User login
Advanced HCC: Immunotherapy vs chemotherapy improves survival
Key clinical point: Immunotherapy was associated with prolonged survival compared with chemotherapy in patients with advanced hepatocellular carcinoma (HCC).
Major finding: After adjusting for confounding variables, immunotherapy was independently associated with improved overall survival (adjusted hazard ratio 0.76; 95% CI 0.65-0.88) compared with chemotherapy.
Study details: Findings are from a retrospective cohort study that included 3990 patients with advanced HCC (tumor-node-metastasis stage III or IV) from the National Cancer Database who received chemotherapy (n = 3248) or immunotherapy (n = 742) as the first-line systemic treatment.
Disclosures: No funding source was reported. Some authors declared serving as consultants or advisors or receiving institutional research support from various organizations.
Source: Ahn JC et al. Racial and ethnic disparities in early treatment with immunotherapy for advanced HCC in the United States. Hepatology. 2022 (Apr 16). Doi: 10.1002/hep.32527
Key clinical point: Immunotherapy was associated with prolonged survival compared with chemotherapy in patients with advanced hepatocellular carcinoma (HCC).
Major finding: After adjusting for confounding variables, immunotherapy was independently associated with improved overall survival (adjusted hazard ratio 0.76; 95% CI 0.65-0.88) compared with chemotherapy.
Study details: Findings are from a retrospective cohort study that included 3990 patients with advanced HCC (tumor-node-metastasis stage III or IV) from the National Cancer Database who received chemotherapy (n = 3248) or immunotherapy (n = 742) as the first-line systemic treatment.
Disclosures: No funding source was reported. Some authors declared serving as consultants or advisors or receiving institutional research support from various organizations.
Source: Ahn JC et al. Racial and ethnic disparities in early treatment with immunotherapy for advanced HCC in the United States. Hepatology. 2022 (Apr 16). Doi: 10.1002/hep.32527
Key clinical point: Immunotherapy was associated with prolonged survival compared with chemotherapy in patients with advanced hepatocellular carcinoma (HCC).
Major finding: After adjusting for confounding variables, immunotherapy was independently associated with improved overall survival (adjusted hazard ratio 0.76; 95% CI 0.65-0.88) compared with chemotherapy.
Study details: Findings are from a retrospective cohort study that included 3990 patients with advanced HCC (tumor-node-metastasis stage III or IV) from the National Cancer Database who received chemotherapy (n = 3248) or immunotherapy (n = 742) as the first-line systemic treatment.
Disclosures: No funding source was reported. Some authors declared serving as consultants or advisors or receiving institutional research support from various organizations.
Source: Ahn JC et al. Racial and ethnic disparities in early treatment with immunotherapy for advanced HCC in the United States. Hepatology. 2022 (Apr 16). Doi: 10.1002/hep.32527
Laparoscopic anatomic hepatectomy achieves better follow-up outcomes than non-anatomical hepatectomy in HCC
Key clinical point: As laparoscopic anatomical hepatectomy (LAH) is associated with increased disease-free survival (DFS) and comparable long-term overall survival (OS) and postoperative complications compared with non-anatomical hepatectomy (LNAH), it is recommended over LNAH for selected patients with HCC.
Major finding: Patients who underwent LAH vs LNAH showed significantly higher 5-year DFS rate (33.9% vs 30.1%; P = .009) and comparable long-term OS (43.2% vs 35.2%; P = .054) and postoperative complication (8.9% vs 12.4%; P = .255) rates.
Study details: Findings are from a single-center, prospective randomized controlled trial including 385 adult patients with HCC (single tumor ≤10 cm in size) who were randomly assigned to undergo LAH (n = 192) or LNAH (n = 193).
Disclosures: The study was sponsored by the National Natural Science Foundation of China and Project of Chongqing Municipality. The authors reported no conflicts of interest.
Source: Liao K et al. Laparoscopic anatomical versus non-anatomical hepatectomy in the treatment of hepatocellular carcinoma: A randomised controlled trial. Int J Surg. 2022;102:106652 (May 4). Doi: 10.1016/j.ijsu.2022.106652
Key clinical point: As laparoscopic anatomical hepatectomy (LAH) is associated with increased disease-free survival (DFS) and comparable long-term overall survival (OS) and postoperative complications compared with non-anatomical hepatectomy (LNAH), it is recommended over LNAH for selected patients with HCC.
Major finding: Patients who underwent LAH vs LNAH showed significantly higher 5-year DFS rate (33.9% vs 30.1%; P = .009) and comparable long-term OS (43.2% vs 35.2%; P = .054) and postoperative complication (8.9% vs 12.4%; P = .255) rates.
Study details: Findings are from a single-center, prospective randomized controlled trial including 385 adult patients with HCC (single tumor ≤10 cm in size) who were randomly assigned to undergo LAH (n = 192) or LNAH (n = 193).
Disclosures: The study was sponsored by the National Natural Science Foundation of China and Project of Chongqing Municipality. The authors reported no conflicts of interest.
Source: Liao K et al. Laparoscopic anatomical versus non-anatomical hepatectomy in the treatment of hepatocellular carcinoma: A randomised controlled trial. Int J Surg. 2022;102:106652 (May 4). Doi: 10.1016/j.ijsu.2022.106652
Key clinical point: As laparoscopic anatomical hepatectomy (LAH) is associated with increased disease-free survival (DFS) and comparable long-term overall survival (OS) and postoperative complications compared with non-anatomical hepatectomy (LNAH), it is recommended over LNAH for selected patients with HCC.
Major finding: Patients who underwent LAH vs LNAH showed significantly higher 5-year DFS rate (33.9% vs 30.1%; P = .009) and comparable long-term OS (43.2% vs 35.2%; P = .054) and postoperative complication (8.9% vs 12.4%; P = .255) rates.
Study details: Findings are from a single-center, prospective randomized controlled trial including 385 adult patients with HCC (single tumor ≤10 cm in size) who were randomly assigned to undergo LAH (n = 192) or LNAH (n = 193).
Disclosures: The study was sponsored by the National Natural Science Foundation of China and Project of Chongqing Municipality. The authors reported no conflicts of interest.
Source: Liao K et al. Laparoscopic anatomical versus non-anatomical hepatectomy in the treatment of hepatocellular carcinoma: A randomised controlled trial. Int J Surg. 2022;102:106652 (May 4). Doi: 10.1016/j.ijsu.2022.106652
Does imaging surveillance intensity govern clinical outcomes in HCC?
Key clinical point: Compared with the standard ultrasonography (US)-based imaging surveillance for hepatocellular carcinoma (HCC), intensive surveillance using alternative computed tomography (CT)/magnetic resonance imaging (MRI) in addition to US may facilitate the diagnosis of very early-stage HCC without providing any survival advantage.
Major finding: Diagnosis of very early-stage HCC was better in the low- (adjusted odds ratio [aOR] 0.44; P = .034) and high- (aOR 0.40; P = .014) intensive surveillance groups than in the standard surveillance group. However, overall survival remained unaffected by the surveillance intensity (P > .05).
Study details: This was a retrospective cohort study including 529 patients with newly diagnosed HCC who were on regular surveillance and were monitored using only US (standard group; n = 62) or CT/MRI plus US (categorized into low-intensive group [n = 232] and high-intensive group [n = 235] based on the median percentage of CT/MRI investigations [cut-off, 27%]).
Disclosures: The study did not receive any funding. The authors disclosed no conflicts of interest.
Source: Hwang JA et al. Association between intensity of imaging surveillance and clinical outcomes in patients with hepatocellular carcinoma. Eur J Radiol. 2022;151:110328 (Apr 21). Doi: 10.1016/j.ejrad.2022.110328
Key clinical point: Compared with the standard ultrasonography (US)-based imaging surveillance for hepatocellular carcinoma (HCC), intensive surveillance using alternative computed tomography (CT)/magnetic resonance imaging (MRI) in addition to US may facilitate the diagnosis of very early-stage HCC without providing any survival advantage.
Major finding: Diagnosis of very early-stage HCC was better in the low- (adjusted odds ratio [aOR] 0.44; P = .034) and high- (aOR 0.40; P = .014) intensive surveillance groups than in the standard surveillance group. However, overall survival remained unaffected by the surveillance intensity (P > .05).
Study details: This was a retrospective cohort study including 529 patients with newly diagnosed HCC who were on regular surveillance and were monitored using only US (standard group; n = 62) or CT/MRI plus US (categorized into low-intensive group [n = 232] and high-intensive group [n = 235] based on the median percentage of CT/MRI investigations [cut-off, 27%]).
Disclosures: The study did not receive any funding. The authors disclosed no conflicts of interest.
Source: Hwang JA et al. Association between intensity of imaging surveillance and clinical outcomes in patients with hepatocellular carcinoma. Eur J Radiol. 2022;151:110328 (Apr 21). Doi: 10.1016/j.ejrad.2022.110328
Key clinical point: Compared with the standard ultrasonography (US)-based imaging surveillance for hepatocellular carcinoma (HCC), intensive surveillance using alternative computed tomography (CT)/magnetic resonance imaging (MRI) in addition to US may facilitate the diagnosis of very early-stage HCC without providing any survival advantage.
Major finding: Diagnosis of very early-stage HCC was better in the low- (adjusted odds ratio [aOR] 0.44; P = .034) and high- (aOR 0.40; P = .014) intensive surveillance groups than in the standard surveillance group. However, overall survival remained unaffected by the surveillance intensity (P > .05).
Study details: This was a retrospective cohort study including 529 patients with newly diagnosed HCC who were on regular surveillance and were monitored using only US (standard group; n = 62) or CT/MRI plus US (categorized into low-intensive group [n = 232] and high-intensive group [n = 235] based on the median percentage of CT/MRI investigations [cut-off, 27%]).
Disclosures: The study did not receive any funding. The authors disclosed no conflicts of interest.
Source: Hwang JA et al. Association between intensity of imaging surveillance and clinical outcomes in patients with hepatocellular carcinoma. Eur J Radiol. 2022;151:110328 (Apr 21). Doi: 10.1016/j.ejrad.2022.110328
Subsequent anticancer therapy after ICI treatment prolongs survival in HCC
Key clinical point: Compared with best supportive care (BSC), treatment with any type of anticancer therapy after immune checkpoint inhibitor (ICI) therapy discontinuation in hepatocellular carcinoma (HCC) is associated with a significant improvement in overall survival.
Major finding: After ICI therapy discontinuation, patients who received anticancer therapy vs. BSC showed a significantly improved median overall survival (12.2 vs 3.2 months; hazard ratio 0.4; P < .001).
Study details: This was a retrospective, multicenter study that included 420 patients with HCC who were treated with ICI followed by subsequent anticancer treatment (n = 163) or BSC (n = 152).
Disclosures: The study was supported by the Wellcome Trust Strategic Fund, UK. Some authors declared serving as advisors, consultants, or speakers for and receiving grants from various sources.
Source: Sharma R et al. Patterns and outcomes of subsequent therapy after immune checkpoint inhibitor discontinuation in HCC. Hepatol Commun. 2022 (Apr 28). Doi: 10.1002/hep4.1927
Key clinical point: Compared with best supportive care (BSC), treatment with any type of anticancer therapy after immune checkpoint inhibitor (ICI) therapy discontinuation in hepatocellular carcinoma (HCC) is associated with a significant improvement in overall survival.
Major finding: After ICI therapy discontinuation, patients who received anticancer therapy vs. BSC showed a significantly improved median overall survival (12.2 vs 3.2 months; hazard ratio 0.4; P < .001).
Study details: This was a retrospective, multicenter study that included 420 patients with HCC who were treated with ICI followed by subsequent anticancer treatment (n = 163) or BSC (n = 152).
Disclosures: The study was supported by the Wellcome Trust Strategic Fund, UK. Some authors declared serving as advisors, consultants, or speakers for and receiving grants from various sources.
Source: Sharma R et al. Patterns and outcomes of subsequent therapy after immune checkpoint inhibitor discontinuation in HCC. Hepatol Commun. 2022 (Apr 28). Doi: 10.1002/hep4.1927
Key clinical point: Compared with best supportive care (BSC), treatment with any type of anticancer therapy after immune checkpoint inhibitor (ICI) therapy discontinuation in hepatocellular carcinoma (HCC) is associated with a significant improvement in overall survival.
Major finding: After ICI therapy discontinuation, patients who received anticancer therapy vs. BSC showed a significantly improved median overall survival (12.2 vs 3.2 months; hazard ratio 0.4; P < .001).
Study details: This was a retrospective, multicenter study that included 420 patients with HCC who were treated with ICI followed by subsequent anticancer treatment (n = 163) or BSC (n = 152).
Disclosures: The study was supported by the Wellcome Trust Strategic Fund, UK. Some authors declared serving as advisors, consultants, or speakers for and receiving grants from various sources.
Source: Sharma R et al. Patterns and outcomes of subsequent therapy after immune checkpoint inhibitor discontinuation in HCC. Hepatol Commun. 2022 (Apr 28). Doi: 10.1002/hep4.1927
Pembrolizumab monotherapy shows promise for untreated advanced HCC in phase 2
Key clinical point: Pembrolizumab monotherapy showed favorable efficacy in systemic therapy-naive patients with advanced hepatocellular carcinoma (aHCC), with no new safety signals in addition to those observed for pembrolizumab in aHCC in a second-line setting.
Major finding: The objective response rate was 16% (95% CI 7%-29%), and the median duration of response was 16 months. The median progression-free and overall survival were 4 (95% CI 2-8) and 17 (95% CI 8-23) months, respectively. Only 16% of patients experienced grade ≥3 treatment-related adverse events.
Study details: Findings are from the phase 2 KEYNOTE-224 trial including 51 adult, systemic therapy-naive patients with aHCC who received 200 mg pembrolizumab intravenously every 3 weeks for up to 35 cycles.
Disclosures: The study was sponsored by Merck Sharp & Dohme Corp. (MSD), a subsidiary of Merck & Co., Inc., USA. Some authors reported being advisory board members, consultants, or advisors or receiving research grants, speaker honoraria, or travel and accommodation expenses from various sources, including MSD. The other authors are employees and stock owners of MSD.
Source: Verset G et al. Pembrolizumab monotherapy for previously untreated advanced hepatocellular carcinoma: Data from the open-label, phase II KEYNOTE-224 trial. Clin Cancer Res. 2022 (Apr 14). Doi: 10.1158/1078-0432.CCR-21-3807
Key clinical point: Pembrolizumab monotherapy showed favorable efficacy in systemic therapy-naive patients with advanced hepatocellular carcinoma (aHCC), with no new safety signals in addition to those observed for pembrolizumab in aHCC in a second-line setting.
Major finding: The objective response rate was 16% (95% CI 7%-29%), and the median duration of response was 16 months. The median progression-free and overall survival were 4 (95% CI 2-8) and 17 (95% CI 8-23) months, respectively. Only 16% of patients experienced grade ≥3 treatment-related adverse events.
Study details: Findings are from the phase 2 KEYNOTE-224 trial including 51 adult, systemic therapy-naive patients with aHCC who received 200 mg pembrolizumab intravenously every 3 weeks for up to 35 cycles.
Disclosures: The study was sponsored by Merck Sharp & Dohme Corp. (MSD), a subsidiary of Merck & Co., Inc., USA. Some authors reported being advisory board members, consultants, or advisors or receiving research grants, speaker honoraria, or travel and accommodation expenses from various sources, including MSD. The other authors are employees and stock owners of MSD.
Source: Verset G et al. Pembrolizumab monotherapy for previously untreated advanced hepatocellular carcinoma: Data from the open-label, phase II KEYNOTE-224 trial. Clin Cancer Res. 2022 (Apr 14). Doi: 10.1158/1078-0432.CCR-21-3807
Key clinical point: Pembrolizumab monotherapy showed favorable efficacy in systemic therapy-naive patients with advanced hepatocellular carcinoma (aHCC), with no new safety signals in addition to those observed for pembrolizumab in aHCC in a second-line setting.
Major finding: The objective response rate was 16% (95% CI 7%-29%), and the median duration of response was 16 months. The median progression-free and overall survival were 4 (95% CI 2-8) and 17 (95% CI 8-23) months, respectively. Only 16% of patients experienced grade ≥3 treatment-related adverse events.
Study details: Findings are from the phase 2 KEYNOTE-224 trial including 51 adult, systemic therapy-naive patients with aHCC who received 200 mg pembrolizumab intravenously every 3 weeks for up to 35 cycles.
Disclosures: The study was sponsored by Merck Sharp & Dohme Corp. (MSD), a subsidiary of Merck & Co., Inc., USA. Some authors reported being advisory board members, consultants, or advisors or receiving research grants, speaker honoraria, or travel and accommodation expenses from various sources, including MSD. The other authors are employees and stock owners of MSD.
Source: Verset G et al. Pembrolizumab monotherapy for previously untreated advanced hepatocellular carcinoma: Data from the open-label, phase II KEYNOTE-224 trial. Clin Cancer Res. 2022 (Apr 14). Doi: 10.1158/1078-0432.CCR-21-3807
Early-stage HCC: OLT offers better survival outcomes than ablative therapies in the elderly
Key clinical point: Compared with liver-directed ablative therapies, orthotopic liver transplantation (OLT) offers a survival advantage for elderly patients with early-stage hepatocellular carcinoma (HCC) and alpha-fetoprotein (AFP) levels <500 ng/mL.
Major finding: Multivariable analysis revealed a significant survival benefit of OLT compared with ablative therapy alone (adjusted hazard ratio [aHR] 0.31; P < .001), with OLT being associated with better survival even after adjusting for imbalanced factors after propensity matching (aHR 0.35; P < .001).
Study details: The data come from a retrospective review study that propensity score matched patients aged ≥70 years with stage I-II HCC and AFP levels of <500 ng/mL receiving OLT (n = 170) with those undergoing liver-directed ablative therapy (n = 170).
Disclosures: No source of funding or conflicts of interest was declared by the authors.
Source: Shah MB et al. Outcomes in elderly patients undergoing liver transplantation compared with liver-directed ablative therapy in early-stage hepatocellular carcinoma. J Am Coll Surg. 2022;234(5):892-899 (Apr 15). Doi: 10.1097/XCS.0000000000000135
Key clinical point: Compared with liver-directed ablative therapies, orthotopic liver transplantation (OLT) offers a survival advantage for elderly patients with early-stage hepatocellular carcinoma (HCC) and alpha-fetoprotein (AFP) levels <500 ng/mL.
Major finding: Multivariable analysis revealed a significant survival benefit of OLT compared with ablative therapy alone (adjusted hazard ratio [aHR] 0.31; P < .001), with OLT being associated with better survival even after adjusting for imbalanced factors after propensity matching (aHR 0.35; P < .001).
Study details: The data come from a retrospective review study that propensity score matched patients aged ≥70 years with stage I-II HCC and AFP levels of <500 ng/mL receiving OLT (n = 170) with those undergoing liver-directed ablative therapy (n = 170).
Disclosures: No source of funding or conflicts of interest was declared by the authors.
Source: Shah MB et al. Outcomes in elderly patients undergoing liver transplantation compared with liver-directed ablative therapy in early-stage hepatocellular carcinoma. J Am Coll Surg. 2022;234(5):892-899 (Apr 15). Doi: 10.1097/XCS.0000000000000135
Key clinical point: Compared with liver-directed ablative therapies, orthotopic liver transplantation (OLT) offers a survival advantage for elderly patients with early-stage hepatocellular carcinoma (HCC) and alpha-fetoprotein (AFP) levels <500 ng/mL.
Major finding: Multivariable analysis revealed a significant survival benefit of OLT compared with ablative therapy alone (adjusted hazard ratio [aHR] 0.31; P < .001), with OLT being associated with better survival even after adjusting for imbalanced factors after propensity matching (aHR 0.35; P < .001).
Study details: The data come from a retrospective review study that propensity score matched patients aged ≥70 years with stage I-II HCC and AFP levels of <500 ng/mL receiving OLT (n = 170) with those undergoing liver-directed ablative therapy (n = 170).
Disclosures: No source of funding or conflicts of interest was declared by the authors.
Source: Shah MB et al. Outcomes in elderly patients undergoing liver transplantation compared with liver-directed ablative therapy in early-stage hepatocellular carcinoma. J Am Coll Surg. 2022;234(5):892-899 (Apr 15). Doi: 10.1097/XCS.0000000000000135
Psychosocial Barriers and Their Impact on Hepatocellular Carcinoma Care in US Veterans: Tumor Board Model of Care
Hepatocellular carcinoma (HCC) remains a major global health problem and is the third leading cause of cancer-related mortality worldwide.1 Management of HCC is complex; as it largely occurs in the background of chronic liver disease, its management must simultaneously address challenges related to the patient’s tumor burden, as well as their underlying liver dysfunction and performance status. HCC is universally fatal without treatment, with a 5-year survival < 10%.2 However, if detected early HCC is potentially curable, with treatments such as hepatic resection, ablation, and/or liver transplantation, which are associated with 5-year survival rates as high as 70%.2 HCC-specific palliative treatments, including intra-arterial therapies (eg, trans-arterial chemoembolization, radioembolization) and systemic chemotherapy, have also been shown to prolong survival in patients with advanced HCC. Therefore, a key driver of patient survival is receipt of HCC-specific therapy.
There is rising incidence and mortality related to HCC in the US veteran population, largely attributed to acquisition of chronic hepatitis C virus (HCV) infection decades prior.3 There is also a high prevalence of psychosocial barriers in this population, such as low socioeconomic status, homelessness, alcohol and substance use disorders, and psychiatric disorders which can negatively influence receipt of medical treatment, including cancer care.4,5 Given the complexity of managing HCC, as well as the plethora of potential treatment options available, it is widely accepted that a multidisciplinary team approach, such as the multidisciplinary tumor board (MDTB) provides optimal care to patients with HCC.2,6 The aim of the present study was to identify in a population of veterans diagnosed with HCC the prevalence of psychosocial barriers to care and assess their impact and the role of an MDTB on receipt of HCC-specific care.
Methods
In June 2007, a joint institutional MDTB was established for patients with primary liver tumors receiving care at the William S. Middleton Memorial Veterans’ Hospital (WSMMVH) in Madison, Wisconsin. As we have described elsewhere, individual cases with their corresponding imaging studies were reviewed at a weekly conference attended by transplant hepatologists, medical oncologists, hepatobiliary and transplant surgeons, pathologists, diagnostic and interventional radiologists, and nurse coordinators.6 Potential therapies offered included surgical resection, liver transplantation (LT), thermal ablation, intra-arterial therapies (chemo and/or radioembolization), systemic chemotherapy, stereotactic radiation, and best supportive care. Decisions regarding the appropriate treatment modality were made based on patient factors, review of their cross-sectional imaging studies and/or histopathology, and context of their underlying liver dysfunction. The tumor board discussion was summarized in meeting minutes as well as tumor board encounters recorded in each patient’s health record. Although patients with benign tumors were presented at the MDTB, only patients with a diagnosis of HCC were included in this study.
A database analysis was conducted of all veteran patients with HCC managed through the WSMMVH MDTB, since its inception up to December 31, 2016, with follow-up until December 31, 2018. Data for analysis included demographics, laboratory parameters at time of diagnosis and treatment, imaging findings, histopathology and/or surgical pathology, treatment rendered, and follow-up information. The primary outcome measured in this study included receipt of any therapy and secondarily, patient survival.
Discrete variables were analyzed with χ2 statistics or Fisher exact test and continuous variables with the student t test. Multivariable analyses were carried out with logistic regression. Variables with a P < .05 were considered statistically significant. Analyses were carried out using IBM SPSS v24.0.
As a quality-improvement initiative for the care and management of veterans with HCC, this study was determined to be exempt from review by the WSMMVH and University of Wisconsin School of Medicine and Public Health Institutional Review Board.
Results
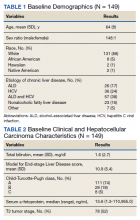
From January 1, 2007, through December 31, 2016, 149 patients with HCC were managed through the MDTB. Baseline demographic data, Model for End-stage Liver Disease (MELD) score and Child-Turcotte-Pugh class, and baseline HCC characteristics of the cohort are shown in Tables 1 and 2.
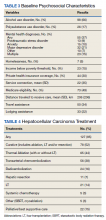
There was a high prevalence of psychosocial barriers in our study cohort, including alcohol or substance use disorder, mental illness diagnosis, and low socioeconomic status (Table 3). The mean distance traveled to WSMMVH for HCC-specific care was 206 km. Fifty patients in the cohort utilized travel assistance and 33 patients accessed lodging assistance.
HCC Treatments
There was a high rate of receipt of treatment in our study cohort with 127 (85%) patients receiving at least one HCC-specific therapy. Care was individualized and coordinated through our institutional MDTB, with both curative and palliative treatment modalities utilized (Table 4).
Curative treatment, which includes LT, ablation, or resection, was offered to 78 (52%) patients who were within T2 stage. Of these 78 patients who were potential candidates for LT as a curative treatment for HCC, 31 were not deemed suitable transplant candidates. Psychosocial barriers precluded consideration for LT in 7 of the 31 patients due to active substance use, homelessness in 1 patient, and severe mental illness in 3 patients. Medical comorbidities, advanced patient age, and patient preference accounted for the remainder.
In a univariate analysis of the cohort of 149 patients, factors that decreased the likelihood of receipt of curative HCC therapy included T2 stage or higher at diagnosis and a diagnosis of depression, whereas provision for lodging was associated with increased likelihood of receiving HCC-specific care (Table 5). Other factors that influenced receipt of any treatment included patient’s MELD score, total bilirubin, and serum α-fetoprotein, a surrogate marker for tumor stage. In the multivariable analysis, predictors of receiving curative therapy included absence of substance use, within T2 stage of tumor, and Child-Turcotte-Pugh class A cirrhosis. The presence of psychosocial barriers apart from substance use did not predict a lower chance of receiving curative HCC therapy (including homelessness, distance traveled to center, mental health disorder, and low income).
Median survival was 727 (95% CI, 488-966) days from diagnosis. Survival from HCC diagnosis in study cohort was 72% at 1 year, 50% at 2 years, 39% at 3 years, and 36% at 5 years. Death occurred in 71 (48%) patients; HCC accounted for death in 52 (73%) patients, complications of end-stage liver disease in 13 (18%) patients, and other causes for the remainder of patients.
Discussion
Increases in prevalence and mortality related to cirrhosis and HCC have been reported among the US veteran population.3 This is in large part attributable to the burden of chronic HCV infection in this population. As mirrored in the US population in general, we may be at a turning point regarding the gradual increase in prevalence in HCC.7 The prevalence of cirrhosis and viral-related HCC related to HCV infection will decline with availability of effective antiviral therapy. Alcoholic liver disease remains a main etiological factor for development of cirrhosis and HCC. Nonalcoholic fatty liver disease is becoming a more prevalent cause for development of cirrhosis, indication for liver transplantation, and development of HCC, and indeed may lead to HCC even in the absence of cirrhosis.8
HCC remains a challenging clinical problem.2 As the vast majority of cases arise in the context of cirrhosis, management of HCC not only must address the cancer stage at diagnosis, but also the patient’s underlying liver dysfunction and performance status. Receipt of HCC-specific therapy is a key driver of patient outcome, with curative therapies available for those diagnosed with early-stage disease. We and others have shown that a multidisciplinary approach to coordinate, individualize, and optimize care for these complex patients can improve the rate of treatment utilization, reduce treatment delays, and improve patient survival.6,9,10
Patient psychosocial barriers, such as low socioeconomic status, homelessness, alcohol and substance use, and psychiatric disorders, are more prevalent among the veteran population and have the potential to negatively influence successful health care delivery. One retrospective study of 100 veterans at a US Department of Veterans Affairs (VA) medical center treated for HCC from 2009 to 2014 showed a majority of the patients lived on a meager income, a high prevalence of homelessness, substance use history in 96% of their cohort, and psychiatric illness in 65% of patients.11 Other studies have documented similar findings in the veteran population, with alcohol, substance use, as well as other uncontrolled comorbidities as barriers to providing care, such as antiviral therapy for chronic HCV infection.12
Herein, we present a cohort of veterans with HCC managed through our MDTB from 2007 to 2016, for whom chronic HCV infection and/or alcoholic liver disease were the main causes of cirrhosis. Our cohort had a high burden of alcohol and substance use disorders while other psychiatric illnesses were also common. Our cohort includes patients who were poor, and even some veterans who lacked a stable home. This profile of poverty and social deprivation among veterans is matched in national data.13-15 Using a tumor board model of nurse navigation and multidisciplinary care, we were able to provide travel and lodging assistance to 50 (34%) and 33 (22%) patients, respectively, in order to facilitate their care.
Our data demonstrate that the impact of psychosocial barriers on our capacity to deliver care varies with the nature of the treatment under consideration: curative vs cancer control. For example, active substance use disorder, homelessness, and severe established mental illness were often considered insurmountable when the treatment in question was LT. Nevertheless, despite the high prevalence in our study group of barriers, such as lack of transport while living far from a VA medical center, or alcohol use disorder, a curative treatment with either LT, tumor ablation, or resection could be offered to over half of our cohort. When noncurative therapies are included, most patients (85%) received HCC-specific care, with good relative survival.
Our reported high receipt of HCC-specific care and patient survival is in contrast to previously reported low rates of HCC-specific care in in a national survey of management of 1296 veteran patients infected with HCV who developed HCC from 1998 to 2006. In this population, HCC-specific treatment was provided to 34%.16 However our data are consistent with our previously published data of patients with HCC managed through an institutional MDTB.6 Indeed, as shown by a univariate analysis in our present study, individualizing care to address modifiable patient barriers, such as providing provisions for lodging if needed, was associated with an increased likelihood of receiving HCC-specific care. On the other hand, advanced tumor stage (> T2) at diagnosis and a diagnosis of depression, which was the most common psychiatric diagnosis in our cohort, were both associated with decreased likelihood of receiving HCC-specific care. Clinical factors such as MELD score, total bilirubin, and serum AFP all affected the likelihood of providing HCC-specific care. In a multivariate analysis, factors that predicted ability to receive curative therapy included absence of substance use, T2 stage of tumor, and Child-Turcotte-Pugh class A cirrhosis. This is expected as patients with HCC within T2 stage (or Milan criteria) with compensated cirrhosis are most likely to receive curative therapies, such as resection, ablation, or LT.2
Conclusions
Our study demonstrates a high burden of psychosocial challenges in veterans with HCC. These accounted for a significant barrier to receive HCC-specific care. Despite the presence of these patient barriers, high rates of HCC-specific treatment are attainable through individualization and coordination of patient care in the context of a MDTB model with nurse navigation. Provision of targeted social support to ameliorate these modifiable factors improves patient outcomes.
1. McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of hepatocellular carcinoma. Hepatology. 2021;73(suppl 1):4-13. doi:10.1002/hep.31288.
2. Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68(2):723-750. doi:10.1002/hep.29913
3. Beste LA, Leipertz SL, Green PK, Dominitz JA, Ross D, Ioannou GN. Trends in burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US veterans, 2001-2013. Gastroenterology. 2015;149(6):1471-e18. doi:10.1053/j.gastro.2015.07.056
4. Kazis LE, Miller DR, Clark J, et al. Health-related quality of life in patients served by the Department of Veterans Affairs: results from the Veterans Health Study. Arch Intern Med. 1998;158(6):626-632. doi:10.1001/archinte.158.6.626
5. Slind LM, Keating TM, Fisher AG, Rose TG. A patient navigation model for veterans traveling for cancer care. Fed Pract. 2016;33(suppl 1):40S-45S.
6. Agarwal PD, Phillips P, Hillman L, et al. Multidisciplinary management of hepatocellular carcinoma improves access to therapy and patient survival. J Clin Gastroenterol. 2017;51(9):845-849. doi:10.1097/MCG.0000000000000825
7. White DL, Thrift AP, Kanwal F, Davila J, El-Serag HB. Incidence of hepatocellular carcinoma in all 50 United States, From 2000 Through 2012. Gastroenterology. 2017;152(4):812-820.e5. doi:10.1053/j.gastro.2016.11.020
8. Kanwal F, Kramer JR, Mapakshi S, et al. Risk of hepatocellular cancer in patients with non-alcoholic fatty liver disease. Gastroenterology. 2018;155(6):1828-1837.e2. doi:10.1053/j.gastro.2018.08.024
9. Yopp AC, Mansour JC, Beg MS, et al. Establishment of a multidisciplinary hepatocellular carcinoma clinic is associated with improved clinical outcome. Ann Surg Oncol. 2014;21(4):1287-1295. doi:10.1245/s10434-013-3413-8
10. Chang TT, Sawhney R, Monto A, et al. Implementation of a multidisciplinary treatment team for hepatocellular cancer at a Veterans Affairs Medical Center improves survival. HPB (Oxford). 2008;10(6):405-411. doi:10.1080/13651820802356572
11. Hwa KJ, Dua MM, Wren SM, Visser BC. Missing the obvious: psychosocial obstacles in veterans with hepatocellular carcinoma. HPB (Oxford). 2015;17(12):1124-1129. doi:10.1111/hpb.12508
12. Taylor J, Carr-Lopez S, Robinson A, et al. Determinants of treatment eligibility in veterans with hepatitis C viral infection. Clin Ther. 2017;39(1):130-137. doi:10.1016/j.clinthera.2016.11.019
13. Fargo J, Metraux S, Byrne T, et al. Prevalence and risk of homelessness among US veterans. Prev Chronic Dis. 2012;9:E45.
14. Tsai J, Rosenheck RA. Risk factors for homelessness among US veterans. Epidemiol Rev. 2015;37:177-195. doi:10.1093/epirev/mxu004
15. Tsai J, Link B, Rosenheck RA, Pietrzak RH. Homelessness among a nationally representative sample of US veterans: prevalence, service utilization, and correlates. Soc Psychiatry Psychiatr Epidemiol. 2016;51(6):907-916. doi:10.1007/s00127-016-1210-y
16. Davila JA, Kramer JR, Duan Z, et al. Referral and receipt of treatment for hepatocellular carcinoma in United States veterans: effect of patient and nonpatient factors. Hepatology. 2013;57(5):1858-1868. doi:10.1002/hep.26287
Hepatocellular carcinoma (HCC) remains a major global health problem and is the third leading cause of cancer-related mortality worldwide.1 Management of HCC is complex; as it largely occurs in the background of chronic liver disease, its management must simultaneously address challenges related to the patient’s tumor burden, as well as their underlying liver dysfunction and performance status. HCC is universally fatal without treatment, with a 5-year survival < 10%.2 However, if detected early HCC is potentially curable, with treatments such as hepatic resection, ablation, and/or liver transplantation, which are associated with 5-year survival rates as high as 70%.2 HCC-specific palliative treatments, including intra-arterial therapies (eg, trans-arterial chemoembolization, radioembolization) and systemic chemotherapy, have also been shown to prolong survival in patients with advanced HCC. Therefore, a key driver of patient survival is receipt of HCC-specific therapy.
There is rising incidence and mortality related to HCC in the US veteran population, largely attributed to acquisition of chronic hepatitis C virus (HCV) infection decades prior.3 There is also a high prevalence of psychosocial barriers in this population, such as low socioeconomic status, homelessness, alcohol and substance use disorders, and psychiatric disorders which can negatively influence receipt of medical treatment, including cancer care.4,5 Given the complexity of managing HCC, as well as the plethora of potential treatment options available, it is widely accepted that a multidisciplinary team approach, such as the multidisciplinary tumor board (MDTB) provides optimal care to patients with HCC.2,6 The aim of the present study was to identify in a population of veterans diagnosed with HCC the prevalence of psychosocial barriers to care and assess their impact and the role of an MDTB on receipt of HCC-specific care.
Methods
In June 2007, a joint institutional MDTB was established for patients with primary liver tumors receiving care at the William S. Middleton Memorial Veterans’ Hospital (WSMMVH) in Madison, Wisconsin. As we have described elsewhere, individual cases with their corresponding imaging studies were reviewed at a weekly conference attended by transplant hepatologists, medical oncologists, hepatobiliary and transplant surgeons, pathologists, diagnostic and interventional radiologists, and nurse coordinators.6 Potential therapies offered included surgical resection, liver transplantation (LT), thermal ablation, intra-arterial therapies (chemo and/or radioembolization), systemic chemotherapy, stereotactic radiation, and best supportive care. Decisions regarding the appropriate treatment modality were made based on patient factors, review of their cross-sectional imaging studies and/or histopathology, and context of their underlying liver dysfunction. The tumor board discussion was summarized in meeting minutes as well as tumor board encounters recorded in each patient’s health record. Although patients with benign tumors were presented at the MDTB, only patients with a diagnosis of HCC were included in this study.
A database analysis was conducted of all veteran patients with HCC managed through the WSMMVH MDTB, since its inception up to December 31, 2016, with follow-up until December 31, 2018. Data for analysis included demographics, laboratory parameters at time of diagnosis and treatment, imaging findings, histopathology and/or surgical pathology, treatment rendered, and follow-up information. The primary outcome measured in this study included receipt of any therapy and secondarily, patient survival.
Discrete variables were analyzed with χ2 statistics or Fisher exact test and continuous variables with the student t test. Multivariable analyses were carried out with logistic regression. Variables with a P < .05 were considered statistically significant. Analyses were carried out using IBM SPSS v24.0.
As a quality-improvement initiative for the care and management of veterans with HCC, this study was determined to be exempt from review by the WSMMVH and University of Wisconsin School of Medicine and Public Health Institutional Review Board.
Results
From January 1, 2007, through December 31, 2016, 149 patients with HCC were managed through the MDTB. Baseline demographic data, Model for End-stage Liver Disease (MELD) score and Child-Turcotte-Pugh class, and baseline HCC characteristics of the cohort are shown in Tables 1 and 2.
There was a high prevalence of psychosocial barriers in our study cohort, including alcohol or substance use disorder, mental illness diagnosis, and low socioeconomic status (Table 3). The mean distance traveled to WSMMVH for HCC-specific care was 206 km. Fifty patients in the cohort utilized travel assistance and 33 patients accessed lodging assistance.
HCC Treatments
There was a high rate of receipt of treatment in our study cohort with 127 (85%) patients receiving at least one HCC-specific therapy. Care was individualized and coordinated through our institutional MDTB, with both curative and palliative treatment modalities utilized (Table 4).
Curative treatment, which includes LT, ablation, or resection, was offered to 78 (52%) patients who were within T2 stage. Of these 78 patients who were potential candidates for LT as a curative treatment for HCC, 31 were not deemed suitable transplant candidates. Psychosocial barriers precluded consideration for LT in 7 of the 31 patients due to active substance use, homelessness in 1 patient, and severe mental illness in 3 patients. Medical comorbidities, advanced patient age, and patient preference accounted for the remainder.
In a univariate analysis of the cohort of 149 patients, factors that decreased the likelihood of receipt of curative HCC therapy included T2 stage or higher at diagnosis and a diagnosis of depression, whereas provision for lodging was associated with increased likelihood of receiving HCC-specific care (Table 5). Other factors that influenced receipt of any treatment included patient’s MELD score, total bilirubin, and serum α-fetoprotein, a surrogate marker for tumor stage. In the multivariable analysis, predictors of receiving curative therapy included absence of substance use, within T2 stage of tumor, and Child-Turcotte-Pugh class A cirrhosis. The presence of psychosocial barriers apart from substance use did not predict a lower chance of receiving curative HCC therapy (including homelessness, distance traveled to center, mental health disorder, and low income).
Median survival was 727 (95% CI, 488-966) days from diagnosis. Survival from HCC diagnosis in study cohort was 72% at 1 year, 50% at 2 years, 39% at 3 years, and 36% at 5 years. Death occurred in 71 (48%) patients; HCC accounted for death in 52 (73%) patients, complications of end-stage liver disease in 13 (18%) patients, and other causes for the remainder of patients.
Discussion
Increases in prevalence and mortality related to cirrhosis and HCC have been reported among the US veteran population.3 This is in large part attributable to the burden of chronic HCV infection in this population. As mirrored in the US population in general, we may be at a turning point regarding the gradual increase in prevalence in HCC.7 The prevalence of cirrhosis and viral-related HCC related to HCV infection will decline with availability of effective antiviral therapy. Alcoholic liver disease remains a main etiological factor for development of cirrhosis and HCC. Nonalcoholic fatty liver disease is becoming a more prevalent cause for development of cirrhosis, indication for liver transplantation, and development of HCC, and indeed may lead to HCC even in the absence of cirrhosis.8
HCC remains a challenging clinical problem.2 As the vast majority of cases arise in the context of cirrhosis, management of HCC not only must address the cancer stage at diagnosis, but also the patient’s underlying liver dysfunction and performance status. Receipt of HCC-specific therapy is a key driver of patient outcome, with curative therapies available for those diagnosed with early-stage disease. We and others have shown that a multidisciplinary approach to coordinate, individualize, and optimize care for these complex patients can improve the rate of treatment utilization, reduce treatment delays, and improve patient survival.6,9,10
Patient psychosocial barriers, such as low socioeconomic status, homelessness, alcohol and substance use, and psychiatric disorders, are more prevalent among the veteran population and have the potential to negatively influence successful health care delivery. One retrospective study of 100 veterans at a US Department of Veterans Affairs (VA) medical center treated for HCC from 2009 to 2014 showed a majority of the patients lived on a meager income, a high prevalence of homelessness, substance use history in 96% of their cohort, and psychiatric illness in 65% of patients.11 Other studies have documented similar findings in the veteran population, with alcohol, substance use, as well as other uncontrolled comorbidities as barriers to providing care, such as antiviral therapy for chronic HCV infection.12
Herein, we present a cohort of veterans with HCC managed through our MDTB from 2007 to 2016, for whom chronic HCV infection and/or alcoholic liver disease were the main causes of cirrhosis. Our cohort had a high burden of alcohol and substance use disorders while other psychiatric illnesses were also common. Our cohort includes patients who were poor, and even some veterans who lacked a stable home. This profile of poverty and social deprivation among veterans is matched in national data.13-15 Using a tumor board model of nurse navigation and multidisciplinary care, we were able to provide travel and lodging assistance to 50 (34%) and 33 (22%) patients, respectively, in order to facilitate their care.
Our data demonstrate that the impact of psychosocial barriers on our capacity to deliver care varies with the nature of the treatment under consideration: curative vs cancer control. For example, active substance use disorder, homelessness, and severe established mental illness were often considered insurmountable when the treatment in question was LT. Nevertheless, despite the high prevalence in our study group of barriers, such as lack of transport while living far from a VA medical center, or alcohol use disorder, a curative treatment with either LT, tumor ablation, or resection could be offered to over half of our cohort. When noncurative therapies are included, most patients (85%) received HCC-specific care, with good relative survival.
Our reported high receipt of HCC-specific care and patient survival is in contrast to previously reported low rates of HCC-specific care in in a national survey of management of 1296 veteran patients infected with HCV who developed HCC from 1998 to 2006. In this population, HCC-specific treatment was provided to 34%.16 However our data are consistent with our previously published data of patients with HCC managed through an institutional MDTB.6 Indeed, as shown by a univariate analysis in our present study, individualizing care to address modifiable patient barriers, such as providing provisions for lodging if needed, was associated with an increased likelihood of receiving HCC-specific care. On the other hand, advanced tumor stage (> T2) at diagnosis and a diagnosis of depression, which was the most common psychiatric diagnosis in our cohort, were both associated with decreased likelihood of receiving HCC-specific care. Clinical factors such as MELD score, total bilirubin, and serum AFP all affected the likelihood of providing HCC-specific care. In a multivariate analysis, factors that predicted ability to receive curative therapy included absence of substance use, T2 stage of tumor, and Child-Turcotte-Pugh class A cirrhosis. This is expected as patients with HCC within T2 stage (or Milan criteria) with compensated cirrhosis are most likely to receive curative therapies, such as resection, ablation, or LT.2
Conclusions
Our study demonstrates a high burden of psychosocial challenges in veterans with HCC. These accounted for a significant barrier to receive HCC-specific care. Despite the presence of these patient barriers, high rates of HCC-specific treatment are attainable through individualization and coordination of patient care in the context of a MDTB model with nurse navigation. Provision of targeted social support to ameliorate these modifiable factors improves patient outcomes.
Hepatocellular carcinoma (HCC) remains a major global health problem and is the third leading cause of cancer-related mortality worldwide.1 Management of HCC is complex; as it largely occurs in the background of chronic liver disease, its management must simultaneously address challenges related to the patient’s tumor burden, as well as their underlying liver dysfunction and performance status. HCC is universally fatal without treatment, with a 5-year survival < 10%.2 However, if detected early HCC is potentially curable, with treatments such as hepatic resection, ablation, and/or liver transplantation, which are associated with 5-year survival rates as high as 70%.2 HCC-specific palliative treatments, including intra-arterial therapies (eg, trans-arterial chemoembolization, radioembolization) and systemic chemotherapy, have also been shown to prolong survival in patients with advanced HCC. Therefore, a key driver of patient survival is receipt of HCC-specific therapy.
There is rising incidence and mortality related to HCC in the US veteran population, largely attributed to acquisition of chronic hepatitis C virus (HCV) infection decades prior.3 There is also a high prevalence of psychosocial barriers in this population, such as low socioeconomic status, homelessness, alcohol and substance use disorders, and psychiatric disorders which can negatively influence receipt of medical treatment, including cancer care.4,5 Given the complexity of managing HCC, as well as the plethora of potential treatment options available, it is widely accepted that a multidisciplinary team approach, such as the multidisciplinary tumor board (MDTB) provides optimal care to patients with HCC.2,6 The aim of the present study was to identify in a population of veterans diagnosed with HCC the prevalence of psychosocial barriers to care and assess their impact and the role of an MDTB on receipt of HCC-specific care.
Methods
In June 2007, a joint institutional MDTB was established for patients with primary liver tumors receiving care at the William S. Middleton Memorial Veterans’ Hospital (WSMMVH) in Madison, Wisconsin. As we have described elsewhere, individual cases with their corresponding imaging studies were reviewed at a weekly conference attended by transplant hepatologists, medical oncologists, hepatobiliary and transplant surgeons, pathologists, diagnostic and interventional radiologists, and nurse coordinators.6 Potential therapies offered included surgical resection, liver transplantation (LT), thermal ablation, intra-arterial therapies (chemo and/or radioembolization), systemic chemotherapy, stereotactic radiation, and best supportive care. Decisions regarding the appropriate treatment modality were made based on patient factors, review of their cross-sectional imaging studies and/or histopathology, and context of their underlying liver dysfunction. The tumor board discussion was summarized in meeting minutes as well as tumor board encounters recorded in each patient’s health record. Although patients with benign tumors were presented at the MDTB, only patients with a diagnosis of HCC were included in this study.
A database analysis was conducted of all veteran patients with HCC managed through the WSMMVH MDTB, since its inception up to December 31, 2016, with follow-up until December 31, 2018. Data for analysis included demographics, laboratory parameters at time of diagnosis and treatment, imaging findings, histopathology and/or surgical pathology, treatment rendered, and follow-up information. The primary outcome measured in this study included receipt of any therapy and secondarily, patient survival.
Discrete variables were analyzed with χ2 statistics or Fisher exact test and continuous variables with the student t test. Multivariable analyses were carried out with logistic regression. Variables with a P < .05 were considered statistically significant. Analyses were carried out using IBM SPSS v24.0.
As a quality-improvement initiative for the care and management of veterans with HCC, this study was determined to be exempt from review by the WSMMVH and University of Wisconsin School of Medicine and Public Health Institutional Review Board.
Results
From January 1, 2007, through December 31, 2016, 149 patients with HCC were managed through the MDTB. Baseline demographic data, Model for End-stage Liver Disease (MELD) score and Child-Turcotte-Pugh class, and baseline HCC characteristics of the cohort are shown in Tables 1 and 2.
There was a high prevalence of psychosocial barriers in our study cohort, including alcohol or substance use disorder, mental illness diagnosis, and low socioeconomic status (Table 3). The mean distance traveled to WSMMVH for HCC-specific care was 206 km. Fifty patients in the cohort utilized travel assistance and 33 patients accessed lodging assistance.
HCC Treatments
There was a high rate of receipt of treatment in our study cohort with 127 (85%) patients receiving at least one HCC-specific therapy. Care was individualized and coordinated through our institutional MDTB, with both curative and palliative treatment modalities utilized (Table 4).
Curative treatment, which includes LT, ablation, or resection, was offered to 78 (52%) patients who were within T2 stage. Of these 78 patients who were potential candidates for LT as a curative treatment for HCC, 31 were not deemed suitable transplant candidates. Psychosocial barriers precluded consideration for LT in 7 of the 31 patients due to active substance use, homelessness in 1 patient, and severe mental illness in 3 patients. Medical comorbidities, advanced patient age, and patient preference accounted for the remainder.
In a univariate analysis of the cohort of 149 patients, factors that decreased the likelihood of receipt of curative HCC therapy included T2 stage or higher at diagnosis and a diagnosis of depression, whereas provision for lodging was associated with increased likelihood of receiving HCC-specific care (Table 5). Other factors that influenced receipt of any treatment included patient’s MELD score, total bilirubin, and serum α-fetoprotein, a surrogate marker for tumor stage. In the multivariable analysis, predictors of receiving curative therapy included absence of substance use, within T2 stage of tumor, and Child-Turcotte-Pugh class A cirrhosis. The presence of psychosocial barriers apart from substance use did not predict a lower chance of receiving curative HCC therapy (including homelessness, distance traveled to center, mental health disorder, and low income).
Median survival was 727 (95% CI, 488-966) days from diagnosis. Survival from HCC diagnosis in study cohort was 72% at 1 year, 50% at 2 years, 39% at 3 years, and 36% at 5 years. Death occurred in 71 (48%) patients; HCC accounted for death in 52 (73%) patients, complications of end-stage liver disease in 13 (18%) patients, and other causes for the remainder of patients.
Discussion
Increases in prevalence and mortality related to cirrhosis and HCC have been reported among the US veteran population.3 This is in large part attributable to the burden of chronic HCV infection in this population. As mirrored in the US population in general, we may be at a turning point regarding the gradual increase in prevalence in HCC.7 The prevalence of cirrhosis and viral-related HCC related to HCV infection will decline with availability of effective antiviral therapy. Alcoholic liver disease remains a main etiological factor for development of cirrhosis and HCC. Nonalcoholic fatty liver disease is becoming a more prevalent cause for development of cirrhosis, indication for liver transplantation, and development of HCC, and indeed may lead to HCC even in the absence of cirrhosis.8
HCC remains a challenging clinical problem.2 As the vast majority of cases arise in the context of cirrhosis, management of HCC not only must address the cancer stage at diagnosis, but also the patient’s underlying liver dysfunction and performance status. Receipt of HCC-specific therapy is a key driver of patient outcome, with curative therapies available for those diagnosed with early-stage disease. We and others have shown that a multidisciplinary approach to coordinate, individualize, and optimize care for these complex patients can improve the rate of treatment utilization, reduce treatment delays, and improve patient survival.6,9,10
Patient psychosocial barriers, such as low socioeconomic status, homelessness, alcohol and substance use, and psychiatric disorders, are more prevalent among the veteran population and have the potential to negatively influence successful health care delivery. One retrospective study of 100 veterans at a US Department of Veterans Affairs (VA) medical center treated for HCC from 2009 to 2014 showed a majority of the patients lived on a meager income, a high prevalence of homelessness, substance use history in 96% of their cohort, and psychiatric illness in 65% of patients.11 Other studies have documented similar findings in the veteran population, with alcohol, substance use, as well as other uncontrolled comorbidities as barriers to providing care, such as antiviral therapy for chronic HCV infection.12
Herein, we present a cohort of veterans with HCC managed through our MDTB from 2007 to 2016, for whom chronic HCV infection and/or alcoholic liver disease were the main causes of cirrhosis. Our cohort had a high burden of alcohol and substance use disorders while other psychiatric illnesses were also common. Our cohort includes patients who were poor, and even some veterans who lacked a stable home. This profile of poverty and social deprivation among veterans is matched in national data.13-15 Using a tumor board model of nurse navigation and multidisciplinary care, we were able to provide travel and lodging assistance to 50 (34%) and 33 (22%) patients, respectively, in order to facilitate their care.
Our data demonstrate that the impact of psychosocial barriers on our capacity to deliver care varies with the nature of the treatment under consideration: curative vs cancer control. For example, active substance use disorder, homelessness, and severe established mental illness were often considered insurmountable when the treatment in question was LT. Nevertheless, despite the high prevalence in our study group of barriers, such as lack of transport while living far from a VA medical center, or alcohol use disorder, a curative treatment with either LT, tumor ablation, or resection could be offered to over half of our cohort. When noncurative therapies are included, most patients (85%) received HCC-specific care, with good relative survival.
Our reported high receipt of HCC-specific care and patient survival is in contrast to previously reported low rates of HCC-specific care in in a national survey of management of 1296 veteran patients infected with HCV who developed HCC from 1998 to 2006. In this population, HCC-specific treatment was provided to 34%.16 However our data are consistent with our previously published data of patients with HCC managed through an institutional MDTB.6 Indeed, as shown by a univariate analysis in our present study, individualizing care to address modifiable patient barriers, such as providing provisions for lodging if needed, was associated with an increased likelihood of receiving HCC-specific care. On the other hand, advanced tumor stage (> T2) at diagnosis and a diagnosis of depression, which was the most common psychiatric diagnosis in our cohort, were both associated with decreased likelihood of receiving HCC-specific care. Clinical factors such as MELD score, total bilirubin, and serum AFP all affected the likelihood of providing HCC-specific care. In a multivariate analysis, factors that predicted ability to receive curative therapy included absence of substance use, T2 stage of tumor, and Child-Turcotte-Pugh class A cirrhosis. This is expected as patients with HCC within T2 stage (or Milan criteria) with compensated cirrhosis are most likely to receive curative therapies, such as resection, ablation, or LT.2
Conclusions
Our study demonstrates a high burden of psychosocial challenges in veterans with HCC. These accounted for a significant barrier to receive HCC-specific care. Despite the presence of these patient barriers, high rates of HCC-specific treatment are attainable through individualization and coordination of patient care in the context of a MDTB model with nurse navigation. Provision of targeted social support to ameliorate these modifiable factors improves patient outcomes.
1. McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of hepatocellular carcinoma. Hepatology. 2021;73(suppl 1):4-13. doi:10.1002/hep.31288.
2. Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68(2):723-750. doi:10.1002/hep.29913
3. Beste LA, Leipertz SL, Green PK, Dominitz JA, Ross D, Ioannou GN. Trends in burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US veterans, 2001-2013. Gastroenterology. 2015;149(6):1471-e18. doi:10.1053/j.gastro.2015.07.056
4. Kazis LE, Miller DR, Clark J, et al. Health-related quality of life in patients served by the Department of Veterans Affairs: results from the Veterans Health Study. Arch Intern Med. 1998;158(6):626-632. doi:10.1001/archinte.158.6.626
5. Slind LM, Keating TM, Fisher AG, Rose TG. A patient navigation model for veterans traveling for cancer care. Fed Pract. 2016;33(suppl 1):40S-45S.
6. Agarwal PD, Phillips P, Hillman L, et al. Multidisciplinary management of hepatocellular carcinoma improves access to therapy and patient survival. J Clin Gastroenterol. 2017;51(9):845-849. doi:10.1097/MCG.0000000000000825
7. White DL, Thrift AP, Kanwal F, Davila J, El-Serag HB. Incidence of hepatocellular carcinoma in all 50 United States, From 2000 Through 2012. Gastroenterology. 2017;152(4):812-820.e5. doi:10.1053/j.gastro.2016.11.020
8. Kanwal F, Kramer JR, Mapakshi S, et al. Risk of hepatocellular cancer in patients with non-alcoholic fatty liver disease. Gastroenterology. 2018;155(6):1828-1837.e2. doi:10.1053/j.gastro.2018.08.024
9. Yopp AC, Mansour JC, Beg MS, et al. Establishment of a multidisciplinary hepatocellular carcinoma clinic is associated with improved clinical outcome. Ann Surg Oncol. 2014;21(4):1287-1295. doi:10.1245/s10434-013-3413-8
10. Chang TT, Sawhney R, Monto A, et al. Implementation of a multidisciplinary treatment team for hepatocellular cancer at a Veterans Affairs Medical Center improves survival. HPB (Oxford). 2008;10(6):405-411. doi:10.1080/13651820802356572
11. Hwa KJ, Dua MM, Wren SM, Visser BC. Missing the obvious: psychosocial obstacles in veterans with hepatocellular carcinoma. HPB (Oxford). 2015;17(12):1124-1129. doi:10.1111/hpb.12508
12. Taylor J, Carr-Lopez S, Robinson A, et al. Determinants of treatment eligibility in veterans with hepatitis C viral infection. Clin Ther. 2017;39(1):130-137. doi:10.1016/j.clinthera.2016.11.019
13. Fargo J, Metraux S, Byrne T, et al. Prevalence and risk of homelessness among US veterans. Prev Chronic Dis. 2012;9:E45.
14. Tsai J, Rosenheck RA. Risk factors for homelessness among US veterans. Epidemiol Rev. 2015;37:177-195. doi:10.1093/epirev/mxu004
15. Tsai J, Link B, Rosenheck RA, Pietrzak RH. Homelessness among a nationally representative sample of US veterans: prevalence, service utilization, and correlates. Soc Psychiatry Psychiatr Epidemiol. 2016;51(6):907-916. doi:10.1007/s00127-016-1210-y
16. Davila JA, Kramer JR, Duan Z, et al. Referral and receipt of treatment for hepatocellular carcinoma in United States veterans: effect of patient and nonpatient factors. Hepatology. 2013;57(5):1858-1868. doi:10.1002/hep.26287
1. McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of hepatocellular carcinoma. Hepatology. 2021;73(suppl 1):4-13. doi:10.1002/hep.31288.
2. Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68(2):723-750. doi:10.1002/hep.29913
3. Beste LA, Leipertz SL, Green PK, Dominitz JA, Ross D, Ioannou GN. Trends in burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US veterans, 2001-2013. Gastroenterology. 2015;149(6):1471-e18. doi:10.1053/j.gastro.2015.07.056
4. Kazis LE, Miller DR, Clark J, et al. Health-related quality of life in patients served by the Department of Veterans Affairs: results from the Veterans Health Study. Arch Intern Med. 1998;158(6):626-632. doi:10.1001/archinte.158.6.626
5. Slind LM, Keating TM, Fisher AG, Rose TG. A patient navigation model for veterans traveling for cancer care. Fed Pract. 2016;33(suppl 1):40S-45S.
6. Agarwal PD, Phillips P, Hillman L, et al. Multidisciplinary management of hepatocellular carcinoma improves access to therapy and patient survival. J Clin Gastroenterol. 2017;51(9):845-849. doi:10.1097/MCG.0000000000000825
7. White DL, Thrift AP, Kanwal F, Davila J, El-Serag HB. Incidence of hepatocellular carcinoma in all 50 United States, From 2000 Through 2012. Gastroenterology. 2017;152(4):812-820.e5. doi:10.1053/j.gastro.2016.11.020
8. Kanwal F, Kramer JR, Mapakshi S, et al. Risk of hepatocellular cancer in patients with non-alcoholic fatty liver disease. Gastroenterology. 2018;155(6):1828-1837.e2. doi:10.1053/j.gastro.2018.08.024
9. Yopp AC, Mansour JC, Beg MS, et al. Establishment of a multidisciplinary hepatocellular carcinoma clinic is associated with improved clinical outcome. Ann Surg Oncol. 2014;21(4):1287-1295. doi:10.1245/s10434-013-3413-8
10. Chang TT, Sawhney R, Monto A, et al. Implementation of a multidisciplinary treatment team for hepatocellular cancer at a Veterans Affairs Medical Center improves survival. HPB (Oxford). 2008;10(6):405-411. doi:10.1080/13651820802356572
11. Hwa KJ, Dua MM, Wren SM, Visser BC. Missing the obvious: psychosocial obstacles in veterans with hepatocellular carcinoma. HPB (Oxford). 2015;17(12):1124-1129. doi:10.1111/hpb.12508
12. Taylor J, Carr-Lopez S, Robinson A, et al. Determinants of treatment eligibility in veterans with hepatitis C viral infection. Clin Ther. 2017;39(1):130-137. doi:10.1016/j.clinthera.2016.11.019
13. Fargo J, Metraux S, Byrne T, et al. Prevalence and risk of homelessness among US veterans. Prev Chronic Dis. 2012;9:E45.
14. Tsai J, Rosenheck RA. Risk factors for homelessness among US veterans. Epidemiol Rev. 2015;37:177-195. doi:10.1093/epirev/mxu004
15. Tsai J, Link B, Rosenheck RA, Pietrzak RH. Homelessness among a nationally representative sample of US veterans: prevalence, service utilization, and correlates. Soc Psychiatry Psychiatr Epidemiol. 2016;51(6):907-916. doi:10.1007/s00127-016-1210-y
16. Davila JA, Kramer JR, Duan Z, et al. Referral and receipt of treatment for hepatocellular carcinoma in United States veterans: effect of patient and nonpatient factors. Hepatology. 2013;57(5):1858-1868. doi:10.1002/hep.26287
Clinical Edge Journal Scan Commentary: HCC May 2022
Clinical trials for unresectable HCC (uHCC) have mandated excellent underlying liver function. Patients with Child-Pugh (CP) A cirrhosis do not have cirrhosis as their most life-limiting disease. In clinical practice, there are many patients with uHCC who are functionally well yet have CP-B cirrhosis. D'Alessio and colleagues undertook a retrospective evaluation of 202 patients with either CP-A or CP-B cirrhosis who received atezolizumab and bevacizumab as first-line treatment of uHCC. The majority, 154 patients (76%), had CP-A cirrhosis, whereas 48 (24%) had CP-B, including 21 B7, 21 B8, and 6 B9. The authors found that in the overall population, median overall survival (mOS) was 14.9 months (95% CI 13.6-16.3), with patients with CP-A mOS of 16.8 months (95% CI 14.1-23.9), and CP-B mOS of 6.7 months (95% CI 4.3-15.6; P = .0003). Overall response rates (ORR) were comparable, with an ORR of 26% in CP-A and 21% in CP-B, not influenced by Barcelona Clinic Liver Cancer (BCLC) stage, performance status, etiology (viral vs nonviral), portal vein thrombosis (PVT), or extrahepatic spread (P > .05 for all associations). The investigators concluded that atezolizumab and bevacizumab in patients with CP-B was well tolerated, with no relevant difference in terms of clinically significant treatment-related adverse events compared with patients with CP-A.
Shi and colleagues reported a randomized controlled trial of patients with HCC and microvascular invasion (MVI) who underwent suboptimal resection (distance from tumor edge to the cut surface < 1 mm), followed by either stereotactic body radiotherapy (SBRT) or observation. From August 2015 to December 2016, 76 patients with BCLC stage 0/A liver disease, MVI, and no macroscopic vascular invasion were randomized after partial hepatectomy to either observation or SBRT (35 Gy delivered in a week). The 1-, 3-, and 5-year disease free survival (DFS) rates were 92.1%, 65.8%, and 56.1% in the SBRT group vs 76.3%, 36.8%, and 26.3% in the surgery alone group, respectively (P = .005). The 1-, 3-, and 5-year overall survival (OS) rates were 100%, 89.5%, and 75.0% in SBRT group vs 100.0%, 68.4%, and 53.7% in the surgery alone group, respectively (P = .053). The authors concluded that SBRT eradicates residual tumor cells present at the margin and improves surgical outcomes.
Roth and colleagues evaluated the safety and efficacy of transarterial chemoembolization (TACE) in older (> 70 years) patients with intermediate HCC. Out of 271 patients evaluated, 88 were older patients. 20.5% of older patients experienced serious adverse events vs 21.3% of younger patients (P = .87). The predictive factors of serious adverse events were CP stage ≥ B7 (P < .0001), Eastern Cooperative Oncology Group (ECOG) scale ≥ 1 (P = .0019), and Model for End-stage Liver Disease (MELD) score ≥ 9 (P = .0415). The serious adverse event rate was not increased with age (P = .87). The authors concluded that age should not be an exclusionary factor when considering TACE.
Clinical trials for unresectable HCC (uHCC) have mandated excellent underlying liver function. Patients with Child-Pugh (CP) A cirrhosis do not have cirrhosis as their most life-limiting disease. In clinical practice, there are many patients with uHCC who are functionally well yet have CP-B cirrhosis. D'Alessio and colleagues undertook a retrospective evaluation of 202 patients with either CP-A or CP-B cirrhosis who received atezolizumab and bevacizumab as first-line treatment of uHCC. The majority, 154 patients (76%), had CP-A cirrhosis, whereas 48 (24%) had CP-B, including 21 B7, 21 B8, and 6 B9. The authors found that in the overall population, median overall survival (mOS) was 14.9 months (95% CI 13.6-16.3), with patients with CP-A mOS of 16.8 months (95% CI 14.1-23.9), and CP-B mOS of 6.7 months (95% CI 4.3-15.6; P = .0003). Overall response rates (ORR) were comparable, with an ORR of 26% in CP-A and 21% in CP-B, not influenced by Barcelona Clinic Liver Cancer (BCLC) stage, performance status, etiology (viral vs nonviral), portal vein thrombosis (PVT), or extrahepatic spread (P > .05 for all associations). The investigators concluded that atezolizumab and bevacizumab in patients with CP-B was well tolerated, with no relevant difference in terms of clinically significant treatment-related adverse events compared with patients with CP-A.
Shi and colleagues reported a randomized controlled trial of patients with HCC and microvascular invasion (MVI) who underwent suboptimal resection (distance from tumor edge to the cut surface < 1 mm), followed by either stereotactic body radiotherapy (SBRT) or observation. From August 2015 to December 2016, 76 patients with BCLC stage 0/A liver disease, MVI, and no macroscopic vascular invasion were randomized after partial hepatectomy to either observation or SBRT (35 Gy delivered in a week). The 1-, 3-, and 5-year disease free survival (DFS) rates were 92.1%, 65.8%, and 56.1% in the SBRT group vs 76.3%, 36.8%, and 26.3% in the surgery alone group, respectively (P = .005). The 1-, 3-, and 5-year overall survival (OS) rates were 100%, 89.5%, and 75.0% in SBRT group vs 100.0%, 68.4%, and 53.7% in the surgery alone group, respectively (P = .053). The authors concluded that SBRT eradicates residual tumor cells present at the margin and improves surgical outcomes.
Roth and colleagues evaluated the safety and efficacy of transarterial chemoembolization (TACE) in older (> 70 years) patients with intermediate HCC. Out of 271 patients evaluated, 88 were older patients. 20.5% of older patients experienced serious adverse events vs 21.3% of younger patients (P = .87). The predictive factors of serious adverse events were CP stage ≥ B7 (P < .0001), Eastern Cooperative Oncology Group (ECOG) scale ≥ 1 (P = .0019), and Model for End-stage Liver Disease (MELD) score ≥ 9 (P = .0415). The serious adverse event rate was not increased with age (P = .87). The authors concluded that age should not be an exclusionary factor when considering TACE.
Clinical trials for unresectable HCC (uHCC) have mandated excellent underlying liver function. Patients with Child-Pugh (CP) A cirrhosis do not have cirrhosis as their most life-limiting disease. In clinical practice, there are many patients with uHCC who are functionally well yet have CP-B cirrhosis. D'Alessio and colleagues undertook a retrospective evaluation of 202 patients with either CP-A or CP-B cirrhosis who received atezolizumab and bevacizumab as first-line treatment of uHCC. The majority, 154 patients (76%), had CP-A cirrhosis, whereas 48 (24%) had CP-B, including 21 B7, 21 B8, and 6 B9. The authors found that in the overall population, median overall survival (mOS) was 14.9 months (95% CI 13.6-16.3), with patients with CP-A mOS of 16.8 months (95% CI 14.1-23.9), and CP-B mOS of 6.7 months (95% CI 4.3-15.6; P = .0003). Overall response rates (ORR) were comparable, with an ORR of 26% in CP-A and 21% in CP-B, not influenced by Barcelona Clinic Liver Cancer (BCLC) stage, performance status, etiology (viral vs nonviral), portal vein thrombosis (PVT), or extrahepatic spread (P > .05 for all associations). The investigators concluded that atezolizumab and bevacizumab in patients with CP-B was well tolerated, with no relevant difference in terms of clinically significant treatment-related adverse events compared with patients with CP-A.
Shi and colleagues reported a randomized controlled trial of patients with HCC and microvascular invasion (MVI) who underwent suboptimal resection (distance from tumor edge to the cut surface < 1 mm), followed by either stereotactic body radiotherapy (SBRT) or observation. From August 2015 to December 2016, 76 patients with BCLC stage 0/A liver disease, MVI, and no macroscopic vascular invasion were randomized after partial hepatectomy to either observation or SBRT (35 Gy delivered in a week). The 1-, 3-, and 5-year disease free survival (DFS) rates were 92.1%, 65.8%, and 56.1% in the SBRT group vs 76.3%, 36.8%, and 26.3% in the surgery alone group, respectively (P = .005). The 1-, 3-, and 5-year overall survival (OS) rates were 100%, 89.5%, and 75.0% in SBRT group vs 100.0%, 68.4%, and 53.7% in the surgery alone group, respectively (P = .053). The authors concluded that SBRT eradicates residual tumor cells present at the margin and improves surgical outcomes.
Roth and colleagues evaluated the safety and efficacy of transarterial chemoembolization (TACE) in older (> 70 years) patients with intermediate HCC. Out of 271 patients evaluated, 88 were older patients. 20.5% of older patients experienced serious adverse events vs 21.3% of younger patients (P = .87). The predictive factors of serious adverse events were CP stage ≥ B7 (P < .0001), Eastern Cooperative Oncology Group (ECOG) scale ≥ 1 (P = .0019), and Model for End-stage Liver Disease (MELD) score ≥ 9 (P = .0415). The serious adverse event rate was not increased with age (P = .87). The authors concluded that age should not be an exclusionary factor when considering TACE.
Clinical Edge Journal Scan Commentary: HCC May 2022
Clinical trials for unresectable HCC (uHCC) have mandated excellent underlying liver function. Patients with Child-Pugh (CP) A cirrhosis do not have cirrhosis as their most life-limiting disease. In clinical practice, there are many patients with uHCC who are functionally well yet have CP-B cirrhosis. D'Alessio and colleagues undertook a retrospective evaluation of 202 patients with either CP-A or CP-B cirrhosis who received atezolizumab and bevacizumab as first-line treatment of uHCC. The majority, 154 patients (76%), had CP-A cirrhosis, whereas 48 (24%) had CP-B, including 21 B7, 21 B8, and 6 B9. The authors found that in the overall population, median overall survival (mOS) was 14.9 months (95% CI 13.6-16.3), with patients with CP-A mOS of 16.8 months (95% CI 14.1-23.9), and CP-B mOS of 6.7 months (95% CI 4.3-15.6; P = .0003). Overall response rates (ORR) were comparable, with an ORR of 26% in CP-A and 21% in CP-B, not influenced by Barcelona Clinic Liver Cancer (BCLC) stage, performance status, etiology (viral vs nonviral), portal vein thrombosis (PVT), or extrahepatic spread (P > .05 for all associations). The investigators concluded that atezolizumab and bevacizumab in patients with CP-B was well tolerated, with no relevant difference in terms of clinically significant treatment-related adverse events compared with patients with CP-A.
Shi and colleagues reported a randomized controlled trial of patients with HCC and microvascular invasion (MVI) who underwent suboptimal resection (distance from tumor edge to the cut surface < 1 mm), followed by either stereotactic body radiotherapy (SBRT) or observation. From August 2015 to December 2016, 76 patients with BCLC stage 0/A liver disease, MVI, and no macroscopic vascular invasion were randomized after partial hepatectomy to either observation or SBRT (35 Gy delivered in a week). The 1-, 3-, and 5-year disease free survival (DFS) rates were 92.1%, 65.8%, and 56.1% in the SBRT group vs 76.3%, 36.8%, and 26.3% in the surgery alone group, respectively (P = .005). The 1-, 3-, and 5-year overall survival (OS) rates were 100%, 89.5%, and 75.0% in SBRT group vs 100.0%, 68.4%, and 53.7% in the surgery alone group, respectively (P = .053). The authors concluded that SBRT eradicates residual tumor cells present at the margin and improves surgical outcomes.
Roth and colleagues evaluated the safety and efficacy of transarterial chemoembolization (TACE) in older (> 70 years) patients with intermediate HCC. Out of 271 patients evaluated, 88 were older patients. 20.5% of older patients experienced serious adverse events vs 21.3% of younger patients (P = .87). The predictive factors of serious adverse events were CP stage ≥ B7 (P < .0001), Eastern Cooperative Oncology Group (ECOG) scale ≥ 1 (P = .0019), and Model for End-stage Liver Disease (MELD) score ≥ 9 (P = .0415). The serious adverse event rate was not increased with age (P = .87). The authors concluded that age should not be an exclusionary factor when considering TACE.
Clinical trials for unresectable HCC (uHCC) have mandated excellent underlying liver function. Patients with Child-Pugh (CP) A cirrhosis do not have cirrhosis as their most life-limiting disease. In clinical practice, there are many patients with uHCC who are functionally well yet have CP-B cirrhosis. D'Alessio and colleagues undertook a retrospective evaluation of 202 patients with either CP-A or CP-B cirrhosis who received atezolizumab and bevacizumab as first-line treatment of uHCC. The majority, 154 patients (76%), had CP-A cirrhosis, whereas 48 (24%) had CP-B, including 21 B7, 21 B8, and 6 B9. The authors found that in the overall population, median overall survival (mOS) was 14.9 months (95% CI 13.6-16.3), with patients with CP-A mOS of 16.8 months (95% CI 14.1-23.9), and CP-B mOS of 6.7 months (95% CI 4.3-15.6; P = .0003). Overall response rates (ORR) were comparable, with an ORR of 26% in CP-A and 21% in CP-B, not influenced by Barcelona Clinic Liver Cancer (BCLC) stage, performance status, etiology (viral vs nonviral), portal vein thrombosis (PVT), or extrahepatic spread (P > .05 for all associations). The investigators concluded that atezolizumab and bevacizumab in patients with CP-B was well tolerated, with no relevant difference in terms of clinically significant treatment-related adverse events compared with patients with CP-A.
Shi and colleagues reported a randomized controlled trial of patients with HCC and microvascular invasion (MVI) who underwent suboptimal resection (distance from tumor edge to the cut surface < 1 mm), followed by either stereotactic body radiotherapy (SBRT) or observation. From August 2015 to December 2016, 76 patients with BCLC stage 0/A liver disease, MVI, and no macroscopic vascular invasion were randomized after partial hepatectomy to either observation or SBRT (35 Gy delivered in a week). The 1-, 3-, and 5-year disease free survival (DFS) rates were 92.1%, 65.8%, and 56.1% in the SBRT group vs 76.3%, 36.8%, and 26.3% in the surgery alone group, respectively (P = .005). The 1-, 3-, and 5-year overall survival (OS) rates were 100%, 89.5%, and 75.0% in SBRT group vs 100.0%, 68.4%, and 53.7% in the surgery alone group, respectively (P = .053). The authors concluded that SBRT eradicates residual tumor cells present at the margin and improves surgical outcomes.
Roth and colleagues evaluated the safety and efficacy of transarterial chemoembolization (TACE) in older (> 70 years) patients with intermediate HCC. Out of 271 patients evaluated, 88 were older patients. 20.5% of older patients experienced serious adverse events vs 21.3% of younger patients (P = .87). The predictive factors of serious adverse events were CP stage ≥ B7 (P < .0001), Eastern Cooperative Oncology Group (ECOG) scale ≥ 1 (P = .0019), and Model for End-stage Liver Disease (MELD) score ≥ 9 (P = .0415). The serious adverse event rate was not increased with age (P = .87). The authors concluded that age should not be an exclusionary factor when considering TACE.
Clinical trials for unresectable HCC (uHCC) have mandated excellent underlying liver function. Patients with Child-Pugh (CP) A cirrhosis do not have cirrhosis as their most life-limiting disease. In clinical practice, there are many patients with uHCC who are functionally well yet have CP-B cirrhosis. D'Alessio and colleagues undertook a retrospective evaluation of 202 patients with either CP-A or CP-B cirrhosis who received atezolizumab and bevacizumab as first-line treatment of uHCC. The majority, 154 patients (76%), had CP-A cirrhosis, whereas 48 (24%) had CP-B, including 21 B7, 21 B8, and 6 B9. The authors found that in the overall population, median overall survival (mOS) was 14.9 months (95% CI 13.6-16.3), with patients with CP-A mOS of 16.8 months (95% CI 14.1-23.9), and CP-B mOS of 6.7 months (95% CI 4.3-15.6; P = .0003). Overall response rates (ORR) were comparable, with an ORR of 26% in CP-A and 21% in CP-B, not influenced by Barcelona Clinic Liver Cancer (BCLC) stage, performance status, etiology (viral vs nonviral), portal vein thrombosis (PVT), or extrahepatic spread (P > .05 for all associations). The investigators concluded that atezolizumab and bevacizumab in patients with CP-B was well tolerated, with no relevant difference in terms of clinically significant treatment-related adverse events compared with patients with CP-A.
Shi and colleagues reported a randomized controlled trial of patients with HCC and microvascular invasion (MVI) who underwent suboptimal resection (distance from tumor edge to the cut surface < 1 mm), followed by either stereotactic body radiotherapy (SBRT) or observation. From August 2015 to December 2016, 76 patients with BCLC stage 0/A liver disease, MVI, and no macroscopic vascular invasion were randomized after partial hepatectomy to either observation or SBRT (35 Gy delivered in a week). The 1-, 3-, and 5-year disease free survival (DFS) rates were 92.1%, 65.8%, and 56.1% in the SBRT group vs 76.3%, 36.8%, and 26.3% in the surgery alone group, respectively (P = .005). The 1-, 3-, and 5-year overall survival (OS) rates were 100%, 89.5%, and 75.0% in SBRT group vs 100.0%, 68.4%, and 53.7% in the surgery alone group, respectively (P = .053). The authors concluded that SBRT eradicates residual tumor cells present at the margin and improves surgical outcomes.
Roth and colleagues evaluated the safety and efficacy of transarterial chemoembolization (TACE) in older (> 70 years) patients with intermediate HCC. Out of 271 patients evaluated, 88 were older patients. 20.5% of older patients experienced serious adverse events vs 21.3% of younger patients (P = .87). The predictive factors of serious adverse events were CP stage ≥ B7 (P < .0001), Eastern Cooperative Oncology Group (ECOG) scale ≥ 1 (P = .0019), and Model for End-stage Liver Disease (MELD) score ≥ 9 (P = .0415). The serious adverse event rate was not increased with age (P = .87). The authors concluded that age should not be an exclusionary factor when considering TACE.
Post-LT HCC recurrence unaffected by donor sex
Key clinical point: Donor sex did not affect post-liver transplantation (LT) recurrence of hepatocellular carcinoma (HCC) and need not be considered during donor selection or organ allocation.
Major finding: After propensity score matching, the female donor (F-D) and male donor (M-D) groups showed comparable 5-year overall recurrence rates (15% vs. 14%; P = .63) and graft recurrence rates (5% vs. 5%; P = .94). Donor sex was not identified as a significant risk factor for HCC recurrence by either univariate or multivariate analysis.
Study details: This study evaluated 1118 patients with HCC who underwent LT receiving a liver graft from the F-D (n = 446) or M-D (n = 672) groups.
Disclosures: The authors did not declare any funding source or conflicts of interest.
Source: Taura K et al. No impact of donor sex on the recurrence of hepatocellular carcinoma after liver transplantation. J Hepatobiliary Pancreat Sci. 2022 (Mar 13). Doi: 10.1002/jhbp.1134
Key clinical point: Donor sex did not affect post-liver transplantation (LT) recurrence of hepatocellular carcinoma (HCC) and need not be considered during donor selection or organ allocation.
Major finding: After propensity score matching, the female donor (F-D) and male donor (M-D) groups showed comparable 5-year overall recurrence rates (15% vs. 14%; P = .63) and graft recurrence rates (5% vs. 5%; P = .94). Donor sex was not identified as a significant risk factor for HCC recurrence by either univariate or multivariate analysis.
Study details: This study evaluated 1118 patients with HCC who underwent LT receiving a liver graft from the F-D (n = 446) or M-D (n = 672) groups.
Disclosures: The authors did not declare any funding source or conflicts of interest.
Source: Taura K et al. No impact of donor sex on the recurrence of hepatocellular carcinoma after liver transplantation. J Hepatobiliary Pancreat Sci. 2022 (Mar 13). Doi: 10.1002/jhbp.1134
Key clinical point: Donor sex did not affect post-liver transplantation (LT) recurrence of hepatocellular carcinoma (HCC) and need not be considered during donor selection or organ allocation.
Major finding: After propensity score matching, the female donor (F-D) and male donor (M-D) groups showed comparable 5-year overall recurrence rates (15% vs. 14%; P = .63) and graft recurrence rates (5% vs. 5%; P = .94). Donor sex was not identified as a significant risk factor for HCC recurrence by either univariate or multivariate analysis.
Study details: This study evaluated 1118 patients with HCC who underwent LT receiving a liver graft from the F-D (n = 446) or M-D (n = 672) groups.
Disclosures: The authors did not declare any funding source or conflicts of interest.
Source: Taura K et al. No impact of donor sex on the recurrence of hepatocellular carcinoma after liver transplantation. J Hepatobiliary Pancreat Sci. 2022 (Mar 13). Doi: 10.1002/jhbp.1134