User login
Analysis of Epilepsy Self-Management Skills
Although patients with epilepsy can benefit from self-management programs, a recent analysis from the Centers for Disease Control and Prevention has found that competency in self-management skills varies considerably across behavioral domains.
- Data from the Prevention Managing Epilepsy Well Network found that competencies in information and lifestyle management were considerably weaker than competencies in medication, safety, and seizure management.
- The Managing Epilepsy Well database analysis included 436 patients with epilepsy from 5 studies in the United States.
- Self-management behavioral skills were stronger in females and among patients with less education.
- The same skills were weaker in patients with depression and in those who reported a lower quality of life.
Begley C, Shegog R, Liu H, et al. Correlates of epilepsy self-management in MEW Network participants: From the Centers for Disease Control and Prevention Managing Epilepsy Well Network. Epilepsy Behav. 2018;85:243-247.
Although patients with epilepsy can benefit from self-management programs, a recent analysis from the Centers for Disease Control and Prevention has found that competency in self-management skills varies considerably across behavioral domains.
- Data from the Prevention Managing Epilepsy Well Network found that competencies in information and lifestyle management were considerably weaker than competencies in medication, safety, and seizure management.
- The Managing Epilepsy Well database analysis included 436 patients with epilepsy from 5 studies in the United States.
- Self-management behavioral skills were stronger in females and among patients with less education.
- The same skills were weaker in patients with depression and in those who reported a lower quality of life.
Begley C, Shegog R, Liu H, et al. Correlates of epilepsy self-management in MEW Network participants: From the Centers for Disease Control and Prevention Managing Epilepsy Well Network. Epilepsy Behav. 2018;85:243-247.
Although patients with epilepsy can benefit from self-management programs, a recent analysis from the Centers for Disease Control and Prevention has found that competency in self-management skills varies considerably across behavioral domains.
- Data from the Prevention Managing Epilepsy Well Network found that competencies in information and lifestyle management were considerably weaker than competencies in medication, safety, and seizure management.
- The Managing Epilepsy Well database analysis included 436 patients with epilepsy from 5 studies in the United States.
- Self-management behavioral skills were stronger in females and among patients with less education.
- The same skills were weaker in patients with depression and in those who reported a lower quality of life.
Begley C, Shegog R, Liu H, et al. Correlates of epilepsy self-management in MEW Network participants: From the Centers for Disease Control and Prevention Managing Epilepsy Well Network. Epilepsy Behav. 2018;85:243-247.
Novel cEEG-based scoring system predicts inpatient seizure risk
LOS ANGELES – A novel scoring system based on six readily available seizure risk factors from a patient’s history and continuous electroencephalogram (cEEG) monitoring appears to accurately predict seizures in acutely ill hospitalized patients.
The final model of the system, dubbed the 2HELPS2B score, has an area under the curve (AUC) of 0.821, suggesting a “good overall fit,” Aaron Struck, MD, reported at the annual meeting of the American Academy of Neurology.
However, more relevant than the AUC and suggestive of high classification accuracy is the low calibration error of 2.7%, which shows that the actual incidence of seizures within a particular risk group is, on average, within 2.7% of predicted incidence, Dr. Struck of the University of Wisconsin, Madison, explained in an interview.
The use of cEEG has expanded, largely because of a high incidence of subclinical seizures in hospitalized patients with encephalopathy; EEG features believed to predict seizures include epileptiform discharges and periodic discharges, but the ways in which these variables may jointly affect seizure risk have not been studied, he said.
He and his colleagues used a prospective database to derive a dataset containing 24 clinical and electroencephalographic variables for 5,427 cEEG sessions of at least 24 hours each, and then, using a machine-learning method known as RiskSLIM, created a scoring system model to estimate seizure risk in patients undergoing cEEG.
The name of the scoring system – 2HELPS2B – represents the six variables included in the final model:
- 2 H is for frequency greater than 2.0 Hz for any periodic rhythmic pattern (1 point).
- E is for sporadic epileptiform discharges (1 point).
- L is for the presence of lateralized periodic discharges, lateralized rhythmic delta activity, or bilateral independent periodic discharges (1 point).
- P is for the presence of “plus” features, including superimposed, rhythmic, sharp, or fast activity (1 point).
- S is for prior seizure (1 point).
- 2B is for brief, potentially ictal, rhythmic discharges (2 points).
The predicted seizure risk rose with score, such that the seizure risk was less than 5% for a score of 0, 12% for 1, 27% for 2, 50% for 3, 73% for 4, 88% for 5, and greater than 95% for 6-7, Dr. Struck said. “Really, anything over 2 points, you’re at substantial risk for having seizures.”
Limitations of the study, which are being addressed in an ongoing, multicenter, prospective validation trial through the Critical Care EEG Monitoring Research Consortium, are mainly related to the constraints of the database; the duration of EEG needed to accurately calculate the 2HELPS2B score wasn’t defined, and cEEGs were of varying length.
“So in our validation study moving forward, these are two things we will address,” he said. “We also want to show that this is something that’s useful on a day-to-day basis – that it accurately gauges the degree of variability or potential severity of the ictal-interictal continuum pattern.”
With validation, Dr. Struck said that the 2HELPS2B score could ultimately be used to rapidly communicate seizure potential based on EEG severity and to guide decision making with respect to initiation of empiric antiseizure medication.
Findings from the validation study are “trending in the right direction,” but the confidence intervals are wide, as only 404 patients have been included at this point, Dr. Struck said.
This study was supported by a research infrastructure award from the American Epilepsy Society and the Epilepsy Foundation.
SOURCE: Struck A et al. Neurology. 2018 Apr 90(15 Suppl.):S11.002.
LOS ANGELES – A novel scoring system based on six readily available seizure risk factors from a patient’s history and continuous electroencephalogram (cEEG) monitoring appears to accurately predict seizures in acutely ill hospitalized patients.
The final model of the system, dubbed the 2HELPS2B score, has an area under the curve (AUC) of 0.821, suggesting a “good overall fit,” Aaron Struck, MD, reported at the annual meeting of the American Academy of Neurology.
However, more relevant than the AUC and suggestive of high classification accuracy is the low calibration error of 2.7%, which shows that the actual incidence of seizures within a particular risk group is, on average, within 2.7% of predicted incidence, Dr. Struck of the University of Wisconsin, Madison, explained in an interview.
The use of cEEG has expanded, largely because of a high incidence of subclinical seizures in hospitalized patients with encephalopathy; EEG features believed to predict seizures include epileptiform discharges and periodic discharges, but the ways in which these variables may jointly affect seizure risk have not been studied, he said.
He and his colleagues used a prospective database to derive a dataset containing 24 clinical and electroencephalographic variables for 5,427 cEEG sessions of at least 24 hours each, and then, using a machine-learning method known as RiskSLIM, created a scoring system model to estimate seizure risk in patients undergoing cEEG.
The name of the scoring system – 2HELPS2B – represents the six variables included in the final model:
- 2 H is for frequency greater than 2.0 Hz for any periodic rhythmic pattern (1 point).
- E is for sporadic epileptiform discharges (1 point).
- L is for the presence of lateralized periodic discharges, lateralized rhythmic delta activity, or bilateral independent periodic discharges (1 point).
- P is for the presence of “plus” features, including superimposed, rhythmic, sharp, or fast activity (1 point).
- S is for prior seizure (1 point).
- 2B is for brief, potentially ictal, rhythmic discharges (2 points).
The predicted seizure risk rose with score, such that the seizure risk was less than 5% for a score of 0, 12% for 1, 27% for 2, 50% for 3, 73% for 4, 88% for 5, and greater than 95% for 6-7, Dr. Struck said. “Really, anything over 2 points, you’re at substantial risk for having seizures.”
Limitations of the study, which are being addressed in an ongoing, multicenter, prospective validation trial through the Critical Care EEG Monitoring Research Consortium, are mainly related to the constraints of the database; the duration of EEG needed to accurately calculate the 2HELPS2B score wasn’t defined, and cEEGs were of varying length.
“So in our validation study moving forward, these are two things we will address,” he said. “We also want to show that this is something that’s useful on a day-to-day basis – that it accurately gauges the degree of variability or potential severity of the ictal-interictal continuum pattern.”
With validation, Dr. Struck said that the 2HELPS2B score could ultimately be used to rapidly communicate seizure potential based on EEG severity and to guide decision making with respect to initiation of empiric antiseizure medication.
Findings from the validation study are “trending in the right direction,” but the confidence intervals are wide, as only 404 patients have been included at this point, Dr. Struck said.
This study was supported by a research infrastructure award from the American Epilepsy Society and the Epilepsy Foundation.
SOURCE: Struck A et al. Neurology. 2018 Apr 90(15 Suppl.):S11.002.
LOS ANGELES – A novel scoring system based on six readily available seizure risk factors from a patient’s history and continuous electroencephalogram (cEEG) monitoring appears to accurately predict seizures in acutely ill hospitalized patients.
The final model of the system, dubbed the 2HELPS2B score, has an area under the curve (AUC) of 0.821, suggesting a “good overall fit,” Aaron Struck, MD, reported at the annual meeting of the American Academy of Neurology.
However, more relevant than the AUC and suggestive of high classification accuracy is the low calibration error of 2.7%, which shows that the actual incidence of seizures within a particular risk group is, on average, within 2.7% of predicted incidence, Dr. Struck of the University of Wisconsin, Madison, explained in an interview.
The use of cEEG has expanded, largely because of a high incidence of subclinical seizures in hospitalized patients with encephalopathy; EEG features believed to predict seizures include epileptiform discharges and periodic discharges, but the ways in which these variables may jointly affect seizure risk have not been studied, he said.
He and his colleagues used a prospective database to derive a dataset containing 24 clinical and electroencephalographic variables for 5,427 cEEG sessions of at least 24 hours each, and then, using a machine-learning method known as RiskSLIM, created a scoring system model to estimate seizure risk in patients undergoing cEEG.
The name of the scoring system – 2HELPS2B – represents the six variables included in the final model:
- 2 H is for frequency greater than 2.0 Hz for any periodic rhythmic pattern (1 point).
- E is for sporadic epileptiform discharges (1 point).
- L is for the presence of lateralized periodic discharges, lateralized rhythmic delta activity, or bilateral independent periodic discharges (1 point).
- P is for the presence of “plus” features, including superimposed, rhythmic, sharp, or fast activity (1 point).
- S is for prior seizure (1 point).
- 2B is for brief, potentially ictal, rhythmic discharges (2 points).
The predicted seizure risk rose with score, such that the seizure risk was less than 5% for a score of 0, 12% for 1, 27% for 2, 50% for 3, 73% for 4, 88% for 5, and greater than 95% for 6-7, Dr. Struck said. “Really, anything over 2 points, you’re at substantial risk for having seizures.”
Limitations of the study, which are being addressed in an ongoing, multicenter, prospective validation trial through the Critical Care EEG Monitoring Research Consortium, are mainly related to the constraints of the database; the duration of EEG needed to accurately calculate the 2HELPS2B score wasn’t defined, and cEEGs were of varying length.
“So in our validation study moving forward, these are two things we will address,” he said. “We also want to show that this is something that’s useful on a day-to-day basis – that it accurately gauges the degree of variability or potential severity of the ictal-interictal continuum pattern.”
With validation, Dr. Struck said that the 2HELPS2B score could ultimately be used to rapidly communicate seizure potential based on EEG severity and to guide decision making with respect to initiation of empiric antiseizure medication.
Findings from the validation study are “trending in the right direction,” but the confidence intervals are wide, as only 404 patients have been included at this point, Dr. Struck said.
This study was supported by a research infrastructure award from the American Epilepsy Society and the Epilepsy Foundation.
SOURCE: Struck A et al. Neurology. 2018 Apr 90(15 Suppl.):S11.002.
REPORTING FROM AAN 2018
Key clinical point:
Major finding: The 2HELPS2B score has an AUC of 0.821 and calibration error of 2.7%.
Study details: An analysis of 5,427 cEEG sessions to develop a risk scoring system model.
Disclosures: This study was supported by a research infrastructure award from the American Epilepsy Society and the Epilepsy Foundation.
Source: Struck A et al. Neurology. 2018 Apr 90(15 Suppl.):S11.002.
Data support universal adoption of neuroimaging in early-life epilepsy
There is broad acceptance for the use of guideline-endorsed neuroimaging studies as the standard of care in evaluating early-life epilepsy across U.S pediatric centers, an observational study shows.
Furthermore, the study authors reported, the use of neuroimaging is supported by their data, which showed that a brain MRI obtained up to 1 year after an early-life epilepsy (ELE) diagnosis provides a high diagnostic yield, regardless of clinical factors – including seizure type or development.
“Neuroimaging, and MRI in particular, frequently identifies an etiology for ELE, enhancing the ability of neurologists to provide a precise diagnosis, offer anticipatory guidance, and consider the full array of available therapies,” wrote Jason Coryell, MD, a child neurologist, and his colleagues Aug. 8 in Pediatrics.
Early childhood epilepsy occurs in 1-2 out of every 1,000 children under age 3, Dr. Coryell and his colleagues said. Consensus guidelines recommend MRI for the evaluation of ELE, but no data evaluate whether it is being used in clinical practice, said Dr. Coryell of the Oregon Health & Science University, Portland, and his colleagues. The research team, therefore, set out to identify the yield and findings of neuroimaging in incident cases of epilepsy in 775 children with newly diagnosed ELE (diagnosed before age 3 years) seen at 17 U.S. pediatric epilepsy centers between 2012 and 2015.
They found that the use of neuroimaging was high, with 725 children (93.5%) having had a neuroimaging study. Of those, 714 had an MRI as recommended in current guidelines (87% with seizure protocols), and 11 had computed tomography or ultrasound only.
According to Dr. Coryell and his colleagues, the high use of MRI in the cohort likely could be attributed to three factors: neuroimaging is recommended in guidelines, the improved accessibility of imaging, and increased physician familiarity with the role of MRI in epilepsy.
These included an acquired injury in 97 (13.4%), malformations of cortical development in 56 (7.7%), and other diffuse disorders of brain development in 51 (7.0%).
Neuroimaging was abnormal in 61% (160 of 262) of children with abnormal development at diagnosis, compared with 24% (113 of 463) with typical development.
Structural abnormalities also were high and most common in children with focal seizure semiology (40%), spasms (47%), or unclear semiology (42%).
In children without spasms or focal semiology with typical development, 16% (29 of 185 children) had imaging abnormalities. Pathogenic genetic variants were identified in 44% (53 of 121) of children with abnormal neuroimaging in whom genetic testing was performed.
The research team concluded that their data supported the universal adoption of imaging guidelines for ELE, because the yield was substantially high – even in the lowest-risk group.
“In our cohort 1 in 6 children presenting with typical development and without either focal seizures or spasms still had abnormal neuroimaging,” Dr. Coryell and his coauthors wrote. “This supports the continued adherence to guidelines.”
They advised that next steps include standardization of ELE MRI protocol. “Although there has been widespread adoption of epilepsy protocols, these have been geared toward identification of hippocampal abnormalities in older children and adults rather than toward the young, myelinating brain,” the researchers added.
The team noted several limitations. One limitation cited is the study’s identification of patients by using tertiary centers that might provide care for children who are more severely affected than those in the general population.
The Pediatric Epilepsy Research Foundation in Dallas funded the research. Dr. Coryell and his colleagues said they have no financial disclosures. Several of the researchers declared potential conflicts of interest relating to consulting fees.
SOURCE: Coryell J et al. Pediatrics. 2018 Aug 8. doi: 10.1542/peds.2018.0672.
There is broad acceptance for the use of guideline-endorsed neuroimaging studies as the standard of care in evaluating early-life epilepsy across U.S pediatric centers, an observational study shows.
Furthermore, the study authors reported, the use of neuroimaging is supported by their data, which showed that a brain MRI obtained up to 1 year after an early-life epilepsy (ELE) diagnosis provides a high diagnostic yield, regardless of clinical factors – including seizure type or development.
“Neuroimaging, and MRI in particular, frequently identifies an etiology for ELE, enhancing the ability of neurologists to provide a precise diagnosis, offer anticipatory guidance, and consider the full array of available therapies,” wrote Jason Coryell, MD, a child neurologist, and his colleagues Aug. 8 in Pediatrics.
Early childhood epilepsy occurs in 1-2 out of every 1,000 children under age 3, Dr. Coryell and his colleagues said. Consensus guidelines recommend MRI for the evaluation of ELE, but no data evaluate whether it is being used in clinical practice, said Dr. Coryell of the Oregon Health & Science University, Portland, and his colleagues. The research team, therefore, set out to identify the yield and findings of neuroimaging in incident cases of epilepsy in 775 children with newly diagnosed ELE (diagnosed before age 3 years) seen at 17 U.S. pediatric epilepsy centers between 2012 and 2015.
They found that the use of neuroimaging was high, with 725 children (93.5%) having had a neuroimaging study. Of those, 714 had an MRI as recommended in current guidelines (87% with seizure protocols), and 11 had computed tomography or ultrasound only.
According to Dr. Coryell and his colleagues, the high use of MRI in the cohort likely could be attributed to three factors: neuroimaging is recommended in guidelines, the improved accessibility of imaging, and increased physician familiarity with the role of MRI in epilepsy.
These included an acquired injury in 97 (13.4%), malformations of cortical development in 56 (7.7%), and other diffuse disorders of brain development in 51 (7.0%).
Neuroimaging was abnormal in 61% (160 of 262) of children with abnormal development at diagnosis, compared with 24% (113 of 463) with typical development.
Structural abnormalities also were high and most common in children with focal seizure semiology (40%), spasms (47%), or unclear semiology (42%).
In children without spasms or focal semiology with typical development, 16% (29 of 185 children) had imaging abnormalities. Pathogenic genetic variants were identified in 44% (53 of 121) of children with abnormal neuroimaging in whom genetic testing was performed.
The research team concluded that their data supported the universal adoption of imaging guidelines for ELE, because the yield was substantially high – even in the lowest-risk group.
“In our cohort 1 in 6 children presenting with typical development and without either focal seizures or spasms still had abnormal neuroimaging,” Dr. Coryell and his coauthors wrote. “This supports the continued adherence to guidelines.”
They advised that next steps include standardization of ELE MRI protocol. “Although there has been widespread adoption of epilepsy protocols, these have been geared toward identification of hippocampal abnormalities in older children and adults rather than toward the young, myelinating brain,” the researchers added.
The team noted several limitations. One limitation cited is the study’s identification of patients by using tertiary centers that might provide care for children who are more severely affected than those in the general population.
The Pediatric Epilepsy Research Foundation in Dallas funded the research. Dr. Coryell and his colleagues said they have no financial disclosures. Several of the researchers declared potential conflicts of interest relating to consulting fees.
SOURCE: Coryell J et al. Pediatrics. 2018 Aug 8. doi: 10.1542/peds.2018.0672.
There is broad acceptance for the use of guideline-endorsed neuroimaging studies as the standard of care in evaluating early-life epilepsy across U.S pediatric centers, an observational study shows.
Furthermore, the study authors reported, the use of neuroimaging is supported by their data, which showed that a brain MRI obtained up to 1 year after an early-life epilepsy (ELE) diagnosis provides a high diagnostic yield, regardless of clinical factors – including seizure type or development.
“Neuroimaging, and MRI in particular, frequently identifies an etiology for ELE, enhancing the ability of neurologists to provide a precise diagnosis, offer anticipatory guidance, and consider the full array of available therapies,” wrote Jason Coryell, MD, a child neurologist, and his colleagues Aug. 8 in Pediatrics.
Early childhood epilepsy occurs in 1-2 out of every 1,000 children under age 3, Dr. Coryell and his colleagues said. Consensus guidelines recommend MRI for the evaluation of ELE, but no data evaluate whether it is being used in clinical practice, said Dr. Coryell of the Oregon Health & Science University, Portland, and his colleagues. The research team, therefore, set out to identify the yield and findings of neuroimaging in incident cases of epilepsy in 775 children with newly diagnosed ELE (diagnosed before age 3 years) seen at 17 U.S. pediatric epilepsy centers between 2012 and 2015.
They found that the use of neuroimaging was high, with 725 children (93.5%) having had a neuroimaging study. Of those, 714 had an MRI as recommended in current guidelines (87% with seizure protocols), and 11 had computed tomography or ultrasound only.
According to Dr. Coryell and his colleagues, the high use of MRI in the cohort likely could be attributed to three factors: neuroimaging is recommended in guidelines, the improved accessibility of imaging, and increased physician familiarity with the role of MRI in epilepsy.
These included an acquired injury in 97 (13.4%), malformations of cortical development in 56 (7.7%), and other diffuse disorders of brain development in 51 (7.0%).
Neuroimaging was abnormal in 61% (160 of 262) of children with abnormal development at diagnosis, compared with 24% (113 of 463) with typical development.
Structural abnormalities also were high and most common in children with focal seizure semiology (40%), spasms (47%), or unclear semiology (42%).
In children without spasms or focal semiology with typical development, 16% (29 of 185 children) had imaging abnormalities. Pathogenic genetic variants were identified in 44% (53 of 121) of children with abnormal neuroimaging in whom genetic testing was performed.
The research team concluded that their data supported the universal adoption of imaging guidelines for ELE, because the yield was substantially high – even in the lowest-risk group.
“In our cohort 1 in 6 children presenting with typical development and without either focal seizures or spasms still had abnormal neuroimaging,” Dr. Coryell and his coauthors wrote. “This supports the continued adherence to guidelines.”
They advised that next steps include standardization of ELE MRI protocol. “Although there has been widespread adoption of epilepsy protocols, these have been geared toward identification of hippocampal abnormalities in older children and adults rather than toward the young, myelinating brain,” the researchers added.
The team noted several limitations. One limitation cited is the study’s identification of patients by using tertiary centers that might provide care for children who are more severely affected than those in the general population.
The Pediatric Epilepsy Research Foundation in Dallas funded the research. Dr. Coryell and his colleagues said they have no financial disclosures. Several of the researchers declared potential conflicts of interest relating to consulting fees.
SOURCE: Coryell J et al. Pediatrics. 2018 Aug 8. doi: 10.1542/peds.2018.0672.
FROM PEDIATRICS
Key clinical point: Brain MRI obtained up to 1 year after early-life epilepsy diagnosis provides a high diagnostic yield regardless of clinical factors, including seizure type or development.
Major finding: Most (93.5%) of the study cohort of 775 children with ELE underwent neuroimaging. A 40% rate of etiologically related abnormalities was observed, and structural abnormalities were high and most common in children with focal seizure semiology (40%), spasms (47%), or unclear semiology (42%).
Study details: Prospective study of 775 children (under 3 years old at onset) with a new diagnosis of epilepsy seen at 17 U.S. pediatric epilepsy centers.
Disclosures: The research was funded by the Pediatric Epilepsy Research Foundation in Dallas. Dr. Coryell and the other authors said they have no financial disclosures. Several of the researchers declared potential conflicts of interest relating to consulting fees.
Source: Coryell J et al. Pediatrics. 2018 Aug 8. doi: 10.1542/peds.2018-0672.
Status Epilepticus in Pregnancy
Andrew N. Wilner, MD, FAAN, FACP
Angels Neurological Centers
Abington, MA
Clinical History
A 37-year-old pregnant African American woman with a history of epilepsy and polysubstance abuse was found unresponsive in a hotel room. She had four convulsions en route to the hospital. In transit, she received levetiracetam and phenytoin, resulting in the cessation of the clinical seizures.
According to her mother, seizures began at age 16 during her first pregnancy, which was complicated by hypertension. She was prescribed medications for hypertension and phenytoin for seizures. The patient provided a different history, claiming that her seizures began 2 years ago. She denied taking medication for seizures or other health problems.
The patient has two children, ages 22 and 11 years. Past medical history is otherwise unremarkable. She has no allergies. Social history includes cigarette smoking, and alcohol and substance abuse. She lives with her boyfriend and does not work. She is 25 weeks pregnant. Family history was notable only for migraine in her mother and grandmother.
Physical Examination
In the emergency department, blood pressure was 135/65, pulse 121 beats per minute, and oxygen saturation was 97%. She was oriented only to self and did not follow commands. Pupils were equal and reactive. There was no facial asymmetry. She moved all 4 extremities spontaneously. Reflexes were brisk. Oral mucosa was dry. She had no edema in the lower extremities.
Laboratories
Chest x-ray was normal. EKG revealed tachycardia and nonspecific ST changes. Hemoglobin was 11.1 g/dl, hematocrit 32%, white blood cell count 10,900, and platelets 181,000. Electrolytes were normal except for a low sodium of 132 mmol/l (135-145) and bicarbonate of 17 mmol/l (21-31). Glucose was initially 67 mg/dl and dropped to 46 mg/dl. Total protein was 6 g/dl (6.7-8.2) and albumin was 2.7 g/dl (3.2-5.5). Metabolic panel was otherwise normal. Urinalysis was positive for glucose, ketones, and a small amount of blood and protein. There were no bacteria. Blood and urine cultures were negative. Phenytoin level was undetectable. Urine drug screen was positive for cannabinoids and cocaine.
Hospital Course
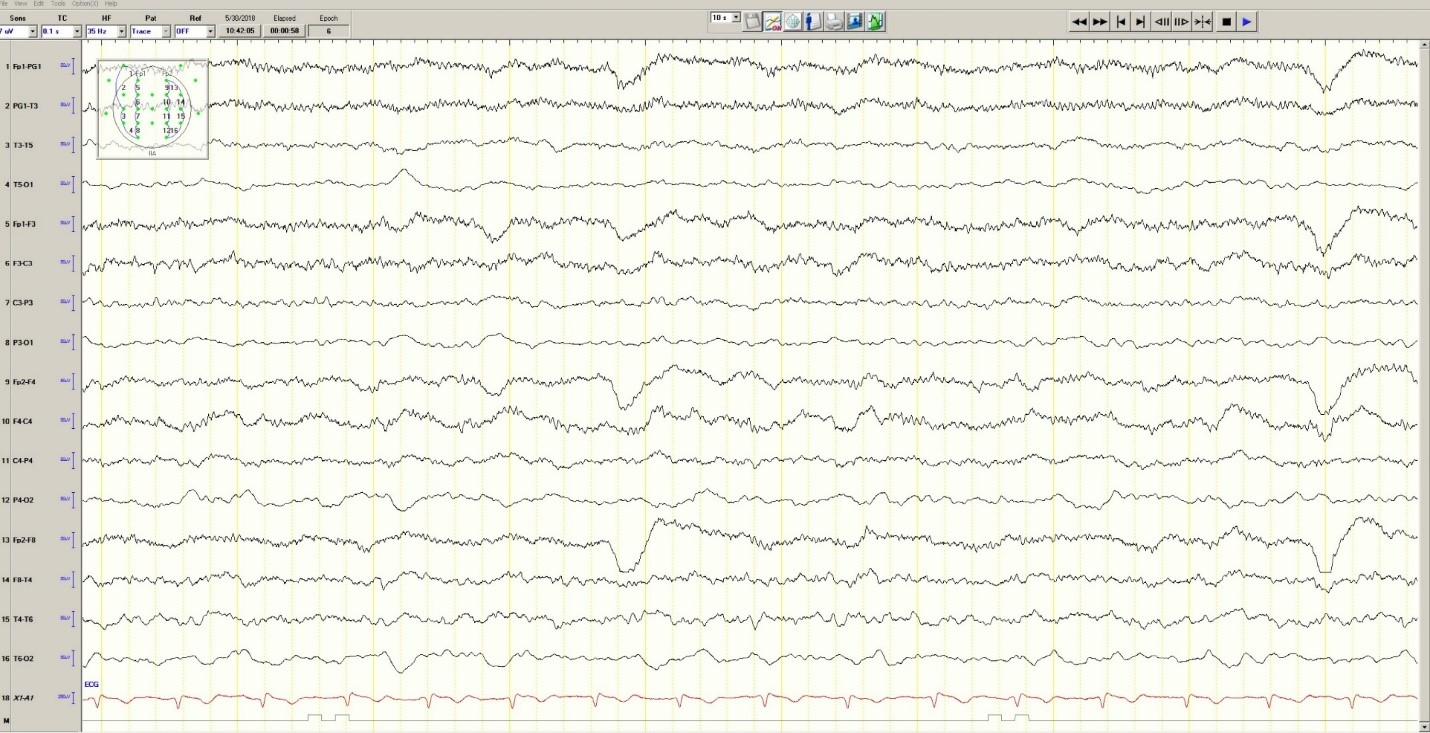
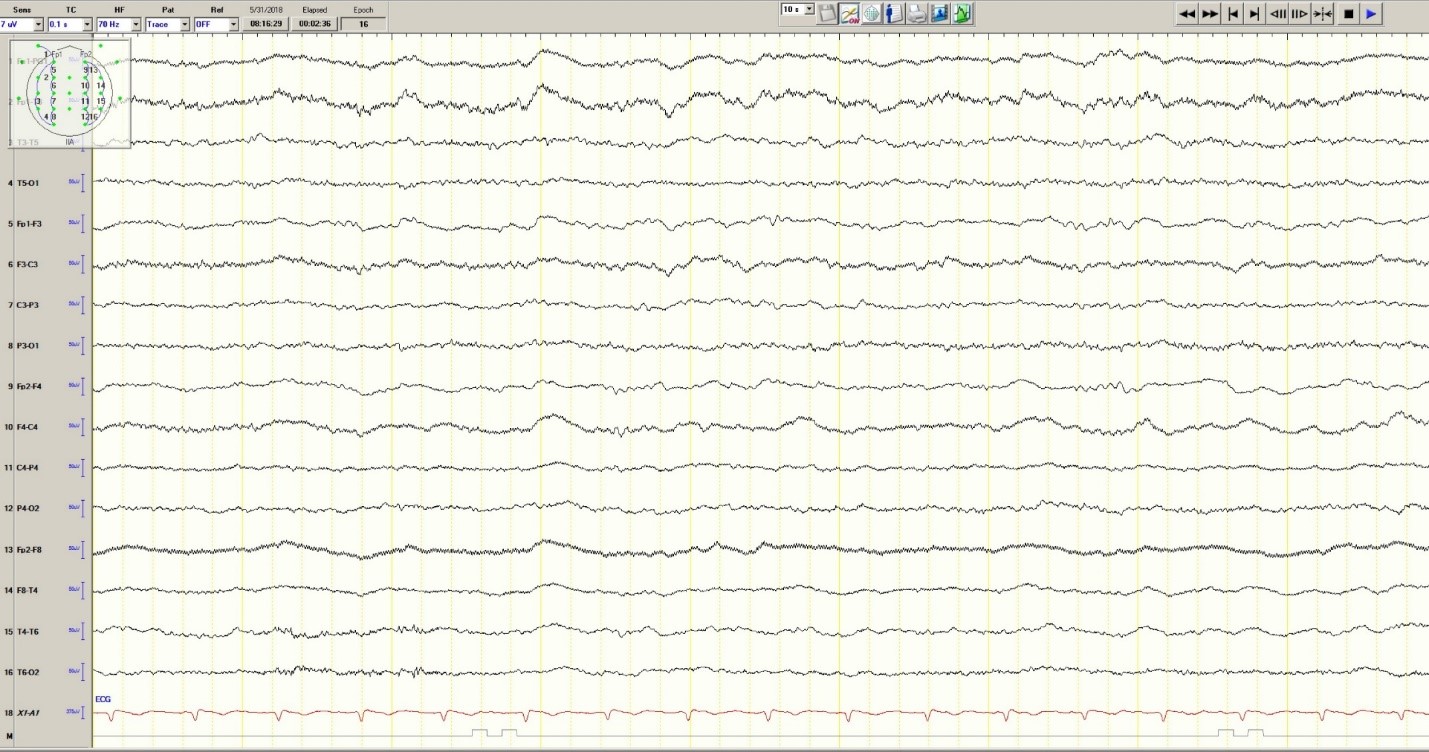
Hypoglycemia was treated with an ampule of D50 and intravenous fluids. On the obstetrics ward, nurses observed several episodes of head and eye deviation to the right accompanied by decreased responsiveness that lasted approximately 30 seconds. The patient was sent to the electrophysiology lab where an EEG revealed a diffusely slow background (Figure 1).
Figure 1. Generalized Slowing
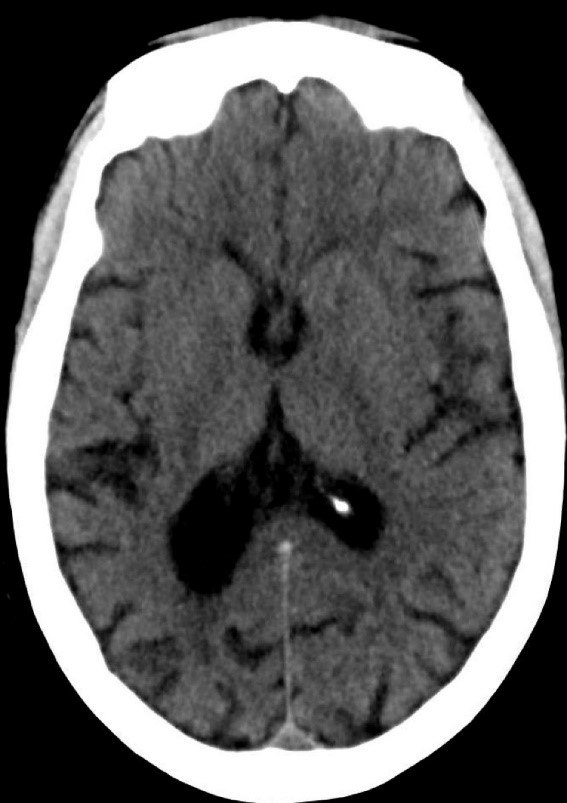
During the 20-minute EEG recording, the patient had six clinical seizures similar to those described by the nurses. These events correlated with an ictal pattern consisting of 11 HZ_sharp activity in the right occipital temporal region that spread to the right parietal and left occipital temporal regions (Figure 2). Head CT revealed mild generalized atrophy and an enlarged right occipital horn, but no acute lesions (Figure 3).
Figure 2. Partial seizure originating in right occipital temporal region
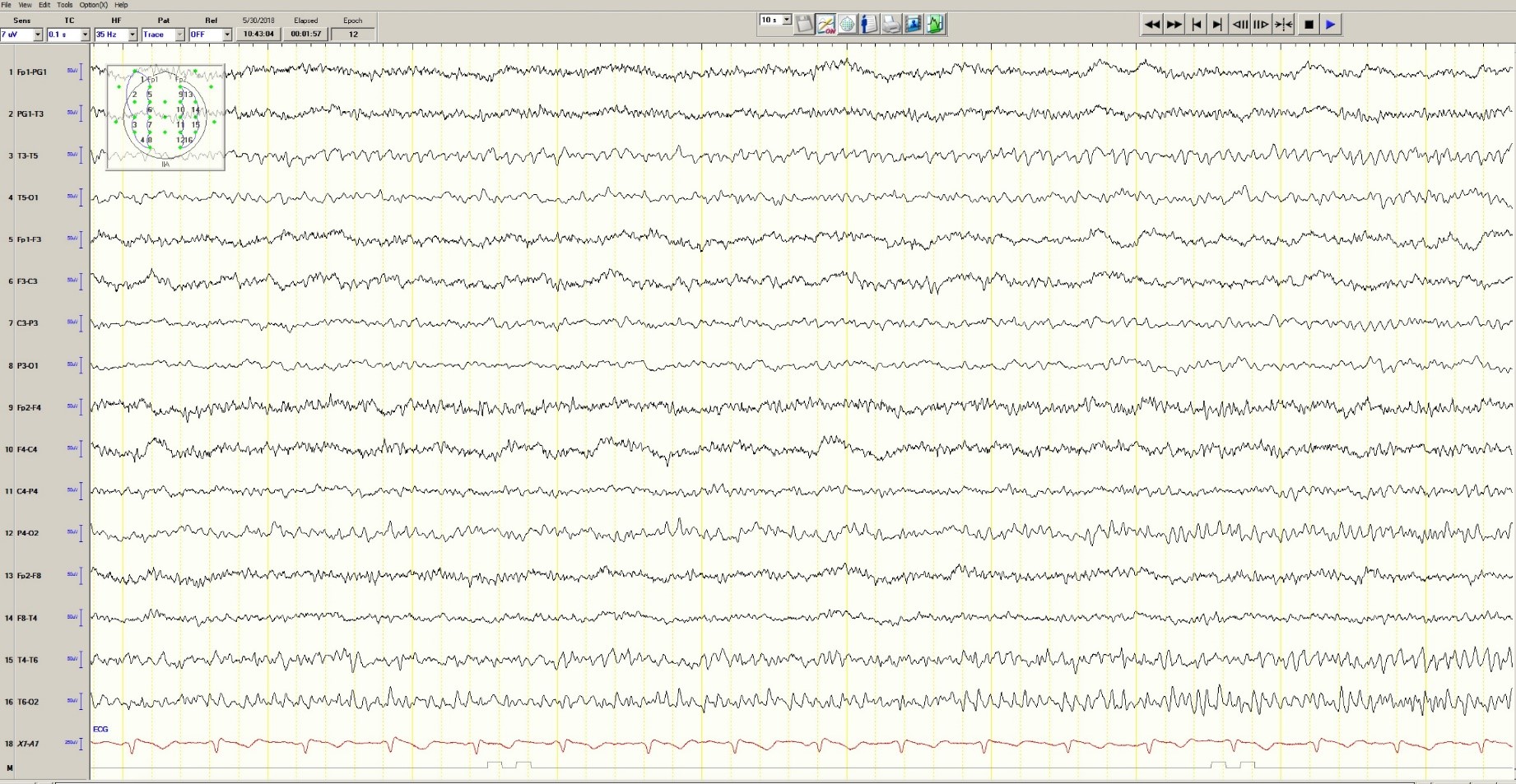
Figure 3. Mild generalized arophy, greater in right hemisphere
The patient was transferred to intensive care and received fosphenytoin. No further clinical and /or electrographic seizures were identified. The following day, an EEG revealed diffuse slowing without focal seizures (Figure 4). The patient gradually became more alert and cooperative over the next 24 hours. However, the next day no fetal heartbeat was detected. Labor was induced and a stillborn baby delivered. The pathology report indicated that the placenta was between the 5th and 10th percentile for gestational age.
Figure 4. Improved generalized slowing
Discussion
Status epilepticus is associated with significant morbidity and mortality (Claassen et al. 2002). This 37-year-old pregnant woman had an episode of focal status epilepticus with impaired awareness likely provoked by nonadherence to antiepileptic drugs (AEDs). Cocaine may have contributed to the episode of status epilepticus (Majlesi et al. 2010). The obstetric service did not diagnose preeclampsia.
The patient’s seizures started in the right occipital region, which was abnormal on neuroimaging. An MRI might have revealed more subtle structural abnormalities such as cortical dysplasia as the etiology of her epilepsy, but she refused the scan.
Women with epilepsy are at increased risk for adverse pregnancy outcomes such as preeclampsia, preterm labor, and stillbirth and should be considered high risk (MacDonald et al. 2015). Serum levels of AEDs such as lamotrigine, levetiracetam and phenytoin may decrease during pregnancy and contribute to breakthrough seizures. Accordingly, monthly measurements of serum levels of AEDs during the entire course of the pregnancy are strongly recommended. These measurements allow for a timely adjustment of AED doses to prevent significant drop in their serum concentrations and minimize the occurrence of breakthrough seizures. In the case of phenytoin, measurement of free and total serum concentrations are recommended. Supplementation with at least 0.4 mg/day to 1 mg /day of folic acid (and up to 4 mg /day) has been recommended (Harden et al. 2009a). Of note, there is no increase in the incidence of status epilepticus due to pregnancy per se (Harden et al. 2009b).
Although the patient survived this episode of status epilepticus, her fetus did not. Antiseizure drug nonadherence and polysubstance abuse probably contributed to fetal demise.
References
Claassen J, Lokin JK, Fitzsimons BFM et al. Predictors of functional disability and mortality after status epilepticus. Neurology. 2002;58:139-142.
Harden CL, Pennell PB, Koppel BS et al. Practice Parameter update: Management issues for women with epilepsy Focus on pregnancy (an evidence-based review): Vitamin K, folic acid, blood levels, and breastfeeding: Neurology 2009a;73:142-149.
Harden CL, Hopp J, Ting TY et al. Practice Parameter update: Management issues for women with epilepsy-focus on pregnancy (an evidence-based review): Obstetrical complications and change in seizure frequency. Neurology 2009b;50(5):1229-36.
MacDonald SC, Bateman BT, McElrath TF, Hernandez-Diaz S. Mortality and morbidity during delivery hospitalization among pregnant women with epilepsy in the United States. JAMA Neurol. 2015;72(9):981-988.
Majlesi N, Shih R, Fiesseler FW et al. Cocaine-associated seizures and incidence of status epilepticus. Western Journal of Emergency Medicine. 2010;XI(2):157-160.
Andrew N. Wilner, MD, FAAN, FACP
Angels Neurological Centers
Abington, MA
Clinical History
A 37-year-old pregnant African American woman with a history of epilepsy and polysubstance abuse was found unresponsive in a hotel room. She had four convulsions en route to the hospital. In transit, she received levetiracetam and phenytoin, resulting in the cessation of the clinical seizures.
According to her mother, seizures began at age 16 during her first pregnancy, which was complicated by hypertension. She was prescribed medications for hypertension and phenytoin for seizures. The patient provided a different history, claiming that her seizures began 2 years ago. She denied taking medication for seizures or other health problems.
The patient has two children, ages 22 and 11 years. Past medical history is otherwise unremarkable. She has no allergies. Social history includes cigarette smoking, and alcohol and substance abuse. She lives with her boyfriend and does not work. She is 25 weeks pregnant. Family history was notable only for migraine in her mother and grandmother.
Physical Examination
In the emergency department, blood pressure was 135/65, pulse 121 beats per minute, and oxygen saturation was 97%. She was oriented only to self and did not follow commands. Pupils were equal and reactive. There was no facial asymmetry. She moved all 4 extremities spontaneously. Reflexes were brisk. Oral mucosa was dry. She had no edema in the lower extremities.
Laboratories
Chest x-ray was normal. EKG revealed tachycardia and nonspecific ST changes. Hemoglobin was 11.1 g/dl, hematocrit 32%, white blood cell count 10,900, and platelets 181,000. Electrolytes were normal except for a low sodium of 132 mmol/l (135-145) and bicarbonate of 17 mmol/l (21-31). Glucose was initially 67 mg/dl and dropped to 46 mg/dl. Total protein was 6 g/dl (6.7-8.2) and albumin was 2.7 g/dl (3.2-5.5). Metabolic panel was otherwise normal. Urinalysis was positive for glucose, ketones, and a small amount of blood and protein. There were no bacteria. Blood and urine cultures were negative. Phenytoin level was undetectable. Urine drug screen was positive for cannabinoids and cocaine.
Hospital Course
Hypoglycemia was treated with an ampule of D50 and intravenous fluids. On the obstetrics ward, nurses observed several episodes of head and eye deviation to the right accompanied by decreased responsiveness that lasted approximately 30 seconds. The patient was sent to the electrophysiology lab where an EEG revealed a diffusely slow background (Figure 1).
Figure 1. Generalized Slowing

During the 20-minute EEG recording, the patient had six clinical seizures similar to those described by the nurses. These events correlated with an ictal pattern consisting of 11 HZ_sharp activity in the right occipital temporal region that spread to the right parietal and left occipital temporal regions (Figure 2). Head CT revealed mild generalized atrophy and an enlarged right occipital horn, but no acute lesions (Figure 3).
Figure 2. Partial seizure originating in right occipital temporal region

Figure 3. Mild generalized arophy, greater in right hemisphere

The patient was transferred to intensive care and received fosphenytoin. No further clinical and /or electrographic seizures were identified. The following day, an EEG revealed diffuse slowing without focal seizures (Figure 4). The patient gradually became more alert and cooperative over the next 24 hours. However, the next day no fetal heartbeat was detected. Labor was induced and a stillborn baby delivered. The pathology report indicated that the placenta was between the 5th and 10th percentile for gestational age.
Figure 4. Improved generalized slowing

Discussion
Status epilepticus is associated with significant morbidity and mortality (Claassen et al. 2002). This 37-year-old pregnant woman had an episode of focal status epilepticus with impaired awareness likely provoked by nonadherence to antiepileptic drugs (AEDs). Cocaine may have contributed to the episode of status epilepticus (Majlesi et al. 2010). The obstetric service did not diagnose preeclampsia.
The patient’s seizures started in the right occipital region, which was abnormal on neuroimaging. An MRI might have revealed more subtle structural abnormalities such as cortical dysplasia as the etiology of her epilepsy, but she refused the scan.
Women with epilepsy are at increased risk for adverse pregnancy outcomes such as preeclampsia, preterm labor, and stillbirth and should be considered high risk (MacDonald et al. 2015). Serum levels of AEDs such as lamotrigine, levetiracetam and phenytoin may decrease during pregnancy and contribute to breakthrough seizures. Accordingly, monthly measurements of serum levels of AEDs during the entire course of the pregnancy are strongly recommended. These measurements allow for a timely adjustment of AED doses to prevent significant drop in their serum concentrations and minimize the occurrence of breakthrough seizures. In the case of phenytoin, measurement of free and total serum concentrations are recommended. Supplementation with at least 0.4 mg/day to 1 mg /day of folic acid (and up to 4 mg /day) has been recommended (Harden et al. 2009a). Of note, there is no increase in the incidence of status epilepticus due to pregnancy per se (Harden et al. 2009b).
Although the patient survived this episode of status epilepticus, her fetus did not. Antiseizure drug nonadherence and polysubstance abuse probably contributed to fetal demise.
References
Claassen J, Lokin JK, Fitzsimons BFM et al. Predictors of functional disability and mortality after status epilepticus. Neurology. 2002;58:139-142.
Harden CL, Pennell PB, Koppel BS et al. Practice Parameter update: Management issues for women with epilepsy Focus on pregnancy (an evidence-based review): Vitamin K, folic acid, blood levels, and breastfeeding: Neurology 2009a;73:142-149.
Harden CL, Hopp J, Ting TY et al. Practice Parameter update: Management issues for women with epilepsy-focus on pregnancy (an evidence-based review): Obstetrical complications and change in seizure frequency. Neurology 2009b;50(5):1229-36.
MacDonald SC, Bateman BT, McElrath TF, Hernandez-Diaz S. Mortality and morbidity during delivery hospitalization among pregnant women with epilepsy in the United States. JAMA Neurol. 2015;72(9):981-988.
Majlesi N, Shih R, Fiesseler FW et al. Cocaine-associated seizures and incidence of status epilepticus. Western Journal of Emergency Medicine. 2010;XI(2):157-160.
Andrew N. Wilner, MD, FAAN, FACP
Angels Neurological Centers
Abington, MA
Clinical History
A 37-year-old pregnant African American woman with a history of epilepsy and polysubstance abuse was found unresponsive in a hotel room. She had four convulsions en route to the hospital. In transit, she received levetiracetam and phenytoin, resulting in the cessation of the clinical seizures.
According to her mother, seizures began at age 16 during her first pregnancy, which was complicated by hypertension. She was prescribed medications for hypertension and phenytoin for seizures. The patient provided a different history, claiming that her seizures began 2 years ago. She denied taking medication for seizures or other health problems.
The patient has two children, ages 22 and 11 years. Past medical history is otherwise unremarkable. She has no allergies. Social history includes cigarette smoking, and alcohol and substance abuse. She lives with her boyfriend and does not work. She is 25 weeks pregnant. Family history was notable only for migraine in her mother and grandmother.
Physical Examination
In the emergency department, blood pressure was 135/65, pulse 121 beats per minute, and oxygen saturation was 97%. She was oriented only to self and did not follow commands. Pupils were equal and reactive. There was no facial asymmetry. She moved all 4 extremities spontaneously. Reflexes were brisk. Oral mucosa was dry. She had no edema in the lower extremities.
Laboratories
Chest x-ray was normal. EKG revealed tachycardia and nonspecific ST changes. Hemoglobin was 11.1 g/dl, hematocrit 32%, white blood cell count 10,900, and platelets 181,000. Electrolytes were normal except for a low sodium of 132 mmol/l (135-145) and bicarbonate of 17 mmol/l (21-31). Glucose was initially 67 mg/dl and dropped to 46 mg/dl. Total protein was 6 g/dl (6.7-8.2) and albumin was 2.7 g/dl (3.2-5.5). Metabolic panel was otherwise normal. Urinalysis was positive for glucose, ketones, and a small amount of blood and protein. There were no bacteria. Blood and urine cultures were negative. Phenytoin level was undetectable. Urine drug screen was positive for cannabinoids and cocaine.
Hospital Course
Hypoglycemia was treated with an ampule of D50 and intravenous fluids. On the obstetrics ward, nurses observed several episodes of head and eye deviation to the right accompanied by decreased responsiveness that lasted approximately 30 seconds. The patient was sent to the electrophysiology lab where an EEG revealed a diffusely slow background (Figure 1).
Figure 1. Generalized Slowing

During the 20-minute EEG recording, the patient had six clinical seizures similar to those described by the nurses. These events correlated with an ictal pattern consisting of 11 HZ_sharp activity in the right occipital temporal region that spread to the right parietal and left occipital temporal regions (Figure 2). Head CT revealed mild generalized atrophy and an enlarged right occipital horn, but no acute lesions (Figure 3).
Figure 2. Partial seizure originating in right occipital temporal region

Figure 3. Mild generalized arophy, greater in right hemisphere

The patient was transferred to intensive care and received fosphenytoin. No further clinical and /or electrographic seizures were identified. The following day, an EEG revealed diffuse slowing without focal seizures (Figure 4). The patient gradually became more alert and cooperative over the next 24 hours. However, the next day no fetal heartbeat was detected. Labor was induced and a stillborn baby delivered. The pathology report indicated that the placenta was between the 5th and 10th percentile for gestational age.
Figure 4. Improved generalized slowing

Discussion
Status epilepticus is associated with significant morbidity and mortality (Claassen et al. 2002). This 37-year-old pregnant woman had an episode of focal status epilepticus with impaired awareness likely provoked by nonadherence to antiepileptic drugs (AEDs). Cocaine may have contributed to the episode of status epilepticus (Majlesi et al. 2010). The obstetric service did not diagnose preeclampsia.
The patient’s seizures started in the right occipital region, which was abnormal on neuroimaging. An MRI might have revealed more subtle structural abnormalities such as cortical dysplasia as the etiology of her epilepsy, but she refused the scan.
Women with epilepsy are at increased risk for adverse pregnancy outcomes such as preeclampsia, preterm labor, and stillbirth and should be considered high risk (MacDonald et al. 2015). Serum levels of AEDs such as lamotrigine, levetiracetam and phenytoin may decrease during pregnancy and contribute to breakthrough seizures. Accordingly, monthly measurements of serum levels of AEDs during the entire course of the pregnancy are strongly recommended. These measurements allow for a timely adjustment of AED doses to prevent significant drop in their serum concentrations and minimize the occurrence of breakthrough seizures. In the case of phenytoin, measurement of free and total serum concentrations are recommended. Supplementation with at least 0.4 mg/day to 1 mg /day of folic acid (and up to 4 mg /day) has been recommended (Harden et al. 2009a). Of note, there is no increase in the incidence of status epilepticus due to pregnancy per se (Harden et al. 2009b).
Although the patient survived this episode of status epilepticus, her fetus did not. Antiseizure drug nonadherence and polysubstance abuse probably contributed to fetal demise.
References
Claassen J, Lokin JK, Fitzsimons BFM et al. Predictors of functional disability and mortality after status epilepticus. Neurology. 2002;58:139-142.
Harden CL, Pennell PB, Koppel BS et al. Practice Parameter update: Management issues for women with epilepsy Focus on pregnancy (an evidence-based review): Vitamin K, folic acid, blood levels, and breastfeeding: Neurology 2009a;73:142-149.
Harden CL, Hopp J, Ting TY et al. Practice Parameter update: Management issues for women with epilepsy-focus on pregnancy (an evidence-based review): Obstetrical complications and change in seizure frequency. Neurology 2009b;50(5):1229-36.
MacDonald SC, Bateman BT, McElrath TF, Hernandez-Diaz S. Mortality and morbidity during delivery hospitalization among pregnant women with epilepsy in the United States. JAMA Neurol. 2015;72(9):981-988.
Majlesi N, Shih R, Fiesseler FW et al. Cocaine-associated seizures and incidence of status epilepticus. Western Journal of Emergency Medicine. 2010;XI(2):157-160.
Role of Astrocyte Glutamine Synthetase in Epilepsy
Astrocyte glutamine synthetase may play an important role in the etiology of mesial temporal lobe epilepsy suggests a recent review in the Journal of Neuroscience Research.
- Investigators from the Yale School of Medicine and Southern Illinois School of Medicine believe that inhibition, loss, or dysfunction of the enzyme in astrocytes may be one of the causative factors responsible for mesial temporal lobe epilepsy.
- Their review of the scientific evidence included a study of astrocyte abnormalities related to aquaporin 4, potassium channel Kir4.1, monocarboxylate transporters MCT1 and MCT2, amino acid transporters EAAT1 and EAA2, and glutamine synthetase.
- Their theory on the role of glutamine synthetase prompted the researchers to suggest that the mechanisms that control the enzyme may be worth consideration as targets for new antiepileptic drugs.
Eid T, Lee TW, Patrylo P, Zaveri HP. Astrocytes and glutamine synthetase in epileptogenesis [published online ahead of print July 18, 2018]. J Neurosci Res. 2018: doi: 10.1002/jnr.24267.
Astrocyte glutamine synthetase may play an important role in the etiology of mesial temporal lobe epilepsy suggests a recent review in the Journal of Neuroscience Research.
- Investigators from the Yale School of Medicine and Southern Illinois School of Medicine believe that inhibition, loss, or dysfunction of the enzyme in astrocytes may be one of the causative factors responsible for mesial temporal lobe epilepsy.
- Their review of the scientific evidence included a study of astrocyte abnormalities related to aquaporin 4, potassium channel Kir4.1, monocarboxylate transporters MCT1 and MCT2, amino acid transporters EAAT1 and EAA2, and glutamine synthetase.
- Their theory on the role of glutamine synthetase prompted the researchers to suggest that the mechanisms that control the enzyme may be worth consideration as targets for new antiepileptic drugs.
Eid T, Lee TW, Patrylo P, Zaveri HP. Astrocytes and glutamine synthetase in epileptogenesis [published online ahead of print July 18, 2018]. J Neurosci Res. 2018: doi: 10.1002/jnr.24267.
Astrocyte glutamine synthetase may play an important role in the etiology of mesial temporal lobe epilepsy suggests a recent review in the Journal of Neuroscience Research.
- Investigators from the Yale School of Medicine and Southern Illinois School of Medicine believe that inhibition, loss, or dysfunction of the enzyme in astrocytes may be one of the causative factors responsible for mesial temporal lobe epilepsy.
- Their review of the scientific evidence included a study of astrocyte abnormalities related to aquaporin 4, potassium channel Kir4.1, monocarboxylate transporters MCT1 and MCT2, amino acid transporters EAAT1 and EAA2, and glutamine synthetase.
- Their theory on the role of glutamine synthetase prompted the researchers to suggest that the mechanisms that control the enzyme may be worth consideration as targets for new antiepileptic drugs.
Eid T, Lee TW, Patrylo P, Zaveri HP. Astrocytes and glutamine synthetase in epileptogenesis [published online ahead of print July 18, 2018]. J Neurosci Res. 2018: doi: 10.1002/jnr.24267.
Interictal Ripples Predict Surgical Outcomes
The presence of interictal ripples in an intracranial EEG may serve as useful biomarkers suggests an analysis of data from 27 children who underwent epilepsy surgery.
- The average rate of onset ripples located inside a resected area of the brain predicted a patient’s outcome (odds ratio, 5.37, P=.02)
- Mean onset ripple rate was associated with the Engel class metric for measuring outcomes.
- Resection of the onset ripple zone was linked to good surgical outcomes (P=.047).
- On the other hand, there was no correlation between spread ripple zone, isolated-ripple zone, or spike zones and outcomes.
Tamilia E, Park EH, Percivati S, et al. Surgical resection of ripple onset predicts outcome in pediatric epilepsy [published online ahead of print July 18, 2018]. Ann Neurol. 2018: doi: 10.1002/ana.25295
The presence of interictal ripples in an intracranial EEG may serve as useful biomarkers suggests an analysis of data from 27 children who underwent epilepsy surgery.
- The average rate of onset ripples located inside a resected area of the brain predicted a patient’s outcome (odds ratio, 5.37, P=.02)
- Mean onset ripple rate was associated with the Engel class metric for measuring outcomes.
- Resection of the onset ripple zone was linked to good surgical outcomes (P=.047).
- On the other hand, there was no correlation between spread ripple zone, isolated-ripple zone, or spike zones and outcomes.
Tamilia E, Park EH, Percivati S, et al. Surgical resection of ripple onset predicts outcome in pediatric epilepsy [published online ahead of print July 18, 2018]. Ann Neurol. 2018: doi: 10.1002/ana.25295
The presence of interictal ripples in an intracranial EEG may serve as useful biomarkers suggests an analysis of data from 27 children who underwent epilepsy surgery.
- The average rate of onset ripples located inside a resected area of the brain predicted a patient’s outcome (odds ratio, 5.37, P=.02)
- Mean onset ripple rate was associated with the Engel class metric for measuring outcomes.
- Resection of the onset ripple zone was linked to good surgical outcomes (P=.047).
- On the other hand, there was no correlation between spread ripple zone, isolated-ripple zone, or spike zones and outcomes.
Tamilia E, Park EH, Percivati S, et al. Surgical resection of ripple onset predicts outcome in pediatric epilepsy [published online ahead of print July 18, 2018]. Ann Neurol. 2018: doi: 10.1002/ana.25295
Magnetoencephalography Offers Clues in Absence Seizures
Magnetoencephalography (MEG) and network-based analyses can help characterize absence epilepsy in children according to a study that looked at 16 patients between ages 6 and 12 years who had absence epilepsy.
- Researchers found functional/anatomical hubs in a network that contained bilateral precuneus, left thalamus, three anterior cerebellar subunits of lobule IV-V, vermis, and lobule III.
- Their analysis suggests that these hubs, which are highly connected brain areas, exist in focal cortical, subcortical, and cerebellar areas during absence seizures.
- The existence of hubs in thalami, precuneus and cingulate cortex suggest bilaterally distributed networks of cortical and subcortical regions that may be responsible for seizures.
- Hubs in the anterior cerebellum may be related to terminating motor automatism seen in absence seizures.
Youssofzadeh, V, Agler W, Tenney JF, Kadis DS. Whole-brain MEG connectivity-based analyses reveals critical hubs in childhood absence epilepsy. Epilepsy Res. 2018;145:102-109.
Magnetoencephalography (MEG) and network-based analyses can help characterize absence epilepsy in children according to a study that looked at 16 patients between ages 6 and 12 years who had absence epilepsy.
- Researchers found functional/anatomical hubs in a network that contained bilateral precuneus, left thalamus, three anterior cerebellar subunits of lobule IV-V, vermis, and lobule III.
- Their analysis suggests that these hubs, which are highly connected brain areas, exist in focal cortical, subcortical, and cerebellar areas during absence seizures.
- The existence of hubs in thalami, precuneus and cingulate cortex suggest bilaterally distributed networks of cortical and subcortical regions that may be responsible for seizures.
- Hubs in the anterior cerebellum may be related to terminating motor automatism seen in absence seizures.
Youssofzadeh, V, Agler W, Tenney JF, Kadis DS. Whole-brain MEG connectivity-based analyses reveals critical hubs in childhood absence epilepsy. Epilepsy Res. 2018;145:102-109.
Magnetoencephalography (MEG) and network-based analyses can help characterize absence epilepsy in children according to a study that looked at 16 patients between ages 6 and 12 years who had absence epilepsy.
- Researchers found functional/anatomical hubs in a network that contained bilateral precuneus, left thalamus, three anterior cerebellar subunits of lobule IV-V, vermis, and lobule III.
- Their analysis suggests that these hubs, which are highly connected brain areas, exist in focal cortical, subcortical, and cerebellar areas during absence seizures.
- The existence of hubs in thalami, precuneus and cingulate cortex suggest bilaterally distributed networks of cortical and subcortical regions that may be responsible for seizures.
- Hubs in the anterior cerebellum may be related to terminating motor automatism seen in absence seizures.
Youssofzadeh, V, Agler W, Tenney JF, Kadis DS. Whole-brain MEG connectivity-based analyses reveals critical hubs in childhood absence epilepsy. Epilepsy Res. 2018;145:102-109.
FDA Approves Deep Brain Stimulation System
The Food and Drug Administration has approved a deep brain stimulation system that has been shown to reduce seizures in a select group of patients with epilepsy.
- Medtronics DBS System for Epilepsy has been cleared as adjunct treatment for patients with partial onset seizures with or without secondary generalization.
- The system is only indicated for patients who have not responded to 3 or more antiepileptic drugs and who have experienced an average of 6 or more seizures each month for the last 3 months.
- The FDA approval also stipulates that the patients’ seizures be no more than 30 days apart.
- The DBS System includes a pulse generator that is implanted in a patient’s chest and 2 lead wires implanted in the brain.
Voelker R. Electrical stimulation for epilepsy. JAMA; 2018;319(21):2164.
The Food and Drug Administration has approved a deep brain stimulation system that has been shown to reduce seizures in a select group of patients with epilepsy.
- Medtronics DBS System for Epilepsy has been cleared as adjunct treatment for patients with partial onset seizures with or without secondary generalization.
- The system is only indicated for patients who have not responded to 3 or more antiepileptic drugs and who have experienced an average of 6 or more seizures each month for the last 3 months.
- The FDA approval also stipulates that the patients’ seizures be no more than 30 days apart.
- The DBS System includes a pulse generator that is implanted in a patient’s chest and 2 lead wires implanted in the brain.
Voelker R. Electrical stimulation for epilepsy. JAMA; 2018;319(21):2164.
The Food and Drug Administration has approved a deep brain stimulation system that has been shown to reduce seizures in a select group of patients with epilepsy.
- Medtronics DBS System for Epilepsy has been cleared as adjunct treatment for patients with partial onset seizures with or without secondary generalization.
- The system is only indicated for patients who have not responded to 3 or more antiepileptic drugs and who have experienced an average of 6 or more seizures each month for the last 3 months.
- The FDA approval also stipulates that the patients’ seizures be no more than 30 days apart.
- The DBS System includes a pulse generator that is implanted in a patient’s chest and 2 lead wires implanted in the brain.
Voelker R. Electrical stimulation for epilepsy. JAMA; 2018;319(21):2164.
Psychiatric Interventions Important for Patients with Epilepsy
Clinicians need to pay more attention to the psychological impact of epilepsy and its treatment according to a report from the International League Against Epilepsy Psychology Task Force.
- The task force identified the best interventions for depression, neurocognitive problems, and medication adherence.
- Several psychological strategies are worth consideration according to the task force, including cognitive behavioral therapy and mindfulness-based treatment.
- These interventions have the potential to improve health-related quality of life among adults and children.
- The recommendations outlined by the League are based primarily on evidence discussed in a recent Cochrane review of randomized clinical trials that evaluated psychological treatment of patients with epilepsy.
Michaelis R, Tang V, Goldstein LH, et al. Psychological treatments for adults and children with epilepsy: Evidence-based recommendations by the International League Against Epilepsy Psychology Task Force. Epilepsia. 2018;59(7):1282-1302.
Clinicians need to pay more attention to the psychological impact of epilepsy and its treatment according to a report from the International League Against Epilepsy Psychology Task Force.
- The task force identified the best interventions for depression, neurocognitive problems, and medication adherence.
- Several psychological strategies are worth consideration according to the task force, including cognitive behavioral therapy and mindfulness-based treatment.
- These interventions have the potential to improve health-related quality of life among adults and children.
- The recommendations outlined by the League are based primarily on evidence discussed in a recent Cochrane review of randomized clinical trials that evaluated psychological treatment of patients with epilepsy.
Michaelis R, Tang V, Goldstein LH, et al. Psychological treatments for adults and children with epilepsy: Evidence-based recommendations by the International League Against Epilepsy Psychology Task Force. Epilepsia. 2018;59(7):1282-1302.
Clinicians need to pay more attention to the psychological impact of epilepsy and its treatment according to a report from the International League Against Epilepsy Psychology Task Force.
- The task force identified the best interventions for depression, neurocognitive problems, and medication adherence.
- Several psychological strategies are worth consideration according to the task force, including cognitive behavioral therapy and mindfulness-based treatment.
- These interventions have the potential to improve health-related quality of life among adults and children.
- The recommendations outlined by the League are based primarily on evidence discussed in a recent Cochrane review of randomized clinical trials that evaluated psychological treatment of patients with epilepsy.
Michaelis R, Tang V, Goldstein LH, et al. Psychological treatments for adults and children with epilepsy: Evidence-based recommendations by the International League Against Epilepsy Psychology Task Force. Epilepsia. 2018;59(7):1282-1302.
No Differences Found Between Generic/Brand Name Epileptic Medication
Switching patients from brand name antiepileptic drugs to generics is generally safe and cost effective according to an analysis published in Epilepsia.
- Researchers looked at data on bioequivalence, health care utilization, and clinical studies on the safety of antiepileptic agents, including a comparison of area under the plasma concentration-time curve (AUC) and peak plasma concentration.
- For most of the drugs that were evaluated, there were negligible differences in AUC and peak plasma concentration between generic drugs and brand name equivalents.
- There were significant increases in health care usage when patients were switched from brand name to generic versions.
- Clinical studies were unable to detect differences in seizure frequency or tolerability.
Holtkamp M, Theodore WH. Generic antiepileptic drugs—safe or harmful in patients with epilepsy? Epilepsia. 2018;59(7):1273-1281.
Switching patients from brand name antiepileptic drugs to generics is generally safe and cost effective according to an analysis published in Epilepsia.
- Researchers looked at data on bioequivalence, health care utilization, and clinical studies on the safety of antiepileptic agents, including a comparison of area under the plasma concentration-time curve (AUC) and peak plasma concentration.
- For most of the drugs that were evaluated, there were negligible differences in AUC and peak plasma concentration between generic drugs and brand name equivalents.
- There were significant increases in health care usage when patients were switched from brand name to generic versions.
- Clinical studies were unable to detect differences in seizure frequency or tolerability.
Holtkamp M, Theodore WH. Generic antiepileptic drugs—safe or harmful in patients with epilepsy? Epilepsia. 2018;59(7):1273-1281.
Switching patients from brand name antiepileptic drugs to generics is generally safe and cost effective according to an analysis published in Epilepsia.
- Researchers looked at data on bioequivalence, health care utilization, and clinical studies on the safety of antiepileptic agents, including a comparison of area under the plasma concentration-time curve (AUC) and peak plasma concentration.
- For most of the drugs that were evaluated, there were negligible differences in AUC and peak plasma concentration between generic drugs and brand name equivalents.
- There were significant increases in health care usage when patients were switched from brand name to generic versions.
- Clinical studies were unable to detect differences in seizure frequency or tolerability.
Holtkamp M, Theodore WH. Generic antiepileptic drugs—safe or harmful in patients with epilepsy? Epilepsia. 2018;59(7):1273-1281.