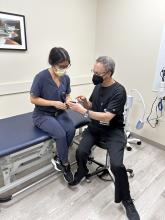
User login
How to get started with prescribing and advising on CGM
Continuous glucose monitoring (CGM) is gaining ground with both patients and providers because of an array of driving forces, including broadening eligibility, insulin price caps, public awareness, and an increasing number of educational initiatives for doctors.
While professional organizations aim to familiarize doctors with this relatively new technology, more patients are learning independently that finger sticks may be optional, leading them to request CGM from their provider, according to Neil Skolnik, MD.
“We in primary care are being shepherded into this space by our patients who have seen an advertisement or talked to a friend about the benefits of CGM, and then asked us to prescribe it,” said Dr. Skolnik, professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Hospital–Jefferson Health.
Systemic factors are also accelerating CGM uptake, he added, highlighting recent Medicare rule changes to expand eligibility, with insurance companies beginning to follow suit.
Warren A. Jones, MD, FAAFP, professor emeritus at the University of Mississippi, Jackson, and past president of the AAFP, said that insulin price regulations have also opened doors to CGM.
“When you had patients trying to determine whether they were going to buy food or pay for high-priced insulin, that was a big challenge,” Dr. Jones said in an interview. “But that barrier has recently been removed, so we’re at the dawn of a new era.”
Like any paradigm shift, however,
Overview of online resources and navigating coverage
The latest learning resource on CGM for physicians comes from the American Academy of Family Physicians in the form of a new online educational hub with a 2-credit, ACCME-accredited course. It offers comprehensive guidance for employing CGM in daily practice. Topics include both medical and practical considerations, from interpretation of curves and glucose goal-setting to choosing a device and navigating coverage.
The AAFP’s new offering joins a growing number of similar educational efforts launched over the past few years by the Association of Diabetes Care & Education Specialists, the American Pharmacists Association, the American Diabetes Association, and the American Association of Clinical Endocrinologists.
Checking for coverage is a key first step when considering CGM for a particular patient, Dr. Jones said, noting that CGM, like any new form of care, presents unique challenges with coding and claims that must be overcome to get reimbursed.
“No margin, no mission,” Dr. Jones said. “If you are not able to pay your bills, you can’t be available for your patients. Our goal at the AAFP is to make sure that physicians get this knowledge [about reimbursement].”
To this end, the AAFP’s new online educational hub and the guide provided by APhA present CGM eligibility criteria for various patient groups, including those with Medicare, Medicaid, private insurance, and without coverage.
Medicare criteria include a diagnosis of diabetes, treatment with three or more daily administrations of insulin or continuous infusion via a pump, frequent adjustment to insulin treatment based on glucose readings, and presentation for diabetes in the past 6 months.
Once these requirements are clearly documented in the patient’s record, providers need to write the script, complete a certificate of medical necessity, and choose a supplier. Medicare covers CGM as a durable medical equipment benefit instead of a pharmacy benefit, according to the AAFP and APhA.
Exact coverage criteria and reimbursement processes for non-Medicare patients follow similar paths, although details vary by state and insurer, so personalized investigation is required.
When exploring coverage, the AAFP recommends paying attention to information needed for prior authorization, the patient’s diabetes type and age, and other medical requirements, such as minimum number of daily finger sticks or insulin doses per day.
Looking ahead, Dr. Jones predicted that authorization obstacles stemming from short-term cost concerns are going to fade as long-term savings are uncovered.
“I think pharmacy benefit managers and payers are going to recognize that we have better patient compliance, and that continuous glucose monitoring is going to bring the cost of care down and decrease the rate of hospitalizations,” Dr. Jones said. “So I think they’re going to be willing to pay clinicians to engage in this more readily over time.”
Patients who fail to qualify for personal CGM can still benefit from professional CGM, in which they borrow necessary equipment on a short-term basis. This avenue typically requires minimal or no insurance authorization. In addition, providers have the “opportunity to cover/exceed expenses by enhancing revenue with separately billable procedures, which can be billed in addition to [evaluation and management] if done on same day,” according to the AAFP guide, which goes on to provide appropriate codes.
Learning CGM through first-hand experience
Getting started with CGM can be intimidating for providers, Dr. Skolnik said, although he offered some reassurance, suggesting that the learning process may be more forgiving than prescribing a new drug for the first time.
“I think the best way to figure out CGM is to prescribe it to a couple of patients and learn with them,” Dr. Skolnik said. “You can’t do that with medicines. With medicines, you need to know what you’re doing before you choose who to give a medicine to.”
Instead of “reading everything under the sun” about CGM, he recommends starting with several of the ADA’s resources focusing on time in range, including an article, webinar, and podcast.
After that, physicians can learn on the job. A beginner’s mindset to CGM is well received by patients, he said, especially if you share your natural curiosity with them.
“Share your patients’ wonder at what they see,” Dr. Skolnik said. “They’ll open the app and you’ll look at their time and range and together you’ll go, ‘Wow, isn’t that something? I wonder why?’ ”
With this approach, providers and patients can join forces to explore trends and troubleshoot anomalous readings.
“Together you’ll go: ‘Hmm, I wonder why on Thursday, that graph is looking so far off from the other days? Wow. And then the patient remembers: they ate out on Thursday. They had a big pasta meal, perhaps. Everyone’s different in how they respond to different carbs. And you’ll both have this epiphany together about: ‘Wow, what I do matters.’ And I think that’s actually the best way to jump in.”
According to the AAFP, ADCES, and APhA resources, providers should first address time below range, as hypoglycemia can be imminently dangerous.
Next, providers should consider time in range, average glucose, and glucose management indicator, the latter of which acts as a surrogate for HbA1c. The first couple weeks of monitoring should be viewed as an information gathering phase, after which specific targets can be addressed through behavioral modifications and insulin adjustments, the AAFP advises.
The ADA guide highlights CGM usage, glucose variability, time in range, time above range, and average glucose as key metrics to monitor and offers corresponding actions when targets are unmet.
Encouraging patients to start CGM
Like providers, patients may also be intimidated by CGM, Dr. Jones said, typically because they don’t know how it works, or it seems complicated. Fortunately, he said, these fears are easily overcome when patients learn that they don’t need to stick themselves, record any of their readings, or really do anything at all for the first few weeks.
“You don’t even worry about it,” Dr. Jones tells his patients, who typically feel “more in control and engaged in their own care” after experiencing CGM for themselves.
Dr. Jones speaks from both professional and personal experience. A member of his family recently started CGM after being discharged from the hospital, and the benefits have been significant for everyone involved.
“I see how effectively we can control [my family member’s] blood pressure and insulin requirements, as opposed to several months ago when we didn’t have it,” Dr. Jones said. “So I’m giving it to you from two perspectives: one, of the clinician who knows, intellectually, what should go on, and two, experientially, from a family trying to take care of someone they love.”
Dr. Skolnik disclosed relationships with AstraZeneca, Teva, Lilly, Boehringer Ingelheim, Sanofi, GSK, Bayer, Genentech, Abbott, Idorsia, Merck, Novartis, Heartland, and Novo Nordisk. Dr Jones disclosed no relevant conflicts of interest.
Continuous glucose monitoring (CGM) is gaining ground with both patients and providers because of an array of driving forces, including broadening eligibility, insulin price caps, public awareness, and an increasing number of educational initiatives for doctors.
While professional organizations aim to familiarize doctors with this relatively new technology, more patients are learning independently that finger sticks may be optional, leading them to request CGM from their provider, according to Neil Skolnik, MD.
“We in primary care are being shepherded into this space by our patients who have seen an advertisement or talked to a friend about the benefits of CGM, and then asked us to prescribe it,” said Dr. Skolnik, professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Hospital–Jefferson Health.
Systemic factors are also accelerating CGM uptake, he added, highlighting recent Medicare rule changes to expand eligibility, with insurance companies beginning to follow suit.
Warren A. Jones, MD, FAAFP, professor emeritus at the University of Mississippi, Jackson, and past president of the AAFP, said that insulin price regulations have also opened doors to CGM.
“When you had patients trying to determine whether they were going to buy food or pay for high-priced insulin, that was a big challenge,” Dr. Jones said in an interview. “But that barrier has recently been removed, so we’re at the dawn of a new era.”
Like any paradigm shift, however,
Overview of online resources and navigating coverage
The latest learning resource on CGM for physicians comes from the American Academy of Family Physicians in the form of a new online educational hub with a 2-credit, ACCME-accredited course. It offers comprehensive guidance for employing CGM in daily practice. Topics include both medical and practical considerations, from interpretation of curves and glucose goal-setting to choosing a device and navigating coverage.
The AAFP’s new offering joins a growing number of similar educational efforts launched over the past few years by the Association of Diabetes Care & Education Specialists, the American Pharmacists Association, the American Diabetes Association, and the American Association of Clinical Endocrinologists.
Checking for coverage is a key first step when considering CGM for a particular patient, Dr. Jones said, noting that CGM, like any new form of care, presents unique challenges with coding and claims that must be overcome to get reimbursed.
“No margin, no mission,” Dr. Jones said. “If you are not able to pay your bills, you can’t be available for your patients. Our goal at the AAFP is to make sure that physicians get this knowledge [about reimbursement].”
To this end, the AAFP’s new online educational hub and the guide provided by APhA present CGM eligibility criteria for various patient groups, including those with Medicare, Medicaid, private insurance, and without coverage.
Medicare criteria include a diagnosis of diabetes, treatment with three or more daily administrations of insulin or continuous infusion via a pump, frequent adjustment to insulin treatment based on glucose readings, and presentation for diabetes in the past 6 months.
Once these requirements are clearly documented in the patient’s record, providers need to write the script, complete a certificate of medical necessity, and choose a supplier. Medicare covers CGM as a durable medical equipment benefit instead of a pharmacy benefit, according to the AAFP and APhA.
Exact coverage criteria and reimbursement processes for non-Medicare patients follow similar paths, although details vary by state and insurer, so personalized investigation is required.
When exploring coverage, the AAFP recommends paying attention to information needed for prior authorization, the patient’s diabetes type and age, and other medical requirements, such as minimum number of daily finger sticks or insulin doses per day.
Looking ahead, Dr. Jones predicted that authorization obstacles stemming from short-term cost concerns are going to fade as long-term savings are uncovered.
“I think pharmacy benefit managers and payers are going to recognize that we have better patient compliance, and that continuous glucose monitoring is going to bring the cost of care down and decrease the rate of hospitalizations,” Dr. Jones said. “So I think they’re going to be willing to pay clinicians to engage in this more readily over time.”
Patients who fail to qualify for personal CGM can still benefit from professional CGM, in which they borrow necessary equipment on a short-term basis. This avenue typically requires minimal or no insurance authorization. In addition, providers have the “opportunity to cover/exceed expenses by enhancing revenue with separately billable procedures, which can be billed in addition to [evaluation and management] if done on same day,” according to the AAFP guide, which goes on to provide appropriate codes.
Learning CGM through first-hand experience
Getting started with CGM can be intimidating for providers, Dr. Skolnik said, although he offered some reassurance, suggesting that the learning process may be more forgiving than prescribing a new drug for the first time.
“I think the best way to figure out CGM is to prescribe it to a couple of patients and learn with them,” Dr. Skolnik said. “You can’t do that with medicines. With medicines, you need to know what you’re doing before you choose who to give a medicine to.”
Instead of “reading everything under the sun” about CGM, he recommends starting with several of the ADA’s resources focusing on time in range, including an article, webinar, and podcast.
After that, physicians can learn on the job. A beginner’s mindset to CGM is well received by patients, he said, especially if you share your natural curiosity with them.
“Share your patients’ wonder at what they see,” Dr. Skolnik said. “They’ll open the app and you’ll look at their time and range and together you’ll go, ‘Wow, isn’t that something? I wonder why?’ ”
With this approach, providers and patients can join forces to explore trends and troubleshoot anomalous readings.
“Together you’ll go: ‘Hmm, I wonder why on Thursday, that graph is looking so far off from the other days? Wow. And then the patient remembers: they ate out on Thursday. They had a big pasta meal, perhaps. Everyone’s different in how they respond to different carbs. And you’ll both have this epiphany together about: ‘Wow, what I do matters.’ And I think that’s actually the best way to jump in.”
According to the AAFP, ADCES, and APhA resources, providers should first address time below range, as hypoglycemia can be imminently dangerous.
Next, providers should consider time in range, average glucose, and glucose management indicator, the latter of which acts as a surrogate for HbA1c. The first couple weeks of monitoring should be viewed as an information gathering phase, after which specific targets can be addressed through behavioral modifications and insulin adjustments, the AAFP advises.
The ADA guide highlights CGM usage, glucose variability, time in range, time above range, and average glucose as key metrics to monitor and offers corresponding actions when targets are unmet.
Encouraging patients to start CGM
Like providers, patients may also be intimidated by CGM, Dr. Jones said, typically because they don’t know how it works, or it seems complicated. Fortunately, he said, these fears are easily overcome when patients learn that they don’t need to stick themselves, record any of their readings, or really do anything at all for the first few weeks.
“You don’t even worry about it,” Dr. Jones tells his patients, who typically feel “more in control and engaged in their own care” after experiencing CGM for themselves.
Dr. Jones speaks from both professional and personal experience. A member of his family recently started CGM after being discharged from the hospital, and the benefits have been significant for everyone involved.
“I see how effectively we can control [my family member’s] blood pressure and insulin requirements, as opposed to several months ago when we didn’t have it,” Dr. Jones said. “So I’m giving it to you from two perspectives: one, of the clinician who knows, intellectually, what should go on, and two, experientially, from a family trying to take care of someone they love.”
Dr. Skolnik disclosed relationships with AstraZeneca, Teva, Lilly, Boehringer Ingelheim, Sanofi, GSK, Bayer, Genentech, Abbott, Idorsia, Merck, Novartis, Heartland, and Novo Nordisk. Dr Jones disclosed no relevant conflicts of interest.
Continuous glucose monitoring (CGM) is gaining ground with both patients and providers because of an array of driving forces, including broadening eligibility, insulin price caps, public awareness, and an increasing number of educational initiatives for doctors.
While professional organizations aim to familiarize doctors with this relatively new technology, more patients are learning independently that finger sticks may be optional, leading them to request CGM from their provider, according to Neil Skolnik, MD.
“We in primary care are being shepherded into this space by our patients who have seen an advertisement or talked to a friend about the benefits of CGM, and then asked us to prescribe it,” said Dr. Skolnik, professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Hospital–Jefferson Health.
Systemic factors are also accelerating CGM uptake, he added, highlighting recent Medicare rule changes to expand eligibility, with insurance companies beginning to follow suit.
Warren A. Jones, MD, FAAFP, professor emeritus at the University of Mississippi, Jackson, and past president of the AAFP, said that insulin price regulations have also opened doors to CGM.
“When you had patients trying to determine whether they were going to buy food or pay for high-priced insulin, that was a big challenge,” Dr. Jones said in an interview. “But that barrier has recently been removed, so we’re at the dawn of a new era.”
Like any paradigm shift, however,
Overview of online resources and navigating coverage
The latest learning resource on CGM for physicians comes from the American Academy of Family Physicians in the form of a new online educational hub with a 2-credit, ACCME-accredited course. It offers comprehensive guidance for employing CGM in daily practice. Topics include both medical and practical considerations, from interpretation of curves and glucose goal-setting to choosing a device and navigating coverage.
The AAFP’s new offering joins a growing number of similar educational efforts launched over the past few years by the Association of Diabetes Care & Education Specialists, the American Pharmacists Association, the American Diabetes Association, and the American Association of Clinical Endocrinologists.
Checking for coverage is a key first step when considering CGM for a particular patient, Dr. Jones said, noting that CGM, like any new form of care, presents unique challenges with coding and claims that must be overcome to get reimbursed.
“No margin, no mission,” Dr. Jones said. “If you are not able to pay your bills, you can’t be available for your patients. Our goal at the AAFP is to make sure that physicians get this knowledge [about reimbursement].”
To this end, the AAFP’s new online educational hub and the guide provided by APhA present CGM eligibility criteria for various patient groups, including those with Medicare, Medicaid, private insurance, and without coverage.
Medicare criteria include a diagnosis of diabetes, treatment with three or more daily administrations of insulin or continuous infusion via a pump, frequent adjustment to insulin treatment based on glucose readings, and presentation for diabetes in the past 6 months.
Once these requirements are clearly documented in the patient’s record, providers need to write the script, complete a certificate of medical necessity, and choose a supplier. Medicare covers CGM as a durable medical equipment benefit instead of a pharmacy benefit, according to the AAFP and APhA.
Exact coverage criteria and reimbursement processes for non-Medicare patients follow similar paths, although details vary by state and insurer, so personalized investigation is required.
When exploring coverage, the AAFP recommends paying attention to information needed for prior authorization, the patient’s diabetes type and age, and other medical requirements, such as minimum number of daily finger sticks or insulin doses per day.
Looking ahead, Dr. Jones predicted that authorization obstacles stemming from short-term cost concerns are going to fade as long-term savings are uncovered.
“I think pharmacy benefit managers and payers are going to recognize that we have better patient compliance, and that continuous glucose monitoring is going to bring the cost of care down and decrease the rate of hospitalizations,” Dr. Jones said. “So I think they’re going to be willing to pay clinicians to engage in this more readily over time.”
Patients who fail to qualify for personal CGM can still benefit from professional CGM, in which they borrow necessary equipment on a short-term basis. This avenue typically requires minimal or no insurance authorization. In addition, providers have the “opportunity to cover/exceed expenses by enhancing revenue with separately billable procedures, which can be billed in addition to [evaluation and management] if done on same day,” according to the AAFP guide, which goes on to provide appropriate codes.
Learning CGM through first-hand experience
Getting started with CGM can be intimidating for providers, Dr. Skolnik said, although he offered some reassurance, suggesting that the learning process may be more forgiving than prescribing a new drug for the first time.
“I think the best way to figure out CGM is to prescribe it to a couple of patients and learn with them,” Dr. Skolnik said. “You can’t do that with medicines. With medicines, you need to know what you’re doing before you choose who to give a medicine to.”
Instead of “reading everything under the sun” about CGM, he recommends starting with several of the ADA’s resources focusing on time in range, including an article, webinar, and podcast.
After that, physicians can learn on the job. A beginner’s mindset to CGM is well received by patients, he said, especially if you share your natural curiosity with them.
“Share your patients’ wonder at what they see,” Dr. Skolnik said. “They’ll open the app and you’ll look at their time and range and together you’ll go, ‘Wow, isn’t that something? I wonder why?’ ”
With this approach, providers and patients can join forces to explore trends and troubleshoot anomalous readings.
“Together you’ll go: ‘Hmm, I wonder why on Thursday, that graph is looking so far off from the other days? Wow. And then the patient remembers: they ate out on Thursday. They had a big pasta meal, perhaps. Everyone’s different in how they respond to different carbs. And you’ll both have this epiphany together about: ‘Wow, what I do matters.’ And I think that’s actually the best way to jump in.”
According to the AAFP, ADCES, and APhA resources, providers should first address time below range, as hypoglycemia can be imminently dangerous.
Next, providers should consider time in range, average glucose, and glucose management indicator, the latter of which acts as a surrogate for HbA1c. The first couple weeks of monitoring should be viewed as an information gathering phase, after which specific targets can be addressed through behavioral modifications and insulin adjustments, the AAFP advises.
The ADA guide highlights CGM usage, glucose variability, time in range, time above range, and average glucose as key metrics to monitor and offers corresponding actions when targets are unmet.
Encouraging patients to start CGM
Like providers, patients may also be intimidated by CGM, Dr. Jones said, typically because they don’t know how it works, or it seems complicated. Fortunately, he said, these fears are easily overcome when patients learn that they don’t need to stick themselves, record any of their readings, or really do anything at all for the first few weeks.
“You don’t even worry about it,” Dr. Jones tells his patients, who typically feel “more in control and engaged in their own care” after experiencing CGM for themselves.
Dr. Jones speaks from both professional and personal experience. A member of his family recently started CGM after being discharged from the hospital, and the benefits have been significant for everyone involved.
“I see how effectively we can control [my family member’s] blood pressure and insulin requirements, as opposed to several months ago when we didn’t have it,” Dr. Jones said. “So I’m giving it to you from two perspectives: one, of the clinician who knows, intellectually, what should go on, and two, experientially, from a family trying to take care of someone they love.”
Dr. Skolnik disclosed relationships with AstraZeneca, Teva, Lilly, Boehringer Ingelheim, Sanofi, GSK, Bayer, Genentech, Abbott, Idorsia, Merck, Novartis, Heartland, and Novo Nordisk. Dr Jones disclosed no relevant conflicts of interest.
Diabetes drug tied to lower dementia risk
new research suggests.
Overall, in a large cohort study from South Korea, patients who took pioglitazone were 16% less likely to develop dementia over an average of 10 years than peers who did not take the drug.
However, the dementia risk reduction was 54% among those with ischemic heart disease and 43% among those with a history of stroke.
“Our study was to see the association between pioglitazone use and incidence of dementia, not how (with what mechanisms) this drug can suppress dementia pathology,” coinvestigator Eosu Kim, MD, PhD, Yonsei University, Seoul, South Korea, said in an interview.
However, “as we found this drug is more effective in diabetic patients who have blood circulation problems in the heart or brain than in those without such problems, we speculate that pioglitazone’s antidementia action may be related to improving blood vessel’s health,” Dr. Kim said.
This finding suggests that pioglitazone could be used as a personalized treatment approach for dementia prevention in this subgroup of patients with diabetes, the researchers noted.
The results were published online in Neurology.
Dose-response relationship
Risk for dementia is doubled in adults with T2DM, the investigators wrote. Prior studies have suggested that pioglitazone may protect against dementia, as well as a first or recurrent stroke, in patients with T2DM.
This led Dr. Kim and colleagues to examine the effects of pioglitazone on dementia risk overall and in relation to stroke and ischemic heart disease.
Using the national Korean health database, the researchers identified 91,218 adults aged 50 and older with new-onset T2DM who did not have dementia. A total of 3,467 were treated with pioglitazone.
Pioglitazone exposure was defined as a total cumulative daily dose of 90 or more calculated from all dispensations during 4 years after T2DM diagnosis, with outcomes assessed after this period.
Over an average of 10 years, 8.3% of pioglitazone users developed dementia, compared with 10.0% of nonusers.
There was a statistically significant 16% lower risk for developing all-cause dementia among pioglitazone users than among nonusers (adjusted hazard ratio, 0.84; 95% confidence interval, 0.75-0.95).
A dose-response relationship was evident; pioglitazone users who received the highest cumulative daily dose were at lower risk for dementia (aHR, 0.72; 95% CI, 0.55-0.94).
Several limitations
The reduced risk for dementia was more pronounced among patients who used pioglitazone for 4 years in comparison with patients who did not use the drug (aHR, 0.63; 95% CI, 0.44-0.90).
The apparent protective effect of pioglitazone with regard to dementia was greater among those with a history of ischemic heart disease (aHR, 0.46; 95% CI, 0.24-0.90) or stroke (aHR, 0.57; 95% CI, 0.38-0.86) before diabetes diagnosis.
The incidence of stroke was also reduced with pioglitazone use (aHR, 0.81; 95% CI, 0.66-1.0).
“These results provide valuable information on who could potentially benefit from pioglitazone use for prevention of dementia,” Dr. Kim said in a news release.
However, “the risk and benefit balance of long-term use of this drug to prevent dementia should be prospectively assessed,” he said in an interview.
The researchers cautioned that the study was observational; hence, the reported associations cannot address causal relationships. Also, because of the use of claims data, drug compliance could not be guaranteed, and exposure may have been overestimated.
There is also the potential for selection bias, and no information on apolipoprotein E was available, they noted.
More data needed
In an accompanying editorial, Colleen J. Maxwell, PhD, University of Waterloo (Ont.), and colleagues wrote that the results “not only support previous studies showing the potential cognitive benefit of pioglitazone but also extend our understanding of this benefit through the mediating effect of reducing ischemic stroke.”
However, because of their associated risks, which include fractures, weight gain, heart failure, and bladder cancer, thiazolidinediones are not currently favored in diabetes management guidelines – and their use has significantly declined since the mid to late 2000s, the editorialists noted.
They agreed that it will be important to reassess the risk-benefit profile of pioglitazone in T2DM as additional findings emerge.
They also noted that sodium-glucose cotransporter-2 inhibitors, which have significant cardiovascular and renal benefits and minimal side effects, may also lower the risk for dementia.
“As both pioglitazone and SGLT-2 inhibitors are second-line options for physicians, the current decision would easily be in favor of SGLT-2 inhibitors given their safety profile,” Dr. Maxwell and colleagues wrote.
For now, pioglitazone “should not be used to prevent dementia in patients with T2DM,” they concluded.
The study was supported by grants from the National Research Foundation of Korea funded by the Korean government and the Ministry of Health and Welfare. The investigators and editorialists report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
new research suggests.
Overall, in a large cohort study from South Korea, patients who took pioglitazone were 16% less likely to develop dementia over an average of 10 years than peers who did not take the drug.
However, the dementia risk reduction was 54% among those with ischemic heart disease and 43% among those with a history of stroke.
“Our study was to see the association between pioglitazone use and incidence of dementia, not how (with what mechanisms) this drug can suppress dementia pathology,” coinvestigator Eosu Kim, MD, PhD, Yonsei University, Seoul, South Korea, said in an interview.
However, “as we found this drug is more effective in diabetic patients who have blood circulation problems in the heart or brain than in those without such problems, we speculate that pioglitazone’s antidementia action may be related to improving blood vessel’s health,” Dr. Kim said.
This finding suggests that pioglitazone could be used as a personalized treatment approach for dementia prevention in this subgroup of patients with diabetes, the researchers noted.
The results were published online in Neurology.
Dose-response relationship
Risk for dementia is doubled in adults with T2DM, the investigators wrote. Prior studies have suggested that pioglitazone may protect against dementia, as well as a first or recurrent stroke, in patients with T2DM.
This led Dr. Kim and colleagues to examine the effects of pioglitazone on dementia risk overall and in relation to stroke and ischemic heart disease.
Using the national Korean health database, the researchers identified 91,218 adults aged 50 and older with new-onset T2DM who did not have dementia. A total of 3,467 were treated with pioglitazone.
Pioglitazone exposure was defined as a total cumulative daily dose of 90 or more calculated from all dispensations during 4 years after T2DM diagnosis, with outcomes assessed after this period.
Over an average of 10 years, 8.3% of pioglitazone users developed dementia, compared with 10.0% of nonusers.
There was a statistically significant 16% lower risk for developing all-cause dementia among pioglitazone users than among nonusers (adjusted hazard ratio, 0.84; 95% confidence interval, 0.75-0.95).
A dose-response relationship was evident; pioglitazone users who received the highest cumulative daily dose were at lower risk for dementia (aHR, 0.72; 95% CI, 0.55-0.94).
Several limitations
The reduced risk for dementia was more pronounced among patients who used pioglitazone for 4 years in comparison with patients who did not use the drug (aHR, 0.63; 95% CI, 0.44-0.90).
The apparent protective effect of pioglitazone with regard to dementia was greater among those with a history of ischemic heart disease (aHR, 0.46; 95% CI, 0.24-0.90) or stroke (aHR, 0.57; 95% CI, 0.38-0.86) before diabetes diagnosis.
The incidence of stroke was also reduced with pioglitazone use (aHR, 0.81; 95% CI, 0.66-1.0).
“These results provide valuable information on who could potentially benefit from pioglitazone use for prevention of dementia,” Dr. Kim said in a news release.
However, “the risk and benefit balance of long-term use of this drug to prevent dementia should be prospectively assessed,” he said in an interview.
The researchers cautioned that the study was observational; hence, the reported associations cannot address causal relationships. Also, because of the use of claims data, drug compliance could not be guaranteed, and exposure may have been overestimated.
There is also the potential for selection bias, and no information on apolipoprotein E was available, they noted.
More data needed
In an accompanying editorial, Colleen J. Maxwell, PhD, University of Waterloo (Ont.), and colleagues wrote that the results “not only support previous studies showing the potential cognitive benefit of pioglitazone but also extend our understanding of this benefit through the mediating effect of reducing ischemic stroke.”
However, because of their associated risks, which include fractures, weight gain, heart failure, and bladder cancer, thiazolidinediones are not currently favored in diabetes management guidelines – and their use has significantly declined since the mid to late 2000s, the editorialists noted.
They agreed that it will be important to reassess the risk-benefit profile of pioglitazone in T2DM as additional findings emerge.
They also noted that sodium-glucose cotransporter-2 inhibitors, which have significant cardiovascular and renal benefits and minimal side effects, may also lower the risk for dementia.
“As both pioglitazone and SGLT-2 inhibitors are second-line options for physicians, the current decision would easily be in favor of SGLT-2 inhibitors given their safety profile,” Dr. Maxwell and colleagues wrote.
For now, pioglitazone “should not be used to prevent dementia in patients with T2DM,” they concluded.
The study was supported by grants from the National Research Foundation of Korea funded by the Korean government and the Ministry of Health and Welfare. The investigators and editorialists report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
new research suggests.
Overall, in a large cohort study from South Korea, patients who took pioglitazone were 16% less likely to develop dementia over an average of 10 years than peers who did not take the drug.
However, the dementia risk reduction was 54% among those with ischemic heart disease and 43% among those with a history of stroke.
“Our study was to see the association between pioglitazone use and incidence of dementia, not how (with what mechanisms) this drug can suppress dementia pathology,” coinvestigator Eosu Kim, MD, PhD, Yonsei University, Seoul, South Korea, said in an interview.
However, “as we found this drug is more effective in diabetic patients who have blood circulation problems in the heart or brain than in those without such problems, we speculate that pioglitazone’s antidementia action may be related to improving blood vessel’s health,” Dr. Kim said.
This finding suggests that pioglitazone could be used as a personalized treatment approach for dementia prevention in this subgroup of patients with diabetes, the researchers noted.
The results were published online in Neurology.
Dose-response relationship
Risk for dementia is doubled in adults with T2DM, the investigators wrote. Prior studies have suggested that pioglitazone may protect against dementia, as well as a first or recurrent stroke, in patients with T2DM.
This led Dr. Kim and colleagues to examine the effects of pioglitazone on dementia risk overall and in relation to stroke and ischemic heart disease.
Using the national Korean health database, the researchers identified 91,218 adults aged 50 and older with new-onset T2DM who did not have dementia. A total of 3,467 were treated with pioglitazone.
Pioglitazone exposure was defined as a total cumulative daily dose of 90 or more calculated from all dispensations during 4 years after T2DM diagnosis, with outcomes assessed after this period.
Over an average of 10 years, 8.3% of pioglitazone users developed dementia, compared with 10.0% of nonusers.
There was a statistically significant 16% lower risk for developing all-cause dementia among pioglitazone users than among nonusers (adjusted hazard ratio, 0.84; 95% confidence interval, 0.75-0.95).
A dose-response relationship was evident; pioglitazone users who received the highest cumulative daily dose were at lower risk for dementia (aHR, 0.72; 95% CI, 0.55-0.94).
Several limitations
The reduced risk for dementia was more pronounced among patients who used pioglitazone for 4 years in comparison with patients who did not use the drug (aHR, 0.63; 95% CI, 0.44-0.90).
The apparent protective effect of pioglitazone with regard to dementia was greater among those with a history of ischemic heart disease (aHR, 0.46; 95% CI, 0.24-0.90) or stroke (aHR, 0.57; 95% CI, 0.38-0.86) before diabetes diagnosis.
The incidence of stroke was also reduced with pioglitazone use (aHR, 0.81; 95% CI, 0.66-1.0).
“These results provide valuable information on who could potentially benefit from pioglitazone use for prevention of dementia,” Dr. Kim said in a news release.
However, “the risk and benefit balance of long-term use of this drug to prevent dementia should be prospectively assessed,” he said in an interview.
The researchers cautioned that the study was observational; hence, the reported associations cannot address causal relationships. Also, because of the use of claims data, drug compliance could not be guaranteed, and exposure may have been overestimated.
There is also the potential for selection bias, and no information on apolipoprotein E was available, they noted.
More data needed
In an accompanying editorial, Colleen J. Maxwell, PhD, University of Waterloo (Ont.), and colleagues wrote that the results “not only support previous studies showing the potential cognitive benefit of pioglitazone but also extend our understanding of this benefit through the mediating effect of reducing ischemic stroke.”
However, because of their associated risks, which include fractures, weight gain, heart failure, and bladder cancer, thiazolidinediones are not currently favored in diabetes management guidelines – and their use has significantly declined since the mid to late 2000s, the editorialists noted.
They agreed that it will be important to reassess the risk-benefit profile of pioglitazone in T2DM as additional findings emerge.
They also noted that sodium-glucose cotransporter-2 inhibitors, which have significant cardiovascular and renal benefits and minimal side effects, may also lower the risk for dementia.
“As both pioglitazone and SGLT-2 inhibitors are second-line options for physicians, the current decision would easily be in favor of SGLT-2 inhibitors given their safety profile,” Dr. Maxwell and colleagues wrote.
For now, pioglitazone “should not be used to prevent dementia in patients with T2DM,” they concluded.
The study was supported by grants from the National Research Foundation of Korea funded by the Korean government and the Ministry of Health and Welfare. The investigators and editorialists report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM NEUROLOGY
Can a hormone shot rescue low libido?
according to results from two small randomized controlled trials.
The data suggest that injections of kisspeptin can boost sexual desire in men and women and can increase penile rigidity in men.
Together, these two studies provide proof of concept for the development of kisspeptin-based therapeutics for men and women with distressing hypoactive sexual desire disorder (HSDD), study investigator Alexander Comninos, MD, PhD, Imperial College London, said in a news release.
One study was published online Feb. 3, 2022, in JAMA Network Open. The other was published in October 2022.
Unmet need
HSDD affects up to 10% of women and 8% of men worldwide and leads to psychological and social harm, the news release noted.
“There is a real unmet need to find new, safer, and more effective therapies for this distressing condition for both women and men seeking treatment,” Dr. Comninos said.
Kisspeptin is a naturally occurring reproductive hormone that serves as a crucial activator of the reproductive system. Emerging evidence from animal models shows that kisspeptin signaling has key roles in modulating reproductive behavior, including sexual motivation and erections.
In a double-blind, placebo-controlled, crossover study, the researchers enrolled 32 healthy heterosexual men (mean age, 37.9 years) who had HSDD.
At the first study visit, the men were given an infusion of kisspeptin-54 (1 nmol/kg per hour) or placebo (saline) over 75 minutes. The participants then crossed over to the other treatment at a second study visit at least 7 days later.
The active treatment significantly increased circulating kisspeptin levels. A steady state was reached after 30-75 minutes of infusion, the researchers reported.
Similar data in men, women
While the men viewed sexual videos, kisspeptin significantly modulated brain activity on fMRI in key structures of the sexual-processing network, compared with placebo (P = .003).
In addition, the treatment led to significant increases in penile tumescence in response to sexual stimuli (by up to 56% more than placebo; P = .02) and behavioral measures of sexual desire – most notably increased happiness about sex (P = .02).
Given the significant stimulatory effect of kisspeptin administration on penile rigidity, coupled with its demonstrated proerectile effect in rodents, future studies should examine the use of kisspeptin for patients with erectile dysfunction, the researchers wrote.
The second study included 32 women with HSDD and had the same design. Its results also showed that kisspeptin restored sexual and attraction brain processing without adverse effects.
“It is highly encouraging to see the same boosting effect in both women and men, although the precise brain pathways were slightly different, as might be expected,” coinvestigator Waljit Dhillo, PhD, Imperial College London, said in the news release.
“Collectively, the results suggest that kisspeptin may offer a safe and much-needed treatment for HSDD that affects millions of people around the world; and we look forward to taking this forward in future larger studies and in other patient groups,” Dr. Dhillo added.
The study was funded by the National Institute for Health and Care Research Imperial Biomedical Research Centre and the Medical Research Council, part of UK Research and Innovation. Dr. Comninos reported no relevant financial relationships. Dr. Dhillo reported receiving consulting fees from Myovant Sciences and KaNDy Therapeutics outside the submitted work.
A version of this article first appeared on Medscape.com.
according to results from two small randomized controlled trials.
The data suggest that injections of kisspeptin can boost sexual desire in men and women and can increase penile rigidity in men.
Together, these two studies provide proof of concept for the development of kisspeptin-based therapeutics for men and women with distressing hypoactive sexual desire disorder (HSDD), study investigator Alexander Comninos, MD, PhD, Imperial College London, said in a news release.
One study was published online Feb. 3, 2022, in JAMA Network Open. The other was published in October 2022.
Unmet need
HSDD affects up to 10% of women and 8% of men worldwide and leads to psychological and social harm, the news release noted.
“There is a real unmet need to find new, safer, and more effective therapies for this distressing condition for both women and men seeking treatment,” Dr. Comninos said.
Kisspeptin is a naturally occurring reproductive hormone that serves as a crucial activator of the reproductive system. Emerging evidence from animal models shows that kisspeptin signaling has key roles in modulating reproductive behavior, including sexual motivation and erections.
In a double-blind, placebo-controlled, crossover study, the researchers enrolled 32 healthy heterosexual men (mean age, 37.9 years) who had HSDD.
At the first study visit, the men were given an infusion of kisspeptin-54 (1 nmol/kg per hour) or placebo (saline) over 75 minutes. The participants then crossed over to the other treatment at a second study visit at least 7 days later.
The active treatment significantly increased circulating kisspeptin levels. A steady state was reached after 30-75 minutes of infusion, the researchers reported.
Similar data in men, women
While the men viewed sexual videos, kisspeptin significantly modulated brain activity on fMRI in key structures of the sexual-processing network, compared with placebo (P = .003).
In addition, the treatment led to significant increases in penile tumescence in response to sexual stimuli (by up to 56% more than placebo; P = .02) and behavioral measures of sexual desire – most notably increased happiness about sex (P = .02).
Given the significant stimulatory effect of kisspeptin administration on penile rigidity, coupled with its demonstrated proerectile effect in rodents, future studies should examine the use of kisspeptin for patients with erectile dysfunction, the researchers wrote.
The second study included 32 women with HSDD and had the same design. Its results also showed that kisspeptin restored sexual and attraction brain processing without adverse effects.
“It is highly encouraging to see the same boosting effect in both women and men, although the precise brain pathways were slightly different, as might be expected,” coinvestigator Waljit Dhillo, PhD, Imperial College London, said in the news release.
“Collectively, the results suggest that kisspeptin may offer a safe and much-needed treatment for HSDD that affects millions of people around the world; and we look forward to taking this forward in future larger studies and in other patient groups,” Dr. Dhillo added.
The study was funded by the National Institute for Health and Care Research Imperial Biomedical Research Centre and the Medical Research Council, part of UK Research and Innovation. Dr. Comninos reported no relevant financial relationships. Dr. Dhillo reported receiving consulting fees from Myovant Sciences and KaNDy Therapeutics outside the submitted work.
A version of this article first appeared on Medscape.com.
according to results from two small randomized controlled trials.
The data suggest that injections of kisspeptin can boost sexual desire in men and women and can increase penile rigidity in men.
Together, these two studies provide proof of concept for the development of kisspeptin-based therapeutics for men and women with distressing hypoactive sexual desire disorder (HSDD), study investigator Alexander Comninos, MD, PhD, Imperial College London, said in a news release.
One study was published online Feb. 3, 2022, in JAMA Network Open. The other was published in October 2022.
Unmet need
HSDD affects up to 10% of women and 8% of men worldwide and leads to psychological and social harm, the news release noted.
“There is a real unmet need to find new, safer, and more effective therapies for this distressing condition for both women and men seeking treatment,” Dr. Comninos said.
Kisspeptin is a naturally occurring reproductive hormone that serves as a crucial activator of the reproductive system. Emerging evidence from animal models shows that kisspeptin signaling has key roles in modulating reproductive behavior, including sexual motivation and erections.
In a double-blind, placebo-controlled, crossover study, the researchers enrolled 32 healthy heterosexual men (mean age, 37.9 years) who had HSDD.
At the first study visit, the men were given an infusion of kisspeptin-54 (1 nmol/kg per hour) or placebo (saline) over 75 minutes. The participants then crossed over to the other treatment at a second study visit at least 7 days later.
The active treatment significantly increased circulating kisspeptin levels. A steady state was reached after 30-75 minutes of infusion, the researchers reported.
Similar data in men, women
While the men viewed sexual videos, kisspeptin significantly modulated brain activity on fMRI in key structures of the sexual-processing network, compared with placebo (P = .003).
In addition, the treatment led to significant increases in penile tumescence in response to sexual stimuli (by up to 56% more than placebo; P = .02) and behavioral measures of sexual desire – most notably increased happiness about sex (P = .02).
Given the significant stimulatory effect of kisspeptin administration on penile rigidity, coupled with its demonstrated proerectile effect in rodents, future studies should examine the use of kisspeptin for patients with erectile dysfunction, the researchers wrote.
The second study included 32 women with HSDD and had the same design. Its results also showed that kisspeptin restored sexual and attraction brain processing without adverse effects.
“It is highly encouraging to see the same boosting effect in both women and men, although the precise brain pathways were slightly different, as might be expected,” coinvestigator Waljit Dhillo, PhD, Imperial College London, said in the news release.
“Collectively, the results suggest that kisspeptin may offer a safe and much-needed treatment for HSDD that affects millions of people around the world; and we look forward to taking this forward in future larger studies and in other patient groups,” Dr. Dhillo added.
The study was funded by the National Institute for Health and Care Research Imperial Biomedical Research Centre and the Medical Research Council, part of UK Research and Innovation. Dr. Comninos reported no relevant financial relationships. Dr. Dhillo reported receiving consulting fees from Myovant Sciences and KaNDy Therapeutics outside the submitted work.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Study documents link between preadolescent acne and elevated BMI
The that used age- and sex-matched controls.
The investigators also identified “a potential association” with precocious puberty that they said “should be considered, especially among those presenting [with acne] under 8 or 9 years old.” The study was published in Pediatric Dermatology .
Senior author Megha M. Tollefson, MD, and coauthors used resources of the Rochester Epidemiology Project to identify all residents of Olmstead County, Minn., who were diagnosed with acne between the ages of 7 and 12 years during 2010-2018. They then randomly selected two age and sex-matched community controls in order to evaluate the relationship of preadolescent acne and BMI.
They confirmed 643 acne cases, and calculated an annual age- and sex-adjusted incidence rate for ages 7-12 of 58 per 10,000 person-years (95% confidence interval, 53.5-62.5). The incidence rate was significantly higher in females than males (89.2 vs. 28.2 per 10,000 person-years; P < .001), and it significantly increased with age (incidence rates of 4.3, 24.4, and 144.3 per 10,000 person-years among those ages 7-8, 9-10, and 11-12 years, respectively).
The median BMI percentile among children with acne was significantly higher than those without an acne diagnosis (75.0 vs. 65.0; P <.001). They also were much more likely to be obese: 16.7% of the children with acne had a BMI in at least the 95th percentile, compared with 12.2% among controls with no acne diagnosis (P = .01). (The qualifying 581 acne cases for this analysis had BMIs recorded within 8 months of the index data, in addition to not having pre-existing acne-relevant endocrine disorders.)
“High BMI is a strong risk factor for acne development and severity in adults, but until now pediatric studies have revealed mixed information ... [and have been] largely retrospective reviews without controls,” Dr. Tollefson, professor of pediatrics and dermatology at the Mayo Clinic, Rochester, Minn., and colleagues wrote.
‘Valuable’ data
Leah Lalor, MD, a pediatric dermatologist not involved with the research, said she is happy to see it. “It’s really valuable,” she said in an interview. “It’s actually the first study that gives us incidence data for preadolescent acne. We all have [had our estimates], but this study quantifies it ... and it will set the stage for further studies of preadolescents in the future.”
The study also documents that “girls are more likely to present to the clinic with acne, and to do so at younger ages, which we’ve suspected and which makes physiologic sense since girls tend to go through puberty earlier than boys,” said Dr. Lalor, assistant professor of dermatology and pediatrics at the Medical College of Wisconsin and the Children’s Wisconsin Clinics, both in Milwaukee. “And most interestingly, it really reveals that BMI is higher among preadolescents with acne than those without.”
The important caveat, she emphasized, is that the study population in Olmstead County, Minn. has a relatively higher level of education, wealth, and employment than the rest of the United States.
The investigators also found that use of systemic acne medications increased with increasing BMI (odds ratio, 1.43 per 5 kg/m2 increase in BMI; 95% CI, 1.07-1.92; P = .015). Approximately 5% of underweight or normal children were prescribed systemic acne medications, compared with 8.1% of overweight children, and 10.3% of those who were obese – data that suggest that most preadolescents with acne had mild to moderate disease and that more severe acne may be associated with increasing BMI percentiles, the authors wrote.
Approximately 4% of the 643 preadolescents with acne were diagnosed with an acne-relevant endocrine disorder prior to or at the time of acne diagnosis – most commonly precocious puberty. Of the 24 diagnoses of precocious puberty, 22 were in females, with a mean age at diagnosis of 7.3 years.
Puberty before age 8 in girls and 9 in boys is classified as precocious puberty. “Thus, a thorough review of systems and exam should be done in this population [with acne] to look for precocious puberty with a low threshold for systemic evaluation if indicated,” the authors wrote, also noting that 19 or the 482 female patients with acne were subsequently diagnosed with polycystic ovary syndrome.
Dr. Lalor said she “automatically” refers children with acne who are younger than 7 for an endocrine workup, but not necessarily children ages 7, 8, or 9 because “that’s considered within the normal realm of starting to get some acne.” Acne in the context of other symptoms such as body odor, hair, or thelarche may prompt referral in these ages, however, she said.
Future research
Obesity may influence preadolescent acne development through its effect on puberty, as overweight and obese girls achieve puberty earlier than those with normal BMI. And “insulin resistance, which may be related to obesity, has been implicated with inducing or worsening acne potentially related to shifts in IGF-1 [insulin-like growth factor 1] signaling and hyperandrogenemia,” Dr. Tollefson and colleagues wrote. Nutrition is also a possible confounder in the study.
“Patients and families have long felt that certain foods or practices contribute to acne, though this has been difficult to prove,” Dr. Lalor said. “We know that excess skim milk seems to contribute ... and there’s a correlation between high glycemic load diets [and acne].”
Assessing dietary habits in conjunction with BMI, and acne incidence and severity, would be valuable. So would research to determine “if decreasing the BMI percentile [in children with acne] would improve or prevent acne, without doing any acne treatments,” she said.
The study was supported by the National Institute on Aging and the Rochester Epidemiology Project. The authors reported no conflicts of interest. Dr. Lalor also reported no conflicts of interest.
The that used age- and sex-matched controls.
The investigators also identified “a potential association” with precocious puberty that they said “should be considered, especially among those presenting [with acne] under 8 or 9 years old.” The study was published in Pediatric Dermatology .
Senior author Megha M. Tollefson, MD, and coauthors used resources of the Rochester Epidemiology Project to identify all residents of Olmstead County, Minn., who were diagnosed with acne between the ages of 7 and 12 years during 2010-2018. They then randomly selected two age and sex-matched community controls in order to evaluate the relationship of preadolescent acne and BMI.
They confirmed 643 acne cases, and calculated an annual age- and sex-adjusted incidence rate for ages 7-12 of 58 per 10,000 person-years (95% confidence interval, 53.5-62.5). The incidence rate was significantly higher in females than males (89.2 vs. 28.2 per 10,000 person-years; P < .001), and it significantly increased with age (incidence rates of 4.3, 24.4, and 144.3 per 10,000 person-years among those ages 7-8, 9-10, and 11-12 years, respectively).
The median BMI percentile among children with acne was significantly higher than those without an acne diagnosis (75.0 vs. 65.0; P <.001). They also were much more likely to be obese: 16.7% of the children with acne had a BMI in at least the 95th percentile, compared with 12.2% among controls with no acne diagnosis (P = .01). (The qualifying 581 acne cases for this analysis had BMIs recorded within 8 months of the index data, in addition to not having pre-existing acne-relevant endocrine disorders.)
“High BMI is a strong risk factor for acne development and severity in adults, but until now pediatric studies have revealed mixed information ... [and have been] largely retrospective reviews without controls,” Dr. Tollefson, professor of pediatrics and dermatology at the Mayo Clinic, Rochester, Minn., and colleagues wrote.
‘Valuable’ data
Leah Lalor, MD, a pediatric dermatologist not involved with the research, said she is happy to see it. “It’s really valuable,” she said in an interview. “It’s actually the first study that gives us incidence data for preadolescent acne. We all have [had our estimates], but this study quantifies it ... and it will set the stage for further studies of preadolescents in the future.”
The study also documents that “girls are more likely to present to the clinic with acne, and to do so at younger ages, which we’ve suspected and which makes physiologic sense since girls tend to go through puberty earlier than boys,” said Dr. Lalor, assistant professor of dermatology and pediatrics at the Medical College of Wisconsin and the Children’s Wisconsin Clinics, both in Milwaukee. “And most interestingly, it really reveals that BMI is higher among preadolescents with acne than those without.”
The important caveat, she emphasized, is that the study population in Olmstead County, Minn. has a relatively higher level of education, wealth, and employment than the rest of the United States.
The investigators also found that use of systemic acne medications increased with increasing BMI (odds ratio, 1.43 per 5 kg/m2 increase in BMI; 95% CI, 1.07-1.92; P = .015). Approximately 5% of underweight or normal children were prescribed systemic acne medications, compared with 8.1% of overweight children, and 10.3% of those who were obese – data that suggest that most preadolescents with acne had mild to moderate disease and that more severe acne may be associated with increasing BMI percentiles, the authors wrote.
Approximately 4% of the 643 preadolescents with acne were diagnosed with an acne-relevant endocrine disorder prior to or at the time of acne diagnosis – most commonly precocious puberty. Of the 24 diagnoses of precocious puberty, 22 were in females, with a mean age at diagnosis of 7.3 years.
Puberty before age 8 in girls and 9 in boys is classified as precocious puberty. “Thus, a thorough review of systems and exam should be done in this population [with acne] to look for precocious puberty with a low threshold for systemic evaluation if indicated,” the authors wrote, also noting that 19 or the 482 female patients with acne were subsequently diagnosed with polycystic ovary syndrome.
Dr. Lalor said she “automatically” refers children with acne who are younger than 7 for an endocrine workup, but not necessarily children ages 7, 8, or 9 because “that’s considered within the normal realm of starting to get some acne.” Acne in the context of other symptoms such as body odor, hair, or thelarche may prompt referral in these ages, however, she said.
Future research
Obesity may influence preadolescent acne development through its effect on puberty, as overweight and obese girls achieve puberty earlier than those with normal BMI. And “insulin resistance, which may be related to obesity, has been implicated with inducing or worsening acne potentially related to shifts in IGF-1 [insulin-like growth factor 1] signaling and hyperandrogenemia,” Dr. Tollefson and colleagues wrote. Nutrition is also a possible confounder in the study.
“Patients and families have long felt that certain foods or practices contribute to acne, though this has been difficult to prove,” Dr. Lalor said. “We know that excess skim milk seems to contribute ... and there’s a correlation between high glycemic load diets [and acne].”
Assessing dietary habits in conjunction with BMI, and acne incidence and severity, would be valuable. So would research to determine “if decreasing the BMI percentile [in children with acne] would improve or prevent acne, without doing any acne treatments,” she said.
The study was supported by the National Institute on Aging and the Rochester Epidemiology Project. The authors reported no conflicts of interest. Dr. Lalor also reported no conflicts of interest.
The that used age- and sex-matched controls.
The investigators also identified “a potential association” with precocious puberty that they said “should be considered, especially among those presenting [with acne] under 8 or 9 years old.” The study was published in Pediatric Dermatology .
Senior author Megha M. Tollefson, MD, and coauthors used resources of the Rochester Epidemiology Project to identify all residents of Olmstead County, Minn., who were diagnosed with acne between the ages of 7 and 12 years during 2010-2018. They then randomly selected two age and sex-matched community controls in order to evaluate the relationship of preadolescent acne and BMI.
They confirmed 643 acne cases, and calculated an annual age- and sex-adjusted incidence rate for ages 7-12 of 58 per 10,000 person-years (95% confidence interval, 53.5-62.5). The incidence rate was significantly higher in females than males (89.2 vs. 28.2 per 10,000 person-years; P < .001), and it significantly increased with age (incidence rates of 4.3, 24.4, and 144.3 per 10,000 person-years among those ages 7-8, 9-10, and 11-12 years, respectively).
The median BMI percentile among children with acne was significantly higher than those without an acne diagnosis (75.0 vs. 65.0; P <.001). They also were much more likely to be obese: 16.7% of the children with acne had a BMI in at least the 95th percentile, compared with 12.2% among controls with no acne diagnosis (P = .01). (The qualifying 581 acne cases for this analysis had BMIs recorded within 8 months of the index data, in addition to not having pre-existing acne-relevant endocrine disorders.)
“High BMI is a strong risk factor for acne development and severity in adults, but until now pediatric studies have revealed mixed information ... [and have been] largely retrospective reviews without controls,” Dr. Tollefson, professor of pediatrics and dermatology at the Mayo Clinic, Rochester, Minn., and colleagues wrote.
‘Valuable’ data
Leah Lalor, MD, a pediatric dermatologist not involved with the research, said she is happy to see it. “It’s really valuable,” she said in an interview. “It’s actually the first study that gives us incidence data for preadolescent acne. We all have [had our estimates], but this study quantifies it ... and it will set the stage for further studies of preadolescents in the future.”
The study also documents that “girls are more likely to present to the clinic with acne, and to do so at younger ages, which we’ve suspected and which makes physiologic sense since girls tend to go through puberty earlier than boys,” said Dr. Lalor, assistant professor of dermatology and pediatrics at the Medical College of Wisconsin and the Children’s Wisconsin Clinics, both in Milwaukee. “And most interestingly, it really reveals that BMI is higher among preadolescents with acne than those without.”
The important caveat, she emphasized, is that the study population in Olmstead County, Minn. has a relatively higher level of education, wealth, and employment than the rest of the United States.
The investigators also found that use of systemic acne medications increased with increasing BMI (odds ratio, 1.43 per 5 kg/m2 increase in BMI; 95% CI, 1.07-1.92; P = .015). Approximately 5% of underweight or normal children were prescribed systemic acne medications, compared with 8.1% of overweight children, and 10.3% of those who were obese – data that suggest that most preadolescents with acne had mild to moderate disease and that more severe acne may be associated with increasing BMI percentiles, the authors wrote.
Approximately 4% of the 643 preadolescents with acne were diagnosed with an acne-relevant endocrine disorder prior to or at the time of acne diagnosis – most commonly precocious puberty. Of the 24 diagnoses of precocious puberty, 22 were in females, with a mean age at diagnosis of 7.3 years.
Puberty before age 8 in girls and 9 in boys is classified as precocious puberty. “Thus, a thorough review of systems and exam should be done in this population [with acne] to look for precocious puberty with a low threshold for systemic evaluation if indicated,” the authors wrote, also noting that 19 or the 482 female patients with acne were subsequently diagnosed with polycystic ovary syndrome.
Dr. Lalor said she “automatically” refers children with acne who are younger than 7 for an endocrine workup, but not necessarily children ages 7, 8, or 9 because “that’s considered within the normal realm of starting to get some acne.” Acne in the context of other symptoms such as body odor, hair, or thelarche may prompt referral in these ages, however, she said.
Future research
Obesity may influence preadolescent acne development through its effect on puberty, as overweight and obese girls achieve puberty earlier than those with normal BMI. And “insulin resistance, which may be related to obesity, has been implicated with inducing or worsening acne potentially related to shifts in IGF-1 [insulin-like growth factor 1] signaling and hyperandrogenemia,” Dr. Tollefson and colleagues wrote. Nutrition is also a possible confounder in the study.
“Patients and families have long felt that certain foods or practices contribute to acne, though this has been difficult to prove,” Dr. Lalor said. “We know that excess skim milk seems to contribute ... and there’s a correlation between high glycemic load diets [and acne].”
Assessing dietary habits in conjunction with BMI, and acne incidence and severity, would be valuable. So would research to determine “if decreasing the BMI percentile [in children with acne] would improve or prevent acne, without doing any acne treatments,” she said.
The study was supported by the National Institute on Aging and the Rochester Epidemiology Project. The authors reported no conflicts of interest. Dr. Lalor also reported no conflicts of interest.
FROM PEDIATRIC DERMATOLOGY
In adults with prediabetes, vitamin D cuts diabetes risk
Results of the analysis, led by Anastassios G. Pittas, MD, MS, with the division of endocrinology, diabetes, and metabolism at Tufts Medical Center, in Boston, were published online in Annals of Internal Medicine (2023 Feb 7. doi: 10.7326/M22-3018).
All three eligible trials included in the analysis were randomized, double blinded, and placebo controlled. The three eligible trials tested three oral formulations of Vitamin D: cholecalciferol, 20,000 IU (500 mcg) weekly; cholecalciferol, 4,000 IU (100 mcg) daily; or eldecalcitol, 0.75 mcg daily, against placebos.
The authors of the new paper found that vitamin D reduced the risk for diabetes in people with prediabetes by a statistically significant 15% in adjusted analyses. The 3-year absolute risk reduction was 3.3%.
They found no difference in the rate ratios for adverse events (kidney stones, 1.17, 95% confidence interval, 0.69-1.99; hypercalcemia, 2.34; 95% CI, 0.83-6.66]; hypercalciuria, 1.65; 95% CI, 0.83-3.28]; death, 0.85; 95% CI, 0.31-2.36]) when study participants got vitamin D instead of placebo.
Differences from previous analyses
The relationship between vitamin D levels and risk for type 2 diabetes has been studied in previous trials and results have been mixed.
The authors note that two previous meta-analyses included trials “that had relatively short durations for assessment of diabetes risk (for example, ≤ 1 year), had high risk of bias (for example, open-label trials), or were not specifically designed and conducted for primary prevention of type 2 diabetes, potentially undermining the validity of the results.”
Each of the trials in this meta-analysis had a low risk of bias as determined by the Cochrane risk-of-bias tool, Dr. Pittas and colleagues said.
“The present study does not reach an opposite conclusion from the D2d study,” said Dr. Pittas, who coauthored that paper as well. “Rather, it confirms the results of the D2d study. In D2d and two other similar vitamin D and diabetes prevention trials (one in Norway and one in Japan), vitamin D reduced the rate of progression to diabetes in adults with prediabetes, but the observed differences were not statistically significant because the reported relative risk reductions (10%-13%) were smaller than each trial was powered to detect (25%-36%).”
“Individual participant data meta-analyses increase the statistical power to detect an effect. After combining data, we found that vitamin D reduced the risk of progression from prediabetes to diabetes by 15% and this result was statistically significant. So, the conclusion of the meta-analysis is essentially the same conclusion as in D2d and the other two trials. The difference is that the result is now statistically significant,” Dr. Pittas added.
Small reduction but large population
The authors acknowledged that the absolute risk reduction number is small, especially when compared with the risk reduction seen with intensive lifestyle changes (58%) and metformin (31%), as reported in an article published in the New England of Journal of Medicine (2002 Feb 7;346:393-403). But “extrapolating to the more than 374 million adults worldwide who have prediabetes suggests that inexpensive vitamin D supplementation could delay the development of diabetes in more than 10 million people,” they said.
As for how high vitamin D levels need to be, the authors write that their research indicates that the optimal level of vitamin D in the blood needed to reduce diabetes risk may be higher than an Institute of Medicine committee recommendation in 2011.
“The blood 25-hydroxy vitamin D level needed to optimally reduce diabetes risk may be near and possibly above the range of 125-150 nmol/L (50-60 ng/mL) that the 2011 Institute of Medicine Committee to Review Dietary Reference Intakes for Calcium and Vitamin D provided as the range corresponding to the tolerable upper intake level (UL) of 4,000 IU/d for vitamin D,” the authors of the new paper said.
Editorialists urge caution
In an accompanying editorial also published in the Annals of Internal Medicine, Malachi J. McKenna, MD, with the department of clinical chemistry, at St. Vincent’s University Hospital, and Mary A.T. Flynn, PhD, RD, with the Food Safety Authority of Ireland in Dublin, urge caution regarding vitamin D dosing.
They write that there are important distinctions between vitamin D supplements and vitamin D therapy, and the potential harms of high-dose vitamin D are still unclear.
“Vitamin D supplementation of 10 to 20 mcg (400 to 800 IU) daily can be applied safely at the population level to prevent skeletal and possibly nonskeletal disease. Very-high-dose vitamin D therapy might prevent type 2 diabetes in some patients but may also cause harm,” they note.
Dr. Pittas said in an interview that there have been some studies with high-dose vitamin D (up to 500,000 IU a year in one study) that reported an increased fall risk in older adults who had high fall risk. “However, these findings are not generalizable to other populations that are younger and at low or average fall risk, such as the prediabetes population to which the results of this meta-analysis apply,” he noted.
“The benefit-to-risk ratio for vitamin D depends on the target population and medical condition,” Dr. Pittas said. “The editorial refers to the NAM (National Academy of Medicine) vitamin D guidelines for the general, healthy population to promote bone health. The guidelines should not be extrapolated to specific populations, for example [patients with] prediabetes,” where the vitamin D benefit-to-risk ratio would be different from that in the general population.
Dr. Pittas and colleagues caution that the people studied in this meta-analysis were at high risk for type 2 diabetes, so these results do not apply to the general healthy population. The results also should not be extrapolated to people at average risk for any type of diabetes, they add.
Several physicians either declined to comment or did not respond to requests for comment on this research.
Dr. Pittas reports the National Institutes of Health and the American Diabetes Association made payments to his institution to conduct Vitamin D-related research. He is an unpaid cochair of the Endocrine Society’s Evaluation, Treatment and Prevention of Vitamin D Deficiency Clinical Practice Guideline team.
Coauthor Dr. Jorde reports grants from Novo Nordisk Foundation, North Norwegian Regional Health Authorities, and the Research Council of Norway.
Dr. Dawson-Hughes reports she is on the DSMB for AgNovos Healthcare. AgNovos is developing a bone implant to reduce hip fracture risk and she gets a stipend from the company. She reports Helsinn Therapeutics provided anamorelin and matching placebo for an NIH-funded clinical trial.
Dr. Trikalinos was supported by the D2d study. He is a technical methodological consultant to Latham and Watkins, who is retained by Pacira Pharmaceuticals.
Dr. Angellotti has been employed by Takeda and owns stock in the company.
The editorialists report no relevant financial relationships.
Results of the analysis, led by Anastassios G. Pittas, MD, MS, with the division of endocrinology, diabetes, and metabolism at Tufts Medical Center, in Boston, were published online in Annals of Internal Medicine (2023 Feb 7. doi: 10.7326/M22-3018).
All three eligible trials included in the analysis were randomized, double blinded, and placebo controlled. The three eligible trials tested three oral formulations of Vitamin D: cholecalciferol, 20,000 IU (500 mcg) weekly; cholecalciferol, 4,000 IU (100 mcg) daily; or eldecalcitol, 0.75 mcg daily, against placebos.
The authors of the new paper found that vitamin D reduced the risk for diabetes in people with prediabetes by a statistically significant 15% in adjusted analyses. The 3-year absolute risk reduction was 3.3%.
They found no difference in the rate ratios for adverse events (kidney stones, 1.17, 95% confidence interval, 0.69-1.99; hypercalcemia, 2.34; 95% CI, 0.83-6.66]; hypercalciuria, 1.65; 95% CI, 0.83-3.28]; death, 0.85; 95% CI, 0.31-2.36]) when study participants got vitamin D instead of placebo.
Differences from previous analyses
The relationship between vitamin D levels and risk for type 2 diabetes has been studied in previous trials and results have been mixed.
The authors note that two previous meta-analyses included trials “that had relatively short durations for assessment of diabetes risk (for example, ≤ 1 year), had high risk of bias (for example, open-label trials), or were not specifically designed and conducted for primary prevention of type 2 diabetes, potentially undermining the validity of the results.”
Each of the trials in this meta-analysis had a low risk of bias as determined by the Cochrane risk-of-bias tool, Dr. Pittas and colleagues said.
“The present study does not reach an opposite conclusion from the D2d study,” said Dr. Pittas, who coauthored that paper as well. “Rather, it confirms the results of the D2d study. In D2d and two other similar vitamin D and diabetes prevention trials (one in Norway and one in Japan), vitamin D reduced the rate of progression to diabetes in adults with prediabetes, but the observed differences were not statistically significant because the reported relative risk reductions (10%-13%) were smaller than each trial was powered to detect (25%-36%).”
“Individual participant data meta-analyses increase the statistical power to detect an effect. After combining data, we found that vitamin D reduced the risk of progression from prediabetes to diabetes by 15% and this result was statistically significant. So, the conclusion of the meta-analysis is essentially the same conclusion as in D2d and the other two trials. The difference is that the result is now statistically significant,” Dr. Pittas added.
Small reduction but large population
The authors acknowledged that the absolute risk reduction number is small, especially when compared with the risk reduction seen with intensive lifestyle changes (58%) and metformin (31%), as reported in an article published in the New England of Journal of Medicine (2002 Feb 7;346:393-403). But “extrapolating to the more than 374 million adults worldwide who have prediabetes suggests that inexpensive vitamin D supplementation could delay the development of diabetes in more than 10 million people,” they said.
As for how high vitamin D levels need to be, the authors write that their research indicates that the optimal level of vitamin D in the blood needed to reduce diabetes risk may be higher than an Institute of Medicine committee recommendation in 2011.
“The blood 25-hydroxy vitamin D level needed to optimally reduce diabetes risk may be near and possibly above the range of 125-150 nmol/L (50-60 ng/mL) that the 2011 Institute of Medicine Committee to Review Dietary Reference Intakes for Calcium and Vitamin D provided as the range corresponding to the tolerable upper intake level (UL) of 4,000 IU/d for vitamin D,” the authors of the new paper said.
Editorialists urge caution
In an accompanying editorial also published in the Annals of Internal Medicine, Malachi J. McKenna, MD, with the department of clinical chemistry, at St. Vincent’s University Hospital, and Mary A.T. Flynn, PhD, RD, with the Food Safety Authority of Ireland in Dublin, urge caution regarding vitamin D dosing.
They write that there are important distinctions between vitamin D supplements and vitamin D therapy, and the potential harms of high-dose vitamin D are still unclear.
“Vitamin D supplementation of 10 to 20 mcg (400 to 800 IU) daily can be applied safely at the population level to prevent skeletal and possibly nonskeletal disease. Very-high-dose vitamin D therapy might prevent type 2 diabetes in some patients but may also cause harm,” they note.
Dr. Pittas said in an interview that there have been some studies with high-dose vitamin D (up to 500,000 IU a year in one study) that reported an increased fall risk in older adults who had high fall risk. “However, these findings are not generalizable to other populations that are younger and at low or average fall risk, such as the prediabetes population to which the results of this meta-analysis apply,” he noted.
“The benefit-to-risk ratio for vitamin D depends on the target population and medical condition,” Dr. Pittas said. “The editorial refers to the NAM (National Academy of Medicine) vitamin D guidelines for the general, healthy population to promote bone health. The guidelines should not be extrapolated to specific populations, for example [patients with] prediabetes,” where the vitamin D benefit-to-risk ratio would be different from that in the general population.
Dr. Pittas and colleagues caution that the people studied in this meta-analysis were at high risk for type 2 diabetes, so these results do not apply to the general healthy population. The results also should not be extrapolated to people at average risk for any type of diabetes, they add.
Several physicians either declined to comment or did not respond to requests for comment on this research.
Dr. Pittas reports the National Institutes of Health and the American Diabetes Association made payments to his institution to conduct Vitamin D-related research. He is an unpaid cochair of the Endocrine Society’s Evaluation, Treatment and Prevention of Vitamin D Deficiency Clinical Practice Guideline team.
Coauthor Dr. Jorde reports grants from Novo Nordisk Foundation, North Norwegian Regional Health Authorities, and the Research Council of Norway.
Dr. Dawson-Hughes reports she is on the DSMB for AgNovos Healthcare. AgNovos is developing a bone implant to reduce hip fracture risk and she gets a stipend from the company. She reports Helsinn Therapeutics provided anamorelin and matching placebo for an NIH-funded clinical trial.
Dr. Trikalinos was supported by the D2d study. He is a technical methodological consultant to Latham and Watkins, who is retained by Pacira Pharmaceuticals.
Dr. Angellotti has been employed by Takeda and owns stock in the company.
The editorialists report no relevant financial relationships.
Results of the analysis, led by Anastassios G. Pittas, MD, MS, with the division of endocrinology, diabetes, and metabolism at Tufts Medical Center, in Boston, were published online in Annals of Internal Medicine (2023 Feb 7. doi: 10.7326/M22-3018).
All three eligible trials included in the analysis were randomized, double blinded, and placebo controlled. The three eligible trials tested three oral formulations of Vitamin D: cholecalciferol, 20,000 IU (500 mcg) weekly; cholecalciferol, 4,000 IU (100 mcg) daily; or eldecalcitol, 0.75 mcg daily, against placebos.
The authors of the new paper found that vitamin D reduced the risk for diabetes in people with prediabetes by a statistically significant 15% in adjusted analyses. The 3-year absolute risk reduction was 3.3%.
They found no difference in the rate ratios for adverse events (kidney stones, 1.17, 95% confidence interval, 0.69-1.99; hypercalcemia, 2.34; 95% CI, 0.83-6.66]; hypercalciuria, 1.65; 95% CI, 0.83-3.28]; death, 0.85; 95% CI, 0.31-2.36]) when study participants got vitamin D instead of placebo.
Differences from previous analyses
The relationship between vitamin D levels and risk for type 2 diabetes has been studied in previous trials and results have been mixed.
The authors note that two previous meta-analyses included trials “that had relatively short durations for assessment of diabetes risk (for example, ≤ 1 year), had high risk of bias (for example, open-label trials), or were not specifically designed and conducted for primary prevention of type 2 diabetes, potentially undermining the validity of the results.”
Each of the trials in this meta-analysis had a low risk of bias as determined by the Cochrane risk-of-bias tool, Dr. Pittas and colleagues said.
“The present study does not reach an opposite conclusion from the D2d study,” said Dr. Pittas, who coauthored that paper as well. “Rather, it confirms the results of the D2d study. In D2d and two other similar vitamin D and diabetes prevention trials (one in Norway and one in Japan), vitamin D reduced the rate of progression to diabetes in adults with prediabetes, but the observed differences were not statistically significant because the reported relative risk reductions (10%-13%) were smaller than each trial was powered to detect (25%-36%).”
“Individual participant data meta-analyses increase the statistical power to detect an effect. After combining data, we found that vitamin D reduced the risk of progression from prediabetes to diabetes by 15% and this result was statistically significant. So, the conclusion of the meta-analysis is essentially the same conclusion as in D2d and the other two trials. The difference is that the result is now statistically significant,” Dr. Pittas added.
Small reduction but large population
The authors acknowledged that the absolute risk reduction number is small, especially when compared with the risk reduction seen with intensive lifestyle changes (58%) and metformin (31%), as reported in an article published in the New England of Journal of Medicine (2002 Feb 7;346:393-403). But “extrapolating to the more than 374 million adults worldwide who have prediabetes suggests that inexpensive vitamin D supplementation could delay the development of diabetes in more than 10 million people,” they said.
As for how high vitamin D levels need to be, the authors write that their research indicates that the optimal level of vitamin D in the blood needed to reduce diabetes risk may be higher than an Institute of Medicine committee recommendation in 2011.
“The blood 25-hydroxy vitamin D level needed to optimally reduce diabetes risk may be near and possibly above the range of 125-150 nmol/L (50-60 ng/mL) that the 2011 Institute of Medicine Committee to Review Dietary Reference Intakes for Calcium and Vitamin D provided as the range corresponding to the tolerable upper intake level (UL) of 4,000 IU/d for vitamin D,” the authors of the new paper said.
Editorialists urge caution
In an accompanying editorial also published in the Annals of Internal Medicine, Malachi J. McKenna, MD, with the department of clinical chemistry, at St. Vincent’s University Hospital, and Mary A.T. Flynn, PhD, RD, with the Food Safety Authority of Ireland in Dublin, urge caution regarding vitamin D dosing.
They write that there are important distinctions between vitamin D supplements and vitamin D therapy, and the potential harms of high-dose vitamin D are still unclear.
“Vitamin D supplementation of 10 to 20 mcg (400 to 800 IU) daily can be applied safely at the population level to prevent skeletal and possibly nonskeletal disease. Very-high-dose vitamin D therapy might prevent type 2 diabetes in some patients but may also cause harm,” they note.
Dr. Pittas said in an interview that there have been some studies with high-dose vitamin D (up to 500,000 IU a year in one study) that reported an increased fall risk in older adults who had high fall risk. “However, these findings are not generalizable to other populations that are younger and at low or average fall risk, such as the prediabetes population to which the results of this meta-analysis apply,” he noted.
“The benefit-to-risk ratio for vitamin D depends on the target population and medical condition,” Dr. Pittas said. “The editorial refers to the NAM (National Academy of Medicine) vitamin D guidelines for the general, healthy population to promote bone health. The guidelines should not be extrapolated to specific populations, for example [patients with] prediabetes,” where the vitamin D benefit-to-risk ratio would be different from that in the general population.
Dr. Pittas and colleagues caution that the people studied in this meta-analysis were at high risk for type 2 diabetes, so these results do not apply to the general healthy population. The results also should not be extrapolated to people at average risk for any type of diabetes, they add.
Several physicians either declined to comment or did not respond to requests for comment on this research.
Dr. Pittas reports the National Institutes of Health and the American Diabetes Association made payments to his institution to conduct Vitamin D-related research. He is an unpaid cochair of the Endocrine Society’s Evaluation, Treatment and Prevention of Vitamin D Deficiency Clinical Practice Guideline team.
Coauthor Dr. Jorde reports grants from Novo Nordisk Foundation, North Norwegian Regional Health Authorities, and the Research Council of Norway.
Dr. Dawson-Hughes reports she is on the DSMB for AgNovos Healthcare. AgNovos is developing a bone implant to reduce hip fracture risk and she gets a stipend from the company. She reports Helsinn Therapeutics provided anamorelin and matching placebo for an NIH-funded clinical trial.
Dr. Trikalinos was supported by the D2d study. He is a technical methodological consultant to Latham and Watkins, who is retained by Pacira Pharmaceuticals.
Dr. Angellotti has been employed by Takeda and owns stock in the company.
The editorialists report no relevant financial relationships.
FROM ANNALS OF INTERNAL MEDICINE
Almonds may be a good diet option
according to researchers at the University of South Australia’s Alliance for Research in Exercise, Nutrition and Activity.
What to know
People who consume as few as 30-50 g of almonds, as opposed to an energy-equivalent carbohydrate snack, can lower their energy intake significantly at the subsequent meal.
People who eat almonds can experience changes in their appetite-regulating hormones that may contribute to less food intake.
Almond consumption can lower C-peptide responses, which can improve insulin sensitivity and reduce the risk of developing diabetes and cardiovascular disease.
Eating almonds can raise levels of glucose-dependent insulinotropic polypeptide glucagon, which can send satiety signals to the brain, and pancreatic polypeptide, which slows digestion, which may reduce food intake, supporting weight loss.
Almonds are high in protein, fiber, and unsaturated fatty acids, which may contribute to their satiating properties and help explain why fewer calories are consumed.
A version of this article originally appeared on Medscape.com.
This is a summary of the article “Acute Feeding With Almonds Compared to a Carbohydrate-Based Snack Improves Appetite-Regulating Hormones With No Effect on Self-reported Appetite Sensations: A Randomised Controlled Trial,” published in the European Journal of Nutrition on Oct. 11, 2022. The full article can be found on link.springer.com.
according to researchers at the University of South Australia’s Alliance for Research in Exercise, Nutrition and Activity.
What to know
People who consume as few as 30-50 g of almonds, as opposed to an energy-equivalent carbohydrate snack, can lower their energy intake significantly at the subsequent meal.
People who eat almonds can experience changes in their appetite-regulating hormones that may contribute to less food intake.
Almond consumption can lower C-peptide responses, which can improve insulin sensitivity and reduce the risk of developing diabetes and cardiovascular disease.
Eating almonds can raise levels of glucose-dependent insulinotropic polypeptide glucagon, which can send satiety signals to the brain, and pancreatic polypeptide, which slows digestion, which may reduce food intake, supporting weight loss.
Almonds are high in protein, fiber, and unsaturated fatty acids, which may contribute to their satiating properties and help explain why fewer calories are consumed.
A version of this article originally appeared on Medscape.com.
This is a summary of the article “Acute Feeding With Almonds Compared to a Carbohydrate-Based Snack Improves Appetite-Regulating Hormones With No Effect on Self-reported Appetite Sensations: A Randomised Controlled Trial,” published in the European Journal of Nutrition on Oct. 11, 2022. The full article can be found on link.springer.com.
according to researchers at the University of South Australia’s Alliance for Research in Exercise, Nutrition and Activity.
What to know
People who consume as few as 30-50 g of almonds, as opposed to an energy-equivalent carbohydrate snack, can lower their energy intake significantly at the subsequent meal.
People who eat almonds can experience changes in their appetite-regulating hormones that may contribute to less food intake.
Almond consumption can lower C-peptide responses, which can improve insulin sensitivity and reduce the risk of developing diabetes and cardiovascular disease.
Eating almonds can raise levels of glucose-dependent insulinotropic polypeptide glucagon, which can send satiety signals to the brain, and pancreatic polypeptide, which slows digestion, which may reduce food intake, supporting weight loss.
Almonds are high in protein, fiber, and unsaturated fatty acids, which may contribute to their satiating properties and help explain why fewer calories are consumed.
A version of this article originally appeared on Medscape.com.
This is a summary of the article “Acute Feeding With Almonds Compared to a Carbohydrate-Based Snack Improves Appetite-Regulating Hormones With No Effect on Self-reported Appetite Sensations: A Randomised Controlled Trial,” published in the European Journal of Nutrition on Oct. 11, 2022. The full article can be found on link.springer.com.
FROM THE EUROPEAN JOURNAL OF NUTRITION
Commenting on weight’s not rude. It’s dangerous.
It was the start of the fall semester of my sophomore year of college.
At my small women’s college, the previous semester’s gossip had been about our classmate, S*. She had gone from being very thin to noticeably gaining a lot of weight in a few months. The rumors were that S was pregnant and gave birth over summer break. As a busy biology premed major, this was my first time hearing the news. So when I saw her standing in the hallway, back to her previous weight, I was excited for her.
In true extravert fashion, I commented on the baby and her new size. But no sooner had the words left my mouth than I regretted them.
The hall grew awkwardly silent as S’s face flushed and she asked, “Excuse me?!” Instantly I knew that the rumors weren’t true.
Thankfully, at that moment, the classroom opened and we walked in. Whew! After class, S asked if we could talk. She explained that she had a thyroid tumor and struggled to adjust to the treatments, which caused her weight fluctuations. She had never been pregnant.
My awkward statement had been the first time anyone on campus had directly mentioned her weight, though she suspected that people were talking about her. We became fast friends after this rocky beginning. Although we lost touch after college, S taught me an invaluable lesson about making assumptions about people’s weight: Ask before you assume.
Now, years later, as an internist and obesity specialist, this lesson continues to be reinforced daily.
In daily life, comments about weight can be perceived as rude. In the clinical setting, however, assumptions about weight are a form of weight bias. Weight bias can lead to weight stigma and even be dangerous to health care.
Let’s discuss the insidious influence of weight bias in health care through two commonly used phrases and then look at a few solutions to address weight bias in health care individually and systematically.
Common weight bias assumptions
“Great job, you lost weight!” In checking your patient’s vital signs, you notice that this patient with obesity has a significant weight change. You congratulate them upon entering the room. Unfortunately, their weight loss was a result of minimal eating after losing a loved one. This isn’t healthy weight loss. One of the adverse effects of weight bias is that it infers that weight loss is always a good thing, especially in people with larger bodies. This is a dangerous presumption. Let’s remember that the body favors fat storage, hence why “unintentional weight loss” is a recognized medical condition prompting evaluation. We have to be careful not to celebrate weight loss “at all costs,” such as fad diets that haven’t been shown to improve health outcomes.
Furthermore, patients who lose weight quickly (more than 4-8 lb/month) require closer follow-up and evaluation for secondary causes of weight loss. Patients may lose weight at a faster rate with the new antiobesity medications, but clinicians still should ensure that age-appropriate health maintenance screening is done and be vigilant for secondary causes of weight changes.
“Have you tried losing weight yet?” Three times. That’s how many times Chanté Burkett went to her doctor about her painful, enlarging firm stomach. She was advised to continue working on weight loss, which she did diligently. But Ms. Burkett’s abdomen kept growing and her concerns were dismissed. A visit to urgent care and a CT scan revealed that Ms. Burkett’s excess abdominal “fat” was a 13-lb mucinous cystadenoma. Sadly, cases like hers aren’t rare, isolated events. Weight bias can cause anchoring on one diagnosis, preventing consideration of other diagnostic possibilities. Even worse, anchoring will lead to the wrong intervention, such as prescribing weight loss for presumed increased adiposity instead of ordering the appropriate testing.
It’s also essential to recognize that, even if someone does have the disease of obesity, weight loss isn’t the solution to every medical concern. Even if weight loss is helpful, other, more pressing treatments may still be necessary. Telling a person with obesity who has an acute complaint to “just lose weight” is comparable to telling a patient with coronary artery disease who presents with an 80% vessel occlusion and chest pain to follow a low-fat diet. In both cases, you need to address the acute concern appropriately, then focus on the chronic treatment.
Ways to reduce clinical weight bias
How do you reduce clinical weight bias?
Ask, don’t assume. The information from the scale is simply data. Instead of judging it positively or negatively and creating a story, ask the patient. An unbiased way to approach the conversation is to say, “Great to see you. You seem [positive adjective of choice]. How have you been?” Wait until the vitals section to objectively discuss weight unless the patient offers the discussion earlier or their chief complaint lists a weight-related concern.
Order necessary tests to evaluate weight. Weight is the vital sign that people wear externally, so we feel that we can readily interpret it without any further assessment. However, resist the urge to interpret scale data without context. Keeping an open mind helps prevent anchoring and missing critical clues in the clinical history.
Address weight changes effectively. Sometimes there is an indication to prescribe weight loss as part of the treatment plan. However, remember that weight loss isn’t simply “calories in vs. calories out.” Obesity is a complex medical disease that requires a multimodal treatment approach. As clinicians, we have access to the most powerful tools for weight loss. Unfortunately, weight bias contributes to limited prescribing of metabolic medications (“antiobesity medications” or AOMs). In addition, systemic weight bias prevents insurance coverage of AOMs. The Treat and Reduce Obesity Act has been introduced into Congress to help improve life-transforming access to AOMs.
Acknowledge your bias. Our experiences make us all susceptible to bias. The Harvard Weight Implicit Association Test is free and a helpful way to assess your level of weight bias. I take it annually to ensure that I remain objective in my practice.
Addressing weight bias needs to extend beyond the individual level.
Systemically, health care needs to address the following:
Language. Use people-centered language. For example, “People aren’t obese. They have obesity.”
Accessibility. Health care settings must be comfortable and accessible for people of all sizes. Furthermore, improvements to access the services that comprehensive obesity care requires, such as AOMs, bariatric procedures and bariatric surgery, mental health care, nutrition, fitness specialists, health coaches, and more, are needed.
Education. Medical students and trainees have to learn the newest obesity science and know how to treat obesity effectively. Acknowledge and address biased tools. Recent data have shown that some of our screening tools, such as body mass index, have inherent bias. It’s time to focus on using improved diagnostic tools and personalized treatments.
We are at a pivotal time in our scientific understanding of body weight regulation and the disease of obesity. Clinical weight bias is primarily rooted in flawed science influenced by biased cultural norms and other forms of discrimination, such as racial and gender bias. We must move past assumptions to give our patients the optimal individualized care they need. So next time you observe a weight change, instead of commenting on their weight, say, “Great to see you! How have you been?”
S*: Initial has been changed to protect privacy.
Dr. Gonsahn-Bollie is an integrative obesity specialist focused on individualized solutions for emotional and biological overeating. Connect with her at www.embraceyouweightloss.com or on Instagram @embraceyoumd. Her bestselling book, “Embrace You: Your Guide to Transforming Weight Loss Misconceptions Into Lifelong Wellness”, was Healthline.com’s Best Overall Weight Loss Book of 2022 and one of Livestrong.com’s 8 Best Weight-Loss Books to Read in 2022. She has disclosed no relevant financial relationships. A version of this article originally appeared on Medscape.com.
It was the start of the fall semester of my sophomore year of college.
At my small women’s college, the previous semester’s gossip had been about our classmate, S*. She had gone from being very thin to noticeably gaining a lot of weight in a few months. The rumors were that S was pregnant and gave birth over summer break. As a busy biology premed major, this was my first time hearing the news. So when I saw her standing in the hallway, back to her previous weight, I was excited for her.
In true extravert fashion, I commented on the baby and her new size. But no sooner had the words left my mouth than I regretted them.
The hall grew awkwardly silent as S’s face flushed and she asked, “Excuse me?!” Instantly I knew that the rumors weren’t true.
Thankfully, at that moment, the classroom opened and we walked in. Whew! After class, S asked if we could talk. She explained that she had a thyroid tumor and struggled to adjust to the treatments, which caused her weight fluctuations. She had never been pregnant.
My awkward statement had been the first time anyone on campus had directly mentioned her weight, though she suspected that people were talking about her. We became fast friends after this rocky beginning. Although we lost touch after college, S taught me an invaluable lesson about making assumptions about people’s weight: Ask before you assume.
Now, years later, as an internist and obesity specialist, this lesson continues to be reinforced daily.
In daily life, comments about weight can be perceived as rude. In the clinical setting, however, assumptions about weight are a form of weight bias. Weight bias can lead to weight stigma and even be dangerous to health care.
Let’s discuss the insidious influence of weight bias in health care through two commonly used phrases and then look at a few solutions to address weight bias in health care individually and systematically.
Common weight bias assumptions
“Great job, you lost weight!” In checking your patient’s vital signs, you notice that this patient with obesity has a significant weight change. You congratulate them upon entering the room. Unfortunately, their weight loss was a result of minimal eating after losing a loved one. This isn’t healthy weight loss. One of the adverse effects of weight bias is that it infers that weight loss is always a good thing, especially in people with larger bodies. This is a dangerous presumption. Let’s remember that the body favors fat storage, hence why “unintentional weight loss” is a recognized medical condition prompting evaluation. We have to be careful not to celebrate weight loss “at all costs,” such as fad diets that haven’t been shown to improve health outcomes.
Furthermore, patients who lose weight quickly (more than 4-8 lb/month) require closer follow-up and evaluation for secondary causes of weight loss. Patients may lose weight at a faster rate with the new antiobesity medications, but clinicians still should ensure that age-appropriate health maintenance screening is done and be vigilant for secondary causes of weight changes.
“Have you tried losing weight yet?” Three times. That’s how many times Chanté Burkett went to her doctor about her painful, enlarging firm stomach. She was advised to continue working on weight loss, which she did diligently. But Ms. Burkett’s abdomen kept growing and her concerns were dismissed. A visit to urgent care and a CT scan revealed that Ms. Burkett’s excess abdominal “fat” was a 13-lb mucinous cystadenoma. Sadly, cases like hers aren’t rare, isolated events. Weight bias can cause anchoring on one diagnosis, preventing consideration of other diagnostic possibilities. Even worse, anchoring will lead to the wrong intervention, such as prescribing weight loss for presumed increased adiposity instead of ordering the appropriate testing.
It’s also essential to recognize that, even if someone does have the disease of obesity, weight loss isn’t the solution to every medical concern. Even if weight loss is helpful, other, more pressing treatments may still be necessary. Telling a person with obesity who has an acute complaint to “just lose weight” is comparable to telling a patient with coronary artery disease who presents with an 80% vessel occlusion and chest pain to follow a low-fat diet. In both cases, you need to address the acute concern appropriately, then focus on the chronic treatment.
Ways to reduce clinical weight bias
How do you reduce clinical weight bias?
Ask, don’t assume. The information from the scale is simply data. Instead of judging it positively or negatively and creating a story, ask the patient. An unbiased way to approach the conversation is to say, “Great to see you. You seem [positive adjective of choice]. How have you been?” Wait until the vitals section to objectively discuss weight unless the patient offers the discussion earlier or their chief complaint lists a weight-related concern.
Order necessary tests to evaluate weight. Weight is the vital sign that people wear externally, so we feel that we can readily interpret it without any further assessment. However, resist the urge to interpret scale data without context. Keeping an open mind helps prevent anchoring and missing critical clues in the clinical history.
Address weight changes effectively. Sometimes there is an indication to prescribe weight loss as part of the treatment plan. However, remember that weight loss isn’t simply “calories in vs. calories out.” Obesity is a complex medical disease that requires a multimodal treatment approach. As clinicians, we have access to the most powerful tools for weight loss. Unfortunately, weight bias contributes to limited prescribing of metabolic medications (“antiobesity medications” or AOMs). In addition, systemic weight bias prevents insurance coverage of AOMs. The Treat and Reduce Obesity Act has been introduced into Congress to help improve life-transforming access to AOMs.
Acknowledge your bias. Our experiences make us all susceptible to bias. The Harvard Weight Implicit Association Test is free and a helpful way to assess your level of weight bias. I take it annually to ensure that I remain objective in my practice.
Addressing weight bias needs to extend beyond the individual level.
Systemically, health care needs to address the following:
Language. Use people-centered language. For example, “People aren’t obese. They have obesity.”
Accessibility. Health care settings must be comfortable and accessible for people of all sizes. Furthermore, improvements to access the services that comprehensive obesity care requires, such as AOMs, bariatric procedures and bariatric surgery, mental health care, nutrition, fitness specialists, health coaches, and more, are needed.
Education. Medical students and trainees have to learn the newest obesity science and know how to treat obesity effectively. Acknowledge and address biased tools. Recent data have shown that some of our screening tools, such as body mass index, have inherent bias. It’s time to focus on using improved diagnostic tools and personalized treatments.
We are at a pivotal time in our scientific understanding of body weight regulation and the disease of obesity. Clinical weight bias is primarily rooted in flawed science influenced by biased cultural norms and other forms of discrimination, such as racial and gender bias. We must move past assumptions to give our patients the optimal individualized care they need. So next time you observe a weight change, instead of commenting on their weight, say, “Great to see you! How have you been?”
S*: Initial has been changed to protect privacy.
Dr. Gonsahn-Bollie is an integrative obesity specialist focused on individualized solutions for emotional and biological overeating. Connect with her at www.embraceyouweightloss.com or on Instagram @embraceyoumd. Her bestselling book, “Embrace You: Your Guide to Transforming Weight Loss Misconceptions Into Lifelong Wellness”, was Healthline.com’s Best Overall Weight Loss Book of 2022 and one of Livestrong.com’s 8 Best Weight-Loss Books to Read in 2022. She has disclosed no relevant financial relationships. A version of this article originally appeared on Medscape.com.
It was the start of the fall semester of my sophomore year of college.
At my small women’s college, the previous semester’s gossip had been about our classmate, S*. She had gone from being very thin to noticeably gaining a lot of weight in a few months. The rumors were that S was pregnant and gave birth over summer break. As a busy biology premed major, this was my first time hearing the news. So when I saw her standing in the hallway, back to her previous weight, I was excited for her.
In true extravert fashion, I commented on the baby and her new size. But no sooner had the words left my mouth than I regretted them.
The hall grew awkwardly silent as S’s face flushed and she asked, “Excuse me?!” Instantly I knew that the rumors weren’t true.
Thankfully, at that moment, the classroom opened and we walked in. Whew! After class, S asked if we could talk. She explained that she had a thyroid tumor and struggled to adjust to the treatments, which caused her weight fluctuations. She had never been pregnant.
My awkward statement had been the first time anyone on campus had directly mentioned her weight, though she suspected that people were talking about her. We became fast friends after this rocky beginning. Although we lost touch after college, S taught me an invaluable lesson about making assumptions about people’s weight: Ask before you assume.
Now, years later, as an internist and obesity specialist, this lesson continues to be reinforced daily.
In daily life, comments about weight can be perceived as rude. In the clinical setting, however, assumptions about weight are a form of weight bias. Weight bias can lead to weight stigma and even be dangerous to health care.
Let’s discuss the insidious influence of weight bias in health care through two commonly used phrases and then look at a few solutions to address weight bias in health care individually and systematically.
Common weight bias assumptions
“Great job, you lost weight!” In checking your patient’s vital signs, you notice that this patient with obesity has a significant weight change. You congratulate them upon entering the room. Unfortunately, their weight loss was a result of minimal eating after losing a loved one. This isn’t healthy weight loss. One of the adverse effects of weight bias is that it infers that weight loss is always a good thing, especially in people with larger bodies. This is a dangerous presumption. Let’s remember that the body favors fat storage, hence why “unintentional weight loss” is a recognized medical condition prompting evaluation. We have to be careful not to celebrate weight loss “at all costs,” such as fad diets that haven’t been shown to improve health outcomes.
Furthermore, patients who lose weight quickly (more than 4-8 lb/month) require closer follow-up and evaluation for secondary causes of weight loss. Patients may lose weight at a faster rate with the new antiobesity medications, but clinicians still should ensure that age-appropriate health maintenance screening is done and be vigilant for secondary causes of weight changes.
“Have you tried losing weight yet?” Three times. That’s how many times Chanté Burkett went to her doctor about her painful, enlarging firm stomach. She was advised to continue working on weight loss, which she did diligently. But Ms. Burkett’s abdomen kept growing and her concerns were dismissed. A visit to urgent care and a CT scan revealed that Ms. Burkett’s excess abdominal “fat” was a 13-lb mucinous cystadenoma. Sadly, cases like hers aren’t rare, isolated events. Weight bias can cause anchoring on one diagnosis, preventing consideration of other diagnostic possibilities. Even worse, anchoring will lead to the wrong intervention, such as prescribing weight loss for presumed increased adiposity instead of ordering the appropriate testing.
It’s also essential to recognize that, even if someone does have the disease of obesity, weight loss isn’t the solution to every medical concern. Even if weight loss is helpful, other, more pressing treatments may still be necessary. Telling a person with obesity who has an acute complaint to “just lose weight” is comparable to telling a patient with coronary artery disease who presents with an 80% vessel occlusion and chest pain to follow a low-fat diet. In both cases, you need to address the acute concern appropriately, then focus on the chronic treatment.
Ways to reduce clinical weight bias
How do you reduce clinical weight bias?
Ask, don’t assume. The information from the scale is simply data. Instead of judging it positively or negatively and creating a story, ask the patient. An unbiased way to approach the conversation is to say, “Great to see you. You seem [positive adjective of choice]. How have you been?” Wait until the vitals section to objectively discuss weight unless the patient offers the discussion earlier or their chief complaint lists a weight-related concern.
Order necessary tests to evaluate weight. Weight is the vital sign that people wear externally, so we feel that we can readily interpret it without any further assessment. However, resist the urge to interpret scale data without context. Keeping an open mind helps prevent anchoring and missing critical clues in the clinical history.
Address weight changes effectively. Sometimes there is an indication to prescribe weight loss as part of the treatment plan. However, remember that weight loss isn’t simply “calories in vs. calories out.” Obesity is a complex medical disease that requires a multimodal treatment approach. As clinicians, we have access to the most powerful tools for weight loss. Unfortunately, weight bias contributes to limited prescribing of metabolic medications (“antiobesity medications” or AOMs). In addition, systemic weight bias prevents insurance coverage of AOMs. The Treat and Reduce Obesity Act has been introduced into Congress to help improve life-transforming access to AOMs.
Acknowledge your bias. Our experiences make us all susceptible to bias. The Harvard Weight Implicit Association Test is free and a helpful way to assess your level of weight bias. I take it annually to ensure that I remain objective in my practice.
Addressing weight bias needs to extend beyond the individual level.
Systemically, health care needs to address the following:
Language. Use people-centered language. For example, “People aren’t obese. They have obesity.”
Accessibility. Health care settings must be comfortable and accessible for people of all sizes. Furthermore, improvements to access the services that comprehensive obesity care requires, such as AOMs, bariatric procedures and bariatric surgery, mental health care, nutrition, fitness specialists, health coaches, and more, are needed.
Education. Medical students and trainees have to learn the newest obesity science and know how to treat obesity effectively. Acknowledge and address biased tools. Recent data have shown that some of our screening tools, such as body mass index, have inherent bias. It’s time to focus on using improved diagnostic tools and personalized treatments.
We are at a pivotal time in our scientific understanding of body weight regulation and the disease of obesity. Clinical weight bias is primarily rooted in flawed science influenced by biased cultural norms and other forms of discrimination, such as racial and gender bias. We must move past assumptions to give our patients the optimal individualized care they need. So next time you observe a weight change, instead of commenting on their weight, say, “Great to see you! How have you been?”
S*: Initial has been changed to protect privacy.
Dr. Gonsahn-Bollie is an integrative obesity specialist focused on individualized solutions for emotional and biological overeating. Connect with her at www.embraceyouweightloss.com or on Instagram @embraceyoumd. Her bestselling book, “Embrace You: Your Guide to Transforming Weight Loss Misconceptions Into Lifelong Wellness”, was Healthline.com’s Best Overall Weight Loss Book of 2022 and one of Livestrong.com’s 8 Best Weight-Loss Books to Read in 2022. She has disclosed no relevant financial relationships. A version of this article originally appeared on Medscape.com.
Exercise halves T2D risk in adults with obesity
“Physical exercise combined with diet restriction has been proven to be effective in prevention of diabetes. However, the long-term effect of exercise on prevention of diabetes, and the difference of exercise intensity in prevention of diabetes have not been well studied,” said corresponding author Xiaoying Li, MD, of Zhongshan Hospital, Fudan University, Shanghai, in an interview.
In the research letter published in JAMA Internal Medicine, Dr. Li and colleagues analyzed the results of a study of 220 adults with central obesity and nonalcoholic fatty liver disease, but no incident diabetes, randomized to a 12-month program of vigorous exercise (73 patients), moderate aerobic exercise (73 patients) or no exercise (74 patients).
A total of 208 participants completed the 1-year intervention; of these, 195 and 178 remained to provide data at 2 years and 10 years, respectively. The mean age of the participants was 53.9 years, 32.3% were male, and the mean waist circumference was 96.1 cm at baseline.
The cumulative incidence of type 2 diabetes in the vigorous exercise, moderate exercise, and nonexercise groups was 2.1 per 100 person-years 1.9 per 100 person-years, and 4.1 per 100 person-years, respectively, over the 10-year follow-up period. This translated to a reduction in type 2 diabetes risk of 49% in the vigorous exercise group and 53% in the moderate exercise group compared with the nonexercise group.
In addition, individuals in the vigorous and moderate exercise groups significantly reduced their HbA1c and waist circumference compared with the nonexercisers. Levels of plasma fasting glucose and weight regain were lower in both exercise groups compared with nonexercisers, but these differences were not significant.
The exercise intervention was described in a 2016 study, which was also published in JAMA Internal Medicine. That study’s purpose was to compare the effects of exercise on patients with nonalcoholic fatty liver disease. Participants were coached and supervised for their exercise programs. The program for the vigorous group involved jogging for 150 minutes per week at 65%-80% of maximum heart rate for 6 months and brisk walking 150 minutes per week at 45%-55% of maximum heart rate for another 6 months. The program for the moderate exercise group involved brisk walking 150 minutes per week for 12 months.
Both exercise groups showed a trend towards higher levels of leisure time physical activity after 10 years compared with the nonexercise groups, although the difference was not significant.
The main limitation of the study was that incident prediabetes was not prespecified, which may have led to some confounding, the researchers noted. In addition, the participants were highly supervised for a 12-month program only. However, the results support the long-term value of physical exercise as a method of obesity management and to delay progression to type 2 diabetes in obese individuals, they said. Vigorous and moderate aerobic exercise programs could be implemented for this patient population, they concluded.
“Surprisingly, our findings demonstrated that a 12-month vigorous aerobic exercise or moderate aerobic exercise could significantly reduce the risk of incident diabetes by 50% over the 10-year follow-up,” Dr. Li said in an interview. The results suggest that physical exercise for some period of time can produce a long-term beneficial effect in prevention of type 2 diabetes, he said.
Potential barriers to the routine use of an exercise intervention in patients with obesity include the unwillingness of this population to engage in vigorous exercise, and the potential for musculoskeletal injury, said Dr. Li. In these cases, obese patients should be encouraged to pursue moderate exercise, Dr. Li said.
Looking ahead, more research is needed to examine the potential mechanism behind the effect of exercise on diabetes prevention, said Dr. Li.
Findings fill gap in long-term outcome data
The current study is important because of the long-term follow-up data, said Jill Kanaley, PhD, professor and interim chair of nutrition and exercise physiology at the University of Missouri, in an interview. “We seldom follow up on our training studies, thus it is important to see if there is any long-term impact of these interventions,” she said.
Dr. Kanaley said she was surprised to see the residual benefits of the exercise intervention 10 years later.
“We often wonder how long the impact of the exercise training will stay with someone so that they continue to exercise and watch their weight; this study seems to indicate that there is an educational component that stays with them,” she said.
The main clinical takeaway from the current study was the minimal weight gain over time, Dr. Kanaley said.
Although time may be a barrier to the routine use of an exercise intervention, patients have to realize that they can usually find the time, especially given the multiple benefits, said Dr. Kanaley. “The exercise interventions provide more benefits than just weight control and glucose levels,” she said.
“The 30-60 minutes of exercise does not have to come all at the same time,” Dr. Kanaley noted. “It could be three 15-minute bouts of exercise/physical activity to get their 45 minutes in,” she noted. Exercise does not have to be heavy vigorous exercise, even walking is beneficial, she said. For people who complain of boredom with an exercise routine, Dr. Kanaley encourages mixing it up, with activities such as different exercise classes, running, or walking on a different day of any given week.
Although the current study was conducted in China, the findings may translate to a U.S. population, Dr. Kanaley said in an interview. However, “frequently our Western diet is less healthy than the traditional Chinese diet. This may have provided an immeasurable benefit to these subjects,” although study participants did not make specific adjustments to their diets, she said.
Additional research is needed to confirm the findings, said Dr. Kanaley. “Ideally, the study should be repeated in a population with a Western diet,” she noted.
Next steps for research include maintenance of activity
Evidence on the long-term benefits of exercise programs is limited, said Amanda Paluch, PhD, a physical activity epidemiologist at the University of Massachusetts, Amherst, in an interview.
“Chronic diseases such as diabetes can take years to develop, so understanding these important health outcomes requires years of follow-up. This study followed their study participants for 10 years, which gives us a nice glimpse of the long-term benefits of exercise training on diabetes prevention,” she said.
Data from previous observational studies of individuals’ current activity levels (without an intervention) suggest that adults who are more physically active have a lower risk of diabetes over time, said Dr. Paluch. However, the current study is one of the few with rigorous exercise interventions with extensive follow-up on diabetes risk, and it provides important evidence that a 12-month structured exercise program in inactive adults with obesity can result in meaningful long-term health benefits by lowering the risk of diabetes, she said.
“The individuals in the current study participated in a structured exercise program where their exercise sessions were supervised and coached,” Dr. Paluch noted. “Having a personalized coach may not be within the budget or time constraints for many people,” she said. Her message to clinicians for their patients: “When looking to start an exercise routine, identify an activity you enjoy and find feasible to fit into your existing life and schedule,” she said.
“Although this study was conducted in China, the results are meaningful for the U.S. population, as we would expect the physiological benefit of exercise to be consistent across various populations,” Dr. Paluch said. “However, there are certainly differences across countries at the individual level to the larger community-wide level that may influence a person’s ability to maintain physical activity and prevent diabetes, so replicating similar studies in other countries, including the U.S., would be of value.”
“Additionally, we need more research on how to encourage maintenance of physical activity in the long-term, after the initial exercise program is over,” she said.
“From this current study, we cannot tease out whether diabetes risk is reduced because of the 12-month exercise intervention or the benefit is from maintaining physical activity regularly over the 10 years of follow-up, or a combination of the two,” said Dr. Paluch. Future studies should consider teasing out participants who were only active during the exercise intervention, then ceased being active vs. participants who continued with vigorous activity long-term, she said.
The study was supported by the National Nature Science Foundation, the National Key Research and Development Program of China, and the Shanghai Municipal Science and Technology Major Project. The researchers, Dr. Kanaley, and Dr. Paluch had no financial conflicts to disclose.
“Physical exercise combined with diet restriction has been proven to be effective in prevention of diabetes. However, the long-term effect of exercise on prevention of diabetes, and the difference of exercise intensity in prevention of diabetes have not been well studied,” said corresponding author Xiaoying Li, MD, of Zhongshan Hospital, Fudan University, Shanghai, in an interview.
In the research letter published in JAMA Internal Medicine, Dr. Li and colleagues analyzed the results of a study of 220 adults with central obesity and nonalcoholic fatty liver disease, but no incident diabetes, randomized to a 12-month program of vigorous exercise (73 patients), moderate aerobic exercise (73 patients) or no exercise (74 patients).
A total of 208 participants completed the 1-year intervention; of these, 195 and 178 remained to provide data at 2 years and 10 years, respectively. The mean age of the participants was 53.9 years, 32.3% were male, and the mean waist circumference was 96.1 cm at baseline.
The cumulative incidence of type 2 diabetes in the vigorous exercise, moderate exercise, and nonexercise groups was 2.1 per 100 person-years 1.9 per 100 person-years, and 4.1 per 100 person-years, respectively, over the 10-year follow-up period. This translated to a reduction in type 2 diabetes risk of 49% in the vigorous exercise group and 53% in the moderate exercise group compared with the nonexercise group.
In addition, individuals in the vigorous and moderate exercise groups significantly reduced their HbA1c and waist circumference compared with the nonexercisers. Levels of plasma fasting glucose and weight regain were lower in both exercise groups compared with nonexercisers, but these differences were not significant.
The exercise intervention was described in a 2016 study, which was also published in JAMA Internal Medicine. That study’s purpose was to compare the effects of exercise on patients with nonalcoholic fatty liver disease. Participants were coached and supervised for their exercise programs. The program for the vigorous group involved jogging for 150 minutes per week at 65%-80% of maximum heart rate for 6 months and brisk walking 150 minutes per week at 45%-55% of maximum heart rate for another 6 months. The program for the moderate exercise group involved brisk walking 150 minutes per week for 12 months.
Both exercise groups showed a trend towards higher levels of leisure time physical activity after 10 years compared with the nonexercise groups, although the difference was not significant.
The main limitation of the study was that incident prediabetes was not prespecified, which may have led to some confounding, the researchers noted. In addition, the participants were highly supervised for a 12-month program only. However, the results support the long-term value of physical exercise as a method of obesity management and to delay progression to type 2 diabetes in obese individuals, they said. Vigorous and moderate aerobic exercise programs could be implemented for this patient population, they concluded.
“Surprisingly, our findings demonstrated that a 12-month vigorous aerobic exercise or moderate aerobic exercise could significantly reduce the risk of incident diabetes by 50% over the 10-year follow-up,” Dr. Li said in an interview. The results suggest that physical exercise for some period of time can produce a long-term beneficial effect in prevention of type 2 diabetes, he said.
Potential barriers to the routine use of an exercise intervention in patients with obesity include the unwillingness of this population to engage in vigorous exercise, and the potential for musculoskeletal injury, said Dr. Li. In these cases, obese patients should be encouraged to pursue moderate exercise, Dr. Li said.
Looking ahead, more research is needed to examine the potential mechanism behind the effect of exercise on diabetes prevention, said Dr. Li.
Findings fill gap in long-term outcome data
The current study is important because of the long-term follow-up data, said Jill Kanaley, PhD, professor and interim chair of nutrition and exercise physiology at the University of Missouri, in an interview. “We seldom follow up on our training studies, thus it is important to see if there is any long-term impact of these interventions,” she said.
Dr. Kanaley said she was surprised to see the residual benefits of the exercise intervention 10 years later.
“We often wonder how long the impact of the exercise training will stay with someone so that they continue to exercise and watch their weight; this study seems to indicate that there is an educational component that stays with them,” she said.
The main clinical takeaway from the current study was the minimal weight gain over time, Dr. Kanaley said.
Although time may be a barrier to the routine use of an exercise intervention, patients have to realize that they can usually find the time, especially given the multiple benefits, said Dr. Kanaley. “The exercise interventions provide more benefits than just weight control and glucose levels,” she said.
“The 30-60 minutes of exercise does not have to come all at the same time,” Dr. Kanaley noted. “It could be three 15-minute bouts of exercise/physical activity to get their 45 minutes in,” she noted. Exercise does not have to be heavy vigorous exercise, even walking is beneficial, she said. For people who complain of boredom with an exercise routine, Dr. Kanaley encourages mixing it up, with activities such as different exercise classes, running, or walking on a different day of any given week.
Although the current study was conducted in China, the findings may translate to a U.S. population, Dr. Kanaley said in an interview. However, “frequently our Western diet is less healthy than the traditional Chinese diet. This may have provided an immeasurable benefit to these subjects,” although study participants did not make specific adjustments to their diets, she said.
Additional research is needed to confirm the findings, said Dr. Kanaley. “Ideally, the study should be repeated in a population with a Western diet,” she noted.
Next steps for research include maintenance of activity
Evidence on the long-term benefits of exercise programs is limited, said Amanda Paluch, PhD, a physical activity epidemiologist at the University of Massachusetts, Amherst, in an interview.
“Chronic diseases such as diabetes can take years to develop, so understanding these important health outcomes requires years of follow-up. This study followed their study participants for 10 years, which gives us a nice glimpse of the long-term benefits of exercise training on diabetes prevention,” she said.
Data from previous observational studies of individuals’ current activity levels (without an intervention) suggest that adults who are more physically active have a lower risk of diabetes over time, said Dr. Paluch. However, the current study is one of the few with rigorous exercise interventions with extensive follow-up on diabetes risk, and it provides important evidence that a 12-month structured exercise program in inactive adults with obesity can result in meaningful long-term health benefits by lowering the risk of diabetes, she said.
“The individuals in the current study participated in a structured exercise program where their exercise sessions were supervised and coached,” Dr. Paluch noted. “Having a personalized coach may not be within the budget or time constraints for many people,” she said. Her message to clinicians for their patients: “When looking to start an exercise routine, identify an activity you enjoy and find feasible to fit into your existing life and schedule,” she said.
“Although this study was conducted in China, the results are meaningful for the U.S. population, as we would expect the physiological benefit of exercise to be consistent across various populations,” Dr. Paluch said. “However, there are certainly differences across countries at the individual level to the larger community-wide level that may influence a person’s ability to maintain physical activity and prevent diabetes, so replicating similar studies in other countries, including the U.S., would be of value.”
“Additionally, we need more research on how to encourage maintenance of physical activity in the long-term, after the initial exercise program is over,” she said.
“From this current study, we cannot tease out whether diabetes risk is reduced because of the 12-month exercise intervention or the benefit is from maintaining physical activity regularly over the 10 years of follow-up, or a combination of the two,” said Dr. Paluch. Future studies should consider teasing out participants who were only active during the exercise intervention, then ceased being active vs. participants who continued with vigorous activity long-term, she said.
The study was supported by the National Nature Science Foundation, the National Key Research and Development Program of China, and the Shanghai Municipal Science and Technology Major Project. The researchers, Dr. Kanaley, and Dr. Paluch had no financial conflicts to disclose.
“Physical exercise combined with diet restriction has been proven to be effective in prevention of diabetes. However, the long-term effect of exercise on prevention of diabetes, and the difference of exercise intensity in prevention of diabetes have not been well studied,” said corresponding author Xiaoying Li, MD, of Zhongshan Hospital, Fudan University, Shanghai, in an interview.
In the research letter published in JAMA Internal Medicine, Dr. Li and colleagues analyzed the results of a study of 220 adults with central obesity and nonalcoholic fatty liver disease, but no incident diabetes, randomized to a 12-month program of vigorous exercise (73 patients), moderate aerobic exercise (73 patients) or no exercise (74 patients).
A total of 208 participants completed the 1-year intervention; of these, 195 and 178 remained to provide data at 2 years and 10 years, respectively. The mean age of the participants was 53.9 years, 32.3% were male, and the mean waist circumference was 96.1 cm at baseline.
The cumulative incidence of type 2 diabetes in the vigorous exercise, moderate exercise, and nonexercise groups was 2.1 per 100 person-years 1.9 per 100 person-years, and 4.1 per 100 person-years, respectively, over the 10-year follow-up period. This translated to a reduction in type 2 diabetes risk of 49% in the vigorous exercise group and 53% in the moderate exercise group compared with the nonexercise group.
In addition, individuals in the vigorous and moderate exercise groups significantly reduced their HbA1c and waist circumference compared with the nonexercisers. Levels of plasma fasting glucose and weight regain were lower in both exercise groups compared with nonexercisers, but these differences were not significant.
The exercise intervention was described in a 2016 study, which was also published in JAMA Internal Medicine. That study’s purpose was to compare the effects of exercise on patients with nonalcoholic fatty liver disease. Participants were coached and supervised for their exercise programs. The program for the vigorous group involved jogging for 150 minutes per week at 65%-80% of maximum heart rate for 6 months and brisk walking 150 minutes per week at 45%-55% of maximum heart rate for another 6 months. The program for the moderate exercise group involved brisk walking 150 minutes per week for 12 months.
Both exercise groups showed a trend towards higher levels of leisure time physical activity after 10 years compared with the nonexercise groups, although the difference was not significant.
The main limitation of the study was that incident prediabetes was not prespecified, which may have led to some confounding, the researchers noted. In addition, the participants were highly supervised for a 12-month program only. However, the results support the long-term value of physical exercise as a method of obesity management and to delay progression to type 2 diabetes in obese individuals, they said. Vigorous and moderate aerobic exercise programs could be implemented for this patient population, they concluded.
“Surprisingly, our findings demonstrated that a 12-month vigorous aerobic exercise or moderate aerobic exercise could significantly reduce the risk of incident diabetes by 50% over the 10-year follow-up,” Dr. Li said in an interview. The results suggest that physical exercise for some period of time can produce a long-term beneficial effect in prevention of type 2 diabetes, he said.
Potential barriers to the routine use of an exercise intervention in patients with obesity include the unwillingness of this population to engage in vigorous exercise, and the potential for musculoskeletal injury, said Dr. Li. In these cases, obese patients should be encouraged to pursue moderate exercise, Dr. Li said.
Looking ahead, more research is needed to examine the potential mechanism behind the effect of exercise on diabetes prevention, said Dr. Li.
Findings fill gap in long-term outcome data
The current study is important because of the long-term follow-up data, said Jill Kanaley, PhD, professor and interim chair of nutrition and exercise physiology at the University of Missouri, in an interview. “We seldom follow up on our training studies, thus it is important to see if there is any long-term impact of these interventions,” she said.
Dr. Kanaley said she was surprised to see the residual benefits of the exercise intervention 10 years later.
“We often wonder how long the impact of the exercise training will stay with someone so that they continue to exercise and watch their weight; this study seems to indicate that there is an educational component that stays with them,” she said.
The main clinical takeaway from the current study was the minimal weight gain over time, Dr. Kanaley said.
Although time may be a barrier to the routine use of an exercise intervention, patients have to realize that they can usually find the time, especially given the multiple benefits, said Dr. Kanaley. “The exercise interventions provide more benefits than just weight control and glucose levels,” she said.
“The 30-60 minutes of exercise does not have to come all at the same time,” Dr. Kanaley noted. “It could be three 15-minute bouts of exercise/physical activity to get their 45 minutes in,” she noted. Exercise does not have to be heavy vigorous exercise, even walking is beneficial, she said. For people who complain of boredom with an exercise routine, Dr. Kanaley encourages mixing it up, with activities such as different exercise classes, running, or walking on a different day of any given week.
Although the current study was conducted in China, the findings may translate to a U.S. population, Dr. Kanaley said in an interview. However, “frequently our Western diet is less healthy than the traditional Chinese diet. This may have provided an immeasurable benefit to these subjects,” although study participants did not make specific adjustments to their diets, she said.
Additional research is needed to confirm the findings, said Dr. Kanaley. “Ideally, the study should be repeated in a population with a Western diet,” she noted.
Next steps for research include maintenance of activity
Evidence on the long-term benefits of exercise programs is limited, said Amanda Paluch, PhD, a physical activity epidemiologist at the University of Massachusetts, Amherst, in an interview.
“Chronic diseases such as diabetes can take years to develop, so understanding these important health outcomes requires years of follow-up. This study followed their study participants for 10 years, which gives us a nice glimpse of the long-term benefits of exercise training on diabetes prevention,” she said.
Data from previous observational studies of individuals’ current activity levels (without an intervention) suggest that adults who are more physically active have a lower risk of diabetes over time, said Dr. Paluch. However, the current study is one of the few with rigorous exercise interventions with extensive follow-up on diabetes risk, and it provides important evidence that a 12-month structured exercise program in inactive adults with obesity can result in meaningful long-term health benefits by lowering the risk of diabetes, she said.
“The individuals in the current study participated in a structured exercise program where their exercise sessions were supervised and coached,” Dr. Paluch noted. “Having a personalized coach may not be within the budget or time constraints for many people,” she said. Her message to clinicians for their patients: “When looking to start an exercise routine, identify an activity you enjoy and find feasible to fit into your existing life and schedule,” she said.
“Although this study was conducted in China, the results are meaningful for the U.S. population, as we would expect the physiological benefit of exercise to be consistent across various populations,” Dr. Paluch said. “However, there are certainly differences across countries at the individual level to the larger community-wide level that may influence a person’s ability to maintain physical activity and prevent diabetes, so replicating similar studies in other countries, including the U.S., would be of value.”
“Additionally, we need more research on how to encourage maintenance of physical activity in the long-term, after the initial exercise program is over,” she said.
“From this current study, we cannot tease out whether diabetes risk is reduced because of the 12-month exercise intervention or the benefit is from maintaining physical activity regularly over the 10 years of follow-up, or a combination of the two,” said Dr. Paluch. Future studies should consider teasing out participants who were only active during the exercise intervention, then ceased being active vs. participants who continued with vigorous activity long-term, she said.
The study was supported by the National Nature Science Foundation, the National Key Research and Development Program of China, and the Shanghai Municipal Science and Technology Major Project. The researchers, Dr. Kanaley, and Dr. Paluch had no financial conflicts to disclose.
FROM JAMA INTERNAL MEDICINE
EMR screening in emergency department tags undiagnosed diabetes
A diabetes screening program built into an electronic medical records system identified diabetes or prediabetes in 52% of individuals flagged for abnormal hemoglobin A1c, based on data from more than 2,000 adults.
“Despite the best efforts of clinicians, researchers, and educators, the number of patients living with undiagnosed diabetes is still rising and is currently at approximately 8.5 million, and the number of people unaware of their prediabetes is approximately 77 million,” lead investigator Kristie K. Danielson, PhD, said in an interview. Screening for diabetes is critical to start treatment early, to potentially reverse prediabetes, and to prevent the long-term complications of diabetes and reduced life expectancy.
In a pilot study published in JAMA Network Open, Dr. Danielson and colleagues reviewed data from 8,441 adults who visited a single emergency department in Chicago during February–April 2021.
The EMR at the hospital contained a built-in best practice alert (BPA) that flagged patients as being at risk for type 2 diabetes based the American Diabetes Association recommendations; the identification algorithm included age 45 years and older, or those aged 18-44 years with a body mass index of 25 kg/m2 or higher, no previous history of diabetes, and no A1c measure in the last 3 years, according to the EMR.
A total of 8,441 adult patients visited the ED during the study period; 2,576 triggered BPA tests, and 2,074 had A1c results for review. Among the patients with A1c results, 52% had elevated values of 5.7% or higher. Of these, a total of 758 individuals were identified with prediabetes (A1c, 5.7%-6.4%), 265 with diabetes (A1c, 6.5%-9.9%), and 62 with severe diabetes (A1c, 10% or higher).
After testing, 352 patients with elevated A1c were contacted by the researchers. The mean age of this group was 52.2 years, 54.5% were women, and nearly two-thirds (64.8%) were non-Hispanic Black. The median income of those contacted was in the 44th percentile, and 50% had public insurance.
Most of those contacted (264 patients) were not aware of a previous diagnosis of prediabetes or diabetes; the remaining 88 had a previous diagnosis, but only 51 self-reported receiving treatment, the researchers noted.
Although the screening program successfully identified a significant number of previously undiagnosed individuals with diabetes, prediabetes, or poorly controlled diabetes, its feasibility in routine practice requires further study, the researchers wrote.
The findings were limited by several factors including the identification of patients previously diagnosed with diabetes but who were not being treated, and the potential bias toward individuals of higher socioeconomic status, the researchers noted. However, the results support further exploration of the program as a way to identify undiagnosed diabetes, especially in underserved populations.
Diabetes in underserved groups goes undetected
“We were surprised by the sheer number of people newly diagnosed with diabetes or prediabetes,” which was far greater than expected, commented Dr. Danielson of the University of Illinois at Chicago. “Clearly, we tapped into a new population that has not often been seen by primary care providers or endocrinologists, as is often the case for underserved and vulnerable individuals who visit the emergency department as a first line for health care.”
The screening alert system is straightforward to build into an existing EMR, with technical support, Dr. Danielson said. “In theory, it should be able to be incorporated into other clinical centers and emergency departments. One of the current limitations that we are seeing is that the EMR is still flagging some people already diagnosed with diabetes to be screened for diabetes.” However, “because of this, we also see this as an opportunity to identify and reach out to those with diabetes who are still underserved and not receiving the appropriate diabetes care they need.”
The study results have broader public health implications, Dr. Danielson added. “We have identified a new, large population of people with diabetes who need medical care and diabetes education. This will further add to the burden of health care and costs, and it raises the ethical question of screening and not having full resources readily available to help.
“In my opinion, the study sheds light on a significant issue that will hopefully help drive change at both a health systems and public health level locally and nationally,” she added.
“One of the significant research gaps that has emerged now is how to link these new patients to health care and diabetes education at our institution after they leave the emergency department,” said Dr. Danielson. Diabetes screening in the ED setting is “a very novel area for health system scientists, social workers, and others to now come to the table and collaborate on next steps to help our patients.”
The study was initiated by the investigators, but was supported by a grant from Novo Nordisk to two coauthors. Dr. Danielson also disclosed grant funding from Novo Nordisk during the conduct of the study.
A diabetes screening program built into an electronic medical records system identified diabetes or prediabetes in 52% of individuals flagged for abnormal hemoglobin A1c, based on data from more than 2,000 adults.
“Despite the best efforts of clinicians, researchers, and educators, the number of patients living with undiagnosed diabetes is still rising and is currently at approximately 8.5 million, and the number of people unaware of their prediabetes is approximately 77 million,” lead investigator Kristie K. Danielson, PhD, said in an interview. Screening for diabetes is critical to start treatment early, to potentially reverse prediabetes, and to prevent the long-term complications of diabetes and reduced life expectancy.
In a pilot study published in JAMA Network Open, Dr. Danielson and colleagues reviewed data from 8,441 adults who visited a single emergency department in Chicago during February–April 2021.
The EMR at the hospital contained a built-in best practice alert (BPA) that flagged patients as being at risk for type 2 diabetes based the American Diabetes Association recommendations; the identification algorithm included age 45 years and older, or those aged 18-44 years with a body mass index of 25 kg/m2 or higher, no previous history of diabetes, and no A1c measure in the last 3 years, according to the EMR.
A total of 8,441 adult patients visited the ED during the study period; 2,576 triggered BPA tests, and 2,074 had A1c results for review. Among the patients with A1c results, 52% had elevated values of 5.7% or higher. Of these, a total of 758 individuals were identified with prediabetes (A1c, 5.7%-6.4%), 265 with diabetes (A1c, 6.5%-9.9%), and 62 with severe diabetes (A1c, 10% or higher).
After testing, 352 patients with elevated A1c were contacted by the researchers. The mean age of this group was 52.2 years, 54.5% were women, and nearly two-thirds (64.8%) were non-Hispanic Black. The median income of those contacted was in the 44th percentile, and 50% had public insurance.
Most of those contacted (264 patients) were not aware of a previous diagnosis of prediabetes or diabetes; the remaining 88 had a previous diagnosis, but only 51 self-reported receiving treatment, the researchers noted.
Although the screening program successfully identified a significant number of previously undiagnosed individuals with diabetes, prediabetes, or poorly controlled diabetes, its feasibility in routine practice requires further study, the researchers wrote.
The findings were limited by several factors including the identification of patients previously diagnosed with diabetes but who were not being treated, and the potential bias toward individuals of higher socioeconomic status, the researchers noted. However, the results support further exploration of the program as a way to identify undiagnosed diabetes, especially in underserved populations.
Diabetes in underserved groups goes undetected
“We were surprised by the sheer number of people newly diagnosed with diabetes or prediabetes,” which was far greater than expected, commented Dr. Danielson of the University of Illinois at Chicago. “Clearly, we tapped into a new population that has not often been seen by primary care providers or endocrinologists, as is often the case for underserved and vulnerable individuals who visit the emergency department as a first line for health care.”
The screening alert system is straightforward to build into an existing EMR, with technical support, Dr. Danielson said. “In theory, it should be able to be incorporated into other clinical centers and emergency departments. One of the current limitations that we are seeing is that the EMR is still flagging some people already diagnosed with diabetes to be screened for diabetes.” However, “because of this, we also see this as an opportunity to identify and reach out to those with diabetes who are still underserved and not receiving the appropriate diabetes care they need.”
The study results have broader public health implications, Dr. Danielson added. “We have identified a new, large population of people with diabetes who need medical care and diabetes education. This will further add to the burden of health care and costs, and it raises the ethical question of screening and not having full resources readily available to help.
“In my opinion, the study sheds light on a significant issue that will hopefully help drive change at both a health systems and public health level locally and nationally,” she added.
“One of the significant research gaps that has emerged now is how to link these new patients to health care and diabetes education at our institution after they leave the emergency department,” said Dr. Danielson. Diabetes screening in the ED setting is “a very novel area for health system scientists, social workers, and others to now come to the table and collaborate on next steps to help our patients.”
The study was initiated by the investigators, but was supported by a grant from Novo Nordisk to two coauthors. Dr. Danielson also disclosed grant funding from Novo Nordisk during the conduct of the study.
A diabetes screening program built into an electronic medical records system identified diabetes or prediabetes in 52% of individuals flagged for abnormal hemoglobin A1c, based on data from more than 2,000 adults.
“Despite the best efforts of clinicians, researchers, and educators, the number of patients living with undiagnosed diabetes is still rising and is currently at approximately 8.5 million, and the number of people unaware of their prediabetes is approximately 77 million,” lead investigator Kristie K. Danielson, PhD, said in an interview. Screening for diabetes is critical to start treatment early, to potentially reverse prediabetes, and to prevent the long-term complications of diabetes and reduced life expectancy.
In a pilot study published in JAMA Network Open, Dr. Danielson and colleagues reviewed data from 8,441 adults who visited a single emergency department in Chicago during February–April 2021.
The EMR at the hospital contained a built-in best practice alert (BPA) that flagged patients as being at risk for type 2 diabetes based the American Diabetes Association recommendations; the identification algorithm included age 45 years and older, or those aged 18-44 years with a body mass index of 25 kg/m2 or higher, no previous history of diabetes, and no A1c measure in the last 3 years, according to the EMR.
A total of 8,441 adult patients visited the ED during the study period; 2,576 triggered BPA tests, and 2,074 had A1c results for review. Among the patients with A1c results, 52% had elevated values of 5.7% or higher. Of these, a total of 758 individuals were identified with prediabetes (A1c, 5.7%-6.4%), 265 with diabetes (A1c, 6.5%-9.9%), and 62 with severe diabetes (A1c, 10% or higher).
After testing, 352 patients with elevated A1c were contacted by the researchers. The mean age of this group was 52.2 years, 54.5% were women, and nearly two-thirds (64.8%) were non-Hispanic Black. The median income of those contacted was in the 44th percentile, and 50% had public insurance.
Most of those contacted (264 patients) were not aware of a previous diagnosis of prediabetes or diabetes; the remaining 88 had a previous diagnosis, but only 51 self-reported receiving treatment, the researchers noted.
Although the screening program successfully identified a significant number of previously undiagnosed individuals with diabetes, prediabetes, or poorly controlled diabetes, its feasibility in routine practice requires further study, the researchers wrote.
The findings were limited by several factors including the identification of patients previously diagnosed with diabetes but who were not being treated, and the potential bias toward individuals of higher socioeconomic status, the researchers noted. However, the results support further exploration of the program as a way to identify undiagnosed diabetes, especially in underserved populations.
Diabetes in underserved groups goes undetected
“We were surprised by the sheer number of people newly diagnosed with diabetes or prediabetes,” which was far greater than expected, commented Dr. Danielson of the University of Illinois at Chicago. “Clearly, we tapped into a new population that has not often been seen by primary care providers or endocrinologists, as is often the case for underserved and vulnerable individuals who visit the emergency department as a first line for health care.”
The screening alert system is straightforward to build into an existing EMR, with technical support, Dr. Danielson said. “In theory, it should be able to be incorporated into other clinical centers and emergency departments. One of the current limitations that we are seeing is that the EMR is still flagging some people already diagnosed with diabetes to be screened for diabetes.” However, “because of this, we also see this as an opportunity to identify and reach out to those with diabetes who are still underserved and not receiving the appropriate diabetes care they need.”
The study results have broader public health implications, Dr. Danielson added. “We have identified a new, large population of people with diabetes who need medical care and diabetes education. This will further add to the burden of health care and costs, and it raises the ethical question of screening and not having full resources readily available to help.
“In my opinion, the study sheds light on a significant issue that will hopefully help drive change at both a health systems and public health level locally and nationally,” she added.
“One of the significant research gaps that has emerged now is how to link these new patients to health care and diabetes education at our institution after they leave the emergency department,” said Dr. Danielson. Diabetes screening in the ED setting is “a very novel area for health system scientists, social workers, and others to now come to the table and collaborate on next steps to help our patients.”
The study was initiated by the investigators, but was supported by a grant from Novo Nordisk to two coauthors. Dr. Danielson also disclosed grant funding from Novo Nordisk during the conduct of the study.
FROM JAMA NETWORK OPEN
HRT may prevent Alzheimer’s in high-risk women
new research suggests.
Results from a cohort study of almost 1,200 women showed that use of HRT was associated with higher delayed memory scores and larger entorhinal and hippocampal brain volumes – areas that are affected early by Alzheimer’s disease (AD) pathology.
HRT was also found to be most effective, as seen by larger hippocampal volume, when introduced during early perimenopause.
“Clinicians are very much aware of the susceptibility of women to cognitive disturbances during menopause,” lead author Rasha Saleh, MD, senior research associate, University of East Anglia (England), said in an interview.
“Identifying the at-risk APOE4 women and early HRT introduction can be of benefit. Confirming our findings in a clinical trial would be the next step forward,” Dr. Saleh said.
The findings were published online in Alzheimer’s Research and Therapy.
Personalized approaches
Dr. Saleh noted that estrogen receptors are localized in various areas of the brain, including cognition-related areas. Estrogen regulates such things as neuroinflammatory status, glucose utilization, and lipid metabolism.
“The decline of estrogen during menopause can lead to disturbance in these functions, which can accelerate AD-related pathology,” she said.
HRT during the menopausal transition and afterward is “being considered as a strategy to mitigate cognitive decline,” the investigators wrote. Early observational studies have suggested that oral estrogen “may be protective against dementia,” but results of clinical trials have been inconsistent, and some have even shown “harmful effects.”
The current researchers were “interested in the personalized approaches in the prevention of AD,” Dr. Saleh said. Preclinical and pilot data from her group have shown that women with APOE4 have “better cognitive test scores with nutritional and hormonal interventions.”
This led Dr. Saleh to hypothesize that HRT would be of more cognitive benefit for those with versus without APOE4, particularly when introduced early during the menopausal transition.
To investigate this hypothesis, the researchers analyzed baseline data from participants in the European Prevention of Alzheimer’s Dementia (EPAD) cohort. This project was initiated in 2015 with the aim of developing longitudinal models over the entire course of AD prior to dementia clinical diagnosis.
Participants were recruited from 10 European countries. All were required to be at least 50 years old, to have not been diagnosed with dementia at baseline, and to have no medical or psychiatric illness that could potentially exclude them from further research.
The current study included 1,178 women (mean age, 65.1 years), who were divided by genotype into non-APOE4 and APOE4 groups. HRT treatment for current or previous users included estrogen alone or estrogen plus progestogens via oral or transdermal administration routes, and at different doses.
The four tests used to assess cognition were the Mini-Mental State Examination dot counting to evaluate verbal working memory, the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) total score, the Four Mountain Test, and the supermarket trolley virtual reality test.
Brain MRI data were collected. The researchers focused on the medial temporal lobe as the “main brain region regulating cognition and memory processing.” This lobe includes the hippocampus, the parahippocampus, the entorhinal cortex, and the amygdala.
‘Critical window’
The researchers found a “trend” toward an APOE-HRT interaction (P-interaction = .097) for the total RBANS score. In particular, it was significant for the RBANS delayed memory index, where scores were consistently higher for women with APOE4 who had received HRT, compared with all other groups (P-interaction = .009).
Within-genotype group comparisons showed that HRT users had a higher RBANS total scale score and delayed memory index (P = .045 and P = .002, respectively), but only among APOE4 carriers. Effect size analyses showed a large effect of HRT use on the Four Mountain Test score and the supermarket trolley virtual reality test score (Cohen’s d = 0.988 and 1.2, respectively).
“This large effect was found only in APOE4 carriers,” the investigators noted.
Similarly, a moderate to large effect of HRT on the left entorhinal volume was observed in APOE4 carriers (Cohen’s d = 0.63).
In members of the APOE4 group who received HRT, the left entorhinal and left and right amygdala volumes were larger, compared with both no-APOE4 and non-HRT users (P-interaction = .002, .003, and .005, respectively). Similar trends were observed for the right entorhinal volume (P = .074).
In addition, among HRT users, the left entorhinal volume was larger (P = .03); the right and left anterior cingulate gyrus volumes were smaller (P = .003 and .062, respectively); and the left superior frontal gyrus volume was larger (P = .009) in comparison with women who did not receive HRT, independently of their APOE genotype.
Early use of HRT among APOE4 carriers was associated with larger right and left hippocampal volume (P = .035 and P = .028, respectively) – an association not found in non-APOE4 carriers. The association was also not significant when participants were not stratified by APOE genotype.
“The key important point here is the timing, or the ‘critical window,’ when HRT can be of most benefit,” Dr. Saleh said. “This is most beneficial when introduced early, before the neuropathology becomes irreversible.”
Study limitations include its cross-sectional design, which precludes the establishment of a causal relationship, and the fact that information regarding the type and dose of estrogen was not available for all participants.
HRT is not without risk, Dr. Saleh noted. She recommended that clinicians “carry out various screening tests to make sure that a woman is eligible for HRT and not at risk of hypercoagulability, for instance.”
Risk-benefit ratio
In a comment, Howard Fillit, MD, cofounder and chief science officer at the Alzheimer’s Drug Discovery Foundation, called the study “exactly the kind of work that needs to be done.”
Dr. Fillit, who was not involved with the current research, is a clinical professor of geriatric medicine, palliative care medicine, and neuroscience at Mount Sinai Hospital, New York.
He compared the process with that of osteoporosis. “We know that if women are treated [with HRT] at the time of the menopause, you can prevent the rapid bone loss that occurs with rapid estrogen loss. But if you wait 5, 10 years out, once the bone loss has occurred, the HRT doesn’t really have any impact on osteoporosis risk because the horse is already out of the barn,” he said.
Although HRT carries risks, “they can clearly be managed; and if it’s proven that estrogen or hormone replacement around the time of the menopause can be protective [against AD], the risk-benefit ratio of HRT could be in favor of treatment,” Dr. Fillit added.
The study was conducted as part of the Medical Research Council NuBrain Consortium. The investigators and Dr. Fillit reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests.
Results from a cohort study of almost 1,200 women showed that use of HRT was associated with higher delayed memory scores and larger entorhinal and hippocampal brain volumes – areas that are affected early by Alzheimer’s disease (AD) pathology.
HRT was also found to be most effective, as seen by larger hippocampal volume, when introduced during early perimenopause.
“Clinicians are very much aware of the susceptibility of women to cognitive disturbances during menopause,” lead author Rasha Saleh, MD, senior research associate, University of East Anglia (England), said in an interview.
“Identifying the at-risk APOE4 women and early HRT introduction can be of benefit. Confirming our findings in a clinical trial would be the next step forward,” Dr. Saleh said.
The findings were published online in Alzheimer’s Research and Therapy.
Personalized approaches
Dr. Saleh noted that estrogen receptors are localized in various areas of the brain, including cognition-related areas. Estrogen regulates such things as neuroinflammatory status, glucose utilization, and lipid metabolism.
“The decline of estrogen during menopause can lead to disturbance in these functions, which can accelerate AD-related pathology,” she said.
HRT during the menopausal transition and afterward is “being considered as a strategy to mitigate cognitive decline,” the investigators wrote. Early observational studies have suggested that oral estrogen “may be protective against dementia,” but results of clinical trials have been inconsistent, and some have even shown “harmful effects.”
The current researchers were “interested in the personalized approaches in the prevention of AD,” Dr. Saleh said. Preclinical and pilot data from her group have shown that women with APOE4 have “better cognitive test scores with nutritional and hormonal interventions.”
This led Dr. Saleh to hypothesize that HRT would be of more cognitive benefit for those with versus without APOE4, particularly when introduced early during the menopausal transition.
To investigate this hypothesis, the researchers analyzed baseline data from participants in the European Prevention of Alzheimer’s Dementia (EPAD) cohort. This project was initiated in 2015 with the aim of developing longitudinal models over the entire course of AD prior to dementia clinical diagnosis.
Participants were recruited from 10 European countries. All were required to be at least 50 years old, to have not been diagnosed with dementia at baseline, and to have no medical or psychiatric illness that could potentially exclude them from further research.
The current study included 1,178 women (mean age, 65.1 years), who were divided by genotype into non-APOE4 and APOE4 groups. HRT treatment for current or previous users included estrogen alone or estrogen plus progestogens via oral or transdermal administration routes, and at different doses.
The four tests used to assess cognition were the Mini-Mental State Examination dot counting to evaluate verbal working memory, the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) total score, the Four Mountain Test, and the supermarket trolley virtual reality test.
Brain MRI data were collected. The researchers focused on the medial temporal lobe as the “main brain region regulating cognition and memory processing.” This lobe includes the hippocampus, the parahippocampus, the entorhinal cortex, and the amygdala.
‘Critical window’
The researchers found a “trend” toward an APOE-HRT interaction (P-interaction = .097) for the total RBANS score. In particular, it was significant for the RBANS delayed memory index, where scores were consistently higher for women with APOE4 who had received HRT, compared with all other groups (P-interaction = .009).
Within-genotype group comparisons showed that HRT users had a higher RBANS total scale score and delayed memory index (P = .045 and P = .002, respectively), but only among APOE4 carriers. Effect size analyses showed a large effect of HRT use on the Four Mountain Test score and the supermarket trolley virtual reality test score (Cohen’s d = 0.988 and 1.2, respectively).
“This large effect was found only in APOE4 carriers,” the investigators noted.
Similarly, a moderate to large effect of HRT on the left entorhinal volume was observed in APOE4 carriers (Cohen’s d = 0.63).
In members of the APOE4 group who received HRT, the left entorhinal and left and right amygdala volumes were larger, compared with both no-APOE4 and non-HRT users (P-interaction = .002, .003, and .005, respectively). Similar trends were observed for the right entorhinal volume (P = .074).
In addition, among HRT users, the left entorhinal volume was larger (P = .03); the right and left anterior cingulate gyrus volumes were smaller (P = .003 and .062, respectively); and the left superior frontal gyrus volume was larger (P = .009) in comparison with women who did not receive HRT, independently of their APOE genotype.
Early use of HRT among APOE4 carriers was associated with larger right and left hippocampal volume (P = .035 and P = .028, respectively) – an association not found in non-APOE4 carriers. The association was also not significant when participants were not stratified by APOE genotype.
“The key important point here is the timing, or the ‘critical window,’ when HRT can be of most benefit,” Dr. Saleh said. “This is most beneficial when introduced early, before the neuropathology becomes irreversible.”
Study limitations include its cross-sectional design, which precludes the establishment of a causal relationship, and the fact that information regarding the type and dose of estrogen was not available for all participants.
HRT is not without risk, Dr. Saleh noted. She recommended that clinicians “carry out various screening tests to make sure that a woman is eligible for HRT and not at risk of hypercoagulability, for instance.”
Risk-benefit ratio
In a comment, Howard Fillit, MD, cofounder and chief science officer at the Alzheimer’s Drug Discovery Foundation, called the study “exactly the kind of work that needs to be done.”
Dr. Fillit, who was not involved with the current research, is a clinical professor of geriatric medicine, palliative care medicine, and neuroscience at Mount Sinai Hospital, New York.
He compared the process with that of osteoporosis. “We know that if women are treated [with HRT] at the time of the menopause, you can prevent the rapid bone loss that occurs with rapid estrogen loss. But if you wait 5, 10 years out, once the bone loss has occurred, the HRT doesn’t really have any impact on osteoporosis risk because the horse is already out of the barn,” he said.
Although HRT carries risks, “they can clearly be managed; and if it’s proven that estrogen or hormone replacement around the time of the menopause can be protective [against AD], the risk-benefit ratio of HRT could be in favor of treatment,” Dr. Fillit added.
The study was conducted as part of the Medical Research Council NuBrain Consortium. The investigators and Dr. Fillit reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests.
Results from a cohort study of almost 1,200 women showed that use of HRT was associated with higher delayed memory scores and larger entorhinal and hippocampal brain volumes – areas that are affected early by Alzheimer’s disease (AD) pathology.
HRT was also found to be most effective, as seen by larger hippocampal volume, when introduced during early perimenopause.
“Clinicians are very much aware of the susceptibility of women to cognitive disturbances during menopause,” lead author Rasha Saleh, MD, senior research associate, University of East Anglia (England), said in an interview.
“Identifying the at-risk APOE4 women and early HRT introduction can be of benefit. Confirming our findings in a clinical trial would be the next step forward,” Dr. Saleh said.
The findings were published online in Alzheimer’s Research and Therapy.
Personalized approaches
Dr. Saleh noted that estrogen receptors are localized in various areas of the brain, including cognition-related areas. Estrogen regulates such things as neuroinflammatory status, glucose utilization, and lipid metabolism.
“The decline of estrogen during menopause can lead to disturbance in these functions, which can accelerate AD-related pathology,” she said.
HRT during the menopausal transition and afterward is “being considered as a strategy to mitigate cognitive decline,” the investigators wrote. Early observational studies have suggested that oral estrogen “may be protective against dementia,” but results of clinical trials have been inconsistent, and some have even shown “harmful effects.”
The current researchers were “interested in the personalized approaches in the prevention of AD,” Dr. Saleh said. Preclinical and pilot data from her group have shown that women with APOE4 have “better cognitive test scores with nutritional and hormonal interventions.”
This led Dr. Saleh to hypothesize that HRT would be of more cognitive benefit for those with versus without APOE4, particularly when introduced early during the menopausal transition.
To investigate this hypothesis, the researchers analyzed baseline data from participants in the European Prevention of Alzheimer’s Dementia (EPAD) cohort. This project was initiated in 2015 with the aim of developing longitudinal models over the entire course of AD prior to dementia clinical diagnosis.
Participants were recruited from 10 European countries. All were required to be at least 50 years old, to have not been diagnosed with dementia at baseline, and to have no medical or psychiatric illness that could potentially exclude them from further research.
The current study included 1,178 women (mean age, 65.1 years), who were divided by genotype into non-APOE4 and APOE4 groups. HRT treatment for current or previous users included estrogen alone or estrogen plus progestogens via oral or transdermal administration routes, and at different doses.
The four tests used to assess cognition were the Mini-Mental State Examination dot counting to evaluate verbal working memory, the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) total score, the Four Mountain Test, and the supermarket trolley virtual reality test.
Brain MRI data were collected. The researchers focused on the medial temporal lobe as the “main brain region regulating cognition and memory processing.” This lobe includes the hippocampus, the parahippocampus, the entorhinal cortex, and the amygdala.
‘Critical window’
The researchers found a “trend” toward an APOE-HRT interaction (P-interaction = .097) for the total RBANS score. In particular, it was significant for the RBANS delayed memory index, where scores were consistently higher for women with APOE4 who had received HRT, compared with all other groups (P-interaction = .009).
Within-genotype group comparisons showed that HRT users had a higher RBANS total scale score and delayed memory index (P = .045 and P = .002, respectively), but only among APOE4 carriers. Effect size analyses showed a large effect of HRT use on the Four Mountain Test score and the supermarket trolley virtual reality test score (Cohen’s d = 0.988 and 1.2, respectively).
“This large effect was found only in APOE4 carriers,” the investigators noted.
Similarly, a moderate to large effect of HRT on the left entorhinal volume was observed in APOE4 carriers (Cohen’s d = 0.63).
In members of the APOE4 group who received HRT, the left entorhinal and left and right amygdala volumes were larger, compared with both no-APOE4 and non-HRT users (P-interaction = .002, .003, and .005, respectively). Similar trends were observed for the right entorhinal volume (P = .074).
In addition, among HRT users, the left entorhinal volume was larger (P = .03); the right and left anterior cingulate gyrus volumes were smaller (P = .003 and .062, respectively); and the left superior frontal gyrus volume was larger (P = .009) in comparison with women who did not receive HRT, independently of their APOE genotype.
Early use of HRT among APOE4 carriers was associated with larger right and left hippocampal volume (P = .035 and P = .028, respectively) – an association not found in non-APOE4 carriers. The association was also not significant when participants were not stratified by APOE genotype.
“The key important point here is the timing, or the ‘critical window,’ when HRT can be of most benefit,” Dr. Saleh said. “This is most beneficial when introduced early, before the neuropathology becomes irreversible.”
Study limitations include its cross-sectional design, which precludes the establishment of a causal relationship, and the fact that information regarding the type and dose of estrogen was not available for all participants.
HRT is not without risk, Dr. Saleh noted. She recommended that clinicians “carry out various screening tests to make sure that a woman is eligible for HRT and not at risk of hypercoagulability, for instance.”
Risk-benefit ratio
In a comment, Howard Fillit, MD, cofounder and chief science officer at the Alzheimer’s Drug Discovery Foundation, called the study “exactly the kind of work that needs to be done.”
Dr. Fillit, who was not involved with the current research, is a clinical professor of geriatric medicine, palliative care medicine, and neuroscience at Mount Sinai Hospital, New York.
He compared the process with that of osteoporosis. “We know that if women are treated [with HRT] at the time of the menopause, you can prevent the rapid bone loss that occurs with rapid estrogen loss. But if you wait 5, 10 years out, once the bone loss has occurred, the HRT doesn’t really have any impact on osteoporosis risk because the horse is already out of the barn,” he said.
Although HRT carries risks, “they can clearly be managed; and if it’s proven that estrogen or hormone replacement around the time of the menopause can be protective [against AD], the risk-benefit ratio of HRT could be in favor of treatment,” Dr. Fillit added.
The study was conducted as part of the Medical Research Council NuBrain Consortium. The investigators and Dr. Fillit reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ALZHEIMER’S RESEARCH AND THERAPY