User login
Leveraging the Care Team to Optimize Disposition Planning
Is this patient a good candidate? In medicine, we subconsciously answer this question for every clinical decision we make. Occasionally, though, a clinical scenario is so complex that it cannot or should not be answered by a single individual. One example is the decision on whether a patient should receive an organ transplant. In this situation, a multidisciplinary committee weighs the complex ethical, clinical, and financial implications of the decision before coming to a verdict. Together, team members discuss the risks and benefits of each patient’s candidacy and, in a united fashion, decide the best course of care. For hospitalists, a far more common question occurs every day and is similarly fraught with multifaceted implications: Is my patient a good candidate for a skilled nursing facility (SNF)? We often rely on a single individual to make the final call, but should we instead be leveraging the expertise of other care team members to assist with this decision?
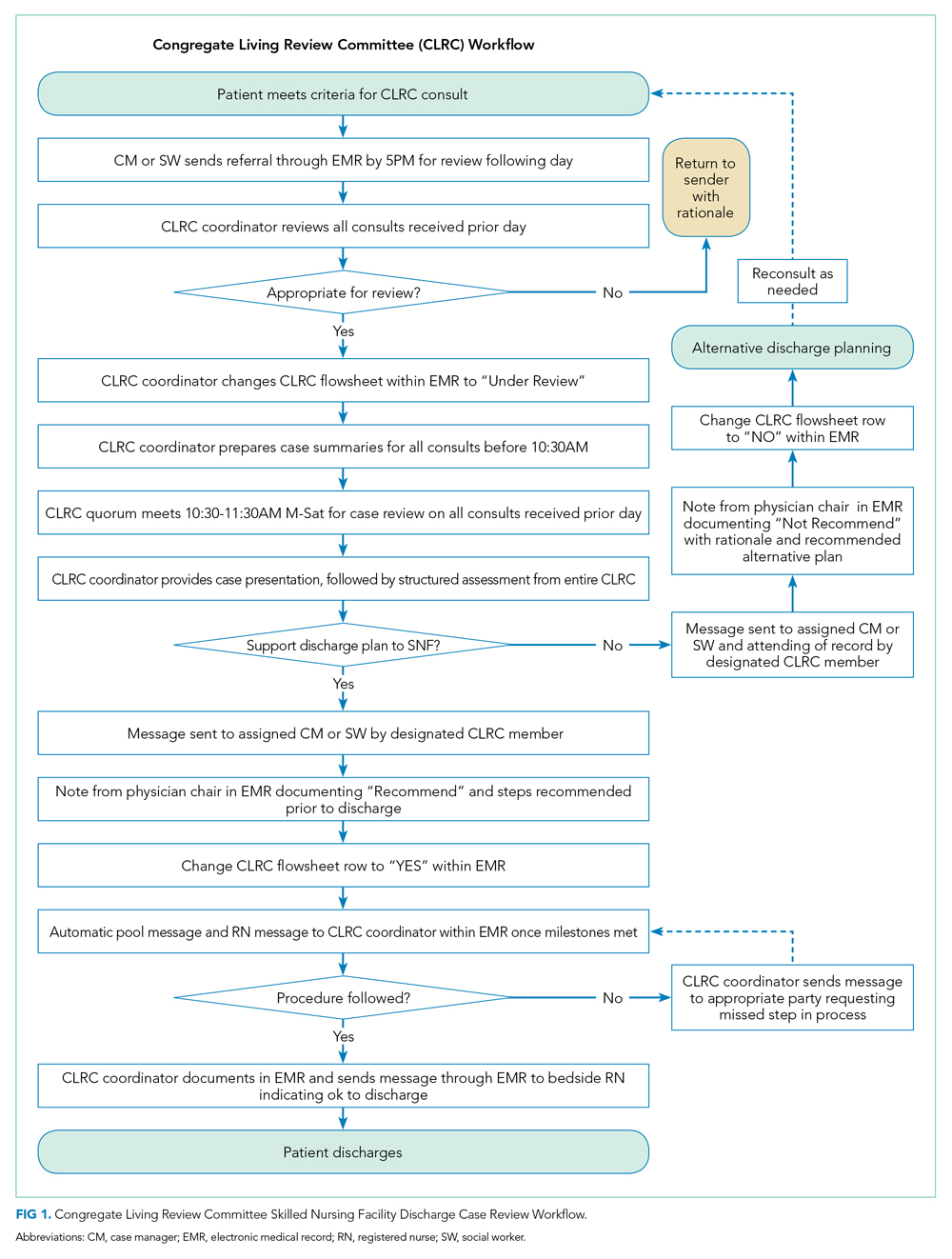
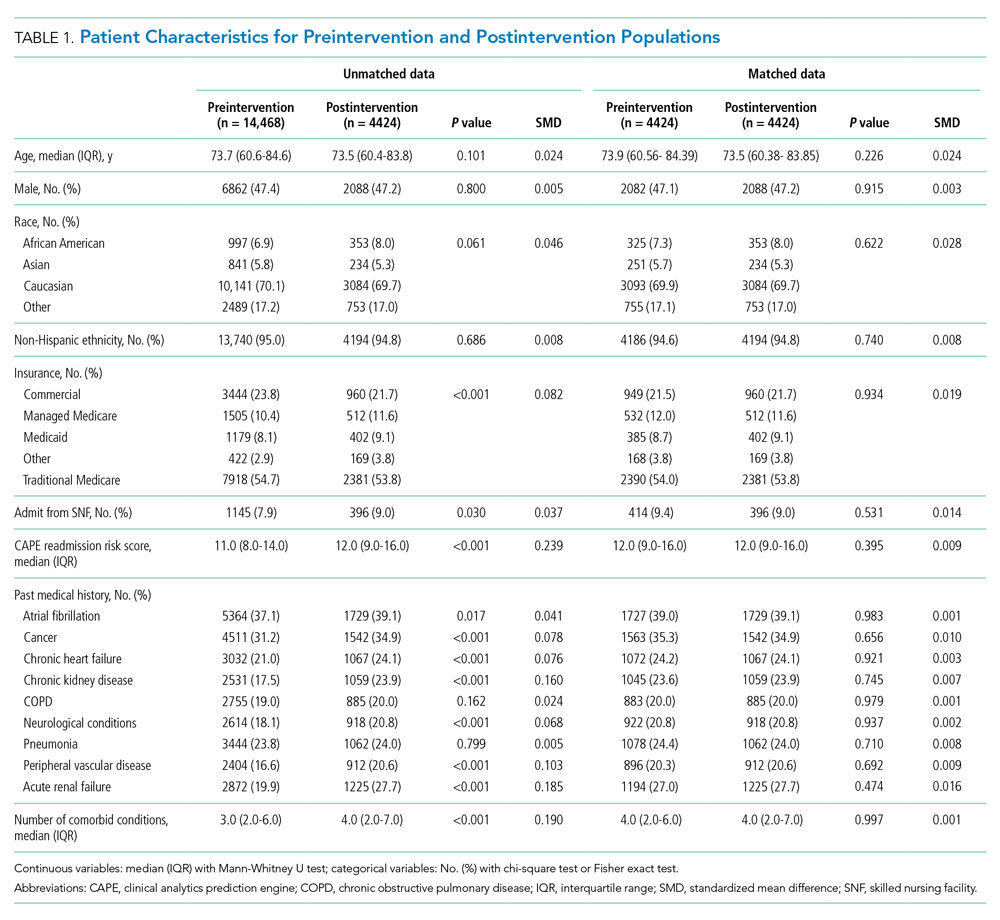
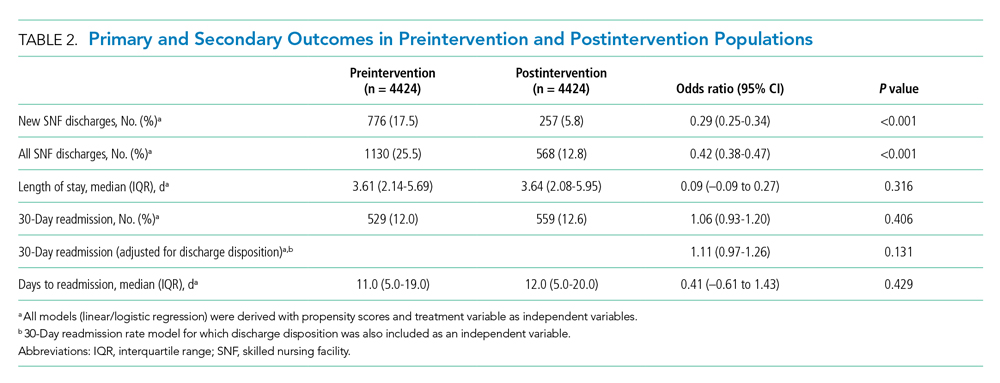
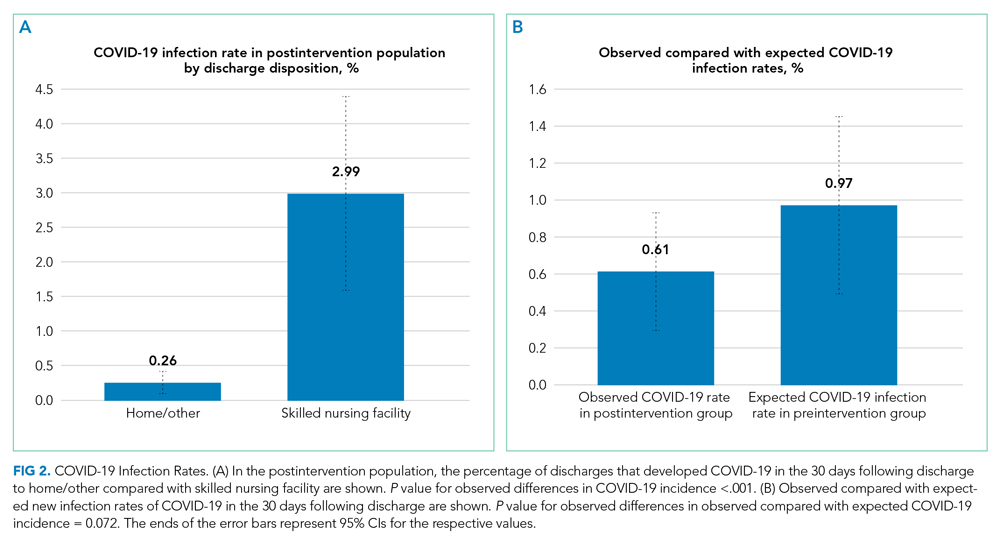
In this issue, Boyle et al1 describe the implementation of a multidisciplinary team consisting of physicians, case managers, social workers, physical and occupational therapists, and home-health representatives that reviewed all patients with an expected discharge to a SNF. Case managers or social workers began the process by referring eligible patients to the committee for review. If deemed appropriate, the committee discussed each case and reached a consensus recommendation as to whether a SNF was an appropriate discharge destination. The investigators used a matched, preintervention sample as a comparison group, with a primary outcome of total discharges to SNFs, and secondary outcomes consisting of readmissions, time to readmission, and median length of stay. The authors observed a 49.7% relative reduction in total SNF discharges (25.5% of preintervention patients discharged to a SNF vs 12.8% postintervention), as well as a 66.9% relative reduction in new SNF discharges. Despite the significant reduction in SNF utilization, no differences were noted in readmissions, time to readmission, or readmission length of stay.
While this study was performed during the COVID-19 pandemic, several characteristics make its findings applicable beyond this period. First, the structure and workflow of the team are extensively detailed and make the intervention easily generalizable to most hospitals. Second, while not specifically examined, the outcome of SNF reduction likely corresponds to an increase in the patient’s time at home—an important patient-centered target for most posthospitalization plans.2 Finally, the intervention used existing infrastructure and individuals, and did not require new resources to improve patient care, which increases the feasibility of implementation at other institutions.
These findings also reveal potential overutilization of SNFs in the discharge process. On average, a typical SNF stay costs the health system more than $11,000.3 A simple intervention could lead to substantial savings for individuals and the healthcare system. With a nearly 50% reduction in SNF use, understanding why patients who were eligible to go home were ultimately discharged to a SNF will be a crucial question to answer. Are there barriers to patient or family education? Is there a perceived safety difference between a SNF and home for nonskilled nursing needs? Additionally, care should be taken to ensure that decreases in SNF utilization do not disproportionately affect certain populations. Further work should assess the performance of similar models in a non-COVID era and among multiple institutions to verify potential scalability and generalizability.
Like organ transplant committees, Boyle et al’s multidisciplinary approach to reduce SNF discharges had to include thoughtful and intentional decisions. Perhaps it is time we use this same model to transplant patients back into their homes as safely and efficiently as possible.
1. Boyle CA, Ravichandran U, Hankamp V, et al. Safe transitions and congregate living in the age of COVID-19: a retrospective cohort study. J Hosp Med. 2021;16(9):524-530. https://doi.org/10.12788/jhm.3657
2. Barnett ML, Grabowski DC, Mehrotra A. Home-to-home time—measuring what matters to patients and payers. N Engl J Med. 2017;377(1):4-6. https://doi.org/10.1056/NEJMp1703423
3. Werner RM, Coe NB, Qi M, Konetzka RT. Patient outcomes after hospital discharge to home with home health care vs to a skilled nursing facility. JAMA Intern Med. 2019;179(5):617-623. https://doi.org/10.1001/jamainternmed.2018.7998
Is this patient a good candidate? In medicine, we subconsciously answer this question for every clinical decision we make. Occasionally, though, a clinical scenario is so complex that it cannot or should not be answered by a single individual. One example is the decision on whether a patient should receive an organ transplant. In this situation, a multidisciplinary committee weighs the complex ethical, clinical, and financial implications of the decision before coming to a verdict. Together, team members discuss the risks and benefits of each patient’s candidacy and, in a united fashion, decide the best course of care. For hospitalists, a far more common question occurs every day and is similarly fraught with multifaceted implications: Is my patient a good candidate for a skilled nursing facility (SNF)? We often rely on a single individual to make the final call, but should we instead be leveraging the expertise of other care team members to assist with this decision?
In this issue, Boyle et al1 describe the implementation of a multidisciplinary team consisting of physicians, case managers, social workers, physical and occupational therapists, and home-health representatives that reviewed all patients with an expected discharge to a SNF. Case managers or social workers began the process by referring eligible patients to the committee for review. If deemed appropriate, the committee discussed each case and reached a consensus recommendation as to whether a SNF was an appropriate discharge destination. The investigators used a matched, preintervention sample as a comparison group, with a primary outcome of total discharges to SNFs, and secondary outcomes consisting of readmissions, time to readmission, and median length of stay. The authors observed a 49.7% relative reduction in total SNF discharges (25.5% of preintervention patients discharged to a SNF vs 12.8% postintervention), as well as a 66.9% relative reduction in new SNF discharges. Despite the significant reduction in SNF utilization, no differences were noted in readmissions, time to readmission, or readmission length of stay.
While this study was performed during the COVID-19 pandemic, several characteristics make its findings applicable beyond this period. First, the structure and workflow of the team are extensively detailed and make the intervention easily generalizable to most hospitals. Second, while not specifically examined, the outcome of SNF reduction likely corresponds to an increase in the patient’s time at home—an important patient-centered target for most posthospitalization plans.2 Finally, the intervention used existing infrastructure and individuals, and did not require new resources to improve patient care, which increases the feasibility of implementation at other institutions.
These findings also reveal potential overutilization of SNFs in the discharge process. On average, a typical SNF stay costs the health system more than $11,000.3 A simple intervention could lead to substantial savings for individuals and the healthcare system. With a nearly 50% reduction in SNF use, understanding why patients who were eligible to go home were ultimately discharged to a SNF will be a crucial question to answer. Are there barriers to patient or family education? Is there a perceived safety difference between a SNF and home for nonskilled nursing needs? Additionally, care should be taken to ensure that decreases in SNF utilization do not disproportionately affect certain populations. Further work should assess the performance of similar models in a non-COVID era and among multiple institutions to verify potential scalability and generalizability.
Like organ transplant committees, Boyle et al’s multidisciplinary approach to reduce SNF discharges had to include thoughtful and intentional decisions. Perhaps it is time we use this same model to transplant patients back into their homes as safely and efficiently as possible.
Is this patient a good candidate? In medicine, we subconsciously answer this question for every clinical decision we make. Occasionally, though, a clinical scenario is so complex that it cannot or should not be answered by a single individual. One example is the decision on whether a patient should receive an organ transplant. In this situation, a multidisciplinary committee weighs the complex ethical, clinical, and financial implications of the decision before coming to a verdict. Together, team members discuss the risks and benefits of each patient’s candidacy and, in a united fashion, decide the best course of care. For hospitalists, a far more common question occurs every day and is similarly fraught with multifaceted implications: Is my patient a good candidate for a skilled nursing facility (SNF)? We often rely on a single individual to make the final call, but should we instead be leveraging the expertise of other care team members to assist with this decision?
In this issue, Boyle et al1 describe the implementation of a multidisciplinary team consisting of physicians, case managers, social workers, physical and occupational therapists, and home-health representatives that reviewed all patients with an expected discharge to a SNF. Case managers or social workers began the process by referring eligible patients to the committee for review. If deemed appropriate, the committee discussed each case and reached a consensus recommendation as to whether a SNF was an appropriate discharge destination. The investigators used a matched, preintervention sample as a comparison group, with a primary outcome of total discharges to SNFs, and secondary outcomes consisting of readmissions, time to readmission, and median length of stay. The authors observed a 49.7% relative reduction in total SNF discharges (25.5% of preintervention patients discharged to a SNF vs 12.8% postintervention), as well as a 66.9% relative reduction in new SNF discharges. Despite the significant reduction in SNF utilization, no differences were noted in readmissions, time to readmission, or readmission length of stay.
While this study was performed during the COVID-19 pandemic, several characteristics make its findings applicable beyond this period. First, the structure and workflow of the team are extensively detailed and make the intervention easily generalizable to most hospitals. Second, while not specifically examined, the outcome of SNF reduction likely corresponds to an increase in the patient’s time at home—an important patient-centered target for most posthospitalization plans.2 Finally, the intervention used existing infrastructure and individuals, and did not require new resources to improve patient care, which increases the feasibility of implementation at other institutions.
These findings also reveal potential overutilization of SNFs in the discharge process. On average, a typical SNF stay costs the health system more than $11,000.3 A simple intervention could lead to substantial savings for individuals and the healthcare system. With a nearly 50% reduction in SNF use, understanding why patients who were eligible to go home were ultimately discharged to a SNF will be a crucial question to answer. Are there barriers to patient or family education? Is there a perceived safety difference between a SNF and home for nonskilled nursing needs? Additionally, care should be taken to ensure that decreases in SNF utilization do not disproportionately affect certain populations. Further work should assess the performance of similar models in a non-COVID era and among multiple institutions to verify potential scalability and generalizability.
Like organ transplant committees, Boyle et al’s multidisciplinary approach to reduce SNF discharges had to include thoughtful and intentional decisions. Perhaps it is time we use this same model to transplant patients back into their homes as safely and efficiently as possible.
1. Boyle CA, Ravichandran U, Hankamp V, et al. Safe transitions and congregate living in the age of COVID-19: a retrospective cohort study. J Hosp Med. 2021;16(9):524-530. https://doi.org/10.12788/jhm.3657
2. Barnett ML, Grabowski DC, Mehrotra A. Home-to-home time—measuring what matters to patients and payers. N Engl J Med. 2017;377(1):4-6. https://doi.org/10.1056/NEJMp1703423
3. Werner RM, Coe NB, Qi M, Konetzka RT. Patient outcomes after hospital discharge to home with home health care vs to a skilled nursing facility. JAMA Intern Med. 2019;179(5):617-623. https://doi.org/10.1001/jamainternmed.2018.7998
1. Boyle CA, Ravichandran U, Hankamp V, et al. Safe transitions and congregate living in the age of COVID-19: a retrospective cohort study. J Hosp Med. 2021;16(9):524-530. https://doi.org/10.12788/jhm.3657
2. Barnett ML, Grabowski DC, Mehrotra A. Home-to-home time—measuring what matters to patients and payers. N Engl J Med. 2017;377(1):4-6. https://doi.org/10.1056/NEJMp1703423
3. Werner RM, Coe NB, Qi M, Konetzka RT. Patient outcomes after hospital discharge to home with home health care vs to a skilled nursing facility. JAMA Intern Med. 2019;179(5):617-623. https://doi.org/10.1001/jamainternmed.2018.7998
© 2021 Society of Hospital Medicine
A More Intentional Analysis of Race and Racism in Research
Earlier this year, the Journal of Hospital Medicine updated its author guidelines to include recommendations on addressing race and racism.1 These recommendations include explicitly naming racism (rather than race) as a determinant of health. Operationalizing these recommendations into manuscripts represents a fundamental shift in how we ask research questions, structure analyses, and interpret results.
In this issue, Maxwell et al2 illustrate how to disseminate research through this lens in their retrospective cohort study of children with type 1 diabetes hospitalized with diabetic ketoacidosis (DKA). Using 6 years of data from a major academic pediatric medical center, the authors examine the association between risk for DKA admission and three factors: neighborhood poverty level, race, and type of insurance (public or private). Secondary outcomes include DKA severity and length of stay. In their unadjusted model, poverty, race, and insurance were all associated with increased hospitalizations. However, following adjustment, the association between race and hospitalizations disappeared.In line with the journal’s new guidelines, the authors point out that the statistically significant associations of poverty and insurance type with clinical outcomes suggest that racism, rather than race, is a social factor at work in their population. The authors provide further context regarding structural racism in the United States and the history of redlining, which has helped shape a society in which Black individuals are more likely to live in areas of concentrated poverty and be publicly insured.
Two other findings related to the impact of racism are notable. First, in both their univariate and multivariate models, the authors found significant A1c differences between Black and White children—higher than those of previous reports.3 These findings suggest the existence of structural factors at work in the health of their patients. Second, Black patients had longer lengths of stay when compared to White patients with the same severity of DKA. Neither poverty level nor insurance status were significantly associated with length of stay. While the analysis was limited to detecting this difference, rather than identifying its causes, the authors suggest factors at both individual and structural levels that may be impacting outcomes. Specifically, care team bias may impact discharge decisions, and factors such as less flexible times to complete diabetes education, transportation barriers, and childcare challenges could also impact discharge timing.
This work provides a template for how to address the impact of racism on health with intentionality. Moreover, individuals’ lived environments should be considered through alternative economic measurements and neighborhood definitions. The proportion of people within a census tract living below the federal poverty line is just one measure of the complex dynamics that contribute to an individual’s socioeconomic status. An alternative measure is the area deprivation index, which incorporates 17 indicators at the more granular census block group level to describe an individual’s environment4 and could be useful in this area of research.
Perhaps most relevant is the use of public insurance as a marker of socioeconomic status. Medicaid, although not without its flaws, provides fairly comprehensive coverage. However, many Americans have incomes too high to qualify for public insurance but too low to afford adequate insurance coverage. Theoretically, these individuals qualify for subsidies through the Affordable Care Act, yet underinsurance remains a significant issue.5 Future analyses to further understand and describe clinical outcomes could include this population of underinsured children as a distinct at-risk group. Maxwell et al2 provide an excellent example of how we should address race and racism in disseminated literature. Although initially challenging, writing with intentionality regarding this fundamental determinant of health can provide rich and actionable information for practitioners and policy-makers.
1. Andrews AL, Unaka N, Shah SS. New author guidelines for addressing race and racism in the Journal of Hospital Medicine. J Hosp Med. 2021;16(4):197. https://doi.org/10.12788/jhm.3598
2. Maxwell AR, Jones NHY, Taylor S, et al. Socioeconomic and racial disparities in diabetic ketoacidosis admissions in youth with type 1 diabetes. J Hosp Med. 2021;16(9):517-523. https://doi.org/10.12788/jhm.3664
3. Bergenstal RM, Gal RL, Connor CG, et al. Racial differences in the relationship of glucose concentrations and hemoglobin A1c levels. Ann Intern Med. 2017;167(2):95-102. https://doi.org/10.7326/M16-2596
4. Kind AJH, Jencks S, Brock J, et al. Neighborhood socioeconomic disadvantage and 30 day rehospitalization: a retrospective cohort study. Ann Intern Med. 2014(11);161:765-774. https://doi.org/10.7326/M13-2946
5. Strane D, Rosenquist R, Rubin D. Leveraging health care reform to address underinsurance in working families. Health Affairs. June 15, 2021. Accessed August 23, 2021. www.healthaffairs.org/do/10.1377/hblog20210611.153918/full/
Earlier this year, the Journal of Hospital Medicine updated its author guidelines to include recommendations on addressing race and racism.1 These recommendations include explicitly naming racism (rather than race) as a determinant of health. Operationalizing these recommendations into manuscripts represents a fundamental shift in how we ask research questions, structure analyses, and interpret results.
In this issue, Maxwell et al2 illustrate how to disseminate research through this lens in their retrospective cohort study of children with type 1 diabetes hospitalized with diabetic ketoacidosis (DKA). Using 6 years of data from a major academic pediatric medical center, the authors examine the association between risk for DKA admission and three factors: neighborhood poverty level, race, and type of insurance (public or private). Secondary outcomes include DKA severity and length of stay. In their unadjusted model, poverty, race, and insurance were all associated with increased hospitalizations. However, following adjustment, the association between race and hospitalizations disappeared.In line with the journal’s new guidelines, the authors point out that the statistically significant associations of poverty and insurance type with clinical outcomes suggest that racism, rather than race, is a social factor at work in their population. The authors provide further context regarding structural racism in the United States and the history of redlining, which has helped shape a society in which Black individuals are more likely to live in areas of concentrated poverty and be publicly insured.
Two other findings related to the impact of racism are notable. First, in both their univariate and multivariate models, the authors found significant A1c differences between Black and White children—higher than those of previous reports.3 These findings suggest the existence of structural factors at work in the health of their patients. Second, Black patients had longer lengths of stay when compared to White patients with the same severity of DKA. Neither poverty level nor insurance status were significantly associated with length of stay. While the analysis was limited to detecting this difference, rather than identifying its causes, the authors suggest factors at both individual and structural levels that may be impacting outcomes. Specifically, care team bias may impact discharge decisions, and factors such as less flexible times to complete diabetes education, transportation barriers, and childcare challenges could also impact discharge timing.
This work provides a template for how to address the impact of racism on health with intentionality. Moreover, individuals’ lived environments should be considered through alternative economic measurements and neighborhood definitions. The proportion of people within a census tract living below the federal poverty line is just one measure of the complex dynamics that contribute to an individual’s socioeconomic status. An alternative measure is the area deprivation index, which incorporates 17 indicators at the more granular census block group level to describe an individual’s environment4 and could be useful in this area of research.
Perhaps most relevant is the use of public insurance as a marker of socioeconomic status. Medicaid, although not without its flaws, provides fairly comprehensive coverage. However, many Americans have incomes too high to qualify for public insurance but too low to afford adequate insurance coverage. Theoretically, these individuals qualify for subsidies through the Affordable Care Act, yet underinsurance remains a significant issue.5 Future analyses to further understand and describe clinical outcomes could include this population of underinsured children as a distinct at-risk group. Maxwell et al2 provide an excellent example of how we should address race and racism in disseminated literature. Although initially challenging, writing with intentionality regarding this fundamental determinant of health can provide rich and actionable information for practitioners and policy-makers.
Earlier this year, the Journal of Hospital Medicine updated its author guidelines to include recommendations on addressing race and racism.1 These recommendations include explicitly naming racism (rather than race) as a determinant of health. Operationalizing these recommendations into manuscripts represents a fundamental shift in how we ask research questions, structure analyses, and interpret results.
In this issue, Maxwell et al2 illustrate how to disseminate research through this lens in their retrospective cohort study of children with type 1 diabetes hospitalized with diabetic ketoacidosis (DKA). Using 6 years of data from a major academic pediatric medical center, the authors examine the association between risk for DKA admission and three factors: neighborhood poverty level, race, and type of insurance (public or private). Secondary outcomes include DKA severity and length of stay. In their unadjusted model, poverty, race, and insurance were all associated with increased hospitalizations. However, following adjustment, the association between race and hospitalizations disappeared.In line with the journal’s new guidelines, the authors point out that the statistically significant associations of poverty and insurance type with clinical outcomes suggest that racism, rather than race, is a social factor at work in their population. The authors provide further context regarding structural racism in the United States and the history of redlining, which has helped shape a society in which Black individuals are more likely to live in areas of concentrated poverty and be publicly insured.
Two other findings related to the impact of racism are notable. First, in both their univariate and multivariate models, the authors found significant A1c differences between Black and White children—higher than those of previous reports.3 These findings suggest the existence of structural factors at work in the health of their patients. Second, Black patients had longer lengths of stay when compared to White patients with the same severity of DKA. Neither poverty level nor insurance status were significantly associated with length of stay. While the analysis was limited to detecting this difference, rather than identifying its causes, the authors suggest factors at both individual and structural levels that may be impacting outcomes. Specifically, care team bias may impact discharge decisions, and factors such as less flexible times to complete diabetes education, transportation barriers, and childcare challenges could also impact discharge timing.
This work provides a template for how to address the impact of racism on health with intentionality. Moreover, individuals’ lived environments should be considered through alternative economic measurements and neighborhood definitions. The proportion of people within a census tract living below the federal poverty line is just one measure of the complex dynamics that contribute to an individual’s socioeconomic status. An alternative measure is the area deprivation index, which incorporates 17 indicators at the more granular census block group level to describe an individual’s environment4 and could be useful in this area of research.
Perhaps most relevant is the use of public insurance as a marker of socioeconomic status. Medicaid, although not without its flaws, provides fairly comprehensive coverage. However, many Americans have incomes too high to qualify for public insurance but too low to afford adequate insurance coverage. Theoretically, these individuals qualify for subsidies through the Affordable Care Act, yet underinsurance remains a significant issue.5 Future analyses to further understand and describe clinical outcomes could include this population of underinsured children as a distinct at-risk group. Maxwell et al2 provide an excellent example of how we should address race and racism in disseminated literature. Although initially challenging, writing with intentionality regarding this fundamental determinant of health can provide rich and actionable information for practitioners and policy-makers.
1. Andrews AL, Unaka N, Shah SS. New author guidelines for addressing race and racism in the Journal of Hospital Medicine. J Hosp Med. 2021;16(4):197. https://doi.org/10.12788/jhm.3598
2. Maxwell AR, Jones NHY, Taylor S, et al. Socioeconomic and racial disparities in diabetic ketoacidosis admissions in youth with type 1 diabetes. J Hosp Med. 2021;16(9):517-523. https://doi.org/10.12788/jhm.3664
3. Bergenstal RM, Gal RL, Connor CG, et al. Racial differences in the relationship of glucose concentrations and hemoglobin A1c levels. Ann Intern Med. 2017;167(2):95-102. https://doi.org/10.7326/M16-2596
4. Kind AJH, Jencks S, Brock J, et al. Neighborhood socioeconomic disadvantage and 30 day rehospitalization: a retrospective cohort study. Ann Intern Med. 2014(11);161:765-774. https://doi.org/10.7326/M13-2946
5. Strane D, Rosenquist R, Rubin D. Leveraging health care reform to address underinsurance in working families. Health Affairs. June 15, 2021. Accessed August 23, 2021. www.healthaffairs.org/do/10.1377/hblog20210611.153918/full/
1. Andrews AL, Unaka N, Shah SS. New author guidelines for addressing race and racism in the Journal of Hospital Medicine. J Hosp Med. 2021;16(4):197. https://doi.org/10.12788/jhm.3598
2. Maxwell AR, Jones NHY, Taylor S, et al. Socioeconomic and racial disparities in diabetic ketoacidosis admissions in youth with type 1 diabetes. J Hosp Med. 2021;16(9):517-523. https://doi.org/10.12788/jhm.3664
3. Bergenstal RM, Gal RL, Connor CG, et al. Racial differences in the relationship of glucose concentrations and hemoglobin A1c levels. Ann Intern Med. 2017;167(2):95-102. https://doi.org/10.7326/M16-2596
4. Kind AJH, Jencks S, Brock J, et al. Neighborhood socioeconomic disadvantage and 30 day rehospitalization: a retrospective cohort study. Ann Intern Med. 2014(11);161:765-774. https://doi.org/10.7326/M13-2946
5. Strane D, Rosenquist R, Rubin D. Leveraging health care reform to address underinsurance in working families. Health Affairs. June 15, 2021. Accessed August 23, 2021. www.healthaffairs.org/do/10.1377/hblog20210611.153918/full/
© 2021 Society of Hospital Medicine
Comprehensive and Equitable Care for Vulnerable Veterans With Integrated Palliative, Psychology, and Oncology Care
Veterans living with cancer need comprehensive assessment that includes supportive psychosocial care. The National Comprehensive Cancer Network (NCCN) and American College of Surgeons Commission on Cancer require accredited cancer centers to evaluate psychosocial distress and provide appropriate triage and treatment for all patients.1-3 Implementing psychosocial distress screening can be difficult because of procedural barriers and time constraints, clinic and supportive care resources, and lack of knowledge about how to access supportive services.
Distress screening protocols must be designed to address the specific needs of each population. To improve screening for cancer-related distress, deliver effective supportive services, and gain agreement on distress screening standards of care, the Coleman Foundation supported development of the Coleman Supportive Oncology Collaborative (CSOC), a project of 135 interdisciplinary health care professionals from 25 Chicago-area cancer care institutions.4
The Jesse Brown US Department of Veterans Affairs (VA) Medical Center (JBVAMC) was chosen to assess cancer-related concerns among veterans using the CSOC screening tool and to improve access to supportive oncology. JBVAMC provides care to approximately 49,000 veterans in Chicago, Illinois, and northwestern Indiana. The JBVAMC patient population includes a large number of veterans with dual diagnoses (co-occurring substance use and mental health disorders) and veterans experiencing homelessness.
Delivering integrated screening and oncologic care that is culture and age appropriate is particularly important for veterans given their unique risk factors. The veteran population is considered vulnerable in terms of health status, psychological functioning, and social context. Veterans who use the VA health system as a principal source of care have poorer health, greater comorbid medical conditions, and an increased risk of mortality and suicide compared with the general population.5,6 Poorer health status in veterans also may relate to old age, low income, poor education, psychological health, and minority race.7-9
Past studies point to unique risk factors for cancer and poor cancer adjustment among veterans, which may complicate cancer treatment and end-of-life/survivorship care. Veteran-specific risk factors include military-related exposures, particularly Agent Orange and morbidity/mortality secondary to comorbid medical and psychiatric conditions (eg, chronic obstructive pulmonary disease, diabetes mellitus, and posttraumatic stress disorder [PTSD]).10-12 Moreover, the geriatric veteran population continues to grow,with increasing rates of cancer that require unique considerations for effective cancer care.13,14 Despite this, there are minimal data to inform best practices and supportive care approaches for veterans with cancer. Lack of guidelines specific to veterans and other populations with increased psychosocial challenges may impede successful cancer care, making distress screening procedures particularly important. This is especially the case for the JBVAMC, which serves primarily African American urban-dwelling veterans who experience high rates of cancer disparities, including increased rates of mortality and increased levels of psychosocial distress.15,16
The goals of this program were to (1) examine levels of psychological, physical, financial, and treatment-related distress in a large sample of urban-dwelling veterans; (2) create a streamlined, sustainable process to screen a large number of veterans receiving cancer care in the outpatient setting and connect them with available supportive services; and (3) educate oncology physicians, nurses, and other staff about cancer-related distress and concerns using in-service trainings and interpersonal interactions to improve patient care. Our program was based on a Primary Care Mental Health Integration (PCMHI) model that embeds health psychologists in general medical clinics to better reach veterans dealing with mental health issues. We tailored for palliative care involvement.
Studies of this model have shown that mental health integration improves access to mental health services and mental health treatment outcomes and has higher patient and provider satisfaction.17 We were also influenced by the construct of the patient aligned care team (PACT) social worker who, in this veteran-centered approach, often functions as a care coordinator. Social work responsibilities include assessment of patients’ stressors including adjusting to the medical conditions, identifying untreated or undertreated mental health or substance abuse issues, economic instability, legal problems, and inadequate housing and transportation, which can often be exacerbated during cancer treatment.18
We screened for distress-related needs that included mental health concerns, physical needs including uncontrolled symptoms or adverse effects of cancer treatment, physical function complaints (eg, pain and fatigue), nutrition concerns, treatment or care related concerns, family and caregiver needs, along with financial challenges (housing and food) and insurance-related support. The goal of this article is to describe the development and implementation of this VA-specific distress screening program and reflect on the lessons learned for the application of streamlined distress screening and triage in similar settings throughout the VA health system and other similar settings.
Methods
This institutional review board at JBVAMC reviewed and exempted this quality improvement program using the SQUIRE framework.19 It was led by a group of palliative care clinicians, psychologists, and administrators who have worked with the oncology service for many years, primarily in the care of hospitalized patients. Common palliative care services include providing care for patients with serious illness diagnosis through the illness trajectory.
Setting
At the start of this program, we assessed the current clinic workflow to determine how to best screen and assist veterans experiencing distress. We met with team members individually to identify the best method of clinic integration, including attending medical oncologists, medical oncology fellows, psychology interns, oncology nursing staff, the oncology nurse coordinator, and clinic clerks.
The JBVAMC provides cancer care through 4 half-day medical hematology-oncology clinics that serve about 50 patients per half-day clinic. The clinics are staffed by hematology-oncology fellows supervised by hematology-oncology attending physicians, who are affiliated with 2 academic medical centers. These clinics are staffed by 3 registered nurses (RNs) and a licensed practical nurse (LPN) and are adjacent to a chemotherapy infusion clinic with unique nursing staff. The JBVAMC also provides a variety of supportive care services, including extensive mental health and substance use treatment, physical and occupational therapy, acupuncture, nutrition, social work, and housing services. Following our assessment, it was evident that there were a low number of referrals from oncology clinics to supportive care services, mostly due to lack of knowledge of resources and unclear referral procedures.
Based on clinical volume, we determined that our screening program could best be implemented through a stepped approach beginning in one clinic and expanding thereafter. We began by having a palliative care physician and health psychology intern embedded in 1 weekly half-day clinic and a health psychology intern embedded in a second weekly half-day clinic. Our program included 2 health psychology interns (for each academic year of the program) who were supervised by a JBVA health psychologist.
About 15 months after successful integration within the first 2 half-day clinics, we expanded the screening program to staff an additional half-day medical oncology clinic with a palliative care APRN. This allowed us to expand the screening tool distribution and collection to 3 of 4 of the weekly half-day oncology clinics as well as to meet individually with veterans experiencing high levels of distress. Veterans were flagged as having high distress levels by either the results of their completed screening tool or by referral from a medical oncology physician. We initially established screening in clinics that were sufficiently staffed to ensure that screens were appropriately distributed and reviewed. Patients seen in nonparticipating clinics were referred to outpatient social work, mental health and/or outpatient palliative care according to oncology fellows’ clinical assessments of the patient. All oncology fellows received education about distress screening and methods for referring to supportive care. Our clinic screening program extended from February 2017 through January 2020.
Screening
Program staff screened patients with new cancer diagnoses, then identified patients for follow-up screens. This tracking allowed staff to identify patients with oncology appointments that day and cross-reference patients needing a follow-up screen.
Following feedback from the clinic nurses, we determined that nurses would provide the distress tool to patients in paper form after they completed their assessment of vitals and waited to be seen by their medical oncologist. The patient would then deliver their completed form to the nurse who would combine it with the patient’s clinic notes for the oncologist to review.
Veterans referred for supportive care services were contacted by the relevant clinical administrator by phone to schedule an intake; for social work referrals, patients were either seen in a walk-in office located in a colocated building or contacted by a social worker by phone.
Our screening tool was the Coleman Foundation Supportive Oncology Collaborative Screening Tool, compiled from validated instruments. Patients completed this screening tool, which includes the PHQ-4, NCCN problem list concerns, adapted Mini Nutrition Assessment and PROMIS Pain and Fatigue measure (eAppendix B available at doi:10.12788/fp.0158).20-22
We also worked with the VA Computerized Patient Record System (CPRS) to create an electronic template for the screening tool. Completed screening tools were manually entered by the physician, psychologists, or APRN into the CPRS chart.
We analyzed the different supportive care services available at the JBVAMC and noticed that many supportive services were available, yet these services were often separated. Therefore, we created a consult flowsheet to assist oncologists in placing referrals. These supportive care services include mental health services, a cancer support group, home health care, social services, nutrition, physical medicine and rehabilitation, and other specialty services.
Patient Education
The psychology and nursing staff created a patient information bulletin board where patients could access information about supportive services available at JBVAMC. This board required frequent replenishment of handouts because patients consulted the board regularly. Handouts and folders about common clinical issues also were placed in the clinic treatment rooms. We partnered with 2 local cancer support centers, Gilda’s Club and the Cancer Support Center, to make referrals for family members and/or caregivers who would benefit from additional support.
We provided in-service trainings for oncology fellows, including trainings on PTSD and substance abuse and their relationship to cancer care at the VA. These topics were chosen based on the feedback program staff received about perceived knowledge gaps from the oncology fellows. This program allowed for multiple informal conversations between that program staff and oncology fellows about overall patient care. We held trainings with the cancer coordinator and clinical nursing staff on strategies to identify and follow-up on cancer-related distress, and with oncology fellows to review the importance of distress screening and to instruct fellows on instructions for the consult flowsheet.
Funding
This program was funded by the Chicago-based Coleman Foundation as part of the CSOC. Funding was used to support a portion of time for administrative and clinical work of program staff, as well as data collection and analysis.
Results
We established 3 half-day integrated clinics where patients were screened and referred for services based on supportive oncology needs. In addition to our primary activities to screen and refer veterans, we held multiple educational sessions for colleagues, developed a workflow template, and integrated patient education materials into the clinics.
Screening
Veterans completed 1010 distress screens in 3 of 4 half-day oncology clinics over the 2.5-year project period. Veterans were screened at initial diagnosis and every 3 months, or during changes in their clinical care or disease status. As a result, 579 patients completed screening, with some patients doing several follow-up screens during their care. Integration of palliative care providers and health psychologists was instrumental in facilitating screening in these busy general medical oncology clinics. Most veterans were receptive to completing surveys with few refusing to fill out the survey.23 Medical oncology fellows often used the completed screener to inform their review of systems (by reviewing the Coleman screener Physical and Other Concerns section) and connect with the supportive care staff present in clinic for patient’s identifying severe needs (ie, mental health distress or complex psychosocial needs). Veterans’ rates of distress needs and successfuloutcomes of integration with mental health and social work services have been reported elsewhere.23
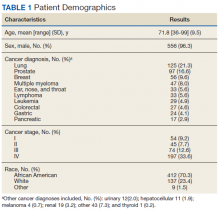
The mean (SD) age for veterans in this cohort was 72 (9.5) years. Participants were primarily African American veterans (70%), with mostly advanced disease (Table 1). Participants endorsed elevated distress needs compared with other patient populations screened in Chicago through the CSOC for depressed mood, pain, housing, transportation, and physical, nutrition, and treatment concerns.23 Elevated presence of needs was especially prominent for food, housing and insurance/medical needs; physical concerns; nutrition, and treatment- or care-related concerns. Veterans in this cohort reported extensive financial and housing concerns: 10.4% reported food and housing concerns, 18.6% reported transportation concerns, and 9.0% reported issues paying for medical care or medications (Table 2).20 Anecdotally, many experienced job loss or strain with their cancer diagnosis or were living at the poverty level before their diagnosis.
Social work referrals were often triggered due to transportation barriers to appointments/medication access, and food and/or housing insecurity. Social workers assisted with referrals for housing, transportation, financial reimbursement, on-site or community-based food banks, home health support, familial support, and hospice services. Social work consults increased 166% from 2016 (the year before the program start date) to the end of 2019.
Based on this increased volume of referrals for social work in our oncology clinics, an oncology-specific social worker was hired at the completion of our program to be based in all 4 half-day oncology clinics in response to results of our quality improvement intervention. The social worker currently sees all patients with a new cancer diagnosis and supports oncology fellows to identify veterans needing a palliative care referral or referrals to other supportive services.
Throughout program implementation, traditional areas of palliative care focus were particularly important as veterans reported significant concerns with understanding their illness (67.4%), wanting to understand their prognosis (71.3%), and having questions about their treatment options (55.1%).20 The palliative care providers spent time educating patients about their disease, coordinating goals of care conversations, promoting patients’ engagement in decision making, and making a large number of referrals to hospice and home health to support veterans at home.
Discussion
This project created a successful program to screen veterans for psychosocial distress and triage them to appropriate services. During the project, patients in VA-outpatient oncology clinics reported significant cancer-related distress due to baseline psychosocial needs, changes in emotional and physical functioning, logistical and financial challenges of receiving cancer care, and lack of instrumental support.23
Staff education supported successful buy-in, development and implementation of supportive oncology programs. We used a combination of in-service trainings, online trainings, and handouts to provide evidence for distress screening.24 Highlighting the evidence-base that demonstrates how cancer-related distress screening improves cancer and quality of life outcomes helped to address physician reluctance to accept the additional requirements needed to address veterans’ psychosocial needs and care concerns. To increase buy-in and collaboration among team members and foster heightened understanding between providers and patients, we recommend creating accessible education for all staff levels.
One specific area of education we focused on was primary palliative care, which includes the core competencies of communication and symptom management recommended for generalists and specialists of all disciplines.25 Program staff supported oncology fellows in developing their primary palliative care skills by being available to discuss basic symptom management and communication issues. VA cancer care programs could benefit from ongoing palliative care education of oncology staff to facilitate primary palliative care as well as earlier integration of secondary palliative care when needed.26 Secondary palliative care or care provided directly by the palliative care team assists with complex symptom management or communication issues. For these needs, oncology fellows were encouraged to refer to either the palliative care staff available in one of the half-day clinics or to the outpatient palliative care clinic. As a unique strength, the VA allows veterans to receive concurrent cancer-directed therapy and hospice care, which enables earlier referrals to hospice care and higher quality end-of-life care and emphasizes the need for primary palliative care in oncology.27,28
Integrating supportive oncology team members, such as licensed clinical social worker and psychology interns, was successful. This was modeled on the VA PACT, which focuses on prevention, health promotion, coordination and chronic disease management.29 Social determinants of health have a major impact on health outcomes especially in veteran-specific and African American populations, making screening for distress critical.30-32 The VA Office of Health Equity actively addresses health inequities by supporting initiation of screening programs for social determinants of health, including education, employment, exposure to abuse and violence, food insecurity, housing instability, legal needs, social isolation, transportation needs, and utility needs. This is especially needed for African-American individuals who are not only more likely to experience cancer, but also more likely to be negatively impacted by the consequences of cancer diagnosis/treatment, such as complications related to one’s job security, access to care, adverse effects, and other highly distressing needs.33,34
Our program found that veterans with cancer often had concerns associated with food and housing insecurity, transportation and paying for medication or medical care, and screening allowed health care providers to detect and address these social determinants of health through referrals to VA and community-specific programs. Social workers integrated
Our ability to roll out distress screening was scaffolded by technological integration into existing VA systems (eg, screening results in CPRS and electronic referrals). Screening procedures could have been even more efficient with improved technology (Table 3). For example, technological limitations made it challenging to easily identify patients due for screening, requiring a cumbersome process of tracking, collecting and entering patients’ paper forms. Health care providers seeking to develop a distress screening program should consider investing in technology that allows for identification of patients requiring screening at a predetermined interval, completion of screening via tablet or personal device, integration of screening responses into the electronic health record, and automatic generation of notifications to the treating physician and appropriate support services.
We also established partnerships with community cancer support groups to offer both referral pathways and in-house programming. Veterans’ cancer care programs could benefit from identifying and securing community partnerships to capitalize on readily available low-cost or no-cost options for supportive oncology in the community. Further, as was the case in our program, cancer support centers may be willing to collaborate with VA hospitals to provide services on site (eg, support groups, art therapy). This would extend the reach of these supportive services while allowing VA employees to address the extensive psychosocial needs of individual veterans.
Conclusions
Veterans with cancer benefited from enhanced screening and psychosocial service availability, similar to a PCMHI model. Robust screening programs helped advocate for veterans dealing with the effects of poverty through identification of need and referral to existing VA programs and services quickly and efficiently. Providing comprehensive care within ambulatory cancer clinics can address cancer-related distress and any potential barriers to care in real time. VA hospitals typically offer an array of supportive services to address veterans’ psychosocial needs, yet these services tend to be siloed. Integrated referrals can help to resolve such access barriers. Since many veterans with burdensome cancers are not able to see their VA primary care physician regularly, offering comprehensive care within medical oncology ensures complete and integrated care that includes psychosocial screening.
We believe that this program is an example of a mechanism for oncologists and palliative care clinicians to integrate their care in a way that identifies needs and triages services for vulnerable veterans. As colleagues have written, “it is fundamental to our commitment to veterans that we ensure comparable, high quality care regardless of a veteran’s gender, race, or where they live.”34 Health care providers may underestimate the extensive change a cancer diagnosis can have on a patient’s quality of life. Cancer diagnosis and treatment have a large impact on all individuals, but this impact may be greater for individuals in poverty due to inability to work from home, inflexible work hours, and limited support structures. By creating screening programs with psychosocial integration in oncology clinics such as we have described, we hope to improve access to more equitable care for vulnerable veterans.
1. National Comprehensive Cancer Network. NCCN guidelines distress management. Version 2.2021. Updated January 5, 2021. Accessed July 8, 2021. http://www.nccn.org/professionals/physician_gls/pdf/distress.pdf
2. American College of Surgeons, Commission on Cancer. Cancer program standards 2012: ensuring patient-centered care. Version 1.2.1. Published 2021. Accessed July 8, 2021. https://www.facs.org/~/media/files/quality%20programs/cancer/coc/programstandards2012.ashx
3. Jacobsen PB, Ransom S. Implementation of NCCN distress management guidelines by member institutions. J Natl Compr Canc Netw. 2007;5(1):99-103. doi:10.6004/jnccn.2007.0010
4. The Coleman Supportive Oncology Collaborative. Training tools. Accessed July 14, 2021. https://www.supportiveoncologycollaborative.org/training-tools
5. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257. doi:10.1001/archinte.160.21.3252
6. Bullman T, Schneiderman A, Gradus JL. Relative importance of posttraumatic stress disorder and depression in predicting risk of suicide among a cohort of Vietnam veterans. Suicide Life Threat Behav. 2019;49(3):838-845. doi:10.1111/sltb.12482
7. Kazis LE, Miller DR, Clark J, et al. Health-related quality of life in patients served by the Department of Veterans Affairs: results from the Veterans Health Study. Arch Intern Med. 1998;158(6):626-632. doi:10.1001/archinte.158.6.626
8. O’Toole BI, Marshall RP, Grayson DA, et al. The Australian Vietnam Veterans Health Study: III. Psychological health of Australian Vietnam veterans and its relationship to combat. Int J Epidemiol. 1996;25(2):331-340. doi:10.1093/ije/25.2.331
9. Vincent C, Chamberlain K, Long N. Mental and physical health status in a community sample of New Zealand Vietnam War veterans. Aust J Public Health. 1994;18(1):58-62. doi:10.1111/j.1753-6405.1994.tb00196.x
10. US Department of Veterans Affairs. Veterans’ diseases associated with Agent Orange. Updated June 16, 2021. Accessed July 8, 2021. http://www.publichealth.va.gov/exposures/agentorange/diseases.asp#veterans
11. Hwa KJ, Dua MM, Wren SM, Visser BC. Missing the obvious: psychosocial obstacles in Veterans with hepatocellular carcinoma. HBP (Oxford). 2015;17(12):1124-1129. doi:10.1111/hpb.12508
12. Saha S, Freeman M, Toure J, Tippens KM, Weeks C, Ibrahim S. Racial and ethnic disparities in the VA health care system: a systematic review. J Gen Intern Med. 2008;23(5):654-671. doi:10.1007/s11606-008-0521-4
13. Amaral EFL, Pollard MS, Mendelsohn J, Cefalu M. Current and future demographics of the veteran population, 2014-2024. Popul Rev. 2018;57(1):28-60. doi:10.1353/prv.2018.0002
14. Mohile SG, Dale W, Somerfield MR, et al. Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO guideline for geriatric oncology. J Clin Oncol. 2018;36(22):2326-2347. doi:10.1200/JCO.2018.78.8687
15. Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61(4):212-236. doi:10.3322/caac.20121
16. Cimino T, Said K, Safier L, Harris H, Kinderman A. Psychosocial distress among oncology patients in the safety net. Psychooncology. 2020;29(11):1927-1935. doi:10.1002/pon.5525
17. Molander R, Hodgkins K, Johnson C, White A, Frazier E, Krahn D. Interprofessional education in patient aligned care team primary care-mental health integration. Fed Pract. 2017;34(6):40-48.
18. Parikh DA, Ragavan M, Dutta R, et al. Financial toxicity of cancer care: an analysis of financial burden in three distinct health care systems [published online ahead of print, 2021 Apr 7]. JCO Oncol Pract. 2021;OP2000890. doi:10.1200/OP.20.00890
19. Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986-992. doi:10.1136/bmjqs-2015-004411
20. Weldon CB, Gerhart JI, Penedo FJ, et al. Correlates of distress for cancer patients: results from multi-institution use of holistic patient-reported screening tool. J Clin Oncol. 2019;37(15)(suppl):11587-11587. doi:10.1200/JCO.2019.37.15_suppl.11587
21. Kroenke K, Spitzer RL, Williams JB, Löwe B. The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: a systematic review. Gen Hosp Psychiatry. 2010;32(4):345-359. doi:10.1016/j.genhosppsych.2010.03.006
22. Kaiser MJ, Bauer JM, Ramsch C, et al. Validation of the Mini Nutritional Assessment short-form (MNA-SF): a practical tool for identification of nutritional status. J Nutr Health Aging. 2009;13(9):782-788. doi:10.1007/s12603-009-0214-7
23. Azizoddin DR, Lakin JR, Hauser J, et al. Meeting the guidelines: implementing a distress screening intervention for veterans with cancer. Psychooncology. 2020;29(12):2067-2074. doi:10.1002/pon.5565
24. Carlson LE, Waller A, Mitchell AJ. Screening for distress and unmet needs in patients with cancer: review and recommendations. J Clin Oncol. 2012;30(11):1160-1177. doi:10.1200/JCO.2011.39.5509
25. Quill TE, Abernethy AP. Generalist plus specialist palliative care—creating a more sustainable model. N Engl J Med. 2013;368(13):1173-1175. doi:10.1056/NEJMp1215620
26. Weissman DE, Meier DE. Identifying patients in need of a palliative care assessment in the hospital setting: a consensus report from the Center to Advance Palliative Care. J Palliat Med. 2011;14(1):17-23. doi:10.1089/jpm.2010.0347
27. Kumar P, Wright AA, Hatfield LA, Temel JS, Keating NL. Family perspectives on hospice care experiences of patients with cancer. J Clin Oncol. 2017;35(4):432-439. doi:10.1200/JCO.2016.68.9257
28. Mor V, Joyce NR, Coté DL, et al. The rise of concurrent care for veterans with advanced cancer at the end of life. Cancer. 2016;122(5):782-790. doi:10.1002/cncr.29827
29. US Department of Veterans Affairs. Patient care services: Patient aligned care team (PACT). Updated November 5, 2020. Accessed July 8, 2021. https://www.patientcare.va.gov/primarycare/PACT.asp
30. US Department of Veterans Affairs, Veterans Health Administration. VHA health equity action plan. Published September 27, 2019. Accessed July 8, 2021. https://www.va.gov/HEALTHEQUITY/docs/Health_Equity_Action_Plan_Final_022020.pdf
31. Alcaraz KI, Wiedt TL, Daniels EC, Yabroff KR, Guerra CE, Wender RC. Understanding and addressing social determinants to advance cancer health equity in the United States: a blueprint for practice, research, and policy. CA Cancer J Clin. 2020;70(1):31-46. doi:10.3322/caac.21586
32. Atkins D, Kilbourne A, Lipson L. Health equity research in the Veterans Health Administration: we’ve come far but aren’t there yet. Am J Public Health. 2014;104(suppl 4):S525-526. doi:10.2105/AJPH.2014.302216
33. American Cancer Society. Cancer Facts & Figures for African Americans 2019-2021. Atlanta: American Cancer Society; 2019.
34. Hastert TA, Kirchhoff AC, Banegas MP, et al. Work changes and individual, cancer-related, and work-related predictors of decreased work participation among African American cancer survivors. Cancer Med. 2020;9(23):9168-9177. doi:10.1002/cam4.3512
35. Bekelman DB, Nowels CT, Allen LA, Shakar S, Kutner JS, Matlock DD. Outpatient palliative care for chronic heart failure: a case series. J Palliat Med. 2011;14(7):815-821. doi:10.1089/jpm.2010.050
Veterans living with cancer need comprehensive assessment that includes supportive psychosocial care. The National Comprehensive Cancer Network (NCCN) and American College of Surgeons Commission on Cancer require accredited cancer centers to evaluate psychosocial distress and provide appropriate triage and treatment for all patients.1-3 Implementing psychosocial distress screening can be difficult because of procedural barriers and time constraints, clinic and supportive care resources, and lack of knowledge about how to access supportive services.
Distress screening protocols must be designed to address the specific needs of each population. To improve screening for cancer-related distress, deliver effective supportive services, and gain agreement on distress screening standards of care, the Coleman Foundation supported development of the Coleman Supportive Oncology Collaborative (CSOC), a project of 135 interdisciplinary health care professionals from 25 Chicago-area cancer care institutions.4
The Jesse Brown US Department of Veterans Affairs (VA) Medical Center (JBVAMC) was chosen to assess cancer-related concerns among veterans using the CSOC screening tool and to improve access to supportive oncology. JBVAMC provides care to approximately 49,000 veterans in Chicago, Illinois, and northwestern Indiana. The JBVAMC patient population includes a large number of veterans with dual diagnoses (co-occurring substance use and mental health disorders) and veterans experiencing homelessness.
Delivering integrated screening and oncologic care that is culture and age appropriate is particularly important for veterans given their unique risk factors. The veteran population is considered vulnerable in terms of health status, psychological functioning, and social context. Veterans who use the VA health system as a principal source of care have poorer health, greater comorbid medical conditions, and an increased risk of mortality and suicide compared with the general population.5,6 Poorer health status in veterans also may relate to old age, low income, poor education, psychological health, and minority race.7-9
Past studies point to unique risk factors for cancer and poor cancer adjustment among veterans, which may complicate cancer treatment and end-of-life/survivorship care. Veteran-specific risk factors include military-related exposures, particularly Agent Orange and morbidity/mortality secondary to comorbid medical and psychiatric conditions (eg, chronic obstructive pulmonary disease, diabetes mellitus, and posttraumatic stress disorder [PTSD]).10-12 Moreover, the geriatric veteran population continues to grow,with increasing rates of cancer that require unique considerations for effective cancer care.13,14 Despite this, there are minimal data to inform best practices and supportive care approaches for veterans with cancer. Lack of guidelines specific to veterans and other populations with increased psychosocial challenges may impede successful cancer care, making distress screening procedures particularly important. This is especially the case for the JBVAMC, which serves primarily African American urban-dwelling veterans who experience high rates of cancer disparities, including increased rates of mortality and increased levels of psychosocial distress.15,16
The goals of this program were to (1) examine levels of psychological, physical, financial, and treatment-related distress in a large sample of urban-dwelling veterans; (2) create a streamlined, sustainable process to screen a large number of veterans receiving cancer care in the outpatient setting and connect them with available supportive services; and (3) educate oncology physicians, nurses, and other staff about cancer-related distress and concerns using in-service trainings and interpersonal interactions to improve patient care. Our program was based on a Primary Care Mental Health Integration (PCMHI) model that embeds health psychologists in general medical clinics to better reach veterans dealing with mental health issues. We tailored for palliative care involvement.
Studies of this model have shown that mental health integration improves access to mental health services and mental health treatment outcomes and has higher patient and provider satisfaction.17 We were also influenced by the construct of the patient aligned care team (PACT) social worker who, in this veteran-centered approach, often functions as a care coordinator. Social work responsibilities include assessment of patients’ stressors including adjusting to the medical conditions, identifying untreated or undertreated mental health or substance abuse issues, economic instability, legal problems, and inadequate housing and transportation, which can often be exacerbated during cancer treatment.18
We screened for distress-related needs that included mental health concerns, physical needs including uncontrolled symptoms or adverse effects of cancer treatment, physical function complaints (eg, pain and fatigue), nutrition concerns, treatment or care related concerns, family and caregiver needs, along with financial challenges (housing and food) and insurance-related support. The goal of this article is to describe the development and implementation of this VA-specific distress screening program and reflect on the lessons learned for the application of streamlined distress screening and triage in similar settings throughout the VA health system and other similar settings.
Methods
This institutional review board at JBVAMC reviewed and exempted this quality improvement program using the SQUIRE framework.19 It was led by a group of palliative care clinicians, psychologists, and administrators who have worked with the oncology service for many years, primarily in the care of hospitalized patients. Common palliative care services include providing care for patients with serious illness diagnosis through the illness trajectory.
Setting
At the start of this program, we assessed the current clinic workflow to determine how to best screen and assist veterans experiencing distress. We met with team members individually to identify the best method of clinic integration, including attending medical oncologists, medical oncology fellows, psychology interns, oncology nursing staff, the oncology nurse coordinator, and clinic clerks.
The JBVAMC provides cancer care through 4 half-day medical hematology-oncology clinics that serve about 50 patients per half-day clinic. The clinics are staffed by hematology-oncology fellows supervised by hematology-oncology attending physicians, who are affiliated with 2 academic medical centers. These clinics are staffed by 3 registered nurses (RNs) and a licensed practical nurse (LPN) and are adjacent to a chemotherapy infusion clinic with unique nursing staff. The JBVAMC also provides a variety of supportive care services, including extensive mental health and substance use treatment, physical and occupational therapy, acupuncture, nutrition, social work, and housing services. Following our assessment, it was evident that there were a low number of referrals from oncology clinics to supportive care services, mostly due to lack of knowledge of resources and unclear referral procedures.
Based on clinical volume, we determined that our screening program could best be implemented through a stepped approach beginning in one clinic and expanding thereafter. We began by having a palliative care physician and health psychology intern embedded in 1 weekly half-day clinic and a health psychology intern embedded in a second weekly half-day clinic. Our program included 2 health psychology interns (for each academic year of the program) who were supervised by a JBVA health psychologist.
About 15 months after successful integration within the first 2 half-day clinics, we expanded the screening program to staff an additional half-day medical oncology clinic with a palliative care APRN. This allowed us to expand the screening tool distribution and collection to 3 of 4 of the weekly half-day oncology clinics as well as to meet individually with veterans experiencing high levels of distress. Veterans were flagged as having high distress levels by either the results of their completed screening tool or by referral from a medical oncology physician. We initially established screening in clinics that were sufficiently staffed to ensure that screens were appropriately distributed and reviewed. Patients seen in nonparticipating clinics were referred to outpatient social work, mental health and/or outpatient palliative care according to oncology fellows’ clinical assessments of the patient. All oncology fellows received education about distress screening and methods for referring to supportive care. Our clinic screening program extended from February 2017 through January 2020.
Screening
Program staff screened patients with new cancer diagnoses, then identified patients for follow-up screens. This tracking allowed staff to identify patients with oncology appointments that day and cross-reference patients needing a follow-up screen.
Following feedback from the clinic nurses, we determined that nurses would provide the distress tool to patients in paper form after they completed their assessment of vitals and waited to be seen by their medical oncologist. The patient would then deliver their completed form to the nurse who would combine it with the patient’s clinic notes for the oncologist to review.
Veterans referred for supportive care services were contacted by the relevant clinical administrator by phone to schedule an intake; for social work referrals, patients were either seen in a walk-in office located in a colocated building or contacted by a social worker by phone.
Our screening tool was the Coleman Foundation Supportive Oncology Collaborative Screening Tool, compiled from validated instruments. Patients completed this screening tool, which includes the PHQ-4, NCCN problem list concerns, adapted Mini Nutrition Assessment and PROMIS Pain and Fatigue measure (eAppendix B available at doi:10.12788/fp.0158).20-22
We also worked with the VA Computerized Patient Record System (CPRS) to create an electronic template for the screening tool. Completed screening tools were manually entered by the physician, psychologists, or APRN into the CPRS chart.
We analyzed the different supportive care services available at the JBVAMC and noticed that many supportive services were available, yet these services were often separated. Therefore, we created a consult flowsheet to assist oncologists in placing referrals. These supportive care services include mental health services, a cancer support group, home health care, social services, nutrition, physical medicine and rehabilitation, and other specialty services.
Patient Education
The psychology and nursing staff created a patient information bulletin board where patients could access information about supportive services available at JBVAMC. This board required frequent replenishment of handouts because patients consulted the board regularly. Handouts and folders about common clinical issues also were placed in the clinic treatment rooms. We partnered with 2 local cancer support centers, Gilda’s Club and the Cancer Support Center, to make referrals for family members and/or caregivers who would benefit from additional support.
We provided in-service trainings for oncology fellows, including trainings on PTSD and substance abuse and their relationship to cancer care at the VA. These topics were chosen based on the feedback program staff received about perceived knowledge gaps from the oncology fellows. This program allowed for multiple informal conversations between that program staff and oncology fellows about overall patient care. We held trainings with the cancer coordinator and clinical nursing staff on strategies to identify and follow-up on cancer-related distress, and with oncology fellows to review the importance of distress screening and to instruct fellows on instructions for the consult flowsheet.
Funding
This program was funded by the Chicago-based Coleman Foundation as part of the CSOC. Funding was used to support a portion of time for administrative and clinical work of program staff, as well as data collection and analysis.
Results
We established 3 half-day integrated clinics where patients were screened and referred for services based on supportive oncology needs. In addition to our primary activities to screen and refer veterans, we held multiple educational sessions for colleagues, developed a workflow template, and integrated patient education materials into the clinics.
Screening
Veterans completed 1010 distress screens in 3 of 4 half-day oncology clinics over the 2.5-year project period. Veterans were screened at initial diagnosis and every 3 months, or during changes in their clinical care or disease status. As a result, 579 patients completed screening, with some patients doing several follow-up screens during their care. Integration of palliative care providers and health psychologists was instrumental in facilitating screening in these busy general medical oncology clinics. Most veterans were receptive to completing surveys with few refusing to fill out the survey.23 Medical oncology fellows often used the completed screener to inform their review of systems (by reviewing the Coleman screener Physical and Other Concerns section) and connect with the supportive care staff present in clinic for patient’s identifying severe needs (ie, mental health distress or complex psychosocial needs). Veterans’ rates of distress needs and successfuloutcomes of integration with mental health and social work services have been reported elsewhere.23
The mean (SD) age for veterans in this cohort was 72 (9.5) years. Participants were primarily African American veterans (70%), with mostly advanced disease (Table 1). Participants endorsed elevated distress needs compared with other patient populations screened in Chicago through the CSOC for depressed mood, pain, housing, transportation, and physical, nutrition, and treatment concerns.23 Elevated presence of needs was especially prominent for food, housing and insurance/medical needs; physical concerns; nutrition, and treatment- or care-related concerns. Veterans in this cohort reported extensive financial and housing concerns: 10.4% reported food and housing concerns, 18.6% reported transportation concerns, and 9.0% reported issues paying for medical care or medications (Table 2).20 Anecdotally, many experienced job loss or strain with their cancer diagnosis or were living at the poverty level before their diagnosis.
Social work referrals were often triggered due to transportation barriers to appointments/medication access, and food and/or housing insecurity. Social workers assisted with referrals for housing, transportation, financial reimbursement, on-site or community-based food banks, home health support, familial support, and hospice services. Social work consults increased 166% from 2016 (the year before the program start date) to the end of 2019.
Based on this increased volume of referrals for social work in our oncology clinics, an oncology-specific social worker was hired at the completion of our program to be based in all 4 half-day oncology clinics in response to results of our quality improvement intervention. The social worker currently sees all patients with a new cancer diagnosis and supports oncology fellows to identify veterans needing a palliative care referral or referrals to other supportive services.
Throughout program implementation, traditional areas of palliative care focus were particularly important as veterans reported significant concerns with understanding their illness (67.4%), wanting to understand their prognosis (71.3%), and having questions about their treatment options (55.1%).20 The palliative care providers spent time educating patients about their disease, coordinating goals of care conversations, promoting patients’ engagement in decision making, and making a large number of referrals to hospice and home health to support veterans at home.
Discussion
This project created a successful program to screen veterans for psychosocial distress and triage them to appropriate services. During the project, patients in VA-outpatient oncology clinics reported significant cancer-related distress due to baseline psychosocial needs, changes in emotional and physical functioning, logistical and financial challenges of receiving cancer care, and lack of instrumental support.23
Staff education supported successful buy-in, development and implementation of supportive oncology programs. We used a combination of in-service trainings, online trainings, and handouts to provide evidence for distress screening.24 Highlighting the evidence-base that demonstrates how cancer-related distress screening improves cancer and quality of life outcomes helped to address physician reluctance to accept the additional requirements needed to address veterans’ psychosocial needs and care concerns. To increase buy-in and collaboration among team members and foster heightened understanding between providers and patients, we recommend creating accessible education for all staff levels.
One specific area of education we focused on was primary palliative care, which includes the core competencies of communication and symptom management recommended for generalists and specialists of all disciplines.25 Program staff supported oncology fellows in developing their primary palliative care skills by being available to discuss basic symptom management and communication issues. VA cancer care programs could benefit from ongoing palliative care education of oncology staff to facilitate primary palliative care as well as earlier integration of secondary palliative care when needed.26 Secondary palliative care or care provided directly by the palliative care team assists with complex symptom management or communication issues. For these needs, oncology fellows were encouraged to refer to either the palliative care staff available in one of the half-day clinics or to the outpatient palliative care clinic. As a unique strength, the VA allows veterans to receive concurrent cancer-directed therapy and hospice care, which enables earlier referrals to hospice care and higher quality end-of-life care and emphasizes the need for primary palliative care in oncology.27,28
Integrating supportive oncology team members, such as licensed clinical social worker and psychology interns, was successful. This was modeled on the VA PACT, which focuses on prevention, health promotion, coordination and chronic disease management.29 Social determinants of health have a major impact on health outcomes especially in veteran-specific and African American populations, making screening for distress critical.30-32 The VA Office of Health Equity actively addresses health inequities by supporting initiation of screening programs for social determinants of health, including education, employment, exposure to abuse and violence, food insecurity, housing instability, legal needs, social isolation, transportation needs, and utility needs. This is especially needed for African-American individuals who are not only more likely to experience cancer, but also more likely to be negatively impacted by the consequences of cancer diagnosis/treatment, such as complications related to one’s job security, access to care, adverse effects, and other highly distressing needs.33,34
Our program found that veterans with cancer often had concerns associated with food and housing insecurity, transportation and paying for medication or medical care, and screening allowed health care providers to detect and address these social determinants of health through referrals to VA and community-specific programs. Social workers integrated
Our ability to roll out distress screening was scaffolded by technological integration into existing VA systems (eg, screening results in CPRS and electronic referrals). Screening procedures could have been even more efficient with improved technology (Table 3). For example, technological limitations made it challenging to easily identify patients due for screening, requiring a cumbersome process of tracking, collecting and entering patients’ paper forms. Health care providers seeking to develop a distress screening program should consider investing in technology that allows for identification of patients requiring screening at a predetermined interval, completion of screening via tablet or personal device, integration of screening responses into the electronic health record, and automatic generation of notifications to the treating physician and appropriate support services.
We also established partnerships with community cancer support groups to offer both referral pathways and in-house programming. Veterans’ cancer care programs could benefit from identifying and securing community partnerships to capitalize on readily available low-cost or no-cost options for supportive oncology in the community. Further, as was the case in our program, cancer support centers may be willing to collaborate with VA hospitals to provide services on site (eg, support groups, art therapy). This would extend the reach of these supportive services while allowing VA employees to address the extensive psychosocial needs of individual veterans.
Conclusions
Veterans with cancer benefited from enhanced screening and psychosocial service availability, similar to a PCMHI model. Robust screening programs helped advocate for veterans dealing with the effects of poverty through identification of need and referral to existing VA programs and services quickly and efficiently. Providing comprehensive care within ambulatory cancer clinics can address cancer-related distress and any potential barriers to care in real time. VA hospitals typically offer an array of supportive services to address veterans’ psychosocial needs, yet these services tend to be siloed. Integrated referrals can help to resolve such access barriers. Since many veterans with burdensome cancers are not able to see their VA primary care physician regularly, offering comprehensive care within medical oncology ensures complete and integrated care that includes psychosocial screening.
We believe that this program is an example of a mechanism for oncologists and palliative care clinicians to integrate their care in a way that identifies needs and triages services for vulnerable veterans. As colleagues have written, “it is fundamental to our commitment to veterans that we ensure comparable, high quality care regardless of a veteran’s gender, race, or where they live.”34 Health care providers may underestimate the extensive change a cancer diagnosis can have on a patient’s quality of life. Cancer diagnosis and treatment have a large impact on all individuals, but this impact may be greater for individuals in poverty due to inability to work from home, inflexible work hours, and limited support structures. By creating screening programs with psychosocial integration in oncology clinics such as we have described, we hope to improve access to more equitable care for vulnerable veterans.
Veterans living with cancer need comprehensive assessment that includes supportive psychosocial care. The National Comprehensive Cancer Network (NCCN) and American College of Surgeons Commission on Cancer require accredited cancer centers to evaluate psychosocial distress and provide appropriate triage and treatment for all patients.1-3 Implementing psychosocial distress screening can be difficult because of procedural barriers and time constraints, clinic and supportive care resources, and lack of knowledge about how to access supportive services.
Distress screening protocols must be designed to address the specific needs of each population. To improve screening for cancer-related distress, deliver effective supportive services, and gain agreement on distress screening standards of care, the Coleman Foundation supported development of the Coleman Supportive Oncology Collaborative (CSOC), a project of 135 interdisciplinary health care professionals from 25 Chicago-area cancer care institutions.4
The Jesse Brown US Department of Veterans Affairs (VA) Medical Center (JBVAMC) was chosen to assess cancer-related concerns among veterans using the CSOC screening tool and to improve access to supportive oncology. JBVAMC provides care to approximately 49,000 veterans in Chicago, Illinois, and northwestern Indiana. The JBVAMC patient population includes a large number of veterans with dual diagnoses (co-occurring substance use and mental health disorders) and veterans experiencing homelessness.
Delivering integrated screening and oncologic care that is culture and age appropriate is particularly important for veterans given their unique risk factors. The veteran population is considered vulnerable in terms of health status, psychological functioning, and social context. Veterans who use the VA health system as a principal source of care have poorer health, greater comorbid medical conditions, and an increased risk of mortality and suicide compared with the general population.5,6 Poorer health status in veterans also may relate to old age, low income, poor education, psychological health, and minority race.7-9
Past studies point to unique risk factors for cancer and poor cancer adjustment among veterans, which may complicate cancer treatment and end-of-life/survivorship care. Veteran-specific risk factors include military-related exposures, particularly Agent Orange and morbidity/mortality secondary to comorbid medical and psychiatric conditions (eg, chronic obstructive pulmonary disease, diabetes mellitus, and posttraumatic stress disorder [PTSD]).10-12 Moreover, the geriatric veteran population continues to grow,with increasing rates of cancer that require unique considerations for effective cancer care.13,14 Despite this, there are minimal data to inform best practices and supportive care approaches for veterans with cancer. Lack of guidelines specific to veterans and other populations with increased psychosocial challenges may impede successful cancer care, making distress screening procedures particularly important. This is especially the case for the JBVAMC, which serves primarily African American urban-dwelling veterans who experience high rates of cancer disparities, including increased rates of mortality and increased levels of psychosocial distress.15,16
The goals of this program were to (1) examine levels of psychological, physical, financial, and treatment-related distress in a large sample of urban-dwelling veterans; (2) create a streamlined, sustainable process to screen a large number of veterans receiving cancer care in the outpatient setting and connect them with available supportive services; and (3) educate oncology physicians, nurses, and other staff about cancer-related distress and concerns using in-service trainings and interpersonal interactions to improve patient care. Our program was based on a Primary Care Mental Health Integration (PCMHI) model that embeds health psychologists in general medical clinics to better reach veterans dealing with mental health issues. We tailored for palliative care involvement.
Studies of this model have shown that mental health integration improves access to mental health services and mental health treatment outcomes and has higher patient and provider satisfaction.17 We were also influenced by the construct of the patient aligned care team (PACT) social worker who, in this veteran-centered approach, often functions as a care coordinator. Social work responsibilities include assessment of patients’ stressors including adjusting to the medical conditions, identifying untreated or undertreated mental health or substance abuse issues, economic instability, legal problems, and inadequate housing and transportation, which can often be exacerbated during cancer treatment.18
We screened for distress-related needs that included mental health concerns, physical needs including uncontrolled symptoms or adverse effects of cancer treatment, physical function complaints (eg, pain and fatigue), nutrition concerns, treatment or care related concerns, family and caregiver needs, along with financial challenges (housing and food) and insurance-related support. The goal of this article is to describe the development and implementation of this VA-specific distress screening program and reflect on the lessons learned for the application of streamlined distress screening and triage in similar settings throughout the VA health system and other similar settings.
Methods
This institutional review board at JBVAMC reviewed and exempted this quality improvement program using the SQUIRE framework.19 It was led by a group of palliative care clinicians, psychologists, and administrators who have worked with the oncology service for many years, primarily in the care of hospitalized patients. Common palliative care services include providing care for patients with serious illness diagnosis through the illness trajectory.
Setting
At the start of this program, we assessed the current clinic workflow to determine how to best screen and assist veterans experiencing distress. We met with team members individually to identify the best method of clinic integration, including attending medical oncologists, medical oncology fellows, psychology interns, oncology nursing staff, the oncology nurse coordinator, and clinic clerks.
The JBVAMC provides cancer care through 4 half-day medical hematology-oncology clinics that serve about 50 patients per half-day clinic. The clinics are staffed by hematology-oncology fellows supervised by hematology-oncology attending physicians, who are affiliated with 2 academic medical centers. These clinics are staffed by 3 registered nurses (RNs) and a licensed practical nurse (LPN) and are adjacent to a chemotherapy infusion clinic with unique nursing staff. The JBVAMC also provides a variety of supportive care services, including extensive mental health and substance use treatment, physical and occupational therapy, acupuncture, nutrition, social work, and housing services. Following our assessment, it was evident that there were a low number of referrals from oncology clinics to supportive care services, mostly due to lack of knowledge of resources and unclear referral procedures.
Based on clinical volume, we determined that our screening program could best be implemented through a stepped approach beginning in one clinic and expanding thereafter. We began by having a palliative care physician and health psychology intern embedded in 1 weekly half-day clinic and a health psychology intern embedded in a second weekly half-day clinic. Our program included 2 health psychology interns (for each academic year of the program) who were supervised by a JBVA health psychologist.
About 15 months after successful integration within the first 2 half-day clinics, we expanded the screening program to staff an additional half-day medical oncology clinic with a palliative care APRN. This allowed us to expand the screening tool distribution and collection to 3 of 4 of the weekly half-day oncology clinics as well as to meet individually with veterans experiencing high levels of distress. Veterans were flagged as having high distress levels by either the results of their completed screening tool or by referral from a medical oncology physician. We initially established screening in clinics that were sufficiently staffed to ensure that screens were appropriately distributed and reviewed. Patients seen in nonparticipating clinics were referred to outpatient social work, mental health and/or outpatient palliative care according to oncology fellows’ clinical assessments of the patient. All oncology fellows received education about distress screening and methods for referring to supportive care. Our clinic screening program extended from February 2017 through January 2020.
Screening
Program staff screened patients with new cancer diagnoses, then identified patients for follow-up screens. This tracking allowed staff to identify patients with oncology appointments that day and cross-reference patients needing a follow-up screen.
Following feedback from the clinic nurses, we determined that nurses would provide the distress tool to patients in paper form after they completed their assessment of vitals and waited to be seen by their medical oncologist. The patient would then deliver their completed form to the nurse who would combine it with the patient’s clinic notes for the oncologist to review.
Veterans referred for supportive care services were contacted by the relevant clinical administrator by phone to schedule an intake; for social work referrals, patients were either seen in a walk-in office located in a colocated building or contacted by a social worker by phone.
Our screening tool was the Coleman Foundation Supportive Oncology Collaborative Screening Tool, compiled from validated instruments. Patients completed this screening tool, which includes the PHQ-4, NCCN problem list concerns, adapted Mini Nutrition Assessment and PROMIS Pain and Fatigue measure (eAppendix B available at doi:10.12788/fp.0158).20-22
We also worked with the VA Computerized Patient Record System (CPRS) to create an electronic template for the screening tool. Completed screening tools were manually entered by the physician, psychologists, or APRN into the CPRS chart.
We analyzed the different supportive care services available at the JBVAMC and noticed that many supportive services were available, yet these services were often separated. Therefore, we created a consult flowsheet to assist oncologists in placing referrals. These supportive care services include mental health services, a cancer support group, home health care, social services, nutrition, physical medicine and rehabilitation, and other specialty services.
Patient Education
The psychology and nursing staff created a patient information bulletin board where patients could access information about supportive services available at JBVAMC. This board required frequent replenishment of handouts because patients consulted the board regularly. Handouts and folders about common clinical issues also were placed in the clinic treatment rooms. We partnered with 2 local cancer support centers, Gilda’s Club and the Cancer Support Center, to make referrals for family members and/or caregivers who would benefit from additional support.
We provided in-service trainings for oncology fellows, including trainings on PTSD and substance abuse and their relationship to cancer care at the VA. These topics were chosen based on the feedback program staff received about perceived knowledge gaps from the oncology fellows. This program allowed for multiple informal conversations between that program staff and oncology fellows about overall patient care. We held trainings with the cancer coordinator and clinical nursing staff on strategies to identify and follow-up on cancer-related distress, and with oncology fellows to review the importance of distress screening and to instruct fellows on instructions for the consult flowsheet.
Funding
This program was funded by the Chicago-based Coleman Foundation as part of the CSOC. Funding was used to support a portion of time for administrative and clinical work of program staff, as well as data collection and analysis.
Results
We established 3 half-day integrated clinics where patients were screened and referred for services based on supportive oncology needs. In addition to our primary activities to screen and refer veterans, we held multiple educational sessions for colleagues, developed a workflow template, and integrated patient education materials into the clinics.
Screening
Veterans completed 1010 distress screens in 3 of 4 half-day oncology clinics over the 2.5-year project period. Veterans were screened at initial diagnosis and every 3 months, or during changes in their clinical care or disease status. As a result, 579 patients completed screening, with some patients doing several follow-up screens during their care. Integration of palliative care providers and health psychologists was instrumental in facilitating screening in these busy general medical oncology clinics. Most veterans were receptive to completing surveys with few refusing to fill out the survey.23 Medical oncology fellows often used the completed screener to inform their review of systems (by reviewing the Coleman screener Physical and Other Concerns section) and connect with the supportive care staff present in clinic for patient’s identifying severe needs (ie, mental health distress or complex psychosocial needs). Veterans’ rates of distress needs and successfuloutcomes of integration with mental health and social work services have been reported elsewhere.23
The mean (SD) age for veterans in this cohort was 72 (9.5) years. Participants were primarily African American veterans (70%), with mostly advanced disease (Table 1). Participants endorsed elevated distress needs compared with other patient populations screened in Chicago through the CSOC for depressed mood, pain, housing, transportation, and physical, nutrition, and treatment concerns.23 Elevated presence of needs was especially prominent for food, housing and insurance/medical needs; physical concerns; nutrition, and treatment- or care-related concerns. Veterans in this cohort reported extensive financial and housing concerns: 10.4% reported food and housing concerns, 18.6% reported transportation concerns, and 9.0% reported issues paying for medical care or medications (Table 2).20 Anecdotally, many experienced job loss or strain with their cancer diagnosis or were living at the poverty level before their diagnosis.
Social work referrals were often triggered due to transportation barriers to appointments/medication access, and food and/or housing insecurity. Social workers assisted with referrals for housing, transportation, financial reimbursement, on-site or community-based food banks, home health support, familial support, and hospice services. Social work consults increased 166% from 2016 (the year before the program start date) to the end of 2019.
Based on this increased volume of referrals for social work in our oncology clinics, an oncology-specific social worker was hired at the completion of our program to be based in all 4 half-day oncology clinics in response to results of our quality improvement intervention. The social worker currently sees all patients with a new cancer diagnosis and supports oncology fellows to identify veterans needing a palliative care referral or referrals to other supportive services.
Throughout program implementation, traditional areas of palliative care focus were particularly important as veterans reported significant concerns with understanding their illness (67.4%), wanting to understand their prognosis (71.3%), and having questions about their treatment options (55.1%).20 The palliative care providers spent time educating patients about their disease, coordinating goals of care conversations, promoting patients’ engagement in decision making, and making a large number of referrals to hospice and home health to support veterans at home.
Discussion
This project created a successful program to screen veterans for psychosocial distress and triage them to appropriate services. During the project, patients in VA-outpatient oncology clinics reported significant cancer-related distress due to baseline psychosocial needs, changes in emotional and physical functioning, logistical and financial challenges of receiving cancer care, and lack of instrumental support.23
Staff education supported successful buy-in, development and implementation of supportive oncology programs. We used a combination of in-service trainings, online trainings, and handouts to provide evidence for distress screening.24 Highlighting the evidence-base that demonstrates how cancer-related distress screening improves cancer and quality of life outcomes helped to address physician reluctance to accept the additional requirements needed to address veterans’ psychosocial needs and care concerns. To increase buy-in and collaboration among team members and foster heightened understanding between providers and patients, we recommend creating accessible education for all staff levels.
One specific area of education we focused on was primary palliative care, which includes the core competencies of communication and symptom management recommended for generalists and specialists of all disciplines.25 Program staff supported oncology fellows in developing their primary palliative care skills by being available to discuss basic symptom management and communication issues. VA cancer care programs could benefit from ongoing palliative care education of oncology staff to facilitate primary palliative care as well as earlier integration of secondary palliative care when needed.26 Secondary palliative care or care provided directly by the palliative care team assists with complex symptom management or communication issues. For these needs, oncology fellows were encouraged to refer to either the palliative care staff available in one of the half-day clinics or to the outpatient palliative care clinic. As a unique strength, the VA allows veterans to receive concurrent cancer-directed therapy and hospice care, which enables earlier referrals to hospice care and higher quality end-of-life care and emphasizes the need for primary palliative care in oncology.27,28
Integrating supportive oncology team members, such as licensed clinical social worker and psychology interns, was successful. This was modeled on the VA PACT, which focuses on prevention, health promotion, coordination and chronic disease management.29 Social determinants of health have a major impact on health outcomes especially in veteran-specific and African American populations, making screening for distress critical.30-32 The VA Office of Health Equity actively addresses health inequities by supporting initiation of screening programs for social determinants of health, including education, employment, exposure to abuse and violence, food insecurity, housing instability, legal needs, social isolation, transportation needs, and utility needs. This is especially needed for African-American individuals who are not only more likely to experience cancer, but also more likely to be negatively impacted by the consequences of cancer diagnosis/treatment, such as complications related to one’s job security, access to care, adverse effects, and other highly distressing needs.33,34
Our program found that veterans with cancer often had concerns associated with food and housing insecurity, transportation and paying for medication or medical care, and screening allowed health care providers to detect and address these social determinants of health through referrals to VA and community-specific programs. Social workers integrated
Our ability to roll out distress screening was scaffolded by technological integration into existing VA systems (eg, screening results in CPRS and electronic referrals). Screening procedures could have been even more efficient with improved technology (Table 3). For example, technological limitations made it challenging to easily identify patients due for screening, requiring a cumbersome process of tracking, collecting and entering patients’ paper forms. Health care providers seeking to develop a distress screening program should consider investing in technology that allows for identification of patients requiring screening at a predetermined interval, completion of screening via tablet or personal device, integration of screening responses into the electronic health record, and automatic generation of notifications to the treating physician and appropriate support services.
We also established partnerships with community cancer support groups to offer both referral pathways and in-house programming. Veterans’ cancer care programs could benefit from identifying and securing community partnerships to capitalize on readily available low-cost or no-cost options for supportive oncology in the community. Further, as was the case in our program, cancer support centers may be willing to collaborate with VA hospitals to provide services on site (eg, support groups, art therapy). This would extend the reach of these supportive services while allowing VA employees to address the extensive psychosocial needs of individual veterans.
Conclusions
Veterans with cancer benefited from enhanced screening and psychosocial service availability, similar to a PCMHI model. Robust screening programs helped advocate for veterans dealing with the effects of poverty through identification of need and referral to existing VA programs and services quickly and efficiently. Providing comprehensive care within ambulatory cancer clinics can address cancer-related distress and any potential barriers to care in real time. VA hospitals typically offer an array of supportive services to address veterans’ psychosocial needs, yet these services tend to be siloed. Integrated referrals can help to resolve such access barriers. Since many veterans with burdensome cancers are not able to see their VA primary care physician regularly, offering comprehensive care within medical oncology ensures complete and integrated care that includes psychosocial screening.
We believe that this program is an example of a mechanism for oncologists and palliative care clinicians to integrate their care in a way that identifies needs and triages services for vulnerable veterans. As colleagues have written, “it is fundamental to our commitment to veterans that we ensure comparable, high quality care regardless of a veteran’s gender, race, or where they live.”34 Health care providers may underestimate the extensive change a cancer diagnosis can have on a patient’s quality of life. Cancer diagnosis and treatment have a large impact on all individuals, but this impact may be greater for individuals in poverty due to inability to work from home, inflexible work hours, and limited support structures. By creating screening programs with psychosocial integration in oncology clinics such as we have described, we hope to improve access to more equitable care for vulnerable veterans.
1. National Comprehensive Cancer Network. NCCN guidelines distress management. Version 2.2021. Updated January 5, 2021. Accessed July 8, 2021. http://www.nccn.org/professionals/physician_gls/pdf/distress.pdf
2. American College of Surgeons, Commission on Cancer. Cancer program standards 2012: ensuring patient-centered care. Version 1.2.1. Published 2021. Accessed July 8, 2021. https://www.facs.org/~/media/files/quality%20programs/cancer/coc/programstandards2012.ashx
3. Jacobsen PB, Ransom S. Implementation of NCCN distress management guidelines by member institutions. J Natl Compr Canc Netw. 2007;5(1):99-103. doi:10.6004/jnccn.2007.0010
4. The Coleman Supportive Oncology Collaborative. Training tools. Accessed July 14, 2021. https://www.supportiveoncologycollaborative.org/training-tools
5. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257. doi:10.1001/archinte.160.21.3252
6. Bullman T, Schneiderman A, Gradus JL. Relative importance of posttraumatic stress disorder and depression in predicting risk of suicide among a cohort of Vietnam veterans. Suicide Life Threat Behav. 2019;49(3):838-845. doi:10.1111/sltb.12482
7. Kazis LE, Miller DR, Clark J, et al. Health-related quality of life in patients served by the Department of Veterans Affairs: results from the Veterans Health Study. Arch Intern Med. 1998;158(6):626-632. doi:10.1001/archinte.158.6.626
8. O’Toole BI, Marshall RP, Grayson DA, et al. The Australian Vietnam Veterans Health Study: III. Psychological health of Australian Vietnam veterans and its relationship to combat. Int J Epidemiol. 1996;25(2):331-340. doi:10.1093/ije/25.2.331
9. Vincent C, Chamberlain K, Long N. Mental and physical health status in a community sample of New Zealand Vietnam War veterans. Aust J Public Health. 1994;18(1):58-62. doi:10.1111/j.1753-6405.1994.tb00196.x
10. US Department of Veterans Affairs. Veterans’ diseases associated with Agent Orange. Updated June 16, 2021. Accessed July 8, 2021. http://www.publichealth.va.gov/exposures/agentorange/diseases.asp#veterans
11. Hwa KJ, Dua MM, Wren SM, Visser BC. Missing the obvious: psychosocial obstacles in Veterans with hepatocellular carcinoma. HBP (Oxford). 2015;17(12):1124-1129. doi:10.1111/hpb.12508
12. Saha S, Freeman M, Toure J, Tippens KM, Weeks C, Ibrahim S. Racial and ethnic disparities in the VA health care system: a systematic review. J Gen Intern Med. 2008;23(5):654-671. doi:10.1007/s11606-008-0521-4
13. Amaral EFL, Pollard MS, Mendelsohn J, Cefalu M. Current and future demographics of the veteran population, 2014-2024. Popul Rev. 2018;57(1):28-60. doi:10.1353/prv.2018.0002
14. Mohile SG, Dale W, Somerfield MR, et al. Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO guideline for geriatric oncology. J Clin Oncol. 2018;36(22):2326-2347. doi:10.1200/JCO.2018.78.8687
15. Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61(4):212-236. doi:10.3322/caac.20121
16. Cimino T, Said K, Safier L, Harris H, Kinderman A. Psychosocial distress among oncology patients in the safety net. Psychooncology. 2020;29(11):1927-1935. doi:10.1002/pon.5525
17. Molander R, Hodgkins K, Johnson C, White A, Frazier E, Krahn D. Interprofessional education in patient aligned care team primary care-mental health integration. Fed Pract. 2017;34(6):40-48.
18. Parikh DA, Ragavan M, Dutta R, et al. Financial toxicity of cancer care: an analysis of financial burden in three distinct health care systems [published online ahead of print, 2021 Apr 7]. JCO Oncol Pract. 2021;OP2000890. doi:10.1200/OP.20.00890
19. Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986-992. doi:10.1136/bmjqs-2015-004411
20. Weldon CB, Gerhart JI, Penedo FJ, et al. Correlates of distress for cancer patients: results from multi-institution use of holistic patient-reported screening tool. J Clin Oncol. 2019;37(15)(suppl):11587-11587. doi:10.1200/JCO.2019.37.15_suppl.11587
21. Kroenke K, Spitzer RL, Williams JB, Löwe B. The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: a systematic review. Gen Hosp Psychiatry. 2010;32(4):345-359. doi:10.1016/j.genhosppsych.2010.03.006
22. Kaiser MJ, Bauer JM, Ramsch C, et al. Validation of the Mini Nutritional Assessment short-form (MNA-SF): a practical tool for identification of nutritional status. J Nutr Health Aging. 2009;13(9):782-788. doi:10.1007/s12603-009-0214-7
23. Azizoddin DR, Lakin JR, Hauser J, et al. Meeting the guidelines: implementing a distress screening intervention for veterans with cancer. Psychooncology. 2020;29(12):2067-2074. doi:10.1002/pon.5565
24. Carlson LE, Waller A, Mitchell AJ. Screening for distress and unmet needs in patients with cancer: review and recommendations. J Clin Oncol. 2012;30(11):1160-1177. doi:10.1200/JCO.2011.39.5509
25. Quill TE, Abernethy AP. Generalist plus specialist palliative care—creating a more sustainable model. N Engl J Med. 2013;368(13):1173-1175. doi:10.1056/NEJMp1215620
26. Weissman DE, Meier DE. Identifying patients in need of a palliative care assessment in the hospital setting: a consensus report from the Center to Advance Palliative Care. J Palliat Med. 2011;14(1):17-23. doi:10.1089/jpm.2010.0347
27. Kumar P, Wright AA, Hatfield LA, Temel JS, Keating NL. Family perspectives on hospice care experiences of patients with cancer. J Clin Oncol. 2017;35(4):432-439. doi:10.1200/JCO.2016.68.9257
28. Mor V, Joyce NR, Coté DL, et al. The rise of concurrent care for veterans with advanced cancer at the end of life. Cancer. 2016;122(5):782-790. doi:10.1002/cncr.29827
29. US Department of Veterans Affairs. Patient care services: Patient aligned care team (PACT). Updated November 5, 2020. Accessed July 8, 2021. https://www.patientcare.va.gov/primarycare/PACT.asp
30. US Department of Veterans Affairs, Veterans Health Administration. VHA health equity action plan. Published September 27, 2019. Accessed July 8, 2021. https://www.va.gov/HEALTHEQUITY/docs/Health_Equity_Action_Plan_Final_022020.pdf
31. Alcaraz KI, Wiedt TL, Daniels EC, Yabroff KR, Guerra CE, Wender RC. Understanding and addressing social determinants to advance cancer health equity in the United States: a blueprint for practice, research, and policy. CA Cancer J Clin. 2020;70(1):31-46. doi:10.3322/caac.21586
32. Atkins D, Kilbourne A, Lipson L. Health equity research in the Veterans Health Administration: we’ve come far but aren’t there yet. Am J Public Health. 2014;104(suppl 4):S525-526. doi:10.2105/AJPH.2014.302216
33. American Cancer Society. Cancer Facts & Figures for African Americans 2019-2021. Atlanta: American Cancer Society; 2019.
34. Hastert TA, Kirchhoff AC, Banegas MP, et al. Work changes and individual, cancer-related, and work-related predictors of decreased work participation among African American cancer survivors. Cancer Med. 2020;9(23):9168-9177. doi:10.1002/cam4.3512
35. Bekelman DB, Nowels CT, Allen LA, Shakar S, Kutner JS, Matlock DD. Outpatient palliative care for chronic heart failure: a case series. J Palliat Med. 2011;14(7):815-821. doi:10.1089/jpm.2010.050
1. National Comprehensive Cancer Network. NCCN guidelines distress management. Version 2.2021. Updated January 5, 2021. Accessed July 8, 2021. http://www.nccn.org/professionals/physician_gls/pdf/distress.pdf
2. American College of Surgeons, Commission on Cancer. Cancer program standards 2012: ensuring patient-centered care. Version 1.2.1. Published 2021. Accessed July 8, 2021. https://www.facs.org/~/media/files/quality%20programs/cancer/coc/programstandards2012.ashx
3. Jacobsen PB, Ransom S. Implementation of NCCN distress management guidelines by member institutions. J Natl Compr Canc Netw. 2007;5(1):99-103. doi:10.6004/jnccn.2007.0010
4. The Coleman Supportive Oncology Collaborative. Training tools. Accessed July 14, 2021. https://www.supportiveoncologycollaborative.org/training-tools
5. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257. doi:10.1001/archinte.160.21.3252
6. Bullman T, Schneiderman A, Gradus JL. Relative importance of posttraumatic stress disorder and depression in predicting risk of suicide among a cohort of Vietnam veterans. Suicide Life Threat Behav. 2019;49(3):838-845. doi:10.1111/sltb.12482
7. Kazis LE, Miller DR, Clark J, et al. Health-related quality of life in patients served by the Department of Veterans Affairs: results from the Veterans Health Study. Arch Intern Med. 1998;158(6):626-632. doi:10.1001/archinte.158.6.626
8. O’Toole BI, Marshall RP, Grayson DA, et al. The Australian Vietnam Veterans Health Study: III. Psychological health of Australian Vietnam veterans and its relationship to combat. Int J Epidemiol. 1996;25(2):331-340. doi:10.1093/ije/25.2.331
9. Vincent C, Chamberlain K, Long N. Mental and physical health status in a community sample of New Zealand Vietnam War veterans. Aust J Public Health. 1994;18(1):58-62. doi:10.1111/j.1753-6405.1994.tb00196.x
10. US Department of Veterans Affairs. Veterans’ diseases associated with Agent Orange. Updated June 16, 2021. Accessed July 8, 2021. http://www.publichealth.va.gov/exposures/agentorange/diseases.asp#veterans
11. Hwa KJ, Dua MM, Wren SM, Visser BC. Missing the obvious: psychosocial obstacles in Veterans with hepatocellular carcinoma. HBP (Oxford). 2015;17(12):1124-1129. doi:10.1111/hpb.12508
12. Saha S, Freeman M, Toure J, Tippens KM, Weeks C, Ibrahim S. Racial and ethnic disparities in the VA health care system: a systematic review. J Gen Intern Med. 2008;23(5):654-671. doi:10.1007/s11606-008-0521-4
13. Amaral EFL, Pollard MS, Mendelsohn J, Cefalu M. Current and future demographics of the veteran population, 2014-2024. Popul Rev. 2018;57(1):28-60. doi:10.1353/prv.2018.0002
14. Mohile SG, Dale W, Somerfield MR, et al. Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO guideline for geriatric oncology. J Clin Oncol. 2018;36(22):2326-2347. doi:10.1200/JCO.2018.78.8687
15. Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61(4):212-236. doi:10.3322/caac.20121
16. Cimino T, Said K, Safier L, Harris H, Kinderman A. Psychosocial distress among oncology patients in the safety net. Psychooncology. 2020;29(11):1927-1935. doi:10.1002/pon.5525
17. Molander R, Hodgkins K, Johnson C, White A, Frazier E, Krahn D. Interprofessional education in patient aligned care team primary care-mental health integration. Fed Pract. 2017;34(6):40-48.
18. Parikh DA, Ragavan M, Dutta R, et al. Financial toxicity of cancer care: an analysis of financial burden in three distinct health care systems [published online ahead of print, 2021 Apr 7]. JCO Oncol Pract. 2021;OP2000890. doi:10.1200/OP.20.00890
19. Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986-992. doi:10.1136/bmjqs-2015-004411
20. Weldon CB, Gerhart JI, Penedo FJ, et al. Correlates of distress for cancer patients: results from multi-institution use of holistic patient-reported screening tool. J Clin Oncol. 2019;37(15)(suppl):11587-11587. doi:10.1200/JCO.2019.37.15_suppl.11587
21. Kroenke K, Spitzer RL, Williams JB, Löwe B. The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: a systematic review. Gen Hosp Psychiatry. 2010;32(4):345-359. doi:10.1016/j.genhosppsych.2010.03.006
22. Kaiser MJ, Bauer JM, Ramsch C, et al. Validation of the Mini Nutritional Assessment short-form (MNA-SF): a practical tool for identification of nutritional status. J Nutr Health Aging. 2009;13(9):782-788. doi:10.1007/s12603-009-0214-7
23. Azizoddin DR, Lakin JR, Hauser J, et al. Meeting the guidelines: implementing a distress screening intervention for veterans with cancer. Psychooncology. 2020;29(12):2067-2074. doi:10.1002/pon.5565
24. Carlson LE, Waller A, Mitchell AJ. Screening for distress and unmet needs in patients with cancer: review and recommendations. J Clin Oncol. 2012;30(11):1160-1177. doi:10.1200/JCO.2011.39.5509
25. Quill TE, Abernethy AP. Generalist plus specialist palliative care—creating a more sustainable model. N Engl J Med. 2013;368(13):1173-1175. doi:10.1056/NEJMp1215620
26. Weissman DE, Meier DE. Identifying patients in need of a palliative care assessment in the hospital setting: a consensus report from the Center to Advance Palliative Care. J Palliat Med. 2011;14(1):17-23. doi:10.1089/jpm.2010.0347
27. Kumar P, Wright AA, Hatfield LA, Temel JS, Keating NL. Family perspectives on hospice care experiences of patients with cancer. J Clin Oncol. 2017;35(4):432-439. doi:10.1200/JCO.2016.68.9257
28. Mor V, Joyce NR, Coté DL, et al. The rise of concurrent care for veterans with advanced cancer at the end of life. Cancer. 2016;122(5):782-790. doi:10.1002/cncr.29827
29. US Department of Veterans Affairs. Patient care services: Patient aligned care team (PACT). Updated November 5, 2020. Accessed July 8, 2021. https://www.patientcare.va.gov/primarycare/PACT.asp
30. US Department of Veterans Affairs, Veterans Health Administration. VHA health equity action plan. Published September 27, 2019. Accessed July 8, 2021. https://www.va.gov/HEALTHEQUITY/docs/Health_Equity_Action_Plan_Final_022020.pdf
31. Alcaraz KI, Wiedt TL, Daniels EC, Yabroff KR, Guerra CE, Wender RC. Understanding and addressing social determinants to advance cancer health equity in the United States: a blueprint for practice, research, and policy. CA Cancer J Clin. 2020;70(1):31-46. doi:10.3322/caac.21586
32. Atkins D, Kilbourne A, Lipson L. Health equity research in the Veterans Health Administration: we’ve come far but aren’t there yet. Am J Public Health. 2014;104(suppl 4):S525-526. doi:10.2105/AJPH.2014.302216
33. American Cancer Society. Cancer Facts & Figures for African Americans 2019-2021. Atlanta: American Cancer Society; 2019.
34. Hastert TA, Kirchhoff AC, Banegas MP, et al. Work changes and individual, cancer-related, and work-related predictors of decreased work participation among African American cancer survivors. Cancer Med. 2020;9(23):9168-9177. doi:10.1002/cam4.3512
35. Bekelman DB, Nowels CT, Allen LA, Shakar S, Kutner JS, Matlock DD. Outpatient palliative care for chronic heart failure: a case series. J Palliat Med. 2011;14(7):815-821. doi:10.1089/jpm.2010.050
Safe Transitions and Congregate Living in the Age of COVID-19: A Retrospective Cohort Study
The COVID-19 outbreak in February 2020 at a congregate living facility near Seattle, Washington, signaled the beginning of the pandemic in the United States. In that facility, infected residents had a 54.5% hospitalization rate and 33.7% case-fatality rate.1 Similar to the experience in Washington, all congregate living facilities have proved particularly vulnerable to the effects of COVID-19,2-7 with residents at increased risk for disease severity and mortality.2-7
Due to the COVID-19 emergency, NorthShore University HealthSystem (NUHS), a multihospital, integrated health system in northern Illinois, established a best practice for appropriate use of congregate living facilities after hospitalization. This focused on the safety of discharged patients and mitigation of COVID-19 by putting in place a referral process to a newly established congregate living review committee (CLRC) for review prior to discharge. Although all discharges to congregate living settings are at high risk,2 new placements to skilled nursing facilities (SNFs) were the primary focus of the committee and the sole focus of this study. In this study, we sought to determine whether establishment of the CLRC was associated with a reduction in SNF utilization, whether this was safe and efficient, and whether it was associated with a reduction in COVID-19 incidence in the 30 days following discharge.
METHODS
Setting and Case Review Intervention
We conducted a retrospective cohort study for patients hospitalized within NUHS from March 19, 2019 to July 16, 2020, designed as an interrupted time series. The study was approved by the NUHS Institutional Review Board (EH21-022).
The study exposure was creation of a referral and review process for all patients with expected discharge to a SNF and was implemented as part of usual discharge planning during the COVID-19 pandemic. The key intervention was to establish a multidisciplinary committee, the CLRC, to review all potential discharges to SNFs. The CLRC had dual goals of preventing COVID-19 spread in facilities by limiting placement of new residents and protecting a vulnerable population from a setting that conferred a higher risk of acquiring COVID-19. The CLRC was organized as a multidisciplinary committee with physicians, case managers, social workers, physical therapists, occupational therapists, and the director of NUHS home health agency. Physician members were evenly split as half hospitalists and half ambulatory physicians. The CLRC review was initiated by a patient’s assigned case manager or social worker by consult through a referral in the electronic medical record (EMR). Each case was summarized and then presented to the full CLRC. The CLRC met for 1 hour per day, 6 days per week, to review all planned discharges that met criteria for review. A committee physician chaired each meeting. Three other members were needed for a quorum, with one other member with a title of director or higher. Time required was the 1-hour daily meeting, as well as one full-time position for case review, preparation, and program administration. The case presentation included a clinical summary of the hospitalization as well as COVID-19 status and testing history, previous living situation, level of home support, functional level, psychosocial needs, barrier(s) to discharging home, and long-term residential plans. A structured assessment was then made by each CLRC member in accordance with their professional expertise. Unanimous consensus would be reached before finalizing any recommended adjustments to the discharge, which would be communicated to the inpatient care team via a structured note within the EMR, along with direct communication to the assigned case manager or social worker. When the CLRC suggested adjustments to the discharge, they would work with the assigned case manager or social worker to communicate an appropriate post–acute care plan with the patient or appropriate representative. If there was disagreement or the recommendations could not be followed, the case manager or social worker would place a new referral with additional information for reconsideration. Following a recommendation for SNF, verification would be completed by the CLRC prior to discharge. This process is detailed in Figure 1.
Patient Population
Inclusion criteria for the study were: (1) inpatient hospitalization and (2) eligibility for risk scoring via the organization’s clinical analytics prediction engine (CAPE).8 CAPE is a validated predictive model that includes risk of readmission, in-hospital mortality, and out-of-hospital mortality,8 with extensive adoption at NUHS. CAPE score eligibility was used as an inclusion criterion so that CAPE could be applied for derivation of a matched control. CAPE eligibility criteria include admission age of at least 18 years and that hospitalization is not psychiatric, rehabilitative, or obstetric. Patients must not be enrolled in hospice and must be discharged alive.
Exclusions were patients who tested positive for SARS-CoV-2 prior to or during index hospitalization. Excluding COVID-19 patients from the analysis eliminated a confounder not present in the preintervention group.
For patients with multiple inpatient admissions, the first admission was the only admission used for analysis. Additionally, if a patient had an admission that occurred in both the preintervention and postintervention periods, they were included only in the postintervention period. This was done to avoid any within-subject correlation and ensure unique patients in each group. Confounding from this approach was mitigated through the process of deriving a matched control.
Outcomes Measurement
The primary outcome of interest was total discharges to SNF across NUHS facilities after hospital admission. Patients were identified as discharging to a SNF if discharge destination codes 03, 64, or 83 appeared on the hospital bill. Additionally, new discharges to SNFs were assessed and identified if documentation indicated that the patient’s living arrangement prior to admission was not a SNF but discharge billing destination codes 03, 64, or 83 appeared on the hospital bill.
Secondary outcomes were measurement of readmissions, days to readmission, and median length of stay (LOS). Readmissions and LOS were balancing measures for the primary outcome, with readmissions measured to evaluate the safety of the CLRC process and LOS measured to evaluate its efficiency. A readmission was any patient who had an unplanned inpatient admission at an NUHS facility within 30 days after an index admission. LOS was measured in days from arrival on a hospital unit to time of discharge.
Additional analysis was done to estimate the effect of the intervention on the incidence of COVID-19 in the 30 days following discharge by comparing the observed to expected incidence of COVID-19 by discharge destination. The expected values were derived by estimating COVID-19 cases that would have been expected to occur with rates of preintervention SNF utilization. This was accomplished by multiplying the observed incidence of COVID-19 in the 30 days following discharge by the number of patients who were discharged to SNFs or home/other in the preintervention period. This expected value was then compared with the observed values to estimate the effect size of the intervention on COVID-19 incidence following discharge. This method of deriving an expected value from the observed incidence was utilized because the preintervention period was before COVID-19 was widespread in the community. It was therefore not possible to directly measure COVID-19 incidence in the preintervention period.
Data Source
Data were retrieved from the NUHS Enterprise Data Warehouse, NUHS’s central data repository, which contains a nightly upload of clinical and financial data from the EMR. Data were collected between March 19, 2019, and July 16, 2020.
The preintervention period was defined as March 19, 2019, to March 18, 2020. Data from that interval were compared with the postintervention period, which was from March 19, 2020, to July 16, 2020. The preintervention period, 1 year immediately prior to the intervention, was chosen to limit any effect of temporal trends while also providing a large sample size. The postintervention period began on the first day NUHS implemented the revised approach to SNF use and ended on the last day before the review process was modified.
Data Analysis
An interrupted time series was used to measure the impact of adoption of the CLRC protocol. A matched control was derived from the preintervention population. To derive this matched control, there was an assessment of covariates in the preintervention and postintervention groups using a standardized mean difference (SMD)9 that indicated an imbalance (SMD ≥ 0.1) in some covariates. A propensity score–matching technique10 was applied to address this imbalance and lack of randomization.
The candidate variables for propensity matching were chosen if they had an association with 30-day readmission. Readmission was chosen to find candidate variables because, of the possible outcomes, this was the only one that was not directly impacted by any CLRC decision. Each covariate was assessed using a logistic regression model while controlling for the postintervention group. If there was an association between a covariate and the outcome, it was chosen for propensity matching. Propensity scores were calculated using a logistic regression model with the treatment (1/0) variable as the dependent variable and the chosen covariates as predictors.
There were no indications of strong multicollinearity. The propensity scores generated were then used to derive a matched control using paired matching. MatchIt package in R (R Foundation for Statistical Computing) was used to create a matched dataset with a logit distance and standard caliper of 0.2 times the standard deviations of the logit of the propensity score. If a match was not found within the caliper, the nearest available match was used.
Regression adjustment11 was then performed using multivariate linear/logistic regression with LOS, readmission rate, days to readmission, total SNF discharges, and new SNF discharges as the outcomes. Treatment (1/0) variable and propensity score were used as the predictors. The adjusted coefficients or odds ratios (ORs) of the intervention variable were thus derived, and their associated P values were used to assess the impact of the intervention on the respective outcomes.
RESULTS
The unmatched preintervention population included 14,468 patients, with 4424 patients in the postintervention population. A matched population was derived and, after matching, the population sizes for pre and post intervention were 4424 each. In the matched population, all measured preintervention characteristics had SMDs and P values that were statistically equivalent. Patient characteristics for the unmatched and matched populations are detailed in Table 1.
During the preintervention period, 1130 (25.5%) patients were discharged to a SNF, with 776 (17.5%) patients being new SNF discharges. In the postintervention period, 568 (12.8%) patients were discharged to a SNF, with 257 (5.8%) patients being new SNF discharges. Total SNF discharges postintervention saw a 49.7% relative reduction (OR, 0.42; 95% CI, 0.38-0.47), while new SNF discharges saw a 66.9% relative reduction (OR, 0.29; 95% CI, 0.25-0.34). These results for both total and new SNF discharges were statistically significant, with P values of <.001, respectively.
Readmissions in the preintervention period were 529 (12.0%) patients, compared with 559 (12.6%) patients in the postintervention period (OR, 1.06; 95% CI, 0.93-1.20; P =.406). An OR was also calculated for readmissions, adjusting for discharge disposition, to account for changes observed in SNF use in the postintervention period. This OR was 1.11 (95% CI, 0.97-1.26; P = .131). Days to readmission in the preintervention and postintervention groups were 11.0 days and 12.0 days, respectively (OR, 0.41; 95% CI, –0.61 to 1.43; P = .429).
LOS was 3.61 days in the preintervention group and 3.64 days in the postintervention group, with an interquartile range (IQR) of 2.14 to 5.69 days in the preintervention group and 2.08 to 5.95 in the postintervention group (OR, 0.09; 95% CI, –0.09 to 0.27; P =.316). These results are summarized in Table 2.
DISCUSSION
A COVID-19 outbreak in a SNF presents a grave risk to residents and patients discharged to these facilities. It is critical for healthcare systems to do the utmost to protect the health of this vulnerable population and the public in efforts to limit COVID-19 within SNFs.12-14
In this study, we observed that at NUHS, establishing a multidisciplinary review committee, the CLRC, to assess the appropriateness of discharge to a SNF after hospitalization resulted in a nearly 50% reduction in total SNF discharges and a greater than two-thirds reduction in new SNF discharges, without any increase in LOS or readmissions. Additionally, it was observed that discharging to settings other than a SNF greatly reduced a patient’s risk of being diagnosed with COVID-19 within 30 days, a result that reached statistical significance. Based on the observed 37.2% relative reduction in COVID-19 cases, we estimate that there may have been one COVID-19 infection prevented every 5.6 days from this intervention. Based on published COVID-19 mortality rates for SNF residents,1 the intervention may have prevented one death every 2.6 weeks. Beyond the risk of COVID-19, other benefits of reducing SNF use are patient and family well-being. Although not measured in this study, others have published about the significant psychological burdens placed on SNF residents, who were at high risk for social isolation, anxiety, and depression during the COVID-19 pandemic2,15-19 Family members also may have had increased stress, as they were deprived of the opportunity to visit loved ones, advocate for them, and help maintain their identity, humanity, and quality of life.20
Although other hospitals have established a structured approach to reduce COVID-19 in SNFs,21 to the best of the authors’ knowledge, the approach described in this article is a unique response to the COVID-19 pandemic. As we have demonstrated, it is highly effective and safe and likely prevented many COVID-19 cases and deaths.
Furthermore, a review committee, such as the one we have described, has value well beyond the COVID-19 pandemic. The health and affordability of care for patients, provider success in value-based care models, and the long-term sustainability of the US healthcare system require close attention to appropriate use of expensive services and to ensuring that their use creates high value. SNF use after a hospitalization is one such service that is frequently targeted and thought to contribute to a substantial portion of wasteful medical spending.22,23 Additionally, SNFs are known to be high risk for communicable disease outbreaks other than COVID-19,24,25 as well as a high-risk environment for many other preventable adverse events.25,26 This review committee ultimately serves to help determine the most appropriate postacute setting for patients being discharged with a determination made through considerations for patient safety, rehabilitation potential, and mental and physical well-being. From a population health perspective, this can lead to better outcomes and lower costs.22,23 Therefore, although the risks of COVID-19 infection in SNFs are expected to subside, the work of evaluating appropriate use of SNFs after hospitalization at our institution continues. The broader focus now extends beyond postacute level of service toward ensuring a high-value discharge that results in both appropriate resource use and safe patient care transitions.
Limitations of this study include its retrospective nature, results from a single center, and a number of potentially unmeasured confounders that the COVID-19 pandemic created. One possible confounder is that the reduction in SNF use we observed was a temporal trend related to changing preferences. In addressing this, we reviewed Medicare claims data from the US Department of Health and Human Services in April 2020 and July 2020 compared with the same period in 2019. These data demonstrated only a modest reduction in spending on SNFs in April 2020 that was smaller than the reduction seen in Part A inpatient hospital spending during that same month.27 By July 2020, the spending from Medicare on SNFs exceeded the levels seen in 2019,27 suggesting that the percentage of acute care admissions discharging to SNFs was no lower for Medicare patients in response to COVD-19. We also considered more stringent SNF admission standards as another potential confounder; however, this was not seen at the SNFs in the NUHS geography, where the referral process became less stringent because of COVID-19 waivers for a qualifying stay or skilled need from the Centers for Medicare and Medicaid Services. We were also not able to account for readmissions outside of NUHS, and therefore there may have been differences in the readmission rate that were unmeasured. To address this limitation, we reviewed a data extract from the Illinois Health and Hospital Association and found that the percentage of patients who returned for readmission to a NUHS facility in the year prior to the intervention and during the intervention period were 92.8% and 95.3%, respectively. From this we concluded the unmeasured readmission rate appears to be low, stable, and unlikely to have altered the results of this study. Additionally, when calculating potential COVID-19 cases avoided, the expected number was, by necessity, derived from the observed outcome, given the absence of COVID-19 in the preintervention population. This may have introduced unmeasured confounders, limiting the ability to precisely measure the effect size or draw conclusions on causation. Finally, there may be limitations to the generalizability of these results based on the payor mix of the population at NUHS, which is predominantly insured through Medicare or commercial payors.
CONCLUSION
We believe this model is replicable and the results generalizable and could serve as both a template for reducing the risks of COVID-19 in SNFs and as part of a larger infection-control strategy to mitigate disease spread in vulnerable populations. It could also be applied as a component of value-improvement programs to foster appropriate use of postacute services after an acute care hospitalization, ensuring safe transitions of care through promotion of high-value care practices.
Acknowledgment
The authors thank Wei Ning Chi for editorial assistance.
1. McMichael TM, Currie DW, Clark S, et al. Epidemiology of Covid-19 in a long-term care facility in King County, Washington. N Engl J Med. 2020;382(21):2005-2011. https://doi.org/10.1056/NEJMoa2005412
2. Ouslander JG, Grabowski DC. COVID-19 in nursing homes: calming the perfect storm. J Am Geriatr Soc. 2020;68(10):2153-2162. https://doi.org/10.1111/jgs.16784
3. CDC COVID-19 Response Team. Severe outcomes among patients with coronavirus disease 2019 (COVID-19) - United States, February 12-March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(12):343-346. https://doi.org/10.15585/mmwr.mm6912e2
4. Ko JY, Danielson ML, Town M, et al. Risk factors for coronavirus disease 2019 (COVID-19)-associated hospitalization: COVID-19-Associated Hospitalization Surveillance Network and Behavioral Risk Factor Surveillance System. Clin Infect Dis. 2020;72(11):e695-e703. https://doi.org/10.1093/cid/ciaa1419
5. Davidson PM, Szanton SL. Nursing homes and COVID-19: we can and should do better. J Clin Nurs. 2020;29(15-16):2758-2759. https://doi.org/10.1111/jocn.15297
6. Dosa D, Jump RLP, LaPlante K, Gravenstein S. Long-term care facilities and the coronavirus epidemic: practical guidelines for a population at highest risk. J Am Med Dir Assoc. 2020;21(5):569-571. https://doi.org/10.1016/j.jamda.2020.03.004
7. Fallon A, Dukelow T, Kennelly SP, O’Neill D. COVID-19 in nursing homes. QJM. 2020;113(6):391-392. https://doi.org/10.1093/qjmed/hcaa136
8. Shah N, Konchak C, Chertok D, et al. Clinical Analytics Prediction Engine (CAPE): development, electronic health record integration and prospective validation of hospital mortality, 180-day mortality and 30-day readmission risk prediction models. PLoS One. 2020;15(8):e0238065. https://doi.org/10.1371/journal.pone.0238065
9. Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput. 2009;38(6):1228-1234. https://doi.org/10.1080/03610910902859574
10. Rosenbaum PR, Rubin DB. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat. 1985;39(1):33-38. https://doi.org/10.2307/2683903
11. Myers JA, Louis TA. Regression adjustment and stratification by propensity score in treatment effect estimation. Johns Hopkins University, Dept of Biostatistics Working Papers. 2010 203(Working Papers):1-27.
12. Lansbury LE, Brown CS, Nguyen-Van-Tam JS. Influenza in long-term care facilities. Influenza Other Respir Viruses. 2017;11(5):356-366. https://doi.org/10.1111/irv.12464
13. Sáez-López E, Marques R, Rodrigues N, et al. Lessons learned from a prolonged norovirus GII.P16-GII.4 Sydney 2012 variant outbreak in a long-term care facility in Portugal, 2017. Infect Control Hosp Epidemiol. 2019;40(10):1164-1169. https://doi.org/10.1017/ice.2019.201
14. Gaspard P, Mosnier A, Stoll-Keller F, Roth C, Larocca S, Bertrand X. Influenza prevention in nursing homes: great significance of seasonal variability and spatio-temporal pattern. Presse Med. 2015;44(10):e311-e319. https://doi.org/10.1016/j.lpm.2015.04.041
15. Pfefferbaum B, North CS. Mental health and the Covid-19 pandemic. N Engl J Med. 2020;383(6):510-512. https://doi.org/10.1056/NEJMp2008017
16. Galea S, Merchant RM, Lurie N. The mental health consequences of COVID-19 and physical distancing: the need for prevention and early intervention. JAMA Intern Med. 2020;180(6):817-818. https://doi.org/10.1001/jamainternmed.2020.1562
17. Armitage R, Nellums LB. COVID-19 and the consequences of isolating the elderly. Lancet Public Health. 2020;5(5):e256. https://doi.org/10.1016/s2468-2667(20)30061-x
18. El Haj M, Altintas E, Chapelet G, Kapogiannis D, Gallouj K. High depression and anxiety in people with Alzheimer’s disease living in retirement homes during the covid-19 crisis. Psychiatry Res. 2020;291:113294. https://doi.org/10.1016/j.psychres.2020.113294
19. Santini ZI, Jose PE, York Cornwell E, et al. Social disconnectedness, perceived isolation, and symptoms of depression and anxiety among older Americans (NSHAP): a longitudinal mediation analysis. Lancet Public Health. 2020;5(1):e62-e70. https://doi.org/10.1016/s2468-2667(19)30230-0
20. Gaugler JE, Anderson KA, Zarit SH, Pearlin LI. Family involvement in nursing homes: effects on stress and well-being. Aging Ment Health. 2004;8(1):65-75. https://doi.org/10.1080/13607860310001613356
21. Kim G, Wang M, Pan H, et al. A health system response to COVID-19 in long-term care and post-acute care: a three-phase approach. J Am Geriatr Soc. 2020;68(6):1155-1161. https://doi.org/10.1111/jgs.16513
22. McWilliams JM, Gilstrap LG, Stevenson DG, Chernew ME, Huskamp HA, Grabowski DC. Changes in postacute care in the Medicare Shared Savings Program. JAMA Intern Med. 2017;177(4):518-526. https://doi.org/10.1001/jamainternmed.2016.9115
23. Ackerly DC, Grabowski DC. Post-acute care reform--beyond the ACA. N Engl J Med. 2014;370(8):689-691. https://doi.org/10.1056/NEJMp1315350
24. Strausbaugh LJ, Sukumar SR, Joseph CL. Infectious disease outbreaks in nursing homes: an unappreciated hazard for frail elderly persons. Clin Infect Dis. 2003;36(7):870-876. https://doi.org/10.1086/368197
25. Kapoor A, Field T, Handler S, et al. Adverse events in long-term care residents transitioning from hospital back to nursing home. JAMA Intern Med. 2019;179(9):1254-1261. https://doi.org/10.1001/jamainternmed.2019.2005
26. Adverse Events in Skilled Nursing Facilities: National Incidence Among Medicare Beneficiaries. Office of Inspector General, US Dept of Health & Human Services; 2014.
27. The Impact of the COVID-19 Pandemic on Medicare Beneficiary Use of Health Care Services and Payments to Providers: Early Data for the First 6 Months of 2020. Office of the Assistant Secretary for Planning and Evaluation, US Dept of Health & Human Services; 2020.
The COVID-19 outbreak in February 2020 at a congregate living facility near Seattle, Washington, signaled the beginning of the pandemic in the United States. In that facility, infected residents had a 54.5% hospitalization rate and 33.7% case-fatality rate.1 Similar to the experience in Washington, all congregate living facilities have proved particularly vulnerable to the effects of COVID-19,2-7 with residents at increased risk for disease severity and mortality.2-7
Due to the COVID-19 emergency, NorthShore University HealthSystem (NUHS), a multihospital, integrated health system in northern Illinois, established a best practice for appropriate use of congregate living facilities after hospitalization. This focused on the safety of discharged patients and mitigation of COVID-19 by putting in place a referral process to a newly established congregate living review committee (CLRC) for review prior to discharge. Although all discharges to congregate living settings are at high risk,2 new placements to skilled nursing facilities (SNFs) were the primary focus of the committee and the sole focus of this study. In this study, we sought to determine whether establishment of the CLRC was associated with a reduction in SNF utilization, whether this was safe and efficient, and whether it was associated with a reduction in COVID-19 incidence in the 30 days following discharge.
METHODS
Setting and Case Review Intervention
We conducted a retrospective cohort study for patients hospitalized within NUHS from March 19, 2019 to July 16, 2020, designed as an interrupted time series. The study was approved by the NUHS Institutional Review Board (EH21-022).
The study exposure was creation of a referral and review process for all patients with expected discharge to a SNF and was implemented as part of usual discharge planning during the COVID-19 pandemic. The key intervention was to establish a multidisciplinary committee, the CLRC, to review all potential discharges to SNFs. The CLRC had dual goals of preventing COVID-19 spread in facilities by limiting placement of new residents and protecting a vulnerable population from a setting that conferred a higher risk of acquiring COVID-19. The CLRC was organized as a multidisciplinary committee with physicians, case managers, social workers, physical therapists, occupational therapists, and the director of NUHS home health agency. Physician members were evenly split as half hospitalists and half ambulatory physicians. The CLRC review was initiated by a patient’s assigned case manager or social worker by consult through a referral in the electronic medical record (EMR). Each case was summarized and then presented to the full CLRC. The CLRC met for 1 hour per day, 6 days per week, to review all planned discharges that met criteria for review. A committee physician chaired each meeting. Three other members were needed for a quorum, with one other member with a title of director or higher. Time required was the 1-hour daily meeting, as well as one full-time position for case review, preparation, and program administration. The case presentation included a clinical summary of the hospitalization as well as COVID-19 status and testing history, previous living situation, level of home support, functional level, psychosocial needs, barrier(s) to discharging home, and long-term residential plans. A structured assessment was then made by each CLRC member in accordance with their professional expertise. Unanimous consensus would be reached before finalizing any recommended adjustments to the discharge, which would be communicated to the inpatient care team via a structured note within the EMR, along with direct communication to the assigned case manager or social worker. When the CLRC suggested adjustments to the discharge, they would work with the assigned case manager or social worker to communicate an appropriate post–acute care plan with the patient or appropriate representative. If there was disagreement or the recommendations could not be followed, the case manager or social worker would place a new referral with additional information for reconsideration. Following a recommendation for SNF, verification would be completed by the CLRC prior to discharge. This process is detailed in Figure 1.
Patient Population
Inclusion criteria for the study were: (1) inpatient hospitalization and (2) eligibility for risk scoring via the organization’s clinical analytics prediction engine (CAPE).8 CAPE is a validated predictive model that includes risk of readmission, in-hospital mortality, and out-of-hospital mortality,8 with extensive adoption at NUHS. CAPE score eligibility was used as an inclusion criterion so that CAPE could be applied for derivation of a matched control. CAPE eligibility criteria include admission age of at least 18 years and that hospitalization is not psychiatric, rehabilitative, or obstetric. Patients must not be enrolled in hospice and must be discharged alive.
Exclusions were patients who tested positive for SARS-CoV-2 prior to or during index hospitalization. Excluding COVID-19 patients from the analysis eliminated a confounder not present in the preintervention group.
For patients with multiple inpatient admissions, the first admission was the only admission used for analysis. Additionally, if a patient had an admission that occurred in both the preintervention and postintervention periods, they were included only in the postintervention period. This was done to avoid any within-subject correlation and ensure unique patients in each group. Confounding from this approach was mitigated through the process of deriving a matched control.
Outcomes Measurement
The primary outcome of interest was total discharges to SNF across NUHS facilities after hospital admission. Patients were identified as discharging to a SNF if discharge destination codes 03, 64, or 83 appeared on the hospital bill. Additionally, new discharges to SNFs were assessed and identified if documentation indicated that the patient’s living arrangement prior to admission was not a SNF but discharge billing destination codes 03, 64, or 83 appeared on the hospital bill.
Secondary outcomes were measurement of readmissions, days to readmission, and median length of stay (LOS). Readmissions and LOS were balancing measures for the primary outcome, with readmissions measured to evaluate the safety of the CLRC process and LOS measured to evaluate its efficiency. A readmission was any patient who had an unplanned inpatient admission at an NUHS facility within 30 days after an index admission. LOS was measured in days from arrival on a hospital unit to time of discharge.
Additional analysis was done to estimate the effect of the intervention on the incidence of COVID-19 in the 30 days following discharge by comparing the observed to expected incidence of COVID-19 by discharge destination. The expected values were derived by estimating COVID-19 cases that would have been expected to occur with rates of preintervention SNF utilization. This was accomplished by multiplying the observed incidence of COVID-19 in the 30 days following discharge by the number of patients who were discharged to SNFs or home/other in the preintervention period. This expected value was then compared with the observed values to estimate the effect size of the intervention on COVID-19 incidence following discharge. This method of deriving an expected value from the observed incidence was utilized because the preintervention period was before COVID-19 was widespread in the community. It was therefore not possible to directly measure COVID-19 incidence in the preintervention period.
Data Source
Data were retrieved from the NUHS Enterprise Data Warehouse, NUHS’s central data repository, which contains a nightly upload of clinical and financial data from the EMR. Data were collected between March 19, 2019, and July 16, 2020.
The preintervention period was defined as March 19, 2019, to March 18, 2020. Data from that interval were compared with the postintervention period, which was from March 19, 2020, to July 16, 2020. The preintervention period, 1 year immediately prior to the intervention, was chosen to limit any effect of temporal trends while also providing a large sample size. The postintervention period began on the first day NUHS implemented the revised approach to SNF use and ended on the last day before the review process was modified.
Data Analysis
An interrupted time series was used to measure the impact of adoption of the CLRC protocol. A matched control was derived from the preintervention population. To derive this matched control, there was an assessment of covariates in the preintervention and postintervention groups using a standardized mean difference (SMD)9 that indicated an imbalance (SMD ≥ 0.1) in some covariates. A propensity score–matching technique10 was applied to address this imbalance and lack of randomization.
The candidate variables for propensity matching were chosen if they had an association with 30-day readmission. Readmission was chosen to find candidate variables because, of the possible outcomes, this was the only one that was not directly impacted by any CLRC decision. Each covariate was assessed using a logistic regression model while controlling for the postintervention group. If there was an association between a covariate and the outcome, it was chosen for propensity matching. Propensity scores were calculated using a logistic regression model with the treatment (1/0) variable as the dependent variable and the chosen covariates as predictors.
There were no indications of strong multicollinearity. The propensity scores generated were then used to derive a matched control using paired matching. MatchIt package in R (R Foundation for Statistical Computing) was used to create a matched dataset with a logit distance and standard caliper of 0.2 times the standard deviations of the logit of the propensity score. If a match was not found within the caliper, the nearest available match was used.
Regression adjustment11 was then performed using multivariate linear/logistic regression with LOS, readmission rate, days to readmission, total SNF discharges, and new SNF discharges as the outcomes. Treatment (1/0) variable and propensity score were used as the predictors. The adjusted coefficients or odds ratios (ORs) of the intervention variable were thus derived, and their associated P values were used to assess the impact of the intervention on the respective outcomes.
RESULTS
The unmatched preintervention population included 14,468 patients, with 4424 patients in the postintervention population. A matched population was derived and, after matching, the population sizes for pre and post intervention were 4424 each. In the matched population, all measured preintervention characteristics had SMDs and P values that were statistically equivalent. Patient characteristics for the unmatched and matched populations are detailed in Table 1.
During the preintervention period, 1130 (25.5%) patients were discharged to a SNF, with 776 (17.5%) patients being new SNF discharges. In the postintervention period, 568 (12.8%) patients were discharged to a SNF, with 257 (5.8%) patients being new SNF discharges. Total SNF discharges postintervention saw a 49.7% relative reduction (OR, 0.42; 95% CI, 0.38-0.47), while new SNF discharges saw a 66.9% relative reduction (OR, 0.29; 95% CI, 0.25-0.34). These results for both total and new SNF discharges were statistically significant, with P values of <.001, respectively.
Readmissions in the preintervention period were 529 (12.0%) patients, compared with 559 (12.6%) patients in the postintervention period (OR, 1.06; 95% CI, 0.93-1.20; P =.406). An OR was also calculated for readmissions, adjusting for discharge disposition, to account for changes observed in SNF use in the postintervention period. This OR was 1.11 (95% CI, 0.97-1.26; P = .131). Days to readmission in the preintervention and postintervention groups were 11.0 days and 12.0 days, respectively (OR, 0.41; 95% CI, –0.61 to 1.43; P = .429).
LOS was 3.61 days in the preintervention group and 3.64 days in the postintervention group, with an interquartile range (IQR) of 2.14 to 5.69 days in the preintervention group and 2.08 to 5.95 in the postintervention group (OR, 0.09; 95% CI, –0.09 to 0.27; P =.316). These results are summarized in Table 2.
DISCUSSION
A COVID-19 outbreak in a SNF presents a grave risk to residents and patients discharged to these facilities. It is critical for healthcare systems to do the utmost to protect the health of this vulnerable population and the public in efforts to limit COVID-19 within SNFs.12-14
In this study, we observed that at NUHS, establishing a multidisciplinary review committee, the CLRC, to assess the appropriateness of discharge to a SNF after hospitalization resulted in a nearly 50% reduction in total SNF discharges and a greater than two-thirds reduction in new SNF discharges, without any increase in LOS or readmissions. Additionally, it was observed that discharging to settings other than a SNF greatly reduced a patient’s risk of being diagnosed with COVID-19 within 30 days, a result that reached statistical significance. Based on the observed 37.2% relative reduction in COVID-19 cases, we estimate that there may have been one COVID-19 infection prevented every 5.6 days from this intervention. Based on published COVID-19 mortality rates for SNF residents,1 the intervention may have prevented one death every 2.6 weeks. Beyond the risk of COVID-19, other benefits of reducing SNF use are patient and family well-being. Although not measured in this study, others have published about the significant psychological burdens placed on SNF residents, who were at high risk for social isolation, anxiety, and depression during the COVID-19 pandemic2,15-19 Family members also may have had increased stress, as they were deprived of the opportunity to visit loved ones, advocate for them, and help maintain their identity, humanity, and quality of life.20
Although other hospitals have established a structured approach to reduce COVID-19 in SNFs,21 to the best of the authors’ knowledge, the approach described in this article is a unique response to the COVID-19 pandemic. As we have demonstrated, it is highly effective and safe and likely prevented many COVID-19 cases and deaths.
Furthermore, a review committee, such as the one we have described, has value well beyond the COVID-19 pandemic. The health and affordability of care for patients, provider success in value-based care models, and the long-term sustainability of the US healthcare system require close attention to appropriate use of expensive services and to ensuring that their use creates high value. SNF use after a hospitalization is one such service that is frequently targeted and thought to contribute to a substantial portion of wasteful medical spending.22,23 Additionally, SNFs are known to be high risk for communicable disease outbreaks other than COVID-19,24,25 as well as a high-risk environment for many other preventable adverse events.25,26 This review committee ultimately serves to help determine the most appropriate postacute setting for patients being discharged with a determination made through considerations for patient safety, rehabilitation potential, and mental and physical well-being. From a population health perspective, this can lead to better outcomes and lower costs.22,23 Therefore, although the risks of COVID-19 infection in SNFs are expected to subside, the work of evaluating appropriate use of SNFs after hospitalization at our institution continues. The broader focus now extends beyond postacute level of service toward ensuring a high-value discharge that results in both appropriate resource use and safe patient care transitions.
Limitations of this study include its retrospective nature, results from a single center, and a number of potentially unmeasured confounders that the COVID-19 pandemic created. One possible confounder is that the reduction in SNF use we observed was a temporal trend related to changing preferences. In addressing this, we reviewed Medicare claims data from the US Department of Health and Human Services in April 2020 and July 2020 compared with the same period in 2019. These data demonstrated only a modest reduction in spending on SNFs in April 2020 that was smaller than the reduction seen in Part A inpatient hospital spending during that same month.27 By July 2020, the spending from Medicare on SNFs exceeded the levels seen in 2019,27 suggesting that the percentage of acute care admissions discharging to SNFs was no lower for Medicare patients in response to COVD-19. We also considered more stringent SNF admission standards as another potential confounder; however, this was not seen at the SNFs in the NUHS geography, where the referral process became less stringent because of COVID-19 waivers for a qualifying stay or skilled need from the Centers for Medicare and Medicaid Services. We were also not able to account for readmissions outside of NUHS, and therefore there may have been differences in the readmission rate that were unmeasured. To address this limitation, we reviewed a data extract from the Illinois Health and Hospital Association and found that the percentage of patients who returned for readmission to a NUHS facility in the year prior to the intervention and during the intervention period were 92.8% and 95.3%, respectively. From this we concluded the unmeasured readmission rate appears to be low, stable, and unlikely to have altered the results of this study. Additionally, when calculating potential COVID-19 cases avoided, the expected number was, by necessity, derived from the observed outcome, given the absence of COVID-19 in the preintervention population. This may have introduced unmeasured confounders, limiting the ability to precisely measure the effect size or draw conclusions on causation. Finally, there may be limitations to the generalizability of these results based on the payor mix of the population at NUHS, which is predominantly insured through Medicare or commercial payors.
CONCLUSION
We believe this model is replicable and the results generalizable and could serve as both a template for reducing the risks of COVID-19 in SNFs and as part of a larger infection-control strategy to mitigate disease spread in vulnerable populations. It could also be applied as a component of value-improvement programs to foster appropriate use of postacute services after an acute care hospitalization, ensuring safe transitions of care through promotion of high-value care practices.
Acknowledgment
The authors thank Wei Ning Chi for editorial assistance.
The COVID-19 outbreak in February 2020 at a congregate living facility near Seattle, Washington, signaled the beginning of the pandemic in the United States. In that facility, infected residents had a 54.5% hospitalization rate and 33.7% case-fatality rate.1 Similar to the experience in Washington, all congregate living facilities have proved particularly vulnerable to the effects of COVID-19,2-7 with residents at increased risk for disease severity and mortality.2-7
Due to the COVID-19 emergency, NorthShore University HealthSystem (NUHS), a multihospital, integrated health system in northern Illinois, established a best practice for appropriate use of congregate living facilities after hospitalization. This focused on the safety of discharged patients and mitigation of COVID-19 by putting in place a referral process to a newly established congregate living review committee (CLRC) for review prior to discharge. Although all discharges to congregate living settings are at high risk,2 new placements to skilled nursing facilities (SNFs) were the primary focus of the committee and the sole focus of this study. In this study, we sought to determine whether establishment of the CLRC was associated with a reduction in SNF utilization, whether this was safe and efficient, and whether it was associated with a reduction in COVID-19 incidence in the 30 days following discharge.
METHODS
Setting and Case Review Intervention
We conducted a retrospective cohort study for patients hospitalized within NUHS from March 19, 2019 to July 16, 2020, designed as an interrupted time series. The study was approved by the NUHS Institutional Review Board (EH21-022).
The study exposure was creation of a referral and review process for all patients with expected discharge to a SNF and was implemented as part of usual discharge planning during the COVID-19 pandemic. The key intervention was to establish a multidisciplinary committee, the CLRC, to review all potential discharges to SNFs. The CLRC had dual goals of preventing COVID-19 spread in facilities by limiting placement of new residents and protecting a vulnerable population from a setting that conferred a higher risk of acquiring COVID-19. The CLRC was organized as a multidisciplinary committee with physicians, case managers, social workers, physical therapists, occupational therapists, and the director of NUHS home health agency. Physician members were evenly split as half hospitalists and half ambulatory physicians. The CLRC review was initiated by a patient’s assigned case manager or social worker by consult through a referral in the electronic medical record (EMR). Each case was summarized and then presented to the full CLRC. The CLRC met for 1 hour per day, 6 days per week, to review all planned discharges that met criteria for review. A committee physician chaired each meeting. Three other members were needed for a quorum, with one other member with a title of director or higher. Time required was the 1-hour daily meeting, as well as one full-time position for case review, preparation, and program administration. The case presentation included a clinical summary of the hospitalization as well as COVID-19 status and testing history, previous living situation, level of home support, functional level, psychosocial needs, barrier(s) to discharging home, and long-term residential plans. A structured assessment was then made by each CLRC member in accordance with their professional expertise. Unanimous consensus would be reached before finalizing any recommended adjustments to the discharge, which would be communicated to the inpatient care team via a structured note within the EMR, along with direct communication to the assigned case manager or social worker. When the CLRC suggested adjustments to the discharge, they would work with the assigned case manager or social worker to communicate an appropriate post–acute care plan with the patient or appropriate representative. If there was disagreement or the recommendations could not be followed, the case manager or social worker would place a new referral with additional information for reconsideration. Following a recommendation for SNF, verification would be completed by the CLRC prior to discharge. This process is detailed in Figure 1.
Patient Population
Inclusion criteria for the study were: (1) inpatient hospitalization and (2) eligibility for risk scoring via the organization’s clinical analytics prediction engine (CAPE).8 CAPE is a validated predictive model that includes risk of readmission, in-hospital mortality, and out-of-hospital mortality,8 with extensive adoption at NUHS. CAPE score eligibility was used as an inclusion criterion so that CAPE could be applied for derivation of a matched control. CAPE eligibility criteria include admission age of at least 18 years and that hospitalization is not psychiatric, rehabilitative, or obstetric. Patients must not be enrolled in hospice and must be discharged alive.
Exclusions were patients who tested positive for SARS-CoV-2 prior to or during index hospitalization. Excluding COVID-19 patients from the analysis eliminated a confounder not present in the preintervention group.
For patients with multiple inpatient admissions, the first admission was the only admission used for analysis. Additionally, if a patient had an admission that occurred in both the preintervention and postintervention periods, they were included only in the postintervention period. This was done to avoid any within-subject correlation and ensure unique patients in each group. Confounding from this approach was mitigated through the process of deriving a matched control.
Outcomes Measurement
The primary outcome of interest was total discharges to SNF across NUHS facilities after hospital admission. Patients were identified as discharging to a SNF if discharge destination codes 03, 64, or 83 appeared on the hospital bill. Additionally, new discharges to SNFs were assessed and identified if documentation indicated that the patient’s living arrangement prior to admission was not a SNF but discharge billing destination codes 03, 64, or 83 appeared on the hospital bill.
Secondary outcomes were measurement of readmissions, days to readmission, and median length of stay (LOS). Readmissions and LOS were balancing measures for the primary outcome, with readmissions measured to evaluate the safety of the CLRC process and LOS measured to evaluate its efficiency. A readmission was any patient who had an unplanned inpatient admission at an NUHS facility within 30 days after an index admission. LOS was measured in days from arrival on a hospital unit to time of discharge.
Additional analysis was done to estimate the effect of the intervention on the incidence of COVID-19 in the 30 days following discharge by comparing the observed to expected incidence of COVID-19 by discharge destination. The expected values were derived by estimating COVID-19 cases that would have been expected to occur with rates of preintervention SNF utilization. This was accomplished by multiplying the observed incidence of COVID-19 in the 30 days following discharge by the number of patients who were discharged to SNFs or home/other in the preintervention period. This expected value was then compared with the observed values to estimate the effect size of the intervention on COVID-19 incidence following discharge. This method of deriving an expected value from the observed incidence was utilized because the preintervention period was before COVID-19 was widespread in the community. It was therefore not possible to directly measure COVID-19 incidence in the preintervention period.
Data Source
Data were retrieved from the NUHS Enterprise Data Warehouse, NUHS’s central data repository, which contains a nightly upload of clinical and financial data from the EMR. Data were collected between March 19, 2019, and July 16, 2020.
The preintervention period was defined as March 19, 2019, to March 18, 2020. Data from that interval were compared with the postintervention period, which was from March 19, 2020, to July 16, 2020. The preintervention period, 1 year immediately prior to the intervention, was chosen to limit any effect of temporal trends while also providing a large sample size. The postintervention period began on the first day NUHS implemented the revised approach to SNF use and ended on the last day before the review process was modified.
Data Analysis
An interrupted time series was used to measure the impact of adoption of the CLRC protocol. A matched control was derived from the preintervention population. To derive this matched control, there was an assessment of covariates in the preintervention and postintervention groups using a standardized mean difference (SMD)9 that indicated an imbalance (SMD ≥ 0.1) in some covariates. A propensity score–matching technique10 was applied to address this imbalance and lack of randomization.
The candidate variables for propensity matching were chosen if they had an association with 30-day readmission. Readmission was chosen to find candidate variables because, of the possible outcomes, this was the only one that was not directly impacted by any CLRC decision. Each covariate was assessed using a logistic regression model while controlling for the postintervention group. If there was an association between a covariate and the outcome, it was chosen for propensity matching. Propensity scores were calculated using a logistic regression model with the treatment (1/0) variable as the dependent variable and the chosen covariates as predictors.
There were no indications of strong multicollinearity. The propensity scores generated were then used to derive a matched control using paired matching. MatchIt package in R (R Foundation for Statistical Computing) was used to create a matched dataset with a logit distance and standard caliper of 0.2 times the standard deviations of the logit of the propensity score. If a match was not found within the caliper, the nearest available match was used.
Regression adjustment11 was then performed using multivariate linear/logistic regression with LOS, readmission rate, days to readmission, total SNF discharges, and new SNF discharges as the outcomes. Treatment (1/0) variable and propensity score were used as the predictors. The adjusted coefficients or odds ratios (ORs) of the intervention variable were thus derived, and their associated P values were used to assess the impact of the intervention on the respective outcomes.
RESULTS
The unmatched preintervention population included 14,468 patients, with 4424 patients in the postintervention population. A matched population was derived and, after matching, the population sizes for pre and post intervention were 4424 each. In the matched population, all measured preintervention characteristics had SMDs and P values that were statistically equivalent. Patient characteristics for the unmatched and matched populations are detailed in Table 1.
During the preintervention period, 1130 (25.5%) patients were discharged to a SNF, with 776 (17.5%) patients being new SNF discharges. In the postintervention period, 568 (12.8%) patients were discharged to a SNF, with 257 (5.8%) patients being new SNF discharges. Total SNF discharges postintervention saw a 49.7% relative reduction (OR, 0.42; 95% CI, 0.38-0.47), while new SNF discharges saw a 66.9% relative reduction (OR, 0.29; 95% CI, 0.25-0.34). These results for both total and new SNF discharges were statistically significant, with P values of <.001, respectively.
Readmissions in the preintervention period were 529 (12.0%) patients, compared with 559 (12.6%) patients in the postintervention period (OR, 1.06; 95% CI, 0.93-1.20; P =.406). An OR was also calculated for readmissions, adjusting for discharge disposition, to account for changes observed in SNF use in the postintervention period. This OR was 1.11 (95% CI, 0.97-1.26; P = .131). Days to readmission in the preintervention and postintervention groups were 11.0 days and 12.0 days, respectively (OR, 0.41; 95% CI, –0.61 to 1.43; P = .429).
LOS was 3.61 days in the preintervention group and 3.64 days in the postintervention group, with an interquartile range (IQR) of 2.14 to 5.69 days in the preintervention group and 2.08 to 5.95 in the postintervention group (OR, 0.09; 95% CI, –0.09 to 0.27; P =.316). These results are summarized in Table 2.
DISCUSSION
A COVID-19 outbreak in a SNF presents a grave risk to residents and patients discharged to these facilities. It is critical for healthcare systems to do the utmost to protect the health of this vulnerable population and the public in efforts to limit COVID-19 within SNFs.12-14
In this study, we observed that at NUHS, establishing a multidisciplinary review committee, the CLRC, to assess the appropriateness of discharge to a SNF after hospitalization resulted in a nearly 50% reduction in total SNF discharges and a greater than two-thirds reduction in new SNF discharges, without any increase in LOS or readmissions. Additionally, it was observed that discharging to settings other than a SNF greatly reduced a patient’s risk of being diagnosed with COVID-19 within 30 days, a result that reached statistical significance. Based on the observed 37.2% relative reduction in COVID-19 cases, we estimate that there may have been one COVID-19 infection prevented every 5.6 days from this intervention. Based on published COVID-19 mortality rates for SNF residents,1 the intervention may have prevented one death every 2.6 weeks. Beyond the risk of COVID-19, other benefits of reducing SNF use are patient and family well-being. Although not measured in this study, others have published about the significant psychological burdens placed on SNF residents, who were at high risk for social isolation, anxiety, and depression during the COVID-19 pandemic2,15-19 Family members also may have had increased stress, as they were deprived of the opportunity to visit loved ones, advocate for them, and help maintain their identity, humanity, and quality of life.20
Although other hospitals have established a structured approach to reduce COVID-19 in SNFs,21 to the best of the authors’ knowledge, the approach described in this article is a unique response to the COVID-19 pandemic. As we have demonstrated, it is highly effective and safe and likely prevented many COVID-19 cases and deaths.
Furthermore, a review committee, such as the one we have described, has value well beyond the COVID-19 pandemic. The health and affordability of care for patients, provider success in value-based care models, and the long-term sustainability of the US healthcare system require close attention to appropriate use of expensive services and to ensuring that their use creates high value. SNF use after a hospitalization is one such service that is frequently targeted and thought to contribute to a substantial portion of wasteful medical spending.22,23 Additionally, SNFs are known to be high risk for communicable disease outbreaks other than COVID-19,24,25 as well as a high-risk environment for many other preventable adverse events.25,26 This review committee ultimately serves to help determine the most appropriate postacute setting for patients being discharged with a determination made through considerations for patient safety, rehabilitation potential, and mental and physical well-being. From a population health perspective, this can lead to better outcomes and lower costs.22,23 Therefore, although the risks of COVID-19 infection in SNFs are expected to subside, the work of evaluating appropriate use of SNFs after hospitalization at our institution continues. The broader focus now extends beyond postacute level of service toward ensuring a high-value discharge that results in both appropriate resource use and safe patient care transitions.
Limitations of this study include its retrospective nature, results from a single center, and a number of potentially unmeasured confounders that the COVID-19 pandemic created. One possible confounder is that the reduction in SNF use we observed was a temporal trend related to changing preferences. In addressing this, we reviewed Medicare claims data from the US Department of Health and Human Services in April 2020 and July 2020 compared with the same period in 2019. These data demonstrated only a modest reduction in spending on SNFs in April 2020 that was smaller than the reduction seen in Part A inpatient hospital spending during that same month.27 By July 2020, the spending from Medicare on SNFs exceeded the levels seen in 2019,27 suggesting that the percentage of acute care admissions discharging to SNFs was no lower for Medicare patients in response to COVD-19. We also considered more stringent SNF admission standards as another potential confounder; however, this was not seen at the SNFs in the NUHS geography, where the referral process became less stringent because of COVID-19 waivers for a qualifying stay or skilled need from the Centers for Medicare and Medicaid Services. We were also not able to account for readmissions outside of NUHS, and therefore there may have been differences in the readmission rate that were unmeasured. To address this limitation, we reviewed a data extract from the Illinois Health and Hospital Association and found that the percentage of patients who returned for readmission to a NUHS facility in the year prior to the intervention and during the intervention period were 92.8% and 95.3%, respectively. From this we concluded the unmeasured readmission rate appears to be low, stable, and unlikely to have altered the results of this study. Additionally, when calculating potential COVID-19 cases avoided, the expected number was, by necessity, derived from the observed outcome, given the absence of COVID-19 in the preintervention population. This may have introduced unmeasured confounders, limiting the ability to precisely measure the effect size or draw conclusions on causation. Finally, there may be limitations to the generalizability of these results based on the payor mix of the population at NUHS, which is predominantly insured through Medicare or commercial payors.
CONCLUSION
We believe this model is replicable and the results generalizable and could serve as both a template for reducing the risks of COVID-19 in SNFs and as part of a larger infection-control strategy to mitigate disease spread in vulnerable populations. It could also be applied as a component of value-improvement programs to foster appropriate use of postacute services after an acute care hospitalization, ensuring safe transitions of care through promotion of high-value care practices.
Acknowledgment
The authors thank Wei Ning Chi for editorial assistance.
1. McMichael TM, Currie DW, Clark S, et al. Epidemiology of Covid-19 in a long-term care facility in King County, Washington. N Engl J Med. 2020;382(21):2005-2011. https://doi.org/10.1056/NEJMoa2005412
2. Ouslander JG, Grabowski DC. COVID-19 in nursing homes: calming the perfect storm. J Am Geriatr Soc. 2020;68(10):2153-2162. https://doi.org/10.1111/jgs.16784
3. CDC COVID-19 Response Team. Severe outcomes among patients with coronavirus disease 2019 (COVID-19) - United States, February 12-March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(12):343-346. https://doi.org/10.15585/mmwr.mm6912e2
4. Ko JY, Danielson ML, Town M, et al. Risk factors for coronavirus disease 2019 (COVID-19)-associated hospitalization: COVID-19-Associated Hospitalization Surveillance Network and Behavioral Risk Factor Surveillance System. Clin Infect Dis. 2020;72(11):e695-e703. https://doi.org/10.1093/cid/ciaa1419
5. Davidson PM, Szanton SL. Nursing homes and COVID-19: we can and should do better. J Clin Nurs. 2020;29(15-16):2758-2759. https://doi.org/10.1111/jocn.15297
6. Dosa D, Jump RLP, LaPlante K, Gravenstein S. Long-term care facilities and the coronavirus epidemic: practical guidelines for a population at highest risk. J Am Med Dir Assoc. 2020;21(5):569-571. https://doi.org/10.1016/j.jamda.2020.03.004
7. Fallon A, Dukelow T, Kennelly SP, O’Neill D. COVID-19 in nursing homes. QJM. 2020;113(6):391-392. https://doi.org/10.1093/qjmed/hcaa136
8. Shah N, Konchak C, Chertok D, et al. Clinical Analytics Prediction Engine (CAPE): development, electronic health record integration and prospective validation of hospital mortality, 180-day mortality and 30-day readmission risk prediction models. PLoS One. 2020;15(8):e0238065. https://doi.org/10.1371/journal.pone.0238065
9. Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput. 2009;38(6):1228-1234. https://doi.org/10.1080/03610910902859574
10. Rosenbaum PR, Rubin DB. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat. 1985;39(1):33-38. https://doi.org/10.2307/2683903
11. Myers JA, Louis TA. Regression adjustment and stratification by propensity score in treatment effect estimation. Johns Hopkins University, Dept of Biostatistics Working Papers. 2010 203(Working Papers):1-27.
12. Lansbury LE, Brown CS, Nguyen-Van-Tam JS. Influenza in long-term care facilities. Influenza Other Respir Viruses. 2017;11(5):356-366. https://doi.org/10.1111/irv.12464
13. Sáez-López E, Marques R, Rodrigues N, et al. Lessons learned from a prolonged norovirus GII.P16-GII.4 Sydney 2012 variant outbreak in a long-term care facility in Portugal, 2017. Infect Control Hosp Epidemiol. 2019;40(10):1164-1169. https://doi.org/10.1017/ice.2019.201
14. Gaspard P, Mosnier A, Stoll-Keller F, Roth C, Larocca S, Bertrand X. Influenza prevention in nursing homes: great significance of seasonal variability and spatio-temporal pattern. Presse Med. 2015;44(10):e311-e319. https://doi.org/10.1016/j.lpm.2015.04.041
15. Pfefferbaum B, North CS. Mental health and the Covid-19 pandemic. N Engl J Med. 2020;383(6):510-512. https://doi.org/10.1056/NEJMp2008017
16. Galea S, Merchant RM, Lurie N. The mental health consequences of COVID-19 and physical distancing: the need for prevention and early intervention. JAMA Intern Med. 2020;180(6):817-818. https://doi.org/10.1001/jamainternmed.2020.1562
17. Armitage R, Nellums LB. COVID-19 and the consequences of isolating the elderly. Lancet Public Health. 2020;5(5):e256. https://doi.org/10.1016/s2468-2667(20)30061-x
18. El Haj M, Altintas E, Chapelet G, Kapogiannis D, Gallouj K. High depression and anxiety in people with Alzheimer’s disease living in retirement homes during the covid-19 crisis. Psychiatry Res. 2020;291:113294. https://doi.org/10.1016/j.psychres.2020.113294
19. Santini ZI, Jose PE, York Cornwell E, et al. Social disconnectedness, perceived isolation, and symptoms of depression and anxiety among older Americans (NSHAP): a longitudinal mediation analysis. Lancet Public Health. 2020;5(1):e62-e70. https://doi.org/10.1016/s2468-2667(19)30230-0
20. Gaugler JE, Anderson KA, Zarit SH, Pearlin LI. Family involvement in nursing homes: effects on stress and well-being. Aging Ment Health. 2004;8(1):65-75. https://doi.org/10.1080/13607860310001613356
21. Kim G, Wang M, Pan H, et al. A health system response to COVID-19 in long-term care and post-acute care: a three-phase approach. J Am Geriatr Soc. 2020;68(6):1155-1161. https://doi.org/10.1111/jgs.16513
22. McWilliams JM, Gilstrap LG, Stevenson DG, Chernew ME, Huskamp HA, Grabowski DC. Changes in postacute care in the Medicare Shared Savings Program. JAMA Intern Med. 2017;177(4):518-526. https://doi.org/10.1001/jamainternmed.2016.9115
23. Ackerly DC, Grabowski DC. Post-acute care reform--beyond the ACA. N Engl J Med. 2014;370(8):689-691. https://doi.org/10.1056/NEJMp1315350
24. Strausbaugh LJ, Sukumar SR, Joseph CL. Infectious disease outbreaks in nursing homes: an unappreciated hazard for frail elderly persons. Clin Infect Dis. 2003;36(7):870-876. https://doi.org/10.1086/368197
25. Kapoor A, Field T, Handler S, et al. Adverse events in long-term care residents transitioning from hospital back to nursing home. JAMA Intern Med. 2019;179(9):1254-1261. https://doi.org/10.1001/jamainternmed.2019.2005
26. Adverse Events in Skilled Nursing Facilities: National Incidence Among Medicare Beneficiaries. Office of Inspector General, US Dept of Health & Human Services; 2014.
27. The Impact of the COVID-19 Pandemic on Medicare Beneficiary Use of Health Care Services and Payments to Providers: Early Data for the First 6 Months of 2020. Office of the Assistant Secretary for Planning and Evaluation, US Dept of Health & Human Services; 2020.
1. McMichael TM, Currie DW, Clark S, et al. Epidemiology of Covid-19 in a long-term care facility in King County, Washington. N Engl J Med. 2020;382(21):2005-2011. https://doi.org/10.1056/NEJMoa2005412
2. Ouslander JG, Grabowski DC. COVID-19 in nursing homes: calming the perfect storm. J Am Geriatr Soc. 2020;68(10):2153-2162. https://doi.org/10.1111/jgs.16784
3. CDC COVID-19 Response Team. Severe outcomes among patients with coronavirus disease 2019 (COVID-19) - United States, February 12-March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(12):343-346. https://doi.org/10.15585/mmwr.mm6912e2
4. Ko JY, Danielson ML, Town M, et al. Risk factors for coronavirus disease 2019 (COVID-19)-associated hospitalization: COVID-19-Associated Hospitalization Surveillance Network and Behavioral Risk Factor Surveillance System. Clin Infect Dis. 2020;72(11):e695-e703. https://doi.org/10.1093/cid/ciaa1419
5. Davidson PM, Szanton SL. Nursing homes and COVID-19: we can and should do better. J Clin Nurs. 2020;29(15-16):2758-2759. https://doi.org/10.1111/jocn.15297
6. Dosa D, Jump RLP, LaPlante K, Gravenstein S. Long-term care facilities and the coronavirus epidemic: practical guidelines for a population at highest risk. J Am Med Dir Assoc. 2020;21(5):569-571. https://doi.org/10.1016/j.jamda.2020.03.004
7. Fallon A, Dukelow T, Kennelly SP, O’Neill D. COVID-19 in nursing homes. QJM. 2020;113(6):391-392. https://doi.org/10.1093/qjmed/hcaa136
8. Shah N, Konchak C, Chertok D, et al. Clinical Analytics Prediction Engine (CAPE): development, electronic health record integration and prospective validation of hospital mortality, 180-day mortality and 30-day readmission risk prediction models. PLoS One. 2020;15(8):e0238065. https://doi.org/10.1371/journal.pone.0238065
9. Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput. 2009;38(6):1228-1234. https://doi.org/10.1080/03610910902859574
10. Rosenbaum PR, Rubin DB. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat. 1985;39(1):33-38. https://doi.org/10.2307/2683903
11. Myers JA, Louis TA. Regression adjustment and stratification by propensity score in treatment effect estimation. Johns Hopkins University, Dept of Biostatistics Working Papers. 2010 203(Working Papers):1-27.
12. Lansbury LE, Brown CS, Nguyen-Van-Tam JS. Influenza in long-term care facilities. Influenza Other Respir Viruses. 2017;11(5):356-366. https://doi.org/10.1111/irv.12464
13. Sáez-López E, Marques R, Rodrigues N, et al. Lessons learned from a prolonged norovirus GII.P16-GII.4 Sydney 2012 variant outbreak in a long-term care facility in Portugal, 2017. Infect Control Hosp Epidemiol. 2019;40(10):1164-1169. https://doi.org/10.1017/ice.2019.201
14. Gaspard P, Mosnier A, Stoll-Keller F, Roth C, Larocca S, Bertrand X. Influenza prevention in nursing homes: great significance of seasonal variability and spatio-temporal pattern. Presse Med. 2015;44(10):e311-e319. https://doi.org/10.1016/j.lpm.2015.04.041
15. Pfefferbaum B, North CS. Mental health and the Covid-19 pandemic. N Engl J Med. 2020;383(6):510-512. https://doi.org/10.1056/NEJMp2008017
16. Galea S, Merchant RM, Lurie N. The mental health consequences of COVID-19 and physical distancing: the need for prevention and early intervention. JAMA Intern Med. 2020;180(6):817-818. https://doi.org/10.1001/jamainternmed.2020.1562
17. Armitage R, Nellums LB. COVID-19 and the consequences of isolating the elderly. Lancet Public Health. 2020;5(5):e256. https://doi.org/10.1016/s2468-2667(20)30061-x
18. El Haj M, Altintas E, Chapelet G, Kapogiannis D, Gallouj K. High depression and anxiety in people with Alzheimer’s disease living in retirement homes during the covid-19 crisis. Psychiatry Res. 2020;291:113294. https://doi.org/10.1016/j.psychres.2020.113294
19. Santini ZI, Jose PE, York Cornwell E, et al. Social disconnectedness, perceived isolation, and symptoms of depression and anxiety among older Americans (NSHAP): a longitudinal mediation analysis. Lancet Public Health. 2020;5(1):e62-e70. https://doi.org/10.1016/s2468-2667(19)30230-0
20. Gaugler JE, Anderson KA, Zarit SH, Pearlin LI. Family involvement in nursing homes: effects on stress and well-being. Aging Ment Health. 2004;8(1):65-75. https://doi.org/10.1080/13607860310001613356
21. Kim G, Wang M, Pan H, et al. A health system response to COVID-19 in long-term care and post-acute care: a three-phase approach. J Am Geriatr Soc. 2020;68(6):1155-1161. https://doi.org/10.1111/jgs.16513
22. McWilliams JM, Gilstrap LG, Stevenson DG, Chernew ME, Huskamp HA, Grabowski DC. Changes in postacute care in the Medicare Shared Savings Program. JAMA Intern Med. 2017;177(4):518-526. https://doi.org/10.1001/jamainternmed.2016.9115
23. Ackerly DC, Grabowski DC. Post-acute care reform--beyond the ACA. N Engl J Med. 2014;370(8):689-691. https://doi.org/10.1056/NEJMp1315350
24. Strausbaugh LJ, Sukumar SR, Joseph CL. Infectious disease outbreaks in nursing homes: an unappreciated hazard for frail elderly persons. Clin Infect Dis. 2003;36(7):870-876. https://doi.org/10.1086/368197
25. Kapoor A, Field T, Handler S, et al. Adverse events in long-term care residents transitioning from hospital back to nursing home. JAMA Intern Med. 2019;179(9):1254-1261. https://doi.org/10.1001/jamainternmed.2019.2005
26. Adverse Events in Skilled Nursing Facilities: National Incidence Among Medicare Beneficiaries. Office of Inspector General, US Dept of Health & Human Services; 2014.
27. The Impact of the COVID-19 Pandemic on Medicare Beneficiary Use of Health Care Services and Payments to Providers: Early Data for the First 6 Months of 2020. Office of the Assistant Secretary for Planning and Evaluation, US Dept of Health & Human Services; 2020.
© 2021 Society of Hospital Medicine
Things We Do for No Reason™: Tumor Markers CA125, CA19-9, and CEA in the Initial Diagnosis of Malignancy
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 56-year-old woman presents to the emergency department with a 2-week history of abdominal pain associated with nausea and an episode of nonbilious, nonbloody emesis. Her last bowel movement was 2 days prior to her presentation. The patient has tachycardia to 105 beats per minute but otherwise normal vital signs. Findings on her physical examination include dry mucous membranes and increased bowel sounds. A review of systems reveals an unintentional weight loss of 15 kg over the past 4 months and increased fatigue. Computed tomography scan of the abdomen and pelvis with contrast reveals multiple areas of attenuation in the liver and small bowel obstruction. The hospitalist admits the patient to the medicine service for supportive treatment and workup for underlying malignancy. Her admitting team orders serum tumor biomarkers on admission to expedite the diagnosis.
BACKGROUND
When patients present with unexplained weight loss or with metastasis from an unknown primary location, the initial workup often includes imaging and a tumor biomarker panel (eg, cancer antigen 125 [CA125], carbohydrate antigen 19-9 [CA19-9], carcinoembryonic antigen [CEA]). The CA125, CA19-9, and CEA biomarkers are traditionally associated with ovarian, pancreatic, and colorectal cancer, respectively.1 While clinicians initially used these serum biomarkers to monitor for cancer recurrence or treatment response, they have since become widely used in multiple clinical stages of oncological evaluation.
WHY YOU MIGHT THINK CA125, CA19-9, AND CEA ARE HELPFUL IN THE DIAGNOSIS OF CANCER
Hospitalists routinely order biomarkers as part of the malignancy workup. More than a dozen oncology biomarkers are used in the clinical setting to risk stratify, plan treatment, and monitor for recurrence. For example, studies associate elevated preoperative levels of CEA and CA19-9 with metastatic invasion of colorectal2 and gastric3 cancers and with poor prognosis of intrahepatic cholangiocarcinoma. Similarly, CA125 has demonstrated utility in monitoring response to ovarian cancer treatment.4 Specific biomarkers, such as alpha-fetoprotein, improve diagnosis of liver and nonseminomatous testicular tumors.5 Clinicians often apply the same paradigm to other biomarkers due to their widespread availability, noninvasiveness, reproducibility, and ease of use, particularly in acute settings wherein any new information is perceived to be potentially helpful.
WHY YOU SHOULD NOT USE CA125, CA19-9, AND CEA TO DIAGNOSE CANCER
Utilizing these serum biomarkers to diagnose cancer has the potential for diagnostic error and can result in unnecessary patient anxiety and follow-up testing. Since tissue sampling is necessary and remains the gold standard in most cancer diagnoses, obtaining these tumor biomarkers in the early diagnostic stage does not change management and may even lead to harm. Furthermore, due to their poor sensitivity and specificity, these biomarkers cannot rule in or rule out cancer. Elevated CA125, CA19-9, and CEA biomarkers occur in a variety of malignancies, including gastric, gallbladder, hepatocellular, bladder, and breast cancers.1,3,6 In addition, these biomarkers have a very limited role in the workup of cancer of unknown primary origin.7
Even in the setting of a known pelvic mass, the use of CA125 alone has poor sensitivity at a cut-off level of 35 U/mL as a biomarker for the diagnosis of early ovarian cancer.8
Serum CA19-9 is not a useful diagnostic biomarker as elevated CA19-9 can occur in benign conditions, including cirrhosis, chronic pancreatitis, and cholangitis. In a systematic review of patients with histologic confirmation of pancreatic malignancy, the median positive predictive value of CA19-9 was 72% (interquartile range, 41%-95%).9 Additionally, patients with Lewis-null blood type, which is present in 5% to 10% of the Caucasian population, do not produce CA19-9.10 Therefore, CA19-9 will be 0% specific for tumors in this population.
The use of CEA in the diagnosis of colorectal cancer is also questionable. In stage I colorectal cancer, CEA was only 38.1% sensitive at a cut-off level of 2.41 ng/mL; it was 78.3% sensitive in stage IV disease.11 The specificity of CEA is limited since elevated CEA occurs in benign conditions, such as inflammatory bowel disease, smoking, hypothyroidism, pancreatitis, biliary obstruction, peptic ulcers, and cirrhosis—though CEA levels in these conditions are rarely >10 ng/mL.11 Regardless of the results of biomarker testing, definitive diagnosis requires tissue biopsy; therefore, biomarker findings are of little utility in the initial workup.
In addition to variable diagnostic utility, overreliance on these biomarkers has the potential for serious patient harm. In a study examining patients with established rectal cancer, combination CEA and CA19-9 testing alone was insufficient to predict the pathologic stage of disease correctly.2 A cancer misdiagnosis not only traumatizes patients but also erodes their trust in clinicians and creates anxiety during future clinical encounters. Overutilization of these tumor biomarkers is also costly and contributes to waste in the US healthcare system.
WHEN YOU SHOULD USE CA125, CA19-9, AND CEA
There is a role for tumor biomarker testing in specific cancers after the primary source of malignancy has been determined. When evaluating a known pelvic mass, CA125 testing is performed in conjunction with transvaginal ultrasound and assessment of menopausal status in the risk of ovarian malignancy algorithm for prognostication of disease prior to surgery.12 This algorithm takes into account levels of CA125 in addition to levels of human epididymis protein 4 and patient age, yielding an area under the curve as high as 0.93 for ovarian cancer risk classification.8 Beyond the prognostication process, oncologists follow CA125 to monitor response to first-line ovarian cancer treatment. However, CA125 has a less defined role in surveillance for ovarian cancer recurrence.
CA19-9 has demonstrated utility for pancreatic cancer and cholangiocarcinoma survival estimates. A national cohort analysis of patients with established intrahepatic cholangiocarcinoma found that CA19-9 independently predicted increased mortality. Patients with elevated CA19-9 also had significantly more nodal metastases and positive-margin resections.6 A study of 353 patients with pancreatic ductal adenocarcinoma undergoing radical resection further demonstrated the utility of CA19-9. In this study, patients with postoperative CA19-9 normalization had improved survival by almost 12 months when compared to those with consistently elevated CA19-9.13
Last, the literature describes CEA biomarker testing in the surveillance of patients after curative treatment of colon and rectal cancer. The American Society of Colon and Rectal Surgeons recommends regularly tracking this biomarker following curative resection, in conjunction with colonoscopy and chest and liver imaging studies.14 A prospective randomized controlled study that followed this monitoring protocol in cured asymptomatic patients on a bimonthly basis found that early diagnosis of recurrent colorectal cancer improved survival.15 The use of CEA testing as a monitoring tool should therefore be a point of discussion between providers and patients, as its utility varies based on patient comorbidities, their ability to tolerate surgery or chemotherapy, risk factors for recurrence, performance status, compliance, age, and preference.14
WHAT YOU SHOULD DO INSTEAD
The use of CA125, CA19-9, and CEA testing alone as initial diagnostic tools for malignancy are problematic due to their poor sensitivities and/or positive predictive value. Multiple studies have demonstrated their utility as markers of metastasis or malignancy progression rather than as clinically useful markers for the detection of any one type of cancer.1,3,6 In an undiagnosed symptomatic patient with unexplained weight loss or symptoms of a tumor mass, elevated CA125, CA19-9, and CEA add no new information as metastatic pancreatic, colorectal, ovarian, gastric, gallbladder, hepatocellular, bladder, ovarian, and breast cancers all remain in the differential diagnosis. Clinicians should approach the initial diagnosis of cancer in such patients with appropriate imaging studies, a thorough physical examination, and prompt biopsy of abnormal findings, as long as these are consistent with the patient’s goals of care. After establishing a tissue diagnosis, some tumor biomarkers have valid prognostic, staging, and monitoring roles.6,13,14
RECOMMENDATIONS
- Do not routinely order CA125, CA19-9, and CEA tests for the initial diagnostic workup of visceral malignancy of unknown origin regardless of whether imaging studies have been obtained.
- Use appropriate imaging, perform a thorough physical examination, and obtain tissue biopsy in the initial diagnostic workup of a visceral malignancy of unknown origin.
CONCLUSION
Clinicians should use serum biomarkers, like any other diagnostic test, to maximize benefit while preventing patient harm. In general, CA125, CA19-9, and CEA do not have a role in cancer diagnosis. The patient described in our clinical scenario would not benefit from a serum tumor biomarker panel at the time of admission. Regardless of findings from these tests, a tissue sample is required to make a definitive diagnosis of underlying malignancy in this patient.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing [email protected]
1. Yotsukura S, Mamitsuka H. Evaluation of serum-based cancer biomarkers: a brief review from a clinical and computational viewpoint. Crit Rev Oncol Hematol. 2015;93(2):103-115. https://doi.org/10.1016/j.critrevonc.2014.10.002
2. Zhang B, Sun Z, Song M, et al. Ultrasound/CT combined with serum CEA/CA19.9 in the diagnosis and prognosis of rectal cancer. J Buon. 2018;23(3):592-597.
3. Zhou YC, Zhao HJ, Shen LZ. Preoperative serum CEA and CA19-9 in gastric cancer--a single tertiary hospital study of 1,075 cases. Asian Pac J Cancer Prev. 2015;16(7):2685-2691. https://doi.org/10.7314/apjcp.2015.16.7.2685
4. Karam AK, Karlan BY. Ovarian cancer: the duplicity of CA125 measurement. Nat Rev Clin Oncol. 2010;7(6):335-339. https://doi.org/10.1038/nrclinonc.2010.44
5. Gilligan TD, Seidenfeld J, Basch EM, et al; American Society of Clinical Oncology. American Society of Clinical Oncology Clinical Practice Guideline on uses of serum tumor markers in adult males with germ cell tumors. J Clin Oncol. 2010;28(20):3388-3404. https://doi.org/10.1200/jco.2009.26.4481
6. Bergquist JR, Ivanics T, Storlie CB, et al. Implications of CA19-9 elevation for survival, staging, and treatment sequencing in intrahepatic cholangiocarcinoma: a national cohort analysis. J Surg Oncol. 2016;114(4):475-482. https://doi.org/10.1002/jso.24381
7. Milovic M, Popov I, Jelic S. Tumor markers in metastatic disease from cancer of unknown primary origin. Med Sci Monit. 2002;8(2):MT25-MT30.
8. Dochez V, Caillon H, Vaucel E, Dimet J, Winer N. Biomarkers and algorithms for diagnosis of ovarian cancer: CA125, HE4, RMI and ROMA, a review. J Ovarian Res. 2019;12(1):28. https://doi.org/10.1186/s13048-019-0503-7
9. Goonetilleke KS, Siriwardena AK. Systematic review of carbohydrate antigen (CA 19-9) as a biochemical marker in the diagnosis of pancreatic cancer. Eur J Surg Oncol. 2007;33(3):266-270. https://doi.org/10.1016/j.ejso.2006.10.004
10. Loosen SH, Neumann UP, Trautwein C, Roderburg C, Luedde T. Current and future biomarkers for pancreatic adenocarcinoma. Tumour Biol. 2017;39(6):1010428317692231. https://doi.org/10.1177/1010428317692231
11. Polat E, Duman U, Duman M, et al. Diagnostic value of preoperative serum carcinoembryonic antigen and carbohydrate antigen 19-9 in colorectal cancer. Curr Oncol. 2014;21(1):e1-e7. https://doi.org/10.3747/co.21.1711
12. Sölétormos G, Duffy MJ, Othman Abu Hassan S, et al. Clinical use of cancer biomarkers in epithelial ovarian cancer: updated guidelines from the European Group on Tumor Markers. Int J Gynecol Cancer. 2016;26(1):43-51. https://doi.org/10.1097/igc.0000000000000586
13. Xu HX, Liu L, Xiang JF, et al. Postoperative serum CEA and CA125 levels are supplementary to perioperative CA19-9 levels in predicting operative outcomes of pancreatic ductal adenocarcinoma. Surgery. 2017;161(2):373-384. https://doi.org/10.1016/j.surg.2016.08.005
14. Steele SR, Chang GJ, Hendren S, et al. Practice guideline for the surveillance of patients after curative treatment of colon and rectal cancer. Dis Colon Rectum. 2015;58(8):713-725. https://doi.org/10.1097/dcr.0000000000000410
15. Verberne CJ, Zhan Z, van den Heuvel E, et al. Intensified follow-up in colorectal cancer patients using frequent Carcino-Embryonic Antigen (CEA) measurements and CEA-triggered imaging: results of the randomized “CEAwatch” trial. Eur J Surg Oncol. 2015;41(9):1188-1196. https://doi.org/10.1016/j.ejso.2015.06.008
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 56-year-old woman presents to the emergency department with a 2-week history of abdominal pain associated with nausea and an episode of nonbilious, nonbloody emesis. Her last bowel movement was 2 days prior to her presentation. The patient has tachycardia to 105 beats per minute but otherwise normal vital signs. Findings on her physical examination include dry mucous membranes and increased bowel sounds. A review of systems reveals an unintentional weight loss of 15 kg over the past 4 months and increased fatigue. Computed tomography scan of the abdomen and pelvis with contrast reveals multiple areas of attenuation in the liver and small bowel obstruction. The hospitalist admits the patient to the medicine service for supportive treatment and workup for underlying malignancy. Her admitting team orders serum tumor biomarkers on admission to expedite the diagnosis.
BACKGROUND
When patients present with unexplained weight loss or with metastasis from an unknown primary location, the initial workup often includes imaging and a tumor biomarker panel (eg, cancer antigen 125 [CA125], carbohydrate antigen 19-9 [CA19-9], carcinoembryonic antigen [CEA]). The CA125, CA19-9, and CEA biomarkers are traditionally associated with ovarian, pancreatic, and colorectal cancer, respectively.1 While clinicians initially used these serum biomarkers to monitor for cancer recurrence or treatment response, they have since become widely used in multiple clinical stages of oncological evaluation.
WHY YOU MIGHT THINK CA125, CA19-9, AND CEA ARE HELPFUL IN THE DIAGNOSIS OF CANCER
Hospitalists routinely order biomarkers as part of the malignancy workup. More than a dozen oncology biomarkers are used in the clinical setting to risk stratify, plan treatment, and monitor for recurrence. For example, studies associate elevated preoperative levels of CEA and CA19-9 with metastatic invasion of colorectal2 and gastric3 cancers and with poor prognosis of intrahepatic cholangiocarcinoma. Similarly, CA125 has demonstrated utility in monitoring response to ovarian cancer treatment.4 Specific biomarkers, such as alpha-fetoprotein, improve diagnosis of liver and nonseminomatous testicular tumors.5 Clinicians often apply the same paradigm to other biomarkers due to their widespread availability, noninvasiveness, reproducibility, and ease of use, particularly in acute settings wherein any new information is perceived to be potentially helpful.
WHY YOU SHOULD NOT USE CA125, CA19-9, AND CEA TO DIAGNOSE CANCER
Utilizing these serum biomarkers to diagnose cancer has the potential for diagnostic error and can result in unnecessary patient anxiety and follow-up testing. Since tissue sampling is necessary and remains the gold standard in most cancer diagnoses, obtaining these tumor biomarkers in the early diagnostic stage does not change management and may even lead to harm. Furthermore, due to their poor sensitivity and specificity, these biomarkers cannot rule in or rule out cancer. Elevated CA125, CA19-9, and CEA biomarkers occur in a variety of malignancies, including gastric, gallbladder, hepatocellular, bladder, and breast cancers.1,3,6 In addition, these biomarkers have a very limited role in the workup of cancer of unknown primary origin.7
Even in the setting of a known pelvic mass, the use of CA125 alone has poor sensitivity at a cut-off level of 35 U/mL as a biomarker for the diagnosis of early ovarian cancer.8
Serum CA19-9 is not a useful diagnostic biomarker as elevated CA19-9 can occur in benign conditions, including cirrhosis, chronic pancreatitis, and cholangitis. In a systematic review of patients with histologic confirmation of pancreatic malignancy, the median positive predictive value of CA19-9 was 72% (interquartile range, 41%-95%).9 Additionally, patients with Lewis-null blood type, which is present in 5% to 10% of the Caucasian population, do not produce CA19-9.10 Therefore, CA19-9 will be 0% specific for tumors in this population.
The use of CEA in the diagnosis of colorectal cancer is also questionable. In stage I colorectal cancer, CEA was only 38.1% sensitive at a cut-off level of 2.41 ng/mL; it was 78.3% sensitive in stage IV disease.11 The specificity of CEA is limited since elevated CEA occurs in benign conditions, such as inflammatory bowel disease, smoking, hypothyroidism, pancreatitis, biliary obstruction, peptic ulcers, and cirrhosis—though CEA levels in these conditions are rarely >10 ng/mL.11 Regardless of the results of biomarker testing, definitive diagnosis requires tissue biopsy; therefore, biomarker findings are of little utility in the initial workup.
In addition to variable diagnostic utility, overreliance on these biomarkers has the potential for serious patient harm. In a study examining patients with established rectal cancer, combination CEA and CA19-9 testing alone was insufficient to predict the pathologic stage of disease correctly.2 A cancer misdiagnosis not only traumatizes patients but also erodes their trust in clinicians and creates anxiety during future clinical encounters. Overutilization of these tumor biomarkers is also costly and contributes to waste in the US healthcare system.
WHEN YOU SHOULD USE CA125, CA19-9, AND CEA
There is a role for tumor biomarker testing in specific cancers after the primary source of malignancy has been determined. When evaluating a known pelvic mass, CA125 testing is performed in conjunction with transvaginal ultrasound and assessment of menopausal status in the risk of ovarian malignancy algorithm for prognostication of disease prior to surgery.12 This algorithm takes into account levels of CA125 in addition to levels of human epididymis protein 4 and patient age, yielding an area under the curve as high as 0.93 for ovarian cancer risk classification.8 Beyond the prognostication process, oncologists follow CA125 to monitor response to first-line ovarian cancer treatment. However, CA125 has a less defined role in surveillance for ovarian cancer recurrence.
CA19-9 has demonstrated utility for pancreatic cancer and cholangiocarcinoma survival estimates. A national cohort analysis of patients with established intrahepatic cholangiocarcinoma found that CA19-9 independently predicted increased mortality. Patients with elevated CA19-9 also had significantly more nodal metastases and positive-margin resections.6 A study of 353 patients with pancreatic ductal adenocarcinoma undergoing radical resection further demonstrated the utility of CA19-9. In this study, patients with postoperative CA19-9 normalization had improved survival by almost 12 months when compared to those with consistently elevated CA19-9.13
Last, the literature describes CEA biomarker testing in the surveillance of patients after curative treatment of colon and rectal cancer. The American Society of Colon and Rectal Surgeons recommends regularly tracking this biomarker following curative resection, in conjunction with colonoscopy and chest and liver imaging studies.14 A prospective randomized controlled study that followed this monitoring protocol in cured asymptomatic patients on a bimonthly basis found that early diagnosis of recurrent colorectal cancer improved survival.15 The use of CEA testing as a monitoring tool should therefore be a point of discussion between providers and patients, as its utility varies based on patient comorbidities, their ability to tolerate surgery or chemotherapy, risk factors for recurrence, performance status, compliance, age, and preference.14
WHAT YOU SHOULD DO INSTEAD
The use of CA125, CA19-9, and CEA testing alone as initial diagnostic tools for malignancy are problematic due to their poor sensitivities and/or positive predictive value. Multiple studies have demonstrated their utility as markers of metastasis or malignancy progression rather than as clinically useful markers for the detection of any one type of cancer.1,3,6 In an undiagnosed symptomatic patient with unexplained weight loss or symptoms of a tumor mass, elevated CA125, CA19-9, and CEA add no new information as metastatic pancreatic, colorectal, ovarian, gastric, gallbladder, hepatocellular, bladder, ovarian, and breast cancers all remain in the differential diagnosis. Clinicians should approach the initial diagnosis of cancer in such patients with appropriate imaging studies, a thorough physical examination, and prompt biopsy of abnormal findings, as long as these are consistent with the patient’s goals of care. After establishing a tissue diagnosis, some tumor biomarkers have valid prognostic, staging, and monitoring roles.6,13,14
RECOMMENDATIONS
- Do not routinely order CA125, CA19-9, and CEA tests for the initial diagnostic workup of visceral malignancy of unknown origin regardless of whether imaging studies have been obtained.
- Use appropriate imaging, perform a thorough physical examination, and obtain tissue biopsy in the initial diagnostic workup of a visceral malignancy of unknown origin.
CONCLUSION
Clinicians should use serum biomarkers, like any other diagnostic test, to maximize benefit while preventing patient harm. In general, CA125, CA19-9, and CEA do not have a role in cancer diagnosis. The patient described in our clinical scenario would not benefit from a serum tumor biomarker panel at the time of admission. Regardless of findings from these tests, a tissue sample is required to make a definitive diagnosis of underlying malignancy in this patient.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing [email protected]
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 56-year-old woman presents to the emergency department with a 2-week history of abdominal pain associated with nausea and an episode of nonbilious, nonbloody emesis. Her last bowel movement was 2 days prior to her presentation. The patient has tachycardia to 105 beats per minute but otherwise normal vital signs. Findings on her physical examination include dry mucous membranes and increased bowel sounds. A review of systems reveals an unintentional weight loss of 15 kg over the past 4 months and increased fatigue. Computed tomography scan of the abdomen and pelvis with contrast reveals multiple areas of attenuation in the liver and small bowel obstruction. The hospitalist admits the patient to the medicine service for supportive treatment and workup for underlying malignancy. Her admitting team orders serum tumor biomarkers on admission to expedite the diagnosis.
BACKGROUND
When patients present with unexplained weight loss or with metastasis from an unknown primary location, the initial workup often includes imaging and a tumor biomarker panel (eg, cancer antigen 125 [CA125], carbohydrate antigen 19-9 [CA19-9], carcinoembryonic antigen [CEA]). The CA125, CA19-9, and CEA biomarkers are traditionally associated with ovarian, pancreatic, and colorectal cancer, respectively.1 While clinicians initially used these serum biomarkers to monitor for cancer recurrence or treatment response, they have since become widely used in multiple clinical stages of oncological evaluation.
WHY YOU MIGHT THINK CA125, CA19-9, AND CEA ARE HELPFUL IN THE DIAGNOSIS OF CANCER
Hospitalists routinely order biomarkers as part of the malignancy workup. More than a dozen oncology biomarkers are used in the clinical setting to risk stratify, plan treatment, and monitor for recurrence. For example, studies associate elevated preoperative levels of CEA and CA19-9 with metastatic invasion of colorectal2 and gastric3 cancers and with poor prognosis of intrahepatic cholangiocarcinoma. Similarly, CA125 has demonstrated utility in monitoring response to ovarian cancer treatment.4 Specific biomarkers, such as alpha-fetoprotein, improve diagnosis of liver and nonseminomatous testicular tumors.5 Clinicians often apply the same paradigm to other biomarkers due to their widespread availability, noninvasiveness, reproducibility, and ease of use, particularly in acute settings wherein any new information is perceived to be potentially helpful.
WHY YOU SHOULD NOT USE CA125, CA19-9, AND CEA TO DIAGNOSE CANCER
Utilizing these serum biomarkers to diagnose cancer has the potential for diagnostic error and can result in unnecessary patient anxiety and follow-up testing. Since tissue sampling is necessary and remains the gold standard in most cancer diagnoses, obtaining these tumor biomarkers in the early diagnostic stage does not change management and may even lead to harm. Furthermore, due to their poor sensitivity and specificity, these biomarkers cannot rule in or rule out cancer. Elevated CA125, CA19-9, and CEA biomarkers occur in a variety of malignancies, including gastric, gallbladder, hepatocellular, bladder, and breast cancers.1,3,6 In addition, these biomarkers have a very limited role in the workup of cancer of unknown primary origin.7
Even in the setting of a known pelvic mass, the use of CA125 alone has poor sensitivity at a cut-off level of 35 U/mL as a biomarker for the diagnosis of early ovarian cancer.8
Serum CA19-9 is not a useful diagnostic biomarker as elevated CA19-9 can occur in benign conditions, including cirrhosis, chronic pancreatitis, and cholangitis. In a systematic review of patients with histologic confirmation of pancreatic malignancy, the median positive predictive value of CA19-9 was 72% (interquartile range, 41%-95%).9 Additionally, patients with Lewis-null blood type, which is present in 5% to 10% of the Caucasian population, do not produce CA19-9.10 Therefore, CA19-9 will be 0% specific for tumors in this population.
The use of CEA in the diagnosis of colorectal cancer is also questionable. In stage I colorectal cancer, CEA was only 38.1% sensitive at a cut-off level of 2.41 ng/mL; it was 78.3% sensitive in stage IV disease.11 The specificity of CEA is limited since elevated CEA occurs in benign conditions, such as inflammatory bowel disease, smoking, hypothyroidism, pancreatitis, biliary obstruction, peptic ulcers, and cirrhosis—though CEA levels in these conditions are rarely >10 ng/mL.11 Regardless of the results of biomarker testing, definitive diagnosis requires tissue biopsy; therefore, biomarker findings are of little utility in the initial workup.
In addition to variable diagnostic utility, overreliance on these biomarkers has the potential for serious patient harm. In a study examining patients with established rectal cancer, combination CEA and CA19-9 testing alone was insufficient to predict the pathologic stage of disease correctly.2 A cancer misdiagnosis not only traumatizes patients but also erodes their trust in clinicians and creates anxiety during future clinical encounters. Overutilization of these tumor biomarkers is also costly and contributes to waste in the US healthcare system.
WHEN YOU SHOULD USE CA125, CA19-9, AND CEA
There is a role for tumor biomarker testing in specific cancers after the primary source of malignancy has been determined. When evaluating a known pelvic mass, CA125 testing is performed in conjunction with transvaginal ultrasound and assessment of menopausal status in the risk of ovarian malignancy algorithm for prognostication of disease prior to surgery.12 This algorithm takes into account levels of CA125 in addition to levels of human epididymis protein 4 and patient age, yielding an area under the curve as high as 0.93 for ovarian cancer risk classification.8 Beyond the prognostication process, oncologists follow CA125 to monitor response to first-line ovarian cancer treatment. However, CA125 has a less defined role in surveillance for ovarian cancer recurrence.
CA19-9 has demonstrated utility for pancreatic cancer and cholangiocarcinoma survival estimates. A national cohort analysis of patients with established intrahepatic cholangiocarcinoma found that CA19-9 independently predicted increased mortality. Patients with elevated CA19-9 also had significantly more nodal metastases and positive-margin resections.6 A study of 353 patients with pancreatic ductal adenocarcinoma undergoing radical resection further demonstrated the utility of CA19-9. In this study, patients with postoperative CA19-9 normalization had improved survival by almost 12 months when compared to those with consistently elevated CA19-9.13
Last, the literature describes CEA biomarker testing in the surveillance of patients after curative treatment of colon and rectal cancer. The American Society of Colon and Rectal Surgeons recommends regularly tracking this biomarker following curative resection, in conjunction with colonoscopy and chest and liver imaging studies.14 A prospective randomized controlled study that followed this monitoring protocol in cured asymptomatic patients on a bimonthly basis found that early diagnosis of recurrent colorectal cancer improved survival.15 The use of CEA testing as a monitoring tool should therefore be a point of discussion between providers and patients, as its utility varies based on patient comorbidities, their ability to tolerate surgery or chemotherapy, risk factors for recurrence, performance status, compliance, age, and preference.14
WHAT YOU SHOULD DO INSTEAD
The use of CA125, CA19-9, and CEA testing alone as initial diagnostic tools for malignancy are problematic due to their poor sensitivities and/or positive predictive value. Multiple studies have demonstrated their utility as markers of metastasis or malignancy progression rather than as clinically useful markers for the detection of any one type of cancer.1,3,6 In an undiagnosed symptomatic patient with unexplained weight loss or symptoms of a tumor mass, elevated CA125, CA19-9, and CEA add no new information as metastatic pancreatic, colorectal, ovarian, gastric, gallbladder, hepatocellular, bladder, ovarian, and breast cancers all remain in the differential diagnosis. Clinicians should approach the initial diagnosis of cancer in such patients with appropriate imaging studies, a thorough physical examination, and prompt biopsy of abnormal findings, as long as these are consistent with the patient’s goals of care. After establishing a tissue diagnosis, some tumor biomarkers have valid prognostic, staging, and monitoring roles.6,13,14
RECOMMENDATIONS
- Do not routinely order CA125, CA19-9, and CEA tests for the initial diagnostic workup of visceral malignancy of unknown origin regardless of whether imaging studies have been obtained.
- Use appropriate imaging, perform a thorough physical examination, and obtain tissue biopsy in the initial diagnostic workup of a visceral malignancy of unknown origin.
CONCLUSION
Clinicians should use serum biomarkers, like any other diagnostic test, to maximize benefit while preventing patient harm. In general, CA125, CA19-9, and CEA do not have a role in cancer diagnosis. The patient described in our clinical scenario would not benefit from a serum tumor biomarker panel at the time of admission. Regardless of findings from these tests, a tissue sample is required to make a definitive diagnosis of underlying malignancy in this patient.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing [email protected]
1. Yotsukura S, Mamitsuka H. Evaluation of serum-based cancer biomarkers: a brief review from a clinical and computational viewpoint. Crit Rev Oncol Hematol. 2015;93(2):103-115. https://doi.org/10.1016/j.critrevonc.2014.10.002
2. Zhang B, Sun Z, Song M, et al. Ultrasound/CT combined with serum CEA/CA19.9 in the diagnosis and prognosis of rectal cancer. J Buon. 2018;23(3):592-597.
3. Zhou YC, Zhao HJ, Shen LZ. Preoperative serum CEA and CA19-9 in gastric cancer--a single tertiary hospital study of 1,075 cases. Asian Pac J Cancer Prev. 2015;16(7):2685-2691. https://doi.org/10.7314/apjcp.2015.16.7.2685
4. Karam AK, Karlan BY. Ovarian cancer: the duplicity of CA125 measurement. Nat Rev Clin Oncol. 2010;7(6):335-339. https://doi.org/10.1038/nrclinonc.2010.44
5. Gilligan TD, Seidenfeld J, Basch EM, et al; American Society of Clinical Oncology. American Society of Clinical Oncology Clinical Practice Guideline on uses of serum tumor markers in adult males with germ cell tumors. J Clin Oncol. 2010;28(20):3388-3404. https://doi.org/10.1200/jco.2009.26.4481
6. Bergquist JR, Ivanics T, Storlie CB, et al. Implications of CA19-9 elevation for survival, staging, and treatment sequencing in intrahepatic cholangiocarcinoma: a national cohort analysis. J Surg Oncol. 2016;114(4):475-482. https://doi.org/10.1002/jso.24381
7. Milovic M, Popov I, Jelic S. Tumor markers in metastatic disease from cancer of unknown primary origin. Med Sci Monit. 2002;8(2):MT25-MT30.
8. Dochez V, Caillon H, Vaucel E, Dimet J, Winer N. Biomarkers and algorithms for diagnosis of ovarian cancer: CA125, HE4, RMI and ROMA, a review. J Ovarian Res. 2019;12(1):28. https://doi.org/10.1186/s13048-019-0503-7
9. Goonetilleke KS, Siriwardena AK. Systematic review of carbohydrate antigen (CA 19-9) as a biochemical marker in the diagnosis of pancreatic cancer. Eur J Surg Oncol. 2007;33(3):266-270. https://doi.org/10.1016/j.ejso.2006.10.004
10. Loosen SH, Neumann UP, Trautwein C, Roderburg C, Luedde T. Current and future biomarkers for pancreatic adenocarcinoma. Tumour Biol. 2017;39(6):1010428317692231. https://doi.org/10.1177/1010428317692231
11. Polat E, Duman U, Duman M, et al. Diagnostic value of preoperative serum carcinoembryonic antigen and carbohydrate antigen 19-9 in colorectal cancer. Curr Oncol. 2014;21(1):e1-e7. https://doi.org/10.3747/co.21.1711
12. Sölétormos G, Duffy MJ, Othman Abu Hassan S, et al. Clinical use of cancer biomarkers in epithelial ovarian cancer: updated guidelines from the European Group on Tumor Markers. Int J Gynecol Cancer. 2016;26(1):43-51. https://doi.org/10.1097/igc.0000000000000586
13. Xu HX, Liu L, Xiang JF, et al. Postoperative serum CEA and CA125 levels are supplementary to perioperative CA19-9 levels in predicting operative outcomes of pancreatic ductal adenocarcinoma. Surgery. 2017;161(2):373-384. https://doi.org/10.1016/j.surg.2016.08.005
14. Steele SR, Chang GJ, Hendren S, et al. Practice guideline for the surveillance of patients after curative treatment of colon and rectal cancer. Dis Colon Rectum. 2015;58(8):713-725. https://doi.org/10.1097/dcr.0000000000000410
15. Verberne CJ, Zhan Z, van den Heuvel E, et al. Intensified follow-up in colorectal cancer patients using frequent Carcino-Embryonic Antigen (CEA) measurements and CEA-triggered imaging: results of the randomized “CEAwatch” trial. Eur J Surg Oncol. 2015;41(9):1188-1196. https://doi.org/10.1016/j.ejso.2015.06.008
1. Yotsukura S, Mamitsuka H. Evaluation of serum-based cancer biomarkers: a brief review from a clinical and computational viewpoint. Crit Rev Oncol Hematol. 2015;93(2):103-115. https://doi.org/10.1016/j.critrevonc.2014.10.002
2. Zhang B, Sun Z, Song M, et al. Ultrasound/CT combined with serum CEA/CA19.9 in the diagnosis and prognosis of rectal cancer. J Buon. 2018;23(3):592-597.
3. Zhou YC, Zhao HJ, Shen LZ. Preoperative serum CEA and CA19-9 in gastric cancer--a single tertiary hospital study of 1,075 cases. Asian Pac J Cancer Prev. 2015;16(7):2685-2691. https://doi.org/10.7314/apjcp.2015.16.7.2685
4. Karam AK, Karlan BY. Ovarian cancer: the duplicity of CA125 measurement. Nat Rev Clin Oncol. 2010;7(6):335-339. https://doi.org/10.1038/nrclinonc.2010.44
5. Gilligan TD, Seidenfeld J, Basch EM, et al; American Society of Clinical Oncology. American Society of Clinical Oncology Clinical Practice Guideline on uses of serum tumor markers in adult males with germ cell tumors. J Clin Oncol. 2010;28(20):3388-3404. https://doi.org/10.1200/jco.2009.26.4481
6. Bergquist JR, Ivanics T, Storlie CB, et al. Implications of CA19-9 elevation for survival, staging, and treatment sequencing in intrahepatic cholangiocarcinoma: a national cohort analysis. J Surg Oncol. 2016;114(4):475-482. https://doi.org/10.1002/jso.24381
7. Milovic M, Popov I, Jelic S. Tumor markers in metastatic disease from cancer of unknown primary origin. Med Sci Monit. 2002;8(2):MT25-MT30.
8. Dochez V, Caillon H, Vaucel E, Dimet J, Winer N. Biomarkers and algorithms for diagnosis of ovarian cancer: CA125, HE4, RMI and ROMA, a review. J Ovarian Res. 2019;12(1):28. https://doi.org/10.1186/s13048-019-0503-7
9. Goonetilleke KS, Siriwardena AK. Systematic review of carbohydrate antigen (CA 19-9) as a biochemical marker in the diagnosis of pancreatic cancer. Eur J Surg Oncol. 2007;33(3):266-270. https://doi.org/10.1016/j.ejso.2006.10.004
10. Loosen SH, Neumann UP, Trautwein C, Roderburg C, Luedde T. Current and future biomarkers for pancreatic adenocarcinoma. Tumour Biol. 2017;39(6):1010428317692231. https://doi.org/10.1177/1010428317692231
11. Polat E, Duman U, Duman M, et al. Diagnostic value of preoperative serum carcinoembryonic antigen and carbohydrate antigen 19-9 in colorectal cancer. Curr Oncol. 2014;21(1):e1-e7. https://doi.org/10.3747/co.21.1711
12. Sölétormos G, Duffy MJ, Othman Abu Hassan S, et al. Clinical use of cancer biomarkers in epithelial ovarian cancer: updated guidelines from the European Group on Tumor Markers. Int J Gynecol Cancer. 2016;26(1):43-51. https://doi.org/10.1097/igc.0000000000000586
13. Xu HX, Liu L, Xiang JF, et al. Postoperative serum CEA and CA125 levels are supplementary to perioperative CA19-9 levels in predicting operative outcomes of pancreatic ductal adenocarcinoma. Surgery. 2017;161(2):373-384. https://doi.org/10.1016/j.surg.2016.08.005
14. Steele SR, Chang GJ, Hendren S, et al. Practice guideline for the surveillance of patients after curative treatment of colon and rectal cancer. Dis Colon Rectum. 2015;58(8):713-725. https://doi.org/10.1097/dcr.0000000000000410
15. Verberne CJ, Zhan Z, van den Heuvel E, et al. Intensified follow-up in colorectal cancer patients using frequent Carcino-Embryonic Antigen (CEA) measurements and CEA-triggered imaging: results of the randomized “CEAwatch” trial. Eur J Surg Oncol. 2015;41(9):1188-1196. https://doi.org/10.1016/j.ejso.2015.06.008
© 2021 Society of Hospital Medicine
Things We Do For No Reason™: Routinely Holding Metformin in the Hospital
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A hospitalist admits a 29-year-old man with hypertension, obesity, and type 2 diabetes (type 2 DM) for a posterior neck abscess that failed outpatient oral antibiotic therapy. The patient’s medications include metformin monotherapy. Vital signs taken upon admission include a blood pressure of 136/82 mm Hg, heart rate of 98 beats per minute, respiratory rate 18 of breaths per minute, oxygen saturation of 100% on room air, and temperature of 38.5 oC. Laboratory evaluation revealed a glucose level of 212 mg/dL, with a hemoglobin A1c of 8.0%, lactic acid of 1.4 mmol/L, and normal renal and hepatic function. Based on these findings, the hospitalist holds metformin and starts the patient on sliding-scale insulin therapy.
WHY YOU MIGHT THINK ROUTINELY HOLDING METFORMIN IN THE HOSPITAL IS NECESSARY
Following the introduction of metformin in the United States, the US Food and Drug Administration (FDA) received 47 confirmed reports of nonfatal lactic acidosis associated with the use of metformin, all of which involved cardiac disease (specifically congestive heart failure [CHF]), renal insufficiency, hypoxia, or sepsis.2 Consequently, the FDA listed CHF as a contraindication to metformin use; however, it has since changed the use of metformin in CHF from a contraindication to a warning/precaution for lactic acidosis. The FDA also added a warning against the use of metformin in patients with sepsis or in patients older than 80 years who have abnormal creatinine clearance.
Acute kidney injury, a common inpatient condition, occurs in 20% of hospitalized patients and more than 50% of intensive care patients.3 Moreover, a retrospective observational study showed approximately 50% of all patients hospitalized for COVID-19 had AKI.4 Iodinated contrast, a diagnostic media commonly used in the hospital, may also increase the risk of renal dysfunction. The FDA recommends providers discontinue metformin at or before initiating imaging studies with iodinated contrast5 in patients with an estimated glomerular filtration rate (eGFR) between 30 and 60 mL/min/1.73 m2. The FDA also advises that providers not restart metformin until 48 hours after an intra-arterial (IA) or intravenous (IV) contrast study in patients with an eGFR <60 mL/min/1.73 m2 (equivalent to chronic kidney disease [CKD] stage 3 or worse).5 The American Diabetes Association (ADA) recommends the same eGFR cutoff level in its clinical practice recommendations, as well as withholding metformin 48 hours before patients receive IV contrast.6 Given the risk of AKI in hospitalized patients and concerns of increased MALA, clinicians reflexively hold metformin.
Holding metformin is also consistent with professional guidelines. The 2009 American Association of Clinical Endocrinology and ADA Consensus Statement on Inpatient Glycemic Control recommends cautious use of metformin in the inpatient setting “because of the potential development of a contraindication during the hospitalization.”7 Similarly, the 2012 Endocrine Society guidelines recommend withholding metformin in almost all hospitalized patients.8
WHY ROUTINELY HOLDING METFORMIN IN THE HOSPITAL IS NOT BENEFICIAL
Routinely holding metformin in hospitalized patients is unnecessary and potentially harmful. First, MALA is exceedingly rare, and experts question the causal link. Furthermore, iodinated contrast does not place patients with normal renal function at increased risk of MALA. Finally, holding metformin leads to worsened glycemic control and increased use of insulin, both of which may result in adverse patient outcomes.
The concerns about MALA stem from clinical experiences with phenformin, an older and more potent biguanide. Phenformin shares a similar mechanism of action with metformin but causes more lactic acid production. In 1978, following 306 documented cases of phenformin-associated lactic acidosis, the FDA removed this medication from the market.9 Since the initial 47 cases of MALA were reported to the FDA, repeated studies and systematic reviews have disputed the link between metformin and lactic acidosis, particularly in the absence of significant risk factors or in patients with an eGFR ≥30 mL/min/1.73 m2. In fact, a large observational study showed a reduction in acidosis and mortality in outpatients with stage 3a CKD (eGFR, 45-59 mL/min/1.73 m2) who were taking metformin compared to patients taking insulin or other oral hypoglycemics agents.10 In patients with stage 3b CKD (eGFR, 30-44 mL/min/1.73 m2), this study found no difference in the same outcomes.10
Studies show that metformin does not cause elevated lactate levels in patients with stage 4 CKD (eGFR >15mL/min/1.732) or lower stages of CKD as long as doses are adjusted appropriately to reflect renal function.11 These and other investigations reveal that in the absence of other risk factors, metformin does not cause lactic acidosis (Table).10-15 Based on these findings, the Endocrine Society changed the strength of its recommendation to withhold metformin in hospitalized patients to “weak,” with “very low-quality evidence.” The FDA similarly revised its warnings8 to allow metformin use in all patients with an eGFR ≥30 mL/min/1.73 m2. A large community-based cohort study, which demonstrated no association between hospitalization with acidosis and metformin use in patients with stage 3b CKD or lower stages of CKD, supports this change in treatment threshold.15
Published evidence also does not support the practice of routinely holding metformin before contrast administration, despite concerns regarding contrast-induced nephropathy. Retrospective chart reviews and a direct comparison in human models have not shown any significant difference in the risk of AKI between the IV and IA contrast.16 Moreover, evidence suggests no interaction between metformin and contrast media in patients with normal renal function.17 In response, the American College of Radiology, Canadian Association of Radiology, Royal College of Radiologists, and Royal Australian and New Zealand College of Radiologists all recommend continuing metformin in patients with normal renal function (eGFR ≥30 mL/min/1.73m2) receiving IV contrast. They advise holding metformin for 48 hours in patients with renal insufficiency (eGFR <30 mL/min/1.73m2) or those undergoing IA catheter studies that might result in renal artery emboli.18
Finally, continuing metformin maintains steady blood glucose control. The practice of replacing metformin with sliding-scale insulin monotherapy for hospitalized patients significantly increases the risk of hyperglycemia and is associated with an increased length of stay.19 Additionally, unlike insulin, metformin does not increase the risk of hypoglycemia. Finally, a recent matched cohort study comparing the use of oral hypoglycemic agents (metformin, thiazolidines, and sulfonylureas) vs insulin monotherapy in patients undergoing emergency abdominal surgery showed that the patients admitted with sepsis and treated with oral agents had a lower 30-day mortality rate and a shorter length of stay.20 Based on the evidence showing that inpatient oral hypoglycemic agents improve quality metrics and mitigate safety events, the ADA advocates resuming oral antihyperglycemic medications (most commonly metformin) 1 to 2 days before discharge.7
WHAT YOU SHOULD DO INSTEAD
Clinicians should continue metformin in all hospitalized patients who are not at significant risk of developing lactic acidosis. Risk factors for MALA include severe sepsis (in the setting of end-organ damage as defined by systemic inflammatory response syndrome criteria), hypoxia requiring oxygen supplementation, hypoperfusion (as from CHF), AKI, CKD (eGFR <30 mL/min/1.73 m2), and advanced cirrhosis. Given the high rates of hypoxia and AKI in admitted patients with COVID-19, clinicians should hold metformin on admission. Continue metformin for patients receiving IV contrast media with an eGFR >30 mL/min/1.73 m2. For patients undergoing IA catheter studies associated with a risk for renal artery emboli, or in patients with renal insufficiency (eGFR <30 mL/min/1.73 m2), temporarily hold metformin for 48 hours. When held, restart metformin as soon as risk factors resolve.
RECOMMENDATIONS
- Hold metformin in patients with or undergoing the following:
- High risk for or currently suffering from decompensated heart failure, severe sepsis, or other disease states resulting in hypoxia or tissue hypoperfusion;
- An eGFR <30 mL/min/1.73 m2 or AKI; resume metformin when the AKI resolves;
- COVID-19 infection, until the risk of hypoxia has resolved;
- IV contrast study in the presence of acute renal failure or an eGFR <30 mL/min/1.73 m2; resume metformin 48 hours after contrast administration;
- Intra-arterial catheter study that might result in renal artery emboli; resume metformin when renal function normalizes.
- Continue metformin in all hospitalized patients in the absence of the aforementioned disease states or contrast-related indications.
CONCLUSION
Returning to the patient in our clinical scenario, we recommend continuing metformin given the lack of risk factors or disease states associated with increased lactic acidosis. The practice of withholding metformin in hospitalized patients for fear of MALA is based on minimal evidence. Clinicians should, however, hold metformin in patients who have true contraindications, including existing acidosis, hypoperfusion, renal insufficiency, CHF, severe sepsis, hypoxia, advanced cirrhosis, and COVID-19. With regard to iodinated contrast studies, temporarily withhold metformin for 48 hours in patients with an eGFR <30 mL/min/1.73 m2, acute kidney injury, or in patients undergoing an IA catheter study at risk for renal artery emboli. Patients should be restarted on metformin 48 hours after these studies and as renal function normalizes. When withholding metformin during a hospitalization, restart it once risk factors have resolved.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing [email protected]
1. Kopec KT, Kowalski MJ. Metformin-associated lactic acidosis (MALA): case files of the Einstein Medical Center medical toxicology fellowship. J Med Toxicol. 2013;9(1):61-66. https://doi.org/10.1007/s13181-012-0278-3
2. Misbin RI, Green L, Stadel BV, Gueriguian JL, Gubbi A, Fleming GA. Lactic acidosis in patients with diabetes treated with metformin. N Engl J Med. 1998;338(4):265-266. https://doi.org/10.1056/nejm199801223380415
3. Wang HE, Muntner P, Chertow GM, Warnock DG. Acute kidney injury and mortality in hospitalized patients. Am J Nephrol. 2012;35(4):349-355. https://doi.org/10.1159/000337487
4. Chan L, Chaudhary K, Saha A, et al; Mount Sinai COVID Informatics Center (MSCIC), Li L. AKI in hospitalized patients with COVID-19. J Am Soc Nephrol. 2021;32(1):151-160. https://doi.org/10.1681/asn.2020050615
5. US Food and Drug Administration. FDA drug safety communication: FDA revises warnings regarding use of the diabetes medicine metformin in certain patients with reduced kidney function. Updated November 14, 2017. Accessed June 22, 2021. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-revises-warnings-regarding-use-diabetes-medicine-metformin-certain
6. American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes—2019. Diabetes Care. 2019;42 (Suppl 1):S90-S102. https://doi.org/10.2337/dc19-s009
7. Moghissi ES, Korytkowski MT, DiNardo M, et al; American Association of Clinical Endocrinologists; American Diabetes Association. Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care. 2009;32(6):1119-1131. https://doi.org/10.2337/dc09-9029
8. Umpierrez GE, Hellman R, Korytkowski MT, et al; Endocrine Society. Management of hyperglycemia in hospitalized patients in non-critical care setting: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(1):16-38. https://doi.org/10.1210/jc.2011-2098
9. Misbin RI. Phenformin-associated lactic acidosis: pathogenesis and treatment. Ann Intern Med. 1977;87(5):591-595. https://doi.org/10.7326/0003-4819-87-5-591
10. Ekström N, Schiöler L, Svensson AM, et al. Effectiveness and safety of metformin in 51 675 patients with type 2 diabetes and different levels of renal function: a cohort study from the Swedish National Diabetes Register. BMJ Open. 2012;2(4):e001076. https://doi.org/10.1136/bmjopen-2012-001076
11. Lalau JD, Kajbaf F, Bennis Y, Hurtel-Lemaire AS, Belpaire F, De Broe ME. Metformin treatment in patients with type 2 diabetes and chronic kidney disease stages 3A, 3B, or 4. Diabetes Care. 2018;41(3):547-553. https://doi.org/10.2337/dc17-2231
12. Brown JB, Pedula K, Barzilay J, Herson MK, Latare P. Lactic acidosis rates in type 2 diabetes. Diabetes Care. 1998;21(10):1659-1663. https://doi.org/10.2337/diacare.21.10.1659
13. Lalau JD, Race JM. Lactic acidosis in metformin-treated patients. Prognostic value of arterial lactate levels and plasma metformin concentrations. Drug Saf. 1999;20(4):377-384. https://doi.org/10.2165/00002018-199920040-00006
14. Salpeter SR, Greyber E, Pasternak GA, Salpeter Posthumous EE. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database Syst Rev. 2010;(1):CD002967. https://doi.org/10.1002/14651858.cd002967.pub3
15. Lazarus B, Wu A, Shin JI, et al. Association of metformin use with risk of lactic acidosis across the range of kidney function: a community-based cohort study. JAMA Intern Med. 2018;178(7):903-910. https://doi.org/10.1001/jamainternmed.2018.0292
16. McDonald JS, Leake CB, McDonald RJ, et al. Acute kidney injury after intravenous versus intra-arterial contrast material administration in a paired cohort. Invest Radiol. 2016;51(12):804-809. https://doi.org/10.1097/rli.0000000000000298
17. Zeller M, Labalette-Bart M, Juliard JM, et al. Metformin and contrast-induced acute kidney injury in diabetic patients treated with primary percutaneous coronary intervention for ST segment elevation myocardial infarction: a multicenter study. Int J Cardiol. 2016;220:137-142. https://doi.org/10.1016/j.ijcard.2016.06.076
18. Goergen SK, Rumbold G, Compton G, Harris C. Systematic review of current guidelines, and their evidence base, on risk of lactic acidosis after administration of contrast medium for patients receiving metformin. Radiology. 2010;254(1):261-269. https://doi.org/10.1148/radiol.09090690
19. Ambrus DB, O’Connor MJ. Things we do for no reason: sliding-scale insulin as monotherapy for glycemic control in hospitalized patients. J Hosp Med. 2019;14(2):114-116. https://doi.org/10.12788/jhm.3109
20. Haltmeier T, Benjamin E, Beale E, Inaba K, Demetriades D. Insulin-treated patients with diabetes mellitus undergoing emergency abdominal surgery have worse outcomes than patients treated with oral agents. World J Surg. 2016;40(7):1575-1582. https://doi.org/10.1007/s00268-016-3469-2
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A hospitalist admits a 29-year-old man with hypertension, obesity, and type 2 diabetes (type 2 DM) for a posterior neck abscess that failed outpatient oral antibiotic therapy. The patient’s medications include metformin monotherapy. Vital signs taken upon admission include a blood pressure of 136/82 mm Hg, heart rate of 98 beats per minute, respiratory rate 18 of breaths per minute, oxygen saturation of 100% on room air, and temperature of 38.5 oC. Laboratory evaluation revealed a glucose level of 212 mg/dL, with a hemoglobin A1c of 8.0%, lactic acid of 1.4 mmol/L, and normal renal and hepatic function. Based on these findings, the hospitalist holds metformin and starts the patient on sliding-scale insulin therapy.
WHY YOU MIGHT THINK ROUTINELY HOLDING METFORMIN IN THE HOSPITAL IS NECESSARY
Following the introduction of metformin in the United States, the US Food and Drug Administration (FDA) received 47 confirmed reports of nonfatal lactic acidosis associated with the use of metformin, all of which involved cardiac disease (specifically congestive heart failure [CHF]), renal insufficiency, hypoxia, or sepsis.2 Consequently, the FDA listed CHF as a contraindication to metformin use; however, it has since changed the use of metformin in CHF from a contraindication to a warning/precaution for lactic acidosis. The FDA also added a warning against the use of metformin in patients with sepsis or in patients older than 80 years who have abnormal creatinine clearance.
Acute kidney injury, a common inpatient condition, occurs in 20% of hospitalized patients and more than 50% of intensive care patients.3 Moreover, a retrospective observational study showed approximately 50% of all patients hospitalized for COVID-19 had AKI.4 Iodinated contrast, a diagnostic media commonly used in the hospital, may also increase the risk of renal dysfunction. The FDA recommends providers discontinue metformin at or before initiating imaging studies with iodinated contrast5 in patients with an estimated glomerular filtration rate (eGFR) between 30 and 60 mL/min/1.73 m2. The FDA also advises that providers not restart metformin until 48 hours after an intra-arterial (IA) or intravenous (IV) contrast study in patients with an eGFR <60 mL/min/1.73 m2 (equivalent to chronic kidney disease [CKD] stage 3 or worse).5 The American Diabetes Association (ADA) recommends the same eGFR cutoff level in its clinical practice recommendations, as well as withholding metformin 48 hours before patients receive IV contrast.6 Given the risk of AKI in hospitalized patients and concerns of increased MALA, clinicians reflexively hold metformin.
Holding metformin is also consistent with professional guidelines. The 2009 American Association of Clinical Endocrinology and ADA Consensus Statement on Inpatient Glycemic Control recommends cautious use of metformin in the inpatient setting “because of the potential development of a contraindication during the hospitalization.”7 Similarly, the 2012 Endocrine Society guidelines recommend withholding metformin in almost all hospitalized patients.8
WHY ROUTINELY HOLDING METFORMIN IN THE HOSPITAL IS NOT BENEFICIAL
Routinely holding metformin in hospitalized patients is unnecessary and potentially harmful. First, MALA is exceedingly rare, and experts question the causal link. Furthermore, iodinated contrast does not place patients with normal renal function at increased risk of MALA. Finally, holding metformin leads to worsened glycemic control and increased use of insulin, both of which may result in adverse patient outcomes.
The concerns about MALA stem from clinical experiences with phenformin, an older and more potent biguanide. Phenformin shares a similar mechanism of action with metformin but causes more lactic acid production. In 1978, following 306 documented cases of phenformin-associated lactic acidosis, the FDA removed this medication from the market.9 Since the initial 47 cases of MALA were reported to the FDA, repeated studies and systematic reviews have disputed the link between metformin and lactic acidosis, particularly in the absence of significant risk factors or in patients with an eGFR ≥30 mL/min/1.73 m2. In fact, a large observational study showed a reduction in acidosis and mortality in outpatients with stage 3a CKD (eGFR, 45-59 mL/min/1.73 m2) who were taking metformin compared to patients taking insulin or other oral hypoglycemics agents.10 In patients with stage 3b CKD (eGFR, 30-44 mL/min/1.73 m2), this study found no difference in the same outcomes.10
Studies show that metformin does not cause elevated lactate levels in patients with stage 4 CKD (eGFR >15mL/min/1.732) or lower stages of CKD as long as doses are adjusted appropriately to reflect renal function.11 These and other investigations reveal that in the absence of other risk factors, metformin does not cause lactic acidosis (Table).10-15 Based on these findings, the Endocrine Society changed the strength of its recommendation to withhold metformin in hospitalized patients to “weak,” with “very low-quality evidence.” The FDA similarly revised its warnings8 to allow metformin use in all patients with an eGFR ≥30 mL/min/1.73 m2. A large community-based cohort study, which demonstrated no association between hospitalization with acidosis and metformin use in patients with stage 3b CKD or lower stages of CKD, supports this change in treatment threshold.15
Published evidence also does not support the practice of routinely holding metformin before contrast administration, despite concerns regarding contrast-induced nephropathy. Retrospective chart reviews and a direct comparison in human models have not shown any significant difference in the risk of AKI between the IV and IA contrast.16 Moreover, evidence suggests no interaction between metformin and contrast media in patients with normal renal function.17 In response, the American College of Radiology, Canadian Association of Radiology, Royal College of Radiologists, and Royal Australian and New Zealand College of Radiologists all recommend continuing metformin in patients with normal renal function (eGFR ≥30 mL/min/1.73m2) receiving IV contrast. They advise holding metformin for 48 hours in patients with renal insufficiency (eGFR <30 mL/min/1.73m2) or those undergoing IA catheter studies that might result in renal artery emboli.18
Finally, continuing metformin maintains steady blood glucose control. The practice of replacing metformin with sliding-scale insulin monotherapy for hospitalized patients significantly increases the risk of hyperglycemia and is associated with an increased length of stay.19 Additionally, unlike insulin, metformin does not increase the risk of hypoglycemia. Finally, a recent matched cohort study comparing the use of oral hypoglycemic agents (metformin, thiazolidines, and sulfonylureas) vs insulin monotherapy in patients undergoing emergency abdominal surgery showed that the patients admitted with sepsis and treated with oral agents had a lower 30-day mortality rate and a shorter length of stay.20 Based on the evidence showing that inpatient oral hypoglycemic agents improve quality metrics and mitigate safety events, the ADA advocates resuming oral antihyperglycemic medications (most commonly metformin) 1 to 2 days before discharge.7
WHAT YOU SHOULD DO INSTEAD
Clinicians should continue metformin in all hospitalized patients who are not at significant risk of developing lactic acidosis. Risk factors for MALA include severe sepsis (in the setting of end-organ damage as defined by systemic inflammatory response syndrome criteria), hypoxia requiring oxygen supplementation, hypoperfusion (as from CHF), AKI, CKD (eGFR <30 mL/min/1.73 m2), and advanced cirrhosis. Given the high rates of hypoxia and AKI in admitted patients with COVID-19, clinicians should hold metformin on admission. Continue metformin for patients receiving IV contrast media with an eGFR >30 mL/min/1.73 m2. For patients undergoing IA catheter studies associated with a risk for renal artery emboli, or in patients with renal insufficiency (eGFR <30 mL/min/1.73 m2), temporarily hold metformin for 48 hours. When held, restart metformin as soon as risk factors resolve.
RECOMMENDATIONS
- Hold metformin in patients with or undergoing the following:
- High risk for or currently suffering from decompensated heart failure, severe sepsis, or other disease states resulting in hypoxia or tissue hypoperfusion;
- An eGFR <30 mL/min/1.73 m2 or AKI; resume metformin when the AKI resolves;
- COVID-19 infection, until the risk of hypoxia has resolved;
- IV contrast study in the presence of acute renal failure or an eGFR <30 mL/min/1.73 m2; resume metformin 48 hours after contrast administration;
- Intra-arterial catheter study that might result in renal artery emboli; resume metformin when renal function normalizes.
- Continue metformin in all hospitalized patients in the absence of the aforementioned disease states or contrast-related indications.
CONCLUSION
Returning to the patient in our clinical scenario, we recommend continuing metformin given the lack of risk factors or disease states associated with increased lactic acidosis. The practice of withholding metformin in hospitalized patients for fear of MALA is based on minimal evidence. Clinicians should, however, hold metformin in patients who have true contraindications, including existing acidosis, hypoperfusion, renal insufficiency, CHF, severe sepsis, hypoxia, advanced cirrhosis, and COVID-19. With regard to iodinated contrast studies, temporarily withhold metformin for 48 hours in patients with an eGFR <30 mL/min/1.73 m2, acute kidney injury, or in patients undergoing an IA catheter study at risk for renal artery emboli. Patients should be restarted on metformin 48 hours after these studies and as renal function normalizes. When withholding metformin during a hospitalization, restart it once risk factors have resolved.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing [email protected]
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A hospitalist admits a 29-year-old man with hypertension, obesity, and type 2 diabetes (type 2 DM) for a posterior neck abscess that failed outpatient oral antibiotic therapy. The patient’s medications include metformin monotherapy. Vital signs taken upon admission include a blood pressure of 136/82 mm Hg, heart rate of 98 beats per minute, respiratory rate 18 of breaths per minute, oxygen saturation of 100% on room air, and temperature of 38.5 oC. Laboratory evaluation revealed a glucose level of 212 mg/dL, with a hemoglobin A1c of 8.0%, lactic acid of 1.4 mmol/L, and normal renal and hepatic function. Based on these findings, the hospitalist holds metformin and starts the patient on sliding-scale insulin therapy.
WHY YOU MIGHT THINK ROUTINELY HOLDING METFORMIN IN THE HOSPITAL IS NECESSARY
Following the introduction of metformin in the United States, the US Food and Drug Administration (FDA) received 47 confirmed reports of nonfatal lactic acidosis associated with the use of metformin, all of which involved cardiac disease (specifically congestive heart failure [CHF]), renal insufficiency, hypoxia, or sepsis.2 Consequently, the FDA listed CHF as a contraindication to metformin use; however, it has since changed the use of metformin in CHF from a contraindication to a warning/precaution for lactic acidosis. The FDA also added a warning against the use of metformin in patients with sepsis or in patients older than 80 years who have abnormal creatinine clearance.
Acute kidney injury, a common inpatient condition, occurs in 20% of hospitalized patients and more than 50% of intensive care patients.3 Moreover, a retrospective observational study showed approximately 50% of all patients hospitalized for COVID-19 had AKI.4 Iodinated contrast, a diagnostic media commonly used in the hospital, may also increase the risk of renal dysfunction. The FDA recommends providers discontinue metformin at or before initiating imaging studies with iodinated contrast5 in patients with an estimated glomerular filtration rate (eGFR) between 30 and 60 mL/min/1.73 m2. The FDA also advises that providers not restart metformin until 48 hours after an intra-arterial (IA) or intravenous (IV) contrast study in patients with an eGFR <60 mL/min/1.73 m2 (equivalent to chronic kidney disease [CKD] stage 3 or worse).5 The American Diabetes Association (ADA) recommends the same eGFR cutoff level in its clinical practice recommendations, as well as withholding metformin 48 hours before patients receive IV contrast.6 Given the risk of AKI in hospitalized patients and concerns of increased MALA, clinicians reflexively hold metformin.
Holding metformin is also consistent with professional guidelines. The 2009 American Association of Clinical Endocrinology and ADA Consensus Statement on Inpatient Glycemic Control recommends cautious use of metformin in the inpatient setting “because of the potential development of a contraindication during the hospitalization.”7 Similarly, the 2012 Endocrine Society guidelines recommend withholding metformin in almost all hospitalized patients.8
WHY ROUTINELY HOLDING METFORMIN IN THE HOSPITAL IS NOT BENEFICIAL
Routinely holding metformin in hospitalized patients is unnecessary and potentially harmful. First, MALA is exceedingly rare, and experts question the causal link. Furthermore, iodinated contrast does not place patients with normal renal function at increased risk of MALA. Finally, holding metformin leads to worsened glycemic control and increased use of insulin, both of which may result in adverse patient outcomes.
The concerns about MALA stem from clinical experiences with phenformin, an older and more potent biguanide. Phenformin shares a similar mechanism of action with metformin but causes more lactic acid production. In 1978, following 306 documented cases of phenformin-associated lactic acidosis, the FDA removed this medication from the market.9 Since the initial 47 cases of MALA were reported to the FDA, repeated studies and systematic reviews have disputed the link between metformin and lactic acidosis, particularly in the absence of significant risk factors or in patients with an eGFR ≥30 mL/min/1.73 m2. In fact, a large observational study showed a reduction in acidosis and mortality in outpatients with stage 3a CKD (eGFR, 45-59 mL/min/1.73 m2) who were taking metformin compared to patients taking insulin or other oral hypoglycemics agents.10 In patients with stage 3b CKD (eGFR, 30-44 mL/min/1.73 m2), this study found no difference in the same outcomes.10
Studies show that metformin does not cause elevated lactate levels in patients with stage 4 CKD (eGFR >15mL/min/1.732) or lower stages of CKD as long as doses are adjusted appropriately to reflect renal function.11 These and other investigations reveal that in the absence of other risk factors, metformin does not cause lactic acidosis (Table).10-15 Based on these findings, the Endocrine Society changed the strength of its recommendation to withhold metformin in hospitalized patients to “weak,” with “very low-quality evidence.” The FDA similarly revised its warnings8 to allow metformin use in all patients with an eGFR ≥30 mL/min/1.73 m2. A large community-based cohort study, which demonstrated no association between hospitalization with acidosis and metformin use in patients with stage 3b CKD or lower stages of CKD, supports this change in treatment threshold.15
Published evidence also does not support the practice of routinely holding metformin before contrast administration, despite concerns regarding contrast-induced nephropathy. Retrospective chart reviews and a direct comparison in human models have not shown any significant difference in the risk of AKI between the IV and IA contrast.16 Moreover, evidence suggests no interaction between metformin and contrast media in patients with normal renal function.17 In response, the American College of Radiology, Canadian Association of Radiology, Royal College of Radiologists, and Royal Australian and New Zealand College of Radiologists all recommend continuing metformin in patients with normal renal function (eGFR ≥30 mL/min/1.73m2) receiving IV contrast. They advise holding metformin for 48 hours in patients with renal insufficiency (eGFR <30 mL/min/1.73m2) or those undergoing IA catheter studies that might result in renal artery emboli.18
Finally, continuing metformin maintains steady blood glucose control. The practice of replacing metformin with sliding-scale insulin monotherapy for hospitalized patients significantly increases the risk of hyperglycemia and is associated with an increased length of stay.19 Additionally, unlike insulin, metformin does not increase the risk of hypoglycemia. Finally, a recent matched cohort study comparing the use of oral hypoglycemic agents (metformin, thiazolidines, and sulfonylureas) vs insulin monotherapy in patients undergoing emergency abdominal surgery showed that the patients admitted with sepsis and treated with oral agents had a lower 30-day mortality rate and a shorter length of stay.20 Based on the evidence showing that inpatient oral hypoglycemic agents improve quality metrics and mitigate safety events, the ADA advocates resuming oral antihyperglycemic medications (most commonly metformin) 1 to 2 days before discharge.7
WHAT YOU SHOULD DO INSTEAD
Clinicians should continue metformin in all hospitalized patients who are not at significant risk of developing lactic acidosis. Risk factors for MALA include severe sepsis (in the setting of end-organ damage as defined by systemic inflammatory response syndrome criteria), hypoxia requiring oxygen supplementation, hypoperfusion (as from CHF), AKI, CKD (eGFR <30 mL/min/1.73 m2), and advanced cirrhosis. Given the high rates of hypoxia and AKI in admitted patients with COVID-19, clinicians should hold metformin on admission. Continue metformin for patients receiving IV contrast media with an eGFR >30 mL/min/1.73 m2. For patients undergoing IA catheter studies associated with a risk for renal artery emboli, or in patients with renal insufficiency (eGFR <30 mL/min/1.73 m2), temporarily hold metformin for 48 hours. When held, restart metformin as soon as risk factors resolve.
RECOMMENDATIONS
- Hold metformin in patients with or undergoing the following:
- High risk for or currently suffering from decompensated heart failure, severe sepsis, or other disease states resulting in hypoxia or tissue hypoperfusion;
- An eGFR <30 mL/min/1.73 m2 or AKI; resume metformin when the AKI resolves;
- COVID-19 infection, until the risk of hypoxia has resolved;
- IV contrast study in the presence of acute renal failure or an eGFR <30 mL/min/1.73 m2; resume metformin 48 hours after contrast administration;
- Intra-arterial catheter study that might result in renal artery emboli; resume metformin when renal function normalizes.
- Continue metformin in all hospitalized patients in the absence of the aforementioned disease states or contrast-related indications.
CONCLUSION
Returning to the patient in our clinical scenario, we recommend continuing metformin given the lack of risk factors or disease states associated with increased lactic acidosis. The practice of withholding metformin in hospitalized patients for fear of MALA is based on minimal evidence. Clinicians should, however, hold metformin in patients who have true contraindications, including existing acidosis, hypoperfusion, renal insufficiency, CHF, severe sepsis, hypoxia, advanced cirrhosis, and COVID-19. With regard to iodinated contrast studies, temporarily withhold metformin for 48 hours in patients with an eGFR <30 mL/min/1.73 m2, acute kidney injury, or in patients undergoing an IA catheter study at risk for renal artery emboli. Patients should be restarted on metformin 48 hours after these studies and as renal function normalizes. When withholding metformin during a hospitalization, restart it once risk factors have resolved.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing [email protected]
1. Kopec KT, Kowalski MJ. Metformin-associated lactic acidosis (MALA): case files of the Einstein Medical Center medical toxicology fellowship. J Med Toxicol. 2013;9(1):61-66. https://doi.org/10.1007/s13181-012-0278-3
2. Misbin RI, Green L, Stadel BV, Gueriguian JL, Gubbi A, Fleming GA. Lactic acidosis in patients with diabetes treated with metformin. N Engl J Med. 1998;338(4):265-266. https://doi.org/10.1056/nejm199801223380415
3. Wang HE, Muntner P, Chertow GM, Warnock DG. Acute kidney injury and mortality in hospitalized patients. Am J Nephrol. 2012;35(4):349-355. https://doi.org/10.1159/000337487
4. Chan L, Chaudhary K, Saha A, et al; Mount Sinai COVID Informatics Center (MSCIC), Li L. AKI in hospitalized patients with COVID-19. J Am Soc Nephrol. 2021;32(1):151-160. https://doi.org/10.1681/asn.2020050615
5. US Food and Drug Administration. FDA drug safety communication: FDA revises warnings regarding use of the diabetes medicine metformin in certain patients with reduced kidney function. Updated November 14, 2017. Accessed June 22, 2021. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-revises-warnings-regarding-use-diabetes-medicine-metformin-certain
6. American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes—2019. Diabetes Care. 2019;42 (Suppl 1):S90-S102. https://doi.org/10.2337/dc19-s009
7. Moghissi ES, Korytkowski MT, DiNardo M, et al; American Association of Clinical Endocrinologists; American Diabetes Association. Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care. 2009;32(6):1119-1131. https://doi.org/10.2337/dc09-9029
8. Umpierrez GE, Hellman R, Korytkowski MT, et al; Endocrine Society. Management of hyperglycemia in hospitalized patients in non-critical care setting: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(1):16-38. https://doi.org/10.1210/jc.2011-2098
9. Misbin RI. Phenformin-associated lactic acidosis: pathogenesis and treatment. Ann Intern Med. 1977;87(5):591-595. https://doi.org/10.7326/0003-4819-87-5-591
10. Ekström N, Schiöler L, Svensson AM, et al. Effectiveness and safety of metformin in 51 675 patients with type 2 diabetes and different levels of renal function: a cohort study from the Swedish National Diabetes Register. BMJ Open. 2012;2(4):e001076. https://doi.org/10.1136/bmjopen-2012-001076
11. Lalau JD, Kajbaf F, Bennis Y, Hurtel-Lemaire AS, Belpaire F, De Broe ME. Metformin treatment in patients with type 2 diabetes and chronic kidney disease stages 3A, 3B, or 4. Diabetes Care. 2018;41(3):547-553. https://doi.org/10.2337/dc17-2231
12. Brown JB, Pedula K, Barzilay J, Herson MK, Latare P. Lactic acidosis rates in type 2 diabetes. Diabetes Care. 1998;21(10):1659-1663. https://doi.org/10.2337/diacare.21.10.1659
13. Lalau JD, Race JM. Lactic acidosis in metformin-treated patients. Prognostic value of arterial lactate levels and plasma metformin concentrations. Drug Saf. 1999;20(4):377-384. https://doi.org/10.2165/00002018-199920040-00006
14. Salpeter SR, Greyber E, Pasternak GA, Salpeter Posthumous EE. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database Syst Rev. 2010;(1):CD002967. https://doi.org/10.1002/14651858.cd002967.pub3
15. Lazarus B, Wu A, Shin JI, et al. Association of metformin use with risk of lactic acidosis across the range of kidney function: a community-based cohort study. JAMA Intern Med. 2018;178(7):903-910. https://doi.org/10.1001/jamainternmed.2018.0292
16. McDonald JS, Leake CB, McDonald RJ, et al. Acute kidney injury after intravenous versus intra-arterial contrast material administration in a paired cohort. Invest Radiol. 2016;51(12):804-809. https://doi.org/10.1097/rli.0000000000000298
17. Zeller M, Labalette-Bart M, Juliard JM, et al. Metformin and contrast-induced acute kidney injury in diabetic patients treated with primary percutaneous coronary intervention for ST segment elevation myocardial infarction: a multicenter study. Int J Cardiol. 2016;220:137-142. https://doi.org/10.1016/j.ijcard.2016.06.076
18. Goergen SK, Rumbold G, Compton G, Harris C. Systematic review of current guidelines, and their evidence base, on risk of lactic acidosis after administration of contrast medium for patients receiving metformin. Radiology. 2010;254(1):261-269. https://doi.org/10.1148/radiol.09090690
19. Ambrus DB, O’Connor MJ. Things we do for no reason: sliding-scale insulin as monotherapy for glycemic control in hospitalized patients. J Hosp Med. 2019;14(2):114-116. https://doi.org/10.12788/jhm.3109
20. Haltmeier T, Benjamin E, Beale E, Inaba K, Demetriades D. Insulin-treated patients with diabetes mellitus undergoing emergency abdominal surgery have worse outcomes than patients treated with oral agents. World J Surg. 2016;40(7):1575-1582. https://doi.org/10.1007/s00268-016-3469-2
1. Kopec KT, Kowalski MJ. Metformin-associated lactic acidosis (MALA): case files of the Einstein Medical Center medical toxicology fellowship. J Med Toxicol. 2013;9(1):61-66. https://doi.org/10.1007/s13181-012-0278-3
2. Misbin RI, Green L, Stadel BV, Gueriguian JL, Gubbi A, Fleming GA. Lactic acidosis in patients with diabetes treated with metformin. N Engl J Med. 1998;338(4):265-266. https://doi.org/10.1056/nejm199801223380415
3. Wang HE, Muntner P, Chertow GM, Warnock DG. Acute kidney injury and mortality in hospitalized patients. Am J Nephrol. 2012;35(4):349-355. https://doi.org/10.1159/000337487
4. Chan L, Chaudhary K, Saha A, et al; Mount Sinai COVID Informatics Center (MSCIC), Li L. AKI in hospitalized patients with COVID-19. J Am Soc Nephrol. 2021;32(1):151-160. https://doi.org/10.1681/asn.2020050615
5. US Food and Drug Administration. FDA drug safety communication: FDA revises warnings regarding use of the diabetes medicine metformin in certain patients with reduced kidney function. Updated November 14, 2017. Accessed June 22, 2021. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-revises-warnings-regarding-use-diabetes-medicine-metformin-certain
6. American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes—2019. Diabetes Care. 2019;42 (Suppl 1):S90-S102. https://doi.org/10.2337/dc19-s009
7. Moghissi ES, Korytkowski MT, DiNardo M, et al; American Association of Clinical Endocrinologists; American Diabetes Association. Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care. 2009;32(6):1119-1131. https://doi.org/10.2337/dc09-9029
8. Umpierrez GE, Hellman R, Korytkowski MT, et al; Endocrine Society. Management of hyperglycemia in hospitalized patients in non-critical care setting: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(1):16-38. https://doi.org/10.1210/jc.2011-2098
9. Misbin RI. Phenformin-associated lactic acidosis: pathogenesis and treatment. Ann Intern Med. 1977;87(5):591-595. https://doi.org/10.7326/0003-4819-87-5-591
10. Ekström N, Schiöler L, Svensson AM, et al. Effectiveness and safety of metformin in 51 675 patients with type 2 diabetes and different levels of renal function: a cohort study from the Swedish National Diabetes Register. BMJ Open. 2012;2(4):e001076. https://doi.org/10.1136/bmjopen-2012-001076
11. Lalau JD, Kajbaf F, Bennis Y, Hurtel-Lemaire AS, Belpaire F, De Broe ME. Metformin treatment in patients with type 2 diabetes and chronic kidney disease stages 3A, 3B, or 4. Diabetes Care. 2018;41(3):547-553. https://doi.org/10.2337/dc17-2231
12. Brown JB, Pedula K, Barzilay J, Herson MK, Latare P. Lactic acidosis rates in type 2 diabetes. Diabetes Care. 1998;21(10):1659-1663. https://doi.org/10.2337/diacare.21.10.1659
13. Lalau JD, Race JM. Lactic acidosis in metformin-treated patients. Prognostic value of arterial lactate levels and plasma metformin concentrations. Drug Saf. 1999;20(4):377-384. https://doi.org/10.2165/00002018-199920040-00006
14. Salpeter SR, Greyber E, Pasternak GA, Salpeter Posthumous EE. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database Syst Rev. 2010;(1):CD002967. https://doi.org/10.1002/14651858.cd002967.pub3
15. Lazarus B, Wu A, Shin JI, et al. Association of metformin use with risk of lactic acidosis across the range of kidney function: a community-based cohort study. JAMA Intern Med. 2018;178(7):903-910. https://doi.org/10.1001/jamainternmed.2018.0292
16. McDonald JS, Leake CB, McDonald RJ, et al. Acute kidney injury after intravenous versus intra-arterial contrast material administration in a paired cohort. Invest Radiol. 2016;51(12):804-809. https://doi.org/10.1097/rli.0000000000000298
17. Zeller M, Labalette-Bart M, Juliard JM, et al. Metformin and contrast-induced acute kidney injury in diabetic patients treated with primary percutaneous coronary intervention for ST segment elevation myocardial infarction: a multicenter study. Int J Cardiol. 2016;220:137-142. https://doi.org/10.1016/j.ijcard.2016.06.076
18. Goergen SK, Rumbold G, Compton G, Harris C. Systematic review of current guidelines, and their evidence base, on risk of lactic acidosis after administration of contrast medium for patients receiving metformin. Radiology. 2010;254(1):261-269. https://doi.org/10.1148/radiol.09090690
19. Ambrus DB, O’Connor MJ. Things we do for no reason: sliding-scale insulin as monotherapy for glycemic control in hospitalized patients. J Hosp Med. 2019;14(2):114-116. https://doi.org/10.12788/jhm.3109
20. Haltmeier T, Benjamin E, Beale E, Inaba K, Demetriades D. Insulin-treated patients with diabetes mellitus undergoing emergency abdominal surgery have worse outcomes than patients treated with oral agents. World J Surg. 2016;40(7):1575-1582. https://doi.org/10.1007/s00268-016-3469-2
© 2021 Society of Hospital Medicine
An Initiative to Improve 30-Day Readmission Rates Using a Transitions-of-Care Clinic Among a Mixed Urban and Rural Veteran Population
Hospital readmissions are a significant problem in the United States, affecting 15% to 30% of discharges and incurring costs of more than $17 billion annually.1 Timely posthospitalization follow-up visits are critical to ensure the effective transfer of patients to the outpatient setting; such visits reduce readmission rates as well as hospital length of stay and overall health care resource utilization.2-4 Patients who receive inadequate follow-up care (ie, within 4 weeks of discharge) are significantly more likely to be readmitted than those who receive close follow-up care.5
Due to the large clinical and financial consequences associated with hospital readmission, a variety of interventions have been studied, including home visits, telemonitoring, medication management, telephone calls, and postdischarge clinics.6,7 While studies have not shown postdischarge clinics to be universally efficacious in reducing readmission rates, there is increasing evidence of reduced readmission rates in clinics that target high-risk patients (eg, patients with congestive heart failure [CHF]) rather than the total population.2 A study by Hernandez et al that evaluated the relationship between early physician follow-up and 30-day readmissions showed a significantly lower readmission rate among hospitals with higher follow-up rates.8 Similarly, patients with CHF in a large, integrated health system who were seen within 7 days of discharge had an odds ratio (OR) of 0.81 (95% CI, 0.70-0.94) for 30-day readmissions.9
Transitions-of-care clinics (TOCC), designed to provide early postdischarge follow-up to high-risk patients, have been shown to reduce 30-day readmission rates,3,4,10,11 especially in clinics that have same-physician follow-up visits rather than follow-up visits with a community primary care physician (PCP).12 The most pronounced impact of postdischarge follow-up is seen in high-risk patients with high complexity or high severity of disease; however, complex rural patients are less likely to have access to specialty care.13 As a result, since rural residents must travel farther for specialty care, they are seen less frequently than their urban counterparts.14,15
Prior to our TOCC initiative, the Iowa City VA (ICVA) ranked in the fifth quintile of the Veterans Health Administration (VHA)
To meet these challenges, we implemented a TOCC to deliver timely postdischarge care focusing on high-risk and high-complexity patients. To address access-to-care issues of patients living in rural areas within the ICVA, we included virtual follow-up visits as a key component of our intervention.16,17 The aim of this project was to decrease 30-day readmission rates of ICVA patients by 20% within 12 months of implementation.
METHODS
Setting/Study Population
The ICVA serves 184,000 veterans stretched over 50 counties in eastern Iowa, western Illinois, and northern Missouri, with more than 60% of these patients residing in rural areas. Patients were initially eligible for the TOCC if they had an admission diagnosis of CHF and a CAN score > 85 at the time of discharge. The CAN score, developed by the VA to assess the risk of hospital readmission in individual patients, factors in several variables, including demographics, coexisting conditions, vital signs, utilization of services, pharmacy visits, and laboratory results. Patients in the top 5% (95-99) have a readmission rate of 20% at 90 days. Since the CAN is a proprietary tool, it may not be published in full; however, this assessment tool is commonly used and frequently cited in VA research.18-22 The CAN score is expressed as a percentile ranging from 0 (lowest risk) to 99 (highest risk). Patient eligibility was expanded during subsequent Plan-Do-Study-Act (PDSA) cycles, as outlined below. Patient eligibility was expanded during subsequent PDSA cycles (also outlined below). A review by a local institutional review board was obtained, and the study was classified as exempt due to the use of deidentified data. Standards for Quality Improvement Reporting Excellence 2.0 guidelines were used to construct the manuscript.
Magnitude Assessment
The numbers of discharges, readmissions within 30 days, emergency department (ED) visits by all discharged veterans, and veterans discharged with a CHF hospital diagnosis were recorded from February 2017 to February 2018, which were the 12 months immediately preceding the pilot implementation.
Intervention
The primary intervention was referral to the newly formed ICVA TOCC. The multidisciplinary TOCC team consisted of hospitalists, pharmacists, schedulers, and discharge planners/care managers. Patients were identified by the hospitalist team during admission; prior to hospital discharge, these patients were referred to TOCC discharge planners to schedule appropriate follow-up appointments. Virtual follow-up visits were conducted using a patient’s home technology; in cases where a patient lacked adequate technology capabilities (eg, no computer or internet access), the ICVA provided a tablet device with cellular internet capability for temporary use. Specific clinical activities included medication reconciliation by a pharmacist, follow-up of pending laboratory studies, imaging studies, pathology results, medical diagnosis education, counseling regarding dietary restrictions, and contingency planning outside of an ED visit in the event of a change in clinical status. In addition, the TOCC aimed to facilitate a smooth transition of care back to the PCP by arranging follow-up appointments, providing visit summaries, and scheduling consults with specialty care, as appropriate.
Measures
The primary objective measure was the 30-day readmission rate in the ICVA hospital. Secondary measures included the number of VHA ED visits within 30 days of discharge. The main process measures were the number of hospital discharges per month, the number of TOCC referrals, the number of TOCC appointments made, the number of virtual and in-person visits, and the percentage of appointment “no-shows.”
Implementation
The TOCC was piloted from April 2018 to October 2018. During the pilot phase, TOCC enrollment was limited to virtual appointments and to patients with an admission diagnosis of CHF and a CAN score of > 85. The TOCC had staff on-site 2 days a week; this included pharmacists to reconcile medications and hospitalists to address follow-up care needs.
The TOCC clinic was temporarily closed at the end of October 2018 to analyze pilot results. Based on stakeholder feedback, changes made as part of the second PDSA cycle included expanding eligibility criteria to any hospital admission diagnosis and to patients with a CAN score < 85 if the hospitalist team felt the patient was likely to benefit from TOCC follow-up. In addition, on-site clinic staffing was expanded from 2 to 5 days per week to improve access, and the option for an in-person visit was added based on concerns some veterans expressed regarding the use of the technology at home. Finally, a formal resident program was added, and the order set for referrals was simplified. The TOCC was restarted in February 2019, and TOCC metrics were reviewed monthly. By July 2019, we identified issues with TOCC referrals and appointment creation that required additional modifications to the intervention.
A third PDSA cycle was initiated in July 2019 and included major changes, notably the formation of a designated TOCC committee. The committee appointed a dedicated TOCC scheduler whose role was to reduce confusion regarding scheduling, to update the discharge instructions/orders template to lower incidences of “double-booking” that occurred with PCP and TOCC appointments, to modify discharge educational instruction regarding virtual visits and tablet use, to adjust the TOCC-PCP handoff, and to formalize interactions between discharge coordinators and residents to review possible referrals every morning (Appendix Figure 1).
Statistical Analysis
Run charts were constructed by plotting monthly primary outcome values and monthly process metrics (Figure, Appendix Figure 2, Appendix Figure 3). Chi-square tests were used to compare 30-day readmission rates before and after the intervention.
Mean (SD) or counts and percentages were used to describe the distribution of continuous and categorical variables, respectively. Kruskal-Wallis test, t test, or chi-square tests were used, as appropriate, across categories. Generalized linear models with a logistic link function were used to test for differences between patients who kept their appointment at the TOCC and those who did not keep their TOCC appointment (both unadjusted and adjusted for all of the covariates previously mentioned). In addition, generalized linear models were also used to compare outcomes between TOCC patients seen virtually vs those seen in-person (both unadjusted and adjusted for all the covariates previously mentioned). All statistical tests were considered significant at a two-sided P < .05. All analyses were performed using SAS software version 9.4 (SAS Institute Inc).
RESULTS
Magnitude Assessment
During the preimplementation period (February 2017-February 2018), there were 3014 patient discharges from ICVA and 343 readmissions, resulting in a readmission rate of 11.4%. Among patients with a hospital-admission diagnosis of cardiorespiratory disease, which included patients with CHF, there were 381 discharges and 46 readmissions, resulting in a readmission rate of 12.1%.
Primary Outcome
During the pilot phase, which was conducted from April 2018 to October 2018, 142 patients who met inclusion criteria (CHF diagnosis and a CAN score > 85) were discharged from ICVA, and 56 referrals to the TOCC were placed. The readmission rate among the cardiorespiratory cohort of veterans was 9.5%.
During the expansion of the intervention from February 2019 to February 2020, there were 2844 discharges from the ICVA and 291 readmissions, resulting in a readmission rate of 10.2%. However, there was a further decrease in the readmission rate after the third PDSA cycle was initiated in July 2019 (Appendix Figure 1). The readmission rate was 9.2% in the final 6 months of the intervention period, and 7.9% in the final 3 months.
When comparing the 6 months following the third PDSA cycle to the magnitude assessment period, there was a relative readmission reduction of 19.3% (P = .04), and an absolute reduction of 2.2%. If the final 3 months of the intervention period are included, there was an absolute reduction of 3.5% and a relative reduction of 30.7% (P = .01). Notably, before the pilot phase, ICVA was in the fifth quintile for HWR among VA hospitals but improved to the second quintile by the end of the expansion phase.
Process Outcomes
Process metrics for TOCC referrals, the number of patients seen, and the number of virtual and in-person visits over time are shown in Appendix Figure 3. Rates of TOCC referrals and the number of TOCC visits were lower than anticipated during the first 5 months of the intervention. However, TOCC referrals increased significantly after we implemented the previously described changes as part of the third PDSA cycle. As a result, total, virtual, and in-person visits also significantly increased from July 2019 to February 2020. The proportion of patients choosing virtual vs in-person visits fluctuated over time, but virtual visits were generally chosen more often than in-person visits.
Statistical Modeling
Baseline Data
Cohort characteristics are shown in Table 1. The cohort, which reflected the ICVA population, was predominantly male (96%) and White (93%), with a mean age of 67 years. The population was approximately half urban and half rural in composition, and the most common reason for hospital admission was cardiac. Other than a small but statistically significant difference in CAN scores, there were no significant differences between patients who kept their TOCC appointment and those who did not. There were also no differences in baseline characteristics between patients who chose virtual follow-up and patients who chose in-person follow-up, including the proportion of urban and rural patients.
Outcomes
Patients who kept their TOCC appointments had a 30-day readmission rate of 9.6%, which was significantly lower than the 30-day readmission rate of 27% in the group that did not keep their TOCC appointment (P < .001). Similarly, the percentage of patients treated in the ED was 15% in the TOCC group compared to 31.2% in the group that canceled their appointment (P < .001) (Table 1). In the multivariable analysis, patients who were seen in the TOCC group had an OR for 30-day readmission of 0.35 (95% CI, 0.19-0.62, P < .001), and an OR for ED visits of 0.39 (95% CI, 0.23-0.65; P < .001) (Table 2). There was no statistically significant difference in 6-month mortality between the two groups. In the virtual group compared to the in-person group, there were no statistically significant differences in outcomes between the two groups in the unadjusted or adjusted analysis (Table 2).
DISCUSSION
In the expansion phase, eligibility was expanded to include any hospital indication but continued to focus on high-risk patients. Existing literature suggests that providing postdischarge care to all patients, including low- or medium-risk patients, may not be as impactful as enrolling high-risk patients only. For instance, a postdischarge clinic offered to all patients at a VA system in Colorado did not reduce readmission rates compared to PCP follow-up.23 In contrast, a study of more than 10,000 high-risk urban patients demonstrated that postdischarge care resulted in a 9.3% reduction in readmission risk.24 Our data are consistent with the previously published studies, as the average CAN score of patients seen in TOCC was 90, suggesting a high risk of readmission. In the final 12 months of the intervention, 15% of discharged patients were seen at the TOCC clinic, suggesting that targeted intervention within the small subset of high-risk patients was sufficient to achieve our primary aim. Of note, among patients who did not meet the inclusion criteria for TOCC referral (ie, patients not considered high risk [CAN score ≤ 85]), the rate of readmissions was 8.6%.
Most of the available research on the efficacy of postdischarge clinics was conducted in urban environments. Our ICVA population sees a large proportion of rural veterans, who account for just over 50% of the discharge population. In a study of more than 2 million Medicare patients discharged from US hospitals, the 30-day readmission rates and adjusted mortality rates were higher among patients in rural counties, and post–acute care seemed to have a greater impact in rural rather than urban settings.25 Previous studies have demonstrated that virtual visits have the potential to improve readmission rates, especially in patients with CHF26 and in patients at the highest risk for readmission.27 In our study, the pilot phase offered only virtual visits, but we subsequently added an in-person option based on veteran feedback. Interestingly, over the next 12 months, virtual visits were more popular with both urban and rural veterans, and there were no differences in the number of rural patients in the in-person vs the virtual group. These findings suggest factors other than rurality influenced the decision to choose virtual follow-up visits over in-person visits. Future studies should seek to determine the extent to which factors such as age, race, educational level, and socioeconomic circumstances impact veterans’ follow-up decisions. Not only were outcomes among patients who chose virtual visits the same as those of patients who chose in-person visits, but both of these groups had better outcomes compared to the non-TOCC group (Table 2). This finding demonstrating the efficacy of virtual visits among rural and urban patients has taken on increased significance due to the COVID-19 pandemic, as virtual visits offer a safer option, one that minimizes physical contact.
Our quality improvement analysis included a statistical comparison of patients seen vs those not seen at the TOCC. Patients who were referred to the TOCC but chose not to keep their appointment were similar to those seen in TOCC in terms of age, CAN score, rurality, and hospital diagnosis, but readmission rates were substantially higher in this group even after adjustments for covariates (Table 2). Evaluating causality in interventions aimed to reduce hospital readmission rates is complicated.28 Our findings add greater plausibility to the utility of TOCC in accounting for at least a portion of the reported decrease in ICVA 30-day readmissions.
Our study has several strengths, including an observation period longer than 2 years, a large population of discharged veterans within an integrated healthcare system, and a large proportion of patients living in rural areas. Another strength of our study is the innovative nature of the intervention, which features a multidisciplinary team and the option of virtual or in-person visits. Nevertheless, this study also has several important limitations. As a single-center study, our findings may not be generalizable to other institutions, especially those outside the VHA system. Similarly, our study population reflected that of the ICVA, which may limit generalizability to a more diverse population. While we attempted to account in our statistical modeling for baseline differences between referred patients seen by the TOCC and those referred but not seen, we cannot exclude residual confounding between the groups. Specifically, the comparison of patients who did and did not choose TOCC follow-up introduces the possibility of selection bias. Future randomized/controlled studies will need to evaluate whether TOCC is more effective than the standard of care to reduce readmissions. Finally, since the analysis period following the final PDSA cycle was compressed due to the onset of the COVID-19 pandemic in the United States, no data are available regarding the sustained impacts of changes made during this cycle.
CONCLUSION
A multidisciplinary TOCC within the ICVA, featuring both virtual and in-person visits, reduced 30-day readmission rates by 19.3%; this approach to care was especially effective in patients with CHF. Virtual visits were the follow-up mode of choice for both urban and rural veterans, and there was no difference in outcomes between these two follow-up options. Future studies will focus on additional quality metrics, including cost-effectiveness and patient satisfaction.
1. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428. https://doi.org/10.1056/nejmsa0803563
2. Doctoroff L. Postdischarge clinics and hospitalists: a review of the evidence and existing models. J Hosp Med. 2017;12(6):467-471. https://doi.org/10.12788/jhm.2750
3. Koehler BE, Richter KM, Youngblood L, et al. Reduction of 30-day postdischarge hospital readmission or emergency department (ED) visit rates in high-risk elderly medical patients through delivery of a targeted care bundle. J Hosp Med. 2009;4(4):211-218. https://doi.org/10.1002/jhm.427
4. Abrashkin KA, Cho HJ, Torgalkar S, Markoff B. Improving transitions of care from hospital to home: what works? Mt Sinai J Med. 2012;79(5):535-544. https://doi.org/10.1002/msj.21332
5. Misky GJ, Wald HL, Coleman EA. Post-hospitalization transitions: examining the effects of timing of primary care provider follow-up. J Hosp Med. 2010;5(7):392-397. https://doi.org/10.1002/jhm.666
6. Greysen SR, Harrison JD, Kripalani S, et al. Understanding patient-centred readmission factors: a multi-site, mixed-methods study. BMJ Qual Saf. 2017;26(1):33-41. https://doi.org/10.1136/bmjqs-2015-004570
7. Hansen LO, Young RS, Hinami K, Leung A, Williams MV. Interventions to reduce 30-day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520-528. https://doi.org/10.7326/0003-4819-155-8-201110180-00008
8. Hernandez AF, Greiner MA, Fonarow GC, et al. Relationship between early physician follow-up and 30-day readmission among Medicare beneficiaries hospitalized for heart failure. JAMA. 2010;303(17):1716-1722. https://doi.org/10.1001/jama.2010.533
9. Lee KK, Yang J, Hernandez AF, Steimle AE, Go AS. Post-discharge follow-up characteristics associated with 30-day readmission after heart failure hospitalization. Med Care. 2016;54(4):365-372. https://doi.org/10.1097/mlr.0000000000000492
10. Balaban RB, Williams MV. Improving care transitions: hospitalists partnering with primary care. J Hosp Med. 2010;5(7):375-377. https://doi.org/10.1002/jhm.824
11. Rodrigues CR, Harrington AR, Murdock N, et al. Effect of pharmacy-supported transition-of-care interventions on 30-day readmissions: a systematic review and meta-analysis. Ann Pharmacother. 2017;51(10):866-889. https://doi.org/10.1177/1060028017712725
12. van Walraven C, Taljaard M, Etchells E, et al. The independent association of provider and information continuity on outcomes after hospital discharge: implications for hospitalists. J Hosp Med. 2010;5(7):398-405. https://doi.org/10.1002/jhm.716
13. Gruca TS, Pyo TH, Nelson GC. Providing cardiology care in rural areas through vsiting consultant clinics. J Am Heart Assoc. 2016;5(7):e002909. https://doi.org/10.1161/jaha.115.002909
14. Chan L, Hart LG, Goodman DC. Geographic access to health care for rural Medicare beneficiaries. J Rural Health. 2006;22(2):140-146. https://doi.org/10.1111/j.1748-0361.2006.00022.x
15. Burke RE, Jones CD, Coleman EA, Falvey JR, Stevens-Lapsley JE, Ginde AA. Use of post-acute care after hospital discharge in urban and rural hospitals. Am J Accountable Care. 2017;5(1):16-22.
16. Jetty A, Moore MA, Coffman M, Petterson S, Bazemore A. Rural family physicians are twice as likely to use telehealth as urban family physicians. Telemed J E Health. 2018;24(4):268-276. https://doi.org/10.1089/tmj.2017.0161
17. Harrison PL, Hara PA, Pope JE, Young MC, Rula EY. The impact of postdischarge telephonic follow-up on hospital readmissions. Popul Health Manag. 2011;14(1):27-32. https://doi.org/10.1089/pop.2009.0076
18. Wang L, Porter B, Maynard C, et al. Predicting risk of hospitalization or death among patients receiving primary care in the Veterans Health Administration. Med Care. 2013;51(4):368-373. https://doi.org/10.1097/mlr.0b013e31827da95a
19. Spece LJ, Donovan LM, Griffith MF, et al. Initiating low-value inhaled corticosteroids in an inception cohort with chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2020;17(5):589-595. https://doi.org/10.1513/annalsats.201911-854oc
20. Osborne TF, Suarez P, Edwards D, Hernandez-Boussard T, Curtin C. Patient electronic health records score for preoperative risk assessment before total knee arthroplasty. JB JS Open Access. 2020;5(2):e0061. https://doi.org/10.2106/jbjs.oa.19.00061
21. Levy C, Ersek M, Scott W, et al. Life-sustaining treatment decisions initiative: early implementation results of a national Veterans Affairs program to honor veterans’ care preferences. J Gen Intern Med. 2020;35(6):1803-1812. https://doi.org/10.1007/s11606-020-05697-2
22. Ibrahim SA. High-risk patients and utilization of primary care in the US Veterans Affairs health system. JAMA Netw Open. 2020;3(6):e209518. https://doi.org/10.1001/jamanetworkopen.2020.9518
23. Burke RE, Whitfield E, Prochazka AV. Effect of a hospitalist-run postdischarge clinic on outcomes. J Hosp Med. 2014;9(1):7-12. https://doi.org/10.1002/jhm.2099
24. Jenq GY, Doyle MM, Belton BM, Herrin J, Horwitz LI. Quasi-experimental evaluation of the effectiveness of a large-scale readmission reduction program. JAMA Intern Med. 2016;176(5):681-690. https://doi.org/10.1001/jamainternmed.2016.0833
25. Kosar CM, Loomer L, Ferdows NB, Trivedi AN, Panagiotou OA, Rahman M. Assessment of rural-urban differences in postacute care utilization and outcomes among older US adults. JAMA Netw Open. 2020;3(1):e1918738. https://doi.org/10.1001/jamanetworkopen.2019.18738
26. Pandor A, Thokala P, Gomersall T, et al. Home telemonitoring or structured telephone support programmes after recent discharge in patients with heart failure: systematic review and economic evaluation. Health Technol Assess. 2013;17(32):1-207, v-vi. https://doi.org/10.3310/hta17320
27. Low LL, Tan SY, Ng MJM, et al. Applying the integrated practice unit concept to a modified virtual ward model of care for patients at highest risk of readmission: a randomized controlled trial. PloS One. 2017;12(1):e0168757. https://doi.org/10.1371/journal.pone.0168757
28. McIlvennan CK, Eapen ZJ, Allen LA. Hospital readmissions reduction program. Circulation. 2015;131(20):1796-1803. https://doi.org/10.1161/circulationaha.114.010270
Hospital readmissions are a significant problem in the United States, affecting 15% to 30% of discharges and incurring costs of more than $17 billion annually.1 Timely posthospitalization follow-up visits are critical to ensure the effective transfer of patients to the outpatient setting; such visits reduce readmission rates as well as hospital length of stay and overall health care resource utilization.2-4 Patients who receive inadequate follow-up care (ie, within 4 weeks of discharge) are significantly more likely to be readmitted than those who receive close follow-up care.5
Due to the large clinical and financial consequences associated with hospital readmission, a variety of interventions have been studied, including home visits, telemonitoring, medication management, telephone calls, and postdischarge clinics.6,7 While studies have not shown postdischarge clinics to be universally efficacious in reducing readmission rates, there is increasing evidence of reduced readmission rates in clinics that target high-risk patients (eg, patients with congestive heart failure [CHF]) rather than the total population.2 A study by Hernandez et al that evaluated the relationship between early physician follow-up and 30-day readmissions showed a significantly lower readmission rate among hospitals with higher follow-up rates.8 Similarly, patients with CHF in a large, integrated health system who were seen within 7 days of discharge had an odds ratio (OR) of 0.81 (95% CI, 0.70-0.94) for 30-day readmissions.9
Transitions-of-care clinics (TOCC), designed to provide early postdischarge follow-up to high-risk patients, have been shown to reduce 30-day readmission rates,3,4,10,11 especially in clinics that have same-physician follow-up visits rather than follow-up visits with a community primary care physician (PCP).12 The most pronounced impact of postdischarge follow-up is seen in high-risk patients with high complexity or high severity of disease; however, complex rural patients are less likely to have access to specialty care.13 As a result, since rural residents must travel farther for specialty care, they are seen less frequently than their urban counterparts.14,15
Prior to our TOCC initiative, the Iowa City VA (ICVA) ranked in the fifth quintile of the Veterans Health Administration (VHA)
To meet these challenges, we implemented a TOCC to deliver timely postdischarge care focusing on high-risk and high-complexity patients. To address access-to-care issues of patients living in rural areas within the ICVA, we included virtual follow-up visits as a key component of our intervention.16,17 The aim of this project was to decrease 30-day readmission rates of ICVA patients by 20% within 12 months of implementation.
METHODS
Setting/Study Population
The ICVA serves 184,000 veterans stretched over 50 counties in eastern Iowa, western Illinois, and northern Missouri, with more than 60% of these patients residing in rural areas. Patients were initially eligible for the TOCC if they had an admission diagnosis of CHF and a CAN score > 85 at the time of discharge. The CAN score, developed by the VA to assess the risk of hospital readmission in individual patients, factors in several variables, including demographics, coexisting conditions, vital signs, utilization of services, pharmacy visits, and laboratory results. Patients in the top 5% (95-99) have a readmission rate of 20% at 90 days. Since the CAN is a proprietary tool, it may not be published in full; however, this assessment tool is commonly used and frequently cited in VA research.18-22 The CAN score is expressed as a percentile ranging from 0 (lowest risk) to 99 (highest risk). Patient eligibility was expanded during subsequent Plan-Do-Study-Act (PDSA) cycles, as outlined below. Patient eligibility was expanded during subsequent PDSA cycles (also outlined below). A review by a local institutional review board was obtained, and the study was classified as exempt due to the use of deidentified data. Standards for Quality Improvement Reporting Excellence 2.0 guidelines were used to construct the manuscript.
Magnitude Assessment
The numbers of discharges, readmissions within 30 days, emergency department (ED) visits by all discharged veterans, and veterans discharged with a CHF hospital diagnosis were recorded from February 2017 to February 2018, which were the 12 months immediately preceding the pilot implementation.
Intervention
The primary intervention was referral to the newly formed ICVA TOCC. The multidisciplinary TOCC team consisted of hospitalists, pharmacists, schedulers, and discharge planners/care managers. Patients were identified by the hospitalist team during admission; prior to hospital discharge, these patients were referred to TOCC discharge planners to schedule appropriate follow-up appointments. Virtual follow-up visits were conducted using a patient’s home technology; in cases where a patient lacked adequate technology capabilities (eg, no computer or internet access), the ICVA provided a tablet device with cellular internet capability for temporary use. Specific clinical activities included medication reconciliation by a pharmacist, follow-up of pending laboratory studies, imaging studies, pathology results, medical diagnosis education, counseling regarding dietary restrictions, and contingency planning outside of an ED visit in the event of a change in clinical status. In addition, the TOCC aimed to facilitate a smooth transition of care back to the PCP by arranging follow-up appointments, providing visit summaries, and scheduling consults with specialty care, as appropriate.
Measures
The primary objective measure was the 30-day readmission rate in the ICVA hospital. Secondary measures included the number of VHA ED visits within 30 days of discharge. The main process measures were the number of hospital discharges per month, the number of TOCC referrals, the number of TOCC appointments made, the number of virtual and in-person visits, and the percentage of appointment “no-shows.”
Implementation
The TOCC was piloted from April 2018 to October 2018. During the pilot phase, TOCC enrollment was limited to virtual appointments and to patients with an admission diagnosis of CHF and a CAN score of > 85. The TOCC had staff on-site 2 days a week; this included pharmacists to reconcile medications and hospitalists to address follow-up care needs.
The TOCC clinic was temporarily closed at the end of October 2018 to analyze pilot results. Based on stakeholder feedback, changes made as part of the second PDSA cycle included expanding eligibility criteria to any hospital admission diagnosis and to patients with a CAN score < 85 if the hospitalist team felt the patient was likely to benefit from TOCC follow-up. In addition, on-site clinic staffing was expanded from 2 to 5 days per week to improve access, and the option for an in-person visit was added based on concerns some veterans expressed regarding the use of the technology at home. Finally, a formal resident program was added, and the order set for referrals was simplified. The TOCC was restarted in February 2019, and TOCC metrics were reviewed monthly. By July 2019, we identified issues with TOCC referrals and appointment creation that required additional modifications to the intervention.
A third PDSA cycle was initiated in July 2019 and included major changes, notably the formation of a designated TOCC committee. The committee appointed a dedicated TOCC scheduler whose role was to reduce confusion regarding scheduling, to update the discharge instructions/orders template to lower incidences of “double-booking” that occurred with PCP and TOCC appointments, to modify discharge educational instruction regarding virtual visits and tablet use, to adjust the TOCC-PCP handoff, and to formalize interactions between discharge coordinators and residents to review possible referrals every morning (Appendix Figure 1).
Statistical Analysis
Run charts were constructed by plotting monthly primary outcome values and monthly process metrics (Figure, Appendix Figure 2, Appendix Figure 3). Chi-square tests were used to compare 30-day readmission rates before and after the intervention.
Mean (SD) or counts and percentages were used to describe the distribution of continuous and categorical variables, respectively. Kruskal-Wallis test, t test, or chi-square tests were used, as appropriate, across categories. Generalized linear models with a logistic link function were used to test for differences between patients who kept their appointment at the TOCC and those who did not keep their TOCC appointment (both unadjusted and adjusted for all of the covariates previously mentioned). In addition, generalized linear models were also used to compare outcomes between TOCC patients seen virtually vs those seen in-person (both unadjusted and adjusted for all the covariates previously mentioned). All statistical tests were considered significant at a two-sided P < .05. All analyses were performed using SAS software version 9.4 (SAS Institute Inc).
RESULTS
Magnitude Assessment
During the preimplementation period (February 2017-February 2018), there were 3014 patient discharges from ICVA and 343 readmissions, resulting in a readmission rate of 11.4%. Among patients with a hospital-admission diagnosis of cardiorespiratory disease, which included patients with CHF, there were 381 discharges and 46 readmissions, resulting in a readmission rate of 12.1%.
Primary Outcome
During the pilot phase, which was conducted from April 2018 to October 2018, 142 patients who met inclusion criteria (CHF diagnosis and a CAN score > 85) were discharged from ICVA, and 56 referrals to the TOCC were placed. The readmission rate among the cardiorespiratory cohort of veterans was 9.5%.
During the expansion of the intervention from February 2019 to February 2020, there were 2844 discharges from the ICVA and 291 readmissions, resulting in a readmission rate of 10.2%. However, there was a further decrease in the readmission rate after the third PDSA cycle was initiated in July 2019 (Appendix Figure 1). The readmission rate was 9.2% in the final 6 months of the intervention period, and 7.9% in the final 3 months.
When comparing the 6 months following the third PDSA cycle to the magnitude assessment period, there was a relative readmission reduction of 19.3% (P = .04), and an absolute reduction of 2.2%. If the final 3 months of the intervention period are included, there was an absolute reduction of 3.5% and a relative reduction of 30.7% (P = .01). Notably, before the pilot phase, ICVA was in the fifth quintile for HWR among VA hospitals but improved to the second quintile by the end of the expansion phase.
Process Outcomes
Process metrics for TOCC referrals, the number of patients seen, and the number of virtual and in-person visits over time are shown in Appendix Figure 3. Rates of TOCC referrals and the number of TOCC visits were lower than anticipated during the first 5 months of the intervention. However, TOCC referrals increased significantly after we implemented the previously described changes as part of the third PDSA cycle. As a result, total, virtual, and in-person visits also significantly increased from July 2019 to February 2020. The proportion of patients choosing virtual vs in-person visits fluctuated over time, but virtual visits were generally chosen more often than in-person visits.
Statistical Modeling
Baseline Data
Cohort characteristics are shown in Table 1. The cohort, which reflected the ICVA population, was predominantly male (96%) and White (93%), with a mean age of 67 years. The population was approximately half urban and half rural in composition, and the most common reason for hospital admission was cardiac. Other than a small but statistically significant difference in CAN scores, there were no significant differences between patients who kept their TOCC appointment and those who did not. There were also no differences in baseline characteristics between patients who chose virtual follow-up and patients who chose in-person follow-up, including the proportion of urban and rural patients.
Outcomes
Patients who kept their TOCC appointments had a 30-day readmission rate of 9.6%, which was significantly lower than the 30-day readmission rate of 27% in the group that did not keep their TOCC appointment (P < .001). Similarly, the percentage of patients treated in the ED was 15% in the TOCC group compared to 31.2% in the group that canceled their appointment (P < .001) (Table 1). In the multivariable analysis, patients who were seen in the TOCC group had an OR for 30-day readmission of 0.35 (95% CI, 0.19-0.62, P < .001), and an OR for ED visits of 0.39 (95% CI, 0.23-0.65; P < .001) (Table 2). There was no statistically significant difference in 6-month mortality between the two groups. In the virtual group compared to the in-person group, there were no statistically significant differences in outcomes between the two groups in the unadjusted or adjusted analysis (Table 2).
DISCUSSION
In the expansion phase, eligibility was expanded to include any hospital indication but continued to focus on high-risk patients. Existing literature suggests that providing postdischarge care to all patients, including low- or medium-risk patients, may not be as impactful as enrolling high-risk patients only. For instance, a postdischarge clinic offered to all patients at a VA system in Colorado did not reduce readmission rates compared to PCP follow-up.23 In contrast, a study of more than 10,000 high-risk urban patients demonstrated that postdischarge care resulted in a 9.3% reduction in readmission risk.24 Our data are consistent with the previously published studies, as the average CAN score of patients seen in TOCC was 90, suggesting a high risk of readmission. In the final 12 months of the intervention, 15% of discharged patients were seen at the TOCC clinic, suggesting that targeted intervention within the small subset of high-risk patients was sufficient to achieve our primary aim. Of note, among patients who did not meet the inclusion criteria for TOCC referral (ie, patients not considered high risk [CAN score ≤ 85]), the rate of readmissions was 8.6%.
Most of the available research on the efficacy of postdischarge clinics was conducted in urban environments. Our ICVA population sees a large proportion of rural veterans, who account for just over 50% of the discharge population. In a study of more than 2 million Medicare patients discharged from US hospitals, the 30-day readmission rates and adjusted mortality rates were higher among patients in rural counties, and post–acute care seemed to have a greater impact in rural rather than urban settings.25 Previous studies have demonstrated that virtual visits have the potential to improve readmission rates, especially in patients with CHF26 and in patients at the highest risk for readmission.27 In our study, the pilot phase offered only virtual visits, but we subsequently added an in-person option based on veteran feedback. Interestingly, over the next 12 months, virtual visits were more popular with both urban and rural veterans, and there were no differences in the number of rural patients in the in-person vs the virtual group. These findings suggest factors other than rurality influenced the decision to choose virtual follow-up visits over in-person visits. Future studies should seek to determine the extent to which factors such as age, race, educational level, and socioeconomic circumstances impact veterans’ follow-up decisions. Not only were outcomes among patients who chose virtual visits the same as those of patients who chose in-person visits, but both of these groups had better outcomes compared to the non-TOCC group (Table 2). This finding demonstrating the efficacy of virtual visits among rural and urban patients has taken on increased significance due to the COVID-19 pandemic, as virtual visits offer a safer option, one that minimizes physical contact.
Our quality improvement analysis included a statistical comparison of patients seen vs those not seen at the TOCC. Patients who were referred to the TOCC but chose not to keep their appointment were similar to those seen in TOCC in terms of age, CAN score, rurality, and hospital diagnosis, but readmission rates were substantially higher in this group even after adjustments for covariates (Table 2). Evaluating causality in interventions aimed to reduce hospital readmission rates is complicated.28 Our findings add greater plausibility to the utility of TOCC in accounting for at least a portion of the reported decrease in ICVA 30-day readmissions.
Our study has several strengths, including an observation period longer than 2 years, a large population of discharged veterans within an integrated healthcare system, and a large proportion of patients living in rural areas. Another strength of our study is the innovative nature of the intervention, which features a multidisciplinary team and the option of virtual or in-person visits. Nevertheless, this study also has several important limitations. As a single-center study, our findings may not be generalizable to other institutions, especially those outside the VHA system. Similarly, our study population reflected that of the ICVA, which may limit generalizability to a more diverse population. While we attempted to account in our statistical modeling for baseline differences between referred patients seen by the TOCC and those referred but not seen, we cannot exclude residual confounding between the groups. Specifically, the comparison of patients who did and did not choose TOCC follow-up introduces the possibility of selection bias. Future randomized/controlled studies will need to evaluate whether TOCC is more effective than the standard of care to reduce readmissions. Finally, since the analysis period following the final PDSA cycle was compressed due to the onset of the COVID-19 pandemic in the United States, no data are available regarding the sustained impacts of changes made during this cycle.
CONCLUSION
A multidisciplinary TOCC within the ICVA, featuring both virtual and in-person visits, reduced 30-day readmission rates by 19.3%; this approach to care was especially effective in patients with CHF. Virtual visits were the follow-up mode of choice for both urban and rural veterans, and there was no difference in outcomes between these two follow-up options. Future studies will focus on additional quality metrics, including cost-effectiveness and patient satisfaction.
Hospital readmissions are a significant problem in the United States, affecting 15% to 30% of discharges and incurring costs of more than $17 billion annually.1 Timely posthospitalization follow-up visits are critical to ensure the effective transfer of patients to the outpatient setting; such visits reduce readmission rates as well as hospital length of stay and overall health care resource utilization.2-4 Patients who receive inadequate follow-up care (ie, within 4 weeks of discharge) are significantly more likely to be readmitted than those who receive close follow-up care.5
Due to the large clinical and financial consequences associated with hospital readmission, a variety of interventions have been studied, including home visits, telemonitoring, medication management, telephone calls, and postdischarge clinics.6,7 While studies have not shown postdischarge clinics to be universally efficacious in reducing readmission rates, there is increasing evidence of reduced readmission rates in clinics that target high-risk patients (eg, patients with congestive heart failure [CHF]) rather than the total population.2 A study by Hernandez et al that evaluated the relationship between early physician follow-up and 30-day readmissions showed a significantly lower readmission rate among hospitals with higher follow-up rates.8 Similarly, patients with CHF in a large, integrated health system who were seen within 7 days of discharge had an odds ratio (OR) of 0.81 (95% CI, 0.70-0.94) for 30-day readmissions.9
Transitions-of-care clinics (TOCC), designed to provide early postdischarge follow-up to high-risk patients, have been shown to reduce 30-day readmission rates,3,4,10,11 especially in clinics that have same-physician follow-up visits rather than follow-up visits with a community primary care physician (PCP).12 The most pronounced impact of postdischarge follow-up is seen in high-risk patients with high complexity or high severity of disease; however, complex rural patients are less likely to have access to specialty care.13 As a result, since rural residents must travel farther for specialty care, they are seen less frequently than their urban counterparts.14,15
Prior to our TOCC initiative, the Iowa City VA (ICVA) ranked in the fifth quintile of the Veterans Health Administration (VHA)
To meet these challenges, we implemented a TOCC to deliver timely postdischarge care focusing on high-risk and high-complexity patients. To address access-to-care issues of patients living in rural areas within the ICVA, we included virtual follow-up visits as a key component of our intervention.16,17 The aim of this project was to decrease 30-day readmission rates of ICVA patients by 20% within 12 months of implementation.
METHODS
Setting/Study Population
The ICVA serves 184,000 veterans stretched over 50 counties in eastern Iowa, western Illinois, and northern Missouri, with more than 60% of these patients residing in rural areas. Patients were initially eligible for the TOCC if they had an admission diagnosis of CHF and a CAN score > 85 at the time of discharge. The CAN score, developed by the VA to assess the risk of hospital readmission in individual patients, factors in several variables, including demographics, coexisting conditions, vital signs, utilization of services, pharmacy visits, and laboratory results. Patients in the top 5% (95-99) have a readmission rate of 20% at 90 days. Since the CAN is a proprietary tool, it may not be published in full; however, this assessment tool is commonly used and frequently cited in VA research.18-22 The CAN score is expressed as a percentile ranging from 0 (lowest risk) to 99 (highest risk). Patient eligibility was expanded during subsequent Plan-Do-Study-Act (PDSA) cycles, as outlined below. Patient eligibility was expanded during subsequent PDSA cycles (also outlined below). A review by a local institutional review board was obtained, and the study was classified as exempt due to the use of deidentified data. Standards for Quality Improvement Reporting Excellence 2.0 guidelines were used to construct the manuscript.
Magnitude Assessment
The numbers of discharges, readmissions within 30 days, emergency department (ED) visits by all discharged veterans, and veterans discharged with a CHF hospital diagnosis were recorded from February 2017 to February 2018, which were the 12 months immediately preceding the pilot implementation.
Intervention
The primary intervention was referral to the newly formed ICVA TOCC. The multidisciplinary TOCC team consisted of hospitalists, pharmacists, schedulers, and discharge planners/care managers. Patients were identified by the hospitalist team during admission; prior to hospital discharge, these patients were referred to TOCC discharge planners to schedule appropriate follow-up appointments. Virtual follow-up visits were conducted using a patient’s home technology; in cases where a patient lacked adequate technology capabilities (eg, no computer or internet access), the ICVA provided a tablet device with cellular internet capability for temporary use. Specific clinical activities included medication reconciliation by a pharmacist, follow-up of pending laboratory studies, imaging studies, pathology results, medical diagnosis education, counseling regarding dietary restrictions, and contingency planning outside of an ED visit in the event of a change in clinical status. In addition, the TOCC aimed to facilitate a smooth transition of care back to the PCP by arranging follow-up appointments, providing visit summaries, and scheduling consults with specialty care, as appropriate.
Measures
The primary objective measure was the 30-day readmission rate in the ICVA hospital. Secondary measures included the number of VHA ED visits within 30 days of discharge. The main process measures were the number of hospital discharges per month, the number of TOCC referrals, the number of TOCC appointments made, the number of virtual and in-person visits, and the percentage of appointment “no-shows.”
Implementation
The TOCC was piloted from April 2018 to October 2018. During the pilot phase, TOCC enrollment was limited to virtual appointments and to patients with an admission diagnosis of CHF and a CAN score of > 85. The TOCC had staff on-site 2 days a week; this included pharmacists to reconcile medications and hospitalists to address follow-up care needs.
The TOCC clinic was temporarily closed at the end of October 2018 to analyze pilot results. Based on stakeholder feedback, changes made as part of the second PDSA cycle included expanding eligibility criteria to any hospital admission diagnosis and to patients with a CAN score < 85 if the hospitalist team felt the patient was likely to benefit from TOCC follow-up. In addition, on-site clinic staffing was expanded from 2 to 5 days per week to improve access, and the option for an in-person visit was added based on concerns some veterans expressed regarding the use of the technology at home. Finally, a formal resident program was added, and the order set for referrals was simplified. The TOCC was restarted in February 2019, and TOCC metrics were reviewed monthly. By July 2019, we identified issues with TOCC referrals and appointment creation that required additional modifications to the intervention.
A third PDSA cycle was initiated in July 2019 and included major changes, notably the formation of a designated TOCC committee. The committee appointed a dedicated TOCC scheduler whose role was to reduce confusion regarding scheduling, to update the discharge instructions/orders template to lower incidences of “double-booking” that occurred with PCP and TOCC appointments, to modify discharge educational instruction regarding virtual visits and tablet use, to adjust the TOCC-PCP handoff, and to formalize interactions between discharge coordinators and residents to review possible referrals every morning (Appendix Figure 1).
Statistical Analysis
Run charts were constructed by plotting monthly primary outcome values and monthly process metrics (Figure, Appendix Figure 2, Appendix Figure 3). Chi-square tests were used to compare 30-day readmission rates before and after the intervention.
Mean (SD) or counts and percentages were used to describe the distribution of continuous and categorical variables, respectively. Kruskal-Wallis test, t test, or chi-square tests were used, as appropriate, across categories. Generalized linear models with a logistic link function were used to test for differences between patients who kept their appointment at the TOCC and those who did not keep their TOCC appointment (both unadjusted and adjusted for all of the covariates previously mentioned). In addition, generalized linear models were also used to compare outcomes between TOCC patients seen virtually vs those seen in-person (both unadjusted and adjusted for all the covariates previously mentioned). All statistical tests were considered significant at a two-sided P < .05. All analyses were performed using SAS software version 9.4 (SAS Institute Inc).
RESULTS
Magnitude Assessment
During the preimplementation period (February 2017-February 2018), there were 3014 patient discharges from ICVA and 343 readmissions, resulting in a readmission rate of 11.4%. Among patients with a hospital-admission diagnosis of cardiorespiratory disease, which included patients with CHF, there were 381 discharges and 46 readmissions, resulting in a readmission rate of 12.1%.
Primary Outcome
During the pilot phase, which was conducted from April 2018 to October 2018, 142 patients who met inclusion criteria (CHF diagnosis and a CAN score > 85) were discharged from ICVA, and 56 referrals to the TOCC were placed. The readmission rate among the cardiorespiratory cohort of veterans was 9.5%.
During the expansion of the intervention from February 2019 to February 2020, there were 2844 discharges from the ICVA and 291 readmissions, resulting in a readmission rate of 10.2%. However, there was a further decrease in the readmission rate after the third PDSA cycle was initiated in July 2019 (Appendix Figure 1). The readmission rate was 9.2% in the final 6 months of the intervention period, and 7.9% in the final 3 months.
When comparing the 6 months following the third PDSA cycle to the magnitude assessment period, there was a relative readmission reduction of 19.3% (P = .04), and an absolute reduction of 2.2%. If the final 3 months of the intervention period are included, there was an absolute reduction of 3.5% and a relative reduction of 30.7% (P = .01). Notably, before the pilot phase, ICVA was in the fifth quintile for HWR among VA hospitals but improved to the second quintile by the end of the expansion phase.
Process Outcomes
Process metrics for TOCC referrals, the number of patients seen, and the number of virtual and in-person visits over time are shown in Appendix Figure 3. Rates of TOCC referrals and the number of TOCC visits were lower than anticipated during the first 5 months of the intervention. However, TOCC referrals increased significantly after we implemented the previously described changes as part of the third PDSA cycle. As a result, total, virtual, and in-person visits also significantly increased from July 2019 to February 2020. The proportion of patients choosing virtual vs in-person visits fluctuated over time, but virtual visits were generally chosen more often than in-person visits.
Statistical Modeling
Baseline Data
Cohort characteristics are shown in Table 1. The cohort, which reflected the ICVA population, was predominantly male (96%) and White (93%), with a mean age of 67 years. The population was approximately half urban and half rural in composition, and the most common reason for hospital admission was cardiac. Other than a small but statistically significant difference in CAN scores, there were no significant differences between patients who kept their TOCC appointment and those who did not. There were also no differences in baseline characteristics between patients who chose virtual follow-up and patients who chose in-person follow-up, including the proportion of urban and rural patients.
Outcomes
Patients who kept their TOCC appointments had a 30-day readmission rate of 9.6%, which was significantly lower than the 30-day readmission rate of 27% in the group that did not keep their TOCC appointment (P < .001). Similarly, the percentage of patients treated in the ED was 15% in the TOCC group compared to 31.2% in the group that canceled their appointment (P < .001) (Table 1). In the multivariable analysis, patients who were seen in the TOCC group had an OR for 30-day readmission of 0.35 (95% CI, 0.19-0.62, P < .001), and an OR for ED visits of 0.39 (95% CI, 0.23-0.65; P < .001) (Table 2). There was no statistically significant difference in 6-month mortality between the two groups. In the virtual group compared to the in-person group, there were no statistically significant differences in outcomes between the two groups in the unadjusted or adjusted analysis (Table 2).
DISCUSSION
In the expansion phase, eligibility was expanded to include any hospital indication but continued to focus on high-risk patients. Existing literature suggests that providing postdischarge care to all patients, including low- or medium-risk patients, may not be as impactful as enrolling high-risk patients only. For instance, a postdischarge clinic offered to all patients at a VA system in Colorado did not reduce readmission rates compared to PCP follow-up.23 In contrast, a study of more than 10,000 high-risk urban patients demonstrated that postdischarge care resulted in a 9.3% reduction in readmission risk.24 Our data are consistent with the previously published studies, as the average CAN score of patients seen in TOCC was 90, suggesting a high risk of readmission. In the final 12 months of the intervention, 15% of discharged patients were seen at the TOCC clinic, suggesting that targeted intervention within the small subset of high-risk patients was sufficient to achieve our primary aim. Of note, among patients who did not meet the inclusion criteria for TOCC referral (ie, patients not considered high risk [CAN score ≤ 85]), the rate of readmissions was 8.6%.
Most of the available research on the efficacy of postdischarge clinics was conducted in urban environments. Our ICVA population sees a large proportion of rural veterans, who account for just over 50% of the discharge population. In a study of more than 2 million Medicare patients discharged from US hospitals, the 30-day readmission rates and adjusted mortality rates were higher among patients in rural counties, and post–acute care seemed to have a greater impact in rural rather than urban settings.25 Previous studies have demonstrated that virtual visits have the potential to improve readmission rates, especially in patients with CHF26 and in patients at the highest risk for readmission.27 In our study, the pilot phase offered only virtual visits, but we subsequently added an in-person option based on veteran feedback. Interestingly, over the next 12 months, virtual visits were more popular with both urban and rural veterans, and there were no differences in the number of rural patients in the in-person vs the virtual group. These findings suggest factors other than rurality influenced the decision to choose virtual follow-up visits over in-person visits. Future studies should seek to determine the extent to which factors such as age, race, educational level, and socioeconomic circumstances impact veterans’ follow-up decisions. Not only were outcomes among patients who chose virtual visits the same as those of patients who chose in-person visits, but both of these groups had better outcomes compared to the non-TOCC group (Table 2). This finding demonstrating the efficacy of virtual visits among rural and urban patients has taken on increased significance due to the COVID-19 pandemic, as virtual visits offer a safer option, one that minimizes physical contact.
Our quality improvement analysis included a statistical comparison of patients seen vs those not seen at the TOCC. Patients who were referred to the TOCC but chose not to keep their appointment were similar to those seen in TOCC in terms of age, CAN score, rurality, and hospital diagnosis, but readmission rates were substantially higher in this group even after adjustments for covariates (Table 2). Evaluating causality in interventions aimed to reduce hospital readmission rates is complicated.28 Our findings add greater plausibility to the utility of TOCC in accounting for at least a portion of the reported decrease in ICVA 30-day readmissions.
Our study has several strengths, including an observation period longer than 2 years, a large population of discharged veterans within an integrated healthcare system, and a large proportion of patients living in rural areas. Another strength of our study is the innovative nature of the intervention, which features a multidisciplinary team and the option of virtual or in-person visits. Nevertheless, this study also has several important limitations. As a single-center study, our findings may not be generalizable to other institutions, especially those outside the VHA system. Similarly, our study population reflected that of the ICVA, which may limit generalizability to a more diverse population. While we attempted to account in our statistical modeling for baseline differences between referred patients seen by the TOCC and those referred but not seen, we cannot exclude residual confounding between the groups. Specifically, the comparison of patients who did and did not choose TOCC follow-up introduces the possibility of selection bias. Future randomized/controlled studies will need to evaluate whether TOCC is more effective than the standard of care to reduce readmissions. Finally, since the analysis period following the final PDSA cycle was compressed due to the onset of the COVID-19 pandemic in the United States, no data are available regarding the sustained impacts of changes made during this cycle.
CONCLUSION
A multidisciplinary TOCC within the ICVA, featuring both virtual and in-person visits, reduced 30-day readmission rates by 19.3%; this approach to care was especially effective in patients with CHF. Virtual visits were the follow-up mode of choice for both urban and rural veterans, and there was no difference in outcomes between these two follow-up options. Future studies will focus on additional quality metrics, including cost-effectiveness and patient satisfaction.
1. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428. https://doi.org/10.1056/nejmsa0803563
2. Doctoroff L. Postdischarge clinics and hospitalists: a review of the evidence and existing models. J Hosp Med. 2017;12(6):467-471. https://doi.org/10.12788/jhm.2750
3. Koehler BE, Richter KM, Youngblood L, et al. Reduction of 30-day postdischarge hospital readmission or emergency department (ED) visit rates in high-risk elderly medical patients through delivery of a targeted care bundle. J Hosp Med. 2009;4(4):211-218. https://doi.org/10.1002/jhm.427
4. Abrashkin KA, Cho HJ, Torgalkar S, Markoff B. Improving transitions of care from hospital to home: what works? Mt Sinai J Med. 2012;79(5):535-544. https://doi.org/10.1002/msj.21332
5. Misky GJ, Wald HL, Coleman EA. Post-hospitalization transitions: examining the effects of timing of primary care provider follow-up. J Hosp Med. 2010;5(7):392-397. https://doi.org/10.1002/jhm.666
6. Greysen SR, Harrison JD, Kripalani S, et al. Understanding patient-centred readmission factors: a multi-site, mixed-methods study. BMJ Qual Saf. 2017;26(1):33-41. https://doi.org/10.1136/bmjqs-2015-004570
7. Hansen LO, Young RS, Hinami K, Leung A, Williams MV. Interventions to reduce 30-day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520-528. https://doi.org/10.7326/0003-4819-155-8-201110180-00008
8. Hernandez AF, Greiner MA, Fonarow GC, et al. Relationship between early physician follow-up and 30-day readmission among Medicare beneficiaries hospitalized for heart failure. JAMA. 2010;303(17):1716-1722. https://doi.org/10.1001/jama.2010.533
9. Lee KK, Yang J, Hernandez AF, Steimle AE, Go AS. Post-discharge follow-up characteristics associated with 30-day readmission after heart failure hospitalization. Med Care. 2016;54(4):365-372. https://doi.org/10.1097/mlr.0000000000000492
10. Balaban RB, Williams MV. Improving care transitions: hospitalists partnering with primary care. J Hosp Med. 2010;5(7):375-377. https://doi.org/10.1002/jhm.824
11. Rodrigues CR, Harrington AR, Murdock N, et al. Effect of pharmacy-supported transition-of-care interventions on 30-day readmissions: a systematic review and meta-analysis. Ann Pharmacother. 2017;51(10):866-889. https://doi.org/10.1177/1060028017712725
12. van Walraven C, Taljaard M, Etchells E, et al. The independent association of provider and information continuity on outcomes after hospital discharge: implications for hospitalists. J Hosp Med. 2010;5(7):398-405. https://doi.org/10.1002/jhm.716
13. Gruca TS, Pyo TH, Nelson GC. Providing cardiology care in rural areas through vsiting consultant clinics. J Am Heart Assoc. 2016;5(7):e002909. https://doi.org/10.1161/jaha.115.002909
14. Chan L, Hart LG, Goodman DC. Geographic access to health care for rural Medicare beneficiaries. J Rural Health. 2006;22(2):140-146. https://doi.org/10.1111/j.1748-0361.2006.00022.x
15. Burke RE, Jones CD, Coleman EA, Falvey JR, Stevens-Lapsley JE, Ginde AA. Use of post-acute care after hospital discharge in urban and rural hospitals. Am J Accountable Care. 2017;5(1):16-22.
16. Jetty A, Moore MA, Coffman M, Petterson S, Bazemore A. Rural family physicians are twice as likely to use telehealth as urban family physicians. Telemed J E Health. 2018;24(4):268-276. https://doi.org/10.1089/tmj.2017.0161
17. Harrison PL, Hara PA, Pope JE, Young MC, Rula EY. The impact of postdischarge telephonic follow-up on hospital readmissions. Popul Health Manag. 2011;14(1):27-32. https://doi.org/10.1089/pop.2009.0076
18. Wang L, Porter B, Maynard C, et al. Predicting risk of hospitalization or death among patients receiving primary care in the Veterans Health Administration. Med Care. 2013;51(4):368-373. https://doi.org/10.1097/mlr.0b013e31827da95a
19. Spece LJ, Donovan LM, Griffith MF, et al. Initiating low-value inhaled corticosteroids in an inception cohort with chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2020;17(5):589-595. https://doi.org/10.1513/annalsats.201911-854oc
20. Osborne TF, Suarez P, Edwards D, Hernandez-Boussard T, Curtin C. Patient electronic health records score for preoperative risk assessment before total knee arthroplasty. JB JS Open Access. 2020;5(2):e0061. https://doi.org/10.2106/jbjs.oa.19.00061
21. Levy C, Ersek M, Scott W, et al. Life-sustaining treatment decisions initiative: early implementation results of a national Veterans Affairs program to honor veterans’ care preferences. J Gen Intern Med. 2020;35(6):1803-1812. https://doi.org/10.1007/s11606-020-05697-2
22. Ibrahim SA. High-risk patients and utilization of primary care in the US Veterans Affairs health system. JAMA Netw Open. 2020;3(6):e209518. https://doi.org/10.1001/jamanetworkopen.2020.9518
23. Burke RE, Whitfield E, Prochazka AV. Effect of a hospitalist-run postdischarge clinic on outcomes. J Hosp Med. 2014;9(1):7-12. https://doi.org/10.1002/jhm.2099
24. Jenq GY, Doyle MM, Belton BM, Herrin J, Horwitz LI. Quasi-experimental evaluation of the effectiveness of a large-scale readmission reduction program. JAMA Intern Med. 2016;176(5):681-690. https://doi.org/10.1001/jamainternmed.2016.0833
25. Kosar CM, Loomer L, Ferdows NB, Trivedi AN, Panagiotou OA, Rahman M. Assessment of rural-urban differences in postacute care utilization and outcomes among older US adults. JAMA Netw Open. 2020;3(1):e1918738. https://doi.org/10.1001/jamanetworkopen.2019.18738
26. Pandor A, Thokala P, Gomersall T, et al. Home telemonitoring or structured telephone support programmes after recent discharge in patients with heart failure: systematic review and economic evaluation. Health Technol Assess. 2013;17(32):1-207, v-vi. https://doi.org/10.3310/hta17320
27. Low LL, Tan SY, Ng MJM, et al. Applying the integrated practice unit concept to a modified virtual ward model of care for patients at highest risk of readmission: a randomized controlled trial. PloS One. 2017;12(1):e0168757. https://doi.org/10.1371/journal.pone.0168757
28. McIlvennan CK, Eapen ZJ, Allen LA. Hospital readmissions reduction program. Circulation. 2015;131(20):1796-1803. https://doi.org/10.1161/circulationaha.114.010270
1. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428. https://doi.org/10.1056/nejmsa0803563
2. Doctoroff L. Postdischarge clinics and hospitalists: a review of the evidence and existing models. J Hosp Med. 2017;12(6):467-471. https://doi.org/10.12788/jhm.2750
3. Koehler BE, Richter KM, Youngblood L, et al. Reduction of 30-day postdischarge hospital readmission or emergency department (ED) visit rates in high-risk elderly medical patients through delivery of a targeted care bundle. J Hosp Med. 2009;4(4):211-218. https://doi.org/10.1002/jhm.427
4. Abrashkin KA, Cho HJ, Torgalkar S, Markoff B. Improving transitions of care from hospital to home: what works? Mt Sinai J Med. 2012;79(5):535-544. https://doi.org/10.1002/msj.21332
5. Misky GJ, Wald HL, Coleman EA. Post-hospitalization transitions: examining the effects of timing of primary care provider follow-up. J Hosp Med. 2010;5(7):392-397. https://doi.org/10.1002/jhm.666
6. Greysen SR, Harrison JD, Kripalani S, et al. Understanding patient-centred readmission factors: a multi-site, mixed-methods study. BMJ Qual Saf. 2017;26(1):33-41. https://doi.org/10.1136/bmjqs-2015-004570
7. Hansen LO, Young RS, Hinami K, Leung A, Williams MV. Interventions to reduce 30-day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520-528. https://doi.org/10.7326/0003-4819-155-8-201110180-00008
8. Hernandez AF, Greiner MA, Fonarow GC, et al. Relationship between early physician follow-up and 30-day readmission among Medicare beneficiaries hospitalized for heart failure. JAMA. 2010;303(17):1716-1722. https://doi.org/10.1001/jama.2010.533
9. Lee KK, Yang J, Hernandez AF, Steimle AE, Go AS. Post-discharge follow-up characteristics associated with 30-day readmission after heart failure hospitalization. Med Care. 2016;54(4):365-372. https://doi.org/10.1097/mlr.0000000000000492
10. Balaban RB, Williams MV. Improving care transitions: hospitalists partnering with primary care. J Hosp Med. 2010;5(7):375-377. https://doi.org/10.1002/jhm.824
11. Rodrigues CR, Harrington AR, Murdock N, et al. Effect of pharmacy-supported transition-of-care interventions on 30-day readmissions: a systematic review and meta-analysis. Ann Pharmacother. 2017;51(10):866-889. https://doi.org/10.1177/1060028017712725
12. van Walraven C, Taljaard M, Etchells E, et al. The independent association of provider and information continuity on outcomes after hospital discharge: implications for hospitalists. J Hosp Med. 2010;5(7):398-405. https://doi.org/10.1002/jhm.716
13. Gruca TS, Pyo TH, Nelson GC. Providing cardiology care in rural areas through vsiting consultant clinics. J Am Heart Assoc. 2016;5(7):e002909. https://doi.org/10.1161/jaha.115.002909
14. Chan L, Hart LG, Goodman DC. Geographic access to health care for rural Medicare beneficiaries. J Rural Health. 2006;22(2):140-146. https://doi.org/10.1111/j.1748-0361.2006.00022.x
15. Burke RE, Jones CD, Coleman EA, Falvey JR, Stevens-Lapsley JE, Ginde AA. Use of post-acute care after hospital discharge in urban and rural hospitals. Am J Accountable Care. 2017;5(1):16-22.
16. Jetty A, Moore MA, Coffman M, Petterson S, Bazemore A. Rural family physicians are twice as likely to use telehealth as urban family physicians. Telemed J E Health. 2018;24(4):268-276. https://doi.org/10.1089/tmj.2017.0161
17. Harrison PL, Hara PA, Pope JE, Young MC, Rula EY. The impact of postdischarge telephonic follow-up on hospital readmissions. Popul Health Manag. 2011;14(1):27-32. https://doi.org/10.1089/pop.2009.0076
18. Wang L, Porter B, Maynard C, et al. Predicting risk of hospitalization or death among patients receiving primary care in the Veterans Health Administration. Med Care. 2013;51(4):368-373. https://doi.org/10.1097/mlr.0b013e31827da95a
19. Spece LJ, Donovan LM, Griffith MF, et al. Initiating low-value inhaled corticosteroids in an inception cohort with chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2020;17(5):589-595. https://doi.org/10.1513/annalsats.201911-854oc
20. Osborne TF, Suarez P, Edwards D, Hernandez-Boussard T, Curtin C. Patient electronic health records score for preoperative risk assessment before total knee arthroplasty. JB JS Open Access. 2020;5(2):e0061. https://doi.org/10.2106/jbjs.oa.19.00061
21. Levy C, Ersek M, Scott W, et al. Life-sustaining treatment decisions initiative: early implementation results of a national Veterans Affairs program to honor veterans’ care preferences. J Gen Intern Med. 2020;35(6):1803-1812. https://doi.org/10.1007/s11606-020-05697-2
22. Ibrahim SA. High-risk patients and utilization of primary care in the US Veterans Affairs health system. JAMA Netw Open. 2020;3(6):e209518. https://doi.org/10.1001/jamanetworkopen.2020.9518
23. Burke RE, Whitfield E, Prochazka AV. Effect of a hospitalist-run postdischarge clinic on outcomes. J Hosp Med. 2014;9(1):7-12. https://doi.org/10.1002/jhm.2099
24. Jenq GY, Doyle MM, Belton BM, Herrin J, Horwitz LI. Quasi-experimental evaluation of the effectiveness of a large-scale readmission reduction program. JAMA Intern Med. 2016;176(5):681-690. https://doi.org/10.1001/jamainternmed.2016.0833
25. Kosar CM, Loomer L, Ferdows NB, Trivedi AN, Panagiotou OA, Rahman M. Assessment of rural-urban differences in postacute care utilization and outcomes among older US adults. JAMA Netw Open. 2020;3(1):e1918738. https://doi.org/10.1001/jamanetworkopen.2019.18738
26. Pandor A, Thokala P, Gomersall T, et al. Home telemonitoring or structured telephone support programmes after recent discharge in patients with heart failure: systematic review and economic evaluation. Health Technol Assess. 2013;17(32):1-207, v-vi. https://doi.org/10.3310/hta17320
27. Low LL, Tan SY, Ng MJM, et al. Applying the integrated practice unit concept to a modified virtual ward model of care for patients at highest risk of readmission: a randomized controlled trial. PloS One. 2017;12(1):e0168757. https://doi.org/10.1371/journal.pone.0168757
28. McIlvennan CK, Eapen ZJ, Allen LA. Hospital readmissions reduction program. Circulation. 2015;131(20):1796-1803. https://doi.org/10.1161/circulationaha.114.010270
© 2021 Society of Hospital Medicine
Rebuttal: Routine Daily Physical Exam
While we agree that a well-honed physical exam is one of the most important diagnostic tools than an internist can use, we have several responses to Drs Kanjee and McNamara’s point that a routine physical exam is essential for hospitalized patients.1 They argue that this exam might be helpful as a deliberate practice to improve skills for effective diagnostic exams. To this, we have two responses: the first is that the typical “routine” exam—a brief auscultation of the chest and abdomen—is performed frequently enough that additional practice should not be necessary for any practicing hospitalist. Performing a true full exam that would hone infrequently used skills, such as a full neurological exam, an orthopedic knee exam, or fundoscopy, comes at the expense of time spent talking to patients, as well as the potential harm of downstream testing cascades leading to adverse events. Second, we would argue that the real skill being developed is not “recognizing normal” but instead learning how to appropriately use physical diagnostic skills. Knowing precisely what exam maneuvers might be beneficial in a given hospitalized patient is incredibly complex, far more so than charts in evidence-based exam textbooks would suggest. It is this skill, not “recognizing normal,” that requires deliberate practice.
We agree that even during routine hospitalizations, daily exams may help detect complications of therapy, such as a patient with cellulitis on intravenous fluids developing volume overload. We are not against performing physical exams for diagnostic or monitoring purposes. In fact, it may be that most hospitalized patients would benefit from some sort of daily exam. However, rarely performed maneuvers, such as walking with patients or performing a validated delirium screen, are likely to have a higher yield than routine lung auscultation. It may also be true that hospitalized patients would benefit from certain screening exam maneuvers, but again, evidence is lacking, and decades of experience in the outpatient world would suggest the contrary.
Finally, and most ardently, we disagree that performing a routine daily physical exam can somehow inoculate against burnout. That is a view wholly unsupported by any evidence. The physical exam was originally developed as a diagnostic tool, not as a method to connect with patients. However, this traditional “routine” exam has been taught in medical schools as normal ever since, with very little serious interrogation of its utility or downstream effects. Increased cynicism about the exam’s usefulness, in our opinion, reflects physician cognizance of actual disutility of routine exams, rather than pining for a halcyon era that never existed. In fact, we believe a more hypothesis-driven diagnostic use of exams enriches physical diagnosis. For instance, listening to the chest of a patient with cellulitis on intravenous fluids is no longer “just listening,” it is an exercise specifically looking for a finding that affects management. Patient-centered care means tailoring all of our care—including the physical exam—to the needs of the patient. Doing a cursory, routine exam day after day for every patient with the goal of “recognizing normal” is not patient-centered, but rather physician-centered.
We do not doubt the importance of ritual, especially in such a stressful situation as a modern hospitalization. But rather than use a diagnostic procedure with downstream effects, we urge hospitalists to consider instead a ritual dating back to the time of Hippocrates—the compassionate physician sitting at the bedside, laying a hand on the shoulder, and listening to the patient’s concerns. That is authentic human connection rather than performance.
Acknowledgment
The authors of this point-counterpoint to thank Chris Smith, MD, and the members of the BIDMC Internal Medicine Residency Clinician Educator Track for thoughtful discussion around these topics.
1. McNamara LC, Kanjee Z. Counterpoint: routine daily physical exams add value for the hospitalist and patient. J Hosp Med. Published online August 18, 2021. https://doi.org/10.12788/jhm.3671
While we agree that a well-honed physical exam is one of the most important diagnostic tools than an internist can use, we have several responses to Drs Kanjee and McNamara’s point that a routine physical exam is essential for hospitalized patients.1 They argue that this exam might be helpful as a deliberate practice to improve skills for effective diagnostic exams. To this, we have two responses: the first is that the typical “routine” exam—a brief auscultation of the chest and abdomen—is performed frequently enough that additional practice should not be necessary for any practicing hospitalist. Performing a true full exam that would hone infrequently used skills, such as a full neurological exam, an orthopedic knee exam, or fundoscopy, comes at the expense of time spent talking to patients, as well as the potential harm of downstream testing cascades leading to adverse events. Second, we would argue that the real skill being developed is not “recognizing normal” but instead learning how to appropriately use physical diagnostic skills. Knowing precisely what exam maneuvers might be beneficial in a given hospitalized patient is incredibly complex, far more so than charts in evidence-based exam textbooks would suggest. It is this skill, not “recognizing normal,” that requires deliberate practice.
We agree that even during routine hospitalizations, daily exams may help detect complications of therapy, such as a patient with cellulitis on intravenous fluids developing volume overload. We are not against performing physical exams for diagnostic or monitoring purposes. In fact, it may be that most hospitalized patients would benefit from some sort of daily exam. However, rarely performed maneuvers, such as walking with patients or performing a validated delirium screen, are likely to have a higher yield than routine lung auscultation. It may also be true that hospitalized patients would benefit from certain screening exam maneuvers, but again, evidence is lacking, and decades of experience in the outpatient world would suggest the contrary.
Finally, and most ardently, we disagree that performing a routine daily physical exam can somehow inoculate against burnout. That is a view wholly unsupported by any evidence. The physical exam was originally developed as a diagnostic tool, not as a method to connect with patients. However, this traditional “routine” exam has been taught in medical schools as normal ever since, with very little serious interrogation of its utility or downstream effects. Increased cynicism about the exam’s usefulness, in our opinion, reflects physician cognizance of actual disutility of routine exams, rather than pining for a halcyon era that never existed. In fact, we believe a more hypothesis-driven diagnostic use of exams enriches physical diagnosis. For instance, listening to the chest of a patient with cellulitis on intravenous fluids is no longer “just listening,” it is an exercise specifically looking for a finding that affects management. Patient-centered care means tailoring all of our care—including the physical exam—to the needs of the patient. Doing a cursory, routine exam day after day for every patient with the goal of “recognizing normal” is not patient-centered, but rather physician-centered.
We do not doubt the importance of ritual, especially in such a stressful situation as a modern hospitalization. But rather than use a diagnostic procedure with downstream effects, we urge hospitalists to consider instead a ritual dating back to the time of Hippocrates—the compassionate physician sitting at the bedside, laying a hand on the shoulder, and listening to the patient’s concerns. That is authentic human connection rather than performance.
Acknowledgment
The authors of this point-counterpoint to thank Chris Smith, MD, and the members of the BIDMC Internal Medicine Residency Clinician Educator Track for thoughtful discussion around these topics.
While we agree that a well-honed physical exam is one of the most important diagnostic tools than an internist can use, we have several responses to Drs Kanjee and McNamara’s point that a routine physical exam is essential for hospitalized patients.1 They argue that this exam might be helpful as a deliberate practice to improve skills for effective diagnostic exams. To this, we have two responses: the first is that the typical “routine” exam—a brief auscultation of the chest and abdomen—is performed frequently enough that additional practice should not be necessary for any practicing hospitalist. Performing a true full exam that would hone infrequently used skills, such as a full neurological exam, an orthopedic knee exam, or fundoscopy, comes at the expense of time spent talking to patients, as well as the potential harm of downstream testing cascades leading to adverse events. Second, we would argue that the real skill being developed is not “recognizing normal” but instead learning how to appropriately use physical diagnostic skills. Knowing precisely what exam maneuvers might be beneficial in a given hospitalized patient is incredibly complex, far more so than charts in evidence-based exam textbooks would suggest. It is this skill, not “recognizing normal,” that requires deliberate practice.
We agree that even during routine hospitalizations, daily exams may help detect complications of therapy, such as a patient with cellulitis on intravenous fluids developing volume overload. We are not against performing physical exams for diagnostic or monitoring purposes. In fact, it may be that most hospitalized patients would benefit from some sort of daily exam. However, rarely performed maneuvers, such as walking with patients or performing a validated delirium screen, are likely to have a higher yield than routine lung auscultation. It may also be true that hospitalized patients would benefit from certain screening exam maneuvers, but again, evidence is lacking, and decades of experience in the outpatient world would suggest the contrary.
Finally, and most ardently, we disagree that performing a routine daily physical exam can somehow inoculate against burnout. That is a view wholly unsupported by any evidence. The physical exam was originally developed as a diagnostic tool, not as a method to connect with patients. However, this traditional “routine” exam has been taught in medical schools as normal ever since, with very little serious interrogation of its utility or downstream effects. Increased cynicism about the exam’s usefulness, in our opinion, reflects physician cognizance of actual disutility of routine exams, rather than pining for a halcyon era that never existed. In fact, we believe a more hypothesis-driven diagnostic use of exams enriches physical diagnosis. For instance, listening to the chest of a patient with cellulitis on intravenous fluids is no longer “just listening,” it is an exercise specifically looking for a finding that affects management. Patient-centered care means tailoring all of our care—including the physical exam—to the needs of the patient. Doing a cursory, routine exam day after day for every patient with the goal of “recognizing normal” is not patient-centered, but rather physician-centered.
We do not doubt the importance of ritual, especially in such a stressful situation as a modern hospitalization. But rather than use a diagnostic procedure with downstream effects, we urge hospitalists to consider instead a ritual dating back to the time of Hippocrates—the compassionate physician sitting at the bedside, laying a hand on the shoulder, and listening to the patient’s concerns. That is authentic human connection rather than performance.
Acknowledgment
The authors of this point-counterpoint to thank Chris Smith, MD, and the members of the BIDMC Internal Medicine Residency Clinician Educator Track for thoughtful discussion around these topics.
1. McNamara LC, Kanjee Z. Counterpoint: routine daily physical exams add value for the hospitalist and patient. J Hosp Med. Published online August 18, 2021. https://doi.org/10.12788/jhm.3671
1. McNamara LC, Kanjee Z. Counterpoint: routine daily physical exams add value for the hospitalist and patient. J Hosp Med. Published online August 18, 2021. https://doi.org/10.12788/jhm.3671
© 2021 Society of Hospital Medicine
Counterpoint: Routine Daily Physical Exams Add Value for the Hospitalist and Patient
We read with interest the perspective of Drs Rodman and Warnock,1 but disagree with the authors on several points. We find that the routine daily physical examination, often neglected or unappreciated amidst technological advances and new diagnostic testing, provides significant value to both hospitalist and patient in terms of diagnosis, treatment, patient-centered care, and maintaining the patient-doctor relationship.
First, the daily physical exam provides the practice that hospitalists need to develop and maintain necessary diagnostic finesse. We are taught fundamental physical exam maneuvers in medical school, but these skills often atrophy during residency and throughout our careers.2 This leaves us, as practicing physicians, potentially worse at these competencies than when we were students. The daily physical exam, on the other hand, provides frequent and effective practice to keep up and build upon these skills. We gain in part from our repeated normal exams, which help us to skillfully recognize the rare abnormal finding; a hospitalist must likely feel hundreds of normal abdomens to reliably discover a furtive abdominal mass. Our exams also benefit from several forms of prompt and relevant feedback, including that which is provided by subspecialist consultants (like a cardiologist agreeing with your assessment of jugular venous pressure) and other diagnostic tests (like the echocardiogram without the valvulopathy thought to be detected at the bedside). The physical examination is best learned at the bedside, and the daily exam offers an unparalleled opportunity to do so. Such continual skill improvement is necessary for hospitalists to accurately apply data from the evidence-based physical diagnosis literature. Many studies of the utility of various physical exam findings3 involve maneuvers performed by experts; to truly apply their results to our generalist practice, we are required to push ourselves to obtain the diagnostic expertise of the specialist. The daily physical examination, being the most concrete way for hospitalists to do so, is therefore essential to practicing better evidence-based physical diagnosis.
Beyond these larger benefits of the daily physical examination on our own practice and skills, patients in our care benefit diagnostically from these exams as well. Time and again, we see an inadequate or incomplete physical exam leading to errors or adverse patient outcomes.4,5 Even after completion of initial laboratory and imaging tests, laying of hands and stethoscope can lead to dramatic changes in inpatient diagnosis and management.6 The subsequent routine daily physical provides fresh opportunities to reexamine the evidence for or against our own working diagnoses and management plans. The adage that “you don’t know what you don’t know” is especially fitting here. We often do not know to look for and rule a disease process in or out if it is not on our initial differential on hospital day one or two; the daily physical exam allows us to be on the lookout for diagnoses we have not yet considered. The two of us have more than once made a serendipitous discovery of a new rash or other physical finding on hospital day three or four that helps suggest another, and ultimately correct, final diagnosis. Particularly in a setting in which so many inpatient diagnoses are wrong and can lead to patient harm,7 the daily physical examination provides an important check on our own diagnostic reasoning.
Even if we are right about the diagnosis, the daily exam also allows for timely recognition of complications from our management. Listening to each patient’s lungs every day, including those of patients with seemingly unrelated lower-extremity cellulitis, means we will more promptly notice when they retain fluid due to as yet unknown underlying heart failure. Those subtle bibasilar crackles not only become diagnostically useful, but also allow us the possibility of intervening and changing course even before the patient reports shortness of breath or the nurse notes hypoxemia on routine vital signs a day or two later. In an era when our treatment regimens are more complex, with frequent off-target results and side effects, the daily exam is a key screening tool for adverse outcomes in an increasingly ill population. Having a frequently updated and accurate baseline exam is also exceptionally important in the event of sudden neurologic deficits; an inpatient with new facial droop and left-arm weakness at 10
Finally, the daily physical examination is important to patient-centered care and potentially preventing physician burnout.8 Patients have more confidence in us when we conduct a thorough exam. The ritual of the physical exam is also an important contributor to the patient-doctor relationship, and a daily exam can help strengthen that bond each morning.9 Such benefits also extend to physicians. Hospitalists are spending less and less time at the bedside,10 a reality at least partially responsible for rising rates of burnout.11 We all went into clinical medicine to take care of and connect with people. The daily physical examination offers valuable time to show our patients we care about them while also giving us the opportunity to spend time with them, rather than with the “iPatient” that can otherwise become our focus.12 In this way, the daily physical examination can be immensely satisfying and may not only inoculate against burnout but also contribute to a stronger patient-doctor relationship.
For so many reasons, the daily physical exam is of great benefit to hospitalists looking to develop and maintain diagnostic skills, to our patients as we stay on the lookout for unexpected diagnoses and complications, and to the relationships we have with those for whom we care. It is a practice worth not only continuing but celebrating.
1. Rodman A, Warnock S. Point: routine daily physical exams in hospitalized patients are a waste of time. J Hosp Med. Published online August 18, 2021. https://doi.org/10.12788/jhm.3670
2. Vukanovic-Criley JM, Criley S, Warde CM, et al. Competency in cardiac examination skills in medical students, trainees, physicians, and faculty: a multicenter study. Arch Intern Med. 2006;166(6):610-616. https://doi.org/10.1001/archinte.166.6.610
3. McGee S. Evidence-Based Physical Diagnosis. 4th ed. Elsevier; 2018.
4. Verghese A, Charlton B, Kassirer JP, Ramsey M, Ioannidis JPA. Inadequacies of physical examination as a cause of medical errors and adverse events: a collection of vignettes. Am J Med. 2015;128(12):1322-1324.e3. https://doi.org/10.1016/j.amjmed.2015.06.004
5. Singh H, Giardina TD, Meyer AND, Forjuoh SN, Reis MD, Thomas EJ. Types and origins of diagnostic errors in primary care settings. JAMA Intern Med. 2013;173(6):418-425. https://doi.org/10.1001/jamainternmed.2013.2777
6. Reilly BM. Physical examination in the care of medical inpatients: an observational study. Lancet. 2003;362(9390):1100-1105. https://doi.org/10.1016/S0140-6736(03)14464-9
7. Gunderson CG, Bilan VP, Holleck JL, et al. Prevalence of harmful diagnostic errors in hospitalised adults: a systematic review and meta-analysis. BMJ Qual Saf. 2020;29(12):1008-1018. https://doi.org/10.1136/bmjqs-2019-010822
8. Silverman B, Gertz A. Present role of the precordial examination in patient care. Am J Cardiol. 2015;115(2):253-255. https://doi.org/10.1016/j.amjcard.2014.10.031
9. Costanzo C, Verghese A. The physical examination as ritual: social sciences and embodiment in the context of the physical examination. Med Clin North Am. 2018;102(3):425-431. https://doi.org/10.1016/j.mcna.2017.12.004
10. Malkenson D, Siegal EM, Leff JA, Weber R, Struck R. Comparing academic and community-based hospitalists. J Hosp Med. 2010;5(6):349-352. https://doi.org/10.1002/jhm.793
11. Hipp DM, Rialon KL, Nevel K, Kothari AN, Jardine LDA. “Back to bedside”: Residents’ and fellows’ perspectives on finding meaning in work. J Grad Med Educ. 2017;9(2):269-273. https://doi.org/10.4300/JGME-D-17-00136.1
12. Verghese A. Culture shock--patient as icon, icon as patient. N Engl J Med. 2008;359(26):2748-2751. https://doi.org/10.1056/NEJMp0807461
We read with interest the perspective of Drs Rodman and Warnock,1 but disagree with the authors on several points. We find that the routine daily physical examination, often neglected or unappreciated amidst technological advances and new diagnostic testing, provides significant value to both hospitalist and patient in terms of diagnosis, treatment, patient-centered care, and maintaining the patient-doctor relationship.
First, the daily physical exam provides the practice that hospitalists need to develop and maintain necessary diagnostic finesse. We are taught fundamental physical exam maneuvers in medical school, but these skills often atrophy during residency and throughout our careers.2 This leaves us, as practicing physicians, potentially worse at these competencies than when we were students. The daily physical exam, on the other hand, provides frequent and effective practice to keep up and build upon these skills. We gain in part from our repeated normal exams, which help us to skillfully recognize the rare abnormal finding; a hospitalist must likely feel hundreds of normal abdomens to reliably discover a furtive abdominal mass. Our exams also benefit from several forms of prompt and relevant feedback, including that which is provided by subspecialist consultants (like a cardiologist agreeing with your assessment of jugular venous pressure) and other diagnostic tests (like the echocardiogram without the valvulopathy thought to be detected at the bedside). The physical examination is best learned at the bedside, and the daily exam offers an unparalleled opportunity to do so. Such continual skill improvement is necessary for hospitalists to accurately apply data from the evidence-based physical diagnosis literature. Many studies of the utility of various physical exam findings3 involve maneuvers performed by experts; to truly apply their results to our generalist practice, we are required to push ourselves to obtain the diagnostic expertise of the specialist. The daily physical examination, being the most concrete way for hospitalists to do so, is therefore essential to practicing better evidence-based physical diagnosis.
Beyond these larger benefits of the daily physical examination on our own practice and skills, patients in our care benefit diagnostically from these exams as well. Time and again, we see an inadequate or incomplete physical exam leading to errors or adverse patient outcomes.4,5 Even after completion of initial laboratory and imaging tests, laying of hands and stethoscope can lead to dramatic changes in inpatient diagnosis and management.6 The subsequent routine daily physical provides fresh opportunities to reexamine the evidence for or against our own working diagnoses and management plans. The adage that “you don’t know what you don’t know” is especially fitting here. We often do not know to look for and rule a disease process in or out if it is not on our initial differential on hospital day one or two; the daily physical exam allows us to be on the lookout for diagnoses we have not yet considered. The two of us have more than once made a serendipitous discovery of a new rash or other physical finding on hospital day three or four that helps suggest another, and ultimately correct, final diagnosis. Particularly in a setting in which so many inpatient diagnoses are wrong and can lead to patient harm,7 the daily physical examination provides an important check on our own diagnostic reasoning.
Even if we are right about the diagnosis, the daily exam also allows for timely recognition of complications from our management. Listening to each patient’s lungs every day, including those of patients with seemingly unrelated lower-extremity cellulitis, means we will more promptly notice when they retain fluid due to as yet unknown underlying heart failure. Those subtle bibasilar crackles not only become diagnostically useful, but also allow us the possibility of intervening and changing course even before the patient reports shortness of breath or the nurse notes hypoxemia on routine vital signs a day or two later. In an era when our treatment regimens are more complex, with frequent off-target results and side effects, the daily exam is a key screening tool for adverse outcomes in an increasingly ill population. Having a frequently updated and accurate baseline exam is also exceptionally important in the event of sudden neurologic deficits; an inpatient with new facial droop and left-arm weakness at 10
Finally, the daily physical examination is important to patient-centered care and potentially preventing physician burnout.8 Patients have more confidence in us when we conduct a thorough exam. The ritual of the physical exam is also an important contributor to the patient-doctor relationship, and a daily exam can help strengthen that bond each morning.9 Such benefits also extend to physicians. Hospitalists are spending less and less time at the bedside,10 a reality at least partially responsible for rising rates of burnout.11 We all went into clinical medicine to take care of and connect with people. The daily physical examination offers valuable time to show our patients we care about them while also giving us the opportunity to spend time with them, rather than with the “iPatient” that can otherwise become our focus.12 In this way, the daily physical examination can be immensely satisfying and may not only inoculate against burnout but also contribute to a stronger patient-doctor relationship.
For so many reasons, the daily physical exam is of great benefit to hospitalists looking to develop and maintain diagnostic skills, to our patients as we stay on the lookout for unexpected diagnoses and complications, and to the relationships we have with those for whom we care. It is a practice worth not only continuing but celebrating.
We read with interest the perspective of Drs Rodman and Warnock,1 but disagree with the authors on several points. We find that the routine daily physical examination, often neglected or unappreciated amidst technological advances and new diagnostic testing, provides significant value to both hospitalist and patient in terms of diagnosis, treatment, patient-centered care, and maintaining the patient-doctor relationship.
First, the daily physical exam provides the practice that hospitalists need to develop and maintain necessary diagnostic finesse. We are taught fundamental physical exam maneuvers in medical school, but these skills often atrophy during residency and throughout our careers.2 This leaves us, as practicing physicians, potentially worse at these competencies than when we were students. The daily physical exam, on the other hand, provides frequent and effective practice to keep up and build upon these skills. We gain in part from our repeated normal exams, which help us to skillfully recognize the rare abnormal finding; a hospitalist must likely feel hundreds of normal abdomens to reliably discover a furtive abdominal mass. Our exams also benefit from several forms of prompt and relevant feedback, including that which is provided by subspecialist consultants (like a cardiologist agreeing with your assessment of jugular venous pressure) and other diagnostic tests (like the echocardiogram without the valvulopathy thought to be detected at the bedside). The physical examination is best learned at the bedside, and the daily exam offers an unparalleled opportunity to do so. Such continual skill improvement is necessary for hospitalists to accurately apply data from the evidence-based physical diagnosis literature. Many studies of the utility of various physical exam findings3 involve maneuvers performed by experts; to truly apply their results to our generalist practice, we are required to push ourselves to obtain the diagnostic expertise of the specialist. The daily physical examination, being the most concrete way for hospitalists to do so, is therefore essential to practicing better evidence-based physical diagnosis.
Beyond these larger benefits of the daily physical examination on our own practice and skills, patients in our care benefit diagnostically from these exams as well. Time and again, we see an inadequate or incomplete physical exam leading to errors or adverse patient outcomes.4,5 Even after completion of initial laboratory and imaging tests, laying of hands and stethoscope can lead to dramatic changes in inpatient diagnosis and management.6 The subsequent routine daily physical provides fresh opportunities to reexamine the evidence for or against our own working diagnoses and management plans. The adage that “you don’t know what you don’t know” is especially fitting here. We often do not know to look for and rule a disease process in or out if it is not on our initial differential on hospital day one or two; the daily physical exam allows us to be on the lookout for diagnoses we have not yet considered. The two of us have more than once made a serendipitous discovery of a new rash or other physical finding on hospital day three or four that helps suggest another, and ultimately correct, final diagnosis. Particularly in a setting in which so many inpatient diagnoses are wrong and can lead to patient harm,7 the daily physical examination provides an important check on our own diagnostic reasoning.
Even if we are right about the diagnosis, the daily exam also allows for timely recognition of complications from our management. Listening to each patient’s lungs every day, including those of patients with seemingly unrelated lower-extremity cellulitis, means we will more promptly notice when they retain fluid due to as yet unknown underlying heart failure. Those subtle bibasilar crackles not only become diagnostically useful, but also allow us the possibility of intervening and changing course even before the patient reports shortness of breath or the nurse notes hypoxemia on routine vital signs a day or two later. In an era when our treatment regimens are more complex, with frequent off-target results and side effects, the daily exam is a key screening tool for adverse outcomes in an increasingly ill population. Having a frequently updated and accurate baseline exam is also exceptionally important in the event of sudden neurologic deficits; an inpatient with new facial droop and left-arm weakness at 10
Finally, the daily physical examination is important to patient-centered care and potentially preventing physician burnout.8 Patients have more confidence in us when we conduct a thorough exam. The ritual of the physical exam is also an important contributor to the patient-doctor relationship, and a daily exam can help strengthen that bond each morning.9 Such benefits also extend to physicians. Hospitalists are spending less and less time at the bedside,10 a reality at least partially responsible for rising rates of burnout.11 We all went into clinical medicine to take care of and connect with people. The daily physical examination offers valuable time to show our patients we care about them while also giving us the opportunity to spend time with them, rather than with the “iPatient” that can otherwise become our focus.12 In this way, the daily physical examination can be immensely satisfying and may not only inoculate against burnout but also contribute to a stronger patient-doctor relationship.
For so many reasons, the daily physical exam is of great benefit to hospitalists looking to develop and maintain diagnostic skills, to our patients as we stay on the lookout for unexpected diagnoses and complications, and to the relationships we have with those for whom we care. It is a practice worth not only continuing but celebrating.
1. Rodman A, Warnock S. Point: routine daily physical exams in hospitalized patients are a waste of time. J Hosp Med. Published online August 18, 2021. https://doi.org/10.12788/jhm.3670
2. Vukanovic-Criley JM, Criley S, Warde CM, et al. Competency in cardiac examination skills in medical students, trainees, physicians, and faculty: a multicenter study. Arch Intern Med. 2006;166(6):610-616. https://doi.org/10.1001/archinte.166.6.610
3. McGee S. Evidence-Based Physical Diagnosis. 4th ed. Elsevier; 2018.
4. Verghese A, Charlton B, Kassirer JP, Ramsey M, Ioannidis JPA. Inadequacies of physical examination as a cause of medical errors and adverse events: a collection of vignettes. Am J Med. 2015;128(12):1322-1324.e3. https://doi.org/10.1016/j.amjmed.2015.06.004
5. Singh H, Giardina TD, Meyer AND, Forjuoh SN, Reis MD, Thomas EJ. Types and origins of diagnostic errors in primary care settings. JAMA Intern Med. 2013;173(6):418-425. https://doi.org/10.1001/jamainternmed.2013.2777
6. Reilly BM. Physical examination in the care of medical inpatients: an observational study. Lancet. 2003;362(9390):1100-1105. https://doi.org/10.1016/S0140-6736(03)14464-9
7. Gunderson CG, Bilan VP, Holleck JL, et al. Prevalence of harmful diagnostic errors in hospitalised adults: a systematic review and meta-analysis. BMJ Qual Saf. 2020;29(12):1008-1018. https://doi.org/10.1136/bmjqs-2019-010822
8. Silverman B, Gertz A. Present role of the precordial examination in patient care. Am J Cardiol. 2015;115(2):253-255. https://doi.org/10.1016/j.amjcard.2014.10.031
9. Costanzo C, Verghese A. The physical examination as ritual: social sciences and embodiment in the context of the physical examination. Med Clin North Am. 2018;102(3):425-431. https://doi.org/10.1016/j.mcna.2017.12.004
10. Malkenson D, Siegal EM, Leff JA, Weber R, Struck R. Comparing academic and community-based hospitalists. J Hosp Med. 2010;5(6):349-352. https://doi.org/10.1002/jhm.793
11. Hipp DM, Rialon KL, Nevel K, Kothari AN, Jardine LDA. “Back to bedside”: Residents’ and fellows’ perspectives on finding meaning in work. J Grad Med Educ. 2017;9(2):269-273. https://doi.org/10.4300/JGME-D-17-00136.1
12. Verghese A. Culture shock--patient as icon, icon as patient. N Engl J Med. 2008;359(26):2748-2751. https://doi.org/10.1056/NEJMp0807461
1. Rodman A, Warnock S. Point: routine daily physical exams in hospitalized patients are a waste of time. J Hosp Med. Published online August 18, 2021. https://doi.org/10.12788/jhm.3670
2. Vukanovic-Criley JM, Criley S, Warde CM, et al. Competency in cardiac examination skills in medical students, trainees, physicians, and faculty: a multicenter study. Arch Intern Med. 2006;166(6):610-616. https://doi.org/10.1001/archinte.166.6.610
3. McGee S. Evidence-Based Physical Diagnosis. 4th ed. Elsevier; 2018.
4. Verghese A, Charlton B, Kassirer JP, Ramsey M, Ioannidis JPA. Inadequacies of physical examination as a cause of medical errors and adverse events: a collection of vignettes. Am J Med. 2015;128(12):1322-1324.e3. https://doi.org/10.1016/j.amjmed.2015.06.004
5. Singh H, Giardina TD, Meyer AND, Forjuoh SN, Reis MD, Thomas EJ. Types and origins of diagnostic errors in primary care settings. JAMA Intern Med. 2013;173(6):418-425. https://doi.org/10.1001/jamainternmed.2013.2777
6. Reilly BM. Physical examination in the care of medical inpatients: an observational study. Lancet. 2003;362(9390):1100-1105. https://doi.org/10.1016/S0140-6736(03)14464-9
7. Gunderson CG, Bilan VP, Holleck JL, et al. Prevalence of harmful diagnostic errors in hospitalised adults: a systematic review and meta-analysis. BMJ Qual Saf. 2020;29(12):1008-1018. https://doi.org/10.1136/bmjqs-2019-010822
8. Silverman B, Gertz A. Present role of the precordial examination in patient care. Am J Cardiol. 2015;115(2):253-255. https://doi.org/10.1016/j.amjcard.2014.10.031
9. Costanzo C, Verghese A. The physical examination as ritual: social sciences and embodiment in the context of the physical examination. Med Clin North Am. 2018;102(3):425-431. https://doi.org/10.1016/j.mcna.2017.12.004
10. Malkenson D, Siegal EM, Leff JA, Weber R, Struck R. Comparing academic and community-based hospitalists. J Hosp Med. 2010;5(6):349-352. https://doi.org/10.1002/jhm.793
11. Hipp DM, Rialon KL, Nevel K, Kothari AN, Jardine LDA. “Back to bedside”: Residents’ and fellows’ perspectives on finding meaning in work. J Grad Med Educ. 2017;9(2):269-273. https://doi.org/10.4300/JGME-D-17-00136.1
12. Verghese A. Culture shock--patient as icon, icon as patient. N Engl J Med. 2008;359(26):2748-2751. https://doi.org/10.1056/NEJMp0807461
© 2021 Society of Hospital Medicine
Point: Routine Daily Physical Exams in Hospitalized Patients Are a Waste of Time
Every day, physicians engage in an elaborate performance with their patients—the routine complete physical exam. We argue that this purportedly time-tested ritual is at best a waste of time, and at worst potentially harmful.
The modern physical exam evolved throughout the 19th century as the first diagnostic tool in a medical field that was rapidly transforming from its traditional roots to a modern scientific discipline.1 Despite the vast increase in diagnostic tools since then, the physical exam remains one of the most predictive. Several decades of investigation into the “evidence-based” physical exam have attempted to calculate the test characteristics of individual exam findings, confirming that the exam remains as useful a diagnostic tool today as it was for Laënnec or Osler.2
Performing a physical exam for the purposes of diagnosis and prognosis—not only on admission, but also on a daily basis to assess treatment response—remains a fundamental part of a hospitalist’s job. For example, a daily volume assessment, including cardiac auscultation for an S3, evaluation of the jugular venous pulse, and measurement of edema, is essential in managing patients with decompensated heart failure. However, when we stray from these diagnostic purposes, we are no longer using the exam as intended.
The physical exam most frequently performed in the hospital today is the so-called routine daily exam. Generally, this involves passing a stethoscope fleetingly across the chest and abdomen, perhaps with some additional palpation of the abdomen. Cranial nerves II through XII may also occasionally be checked. This routine exam—and by extension, the templated physical exams that fill hospitalists’ documentation—not only lack an evidence base, but also are arguably harmful to patients. Such exams should not be part of a hospitalist’s daily practice.
The most concerning aspect of a routine daily exam is that examination of an asymptomatic patient—for example, auscultation of the lungs of a patient admitted with lower extremity cellulitis—is fundamentally a screening rather than a diagnostic test. While little work has been done in the inpatient setting, decades of studies on outpatient screening exams demonstrate that very few of them are effective.3 For example, a review of commonly used exam maneuvers in wellness visits concluded that “for the asymptomatic, nonpregnant adult of any age, no evidence supports the need for a complete physical exam as traditionally defined,” recommending against such popular maneuvers as lung and heart auscultation and peripheral pulse palpation.4 While the inpatient hospital medicine population has different characteristics that may warrant a routine exam, there is no evidence to support such practice.
It is often argued that the routine physical exam is “cheap” and “quick” and, therefore, should be performed regardless of evidence. While this is certainly true for many diagnostic physical exams, the literature suggests that there is no reason to think that a routine physical exam would be cost-effective.5 Even cost-effective screening physical exam tests, such as an outpatient nurse performing a 1-minute pulse palpation starting at age 55, have incremental costs measuring in the thousands of dollars.6 Furthermore, screening tests can have unexpected downstream effects that are both costly and associated with morbidity and mortality.7 For example, abdominal palpation of a “prominent” aorta can lead to imaging, where incidental findings can trigger procedures that may involve complications.
In addition to potentially adding more risk, the routine daily physical exam represents time that can be better allocated. Medical residents spend the vast majority of their day at the computer, while spending less than 10% of their time at the patient’s bedside.8 Anything that takes up that valuable time, including a “routine exam,” is time spent not talking to the patient, learning about their symptoms, their fears, and who they are as human beings.
It is also true that patients expect a physical exam to be performed, and that additional exam maneuvers, including potentially invasive exams, are associated with increasing patient satisfaction.9 However, these arguments miss much of the nuance of why patients have these expectations. Qualitative research suggests that much of a patient’s desire for unnecessary tests or exams is actually their concern about a lack of validation or empathy from the physician, as well as general skepticism about evidence-based medical decision-making.10 Perhaps spending more face time with patients discussing their issues, rather than idle time performing routine maneuvers, would lead to even greater patient satisfaction.
Finally, one of the most popular arguments in defense of a routine physical exam is that the exam is a “sacred ritual” essential to the patient-physician relationship.11 However, this is an argument not supported by historical interpretation. The physical exam was developed as an explicitly diagnostic procedure in the early 19th century, while the primacy of the doctor-physician relationship dates back millennia, long before the development of the modern physical exam. Furthermore, modern historiography has identified the development of the physical exam as part of a movement to minimize the experience of the patient in their own disease, and to situate the physician as the ultimate source of knowledge about a patient’s body rather than an attempt to strengthen a relationship.12
Ritual is indeed important, and the exam as currently practiced may indeed reinforce the physician-patient relationship. But we should also keep in mind what that relationship entails. Having full access to a patient’s unclothed body and having the ability to perform invasive procedures are far beyond regular social norms—these are powerful diagnostic tools, yes, but they also serve to reinforce an imbalance of power in the relationship. Medical rituals have also changed dramatically over time. Modern evidence suggests that pulse palpation alone, the form of the exam that was dominant for millennia, has profound physiological effects even on critically ill patients.13 Rather than a diagnostic exam that has potential downstream cost implications and consumes valuable time from an encounter, we suggest a return to a more traditional ritual of physical touch: sitting at the patient’s bedside, holding their hand, and speaking to them compassionately about their fears and hopes. This would be a far more valuable “routine” encounter to incorporate into the busy hospitalist’s day.
Acknowledgment
The authors of this point-counterpoint thank Chris Smith, MD, and the members of the BIDMC Internal Medicine Residency Clinician Educator Track for thoughtful discussion around these topics.
1. Laënnec RTH. De l’auscultation médiate ou Traité du Diagnostic des Maladies des Poumon et du Coeur fondé principalement sur ce Nouveau Moyen d’Exploration. Brosson & Chaudé; 1819.
2. McGee S. Evidence-Based Physical Diagnosis. 4th ed. Elsevier; 2018.
3. Bloomfield HE, Wilt TJ. Evidence brief: Role of the annual comprehensive physical examination in the asymptomatic adult. VA Evidence Synthesis Program Evidence Briefs. US Department of Veterans Affairs; October 2011.
4. Oboler SK, LaForce FM. The periodic physical examination in asymptomatic adults. Ann Intern Med. 1989;110(3):214-226. https://doi.org/10.7326/0003-4819-110-3-214
5. Angus S. The cost-effective evaluation of syncope. Med Clin North Am. 2016;100(5):1019-1032. https://doi.org/10.1016/j.mcna.2016.04.010
6. Welton NJ, McAleenan A, Thom HH, et al. Screening strategies for atrial fibrillation: a systematic review and cost-effectiveness analysis. Health Technol Assess. 2017;21(29):1-236. https://doi.org/10.3310/hta21290
7. Rothberg MB. The $50 000 physical. JAMA. 2020;323(17):1682-1683. https://doi.org/10.1001/jama.2020.2866
8. Mamykina L, Vawdrey DK, Hripcsak G. How do residents spend their shift time? A time and motion study with a particular focus on the use of computers. Acad Med. 2016;91(6):827-832. https://doi.org/10.1097/ACM.0000000000001148
9. Duan L, Mukherjee EM, Federman DG. The physical examination: a survey of patient preferences and expectations during primary care visits. Postgrad Med. 2020;132(1):102-108. https://doi.org/10.1080/00325481.2020.1713618
10. Kravitz RL, Callahan EJ. Patients’ perceptions of omitted examinations and tests: a qualitative analysis. J Gen Intern Med. 2000;15(1):38-45. https://doi.org/10.1046/j.1525-1497.2000.12058.x
11. Costanzo C, Verghese A. The physical examination as ritual: social sciences and embodiment in the context of the physical examination. Med Clin North Am. 2018;102(3):425-431. https://doi.org/10.1016/j.mcna.2017.12.004
12. Jewson ND. The disappearance of the sick-man from medical cosmology, 1770–1870. Int J Epidemiol. 2009;38(3):622-633. https://doi.org/10.1093/ije/dyp180
13. Arnold MH, Komesaroff P, Kerridge I. Understanding the ethical implications of the rituals of medicine. Intern Med J. 2020;50(9):1123-1131. https://doi.org/10.1111/imj.14990
Every day, physicians engage in an elaborate performance with their patients—the routine complete physical exam. We argue that this purportedly time-tested ritual is at best a waste of time, and at worst potentially harmful.
The modern physical exam evolved throughout the 19th century as the first diagnostic tool in a medical field that was rapidly transforming from its traditional roots to a modern scientific discipline.1 Despite the vast increase in diagnostic tools since then, the physical exam remains one of the most predictive. Several decades of investigation into the “evidence-based” physical exam have attempted to calculate the test characteristics of individual exam findings, confirming that the exam remains as useful a diagnostic tool today as it was for Laënnec or Osler.2
Performing a physical exam for the purposes of diagnosis and prognosis—not only on admission, but also on a daily basis to assess treatment response—remains a fundamental part of a hospitalist’s job. For example, a daily volume assessment, including cardiac auscultation for an S3, evaluation of the jugular venous pulse, and measurement of edema, is essential in managing patients with decompensated heart failure. However, when we stray from these diagnostic purposes, we are no longer using the exam as intended.
The physical exam most frequently performed in the hospital today is the so-called routine daily exam. Generally, this involves passing a stethoscope fleetingly across the chest and abdomen, perhaps with some additional palpation of the abdomen. Cranial nerves II through XII may also occasionally be checked. This routine exam—and by extension, the templated physical exams that fill hospitalists’ documentation—not only lack an evidence base, but also are arguably harmful to patients. Such exams should not be part of a hospitalist’s daily practice.
The most concerning aspect of a routine daily exam is that examination of an asymptomatic patient—for example, auscultation of the lungs of a patient admitted with lower extremity cellulitis—is fundamentally a screening rather than a diagnostic test. While little work has been done in the inpatient setting, decades of studies on outpatient screening exams demonstrate that very few of them are effective.3 For example, a review of commonly used exam maneuvers in wellness visits concluded that “for the asymptomatic, nonpregnant adult of any age, no evidence supports the need for a complete physical exam as traditionally defined,” recommending against such popular maneuvers as lung and heart auscultation and peripheral pulse palpation.4 While the inpatient hospital medicine population has different characteristics that may warrant a routine exam, there is no evidence to support such practice.
It is often argued that the routine physical exam is “cheap” and “quick” and, therefore, should be performed regardless of evidence. While this is certainly true for many diagnostic physical exams, the literature suggests that there is no reason to think that a routine physical exam would be cost-effective.5 Even cost-effective screening physical exam tests, such as an outpatient nurse performing a 1-minute pulse palpation starting at age 55, have incremental costs measuring in the thousands of dollars.6 Furthermore, screening tests can have unexpected downstream effects that are both costly and associated with morbidity and mortality.7 For example, abdominal palpation of a “prominent” aorta can lead to imaging, where incidental findings can trigger procedures that may involve complications.
In addition to potentially adding more risk, the routine daily physical exam represents time that can be better allocated. Medical residents spend the vast majority of their day at the computer, while spending less than 10% of their time at the patient’s bedside.8 Anything that takes up that valuable time, including a “routine exam,” is time spent not talking to the patient, learning about their symptoms, their fears, and who they are as human beings.
It is also true that patients expect a physical exam to be performed, and that additional exam maneuvers, including potentially invasive exams, are associated with increasing patient satisfaction.9 However, these arguments miss much of the nuance of why patients have these expectations. Qualitative research suggests that much of a patient’s desire for unnecessary tests or exams is actually their concern about a lack of validation or empathy from the physician, as well as general skepticism about evidence-based medical decision-making.10 Perhaps spending more face time with patients discussing their issues, rather than idle time performing routine maneuvers, would lead to even greater patient satisfaction.
Finally, one of the most popular arguments in defense of a routine physical exam is that the exam is a “sacred ritual” essential to the patient-physician relationship.11 However, this is an argument not supported by historical interpretation. The physical exam was developed as an explicitly diagnostic procedure in the early 19th century, while the primacy of the doctor-physician relationship dates back millennia, long before the development of the modern physical exam. Furthermore, modern historiography has identified the development of the physical exam as part of a movement to minimize the experience of the patient in their own disease, and to situate the physician as the ultimate source of knowledge about a patient’s body rather than an attempt to strengthen a relationship.12
Ritual is indeed important, and the exam as currently practiced may indeed reinforce the physician-patient relationship. But we should also keep in mind what that relationship entails. Having full access to a patient’s unclothed body and having the ability to perform invasive procedures are far beyond regular social norms—these are powerful diagnostic tools, yes, but they also serve to reinforce an imbalance of power in the relationship. Medical rituals have also changed dramatically over time. Modern evidence suggests that pulse palpation alone, the form of the exam that was dominant for millennia, has profound physiological effects even on critically ill patients.13 Rather than a diagnostic exam that has potential downstream cost implications and consumes valuable time from an encounter, we suggest a return to a more traditional ritual of physical touch: sitting at the patient’s bedside, holding their hand, and speaking to them compassionately about their fears and hopes. This would be a far more valuable “routine” encounter to incorporate into the busy hospitalist’s day.
Acknowledgment
The authors of this point-counterpoint thank Chris Smith, MD, and the members of the BIDMC Internal Medicine Residency Clinician Educator Track for thoughtful discussion around these topics.
Every day, physicians engage in an elaborate performance with their patients—the routine complete physical exam. We argue that this purportedly time-tested ritual is at best a waste of time, and at worst potentially harmful.
The modern physical exam evolved throughout the 19th century as the first diagnostic tool in a medical field that was rapidly transforming from its traditional roots to a modern scientific discipline.1 Despite the vast increase in diagnostic tools since then, the physical exam remains one of the most predictive. Several decades of investigation into the “evidence-based” physical exam have attempted to calculate the test characteristics of individual exam findings, confirming that the exam remains as useful a diagnostic tool today as it was for Laënnec or Osler.2
Performing a physical exam for the purposes of diagnosis and prognosis—not only on admission, but also on a daily basis to assess treatment response—remains a fundamental part of a hospitalist’s job. For example, a daily volume assessment, including cardiac auscultation for an S3, evaluation of the jugular venous pulse, and measurement of edema, is essential in managing patients with decompensated heart failure. However, when we stray from these diagnostic purposes, we are no longer using the exam as intended.
The physical exam most frequently performed in the hospital today is the so-called routine daily exam. Generally, this involves passing a stethoscope fleetingly across the chest and abdomen, perhaps with some additional palpation of the abdomen. Cranial nerves II through XII may also occasionally be checked. This routine exam—and by extension, the templated physical exams that fill hospitalists’ documentation—not only lack an evidence base, but also are arguably harmful to patients. Such exams should not be part of a hospitalist’s daily practice.
The most concerning aspect of a routine daily exam is that examination of an asymptomatic patient—for example, auscultation of the lungs of a patient admitted with lower extremity cellulitis—is fundamentally a screening rather than a diagnostic test. While little work has been done in the inpatient setting, decades of studies on outpatient screening exams demonstrate that very few of them are effective.3 For example, a review of commonly used exam maneuvers in wellness visits concluded that “for the asymptomatic, nonpregnant adult of any age, no evidence supports the need for a complete physical exam as traditionally defined,” recommending against such popular maneuvers as lung and heart auscultation and peripheral pulse palpation.4 While the inpatient hospital medicine population has different characteristics that may warrant a routine exam, there is no evidence to support such practice.
It is often argued that the routine physical exam is “cheap” and “quick” and, therefore, should be performed regardless of evidence. While this is certainly true for many diagnostic physical exams, the literature suggests that there is no reason to think that a routine physical exam would be cost-effective.5 Even cost-effective screening physical exam tests, such as an outpatient nurse performing a 1-minute pulse palpation starting at age 55, have incremental costs measuring in the thousands of dollars.6 Furthermore, screening tests can have unexpected downstream effects that are both costly and associated with morbidity and mortality.7 For example, abdominal palpation of a “prominent” aorta can lead to imaging, where incidental findings can trigger procedures that may involve complications.
In addition to potentially adding more risk, the routine daily physical exam represents time that can be better allocated. Medical residents spend the vast majority of their day at the computer, while spending less than 10% of their time at the patient’s bedside.8 Anything that takes up that valuable time, including a “routine exam,” is time spent not talking to the patient, learning about their symptoms, their fears, and who they are as human beings.
It is also true that patients expect a physical exam to be performed, and that additional exam maneuvers, including potentially invasive exams, are associated with increasing patient satisfaction.9 However, these arguments miss much of the nuance of why patients have these expectations. Qualitative research suggests that much of a patient’s desire for unnecessary tests or exams is actually their concern about a lack of validation or empathy from the physician, as well as general skepticism about evidence-based medical decision-making.10 Perhaps spending more face time with patients discussing their issues, rather than idle time performing routine maneuvers, would lead to even greater patient satisfaction.
Finally, one of the most popular arguments in defense of a routine physical exam is that the exam is a “sacred ritual” essential to the patient-physician relationship.11 However, this is an argument not supported by historical interpretation. The physical exam was developed as an explicitly diagnostic procedure in the early 19th century, while the primacy of the doctor-physician relationship dates back millennia, long before the development of the modern physical exam. Furthermore, modern historiography has identified the development of the physical exam as part of a movement to minimize the experience of the patient in their own disease, and to situate the physician as the ultimate source of knowledge about a patient’s body rather than an attempt to strengthen a relationship.12
Ritual is indeed important, and the exam as currently practiced may indeed reinforce the physician-patient relationship. But we should also keep in mind what that relationship entails. Having full access to a patient’s unclothed body and having the ability to perform invasive procedures are far beyond regular social norms—these are powerful diagnostic tools, yes, but they also serve to reinforce an imbalance of power in the relationship. Medical rituals have also changed dramatically over time. Modern evidence suggests that pulse palpation alone, the form of the exam that was dominant for millennia, has profound physiological effects even on critically ill patients.13 Rather than a diagnostic exam that has potential downstream cost implications and consumes valuable time from an encounter, we suggest a return to a more traditional ritual of physical touch: sitting at the patient’s bedside, holding their hand, and speaking to them compassionately about their fears and hopes. This would be a far more valuable “routine” encounter to incorporate into the busy hospitalist’s day.
Acknowledgment
The authors of this point-counterpoint thank Chris Smith, MD, and the members of the BIDMC Internal Medicine Residency Clinician Educator Track for thoughtful discussion around these topics.
1. Laënnec RTH. De l’auscultation médiate ou Traité du Diagnostic des Maladies des Poumon et du Coeur fondé principalement sur ce Nouveau Moyen d’Exploration. Brosson & Chaudé; 1819.
2. McGee S. Evidence-Based Physical Diagnosis. 4th ed. Elsevier; 2018.
3. Bloomfield HE, Wilt TJ. Evidence brief: Role of the annual comprehensive physical examination in the asymptomatic adult. VA Evidence Synthesis Program Evidence Briefs. US Department of Veterans Affairs; October 2011.
4. Oboler SK, LaForce FM. The periodic physical examination in asymptomatic adults. Ann Intern Med. 1989;110(3):214-226. https://doi.org/10.7326/0003-4819-110-3-214
5. Angus S. The cost-effective evaluation of syncope. Med Clin North Am. 2016;100(5):1019-1032. https://doi.org/10.1016/j.mcna.2016.04.010
6. Welton NJ, McAleenan A, Thom HH, et al. Screening strategies for atrial fibrillation: a systematic review and cost-effectiveness analysis. Health Technol Assess. 2017;21(29):1-236. https://doi.org/10.3310/hta21290
7. Rothberg MB. The $50 000 physical. JAMA. 2020;323(17):1682-1683. https://doi.org/10.1001/jama.2020.2866
8. Mamykina L, Vawdrey DK, Hripcsak G. How do residents spend their shift time? A time and motion study with a particular focus on the use of computers. Acad Med. 2016;91(6):827-832. https://doi.org/10.1097/ACM.0000000000001148
9. Duan L, Mukherjee EM, Federman DG. The physical examination: a survey of patient preferences and expectations during primary care visits. Postgrad Med. 2020;132(1):102-108. https://doi.org/10.1080/00325481.2020.1713618
10. Kravitz RL, Callahan EJ. Patients’ perceptions of omitted examinations and tests: a qualitative analysis. J Gen Intern Med. 2000;15(1):38-45. https://doi.org/10.1046/j.1525-1497.2000.12058.x
11. Costanzo C, Verghese A. The physical examination as ritual: social sciences and embodiment in the context of the physical examination. Med Clin North Am. 2018;102(3):425-431. https://doi.org/10.1016/j.mcna.2017.12.004
12. Jewson ND. The disappearance of the sick-man from medical cosmology, 1770–1870. Int J Epidemiol. 2009;38(3):622-633. https://doi.org/10.1093/ije/dyp180
13. Arnold MH, Komesaroff P, Kerridge I. Understanding the ethical implications of the rituals of medicine. Intern Med J. 2020;50(9):1123-1131. https://doi.org/10.1111/imj.14990
1. Laënnec RTH. De l’auscultation médiate ou Traité du Diagnostic des Maladies des Poumon et du Coeur fondé principalement sur ce Nouveau Moyen d’Exploration. Brosson & Chaudé; 1819.
2. McGee S. Evidence-Based Physical Diagnosis. 4th ed. Elsevier; 2018.
3. Bloomfield HE, Wilt TJ. Evidence brief: Role of the annual comprehensive physical examination in the asymptomatic adult. VA Evidence Synthesis Program Evidence Briefs. US Department of Veterans Affairs; October 2011.
4. Oboler SK, LaForce FM. The periodic physical examination in asymptomatic adults. Ann Intern Med. 1989;110(3):214-226. https://doi.org/10.7326/0003-4819-110-3-214
5. Angus S. The cost-effective evaluation of syncope. Med Clin North Am. 2016;100(5):1019-1032. https://doi.org/10.1016/j.mcna.2016.04.010
6. Welton NJ, McAleenan A, Thom HH, et al. Screening strategies for atrial fibrillation: a systematic review and cost-effectiveness analysis. Health Technol Assess. 2017;21(29):1-236. https://doi.org/10.3310/hta21290
7. Rothberg MB. The $50 000 physical. JAMA. 2020;323(17):1682-1683. https://doi.org/10.1001/jama.2020.2866
8. Mamykina L, Vawdrey DK, Hripcsak G. How do residents spend their shift time? A time and motion study with a particular focus on the use of computers. Acad Med. 2016;91(6):827-832. https://doi.org/10.1097/ACM.0000000000001148
9. Duan L, Mukherjee EM, Federman DG. The physical examination: a survey of patient preferences and expectations during primary care visits. Postgrad Med. 2020;132(1):102-108. https://doi.org/10.1080/00325481.2020.1713618
10. Kravitz RL, Callahan EJ. Patients’ perceptions of omitted examinations and tests: a qualitative analysis. J Gen Intern Med. 2000;15(1):38-45. https://doi.org/10.1046/j.1525-1497.2000.12058.x
11. Costanzo C, Verghese A. The physical examination as ritual: social sciences and embodiment in the context of the physical examination. Med Clin North Am. 2018;102(3):425-431. https://doi.org/10.1016/j.mcna.2017.12.004
12. Jewson ND. The disappearance of the sick-man from medical cosmology, 1770–1870. Int J Epidemiol. 2009;38(3):622-633. https://doi.org/10.1093/ije/dyp180
13. Arnold MH, Komesaroff P, Kerridge I. Understanding the ethical implications of the rituals of medicine. Intern Med J. 2020;50(9):1123-1131. https://doi.org/10.1111/imj.14990
© 2021 Society of Hospital Medicine