User login
4 new short-acting hormonal contraceptives offer enhancement over earlier options
Short-term hormonal contraceptives remain the most popular class of reversible contraceptives in the United States, despite the availability of longer-acting methods. Oral contraceptives (OCs), contraceptive patches, and contraceptive vaginal rings are extensively used not only because these methods are easy to initiate but also because their ongoing use remains under the control of the woman herself and also provides her with a wide range of important noncontraceptive benefits.
Despite the more than 60 years of innovation that have made hormonal contraceptives safer, more tolerable, and more convenient, there has been room for improvement. Over the last few years, 4 new hormonal methods have been introduced, and each addresses different limitations and problems associated with the existing, often generic, products.
Compared with the traditional norethindrone pill (Micronor and generics), a new drospirenone progestin-only pill (POP) increases ovulation suppression, offers an improved cyclical bleeding profile, and relaxes the tight missed-pill rules that are usually associated with POPs.
In contrast with the older norelgestromin patch (Evra, Xulane), a new contraceptive transdermal patch significantly decreases total estrogen exposure and pairs its estrogen with levonorgestrel, the progestin associated with the lowest venous thromboembolism (VTE) risk in combined hormonal pills.
While existing combination OCs are formulated with the potent estrogen ethinyl estradiol (EE), a new combination pill, formulated with estetrol (E4) and drospirenone, introduces the first new estrogen (estetrol) used in a contraceptive in more than 50 years. Estetrol, a native estrogen, has selective tissue activity with minimal hepatic and breast impacts. Combined with drospirenone, this formulation offers women good contraceptive efficacy and bleeding patterns.
A new contraceptive vaginal ring introduces a new long-acting, specific progestin (segesterone acetate) and pairs it with low-dose EE. These hormones are packaged in a soft vaginal ring that provides up to 13 cycles of contraceptive protection (3 weeks in/1 week out) with one ring, greatly increasing convenience for women.
Each of these new products represents important incremental improvement over existing options.
Continue to: 1. The drospirenone-only OC...
1. The drospirenone-only OC
The new POP with drospirenone 4 mg (Slynd), which received US Food and Drug Administration (FDA) approval in 2019, is packaged in a 24/4 formulation (24 hormonally active tablets followed by 4 inactive tablets). This formulation results in more predictable bleeding than does the 0.35-mg norethindrone POP, which contains 28 hormonally active tablets in each pack. In the US clinical trials of drospirenone 4 mg, scheduled bleeding decreased from 81% in cycle 1 to 20% in cycle 13. Unscheduled spotting and bleeding decreased from 61% to 40% in the same timeframe. Notably, this bleeding pattern was well tolerated; only 0.4% of trial participants discontinued this drospirenone POP due to problems with irregular bleeding or amenorrhea.
In contrast to the continuous norethindrone POP, which is not sufficiently dosed to consistently suppress ovulation, the 4-mg daily dose of drospirenone in this new POP is higher than the 3 mg used in commonly prescribed combination OCs that contain EE and drospirenone. This results in a POP that has more consistent ovulation suppression. Because this drospirenone POP is appropriately dosed and based on a longer-acting progestin, it is more forgiving of inconsistent pill taking. Accordingly, the missed-pill rules for this pill are the same as with combination estrogen-progestin OCs.1 The package labeling cites a first-year failure rate of 4%, but this includes unconfirmed pregnancies. The Pearl Index from the North American trials, based on confirmed pregnancies in nonbreastfeeding women, was 2.9.2
The package labeling for this drospirenone POP includes few contraindications. Conditions that preclude use include the US Medical Eligibility Criteria for contraception Category 4 condition (breast cancer in the last 5 years), renal impairment, and adrenal insufficiency. Other standard contraindications are listed in the prescribing information. Serum potassium levels should be checked (one time only) in the first cycle only for women who chronically use medications that could cause hyperkalemia, such as nonsteroidal anti-inflammatory drugs.
Given the ovulation suppression associated with this drospirenone POP, the safety of a progestin-only method, and the persistent popularity of OC pills, this pill should greatly increase the use of POPs beyond their traditional niche of postpartum and breastfeeding women. The advent of the drospirenone POP means that clinicians now have better options for women who have contraindications to estrogen and desire to control their own contraceptive use. It would be a logical consideration for over-the-counter accessibility.
2. Transdermal patch with ethinyl estradiol/levonorgestrel
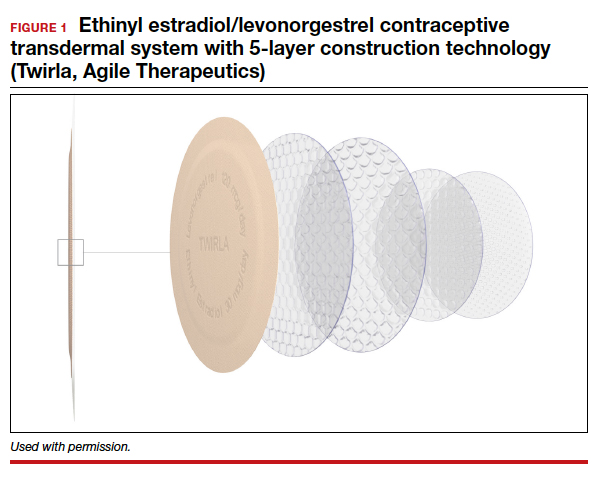
The new EE/levonorgestrel transdermal contraceptive patch (Twirla) is soft and flexible, about the same size as other contraceptive patches, and contains EE 2.3 mg/levonorgestrel 2.6 mg. It provides total estrogen exposure that is similar to that of OCs with EE 30 µg and distinctly lower than estrogen levels seen with the original norelgestromin-containing patch or its 2 subsequent generic versions.3 This EE/levonorgestrel patch uses a new 5-layer drug delivery system that focuses the steroids for absorption beneath the patch; there is no peripheral spread of drug around the patch (FIGURE 1).
Transdermal patches offer the convenience of once-a-week dosing. One patch is used each week for 3 consecutive weeks followed by a patch-free week. Patches can be worn on the abdomen, buttock, or trunk (except breasts). Patches should not be placed consecutively on the same site; after a week’s rest, however, the first site can be reused. All transdermal contraceptive products are indicated for use only by women with a body mass index (BMI) <30 kg/m2.4
While no head-to-head trials have compared this new lower-dose patch with older patches, each patch was compared against a standardized pill, so meaningful comparisons can be made.
In each case, the circulating estrogen levels associated with use of the EE/levonorgestrel patch were considerably lower than those of the comparator pill, while the older norelgestromin patch consistently delivered higher total estrogen levels than its 35-µg comparator pill (TABLE).3 Along these lines, no VTE events occurred in women in the clinical trial of the new patch among women with a BMI <30 kg/m2.4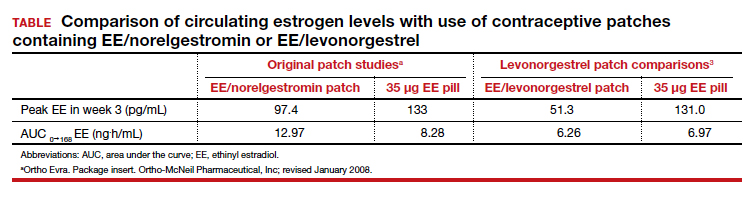
Women with a BMI <25 kg/m2 experienced lower Pearl Index (PI) pregnancy rates (3.5%) compared with women with a BMI between 25 and 30 kg/m2 (5.7%), according to clinical trial data cited in the package labeling. All the modern PI criteria were used to calculate these failure rates. Cycles in which no coitus occurred were excluded. Similarly, cycles in which another contraceptive method (for example, condoms) was added (even once) were excluded. Frequent pregnancy testing was done in the study centers and by the women at home. Bleeding patterns were well accepted; only 2.2% of study participants exited the study early due to menstrual disorders of any kind. Similarly, 3.1% of women discontinued use because of application site disorders. Women should be advised to press down on the patch edges after emerging from water exposure. Replacement patches are rapidly available from the manufacturer should permanent complete patch detachment occur.
Larger-scale phase 4 trials will be conducted to study the impact of this lower-dose patch on VTE rates.
Continue to: 3. A 1-year contraceptive vaginal ring...
3. A 1-year contraceptive vaginal ring
The need to obtain new supplies every month or every 3 months contributes to high rates of contraceptive failure and unintended pregnancy among women using short-acting hormonal contraceptives (pills, patches, and vaginal rings).5 A woman-controlled contraceptive that would provide 1 year of protection against unintended pregnancy represents a step forward. A contraceptive vaginal ring (CVR) that releases the novel progestin segesterone acetate and EE provides woman-controlled contraception for up to 1 year. This CVR (Annovera) received FDA approval in 2018 and has been marketed in the United States since 2020.
The segesterone acetate/EE CVR is a soft, flexible ring that is opaque white in color and fabricated from nonbiodegradable silicone (FIGURE 2). The outside diameter is 5.6 cm, compared with the 5.4-cm outer diameter of the etonogestrel/EE vaginal ring (NuvaRing). The segesterone acetate/EE CVR has 2 channels: one releases segesterone acetate only and the other releases segesterone acetate and EE. In contrast with the etonogestrel/EE CVR, the segesterone acetate/EE CVR does not need to be refrigerated when stored.6
Segesterone is a 19-nor-progesterone derivative that binds in a highly selective fashion to progesterone receptors, and it is potent in suppressing ovulation. During use of the segesterone acetate/EE CVR, mean levels of EE are incrementally higher than those observed with use of the etonogestrel/EE CVR.
Two 13-cycle (1 year) phase 3 clinical trials conducted from 2006 to 2009 enrolled 2,308 women aged 18 to 40 years, including 2,265 women aged 18 to 35 (the age group the FDA considers for efficacy analysis). Trial participants placed the ring vaginally on cycle days 2 to 5 and were asked to keep the ring in place for 21 days, then to remove the CVR for 7 days, during which scheduled bleeding was anticipated. For sexual intercourse, rings could be removed, depending on patient/couple preference, for up to 2 hours.
In the combined trials, the PI was 2.98 per 100 woman-years, a pregnancy rate comparable to those seen in other recent trials of combination estrogen-progestin contraceptives. The incidence of contraceptive failure did not increase over time during the 1-year trials, indicating that contraceptive efficacy of the segesterone acetate/EE was maintained during 1 year of use. While the pregnancy rate was lower in participants who did not report any instances of CVR removal during the 21-day periods of use, the rate was substantially higher among those who reported prolonged episodes of CVR removal.
In the 2 trials, bleeding patterns were similar to those observed with other combination estrogen-progestin contraceptives. Fewer than 2% of trial participants discontinued the trial early due to what they considered unacceptable bleeding.
More than one-half of trial participants reported at least 1 episode of complete or partial CVR expulsion. Most expulsions occurred in the first cycle, suggesting a learning curve with CVR use. Fewer than 2% of participants discontinued trial participation due to expulsions.
Almost 90% of participants reported that they were “highly satisfied” or “satisfied” with the CVR. Although more than two-thirds of participants reported that they never felt the ring during intercourse, if a couple did report feeling the ring during sex, the likelihood of dissatisfaction with the CVR doubled. In addition, feeling the CVR at other times was strongly associated with dissatisfaction. Because a deeply positioned CVR is less likely to be felt by users, these observations underscore the importance of counseling users to place the ring into the upper vagina. Of note, neither prior ring use nor tampon use was associated with CVR satisfaction.
One other important counseling point regarding CVR use relates to the discoloration of the ring that occurs over time. The initially white ring tends to become dark brown during the 1-year usage period. Although this discoloration does not indicate hygiene problems, women who are not advised about this in advance may be put off by the color change.
Four nonfatal VTE events occurred, all in the US trial sites. The overall VTE incidence was higher than expected, particularly among participants with a BMI of 29 kg/m2 or higher. After this association was noted, participants with a BMI >29 kg/m2 were discontinued from the trials. The package labeling for the segesterone acetate/EE CVR states that “Limited data are available in females with a BMI >29.0 kg/m2 because this subpopulation was excluded from the clinical trials after VTEs were reported.”6
A 1-year CVR raises the possibility that users could use their rings in an experimental extended fashion to reduce the frequency of withdrawal bleeding or continuously so as to eliminate withdrawal bleeding. In a randomly chosen sample of CVRs that had been used in the 13-cycle clinical trials, residual steroids in the CVRs were assessed. Sixty percent of segesterone acetate and 80% of EE remained. Using these observations as well as pharmacokinetic data collected from phase 3 trial participants, predicted segesterone acetate levels after 1 year of hypothetical continuous use appear to be sufficient to provide effective contraception.7 These observations suggest that performing clinical trials of extended as well as continuous segesterone acetate/EE CVR use is warranted.
Continue to: 4. An OC with a novel estrogen...
4. An OC with a novel estrogen
Even as use of intrauterine devices and contraceptive implants continues to grow, OCs remain the reversible contraceptive most used by US women. While OCs have been widely studied and represent a safe method of contraception for most reproductive-age women, combination estrogen-progestin OCs are well recognized to increase the risk of VTE. Although the primary role of the progestin component of combination OCs is to suppress ovulation, estrogen is included in combination OCs to stimulate endometrial proliferation, thereby causing predictable bleeding. EE, the potent synthetic estrogen used in the great majority of current OC formulations, induces hepatic production of prothrombotic proteins while inhibiting synthesis of antithrombotic proteins. While the lower EE doses (10–35 µg) in today’s OC formulations are associated with a lower VTE risk than older OCs that contained higher doses of estrogen, VTE continues to represent the principal health risk associated with use of combination OCs. Accordingly, development of a combination OC that has less impact on risk of VTE would be appealing.
In April 2021, the FDA approved an OC formulation that combines 15 mg of the novel estrogen estetrol with 3 mg of drospirenone (Nextstellis). This dose of drospirenone is the same as that used in commonly prescribed EE/drospirenone OC formulations. Also known as E4, estetrol is a natural estrogen synthesized by the fetal liver. Plant-derived E4 is used in this new OC.
Depending on the tissue, E4 acts differently than other estrogens. Similar to other estrogens, E4 acts as an agonist on the nuclear receptor to produce beneficial effects in bone, vaginal mucosa, and heart.8 Unlike other estrogens, E4 inhibits proliferation of mammary gland cells and has a neutral impact on the liver.9
In contrast with EE, E4 is not inhibited by the liver’s P450 enzymes; accordingly, the risk of drug-drug interactions is reduced. Because E4 is primarily excreted through the urine and not through the biliary tract, the risk of gallstone formation may be lower than with an EE OC. Likewise, E4 has substantially less impact on triglycerides, which are increased with EE. Finally, because of E4’s reduced effect on the liver, the impact on clotting parameters is less than that observed with an OC formulated with EE.10 This latter observation raises the possibility that VTE risk is lower with the E4/drospirenone OC than an OC formulated with EE.
A 13-cycle phase 3 trial of the E4/drospirenone OC conducted in the United States and Canada enrolled 1,864 women aged 16 to 50 years, including 1,674 who were aged 16 to 35 years.11 Among women in this latter age group, the PI was 2.65 per 100 woman-years. Bleeding/cycle control patterns were similar to those observed in recent trials of other combination contraceptives. Likewise, the proportion of trial participants who discontinued the study due to adverse effects was similar to or lower than that noted in recent trials of other combination contraceptives. Of particular note, no cases of VTE were noted among trial participants of any BMI, a finding which contrasts with recent phase 3 trials of other combination contraceptives. The result of this pivotal trial suggests that the theoretic advantages of E4 when used in a combination OC formulation may translate into a safer, effective, and well-tolerated contraceptive.
Refinements in hormonal contraceptives continue
The 4 new short-acting hormonal contraceptives we reviewed represent enhancements on existing pills, patches, and rings. We hope that, financially, women will have access to these innovative methods and, in particular, that third-party payers will facilitate women’s access to these enhanced short-acting hormonal contraceptives. ●
- Palacios S, Colli E, Regidor PA. Multicenter, phase III trials on the contraceptive efficacy, tolerability and safety of a new drospirenone-only pill. Acta Obstet Gynecol Scand. 2019;98:1549-1557.
- Kimble T, Burke AE, Barnhart KT, et al. A 1-year prospective, open-label, single-arm, multicenter, phase 3 trial of the contraceptive efficacy and safety of the oral progestin-only pill drospirenone 4 mg using a 24/4-day regimen. Contracept X. 2020;2:100020.
- Archer DF, Stanczyk FZ, Rubin A, et al. Ethinyl estradiol and levonorgestrel pharmacokinetics with a low-dose transdermal contraceptive delivery system, AG200-15: a randomized controlled trial. Contraception. 2012;85:595-601.
- Nelson AL, Kaunitz AM, Kroll R, et al; SECURE Investigators. Efficacy, safety, and tolerability of a levonorgestrel/ethinyl estradiol transdermal delivery system: phase 3 clinical trial results. Contraception. 2021;103:137-143.
- Westhoff CL, Heartwell S, Edwards S, et al. Oral contraceptive discontinuation: do side effects matter? Am J Obstet Gynecol. 2007;196:412.e1-6; discussion 412.e6-7.
- Nelson AL. Comprehensive overview of the recently FDAapproved contraceptive vaginal ring releasing segesterone acetate and ethinylestradiol: a new year-long, patient controlled, reversible birth control method. Expert Rev Clin Pharmacol. 2019;12:953-963.
- Liu JH, Plagianos M, Archer DF, et al. Segesterone acetate serum levels with a regression model of continuous use of the segesterone acetate/ethinyl estradiol contraceptive vaginal system. Contraception. 2021;104:229-234.
- Mawet M, Maillard C, Klipping C, et al. Unique effects on hepatic function, lipid metabolism, bone and growth endocrine parameters of estetrol in combined oral contraceptives. Eur J Contracept Reprod Health Care. 2015;20:463-475.
- Gérard C, Blacher S, Communal L, et al. Estetrol is a weak estrogen antagonizing estradiol-dependent mammary gland proliferation. J Endocrinol. 2015;224:85-95.
- Douxfils J, Klipping C, Duijkers I, et al. Evaluation of the effect of a new oral contraceptive containing estetrol and drospirenone on hemostasis parameters. Contraception. 2020;102:396-402.
- Creinin MD, Westhoff CL, Bouchard C, et al. Estetroldrospirenone combination oral contraceptive: North American phase 3 efficacy and safety results. Contraception. 2021;104:222-228.
Short-term hormonal contraceptives remain the most popular class of reversible contraceptives in the United States, despite the availability of longer-acting methods. Oral contraceptives (OCs), contraceptive patches, and contraceptive vaginal rings are extensively used not only because these methods are easy to initiate but also because their ongoing use remains under the control of the woman herself and also provides her with a wide range of important noncontraceptive benefits.
Despite the more than 60 years of innovation that have made hormonal contraceptives safer, more tolerable, and more convenient, there has been room for improvement. Over the last few years, 4 new hormonal methods have been introduced, and each addresses different limitations and problems associated with the existing, often generic, products.
Compared with the traditional norethindrone pill (Micronor and generics), a new drospirenone progestin-only pill (POP) increases ovulation suppression, offers an improved cyclical bleeding profile, and relaxes the tight missed-pill rules that are usually associated with POPs.
In contrast with the older norelgestromin patch (Evra, Xulane), a new contraceptive transdermal patch significantly decreases total estrogen exposure and pairs its estrogen with levonorgestrel, the progestin associated with the lowest venous thromboembolism (VTE) risk in combined hormonal pills.
While existing combination OCs are formulated with the potent estrogen ethinyl estradiol (EE), a new combination pill, formulated with estetrol (E4) and drospirenone, introduces the first new estrogen (estetrol) used in a contraceptive in more than 50 years. Estetrol, a native estrogen, has selective tissue activity with minimal hepatic and breast impacts. Combined with drospirenone, this formulation offers women good contraceptive efficacy and bleeding patterns.
A new contraceptive vaginal ring introduces a new long-acting, specific progestin (segesterone acetate) and pairs it with low-dose EE. These hormones are packaged in a soft vaginal ring that provides up to 13 cycles of contraceptive protection (3 weeks in/1 week out) with one ring, greatly increasing convenience for women.
Each of these new products represents important incremental improvement over existing options.
Continue to: 1. The drospirenone-only OC...
1. The drospirenone-only OC
The new POP with drospirenone 4 mg (Slynd), which received US Food and Drug Administration (FDA) approval in 2019, is packaged in a 24/4 formulation (24 hormonally active tablets followed by 4 inactive tablets). This formulation results in more predictable bleeding than does the 0.35-mg norethindrone POP, which contains 28 hormonally active tablets in each pack. In the US clinical trials of drospirenone 4 mg, scheduled bleeding decreased from 81% in cycle 1 to 20% in cycle 13. Unscheduled spotting and bleeding decreased from 61% to 40% in the same timeframe. Notably, this bleeding pattern was well tolerated; only 0.4% of trial participants discontinued this drospirenone POP due to problems with irregular bleeding or amenorrhea.
In contrast to the continuous norethindrone POP, which is not sufficiently dosed to consistently suppress ovulation, the 4-mg daily dose of drospirenone in this new POP is higher than the 3 mg used in commonly prescribed combination OCs that contain EE and drospirenone. This results in a POP that has more consistent ovulation suppression. Because this drospirenone POP is appropriately dosed and based on a longer-acting progestin, it is more forgiving of inconsistent pill taking. Accordingly, the missed-pill rules for this pill are the same as with combination estrogen-progestin OCs.1 The package labeling cites a first-year failure rate of 4%, but this includes unconfirmed pregnancies. The Pearl Index from the North American trials, based on confirmed pregnancies in nonbreastfeeding women, was 2.9.2
The package labeling for this drospirenone POP includes few contraindications. Conditions that preclude use include the US Medical Eligibility Criteria for contraception Category 4 condition (breast cancer in the last 5 years), renal impairment, and adrenal insufficiency. Other standard contraindications are listed in the prescribing information. Serum potassium levels should be checked (one time only) in the first cycle only for women who chronically use medications that could cause hyperkalemia, such as nonsteroidal anti-inflammatory drugs.
Given the ovulation suppression associated with this drospirenone POP, the safety of a progestin-only method, and the persistent popularity of OC pills, this pill should greatly increase the use of POPs beyond their traditional niche of postpartum and breastfeeding women. The advent of the drospirenone POP means that clinicians now have better options for women who have contraindications to estrogen and desire to control their own contraceptive use. It would be a logical consideration for over-the-counter accessibility.
2. Transdermal patch with ethinyl estradiol/levonorgestrel
The new EE/levonorgestrel transdermal contraceptive patch (Twirla) is soft and flexible, about the same size as other contraceptive patches, and contains EE 2.3 mg/levonorgestrel 2.6 mg. It provides total estrogen exposure that is similar to that of OCs with EE 30 µg and distinctly lower than estrogen levels seen with the original norelgestromin-containing patch or its 2 subsequent generic versions.3 This EE/levonorgestrel patch uses a new 5-layer drug delivery system that focuses the steroids for absorption beneath the patch; there is no peripheral spread of drug around the patch (FIGURE 1).

Transdermal patches offer the convenience of once-a-week dosing. One patch is used each week for 3 consecutive weeks followed by a patch-free week. Patches can be worn on the abdomen, buttock, or trunk (except breasts). Patches should not be placed consecutively on the same site; after a week’s rest, however, the first site can be reused. All transdermal contraceptive products are indicated for use only by women with a body mass index (BMI) <30 kg/m2.4
While no head-to-head trials have compared this new lower-dose patch with older patches, each patch was compared against a standardized pill, so meaningful comparisons can be made.
In each case, the circulating estrogen levels associated with use of the EE/levonorgestrel patch were considerably lower than those of the comparator pill, while the older norelgestromin patch consistently delivered higher total estrogen levels than its 35-µg comparator pill (TABLE).3 Along these lines, no VTE events occurred in women in the clinical trial of the new patch among women with a BMI <30 kg/m2.4
Women with a BMI <25 kg/m2 experienced lower Pearl Index (PI) pregnancy rates (3.5%) compared with women with a BMI between 25 and 30 kg/m2 (5.7%), according to clinical trial data cited in the package labeling. All the modern PI criteria were used to calculate these failure rates. Cycles in which no coitus occurred were excluded. Similarly, cycles in which another contraceptive method (for example, condoms) was added (even once) were excluded. Frequent pregnancy testing was done in the study centers and by the women at home. Bleeding patterns were well accepted; only 2.2% of study participants exited the study early due to menstrual disorders of any kind. Similarly, 3.1% of women discontinued use because of application site disorders. Women should be advised to press down on the patch edges after emerging from water exposure. Replacement patches are rapidly available from the manufacturer should permanent complete patch detachment occur.
Larger-scale phase 4 trials will be conducted to study the impact of this lower-dose patch on VTE rates.
Continue to: 3. A 1-year contraceptive vaginal ring...
3. A 1-year contraceptive vaginal ring
The need to obtain new supplies every month or every 3 months contributes to high rates of contraceptive failure and unintended pregnancy among women using short-acting hormonal contraceptives (pills, patches, and vaginal rings).5 A woman-controlled contraceptive that would provide 1 year of protection against unintended pregnancy represents a step forward. A contraceptive vaginal ring (CVR) that releases the novel progestin segesterone acetate and EE provides woman-controlled contraception for up to 1 year. This CVR (Annovera) received FDA approval in 2018 and has been marketed in the United States since 2020.
The segesterone acetate/EE CVR is a soft, flexible ring that is opaque white in color and fabricated from nonbiodegradable silicone (FIGURE 2). The outside diameter is 5.6 cm, compared with the 5.4-cm outer diameter of the etonogestrel/EE vaginal ring (NuvaRing). The segesterone acetate/EE CVR has 2 channels: one releases segesterone acetate only and the other releases segesterone acetate and EE. In contrast with the etonogestrel/EE CVR, the segesterone acetate/EE CVR does not need to be refrigerated when stored.6

Segesterone is a 19-nor-progesterone derivative that binds in a highly selective fashion to progesterone receptors, and it is potent in suppressing ovulation. During use of the segesterone acetate/EE CVR, mean levels of EE are incrementally higher than those observed with use of the etonogestrel/EE CVR.
Two 13-cycle (1 year) phase 3 clinical trials conducted from 2006 to 2009 enrolled 2,308 women aged 18 to 40 years, including 2,265 women aged 18 to 35 (the age group the FDA considers for efficacy analysis). Trial participants placed the ring vaginally on cycle days 2 to 5 and were asked to keep the ring in place for 21 days, then to remove the CVR for 7 days, during which scheduled bleeding was anticipated. For sexual intercourse, rings could be removed, depending on patient/couple preference, for up to 2 hours.
In the combined trials, the PI was 2.98 per 100 woman-years, a pregnancy rate comparable to those seen in other recent trials of combination estrogen-progestin contraceptives. The incidence of contraceptive failure did not increase over time during the 1-year trials, indicating that contraceptive efficacy of the segesterone acetate/EE was maintained during 1 year of use. While the pregnancy rate was lower in participants who did not report any instances of CVR removal during the 21-day periods of use, the rate was substantially higher among those who reported prolonged episodes of CVR removal.
In the 2 trials, bleeding patterns were similar to those observed with other combination estrogen-progestin contraceptives. Fewer than 2% of trial participants discontinued the trial early due to what they considered unacceptable bleeding.
More than one-half of trial participants reported at least 1 episode of complete or partial CVR expulsion. Most expulsions occurred in the first cycle, suggesting a learning curve with CVR use. Fewer than 2% of participants discontinued trial participation due to expulsions.
Almost 90% of participants reported that they were “highly satisfied” or “satisfied” with the CVR. Although more than two-thirds of participants reported that they never felt the ring during intercourse, if a couple did report feeling the ring during sex, the likelihood of dissatisfaction with the CVR doubled. In addition, feeling the CVR at other times was strongly associated with dissatisfaction. Because a deeply positioned CVR is less likely to be felt by users, these observations underscore the importance of counseling users to place the ring into the upper vagina. Of note, neither prior ring use nor tampon use was associated with CVR satisfaction.
One other important counseling point regarding CVR use relates to the discoloration of the ring that occurs over time. The initially white ring tends to become dark brown during the 1-year usage period. Although this discoloration does not indicate hygiene problems, women who are not advised about this in advance may be put off by the color change.
Four nonfatal VTE events occurred, all in the US trial sites. The overall VTE incidence was higher than expected, particularly among participants with a BMI of 29 kg/m2 or higher. After this association was noted, participants with a BMI >29 kg/m2 were discontinued from the trials. The package labeling for the segesterone acetate/EE CVR states that “Limited data are available in females with a BMI >29.0 kg/m2 because this subpopulation was excluded from the clinical trials after VTEs were reported.”6
A 1-year CVR raises the possibility that users could use their rings in an experimental extended fashion to reduce the frequency of withdrawal bleeding or continuously so as to eliminate withdrawal bleeding. In a randomly chosen sample of CVRs that had been used in the 13-cycle clinical trials, residual steroids in the CVRs were assessed. Sixty percent of segesterone acetate and 80% of EE remained. Using these observations as well as pharmacokinetic data collected from phase 3 trial participants, predicted segesterone acetate levels after 1 year of hypothetical continuous use appear to be sufficient to provide effective contraception.7 These observations suggest that performing clinical trials of extended as well as continuous segesterone acetate/EE CVR use is warranted.
Continue to: 4. An OC with a novel estrogen...
4. An OC with a novel estrogen
Even as use of intrauterine devices and contraceptive implants continues to grow, OCs remain the reversible contraceptive most used by US women. While OCs have been widely studied and represent a safe method of contraception for most reproductive-age women, combination estrogen-progestin OCs are well recognized to increase the risk of VTE. Although the primary role of the progestin component of combination OCs is to suppress ovulation, estrogen is included in combination OCs to stimulate endometrial proliferation, thereby causing predictable bleeding. EE, the potent synthetic estrogen used in the great majority of current OC formulations, induces hepatic production of prothrombotic proteins while inhibiting synthesis of antithrombotic proteins. While the lower EE doses (10–35 µg) in today’s OC formulations are associated with a lower VTE risk than older OCs that contained higher doses of estrogen, VTE continues to represent the principal health risk associated with use of combination OCs. Accordingly, development of a combination OC that has less impact on risk of VTE would be appealing.
In April 2021, the FDA approved an OC formulation that combines 15 mg of the novel estrogen estetrol with 3 mg of drospirenone (Nextstellis). This dose of drospirenone is the same as that used in commonly prescribed EE/drospirenone OC formulations. Also known as E4, estetrol is a natural estrogen synthesized by the fetal liver. Plant-derived E4 is used in this new OC.
Depending on the tissue, E4 acts differently than other estrogens. Similar to other estrogens, E4 acts as an agonist on the nuclear receptor to produce beneficial effects in bone, vaginal mucosa, and heart.8 Unlike other estrogens, E4 inhibits proliferation of mammary gland cells and has a neutral impact on the liver.9
In contrast with EE, E4 is not inhibited by the liver’s P450 enzymes; accordingly, the risk of drug-drug interactions is reduced. Because E4 is primarily excreted through the urine and not through the biliary tract, the risk of gallstone formation may be lower than with an EE OC. Likewise, E4 has substantially less impact on triglycerides, which are increased with EE. Finally, because of E4’s reduced effect on the liver, the impact on clotting parameters is less than that observed with an OC formulated with EE.10 This latter observation raises the possibility that VTE risk is lower with the E4/drospirenone OC than an OC formulated with EE.
A 13-cycle phase 3 trial of the E4/drospirenone OC conducted in the United States and Canada enrolled 1,864 women aged 16 to 50 years, including 1,674 who were aged 16 to 35 years.11 Among women in this latter age group, the PI was 2.65 per 100 woman-years. Bleeding/cycle control patterns were similar to those observed in recent trials of other combination contraceptives. Likewise, the proportion of trial participants who discontinued the study due to adverse effects was similar to or lower than that noted in recent trials of other combination contraceptives. Of particular note, no cases of VTE were noted among trial participants of any BMI, a finding which contrasts with recent phase 3 trials of other combination contraceptives. The result of this pivotal trial suggests that the theoretic advantages of E4 when used in a combination OC formulation may translate into a safer, effective, and well-tolerated contraceptive.
Refinements in hormonal contraceptives continue
The 4 new short-acting hormonal contraceptives we reviewed represent enhancements on existing pills, patches, and rings. We hope that, financially, women will have access to these innovative methods and, in particular, that third-party payers will facilitate women’s access to these enhanced short-acting hormonal contraceptives. ●
Short-term hormonal contraceptives remain the most popular class of reversible contraceptives in the United States, despite the availability of longer-acting methods. Oral contraceptives (OCs), contraceptive patches, and contraceptive vaginal rings are extensively used not only because these methods are easy to initiate but also because their ongoing use remains under the control of the woman herself and also provides her with a wide range of important noncontraceptive benefits.
Despite the more than 60 years of innovation that have made hormonal contraceptives safer, more tolerable, and more convenient, there has been room for improvement. Over the last few years, 4 new hormonal methods have been introduced, and each addresses different limitations and problems associated with the existing, often generic, products.
Compared with the traditional norethindrone pill (Micronor and generics), a new drospirenone progestin-only pill (POP) increases ovulation suppression, offers an improved cyclical bleeding profile, and relaxes the tight missed-pill rules that are usually associated with POPs.
In contrast with the older norelgestromin patch (Evra, Xulane), a new contraceptive transdermal patch significantly decreases total estrogen exposure and pairs its estrogen with levonorgestrel, the progestin associated with the lowest venous thromboembolism (VTE) risk in combined hormonal pills.
While existing combination OCs are formulated with the potent estrogen ethinyl estradiol (EE), a new combination pill, formulated with estetrol (E4) and drospirenone, introduces the first new estrogen (estetrol) used in a contraceptive in more than 50 years. Estetrol, a native estrogen, has selective tissue activity with minimal hepatic and breast impacts. Combined with drospirenone, this formulation offers women good contraceptive efficacy and bleeding patterns.
A new contraceptive vaginal ring introduces a new long-acting, specific progestin (segesterone acetate) and pairs it with low-dose EE. These hormones are packaged in a soft vaginal ring that provides up to 13 cycles of contraceptive protection (3 weeks in/1 week out) with one ring, greatly increasing convenience for women.
Each of these new products represents important incremental improvement over existing options.
Continue to: 1. The drospirenone-only OC...
1. The drospirenone-only OC
The new POP with drospirenone 4 mg (Slynd), which received US Food and Drug Administration (FDA) approval in 2019, is packaged in a 24/4 formulation (24 hormonally active tablets followed by 4 inactive tablets). This formulation results in more predictable bleeding than does the 0.35-mg norethindrone POP, which contains 28 hormonally active tablets in each pack. In the US clinical trials of drospirenone 4 mg, scheduled bleeding decreased from 81% in cycle 1 to 20% in cycle 13. Unscheduled spotting and bleeding decreased from 61% to 40% in the same timeframe. Notably, this bleeding pattern was well tolerated; only 0.4% of trial participants discontinued this drospirenone POP due to problems with irregular bleeding or amenorrhea.
In contrast to the continuous norethindrone POP, which is not sufficiently dosed to consistently suppress ovulation, the 4-mg daily dose of drospirenone in this new POP is higher than the 3 mg used in commonly prescribed combination OCs that contain EE and drospirenone. This results in a POP that has more consistent ovulation suppression. Because this drospirenone POP is appropriately dosed and based on a longer-acting progestin, it is more forgiving of inconsistent pill taking. Accordingly, the missed-pill rules for this pill are the same as with combination estrogen-progestin OCs.1 The package labeling cites a first-year failure rate of 4%, but this includes unconfirmed pregnancies. The Pearl Index from the North American trials, based on confirmed pregnancies in nonbreastfeeding women, was 2.9.2
The package labeling for this drospirenone POP includes few contraindications. Conditions that preclude use include the US Medical Eligibility Criteria for contraception Category 4 condition (breast cancer in the last 5 years), renal impairment, and adrenal insufficiency. Other standard contraindications are listed in the prescribing information. Serum potassium levels should be checked (one time only) in the first cycle only for women who chronically use medications that could cause hyperkalemia, such as nonsteroidal anti-inflammatory drugs.
Given the ovulation suppression associated with this drospirenone POP, the safety of a progestin-only method, and the persistent popularity of OC pills, this pill should greatly increase the use of POPs beyond their traditional niche of postpartum and breastfeeding women. The advent of the drospirenone POP means that clinicians now have better options for women who have contraindications to estrogen and desire to control their own contraceptive use. It would be a logical consideration for over-the-counter accessibility.
2. Transdermal patch with ethinyl estradiol/levonorgestrel
The new EE/levonorgestrel transdermal contraceptive patch (Twirla) is soft and flexible, about the same size as other contraceptive patches, and contains EE 2.3 mg/levonorgestrel 2.6 mg. It provides total estrogen exposure that is similar to that of OCs with EE 30 µg and distinctly lower than estrogen levels seen with the original norelgestromin-containing patch or its 2 subsequent generic versions.3 This EE/levonorgestrel patch uses a new 5-layer drug delivery system that focuses the steroids for absorption beneath the patch; there is no peripheral spread of drug around the patch (FIGURE 1).

Transdermal patches offer the convenience of once-a-week dosing. One patch is used each week for 3 consecutive weeks followed by a patch-free week. Patches can be worn on the abdomen, buttock, or trunk (except breasts). Patches should not be placed consecutively on the same site; after a week’s rest, however, the first site can be reused. All transdermal contraceptive products are indicated for use only by women with a body mass index (BMI) <30 kg/m2.4
While no head-to-head trials have compared this new lower-dose patch with older patches, each patch was compared against a standardized pill, so meaningful comparisons can be made.
In each case, the circulating estrogen levels associated with use of the EE/levonorgestrel patch were considerably lower than those of the comparator pill, while the older norelgestromin patch consistently delivered higher total estrogen levels than its 35-µg comparator pill (TABLE).3 Along these lines, no VTE events occurred in women in the clinical trial of the new patch among women with a BMI <30 kg/m2.4
Women with a BMI <25 kg/m2 experienced lower Pearl Index (PI) pregnancy rates (3.5%) compared with women with a BMI between 25 and 30 kg/m2 (5.7%), according to clinical trial data cited in the package labeling. All the modern PI criteria were used to calculate these failure rates. Cycles in which no coitus occurred were excluded. Similarly, cycles in which another contraceptive method (for example, condoms) was added (even once) were excluded. Frequent pregnancy testing was done in the study centers and by the women at home. Bleeding patterns were well accepted; only 2.2% of study participants exited the study early due to menstrual disorders of any kind. Similarly, 3.1% of women discontinued use because of application site disorders. Women should be advised to press down on the patch edges after emerging from water exposure. Replacement patches are rapidly available from the manufacturer should permanent complete patch detachment occur.
Larger-scale phase 4 trials will be conducted to study the impact of this lower-dose patch on VTE rates.
Continue to: 3. A 1-year contraceptive vaginal ring...
3. A 1-year contraceptive vaginal ring
The need to obtain new supplies every month or every 3 months contributes to high rates of contraceptive failure and unintended pregnancy among women using short-acting hormonal contraceptives (pills, patches, and vaginal rings).5 A woman-controlled contraceptive that would provide 1 year of protection against unintended pregnancy represents a step forward. A contraceptive vaginal ring (CVR) that releases the novel progestin segesterone acetate and EE provides woman-controlled contraception for up to 1 year. This CVR (Annovera) received FDA approval in 2018 and has been marketed in the United States since 2020.
The segesterone acetate/EE CVR is a soft, flexible ring that is opaque white in color and fabricated from nonbiodegradable silicone (FIGURE 2). The outside diameter is 5.6 cm, compared with the 5.4-cm outer diameter of the etonogestrel/EE vaginal ring (NuvaRing). The segesterone acetate/EE CVR has 2 channels: one releases segesterone acetate only and the other releases segesterone acetate and EE. In contrast with the etonogestrel/EE CVR, the segesterone acetate/EE CVR does not need to be refrigerated when stored.6

Segesterone is a 19-nor-progesterone derivative that binds in a highly selective fashion to progesterone receptors, and it is potent in suppressing ovulation. During use of the segesterone acetate/EE CVR, mean levels of EE are incrementally higher than those observed with use of the etonogestrel/EE CVR.
Two 13-cycle (1 year) phase 3 clinical trials conducted from 2006 to 2009 enrolled 2,308 women aged 18 to 40 years, including 2,265 women aged 18 to 35 (the age group the FDA considers for efficacy analysis). Trial participants placed the ring vaginally on cycle days 2 to 5 and were asked to keep the ring in place for 21 days, then to remove the CVR for 7 days, during which scheduled bleeding was anticipated. For sexual intercourse, rings could be removed, depending on patient/couple preference, for up to 2 hours.
In the combined trials, the PI was 2.98 per 100 woman-years, a pregnancy rate comparable to those seen in other recent trials of combination estrogen-progestin contraceptives. The incidence of contraceptive failure did not increase over time during the 1-year trials, indicating that contraceptive efficacy of the segesterone acetate/EE was maintained during 1 year of use. While the pregnancy rate was lower in participants who did not report any instances of CVR removal during the 21-day periods of use, the rate was substantially higher among those who reported prolonged episodes of CVR removal.
In the 2 trials, bleeding patterns were similar to those observed with other combination estrogen-progestin contraceptives. Fewer than 2% of trial participants discontinued the trial early due to what they considered unacceptable bleeding.
More than one-half of trial participants reported at least 1 episode of complete or partial CVR expulsion. Most expulsions occurred in the first cycle, suggesting a learning curve with CVR use. Fewer than 2% of participants discontinued trial participation due to expulsions.
Almost 90% of participants reported that they were “highly satisfied” or “satisfied” with the CVR. Although more than two-thirds of participants reported that they never felt the ring during intercourse, if a couple did report feeling the ring during sex, the likelihood of dissatisfaction with the CVR doubled. In addition, feeling the CVR at other times was strongly associated with dissatisfaction. Because a deeply positioned CVR is less likely to be felt by users, these observations underscore the importance of counseling users to place the ring into the upper vagina. Of note, neither prior ring use nor tampon use was associated with CVR satisfaction.
One other important counseling point regarding CVR use relates to the discoloration of the ring that occurs over time. The initially white ring tends to become dark brown during the 1-year usage period. Although this discoloration does not indicate hygiene problems, women who are not advised about this in advance may be put off by the color change.
Four nonfatal VTE events occurred, all in the US trial sites. The overall VTE incidence was higher than expected, particularly among participants with a BMI of 29 kg/m2 or higher. After this association was noted, participants with a BMI >29 kg/m2 were discontinued from the trials. The package labeling for the segesterone acetate/EE CVR states that “Limited data are available in females with a BMI >29.0 kg/m2 because this subpopulation was excluded from the clinical trials after VTEs were reported.”6
A 1-year CVR raises the possibility that users could use their rings in an experimental extended fashion to reduce the frequency of withdrawal bleeding or continuously so as to eliminate withdrawal bleeding. In a randomly chosen sample of CVRs that had been used in the 13-cycle clinical trials, residual steroids in the CVRs were assessed. Sixty percent of segesterone acetate and 80% of EE remained. Using these observations as well as pharmacokinetic data collected from phase 3 trial participants, predicted segesterone acetate levels after 1 year of hypothetical continuous use appear to be sufficient to provide effective contraception.7 These observations suggest that performing clinical trials of extended as well as continuous segesterone acetate/EE CVR use is warranted.
Continue to: 4. An OC with a novel estrogen...
4. An OC with a novel estrogen
Even as use of intrauterine devices and contraceptive implants continues to grow, OCs remain the reversible contraceptive most used by US women. While OCs have been widely studied and represent a safe method of contraception for most reproductive-age women, combination estrogen-progestin OCs are well recognized to increase the risk of VTE. Although the primary role of the progestin component of combination OCs is to suppress ovulation, estrogen is included in combination OCs to stimulate endometrial proliferation, thereby causing predictable bleeding. EE, the potent synthetic estrogen used in the great majority of current OC formulations, induces hepatic production of prothrombotic proteins while inhibiting synthesis of antithrombotic proteins. While the lower EE doses (10–35 µg) in today’s OC formulations are associated with a lower VTE risk than older OCs that contained higher doses of estrogen, VTE continues to represent the principal health risk associated with use of combination OCs. Accordingly, development of a combination OC that has less impact on risk of VTE would be appealing.
In April 2021, the FDA approved an OC formulation that combines 15 mg of the novel estrogen estetrol with 3 mg of drospirenone (Nextstellis). This dose of drospirenone is the same as that used in commonly prescribed EE/drospirenone OC formulations. Also known as E4, estetrol is a natural estrogen synthesized by the fetal liver. Plant-derived E4 is used in this new OC.
Depending on the tissue, E4 acts differently than other estrogens. Similar to other estrogens, E4 acts as an agonist on the nuclear receptor to produce beneficial effects in bone, vaginal mucosa, and heart.8 Unlike other estrogens, E4 inhibits proliferation of mammary gland cells and has a neutral impact on the liver.9
In contrast with EE, E4 is not inhibited by the liver’s P450 enzymes; accordingly, the risk of drug-drug interactions is reduced. Because E4 is primarily excreted through the urine and not through the biliary tract, the risk of gallstone formation may be lower than with an EE OC. Likewise, E4 has substantially less impact on triglycerides, which are increased with EE. Finally, because of E4’s reduced effect on the liver, the impact on clotting parameters is less than that observed with an OC formulated with EE.10 This latter observation raises the possibility that VTE risk is lower with the E4/drospirenone OC than an OC formulated with EE.
A 13-cycle phase 3 trial of the E4/drospirenone OC conducted in the United States and Canada enrolled 1,864 women aged 16 to 50 years, including 1,674 who were aged 16 to 35 years.11 Among women in this latter age group, the PI was 2.65 per 100 woman-years. Bleeding/cycle control patterns were similar to those observed in recent trials of other combination contraceptives. Likewise, the proportion of trial participants who discontinued the study due to adverse effects was similar to or lower than that noted in recent trials of other combination contraceptives. Of particular note, no cases of VTE were noted among trial participants of any BMI, a finding which contrasts with recent phase 3 trials of other combination contraceptives. The result of this pivotal trial suggests that the theoretic advantages of E4 when used in a combination OC formulation may translate into a safer, effective, and well-tolerated contraceptive.
Refinements in hormonal contraceptives continue
The 4 new short-acting hormonal contraceptives we reviewed represent enhancements on existing pills, patches, and rings. We hope that, financially, women will have access to these innovative methods and, in particular, that third-party payers will facilitate women’s access to these enhanced short-acting hormonal contraceptives. ●
- Palacios S, Colli E, Regidor PA. Multicenter, phase III trials on the contraceptive efficacy, tolerability and safety of a new drospirenone-only pill. Acta Obstet Gynecol Scand. 2019;98:1549-1557.
- Kimble T, Burke AE, Barnhart KT, et al. A 1-year prospective, open-label, single-arm, multicenter, phase 3 trial of the contraceptive efficacy and safety of the oral progestin-only pill drospirenone 4 mg using a 24/4-day regimen. Contracept X. 2020;2:100020.
- Archer DF, Stanczyk FZ, Rubin A, et al. Ethinyl estradiol and levonorgestrel pharmacokinetics with a low-dose transdermal contraceptive delivery system, AG200-15: a randomized controlled trial. Contraception. 2012;85:595-601.
- Nelson AL, Kaunitz AM, Kroll R, et al; SECURE Investigators. Efficacy, safety, and tolerability of a levonorgestrel/ethinyl estradiol transdermal delivery system: phase 3 clinical trial results. Contraception. 2021;103:137-143.
- Westhoff CL, Heartwell S, Edwards S, et al. Oral contraceptive discontinuation: do side effects matter? Am J Obstet Gynecol. 2007;196:412.e1-6; discussion 412.e6-7.
- Nelson AL. Comprehensive overview of the recently FDAapproved contraceptive vaginal ring releasing segesterone acetate and ethinylestradiol: a new year-long, patient controlled, reversible birth control method. Expert Rev Clin Pharmacol. 2019;12:953-963.
- Liu JH, Plagianos M, Archer DF, et al. Segesterone acetate serum levels with a regression model of continuous use of the segesterone acetate/ethinyl estradiol contraceptive vaginal system. Contraception. 2021;104:229-234.
- Mawet M, Maillard C, Klipping C, et al. Unique effects on hepatic function, lipid metabolism, bone and growth endocrine parameters of estetrol in combined oral contraceptives. Eur J Contracept Reprod Health Care. 2015;20:463-475.
- Gérard C, Blacher S, Communal L, et al. Estetrol is a weak estrogen antagonizing estradiol-dependent mammary gland proliferation. J Endocrinol. 2015;224:85-95.
- Douxfils J, Klipping C, Duijkers I, et al. Evaluation of the effect of a new oral contraceptive containing estetrol and drospirenone on hemostasis parameters. Contraception. 2020;102:396-402.
- Creinin MD, Westhoff CL, Bouchard C, et al. Estetroldrospirenone combination oral contraceptive: North American phase 3 efficacy and safety results. Contraception. 2021;104:222-228.
- Palacios S, Colli E, Regidor PA. Multicenter, phase III trials on the contraceptive efficacy, tolerability and safety of a new drospirenone-only pill. Acta Obstet Gynecol Scand. 2019;98:1549-1557.
- Kimble T, Burke AE, Barnhart KT, et al. A 1-year prospective, open-label, single-arm, multicenter, phase 3 trial of the contraceptive efficacy and safety of the oral progestin-only pill drospirenone 4 mg using a 24/4-day regimen. Contracept X. 2020;2:100020.
- Archer DF, Stanczyk FZ, Rubin A, et al. Ethinyl estradiol and levonorgestrel pharmacokinetics with a low-dose transdermal contraceptive delivery system, AG200-15: a randomized controlled trial. Contraception. 2012;85:595-601.
- Nelson AL, Kaunitz AM, Kroll R, et al; SECURE Investigators. Efficacy, safety, and tolerability of a levonorgestrel/ethinyl estradiol transdermal delivery system: phase 3 clinical trial results. Contraception. 2021;103:137-143.
- Westhoff CL, Heartwell S, Edwards S, et al. Oral contraceptive discontinuation: do side effects matter? Am J Obstet Gynecol. 2007;196:412.e1-6; discussion 412.e6-7.
- Nelson AL. Comprehensive overview of the recently FDAapproved contraceptive vaginal ring releasing segesterone acetate and ethinylestradiol: a new year-long, patient controlled, reversible birth control method. Expert Rev Clin Pharmacol. 2019;12:953-963.
- Liu JH, Plagianos M, Archer DF, et al. Segesterone acetate serum levels with a regression model of continuous use of the segesterone acetate/ethinyl estradiol contraceptive vaginal system. Contraception. 2021;104:229-234.
- Mawet M, Maillard C, Klipping C, et al. Unique effects on hepatic function, lipid metabolism, bone and growth endocrine parameters of estetrol in combined oral contraceptives. Eur J Contracept Reprod Health Care. 2015;20:463-475.
- Gérard C, Blacher S, Communal L, et al. Estetrol is a weak estrogen antagonizing estradiol-dependent mammary gland proliferation. J Endocrinol. 2015;224:85-95.
- Douxfils J, Klipping C, Duijkers I, et al. Evaluation of the effect of a new oral contraceptive containing estetrol and drospirenone on hemostasis parameters. Contraception. 2020;102:396-402.
- Creinin MD, Westhoff CL, Bouchard C, et al. Estetroldrospirenone combination oral contraceptive: North American phase 3 efficacy and safety results. Contraception. 2021;104:222-228.
FDA OKs iPLEDGE change for gender-neutral language
The Food and Drug Administration has approved a modification to the isotretinoin risk-mitigation program to make it more inclusive for transgender patients.
Beginning on Dec. 13, 2021, Previously, there were three risk categories: females of reproductive potential, females not of reproductive potential, and males.
In recent years, dermatologists and others have advocated for the change, hoping to make the process more inclusive and less intrusive for their transgender patients.
Isotretinoin (Accutane, Absorica, Amnesteem, Claravis, others) has a high risk of severe birth defects, and has been linked with other health issues, making it crucial for those with the ability to become pregnant to take contraceptive precautions while on the medication. Under the iPLEDGE program, physicians, patients, and pharmacies prescribing, using, or dispensing the drug must all be registered, with requirements that include the use of two forms of an effective contraceptive and regular pregnancy testing for patients who can become pregnant.
The FDA had given notification in June 2018 that the REMS modification and labeling change would be required, replacing the gender-specific language with gender-neutral language, according to an FDA spokesperson. The change was based on feedback that the gender-specific language can be a barrier to access for some patients. The FDA approved the modification on Oct. 8.
Expert reactions
“This is an exciting and welcome change from the FDA on iPLEDGE that many dermatologists, myself included, have advocated for quite a few years,” Howa Yeung, MD, MSc, assistant professor of dermatology at Emory University, Atlanta, said in an interview.
In a report on the dermatologic care for lesbian, gay, bisexual, and transgender persons published in the Journal of the American Academy of Dermatology, Dr. Yeung and his colleagues noted that more than 10 million lesbian, gay, bisexual and transgender people live in the United States and that improving their health is a public health priority.
“For cisgender patients, nothing has changed – patients will continue to receive appropriate educational material related to isotretinoin based on their pregnancy potential,” Dr. Yeung said. “For transgender and gender diverse patients, this is a huge step forward.”
Under the previous system, doctors were asked to register patients using gender binary categories, “which were confusing when they did not reflect reality” for these patients, Dr. Yeung said. The new system, Dr. Yeung added, “will make my job easier. I no longer have to struggle between respecting the patient’s gender identity and providing medically necessary care for patients with severe acne.”
“The new terminology is not just respectful, it also is simpler and makes more sense,” agreed Joshua D. Safer, MD, executive director of the Center for Transgender Medicine and Surgery at Mount Sinai Health System and professor of medicine at the Icahn School of Medicine at Mount Sinai, New York. “As it stood, a transgender man with his uterus and ovaries in place might be missed in the pregnancy surveillance system because he could simply be labeled a man and not followed further. At the same time, both transgender women and cisgender women who were at no risk of pregnancy could be subject to more medical scrutiny that might have been consider intrusive.”
The change “validates the important point that pregnancy potential is not exclusively defined by sociocultural constructs of gender and allow dermatologists to focus purely on what matters when prescribing isotretinoin – whether an individual is able to become pregnant or not, regardless of their gender identity,” Klint Peebles, MD, a dermatologist at Kaiser Permanente in Washington, D.C., and suburban Maryland, who has also advocated for the change, said in an interview.
FDA elaborates
The modification includes important changes for doctors, pharmacists, and patients alike, according to the FDA.
Health care providers must assign and confirm their currently enrolled patient’s risk category when they first log in to the IPLEDGE REMS website on or after Dec. 13, the effective date. They should be sure any patient whose prescription RMA (iPLEDGE authorization) expires on Dec. 11-12 is told to obtain their prescription before midnight, Eastern time, Dec. 10.
Pharmacists will be affected, too, since the iPLEDGE REMS changed to a new platform vendor and the current “switch” pharmacy management system will be removed as a method to verify authorization to dispense isotretinoin. With these changes, as of Dec. 13, pharmacists can’t use the switch system to obtain a predispense authorization, or RMA (risk management authorization). They will need to obtain an RMA online by accessing the iPLEDGE REMS website or via telephone to the PLEDGE REMS center, 866-495-0654, before dispensing the prescription.
Patients, beginning Dec. 13, will have the option of presenting a unique QR code at the pharmacy on their smartphone rather than providing the iPLEDGE identification number. The code can be accessed by logging into their account on the iPLEDGE REMS website.
Patients with an isotretinoin prescription RMA that expires Dec. 11-12, must obtain the prescription before 11:59 p.m. Eastern time on Dec. 10. If the RMA expires before the prescription is picked up, the patient must begin the authorization process all over again.
Dr. Safer, Dr. Yeung, and Dr. Peebles have no relevant disclosures.
More information on the update and the isotretinoin REMS program is available on the FDA website.
The Food and Drug Administration has approved a modification to the isotretinoin risk-mitigation program to make it more inclusive for transgender patients.
Beginning on Dec. 13, 2021, Previously, there were three risk categories: females of reproductive potential, females not of reproductive potential, and males.
In recent years, dermatologists and others have advocated for the change, hoping to make the process more inclusive and less intrusive for their transgender patients.
Isotretinoin (Accutane, Absorica, Amnesteem, Claravis, others) has a high risk of severe birth defects, and has been linked with other health issues, making it crucial for those with the ability to become pregnant to take contraceptive precautions while on the medication. Under the iPLEDGE program, physicians, patients, and pharmacies prescribing, using, or dispensing the drug must all be registered, with requirements that include the use of two forms of an effective contraceptive and regular pregnancy testing for patients who can become pregnant.
The FDA had given notification in June 2018 that the REMS modification and labeling change would be required, replacing the gender-specific language with gender-neutral language, according to an FDA spokesperson. The change was based on feedback that the gender-specific language can be a barrier to access for some patients. The FDA approved the modification on Oct. 8.
Expert reactions
“This is an exciting and welcome change from the FDA on iPLEDGE that many dermatologists, myself included, have advocated for quite a few years,” Howa Yeung, MD, MSc, assistant professor of dermatology at Emory University, Atlanta, said in an interview.
In a report on the dermatologic care for lesbian, gay, bisexual, and transgender persons published in the Journal of the American Academy of Dermatology, Dr. Yeung and his colleagues noted that more than 10 million lesbian, gay, bisexual and transgender people live in the United States and that improving their health is a public health priority.
“For cisgender patients, nothing has changed – patients will continue to receive appropriate educational material related to isotretinoin based on their pregnancy potential,” Dr. Yeung said. “For transgender and gender diverse patients, this is a huge step forward.”
Under the previous system, doctors were asked to register patients using gender binary categories, “which were confusing when they did not reflect reality” for these patients, Dr. Yeung said. The new system, Dr. Yeung added, “will make my job easier. I no longer have to struggle between respecting the patient’s gender identity and providing medically necessary care for patients with severe acne.”
“The new terminology is not just respectful, it also is simpler and makes more sense,” agreed Joshua D. Safer, MD, executive director of the Center for Transgender Medicine and Surgery at Mount Sinai Health System and professor of medicine at the Icahn School of Medicine at Mount Sinai, New York. “As it stood, a transgender man with his uterus and ovaries in place might be missed in the pregnancy surveillance system because he could simply be labeled a man and not followed further. At the same time, both transgender women and cisgender women who were at no risk of pregnancy could be subject to more medical scrutiny that might have been consider intrusive.”
The change “validates the important point that pregnancy potential is not exclusively defined by sociocultural constructs of gender and allow dermatologists to focus purely on what matters when prescribing isotretinoin – whether an individual is able to become pregnant or not, regardless of their gender identity,” Klint Peebles, MD, a dermatologist at Kaiser Permanente in Washington, D.C., and suburban Maryland, who has also advocated for the change, said in an interview.
FDA elaborates
The modification includes important changes for doctors, pharmacists, and patients alike, according to the FDA.
Health care providers must assign and confirm their currently enrolled patient’s risk category when they first log in to the IPLEDGE REMS website on or after Dec. 13, the effective date. They should be sure any patient whose prescription RMA (iPLEDGE authorization) expires on Dec. 11-12 is told to obtain their prescription before midnight, Eastern time, Dec. 10.
Pharmacists will be affected, too, since the iPLEDGE REMS changed to a new platform vendor and the current “switch” pharmacy management system will be removed as a method to verify authorization to dispense isotretinoin. With these changes, as of Dec. 13, pharmacists can’t use the switch system to obtain a predispense authorization, or RMA (risk management authorization). They will need to obtain an RMA online by accessing the iPLEDGE REMS website or via telephone to the PLEDGE REMS center, 866-495-0654, before dispensing the prescription.
Patients, beginning Dec. 13, will have the option of presenting a unique QR code at the pharmacy on their smartphone rather than providing the iPLEDGE identification number. The code can be accessed by logging into their account on the iPLEDGE REMS website.
Patients with an isotretinoin prescription RMA that expires Dec. 11-12, must obtain the prescription before 11:59 p.m. Eastern time on Dec. 10. If the RMA expires before the prescription is picked up, the patient must begin the authorization process all over again.
Dr. Safer, Dr. Yeung, and Dr. Peebles have no relevant disclosures.
More information on the update and the isotretinoin REMS program is available on the FDA website.
The Food and Drug Administration has approved a modification to the isotretinoin risk-mitigation program to make it more inclusive for transgender patients.
Beginning on Dec. 13, 2021, Previously, there were three risk categories: females of reproductive potential, females not of reproductive potential, and males.
In recent years, dermatologists and others have advocated for the change, hoping to make the process more inclusive and less intrusive for their transgender patients.
Isotretinoin (Accutane, Absorica, Amnesteem, Claravis, others) has a high risk of severe birth defects, and has been linked with other health issues, making it crucial for those with the ability to become pregnant to take contraceptive precautions while on the medication. Under the iPLEDGE program, physicians, patients, and pharmacies prescribing, using, or dispensing the drug must all be registered, with requirements that include the use of two forms of an effective contraceptive and regular pregnancy testing for patients who can become pregnant.
The FDA had given notification in June 2018 that the REMS modification and labeling change would be required, replacing the gender-specific language with gender-neutral language, according to an FDA spokesperson. The change was based on feedback that the gender-specific language can be a barrier to access for some patients. The FDA approved the modification on Oct. 8.
Expert reactions
“This is an exciting and welcome change from the FDA on iPLEDGE that many dermatologists, myself included, have advocated for quite a few years,” Howa Yeung, MD, MSc, assistant professor of dermatology at Emory University, Atlanta, said in an interview.
In a report on the dermatologic care for lesbian, gay, bisexual, and transgender persons published in the Journal of the American Academy of Dermatology, Dr. Yeung and his colleagues noted that more than 10 million lesbian, gay, bisexual and transgender people live in the United States and that improving their health is a public health priority.
“For cisgender patients, nothing has changed – patients will continue to receive appropriate educational material related to isotretinoin based on their pregnancy potential,” Dr. Yeung said. “For transgender and gender diverse patients, this is a huge step forward.”
Under the previous system, doctors were asked to register patients using gender binary categories, “which were confusing when they did not reflect reality” for these patients, Dr. Yeung said. The new system, Dr. Yeung added, “will make my job easier. I no longer have to struggle between respecting the patient’s gender identity and providing medically necessary care for patients with severe acne.”
“The new terminology is not just respectful, it also is simpler and makes more sense,” agreed Joshua D. Safer, MD, executive director of the Center for Transgender Medicine and Surgery at Mount Sinai Health System and professor of medicine at the Icahn School of Medicine at Mount Sinai, New York. “As it stood, a transgender man with his uterus and ovaries in place might be missed in the pregnancy surveillance system because he could simply be labeled a man and not followed further. At the same time, both transgender women and cisgender women who were at no risk of pregnancy could be subject to more medical scrutiny that might have been consider intrusive.”
The change “validates the important point that pregnancy potential is not exclusively defined by sociocultural constructs of gender and allow dermatologists to focus purely on what matters when prescribing isotretinoin – whether an individual is able to become pregnant or not, regardless of their gender identity,” Klint Peebles, MD, a dermatologist at Kaiser Permanente in Washington, D.C., and suburban Maryland, who has also advocated for the change, said in an interview.
FDA elaborates
The modification includes important changes for doctors, pharmacists, and patients alike, according to the FDA.
Health care providers must assign and confirm their currently enrolled patient’s risk category when they first log in to the IPLEDGE REMS website on or after Dec. 13, the effective date. They should be sure any patient whose prescription RMA (iPLEDGE authorization) expires on Dec. 11-12 is told to obtain their prescription before midnight, Eastern time, Dec. 10.
Pharmacists will be affected, too, since the iPLEDGE REMS changed to a new platform vendor and the current “switch” pharmacy management system will be removed as a method to verify authorization to dispense isotretinoin. With these changes, as of Dec. 13, pharmacists can’t use the switch system to obtain a predispense authorization, or RMA (risk management authorization). They will need to obtain an RMA online by accessing the iPLEDGE REMS website or via telephone to the PLEDGE REMS center, 866-495-0654, before dispensing the prescription.
Patients, beginning Dec. 13, will have the option of presenting a unique QR code at the pharmacy on their smartphone rather than providing the iPLEDGE identification number. The code can be accessed by logging into their account on the iPLEDGE REMS website.
Patients with an isotretinoin prescription RMA that expires Dec. 11-12, must obtain the prescription before 11:59 p.m. Eastern time on Dec. 10. If the RMA expires before the prescription is picked up, the patient must begin the authorization process all over again.
Dr. Safer, Dr. Yeung, and Dr. Peebles have no relevant disclosures.
More information on the update and the isotretinoin REMS program is available on the FDA website.
2021 update on contraception
A new contraceptive method should ideally provide improved access or a higher quality and safety option. Although unintended pregnancy rates in the United States are decreasing, significant disparities across race and socioeconomic status remain,1 and these disparities actually doubled from 1994 to 2011 even though the overall unintended pregnancy rate decreased.1-3 Specifically, people of color, those with lower income, and people with lower education levels had higher rates of unintended pregnancies than did White people with higher education and income, suggesting disparate access to contraception services.1 Thus, as new contraceptive methods are introduced, we must assess if they have the potential to address this disparity as well as continue to provide higher quality and safer options.
In this Update, we critically review the phase 3 data on efficacy and safety for 3 new methods that were introduced to the US market over the past year to evaluate their impact on the current contraceptive landscape.
The first method, newly approved by the US Food and Drug Administration (FDA), is a combined oral contraceptive (OC) that contains a novel endogenous estrogen, estetrol, or E4 (Nextstellis). E4 is a natural estrogen produced in the fetal liver that has lower potency and a longer half-life than estradiol. Nextstellis is a monophasic 24/4 OC pill that contains E4 14.2 mg and drospirenone 3 mg in each of the 24 hormone-containing pills. Most combined hormonal contraceptives (CHCs) in the United States today contain synthetically made ethinyl estradiol (EE) due to its high potency and oral bioavailability. Outside of the reproductive system, EE upregulates the production of hepatic proteins and alters procoagulant and anticoagulant factors, which results in an overall increase in venous thromboembolic (VTE) risk among CHC users.2
After widespread use of combined oral contraceptives (COCs) started in the 1960s, data emerged regarding increased VTE risk.3 Subsequent research discovered that the type of estrogen used in CHCs directly correlates with the thrombosis risk due to the hepatic upregulation with both first- and second-pass metabolism. Although this risk was reduced as the EE dose decreased below 50 µg and concurrent VTE risk factors were contraindicated, CHC users still faced a 2-fold increase in VTE risk compared with nonusers.4,5 EE in contraceptive formulations increases VTE risk, likely related to upregulation of procoagulant factors and decreasing anticoagulant proteins.2 By contrast, a phase 2 trial of Nextstellis demonstrated more neutral effects of E4/drospirenone on hemostatic parameters compared with EE/levonorgestrel or EE/drospirenone.6 Furthermore, E4/drospirenone exhibited lower increases in hepatic proteins, such as angiotensinogen, triglycerides, and sex-hormone binding globulin.7 These findings together suggest that this novel CHC pill has a more favorable cardiovascular adverse effect profile compared with currently available CHCs.
The second contraceptive method is a new transdermal patch that contains EE and levonorgestrel (Twirla); this is in contrast to the available EE/norelgestromin contraceptive patch (Xulane). Transdermal contraceptive patches can offer some users easier adherence as compared with a daily OC.8 Until this past year, the only transdermal contraceptive available in the United States was Xulane, which contains a daily dose of EE 35 µg and norelgestromin 150 µg. Norelgestromin is eventually metabolized to levonorgestrel derivatives.9 Twirla is administered in the same manner as Xulane and contains a daily hormone exposure equivalent to a COC containing EE 30 µg and levonorgestrel 120 µg. Similar to EE/norelgestromin, the EE/levonorgestrel patch also is contraindicated in obese patients (body mass index [BMI] ≥30 kg/m2) due to decreased efficacy and increased risk for VTE. Additionally, phase 3 data demonstrated decreasing efficacy of Twirla in overweight users (BMI ≥25–30 kg/m2), perhaps further limiting the population that may benefit from this contraceptive method.10 These issues with efficacy and weight likely are related to the fact that levonorgestrel distribution is weight dependent, with evidence of lower plasma levels in obese individuals.11-13
The third new method is a prescription vaginal contraceptive gel with lactic acid, citric acid, and potassium bitartrate (Phexxi) designed to prevent pregnancy by maintaining an acidic vaginal environment that is inhospitable to sperm. For many decades, vaginal contraceptives, including vaginal spermicidal gels, provided easy access to a nonhormonal and flexible method of moderately effective contraception for many users. Phexxi is a prescription vaginal pH regulator administered as a gel and active for 1 hour after application. All previous vaginal gels sold in the United States are applied similarly, are available over the counter, and include nonoxynol-9, which is a surfactant that damages sperm cell membranes. Recent data from a phase 3 trial demonstrated similar contraceptive effectiveness of Phexxi when compared with available nonoxynol-9 alternatives.14
Continue to: New OC with the novel estrogen E4 demonstrates good safety profile for VTE...
New OC with the novel estrogen E4 demonstrates good safety profile for VTE
Creinin MD, Westhoff CL, Bouchard C, et al. Estetrol-drospirenone combination oral contraceptive: North American phase 3 efficacy and safety results. Contraception. 2021;104:222-228.
The COC E4/drospirenone was evaluated in 2 parallel multinational studies. Here, we review the North American data that are more relevant for the US population; the European-Russian data also are published.15
Study examined 1 year’s use of E4/drospirenone
The US–Canadian trial conducted by Creinin and colleagues enrolled 1,864 participants aged 16 to 50 years to evaluate contraceptive efficacy, bleeding patterns, and adverse events with 1-year use (13 cycles) of E4/drospirenone. The primary efficacy group included 1,524 women aged 16 to 35. This study enrolled healthy, heterosexually active participants with a BMI ≤35 kg/m2 and regular menses from 70 sites in the United States and 7 sites in Canada. The dropout rate was 45%, comparable to that in other contraceptive studies. Participants used E4/drospirenone cyclically, taking 1 hormone-containing pill daily for 24 days followed by 4 days of placebo pills.
Contraceptive efficacious, no VTE observed
The researchers reported efficacy as a Pearl Index (PI) of 2.65 pregnancies per 100 woman-years in participants aged 16 to 35 and an overall 13-cycle life-table pregnancy rate of 2.06%. The PI did not differ among nonobese and obese participants in multivariable analysis. Most users experienced scheduled withdrawal bleeding; only 13% to 18% reported absence of scheduled bleeding. Unscheduled bleeding was typically spotting (55.2%), and this decreased with treatment duration from 30% in cycle 1 to 15% to 20% in cycle 5 and on.
Overall, 28.9% of participants reported treatment-related adverse events (AEs), which most commonly were headache (5.0%), metrorrhagia (4.6%), and nausea (3.8%). Investigators reported a minimal change in mean (SD) BMI of 0.4 (1.7) kg/m2 from baseline after 1 year of E4/drospirenone use, and only 0.5% of participants discontinued use due to weight gain. The most common reasons for AE-related treatment discontinuation included metrorrhagia (0.9%), menorrhagia (0.8%), and vaginal hemorrhage (0.5%). Importantly, no cases of VTE occurred in this study of estetrol despite 23% of participants being obese, a known risk factor for VTE.
Nextstellis provides safe, effective contraception with a PI comparable to that of other available CHCs as well as a favorable bleeding profile in healthy users who are adherent to treatment. Importantly, contraceptive efficacy was maintained in obese users with a BMI up to 35 kg/m2. In contrast to EE or estradiol, E4 demonstrates a lower impact on the hepatic system, and preliminary findings suggest a lower VTE risk compared with other CHCs on the market. The European phase 3 trial of 1,553 participants also demonstrated a low rate of VTE, with only 1 case diagnosed.15 By contrast, similar phase 3 trials of available CHCs demonstrated more frequent VTE events despite low-dose EE formulations (TABLE 1).10,15-18 In general, most US phase 3 trials have 3 to 4 VTE events in the studied population, and the Nextstellis North American trial, of which 92% of participants were from the United States, had 0. However, confirmation of any potential lower VTE risk requires further analysis from large, population-based postmarketing studies.
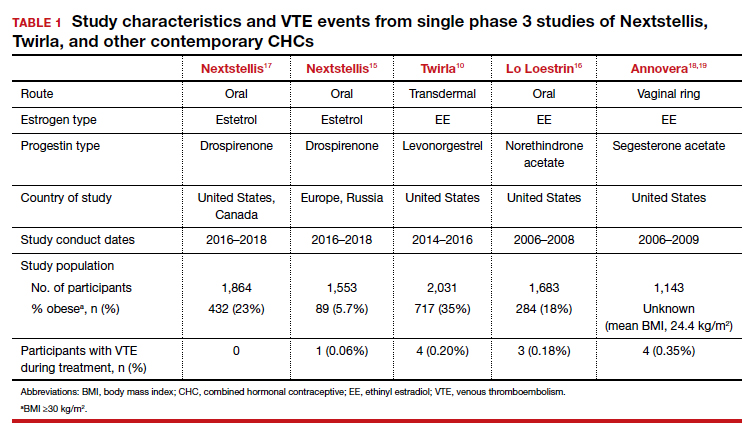
Continue to: Efficacy of a new EE/levonorgestrel transdermal patch may be lower in overweight, obese women...
Efficacy of a new EE/levonorgestrel transdermal patch may be lower in overweight, obese women
Nelson AL, Kaunitz AM, Kroll R; SECURE Investigators. Efficacy, safety, and tolerability of a levonorgestrel/ethinyl estradiol transdermal delivery system: phase 3 clinical trial results. Contraception. 2021;103:137-143.
To assess the contraceptive efficacy, tolerability, and safety of the transdermal patch Twirla (EE/levonorgestrel) over 1 year of treatment (13 cycles), Nelson and colleagues conducted an open-label, multicenter, US-based phase 3 trial of participants aged 18 years and older with regular cycles. There were no restrictions based on BMI. On average, the study population was overweight, with a mean BMI of 28.3 kg/m2 , and 35% of the population was considered obese (BMI ≥30 kg/m2).
Study design
A total of 2,032 participants enrolled in the study, with separate populations defined for specific analysis on safety, contraceptive efficacy, and cycle control. The primary efficacy group included 1,736 participants. Fifty-one percent discontinued the study, most commonly due to “women’s decision” (15%) and lost to follow-up (11%). Users received bleeding diaries and returned periodically throughout the study for evaluation for efficacy, adherence, and adverse events.
Efficacy associated with BMI
The study results demonstrated an overall PI of 5.8 pregnancies per 100 woman-years for users aged younger than 35. TABLE 2 demonstrates the overall trend of efficacy in relation to BMI.10,15-19 Participants with a higher BMI were found to have a higher PI, revealing lower contraceptive efficacy in more overweight and obese patients. The overall cumulative pregnancy rate over 13 cycles was 5.3%
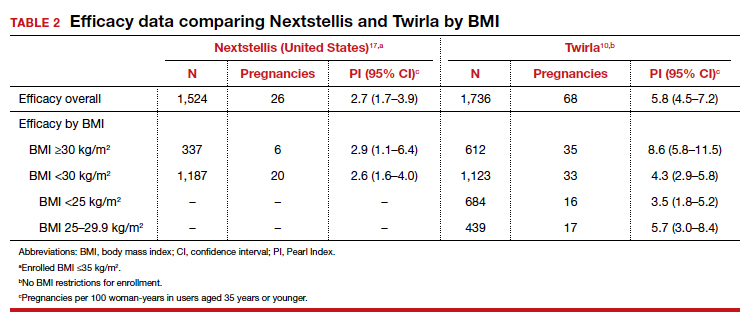
Participants reported decreasing frequency of bleeding/spotting days over the treatment duration of 13 cycles, from a mean (SD) of 6.2 (4.5) days in cycle 1 to 4.9 (3.5) days in cycle 13. Unscheduled bleeding episodes remained high throughout the study period. Initially, 60% of users reported 1 or more days of unscheduled bleeding in cycle 1, and 42% still reported unscheduled bleeding in cycle 13. In light of this, only 45 participants (2.2%) discontinued the study due to bleeding issues, suggesting perhaps that the bleeding was light. Overall, users experienced acceptable wearability of the patch, and the rate of detachment decreased over the study period from 9.9% in cycle 1 to 2.4% in cycle 13. There were also low rates (0.5%) of moderate to severe irritation. Itching at the adhesion site decreased slightly from 13.1% in cycle 2 to 9.6% in cycle 13.
In general, 27.2% of patch users experienced a study-related AE, most reported as mild to moderate. Nausea (4.1%) and headaches (3.6%) were the most common hormone-related AE. Importantly, 4 obese users experienced 5 VTEs (deep vein thrombosis, n = 2; pulmonary embolism, n = 3) between cycle 5 and 13. Three of these users had additional VTE risk factors, such as air travel and a family history of clots. No users who were of normal weight or overweight experienced VTE.
Available data demonstrate that the EE/norelgestromin patch exposes users to higher serum levels compared with the pill or the ring.20 The higher estrogen exposure with the patch may explain higher estrogen-related adverse effects and may result in increased VTE risk. Initial pharmacokinetic data of the EE/levonorgestrel patch showed lower EE concentrations, similar to marketed COCs and lower than EE/norelgestromin.21 Despite this lower estrogen exposure, the phase 3 trial by Nelson and colleagues did not demonstrate a safer profile with respect to thromboembolic events.
Further, the high PI of 5.8 pregnancies per 100 woman-years calls into question the efficacy of this patch compared with already available CHC options. Indeed, the efficacy appears reasonable in normal-weight individuals, with a PI of 3.5 pregnancies per 100 woman-years; however, this is still higher than its contemporary counterpart, Nextstellis, which has a PI of 2.65 pregnancies per 100 woman-years and included users with a BMI of up to 35 kg/m2 (Table 2). Given the evidence of decreased efficacy, clinicians may consider reserving this option for only normal-weight women who cannot use or prefer not to use another CHC method. Obese individuals (BMI ≥30 kg/m2 ) should not use this patch due to decreased efficacy and increased VTE risk. Lastly, although use in overweight individuals (BMI ≥25 kg/m2) is not absolutely contraindicated, clinicians should counsel the overweight patient on the possibility of decreased contraceptive efficacy due to weight, and they may choose to reserve use of this patch in overweight individuals only when no other comparable or more effective method is an option.
Continue to: Novel vaginal pH buffering spermicide is a new Rx-only option...
Novel vaginal pH buffering spermicide is a new Rx-only option
Thomas MA, Chappell BT, Maximos B, et al. A novel vaginal pH regulator: results from the phase 3 AMPOWER contraception clinical trial. Contracept X. 2020;2:100031.
In an open-label phase 3 study, Thomas and colleagues enrolled 1,384 participants aged 18 to 35 with regular cycles at 112 sites in the United States to assess the contraceptive efficacy, safety, and acceptability of Phexxi vaginal gel (lactic acid, citric acid, and potassium bitartrate) over 7 cycles (6 months). Participants were required to have at least 3 episodes of heterosexual vaginal intercourse per cycle and return throughout the treatment duration for study visits. Fifty-three percent of participants did not complete the study, most frequently due to loss to follow-up (18.1%) and participant withdrawal (12.3%). Most participants were White (69%) and had an average (SD) age of 27.7 (4.5) years.
Efficacy and AE rates
The investigators reported a cumulative pregnancy rate of 13.7% over 7 cycles (6 months). In this study, 45.2% of women experienced 1 AE, and most were noted to be mild (23.9%) to moderate (18.7%). The most reported AE was vulvovaginal burning (20.0%), followed by vulvovaginal pruritus (11.2%), urinary tract infection (5.7%), and vulvovaginal pain (3.8%). Less than 2% of participants discontinued the study due to an AE. Burning and itching decreased with time and with decreased frequency of use. When used twice per day compared with once per day, burning rates decreased from 4.6% to 2.1%, and itching rates decreased from 1.0% to 0.7%. Serious AEs were uncommon, occurring in 1.3% of users; only 1, cystitis, was noted to be “probably” related to the treatment. ●
Prior to the approval of Phexxi, all currently available vaginal contraceptive gels in the United States contained nonoxynol-9 as the active ingredient, which is a surfactant that is spermicidal by damaging cell membranes. Although Phexxi provides a novel mechanism of action as a spermicide, the contraceptive efficacy is about the same as available spermicides on the market (see TABLE 3).14,22,23 The FDA calculated a 13-cycle PI to include in the label (27.5 pregnancies per 100 woman-years) based on the results of this study; however, no reliable statistical method exists to calculate a true PI from a 7-cycle study. Thus, we recommend that clinicians counsel patients appropriately based on the 6-month rate noted in the study, and that this rate is similar to that with currently available over-the-counter products. This point is important, as Phexxi is available only by prescription, which may impact patient cost and access.
Equally important is Phexxi’s potential for sexually transmitted infection (STI) prevention. In a US-based randomized controlled trial, Phexxi use demonstrated significant risk reduction in gonorrhea and chlamydia infections among participants aged 18 to 45 years.24 That study showed a relative risk reduction of 50% and 78% for chlamydia and gonorrhea, respectively.24 Future research is planned to evaluate this spermicide as a novel STI prevention method. Ultimately, Phexxi may provide an alternative spermicide for users interested in moderately effective contraception and unable to tolerate available nonoxynol-9 formulations. Interested users will have to rely on a prescription, possibly limiting access to this novel spermicide. Further data are required to determine its potential as an STI prevention agent.
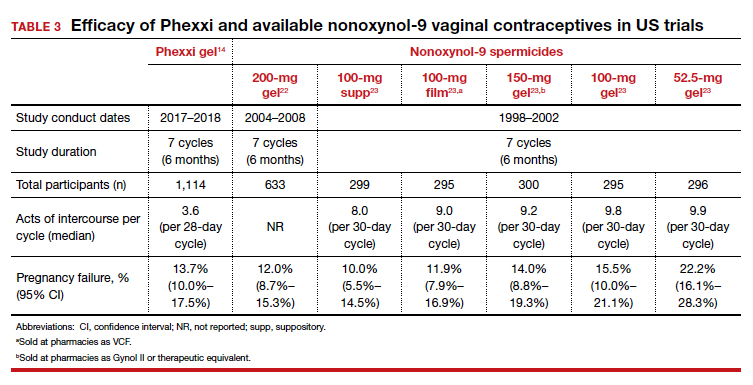
- Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008–2011. N Engl J Med. 2016;374:843-852.
- Meade TW. Oral contraceptives, clotting factors, and thrombosis. Am J Obstet Gynecol. 1982;142(6 pt 2):758-761.
- Royal College of General Practitioners’ Oral Contraception Study. Oral contraceptives, venous thrombosis, and varicose veins. J R Coll Gen Pract. 1978;28:393-399.
- Dinger JC, Heinemann LA, Kühl-Habich D. The safety of a drospirenone-containing oral contraceptive: final results from the European Active Surveillance Study on oral contraceptives based on 142,475 women-years of observation. Contraception. 2007;75:344-354.
- Heinemann LA, Dinger JC. Range of published estimates of venous thromboembolism incidence in young women. Contraception. 2007;75:328-336.
- Douxfils J, Klipping C, Duijkers I, et al. Evaluation of the effect of a new oral contraceptive containing estetrol and drospirenone on hemostasis parameters. Contraception. 2020;102:396-402.
- Klipping C, Duijkers I, Mawet M, et al. Endocrine and metabolic effects of an oral contraceptive containing estetrol and drospirenone. Contraception. 2021;103:213-221.
- Archer DF, Cullins V, Creasy GW, et al. The impact of improved compliance with a weekly contraceptive transdermal system (Ortho Evra) on contraceptive efficacy. Contraception. 2004;69:189-195.
- Stanczyk FZ, Roy S. Metabolism of levonorgestrel, norethindrone, and structurally related contraceptive steroids. Contraception. 1990;42:67-96.
- Nelson AL, Kaunitz AM, Kroll R; SECURE Investigators. Efficacy, safety, and tolerability of a levonorgestrel/ethinyl estradiol transdermal delivery system: phase 3 clinical trial results. Contraception. 2021;103:137-143.
- Natavio M, Stanczyk FZ, Molins EAG, et al. Pharmacokinetics of the 1.5 mg levonorgestrel emergency contraceptive in women with normal, obese and extremely obese body mass index. Contraception. 2019;99:306-311.
- Praditpan P, Hamouie A, Basaraba CN, et al. Pharmacokinetics of levonorgestrel and ulipristal acetate emergency contraception in women with normal and obese body mass index. Contraception. 2017;95:464-469.
- Westhoff CL, Torgal AH, Mayeda ER, et al. Pharmacokinetics of a combined oral contraceptive in obese and normal-weight women. Contraception. 2010;81:474-480.
- Thomas MA, Chappell BT, Maximos B, et al. A novel vaginal pH regulator: results from the phase 3 AMPOWER contraception clinical trial. Contracept X. 2020;2:100031.
- Gemzell-Danielsson K, Apter D, Zatik J, et al. Estetrol-drospirenone combination oral contraceptive: a clinical study of contraceptive efficacy, bleeding pattern, and safety in Europe and Russia. BJOG. 2021. doi: 10.1111/1471-0528.16840.
- Archer DF, Nakajima ST, Sawyer AT, et al. Norethindrone acetate 1.0 milligram and ethinyl estradiol 10 micrograms as an ultra low-dose oral contraceptive. Obstet Gynecol. 2013;122:601-607.
- Creinin MD, Westhoff CL, Bouchard C, et al. Estetrol-drospirenone combination oral contraceptive: North American phase 3 efficacy and safety results. Contraception. 2021;104:222-228.
- Gemzell-Danielsson K, Sitruk-Ware R, Creinin MD, et al. Segesterone acetate/ethinyl estradiol 12-month contraceptive vaginal system safety evaluation. Contraception. 2019;99:323-328.
- Safety and efficacy of a contraceptive vaginal ring delivering Nestorone and ethinyl estradiol. Clinicaltrials.gov identifier: NCT00263341. https://clinicaltrials.gov/ct2/show /NCT00263341. Accessed August 23, 2021.
- van den Heuvel MW, van Bragt AJ, Alnabawy AK, et al. Comparison of ethinylestradiol pharmacokinetics in three hormonal contraceptive formulations: the vaginal ring, the transdermal patch and an oral contraceptive. Contraception. 2005;72:168-174.
- Stanczyk FZ, Rubin A, Flood L, et al. Pharmacokinetics, tolerability and cycle control of three transdermal contraceptive delivery systems containing different doses of ethinyl-estradiol and levonorgestrel. Horm Mol Biol Clin Investig. 2011;6:231-240.
- Burke AE, Barnhart K, Jensen JT, et al. Contraceptive efficacy, acceptability, and safety of C31G and nonoxynol-9 spermicidal gels: a randomized controlled trial. Obstet Gynecol. 2010;116:1265-1273.
- Raymond EG, Chen PL, Luoto J; Spermicidal Trial Group. Contraceptive effectiveness and safety of five nonoxynol-9 spermicides: a randomized trial. Obstet Gynecol. 2004;103:430-439.
- Chappell BT, Mena LA, Maximos B, et al. EVO100 prevents chlamydia and gonorrhea in women at high risk of infection. Am J Obstet Gynecol. 2021;225:162.e1-162.e14.
A new contraceptive method should ideally provide improved access or a higher quality and safety option. Although unintended pregnancy rates in the United States are decreasing, significant disparities across race and socioeconomic status remain,1 and these disparities actually doubled from 1994 to 2011 even though the overall unintended pregnancy rate decreased.1-3 Specifically, people of color, those with lower income, and people with lower education levels had higher rates of unintended pregnancies than did White people with higher education and income, suggesting disparate access to contraception services.1 Thus, as new contraceptive methods are introduced, we must assess if they have the potential to address this disparity as well as continue to provide higher quality and safer options.
In this Update, we critically review the phase 3 data on efficacy and safety for 3 new methods that were introduced to the US market over the past year to evaluate their impact on the current contraceptive landscape.
The first method, newly approved by the US Food and Drug Administration (FDA), is a combined oral contraceptive (OC) that contains a novel endogenous estrogen, estetrol, or E4 (Nextstellis). E4 is a natural estrogen produced in the fetal liver that has lower potency and a longer half-life than estradiol. Nextstellis is a monophasic 24/4 OC pill that contains E4 14.2 mg and drospirenone 3 mg in each of the 24 hormone-containing pills. Most combined hormonal contraceptives (CHCs) in the United States today contain synthetically made ethinyl estradiol (EE) due to its high potency and oral bioavailability. Outside of the reproductive system, EE upregulates the production of hepatic proteins and alters procoagulant and anticoagulant factors, which results in an overall increase in venous thromboembolic (VTE) risk among CHC users.2
After widespread use of combined oral contraceptives (COCs) started in the 1960s, data emerged regarding increased VTE risk.3 Subsequent research discovered that the type of estrogen used in CHCs directly correlates with the thrombosis risk due to the hepatic upregulation with both first- and second-pass metabolism. Although this risk was reduced as the EE dose decreased below 50 µg and concurrent VTE risk factors were contraindicated, CHC users still faced a 2-fold increase in VTE risk compared with nonusers.4,5 EE in contraceptive formulations increases VTE risk, likely related to upregulation of procoagulant factors and decreasing anticoagulant proteins.2 By contrast, a phase 2 trial of Nextstellis demonstrated more neutral effects of E4/drospirenone on hemostatic parameters compared with EE/levonorgestrel or EE/drospirenone.6 Furthermore, E4/drospirenone exhibited lower increases in hepatic proteins, such as angiotensinogen, triglycerides, and sex-hormone binding globulin.7 These findings together suggest that this novel CHC pill has a more favorable cardiovascular adverse effect profile compared with currently available CHCs.
The second contraceptive method is a new transdermal patch that contains EE and levonorgestrel (Twirla); this is in contrast to the available EE/norelgestromin contraceptive patch (Xulane). Transdermal contraceptive patches can offer some users easier adherence as compared with a daily OC.8 Until this past year, the only transdermal contraceptive available in the United States was Xulane, which contains a daily dose of EE 35 µg and norelgestromin 150 µg. Norelgestromin is eventually metabolized to levonorgestrel derivatives.9 Twirla is administered in the same manner as Xulane and contains a daily hormone exposure equivalent to a COC containing EE 30 µg and levonorgestrel 120 µg. Similar to EE/norelgestromin, the EE/levonorgestrel patch also is contraindicated in obese patients (body mass index [BMI] ≥30 kg/m2) due to decreased efficacy and increased risk for VTE. Additionally, phase 3 data demonstrated decreasing efficacy of Twirla in overweight users (BMI ≥25–30 kg/m2), perhaps further limiting the population that may benefit from this contraceptive method.10 These issues with efficacy and weight likely are related to the fact that levonorgestrel distribution is weight dependent, with evidence of lower plasma levels in obese individuals.11-13
The third new method is a prescription vaginal contraceptive gel with lactic acid, citric acid, and potassium bitartrate (Phexxi) designed to prevent pregnancy by maintaining an acidic vaginal environment that is inhospitable to sperm. For many decades, vaginal contraceptives, including vaginal spermicidal gels, provided easy access to a nonhormonal and flexible method of moderately effective contraception for many users. Phexxi is a prescription vaginal pH regulator administered as a gel and active for 1 hour after application. All previous vaginal gels sold in the United States are applied similarly, are available over the counter, and include nonoxynol-9, which is a surfactant that damages sperm cell membranes. Recent data from a phase 3 trial demonstrated similar contraceptive effectiveness of Phexxi when compared with available nonoxynol-9 alternatives.14
Continue to: New OC with the novel estrogen E4 demonstrates good safety profile for VTE...
New OC with the novel estrogen E4 demonstrates good safety profile for VTE
Creinin MD, Westhoff CL, Bouchard C, et al. Estetrol-drospirenone combination oral contraceptive: North American phase 3 efficacy and safety results. Contraception. 2021;104:222-228.
The COC E4/drospirenone was evaluated in 2 parallel multinational studies. Here, we review the North American data that are more relevant for the US population; the European-Russian data also are published.15
Study examined 1 year’s use of E4/drospirenone
The US–Canadian trial conducted by Creinin and colleagues enrolled 1,864 participants aged 16 to 50 years to evaluate contraceptive efficacy, bleeding patterns, and adverse events with 1-year use (13 cycles) of E4/drospirenone. The primary efficacy group included 1,524 women aged 16 to 35. This study enrolled healthy, heterosexually active participants with a BMI ≤35 kg/m2 and regular menses from 70 sites in the United States and 7 sites in Canada. The dropout rate was 45%, comparable to that in other contraceptive studies. Participants used E4/drospirenone cyclically, taking 1 hormone-containing pill daily for 24 days followed by 4 days of placebo pills.
Contraceptive efficacious, no VTE observed
The researchers reported efficacy as a Pearl Index (PI) of 2.65 pregnancies per 100 woman-years in participants aged 16 to 35 and an overall 13-cycle life-table pregnancy rate of 2.06%. The PI did not differ among nonobese and obese participants in multivariable analysis. Most users experienced scheduled withdrawal bleeding; only 13% to 18% reported absence of scheduled bleeding. Unscheduled bleeding was typically spotting (55.2%), and this decreased with treatment duration from 30% in cycle 1 to 15% to 20% in cycle 5 and on.
Overall, 28.9% of participants reported treatment-related adverse events (AEs), which most commonly were headache (5.0%), metrorrhagia (4.6%), and nausea (3.8%). Investigators reported a minimal change in mean (SD) BMI of 0.4 (1.7) kg/m2 from baseline after 1 year of E4/drospirenone use, and only 0.5% of participants discontinued use due to weight gain. The most common reasons for AE-related treatment discontinuation included metrorrhagia (0.9%), menorrhagia (0.8%), and vaginal hemorrhage (0.5%). Importantly, no cases of VTE occurred in this study of estetrol despite 23% of participants being obese, a known risk factor for VTE.
Nextstellis provides safe, effective contraception with a PI comparable to that of other available CHCs as well as a favorable bleeding profile in healthy users who are adherent to treatment. Importantly, contraceptive efficacy was maintained in obese users with a BMI up to 35 kg/m2. In contrast to EE or estradiol, E4 demonstrates a lower impact on the hepatic system, and preliminary findings suggest a lower VTE risk compared with other CHCs on the market. The European phase 3 trial of 1,553 participants also demonstrated a low rate of VTE, with only 1 case diagnosed.15 By contrast, similar phase 3 trials of available CHCs demonstrated more frequent VTE events despite low-dose EE formulations (TABLE 1).10,15-18 In general, most US phase 3 trials have 3 to 4 VTE events in the studied population, and the Nextstellis North American trial, of which 92% of participants were from the United States, had 0. However, confirmation of any potential lower VTE risk requires further analysis from large, population-based postmarketing studies.

Continue to: Efficacy of a new EE/levonorgestrel transdermal patch may be lower in overweight, obese women...
Efficacy of a new EE/levonorgestrel transdermal patch may be lower in overweight, obese women
Nelson AL, Kaunitz AM, Kroll R; SECURE Investigators. Efficacy, safety, and tolerability of a levonorgestrel/ethinyl estradiol transdermal delivery system: phase 3 clinical trial results. Contraception. 2021;103:137-143.
To assess the contraceptive efficacy, tolerability, and safety of the transdermal patch Twirla (EE/levonorgestrel) over 1 year of treatment (13 cycles), Nelson and colleagues conducted an open-label, multicenter, US-based phase 3 trial of participants aged 18 years and older with regular cycles. There were no restrictions based on BMI. On average, the study population was overweight, with a mean BMI of 28.3 kg/m2 , and 35% of the population was considered obese (BMI ≥30 kg/m2).
Study design
A total of 2,032 participants enrolled in the study, with separate populations defined for specific analysis on safety, contraceptive efficacy, and cycle control. The primary efficacy group included 1,736 participants. Fifty-one percent discontinued the study, most commonly due to “women’s decision” (15%) and lost to follow-up (11%). Users received bleeding diaries and returned periodically throughout the study for evaluation for efficacy, adherence, and adverse events.
Efficacy associated with BMI
The study results demonstrated an overall PI of 5.8 pregnancies per 100 woman-years for users aged younger than 35. TABLE 2 demonstrates the overall trend of efficacy in relation to BMI.10,15-19 Participants with a higher BMI were found to have a higher PI, revealing lower contraceptive efficacy in more overweight and obese patients. The overall cumulative pregnancy rate over 13 cycles was 5.3%

Participants reported decreasing frequency of bleeding/spotting days over the treatment duration of 13 cycles, from a mean (SD) of 6.2 (4.5) days in cycle 1 to 4.9 (3.5) days in cycle 13. Unscheduled bleeding episodes remained high throughout the study period. Initially, 60% of users reported 1 or more days of unscheduled bleeding in cycle 1, and 42% still reported unscheduled bleeding in cycle 13. In light of this, only 45 participants (2.2%) discontinued the study due to bleeding issues, suggesting perhaps that the bleeding was light. Overall, users experienced acceptable wearability of the patch, and the rate of detachment decreased over the study period from 9.9% in cycle 1 to 2.4% in cycle 13. There were also low rates (0.5%) of moderate to severe irritation. Itching at the adhesion site decreased slightly from 13.1% in cycle 2 to 9.6% in cycle 13.
In general, 27.2% of patch users experienced a study-related AE, most reported as mild to moderate. Nausea (4.1%) and headaches (3.6%) were the most common hormone-related AE. Importantly, 4 obese users experienced 5 VTEs (deep vein thrombosis, n = 2; pulmonary embolism, n = 3) between cycle 5 and 13. Three of these users had additional VTE risk factors, such as air travel and a family history of clots. No users who were of normal weight or overweight experienced VTE.
Available data demonstrate that the EE/norelgestromin patch exposes users to higher serum levels compared with the pill or the ring.20 The higher estrogen exposure with the patch may explain higher estrogen-related adverse effects and may result in increased VTE risk. Initial pharmacokinetic data of the EE/levonorgestrel patch showed lower EE concentrations, similar to marketed COCs and lower than EE/norelgestromin.21 Despite this lower estrogen exposure, the phase 3 trial by Nelson and colleagues did not demonstrate a safer profile with respect to thromboembolic events.
Further, the high PI of 5.8 pregnancies per 100 woman-years calls into question the efficacy of this patch compared with already available CHC options. Indeed, the efficacy appears reasonable in normal-weight individuals, with a PI of 3.5 pregnancies per 100 woman-years; however, this is still higher than its contemporary counterpart, Nextstellis, which has a PI of 2.65 pregnancies per 100 woman-years and included users with a BMI of up to 35 kg/m2 (Table 2). Given the evidence of decreased efficacy, clinicians may consider reserving this option for only normal-weight women who cannot use or prefer not to use another CHC method. Obese individuals (BMI ≥30 kg/m2 ) should not use this patch due to decreased efficacy and increased VTE risk. Lastly, although use in overweight individuals (BMI ≥25 kg/m2) is not absolutely contraindicated, clinicians should counsel the overweight patient on the possibility of decreased contraceptive efficacy due to weight, and they may choose to reserve use of this patch in overweight individuals only when no other comparable or more effective method is an option.
Continue to: Novel vaginal pH buffering spermicide is a new Rx-only option...
Novel vaginal pH buffering spermicide is a new Rx-only option
Thomas MA, Chappell BT, Maximos B, et al. A novel vaginal pH regulator: results from the phase 3 AMPOWER contraception clinical trial. Contracept X. 2020;2:100031.
In an open-label phase 3 study, Thomas and colleagues enrolled 1,384 participants aged 18 to 35 with regular cycles at 112 sites in the United States to assess the contraceptive efficacy, safety, and acceptability of Phexxi vaginal gel (lactic acid, citric acid, and potassium bitartrate) over 7 cycles (6 months). Participants were required to have at least 3 episodes of heterosexual vaginal intercourse per cycle and return throughout the treatment duration for study visits. Fifty-three percent of participants did not complete the study, most frequently due to loss to follow-up (18.1%) and participant withdrawal (12.3%). Most participants were White (69%) and had an average (SD) age of 27.7 (4.5) years.
Efficacy and AE rates
The investigators reported a cumulative pregnancy rate of 13.7% over 7 cycles (6 months). In this study, 45.2% of women experienced 1 AE, and most were noted to be mild (23.9%) to moderate (18.7%). The most reported AE was vulvovaginal burning (20.0%), followed by vulvovaginal pruritus (11.2%), urinary tract infection (5.7%), and vulvovaginal pain (3.8%). Less than 2% of participants discontinued the study due to an AE. Burning and itching decreased with time and with decreased frequency of use. When used twice per day compared with once per day, burning rates decreased from 4.6% to 2.1%, and itching rates decreased from 1.0% to 0.7%. Serious AEs were uncommon, occurring in 1.3% of users; only 1, cystitis, was noted to be “probably” related to the treatment. ●
Prior to the approval of Phexxi, all currently available vaginal contraceptive gels in the United States contained nonoxynol-9 as the active ingredient, which is a surfactant that is spermicidal by damaging cell membranes. Although Phexxi provides a novel mechanism of action as a spermicide, the contraceptive efficacy is about the same as available spermicides on the market (see TABLE 3).14,22,23 The FDA calculated a 13-cycle PI to include in the label (27.5 pregnancies per 100 woman-years) based on the results of this study; however, no reliable statistical method exists to calculate a true PI from a 7-cycle study. Thus, we recommend that clinicians counsel patients appropriately based on the 6-month rate noted in the study, and that this rate is similar to that with currently available over-the-counter products. This point is important, as Phexxi is available only by prescription, which may impact patient cost and access.
Equally important is Phexxi’s potential for sexually transmitted infection (STI) prevention. In a US-based randomized controlled trial, Phexxi use demonstrated significant risk reduction in gonorrhea and chlamydia infections among participants aged 18 to 45 years.24 That study showed a relative risk reduction of 50% and 78% for chlamydia and gonorrhea, respectively.24 Future research is planned to evaluate this spermicide as a novel STI prevention method. Ultimately, Phexxi may provide an alternative spermicide for users interested in moderately effective contraception and unable to tolerate available nonoxynol-9 formulations. Interested users will have to rely on a prescription, possibly limiting access to this novel spermicide. Further data are required to determine its potential as an STI prevention agent.

A new contraceptive method should ideally provide improved access or a higher quality and safety option. Although unintended pregnancy rates in the United States are decreasing, significant disparities across race and socioeconomic status remain,1 and these disparities actually doubled from 1994 to 2011 even though the overall unintended pregnancy rate decreased.1-3 Specifically, people of color, those with lower income, and people with lower education levels had higher rates of unintended pregnancies than did White people with higher education and income, suggesting disparate access to contraception services.1 Thus, as new contraceptive methods are introduced, we must assess if they have the potential to address this disparity as well as continue to provide higher quality and safer options.
In this Update, we critically review the phase 3 data on efficacy and safety for 3 new methods that were introduced to the US market over the past year to evaluate their impact on the current contraceptive landscape.
The first method, newly approved by the US Food and Drug Administration (FDA), is a combined oral contraceptive (OC) that contains a novel endogenous estrogen, estetrol, or E4 (Nextstellis). E4 is a natural estrogen produced in the fetal liver that has lower potency and a longer half-life than estradiol. Nextstellis is a monophasic 24/4 OC pill that contains E4 14.2 mg and drospirenone 3 mg in each of the 24 hormone-containing pills. Most combined hormonal contraceptives (CHCs) in the United States today contain synthetically made ethinyl estradiol (EE) due to its high potency and oral bioavailability. Outside of the reproductive system, EE upregulates the production of hepatic proteins and alters procoagulant and anticoagulant factors, which results in an overall increase in venous thromboembolic (VTE) risk among CHC users.2
After widespread use of combined oral contraceptives (COCs) started in the 1960s, data emerged regarding increased VTE risk.3 Subsequent research discovered that the type of estrogen used in CHCs directly correlates with the thrombosis risk due to the hepatic upregulation with both first- and second-pass metabolism. Although this risk was reduced as the EE dose decreased below 50 µg and concurrent VTE risk factors were contraindicated, CHC users still faced a 2-fold increase in VTE risk compared with nonusers.4,5 EE in contraceptive formulations increases VTE risk, likely related to upregulation of procoagulant factors and decreasing anticoagulant proteins.2 By contrast, a phase 2 trial of Nextstellis demonstrated more neutral effects of E4/drospirenone on hemostatic parameters compared with EE/levonorgestrel or EE/drospirenone.6 Furthermore, E4/drospirenone exhibited lower increases in hepatic proteins, such as angiotensinogen, triglycerides, and sex-hormone binding globulin.7 These findings together suggest that this novel CHC pill has a more favorable cardiovascular adverse effect profile compared with currently available CHCs.
The second contraceptive method is a new transdermal patch that contains EE and levonorgestrel (Twirla); this is in contrast to the available EE/norelgestromin contraceptive patch (Xulane). Transdermal contraceptive patches can offer some users easier adherence as compared with a daily OC.8 Until this past year, the only transdermal contraceptive available in the United States was Xulane, which contains a daily dose of EE 35 µg and norelgestromin 150 µg. Norelgestromin is eventually metabolized to levonorgestrel derivatives.9 Twirla is administered in the same manner as Xulane and contains a daily hormone exposure equivalent to a COC containing EE 30 µg and levonorgestrel 120 µg. Similar to EE/norelgestromin, the EE/levonorgestrel patch also is contraindicated in obese patients (body mass index [BMI] ≥30 kg/m2) due to decreased efficacy and increased risk for VTE. Additionally, phase 3 data demonstrated decreasing efficacy of Twirla in overweight users (BMI ≥25–30 kg/m2), perhaps further limiting the population that may benefit from this contraceptive method.10 These issues with efficacy and weight likely are related to the fact that levonorgestrel distribution is weight dependent, with evidence of lower plasma levels in obese individuals.11-13
The third new method is a prescription vaginal contraceptive gel with lactic acid, citric acid, and potassium bitartrate (Phexxi) designed to prevent pregnancy by maintaining an acidic vaginal environment that is inhospitable to sperm. For many decades, vaginal contraceptives, including vaginal spermicidal gels, provided easy access to a nonhormonal and flexible method of moderately effective contraception for many users. Phexxi is a prescription vaginal pH regulator administered as a gel and active for 1 hour after application. All previous vaginal gels sold in the United States are applied similarly, are available over the counter, and include nonoxynol-9, which is a surfactant that damages sperm cell membranes. Recent data from a phase 3 trial demonstrated similar contraceptive effectiveness of Phexxi when compared with available nonoxynol-9 alternatives.14
Continue to: New OC with the novel estrogen E4 demonstrates good safety profile for VTE...
New OC with the novel estrogen E4 demonstrates good safety profile for VTE
Creinin MD, Westhoff CL, Bouchard C, et al. Estetrol-drospirenone combination oral contraceptive: North American phase 3 efficacy and safety results. Contraception. 2021;104:222-228.
The COC E4/drospirenone was evaluated in 2 parallel multinational studies. Here, we review the North American data that are more relevant for the US population; the European-Russian data also are published.15
Study examined 1 year’s use of E4/drospirenone
The US–Canadian trial conducted by Creinin and colleagues enrolled 1,864 participants aged 16 to 50 years to evaluate contraceptive efficacy, bleeding patterns, and adverse events with 1-year use (13 cycles) of E4/drospirenone. The primary efficacy group included 1,524 women aged 16 to 35. This study enrolled healthy, heterosexually active participants with a BMI ≤35 kg/m2 and regular menses from 70 sites in the United States and 7 sites in Canada. The dropout rate was 45%, comparable to that in other contraceptive studies. Participants used E4/drospirenone cyclically, taking 1 hormone-containing pill daily for 24 days followed by 4 days of placebo pills.
Contraceptive efficacious, no VTE observed
The researchers reported efficacy as a Pearl Index (PI) of 2.65 pregnancies per 100 woman-years in participants aged 16 to 35 and an overall 13-cycle life-table pregnancy rate of 2.06%. The PI did not differ among nonobese and obese participants in multivariable analysis. Most users experienced scheduled withdrawal bleeding; only 13% to 18% reported absence of scheduled bleeding. Unscheduled bleeding was typically spotting (55.2%), and this decreased with treatment duration from 30% in cycle 1 to 15% to 20% in cycle 5 and on.
Overall, 28.9% of participants reported treatment-related adverse events (AEs), which most commonly were headache (5.0%), metrorrhagia (4.6%), and nausea (3.8%). Investigators reported a minimal change in mean (SD) BMI of 0.4 (1.7) kg/m2 from baseline after 1 year of E4/drospirenone use, and only 0.5% of participants discontinued use due to weight gain. The most common reasons for AE-related treatment discontinuation included metrorrhagia (0.9%), menorrhagia (0.8%), and vaginal hemorrhage (0.5%). Importantly, no cases of VTE occurred in this study of estetrol despite 23% of participants being obese, a known risk factor for VTE.
Nextstellis provides safe, effective contraception with a PI comparable to that of other available CHCs as well as a favorable bleeding profile in healthy users who are adherent to treatment. Importantly, contraceptive efficacy was maintained in obese users with a BMI up to 35 kg/m2. In contrast to EE or estradiol, E4 demonstrates a lower impact on the hepatic system, and preliminary findings suggest a lower VTE risk compared with other CHCs on the market. The European phase 3 trial of 1,553 participants also demonstrated a low rate of VTE, with only 1 case diagnosed.15 By contrast, similar phase 3 trials of available CHCs demonstrated more frequent VTE events despite low-dose EE formulations (TABLE 1).10,15-18 In general, most US phase 3 trials have 3 to 4 VTE events in the studied population, and the Nextstellis North American trial, of which 92% of participants were from the United States, had 0. However, confirmation of any potential lower VTE risk requires further analysis from large, population-based postmarketing studies.

Continue to: Efficacy of a new EE/levonorgestrel transdermal patch may be lower in overweight, obese women...
Efficacy of a new EE/levonorgestrel transdermal patch may be lower in overweight, obese women
Nelson AL, Kaunitz AM, Kroll R; SECURE Investigators. Efficacy, safety, and tolerability of a levonorgestrel/ethinyl estradiol transdermal delivery system: phase 3 clinical trial results. Contraception. 2021;103:137-143.
To assess the contraceptive efficacy, tolerability, and safety of the transdermal patch Twirla (EE/levonorgestrel) over 1 year of treatment (13 cycles), Nelson and colleagues conducted an open-label, multicenter, US-based phase 3 trial of participants aged 18 years and older with regular cycles. There were no restrictions based on BMI. On average, the study population was overweight, with a mean BMI of 28.3 kg/m2 , and 35% of the population was considered obese (BMI ≥30 kg/m2).
Study design
A total of 2,032 participants enrolled in the study, with separate populations defined for specific analysis on safety, contraceptive efficacy, and cycle control. The primary efficacy group included 1,736 participants. Fifty-one percent discontinued the study, most commonly due to “women’s decision” (15%) and lost to follow-up (11%). Users received bleeding diaries and returned periodically throughout the study for evaluation for efficacy, adherence, and adverse events.
Efficacy associated with BMI
The study results demonstrated an overall PI of 5.8 pregnancies per 100 woman-years for users aged younger than 35. TABLE 2 demonstrates the overall trend of efficacy in relation to BMI.10,15-19 Participants with a higher BMI were found to have a higher PI, revealing lower contraceptive efficacy in more overweight and obese patients. The overall cumulative pregnancy rate over 13 cycles was 5.3%

Participants reported decreasing frequency of bleeding/spotting days over the treatment duration of 13 cycles, from a mean (SD) of 6.2 (4.5) days in cycle 1 to 4.9 (3.5) days in cycle 13. Unscheduled bleeding episodes remained high throughout the study period. Initially, 60% of users reported 1 or more days of unscheduled bleeding in cycle 1, and 42% still reported unscheduled bleeding in cycle 13. In light of this, only 45 participants (2.2%) discontinued the study due to bleeding issues, suggesting perhaps that the bleeding was light. Overall, users experienced acceptable wearability of the patch, and the rate of detachment decreased over the study period from 9.9% in cycle 1 to 2.4% in cycle 13. There were also low rates (0.5%) of moderate to severe irritation. Itching at the adhesion site decreased slightly from 13.1% in cycle 2 to 9.6% in cycle 13.
In general, 27.2% of patch users experienced a study-related AE, most reported as mild to moderate. Nausea (4.1%) and headaches (3.6%) were the most common hormone-related AE. Importantly, 4 obese users experienced 5 VTEs (deep vein thrombosis, n = 2; pulmonary embolism, n = 3) between cycle 5 and 13. Three of these users had additional VTE risk factors, such as air travel and a family history of clots. No users who were of normal weight or overweight experienced VTE.
Available data demonstrate that the EE/norelgestromin patch exposes users to higher serum levels compared with the pill or the ring.20 The higher estrogen exposure with the patch may explain higher estrogen-related adverse effects and may result in increased VTE risk. Initial pharmacokinetic data of the EE/levonorgestrel patch showed lower EE concentrations, similar to marketed COCs and lower than EE/norelgestromin.21 Despite this lower estrogen exposure, the phase 3 trial by Nelson and colleagues did not demonstrate a safer profile with respect to thromboembolic events.
Further, the high PI of 5.8 pregnancies per 100 woman-years calls into question the efficacy of this patch compared with already available CHC options. Indeed, the efficacy appears reasonable in normal-weight individuals, with a PI of 3.5 pregnancies per 100 woman-years; however, this is still higher than its contemporary counterpart, Nextstellis, which has a PI of 2.65 pregnancies per 100 woman-years and included users with a BMI of up to 35 kg/m2 (Table 2). Given the evidence of decreased efficacy, clinicians may consider reserving this option for only normal-weight women who cannot use or prefer not to use another CHC method. Obese individuals (BMI ≥30 kg/m2 ) should not use this patch due to decreased efficacy and increased VTE risk. Lastly, although use in overweight individuals (BMI ≥25 kg/m2) is not absolutely contraindicated, clinicians should counsel the overweight patient on the possibility of decreased contraceptive efficacy due to weight, and they may choose to reserve use of this patch in overweight individuals only when no other comparable or more effective method is an option.
Continue to: Novel vaginal pH buffering spermicide is a new Rx-only option...
Novel vaginal pH buffering spermicide is a new Rx-only option
Thomas MA, Chappell BT, Maximos B, et al. A novel vaginal pH regulator: results from the phase 3 AMPOWER contraception clinical trial. Contracept X. 2020;2:100031.
In an open-label phase 3 study, Thomas and colleagues enrolled 1,384 participants aged 18 to 35 with regular cycles at 112 sites in the United States to assess the contraceptive efficacy, safety, and acceptability of Phexxi vaginal gel (lactic acid, citric acid, and potassium bitartrate) over 7 cycles (6 months). Participants were required to have at least 3 episodes of heterosexual vaginal intercourse per cycle and return throughout the treatment duration for study visits. Fifty-three percent of participants did not complete the study, most frequently due to loss to follow-up (18.1%) and participant withdrawal (12.3%). Most participants were White (69%) and had an average (SD) age of 27.7 (4.5) years.
Efficacy and AE rates
The investigators reported a cumulative pregnancy rate of 13.7% over 7 cycles (6 months). In this study, 45.2% of women experienced 1 AE, and most were noted to be mild (23.9%) to moderate (18.7%). The most reported AE was vulvovaginal burning (20.0%), followed by vulvovaginal pruritus (11.2%), urinary tract infection (5.7%), and vulvovaginal pain (3.8%). Less than 2% of participants discontinued the study due to an AE. Burning and itching decreased with time and with decreased frequency of use. When used twice per day compared with once per day, burning rates decreased from 4.6% to 2.1%, and itching rates decreased from 1.0% to 0.7%. Serious AEs were uncommon, occurring in 1.3% of users; only 1, cystitis, was noted to be “probably” related to the treatment. ●
Prior to the approval of Phexxi, all currently available vaginal contraceptive gels in the United States contained nonoxynol-9 as the active ingredient, which is a surfactant that is spermicidal by damaging cell membranes. Although Phexxi provides a novel mechanism of action as a spermicide, the contraceptive efficacy is about the same as available spermicides on the market (see TABLE 3).14,22,23 The FDA calculated a 13-cycle PI to include in the label (27.5 pregnancies per 100 woman-years) based on the results of this study; however, no reliable statistical method exists to calculate a true PI from a 7-cycle study. Thus, we recommend that clinicians counsel patients appropriately based on the 6-month rate noted in the study, and that this rate is similar to that with currently available over-the-counter products. This point is important, as Phexxi is available only by prescription, which may impact patient cost and access.
Equally important is Phexxi’s potential for sexually transmitted infection (STI) prevention. In a US-based randomized controlled trial, Phexxi use demonstrated significant risk reduction in gonorrhea and chlamydia infections among participants aged 18 to 45 years.24 That study showed a relative risk reduction of 50% and 78% for chlamydia and gonorrhea, respectively.24 Future research is planned to evaluate this spermicide as a novel STI prevention method. Ultimately, Phexxi may provide an alternative spermicide for users interested in moderately effective contraception and unable to tolerate available nonoxynol-9 formulations. Interested users will have to rely on a prescription, possibly limiting access to this novel spermicide. Further data are required to determine its potential as an STI prevention agent.

- Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008–2011. N Engl J Med. 2016;374:843-852.
- Meade TW. Oral contraceptives, clotting factors, and thrombosis. Am J Obstet Gynecol. 1982;142(6 pt 2):758-761.
- Royal College of General Practitioners’ Oral Contraception Study. Oral contraceptives, venous thrombosis, and varicose veins. J R Coll Gen Pract. 1978;28:393-399.
- Dinger JC, Heinemann LA, Kühl-Habich D. The safety of a drospirenone-containing oral contraceptive: final results from the European Active Surveillance Study on oral contraceptives based on 142,475 women-years of observation. Contraception. 2007;75:344-354.
- Heinemann LA, Dinger JC. Range of published estimates of venous thromboembolism incidence in young women. Contraception. 2007;75:328-336.
- Douxfils J, Klipping C, Duijkers I, et al. Evaluation of the effect of a new oral contraceptive containing estetrol and drospirenone on hemostasis parameters. Contraception. 2020;102:396-402.
- Klipping C, Duijkers I, Mawet M, et al. Endocrine and metabolic effects of an oral contraceptive containing estetrol and drospirenone. Contraception. 2021;103:213-221.
- Archer DF, Cullins V, Creasy GW, et al. The impact of improved compliance with a weekly contraceptive transdermal system (Ortho Evra) on contraceptive efficacy. Contraception. 2004;69:189-195.
- Stanczyk FZ, Roy S. Metabolism of levonorgestrel, norethindrone, and structurally related contraceptive steroids. Contraception. 1990;42:67-96.
- Nelson AL, Kaunitz AM, Kroll R; SECURE Investigators. Efficacy, safety, and tolerability of a levonorgestrel/ethinyl estradiol transdermal delivery system: phase 3 clinical trial results. Contraception. 2021;103:137-143.
- Natavio M, Stanczyk FZ, Molins EAG, et al. Pharmacokinetics of the 1.5 mg levonorgestrel emergency contraceptive in women with normal, obese and extremely obese body mass index. Contraception. 2019;99:306-311.
- Praditpan P, Hamouie A, Basaraba CN, et al. Pharmacokinetics of levonorgestrel and ulipristal acetate emergency contraception in women with normal and obese body mass index. Contraception. 2017;95:464-469.
- Westhoff CL, Torgal AH, Mayeda ER, et al. Pharmacokinetics of a combined oral contraceptive in obese and normal-weight women. Contraception. 2010;81:474-480.
- Thomas MA, Chappell BT, Maximos B, et al. A novel vaginal pH regulator: results from the phase 3 AMPOWER contraception clinical trial. Contracept X. 2020;2:100031.
- Gemzell-Danielsson K, Apter D, Zatik J, et al. Estetrol-drospirenone combination oral contraceptive: a clinical study of contraceptive efficacy, bleeding pattern, and safety in Europe and Russia. BJOG. 2021. doi: 10.1111/1471-0528.16840.
- Archer DF, Nakajima ST, Sawyer AT, et al. Norethindrone acetate 1.0 milligram and ethinyl estradiol 10 micrograms as an ultra low-dose oral contraceptive. Obstet Gynecol. 2013;122:601-607.
- Creinin MD, Westhoff CL, Bouchard C, et al. Estetrol-drospirenone combination oral contraceptive: North American phase 3 efficacy and safety results. Contraception. 2021;104:222-228.
- Gemzell-Danielsson K, Sitruk-Ware R, Creinin MD, et al. Segesterone acetate/ethinyl estradiol 12-month contraceptive vaginal system safety evaluation. Contraception. 2019;99:323-328.
- Safety and efficacy of a contraceptive vaginal ring delivering Nestorone and ethinyl estradiol. Clinicaltrials.gov identifier: NCT00263341. https://clinicaltrials.gov/ct2/show /NCT00263341. Accessed August 23, 2021.
- van den Heuvel MW, van Bragt AJ, Alnabawy AK, et al. Comparison of ethinylestradiol pharmacokinetics in three hormonal contraceptive formulations: the vaginal ring, the transdermal patch and an oral contraceptive. Contraception. 2005;72:168-174.
- Stanczyk FZ, Rubin A, Flood L, et al. Pharmacokinetics, tolerability and cycle control of three transdermal contraceptive delivery systems containing different doses of ethinyl-estradiol and levonorgestrel. Horm Mol Biol Clin Investig. 2011;6:231-240.
- Burke AE, Barnhart K, Jensen JT, et al. Contraceptive efficacy, acceptability, and safety of C31G and nonoxynol-9 spermicidal gels: a randomized controlled trial. Obstet Gynecol. 2010;116:1265-1273.
- Raymond EG, Chen PL, Luoto J; Spermicidal Trial Group. Contraceptive effectiveness and safety of five nonoxynol-9 spermicides: a randomized trial. Obstet Gynecol. 2004;103:430-439.
- Chappell BT, Mena LA, Maximos B, et al. EVO100 prevents chlamydia and gonorrhea in women at high risk of infection. Am J Obstet Gynecol. 2021;225:162.e1-162.e14.
- Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008–2011. N Engl J Med. 2016;374:843-852.
- Meade TW. Oral contraceptives, clotting factors, and thrombosis. Am J Obstet Gynecol. 1982;142(6 pt 2):758-761.
- Royal College of General Practitioners’ Oral Contraception Study. Oral contraceptives, venous thrombosis, and varicose veins. J R Coll Gen Pract. 1978;28:393-399.
- Dinger JC, Heinemann LA, Kühl-Habich D. The safety of a drospirenone-containing oral contraceptive: final results from the European Active Surveillance Study on oral contraceptives based on 142,475 women-years of observation. Contraception. 2007;75:344-354.
- Heinemann LA, Dinger JC. Range of published estimates of venous thromboembolism incidence in young women. Contraception. 2007;75:328-336.
- Douxfils J, Klipping C, Duijkers I, et al. Evaluation of the effect of a new oral contraceptive containing estetrol and drospirenone on hemostasis parameters. Contraception. 2020;102:396-402.
- Klipping C, Duijkers I, Mawet M, et al. Endocrine and metabolic effects of an oral contraceptive containing estetrol and drospirenone. Contraception. 2021;103:213-221.
- Archer DF, Cullins V, Creasy GW, et al. The impact of improved compliance with a weekly contraceptive transdermal system (Ortho Evra) on contraceptive efficacy. Contraception. 2004;69:189-195.
- Stanczyk FZ, Roy S. Metabolism of levonorgestrel, norethindrone, and structurally related contraceptive steroids. Contraception. 1990;42:67-96.
- Nelson AL, Kaunitz AM, Kroll R; SECURE Investigators. Efficacy, safety, and tolerability of a levonorgestrel/ethinyl estradiol transdermal delivery system: phase 3 clinical trial results. Contraception. 2021;103:137-143.
- Natavio M, Stanczyk FZ, Molins EAG, et al. Pharmacokinetics of the 1.5 mg levonorgestrel emergency contraceptive in women with normal, obese and extremely obese body mass index. Contraception. 2019;99:306-311.
- Praditpan P, Hamouie A, Basaraba CN, et al. Pharmacokinetics of levonorgestrel and ulipristal acetate emergency contraception in women with normal and obese body mass index. Contraception. 2017;95:464-469.
- Westhoff CL, Torgal AH, Mayeda ER, et al. Pharmacokinetics of a combined oral contraceptive in obese and normal-weight women. Contraception. 2010;81:474-480.
- Thomas MA, Chappell BT, Maximos B, et al. A novel vaginal pH regulator: results from the phase 3 AMPOWER contraception clinical trial. Contracept X. 2020;2:100031.
- Gemzell-Danielsson K, Apter D, Zatik J, et al. Estetrol-drospirenone combination oral contraceptive: a clinical study of contraceptive efficacy, bleeding pattern, and safety in Europe and Russia. BJOG. 2021. doi: 10.1111/1471-0528.16840.
- Archer DF, Nakajima ST, Sawyer AT, et al. Norethindrone acetate 1.0 milligram and ethinyl estradiol 10 micrograms as an ultra low-dose oral contraceptive. Obstet Gynecol. 2013;122:601-607.
- Creinin MD, Westhoff CL, Bouchard C, et al. Estetrol-drospirenone combination oral contraceptive: North American phase 3 efficacy and safety results. Contraception. 2021;104:222-228.
- Gemzell-Danielsson K, Sitruk-Ware R, Creinin MD, et al. Segesterone acetate/ethinyl estradiol 12-month contraceptive vaginal system safety evaluation. Contraception. 2019;99:323-328.
- Safety and efficacy of a contraceptive vaginal ring delivering Nestorone and ethinyl estradiol. Clinicaltrials.gov identifier: NCT00263341. https://clinicaltrials.gov/ct2/show /NCT00263341. Accessed August 23, 2021.
- van den Heuvel MW, van Bragt AJ, Alnabawy AK, et al. Comparison of ethinylestradiol pharmacokinetics in three hormonal contraceptive formulations: the vaginal ring, the transdermal patch and an oral contraceptive. Contraception. 2005;72:168-174.
- Stanczyk FZ, Rubin A, Flood L, et al. Pharmacokinetics, tolerability and cycle control of three transdermal contraceptive delivery systems containing different doses of ethinyl-estradiol and levonorgestrel. Horm Mol Biol Clin Investig. 2011;6:231-240.
- Burke AE, Barnhart K, Jensen JT, et al. Contraceptive efficacy, acceptability, and safety of C31G and nonoxynol-9 spermicidal gels: a randomized controlled trial. Obstet Gynecol. 2010;116:1265-1273.
- Raymond EG, Chen PL, Luoto J; Spermicidal Trial Group. Contraceptive effectiveness and safety of five nonoxynol-9 spermicides: a randomized trial. Obstet Gynecol. 2004;103:430-439.
- Chappell BT, Mena LA, Maximos B, et al. EVO100 prevents chlamydia and gonorrhea in women at high risk of infection. Am J Obstet Gynecol. 2021;225:162.e1-162.e14.
Clinical Edge Journal Scan Commentary: Contraception October 2021
Contraception prescription patterns vary by specialty and geography
Access to contraceptive services is dependent on both the local availability of healthcare providers as well as the types of contraception services offered by those providers. Little is known about the national US contraception workforce, which includes any type of provider that offers contraceptive care. In this observational study, three national data sources were combined to construct a comprehensive database of the contraception provider workforce to evaluate Medicaid participation and variation in the supply, distribution, and types of contraceptive services offered. The study found that 73.1% of obstetric and gynecologic medical physicians (OBGYN), 72.6% of nurse-midwives, 51.4% of family medicine physicians, 32.4% of pediatricians, 25.2% of advanced practice nurses, 19.8% of internal medicine physicians, and 19.4% of physician assistants prescribed the contraceptive pill, patch, or ring. Approximately half of OBGYNs and family medicine physicians (50.2% and 52.2%, respectively) provided injectable contraception, compared to 34.7% of internal medicine physicians and 34.1% of pediatricians. Intrauterine devices (IUD) were provided by 92.8% of OBGYNs compared with 16.4% of family physicians, 2.6% of internal medicine physicians, and 0.6% of pediatricians. Contraceptive implants were provided by 56.2% of OBGYNs, compared with 13.7% of family medicine physicians, 1.8% of internal medicine physicians, and 4.0% of pediatricians. The contraception workforce also varied by geography, both in the density and types of providers that different communities depend upon. States ranged from provider-to-population ratios of 27.9 to 74.2 providers per 10,000 women of reproductive age. The availability of different specialties and professions also varied between counties, with 675 of the 1,411 counties lacking either OBGYNs or nurse-midwives prescribing contraception. This study also found variation across states and provider types in the proportion of contraceptive providers who accept Medicaid, with rates of Medicaid acceptance highest amongst OBGYNs and lowest amongst internal medicine physicians. This report highlights that the distribution of the contraception workforce and Medicaid acceptance varies widely by location and specialty and documents large gaps in the provision of highly effective contraceptive services including IUDs and implants. Increasing the number and types of providers that can provide family planning is central to providing comprehensive reproductive healthcare and reducing unintended pregnancies.
US Healthcare provider practices related to Emergency Contraception
Emergency contraception (EC) can prevent pregnancy after sexual encounters in which contraception was not used or used incorrectly. The US Selected Practice Recommendations for Contraceptive Use (US SPR) was initially released in 2013 and includes recommendations for healthcare providers on the initiation of EC, increasing access to EC through advance provision of EC pills, and initiation of regular contraception in conjunction with provision of EC pills. The objective of this study was to assess the percentage of healthcare providers reporting frequent provision of select EC practices around the time of and after the release of the US SPR. Two cross-sectional mailed surveys were conducted using different nationwide samples of office-based physicians and public-sector providers around the time of (2013-2014) and after (2019) the initial US SPR release. Providers were asked to indicate how often in the past year they had: 1) provided an advance prescription of EC pills to a woman not specifically seeking EC; 2) provided an advanced supply of EC pills to a woman not specifically seeking EC; 3) provided or prescribed a contraceptive at the same time as EC pills were provided; and 4) provided a copper IUD as EC. Data was pooled from both surveys, resulting in an overall sample size of 3,480 providers (n = 2,060 for the 2013-2014 survey and n = 1,420 for the 2019 survey). In the 2019 nationwide sample, 16% of respondents frequently provided an advance prescription of EC pills, 7% provided an advanced supply of EC pills, 8% provided the copper IUD as EC, and 41% cfrequently provided regular contraception at the time of EC pills. Overall, there were no significant changes in prevalence of frequently providing or prescribing an advance supply of EC pills between 2013-2014 and 2019, which may reflect changes in provider practices based on availability of over-the-counter levonogestrel EC pills in 2013. An increase in the proportion of providers who frequently provided regular contraception at the same time as EC pills and who provided a copper IUD for EC between 2013-2014 and 2019 was observed. In 2019, providers who reported using the US SPR were more likely to provide contraception at the same time as EC pills and provide the copper IUD for EC compared with those who did not use the US SPR. Wider implementation of the US SPR recommendations and an improved understanding of the barriers faced by providers in implementing these practices may improve access to EC. A recent report found that the levonorgestrel 52 IUD provides EC with efficacy similar to that of the copper IUD and may lead to more widespread placement of IUDs for EC (Turok).
Progestogen-only pill shows promise as a potential non-prescription contraception option for both breastfeeding and non-breastfeeding women
An initiative is currently underway to apply for US Food and Drug Administration (FDA) approval for over-the-counter sales of a progestogen-only contraceptive pill (POP) containing 75 mg/day norgestrel. Although 75 mg/day norgestrel is approved by the FDA for prescription use, this formulation is not currently available in the US as marketing of this product was discontinued in 2005 for reasons not related to safety or effectiveness. The failure rate of the POP is presently reported to be the same as that of combined oral contraceptive pills (COC): 9% typical use and 0.3% perfect use unintended pregnancy rate. The objective of this review is to summarize and present the published data regarding the contraceptive effectiveness of 75 mg/day norgestrel amongst breastfeeding and non-breastfeeding women. A literature search was conducted in 2019 and identified 13 articles that specifically assessed the contraceptive efficacy of 75 mg/day norgestrel. Seven of the 13 studies included a total of 5,258 women who were breastfeeding and six of the 13 studies included a total 3,144 non-breastfeeding women. Taken together, the six studies of 3,144 non-breastfeeding women provide data on 35,319 months of use with a range of overall 12-month failure rates from 0-2.4/hundred woman-years from 75 mg/day norgestrel during typical use with a calculated aggregate Pearl Index of 2.2. Among breastfeeding women, the 12-month life table cumulative pregnancy rates for 75 mg/day norgestrel ranged from 0-3.4. This review concluded that the data support that 75 mg/day norgestrel is highly effective in clinical use, with similar estimates of failure in breastfeeding and non-breastfeeding women, providing support to the case for FDA approval of over-the-counter use of 75 mg/day norgestrel. Most contraindications to use of combination estrogen-progestin contraceptives relate to the estrogen component. Over the counter availability of the norgestrel POP could enhance women’s access to hormonal contraception.
Millions of women view YouTube videos on self-removal of long-acting contraception
This study reviewed 58 YouTube videos related to self-removal of long-acting reversible contraception (LARC)– namely intrauterine devices (IUD) and contraceptive implants. Video content was analyzed to explore demographic characteristics, method and duration of LARC use, and motivations and experiences of self-removal. There were 48 videos (83%) that featured individuals who self-removed an IUD and 10 videos (17%) that featured individuals who self-removed an implant. All videos were uploaded between 2012-2020 and had over 4 million collective views, with the median number of views being 10,473 per video. Although a much smaller proportion of videos featured the self-removal of an implant, these videos had a higher average number of views (median 23,097 vs, 9533) and comments (median 44 vs. 14) compared to videos of IUD self-removals. The video creators of 53% were identified as White, 31% as Black, and 14% as Latina. The top comments for each video were analyzed and three primary themes emerged: positive affirmations; the viewer’s consideration of or attempt at self-removal; and complaints about LARC. There were 25 videos (n = 25/58) that included a comment from a viewer who stated they had either removed their own LARC device after watching the video or intended to do so soon. Three main motivations for self-removal were identified. Roughly half the sample (n = 30/58) described a desire to remove their method at home out of personal preference or convenience (n = 28/48 IUD users and n = 2/10 implant users). Others noted the inconvenience of an in-clinic removal. A large proportion of LARC users described barriers to clinic-based removal, including cost, lack of insurance, and long waiting times for an appointment. Most individuals in the sample (n = 56/58) successfully removed their device and described their experience in positive terms related to the ease of removal. Roughly a third of all video creators encountered challenges, including difficulty grasping the strings of their IUD or challenges removing the implant (n = 17/48 IUD users and n = 3/10 implant users). Positive experiences of self-removal and high levels of viewer engagement with online videos suggest a need for provider counseling on LARC removal at the time of insertion. Providers should clearly describe any procedural or financial requirements of removal prior to LARC placement. Providers may also wish to proactively discuss the risks and best practices for safe self-removal of LARC, including a conversation about the desired length of the IUD strings, risks associated with self-removal, and available resources when the patient encounters barriers to clinic-based removal. This study provides important data about the characteristics, motivations, and experiences of a group of people that are often invisible to researchers and healthcare providers.
References:
Broussard K, Becker A. Self-removal of long-acting reversible contraception: A content analysis of YouTube videos. Contraception. 2021 Aug 13: S0010-7824(21)00346-2 (in press).
Chen C, Strasser J, Banawa R, Luo Q, Bodas M, Castruccio-Prince C, Das K, Pittman P. Who is providing contraception care in the United States? An observational study of the contraception workforce. Am J Obstet Gynecol. 2021 Aug 18: S0002-9378(21)00883-8 (in press).
Contraception prescription patterns vary by specialty and geography
Access to contraceptive services is dependent on both the local availability of healthcare providers as well as the types of contraception services offered by those providers. Little is known about the national US contraception workforce, which includes any type of provider that offers contraceptive care. In this observational study, three national data sources were combined to construct a comprehensive database of the contraception provider workforce to evaluate Medicaid participation and variation in the supply, distribution, and types of contraceptive services offered. The study found that 73.1% of obstetric and gynecologic medical physicians (OBGYN), 72.6% of nurse-midwives, 51.4% of family medicine physicians, 32.4% of pediatricians, 25.2% of advanced practice nurses, 19.8% of internal medicine physicians, and 19.4% of physician assistants prescribed the contraceptive pill, patch, or ring. Approximately half of OBGYNs and family medicine physicians (50.2% and 52.2%, respectively) provided injectable contraception, compared to 34.7% of internal medicine physicians and 34.1% of pediatricians. Intrauterine devices (IUD) were provided by 92.8% of OBGYNs compared with 16.4% of family physicians, 2.6% of internal medicine physicians, and 0.6% of pediatricians. Contraceptive implants were provided by 56.2% of OBGYNs, compared with 13.7% of family medicine physicians, 1.8% of internal medicine physicians, and 4.0% of pediatricians. The contraception workforce also varied by geography, both in the density and types of providers that different communities depend upon. States ranged from provider-to-population ratios of 27.9 to 74.2 providers per 10,000 women of reproductive age. The availability of different specialties and professions also varied between counties, with 675 of the 1,411 counties lacking either OBGYNs or nurse-midwives prescribing contraception. This study also found variation across states and provider types in the proportion of contraceptive providers who accept Medicaid, with rates of Medicaid acceptance highest amongst OBGYNs and lowest amongst internal medicine physicians. This report highlights that the distribution of the contraception workforce and Medicaid acceptance varies widely by location and specialty and documents large gaps in the provision of highly effective contraceptive services including IUDs and implants. Increasing the number and types of providers that can provide family planning is central to providing comprehensive reproductive healthcare and reducing unintended pregnancies.
US Healthcare provider practices related to Emergency Contraception
Emergency contraception (EC) can prevent pregnancy after sexual encounters in which contraception was not used or used incorrectly. The US Selected Practice Recommendations for Contraceptive Use (US SPR) was initially released in 2013 and includes recommendations for healthcare providers on the initiation of EC, increasing access to EC through advance provision of EC pills, and initiation of regular contraception in conjunction with provision of EC pills. The objective of this study was to assess the percentage of healthcare providers reporting frequent provision of select EC practices around the time of and after the release of the US SPR. Two cross-sectional mailed surveys were conducted using different nationwide samples of office-based physicians and public-sector providers around the time of (2013-2014) and after (2019) the initial US SPR release. Providers were asked to indicate how often in the past year they had: 1) provided an advance prescription of EC pills to a woman not specifically seeking EC; 2) provided an advanced supply of EC pills to a woman not specifically seeking EC; 3) provided or prescribed a contraceptive at the same time as EC pills were provided; and 4) provided a copper IUD as EC. Data was pooled from both surveys, resulting in an overall sample size of 3,480 providers (n = 2,060 for the 2013-2014 survey and n = 1,420 for the 2019 survey). In the 2019 nationwide sample, 16% of respondents frequently provided an advance prescription of EC pills, 7% provided an advanced supply of EC pills, 8% provided the copper IUD as EC, and 41% cfrequently provided regular contraception at the time of EC pills. Overall, there were no significant changes in prevalence of frequently providing or prescribing an advance supply of EC pills between 2013-2014 and 2019, which may reflect changes in provider practices based on availability of over-the-counter levonogestrel EC pills in 2013. An increase in the proportion of providers who frequently provided regular contraception at the same time as EC pills and who provided a copper IUD for EC between 2013-2014 and 2019 was observed. In 2019, providers who reported using the US SPR were more likely to provide contraception at the same time as EC pills and provide the copper IUD for EC compared with those who did not use the US SPR. Wider implementation of the US SPR recommendations and an improved understanding of the barriers faced by providers in implementing these practices may improve access to EC. A recent report found that the levonorgestrel 52 IUD provides EC with efficacy similar to that of the copper IUD and may lead to more widespread placement of IUDs for EC (Turok).
Progestogen-only pill shows promise as a potential non-prescription contraception option for both breastfeeding and non-breastfeeding women
An initiative is currently underway to apply for US Food and Drug Administration (FDA) approval for over-the-counter sales of a progestogen-only contraceptive pill (POP) containing 75 mg/day norgestrel. Although 75 mg/day norgestrel is approved by the FDA for prescription use, this formulation is not currently available in the US as marketing of this product was discontinued in 2005 for reasons not related to safety or effectiveness. The failure rate of the POP is presently reported to be the same as that of combined oral contraceptive pills (COC): 9% typical use and 0.3% perfect use unintended pregnancy rate. The objective of this review is to summarize and present the published data regarding the contraceptive effectiveness of 75 mg/day norgestrel amongst breastfeeding and non-breastfeeding women. A literature search was conducted in 2019 and identified 13 articles that specifically assessed the contraceptive efficacy of 75 mg/day norgestrel. Seven of the 13 studies included a total of 5,258 women who were breastfeeding and six of the 13 studies included a total 3,144 non-breastfeeding women. Taken together, the six studies of 3,144 non-breastfeeding women provide data on 35,319 months of use with a range of overall 12-month failure rates from 0-2.4/hundred woman-years from 75 mg/day norgestrel during typical use with a calculated aggregate Pearl Index of 2.2. Among breastfeeding women, the 12-month life table cumulative pregnancy rates for 75 mg/day norgestrel ranged from 0-3.4. This review concluded that the data support that 75 mg/day norgestrel is highly effective in clinical use, with similar estimates of failure in breastfeeding and non-breastfeeding women, providing support to the case for FDA approval of over-the-counter use of 75 mg/day norgestrel. Most contraindications to use of combination estrogen-progestin contraceptives relate to the estrogen component. Over the counter availability of the norgestrel POP could enhance women’s access to hormonal contraception.
Millions of women view YouTube videos on self-removal of long-acting contraception
This study reviewed 58 YouTube videos related to self-removal of long-acting reversible contraception (LARC)– namely intrauterine devices (IUD) and contraceptive implants. Video content was analyzed to explore demographic characteristics, method and duration of LARC use, and motivations and experiences of self-removal. There were 48 videos (83%) that featured individuals who self-removed an IUD and 10 videos (17%) that featured individuals who self-removed an implant. All videos were uploaded between 2012-2020 and had over 4 million collective views, with the median number of views being 10,473 per video. Although a much smaller proportion of videos featured the self-removal of an implant, these videos had a higher average number of views (median 23,097 vs, 9533) and comments (median 44 vs. 14) compared to videos of IUD self-removals. The video creators of 53% were identified as White, 31% as Black, and 14% as Latina. The top comments for each video were analyzed and three primary themes emerged: positive affirmations; the viewer’s consideration of or attempt at self-removal; and complaints about LARC. There were 25 videos (n = 25/58) that included a comment from a viewer who stated they had either removed their own LARC device after watching the video or intended to do so soon. Three main motivations for self-removal were identified. Roughly half the sample (n = 30/58) described a desire to remove their method at home out of personal preference or convenience (n = 28/48 IUD users and n = 2/10 implant users). Others noted the inconvenience of an in-clinic removal. A large proportion of LARC users described barriers to clinic-based removal, including cost, lack of insurance, and long waiting times for an appointment. Most individuals in the sample (n = 56/58) successfully removed their device and described their experience in positive terms related to the ease of removal. Roughly a third of all video creators encountered challenges, including difficulty grasping the strings of their IUD or challenges removing the implant (n = 17/48 IUD users and n = 3/10 implant users). Positive experiences of self-removal and high levels of viewer engagement with online videos suggest a need for provider counseling on LARC removal at the time of insertion. Providers should clearly describe any procedural or financial requirements of removal prior to LARC placement. Providers may also wish to proactively discuss the risks and best practices for safe self-removal of LARC, including a conversation about the desired length of the IUD strings, risks associated with self-removal, and available resources when the patient encounters barriers to clinic-based removal. This study provides important data about the characteristics, motivations, and experiences of a group of people that are often invisible to researchers and healthcare providers.
References:
Broussard K, Becker A. Self-removal of long-acting reversible contraception: A content analysis of YouTube videos. Contraception. 2021 Aug 13: S0010-7824(21)00346-2 (in press).
Chen C, Strasser J, Banawa R, Luo Q, Bodas M, Castruccio-Prince C, Das K, Pittman P. Who is providing contraception care in the United States? An observational study of the contraception workforce. Am J Obstet Gynecol. 2021 Aug 18: S0002-9378(21)00883-8 (in press).
Contraception prescription patterns vary by specialty and geography
Access to contraceptive services is dependent on both the local availability of healthcare providers as well as the types of contraception services offered by those providers. Little is known about the national US contraception workforce, which includes any type of provider that offers contraceptive care. In this observational study, three national data sources were combined to construct a comprehensive database of the contraception provider workforce to evaluate Medicaid participation and variation in the supply, distribution, and types of contraceptive services offered. The study found that 73.1% of obstetric and gynecologic medical physicians (OBGYN), 72.6% of nurse-midwives, 51.4% of family medicine physicians, 32.4% of pediatricians, 25.2% of advanced practice nurses, 19.8% of internal medicine physicians, and 19.4% of physician assistants prescribed the contraceptive pill, patch, or ring. Approximately half of OBGYNs and family medicine physicians (50.2% and 52.2%, respectively) provided injectable contraception, compared to 34.7% of internal medicine physicians and 34.1% of pediatricians. Intrauterine devices (IUD) were provided by 92.8% of OBGYNs compared with 16.4% of family physicians, 2.6% of internal medicine physicians, and 0.6% of pediatricians. Contraceptive implants were provided by 56.2% of OBGYNs, compared with 13.7% of family medicine physicians, 1.8% of internal medicine physicians, and 4.0% of pediatricians. The contraception workforce also varied by geography, both in the density and types of providers that different communities depend upon. States ranged from provider-to-population ratios of 27.9 to 74.2 providers per 10,000 women of reproductive age. The availability of different specialties and professions also varied between counties, with 675 of the 1,411 counties lacking either OBGYNs or nurse-midwives prescribing contraception. This study also found variation across states and provider types in the proportion of contraceptive providers who accept Medicaid, with rates of Medicaid acceptance highest amongst OBGYNs and lowest amongst internal medicine physicians. This report highlights that the distribution of the contraception workforce and Medicaid acceptance varies widely by location and specialty and documents large gaps in the provision of highly effective contraceptive services including IUDs and implants. Increasing the number and types of providers that can provide family planning is central to providing comprehensive reproductive healthcare and reducing unintended pregnancies.
US Healthcare provider practices related to Emergency Contraception
Emergency contraception (EC) can prevent pregnancy after sexual encounters in which contraception was not used or used incorrectly. The US Selected Practice Recommendations for Contraceptive Use (US SPR) was initially released in 2013 and includes recommendations for healthcare providers on the initiation of EC, increasing access to EC through advance provision of EC pills, and initiation of regular contraception in conjunction with provision of EC pills. The objective of this study was to assess the percentage of healthcare providers reporting frequent provision of select EC practices around the time of and after the release of the US SPR. Two cross-sectional mailed surveys were conducted using different nationwide samples of office-based physicians and public-sector providers around the time of (2013-2014) and after (2019) the initial US SPR release. Providers were asked to indicate how often in the past year they had: 1) provided an advance prescription of EC pills to a woman not specifically seeking EC; 2) provided an advanced supply of EC pills to a woman not specifically seeking EC; 3) provided or prescribed a contraceptive at the same time as EC pills were provided; and 4) provided a copper IUD as EC. Data was pooled from both surveys, resulting in an overall sample size of 3,480 providers (n = 2,060 for the 2013-2014 survey and n = 1,420 for the 2019 survey). In the 2019 nationwide sample, 16% of respondents frequently provided an advance prescription of EC pills, 7% provided an advanced supply of EC pills, 8% provided the copper IUD as EC, and 41% cfrequently provided regular contraception at the time of EC pills. Overall, there were no significant changes in prevalence of frequently providing or prescribing an advance supply of EC pills between 2013-2014 and 2019, which may reflect changes in provider practices based on availability of over-the-counter levonogestrel EC pills in 2013. An increase in the proportion of providers who frequently provided regular contraception at the same time as EC pills and who provided a copper IUD for EC between 2013-2014 and 2019 was observed. In 2019, providers who reported using the US SPR were more likely to provide contraception at the same time as EC pills and provide the copper IUD for EC compared with those who did not use the US SPR. Wider implementation of the US SPR recommendations and an improved understanding of the barriers faced by providers in implementing these practices may improve access to EC. A recent report found that the levonorgestrel 52 IUD provides EC with efficacy similar to that of the copper IUD and may lead to more widespread placement of IUDs for EC (Turok).
Progestogen-only pill shows promise as a potential non-prescription contraception option for both breastfeeding and non-breastfeeding women
An initiative is currently underway to apply for US Food and Drug Administration (FDA) approval for over-the-counter sales of a progestogen-only contraceptive pill (POP) containing 75 mg/day norgestrel. Although 75 mg/day norgestrel is approved by the FDA for prescription use, this formulation is not currently available in the US as marketing of this product was discontinued in 2005 for reasons not related to safety or effectiveness. The failure rate of the POP is presently reported to be the same as that of combined oral contraceptive pills (COC): 9% typical use and 0.3% perfect use unintended pregnancy rate. The objective of this review is to summarize and present the published data regarding the contraceptive effectiveness of 75 mg/day norgestrel amongst breastfeeding and non-breastfeeding women. A literature search was conducted in 2019 and identified 13 articles that specifically assessed the contraceptive efficacy of 75 mg/day norgestrel. Seven of the 13 studies included a total of 5,258 women who were breastfeeding and six of the 13 studies included a total 3,144 non-breastfeeding women. Taken together, the six studies of 3,144 non-breastfeeding women provide data on 35,319 months of use with a range of overall 12-month failure rates from 0-2.4/hundred woman-years from 75 mg/day norgestrel during typical use with a calculated aggregate Pearl Index of 2.2. Among breastfeeding women, the 12-month life table cumulative pregnancy rates for 75 mg/day norgestrel ranged from 0-3.4. This review concluded that the data support that 75 mg/day norgestrel is highly effective in clinical use, with similar estimates of failure in breastfeeding and non-breastfeeding women, providing support to the case for FDA approval of over-the-counter use of 75 mg/day norgestrel. Most contraindications to use of combination estrogen-progestin contraceptives relate to the estrogen component. Over the counter availability of the norgestrel POP could enhance women’s access to hormonal contraception.
Millions of women view YouTube videos on self-removal of long-acting contraception
This study reviewed 58 YouTube videos related to self-removal of long-acting reversible contraception (LARC)– namely intrauterine devices (IUD) and contraceptive implants. Video content was analyzed to explore demographic characteristics, method and duration of LARC use, and motivations and experiences of self-removal. There were 48 videos (83%) that featured individuals who self-removed an IUD and 10 videos (17%) that featured individuals who self-removed an implant. All videos were uploaded between 2012-2020 and had over 4 million collective views, with the median number of views being 10,473 per video. Although a much smaller proportion of videos featured the self-removal of an implant, these videos had a higher average number of views (median 23,097 vs, 9533) and comments (median 44 vs. 14) compared to videos of IUD self-removals. The video creators of 53% were identified as White, 31% as Black, and 14% as Latina. The top comments for each video were analyzed and three primary themes emerged: positive affirmations; the viewer’s consideration of or attempt at self-removal; and complaints about LARC. There were 25 videos (n = 25/58) that included a comment from a viewer who stated they had either removed their own LARC device after watching the video or intended to do so soon. Three main motivations for self-removal were identified. Roughly half the sample (n = 30/58) described a desire to remove their method at home out of personal preference or convenience (n = 28/48 IUD users and n = 2/10 implant users). Others noted the inconvenience of an in-clinic removal. A large proportion of LARC users described barriers to clinic-based removal, including cost, lack of insurance, and long waiting times for an appointment. Most individuals in the sample (n = 56/58) successfully removed their device and described their experience in positive terms related to the ease of removal. Roughly a third of all video creators encountered challenges, including difficulty grasping the strings of their IUD or challenges removing the implant (n = 17/48 IUD users and n = 3/10 implant users). Positive experiences of self-removal and high levels of viewer engagement with online videos suggest a need for provider counseling on LARC removal at the time of insertion. Providers should clearly describe any procedural or financial requirements of removal prior to LARC placement. Providers may also wish to proactively discuss the risks and best practices for safe self-removal of LARC, including a conversation about the desired length of the IUD strings, risks associated with self-removal, and available resources when the patient encounters barriers to clinic-based removal. This study provides important data about the characteristics, motivations, and experiences of a group of people that are often invisible to researchers and healthcare providers.
References:
Broussard K, Becker A. Self-removal of long-acting reversible contraception: A content analysis of YouTube videos. Contraception. 2021 Aug 13: S0010-7824(21)00346-2 (in press).
Chen C, Strasser J, Banawa R, Luo Q, Bodas M, Castruccio-Prince C, Das K, Pittman P. Who is providing contraception care in the United States? An observational study of the contraception workforce. Am J Obstet Gynecol. 2021 Aug 18: S0002-9378(21)00883-8 (in press).
Uterine perforation rates remain low after intrauterine device insertion
Key clinical point: The overall rate of uterine perforation after intrauterine device (IUD) insertion was less than 1%, but higher with placement at 4-8 weeks postpartum compared to 9-36 weeks postpartum.
Major finding: After adjusting for multiple variables, perforation rates associated with IUDs were significantly higher when placed at 4-8 weeks vs. 9-36 weeks postpartum (0.78% versus 0.46%, P = .001). Expulsion rates were low and similar between the early and late placement groups (1.02 vs. 1.17).
Study details: The data come from a retrospective cohort study of 24,959 women who underwent insertion of an intrauterine device at a single center. A total of 430 patients had confirmed complications; 157 of these were uterine perforations and 273 were intrauterine device expulsions.
Disclosures: The study received no outside funding. Lead author Dr. Ramos-Rivera had no financial conflicts to disclose. A coauthor is supported by the NIH Eunice Kennedy Shriver National Institute of Child Health & Human Development.
Source: Ramos-Rivera M et al. Am J Obstet Gynecol. 2021 Aug 27. doi: 10.1016/j.ajog.2021.08.028.
Key clinical point: The overall rate of uterine perforation after intrauterine device (IUD) insertion was less than 1%, but higher with placement at 4-8 weeks postpartum compared to 9-36 weeks postpartum.
Major finding: After adjusting for multiple variables, perforation rates associated with IUDs were significantly higher when placed at 4-8 weeks vs. 9-36 weeks postpartum (0.78% versus 0.46%, P = .001). Expulsion rates were low and similar between the early and late placement groups (1.02 vs. 1.17).
Study details: The data come from a retrospective cohort study of 24,959 women who underwent insertion of an intrauterine device at a single center. A total of 430 patients had confirmed complications; 157 of these were uterine perforations and 273 were intrauterine device expulsions.
Disclosures: The study received no outside funding. Lead author Dr. Ramos-Rivera had no financial conflicts to disclose. A coauthor is supported by the NIH Eunice Kennedy Shriver National Institute of Child Health & Human Development.
Source: Ramos-Rivera M et al. Am J Obstet Gynecol. 2021 Aug 27. doi: 10.1016/j.ajog.2021.08.028.
Key clinical point: The overall rate of uterine perforation after intrauterine device (IUD) insertion was less than 1%, but higher with placement at 4-8 weeks postpartum compared to 9-36 weeks postpartum.
Major finding: After adjusting for multiple variables, perforation rates associated with IUDs were significantly higher when placed at 4-8 weeks vs. 9-36 weeks postpartum (0.78% versus 0.46%, P = .001). Expulsion rates were low and similar between the early and late placement groups (1.02 vs. 1.17).
Study details: The data come from a retrospective cohort study of 24,959 women who underwent insertion of an intrauterine device at a single center. A total of 430 patients had confirmed complications; 157 of these were uterine perforations and 273 were intrauterine device expulsions.
Disclosures: The study received no outside funding. Lead author Dr. Ramos-Rivera had no financial conflicts to disclose. A coauthor is supported by the NIH Eunice Kennedy Shriver National Institute of Child Health & Human Development.
Source: Ramos-Rivera M et al. Am J Obstet Gynecol. 2021 Aug 27. doi: 10.1016/j.ajog.2021.08.028.
Most women choose contraception after pregnancy termination
Key clinical point: No significant associations were noted between contraception choice and age, previous pregnancies, or social determinants of health, but nearly 100% of women opted for some form of contraception following a pregnancy termination.
Major finding: Prior to pregnancy termination, 58.5% of women reported not using contraception, and 22.4% reported using a barrier or fertility awareness. After pregnancy termination, 99.7% of women chose a form of contraception, and 95.2% chose a more effective method than what they had been using. After 6 months, 85.8% were still using contraception, and 37.8% were still using a more effective method.
Study details: The data come from a cross-sectional study of 400 women who underwent termination of pregnancy over a 2-year period. Information about contraception choice was collected before pregnancy termination, at the time of termination, and at 6 months following termination.
Disclosures: The study received no outside funding. The researchers had no financial conflicts to disclose.
Source: Smith SN et al. J Obstet Gynaecol Can. 2021 Aug 27. doi: 10.1016/j.jogc.2021.07.012.
Key clinical point: No significant associations were noted between contraception choice and age, previous pregnancies, or social determinants of health, but nearly 100% of women opted for some form of contraception following a pregnancy termination.
Major finding: Prior to pregnancy termination, 58.5% of women reported not using contraception, and 22.4% reported using a barrier or fertility awareness. After pregnancy termination, 99.7% of women chose a form of contraception, and 95.2% chose a more effective method than what they had been using. After 6 months, 85.8% were still using contraception, and 37.8% were still using a more effective method.
Study details: The data come from a cross-sectional study of 400 women who underwent termination of pregnancy over a 2-year period. Information about contraception choice was collected before pregnancy termination, at the time of termination, and at 6 months following termination.
Disclosures: The study received no outside funding. The researchers had no financial conflicts to disclose.
Source: Smith SN et al. J Obstet Gynaecol Can. 2021 Aug 27. doi: 10.1016/j.jogc.2021.07.012.
Key clinical point: No significant associations were noted between contraception choice and age, previous pregnancies, or social determinants of health, but nearly 100% of women opted for some form of contraception following a pregnancy termination.
Major finding: Prior to pregnancy termination, 58.5% of women reported not using contraception, and 22.4% reported using a barrier or fertility awareness. After pregnancy termination, 99.7% of women chose a form of contraception, and 95.2% chose a more effective method than what they had been using. After 6 months, 85.8% were still using contraception, and 37.8% were still using a more effective method.
Study details: The data come from a cross-sectional study of 400 women who underwent termination of pregnancy over a 2-year period. Information about contraception choice was collected before pregnancy termination, at the time of termination, and at 6 months following termination.
Disclosures: The study received no outside funding. The researchers had no financial conflicts to disclose.
Source: Smith SN et al. J Obstet Gynaecol Can. 2021 Aug 27. doi: 10.1016/j.jogc.2021.07.012.
Fewer than half of health care providers offer routine contraception along with emergency contraception
Key clinical point: In 2019, 41% of health care providers prescribed regular contraception at the same time as emergency contraception; this percentage was an increase from 2013-2014, but falls short of the US Selected Practice Recommendations for Contraceptive Use, first released in 2013.
Major finding: Health care providers in 2019 were more likely than health care providers in 2013 and 2014 to prescribe or provide contraception when providing emergency contraception (adjusted prevalence ratio 1.26) and to provide a copper intrauterine device (adjusted prevalence ratio 3.87). A total of 41% frequently provided or prescribed regular contraception at the time of providing emergency contraceptive pills (ECP), 16% of providers in 2019 frequently provided an advance prescription for ECPs, 8% provided a copper intrauterine device as emergency contraception, and 7% provided an advance supply of ECPs.
Study details: The data come from two cross-sectional surveys mailed to office-based physicians and public-sector health care providers in the United States in 2013-14 (2,060 respondents) and 2019 (1,420 respondents).
Disclosures: The study was published on behalf of the Jacobs Institute of Women’s Health. The researchers had no financial conflicts to disclose.
Source: Pagano HP et al. Womens Health Issues. 2021 Sep 9. doi: 10.1016/j.whi.2021.07.006.
Key clinical point: In 2019, 41% of health care providers prescribed regular contraception at the same time as emergency contraception; this percentage was an increase from 2013-2014, but falls short of the US Selected Practice Recommendations for Contraceptive Use, first released in 2013.
Major finding: Health care providers in 2019 were more likely than health care providers in 2013 and 2014 to prescribe or provide contraception when providing emergency contraception (adjusted prevalence ratio 1.26) and to provide a copper intrauterine device (adjusted prevalence ratio 3.87). A total of 41% frequently provided or prescribed regular contraception at the time of providing emergency contraceptive pills (ECP), 16% of providers in 2019 frequently provided an advance prescription for ECPs, 8% provided a copper intrauterine device as emergency contraception, and 7% provided an advance supply of ECPs.
Study details: The data come from two cross-sectional surveys mailed to office-based physicians and public-sector health care providers in the United States in 2013-14 (2,060 respondents) and 2019 (1,420 respondents).
Disclosures: The study was published on behalf of the Jacobs Institute of Women’s Health. The researchers had no financial conflicts to disclose.
Source: Pagano HP et al. Womens Health Issues. 2021 Sep 9. doi: 10.1016/j.whi.2021.07.006.
Key clinical point: In 2019, 41% of health care providers prescribed regular contraception at the same time as emergency contraception; this percentage was an increase from 2013-2014, but falls short of the US Selected Practice Recommendations for Contraceptive Use, first released in 2013.
Major finding: Health care providers in 2019 were more likely than health care providers in 2013 and 2014 to prescribe or provide contraception when providing emergency contraception (adjusted prevalence ratio 1.26) and to provide a copper intrauterine device (adjusted prevalence ratio 3.87). A total of 41% frequently provided or prescribed regular contraception at the time of providing emergency contraceptive pills (ECP), 16% of providers in 2019 frequently provided an advance prescription for ECPs, 8% provided a copper intrauterine device as emergency contraception, and 7% provided an advance supply of ECPs.
Study details: The data come from two cross-sectional surveys mailed to office-based physicians and public-sector health care providers in the United States in 2013-14 (2,060 respondents) and 2019 (1,420 respondents).
Disclosures: The study was published on behalf of the Jacobs Institute of Women’s Health. The researchers had no financial conflicts to disclose.
Source: Pagano HP et al. Womens Health Issues. 2021 Sep 9. doi: 10.1016/j.whi.2021.07.006.
LARCs prompt increase in body mass index among adolescents
Key clinical point: Use of progestin-releasing long-acting reversible contraceptives (LARC) was linked to weight gain in nulliparous adolescents aged 14-19 years; 25% of the study population was obese, and significant interaction effect (P = .017) showed a greater increase in BMI in this subset of participants who used an LARC compared to a copper intrauterine device (IUD).
Major finding: The mean change in body mass index among teen girls who used LARC was an increase of 0.73 kg/m2; BMI increases averaged 0.92 kg/m2 for those who used an etornogestrel subdermal implant plus levonorgestrel intrauterine device (LNG-IUD) and 0.37 kg/m2 in those who used a copper IUD.
Study details: The data come from a retrospective cohort study of 196 adolescents aged 14-19 years who underwent placement of long-acting reversible contraceptive devices.
Disclosures: The study received no outside funding. The researchers had no financial conflicts to disclose.
Source: Scott N et al. J Pediatr Adolesc Gynecol. 2021 Aug 10. doi: 10.1016/j.jpag.2021.08.004.
Key clinical point: Use of progestin-releasing long-acting reversible contraceptives (LARC) was linked to weight gain in nulliparous adolescents aged 14-19 years; 25% of the study population was obese, and significant interaction effect (P = .017) showed a greater increase in BMI in this subset of participants who used an LARC compared to a copper intrauterine device (IUD).
Major finding: The mean change in body mass index among teen girls who used LARC was an increase of 0.73 kg/m2; BMI increases averaged 0.92 kg/m2 for those who used an etornogestrel subdermal implant plus levonorgestrel intrauterine device (LNG-IUD) and 0.37 kg/m2 in those who used a copper IUD.
Study details: The data come from a retrospective cohort study of 196 adolescents aged 14-19 years who underwent placement of long-acting reversible contraceptive devices.
Disclosures: The study received no outside funding. The researchers had no financial conflicts to disclose.
Source: Scott N et al. J Pediatr Adolesc Gynecol. 2021 Aug 10. doi: 10.1016/j.jpag.2021.08.004.
Key clinical point: Use of progestin-releasing long-acting reversible contraceptives (LARC) was linked to weight gain in nulliparous adolescents aged 14-19 years; 25% of the study population was obese, and significant interaction effect (P = .017) showed a greater increase in BMI in this subset of participants who used an LARC compared to a copper intrauterine device (IUD).
Major finding: The mean change in body mass index among teen girls who used LARC was an increase of 0.73 kg/m2; BMI increases averaged 0.92 kg/m2 for those who used an etornogestrel subdermal implant plus levonorgestrel intrauterine device (LNG-IUD) and 0.37 kg/m2 in those who used a copper IUD.
Study details: The data come from a retrospective cohort study of 196 adolescents aged 14-19 years who underwent placement of long-acting reversible contraceptive devices.
Disclosures: The study received no outside funding. The researchers had no financial conflicts to disclose.
Source: Scott N et al. J Pediatr Adolesc Gynecol. 2021 Aug 10. doi: 10.1016/j.jpag.2021.08.004.
Millions of women view YouTube videos on self-removal of long-acting contraception
Key clinical point: A review of 58 YouTube videos on removal of long-acting reversible contraception showed more than 4 million views overall; reasons for removal included negative side effects, fear of side effects, and desire for pregnancy.
Major finding: Most of the women who created the 58 videos were white (53%), 31% were Black, and 14% were Latina. Of these, 56 of 58 successfully removed their device and described the experience as positive in terms of ease of removal.
Study details: The data come from a review of 58 videos on self-removal of long-acting reversible contraception based on YouTube keyword searches. The videos included 48 individuals removing an intrauterine device and 10 removing an implant.
Disclosures: The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and by the National Science Foundation Graduate Research Fellowship Program. The researchers had no financial conflicts to disclose.
Source: Broussard K and Becker A. Contraception. 2021 Aug 13. doi: 10.1016/j.contraception.2021.08.002.
Key clinical point: A review of 58 YouTube videos on removal of long-acting reversible contraception showed more than 4 million views overall; reasons for removal included negative side effects, fear of side effects, and desire for pregnancy.
Major finding: Most of the women who created the 58 videos were white (53%), 31% were Black, and 14% were Latina. Of these, 56 of 58 successfully removed their device and described the experience as positive in terms of ease of removal.
Study details: The data come from a review of 58 videos on self-removal of long-acting reversible contraception based on YouTube keyword searches. The videos included 48 individuals removing an intrauterine device and 10 removing an implant.
Disclosures: The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and by the National Science Foundation Graduate Research Fellowship Program. The researchers had no financial conflicts to disclose.
Source: Broussard K and Becker A. Contraception. 2021 Aug 13. doi: 10.1016/j.contraception.2021.08.002.
Key clinical point: A review of 58 YouTube videos on removal of long-acting reversible contraception showed more than 4 million views overall; reasons for removal included negative side effects, fear of side effects, and desire for pregnancy.
Major finding: Most of the women who created the 58 videos were white (53%), 31% were Black, and 14% were Latina. Of these, 56 of 58 successfully removed their device and described the experience as positive in terms of ease of removal.
Study details: The data come from a review of 58 videos on self-removal of long-acting reversible contraception based on YouTube keyword searches. The videos included 48 individuals removing an intrauterine device and 10 removing an implant.
Disclosures: The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and by the National Science Foundation Graduate Research Fellowship Program. The researchers had no financial conflicts to disclose.
Source: Broussard K and Becker A. Contraception. 2021 Aug 13. doi: 10.1016/j.contraception.2021.08.002.
Counseling promotes contraception in hospitalized adolescents
Key clinical point: Among young women, the average Pregnancy Risk Index (PRI) was 4.75; individuals using teratogenic medication had the lowest PRI (0.32); 88% of these individuals used reversible contraception and 31% used long-acting reversible contraception.
Major finding: Approximately 73% of sexually active young women received contraceptive counseling, which was associated with a significantly increased use of reversible and dual contraception, but not long-acting reversible contraception. At last reported vaginal sex, 65% reported using condoms, 49% reported using reversible contraception, and 12% reported using long-acting reversible contraception.
Study details: The data come from a cross-sectional survey of 177 hospitalized females aged 14 to 21 years at two academic medical centers; the surveys assessed sexual health behaviors, contraceptive use, contraceptive counseling, and pregnancy complications. Researchers calculated the Pregnancy Risk Index (the number per 100 individuals who will become pregnant in the next year).
Disclosures: The study received no outside funding. The researchers had no financial conflicts to disclose.
Source: Hunt JA et al. Hosp Pediatr. 2021 Sep 13. doi: 10.1542/hpeds.2021-005810.
Key clinical point: Among young women, the average Pregnancy Risk Index (PRI) was 4.75; individuals using teratogenic medication had the lowest PRI (0.32); 88% of these individuals used reversible contraception and 31% used long-acting reversible contraception.
Major finding: Approximately 73% of sexually active young women received contraceptive counseling, which was associated with a significantly increased use of reversible and dual contraception, but not long-acting reversible contraception. At last reported vaginal sex, 65% reported using condoms, 49% reported using reversible contraception, and 12% reported using long-acting reversible contraception.
Study details: The data come from a cross-sectional survey of 177 hospitalized females aged 14 to 21 years at two academic medical centers; the surveys assessed sexual health behaviors, contraceptive use, contraceptive counseling, and pregnancy complications. Researchers calculated the Pregnancy Risk Index (the number per 100 individuals who will become pregnant in the next year).
Disclosures: The study received no outside funding. The researchers had no financial conflicts to disclose.
Source: Hunt JA et al. Hosp Pediatr. 2021 Sep 13. doi: 10.1542/hpeds.2021-005810.
Key clinical point: Among young women, the average Pregnancy Risk Index (PRI) was 4.75; individuals using teratogenic medication had the lowest PRI (0.32); 88% of these individuals used reversible contraception and 31% used long-acting reversible contraception.
Major finding: Approximately 73% of sexually active young women received contraceptive counseling, which was associated with a significantly increased use of reversible and dual contraception, but not long-acting reversible contraception. At last reported vaginal sex, 65% reported using condoms, 49% reported using reversible contraception, and 12% reported using long-acting reversible contraception.
Study details: The data come from a cross-sectional survey of 177 hospitalized females aged 14 to 21 years at two academic medical centers; the surveys assessed sexual health behaviors, contraceptive use, contraceptive counseling, and pregnancy complications. Researchers calculated the Pregnancy Risk Index (the number per 100 individuals who will become pregnant in the next year).
Disclosures: The study received no outside funding. The researchers had no financial conflicts to disclose.
Source: Hunt JA et al. Hosp Pediatr. 2021 Sep 13. doi: 10.1542/hpeds.2021-005810.