathways and several resources for implementation support, including educational meetings, quality improvement (QI) training, audit and feedback, and facilitation. Facilitation is a process of interactive problem-solving and support that occurs in the context of a supportive interpersonal relationship and a recognized need for improvement.26 A facilitator, or a “coach”, is an external expert who provides project mentorship and assists the process of making meaningful changes to improve patient care.26 Facilitation was provided by external consultants with QI expertise.
For this qualitative study, facilitators conducted semi-structured interviews with a convenience sample of project leaders from community hospitals participating in PIPA, with some interviews including multiple project leaders (eg, nursing, inpatient, and Emergency Department [ED] leaders). Verbal consent was obtained from all participants. No incentives were provided. This study was approved by the AAP institutional review board.
Data Collection
We used the constructs described in the Consolidated Framework for Implementation Research (CFIR)27 and adapted those salient to pediatric asthma pathways to develop an interview guide that was used with all participants (Appendix 1). The CFIR offers an overarching typology to understand what works where and why across five major domains that influence implementation: intervention characteristics, inner setting (hospital), outer setting (economic, political, and social context of the hospital), characteristics of the individuals involved, and the process of implementation. Data were collected across these domains to inform our analysis of the key determinants of pediatric asthma pathway implementation in community hospitals.
Interviews were conducted by phone from December 2017 to April 2018 (first four months of pathway implementation). Interviews lasted 30-60 minutes and were recorded and transcribed verbatim. Transcripts were edited for accuracy using the audio recordings. As data collection occurred concurrently with analysis, the interview guide was iteratively revised to reflect new insights and patterns that emerged from our analysis. All sites were anonymized in the data analysis. New interviews were coded until thematic saturation was reached.
Analysis
We conducted an inductive thematic analysis using the CFIR as our conceptual framework.28,29 Four investigators (CM, MJ, ES, and SK) performed the initial open coding of the data. Investigators met twice during the open coding process to develop and then finalize a codebook of standard definitions for codes. This codebook facilitated coding consistency through the remainder of the analytic process. Two investigators (CM and MJ) then independently read and coded all data to ensure intercoder reliability. During this process, CM and MJ met every two weeks to compare coding consistency, resolve discrepancies, and discuss preliminary findings. When the coding was complete, all investigators met to explore and develop themes that encompassed related common codes.
The CFIR was used at two stages of the study: (1) developing the interview guide and (2) cross-checking for any potentially important codes that were missing/needed to be explored further. Thus, the investigators maintained an inductive approach grounded in the data. To assure study rigor, we employed investigator triangulation (use of multiple investigators and participants from multiple clinical roles) and reflexivity (ongoing critique and critical reflection of the individual biases of the investigators).30 Coding was performed using Dedoose (version 7.0.23; Los Angeles, California).
RESULTS
A total of 34 community hospitals completed the PIPA project, of which the project leaders of 25 hospitals connected with the facilitators and were approached to participate; 18 (72%) hospitals’ project leaders participated in the study. We analyzed 18 interviews conducted between facilitators and project leaders, which included a total of 32 project leaders (one to five leaders per interview). The hospitals represented were diverse in geographic location and size (range 4-50 pediatric beds per hospital), and the majority of sites (78%) supported the trainees (Table 1).
We identified four overarching themes that described the key determinants of pathway implementation in community hospitals. These themes are presented in order of their frequency of occurrence in the data. They included (1) building an implementation infrastructure, (2) engaging and motivating providers, (3) addressing organizational and resource limitations, and (4) devising implementation solutions with facilitators. Descriptions and exemplary quotations for each theme are provided in Table 2 and Appendix Figure 1.
Building an Implementation Infrastructure
“So, I’m going to sit down with the primary nursing staff and the other four physicians in the group to go over the expectations…We’re not going to have the actual EMR [changes] and we’re not going to have the nursing documentation field built right away but [we want to] make sure that people are documenting the respiratory score in their generic nursing note so that the information is easily accessible.” (Physician leader, Hospital G)
Participants also described the need to deliver education on the evidence supporting changes in practice and skills training specific to pediatric asthma care:
“Once we realized that we were going to be doing this pathway, we started training our nurses on the inpatient side on [pediatric respiratory scoring].” (Nursing leader, Hospital P)
In addition, pathway implementation required modification of clinical workflows via changes to hospital policies or guidelines, electronic medical records (EMR), and/or the physical environment (eg, placing supplies in proximity to care delivery):
“I think it can help if we could get an order set or a nursing protocol where asthmatics over a certain severity can just get steroids in triage.” (Physician leader, Hospital A)
Engaging and Motivating Providers
Another crucial step in pathway implementation was engaging and motivating providers. This included overcoming inertia to practice change, facilitating multidisciplinary collaboration, and handling conflicts regarding practice changes. Participants discussed the excitement of participating in a national collaborative as especially motivating to help drive engagement and overcome barriers to change, particularly the ability to compare local hospital performance to national peers.
“I think everyone is a little competitive. So I think that when we see how we compare to other institutions—both our group and the ER—I think it also adds a little oomph…I think for our nurses too; we’re able to say, ‘[look how we compare to] most of the other hospitals.’ I think that helps.” (Physician leader, Hospital B)
Multidisciplinary collaboration across a wide variety of frontline pediatric and nonpediatric providers was key to understanding current workflows and identifying needed modifications for pathway implementation:
“I do think clearly our biggest obstacles are the fact that we have adult ED providers. We have the opportunity on the inpatient side [with nursing and respiratory therapy], who really do awesome with pediatric changes, to take our wins where we can and make the changes with the ED. In the ED we have an RN educator. She’s very on board with doing the respiratory scoring and getting this whole thing started.” (Physician leader, Hospital L)
Intentional communication and leadership skills also played key roles in engaging hesitant providers and handling conflict:
“Just sitting and talking with our respiratory therapist about the ability to provide this type of service or support and seeing what their reservations have been, at least it’s open to conversation so that we could provide these types of therapies in the future and we’re able to see like what people’s concerns are. I think just basically increasing familiarity with not only these processes, but different types of therapy will hopefully in the future help us provide better care to our patients.” (Physician leader, Hospital Q)
Addressing Organizational and Resource Limitations
Participants recognized organizational and resource limitations, some of which may be unique to community hospitals that prioritize resources for adult care. The limitations described included EMR staff support, healthcare provider staffing/capacity, navigating IRBs, and addressing administrative processes. Competing demands for information technology staff support and lack of prioritization of pediatric-specific initiatives often hindered efforts to modify the EMR.
“Resource wise, we are hoping to implement an order set in our Epic EMR, [but] finding the availability from the Epic team may be a challenge.” (Physician Leader, Hospital A)
Participants also reported that limited staff capacity (eg, nursing, RT) hindered pathway implementation efforts. This limited capacity hindered workflow changes and limited the time available for education and training on pathways:
“[Respiratory scoring for asthma is] an added responsibility for the [nursing] staff and we don’t have patient technicians. So they’re doing everything from changing the sheets to bringing water to all of the medical patients. So, that I think may be a barrier.” (Physician leader, Hospital B)
Across sites, navigating the IRB posed various challenges. Some sites were required to obtain approval from regional IRBs, which did not have resources to devote to pediatric projects. Other sites did not have IRBs at all, but instead required separate approvals for the project from hospital leadership or other entities:
“On the IRB, I contacted the manager of the IRB and she’s said, ‘No, it’s not an IRB project,’ but she sent it to another director for review, and it took forever to be able to get a data agreement with [the local university hospital] so that we can pull the data. I just couldn’t believe it took months to get done.” (Physician Leader, Hospital K)
Finally, administrative barriers such as addressing formulary changes in the context of adult-focused settings were challenging. For example, at one hospital, metered dose inhalers (MDIs) were not used for adult patients, and the hospital administration was resistant to incorporate their use into practice for pediatric patients due to the cost of such changes.
“The [general hospital] didn’t have MDI’s anymore because of cost reasons, and when we started the pediatric work, we really made it a point to get the MDI’s for pediatric patients back in the formulary.” (Physician leader, Hospital A)
Devising Implementation Solutions with Practice Facilitators
Participants often devised pathway implementation solutions with facilitators in-the-moment during meetings. This problem-solving included figuring out work-arounds, proactive coaching by external facilitators, and just-in-time solution building. Furthermore, in meetings that included more than one project leader, leaders would often work with each other to devise solutions. Meetings provided forums that stimulated identification of implementation barriers, brainstorming, and subsequently solution building.
Physician leader: I’m wondering if we could, as an interim solution, try out an algorithm on paper, I don’t know if that’s allowed, until we get Epic approval. Do you know?
Nurse Leader: You mean having an algorithm posted in triage? Yeah, I don’t see why not. (Hospital A)
Next, problem solving was often driven by the facilitator’s experience and knowledge, drawn from their interactions with other collaborative sites or their own prior experiences with asthma, QI, or pathway implementation. The facilitators brought an outside perspective, not bound by that particular hospital’s local culture or structural intricacies. This proactive coaching spurred the identification of creative, yet practical solutions:
Project Leader: We’re still trying to get all our templates [for the EMR]…because [currently they are] all adult templates.
Facilitator: If you’re making templates right now, could you also add the three asterisks? Like smoking or exposure to second hand tobacco smoke or marijuana…then have the three asterisks there and then “Referral made?***”. That would force people to document in a certain place in the template as well.Project Leader: That’s definitely something we could add right now. (Hospital O)
Check-in meetings with facilitators offered an opportunity to trouble shoot, brainstorm work-arounds, devise in-the-moment site-specific solutions to enable successful pathway implementation, and provide ongoing support throughout implementation.
DISCUSSION
Pathways can improve the quality of care for children with asthma.31 However, there is little evidence-based guidance on how to implement pathways and improve pediatric care in community hospitals,17-20 where the majority of children are cared for nationally. This is the first study to our knowledge that details the key determinants of pediatric asthma pathway implementation in community hospital settings. We identified four key determinants of implementation that can help guide others in similar settings. These include building an implementation infrastructure, engaging and motivating multidisciplinary providers, addressing organizational and resource limitations, and using external facilitators to devise implementation solutions.
Existing frameworks such as the CFIR outline the potential determinants of implementation success but do not provide population- or setting-specific guidance.27 There have been prior studies detailing pathway implementation for pediatric populations, but these studies did not focus on community hospitals.32,33 Our findings align with these prior studies, which highlight the importance of identifying implementation champions, engaging and motivating multidisciplinary providers, establishing a QI infrastructure, and addressing organizational and resource limitations, such as EMR integration.32,33 However, our study provides unique insights into issues that are important to successful pathway implementation in community hospitals, including engagement of adult-focused healthcare providers, reprioritization of resources toward the care of children, and the potentially critical role of external facilitators.
Our findings indicate that community hospitals seeking to improve care for children may particularly benefit from using external facilitators and/or partnering with external organizations. We found that external facilitators played a significant and proactive role in community hospitals’ efforts to improve care for children. Facilitators helped devise work-arounds and engaged in just-in-time solution building with local project leaders. For instance, facilitators helped develop strategies for training healthcare providers in performing new clinical tasks, building reminders of pathway recommendations into clinical workflows, and overcoming resource barriers. Thus, community hospitals may uniquely benefit from participation in national learning collaboratives, which often provide avenues for external facilitation.25,34,35 National networks, such as the VIP network, lead national learning collaboratives that provide external facilitation as well as other resources (eg, educational materials, data analysis support) to community hospitals seeking to improve pediatric care.24 Previous work by McDaniel et al. identified that intentional partnerships between children’s and community hospitals can also potentially provide access to resources for education and training in pediatric care and support in navigating organizational and resource challenges.22
Our results characterize the key determinants of pediatric asthma pathway implementation using a national sample of community hospitals that were diverse in geography, size, and structure. This imparts greater transferability of our findings. We also used strategies to promote the rigor of our findings, including triangulation and reflexivity. However, our study has several limitations. First, we analyzed only the meetings that occurred during the early months of pathway implementation. As such, we did not capture any key determinants that may have arisen later in implementation. However, process analyses of implementation indicate that the majority of implementation efforts occurred within these first three to four months.36 Second, we did not elicit input from hospital administration or leadership. The lack of administrative/leadership input probably affected the CFIR themes we found, as no themes from the outer setting were elicited. However, the goal of our study was to characterize the experiences of those leading implementation efforts, and focusing on these leaders allows our work to better guide those doing similar work in the future. Third, we used CFIR to guide the development of our interview guide and as a reference during analysis, which may have skewed our findings to preferentially reflect CFIR constructs. However, our overall analysis was grounded in the primary data and we employed reflexivity during all stages of our analysis. In addition, having the facilitators conduct the qualitative interviews may have biased our findings toward the perspectives of the facilitators; however, the facilitators represented quite diverse clinical and QI backgrounds. Finally, our findings do not necessarily correlate with improvements in clinical outcomes. As such, they are not meant to serve as explicit recommendations for improving patient outcomes, but rather as a characterization of the context, processes, and experiences of implementing pathways in the community setting to inform others doing this important work.
CONCLUSIONS
We identified the key determinants of pediatric asthma pathway implementation in community hospitals, which may help inform QI efforts in these settings. We also identified organizational and resource limitations that are probably unique to these adult-focused hospitals. Participating in national learning collaboratives and/or working with facilitators may support pathway implementation and improved quality of care for children with asthma in community hospitals.
Future work should seek to correlate these and other determinants of pathway implementation with health outcomes for hospitalized children, as well as integrate broader and more diverse samples of community hospitals.
Despite the widespread availability of evidence-based guidelines,1 there is inappropriate variation in the care and outcomes for children with asthma in both the emergency department (ED) and the inpatient setting.2-6 Operational versions of evidence-based guidelines known as “pathways” have been shown to improve adoption of evidence-based guidelines, quality of care, and health outcomes for children with asthma.7-14 However, little is known about how to successfully implement pathways outside of free-standing children’s hospitals.15-19
The majority of children with asthma in the United States are cared for in community hospitals, which provide services for both adults and children.20 However, prior studies of pediatric asthma pathways have largely excluded community hospitals. These studies primarily focused on determining clinical effectiveness, rather than detailing the implementation process. These approaches have left critical gaps that hinder our ability to implement pathways and improve care in community hospitals, which have unique barriers and less resources.21,22 Therefore, understanding the process of pathway implementation in community hospitals is critical to improving care for children.22 Our objective was to identify the key determinants of successful pediatric asthma pathway implementation using a national sample of community hospitals. This knowledge can guide hospital leaders and healthcare providers in efforts to improve pediatric care and outcomes in these settings.
METHODS
Study Setting, Design, and Population
In Fall 2017, the Value in Inpatient Pediatrics (VIP) network launched PIPA, Pathways to Improving Pediatric Asthma care.23 The VIP network, a part of the American Academy of Pediatrics (AAP), aims to improve the value of care delivered to any pediatric patient in a hospital bed, from rural to free-standing children’s hospitals.24 PIPA used a learning collaborative model25 and recruited local project leaders (physicians, nurses, respiratory therapists (RT), and pharmacists) from 89 hospitals around the country. PIPA provided these hospital teams with asthma pathways and several resources for implementation support, including educational meetings, quality improvement (QI) training, audit and feedback, and facilitation. Facilitation is a process of interactive problem-solving and support that occurs in the context of a supportive interpersonal relationship and a recognized need for improvement.26 A facilitator, or a “coach”, is an external expert who provides project mentorship and assists the process of making meaningful changes to improve patient care.26 Facilitation was provided by external consultants with QI expertise.
For this qualitative study, facilitators conducted semi-structured interviews with a convenience sample of project leaders from community hospitals participating in PIPA, with some interviews including multiple project leaders (eg, nursing, inpatient, and Emergency Department [ED] leaders). Verbal consent was obtained from all participants. No incentives were provided. This study was approved by the AAP institutional review board.
Data Collection
We used the constructs described in the Consolidated Framework for Implementation Research (CFIR)27 and adapted those salient to pediatric asthma pathways to develop an interview guide that was used with all participants (Appendix 1). The CFIR offers an overarching typology to understand what works where and why across five major domains that influence implementation: intervention characteristics, inner setting (hospital), outer setting (economic, political, and social context of the hospital), characteristics of the individuals involved, and the process of implementation. Data were collected across these domains to inform our analysis of the key determinants of pediatric asthma pathway implementation in community hospitals.
Interviews were conducted by phone from December 2017 to April 2018 (first four months of pathway implementation). Interviews lasted 30-60 minutes and were recorded and transcribed verbatim. Transcripts were edited for accuracy using the audio recordings. As data collection occurred concurrently with analysis, the interview guide was iteratively revised to reflect new insights and patterns that emerged from our analysis. All sites were anonymized in the data analysis. New interviews were coded until thematic saturation was reached.
Analysis
We conducted an inductive thematic analysis using the CFIR as our conceptual framework.28,29 Four investigators (CM, MJ, ES, and SK) performed the initial open coding of the data. Investigators met twice during the open coding process to develop and then finalize a codebook of standard definitions for codes. This codebook facilitated coding consistency through the remainder of the analytic process. Two investigators (CM and MJ) then independently read and coded all data to ensure intercoder reliability. During this process, CM and MJ met every two weeks to compare coding consistency, resolve discrepancies, and discuss preliminary findings. When the coding was complete, all investigators met to explore and develop themes that encompassed related common codes.
The CFIR was used at two stages of the study: (1) developing the interview guide and (2) cross-checking for any potentially important codes that were missing/needed to be explored further. Thus, the investigators maintained an inductive approach grounded in the data. To assure study rigor, we employed investigator triangulation (use of multiple investigators and participants from multiple clinical roles) and reflexivity (ongoing critique and critical reflection of the individual biases of the investigators).30 Coding was performed using Dedoose (version 7.0.23; Los Angeles, California).
RESULTS
A total of 34 community hospitals completed the PIPA project, of which the project leaders of 25 hospitals connected with the facilitators and were approached to participate; 18 (72%) hospitals’ project leaders participated in the study. We analyzed 18 interviews conducted between facilitators and project leaders, which included a total of 32 project leaders (one to five leaders per interview). The hospitals represented were diverse in geographic location and size (range 4-50 pediatric beds per hospital), and the majority of sites (78%) supported the trainees (Table 1).
We identified four overarching themes that described the key determinants of pathway implementation in community hospitals. These themes are presented in order of their frequency of occurrence in the data. They included (1) building an implementation infrastructure, (2) engaging and motivating providers, (3) addressing organizational and resource limitations, and (4) devising implementation solutions with facilitators. Descriptions and exemplary quotations for each theme are provided in Table 2 and Appendix Figure 1.
Building an Implementation Infrastructure
“So, I’m going to sit down with the primary nursing staff and the other four physicians in the group to go over the expectations…We’re not going to have the actual EMR [changes] and we’re not going to have the nursing documentation field built right away but [we want to] make sure that people are documenting the respiratory score in their generic nursing note so that the information is easily accessible.” (Physician leader, Hospital G)
Participants also described the need to deliver education on the evidence supporting changes in practice and skills training specific to pediatric asthma care:
“Once we realized that we were going to be doing this pathway, we started training our nurses on the inpatient side on [pediatric respiratory scoring].” (Nursing leader, Hospital P)
In addition, pathway implementation required modification of clinical workflows via changes to hospital policies or guidelines, electronic medical records (EMR), and/or the physical environment (eg, placing supplies in proximity to care delivery):
“I think it can help if we could get an order set or a nursing protocol where asthmatics over a certain severity can just get steroids in triage.” (Physician leader, Hospital A)
Engaging and Motivating Providers
Another crucial step in pathway implementation was engaging and motivating providers. This included overcoming inertia to practice change, facilitating multidisciplinary collaboration, and handling conflicts regarding practice changes. Participants discussed the excitement of participating in a national collaborative as especially motivating to help drive engagement and overcome barriers to change, particularly the ability to compare local hospital performance to national peers.
“I think everyone is a little competitive. So I think that when we see how we compare to other institutions—both our group and the ER—I think it also adds a little oomph…I think for our nurses too; we’re able to say, ‘[look how we compare to] most of the other hospitals.’ I think that helps.” (Physician leader, Hospital B)
Multidisciplinary collaboration across a wide variety of frontline pediatric and nonpediatric providers was key to understanding current workflows and identifying needed modifications for pathway implementation:
“I do think clearly our biggest obstacles are the fact that we have adult ED providers. We have the opportunity on the inpatient side [with nursing and respiratory therapy], who really do awesome with pediatric changes, to take our wins where we can and make the changes with the ED. In the ED we have an RN educator. She’s very on board with doing the respiratory scoring and getting this whole thing started.” (Physician leader, Hospital L)
Intentional communication and leadership skills also played key roles in engaging hesitant providers and handling conflict:
“Just sitting and talking with our respiratory therapist about the ability to provide this type of service or support and seeing what their reservations have been, at least it’s open to conversation so that we could provide these types of therapies in the future and we’re able to see like what people’s concerns are. I think just basically increasing familiarity with not only these processes, but different types of therapy will hopefully in the future help us provide better care to our patients.” (Physician leader, Hospital Q)
Addressing Organizational and Resource Limitations
Participants recognized organizational and resource limitations, some of which may be unique to community hospitals that prioritize resources for adult care. The limitations described included EMR staff support, healthcare provider staffing/capacity, navigating IRBs, and addressing administrative processes. Competing demands for information technology staff support and lack of prioritization of pediatric-specific initiatives often hindered efforts to modify the EMR.
“Resource wise, we are hoping to implement an order set in our Epic EMR, [but] finding the availability from the Epic team may be a challenge.” (Physician Leader, Hospital A)
Participants also reported that limited staff capacity (eg, nursing, RT) hindered pathway implementation efforts. This limited capacity hindered workflow changes and limited the time available for education and training on pathways:
“[Respiratory scoring for asthma is] an added responsibility for the [nursing] staff and we don’t have patient technicians. So they’re doing everything from changing the sheets to bringing water to all of the medical patients. So, that I think may be a barrier.” (Physician leader, Hospital B)
Across sites, navigating the IRB posed various challenges. Some sites were required to obtain approval from regional IRBs, which did not have resources to devote to pediatric projects. Other sites did not have IRBs at all, but instead required separate approvals for the project from hospital leadership or other entities:
“On the IRB, I contacted the manager of the IRB and she’s said, ‘No, it’s not an IRB project,’ but she sent it to another director for review, and it took forever to be able to get a data agreement with [the local university hospital] so that we can pull the data. I just couldn’t believe it took months to get done.” (Physician Leader, Hospital K)
Finally, administrative barriers such as addressing formulary changes in the context of adult-focused settings were challenging. For example, at one hospital, metered dose inhalers (MDIs) were not used for adult patients, and the hospital administration was resistant to incorporate their use into practice for pediatric patients due to the cost of such changes.
“The [general hospital] didn’t have MDI’s anymore because of cost reasons, and when we started the pediatric work, we really made it a point to get the MDI’s for pediatric patients back in the formulary.” (Physician leader, Hospital A)
Devising Implementation Solutions with Practice Facilitators
Participants often devised pathway implementation solutions with facilitators in-the-moment during meetings. This problem-solving included figuring out work-arounds, proactive coaching by external facilitators, and just-in-time solution building. Furthermore, in meetings that included more than one project leader, leaders would often work with each other to devise solutions. Meetings provided forums that stimulated identification of implementation barriers, brainstorming, and subsequently solution building.
Physician leader: I’m wondering if we could, as an interim solution, try out an algorithm on paper, I don’t know if that’s allowed, until we get Epic approval. Do you know?
Nurse Leader: You mean having an algorithm posted in triage? Yeah, I don’t see why not. (Hospital A)
Next, problem solving was often driven by the facilitator’s experience and knowledge, drawn from their interactions with other collaborative sites or their own prior experiences with asthma, QI, or pathway implementation. The facilitators brought an outside perspective, not bound by that particular hospital’s local culture or structural intricacies. This proactive coaching spurred the identification of creative, yet practical solutions:
Project Leader: We’re still trying to get all our templates [for the EMR]…because [currently they are] all adult templates.
Facilitator: If you’re making templates right now, could you also add the three asterisks? Like smoking or exposure to second hand tobacco smoke or marijuana…then have the three asterisks there and then “Referral made?***”. That would force people to document in a certain place in the template as well.Project Leader: That’s definitely something we could add right now. (Hospital O)
Check-in meetings with facilitators offered an opportunity to trouble shoot, brainstorm work-arounds, devise in-the-moment site-specific solutions to enable successful pathway implementation, and provide ongoing support throughout implementation.
DISCUSSION
Pathways can improve the quality of care for children with asthma.31 However, there is little evidence-based guidance on how to implement pathways and improve pediatric care in community hospitals,17-20 where the majority of children are cared for nationally. This is the first study to our knowledge that details the key determinants of pediatric asthma pathway implementation in community hospital settings. We identified four key determinants of implementation that can help guide others in similar settings. These include building an implementation infrastructure, engaging and motivating multidisciplinary providers, addressing organizational and resource limitations, and using external facilitators to devise implementation solutions.
Existing frameworks such as the CFIR outline the potential determinants of implementation success but do not provide population- or setting-specific guidance.27 There have been prior studies detailing pathway implementation for pediatric populations, but these studies did not focus on community hospitals.32,33 Our findings align with these prior studies, which highlight the importance of identifying implementation champions, engaging and motivating multidisciplinary providers, establishing a QI infrastructure, and addressing organizational and resource limitations, such as EMR integration.32,33 However, our study provides unique insights into issues that are important to successful pathway implementation in community hospitals, including engagement of adult-focused healthcare providers, reprioritization of resources toward the care of children, and the potentially critical role of external facilitators.
Our findings indicate that community hospitals seeking to improve care for children may particularly benefit from using external facilitators and/or partnering with external organizations. We found that external facilitators played a significant and proactive role in community hospitals’ efforts to improve care for children. Facilitators helped devise work-arounds and engaged in just-in-time solution building with local project leaders. For instance, facilitators helped develop strategies for training healthcare providers in performing new clinical tasks, building reminders of pathway recommendations into clinical workflows, and overcoming resource barriers. Thus, community hospitals may uniquely benefit from participation in national learning collaboratives, which often provide avenues for external facilitation.25,34,35 National networks, such as the VIP network, lead national learning collaboratives that provide external facilitation as well as other resources (eg, educational materials, data analysis support) to community hospitals seeking to improve pediatric care.24 Previous work by McDaniel et al. identified that intentional partnerships between children’s and community hospitals can also potentially provide access to resources for education and training in pediatric care and support in navigating organizational and resource challenges.22
Our results characterize the key determinants of pediatric asthma pathway implementation using a national sample of community hospitals that were diverse in geography, size, and structure. This imparts greater transferability of our findings. We also used strategies to promote the rigor of our findings, including triangulation and reflexivity. However, our study has several limitations. First, we analyzed only the meetings that occurred during the early months of pathway implementation. As such, we did not capture any key determinants that may have arisen later in implementation. However, process analyses of implementation indicate that the majority of implementation efforts occurred within these first three to four months.36 Second, we did not elicit input from hospital administration or leadership. The lack of administrative/leadership input probably affected the CFIR themes we found, as no themes from the outer setting were elicited. However, the goal of our study was to characterize the experiences of those leading implementation efforts, and focusing on these leaders allows our work to better guide those doing similar work in the future. Third, we used CFIR to guide the development of our interview guide and as a reference during analysis, which may have skewed our findings to preferentially reflect CFIR constructs. However, our overall analysis was grounded in the primary data and we employed reflexivity during all stages of our analysis. In addition, having the facilitators conduct the qualitative interviews may have biased our findings toward the perspectives of the facilitators; however, the facilitators represented quite diverse clinical and QI backgrounds. Finally, our findings do not necessarily correlate with improvements in clinical outcomes. As such, they are not meant to serve as explicit recommendations for improving patient outcomes, but rather as a characterization of the context, processes, and experiences of implementing pathways in the community setting to inform others doing this important work.
CONCLUSIONS
We identified the key determinants of pediatric asthma pathway implementation in community hospitals, which may help inform QI efforts in these settings. We also identified organizational and resource limitations that are probably unique to these adult-focused hospitals. Participating in national learning collaboratives and/or working with facilitators may support pathway implementation and improved quality of care for children with asthma in community hospitals.
Future work should seek to correlate these and other determinants of pathway implementation with health outcomes for hospitalized children, as well as integrate broader and more diverse samples of community hospitals.
1. National Asthma E, Prevention P. Expert Panel Report 3 (EPR-3): Guidelines for the diagnosis and management of asthma-summary report 2007. J Allergy Clin Immunol. 2007;120(5):S94-S138. https://doi.org/10.1016/j.jaci.2007.09.043.
2. Bekmezian A, Hersh AL, Maselli JH, Cabana MD. Pediatric emergency departments are more likely than general emergency departments to treat asthma exacerbation with systemic corticosteroids. J Asthma. 2011;48(1):69-74. https://doi.org/10.3109/02770903.2010.535884.
3. Biagini Myers JM, Simmons JM, Kercsmar CM, et al. Heterogeneity in asthma care in a statewide collaborative: the Ohio Pediatric Asthma Repository. Pediatrics. 2015;135(2):271-279. https://doi.org/10.1542/peds.2014-2230.
4. Kharbanda AB, Hall M, Shah SS, et al. Variation in resource utilization across a national sample of pediatric emergency departments. J Pediatr. 2013;163(1):230-236. https://doi.org/10.1016/j.jpeds.2012.12.013.
5. O’Con
6. Lougheed MD, Garvey N, Chapman KR, et al. Variations and gaps in management of acute asthma in Ontario emergency departments. Chest. 2009;135(3):724-736. https://doi.org/10.1378/chest.08-0371.
7. Bekmezian A, Fee C, Weber E. Clinical pathway improves pediatrics asthma management in the emergency department and reduces admissions. J Asthma. 2015;52(8):806-814. https://doi.org/10.3109/02770903.2015.1019086.
8. Chen KH, Chen CC, Liu HE, Tzeng PC, Glasziou PP. Effectiveness of paediatric asthma clinical pathways: a narrative systematic review. J Asthma. 2014;51(5):480-492. https://doi.org/10.3109/02770903.2014.887728.
9. Johnson KB, Blaisdell CJ, Walker A, Eggleston P. Effectiveness of a clinical pathway for inpatient asthma management. Pediatrics. 2000;106(5):1006-1012. https://doi.org/10.1542/peds.106.5.1006.
10. Kelly CS, Andersen CL, Pestian JP, et al. Improved outcomes for hospitalized asthmatic children using a clinical pathway. Ann Allergy Asthma Immunol. 2000;84(5):509-516. https://doi.org/10.1016/S1081-1206(10)62514-8.
11. McDowell KM, Chatburn RL, Myers TR, O’Riordan MA, Kercsmar CM. A cost-saving algorithm for children hospitalized for status asthmaticus. Arch Pediatr Adolesc Med. 1998;152(10):977-984. https://doi.org/10.1001/archpedi.152.10.977.
12. Miller AG, Breslin ME, Pineda LC, Fox JW. An asthma protocol improved adherence to evidence-based guidelines for pediatric subjects with status asthmaticus in the emergency department. Respir Care. 2015;60(12):1759-1764. https://doi.org/10.4187/respcare.04011.
13. Nkoy F, Fassl B, Stone B, et al. Improving pediatric asthma care and outcomes across multiple hospitals. Pediatrics. 2015;136(6):e1602-e1610. https://doi.org/10.1542/peds.2015-0285.
14. Rutman L, Atkins RC, Migita R, et al. Modification of an established pediatric asthma pathway improves evidence-based, efficient care. Pediatrics. 2016;138(6). https://doi.org/10.1542/peds.2016-1248.
15. Glauber JH, Farber HJ, Homer CJ. Asthma clinical pathways: toward what end? Pediatrics. 2001;107(3):590-592. https://doi.org/10.1542/peds.107.3.590.
16. Grimshaw J, Eccles M, Thomas R, et al. Toward evidence-based quality improvement. Evidence (and its limitations) of the effectiveness of guideline dissemination and implementation strategies 1966-1998. J Gen Intern Med. 2006;21(2):S14-S20. https://doi.org/10.1111/j.1525-1497.2006.00357.x.
17. Scott SD, Grimshaw J, Klassen TP, Nettel-Aguirre A, Johnson DW. Understanding implementation processes of clinical pathways and clinical practice guidelines in pediatric contexts: a study protocol. Implement Sci. 2011;6:133. https://doi.org/10.1186/1748-5908-6-133.
18. Walls TA, Hughes NT, Mullan PC, Chamberlain JM, Brown K. Improving pediatric asthma outcomes in a community emergency department. Pediatrics. 2017;139(1). https://doi.org/10.1542/peds.2016-0088.
19. Kaiser SV, Lam R, Cabana MD, et al. Best practices in implementing inpatient pediatric asthma pathways: a qualitative study. J Asthma. 2019:1-11. https://doi.org/10.1080/02770903.2019.1606237.
20. Leyenaar JK, Ralston SL, Shieh MS, Pekow PS, Mangione-Smith R, Lindenauer PK. Epidemiology of pediatric hospitalizations at general hospitals and freestanding children’s hospitals in the United States. J Hosp Med. 2016;11(11):743-749. https://doi.org/10.1002/jhm.2624.
21. Franca UL, McManus ML. Availability of definitive hospital care for children. JAMA Pediatr. 2017;171(9):e171096. https://doi.org/10.1001/jamapediatrics.2017.1096.
22. McDaniel CE, Jennings R, Schroeder AR, Paciorkowski N, Hofmann M, Leyenaar J. Aligning inpatient pediatric research with settings of care: a call to action. Pediatrics. 2019;143(5). https://doi.org/10.1542/peds.2018-2648.
23. Kaiser SV JB. Value in inpatient pediatrics network launches National Asthma Project. In: AAP Quality Connections 2018; 26:8-9. Retrieved from: https://www.aap.org/en-us/Documents/coqips_newsletter_2018_winter_26.pdf
24. Value in Inpatient Pediatrics. https://www.aap.org/en-us/professional-resources/quality-improvement/Pages/Value-in-Inpatient-Pediatrics.aspx. Accessed December 1, 2017.
25. The Breakthrough Series: IHI’s Collaborative Model for Achieving Breakthrough Improvement. IHI Innovation Series white paper. Boston: Institute for Healthcare Improvement; 2003. Retrieved from: www.IHI.org
26. Powell BJ, Waltz TJ, Chinman MJ, et al. A refined compilation of implementation strategies: results from the Expert Recommendations for Implementing Change (ERIC) project. Implement Sci. 2015;10:21. https://doi.org/10.1186/s13012-015-0209-1.
27. Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4:50. https://doi.org/10.1186/1748-5908-4-50.
28. Braun VaC, V. Thematic analysis. In: H. Cooper PC, Long DL, Panter AT, Rindskopf E, Sher KJ, eds. APA handbook of research methods in psychology, Vol 2. Research designs: Quantitative, qualitative, neuropsychologial, and biological. Washington, DC, US: American Psychological Association; 2012. https://doi.org/10.1037/13620-000.
29. Charmaz K. Grounded Theory. 2nd ed. Thousand Oaks, CA: SAGE Publications; 2014.
30. Creswell JW, Poth CNCN CJaP. Qualitative Inquiry and Research Design: Choosing Among Five Approaches. Thousand Oaks, CA: Sage; 2017.
31. Kaiser SV, Rodean J, Bekmezian A, et al. Effectiveness of pediatric asthma pathways for hospitalized children: a multicenter, national analysis. J. Pediatr. 2018;197:165-171. https://doi.org/10.1016/j.jpeds.2018.01.084.
32. Leyenaar JK, Andrews CB, Tyksinski ER, Biondi E, Parikh K, Ralston S. Facilitators of interdepartmental quality improvement: a mixed-methods analysis of a collaborative to improve pediatric community-acquired pneumonia management. BMJ Qual Saf. 2019;28(3):215-222. https://doi.org/10.1136/bmjqs-2018-008065. 33. Ralston SL, Atwood EC, Garber MD, Holmes AV. What works to reduce unnecessary care for bronchiolitis? A qualitative analysis of a national collaborative. Acad Pediatr. 2017;17(2):198-204. https://doi.org/10.1016/j.acap.2016.07.001.
33. Ralston SL, Atwood EC, Garber MD, Holmes AV. What works to reduce unnecessary care for bronchiolitis? A qualitative analysis of a national collaborative. Acad Pediatr. 2017;17(2):198-204. https://doi.org/10.1016/j.acap.2016.07.001.
34. Parikh K, Biondi E, Nazif J, et al. A multicenter collaborative to improve care of community acquired pneumonia in hospitalized children. Pediatrics. 2017;139(2). https://doi.org/10.1542/peds.2016-1411.
35. Ralston S, Garber M, Narang S, et al. Decreasing unnecessary utilization in acute bronchiolitis care: results from the value in inpatient pediatrics network. J Hosp Med. 2013;8(1):25-30. https://doi.org/10.1002/jhm.1982.
36. Gupta N CA, Cabana MD, Jennings B, Parikh K, Kaiser SV. PIPA (Pathways for Improving Pediatric Asthma Care): Process Evaluation of a National Collaborative to Implement Pathways. Platform presented at Pediatric Academic Society National Meeting. Baltimore, Maryland; 2019.
1. National Asthma E, Prevention P. Expert Panel Report 3 (EPR-3): Guidelines for the diagnosis and management of asthma-summary report 2007. J Allergy Clin Immunol. 2007;120(5):S94-S138. https://doi.org/10.1016/j.jaci.2007.09.043.
2. Bekmezian A, Hersh AL, Maselli JH, Cabana MD. Pediatric emergency departments are more likely than general emergency departments to treat asthma exacerbation with systemic corticosteroids. J Asthma. 2011;48(1):69-74. https://doi.org/10.3109/02770903.2010.535884.
3. Biagini Myers JM, Simmons JM, Kercsmar CM, et al. Heterogeneity in asthma care in a statewide collaborative: the Ohio Pediatric Asthma Repository. Pediatrics. 2015;135(2):271-279. https://doi.org/10.1542/peds.2014-2230.
4. Kharbanda AB, Hall M, Shah SS, et al. Variation in resource utilization across a national sample of pediatric emergency departments. J Pediatr. 2013;163(1):230-236. https://doi.org/10.1016/j.jpeds.2012.12.013.
5. O’Con
6. Lougheed MD, Garvey N, Chapman KR, et al. Variations and gaps in management of acute asthma in Ontario emergency departments. Chest. 2009;135(3):724-736. https://doi.org/10.1378/chest.08-0371.
7. Bekmezian A, Fee C, Weber E. Clinical pathway improves pediatrics asthma management in the emergency department and reduces admissions. J Asthma. 2015;52(8):806-814. https://doi.org/10.3109/02770903.2015.1019086.
8. Chen KH, Chen CC, Liu HE, Tzeng PC, Glasziou PP. Effectiveness of paediatric asthma clinical pathways: a narrative systematic review. J Asthma. 2014;51(5):480-492. https://doi.org/10.3109/02770903.2014.887728.
9. Johnson KB, Blaisdell CJ, Walker A, Eggleston P. Effectiveness of a clinical pathway for inpatient asthma management. Pediatrics. 2000;106(5):1006-1012. https://doi.org/10.1542/peds.106.5.1006.
10. Kelly CS, Andersen CL, Pestian JP, et al. Improved outcomes for hospitalized asthmatic children using a clinical pathway. Ann Allergy Asthma Immunol. 2000;84(5):509-516. https://doi.org/10.1016/S1081-1206(10)62514-8.
11. McDowell KM, Chatburn RL, Myers TR, O’Riordan MA, Kercsmar CM. A cost-saving algorithm for children hospitalized for status asthmaticus. Arch Pediatr Adolesc Med. 1998;152(10):977-984. https://doi.org/10.1001/archpedi.152.10.977.
12. Miller AG, Breslin ME, Pineda LC, Fox JW. An asthma protocol improved adherence to evidence-based guidelines for pediatric subjects with status asthmaticus in the emergency department. Respir Care. 2015;60(12):1759-1764. https://doi.org/10.4187/respcare.04011.
13. Nkoy F, Fassl B, Stone B, et al. Improving pediatric asthma care and outcomes across multiple hospitals. Pediatrics. 2015;136(6):e1602-e1610. https://doi.org/10.1542/peds.2015-0285.
14. Rutman L, Atkins RC, Migita R, et al. Modification of an established pediatric asthma pathway improves evidence-based, efficient care. Pediatrics. 2016;138(6). https://doi.org/10.1542/peds.2016-1248.
15. Glauber JH, Farber HJ, Homer CJ. Asthma clinical pathways: toward what end? Pediatrics. 2001;107(3):590-592. https://doi.org/10.1542/peds.107.3.590.
16. Grimshaw J, Eccles M, Thomas R, et al. Toward evidence-based quality improvement. Evidence (and its limitations) of the effectiveness of guideline dissemination and implementation strategies 1966-1998. J Gen Intern Med. 2006;21(2):S14-S20. https://doi.org/10.1111/j.1525-1497.2006.00357.x.
17. Scott SD, Grimshaw J, Klassen TP, Nettel-Aguirre A, Johnson DW. Understanding implementation processes of clinical pathways and clinical practice guidelines in pediatric contexts: a study protocol. Implement Sci. 2011;6:133. https://doi.org/10.1186/1748-5908-6-133.
18. Walls TA, Hughes NT, Mullan PC, Chamberlain JM, Brown K. Improving pediatric asthma outcomes in a community emergency department. Pediatrics. 2017;139(1). https://doi.org/10.1542/peds.2016-0088.
19. Kaiser SV, Lam R, Cabana MD, et al. Best practices in implementing inpatient pediatric asthma pathways: a qualitative study. J Asthma. 2019:1-11. https://doi.org/10.1080/02770903.2019.1606237.
20. Leyenaar JK, Ralston SL, Shieh MS, Pekow PS, Mangione-Smith R, Lindenauer PK. Epidemiology of pediatric hospitalizations at general hospitals and freestanding children’s hospitals in the United States. J Hosp Med. 2016;11(11):743-749. https://doi.org/10.1002/jhm.2624.
21. Franca UL, McManus ML. Availability of definitive hospital care for children. JAMA Pediatr. 2017;171(9):e171096. https://doi.org/10.1001/jamapediatrics.2017.1096.
22. McDaniel CE, Jennings R, Schroeder AR, Paciorkowski N, Hofmann M, Leyenaar J. Aligning inpatient pediatric research with settings of care: a call to action. Pediatrics. 2019;143(5). https://doi.org/10.1542/peds.2018-2648.
23. Kaiser SV JB. Value in inpatient pediatrics network launches National Asthma Project. In: AAP Quality Connections 2018; 26:8-9. Retrieved from: https://www.aap.org/en-us/Documents/coqips_newsletter_2018_winter_26.pdf
24. Value in Inpatient Pediatrics. https://www.aap.org/en-us/professional-resources/quality-improvement/Pages/Value-in-Inpatient-Pediatrics.aspx. Accessed December 1, 2017.
25. The Breakthrough Series: IHI’s Collaborative Model for Achieving Breakthrough Improvement. IHI Innovation Series white paper. Boston: Institute for Healthcare Improvement; 2003. Retrieved from: www.IHI.org
26. Powell BJ, Waltz TJ, Chinman MJ, et al. A refined compilation of implementation strategies: results from the Expert Recommendations for Implementing Change (ERIC) project. Implement Sci. 2015;10:21. https://doi.org/10.1186/s13012-015-0209-1.
27. Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4:50. https://doi.org/10.1186/1748-5908-4-50.
28. Braun VaC, V. Thematic analysis. In: H. Cooper PC, Long DL, Panter AT, Rindskopf E, Sher KJ, eds. APA handbook of research methods in psychology, Vol 2. Research designs: Quantitative, qualitative, neuropsychologial, and biological. Washington, DC, US: American Psychological Association; 2012. https://doi.org/10.1037/13620-000.
29. Charmaz K. Grounded Theory. 2nd ed. Thousand Oaks, CA: SAGE Publications; 2014.
30. Creswell JW, Poth CNCN CJaP. Qualitative Inquiry and Research Design: Choosing Among Five Approaches. Thousand Oaks, CA: Sage; 2017.
31. Kaiser SV, Rodean J, Bekmezian A, et al. Effectiveness of pediatric asthma pathways for hospitalized children: a multicenter, national analysis. J. Pediatr. 2018;197:165-171. https://doi.org/10.1016/j.jpeds.2018.01.084.
32. Leyenaar JK, Andrews CB, Tyksinski ER, Biondi E, Parikh K, Ralston S. Facilitators of interdepartmental quality improvement: a mixed-methods analysis of a collaborative to improve pediatric community-acquired pneumonia management. BMJ Qual Saf. 2019;28(3):215-222. https://doi.org/10.1136/bmjqs-2018-008065. 33. Ralston SL, Atwood EC, Garber MD, Holmes AV. What works to reduce unnecessary care for bronchiolitis? A qualitative analysis of a national collaborative. Acad Pediatr. 2017;17(2):198-204. https://doi.org/10.1016/j.acap.2016.07.001.
33. Ralston SL, Atwood EC, Garber MD, Holmes AV. What works to reduce unnecessary care for bronchiolitis? A qualitative analysis of a national collaborative. Acad Pediatr. 2017;17(2):198-204. https://doi.org/10.1016/j.acap.2016.07.001.
34. Parikh K, Biondi E, Nazif J, et al. A multicenter collaborative to improve care of community acquired pneumonia in hospitalized children. Pediatrics. 2017;139(2). https://doi.org/10.1542/peds.2016-1411.
35. Ralston S, Garber M, Narang S, et al. Decreasing unnecessary utilization in acute bronchiolitis care: results from the value in inpatient pediatrics network. J Hosp Med. 2013;8(1):25-30. https://doi.org/10.1002/jhm.1982.
36. Gupta N CA, Cabana MD, Jennings B, Parikh K, Kaiser SV. PIPA (Pathways for Improving Pediatric Asthma Care): Process Evaluation of a National Collaborative to Implement Pathways. Platform presented at Pediatric Academic Society National Meeting. Baltimore, Maryland; 2019.
© 2020 Society of Hospital Medicine
Things We Do For No Reason: Routine Blood Culture Acquisition for Children Hospitalized with Community-Acquired Pneumonia

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 4-year-old previously healthy, fully immunized boy presented to the emergency department (ED) with three days of worsening cough, fever to 103oF, dyspnea, and decreased oral intake. In the ED, he was febrile, temperature 102.7oF, heart rate 115 beats/min, respiratory rate 30 breaths/min, and O2 saturation 86%. Pertinent findings identified on examination included tachypnea, dry mucous membranes, and decreased breath sounds in the posterior right lung fields. Chest radiograph revealed a right lower lobe opacification concerning for community-acquired pneumonia (CAP). He was admitted to the hospital due to hypoxemia and dehydration. A blood culture was obtained, and treatment with ampicillin was initiated. The following morning, he was afebrile, clinically improved, and no longer hypoxemic, but the blood culture grew Gram-positive cocci. Another blood culture was performed, and he was switched to vancomycin. The next day, penicillin-susceptible Streptococcus pneumoniae was confirmed from the original culture, and he was discharged home on high-dose amoxicillin.
WHY YOU MIGHT THINK A BLOOD CULTURE IS HELPFUL
CAP is a prominent cause of childhood morbidity and among the most common causes for acute childhood hospitalizations in the United States, with 124,900 hospital stays documented in 2012.1 In 2011, the Infectious Diseases Society of America (IDSA) released recommendations for pediatric CAP in immunocompetent children aged >3 months without chronic medical conditions. The recommendations clearly discourage blood cultures in the outpatient setting but are less direct in the inpatient setting. The recommendations state that providers should obtain blood cultures “in children requiring hospitalization for presumed bacterial CAP that is moderate to severe, particularly those with complicated pneumonia.”2 The recommendation is graded as “strong”, though the IDSA acknowledged the “low” quality of supporting evidence. Although the organization provides a classification for “complicated pneumonia,” it does not define what constitutes mild versus moderate or severe pneumonia.
Without clear recommendations, decisions to obtain blood cultures for hospitalized children with CAP vary among providers and institutions, with the reported hospital-to-hospital variation being as large as 0%-78.7%.3 Some believe that any child hospitalized with CAP meets the definition of moderate to severe pneumonia and have implemented projects to increase blood culture acquisition for this population.4 The decision to err on the side of routinely obtaining a blood culture may come from providers’ prevalent worry of “missing” a diagnosis, desire to target antibacterial therapy, and assumption that it will provide additional information for patients lacking improvement.
WHY A ROUTINE BLOOD CULTURE ON PEDIATRIC CAP ADMISSIONS IS NOT HELPFUL
Since the publication of the 2011 IDSA guidelines, new evidence has revealed a decreasing incidence of bacteremia in pediatric populations.5 Moreover, viruses were the most frequently identified pathogens in children hospitalized with CAP in a large study, which were isolated in 66% of patients, whereas typical bacteria (either alone or in combination with a virus) were identified in only 7% of cases.6 When blood cultures are obtained for pediatric CAP, the incidence of a true bacterial bloodstream pathogen is 1.4%-7% of patients in the United States in the conjugate vaccine era.7-11 Given that the practice of obtaining blood cultures varies widely among hospitalized patients and that cultures are often obtained based on perceived severity of presentation,8,9,12 the true incidence of bacteremia in children with CAP would likely be lower if blood cultures were performed in all patients.
Since the introduction of the first conjugated pneumococcal vaccine, the prevalence of penicillin resistance among pneumococcal isolates dramatically declined,13 though with geographic variability.14 Therefore, when we isolate pneumococcus strains, resistance prevalence requires that we alter treatment much less frequently in the majority of patients with CAP receiving IDSA-recommended ampicillin/amoxicillin.2 In a large six-center, geographically dispersed retrospective cohort study, Neuman et al. reported a rate of true bacteremia of 2.53%; 82% of all pathogens and 92% of pneumococcal isolates were susceptible to penicillin. Therefore, the authors estimated that 667 children hospitalized with CAP would need blood cultures to identify one child requiring an antibiotic other than an aminopenicillin.9 Staphylococcus aureus was identified only in 1% (23/2,138) of patients in the EPIC cohort; the pathogen was identified via blood culture in only 26% (6/23) of these patients.15 Therefore, the concern about the possibility of S. aureus may be a common reason for physicians straying from IDSA-recommended therapy, but it is an uncommon cause of CAP and infrequently identified via blood culture.
Blood culture contaminants have been reported to approach the rate of true pathogens in some studies8,9,11 and be equal or exceed the rates in others.7,16 While awaiting bacterial speciation, antibiotic coverage is often broadened, even for contaminants,8 which can result in unnecessary exposure to nephrotoxic agents such as vancomycin, cause rare adverse events such as Stevens-Johnson syndrome, contribute to antibiotic resistance and unnecessary costs, and increase the length of stay and laboratory utilization.17-19
WHEN MIGHT A BLOOD CULTURE BE HELPFUL
Given the low penicillin resistance prevalence among pneumococcal isolates in several parts of the United States, blood cultures should be used to identify patients with nonpneumococcal CAP as these patients are more likely to require antibiotics other than penicillin or aminopenicillin. Children with complicated pneumonia are more likely to have nonpneumococcal etiologies than children with uncomplicated pneumonia.2 Moreover, literature published since the IDSA guidelines continues to indicate that the incidence of bacteremia in complicated pneumonia is significantly higher than that in uncomplicated pneumonia (Table). This further supports the IDSA guideline recommendation for blood culture acquisition in children with complicated pneumonia.2
One difficulty in interpreting these data is that each publication used a different definition of “complicated” pneumonia, probably due to differences in data sources. Neuman et al. incorporated the narrowest definition of severe and complicated pneumonia as patients who were either admitted to an intensive care unit (ICU) or who underwent a pleural drainage procedure.9 Myers’ and Shah’s definitions were similar to each other but much broader than that of Neuman et al. Shah et al. included lung abscess/necrosis, parapneumonic effusion/empyema, or bronchopleural fistula.11 Myers et al. included the same indications but qualified their pleural fluid effusions as “moderate-to-large” and any effusion that required pleural drainage procedure.8 Myers et al. also reported bacteremia in 75% of patients with metastatic complications, including osteomyelitis.8 These definitions of complicated pneumonia may at least partially explain the differences noted in the rates of bacteremia in complicated pneumonia, with the patients in the study of Myers et al. potentially representing the most severe cohort and with the highest rate of bacteremia8,9 (Table).
These studies not only support the definition of complicated pneumonia put forward by the IDSA but also provide further information, though imperfect, on how to define “moderate to severe.” All the patients with bacteremia in the report of Heine et al. had complicated pneumonia and were described on chart review as either toxic-appearing or requiring ICU care.7 This, in addition to the inclusion of ICU care in the definition of complicated pneumonia of Neuman et al.,9 indicates that children with CAP requiring ICU care may be at higher risk of bacteremia. In fact, the British Thoracic Society guidelines do not recommend microbiological investigations of children with CAP, including blood culture, unless a child requires ICU care.20
WHAT YOU SHOULD DO INSTEAD
Given the low rate of bacteremia in CAP, the risk of blood culture contaminants, and the small likelihood that isolation of a pathogen alters treatment for children, we recommend not using hospital admission as the determining factor for blood culture acquisition. Instead, we recommend a more targeted approach. To achieve a higher rate of true-positive bacteremia in immunocompetent children with up-to-date vaccinations, we recommend acquiring a blood culture in children with complicated pneumonia, metastatic complications, or with ICU needs. By initiating the IDSA-recommended ampicillin/amoxicillin in the remaining hospitalized patients and acquiring blood cultures for the minority of patients who do not improve, we can increase the likelihood of isolating penicillin-resistant bacteria.
Attempting to balance the importance of identifying clinically important bacteremia for children hospitalized with CAP and the inherent risks of obtaining blood cultures for all hospitalized patients, Andrews et al. created and analyzed a cost-effectiveness model. The authors compared universal acquisition of blood cultures for hospitalized children with CAP versus a targeted approach with blood cultures obtained in patients with effusion or empyema, requiring ICU care, or who are immunosuppressed. Based on this model, a targeted approach could save more than $187 million annually, reduce the number of cultures needed to result in a meaningful change in antibiotic therapy for one patient from 122 to 42, and would “miss” only approximately one case of bacteremia resulting in treatment failure per 1,400 patients.17
RECOMMENDATIONS
- Do not obtain blood culture routinely for children aged >3 months hospitalized for uncomplicated CAP.
- Obtain a blood culture for the following hospitalized patients with CAP:
a. Patients with complicated CAP as defined by the IDSA, particularly those with empyema, abscess, or fistula, or metastatic complications of pneumonia (Table); or
b. Patients with CAP requiring ICU care20 for the management of shock and/or advanced respiratory support.
c. Patients with CAP judged to need antibiotic treatment with an agent other than the IDSA-recommended ampicillin/penicillin (concern for pathogens other than penicillin-sensitive S. pneumonia, immunocompromised or under-immunized status, or inadequate clinical response to empiric ampicillin therapy).
CONCLUSION
Implementing a more targeted approach to blood culture acquisition for hospitalized children with CAP will hopefully increase the yield of true bacterial pathogens that alter management decisions. A targeted approach for the child in the opening vignette would have saved him from the pain of unnecessary phlebotomy (repeat culture), exposure to vancomycin as a nephrotoxic agent, and an additional hospital day.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?™” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by e-mailing [email protected].
1. Whitney P, Whitt AJW, Elixhauser A. Overview of hospital stays for children in the United States, 2012. Statistical Brief 187. 2014;187. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb187-Hospital-Stays-Children-2012.jsp. Accessed December 21, 2017.
2. Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53(7):e25-e76. https://doi.org/10.1093/cid/cir531.
3. Brogan TV, Hall M, Williams DJ, et al. Variability in processes of care and outcomes among children hospitalized with community-acquired pneumonia. Pediatr Infect Dis J. 2012;31(10):1036-1041. https://doi.org/10.1097/INF.0b013e31825f2b10.
4. Murtagh Kurowski E, Shah SS, Thomson J, et al. Improvement methodology increases guideline recommended blood cultures in children with pneumonia. Pediatrics. 2015;135(4):e1052-e1059. https://doi.org/10.1542/peds.2014-2077.
5. Greenhow TL, Hung YY, Herz A. Bacteremia in children 3 to 36 months old after introduction of conjugated pneumococcal vaccines. Pediatrics. 2017;139(4):e20162098. https://doi.org/10.1542/peds.2016-2098.
6. Jain S, Williams DJ, Arnold SR, et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015;372(9):835-845. https://doi.org/10.1056/NEJMoa1405870.
7. Heine D, Cochran C, Moore M, Titus MO, Andrews AL. The prevalence of bacteremia in pediatric patients with community-acquired pneumonia: guidelines to reduce the frequency of obtaining blood cultures. Hosp Pediatr. 2013;3(2):92-96. https://doi.org/10.1542/hpeds.2012-0050.
8. Myers AL, Hall M, Williams DJ, et al. Prevalence of bacteremia in hospitalized pediatric patients with community-acquired pneumonia. Pediatr Infect Dis J. 2013;32(7):736-740. https://doi.org/10.1097/INF.0b013e318290bf63.
9. Neuman MI, Hall M, Lipsett SC, et al. Utility of blood culture among children hospitalized with community-acquired pneumonia. Pediatrics. 2017;140(3). https://doi.org/10.1542/peds.2017-1013.
10. Sandora TJ, Desai R, Miko BA, Harper MB. Assessing quality indicators for pediatric community-acquired pneumonia. Am J Med Qual. 2009;24(5):419-427. https://doi.org/10.1177/1062860609337900.
11. Shah SS, Dugan MH, Bell LM, et al. Blood cultures in the emergency department evaluation of childhood pneumonia. Pediatr Infect Dis J. 2011;30(6):475-479. https://doi.org/10.1097/INF.0b013e31820a5adb.
12. Davis TR, Evans HR, Murtas J et al. Utility of blood cultures in children admitted to hospital with community-acquired pneumonia. J Paediatr Child Health. 2017;53(3):232-236. https://doi.org/10.1111/jpc.13376.
13. Williams DJ, Shah SS. Community-acquired pneumonia in the conjugate vaccine era. J Pediatr Infect Dis Soc. 2012;1(4):314-328. https://doi.org/10.1093/jpids/pis101.
14. Kyaw MH, Lynfield R, Schaffner W, et al. Effect of introduction of the pneumococcal conjugate vaccine on drug-resistant Streptococcus pneumoniae. N Engl J Med. 2006;354(14):1455-1463. https://doi.org/10.1056/NEJMoa051642.
15. Frush JM, Zhu Y, Edwards KM, et al. Prevalence of Staphylococcus aureus and use of antistaphylococcal therapy in children hospitalized with pneumonia. J Hosp Med. 2018;13(12):848-852. https://doi.org/10.12788/jhm.3093.
16. Mendoza-Paredes A, Bastos J, Leber M, Erickson E, Waseem M. Utility of blood culture in uncomplicated pneumonia in children. Clin Med Insights Pediatr. 2013;7:1-5. https://doi.org/10.4137/CMPed.S8051.
17. Andrews AL, Simpson AN, Heine D, Teufel II RJ. A cost-effectiveness analysis of obtaining blood cultures in children hospitalized for community-acquired pneumonia. J Pediatr. 2015;167(6):1280-1286. https://doi.org/10.1016/j.jpeds.2015.09.025.
18. Leyenaar JK, Lagu T, Shieh MS, Pekow PS, Lindenauer PK. Variation in resource utilization for the management of uncomplicated community-acquired pneumonia across community and children’s hospitals. J Pediatr. 2014;165(3):585-591. https://doi.org/10.1016/j.jpeds.2014.04.062.
19. McCulloh RJ, Koster MP, Yin DE, et al. Evaluating the use of blood cultures in the management of children hospitalized for community-acquired pneumonia. PloS One. 2015;10(2):e0117462. https://doi.org/10.1371/journal.pone.0117462.
20. Harris M, Clark J, Coote N, et al. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011;66(2):ii1-ii23. https://doi.org/10.1136/thoraxjnl-2011-200598.

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 4-year-old previously healthy, fully immunized boy presented to the emergency department (ED) with three days of worsening cough, fever to 103oF, dyspnea, and decreased oral intake. In the ED, he was febrile, temperature 102.7oF, heart rate 115 beats/min, respiratory rate 30 breaths/min, and O2 saturation 86%. Pertinent findings identified on examination included tachypnea, dry mucous membranes, and decreased breath sounds in the posterior right lung fields. Chest radiograph revealed a right lower lobe opacification concerning for community-acquired pneumonia (CAP). He was admitted to the hospital due to hypoxemia and dehydration. A blood culture was obtained, and treatment with ampicillin was initiated. The following morning, he was afebrile, clinically improved, and no longer hypoxemic, but the blood culture grew Gram-positive cocci. Another blood culture was performed, and he was switched to vancomycin. The next day, penicillin-susceptible Streptococcus pneumoniae was confirmed from the original culture, and he was discharged home on high-dose amoxicillin.
WHY YOU MIGHT THINK A BLOOD CULTURE IS HELPFUL
CAP is a prominent cause of childhood morbidity and among the most common causes for acute childhood hospitalizations in the United States, with 124,900 hospital stays documented in 2012.1 In 2011, the Infectious Diseases Society of America (IDSA) released recommendations for pediatric CAP in immunocompetent children aged >3 months without chronic medical conditions. The recommendations clearly discourage blood cultures in the outpatient setting but are less direct in the inpatient setting. The recommendations state that providers should obtain blood cultures “in children requiring hospitalization for presumed bacterial CAP that is moderate to severe, particularly those with complicated pneumonia.”2 The recommendation is graded as “strong”, though the IDSA acknowledged the “low” quality of supporting evidence. Although the organization provides a classification for “complicated pneumonia,” it does not define what constitutes mild versus moderate or severe pneumonia.
Without clear recommendations, decisions to obtain blood cultures for hospitalized children with CAP vary among providers and institutions, with the reported hospital-to-hospital variation being as large as 0%-78.7%.3 Some believe that any child hospitalized with CAP meets the definition of moderate to severe pneumonia and have implemented projects to increase blood culture acquisition for this population.4 The decision to err on the side of routinely obtaining a blood culture may come from providers’ prevalent worry of “missing” a diagnosis, desire to target antibacterial therapy, and assumption that it will provide additional information for patients lacking improvement.
WHY A ROUTINE BLOOD CULTURE ON PEDIATRIC CAP ADMISSIONS IS NOT HELPFUL
Since the publication of the 2011 IDSA guidelines, new evidence has revealed a decreasing incidence of bacteremia in pediatric populations.5 Moreover, viruses were the most frequently identified pathogens in children hospitalized with CAP in a large study, which were isolated in 66% of patients, whereas typical bacteria (either alone or in combination with a virus) were identified in only 7% of cases.6 When blood cultures are obtained for pediatric CAP, the incidence of a true bacterial bloodstream pathogen is 1.4%-7% of patients in the United States in the conjugate vaccine era.7-11 Given that the practice of obtaining blood cultures varies widely among hospitalized patients and that cultures are often obtained based on perceived severity of presentation,8,9,12 the true incidence of bacteremia in children with CAP would likely be lower if blood cultures were performed in all patients.
Since the introduction of the first conjugated pneumococcal vaccine, the prevalence of penicillin resistance among pneumococcal isolates dramatically declined,13 though with geographic variability.14 Therefore, when we isolate pneumococcus strains, resistance prevalence requires that we alter treatment much less frequently in the majority of patients with CAP receiving IDSA-recommended ampicillin/amoxicillin.2 In a large six-center, geographically dispersed retrospective cohort study, Neuman et al. reported a rate of true bacteremia of 2.53%; 82% of all pathogens and 92% of pneumococcal isolates were susceptible to penicillin. Therefore, the authors estimated that 667 children hospitalized with CAP would need blood cultures to identify one child requiring an antibiotic other than an aminopenicillin.9 Staphylococcus aureus was identified only in 1% (23/2,138) of patients in the EPIC cohort; the pathogen was identified via blood culture in only 26% (6/23) of these patients.15 Therefore, the concern about the possibility of S. aureus may be a common reason for physicians straying from IDSA-recommended therapy, but it is an uncommon cause of CAP and infrequently identified via blood culture.
Blood culture contaminants have been reported to approach the rate of true pathogens in some studies8,9,11 and be equal or exceed the rates in others.7,16 While awaiting bacterial speciation, antibiotic coverage is often broadened, even for contaminants,8 which can result in unnecessary exposure to nephrotoxic agents such as vancomycin, cause rare adverse events such as Stevens-Johnson syndrome, contribute to antibiotic resistance and unnecessary costs, and increase the length of stay and laboratory utilization.17-19
WHEN MIGHT A BLOOD CULTURE BE HELPFUL
Given the low penicillin resistance prevalence among pneumococcal isolates in several parts of the United States, blood cultures should be used to identify patients with nonpneumococcal CAP as these patients are more likely to require antibiotics other than penicillin or aminopenicillin. Children with complicated pneumonia are more likely to have nonpneumococcal etiologies than children with uncomplicated pneumonia.2 Moreover, literature published since the IDSA guidelines continues to indicate that the incidence of bacteremia in complicated pneumonia is significantly higher than that in uncomplicated pneumonia (Table). This further supports the IDSA guideline recommendation for blood culture acquisition in children with complicated pneumonia.2
One difficulty in interpreting these data is that each publication used a different definition of “complicated” pneumonia, probably due to differences in data sources. Neuman et al. incorporated the narrowest definition of severe and complicated pneumonia as patients who were either admitted to an intensive care unit (ICU) or who underwent a pleural drainage procedure.9 Myers’ and Shah’s definitions were similar to each other but much broader than that of Neuman et al. Shah et al. included lung abscess/necrosis, parapneumonic effusion/empyema, or bronchopleural fistula.11 Myers et al. included the same indications but qualified their pleural fluid effusions as “moderate-to-large” and any effusion that required pleural drainage procedure.8 Myers et al. also reported bacteremia in 75% of patients with metastatic complications, including osteomyelitis.8 These definitions of complicated pneumonia may at least partially explain the differences noted in the rates of bacteremia in complicated pneumonia, with the patients in the study of Myers et al. potentially representing the most severe cohort and with the highest rate of bacteremia8,9 (Table).
These studies not only support the definition of complicated pneumonia put forward by the IDSA but also provide further information, though imperfect, on how to define “moderate to severe.” All the patients with bacteremia in the report of Heine et al. had complicated pneumonia and were described on chart review as either toxic-appearing or requiring ICU care.7 This, in addition to the inclusion of ICU care in the definition of complicated pneumonia of Neuman et al.,9 indicates that children with CAP requiring ICU care may be at higher risk of bacteremia. In fact, the British Thoracic Society guidelines do not recommend microbiological investigations of children with CAP, including blood culture, unless a child requires ICU care.20
WHAT YOU SHOULD DO INSTEAD
Given the low rate of bacteremia in CAP, the risk of blood culture contaminants, and the small likelihood that isolation of a pathogen alters treatment for children, we recommend not using hospital admission as the determining factor for blood culture acquisition. Instead, we recommend a more targeted approach. To achieve a higher rate of true-positive bacteremia in immunocompetent children with up-to-date vaccinations, we recommend acquiring a blood culture in children with complicated pneumonia, metastatic complications, or with ICU needs. By initiating the IDSA-recommended ampicillin/amoxicillin in the remaining hospitalized patients and acquiring blood cultures for the minority of patients who do not improve, we can increase the likelihood of isolating penicillin-resistant bacteria.
Attempting to balance the importance of identifying clinically important bacteremia for children hospitalized with CAP and the inherent risks of obtaining blood cultures for all hospitalized patients, Andrews et al. created and analyzed a cost-effectiveness model. The authors compared universal acquisition of blood cultures for hospitalized children with CAP versus a targeted approach with blood cultures obtained in patients with effusion or empyema, requiring ICU care, or who are immunosuppressed. Based on this model, a targeted approach could save more than $187 million annually, reduce the number of cultures needed to result in a meaningful change in antibiotic therapy for one patient from 122 to 42, and would “miss” only approximately one case of bacteremia resulting in treatment failure per 1,400 patients.17
RECOMMENDATIONS
- Do not obtain blood culture routinely for children aged >3 months hospitalized for uncomplicated CAP.
- Obtain a blood culture for the following hospitalized patients with CAP:
a. Patients with complicated CAP as defined by the IDSA, particularly those with empyema, abscess, or fistula, or metastatic complications of pneumonia (Table); or
b. Patients with CAP requiring ICU care20 for the management of shock and/or advanced respiratory support.
c. Patients with CAP judged to need antibiotic treatment with an agent other than the IDSA-recommended ampicillin/penicillin (concern for pathogens other than penicillin-sensitive S. pneumonia, immunocompromised or under-immunized status, or inadequate clinical response to empiric ampicillin therapy).
CONCLUSION
Implementing a more targeted approach to blood culture acquisition for hospitalized children with CAP will hopefully increase the yield of true bacterial pathogens that alter management decisions. A targeted approach for the child in the opening vignette would have saved him from the pain of unnecessary phlebotomy (repeat culture), exposure to vancomycin as a nephrotoxic agent, and an additional hospital day.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?™” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by e-mailing [email protected].

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 4-year-old previously healthy, fully immunized boy presented to the emergency department (ED) with three days of worsening cough, fever to 103oF, dyspnea, and decreased oral intake. In the ED, he was febrile, temperature 102.7oF, heart rate 115 beats/min, respiratory rate 30 breaths/min, and O2 saturation 86%. Pertinent findings identified on examination included tachypnea, dry mucous membranes, and decreased breath sounds in the posterior right lung fields. Chest radiograph revealed a right lower lobe opacification concerning for community-acquired pneumonia (CAP). He was admitted to the hospital due to hypoxemia and dehydration. A blood culture was obtained, and treatment with ampicillin was initiated. The following morning, he was afebrile, clinically improved, and no longer hypoxemic, but the blood culture grew Gram-positive cocci. Another blood culture was performed, and he was switched to vancomycin. The next day, penicillin-susceptible Streptococcus pneumoniae was confirmed from the original culture, and he was discharged home on high-dose amoxicillin.
WHY YOU MIGHT THINK A BLOOD CULTURE IS HELPFUL
CAP is a prominent cause of childhood morbidity and among the most common causes for acute childhood hospitalizations in the United States, with 124,900 hospital stays documented in 2012.1 In 2011, the Infectious Diseases Society of America (IDSA) released recommendations for pediatric CAP in immunocompetent children aged >3 months without chronic medical conditions. The recommendations clearly discourage blood cultures in the outpatient setting but are less direct in the inpatient setting. The recommendations state that providers should obtain blood cultures “in children requiring hospitalization for presumed bacterial CAP that is moderate to severe, particularly those with complicated pneumonia.”2 The recommendation is graded as “strong”, though the IDSA acknowledged the “low” quality of supporting evidence. Although the organization provides a classification for “complicated pneumonia,” it does not define what constitutes mild versus moderate or severe pneumonia.
Without clear recommendations, decisions to obtain blood cultures for hospitalized children with CAP vary among providers and institutions, with the reported hospital-to-hospital variation being as large as 0%-78.7%.3 Some believe that any child hospitalized with CAP meets the definition of moderate to severe pneumonia and have implemented projects to increase blood culture acquisition for this population.4 The decision to err on the side of routinely obtaining a blood culture may come from providers’ prevalent worry of “missing” a diagnosis, desire to target antibacterial therapy, and assumption that it will provide additional information for patients lacking improvement.
WHY A ROUTINE BLOOD CULTURE ON PEDIATRIC CAP ADMISSIONS IS NOT HELPFUL
Since the publication of the 2011 IDSA guidelines, new evidence has revealed a decreasing incidence of bacteremia in pediatric populations.5 Moreover, viruses were the most frequently identified pathogens in children hospitalized with CAP in a large study, which were isolated in 66% of patients, whereas typical bacteria (either alone or in combination with a virus) were identified in only 7% of cases.6 When blood cultures are obtained for pediatric CAP, the incidence of a true bacterial bloodstream pathogen is 1.4%-7% of patients in the United States in the conjugate vaccine era.7-11 Given that the practice of obtaining blood cultures varies widely among hospitalized patients and that cultures are often obtained based on perceived severity of presentation,8,9,12 the true incidence of bacteremia in children with CAP would likely be lower if blood cultures were performed in all patients.
Since the introduction of the first conjugated pneumococcal vaccine, the prevalence of penicillin resistance among pneumococcal isolates dramatically declined,13 though with geographic variability.14 Therefore, when we isolate pneumococcus strains, resistance prevalence requires that we alter treatment much less frequently in the majority of patients with CAP receiving IDSA-recommended ampicillin/amoxicillin.2 In a large six-center, geographically dispersed retrospective cohort study, Neuman et al. reported a rate of true bacteremia of 2.53%; 82% of all pathogens and 92% of pneumococcal isolates were susceptible to penicillin. Therefore, the authors estimated that 667 children hospitalized with CAP would need blood cultures to identify one child requiring an antibiotic other than an aminopenicillin.9 Staphylococcus aureus was identified only in 1% (23/2,138) of patients in the EPIC cohort; the pathogen was identified via blood culture in only 26% (6/23) of these patients.15 Therefore, the concern about the possibility of S. aureus may be a common reason for physicians straying from IDSA-recommended therapy, but it is an uncommon cause of CAP and infrequently identified via blood culture.
Blood culture contaminants have been reported to approach the rate of true pathogens in some studies8,9,11 and be equal or exceed the rates in others.7,16 While awaiting bacterial speciation, antibiotic coverage is often broadened, even for contaminants,8 which can result in unnecessary exposure to nephrotoxic agents such as vancomycin, cause rare adverse events such as Stevens-Johnson syndrome, contribute to antibiotic resistance and unnecessary costs, and increase the length of stay and laboratory utilization.17-19
WHEN MIGHT A BLOOD CULTURE BE HELPFUL
Given the low penicillin resistance prevalence among pneumococcal isolates in several parts of the United States, blood cultures should be used to identify patients with nonpneumococcal CAP as these patients are more likely to require antibiotics other than penicillin or aminopenicillin. Children with complicated pneumonia are more likely to have nonpneumococcal etiologies than children with uncomplicated pneumonia.2 Moreover, literature published since the IDSA guidelines continues to indicate that the incidence of bacteremia in complicated pneumonia is significantly higher than that in uncomplicated pneumonia (Table). This further supports the IDSA guideline recommendation for blood culture acquisition in children with complicated pneumonia.2
One difficulty in interpreting these data is that each publication used a different definition of “complicated” pneumonia, probably due to differences in data sources. Neuman et al. incorporated the narrowest definition of severe and complicated pneumonia as patients who were either admitted to an intensive care unit (ICU) or who underwent a pleural drainage procedure.9 Myers’ and Shah’s definitions were similar to each other but much broader than that of Neuman et al. Shah et al. included lung abscess/necrosis, parapneumonic effusion/empyema, or bronchopleural fistula.11 Myers et al. included the same indications but qualified their pleural fluid effusions as “moderate-to-large” and any effusion that required pleural drainage procedure.8 Myers et al. also reported bacteremia in 75% of patients with metastatic complications, including osteomyelitis.8 These definitions of complicated pneumonia may at least partially explain the differences noted in the rates of bacteremia in complicated pneumonia, with the patients in the study of Myers et al. potentially representing the most severe cohort and with the highest rate of bacteremia8,9 (Table).
These studies not only support the definition of complicated pneumonia put forward by the IDSA but also provide further information, though imperfect, on how to define “moderate to severe.” All the patients with bacteremia in the report of Heine et al. had complicated pneumonia and were described on chart review as either toxic-appearing or requiring ICU care.7 This, in addition to the inclusion of ICU care in the definition of complicated pneumonia of Neuman et al.,9 indicates that children with CAP requiring ICU care may be at higher risk of bacteremia. In fact, the British Thoracic Society guidelines do not recommend microbiological investigations of children with CAP, including blood culture, unless a child requires ICU care.20
WHAT YOU SHOULD DO INSTEAD
Given the low rate of bacteremia in CAP, the risk of blood culture contaminants, and the small likelihood that isolation of a pathogen alters treatment for children, we recommend not using hospital admission as the determining factor for blood culture acquisition. Instead, we recommend a more targeted approach. To achieve a higher rate of true-positive bacteremia in immunocompetent children with up-to-date vaccinations, we recommend acquiring a blood culture in children with complicated pneumonia, metastatic complications, or with ICU needs. By initiating the IDSA-recommended ampicillin/amoxicillin in the remaining hospitalized patients and acquiring blood cultures for the minority of patients who do not improve, we can increase the likelihood of isolating penicillin-resistant bacteria.
Attempting to balance the importance of identifying clinically important bacteremia for children hospitalized with CAP and the inherent risks of obtaining blood cultures for all hospitalized patients, Andrews et al. created and analyzed a cost-effectiveness model. The authors compared universal acquisition of blood cultures for hospitalized children with CAP versus a targeted approach with blood cultures obtained in patients with effusion or empyema, requiring ICU care, or who are immunosuppressed. Based on this model, a targeted approach could save more than $187 million annually, reduce the number of cultures needed to result in a meaningful change in antibiotic therapy for one patient from 122 to 42, and would “miss” only approximately one case of bacteremia resulting in treatment failure per 1,400 patients.17
RECOMMENDATIONS
- Do not obtain blood culture routinely for children aged >3 months hospitalized for uncomplicated CAP.
- Obtain a blood culture for the following hospitalized patients with CAP:
a. Patients with complicated CAP as defined by the IDSA, particularly those with empyema, abscess, or fistula, or metastatic complications of pneumonia (Table); or
b. Patients with CAP requiring ICU care20 for the management of shock and/or advanced respiratory support.
c. Patients with CAP judged to need antibiotic treatment with an agent other than the IDSA-recommended ampicillin/penicillin (concern for pathogens other than penicillin-sensitive S. pneumonia, immunocompromised or under-immunized status, or inadequate clinical response to empiric ampicillin therapy).
CONCLUSION
Implementing a more targeted approach to blood culture acquisition for hospitalized children with CAP will hopefully increase the yield of true bacterial pathogens that alter management decisions. A targeted approach for the child in the opening vignette would have saved him from the pain of unnecessary phlebotomy (repeat culture), exposure to vancomycin as a nephrotoxic agent, and an additional hospital day.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?™” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by e-mailing [email protected].
1. Whitney P, Whitt AJW, Elixhauser A. Overview of hospital stays for children in the United States, 2012. Statistical Brief 187. 2014;187. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb187-Hospital-Stays-Children-2012.jsp. Accessed December 21, 2017.
2. Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53(7):e25-e76. https://doi.org/10.1093/cid/cir531.
3. Brogan TV, Hall M, Williams DJ, et al. Variability in processes of care and outcomes among children hospitalized with community-acquired pneumonia. Pediatr Infect Dis J. 2012;31(10):1036-1041. https://doi.org/10.1097/INF.0b013e31825f2b10.
4. Murtagh Kurowski E, Shah SS, Thomson J, et al. Improvement methodology increases guideline recommended blood cultures in children with pneumonia. Pediatrics. 2015;135(4):e1052-e1059. https://doi.org/10.1542/peds.2014-2077.
5. Greenhow TL, Hung YY, Herz A. Bacteremia in children 3 to 36 months old after introduction of conjugated pneumococcal vaccines. Pediatrics. 2017;139(4):e20162098. https://doi.org/10.1542/peds.2016-2098.
6. Jain S, Williams DJ, Arnold SR, et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015;372(9):835-845. https://doi.org/10.1056/NEJMoa1405870.
7. Heine D, Cochran C, Moore M, Titus MO, Andrews AL. The prevalence of bacteremia in pediatric patients with community-acquired pneumonia: guidelines to reduce the frequency of obtaining blood cultures. Hosp Pediatr. 2013;3(2):92-96. https://doi.org/10.1542/hpeds.2012-0050.
8. Myers AL, Hall M, Williams DJ, et al. Prevalence of bacteremia in hospitalized pediatric patients with community-acquired pneumonia. Pediatr Infect Dis J. 2013;32(7):736-740. https://doi.org/10.1097/INF.0b013e318290bf63.
9. Neuman MI, Hall M, Lipsett SC, et al. Utility of blood culture among children hospitalized with community-acquired pneumonia. Pediatrics. 2017;140(3). https://doi.org/10.1542/peds.2017-1013.
10. Sandora TJ, Desai R, Miko BA, Harper MB. Assessing quality indicators for pediatric community-acquired pneumonia. Am J Med Qual. 2009;24(5):419-427. https://doi.org/10.1177/1062860609337900.
11. Shah SS, Dugan MH, Bell LM, et al. Blood cultures in the emergency department evaluation of childhood pneumonia. Pediatr Infect Dis J. 2011;30(6):475-479. https://doi.org/10.1097/INF.0b013e31820a5adb.
12. Davis TR, Evans HR, Murtas J et al. Utility of blood cultures in children admitted to hospital with community-acquired pneumonia. J Paediatr Child Health. 2017;53(3):232-236. https://doi.org/10.1111/jpc.13376.
13. Williams DJ, Shah SS. Community-acquired pneumonia in the conjugate vaccine era. J Pediatr Infect Dis Soc. 2012;1(4):314-328. https://doi.org/10.1093/jpids/pis101.
14. Kyaw MH, Lynfield R, Schaffner W, et al. Effect of introduction of the pneumococcal conjugate vaccine on drug-resistant Streptococcus pneumoniae. N Engl J Med. 2006;354(14):1455-1463. https://doi.org/10.1056/NEJMoa051642.
15. Frush JM, Zhu Y, Edwards KM, et al. Prevalence of Staphylococcus aureus and use of antistaphylococcal therapy in children hospitalized with pneumonia. J Hosp Med. 2018;13(12):848-852. https://doi.org/10.12788/jhm.3093.
16. Mendoza-Paredes A, Bastos J, Leber M, Erickson E, Waseem M. Utility of blood culture in uncomplicated pneumonia in children. Clin Med Insights Pediatr. 2013;7:1-5. https://doi.org/10.4137/CMPed.S8051.
17. Andrews AL, Simpson AN, Heine D, Teufel II RJ. A cost-effectiveness analysis of obtaining blood cultures in children hospitalized for community-acquired pneumonia. J Pediatr. 2015;167(6):1280-1286. https://doi.org/10.1016/j.jpeds.2015.09.025.
18. Leyenaar JK, Lagu T, Shieh MS, Pekow PS, Lindenauer PK. Variation in resource utilization for the management of uncomplicated community-acquired pneumonia across community and children’s hospitals. J Pediatr. 2014;165(3):585-591. https://doi.org/10.1016/j.jpeds.2014.04.062.
19. McCulloh RJ, Koster MP, Yin DE, et al. Evaluating the use of blood cultures in the management of children hospitalized for community-acquired pneumonia. PloS One. 2015;10(2):e0117462. https://doi.org/10.1371/journal.pone.0117462.
20. Harris M, Clark J, Coote N, et al. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011;66(2):ii1-ii23. https://doi.org/10.1136/thoraxjnl-2011-200598.
1. Whitney P, Whitt AJW, Elixhauser A. Overview of hospital stays for children in the United States, 2012. Statistical Brief 187. 2014;187. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb187-Hospital-Stays-Children-2012.jsp. Accessed December 21, 2017.
2. Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53(7):e25-e76. https://doi.org/10.1093/cid/cir531.
3. Brogan TV, Hall M, Williams DJ, et al. Variability in processes of care and outcomes among children hospitalized with community-acquired pneumonia. Pediatr Infect Dis J. 2012;31(10):1036-1041. https://doi.org/10.1097/INF.0b013e31825f2b10.
4. Murtagh Kurowski E, Shah SS, Thomson J, et al. Improvement methodology increases guideline recommended blood cultures in children with pneumonia. Pediatrics. 2015;135(4):e1052-e1059. https://doi.org/10.1542/peds.2014-2077.
5. Greenhow TL, Hung YY, Herz A. Bacteremia in children 3 to 36 months old after introduction of conjugated pneumococcal vaccines. Pediatrics. 2017;139(4):e20162098. https://doi.org/10.1542/peds.2016-2098.
6. Jain S, Williams DJ, Arnold SR, et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015;372(9):835-845. https://doi.org/10.1056/NEJMoa1405870.
7. Heine D, Cochran C, Moore M, Titus MO, Andrews AL. The prevalence of bacteremia in pediatric patients with community-acquired pneumonia: guidelines to reduce the frequency of obtaining blood cultures. Hosp Pediatr. 2013;3(2):92-96. https://doi.org/10.1542/hpeds.2012-0050.
8. Myers AL, Hall M, Williams DJ, et al. Prevalence of bacteremia in hospitalized pediatric patients with community-acquired pneumonia. Pediatr Infect Dis J. 2013;32(7):736-740. https://doi.org/10.1097/INF.0b013e318290bf63.
9. Neuman MI, Hall M, Lipsett SC, et al. Utility of blood culture among children hospitalized with community-acquired pneumonia. Pediatrics. 2017;140(3). https://doi.org/10.1542/peds.2017-1013.
10. Sandora TJ, Desai R, Miko BA, Harper MB. Assessing quality indicators for pediatric community-acquired pneumonia. Am J Med Qual. 2009;24(5):419-427. https://doi.org/10.1177/1062860609337900.
11. Shah SS, Dugan MH, Bell LM, et al. Blood cultures in the emergency department evaluation of childhood pneumonia. Pediatr Infect Dis J. 2011;30(6):475-479. https://doi.org/10.1097/INF.0b013e31820a5adb.
12. Davis TR, Evans HR, Murtas J et al. Utility of blood cultures in children admitted to hospital with community-acquired pneumonia. J Paediatr Child Health. 2017;53(3):232-236. https://doi.org/10.1111/jpc.13376.
13. Williams DJ, Shah SS. Community-acquired pneumonia in the conjugate vaccine era. J Pediatr Infect Dis Soc. 2012;1(4):314-328. https://doi.org/10.1093/jpids/pis101.
14. Kyaw MH, Lynfield R, Schaffner W, et al. Effect of introduction of the pneumococcal conjugate vaccine on drug-resistant Streptococcus pneumoniae. N Engl J Med. 2006;354(14):1455-1463. https://doi.org/10.1056/NEJMoa051642.
15. Frush JM, Zhu Y, Edwards KM, et al. Prevalence of Staphylococcus aureus and use of antistaphylococcal therapy in children hospitalized with pneumonia. J Hosp Med. 2018;13(12):848-852. https://doi.org/10.12788/jhm.3093.
16. Mendoza-Paredes A, Bastos J, Leber M, Erickson E, Waseem M. Utility of blood culture in uncomplicated pneumonia in children. Clin Med Insights Pediatr. 2013;7:1-5. https://doi.org/10.4137/CMPed.S8051.
17. Andrews AL, Simpson AN, Heine D, Teufel II RJ. A cost-effectiveness analysis of obtaining blood cultures in children hospitalized for community-acquired pneumonia. J Pediatr. 2015;167(6):1280-1286. https://doi.org/10.1016/j.jpeds.2015.09.025.
18. Leyenaar JK, Lagu T, Shieh MS, Pekow PS, Lindenauer PK. Variation in resource utilization for the management of uncomplicated community-acquired pneumonia across community and children’s hospitals. J Pediatr. 2014;165(3):585-591. https://doi.org/10.1016/j.jpeds.2014.04.062.
19. McCulloh RJ, Koster MP, Yin DE, et al. Evaluating the use of blood cultures in the management of children hospitalized for community-acquired pneumonia. PloS One. 2015;10(2):e0117462. https://doi.org/10.1371/journal.pone.0117462.
20. Harris M, Clark J, Coote N, et al. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011;66(2):ii1-ii23. https://doi.org/10.1136/thoraxjnl-2011-200598.
© 2020 Society of Hospital Medicine
Clinical Guideline Highlights for the Hospitalist: Diagnosis and Management of Clostridium difficile in Adults
Clostridium difficile, now referred to as Clostridioides difficile (C. difficile), is the most commonly identified cause of healthcare-associated infection among adults in the United States.1 Because C. difficile infection results in significant mortality and inpatient costs, its persistence threatens to undermine patient safety and the value of healthcare delivery.1 A standardized, evidence-based approach to diagnosis and management is crucial. However, inconsistencies remain with regard to the appropriate threshold for testing, the type of diagnostic tests used, and treatment. Knowledge of these areas has progressed since the publication of the previous C. difficile guidelines in 2010. These guidelines contain 53 recommendations across 35 sections based on a systematic weighting of the strength of recommendation and quality of evidence using the Grading of Recommendations Assessment, Development, and Evaluation system. Herein, we have chosen to highlight five of these recommendations most relevant to hospitalists.
KEY RECOMMENDATIONS FOR THE HOSPITALIST
Recommendation 1. Patients with unexplained and new-onset ≥3 unformed stools within 24 hours are the preferred target population for testing for C. difficile infection (weak recommendation, very low quality of evidence). Do not perform repeat testing (within seven days) during the same episode of diarrhea and do not test stool from asymptomatic patients (strong recommendation, moderate quality of evidence).
In the recent past, healthcare facilities employed C. difficile tests with limited sensitivity, leading to frequent and repeat testing of hospitalized patients. Excess testing puts patients at risk for false positive results and unnecessary or prolonged treatment courses. Proper testing requires consideration of pretest probability, including analysis of the alternative causes of diarrhea. Duration of hospitalization and antibiotic exposure are the most significant modifiable risk factors for C. difficile infection in adult inpatients.2 Laxative use within the previous 48 hours, enteral tube feeding, and underlying medical conditions, such as inflammatory bowel disease (IBD), are common causes of improper testing.3 This decision may be difficult, as some underlying causes of diarrhea, such as IBD and enteral tube feeding, also increase the risk of C. difficile infection.3 Laboratories can help by rejecting specimens that are not liquid or soft and employing a multistep algorithm using a combination of nucleic acid testing, antigen testing, and toxin detection to maximize sensitivity and specificity. Because recurrent C. difficile infection is relatively common, repeat testing is appropriate only for recurrence of symptoms following successful treatment and should focus on detection of C. difficile toxin because the persistence of the organism itself can occur after successful treatment.4
Recommendation 2. Either vancomycin (125 mg orally four times per day for 10 days) or fidaxomicin (200 mg twice daily for 10 days) is recommended over metronidazole for an initial episode of nonsevere or severe C. difficile infection (strong recommendation, high quality of evidence). For fulminant C. difficile infection, the regimen of choice is a vancomycin dosage of 500 mg orally four times per day (per rectum every six hours if with ileus) in addition to intravenous metronidazole (strong recommendation, moderate quality of evidence).
For several decades now, metronidazole has been the primary antibiotic agent for initial treatment of nonsevere C. difficile infection. Two recent randomized, placebo-controlled trials, however, have found oral vancomycin to be superior to metronidazole for producing a clinical cure and resolution of diarrhea without recurrence.5,6 Oral vancomycin remains the treatment of choice for severe C. difficile infection. Fidaxomicin, a recently FDA-approved antibiotic, can also be used as initial treatment in place of oral vancomycin. One study found fidaxomicin to be superior to oral vancomycin for producing a sustained clinical response, that is, resolution of diarrhea at the end of treatment without recurrence 25 days later.7 Fulminant disease, which is characterized by hypotension or shock, ileus, or megacolon, requires a higher dose of oral vancomycin (or vancomycin enema if with ileus) in addition to intravenous metronidazole.
Recommendation 3. Treat a first recurrence of C. difficile infection with oral vancomycin as a tapered and pulsed regimen rather than a second standard 10-day course of vancomycin or metronidazole (weak recommendation, low quality of evidence).
Despite the improved treatment response with oral vancomycin, one in four patients will experience recurrence. For a first recurrence of C. difficile infection after a 10-day course of oral vancomycin, an extended taper or pulsed course of vancomycin should be attempted. Various regimens have been tried and found to be effective. For a second recurrence, providers can consider addition of rifaximin following oral vancomycin. Fecal microbiota transplantation is recommended for patients with multiple recurrences of C. difficile infection who have failed these antibiotic treatments.
Recommendation 4. Minimize the frequency and duration of high-risk antibiotic therapy (based on local epidemiology) and the number of antibiotic agents prescribed to reduce C. difficile infection risk (strong recommendation, moderate quality of evidence).
Antibiotic stewardship is a necessary component of any successful effort to reduce C. difficile infections. Antibiotic stewardship programs, which are now commonplace in US hospitals, largely rely on educational initiatives or committee-based order review. Hospitalists should take a structured approach emphasizing the four critical questions of antibiotic prescribing: Does this infection require antibiotics? Have I ordered appropriate cultures and the correct empiric therapy? Can I stop, narrow, or switch to oral agents? Finally, what duration of therapy is needed at discharge?8 Initial efforts should focus on the restriction of fluoroquinolones, clindamycin, and cephalosporins (except for surgical antibiotic prophylaxis) given their known risk to cause C. difficile infection.
Recommendation 5. Contact precautions should be maintained for at least 48 hours after diarrhea has resolved (weak recommendation, low quality of evidence).
Although C. difficile is undetectable in stool samples from most patients by the time diarrhea has resolved, skin and environmental contaminations remain high. No studies demonstrating a benefit to further extending contact precautions beyond 48 hours after resolution of diarrhea are yet available.
CRITIQUE
Methods in Preparing Guidelines
The guideline committee consisted of an interdisciplinary team of healthcare providers with extensive experience in the diagnosis, infection control, treatment, and management of C. difficile. The literature search accessed five different databases (Medline, Embase, Cochrane, Health Technology Assessment, and Database of Abstracts of Reviews and Effects), relevant journals, conference proceedings, and regulatory websites published over the search period of 2009-2016.
A major strength of these guidelines is the extensive work that went into their preparation. The committee reviewed over 14,000 pieces of literature and performed a detailed analysis of each one to determine the quality of evidence in support of each recommendation.
Sources of Potential Conflict of Interest or Bias
To reduce bias, the committee’s work was funded by Infectious Disease Society of America and Society for Healthcare Epidemiology of America. Some authors received funding for work outside of this guideline by companies that manufacture diagnostic assays, vancomycin, and fidaxomicin. These potential conflicts were listed at the end of the article.
Generalizability of the Guideline
Not all studies included in the guideline contain exclusively hospitalized patients, but much of the guideline content is applicable to hospitalized patients. Because C. difficile infection is such a widespread public health problem and these guidelines represent a significant update in knowledge since 2010, the specific recommendations highlighted in this review will impact numerous hospitalists, regardless of the practice setting.
Areas in Need of Future Study
Based on the current literature, as well as statements in the guideline, we expect future guidance around potential screening for and isolation of asymptomatic carriers, including closer guidance on stool transplantation focusing on timing and route, as further data emerge in these areas.
Other Resources
- Grading of Recommendations Assessment, Development, and Evaluation system (http://www.gradeworkinggroup.org)
- Universal Screening for C. difficile in a Tertiary Hospital: risk factors for carriage and clinical disease (Color/Blackhttps://www.clinicalmicrobiologyandinfection.com/article/S1198-743X(19)30048-5/fulltext)
- Effectiveness of Isolating Clostridium Difficile Asymptomatic Carriers on the Incidence of Infections (Color/Blackhttps://clinicaltrials.gov/ct2/show/NCT03223415)
- Effect of Detecting and Isolating Clostridium difficile Carriers at Hospital Admission on the Incidence of C difficile Infections (Color/Blackhttps://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2516765)
- Clinical Trial Testing Fecal Microbiota Transplant for Recurrent Diarrheal Disease Begins (Color/Blackhttps://www.nih.gov/news-events/news-releases/clinical-trial-testing-fecal-microbiota-transplant-recurrent-diarrheal-disease-begins)
1. Dubberke ER, Olsen MA. Burden of Clostridium difficile on the healthcare system. Clin Infect Dis. 2012;55(2):S88-S92. https://doi.org/10.1093/cid/cis335.
2. Loo VG, Bourgault AM, Poirier L, et al. Host and pathogen factors for Clostridium difficile infection and colonization. N Engl J Med. 2011;365(18):1693-703. https://doi.org/10.1056/NEJMoa1012413.
3. O’Keefe SJ. Tube feeding, the microbiota, and Clostridium difficile infection. World J Gastroenterol. 2010;16(2):139-142. https://doi.org/10.3748/wjg.v16.i2.139
4. Zacharioudakis IM, Zervou FN, Pliakos EE, Ziakas PD, Mylonakis E. Colonization with toxinogenic C. difficile upon hospital admission, and risk of infection: a systematic review and meta-analysis. Am J Gastroenterol. 2015;110(3):381-90; quiz 391. https://doi.org/10.1038/ajg.2015.22.
5. Johnson S, Louie TJ, Gerding DN, et al. Vancomycin, metronidazole, or tolevamer for Clostridium difficile infection: results from two multinational, randomized, controlled trials. Clin Infect Dis. 2014;59(3):345-354. https://doi.org/10.1093/cid/ciu313.
6. Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45(3):302-307. https://doi.org/10.1086/519265.
7. Crook DW, Walker AS, Kean Y, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection: meta-analysis of pivotal randomized controlled trials. Clin Infect Dis. 2012;55(2):S93-S103. https://doi.org/10.1093/cid/cis499.
8. Tamma, PD, Miller MA, Cosgrove SE. Rethinking how antibiotics are prescribed: incorporating the 4 moments of antibiotic decision making into clinical practice. JAMA. 2018;321(2):139-140. https://doi.org/10.1001/jama.2018.19509.
Clostridium difficile, now referred to as Clostridioides difficile (C. difficile), is the most commonly identified cause of healthcare-associated infection among adults in the United States.1 Because C. difficile infection results in significant mortality and inpatient costs, its persistence threatens to undermine patient safety and the value of healthcare delivery.1 A standardized, evidence-based approach to diagnosis and management is crucial. However, inconsistencies remain with regard to the appropriate threshold for testing, the type of diagnostic tests used, and treatment. Knowledge of these areas has progressed since the publication of the previous C. difficile guidelines in 2010. These guidelines contain 53 recommendations across 35 sections based on a systematic weighting of the strength of recommendation and quality of evidence using the Grading of Recommendations Assessment, Development, and Evaluation system. Herein, we have chosen to highlight five of these recommendations most relevant to hospitalists.
KEY RECOMMENDATIONS FOR THE HOSPITALIST
Recommendation 1. Patients with unexplained and new-onset ≥3 unformed stools within 24 hours are the preferred target population for testing for C. difficile infection (weak recommendation, very low quality of evidence). Do not perform repeat testing (within seven days) during the same episode of diarrhea and do not test stool from asymptomatic patients (strong recommendation, moderate quality of evidence).
In the recent past, healthcare facilities employed C. difficile tests with limited sensitivity, leading to frequent and repeat testing of hospitalized patients. Excess testing puts patients at risk for false positive results and unnecessary or prolonged treatment courses. Proper testing requires consideration of pretest probability, including analysis of the alternative causes of diarrhea. Duration of hospitalization and antibiotic exposure are the most significant modifiable risk factors for C. difficile infection in adult inpatients.2 Laxative use within the previous 48 hours, enteral tube feeding, and underlying medical conditions, such as inflammatory bowel disease (IBD), are common causes of improper testing.3 This decision may be difficult, as some underlying causes of diarrhea, such as IBD and enteral tube feeding, also increase the risk of C. difficile infection.3 Laboratories can help by rejecting specimens that are not liquid or soft and employing a multistep algorithm using a combination of nucleic acid testing, antigen testing, and toxin detection to maximize sensitivity and specificity. Because recurrent C. difficile infection is relatively common, repeat testing is appropriate only for recurrence of symptoms following successful treatment and should focus on detection of C. difficile toxin because the persistence of the organism itself can occur after successful treatment.4
Recommendation 2. Either vancomycin (125 mg orally four times per day for 10 days) or fidaxomicin (200 mg twice daily for 10 days) is recommended over metronidazole for an initial episode of nonsevere or severe C. difficile infection (strong recommendation, high quality of evidence). For fulminant C. difficile infection, the regimen of choice is a vancomycin dosage of 500 mg orally four times per day (per rectum every six hours if with ileus) in addition to intravenous metronidazole (strong recommendation, moderate quality of evidence).
For several decades now, metronidazole has been the primary antibiotic agent for initial treatment of nonsevere C. difficile infection. Two recent randomized, placebo-controlled trials, however, have found oral vancomycin to be superior to metronidazole for producing a clinical cure and resolution of diarrhea without recurrence.5,6 Oral vancomycin remains the treatment of choice for severe C. difficile infection. Fidaxomicin, a recently FDA-approved antibiotic, can also be used as initial treatment in place of oral vancomycin. One study found fidaxomicin to be superior to oral vancomycin for producing a sustained clinical response, that is, resolution of diarrhea at the end of treatment without recurrence 25 days later.7 Fulminant disease, which is characterized by hypotension or shock, ileus, or megacolon, requires a higher dose of oral vancomycin (or vancomycin enema if with ileus) in addition to intravenous metronidazole.
Recommendation 3. Treat a first recurrence of C. difficile infection with oral vancomycin as a tapered and pulsed regimen rather than a second standard 10-day course of vancomycin or metronidazole (weak recommendation, low quality of evidence).
Despite the improved treatment response with oral vancomycin, one in four patients will experience recurrence. For a first recurrence of C. difficile infection after a 10-day course of oral vancomycin, an extended taper or pulsed course of vancomycin should be attempted. Various regimens have been tried and found to be effective. For a second recurrence, providers can consider addition of rifaximin following oral vancomycin. Fecal microbiota transplantation is recommended for patients with multiple recurrences of C. difficile infection who have failed these antibiotic treatments.
Recommendation 4. Minimize the frequency and duration of high-risk antibiotic therapy (based on local epidemiology) and the number of antibiotic agents prescribed to reduce C. difficile infection risk (strong recommendation, moderate quality of evidence).
Antibiotic stewardship is a necessary component of any successful effort to reduce C. difficile infections. Antibiotic stewardship programs, which are now commonplace in US hospitals, largely rely on educational initiatives or committee-based order review. Hospitalists should take a structured approach emphasizing the four critical questions of antibiotic prescribing: Does this infection require antibiotics? Have I ordered appropriate cultures and the correct empiric therapy? Can I stop, narrow, or switch to oral agents? Finally, what duration of therapy is needed at discharge?8 Initial efforts should focus on the restriction of fluoroquinolones, clindamycin, and cephalosporins (except for surgical antibiotic prophylaxis) given their known risk to cause C. difficile infection.
Recommendation 5. Contact precautions should be maintained for at least 48 hours after diarrhea has resolved (weak recommendation, low quality of evidence).
Although C. difficile is undetectable in stool samples from most patients by the time diarrhea has resolved, skin and environmental contaminations remain high. No studies demonstrating a benefit to further extending contact precautions beyond 48 hours after resolution of diarrhea are yet available.
CRITIQUE
Methods in Preparing Guidelines
The guideline committee consisted of an interdisciplinary team of healthcare providers with extensive experience in the diagnosis, infection control, treatment, and management of C. difficile. The literature search accessed five different databases (Medline, Embase, Cochrane, Health Technology Assessment, and Database of Abstracts of Reviews and Effects), relevant journals, conference proceedings, and regulatory websites published over the search period of 2009-2016.
A major strength of these guidelines is the extensive work that went into their preparation. The committee reviewed over 14,000 pieces of literature and performed a detailed analysis of each one to determine the quality of evidence in support of each recommendation.
Sources of Potential Conflict of Interest or Bias
To reduce bias, the committee’s work was funded by Infectious Disease Society of America and Society for Healthcare Epidemiology of America. Some authors received funding for work outside of this guideline by companies that manufacture diagnostic assays, vancomycin, and fidaxomicin. These potential conflicts were listed at the end of the article.
Generalizability of the Guideline
Not all studies included in the guideline contain exclusively hospitalized patients, but much of the guideline content is applicable to hospitalized patients. Because C. difficile infection is such a widespread public health problem and these guidelines represent a significant update in knowledge since 2010, the specific recommendations highlighted in this review will impact numerous hospitalists, regardless of the practice setting.
Areas in Need of Future Study
Based on the current literature, as well as statements in the guideline, we expect future guidance around potential screening for and isolation of asymptomatic carriers, including closer guidance on stool transplantation focusing on timing and route, as further data emerge in these areas.
Other Resources
- Grading of Recommendations Assessment, Development, and Evaluation system (http://www.gradeworkinggroup.org)
- Universal Screening for C. difficile in a Tertiary Hospital: risk factors for carriage and clinical disease (Color/Blackhttps://www.clinicalmicrobiologyandinfection.com/article/S1198-743X(19)30048-5/fulltext)
- Effectiveness of Isolating Clostridium Difficile Asymptomatic Carriers on the Incidence of Infections (Color/Blackhttps://clinicaltrials.gov/ct2/show/NCT03223415)
- Effect of Detecting and Isolating Clostridium difficile Carriers at Hospital Admission on the Incidence of C difficile Infections (Color/Blackhttps://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2516765)
- Clinical Trial Testing Fecal Microbiota Transplant for Recurrent Diarrheal Disease Begins (Color/Blackhttps://www.nih.gov/news-events/news-releases/clinical-trial-testing-fecal-microbiota-transplant-recurrent-diarrheal-disease-begins)
Clostridium difficile, now referred to as Clostridioides difficile (C. difficile), is the most commonly identified cause of healthcare-associated infection among adults in the United States.1 Because C. difficile infection results in significant mortality and inpatient costs, its persistence threatens to undermine patient safety and the value of healthcare delivery.1 A standardized, evidence-based approach to diagnosis and management is crucial. However, inconsistencies remain with regard to the appropriate threshold for testing, the type of diagnostic tests used, and treatment. Knowledge of these areas has progressed since the publication of the previous C. difficile guidelines in 2010. These guidelines contain 53 recommendations across 35 sections based on a systematic weighting of the strength of recommendation and quality of evidence using the Grading of Recommendations Assessment, Development, and Evaluation system. Herein, we have chosen to highlight five of these recommendations most relevant to hospitalists.
KEY RECOMMENDATIONS FOR THE HOSPITALIST
Recommendation 1. Patients with unexplained and new-onset ≥3 unformed stools within 24 hours are the preferred target population for testing for C. difficile infection (weak recommendation, very low quality of evidence). Do not perform repeat testing (within seven days) during the same episode of diarrhea and do not test stool from asymptomatic patients (strong recommendation, moderate quality of evidence).
In the recent past, healthcare facilities employed C. difficile tests with limited sensitivity, leading to frequent and repeat testing of hospitalized patients. Excess testing puts patients at risk for false positive results and unnecessary or prolonged treatment courses. Proper testing requires consideration of pretest probability, including analysis of the alternative causes of diarrhea. Duration of hospitalization and antibiotic exposure are the most significant modifiable risk factors for C. difficile infection in adult inpatients.2 Laxative use within the previous 48 hours, enteral tube feeding, and underlying medical conditions, such as inflammatory bowel disease (IBD), are common causes of improper testing.3 This decision may be difficult, as some underlying causes of diarrhea, such as IBD and enteral tube feeding, also increase the risk of C. difficile infection.3 Laboratories can help by rejecting specimens that are not liquid or soft and employing a multistep algorithm using a combination of nucleic acid testing, antigen testing, and toxin detection to maximize sensitivity and specificity. Because recurrent C. difficile infection is relatively common, repeat testing is appropriate only for recurrence of symptoms following successful treatment and should focus on detection of C. difficile toxin because the persistence of the organism itself can occur after successful treatment.4
Recommendation 2. Either vancomycin (125 mg orally four times per day for 10 days) or fidaxomicin (200 mg twice daily for 10 days) is recommended over metronidazole for an initial episode of nonsevere or severe C. difficile infection (strong recommendation, high quality of evidence). For fulminant C. difficile infection, the regimen of choice is a vancomycin dosage of 500 mg orally four times per day (per rectum every six hours if with ileus) in addition to intravenous metronidazole (strong recommendation, moderate quality of evidence).
For several decades now, metronidazole has been the primary antibiotic agent for initial treatment of nonsevere C. difficile infection. Two recent randomized, placebo-controlled trials, however, have found oral vancomycin to be superior to metronidazole for producing a clinical cure and resolution of diarrhea without recurrence.5,6 Oral vancomycin remains the treatment of choice for severe C. difficile infection. Fidaxomicin, a recently FDA-approved antibiotic, can also be used as initial treatment in place of oral vancomycin. One study found fidaxomicin to be superior to oral vancomycin for producing a sustained clinical response, that is, resolution of diarrhea at the end of treatment without recurrence 25 days later.7 Fulminant disease, which is characterized by hypotension or shock, ileus, or megacolon, requires a higher dose of oral vancomycin (or vancomycin enema if with ileus) in addition to intravenous metronidazole.
Recommendation 3. Treat a first recurrence of C. difficile infection with oral vancomycin as a tapered and pulsed regimen rather than a second standard 10-day course of vancomycin or metronidazole (weak recommendation, low quality of evidence).
Despite the improved treatment response with oral vancomycin, one in four patients will experience recurrence. For a first recurrence of C. difficile infection after a 10-day course of oral vancomycin, an extended taper or pulsed course of vancomycin should be attempted. Various regimens have been tried and found to be effective. For a second recurrence, providers can consider addition of rifaximin following oral vancomycin. Fecal microbiota transplantation is recommended for patients with multiple recurrences of C. difficile infection who have failed these antibiotic treatments.
Recommendation 4. Minimize the frequency and duration of high-risk antibiotic therapy (based on local epidemiology) and the number of antibiotic agents prescribed to reduce C. difficile infection risk (strong recommendation, moderate quality of evidence).
Antibiotic stewardship is a necessary component of any successful effort to reduce C. difficile infections. Antibiotic stewardship programs, which are now commonplace in US hospitals, largely rely on educational initiatives or committee-based order review. Hospitalists should take a structured approach emphasizing the four critical questions of antibiotic prescribing: Does this infection require antibiotics? Have I ordered appropriate cultures and the correct empiric therapy? Can I stop, narrow, or switch to oral agents? Finally, what duration of therapy is needed at discharge?8 Initial efforts should focus on the restriction of fluoroquinolones, clindamycin, and cephalosporins (except for surgical antibiotic prophylaxis) given their known risk to cause C. difficile infection.
Recommendation 5. Contact precautions should be maintained for at least 48 hours after diarrhea has resolved (weak recommendation, low quality of evidence).
Although C. difficile is undetectable in stool samples from most patients by the time diarrhea has resolved, skin and environmental contaminations remain high. No studies demonstrating a benefit to further extending contact precautions beyond 48 hours after resolution of diarrhea are yet available.
CRITIQUE
Methods in Preparing Guidelines
The guideline committee consisted of an interdisciplinary team of healthcare providers with extensive experience in the diagnosis, infection control, treatment, and management of C. difficile. The literature search accessed five different databases (Medline, Embase, Cochrane, Health Technology Assessment, and Database of Abstracts of Reviews and Effects), relevant journals, conference proceedings, and regulatory websites published over the search period of 2009-2016.
A major strength of these guidelines is the extensive work that went into their preparation. The committee reviewed over 14,000 pieces of literature and performed a detailed analysis of each one to determine the quality of evidence in support of each recommendation.
Sources of Potential Conflict of Interest or Bias
To reduce bias, the committee’s work was funded by Infectious Disease Society of America and Society for Healthcare Epidemiology of America. Some authors received funding for work outside of this guideline by companies that manufacture diagnostic assays, vancomycin, and fidaxomicin. These potential conflicts were listed at the end of the article.
Generalizability of the Guideline
Not all studies included in the guideline contain exclusively hospitalized patients, but much of the guideline content is applicable to hospitalized patients. Because C. difficile infection is such a widespread public health problem and these guidelines represent a significant update in knowledge since 2010, the specific recommendations highlighted in this review will impact numerous hospitalists, regardless of the practice setting.
Areas in Need of Future Study
Based on the current literature, as well as statements in the guideline, we expect future guidance around potential screening for and isolation of asymptomatic carriers, including closer guidance on stool transplantation focusing on timing and route, as further data emerge in these areas.
Other Resources
- Grading of Recommendations Assessment, Development, and Evaluation system (http://www.gradeworkinggroup.org)
- Universal Screening for C. difficile in a Tertiary Hospital: risk factors for carriage and clinical disease (Color/Blackhttps://www.clinicalmicrobiologyandinfection.com/article/S1198-743X(19)30048-5/fulltext)
- Effectiveness of Isolating Clostridium Difficile Asymptomatic Carriers on the Incidence of Infections (Color/Blackhttps://clinicaltrials.gov/ct2/show/NCT03223415)
- Effect of Detecting and Isolating Clostridium difficile Carriers at Hospital Admission on the Incidence of C difficile Infections (Color/Blackhttps://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2516765)
- Clinical Trial Testing Fecal Microbiota Transplant for Recurrent Diarrheal Disease Begins (Color/Blackhttps://www.nih.gov/news-events/news-releases/clinical-trial-testing-fecal-microbiota-transplant-recurrent-diarrheal-disease-begins)
1. Dubberke ER, Olsen MA. Burden of Clostridium difficile on the healthcare system. Clin Infect Dis. 2012;55(2):S88-S92. https://doi.org/10.1093/cid/cis335.
2. Loo VG, Bourgault AM, Poirier L, et al. Host and pathogen factors for Clostridium difficile infection and colonization. N Engl J Med. 2011;365(18):1693-703. https://doi.org/10.1056/NEJMoa1012413.
3. O’Keefe SJ. Tube feeding, the microbiota, and Clostridium difficile infection. World J Gastroenterol. 2010;16(2):139-142. https://doi.org/10.3748/wjg.v16.i2.139
4. Zacharioudakis IM, Zervou FN, Pliakos EE, Ziakas PD, Mylonakis E. Colonization with toxinogenic C. difficile upon hospital admission, and risk of infection: a systematic review and meta-analysis. Am J Gastroenterol. 2015;110(3):381-90; quiz 391. https://doi.org/10.1038/ajg.2015.22.
5. Johnson S, Louie TJ, Gerding DN, et al. Vancomycin, metronidazole, or tolevamer for Clostridium difficile infection: results from two multinational, randomized, controlled trials. Clin Infect Dis. 2014;59(3):345-354. https://doi.org/10.1093/cid/ciu313.
6. Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45(3):302-307. https://doi.org/10.1086/519265.
7. Crook DW, Walker AS, Kean Y, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection: meta-analysis of pivotal randomized controlled trials. Clin Infect Dis. 2012;55(2):S93-S103. https://doi.org/10.1093/cid/cis499.
8. Tamma, PD, Miller MA, Cosgrove SE. Rethinking how antibiotics are prescribed: incorporating the 4 moments of antibiotic decision making into clinical practice. JAMA. 2018;321(2):139-140. https://doi.org/10.1001/jama.2018.19509.
1. Dubberke ER, Olsen MA. Burden of Clostridium difficile on the healthcare system. Clin Infect Dis. 2012;55(2):S88-S92. https://doi.org/10.1093/cid/cis335.
2. Loo VG, Bourgault AM, Poirier L, et al. Host and pathogen factors for Clostridium difficile infection and colonization. N Engl J Med. 2011;365(18):1693-703. https://doi.org/10.1056/NEJMoa1012413.
3. O’Keefe SJ. Tube feeding, the microbiota, and Clostridium difficile infection. World J Gastroenterol. 2010;16(2):139-142. https://doi.org/10.3748/wjg.v16.i2.139
4. Zacharioudakis IM, Zervou FN, Pliakos EE, Ziakas PD, Mylonakis E. Colonization with toxinogenic C. difficile upon hospital admission, and risk of infection: a systematic review and meta-analysis. Am J Gastroenterol. 2015;110(3):381-90; quiz 391. https://doi.org/10.1038/ajg.2015.22.
5. Johnson S, Louie TJ, Gerding DN, et al. Vancomycin, metronidazole, or tolevamer for Clostridium difficile infection: results from two multinational, randomized, controlled trials. Clin Infect Dis. 2014;59(3):345-354. https://doi.org/10.1093/cid/ciu313.
6. Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45(3):302-307. https://doi.org/10.1086/519265.
7. Crook DW, Walker AS, Kean Y, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection: meta-analysis of pivotal randomized controlled trials. Clin Infect Dis. 2012;55(2):S93-S103. https://doi.org/10.1093/cid/cis499.
8. Tamma, PD, Miller MA, Cosgrove SE. Rethinking how antibiotics are prescribed: incorporating the 4 moments of antibiotic decision making into clinical practice. JAMA. 2018;321(2):139-140. https://doi.org/10.1001/jama.2018.19509.
© 2019 Society of Hospital Medicine
All in the Stream
A 67-year-old man presented to the emergency department with four days of nausea, vomiting, and chills. He was originally from the Philippines but lived in the United States for six years. His past medical history was notable for nephrolithiasis for which a ureteral stent had been placed and was subsequently removed three years prior. He reported no abdominal pain, diarrhea, dysuria, urinary frequency, hematuria, cough, headache, or fever. He was a retired high school teacher and a lifelong nonsmoker.
This patient presents with a nonspecific constellation of constitutional and gastrointestinal (GI) symptoms. A system-based approach may be helpful when considering the differential diagnosis. Chills most often suggest infection, especially in older patients. With regard to GI causes, acute gastroenteritis and other food-borne infections can cause nausea, vomiting, and chills, but these are typically accompanied by abdominal pain and diarrhea and often resolve in less than four days. Abdominal pain would also be expected with cholecystitis as well as more life-threatening causes of nausea such as acute pancreatitis, mesenteric ischemia, and bowel obstruction. In contrast, abdominal pain would not be expected with a central nervous system (CNS) infection such as a brain abscess, which may cause nausea from increased intracranial pressure. Headaches occur in a majority of these cases, making CNS etiologies of nausea less likely. Cardiovascular causes, including myocardial ischemia and infarction, may lead to nausea and vomiting, but these are less likely given the absence of chest pain. Genitourinary causes must be considered, especially given his history of both nephrolithiasis and instrumentation. A stricture or recurrence of nephrolithiasis could lead to acute pyelonephritis or perinephric abscess, although both commonly present with urinary tract symptoms. Uremia, possibly from obstructive uropathy given the patient’s history of nephrolithiasis, could also lead to this constellation of symptoms.
On examination, temperature was 101.6 °F, heart rate 126 beats per minute, respiratory rate 18 breaths per minute, blood pressure 120/76 mm Hg, and oxygen saturation 98% on room air. The oral mucosa was moist, heart sounds were normal without murmurs, lungs were clear to auscultation, and abdomen was soft, nontender, and nondistended. There was no flank tenderness, and penile, testicular, and prostate examination findings were normal.
Laboratory studies revealed a serum sodium of 126 mEq/L, potassium 5.0 mEq/L, chloride 98 mEq/L, bicarbonate 15 mEq/L, blood urea nitrogen 88 mg/dL, creatinine 9.0 mg/dL, calcium 8 mg/dL, glucose 110 mg/dL, and albumin 3.3 g/dL. One year prior, serum creatinine was 1.4 mg/dL. His white blood cell (WBC) count was 8.0 k/uL with normal differential, hemoglobin 11.4 g/dL with normal MCV, and platelet count was normal. Serum osmolality was 286 mOsm/kg and serum parathyroid hormone (PTH) level 63 pg/mL (normal, 10-65). The urinalysis revealed cloudy urine with a specific gravity 1.009, 54 red blood cells (RBC), 236 WBC, large leukocyte esterase, negative nitrite, trace protein, and no casts or dysmorphic RBCs. A random urine specimen revealed sodium of 86 mEq/L, potassium 16 mEq/L, chloride 80 mEq/L, and creatinine 70 mg/dL.
Fever and tachycardia support an infectious cause of his symptoms. Absent flank tenderness and a normal genitourinary examination have only moderate negative predictive values for acute pyelonephritis and prostatitis, respectively. The most striking laboratory finding is his azotemia. Acute kidney injury (AKI) is more likely than chronic kidney disease (CKD) given that the PTH level is normal and the serum creatinine from a year ago was near normal. The most useful finding to differentiate AKI from CKD is the presence of atrophic kidneys on imaging. The low bicarbonate level indicates a metabolic acidosis. His serum anion gap is 13 mEq/L, which falls above most normal ranges. A mildly elevated serum anion gap together with a “delta serum anion gap/delta serum bicarbonate” ratio less than one suggest concomitant anion gap metabolic acidosis and non anion gap metabolic acidosis. The latter, coupled with a history of nephrolithiasis, may point to the possibility of renal tubular acidosis and AKI caused by urinary tract obstruction. This could also account for the marked hyponatremia. Moreover, his high fractional excretion of sodium (9%) is not suggestive of prerenal injury, the most common acute renal injury among patients who present to the emergency department. Hematuria carries a broad differential diagnosis, but most common causes include nephrolithiasis, urinary tract infection (UTI), prostatitis, neoplasm, and glomerulonephritis (GN). The lack of casts and dysmorphic RBCs makes GN unlikely. Taken together, his vital signs, examination, and laboratory studies suggest a high likelihood of an upper UTI (acute obstructive pyelonephritis) in the context of AKI due to obstructive uropathy.
Despite both a normal serum WBC count (which has only a moderate negative predictive value) and his low risk of developing life-threating organ dysfunction from sepsis based on a quick Sequential Organ Failure Assessment (qSOFA) score of zero, it is appropriate to start antibiotics after drawing blood and doing urine cultures. The next step should include administration of a broad-spectrum regimen that is appropriately dose-adjusted for renal dysfunction, such as an antipseudomonal carbapenem and vancomycin to cover extended-spectrum beta-lactamase (ESBL)-producing organisms, Pseudomonas aeruginosa, and methicillin-resistant Staphylococcus aureus (MRSA). This broad coverage is indicated for empiric treatment of complicated obstructive pyelonephritis, a condition that may arise from significant urinary obstruction and that carries a high risk of rapid clinical deterioration. He should undergo a rapid bedside test to assess for urethral or bladder outlet obstruction: either a bladder ultrasound or temporary insertion of a bladder catheter. He should also have an urgent computed tomography (CT) of his abdomen and pelvis without intravenous (IV) contrast, looking for evidence of urinary tract obstruction. A CT is preferred over ultrasound of the kidneys and bladder as CT has higher sensitivity and specificity for nephrolithiasis and neoplasm.
A CT of the abdomen and pelvis without IV contrast revealed bilateral hydroureter and hydronephrosis with multiple punctate calcified stones within the right calyces and the distal right ureter (Figure 1, Figure 2). However, these appeared too small to cause the degree of obstruction visualized. There were no stones noted in the left ureter to account for the obstruction, though small stones were noted in the left calyces. The bladder appeared normal.
Rarely are both ureters obstructed proximal to the ureterovesical junctions in the retroperitoneum. When they are, CT scans usually reveal culprit lesions that are extrinsic to the urinary tract, such as masses or retroperitoneal fibrosis, the latter of which can be associated with IgG4-related disease. Intrinsic causes of urinary tract obstructions include ureteral strictures (from infections, nephrolithiasis, instrumentation, prior radiotherapy, or rarely urothelial cancer), blood clots, metastatic ureteral deposits, or nephrolithiasis. While most intrinsic causes are unilateral, the patient is predisposed to strictures given his history of ureteral instrumentation. A preexisting unilateral obstruction due to a stricture may now, therefore, be unmasked by a second intrinsic obstruction in the contralateral ureter. Alternatively, given his remote history of living in the Philippines, a site where Schistosoma haematobium is endemic, chronic genitourinary schistosomiasis may have caused ureteral strictures due to granulomas, fibrosis, or pseudopolyps.
More commonly, bilateral hydroureter with bilateral hydronephrosis is caused by an obstruction of the bladder or urethra. CT scans can reveal prostatic hyperplasia (occasionally with protrusion into the bladder) and increased bladder wall thickness as a result of chronic bladder outlet obstruction, but the negative predictive value of either finding is modest. More revealing is that the patient reported neither an inability to pass urine (in fact, a “random” urine sample was obtained) nor suprapubic discomfort. Both symptoms would be pronounced with acute bladder obstruction but may be minimal with slowly progressive obstruction. In either case, a distended bladder would have been seen on the CT scan.
Regardless of the cause or whether the obstruction is in the upper or lower urinary tract, emergency intervention is needed to relieve the obstruction when AKI presents with bilateral hydronephrosis. A urology consultation should be sought urgently to determine the best strategy to relieve the obstruction. This may include bilateral percutaneous nephrostomy tubes given that the obstruction appears to be above the level of the bladder. Their findings will also direct additional diagnostic workup.
The patient received ceftriaxone and underwent cystoscopy, which revealed a stricture of the distal bulbar urethra. The ureters and bladder could not be completely visualized due to hematuria. The urethral stricture was dilated, and a Foley catheter was placed. In the operating room, the patient had a fever of 103 °F and developed severe hypotension unresponsive to 3 L of intravenous normal saline. Norepinephrine infusion was initiated for refractory hypotension.
Except for transurethral prostate resections, endoscopic urologic procedures rarely lead to a stricture of any segment of the urethra, suggesting that the previous ureteral stent placement and retrieval were not causal. Longstanding Mycobacterium tuberculosis or Schistosoma haematobium infection occasionally causes urethral strictures presenting as bilateral hydronephrosis. The multiple punctate calcified “stones” demonstrated on CT may suggest either diagnosis if they were actually calcified granulomas.
Regardless of the cause, most patients with a urethral stricture have chronic lower urinary tract symptoms such as decreased stream and the feeling of incomplete bladder emptying. Since this patient does not report these symptoms, it is important to consider if the stricture might be merely incidental. The absence of pain is more telling than the absence of chronic or recurrent symptoms. Lack of pain argues strongly against a pure de novo acute obstruction because abrupt stretching of the renal capsules and the walls of the collecting system is usually painful. Slow stretching caused by a progressive stricture may mask the pain of a superimposed acute obstruction. A blood clot, for example, may have precipitated an acute-on-chronic obstruction upon lodging at the urethral stricture.
The worsening systemic response to the procedure may be due to increased intravesical pressure by dilation and cystoscopy, which may have caused subsequent backflow of bacteria from the renal parenchyma into the circulation (pyelorenal backflow). The broad-spectrum antibiotic regimen suggested above and IV crystalloid infusion should be continued with close hemodynamic monitoring.
Treatment for severe sepsis was initiated with empiric piperacillin-tazobactam, ceftriaxone was discontinued, and the patient was transferred to the intensive care unit (ICU). Norepinephrine was discontinued after 24 hours. Despite the indwelling Foley catheter, his kidney function worsened (creatinine increased to 10.5 over the next 36 hours, and he remained oliguric). Therefore, bilateral percutaneous nephrostomy tubes were placed to relieve the ongoing obstruction. In the ICU, he remained febrile, despite receiving piperacillin-tazobactam, through hospital day 7. Serial blood and urine cultures all remained negative. HIV testing was negative. His chest radiograph was unremarkable, and transthoracic echocardiogram was normal. His creatinine improved but plateaued at 2.5 mg/dL by day 7.
Worsening renal function (alongside oliguria or anuria) despite a functioning Foley catheter suggests either intrinsic renal disease or bilateral ureteral obstructions. The initial attempt at relieving the obstruction with a Foley catheter did not take into consideration the bilateral ureteral strictures. As a result, soon thereafter, the insertion of percutaneous nephrostomy tubes was necessary. Given the severity of his illness, underlying obstructive uropathy, and persistent fever, suggesting an ongoing infection, one strategy would be to continue antibiotics with broader coverage than piperacillin-tazobactam. This approach may be reasonable, given the emergence of ESBL organisms and the possibility of MRSA due to instrumentation. However, it is important to note that only sterile pyuria has been identified to date, which raises the possibility of nonbacterial infections. Although chronic infection with Schistosoma haematobium can cause bilateral ureteral strictures, associated fever is limited to the acute phase of infection and not the chronic obstructive phase, unless there is a superimposed infection. Genitourinary Mycobacterium tuberculosis remains a likely possibility, regardless of the unrevealing chest radiograph. Urine nucleic acid amplification and acid-fast bacilli (AFB) smear and culture, the best initial diagnostic test, should be sent. Although less definitive, a tuberculin skin test and an interferon-gamma release assay should also be conducted. Histopathology of the ureters obtained by repeat cystoscopy may be diagnostic, but given the limited visualization during the last cystoscopy and the recent dilation of the urethra, this option should be kept in reserve for now.
Antibiotics were discontinued on day 7, but the patient continued to experience ongoing fever. Urine Histoplasma and serum cryptococcal antigens were negative. His urine AFB smear was 1+ positive. Liver function tests revealed a total protein of 7.0 g/dL, albumin 3.0 g/dL, total bilirubin 1.2 mg/dL, direct bilirubin 0.3 mg/dL, alkaline phosphatase 418 U/L, aspartate aminotransferase 65 U/L, alanine aminotransferase 88 U/L, gamma-glutamyltransferase (GGT) 609 U/L (normal, 3-60), and lactate dehydrogenase 284 U/L (normal, 85-210).
Acid-fast bacillus in the urine strongly suggests Mycobacterium tuberculosis (MTB) with several reports of likelihood ratios greater than 10. Nevertheless, confirmation is needed to rule out nontuberculous mycobacteria because of potential hepatotoxicity from treatment. Up to six urine samples should be sent for mycobacterium culture. However, false negative rates of up to 90% are reported, and final test results can take up to two months, so other methods of confirmation should be simultaneously sought. A nucleic acid amplification test of urine could rule in a pathogenic species within 24 hours. Alternatively, the probability of a nonpathogenic colonizing species would be negligible if a caseating granuloma was found. Biopsy could be obtained from the ureters, as suggested above. Liver biopsy should also be considered, especially if the moderate elevations in alkaline phosphatase and GGT (the most common liver enzyme abnormalities in hepatic tuberculosis) did not merely wax and wane with sepsis.
A CT of the thorax without IV contrast was done to evaluate for evidence of pulmonary disease given the positive urine AFB. This demonstrated bilateral fibrotic upper lobe opacities suggestive of prior granulomatous disease but no cavitary lung lesions (Figure 3). Three sputum smears were negative for AFB, but one sample showed Mycobacterium tuberculosis detected by a polymerase chain reaction (PCR) probe.
Given the concern for genitourinary tuberculosis (GUTB), it is appropriate to place the patient in respiratory isolation to exclude concomitant pulmonary tuberculosis (TB). AFB smears were negative, but the sputum PCR probe was positive, confirming pathogenic MTB. However, the negative AFB smears make the likelihood of pulmonary infectivity low. As a result, contact tracing is often deemed unnecessary by hospital infection control teams. Though his chest radiograph was normal, CT showed bilateral upper lobe fibrotic disease suggestive of prior pulmonary TB, thus making it likely that the current GUTB represents reactivation.
The two-month initiation phase of treatment with four antituberculosis drugs should begin while drug susceptibility tests are pending. Potential hepatotoxicity should be closely monitored, ideally by a clinician with experience treating tuberculosis in patients with existing liver disease. As a general precaution, alcohol should be avoided as should medications such as acetaminophen that are known to be hepatotoxic. Urology follow-up is also needed because about one-third of tuberculous ureteral strictures treated initially with percutaneous nephrostomy do not resolve with antituberculosis therapy.
The patient was started on weight-based antituberculosis treatment with four antimicrobial agents (rifampin, ethambutol, pyrazinamide, and isoniazid). He was seen in the infectious disease clinic two weeks later; his fever had resolved, and his liver function tests showed normalization of AST and LDH as well as a 45% reduction in his GGT and alkaline phosphatase levels. Two months following discharge, a nuclear medicine radionuclide angiogram renal flow scan showed normal right kidney function. The right nephrostomy tube was subsequently removed. He continued to have left kidney outflow obstruction due to a residual ureteral stricture (Figure 4). Repeat cystoscopy and attempted left ureteral stenting was unsuccessful. The left nephrostomy tube remained in place.
DISCUSSION
According to the Centers for Disease Control, in 2017, 10 million people became sick with TB, and there were 1.3 million TB-related deaths worldwide with 9,150 cases reported in the United States. Extrapulmonary TB (EPTB) constitutes 10% of all TB cases globally.1-4 GUTB is the second most common form of EPTB after lymph node TB, and it occurs in up to 20% of all pulmonary TB cases.2,3
Mycobacteria reach the genitourinary (GU) tract via hematogenous spread during primary infection or reactivation of TB. This leads to cortical and medullary lesions, which can heal spontaneously or eventually (average of 22 years) rupture into the tubules and onto the collecting system, ureters, and bladder.5,6 Spread to the ureter and bladder leads to multiple ureteral strictures and contracture of the bladder with disruption of the ureterovesical junction (UVJ), which causes hydroureter and hydronephrosis.7 Unilateral kidney involvement is most common, but bilateral involvement can occur following exacerbated hematogenous spread, particularly in immune deficient patients. Bilateral kidney involvement is also possible from retrograde spread to the good kidney due to bladder contracture and UVJ disruption.8,9 Distal infection can involve all aspects of the male and female genital tracts, but urethral strictures are extremely rare.10,11
GUTB affects males more than females (2:1) and presents insidiously at 40 to 60 years of age.12 Other risk factors for TB include birth in TB endemic areas, prior TB infection, immunosuppression, malnutrition, severe systemic disease, diabetes, and cirrhosis. It is crucial to assess these risk factors when creating and refining differential diagnoses. Many patients have hematuria and sterile pyuria as incidental initial findings. The most common symptoms arise from bladder involvement, including frequency, urgency, and dysuria. Low back pain and gross hematuria are also common, but fever and constitutional symptoms are uncommon.10 Bilateral ureteral strictures can lead to obstructive renal failure, and involvement of the genital tracts can lead to pelvic or scrotal pain, swelling, and fistula formation.10
Diagnosis involves the demonstration of TB bacilli in urine or GU tissue. The urinalysis reveals hematuria and sterile pyuria.13 Urine AFB stains are positive in 52% of cases but are not diagnostic as nontuberculous mycobacteria may also cause a positive test result.13,14 Urine cultures for Mycobacterium tuberculosis are positive in up to 90% of cases after six to eight weeks. As many as three to six morning urine samples are required to achieve diagnostic accuracy.10,14 Urine PCR for Mycobacterium tuberculosis has 96% sensitivity and up to 98% specificity,14 while PCR on GU tissue has a sensitivity of 88% and specificity of 87%.15 The rapid nucleic acid amplification assay Xpert MTB/RIF in urine has a sensitivity of 83%, and specificity of 98%.16 Imaging is required to evaluate for obstruction, and the CT scan is abnormal in up to 90% of cases, showing multiple ureteral stenoses, hydroureter and hydronephrosis, and a contracted bladder.10,17
GUTB is treated with standard antituberculosis regimens.18 Patients with urinary obstruction benefit from ureteral stenting or percutaneous nephrostomy, bladder diversion, or ureteral reconstruction surgery. Unilateral nephrectomy for a nonfunctioning kidney with extensive disease is occasionally required.19 Following treatment, relapse occurs in up to 6% of patients over five years, and long-term follow-up with urine cultures and PCR every six months is recommended.10,20 Appropriate screening and treatment for latent tuberculosis infection greatly reduces the risk of reactivation GUTB.
This patient presented with features of an infection, which, combined with his history of renal stones and his urinalysis, led to an appropriate suspicion of and empiric treatment for an upper UTI. Given the AKI and nephrolithiasis, imaging was done to exclude obstruction. The CT finding of bilateral hydroureters and hydronephrosis absent an obstructing stone or mass or abnormal bladder was the initial clue that this was not a typical bacterial infection and that there was likely an underlying infectious pathologic process such as TB involving the GU tract diffusely. The care team treated the patient as an individual with fever and sterile pyuria in the context of multiple urinary tract strictures and an initial unrevealing infectious diagnostic workup. They recognized that the clues to the ultimate diagnosis of GUTB were all in the stream.
KEY TEACHING POINTS
- GUTB is a significant cause of sterile pyuria.
- In the presence of bilateral hydronephrosis, it is vital to determine the level of obstruction. If the bladder is not distended or contracted, then obstruction is likely at the level of the ureters and initial use of percutaneous nephrostomy tubes to relieve obstruction may be preferred.
- Imaging abnormalities such as multiple ureteral strictures, hydroureter and hydronephrosis (absent an obstructing stone or mass), and the finding of a contracted bladder are highly suggestive of GUTB.
- The mainstay of treatment for GUTB is standard antituberculosis treatment regimens in combination with the relief of urinary obstruction by ureteral stenting, percutaneous nephrostomy or open surgery.
- GUTB can relapse in up to 6% of treated cases over five years, and long-term follow-up and surveillance with urine culture and PCR every six months are recommended.
Disclosures
Benjamin Mba, Nathan Houchens, Marie Jennifer Seares, and Udit Joshi have no financial conflicts of interest and no disclosures.
Funding
Brian P. Lucas receives funding from the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development and Dartmouth SYNERGY, National Institutes of Health, and National Center for Translational Science (UL1TR001086).
1. Forssbohm M, Zwahlen M, Loddenkemper R, Rieder HL. Demographic characteristics of patients with extrapulmonary tuberculosis in Germany. Eur Resp J. 2008;31(1):99-105. https://doi.org/10.1183/09031936.00020607.
2. French CE, Antoine D, Gelb D, Jones JA, Gilbert RL, Watson JM. Tuberculosis in non-UK-born persons, England and Wales, 2001-2003. Int J Tuberc Lung Dis. 2007;11(5):577-584.
3. Peto HM, Pratt RH, Harrington TA, LoBue PA, Armstrong LR. Epidemiology of extrapulmonary tuberculosis in the United States, 1993-2006. Clin Infect Dis. 2009;49(9):1350-1357. https://doi.org/10.1086/605559.
4. Alvarez S, McCabe WR. Extrapulmonary tuberculosis revisited: a review of experience at Boston City and other hospitals. Medicine. 1984;63(1):25-55.
5. Simon HB, Weinstein AJ, Pasternak MS, Swartz MN, Kunz LJ. Genitourinary tuberculosis. Clinical features in a general hospital population. Am J Med. 1977;63(3):410-420. https://doi.org/10.1016/0002-9343(77)90279-0.
6. Christensen WI. Genitourinary tuberculosis: review of 102 cases. Medicine. 1974;53(5):377-390. https://doi.org/10.1016/0002-9343(77)90279-0.
7. Eastwood JB, Corbishley CM, Grange JM. Tuberculosis and the kidney. J Am Soc Nephrol. 2001;12(6):1307-1314.
8. Garcia-Rodriguez JA, Garcia Sanchez JE, Munoz Bellido JL, et al. Genitourinary tuberculosis in Spain: review of 81 cases. Clin Infect Dis.1994;18(4):557-561. https://doi.org/10.1093/clinids/18.4.557.
9. de Figueiredo AA, Lucon AM, Srougi M. Bladder augmentation for the treatment of chronic tuberculous cystitis. Clinical and urodynamic evaluation of 25 patients after long term follow-up. Neurourol Urodyn. 2006;25(5):433-440. https://doi.org/10.1002/nau.20264.
10. Figueiredo AA, Lucon AM, Srougi M. Urogenital Tuberculosis. Microbiol Spectr. 2017;5. https://doi.org/10.1128/microbiolspec.TNMI7-0015-2016.
11. Gupta N, Mandal AK, Singh SK. Tuberculosis of the prostate and urethra: A review. Indian J Urol. 2008;24(3):388-391. https://doi.org/10.4103/0970-1591.42623.
12. Figueiredo AA, Lucon AM, Junior RF, Srougi M. Epidemiology of urogenital tuberculosis worldwide. Int J Urol. 2008;15(9):827-832. https://doi.org/10.1111/j.1442-2042.2008.02099.x.
13. Mortier E, Pouchot J, Girard L, Boussougant Y, Vinceneux P. Assessment of urine analysis for the diagnosis of tuberculosis. BMJ (Clinical research ed). 1996;312:27-28. https://doi.org/10.1136/bmj.312.7022.27.
14. Moussa OM, Eraky I, El-Far MA, et al. Rapid diagnosis of genitourinary tuberculosis by polymerase chain reaction and non-radioactive DNA hybridization. J Urol. 2000;164(2):584-588. https://doi.org/10.1016/S0022-5347(05)67427-7.
15. Chawla A, Chawla K, Reddy S, et al. Can tissue PCR augment the diagnostic accuracy in genitourinary tract tuberculosis? Urol Int. 2012;88(1):34-38. https://doi.org/10.1159/000327039.
16. Kohli M, Schiller I, Dendukuri N, et al. Xpert((R)) MTB/RIF assay for extrapulmonary tuberculosis and rifampicin resistance. Cochrane Database Syst Rev. 2018;8:Cd012768. https://doi.org/10.1002/14651858.CD012768.pub2.
17. Figueiredo AA, Lucon AM, Arvellos AN, et al. A better understanding of urogenital tuberculosis pathophysiology based on radiological findings. Eur J Radiol. 2010;76(2):246-257. https://doi.org/10.1016/j.ejrad.2009.05.049.
18. Treatment of Tuberculosis: Guidelines. 4th edition. Geneva: World Health Organization. 2010.
19. O’Flynn D. Surgical treatment of genito-urinary tuberculosis. A report on 762 cases. Br J Urol. 1970;42(6):667-671. https://doi.org/10.1111/j.1464-410X.1970.tb06789.x.
20. Butler MR, O’Flynn JD. Reactivation of genito-urinary tuberculosis: a retrospective review of 838 cases. Eur Urol. 1975;1:14-17. https://doi.org/10.1159/000455566.
A 67-year-old man presented to the emergency department with four days of nausea, vomiting, and chills. He was originally from the Philippines but lived in the United States for six years. His past medical history was notable for nephrolithiasis for which a ureteral stent had been placed and was subsequently removed three years prior. He reported no abdominal pain, diarrhea, dysuria, urinary frequency, hematuria, cough, headache, or fever. He was a retired high school teacher and a lifelong nonsmoker.
This patient presents with a nonspecific constellation of constitutional and gastrointestinal (GI) symptoms. A system-based approach may be helpful when considering the differential diagnosis. Chills most often suggest infection, especially in older patients. With regard to GI causes, acute gastroenteritis and other food-borne infections can cause nausea, vomiting, and chills, but these are typically accompanied by abdominal pain and diarrhea and often resolve in less than four days. Abdominal pain would also be expected with cholecystitis as well as more life-threatening causes of nausea such as acute pancreatitis, mesenteric ischemia, and bowel obstruction. In contrast, abdominal pain would not be expected with a central nervous system (CNS) infection such as a brain abscess, which may cause nausea from increased intracranial pressure. Headaches occur in a majority of these cases, making CNS etiologies of nausea less likely. Cardiovascular causes, including myocardial ischemia and infarction, may lead to nausea and vomiting, but these are less likely given the absence of chest pain. Genitourinary causes must be considered, especially given his history of both nephrolithiasis and instrumentation. A stricture or recurrence of nephrolithiasis could lead to acute pyelonephritis or perinephric abscess, although both commonly present with urinary tract symptoms. Uremia, possibly from obstructive uropathy given the patient’s history of nephrolithiasis, could also lead to this constellation of symptoms.
On examination, temperature was 101.6 °F, heart rate 126 beats per minute, respiratory rate 18 breaths per minute, blood pressure 120/76 mm Hg, and oxygen saturation 98% on room air. The oral mucosa was moist, heart sounds were normal without murmurs, lungs were clear to auscultation, and abdomen was soft, nontender, and nondistended. There was no flank tenderness, and penile, testicular, and prostate examination findings were normal.
Laboratory studies revealed a serum sodium of 126 mEq/L, potassium 5.0 mEq/L, chloride 98 mEq/L, bicarbonate 15 mEq/L, blood urea nitrogen 88 mg/dL, creatinine 9.0 mg/dL, calcium 8 mg/dL, glucose 110 mg/dL, and albumin 3.3 g/dL. One year prior, serum creatinine was 1.4 mg/dL. His white blood cell (WBC) count was 8.0 k/uL with normal differential, hemoglobin 11.4 g/dL with normal MCV, and platelet count was normal. Serum osmolality was 286 mOsm/kg and serum parathyroid hormone (PTH) level 63 pg/mL (normal, 10-65). The urinalysis revealed cloudy urine with a specific gravity 1.009, 54 red blood cells (RBC), 236 WBC, large leukocyte esterase, negative nitrite, trace protein, and no casts or dysmorphic RBCs. A random urine specimen revealed sodium of 86 mEq/L, potassium 16 mEq/L, chloride 80 mEq/L, and creatinine 70 mg/dL.
Fever and tachycardia support an infectious cause of his symptoms. Absent flank tenderness and a normal genitourinary examination have only moderate negative predictive values for acute pyelonephritis and prostatitis, respectively. The most striking laboratory finding is his azotemia. Acute kidney injury (AKI) is more likely than chronic kidney disease (CKD) given that the PTH level is normal and the serum creatinine from a year ago was near normal. The most useful finding to differentiate AKI from CKD is the presence of atrophic kidneys on imaging. The low bicarbonate level indicates a metabolic acidosis. His serum anion gap is 13 mEq/L, which falls above most normal ranges. A mildly elevated serum anion gap together with a “delta serum anion gap/delta serum bicarbonate” ratio less than one suggest concomitant anion gap metabolic acidosis and non anion gap metabolic acidosis. The latter, coupled with a history of nephrolithiasis, may point to the possibility of renal tubular acidosis and AKI caused by urinary tract obstruction. This could also account for the marked hyponatremia. Moreover, his high fractional excretion of sodium (9%) is not suggestive of prerenal injury, the most common acute renal injury among patients who present to the emergency department. Hematuria carries a broad differential diagnosis, but most common causes include nephrolithiasis, urinary tract infection (UTI), prostatitis, neoplasm, and glomerulonephritis (GN). The lack of casts and dysmorphic RBCs makes GN unlikely. Taken together, his vital signs, examination, and laboratory studies suggest a high likelihood of an upper UTI (acute obstructive pyelonephritis) in the context of AKI due to obstructive uropathy.
Despite both a normal serum WBC count (which has only a moderate negative predictive value) and his low risk of developing life-threating organ dysfunction from sepsis based on a quick Sequential Organ Failure Assessment (qSOFA) score of zero, it is appropriate to start antibiotics after drawing blood and doing urine cultures. The next step should include administration of a broad-spectrum regimen that is appropriately dose-adjusted for renal dysfunction, such as an antipseudomonal carbapenem and vancomycin to cover extended-spectrum beta-lactamase (ESBL)-producing organisms, Pseudomonas aeruginosa, and methicillin-resistant Staphylococcus aureus (MRSA). This broad coverage is indicated for empiric treatment of complicated obstructive pyelonephritis, a condition that may arise from significant urinary obstruction and that carries a high risk of rapid clinical deterioration. He should undergo a rapid bedside test to assess for urethral or bladder outlet obstruction: either a bladder ultrasound or temporary insertion of a bladder catheter. He should also have an urgent computed tomography (CT) of his abdomen and pelvis without intravenous (IV) contrast, looking for evidence of urinary tract obstruction. A CT is preferred over ultrasound of the kidneys and bladder as CT has higher sensitivity and specificity for nephrolithiasis and neoplasm.
A CT of the abdomen and pelvis without IV contrast revealed bilateral hydroureter and hydronephrosis with multiple punctate calcified stones within the right calyces and the distal right ureter (Figure 1, Figure 2). However, these appeared too small to cause the degree of obstruction visualized. There were no stones noted in the left ureter to account for the obstruction, though small stones were noted in the left calyces. The bladder appeared normal.
Rarely are both ureters obstructed proximal to the ureterovesical junctions in the retroperitoneum. When they are, CT scans usually reveal culprit lesions that are extrinsic to the urinary tract, such as masses or retroperitoneal fibrosis, the latter of which can be associated with IgG4-related disease. Intrinsic causes of urinary tract obstructions include ureteral strictures (from infections, nephrolithiasis, instrumentation, prior radiotherapy, or rarely urothelial cancer), blood clots, metastatic ureteral deposits, or nephrolithiasis. While most intrinsic causes are unilateral, the patient is predisposed to strictures given his history of ureteral instrumentation. A preexisting unilateral obstruction due to a stricture may now, therefore, be unmasked by a second intrinsic obstruction in the contralateral ureter. Alternatively, given his remote history of living in the Philippines, a site where Schistosoma haematobium is endemic, chronic genitourinary schistosomiasis may have caused ureteral strictures due to granulomas, fibrosis, or pseudopolyps.
More commonly, bilateral hydroureter with bilateral hydronephrosis is caused by an obstruction of the bladder or urethra. CT scans can reveal prostatic hyperplasia (occasionally with protrusion into the bladder) and increased bladder wall thickness as a result of chronic bladder outlet obstruction, but the negative predictive value of either finding is modest. More revealing is that the patient reported neither an inability to pass urine (in fact, a “random” urine sample was obtained) nor suprapubic discomfort. Both symptoms would be pronounced with acute bladder obstruction but may be minimal with slowly progressive obstruction. In either case, a distended bladder would have been seen on the CT scan.
Regardless of the cause or whether the obstruction is in the upper or lower urinary tract, emergency intervention is needed to relieve the obstruction when AKI presents with bilateral hydronephrosis. A urology consultation should be sought urgently to determine the best strategy to relieve the obstruction. This may include bilateral percutaneous nephrostomy tubes given that the obstruction appears to be above the level of the bladder. Their findings will also direct additional diagnostic workup.
The patient received ceftriaxone and underwent cystoscopy, which revealed a stricture of the distal bulbar urethra. The ureters and bladder could not be completely visualized due to hematuria. The urethral stricture was dilated, and a Foley catheter was placed. In the operating room, the patient had a fever of 103 °F and developed severe hypotension unresponsive to 3 L of intravenous normal saline. Norepinephrine infusion was initiated for refractory hypotension.
Except for transurethral prostate resections, endoscopic urologic procedures rarely lead to a stricture of any segment of the urethra, suggesting that the previous ureteral stent placement and retrieval were not causal. Longstanding Mycobacterium tuberculosis or Schistosoma haematobium infection occasionally causes urethral strictures presenting as bilateral hydronephrosis. The multiple punctate calcified “stones” demonstrated on CT may suggest either diagnosis if they were actually calcified granulomas.
Regardless of the cause, most patients with a urethral stricture have chronic lower urinary tract symptoms such as decreased stream and the feeling of incomplete bladder emptying. Since this patient does not report these symptoms, it is important to consider if the stricture might be merely incidental. The absence of pain is more telling than the absence of chronic or recurrent symptoms. Lack of pain argues strongly against a pure de novo acute obstruction because abrupt stretching of the renal capsules and the walls of the collecting system is usually painful. Slow stretching caused by a progressive stricture may mask the pain of a superimposed acute obstruction. A blood clot, for example, may have precipitated an acute-on-chronic obstruction upon lodging at the urethral stricture.
The worsening systemic response to the procedure may be due to increased intravesical pressure by dilation and cystoscopy, which may have caused subsequent backflow of bacteria from the renal parenchyma into the circulation (pyelorenal backflow). The broad-spectrum antibiotic regimen suggested above and IV crystalloid infusion should be continued with close hemodynamic monitoring.
Treatment for severe sepsis was initiated with empiric piperacillin-tazobactam, ceftriaxone was discontinued, and the patient was transferred to the intensive care unit (ICU). Norepinephrine was discontinued after 24 hours. Despite the indwelling Foley catheter, his kidney function worsened (creatinine increased to 10.5 over the next 36 hours, and he remained oliguric). Therefore, bilateral percutaneous nephrostomy tubes were placed to relieve the ongoing obstruction. In the ICU, he remained febrile, despite receiving piperacillin-tazobactam, through hospital day 7. Serial blood and urine cultures all remained negative. HIV testing was negative. His chest radiograph was unremarkable, and transthoracic echocardiogram was normal. His creatinine improved but plateaued at 2.5 mg/dL by day 7.
Worsening renal function (alongside oliguria or anuria) despite a functioning Foley catheter suggests either intrinsic renal disease or bilateral ureteral obstructions. The initial attempt at relieving the obstruction with a Foley catheter did not take into consideration the bilateral ureteral strictures. As a result, soon thereafter, the insertion of percutaneous nephrostomy tubes was necessary. Given the severity of his illness, underlying obstructive uropathy, and persistent fever, suggesting an ongoing infection, one strategy would be to continue antibiotics with broader coverage than piperacillin-tazobactam. This approach may be reasonable, given the emergence of ESBL organisms and the possibility of MRSA due to instrumentation. However, it is important to note that only sterile pyuria has been identified to date, which raises the possibility of nonbacterial infections. Although chronic infection with Schistosoma haematobium can cause bilateral ureteral strictures, associated fever is limited to the acute phase of infection and not the chronic obstructive phase, unless there is a superimposed infection. Genitourinary Mycobacterium tuberculosis remains a likely possibility, regardless of the unrevealing chest radiograph. Urine nucleic acid amplification and acid-fast bacilli (AFB) smear and culture, the best initial diagnostic test, should be sent. Although less definitive, a tuberculin skin test and an interferon-gamma release assay should also be conducted. Histopathology of the ureters obtained by repeat cystoscopy may be diagnostic, but given the limited visualization during the last cystoscopy and the recent dilation of the urethra, this option should be kept in reserve for now.
Antibiotics were discontinued on day 7, but the patient continued to experience ongoing fever. Urine Histoplasma and serum cryptococcal antigens were negative. His urine AFB smear was 1+ positive. Liver function tests revealed a total protein of 7.0 g/dL, albumin 3.0 g/dL, total bilirubin 1.2 mg/dL, direct bilirubin 0.3 mg/dL, alkaline phosphatase 418 U/L, aspartate aminotransferase 65 U/L, alanine aminotransferase 88 U/L, gamma-glutamyltransferase (GGT) 609 U/L (normal, 3-60), and lactate dehydrogenase 284 U/L (normal, 85-210).
Acid-fast bacillus in the urine strongly suggests Mycobacterium tuberculosis (MTB) with several reports of likelihood ratios greater than 10. Nevertheless, confirmation is needed to rule out nontuberculous mycobacteria because of potential hepatotoxicity from treatment. Up to six urine samples should be sent for mycobacterium culture. However, false negative rates of up to 90% are reported, and final test results can take up to two months, so other methods of confirmation should be simultaneously sought. A nucleic acid amplification test of urine could rule in a pathogenic species within 24 hours. Alternatively, the probability of a nonpathogenic colonizing species would be negligible if a caseating granuloma was found. Biopsy could be obtained from the ureters, as suggested above. Liver biopsy should also be considered, especially if the moderate elevations in alkaline phosphatase and GGT (the most common liver enzyme abnormalities in hepatic tuberculosis) did not merely wax and wane with sepsis.
A CT of the thorax without IV contrast was done to evaluate for evidence of pulmonary disease given the positive urine AFB. This demonstrated bilateral fibrotic upper lobe opacities suggestive of prior granulomatous disease but no cavitary lung lesions (Figure 3). Three sputum smears were negative for AFB, but one sample showed Mycobacterium tuberculosis detected by a polymerase chain reaction (PCR) probe.
Given the concern for genitourinary tuberculosis (GUTB), it is appropriate to place the patient in respiratory isolation to exclude concomitant pulmonary tuberculosis (TB). AFB smears were negative, but the sputum PCR probe was positive, confirming pathogenic MTB. However, the negative AFB smears make the likelihood of pulmonary infectivity low. As a result, contact tracing is often deemed unnecessary by hospital infection control teams. Though his chest radiograph was normal, CT showed bilateral upper lobe fibrotic disease suggestive of prior pulmonary TB, thus making it likely that the current GUTB represents reactivation.
The two-month initiation phase of treatment with four antituberculosis drugs should begin while drug susceptibility tests are pending. Potential hepatotoxicity should be closely monitored, ideally by a clinician with experience treating tuberculosis in patients with existing liver disease. As a general precaution, alcohol should be avoided as should medications such as acetaminophen that are known to be hepatotoxic. Urology follow-up is also needed because about one-third of tuberculous ureteral strictures treated initially with percutaneous nephrostomy do not resolve with antituberculosis therapy.
The patient was started on weight-based antituberculosis treatment with four antimicrobial agents (rifampin, ethambutol, pyrazinamide, and isoniazid). He was seen in the infectious disease clinic two weeks later; his fever had resolved, and his liver function tests showed normalization of AST and LDH as well as a 45% reduction in his GGT and alkaline phosphatase levels. Two months following discharge, a nuclear medicine radionuclide angiogram renal flow scan showed normal right kidney function. The right nephrostomy tube was subsequently removed. He continued to have left kidney outflow obstruction due to a residual ureteral stricture (Figure 4). Repeat cystoscopy and attempted left ureteral stenting was unsuccessful. The left nephrostomy tube remained in place.
DISCUSSION
According to the Centers for Disease Control, in 2017, 10 million people became sick with TB, and there were 1.3 million TB-related deaths worldwide with 9,150 cases reported in the United States. Extrapulmonary TB (EPTB) constitutes 10% of all TB cases globally.1-4 GUTB is the second most common form of EPTB after lymph node TB, and it occurs in up to 20% of all pulmonary TB cases.2,3
Mycobacteria reach the genitourinary (GU) tract via hematogenous spread during primary infection or reactivation of TB. This leads to cortical and medullary lesions, which can heal spontaneously or eventually (average of 22 years) rupture into the tubules and onto the collecting system, ureters, and bladder.5,6 Spread to the ureter and bladder leads to multiple ureteral strictures and contracture of the bladder with disruption of the ureterovesical junction (UVJ), which causes hydroureter and hydronephrosis.7 Unilateral kidney involvement is most common, but bilateral involvement can occur following exacerbated hematogenous spread, particularly in immune deficient patients. Bilateral kidney involvement is also possible from retrograde spread to the good kidney due to bladder contracture and UVJ disruption.8,9 Distal infection can involve all aspects of the male and female genital tracts, but urethral strictures are extremely rare.10,11
GUTB affects males more than females (2:1) and presents insidiously at 40 to 60 years of age.12 Other risk factors for TB include birth in TB endemic areas, prior TB infection, immunosuppression, malnutrition, severe systemic disease, diabetes, and cirrhosis. It is crucial to assess these risk factors when creating and refining differential diagnoses. Many patients have hematuria and sterile pyuria as incidental initial findings. The most common symptoms arise from bladder involvement, including frequency, urgency, and dysuria. Low back pain and gross hematuria are also common, but fever and constitutional symptoms are uncommon.10 Bilateral ureteral strictures can lead to obstructive renal failure, and involvement of the genital tracts can lead to pelvic or scrotal pain, swelling, and fistula formation.10
Diagnosis involves the demonstration of TB bacilli in urine or GU tissue. The urinalysis reveals hematuria and sterile pyuria.13 Urine AFB stains are positive in 52% of cases but are not diagnostic as nontuberculous mycobacteria may also cause a positive test result.13,14 Urine cultures for Mycobacterium tuberculosis are positive in up to 90% of cases after six to eight weeks. As many as three to six morning urine samples are required to achieve diagnostic accuracy.10,14 Urine PCR for Mycobacterium tuberculosis has 96% sensitivity and up to 98% specificity,14 while PCR on GU tissue has a sensitivity of 88% and specificity of 87%.15 The rapid nucleic acid amplification assay Xpert MTB/RIF in urine has a sensitivity of 83%, and specificity of 98%.16 Imaging is required to evaluate for obstruction, and the CT scan is abnormal in up to 90% of cases, showing multiple ureteral stenoses, hydroureter and hydronephrosis, and a contracted bladder.10,17
GUTB is treated with standard antituberculosis regimens.18 Patients with urinary obstruction benefit from ureteral stenting or percutaneous nephrostomy, bladder diversion, or ureteral reconstruction surgery. Unilateral nephrectomy for a nonfunctioning kidney with extensive disease is occasionally required.19 Following treatment, relapse occurs in up to 6% of patients over five years, and long-term follow-up with urine cultures and PCR every six months is recommended.10,20 Appropriate screening and treatment for latent tuberculosis infection greatly reduces the risk of reactivation GUTB.
This patient presented with features of an infection, which, combined with his history of renal stones and his urinalysis, led to an appropriate suspicion of and empiric treatment for an upper UTI. Given the AKI and nephrolithiasis, imaging was done to exclude obstruction. The CT finding of bilateral hydroureters and hydronephrosis absent an obstructing stone or mass or abnormal bladder was the initial clue that this was not a typical bacterial infection and that there was likely an underlying infectious pathologic process such as TB involving the GU tract diffusely. The care team treated the patient as an individual with fever and sterile pyuria in the context of multiple urinary tract strictures and an initial unrevealing infectious diagnostic workup. They recognized that the clues to the ultimate diagnosis of GUTB were all in the stream.
KEY TEACHING POINTS
- GUTB is a significant cause of sterile pyuria.
- In the presence of bilateral hydronephrosis, it is vital to determine the level of obstruction. If the bladder is not distended or contracted, then obstruction is likely at the level of the ureters and initial use of percutaneous nephrostomy tubes to relieve obstruction may be preferred.
- Imaging abnormalities such as multiple ureteral strictures, hydroureter and hydronephrosis (absent an obstructing stone or mass), and the finding of a contracted bladder are highly suggestive of GUTB.
- The mainstay of treatment for GUTB is standard antituberculosis treatment regimens in combination with the relief of urinary obstruction by ureteral stenting, percutaneous nephrostomy or open surgery.
- GUTB can relapse in up to 6% of treated cases over five years, and long-term follow-up and surveillance with urine culture and PCR every six months are recommended.
Disclosures
Benjamin Mba, Nathan Houchens, Marie Jennifer Seares, and Udit Joshi have no financial conflicts of interest and no disclosures.
Funding
Brian P. Lucas receives funding from the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development and Dartmouth SYNERGY, National Institutes of Health, and National Center for Translational Science (UL1TR001086).
A 67-year-old man presented to the emergency department with four days of nausea, vomiting, and chills. He was originally from the Philippines but lived in the United States for six years. His past medical history was notable for nephrolithiasis for which a ureteral stent had been placed and was subsequently removed three years prior. He reported no abdominal pain, diarrhea, dysuria, urinary frequency, hematuria, cough, headache, or fever. He was a retired high school teacher and a lifelong nonsmoker.
This patient presents with a nonspecific constellation of constitutional and gastrointestinal (GI) symptoms. A system-based approach may be helpful when considering the differential diagnosis. Chills most often suggest infection, especially in older patients. With regard to GI causes, acute gastroenteritis and other food-borne infections can cause nausea, vomiting, and chills, but these are typically accompanied by abdominal pain and diarrhea and often resolve in less than four days. Abdominal pain would also be expected with cholecystitis as well as more life-threatening causes of nausea such as acute pancreatitis, mesenteric ischemia, and bowel obstruction. In contrast, abdominal pain would not be expected with a central nervous system (CNS) infection such as a brain abscess, which may cause nausea from increased intracranial pressure. Headaches occur in a majority of these cases, making CNS etiologies of nausea less likely. Cardiovascular causes, including myocardial ischemia and infarction, may lead to nausea and vomiting, but these are less likely given the absence of chest pain. Genitourinary causes must be considered, especially given his history of both nephrolithiasis and instrumentation. A stricture or recurrence of nephrolithiasis could lead to acute pyelonephritis or perinephric abscess, although both commonly present with urinary tract symptoms. Uremia, possibly from obstructive uropathy given the patient’s history of nephrolithiasis, could also lead to this constellation of symptoms.
On examination, temperature was 101.6 °F, heart rate 126 beats per minute, respiratory rate 18 breaths per minute, blood pressure 120/76 mm Hg, and oxygen saturation 98% on room air. The oral mucosa was moist, heart sounds were normal without murmurs, lungs were clear to auscultation, and abdomen was soft, nontender, and nondistended. There was no flank tenderness, and penile, testicular, and prostate examination findings were normal.
Laboratory studies revealed a serum sodium of 126 mEq/L, potassium 5.0 mEq/L, chloride 98 mEq/L, bicarbonate 15 mEq/L, blood urea nitrogen 88 mg/dL, creatinine 9.0 mg/dL, calcium 8 mg/dL, glucose 110 mg/dL, and albumin 3.3 g/dL. One year prior, serum creatinine was 1.4 mg/dL. His white blood cell (WBC) count was 8.0 k/uL with normal differential, hemoglobin 11.4 g/dL with normal MCV, and platelet count was normal. Serum osmolality was 286 mOsm/kg and serum parathyroid hormone (PTH) level 63 pg/mL (normal, 10-65). The urinalysis revealed cloudy urine with a specific gravity 1.009, 54 red blood cells (RBC), 236 WBC, large leukocyte esterase, negative nitrite, trace protein, and no casts or dysmorphic RBCs. A random urine specimen revealed sodium of 86 mEq/L, potassium 16 mEq/L, chloride 80 mEq/L, and creatinine 70 mg/dL.
Fever and tachycardia support an infectious cause of his symptoms. Absent flank tenderness and a normal genitourinary examination have only moderate negative predictive values for acute pyelonephritis and prostatitis, respectively. The most striking laboratory finding is his azotemia. Acute kidney injury (AKI) is more likely than chronic kidney disease (CKD) given that the PTH level is normal and the serum creatinine from a year ago was near normal. The most useful finding to differentiate AKI from CKD is the presence of atrophic kidneys on imaging. The low bicarbonate level indicates a metabolic acidosis. His serum anion gap is 13 mEq/L, which falls above most normal ranges. A mildly elevated serum anion gap together with a “delta serum anion gap/delta serum bicarbonate” ratio less than one suggest concomitant anion gap metabolic acidosis and non anion gap metabolic acidosis. The latter, coupled with a history of nephrolithiasis, may point to the possibility of renal tubular acidosis and AKI caused by urinary tract obstruction. This could also account for the marked hyponatremia. Moreover, his high fractional excretion of sodium (9%) is not suggestive of prerenal injury, the most common acute renal injury among patients who present to the emergency department. Hematuria carries a broad differential diagnosis, but most common causes include nephrolithiasis, urinary tract infection (UTI), prostatitis, neoplasm, and glomerulonephritis (GN). The lack of casts and dysmorphic RBCs makes GN unlikely. Taken together, his vital signs, examination, and laboratory studies suggest a high likelihood of an upper UTI (acute obstructive pyelonephritis) in the context of AKI due to obstructive uropathy.
Despite both a normal serum WBC count (which has only a moderate negative predictive value) and his low risk of developing life-threating organ dysfunction from sepsis based on a quick Sequential Organ Failure Assessment (qSOFA) score of zero, it is appropriate to start antibiotics after drawing blood and doing urine cultures. The next step should include administration of a broad-spectrum regimen that is appropriately dose-adjusted for renal dysfunction, such as an antipseudomonal carbapenem and vancomycin to cover extended-spectrum beta-lactamase (ESBL)-producing organisms, Pseudomonas aeruginosa, and methicillin-resistant Staphylococcus aureus (MRSA). This broad coverage is indicated for empiric treatment of complicated obstructive pyelonephritis, a condition that may arise from significant urinary obstruction and that carries a high risk of rapid clinical deterioration. He should undergo a rapid bedside test to assess for urethral or bladder outlet obstruction: either a bladder ultrasound or temporary insertion of a bladder catheter. He should also have an urgent computed tomography (CT) of his abdomen and pelvis without intravenous (IV) contrast, looking for evidence of urinary tract obstruction. A CT is preferred over ultrasound of the kidneys and bladder as CT has higher sensitivity and specificity for nephrolithiasis and neoplasm.
A CT of the abdomen and pelvis without IV contrast revealed bilateral hydroureter and hydronephrosis with multiple punctate calcified stones within the right calyces and the distal right ureter (Figure 1, Figure 2). However, these appeared too small to cause the degree of obstruction visualized. There were no stones noted in the left ureter to account for the obstruction, though small stones were noted in the left calyces. The bladder appeared normal.
Rarely are both ureters obstructed proximal to the ureterovesical junctions in the retroperitoneum. When they are, CT scans usually reveal culprit lesions that are extrinsic to the urinary tract, such as masses or retroperitoneal fibrosis, the latter of which can be associated with IgG4-related disease. Intrinsic causes of urinary tract obstructions include ureteral strictures (from infections, nephrolithiasis, instrumentation, prior radiotherapy, or rarely urothelial cancer), blood clots, metastatic ureteral deposits, or nephrolithiasis. While most intrinsic causes are unilateral, the patient is predisposed to strictures given his history of ureteral instrumentation. A preexisting unilateral obstruction due to a stricture may now, therefore, be unmasked by a second intrinsic obstruction in the contralateral ureter. Alternatively, given his remote history of living in the Philippines, a site where Schistosoma haematobium is endemic, chronic genitourinary schistosomiasis may have caused ureteral strictures due to granulomas, fibrosis, or pseudopolyps.
More commonly, bilateral hydroureter with bilateral hydronephrosis is caused by an obstruction of the bladder or urethra. CT scans can reveal prostatic hyperplasia (occasionally with protrusion into the bladder) and increased bladder wall thickness as a result of chronic bladder outlet obstruction, but the negative predictive value of either finding is modest. More revealing is that the patient reported neither an inability to pass urine (in fact, a “random” urine sample was obtained) nor suprapubic discomfort. Both symptoms would be pronounced with acute bladder obstruction but may be minimal with slowly progressive obstruction. In either case, a distended bladder would have been seen on the CT scan.
Regardless of the cause or whether the obstruction is in the upper or lower urinary tract, emergency intervention is needed to relieve the obstruction when AKI presents with bilateral hydronephrosis. A urology consultation should be sought urgently to determine the best strategy to relieve the obstruction. This may include bilateral percutaneous nephrostomy tubes given that the obstruction appears to be above the level of the bladder. Their findings will also direct additional diagnostic workup.
The patient received ceftriaxone and underwent cystoscopy, which revealed a stricture of the distal bulbar urethra. The ureters and bladder could not be completely visualized due to hematuria. The urethral stricture was dilated, and a Foley catheter was placed. In the operating room, the patient had a fever of 103 °F and developed severe hypotension unresponsive to 3 L of intravenous normal saline. Norepinephrine infusion was initiated for refractory hypotension.
Except for transurethral prostate resections, endoscopic urologic procedures rarely lead to a stricture of any segment of the urethra, suggesting that the previous ureteral stent placement and retrieval were not causal. Longstanding Mycobacterium tuberculosis or Schistosoma haematobium infection occasionally causes urethral strictures presenting as bilateral hydronephrosis. The multiple punctate calcified “stones” demonstrated on CT may suggest either diagnosis if they were actually calcified granulomas.
Regardless of the cause, most patients with a urethral stricture have chronic lower urinary tract symptoms such as decreased stream and the feeling of incomplete bladder emptying. Since this patient does not report these symptoms, it is important to consider if the stricture might be merely incidental. The absence of pain is more telling than the absence of chronic or recurrent symptoms. Lack of pain argues strongly against a pure de novo acute obstruction because abrupt stretching of the renal capsules and the walls of the collecting system is usually painful. Slow stretching caused by a progressive stricture may mask the pain of a superimposed acute obstruction. A blood clot, for example, may have precipitated an acute-on-chronic obstruction upon lodging at the urethral stricture.
The worsening systemic response to the procedure may be due to increased intravesical pressure by dilation and cystoscopy, which may have caused subsequent backflow of bacteria from the renal parenchyma into the circulation (pyelorenal backflow). The broad-spectrum antibiotic regimen suggested above and IV crystalloid infusion should be continued with close hemodynamic monitoring.
Treatment for severe sepsis was initiated with empiric piperacillin-tazobactam, ceftriaxone was discontinued, and the patient was transferred to the intensive care unit (ICU). Norepinephrine was discontinued after 24 hours. Despite the indwelling Foley catheter, his kidney function worsened (creatinine increased to 10.5 over the next 36 hours, and he remained oliguric). Therefore, bilateral percutaneous nephrostomy tubes were placed to relieve the ongoing obstruction. In the ICU, he remained febrile, despite receiving piperacillin-tazobactam, through hospital day 7. Serial blood and urine cultures all remained negative. HIV testing was negative. His chest radiograph was unremarkable, and transthoracic echocardiogram was normal. His creatinine improved but plateaued at 2.5 mg/dL by day 7.
Worsening renal function (alongside oliguria or anuria) despite a functioning Foley catheter suggests either intrinsic renal disease or bilateral ureteral obstructions. The initial attempt at relieving the obstruction with a Foley catheter did not take into consideration the bilateral ureteral strictures. As a result, soon thereafter, the insertion of percutaneous nephrostomy tubes was necessary. Given the severity of his illness, underlying obstructive uropathy, and persistent fever, suggesting an ongoing infection, one strategy would be to continue antibiotics with broader coverage than piperacillin-tazobactam. This approach may be reasonable, given the emergence of ESBL organisms and the possibility of MRSA due to instrumentation. However, it is important to note that only sterile pyuria has been identified to date, which raises the possibility of nonbacterial infections. Although chronic infection with Schistosoma haematobium can cause bilateral ureteral strictures, associated fever is limited to the acute phase of infection and not the chronic obstructive phase, unless there is a superimposed infection. Genitourinary Mycobacterium tuberculosis remains a likely possibility, regardless of the unrevealing chest radiograph. Urine nucleic acid amplification and acid-fast bacilli (AFB) smear and culture, the best initial diagnostic test, should be sent. Although less definitive, a tuberculin skin test and an interferon-gamma release assay should also be conducted. Histopathology of the ureters obtained by repeat cystoscopy may be diagnostic, but given the limited visualization during the last cystoscopy and the recent dilation of the urethra, this option should be kept in reserve for now.
Antibiotics were discontinued on day 7, but the patient continued to experience ongoing fever. Urine Histoplasma and serum cryptococcal antigens were negative. His urine AFB smear was 1+ positive. Liver function tests revealed a total protein of 7.0 g/dL, albumin 3.0 g/dL, total bilirubin 1.2 mg/dL, direct bilirubin 0.3 mg/dL, alkaline phosphatase 418 U/L, aspartate aminotransferase 65 U/L, alanine aminotransferase 88 U/L, gamma-glutamyltransferase (GGT) 609 U/L (normal, 3-60), and lactate dehydrogenase 284 U/L (normal, 85-210).
Acid-fast bacillus in the urine strongly suggests Mycobacterium tuberculosis (MTB) with several reports of likelihood ratios greater than 10. Nevertheless, confirmation is needed to rule out nontuberculous mycobacteria because of potential hepatotoxicity from treatment. Up to six urine samples should be sent for mycobacterium culture. However, false negative rates of up to 90% are reported, and final test results can take up to two months, so other methods of confirmation should be simultaneously sought. A nucleic acid amplification test of urine could rule in a pathogenic species within 24 hours. Alternatively, the probability of a nonpathogenic colonizing species would be negligible if a caseating granuloma was found. Biopsy could be obtained from the ureters, as suggested above. Liver biopsy should also be considered, especially if the moderate elevations in alkaline phosphatase and GGT (the most common liver enzyme abnormalities in hepatic tuberculosis) did not merely wax and wane with sepsis.
A CT of the thorax without IV contrast was done to evaluate for evidence of pulmonary disease given the positive urine AFB. This demonstrated bilateral fibrotic upper lobe opacities suggestive of prior granulomatous disease but no cavitary lung lesions (Figure 3). Three sputum smears were negative for AFB, but one sample showed Mycobacterium tuberculosis detected by a polymerase chain reaction (PCR) probe.
Given the concern for genitourinary tuberculosis (GUTB), it is appropriate to place the patient in respiratory isolation to exclude concomitant pulmonary tuberculosis (TB). AFB smears were negative, but the sputum PCR probe was positive, confirming pathogenic MTB. However, the negative AFB smears make the likelihood of pulmonary infectivity low. As a result, contact tracing is often deemed unnecessary by hospital infection control teams. Though his chest radiograph was normal, CT showed bilateral upper lobe fibrotic disease suggestive of prior pulmonary TB, thus making it likely that the current GUTB represents reactivation.
The two-month initiation phase of treatment with four antituberculosis drugs should begin while drug susceptibility tests are pending. Potential hepatotoxicity should be closely monitored, ideally by a clinician with experience treating tuberculosis in patients with existing liver disease. As a general precaution, alcohol should be avoided as should medications such as acetaminophen that are known to be hepatotoxic. Urology follow-up is also needed because about one-third of tuberculous ureteral strictures treated initially with percutaneous nephrostomy do not resolve with antituberculosis therapy.
The patient was started on weight-based antituberculosis treatment with four antimicrobial agents (rifampin, ethambutol, pyrazinamide, and isoniazid). He was seen in the infectious disease clinic two weeks later; his fever had resolved, and his liver function tests showed normalization of AST and LDH as well as a 45% reduction in his GGT and alkaline phosphatase levels. Two months following discharge, a nuclear medicine radionuclide angiogram renal flow scan showed normal right kidney function. The right nephrostomy tube was subsequently removed. He continued to have left kidney outflow obstruction due to a residual ureteral stricture (Figure 4). Repeat cystoscopy and attempted left ureteral stenting was unsuccessful. The left nephrostomy tube remained in place.
DISCUSSION
According to the Centers for Disease Control, in 2017, 10 million people became sick with TB, and there were 1.3 million TB-related deaths worldwide with 9,150 cases reported in the United States. Extrapulmonary TB (EPTB) constitutes 10% of all TB cases globally.1-4 GUTB is the second most common form of EPTB after lymph node TB, and it occurs in up to 20% of all pulmonary TB cases.2,3
Mycobacteria reach the genitourinary (GU) tract via hematogenous spread during primary infection or reactivation of TB. This leads to cortical and medullary lesions, which can heal spontaneously or eventually (average of 22 years) rupture into the tubules and onto the collecting system, ureters, and bladder.5,6 Spread to the ureter and bladder leads to multiple ureteral strictures and contracture of the bladder with disruption of the ureterovesical junction (UVJ), which causes hydroureter and hydronephrosis.7 Unilateral kidney involvement is most common, but bilateral involvement can occur following exacerbated hematogenous spread, particularly in immune deficient patients. Bilateral kidney involvement is also possible from retrograde spread to the good kidney due to bladder contracture and UVJ disruption.8,9 Distal infection can involve all aspects of the male and female genital tracts, but urethral strictures are extremely rare.10,11
GUTB affects males more than females (2:1) and presents insidiously at 40 to 60 years of age.12 Other risk factors for TB include birth in TB endemic areas, prior TB infection, immunosuppression, malnutrition, severe systemic disease, diabetes, and cirrhosis. It is crucial to assess these risk factors when creating and refining differential diagnoses. Many patients have hematuria and sterile pyuria as incidental initial findings. The most common symptoms arise from bladder involvement, including frequency, urgency, and dysuria. Low back pain and gross hematuria are also common, but fever and constitutional symptoms are uncommon.10 Bilateral ureteral strictures can lead to obstructive renal failure, and involvement of the genital tracts can lead to pelvic or scrotal pain, swelling, and fistula formation.10
Diagnosis involves the demonstration of TB bacilli in urine or GU tissue. The urinalysis reveals hematuria and sterile pyuria.13 Urine AFB stains are positive in 52% of cases but are not diagnostic as nontuberculous mycobacteria may also cause a positive test result.13,14 Urine cultures for Mycobacterium tuberculosis are positive in up to 90% of cases after six to eight weeks. As many as three to six morning urine samples are required to achieve diagnostic accuracy.10,14 Urine PCR for Mycobacterium tuberculosis has 96% sensitivity and up to 98% specificity,14 while PCR on GU tissue has a sensitivity of 88% and specificity of 87%.15 The rapid nucleic acid amplification assay Xpert MTB/RIF in urine has a sensitivity of 83%, and specificity of 98%.16 Imaging is required to evaluate for obstruction, and the CT scan is abnormal in up to 90% of cases, showing multiple ureteral stenoses, hydroureter and hydronephrosis, and a contracted bladder.10,17
GUTB is treated with standard antituberculosis regimens.18 Patients with urinary obstruction benefit from ureteral stenting or percutaneous nephrostomy, bladder diversion, or ureteral reconstruction surgery. Unilateral nephrectomy for a nonfunctioning kidney with extensive disease is occasionally required.19 Following treatment, relapse occurs in up to 6% of patients over five years, and long-term follow-up with urine cultures and PCR every six months is recommended.10,20 Appropriate screening and treatment for latent tuberculosis infection greatly reduces the risk of reactivation GUTB.
This patient presented with features of an infection, which, combined with his history of renal stones and his urinalysis, led to an appropriate suspicion of and empiric treatment for an upper UTI. Given the AKI and nephrolithiasis, imaging was done to exclude obstruction. The CT finding of bilateral hydroureters and hydronephrosis absent an obstructing stone or mass or abnormal bladder was the initial clue that this was not a typical bacterial infection and that there was likely an underlying infectious pathologic process such as TB involving the GU tract diffusely. The care team treated the patient as an individual with fever and sterile pyuria in the context of multiple urinary tract strictures and an initial unrevealing infectious diagnostic workup. They recognized that the clues to the ultimate diagnosis of GUTB were all in the stream.
KEY TEACHING POINTS
- GUTB is a significant cause of sterile pyuria.
- In the presence of bilateral hydronephrosis, it is vital to determine the level of obstruction. If the bladder is not distended or contracted, then obstruction is likely at the level of the ureters and initial use of percutaneous nephrostomy tubes to relieve obstruction may be preferred.
- Imaging abnormalities such as multiple ureteral strictures, hydroureter and hydronephrosis (absent an obstructing stone or mass), and the finding of a contracted bladder are highly suggestive of GUTB.
- The mainstay of treatment for GUTB is standard antituberculosis treatment regimens in combination with the relief of urinary obstruction by ureteral stenting, percutaneous nephrostomy or open surgery.
- GUTB can relapse in up to 6% of treated cases over five years, and long-term follow-up and surveillance with urine culture and PCR every six months are recommended.
Disclosures
Benjamin Mba, Nathan Houchens, Marie Jennifer Seares, and Udit Joshi have no financial conflicts of interest and no disclosures.
Funding
Brian P. Lucas receives funding from the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development and Dartmouth SYNERGY, National Institutes of Health, and National Center for Translational Science (UL1TR001086).
1. Forssbohm M, Zwahlen M, Loddenkemper R, Rieder HL. Demographic characteristics of patients with extrapulmonary tuberculosis in Germany. Eur Resp J. 2008;31(1):99-105. https://doi.org/10.1183/09031936.00020607.
2. French CE, Antoine D, Gelb D, Jones JA, Gilbert RL, Watson JM. Tuberculosis in non-UK-born persons, England and Wales, 2001-2003. Int J Tuberc Lung Dis. 2007;11(5):577-584.
3. Peto HM, Pratt RH, Harrington TA, LoBue PA, Armstrong LR. Epidemiology of extrapulmonary tuberculosis in the United States, 1993-2006. Clin Infect Dis. 2009;49(9):1350-1357. https://doi.org/10.1086/605559.
4. Alvarez S, McCabe WR. Extrapulmonary tuberculosis revisited: a review of experience at Boston City and other hospitals. Medicine. 1984;63(1):25-55.
5. Simon HB, Weinstein AJ, Pasternak MS, Swartz MN, Kunz LJ. Genitourinary tuberculosis. Clinical features in a general hospital population. Am J Med. 1977;63(3):410-420. https://doi.org/10.1016/0002-9343(77)90279-0.
6. Christensen WI. Genitourinary tuberculosis: review of 102 cases. Medicine. 1974;53(5):377-390. https://doi.org/10.1016/0002-9343(77)90279-0.
7. Eastwood JB, Corbishley CM, Grange JM. Tuberculosis and the kidney. J Am Soc Nephrol. 2001;12(6):1307-1314.
8. Garcia-Rodriguez JA, Garcia Sanchez JE, Munoz Bellido JL, et al. Genitourinary tuberculosis in Spain: review of 81 cases. Clin Infect Dis.1994;18(4):557-561. https://doi.org/10.1093/clinids/18.4.557.
9. de Figueiredo AA, Lucon AM, Srougi M. Bladder augmentation for the treatment of chronic tuberculous cystitis. Clinical and urodynamic evaluation of 25 patients after long term follow-up. Neurourol Urodyn. 2006;25(5):433-440. https://doi.org/10.1002/nau.20264.
10. Figueiredo AA, Lucon AM, Srougi M. Urogenital Tuberculosis. Microbiol Spectr. 2017;5. https://doi.org/10.1128/microbiolspec.TNMI7-0015-2016.
11. Gupta N, Mandal AK, Singh SK. Tuberculosis of the prostate and urethra: A review. Indian J Urol. 2008;24(3):388-391. https://doi.org/10.4103/0970-1591.42623.
12. Figueiredo AA, Lucon AM, Junior RF, Srougi M. Epidemiology of urogenital tuberculosis worldwide. Int J Urol. 2008;15(9):827-832. https://doi.org/10.1111/j.1442-2042.2008.02099.x.
13. Mortier E, Pouchot J, Girard L, Boussougant Y, Vinceneux P. Assessment of urine analysis for the diagnosis of tuberculosis. BMJ (Clinical research ed). 1996;312:27-28. https://doi.org/10.1136/bmj.312.7022.27.
14. Moussa OM, Eraky I, El-Far MA, et al. Rapid diagnosis of genitourinary tuberculosis by polymerase chain reaction and non-radioactive DNA hybridization. J Urol. 2000;164(2):584-588. https://doi.org/10.1016/S0022-5347(05)67427-7.
15. Chawla A, Chawla K, Reddy S, et al. Can tissue PCR augment the diagnostic accuracy in genitourinary tract tuberculosis? Urol Int. 2012;88(1):34-38. https://doi.org/10.1159/000327039.
16. Kohli M, Schiller I, Dendukuri N, et al. Xpert((R)) MTB/RIF assay for extrapulmonary tuberculosis and rifampicin resistance. Cochrane Database Syst Rev. 2018;8:Cd012768. https://doi.org/10.1002/14651858.CD012768.pub2.
17. Figueiredo AA, Lucon AM, Arvellos AN, et al. A better understanding of urogenital tuberculosis pathophysiology based on radiological findings. Eur J Radiol. 2010;76(2):246-257. https://doi.org/10.1016/j.ejrad.2009.05.049.
18. Treatment of Tuberculosis: Guidelines. 4th edition. Geneva: World Health Organization. 2010.
19. O’Flynn D. Surgical treatment of genito-urinary tuberculosis. A report on 762 cases. Br J Urol. 1970;42(6):667-671. https://doi.org/10.1111/j.1464-410X.1970.tb06789.x.
20. Butler MR, O’Flynn JD. Reactivation of genito-urinary tuberculosis: a retrospective review of 838 cases. Eur Urol. 1975;1:14-17. https://doi.org/10.1159/000455566.
1. Forssbohm M, Zwahlen M, Loddenkemper R, Rieder HL. Demographic characteristics of patients with extrapulmonary tuberculosis in Germany. Eur Resp J. 2008;31(1):99-105. https://doi.org/10.1183/09031936.00020607.
2. French CE, Antoine D, Gelb D, Jones JA, Gilbert RL, Watson JM. Tuberculosis in non-UK-born persons, England and Wales, 2001-2003. Int J Tuberc Lung Dis. 2007;11(5):577-584.
3. Peto HM, Pratt RH, Harrington TA, LoBue PA, Armstrong LR. Epidemiology of extrapulmonary tuberculosis in the United States, 1993-2006. Clin Infect Dis. 2009;49(9):1350-1357. https://doi.org/10.1086/605559.
4. Alvarez S, McCabe WR. Extrapulmonary tuberculosis revisited: a review of experience at Boston City and other hospitals. Medicine. 1984;63(1):25-55.
5. Simon HB, Weinstein AJ, Pasternak MS, Swartz MN, Kunz LJ. Genitourinary tuberculosis. Clinical features in a general hospital population. Am J Med. 1977;63(3):410-420. https://doi.org/10.1016/0002-9343(77)90279-0.
6. Christensen WI. Genitourinary tuberculosis: review of 102 cases. Medicine. 1974;53(5):377-390. https://doi.org/10.1016/0002-9343(77)90279-0.
7. Eastwood JB, Corbishley CM, Grange JM. Tuberculosis and the kidney. J Am Soc Nephrol. 2001;12(6):1307-1314.
8. Garcia-Rodriguez JA, Garcia Sanchez JE, Munoz Bellido JL, et al. Genitourinary tuberculosis in Spain: review of 81 cases. Clin Infect Dis.1994;18(4):557-561. https://doi.org/10.1093/clinids/18.4.557.
9. de Figueiredo AA, Lucon AM, Srougi M. Bladder augmentation for the treatment of chronic tuberculous cystitis. Clinical and urodynamic evaluation of 25 patients after long term follow-up. Neurourol Urodyn. 2006;25(5):433-440. https://doi.org/10.1002/nau.20264.
10. Figueiredo AA, Lucon AM, Srougi M. Urogenital Tuberculosis. Microbiol Spectr. 2017;5. https://doi.org/10.1128/microbiolspec.TNMI7-0015-2016.
11. Gupta N, Mandal AK, Singh SK. Tuberculosis of the prostate and urethra: A review. Indian J Urol. 2008;24(3):388-391. https://doi.org/10.4103/0970-1591.42623.
12. Figueiredo AA, Lucon AM, Junior RF, Srougi M. Epidemiology of urogenital tuberculosis worldwide. Int J Urol. 2008;15(9):827-832. https://doi.org/10.1111/j.1442-2042.2008.02099.x.
13. Mortier E, Pouchot J, Girard L, Boussougant Y, Vinceneux P. Assessment of urine analysis for the diagnosis of tuberculosis. BMJ (Clinical research ed). 1996;312:27-28. https://doi.org/10.1136/bmj.312.7022.27.
14. Moussa OM, Eraky I, El-Far MA, et al. Rapid diagnosis of genitourinary tuberculosis by polymerase chain reaction and non-radioactive DNA hybridization. J Urol. 2000;164(2):584-588. https://doi.org/10.1016/S0022-5347(05)67427-7.
15. Chawla A, Chawla K, Reddy S, et al. Can tissue PCR augment the diagnostic accuracy in genitourinary tract tuberculosis? Urol Int. 2012;88(1):34-38. https://doi.org/10.1159/000327039.
16. Kohli M, Schiller I, Dendukuri N, et al. Xpert((R)) MTB/RIF assay for extrapulmonary tuberculosis and rifampicin resistance. Cochrane Database Syst Rev. 2018;8:Cd012768. https://doi.org/10.1002/14651858.CD012768.pub2.
17. Figueiredo AA, Lucon AM, Arvellos AN, et al. A better understanding of urogenital tuberculosis pathophysiology based on radiological findings. Eur J Radiol. 2010;76(2):246-257. https://doi.org/10.1016/j.ejrad.2009.05.049.
18. Treatment of Tuberculosis: Guidelines. 4th edition. Geneva: World Health Organization. 2010.
19. O’Flynn D. Surgical treatment of genito-urinary tuberculosis. A report on 762 cases. Br J Urol. 1970;42(6):667-671. https://doi.org/10.1111/j.1464-410X.1970.tb06789.x.
20. Butler MR, O’Flynn JD. Reactivation of genito-urinary tuberculosis: a retrospective review of 838 cases. Eur Urol. 1975;1:14-17. https://doi.org/10.1159/000455566.
© 2019 Society of Hospital Medicine