User login
Perceived safety and value of inpatient “very important person” services
Recent publications in the medical literature and lay press have stirred controversy regarding the use of inpatient ‘very important person’ (VIP) services.1-3 The term “VIP services” often refers to select conveniences offered in addition to the assumed basic level of care and services provided by a hospital. Examples include additional space, enhanced facilities, specific comforts, or personal support. In some instances, these amenities may only be provided to patients who have close financial, social, or professional relationships with the hospital.
How VIP patients interact with their health system to obtain VIP services has raised unique concerns. Some have speculated that the presence of a VIP patient may be disruptive to the care of non-VIP patients, while others have cautioned physicians about potential dangers to the VIP patients themselves.4-6 Despite much being written on the topics of VIP patients and services in both the lay and academic press, our literature review identified only 1 study on the topic, which cataloged the preferential treatment of VIP patients in the emergency department.6 We are unaware of any investigations of VIP-service use in the inpatient setting. Through a multisite survey of hospital medicine physicians, we assessed physician viewpoints and behavior regarding VIP services.
METHODS
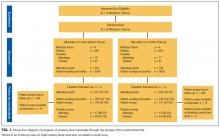
The Hospital Medicine Reengineering Network (HOMERuN) is a nation-wide learning organization focused on measuring and improving the outcomes of hospitalized patients.7 We surveyed hospitalists from 8 HOMERuN hospitals (Appendix 1). The survey instrument contained 4 sections: nonidentifying respondent demographics, local use of VIP services, reported physician perceptions of VIP services, and case-based assessments (Appendix 2). Survey questions and individual cases were developed by study authors and based on real scenarios and concerns provided by front-line clinical providers. Content, length, and reliability of physician understanding were assessed by a 5-person focus group consisting of physicians not included in the survey population.
Subjects were identified via administrative rosters from each HOMERuN site. Surveys were administered via SurveyMonkey, and results were analyzed descriptively. Populations were compared via the Fisher exact test. “VIP services” were defined as conveniences provided in addition to the assumed basic level of care and services (eg, private or luxury-style rooms, access to a special menu, better views, dedicated personal care attendants, hospital liaisons). VIP patients were defined as those patients receiving VIP services. A hospital was identified as providing VIP services if 50% or more of respondents from that site reported the presence of VIP services.
RESULTS
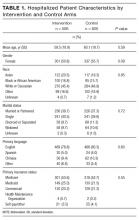
Of 366 hospitalists contacted, 160 completed the survey (44%). Respondent characteristics and reported prevalence of VIP services are demonstrated in Table 1. In total, 78 respondents (45%) reported the presence of VIP services at their hospital. Of the 8 sites surveyed, a majority of physicians at 4 sites (50%) reported presence of VIP services.
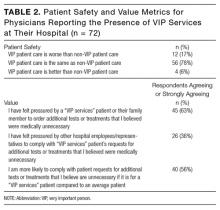
Of respondents reporting the presence of VIP services at their hospital, a majority felt that, from a patient safety perspective, the care received by VIP patients was the same as care received by non-VIP patients (Table 2). A majority reported they had felt pressured by a VIP patient or a family member to order additional tests or treatments that the physician believed were medically unnecessary and that they would be more likely to comply with VIP patient’s requests for tests or treatments they felt were unnecessary. More than one-third (36%) felt pressured by other hospital employees or representatives to comply with VIP services patient’s requests for additional tests or treatments that the physicians believed were medically unnecessary.
When presented the case of a VIP patient with community-acquired pneumonia who is clinically stable for discharge but expressing concerns about leaving the hospital, 61 (38%) respondents reported they would not discharge this patient home: 39 of 70 (55.7%) who reported the presence of VIP services at their hospital, and 22 of 91 (24.2%) who reported the absence of VIP services (P < 0.001). Of those who reported they would not discharge this patient home, 37 (61%) reported the reason for this related to the patient’s connection to the Board of Trustees; 48 (79%) reported the reason for this related to the patient’s concerns; 9 (15%) reported the reason for this related to their own concerns regarding medical details of the patient’s case (respondents could select more than 1 reason).
When presented the case of a VIP patient with acute pulmonary embolism who is medically ready for discharge with primary care physician-approved anticoagulation and discharge plans but for whom their family requests additional consultations and inpatient hypercoagulable workup, 33 (21%) respondents reported they would order additional testing and specialist consultation: 17 of 69 (24.6%) who reported the presence of VIP services their hospital, and 16 of 91 (17.6%) who reported the absence of VIP services (P = 0.33). Of those who reported they would order additional testing and specialist consultation, 14 (42%) reported the reason for this related to the family’s financial connections to the hospital; 30 (91%) reported the reason for this related to the family’s concerns; 3 (9%) reported the reason for this related to their own concerns about the medical details of the patient’s case (respondents could select more than 1 reason).
DISCUSSION
In our study, a majority of physicians who reported the presence of VIP services at their hospital felt pressured by VIP patients or their family members to perform unnecessary testing or treatment. While this study was not designed to quantify the burden of unnecessary care for VIP patients, our results have implications for individual patients and public health, including potential effects on resource availability, the identification of clinically irrelevant incidental findings, and short- and long-term medical complications of procedures, testing and radiation exposure.
Prior publications have advocated that physicians and hospitals should not allow VIP status to influence management decisions.3,5 We found that more than one-third of physicians who reported the presence of VIP services at their hospital also reported receiving pressure from hospital representatives to provide care to VIP patients that was not medically indicated. These findings highlight an example of the tension faced by physicians who are caught between patient requests and the delivery of value-based care. This potential conflict may be amplified particularly for those patients with close financial, social, or professional ties to the hospitals (and physicians) providing their care. These results suggest the need for physicians, administrators, and patients to work together to address the potential blurring of ethical boundaries created by VIP relationships. Prevention of harm and avoidance of placing physicians in morally distressing situations are common goals for all involved parties.
Efforts to reduce unnecessary care have predominantly focused on structural and knowledge-based drivers.4,8,9 Our results highlight the presence of additional forces. A majority of physician respondents who reported the presence of VIP services at their hospital also reported that they would be more likely to comply with requests for unnecessary care for a VIP patient as compared to a non-VIP patient. Furthermore, in case-based questions about the requests of a VIP patient and their family for additional unnecessary care, a significant portion of physicians who reported they would comply with these requests listed the VIP status of the patient or family as a factor underlying this decision. Only a minority of physicians reported their decision to provide additional care was the result of their own medically-based concerns. Because these cases were hypothetical and we did not include comparator cases involving non-VIP patients, it remains uncertain whether the observed perceptions accurately reflect real-world differences in the care of VIP and non-VIP patients. Nonetheless, our findings emphasize the importance of better understanding the social drivers of overuse and physician communication strategies related to medically inappropriate tests.10,11
Demand for unnecessary testing may be driven by the mentality that “more is better.”12 Contrary to this belief, provision of unnecessary care can increase the risk of patient harm.13 Despite physician respondents reporting that VIP patients requested and/or received additional unnecessary care, a majority of respondents felt that patient safety for VIP patients was equivalent to that for non-VIP patients. As we assessed only physician perceptions of safety, which may not necessarily correlate with actual safety, further research in this area is needed.
Our study was limited by several factors. While our study population included hospitalists from 8 geographically broad hospitals, including university, safety net, and community hospitals, study responses may not be reflective of nationwide trends. Our response rate may limit our ability to generalize conclusions beyond respondents. Second, our study captured physician perceptions of behavior and safety rather than actually measuring practice and outcomes. Studies comparing physician practice patterns and outcomes between VIP and non-VIP patients would be informative. Additionally, despite our inclusive survey design process, our survey was not validated, and it is possible that our questions were not interpreted as intended. Lastly, despite the anonymous nature of our survey, physicians may have felt compelled to respond in a particular way due to conflicting professional, financial, or social factors.
Our findings provide initial insight into how care for the VIP patient may present unique challenges for physicians, hospitals, and society by systematizing care inequities, as well as potentially incentivizing low-value care practices. Whether these imbalances produce clinical harms or benefits remains worthy of future studies.
Disclosure
Nothing to report.
1. Bernstein N. Chefs, butlers, marble baths: Hospitals vie for the affluent. New York Times. January 21, 2012. http://www.nytimes.com/2012/01/22/nyregion/chefs-butlers-and-marble-baths-not-your-average-hospital-room.html. Accessed February 1, 2017.
2. Kennedy DW, Kagan SH, Abramson KB, Boberick C, Kaiser LR. Academic medicine amenities unit: developing a model to integrate academic medical care with luxury hotel services. Acad Med. 2009;84(2):185-191. PubMed
3. Alfandre D, Clever S, Farber NJ, Hughes MT, Redstone P, Lehmann LS. Caring for ‘very important patients’--ethical dilemmas and suggestions for practical management. Am J Med. 2016;129(2):143-147. PubMed
4. Bulger J, Nickel W, Messler J, et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):486-492. PubMed
5. Martin A, Bostic JQ, Pruett K. The V.I.P.: hazard and promise in treating “special” patients. J Am Acad Child Adolesc Psychiatry. 2004;43(3):366-369. PubMed
6. Smally AJ, Carroll B, Carius M, Tilden F, Werdmann M. Treatment of VIPs. Ann Emerg Med. 2011;58(4):397-398. PubMed
7. Auerbach AD, Patel MS, Metlay JP, et al. The hospital medicine reengineering network (HOMERuN): a learning organization focused on improving hospital care. Acad Med. 2014;89(3):415-420. PubMed
8. Caverly TJ, Combs BP, Moriates C, Shah N, Grady D. Too much medicine happens too often: the teachable moment and a call for manuscripts from clinical trainees. JAMA Intern Med. 2014;174(1):8-9. PubMed
9. Schwartz AL, Chernew ME, Landon BE, McWilliams JM. Changes in low-value services in year 1 of the Medicare pioneer accountable care organization program. JAMA Intern Med. 2015;175(11):1815-1825. PubMed
10. Paterniti DA, Fancher TL, Cipri CS, Timmermans S, Heritage J, Kravitz RL. Getting to “no”: strategies primary care physicians use to deny patient requests. Arch Intern Med. 2010;170(4):381-388. PubMed
11. Veroff D, Marr A, Wennberg DE. Enhanced support for shared decision making reduced costs of care for patients with preference-sensitive conditions. Health Aff (Millwood). 2013;32(2):285-293. PubMed
12. Korenstein D. Patient perception of benefits and harms: the Achilles heel of high-value care. JAMA Intern Med. 2015;175(2):287-288. PubMed
13. Moynihan R, Doust J, Henry D. Preventing overdiagnosis: how to stop harming the healthy. BMJ. 2012;344:e3502. PubMed
Recent publications in the medical literature and lay press have stirred controversy regarding the use of inpatient ‘very important person’ (VIP) services.1-3 The term “VIP services” often refers to select conveniences offered in addition to the assumed basic level of care and services provided by a hospital. Examples include additional space, enhanced facilities, specific comforts, or personal support. In some instances, these amenities may only be provided to patients who have close financial, social, or professional relationships with the hospital.
How VIP patients interact with their health system to obtain VIP services has raised unique concerns. Some have speculated that the presence of a VIP patient may be disruptive to the care of non-VIP patients, while others have cautioned physicians about potential dangers to the VIP patients themselves.4-6 Despite much being written on the topics of VIP patients and services in both the lay and academic press, our literature review identified only 1 study on the topic, which cataloged the preferential treatment of VIP patients in the emergency department.6 We are unaware of any investigations of VIP-service use in the inpatient setting. Through a multisite survey of hospital medicine physicians, we assessed physician viewpoints and behavior regarding VIP services.
METHODS
The Hospital Medicine Reengineering Network (HOMERuN) is a nation-wide learning organization focused on measuring and improving the outcomes of hospitalized patients.7 We surveyed hospitalists from 8 HOMERuN hospitals (Appendix 1). The survey instrument contained 4 sections: nonidentifying respondent demographics, local use of VIP services, reported physician perceptions of VIP services, and case-based assessments (Appendix 2). Survey questions and individual cases were developed by study authors and based on real scenarios and concerns provided by front-line clinical providers. Content, length, and reliability of physician understanding were assessed by a 5-person focus group consisting of physicians not included in the survey population.
Subjects were identified via administrative rosters from each HOMERuN site. Surveys were administered via SurveyMonkey, and results were analyzed descriptively. Populations were compared via the Fisher exact test. “VIP services” were defined as conveniences provided in addition to the assumed basic level of care and services (eg, private or luxury-style rooms, access to a special menu, better views, dedicated personal care attendants, hospital liaisons). VIP patients were defined as those patients receiving VIP services. A hospital was identified as providing VIP services if 50% or more of respondents from that site reported the presence of VIP services.
RESULTS
Of 366 hospitalists contacted, 160 completed the survey (44%). Respondent characteristics and reported prevalence of VIP services are demonstrated in Table 1. In total, 78 respondents (45%) reported the presence of VIP services at their hospital. Of the 8 sites surveyed, a majority of physicians at 4 sites (50%) reported presence of VIP services.
Of respondents reporting the presence of VIP services at their hospital, a majority felt that, from a patient safety perspective, the care received by VIP patients was the same as care received by non-VIP patients (Table 2). A majority reported they had felt pressured by a VIP patient or a family member to order additional tests or treatments that the physician believed were medically unnecessary and that they would be more likely to comply with VIP patient’s requests for tests or treatments they felt were unnecessary. More than one-third (36%) felt pressured by other hospital employees or representatives to comply with VIP services patient’s requests for additional tests or treatments that the physicians believed were medically unnecessary.
When presented the case of a VIP patient with community-acquired pneumonia who is clinically stable for discharge but expressing concerns about leaving the hospital, 61 (38%) respondents reported they would not discharge this patient home: 39 of 70 (55.7%) who reported the presence of VIP services at their hospital, and 22 of 91 (24.2%) who reported the absence of VIP services (P < 0.001). Of those who reported they would not discharge this patient home, 37 (61%) reported the reason for this related to the patient’s connection to the Board of Trustees; 48 (79%) reported the reason for this related to the patient’s concerns; 9 (15%) reported the reason for this related to their own concerns regarding medical details of the patient’s case (respondents could select more than 1 reason).
When presented the case of a VIP patient with acute pulmonary embolism who is medically ready for discharge with primary care physician-approved anticoagulation and discharge plans but for whom their family requests additional consultations and inpatient hypercoagulable workup, 33 (21%) respondents reported they would order additional testing and specialist consultation: 17 of 69 (24.6%) who reported the presence of VIP services their hospital, and 16 of 91 (17.6%) who reported the absence of VIP services (P = 0.33). Of those who reported they would order additional testing and specialist consultation, 14 (42%) reported the reason for this related to the family’s financial connections to the hospital; 30 (91%) reported the reason for this related to the family’s concerns; 3 (9%) reported the reason for this related to their own concerns about the medical details of the patient’s case (respondents could select more than 1 reason).
DISCUSSION
In our study, a majority of physicians who reported the presence of VIP services at their hospital felt pressured by VIP patients or their family members to perform unnecessary testing or treatment. While this study was not designed to quantify the burden of unnecessary care for VIP patients, our results have implications for individual patients and public health, including potential effects on resource availability, the identification of clinically irrelevant incidental findings, and short- and long-term medical complications of procedures, testing and radiation exposure.
Prior publications have advocated that physicians and hospitals should not allow VIP status to influence management decisions.3,5 We found that more than one-third of physicians who reported the presence of VIP services at their hospital also reported receiving pressure from hospital representatives to provide care to VIP patients that was not medically indicated. These findings highlight an example of the tension faced by physicians who are caught between patient requests and the delivery of value-based care. This potential conflict may be amplified particularly for those patients with close financial, social, or professional ties to the hospitals (and physicians) providing their care. These results suggest the need for physicians, administrators, and patients to work together to address the potential blurring of ethical boundaries created by VIP relationships. Prevention of harm and avoidance of placing physicians in morally distressing situations are common goals for all involved parties.
Efforts to reduce unnecessary care have predominantly focused on structural and knowledge-based drivers.4,8,9 Our results highlight the presence of additional forces. A majority of physician respondents who reported the presence of VIP services at their hospital also reported that they would be more likely to comply with requests for unnecessary care for a VIP patient as compared to a non-VIP patient. Furthermore, in case-based questions about the requests of a VIP patient and their family for additional unnecessary care, a significant portion of physicians who reported they would comply with these requests listed the VIP status of the patient or family as a factor underlying this decision. Only a minority of physicians reported their decision to provide additional care was the result of their own medically-based concerns. Because these cases were hypothetical and we did not include comparator cases involving non-VIP patients, it remains uncertain whether the observed perceptions accurately reflect real-world differences in the care of VIP and non-VIP patients. Nonetheless, our findings emphasize the importance of better understanding the social drivers of overuse and physician communication strategies related to medically inappropriate tests.10,11
Demand for unnecessary testing may be driven by the mentality that “more is better.”12 Contrary to this belief, provision of unnecessary care can increase the risk of patient harm.13 Despite physician respondents reporting that VIP patients requested and/or received additional unnecessary care, a majority of respondents felt that patient safety for VIP patients was equivalent to that for non-VIP patients. As we assessed only physician perceptions of safety, which may not necessarily correlate with actual safety, further research in this area is needed.
Our study was limited by several factors. While our study population included hospitalists from 8 geographically broad hospitals, including university, safety net, and community hospitals, study responses may not be reflective of nationwide trends. Our response rate may limit our ability to generalize conclusions beyond respondents. Second, our study captured physician perceptions of behavior and safety rather than actually measuring practice and outcomes. Studies comparing physician practice patterns and outcomes between VIP and non-VIP patients would be informative. Additionally, despite our inclusive survey design process, our survey was not validated, and it is possible that our questions were not interpreted as intended. Lastly, despite the anonymous nature of our survey, physicians may have felt compelled to respond in a particular way due to conflicting professional, financial, or social factors.
Our findings provide initial insight into how care for the VIP patient may present unique challenges for physicians, hospitals, and society by systematizing care inequities, as well as potentially incentivizing low-value care practices. Whether these imbalances produce clinical harms or benefits remains worthy of future studies.
Disclosure
Nothing to report.
Recent publications in the medical literature and lay press have stirred controversy regarding the use of inpatient ‘very important person’ (VIP) services.1-3 The term “VIP services” often refers to select conveniences offered in addition to the assumed basic level of care and services provided by a hospital. Examples include additional space, enhanced facilities, specific comforts, or personal support. In some instances, these amenities may only be provided to patients who have close financial, social, or professional relationships with the hospital.
How VIP patients interact with their health system to obtain VIP services has raised unique concerns. Some have speculated that the presence of a VIP patient may be disruptive to the care of non-VIP patients, while others have cautioned physicians about potential dangers to the VIP patients themselves.4-6 Despite much being written on the topics of VIP patients and services in both the lay and academic press, our literature review identified only 1 study on the topic, which cataloged the preferential treatment of VIP patients in the emergency department.6 We are unaware of any investigations of VIP-service use in the inpatient setting. Through a multisite survey of hospital medicine physicians, we assessed physician viewpoints and behavior regarding VIP services.
METHODS
The Hospital Medicine Reengineering Network (HOMERuN) is a nation-wide learning organization focused on measuring and improving the outcomes of hospitalized patients.7 We surveyed hospitalists from 8 HOMERuN hospitals (Appendix 1). The survey instrument contained 4 sections: nonidentifying respondent demographics, local use of VIP services, reported physician perceptions of VIP services, and case-based assessments (Appendix 2). Survey questions and individual cases were developed by study authors and based on real scenarios and concerns provided by front-line clinical providers. Content, length, and reliability of physician understanding were assessed by a 5-person focus group consisting of physicians not included in the survey population.
Subjects were identified via administrative rosters from each HOMERuN site. Surveys were administered via SurveyMonkey, and results were analyzed descriptively. Populations were compared via the Fisher exact test. “VIP services” were defined as conveniences provided in addition to the assumed basic level of care and services (eg, private or luxury-style rooms, access to a special menu, better views, dedicated personal care attendants, hospital liaisons). VIP patients were defined as those patients receiving VIP services. A hospital was identified as providing VIP services if 50% or more of respondents from that site reported the presence of VIP services.
RESULTS
Of 366 hospitalists contacted, 160 completed the survey (44%). Respondent characteristics and reported prevalence of VIP services are demonstrated in Table 1. In total, 78 respondents (45%) reported the presence of VIP services at their hospital. Of the 8 sites surveyed, a majority of physicians at 4 sites (50%) reported presence of VIP services.
Of respondents reporting the presence of VIP services at their hospital, a majority felt that, from a patient safety perspective, the care received by VIP patients was the same as care received by non-VIP patients (Table 2). A majority reported they had felt pressured by a VIP patient or a family member to order additional tests or treatments that the physician believed were medically unnecessary and that they would be more likely to comply with VIP patient’s requests for tests or treatments they felt were unnecessary. More than one-third (36%) felt pressured by other hospital employees or representatives to comply with VIP services patient’s requests for additional tests or treatments that the physicians believed were medically unnecessary.
When presented the case of a VIP patient with community-acquired pneumonia who is clinically stable for discharge but expressing concerns about leaving the hospital, 61 (38%) respondents reported they would not discharge this patient home: 39 of 70 (55.7%) who reported the presence of VIP services at their hospital, and 22 of 91 (24.2%) who reported the absence of VIP services (P < 0.001). Of those who reported they would not discharge this patient home, 37 (61%) reported the reason for this related to the patient’s connection to the Board of Trustees; 48 (79%) reported the reason for this related to the patient’s concerns; 9 (15%) reported the reason for this related to their own concerns regarding medical details of the patient’s case (respondents could select more than 1 reason).
When presented the case of a VIP patient with acute pulmonary embolism who is medically ready for discharge with primary care physician-approved anticoagulation and discharge plans but for whom their family requests additional consultations and inpatient hypercoagulable workup, 33 (21%) respondents reported they would order additional testing and specialist consultation: 17 of 69 (24.6%) who reported the presence of VIP services their hospital, and 16 of 91 (17.6%) who reported the absence of VIP services (P = 0.33). Of those who reported they would order additional testing and specialist consultation, 14 (42%) reported the reason for this related to the family’s financial connections to the hospital; 30 (91%) reported the reason for this related to the family’s concerns; 3 (9%) reported the reason for this related to their own concerns about the medical details of the patient’s case (respondents could select more than 1 reason).
DISCUSSION
In our study, a majority of physicians who reported the presence of VIP services at their hospital felt pressured by VIP patients or their family members to perform unnecessary testing or treatment. While this study was not designed to quantify the burden of unnecessary care for VIP patients, our results have implications for individual patients and public health, including potential effects on resource availability, the identification of clinically irrelevant incidental findings, and short- and long-term medical complications of procedures, testing and radiation exposure.
Prior publications have advocated that physicians and hospitals should not allow VIP status to influence management decisions.3,5 We found that more than one-third of physicians who reported the presence of VIP services at their hospital also reported receiving pressure from hospital representatives to provide care to VIP patients that was not medically indicated. These findings highlight an example of the tension faced by physicians who are caught between patient requests and the delivery of value-based care. This potential conflict may be amplified particularly for those patients with close financial, social, or professional ties to the hospitals (and physicians) providing their care. These results suggest the need for physicians, administrators, and patients to work together to address the potential blurring of ethical boundaries created by VIP relationships. Prevention of harm and avoidance of placing physicians in morally distressing situations are common goals for all involved parties.
Efforts to reduce unnecessary care have predominantly focused on structural and knowledge-based drivers.4,8,9 Our results highlight the presence of additional forces. A majority of physician respondents who reported the presence of VIP services at their hospital also reported that they would be more likely to comply with requests for unnecessary care for a VIP patient as compared to a non-VIP patient. Furthermore, in case-based questions about the requests of a VIP patient and their family for additional unnecessary care, a significant portion of physicians who reported they would comply with these requests listed the VIP status of the patient or family as a factor underlying this decision. Only a minority of physicians reported their decision to provide additional care was the result of their own medically-based concerns. Because these cases were hypothetical and we did not include comparator cases involving non-VIP patients, it remains uncertain whether the observed perceptions accurately reflect real-world differences in the care of VIP and non-VIP patients. Nonetheless, our findings emphasize the importance of better understanding the social drivers of overuse and physician communication strategies related to medically inappropriate tests.10,11
Demand for unnecessary testing may be driven by the mentality that “more is better.”12 Contrary to this belief, provision of unnecessary care can increase the risk of patient harm.13 Despite physician respondents reporting that VIP patients requested and/or received additional unnecessary care, a majority of respondents felt that patient safety for VIP patients was equivalent to that for non-VIP patients. As we assessed only physician perceptions of safety, which may not necessarily correlate with actual safety, further research in this area is needed.
Our study was limited by several factors. While our study population included hospitalists from 8 geographically broad hospitals, including university, safety net, and community hospitals, study responses may not be reflective of nationwide trends. Our response rate may limit our ability to generalize conclusions beyond respondents. Second, our study captured physician perceptions of behavior and safety rather than actually measuring practice and outcomes. Studies comparing physician practice patterns and outcomes between VIP and non-VIP patients would be informative. Additionally, despite our inclusive survey design process, our survey was not validated, and it is possible that our questions were not interpreted as intended. Lastly, despite the anonymous nature of our survey, physicians may have felt compelled to respond in a particular way due to conflicting professional, financial, or social factors.
Our findings provide initial insight into how care for the VIP patient may present unique challenges for physicians, hospitals, and society by systematizing care inequities, as well as potentially incentivizing low-value care practices. Whether these imbalances produce clinical harms or benefits remains worthy of future studies.
Disclosure
Nothing to report.
1. Bernstein N. Chefs, butlers, marble baths: Hospitals vie for the affluent. New York Times. January 21, 2012. http://www.nytimes.com/2012/01/22/nyregion/chefs-butlers-and-marble-baths-not-your-average-hospital-room.html. Accessed February 1, 2017.
2. Kennedy DW, Kagan SH, Abramson KB, Boberick C, Kaiser LR. Academic medicine amenities unit: developing a model to integrate academic medical care with luxury hotel services. Acad Med. 2009;84(2):185-191. PubMed
3. Alfandre D, Clever S, Farber NJ, Hughes MT, Redstone P, Lehmann LS. Caring for ‘very important patients’--ethical dilemmas and suggestions for practical management. Am J Med. 2016;129(2):143-147. PubMed
4. Bulger J, Nickel W, Messler J, et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):486-492. PubMed
5. Martin A, Bostic JQ, Pruett K. The V.I.P.: hazard and promise in treating “special” patients. J Am Acad Child Adolesc Psychiatry. 2004;43(3):366-369. PubMed
6. Smally AJ, Carroll B, Carius M, Tilden F, Werdmann M. Treatment of VIPs. Ann Emerg Med. 2011;58(4):397-398. PubMed
7. Auerbach AD, Patel MS, Metlay JP, et al. The hospital medicine reengineering network (HOMERuN): a learning organization focused on improving hospital care. Acad Med. 2014;89(3):415-420. PubMed
8. Caverly TJ, Combs BP, Moriates C, Shah N, Grady D. Too much medicine happens too often: the teachable moment and a call for manuscripts from clinical trainees. JAMA Intern Med. 2014;174(1):8-9. PubMed
9. Schwartz AL, Chernew ME, Landon BE, McWilliams JM. Changes in low-value services in year 1 of the Medicare pioneer accountable care organization program. JAMA Intern Med. 2015;175(11):1815-1825. PubMed
10. Paterniti DA, Fancher TL, Cipri CS, Timmermans S, Heritage J, Kravitz RL. Getting to “no”: strategies primary care physicians use to deny patient requests. Arch Intern Med. 2010;170(4):381-388. PubMed
11. Veroff D, Marr A, Wennberg DE. Enhanced support for shared decision making reduced costs of care for patients with preference-sensitive conditions. Health Aff (Millwood). 2013;32(2):285-293. PubMed
12. Korenstein D. Patient perception of benefits and harms: the Achilles heel of high-value care. JAMA Intern Med. 2015;175(2):287-288. PubMed
13. Moynihan R, Doust J, Henry D. Preventing overdiagnosis: how to stop harming the healthy. BMJ. 2012;344:e3502. PubMed
1. Bernstein N. Chefs, butlers, marble baths: Hospitals vie for the affluent. New York Times. January 21, 2012. http://www.nytimes.com/2012/01/22/nyregion/chefs-butlers-and-marble-baths-not-your-average-hospital-room.html. Accessed February 1, 2017.
2. Kennedy DW, Kagan SH, Abramson KB, Boberick C, Kaiser LR. Academic medicine amenities unit: developing a model to integrate academic medical care with luxury hotel services. Acad Med. 2009;84(2):185-191. PubMed
3. Alfandre D, Clever S, Farber NJ, Hughes MT, Redstone P, Lehmann LS. Caring for ‘very important patients’--ethical dilemmas and suggestions for practical management. Am J Med. 2016;129(2):143-147. PubMed
4. Bulger J, Nickel W, Messler J, et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):486-492. PubMed
5. Martin A, Bostic JQ, Pruett K. The V.I.P.: hazard and promise in treating “special” patients. J Am Acad Child Adolesc Psychiatry. 2004;43(3):366-369. PubMed
6. Smally AJ, Carroll B, Carius M, Tilden F, Werdmann M. Treatment of VIPs. Ann Emerg Med. 2011;58(4):397-398. PubMed
7. Auerbach AD, Patel MS, Metlay JP, et al. The hospital medicine reengineering network (HOMERuN): a learning organization focused on improving hospital care. Acad Med. 2014;89(3):415-420. PubMed
8. Caverly TJ, Combs BP, Moriates C, Shah N, Grady D. Too much medicine happens too often: the teachable moment and a call for manuscripts from clinical trainees. JAMA Intern Med. 2014;174(1):8-9. PubMed
9. Schwartz AL, Chernew ME, Landon BE, McWilliams JM. Changes in low-value services in year 1 of the Medicare pioneer accountable care organization program. JAMA Intern Med. 2015;175(11):1815-1825. PubMed
10. Paterniti DA, Fancher TL, Cipri CS, Timmermans S, Heritage J, Kravitz RL. Getting to “no”: strategies primary care physicians use to deny patient requests. Arch Intern Med. 2010;170(4):381-388. PubMed
11. Veroff D, Marr A, Wennberg DE. Enhanced support for shared decision making reduced costs of care for patients with preference-sensitive conditions. Health Aff (Millwood). 2013;32(2):285-293. PubMed
12. Korenstein D. Patient perception of benefits and harms: the Achilles heel of high-value care. JAMA Intern Med. 2015;175(2):287-288. PubMed
13. Moynihan R, Doust J, Henry D. Preventing overdiagnosis: how to stop harming the healthy. BMJ. 2012;344:e3502. PubMed
© 2017 Society of Hospital Medicine
A time and motion study of pharmacists and pharmacy technicians obtaining admission medication histories
Using pharmacists to obtain admission medication histories (AMHs) reduces medication errors by 70% to 83% and resultant adverse drug events (ADEs) by 15%.1-3 Dissemination of this practice has been limited by several factors, including clinician practice models, staff availability, confusion in provider roles and accountability, and absence of standardized best practices.4-5 This paper assesses one of these barriers: the high cost of utilizing pharmacists. Third-person observer time and motion analysis shows that pharmacists require 46 and 92 minutes to obtain AMHs from medical and geriatric patients,6 respectively, resulting in pharmacist costs of $44 to $88 per patient, based on 2015 US Bureau of Labor Statistics (BLS) hourly wage data for pharmacists ($57.34).7
Ph
METHODS
This study originated as part of a randomized, controlled trial conducted during January-February 2014 at Cedars-Sinai Medical Center (CSMC), an 896-bed, university-affiliated, not-for-profit hospital.9 Pharmacy staff included pharmacists, PGY-1 pharmacy residents, and pharmacy technicians, each of whom received standardized didactic and experiential training (Appendix 1).
The pharmacists’ AMH and general pharmacy experience ranged from <1 to 3 years and <1 to 5 years, respectively. For PSPTs, AMH and general pharmacy experience ranged from <1 to 2 years and 1 to 17 years, respectively. Three additional pharmacists were involved in supervising PSPTs, and their experience fell within the aforementioned ranges, except for one pharmacist with general pharmacy experience of 16 years. The CSMC Institutional Review Board approved this study with oral consent from pharmacy staff.
For the trial, pharmacists and PSPTs obtained AMHs from 185 patients identified as high-risk for ADEs in the CSMC Emergency Department (ED). Patients were randomized into each arm using RANDI2 software11 if they met one of the trial inclusion criteria, accessed via electronic health record (EHR) (Appendix 2). For several days during this trial, a trained research nurse shadowed pharmacists and PSPTs to record tasks performed, as well as the actual time, including start and end times, dedicated to each task.
After excluding AMHs with incomplete data, we calculated mean AMH times and component task times (Table). We compared mean times for pharmacists and PSPTs using two sample t tests (Table). We calculated mean times of tasks across only AMHs that required the task, mean times of tasks across all AMHs studied, regardless of whether the AMH required the task or not (assigning 0 minutes for the task if it was not required), and percent mean time of task per patient for providers combined (Table).
We calculated Pearson product-moment correlation estimates between AMH time and these continuous variables: patient age; total number of EHR medications; number of chronic EHR medications; years of provider AMH experience; and years of provider general pharmacy experience. Using two sample t tests, we also checked for associations between AMH time and the following categorical variables: sex; presence of a patient-provided medication list; caregiver availability; and altered mental status, as determined by review of the ED physician’s note. Caregiver availability was defined as the availability of a family member, caregiver, or medication administration record (MAR) for patients residing at a skilled nursing facility (SNF). The rationale for combining these variables is that SNF nurses are the primary caregivers responsible for administering medications, and the MAR is reflective of their actions.
After reviewing our initial data, we decided to increase our sample size from 20 to 30 complete AMHs. Because the trial had concluded, we selected 10 additional patients who met trial criteria and who would already have an AMH obtained by pharmacy staff for operational reasons. The only difference with the second set of patients (n = 10) is that we did not randomize patients into each arm, but chose to focus on AMHs obtained by PSPTs, as there is a greater need in the literature to study PSPTs. After finalizing data collection, the aforementioned analyses were conducted on the complete data set.
Lastly, we estimated the mean labor cost for pharmacists and PSPTs to obtain an AMH by using 2015 US BLS hourly wage data for pharmacists ($57.34) and pharmacy technicians ($15.23).7 The cost for a pharmacist-obtained AMH was calculated by multiplying the measured mean time a pharmacist needed to obtain an AMH by $57.34 per hour. The cost for a PSPT-obtained AMH was the sum of the PSPT’s measured mean time to obtain an AMH multiplied by $15.23 per hour and the measured mean pharmacist supervisory time multiplied by $57.34 per hour.
RESULTS
Of the 37 observed AMHs, 30 had complete data. Seven AMHs were excluded because not all task times were recorded, due to the schedule restraints of the research nurse. Pharmacists and PSPTs obtained 12 and 18 AMHs, respectively. Mean patient ages were 83.3 (95% confidence interval [CI], 77.3-89.2) and 79.8 (95% CI, 71.5-88.0), for pharmacists and PSPTs, respectively (P = 0.55). Patient’s EHRs contained a mean of 14.3 (95% CI, 11.2-17.5) and 16.3 (95% CI, 13.2-19.5) medications, prior to pharmacists and PSPTs obtaining an AMH, respectively (P = 0.41).
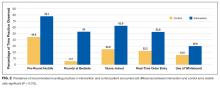
The mean time pharmacists and PSPTs needed to obtain an AMH was 58.5 (95% CI, 46.9-70.1) and 79.4 (95% CI, 59.1-99.8) minutes, respectively (P = 0.14). Summary time data per provider is reported in the Figure. The mean time for pharmacist supervision of technicians was 26 (95% CI, 14.9-37.1) minutes. Mean times of tasks and comparisons of these means times between providers are reported in the Table. The percent mean time for each task per patient for providers combined is also reported in the Table, in which utilizing the EHR was associated with the greatest percentage of time spent at 42.8% (95% CI, 37.4-48.2).
In the 18 cases for which a caregiver (or SNF medication list) was available, providers needed only 58.1 (95% CI, 44.1-72.1) minutes to obtain an AMH, as compared with 90.5 (95% CI, 67.9-113.1) minutes for the 12 cases lacking these resources (P = 0.02). We also found that among PSPTs, years of AMH experience were positively correlated with AMH time (coefficient of correlation 0.49, P = 0.04). No other studied variables were correlated with or associated with differential AMH times.
We estimated mean labor costs for pharmacists and PSPTs to obtain AMHs as $55.91 (95% CI, 44.9-67.0) and $45.00 (95% CI, 29.7-60.4) per patient, respectively (P = 0.32). In the latter case, $24.85 (95% CI, 14.3-35.4) of the $45.00 would be needed for pharmacist supervisory time. The labor cost for a PSPT-obtained AMH ($45.00) was the sum of the PSPT’s mean time (79.4 minutes) multiplied by technician wage data ($15.23/hour) and supervising pharmacist’s mean time (26.0 minutes) multiplied by pharmacist wage data ($57.34/hour).
DISCUSSION
Although limited by sample size, we observed no difference in time or costs of obtaining AMHs between pharmacists and PSPTs. Several prior studies reported that pharmacists and technicians needed less time to obtain AMHs (20-40 minutes), as compared with our findings.12-14 However, most prior studies used younger, healthier patients. Additionally, they used clinician self-reporting instead of third-person observer time and motion methodology. Indeed, the pharmacist times we observed in this study were consistent with prior findings6 that used accepted third-person observer time and motion methodology.10
We observed more variation in time to obtain AMHs among PSPTs than among pharmacists. While variation may be at least in part to the greater number of technicians studied, variation also points to the need for training and oversight of PSPTs. Selection of PSPTs with prior experience interacting with patients and functioning with higher levels of autonomy, standardized training of PSPTs, and consistent dedication of trained PSPTs to AMH functions to maintain their skills, may help to minimize such variation.
Limitations include the use of a single center and a small sample size. As such, the study may be underpowered to demonstrate statistically significant differences between providers. Furthermore, 7 AMHs (19%) had to be excluded because complete task times were missing. This was exclusively because the workday of the research nurse ended before the AMH had been completed. Another limitation was that the tasks observed could have been dissected further to identify even more specific factors that could be targeted to decrease AMH times. We recommend that future studies be larger, investigate in more depth various factors associated with time needed to obtain AMHs, consider which patients would most likely benefit from PSPTs, and use a measure of value (eg, number of history errors prevented/dollar spent).
In summary, we found that PSPTs can obtain AMHs for similar cost to pharmacists. It will be especially important to know whether PSPTs maintain the accuracy documented in prior studies.8-9 If that continues to be the case, we expect our findings to allow many hospitals to implement programs using PSPTs to obtain accurate AMHs.
Acknowledgment
The authors thank Katherine M. Abdel-Razek for her role in data collection.
Disclosure
This research was supported by NIH/National Center for Advancing Translational Science UCLA CTSI Grant Number KL2TR000122 and National Institute on Aging Grant Number K23 AG049181-01 (Pevnick). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The investigators retained full independence in the conduct of this research.
1. Mergenhagen KA, Blum SS, Kugler A, et al. Pharmacist- versus physician-initiated admission medication reconciliation: impact on adverse drug events. Am J Geriatr Pharmacother. 2012;10(4):242-250. PubMed
2. Mills PR, McGuffie AC. Formal medication reconciliation within the emergency department reduces the medication error rates for emergency admissions. Emerg Med J. 2010;27(12):911-915. PubMed
3. Boockvar KS, LaCorte HC, Giambanco V, Fridman B, Siu A. Medication reconciliation for reducing drug-discrepancy adverse events. Am J Geriatr Pharmacother. 2006;4(3):236-243. PubMed
4. Mueller SK, Sponsler KC, Kripalani S, Schnipper JL. Hospital-based medication reconciliation practices: a systematic review. Arch Intern Med. 2012;172(14):1057-1069. PubMed
5. Lee KP, Hartridge C, Corbett K, Vittinghoff E, Auerbach AD. “Whose job is it, really?” Physicians’, nurses’, and pharmacists’ perspectives on completing inpatient medication reconciliation. J Hosp Med. 2015;10(3):184-186. PubMed
6. Meguerditchian AN, Krotneva S, Reidel K, Huang A, Tamblyn R. Medication reconciliation at admission and discharge: a time and motion study. BMC Health Serv Res. 2013;13:485. PubMed
7. Bureau of Labor Statistics, US Department of Labor, Occupational Employment Statistics, May 2015. Pharmacists and Pharmacy Technicians. http://www.bls.gov/oes/. Accessed July 15, 2016.
8. Johnston R, Saulnier L, Gould O. Best possible medication history in the emergency department: comparing pharmacy technicians and pharmacists. Can J Hosp Pharm. 2010;63(5):359-365. PubMed
9. Pevnick JM, Nguyen CB, Jackevicius CA, et al. Minimizing medication histories errors for patients admitted to the hospital through the emergency department: a three-arm pragmatic randomized controlled trial of adding admission medication history interviews by pharmacists or pharmacist-supervised pharmacy technicians to usual care. J Patient Cent Res Rev. 2015;2:93.
10. Zheng K, Guo MH, Hanauer DA. Using the time and motion method to study clinical work processes and workflow: methodological inconsistencies and a call for standardized research. J Am Med Inform Assoc. 2011;18(5):704-710. PubMed
11. Schrimpf D, Plotnicki L, Pilz LR. Web-based open source application for the randomization process in clinical trials: RANDI2. Int J Clin Pharmacol Ther. 2010;48(7):465-467. PubMed
12. American Society of Health-System Pharmacists and the American Pharmacists Association. ASHP-APhA medication management in care transitions best practices. http://media.pharmacist.com/practice/ASHP_APhA_MedicationManagementinCareTransitionsBestPracticesReport2_2013.pdf. Accessed January 15, 2016.
13. Kent AJ, Harrington L, Skinner J. Medication reconciliation by a pharmacist in the emergency department: a pilot project. Can J Hosp Pharm. 2009;62(3):238-242. PubMed
14. Sen S, Siemianowski L, Murphy M, McAllister SC. Implementation of a pharmacy technician-centered medication reconciliation program at an urban teaching medical center. Am J Health Syst Pharm. 2014;71(1):51-56. PubMed
Using pharmacists to obtain admission medication histories (AMHs) reduces medication errors by 70% to 83% and resultant adverse drug events (ADEs) by 15%.1-3 Dissemination of this practice has been limited by several factors, including clinician practice models, staff availability, confusion in provider roles and accountability, and absence of standardized best practices.4-5 This paper assesses one of these barriers: the high cost of utilizing pharmacists. Third-person observer time and motion analysis shows that pharmacists require 46 and 92 minutes to obtain AMHs from medical and geriatric patients,6 respectively, resulting in pharmacist costs of $44 to $88 per patient, based on 2015 US Bureau of Labor Statistics (BLS) hourly wage data for pharmacists ($57.34).7
Ph
METHODS
This study originated as part of a randomized, controlled trial conducted during January-February 2014 at Cedars-Sinai Medical Center (CSMC), an 896-bed, university-affiliated, not-for-profit hospital.9 Pharmacy staff included pharmacists, PGY-1 pharmacy residents, and pharmacy technicians, each of whom received standardized didactic and experiential training (Appendix 1).
The pharmacists’ AMH and general pharmacy experience ranged from <1 to 3 years and <1 to 5 years, respectively. For PSPTs, AMH and general pharmacy experience ranged from <1 to 2 years and 1 to 17 years, respectively. Three additional pharmacists were involved in supervising PSPTs, and their experience fell within the aforementioned ranges, except for one pharmacist with general pharmacy experience of 16 years. The CSMC Institutional Review Board approved this study with oral consent from pharmacy staff.
For the trial, pharmacists and PSPTs obtained AMHs from 185 patients identified as high-risk for ADEs in the CSMC Emergency Department (ED). Patients were randomized into each arm using RANDI2 software11 if they met one of the trial inclusion criteria, accessed via electronic health record (EHR) (Appendix 2). For several days during this trial, a trained research nurse shadowed pharmacists and PSPTs to record tasks performed, as well as the actual time, including start and end times, dedicated to each task.
After excluding AMHs with incomplete data, we calculated mean AMH times and component task times (Table). We compared mean times for pharmacists and PSPTs using two sample t tests (Table). We calculated mean times of tasks across only AMHs that required the task, mean times of tasks across all AMHs studied, regardless of whether the AMH required the task or not (assigning 0 minutes for the task if it was not required), and percent mean time of task per patient for providers combined (Table).
We calculated Pearson product-moment correlation estimates between AMH time and these continuous variables: patient age; total number of EHR medications; number of chronic EHR medications; years of provider AMH experience; and years of provider general pharmacy experience. Using two sample t tests, we also checked for associations between AMH time and the following categorical variables: sex; presence of a patient-provided medication list; caregiver availability; and altered mental status, as determined by review of the ED physician’s note. Caregiver availability was defined as the availability of a family member, caregiver, or medication administration record (MAR) for patients residing at a skilled nursing facility (SNF). The rationale for combining these variables is that SNF nurses are the primary caregivers responsible for administering medications, and the MAR is reflective of their actions.
After reviewing our initial data, we decided to increase our sample size from 20 to 30 complete AMHs. Because the trial had concluded, we selected 10 additional patients who met trial criteria and who would already have an AMH obtained by pharmacy staff for operational reasons. The only difference with the second set of patients (n = 10) is that we did not randomize patients into each arm, but chose to focus on AMHs obtained by PSPTs, as there is a greater need in the literature to study PSPTs. After finalizing data collection, the aforementioned analyses were conducted on the complete data set.
Lastly, we estimated the mean labor cost for pharmacists and PSPTs to obtain an AMH by using 2015 US BLS hourly wage data for pharmacists ($57.34) and pharmacy technicians ($15.23).7 The cost for a pharmacist-obtained AMH was calculated by multiplying the measured mean time a pharmacist needed to obtain an AMH by $57.34 per hour. The cost for a PSPT-obtained AMH was the sum of the PSPT’s measured mean time to obtain an AMH multiplied by $15.23 per hour and the measured mean pharmacist supervisory time multiplied by $57.34 per hour.
RESULTS
Of the 37 observed AMHs, 30 had complete data. Seven AMHs were excluded because not all task times were recorded, due to the schedule restraints of the research nurse. Pharmacists and PSPTs obtained 12 and 18 AMHs, respectively. Mean patient ages were 83.3 (95% confidence interval [CI], 77.3-89.2) and 79.8 (95% CI, 71.5-88.0), for pharmacists and PSPTs, respectively (P = 0.55). Patient’s EHRs contained a mean of 14.3 (95% CI, 11.2-17.5) and 16.3 (95% CI, 13.2-19.5) medications, prior to pharmacists and PSPTs obtaining an AMH, respectively (P = 0.41).
The mean time pharmacists and PSPTs needed to obtain an AMH was 58.5 (95% CI, 46.9-70.1) and 79.4 (95% CI, 59.1-99.8) minutes, respectively (P = 0.14). Summary time data per provider is reported in the Figure. The mean time for pharmacist supervision of technicians was 26 (95% CI, 14.9-37.1) minutes. Mean times of tasks and comparisons of these means times between providers are reported in the Table. The percent mean time for each task per patient for providers combined is also reported in the Table, in which utilizing the EHR was associated with the greatest percentage of time spent at 42.8% (95% CI, 37.4-48.2).
In the 18 cases for which a caregiver (or SNF medication list) was available, providers needed only 58.1 (95% CI, 44.1-72.1) minutes to obtain an AMH, as compared with 90.5 (95% CI, 67.9-113.1) minutes for the 12 cases lacking these resources (P = 0.02). We also found that among PSPTs, years of AMH experience were positively correlated with AMH time (coefficient of correlation 0.49, P = 0.04). No other studied variables were correlated with or associated with differential AMH times.
We estimated mean labor costs for pharmacists and PSPTs to obtain AMHs as $55.91 (95% CI, 44.9-67.0) and $45.00 (95% CI, 29.7-60.4) per patient, respectively (P = 0.32). In the latter case, $24.85 (95% CI, 14.3-35.4) of the $45.00 would be needed for pharmacist supervisory time. The labor cost for a PSPT-obtained AMH ($45.00) was the sum of the PSPT’s mean time (79.4 minutes) multiplied by technician wage data ($15.23/hour) and supervising pharmacist’s mean time (26.0 minutes) multiplied by pharmacist wage data ($57.34/hour).
DISCUSSION
Although limited by sample size, we observed no difference in time or costs of obtaining AMHs between pharmacists and PSPTs. Several prior studies reported that pharmacists and technicians needed less time to obtain AMHs (20-40 minutes), as compared with our findings.12-14 However, most prior studies used younger, healthier patients. Additionally, they used clinician self-reporting instead of third-person observer time and motion methodology. Indeed, the pharmacist times we observed in this study were consistent with prior findings6 that used accepted third-person observer time and motion methodology.10
We observed more variation in time to obtain AMHs among PSPTs than among pharmacists. While variation may be at least in part to the greater number of technicians studied, variation also points to the need for training and oversight of PSPTs. Selection of PSPTs with prior experience interacting with patients and functioning with higher levels of autonomy, standardized training of PSPTs, and consistent dedication of trained PSPTs to AMH functions to maintain their skills, may help to minimize such variation.
Limitations include the use of a single center and a small sample size. As such, the study may be underpowered to demonstrate statistically significant differences between providers. Furthermore, 7 AMHs (19%) had to be excluded because complete task times were missing. This was exclusively because the workday of the research nurse ended before the AMH had been completed. Another limitation was that the tasks observed could have been dissected further to identify even more specific factors that could be targeted to decrease AMH times. We recommend that future studies be larger, investigate in more depth various factors associated with time needed to obtain AMHs, consider which patients would most likely benefit from PSPTs, and use a measure of value (eg, number of history errors prevented/dollar spent).
In summary, we found that PSPTs can obtain AMHs for similar cost to pharmacists. It will be especially important to know whether PSPTs maintain the accuracy documented in prior studies.8-9 If that continues to be the case, we expect our findings to allow many hospitals to implement programs using PSPTs to obtain accurate AMHs.
Acknowledgment
The authors thank Katherine M. Abdel-Razek for her role in data collection.
Disclosure
This research was supported by NIH/National Center for Advancing Translational Science UCLA CTSI Grant Number KL2TR000122 and National Institute on Aging Grant Number K23 AG049181-01 (Pevnick). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The investigators retained full independence in the conduct of this research.
Using pharmacists to obtain admission medication histories (AMHs) reduces medication errors by 70% to 83% and resultant adverse drug events (ADEs) by 15%.1-3 Dissemination of this practice has been limited by several factors, including clinician practice models, staff availability, confusion in provider roles and accountability, and absence of standardized best practices.4-5 This paper assesses one of these barriers: the high cost of utilizing pharmacists. Third-person observer time and motion analysis shows that pharmacists require 46 and 92 minutes to obtain AMHs from medical and geriatric patients,6 respectively, resulting in pharmacist costs of $44 to $88 per patient, based on 2015 US Bureau of Labor Statistics (BLS) hourly wage data for pharmacists ($57.34).7
Ph
METHODS
This study originated as part of a randomized, controlled trial conducted during January-February 2014 at Cedars-Sinai Medical Center (CSMC), an 896-bed, university-affiliated, not-for-profit hospital.9 Pharmacy staff included pharmacists, PGY-1 pharmacy residents, and pharmacy technicians, each of whom received standardized didactic and experiential training (Appendix 1).
The pharmacists’ AMH and general pharmacy experience ranged from <1 to 3 years and <1 to 5 years, respectively. For PSPTs, AMH and general pharmacy experience ranged from <1 to 2 years and 1 to 17 years, respectively. Three additional pharmacists were involved in supervising PSPTs, and their experience fell within the aforementioned ranges, except for one pharmacist with general pharmacy experience of 16 years. The CSMC Institutional Review Board approved this study with oral consent from pharmacy staff.
For the trial, pharmacists and PSPTs obtained AMHs from 185 patients identified as high-risk for ADEs in the CSMC Emergency Department (ED). Patients were randomized into each arm using RANDI2 software11 if they met one of the trial inclusion criteria, accessed via electronic health record (EHR) (Appendix 2). For several days during this trial, a trained research nurse shadowed pharmacists and PSPTs to record tasks performed, as well as the actual time, including start and end times, dedicated to each task.
After excluding AMHs with incomplete data, we calculated mean AMH times and component task times (Table). We compared mean times for pharmacists and PSPTs using two sample t tests (Table). We calculated mean times of tasks across only AMHs that required the task, mean times of tasks across all AMHs studied, regardless of whether the AMH required the task or not (assigning 0 minutes for the task if it was not required), and percent mean time of task per patient for providers combined (Table).
We calculated Pearson product-moment correlation estimates between AMH time and these continuous variables: patient age; total number of EHR medications; number of chronic EHR medications; years of provider AMH experience; and years of provider general pharmacy experience. Using two sample t tests, we also checked for associations between AMH time and the following categorical variables: sex; presence of a patient-provided medication list; caregiver availability; and altered mental status, as determined by review of the ED physician’s note. Caregiver availability was defined as the availability of a family member, caregiver, or medication administration record (MAR) for patients residing at a skilled nursing facility (SNF). The rationale for combining these variables is that SNF nurses are the primary caregivers responsible for administering medications, and the MAR is reflective of their actions.
After reviewing our initial data, we decided to increase our sample size from 20 to 30 complete AMHs. Because the trial had concluded, we selected 10 additional patients who met trial criteria and who would already have an AMH obtained by pharmacy staff for operational reasons. The only difference with the second set of patients (n = 10) is that we did not randomize patients into each arm, but chose to focus on AMHs obtained by PSPTs, as there is a greater need in the literature to study PSPTs. After finalizing data collection, the aforementioned analyses were conducted on the complete data set.
Lastly, we estimated the mean labor cost for pharmacists and PSPTs to obtain an AMH by using 2015 US BLS hourly wage data for pharmacists ($57.34) and pharmacy technicians ($15.23).7 The cost for a pharmacist-obtained AMH was calculated by multiplying the measured mean time a pharmacist needed to obtain an AMH by $57.34 per hour. The cost for a PSPT-obtained AMH was the sum of the PSPT’s measured mean time to obtain an AMH multiplied by $15.23 per hour and the measured mean pharmacist supervisory time multiplied by $57.34 per hour.
RESULTS
Of the 37 observed AMHs, 30 had complete data. Seven AMHs were excluded because not all task times were recorded, due to the schedule restraints of the research nurse. Pharmacists and PSPTs obtained 12 and 18 AMHs, respectively. Mean patient ages were 83.3 (95% confidence interval [CI], 77.3-89.2) and 79.8 (95% CI, 71.5-88.0), for pharmacists and PSPTs, respectively (P = 0.55). Patient’s EHRs contained a mean of 14.3 (95% CI, 11.2-17.5) and 16.3 (95% CI, 13.2-19.5) medications, prior to pharmacists and PSPTs obtaining an AMH, respectively (P = 0.41).
The mean time pharmacists and PSPTs needed to obtain an AMH was 58.5 (95% CI, 46.9-70.1) and 79.4 (95% CI, 59.1-99.8) minutes, respectively (P = 0.14). Summary time data per provider is reported in the Figure. The mean time for pharmacist supervision of technicians was 26 (95% CI, 14.9-37.1) minutes. Mean times of tasks and comparisons of these means times between providers are reported in the Table. The percent mean time for each task per patient for providers combined is also reported in the Table, in which utilizing the EHR was associated with the greatest percentage of time spent at 42.8% (95% CI, 37.4-48.2).
In the 18 cases for which a caregiver (or SNF medication list) was available, providers needed only 58.1 (95% CI, 44.1-72.1) minutes to obtain an AMH, as compared with 90.5 (95% CI, 67.9-113.1) minutes for the 12 cases lacking these resources (P = 0.02). We also found that among PSPTs, years of AMH experience were positively correlated with AMH time (coefficient of correlation 0.49, P = 0.04). No other studied variables were correlated with or associated with differential AMH times.
We estimated mean labor costs for pharmacists and PSPTs to obtain AMHs as $55.91 (95% CI, 44.9-67.0) and $45.00 (95% CI, 29.7-60.4) per patient, respectively (P = 0.32). In the latter case, $24.85 (95% CI, 14.3-35.4) of the $45.00 would be needed for pharmacist supervisory time. The labor cost for a PSPT-obtained AMH ($45.00) was the sum of the PSPT’s mean time (79.4 minutes) multiplied by technician wage data ($15.23/hour) and supervising pharmacist’s mean time (26.0 minutes) multiplied by pharmacist wage data ($57.34/hour).
DISCUSSION
Although limited by sample size, we observed no difference in time or costs of obtaining AMHs between pharmacists and PSPTs. Several prior studies reported that pharmacists and technicians needed less time to obtain AMHs (20-40 minutes), as compared with our findings.12-14 However, most prior studies used younger, healthier patients. Additionally, they used clinician self-reporting instead of third-person observer time and motion methodology. Indeed, the pharmacist times we observed in this study were consistent with prior findings6 that used accepted third-person observer time and motion methodology.10
We observed more variation in time to obtain AMHs among PSPTs than among pharmacists. While variation may be at least in part to the greater number of technicians studied, variation also points to the need for training and oversight of PSPTs. Selection of PSPTs with prior experience interacting with patients and functioning with higher levels of autonomy, standardized training of PSPTs, and consistent dedication of trained PSPTs to AMH functions to maintain their skills, may help to minimize such variation.
Limitations include the use of a single center and a small sample size. As such, the study may be underpowered to demonstrate statistically significant differences between providers. Furthermore, 7 AMHs (19%) had to be excluded because complete task times were missing. This was exclusively because the workday of the research nurse ended before the AMH had been completed. Another limitation was that the tasks observed could have been dissected further to identify even more specific factors that could be targeted to decrease AMH times. We recommend that future studies be larger, investigate in more depth various factors associated with time needed to obtain AMHs, consider which patients would most likely benefit from PSPTs, and use a measure of value (eg, number of history errors prevented/dollar spent).
In summary, we found that PSPTs can obtain AMHs for similar cost to pharmacists. It will be especially important to know whether PSPTs maintain the accuracy documented in prior studies.8-9 If that continues to be the case, we expect our findings to allow many hospitals to implement programs using PSPTs to obtain accurate AMHs.
Acknowledgment
The authors thank Katherine M. Abdel-Razek for her role in data collection.
Disclosure
This research was supported by NIH/National Center for Advancing Translational Science UCLA CTSI Grant Number KL2TR000122 and National Institute on Aging Grant Number K23 AG049181-01 (Pevnick). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The investigators retained full independence in the conduct of this research.
1. Mergenhagen KA, Blum SS, Kugler A, et al. Pharmacist- versus physician-initiated admission medication reconciliation: impact on adverse drug events. Am J Geriatr Pharmacother. 2012;10(4):242-250. PubMed
2. Mills PR, McGuffie AC. Formal medication reconciliation within the emergency department reduces the medication error rates for emergency admissions. Emerg Med J. 2010;27(12):911-915. PubMed
3. Boockvar KS, LaCorte HC, Giambanco V, Fridman B, Siu A. Medication reconciliation for reducing drug-discrepancy adverse events. Am J Geriatr Pharmacother. 2006;4(3):236-243. PubMed
4. Mueller SK, Sponsler KC, Kripalani S, Schnipper JL. Hospital-based medication reconciliation practices: a systematic review. Arch Intern Med. 2012;172(14):1057-1069. PubMed
5. Lee KP, Hartridge C, Corbett K, Vittinghoff E, Auerbach AD. “Whose job is it, really?” Physicians’, nurses’, and pharmacists’ perspectives on completing inpatient medication reconciliation. J Hosp Med. 2015;10(3):184-186. PubMed
6. Meguerditchian AN, Krotneva S, Reidel K, Huang A, Tamblyn R. Medication reconciliation at admission and discharge: a time and motion study. BMC Health Serv Res. 2013;13:485. PubMed
7. Bureau of Labor Statistics, US Department of Labor, Occupational Employment Statistics, May 2015. Pharmacists and Pharmacy Technicians. http://www.bls.gov/oes/. Accessed July 15, 2016.
8. Johnston R, Saulnier L, Gould O. Best possible medication history in the emergency department: comparing pharmacy technicians and pharmacists. Can J Hosp Pharm. 2010;63(5):359-365. PubMed
9. Pevnick JM, Nguyen CB, Jackevicius CA, et al. Minimizing medication histories errors for patients admitted to the hospital through the emergency department: a three-arm pragmatic randomized controlled trial of adding admission medication history interviews by pharmacists or pharmacist-supervised pharmacy technicians to usual care. J Patient Cent Res Rev. 2015;2:93.
10. Zheng K, Guo MH, Hanauer DA. Using the time and motion method to study clinical work processes and workflow: methodological inconsistencies and a call for standardized research. J Am Med Inform Assoc. 2011;18(5):704-710. PubMed
11. Schrimpf D, Plotnicki L, Pilz LR. Web-based open source application for the randomization process in clinical trials: RANDI2. Int J Clin Pharmacol Ther. 2010;48(7):465-467. PubMed
12. American Society of Health-System Pharmacists and the American Pharmacists Association. ASHP-APhA medication management in care transitions best practices. http://media.pharmacist.com/practice/ASHP_APhA_MedicationManagementinCareTransitionsBestPracticesReport2_2013.pdf. Accessed January 15, 2016.
13. Kent AJ, Harrington L, Skinner J. Medication reconciliation by a pharmacist in the emergency department: a pilot project. Can J Hosp Pharm. 2009;62(3):238-242. PubMed
14. Sen S, Siemianowski L, Murphy M, McAllister SC. Implementation of a pharmacy technician-centered medication reconciliation program at an urban teaching medical center. Am J Health Syst Pharm. 2014;71(1):51-56. PubMed
1. Mergenhagen KA, Blum SS, Kugler A, et al. Pharmacist- versus physician-initiated admission medication reconciliation: impact on adverse drug events. Am J Geriatr Pharmacother. 2012;10(4):242-250. PubMed
2. Mills PR, McGuffie AC. Formal medication reconciliation within the emergency department reduces the medication error rates for emergency admissions. Emerg Med J. 2010;27(12):911-915. PubMed
3. Boockvar KS, LaCorte HC, Giambanco V, Fridman B, Siu A. Medication reconciliation for reducing drug-discrepancy adverse events. Am J Geriatr Pharmacother. 2006;4(3):236-243. PubMed
4. Mueller SK, Sponsler KC, Kripalani S, Schnipper JL. Hospital-based medication reconciliation practices: a systematic review. Arch Intern Med. 2012;172(14):1057-1069. PubMed
5. Lee KP, Hartridge C, Corbett K, Vittinghoff E, Auerbach AD. “Whose job is it, really?” Physicians’, nurses’, and pharmacists’ perspectives on completing inpatient medication reconciliation. J Hosp Med. 2015;10(3):184-186. PubMed
6. Meguerditchian AN, Krotneva S, Reidel K, Huang A, Tamblyn R. Medication reconciliation at admission and discharge: a time and motion study. BMC Health Serv Res. 2013;13:485. PubMed
7. Bureau of Labor Statistics, US Department of Labor, Occupational Employment Statistics, May 2015. Pharmacists and Pharmacy Technicians. http://www.bls.gov/oes/. Accessed July 15, 2016.
8. Johnston R, Saulnier L, Gould O. Best possible medication history in the emergency department: comparing pharmacy technicians and pharmacists. Can J Hosp Pharm. 2010;63(5):359-365. PubMed
9. Pevnick JM, Nguyen CB, Jackevicius CA, et al. Minimizing medication histories errors for patients admitted to the hospital through the emergency department: a three-arm pragmatic randomized controlled trial of adding admission medication history interviews by pharmacists or pharmacist-supervised pharmacy technicians to usual care. J Patient Cent Res Rev. 2015;2:93.
10. Zheng K, Guo MH, Hanauer DA. Using the time and motion method to study clinical work processes and workflow: methodological inconsistencies and a call for standardized research. J Am Med Inform Assoc. 2011;18(5):704-710. PubMed
11. Schrimpf D, Plotnicki L, Pilz LR. Web-based open source application for the randomization process in clinical trials: RANDI2. Int J Clin Pharmacol Ther. 2010;48(7):465-467. PubMed
12. American Society of Health-System Pharmacists and the American Pharmacists Association. ASHP-APhA medication management in care transitions best practices. http://media.pharmacist.com/practice/ASHP_APhA_MedicationManagementinCareTransitionsBestPracticesReport2_2013.pdf. Accessed January 15, 2016.
13. Kent AJ, Harrington L, Skinner J. Medication reconciliation by a pharmacist in the emergency department: a pilot project. Can J Hosp Pharm. 2009;62(3):238-242. PubMed
14. Sen S, Siemianowski L, Murphy M, McAllister SC. Implementation of a pharmacy technician-centered medication reconciliation program at an urban teaching medical center. Am J Health Syst Pharm. 2014;71(1):51-56. PubMed
© 2017 Society of Hospital Medicine
Nondirected testing for inpatients with severe liver injury
The “Things We Do for No Reason” series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CASE REPORT
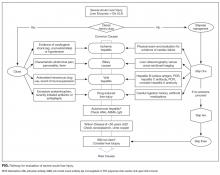
A 68-year-old woman with ischemic cardiomyopathy was admitted with abdominal cramping, diarrhea, and nausea, which had left her unable to keep food and liquids down for 2 days. She had been taking diuretics and had a remote history of intravenous drug use. On admission, she was afebrile and had blood pressure of 100/60 mm Hg and a heart rate of 100 bpm. Her extremities were cool and clammy. Blood test results showed an alanine aminotransferase (ALT) level of 1510 IU/L and an aspartate aminotransferase (AST) level of 1643 IU/L. The patient’s clinician did not know her baseline ALT and AST levels and thought the best approach was to identify the cause of the transaminase elevation.
Severe acute liver injury (liver enzymes, >10 × upper limit of normal [ULN], usually 40 IU/L) is a common presentation among hospitalized patients. Between 1997 and 2015, 1.5% of patients admitted to our hospital had severe liver injury. In another large cohort of hospitalized patients,1 0.6% had an ALT level higher than 1000 IU/L (~20 × ULN). A precise diagnosis is often needed to direct appropriate therapy, and serologic tests are available for many conditions, both common and rare (Table). Given the relative ease of bundled blood testing, nondirected testing has emerged as a popular, if reflexive, strategy.2-5 In this approach, clinicians evaluate each patient for the set of testable diseases all at once—in contrast to taking a directed, stepwise testing approach guided by the patient’s history.
Use of nondirected testing is common in patients with severe acute liver injury. Of the 5795 such patients treated at our hospital between 2000 and 2015, within the same day of service 53% were tested for hepatitis C virus antibody, 38% for hemochromatosis (ferritin test), 28% for autoimmune hepatitis (antinuclear antibody test), and 15% for primary biliary cholangitis (antimitochondrial antibody test) by our clinical laboratory. Of the 5023 patients who had send-out tests performed for Wilson disease (ceruloplasmin), 81% were queried for hepatitis B virus infection, 76% for hepatitis C virus infection, 75% for autoimmune hepatitis, and 73.1% for hemochromatosis.2 Similar trends were found for patients with severe liver injury tested for α1-antitrypsin (AAT) deficiency.3 In sum, these data showed that each patient with severe liver injury was tested out of concern about diseases with markedly different epidemiology and clinical presentations (Table).
WHY YOU MIGHT THINK NONDIRECTED TESTING IS HELPFUL
Use of nondirected testing may reflect perceived urgency, convenience, and thoroughness.2-6 Alternatively, it may simply involve following a consultant’s recommendations.4 As severe acute liver injury is often associated with tremendous morbidity, clinicians seeking answers may perceive directed, stepwise testing as inappropriately slow given the urgency of the presentation; they may think that nondirected testing can reduce hospital length of stay.
WHY NONDIRECTED TESTING IS NOT HELPFUL
Nondirected testing is a problem for at least 4 reasons: limited benefit of reflexive testing for rare diseases, no meaningful impact on outcomes, false positives, and financial cost.
First, immediately testing for rare causes of liver disease is unlikely to benefit patients with severe liver injury. The underlying etiologies of severe liver injury are relatively well circumscribed (Table). Overall, 42% of patients with severe liver injury and 57% of those with an ALT level higher than 1000 IU/L have ischemic hepatitis.7 Accounting for a significant percentage of severe liver injury cases are acute biliary obstruction (24%), drug-induced injury (10%-13%), and viral hepatitis (4%-7%).1,8 Of the small subset of patients with severe liver injury that progresses to acute liver failure (ALF; encephalopathy, coagulopathy), 0.5% have autoimmune hepatitis and 0.1% have Wilson disease.9 Furthermore, many patients are tested for AAT deficiency, hemochromatosis, and primary biliary cholangitis, but these are never causes of severe acute liver injury (Table).
Second, diagnosing a rarer cause of acute liver injury modestly earlier has no meaningful impact on outcome. Work-up for more common etiologies can usually be completed within 2 or 3 days. This is true even for patients with ALF. Specific therapies generally are lacking for ALF, save for use of N-acetylcysteine for acetaminophen overdose and antiviral therapy for hepatitis B virus infection.9,10 Furthermore, although effective therapies are available for both autoimmune hepatitis and Wilson disease, the potential benefit stems from altering the longer term course of disease. Initial management, even for these rare conditions, is no different from that for other etiologies. Conversely, acute liver injury caused by ischemic hepatitis, biliary disease, or drug-induced liver injury requires swift corrective action. Even if normotensive, patients with ischemic hepatitis are often in cardiogenic shock and benefit from careful monitoring and critical care.7 Patients with acute biliary obstruction may need therapeutic endoscopy. Last, patients with drug-induced liver injury benefit from immediate discontinuation of the offending drug.
Third, in the testing of patients with low pretest probabilities, false positives are common. For example, at our institution and at an institution in Austria, severe liver injury patients with a low ceruloplasmin level have a 95.1% to 98.1% chance of a false-positive result (they have a low ceruloplasmin level but do not have Wilson disease).3,4 Furthermore, 91% of positive tests are never confirmed,3 indicating either that clinicians never valued the initial test or that other diagnoses were much more likely. Even worse, as was the case in 65% of patients with low AAT levels,2,3 genetic diagnoses were based on unconfirmed, potentially false-positive serologic tests.
Fourth, although the financial cost for each individual test is small, at the population level the cost of nondirected testing is significant. For example, although each reflects testing for conditions that do not cause acute liver injury, the costs of ferritin, AAT, and antimitochondrial antibody tests are $13, $16, and $37, respectively (Medicare/Medicaid reimbursements in 2016 $US).11 About 1.5% of admitted patients are found to have severe liver injury. If this proportion holds true for the roughly 40 million discharges from US hospitals each year, then there would be an annual cost of about $40 million if all 3 tests were performed for each patient with severe liver injury. In addition, although nondirected testing may seem clinically expedient, there are no data suggesting it reduces length of stay. In fact, ceruloplasmin, AAT, and many other tests are sent to external laboratories and are unlikely to be returned before discharge. If clinicians delay discharge for results, then nondirected testing would increase rather than decrease length of stay.
WHAT YOU SHOULD DO INSTEAD
In this era of increasing cost-consciousness, nondirected testing has escaped relatively unscathed. Indeed, nondirected testing is prevalent, yet has pitfalls similar to those of serologic testing (eg, vasculitis or arthritis,6 acute renal injury, infectious disease12). The alternative is deliberate, empirical, patient-centered testing that is attentive to the patient’s presentation and the harms of false positives. The idea is to select tests for each patient with acute liver injury according to presentation and the most likely corresponding diagnoses (Table, Figure).
The “one-stop shopping” in providers’ electronic order entry systems makes it too easy to over-order tests. Fortunately, these systems’ simple and effective decision supports can force pauses in the ordering process, create barriers to waste, and provide education about test characteristics and costs.4,5,13 Our medical center’s volume of ceruloplasmin orders decreased by 80% after a change was made to its ordering system; the ordering of a ceruloplasmin test is now interrupted by a pop-up screen that displays test characteristics and an option to continue or cancel the order.4,5 Hospitals should consider implementing clinical decision supports in this area. Successful interventions provide electronic rather than paper-based support as part of the clinical workflow, during the ordering process, and recommendations rather than assessments.13
RECOMMENDATIONS
- For each patient with severe acute liver injury, select tests on the basis of the presentation (Figure). Testing for rare diseases should be performed only after common diseases have been excluded.
- Avoid testing for hemochromatosis (iron indices, genetic tests), AAT deficiency (AAT levels or phenotypes), and primary biliary cholangitis (antimitochondrial antibodies) in patients with severe acute liver injury.
- Consider implementing decision supports that can curb nondirected testing in areas in which it is common.
CONCLUSION
Nondirected testing is associated with false positives and increased costs in the evaluation and management of severe acute liver injury. The alternative is deliberate, epidemiologically and clinically driven directed testing. Electronic ordering system decision supports can be useful in curtailing nondirected testing.
Disclosure
Nothing to report.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason”? Let us know what you do in your practice and propose ideas for other “Things We Do for No Reason” topics. Please join in the conversation online at Twitter (#TWDFNR)/Facebook and don’t forget to “Like It” on Facebook or retweet it on Twitter.
1. Johnson RD, O’Connor ML, Kerr RM. Extreme serum elevations of aspartate aminotransferase. Am J Gastroenterol. 1995;90(8):1244-1245. PubMed
2. Tapper EB, Patwardhan VR, Curry M. Low yield and utilization of confirmatory testing in a cohort of patients with liver disease assessed for alpha-1 antitrypsin deficiency. Dig Dis Sci. 2015;60(6):1589-1594. PubMed
3. Tapper EB, Rahni DO, Arnaout R, Lai M. The overuse of serum ceruloplasmin measurement. Am J Med. 2013;126(10):926.e1-e5. PubMed
4. Tapper EB, Sengupta N, Lai M, Horowitz G. Understanding and reducing ceruloplasmin overuse with a decision support intervention for liver disease evaluation. Am J Med. 2016;129(1):115.e17-e22. PubMed
5. Tapper EB, Sengupta N, Lai M, Horowitz G. A decision support tool to reduce overtesting for ceruloplasmin and improve adherence with clinical guidelines. JAMA Intern Med. 2015;175(9):1561-1562. PubMed
6. Lichtenstein MJ, Pincus T. How useful are combinations of blood tests in “rheumatic panels” in diagnosis of rheumatic diseases? J Gen Intern Med. 1988;3(5):435-442. PubMed
7. Tapper EB, Sengupta N, Bonder A. The incidence and outcomes of ischemic hepatitis: a systematic review with meta-analysis. Am J Med. 2015;128(12):1314-1321. PubMed
8. Whitehead MW, Hawkes ND, Hainsworth I, Kingham JG. A prospective study of the causes of notably raised aspartate aminotransferase of liver origin. Gut. 1999;45(1):129-133. PubMed
9. Fontana RJ. Acute liver failure including acetaminophen overdose. Med Clin North Am. 2008;92(4):761-794. PubMed
10. Lee WM, Larson AM, Stravitz RT. AASLD Position Paper: The Management of Acute Liver Failure: Update 2011. American Association for the Study of Liver Diseases website. https://www.aasld.org/sites/default/files/guideline_documents/alfenhanced.pdf. Published 2011. Accessed January 26, 2017.
11. Green RM, Flamm S. AGA technical review on the evaluation of liver chemistry tests. Gastroenterology. 2002;123(4):1367-1384. PubMed
12. Aesif SW, Parenti DM, Lesky L, Keiser JF. A cost-effective interdisciplinary approach to microbiologic send-out test use. Arch Pathol Lab Med. 2015;139(2):194-198. PubMed
13. Kawamoto K, Houlihan CA, Balas EA, Lobach DF. Improving clinical practice using clinical decision support systems: a systematic review of trials to identify features critical to success. BMJ. 2005;330(7494):765. PubMed
14. Boberg KM. Prevalence and epidemiology of autoimmune hepatitis. Clin Liver Dis. 2002;6(3):635-647. PubMed
15. Bacon BR, Adams PC, Kowdley KV, Powell LW, Tavill AS; American Association for the Study of Liver Diseases. Diagnosis and management of hemochromatosis: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology. 2011;54(1):328-343. PubMed
16. Boonstra K, Beuers U, Ponsioen CY. Epidemiology of primary sclerosing cholangitis and primary biliary cirrhosis: a systematic review. J Hepatol. 2012;56(5):1181-1188. PubMed
The “Things We Do for No Reason” series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CASE REPORT
A 68-year-old woman with ischemic cardiomyopathy was admitted with abdominal cramping, diarrhea, and nausea, which had left her unable to keep food and liquids down for 2 days. She had been taking diuretics and had a remote history of intravenous drug use. On admission, she was afebrile and had blood pressure of 100/60 mm Hg and a heart rate of 100 bpm. Her extremities were cool and clammy. Blood test results showed an alanine aminotransferase (ALT) level of 1510 IU/L and an aspartate aminotransferase (AST) level of 1643 IU/L. The patient’s clinician did not know her baseline ALT and AST levels and thought the best approach was to identify the cause of the transaminase elevation.
Severe acute liver injury (liver enzymes, >10 × upper limit of normal [ULN], usually 40 IU/L) is a common presentation among hospitalized patients. Between 1997 and 2015, 1.5% of patients admitted to our hospital had severe liver injury. In another large cohort of hospitalized patients,1 0.6% had an ALT level higher than 1000 IU/L (~20 × ULN). A precise diagnosis is often needed to direct appropriate therapy, and serologic tests are available for many conditions, both common and rare (Table). Given the relative ease of bundled blood testing, nondirected testing has emerged as a popular, if reflexive, strategy.2-5 In this approach, clinicians evaluate each patient for the set of testable diseases all at once—in contrast to taking a directed, stepwise testing approach guided by the patient’s history.
Use of nondirected testing is common in patients with severe acute liver injury. Of the 5795 such patients treated at our hospital between 2000 and 2015, within the same day of service 53% were tested for hepatitis C virus antibody, 38% for hemochromatosis (ferritin test), 28% for autoimmune hepatitis (antinuclear antibody test), and 15% for primary biliary cholangitis (antimitochondrial antibody test) by our clinical laboratory. Of the 5023 patients who had send-out tests performed for Wilson disease (ceruloplasmin), 81% were queried for hepatitis B virus infection, 76% for hepatitis C virus infection, 75% for autoimmune hepatitis, and 73.1% for hemochromatosis.2 Similar trends were found for patients with severe liver injury tested for α1-antitrypsin (AAT) deficiency.3 In sum, these data showed that each patient with severe liver injury was tested out of concern about diseases with markedly different epidemiology and clinical presentations (Table).
WHY YOU MIGHT THINK NONDIRECTED TESTING IS HELPFUL
Use of nondirected testing may reflect perceived urgency, convenience, and thoroughness.2-6 Alternatively, it may simply involve following a consultant’s recommendations.4 As severe acute liver injury is often associated with tremendous morbidity, clinicians seeking answers may perceive directed, stepwise testing as inappropriately slow given the urgency of the presentation; they may think that nondirected testing can reduce hospital length of stay.
WHY NONDIRECTED TESTING IS NOT HELPFUL
Nondirected testing is a problem for at least 4 reasons: limited benefit of reflexive testing for rare diseases, no meaningful impact on outcomes, false positives, and financial cost.
First, immediately testing for rare causes of liver disease is unlikely to benefit patients with severe liver injury. The underlying etiologies of severe liver injury are relatively well circumscribed (Table). Overall, 42% of patients with severe liver injury and 57% of those with an ALT level higher than 1000 IU/L have ischemic hepatitis.7 Accounting for a significant percentage of severe liver injury cases are acute biliary obstruction (24%), drug-induced injury (10%-13%), and viral hepatitis (4%-7%).1,8 Of the small subset of patients with severe liver injury that progresses to acute liver failure (ALF; encephalopathy, coagulopathy), 0.5% have autoimmune hepatitis and 0.1% have Wilson disease.9 Furthermore, many patients are tested for AAT deficiency, hemochromatosis, and primary biliary cholangitis, but these are never causes of severe acute liver injury (Table).
Second, diagnosing a rarer cause of acute liver injury modestly earlier has no meaningful impact on outcome. Work-up for more common etiologies can usually be completed within 2 or 3 days. This is true even for patients with ALF. Specific therapies generally are lacking for ALF, save for use of N-acetylcysteine for acetaminophen overdose and antiviral therapy for hepatitis B virus infection.9,10 Furthermore, although effective therapies are available for both autoimmune hepatitis and Wilson disease, the potential benefit stems from altering the longer term course of disease. Initial management, even for these rare conditions, is no different from that for other etiologies. Conversely, acute liver injury caused by ischemic hepatitis, biliary disease, or drug-induced liver injury requires swift corrective action. Even if normotensive, patients with ischemic hepatitis are often in cardiogenic shock and benefit from careful monitoring and critical care.7 Patients with acute biliary obstruction may need therapeutic endoscopy. Last, patients with drug-induced liver injury benefit from immediate discontinuation of the offending drug.
Third, in the testing of patients with low pretest probabilities, false positives are common. For example, at our institution and at an institution in Austria, severe liver injury patients with a low ceruloplasmin level have a 95.1% to 98.1% chance of a false-positive result (they have a low ceruloplasmin level but do not have Wilson disease).3,4 Furthermore, 91% of positive tests are never confirmed,3 indicating either that clinicians never valued the initial test or that other diagnoses were much more likely. Even worse, as was the case in 65% of patients with low AAT levels,2,3 genetic diagnoses were based on unconfirmed, potentially false-positive serologic tests.
Fourth, although the financial cost for each individual test is small, at the population level the cost of nondirected testing is significant. For example, although each reflects testing for conditions that do not cause acute liver injury, the costs of ferritin, AAT, and antimitochondrial antibody tests are $13, $16, and $37, respectively (Medicare/Medicaid reimbursements in 2016 $US).11 About 1.5% of admitted patients are found to have severe liver injury. If this proportion holds true for the roughly 40 million discharges from US hospitals each year, then there would be an annual cost of about $40 million if all 3 tests were performed for each patient with severe liver injury. In addition, although nondirected testing may seem clinically expedient, there are no data suggesting it reduces length of stay. In fact, ceruloplasmin, AAT, and many other tests are sent to external laboratories and are unlikely to be returned before discharge. If clinicians delay discharge for results, then nondirected testing would increase rather than decrease length of stay.
WHAT YOU SHOULD DO INSTEAD
In this era of increasing cost-consciousness, nondirected testing has escaped relatively unscathed. Indeed, nondirected testing is prevalent, yet has pitfalls similar to those of serologic testing (eg, vasculitis or arthritis,6 acute renal injury, infectious disease12). The alternative is deliberate, empirical, patient-centered testing that is attentive to the patient’s presentation and the harms of false positives. The idea is to select tests for each patient with acute liver injury according to presentation and the most likely corresponding diagnoses (Table, Figure).
The “one-stop shopping” in providers’ electronic order entry systems makes it too easy to over-order tests. Fortunately, these systems’ simple and effective decision supports can force pauses in the ordering process, create barriers to waste, and provide education about test characteristics and costs.4,5,13 Our medical center’s volume of ceruloplasmin orders decreased by 80% after a change was made to its ordering system; the ordering of a ceruloplasmin test is now interrupted by a pop-up screen that displays test characteristics and an option to continue or cancel the order.4,5 Hospitals should consider implementing clinical decision supports in this area. Successful interventions provide electronic rather than paper-based support as part of the clinical workflow, during the ordering process, and recommendations rather than assessments.13
RECOMMENDATIONS
- For each patient with severe acute liver injury, select tests on the basis of the presentation (Figure). Testing for rare diseases should be performed only after common diseases have been excluded.
- Avoid testing for hemochromatosis (iron indices, genetic tests), AAT deficiency (AAT levels or phenotypes), and primary biliary cholangitis (antimitochondrial antibodies) in patients with severe acute liver injury.
- Consider implementing decision supports that can curb nondirected testing in areas in which it is common.
CONCLUSION
Nondirected testing is associated with false positives and increased costs in the evaluation and management of severe acute liver injury. The alternative is deliberate, epidemiologically and clinically driven directed testing. Electronic ordering system decision supports can be useful in curtailing nondirected testing.
Disclosure
Nothing to report.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason”? Let us know what you do in your practice and propose ideas for other “Things We Do for No Reason” topics. Please join in the conversation online at Twitter (#TWDFNR)/Facebook and don’t forget to “Like It” on Facebook or retweet it on Twitter.
The “Things We Do for No Reason” series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CASE REPORT
A 68-year-old woman with ischemic cardiomyopathy was admitted with abdominal cramping, diarrhea, and nausea, which had left her unable to keep food and liquids down for 2 days. She had been taking diuretics and had a remote history of intravenous drug use. On admission, she was afebrile and had blood pressure of 100/60 mm Hg and a heart rate of 100 bpm. Her extremities were cool and clammy. Blood test results showed an alanine aminotransferase (ALT) level of 1510 IU/L and an aspartate aminotransferase (AST) level of 1643 IU/L. The patient’s clinician did not know her baseline ALT and AST levels and thought the best approach was to identify the cause of the transaminase elevation.
Severe acute liver injury (liver enzymes, >10 × upper limit of normal [ULN], usually 40 IU/L) is a common presentation among hospitalized patients. Between 1997 and 2015, 1.5% of patients admitted to our hospital had severe liver injury. In another large cohort of hospitalized patients,1 0.6% had an ALT level higher than 1000 IU/L (~20 × ULN). A precise diagnosis is often needed to direct appropriate therapy, and serologic tests are available for many conditions, both common and rare (Table). Given the relative ease of bundled blood testing, nondirected testing has emerged as a popular, if reflexive, strategy.2-5 In this approach, clinicians evaluate each patient for the set of testable diseases all at once—in contrast to taking a directed, stepwise testing approach guided by the patient’s history.
Use of nondirected testing is common in patients with severe acute liver injury. Of the 5795 such patients treated at our hospital between 2000 and 2015, within the same day of service 53% were tested for hepatitis C virus antibody, 38% for hemochromatosis (ferritin test), 28% for autoimmune hepatitis (antinuclear antibody test), and 15% for primary biliary cholangitis (antimitochondrial antibody test) by our clinical laboratory. Of the 5023 patients who had send-out tests performed for Wilson disease (ceruloplasmin), 81% were queried for hepatitis B virus infection, 76% for hepatitis C virus infection, 75% for autoimmune hepatitis, and 73.1% for hemochromatosis.2 Similar trends were found for patients with severe liver injury tested for α1-antitrypsin (AAT) deficiency.3 In sum, these data showed that each patient with severe liver injury was tested out of concern about diseases with markedly different epidemiology and clinical presentations (Table).
WHY YOU MIGHT THINK NONDIRECTED TESTING IS HELPFUL
Use of nondirected testing may reflect perceived urgency, convenience, and thoroughness.2-6 Alternatively, it may simply involve following a consultant’s recommendations.4 As severe acute liver injury is often associated with tremendous morbidity, clinicians seeking answers may perceive directed, stepwise testing as inappropriately slow given the urgency of the presentation; they may think that nondirected testing can reduce hospital length of stay.
WHY NONDIRECTED TESTING IS NOT HELPFUL
Nondirected testing is a problem for at least 4 reasons: limited benefit of reflexive testing for rare diseases, no meaningful impact on outcomes, false positives, and financial cost.
First, immediately testing for rare causes of liver disease is unlikely to benefit patients with severe liver injury. The underlying etiologies of severe liver injury are relatively well circumscribed (Table). Overall, 42% of patients with severe liver injury and 57% of those with an ALT level higher than 1000 IU/L have ischemic hepatitis.7 Accounting for a significant percentage of severe liver injury cases are acute biliary obstruction (24%), drug-induced injury (10%-13%), and viral hepatitis (4%-7%).1,8 Of the small subset of patients with severe liver injury that progresses to acute liver failure (ALF; encephalopathy, coagulopathy), 0.5% have autoimmune hepatitis and 0.1% have Wilson disease.9 Furthermore, many patients are tested for AAT deficiency, hemochromatosis, and primary biliary cholangitis, but these are never causes of severe acute liver injury (Table).
Second, diagnosing a rarer cause of acute liver injury modestly earlier has no meaningful impact on outcome. Work-up for more common etiologies can usually be completed within 2 or 3 days. This is true even for patients with ALF. Specific therapies generally are lacking for ALF, save for use of N-acetylcysteine for acetaminophen overdose and antiviral therapy for hepatitis B virus infection.9,10 Furthermore, although effective therapies are available for both autoimmune hepatitis and Wilson disease, the potential benefit stems from altering the longer term course of disease. Initial management, even for these rare conditions, is no different from that for other etiologies. Conversely, acute liver injury caused by ischemic hepatitis, biliary disease, or drug-induced liver injury requires swift corrective action. Even if normotensive, patients with ischemic hepatitis are often in cardiogenic shock and benefit from careful monitoring and critical care.7 Patients with acute biliary obstruction may need therapeutic endoscopy. Last, patients with drug-induced liver injury benefit from immediate discontinuation of the offending drug.
Third, in the testing of patients with low pretest probabilities, false positives are common. For example, at our institution and at an institution in Austria, severe liver injury patients with a low ceruloplasmin level have a 95.1% to 98.1% chance of a false-positive result (they have a low ceruloplasmin level but do not have Wilson disease).3,4 Furthermore, 91% of positive tests are never confirmed,3 indicating either that clinicians never valued the initial test or that other diagnoses were much more likely. Even worse, as was the case in 65% of patients with low AAT levels,2,3 genetic diagnoses were based on unconfirmed, potentially false-positive serologic tests.
Fourth, although the financial cost for each individual test is small, at the population level the cost of nondirected testing is significant. For example, although each reflects testing for conditions that do not cause acute liver injury, the costs of ferritin, AAT, and antimitochondrial antibody tests are $13, $16, and $37, respectively (Medicare/Medicaid reimbursements in 2016 $US).11 About 1.5% of admitted patients are found to have severe liver injury. If this proportion holds true for the roughly 40 million discharges from US hospitals each year, then there would be an annual cost of about $40 million if all 3 tests were performed for each patient with severe liver injury. In addition, although nondirected testing may seem clinically expedient, there are no data suggesting it reduces length of stay. In fact, ceruloplasmin, AAT, and many other tests are sent to external laboratories and are unlikely to be returned before discharge. If clinicians delay discharge for results, then nondirected testing would increase rather than decrease length of stay.
WHAT YOU SHOULD DO INSTEAD
In this era of increasing cost-consciousness, nondirected testing has escaped relatively unscathed. Indeed, nondirected testing is prevalent, yet has pitfalls similar to those of serologic testing (eg, vasculitis or arthritis,6 acute renal injury, infectious disease12). The alternative is deliberate, empirical, patient-centered testing that is attentive to the patient’s presentation and the harms of false positives. The idea is to select tests for each patient with acute liver injury according to presentation and the most likely corresponding diagnoses (Table, Figure).
The “one-stop shopping” in providers’ electronic order entry systems makes it too easy to over-order tests. Fortunately, these systems’ simple and effective decision supports can force pauses in the ordering process, create barriers to waste, and provide education about test characteristics and costs.4,5,13 Our medical center’s volume of ceruloplasmin orders decreased by 80% after a change was made to its ordering system; the ordering of a ceruloplasmin test is now interrupted by a pop-up screen that displays test characteristics and an option to continue or cancel the order.4,5 Hospitals should consider implementing clinical decision supports in this area. Successful interventions provide electronic rather than paper-based support as part of the clinical workflow, during the ordering process, and recommendations rather than assessments.13
RECOMMENDATIONS
- For each patient with severe acute liver injury, select tests on the basis of the presentation (Figure). Testing for rare diseases should be performed only after common diseases have been excluded.
- Avoid testing for hemochromatosis (iron indices, genetic tests), AAT deficiency (AAT levels or phenotypes), and primary biliary cholangitis (antimitochondrial antibodies) in patients with severe acute liver injury.
- Consider implementing decision supports that can curb nondirected testing in areas in which it is common.
CONCLUSION
Nondirected testing is associated with false positives and increased costs in the evaluation and management of severe acute liver injury. The alternative is deliberate, epidemiologically and clinically driven directed testing. Electronic ordering system decision supports can be useful in curtailing nondirected testing.
Disclosure
Nothing to report.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason”? Let us know what you do in your practice and propose ideas for other “Things We Do for No Reason” topics. Please join in the conversation online at Twitter (#TWDFNR)/Facebook and don’t forget to “Like It” on Facebook or retweet it on Twitter.
1. Johnson RD, O’Connor ML, Kerr RM. Extreme serum elevations of aspartate aminotransferase. Am J Gastroenterol. 1995;90(8):1244-1245. PubMed
2. Tapper EB, Patwardhan VR, Curry M. Low yield and utilization of confirmatory testing in a cohort of patients with liver disease assessed for alpha-1 antitrypsin deficiency. Dig Dis Sci. 2015;60(6):1589-1594. PubMed
3. Tapper EB, Rahni DO, Arnaout R, Lai M. The overuse of serum ceruloplasmin measurement. Am J Med. 2013;126(10):926.e1-e5. PubMed
4. Tapper EB, Sengupta N, Lai M, Horowitz G. Understanding and reducing ceruloplasmin overuse with a decision support intervention for liver disease evaluation. Am J Med. 2016;129(1):115.e17-e22. PubMed
5. Tapper EB, Sengupta N, Lai M, Horowitz G. A decision support tool to reduce overtesting for ceruloplasmin and improve adherence with clinical guidelines. JAMA Intern Med. 2015;175(9):1561-1562. PubMed
6. Lichtenstein MJ, Pincus T. How useful are combinations of blood tests in “rheumatic panels” in diagnosis of rheumatic diseases? J Gen Intern Med. 1988;3(5):435-442. PubMed
7. Tapper EB, Sengupta N, Bonder A. The incidence and outcomes of ischemic hepatitis: a systematic review with meta-analysis. Am J Med. 2015;128(12):1314-1321. PubMed
8. Whitehead MW, Hawkes ND, Hainsworth I, Kingham JG. A prospective study of the causes of notably raised aspartate aminotransferase of liver origin. Gut. 1999;45(1):129-133. PubMed
9. Fontana RJ. Acute liver failure including acetaminophen overdose. Med Clin North Am. 2008;92(4):761-794. PubMed
10. Lee WM, Larson AM, Stravitz RT. AASLD Position Paper: The Management of Acute Liver Failure: Update 2011. American Association for the Study of Liver Diseases website. https://www.aasld.org/sites/default/files/guideline_documents/alfenhanced.pdf. Published 2011. Accessed January 26, 2017.
11. Green RM, Flamm S. AGA technical review on the evaluation of liver chemistry tests. Gastroenterology. 2002;123(4):1367-1384. PubMed
12. Aesif SW, Parenti DM, Lesky L, Keiser JF. A cost-effective interdisciplinary approach to microbiologic send-out test use. Arch Pathol Lab Med. 2015;139(2):194-198. PubMed
13. Kawamoto K, Houlihan CA, Balas EA, Lobach DF. Improving clinical practice using clinical decision support systems: a systematic review of trials to identify features critical to success. BMJ. 2005;330(7494):765. PubMed
14. Boberg KM. Prevalence and epidemiology of autoimmune hepatitis. Clin Liver Dis. 2002;6(3):635-647. PubMed
15. Bacon BR, Adams PC, Kowdley KV, Powell LW, Tavill AS; American Association for the Study of Liver Diseases. Diagnosis and management of hemochromatosis: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology. 2011;54(1):328-343. PubMed
16. Boonstra K, Beuers U, Ponsioen CY. Epidemiology of primary sclerosing cholangitis and primary biliary cirrhosis: a systematic review. J Hepatol. 2012;56(5):1181-1188. PubMed
1. Johnson RD, O’Connor ML, Kerr RM. Extreme serum elevations of aspartate aminotransferase. Am J Gastroenterol. 1995;90(8):1244-1245. PubMed
2. Tapper EB, Patwardhan VR, Curry M. Low yield and utilization of confirmatory testing in a cohort of patients with liver disease assessed for alpha-1 antitrypsin deficiency. Dig Dis Sci. 2015;60(6):1589-1594. PubMed
3. Tapper EB, Rahni DO, Arnaout R, Lai M. The overuse of serum ceruloplasmin measurement. Am J Med. 2013;126(10):926.e1-e5. PubMed
4. Tapper EB, Sengupta N, Lai M, Horowitz G. Understanding and reducing ceruloplasmin overuse with a decision support intervention for liver disease evaluation. Am J Med. 2016;129(1):115.e17-e22. PubMed
5. Tapper EB, Sengupta N, Lai M, Horowitz G. A decision support tool to reduce overtesting for ceruloplasmin and improve adherence with clinical guidelines. JAMA Intern Med. 2015;175(9):1561-1562. PubMed
6. Lichtenstein MJ, Pincus T. How useful are combinations of blood tests in “rheumatic panels” in diagnosis of rheumatic diseases? J Gen Intern Med. 1988;3(5):435-442. PubMed
7. Tapper EB, Sengupta N, Bonder A. The incidence and outcomes of ischemic hepatitis: a systematic review with meta-analysis. Am J Med. 2015;128(12):1314-1321. PubMed
8. Whitehead MW, Hawkes ND, Hainsworth I, Kingham JG. A prospective study of the causes of notably raised aspartate aminotransferase of liver origin. Gut. 1999;45(1):129-133. PubMed
9. Fontana RJ. Acute liver failure including acetaminophen overdose. Med Clin North Am. 2008;92(4):761-794. PubMed
10. Lee WM, Larson AM, Stravitz RT. AASLD Position Paper: The Management of Acute Liver Failure: Update 2011. American Association for the Study of Liver Diseases website. https://www.aasld.org/sites/default/files/guideline_documents/alfenhanced.pdf. Published 2011. Accessed January 26, 2017.
11. Green RM, Flamm S. AGA technical review on the evaluation of liver chemistry tests. Gastroenterology. 2002;123(4):1367-1384. PubMed
12. Aesif SW, Parenti DM, Lesky L, Keiser JF. A cost-effective interdisciplinary approach to microbiologic send-out test use. Arch Pathol Lab Med. 2015;139(2):194-198. PubMed
13. Kawamoto K, Houlihan CA, Balas EA, Lobach DF. Improving clinical practice using clinical decision support systems: a systematic review of trials to identify features critical to success. BMJ. 2005;330(7494):765. PubMed
14. Boberg KM. Prevalence and epidemiology of autoimmune hepatitis. Clin Liver Dis. 2002;6(3):635-647. PubMed
15. Bacon BR, Adams PC, Kowdley KV, Powell LW, Tavill AS; American Association for the Study of Liver Diseases. Diagnosis and management of hemochromatosis: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology. 2011;54(1):328-343. PubMed
16. Boonstra K, Beuers U, Ponsioen CY. Epidemiology of primary sclerosing cholangitis and primary biliary cirrhosis: a systematic review. J Hepatol. 2012;56(5):1181-1188. PubMed
© 2017 Society of Hospital Medicine
Forging ahead
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient’s case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant. The bolded text represents the patient’s case. Each paragraph that follows represents the discussant’s thoughts.
A 45-year-old woman presented to the emergency department with 2 days of generalized, progressive weakness. Her ability to walk and perform daily chores was increasingly limited. On the morning of her presentation, she was unable to stand up without falling.
A complaint of weakness must be classified as either functional weakness related to a systemic process or true neurologic weakness from dysfunction of the central nervous system (eg, brain, spinal cord) or peripheral nervous system (eg, anterior horn cell, nerve, neuromuscular junction, or muscle). More information on her clinical course and a detailed neurologic exam will help clarify this key branch point.
She was 2 weeks status-post laparoscopic Roux-en-Y gastric bypass and gastric band removal performed in Europe. Immediately following surgery, she experienced abdominal discomfort and nausea with occasional nonbloody, nonbilious emesis, attributed to expected postoperative anatomical changes. She developed a postoperative pneumonia treated with amoxicillin-clavulanate. She tolerated her flight back to the United States, but her abdominal discomfort persisted and she had minimal oral intake due to her nausea.
Functional weakness may stem from hypovolemia from insufficient oral intake, anemia related to the recent surgery, electrolyte abnormalities, chronic nutritional issues associated with obesity and weight-reduction surgery, and pneumonia. Prolonged air travel, obesity, and recent surgery place her at risk for venous thromboembolism, which may manifest as reduced exercise tolerance. Nausea, vomiting, and abdominal pain persisting for 2 weeks after a Roux-en-Y gastric bypass surgery raises several concerns, including gastric remnant distension (although hiccups are often prominent); stomal stenosis, which typically presents several weeks after surgery; marginal ulceration; or infection at the surgical site or from an anastomotic leak. She may also have a surgery- or medication-related myopathy.
The patient had a history of obesity, hypertension, hyperlipidemia, migraine headaches, and nonalcoholic steatohepatitis. Four years previously, she had undergone gastric banding complicated by band migration and ulceration at the banding site. Her medications were amlodipine, losartan, ranitidine, acetaminophen, and nadroparin for venous thromboembolism prophylaxis during her flight. She denied alcohol, tobacco, or illicit drug use. On further questioning, she reported diaphoresis, mild dyspnea, loose stools, and a sensation of numbness and “heaviness” in her arms. Her abdominal pain was limited to the surgical incision and was controlled with acetaminophen. She denied fevers, cough, chest pain, diplopia, or dysphagia.
Heaviness in both arms could result from an acutely presenting myopathic or neuropathic process, while the coexistence of numbness suggests a sensorimotor polyneuropathy. Obesity and gastric bypass surgery increase her nutritional risk, and thiamine deficiency may present as an acute axonal polyneuropathy (ie, beriberi). Unlike vitamin B12 deficiency, which may take years to develop, thiamine deficiency can present within 4 weeks of gastric bypass surgery. Her dyspnea may be a manifestation of diaphragmatic weakness, although her ostensibly treated pneumonia or as of yet unproven postoperative anemia may be contributing. Chemoprophylaxis mitigates her risk of venous thromboembolism, which is, nonetheless, unlikely to account for the gastrointestinal symptoms and upper extremity weakness. If she is continuing to take amlodipine and losartan but has become volume-depleted, hypotension may be contributing to the generalized weakness.
Physical examination revealed an obese, pale and diaphoretic woman. Her temperature was 36.9°C, heart rate 77 beats per minute, blood pressure 158/90 mm Hg, respiratory rate 28 breaths per minute, and O2 saturation 99% on ambient air. She had no cervical lymphadenopathy and a normal thyroid exam. There were no murmurs on cardiac examination, and jugular venous pressure was estimated at 10 cm of water. Her lung sounds were clear. Her abdomen was soft, nondistended, with localized tenderness and fluctuance around the midline surgical incision with a small amount of purulent drainage. She was alert and oriented to name, date, place, and situation. Cranial nerves II through XII were grossly intact. Strength was 4/5 in bilateral biceps, triceps and distal hand and finger extensors, 3/5 in bilateral deltoids. Strength in hip flexors was 4/5 and it was 5/5 in distal lower extremities. Sensation was intact to pinprick in upper and lower extremities. Biceps reflexes were absent; patellar and ankle reflexes were 1+ and symmetric. The remainder of the physical exam was unremarkable.
The patient has symmetric proximal muscle weakness with upper extremity predominance and preserved strength in her distal lower extremities. A myopathy could explain this pattern of weakness, further substantiated by absent reflexes and reportedly intact sensation. Subacute causes of myopathy include hypokalemia, hyperkalemia, toxic myopathies from medications, or infection-induced rhabdomyolysis. However, she does not report muscle pain, and the loss of reflexes is faster than would be expected with a myopathy. A more thorough sensory examination would inform the assessment of potential neuropathic processes. Guillain-Barré syndrome (GBS) is possible; it most commonly presents as an ascending, distally predominant acute inflammatory demyelinating polyneuropathy (AIDP), although her upper extremity weakness predominates and there are no clear sensory changes. It remains to be determined how her wound infection might relate to her overall presentation.
Her white blood cell count was 12,600/μL (reference range: 3,400-10,000/μL), hemoglobin was 10.2 g/dL, and platelet count was 698,000/μL. Mean corpuscular volume was 86 fL. Serum chemistries were: sodium 138 mEq/L, potassium 3.8 mEq/L, chloride 106 mmol/L, bicarbonate 15 mmol/L, blood urea nitrogen 5 mg/dL, creatinine 0.65 mg/dL, glucose 125 mg/dL, calcium 8.3 mg/dL, magnesium 1.9 mg/dL, phosphorous 2.4 mg/dL, and lactate 1.8 mmol/L (normal: < 2.0 mmol/L). Creatinine kinase (CK), liver function tests, and coagulation panel were normal. Total protein was 6.4 g/dL, and albumin was 2.7 g/dL. Venous blood gas was: pH 7.39 and PCO2 25 mmHg. Urinalysis revealed ketones. Blood and wound cultures were sent for evaluation. A chest x-ray was unremarkable. An electrocardiogram showed normal sinus rhythm. Computed tomography (CT) of the abdomen and pelvis revealed a multiloculated rim-enhancing fluid collection in the anterior abdominal wall (Figure 1).
She does not have any notable electrolyte derangements that would account for her weakness, and the normal creatinine kinase lowers the probability of a myopathy and excludes rhabdomyolysis. Progression of weakness from proximal to distal muscles in a symmetric fashion is consistent with botulism, and she has an intra-abdominal wound infection that could be harboring Clostridium botulinum. Nonetheless, the normal cranial nerve exam and the rarity of botulism occurring with surgical wounds argue against this diagnosis. She should receive intravenous (IV) thiamine for the possibility of beriberi. A lumbar puncture should be performed to assess for albuminocytologic dissociation, which can be seen in patients with GBS.
The patient received high-dose IV thiamine, IV vancomycin, IV piperacillin-tazobactam, and acetaminophen. Over the subsequent 4 hours, her anion gap acidosis worsened. She declined arterial puncture. Repeat venous blood gas was: pH 7.22, PCO2 28 mmHg, and bicarbonate 11 mmol/L. Lactate and glucose were normal. Serum osmolarity was 292 mmol/kg (reference range: 283-301 mmol/kg). She was started on an IV sodium bicarbonate infusion without improvement in her acidemia.
An acute anion gap metabolic acidosis suggests a limited differential diagnosis that includes lactic acidosis, D-lactic acidosis, severe starvation ketoacidosis, acute renal failure, salicylate, or other drug or poison ingestion. Starvation ketoacidosis may be contributing, but a bicarbonate value this low would be unusual. There is no history of alcohol use or other ingestions, and the normal serum osmolality and low osmolal gap (less than 10 mOsm/kg) argue against a poisoning with ethanol, ethylene glycol, or methanol. The initial combined anion gap metabolic acidosis and respiratory alkalosis is consistent with salicylate toxicity, but she does not report aspirin ingestion. Acetaminophen use in the setting of malnutrition or starvation physiology raises the possibility of 5-oxoproline accumulation.
Routine serum lactate does not detect D-lactate, which is produced by colonic bacteria and has been reported in short bowel syndrome and following intestinal bypass surgery. This may occur weeks to months after intestinal procedures, following ingestion of a heavy carbohydrate load, and almost invariably presents with altered mental status and increased anion gap metabolic acidosis, although generalized weakness has been reported.
A surgical consultant drained her wound infection. Fluid Gram stain was negative. D-lactate, salicylate and acetaminophen levels were undetectable. Thiamine pyrophosphate level was 229 nmol/L (reference range: 78-185 nmol/L). Acetaminophen was discontinued and N-acetylcysteine infusion was started for possible 5-oxoprolinemia. Her anion gap acidosis rapidly improved. Twelve hours after admission, she reported sudden onset of blurry vision. Her vital signs were: temperature 37oC, heart rate 110 beats per minute, respiratory rate 40 breaths per minute, blood pressure 168/90, and oxygen saturation 100% on ambient air. Telemetry showed ventricular bigeminy. On examination, she was unable to abduct her right eye; muscle strength was 1/5 in all extremities; biceps, ankle, and patellar reflexes were absent.
Her neurological deficits have progressed over hours to near complete paralysis, asymmetric cranial nerve paresis, and areflexia. Although botulism can cause blurred vision and absent deep tendon reflexes, patients almost always have symmetrical bulbar findings followed by descending paralysis. Should the “numbness” in her arms reported earlier represent undetected sensory deficits, this, too would be inconsistent with botulism.
A diagnosis of GBS ties together several aspects of her presentation and clinical course. Several variants show different patterns of weakness and may involve cranial nerves. Her tachypnea and dyspnea are concerning signs of potential impending respiratory failure. The ventricular bigeminy and mild hypertension could represent autonomic dysfunction that is seen in many cases of GBS.
She was intubated for airway protection. Computed tomography angiography and magnetic resonance imaging of her brain were normal. Cerebral spinal fluid analysis obtained through lumbar puncture showed the following: white blood cell count 3/μL, red blood cell count 11/μL, protein 63 mg/dL (reference range: 15-60mg/dL), and glucose 128 mg/dL (reference range: 40-80mg/dL).
The lumbar puncture is consistent with GBS given the slightly elevated protein and cell count well below 50/μL. Given the severity of her symptoms, treatment with IV immunoglobulin or plasmapheresis should be initiated. Nerve conduction studies (NCS) and electromyography (EMG) are indicated for diagnostic confirmation.
EMG and NCS revealed a severe sensorimotor polyneuropathy with demyelinating features including a conduction block at a noncompressible site, consistent with AIDP. Left sural nerve biopsy confirmed acute demyelinating and mild axonal neuropathy (Figure 2). On hospital day 2, treatment with IV immunoglobulins (IVIG) was initiated; however, she developed anaphylaxis following her second administration and subsequently received plasmapheresis. A tracheostomy was performed for respiratory muscle weakness, and she was discharged to a nursing facility. C. botulinum cultures from the wound eventually returned negative. Following her hospitalization, a serum 5-oxoproline level sent 10 hours after admission returned as elevated, confirming the additional diagnosis of 5-oxoprolinemia. On follow-up, she can sit up and feed herself without assistance, and her gait continues to improve with physical therapy.
DISCUSSION
This patient presented with rapidly progressive weakness that developed in the 2 weeks following bariatric surgery. In the postsurgical setting, patient complaints of weakness are commonly encountered and can pose a diagnostic challenge. Asthenia (ie, general loss of strength or energy) is frequently reported in the immediate postoperative period, and may result from the stress of surgery, pain, deconditioning, or infection. This must be distinguished from true neurologic weakness, which results from dysfunction of the brain, spinal cord, nerve, neuromuscular junction, or muscle. The initial history can help elucidate the inciting events such as preceding surgery, infections or ingestions, and can also categorize the pattern of weakness. The neurologic examination can localize the pathology within the neuraxis. EMG and NCS can distinguish neuropathy from radiculopathy, and categorize the process as axonal, demyelinating, or mixed. In this case, the oculomotor weakness, sensory abnormalities and areflexia signaled a severe sensorimotor polyneuropathy, and EMG/NCS confirmed a demyelinating process consistent with GBS.
Guillain-Barré syndrome is an acute, immune-mediated polyneuropathy. Patients with GBS often present with a preceding respiratory or diarrheal illness; however, the stress of a recent surgery can serve as an inciting event. The syndrome, acute postgastric reduction surgery (APGARS) neuropathy, was introduced in the literature in 2002, describing 3 patients who presented with progressive vomiting, weakness, and hyporeflexia following bariatric surgery.1 The term has been used to describe bariatric surgery patients who developed postoperative quadriparesis, cranial nerve deficits, and respiratory compromise.2 Given the clinical heterogeneity in the literature with relation to APGARS, it is probable that the cases described could result from multiple etiologies. While GBS is purely immune-mediated and can be precipitated by the stress of surgery itself, postbariatric surgery patients are susceptible to many nutritional deficiencies that can lead to similar presentations.3 For example, thiamine (vitamin B1) and cobalamin (vitamin B12) deficiencies cause distinct postbariatric surgery neuropathies.4 Thiamine deficiency may manifest weeks to months after surgery and can rapidly progress, whereas cobalamin deficiency generally develops over 3 to 5 years. Both of these syndromes demonstrate an axonal pattern of nerve injury on EMG/NCS, in contrast to the demyelinating pattern typically seen in GBS. In addition, bariatric surgery patients are at higher risk for copper deficiency, which usually presents as a myeloneuropathy with subacute gait decline and upper motor neuron signs including spasticity.
Although GBS classically presents with symmetric ascending weakness and sensory abnormalities, it may manifest in myriad ways. Factors influencing the presentation include the types of nerve fibers involved (motor, sensory, cranial or autonomic), the predominant mode of injury (axonal vs demyelinating), and the presence or absence of alteration in consciousness.5 The most common form of GBS is AIDP. The classic presentation involves paresthesias in the fingertips and toes followed by lower extremity weakness that ascends over hours to days to involve the arms and potentially the muscles of respiration. A minority of patients with GBS first experience weakness in the upper extremities or facial muscles, and oculomotor involvement is rare.5 Pain is common and often severe.6 Dysautonomia affects most patients with GBS and may manifest as labile blood pressure or arrhythmias.5 Several variant GBS presentation patterns have been described, including acute motor axonal neuropathy, a pure motor form of GBS; ophthalmoplegia, ataxia, and areflexia in Miller Fisher syndrome; and alteration in consciousness, hyperreflexia, ataxia, and ophthalmoparesis in Bickerstaff’s brain stem encephalitis.5
Patients with GBS can progress rapidly to respiratory failure. Serial neurologic exams may signal the diagnosis and inform triage to the appropriate level of care. Measurement of bedside pulmonary function, including mean inspiratory force and functional vital capacity, help to determine if there is weakness of diaphragmatic muscles. Patients with signs or symptoms of diaphragmatic weakness require monitoring in an intensive care unit and potentially early intubation. Treatment with IVIG or plasmapheresis has been found to hasten recovery from GBS, including earlier improvement in muscle strength and a reduced need for mechanical ventilation.7 Treatment selection is based on available resources as both modalities are felt to be equivalent.The majority of patients with GBS make a full recovery over a period of weeks to months, although many have persistent motor weakness. Despite immunotherapy, up to 20% of patients remain severely disabled and approximately 5% die.8 Advanced age, rapid progression of weakness over a period of less than 72 hours, need for mechanical ventilation, and absent compound muscle action potentials on NCS are all associated with prolonged and incomplete recovery.9
This patient developed respiratory failure within 12 hours of hospitalization, prior to being diagnosed with GBS. Even in that short time, the treating clinicians encountered a series of clinical diversions. The initial proximal pattern of muscle weakness suggested a possible myopathic process; the wound infection introduced the possibility of botulism; obesity and recent bariatric surgery triggered concern for thiamine deficiency; and the anion gap acidosis from 5-oxoprolinemia created yet another clinical detour. While the path from presentation to diagnosis is seldom a straight line, when faced with rapidly progressive weakness, it is paramount to forge ahead with an efficient diagnostic evaluation and timely therapeutic intervention.
KEY TEACHING POINTS
- A complaint of general weakness requires distinction between asthenia (ie, general loss of strength or energy) and true neuromuscular weakness from dysfunction of the brain, spinal cord, nerve, neuromuscular junction, and/or muscle.
- Guillain-Barré syndrome may present in a variety of atypical fashions not limited to ascending, distally predominant weakness.
- Acute postgastric reduction surgery neuropathy should be considered in patients presenting with weakness, vomiting, or hyporeflexia after bariatric surgery.
- Acute inflammatory demyelinating polyneuropathy may rapidly progress to respiratory failure, and warrants serial neurologic examinations, monitoring of pulmonary function, and an expedited diagnostic evaluation.
Disclosure
Nothing to report.
1. Akhtar M, Collins MP, Kissel JT. Acute postgastric reduction surgery (APGARS) Neuropathy: A polynutritional, multisystem disorder. Neurology. 2002;58:A68. PubMed
2. Chang CG, Adams-Huet B, Provost DA. Acute post-gastric reduction surgery (APGARS) neuropathy. Obes Surg. 2004;14(2):182-189. PubMed
3. Chang CG, Helling TS, Black WE, Rymer MM. Weakness after gastric bypass. Obes Surg. 2002;12(4):592-597. PubMed
4. Shankar P, Boylan M, Sriram K. Micronutrient deficiencies after bariatric surgery. Nutrition. 2010;26(11-12):1031-1037. PubMed
5. Dimachkie MM, Barohn RJ. Guillain-Barré syndrome and variants. Neurol Clin. 2013;31(2):491-510. PubMed
6. Ruts L, Drenthen J, Jongen JL, et al. Pain in Guillain-Barré syndrome: a long-term follow-up study. Neurology. 2010;75(16):1439-1447. PubMed
7. Hughes RAC, Wijdicks EFM, Barohn R, et al: Quality Standards Subcommittee of the American Academy of Neurology. Practice parameter: immunotherapy for Guillain-Barré syndrome: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2003;61:736-740. PubMed
8. Hughes RA, Swan AV, Raphaël JC, Annane D, van Koningsveld R, van Doorn PA. Immunotherapy for Guillain-Barré syndrome: a systematic review. Brain. 2007;130(Pt 9):2245-2257. PubMed
9. Rajabally YA, Uncini A. Outcome and predictors in Guillain-Barré syndrome. J Neurol Neurosurg Psychiatry. 2012;83(7):711-718. PubMed
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient’s case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant. The bolded text represents the patient’s case. Each paragraph that follows represents the discussant’s thoughts.
A 45-year-old woman presented to the emergency department with 2 days of generalized, progressive weakness. Her ability to walk and perform daily chores was increasingly limited. On the morning of her presentation, she was unable to stand up without falling.
A complaint of weakness must be classified as either functional weakness related to a systemic process or true neurologic weakness from dysfunction of the central nervous system (eg, brain, spinal cord) or peripheral nervous system (eg, anterior horn cell, nerve, neuromuscular junction, or muscle). More information on her clinical course and a detailed neurologic exam will help clarify this key branch point.
She was 2 weeks status-post laparoscopic Roux-en-Y gastric bypass and gastric band removal performed in Europe. Immediately following surgery, she experienced abdominal discomfort and nausea with occasional nonbloody, nonbilious emesis, attributed to expected postoperative anatomical changes. She developed a postoperative pneumonia treated with amoxicillin-clavulanate. She tolerated her flight back to the United States, but her abdominal discomfort persisted and she had minimal oral intake due to her nausea.
Functional weakness may stem from hypovolemia from insufficient oral intake, anemia related to the recent surgery, electrolyte abnormalities, chronic nutritional issues associated with obesity and weight-reduction surgery, and pneumonia. Prolonged air travel, obesity, and recent surgery place her at risk for venous thromboembolism, which may manifest as reduced exercise tolerance. Nausea, vomiting, and abdominal pain persisting for 2 weeks after a Roux-en-Y gastric bypass surgery raises several concerns, including gastric remnant distension (although hiccups are often prominent); stomal stenosis, which typically presents several weeks after surgery; marginal ulceration; or infection at the surgical site or from an anastomotic leak. She may also have a surgery- or medication-related myopathy.
The patient had a history of obesity, hypertension, hyperlipidemia, migraine headaches, and nonalcoholic steatohepatitis. Four years previously, she had undergone gastric banding complicated by band migration and ulceration at the banding site. Her medications were amlodipine, losartan, ranitidine, acetaminophen, and nadroparin for venous thromboembolism prophylaxis during her flight. She denied alcohol, tobacco, or illicit drug use. On further questioning, she reported diaphoresis, mild dyspnea, loose stools, and a sensation of numbness and “heaviness” in her arms. Her abdominal pain was limited to the surgical incision and was controlled with acetaminophen. She denied fevers, cough, chest pain, diplopia, or dysphagia.
Heaviness in both arms could result from an acutely presenting myopathic or neuropathic process, while the coexistence of numbness suggests a sensorimotor polyneuropathy. Obesity and gastric bypass surgery increase her nutritional risk, and thiamine deficiency may present as an acute axonal polyneuropathy (ie, beriberi). Unlike vitamin B12 deficiency, which may take years to develop, thiamine deficiency can present within 4 weeks of gastric bypass surgery. Her dyspnea may be a manifestation of diaphragmatic weakness, although her ostensibly treated pneumonia or as of yet unproven postoperative anemia may be contributing. Chemoprophylaxis mitigates her risk of venous thromboembolism, which is, nonetheless, unlikely to account for the gastrointestinal symptoms and upper extremity weakness. If she is continuing to take amlodipine and losartan but has become volume-depleted, hypotension may be contributing to the generalized weakness.
Physical examination revealed an obese, pale and diaphoretic woman. Her temperature was 36.9°C, heart rate 77 beats per minute, blood pressure 158/90 mm Hg, respiratory rate 28 breaths per minute, and O2 saturation 99% on ambient air. She had no cervical lymphadenopathy and a normal thyroid exam. There were no murmurs on cardiac examination, and jugular venous pressure was estimated at 10 cm of water. Her lung sounds were clear. Her abdomen was soft, nondistended, with localized tenderness and fluctuance around the midline surgical incision with a small amount of purulent drainage. She was alert and oriented to name, date, place, and situation. Cranial nerves II through XII were grossly intact. Strength was 4/5 in bilateral biceps, triceps and distal hand and finger extensors, 3/5 in bilateral deltoids. Strength in hip flexors was 4/5 and it was 5/5 in distal lower extremities. Sensation was intact to pinprick in upper and lower extremities. Biceps reflexes were absent; patellar and ankle reflexes were 1+ and symmetric. The remainder of the physical exam was unremarkable.
The patient has symmetric proximal muscle weakness with upper extremity predominance and preserved strength in her distal lower extremities. A myopathy could explain this pattern of weakness, further substantiated by absent reflexes and reportedly intact sensation. Subacute causes of myopathy include hypokalemia, hyperkalemia, toxic myopathies from medications, or infection-induced rhabdomyolysis. However, she does not report muscle pain, and the loss of reflexes is faster than would be expected with a myopathy. A more thorough sensory examination would inform the assessment of potential neuropathic processes. Guillain-Barré syndrome (GBS) is possible; it most commonly presents as an ascending, distally predominant acute inflammatory demyelinating polyneuropathy (AIDP), although her upper extremity weakness predominates and there are no clear sensory changes. It remains to be determined how her wound infection might relate to her overall presentation.
Her white blood cell count was 12,600/μL (reference range: 3,400-10,000/μL), hemoglobin was 10.2 g/dL, and platelet count was 698,000/μL. Mean corpuscular volume was 86 fL. Serum chemistries were: sodium 138 mEq/L, potassium 3.8 mEq/L, chloride 106 mmol/L, bicarbonate 15 mmol/L, blood urea nitrogen 5 mg/dL, creatinine 0.65 mg/dL, glucose 125 mg/dL, calcium 8.3 mg/dL, magnesium 1.9 mg/dL, phosphorous 2.4 mg/dL, and lactate 1.8 mmol/L (normal: < 2.0 mmol/L). Creatinine kinase (CK), liver function tests, and coagulation panel were normal. Total protein was 6.4 g/dL, and albumin was 2.7 g/dL. Venous blood gas was: pH 7.39 and PCO2 25 mmHg. Urinalysis revealed ketones. Blood and wound cultures were sent for evaluation. A chest x-ray was unremarkable. An electrocardiogram showed normal sinus rhythm. Computed tomography (CT) of the abdomen and pelvis revealed a multiloculated rim-enhancing fluid collection in the anterior abdominal wall (Figure 1).
She does not have any notable electrolyte derangements that would account for her weakness, and the normal creatinine kinase lowers the probability of a myopathy and excludes rhabdomyolysis. Progression of weakness from proximal to distal muscles in a symmetric fashion is consistent with botulism, and she has an intra-abdominal wound infection that could be harboring Clostridium botulinum. Nonetheless, the normal cranial nerve exam and the rarity of botulism occurring with surgical wounds argue against this diagnosis. She should receive intravenous (IV) thiamine for the possibility of beriberi. A lumbar puncture should be performed to assess for albuminocytologic dissociation, which can be seen in patients with GBS.
The patient received high-dose IV thiamine, IV vancomycin, IV piperacillin-tazobactam, and acetaminophen. Over the subsequent 4 hours, her anion gap acidosis worsened. She declined arterial puncture. Repeat venous blood gas was: pH 7.22, PCO2 28 mmHg, and bicarbonate 11 mmol/L. Lactate and glucose were normal. Serum osmolarity was 292 mmol/kg (reference range: 283-301 mmol/kg). She was started on an IV sodium bicarbonate infusion without improvement in her acidemia.
An acute anion gap metabolic acidosis suggests a limited differential diagnosis that includes lactic acidosis, D-lactic acidosis, severe starvation ketoacidosis, acute renal failure, salicylate, or other drug or poison ingestion. Starvation ketoacidosis may be contributing, but a bicarbonate value this low would be unusual. There is no history of alcohol use or other ingestions, and the normal serum osmolality and low osmolal gap (less than 10 mOsm/kg) argue against a poisoning with ethanol, ethylene glycol, or methanol. The initial combined anion gap metabolic acidosis and respiratory alkalosis is consistent with salicylate toxicity, but she does not report aspirin ingestion. Acetaminophen use in the setting of malnutrition or starvation physiology raises the possibility of 5-oxoproline accumulation.
Routine serum lactate does not detect D-lactate, which is produced by colonic bacteria and has been reported in short bowel syndrome and following intestinal bypass surgery. This may occur weeks to months after intestinal procedures, following ingestion of a heavy carbohydrate load, and almost invariably presents with altered mental status and increased anion gap metabolic acidosis, although generalized weakness has been reported.
A surgical consultant drained her wound infection. Fluid Gram stain was negative. D-lactate, salicylate and acetaminophen levels were undetectable. Thiamine pyrophosphate level was 229 nmol/L (reference range: 78-185 nmol/L). Acetaminophen was discontinued and N-acetylcysteine infusion was started for possible 5-oxoprolinemia. Her anion gap acidosis rapidly improved. Twelve hours after admission, she reported sudden onset of blurry vision. Her vital signs were: temperature 37oC, heart rate 110 beats per minute, respiratory rate 40 breaths per minute, blood pressure 168/90, and oxygen saturation 100% on ambient air. Telemetry showed ventricular bigeminy. On examination, she was unable to abduct her right eye; muscle strength was 1/5 in all extremities; biceps, ankle, and patellar reflexes were absent.
Her neurological deficits have progressed over hours to near complete paralysis, asymmetric cranial nerve paresis, and areflexia. Although botulism can cause blurred vision and absent deep tendon reflexes, patients almost always have symmetrical bulbar findings followed by descending paralysis. Should the “numbness” in her arms reported earlier represent undetected sensory deficits, this, too would be inconsistent with botulism.
A diagnosis of GBS ties together several aspects of her presentation and clinical course. Several variants show different patterns of weakness and may involve cranial nerves. Her tachypnea and dyspnea are concerning signs of potential impending respiratory failure. The ventricular bigeminy and mild hypertension could represent autonomic dysfunction that is seen in many cases of GBS.
She was intubated for airway protection. Computed tomography angiography and magnetic resonance imaging of her brain were normal. Cerebral spinal fluid analysis obtained through lumbar puncture showed the following: white blood cell count 3/μL, red blood cell count 11/μL, protein 63 mg/dL (reference range: 15-60mg/dL), and glucose 128 mg/dL (reference range: 40-80mg/dL).
The lumbar puncture is consistent with GBS given the slightly elevated protein and cell count well below 50/μL. Given the severity of her symptoms, treatment with IV immunoglobulin or plasmapheresis should be initiated. Nerve conduction studies (NCS) and electromyography (EMG) are indicated for diagnostic confirmation.
EMG and NCS revealed a severe sensorimotor polyneuropathy with demyelinating features including a conduction block at a noncompressible site, consistent with AIDP. Left sural nerve biopsy confirmed acute demyelinating and mild axonal neuropathy (Figure 2). On hospital day 2, treatment with IV immunoglobulins (IVIG) was initiated; however, she developed anaphylaxis following her second administration and subsequently received plasmapheresis. A tracheostomy was performed for respiratory muscle weakness, and she was discharged to a nursing facility. C. botulinum cultures from the wound eventually returned negative. Following her hospitalization, a serum 5-oxoproline level sent 10 hours after admission returned as elevated, confirming the additional diagnosis of 5-oxoprolinemia. On follow-up, she can sit up and feed herself without assistance, and her gait continues to improve with physical therapy.
DISCUSSION
This patient presented with rapidly progressive weakness that developed in the 2 weeks following bariatric surgery. In the postsurgical setting, patient complaints of weakness are commonly encountered and can pose a diagnostic challenge. Asthenia (ie, general loss of strength or energy) is frequently reported in the immediate postoperative period, and may result from the stress of surgery, pain, deconditioning, or infection. This must be distinguished from true neurologic weakness, which results from dysfunction of the brain, spinal cord, nerve, neuromuscular junction, or muscle. The initial history can help elucidate the inciting events such as preceding surgery, infections or ingestions, and can also categorize the pattern of weakness. The neurologic examination can localize the pathology within the neuraxis. EMG and NCS can distinguish neuropathy from radiculopathy, and categorize the process as axonal, demyelinating, or mixed. In this case, the oculomotor weakness, sensory abnormalities and areflexia signaled a severe sensorimotor polyneuropathy, and EMG/NCS confirmed a demyelinating process consistent with GBS.
Guillain-Barré syndrome is an acute, immune-mediated polyneuropathy. Patients with GBS often present with a preceding respiratory or diarrheal illness; however, the stress of a recent surgery can serve as an inciting event. The syndrome, acute postgastric reduction surgery (APGARS) neuropathy, was introduced in the literature in 2002, describing 3 patients who presented with progressive vomiting, weakness, and hyporeflexia following bariatric surgery.1 The term has been used to describe bariatric surgery patients who developed postoperative quadriparesis, cranial nerve deficits, and respiratory compromise.2 Given the clinical heterogeneity in the literature with relation to APGARS, it is probable that the cases described could result from multiple etiologies. While GBS is purely immune-mediated and can be precipitated by the stress of surgery itself, postbariatric surgery patients are susceptible to many nutritional deficiencies that can lead to similar presentations.3 For example, thiamine (vitamin B1) and cobalamin (vitamin B12) deficiencies cause distinct postbariatric surgery neuropathies.4 Thiamine deficiency may manifest weeks to months after surgery and can rapidly progress, whereas cobalamin deficiency generally develops over 3 to 5 years. Both of these syndromes demonstrate an axonal pattern of nerve injury on EMG/NCS, in contrast to the demyelinating pattern typically seen in GBS. In addition, bariatric surgery patients are at higher risk for copper deficiency, which usually presents as a myeloneuropathy with subacute gait decline and upper motor neuron signs including spasticity.
Although GBS classically presents with symmetric ascending weakness and sensory abnormalities, it may manifest in myriad ways. Factors influencing the presentation include the types of nerve fibers involved (motor, sensory, cranial or autonomic), the predominant mode of injury (axonal vs demyelinating), and the presence or absence of alteration in consciousness.5 The most common form of GBS is AIDP. The classic presentation involves paresthesias in the fingertips and toes followed by lower extremity weakness that ascends over hours to days to involve the arms and potentially the muscles of respiration. A minority of patients with GBS first experience weakness in the upper extremities or facial muscles, and oculomotor involvement is rare.5 Pain is common and often severe.6 Dysautonomia affects most patients with GBS and may manifest as labile blood pressure or arrhythmias.5 Several variant GBS presentation patterns have been described, including acute motor axonal neuropathy, a pure motor form of GBS; ophthalmoplegia, ataxia, and areflexia in Miller Fisher syndrome; and alteration in consciousness, hyperreflexia, ataxia, and ophthalmoparesis in Bickerstaff’s brain stem encephalitis.5
Patients with GBS can progress rapidly to respiratory failure. Serial neurologic exams may signal the diagnosis and inform triage to the appropriate level of care. Measurement of bedside pulmonary function, including mean inspiratory force and functional vital capacity, help to determine if there is weakness of diaphragmatic muscles. Patients with signs or symptoms of diaphragmatic weakness require monitoring in an intensive care unit and potentially early intubation. Treatment with IVIG or plasmapheresis has been found to hasten recovery from GBS, including earlier improvement in muscle strength and a reduced need for mechanical ventilation.7 Treatment selection is based on available resources as both modalities are felt to be equivalent.The majority of patients with GBS make a full recovery over a period of weeks to months, although many have persistent motor weakness. Despite immunotherapy, up to 20% of patients remain severely disabled and approximately 5% die.8 Advanced age, rapid progression of weakness over a period of less than 72 hours, need for mechanical ventilation, and absent compound muscle action potentials on NCS are all associated with prolonged and incomplete recovery.9
This patient developed respiratory failure within 12 hours of hospitalization, prior to being diagnosed with GBS. Even in that short time, the treating clinicians encountered a series of clinical diversions. The initial proximal pattern of muscle weakness suggested a possible myopathic process; the wound infection introduced the possibility of botulism; obesity and recent bariatric surgery triggered concern for thiamine deficiency; and the anion gap acidosis from 5-oxoprolinemia created yet another clinical detour. While the path from presentation to diagnosis is seldom a straight line, when faced with rapidly progressive weakness, it is paramount to forge ahead with an efficient diagnostic evaluation and timely therapeutic intervention.
KEY TEACHING POINTS
- A complaint of general weakness requires distinction between asthenia (ie, general loss of strength or energy) and true neuromuscular weakness from dysfunction of the brain, spinal cord, nerve, neuromuscular junction, and/or muscle.
- Guillain-Barré syndrome may present in a variety of atypical fashions not limited to ascending, distally predominant weakness.
- Acute postgastric reduction surgery neuropathy should be considered in patients presenting with weakness, vomiting, or hyporeflexia after bariatric surgery.
- Acute inflammatory demyelinating polyneuropathy may rapidly progress to respiratory failure, and warrants serial neurologic examinations, monitoring of pulmonary function, and an expedited diagnostic evaluation.
Disclosure
Nothing to report.
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient’s case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant. The bolded text represents the patient’s case. Each paragraph that follows represents the discussant’s thoughts.
A 45-year-old woman presented to the emergency department with 2 days of generalized, progressive weakness. Her ability to walk and perform daily chores was increasingly limited. On the morning of her presentation, she was unable to stand up without falling.
A complaint of weakness must be classified as either functional weakness related to a systemic process or true neurologic weakness from dysfunction of the central nervous system (eg, brain, spinal cord) or peripheral nervous system (eg, anterior horn cell, nerve, neuromuscular junction, or muscle). More information on her clinical course and a detailed neurologic exam will help clarify this key branch point.
She was 2 weeks status-post laparoscopic Roux-en-Y gastric bypass and gastric band removal performed in Europe. Immediately following surgery, she experienced abdominal discomfort and nausea with occasional nonbloody, nonbilious emesis, attributed to expected postoperative anatomical changes. She developed a postoperative pneumonia treated with amoxicillin-clavulanate. She tolerated her flight back to the United States, but her abdominal discomfort persisted and she had minimal oral intake due to her nausea.
Functional weakness may stem from hypovolemia from insufficient oral intake, anemia related to the recent surgery, electrolyte abnormalities, chronic nutritional issues associated with obesity and weight-reduction surgery, and pneumonia. Prolonged air travel, obesity, and recent surgery place her at risk for venous thromboembolism, which may manifest as reduced exercise tolerance. Nausea, vomiting, and abdominal pain persisting for 2 weeks after a Roux-en-Y gastric bypass surgery raises several concerns, including gastric remnant distension (although hiccups are often prominent); stomal stenosis, which typically presents several weeks after surgery; marginal ulceration; or infection at the surgical site or from an anastomotic leak. She may also have a surgery- or medication-related myopathy.
The patient had a history of obesity, hypertension, hyperlipidemia, migraine headaches, and nonalcoholic steatohepatitis. Four years previously, she had undergone gastric banding complicated by band migration and ulceration at the banding site. Her medications were amlodipine, losartan, ranitidine, acetaminophen, and nadroparin for venous thromboembolism prophylaxis during her flight. She denied alcohol, tobacco, or illicit drug use. On further questioning, she reported diaphoresis, mild dyspnea, loose stools, and a sensation of numbness and “heaviness” in her arms. Her abdominal pain was limited to the surgical incision and was controlled with acetaminophen. She denied fevers, cough, chest pain, diplopia, or dysphagia.
Heaviness in both arms could result from an acutely presenting myopathic or neuropathic process, while the coexistence of numbness suggests a sensorimotor polyneuropathy. Obesity and gastric bypass surgery increase her nutritional risk, and thiamine deficiency may present as an acute axonal polyneuropathy (ie, beriberi). Unlike vitamin B12 deficiency, which may take years to develop, thiamine deficiency can present within 4 weeks of gastric bypass surgery. Her dyspnea may be a manifestation of diaphragmatic weakness, although her ostensibly treated pneumonia or as of yet unproven postoperative anemia may be contributing. Chemoprophylaxis mitigates her risk of venous thromboembolism, which is, nonetheless, unlikely to account for the gastrointestinal symptoms and upper extremity weakness. If she is continuing to take amlodipine and losartan but has become volume-depleted, hypotension may be contributing to the generalized weakness.
Physical examination revealed an obese, pale and diaphoretic woman. Her temperature was 36.9°C, heart rate 77 beats per minute, blood pressure 158/90 mm Hg, respiratory rate 28 breaths per minute, and O2 saturation 99% on ambient air. She had no cervical lymphadenopathy and a normal thyroid exam. There were no murmurs on cardiac examination, and jugular venous pressure was estimated at 10 cm of water. Her lung sounds were clear. Her abdomen was soft, nondistended, with localized tenderness and fluctuance around the midline surgical incision with a small amount of purulent drainage. She was alert and oriented to name, date, place, and situation. Cranial nerves II through XII were grossly intact. Strength was 4/5 in bilateral biceps, triceps and distal hand and finger extensors, 3/5 in bilateral deltoids. Strength in hip flexors was 4/5 and it was 5/5 in distal lower extremities. Sensation was intact to pinprick in upper and lower extremities. Biceps reflexes were absent; patellar and ankle reflexes were 1+ and symmetric. The remainder of the physical exam was unremarkable.
The patient has symmetric proximal muscle weakness with upper extremity predominance and preserved strength in her distal lower extremities. A myopathy could explain this pattern of weakness, further substantiated by absent reflexes and reportedly intact sensation. Subacute causes of myopathy include hypokalemia, hyperkalemia, toxic myopathies from medications, or infection-induced rhabdomyolysis. However, she does not report muscle pain, and the loss of reflexes is faster than would be expected with a myopathy. A more thorough sensory examination would inform the assessment of potential neuropathic processes. Guillain-Barré syndrome (GBS) is possible; it most commonly presents as an ascending, distally predominant acute inflammatory demyelinating polyneuropathy (AIDP), although her upper extremity weakness predominates and there are no clear sensory changes. It remains to be determined how her wound infection might relate to her overall presentation.
Her white blood cell count was 12,600/μL (reference range: 3,400-10,000/μL), hemoglobin was 10.2 g/dL, and platelet count was 698,000/μL. Mean corpuscular volume was 86 fL. Serum chemistries were: sodium 138 mEq/L, potassium 3.8 mEq/L, chloride 106 mmol/L, bicarbonate 15 mmol/L, blood urea nitrogen 5 mg/dL, creatinine 0.65 mg/dL, glucose 125 mg/dL, calcium 8.3 mg/dL, magnesium 1.9 mg/dL, phosphorous 2.4 mg/dL, and lactate 1.8 mmol/L (normal: < 2.0 mmol/L). Creatinine kinase (CK), liver function tests, and coagulation panel were normal. Total protein was 6.4 g/dL, and albumin was 2.7 g/dL. Venous blood gas was: pH 7.39 and PCO2 25 mmHg. Urinalysis revealed ketones. Blood and wound cultures were sent for evaluation. A chest x-ray was unremarkable. An electrocardiogram showed normal sinus rhythm. Computed tomography (CT) of the abdomen and pelvis revealed a multiloculated rim-enhancing fluid collection in the anterior abdominal wall (Figure 1).
She does not have any notable electrolyte derangements that would account for her weakness, and the normal creatinine kinase lowers the probability of a myopathy and excludes rhabdomyolysis. Progression of weakness from proximal to distal muscles in a symmetric fashion is consistent with botulism, and she has an intra-abdominal wound infection that could be harboring Clostridium botulinum. Nonetheless, the normal cranial nerve exam and the rarity of botulism occurring with surgical wounds argue against this diagnosis. She should receive intravenous (IV) thiamine for the possibility of beriberi. A lumbar puncture should be performed to assess for albuminocytologic dissociation, which can be seen in patients with GBS.
The patient received high-dose IV thiamine, IV vancomycin, IV piperacillin-tazobactam, and acetaminophen. Over the subsequent 4 hours, her anion gap acidosis worsened. She declined arterial puncture. Repeat venous blood gas was: pH 7.22, PCO2 28 mmHg, and bicarbonate 11 mmol/L. Lactate and glucose were normal. Serum osmolarity was 292 mmol/kg (reference range: 283-301 mmol/kg). She was started on an IV sodium bicarbonate infusion without improvement in her acidemia.
An acute anion gap metabolic acidosis suggests a limited differential diagnosis that includes lactic acidosis, D-lactic acidosis, severe starvation ketoacidosis, acute renal failure, salicylate, or other drug or poison ingestion. Starvation ketoacidosis may be contributing, but a bicarbonate value this low would be unusual. There is no history of alcohol use or other ingestions, and the normal serum osmolality and low osmolal gap (less than 10 mOsm/kg) argue against a poisoning with ethanol, ethylene glycol, or methanol. The initial combined anion gap metabolic acidosis and respiratory alkalosis is consistent with salicylate toxicity, but she does not report aspirin ingestion. Acetaminophen use in the setting of malnutrition or starvation physiology raises the possibility of 5-oxoproline accumulation.
Routine serum lactate does not detect D-lactate, which is produced by colonic bacteria and has been reported in short bowel syndrome and following intestinal bypass surgery. This may occur weeks to months after intestinal procedures, following ingestion of a heavy carbohydrate load, and almost invariably presents with altered mental status and increased anion gap metabolic acidosis, although generalized weakness has been reported.
A surgical consultant drained her wound infection. Fluid Gram stain was negative. D-lactate, salicylate and acetaminophen levels were undetectable. Thiamine pyrophosphate level was 229 nmol/L (reference range: 78-185 nmol/L). Acetaminophen was discontinued and N-acetylcysteine infusion was started for possible 5-oxoprolinemia. Her anion gap acidosis rapidly improved. Twelve hours after admission, she reported sudden onset of blurry vision. Her vital signs were: temperature 37oC, heart rate 110 beats per minute, respiratory rate 40 breaths per minute, blood pressure 168/90, and oxygen saturation 100% on ambient air. Telemetry showed ventricular bigeminy. On examination, she was unable to abduct her right eye; muscle strength was 1/5 in all extremities; biceps, ankle, and patellar reflexes were absent.
Her neurological deficits have progressed over hours to near complete paralysis, asymmetric cranial nerve paresis, and areflexia. Although botulism can cause blurred vision and absent deep tendon reflexes, patients almost always have symmetrical bulbar findings followed by descending paralysis. Should the “numbness” in her arms reported earlier represent undetected sensory deficits, this, too would be inconsistent with botulism.
A diagnosis of GBS ties together several aspects of her presentation and clinical course. Several variants show different patterns of weakness and may involve cranial nerves. Her tachypnea and dyspnea are concerning signs of potential impending respiratory failure. The ventricular bigeminy and mild hypertension could represent autonomic dysfunction that is seen in many cases of GBS.
She was intubated for airway protection. Computed tomography angiography and magnetic resonance imaging of her brain were normal. Cerebral spinal fluid analysis obtained through lumbar puncture showed the following: white blood cell count 3/μL, red blood cell count 11/μL, protein 63 mg/dL (reference range: 15-60mg/dL), and glucose 128 mg/dL (reference range: 40-80mg/dL).
The lumbar puncture is consistent with GBS given the slightly elevated protein and cell count well below 50/μL. Given the severity of her symptoms, treatment with IV immunoglobulin or plasmapheresis should be initiated. Nerve conduction studies (NCS) and electromyography (EMG) are indicated for diagnostic confirmation.
EMG and NCS revealed a severe sensorimotor polyneuropathy with demyelinating features including a conduction block at a noncompressible site, consistent with AIDP. Left sural nerve biopsy confirmed acute demyelinating and mild axonal neuropathy (Figure 2). On hospital day 2, treatment with IV immunoglobulins (IVIG) was initiated; however, she developed anaphylaxis following her second administration and subsequently received plasmapheresis. A tracheostomy was performed for respiratory muscle weakness, and she was discharged to a nursing facility. C. botulinum cultures from the wound eventually returned negative. Following her hospitalization, a serum 5-oxoproline level sent 10 hours after admission returned as elevated, confirming the additional diagnosis of 5-oxoprolinemia. On follow-up, she can sit up and feed herself without assistance, and her gait continues to improve with physical therapy.
DISCUSSION
This patient presented with rapidly progressive weakness that developed in the 2 weeks following bariatric surgery. In the postsurgical setting, patient complaints of weakness are commonly encountered and can pose a diagnostic challenge. Asthenia (ie, general loss of strength or energy) is frequently reported in the immediate postoperative period, and may result from the stress of surgery, pain, deconditioning, or infection. This must be distinguished from true neurologic weakness, which results from dysfunction of the brain, spinal cord, nerve, neuromuscular junction, or muscle. The initial history can help elucidate the inciting events such as preceding surgery, infections or ingestions, and can also categorize the pattern of weakness. The neurologic examination can localize the pathology within the neuraxis. EMG and NCS can distinguish neuropathy from radiculopathy, and categorize the process as axonal, demyelinating, or mixed. In this case, the oculomotor weakness, sensory abnormalities and areflexia signaled a severe sensorimotor polyneuropathy, and EMG/NCS confirmed a demyelinating process consistent with GBS.
Guillain-Barré syndrome is an acute, immune-mediated polyneuropathy. Patients with GBS often present with a preceding respiratory or diarrheal illness; however, the stress of a recent surgery can serve as an inciting event. The syndrome, acute postgastric reduction surgery (APGARS) neuropathy, was introduced in the literature in 2002, describing 3 patients who presented with progressive vomiting, weakness, and hyporeflexia following bariatric surgery.1 The term has been used to describe bariatric surgery patients who developed postoperative quadriparesis, cranial nerve deficits, and respiratory compromise.2 Given the clinical heterogeneity in the literature with relation to APGARS, it is probable that the cases described could result from multiple etiologies. While GBS is purely immune-mediated and can be precipitated by the stress of surgery itself, postbariatric surgery patients are susceptible to many nutritional deficiencies that can lead to similar presentations.3 For example, thiamine (vitamin B1) and cobalamin (vitamin B12) deficiencies cause distinct postbariatric surgery neuropathies.4 Thiamine deficiency may manifest weeks to months after surgery and can rapidly progress, whereas cobalamin deficiency generally develops over 3 to 5 years. Both of these syndromes demonstrate an axonal pattern of nerve injury on EMG/NCS, in contrast to the demyelinating pattern typically seen in GBS. In addition, bariatric surgery patients are at higher risk for copper deficiency, which usually presents as a myeloneuropathy with subacute gait decline and upper motor neuron signs including spasticity.
Although GBS classically presents with symmetric ascending weakness and sensory abnormalities, it may manifest in myriad ways. Factors influencing the presentation include the types of nerve fibers involved (motor, sensory, cranial or autonomic), the predominant mode of injury (axonal vs demyelinating), and the presence or absence of alteration in consciousness.5 The most common form of GBS is AIDP. The classic presentation involves paresthesias in the fingertips and toes followed by lower extremity weakness that ascends over hours to days to involve the arms and potentially the muscles of respiration. A minority of patients with GBS first experience weakness in the upper extremities or facial muscles, and oculomotor involvement is rare.5 Pain is common and often severe.6 Dysautonomia affects most patients with GBS and may manifest as labile blood pressure or arrhythmias.5 Several variant GBS presentation patterns have been described, including acute motor axonal neuropathy, a pure motor form of GBS; ophthalmoplegia, ataxia, and areflexia in Miller Fisher syndrome; and alteration in consciousness, hyperreflexia, ataxia, and ophthalmoparesis in Bickerstaff’s brain stem encephalitis.5
Patients with GBS can progress rapidly to respiratory failure. Serial neurologic exams may signal the diagnosis and inform triage to the appropriate level of care. Measurement of bedside pulmonary function, including mean inspiratory force and functional vital capacity, help to determine if there is weakness of diaphragmatic muscles. Patients with signs or symptoms of diaphragmatic weakness require monitoring in an intensive care unit and potentially early intubation. Treatment with IVIG or plasmapheresis has been found to hasten recovery from GBS, including earlier improvement in muscle strength and a reduced need for mechanical ventilation.7 Treatment selection is based on available resources as both modalities are felt to be equivalent.The majority of patients with GBS make a full recovery over a period of weeks to months, although many have persistent motor weakness. Despite immunotherapy, up to 20% of patients remain severely disabled and approximately 5% die.8 Advanced age, rapid progression of weakness over a period of less than 72 hours, need for mechanical ventilation, and absent compound muscle action potentials on NCS are all associated with prolonged and incomplete recovery.9
This patient developed respiratory failure within 12 hours of hospitalization, prior to being diagnosed with GBS. Even in that short time, the treating clinicians encountered a series of clinical diversions. The initial proximal pattern of muscle weakness suggested a possible myopathic process; the wound infection introduced the possibility of botulism; obesity and recent bariatric surgery triggered concern for thiamine deficiency; and the anion gap acidosis from 5-oxoprolinemia created yet another clinical detour. While the path from presentation to diagnosis is seldom a straight line, when faced with rapidly progressive weakness, it is paramount to forge ahead with an efficient diagnostic evaluation and timely therapeutic intervention.
KEY TEACHING POINTS
- A complaint of general weakness requires distinction between asthenia (ie, general loss of strength or energy) and true neuromuscular weakness from dysfunction of the brain, spinal cord, nerve, neuromuscular junction, and/or muscle.
- Guillain-Barré syndrome may present in a variety of atypical fashions not limited to ascending, distally predominant weakness.
- Acute postgastric reduction surgery neuropathy should be considered in patients presenting with weakness, vomiting, or hyporeflexia after bariatric surgery.
- Acute inflammatory demyelinating polyneuropathy may rapidly progress to respiratory failure, and warrants serial neurologic examinations, monitoring of pulmonary function, and an expedited diagnostic evaluation.
Disclosure
Nothing to report.
1. Akhtar M, Collins MP, Kissel JT. Acute postgastric reduction surgery (APGARS) Neuropathy: A polynutritional, multisystem disorder. Neurology. 2002;58:A68. PubMed
2. Chang CG, Adams-Huet B, Provost DA. Acute post-gastric reduction surgery (APGARS) neuropathy. Obes Surg. 2004;14(2):182-189. PubMed
3. Chang CG, Helling TS, Black WE, Rymer MM. Weakness after gastric bypass. Obes Surg. 2002;12(4):592-597. PubMed
4. Shankar P, Boylan M, Sriram K. Micronutrient deficiencies after bariatric surgery. Nutrition. 2010;26(11-12):1031-1037. PubMed
5. Dimachkie MM, Barohn RJ. Guillain-Barré syndrome and variants. Neurol Clin. 2013;31(2):491-510. PubMed
6. Ruts L, Drenthen J, Jongen JL, et al. Pain in Guillain-Barré syndrome: a long-term follow-up study. Neurology. 2010;75(16):1439-1447. PubMed
7. Hughes RAC, Wijdicks EFM, Barohn R, et al: Quality Standards Subcommittee of the American Academy of Neurology. Practice parameter: immunotherapy for Guillain-Barré syndrome: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2003;61:736-740. PubMed
8. Hughes RA, Swan AV, Raphaël JC, Annane D, van Koningsveld R, van Doorn PA. Immunotherapy for Guillain-Barré syndrome: a systematic review. Brain. 2007;130(Pt 9):2245-2257. PubMed
9. Rajabally YA, Uncini A. Outcome and predictors in Guillain-Barré syndrome. J Neurol Neurosurg Psychiatry. 2012;83(7):711-718. PubMed
1. Akhtar M, Collins MP, Kissel JT. Acute postgastric reduction surgery (APGARS) Neuropathy: A polynutritional, multisystem disorder. Neurology. 2002;58:A68. PubMed
2. Chang CG, Adams-Huet B, Provost DA. Acute post-gastric reduction surgery (APGARS) neuropathy. Obes Surg. 2004;14(2):182-189. PubMed
3. Chang CG, Helling TS, Black WE, Rymer MM. Weakness after gastric bypass. Obes Surg. 2002;12(4):592-597. PubMed
4. Shankar P, Boylan M, Sriram K. Micronutrient deficiencies after bariatric surgery. Nutrition. 2010;26(11-12):1031-1037. PubMed
5. Dimachkie MM, Barohn RJ. Guillain-Barré syndrome and variants. Neurol Clin. 2013;31(2):491-510. PubMed
6. Ruts L, Drenthen J, Jongen JL, et al. Pain in Guillain-Barré syndrome: a long-term follow-up study. Neurology. 2010;75(16):1439-1447. PubMed
7. Hughes RAC, Wijdicks EFM, Barohn R, et al: Quality Standards Subcommittee of the American Academy of Neurology. Practice parameter: immunotherapy for Guillain-Barré syndrome: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2003;61:736-740. PubMed
8. Hughes RA, Swan AV, Raphaël JC, Annane D, van Koningsveld R, van Doorn PA. Immunotherapy for Guillain-Barré syndrome: a systematic review. Brain. 2007;130(Pt 9):2245-2257. PubMed
9. Rajabally YA, Uncini A. Outcome and predictors in Guillain-Barré syndrome. J Neurol Neurosurg Psychiatry. 2012;83(7):711-718. PubMed
© 2017 Society of Hospital Medicine
Health information exchange in US hospitals: The current landscape and a path to improved information sharing
The US healthcare system is highly fragmented, with patients typically receiving treatment from multiple providers during an episode of care and from many more providers over their lifetime.1,2 As patients move between care delivery settings, whether and how their information follows them is determined by a haphazard and error-prone patchwork of telephone, fax, and electronic communication channels.3 The existence of more robust electronic communication channels is often dictated by factors such as which providers share the same electronic health record (EHR) vendor rather than which providers share the highest volume of patients. As a result, providers often make clinical decisions with incomplete information, increasing the chances of misdiagnosis, unsafe or suboptimal treatment, and duplicative utilization.
Providers across the continuum of care encounter challenges to optimal clinical decision-making as a result of incomplete information. These are particularly problematic among clinicians in hospitals and emergency departments (EDs). Clinical decision-making in EDs often involves urgent and critical conditions in which decisions are made under pressure. Time constraints limit provider ability to find key clinical information to accurately diagnose and safely treat patients.4-6 Even for planned inpatient care, providers are often unfamiliar with patients, and they make safer decisions when they have full access to information from outside providers.7,8
Transitions of care between hospitals and primary care settings are also fraught with gaps in information sharing. Clinical decisions made in primary care can set patients on treatment trajectories that are greatly affected by the quality of information available to the care team at the time of initial diagnosis as well as in their subsequent treatment. Primary care physicians are not universally notified when their patients are hospitalized and may not have access to detailed information about the hospitalization, which can impair their ability to provide high quality care.9-11
Widespread and effective electronic health information exchange (HIE) holds the potential to address these challenges.3 With robust, interconnected electronic systems, key pieces of a patient’s health record can be electronically accessed and reconciled during planned and unplanned care transitions. The concept of HIE is simple—make all relevant patient data available to the clinical care team at the point of care, regardless of where that information was generated. The estimated value of nationwide interoperable EHR adoption suggests large savings from the more efficient, less duplicative, and higher quality care that likely results.12,13
There has been substantial funding and activity at federal, state, and local levels to promote the development of HIE in the US. The 2009 Health Information Technology for Economic and Clinical Health (HITECH) Act has the specific goal of accelerating adoption and use of certified EHR technology coupled with the ability to exchange clinical information to support patient care.14 The HITECH programs supported specific types of HIE that were believed to be particularly critical to improving patient care and included them in the federally-defined criteria for Meaningful Use (MU) of EHRs (ie, providers receive financial incentives for achieving specific objectives). The MU criteria evolve, moving from data capture in stage 1 to improved patient outcomes in stage 3.15 The HIE criteria focus on sending and receiving summary-of-care records during care transitions.
Despite the clear benefits of HIE and substantial support stated in policy initiatives, the spread of national HIE has been slow. Today, HIE in the US is highly heterogeneous: as a result of multiple federal-, state-, community-, enterprise- and EHR vendor-level efforts, only some provider organizations are able to engage in HIE with the other provider organizations with which they routinely share patients. In this review, we offer a framework and a corresponding set of definitions to understand the current state of HIE in the US. We describe key challenges to HIE progress and offer insights into the likely path to ensure that clinicians have routine, electronic access to patient information.
FOUR KEY DIMENSIONS OF HEALTH INFORMATION EXCHANGE
While the concept of HIE is simple—electronic access to clinical information across healthcare settings—the operationalization of HIE occurs in many different ways.16 While the terms “health information exchange” and “interoperability” are often used interchangeably, they can have different meanings. In this section, we describe 4 important dimensions that serve as a framework for understanding any given effort to enable HIE (Table).
(1) What Is Exchanged? Types of Information
The term “health information exchange” is ambiguous with respect to the type(s) of information that are accessible. Health information exchange may refer to the process of 2 providers electronically sharing a wide range of data, from a single type of information (eg, lab test results), summary of care records, to complete patient records.17 Part of this ambiguity may stem from uncertainty about the scope of information that should be shared, and how this varies based on the type of clinical encounter. For example, critical types of information in the ED setting may differ from those relevant to a primary care team after a referral. While the ability to access only particular types of information will not address all information gaps, providing access to complete patient records may result in information overload that inhibits the ability to find the subset of information relevant in a given clinical encounter.
(2) Who is Exchanging? Relationship Between Provider Organizations
The types of information accessed electronically are effectively agnostic to the relationship between the provider organizations that are sharing information. Traditionally, HIE has been considered as information that is electronically shared among 2 or more unaffiliated organizations. However, there is increasing recognition that some providers may not have electronic access to all information about their patients that exists within their organization, often after a merger or acquisition between 2 providers with different EHR systems.18,19 In these cases, a primary care team in a large integrated delivery system may have as many information gaps as a primary care team in a small, independent practice. Fulfilling clinical information needs may require both intra- and interorganizational HIE, which complicates the design of HIE processes and how the care team approaches incorporating information from both types of organizations into their decision-making. It is also important to recognize that some provider organizations, particularly small, rural practices, may not have the information technology and connectivity infrastructure required to engage in HIE.
(3) How Is Information Exchanged? Types of Electronic Access: Push vs Pull Exchange
To minimize information gaps, electronic access to information from external settings needs to offer both “push” and “pull” options. Push exchange, which can direct information electronically to a targeted recipient, works in scenarios in which there is a known information gap and known information source. The classic use for push exchange is care coordination, such as primary care physician-specialist referrals or hospital-primary care physician transitions postdischarge. Pull exchange accommodates scenarios in which there is a known information gap but the source(s) of information are unknown; it requires that clinical care teams search for and locate the clinical information that exists about the patient in external settings. Here, the classic use is emergency care in which the care team may encounter a new patient and want to retrieve records.
Widespread use of provider portals that offer view-only access into EHRs and other clinical data repositories maintained by external organizations complicate the picture. Portals are commonly used by hospitals to enable community providers to view information from a hospitalization.21 While this does not fall under the commonly held notion of HIE because no exchange occurs, portals support a pull approach to accessing information electronically among care settings that treat the same patients but use different EHRs.
Regardless of whether information is pushed or pulled, this may happen with varying degrees of human effort. This distinction gives rise to the difference between HIE and interoperability. Health information exchange reflects the ability of EHRs to exchange information, while interoperability additionally requires that EHRs be able to use exchanged information. From an operational perspective, the key distinction between HIE and interoperability is the extent of human involvement. Health information exchange requires that a human read and decide how to enter information from external settings (eg, a chart in PDF format sent between 2 EHRs), while interoperability enables the EHR that receives the information to understand the content and automatically triage or reconcile information, such as a medication list, without any human action.21 Health information exchange, therefore, relies on the diligence of the receiving clinician, while interoperability does not.
(4) What Governance Entity Defines the “Rules” of Exchange?
When more than 1 provider organization shares patient-identified data, a governance entity must specify the framework that governs the exchange. While the specifics of HIE governance vary, there are 3 predominant types of HIE networks, based on the type of organization that governs exchange: enterprise HIE networks, EHR vendor HIE networks or community HIE networks.
Enterprise HIE networks exist when 1 or more provider organizations electronically share clinical information to support patient care with some restriction, beyond geography, that dictates which organizations are involved. Typically, restrictions are driven by strategic, proprietary interests.22,23 Although broad-based information access across settings would be in the best interest of the patient, provider organizations are sensitive to the competitive implications of sharing data and may pursue such sharing in a strategic way.24 A common scenario is when hospitals choose to strategically affiliate with select ambulatory providers and exclusively exchange information with them. This should facilitate better care coordination for patients shared by the hospital and those providers but can also benefit the hospital by increasing the referrals from those providers. While there is little direct evidence quantifying the extent to which this type of strategic sharing takes place, there have been anecdotal reports as well as indirect findings that for-profit hospitals in competitive markets are less likely to share patient data.19,25
EHR vendor HIE networks exist when exchange occurs within a community of provider organizations that use an EHR from the same vendor. A subset of EHR vendors have made this capability available; EPIC’s CareEverywhere solution27 is the best-known example. Providers with an EPIC EHR are able to query for and retrieve summary of care records and other documents from any provider organization with EPIC that has activated this functionality. There are also multivendor efforts, such as CommonWell27 and the Sequoia Project’s Carequality collaborative,28 which are initiatives that seek to provide a common interoperability framework across a diverse set of stakeholders, including provider organizations with different EHR systems, in a similar fashion to HIE modules like CareEverywhere. To date, growth in these cross-vendor collaborations has been slow, and they have limited participation. While HIE networks that involve EHR vendors are likely to grow, it is difficult to predict how quickly because they are still in an early phase of development, and face nontechnical barriers such as patient consent policies that vary between providers and across states.
Community HIE networks—also referred to as health information organizations (HIOs) or regional health information organizations (RHIOs)—exist when provider organizations in a community, frequently state-level organizations that were funded through HITECH grants,14 set up the technical infrastructure and governance approach to engage in HIE to improve patient care. In contrast to enterprise or vendor HIE networks that have pursued HIE in ways that appear strategically beneficial, the only restriction on participation in community and state HIE networks is usually geography because they view information exchange as a public good. Seventyone percent of hospital service areas (HSAs) are covered by at least 1 of the 106 operational HIOs, with 309,793 clinicians (licensed prescribers) participating in those exchange networks. Even with early infusions of public and other grant-funding, community HIE networks have experienced significant challenges to sustained operation, and many have ceased operating.29
Thus, for any given provider organization, available HIE networks are primarily shaped by 3 factors:
1. Geographic location, which determines the available community and state HIE networks (as well as other basic information technology and connectivity infrastructure); providers located outside the service areas covered by an operational HIE have little incentive to participate because they do not connect them to providers with whom they share patients. Providers in rural areas may simply not have the needed infrastructure to pursue HIE.
2. Type of organization to which they belong, which determines the available enterprise HIE networks; providers who are not members of large health systems may be excluded from participation in these types of networks.
3. EHR vendor, which determines whether they have access to an EHR vendor HIE network.
ONGOING CHALLENGES
Despite agreement about the substantial potential of HIE to reduce costs and increase the quality of care delivered across a broad range of providers, HIE progress has been slow. While HITECH has successfully increased EHR adoption in hospitals and ambulatory practices,30 HIE has lagged. This is largely because many complex, intertwined barriers must be addressed for HIE to be widespread.
Lack of a Defined Goal
The cost and complexity associated with the exchange of a single type of data (eg, medications) is substantially less than the cost and complexity of sharing complete patient records. There has been little industry consensus on the target goal—do we need to enable sharing of complete patient records across all providers, or will summary of care records suffice? If the latter, as is the focus of the current MU criteria, what types of information should be included in a summary of care record, and should content and/or structure vary depending on the type of care transition? While the MU criteria require the exchange of a summary of care record with defined data fields, it remains unclear whether this is the end state or whether we should continue to push towards broad-based sharing of all patient data as structured elements. Without a clear picture of the ideal end state, there has been significant heterogeneity in the development of HIE capabilities across providers and vendors, and difficulty coordinating efforts to continue to advance towards a nationwide approach. Addressing this issue also requires progress to define HIE usability, that is, how information from external organizations should be presented and integrated into clinical workflow and clinical decisions. Currently, where HIE is occurring and clinicians are receiving summary of care records, they find them long, cluttered, and difficult to locate key information.
Numerous, Complex Barriers Spanning Multiple Stakeholders
In the context of any individual HIE effort, even after the goal is defined, there are a myriad of challenges. In a recent survey of HIO efforts, many identified the following barriers as substantially impeding their development: establishing a sustainable business model, lack of funding, integration of HIE into provider workflow, limitations of current data standards, and working with governmental policy and mandates.30 What is notable about this list is that the barriers span an array of areas, including financial incentives and identifying a sustainable business model, technical barriers such as working within the limitations of data standards, and regulatory issues such as state laws that govern the requirements for patient consent to exchange personal health information. Overcoming any of these issues is challenging, but trying to tackle all of them simultaneously clearly reveals why progress has been slow. Further, resolving many of the issues involve different groups of stakeholders. For example, implementing appropriate patient consent procedures can require engaging with and harmonizing the regulations of multiple states, as well as the Health Insurance Portability and Accountability Act (HIPAA) and regulations specific to substance abuse data.
Weak or Misaligned Incentives
Among the top barriers to HIE efforts are those related to funding and lack of a sustainable business model. This reflects the fact that economic incentives in the current market have not promoted provider engagement in HIE. Traditional fee-for-service payment structures do not reward providers for avoiding duplicative care.31 Further, hospitals perceive patient data as a “key strategic asset, tying physicians and patients to their organization,”24 and are reluctant to share data with competitors. Compounding the problem is that EHR vendors have a business interest in using HIE as a lever to increase revenue. In the short-term, they can charge high fees for interfaces and other HIE-related functionality. In the long-run, vendors may try to influence provider choice of system by making it difficult to engage in cross-vendor exchange.32 Information blocking—when providers or vendors knowingly interfere with HIE33—reflects not only weak incentives, but perverse incentives. While not all providers and vendors experience perverse incentives, the combination of weak and perverse incentives suggests the need to strengthen incentives, so that both types of stakeholders are motivated to tackle the barriers to HIE development. Key to strengthening incentives are payers, who are thought to be the largest beneficiaries of HIE. Payers have been reluctant to make significant investments in HIE without a more active voice in its implementation,34 but a shift to value-based payment may increase their engagement.
THE PATH FORWARD
Despite the continued challenges to nationwide HIE, several policy and technology developments show promise. Stage 3 meaningful use criteria continue to build on previous stages in increasing HIE requirements, raising the threshold for electronic exchange and EHR integration of summary of care documentation in patient transitions. The recently released Medicare Access and CHIP Reauthorization Act (MACRA) Merit-based Incentive Payment System (MIPS) proposed rule replaces stage 3 meaningful use for Medicare-eligible providers with advancing care information (ACI), which accounts for 25% of a provider’s overall incentive reimbursement and includes multiple HIE criteria for providers to report as part of the base and performance score, and follows a very similar framework to stage 3 MU with its criteria regarding HIE.35 While the Centers for Medicare and Medicaid Services (CMS) has not publicly declared that stage 3 MU will be replaced by ACI for hospitals and Medicaid providers, it is likely it will align those programs with the newly announced Medicare incentives.
MACRA also included changes to the Office of the National Coordinator (ONC) EHR certification program in an attempt to further encourage HIE. Vendors and providers must attest that they do not engage in information blocking and will cooperate with the Office’s surveillance programs to that effect. They also must attest that, to the greatest degree possible, their EHR systems allow for bi-directional interoperability with other providers, including those with different EHR vendors, and timely access for patients to view, download, and transmit their health data. In addition, there are emerging federal efforts to pursue a more standardized approach to patient matching and harmonize consent policies across states. These types of new policy initiatives indicate a continued interest in prioritizing HIE and interoperability.21
New technologies may also help spur HIE progress. The newest policy initiatives from CMS, including stage 3 MU and MACRA, have looked to incentivize the creation of application program interfaces (APIs), a set of publicly available tools from EHR vendors to allow developers to build applications that can directly interface with, and retrieve data from, their EHRs. While most patient access to electronic health data to date has been accomplished via patient portals, open APIs would enable developers to build an array of programs for consumers to view, download, and transmit their health data.
Even more promising is the development of the newest Health Level 7 data transmission standard, Fast Healthcare Interoperability Resources (FHIR), which promises to dramatically simplify the technical aspects of interoperability. FHIR utilizes a human-readable, easy to implement modular “resources” standard that may alleviate many technical challenges that come with implementation of an HIE system, enabling cheaper and simpler interoperability.36 A consortium of EHR vendors are working together to test these standards.28 The new FHIR standards also work in conjunction with APIs to allow easier development of consumer-facing applications37 that may empower patients to take ownership of their health data.
CONCLUSION
While HIE holds great promise to reduce the cost and improve the quality of care, progress towards a nationally interoperable health system has been slow. Simply defining HIE and what types of HIE are needed in different clinical scenarios has proven challenging. The additional challenges to implementing HIE in complex technology, legal/regulatory, governance, and incentive environment are not without solutions. Continued policy interventions, private sector collaborations, and new technologies may hold the keys to realizing the vast potential of electronic HIE.
Disclosure
Nothing to report.
1. Pham HH, Schrag D, O’Malley AS, Wu B, Bach PB. Care patterns in Medicare and their implications for pay for performance. N Engl J Med. 2007;356(11):1130-1139. PubMed
2. Finnell JT, Overhage JM, Dexter PR, Perkins SM, Lane KA, McDonald CJ. Community clinical data exchange for emergency medicine patients. Paper presented at: AMIA Annual Symposium Proceedings 2003. PubMed
3. Bodenheimer T. Coordinating care-a perilous journey through the health care system. N Engl J Med. 2008;358(10):1064-1071. PubMed
4. Franczak MJ, Klein M, Raslau F, Bergholte J, Mark LP, Ulmer JL. In emergency departments, radiologists’ access to EHRs may influence interpretations and medical management. Health Aff (Millwood). 2014;33(5):800-806. PubMed
5. Shapiro JS, Kannry J, Kushniruk AW, Kuperman G; New York Clinical Information Exchange (NYCLIX) Clinical Advisory Subcommittee. Emergency physicians’ perceptions of health information exchange. J Am Med Inform Assoc. 2007;14(6):700-705. PubMed
6. Shapiro JS, Kannry J, Lipton M, et al. Approaches to patient health information exchange and their impact on emergency medicine. Ann Emerg Med. 2006;48(4):426-432. PubMed
7. Sutcliffe KM, Lewton E, Rosenthal MM. Communication failures: an insidious contributor to medical mishaps. Acad Med.. 2004;79(2):186-194. PubMed
8. Kaelber DC, Bates DW. Health information exchange and patient safety. J Biomed Inform. 2007;40(suppl 6):S40-S45. PubMed
9. Smith PC, Araya-Guerra R, Bublitz C, et al. MIssing clinical information during primary care visits. JAMA. 2005;293(5):565-571. PubMed
10. Bell CM, Schnipper JL, Auerbach AD, et al. Association of communication between hospital-based physicians and primary care providers with patient outcomes. J Gen Intern Med. 2009;24(3):381-386. PubMed
11. van Walraven C, Taljaard M, Bell CM, et al. A prospective cohort study found that provider and information continuity was low after patient discharge from hospital. J Clin Epidemiol. 2010;63(9):1000-1010. PubMed
12. Walker J, Pan E, Johnston D, Adler-Milstein J, Bates DW, Middleton B. The value of health care information exchange and interoperability. Health Aff (Millwood). 2005:(suppl)W5-10-W5-18. PubMed
13. Shekelle PG, Morton SC, Keeler EB. Costs and benefits of health information technology. Evid Rep Technol Assess (Full Rep). 2006;132:1-71. PubMed
14. Blumenthal D. Launching HITECH. N Engl J Med. 2010;362(5):382-385. PubMed
15. Blumenthal D, Tavenner M. The “meaningful use” regulation for electronic health records. N Engl J Med. 2010;363(6):501-504. PubMed
16. Kuperman G, McGowan J. Potential unintended consequences of health information exchange. J Gen Intern Med. 2013;28(12):1663-1666. PubMed
17. Mathematica Policy Research and Harvard School of Public Health. DesRoches CM, Painter MW, Jha AK, eds. Health Information Technology in the United States, 2015: Transition to a Post-HITECH World (Executive Summary). September 18, 2015. Princeton, NJ: Robert Wood Johnson Foundation; 2015.
18. O’Malley AS, Anglin G, Bond AM, Cunningham PJ, Stark LB, Yee T. Greenville & Spartanburg: Surging Hospital Employment of Physicians Poses Opportunities and Challenges. Washington, DC: Center for Studying Health System Change (HSC); February 2011. 6.
19. Katz A, Bond AM, Carrier E, Docteur E, Quach CW, Yee T. Cleveland Hospital Systems Expand Despite Weak Economy. Washington, DC: Center for Studying Health System Change (HSC); September 2010. 2.
20. Grossman JM, Bodenheimer TS, McKenzie K. Hospital-physician portals: the role of competition in driving clinical data exchange. Health Aff (Millwood). 2006;25(6):1629-1636. PubMed
21. De Salvo KB, Galvez E. Connecting Health and Care for the Nation A Shared Nationwide Interoperability Roadmap - Version 1.0. In: Office of the National Coordinator for Health Information Technology. ed 2015. https://www.healthit.gov/buzz-blog/electronic-health-and-medical-records/interoperability-electronic-health-and-medical-records/connecting-health-care-nation-shared-nationwide-interoperability-roadmap-version-10/. Accessed September 3, 2016.
22. Adler-Milstein J, DesRoches C, Jha AK. Health information exchange among US hospitals. Am J Manag Care. 2011;17(11):761-768. PubMed
23. Vest JR. More than just a question of technology: factors related to hospitals’ adoption and implementation of health information exchange. Int J Med Inform. 2010;79(12):797-806. PubMed
24. Grossman JM, Kushner KL, November EA. Creating sustainable local health information exchanges: can barriers to stakeholder participation be overcome? Res Brief. 2008;2:1-12. PubMed
25. Grossman JM, Cohen G. Despite regulatory changes, hospitals cautious in helping physicians purchase electronic medical records. Issue Brief Cent Stud Health Syst Change 2008;123:1-4. PubMed
26. Kaelber DC, Waheed R, Einstadter D, Love TE, Cebul RD. Use and perceived value of health information exchange: one public healthcare system’s experience. Am J Manag Care. 2013;19(10 spec no):SP337-SP343. PubMed
27. Commonwell Health Alliance. http://www.commonwellalliance.org/, 2016. Accessed September 3, 2016.
28. Carequality. http://sequoiaproject.org/carequality/, 2016. Accessed September 3, 2016.
29. Adler-Milstein J, Lin SC, Jha AK. The number of health information exchange efforts is declining, leaving the viability of broad clinical data exchange uncertain. Health Aff (Millwood). 2016;35(7):1278-1285. PubMed
30. Adler-Milstein J, DesRoches CM, Kralovec P, et al. Electronic health record adoption in US hospitals: progress continues, but challenges persist. Health Aff (Millwood). 2015:34(12):2174-2180. PubMed
31. Health IT Policy Committee Report to Congress: Challenges and Barriers to Interoperability. 2015. https://www.healthit.gov/facas/health-it-policy-committee/health-it-policy-committee-recommendations-national-coordinator-health-it. Accessed September 3, 2016.
32. Everson J, Adler-Milstein J. Engagement in hospital health information exchange is associated with vendor marketplace dominance. Health Aff (MIllwood). 2016;35(7):1286-1293. PubMed
33. Downing K, Mason J. ONC targets information blocking. J AHIMA. 2015;86(7):36-38. PubMed
34. Cross DA, Lin SC, Adler-Milstein J. Assessing payer perspectives on health information exchange. J Am Med Inform Assoc. 2016;23(2):297-303. PubMed
35. Centers for Medicare & Medicaid Services. MACRA: MIPS and APMs. 2016; https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Value-Based-Programs/MACRA-MIPS-and-APMs/MACRA-MIPS-and-APMs.html. Accessed September 3, 2016.
36. Raths D. Trend: standards development. Catching FHIR. A new HL7 draft standard may boost web services development in healthcare. Healthc Inform. 2014;31(2):13,16. PubMed
37. Alterovitz G, Warner J, Zhang P, et al. SMART on FHIR genomics: facilitating
The US healthcare system is highly fragmented, with patients typically receiving treatment from multiple providers during an episode of care and from many more providers over their lifetime.1,2 As patients move between care delivery settings, whether and how their information follows them is determined by a haphazard and error-prone patchwork of telephone, fax, and electronic communication channels.3 The existence of more robust electronic communication channels is often dictated by factors such as which providers share the same electronic health record (EHR) vendor rather than which providers share the highest volume of patients. As a result, providers often make clinical decisions with incomplete information, increasing the chances of misdiagnosis, unsafe or suboptimal treatment, and duplicative utilization.
Providers across the continuum of care encounter challenges to optimal clinical decision-making as a result of incomplete information. These are particularly problematic among clinicians in hospitals and emergency departments (EDs). Clinical decision-making in EDs often involves urgent and critical conditions in which decisions are made under pressure. Time constraints limit provider ability to find key clinical information to accurately diagnose and safely treat patients.4-6 Even for planned inpatient care, providers are often unfamiliar with patients, and they make safer decisions when they have full access to information from outside providers.7,8
Transitions of care between hospitals and primary care settings are also fraught with gaps in information sharing. Clinical decisions made in primary care can set patients on treatment trajectories that are greatly affected by the quality of information available to the care team at the time of initial diagnosis as well as in their subsequent treatment. Primary care physicians are not universally notified when their patients are hospitalized and may not have access to detailed information about the hospitalization, which can impair their ability to provide high quality care.9-11
Widespread and effective electronic health information exchange (HIE) holds the potential to address these challenges.3 With robust, interconnected electronic systems, key pieces of a patient’s health record can be electronically accessed and reconciled during planned and unplanned care transitions. The concept of HIE is simple—make all relevant patient data available to the clinical care team at the point of care, regardless of where that information was generated. The estimated value of nationwide interoperable EHR adoption suggests large savings from the more efficient, less duplicative, and higher quality care that likely results.12,13
There has been substantial funding and activity at federal, state, and local levels to promote the development of HIE in the US. The 2009 Health Information Technology for Economic and Clinical Health (HITECH) Act has the specific goal of accelerating adoption and use of certified EHR technology coupled with the ability to exchange clinical information to support patient care.14 The HITECH programs supported specific types of HIE that were believed to be particularly critical to improving patient care and included them in the federally-defined criteria for Meaningful Use (MU) of EHRs (ie, providers receive financial incentives for achieving specific objectives). The MU criteria evolve, moving from data capture in stage 1 to improved patient outcomes in stage 3.15 The HIE criteria focus on sending and receiving summary-of-care records during care transitions.
Despite the clear benefits of HIE and substantial support stated in policy initiatives, the spread of national HIE has been slow. Today, HIE in the US is highly heterogeneous: as a result of multiple federal-, state-, community-, enterprise- and EHR vendor-level efforts, only some provider organizations are able to engage in HIE with the other provider organizations with which they routinely share patients. In this review, we offer a framework and a corresponding set of definitions to understand the current state of HIE in the US. We describe key challenges to HIE progress and offer insights into the likely path to ensure that clinicians have routine, electronic access to patient information.
FOUR KEY DIMENSIONS OF HEALTH INFORMATION EXCHANGE
While the concept of HIE is simple—electronic access to clinical information across healthcare settings—the operationalization of HIE occurs in many different ways.16 While the terms “health information exchange” and “interoperability” are often used interchangeably, they can have different meanings. In this section, we describe 4 important dimensions that serve as a framework for understanding any given effort to enable HIE (Table).
(1) What Is Exchanged? Types of Information
The term “health information exchange” is ambiguous with respect to the type(s) of information that are accessible. Health information exchange may refer to the process of 2 providers electronically sharing a wide range of data, from a single type of information (eg, lab test results), summary of care records, to complete patient records.17 Part of this ambiguity may stem from uncertainty about the scope of information that should be shared, and how this varies based on the type of clinical encounter. For example, critical types of information in the ED setting may differ from those relevant to a primary care team after a referral. While the ability to access only particular types of information will not address all information gaps, providing access to complete patient records may result in information overload that inhibits the ability to find the subset of information relevant in a given clinical encounter.
(2) Who is Exchanging? Relationship Between Provider Organizations
The types of information accessed electronically are effectively agnostic to the relationship between the provider organizations that are sharing information. Traditionally, HIE has been considered as information that is electronically shared among 2 or more unaffiliated organizations. However, there is increasing recognition that some providers may not have electronic access to all information about their patients that exists within their organization, often after a merger or acquisition between 2 providers with different EHR systems.18,19 In these cases, a primary care team in a large integrated delivery system may have as many information gaps as a primary care team in a small, independent practice. Fulfilling clinical information needs may require both intra- and interorganizational HIE, which complicates the design of HIE processes and how the care team approaches incorporating information from both types of organizations into their decision-making. It is also important to recognize that some provider organizations, particularly small, rural practices, may not have the information technology and connectivity infrastructure required to engage in HIE.
(3) How Is Information Exchanged? Types of Electronic Access: Push vs Pull Exchange
To minimize information gaps, electronic access to information from external settings needs to offer both “push” and “pull” options. Push exchange, which can direct information electronically to a targeted recipient, works in scenarios in which there is a known information gap and known information source. The classic use for push exchange is care coordination, such as primary care physician-specialist referrals or hospital-primary care physician transitions postdischarge. Pull exchange accommodates scenarios in which there is a known information gap but the source(s) of information are unknown; it requires that clinical care teams search for and locate the clinical information that exists about the patient in external settings. Here, the classic use is emergency care in which the care team may encounter a new patient and want to retrieve records.
Widespread use of provider portals that offer view-only access into EHRs and other clinical data repositories maintained by external organizations complicate the picture. Portals are commonly used by hospitals to enable community providers to view information from a hospitalization.21 While this does not fall under the commonly held notion of HIE because no exchange occurs, portals support a pull approach to accessing information electronically among care settings that treat the same patients but use different EHRs.
Regardless of whether information is pushed or pulled, this may happen with varying degrees of human effort. This distinction gives rise to the difference between HIE and interoperability. Health information exchange reflects the ability of EHRs to exchange information, while interoperability additionally requires that EHRs be able to use exchanged information. From an operational perspective, the key distinction between HIE and interoperability is the extent of human involvement. Health information exchange requires that a human read and decide how to enter information from external settings (eg, a chart in PDF format sent between 2 EHRs), while interoperability enables the EHR that receives the information to understand the content and automatically triage or reconcile information, such as a medication list, without any human action.21 Health information exchange, therefore, relies on the diligence of the receiving clinician, while interoperability does not.
(4) What Governance Entity Defines the “Rules” of Exchange?
When more than 1 provider organization shares patient-identified data, a governance entity must specify the framework that governs the exchange. While the specifics of HIE governance vary, there are 3 predominant types of HIE networks, based on the type of organization that governs exchange: enterprise HIE networks, EHR vendor HIE networks or community HIE networks.
Enterprise HIE networks exist when 1 or more provider organizations electronically share clinical information to support patient care with some restriction, beyond geography, that dictates which organizations are involved. Typically, restrictions are driven by strategic, proprietary interests.22,23 Although broad-based information access across settings would be in the best interest of the patient, provider organizations are sensitive to the competitive implications of sharing data and may pursue such sharing in a strategic way.24 A common scenario is when hospitals choose to strategically affiliate with select ambulatory providers and exclusively exchange information with them. This should facilitate better care coordination for patients shared by the hospital and those providers but can also benefit the hospital by increasing the referrals from those providers. While there is little direct evidence quantifying the extent to which this type of strategic sharing takes place, there have been anecdotal reports as well as indirect findings that for-profit hospitals in competitive markets are less likely to share patient data.19,25
EHR vendor HIE networks exist when exchange occurs within a community of provider organizations that use an EHR from the same vendor. A subset of EHR vendors have made this capability available; EPIC’s CareEverywhere solution27 is the best-known example. Providers with an EPIC EHR are able to query for and retrieve summary of care records and other documents from any provider organization with EPIC that has activated this functionality. There are also multivendor efforts, such as CommonWell27 and the Sequoia Project’s Carequality collaborative,28 which are initiatives that seek to provide a common interoperability framework across a diverse set of stakeholders, including provider organizations with different EHR systems, in a similar fashion to HIE modules like CareEverywhere. To date, growth in these cross-vendor collaborations has been slow, and they have limited participation. While HIE networks that involve EHR vendors are likely to grow, it is difficult to predict how quickly because they are still in an early phase of development, and face nontechnical barriers such as patient consent policies that vary between providers and across states.
Community HIE networks—also referred to as health information organizations (HIOs) or regional health information organizations (RHIOs)—exist when provider organizations in a community, frequently state-level organizations that were funded through HITECH grants,14 set up the technical infrastructure and governance approach to engage in HIE to improve patient care. In contrast to enterprise or vendor HIE networks that have pursued HIE in ways that appear strategically beneficial, the only restriction on participation in community and state HIE networks is usually geography because they view information exchange as a public good. Seventyone percent of hospital service areas (HSAs) are covered by at least 1 of the 106 operational HIOs, with 309,793 clinicians (licensed prescribers) participating in those exchange networks. Even with early infusions of public and other grant-funding, community HIE networks have experienced significant challenges to sustained operation, and many have ceased operating.29
Thus, for any given provider organization, available HIE networks are primarily shaped by 3 factors:
1. Geographic location, which determines the available community and state HIE networks (as well as other basic information technology and connectivity infrastructure); providers located outside the service areas covered by an operational HIE have little incentive to participate because they do not connect them to providers with whom they share patients. Providers in rural areas may simply not have the needed infrastructure to pursue HIE.
2. Type of organization to which they belong, which determines the available enterprise HIE networks; providers who are not members of large health systems may be excluded from participation in these types of networks.
3. EHR vendor, which determines whether they have access to an EHR vendor HIE network.
ONGOING CHALLENGES
Despite agreement about the substantial potential of HIE to reduce costs and increase the quality of care delivered across a broad range of providers, HIE progress has been slow. While HITECH has successfully increased EHR adoption in hospitals and ambulatory practices,30 HIE has lagged. This is largely because many complex, intertwined barriers must be addressed for HIE to be widespread.
Lack of a Defined Goal
The cost and complexity associated with the exchange of a single type of data (eg, medications) is substantially less than the cost and complexity of sharing complete patient records. There has been little industry consensus on the target goal—do we need to enable sharing of complete patient records across all providers, or will summary of care records suffice? If the latter, as is the focus of the current MU criteria, what types of information should be included in a summary of care record, and should content and/or structure vary depending on the type of care transition? While the MU criteria require the exchange of a summary of care record with defined data fields, it remains unclear whether this is the end state or whether we should continue to push towards broad-based sharing of all patient data as structured elements. Without a clear picture of the ideal end state, there has been significant heterogeneity in the development of HIE capabilities across providers and vendors, and difficulty coordinating efforts to continue to advance towards a nationwide approach. Addressing this issue also requires progress to define HIE usability, that is, how information from external organizations should be presented and integrated into clinical workflow and clinical decisions. Currently, where HIE is occurring and clinicians are receiving summary of care records, they find them long, cluttered, and difficult to locate key information.
Numerous, Complex Barriers Spanning Multiple Stakeholders
In the context of any individual HIE effort, even after the goal is defined, there are a myriad of challenges. In a recent survey of HIO efforts, many identified the following barriers as substantially impeding their development: establishing a sustainable business model, lack of funding, integration of HIE into provider workflow, limitations of current data standards, and working with governmental policy and mandates.30 What is notable about this list is that the barriers span an array of areas, including financial incentives and identifying a sustainable business model, technical barriers such as working within the limitations of data standards, and regulatory issues such as state laws that govern the requirements for patient consent to exchange personal health information. Overcoming any of these issues is challenging, but trying to tackle all of them simultaneously clearly reveals why progress has been slow. Further, resolving many of the issues involve different groups of stakeholders. For example, implementing appropriate patient consent procedures can require engaging with and harmonizing the regulations of multiple states, as well as the Health Insurance Portability and Accountability Act (HIPAA) and regulations specific to substance abuse data.
Weak or Misaligned Incentives
Among the top barriers to HIE efforts are those related to funding and lack of a sustainable business model. This reflects the fact that economic incentives in the current market have not promoted provider engagement in HIE. Traditional fee-for-service payment structures do not reward providers for avoiding duplicative care.31 Further, hospitals perceive patient data as a “key strategic asset, tying physicians and patients to their organization,”24 and are reluctant to share data with competitors. Compounding the problem is that EHR vendors have a business interest in using HIE as a lever to increase revenue. In the short-term, they can charge high fees for interfaces and other HIE-related functionality. In the long-run, vendors may try to influence provider choice of system by making it difficult to engage in cross-vendor exchange.32 Information blocking—when providers or vendors knowingly interfere with HIE33—reflects not only weak incentives, but perverse incentives. While not all providers and vendors experience perverse incentives, the combination of weak and perverse incentives suggests the need to strengthen incentives, so that both types of stakeholders are motivated to tackle the barriers to HIE development. Key to strengthening incentives are payers, who are thought to be the largest beneficiaries of HIE. Payers have been reluctant to make significant investments in HIE without a more active voice in its implementation,34 but a shift to value-based payment may increase their engagement.
THE PATH FORWARD
Despite the continued challenges to nationwide HIE, several policy and technology developments show promise. Stage 3 meaningful use criteria continue to build on previous stages in increasing HIE requirements, raising the threshold for electronic exchange and EHR integration of summary of care documentation in patient transitions. The recently released Medicare Access and CHIP Reauthorization Act (MACRA) Merit-based Incentive Payment System (MIPS) proposed rule replaces stage 3 meaningful use for Medicare-eligible providers with advancing care information (ACI), which accounts for 25% of a provider’s overall incentive reimbursement and includes multiple HIE criteria for providers to report as part of the base and performance score, and follows a very similar framework to stage 3 MU with its criteria regarding HIE.35 While the Centers for Medicare and Medicaid Services (CMS) has not publicly declared that stage 3 MU will be replaced by ACI for hospitals and Medicaid providers, it is likely it will align those programs with the newly announced Medicare incentives.
MACRA also included changes to the Office of the National Coordinator (ONC) EHR certification program in an attempt to further encourage HIE. Vendors and providers must attest that they do not engage in information blocking and will cooperate with the Office’s surveillance programs to that effect. They also must attest that, to the greatest degree possible, their EHR systems allow for bi-directional interoperability with other providers, including those with different EHR vendors, and timely access for patients to view, download, and transmit their health data. In addition, there are emerging federal efforts to pursue a more standardized approach to patient matching and harmonize consent policies across states. These types of new policy initiatives indicate a continued interest in prioritizing HIE and interoperability.21
New technologies may also help spur HIE progress. The newest policy initiatives from CMS, including stage 3 MU and MACRA, have looked to incentivize the creation of application program interfaces (APIs), a set of publicly available tools from EHR vendors to allow developers to build applications that can directly interface with, and retrieve data from, their EHRs. While most patient access to electronic health data to date has been accomplished via patient portals, open APIs would enable developers to build an array of programs for consumers to view, download, and transmit their health data.
Even more promising is the development of the newest Health Level 7 data transmission standard, Fast Healthcare Interoperability Resources (FHIR), which promises to dramatically simplify the technical aspects of interoperability. FHIR utilizes a human-readable, easy to implement modular “resources” standard that may alleviate many technical challenges that come with implementation of an HIE system, enabling cheaper and simpler interoperability.36 A consortium of EHR vendors are working together to test these standards.28 The new FHIR standards also work in conjunction with APIs to allow easier development of consumer-facing applications37 that may empower patients to take ownership of their health data.
CONCLUSION
While HIE holds great promise to reduce the cost and improve the quality of care, progress towards a nationally interoperable health system has been slow. Simply defining HIE and what types of HIE are needed in different clinical scenarios has proven challenging. The additional challenges to implementing HIE in complex technology, legal/regulatory, governance, and incentive environment are not without solutions. Continued policy interventions, private sector collaborations, and new technologies may hold the keys to realizing the vast potential of electronic HIE.
Disclosure
Nothing to report.
The US healthcare system is highly fragmented, with patients typically receiving treatment from multiple providers during an episode of care and from many more providers over their lifetime.1,2 As patients move between care delivery settings, whether and how their information follows them is determined by a haphazard and error-prone patchwork of telephone, fax, and electronic communication channels.3 The existence of more robust electronic communication channels is often dictated by factors such as which providers share the same electronic health record (EHR) vendor rather than which providers share the highest volume of patients. As a result, providers often make clinical decisions with incomplete information, increasing the chances of misdiagnosis, unsafe or suboptimal treatment, and duplicative utilization.
Providers across the continuum of care encounter challenges to optimal clinical decision-making as a result of incomplete information. These are particularly problematic among clinicians in hospitals and emergency departments (EDs). Clinical decision-making in EDs often involves urgent and critical conditions in which decisions are made under pressure. Time constraints limit provider ability to find key clinical information to accurately diagnose and safely treat patients.4-6 Even for planned inpatient care, providers are often unfamiliar with patients, and they make safer decisions when they have full access to information from outside providers.7,8
Transitions of care between hospitals and primary care settings are also fraught with gaps in information sharing. Clinical decisions made in primary care can set patients on treatment trajectories that are greatly affected by the quality of information available to the care team at the time of initial diagnosis as well as in their subsequent treatment. Primary care physicians are not universally notified when their patients are hospitalized and may not have access to detailed information about the hospitalization, which can impair their ability to provide high quality care.9-11
Widespread and effective electronic health information exchange (HIE) holds the potential to address these challenges.3 With robust, interconnected electronic systems, key pieces of a patient’s health record can be electronically accessed and reconciled during planned and unplanned care transitions. The concept of HIE is simple—make all relevant patient data available to the clinical care team at the point of care, regardless of where that information was generated. The estimated value of nationwide interoperable EHR adoption suggests large savings from the more efficient, less duplicative, and higher quality care that likely results.12,13
There has been substantial funding and activity at federal, state, and local levels to promote the development of HIE in the US. The 2009 Health Information Technology for Economic and Clinical Health (HITECH) Act has the specific goal of accelerating adoption and use of certified EHR technology coupled with the ability to exchange clinical information to support patient care.14 The HITECH programs supported specific types of HIE that were believed to be particularly critical to improving patient care and included them in the federally-defined criteria for Meaningful Use (MU) of EHRs (ie, providers receive financial incentives for achieving specific objectives). The MU criteria evolve, moving from data capture in stage 1 to improved patient outcomes in stage 3.15 The HIE criteria focus on sending and receiving summary-of-care records during care transitions.
Despite the clear benefits of HIE and substantial support stated in policy initiatives, the spread of national HIE has been slow. Today, HIE in the US is highly heterogeneous: as a result of multiple federal-, state-, community-, enterprise- and EHR vendor-level efforts, only some provider organizations are able to engage in HIE with the other provider organizations with which they routinely share patients. In this review, we offer a framework and a corresponding set of definitions to understand the current state of HIE in the US. We describe key challenges to HIE progress and offer insights into the likely path to ensure that clinicians have routine, electronic access to patient information.
FOUR KEY DIMENSIONS OF HEALTH INFORMATION EXCHANGE
While the concept of HIE is simple—electronic access to clinical information across healthcare settings—the operationalization of HIE occurs in many different ways.16 While the terms “health information exchange” and “interoperability” are often used interchangeably, they can have different meanings. In this section, we describe 4 important dimensions that serve as a framework for understanding any given effort to enable HIE (Table).
(1) What Is Exchanged? Types of Information
The term “health information exchange” is ambiguous with respect to the type(s) of information that are accessible. Health information exchange may refer to the process of 2 providers electronically sharing a wide range of data, from a single type of information (eg, lab test results), summary of care records, to complete patient records.17 Part of this ambiguity may stem from uncertainty about the scope of information that should be shared, and how this varies based on the type of clinical encounter. For example, critical types of information in the ED setting may differ from those relevant to a primary care team after a referral. While the ability to access only particular types of information will not address all information gaps, providing access to complete patient records may result in information overload that inhibits the ability to find the subset of information relevant in a given clinical encounter.
(2) Who is Exchanging? Relationship Between Provider Organizations
The types of information accessed electronically are effectively agnostic to the relationship between the provider organizations that are sharing information. Traditionally, HIE has been considered as information that is electronically shared among 2 or more unaffiliated organizations. However, there is increasing recognition that some providers may not have electronic access to all information about their patients that exists within their organization, often after a merger or acquisition between 2 providers with different EHR systems.18,19 In these cases, a primary care team in a large integrated delivery system may have as many information gaps as a primary care team in a small, independent practice. Fulfilling clinical information needs may require both intra- and interorganizational HIE, which complicates the design of HIE processes and how the care team approaches incorporating information from both types of organizations into their decision-making. It is also important to recognize that some provider organizations, particularly small, rural practices, may not have the information technology and connectivity infrastructure required to engage in HIE.
(3) How Is Information Exchanged? Types of Electronic Access: Push vs Pull Exchange
To minimize information gaps, electronic access to information from external settings needs to offer both “push” and “pull” options. Push exchange, which can direct information electronically to a targeted recipient, works in scenarios in which there is a known information gap and known information source. The classic use for push exchange is care coordination, such as primary care physician-specialist referrals or hospital-primary care physician transitions postdischarge. Pull exchange accommodates scenarios in which there is a known information gap but the source(s) of information are unknown; it requires that clinical care teams search for and locate the clinical information that exists about the patient in external settings. Here, the classic use is emergency care in which the care team may encounter a new patient and want to retrieve records.
Widespread use of provider portals that offer view-only access into EHRs and other clinical data repositories maintained by external organizations complicate the picture. Portals are commonly used by hospitals to enable community providers to view information from a hospitalization.21 While this does not fall under the commonly held notion of HIE because no exchange occurs, portals support a pull approach to accessing information electronically among care settings that treat the same patients but use different EHRs.
Regardless of whether information is pushed or pulled, this may happen with varying degrees of human effort. This distinction gives rise to the difference between HIE and interoperability. Health information exchange reflects the ability of EHRs to exchange information, while interoperability additionally requires that EHRs be able to use exchanged information. From an operational perspective, the key distinction between HIE and interoperability is the extent of human involvement. Health information exchange requires that a human read and decide how to enter information from external settings (eg, a chart in PDF format sent between 2 EHRs), while interoperability enables the EHR that receives the information to understand the content and automatically triage or reconcile information, such as a medication list, without any human action.21 Health information exchange, therefore, relies on the diligence of the receiving clinician, while interoperability does not.
(4) What Governance Entity Defines the “Rules” of Exchange?
When more than 1 provider organization shares patient-identified data, a governance entity must specify the framework that governs the exchange. While the specifics of HIE governance vary, there are 3 predominant types of HIE networks, based on the type of organization that governs exchange: enterprise HIE networks, EHR vendor HIE networks or community HIE networks.
Enterprise HIE networks exist when 1 or more provider organizations electronically share clinical information to support patient care with some restriction, beyond geography, that dictates which organizations are involved. Typically, restrictions are driven by strategic, proprietary interests.22,23 Although broad-based information access across settings would be in the best interest of the patient, provider organizations are sensitive to the competitive implications of sharing data and may pursue such sharing in a strategic way.24 A common scenario is when hospitals choose to strategically affiliate with select ambulatory providers and exclusively exchange information with them. This should facilitate better care coordination for patients shared by the hospital and those providers but can also benefit the hospital by increasing the referrals from those providers. While there is little direct evidence quantifying the extent to which this type of strategic sharing takes place, there have been anecdotal reports as well as indirect findings that for-profit hospitals in competitive markets are less likely to share patient data.19,25
EHR vendor HIE networks exist when exchange occurs within a community of provider organizations that use an EHR from the same vendor. A subset of EHR vendors have made this capability available; EPIC’s CareEverywhere solution27 is the best-known example. Providers with an EPIC EHR are able to query for and retrieve summary of care records and other documents from any provider organization with EPIC that has activated this functionality. There are also multivendor efforts, such as CommonWell27 and the Sequoia Project’s Carequality collaborative,28 which are initiatives that seek to provide a common interoperability framework across a diverse set of stakeholders, including provider organizations with different EHR systems, in a similar fashion to HIE modules like CareEverywhere. To date, growth in these cross-vendor collaborations has been slow, and they have limited participation. While HIE networks that involve EHR vendors are likely to grow, it is difficult to predict how quickly because they are still in an early phase of development, and face nontechnical barriers such as patient consent policies that vary between providers and across states.
Community HIE networks—also referred to as health information organizations (HIOs) or regional health information organizations (RHIOs)—exist when provider organizations in a community, frequently state-level organizations that were funded through HITECH grants,14 set up the technical infrastructure and governance approach to engage in HIE to improve patient care. In contrast to enterprise or vendor HIE networks that have pursued HIE in ways that appear strategically beneficial, the only restriction on participation in community and state HIE networks is usually geography because they view information exchange as a public good. Seventyone percent of hospital service areas (HSAs) are covered by at least 1 of the 106 operational HIOs, with 309,793 clinicians (licensed prescribers) participating in those exchange networks. Even with early infusions of public and other grant-funding, community HIE networks have experienced significant challenges to sustained operation, and many have ceased operating.29
Thus, for any given provider organization, available HIE networks are primarily shaped by 3 factors:
1. Geographic location, which determines the available community and state HIE networks (as well as other basic information technology and connectivity infrastructure); providers located outside the service areas covered by an operational HIE have little incentive to participate because they do not connect them to providers with whom they share patients. Providers in rural areas may simply not have the needed infrastructure to pursue HIE.
2. Type of organization to which they belong, which determines the available enterprise HIE networks; providers who are not members of large health systems may be excluded from participation in these types of networks.
3. EHR vendor, which determines whether they have access to an EHR vendor HIE network.
ONGOING CHALLENGES
Despite agreement about the substantial potential of HIE to reduce costs and increase the quality of care delivered across a broad range of providers, HIE progress has been slow. While HITECH has successfully increased EHR adoption in hospitals and ambulatory practices,30 HIE has lagged. This is largely because many complex, intertwined barriers must be addressed for HIE to be widespread.
Lack of a Defined Goal
The cost and complexity associated with the exchange of a single type of data (eg, medications) is substantially less than the cost and complexity of sharing complete patient records. There has been little industry consensus on the target goal—do we need to enable sharing of complete patient records across all providers, or will summary of care records suffice? If the latter, as is the focus of the current MU criteria, what types of information should be included in a summary of care record, and should content and/or structure vary depending on the type of care transition? While the MU criteria require the exchange of a summary of care record with defined data fields, it remains unclear whether this is the end state or whether we should continue to push towards broad-based sharing of all patient data as structured elements. Without a clear picture of the ideal end state, there has been significant heterogeneity in the development of HIE capabilities across providers and vendors, and difficulty coordinating efforts to continue to advance towards a nationwide approach. Addressing this issue also requires progress to define HIE usability, that is, how information from external organizations should be presented and integrated into clinical workflow and clinical decisions. Currently, where HIE is occurring and clinicians are receiving summary of care records, they find them long, cluttered, and difficult to locate key information.
Numerous, Complex Barriers Spanning Multiple Stakeholders
In the context of any individual HIE effort, even after the goal is defined, there are a myriad of challenges. In a recent survey of HIO efforts, many identified the following barriers as substantially impeding their development: establishing a sustainable business model, lack of funding, integration of HIE into provider workflow, limitations of current data standards, and working with governmental policy and mandates.30 What is notable about this list is that the barriers span an array of areas, including financial incentives and identifying a sustainable business model, technical barriers such as working within the limitations of data standards, and regulatory issues such as state laws that govern the requirements for patient consent to exchange personal health information. Overcoming any of these issues is challenging, but trying to tackle all of them simultaneously clearly reveals why progress has been slow. Further, resolving many of the issues involve different groups of stakeholders. For example, implementing appropriate patient consent procedures can require engaging with and harmonizing the regulations of multiple states, as well as the Health Insurance Portability and Accountability Act (HIPAA) and regulations specific to substance abuse data.
Weak or Misaligned Incentives
Among the top barriers to HIE efforts are those related to funding and lack of a sustainable business model. This reflects the fact that economic incentives in the current market have not promoted provider engagement in HIE. Traditional fee-for-service payment structures do not reward providers for avoiding duplicative care.31 Further, hospitals perceive patient data as a “key strategic asset, tying physicians and patients to their organization,”24 and are reluctant to share data with competitors. Compounding the problem is that EHR vendors have a business interest in using HIE as a lever to increase revenue. In the short-term, they can charge high fees for interfaces and other HIE-related functionality. In the long-run, vendors may try to influence provider choice of system by making it difficult to engage in cross-vendor exchange.32 Information blocking—when providers or vendors knowingly interfere with HIE33—reflects not only weak incentives, but perverse incentives. While not all providers and vendors experience perverse incentives, the combination of weak and perverse incentives suggests the need to strengthen incentives, so that both types of stakeholders are motivated to tackle the barriers to HIE development. Key to strengthening incentives are payers, who are thought to be the largest beneficiaries of HIE. Payers have been reluctant to make significant investments in HIE without a more active voice in its implementation,34 but a shift to value-based payment may increase their engagement.
THE PATH FORWARD
Despite the continued challenges to nationwide HIE, several policy and technology developments show promise. Stage 3 meaningful use criteria continue to build on previous stages in increasing HIE requirements, raising the threshold for electronic exchange and EHR integration of summary of care documentation in patient transitions. The recently released Medicare Access and CHIP Reauthorization Act (MACRA) Merit-based Incentive Payment System (MIPS) proposed rule replaces stage 3 meaningful use for Medicare-eligible providers with advancing care information (ACI), which accounts for 25% of a provider’s overall incentive reimbursement and includes multiple HIE criteria for providers to report as part of the base and performance score, and follows a very similar framework to stage 3 MU with its criteria regarding HIE.35 While the Centers for Medicare and Medicaid Services (CMS) has not publicly declared that stage 3 MU will be replaced by ACI for hospitals and Medicaid providers, it is likely it will align those programs with the newly announced Medicare incentives.
MACRA also included changes to the Office of the National Coordinator (ONC) EHR certification program in an attempt to further encourage HIE. Vendors and providers must attest that they do not engage in information blocking and will cooperate with the Office’s surveillance programs to that effect. They also must attest that, to the greatest degree possible, their EHR systems allow for bi-directional interoperability with other providers, including those with different EHR vendors, and timely access for patients to view, download, and transmit their health data. In addition, there are emerging federal efforts to pursue a more standardized approach to patient matching and harmonize consent policies across states. These types of new policy initiatives indicate a continued interest in prioritizing HIE and interoperability.21
New technologies may also help spur HIE progress. The newest policy initiatives from CMS, including stage 3 MU and MACRA, have looked to incentivize the creation of application program interfaces (APIs), a set of publicly available tools from EHR vendors to allow developers to build applications that can directly interface with, and retrieve data from, their EHRs. While most patient access to electronic health data to date has been accomplished via patient portals, open APIs would enable developers to build an array of programs for consumers to view, download, and transmit their health data.
Even more promising is the development of the newest Health Level 7 data transmission standard, Fast Healthcare Interoperability Resources (FHIR), which promises to dramatically simplify the technical aspects of interoperability. FHIR utilizes a human-readable, easy to implement modular “resources” standard that may alleviate many technical challenges that come with implementation of an HIE system, enabling cheaper and simpler interoperability.36 A consortium of EHR vendors are working together to test these standards.28 The new FHIR standards also work in conjunction with APIs to allow easier development of consumer-facing applications37 that may empower patients to take ownership of their health data.
CONCLUSION
While HIE holds great promise to reduce the cost and improve the quality of care, progress towards a nationally interoperable health system has been slow. Simply defining HIE and what types of HIE are needed in different clinical scenarios has proven challenging. The additional challenges to implementing HIE in complex technology, legal/regulatory, governance, and incentive environment are not without solutions. Continued policy interventions, private sector collaborations, and new technologies may hold the keys to realizing the vast potential of electronic HIE.
Disclosure
Nothing to report.
1. Pham HH, Schrag D, O’Malley AS, Wu B, Bach PB. Care patterns in Medicare and their implications for pay for performance. N Engl J Med. 2007;356(11):1130-1139. PubMed
2. Finnell JT, Overhage JM, Dexter PR, Perkins SM, Lane KA, McDonald CJ. Community clinical data exchange for emergency medicine patients. Paper presented at: AMIA Annual Symposium Proceedings 2003. PubMed
3. Bodenheimer T. Coordinating care-a perilous journey through the health care system. N Engl J Med. 2008;358(10):1064-1071. PubMed
4. Franczak MJ, Klein M, Raslau F, Bergholte J, Mark LP, Ulmer JL. In emergency departments, radiologists’ access to EHRs may influence interpretations and medical management. Health Aff (Millwood). 2014;33(5):800-806. PubMed
5. Shapiro JS, Kannry J, Kushniruk AW, Kuperman G; New York Clinical Information Exchange (NYCLIX) Clinical Advisory Subcommittee. Emergency physicians’ perceptions of health information exchange. J Am Med Inform Assoc. 2007;14(6):700-705. PubMed
6. Shapiro JS, Kannry J, Lipton M, et al. Approaches to patient health information exchange and their impact on emergency medicine. Ann Emerg Med. 2006;48(4):426-432. PubMed
7. Sutcliffe KM, Lewton E, Rosenthal MM. Communication failures: an insidious contributor to medical mishaps. Acad Med.. 2004;79(2):186-194. PubMed
8. Kaelber DC, Bates DW. Health information exchange and patient safety. J Biomed Inform. 2007;40(suppl 6):S40-S45. PubMed
9. Smith PC, Araya-Guerra R, Bublitz C, et al. MIssing clinical information during primary care visits. JAMA. 2005;293(5):565-571. PubMed
10. Bell CM, Schnipper JL, Auerbach AD, et al. Association of communication between hospital-based physicians and primary care providers with patient outcomes. J Gen Intern Med. 2009;24(3):381-386. PubMed
11. van Walraven C, Taljaard M, Bell CM, et al. A prospective cohort study found that provider and information continuity was low after patient discharge from hospital. J Clin Epidemiol. 2010;63(9):1000-1010. PubMed
12. Walker J, Pan E, Johnston D, Adler-Milstein J, Bates DW, Middleton B. The value of health care information exchange and interoperability. Health Aff (Millwood). 2005:(suppl)W5-10-W5-18. PubMed
13. Shekelle PG, Morton SC, Keeler EB. Costs and benefits of health information technology. Evid Rep Technol Assess (Full Rep). 2006;132:1-71. PubMed
14. Blumenthal D. Launching HITECH. N Engl J Med. 2010;362(5):382-385. PubMed
15. Blumenthal D, Tavenner M. The “meaningful use” regulation for electronic health records. N Engl J Med. 2010;363(6):501-504. PubMed
16. Kuperman G, McGowan J. Potential unintended consequences of health information exchange. J Gen Intern Med. 2013;28(12):1663-1666. PubMed
17. Mathematica Policy Research and Harvard School of Public Health. DesRoches CM, Painter MW, Jha AK, eds. Health Information Technology in the United States, 2015: Transition to a Post-HITECH World (Executive Summary). September 18, 2015. Princeton, NJ: Robert Wood Johnson Foundation; 2015.
18. O’Malley AS, Anglin G, Bond AM, Cunningham PJ, Stark LB, Yee T. Greenville & Spartanburg: Surging Hospital Employment of Physicians Poses Opportunities and Challenges. Washington, DC: Center for Studying Health System Change (HSC); February 2011. 6.
19. Katz A, Bond AM, Carrier E, Docteur E, Quach CW, Yee T. Cleveland Hospital Systems Expand Despite Weak Economy. Washington, DC: Center for Studying Health System Change (HSC); September 2010. 2.
20. Grossman JM, Bodenheimer TS, McKenzie K. Hospital-physician portals: the role of competition in driving clinical data exchange. Health Aff (Millwood). 2006;25(6):1629-1636. PubMed
21. De Salvo KB, Galvez E. Connecting Health and Care for the Nation A Shared Nationwide Interoperability Roadmap - Version 1.0. In: Office of the National Coordinator for Health Information Technology. ed 2015. https://www.healthit.gov/buzz-blog/electronic-health-and-medical-records/interoperability-electronic-health-and-medical-records/connecting-health-care-nation-shared-nationwide-interoperability-roadmap-version-10/. Accessed September 3, 2016.
22. Adler-Milstein J, DesRoches C, Jha AK. Health information exchange among US hospitals. Am J Manag Care. 2011;17(11):761-768. PubMed
23. Vest JR. More than just a question of technology: factors related to hospitals’ adoption and implementation of health information exchange. Int J Med Inform. 2010;79(12):797-806. PubMed
24. Grossman JM, Kushner KL, November EA. Creating sustainable local health information exchanges: can barriers to stakeholder participation be overcome? Res Brief. 2008;2:1-12. PubMed
25. Grossman JM, Cohen G. Despite regulatory changes, hospitals cautious in helping physicians purchase electronic medical records. Issue Brief Cent Stud Health Syst Change 2008;123:1-4. PubMed
26. Kaelber DC, Waheed R, Einstadter D, Love TE, Cebul RD. Use and perceived value of health information exchange: one public healthcare system’s experience. Am J Manag Care. 2013;19(10 spec no):SP337-SP343. PubMed
27. Commonwell Health Alliance. http://www.commonwellalliance.org/, 2016. Accessed September 3, 2016.
28. Carequality. http://sequoiaproject.org/carequality/, 2016. Accessed September 3, 2016.
29. Adler-Milstein J, Lin SC, Jha AK. The number of health information exchange efforts is declining, leaving the viability of broad clinical data exchange uncertain. Health Aff (Millwood). 2016;35(7):1278-1285. PubMed
30. Adler-Milstein J, DesRoches CM, Kralovec P, et al. Electronic health record adoption in US hospitals: progress continues, but challenges persist. Health Aff (Millwood). 2015:34(12):2174-2180. PubMed
31. Health IT Policy Committee Report to Congress: Challenges and Barriers to Interoperability. 2015. https://www.healthit.gov/facas/health-it-policy-committee/health-it-policy-committee-recommendations-national-coordinator-health-it. Accessed September 3, 2016.
32. Everson J, Adler-Milstein J. Engagement in hospital health information exchange is associated with vendor marketplace dominance. Health Aff (MIllwood). 2016;35(7):1286-1293. PubMed
33. Downing K, Mason J. ONC targets information blocking. J AHIMA. 2015;86(7):36-38. PubMed
34. Cross DA, Lin SC, Adler-Milstein J. Assessing payer perspectives on health information exchange. J Am Med Inform Assoc. 2016;23(2):297-303. PubMed
35. Centers for Medicare & Medicaid Services. MACRA: MIPS and APMs. 2016; https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Value-Based-Programs/MACRA-MIPS-and-APMs/MACRA-MIPS-and-APMs.html. Accessed September 3, 2016.
36. Raths D. Trend: standards development. Catching FHIR. A new HL7 draft standard may boost web services development in healthcare. Healthc Inform. 2014;31(2):13,16. PubMed
37. Alterovitz G, Warner J, Zhang P, et al. SMART on FHIR genomics: facilitating
1. Pham HH, Schrag D, O’Malley AS, Wu B, Bach PB. Care patterns in Medicare and their implications for pay for performance. N Engl J Med. 2007;356(11):1130-1139. PubMed
2. Finnell JT, Overhage JM, Dexter PR, Perkins SM, Lane KA, McDonald CJ. Community clinical data exchange for emergency medicine patients. Paper presented at: AMIA Annual Symposium Proceedings 2003. PubMed
3. Bodenheimer T. Coordinating care-a perilous journey through the health care system. N Engl J Med. 2008;358(10):1064-1071. PubMed
4. Franczak MJ, Klein M, Raslau F, Bergholte J, Mark LP, Ulmer JL. In emergency departments, radiologists’ access to EHRs may influence interpretations and medical management. Health Aff (Millwood). 2014;33(5):800-806. PubMed
5. Shapiro JS, Kannry J, Kushniruk AW, Kuperman G; New York Clinical Information Exchange (NYCLIX) Clinical Advisory Subcommittee. Emergency physicians’ perceptions of health information exchange. J Am Med Inform Assoc. 2007;14(6):700-705. PubMed
6. Shapiro JS, Kannry J, Lipton M, et al. Approaches to patient health information exchange and their impact on emergency medicine. Ann Emerg Med. 2006;48(4):426-432. PubMed
7. Sutcliffe KM, Lewton E, Rosenthal MM. Communication failures: an insidious contributor to medical mishaps. Acad Med.. 2004;79(2):186-194. PubMed
8. Kaelber DC, Bates DW. Health information exchange and patient safety. J Biomed Inform. 2007;40(suppl 6):S40-S45. PubMed
9. Smith PC, Araya-Guerra R, Bublitz C, et al. MIssing clinical information during primary care visits. JAMA. 2005;293(5):565-571. PubMed
10. Bell CM, Schnipper JL, Auerbach AD, et al. Association of communication between hospital-based physicians and primary care providers with patient outcomes. J Gen Intern Med. 2009;24(3):381-386. PubMed
11. van Walraven C, Taljaard M, Bell CM, et al. A prospective cohort study found that provider and information continuity was low after patient discharge from hospital. J Clin Epidemiol. 2010;63(9):1000-1010. PubMed
12. Walker J, Pan E, Johnston D, Adler-Milstein J, Bates DW, Middleton B. The value of health care information exchange and interoperability. Health Aff (Millwood). 2005:(suppl)W5-10-W5-18. PubMed
13. Shekelle PG, Morton SC, Keeler EB. Costs and benefits of health information technology. Evid Rep Technol Assess (Full Rep). 2006;132:1-71. PubMed
14. Blumenthal D. Launching HITECH. N Engl J Med. 2010;362(5):382-385. PubMed
15. Blumenthal D, Tavenner M. The “meaningful use” regulation for electronic health records. N Engl J Med. 2010;363(6):501-504. PubMed
16. Kuperman G, McGowan J. Potential unintended consequences of health information exchange. J Gen Intern Med. 2013;28(12):1663-1666. PubMed
17. Mathematica Policy Research and Harvard School of Public Health. DesRoches CM, Painter MW, Jha AK, eds. Health Information Technology in the United States, 2015: Transition to a Post-HITECH World (Executive Summary). September 18, 2015. Princeton, NJ: Robert Wood Johnson Foundation; 2015.
18. O’Malley AS, Anglin G, Bond AM, Cunningham PJ, Stark LB, Yee T. Greenville & Spartanburg: Surging Hospital Employment of Physicians Poses Opportunities and Challenges. Washington, DC: Center for Studying Health System Change (HSC); February 2011. 6.
19. Katz A, Bond AM, Carrier E, Docteur E, Quach CW, Yee T. Cleveland Hospital Systems Expand Despite Weak Economy. Washington, DC: Center for Studying Health System Change (HSC); September 2010. 2.
20. Grossman JM, Bodenheimer TS, McKenzie K. Hospital-physician portals: the role of competition in driving clinical data exchange. Health Aff (Millwood). 2006;25(6):1629-1636. PubMed
21. De Salvo KB, Galvez E. Connecting Health and Care for the Nation A Shared Nationwide Interoperability Roadmap - Version 1.0. In: Office of the National Coordinator for Health Information Technology. ed 2015. https://www.healthit.gov/buzz-blog/electronic-health-and-medical-records/interoperability-electronic-health-and-medical-records/connecting-health-care-nation-shared-nationwide-interoperability-roadmap-version-10/. Accessed September 3, 2016.
22. Adler-Milstein J, DesRoches C, Jha AK. Health information exchange among US hospitals. Am J Manag Care. 2011;17(11):761-768. PubMed
23. Vest JR. More than just a question of technology: factors related to hospitals’ adoption and implementation of health information exchange. Int J Med Inform. 2010;79(12):797-806. PubMed
24. Grossman JM, Kushner KL, November EA. Creating sustainable local health information exchanges: can barriers to stakeholder participation be overcome? Res Brief. 2008;2:1-12. PubMed
25. Grossman JM, Cohen G. Despite regulatory changes, hospitals cautious in helping physicians purchase electronic medical records. Issue Brief Cent Stud Health Syst Change 2008;123:1-4. PubMed
26. Kaelber DC, Waheed R, Einstadter D, Love TE, Cebul RD. Use and perceived value of health information exchange: one public healthcare system’s experience. Am J Manag Care. 2013;19(10 spec no):SP337-SP343. PubMed
27. Commonwell Health Alliance. http://www.commonwellalliance.org/, 2016. Accessed September 3, 2016.
28. Carequality. http://sequoiaproject.org/carequality/, 2016. Accessed September 3, 2016.
29. Adler-Milstein J, Lin SC, Jha AK. The number of health information exchange efforts is declining, leaving the viability of broad clinical data exchange uncertain. Health Aff (Millwood). 2016;35(7):1278-1285. PubMed
30. Adler-Milstein J, DesRoches CM, Kralovec P, et al. Electronic health record adoption in US hospitals: progress continues, but challenges persist. Health Aff (Millwood). 2015:34(12):2174-2180. PubMed
31. Health IT Policy Committee Report to Congress: Challenges and Barriers to Interoperability. 2015. https://www.healthit.gov/facas/health-it-policy-committee/health-it-policy-committee-recommendations-national-coordinator-health-it. Accessed September 3, 2016.
32. Everson J, Adler-Milstein J. Engagement in hospital health information exchange is associated with vendor marketplace dominance. Health Aff (MIllwood). 2016;35(7):1286-1293. PubMed
33. Downing K, Mason J. ONC targets information blocking. J AHIMA. 2015;86(7):36-38. PubMed
34. Cross DA, Lin SC, Adler-Milstein J. Assessing payer perspectives on health information exchange. J Am Med Inform Assoc. 2016;23(2):297-303. PubMed
35. Centers for Medicare & Medicaid Services. MACRA: MIPS and APMs. 2016; https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Value-Based-Programs/MACRA-MIPS-and-APMs/MACRA-MIPS-and-APMs.html. Accessed September 3, 2016.
36. Raths D. Trend: standards development. Catching FHIR. A new HL7 draft standard may boost web services development in healthcare. Healthc Inform. 2014;31(2):13,16. PubMed
37. Alterovitz G, Warner J, Zhang P, et al. SMART on FHIR genomics: facilitating
© 2017 Society of Hospital Medicine
Medicare and the 3-inpatient midnight requirement: A statute in need of modernization
On July 30, 1965, Lyndon B. Johnson signed H.R. 6675 into law, establishing Medicare and Medicaid as Title XVIII and Title XIX of the Social Security Act.1 Shortly after, Medicare’s “extended care benefit” began, offering Medicare beneficiaries skilled nursing facility (SNF) care after a qualifying stay of 3 or more consecutive inpatient midnights.2 Fifty years later, the word “inpatient” remains embedded in statute, limiting SNF coverage for Medicare beneficiaries hospitalized as outpatients under observation for part or all of a 3-midnight stay.3
At the individual Medicare beneficiary level, the financial impact of this policy is clear. The Office of Inspector General (OIG) reported a $10,503 beneficiary out-of-pocket cost per uncovered SNF stay following an observation hospitalization in 2012.4 But the actual number of Medicare beneficiaries impacted by this coverage gap is unknown. Using 2009 claims data, Feng et al.5 estimated that 0.75% of previously community dwelling Medicare beneficiaries are discharged to a SNF following an observation hospitalization, and the OIG reported 617,702 beneficiary hospital stays of 3 or more midnights not meeting the 3-midnight inpatient requirement in 2012, with 4% of these beneficiaries discharging to SNFs.4 Yet these studies based on Medicare claims data only capture actual SNF utilization, failing to answer the critical question: How many Medicare beneficiaries need, but forgo, SNF care following a non-qualifying observation hospital stay? In this issue of the Journal of Hospital Medicine, Goldstein et al.6 provide insight to that question. Using chart review of physical therapy and case management recommendations for post-acute SNF care, Goldstein et al.6 compare actual discharge rate to SNF or acute inpatient rehabilitation following an observation stay when such disposition is recommended. In their two-hospital system, fewer than 20% of previously community-dwelling hospitalist patients followed recommendation for post-acute facility stay after observation hospitalization, and more than 40% cited financial concerns as the reason for declining. Patients recommended for SNF also were more likely to be rehospitalized in the subsequent 30 days after discharge, confirming this as a vulnerable patient population. Given Medicare’s original intent to improve health care access for seniors, the case for change seems clear, and the repercussions of not addressing the plight of patients hospitalized under observation is having negative financial and overall detrimental health impacts.
But there are other compelling reasons why this 50-year-old law needs to be improved. Hospital care today is vastly different than when Medicare became law. Average hospital length of stay for patients 65 years and older was 14.2 days in 19657 compared to 5.2 days today,8 clearly a shift in what 3 days of hospital care means. Most importantly, observation stays have become a major part of hospital care. Between 2006 and 2014, per-beneficiary outpatient visits (which include all observation stays) increased 44.2% nationally, while inpatient discharges decreased 19.9%.9 In 2012, the Centers for Medicare & Medicaid Services (CMS) received 1.7 million outpatient observation claims and an additional 700,000 inpatient claims that started with observation days.10 CMS also expected the 2-midnight rule to reduce outpatient observation stays,4 but a recent OIG report11 found that outpatient stays increased 8.1% in the first year (FY 2014) under the new rule, and there were still 748,337 long observation stays (those lasting 2 midnights or longer) in 2014, only a small (2.8%) decrease from the prior year. These factors limit Medicare beneficiary post–acute SNF eligibility in ways that could not have been anticipated when the extended care benefit was created to help seniors access needed health care.
Policymakers must consider cost when considering statutory change. Waiver programs in the 1980s suspending the 3-midnight requirement raised concerns over potential increase in both SNF utilization and associated costs.12 However, more recent data suggest that altering the 3-midnight requirement may not increase post-acute SNF utilization. From 2006 to 2010, Medicare Advantage programs that waived the 3-midnight requirement saw a decrease in hospital length of stay without increased SNF utilization or SNF length of stay, indicating that access to the right level of care at the right time could be cost-saving.13 Recent data from the Bundled Payments for Care Improvement (BPCI) program found savings were largely related to decreased SNF utilization when payments were episode-based,14 a trend that may continue as Medicare moves away from fee-for-service towards bundled payments for more conditions. And although neither example directly tests changing the 3-midnight requirement to include observation midnights, both studies suggest that innovative health care delivery and modification of SNF access did not result in increased SNF utilization or greater post-acute costs. In fact, as Goldstein et al.6 showed, patients recommended for post-acute SNF following observation stay were more likely to be rehospitalized within 30 days, an additional cost that could potentially be avoided if these patients had SNF access. We believe that these correlations strongly support rescinding the 3-
That being said, what can be done? In 2015, the Medicare Payment Advisory Commission (MedPAC) recommended changing the 3-night requirement to require just one of 3 midnights to be inpatient to make a qualifying stay.10 Although an improvement over current law, this proposal would not help the majority of beneficiaries who are exclusively hospitalized under observation status. The “Improving Access to Medicare Coverage Act of 2015”, to be reintroduced in Congress in the coming weeks, would count any midnight spent in the hospital towards the 3-midnight stay requirement, and has bipartisan, bicameral support and cosponsorship.15 In 2015, through unanimous bipartisan, bicameral support, Congress passed the NOTICE Act (PL 114-42), which requires hospitals to inform Medicare beneficiaries hospitalized under observation.16 We believe that the data are clear to both sides of the aisle that Congress should now work together using scientifically-supported research to improve the exact observation policies they felt patients should be informed of. Passing the Improving Access to Medicare Coverage Act is the logical next step in this arena.
Medicare was intended to give seniors access to the healthcare they need. Growth in hospital-based observation care begs for modernization of the statutory 3-inpatient midnight rule. Counting all midnights towards the 3-midnight requirement, whether those midnights are outpatient observation or inpatient, is the right first step.
Disclosures
Representative Courtney is the bill sponsor of the Improving Access to Medicare Coverage Act. The authors report no other conflicts.
1. Medicare & Medicaid Milestones 1937-2015. https://www.cms.gov/About-CMS/Agency-Information/History/Downloads/Medicare-and-Medicaid-Milestones-1937-2015.pdf . Accessed September 25, 2016.
2. Loewenstein R. Early effects of Medicare on the health care of the aged. https://www.ssa.gov/policy/docs/ssb/v34n4/v34n4p3.pdf. Accessed September 25, 2016.
3. US Social Security Act, Sec. 1861 (i). [42 U.S.C. 1395x]. https://www.ssa.gov/OP_Home/ssact/title18/1861.htm. Accessed September 25, 2016.
4. Department of Health and Human Services Office of Inspector General. Hospitals’ use of observation stays and short inpatient stays for Medicare beneficiaries, OEI-02-12-00040. Available at: https://oig.hhs.gov/oei/reports/oei-02-12-00040.pdf. Accessed September 25, 2016.
5. Feng Z, Jung H-Y, Wright B, Mor V. The origin and disposition of Medicare observation stays. Med Care 2014;52:796-800. PubMed
6. Goldstein JN, Schwartz JS, McGraw P, Banks TL, Hicks LS. The unmet need for postacute rehabilitation among medicare observation patients: a single-center study. J Hosp Med. 2017;12(3):168-172.
7. Vital and Health Statistics. Trends in hospital utilization: United States, 1965-1986. https://www.cdc.gov/nchs/data/series/sr_13/sr13_101.pdf. Accessed September 25, 2016.
8. Healthcare Cost and Utilization Project (HCUP). Statistical brief #180. Overview of hospital stays in the United States, 2012. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb180-Hospitalizations-United-States-2012.pdf. Accessed September 25, 2016.
9. MedPAC March 2016 Report to the Congress. Chapter 3. Hospital inpatient and outpatient services. http://www.medpac.gov/docs/default-source/reports/march-2016-report-to-the-congress-medicare-payment-policy.pdf?sfvrsn=0. Accessed September 25, 2016.
10. MedPAC. June 2015 Report to the Congress. Chapter 7: Hospital short-stay policy issues. http://www.medpac.gov/docs/default-source/reports/chapter-7-hospital-short-stay-policy-issues-june-2015-report-.pdf?sfvrsn=0 Accessed September 25, 2016.
11. Department of Health and Human Services Office of Inspector General. Vulnerabilities remain under Medicare’s 2-midnight hospital policy, OEI-02-15-00020. https://oig.hhs.gov/oei/reports/oei-02-15-00020.pdf. Accessed February 19, 2017.
12. Lipsitz L. The 3-night hospital stay and Medicare coverage for skilled nursing care. JAMA. 2013;310: 1441-1442. PubMed
13. Grebela R, Keohane L Lee Y, Lipsitz L, Rahman M, Trevedi A. Waiving the three-day rule: admissions and length-of-stay at hospitals and skilled nursing facilities did not increase. Health Affairs. 2015;34:1324-1330. PubMed
14. Dummit L, Kahvecioglu D, Marrufo G, et al. Association between hospital participation in a Medicare bundled payment initiative and payments and quality outcomes for lower extremity joint replacement episodes. JAMA. 2016;316(12):1267-1278. PubMed
15. HR. 1571 Improving Access to Medicare Coverage Act of 2015. https://www.govtrack.us/congress/bills/114/hr1571/text. Accessed September 25, 2016.
16. PL 114-42. The NOTICE Act. https://www.govtrack.us/congress/bills/114/hr876. Accessed September 25, 2016.
On July 30, 1965, Lyndon B. Johnson signed H.R. 6675 into law, establishing Medicare and Medicaid as Title XVIII and Title XIX of the Social Security Act.1 Shortly after, Medicare’s “extended care benefit” began, offering Medicare beneficiaries skilled nursing facility (SNF) care after a qualifying stay of 3 or more consecutive inpatient midnights.2 Fifty years later, the word “inpatient” remains embedded in statute, limiting SNF coverage for Medicare beneficiaries hospitalized as outpatients under observation for part or all of a 3-midnight stay.3
At the individual Medicare beneficiary level, the financial impact of this policy is clear. The Office of Inspector General (OIG) reported a $10,503 beneficiary out-of-pocket cost per uncovered SNF stay following an observation hospitalization in 2012.4 But the actual number of Medicare beneficiaries impacted by this coverage gap is unknown. Using 2009 claims data, Feng et al.5 estimated that 0.75% of previously community dwelling Medicare beneficiaries are discharged to a SNF following an observation hospitalization, and the OIG reported 617,702 beneficiary hospital stays of 3 or more midnights not meeting the 3-midnight inpatient requirement in 2012, with 4% of these beneficiaries discharging to SNFs.4 Yet these studies based on Medicare claims data only capture actual SNF utilization, failing to answer the critical question: How many Medicare beneficiaries need, but forgo, SNF care following a non-qualifying observation hospital stay? In this issue of the Journal of Hospital Medicine, Goldstein et al.6 provide insight to that question. Using chart review of physical therapy and case management recommendations for post-acute SNF care, Goldstein et al.6 compare actual discharge rate to SNF or acute inpatient rehabilitation following an observation stay when such disposition is recommended. In their two-hospital system, fewer than 20% of previously community-dwelling hospitalist patients followed recommendation for post-acute facility stay after observation hospitalization, and more than 40% cited financial concerns as the reason for declining. Patients recommended for SNF also were more likely to be rehospitalized in the subsequent 30 days after discharge, confirming this as a vulnerable patient population. Given Medicare’s original intent to improve health care access for seniors, the case for change seems clear, and the repercussions of not addressing the plight of patients hospitalized under observation is having negative financial and overall detrimental health impacts.
But there are other compelling reasons why this 50-year-old law needs to be improved. Hospital care today is vastly different than when Medicare became law. Average hospital length of stay for patients 65 years and older was 14.2 days in 19657 compared to 5.2 days today,8 clearly a shift in what 3 days of hospital care means. Most importantly, observation stays have become a major part of hospital care. Between 2006 and 2014, per-beneficiary outpatient visits (which include all observation stays) increased 44.2% nationally, while inpatient discharges decreased 19.9%.9 In 2012, the Centers for Medicare & Medicaid Services (CMS) received 1.7 million outpatient observation claims and an additional 700,000 inpatient claims that started with observation days.10 CMS also expected the 2-midnight rule to reduce outpatient observation stays,4 but a recent OIG report11 found that outpatient stays increased 8.1% in the first year (FY 2014) under the new rule, and there were still 748,337 long observation stays (those lasting 2 midnights or longer) in 2014, only a small (2.8%) decrease from the prior year. These factors limit Medicare beneficiary post–acute SNF eligibility in ways that could not have been anticipated when the extended care benefit was created to help seniors access needed health care.
Policymakers must consider cost when considering statutory change. Waiver programs in the 1980s suspending the 3-midnight requirement raised concerns over potential increase in both SNF utilization and associated costs.12 However, more recent data suggest that altering the 3-midnight requirement may not increase post-acute SNF utilization. From 2006 to 2010, Medicare Advantage programs that waived the 3-midnight requirement saw a decrease in hospital length of stay without increased SNF utilization or SNF length of stay, indicating that access to the right level of care at the right time could be cost-saving.13 Recent data from the Bundled Payments for Care Improvement (BPCI) program found savings were largely related to decreased SNF utilization when payments were episode-based,14 a trend that may continue as Medicare moves away from fee-for-service towards bundled payments for more conditions. And although neither example directly tests changing the 3-midnight requirement to include observation midnights, both studies suggest that innovative health care delivery and modification of SNF access did not result in increased SNF utilization or greater post-acute costs. In fact, as Goldstein et al.6 showed, patients recommended for post-acute SNF following observation stay were more likely to be rehospitalized within 30 days, an additional cost that could potentially be avoided if these patients had SNF access. We believe that these correlations strongly support rescinding the 3-
That being said, what can be done? In 2015, the Medicare Payment Advisory Commission (MedPAC) recommended changing the 3-night requirement to require just one of 3 midnights to be inpatient to make a qualifying stay.10 Although an improvement over current law, this proposal would not help the majority of beneficiaries who are exclusively hospitalized under observation status. The “Improving Access to Medicare Coverage Act of 2015”, to be reintroduced in Congress in the coming weeks, would count any midnight spent in the hospital towards the 3-midnight stay requirement, and has bipartisan, bicameral support and cosponsorship.15 In 2015, through unanimous bipartisan, bicameral support, Congress passed the NOTICE Act (PL 114-42), which requires hospitals to inform Medicare beneficiaries hospitalized under observation.16 We believe that the data are clear to both sides of the aisle that Congress should now work together using scientifically-supported research to improve the exact observation policies they felt patients should be informed of. Passing the Improving Access to Medicare Coverage Act is the logical next step in this arena.
Medicare was intended to give seniors access to the healthcare they need. Growth in hospital-based observation care begs for modernization of the statutory 3-inpatient midnight rule. Counting all midnights towards the 3-midnight requirement, whether those midnights are outpatient observation or inpatient, is the right first step.
Disclosures
Representative Courtney is the bill sponsor of the Improving Access to Medicare Coverage Act. The authors report no other conflicts.
On July 30, 1965, Lyndon B. Johnson signed H.R. 6675 into law, establishing Medicare and Medicaid as Title XVIII and Title XIX of the Social Security Act.1 Shortly after, Medicare’s “extended care benefit” began, offering Medicare beneficiaries skilled nursing facility (SNF) care after a qualifying stay of 3 or more consecutive inpatient midnights.2 Fifty years later, the word “inpatient” remains embedded in statute, limiting SNF coverage for Medicare beneficiaries hospitalized as outpatients under observation for part or all of a 3-midnight stay.3
At the individual Medicare beneficiary level, the financial impact of this policy is clear. The Office of Inspector General (OIG) reported a $10,503 beneficiary out-of-pocket cost per uncovered SNF stay following an observation hospitalization in 2012.4 But the actual number of Medicare beneficiaries impacted by this coverage gap is unknown. Using 2009 claims data, Feng et al.5 estimated that 0.75% of previously community dwelling Medicare beneficiaries are discharged to a SNF following an observation hospitalization, and the OIG reported 617,702 beneficiary hospital stays of 3 or more midnights not meeting the 3-midnight inpatient requirement in 2012, with 4% of these beneficiaries discharging to SNFs.4 Yet these studies based on Medicare claims data only capture actual SNF utilization, failing to answer the critical question: How many Medicare beneficiaries need, but forgo, SNF care following a non-qualifying observation hospital stay? In this issue of the Journal of Hospital Medicine, Goldstein et al.6 provide insight to that question. Using chart review of physical therapy and case management recommendations for post-acute SNF care, Goldstein et al.6 compare actual discharge rate to SNF or acute inpatient rehabilitation following an observation stay when such disposition is recommended. In their two-hospital system, fewer than 20% of previously community-dwelling hospitalist patients followed recommendation for post-acute facility stay after observation hospitalization, and more than 40% cited financial concerns as the reason for declining. Patients recommended for SNF also were more likely to be rehospitalized in the subsequent 30 days after discharge, confirming this as a vulnerable patient population. Given Medicare’s original intent to improve health care access for seniors, the case for change seems clear, and the repercussions of not addressing the plight of patients hospitalized under observation is having negative financial and overall detrimental health impacts.
But there are other compelling reasons why this 50-year-old law needs to be improved. Hospital care today is vastly different than when Medicare became law. Average hospital length of stay for patients 65 years and older was 14.2 days in 19657 compared to 5.2 days today,8 clearly a shift in what 3 days of hospital care means. Most importantly, observation stays have become a major part of hospital care. Between 2006 and 2014, per-beneficiary outpatient visits (which include all observation stays) increased 44.2% nationally, while inpatient discharges decreased 19.9%.9 In 2012, the Centers for Medicare & Medicaid Services (CMS) received 1.7 million outpatient observation claims and an additional 700,000 inpatient claims that started with observation days.10 CMS also expected the 2-midnight rule to reduce outpatient observation stays,4 but a recent OIG report11 found that outpatient stays increased 8.1% in the first year (FY 2014) under the new rule, and there were still 748,337 long observation stays (those lasting 2 midnights or longer) in 2014, only a small (2.8%) decrease from the prior year. These factors limit Medicare beneficiary post–acute SNF eligibility in ways that could not have been anticipated when the extended care benefit was created to help seniors access needed health care.
Policymakers must consider cost when considering statutory change. Waiver programs in the 1980s suspending the 3-midnight requirement raised concerns over potential increase in both SNF utilization and associated costs.12 However, more recent data suggest that altering the 3-midnight requirement may not increase post-acute SNF utilization. From 2006 to 2010, Medicare Advantage programs that waived the 3-midnight requirement saw a decrease in hospital length of stay without increased SNF utilization or SNF length of stay, indicating that access to the right level of care at the right time could be cost-saving.13 Recent data from the Bundled Payments for Care Improvement (BPCI) program found savings were largely related to decreased SNF utilization when payments were episode-based,14 a trend that may continue as Medicare moves away from fee-for-service towards bundled payments for more conditions. And although neither example directly tests changing the 3-midnight requirement to include observation midnights, both studies suggest that innovative health care delivery and modification of SNF access did not result in increased SNF utilization or greater post-acute costs. In fact, as Goldstein et al.6 showed, patients recommended for post-acute SNF following observation stay were more likely to be rehospitalized within 30 days, an additional cost that could potentially be avoided if these patients had SNF access. We believe that these correlations strongly support rescinding the 3-
That being said, what can be done? In 2015, the Medicare Payment Advisory Commission (MedPAC) recommended changing the 3-night requirement to require just one of 3 midnights to be inpatient to make a qualifying stay.10 Although an improvement over current law, this proposal would not help the majority of beneficiaries who are exclusively hospitalized under observation status. The “Improving Access to Medicare Coverage Act of 2015”, to be reintroduced in Congress in the coming weeks, would count any midnight spent in the hospital towards the 3-midnight stay requirement, and has bipartisan, bicameral support and cosponsorship.15 In 2015, through unanimous bipartisan, bicameral support, Congress passed the NOTICE Act (PL 114-42), which requires hospitals to inform Medicare beneficiaries hospitalized under observation.16 We believe that the data are clear to both sides of the aisle that Congress should now work together using scientifically-supported research to improve the exact observation policies they felt patients should be informed of. Passing the Improving Access to Medicare Coverage Act is the logical next step in this arena.
Medicare was intended to give seniors access to the healthcare they need. Growth in hospital-based observation care begs for modernization of the statutory 3-inpatient midnight rule. Counting all midnights towards the 3-midnight requirement, whether those midnights are outpatient observation or inpatient, is the right first step.
Disclosures
Representative Courtney is the bill sponsor of the Improving Access to Medicare Coverage Act. The authors report no other conflicts.
1. Medicare & Medicaid Milestones 1937-2015. https://www.cms.gov/About-CMS/Agency-Information/History/Downloads/Medicare-and-Medicaid-Milestones-1937-2015.pdf . Accessed September 25, 2016.
2. Loewenstein R. Early effects of Medicare on the health care of the aged. https://www.ssa.gov/policy/docs/ssb/v34n4/v34n4p3.pdf. Accessed September 25, 2016.
3. US Social Security Act, Sec. 1861 (i). [42 U.S.C. 1395x]. https://www.ssa.gov/OP_Home/ssact/title18/1861.htm. Accessed September 25, 2016.
4. Department of Health and Human Services Office of Inspector General. Hospitals’ use of observation stays and short inpatient stays for Medicare beneficiaries, OEI-02-12-00040. Available at: https://oig.hhs.gov/oei/reports/oei-02-12-00040.pdf. Accessed September 25, 2016.
5. Feng Z, Jung H-Y, Wright B, Mor V. The origin and disposition of Medicare observation stays. Med Care 2014;52:796-800. PubMed
6. Goldstein JN, Schwartz JS, McGraw P, Banks TL, Hicks LS. The unmet need for postacute rehabilitation among medicare observation patients: a single-center study. J Hosp Med. 2017;12(3):168-172.
7. Vital and Health Statistics. Trends in hospital utilization: United States, 1965-1986. https://www.cdc.gov/nchs/data/series/sr_13/sr13_101.pdf. Accessed September 25, 2016.
8. Healthcare Cost and Utilization Project (HCUP). Statistical brief #180. Overview of hospital stays in the United States, 2012. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb180-Hospitalizations-United-States-2012.pdf. Accessed September 25, 2016.
9. MedPAC March 2016 Report to the Congress. Chapter 3. Hospital inpatient and outpatient services. http://www.medpac.gov/docs/default-source/reports/march-2016-report-to-the-congress-medicare-payment-policy.pdf?sfvrsn=0. Accessed September 25, 2016.
10. MedPAC. June 2015 Report to the Congress. Chapter 7: Hospital short-stay policy issues. http://www.medpac.gov/docs/default-source/reports/chapter-7-hospital-short-stay-policy-issues-june-2015-report-.pdf?sfvrsn=0 Accessed September 25, 2016.
11. Department of Health and Human Services Office of Inspector General. Vulnerabilities remain under Medicare’s 2-midnight hospital policy, OEI-02-15-00020. https://oig.hhs.gov/oei/reports/oei-02-15-00020.pdf. Accessed February 19, 2017.
12. Lipsitz L. The 3-night hospital stay and Medicare coverage for skilled nursing care. JAMA. 2013;310: 1441-1442. PubMed
13. Grebela R, Keohane L Lee Y, Lipsitz L, Rahman M, Trevedi A. Waiving the three-day rule: admissions and length-of-stay at hospitals and skilled nursing facilities did not increase. Health Affairs. 2015;34:1324-1330. PubMed
14. Dummit L, Kahvecioglu D, Marrufo G, et al. Association between hospital participation in a Medicare bundled payment initiative and payments and quality outcomes for lower extremity joint replacement episodes. JAMA. 2016;316(12):1267-1278. PubMed
15. HR. 1571 Improving Access to Medicare Coverage Act of 2015. https://www.govtrack.us/congress/bills/114/hr1571/text. Accessed September 25, 2016.
16. PL 114-42. The NOTICE Act. https://www.govtrack.us/congress/bills/114/hr876. Accessed September 25, 2016.
1. Medicare & Medicaid Milestones 1937-2015. https://www.cms.gov/About-CMS/Agency-Information/History/Downloads/Medicare-and-Medicaid-Milestones-1937-2015.pdf . Accessed September 25, 2016.
2. Loewenstein R. Early effects of Medicare on the health care of the aged. https://www.ssa.gov/policy/docs/ssb/v34n4/v34n4p3.pdf. Accessed September 25, 2016.
3. US Social Security Act, Sec. 1861 (i). [42 U.S.C. 1395x]. https://www.ssa.gov/OP_Home/ssact/title18/1861.htm. Accessed September 25, 2016.
4. Department of Health and Human Services Office of Inspector General. Hospitals’ use of observation stays and short inpatient stays for Medicare beneficiaries, OEI-02-12-00040. Available at: https://oig.hhs.gov/oei/reports/oei-02-12-00040.pdf. Accessed September 25, 2016.
5. Feng Z, Jung H-Y, Wright B, Mor V. The origin and disposition of Medicare observation stays. Med Care 2014;52:796-800. PubMed
6. Goldstein JN, Schwartz JS, McGraw P, Banks TL, Hicks LS. The unmet need for postacute rehabilitation among medicare observation patients: a single-center study. J Hosp Med. 2017;12(3):168-172.
7. Vital and Health Statistics. Trends in hospital utilization: United States, 1965-1986. https://www.cdc.gov/nchs/data/series/sr_13/sr13_101.pdf. Accessed September 25, 2016.
8. Healthcare Cost and Utilization Project (HCUP). Statistical brief #180. Overview of hospital stays in the United States, 2012. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb180-Hospitalizations-United-States-2012.pdf. Accessed September 25, 2016.
9. MedPAC March 2016 Report to the Congress. Chapter 3. Hospital inpatient and outpatient services. http://www.medpac.gov/docs/default-source/reports/march-2016-report-to-the-congress-medicare-payment-policy.pdf?sfvrsn=0. Accessed September 25, 2016.
10. MedPAC. June 2015 Report to the Congress. Chapter 7: Hospital short-stay policy issues. http://www.medpac.gov/docs/default-source/reports/chapter-7-hospital-short-stay-policy-issues-june-2015-report-.pdf?sfvrsn=0 Accessed September 25, 2016.
11. Department of Health and Human Services Office of Inspector General. Vulnerabilities remain under Medicare’s 2-midnight hospital policy, OEI-02-15-00020. https://oig.hhs.gov/oei/reports/oei-02-15-00020.pdf. Accessed February 19, 2017.
12. Lipsitz L. The 3-night hospital stay and Medicare coverage for skilled nursing care. JAMA. 2013;310: 1441-1442. PubMed
13. Grebela R, Keohane L Lee Y, Lipsitz L, Rahman M, Trevedi A. Waiving the three-day rule: admissions and length-of-stay at hospitals and skilled nursing facilities did not increase. Health Affairs. 2015;34:1324-1330. PubMed
14. Dummit L, Kahvecioglu D, Marrufo G, et al. Association between hospital participation in a Medicare bundled payment initiative and payments and quality outcomes for lower extremity joint replacement episodes. JAMA. 2016;316(12):1267-1278. PubMed
15. HR. 1571 Improving Access to Medicare Coverage Act of 2015. https://www.govtrack.us/congress/bills/114/hr1571/text. Accessed September 25, 2016.
16. PL 114-42. The NOTICE Act. https://www.govtrack.us/congress/bills/114/hr876. Accessed September 25, 2016.
© 2017 Society of Hospital Medicine
In reference to “When personality is the problem: Managing patients with difficult personalities on the acute care unit"
In the article by Riddle et al,1 the authors state that in the example of Cluster A type personality disorder, the elderly male patient’s paranoid disorder should be ignored, rather than confronting the paranoia. We do not need to confront the paranoia, but we need to treat the paranoid disorder. The symptom of paranoia extends beyond the single diagnostic category of delusional disorder and has been noted in many elderly patients with other underlying disorders.2 This patient needs early psychiatric consultation and therapy.
They also give recommendations regarding Ms. B for her ever-increasing need of opiates. I find it too naïve for me to offer this patient “…choices, such as walking with her around the unit or listen to the music.” This patient needs pain physician consultations and aggressive interventional pain control.3
1. Riddle MR, Meeks T, Alvarez C, Dubovsky A. When personality is the problem: Managing patients with difficult personalities on the acute care unit. J Hosp Med. 2016:11(12):873-878. PubMed
2. Targum SD. Treating psychotic symptoms in elderly patients. Prim Care Companion J Clin Psychiatry. 2001;3(4):156-163. PubMed
3. Karmakar MK, Ho AM. Acute pain management of patients with multiple fractured ribs. J Trauma. 2003;54(3):615-625. PubMed
In the article by Riddle et al,1 the authors state that in the example of Cluster A type personality disorder, the elderly male patient’s paranoid disorder should be ignored, rather than confronting the paranoia. We do not need to confront the paranoia, but we need to treat the paranoid disorder. The symptom of paranoia extends beyond the single diagnostic category of delusional disorder and has been noted in many elderly patients with other underlying disorders.2 This patient needs early psychiatric consultation and therapy.
They also give recommendations regarding Ms. B for her ever-increasing need of opiates. I find it too naïve for me to offer this patient “…choices, such as walking with her around the unit or listen to the music.” This patient needs pain physician consultations and aggressive interventional pain control.3
In the article by Riddle et al,1 the authors state that in the example of Cluster A type personality disorder, the elderly male patient’s paranoid disorder should be ignored, rather than confronting the paranoia. We do not need to confront the paranoia, but we need to treat the paranoid disorder. The symptom of paranoia extends beyond the single diagnostic category of delusional disorder and has been noted in many elderly patients with other underlying disorders.2 This patient needs early psychiatric consultation and therapy.
They also give recommendations regarding Ms. B for her ever-increasing need of opiates. I find it too naïve for me to offer this patient “…choices, such as walking with her around the unit or listen to the music.” This patient needs pain physician consultations and aggressive interventional pain control.3
1. Riddle MR, Meeks T, Alvarez C, Dubovsky A. When personality is the problem: Managing patients with difficult personalities on the acute care unit. J Hosp Med. 2016:11(12):873-878. PubMed
2. Targum SD. Treating psychotic symptoms in elderly patients. Prim Care Companion J Clin Psychiatry. 2001;3(4):156-163. PubMed
3. Karmakar MK, Ho AM. Acute pain management of patients with multiple fractured ribs. J Trauma. 2003;54(3):615-625. PubMed
1. Riddle MR, Meeks T, Alvarez C, Dubovsky A. When personality is the problem: Managing patients with difficult personalities on the acute care unit. J Hosp Med. 2016:11(12):873-878. PubMed
2. Targum SD. Treating psychotic symptoms in elderly patients. Prim Care Companion J Clin Psychiatry. 2001;3(4):156-163. PubMed
3. Karmakar MK, Ho AM. Acute pain management of patients with multiple fractured ribs. J Trauma. 2003;54(3):615-625. PubMed
© 2017 Society of Hospital Medicine
Paid Sick Days Help Reduce Flu Exposure (For Some)
Research has confirmed what many people know from experience: that paid sick days can make the workplace healthier. In 1 study, researchers found universal access to paid sick days (PSD) reduced influenza in the workplace by 6%. To drill down on the influence PSD access has on decisions to stay home from work, or to stay home with a child who has flu or influenza-like-illness, researchers from University of Pittsburgh analyzed data from the 2009 Medical Expenditure Panel Survey for 12,901 households and 12,044 employees. They chose the 2009 survey because the numbers of influenza-like-illness and influenza cases were likely to have been higher due to the 2009 H1N1 pandemic.
Of the workers surveyed, 64% had access to PSD. Access was associated significantly with gender, race/ethnicity, income, education, and number of employees in the workplace. In the group of 4,911 employees who had children, 68% had PSD.
The study highlighted some subgroups that face barriers to following CDC recommendations, such as staying home for up to 24 hours after symptoms subside. Hispanics, for instance, were significantly less likely to stay home when ill, but this was not necessarily an ethnic difference, the researchers say. Rather, they suggest, it may have had more to do with job security and workplace culture. They cite a survey done during the 2009 H1N1 pandemic, in which Hispanics reported fewer resources at work than non-Hispanic whites, including paid sick leave, job security, and ability to work from home.
Women tend to be the main caregivers for children. In this study, women had a higher prevalence of staying home for a child’s illness than men, even after the researchers controlled for PSD access. Yet, in a different survey women also were more likely to report for work when ill, or when a child was ill. The researchers called this “presenteeism.”
The researchers underscore the importance of PSD laws in reducing the economic burden of healthy behavior in families. They note that in 2015, 35% of employees did not have access to PSD, and those employees were usually people with low income. Only 34% of those in the lowest-income group had access to PSD, compared with 89% in the highest income groups.
Source:
Piper K, Youk A, James AE, III, Kumar S. PLoS ONE. 2017;12(2): e0170698.
doi:10.1371/journal.pone.0170698
Research has confirmed what many people know from experience: that paid sick days can make the workplace healthier. In 1 study, researchers found universal access to paid sick days (PSD) reduced influenza in the workplace by 6%. To drill down on the influence PSD access has on decisions to stay home from work, or to stay home with a child who has flu or influenza-like-illness, researchers from University of Pittsburgh analyzed data from the 2009 Medical Expenditure Panel Survey for 12,901 households and 12,044 employees. They chose the 2009 survey because the numbers of influenza-like-illness and influenza cases were likely to have been higher due to the 2009 H1N1 pandemic.
Of the workers surveyed, 64% had access to PSD. Access was associated significantly with gender, race/ethnicity, income, education, and number of employees in the workplace. In the group of 4,911 employees who had children, 68% had PSD.
The study highlighted some subgroups that face barriers to following CDC recommendations, such as staying home for up to 24 hours after symptoms subside. Hispanics, for instance, were significantly less likely to stay home when ill, but this was not necessarily an ethnic difference, the researchers say. Rather, they suggest, it may have had more to do with job security and workplace culture. They cite a survey done during the 2009 H1N1 pandemic, in which Hispanics reported fewer resources at work than non-Hispanic whites, including paid sick leave, job security, and ability to work from home.
Women tend to be the main caregivers for children. In this study, women had a higher prevalence of staying home for a child’s illness than men, even after the researchers controlled for PSD access. Yet, in a different survey women also were more likely to report for work when ill, or when a child was ill. The researchers called this “presenteeism.”
The researchers underscore the importance of PSD laws in reducing the economic burden of healthy behavior in families. They note that in 2015, 35% of employees did not have access to PSD, and those employees were usually people with low income. Only 34% of those in the lowest-income group had access to PSD, compared with 89% in the highest income groups.
Source:
Piper K, Youk A, James AE, III, Kumar S. PLoS ONE. 2017;12(2): e0170698.
doi:10.1371/journal.pone.0170698
Research has confirmed what many people know from experience: that paid sick days can make the workplace healthier. In 1 study, researchers found universal access to paid sick days (PSD) reduced influenza in the workplace by 6%. To drill down on the influence PSD access has on decisions to stay home from work, or to stay home with a child who has flu or influenza-like-illness, researchers from University of Pittsburgh analyzed data from the 2009 Medical Expenditure Panel Survey for 12,901 households and 12,044 employees. They chose the 2009 survey because the numbers of influenza-like-illness and influenza cases were likely to have been higher due to the 2009 H1N1 pandemic.
Of the workers surveyed, 64% had access to PSD. Access was associated significantly with gender, race/ethnicity, income, education, and number of employees in the workplace. In the group of 4,911 employees who had children, 68% had PSD.
The study highlighted some subgroups that face barriers to following CDC recommendations, such as staying home for up to 24 hours after symptoms subside. Hispanics, for instance, were significantly less likely to stay home when ill, but this was not necessarily an ethnic difference, the researchers say. Rather, they suggest, it may have had more to do with job security and workplace culture. They cite a survey done during the 2009 H1N1 pandemic, in which Hispanics reported fewer resources at work than non-Hispanic whites, including paid sick leave, job security, and ability to work from home.
Women tend to be the main caregivers for children. In this study, women had a higher prevalence of staying home for a child’s illness than men, even after the researchers controlled for PSD access. Yet, in a different survey women also were more likely to report for work when ill, or when a child was ill. The researchers called this “presenteeism.”
The researchers underscore the importance of PSD laws in reducing the economic burden of healthy behavior in families. They note that in 2015, 35% of employees did not have access to PSD, and those employees were usually people with low income. Only 34% of those in the lowest-income group had access to PSD, compared with 89% in the highest income groups.
Source:
Piper K, Youk A, James AE, III, Kumar S. PLoS ONE. 2017;12(2): e0170698.
doi:10.1371/journal.pone.0170698
Allergic Reaction to Phenylephrine
Phenylephrine, a sympathomimetic drug, is commonly used in eye exams to dilate the pupil of the eye and to differentiate scleritis from episcleritis. Common adverse effects (AEs) of phenylephrine include subjective burning, stinging with lacrimation, rebound hyperemia, and liberation of iris pigment into the anterior chamber. Less common, systemic AEs include tachycardia and elevation of systemic blood pressure. Although instances of allergic reactions are rare, phenylephrine has been reported to cause contact dermatitis, blepharoconjunctivitis, and as in this case, keratoconjunctivitis.
Case Report
An 83-year-old white male presented for a red eye evaluation 2 days after having undergone a comprehensive eye exam with dilation at the Malcom Randall VAMC clinic in Gainesville, Florida. The patient reported onset of blurred vision, which he described as looking through a fog. He further compared the feeling to pins sticking in his eyes. The patient noted he had experienced similar symptoms on a few other occasions following eye exams. At the most recent eye exam, proparacaine and fluorescein had been used for tonometry, and phenylephrine 2.5% and tropicamide 0.5% had been used for pupillary dilation.
The patient’s best-corrected visual acuity was counting fingers at 2 feet in the right eye (OD) and left eye (OS). The best-corrected visual acuity 2 days prior had been 20/20 OD and OS. Pupils and extraocular motilities were unremarkable. Intraocular pressures were not obtained due to concern for a possible adverse reaction to proparacaine.
Slit-lamp evaluation revealed the lids to be lax, erythematous, and edematous in both eyes (Figure 1).
The initial diagnosis was acute chemical conjunctivitis most likely due to an AE to proparacaine. The plan was to start the patient on antibiotic eye drops qid OU, prednisolone qid OU, and artificial tears every hour OU. The patient was scheduled to return to clinic 4 days later for an anterior segment follow-up.
At the follow-up visit, the patient reported significant visual improvement. His best-corrected visual acuity was 20/40-2 without improvement on pinhole OD and 20/50-2 with improvement to 20/30+ on pinhole OS. Slit-lamp evaluation revealed 1+ bulbar conjunctival injection OU, intact corneal epithelium OU, and no cells or flare in the anterior chambers OU. Due to improving punctate epitheliopathy, the frequency of the antibiotic drops, the prednisolone, and the artificial tears was reduced to bid. After 3 days, he was instructed to discontinue them. The patient was scheduled to return in 2 weeks for an anterior segment follow-up.
At the next follow-up visit, the patient reported that his vision had returned to normal, and he had no further ocular AEs. His best-corrected visual acuity was 20/20-2 OD and 20/20 OS. Slit-lamp evaluation revealed mild blepharitis OU, trace bulbar conjunctival injection OU, and complete resolution of the keratitis OU. The assessment was acute allergic conjunctivitis thought to be secondary to an AE to proparacaine OU, yet the need to rule out hypersensitivity to tropicamide and/or phenylephrine remained. The plan was to educate the patient of the possibility of allergic reaction on future visits and to recommend continued use of artificial tears as needed.
Through a careful and extensive chart review of all past visits, it was suspected that phenylephrine might be to blame rather than proparacaine. At the subsequent visit, the patient agreed to undergo testing to determine the culprit via instillation of proparacaine in one eye and tropicamide in the other. The patient had no reaction to either drop (checked 45 minutes after instillation and the following day). By process of elimination, phenylephrine was determined to be the offending agent.
Discussion
Following a thorough review of the patient’s chart, it was found that on other occasions he had presented with suspected allergic reactions following routine eye examinations. The patient reported he had experienced a reaction in 2007 but could not recall what drops were instilled in his eyes at the time. In addition, there was no documentation in his medical record of the subsequent reaction following that visit. Another reaction occurred in July 2010 with instillation of tropicamide 1%, phenylephrine 2.5%, and Fluress (fluorescein sodium and benoxinate hydrochloride ophthalmic solution USP). In October 2013, when tropicamide 0.5%, proparacaine, and fluorescein strips were instilled, there was no reaction. The next reaction occurred in October 2014, when tropicamide 0.5%, phenylephrine 2.5%, proparacaine, and fluorescein strips were instilled.
This careful review of past exam notes revealed that phenylephrine and Fluress were the only drops that had not been instilled at the October 2013 visit when no AE was reported. However, Fluress was an unlikely culprit since it was not instilled in October 2014, and the patient still experienced an AE. Therefore, the agent most likely responsible for the allergic reaction in the patient, as confirmed by a review of the past notes and by the aforementioned pharmacologic test, was deemed to be phenylephrine (Table).
Adverse reactions to topical ocular medications and specifically to diagnostic eye drops have long been recognized. Mathias, Camarasa, Barber, Ducombs, and Monsálvezhave reported on variations of conjunctivitis and periorbital erythema with positive patch testing to phenylephrine.1-5 Geyer and colleagues reported on a study of 21 patients who had blepharoconjunctivitis after instillation of phenylephrine.6 In this case study patient, severe keratoconjunctivitis was the clinical manifestation observed.
Villarreal and colleagues studied 31 patients who had a previous reaction to mydriatic drops. The study found that phenylephrine was the drug that most frequently caused an AE (93.5%).7 One patient reacted to the preservative thimerosal, and 1 patient reacted to benoxiprocaine. Tropicamide was demonstrated to be very well tolerated as none of the patients tested positive on either the patch test or the pharmacologic test.
Tropicamide is a nonselective muscarinic antagonist commonly used for mydriasis due to its fast onset and short duration.8 Adverse reactions to tropicamide are rare. Three studies reported on patients who had a positive patch test to tropicamide.9-11 However, the reaction was not provoked by direct instillation of tropicamide into the eye.
Common in-office topical anesthetics, proparacaine, tetracaine, benoxinate, and lidocaine also can cause AEs. Corneal toxicity is a well-known complication with topical anesthetic abuse, whereas allergic reactions are considered rare. The most common symptoms include stingingand discomfort upon instillation. Common signs include punctate corneal epithelial erosionsresulting indirectly from a decrease in reflex tearing, infrequent blinking, and increased tear evaporation.12 Topical anesthetics also inhibit the migration of corneal epithelial cells and cause direct damage to the cells that are present, leading to impaired healing and epithelial defects.13
Manifestations of allergic reaction to topical anesthetics can include conjunctival hyperemia and edema, edematous eyelids, and lacrimation. One published case described a 60-year-old woman who developed eczematous dermatitis of the eyelids after ophthalmic anesthetic drops were instilled prior to laser surgery. Patch testing showed a positive response to benzocaine 5%, proparacaine, and tetracaine 0.5%.14
Preservatives, in general, can cause an allergic reaction. Benzalkonium chloride’s (BAK) cytotoxic sequelae include possible trabecular cell death in glaucoma patients, disruption of tear film stability (even at low concentrations), and immune-allergenic properties. One article reported BAK as one of the 30 most frequent allergens causing allergic periorbital dermatitis.15 Benzalkonium chloride is used in most brands of phenylephrine. However, preservatives in this patient’s case were ruled out as instigating agents since both phenylephrine and tropicamide contain the same preservative, BAK 0.01%, yet this patient did not develop a reaction to tropicamide when used without phenylephrine. Expired medications also were not considered to be a factor as none of the medications used on the patient were indeed expired (the Malcom Randall VAMC clinic maintains a strict policy of discarding medications 28 days after being opened).
Although uncommon, phenylephrine sometimes has been found to cause a type 4 hypersensitivity reaction, also known as cell-mediated or delayed-type hypersensitivity.16 First, helper T cells secrete cytokines. Activation of cytokines recruits and activates cytotoxic T cells, monocytes, and macrophages, leading to inflammation of the surrounding tissue. Examples of cell-mediated hypersensitivity include reactions to the tuberculin skin test and to poison ivy.
Type 1 hypersensitivity reactions, also known as immediate or anaphylactic hypersensitivity reactions, are not triggered by phenylephrine. In this type of reaction, IgE binds to the mast cell on initial exposure to an allergen. On second exposure, the allergen binds to the IgE, causing the mast cell to release mediators of inflammation, triggering physiologic responses. Examples of this type of hypersensitivity include those seen with penicillin, bee stings, hay fever, bronchial asthma, and food allergies, for example, to shellfish.
A toxic reaction’s mechanism differs from that of a type 4 hypersensitivity reaction. Toxic reactions occur due to direct cytotoxicity of a drug caused by a low or high pH and either hyper- or hypo-osmolarity. Toxicity can lead to corneal and conjunctival cell necrosis or induce apoptosis, stimulating inflammatory reactions. Clinically, toxic reactions will present with follicles, whereas allergic reactions will present with papillae.
The definitive diagnostic methods used to determine the allergic agent causing ocular or periocular AEs are patch testing and conjunctival challenge.7 Mathias, Camarasa, Barber, Ducombs,and Monsálvezused patch testing to confirm phenylephrine as the allergic agent in their series of cases. Patch testing entails the application of a small amount of an allergic agent that is taped onto the skin. The allergic agent is confirmed if the patient has a dermal reaction, wherein the area patched will become erythematous. When patch testing is negative or inconclusive, a conjunctival challenge is performed by instillation of the suspected allergic agent into the eye with subsequent observation to determine whether a reaction occurs. The sequelae found in Villarreal’s study included itching, lacrimation, edema, erythema, and sometimes blepharitis.7
A direct conjunctival challenge with the suspected culprit was not pursued in this patient’s case due to the known severity of the potential resulting reaction. The authors instead chose an indirect method of determining the implicating agent and used the process of elimination to whittle down the most likely suspect. A challenge with the medications suspected not to be likely offenders was undertaken. This spared the patient a likely repeat of the AE he had just recovered from.
Management
Allergic reactions can resolve without medical intervention. The first step is to remove the allergen. For delayed hypersensitivity reactions, treatments may include topical decongestants, cool compresses, and corticosteroids.8 The treatment for immediate hypersensitivity reaction differs from that of delayed hypersensitivity reaction in that antihistamines are used.17,18
This patient reported receiving no treatment for his ocular symptoms following eye examinations in the past, yet he experienced complete resolution after each AE. In this case, both a steroid and a prophylactic antibiotic to facilitate a more rapid improvement were used.
Conclusion
Although uncommon, cases of allergic reaction to phenylephrine can occur. The incidence of phenylephrine allergy is 0.6%.6 The case study patient presented with a severe keratoconjunctivitis following routine eye examination with an accompanying history of adverse ocular signs and symptoms following multiple past exams.
It is important for all eye care clinicians to realize that AEs to diagnostic eye drops are possible and can occur following the most routine of visits. Such reactions can be caused by dilating agents, anesthetics, or preservatives, and these may be allergic or toxic. Clinicians should take special care to identify the instigating agent, and if possible, to avoid using such agents on patients during future exams. Clinicians also should understand how best to manage iatrogenic AEs when they encounter them in order to restore a patient’s visual function as quickly as possible.
1. Mathias CG, Maibach HI, Irvine A, Adler W. Allergic contact dermatitis to echothiophate iodide and phenylephrine. Arch Ophthalmol. 1979;97(2):286-287.
2. Camarasa JG. Contact dermatitis to phenylephrine. Contact Dermatitis. 1984;10(3):182.
3. Barber K. Allergic contact eczema to phenylephrine. Contact Dermatitis. 1983;9(4):274-277.
4. Ducombs G, de Casamayor J, Verin P, Maleville J. Allergic contact dermatitis to phenylephrine. Contact Dermatitis. 1986;15(2):107-108.
5. Monsálvez V, Fuertes L, García-Cano I, Vanaclocha F, Ortez de Frutos J. Blepharoconjunctivitis due to phenylephrine [in Spanish]. Actas Dermosifiliogr. 2010;101(5):466-467.
6. Geyer O, Yust I, Lazar M. Allergic blepharoconjunctivitis due to phenylephrine. J Ocul Pharmacol. 1988;4(2):123-126.
7. Villarreal O. Reliability of diagnostic tests for contact allergy to mydriatic eyedrops. Contact Dermatitis. 1998;38(3):150-154.
8. Frazier M, Jaanus SD. Cycloplegics. In: Bartlett JD, Jaanus SD. Clinical Ocular Pharmacology. 5th ed. St. Louis, MO: Butterworth-Heinemann; 2009:125-138.
9. Decraene T, Goossens A. Contact allergy to atropine and other mydriatic agents in eye drops. Contact Dermatitis. 2001;45(5):309-310.
10. Boukhman MP, Maibach HI. Allergic contact dermatitis from tropicamide ophthalmic solution. Contact Dermatitis. 1999;41(1):47-48.
11. Yoshikawa K, Kawahara S. Contact allergy to atropine and other mydriatic agents. Contact Dermatitis. 1985;12(1):56-57.
12. Mcgee HT, Fraunfelder FW. Toxicities of topical ophthalmic anesthetics. Expert Opin Drug Saf. 2007;6(6):637-640.
13. Dass BA, Soong HK, Lee B. Effects of proparacaine of actin cytoskeleton of corneal epithelium. J Ocul Pharmacol. 1988;4(3):187-194.
14. Dannaker CJ, Maibach HI, Austin E. Allergic contact dermatitis to proparacaine with subsequent cross-sensitization to tetracaine from ophthalmic preparations. Am J Contact Dermat. 2001;12(3):177-179.
15. Hong J, Bielory L. Allergy to ophthalmic preservatives. Curr Opin Allergy Clin Immunol. 2009;9(5):447-453.
16. Gonzalo-Garijo MA, Pérez-Calderón R, de Argila D, Rodríguez-Nevado I. Erythrodermia to pseudoephedrine in a patient with contact allergy to phenylephrine. Allergol Immunopathol (Madr). 2002;30(4):239-242.
17. Platts-Mills TAE. Immediate hypersensitivity (Type I). In: Male D, Brostoff J, Roth DB, Roitt I. Immunology. 7th ed. Canada: Elsevier Limited; 2006:423-446.
18. Britton W. Type IV hypersensitivity. In: Male D, Brostoff J, Roth DB, Roitt I. Immunology. 7th ed. Canada: Elsevier Limited; 2006:477-491.
Phenylephrine, a sympathomimetic drug, is commonly used in eye exams to dilate the pupil of the eye and to differentiate scleritis from episcleritis. Common adverse effects (AEs) of phenylephrine include subjective burning, stinging with lacrimation, rebound hyperemia, and liberation of iris pigment into the anterior chamber. Less common, systemic AEs include tachycardia and elevation of systemic blood pressure. Although instances of allergic reactions are rare, phenylephrine has been reported to cause contact dermatitis, blepharoconjunctivitis, and as in this case, keratoconjunctivitis.
Case Report
An 83-year-old white male presented for a red eye evaluation 2 days after having undergone a comprehensive eye exam with dilation at the Malcom Randall VAMC clinic in Gainesville, Florida. The patient reported onset of blurred vision, which he described as looking through a fog. He further compared the feeling to pins sticking in his eyes. The patient noted he had experienced similar symptoms on a few other occasions following eye exams. At the most recent eye exam, proparacaine and fluorescein had been used for tonometry, and phenylephrine 2.5% and tropicamide 0.5% had been used for pupillary dilation.
The patient’s best-corrected visual acuity was counting fingers at 2 feet in the right eye (OD) and left eye (OS). The best-corrected visual acuity 2 days prior had been 20/20 OD and OS. Pupils and extraocular motilities were unremarkable. Intraocular pressures were not obtained due to concern for a possible adverse reaction to proparacaine.
Slit-lamp evaluation revealed the lids to be lax, erythematous, and edematous in both eyes (Figure 1).
The initial diagnosis was acute chemical conjunctivitis most likely due to an AE to proparacaine. The plan was to start the patient on antibiotic eye drops qid OU, prednisolone qid OU, and artificial tears every hour OU. The patient was scheduled to return to clinic 4 days later for an anterior segment follow-up.
At the follow-up visit, the patient reported significant visual improvement. His best-corrected visual acuity was 20/40-2 without improvement on pinhole OD and 20/50-2 with improvement to 20/30+ on pinhole OS. Slit-lamp evaluation revealed 1+ bulbar conjunctival injection OU, intact corneal epithelium OU, and no cells or flare in the anterior chambers OU. Due to improving punctate epitheliopathy, the frequency of the antibiotic drops, the prednisolone, and the artificial tears was reduced to bid. After 3 days, he was instructed to discontinue them. The patient was scheduled to return in 2 weeks for an anterior segment follow-up.
At the next follow-up visit, the patient reported that his vision had returned to normal, and he had no further ocular AEs. His best-corrected visual acuity was 20/20-2 OD and 20/20 OS. Slit-lamp evaluation revealed mild blepharitis OU, trace bulbar conjunctival injection OU, and complete resolution of the keratitis OU. The assessment was acute allergic conjunctivitis thought to be secondary to an AE to proparacaine OU, yet the need to rule out hypersensitivity to tropicamide and/or phenylephrine remained. The plan was to educate the patient of the possibility of allergic reaction on future visits and to recommend continued use of artificial tears as needed.
Through a careful and extensive chart review of all past visits, it was suspected that phenylephrine might be to blame rather than proparacaine. At the subsequent visit, the patient agreed to undergo testing to determine the culprit via instillation of proparacaine in one eye and tropicamide in the other. The patient had no reaction to either drop (checked 45 minutes after instillation and the following day). By process of elimination, phenylephrine was determined to be the offending agent.
Discussion
Following a thorough review of the patient’s chart, it was found that on other occasions he had presented with suspected allergic reactions following routine eye examinations. The patient reported he had experienced a reaction in 2007 but could not recall what drops were instilled in his eyes at the time. In addition, there was no documentation in his medical record of the subsequent reaction following that visit. Another reaction occurred in July 2010 with instillation of tropicamide 1%, phenylephrine 2.5%, and Fluress (fluorescein sodium and benoxinate hydrochloride ophthalmic solution USP). In October 2013, when tropicamide 0.5%, proparacaine, and fluorescein strips were instilled, there was no reaction. The next reaction occurred in October 2014, when tropicamide 0.5%, phenylephrine 2.5%, proparacaine, and fluorescein strips were instilled.
This careful review of past exam notes revealed that phenylephrine and Fluress were the only drops that had not been instilled at the October 2013 visit when no AE was reported. However, Fluress was an unlikely culprit since it was not instilled in October 2014, and the patient still experienced an AE. Therefore, the agent most likely responsible for the allergic reaction in the patient, as confirmed by a review of the past notes and by the aforementioned pharmacologic test, was deemed to be phenylephrine (Table).
Adverse reactions to topical ocular medications and specifically to diagnostic eye drops have long been recognized. Mathias, Camarasa, Barber, Ducombs, and Monsálvezhave reported on variations of conjunctivitis and periorbital erythema with positive patch testing to phenylephrine.1-5 Geyer and colleagues reported on a study of 21 patients who had blepharoconjunctivitis after instillation of phenylephrine.6 In this case study patient, severe keratoconjunctivitis was the clinical manifestation observed.
Villarreal and colleagues studied 31 patients who had a previous reaction to mydriatic drops. The study found that phenylephrine was the drug that most frequently caused an AE (93.5%).7 One patient reacted to the preservative thimerosal, and 1 patient reacted to benoxiprocaine. Tropicamide was demonstrated to be very well tolerated as none of the patients tested positive on either the patch test or the pharmacologic test.
Tropicamide is a nonselective muscarinic antagonist commonly used for mydriasis due to its fast onset and short duration.8 Adverse reactions to tropicamide are rare. Three studies reported on patients who had a positive patch test to tropicamide.9-11 However, the reaction was not provoked by direct instillation of tropicamide into the eye.
Common in-office topical anesthetics, proparacaine, tetracaine, benoxinate, and lidocaine also can cause AEs. Corneal toxicity is a well-known complication with topical anesthetic abuse, whereas allergic reactions are considered rare. The most common symptoms include stingingand discomfort upon instillation. Common signs include punctate corneal epithelial erosionsresulting indirectly from a decrease in reflex tearing, infrequent blinking, and increased tear evaporation.12 Topical anesthetics also inhibit the migration of corneal epithelial cells and cause direct damage to the cells that are present, leading to impaired healing and epithelial defects.13
Manifestations of allergic reaction to topical anesthetics can include conjunctival hyperemia and edema, edematous eyelids, and lacrimation. One published case described a 60-year-old woman who developed eczematous dermatitis of the eyelids after ophthalmic anesthetic drops were instilled prior to laser surgery. Patch testing showed a positive response to benzocaine 5%, proparacaine, and tetracaine 0.5%.14
Preservatives, in general, can cause an allergic reaction. Benzalkonium chloride’s (BAK) cytotoxic sequelae include possible trabecular cell death in glaucoma patients, disruption of tear film stability (even at low concentrations), and immune-allergenic properties. One article reported BAK as one of the 30 most frequent allergens causing allergic periorbital dermatitis.15 Benzalkonium chloride is used in most brands of phenylephrine. However, preservatives in this patient’s case were ruled out as instigating agents since both phenylephrine and tropicamide contain the same preservative, BAK 0.01%, yet this patient did not develop a reaction to tropicamide when used without phenylephrine. Expired medications also were not considered to be a factor as none of the medications used on the patient were indeed expired (the Malcom Randall VAMC clinic maintains a strict policy of discarding medications 28 days after being opened).
Although uncommon, phenylephrine sometimes has been found to cause a type 4 hypersensitivity reaction, also known as cell-mediated or delayed-type hypersensitivity.16 First, helper T cells secrete cytokines. Activation of cytokines recruits and activates cytotoxic T cells, monocytes, and macrophages, leading to inflammation of the surrounding tissue. Examples of cell-mediated hypersensitivity include reactions to the tuberculin skin test and to poison ivy.
Type 1 hypersensitivity reactions, also known as immediate or anaphylactic hypersensitivity reactions, are not triggered by phenylephrine. In this type of reaction, IgE binds to the mast cell on initial exposure to an allergen. On second exposure, the allergen binds to the IgE, causing the mast cell to release mediators of inflammation, triggering physiologic responses. Examples of this type of hypersensitivity include those seen with penicillin, bee stings, hay fever, bronchial asthma, and food allergies, for example, to shellfish.
A toxic reaction’s mechanism differs from that of a type 4 hypersensitivity reaction. Toxic reactions occur due to direct cytotoxicity of a drug caused by a low or high pH and either hyper- or hypo-osmolarity. Toxicity can lead to corneal and conjunctival cell necrosis or induce apoptosis, stimulating inflammatory reactions. Clinically, toxic reactions will present with follicles, whereas allergic reactions will present with papillae.
The definitive diagnostic methods used to determine the allergic agent causing ocular or periocular AEs are patch testing and conjunctival challenge.7 Mathias, Camarasa, Barber, Ducombs,and Monsálvezused patch testing to confirm phenylephrine as the allergic agent in their series of cases. Patch testing entails the application of a small amount of an allergic agent that is taped onto the skin. The allergic agent is confirmed if the patient has a dermal reaction, wherein the area patched will become erythematous. When patch testing is negative or inconclusive, a conjunctival challenge is performed by instillation of the suspected allergic agent into the eye with subsequent observation to determine whether a reaction occurs. The sequelae found in Villarreal’s study included itching, lacrimation, edema, erythema, and sometimes blepharitis.7
A direct conjunctival challenge with the suspected culprit was not pursued in this patient’s case due to the known severity of the potential resulting reaction. The authors instead chose an indirect method of determining the implicating agent and used the process of elimination to whittle down the most likely suspect. A challenge with the medications suspected not to be likely offenders was undertaken. This spared the patient a likely repeat of the AE he had just recovered from.
Management
Allergic reactions can resolve without medical intervention. The first step is to remove the allergen. For delayed hypersensitivity reactions, treatments may include topical decongestants, cool compresses, and corticosteroids.8 The treatment for immediate hypersensitivity reaction differs from that of delayed hypersensitivity reaction in that antihistamines are used.17,18
This patient reported receiving no treatment for his ocular symptoms following eye examinations in the past, yet he experienced complete resolution after each AE. In this case, both a steroid and a prophylactic antibiotic to facilitate a more rapid improvement were used.
Conclusion
Although uncommon, cases of allergic reaction to phenylephrine can occur. The incidence of phenylephrine allergy is 0.6%.6 The case study patient presented with a severe keratoconjunctivitis following routine eye examination with an accompanying history of adverse ocular signs and symptoms following multiple past exams.
It is important for all eye care clinicians to realize that AEs to diagnostic eye drops are possible and can occur following the most routine of visits. Such reactions can be caused by dilating agents, anesthetics, or preservatives, and these may be allergic or toxic. Clinicians should take special care to identify the instigating agent, and if possible, to avoid using such agents on patients during future exams. Clinicians also should understand how best to manage iatrogenic AEs when they encounter them in order to restore a patient’s visual function as quickly as possible.
Phenylephrine, a sympathomimetic drug, is commonly used in eye exams to dilate the pupil of the eye and to differentiate scleritis from episcleritis. Common adverse effects (AEs) of phenylephrine include subjective burning, stinging with lacrimation, rebound hyperemia, and liberation of iris pigment into the anterior chamber. Less common, systemic AEs include tachycardia and elevation of systemic blood pressure. Although instances of allergic reactions are rare, phenylephrine has been reported to cause contact dermatitis, blepharoconjunctivitis, and as in this case, keratoconjunctivitis.
Case Report
An 83-year-old white male presented for a red eye evaluation 2 days after having undergone a comprehensive eye exam with dilation at the Malcom Randall VAMC clinic in Gainesville, Florida. The patient reported onset of blurred vision, which he described as looking through a fog. He further compared the feeling to pins sticking in his eyes. The patient noted he had experienced similar symptoms on a few other occasions following eye exams. At the most recent eye exam, proparacaine and fluorescein had been used for tonometry, and phenylephrine 2.5% and tropicamide 0.5% had been used for pupillary dilation.
The patient’s best-corrected visual acuity was counting fingers at 2 feet in the right eye (OD) and left eye (OS). The best-corrected visual acuity 2 days prior had been 20/20 OD and OS. Pupils and extraocular motilities were unremarkable. Intraocular pressures were not obtained due to concern for a possible adverse reaction to proparacaine.
Slit-lamp evaluation revealed the lids to be lax, erythematous, and edematous in both eyes (Figure 1).
The initial diagnosis was acute chemical conjunctivitis most likely due to an AE to proparacaine. The plan was to start the patient on antibiotic eye drops qid OU, prednisolone qid OU, and artificial tears every hour OU. The patient was scheduled to return to clinic 4 days later for an anterior segment follow-up.
At the follow-up visit, the patient reported significant visual improvement. His best-corrected visual acuity was 20/40-2 without improvement on pinhole OD and 20/50-2 with improvement to 20/30+ on pinhole OS. Slit-lamp evaluation revealed 1+ bulbar conjunctival injection OU, intact corneal epithelium OU, and no cells or flare in the anterior chambers OU. Due to improving punctate epitheliopathy, the frequency of the antibiotic drops, the prednisolone, and the artificial tears was reduced to bid. After 3 days, he was instructed to discontinue them. The patient was scheduled to return in 2 weeks for an anterior segment follow-up.
At the next follow-up visit, the patient reported that his vision had returned to normal, and he had no further ocular AEs. His best-corrected visual acuity was 20/20-2 OD and 20/20 OS. Slit-lamp evaluation revealed mild blepharitis OU, trace bulbar conjunctival injection OU, and complete resolution of the keratitis OU. The assessment was acute allergic conjunctivitis thought to be secondary to an AE to proparacaine OU, yet the need to rule out hypersensitivity to tropicamide and/or phenylephrine remained. The plan was to educate the patient of the possibility of allergic reaction on future visits and to recommend continued use of artificial tears as needed.
Through a careful and extensive chart review of all past visits, it was suspected that phenylephrine might be to blame rather than proparacaine. At the subsequent visit, the patient agreed to undergo testing to determine the culprit via instillation of proparacaine in one eye and tropicamide in the other. The patient had no reaction to either drop (checked 45 minutes after instillation and the following day). By process of elimination, phenylephrine was determined to be the offending agent.
Discussion
Following a thorough review of the patient’s chart, it was found that on other occasions he had presented with suspected allergic reactions following routine eye examinations. The patient reported he had experienced a reaction in 2007 but could not recall what drops were instilled in his eyes at the time. In addition, there was no documentation in his medical record of the subsequent reaction following that visit. Another reaction occurred in July 2010 with instillation of tropicamide 1%, phenylephrine 2.5%, and Fluress (fluorescein sodium and benoxinate hydrochloride ophthalmic solution USP). In October 2013, when tropicamide 0.5%, proparacaine, and fluorescein strips were instilled, there was no reaction. The next reaction occurred in October 2014, when tropicamide 0.5%, phenylephrine 2.5%, proparacaine, and fluorescein strips were instilled.
This careful review of past exam notes revealed that phenylephrine and Fluress were the only drops that had not been instilled at the October 2013 visit when no AE was reported. However, Fluress was an unlikely culprit since it was not instilled in October 2014, and the patient still experienced an AE. Therefore, the agent most likely responsible for the allergic reaction in the patient, as confirmed by a review of the past notes and by the aforementioned pharmacologic test, was deemed to be phenylephrine (Table).
Adverse reactions to topical ocular medications and specifically to diagnostic eye drops have long been recognized. Mathias, Camarasa, Barber, Ducombs, and Monsálvezhave reported on variations of conjunctivitis and periorbital erythema with positive patch testing to phenylephrine.1-5 Geyer and colleagues reported on a study of 21 patients who had blepharoconjunctivitis after instillation of phenylephrine.6 In this case study patient, severe keratoconjunctivitis was the clinical manifestation observed.
Villarreal and colleagues studied 31 patients who had a previous reaction to mydriatic drops. The study found that phenylephrine was the drug that most frequently caused an AE (93.5%).7 One patient reacted to the preservative thimerosal, and 1 patient reacted to benoxiprocaine. Tropicamide was demonstrated to be very well tolerated as none of the patients tested positive on either the patch test or the pharmacologic test.
Tropicamide is a nonselective muscarinic antagonist commonly used for mydriasis due to its fast onset and short duration.8 Adverse reactions to tropicamide are rare. Three studies reported on patients who had a positive patch test to tropicamide.9-11 However, the reaction was not provoked by direct instillation of tropicamide into the eye.
Common in-office topical anesthetics, proparacaine, tetracaine, benoxinate, and lidocaine also can cause AEs. Corneal toxicity is a well-known complication with topical anesthetic abuse, whereas allergic reactions are considered rare. The most common symptoms include stingingand discomfort upon instillation. Common signs include punctate corneal epithelial erosionsresulting indirectly from a decrease in reflex tearing, infrequent blinking, and increased tear evaporation.12 Topical anesthetics also inhibit the migration of corneal epithelial cells and cause direct damage to the cells that are present, leading to impaired healing and epithelial defects.13
Manifestations of allergic reaction to topical anesthetics can include conjunctival hyperemia and edema, edematous eyelids, and lacrimation. One published case described a 60-year-old woman who developed eczematous dermatitis of the eyelids after ophthalmic anesthetic drops were instilled prior to laser surgery. Patch testing showed a positive response to benzocaine 5%, proparacaine, and tetracaine 0.5%.14
Preservatives, in general, can cause an allergic reaction. Benzalkonium chloride’s (BAK) cytotoxic sequelae include possible trabecular cell death in glaucoma patients, disruption of tear film stability (even at low concentrations), and immune-allergenic properties. One article reported BAK as one of the 30 most frequent allergens causing allergic periorbital dermatitis.15 Benzalkonium chloride is used in most brands of phenylephrine. However, preservatives in this patient’s case were ruled out as instigating agents since both phenylephrine and tropicamide contain the same preservative, BAK 0.01%, yet this patient did not develop a reaction to tropicamide when used without phenylephrine. Expired medications also were not considered to be a factor as none of the medications used on the patient were indeed expired (the Malcom Randall VAMC clinic maintains a strict policy of discarding medications 28 days after being opened).
Although uncommon, phenylephrine sometimes has been found to cause a type 4 hypersensitivity reaction, also known as cell-mediated or delayed-type hypersensitivity.16 First, helper T cells secrete cytokines. Activation of cytokines recruits and activates cytotoxic T cells, monocytes, and macrophages, leading to inflammation of the surrounding tissue. Examples of cell-mediated hypersensitivity include reactions to the tuberculin skin test and to poison ivy.
Type 1 hypersensitivity reactions, also known as immediate or anaphylactic hypersensitivity reactions, are not triggered by phenylephrine. In this type of reaction, IgE binds to the mast cell on initial exposure to an allergen. On second exposure, the allergen binds to the IgE, causing the mast cell to release mediators of inflammation, triggering physiologic responses. Examples of this type of hypersensitivity include those seen with penicillin, bee stings, hay fever, bronchial asthma, and food allergies, for example, to shellfish.
A toxic reaction’s mechanism differs from that of a type 4 hypersensitivity reaction. Toxic reactions occur due to direct cytotoxicity of a drug caused by a low or high pH and either hyper- or hypo-osmolarity. Toxicity can lead to corneal and conjunctival cell necrosis or induce apoptosis, stimulating inflammatory reactions. Clinically, toxic reactions will present with follicles, whereas allergic reactions will present with papillae.
The definitive diagnostic methods used to determine the allergic agent causing ocular or periocular AEs are patch testing and conjunctival challenge.7 Mathias, Camarasa, Barber, Ducombs,and Monsálvezused patch testing to confirm phenylephrine as the allergic agent in their series of cases. Patch testing entails the application of a small amount of an allergic agent that is taped onto the skin. The allergic agent is confirmed if the patient has a dermal reaction, wherein the area patched will become erythematous. When patch testing is negative or inconclusive, a conjunctival challenge is performed by instillation of the suspected allergic agent into the eye with subsequent observation to determine whether a reaction occurs. The sequelae found in Villarreal’s study included itching, lacrimation, edema, erythema, and sometimes blepharitis.7
A direct conjunctival challenge with the suspected culprit was not pursued in this patient’s case due to the known severity of the potential resulting reaction. The authors instead chose an indirect method of determining the implicating agent and used the process of elimination to whittle down the most likely suspect. A challenge with the medications suspected not to be likely offenders was undertaken. This spared the patient a likely repeat of the AE he had just recovered from.
Management
Allergic reactions can resolve without medical intervention. The first step is to remove the allergen. For delayed hypersensitivity reactions, treatments may include topical decongestants, cool compresses, and corticosteroids.8 The treatment for immediate hypersensitivity reaction differs from that of delayed hypersensitivity reaction in that antihistamines are used.17,18
This patient reported receiving no treatment for his ocular symptoms following eye examinations in the past, yet he experienced complete resolution after each AE. In this case, both a steroid and a prophylactic antibiotic to facilitate a more rapid improvement were used.
Conclusion
Although uncommon, cases of allergic reaction to phenylephrine can occur. The incidence of phenylephrine allergy is 0.6%.6 The case study patient presented with a severe keratoconjunctivitis following routine eye examination with an accompanying history of adverse ocular signs and symptoms following multiple past exams.
It is important for all eye care clinicians to realize that AEs to diagnostic eye drops are possible and can occur following the most routine of visits. Such reactions can be caused by dilating agents, anesthetics, or preservatives, and these may be allergic or toxic. Clinicians should take special care to identify the instigating agent, and if possible, to avoid using such agents on patients during future exams. Clinicians also should understand how best to manage iatrogenic AEs when they encounter them in order to restore a patient’s visual function as quickly as possible.
1. Mathias CG, Maibach HI, Irvine A, Adler W. Allergic contact dermatitis to echothiophate iodide and phenylephrine. Arch Ophthalmol. 1979;97(2):286-287.
2. Camarasa JG. Contact dermatitis to phenylephrine. Contact Dermatitis. 1984;10(3):182.
3. Barber K. Allergic contact eczema to phenylephrine. Contact Dermatitis. 1983;9(4):274-277.
4. Ducombs G, de Casamayor J, Verin P, Maleville J. Allergic contact dermatitis to phenylephrine. Contact Dermatitis. 1986;15(2):107-108.
5. Monsálvez V, Fuertes L, García-Cano I, Vanaclocha F, Ortez de Frutos J. Blepharoconjunctivitis due to phenylephrine [in Spanish]. Actas Dermosifiliogr. 2010;101(5):466-467.
6. Geyer O, Yust I, Lazar M. Allergic blepharoconjunctivitis due to phenylephrine. J Ocul Pharmacol. 1988;4(2):123-126.
7. Villarreal O. Reliability of diagnostic tests for contact allergy to mydriatic eyedrops. Contact Dermatitis. 1998;38(3):150-154.
8. Frazier M, Jaanus SD. Cycloplegics. In: Bartlett JD, Jaanus SD. Clinical Ocular Pharmacology. 5th ed. St. Louis, MO: Butterworth-Heinemann; 2009:125-138.
9. Decraene T, Goossens A. Contact allergy to atropine and other mydriatic agents in eye drops. Contact Dermatitis. 2001;45(5):309-310.
10. Boukhman MP, Maibach HI. Allergic contact dermatitis from tropicamide ophthalmic solution. Contact Dermatitis. 1999;41(1):47-48.
11. Yoshikawa K, Kawahara S. Contact allergy to atropine and other mydriatic agents. Contact Dermatitis. 1985;12(1):56-57.
12. Mcgee HT, Fraunfelder FW. Toxicities of topical ophthalmic anesthetics. Expert Opin Drug Saf. 2007;6(6):637-640.
13. Dass BA, Soong HK, Lee B. Effects of proparacaine of actin cytoskeleton of corneal epithelium. J Ocul Pharmacol. 1988;4(3):187-194.
14. Dannaker CJ, Maibach HI, Austin E. Allergic contact dermatitis to proparacaine with subsequent cross-sensitization to tetracaine from ophthalmic preparations. Am J Contact Dermat. 2001;12(3):177-179.
15. Hong J, Bielory L. Allergy to ophthalmic preservatives. Curr Opin Allergy Clin Immunol. 2009;9(5):447-453.
16. Gonzalo-Garijo MA, Pérez-Calderón R, de Argila D, Rodríguez-Nevado I. Erythrodermia to pseudoephedrine in a patient with contact allergy to phenylephrine. Allergol Immunopathol (Madr). 2002;30(4):239-242.
17. Platts-Mills TAE. Immediate hypersensitivity (Type I). In: Male D, Brostoff J, Roth DB, Roitt I. Immunology. 7th ed. Canada: Elsevier Limited; 2006:423-446.
18. Britton W. Type IV hypersensitivity. In: Male D, Brostoff J, Roth DB, Roitt I. Immunology. 7th ed. Canada: Elsevier Limited; 2006:477-491.
1. Mathias CG, Maibach HI, Irvine A, Adler W. Allergic contact dermatitis to echothiophate iodide and phenylephrine. Arch Ophthalmol. 1979;97(2):286-287.
2. Camarasa JG. Contact dermatitis to phenylephrine. Contact Dermatitis. 1984;10(3):182.
3. Barber K. Allergic contact eczema to phenylephrine. Contact Dermatitis. 1983;9(4):274-277.
4. Ducombs G, de Casamayor J, Verin P, Maleville J. Allergic contact dermatitis to phenylephrine. Contact Dermatitis. 1986;15(2):107-108.
5. Monsálvez V, Fuertes L, García-Cano I, Vanaclocha F, Ortez de Frutos J. Blepharoconjunctivitis due to phenylephrine [in Spanish]. Actas Dermosifiliogr. 2010;101(5):466-467.
6. Geyer O, Yust I, Lazar M. Allergic blepharoconjunctivitis due to phenylephrine. J Ocul Pharmacol. 1988;4(2):123-126.
7. Villarreal O. Reliability of diagnostic tests for contact allergy to mydriatic eyedrops. Contact Dermatitis. 1998;38(3):150-154.
8. Frazier M, Jaanus SD. Cycloplegics. In: Bartlett JD, Jaanus SD. Clinical Ocular Pharmacology. 5th ed. St. Louis, MO: Butterworth-Heinemann; 2009:125-138.
9. Decraene T, Goossens A. Contact allergy to atropine and other mydriatic agents in eye drops. Contact Dermatitis. 2001;45(5):309-310.
10. Boukhman MP, Maibach HI. Allergic contact dermatitis from tropicamide ophthalmic solution. Contact Dermatitis. 1999;41(1):47-48.
11. Yoshikawa K, Kawahara S. Contact allergy to atropine and other mydriatic agents. Contact Dermatitis. 1985;12(1):56-57.
12. Mcgee HT, Fraunfelder FW. Toxicities of topical ophthalmic anesthetics. Expert Opin Drug Saf. 2007;6(6):637-640.
13. Dass BA, Soong HK, Lee B. Effects of proparacaine of actin cytoskeleton of corneal epithelium. J Ocul Pharmacol. 1988;4(3):187-194.
14. Dannaker CJ, Maibach HI, Austin E. Allergic contact dermatitis to proparacaine with subsequent cross-sensitization to tetracaine from ophthalmic preparations. Am J Contact Dermat. 2001;12(3):177-179.
15. Hong J, Bielory L. Allergy to ophthalmic preservatives. Curr Opin Allergy Clin Immunol. 2009;9(5):447-453.
16. Gonzalo-Garijo MA, Pérez-Calderón R, de Argila D, Rodríguez-Nevado I. Erythrodermia to pseudoephedrine in a patient with contact allergy to phenylephrine. Allergol Immunopathol (Madr). 2002;30(4):239-242.
17. Platts-Mills TAE. Immediate hypersensitivity (Type I). In: Male D, Brostoff J, Roth DB, Roitt I. Immunology. 7th ed. Canada: Elsevier Limited; 2006:423-446.
18. Britton W. Type IV hypersensitivity. In: Male D, Brostoff J, Roth DB, Roitt I. Immunology. 7th ed. Canada: Elsevier Limited; 2006:477-491.
Standardized attending rounds to improve the patient experience: A pragmatic cluster randomized controlled trial
Patient experience has recently received heightened attention given evidence supporting an association between patient experience and quality of care,1 and the coupling of patient satisfaction to reimbursement rates for Medicare patients.2 Patient experience is often assessed through surveys of patient satisfaction, which correlates with patient perceptions of nurse and physician communication.3 Teaching hospitals introduce variables that may impact communication, including the involvement of multiple levels of care providers and competing patient care vs. educational priorities. Patients admitted to teaching services express decreased satisfaction with coordination and overall care compared with patients on nonteaching services.4
Clinical supervision of trainees on teaching services is primarily achieved through attending rounds (AR), where patients’ clinical presentations and management are discussed with an attending physician. Poor communication during AR may negatively affect the patient experience through inefficient care coordination among the inter-professional care team or through implementation of interventions without patients’ knowledge or input.5-11 Although patient engagement in rounds has been associated with higher patient satisfaction with rounds,12-19 AR and case presentations often occur at a distance from the patient’s bedside.20,21 Furthermore, AR vary in the time allotted per patient and the extent of participation of nurses and other allied health professionals. Standardized bedside rounding processes have been shown to improve efficiency, decrease daily resident work hours,22 and improve nurse-physician teamwork.23
Despite these benefits, recent prospective studies of bedside AR interventions have not improved patient satisfaction with rounds. One involved the implementation of interprofessional patient-centered bedside rounds on a nonteaching service,24 while the other evaluated the impact of integrating athletic principles into multidisciplinary work rounds.25 Work at our institution had sought to develop AR practice recommendations to foster an optimal patient experience, while maintaining provider workflow efficiency, facilitating interdisciplinary communication, and advancing trainee education.26 Using these AR recommendations, we conducted a prospective randomized controlled trial to evaluate the impact of implementing a standardized bedside AR model on patient satisfaction with rounds. We also assessed attending physician and trainee satisfaction with rounds, and perceived and actual AR duration.
METHODS
Setting and Participants
This trial was conducted on the internal medicine teaching service of the University of California San Francisco Medical Center from September 3, 2013 to November 27, 2013. The service is comprised of 8 teams, with a total average daily census of 80 to 90 patients. Teams are comprised of an attending physician, a senior resident (in the second or third year of residency training), 2 interns, and a third- and/or fourth-year medical student.
This trial, which was approved by the University of California, San Francisco Committee on Human Research (UCSF CHR) and was registered with ClinicalTrials.gov (NCT01931553), was classified under Quality Improvement and did not require informed consent of patients or providers.
Intervention Description
We conducted a cluster randomized trial to evaluate the impact of a bundled set of 5 AR practice recommendations, adapted from published work,26 on patient experience, as well as on attending and trainee satisfaction: 1) huddling to establish the rounding schedule and priorities; 2) conducting bedside rounds; 3) integrating bedside nurses; 4) completing real-time order entry using bedside computers; 5) updating the whiteboard in each patient’s room with care plan information.
At the beginning of each month, study investigators (Nader Najafi and Bradley Monash) led a 1.5-hour workshop to train attending physicians and trainees allocated to the intervention arm on the recommended AR practices. Participants also received informational handouts to be referenced during AR. Attending physicians and trainees randomized to the control arm continued usual rounding practices. Control teams were notified that there would be observers on rounds but were not informed of the study aims.
Randomization and Team Assignments
The medicine service was divided into 2 arms, each comprised of 4 teams. Using a coin flip, Cluster 1 (Teams A, B, C and D) was randomized to the intervention, and Cluster 2 (Teams E, F, G and H) was randomized to the control. This design was pragmatically chosen to ensure that 1 team from each arm would admit patients daily. Allocation concealment of attending physicians and trainees was not possible given the nature of the intervention. Patients were blinded to study arm allocation.
MEASURES AND OUTCOMES
Adherence to Practice Recommendations
Thirty premedical students served as volunteer AR auditors. Each auditor received orientation and training in data collection techniques during a single 2-hour workshop. The auditors, blinded to study arm allocation, independently observed morning AR during weekdays and recorded the completion of the following elements as a dichotomous (yes/no) outcome: pre-rounds huddle, participation of nurse in AR, real-time order entry, and whiteboard use. They recorded the duration of AR per day for each team (minutes) and the rounding model for each patient rounding encounter during AR (bedside, hallway, or card flip).23 Bedside rounds were defined as presentation and discussion of the patient care plan in the presence of the patient. Hallway rounds were defined as presentation and discussion of the patient care plan partially outside the patient’s room and partially in the presence of the patient. Card-flip rounds were defined as presentation and discussion of the patient care plan entirely outside of the patient’s room without the team seeing the patient together. Two auditors simultaneously observed a random subset of patient-rounding encounters to evaluate inter-rater reliability, and the concordance between auditor observations was good (Pearson correlation = 0.66).27
Patient-Related Outcomes
The primary outcome was patient satisfaction with AR, assessed using a survey adapted from published work.12,14,28,29 Patients were approached to complete the questionnaire after they had experienced at least 1 AR. Patients were excluded if they were non-English-speaking, unavailable (eg, off the unit for testing or treatment), in isolation, or had impaired mental status. For patients admitted multiple times during the study period, only the first questionnaire was used. Survey questions included patient involvement in decision-making, quality of communication between patient and medicine team, and the perception that the medicine team cared about the patient. Patients were asked to state their level of agreement with each item on a 5-point Likert scale. We obtained data on patient demographics from administrative datasets.
Healthcare Provider Outcomes
Attending physicians and trainees on service for at least 7 consecutive days were sent an electronic survey, adapted from published work.25,30 Questions assessed satisfaction with AR, perceived value of bedside rounds, and extent of patient and nursing involvement.Level of agreement with each item was captured on a continuous scale; 0 = strongly disagree to 100 = strongly agree, or from 0 (far too little) to 100 (far too much), with 50 equating to “about right.” Attending physicians and trainees were also asked to estimate the average duration of AR (in minutes).
Statistical Analyses
Analyses were blinded to study arm allocation and followed intention-to-treat principles. One attending physician crossed over from intervention to control arm; patient surveys associated with this attending (n = 4) were excluded to avoid contamination. No trainees crossed over.
Demographic and clinical characteristics of patients who completed the survey are reported (Appendix). To compare patient satisfaction scores, we used a random-effects regression model to account for correlation among responses within teams within randomized clusters, defining teams by attending physician. As this correlation was negligible and not statistically significant, we did not adjust ordinary linear regression models for clustering. Given observed differences in patient characteristics, we adjusted for a number of covariates (eg, age, gender, insurance payer, race, marital status, trial group arm).
We conducted simple linear regression for attending and trainee satisfaction comparisons between arms, adjusting only for trainee type (eg, resident, intern, and medical student).
We compared the frequency with which intervention and control teams adhered to the 5 recommended AR practices using chi-square tests. We used independent Student’s t tests to compare total duration of AR by teams within each arm, as well as mean time spent per patient.
This trial had a fixed number of arms (n = 2), each of fixed size (n = 600), based on the average monthly inpatient census on the medicine service. This fixed sample size, with 80% power and α = 0.05, will be able to detect a 0.16 difference in patient satisfaction scores between groups.
All analyses were conducted using SAS® v 9.4 (SAS Institute, Inc., Cary, NC).
RESULTS
We observed 241 AR involving 1855 patient rounding encounters in the intervention arm and 264 AR involving 1903 patient rounding encounters in the control arm (response rates shown in Figure 1).
Patient Satisfaction and Clinical Outcomes
Five hundred ninety-five patients were allocated to the intervention arm and 605 were allocated to the control arm (Figure 1). Mean age, gender, race, marital status, primary language, and insurance provider did not differ between intervention and control arms (Table 1).
Patients in the intervention arm reported significantly higher satisfaction with AR and felt more cared for by their medicine team (Table 2).
Actual and Perceived Duration of Attending Rounds
The intervention shortened the total duration of AR by 8 minutes on average (143 vs. 151 minutes, P = 0.052) and the time spent per patient by 4 minutes on average (19 vs. 23 minutes, P < 0.001). Despite this, trainees in the intervention arm perceived AR to last longer (mean estimated time: 167 min vs. 152 min, P < 0.001).
Healthcare Provider Outcomes
We observed 79 attending physicians and trainees in the intervention arm and 78 in the control arm, with survey response rates shown in Figure 1. Attending physicians in the intervention and the control arms reported high levels of satisfaction with the quality of AR (Table 2). Attending physicians in the intervention arm were more likely to report an appropriate level of patient involvement and nurse involvement.
Although trainees in the intervention and control arms reported high levels of satisfaction with the quality of AR, trainees in the intervention arm reported lower satisfaction with AR compared with control arm trainees (Table 2). Trainees in the intervention arm reported that AR involved less autonomy, efficiency, and teaching. Trainees in the intervention arm also scored patient involvement more towards the “far too much” end of the scale compared with “about right” in the control arm. However, trainees in the intervention arm perceived nurse involvement closer to “about right,” as opposed to “far too little” in the control arm.
CONCLUSION/DISCUSSION
Training internal medicine teams to adhere to 5 recommended AR practices increased patient satisfaction with AR and the perception that patients were more cared for by their medicine team. Despite the intervention potentially shortening the duration of AR, attending physicians and trainees perceived AR to last longer, and trainee satisfaction with AR decreased.
Teams in the intervention arm adhered to all recommended rounding practices at higher rates than the control teams. Although intervention teams rounded at the bedside 53% of the time, they were encouraged to bedside round only on patients who desired to participate in rounds, were not altered, and for whom the clinical discussion was not too sensitive to occur at the bedside. Of the recommended rounding behaviors, the lowest adherence was seen with whiteboard use.
A major component of the intervention was to move the clinical presentation to the patient’s bedside. Most patients prefer being included in rounds and partaking in trainee education.12-19,28,29,31-33 Patients may also perceive that more time is spent with them during bedside case presentations,14,28 and exposure to providers conferring on their care may enhance patient confidence in the care being delivered.12 Although a recent study of patient-centered bedside rounding on a nonteaching service did not result in increased patient satisfaction,24 teaching services may offer more opportunities for improvement in care coordination and communication.4
Other aspects of the intervention may have contributed to increased patient satisfaction with AR. The pre-rounds huddle may have helped teams prioritize which patients required more time or would benefit most from bedside rounds. The involvement of nurses in AR may have bolstered communication and team dynamics, enhancing the patient’s perception of interprofessional collaboration. Real-time order entry might have led to more efficient implementation of the care plan, and whiteboard use may have helped to keep patients abreast of the care plan.
Patients in the intervention arm felt more cared for by their medicine teams but did not report improvements in communication or in shared decision-making. Prior work highlights that limited patient engagement, activation, and shared decision-making may occur during AR.24,34 Patient-physician communication during AR is challenged by time pressures and competing priorities, including the “need” for trainees to demonstrate their medical knowledge and clinical skills. Efforts that encourage bedside rounding should include communication training with respect to patient engagement and shared decision-making.
Attending physicians reported positive attitudes toward bedside rounding, consistent with prior studies.13,21,31 However, trainees in the intervention arm expressed decreased satisfaction with AR, estimating that AR took longer and reporting too much patient involvement. Prior studies reflect similar bedside-rounding concerns, including perceived workflow inefficiencies, infringement on teaching opportunities, and time constraints.12,20,35 Trainees are under intense time pressures to complete their work, attend educational conferences, and leave the hospital to attend afternoon clinic or to comply with duty-hour restrictions. Trainees value succinctness,12,35,36 so the perception that intervention AR lasted longer likely contributed to trainee dissatisfaction.
Reduced trainee satisfaction with intervention AR may have also stemmed from the perception of decreased autonomy and less teaching, both valued by trainees.20,35,36 The intervention itself reduced trainee autonomy because usual practice at our hospital involves residents deciding where and how to round. Attending physician presence at the bedside during rounds may have further infringed on trainee autonomy if the patient looked to the attending for answers, or if the attending was seen as the AR leader. Attending physicians may mitigate the risk of compromising trainee autonomy by allowing the trainee to speak first, ensuring the trainee is positioned closer to, and at eye level with, the patient, and redirecting patient questions to the trainee as appropriate. Optimizing trainee experience with bedside AR requires preparation and training of attending physicians, who may feel inadequately prepared to lead bedside rounds and conduct bedside teaching.37 Faculty must learn how to preserve team efficiency, create a safe, nonpunitive bedside environment that fosters the trainee-patient relationship, and ensure rounds remain educational.36,38,39
The intervention reduced the average time spent on AR and time spent per patient. Studies examining the relationship between bedside rounding and duration of rounds have yielded mixed results: some have demonstrated no effect of bedside rounds on rounding time,28,40 while others report longer rounding times.37 The pre-rounds huddle and real-time order writing may have enhanced workflow efficiency.
Our study has several limitations. These results reflect the experience of a single large academic medical center and may not be generalizable to other settings. Although overall patient response to the survey was low and may not be representative of the entire patient population, response rates in the intervention and control arms were equivalent. Non-English speaking patients may have preferences that were not reflected in our survey results, and we did not otherwise quantify individual reasons for survey noncompletion. The presence of auditors on AR may have introduced observer bias. There may have been crossover effect; however, observed prevalence of individual practices remained low in the control arm. The 1.5-hour workshop may have inadequately equipped trainees with the complex skills required to lead and participate in bedside rounding, and more training, experience, and feedback may have yielded different results. For instance, residents with more exposure to bedside rounding express greater appreciation of its role in education and patient care.20 While adherence to some of the recommended practices remained low, we did not employ a full range of change-management techniques. Instead, we opted for a “low intensity” intervention (eg, single workshop, handouts) that relied on voluntary adoption by medicine teams and that we hoped other institutions could reproduce. Finally, we did not assess the relative impact of individual rounding behaviors on the measured outcomes.
In conclusion, training medicine teams to adhere to a standardized bedside AR model increased patient satisfaction with rounds. Concomitant trainee dissatisfaction may require further experience and training of attending physicians and trainees to ensure successful adoption.
Acknowledgements
We would like to thank all patients, providers, and trainees who participated in this study. We would also like to acknowledge the following volunteer auditors who observed teams daily: Arianna Abundo, Elahhe Afkhamnejad, Yolanda Banuelos, Laila Fozoun, Soe Yupar Khin, Tam Thien Le, Wing Sum Li, Yaqiao Li, Mengyao Liu, Tzyy-Harn Lo, Shynh-Herng Lo, David Lowe, Danoush Paborji, Sa Nan Park, Urmila Powale, Redha Fouad Qabazard, Monique Quiroz, John-Luke Marcelo Rivera, Manfred Roy Luna Salvador, Tobias Gowen Squier-Roper, Flora Yan Ting, Francesca Natasha T. Tizon, Emily Claire Trautner, Stephen Weiner, Alice Wilson, Kimberly Woo, Bingling J Wu, Johnny Wu, Brenda Yee. Statistical expertise was provided by Joan Hilton from the UCSF Clinical and Translational Science Institute (CTSI), which is supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number UL1 TR000004. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. Thanks also to Oralia Schatzman, Andrea Mazzini, and Erika Huie for their administrative support, and John Hillman for data-related support. Special thanks to Kirsten Kangelaris and Andrew Auerbach for their valuable feedback throughout, and to Maria Novelero and Robert Wachter for their divisional support of this project.
Disclosure
The authors report no financial conflicts of interest.
1. Doyle C, Lennox L, Bell D. A systematic review of evidence on the links between patient experience and clinical safety and effectiveness. BMJ Open. 2013;3(1):1-18. PubMed
2. Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) Fact Sheet. August 2013. Centers for Medicare and Medicaid Services (CMS). Baltimore, MD.http://www.hcahpsonline.org/files/August_2013_HCAHPS_Fact_Sheet3.pdf. Accessed December 1, 2015.
3. Boulding W, Glickman SW, Manary MP, Schulman KA, Staelin R. Relationship between patient satisfaction with inpatient care and hospital readmission within 30 days. Am J Manag Care. 2011;17:41-48. PubMed
4. Wray CM, Flores A, Padula WV, Prochaska MT, Meltzer DO, Arora VM. Measuring patient experiences on hospitalist and teaching services: Patient responses to a 30-day postdischarge questionnaire. J Hosp Med. 2016;11(2):99-104. PubMed
5. Bharwani AM, Harris GC, Southwick FS. Perspective: A business school view of medical interprofessional rounds: transforming rounding groups into rounding teams. Acad Med. 2012;87(12):1768-1771. PubMed
6. Chand DV. Observational study using the tools of lean six sigma to improve the efficiency of the resident rounding process. J Grad Med Educ. 2011;3(2):144-150. PubMed
7. Stickrath C, Noble M, Prochazka A, et al. Attending rounds in the current era: what is and is not happening. JAMA Intern Med. 2013;173(12):1084-1089. PubMed
8. Weber H, Stöckli M, Nübling M, Langewitz WA. Communication during ward rounds in internal medicine. An analysis of patient-nurse-physician interactions using RIAS. Patient Educ Couns. 2007;67(3):343-348. PubMed
9. McMahon GT, Katz JT, Thorndike ME, Levy BD, Loscalzo J. Evaluation of a redesign initiative in an internal-medicine residency. N Engl J Med. 2010;362(14):1304-1311. PubMed
10. Amoss J. Attending rounds: where do we go from here?: comment on “Attending rounds in the current era”. JAMA Intern Med. 2013;173(12):1089-1090. PubMed
11. Curley C, McEachern JE, Speroff T. A firm trial of interdisciplinary rounds on the inpatient medical wards: an intervention designed using continuous quality improvement. Med Care. 1998;36(suppl 8):AS4-A12. PubMed
12. Wang-Cheng RM, Barnas GP, Sigmann P, Riendl PA, Young MJ. Bedside case presentations: why patients like them but learners don’t. J Gen Intern Med. 1989;4(4):284-287. PubMed
13. Chauke, HL, Pattinson RC. Ward rounds—bedside or conference room? S Afr Med J. 2006;96(5):398-400. PubMed
14. Lehmann LS, Brancati FL, Chen MC, Roter D, Dobs AS. The effect of bedside case presentations on patients’ perceptions of their medical care. N Engl J Med. 1997;336(16):336, 1150-1155. PubMed
15. Simons RJ, Baily RG, Zelis R, Zwillich CW. The physiologic and psychological effects of the bedside presentation. N Engl J Med. 1989;321(18):1273-1275. PubMed
16. Wise TN, Feldheim D, Mann LS, Boyle E, Rustgi VK. Patients’ reactions to house staff work rounds. Psychosomatics. 1985;26(8):669-672. PubMed
17. Linfors EW, Neelon FA. Sounding Boards. The case of bedside rounds. N Engl J Med. 1980;303(21):1230-1233. PubMed
18. Nair BR, Coughlan JL, Hensley MJ. Student and patient perspectives on bedside teaching. Med Educ. 1997;31(5):341-346. PubMed
19. Romano J. Patients’ attitudes and behavior in ward round teaching. JAMA. 1941;117(9):664-667.
20. Gonzalo JD, Masters PA, Simons RJ, Chuang CH. Attending rounds and bedside case presentations: medical student and medicine resident experiences and attitudes. Teach Learn Med. 2009;21(2):105-110. PubMed
21. Shoeb M, Khanna R, Fang M, et al. Internal medicine rounding practices and the Accreditation Council for Graduate Medical Education core competencies. J Hosp Med. 2014;9(4):239-243. PubMed
22. Calderon AS, Blackmore CC, Williams BL, et al. Transforming ward rounds through rounding-in-flow. J Grad Med Educ. 2014;6(4):750-755. PubMed
23. Henkin S, Chon TY, Christopherson ML, Halvorsen AJ, Worden LM, Ratelle JT. Improving nurse-physician teamwork through interprofessional bedside rounding. J Multidiscip Healthc. 2016;9:201-205. PubMed
24. O’Leary KJ, Killarney A, Hansen LO, et al. Effect of patient-centred bedside rounds on hospitalised patients’ decision control, activation and satisfaction with care. BMJ Qual Saf. 2016;25:921-928. PubMed
25. Southwick F, Lewis M, Treloar D, et al. Applying athletic principles to medical rounds to improve teaching and patient care. Acad Med. 2014;89(7):1018-1023. PubMed
26. Najafi N, Monash B, Mourad M, et al. Improving attending rounds: Qualitative reflections from multidisciplinary providers. Hosp Pract (1995). 2015;43(3):186-190. PubMed
27. Altman DG. Practical Statistics For Medical Research. Boca Raton, FL: Chapman & Hall/CRC; 2006.
28. Gonzalo JD, Chuang CH, Huang G, Smith C. The return of bedside rounds: an educational intervention. J Gen Intern Med. 2010;25(8):792-798. PubMed
29. Fletcher KE, Rankey DS, Stern DT. Bedside interactions from the other side of the bedrail. J Gen Intern Med. 2005;20(1):58-61. PubMed
30. Gatorounds: Applying Championship Athletic Principles to Healthcare. University of Florida Health. http://gatorounds.med.ufl.edu/surveys/. Accessed March 1, 2013.
31. Gonzalo JD, Heist BS, Duffy BL, et al. The value of bedside rounds: a multicenter qualitative study. Teach Learn Med. 2013;25(4):326-333. PubMed
32. Rogers HD, Carline JD, Paauw DS. Examination room presentations in general internal medicine clinic: patients’ and students’ perceptions. Acad Med. 2003;78(9):945-949. PubMed
33. Fletcher KE, Furney SL, Stern DT. Patients speak: what’s really important about bedside interactions with physician teams. Teach Learn Med. 2007;19(2):120-127. PubMed
34. Satterfield JM, Bereknyei S, Hilton JF, et al. The prevalence of social and behavioral topics and related educational opportunities during attending rounds. Acad Med. 2014; 89(11):1548-1557. PubMed
35. Kroenke K, Simmons JO, Copley JB, Smith C. Attending rounds: a survey of physician attitudes. J Gen Intern Med. 1990;5(3):229-233. PubMed
36. Castiglioni A, Shewchuk RM, Willett LL, Heudebert GR, Centor RM. A pilot study using nominal group technique to assess residents’ perceptions of successful attending rounds. J Gen Intern Med. 2008;23(7):1060-1065. PubMed
37. Crumlish CM, Yialamas MA, McMahon GT. Quantification of bedside teaching by an academic hospitalist group. J Hosp Med. 2009;4(5):304-307. PubMed
38. Gonzalo JD, Wolpaw DR, Lehman E, Chuang CH. Patient-centered interprofessional collaborative care: factors associated with bedside interprofessional rounds. J Gen Intern Med. 2014;29(7):1040-1047. PubMed
39. Roy B, Castiglioni A, Kraemer RR, et al. Using cognitive mapping to define key domains for successful attending rounds. J Gen Intern Med. 2012;27(11):1492-1498. PubMed
40. Bhansali P, Birch S, Campbell JK, et al. A time-motion study of inpatient rounds using a family-centered rounds model. Hosp Pediatr. 2013;3(1):31-38. PubMed
Patient experience has recently received heightened attention given evidence supporting an association between patient experience and quality of care,1 and the coupling of patient satisfaction to reimbursement rates for Medicare patients.2 Patient experience is often assessed through surveys of patient satisfaction, which correlates with patient perceptions of nurse and physician communication.3 Teaching hospitals introduce variables that may impact communication, including the involvement of multiple levels of care providers and competing patient care vs. educational priorities. Patients admitted to teaching services express decreased satisfaction with coordination and overall care compared with patients on nonteaching services.4
Clinical supervision of trainees on teaching services is primarily achieved through attending rounds (AR), where patients’ clinical presentations and management are discussed with an attending physician. Poor communication during AR may negatively affect the patient experience through inefficient care coordination among the inter-professional care team or through implementation of interventions without patients’ knowledge or input.5-11 Although patient engagement in rounds has been associated with higher patient satisfaction with rounds,12-19 AR and case presentations often occur at a distance from the patient’s bedside.20,21 Furthermore, AR vary in the time allotted per patient and the extent of participation of nurses and other allied health professionals. Standardized bedside rounding processes have been shown to improve efficiency, decrease daily resident work hours,22 and improve nurse-physician teamwork.23
Despite these benefits, recent prospective studies of bedside AR interventions have not improved patient satisfaction with rounds. One involved the implementation of interprofessional patient-centered bedside rounds on a nonteaching service,24 while the other evaluated the impact of integrating athletic principles into multidisciplinary work rounds.25 Work at our institution had sought to develop AR practice recommendations to foster an optimal patient experience, while maintaining provider workflow efficiency, facilitating interdisciplinary communication, and advancing trainee education.26 Using these AR recommendations, we conducted a prospective randomized controlled trial to evaluate the impact of implementing a standardized bedside AR model on patient satisfaction with rounds. We also assessed attending physician and trainee satisfaction with rounds, and perceived and actual AR duration.
METHODS
Setting and Participants
This trial was conducted on the internal medicine teaching service of the University of California San Francisco Medical Center from September 3, 2013 to November 27, 2013. The service is comprised of 8 teams, with a total average daily census of 80 to 90 patients. Teams are comprised of an attending physician, a senior resident (in the second or third year of residency training), 2 interns, and a third- and/or fourth-year medical student.
This trial, which was approved by the University of California, San Francisco Committee on Human Research (UCSF CHR) and was registered with ClinicalTrials.gov (NCT01931553), was classified under Quality Improvement and did not require informed consent of patients or providers.
Intervention Description
We conducted a cluster randomized trial to evaluate the impact of a bundled set of 5 AR practice recommendations, adapted from published work,26 on patient experience, as well as on attending and trainee satisfaction: 1) huddling to establish the rounding schedule and priorities; 2) conducting bedside rounds; 3) integrating bedside nurses; 4) completing real-time order entry using bedside computers; 5) updating the whiteboard in each patient’s room with care plan information.
At the beginning of each month, study investigators (Nader Najafi and Bradley Monash) led a 1.5-hour workshop to train attending physicians and trainees allocated to the intervention arm on the recommended AR practices. Participants also received informational handouts to be referenced during AR. Attending physicians and trainees randomized to the control arm continued usual rounding practices. Control teams were notified that there would be observers on rounds but were not informed of the study aims.
Randomization and Team Assignments
The medicine service was divided into 2 arms, each comprised of 4 teams. Using a coin flip, Cluster 1 (Teams A, B, C and D) was randomized to the intervention, and Cluster 2 (Teams E, F, G and H) was randomized to the control. This design was pragmatically chosen to ensure that 1 team from each arm would admit patients daily. Allocation concealment of attending physicians and trainees was not possible given the nature of the intervention. Patients were blinded to study arm allocation.
MEASURES AND OUTCOMES
Adherence to Practice Recommendations
Thirty premedical students served as volunteer AR auditors. Each auditor received orientation and training in data collection techniques during a single 2-hour workshop. The auditors, blinded to study arm allocation, independently observed morning AR during weekdays and recorded the completion of the following elements as a dichotomous (yes/no) outcome: pre-rounds huddle, participation of nurse in AR, real-time order entry, and whiteboard use. They recorded the duration of AR per day for each team (minutes) and the rounding model for each patient rounding encounter during AR (bedside, hallway, or card flip).23 Bedside rounds were defined as presentation and discussion of the patient care plan in the presence of the patient. Hallway rounds were defined as presentation and discussion of the patient care plan partially outside the patient’s room and partially in the presence of the patient. Card-flip rounds were defined as presentation and discussion of the patient care plan entirely outside of the patient’s room without the team seeing the patient together. Two auditors simultaneously observed a random subset of patient-rounding encounters to evaluate inter-rater reliability, and the concordance between auditor observations was good (Pearson correlation = 0.66).27
Patient-Related Outcomes
The primary outcome was patient satisfaction with AR, assessed using a survey adapted from published work.12,14,28,29 Patients were approached to complete the questionnaire after they had experienced at least 1 AR. Patients were excluded if they were non-English-speaking, unavailable (eg, off the unit for testing or treatment), in isolation, or had impaired mental status. For patients admitted multiple times during the study period, only the first questionnaire was used. Survey questions included patient involvement in decision-making, quality of communication between patient and medicine team, and the perception that the medicine team cared about the patient. Patients were asked to state their level of agreement with each item on a 5-point Likert scale. We obtained data on patient demographics from administrative datasets.
Healthcare Provider Outcomes
Attending physicians and trainees on service for at least 7 consecutive days were sent an electronic survey, adapted from published work.25,30 Questions assessed satisfaction with AR, perceived value of bedside rounds, and extent of patient and nursing involvement.Level of agreement with each item was captured on a continuous scale; 0 = strongly disagree to 100 = strongly agree, or from 0 (far too little) to 100 (far too much), with 50 equating to “about right.” Attending physicians and trainees were also asked to estimate the average duration of AR (in minutes).
Statistical Analyses
Analyses were blinded to study arm allocation and followed intention-to-treat principles. One attending physician crossed over from intervention to control arm; patient surveys associated with this attending (n = 4) were excluded to avoid contamination. No trainees crossed over.
Demographic and clinical characteristics of patients who completed the survey are reported (Appendix). To compare patient satisfaction scores, we used a random-effects regression model to account for correlation among responses within teams within randomized clusters, defining teams by attending physician. As this correlation was negligible and not statistically significant, we did not adjust ordinary linear regression models for clustering. Given observed differences in patient characteristics, we adjusted for a number of covariates (eg, age, gender, insurance payer, race, marital status, trial group arm).
We conducted simple linear regression for attending and trainee satisfaction comparisons between arms, adjusting only for trainee type (eg, resident, intern, and medical student).
We compared the frequency with which intervention and control teams adhered to the 5 recommended AR practices using chi-square tests. We used independent Student’s t tests to compare total duration of AR by teams within each arm, as well as mean time spent per patient.
This trial had a fixed number of arms (n = 2), each of fixed size (n = 600), based on the average monthly inpatient census on the medicine service. This fixed sample size, with 80% power and α = 0.05, will be able to detect a 0.16 difference in patient satisfaction scores between groups.
All analyses were conducted using SAS® v 9.4 (SAS Institute, Inc., Cary, NC).
RESULTS
We observed 241 AR involving 1855 patient rounding encounters in the intervention arm and 264 AR involving 1903 patient rounding encounters in the control arm (response rates shown in Figure 1).
Patient Satisfaction and Clinical Outcomes
Five hundred ninety-five patients were allocated to the intervention arm and 605 were allocated to the control arm (Figure 1). Mean age, gender, race, marital status, primary language, and insurance provider did not differ between intervention and control arms (Table 1).
Patients in the intervention arm reported significantly higher satisfaction with AR and felt more cared for by their medicine team (Table 2).
Actual and Perceived Duration of Attending Rounds
The intervention shortened the total duration of AR by 8 minutes on average (143 vs. 151 minutes, P = 0.052) and the time spent per patient by 4 minutes on average (19 vs. 23 minutes, P < 0.001). Despite this, trainees in the intervention arm perceived AR to last longer (mean estimated time: 167 min vs. 152 min, P < 0.001).
Healthcare Provider Outcomes
We observed 79 attending physicians and trainees in the intervention arm and 78 in the control arm, with survey response rates shown in Figure 1. Attending physicians in the intervention and the control arms reported high levels of satisfaction with the quality of AR (Table 2). Attending physicians in the intervention arm were more likely to report an appropriate level of patient involvement and nurse involvement.
Although trainees in the intervention and control arms reported high levels of satisfaction with the quality of AR, trainees in the intervention arm reported lower satisfaction with AR compared with control arm trainees (Table 2). Trainees in the intervention arm reported that AR involved less autonomy, efficiency, and teaching. Trainees in the intervention arm also scored patient involvement more towards the “far too much” end of the scale compared with “about right” in the control arm. However, trainees in the intervention arm perceived nurse involvement closer to “about right,” as opposed to “far too little” in the control arm.
CONCLUSION/DISCUSSION
Training internal medicine teams to adhere to 5 recommended AR practices increased patient satisfaction with AR and the perception that patients were more cared for by their medicine team. Despite the intervention potentially shortening the duration of AR, attending physicians and trainees perceived AR to last longer, and trainee satisfaction with AR decreased.
Teams in the intervention arm adhered to all recommended rounding practices at higher rates than the control teams. Although intervention teams rounded at the bedside 53% of the time, they were encouraged to bedside round only on patients who desired to participate in rounds, were not altered, and for whom the clinical discussion was not too sensitive to occur at the bedside. Of the recommended rounding behaviors, the lowest adherence was seen with whiteboard use.
A major component of the intervention was to move the clinical presentation to the patient’s bedside. Most patients prefer being included in rounds and partaking in trainee education.12-19,28,29,31-33 Patients may also perceive that more time is spent with them during bedside case presentations,14,28 and exposure to providers conferring on their care may enhance patient confidence in the care being delivered.12 Although a recent study of patient-centered bedside rounding on a nonteaching service did not result in increased patient satisfaction,24 teaching services may offer more opportunities for improvement in care coordination and communication.4
Other aspects of the intervention may have contributed to increased patient satisfaction with AR. The pre-rounds huddle may have helped teams prioritize which patients required more time or would benefit most from bedside rounds. The involvement of nurses in AR may have bolstered communication and team dynamics, enhancing the patient’s perception of interprofessional collaboration. Real-time order entry might have led to more efficient implementation of the care plan, and whiteboard use may have helped to keep patients abreast of the care plan.
Patients in the intervention arm felt more cared for by their medicine teams but did not report improvements in communication or in shared decision-making. Prior work highlights that limited patient engagement, activation, and shared decision-making may occur during AR.24,34 Patient-physician communication during AR is challenged by time pressures and competing priorities, including the “need” for trainees to demonstrate their medical knowledge and clinical skills. Efforts that encourage bedside rounding should include communication training with respect to patient engagement and shared decision-making.
Attending physicians reported positive attitudes toward bedside rounding, consistent with prior studies.13,21,31 However, trainees in the intervention arm expressed decreased satisfaction with AR, estimating that AR took longer and reporting too much patient involvement. Prior studies reflect similar bedside-rounding concerns, including perceived workflow inefficiencies, infringement on teaching opportunities, and time constraints.12,20,35 Trainees are under intense time pressures to complete their work, attend educational conferences, and leave the hospital to attend afternoon clinic or to comply with duty-hour restrictions. Trainees value succinctness,12,35,36 so the perception that intervention AR lasted longer likely contributed to trainee dissatisfaction.
Reduced trainee satisfaction with intervention AR may have also stemmed from the perception of decreased autonomy and less teaching, both valued by trainees.20,35,36 The intervention itself reduced trainee autonomy because usual practice at our hospital involves residents deciding where and how to round. Attending physician presence at the bedside during rounds may have further infringed on trainee autonomy if the patient looked to the attending for answers, or if the attending was seen as the AR leader. Attending physicians may mitigate the risk of compromising trainee autonomy by allowing the trainee to speak first, ensuring the trainee is positioned closer to, and at eye level with, the patient, and redirecting patient questions to the trainee as appropriate. Optimizing trainee experience with bedside AR requires preparation and training of attending physicians, who may feel inadequately prepared to lead bedside rounds and conduct bedside teaching.37 Faculty must learn how to preserve team efficiency, create a safe, nonpunitive bedside environment that fosters the trainee-patient relationship, and ensure rounds remain educational.36,38,39
The intervention reduced the average time spent on AR and time spent per patient. Studies examining the relationship between bedside rounding and duration of rounds have yielded mixed results: some have demonstrated no effect of bedside rounds on rounding time,28,40 while others report longer rounding times.37 The pre-rounds huddle and real-time order writing may have enhanced workflow efficiency.
Our study has several limitations. These results reflect the experience of a single large academic medical center and may not be generalizable to other settings. Although overall patient response to the survey was low and may not be representative of the entire patient population, response rates in the intervention and control arms were equivalent. Non-English speaking patients may have preferences that were not reflected in our survey results, and we did not otherwise quantify individual reasons for survey noncompletion. The presence of auditors on AR may have introduced observer bias. There may have been crossover effect; however, observed prevalence of individual practices remained low in the control arm. The 1.5-hour workshop may have inadequately equipped trainees with the complex skills required to lead and participate in bedside rounding, and more training, experience, and feedback may have yielded different results. For instance, residents with more exposure to bedside rounding express greater appreciation of its role in education and patient care.20 While adherence to some of the recommended practices remained low, we did not employ a full range of change-management techniques. Instead, we opted for a “low intensity” intervention (eg, single workshop, handouts) that relied on voluntary adoption by medicine teams and that we hoped other institutions could reproduce. Finally, we did not assess the relative impact of individual rounding behaviors on the measured outcomes.
In conclusion, training medicine teams to adhere to a standardized bedside AR model increased patient satisfaction with rounds. Concomitant trainee dissatisfaction may require further experience and training of attending physicians and trainees to ensure successful adoption.
Acknowledgements
We would like to thank all patients, providers, and trainees who participated in this study. We would also like to acknowledge the following volunteer auditors who observed teams daily: Arianna Abundo, Elahhe Afkhamnejad, Yolanda Banuelos, Laila Fozoun, Soe Yupar Khin, Tam Thien Le, Wing Sum Li, Yaqiao Li, Mengyao Liu, Tzyy-Harn Lo, Shynh-Herng Lo, David Lowe, Danoush Paborji, Sa Nan Park, Urmila Powale, Redha Fouad Qabazard, Monique Quiroz, John-Luke Marcelo Rivera, Manfred Roy Luna Salvador, Tobias Gowen Squier-Roper, Flora Yan Ting, Francesca Natasha T. Tizon, Emily Claire Trautner, Stephen Weiner, Alice Wilson, Kimberly Woo, Bingling J Wu, Johnny Wu, Brenda Yee. Statistical expertise was provided by Joan Hilton from the UCSF Clinical and Translational Science Institute (CTSI), which is supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number UL1 TR000004. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. Thanks also to Oralia Schatzman, Andrea Mazzini, and Erika Huie for their administrative support, and John Hillman for data-related support. Special thanks to Kirsten Kangelaris and Andrew Auerbach for their valuable feedback throughout, and to Maria Novelero and Robert Wachter for their divisional support of this project.
Disclosure
The authors report no financial conflicts of interest.
Patient experience has recently received heightened attention given evidence supporting an association between patient experience and quality of care,1 and the coupling of patient satisfaction to reimbursement rates for Medicare patients.2 Patient experience is often assessed through surveys of patient satisfaction, which correlates with patient perceptions of nurse and physician communication.3 Teaching hospitals introduce variables that may impact communication, including the involvement of multiple levels of care providers and competing patient care vs. educational priorities. Patients admitted to teaching services express decreased satisfaction with coordination and overall care compared with patients on nonteaching services.4
Clinical supervision of trainees on teaching services is primarily achieved through attending rounds (AR), where patients’ clinical presentations and management are discussed with an attending physician. Poor communication during AR may negatively affect the patient experience through inefficient care coordination among the inter-professional care team or through implementation of interventions without patients’ knowledge or input.5-11 Although patient engagement in rounds has been associated with higher patient satisfaction with rounds,12-19 AR and case presentations often occur at a distance from the patient’s bedside.20,21 Furthermore, AR vary in the time allotted per patient and the extent of participation of nurses and other allied health professionals. Standardized bedside rounding processes have been shown to improve efficiency, decrease daily resident work hours,22 and improve nurse-physician teamwork.23
Despite these benefits, recent prospective studies of bedside AR interventions have not improved patient satisfaction with rounds. One involved the implementation of interprofessional patient-centered bedside rounds on a nonteaching service,24 while the other evaluated the impact of integrating athletic principles into multidisciplinary work rounds.25 Work at our institution had sought to develop AR practice recommendations to foster an optimal patient experience, while maintaining provider workflow efficiency, facilitating interdisciplinary communication, and advancing trainee education.26 Using these AR recommendations, we conducted a prospective randomized controlled trial to evaluate the impact of implementing a standardized bedside AR model on patient satisfaction with rounds. We also assessed attending physician and trainee satisfaction with rounds, and perceived and actual AR duration.
METHODS
Setting and Participants
This trial was conducted on the internal medicine teaching service of the University of California San Francisco Medical Center from September 3, 2013 to November 27, 2013. The service is comprised of 8 teams, with a total average daily census of 80 to 90 patients. Teams are comprised of an attending physician, a senior resident (in the second or third year of residency training), 2 interns, and a third- and/or fourth-year medical student.
This trial, which was approved by the University of California, San Francisco Committee on Human Research (UCSF CHR) and was registered with ClinicalTrials.gov (NCT01931553), was classified under Quality Improvement and did not require informed consent of patients or providers.
Intervention Description
We conducted a cluster randomized trial to evaluate the impact of a bundled set of 5 AR practice recommendations, adapted from published work,26 on patient experience, as well as on attending and trainee satisfaction: 1) huddling to establish the rounding schedule and priorities; 2) conducting bedside rounds; 3) integrating bedside nurses; 4) completing real-time order entry using bedside computers; 5) updating the whiteboard in each patient’s room with care plan information.
At the beginning of each month, study investigators (Nader Najafi and Bradley Monash) led a 1.5-hour workshop to train attending physicians and trainees allocated to the intervention arm on the recommended AR practices. Participants also received informational handouts to be referenced during AR. Attending physicians and trainees randomized to the control arm continued usual rounding practices. Control teams were notified that there would be observers on rounds but were not informed of the study aims.
Randomization and Team Assignments
The medicine service was divided into 2 arms, each comprised of 4 teams. Using a coin flip, Cluster 1 (Teams A, B, C and D) was randomized to the intervention, and Cluster 2 (Teams E, F, G and H) was randomized to the control. This design was pragmatically chosen to ensure that 1 team from each arm would admit patients daily. Allocation concealment of attending physicians and trainees was not possible given the nature of the intervention. Patients were blinded to study arm allocation.
MEASURES AND OUTCOMES
Adherence to Practice Recommendations
Thirty premedical students served as volunteer AR auditors. Each auditor received orientation and training in data collection techniques during a single 2-hour workshop. The auditors, blinded to study arm allocation, independently observed morning AR during weekdays and recorded the completion of the following elements as a dichotomous (yes/no) outcome: pre-rounds huddle, participation of nurse in AR, real-time order entry, and whiteboard use. They recorded the duration of AR per day for each team (minutes) and the rounding model for each patient rounding encounter during AR (bedside, hallway, or card flip).23 Bedside rounds were defined as presentation and discussion of the patient care plan in the presence of the patient. Hallway rounds were defined as presentation and discussion of the patient care plan partially outside the patient’s room and partially in the presence of the patient. Card-flip rounds were defined as presentation and discussion of the patient care plan entirely outside of the patient’s room without the team seeing the patient together. Two auditors simultaneously observed a random subset of patient-rounding encounters to evaluate inter-rater reliability, and the concordance between auditor observations was good (Pearson correlation = 0.66).27
Patient-Related Outcomes
The primary outcome was patient satisfaction with AR, assessed using a survey adapted from published work.12,14,28,29 Patients were approached to complete the questionnaire after they had experienced at least 1 AR. Patients were excluded if they were non-English-speaking, unavailable (eg, off the unit for testing or treatment), in isolation, or had impaired mental status. For patients admitted multiple times during the study period, only the first questionnaire was used. Survey questions included patient involvement in decision-making, quality of communication between patient and medicine team, and the perception that the medicine team cared about the patient. Patients were asked to state their level of agreement with each item on a 5-point Likert scale. We obtained data on patient demographics from administrative datasets.
Healthcare Provider Outcomes
Attending physicians and trainees on service for at least 7 consecutive days were sent an electronic survey, adapted from published work.25,30 Questions assessed satisfaction with AR, perceived value of bedside rounds, and extent of patient and nursing involvement.Level of agreement with each item was captured on a continuous scale; 0 = strongly disagree to 100 = strongly agree, or from 0 (far too little) to 100 (far too much), with 50 equating to “about right.” Attending physicians and trainees were also asked to estimate the average duration of AR (in minutes).
Statistical Analyses
Analyses were blinded to study arm allocation and followed intention-to-treat principles. One attending physician crossed over from intervention to control arm; patient surveys associated with this attending (n = 4) were excluded to avoid contamination. No trainees crossed over.
Demographic and clinical characteristics of patients who completed the survey are reported (Appendix). To compare patient satisfaction scores, we used a random-effects regression model to account for correlation among responses within teams within randomized clusters, defining teams by attending physician. As this correlation was negligible and not statistically significant, we did not adjust ordinary linear regression models for clustering. Given observed differences in patient characteristics, we adjusted for a number of covariates (eg, age, gender, insurance payer, race, marital status, trial group arm).
We conducted simple linear regression for attending and trainee satisfaction comparisons between arms, adjusting only for trainee type (eg, resident, intern, and medical student).
We compared the frequency with which intervention and control teams adhered to the 5 recommended AR practices using chi-square tests. We used independent Student’s t tests to compare total duration of AR by teams within each arm, as well as mean time spent per patient.
This trial had a fixed number of arms (n = 2), each of fixed size (n = 600), based on the average monthly inpatient census on the medicine service. This fixed sample size, with 80% power and α = 0.05, will be able to detect a 0.16 difference in patient satisfaction scores between groups.
All analyses were conducted using SAS® v 9.4 (SAS Institute, Inc., Cary, NC).
RESULTS
We observed 241 AR involving 1855 patient rounding encounters in the intervention arm and 264 AR involving 1903 patient rounding encounters in the control arm (response rates shown in Figure 1).
Patient Satisfaction and Clinical Outcomes
Five hundred ninety-five patients were allocated to the intervention arm and 605 were allocated to the control arm (Figure 1). Mean age, gender, race, marital status, primary language, and insurance provider did not differ between intervention and control arms (Table 1).
Patients in the intervention arm reported significantly higher satisfaction with AR and felt more cared for by their medicine team (Table 2).
Actual and Perceived Duration of Attending Rounds
The intervention shortened the total duration of AR by 8 minutes on average (143 vs. 151 minutes, P = 0.052) and the time spent per patient by 4 minutes on average (19 vs. 23 minutes, P < 0.001). Despite this, trainees in the intervention arm perceived AR to last longer (mean estimated time: 167 min vs. 152 min, P < 0.001).
Healthcare Provider Outcomes
We observed 79 attending physicians and trainees in the intervention arm and 78 in the control arm, with survey response rates shown in Figure 1. Attending physicians in the intervention and the control arms reported high levels of satisfaction with the quality of AR (Table 2). Attending physicians in the intervention arm were more likely to report an appropriate level of patient involvement and nurse involvement.
Although trainees in the intervention and control arms reported high levels of satisfaction with the quality of AR, trainees in the intervention arm reported lower satisfaction with AR compared with control arm trainees (Table 2). Trainees in the intervention arm reported that AR involved less autonomy, efficiency, and teaching. Trainees in the intervention arm also scored patient involvement more towards the “far too much” end of the scale compared with “about right” in the control arm. However, trainees in the intervention arm perceived nurse involvement closer to “about right,” as opposed to “far too little” in the control arm.
CONCLUSION/DISCUSSION
Training internal medicine teams to adhere to 5 recommended AR practices increased patient satisfaction with AR and the perception that patients were more cared for by their medicine team. Despite the intervention potentially shortening the duration of AR, attending physicians and trainees perceived AR to last longer, and trainee satisfaction with AR decreased.
Teams in the intervention arm adhered to all recommended rounding practices at higher rates than the control teams. Although intervention teams rounded at the bedside 53% of the time, they were encouraged to bedside round only on patients who desired to participate in rounds, were not altered, and for whom the clinical discussion was not too sensitive to occur at the bedside. Of the recommended rounding behaviors, the lowest adherence was seen with whiteboard use.
A major component of the intervention was to move the clinical presentation to the patient’s bedside. Most patients prefer being included in rounds and partaking in trainee education.12-19,28,29,31-33 Patients may also perceive that more time is spent with them during bedside case presentations,14,28 and exposure to providers conferring on their care may enhance patient confidence in the care being delivered.12 Although a recent study of patient-centered bedside rounding on a nonteaching service did not result in increased patient satisfaction,24 teaching services may offer more opportunities for improvement in care coordination and communication.4
Other aspects of the intervention may have contributed to increased patient satisfaction with AR. The pre-rounds huddle may have helped teams prioritize which patients required more time or would benefit most from bedside rounds. The involvement of nurses in AR may have bolstered communication and team dynamics, enhancing the patient’s perception of interprofessional collaboration. Real-time order entry might have led to more efficient implementation of the care plan, and whiteboard use may have helped to keep patients abreast of the care plan.
Patients in the intervention arm felt more cared for by their medicine teams but did not report improvements in communication or in shared decision-making. Prior work highlights that limited patient engagement, activation, and shared decision-making may occur during AR.24,34 Patient-physician communication during AR is challenged by time pressures and competing priorities, including the “need” for trainees to demonstrate their medical knowledge and clinical skills. Efforts that encourage bedside rounding should include communication training with respect to patient engagement and shared decision-making.
Attending physicians reported positive attitudes toward bedside rounding, consistent with prior studies.13,21,31 However, trainees in the intervention arm expressed decreased satisfaction with AR, estimating that AR took longer and reporting too much patient involvement. Prior studies reflect similar bedside-rounding concerns, including perceived workflow inefficiencies, infringement on teaching opportunities, and time constraints.12,20,35 Trainees are under intense time pressures to complete their work, attend educational conferences, and leave the hospital to attend afternoon clinic or to comply with duty-hour restrictions. Trainees value succinctness,12,35,36 so the perception that intervention AR lasted longer likely contributed to trainee dissatisfaction.
Reduced trainee satisfaction with intervention AR may have also stemmed from the perception of decreased autonomy and less teaching, both valued by trainees.20,35,36 The intervention itself reduced trainee autonomy because usual practice at our hospital involves residents deciding where and how to round. Attending physician presence at the bedside during rounds may have further infringed on trainee autonomy if the patient looked to the attending for answers, or if the attending was seen as the AR leader. Attending physicians may mitigate the risk of compromising trainee autonomy by allowing the trainee to speak first, ensuring the trainee is positioned closer to, and at eye level with, the patient, and redirecting patient questions to the trainee as appropriate. Optimizing trainee experience with bedside AR requires preparation and training of attending physicians, who may feel inadequately prepared to lead bedside rounds and conduct bedside teaching.37 Faculty must learn how to preserve team efficiency, create a safe, nonpunitive bedside environment that fosters the trainee-patient relationship, and ensure rounds remain educational.36,38,39
The intervention reduced the average time spent on AR and time spent per patient. Studies examining the relationship between bedside rounding and duration of rounds have yielded mixed results: some have demonstrated no effect of bedside rounds on rounding time,28,40 while others report longer rounding times.37 The pre-rounds huddle and real-time order writing may have enhanced workflow efficiency.
Our study has several limitations. These results reflect the experience of a single large academic medical center and may not be generalizable to other settings. Although overall patient response to the survey was low and may not be representative of the entire patient population, response rates in the intervention and control arms were equivalent. Non-English speaking patients may have preferences that were not reflected in our survey results, and we did not otherwise quantify individual reasons for survey noncompletion. The presence of auditors on AR may have introduced observer bias. There may have been crossover effect; however, observed prevalence of individual practices remained low in the control arm. The 1.5-hour workshop may have inadequately equipped trainees with the complex skills required to lead and participate in bedside rounding, and more training, experience, and feedback may have yielded different results. For instance, residents with more exposure to bedside rounding express greater appreciation of its role in education and patient care.20 While adherence to some of the recommended practices remained low, we did not employ a full range of change-management techniques. Instead, we opted for a “low intensity” intervention (eg, single workshop, handouts) that relied on voluntary adoption by medicine teams and that we hoped other institutions could reproduce. Finally, we did not assess the relative impact of individual rounding behaviors on the measured outcomes.
In conclusion, training medicine teams to adhere to a standardized bedside AR model increased patient satisfaction with rounds. Concomitant trainee dissatisfaction may require further experience and training of attending physicians and trainees to ensure successful adoption.
Acknowledgements
We would like to thank all patients, providers, and trainees who participated in this study. We would also like to acknowledge the following volunteer auditors who observed teams daily: Arianna Abundo, Elahhe Afkhamnejad, Yolanda Banuelos, Laila Fozoun, Soe Yupar Khin, Tam Thien Le, Wing Sum Li, Yaqiao Li, Mengyao Liu, Tzyy-Harn Lo, Shynh-Herng Lo, David Lowe, Danoush Paborji, Sa Nan Park, Urmila Powale, Redha Fouad Qabazard, Monique Quiroz, John-Luke Marcelo Rivera, Manfred Roy Luna Salvador, Tobias Gowen Squier-Roper, Flora Yan Ting, Francesca Natasha T. Tizon, Emily Claire Trautner, Stephen Weiner, Alice Wilson, Kimberly Woo, Bingling J Wu, Johnny Wu, Brenda Yee. Statistical expertise was provided by Joan Hilton from the UCSF Clinical and Translational Science Institute (CTSI), which is supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number UL1 TR000004. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. Thanks also to Oralia Schatzman, Andrea Mazzini, and Erika Huie for their administrative support, and John Hillman for data-related support. Special thanks to Kirsten Kangelaris and Andrew Auerbach for their valuable feedback throughout, and to Maria Novelero and Robert Wachter for their divisional support of this project.
Disclosure
The authors report no financial conflicts of interest.
1. Doyle C, Lennox L, Bell D. A systematic review of evidence on the links between patient experience and clinical safety and effectiveness. BMJ Open. 2013;3(1):1-18. PubMed
2. Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) Fact Sheet. August 2013. Centers for Medicare and Medicaid Services (CMS). Baltimore, MD.http://www.hcahpsonline.org/files/August_2013_HCAHPS_Fact_Sheet3.pdf. Accessed December 1, 2015.
3. Boulding W, Glickman SW, Manary MP, Schulman KA, Staelin R. Relationship between patient satisfaction with inpatient care and hospital readmission within 30 days. Am J Manag Care. 2011;17:41-48. PubMed
4. Wray CM, Flores A, Padula WV, Prochaska MT, Meltzer DO, Arora VM. Measuring patient experiences on hospitalist and teaching services: Patient responses to a 30-day postdischarge questionnaire. J Hosp Med. 2016;11(2):99-104. PubMed
5. Bharwani AM, Harris GC, Southwick FS. Perspective: A business school view of medical interprofessional rounds: transforming rounding groups into rounding teams. Acad Med. 2012;87(12):1768-1771. PubMed
6. Chand DV. Observational study using the tools of lean six sigma to improve the efficiency of the resident rounding process. J Grad Med Educ. 2011;3(2):144-150. PubMed
7. Stickrath C, Noble M, Prochazka A, et al. Attending rounds in the current era: what is and is not happening. JAMA Intern Med. 2013;173(12):1084-1089. PubMed
8. Weber H, Stöckli M, Nübling M, Langewitz WA. Communication during ward rounds in internal medicine. An analysis of patient-nurse-physician interactions using RIAS. Patient Educ Couns. 2007;67(3):343-348. PubMed
9. McMahon GT, Katz JT, Thorndike ME, Levy BD, Loscalzo J. Evaluation of a redesign initiative in an internal-medicine residency. N Engl J Med. 2010;362(14):1304-1311. PubMed
10. Amoss J. Attending rounds: where do we go from here?: comment on “Attending rounds in the current era”. JAMA Intern Med. 2013;173(12):1089-1090. PubMed
11. Curley C, McEachern JE, Speroff T. A firm trial of interdisciplinary rounds on the inpatient medical wards: an intervention designed using continuous quality improvement. Med Care. 1998;36(suppl 8):AS4-A12. PubMed
12. Wang-Cheng RM, Barnas GP, Sigmann P, Riendl PA, Young MJ. Bedside case presentations: why patients like them but learners don’t. J Gen Intern Med. 1989;4(4):284-287. PubMed
13. Chauke, HL, Pattinson RC. Ward rounds—bedside or conference room? S Afr Med J. 2006;96(5):398-400. PubMed
14. Lehmann LS, Brancati FL, Chen MC, Roter D, Dobs AS. The effect of bedside case presentations on patients’ perceptions of their medical care. N Engl J Med. 1997;336(16):336, 1150-1155. PubMed
15. Simons RJ, Baily RG, Zelis R, Zwillich CW. The physiologic and psychological effects of the bedside presentation. N Engl J Med. 1989;321(18):1273-1275. PubMed
16. Wise TN, Feldheim D, Mann LS, Boyle E, Rustgi VK. Patients’ reactions to house staff work rounds. Psychosomatics. 1985;26(8):669-672. PubMed
17. Linfors EW, Neelon FA. Sounding Boards. The case of bedside rounds. N Engl J Med. 1980;303(21):1230-1233. PubMed
18. Nair BR, Coughlan JL, Hensley MJ. Student and patient perspectives on bedside teaching. Med Educ. 1997;31(5):341-346. PubMed
19. Romano J. Patients’ attitudes and behavior in ward round teaching. JAMA. 1941;117(9):664-667.
20. Gonzalo JD, Masters PA, Simons RJ, Chuang CH. Attending rounds and bedside case presentations: medical student and medicine resident experiences and attitudes. Teach Learn Med. 2009;21(2):105-110. PubMed
21. Shoeb M, Khanna R, Fang M, et al. Internal medicine rounding practices and the Accreditation Council for Graduate Medical Education core competencies. J Hosp Med. 2014;9(4):239-243. PubMed
22. Calderon AS, Blackmore CC, Williams BL, et al. Transforming ward rounds through rounding-in-flow. J Grad Med Educ. 2014;6(4):750-755. PubMed
23. Henkin S, Chon TY, Christopherson ML, Halvorsen AJ, Worden LM, Ratelle JT. Improving nurse-physician teamwork through interprofessional bedside rounding. J Multidiscip Healthc. 2016;9:201-205. PubMed
24. O’Leary KJ, Killarney A, Hansen LO, et al. Effect of patient-centred bedside rounds on hospitalised patients’ decision control, activation and satisfaction with care. BMJ Qual Saf. 2016;25:921-928. PubMed
25. Southwick F, Lewis M, Treloar D, et al. Applying athletic principles to medical rounds to improve teaching and patient care. Acad Med. 2014;89(7):1018-1023. PubMed
26. Najafi N, Monash B, Mourad M, et al. Improving attending rounds: Qualitative reflections from multidisciplinary providers. Hosp Pract (1995). 2015;43(3):186-190. PubMed
27. Altman DG. Practical Statistics For Medical Research. Boca Raton, FL: Chapman & Hall/CRC; 2006.
28. Gonzalo JD, Chuang CH, Huang G, Smith C. The return of bedside rounds: an educational intervention. J Gen Intern Med. 2010;25(8):792-798. PubMed
29. Fletcher KE, Rankey DS, Stern DT. Bedside interactions from the other side of the bedrail. J Gen Intern Med. 2005;20(1):58-61. PubMed
30. Gatorounds: Applying Championship Athletic Principles to Healthcare. University of Florida Health. http://gatorounds.med.ufl.edu/surveys/. Accessed March 1, 2013.
31. Gonzalo JD, Heist BS, Duffy BL, et al. The value of bedside rounds: a multicenter qualitative study. Teach Learn Med. 2013;25(4):326-333. PubMed
32. Rogers HD, Carline JD, Paauw DS. Examination room presentations in general internal medicine clinic: patients’ and students’ perceptions. Acad Med. 2003;78(9):945-949. PubMed
33. Fletcher KE, Furney SL, Stern DT. Patients speak: what’s really important about bedside interactions with physician teams. Teach Learn Med. 2007;19(2):120-127. PubMed
34. Satterfield JM, Bereknyei S, Hilton JF, et al. The prevalence of social and behavioral topics and related educational opportunities during attending rounds. Acad Med. 2014; 89(11):1548-1557. PubMed
35. Kroenke K, Simmons JO, Copley JB, Smith C. Attending rounds: a survey of physician attitudes. J Gen Intern Med. 1990;5(3):229-233. PubMed
36. Castiglioni A, Shewchuk RM, Willett LL, Heudebert GR, Centor RM. A pilot study using nominal group technique to assess residents’ perceptions of successful attending rounds. J Gen Intern Med. 2008;23(7):1060-1065. PubMed
37. Crumlish CM, Yialamas MA, McMahon GT. Quantification of bedside teaching by an academic hospitalist group. J Hosp Med. 2009;4(5):304-307. PubMed
38. Gonzalo JD, Wolpaw DR, Lehman E, Chuang CH. Patient-centered interprofessional collaborative care: factors associated with bedside interprofessional rounds. J Gen Intern Med. 2014;29(7):1040-1047. PubMed
39. Roy B, Castiglioni A, Kraemer RR, et al. Using cognitive mapping to define key domains for successful attending rounds. J Gen Intern Med. 2012;27(11):1492-1498. PubMed
40. Bhansali P, Birch S, Campbell JK, et al. A time-motion study of inpatient rounds using a family-centered rounds model. Hosp Pediatr. 2013;3(1):31-38. PubMed
1. Doyle C, Lennox L, Bell D. A systematic review of evidence on the links between patient experience and clinical safety and effectiveness. BMJ Open. 2013;3(1):1-18. PubMed
2. Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) Fact Sheet. August 2013. Centers for Medicare and Medicaid Services (CMS). Baltimore, MD.http://www.hcahpsonline.org/files/August_2013_HCAHPS_Fact_Sheet3.pdf. Accessed December 1, 2015.
3. Boulding W, Glickman SW, Manary MP, Schulman KA, Staelin R. Relationship between patient satisfaction with inpatient care and hospital readmission within 30 days. Am J Manag Care. 2011;17:41-48. PubMed
4. Wray CM, Flores A, Padula WV, Prochaska MT, Meltzer DO, Arora VM. Measuring patient experiences on hospitalist and teaching services: Patient responses to a 30-day postdischarge questionnaire. J Hosp Med. 2016;11(2):99-104. PubMed
5. Bharwani AM, Harris GC, Southwick FS. Perspective: A business school view of medical interprofessional rounds: transforming rounding groups into rounding teams. Acad Med. 2012;87(12):1768-1771. PubMed
6. Chand DV. Observational study using the tools of lean six sigma to improve the efficiency of the resident rounding process. J Grad Med Educ. 2011;3(2):144-150. PubMed
7. Stickrath C, Noble M, Prochazka A, et al. Attending rounds in the current era: what is and is not happening. JAMA Intern Med. 2013;173(12):1084-1089. PubMed
8. Weber H, Stöckli M, Nübling M, Langewitz WA. Communication during ward rounds in internal medicine. An analysis of patient-nurse-physician interactions using RIAS. Patient Educ Couns. 2007;67(3):343-348. PubMed
9. McMahon GT, Katz JT, Thorndike ME, Levy BD, Loscalzo J. Evaluation of a redesign initiative in an internal-medicine residency. N Engl J Med. 2010;362(14):1304-1311. PubMed
10. Amoss J. Attending rounds: where do we go from here?: comment on “Attending rounds in the current era”. JAMA Intern Med. 2013;173(12):1089-1090. PubMed
11. Curley C, McEachern JE, Speroff T. A firm trial of interdisciplinary rounds on the inpatient medical wards: an intervention designed using continuous quality improvement. Med Care. 1998;36(suppl 8):AS4-A12. PubMed
12. Wang-Cheng RM, Barnas GP, Sigmann P, Riendl PA, Young MJ. Bedside case presentations: why patients like them but learners don’t. J Gen Intern Med. 1989;4(4):284-287. PubMed
13. Chauke, HL, Pattinson RC. Ward rounds—bedside or conference room? S Afr Med J. 2006;96(5):398-400. PubMed
14. Lehmann LS, Brancati FL, Chen MC, Roter D, Dobs AS. The effect of bedside case presentations on patients’ perceptions of their medical care. N Engl J Med. 1997;336(16):336, 1150-1155. PubMed
15. Simons RJ, Baily RG, Zelis R, Zwillich CW. The physiologic and psychological effects of the bedside presentation. N Engl J Med. 1989;321(18):1273-1275. PubMed
16. Wise TN, Feldheim D, Mann LS, Boyle E, Rustgi VK. Patients’ reactions to house staff work rounds. Psychosomatics. 1985;26(8):669-672. PubMed
17. Linfors EW, Neelon FA. Sounding Boards. The case of bedside rounds. N Engl J Med. 1980;303(21):1230-1233. PubMed
18. Nair BR, Coughlan JL, Hensley MJ. Student and patient perspectives on bedside teaching. Med Educ. 1997;31(5):341-346. PubMed
19. Romano J. Patients’ attitudes and behavior in ward round teaching. JAMA. 1941;117(9):664-667.
20. Gonzalo JD, Masters PA, Simons RJ, Chuang CH. Attending rounds and bedside case presentations: medical student and medicine resident experiences and attitudes. Teach Learn Med. 2009;21(2):105-110. PubMed
21. Shoeb M, Khanna R, Fang M, et al. Internal medicine rounding practices and the Accreditation Council for Graduate Medical Education core competencies. J Hosp Med. 2014;9(4):239-243. PubMed
22. Calderon AS, Blackmore CC, Williams BL, et al. Transforming ward rounds through rounding-in-flow. J Grad Med Educ. 2014;6(4):750-755. PubMed
23. Henkin S, Chon TY, Christopherson ML, Halvorsen AJ, Worden LM, Ratelle JT. Improving nurse-physician teamwork through interprofessional bedside rounding. J Multidiscip Healthc. 2016;9:201-205. PubMed
24. O’Leary KJ, Killarney A, Hansen LO, et al. Effect of patient-centred bedside rounds on hospitalised patients’ decision control, activation and satisfaction with care. BMJ Qual Saf. 2016;25:921-928. PubMed
25. Southwick F, Lewis M, Treloar D, et al. Applying athletic principles to medical rounds to improve teaching and patient care. Acad Med. 2014;89(7):1018-1023. PubMed
26. Najafi N, Monash B, Mourad M, et al. Improving attending rounds: Qualitative reflections from multidisciplinary providers. Hosp Pract (1995). 2015;43(3):186-190. PubMed
27. Altman DG. Practical Statistics For Medical Research. Boca Raton, FL: Chapman & Hall/CRC; 2006.
28. Gonzalo JD, Chuang CH, Huang G, Smith C. The return of bedside rounds: an educational intervention. J Gen Intern Med. 2010;25(8):792-798. PubMed
29. Fletcher KE, Rankey DS, Stern DT. Bedside interactions from the other side of the bedrail. J Gen Intern Med. 2005;20(1):58-61. PubMed
30. Gatorounds: Applying Championship Athletic Principles to Healthcare. University of Florida Health. http://gatorounds.med.ufl.edu/surveys/. Accessed March 1, 2013.
31. Gonzalo JD, Heist BS, Duffy BL, et al. The value of bedside rounds: a multicenter qualitative study. Teach Learn Med. 2013;25(4):326-333. PubMed
32. Rogers HD, Carline JD, Paauw DS. Examination room presentations in general internal medicine clinic: patients’ and students’ perceptions. Acad Med. 2003;78(9):945-949. PubMed
33. Fletcher KE, Furney SL, Stern DT. Patients speak: what’s really important about bedside interactions with physician teams. Teach Learn Med. 2007;19(2):120-127. PubMed
34. Satterfield JM, Bereknyei S, Hilton JF, et al. The prevalence of social and behavioral topics and related educational opportunities during attending rounds. Acad Med. 2014; 89(11):1548-1557. PubMed
35. Kroenke K, Simmons JO, Copley JB, Smith C. Attending rounds: a survey of physician attitudes. J Gen Intern Med. 1990;5(3):229-233. PubMed
36. Castiglioni A, Shewchuk RM, Willett LL, Heudebert GR, Centor RM. A pilot study using nominal group technique to assess residents’ perceptions of successful attending rounds. J Gen Intern Med. 2008;23(7):1060-1065. PubMed
37. Crumlish CM, Yialamas MA, McMahon GT. Quantification of bedside teaching by an academic hospitalist group. J Hosp Med. 2009;4(5):304-307. PubMed
38. Gonzalo JD, Wolpaw DR, Lehman E, Chuang CH. Patient-centered interprofessional collaborative care: factors associated with bedside interprofessional rounds. J Gen Intern Med. 2014;29(7):1040-1047. PubMed
39. Roy B, Castiglioni A, Kraemer RR, et al. Using cognitive mapping to define key domains for successful attending rounds. J Gen Intern Med. 2012;27(11):1492-1498. PubMed
40. Bhansali P, Birch S, Campbell JK, et al. A time-motion study of inpatient rounds using a family-centered rounds model. Hosp Pediatr. 2013;3(1):31-38. PubMed
© 2017 Society of Hospital Medicine