User login
Combination arsenic emulsion in TACE and apatinib benefits advanced HCC patients
Key clinical point: Arsenic trioxide (ATO)/lipiodol emulsion in the transcatheter arterial chemoembolization (TACE) combined with apatinib was safe and effective in patients with advanced HCC.
Major finding: At one week after the aTACE plus apatinib procedure, levels of AST and ALT were significantly elevated compared with pre-treatment levels, indicating treatment response (65.84 U/L vs 54.15 U/L and 63.44 U/L vs 51.60 U/L, respectively). Median progression-free survival was 10.2 months, and median overall survival was 23.3 months, with longer survival in patients without portal vein tumor thrombus.
Study details: The data come from 87 consecutive adults with advanced hepatocellular carcinoma who underwent arsenic trioxide (ATO)/lipiodol emulsion in the transcatheter arterial chemoembolization (TACE) combined with apatinib for advanced hepatocellular carcinoma between December 2015 and February 2017.
Disclosures: The study received no outside funding. The researchers had no financial conflicts to disclose.
Source: Li Z et al. Can J Gastroenterol Hepatol. 2021 Aug 19. doi: 10.1155/2021/5565793.
Key clinical point: Arsenic trioxide (ATO)/lipiodol emulsion in the transcatheter arterial chemoembolization (TACE) combined with apatinib was safe and effective in patients with advanced HCC.
Major finding: At one week after the aTACE plus apatinib procedure, levels of AST and ALT were significantly elevated compared with pre-treatment levels, indicating treatment response (65.84 U/L vs 54.15 U/L and 63.44 U/L vs 51.60 U/L, respectively). Median progression-free survival was 10.2 months, and median overall survival was 23.3 months, with longer survival in patients without portal vein tumor thrombus.
Study details: The data come from 87 consecutive adults with advanced hepatocellular carcinoma who underwent arsenic trioxide (ATO)/lipiodol emulsion in the transcatheter arterial chemoembolization (TACE) combined with apatinib for advanced hepatocellular carcinoma between December 2015 and February 2017.
Disclosures: The study received no outside funding. The researchers had no financial conflicts to disclose.
Source: Li Z et al. Can J Gastroenterol Hepatol. 2021 Aug 19. doi: 10.1155/2021/5565793.
Key clinical point: Arsenic trioxide (ATO)/lipiodol emulsion in the transcatheter arterial chemoembolization (TACE) combined with apatinib was safe and effective in patients with advanced HCC.
Major finding: At one week after the aTACE plus apatinib procedure, levels of AST and ALT were significantly elevated compared with pre-treatment levels, indicating treatment response (65.84 U/L vs 54.15 U/L and 63.44 U/L vs 51.60 U/L, respectively). Median progression-free survival was 10.2 months, and median overall survival was 23.3 months, with longer survival in patients without portal vein tumor thrombus.
Study details: The data come from 87 consecutive adults with advanced hepatocellular carcinoma who underwent arsenic trioxide (ATO)/lipiodol emulsion in the transcatheter arterial chemoembolization (TACE) combined with apatinib for advanced hepatocellular carcinoma between December 2015 and February 2017.
Disclosures: The study received no outside funding. The researchers had no financial conflicts to disclose.
Source: Li Z et al. Can J Gastroenterol Hepatol. 2021 Aug 19. doi: 10.1155/2021/5565793.
New predictive markers for risk of HCC in cirrhotic chronic hepatitis B
Key clinical point: Serum Prothrombin Induced by Vitamin K Absence or Antagonist-II (PIVKA-II) and alpha-fetoprotein were significant predictors of hepatocellular carcionoma and death in patients with chronic hepatitis B-related cirrhosis.
Major finding: Alpha-fetoprotein levels greater than 7 ng/mL (hazard ratio [HR]: 2.84, 95% confidence interval [CI]: 1.73–4.67) and PIVKA-II levels greater than 50 mAU/mL at virological remission significantly predicted the development of HCC (hazard ratios 2.84 and 2.46, respectively).
Study details: The data come from 293 adults with chronic hepatitis B-related cirrhosis; after an average follow-up of 78 months, 76 patient developed HCC and 19 died.
Disclosures: The study was supported by the Ministry of Science and Technology, National Taiwan University Hospital, and the Liver Disease Prevention & Treatment Research Foundation, Taiwan. Lead author Dr. Su disclosed research grant from Gilead Sciences, and serving on speaker's bureaus for AbbVie, Bayer, Bristol-Myers Squibb, Gilead Sciences, Merck Sharp and Dohme, and Takeda.
Source: Su T-H et al. J Formos Med Assoc. 2021 Aug 24. doi: 10.1016/j.jfma.2021.08.003.
Key clinical point: Serum Prothrombin Induced by Vitamin K Absence or Antagonist-II (PIVKA-II) and alpha-fetoprotein were significant predictors of hepatocellular carcionoma and death in patients with chronic hepatitis B-related cirrhosis.
Major finding: Alpha-fetoprotein levels greater than 7 ng/mL (hazard ratio [HR]: 2.84, 95% confidence interval [CI]: 1.73–4.67) and PIVKA-II levels greater than 50 mAU/mL at virological remission significantly predicted the development of HCC (hazard ratios 2.84 and 2.46, respectively).
Study details: The data come from 293 adults with chronic hepatitis B-related cirrhosis; after an average follow-up of 78 months, 76 patient developed HCC and 19 died.
Disclosures: The study was supported by the Ministry of Science and Technology, National Taiwan University Hospital, and the Liver Disease Prevention & Treatment Research Foundation, Taiwan. Lead author Dr. Su disclosed research grant from Gilead Sciences, and serving on speaker's bureaus for AbbVie, Bayer, Bristol-Myers Squibb, Gilead Sciences, Merck Sharp and Dohme, and Takeda.
Source: Su T-H et al. J Formos Med Assoc. 2021 Aug 24. doi: 10.1016/j.jfma.2021.08.003.
Key clinical point: Serum Prothrombin Induced by Vitamin K Absence or Antagonist-II (PIVKA-II) and alpha-fetoprotein were significant predictors of hepatocellular carcionoma and death in patients with chronic hepatitis B-related cirrhosis.
Major finding: Alpha-fetoprotein levels greater than 7 ng/mL (hazard ratio [HR]: 2.84, 95% confidence interval [CI]: 1.73–4.67) and PIVKA-II levels greater than 50 mAU/mL at virological remission significantly predicted the development of HCC (hazard ratios 2.84 and 2.46, respectively).
Study details: The data come from 293 adults with chronic hepatitis B-related cirrhosis; after an average follow-up of 78 months, 76 patient developed HCC and 19 died.
Disclosures: The study was supported by the Ministry of Science and Technology, National Taiwan University Hospital, and the Liver Disease Prevention & Treatment Research Foundation, Taiwan. Lead author Dr. Su disclosed research grant from Gilead Sciences, and serving on speaker's bureaus for AbbVie, Bayer, Bristol-Myers Squibb, Gilead Sciences, Merck Sharp and Dohme, and Takeda.
Source: Su T-H et al. J Formos Med Assoc. 2021 Aug 24. doi: 10.1016/j.jfma.2021.08.003.
Adding pembrolizumab shows promise for treating unresectable HCC
Key clinical point: After a median of 27 months’ follow-up, the rates of conversion therapy and both overall and progression-free survival were significantly higher in HCC patients treated with pembrolizumab-lenvatinib-TACE compared to those treated with lenvatinib-TACE.
Major finding: The rate of conversion therapy at the last follow-up was 25.7% in the pembrolizumab-lenvatinib-TACE group vs 11.1% in the lenvatinib-TACE group; median overall survival was 18.1 months in the pembrolizumab-lenvatinib-TACE group vs 14.1 months in the lenvatinib-TACE group, and median progression-free survival interval was 9.2 months in the pembrolizumab-lenvatinib-TACE group vs 5.5 months in the lenvatinib-TACE group.
Study details: The data come from a retrospective study of 142 consecutive adult patients with programmed cell death ligand-1 (PD-L1)-positive unresectable hepatocellular carcinoma who were treated with either pembrolizumab-lenvatinib-transarterial chemoembolization (TACE) or lenvatinib-TACE sequential therapy.
Disclosures: The study received no outside funding. The researchers had no financial conflicts to disclose.
Source: Chen S et al. J Cancer Res Clin Oncol. 2021 Aug 28. doi: 10.1007/s00432-021-03767-4.
Key clinical point: After a median of 27 months’ follow-up, the rates of conversion therapy and both overall and progression-free survival were significantly higher in HCC patients treated with pembrolizumab-lenvatinib-TACE compared to those treated with lenvatinib-TACE.
Major finding: The rate of conversion therapy at the last follow-up was 25.7% in the pembrolizumab-lenvatinib-TACE group vs 11.1% in the lenvatinib-TACE group; median overall survival was 18.1 months in the pembrolizumab-lenvatinib-TACE group vs 14.1 months in the lenvatinib-TACE group, and median progression-free survival interval was 9.2 months in the pembrolizumab-lenvatinib-TACE group vs 5.5 months in the lenvatinib-TACE group.
Study details: The data come from a retrospective study of 142 consecutive adult patients with programmed cell death ligand-1 (PD-L1)-positive unresectable hepatocellular carcinoma who were treated with either pembrolizumab-lenvatinib-transarterial chemoembolization (TACE) or lenvatinib-TACE sequential therapy.
Disclosures: The study received no outside funding. The researchers had no financial conflicts to disclose.
Source: Chen S et al. J Cancer Res Clin Oncol. 2021 Aug 28. doi: 10.1007/s00432-021-03767-4.
Key clinical point: After a median of 27 months’ follow-up, the rates of conversion therapy and both overall and progression-free survival were significantly higher in HCC patients treated with pembrolizumab-lenvatinib-TACE compared to those treated with lenvatinib-TACE.
Major finding: The rate of conversion therapy at the last follow-up was 25.7% in the pembrolizumab-lenvatinib-TACE group vs 11.1% in the lenvatinib-TACE group; median overall survival was 18.1 months in the pembrolizumab-lenvatinib-TACE group vs 14.1 months in the lenvatinib-TACE group, and median progression-free survival interval was 9.2 months in the pembrolizumab-lenvatinib-TACE group vs 5.5 months in the lenvatinib-TACE group.
Study details: The data come from a retrospective study of 142 consecutive adult patients with programmed cell death ligand-1 (PD-L1)-positive unresectable hepatocellular carcinoma who were treated with either pembrolizumab-lenvatinib-transarterial chemoembolization (TACE) or lenvatinib-TACE sequential therapy.
Disclosures: The study received no outside funding. The researchers had no financial conflicts to disclose.
Source: Chen S et al. J Cancer Res Clin Oncol. 2021 Aug 28. doi: 10.1007/s00432-021-03767-4.
Antiviral Therapy Improves Hepatocellular Cancer Survival
Hepatocellular cancer (HCC) is the most common type of hepatic cancers, accounting for 65% of all hepatic cancers.1 Among all cancers, HCC is one of the fastest growing causes of death in the United States, and the rate of new HCC cases are on the rise over several decades.2 There are many risk factors leading to HCC, including alcohol use, obesity, and smoking. Infection with hepatitis C virus (HCV) poses a significant risk.1
The pathogenesis of HCV-induced carcinogenesis is mediated by a unique host-induced immunologic response. Viral replication induces production of inflammatory factors, such as tumor necrosis factor (TNF-α), interferon (IFN), and oxidative stress on hepatocytes, resulting in cell injury, death, and regeneration. Repetitive cycles of cellular death and regeneration induce fibrosis, which may lead to cirrhosis.3 Hence, early treatment of HCV infection and achieving sustained virologic response (SVR) may lead to decreased incidence and mortality associated with HCC.
Treatment of HCV infection has become more effective with the development of direct-acting antivirals (DAAs) leading to SVR in > 90% of patients compared with 40 to 50% with IFN-based treatment.4,5 DAAs have been proved safe and highly effective in eradicating HCV infection even in patients with advanced liver disease with decompensated cirrhosis.6 Although achieving SVR indicates a complete cure from chronic HCV infection, several studies have shown subsequent risk of developing HCC persists even after successful HCV treatment.7-9 Some studies show that using DAAs to achieve SVR in patients with HCV infection leads to a decreased relative risk of HCC development compared with patients who do not receive treatment.10-12 But data on HCC risk following DAA-induced SVR vs IFN-induced SVR are somewhat conflicting.
Much of the information regarding the association between SVR and HCC has been gleaned from large data banks without accounting for individual patient characteristics that can be obtained through full chart review. Due to small sample sizes in many chart review studies, the impact that SVR from DAA therapy has on the progression and severity of HCC is not entirely clear. The aim of our study is to evaluate the effect of HCV treatment and SVR status on overall survival (OS) in patients with HCC. Second, we aim to compare survival benefits, if any exist, among the 2 major HCV treatment modalities (IFN vs DAA).
Methods
We performed a retrospective review of patients at Memphis Veterans Affairs Medical Center (VAMC) in Tennessee to determine whether treatment for HCV infection in general, and achieving SVR in particular, makes a difference in progression, recurrence, or OS among patients with HCV infection who develop HCC. We identified 111 patients with a diagnosis of both HCV and new or recurrent HCC lesions from November 2008 to March 2019 (Table 1). We divided these patients based on their HCV treatment status, SVR status, and treatment types (IFN vs DAA).
The inclusion criteria were patients aged > 18 years treated at the Memphis VAMC who have HCV infection and developed HCC. Exclusion criteria were patients who developed HCC from other causes such as alcoholic steatohepatitis, hepatitis B virus infection, hemochromatosis, patients without HCV infection, and patients who were not established at the Memphis VAMC. This protocol was approved by the Memphis VAMC Institutional Review Board.
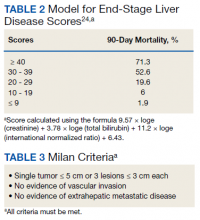
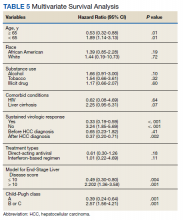
HCC diagnosis was determined using International Classification of Diseases codes (9th revision: 155 and 155.2; 10th revision: CD 22 and 22.9). We also used records of multidisciplinary gastrointestinal malignancy tumor conferences to identify patient who had been diagnosed and treated for HCV infection. We identified patients who were treated with DAA vs IFN as well as patients who had achieved SVR (classified as having negative HCV RNA tests at the end of DAA treatment). We were unable to evaluate Barcelona Clinic Liver Cancer staging since this required documented performance status that was not available in many patient records. We selected cases consistent with both treatment for HCV infection and subsequent development of HCC. Patient data included age; OS time; HIV status HCV genotype; time and status of progression to HCC; type and duration of treatment; and alcohol, tobacco, and drug use. Disease status was measured using the Model for End-Stage Liver Disease (MELD) score (Table 2), Milan criteria (Table 3), and Child-Pugh score (Table 4).
Statistical Analysis
OS was measured from the date of HCC diagnosis to the date of death or last follow-up. Progression-free survival (PFS) was defined from the date of HCC treatment initiation to the date of first HCC recurrence. We compared survival data for the SVR and non-SVR subgroups, the HCV treatment vs non-HCV treatment subgroups, and the IFN therapy vs DAA therapy subgroups, using the Kaplan-Meier method. The differences between subgroups were assessed using a log-rank test. Multivariate analysis using Cox proportional hazards regression model was used to identify factors that had significant impact on OS. Those factors included age; race; alcohol, tobacco, and illicit drug use; SVR status; HCV treatment status; IFN-based regimen vs DAA; MELD, and Child-Pugh scores. The results were expressed as hazard ratios (HRs) and 95% CI. Calculations were made using Statistical Analysis SAS and IBM SPSS software.
Results
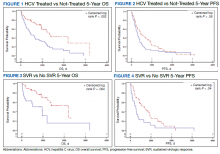
The study included 111 patients. The mean age was 65.7 years; all were male and half of were Black patients. The gender imbalance was due to the predominantly male patient population at Memphis VAMC. Among 111 patients with HCV infection and HCC, 68 patients were treated for HCV infection and had significantly improved OS and PFS compared with the nontreatment group. The median 5-year OS was 44.6 months (95% CI, 966-3202) in the treated HCV infection group compared with 15.1 months in the untreated HCV infection group with a Wilcoxon P = .0005 (Figure 1). Similarly, patients treated for HCV infection had a significantly better 5-year PFS of 15.3 months (95% CI, 294-726) compared with the nontreatment group 9.5 months (95% CI, 205-405) with a Wilcoxon P = .04 (Figure 2).
Among 68 patients treated for HCV infection, 51 achieved SVR, and 34 achieved SVR after the diagnosis of HCC. Patients who achieved SVR had an improved 5-year OS when compared with patients who did not achieve SVR (median 65.8 months [95% CI, 1222-NA] vs 15.7 months [95% CI, 242-853], Wilcoxon P < .001) (Figure 3). Similarly, patients with SVR had improved 5-year PFS when compared with the non-SVR group (median 20.5 months [95% CI, 431-914] vs 8.9 months [95% CI, 191-340], Wilcoxon P = .007 (Figure 4). Achievement of SVR after HCC diagnosis suggests a significantly improved OS (HR 0.37) compared with achievement prior to HCC diagnosis (HR, 0.65; 95% CI, 0.23-1.82, P = .41)
Multivariate Cox regression was used to determine factors with significant survival impact. Advanced age at diagnosis (aged ≥ 65 years) (HR, 0.53; 95% CI, 0.320-0.880; P = .01), SVR status (HR, 0.33; 95% CI, 0.190-0.587; P < .001), achieving SVR after HCC diagnosis (HR, 0.37; 95% CI, 0.20-0.71; P = .002), low MELD score (< 10) (HR, 0.49; 95% CI, 0.30-0.80; P = .004) and low Child-Pugh score (class A) (HR, 0.39; 95% CI, 0.24-0.64; P = .001) have a significant positive impact on OS. Survival was not significantly influenced by race, tobacco, drug use, HIV or cirrhosis status, or HCV treatment type. In addition, higher Child-Pugh class (B or C), higher MELD score (> 10), and younger age at diagnosis (< 65 years) have a negative impact on survival outcome (Table 5).
Discussion
The survival benefit of HCV eradication and achieving SVR status has been well established in patients with HCC.13 In a retrospective cohort study of 250 patients with HCV infection who had received curative treatment for HCC, multivariate analysis demonstrated that achieving SVR is an independent predictor of OS.14 The 3-year and 5-year OS rates were 97% and 94% for the SVR group, and 91% and 60% for the non‐SVR group, respectively (P < .001). Similarly, according to Sou and colleagues, of 122 patients with HCV-related HCC, patients with SVR had longer OS than patients with no SVR (P = .04).15 One of the hypotheses that could explain the survival benefit in patients who achieved SVR is the effect of achieving SVR in reducing persistent liver inflammation and associated liver mortality, and therefore lowering risks of complication in patients with HCC.16 In our study, multivariate analysis shows that achieving SVR is associated with significant improved OS (HR, 0.33). In contrast, patients with HCC who have not achieved SVR are associated with worse survival (HR, 3.24). This finding supports early treatment of HCV to obtain SVR in HCV-related patients with HCC, even after development of HCC.
Among 68 patients treated for HCV infection, 45 patients were treated after HCC diagnosis, and 34 patients achieved SVR after HCC diagnosis. The average time between HCV infection treatment after HCC diagnosis was 6 months. Our data suggested that achievement of SVR after HCC diagnosis suggests an improved OS (HR, 0.37) compared with achievement prior to HCC diagnosis (HR, 0.65; 95% CI,0.23-1.82; P = .41). This lack of statistical significance is likely due to small sample size of patients achieving SVR prior to HCC diagnosis. Our results are consistent with the findings regarding the efficacy and timing of DAA treatment in patients with active HCC. According to Singal and colleagues, achieving SVR after DAA therapy may result in improved liver function and facilitate additional HCC-directed therapy, which potentially improves survival.17-19
Nagaoki and colleagues found that there was no significant difference in OS in patients with HCC between the DAA and IFN groups. According to the study, the 3-year and 5-year OS rates were 96% and 96% for DAA patients and 93% and 73% for IFN patients, respectively (P = .16).14 This finding is consistent with the results of our study. HCV treatment type (IFN vs DAA) was not found to be associated with either OS or PFS time, regardless of time period.
A higher MELD score (> 10) and a higher Child-Pugh class (B or C) score are associated with worse survival outcome regardless of SVR status. While patients with a low MELD score (≤ 10) have a better survival rate (HR 0.49), a higher MELD score has a significantly higher HR and therefore worse survival outcomes (HR, 2.20). Similarly, patients with Child-Pugh A (HR, 0.39) have a better survival outcome compared with those patients with Child-Pugh class B or C (HR, 2.57). This finding is consistent with results of multiple studies indicating that advanced liver disease, as measured by a high MELD score and Child-Pugh class score, can be used to predict the survival outcome in patients with HCV-related HCC.20-22
Unlike other studies that look at a single prognostic variable, our study evaluated prognostic impacts of multiple variables (age, SVR status, the order of SVR in relation to HCC development, HCV treatment type, MELD score and Child-Pugh class) in patients with HCC. The study included patients treated for HCV after development of HCC along with other multiple variables leading to OS benefit. It is one of the only studies in the United States that compared 5-year OS and PFS among patients with HCC treated for HCV and achieved SVR. The studies by Nagaoki and colleagues and Sou and colleagues were conducted in Japan, and some of their subset analyses were univariate. Among our study population of veterans, 50% were African American patients, suggesting that they may have similar OS benefit when compared to White patients with HCC and HCV treatment.
Limitations
Our findings were limited in that our study population is too small to conduct further subset analysis that would allow statistical significance of those subsets, such as the suggested benefit of SVR in patients who presented with HCC after antiviral therapy. Another limitation is the all-male population, likely a result of the older veteran population at the Memphis VAMC. The mean age at diagnosis was 65 years, which is slightly higher than the general population. Compared to the SEER database, HCC is most frequently diagnosed among people aged 55 to 64 years.23 The age difference was likely due to our aging veteran population.
Further studies are needed to determine the significance of SVR on HCC recurrence and treatment. Immunotherapy is now first-line treatment for patients with local advanced HCC. All the immunotherapy studies excluded patients with active HCV infection. Hence, we need more data on HCV treatment timing among patients scheduled to start treatment with immunotherapy.
Conclusions
In a population of older veterans, treatment of HCV infection leads to OS benefit among patients with HCC. In addition, patients with HCV infection who achieve SVR have an OS benefit over patients unable to achieve SVR. The type of treatment, DAA vs IFN-based regimen, did not show significant survival benefit.
1. Ghouri YA, Mian I, Rowe JH. Review of hepatocellular carcinoma: epidemiology, etiology, and carcinogenesis. J Carcinog. 2017;16:1. Published 2017 May 29. doi:10.4103/jcar.JCar_9_16
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424. doi:10.3322/caac.21492
3. Farazi PA, DePinho RA. Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer. 2006;6(9):674-687. doi:10.1038/nrc1934
4. Falade-Nwulia O, Suarez-Cuervo C, Nelson DR, Fried MW, Segal JB, Sulkowski MS. Oral direct-acting agent therapy for hepatitis c virus infection: a systematic review. Ann Intern Med. 2017;166(9):637-648. doi:10.7326/M16-2575
5. Kouris G, Hydery T, Greenwood BC, et al. Effectiveness of Ledipasvir/Sofosbuvir and predictors of treatment failure in members with hepatitis C genotype 1 infection: a retrospective cohort study in a medicaid population. J Manag Care Spec Pharm. 2018;24(7):591-597. doi:10.18553/jmcp.2018.24.7.591
6. Jacobson IM, Lawitz E, Kwo PY, et al. Safety and efficacy of elbasvir/grazoprevir in patients with hepatitis c virus infection and compensated cirrhosis: an integrated analysis. Gastroenterology. 2017;152(6):1372-1382.e2. doi:10.1053/j.gastro.2017.01.050
7. Nahon P, Layese R, Bourcier V, et al. Incidence of hepatocellular carcinoma after direct antiviral therapy for HCV in patients with cirrhosis included in surveillance programs. Gastroenterology. 2018;155(5):1436-1450.e6. doi:10.1053/j.gastro.2018.07.01510.
8. Innes H, Barclay ST, Hayes PC, et al. The risk of hepatocellular carcinoma in cirrhotic patients with hepatitis C and sustained viral response: role of the treatment regimen. J Hepatol. 2018;68(4):646-654. doi:10.1016/j.jhep.2017.10.033
9. Romano A, Angeli P, Piovesan S, et al. Newly diagnosed hepatocellular carcinoma in patients with advanced hepatitis C treated with DAAs: a prospective population study. J Hepatol. 2018;69(2):345-352. doi:10.1016/j.jhep.2018.03.009
10. Kanwal F, Kramer J, Asch SM, Chayanupatkul M, Cao Y, El-Serag HB. Risk of hepatocellular cancer in HCV patients treated with direct-acting antiviral agents. Gastroenterology. 2017;153(4):996-1005.e1. doi:10.1053/j.gastro.2017.06.0122
11. Singh S, Nautiyal A, Loke YK. Oral direct-acting antivirals and the incidence or recurrence of hepatocellular carcinoma: a systematic review and meta-analysis. Frontline Gastroenterol. 2018;9(4):262-270. doi:10.1136/flgastro-2018-101017
12. Kuftinec G, Loehfelm T, Corwin M, et al. De novo hepatocellular carcinoma occurrence in hepatitis C cirrhotics treated with direct-acting antiviral agents. Hepat Oncol. 2018;5(1):HEP06. Published 2018 Jul 25. doi:10.2217/hep-2018-00033
13. Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med. 2013;158(5 Pt 1):329-337. doi:10.7326/0003-4819-158-5-201303050-00005
14. Nagaoki Y, Imamura M, Nishida Y, et al. The impact of interferon-free direct-acting antivirals on clinical outcome after curative treatment for hepatitis C virus-associated hepatocellular carcinoma: comparison with interferon-based therapy. J Med Virol. 2019;91(4):650-658. doi:10.1002/jmv.25352
15. Sou FM, Wu CK, Chang KC, et al. Clinical characteristics and prognosis of HCC occurrence after antiviral therapy for HCV patients between sustained and non-sustained responders. J Formos Med Assoc. 2019;118(1 Pt 3):504-513. doi:10.1016/j.jfma.2018.10.017
16. Roche B, Coilly A, Duclos-Vallee JC, Samuel D. The impact of treatment of hepatitis C with DAAs on the occurrence of HCC. Liver Int. 2018;38(suppl 1):139-145. doi:10.1111/liv.13659
17. Singal AG, Lim JK, Kanwal F. AGA clinical practice update on interaction between oral direct-acting antivirals for chronic hepatitis C infection and hepatocellular carcinoma: expert review. Gastroenterology. 2019;156(8):2149-2157. doi:10.1053/j.gastro.2019.02.046
18. Toyoda H, Kumada T, Hayashi K, et al. Characteristics and prognosis of hepatocellular carcinoma detected in sustained responders to interferon therapy for chronic hepatitis C. Cancer Detect Prev. 2003;27(6):498-502. doi:10.1016/j.cdp.2003.09.00719. Okamura Y, Sugiura T, Ito T, et al. The achievement of a sustained virological response either before or after hepatectomy improves the prognosis of patients with primary hepatitis C virus-related hepatocellular carcinoma. Ann Surg Oncol. 2019; 26(13):4566-4575. doi:10.1245/s10434-019-07911-w
20. Wray CJ, Harvin JA, Silberfein EJ, Ko TC, Kao LS. Pilot prognostic model of extremely poor survival among high-risk hepatocellular carcinoma patients. Cancer. 2012;118(24):6118-6125. doi:10.1002/cncr.27649
21. Kim JH, Kim JH, Choi JH, et al. Value of the model for end-stage liver disease for predicting survival in hepatocellular carcinoma patients treated with transarterial chemoembolization. Scand J Gastroenterol. 2009;44(3):346-357. doi:10.1080/00365520802530838
22. Vogeler M, Mohr I, Pfeiffenberger J, et al. Applicability of scoring systems predicting outcome of transarterial chemoembolization for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2020;146(4):1033-1050. doi:10.1007/s00432-020-03135-8
23. National Institutes of Health, Surveillance, Epidemiology, and End Results. Cancer stat facts: cancer of the liver and intrahepatic bile duct. Accessed July 15, 2021. https://seer.cancer.gov/statfacts/html/livibd.html
24. Singal AK, Kamath PS. Model for End-stage Liver Disease. J Clin Exp Hepatol. 2013;3(1):50-60. doi:10.1016/j.jceh.2012.11.002
Hepatocellular cancer (HCC) is the most common type of hepatic cancers, accounting for 65% of all hepatic cancers.1 Among all cancers, HCC is one of the fastest growing causes of death in the United States, and the rate of new HCC cases are on the rise over several decades.2 There are many risk factors leading to HCC, including alcohol use, obesity, and smoking. Infection with hepatitis C virus (HCV) poses a significant risk.1
The pathogenesis of HCV-induced carcinogenesis is mediated by a unique host-induced immunologic response. Viral replication induces production of inflammatory factors, such as tumor necrosis factor (TNF-α), interferon (IFN), and oxidative stress on hepatocytes, resulting in cell injury, death, and regeneration. Repetitive cycles of cellular death and regeneration induce fibrosis, which may lead to cirrhosis.3 Hence, early treatment of HCV infection and achieving sustained virologic response (SVR) may lead to decreased incidence and mortality associated with HCC.
Treatment of HCV infection has become more effective with the development of direct-acting antivirals (DAAs) leading to SVR in > 90% of patients compared with 40 to 50% with IFN-based treatment.4,5 DAAs have been proved safe and highly effective in eradicating HCV infection even in patients with advanced liver disease with decompensated cirrhosis.6 Although achieving SVR indicates a complete cure from chronic HCV infection, several studies have shown subsequent risk of developing HCC persists even after successful HCV treatment.7-9 Some studies show that using DAAs to achieve SVR in patients with HCV infection leads to a decreased relative risk of HCC development compared with patients who do not receive treatment.10-12 But data on HCC risk following DAA-induced SVR vs IFN-induced SVR are somewhat conflicting.
Much of the information regarding the association between SVR and HCC has been gleaned from large data banks without accounting for individual patient characteristics that can be obtained through full chart review. Due to small sample sizes in many chart review studies, the impact that SVR from DAA therapy has on the progression and severity of HCC is not entirely clear. The aim of our study is to evaluate the effect of HCV treatment and SVR status on overall survival (OS) in patients with HCC. Second, we aim to compare survival benefits, if any exist, among the 2 major HCV treatment modalities (IFN vs DAA).
Methods
We performed a retrospective review of patients at Memphis Veterans Affairs Medical Center (VAMC) in Tennessee to determine whether treatment for HCV infection in general, and achieving SVR in particular, makes a difference in progression, recurrence, or OS among patients with HCV infection who develop HCC. We identified 111 patients with a diagnosis of both HCV and new or recurrent HCC lesions from November 2008 to March 2019 (Table 1). We divided these patients based on their HCV treatment status, SVR status, and treatment types (IFN vs DAA).
The inclusion criteria were patients aged > 18 years treated at the Memphis VAMC who have HCV infection and developed HCC. Exclusion criteria were patients who developed HCC from other causes such as alcoholic steatohepatitis, hepatitis B virus infection, hemochromatosis, patients without HCV infection, and patients who were not established at the Memphis VAMC. This protocol was approved by the Memphis VAMC Institutional Review Board.
HCC diagnosis was determined using International Classification of Diseases codes (9th revision: 155 and 155.2; 10th revision: CD 22 and 22.9). We also used records of multidisciplinary gastrointestinal malignancy tumor conferences to identify patient who had been diagnosed and treated for HCV infection. We identified patients who were treated with DAA vs IFN as well as patients who had achieved SVR (classified as having negative HCV RNA tests at the end of DAA treatment). We were unable to evaluate Barcelona Clinic Liver Cancer staging since this required documented performance status that was not available in many patient records. We selected cases consistent with both treatment for HCV infection and subsequent development of HCC. Patient data included age; OS time; HIV status HCV genotype; time and status of progression to HCC; type and duration of treatment; and alcohol, tobacco, and drug use. Disease status was measured using the Model for End-Stage Liver Disease (MELD) score (Table 2), Milan criteria (Table 3), and Child-Pugh score (Table 4).
Statistical Analysis
OS was measured from the date of HCC diagnosis to the date of death or last follow-up. Progression-free survival (PFS) was defined from the date of HCC treatment initiation to the date of first HCC recurrence. We compared survival data for the SVR and non-SVR subgroups, the HCV treatment vs non-HCV treatment subgroups, and the IFN therapy vs DAA therapy subgroups, using the Kaplan-Meier method. The differences between subgroups were assessed using a log-rank test. Multivariate analysis using Cox proportional hazards regression model was used to identify factors that had significant impact on OS. Those factors included age; race; alcohol, tobacco, and illicit drug use; SVR status; HCV treatment status; IFN-based regimen vs DAA; MELD, and Child-Pugh scores. The results were expressed as hazard ratios (HRs) and 95% CI. Calculations were made using Statistical Analysis SAS and IBM SPSS software.
Results
The study included 111 patients. The mean age was 65.7 years; all were male and half of were Black patients. The gender imbalance was due to the predominantly male patient population at Memphis VAMC. Among 111 patients with HCV infection and HCC, 68 patients were treated for HCV infection and had significantly improved OS and PFS compared with the nontreatment group. The median 5-year OS was 44.6 months (95% CI, 966-3202) in the treated HCV infection group compared with 15.1 months in the untreated HCV infection group with a Wilcoxon P = .0005 (Figure 1). Similarly, patients treated for HCV infection had a significantly better 5-year PFS of 15.3 months (95% CI, 294-726) compared with the nontreatment group 9.5 months (95% CI, 205-405) with a Wilcoxon P = .04 (Figure 2).
Among 68 patients treated for HCV infection, 51 achieved SVR, and 34 achieved SVR after the diagnosis of HCC. Patients who achieved SVR had an improved 5-year OS when compared with patients who did not achieve SVR (median 65.8 months [95% CI, 1222-NA] vs 15.7 months [95% CI, 242-853], Wilcoxon P < .001) (Figure 3). Similarly, patients with SVR had improved 5-year PFS when compared with the non-SVR group (median 20.5 months [95% CI, 431-914] vs 8.9 months [95% CI, 191-340], Wilcoxon P = .007 (Figure 4). Achievement of SVR after HCC diagnosis suggests a significantly improved OS (HR 0.37) compared with achievement prior to HCC diagnosis (HR, 0.65; 95% CI, 0.23-1.82, P = .41)
Multivariate Cox regression was used to determine factors with significant survival impact. Advanced age at diagnosis (aged ≥ 65 years) (HR, 0.53; 95% CI, 0.320-0.880; P = .01), SVR status (HR, 0.33; 95% CI, 0.190-0.587; P < .001), achieving SVR after HCC diagnosis (HR, 0.37; 95% CI, 0.20-0.71; P = .002), low MELD score (< 10) (HR, 0.49; 95% CI, 0.30-0.80; P = .004) and low Child-Pugh score (class A) (HR, 0.39; 95% CI, 0.24-0.64; P = .001) have a significant positive impact on OS. Survival was not significantly influenced by race, tobacco, drug use, HIV or cirrhosis status, or HCV treatment type. In addition, higher Child-Pugh class (B or C), higher MELD score (> 10), and younger age at diagnosis (< 65 years) have a negative impact on survival outcome (Table 5).
Discussion
The survival benefit of HCV eradication and achieving SVR status has been well established in patients with HCC.13 In a retrospective cohort study of 250 patients with HCV infection who had received curative treatment for HCC, multivariate analysis demonstrated that achieving SVR is an independent predictor of OS.14 The 3-year and 5-year OS rates were 97% and 94% for the SVR group, and 91% and 60% for the non‐SVR group, respectively (P < .001). Similarly, according to Sou and colleagues, of 122 patients with HCV-related HCC, patients with SVR had longer OS than patients with no SVR (P = .04).15 One of the hypotheses that could explain the survival benefit in patients who achieved SVR is the effect of achieving SVR in reducing persistent liver inflammation and associated liver mortality, and therefore lowering risks of complication in patients with HCC.16 In our study, multivariate analysis shows that achieving SVR is associated with significant improved OS (HR, 0.33). In contrast, patients with HCC who have not achieved SVR are associated with worse survival (HR, 3.24). This finding supports early treatment of HCV to obtain SVR in HCV-related patients with HCC, even after development of HCC.
Among 68 patients treated for HCV infection, 45 patients were treated after HCC diagnosis, and 34 patients achieved SVR after HCC diagnosis. The average time between HCV infection treatment after HCC diagnosis was 6 months. Our data suggested that achievement of SVR after HCC diagnosis suggests an improved OS (HR, 0.37) compared with achievement prior to HCC diagnosis (HR, 0.65; 95% CI,0.23-1.82; P = .41). This lack of statistical significance is likely due to small sample size of patients achieving SVR prior to HCC diagnosis. Our results are consistent with the findings regarding the efficacy and timing of DAA treatment in patients with active HCC. According to Singal and colleagues, achieving SVR after DAA therapy may result in improved liver function and facilitate additional HCC-directed therapy, which potentially improves survival.17-19
Nagaoki and colleagues found that there was no significant difference in OS in patients with HCC between the DAA and IFN groups. According to the study, the 3-year and 5-year OS rates were 96% and 96% for DAA patients and 93% and 73% for IFN patients, respectively (P = .16).14 This finding is consistent with the results of our study. HCV treatment type (IFN vs DAA) was not found to be associated with either OS or PFS time, regardless of time period.
A higher MELD score (> 10) and a higher Child-Pugh class (B or C) score are associated with worse survival outcome regardless of SVR status. While patients with a low MELD score (≤ 10) have a better survival rate (HR 0.49), a higher MELD score has a significantly higher HR and therefore worse survival outcomes (HR, 2.20). Similarly, patients with Child-Pugh A (HR, 0.39) have a better survival outcome compared with those patients with Child-Pugh class B or C (HR, 2.57). This finding is consistent with results of multiple studies indicating that advanced liver disease, as measured by a high MELD score and Child-Pugh class score, can be used to predict the survival outcome in patients with HCV-related HCC.20-22
Unlike other studies that look at a single prognostic variable, our study evaluated prognostic impacts of multiple variables (age, SVR status, the order of SVR in relation to HCC development, HCV treatment type, MELD score and Child-Pugh class) in patients with HCC. The study included patients treated for HCV after development of HCC along with other multiple variables leading to OS benefit. It is one of the only studies in the United States that compared 5-year OS and PFS among patients with HCC treated for HCV and achieved SVR. The studies by Nagaoki and colleagues and Sou and colleagues were conducted in Japan, and some of their subset analyses were univariate. Among our study population of veterans, 50% were African American patients, suggesting that they may have similar OS benefit when compared to White patients with HCC and HCV treatment.
Limitations
Our findings were limited in that our study population is too small to conduct further subset analysis that would allow statistical significance of those subsets, such as the suggested benefit of SVR in patients who presented with HCC after antiviral therapy. Another limitation is the all-male population, likely a result of the older veteran population at the Memphis VAMC. The mean age at diagnosis was 65 years, which is slightly higher than the general population. Compared to the SEER database, HCC is most frequently diagnosed among people aged 55 to 64 years.23 The age difference was likely due to our aging veteran population.
Further studies are needed to determine the significance of SVR on HCC recurrence and treatment. Immunotherapy is now first-line treatment for patients with local advanced HCC. All the immunotherapy studies excluded patients with active HCV infection. Hence, we need more data on HCV treatment timing among patients scheduled to start treatment with immunotherapy.
Conclusions
In a population of older veterans, treatment of HCV infection leads to OS benefit among patients with HCC. In addition, patients with HCV infection who achieve SVR have an OS benefit over patients unable to achieve SVR. The type of treatment, DAA vs IFN-based regimen, did not show significant survival benefit.
Hepatocellular cancer (HCC) is the most common type of hepatic cancers, accounting for 65% of all hepatic cancers.1 Among all cancers, HCC is one of the fastest growing causes of death in the United States, and the rate of new HCC cases are on the rise over several decades.2 There are many risk factors leading to HCC, including alcohol use, obesity, and smoking. Infection with hepatitis C virus (HCV) poses a significant risk.1
The pathogenesis of HCV-induced carcinogenesis is mediated by a unique host-induced immunologic response. Viral replication induces production of inflammatory factors, such as tumor necrosis factor (TNF-α), interferon (IFN), and oxidative stress on hepatocytes, resulting in cell injury, death, and regeneration. Repetitive cycles of cellular death and regeneration induce fibrosis, which may lead to cirrhosis.3 Hence, early treatment of HCV infection and achieving sustained virologic response (SVR) may lead to decreased incidence and mortality associated with HCC.
Treatment of HCV infection has become more effective with the development of direct-acting antivirals (DAAs) leading to SVR in > 90% of patients compared with 40 to 50% with IFN-based treatment.4,5 DAAs have been proved safe and highly effective in eradicating HCV infection even in patients with advanced liver disease with decompensated cirrhosis.6 Although achieving SVR indicates a complete cure from chronic HCV infection, several studies have shown subsequent risk of developing HCC persists even after successful HCV treatment.7-9 Some studies show that using DAAs to achieve SVR in patients with HCV infection leads to a decreased relative risk of HCC development compared with patients who do not receive treatment.10-12 But data on HCC risk following DAA-induced SVR vs IFN-induced SVR are somewhat conflicting.
Much of the information regarding the association between SVR and HCC has been gleaned from large data banks without accounting for individual patient characteristics that can be obtained through full chart review. Due to small sample sizes in many chart review studies, the impact that SVR from DAA therapy has on the progression and severity of HCC is not entirely clear. The aim of our study is to evaluate the effect of HCV treatment and SVR status on overall survival (OS) in patients with HCC. Second, we aim to compare survival benefits, if any exist, among the 2 major HCV treatment modalities (IFN vs DAA).
Methods
We performed a retrospective review of patients at Memphis Veterans Affairs Medical Center (VAMC) in Tennessee to determine whether treatment for HCV infection in general, and achieving SVR in particular, makes a difference in progression, recurrence, or OS among patients with HCV infection who develop HCC. We identified 111 patients with a diagnosis of both HCV and new or recurrent HCC lesions from November 2008 to March 2019 (Table 1). We divided these patients based on their HCV treatment status, SVR status, and treatment types (IFN vs DAA).
The inclusion criteria were patients aged > 18 years treated at the Memphis VAMC who have HCV infection and developed HCC. Exclusion criteria were patients who developed HCC from other causes such as alcoholic steatohepatitis, hepatitis B virus infection, hemochromatosis, patients without HCV infection, and patients who were not established at the Memphis VAMC. This protocol was approved by the Memphis VAMC Institutional Review Board.
HCC diagnosis was determined using International Classification of Diseases codes (9th revision: 155 and 155.2; 10th revision: CD 22 and 22.9). We also used records of multidisciplinary gastrointestinal malignancy tumor conferences to identify patient who had been diagnosed and treated for HCV infection. We identified patients who were treated with DAA vs IFN as well as patients who had achieved SVR (classified as having negative HCV RNA tests at the end of DAA treatment). We were unable to evaluate Barcelona Clinic Liver Cancer staging since this required documented performance status that was not available in many patient records. We selected cases consistent with both treatment for HCV infection and subsequent development of HCC. Patient data included age; OS time; HIV status HCV genotype; time and status of progression to HCC; type and duration of treatment; and alcohol, tobacco, and drug use. Disease status was measured using the Model for End-Stage Liver Disease (MELD) score (Table 2), Milan criteria (Table 3), and Child-Pugh score (Table 4).
Statistical Analysis
OS was measured from the date of HCC diagnosis to the date of death or last follow-up. Progression-free survival (PFS) was defined from the date of HCC treatment initiation to the date of first HCC recurrence. We compared survival data for the SVR and non-SVR subgroups, the HCV treatment vs non-HCV treatment subgroups, and the IFN therapy vs DAA therapy subgroups, using the Kaplan-Meier method. The differences between subgroups were assessed using a log-rank test. Multivariate analysis using Cox proportional hazards regression model was used to identify factors that had significant impact on OS. Those factors included age; race; alcohol, tobacco, and illicit drug use; SVR status; HCV treatment status; IFN-based regimen vs DAA; MELD, and Child-Pugh scores. The results were expressed as hazard ratios (HRs) and 95% CI. Calculations were made using Statistical Analysis SAS and IBM SPSS software.
Results
The study included 111 patients. The mean age was 65.7 years; all were male and half of were Black patients. The gender imbalance was due to the predominantly male patient population at Memphis VAMC. Among 111 patients with HCV infection and HCC, 68 patients were treated for HCV infection and had significantly improved OS and PFS compared with the nontreatment group. The median 5-year OS was 44.6 months (95% CI, 966-3202) in the treated HCV infection group compared with 15.1 months in the untreated HCV infection group with a Wilcoxon P = .0005 (Figure 1). Similarly, patients treated for HCV infection had a significantly better 5-year PFS of 15.3 months (95% CI, 294-726) compared with the nontreatment group 9.5 months (95% CI, 205-405) with a Wilcoxon P = .04 (Figure 2).
Among 68 patients treated for HCV infection, 51 achieved SVR, and 34 achieved SVR after the diagnosis of HCC. Patients who achieved SVR had an improved 5-year OS when compared with patients who did not achieve SVR (median 65.8 months [95% CI, 1222-NA] vs 15.7 months [95% CI, 242-853], Wilcoxon P < .001) (Figure 3). Similarly, patients with SVR had improved 5-year PFS when compared with the non-SVR group (median 20.5 months [95% CI, 431-914] vs 8.9 months [95% CI, 191-340], Wilcoxon P = .007 (Figure 4). Achievement of SVR after HCC diagnosis suggests a significantly improved OS (HR 0.37) compared with achievement prior to HCC diagnosis (HR, 0.65; 95% CI, 0.23-1.82, P = .41)
Multivariate Cox regression was used to determine factors with significant survival impact. Advanced age at diagnosis (aged ≥ 65 years) (HR, 0.53; 95% CI, 0.320-0.880; P = .01), SVR status (HR, 0.33; 95% CI, 0.190-0.587; P < .001), achieving SVR after HCC diagnosis (HR, 0.37; 95% CI, 0.20-0.71; P = .002), low MELD score (< 10) (HR, 0.49; 95% CI, 0.30-0.80; P = .004) and low Child-Pugh score (class A) (HR, 0.39; 95% CI, 0.24-0.64; P = .001) have a significant positive impact on OS. Survival was not significantly influenced by race, tobacco, drug use, HIV or cirrhosis status, or HCV treatment type. In addition, higher Child-Pugh class (B or C), higher MELD score (> 10), and younger age at diagnosis (< 65 years) have a negative impact on survival outcome (Table 5).
Discussion
The survival benefit of HCV eradication and achieving SVR status has been well established in patients with HCC.13 In a retrospective cohort study of 250 patients with HCV infection who had received curative treatment for HCC, multivariate analysis demonstrated that achieving SVR is an independent predictor of OS.14 The 3-year and 5-year OS rates were 97% and 94% for the SVR group, and 91% and 60% for the non‐SVR group, respectively (P < .001). Similarly, according to Sou and colleagues, of 122 patients with HCV-related HCC, patients with SVR had longer OS than patients with no SVR (P = .04).15 One of the hypotheses that could explain the survival benefit in patients who achieved SVR is the effect of achieving SVR in reducing persistent liver inflammation and associated liver mortality, and therefore lowering risks of complication in patients with HCC.16 In our study, multivariate analysis shows that achieving SVR is associated with significant improved OS (HR, 0.33). In contrast, patients with HCC who have not achieved SVR are associated with worse survival (HR, 3.24). This finding supports early treatment of HCV to obtain SVR in HCV-related patients with HCC, even after development of HCC.
Among 68 patients treated for HCV infection, 45 patients were treated after HCC diagnosis, and 34 patients achieved SVR after HCC diagnosis. The average time between HCV infection treatment after HCC diagnosis was 6 months. Our data suggested that achievement of SVR after HCC diagnosis suggests an improved OS (HR, 0.37) compared with achievement prior to HCC diagnosis (HR, 0.65; 95% CI,0.23-1.82; P = .41). This lack of statistical significance is likely due to small sample size of patients achieving SVR prior to HCC diagnosis. Our results are consistent with the findings regarding the efficacy and timing of DAA treatment in patients with active HCC. According to Singal and colleagues, achieving SVR after DAA therapy may result in improved liver function and facilitate additional HCC-directed therapy, which potentially improves survival.17-19
Nagaoki and colleagues found that there was no significant difference in OS in patients with HCC between the DAA and IFN groups. According to the study, the 3-year and 5-year OS rates were 96% and 96% for DAA patients and 93% and 73% for IFN patients, respectively (P = .16).14 This finding is consistent with the results of our study. HCV treatment type (IFN vs DAA) was not found to be associated with either OS or PFS time, regardless of time period.
A higher MELD score (> 10) and a higher Child-Pugh class (B or C) score are associated with worse survival outcome regardless of SVR status. While patients with a low MELD score (≤ 10) have a better survival rate (HR 0.49), a higher MELD score has a significantly higher HR and therefore worse survival outcomes (HR, 2.20). Similarly, patients with Child-Pugh A (HR, 0.39) have a better survival outcome compared with those patients with Child-Pugh class B or C (HR, 2.57). This finding is consistent with results of multiple studies indicating that advanced liver disease, as measured by a high MELD score and Child-Pugh class score, can be used to predict the survival outcome in patients with HCV-related HCC.20-22
Unlike other studies that look at a single prognostic variable, our study evaluated prognostic impacts of multiple variables (age, SVR status, the order of SVR in relation to HCC development, HCV treatment type, MELD score and Child-Pugh class) in patients with HCC. The study included patients treated for HCV after development of HCC along with other multiple variables leading to OS benefit. It is one of the only studies in the United States that compared 5-year OS and PFS among patients with HCC treated for HCV and achieved SVR. The studies by Nagaoki and colleagues and Sou and colleagues were conducted in Japan, and some of their subset analyses were univariate. Among our study population of veterans, 50% were African American patients, suggesting that they may have similar OS benefit when compared to White patients with HCC and HCV treatment.
Limitations
Our findings were limited in that our study population is too small to conduct further subset analysis that would allow statistical significance of those subsets, such as the suggested benefit of SVR in patients who presented with HCC after antiviral therapy. Another limitation is the all-male population, likely a result of the older veteran population at the Memphis VAMC. The mean age at diagnosis was 65 years, which is slightly higher than the general population. Compared to the SEER database, HCC is most frequently diagnosed among people aged 55 to 64 years.23 The age difference was likely due to our aging veteran population.
Further studies are needed to determine the significance of SVR on HCC recurrence and treatment. Immunotherapy is now first-line treatment for patients with local advanced HCC. All the immunotherapy studies excluded patients with active HCV infection. Hence, we need more data on HCV treatment timing among patients scheduled to start treatment with immunotherapy.
Conclusions
In a population of older veterans, treatment of HCV infection leads to OS benefit among patients with HCC. In addition, patients with HCV infection who achieve SVR have an OS benefit over patients unable to achieve SVR. The type of treatment, DAA vs IFN-based regimen, did not show significant survival benefit.
1. Ghouri YA, Mian I, Rowe JH. Review of hepatocellular carcinoma: epidemiology, etiology, and carcinogenesis. J Carcinog. 2017;16:1. Published 2017 May 29. doi:10.4103/jcar.JCar_9_16
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424. doi:10.3322/caac.21492
3. Farazi PA, DePinho RA. Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer. 2006;6(9):674-687. doi:10.1038/nrc1934
4. Falade-Nwulia O, Suarez-Cuervo C, Nelson DR, Fried MW, Segal JB, Sulkowski MS. Oral direct-acting agent therapy for hepatitis c virus infection: a systematic review. Ann Intern Med. 2017;166(9):637-648. doi:10.7326/M16-2575
5. Kouris G, Hydery T, Greenwood BC, et al. Effectiveness of Ledipasvir/Sofosbuvir and predictors of treatment failure in members with hepatitis C genotype 1 infection: a retrospective cohort study in a medicaid population. J Manag Care Spec Pharm. 2018;24(7):591-597. doi:10.18553/jmcp.2018.24.7.591
6. Jacobson IM, Lawitz E, Kwo PY, et al. Safety and efficacy of elbasvir/grazoprevir in patients with hepatitis c virus infection and compensated cirrhosis: an integrated analysis. Gastroenterology. 2017;152(6):1372-1382.e2. doi:10.1053/j.gastro.2017.01.050
7. Nahon P, Layese R, Bourcier V, et al. Incidence of hepatocellular carcinoma after direct antiviral therapy for HCV in patients with cirrhosis included in surveillance programs. Gastroenterology. 2018;155(5):1436-1450.e6. doi:10.1053/j.gastro.2018.07.01510.
8. Innes H, Barclay ST, Hayes PC, et al. The risk of hepatocellular carcinoma in cirrhotic patients with hepatitis C and sustained viral response: role of the treatment regimen. J Hepatol. 2018;68(4):646-654. doi:10.1016/j.jhep.2017.10.033
9. Romano A, Angeli P, Piovesan S, et al. Newly diagnosed hepatocellular carcinoma in patients with advanced hepatitis C treated with DAAs: a prospective population study. J Hepatol. 2018;69(2):345-352. doi:10.1016/j.jhep.2018.03.009
10. Kanwal F, Kramer J, Asch SM, Chayanupatkul M, Cao Y, El-Serag HB. Risk of hepatocellular cancer in HCV patients treated with direct-acting antiviral agents. Gastroenterology. 2017;153(4):996-1005.e1. doi:10.1053/j.gastro.2017.06.0122
11. Singh S, Nautiyal A, Loke YK. Oral direct-acting antivirals and the incidence or recurrence of hepatocellular carcinoma: a systematic review and meta-analysis. Frontline Gastroenterol. 2018;9(4):262-270. doi:10.1136/flgastro-2018-101017
12. Kuftinec G, Loehfelm T, Corwin M, et al. De novo hepatocellular carcinoma occurrence in hepatitis C cirrhotics treated with direct-acting antiviral agents. Hepat Oncol. 2018;5(1):HEP06. Published 2018 Jul 25. doi:10.2217/hep-2018-00033
13. Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med. 2013;158(5 Pt 1):329-337. doi:10.7326/0003-4819-158-5-201303050-00005
14. Nagaoki Y, Imamura M, Nishida Y, et al. The impact of interferon-free direct-acting antivirals on clinical outcome after curative treatment for hepatitis C virus-associated hepatocellular carcinoma: comparison with interferon-based therapy. J Med Virol. 2019;91(4):650-658. doi:10.1002/jmv.25352
15. Sou FM, Wu CK, Chang KC, et al. Clinical characteristics and prognosis of HCC occurrence after antiviral therapy for HCV patients between sustained and non-sustained responders. J Formos Med Assoc. 2019;118(1 Pt 3):504-513. doi:10.1016/j.jfma.2018.10.017
16. Roche B, Coilly A, Duclos-Vallee JC, Samuel D. The impact of treatment of hepatitis C with DAAs on the occurrence of HCC. Liver Int. 2018;38(suppl 1):139-145. doi:10.1111/liv.13659
17. Singal AG, Lim JK, Kanwal F. AGA clinical practice update on interaction between oral direct-acting antivirals for chronic hepatitis C infection and hepatocellular carcinoma: expert review. Gastroenterology. 2019;156(8):2149-2157. doi:10.1053/j.gastro.2019.02.046
18. Toyoda H, Kumada T, Hayashi K, et al. Characteristics and prognosis of hepatocellular carcinoma detected in sustained responders to interferon therapy for chronic hepatitis C. Cancer Detect Prev. 2003;27(6):498-502. doi:10.1016/j.cdp.2003.09.00719. Okamura Y, Sugiura T, Ito T, et al. The achievement of a sustained virological response either before or after hepatectomy improves the prognosis of patients with primary hepatitis C virus-related hepatocellular carcinoma. Ann Surg Oncol. 2019; 26(13):4566-4575. doi:10.1245/s10434-019-07911-w
20. Wray CJ, Harvin JA, Silberfein EJ, Ko TC, Kao LS. Pilot prognostic model of extremely poor survival among high-risk hepatocellular carcinoma patients. Cancer. 2012;118(24):6118-6125. doi:10.1002/cncr.27649
21. Kim JH, Kim JH, Choi JH, et al. Value of the model for end-stage liver disease for predicting survival in hepatocellular carcinoma patients treated with transarterial chemoembolization. Scand J Gastroenterol. 2009;44(3):346-357. doi:10.1080/00365520802530838
22. Vogeler M, Mohr I, Pfeiffenberger J, et al. Applicability of scoring systems predicting outcome of transarterial chemoembolization for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2020;146(4):1033-1050. doi:10.1007/s00432-020-03135-8
23. National Institutes of Health, Surveillance, Epidemiology, and End Results. Cancer stat facts: cancer of the liver and intrahepatic bile duct. Accessed July 15, 2021. https://seer.cancer.gov/statfacts/html/livibd.html
24. Singal AK, Kamath PS. Model for End-stage Liver Disease. J Clin Exp Hepatol. 2013;3(1):50-60. doi:10.1016/j.jceh.2012.11.002
1. Ghouri YA, Mian I, Rowe JH. Review of hepatocellular carcinoma: epidemiology, etiology, and carcinogenesis. J Carcinog. 2017;16:1. Published 2017 May 29. doi:10.4103/jcar.JCar_9_16
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424. doi:10.3322/caac.21492
3. Farazi PA, DePinho RA. Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer. 2006;6(9):674-687. doi:10.1038/nrc1934
4. Falade-Nwulia O, Suarez-Cuervo C, Nelson DR, Fried MW, Segal JB, Sulkowski MS. Oral direct-acting agent therapy for hepatitis c virus infection: a systematic review. Ann Intern Med. 2017;166(9):637-648. doi:10.7326/M16-2575
5. Kouris G, Hydery T, Greenwood BC, et al. Effectiveness of Ledipasvir/Sofosbuvir and predictors of treatment failure in members with hepatitis C genotype 1 infection: a retrospective cohort study in a medicaid population. J Manag Care Spec Pharm. 2018;24(7):591-597. doi:10.18553/jmcp.2018.24.7.591
6. Jacobson IM, Lawitz E, Kwo PY, et al. Safety and efficacy of elbasvir/grazoprevir in patients with hepatitis c virus infection and compensated cirrhosis: an integrated analysis. Gastroenterology. 2017;152(6):1372-1382.e2. doi:10.1053/j.gastro.2017.01.050
7. Nahon P, Layese R, Bourcier V, et al. Incidence of hepatocellular carcinoma after direct antiviral therapy for HCV in patients with cirrhosis included in surveillance programs. Gastroenterology. 2018;155(5):1436-1450.e6. doi:10.1053/j.gastro.2018.07.01510.
8. Innes H, Barclay ST, Hayes PC, et al. The risk of hepatocellular carcinoma in cirrhotic patients with hepatitis C and sustained viral response: role of the treatment regimen. J Hepatol. 2018;68(4):646-654. doi:10.1016/j.jhep.2017.10.033
9. Romano A, Angeli P, Piovesan S, et al. Newly diagnosed hepatocellular carcinoma in patients with advanced hepatitis C treated with DAAs: a prospective population study. J Hepatol. 2018;69(2):345-352. doi:10.1016/j.jhep.2018.03.009
10. Kanwal F, Kramer J, Asch SM, Chayanupatkul M, Cao Y, El-Serag HB. Risk of hepatocellular cancer in HCV patients treated with direct-acting antiviral agents. Gastroenterology. 2017;153(4):996-1005.e1. doi:10.1053/j.gastro.2017.06.0122
11. Singh S, Nautiyal A, Loke YK. Oral direct-acting antivirals and the incidence or recurrence of hepatocellular carcinoma: a systematic review and meta-analysis. Frontline Gastroenterol. 2018;9(4):262-270. doi:10.1136/flgastro-2018-101017
12. Kuftinec G, Loehfelm T, Corwin M, et al. De novo hepatocellular carcinoma occurrence in hepatitis C cirrhotics treated with direct-acting antiviral agents. Hepat Oncol. 2018;5(1):HEP06. Published 2018 Jul 25. doi:10.2217/hep-2018-00033
13. Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med. 2013;158(5 Pt 1):329-337. doi:10.7326/0003-4819-158-5-201303050-00005
14. Nagaoki Y, Imamura M, Nishida Y, et al. The impact of interferon-free direct-acting antivirals on clinical outcome after curative treatment for hepatitis C virus-associated hepatocellular carcinoma: comparison with interferon-based therapy. J Med Virol. 2019;91(4):650-658. doi:10.1002/jmv.25352
15. Sou FM, Wu CK, Chang KC, et al. Clinical characteristics and prognosis of HCC occurrence after antiviral therapy for HCV patients between sustained and non-sustained responders. J Formos Med Assoc. 2019;118(1 Pt 3):504-513. doi:10.1016/j.jfma.2018.10.017
16. Roche B, Coilly A, Duclos-Vallee JC, Samuel D. The impact of treatment of hepatitis C with DAAs on the occurrence of HCC. Liver Int. 2018;38(suppl 1):139-145. doi:10.1111/liv.13659
17. Singal AG, Lim JK, Kanwal F. AGA clinical practice update on interaction between oral direct-acting antivirals for chronic hepatitis C infection and hepatocellular carcinoma: expert review. Gastroenterology. 2019;156(8):2149-2157. doi:10.1053/j.gastro.2019.02.046
18. Toyoda H, Kumada T, Hayashi K, et al. Characteristics and prognosis of hepatocellular carcinoma detected in sustained responders to interferon therapy for chronic hepatitis C. Cancer Detect Prev. 2003;27(6):498-502. doi:10.1016/j.cdp.2003.09.00719. Okamura Y, Sugiura T, Ito T, et al. The achievement of a sustained virological response either before or after hepatectomy improves the prognosis of patients with primary hepatitis C virus-related hepatocellular carcinoma. Ann Surg Oncol. 2019; 26(13):4566-4575. doi:10.1245/s10434-019-07911-w
20. Wray CJ, Harvin JA, Silberfein EJ, Ko TC, Kao LS. Pilot prognostic model of extremely poor survival among high-risk hepatocellular carcinoma patients. Cancer. 2012;118(24):6118-6125. doi:10.1002/cncr.27649
21. Kim JH, Kim JH, Choi JH, et al. Value of the model for end-stage liver disease for predicting survival in hepatocellular carcinoma patients treated with transarterial chemoembolization. Scand J Gastroenterol. 2009;44(3):346-357. doi:10.1080/00365520802530838
22. Vogeler M, Mohr I, Pfeiffenberger J, et al. Applicability of scoring systems predicting outcome of transarterial chemoembolization for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2020;146(4):1033-1050. doi:10.1007/s00432-020-03135-8
23. National Institutes of Health, Surveillance, Epidemiology, and End Results. Cancer stat facts: cancer of the liver and intrahepatic bile duct. Accessed July 15, 2021. https://seer.cancer.gov/statfacts/html/livibd.html
24. Singal AK, Kamath PS. Model for End-stage Liver Disease. J Clin Exp Hepatol. 2013;3(1):50-60. doi:10.1016/j.jceh.2012.11.002
Clinical Edge Journal Scan Commentary: HCC September 2021
Patients with hepatocellular carcinoma (HCC) that has not spread outside the liver have several treatment options available. This month we will review articles that analyze outcomes after liver transplantation, liver resection, as well as radiofrequency ablation.
In HCC patients within the Milan criteria, the 5-year overall survival rate after transplant is about 70%, and the 5-year HCC recurrence rate is about 10%. Patients with tumors beyond the Milan criteria are frequently down staged with locoregional therapies to fall within the Milan criteria. The incidence of HCC recurrence in these patients is around 15.5% at 5 years. In this study, Lee et al demonstrated that statin use substantially reduced the risk of HCC recurrence. In this retrospective analysis of a longitudinal cohort of 430 patients transplanted between September 1995 and December 2019, 323 patients (75.1%) were statin non-users and 107 (24.9%) were statin users. Statin use was defined as at least 90 days of statin therapy, prescribed according to the treatment guidelines for dyslipidemia for primary or secondary prevention of CVD. At a median follow-up of 64.9 months, HCC recurred in 79 patients (18.4%), including 72 (22.3%) in the statin non-user group and 7 (6.5%) in the statin user group. Of those, 61 (77.2%) patients had HCC recurrence that initially presented at extrahepatic site regardless of the presence of intrahepatic tumors. Sixty-three patients (79.7%) had recurrence within 2 years of liver transplantation. The cumulative incidence of HCC recurrence at 2 and 5 years was 18.9% and 22.3% in the statin non-user group, and 3.8% and 5.7% in the statin user group, respectively (P < 0.001).
Liang et al evaluated the importance of tumor size in predicting the likelihood of HCC recurrence following surgical resection. In this retrospective study, a total of 813 cirrhotic patients who underwent curative-intent hepatectomy for solitary HCC without macrovascular invasion between 2001 and 2014 were evaluated. Overall, 464 patients had tumor size ≤ 5 cm, and 349 had tumor size > 5 cm. The 5-year RFS and OS rates were 38.3% and 61.5% in the ≤ 5 cm group, compared with 25.1% and 59.9% in the > 5 cm group. Long-term survival outcomes were significantly worse as tumor size increased. Multivariate analysis indicated that tumor size > 5 cm was an independent risk factor for tumor recurrence and long-term survival.
Finally, Sulaiman et al reported the survival rate of the early and intermediate stage HCC patients who underwent radiofrequency ablation (RFA). In this retrospective analysis, patients with BCLC A and B HCC who underwent RFA treatments between January 2015 to December 2017 were evaluated. Out of 62 patients 46 (74.2%) were reported to have RFA as their only first line of treatment, while 12 (25.8%) were reported to have a combination of RFA and other therapeutic modalities. At a mean follow up of 27 months, the survival rate at 12 and 36 months in patients who received RFA was 82.3% and 57.8%, respectively. A relatively high 36-month survival rate was seen in patients who had a response to RFA compared to the non-response group (100% vs 44.8%, P = 0.021). In terms of prognosis, BCLC staging of liver cancer and response after RFA were significantly associated with survival.
Patients with hepatocellular carcinoma (HCC) that has not spread outside the liver have several treatment options available. This month we will review articles that analyze outcomes after liver transplantation, liver resection, as well as radiofrequency ablation.
In HCC patients within the Milan criteria, the 5-year overall survival rate after transplant is about 70%, and the 5-year HCC recurrence rate is about 10%. Patients with tumors beyond the Milan criteria are frequently down staged with locoregional therapies to fall within the Milan criteria. The incidence of HCC recurrence in these patients is around 15.5% at 5 years. In this study, Lee et al demonstrated that statin use substantially reduced the risk of HCC recurrence. In this retrospective analysis of a longitudinal cohort of 430 patients transplanted between September 1995 and December 2019, 323 patients (75.1%) were statin non-users and 107 (24.9%) were statin users. Statin use was defined as at least 90 days of statin therapy, prescribed according to the treatment guidelines for dyslipidemia for primary or secondary prevention of CVD. At a median follow-up of 64.9 months, HCC recurred in 79 patients (18.4%), including 72 (22.3%) in the statin non-user group and 7 (6.5%) in the statin user group. Of those, 61 (77.2%) patients had HCC recurrence that initially presented at extrahepatic site regardless of the presence of intrahepatic tumors. Sixty-three patients (79.7%) had recurrence within 2 years of liver transplantation. The cumulative incidence of HCC recurrence at 2 and 5 years was 18.9% and 22.3% in the statin non-user group, and 3.8% and 5.7% in the statin user group, respectively (P < 0.001).
Liang et al evaluated the importance of tumor size in predicting the likelihood of HCC recurrence following surgical resection. In this retrospective study, a total of 813 cirrhotic patients who underwent curative-intent hepatectomy for solitary HCC without macrovascular invasion between 2001 and 2014 were evaluated. Overall, 464 patients had tumor size ≤ 5 cm, and 349 had tumor size > 5 cm. The 5-year RFS and OS rates were 38.3% and 61.5% in the ≤ 5 cm group, compared with 25.1% and 59.9% in the > 5 cm group. Long-term survival outcomes were significantly worse as tumor size increased. Multivariate analysis indicated that tumor size > 5 cm was an independent risk factor for tumor recurrence and long-term survival.
Finally, Sulaiman et al reported the survival rate of the early and intermediate stage HCC patients who underwent radiofrequency ablation (RFA). In this retrospective analysis, patients with BCLC A and B HCC who underwent RFA treatments between January 2015 to December 2017 were evaluated. Out of 62 patients 46 (74.2%) were reported to have RFA as their only first line of treatment, while 12 (25.8%) were reported to have a combination of RFA and other therapeutic modalities. At a mean follow up of 27 months, the survival rate at 12 and 36 months in patients who received RFA was 82.3% and 57.8%, respectively. A relatively high 36-month survival rate was seen in patients who had a response to RFA compared to the non-response group (100% vs 44.8%, P = 0.021). In terms of prognosis, BCLC staging of liver cancer and response after RFA were significantly associated with survival.
Patients with hepatocellular carcinoma (HCC) that has not spread outside the liver have several treatment options available. This month we will review articles that analyze outcomes after liver transplantation, liver resection, as well as radiofrequency ablation.
In HCC patients within the Milan criteria, the 5-year overall survival rate after transplant is about 70%, and the 5-year HCC recurrence rate is about 10%. Patients with tumors beyond the Milan criteria are frequently down staged with locoregional therapies to fall within the Milan criteria. The incidence of HCC recurrence in these patients is around 15.5% at 5 years. In this study, Lee et al demonstrated that statin use substantially reduced the risk of HCC recurrence. In this retrospective analysis of a longitudinal cohort of 430 patients transplanted between September 1995 and December 2019, 323 patients (75.1%) were statin non-users and 107 (24.9%) were statin users. Statin use was defined as at least 90 days of statin therapy, prescribed according to the treatment guidelines for dyslipidemia for primary or secondary prevention of CVD. At a median follow-up of 64.9 months, HCC recurred in 79 patients (18.4%), including 72 (22.3%) in the statin non-user group and 7 (6.5%) in the statin user group. Of those, 61 (77.2%) patients had HCC recurrence that initially presented at extrahepatic site regardless of the presence of intrahepatic tumors. Sixty-three patients (79.7%) had recurrence within 2 years of liver transplantation. The cumulative incidence of HCC recurrence at 2 and 5 years was 18.9% and 22.3% in the statin non-user group, and 3.8% and 5.7% in the statin user group, respectively (P < 0.001).
Liang et al evaluated the importance of tumor size in predicting the likelihood of HCC recurrence following surgical resection. In this retrospective study, a total of 813 cirrhotic patients who underwent curative-intent hepatectomy for solitary HCC without macrovascular invasion between 2001 and 2014 were evaluated. Overall, 464 patients had tumor size ≤ 5 cm, and 349 had tumor size > 5 cm. The 5-year RFS and OS rates were 38.3% and 61.5% in the ≤ 5 cm group, compared with 25.1% and 59.9% in the > 5 cm group. Long-term survival outcomes were significantly worse as tumor size increased. Multivariate analysis indicated that tumor size > 5 cm was an independent risk factor for tumor recurrence and long-term survival.
Finally, Sulaiman et al reported the survival rate of the early and intermediate stage HCC patients who underwent radiofrequency ablation (RFA). In this retrospective analysis, patients with BCLC A and B HCC who underwent RFA treatments between January 2015 to December 2017 were evaluated. Out of 62 patients 46 (74.2%) were reported to have RFA as their only first line of treatment, while 12 (25.8%) were reported to have a combination of RFA and other therapeutic modalities. At a mean follow up of 27 months, the survival rate at 12 and 36 months in patients who received RFA was 82.3% and 57.8%, respectively. A relatively high 36-month survival rate was seen in patients who had a response to RFA compared to the non-response group (100% vs 44.8%, P = 0.021). In terms of prognosis, BCLC staging of liver cancer and response after RFA were significantly associated with survival.
Bone resorption inhibitors extend OS in castration-resistant prostate cancer
Key clinical point: The addition of bone resorption inhibitors (BRIs) to abiraterone acetate with prednisone prolongs overall survival (OS) in patients with metastatic castration-resistant prostate cancer (CRPC) and bone metastases. The OS was similar regardless of the BRI type used.
Major finding: The median follow-up was 23.5 months. Concomitant use of BRIs was reported in 29.0% of patients. BRI use was associated with OS improvement (31.8 months vs 23.0 months; hazard ratio [HR], 0.65; P less than .001) and shorter time to the first skeletal-related event (32.4 months vs 42.7 months; HR, 1.27; P = .04). Both denosumab and zoledronic acid were associated with similar OS (P = .79).
Study details: A retrospective study of 745 consecutive patients with metastatic CRPC and bone metastases who received abiraterone acetate plus prednisone between 2013 and 2016.
Disclosures: No funding source was reported. The authors received grants, personal fees, travel expenses, and research funding from and/or owned stocks in pharmaceutical companies outside the submitted work.
Source: Francini E et al. JAMA Netw Open. 2021 Jul 22. doi: 10.1001/jamanetworkopen.2021.16536.
Key clinical point: The addition of bone resorption inhibitors (BRIs) to abiraterone acetate with prednisone prolongs overall survival (OS) in patients with metastatic castration-resistant prostate cancer (CRPC) and bone metastases. The OS was similar regardless of the BRI type used.
Major finding: The median follow-up was 23.5 months. Concomitant use of BRIs was reported in 29.0% of patients. BRI use was associated with OS improvement (31.8 months vs 23.0 months; hazard ratio [HR], 0.65; P less than .001) and shorter time to the first skeletal-related event (32.4 months vs 42.7 months; HR, 1.27; P = .04). Both denosumab and zoledronic acid were associated with similar OS (P = .79).
Study details: A retrospective study of 745 consecutive patients with metastatic CRPC and bone metastases who received abiraterone acetate plus prednisone between 2013 and 2016.
Disclosures: No funding source was reported. The authors received grants, personal fees, travel expenses, and research funding from and/or owned stocks in pharmaceutical companies outside the submitted work.
Source: Francini E et al. JAMA Netw Open. 2021 Jul 22. doi: 10.1001/jamanetworkopen.2021.16536.
Key clinical point: The addition of bone resorption inhibitors (BRIs) to abiraterone acetate with prednisone prolongs overall survival (OS) in patients with metastatic castration-resistant prostate cancer (CRPC) and bone metastases. The OS was similar regardless of the BRI type used.
Major finding: The median follow-up was 23.5 months. Concomitant use of BRIs was reported in 29.0% of patients. BRI use was associated with OS improvement (31.8 months vs 23.0 months; hazard ratio [HR], 0.65; P less than .001) and shorter time to the first skeletal-related event (32.4 months vs 42.7 months; HR, 1.27; P = .04). Both denosumab and zoledronic acid were associated with similar OS (P = .79).
Study details: A retrospective study of 745 consecutive patients with metastatic CRPC and bone metastases who received abiraterone acetate plus prednisone between 2013 and 2016.
Disclosures: No funding source was reported. The authors received grants, personal fees, travel expenses, and research funding from and/or owned stocks in pharmaceutical companies outside the submitted work.
Source: Francini E et al. JAMA Netw Open. 2021 Jul 22. doi: 10.1001/jamanetworkopen.2021.16536.
Prostate cancer: PDE-5 inhibitor use linked to survival benefit
Key clinical point: In patients with localized prostate cancer, the use of phosphodiesterase (PDE)-5 inhibitors improve survival.
Major finding: The patients who received PDE-5 inhibitors vs those who did not showed a significant improvement in biochemical recurrence-free (BCRF) survival (hazard ratio [HR], 0.44; 95% confidence interval [CI], 0.34-0.56) and overall survival (OS; HR, 0.65; 95% CI, 0.45-0.94) at 10 years. In PDE-5 inhibitors users vs nonusers, BCRF survival was 93.2% vs 85.3% and OS was 95.8% vs 94.5%.
Study details: A retrospective cohort study of 3,100 patients with prostate cancer who underwent radical prostatectomy between 2003 and 2015; 1,372 patients received PDE-5 inhibitors.
Disclosures: The study was supported by National Cancer Institute. The authors declared no conflict of interests.
Source: Danley KT et al. Urol Oncol. 2021 Jul 18. doi: 10.1016/j.urolonc.2021.05.031.
Key clinical point: In patients with localized prostate cancer, the use of phosphodiesterase (PDE)-5 inhibitors improve survival.
Major finding: The patients who received PDE-5 inhibitors vs those who did not showed a significant improvement in biochemical recurrence-free (BCRF) survival (hazard ratio [HR], 0.44; 95% confidence interval [CI], 0.34-0.56) and overall survival (OS; HR, 0.65; 95% CI, 0.45-0.94) at 10 years. In PDE-5 inhibitors users vs nonusers, BCRF survival was 93.2% vs 85.3% and OS was 95.8% vs 94.5%.
Study details: A retrospective cohort study of 3,100 patients with prostate cancer who underwent radical prostatectomy between 2003 and 2015; 1,372 patients received PDE-5 inhibitors.
Disclosures: The study was supported by National Cancer Institute. The authors declared no conflict of interests.
Source: Danley KT et al. Urol Oncol. 2021 Jul 18. doi: 10.1016/j.urolonc.2021.05.031.
Key clinical point: In patients with localized prostate cancer, the use of phosphodiesterase (PDE)-5 inhibitors improve survival.
Major finding: The patients who received PDE-5 inhibitors vs those who did not showed a significant improvement in biochemical recurrence-free (BCRF) survival (hazard ratio [HR], 0.44; 95% confidence interval [CI], 0.34-0.56) and overall survival (OS; HR, 0.65; 95% CI, 0.45-0.94) at 10 years. In PDE-5 inhibitors users vs nonusers, BCRF survival was 93.2% vs 85.3% and OS was 95.8% vs 94.5%.
Study details: A retrospective cohort study of 3,100 patients with prostate cancer who underwent radical prostatectomy between 2003 and 2015; 1,372 patients received PDE-5 inhibitors.
Disclosures: The study was supported by National Cancer Institute. The authors declared no conflict of interests.
Source: Danley KT et al. Urol Oncol. 2021 Jul 18. doi: 10.1016/j.urolonc.2021.05.031.
Positive PSMA PET indicates worse metastasis-free survival in recurrent prostate cancer
Key clinical point: Positive prostate-specific membrane antigen positron emission tomography (PSMA PET) findings prior to salvage radiotherapy (SRT) are associated with worse metastasis-free survival (MFS) in patients with recurrent prostate cancer.
Major finding: In 155 patients who underwent PSMA PET prior to SRT, 31.6% had positive PSMA PET. After propensity score matching, 5-year MFS was significantly lower in patients with “positive PSMA PET” vs those who did not undergo PSMA PET (48.5% vs 92.3%; P < .001). Positive PSMA PET imaging was associated with worse MFS compared with “no PSMA PET” (hazard ratio, 13.8; P < .001).
Study design: A retrospective study of 1,599 patients with recurrent prostate cancer who received salvage radiotherapy after biochemical recurrence.
Disclosures: The study received no funding. The authors declared no conflict of interests.
Source: Wenzel M et al. Urol Oncol. 2021 Jul 31. doi: 10.1016/j.urolonc.2021.06.008.
Key clinical point: Positive prostate-specific membrane antigen positron emission tomography (PSMA PET) findings prior to salvage radiotherapy (SRT) are associated with worse metastasis-free survival (MFS) in patients with recurrent prostate cancer.
Major finding: In 155 patients who underwent PSMA PET prior to SRT, 31.6% had positive PSMA PET. After propensity score matching, 5-year MFS was significantly lower in patients with “positive PSMA PET” vs those who did not undergo PSMA PET (48.5% vs 92.3%; P < .001). Positive PSMA PET imaging was associated with worse MFS compared with “no PSMA PET” (hazard ratio, 13.8; P < .001).
Study design: A retrospective study of 1,599 patients with recurrent prostate cancer who received salvage radiotherapy after biochemical recurrence.
Disclosures: The study received no funding. The authors declared no conflict of interests.
Source: Wenzel M et al. Urol Oncol. 2021 Jul 31. doi: 10.1016/j.urolonc.2021.06.008.
Key clinical point: Positive prostate-specific membrane antigen positron emission tomography (PSMA PET) findings prior to salvage radiotherapy (SRT) are associated with worse metastasis-free survival (MFS) in patients with recurrent prostate cancer.
Major finding: In 155 patients who underwent PSMA PET prior to SRT, 31.6% had positive PSMA PET. After propensity score matching, 5-year MFS was significantly lower in patients with “positive PSMA PET” vs those who did not undergo PSMA PET (48.5% vs 92.3%; P < .001). Positive PSMA PET imaging was associated with worse MFS compared with “no PSMA PET” (hazard ratio, 13.8; P < .001).
Study design: A retrospective study of 1,599 patients with recurrent prostate cancer who received salvage radiotherapy after biochemical recurrence.
Disclosures: The study received no funding. The authors declared no conflict of interests.
Source: Wenzel M et al. Urol Oncol. 2021 Jul 31. doi: 10.1016/j.urolonc.2021.06.008.
High-grade prostate cancer: Elevated PSA is linked to high mortality risk
Key clinical point: In patients with Gleason grade 3+4 prostate cancer treated with brachytherapy, high prostate-specific antigen (PSA) levels are associated with an elevated risk for prostate cancer-specific mortality (PCSM).
Major finding: The median follow-up was 7.8 years. The PSA levels of 10.1-20.0 ng/mL vs 4.0-10.0 ng/mL were associated with higher PCSM in patients who received brachytherapy alone (adjusted hazard ratio [aHR], 5.55; P < .001) and those who received brachytherapy with androgen deprivation treatment (ADT; aHR, 4.17; P = .02).
Study details: A retrospective study of 1,920 patients with Gleason grade 3+4 prostate cancer who received brachytherapy with or without ADT.
Disclosures: No funding source was reported. The authors did not report any conflict of interests.
Source: Yang DD et al. Urol Oncol. 2021 Jul 24. doi: 10.1016/j.urolonc.2021.06.022.
Key clinical point: In patients with Gleason grade 3+4 prostate cancer treated with brachytherapy, high prostate-specific antigen (PSA) levels are associated with an elevated risk for prostate cancer-specific mortality (PCSM).
Major finding: The median follow-up was 7.8 years. The PSA levels of 10.1-20.0 ng/mL vs 4.0-10.0 ng/mL were associated with higher PCSM in patients who received brachytherapy alone (adjusted hazard ratio [aHR], 5.55; P < .001) and those who received brachytherapy with androgen deprivation treatment (ADT; aHR, 4.17; P = .02).
Study details: A retrospective study of 1,920 patients with Gleason grade 3+4 prostate cancer who received brachytherapy with or without ADT.
Disclosures: No funding source was reported. The authors did not report any conflict of interests.
Source: Yang DD et al. Urol Oncol. 2021 Jul 24. doi: 10.1016/j.urolonc.2021.06.022.
Key clinical point: In patients with Gleason grade 3+4 prostate cancer treated with brachytherapy, high prostate-specific antigen (PSA) levels are associated with an elevated risk for prostate cancer-specific mortality (PCSM).
Major finding: The median follow-up was 7.8 years. The PSA levels of 10.1-20.0 ng/mL vs 4.0-10.0 ng/mL were associated with higher PCSM in patients who received brachytherapy alone (adjusted hazard ratio [aHR], 5.55; P < .001) and those who received brachytherapy with androgen deprivation treatment (ADT; aHR, 4.17; P = .02).
Study details: A retrospective study of 1,920 patients with Gleason grade 3+4 prostate cancer who received brachytherapy with or without ADT.
Disclosures: No funding source was reported. The authors did not report any conflict of interests.
Source: Yang DD et al. Urol Oncol. 2021 Jul 24. doi: 10.1016/j.urolonc.2021.06.022.
CRPC: Nomograms predict outcomes with targeted radionuclide therapy
Key clinical point: Nomograms to predict survival and prostate-specific antigen (PSA) response in patients with metastatic castration-resistant prostate cancer (mCRPC) receiving lutetium-177 prostate-specific membrane antigen (¹⁷⁷Lu-PSMA) radionuclide treatment have been developed and externally validated.
Major finding: The median follow-up was 21.5 months. The concordance index of the overall survival model was 0.71 and PSA-progression-free survival model was 0.70. The model for PSA decline of 50% or more had a sensitivity of 94%, negative predictive value of 89%, and specificity of 38%.
Study details: Nomograms for predicting outcomes after Lu-PSMA treatment were developed (n=196) and validated (n=74) using clinical trial and real-world data of patients with late-stage mCRPC.
Disclosures: The study was supported by Prostate Cancer Foundation. The authors declared consulting, personal fees, travel fees, grants, honoraria, nonfinancial support, and patents outside this work. Dr. J Czernin reported being a founder and board member of and holding equity in Sofie Biosciences and Trethera Therapeutics.
Source: Gafita A et al. Lancet Oncol. 2021 Jul 8. doi: 10.1016/S1470-2045(21)00274-6.
Key clinical point: Nomograms to predict survival and prostate-specific antigen (PSA) response in patients with metastatic castration-resistant prostate cancer (mCRPC) receiving lutetium-177 prostate-specific membrane antigen (¹⁷⁷Lu-PSMA) radionuclide treatment have been developed and externally validated.
Major finding: The median follow-up was 21.5 months. The concordance index of the overall survival model was 0.71 and PSA-progression-free survival model was 0.70. The model for PSA decline of 50% or more had a sensitivity of 94%, negative predictive value of 89%, and specificity of 38%.
Study details: Nomograms for predicting outcomes after Lu-PSMA treatment were developed (n=196) and validated (n=74) using clinical trial and real-world data of patients with late-stage mCRPC.
Disclosures: The study was supported by Prostate Cancer Foundation. The authors declared consulting, personal fees, travel fees, grants, honoraria, nonfinancial support, and patents outside this work. Dr. J Czernin reported being a founder and board member of and holding equity in Sofie Biosciences and Trethera Therapeutics.
Source: Gafita A et al. Lancet Oncol. 2021 Jul 8. doi: 10.1016/S1470-2045(21)00274-6.
Key clinical point: Nomograms to predict survival and prostate-specific antigen (PSA) response in patients with metastatic castration-resistant prostate cancer (mCRPC) receiving lutetium-177 prostate-specific membrane antigen (¹⁷⁷Lu-PSMA) radionuclide treatment have been developed and externally validated.
Major finding: The median follow-up was 21.5 months. The concordance index of the overall survival model was 0.71 and PSA-progression-free survival model was 0.70. The model for PSA decline of 50% or more had a sensitivity of 94%, negative predictive value of 89%, and specificity of 38%.
Study details: Nomograms for predicting outcomes after Lu-PSMA treatment were developed (n=196) and validated (n=74) using clinical trial and real-world data of patients with late-stage mCRPC.
Disclosures: The study was supported by Prostate Cancer Foundation. The authors declared consulting, personal fees, travel fees, grants, honoraria, nonfinancial support, and patents outside this work. Dr. J Czernin reported being a founder and board member of and holding equity in Sofie Biosciences and Trethera Therapeutics.
Source: Gafita A et al. Lancet Oncol. 2021 Jul 8. doi: 10.1016/S1470-2045(21)00274-6.