User login
In COPD, tai chi confers long-term benefit
and seems to confer better long-term improvement, suggests a study published online in the journal CHEST®.
Following 12 weeks of participation in tai chi or pulmonary rehabilitation, patients improved in most of the measurements taken, although no significant between-group differences were observed at that time. However, further improvements were observed in the tai chi group 12 weeks after the intervention had ended. These improvements manifested as a statistically significant 4.5 between-group difference in St. George’s Respiratory Questionnaire points in favor of tai chi (P less than .001)
“This observation, supported also by improvements in dyspnea and exercise performance, suggests that tai chi could be substituted for PR [pulmonary rehabilitation] in the treatment of COPD with greater convenience for patients,” the researchers concluded.
SOURCE: Polkey MI et al. CHEST. 2018 May;153[5]:1116-24.
and seems to confer better long-term improvement, suggests a study published online in the journal CHEST®.
Following 12 weeks of participation in tai chi or pulmonary rehabilitation, patients improved in most of the measurements taken, although no significant between-group differences were observed at that time. However, further improvements were observed in the tai chi group 12 weeks after the intervention had ended. These improvements manifested as a statistically significant 4.5 between-group difference in St. George’s Respiratory Questionnaire points in favor of tai chi (P less than .001)
“This observation, supported also by improvements in dyspnea and exercise performance, suggests that tai chi could be substituted for PR [pulmonary rehabilitation] in the treatment of COPD with greater convenience for patients,” the researchers concluded.
SOURCE: Polkey MI et al. CHEST. 2018 May;153[5]:1116-24.
and seems to confer better long-term improvement, suggests a study published online in the journal CHEST®.
Following 12 weeks of participation in tai chi or pulmonary rehabilitation, patients improved in most of the measurements taken, although no significant between-group differences were observed at that time. However, further improvements were observed in the tai chi group 12 weeks after the intervention had ended. These improvements manifested as a statistically significant 4.5 between-group difference in St. George’s Respiratory Questionnaire points in favor of tai chi (P less than .001)
“This observation, supported also by improvements in dyspnea and exercise performance, suggests that tai chi could be substituted for PR [pulmonary rehabilitation] in the treatment of COPD with greater convenience for patients,” the researchers concluded.
SOURCE: Polkey MI et al. CHEST. 2018 May;153[5]:1116-24.
FROM THE JOURNAL CHEST®
FDA: More COPD patients can use triple therapy
The Food and Drug Administration has approved a new indication for the chronic obstructive pulmonary disease (COPD) therapy fluticasone furoate/umeclidinium/vilanterol (Trelegy Ellipta), which allows physicians to prescribe the drug to a broader class of COPD patients, according to a statement from two pharmaceutical companies.
“Following the initial approval of Trelegy Ellipta in September, we have analysed the data from the IMPACT study and identified additional benefits that this important medicine offers patients with [COPD],” said Hal Barron, MD, chief scientific officer and president of research and development at GlaxoSmithKline, in the statement. “We are pleased that the robust data from the IMPACT study has enabled the expanded indication announced today and the FDA action has been taken so swiftly.”
The results of the IMPACT trial, which was the first study to compare a single-inhaler triple therapy with two dual therapies, were published on April 18 (N Engl J Med 2018. doi: 10.1056/NEJMoa1713901).
This study randomized patients to 52 weeks of either triple inhaled therapy involving a once-daily combination of 100 mcg fluticasone furoate, 62.5 mcg of umeclidinium, and 25 mcg of vilanterol; or dual inhaled therapy involving either 100 mcg fluticasone furoate plus 25 mcg of vilanterol, or 62.5 mcg of umeclidinium plus 25 mcg of vilanterol.
After 1 year, the rate of moderate to severe COPD exacerbations in the triple-therapy group was 0.91 per year, compared with 1.07 in the fluticasone furoate–vilanterol group and 1.21 in the vilanterol-umeclidinium group. This translated to a 15% reduction with triple therapy compared with fluticasone furoate–vilanterol and a 25% reduction, compared with vilanterol-umeclidinium (P less than .001 for both).
The Food and Drug Administration has approved a new indication for the chronic obstructive pulmonary disease (COPD) therapy fluticasone furoate/umeclidinium/vilanterol (Trelegy Ellipta), which allows physicians to prescribe the drug to a broader class of COPD patients, according to a statement from two pharmaceutical companies.
“Following the initial approval of Trelegy Ellipta in September, we have analysed the data from the IMPACT study and identified additional benefits that this important medicine offers patients with [COPD],” said Hal Barron, MD, chief scientific officer and president of research and development at GlaxoSmithKline, in the statement. “We are pleased that the robust data from the IMPACT study has enabled the expanded indication announced today and the FDA action has been taken so swiftly.”
The results of the IMPACT trial, which was the first study to compare a single-inhaler triple therapy with two dual therapies, were published on April 18 (N Engl J Med 2018. doi: 10.1056/NEJMoa1713901).
This study randomized patients to 52 weeks of either triple inhaled therapy involving a once-daily combination of 100 mcg fluticasone furoate, 62.5 mcg of umeclidinium, and 25 mcg of vilanterol; or dual inhaled therapy involving either 100 mcg fluticasone furoate plus 25 mcg of vilanterol, or 62.5 mcg of umeclidinium plus 25 mcg of vilanterol.
After 1 year, the rate of moderate to severe COPD exacerbations in the triple-therapy group was 0.91 per year, compared with 1.07 in the fluticasone furoate–vilanterol group and 1.21 in the vilanterol-umeclidinium group. This translated to a 15% reduction with triple therapy compared with fluticasone furoate–vilanterol and a 25% reduction, compared with vilanterol-umeclidinium (P less than .001 for both).
The Food and Drug Administration has approved a new indication for the chronic obstructive pulmonary disease (COPD) therapy fluticasone furoate/umeclidinium/vilanterol (Trelegy Ellipta), which allows physicians to prescribe the drug to a broader class of COPD patients, according to a statement from two pharmaceutical companies.
“Following the initial approval of Trelegy Ellipta in September, we have analysed the data from the IMPACT study and identified additional benefits that this important medicine offers patients with [COPD],” said Hal Barron, MD, chief scientific officer and president of research and development at GlaxoSmithKline, in the statement. “We are pleased that the robust data from the IMPACT study has enabled the expanded indication announced today and the FDA action has been taken so swiftly.”
The results of the IMPACT trial, which was the first study to compare a single-inhaler triple therapy with two dual therapies, were published on April 18 (N Engl J Med 2018. doi: 10.1056/NEJMoa1713901).
This study randomized patients to 52 weeks of either triple inhaled therapy involving a once-daily combination of 100 mcg fluticasone furoate, 62.5 mcg of umeclidinium, and 25 mcg of vilanterol; or dual inhaled therapy involving either 100 mcg fluticasone furoate plus 25 mcg of vilanterol, or 62.5 mcg of umeclidinium plus 25 mcg of vilanterol.
After 1 year, the rate of moderate to severe COPD exacerbations in the triple-therapy group was 0.91 per year, compared with 1.07 in the fluticasone furoate–vilanterol group and 1.21 in the vilanterol-umeclidinium group. This translated to a 15% reduction with triple therapy compared with fluticasone furoate–vilanterol and a 25% reduction, compared with vilanterol-umeclidinium (P less than .001 for both).
Triple-therapy cuts COPD exacerbations
Triple therapy for chronic obstructive pulmonary disease (COPD) achieved reductions in moderate to severe exacerbations when compared with two kinds of dual therapy, in a study published online in the New England Journal of Medicine.
The trial compared the outcomes of COPD patients using an inhaled therapy comprising a corticosteroid, a long-acting muscarinic antagonist (LAMA), and a long-acting beta2-agonist (LABA) with the outcomes of similar patients taking one of two other therapy combinations – a corticosteroid and a LABA, or a LABA and a LAMA. This trial – Informing the Pathway of COPD Treatment (IMPACT) – included 10,355 patients with symptomatic COPD in 37 countries, according to David A. Lipson, MD, and his colleagues (N Engl J Med. 2018 Apr 18. doi: 10.1056/NEJMoa1713901).
The study randomized patients to 52 weeks of either triple inhaled therapy involving a once-daily combination of 100 mcg fluticasone furoate (a corticosteroid), 62.5 mcg of the LAMA umeclidinium and 25 mcg of the LABA vilanterol; or dual inhaled therapy involving either 100 mcg fluticasone furoate plus 25 mcg of vilanterol, or 62.5 mcg of umeclidinium plus 25 mcg of vilanterol.
After 1 year, the rate of moderate to severe COPD exacerbations in the triple-therapy group was 0.91 per year, compared with 1.07 in the fluticasone furoate–vilanterol group and 1.21 in the vilanterol-umeclidinium group. This translated to a 15% reduction with triple therapy compared with fluticasone furoate–vilanterol and a 25% reduction compared with vilanterol-umeclidinium (P less than .001 for both).
When the analysis was limited to severe exacerbations alone, the difference was significant only between the triple therapy, which GSK is marketing as Trelegy Ellipta, and the vilanterol-umeclidinium dual therapy.
Dr. Lipson, of GSK and the University of Pennsylvania, and his coauthors noted that their finding of a greater benefit with the glucocorticoid-containing dual-therapy compared with the LABA-LAMA vilanterol-umeclidinium combination contradicted the findings of the earlier FLAME trial. This was likely due to differences in patient populations and design, as all patients in the FLAME trial had a 1-month run-in treatment with the bronchodilator tiotropium, the researchers explained.
“Therefore any patients who would require an inhaled glucocorticoid may have had an increase in exacerbations and a decrease in lung function during the run-in period and would have been forced to leave the trial,” they wrote.
Patients with higher eosinophil levels seemed to do even better with triple therapy. In those with eosinophil levels of 150 cells per microliter or above, the annual rate of moderate to severe exacerbations was 0.95 with triple therapy, 1.08 with fluticasone furoate–vilanterol, and 1.39 with vilanterol-umeclidinium.
Triple therapy also was associated with a significantly longer time to first event and greater improvements in quality of life, compared with the dual therapies.
Overall, the adverse event profile of triple therapy was similar to that of dual therapy. Contrasting that finding were differences in the incidences of physician-diagnosed pneumonia between the treatment groups. Physician-diagnosed pneumonia was 53% higher among patients who received fluticasone furoate – either in dual or triple therapy combinations. Eight percent of patients in the triple therapy group experienced pneumonia, compared with 7% of patients in the fluticasone furoate–vilanterol group and 5% in the vilanterol-umeclidinium group.
All-cause mortality was significantly lower in patients who received the inhaled glucocorticoid, although the authors said this finding was “fragile” and needed further investigation.
The rate of discontinuation or withdrawal from the trial was 6% for the triple therapy group, 8% for the fluticasone furoate–vilanterol group, and 9% for the vilanterol-umeclidinium group. The rates of serious adverse events in each group were 22%, 21%, and 23%, respectively.
At trial entry, 38% of patients were already receiving triple therapy and 29% were taking an inhaled glucocorticoid. The authors noted that any patients taking an inhaled glucocorticoid who were randomized to the vilanterol-umeclidinium group would have had to abruptly stop taking their inhaled glucocorticoids.
“It is unknown whether the abrupt discontinuation of inhaled glucocorticoids would have contributed to our finding of a lower rate of exacerbations in the inhaled glucocorticoid groups than in the LAMA-LABA group,” they wrote.
Fernando Martinez, MD, chief of the division of pulmonary and critical care medicine at New York–Presbyterian Hospital/Weill Cornell Medical Center, said the study advanced the understanding of COPD management by addressing some key evidence gaps, in a statement issued by GSK.
“By comparing various combinations of effective medications in the same device the study clarifies which type of patient gains greatest benefit from each class of medicine,” Dr Martinez said in the statement. “As many patients experience frequent exacerbations or ‘flare ups,’ which can often result in hospitalization, these data will be highly relevant to patients and clinicians as they consider the optimal treatment.”
The study was funded by GSK, which manufactures Trelegy Ellipta triple therapy for COPD. Eight authors were employees of GSK and two were on advisory boards for the company. Seven authors declared funding from a range of pharmaceutical companies including GSK. One author had no conflicts of interest to declare.
SOURCE: Lipson D et al. N Engl J Med. 2018 Apr 18. doi: 10.1056/NEJMoa1713901.
The data from the IMPACT study fills a gap in the evidence supporting a step-up from dual to triple inhaled therapy for COPD, which so far has been recommended only for patients with severe loss of lung function and those with frequent exacerbations despite maximum bronchodilator treatment. The study has the strengths of comparing the step-up to triple therapy with the GOLD guideline–recommended dual therapies and using the same dosages in the triple therapy as in the dual therapy
However, it is important to note that nearly 40% of patients enrolled in the trial were already being treated with triple therapy, 70% were receiving a glucocorticoid, and patients with a history of asthma were not excluded. This means patients assigned to the dual therapy without glucocorticoids would have had an abrupt cessation of their glucocorticoid therapy, which may explain a rapid surge in exacerbations in the first month and the lower rate of exacerbations in the dual-therapy group that did include glucocorticoids. The choice of patients for the study could potentially have artificially inflated the observed effectiveness of triple therapy over dual bronchodilator treatment.
As such, we suggest clinicians stick with the GOLD 2017 recommendations that escalation to triple therapy only occur after maximization of bronchodilator treatment.
Dr. Samy Suissa (PhD) is with the Center for Clinical Epidemiology at Lady Davis Institute–Jewish General Hospital, and the departments of epidemiology and biostatistics and medicine at McGill University, Montreal. Dr. Jeffrey M. Drazen is editor-in-chief of the New England Journal of Medicine. These comments are taken from an editorial (N Engl J Med. 2018 Apr 18. doi: 10.1056/NEJMe1716802 ). Dr. Suissa declared personal fees and grants from the pharmaceutical industry outside the submitted work.
The data from the IMPACT study fills a gap in the evidence supporting a step-up from dual to triple inhaled therapy for COPD, which so far has been recommended only for patients with severe loss of lung function and those with frequent exacerbations despite maximum bronchodilator treatment. The study has the strengths of comparing the step-up to triple therapy with the GOLD guideline–recommended dual therapies and using the same dosages in the triple therapy as in the dual therapy
However, it is important to note that nearly 40% of patients enrolled in the trial were already being treated with triple therapy, 70% were receiving a glucocorticoid, and patients with a history of asthma were not excluded. This means patients assigned to the dual therapy without glucocorticoids would have had an abrupt cessation of their glucocorticoid therapy, which may explain a rapid surge in exacerbations in the first month and the lower rate of exacerbations in the dual-therapy group that did include glucocorticoids. The choice of patients for the study could potentially have artificially inflated the observed effectiveness of triple therapy over dual bronchodilator treatment.
As such, we suggest clinicians stick with the GOLD 2017 recommendations that escalation to triple therapy only occur after maximization of bronchodilator treatment.
Dr. Samy Suissa (PhD) is with the Center for Clinical Epidemiology at Lady Davis Institute–Jewish General Hospital, and the departments of epidemiology and biostatistics and medicine at McGill University, Montreal. Dr. Jeffrey M. Drazen is editor-in-chief of the New England Journal of Medicine. These comments are taken from an editorial (N Engl J Med. 2018 Apr 18. doi: 10.1056/NEJMe1716802 ). Dr. Suissa declared personal fees and grants from the pharmaceutical industry outside the submitted work.
The data from the IMPACT study fills a gap in the evidence supporting a step-up from dual to triple inhaled therapy for COPD, which so far has been recommended only for patients with severe loss of lung function and those with frequent exacerbations despite maximum bronchodilator treatment. The study has the strengths of comparing the step-up to triple therapy with the GOLD guideline–recommended dual therapies and using the same dosages in the triple therapy as in the dual therapy
However, it is important to note that nearly 40% of patients enrolled in the trial were already being treated with triple therapy, 70% were receiving a glucocorticoid, and patients with a history of asthma were not excluded. This means patients assigned to the dual therapy without glucocorticoids would have had an abrupt cessation of their glucocorticoid therapy, which may explain a rapid surge in exacerbations in the first month and the lower rate of exacerbations in the dual-therapy group that did include glucocorticoids. The choice of patients for the study could potentially have artificially inflated the observed effectiveness of triple therapy over dual bronchodilator treatment.
As such, we suggest clinicians stick with the GOLD 2017 recommendations that escalation to triple therapy only occur after maximization of bronchodilator treatment.
Dr. Samy Suissa (PhD) is with the Center for Clinical Epidemiology at Lady Davis Institute–Jewish General Hospital, and the departments of epidemiology and biostatistics and medicine at McGill University, Montreal. Dr. Jeffrey M. Drazen is editor-in-chief of the New England Journal of Medicine. These comments are taken from an editorial (N Engl J Med. 2018 Apr 18. doi: 10.1056/NEJMe1716802 ). Dr. Suissa declared personal fees and grants from the pharmaceutical industry outside the submitted work.
Triple therapy for chronic obstructive pulmonary disease (COPD) achieved reductions in moderate to severe exacerbations when compared with two kinds of dual therapy, in a study published online in the New England Journal of Medicine.
The trial compared the outcomes of COPD patients using an inhaled therapy comprising a corticosteroid, a long-acting muscarinic antagonist (LAMA), and a long-acting beta2-agonist (LABA) with the outcomes of similar patients taking one of two other therapy combinations – a corticosteroid and a LABA, or a LABA and a LAMA. This trial – Informing the Pathway of COPD Treatment (IMPACT) – included 10,355 patients with symptomatic COPD in 37 countries, according to David A. Lipson, MD, and his colleagues (N Engl J Med. 2018 Apr 18. doi: 10.1056/NEJMoa1713901).
The study randomized patients to 52 weeks of either triple inhaled therapy involving a once-daily combination of 100 mcg fluticasone furoate (a corticosteroid), 62.5 mcg of the LAMA umeclidinium and 25 mcg of the LABA vilanterol; or dual inhaled therapy involving either 100 mcg fluticasone furoate plus 25 mcg of vilanterol, or 62.5 mcg of umeclidinium plus 25 mcg of vilanterol.
After 1 year, the rate of moderate to severe COPD exacerbations in the triple-therapy group was 0.91 per year, compared with 1.07 in the fluticasone furoate–vilanterol group and 1.21 in the vilanterol-umeclidinium group. This translated to a 15% reduction with triple therapy compared with fluticasone furoate–vilanterol and a 25% reduction compared with vilanterol-umeclidinium (P less than .001 for both).
When the analysis was limited to severe exacerbations alone, the difference was significant only between the triple therapy, which GSK is marketing as Trelegy Ellipta, and the vilanterol-umeclidinium dual therapy.
Dr. Lipson, of GSK and the University of Pennsylvania, and his coauthors noted that their finding of a greater benefit with the glucocorticoid-containing dual-therapy compared with the LABA-LAMA vilanterol-umeclidinium combination contradicted the findings of the earlier FLAME trial. This was likely due to differences in patient populations and design, as all patients in the FLAME trial had a 1-month run-in treatment with the bronchodilator tiotropium, the researchers explained.
“Therefore any patients who would require an inhaled glucocorticoid may have had an increase in exacerbations and a decrease in lung function during the run-in period and would have been forced to leave the trial,” they wrote.
Patients with higher eosinophil levels seemed to do even better with triple therapy. In those with eosinophil levels of 150 cells per microliter or above, the annual rate of moderate to severe exacerbations was 0.95 with triple therapy, 1.08 with fluticasone furoate–vilanterol, and 1.39 with vilanterol-umeclidinium.
Triple therapy also was associated with a significantly longer time to first event and greater improvements in quality of life, compared with the dual therapies.
Overall, the adverse event profile of triple therapy was similar to that of dual therapy. Contrasting that finding were differences in the incidences of physician-diagnosed pneumonia between the treatment groups. Physician-diagnosed pneumonia was 53% higher among patients who received fluticasone furoate – either in dual or triple therapy combinations. Eight percent of patients in the triple therapy group experienced pneumonia, compared with 7% of patients in the fluticasone furoate–vilanterol group and 5% in the vilanterol-umeclidinium group.
All-cause mortality was significantly lower in patients who received the inhaled glucocorticoid, although the authors said this finding was “fragile” and needed further investigation.
The rate of discontinuation or withdrawal from the trial was 6% for the triple therapy group, 8% for the fluticasone furoate–vilanterol group, and 9% for the vilanterol-umeclidinium group. The rates of serious adverse events in each group were 22%, 21%, and 23%, respectively.
At trial entry, 38% of patients were already receiving triple therapy and 29% were taking an inhaled glucocorticoid. The authors noted that any patients taking an inhaled glucocorticoid who were randomized to the vilanterol-umeclidinium group would have had to abruptly stop taking their inhaled glucocorticoids.
“It is unknown whether the abrupt discontinuation of inhaled glucocorticoids would have contributed to our finding of a lower rate of exacerbations in the inhaled glucocorticoid groups than in the LAMA-LABA group,” they wrote.
Fernando Martinez, MD, chief of the division of pulmonary and critical care medicine at New York–Presbyterian Hospital/Weill Cornell Medical Center, said the study advanced the understanding of COPD management by addressing some key evidence gaps, in a statement issued by GSK.
“By comparing various combinations of effective medications in the same device the study clarifies which type of patient gains greatest benefit from each class of medicine,” Dr Martinez said in the statement. “As many patients experience frequent exacerbations or ‘flare ups,’ which can often result in hospitalization, these data will be highly relevant to patients and clinicians as they consider the optimal treatment.”
The study was funded by GSK, which manufactures Trelegy Ellipta triple therapy for COPD. Eight authors were employees of GSK and two were on advisory boards for the company. Seven authors declared funding from a range of pharmaceutical companies including GSK. One author had no conflicts of interest to declare.
SOURCE: Lipson D et al. N Engl J Med. 2018 Apr 18. doi: 10.1056/NEJMoa1713901.
Triple therapy for chronic obstructive pulmonary disease (COPD) achieved reductions in moderate to severe exacerbations when compared with two kinds of dual therapy, in a study published online in the New England Journal of Medicine.
The trial compared the outcomes of COPD patients using an inhaled therapy comprising a corticosteroid, a long-acting muscarinic antagonist (LAMA), and a long-acting beta2-agonist (LABA) with the outcomes of similar patients taking one of two other therapy combinations – a corticosteroid and a LABA, or a LABA and a LAMA. This trial – Informing the Pathway of COPD Treatment (IMPACT) – included 10,355 patients with symptomatic COPD in 37 countries, according to David A. Lipson, MD, and his colleagues (N Engl J Med. 2018 Apr 18. doi: 10.1056/NEJMoa1713901).
The study randomized patients to 52 weeks of either triple inhaled therapy involving a once-daily combination of 100 mcg fluticasone furoate (a corticosteroid), 62.5 mcg of the LAMA umeclidinium and 25 mcg of the LABA vilanterol; or dual inhaled therapy involving either 100 mcg fluticasone furoate plus 25 mcg of vilanterol, or 62.5 mcg of umeclidinium plus 25 mcg of vilanterol.
After 1 year, the rate of moderate to severe COPD exacerbations in the triple-therapy group was 0.91 per year, compared with 1.07 in the fluticasone furoate–vilanterol group and 1.21 in the vilanterol-umeclidinium group. This translated to a 15% reduction with triple therapy compared with fluticasone furoate–vilanterol and a 25% reduction compared with vilanterol-umeclidinium (P less than .001 for both).
When the analysis was limited to severe exacerbations alone, the difference was significant only between the triple therapy, which GSK is marketing as Trelegy Ellipta, and the vilanterol-umeclidinium dual therapy.
Dr. Lipson, of GSK and the University of Pennsylvania, and his coauthors noted that their finding of a greater benefit with the glucocorticoid-containing dual-therapy compared with the LABA-LAMA vilanterol-umeclidinium combination contradicted the findings of the earlier FLAME trial. This was likely due to differences in patient populations and design, as all patients in the FLAME trial had a 1-month run-in treatment with the bronchodilator tiotropium, the researchers explained.
“Therefore any patients who would require an inhaled glucocorticoid may have had an increase in exacerbations and a decrease in lung function during the run-in period and would have been forced to leave the trial,” they wrote.
Patients with higher eosinophil levels seemed to do even better with triple therapy. In those with eosinophil levels of 150 cells per microliter or above, the annual rate of moderate to severe exacerbations was 0.95 with triple therapy, 1.08 with fluticasone furoate–vilanterol, and 1.39 with vilanterol-umeclidinium.
Triple therapy also was associated with a significantly longer time to first event and greater improvements in quality of life, compared with the dual therapies.
Overall, the adverse event profile of triple therapy was similar to that of dual therapy. Contrasting that finding were differences in the incidences of physician-diagnosed pneumonia between the treatment groups. Physician-diagnosed pneumonia was 53% higher among patients who received fluticasone furoate – either in dual or triple therapy combinations. Eight percent of patients in the triple therapy group experienced pneumonia, compared with 7% of patients in the fluticasone furoate–vilanterol group and 5% in the vilanterol-umeclidinium group.
All-cause mortality was significantly lower in patients who received the inhaled glucocorticoid, although the authors said this finding was “fragile” and needed further investigation.
The rate of discontinuation or withdrawal from the trial was 6% for the triple therapy group, 8% for the fluticasone furoate–vilanterol group, and 9% for the vilanterol-umeclidinium group. The rates of serious adverse events in each group were 22%, 21%, and 23%, respectively.
At trial entry, 38% of patients were already receiving triple therapy and 29% were taking an inhaled glucocorticoid. The authors noted that any patients taking an inhaled glucocorticoid who were randomized to the vilanterol-umeclidinium group would have had to abruptly stop taking their inhaled glucocorticoids.
“It is unknown whether the abrupt discontinuation of inhaled glucocorticoids would have contributed to our finding of a lower rate of exacerbations in the inhaled glucocorticoid groups than in the LAMA-LABA group,” they wrote.
Fernando Martinez, MD, chief of the division of pulmonary and critical care medicine at New York–Presbyterian Hospital/Weill Cornell Medical Center, said the study advanced the understanding of COPD management by addressing some key evidence gaps, in a statement issued by GSK.
“By comparing various combinations of effective medications in the same device the study clarifies which type of patient gains greatest benefit from each class of medicine,” Dr Martinez said in the statement. “As many patients experience frequent exacerbations or ‘flare ups,’ which can often result in hospitalization, these data will be highly relevant to patients and clinicians as they consider the optimal treatment.”
The study was funded by GSK, which manufactures Trelegy Ellipta triple therapy for COPD. Eight authors were employees of GSK and two were on advisory boards for the company. Seven authors declared funding from a range of pharmaceutical companies including GSK. One author had no conflicts of interest to declare.
SOURCE: Lipson D et al. N Engl J Med. 2018 Apr 18. doi: 10.1056/NEJMoa1713901.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Triple COPD therapy shows fewer exacerbations than does dual therapy.
Major finding: Triple COPD therapy achieves a 15%-25% greater reduction in exacerbations compared with dual therapy.
Study details: Randomized controlled trial of 10,355 patients with symptomatic COPD.
Disclosures: The study was funded by GlaxoSmithKline, which manufactures Trelegy Ellipta triple therapy for COPD. Eight authors were employees of GlaxoSmithKline and two were on advisory boards for the company. Seven authors declared funding from a range of pharmaceutical companies including GlaxoSmithKline. One author had no conflicts of interest to declare.
Source: Lipson D et al. N Engl J Med. 2018 Apr 18. doi: 10.1056/NEJMoa1713901.
Life and health are not even across the U.S.
While U.S. death rates have declined overall, marked geographic disparities exist at the state level in burden of disease, injuries, and risk factors, according to a comprehensive analysis.
Life expectancy varies substantially, for example, ranging from a high of 81.3 years in Hawaii to a low of 74.7 years in Mississippi, according to results from the analysis of data from the Global Burden of Disease (GBD) study (JAMA. 2018;319[14]:1444-72).
Previously decreasing death rates for adults have reversed in 19 states, according to the analysis, which covers the years 1990 to 2016.
Hardest hit were Kentucky, New Mexico, Oklahoma, West Virginia, and Wyoming, which had mortality increases of more than 10% among adults aged 20-55 years. Those increases were largely due to causes such as substance use disorders, self-harm, and cirrhosis, according to the US Burden of Disease Collaborators, who authored the report.
“These findings should be used to examine the causes of health variations and to plan, develop, and implement programs and policies to improve health overall and eliminate disparities in the United States,” the authors wrote.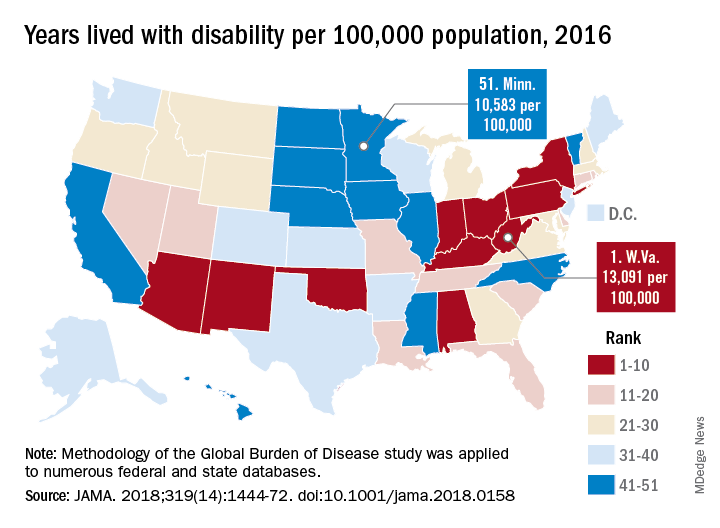
Overall, U.S. death rates have declined from 745.2 per 100,000 persons in 1990 to 578.0 per 100,000 persons in 2016, according to the report.
Likewise, health outcomes throughout the United States have improved over time for some conditions, such as ischemic heart disease, lung cancer, and neonatal preterm complications, the report says.
However, those gains are offset by rising death rates due to drug-use disorders, chronic kidney disease, cirrhosis, chronic obstructive pulmonary disease, hypertension, and self-harm.

The three most important risk factors in the United States are high body mass index, smoking, and high fasting plasma glucose, the analysis showed. Of those risk factors, only smoking is decreasing, authors noted.
Many risk factors contributing to disparities in burden among states are amenable to medical treatment that emphasizes supportive behavioral and lifestyle changes, according to the authors.
“Expanding health coverage for certain conditions and medications should be considered and adopted to reduce burden,” they said.
Substance abuse disorders, cirrhosis, and self-harm, the causes of the mortality reversal in Kentucky, New Mexico, and other states, could be addressed via a wide range of interventions, according to the investigators.
Prevention programs could address the root causes of substance use and causes of relapse, while physicians can play a “major role” in addiction control through counseling of patients on pain control medication, they said.
Interventions to treat hepatitis C and decrease excessive alcohol consumption could help address cirrhosis, while for self-harm, the most promising approaches focus on restricting access to lethal means, they said, noting that a large proportion of U.S. suicides are due to firearms.
“While multiple strategies are available for dealing with these problems, they have not until very recently garnered attention,” investigators wrote.
The study was supported in part by the National Institute of Environmental Health Sciences and the Bill and Melinda Gates Foundation. Some individual study collaborators reported disclosures related to Savient, Takeda, Crealta/Horizon, Regeneron, Allergan, and others.
SOURCE: The US Burden of Disease Collaborators. JAMA 2018;319(14):1444-72.
This report on Global Burden of Disease (GBD) study data profoundly and powerfully illuminates U.S. health trends over time and by geography. There is much unfinished business for us, nationally and at the state level.
Clinicians and policy makers can use the rankings to evaluate why many individuals are still experiencing injury, disease, and deaths that are preventable; in doing so, the entire nation could move closely resemble a United States of health.
Clinicians could use the results to help guide patients through evidence-based disease prevention and early intervention, a strategy that has led to decreases in death due to cancer and cardiovascular disease over the past few decades.
At the same time, policy makers could use GBD 2016 results to reevaluate current national attitudes toward disease prevention.
Howard K. Koh, MD, MPH, is with the Harvard T.H. Chan School of Public Health, Boston. Anand K. Parekh, MD, MPH, is with the Bipartisan Policy Center in Washington. The comments above are derived from an editorial accompanying the report from the US Burden of Disease Collaborators ( JAMA. 2018;319[14]:1438-40 ). Dr. Koh and Dr. Parekh reported no conflicts of interest related to the editorial.
This report on Global Burden of Disease (GBD) study data profoundly and powerfully illuminates U.S. health trends over time and by geography. There is much unfinished business for us, nationally and at the state level.
Clinicians and policy makers can use the rankings to evaluate why many individuals are still experiencing injury, disease, and deaths that are preventable; in doing so, the entire nation could move closely resemble a United States of health.
Clinicians could use the results to help guide patients through evidence-based disease prevention and early intervention, a strategy that has led to decreases in death due to cancer and cardiovascular disease over the past few decades.
At the same time, policy makers could use GBD 2016 results to reevaluate current national attitudes toward disease prevention.
Howard K. Koh, MD, MPH, is with the Harvard T.H. Chan School of Public Health, Boston. Anand K. Parekh, MD, MPH, is with the Bipartisan Policy Center in Washington. The comments above are derived from an editorial accompanying the report from the US Burden of Disease Collaborators ( JAMA. 2018;319[14]:1438-40 ). Dr. Koh and Dr. Parekh reported no conflicts of interest related to the editorial.
This report on Global Burden of Disease (GBD) study data profoundly and powerfully illuminates U.S. health trends over time and by geography. There is much unfinished business for us, nationally and at the state level.
Clinicians and policy makers can use the rankings to evaluate why many individuals are still experiencing injury, disease, and deaths that are preventable; in doing so, the entire nation could move closely resemble a United States of health.
Clinicians could use the results to help guide patients through evidence-based disease prevention and early intervention, a strategy that has led to decreases in death due to cancer and cardiovascular disease over the past few decades.
At the same time, policy makers could use GBD 2016 results to reevaluate current national attitudes toward disease prevention.
Howard K. Koh, MD, MPH, is with the Harvard T.H. Chan School of Public Health, Boston. Anand K. Parekh, MD, MPH, is with the Bipartisan Policy Center in Washington. The comments above are derived from an editorial accompanying the report from the US Burden of Disease Collaborators ( JAMA. 2018;319[14]:1438-40 ). Dr. Koh and Dr. Parekh reported no conflicts of interest related to the editorial.
While U.S. death rates have declined overall, marked geographic disparities exist at the state level in burden of disease, injuries, and risk factors, according to a comprehensive analysis.
Life expectancy varies substantially, for example, ranging from a high of 81.3 years in Hawaii to a low of 74.7 years in Mississippi, according to results from the analysis of data from the Global Burden of Disease (GBD) study (JAMA. 2018;319[14]:1444-72).
Previously decreasing death rates for adults have reversed in 19 states, according to the analysis, which covers the years 1990 to 2016.
Hardest hit were Kentucky, New Mexico, Oklahoma, West Virginia, and Wyoming, which had mortality increases of more than 10% among adults aged 20-55 years. Those increases were largely due to causes such as substance use disorders, self-harm, and cirrhosis, according to the US Burden of Disease Collaborators, who authored the report.
“These findings should be used to examine the causes of health variations and to plan, develop, and implement programs and policies to improve health overall and eliminate disparities in the United States,” the authors wrote.
Overall, U.S. death rates have declined from 745.2 per 100,000 persons in 1990 to 578.0 per 100,000 persons in 2016, according to the report.
Likewise, health outcomes throughout the United States have improved over time for some conditions, such as ischemic heart disease, lung cancer, and neonatal preterm complications, the report says.
However, those gains are offset by rising death rates due to drug-use disorders, chronic kidney disease, cirrhosis, chronic obstructive pulmonary disease, hypertension, and self-harm.

The three most important risk factors in the United States are high body mass index, smoking, and high fasting plasma glucose, the analysis showed. Of those risk factors, only smoking is decreasing, authors noted.
Many risk factors contributing to disparities in burden among states are amenable to medical treatment that emphasizes supportive behavioral and lifestyle changes, according to the authors.
“Expanding health coverage for certain conditions and medications should be considered and adopted to reduce burden,” they said.
Substance abuse disorders, cirrhosis, and self-harm, the causes of the mortality reversal in Kentucky, New Mexico, and other states, could be addressed via a wide range of interventions, according to the investigators.
Prevention programs could address the root causes of substance use and causes of relapse, while physicians can play a “major role” in addiction control through counseling of patients on pain control medication, they said.
Interventions to treat hepatitis C and decrease excessive alcohol consumption could help address cirrhosis, while for self-harm, the most promising approaches focus on restricting access to lethal means, they said, noting that a large proportion of U.S. suicides are due to firearms.
“While multiple strategies are available for dealing with these problems, they have not until very recently garnered attention,” investigators wrote.
The study was supported in part by the National Institute of Environmental Health Sciences and the Bill and Melinda Gates Foundation. Some individual study collaborators reported disclosures related to Savient, Takeda, Crealta/Horizon, Regeneron, Allergan, and others.
SOURCE: The US Burden of Disease Collaborators. JAMA 2018;319(14):1444-72.
While U.S. death rates have declined overall, marked geographic disparities exist at the state level in burden of disease, injuries, and risk factors, according to a comprehensive analysis.
Life expectancy varies substantially, for example, ranging from a high of 81.3 years in Hawaii to a low of 74.7 years in Mississippi, according to results from the analysis of data from the Global Burden of Disease (GBD) study (JAMA. 2018;319[14]:1444-72).
Previously decreasing death rates for adults have reversed in 19 states, according to the analysis, which covers the years 1990 to 2016.
Hardest hit were Kentucky, New Mexico, Oklahoma, West Virginia, and Wyoming, which had mortality increases of more than 10% among adults aged 20-55 years. Those increases were largely due to causes such as substance use disorders, self-harm, and cirrhosis, according to the US Burden of Disease Collaborators, who authored the report.
“These findings should be used to examine the causes of health variations and to plan, develop, and implement programs and policies to improve health overall and eliminate disparities in the United States,” the authors wrote.
Overall, U.S. death rates have declined from 745.2 per 100,000 persons in 1990 to 578.0 per 100,000 persons in 2016, according to the report.
Likewise, health outcomes throughout the United States have improved over time for some conditions, such as ischemic heart disease, lung cancer, and neonatal preterm complications, the report says.
However, those gains are offset by rising death rates due to drug-use disorders, chronic kidney disease, cirrhosis, chronic obstructive pulmonary disease, hypertension, and self-harm.

The three most important risk factors in the United States are high body mass index, smoking, and high fasting plasma glucose, the analysis showed. Of those risk factors, only smoking is decreasing, authors noted.
Many risk factors contributing to disparities in burden among states are amenable to medical treatment that emphasizes supportive behavioral and lifestyle changes, according to the authors.
“Expanding health coverage for certain conditions and medications should be considered and adopted to reduce burden,” they said.
Substance abuse disorders, cirrhosis, and self-harm, the causes of the mortality reversal in Kentucky, New Mexico, and other states, could be addressed via a wide range of interventions, according to the investigators.
Prevention programs could address the root causes of substance use and causes of relapse, while physicians can play a “major role” in addiction control through counseling of patients on pain control medication, they said.
Interventions to treat hepatitis C and decrease excessive alcohol consumption could help address cirrhosis, while for self-harm, the most promising approaches focus on restricting access to lethal means, they said, noting that a large proportion of U.S. suicides are due to firearms.
“While multiple strategies are available for dealing with these problems, they have not until very recently garnered attention,” investigators wrote.
The study was supported in part by the National Institute of Environmental Health Sciences and the Bill and Melinda Gates Foundation. Some individual study collaborators reported disclosures related to Savient, Takeda, Crealta/Horizon, Regeneron, Allergan, and others.
SOURCE: The US Burden of Disease Collaborators. JAMA 2018;319(14):1444-72.
FROM JAMA
Key clinical point: While U.S. death rates have declined overall, marked geographic disparities exist at the state level in burden of disease, injuries, and risk factors.
Major finding: Life expectancy ranged from a high of 81.3 years in Hawaii to a low of 74.7 years in Mississippi, and previously decreasing death rates for adults have reversed in 19 states.
Study details: A U.S. state-level analysis of results from the Global Burden of Disease (GBD) study illustrating trends in diseases, injuries, risk factors, and deaths from 1990 to 2016.
Disclosures: The study was supported in part by the National Institute of Environmental Health Sciences and the Bill and Melinda Gates Foundation. Study authors reported disclosures related to Savient, Takeda, Crealta/Horizon, Regeneron, Allergan, and others.
Source: The US Burden of Disease Collaborators. JAMA 2018;319(14):1444-1472.
The outcomes of “GOLD 2017”
After the Global Initiative for Chronic Obstructive Lung Disease released updated recommendations for grading COPD patients’ level of disease in November of 2016, Imran Iftikhar, MD, tried to incorporate them into his practice, but he encountered problems.
For one thing, the new classification system, which became known as GOLD 2017, uncoupled spirometry results from the ABCD treatment algorithm. “I found it wasn’t really helping me in terms of prognostication or COPD management,” said Dr. Iftikhar, section chief of pulmonary and critical care at Emory Saint Joseph’s Hospital, Atlanta. “Although the purpose of the GOLD classification was not really meant for prognostication, most practicing physicians are frequently asked about prognosis by patients, and I am not sure if the 2017 reclassification really helps with that.”
The GOLD 2017 classification simplified the chronic obstructive pulmonary disease staging that was available from 2011 to 2015 from three variables (spirometry thresholds, exacerbation risk, and dyspnea scale) to two variables (exacerbation risk and dyspnea scale). In the 2017 report, authors of the new guidelines characterized forced expiratory volume in 1 second (FEV1) as “a poor predictor of disease status” and proposed that clinicians derive ABCD groups exclusively from patient symptoms and their exacerbations. FEV1 is an “important parameter at the population level” in predicting hospitalization and mortality, the authors wrote, but keeping results separate “acknowledges the limitations of FEV1 in making treatment decisions for individualized patient care and highlights the importance of patient symptoms and exacerbation risks in guiding therapies in COPD.”
According to Meilan Han, MD, MS, a member of the GOLD Science Committee, since release of the 2017 guidelines, “clinicians have indicated that they like the flexibility the system provides in separating spirometry, symptoms, and exacerbation risk as this more accurately reflects the heterogeneity we see in the COPD patient population.” Nevertheless, how this approach influences long-term outcomes remains unclear.
Daniel Ouellette, MD, FCCP, a pulmonologist with the Henry Ford Health System in Detroit, described the GOLD 2017 criteria as “a good step forward” but said he wasn’t sure if the optimal or perfect tool exists for categorizing COPD patients’ level of disease.
“I think what we see is an effort to use all of these criteria to help us better treat our patients. I think ,” he said in an interview.
“All guidelines need to be modified as further research becomes available. I think that the frontiers of this area are going to be to incorporate new elements such as tobacco history, more emphasis on clinical signs and symptoms, and use of markers other than spirometry, such as eosinophil count, to categorize patients with COPD,” Dr. Ouellette added.
In an analysis of the GOLD 2017 criteria applied to 819 COPD patients in Spain and the United States, published online Nov. 3, 2017, in the American Journal of Respiratory and Critical Care Medicine, Carlos Cabrera López, MD, and his colleagues concluded that the mortality risk was better predicted by the 2015 GOLD classification system than by the 2017 iteration (Am J Respir Crit Care Med. 2018 Feb. doi: 10.101164/rccm.201707-1363OC).
The distribution of Charlson index scores also changed. Whereas group D was higher than B in 2015, they became similar in the 2017 system. For her part, Dr. Han emphasized that the primary goal of the GOLD ABCD classification system is to categorize patients with respect to treatment groups. “Current therapy targets symptoms and exacerbations, which are the key current elements of the classification schema,” she said in an interview. “The results of the Cabrera Lopez analysis are not necessarily unexpected, as FEV1 is associated with mortality.”
In a prospective, multicenter analysis, Portuguese researchers compared the performance of GOLD 2011 and 2017 in terms of how 200 COPD patients were reclassified, the level of agreement between the two iterations, and the performance of each to predict future exacerbations (COPD. 2018 Feb;15[1]; 21-6). They found that about half of patients classified as GOLD D under the 2011 guidelines became classified as GOLD B when the 2017 version was used, and the extent of agreement between the two iterations was moderate (P less than .001). They also found that the two versions of the guidelines were equivalently effective at predicting exacerbations (69.7% vs. 67.6% in the 2011 and 2017 iterations, respectively). In addition, patients who met the criteria for a GOLD B grouping in the 2017 iteration exacerbated 17% more often and had a lower percent predicted post bronchodilator FEV1 than did those who met the criteria for a GOLD B classification under the 2011 guidelines.
Dr. Han, who is also an associate professor of medicine at the University of Michigan Hospital, acknowledged that GOLD 2017 has resulted in the reclassification of some previously group D patients as group B patients. “Our primary goal is to aid clinicians with the diagnosis and management of patients with COPD,” she said. “We look forward to additional data coming in from ongoing clinical trials that will provide longer term data to further refine treatment algorithms.”
In a recent study of more than 33,000 Danish patients older than age 30 with COPD, researchers led by Anne Gedebjerg, MD, found that the GOLD 2017 ABCD classification did not predict all-cause and respiratory mortality more accurately than previous GOLD iterations from 2007 and 2011. Area under the curve for all-cause mortality was 0.61 for GOLD 2007, 0.61 for GOLD 2011, and 0.63 for GOLD 2017, while the area under the curve for respiratory mortality was 0.64 for GOLD 2007, 0.63 for GOLD 2011, and 0.65 for GOLD 2017 (Lancet Respir Med. 2018 Jan;6[3]:204-12).
However, when the spirometric stages 1-4 were combined with the A to D groupings based on symptoms and exacerbations, the 2017 classification predicted mortality with greater accuracy, compared with previous iterations (P less than .0001). “My practice is very much like this paper,” Dr. Iftikhar said. “I use both the spirometric grade and the ABCD grouping to specify which ‘group’ and ‘grade’ my patient belongs to. I think future investigators need to combine ABCD with spirometry classification to see how we can improve the classification system.”
In a commentary published in the same issue of the Lancet Respiratory Medicine as the large Danish study, Joan B. Soriano, MD, PhD, wrote that the 2011 GOLD guideline’s collapse of four spirometric thresholds (greater than 80%, 50%-80%, 30%-50%, and less than 30%) into just two (greater than 50% or 50% or less) “reduced the system’s ability to inform and predict mortality from the short term up to 10 years” (Lancet Respir Med. 2018 Jan;6[3]:165-6).
Lung function remains the best available biomarker for life expectancy in both patients with COPD and the general population,” wrote Dr. Soriano, a respiratory medicine researcher based in Madrid, Spain.
Additional important outcomes
Dr. Ouellette noted that while mortality is an important outcome for COPD patients, it’s not the only outcome of interest. “In addition to [trying to] help people live longer, which is certainly a desirable goal, we also want to make people be able to be more functional during their life, have fewer hospitalizations, and have less of a need of other types of supportive medical care for worsening of their disease,” he said. “The fact that the current guidelines don’t improve mortality more than the previous ones may not be a negative thing. It may tell us that the previous guidelines already did a pretty good job of helping us to improve mortality.”
Dr. Ouellette was quick to add that none of inhaled drugs currently available to treat COPD have been conclusively shown to improve mortality. “The only things we know that improve mortality for COPD patients are quitting smoking and using oxygen if a patient meets predefined goals for oxygen,” he said. “So the fact that GOLD criteria doesn’t improve mortality shouldn’t make us think that it’s not a useful tool. We already know that the medicines may not help people live longer.”
Dr. Han pointed out that spirometry “is still used to further clarify the choice of therapy recommended based on the nature and degree of airflow obstruction in light of severity of patient symptoms. The data are still designed to be used in conjunction to personalize therapy for patients.”
She added that the GOLD Science Committee “welcomes additional data analyses so that future recommendations can be further refined.”
Dr. Han disclosed that she has consulted for Boehringer Ingelheim, AstraZeneca, and GlaxoSmithKline. She has also received in-kind research support from Novartis and Sunovion.
Dr. Iftikhar reported having no financial disclosures. Dr. Ouellette is a member of CHEST® Physician’s editorial advisory board. He disclosed being part of a federally funded study being carried out by the Patient-Centered Outcomes Research Institute.
There was no industry involvement in the GOLD 2017 report, but many of its authors and board members had pharmaceutical company ties, and GOLD’s treatment advice relies on data from industry-sponsored studies.
After the Global Initiative for Chronic Obstructive Lung Disease released updated recommendations for grading COPD patients’ level of disease in November of 2016, Imran Iftikhar, MD, tried to incorporate them into his practice, but he encountered problems.
For one thing, the new classification system, which became known as GOLD 2017, uncoupled spirometry results from the ABCD treatment algorithm. “I found it wasn’t really helping me in terms of prognostication or COPD management,” said Dr. Iftikhar, section chief of pulmonary and critical care at Emory Saint Joseph’s Hospital, Atlanta. “Although the purpose of the GOLD classification was not really meant for prognostication, most practicing physicians are frequently asked about prognosis by patients, and I am not sure if the 2017 reclassification really helps with that.”
The GOLD 2017 classification simplified the chronic obstructive pulmonary disease staging that was available from 2011 to 2015 from three variables (spirometry thresholds, exacerbation risk, and dyspnea scale) to two variables (exacerbation risk and dyspnea scale). In the 2017 report, authors of the new guidelines characterized forced expiratory volume in 1 second (FEV1) as “a poor predictor of disease status” and proposed that clinicians derive ABCD groups exclusively from patient symptoms and their exacerbations. FEV1 is an “important parameter at the population level” in predicting hospitalization and mortality, the authors wrote, but keeping results separate “acknowledges the limitations of FEV1 in making treatment decisions for individualized patient care and highlights the importance of patient symptoms and exacerbation risks in guiding therapies in COPD.”
According to Meilan Han, MD, MS, a member of the GOLD Science Committee, since release of the 2017 guidelines, “clinicians have indicated that they like the flexibility the system provides in separating spirometry, symptoms, and exacerbation risk as this more accurately reflects the heterogeneity we see in the COPD patient population.” Nevertheless, how this approach influences long-term outcomes remains unclear.
Daniel Ouellette, MD, FCCP, a pulmonologist with the Henry Ford Health System in Detroit, described the GOLD 2017 criteria as “a good step forward” but said he wasn’t sure if the optimal or perfect tool exists for categorizing COPD patients’ level of disease.
“I think what we see is an effort to use all of these criteria to help us better treat our patients. I think ,” he said in an interview.
“All guidelines need to be modified as further research becomes available. I think that the frontiers of this area are going to be to incorporate new elements such as tobacco history, more emphasis on clinical signs and symptoms, and use of markers other than spirometry, such as eosinophil count, to categorize patients with COPD,” Dr. Ouellette added.
In an analysis of the GOLD 2017 criteria applied to 819 COPD patients in Spain and the United States, published online Nov. 3, 2017, in the American Journal of Respiratory and Critical Care Medicine, Carlos Cabrera López, MD, and his colleagues concluded that the mortality risk was better predicted by the 2015 GOLD classification system than by the 2017 iteration (Am J Respir Crit Care Med. 2018 Feb. doi: 10.101164/rccm.201707-1363OC).
The distribution of Charlson index scores also changed. Whereas group D was higher than B in 2015, they became similar in the 2017 system. For her part, Dr. Han emphasized that the primary goal of the GOLD ABCD classification system is to categorize patients with respect to treatment groups. “Current therapy targets symptoms and exacerbations, which are the key current elements of the classification schema,” she said in an interview. “The results of the Cabrera Lopez analysis are not necessarily unexpected, as FEV1 is associated with mortality.”
In a prospective, multicenter analysis, Portuguese researchers compared the performance of GOLD 2011 and 2017 in terms of how 200 COPD patients were reclassified, the level of agreement between the two iterations, and the performance of each to predict future exacerbations (COPD. 2018 Feb;15[1]; 21-6). They found that about half of patients classified as GOLD D under the 2011 guidelines became classified as GOLD B when the 2017 version was used, and the extent of agreement between the two iterations was moderate (P less than .001). They also found that the two versions of the guidelines were equivalently effective at predicting exacerbations (69.7% vs. 67.6% in the 2011 and 2017 iterations, respectively). In addition, patients who met the criteria for a GOLD B grouping in the 2017 iteration exacerbated 17% more often and had a lower percent predicted post bronchodilator FEV1 than did those who met the criteria for a GOLD B classification under the 2011 guidelines.
Dr. Han, who is also an associate professor of medicine at the University of Michigan Hospital, acknowledged that GOLD 2017 has resulted in the reclassification of some previously group D patients as group B patients. “Our primary goal is to aid clinicians with the diagnosis and management of patients with COPD,” she said. “We look forward to additional data coming in from ongoing clinical trials that will provide longer term data to further refine treatment algorithms.”
In a recent study of more than 33,000 Danish patients older than age 30 with COPD, researchers led by Anne Gedebjerg, MD, found that the GOLD 2017 ABCD classification did not predict all-cause and respiratory mortality more accurately than previous GOLD iterations from 2007 and 2011. Area under the curve for all-cause mortality was 0.61 for GOLD 2007, 0.61 for GOLD 2011, and 0.63 for GOLD 2017, while the area under the curve for respiratory mortality was 0.64 for GOLD 2007, 0.63 for GOLD 2011, and 0.65 for GOLD 2017 (Lancet Respir Med. 2018 Jan;6[3]:204-12).
However, when the spirometric stages 1-4 were combined with the A to D groupings based on symptoms and exacerbations, the 2017 classification predicted mortality with greater accuracy, compared with previous iterations (P less than .0001). “My practice is very much like this paper,” Dr. Iftikhar said. “I use both the spirometric grade and the ABCD grouping to specify which ‘group’ and ‘grade’ my patient belongs to. I think future investigators need to combine ABCD with spirometry classification to see how we can improve the classification system.”
In a commentary published in the same issue of the Lancet Respiratory Medicine as the large Danish study, Joan B. Soriano, MD, PhD, wrote that the 2011 GOLD guideline’s collapse of four spirometric thresholds (greater than 80%, 50%-80%, 30%-50%, and less than 30%) into just two (greater than 50% or 50% or less) “reduced the system’s ability to inform and predict mortality from the short term up to 10 years” (Lancet Respir Med. 2018 Jan;6[3]:165-6).
Lung function remains the best available biomarker for life expectancy in both patients with COPD and the general population,” wrote Dr. Soriano, a respiratory medicine researcher based in Madrid, Spain.
Additional important outcomes
Dr. Ouellette noted that while mortality is an important outcome for COPD patients, it’s not the only outcome of interest. “In addition to [trying to] help people live longer, which is certainly a desirable goal, we also want to make people be able to be more functional during their life, have fewer hospitalizations, and have less of a need of other types of supportive medical care for worsening of their disease,” he said. “The fact that the current guidelines don’t improve mortality more than the previous ones may not be a negative thing. It may tell us that the previous guidelines already did a pretty good job of helping us to improve mortality.”
Dr. Ouellette was quick to add that none of inhaled drugs currently available to treat COPD have been conclusively shown to improve mortality. “The only things we know that improve mortality for COPD patients are quitting smoking and using oxygen if a patient meets predefined goals for oxygen,” he said. “So the fact that GOLD criteria doesn’t improve mortality shouldn’t make us think that it’s not a useful tool. We already know that the medicines may not help people live longer.”
Dr. Han pointed out that spirometry “is still used to further clarify the choice of therapy recommended based on the nature and degree of airflow obstruction in light of severity of patient symptoms. The data are still designed to be used in conjunction to personalize therapy for patients.”
She added that the GOLD Science Committee “welcomes additional data analyses so that future recommendations can be further refined.”
Dr. Han disclosed that she has consulted for Boehringer Ingelheim, AstraZeneca, and GlaxoSmithKline. She has also received in-kind research support from Novartis and Sunovion.
Dr. Iftikhar reported having no financial disclosures. Dr. Ouellette is a member of CHEST® Physician’s editorial advisory board. He disclosed being part of a federally funded study being carried out by the Patient-Centered Outcomes Research Institute.
There was no industry involvement in the GOLD 2017 report, but many of its authors and board members had pharmaceutical company ties, and GOLD’s treatment advice relies on data from industry-sponsored studies.
After the Global Initiative for Chronic Obstructive Lung Disease released updated recommendations for grading COPD patients’ level of disease in November of 2016, Imran Iftikhar, MD, tried to incorporate them into his practice, but he encountered problems.
For one thing, the new classification system, which became known as GOLD 2017, uncoupled spirometry results from the ABCD treatment algorithm. “I found it wasn’t really helping me in terms of prognostication or COPD management,” said Dr. Iftikhar, section chief of pulmonary and critical care at Emory Saint Joseph’s Hospital, Atlanta. “Although the purpose of the GOLD classification was not really meant for prognostication, most practicing physicians are frequently asked about prognosis by patients, and I am not sure if the 2017 reclassification really helps with that.”
The GOLD 2017 classification simplified the chronic obstructive pulmonary disease staging that was available from 2011 to 2015 from three variables (spirometry thresholds, exacerbation risk, and dyspnea scale) to two variables (exacerbation risk and dyspnea scale). In the 2017 report, authors of the new guidelines characterized forced expiratory volume in 1 second (FEV1) as “a poor predictor of disease status” and proposed that clinicians derive ABCD groups exclusively from patient symptoms and their exacerbations. FEV1 is an “important parameter at the population level” in predicting hospitalization and mortality, the authors wrote, but keeping results separate “acknowledges the limitations of FEV1 in making treatment decisions for individualized patient care and highlights the importance of patient symptoms and exacerbation risks in guiding therapies in COPD.”
According to Meilan Han, MD, MS, a member of the GOLD Science Committee, since release of the 2017 guidelines, “clinicians have indicated that they like the flexibility the system provides in separating spirometry, symptoms, and exacerbation risk as this more accurately reflects the heterogeneity we see in the COPD patient population.” Nevertheless, how this approach influences long-term outcomes remains unclear.
Daniel Ouellette, MD, FCCP, a pulmonologist with the Henry Ford Health System in Detroit, described the GOLD 2017 criteria as “a good step forward” but said he wasn’t sure if the optimal or perfect tool exists for categorizing COPD patients’ level of disease.
“I think what we see is an effort to use all of these criteria to help us better treat our patients. I think ,” he said in an interview.
“All guidelines need to be modified as further research becomes available. I think that the frontiers of this area are going to be to incorporate new elements such as tobacco history, more emphasis on clinical signs and symptoms, and use of markers other than spirometry, such as eosinophil count, to categorize patients with COPD,” Dr. Ouellette added.
In an analysis of the GOLD 2017 criteria applied to 819 COPD patients in Spain and the United States, published online Nov. 3, 2017, in the American Journal of Respiratory and Critical Care Medicine, Carlos Cabrera López, MD, and his colleagues concluded that the mortality risk was better predicted by the 2015 GOLD classification system than by the 2017 iteration (Am J Respir Crit Care Med. 2018 Feb. doi: 10.101164/rccm.201707-1363OC).
The distribution of Charlson index scores also changed. Whereas group D was higher than B in 2015, they became similar in the 2017 system. For her part, Dr. Han emphasized that the primary goal of the GOLD ABCD classification system is to categorize patients with respect to treatment groups. “Current therapy targets symptoms and exacerbations, which are the key current elements of the classification schema,” she said in an interview. “The results of the Cabrera Lopez analysis are not necessarily unexpected, as FEV1 is associated with mortality.”
In a prospective, multicenter analysis, Portuguese researchers compared the performance of GOLD 2011 and 2017 in terms of how 200 COPD patients were reclassified, the level of agreement between the two iterations, and the performance of each to predict future exacerbations (COPD. 2018 Feb;15[1]; 21-6). They found that about half of patients classified as GOLD D under the 2011 guidelines became classified as GOLD B when the 2017 version was used, and the extent of agreement between the two iterations was moderate (P less than .001). They also found that the two versions of the guidelines were equivalently effective at predicting exacerbations (69.7% vs. 67.6% in the 2011 and 2017 iterations, respectively). In addition, patients who met the criteria for a GOLD B grouping in the 2017 iteration exacerbated 17% more often and had a lower percent predicted post bronchodilator FEV1 than did those who met the criteria for a GOLD B classification under the 2011 guidelines.
Dr. Han, who is also an associate professor of medicine at the University of Michigan Hospital, acknowledged that GOLD 2017 has resulted in the reclassification of some previously group D patients as group B patients. “Our primary goal is to aid clinicians with the diagnosis and management of patients with COPD,” she said. “We look forward to additional data coming in from ongoing clinical trials that will provide longer term data to further refine treatment algorithms.”
In a recent study of more than 33,000 Danish patients older than age 30 with COPD, researchers led by Anne Gedebjerg, MD, found that the GOLD 2017 ABCD classification did not predict all-cause and respiratory mortality more accurately than previous GOLD iterations from 2007 and 2011. Area under the curve for all-cause mortality was 0.61 for GOLD 2007, 0.61 for GOLD 2011, and 0.63 for GOLD 2017, while the area under the curve for respiratory mortality was 0.64 for GOLD 2007, 0.63 for GOLD 2011, and 0.65 for GOLD 2017 (Lancet Respir Med. 2018 Jan;6[3]:204-12).
However, when the spirometric stages 1-4 were combined with the A to D groupings based on symptoms and exacerbations, the 2017 classification predicted mortality with greater accuracy, compared with previous iterations (P less than .0001). “My practice is very much like this paper,” Dr. Iftikhar said. “I use both the spirometric grade and the ABCD grouping to specify which ‘group’ and ‘grade’ my patient belongs to. I think future investigators need to combine ABCD with spirometry classification to see how we can improve the classification system.”
In a commentary published in the same issue of the Lancet Respiratory Medicine as the large Danish study, Joan B. Soriano, MD, PhD, wrote that the 2011 GOLD guideline’s collapse of four spirometric thresholds (greater than 80%, 50%-80%, 30%-50%, and less than 30%) into just two (greater than 50% or 50% or less) “reduced the system’s ability to inform and predict mortality from the short term up to 10 years” (Lancet Respir Med. 2018 Jan;6[3]:165-6).
Lung function remains the best available biomarker for life expectancy in both patients with COPD and the general population,” wrote Dr. Soriano, a respiratory medicine researcher based in Madrid, Spain.
Additional important outcomes
Dr. Ouellette noted that while mortality is an important outcome for COPD patients, it’s not the only outcome of interest. “In addition to [trying to] help people live longer, which is certainly a desirable goal, we also want to make people be able to be more functional during their life, have fewer hospitalizations, and have less of a need of other types of supportive medical care for worsening of their disease,” he said. “The fact that the current guidelines don’t improve mortality more than the previous ones may not be a negative thing. It may tell us that the previous guidelines already did a pretty good job of helping us to improve mortality.”
Dr. Ouellette was quick to add that none of inhaled drugs currently available to treat COPD have been conclusively shown to improve mortality. “The only things we know that improve mortality for COPD patients are quitting smoking and using oxygen if a patient meets predefined goals for oxygen,” he said. “So the fact that GOLD criteria doesn’t improve mortality shouldn’t make us think that it’s not a useful tool. We already know that the medicines may not help people live longer.”
Dr. Han pointed out that spirometry “is still used to further clarify the choice of therapy recommended based on the nature and degree of airflow obstruction in light of severity of patient symptoms. The data are still designed to be used in conjunction to personalize therapy for patients.”
She added that the GOLD Science Committee “welcomes additional data analyses so that future recommendations can be further refined.”
Dr. Han disclosed that she has consulted for Boehringer Ingelheim, AstraZeneca, and GlaxoSmithKline. She has also received in-kind research support from Novartis and Sunovion.
Dr. Iftikhar reported having no financial disclosures. Dr. Ouellette is a member of CHEST® Physician’s editorial advisory board. He disclosed being part of a federally funded study being carried out by the Patient-Centered Outcomes Research Institute.
There was no industry involvement in the GOLD 2017 report, but many of its authors and board members had pharmaceutical company ties, and GOLD’s treatment advice relies on data from industry-sponsored studies.
Good definitions, research lacking for COPD-asthma overlap
ORLANDO – to give clinicians data they can actually use.
The topic is even more pressing given the growing interest and research into biological treatments for asthma and consideration of their possible use in COPD, experts said at the joint congress of the American Academy of Allergy, Asthma, and Immunology and the World Asthma Organization. Their remarks came in what was ostensibly a “debate” on whether ACOS is a distinct entity requiring special treatment but largely turned into a discussion about gaps in knowledge on the topic.
“The problem here is that it has not been defined in a way that everyone agrees on – that does create a problem because, if there’s no consensus on the diagnostic criteria, then it may be difficult to study this overlap,” said Donald Tashkin, MD, director of the pulmonary function laboratories at the University of California, Los Angeles. “Because there is no agreement on how to diagnose ACOS, it hasn’t been studied with respect to its responsiveness to different treatment options.”R. Stokes Peebles Jr., MD, professor of allergy, pulmonary, and critical care medicine at Vanderbilt University Medical Center, Nashville, Tenn., said that, although the number of published articles on ACOS has skyrocketed over the last several years, review articles have outnumbered original research articles.
There is disagreement in published definitions: One set of definitions includes a criterion of fractional exhaled nitric oxide not seen in any other definitions, whereas some other definitions require a history of smoking while others don’t, he said.
“How does one manage a disease without a definition and without clinical studies? It’s impossible for me to know,” Dr. Peebles said.
Jeffrey Drazen, MD, the Distinguished Parker B. Francis Professor of Medicine at Harvard Medical School, Boston, and the editor of the New England Journal of Medicine, also lamented the polar nature of the research.
“We all treat patients in the middle, everybody does, all the time – and we would love more guidance,” he said. “One of the reasons the number of articles has gone up is that there have been lots of case definitions. But we can’t get consensus. So who do we have to bring to the table to get a consensus definition so we can get the funding we need from the drug companies or governmental bodies to do the research that we all want?”
Dr. Tashkin said the way forward could be to draw on the points of consensus that do exist on certain criteria.
Dr. Peebles said an international panel is needed to draw up a consensus guideline, with the panel including both pulmonologists and allergists – “people experienced with clinical trials, who take care of a lot of patients.”
ORLANDO – to give clinicians data they can actually use.
The topic is even more pressing given the growing interest and research into biological treatments for asthma and consideration of their possible use in COPD, experts said at the joint congress of the American Academy of Allergy, Asthma, and Immunology and the World Asthma Organization. Their remarks came in what was ostensibly a “debate” on whether ACOS is a distinct entity requiring special treatment but largely turned into a discussion about gaps in knowledge on the topic.
“The problem here is that it has not been defined in a way that everyone agrees on – that does create a problem because, if there’s no consensus on the diagnostic criteria, then it may be difficult to study this overlap,” said Donald Tashkin, MD, director of the pulmonary function laboratories at the University of California, Los Angeles. “Because there is no agreement on how to diagnose ACOS, it hasn’t been studied with respect to its responsiveness to different treatment options.”R. Stokes Peebles Jr., MD, professor of allergy, pulmonary, and critical care medicine at Vanderbilt University Medical Center, Nashville, Tenn., said that, although the number of published articles on ACOS has skyrocketed over the last several years, review articles have outnumbered original research articles.
There is disagreement in published definitions: One set of definitions includes a criterion of fractional exhaled nitric oxide not seen in any other definitions, whereas some other definitions require a history of smoking while others don’t, he said.
“How does one manage a disease without a definition and without clinical studies? It’s impossible for me to know,” Dr. Peebles said.
Jeffrey Drazen, MD, the Distinguished Parker B. Francis Professor of Medicine at Harvard Medical School, Boston, and the editor of the New England Journal of Medicine, also lamented the polar nature of the research.
“We all treat patients in the middle, everybody does, all the time – and we would love more guidance,” he said. “One of the reasons the number of articles has gone up is that there have been lots of case definitions. But we can’t get consensus. So who do we have to bring to the table to get a consensus definition so we can get the funding we need from the drug companies or governmental bodies to do the research that we all want?”
Dr. Tashkin said the way forward could be to draw on the points of consensus that do exist on certain criteria.
Dr. Peebles said an international panel is needed to draw up a consensus guideline, with the panel including both pulmonologists and allergists – “people experienced with clinical trials, who take care of a lot of patients.”
ORLANDO – to give clinicians data they can actually use.
The topic is even more pressing given the growing interest and research into biological treatments for asthma and consideration of their possible use in COPD, experts said at the joint congress of the American Academy of Allergy, Asthma, and Immunology and the World Asthma Organization. Their remarks came in what was ostensibly a “debate” on whether ACOS is a distinct entity requiring special treatment but largely turned into a discussion about gaps in knowledge on the topic.
“The problem here is that it has not been defined in a way that everyone agrees on – that does create a problem because, if there’s no consensus on the diagnostic criteria, then it may be difficult to study this overlap,” said Donald Tashkin, MD, director of the pulmonary function laboratories at the University of California, Los Angeles. “Because there is no agreement on how to diagnose ACOS, it hasn’t been studied with respect to its responsiveness to different treatment options.”R. Stokes Peebles Jr., MD, professor of allergy, pulmonary, and critical care medicine at Vanderbilt University Medical Center, Nashville, Tenn., said that, although the number of published articles on ACOS has skyrocketed over the last several years, review articles have outnumbered original research articles.
There is disagreement in published definitions: One set of definitions includes a criterion of fractional exhaled nitric oxide not seen in any other definitions, whereas some other definitions require a history of smoking while others don’t, he said.
“How does one manage a disease without a definition and without clinical studies? It’s impossible for me to know,” Dr. Peebles said.
Jeffrey Drazen, MD, the Distinguished Parker B. Francis Professor of Medicine at Harvard Medical School, Boston, and the editor of the New England Journal of Medicine, also lamented the polar nature of the research.
“We all treat patients in the middle, everybody does, all the time – and we would love more guidance,” he said. “One of the reasons the number of articles has gone up is that there have been lots of case definitions. But we can’t get consensus. So who do we have to bring to the table to get a consensus definition so we can get the funding we need from the drug companies or governmental bodies to do the research that we all want?”
Dr. Tashkin said the way forward could be to draw on the points of consensus that do exist on certain criteria.
Dr. Peebles said an international panel is needed to draw up a consensus guideline, with the panel including both pulmonologists and allergists – “people experienced with clinical trials, who take care of a lot of patients.”
EXPERT ANALYSIS FROM AAAAI/WAO JOINT CONGRESS
House cleaning linked to lung function decline
that has found accelerated decline in lung function among women regularly engaged in cleaning activities.
The longitudinal population-based cohort study, published online Feb. 16 in the American Journal of Respiratory and Critical Care Medicine, looked at the lung health of 6,230 people who were followed for more than 20 years as part of the European Community Respiratory Health Survey.
Analysis based on questionnaires about cleaning practices revealed that women who were responsible for cleaning at home or who worked as professional cleaners showed significantly greater declines in maximum forced vital capacity (FVC) and maximum forced expiratory volume in 1 second (FEV1), compared with women who said they did not regularly clean.
However, there was no association between cleaning practices in men – either professional or domestic – and accelerated lung function decline. The authors suggested that the exposures experienced by men who worked as cleaners may have been different from the exposures experienced by women. They also noted that the small numbers of male cleaners meant the study wasn’t powered to pick up greater declines in lung function.
The study also showed a significant association between use of cleaning products and decline in lung function. Women who used sprays or other cleaning agents at least once a week showed significantly greater declines in FEV1 and FVC, compared with women who didn’t use cleaning products. Again, this effect was not significant in men.
“One possible mechanism for the accelerated decline in cleaners is the repetitive exposure to low-grade irritative cleaning agents over time, thereby causing persistent changes in the airways,” the authors wrote. “Repeated exposure could lead to remodelling of the airways, thereby over time causing an accelerated decline in FVC and FEV1.”
The analysis found no significant increases in the incidence of chronic airway obstruction among regular cleaners, nor among those who used cleaning products. The authors noted that while previous studies had suggested an increase in chronic obstructive pulmonary disease among occupational cleaners, their study reported relatively few cases of COPD.
While the prevalence of asthma was slightly higher in the two groups of women exposed to regular cleaning (12.3% and 13.7%, versus 9.6%), adjustment for asthma in the analysis did not change the associations. This suggests that the declines in lung function seen in regular cleaners were not mediated by cleaning-related asthma, the researchers noted.
They also noted that the women who reported not engaging in any cleaning may represent a particular socioeconomic group, but adjustment for socioeconomic status did not alter the associations.
The European Community Respiratory Health Survey is supported by the European Union, the European Commission, and the Medical Research Council. No conflicts of interest were reported.
SOURCE: Svanes Ø et al. Am J Resp Crit Care Med. 2018 Feb 16. doi: 10.1164/rccm.201706-1311OC.
that has found accelerated decline in lung function among women regularly engaged in cleaning activities.
The longitudinal population-based cohort study, published online Feb. 16 in the American Journal of Respiratory and Critical Care Medicine, looked at the lung health of 6,230 people who were followed for more than 20 years as part of the European Community Respiratory Health Survey.
Analysis based on questionnaires about cleaning practices revealed that women who were responsible for cleaning at home or who worked as professional cleaners showed significantly greater declines in maximum forced vital capacity (FVC) and maximum forced expiratory volume in 1 second (FEV1), compared with women who said they did not regularly clean.
However, there was no association between cleaning practices in men – either professional or domestic – and accelerated lung function decline. The authors suggested that the exposures experienced by men who worked as cleaners may have been different from the exposures experienced by women. They also noted that the small numbers of male cleaners meant the study wasn’t powered to pick up greater declines in lung function.
The study also showed a significant association between use of cleaning products and decline in lung function. Women who used sprays or other cleaning agents at least once a week showed significantly greater declines in FEV1 and FVC, compared with women who didn’t use cleaning products. Again, this effect was not significant in men.
“One possible mechanism for the accelerated decline in cleaners is the repetitive exposure to low-grade irritative cleaning agents over time, thereby causing persistent changes in the airways,” the authors wrote. “Repeated exposure could lead to remodelling of the airways, thereby over time causing an accelerated decline in FVC and FEV1.”
The analysis found no significant increases in the incidence of chronic airway obstruction among regular cleaners, nor among those who used cleaning products. The authors noted that while previous studies had suggested an increase in chronic obstructive pulmonary disease among occupational cleaners, their study reported relatively few cases of COPD.
While the prevalence of asthma was slightly higher in the two groups of women exposed to regular cleaning (12.3% and 13.7%, versus 9.6%), adjustment for asthma in the analysis did not change the associations. This suggests that the declines in lung function seen in regular cleaners were not mediated by cleaning-related asthma, the researchers noted.
They also noted that the women who reported not engaging in any cleaning may represent a particular socioeconomic group, but adjustment for socioeconomic status did not alter the associations.
The European Community Respiratory Health Survey is supported by the European Union, the European Commission, and the Medical Research Council. No conflicts of interest were reported.
SOURCE: Svanes Ø et al. Am J Resp Crit Care Med. 2018 Feb 16. doi: 10.1164/rccm.201706-1311OC.
that has found accelerated decline in lung function among women regularly engaged in cleaning activities.
The longitudinal population-based cohort study, published online Feb. 16 in the American Journal of Respiratory and Critical Care Medicine, looked at the lung health of 6,230 people who were followed for more than 20 years as part of the European Community Respiratory Health Survey.
Analysis based on questionnaires about cleaning practices revealed that women who were responsible for cleaning at home or who worked as professional cleaners showed significantly greater declines in maximum forced vital capacity (FVC) and maximum forced expiratory volume in 1 second (FEV1), compared with women who said they did not regularly clean.
However, there was no association between cleaning practices in men – either professional or domestic – and accelerated lung function decline. The authors suggested that the exposures experienced by men who worked as cleaners may have been different from the exposures experienced by women. They also noted that the small numbers of male cleaners meant the study wasn’t powered to pick up greater declines in lung function.
The study also showed a significant association between use of cleaning products and decline in lung function. Women who used sprays or other cleaning agents at least once a week showed significantly greater declines in FEV1 and FVC, compared with women who didn’t use cleaning products. Again, this effect was not significant in men.
“One possible mechanism for the accelerated decline in cleaners is the repetitive exposure to low-grade irritative cleaning agents over time, thereby causing persistent changes in the airways,” the authors wrote. “Repeated exposure could lead to remodelling of the airways, thereby over time causing an accelerated decline in FVC and FEV1.”
The analysis found no significant increases in the incidence of chronic airway obstruction among regular cleaners, nor among those who used cleaning products. The authors noted that while previous studies had suggested an increase in chronic obstructive pulmonary disease among occupational cleaners, their study reported relatively few cases of COPD.
While the prevalence of asthma was slightly higher in the two groups of women exposed to regular cleaning (12.3% and 13.7%, versus 9.6%), adjustment for asthma in the analysis did not change the associations. This suggests that the declines in lung function seen in regular cleaners were not mediated by cleaning-related asthma, the researchers noted.
They also noted that the women who reported not engaging in any cleaning may represent a particular socioeconomic group, but adjustment for socioeconomic status did not alter the associations.
The European Community Respiratory Health Survey is supported by the European Union, the European Commission, and the Medical Research Council. No conflicts of interest were reported.
SOURCE: Svanes Ø et al. Am J Resp Crit Care Med. 2018 Feb 16. doi: 10.1164/rccm.201706-1311OC.
FROM AMERICAN JOURNAL OF RESPIRATORY AND CRITICAL CARE MEDICINE
Key clinical point: Women – but not men – who regularly clean homes either domestically or professionally show accelerated declines in lung function.
Major finding: Women who work as cleaners or clean their own homes regularly show greater declines in FEV1 and FVC, compared with women who do not clean regularly.
Data source: Longitudinal cohort study of 6,230 individuals in the European Community Respiratory Health Survey.
Disclosures: The European Community Respiratory Health Survey is supported by the European Union, the European Commission, and the Medical Research Council. No conflicts of interest were provided.
Source: Svanes Ø et al. Am J Resp Crit Care Med. 2018 Feb. 16. doi: 10.1164/rccm.201706-1311OC.
FDA’s standards for approving generics are questioned
TORONTO – The Food and Drug Administration’s standards for demonstrating pharmacokinetic bioequivalence between two inhaled products, which allow for single batch comparisons of approved and generic candidate products, need to be revised to address batch to batch variability, suggested a presenter at the CHEST annual meeting.
Marketing approval of a new generic drug in the United States, including orally inhaled products, generally requires a demonstration of pharmacokinetic bioequivalence to a reference listed product. The standard criterion for statistical bioequivalence applied by the FDA requires the pharmacokinetics of the generic to be within about 10% of the branded product.
In early pharmacokinetic bioequivalence studies, Elise Burmeister Getz, PhD, and her colleagues compared single batches of their generic candidate OT329 Solis 100/50 to single batches of Advair Diskus 100/50 in five individual studies and single batches of Advair Diskus 100/50 to single batches of the same drug. They also found Advair Diskus 100/50 batches that were more than 30% different from each other.
“When patients differ from one another, we put many patients in the trial. And when batches differ from one another, we should be putting many batches in the trial,” Dr. Burmeister Getz, director of clinical pharmacology at Oriel Therapeutics, said at the CHEST meeting. “If we want a robust assessment of bioequivalence and not just a check the box exercise, we really need to have product sampling that’s aligned with product variability.”
When the researchers combined the data in a meta-analysis, bioequivalence was demonstrated, but the pooled analysis could not be used for FDA registration because of its retrospective nature.
They later conducted a prospective study with multiple batches of both the generic and branded drugs. This multiple-batch bioequivalence study involved 96 healthy subjects using 16 batches each of Advair Diskus and Oriel’s OT329 Solis 100/50. A single inhalation was administered to healthy adult subjects in a randomized crossover design and blood samples were collected pre dose and up to 48 hours after inhalation.
With the FDA’s definition of bioequivalence, the generic candidate fell within the bioequivalence goalposts, Dr. Burmeister Getz noted.
The issue of pharmacokinetic variance is not unique to Advair Diskus, but she and her colleagues don’t understand why different batches show such wide variability, Dr. Burmeister Getz noted.
“The advantage of this multibatch approach is that the results of the bioequivalence assessment aren’t dependent on the single batch that happened to be chosen for the study. They are generalizable to the product because the product has been robustly represented in the study,” Dr. Burmeister Getz told attendees.
Oriel makes OT329 Solis 100/50, a fully substitutable generic to Advair Diskus 100/50, which is indicated for treating asthma. Both are multidose dry powder oral inhalation products containing fluticasone propionate, to reduce inflammation in the lungs, and salmeterol, to relax muscles in the airways, for the maintenance treatment of asthma. Advair Diskus at higher doses is indicated for asthma and COPD.
An FDA response?
Asked what the FDA makes of the batch-to-batch variability data, Dr. Burmeister Getz answered simply, “We don’t know.” Before she and her colleagues ran the 16 batch per product study, they submitted their protocol to the FDA for review, but 1 year later, they still hadn’t heard any response.
“Sponsors are apparently allowed to simply pick their batch in a careful and, dare I say manipulative way, to gain the result they want. With a single batch study the selection of batch will absolutely determine the outcome of the study.”
In vitro bioequivalence studies are already required to use multiple batches, she noted.
This research was funded by Oriel Therapeutics, an indirect wholly-owned subsidiary of Novartis AG.
TORONTO – The Food and Drug Administration’s standards for demonstrating pharmacokinetic bioequivalence between two inhaled products, which allow for single batch comparisons of approved and generic candidate products, need to be revised to address batch to batch variability, suggested a presenter at the CHEST annual meeting.
Marketing approval of a new generic drug in the United States, including orally inhaled products, generally requires a demonstration of pharmacokinetic bioequivalence to a reference listed product. The standard criterion for statistical bioequivalence applied by the FDA requires the pharmacokinetics of the generic to be within about 10% of the branded product.
In early pharmacokinetic bioequivalence studies, Elise Burmeister Getz, PhD, and her colleagues compared single batches of their generic candidate OT329 Solis 100/50 to single batches of Advair Diskus 100/50 in five individual studies and single batches of Advair Diskus 100/50 to single batches of the same drug. They also found Advair Diskus 100/50 batches that were more than 30% different from each other.
“When patients differ from one another, we put many patients in the trial. And when batches differ from one another, we should be putting many batches in the trial,” Dr. Burmeister Getz, director of clinical pharmacology at Oriel Therapeutics, said at the CHEST meeting. “If we want a robust assessment of bioequivalence and not just a check the box exercise, we really need to have product sampling that’s aligned with product variability.”
When the researchers combined the data in a meta-analysis, bioequivalence was demonstrated, but the pooled analysis could not be used for FDA registration because of its retrospective nature.
They later conducted a prospective study with multiple batches of both the generic and branded drugs. This multiple-batch bioequivalence study involved 96 healthy subjects using 16 batches each of Advair Diskus and Oriel’s OT329 Solis 100/50. A single inhalation was administered to healthy adult subjects in a randomized crossover design and blood samples were collected pre dose and up to 48 hours after inhalation.
With the FDA’s definition of bioequivalence, the generic candidate fell within the bioequivalence goalposts, Dr. Burmeister Getz noted.
The issue of pharmacokinetic variance is not unique to Advair Diskus, but she and her colleagues don’t understand why different batches show such wide variability, Dr. Burmeister Getz noted.
“The advantage of this multibatch approach is that the results of the bioequivalence assessment aren’t dependent on the single batch that happened to be chosen for the study. They are generalizable to the product because the product has been robustly represented in the study,” Dr. Burmeister Getz told attendees.
Oriel makes OT329 Solis 100/50, a fully substitutable generic to Advair Diskus 100/50, which is indicated for treating asthma. Both are multidose dry powder oral inhalation products containing fluticasone propionate, to reduce inflammation in the lungs, and salmeterol, to relax muscles in the airways, for the maintenance treatment of asthma. Advair Diskus at higher doses is indicated for asthma and COPD.
An FDA response?
Asked what the FDA makes of the batch-to-batch variability data, Dr. Burmeister Getz answered simply, “We don’t know.” Before she and her colleagues ran the 16 batch per product study, they submitted their protocol to the FDA for review, but 1 year later, they still hadn’t heard any response.
“Sponsors are apparently allowed to simply pick their batch in a careful and, dare I say manipulative way, to gain the result they want. With a single batch study the selection of batch will absolutely determine the outcome of the study.”
In vitro bioequivalence studies are already required to use multiple batches, she noted.
This research was funded by Oriel Therapeutics, an indirect wholly-owned subsidiary of Novartis AG.
TORONTO – The Food and Drug Administration’s standards for demonstrating pharmacokinetic bioequivalence between two inhaled products, which allow for single batch comparisons of approved and generic candidate products, need to be revised to address batch to batch variability, suggested a presenter at the CHEST annual meeting.
Marketing approval of a new generic drug in the United States, including orally inhaled products, generally requires a demonstration of pharmacokinetic bioequivalence to a reference listed product. The standard criterion for statistical bioequivalence applied by the FDA requires the pharmacokinetics of the generic to be within about 10% of the branded product.
In early pharmacokinetic bioequivalence studies, Elise Burmeister Getz, PhD, and her colleagues compared single batches of their generic candidate OT329 Solis 100/50 to single batches of Advair Diskus 100/50 in five individual studies and single batches of Advair Diskus 100/50 to single batches of the same drug. They also found Advair Diskus 100/50 batches that were more than 30% different from each other.
“When patients differ from one another, we put many patients in the trial. And when batches differ from one another, we should be putting many batches in the trial,” Dr. Burmeister Getz, director of clinical pharmacology at Oriel Therapeutics, said at the CHEST meeting. “If we want a robust assessment of bioequivalence and not just a check the box exercise, we really need to have product sampling that’s aligned with product variability.”
When the researchers combined the data in a meta-analysis, bioequivalence was demonstrated, but the pooled analysis could not be used for FDA registration because of its retrospective nature.
They later conducted a prospective study with multiple batches of both the generic and branded drugs. This multiple-batch bioequivalence study involved 96 healthy subjects using 16 batches each of Advair Diskus and Oriel’s OT329 Solis 100/50. A single inhalation was administered to healthy adult subjects in a randomized crossover design and blood samples were collected pre dose and up to 48 hours after inhalation.
With the FDA’s definition of bioequivalence, the generic candidate fell within the bioequivalence goalposts, Dr. Burmeister Getz noted.
The issue of pharmacokinetic variance is not unique to Advair Diskus, but she and her colleagues don’t understand why different batches show such wide variability, Dr. Burmeister Getz noted.
“The advantage of this multibatch approach is that the results of the bioequivalence assessment aren’t dependent on the single batch that happened to be chosen for the study. They are generalizable to the product because the product has been robustly represented in the study,” Dr. Burmeister Getz told attendees.
Oriel makes OT329 Solis 100/50, a fully substitutable generic to Advair Diskus 100/50, which is indicated for treating asthma. Both are multidose dry powder oral inhalation products containing fluticasone propionate, to reduce inflammation in the lungs, and salmeterol, to relax muscles in the airways, for the maintenance treatment of asthma. Advair Diskus at higher doses is indicated for asthma and COPD.
An FDA response?
Asked what the FDA makes of the batch-to-batch variability data, Dr. Burmeister Getz answered simply, “We don’t know.” Before she and her colleagues ran the 16 batch per product study, they submitted their protocol to the FDA for review, but 1 year later, they still hadn’t heard any response.
“Sponsors are apparently allowed to simply pick their batch in a careful and, dare I say manipulative way, to gain the result they want. With a single batch study the selection of batch will absolutely determine the outcome of the study.”
In vitro bioequivalence studies are already required to use multiple batches, she noted.
This research was funded by Oriel Therapeutics, an indirect wholly-owned subsidiary of Novartis AG.
AT CHEST 2017
Key clinical point: The FDA’s standards for demonstrating pharmacokinetic bioequivalence between two inhaled products need to be revised to address batch to batch variability.
Major finding: Investigators found Advair Diskus 100/50 batches that were more than 30% different from each other.
Data source: Pharmacokinetic bioequivalence studies comparing batches of Advair Diskus 100/50 to each other, and to batches of the generic candidate OT329 Solis 100/50.
Disclosures: This research was funded by Oriel Therapeutics, an indirect wholly-owned subsidiary of Novartis AG. Dr. Burmeister Getz is director of clinical pharmacology at Oriel Therapeutics.
FDA approves starting dose of roflumilast
The Food and Drug Administration has approved the use of a 250-mcg dose of roflumilast for patients with chronic obstructive pulmonary disease (COPD) for 4 weeks, followed by the use of 500-mcg therapeutic doses, according to a statement from the drug’s marketer, AstraZeneca.
The larger doses of roflumilast (Daliresp) are currently indicated for reducing the risk of COPD exacerbations in patients with severe COPD associated with chronic bronchitis and a history of exacerbations, according to the statement. The selective phosphodiesterase-4 inhibitor, roflumilast, was approved for this use in 500-mcg doses in 2011. The new smaller doses of the drug are being offered to help reduce the rate of treatment discontinuation with use of the higher therapeutic dosing. The 250-mcg doses of roflumilast are not to be used as treatment for COPD.
“As the only once-daily tablet to provide enhanced protection against COPD exacerbations when added to current bronchodilator therapy, this is an important new dosing option to help patients start and stay on treatment. Exacerbations are associated with hospitalizations and an accelerated decline in lung function, and these patients living with COPD need effective treatment options,” Tosh Butt, vice president, respiratory, at AstraZeneca, said in the press release.
The approval of use of the 250-mcg doses was based on data from the OPTIMIZE study (Evaluation of Tolerability and Pharmacokinetics of Roflumilast trial, 250 mcg and 500 mcg, as an add-on to Standard COPD Treatment to Treat Severe COPD), according to the statement.
Over 12 weeks, the percentage of patients stopping treatment was significantly lower in those first given 250 mcg of roflumilast daily for 4 weeks, followed by 500 mcg once a week for 8 weeks (18.4%), compared with those given 500 mcg of roflumilast daily for 12 weeks (24.6%; odds ratio, 0.66; 95% confidence interval, 0.47-0.93; P = .017).
In eight controlled clinical trials, the most common adverse effects were diarrhea, weight loss, nausea, headache, back pain, influenza, insomnia, dizziness, and decreased appetite.
The Food and Drug Administration has approved the use of a 250-mcg dose of roflumilast for patients with chronic obstructive pulmonary disease (COPD) for 4 weeks, followed by the use of 500-mcg therapeutic doses, according to a statement from the drug’s marketer, AstraZeneca.
The larger doses of roflumilast (Daliresp) are currently indicated for reducing the risk of COPD exacerbations in patients with severe COPD associated with chronic bronchitis and a history of exacerbations, according to the statement. The selective phosphodiesterase-4 inhibitor, roflumilast, was approved for this use in 500-mcg doses in 2011. The new smaller doses of the drug are being offered to help reduce the rate of treatment discontinuation with use of the higher therapeutic dosing. The 250-mcg doses of roflumilast are not to be used as treatment for COPD.
“As the only once-daily tablet to provide enhanced protection against COPD exacerbations when added to current bronchodilator therapy, this is an important new dosing option to help patients start and stay on treatment. Exacerbations are associated with hospitalizations and an accelerated decline in lung function, and these patients living with COPD need effective treatment options,” Tosh Butt, vice president, respiratory, at AstraZeneca, said in the press release.
The approval of use of the 250-mcg doses was based on data from the OPTIMIZE study (Evaluation of Tolerability and Pharmacokinetics of Roflumilast trial, 250 mcg and 500 mcg, as an add-on to Standard COPD Treatment to Treat Severe COPD), according to the statement.
Over 12 weeks, the percentage of patients stopping treatment was significantly lower in those first given 250 mcg of roflumilast daily for 4 weeks, followed by 500 mcg once a week for 8 weeks (18.4%), compared with those given 500 mcg of roflumilast daily for 12 weeks (24.6%; odds ratio, 0.66; 95% confidence interval, 0.47-0.93; P = .017).
In eight controlled clinical trials, the most common adverse effects were diarrhea, weight loss, nausea, headache, back pain, influenza, insomnia, dizziness, and decreased appetite.
The Food and Drug Administration has approved the use of a 250-mcg dose of roflumilast for patients with chronic obstructive pulmonary disease (COPD) for 4 weeks, followed by the use of 500-mcg therapeutic doses, according to a statement from the drug’s marketer, AstraZeneca.
The larger doses of roflumilast (Daliresp) are currently indicated for reducing the risk of COPD exacerbations in patients with severe COPD associated with chronic bronchitis and a history of exacerbations, according to the statement. The selective phosphodiesterase-4 inhibitor, roflumilast, was approved for this use in 500-mcg doses in 2011. The new smaller doses of the drug are being offered to help reduce the rate of treatment discontinuation with use of the higher therapeutic dosing. The 250-mcg doses of roflumilast are not to be used as treatment for COPD.
“As the only once-daily tablet to provide enhanced protection against COPD exacerbations when added to current bronchodilator therapy, this is an important new dosing option to help patients start and stay on treatment. Exacerbations are associated with hospitalizations and an accelerated decline in lung function, and these patients living with COPD need effective treatment options,” Tosh Butt, vice president, respiratory, at AstraZeneca, said in the press release.
The approval of use of the 250-mcg doses was based on data from the OPTIMIZE study (Evaluation of Tolerability and Pharmacokinetics of Roflumilast trial, 250 mcg and 500 mcg, as an add-on to Standard COPD Treatment to Treat Severe COPD), according to the statement.
Over 12 weeks, the percentage of patients stopping treatment was significantly lower in those first given 250 mcg of roflumilast daily for 4 weeks, followed by 500 mcg once a week for 8 weeks (18.4%), compared with those given 500 mcg of roflumilast daily for 12 weeks (24.6%; odds ratio, 0.66; 95% confidence interval, 0.47-0.93; P = .017).
In eight controlled clinical trials, the most common adverse effects were diarrhea, weight loss, nausea, headache, back pain, influenza, insomnia, dizziness, and decreased appetite.
First month of LABA/LAMA ups cardiovascular risk
New use of inhaled long-acting beta-2 agonists (LABAs) or long-acting antimuscarinic antagonists (LAMAs) was associated with a 1.5-fold increased cardiovascular risk within 30 days of initiation in patients with chronic obstructive pulmonary disease, irrespective of prior cardiovascular disease status and history of exacerbations, according to a review of more than 280,000 COPD patients in Taiwan.
The relationship between cardiovascular disease (CVD) and LABAs and LAMAs in chronic obstructive pulmonary disease (COPD) has long been debated. The new study addressed some limitations of previous studies, which had found conflicting results ranging from no increased risk to up to a 4.5-fold increased risk of cardiovascular events when the medications were used for COPD.
Previous randomized trials haven’t raised much concern, but they included prior users who may have developed tolerance to the heart effects and excluded patients with baseline CVD. “We caution physicians to closely monitor new users of LABAs or LAMAs for cardiovascular symptoms.” Health care professionals should be vigilant for any cardiovascular symptoms during the first 30 days of inhalation therapy, said investigators led by Meng-Ting Wang, PhD, of the National Defense Medical Center, Taipei.
“We suspect that there may exist a subgroup of patients with COPD who are particularly at risk of CVD with initial exposure to LABAs or LAMAs ... we suggest that the use of inhaled long-acting bronchodilators in COPD needs to be carefully assessed, and a thorough cardiovascular physical examination, especially heart rate measurement and electrocardiograms, needs to be performed” before prescribing LABAs and LAMAs, they wrote in an article in JAMA Internal Medicine.
The team identified 284,220 COPD patients in the Taiwan National Health Insurance Research Database during 2007-2011 who were new to the medications. During a mean follow-up of 2 years, 37,719 developed severe CVD requiring hospitalization or emergency care, including coronary artery disease, heart failure, ischemic stroke, and arrhythmia.
The team compared their CVD subjects with controls who did not have a heart event and found that new LABA and LAMA use in COPD was associated with a 1.50-fold (95% confidence interval, 1.35-1.67; P less than .001) and a 1.52-fold (95% CI, 1.28-1.80; P less than .001) increased cardiovascular risk within 30 days of initiation, respectively.
One severe CVD event requiring hospitalization or ED care occurred for every 406 (95% CI, 303-580) new LABA users and 391 (95% CI, 254-725) new LAMA users during the first 30 days of therapy.
The LABA- and LAMA-associated CVD risk remained significant, regardless of patients’ CVD history and COPD exacerbations. Analyses of individual CVD outcomes revealed increased risks of coronary artery disease and heart failure with LABA and LAMA treatment, and an increased risk for cardiac arrhythmias with LAMA therapy.
The cardiovascular risks peaked at around the 30th day of treatment, waned from 31-60 days of treatment, and reduced to a level lower than the baseline risk from 71-240 days.
“Given that CVD is highly prevalent among patients with COPD, clinicians should also pay attention to the management of CVD risk factors throughout the duration of LABA or LAMA therapy ... if needed, a preventive therapy for CVD should be considered during the initial treatment of inhaled long-acting bronchodilators,” the investigators said.
LABAs and LAMAs are believed to cause sympathetic overactivation by activating sympathetic beta-2 adrenergic receptors and suppressing parasympathetic muscarinic-3 receptors, which could contribute to the CVD risk. Also, LABA and LAMA use in COPD has been observed to increase inflammatory cytokine levels, which might also play a role.
The subjects were 40 years or older; the mean age was 71.4 years and 68.9% of the participants were men.
The work was supported by Taiwan’s Ministry of Science and Technology. The investigators had no disclosures.
Eli Zimmerman contributed to this report.
SOURCE: Wang MT et al. JAMA Intern Med. 2018 Jan 2. doi: 10.1001/jamainternmed.2017.7720.
Daniel R. Ouellette, MD, FCCP, comments: Long acting beta agonists (LABA) and long acting muscarinic antagonists (LAMA) are agents commonly used to treat patients with chronic obstructive pulmonary disease (COPD). These inhaled medications have been generally considered to be safe and have a favorable side-effect profile. Although there has been some speculative data that suggest that these agents may be associated with increased cardiovascular risk, prospective, controlled studies have generally suggested that the cardiovascular risk is not increased with the use of these medicines.
One strength of this study is the size of the database, which is robust, and the novel treatment that this study uses to address the research question. Weaknesses include the study's necessarily retrospective design, and the fact that the population is from a single geographic area. Further research will be needed to understand whether or not the initiation of LABA and LAMA medications in COPD patients is associated with increased cardiovascular risk.
Daniel R. Ouellette, MD, FCCP, comments: Long acting beta agonists (LABA) and long acting muscarinic antagonists (LAMA) are agents commonly used to treat patients with chronic obstructive pulmonary disease (COPD). These inhaled medications have been generally considered to be safe and have a favorable side-effect profile. Although there has been some speculative data that suggest that these agents may be associated with increased cardiovascular risk, prospective, controlled studies have generally suggested that the cardiovascular risk is not increased with the use of these medicines.
One strength of this study is the size of the database, which is robust, and the novel treatment that this study uses to address the research question. Weaknesses include the study's necessarily retrospective design, and the fact that the population is from a single geographic area. Further research will be needed to understand whether or not the initiation of LABA and LAMA medications in COPD patients is associated with increased cardiovascular risk.
Daniel R. Ouellette, MD, FCCP, comments: Long acting beta agonists (LABA) and long acting muscarinic antagonists (LAMA) are agents commonly used to treat patients with chronic obstructive pulmonary disease (COPD). These inhaled medications have been generally considered to be safe and have a favorable side-effect profile. Although there has been some speculative data that suggest that these agents may be associated with increased cardiovascular risk, prospective, controlled studies have generally suggested that the cardiovascular risk is not increased with the use of these medicines.
One strength of this study is the size of the database, which is robust, and the novel treatment that this study uses to address the research question. Weaknesses include the study's necessarily retrospective design, and the fact that the population is from a single geographic area. Further research will be needed to understand whether or not the initiation of LABA and LAMA medications in COPD patients is associated with increased cardiovascular risk.
New use of inhaled long-acting beta-2 agonists (LABAs) or long-acting antimuscarinic antagonists (LAMAs) was associated with a 1.5-fold increased cardiovascular risk within 30 days of initiation in patients with chronic obstructive pulmonary disease, irrespective of prior cardiovascular disease status and history of exacerbations, according to a review of more than 280,000 COPD patients in Taiwan.
The relationship between cardiovascular disease (CVD) and LABAs and LAMAs in chronic obstructive pulmonary disease (COPD) has long been debated. The new study addressed some limitations of previous studies, which had found conflicting results ranging from no increased risk to up to a 4.5-fold increased risk of cardiovascular events when the medications were used for COPD.
Previous randomized trials haven’t raised much concern, but they included prior users who may have developed tolerance to the heart effects and excluded patients with baseline CVD. “We caution physicians to closely monitor new users of LABAs or LAMAs for cardiovascular symptoms.” Health care professionals should be vigilant for any cardiovascular symptoms during the first 30 days of inhalation therapy, said investigators led by Meng-Ting Wang, PhD, of the National Defense Medical Center, Taipei.
“We suspect that there may exist a subgroup of patients with COPD who are particularly at risk of CVD with initial exposure to LABAs or LAMAs ... we suggest that the use of inhaled long-acting bronchodilators in COPD needs to be carefully assessed, and a thorough cardiovascular physical examination, especially heart rate measurement and electrocardiograms, needs to be performed” before prescribing LABAs and LAMAs, they wrote in an article in JAMA Internal Medicine.
The team identified 284,220 COPD patients in the Taiwan National Health Insurance Research Database during 2007-2011 who were new to the medications. During a mean follow-up of 2 years, 37,719 developed severe CVD requiring hospitalization or emergency care, including coronary artery disease, heart failure, ischemic stroke, and arrhythmia.
The team compared their CVD subjects with controls who did not have a heart event and found that new LABA and LAMA use in COPD was associated with a 1.50-fold (95% confidence interval, 1.35-1.67; P less than .001) and a 1.52-fold (95% CI, 1.28-1.80; P less than .001) increased cardiovascular risk within 30 days of initiation, respectively.
One severe CVD event requiring hospitalization or ED care occurred for every 406 (95% CI, 303-580) new LABA users and 391 (95% CI, 254-725) new LAMA users during the first 30 days of therapy.
The LABA- and LAMA-associated CVD risk remained significant, regardless of patients’ CVD history and COPD exacerbations. Analyses of individual CVD outcomes revealed increased risks of coronary artery disease and heart failure with LABA and LAMA treatment, and an increased risk for cardiac arrhythmias with LAMA therapy.
The cardiovascular risks peaked at around the 30th day of treatment, waned from 31-60 days of treatment, and reduced to a level lower than the baseline risk from 71-240 days.
“Given that CVD is highly prevalent among patients with COPD, clinicians should also pay attention to the management of CVD risk factors throughout the duration of LABA or LAMA therapy ... if needed, a preventive therapy for CVD should be considered during the initial treatment of inhaled long-acting bronchodilators,” the investigators said.
LABAs and LAMAs are believed to cause sympathetic overactivation by activating sympathetic beta-2 adrenergic receptors and suppressing parasympathetic muscarinic-3 receptors, which could contribute to the CVD risk. Also, LABA and LAMA use in COPD has been observed to increase inflammatory cytokine levels, which might also play a role.
The subjects were 40 years or older; the mean age was 71.4 years and 68.9% of the participants were men.
The work was supported by Taiwan’s Ministry of Science and Technology. The investigators had no disclosures.
Eli Zimmerman contributed to this report.
SOURCE: Wang MT et al. JAMA Intern Med. 2018 Jan 2. doi: 10.1001/jamainternmed.2017.7720.
New use of inhaled long-acting beta-2 agonists (LABAs) or long-acting antimuscarinic antagonists (LAMAs) was associated with a 1.5-fold increased cardiovascular risk within 30 days of initiation in patients with chronic obstructive pulmonary disease, irrespective of prior cardiovascular disease status and history of exacerbations, according to a review of more than 280,000 COPD patients in Taiwan.
The relationship between cardiovascular disease (CVD) and LABAs and LAMAs in chronic obstructive pulmonary disease (COPD) has long been debated. The new study addressed some limitations of previous studies, which had found conflicting results ranging from no increased risk to up to a 4.5-fold increased risk of cardiovascular events when the medications were used for COPD.
Previous randomized trials haven’t raised much concern, but they included prior users who may have developed tolerance to the heart effects and excluded patients with baseline CVD. “We caution physicians to closely monitor new users of LABAs or LAMAs for cardiovascular symptoms.” Health care professionals should be vigilant for any cardiovascular symptoms during the first 30 days of inhalation therapy, said investigators led by Meng-Ting Wang, PhD, of the National Defense Medical Center, Taipei.
“We suspect that there may exist a subgroup of patients with COPD who are particularly at risk of CVD with initial exposure to LABAs or LAMAs ... we suggest that the use of inhaled long-acting bronchodilators in COPD needs to be carefully assessed, and a thorough cardiovascular physical examination, especially heart rate measurement and electrocardiograms, needs to be performed” before prescribing LABAs and LAMAs, they wrote in an article in JAMA Internal Medicine.
The team identified 284,220 COPD patients in the Taiwan National Health Insurance Research Database during 2007-2011 who were new to the medications. During a mean follow-up of 2 years, 37,719 developed severe CVD requiring hospitalization or emergency care, including coronary artery disease, heart failure, ischemic stroke, and arrhythmia.
The team compared their CVD subjects with controls who did not have a heart event and found that new LABA and LAMA use in COPD was associated with a 1.50-fold (95% confidence interval, 1.35-1.67; P less than .001) and a 1.52-fold (95% CI, 1.28-1.80; P less than .001) increased cardiovascular risk within 30 days of initiation, respectively.
One severe CVD event requiring hospitalization or ED care occurred for every 406 (95% CI, 303-580) new LABA users and 391 (95% CI, 254-725) new LAMA users during the first 30 days of therapy.
The LABA- and LAMA-associated CVD risk remained significant, regardless of patients’ CVD history and COPD exacerbations. Analyses of individual CVD outcomes revealed increased risks of coronary artery disease and heart failure with LABA and LAMA treatment, and an increased risk for cardiac arrhythmias with LAMA therapy.
The cardiovascular risks peaked at around the 30th day of treatment, waned from 31-60 days of treatment, and reduced to a level lower than the baseline risk from 71-240 days.
“Given that CVD is highly prevalent among patients with COPD, clinicians should also pay attention to the management of CVD risk factors throughout the duration of LABA or LAMA therapy ... if needed, a preventive therapy for CVD should be considered during the initial treatment of inhaled long-acting bronchodilators,” the investigators said.
LABAs and LAMAs are believed to cause sympathetic overactivation by activating sympathetic beta-2 adrenergic receptors and suppressing parasympathetic muscarinic-3 receptors, which could contribute to the CVD risk. Also, LABA and LAMA use in COPD has been observed to increase inflammatory cytokine levels, which might also play a role.
The subjects were 40 years or older; the mean age was 71.4 years and 68.9% of the participants were men.
The work was supported by Taiwan’s Ministry of Science and Technology. The investigators had no disclosures.
Eli Zimmerman contributed to this report.
SOURCE: Wang MT et al. JAMA Intern Med. 2018 Jan 2. doi: 10.1001/jamainternmed.2017.7720.
FROM JAMA INTERNAL MEDICINE
Key clinical point: Researchers recommend patients receive a thorough cardiovascular physical examination before they are prescribed LABAs and LAMAs.
Major finding: New irrespective of prior cardiovascular disease status and history of exacerbations.
Study details: The findings are from a review of 284,220 COPD patients in the Taiwan National Health Insurance Research Database.
Disclosures: The work was supported by Taiwan’s Ministry of Science and Technology. The investigators had no disclosures.
Source: Wang MT et al. JAMA Intern Med. 2018 Jan 2. doi: 10.1001/jamainternmed.2017.7720.














