User login
Etonogestrel implants may be bent, fractured by trauma or during sports
In 2017, Global Pediatric Health published a case report series associated with the use of long-acting reversible contraceptives, specifically the etonogestrel implant.
In November 2020, the makers of the etonogestrel implant (Merck) recommended a change in practice with the release of a notice to health care providers certified in the training of this product. This mass marketing blast included an updated warning and cautions for prescribers as well as patient information on the potential risks of migration, fracture, and bent devices attributable to trauma or sports. “Broken or Bent Implant (Section 5.16). The addition of the following underlined language: “There have been reports of broken or bent implants, which may be related to external forces (e.g., manipulation of the implant or contact sports) while in the patient’s arm. There have also been reports of migration of a broken implant fragment within the arm.”
Clearly the etonogestrel subdermal hormonal implant is an effective form of contraception and particularly beneficial in nonadherent sexually active teens who struggle to remember oral contraceptives. But it is important to be aware of this alert. Little is known about the type of trauma or rate of external force required to cause migration, fracture, or bend implants. This update requires adequate counseling of potential risks and complications of the etonogestrel implant, including the risk of migration, fracture, or bent devices specifically in the event of contact sports and trauma.
Ms. Thew is medical director of the department of adolescent medicine at Children’s Wisconsin in Milwaukee. She is a member of the Pediatric News editorial advisory board. She had no relevant financial disclosures. Email Ms. Thew at [email protected].
In 2017, Global Pediatric Health published a case report series associated with the use of long-acting reversible contraceptives, specifically the etonogestrel implant.
In November 2020, the makers of the etonogestrel implant (Merck) recommended a change in practice with the release of a notice to health care providers certified in the training of this product. This mass marketing blast included an updated warning and cautions for prescribers as well as patient information on the potential risks of migration, fracture, and bent devices attributable to trauma or sports. “Broken or Bent Implant (Section 5.16). The addition of the following underlined language: “There have been reports of broken or bent implants, which may be related to external forces (e.g., manipulation of the implant or contact sports) while in the patient’s arm. There have also been reports of migration of a broken implant fragment within the arm.”
Clearly the etonogestrel subdermal hormonal implant is an effective form of contraception and particularly beneficial in nonadherent sexually active teens who struggle to remember oral contraceptives. But it is important to be aware of this alert. Little is known about the type of trauma or rate of external force required to cause migration, fracture, or bend implants. This update requires adequate counseling of potential risks and complications of the etonogestrel implant, including the risk of migration, fracture, or bent devices specifically in the event of contact sports and trauma.
Ms. Thew is medical director of the department of adolescent medicine at Children’s Wisconsin in Milwaukee. She is a member of the Pediatric News editorial advisory board. She had no relevant financial disclosures. Email Ms. Thew at [email protected].
In 2017, Global Pediatric Health published a case report series associated with the use of long-acting reversible contraceptives, specifically the etonogestrel implant.
In November 2020, the makers of the etonogestrel implant (Merck) recommended a change in practice with the release of a notice to health care providers certified in the training of this product. This mass marketing blast included an updated warning and cautions for prescribers as well as patient information on the potential risks of migration, fracture, and bent devices attributable to trauma or sports. “Broken or Bent Implant (Section 5.16). The addition of the following underlined language: “There have been reports of broken or bent implants, which may be related to external forces (e.g., manipulation of the implant or contact sports) while in the patient’s arm. There have also been reports of migration of a broken implant fragment within the arm.”
Clearly the etonogestrel subdermal hormonal implant is an effective form of contraception and particularly beneficial in nonadherent sexually active teens who struggle to remember oral contraceptives. But it is important to be aware of this alert. Little is known about the type of trauma or rate of external force required to cause migration, fracture, or bend implants. This update requires adequate counseling of potential risks and complications of the etonogestrel implant, including the risk of migration, fracture, or bent devices specifically in the event of contact sports and trauma.
Ms. Thew is medical director of the department of adolescent medicine at Children’s Wisconsin in Milwaukee. She is a member of the Pediatric News editorial advisory board. She had no relevant financial disclosures. Email Ms. Thew at [email protected].
The pill toolbox: How to choose a combined oral contraceptive
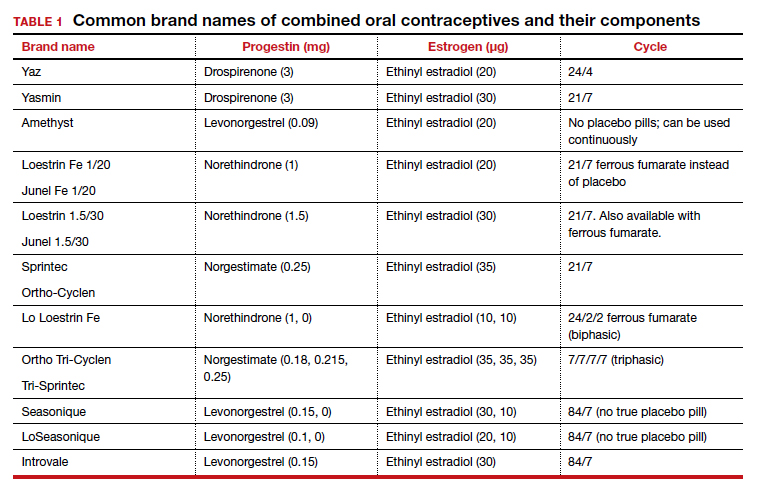
In the era of long-acting reversible contraceptives (LARCs), the pill can seem obsolete. However, it is still the second most commonly used birth control method in the United States, chosen by 19% of female contraceptive users as of 2015–2017.1 It also has noncontraceptive benefits, so it is important that obstetrician-gynecologists are well-versed in its uses. In this article, I will focus on combined oral contraceptives (COCs; TABLE 1), reviewing the major risks, benefits, and adverse effects of COCs before focusing on recommendations for particular formulations of COCs for various patient populations.
Benefits and risks
There are numerous noncontraceptive benefits of COCs, including menstrual cycle regulation; reduced risk of ovarian, endometrial, and colorectal cancer; and treatment of menorrhagia, dysmenorrhea, acne, menstrual migraine, premenstrual syndrome and premenstrual dysphoric disorder, pelvic pain due to endometriosis, and hirsutism.
Common patient concerns
In terms of adverse effects, there are more potential unwanted effects of concern to women than there are ones validated in the literature. Accepted adverse effects include nausea, breast tenderness, and decreased libido. However, one of the most common concerns voiced during contraceptive counseling is that COCs will cause weight gain. A 2014 Cochrane review identified 49 trials studying the weight gain question.2 Of those, only 4 had a placebo or nonintervention group. Of these 4, there was no significant difference in weight change between the COC-receiving group and the control group. When patients bring up their concerns, it may help to remind them that women tend to gain weight over time whether or not they are taking a COC.
Another common concern is that COCs cause mood changes. A 2016 review by Schaffir and colleagues sheds some light on this topic,3 albeit limited by the paucity of prospective studies. This review identified only 1 randomized controlled trial comparing depression incidence among women initiating a COC versus a placebo. There was no difference in the incidence of depression among the groups at 3 months. Among 4 large retrospective studies of women using COCs, the agents either had no or a beneficial effect on mood. Schaffir’s review reports that there may be greater mood adverse effects with COCs among women with underlying mood disorders.
Patients may worry that COC use will permanently impair their fertility or delay return to fertility after discontinuation. Research does indicate that return of fertility after stopping COCs often takes several months (compared with immediate fertility after discontinuing a barrier method). However, there still seem to be comparable conception rates within 12 months after discontinuing COCs as there are after discontinuing other common nonhormonal or hormonal contraceptive methods. Fertility is not impacted by the duration of COC use. In addition, return to fertility seems to be comparable after discontinuation of extended cycle or continuous COCs compared with traditional-cycle COCs.4
COC safety
Known major risks of COCs include venous thromboembolism (VTE). The risk of VTE is about double among COC users than among nonpregnant nonusers: 3–9 per 10,000 woman-years compared with 1–5.5 In a study by the US Food and Drug Administration, drospirenone-containing COCs had double the risk of VTE than other COCs. However, the position of the American College of Obstetricians and Gynecologists on this increased risk of VTE with drospirenone-containing pills is that it is “possible” and “minimal.”5 It is important to remember that an alternative to COC use is pregnancy, in which the VTE risk is about double that among COC users, at 5–20 per 10,000 woman-years. This risk increases further in the postpartum period, to 40–65 per 10,000 woman-years.5
Another known major risk of COCs is arterial embolic disease, including cerebrovascular accidents and myocardial infarctions. Women at increased risk for these complications include those with hypertension, diabetes, and/or obesity and women who are aged 35 or older and smoke. Interestingly, women with migraines with aura are at increased risk for stroke but not for myocardial infarction. These women increase their risk of stroke 2- to 4-fold if they use COCs.
Continue to: Different pills for different problems...
Different pills for different problems
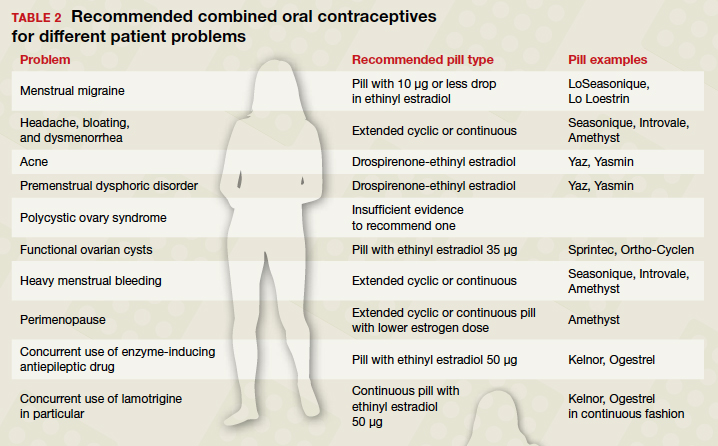
With so many pills on the market, it is important for clinicians to know how to choose a particular pill for a particular patient. The following discussion assumes that the patient in question desires a COC for contraception, then offers guidance on how to choose a pill with patient-specific noncontraceptive benefits (TABLE 2).
When HMB is a concern. Patients with heavy menstrual bleeding may experience fewer bleeding and/or spotting days with extended cyclic or continuous use of a COC rather than with traditional cyclic use.6 Examples of such COC options include:
- Introvale and Seasonique, both extended-cycle formulations
- Amethyst, which is formulated without placebo pills so that it can be used continuously
- any other COC prescribed with instructions for the patient to skip placebo pills.
An extrapolated benefit to extended-cycle or continuous COCs use for heavy menstrual bleeding is addressing anemia.
For premenstrual dysphoric disorder, the only randomized controlled trials showing improvement involve drospirenone-ethinyl estradiol pills (Yaz and Yasmin).7 There is also evidence that extended cyclic or continuous use of these formulations is more impactful for premenstrual dysphoric disorder than a traditional cycle.8
Keeping migraine avoidance and prevention in mind. Various studies have looked at the impact of different COC formulations on menstrual-related symptoms. There is evidence of greater improvement in headache, bloating, and dysmenorrhea with extended cyclic or continuous use compared with traditional cyclic use.6
In terms of headache, let us delve into menstrual migraine in particular. Menstrual migraines occur sometime between 2 days prior to 2 days after the first day of menses and are linked to a sharp drop in estrogen levels. COCs are contraindicated in women with menstrual migraines with aura because of the increased stroke risk. For women with menstrual migraines without aura, COCs can prevent migraines. Prevention depends on minimizing fluctuations in estrogen levels; any change in estrogen level greater than 10 µg of ethinyl estradiol may trigger an estrogen-related migraine. All currently available regimens of COCs that comprise 21 days of active pills and 7 days of placebo involve a drop of more than 10 µg. Options that involve a drop of 10 µg or less include any continuous formulation, the extended formulation LoSeasonique (levonorgestrel 0.1 mg and ethinyl estradiol 20 µg for 84 days, then ethinyl estradiol 10 µg for 7 days), and Lo Loestrin (ethinyl estradiol 10 µg and norethindrone 1 mg for 24 days, then ethinyl estradiol 10 µg for 2 days, then placebo for 2 days).9
What’s best for acne-prone patients? All COCs should improve acne by increasing levels of sex hormone binding globulin. However, some comparative studies have shown drospirenone-containing COCs to be the most effective for acne. This finding makes sense in light of studies demonstrating antiandrogenic effects of drospirenone.10
Managing PCOS symptoms. It seems logical, by extension, that drospirenone-containing COCs would be particularly beneficial for treating hirsutism associated with polycystic ovary syndrome (PCOS). Other low‒androgenic-potential progestins, such as a third-generation progestin (norgestimate or desogestrel), might similarly be hypothesized to be advantageous. However, there is currently insufficient evidence to recommend any one COC formulation over another for the indication of PCOS.11
Ovarian cysts: Can COCs be helpful? COCs are commonly prescribed by gynecologists for patients with functional ovarian cysts. It is important to note that COCs have not been found to hasten the resolution of existing cysts, so they should not be used for this purpose.12 Studies of early COCs, which had high doses of estrogen (on the order of 50 µg), showed lower rates of cysts among users. This effect seems to be attenuated with the lower-estrogen-dose pills that are currently available, but there still appears to be benefit. Therefore, for a patient prone to cysts who desires an oral contraceptive, a COC containing estrogen 35 µg is likely to be the most beneficial of COCs currently on the market.13,14
Lower-dosage COCs in perimenopause may be beneficial. COCs can ameliorate perimenopausal symptoms including abnormal uterine bleeding and vasomotor symptoms. Clinicians are often hesitant to prescribe COCs for perimenopausal women because of increased risk of VTE, stroke, myocardial infarction, and breast cancer with increasing age. However, age alone is not a contraindication to any contraceptive method. An extended cyclic or continuous regimen COC may be the best choice for a perimenopausal woman in order to avoid vasomotor symptoms that occur during hormone-free intervals. In addition, given the increasing risk of adverse effects like VTE with estrogen dose, a lower estrogen formulation is advisable.15
Patients with epilepsy who are taking antiepileptic drugs (AEDs) are a special population when it comes to COCs. Certain AEDs induce hepatic enzymes involved in the metabolism and protein binding of COCs, which can result in contraceptive failure. Strong inducers are carbamazepine, oxcarbazepine, perampanel, phenobarbital, phenytoin, and primidone. Weak inducers are clobazam, eslicarbazepine, felbamate, lamotrigine, rufinamide, and topiramate. Women taking any of the above AEDs are recommended to choose a different form of contraception than a COC. However, if they are limited to COCs for some reason, a preparation containing estrogen 50 µg is recommended. It is speculated that the efficacy and adverse effects of COCs with increased hormone doses, used in combination with enzyme-inducing AEDs, should be comparable to those with standard doses when not combined with AEDs; however, this speculation is unproven.16 There are few COCs on the market with estrogen doses of 50 µg, but a couple of examples are Kelnor and Ogestrel.
Additional factors have to be considered with concurrent COC use with the AED lamotrigine since COCs increase clearance of this agent. Therefore, patients taking lamotrigine who start COCs will need an increase in lamotrigine dose. To avoid fluctuations in lamotrigine serum levels, use of a continuous COC is recommended.17
Continue to: Pill types to minimize adverse effects or risks...
Pill types to minimize adverse effects or risks
For women who desire to use a COC for contraception but who are at risk for a particular complication or are bothered by a particular adverse effect, ObGyns can optimize the choice of pill (TABLE 3). For example, women who have adverse effects of nausea and/or breast tenderness may benefit from reducing the estrogen dose to 20 µg or lower.18

Considering VTE
As discussed previously, VTE is a risk with all COCs, but some pills confer greater risk than others. For one, VTE risk increases with estrogen dose. In addition, VTE risk depends on the type of progestin. Drospirenone and third-generation progestins (norgestimate, gestodene, and desogestrel) confer a higher risk of VTE than first- or second-generation progestins. For example, a pill with estradiol 30 µg and either a third-generation progestin or drospirenone has a 50% to 80% higher risk of VTE compared with a pill with estradiol 30 µg and levonorgestrel.
For patients at particularly high risk for VTE, COCs are contraindicated. For patients for whom COCs are considered medically appropriate but who are at higher risk (eg, obese women), it is wise to use a pill containing a first-generation (norethindrone) or second-generation progestin (levonorgestrel) combined with the lowest dose of estrogen that has tolerable adverse effects.19
What about hypertension concerns?
Let us turn our attention briefly to hypertension and its relation to COC use. While the American College of Cardiology and the American Heart Association redefined hypertension in 2017 using a threshold of 130/80 mm Hg, the American College of Obstetricians and Gynecologists (ACOG) considers hypertension to be 140/90 mm Hg in terms of safety of using COCs. ACOG states, “women with blood pressure below 140/90 mm Hg may use any hormonal contraceptive method.”20 In women with hypertension in the range of 140‒159 mm Hg systolic or 90‒99 mm Hg diastolic, COCs are category 3 according to the US Medical Eligibility Criteria for Contraceptive Use, meaning that the risks usually outweigh the benefits. For women with blood pressures of 160/110 mm Hg or greater, COCs are category 4 (contraindicated). If a woman with mild hypertension is started on a COC, a drospirenone-containing pill may be the best choice because of its diuretic effects. While other contemporary COCs have been associated with a mild increase in blood pressure, drospirenone-containing pills have not shown this association.21
Continue to: At issue: Break-through bleeding, mood, and weight gain...
At issue: Break-through bleeding, mood, and weight gain
For women bothered by intermenstrual bleeding, use of a COC with a third-generation progestin may be preferable to use of one with a first- or second-generation. It may be because of decreased abnormal bleeding that COCs with third-generation progestins have lower discontinuation rates.22 In addition, COCs containing estrogen 20 µg or less are associated with more intermenstrual bleeding than those with more than 20 µg estrogen.23 Keep in mind that it is common with any COC to have intermenstrual bleeding for the first several months.
For women with pre-existing mood disorders or who report mood changes with COCs, it appears that fluctuations in hormone levels are problematic. Consistently, there is evidence that monophasic pills are preferable to multiphasic and that extended cyclic or continuous use is preferable to traditional cyclic use for mitigating mood adverse effects. There is mixed evidence on whether a low dose of ethinyl estradiol is better for mood.3
Although it is discussed above that randomized controlled trials have not shown an association between COC use and weight gain, many women remain concerned. For these women, a drospirenone-containing COC may be the best choice. Drospirenone has antimineralocorticoid activity, so it may help prevent water retention.
A brief word about multiphasic COCs. While these pills were designed to mimic physiologic hormone fluctuations and minimize hormonal adverse effects, there is insufficient evidence to compare their effects to those of monophasic pills.24 Without such evidence, there is little reason to recommend a multiphasic pill to a patient over the more straightforward monophasic formulation.
Conclusion
There are more nuances to prescribing an optimal COC for a patient than may initially come to mind. It is useful to remember that any formulation of pill may be prescribed in an extended or continuous fashion, and there are benefits for such use for premenstrual dysphoric disorder, heavy menstrual bleeding, perimenopause, and menstrual symptoms. Although there are numerous brands of COCs available, a small cadre will suffice for almost all purposes. Such a “toolbox” of pills could include a pill formatted for continuous use (Seasonique), a low estrogen pill (Loestrin), a drospirenone-containing pill (Yaz), and a pill containing a third-generation progestin and a higher dose of estrogen (Sprintec). ●
- Daniels K, Abma JC. Current contraceptive status among women aged 15-49: United States, 2015-2017. NCHS Data Brief, no 327. Hyattsville, MD; 2018.
- Gallo MF, Lopez LM, Grimes DA, et al. Combination contraceptives: effects on weight. Cochrane Database Syst Rev. 2014:CD003987.
- Schaffir J, Worly BL, Gur TL. Combined hormonal contraception and its effects on mood: a critical review. Eur J Contracept Reprod Health Care. 2016;21:347-355.
- Barnhart KT, Schreiber CA. Return to fertility following discontinuation of oral contraceptives. Fertil Steril. 2009;91:659-663.
- American College of Obstetricians and Gynecologists. Committee Opinion #540: Risk of Venous Thromboembolism Among Users of Drospirenone-Containing Oral Contraceptive Pills. Obstet Gynecol. 2012;120:1239-1242.
- Edelman A, Micks E, Gallo MF, et al. Continuous or extended cycle vs. cyclic use of combined hormonal contraceptives for contraception. Cochrane Database Syst Rev. 2014:CD004695.
- American College of Obstetricians and Gynecologists. Practice Bulletin #110: Noncontraceptive Uses of Hormonal Contraceptives. Obstet Gynecol. 2010:206-218.
- Coffee AL, Kuehl TJ, Willis S, et al. Oral contraceptives and premenstrual symptoms: comparison of a 21/7 and extended regimen. Am J Obstet Gynecol. 2006;195:1311-1319.
- Calhoun AH, Batur P. Combined hormonal contraceptives and migraine: an update on the evidence. Cleve Clin J Med. 2017;84:631-638.
- Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. 2012:CD004425.
- McCartney CR, Marshall JC. CLINICAL PRACTICE. Polycystic Ovary Syndrome. N Engl J Med. 2016;375:54-64.
- Grimes DA, Jones LB, Lopez LM, et al. Oral contraceptives for functional ovarian cysts. Cochrane Database Syst Rev. 2014:CD006134.
- Grimes DA, Godwin AJ, Rubin A, et al. Ovulation and follicular development associated with three low-dose oral contraceptives: a randomized controlled trial. Obstet Gynecol. 1994;83:29-34.
- Christensen JT, Boldsen JL, Westergaard JG. Functional ovarian cysts in premenopausal and gynecologically healthy women. Contraception. 2002;66:153-157.
- Hardman SM, Gebbie AE. Hormonal contraceptive regimens in the perimenopause. Maturitas. 2009;63:204-212.
- Zupanc ML. Antiepileptic drugs and hormonal contraceptives in adolescent women with epilepsy. Neurology. 2006;66 (6 suppl 3):S37-S45.
- Wegner I, Edelbroek PM, Bulk S, et al. Lamotrigine kinetics within the menstrual cycle, after menopause, and with oral contraceptives. Neurology. 2009;73:1388-1393.
- Stewart M, Black K. Choosing a combined oral contraceptive pill. Australian Prescriber. 2015;38:6-11.
- de Bastos M, Stegeman BH, Rosendaal FR, et al. Combined oral contraceptives: venous thrombosis. Cochrane Database Syst Rev. 2014:CD010813.
- American College of Obstetricians and Gynecologists. Practice Bulletin #206: use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol. 2019;133:e128-e150.
- de Morais TL, Giribela C, Nisenbaum MG, et al. Effects of a contraceptive containing drospirenone and ethinylestradiol on blood pressure, metabolic profile and neurohumoral axis in hypertensive women at reproductive age. Eur J Obstet Gynecol Reprod Biol. 2014;182:113-117.
- Lawrie TA, Helmerhorst FM, Maitra NK, et al. Types of progestogens in combined oral contraception: effectiveness and side-effects. Cochrane Database Syst Rev. 2011:CD004861.
- Gallo MF, Nanda K, Grimes DA, et al. 20 µg versus >20 µg estrogen combined oral contraceptives for contraception. Cochrane Database Syst Rev. 2013:CD003989.
- van Vliet HA, Grimes DA, Lopez LM, et al. Triphasic versus monophasic oral contraceptives for contraception. Cochrane Database Syst Rev. 2006:CD003553
In the era of long-acting reversible contraceptives (LARCs), the pill can seem obsolete. However, it is still the second most commonly used birth control method in the United States, chosen by 19% of female contraceptive users as of 2015–2017.1 It also has noncontraceptive benefits, so it is important that obstetrician-gynecologists are well-versed in its uses. In this article, I will focus on combined oral contraceptives (COCs; TABLE 1), reviewing the major risks, benefits, and adverse effects of COCs before focusing on recommendations for particular formulations of COCs for various patient populations.

Benefits and risks
There are numerous noncontraceptive benefits of COCs, including menstrual cycle regulation; reduced risk of ovarian, endometrial, and colorectal cancer; and treatment of menorrhagia, dysmenorrhea, acne, menstrual migraine, premenstrual syndrome and premenstrual dysphoric disorder, pelvic pain due to endometriosis, and hirsutism.
Common patient concerns
In terms of adverse effects, there are more potential unwanted effects of concern to women than there are ones validated in the literature. Accepted adverse effects include nausea, breast tenderness, and decreased libido. However, one of the most common concerns voiced during contraceptive counseling is that COCs will cause weight gain. A 2014 Cochrane review identified 49 trials studying the weight gain question.2 Of those, only 4 had a placebo or nonintervention group. Of these 4, there was no significant difference in weight change between the COC-receiving group and the control group. When patients bring up their concerns, it may help to remind them that women tend to gain weight over time whether or not they are taking a COC.
Another common concern is that COCs cause mood changes. A 2016 review by Schaffir and colleagues sheds some light on this topic,3 albeit limited by the paucity of prospective studies. This review identified only 1 randomized controlled trial comparing depression incidence among women initiating a COC versus a placebo. There was no difference in the incidence of depression among the groups at 3 months. Among 4 large retrospective studies of women using COCs, the agents either had no or a beneficial effect on mood. Schaffir’s review reports that there may be greater mood adverse effects with COCs among women with underlying mood disorders.
Patients may worry that COC use will permanently impair their fertility or delay return to fertility after discontinuation. Research does indicate that return of fertility after stopping COCs often takes several months (compared with immediate fertility after discontinuing a barrier method). However, there still seem to be comparable conception rates within 12 months after discontinuing COCs as there are after discontinuing other common nonhormonal or hormonal contraceptive methods. Fertility is not impacted by the duration of COC use. In addition, return to fertility seems to be comparable after discontinuation of extended cycle or continuous COCs compared with traditional-cycle COCs.4
COC safety
Known major risks of COCs include venous thromboembolism (VTE). The risk of VTE is about double among COC users than among nonpregnant nonusers: 3–9 per 10,000 woman-years compared with 1–5.5 In a study by the US Food and Drug Administration, drospirenone-containing COCs had double the risk of VTE than other COCs. However, the position of the American College of Obstetricians and Gynecologists on this increased risk of VTE with drospirenone-containing pills is that it is “possible” and “minimal.”5 It is important to remember that an alternative to COC use is pregnancy, in which the VTE risk is about double that among COC users, at 5–20 per 10,000 woman-years. This risk increases further in the postpartum period, to 40–65 per 10,000 woman-years.5
Another known major risk of COCs is arterial embolic disease, including cerebrovascular accidents and myocardial infarctions. Women at increased risk for these complications include those with hypertension, diabetes, and/or obesity and women who are aged 35 or older and smoke. Interestingly, women with migraines with aura are at increased risk for stroke but not for myocardial infarction. These women increase their risk of stroke 2- to 4-fold if they use COCs.
Continue to: Different pills for different problems...
Different pills for different problems
With so many pills on the market, it is important for clinicians to know how to choose a particular pill for a particular patient. The following discussion assumes that the patient in question desires a COC for contraception, then offers guidance on how to choose a pill with patient-specific noncontraceptive benefits (TABLE 2).

When HMB is a concern. Patients with heavy menstrual bleeding may experience fewer bleeding and/or spotting days with extended cyclic or continuous use of a COC rather than with traditional cyclic use.6 Examples of such COC options include:
- Introvale and Seasonique, both extended-cycle formulations
- Amethyst, which is formulated without placebo pills so that it can be used continuously
- any other COC prescribed with instructions for the patient to skip placebo pills.
An extrapolated benefit to extended-cycle or continuous COCs use for heavy menstrual bleeding is addressing anemia.
For premenstrual dysphoric disorder, the only randomized controlled trials showing improvement involve drospirenone-ethinyl estradiol pills (Yaz and Yasmin).7 There is also evidence that extended cyclic or continuous use of these formulations is more impactful for premenstrual dysphoric disorder than a traditional cycle.8
Keeping migraine avoidance and prevention in mind. Various studies have looked at the impact of different COC formulations on menstrual-related symptoms. There is evidence of greater improvement in headache, bloating, and dysmenorrhea with extended cyclic or continuous use compared with traditional cyclic use.6
In terms of headache, let us delve into menstrual migraine in particular. Menstrual migraines occur sometime between 2 days prior to 2 days after the first day of menses and are linked to a sharp drop in estrogen levels. COCs are contraindicated in women with menstrual migraines with aura because of the increased stroke risk. For women with menstrual migraines without aura, COCs can prevent migraines. Prevention depends on minimizing fluctuations in estrogen levels; any change in estrogen level greater than 10 µg of ethinyl estradiol may trigger an estrogen-related migraine. All currently available regimens of COCs that comprise 21 days of active pills and 7 days of placebo involve a drop of more than 10 µg. Options that involve a drop of 10 µg or less include any continuous formulation, the extended formulation LoSeasonique (levonorgestrel 0.1 mg and ethinyl estradiol 20 µg for 84 days, then ethinyl estradiol 10 µg for 7 days), and Lo Loestrin (ethinyl estradiol 10 µg and norethindrone 1 mg for 24 days, then ethinyl estradiol 10 µg for 2 days, then placebo for 2 days).9
What’s best for acne-prone patients? All COCs should improve acne by increasing levels of sex hormone binding globulin. However, some comparative studies have shown drospirenone-containing COCs to be the most effective for acne. This finding makes sense in light of studies demonstrating antiandrogenic effects of drospirenone.10
Managing PCOS symptoms. It seems logical, by extension, that drospirenone-containing COCs would be particularly beneficial for treating hirsutism associated with polycystic ovary syndrome (PCOS). Other low‒androgenic-potential progestins, such as a third-generation progestin (norgestimate or desogestrel), might similarly be hypothesized to be advantageous. However, there is currently insufficient evidence to recommend any one COC formulation over another for the indication of PCOS.11
Ovarian cysts: Can COCs be helpful? COCs are commonly prescribed by gynecologists for patients with functional ovarian cysts. It is important to note that COCs have not been found to hasten the resolution of existing cysts, so they should not be used for this purpose.12 Studies of early COCs, which had high doses of estrogen (on the order of 50 µg), showed lower rates of cysts among users. This effect seems to be attenuated with the lower-estrogen-dose pills that are currently available, but there still appears to be benefit. Therefore, for a patient prone to cysts who desires an oral contraceptive, a COC containing estrogen 35 µg is likely to be the most beneficial of COCs currently on the market.13,14
Lower-dosage COCs in perimenopause may be beneficial. COCs can ameliorate perimenopausal symptoms including abnormal uterine bleeding and vasomotor symptoms. Clinicians are often hesitant to prescribe COCs for perimenopausal women because of increased risk of VTE, stroke, myocardial infarction, and breast cancer with increasing age. However, age alone is not a contraindication to any contraceptive method. An extended cyclic or continuous regimen COC may be the best choice for a perimenopausal woman in order to avoid vasomotor symptoms that occur during hormone-free intervals. In addition, given the increasing risk of adverse effects like VTE with estrogen dose, a lower estrogen formulation is advisable.15
Patients with epilepsy who are taking antiepileptic drugs (AEDs) are a special population when it comes to COCs. Certain AEDs induce hepatic enzymes involved in the metabolism and protein binding of COCs, which can result in contraceptive failure. Strong inducers are carbamazepine, oxcarbazepine, perampanel, phenobarbital, phenytoin, and primidone. Weak inducers are clobazam, eslicarbazepine, felbamate, lamotrigine, rufinamide, and topiramate. Women taking any of the above AEDs are recommended to choose a different form of contraception than a COC. However, if they are limited to COCs for some reason, a preparation containing estrogen 50 µg is recommended. It is speculated that the efficacy and adverse effects of COCs with increased hormone doses, used in combination with enzyme-inducing AEDs, should be comparable to those with standard doses when not combined with AEDs; however, this speculation is unproven.16 There are few COCs on the market with estrogen doses of 50 µg, but a couple of examples are Kelnor and Ogestrel.
Additional factors have to be considered with concurrent COC use with the AED lamotrigine since COCs increase clearance of this agent. Therefore, patients taking lamotrigine who start COCs will need an increase in lamotrigine dose. To avoid fluctuations in lamotrigine serum levels, use of a continuous COC is recommended.17
Continue to: Pill types to minimize adverse effects or risks...
Pill types to minimize adverse effects or risks
For women who desire to use a COC for contraception but who are at risk for a particular complication or are bothered by a particular adverse effect, ObGyns can optimize the choice of pill (TABLE 3). For example, women who have adverse effects of nausea and/or breast tenderness may benefit from reducing the estrogen dose to 20 µg or lower.18

Considering VTE
As discussed previously, VTE is a risk with all COCs, but some pills confer greater risk than others. For one, VTE risk increases with estrogen dose. In addition, VTE risk depends on the type of progestin. Drospirenone and third-generation progestins (norgestimate, gestodene, and desogestrel) confer a higher risk of VTE than first- or second-generation progestins. For example, a pill with estradiol 30 µg and either a third-generation progestin or drospirenone has a 50% to 80% higher risk of VTE compared with a pill with estradiol 30 µg and levonorgestrel.
For patients at particularly high risk for VTE, COCs are contraindicated. For patients for whom COCs are considered medically appropriate but who are at higher risk (eg, obese women), it is wise to use a pill containing a first-generation (norethindrone) or second-generation progestin (levonorgestrel) combined with the lowest dose of estrogen that has tolerable adverse effects.19
What about hypertension concerns?
Let us turn our attention briefly to hypertension and its relation to COC use. While the American College of Cardiology and the American Heart Association redefined hypertension in 2017 using a threshold of 130/80 mm Hg, the American College of Obstetricians and Gynecologists (ACOG) considers hypertension to be 140/90 mm Hg in terms of safety of using COCs. ACOG states, “women with blood pressure below 140/90 mm Hg may use any hormonal contraceptive method.”20 In women with hypertension in the range of 140‒159 mm Hg systolic or 90‒99 mm Hg diastolic, COCs are category 3 according to the US Medical Eligibility Criteria for Contraceptive Use, meaning that the risks usually outweigh the benefits. For women with blood pressures of 160/110 mm Hg or greater, COCs are category 4 (contraindicated). If a woman with mild hypertension is started on a COC, a drospirenone-containing pill may be the best choice because of its diuretic effects. While other contemporary COCs have been associated with a mild increase in blood pressure, drospirenone-containing pills have not shown this association.21
Continue to: At issue: Break-through bleeding, mood, and weight gain...
At issue: Break-through bleeding, mood, and weight gain
For women bothered by intermenstrual bleeding, use of a COC with a third-generation progestin may be preferable to use of one with a first- or second-generation. It may be because of decreased abnormal bleeding that COCs with third-generation progestins have lower discontinuation rates.22 In addition, COCs containing estrogen 20 µg or less are associated with more intermenstrual bleeding than those with more than 20 µg estrogen.23 Keep in mind that it is common with any COC to have intermenstrual bleeding for the first several months.
For women with pre-existing mood disorders or who report mood changes with COCs, it appears that fluctuations in hormone levels are problematic. Consistently, there is evidence that monophasic pills are preferable to multiphasic and that extended cyclic or continuous use is preferable to traditional cyclic use for mitigating mood adverse effects. There is mixed evidence on whether a low dose of ethinyl estradiol is better for mood.3
Although it is discussed above that randomized controlled trials have not shown an association between COC use and weight gain, many women remain concerned. For these women, a drospirenone-containing COC may be the best choice. Drospirenone has antimineralocorticoid activity, so it may help prevent water retention.
A brief word about multiphasic COCs. While these pills were designed to mimic physiologic hormone fluctuations and minimize hormonal adverse effects, there is insufficient evidence to compare their effects to those of monophasic pills.24 Without such evidence, there is little reason to recommend a multiphasic pill to a patient over the more straightforward monophasic formulation.
Conclusion
There are more nuances to prescribing an optimal COC for a patient than may initially come to mind. It is useful to remember that any formulation of pill may be prescribed in an extended or continuous fashion, and there are benefits for such use for premenstrual dysphoric disorder, heavy menstrual bleeding, perimenopause, and menstrual symptoms. Although there are numerous brands of COCs available, a small cadre will suffice for almost all purposes. Such a “toolbox” of pills could include a pill formatted for continuous use (Seasonique), a low estrogen pill (Loestrin), a drospirenone-containing pill (Yaz), and a pill containing a third-generation progestin and a higher dose of estrogen (Sprintec). ●
In the era of long-acting reversible contraceptives (LARCs), the pill can seem obsolete. However, it is still the second most commonly used birth control method in the United States, chosen by 19% of female contraceptive users as of 2015–2017.1 It also has noncontraceptive benefits, so it is important that obstetrician-gynecologists are well-versed in its uses. In this article, I will focus on combined oral contraceptives (COCs; TABLE 1), reviewing the major risks, benefits, and adverse effects of COCs before focusing on recommendations for particular formulations of COCs for various patient populations.

Benefits and risks
There are numerous noncontraceptive benefits of COCs, including menstrual cycle regulation; reduced risk of ovarian, endometrial, and colorectal cancer; and treatment of menorrhagia, dysmenorrhea, acne, menstrual migraine, premenstrual syndrome and premenstrual dysphoric disorder, pelvic pain due to endometriosis, and hirsutism.
Common patient concerns
In terms of adverse effects, there are more potential unwanted effects of concern to women than there are ones validated in the literature. Accepted adverse effects include nausea, breast tenderness, and decreased libido. However, one of the most common concerns voiced during contraceptive counseling is that COCs will cause weight gain. A 2014 Cochrane review identified 49 trials studying the weight gain question.2 Of those, only 4 had a placebo or nonintervention group. Of these 4, there was no significant difference in weight change between the COC-receiving group and the control group. When patients bring up their concerns, it may help to remind them that women tend to gain weight over time whether or not they are taking a COC.
Another common concern is that COCs cause mood changes. A 2016 review by Schaffir and colleagues sheds some light on this topic,3 albeit limited by the paucity of prospective studies. This review identified only 1 randomized controlled trial comparing depression incidence among women initiating a COC versus a placebo. There was no difference in the incidence of depression among the groups at 3 months. Among 4 large retrospective studies of women using COCs, the agents either had no or a beneficial effect on mood. Schaffir’s review reports that there may be greater mood adverse effects with COCs among women with underlying mood disorders.
Patients may worry that COC use will permanently impair their fertility or delay return to fertility after discontinuation. Research does indicate that return of fertility after stopping COCs often takes several months (compared with immediate fertility after discontinuing a barrier method). However, there still seem to be comparable conception rates within 12 months after discontinuing COCs as there are after discontinuing other common nonhormonal or hormonal contraceptive methods. Fertility is not impacted by the duration of COC use. In addition, return to fertility seems to be comparable after discontinuation of extended cycle or continuous COCs compared with traditional-cycle COCs.4
COC safety
Known major risks of COCs include venous thromboembolism (VTE). The risk of VTE is about double among COC users than among nonpregnant nonusers: 3–9 per 10,000 woman-years compared with 1–5.5 In a study by the US Food and Drug Administration, drospirenone-containing COCs had double the risk of VTE than other COCs. However, the position of the American College of Obstetricians and Gynecologists on this increased risk of VTE with drospirenone-containing pills is that it is “possible” and “minimal.”5 It is important to remember that an alternative to COC use is pregnancy, in which the VTE risk is about double that among COC users, at 5–20 per 10,000 woman-years. This risk increases further in the postpartum period, to 40–65 per 10,000 woman-years.5
Another known major risk of COCs is arterial embolic disease, including cerebrovascular accidents and myocardial infarctions. Women at increased risk for these complications include those with hypertension, diabetes, and/or obesity and women who are aged 35 or older and smoke. Interestingly, women with migraines with aura are at increased risk for stroke but not for myocardial infarction. These women increase their risk of stroke 2- to 4-fold if they use COCs.
Continue to: Different pills for different problems...
Different pills for different problems
With so many pills on the market, it is important for clinicians to know how to choose a particular pill for a particular patient. The following discussion assumes that the patient in question desires a COC for contraception, then offers guidance on how to choose a pill with patient-specific noncontraceptive benefits (TABLE 2).

When HMB is a concern. Patients with heavy menstrual bleeding may experience fewer bleeding and/or spotting days with extended cyclic or continuous use of a COC rather than with traditional cyclic use.6 Examples of such COC options include:
- Introvale and Seasonique, both extended-cycle formulations
- Amethyst, which is formulated without placebo pills so that it can be used continuously
- any other COC prescribed with instructions for the patient to skip placebo pills.
An extrapolated benefit to extended-cycle or continuous COCs use for heavy menstrual bleeding is addressing anemia.
For premenstrual dysphoric disorder, the only randomized controlled trials showing improvement involve drospirenone-ethinyl estradiol pills (Yaz and Yasmin).7 There is also evidence that extended cyclic or continuous use of these formulations is more impactful for premenstrual dysphoric disorder than a traditional cycle.8
Keeping migraine avoidance and prevention in mind. Various studies have looked at the impact of different COC formulations on menstrual-related symptoms. There is evidence of greater improvement in headache, bloating, and dysmenorrhea with extended cyclic or continuous use compared with traditional cyclic use.6
In terms of headache, let us delve into menstrual migraine in particular. Menstrual migraines occur sometime between 2 days prior to 2 days after the first day of menses and are linked to a sharp drop in estrogen levels. COCs are contraindicated in women with menstrual migraines with aura because of the increased stroke risk. For women with menstrual migraines without aura, COCs can prevent migraines. Prevention depends on minimizing fluctuations in estrogen levels; any change in estrogen level greater than 10 µg of ethinyl estradiol may trigger an estrogen-related migraine. All currently available regimens of COCs that comprise 21 days of active pills and 7 days of placebo involve a drop of more than 10 µg. Options that involve a drop of 10 µg or less include any continuous formulation, the extended formulation LoSeasonique (levonorgestrel 0.1 mg and ethinyl estradiol 20 µg for 84 days, then ethinyl estradiol 10 µg for 7 days), and Lo Loestrin (ethinyl estradiol 10 µg and norethindrone 1 mg for 24 days, then ethinyl estradiol 10 µg for 2 days, then placebo for 2 days).9
What’s best for acne-prone patients? All COCs should improve acne by increasing levels of sex hormone binding globulin. However, some comparative studies have shown drospirenone-containing COCs to be the most effective for acne. This finding makes sense in light of studies demonstrating antiandrogenic effects of drospirenone.10
Managing PCOS symptoms. It seems logical, by extension, that drospirenone-containing COCs would be particularly beneficial for treating hirsutism associated with polycystic ovary syndrome (PCOS). Other low‒androgenic-potential progestins, such as a third-generation progestin (norgestimate or desogestrel), might similarly be hypothesized to be advantageous. However, there is currently insufficient evidence to recommend any one COC formulation over another for the indication of PCOS.11
Ovarian cysts: Can COCs be helpful? COCs are commonly prescribed by gynecologists for patients with functional ovarian cysts. It is important to note that COCs have not been found to hasten the resolution of existing cysts, so they should not be used for this purpose.12 Studies of early COCs, which had high doses of estrogen (on the order of 50 µg), showed lower rates of cysts among users. This effect seems to be attenuated with the lower-estrogen-dose pills that are currently available, but there still appears to be benefit. Therefore, for a patient prone to cysts who desires an oral contraceptive, a COC containing estrogen 35 µg is likely to be the most beneficial of COCs currently on the market.13,14
Lower-dosage COCs in perimenopause may be beneficial. COCs can ameliorate perimenopausal symptoms including abnormal uterine bleeding and vasomotor symptoms. Clinicians are often hesitant to prescribe COCs for perimenopausal women because of increased risk of VTE, stroke, myocardial infarction, and breast cancer with increasing age. However, age alone is not a contraindication to any contraceptive method. An extended cyclic or continuous regimen COC may be the best choice for a perimenopausal woman in order to avoid vasomotor symptoms that occur during hormone-free intervals. In addition, given the increasing risk of adverse effects like VTE with estrogen dose, a lower estrogen formulation is advisable.15
Patients with epilepsy who are taking antiepileptic drugs (AEDs) are a special population when it comes to COCs. Certain AEDs induce hepatic enzymes involved in the metabolism and protein binding of COCs, which can result in contraceptive failure. Strong inducers are carbamazepine, oxcarbazepine, perampanel, phenobarbital, phenytoin, and primidone. Weak inducers are clobazam, eslicarbazepine, felbamate, lamotrigine, rufinamide, and topiramate. Women taking any of the above AEDs are recommended to choose a different form of contraception than a COC. However, if they are limited to COCs for some reason, a preparation containing estrogen 50 µg is recommended. It is speculated that the efficacy and adverse effects of COCs with increased hormone doses, used in combination with enzyme-inducing AEDs, should be comparable to those with standard doses when not combined with AEDs; however, this speculation is unproven.16 There are few COCs on the market with estrogen doses of 50 µg, but a couple of examples are Kelnor and Ogestrel.
Additional factors have to be considered with concurrent COC use with the AED lamotrigine since COCs increase clearance of this agent. Therefore, patients taking lamotrigine who start COCs will need an increase in lamotrigine dose. To avoid fluctuations in lamotrigine serum levels, use of a continuous COC is recommended.17
Continue to: Pill types to minimize adverse effects or risks...
Pill types to minimize adverse effects or risks
For women who desire to use a COC for contraception but who are at risk for a particular complication or are bothered by a particular adverse effect, ObGyns can optimize the choice of pill (TABLE 3). For example, women who have adverse effects of nausea and/or breast tenderness may benefit from reducing the estrogen dose to 20 µg or lower.18

Considering VTE
As discussed previously, VTE is a risk with all COCs, but some pills confer greater risk than others. For one, VTE risk increases with estrogen dose. In addition, VTE risk depends on the type of progestin. Drospirenone and third-generation progestins (norgestimate, gestodene, and desogestrel) confer a higher risk of VTE than first- or second-generation progestins. For example, a pill with estradiol 30 µg and either a third-generation progestin or drospirenone has a 50% to 80% higher risk of VTE compared with a pill with estradiol 30 µg and levonorgestrel.
For patients at particularly high risk for VTE, COCs are contraindicated. For patients for whom COCs are considered medically appropriate but who are at higher risk (eg, obese women), it is wise to use a pill containing a first-generation (norethindrone) or second-generation progestin (levonorgestrel) combined with the lowest dose of estrogen that has tolerable adverse effects.19
What about hypertension concerns?
Let us turn our attention briefly to hypertension and its relation to COC use. While the American College of Cardiology and the American Heart Association redefined hypertension in 2017 using a threshold of 130/80 mm Hg, the American College of Obstetricians and Gynecologists (ACOG) considers hypertension to be 140/90 mm Hg in terms of safety of using COCs. ACOG states, “women with blood pressure below 140/90 mm Hg may use any hormonal contraceptive method.”20 In women with hypertension in the range of 140‒159 mm Hg systolic or 90‒99 mm Hg diastolic, COCs are category 3 according to the US Medical Eligibility Criteria for Contraceptive Use, meaning that the risks usually outweigh the benefits. For women with blood pressures of 160/110 mm Hg or greater, COCs are category 4 (contraindicated). If a woman with mild hypertension is started on a COC, a drospirenone-containing pill may be the best choice because of its diuretic effects. While other contemporary COCs have been associated with a mild increase in blood pressure, drospirenone-containing pills have not shown this association.21
Continue to: At issue: Break-through bleeding, mood, and weight gain...
At issue: Break-through bleeding, mood, and weight gain
For women bothered by intermenstrual bleeding, use of a COC with a third-generation progestin may be preferable to use of one with a first- or second-generation. It may be because of decreased abnormal bleeding that COCs with third-generation progestins have lower discontinuation rates.22 In addition, COCs containing estrogen 20 µg or less are associated with more intermenstrual bleeding than those with more than 20 µg estrogen.23 Keep in mind that it is common with any COC to have intermenstrual bleeding for the first several months.
For women with pre-existing mood disorders or who report mood changes with COCs, it appears that fluctuations in hormone levels are problematic. Consistently, there is evidence that monophasic pills are preferable to multiphasic and that extended cyclic or continuous use is preferable to traditional cyclic use for mitigating mood adverse effects. There is mixed evidence on whether a low dose of ethinyl estradiol is better for mood.3
Although it is discussed above that randomized controlled trials have not shown an association between COC use and weight gain, many women remain concerned. For these women, a drospirenone-containing COC may be the best choice. Drospirenone has antimineralocorticoid activity, so it may help prevent water retention.
A brief word about multiphasic COCs. While these pills were designed to mimic physiologic hormone fluctuations and minimize hormonal adverse effects, there is insufficient evidence to compare their effects to those of monophasic pills.24 Without such evidence, there is little reason to recommend a multiphasic pill to a patient over the more straightforward monophasic formulation.
Conclusion
There are more nuances to prescribing an optimal COC for a patient than may initially come to mind. It is useful to remember that any formulation of pill may be prescribed in an extended or continuous fashion, and there are benefits for such use for premenstrual dysphoric disorder, heavy menstrual bleeding, perimenopause, and menstrual symptoms. Although there are numerous brands of COCs available, a small cadre will suffice for almost all purposes. Such a “toolbox” of pills could include a pill formatted for continuous use (Seasonique), a low estrogen pill (Loestrin), a drospirenone-containing pill (Yaz), and a pill containing a third-generation progestin and a higher dose of estrogen (Sprintec). ●
- Daniels K, Abma JC. Current contraceptive status among women aged 15-49: United States, 2015-2017. NCHS Data Brief, no 327. Hyattsville, MD; 2018.
- Gallo MF, Lopez LM, Grimes DA, et al. Combination contraceptives: effects on weight. Cochrane Database Syst Rev. 2014:CD003987.
- Schaffir J, Worly BL, Gur TL. Combined hormonal contraception and its effects on mood: a critical review. Eur J Contracept Reprod Health Care. 2016;21:347-355.
- Barnhart KT, Schreiber CA. Return to fertility following discontinuation of oral contraceptives. Fertil Steril. 2009;91:659-663.
- American College of Obstetricians and Gynecologists. Committee Opinion #540: Risk of Venous Thromboembolism Among Users of Drospirenone-Containing Oral Contraceptive Pills. Obstet Gynecol. 2012;120:1239-1242.
- Edelman A, Micks E, Gallo MF, et al. Continuous or extended cycle vs. cyclic use of combined hormonal contraceptives for contraception. Cochrane Database Syst Rev. 2014:CD004695.
- American College of Obstetricians and Gynecologists. Practice Bulletin #110: Noncontraceptive Uses of Hormonal Contraceptives. Obstet Gynecol. 2010:206-218.
- Coffee AL, Kuehl TJ, Willis S, et al. Oral contraceptives and premenstrual symptoms: comparison of a 21/7 and extended regimen. Am J Obstet Gynecol. 2006;195:1311-1319.
- Calhoun AH, Batur P. Combined hormonal contraceptives and migraine: an update on the evidence. Cleve Clin J Med. 2017;84:631-638.
- Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. 2012:CD004425.
- McCartney CR, Marshall JC. CLINICAL PRACTICE. Polycystic Ovary Syndrome. N Engl J Med. 2016;375:54-64.
- Grimes DA, Jones LB, Lopez LM, et al. Oral contraceptives for functional ovarian cysts. Cochrane Database Syst Rev. 2014:CD006134.
- Grimes DA, Godwin AJ, Rubin A, et al. Ovulation and follicular development associated with three low-dose oral contraceptives: a randomized controlled trial. Obstet Gynecol. 1994;83:29-34.
- Christensen JT, Boldsen JL, Westergaard JG. Functional ovarian cysts in premenopausal and gynecologically healthy women. Contraception. 2002;66:153-157.
- Hardman SM, Gebbie AE. Hormonal contraceptive regimens in the perimenopause. Maturitas. 2009;63:204-212.
- Zupanc ML. Antiepileptic drugs and hormonal contraceptives in adolescent women with epilepsy. Neurology. 2006;66 (6 suppl 3):S37-S45.
- Wegner I, Edelbroek PM, Bulk S, et al. Lamotrigine kinetics within the menstrual cycle, after menopause, and with oral contraceptives. Neurology. 2009;73:1388-1393.
- Stewart M, Black K. Choosing a combined oral contraceptive pill. Australian Prescriber. 2015;38:6-11.
- de Bastos M, Stegeman BH, Rosendaal FR, et al. Combined oral contraceptives: venous thrombosis. Cochrane Database Syst Rev. 2014:CD010813.
- American College of Obstetricians and Gynecologists. Practice Bulletin #206: use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol. 2019;133:e128-e150.
- de Morais TL, Giribela C, Nisenbaum MG, et al. Effects of a contraceptive containing drospirenone and ethinylestradiol on blood pressure, metabolic profile and neurohumoral axis in hypertensive women at reproductive age. Eur J Obstet Gynecol Reprod Biol. 2014;182:113-117.
- Lawrie TA, Helmerhorst FM, Maitra NK, et al. Types of progestogens in combined oral contraception: effectiveness and side-effects. Cochrane Database Syst Rev. 2011:CD004861.
- Gallo MF, Nanda K, Grimes DA, et al. 20 µg versus >20 µg estrogen combined oral contraceptives for contraception. Cochrane Database Syst Rev. 2013:CD003989.
- van Vliet HA, Grimes DA, Lopez LM, et al. Triphasic versus monophasic oral contraceptives for contraception. Cochrane Database Syst Rev. 2006:CD003553
- Daniels K, Abma JC. Current contraceptive status among women aged 15-49: United States, 2015-2017. NCHS Data Brief, no 327. Hyattsville, MD; 2018.
- Gallo MF, Lopez LM, Grimes DA, et al. Combination contraceptives: effects on weight. Cochrane Database Syst Rev. 2014:CD003987.
- Schaffir J, Worly BL, Gur TL. Combined hormonal contraception and its effects on mood: a critical review. Eur J Contracept Reprod Health Care. 2016;21:347-355.
- Barnhart KT, Schreiber CA. Return to fertility following discontinuation of oral contraceptives. Fertil Steril. 2009;91:659-663.
- American College of Obstetricians and Gynecologists. Committee Opinion #540: Risk of Venous Thromboembolism Among Users of Drospirenone-Containing Oral Contraceptive Pills. Obstet Gynecol. 2012;120:1239-1242.
- Edelman A, Micks E, Gallo MF, et al. Continuous or extended cycle vs. cyclic use of combined hormonal contraceptives for contraception. Cochrane Database Syst Rev. 2014:CD004695.
- American College of Obstetricians and Gynecologists. Practice Bulletin #110: Noncontraceptive Uses of Hormonal Contraceptives. Obstet Gynecol. 2010:206-218.
- Coffee AL, Kuehl TJ, Willis S, et al. Oral contraceptives and premenstrual symptoms: comparison of a 21/7 and extended regimen. Am J Obstet Gynecol. 2006;195:1311-1319.
- Calhoun AH, Batur P. Combined hormonal contraceptives and migraine: an update on the evidence. Cleve Clin J Med. 2017;84:631-638.
- Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. 2012:CD004425.
- McCartney CR, Marshall JC. CLINICAL PRACTICE. Polycystic Ovary Syndrome. N Engl J Med. 2016;375:54-64.
- Grimes DA, Jones LB, Lopez LM, et al. Oral contraceptives for functional ovarian cysts. Cochrane Database Syst Rev. 2014:CD006134.
- Grimes DA, Godwin AJ, Rubin A, et al. Ovulation and follicular development associated with three low-dose oral contraceptives: a randomized controlled trial. Obstet Gynecol. 1994;83:29-34.
- Christensen JT, Boldsen JL, Westergaard JG. Functional ovarian cysts in premenopausal and gynecologically healthy women. Contraception. 2002;66:153-157.
- Hardman SM, Gebbie AE. Hormonal contraceptive regimens in the perimenopause. Maturitas. 2009;63:204-212.
- Zupanc ML. Antiepileptic drugs and hormonal contraceptives in adolescent women with epilepsy. Neurology. 2006;66 (6 suppl 3):S37-S45.
- Wegner I, Edelbroek PM, Bulk S, et al. Lamotrigine kinetics within the menstrual cycle, after menopause, and with oral contraceptives. Neurology. 2009;73:1388-1393.
- Stewart M, Black K. Choosing a combined oral contraceptive pill. Australian Prescriber. 2015;38:6-11.
- de Bastos M, Stegeman BH, Rosendaal FR, et al. Combined oral contraceptives: venous thrombosis. Cochrane Database Syst Rev. 2014:CD010813.
- American College of Obstetricians and Gynecologists. Practice Bulletin #206: use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol. 2019;133:e128-e150.
- de Morais TL, Giribela C, Nisenbaum MG, et al. Effects of a contraceptive containing drospirenone and ethinylestradiol on blood pressure, metabolic profile and neurohumoral axis in hypertensive women at reproductive age. Eur J Obstet Gynecol Reprod Biol. 2014;182:113-117.
- Lawrie TA, Helmerhorst FM, Maitra NK, et al. Types of progestogens in combined oral contraception: effectiveness and side-effects. Cochrane Database Syst Rev. 2011:CD004861.
- Gallo MF, Nanda K, Grimes DA, et al. 20 µg versus >20 µg estrogen combined oral contraceptives for contraception. Cochrane Database Syst Rev. 2013:CD003989.
- van Vliet HA, Grimes DA, Lopez LM, et al. Triphasic versus monophasic oral contraceptives for contraception. Cochrane Database Syst Rev. 2006:CD003553
COVID-19 impacts women’s contraception choices
The rate of unintended pregnancies in the United States has decreased to approximately 45%, based on data published in 2016, and “for the first time in many years, this decrease affected women of all race/ethnicity, income levels, and education levels,” Eve Espey, MD, said at the 2020 virtual meeting of the American College of Obstetricians and Gynecologists.
Changes in contraceptive choices drove much of this decrease, said Dr. Espey, professor of obstetrics and gynecology at the University of New Mexico, Albuquerque.
“What is really striking is the very large increase in use of the IUD,” she noted. However, the increased use of IUDs has raised concerns about coercive tactics being used to push for IUD use in communities of color.
“The focus we should have is on reproductive autonomy and not on unintended pregnancy, a metric that is classist and racist and may value the reproduction of some groups over others,” Dr. Espey said. Previous studies have suggested that providers are biased in how they promote long-acting reversible contraception (LARC), and reports from patients suggest that women and people of color particularly may feel marginalized, not heard, and coerced, she noted.
Help patients feel empowered
Overall, the goal of contraception should be to empower women and people to make the reproductive decisions that are best for them. “My own approach to contraceptive counseling has changed over the years; I currently start by asking if the patient wants to talk about contraception,” Dr. Espey said.
The COVID-19 pandemic has impacted many women’s reproductive options and plans, she said.
A survey showed that after COVID-19, more than 40% of women reported changing their plans about childbearing, 34% wanted to get pregnant later, and 33% reported trouble getting birth control or getting an appointment with a health care provider, she said.
ACOG issued a statement in March 2020 about the provision of contraception and how contraception is an essential component of comprehensive health care. The COVID-19 ACOG guidance on contraception includes use of telehealth for services including screening new patients, offering prescriptions and refills as appropriate, and managing side effects. Providers can counsel patients on the use of emergency contraception and provide advance prescriptions for ulipristal acetate, and ideally provide a year’s worth of prescription refills to reduce pharmacy visits, although not all insurance companies allow this, Dr. Espey noted.
ACOG’s COVID-19 guidance on the use of LARCs includes preserving access when possible, and focusing on postpartum contraception as a key access point.
“The postpartum period is a very convenient time for patients who want contraceptives, including LARC,” especially since they are already in the hospital setting, Dr. Espey said. However, it is important to preserve patients’ reproductive autonomy and avoid placing barriers to LARC removal for those who request it, she emphasized.
Consider MEC categories for contraception
When advising patients about contraception, Dr. Espey noted the development of a simple app with the U.S. Medical Eligibility Criteria (MEC) as a useful tool. The app includes the four MEC categories based on the latest evidence-based guidance from the Centers for Disease Control and Prevention on contraceptive practice.
Patients in category 1 have no restriction on the use of a particular contraceptive method; category 2 means that “advantages generally outweigh the theoretical or proven risks”; in category 3, these risks usually outweigh the advantages; and category 4 indicates “unacceptable health risk if the contraceptive method is used,” according to the MEC.
“What complicates category 3 is that many patients have a condition that is associated with adverse outcomes in pregnancy,” Dr. Espey noted, “So it is even more important that category 3 options only be considered if other options are not available or not acceptable to the patient,”
For example, a patient with complicated diabetes who wants depot medroxyprogesterone acetate (DMPA) for contraception for a year must weigh the benefits with the theoretical risk of thromboembolic disease related to a higher dose progestin, and the fact that the injection is not reversible in the case of an adverse event. “Close follow-up is recommended for patients using contraception with category 3 recommendations,” Dr. Espey emphasized.
Some new elements of contraception that are ongoing in the pandemic health care setting include increased pharmacist prescribing of hormonal contraception, Dr. Espey said. Over-the-counter access to contraception is not yet an option, but a progestin-only pill will likely be the first, she added.
Although the Essure birth control implant is no longer available in the United States, new options for a contraceptive patch (Twirla [ethinyl estradiol and levonorgestrel] and Xulane [ethinyl estradiol and norelgestromin]) offer weekly contraceptive options for women with a body mass index less than 30 kg/m2.
Annovera offers more options
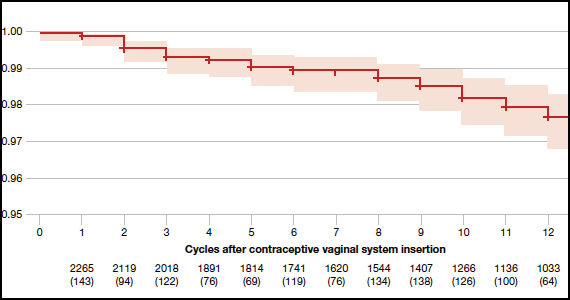
The newest choice on the market is Annovera, a flexible ring that delivers 150 mcg/day of segesterone acetate and 13 mcg/day of ethinyl estradiol. The ring is meant to remain in place for 21 days, with 7 days out, to repeat for a year.
During the question-and-answer session, Dr. Espey was asked whether it would be an off-label use to leave Annovera in continuously. Although this has not been studied, there is no biologically plausible reason not to leave it in for a year without taking it out. In either case, this is a patient-controlled LARC, she said.
Overall, “it remains to be seen how Annovera will do, as a potentially exciting, new, long-acting option” she said. “A major advantage is that it is controlled by the user,” she noted. However, “the price point will be very important as well.”
As for the off-label use by women with a BMI greater than 29 kg/m2, it is complicated. Two women with higher BMIs enrolled in clinical trials developed venous thromboembolisms, so an increased risk can’t be ruled out, although the good news is that BMI has not been shown to impact effectiveness of the product, she added.
IUDs appropriate for younger women
When asked for her guidelines about IUD options in the absence of head-to-head trials, Dr. Espey said that she often recommends either Mirena and Liletta. These levonorgestrel-releasing IUDS are essentially the same, can be used off label for 7 years (both are currently Food and Drug Administration approved for 6 years), and have a favorable bleeding profile. Other IUDs are marketed as having a smaller diameter designed for increased patient comfort with insertion, but she views this as less important than bleeding profile and duration given the length of time the device is in place.
Dr. Espey added that she doesn’t see age as a barrier to IUD use, and that the evidence does not support an increased risk of infertility. In fact, “we are seeing a higher demand among younger and nulliparous women.”
“We should respect the reproductive autonomy and the choices that our patients make,” Dr. Espey concluded.
Dr. Espey had no relevant financial disclosures. She is a member of the Ob.Gyn. News editorial advisory board.
The rate of unintended pregnancies in the United States has decreased to approximately 45%, based on data published in 2016, and “for the first time in many years, this decrease affected women of all race/ethnicity, income levels, and education levels,” Eve Espey, MD, said at the 2020 virtual meeting of the American College of Obstetricians and Gynecologists.
Changes in contraceptive choices drove much of this decrease, said Dr. Espey, professor of obstetrics and gynecology at the University of New Mexico, Albuquerque.
“What is really striking is the very large increase in use of the IUD,” she noted. However, the increased use of IUDs has raised concerns about coercive tactics being used to push for IUD use in communities of color.
“The focus we should have is on reproductive autonomy and not on unintended pregnancy, a metric that is classist and racist and may value the reproduction of some groups over others,” Dr. Espey said. Previous studies have suggested that providers are biased in how they promote long-acting reversible contraception (LARC), and reports from patients suggest that women and people of color particularly may feel marginalized, not heard, and coerced, she noted.
Help patients feel empowered
Overall, the goal of contraception should be to empower women and people to make the reproductive decisions that are best for them. “My own approach to contraceptive counseling has changed over the years; I currently start by asking if the patient wants to talk about contraception,” Dr. Espey said.
The COVID-19 pandemic has impacted many women’s reproductive options and plans, she said.
A survey showed that after COVID-19, more than 40% of women reported changing their plans about childbearing, 34% wanted to get pregnant later, and 33% reported trouble getting birth control or getting an appointment with a health care provider, she said.
ACOG issued a statement in March 2020 about the provision of contraception and how contraception is an essential component of comprehensive health care. The COVID-19 ACOG guidance on contraception includes use of telehealth for services including screening new patients, offering prescriptions and refills as appropriate, and managing side effects. Providers can counsel patients on the use of emergency contraception and provide advance prescriptions for ulipristal acetate, and ideally provide a year’s worth of prescription refills to reduce pharmacy visits, although not all insurance companies allow this, Dr. Espey noted.
ACOG’s COVID-19 guidance on the use of LARCs includes preserving access when possible, and focusing on postpartum contraception as a key access point.
“The postpartum period is a very convenient time for patients who want contraceptives, including LARC,” especially since they are already in the hospital setting, Dr. Espey said. However, it is important to preserve patients’ reproductive autonomy and avoid placing barriers to LARC removal for those who request it, she emphasized.
Consider MEC categories for contraception
When advising patients about contraception, Dr. Espey noted the development of a simple app with the U.S. Medical Eligibility Criteria (MEC) as a useful tool. The app includes the four MEC categories based on the latest evidence-based guidance from the Centers for Disease Control and Prevention on contraceptive practice.
Patients in category 1 have no restriction on the use of a particular contraceptive method; category 2 means that “advantages generally outweigh the theoretical or proven risks”; in category 3, these risks usually outweigh the advantages; and category 4 indicates “unacceptable health risk if the contraceptive method is used,” according to the MEC.
“What complicates category 3 is that many patients have a condition that is associated with adverse outcomes in pregnancy,” Dr. Espey noted, “So it is even more important that category 3 options only be considered if other options are not available or not acceptable to the patient,”
For example, a patient with complicated diabetes who wants depot medroxyprogesterone acetate (DMPA) for contraception for a year must weigh the benefits with the theoretical risk of thromboembolic disease related to a higher dose progestin, and the fact that the injection is not reversible in the case of an adverse event. “Close follow-up is recommended for patients using contraception with category 3 recommendations,” Dr. Espey emphasized.
Some new elements of contraception that are ongoing in the pandemic health care setting include increased pharmacist prescribing of hormonal contraception, Dr. Espey said. Over-the-counter access to contraception is not yet an option, but a progestin-only pill will likely be the first, she added.
Although the Essure birth control implant is no longer available in the United States, new options for a contraceptive patch (Twirla [ethinyl estradiol and levonorgestrel] and Xulane [ethinyl estradiol and norelgestromin]) offer weekly contraceptive options for women with a body mass index less than 30 kg/m2.
Annovera offers more options
The newest choice on the market is Annovera, a flexible ring that delivers 150 mcg/day of segesterone acetate and 13 mcg/day of ethinyl estradiol. The ring is meant to remain in place for 21 days, with 7 days out, to repeat for a year.
During the question-and-answer session, Dr. Espey was asked whether it would be an off-label use to leave Annovera in continuously. Although this has not been studied, there is no biologically plausible reason not to leave it in for a year without taking it out. In either case, this is a patient-controlled LARC, she said.
Overall, “it remains to be seen how Annovera will do, as a potentially exciting, new, long-acting option” she said. “A major advantage is that it is controlled by the user,” she noted. However, “the price point will be very important as well.”
As for the off-label use by women with a BMI greater than 29 kg/m2, it is complicated. Two women with higher BMIs enrolled in clinical trials developed venous thromboembolisms, so an increased risk can’t be ruled out, although the good news is that BMI has not been shown to impact effectiveness of the product, she added.
IUDs appropriate for younger women
When asked for her guidelines about IUD options in the absence of head-to-head trials, Dr. Espey said that she often recommends either Mirena and Liletta. These levonorgestrel-releasing IUDS are essentially the same, can be used off label for 7 years (both are currently Food and Drug Administration approved for 6 years), and have a favorable bleeding profile. Other IUDs are marketed as having a smaller diameter designed for increased patient comfort with insertion, but she views this as less important than bleeding profile and duration given the length of time the device is in place.
Dr. Espey added that she doesn’t see age as a barrier to IUD use, and that the evidence does not support an increased risk of infertility. In fact, “we are seeing a higher demand among younger and nulliparous women.”
“We should respect the reproductive autonomy and the choices that our patients make,” Dr. Espey concluded.
Dr. Espey had no relevant financial disclosures. She is a member of the Ob.Gyn. News editorial advisory board.
The rate of unintended pregnancies in the United States has decreased to approximately 45%, based on data published in 2016, and “for the first time in many years, this decrease affected women of all race/ethnicity, income levels, and education levels,” Eve Espey, MD, said at the 2020 virtual meeting of the American College of Obstetricians and Gynecologists.
Changes in contraceptive choices drove much of this decrease, said Dr. Espey, professor of obstetrics and gynecology at the University of New Mexico, Albuquerque.
“What is really striking is the very large increase in use of the IUD,” she noted. However, the increased use of IUDs has raised concerns about coercive tactics being used to push for IUD use in communities of color.
“The focus we should have is on reproductive autonomy and not on unintended pregnancy, a metric that is classist and racist and may value the reproduction of some groups over others,” Dr. Espey said. Previous studies have suggested that providers are biased in how they promote long-acting reversible contraception (LARC), and reports from patients suggest that women and people of color particularly may feel marginalized, not heard, and coerced, she noted.
Help patients feel empowered
Overall, the goal of contraception should be to empower women and people to make the reproductive decisions that are best for them. “My own approach to contraceptive counseling has changed over the years; I currently start by asking if the patient wants to talk about contraception,” Dr. Espey said.
The COVID-19 pandemic has impacted many women’s reproductive options and plans, she said.
A survey showed that after COVID-19, more than 40% of women reported changing their plans about childbearing, 34% wanted to get pregnant later, and 33% reported trouble getting birth control or getting an appointment with a health care provider, she said.
ACOG issued a statement in March 2020 about the provision of contraception and how contraception is an essential component of comprehensive health care. The COVID-19 ACOG guidance on contraception includes use of telehealth for services including screening new patients, offering prescriptions and refills as appropriate, and managing side effects. Providers can counsel patients on the use of emergency contraception and provide advance prescriptions for ulipristal acetate, and ideally provide a year’s worth of prescription refills to reduce pharmacy visits, although not all insurance companies allow this, Dr. Espey noted.
ACOG’s COVID-19 guidance on the use of LARCs includes preserving access when possible, and focusing on postpartum contraception as a key access point.
“The postpartum period is a very convenient time for patients who want contraceptives, including LARC,” especially since they are already in the hospital setting, Dr. Espey said. However, it is important to preserve patients’ reproductive autonomy and avoid placing barriers to LARC removal for those who request it, she emphasized.
Consider MEC categories for contraception
When advising patients about contraception, Dr. Espey noted the development of a simple app with the U.S. Medical Eligibility Criteria (MEC) as a useful tool. The app includes the four MEC categories based on the latest evidence-based guidance from the Centers for Disease Control and Prevention on contraceptive practice.
Patients in category 1 have no restriction on the use of a particular contraceptive method; category 2 means that “advantages generally outweigh the theoretical or proven risks”; in category 3, these risks usually outweigh the advantages; and category 4 indicates “unacceptable health risk if the contraceptive method is used,” according to the MEC.
“What complicates category 3 is that many patients have a condition that is associated with adverse outcomes in pregnancy,” Dr. Espey noted, “So it is even more important that category 3 options only be considered if other options are not available or not acceptable to the patient,”
For example, a patient with complicated diabetes who wants depot medroxyprogesterone acetate (DMPA) for contraception for a year must weigh the benefits with the theoretical risk of thromboembolic disease related to a higher dose progestin, and the fact that the injection is not reversible in the case of an adverse event. “Close follow-up is recommended for patients using contraception with category 3 recommendations,” Dr. Espey emphasized.
Some new elements of contraception that are ongoing in the pandemic health care setting include increased pharmacist prescribing of hormonal contraception, Dr. Espey said. Over-the-counter access to contraception is not yet an option, but a progestin-only pill will likely be the first, she added.
Although the Essure birth control implant is no longer available in the United States, new options for a contraceptive patch (Twirla [ethinyl estradiol and levonorgestrel] and Xulane [ethinyl estradiol and norelgestromin]) offer weekly contraceptive options for women with a body mass index less than 30 kg/m2.
Annovera offers more options
The newest choice on the market is Annovera, a flexible ring that delivers 150 mcg/day of segesterone acetate and 13 mcg/day of ethinyl estradiol. The ring is meant to remain in place for 21 days, with 7 days out, to repeat for a year.
During the question-and-answer session, Dr. Espey was asked whether it would be an off-label use to leave Annovera in continuously. Although this has not been studied, there is no biologically plausible reason not to leave it in for a year without taking it out. In either case, this is a patient-controlled LARC, she said.
Overall, “it remains to be seen how Annovera will do, as a potentially exciting, new, long-acting option” she said. “A major advantage is that it is controlled by the user,” she noted. However, “the price point will be very important as well.”
As for the off-label use by women with a BMI greater than 29 kg/m2, it is complicated. Two women with higher BMIs enrolled in clinical trials developed venous thromboembolisms, so an increased risk can’t be ruled out, although the good news is that BMI has not been shown to impact effectiveness of the product, she added.
IUDs appropriate for younger women
When asked for her guidelines about IUD options in the absence of head-to-head trials, Dr. Espey said that she often recommends either Mirena and Liletta. These levonorgestrel-releasing IUDS are essentially the same, can be used off label for 7 years (both are currently Food and Drug Administration approved for 6 years), and have a favorable bleeding profile. Other IUDs are marketed as having a smaller diameter designed for increased patient comfort with insertion, but she views this as less important than bleeding profile and duration given the length of time the device is in place.
Dr. Espey added that she doesn’t see age as a barrier to IUD use, and that the evidence does not support an increased risk of infertility. In fact, “we are seeing a higher demand among younger and nulliparous women.”
“We should respect the reproductive autonomy and the choices that our patients make,” Dr. Espey concluded.
Dr. Espey had no relevant financial disclosures. She is a member of the Ob.Gyn. News editorial advisory board.
EXPERT ANALYSIS FROM ACOG 2020
Menstrual cup use increases risk of IUD expulsion
Menstrual cup use is becoming increasingly popular as an option for menstrual hygiene among women in the United States, but little is known about the potential for IUD expulsion with menstrual cup use, Jill Long, MD, said at the 2020 virtual meeting of the American College of Obstetricians and Gynecologists.
Dr. Long cited a 2019 Internet survey of IUD users in which 11% reported menstrual cup use; reports of IUD expulsion were approximately three times higher among menstrual cup users. The results of the survey were limited by the self-reported responses and lack of data on when the expulsions occurred related to menstrual cup use.
Similar concerns about expulsion surfaced in an ongoing phase 3, randomized trial designed to support the marketing application of the Mona Lisa NT Cu380 Mini IUD, which is not currently approved in the United States.
“Nine months into the study, more expulsions were observed than expected, particularly among menstrual cup users,” said Dr. Long, medical officer and project officer for the Contraceptive Clinical Trials Network at the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The researchers then began to advise study participants to avoid menstrual cup use, and to collect data on use of menstrual hygiene products.
The preliminary study results reported by Dr. Long included 1,092 women assigned in a 4:1 ratio to receive the Mona Lisa NT Cu380 Mini and ParaGard, an IUD approved in the United States. Participants had follow-up visits at 6 weeks and at 3, 6, 12, 24, and 36 months after placement to verify the IUD position.
At 34 months, the overall rate of IUD expulsion was 9%. Of 277 women who reported menstrual cup use, the expulsion rate was 18%, compared with a 6% expulsion rates among the 704 women who reported tampon use.
Patient behavior persists despite advisory
Overall IUD expulsion was not significantly different among patients enrolled before and after the advisory against menstrual cup use (9.8% vs. 8.8%, respectively). In addition, menstrual cup use did not decrease after the implementation of the advisory (24% preadvisory vs. 28% post advisory).
The expulsion rates between menstrual cup users and nonusers were significantly different at both 12 months (14% vs. 5%) and 24 months (21% vs. 6%).
In addition, the researchers created a category of accidental self-removal, defined as the percent of expulsions occurring at the time the menstrual hygiene product was removed. Accidental self-removal was significantly higher among menstrual cup users, compared with tampon users (43% vs. 10%).
Study limitations included study blinding with regard to IUD type, parity, and age, so the impact of these factors on expulsion remains unclear, Dr. Long said. In addition, data on menstrual hygiene product use was collected retrospectively for the first 9 months, and no data were available on the impact on expulsion of combined use of menstrual cups and tampons.
Despite the apparent lack of impact of counseling, “women should be informed of the increased risk of expulsion if they choose to use menstrual cups concurrently with copper IUDs,” Dr. Long concluded.
More data to come from further analysis
During a question-and-answer session following the presentation, Dr. Long was asked whether suction might contribute to expulsion. “We advised subjects to break the seal on the menstrual cup prior to removal, but we did not see a decrease in expulsion rates with menstrual cup use after this advisory,” she said.
“The type of menstrual cup may be a factor,” she added. “We still need to analyze the data by the type of menstrual cup used as different cups have different degrees of suction.”
When asked about the potential role of coital activity on expulsion, Dr. Long said that the researchers had not yet reviewed coital activity logs to compare expulsion data with the timing of sexual activity.
Any increased pregnancy rates among menstrual cup users are “unlikely, but we don’t know for sure,” she added.
The current study also did not evaluate quantitative differences in bleeding, and participants are using bleeding diaries, Dr. Long noted. She added that participants are able to report problems with bleeding to the study sites, and that these are captured as adverse events.
The study was supported by a partnership between NICHD and FHI 360, a nonprofit human development organization based in North Carolina, with FHI 360 funding from the Bill and Melinda Gates Foundation. Dr. Long had no financial conflicts to disclose.
Menstrual cup use is becoming increasingly popular as an option for menstrual hygiene among women in the United States, but little is known about the potential for IUD expulsion with menstrual cup use, Jill Long, MD, said at the 2020 virtual meeting of the American College of Obstetricians and Gynecologists.
Dr. Long cited a 2019 Internet survey of IUD users in which 11% reported menstrual cup use; reports of IUD expulsion were approximately three times higher among menstrual cup users. The results of the survey were limited by the self-reported responses and lack of data on when the expulsions occurred related to menstrual cup use.
Similar concerns about expulsion surfaced in an ongoing phase 3, randomized trial designed to support the marketing application of the Mona Lisa NT Cu380 Mini IUD, which is not currently approved in the United States.
“Nine months into the study, more expulsions were observed than expected, particularly among menstrual cup users,” said Dr. Long, medical officer and project officer for the Contraceptive Clinical Trials Network at the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The researchers then began to advise study participants to avoid menstrual cup use, and to collect data on use of menstrual hygiene products.
The preliminary study results reported by Dr. Long included 1,092 women assigned in a 4:1 ratio to receive the Mona Lisa NT Cu380 Mini and ParaGard, an IUD approved in the United States. Participants had follow-up visits at 6 weeks and at 3, 6, 12, 24, and 36 months after placement to verify the IUD position.
At 34 months, the overall rate of IUD expulsion was 9%. Of 277 women who reported menstrual cup use, the expulsion rate was 18%, compared with a 6% expulsion rates among the 704 women who reported tampon use.
Patient behavior persists despite advisory
Overall IUD expulsion was not significantly different among patients enrolled before and after the advisory against menstrual cup use (9.8% vs. 8.8%, respectively). In addition, menstrual cup use did not decrease after the implementation of the advisory (24% preadvisory vs. 28% post advisory).
The expulsion rates between menstrual cup users and nonusers were significantly different at both 12 months (14% vs. 5%) and 24 months (21% vs. 6%).
In addition, the researchers created a category of accidental self-removal, defined as the percent of expulsions occurring at the time the menstrual hygiene product was removed. Accidental self-removal was significantly higher among menstrual cup users, compared with tampon users (43% vs. 10%).
Study limitations included study blinding with regard to IUD type, parity, and age, so the impact of these factors on expulsion remains unclear, Dr. Long said. In addition, data on menstrual hygiene product use was collected retrospectively for the first 9 months, and no data were available on the impact on expulsion of combined use of menstrual cups and tampons.
Despite the apparent lack of impact of counseling, “women should be informed of the increased risk of expulsion if they choose to use menstrual cups concurrently with copper IUDs,” Dr. Long concluded.
More data to come from further analysis
During a question-and-answer session following the presentation, Dr. Long was asked whether suction might contribute to expulsion. “We advised subjects to break the seal on the menstrual cup prior to removal, but we did not see a decrease in expulsion rates with menstrual cup use after this advisory,” she said.
“The type of menstrual cup may be a factor,” she added. “We still need to analyze the data by the type of menstrual cup used as different cups have different degrees of suction.”
When asked about the potential role of coital activity on expulsion, Dr. Long said that the researchers had not yet reviewed coital activity logs to compare expulsion data with the timing of sexual activity.
Any increased pregnancy rates among menstrual cup users are “unlikely, but we don’t know for sure,” she added.
The current study also did not evaluate quantitative differences in bleeding, and participants are using bleeding diaries, Dr. Long noted. She added that participants are able to report problems with bleeding to the study sites, and that these are captured as adverse events.
The study was supported by a partnership between NICHD and FHI 360, a nonprofit human development organization based in North Carolina, with FHI 360 funding from the Bill and Melinda Gates Foundation. Dr. Long had no financial conflicts to disclose.
Menstrual cup use is becoming increasingly popular as an option for menstrual hygiene among women in the United States, but little is known about the potential for IUD expulsion with menstrual cup use, Jill Long, MD, said at the 2020 virtual meeting of the American College of Obstetricians and Gynecologists.
Dr. Long cited a 2019 Internet survey of IUD users in which 11% reported menstrual cup use; reports of IUD expulsion were approximately three times higher among menstrual cup users. The results of the survey were limited by the self-reported responses and lack of data on when the expulsions occurred related to menstrual cup use.
Similar concerns about expulsion surfaced in an ongoing phase 3, randomized trial designed to support the marketing application of the Mona Lisa NT Cu380 Mini IUD, which is not currently approved in the United States.
“Nine months into the study, more expulsions were observed than expected, particularly among menstrual cup users,” said Dr. Long, medical officer and project officer for the Contraceptive Clinical Trials Network at the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The researchers then began to advise study participants to avoid menstrual cup use, and to collect data on use of menstrual hygiene products.
The preliminary study results reported by Dr. Long included 1,092 women assigned in a 4:1 ratio to receive the Mona Lisa NT Cu380 Mini and ParaGard, an IUD approved in the United States. Participants had follow-up visits at 6 weeks and at 3, 6, 12, 24, and 36 months after placement to verify the IUD position.
At 34 months, the overall rate of IUD expulsion was 9%. Of 277 women who reported menstrual cup use, the expulsion rate was 18%, compared with a 6% expulsion rates among the 704 women who reported tampon use.
Patient behavior persists despite advisory
Overall IUD expulsion was not significantly different among patients enrolled before and after the advisory against menstrual cup use (9.8% vs. 8.8%, respectively). In addition, menstrual cup use did not decrease after the implementation of the advisory (24% preadvisory vs. 28% post advisory).
The expulsion rates between menstrual cup users and nonusers were significantly different at both 12 months (14% vs. 5%) and 24 months (21% vs. 6%).
In addition, the researchers created a category of accidental self-removal, defined as the percent of expulsions occurring at the time the menstrual hygiene product was removed. Accidental self-removal was significantly higher among menstrual cup users, compared with tampon users (43% vs. 10%).
Study limitations included study blinding with regard to IUD type, parity, and age, so the impact of these factors on expulsion remains unclear, Dr. Long said. In addition, data on menstrual hygiene product use was collected retrospectively for the first 9 months, and no data were available on the impact on expulsion of combined use of menstrual cups and tampons.
Despite the apparent lack of impact of counseling, “women should be informed of the increased risk of expulsion if they choose to use menstrual cups concurrently with copper IUDs,” Dr. Long concluded.
More data to come from further analysis
During a question-and-answer session following the presentation, Dr. Long was asked whether suction might contribute to expulsion. “We advised subjects to break the seal on the menstrual cup prior to removal, but we did not see a decrease in expulsion rates with menstrual cup use after this advisory,” she said.
“The type of menstrual cup may be a factor,” she added. “We still need to analyze the data by the type of menstrual cup used as different cups have different degrees of suction.”
When asked about the potential role of coital activity on expulsion, Dr. Long said that the researchers had not yet reviewed coital activity logs to compare expulsion data with the timing of sexual activity.
Any increased pregnancy rates among menstrual cup users are “unlikely, but we don’t know for sure,” she added.
The current study also did not evaluate quantitative differences in bleeding, and participants are using bleeding diaries, Dr. Long noted. She added that participants are able to report problems with bleeding to the study sites, and that these are captured as adverse events.
The study was supported by a partnership between NICHD and FHI 360, a nonprofit human development organization based in North Carolina, with FHI 360 funding from the Bill and Melinda Gates Foundation. Dr. Long had no financial conflicts to disclose.
FROM ACOG 2020
Fertility delay varied with contraceptive method in study
according to a new prospective cohort study.
Women who used hormonal intrauterine devices, copper intrauterine devices, and implants had the shortest delays, based on the same research project, which involved analyzing data from approximately 18,000 women in North America and Denmark.
“Most research on the use of contraceptives and fertility has focused on the effect of oral contraceptives on fecundability,” and data on the association between fertility and other contraceptive methods are limited, wrote Jennifer J. Yland, MS, of Boston University School of Public Health and colleagues.
“Given the increasing popularity of long acting reversible contraceptive methods and other alternatives to oral contraceptives, more research into their short- and long-term effects on fertility is needed,” the researchers noted.
In the study, which was published in the BMJ, the researchers reviewed data from a total of 17,954 women from three cohort studies of individuals planning pregnancies between 2007 and 2019. Participants reported their contraceptive use and typical menstrual cycle at baseline, then responded to questionnaires every 2 months for up to a year or until pregnancy.
On average, users of injectable contraceptives had the longest delay in return of normal fertility (five to eight menstrual cycles), compared with four cycles for patch contraceptives, three cycles for oral and ring contraceptives, and two cycles for hormonal and copper intrauterine devices and implants.
A total of 10,729 pregnancies were reported within 66,759 menstrual cycles; approximately 77% of the women conceived within 12 months, and 56% conceived within 6 months.
Oral contraceptives were the most common method of contraception (38%), followed by barrier methods (31%), natural methods (15%), and long-acting reversible contraceptives (13%). Intrauterine devices were the most frequently used of long-acting reversible contraceptives (8% hormonal, 4% copper).
The time until fertility returned after discontinuing contraceptives was not associated with duration of contraceptive use.
The study findings were limited by several factors including the potential misclassification of menstrual cycles and the use of self-reports for the time of contraceptive discontinuation, especially for users of injectable contraceptives, the researchers noted.
However, the results were strengthened by the large study size and show “little or no lasting effect” of long-term use of any of the reported contraceptive methods on fertility, the researchers noted. “Understanding the comparative effects of different contraceptives on fecundity is essential for family planning, counseling for contraception, and management of infertility,” they said.
Comparison of contraceptives can inform counseling
The study is important because the use of long-acting reversible contraceptive (LARC) methods (IUDs, implants, patches, and injectable contraceptives) has become increasingly common worldwide, corresponding author Jennifer J. Yland, MS, said in an interview. “Many women are concerned about the potential effects of contraception on future fertility. However, previous research on this topic has focused mostly on oral contraceptives,” she said.
Ms. Yland said that the findings on oral and injectable contraceptives were consistent with previous publications. However, “we were surprised to find that women who had recently used the hormonal IUD had a shorter time to pregnancy, compared with women who used barrier methods,” she said.
The take-home message for clinicians is that delays in the return to normal fertility were temporary for all hormonal contraceptive methods, Ms. Yland emphasized. “However, delays in the return of fertility after discontinuing certain hormonal methods, such as injectables, were considerably longer than that shown for oral contraceptives. These findings should be taken into account when women are considering contraceptive choice in the context of family planning and infertility management,” she noted.
“Future research should evaluate the potential associations between recent use of hormonal contraceptives and perinatal outcomes,” she added.
Managing expectations helps patients plan
“The question of return to fertility is one that many patients who use contraception have unless they have completed their child bearing,” said Sarah W. Prager, MD, of the University of Washington, Seattle, in an interview. “For patients who want to plan a pregnancy, knowing what to expect in terms of return to fertility is important so they can make sure they are in the space and place they want to be with their health, life, job, and partner,” she said.
Dr. Prager said she was not surprised by the study findings because they agree with previously published data. “Overall, except for the injection, people using any form of contraception are back to their baseline fertility within a few months,” she noted. “It also makes perfect sense for return to fertility to be longer with the injection, as it is designed to prevent pregnancy for 16 weeks after the injection is given. Unlike all the other methods, it cannot be removed from the body once given,” she said.
“Clinicians should continue to advise patients that their return to baseline fertility is relatively rapid with any contraception other than the Depo-Provera injection,” said Dr. Prager. “There are no data to support a benefit in switching from an IUD or implant to a combination hormonal method (pills, patch, ring) before starting to try to conceive,” she said.
“This study tries to account for differences in baseline fertility for people using the different methods, but since the choice of method was not randomized, there could still be baseline differences that were not measured or accounted for,” Dr. Prager noted. “A randomized study would certainly eliminate some of these biases; however, I don’t think the differences found in this study are so profound as to require such study,” she said. “Generally speaking, almost 80% of people using any form of contraception were able to conceive within 1 year of trying, which has been the stated fertility data for decades,” she said.
The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health. Lead author Ms. Yland had no financial conflicts to disclose. Dr. Prager had no financial conflicts to disclose.
SOURCE: Yland JJ et al. BMJ. 2020 Nov 12. doi: 10.1136/bmj.m3966.
according to a new prospective cohort study.
Women who used hormonal intrauterine devices, copper intrauterine devices, and implants had the shortest delays, based on the same research project, which involved analyzing data from approximately 18,000 women in North America and Denmark.
“Most research on the use of contraceptives and fertility has focused on the effect of oral contraceptives on fecundability,” and data on the association between fertility and other contraceptive methods are limited, wrote Jennifer J. Yland, MS, of Boston University School of Public Health and colleagues.
“Given the increasing popularity of long acting reversible contraceptive methods and other alternatives to oral contraceptives, more research into their short- and long-term effects on fertility is needed,” the researchers noted.
In the study, which was published in the BMJ, the researchers reviewed data from a total of 17,954 women from three cohort studies of individuals planning pregnancies between 2007 and 2019. Participants reported their contraceptive use and typical menstrual cycle at baseline, then responded to questionnaires every 2 months for up to a year or until pregnancy.
On average, users of injectable contraceptives had the longest delay in return of normal fertility (five to eight menstrual cycles), compared with four cycles for patch contraceptives, three cycles for oral and ring contraceptives, and two cycles for hormonal and copper intrauterine devices and implants.
A total of 10,729 pregnancies were reported within 66,759 menstrual cycles; approximately 77% of the women conceived within 12 months, and 56% conceived within 6 months.
Oral contraceptives were the most common method of contraception (38%), followed by barrier methods (31%), natural methods (15%), and long-acting reversible contraceptives (13%). Intrauterine devices were the most frequently used of long-acting reversible contraceptives (8% hormonal, 4% copper).
The time until fertility returned after discontinuing contraceptives was not associated with duration of contraceptive use.
The study findings were limited by several factors including the potential misclassification of menstrual cycles and the use of self-reports for the time of contraceptive discontinuation, especially for users of injectable contraceptives, the researchers noted.
However, the results were strengthened by the large study size and show “little or no lasting effect” of long-term use of any of the reported contraceptive methods on fertility, the researchers noted. “Understanding the comparative effects of different contraceptives on fecundity is essential for family planning, counseling for contraception, and management of infertility,” they said.
Comparison of contraceptives can inform counseling
The study is important because the use of long-acting reversible contraceptive (LARC) methods (IUDs, implants, patches, and injectable contraceptives) has become increasingly common worldwide, corresponding author Jennifer J. Yland, MS, said in an interview. “Many women are concerned about the potential effects of contraception on future fertility. However, previous research on this topic has focused mostly on oral contraceptives,” she said.
Ms. Yland said that the findings on oral and injectable contraceptives were consistent with previous publications. However, “we were surprised to find that women who had recently used the hormonal IUD had a shorter time to pregnancy, compared with women who used barrier methods,” she said.
The take-home message for clinicians is that delays in the return to normal fertility were temporary for all hormonal contraceptive methods, Ms. Yland emphasized. “However, delays in the return of fertility after discontinuing certain hormonal methods, such as injectables, were considerably longer than that shown for oral contraceptives. These findings should be taken into account when women are considering contraceptive choice in the context of family planning and infertility management,” she noted.
“Future research should evaluate the potential associations between recent use of hormonal contraceptives and perinatal outcomes,” she added.
Managing expectations helps patients plan
“The question of return to fertility is one that many patients who use contraception have unless they have completed their child bearing,” said Sarah W. Prager, MD, of the University of Washington, Seattle, in an interview. “For patients who want to plan a pregnancy, knowing what to expect in terms of return to fertility is important so they can make sure they are in the space and place they want to be with their health, life, job, and partner,” she said.
Dr. Prager said she was not surprised by the study findings because they agree with previously published data. “Overall, except for the injection, people using any form of contraception are back to their baseline fertility within a few months,” she noted. “It also makes perfect sense for return to fertility to be longer with the injection, as it is designed to prevent pregnancy for 16 weeks after the injection is given. Unlike all the other methods, it cannot be removed from the body once given,” she said.
“Clinicians should continue to advise patients that their return to baseline fertility is relatively rapid with any contraception other than the Depo-Provera injection,” said Dr. Prager. “There are no data to support a benefit in switching from an IUD or implant to a combination hormonal method (pills, patch, ring) before starting to try to conceive,” she said.
“This study tries to account for differences in baseline fertility for people using the different methods, but since the choice of method was not randomized, there could still be baseline differences that were not measured or accounted for,” Dr. Prager noted. “A randomized study would certainly eliminate some of these biases; however, I don’t think the differences found in this study are so profound as to require such study,” she said. “Generally speaking, almost 80% of people using any form of contraception were able to conceive within 1 year of trying, which has been the stated fertility data for decades,” she said.
The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health. Lead author Ms. Yland had no financial conflicts to disclose. Dr. Prager had no financial conflicts to disclose.
SOURCE: Yland JJ et al. BMJ. 2020 Nov 12. doi: 10.1136/bmj.m3966.
according to a new prospective cohort study.
Women who used hormonal intrauterine devices, copper intrauterine devices, and implants had the shortest delays, based on the same research project, which involved analyzing data from approximately 18,000 women in North America and Denmark.
“Most research on the use of contraceptives and fertility has focused on the effect of oral contraceptives on fecundability,” and data on the association between fertility and other contraceptive methods are limited, wrote Jennifer J. Yland, MS, of Boston University School of Public Health and colleagues.
“Given the increasing popularity of long acting reversible contraceptive methods and other alternatives to oral contraceptives, more research into their short- and long-term effects on fertility is needed,” the researchers noted.
In the study, which was published in the BMJ, the researchers reviewed data from a total of 17,954 women from three cohort studies of individuals planning pregnancies between 2007 and 2019. Participants reported their contraceptive use and typical menstrual cycle at baseline, then responded to questionnaires every 2 months for up to a year or until pregnancy.
On average, users of injectable contraceptives had the longest delay in return of normal fertility (five to eight menstrual cycles), compared with four cycles for patch contraceptives, three cycles for oral and ring contraceptives, and two cycles for hormonal and copper intrauterine devices and implants.
A total of 10,729 pregnancies were reported within 66,759 menstrual cycles; approximately 77% of the women conceived within 12 months, and 56% conceived within 6 months.
Oral contraceptives were the most common method of contraception (38%), followed by barrier methods (31%), natural methods (15%), and long-acting reversible contraceptives (13%). Intrauterine devices were the most frequently used of long-acting reversible contraceptives (8% hormonal, 4% copper).
The time until fertility returned after discontinuing contraceptives was not associated with duration of contraceptive use.
The study findings were limited by several factors including the potential misclassification of menstrual cycles and the use of self-reports for the time of contraceptive discontinuation, especially for users of injectable contraceptives, the researchers noted.
However, the results were strengthened by the large study size and show “little or no lasting effect” of long-term use of any of the reported contraceptive methods on fertility, the researchers noted. “Understanding the comparative effects of different contraceptives on fecundity is essential for family planning, counseling for contraception, and management of infertility,” they said.
Comparison of contraceptives can inform counseling
The study is important because the use of long-acting reversible contraceptive (LARC) methods (IUDs, implants, patches, and injectable contraceptives) has become increasingly common worldwide, corresponding author Jennifer J. Yland, MS, said in an interview. “Many women are concerned about the potential effects of contraception on future fertility. However, previous research on this topic has focused mostly on oral contraceptives,” she said.
Ms. Yland said that the findings on oral and injectable contraceptives were consistent with previous publications. However, “we were surprised to find that women who had recently used the hormonal IUD had a shorter time to pregnancy, compared with women who used barrier methods,” she said.
The take-home message for clinicians is that delays in the return to normal fertility were temporary for all hormonal contraceptive methods, Ms. Yland emphasized. “However, delays in the return of fertility after discontinuing certain hormonal methods, such as injectables, were considerably longer than that shown for oral contraceptives. These findings should be taken into account when women are considering contraceptive choice in the context of family planning and infertility management,” she noted.
“Future research should evaluate the potential associations between recent use of hormonal contraceptives and perinatal outcomes,” she added.
Managing expectations helps patients plan
“The question of return to fertility is one that many patients who use contraception have unless they have completed their child bearing,” said Sarah W. Prager, MD, of the University of Washington, Seattle, in an interview. “For patients who want to plan a pregnancy, knowing what to expect in terms of return to fertility is important so they can make sure they are in the space and place they want to be with their health, life, job, and partner,” she said.
Dr. Prager said she was not surprised by the study findings because they agree with previously published data. “Overall, except for the injection, people using any form of contraception are back to their baseline fertility within a few months,” she noted. “It also makes perfect sense for return to fertility to be longer with the injection, as it is designed to prevent pregnancy for 16 weeks after the injection is given. Unlike all the other methods, it cannot be removed from the body once given,” she said.
“Clinicians should continue to advise patients that their return to baseline fertility is relatively rapid with any contraception other than the Depo-Provera injection,” said Dr. Prager. “There are no data to support a benefit in switching from an IUD or implant to a combination hormonal method (pills, patch, ring) before starting to try to conceive,” she said.
“This study tries to account for differences in baseline fertility for people using the different methods, but since the choice of method was not randomized, there could still be baseline differences that were not measured or accounted for,” Dr. Prager noted. “A randomized study would certainly eliminate some of these biases; however, I don’t think the differences found in this study are so profound as to require such study,” she said. “Generally speaking, almost 80% of people using any form of contraception were able to conceive within 1 year of trying, which has been the stated fertility data for decades,” she said.
The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health. Lead author Ms. Yland had no financial conflicts to disclose. Dr. Prager had no financial conflicts to disclose.
SOURCE: Yland JJ et al. BMJ. 2020 Nov 12. doi: 10.1136/bmj.m3966.
FROM THE BMJ
2020 Update on contraception
A vaginal ring that can be reused for up to 1 year and a progestin-only pill (POP) with a wider window for missed pills are 2 of the novel contraceptive products introduced to the market this year. In addition, an ongoing study of the levonorgestrel 52-mg intrauterine system (IUS) continues to provide evidence on its extended duration of use, now approved through 6 years.
The segesterone acetate (SA) and ethinyl estradiol (EE) vaginal ring (Annovera) is new among contraceptive options. Segesterone acetate is a novel progestin that can be used only via nonoral routes; it binds specifically to progesterone receptors without estrogenic or antiandrogen effects.1 Unlike the etonogestrel and ethinyl estradiol ring (NuvaRing; for which generic products became available this past year), which is used for 1 cycle and then thrown away, the SA/EE ring is effective for 13 consecutive cycles. It does not require refrigeration when not in use.2 Because a single ring can be used for 13 cycles, users in locations without laws that mandate a 12-month supply of pills, patches, and rings need less frequent visits to the pharmacy or clinic.
Progestin-only contraceptive pills are an important option for patients who desire hormonal contraception and have contraindications to estrogen, such as migraines with aura, cardiovascular risk factors, and being in the early postpartum period.3 In the United States, current POPs contain norethindrone, which has a 3-hour window for missed pills4; a desogestrel-only pill available outside the United States has a 12-hour window.5 Both are provided as a 28-day pill pack for continuous use, and both result in undesirable bleeding patterns in some users.
The prolonged half-life of drospirenone, another progestin, gives it the potential to increase reliability in the setting of missed or delayed pills and improve bleeding patterns. A new POP contraceptive contains drospirenone (Slynd) and is available in a 28-day pack with a 24-day supply of hormone and a 4-day supply of placebo; it provides a window for missed pill use similar to that for combined hormonal contraception (CHC) as well as a placebo period for a timed withdrawal bleed.6,7
Liletta is a well-known levonorgestrel 52-mg IUS that was first approved by the US Food and Drug Administration (FDA) in 2015. An ongoing clinical trial has been the basis for approval of this IUS for use in increasing durations, from 3 years initially to 4 and then 5 years. The newest data indicate efficacy up to 6 years.8
Continue to: Combined hormonal vaginal system provides a year's contraception with an acceptable safety profile...
Combined hormonal vaginal system provides a year's contraception with an acceptable safety profile
Archer DF, Merkatz RB, Bahamondes L, et al. Efficacy of the 1-year (13-cycle) segesterone acetate and ethinylestradiol contraceptive vaginal system: results of two multicentre, open-label, single-arm, phase 3 trials. Lancet Glob Health. 2019;7:e1054-e1064.
Gemzell-Danielsson K, Sitruk-Ware R, Creinin MD, et al. Segesterone acetate/ethinyl estradiol 12-month contraceptive vaginal system safety evaluation. Contraception. 2019;99:323-328.
Archer and colleagues reported the results of 2 pivotal multicenter, open-label, phase 3 trials, which included 2,265 users, conducted to evaluate efficacy and return to menses or pregnancy after use of the 1-year (13 cycles) SA/EE contraceptive vaginal system (CVS).
Details of the efficacy study
The study included 1,130 women in a US-only study and 1,135 women in an international study with sites in the United States, Australia, Brazil, Chile, Dominican Republic, Finland, Hungary, and Sweden. Participants used the CVS for 21 days followed by a 7-day use-free interval for up to 13 consecutive cycles; they were instructed not to remove the CVS for more than 2 hours during the 21 days of use.
Primary and secondary efficacy outcomes were calculated using the Pearl Index and an intention-to-treat Kaplan-Meier life table, respectively. At the end of the study, users who desired not to continue hormonal contraception or to become pregnant were followed up for 6 months to evaluate return to menses or pregnancy.
Year-long effectiveness
The investigators reported an overall Pearl index of 2.98 (95% confidence interval [CI], 2.13-4.06) and a Kaplan-Meier life table cumulative efficacy rate of 97.5% (FIGURE 1), consistent with other recently approved CHC methods. Women from non-European sites, who primarily were US participants, had a Pearl Index of 3.25 (95% CI, 2.35-4.37), and participants from the European sites had a Pearl Index of 0.47 (95% CI, 0.03-2.07). Importantly, CVS removal had a significant impact on efficacy, with a Pearl Index of 5.98 (95% CI, 2.46-9.27) in users reporting CVS removals for longer than 2 hours, suggesting escape ovulation with improper use. The Pearl Index was highest in users aged 18 to 19 years and was not affected by body mass index (BMI), although 91% of users had a BMI of 29.0 kg/m2 or lower.
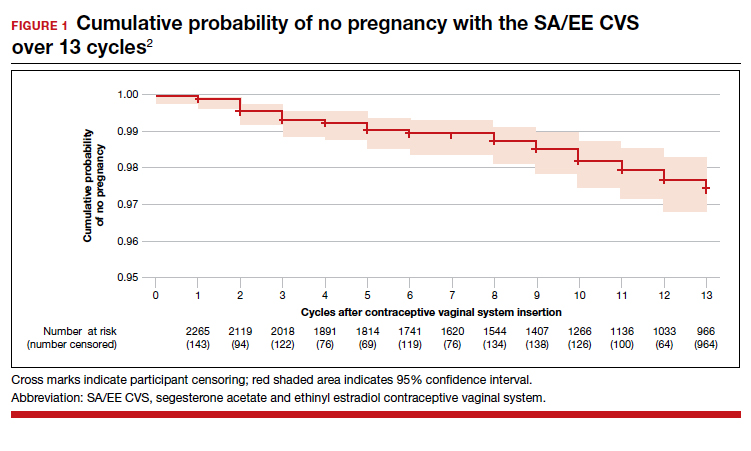
There was no trend for a change in pregnancy risk across 13 cycles, providing evidence of CVS efficacy throughout a full year's use. The follow-up portion of the study included 290 users who were not continuing hormonal contraception at study end; all follow-up participants reported return to menses after method discontinuation.
Clinical safety data
To evaluate safety outcomes from clinical studies on the CVS containing SA/EE, Gemzell-Danielsson and colleagues analyzed 9 studies. Most of the data were derived from 2 phase 3, multicenter trials (as discussed above), with supporting evidence from 7 other studies.
Adverse events reported
Among 2,308 CVS users in the phase 3 trials, 87% reported at least 1 adverse effect, with most of mild or moderate severity. These included headache, 26%; nausea, 18%; vaginal discharge, 10%; and metrorrhagia, 7%. Overall, 12% of CVS users discontinued use due to an adverse effect. Two percent of users experienced severe adverse effects, including venous thromboembolism (VTE), allergic reaction, gallbladder disease, and spontaneous abortion.
In the US-only phase 3 trial, 2 VTE events occurred in the first 6 months in women with baseline BMI greater than 29.0 kg/m2; therefore, enrollment of patients with a BMI greater than 29.0 kg/m2 was halted and current users meeting that criteria were discontinued. Notably, no cases of VTE occurred in studies with a segesterone acetate-only CVS; this suggests that risk can be attributed to the estrogen component. Overall, 4 nonfatal VTEs occurred, all among the 1,536 women enrolled in the phase 3 trials (4 of 1,536 [0.3%]); at least 3 of these cases occurred in users with VTE risk factors (TABLE 1). The estimated VTE rate in CVS users with a BMI greater than 29.0 kg/m2 is 10.8/10,000 women-years (95% CI, 8.9-13.1).
Complete expulsion of the CVS occurred in 7% of cycles and partial expulsion in 19.5% of cycles; users reported expulsion more frequently in the first cycle, most (about 70%) of which were partial expulsions. Of the laboratory values and vital signs studied, including weight, users had no clinically relevant changes from baseline.
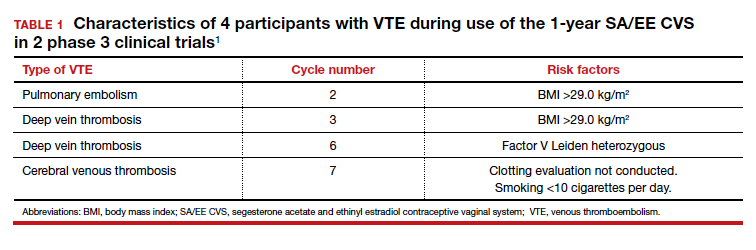
The 13-cycle efficacy and general adverse events rates of the new SA/EE CVS are consistent with those of other CHCs. However, the efficacy and safety findings are not necessarily generalizable to all patients. Because users with a BMI greater than 29.0 kg/m2 were excluded following 2 early VTE events in women with a BMI of 29.1 and 30.8 kg/m2 , only 9% of the phase 3 study population had a BMI greater than 29.0 kg/m2 . Clinicians may question whether the 1-year SA/EE CVS is an acceptable method for obese users. We know that EE causes similar changes in hemostatic factors regardless of oral or vaginal route,9 but these studies as well as pharmacokinetic studies typically include relatively few participants. While studies demonstrate that the SA/EE CVS delivers EE 13 µg daily,1 individual hormone absorption can vary. It is possible that the amount of EE in the CVS (17.4 mg) could, in a person predisposed to higher absorption, increase VTE risk. We do not know if this potential or actual risk is different for nonobese and obese users. To be fair, most of the EE-containing combined hormonal contraceptives were approved with study data that did not include obese women; the FDA first discussed the importance of including obese women in contraceptive approval studies in 2007.10 Thus, we do not know if this CVS has a significantly higher VTE risk in obese users than other methods.
All available information is based on cyclic CVS use (28-day cycles with a 7-day use-free interval). No data are available on drug levels, safety, or efficacy over extended periods of continuous use with the same CVS. During counseling, special emphasis should be placed on the increased pregnancy risk for patients who remove the ring for more than 2 hours.
Continue to: New drospirenone pill is an effective POP option...
New drospirenone pill is an effective POP option
Kimble T, Burke AE, Barnhart KT, et al. A 1-year prospective, open-label, single-arm, multicenter, phase 3 trial of the contraceptive efficacy and safety of the oral progestin-only pill drospirenone 4 mg using a 24/4-day regimen. Contracept X. 2020;2:100020.
Palacios S, Colli E, Regidor PA. Multicenter, phase III trials on the contraceptive efficacy, tolerability and safety of a new drospirenone-only pill. Acta Obstet Gynecol Scand. 2019;98:1549-1557.
In a prospective, single-arm, multicenter phase 3 trial in the United States, Kimble and colleagues evaluated the efficacy and safety of an oral drospirenone POP in a cyclic 24-day hormone/4-day placebo regimen. The trial included 1,006 users. No BMI cutoff was used, and about one-third of study participants were obese (BMI >30.0 kg/m2). Women were instructed to take a missed tablet as soon as remembered if within 24 hours or with the next scheduled dose if more than 24 hours late.
Contraceptive effectiveness
The Pearl Index for nonbreastfeeding users aged 35 years or younger with pregnancies confirmed by a quantitative serum ß-human chorionic gonadotropin test (915 users) was 2.9 (95% CI, 1.5-5.1). Of note, 2 out of 15 on-treatment pregnancies were excluded from this calculation because of protocol site violations, as were 3 pregnancies that were unconfirmed. In the modified full analysis set of 915 users, 36% were obese (BMI≥30 kg/m2), and the Pearl Index was noted to be unaffected by BMI (TABLE 2).

While 61% of women reported adverse effects, more than 95% of these were mild or moderate in intensity, including headache, nausea, dysmenorrhea, metrorrhagia, and breast pain. No VTE occurred. The frequency of hyperkalemia was 0.5%, and there was no evidence of hypotension, which is significant due to the antimineralocorticoid activity of drospirenone. All cases of hyperkalemia were considered mild, and all women were asymptomatic. There were no clinically relevant changes in body weight, gynecologic exam, or other laboratory values.
With increased cycles of use, the number of days with bleeding or spotting generally decreased and amenorrhea increased. However, in cycles 11 to 13, 41.6% of users still had unscheduled bleeding (reduced from 57.0% at cycles 2-4), and 29.0% had scheduled bleeding (decreased from 44% at cycles 2-4) (FIGURE 2). With these bleeding patterns, 86.2% of users agreed or strongly agreed that they were satisfied with the product.
European multicenter study of drospironene
In a European investigation, Palacios and colleagues pooled and analyzed data from 2 phase 3 multicenter trials to assess the efficacy, tolerability, and safety of the same drospirenone-only pill (24 days of drospironene 4 mg and 4 days of placebo) in 1,571 users. No BMI cutoff was used, but overall only 71 participants (4.6%) were obese. One study included desogestrel 0.075 mg (in a regimen of 28 active pills) as a comparator for safety.
The overall Pearl Index for users 35 years or younger (1,251 users) was 1.0 (95% CI, 0.4-2.0). The "method failure Pearl Index" in users 35 years or younger, which included all pregnancies during "perfect medication cycles," was 1.3 (95% CI, 0.5-2.5).
The most common adverse effects were acne (6.6% in study 1 and 4.4% in study 2), headache (4.5% in study 1), and irregular bleeding (4.4% in study 2). No cases of VTE occurred; there was 1 case of asymptomatic hyperkalemia. Additional laboratory values and vital signs showed no significant changes. The trend in bleeding was similar to that in the US studies, but it is interesting to note that there were significantly lower rates of unscheduled bleeding or spotting in drospirenone users than in desogestrel users (67.9% vs 86.5%, respectively; P<.001).
In the US study, the higher Pearl Index compared with that found in the European study (2.9 vs 1.0) likely reflects an increased proportion of study participants with a BMI of 30 kg/m2 or higher, a younger average age of participants, and a historical tendency toward better contraceptive efficacy in European than in US study participants. Kimble and colleagues’ finding of a Pearl Index of 2.9 is similar to that seen with other CHCs and POPs, and the data from the US study are potentially more generalizable.
Among the 2,257 participants in 3 studies, 423 (19%) were obese. No VTE events occurred with drospirenone use, as compared with 4 events in the SA/EE CVS study with 2,308 participants in the phase 3 studies.
Historically, POPs were associated with more days of bleeding than CHCs and require stricter adherence to daily use within a narrow window for missed pills. The new drospirenone-only pill may provide women with more flexibility since it maintains contraceptive efficacy even with 24-hour delayed or missed-pill errors. Although intermenstrual bleeding rates are high, participants still had a very favorable assessment, and the profile may be more tolerable compared with other POPs. Clinicians prescribing this new POP should counsel patients that the cyclic regimen does not always result in regular bleeding patterns.
Continue to: Evidence supports 6 years' use of a levonorgestrel 52-mg IUS...
Evidence supports 6 years' use of a levonorgestrel 52-mg IUS
Westhoff CL, Keder LM, Gangestad A, et al. Six-year contraceptive efficacy and continued safety of a levonorgestrel 52-mg intrauterine system. Contraception. 2020;101:159-161.
Two levonorgestrel 52-mg IUS products are on the market, both of which were approved for 5 years of use. The ACCESS IUS study (A Comprehensive Contraceptive Efficacy and Safety Study of an IUS) is an ongoing phase 3 trial to assess the safety and efficacy of a levonorgestrel 52-mg IUS (Liletta) for up to 10 years of use in US women. Westhoff and colleagues presented the data used for this IUS to gain approval for 6 years of use as of October 2019. The report included safety information for all users, with use exceeding 8 years in 122 participants.
In year 6 of the ongoing trial, there were no on-treatment pregnancies with a 6-year life table pregnancy rate of 0.87 (95% CI, 0.44-1.70). Forty percent of users reported amenorrhea in the 90 days preceding the end of year 6, consistent with prior data after 3 years of use (FIGURE 3). The most common adverse effects over 6 or more years of use were bacterial vulvovaginal infections and urinary tract infections.
Long-term IUS effectiveness
Overall, in users aged 16 to 35 years, 72% discontinued study participation, most frequently due to an adverse event (19.2%) or to seeking pregnancy (15.5%). Through 6 or more years of use, overall discontinuation rates for expulsion (4.0%) and bleeding symptoms (2.3%) were very low, with 2 expulsions occurring in year 6 and only 1 participant discontinuing in year 6 for a bleeding symptom. These findings are consistent with those found at 5 years of IUS use and are representative of continued efficacy as well as overall low frequency of new significant events with extended use.11
Clinicians and patients should be aware of data that support the continued use of levonorgestrel 52-mg IUS products for 6 years, and likely even longer. A low incidence of new significant events and a steady state of amenorrhea are also indications that users who like using a hormonal IUS will likely continue to do so for an extended time, if recommended. This extension, as well as continued study up to 10 years, will allow users who desire reversible long-acting hormonal contraception to have fewer removals and reinsertions; this in turn will decrease the risks and pain associated with IUS insertion and removal as well as health care costs.
- Gemzell-Danielsson K, Sitruk-Ware R, Creinin MD, et al. Segesterone acetate/ethinyl estradiol 12-month contraceptive vaginal system safety evaluation. Contraception. 2019;99:323-328.
- Archer DF, Merkatz RB, Bahamondes L, et al. Efficacy of the 1-year (13-cycle) segesterone acetate and ethinylestradiol contraceptive vaginal system: results of two multicentre, open-label, single-arm, phase 3 trials. Lancet Glob Health. 2019;7:e1054-e1064.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin No. 206. Use of hormonal contraception in women with coexisiting medical conditions. Obstet Gynecol. 2019;133:e128-e150.
- Ortho Micronor [package insert]. Raritan, NJ: Ortho-McNeil Pharmaceutical Inc; 2008.
- Cerazette [package insert]. Oss, Netherlands: Merck Sharp & Dohme Limited; 2019.
- Kimble T, Burke AE, Barnhart KT, et al. A 1-year prospective, open-label, single-arm, multicenter, phase 3 trial of the contraceptive efficacy and safety of the oral progestin-only pill drospirenone 4 mg using a 24/4-day regimen. Contracept X. 2020;2:100020.
- Palacios S, Colli E, Regidor PA. Multicenter, phase III trials on the contraceptive efficacy, tolerability and safety of a new drospirenone-only pill. Acta Obstet Gynecol Scand. 2019;98:1549-1557.
- Westhoff CL, Keder LM, Gangestad A, et al. Six-year contraceptive efficacy and continued safety of a levonorgestrel 52-mg intrauterine system. Contraception. 2020;101:159-161.
- Sitruk-Ware R, Plu-Bureau G, Menard J, et al. Effects of oral and transvaginal ethinyl estradiol on hemostatic factors and hepatic proteins in a randomized, crossover study. J Clin Endocrinol Metab. 2007;92:2074-2079.
- Food and Drug Administration Advisory Committee for Reproductive Health Drugs meeting. Final summary minutes, January 23-24, 2007. https://wayback.archive-it.org/7993/20170404050830/https://www.fda.gov/ohrms/dockets/ac/07/minutes/2007-4274m1.pdf. Accessed July 28, 2020.
- Teal SB, Turok DK, Chen BA, et al. Five-year contraceptive efficacy and safety of a levonorgestrel 52-mg intrauterine system. Obstet Gynecol. 2019;133:63-70.
A vaginal ring that can be reused for up to 1 year and a progestin-only pill (POP) with a wider window for missed pills are 2 of the novel contraceptive products introduced to the market this year. In addition, an ongoing study of the levonorgestrel 52-mg intrauterine system (IUS) continues to provide evidence on its extended duration of use, now approved through 6 years.
The segesterone acetate (SA) and ethinyl estradiol (EE) vaginal ring (Annovera) is new among contraceptive options. Segesterone acetate is a novel progestin that can be used only via nonoral routes; it binds specifically to progesterone receptors without estrogenic or antiandrogen effects.1 Unlike the etonogestrel and ethinyl estradiol ring (NuvaRing; for which generic products became available this past year), which is used for 1 cycle and then thrown away, the SA/EE ring is effective for 13 consecutive cycles. It does not require refrigeration when not in use.2 Because a single ring can be used for 13 cycles, users in locations without laws that mandate a 12-month supply of pills, patches, and rings need less frequent visits to the pharmacy or clinic.
Progestin-only contraceptive pills are an important option for patients who desire hormonal contraception and have contraindications to estrogen, such as migraines with aura, cardiovascular risk factors, and being in the early postpartum period.3 In the United States, current POPs contain norethindrone, which has a 3-hour window for missed pills4; a desogestrel-only pill available outside the United States has a 12-hour window.5 Both are provided as a 28-day pill pack for continuous use, and both result in undesirable bleeding patterns in some users.
The prolonged half-life of drospirenone, another progestin, gives it the potential to increase reliability in the setting of missed or delayed pills and improve bleeding patterns. A new POP contraceptive contains drospirenone (Slynd) and is available in a 28-day pack with a 24-day supply of hormone and a 4-day supply of placebo; it provides a window for missed pill use similar to that for combined hormonal contraception (CHC) as well as a placebo period for a timed withdrawal bleed.6,7
Liletta is a well-known levonorgestrel 52-mg IUS that was first approved by the US Food and Drug Administration (FDA) in 2015. An ongoing clinical trial has been the basis for approval of this IUS for use in increasing durations, from 3 years initially to 4 and then 5 years. The newest data indicate efficacy up to 6 years.8
Continue to: Combined hormonal vaginal system provides a year's contraception with an acceptable safety profile...
Combined hormonal vaginal system provides a year's contraception with an acceptable safety profile
Archer DF, Merkatz RB, Bahamondes L, et al. Efficacy of the 1-year (13-cycle) segesterone acetate and ethinylestradiol contraceptive vaginal system: results of two multicentre, open-label, single-arm, phase 3 trials. Lancet Glob Health. 2019;7:e1054-e1064.
Gemzell-Danielsson K, Sitruk-Ware R, Creinin MD, et al. Segesterone acetate/ethinyl estradiol 12-month contraceptive vaginal system safety evaluation. Contraception. 2019;99:323-328.
Archer and colleagues reported the results of 2 pivotal multicenter, open-label, phase 3 trials, which included 2,265 users, conducted to evaluate efficacy and return to menses or pregnancy after use of the 1-year (13 cycles) SA/EE contraceptive vaginal system (CVS).
Details of the efficacy study
The study included 1,130 women in a US-only study and 1,135 women in an international study with sites in the United States, Australia, Brazil, Chile, Dominican Republic, Finland, Hungary, and Sweden. Participants used the CVS for 21 days followed by a 7-day use-free interval for up to 13 consecutive cycles; they were instructed not to remove the CVS for more than 2 hours during the 21 days of use.
Primary and secondary efficacy outcomes were calculated using the Pearl Index and an intention-to-treat Kaplan-Meier life table, respectively. At the end of the study, users who desired not to continue hormonal contraception or to become pregnant were followed up for 6 months to evaluate return to menses or pregnancy.
Year-long effectiveness
The investigators reported an overall Pearl index of 2.98 (95% confidence interval [CI], 2.13-4.06) and a Kaplan-Meier life table cumulative efficacy rate of 97.5% (FIGURE 1), consistent with other recently approved CHC methods. Women from non-European sites, who primarily were US participants, had a Pearl Index of 3.25 (95% CI, 2.35-4.37), and participants from the European sites had a Pearl Index of 0.47 (95% CI, 0.03-2.07). Importantly, CVS removal had a significant impact on efficacy, with a Pearl Index of 5.98 (95% CI, 2.46-9.27) in users reporting CVS removals for longer than 2 hours, suggesting escape ovulation with improper use. The Pearl Index was highest in users aged 18 to 19 years and was not affected by body mass index (BMI), although 91% of users had a BMI of 29.0 kg/m2 or lower.

There was no trend for a change in pregnancy risk across 13 cycles, providing evidence of CVS efficacy throughout a full year's use. The follow-up portion of the study included 290 users who were not continuing hormonal contraception at study end; all follow-up participants reported return to menses after method discontinuation.
Clinical safety data
To evaluate safety outcomes from clinical studies on the CVS containing SA/EE, Gemzell-Danielsson and colleagues analyzed 9 studies. Most of the data were derived from 2 phase 3, multicenter trials (as discussed above), with supporting evidence from 7 other studies.
Adverse events reported
Among 2,308 CVS users in the phase 3 trials, 87% reported at least 1 adverse effect, with most of mild or moderate severity. These included headache, 26%; nausea, 18%; vaginal discharge, 10%; and metrorrhagia, 7%. Overall, 12% of CVS users discontinued use due to an adverse effect. Two percent of users experienced severe adverse effects, including venous thromboembolism (VTE), allergic reaction, gallbladder disease, and spontaneous abortion.
In the US-only phase 3 trial, 2 VTE events occurred in the first 6 months in women with baseline BMI greater than 29.0 kg/m2; therefore, enrollment of patients with a BMI greater than 29.0 kg/m2 was halted and current users meeting that criteria were discontinued. Notably, no cases of VTE occurred in studies with a segesterone acetate-only CVS; this suggests that risk can be attributed to the estrogen component. Overall, 4 nonfatal VTEs occurred, all among the 1,536 women enrolled in the phase 3 trials (4 of 1,536 [0.3%]); at least 3 of these cases occurred in users with VTE risk factors (TABLE 1). The estimated VTE rate in CVS users with a BMI greater than 29.0 kg/m2 is 10.8/10,000 women-years (95% CI, 8.9-13.1).
Complete expulsion of the CVS occurred in 7% of cycles and partial expulsion in 19.5% of cycles; users reported expulsion more frequently in the first cycle, most (about 70%) of which were partial expulsions. Of the laboratory values and vital signs studied, including weight, users had no clinically relevant changes from baseline.

The 13-cycle efficacy and general adverse events rates of the new SA/EE CVS are consistent with those of other CHCs. However, the efficacy and safety findings are not necessarily generalizable to all patients. Because users with a BMI greater than 29.0 kg/m2 were excluded following 2 early VTE events in women with a BMI of 29.1 and 30.8 kg/m2 , only 9% of the phase 3 study population had a BMI greater than 29.0 kg/m2 . Clinicians may question whether the 1-year SA/EE CVS is an acceptable method for obese users. We know that EE causes similar changes in hemostatic factors regardless of oral or vaginal route,9 but these studies as well as pharmacokinetic studies typically include relatively few participants. While studies demonstrate that the SA/EE CVS delivers EE 13 µg daily,1 individual hormone absorption can vary. It is possible that the amount of EE in the CVS (17.4 mg) could, in a person predisposed to higher absorption, increase VTE risk. We do not know if this potential or actual risk is different for nonobese and obese users. To be fair, most of the EE-containing combined hormonal contraceptives were approved with study data that did not include obese women; the FDA first discussed the importance of including obese women in contraceptive approval studies in 2007.10 Thus, we do not know if this CVS has a significantly higher VTE risk in obese users than other methods.
All available information is based on cyclic CVS use (28-day cycles with a 7-day use-free interval). No data are available on drug levels, safety, or efficacy over extended periods of continuous use with the same CVS. During counseling, special emphasis should be placed on the increased pregnancy risk for patients who remove the ring for more than 2 hours.
Continue to: New drospirenone pill is an effective POP option...
New drospirenone pill is an effective POP option
Kimble T, Burke AE, Barnhart KT, et al. A 1-year prospective, open-label, single-arm, multicenter, phase 3 trial of the contraceptive efficacy and safety of the oral progestin-only pill drospirenone 4 mg using a 24/4-day regimen. Contracept X. 2020;2:100020.
Palacios S, Colli E, Regidor PA. Multicenter, phase III trials on the contraceptive efficacy, tolerability and safety of a new drospirenone-only pill. Acta Obstet Gynecol Scand. 2019;98:1549-1557.
In a prospective, single-arm, multicenter phase 3 trial in the United States, Kimble and colleagues evaluated the efficacy and safety of an oral drospirenone POP in a cyclic 24-day hormone/4-day placebo regimen. The trial included 1,006 users. No BMI cutoff was used, and about one-third of study participants were obese (BMI >30.0 kg/m2). Women were instructed to take a missed tablet as soon as remembered if within 24 hours or with the next scheduled dose if more than 24 hours late.
Contraceptive effectiveness
The Pearl Index for nonbreastfeeding users aged 35 years or younger with pregnancies confirmed by a quantitative serum ß-human chorionic gonadotropin test (915 users) was 2.9 (95% CI, 1.5-5.1). Of note, 2 out of 15 on-treatment pregnancies were excluded from this calculation because of protocol site violations, as were 3 pregnancies that were unconfirmed. In the modified full analysis set of 915 users, 36% were obese (BMI≥30 kg/m2), and the Pearl Index was noted to be unaffected by BMI (TABLE 2).

While 61% of women reported adverse effects, more than 95% of these were mild or moderate in intensity, including headache, nausea, dysmenorrhea, metrorrhagia, and breast pain. No VTE occurred. The frequency of hyperkalemia was 0.5%, and there was no evidence of hypotension, which is significant due to the antimineralocorticoid activity of drospirenone. All cases of hyperkalemia were considered mild, and all women were asymptomatic. There were no clinically relevant changes in body weight, gynecologic exam, or other laboratory values.
With increased cycles of use, the number of days with bleeding or spotting generally decreased and amenorrhea increased. However, in cycles 11 to 13, 41.6% of users still had unscheduled bleeding (reduced from 57.0% at cycles 2-4), and 29.0% had scheduled bleeding (decreased from 44% at cycles 2-4) (FIGURE 2). With these bleeding patterns, 86.2% of users agreed or strongly agreed that they were satisfied with the product.

European multicenter study of drospironene
In a European investigation, Palacios and colleagues pooled and analyzed data from 2 phase 3 multicenter trials to assess the efficacy, tolerability, and safety of the same drospirenone-only pill (24 days of drospironene 4 mg and 4 days of placebo) in 1,571 users. No BMI cutoff was used, but overall only 71 participants (4.6%) were obese. One study included desogestrel 0.075 mg (in a regimen of 28 active pills) as a comparator for safety.
The overall Pearl Index for users 35 years or younger (1,251 users) was 1.0 (95% CI, 0.4-2.0). The "method failure Pearl Index" in users 35 years or younger, which included all pregnancies during "perfect medication cycles," was 1.3 (95% CI, 0.5-2.5).
The most common adverse effects were acne (6.6% in study 1 and 4.4% in study 2), headache (4.5% in study 1), and irregular bleeding (4.4% in study 2). No cases of VTE occurred; there was 1 case of asymptomatic hyperkalemia. Additional laboratory values and vital signs showed no significant changes. The trend in bleeding was similar to that in the US studies, but it is interesting to note that there were significantly lower rates of unscheduled bleeding or spotting in drospirenone users than in desogestrel users (67.9% vs 86.5%, respectively; P<.001).
In the US study, the higher Pearl Index compared with that found in the European study (2.9 vs 1.0) likely reflects an increased proportion of study participants with a BMI of 30 kg/m2 or higher, a younger average age of participants, and a historical tendency toward better contraceptive efficacy in European than in US study participants. Kimble and colleagues’ finding of a Pearl Index of 2.9 is similar to that seen with other CHCs and POPs, and the data from the US study are potentially more generalizable.
Among the 2,257 participants in 3 studies, 423 (19%) were obese. No VTE events occurred with drospirenone use, as compared with 4 events in the SA/EE CVS study with 2,308 participants in the phase 3 studies.
Historically, POPs were associated with more days of bleeding than CHCs and require stricter adherence to daily use within a narrow window for missed pills. The new drospirenone-only pill may provide women with more flexibility since it maintains contraceptive efficacy even with 24-hour delayed or missed-pill errors. Although intermenstrual bleeding rates are high, participants still had a very favorable assessment, and the profile may be more tolerable compared with other POPs. Clinicians prescribing this new POP should counsel patients that the cyclic regimen does not always result in regular bleeding patterns.
Continue to: Evidence supports 6 years' use of a levonorgestrel 52-mg IUS...
Evidence supports 6 years' use of a levonorgestrel 52-mg IUS
Westhoff CL, Keder LM, Gangestad A, et al. Six-year contraceptive efficacy and continued safety of a levonorgestrel 52-mg intrauterine system. Contraception. 2020;101:159-161.
Two levonorgestrel 52-mg IUS products are on the market, both of which were approved for 5 years of use. The ACCESS IUS study (A Comprehensive Contraceptive Efficacy and Safety Study of an IUS) is an ongoing phase 3 trial to assess the safety and efficacy of a levonorgestrel 52-mg IUS (Liletta) for up to 10 years of use in US women. Westhoff and colleagues presented the data used for this IUS to gain approval for 6 years of use as of October 2019. The report included safety information for all users, with use exceeding 8 years in 122 participants.
In year 6 of the ongoing trial, there were no on-treatment pregnancies with a 6-year life table pregnancy rate of 0.87 (95% CI, 0.44-1.70). Forty percent of users reported amenorrhea in the 90 days preceding the end of year 6, consistent with prior data after 3 years of use (FIGURE 3). The most common adverse effects over 6 or more years of use were bacterial vulvovaginal infections and urinary tract infections.

Long-term IUS effectiveness
Overall, in users aged 16 to 35 years, 72% discontinued study participation, most frequently due to an adverse event (19.2%) or to seeking pregnancy (15.5%). Through 6 or more years of use, overall discontinuation rates for expulsion (4.0%) and bleeding symptoms (2.3%) were very low, with 2 expulsions occurring in year 6 and only 1 participant discontinuing in year 6 for a bleeding symptom. These findings are consistent with those found at 5 years of IUS use and are representative of continued efficacy as well as overall low frequency of new significant events with extended use.11
Clinicians and patients should be aware of data that support the continued use of levonorgestrel 52-mg IUS products for 6 years, and likely even longer. A low incidence of new significant events and a steady state of amenorrhea are also indications that users who like using a hormonal IUS will likely continue to do so for an extended time, if recommended. This extension, as well as continued study up to 10 years, will allow users who desire reversible long-acting hormonal contraception to have fewer removals and reinsertions; this in turn will decrease the risks and pain associated with IUS insertion and removal as well as health care costs.
A vaginal ring that can be reused for up to 1 year and a progestin-only pill (POP) with a wider window for missed pills are 2 of the novel contraceptive products introduced to the market this year. In addition, an ongoing study of the levonorgestrel 52-mg intrauterine system (IUS) continues to provide evidence on its extended duration of use, now approved through 6 years.
The segesterone acetate (SA) and ethinyl estradiol (EE) vaginal ring (Annovera) is new among contraceptive options. Segesterone acetate is a novel progestin that can be used only via nonoral routes; it binds specifically to progesterone receptors without estrogenic or antiandrogen effects.1 Unlike the etonogestrel and ethinyl estradiol ring (NuvaRing; for which generic products became available this past year), which is used for 1 cycle and then thrown away, the SA/EE ring is effective for 13 consecutive cycles. It does not require refrigeration when not in use.2 Because a single ring can be used for 13 cycles, users in locations without laws that mandate a 12-month supply of pills, patches, and rings need less frequent visits to the pharmacy or clinic.
Progestin-only contraceptive pills are an important option for patients who desire hormonal contraception and have contraindications to estrogen, such as migraines with aura, cardiovascular risk factors, and being in the early postpartum period.3 In the United States, current POPs contain norethindrone, which has a 3-hour window for missed pills4; a desogestrel-only pill available outside the United States has a 12-hour window.5 Both are provided as a 28-day pill pack for continuous use, and both result in undesirable bleeding patterns in some users.
The prolonged half-life of drospirenone, another progestin, gives it the potential to increase reliability in the setting of missed or delayed pills and improve bleeding patterns. A new POP contraceptive contains drospirenone (Slynd) and is available in a 28-day pack with a 24-day supply of hormone and a 4-day supply of placebo; it provides a window for missed pill use similar to that for combined hormonal contraception (CHC) as well as a placebo period for a timed withdrawal bleed.6,7
Liletta is a well-known levonorgestrel 52-mg IUS that was first approved by the US Food and Drug Administration (FDA) in 2015. An ongoing clinical trial has been the basis for approval of this IUS for use in increasing durations, from 3 years initially to 4 and then 5 years. The newest data indicate efficacy up to 6 years.8
Continue to: Combined hormonal vaginal system provides a year's contraception with an acceptable safety profile...
Combined hormonal vaginal system provides a year's contraception with an acceptable safety profile
Archer DF, Merkatz RB, Bahamondes L, et al. Efficacy of the 1-year (13-cycle) segesterone acetate and ethinylestradiol contraceptive vaginal system: results of two multicentre, open-label, single-arm, phase 3 trials. Lancet Glob Health. 2019;7:e1054-e1064.
Gemzell-Danielsson K, Sitruk-Ware R, Creinin MD, et al. Segesterone acetate/ethinyl estradiol 12-month contraceptive vaginal system safety evaluation. Contraception. 2019;99:323-328.
Archer and colleagues reported the results of 2 pivotal multicenter, open-label, phase 3 trials, which included 2,265 users, conducted to evaluate efficacy and return to menses or pregnancy after use of the 1-year (13 cycles) SA/EE contraceptive vaginal system (CVS).
Details of the efficacy study
The study included 1,130 women in a US-only study and 1,135 women in an international study with sites in the United States, Australia, Brazil, Chile, Dominican Republic, Finland, Hungary, and Sweden. Participants used the CVS for 21 days followed by a 7-day use-free interval for up to 13 consecutive cycles; they were instructed not to remove the CVS for more than 2 hours during the 21 days of use.
Primary and secondary efficacy outcomes were calculated using the Pearl Index and an intention-to-treat Kaplan-Meier life table, respectively. At the end of the study, users who desired not to continue hormonal contraception or to become pregnant were followed up for 6 months to evaluate return to menses or pregnancy.
Year-long effectiveness
The investigators reported an overall Pearl index of 2.98 (95% confidence interval [CI], 2.13-4.06) and a Kaplan-Meier life table cumulative efficacy rate of 97.5% (FIGURE 1), consistent with other recently approved CHC methods. Women from non-European sites, who primarily were US participants, had a Pearl Index of 3.25 (95% CI, 2.35-4.37), and participants from the European sites had a Pearl Index of 0.47 (95% CI, 0.03-2.07). Importantly, CVS removal had a significant impact on efficacy, with a Pearl Index of 5.98 (95% CI, 2.46-9.27) in users reporting CVS removals for longer than 2 hours, suggesting escape ovulation with improper use. The Pearl Index was highest in users aged 18 to 19 years and was not affected by body mass index (BMI), although 91% of users had a BMI of 29.0 kg/m2 or lower.

There was no trend for a change in pregnancy risk across 13 cycles, providing evidence of CVS efficacy throughout a full year's use. The follow-up portion of the study included 290 users who were not continuing hormonal contraception at study end; all follow-up participants reported return to menses after method discontinuation.
Clinical safety data
To evaluate safety outcomes from clinical studies on the CVS containing SA/EE, Gemzell-Danielsson and colleagues analyzed 9 studies. Most of the data were derived from 2 phase 3, multicenter trials (as discussed above), with supporting evidence from 7 other studies.
Adverse events reported
Among 2,308 CVS users in the phase 3 trials, 87% reported at least 1 adverse effect, with most of mild or moderate severity. These included headache, 26%; nausea, 18%; vaginal discharge, 10%; and metrorrhagia, 7%. Overall, 12% of CVS users discontinued use due to an adverse effect. Two percent of users experienced severe adverse effects, including venous thromboembolism (VTE), allergic reaction, gallbladder disease, and spontaneous abortion.
In the US-only phase 3 trial, 2 VTE events occurred in the first 6 months in women with baseline BMI greater than 29.0 kg/m2; therefore, enrollment of patients with a BMI greater than 29.0 kg/m2 was halted and current users meeting that criteria were discontinued. Notably, no cases of VTE occurred in studies with a segesterone acetate-only CVS; this suggests that risk can be attributed to the estrogen component. Overall, 4 nonfatal VTEs occurred, all among the 1,536 women enrolled in the phase 3 trials (4 of 1,536 [0.3%]); at least 3 of these cases occurred in users with VTE risk factors (TABLE 1). The estimated VTE rate in CVS users with a BMI greater than 29.0 kg/m2 is 10.8/10,000 women-years (95% CI, 8.9-13.1).
Complete expulsion of the CVS occurred in 7% of cycles and partial expulsion in 19.5% of cycles; users reported expulsion more frequently in the first cycle, most (about 70%) of which were partial expulsions. Of the laboratory values and vital signs studied, including weight, users had no clinically relevant changes from baseline.

The 13-cycle efficacy and general adverse events rates of the new SA/EE CVS are consistent with those of other CHCs. However, the efficacy and safety findings are not necessarily generalizable to all patients. Because users with a BMI greater than 29.0 kg/m2 were excluded following 2 early VTE events in women with a BMI of 29.1 and 30.8 kg/m2 , only 9% of the phase 3 study population had a BMI greater than 29.0 kg/m2 . Clinicians may question whether the 1-year SA/EE CVS is an acceptable method for obese users. We know that EE causes similar changes in hemostatic factors regardless of oral or vaginal route,9 but these studies as well as pharmacokinetic studies typically include relatively few participants. While studies demonstrate that the SA/EE CVS delivers EE 13 µg daily,1 individual hormone absorption can vary. It is possible that the amount of EE in the CVS (17.4 mg) could, in a person predisposed to higher absorption, increase VTE risk. We do not know if this potential or actual risk is different for nonobese and obese users. To be fair, most of the EE-containing combined hormonal contraceptives were approved with study data that did not include obese women; the FDA first discussed the importance of including obese women in contraceptive approval studies in 2007.10 Thus, we do not know if this CVS has a significantly higher VTE risk in obese users than other methods.
All available information is based on cyclic CVS use (28-day cycles with a 7-day use-free interval). No data are available on drug levels, safety, or efficacy over extended periods of continuous use with the same CVS. During counseling, special emphasis should be placed on the increased pregnancy risk for patients who remove the ring for more than 2 hours.
Continue to: New drospirenone pill is an effective POP option...
New drospirenone pill is an effective POP option
Kimble T, Burke AE, Barnhart KT, et al. A 1-year prospective, open-label, single-arm, multicenter, phase 3 trial of the contraceptive efficacy and safety of the oral progestin-only pill drospirenone 4 mg using a 24/4-day regimen. Contracept X. 2020;2:100020.
Palacios S, Colli E, Regidor PA. Multicenter, phase III trials on the contraceptive efficacy, tolerability and safety of a new drospirenone-only pill. Acta Obstet Gynecol Scand. 2019;98:1549-1557.
In a prospective, single-arm, multicenter phase 3 trial in the United States, Kimble and colleagues evaluated the efficacy and safety of an oral drospirenone POP in a cyclic 24-day hormone/4-day placebo regimen. The trial included 1,006 users. No BMI cutoff was used, and about one-third of study participants were obese (BMI >30.0 kg/m2). Women were instructed to take a missed tablet as soon as remembered if within 24 hours or with the next scheduled dose if more than 24 hours late.
Contraceptive effectiveness
The Pearl Index for nonbreastfeeding users aged 35 years or younger with pregnancies confirmed by a quantitative serum ß-human chorionic gonadotropin test (915 users) was 2.9 (95% CI, 1.5-5.1). Of note, 2 out of 15 on-treatment pregnancies were excluded from this calculation because of protocol site violations, as were 3 pregnancies that were unconfirmed. In the modified full analysis set of 915 users, 36% were obese (BMI≥30 kg/m2), and the Pearl Index was noted to be unaffected by BMI (TABLE 2).

While 61% of women reported adverse effects, more than 95% of these were mild or moderate in intensity, including headache, nausea, dysmenorrhea, metrorrhagia, and breast pain. No VTE occurred. The frequency of hyperkalemia was 0.5%, and there was no evidence of hypotension, which is significant due to the antimineralocorticoid activity of drospirenone. All cases of hyperkalemia were considered mild, and all women were asymptomatic. There were no clinically relevant changes in body weight, gynecologic exam, or other laboratory values.
With increased cycles of use, the number of days with bleeding or spotting generally decreased and amenorrhea increased. However, in cycles 11 to 13, 41.6% of users still had unscheduled bleeding (reduced from 57.0% at cycles 2-4), and 29.0% had scheduled bleeding (decreased from 44% at cycles 2-4) (FIGURE 2). With these bleeding patterns, 86.2% of users agreed or strongly agreed that they were satisfied with the product.

European multicenter study of drospironene
In a European investigation, Palacios and colleagues pooled and analyzed data from 2 phase 3 multicenter trials to assess the efficacy, tolerability, and safety of the same drospirenone-only pill (24 days of drospironene 4 mg and 4 days of placebo) in 1,571 users. No BMI cutoff was used, but overall only 71 participants (4.6%) were obese. One study included desogestrel 0.075 mg (in a regimen of 28 active pills) as a comparator for safety.
The overall Pearl Index for users 35 years or younger (1,251 users) was 1.0 (95% CI, 0.4-2.0). The "method failure Pearl Index" in users 35 years or younger, which included all pregnancies during "perfect medication cycles," was 1.3 (95% CI, 0.5-2.5).
The most common adverse effects were acne (6.6% in study 1 and 4.4% in study 2), headache (4.5% in study 1), and irregular bleeding (4.4% in study 2). No cases of VTE occurred; there was 1 case of asymptomatic hyperkalemia. Additional laboratory values and vital signs showed no significant changes. The trend in bleeding was similar to that in the US studies, but it is interesting to note that there were significantly lower rates of unscheduled bleeding or spotting in drospirenone users than in desogestrel users (67.9% vs 86.5%, respectively; P<.001).
In the US study, the higher Pearl Index compared with that found in the European study (2.9 vs 1.0) likely reflects an increased proportion of study participants with a BMI of 30 kg/m2 or higher, a younger average age of participants, and a historical tendency toward better contraceptive efficacy in European than in US study participants. Kimble and colleagues’ finding of a Pearl Index of 2.9 is similar to that seen with other CHCs and POPs, and the data from the US study are potentially more generalizable.
Among the 2,257 participants in 3 studies, 423 (19%) were obese. No VTE events occurred with drospirenone use, as compared with 4 events in the SA/EE CVS study with 2,308 participants in the phase 3 studies.
Historically, POPs were associated with more days of bleeding than CHCs and require stricter adherence to daily use within a narrow window for missed pills. The new drospirenone-only pill may provide women with more flexibility since it maintains contraceptive efficacy even with 24-hour delayed or missed-pill errors. Although intermenstrual bleeding rates are high, participants still had a very favorable assessment, and the profile may be more tolerable compared with other POPs. Clinicians prescribing this new POP should counsel patients that the cyclic regimen does not always result in regular bleeding patterns.
Continue to: Evidence supports 6 years' use of a levonorgestrel 52-mg IUS...
Evidence supports 6 years' use of a levonorgestrel 52-mg IUS
Westhoff CL, Keder LM, Gangestad A, et al. Six-year contraceptive efficacy and continued safety of a levonorgestrel 52-mg intrauterine system. Contraception. 2020;101:159-161.
Two levonorgestrel 52-mg IUS products are on the market, both of which were approved for 5 years of use. The ACCESS IUS study (A Comprehensive Contraceptive Efficacy and Safety Study of an IUS) is an ongoing phase 3 trial to assess the safety and efficacy of a levonorgestrel 52-mg IUS (Liletta) for up to 10 years of use in US women. Westhoff and colleagues presented the data used for this IUS to gain approval for 6 years of use as of October 2019. The report included safety information for all users, with use exceeding 8 years in 122 participants.
In year 6 of the ongoing trial, there were no on-treatment pregnancies with a 6-year life table pregnancy rate of 0.87 (95% CI, 0.44-1.70). Forty percent of users reported amenorrhea in the 90 days preceding the end of year 6, consistent with prior data after 3 years of use (FIGURE 3). The most common adverse effects over 6 or more years of use were bacterial vulvovaginal infections and urinary tract infections.

Long-term IUS effectiveness
Overall, in users aged 16 to 35 years, 72% discontinued study participation, most frequently due to an adverse event (19.2%) or to seeking pregnancy (15.5%). Through 6 or more years of use, overall discontinuation rates for expulsion (4.0%) and bleeding symptoms (2.3%) were very low, with 2 expulsions occurring in year 6 and only 1 participant discontinuing in year 6 for a bleeding symptom. These findings are consistent with those found at 5 years of IUS use and are representative of continued efficacy as well as overall low frequency of new significant events with extended use.11
Clinicians and patients should be aware of data that support the continued use of levonorgestrel 52-mg IUS products for 6 years, and likely even longer. A low incidence of new significant events and a steady state of amenorrhea are also indications that users who like using a hormonal IUS will likely continue to do so for an extended time, if recommended. This extension, as well as continued study up to 10 years, will allow users who desire reversible long-acting hormonal contraception to have fewer removals and reinsertions; this in turn will decrease the risks and pain associated with IUS insertion and removal as well as health care costs.
- Gemzell-Danielsson K, Sitruk-Ware R, Creinin MD, et al. Segesterone acetate/ethinyl estradiol 12-month contraceptive vaginal system safety evaluation. Contraception. 2019;99:323-328.
- Archer DF, Merkatz RB, Bahamondes L, et al. Efficacy of the 1-year (13-cycle) segesterone acetate and ethinylestradiol contraceptive vaginal system: results of two multicentre, open-label, single-arm, phase 3 trials. Lancet Glob Health. 2019;7:e1054-e1064.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin No. 206. Use of hormonal contraception in women with coexisiting medical conditions. Obstet Gynecol. 2019;133:e128-e150.
- Ortho Micronor [package insert]. Raritan, NJ: Ortho-McNeil Pharmaceutical Inc; 2008.
- Cerazette [package insert]. Oss, Netherlands: Merck Sharp & Dohme Limited; 2019.
- Kimble T, Burke AE, Barnhart KT, et al. A 1-year prospective, open-label, single-arm, multicenter, phase 3 trial of the contraceptive efficacy and safety of the oral progestin-only pill drospirenone 4 mg using a 24/4-day regimen. Contracept X. 2020;2:100020.
- Palacios S, Colli E, Regidor PA. Multicenter, phase III trials on the contraceptive efficacy, tolerability and safety of a new drospirenone-only pill. Acta Obstet Gynecol Scand. 2019;98:1549-1557.
- Westhoff CL, Keder LM, Gangestad A, et al. Six-year contraceptive efficacy and continued safety of a levonorgestrel 52-mg intrauterine system. Contraception. 2020;101:159-161.
- Sitruk-Ware R, Plu-Bureau G, Menard J, et al. Effects of oral and transvaginal ethinyl estradiol on hemostatic factors and hepatic proteins in a randomized, crossover study. J Clin Endocrinol Metab. 2007;92:2074-2079.
- Food and Drug Administration Advisory Committee for Reproductive Health Drugs meeting. Final summary minutes, January 23-24, 2007. https://wayback.archive-it.org/7993/20170404050830/https://www.fda.gov/ohrms/dockets/ac/07/minutes/2007-4274m1.pdf. Accessed July 28, 2020.
- Teal SB, Turok DK, Chen BA, et al. Five-year contraceptive efficacy and safety of a levonorgestrel 52-mg intrauterine system. Obstet Gynecol. 2019;133:63-70.
- Gemzell-Danielsson K, Sitruk-Ware R, Creinin MD, et al. Segesterone acetate/ethinyl estradiol 12-month contraceptive vaginal system safety evaluation. Contraception. 2019;99:323-328.
- Archer DF, Merkatz RB, Bahamondes L, et al. Efficacy of the 1-year (13-cycle) segesterone acetate and ethinylestradiol contraceptive vaginal system: results of two multicentre, open-label, single-arm, phase 3 trials. Lancet Glob Health. 2019;7:e1054-e1064.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin No. 206. Use of hormonal contraception in women with coexisiting medical conditions. Obstet Gynecol. 2019;133:e128-e150.
- Ortho Micronor [package insert]. Raritan, NJ: Ortho-McNeil Pharmaceutical Inc; 2008.
- Cerazette [package insert]. Oss, Netherlands: Merck Sharp & Dohme Limited; 2019.
- Kimble T, Burke AE, Barnhart KT, et al. A 1-year prospective, open-label, single-arm, multicenter, phase 3 trial of the contraceptive efficacy and safety of the oral progestin-only pill drospirenone 4 mg using a 24/4-day regimen. Contracept X. 2020;2:100020.
- Palacios S, Colli E, Regidor PA. Multicenter, phase III trials on the contraceptive efficacy, tolerability and safety of a new drospirenone-only pill. Acta Obstet Gynecol Scand. 2019;98:1549-1557.
- Westhoff CL, Keder LM, Gangestad A, et al. Six-year contraceptive efficacy and continued safety of a levonorgestrel 52-mg intrauterine system. Contraception. 2020;101:159-161.
- Sitruk-Ware R, Plu-Bureau G, Menard J, et al. Effects of oral and transvaginal ethinyl estradiol on hemostatic factors and hepatic proteins in a randomized, crossover study. J Clin Endocrinol Metab. 2007;92:2074-2079.
- Food and Drug Administration Advisory Committee for Reproductive Health Drugs meeting. Final summary minutes, January 23-24, 2007. https://wayback.archive-it.org/7993/20170404050830/https://www.fda.gov/ohrms/dockets/ac/07/minutes/2007-4274m1.pdf. Accessed July 28, 2020.
- Teal SB, Turok DK, Chen BA, et al. Five-year contraceptive efficacy and safety of a levonorgestrel 52-mg intrauterine system. Obstet Gynecol. 2019;133:63-70.
The latest US Supreme Court decisions on contraception, transgender discrimination, more
The 2019-2020 term of the US Supreme Court was remarkable by any standard. An extraordinary number of important cases made it “a buffet of blockbusters.”1
We look first at several cases that will be of particular interest to ObGyns. Then we look briefly at a number of other important cases that affect the medical profession as a whole and the direction of the country (see “Other significant US Supreme Court decisions”), and finally we conclude with an analysis of this term and a forecast for the next.
We chose cases in which specialty organizations, such as the American College of Obstetricians and Gynecologists (ACOG), or organized medicine (the American Medical Association [AMA], the Association of American Medical Colleges [AAMC], or the American Hospital Association [AHA]), took a special interest by filing “amicus curiae” (friend of the court) briefs with the Supreme Court. These briefs are filed by an organization or person who is not a party to the case but who may have important information to convey to the Court. Because these briefs represent a significant commitment of money, time, and effort, they are usually not undertaken lightly.
Decisions concerning abortion
June v Russo
Decided June 29, 2020, June v Russo involved a Louisiana statute that required abortion providers have “active admitting privileges at a hospital” within 30 miles of where the abortion is performed.2 The Court decided a case in 2016 (from Texas) that involved almost the same statutory provision, so it might seem like an easy ruling.3 But Justice Kennedy (the deciding vote in 2016) has been replaced by Justice Gorsuch, so the outcome was uncertain. It was a difficult case, with a total of 5 opinions covering 138 pages and a “surprise” from the Chief Justice.
The Court, in a 5-4 decision, struck down the Louisiana law, but there was no majority opinion. Four justices in the plurality emphasized that the Louisiana law (like the Texas law) substantially burdened the right to abortion without any corresponding benefit to the health of the women seeking abortions. (Under earlier Court precedents, “undue burdens” on abortion are unconstitutional.4) Justice Breyer noted that the state could not present even one example in which a woman would have had better treatment if her doctor had admitting privileges. For a variety of reasons, admitting privileges were cumbersome for abortion providers to obtain; therefore, enforcing the law had little or no benefit, but significant risk of reduced availability of abortion services.
In June v Russo, Chief Justice Roberts literally became the “swing vote”—the fifth vote to strike down the Louisiana law. In 2016, he had voted the other way—to uphold essentially the same law (in Texas) that he struck down here. He attributed his switch to precedent (the general obligation of courts to follow prior decisions). He disagreed with the earlier decision, but felt bound by it.
This should be the end of the abortion provider “hospital privileges requirements” that a number of states have passed. States seeking to nibble away at abortion rights will undoubtedly look elsewhere. Beyond that, it is difficult, from this case, to discern the future of abortion rights.
ACOG was the lead in amicus briefs urging the Court to strike down the Louisiana law. ACOG (with others) was one of only a handful of organizations filing a brief urging the Court to agree to hear the case.5 When the Court did agree to hear the case (“granted certiorari”), ACOG and a number of other medical organizations filed a formal amicus brief on the merits of the case.6 The brief made 2 arguments: First, that this case was essentially decided in Whole Woman’s Health in 2016 (the Texas case) and, second, that “an admitting privileges requirement is not medically necessary” and “clinicians who provide abortions are unable to obtain admitting privileges for reasons unrelated to their ability to safely and competently perform abortions.” Justice Breyer cited the ACOG brief twice.
The American Association of Pro-Life Obstetricians and Gynecologists also filed an amicus brief.7 The brief was directed solely at arguing that ACOG was not presenting reliable science. It summarized, “The American College of Obstetricians and Gynecologists has always presented itself to the Court as a source of objective medical knowledge. However, when it comes to abortion, the College today is primarily a pro-abortion political advocacy organization.” That brief concluded that the “Court should read ACOG’s amicus brief not as an authoritative recitation of settled science, but as a partisan advocacy paper on behalf of a mere subset of American obstetricians and gynecologists.”
The Association of American Physicians and Surgeons (which should not be confused with the “National Board of Physicians and Surgeons”) also filed an amicus brief. The brief argued, “Abortion, like other outpatient surgical procedures, sometimes results in patient hospitalization. Requiring abortion providers to maintain admitting privileges will improve communication between physicians in the transfer of patients to the hospital and allow them to participate in the care of their patients while in the hospital, in line with their ethical duty to ensure their patients’ continuity of care.”8
Continue to: Ultrasonography requirement for abortion...
Ultrasonography requirement for abortion
In another abortion case, the Court was asked to review a Kentucky abortion statute requiring that an ultrasound image be shown to the woman as part of informed consent for an abortion.9 ACOG filed an amicus brief in favor of a review, but the Court declined to hear the case.10,11
Contraception considerations
The Affordable Care Act (ACA) has an ambiguous provision regarding no-cost “preventive care and screenings” for women. The ACA does not, however, specify contraceptive coverage.12 Several departments and the Health Resources and Services Administration (collectively referred to as “HRSA”) interpreted the provision to include contraception, but from the start there were religious objections. HRSA eventually provided an exemption regarding contraception for employers (nonprofits and for-profits with no publicly traded components) that had “sincerely held moral” objections to providing forms of contraceptive coverage. That regulation was again before the Court this term in Little Sisters of the Poor Saints Peter and Paul Home v Pennsylvania.13
In a 7-2 decision, the Court held that the ACA gave HRSA authority to adopt regulations related to the undefined term “preventive care.” Therefore, it found that HRSA could exempt those with religious objections from participation in providing contraceptive coverage. ACOG and other medical groups filed an amicus brief arguing that contraception is an essential preventive service. “Contraception not only helps to prevent unintended pregnancy, but also helps to protect the health and well-being of women and their children.”14 It was cited only by Justice Ginsburg in her dissent.15
Deferred Action for Childhood Arrivals (DACA)
The AAMC, ACOG, AMA, and many other organizations filed an amicus brief16 in Department of Homeland Security v Regents of University of California.17 The case raised the question of whether a decision to end the DACA program followed the appropriate administrative procedures. In 2012, the Obama administration issued a “memorandum” establishing DACA (without congressional approval or formal rulemaking). A lower court decision barring implementation of DACA was upheld by the Supreme Court in 2016 on a 4-4 vote.18 In 2017, the Trump administration moved to end DACA.
In a 5-4 decision, the Court held that the explanation for ending DACA was inadequate, and violated the Administrative Procedures Act, so DACA could continue until the administration redid the repeal, following the proper procedures. The decision of the Court dealt solely with the process by which the rescission took place—there was general agreement that the administration had the right to rescind it if the procedure (with legitimate reasons) was proper.
The brief for the medical groups argued that the failure of the regulation to consider “reliance interests” would have especially difficult consequences in the medical fields. It noted, “At this moment, an estimated 27,000 health care workers and support staff depend on DACA for their authorization to work in the United States. Among those 27,000 are nurses, dentists, pharmacists, physician assistants, home health aides, technicians, and others. The number also includes nearly 200 medical students, medical residents, and physicians who depend on DACA for their eligibility to practice medicine.”16 The brief was not cited by the Court, but the reliance interest the brief spoke about was an important part of the case.
Continue to: Employment discrimination against gay and transgender employees...
Employment discrimination against gay and transgender employees
Federal law (“Title VII”) makes it illegal for an employer to “discriminate against any individual because of race, color, religion, sex, or national origin.”19 The question this term was whether discrimination based on sexual orientation or sexual identity is within the statute’s meaning of “sex.” By a 6-3 majority, the Court held that Title VII applies both to orientation and identity. (This was an interpretation of the statute, not a broad constitutional ruling.)
The majority reasoned that “it is impossible to discriminate against a person for being homosexual or transgender without discriminating against that individual based on sex. Consider, for example, an employer with 2 employees, both of whom are attracted to men.” If the employer fires the gay employee, “the employer discriminates against him for traits or actions it tolerates in his female colleague.”20
AMA and a number of other medical organizations filed an amicus brief in the case.21 The core of the argument of the brief was, “Employment discrimination against transgender people frustrates the treatment of gender dysphoria by preventing transgender individuals from living openly in accordance with their true gender identity and impeding access to needed medical care. Experiencing discrimination in one of the most important aspects of adult life—employment—makes it nearly impossible to live in full congruence with one’s gender identity. The fear of facing such discrimination alone can prompt transgender individuals to hide their gender identity, directly thwarting the goal of social transition…. Lack of treatment, in turn, increases the rate of negative mental health outcomes, substance abuse, and suicide.” The brief was not cited in the opinions in the case.
This decision is likely to have great impact on many aspects of American life. In the employment area, it is now a matter of course that employers may not discriminate based on orientation or identity in any employment decisions including hiring, firing, compensation, fringe benefits, etc. Harassment based on identity or orientation may similarly be an employment law violation. The decision also likely means that giving employment preferences to gay employees would now be as illegal as would be giving preferences to straight employees. (Limited exceptions, notably to some religious organization employees, are not included in anti-discrimination laws.)22
The importance of the decision goes well beyond employment, however. More than 100 federal statutes are in place that prohibit “discrimination because of sex.” It is now likely that these statutes will be interpreted as prohibiting discrimination related to sexual orientation and identification.
Additional cases of interest
HIV/AIDS International Program
A major US program fighting HIV/AIDS worldwide—the United States Leadership Against HIV/AIDS, Tuberculosis, and Malaria Act (aka the Leadership Act)—has provided billions of dollars to agencies abroad.23 Nongovernmental organizations (NGOs) receiving funds under the program must agree to have a “policy explicitly opposing prostitution and sex trafficking” (known as the “Policy Requirement”). Some grant recipients in foreign countries, generally affiliates of US NGOs, do not want to have such a policy and challenged the policy requirement as a violation of First Amendment right of free speech. The Court held that it is a well-settled principle that “foreign citizens outside US territory do not possess rights under the US Constitution.”24 Nor do organizations become entitled to such rights as a result of an affiliation with US organizations. This decision means that foreign organizations are free not to have the required policies, but they will be ineligible for funds under the Leadership Act.
Continue to: ACA government debts edition...
ACA government debts edition
The ACA was before the Court, yet again. To encourage private insurers to participate in online health insurance exchanges, the ACA provided that the federal government would share in insurance company losses for 3 years.25 The Act, however, did not appropriate any money for these “risk corridors,” and insurance companies lost $12 billion.
Congress (after the 2010 election) prohibited any appropriated funds from being used to pay insurance companies for their risk corridor losses. Four insurance companies sued the United States, seeking reimbursements for their losses. This term the Court held that the government must pay for their losses under the ACA.26 The Court said that Congress could have expressly repealed the risk corridor obligation (in the appropriation bill), but instead had only prohibited the expenditure of the money, which the Court said did not amount to an implied repeal of the obligation. We will see that ACA will be back before the Court again next term in California v Texas (discussed below).
Child custody and international abduction
The Hague Convention on the Civil Aspects of International Child Abduction (to which the United States is a party) provides that the courts of the country where the child has “habitual residence” have jurisdiction to decide custody.27 If a parent takes the child to another country, that country is obligated to return the child to the country of “habitual residence.”
This term the Court was called upon to define “habitual residence.” The Court held that determining habitual residence depends on the “totality of the circumstances,” and that “locating a child’s home is a fact-driven inquiry,” and that “courts must be sensitive to the unique circumstances of the case and informed by common sense.”28 An exception to the Convention’s obligation to return a child to the country of habitual residence is where “there is a grave risk that [the] return would expose the child to physical or psychological harm or otherwise place the child in an intolerable situation.”29 Who the parent is can affect many aspects of legal authority over the child, including consent to medical care, and the right to receive information concerning care.
Analysis of the term
The term began October 7, 2019, and adjourned July 9, 2020, somewhat later than usual because of coronavirus disease 2019 (COVID-19). During the term, the Court decided 60 cases, including 53 “signed” merit opinions after oral argument—the lowest number of decided cases in many years.30 Of those 60 cases, 22 (35%) were unanimous, and 13 (22%) resulted in a 5-4 split.30 Ten-year averages are 48% unanimous and 20% with 5-4 decisions.30
Chief Justice Roberts was the central focus of the term. He presided over the impeachment trial of President Trump in the Senate early in the term. He also presided over the Court’s accommodations of the COVID-19 pandemic. He is the “median,” or “swing,” justice. He was in the majority in 12 of the 13 cases with 5-4 decisions.30 He was in the majority in 97% of all cases and in 95% of “divided cases”—the highest of any of the justices this term.30 In some of the most critical decisions, Chief Justice Roberts sided with the “liberal” wing, including on cases concerning abortion, gay and transgender employment, DACA, and 2 Presidential subpoena cases. More often (in 9 of the 5-4 decisions), however, he sided with the more conservative justices.30 Justice Kavanaugh agreed with Chief Justice Roberts most often (in 93% of all cases).30 Among the others, these justices agreed with each other 90% or more of the time: Justices Ginsburg and Breyer (93%), Justices Alito and Thomas (92%), and Justices Breyer and Kagan (90%).30
Continue to: COVID-19 and the Court...
COVID-19 and the Court
Some of the biggest news of the term came not from the law, but from medicine in the form of COVID-19. The Court was in the process of preparing a final period of important arguments when, on March 16, it announced that it was postponing further arguments. The Court rescheduled 10 oral arguments that were held by telephone (other cases were held over to the next term). The phone arguments, during the first 2 weeks of May, necessitated a change in format. Each justice was called on (in order of seniority) by the Chief Justice to ask questions. This was in contrast to the free-for-all questions that usually characterize in-person arguments. These arguments were broadcast live—something that had never been done before. Public access was, on balance, a good thing. There were a couple failures to unmute, and there was “the flush heard round the world” in the middle of one argument, but otherwise the arguments went off with few hitches.31
Looking ahead
By the end of the term, no justice had announced an intention to retire from the Court. On September 18, however, Justice Ruth Bader Ginsburg passed away. In 2009, she had been diagnosed with early-stage pancreatic cancer. This term she had been hospitalized twice, and at the end of the term, she announced a recurrence of pancreatic cancer, which was being treated with chemotherapy. See “RBG: The woman, the legacy” for a tribute to this remarkable woman, lawyer, and justice.
Justice Ginsburg’s death, occurring in the middle of a presidential campaign, ignited a political firestorm concerning her successor. The outcome of selecting and confirming her successor and the political fallout were not immediately apparent. Justice Ginsburg was confirmed just 7 weeks after her nomination by President Clinton, by a vote of 96-3. But those days of Senate consensus are not the current norm.
The next term (called the “October 2020 Term”) will begin on October 5, 2020. The Court will begin with 8 justices and, depending on the nomination process, may operate with 8 justices for some time. When there is a “tie” vote in the Court, the lower court decision is upheld. The Court has been short-handed several times in the past and, with few exceptions, has managed the cases successfully.
The Court has announced that initial arguments will be telephonic. It already has taken a number of cases. The constitutionality of the individual mandate (coverage) in the ACA will once again be before the Court, and that already has produced a flood of amicus briefs from health-related organizations.32 Among other upcoming issues are cases related to state regulation of pharmacy benefit managers, gay rights and foster care, sentencing of juveniles to life in prison without the possibility of parole, a face-off between Google and Oracle on software copyrights, and arbitration. In addition, some of the issues we saw this term will reappear, with more on robocalls, religious freedom and Catholic charities, and immigration and removal cases.
Ruth Bader Ginsburg, as a law student, law professor, lawyer, judge, and justice, was a leading advocate for the rights of women. There were only a few women in law school when she attended, but she graduated tied for first in her class. Although she found it difficult to be hired as a lawyer, as a law professor and lawyer she helped map a strategy to expand legal rights for women, arguing 6 cases before the Supreme Court and winning 5 of them. She served as a federal appeals court judge and then was appointed to the Supreme Court in 1993. She was the second woman to serve on the Court.
As a justice, she was known during much of her tenure on the Court as the leader of the liberal justices, although her jurisprudence was more complex than that simple statement. She was always a strong advocate for the rights of women (and equal rights of men) during her time on the Court. She was a very clear writer; her opinions were direct and easy to understand. She was also fast—she routinely had the record of announcing opinions faster than any of the other current justices. She was 87 when she passed away, having served on the Court for 27 years.
Justice Ginsburg was also something of a cultural phenomenon. In later years she was sometimes known as “the Notorious RBG.” Books, movies, songs, and even workout videos were made about her. In groups she seemed almost shy, but she was thoughtful, kind, and funny (sometimes wickedly so). The outpouring of affection and sympathy at her death was a symbol of the place she held in America. She loved the opera, a passion she shared with her friend, Justice Antonin Scalia. Despite their considerable disagreements on legal matters, Justices Ginsburg and Scalia were close friends. They attended opera with one another, and their families usually spent New Year’s Eves together. They were the 2 most recent justices to pass away while serving on the Court.
The Court heard and ruled on a large number of other significant cases that will have consequences for many years to come. Highlights include:
- In 2 cases involving subpoenas for the President’s personal records, the Court suggested some balance between “nobody is above the law” and not unnecessarily hectoring or interfering with fulfilling the office of President. The Court held that Congress may subpoena a President’s personal and family records, while the President is still in office.1 It instructed lower courts to assess whether the papers are necessary, the subpoena is limited in scope, there is legitimate legislative purpose, whether the burden it imposes on the President is reasonable, and whether the subpoena would unduly interfere with the ability to do the work required as President.
- Similarly, local (state) grand juries may subpoena such personal records, but the President will have the opportunity to raise specific objections to the subpoenas—undue burden, bad faith, or overbreadth. In addition, the respect owed to the office should inform the conduct regarding the subpoena.2
- The Court upheld a federal law that prohibits most robocalls.3 It struck down an amendment that allowed robocalls made to collect debts owed to or guaranteed by the federal government.
- The Court held that a single-director federal agency, whose director cannot be removed by the President (at will), violates the Constitution.4 The Consumer Financial Protection Bureau (created by the Dodd-Frank law) has such a single, no-removal director and that will have to be modified.
- The Court held that the eastern half of Oklahoma (including Tulsa) is part of a Creek Nation reservation.5 This was a question of criminal law jurisdiction, not property ownership. The practical effect is that for crimes involving Native Americans, serious crimes will have to be tried in federal court, while lesser crimes may be tried in tribal courts.
- The Court determined that it was unconstitutional for a state program providing tuition assistance to parents who send their children to private schools, to prohibit students attending religious private schools from participating in the program. That is a burden on the “free exercise” of religion.6
- The Court considered whether there can be civil liability for damages caused by a federal official in the United States harming a foreign national in another country. In this case, a border patrol agent standing in the US shot and killed a Mexican juvenile who was just across the border in Mexico.7 The issue was whether the parents of the Mexican national could sue the US officials for damages. The Court declined to expand liability to include those injured outside the US. Ultimately, the Court was reluctant to impose liability because this liability is not authorized by Congress.
- In a COVID-19 religion case, the Court refused to stop the enforcement of a governor’s COVID-19 order that allowed churches to operate with <100 attendees or 25% occupancy (whichever was lower).8 Meanwhile, businesses, malls, and stores were allowed to reopen without these stringent limitations. The church objected that greater burdens were placed on religion than secular activity. The Court denied the church’s request for an injunction.
- The Court unanimously held that a state may punish or remove a “faithless elector.” Electors cast votes on behalf of their states in the Electoral College—where Presidents are technically selected. Electors are generally pledged to vote for the winner of a state’s vote for President. A few have violated that pledge and voted for someone else. As a practical matter, that could cause real disruption, and the Court upheld state laws that take action against these “faithless” electors.9
- Several days after the Court had officially adjourned for the term, it received several petitions to delay the execution of federal prisoners. One case was based on the method of execution (use of pentobarbital),10 and another was based on the claim that a prisoner had become so mentally incompetent that it was improper to execute him.11 The Court turned down these appeals, allowing the executions to proceed. These were the first federal government executions in 17 years.
References
- Trump v Mazars USA, LLP, 140 S. Ct. 2019 (2020).
- Trump v Vance, 140 S. Ct. 2412 (2020).
- Barr v American Association of Political Consultants, Inc, 140 S. Ct. 2335 (2020).
- Seila Law LLC v Consumer Financial Protection Bureau, 140 S. Ct. 2183 (2020).
- McGirt v Oklahoma, 140 S. Ct. 2452 (2020).
- Espinoza v Montana Department of Revenue, 140 S. Ct. 2246 (2020).
- Hernández v Mesa, 140 S. Ct. 735, 206 L. Ed. 2d 29 (2020).
- South Bay United Pentecostal Church v Newsom, 140 S. Ct. 1613, 207 L. Ed. 2d 154 (2020).
- Chiafalo v Washington, 140 S. Ct. 2316 (2020).
- Barr v Lee, ____ S. Ct. ____ (2020).
- Barr v Purkey, ____ S. Ct. ____ (2020).
1. Liptak A. In a term full of major cases, the Supreme Court tacked to the center. The New York Times. July 10, 2020.
2. June Medical Services LLC v Russo, 591 US 140 S. Ct. 2103, 2112 (2020).
3. Whole Woman’s Health v Hellerstedt, 579 US ___ (2016).
4. Planned Parenthood of Southeastern PA v Casey, 505 US 833, 874 (1992).
5. Brief of the American College of Obstetricians and Gynecologists, the American Academy of Family Physicians, the American Academy of Pediatrics, the American College of Nurse-Midwives, the American College of Osteopathic Obstetricians and Gynecologists, the American College of Physicians, the American Society for Reproductive Medicine, the National Association of Nurse Practitioners in Women’s Health, the North American Society for Pediatric and Adolescent Gynecology, and the Society for Maternal-Fetal Medicine Amici Curiae In Support of Petitioners, June Medical Services v Russo. May 20, 2019. https://www.supremecourt.gov/DocketPDF/18/18-1323/100434/20190520175434029_18-1323%20ACOG%20et%20al.%20cert.%20amicus%20brief.pdf. Accessed August 31, 2020.
6. Brief of Amici Curiae American College of Obstetricians and Gynecologists, American Medical Association, American Academy of Family Physicians, American Academy of Nursing, American Academy of Pediatrics, American College of Nurse-Midwives, American College of Osteopathic Obstetricians and Gynecologists, American College of Physicians, American Osteopathic Association, American Public Health Association, American Society for Reproductive Medicine, North American Society for Pediatric and Adolescent Gynecology, Society for Maternal-Fetal Medicine, and the Society of Ob/Gyn Hospitalists, In Support of June Medical Services, June Medical Services v Russo. December 2, 2019. https://www.supremecourt.gov/DocketPDF/18/18-1323/124091/20191202145531124_18-1323%2018-1460%20tsac%20American%20College%20of%20Obstetricians%20and%20
Gynecologists%20et%20al.pdf [https://perma.cc/8T8V-4D6S]. Accessed August 31, 2020.
7. Brief of Amicus Curiae American Association of Pro-Life Obstetricians and Gynecologists In Support of [Russo] Louisiana Department of Health and Hospitals, June Medical Services v Russo. December 27, 2019. https://www.supremecourt.gov/DocketPDF/18/18-1323/126927/20191227154424488_AAPLOG%20Amicus%20Brief.pdf. Accessed August 31, 2020.
8. Brief of Association of American Physicians and Surgeons as Amicus Curiae in Support of Respondent–Cross-Petitioner, June Medical Services v Russo 2. December 27, 2019. https://www.supremecourt.gov/DocketPDF/18/18-1323/126828/20191227104605915_18-1323%20-1460%20bsac%20AAPS--PDFA.pdf. Accessed August 31, 2020.
9. Ky. Rev. Stat. § 311.727(2).
10. Brief for the American College of Obstetricians and Gynecologists, the American Medical Association, the North American Society for Pediatric and Adolescent Gynecology, the American College of Osteopathic Obstetricians and Gynecologists, and the American Academy of Family Physicians Amici Curiae Supporting Petitioners, EMW Women’s Surgical Center v Meier. October 28, 2019. https://www.supremecourt.gov/DocketPDF/19/19-417/120550/20191028184956458_19-417%20ACOG%20et%20al.%20-%20cert.%20amicus%20brief.pdf. Accessed August 31, 2020.
11. EMW Women’s Surgical Center, PSC v Meier, 140 S. Ct. 655 (2019).
12. Codified at 26 U. S. C. §5000A(f )(2); §§4980H(a), (c)(2) requires employers to provide women with “preventive care and screenings” without “any cost sharing requirements.”
13. Little Sisters of the Poor Saints Peter and Paul Home v Pennsylvania, 140 S. Ct. 2367 (2020).
14. Brief of Amici Curiae American College of Obstetricians and Gynecologists, American Nurses Association, American Academy of Nursing, Physicians for Reproductive Health, and Nurses for Sexual and Reproductive Health, In Support of Respondents and Affirmance, Little Sisters of the Poor Saints Peter and Paul Home v Pennsylvania. April 8, 2020. https://www.supremecourt.gov/DocketPDF/19/19-431/141177/20200408152340136_19-431%20and%2019-454%20Amici%20Curiae.pdf. Accessed August 31, 2020.
15. Little Sisters of the Poor Saints Peter and Paul Home v Pennsylvania, 140 S. Ct. 2367, 2400–12 (2020).
16. Brief for the Association of American Medical Colleges (and more than 30 other organizations, including the American Medical Association, and the American College of Obstetricians and Gynecologists) Amici Curiae, In Support of Respondents, Department of Homeland Security v Regents of University of California. October 4, 2019. https://www.supremecourt.gov/DocketPDF/18/18-587/118129/20191004130646281_Brief%20for%20AAMC%20et%20al%20
Supporting%20Respondents.pdf. Accessed August 31, 2020.
17. Department of Homeland Security v Regents of The University of California, 140 S. Ct. 1891 (2020).
18. United States v Texas, 136 S. Ct. 2271 (2016).
19. Title VII of the Civil Rights Act of 1964, 42 U.S.C §2000e–2(a)(1).
20. Bostock v Clayton County, 140 S. Ct. 1731, 1741 (2020).
21. Brief of the American Medical Association, the American College of Physicians, and 14 additional medical, mental health, and health care organizations as Amici Curiae In Support of the Employees, Bostock v Clayton County. July 3, 2019. https://www.supremecourt.gov/DocketPDF/17/17-1618/107177/20190703172548842_Amicus%20Brief.pdf. Accessed August 31, 2020.
22. Our Lady of Guadalupe School v Morrissey-Berru, 140 S. Ct. 2049 (2020).
23. United States Leadership Against HIV/AIDS, Tuberculosis, and Malaria Act (“the Leadership Act”), 22 U. S. C. §7601 et seq.
24. Agency for International Development v Alliance for Open Society, 140 S. Ct. 2082, 2086 (2020).
25. 42 U.S.C. §1342, §18063.
26. Maine Community Health Options v United States, 140 S. Ct. 1308 (2020).
27. Hague Convention on the Civil Aspects of International Child Abduction (Hague Convention or Convention), implemented in the United States by the International Child Abduction Remedies Act, 22 U. S. C. §9001 et seq.
28. Monasky v Taglieri, 140 S. Ct. 719 (2020).
29. Monasky v Taglieri, 140 S. Ct. 719, 723, 729 (2020).
30. Feldman A. Final stat pack for October term 2019 (upated). July 10, 2020. https://www.scotusblog.com/2020/07/final-stat-pack-for-october-term-2019/. Accessed August 31, 2020.
31. Hejmanowski D. Flush heard around the world. Delaware Gazette. May 8, 2020. https://www.delgazette.com/opinion/columns/83610/flush-heard-around-the-world. Accessed August 31, 2020.
32. SCOTUSblog.com. California v Texas. https://www.scotusblog.com/case-files/cases/california-v-texas/. Accessed August 31, 2020.
The 2019-2020 term of the US Supreme Court was remarkable by any standard. An extraordinary number of important cases made it “a buffet of blockbusters.”1
We look first at several cases that will be of particular interest to ObGyns. Then we look briefly at a number of other important cases that affect the medical profession as a whole and the direction of the country (see “Other significant US Supreme Court decisions”), and finally we conclude with an analysis of this term and a forecast for the next.
We chose cases in which specialty organizations, such as the American College of Obstetricians and Gynecologists (ACOG), or organized medicine (the American Medical Association [AMA], the Association of American Medical Colleges [AAMC], or the American Hospital Association [AHA]), took a special interest by filing “amicus curiae” (friend of the court) briefs with the Supreme Court. These briefs are filed by an organization or person who is not a party to the case but who may have important information to convey to the Court. Because these briefs represent a significant commitment of money, time, and effort, they are usually not undertaken lightly.
Decisions concerning abortion
June v Russo
Decided June 29, 2020, June v Russo involved a Louisiana statute that required abortion providers have “active admitting privileges at a hospital” within 30 miles of where the abortion is performed.2 The Court decided a case in 2016 (from Texas) that involved almost the same statutory provision, so it might seem like an easy ruling.3 But Justice Kennedy (the deciding vote in 2016) has been replaced by Justice Gorsuch, so the outcome was uncertain. It was a difficult case, with a total of 5 opinions covering 138 pages and a “surprise” from the Chief Justice.
The Court, in a 5-4 decision, struck down the Louisiana law, but there was no majority opinion. Four justices in the plurality emphasized that the Louisiana law (like the Texas law) substantially burdened the right to abortion without any corresponding benefit to the health of the women seeking abortions. (Under earlier Court precedents, “undue burdens” on abortion are unconstitutional.4) Justice Breyer noted that the state could not present even one example in which a woman would have had better treatment if her doctor had admitting privileges. For a variety of reasons, admitting privileges were cumbersome for abortion providers to obtain; therefore, enforcing the law had little or no benefit, but significant risk of reduced availability of abortion services.
In June v Russo, Chief Justice Roberts literally became the “swing vote”—the fifth vote to strike down the Louisiana law. In 2016, he had voted the other way—to uphold essentially the same law (in Texas) that he struck down here. He attributed his switch to precedent (the general obligation of courts to follow prior decisions). He disagreed with the earlier decision, but felt bound by it.
This should be the end of the abortion provider “hospital privileges requirements” that a number of states have passed. States seeking to nibble away at abortion rights will undoubtedly look elsewhere. Beyond that, it is difficult, from this case, to discern the future of abortion rights.
ACOG was the lead in amicus briefs urging the Court to strike down the Louisiana law. ACOG (with others) was one of only a handful of organizations filing a brief urging the Court to agree to hear the case.5 When the Court did agree to hear the case (“granted certiorari”), ACOG and a number of other medical organizations filed a formal amicus brief on the merits of the case.6 The brief made 2 arguments: First, that this case was essentially decided in Whole Woman’s Health in 2016 (the Texas case) and, second, that “an admitting privileges requirement is not medically necessary” and “clinicians who provide abortions are unable to obtain admitting privileges for reasons unrelated to their ability to safely and competently perform abortions.” Justice Breyer cited the ACOG brief twice.
The American Association of Pro-Life Obstetricians and Gynecologists also filed an amicus brief.7 The brief was directed solely at arguing that ACOG was not presenting reliable science. It summarized, “The American College of Obstetricians and Gynecologists has always presented itself to the Court as a source of objective medical knowledge. However, when it comes to abortion, the College today is primarily a pro-abortion political advocacy organization.” That brief concluded that the “Court should read ACOG’s amicus brief not as an authoritative recitation of settled science, but as a partisan advocacy paper on behalf of a mere subset of American obstetricians and gynecologists.”
The Association of American Physicians and Surgeons (which should not be confused with the “National Board of Physicians and Surgeons”) also filed an amicus brief. The brief argued, “Abortion, like other outpatient surgical procedures, sometimes results in patient hospitalization. Requiring abortion providers to maintain admitting privileges will improve communication between physicians in the transfer of patients to the hospital and allow them to participate in the care of their patients while in the hospital, in line with their ethical duty to ensure their patients’ continuity of care.”8
Continue to: Ultrasonography requirement for abortion...
Ultrasonography requirement for abortion
In another abortion case, the Court was asked to review a Kentucky abortion statute requiring that an ultrasound image be shown to the woman as part of informed consent for an abortion.9 ACOG filed an amicus brief in favor of a review, but the Court declined to hear the case.10,11
Contraception considerations
The Affordable Care Act (ACA) has an ambiguous provision regarding no-cost “preventive care and screenings” for women. The ACA does not, however, specify contraceptive coverage.12 Several departments and the Health Resources and Services Administration (collectively referred to as “HRSA”) interpreted the provision to include contraception, but from the start there were religious objections. HRSA eventually provided an exemption regarding contraception for employers (nonprofits and for-profits with no publicly traded components) that had “sincerely held moral” objections to providing forms of contraceptive coverage. That regulation was again before the Court this term in Little Sisters of the Poor Saints Peter and Paul Home v Pennsylvania.13
In a 7-2 decision, the Court held that the ACA gave HRSA authority to adopt regulations related to the undefined term “preventive care.” Therefore, it found that HRSA could exempt those with religious objections from participation in providing contraceptive coverage. ACOG and other medical groups filed an amicus brief arguing that contraception is an essential preventive service. “Contraception not only helps to prevent unintended pregnancy, but also helps to protect the health and well-being of women and their children.”14 It was cited only by Justice Ginsburg in her dissent.15
Deferred Action for Childhood Arrivals (DACA)
The AAMC, ACOG, AMA, and many other organizations filed an amicus brief16 in Department of Homeland Security v Regents of University of California.17 The case raised the question of whether a decision to end the DACA program followed the appropriate administrative procedures. In 2012, the Obama administration issued a “memorandum” establishing DACA (without congressional approval or formal rulemaking). A lower court decision barring implementation of DACA was upheld by the Supreme Court in 2016 on a 4-4 vote.18 In 2017, the Trump administration moved to end DACA.
In a 5-4 decision, the Court held that the explanation for ending DACA was inadequate, and violated the Administrative Procedures Act, so DACA could continue until the administration redid the repeal, following the proper procedures. The decision of the Court dealt solely with the process by which the rescission took place—there was general agreement that the administration had the right to rescind it if the procedure (with legitimate reasons) was proper.
The brief for the medical groups argued that the failure of the regulation to consider “reliance interests” would have especially difficult consequences in the medical fields. It noted, “At this moment, an estimated 27,000 health care workers and support staff depend on DACA for their authorization to work in the United States. Among those 27,000 are nurses, dentists, pharmacists, physician assistants, home health aides, technicians, and others. The number also includes nearly 200 medical students, medical residents, and physicians who depend on DACA for their eligibility to practice medicine.”16 The brief was not cited by the Court, but the reliance interest the brief spoke about was an important part of the case.
Continue to: Employment discrimination against gay and transgender employees...
Employment discrimination against gay and transgender employees
Federal law (“Title VII”) makes it illegal for an employer to “discriminate against any individual because of race, color, religion, sex, or national origin.”19 The question this term was whether discrimination based on sexual orientation or sexual identity is within the statute’s meaning of “sex.” By a 6-3 majority, the Court held that Title VII applies both to orientation and identity. (This was an interpretation of the statute, not a broad constitutional ruling.)
The majority reasoned that “it is impossible to discriminate against a person for being homosexual or transgender without discriminating against that individual based on sex. Consider, for example, an employer with 2 employees, both of whom are attracted to men.” If the employer fires the gay employee, “the employer discriminates against him for traits or actions it tolerates in his female colleague.”20
AMA and a number of other medical organizations filed an amicus brief in the case.21 The core of the argument of the brief was, “Employment discrimination against transgender people frustrates the treatment of gender dysphoria by preventing transgender individuals from living openly in accordance with their true gender identity and impeding access to needed medical care. Experiencing discrimination in one of the most important aspects of adult life—employment—makes it nearly impossible to live in full congruence with one’s gender identity. The fear of facing such discrimination alone can prompt transgender individuals to hide their gender identity, directly thwarting the goal of social transition…. Lack of treatment, in turn, increases the rate of negative mental health outcomes, substance abuse, and suicide.” The brief was not cited in the opinions in the case.
This decision is likely to have great impact on many aspects of American life. In the employment area, it is now a matter of course that employers may not discriminate based on orientation or identity in any employment decisions including hiring, firing, compensation, fringe benefits, etc. Harassment based on identity or orientation may similarly be an employment law violation. The decision also likely means that giving employment preferences to gay employees would now be as illegal as would be giving preferences to straight employees. (Limited exceptions, notably to some religious organization employees, are not included in anti-discrimination laws.)22
The importance of the decision goes well beyond employment, however. More than 100 federal statutes are in place that prohibit “discrimination because of sex.” It is now likely that these statutes will be interpreted as prohibiting discrimination related to sexual orientation and identification.
Additional cases of interest
HIV/AIDS International Program
A major US program fighting HIV/AIDS worldwide—the United States Leadership Against HIV/AIDS, Tuberculosis, and Malaria Act (aka the Leadership Act)—has provided billions of dollars to agencies abroad.23 Nongovernmental organizations (NGOs) receiving funds under the program must agree to have a “policy explicitly opposing prostitution and sex trafficking” (known as the “Policy Requirement”). Some grant recipients in foreign countries, generally affiliates of US NGOs, do not want to have such a policy and challenged the policy requirement as a violation of First Amendment right of free speech. The Court held that it is a well-settled principle that “foreign citizens outside US territory do not possess rights under the US Constitution.”24 Nor do organizations become entitled to such rights as a result of an affiliation with US organizations. This decision means that foreign organizations are free not to have the required policies, but they will be ineligible for funds under the Leadership Act.
Continue to: ACA government debts edition...
ACA government debts edition
The ACA was before the Court, yet again. To encourage private insurers to participate in online health insurance exchanges, the ACA provided that the federal government would share in insurance company losses for 3 years.25 The Act, however, did not appropriate any money for these “risk corridors,” and insurance companies lost $12 billion.
Congress (after the 2010 election) prohibited any appropriated funds from being used to pay insurance companies for their risk corridor losses. Four insurance companies sued the United States, seeking reimbursements for their losses. This term the Court held that the government must pay for their losses under the ACA.26 The Court said that Congress could have expressly repealed the risk corridor obligation (in the appropriation bill), but instead had only prohibited the expenditure of the money, which the Court said did not amount to an implied repeal of the obligation. We will see that ACA will be back before the Court again next term in California v Texas (discussed below).
Child custody and international abduction
The Hague Convention on the Civil Aspects of International Child Abduction (to which the United States is a party) provides that the courts of the country where the child has “habitual residence” have jurisdiction to decide custody.27 If a parent takes the child to another country, that country is obligated to return the child to the country of “habitual residence.”
This term the Court was called upon to define “habitual residence.” The Court held that determining habitual residence depends on the “totality of the circumstances,” and that “locating a child’s home is a fact-driven inquiry,” and that “courts must be sensitive to the unique circumstances of the case and informed by common sense.”28 An exception to the Convention’s obligation to return a child to the country of habitual residence is where “there is a grave risk that [the] return would expose the child to physical or psychological harm or otherwise place the child in an intolerable situation.”29 Who the parent is can affect many aspects of legal authority over the child, including consent to medical care, and the right to receive information concerning care.
Analysis of the term
The term began October 7, 2019, and adjourned July 9, 2020, somewhat later than usual because of coronavirus disease 2019 (COVID-19). During the term, the Court decided 60 cases, including 53 “signed” merit opinions after oral argument—the lowest number of decided cases in many years.30 Of those 60 cases, 22 (35%) were unanimous, and 13 (22%) resulted in a 5-4 split.30 Ten-year averages are 48% unanimous and 20% with 5-4 decisions.30
Chief Justice Roberts was the central focus of the term. He presided over the impeachment trial of President Trump in the Senate early in the term. He also presided over the Court’s accommodations of the COVID-19 pandemic. He is the “median,” or “swing,” justice. He was in the majority in 12 of the 13 cases with 5-4 decisions.30 He was in the majority in 97% of all cases and in 95% of “divided cases”—the highest of any of the justices this term.30 In some of the most critical decisions, Chief Justice Roberts sided with the “liberal” wing, including on cases concerning abortion, gay and transgender employment, DACA, and 2 Presidential subpoena cases. More often (in 9 of the 5-4 decisions), however, he sided with the more conservative justices.30 Justice Kavanaugh agreed with Chief Justice Roberts most often (in 93% of all cases).30 Among the others, these justices agreed with each other 90% or more of the time: Justices Ginsburg and Breyer (93%), Justices Alito and Thomas (92%), and Justices Breyer and Kagan (90%).30
Continue to: COVID-19 and the Court...
COVID-19 and the Court
Some of the biggest news of the term came not from the law, but from medicine in the form of COVID-19. The Court was in the process of preparing a final period of important arguments when, on March 16, it announced that it was postponing further arguments. The Court rescheduled 10 oral arguments that were held by telephone (other cases were held over to the next term). The phone arguments, during the first 2 weeks of May, necessitated a change in format. Each justice was called on (in order of seniority) by the Chief Justice to ask questions. This was in contrast to the free-for-all questions that usually characterize in-person arguments. These arguments were broadcast live—something that had never been done before. Public access was, on balance, a good thing. There were a couple failures to unmute, and there was “the flush heard round the world” in the middle of one argument, but otherwise the arguments went off with few hitches.31
Looking ahead
By the end of the term, no justice had announced an intention to retire from the Court. On September 18, however, Justice Ruth Bader Ginsburg passed away. In 2009, she had been diagnosed with early-stage pancreatic cancer. This term she had been hospitalized twice, and at the end of the term, she announced a recurrence of pancreatic cancer, which was being treated with chemotherapy. See “RBG: The woman, the legacy” for a tribute to this remarkable woman, lawyer, and justice.
Justice Ginsburg’s death, occurring in the middle of a presidential campaign, ignited a political firestorm concerning her successor. The outcome of selecting and confirming her successor and the political fallout were not immediately apparent. Justice Ginsburg was confirmed just 7 weeks after her nomination by President Clinton, by a vote of 96-3. But those days of Senate consensus are not the current norm.
The next term (called the “October 2020 Term”) will begin on October 5, 2020. The Court will begin with 8 justices and, depending on the nomination process, may operate with 8 justices for some time. When there is a “tie” vote in the Court, the lower court decision is upheld. The Court has been short-handed several times in the past and, with few exceptions, has managed the cases successfully.
The Court has announced that initial arguments will be telephonic. It already has taken a number of cases. The constitutionality of the individual mandate (coverage) in the ACA will once again be before the Court, and that already has produced a flood of amicus briefs from health-related organizations.32 Among other upcoming issues are cases related to state regulation of pharmacy benefit managers, gay rights and foster care, sentencing of juveniles to life in prison without the possibility of parole, a face-off between Google and Oracle on software copyrights, and arbitration. In addition, some of the issues we saw this term will reappear, with more on robocalls, religious freedom and Catholic charities, and immigration and removal cases.
Ruth Bader Ginsburg, as a law student, law professor, lawyer, judge, and justice, was a leading advocate for the rights of women. There were only a few women in law school when she attended, but she graduated tied for first in her class. Although she found it difficult to be hired as a lawyer, as a law professor and lawyer she helped map a strategy to expand legal rights for women, arguing 6 cases before the Supreme Court and winning 5 of them. She served as a federal appeals court judge and then was appointed to the Supreme Court in 1993. She was the second woman to serve on the Court.
As a justice, she was known during much of her tenure on the Court as the leader of the liberal justices, although her jurisprudence was more complex than that simple statement. She was always a strong advocate for the rights of women (and equal rights of men) during her time on the Court. She was a very clear writer; her opinions were direct and easy to understand. She was also fast—she routinely had the record of announcing opinions faster than any of the other current justices. She was 87 when she passed away, having served on the Court for 27 years.
Justice Ginsburg was also something of a cultural phenomenon. In later years she was sometimes known as “the Notorious RBG.” Books, movies, songs, and even workout videos were made about her. In groups she seemed almost shy, but she was thoughtful, kind, and funny (sometimes wickedly so). The outpouring of affection and sympathy at her death was a symbol of the place she held in America. She loved the opera, a passion she shared with her friend, Justice Antonin Scalia. Despite their considerable disagreements on legal matters, Justices Ginsburg and Scalia were close friends. They attended opera with one another, and their families usually spent New Year’s Eves together. They were the 2 most recent justices to pass away while serving on the Court.
The Court heard and ruled on a large number of other significant cases that will have consequences for many years to come. Highlights include:
- In 2 cases involving subpoenas for the President’s personal records, the Court suggested some balance between “nobody is above the law” and not unnecessarily hectoring or interfering with fulfilling the office of President. The Court held that Congress may subpoena a President’s personal and family records, while the President is still in office.1 It instructed lower courts to assess whether the papers are necessary, the subpoena is limited in scope, there is legitimate legislative purpose, whether the burden it imposes on the President is reasonable, and whether the subpoena would unduly interfere with the ability to do the work required as President.
- Similarly, local (state) grand juries may subpoena such personal records, but the President will have the opportunity to raise specific objections to the subpoenas—undue burden, bad faith, or overbreadth. In addition, the respect owed to the office should inform the conduct regarding the subpoena.2
- The Court upheld a federal law that prohibits most robocalls.3 It struck down an amendment that allowed robocalls made to collect debts owed to or guaranteed by the federal government.
- The Court held that a single-director federal agency, whose director cannot be removed by the President (at will), violates the Constitution.4 The Consumer Financial Protection Bureau (created by the Dodd-Frank law) has such a single, no-removal director and that will have to be modified.
- The Court held that the eastern half of Oklahoma (including Tulsa) is part of a Creek Nation reservation.5 This was a question of criminal law jurisdiction, not property ownership. The practical effect is that for crimes involving Native Americans, serious crimes will have to be tried in federal court, while lesser crimes may be tried in tribal courts.
- The Court determined that it was unconstitutional for a state program providing tuition assistance to parents who send their children to private schools, to prohibit students attending religious private schools from participating in the program. That is a burden on the “free exercise” of religion.6
- The Court considered whether there can be civil liability for damages caused by a federal official in the United States harming a foreign national in another country. In this case, a border patrol agent standing in the US shot and killed a Mexican juvenile who was just across the border in Mexico.7 The issue was whether the parents of the Mexican national could sue the US officials for damages. The Court declined to expand liability to include those injured outside the US. Ultimately, the Court was reluctant to impose liability because this liability is not authorized by Congress.
- In a COVID-19 religion case, the Court refused to stop the enforcement of a governor’s COVID-19 order that allowed churches to operate with <100 attendees or 25% occupancy (whichever was lower).8 Meanwhile, businesses, malls, and stores were allowed to reopen without these stringent limitations. The church objected that greater burdens were placed on religion than secular activity. The Court denied the church’s request for an injunction.
- The Court unanimously held that a state may punish or remove a “faithless elector.” Electors cast votes on behalf of their states in the Electoral College—where Presidents are technically selected. Electors are generally pledged to vote for the winner of a state’s vote for President. A few have violated that pledge and voted for someone else. As a practical matter, that could cause real disruption, and the Court upheld state laws that take action against these “faithless” electors.9
- Several days after the Court had officially adjourned for the term, it received several petitions to delay the execution of federal prisoners. One case was based on the method of execution (use of pentobarbital),10 and another was based on the claim that a prisoner had become so mentally incompetent that it was improper to execute him.11 The Court turned down these appeals, allowing the executions to proceed. These were the first federal government executions in 17 years.
References
- Trump v Mazars USA, LLP, 140 S. Ct. 2019 (2020).
- Trump v Vance, 140 S. Ct. 2412 (2020).
- Barr v American Association of Political Consultants, Inc, 140 S. Ct. 2335 (2020).
- Seila Law LLC v Consumer Financial Protection Bureau, 140 S. Ct. 2183 (2020).
- McGirt v Oklahoma, 140 S. Ct. 2452 (2020).
- Espinoza v Montana Department of Revenue, 140 S. Ct. 2246 (2020).
- Hernández v Mesa, 140 S. Ct. 735, 206 L. Ed. 2d 29 (2020).
- South Bay United Pentecostal Church v Newsom, 140 S. Ct. 1613, 207 L. Ed. 2d 154 (2020).
- Chiafalo v Washington, 140 S. Ct. 2316 (2020).
- Barr v Lee, ____ S. Ct. ____ (2020).
- Barr v Purkey, ____ S. Ct. ____ (2020).
The 2019-2020 term of the US Supreme Court was remarkable by any standard. An extraordinary number of important cases made it “a buffet of blockbusters.”1
We look first at several cases that will be of particular interest to ObGyns. Then we look briefly at a number of other important cases that affect the medical profession as a whole and the direction of the country (see “Other significant US Supreme Court decisions”), and finally we conclude with an analysis of this term and a forecast for the next.
We chose cases in which specialty organizations, such as the American College of Obstetricians and Gynecologists (ACOG), or organized medicine (the American Medical Association [AMA], the Association of American Medical Colleges [AAMC], or the American Hospital Association [AHA]), took a special interest by filing “amicus curiae” (friend of the court) briefs with the Supreme Court. These briefs are filed by an organization or person who is not a party to the case but who may have important information to convey to the Court. Because these briefs represent a significant commitment of money, time, and effort, they are usually not undertaken lightly.
Decisions concerning abortion
June v Russo
Decided June 29, 2020, June v Russo involved a Louisiana statute that required abortion providers have “active admitting privileges at a hospital” within 30 miles of where the abortion is performed.2 The Court decided a case in 2016 (from Texas) that involved almost the same statutory provision, so it might seem like an easy ruling.3 But Justice Kennedy (the deciding vote in 2016) has been replaced by Justice Gorsuch, so the outcome was uncertain. It was a difficult case, with a total of 5 opinions covering 138 pages and a “surprise” from the Chief Justice.
The Court, in a 5-4 decision, struck down the Louisiana law, but there was no majority opinion. Four justices in the plurality emphasized that the Louisiana law (like the Texas law) substantially burdened the right to abortion without any corresponding benefit to the health of the women seeking abortions. (Under earlier Court precedents, “undue burdens” on abortion are unconstitutional.4) Justice Breyer noted that the state could not present even one example in which a woman would have had better treatment if her doctor had admitting privileges. For a variety of reasons, admitting privileges were cumbersome for abortion providers to obtain; therefore, enforcing the law had little or no benefit, but significant risk of reduced availability of abortion services.
In June v Russo, Chief Justice Roberts literally became the “swing vote”—the fifth vote to strike down the Louisiana law. In 2016, he had voted the other way—to uphold essentially the same law (in Texas) that he struck down here. He attributed his switch to precedent (the general obligation of courts to follow prior decisions). He disagreed with the earlier decision, but felt bound by it.
This should be the end of the abortion provider “hospital privileges requirements” that a number of states have passed. States seeking to nibble away at abortion rights will undoubtedly look elsewhere. Beyond that, it is difficult, from this case, to discern the future of abortion rights.
ACOG was the lead in amicus briefs urging the Court to strike down the Louisiana law. ACOG (with others) was one of only a handful of organizations filing a brief urging the Court to agree to hear the case.5 When the Court did agree to hear the case (“granted certiorari”), ACOG and a number of other medical organizations filed a formal amicus brief on the merits of the case.6 The brief made 2 arguments: First, that this case was essentially decided in Whole Woman’s Health in 2016 (the Texas case) and, second, that “an admitting privileges requirement is not medically necessary” and “clinicians who provide abortions are unable to obtain admitting privileges for reasons unrelated to their ability to safely and competently perform abortions.” Justice Breyer cited the ACOG brief twice.
The American Association of Pro-Life Obstetricians and Gynecologists also filed an amicus brief.7 The brief was directed solely at arguing that ACOG was not presenting reliable science. It summarized, “The American College of Obstetricians and Gynecologists has always presented itself to the Court as a source of objective medical knowledge. However, when it comes to abortion, the College today is primarily a pro-abortion political advocacy organization.” That brief concluded that the “Court should read ACOG’s amicus brief not as an authoritative recitation of settled science, but as a partisan advocacy paper on behalf of a mere subset of American obstetricians and gynecologists.”
The Association of American Physicians and Surgeons (which should not be confused with the “National Board of Physicians and Surgeons”) also filed an amicus brief. The brief argued, “Abortion, like other outpatient surgical procedures, sometimes results in patient hospitalization. Requiring abortion providers to maintain admitting privileges will improve communication between physicians in the transfer of patients to the hospital and allow them to participate in the care of their patients while in the hospital, in line with their ethical duty to ensure their patients’ continuity of care.”8
Continue to: Ultrasonography requirement for abortion...
Ultrasonography requirement for abortion
In another abortion case, the Court was asked to review a Kentucky abortion statute requiring that an ultrasound image be shown to the woman as part of informed consent for an abortion.9 ACOG filed an amicus brief in favor of a review, but the Court declined to hear the case.10,11
Contraception considerations
The Affordable Care Act (ACA) has an ambiguous provision regarding no-cost “preventive care and screenings” for women. The ACA does not, however, specify contraceptive coverage.12 Several departments and the Health Resources and Services Administration (collectively referred to as “HRSA”) interpreted the provision to include contraception, but from the start there were religious objections. HRSA eventually provided an exemption regarding contraception for employers (nonprofits and for-profits with no publicly traded components) that had “sincerely held moral” objections to providing forms of contraceptive coverage. That regulation was again before the Court this term in Little Sisters of the Poor Saints Peter and Paul Home v Pennsylvania.13
In a 7-2 decision, the Court held that the ACA gave HRSA authority to adopt regulations related to the undefined term “preventive care.” Therefore, it found that HRSA could exempt those with religious objections from participation in providing contraceptive coverage. ACOG and other medical groups filed an amicus brief arguing that contraception is an essential preventive service. “Contraception not only helps to prevent unintended pregnancy, but also helps to protect the health and well-being of women and their children.”14 It was cited only by Justice Ginsburg in her dissent.15
Deferred Action for Childhood Arrivals (DACA)
The AAMC, ACOG, AMA, and many other organizations filed an amicus brief16 in Department of Homeland Security v Regents of University of California.17 The case raised the question of whether a decision to end the DACA program followed the appropriate administrative procedures. In 2012, the Obama administration issued a “memorandum” establishing DACA (without congressional approval or formal rulemaking). A lower court decision barring implementation of DACA was upheld by the Supreme Court in 2016 on a 4-4 vote.18 In 2017, the Trump administration moved to end DACA.
In a 5-4 decision, the Court held that the explanation for ending DACA was inadequate, and violated the Administrative Procedures Act, so DACA could continue until the administration redid the repeal, following the proper procedures. The decision of the Court dealt solely with the process by which the rescission took place—there was general agreement that the administration had the right to rescind it if the procedure (with legitimate reasons) was proper.
The brief for the medical groups argued that the failure of the regulation to consider “reliance interests” would have especially difficult consequences in the medical fields. It noted, “At this moment, an estimated 27,000 health care workers and support staff depend on DACA for their authorization to work in the United States. Among those 27,000 are nurses, dentists, pharmacists, physician assistants, home health aides, technicians, and others. The number also includes nearly 200 medical students, medical residents, and physicians who depend on DACA for their eligibility to practice medicine.”16 The brief was not cited by the Court, but the reliance interest the brief spoke about was an important part of the case.
Continue to: Employment discrimination against gay and transgender employees...
Employment discrimination against gay and transgender employees
Federal law (“Title VII”) makes it illegal for an employer to “discriminate against any individual because of race, color, religion, sex, or national origin.”19 The question this term was whether discrimination based on sexual orientation or sexual identity is within the statute’s meaning of “sex.” By a 6-3 majority, the Court held that Title VII applies both to orientation and identity. (This was an interpretation of the statute, not a broad constitutional ruling.)
The majority reasoned that “it is impossible to discriminate against a person for being homosexual or transgender without discriminating against that individual based on sex. Consider, for example, an employer with 2 employees, both of whom are attracted to men.” If the employer fires the gay employee, “the employer discriminates against him for traits or actions it tolerates in his female colleague.”20
AMA and a number of other medical organizations filed an amicus brief in the case.21 The core of the argument of the brief was, “Employment discrimination against transgender people frustrates the treatment of gender dysphoria by preventing transgender individuals from living openly in accordance with their true gender identity and impeding access to needed medical care. Experiencing discrimination in one of the most important aspects of adult life—employment—makes it nearly impossible to live in full congruence with one’s gender identity. The fear of facing such discrimination alone can prompt transgender individuals to hide their gender identity, directly thwarting the goal of social transition…. Lack of treatment, in turn, increases the rate of negative mental health outcomes, substance abuse, and suicide.” The brief was not cited in the opinions in the case.
This decision is likely to have great impact on many aspects of American life. In the employment area, it is now a matter of course that employers may not discriminate based on orientation or identity in any employment decisions including hiring, firing, compensation, fringe benefits, etc. Harassment based on identity or orientation may similarly be an employment law violation. The decision also likely means that giving employment preferences to gay employees would now be as illegal as would be giving preferences to straight employees. (Limited exceptions, notably to some religious organization employees, are not included in anti-discrimination laws.)22
The importance of the decision goes well beyond employment, however. More than 100 federal statutes are in place that prohibit “discrimination because of sex.” It is now likely that these statutes will be interpreted as prohibiting discrimination related to sexual orientation and identification.
Additional cases of interest
HIV/AIDS International Program
A major US program fighting HIV/AIDS worldwide—the United States Leadership Against HIV/AIDS, Tuberculosis, and Malaria Act (aka the Leadership Act)—has provided billions of dollars to agencies abroad.23 Nongovernmental organizations (NGOs) receiving funds under the program must agree to have a “policy explicitly opposing prostitution and sex trafficking” (known as the “Policy Requirement”). Some grant recipients in foreign countries, generally affiliates of US NGOs, do not want to have such a policy and challenged the policy requirement as a violation of First Amendment right of free speech. The Court held that it is a well-settled principle that “foreign citizens outside US territory do not possess rights under the US Constitution.”24 Nor do organizations become entitled to such rights as a result of an affiliation with US organizations. This decision means that foreign organizations are free not to have the required policies, but they will be ineligible for funds under the Leadership Act.
Continue to: ACA government debts edition...
ACA government debts edition
The ACA was before the Court, yet again. To encourage private insurers to participate in online health insurance exchanges, the ACA provided that the federal government would share in insurance company losses for 3 years.25 The Act, however, did not appropriate any money for these “risk corridors,” and insurance companies lost $12 billion.
Congress (after the 2010 election) prohibited any appropriated funds from being used to pay insurance companies for their risk corridor losses. Four insurance companies sued the United States, seeking reimbursements for their losses. This term the Court held that the government must pay for their losses under the ACA.26 The Court said that Congress could have expressly repealed the risk corridor obligation (in the appropriation bill), but instead had only prohibited the expenditure of the money, which the Court said did not amount to an implied repeal of the obligation. We will see that ACA will be back before the Court again next term in California v Texas (discussed below).
Child custody and international abduction
The Hague Convention on the Civil Aspects of International Child Abduction (to which the United States is a party) provides that the courts of the country where the child has “habitual residence” have jurisdiction to decide custody.27 If a parent takes the child to another country, that country is obligated to return the child to the country of “habitual residence.”
This term the Court was called upon to define “habitual residence.” The Court held that determining habitual residence depends on the “totality of the circumstances,” and that “locating a child’s home is a fact-driven inquiry,” and that “courts must be sensitive to the unique circumstances of the case and informed by common sense.”28 An exception to the Convention’s obligation to return a child to the country of habitual residence is where “there is a grave risk that [the] return would expose the child to physical or psychological harm or otherwise place the child in an intolerable situation.”29 Who the parent is can affect many aspects of legal authority over the child, including consent to medical care, and the right to receive information concerning care.
Analysis of the term
The term began October 7, 2019, and adjourned July 9, 2020, somewhat later than usual because of coronavirus disease 2019 (COVID-19). During the term, the Court decided 60 cases, including 53 “signed” merit opinions after oral argument—the lowest number of decided cases in many years.30 Of those 60 cases, 22 (35%) were unanimous, and 13 (22%) resulted in a 5-4 split.30 Ten-year averages are 48% unanimous and 20% with 5-4 decisions.30
Chief Justice Roberts was the central focus of the term. He presided over the impeachment trial of President Trump in the Senate early in the term. He also presided over the Court’s accommodations of the COVID-19 pandemic. He is the “median,” or “swing,” justice. He was in the majority in 12 of the 13 cases with 5-4 decisions.30 He was in the majority in 97% of all cases and in 95% of “divided cases”—the highest of any of the justices this term.30 In some of the most critical decisions, Chief Justice Roberts sided with the “liberal” wing, including on cases concerning abortion, gay and transgender employment, DACA, and 2 Presidential subpoena cases. More often (in 9 of the 5-4 decisions), however, he sided with the more conservative justices.30 Justice Kavanaugh agreed with Chief Justice Roberts most often (in 93% of all cases).30 Among the others, these justices agreed with each other 90% or more of the time: Justices Ginsburg and Breyer (93%), Justices Alito and Thomas (92%), and Justices Breyer and Kagan (90%).30
Continue to: COVID-19 and the Court...
COVID-19 and the Court
Some of the biggest news of the term came not from the law, but from medicine in the form of COVID-19. The Court was in the process of preparing a final period of important arguments when, on March 16, it announced that it was postponing further arguments. The Court rescheduled 10 oral arguments that were held by telephone (other cases were held over to the next term). The phone arguments, during the first 2 weeks of May, necessitated a change in format. Each justice was called on (in order of seniority) by the Chief Justice to ask questions. This was in contrast to the free-for-all questions that usually characterize in-person arguments. These arguments were broadcast live—something that had never been done before. Public access was, on balance, a good thing. There were a couple failures to unmute, and there was “the flush heard round the world” in the middle of one argument, but otherwise the arguments went off with few hitches.31
Looking ahead
By the end of the term, no justice had announced an intention to retire from the Court. On September 18, however, Justice Ruth Bader Ginsburg passed away. In 2009, she had been diagnosed with early-stage pancreatic cancer. This term she had been hospitalized twice, and at the end of the term, she announced a recurrence of pancreatic cancer, which was being treated with chemotherapy. See “RBG: The woman, the legacy” for a tribute to this remarkable woman, lawyer, and justice.
Justice Ginsburg’s death, occurring in the middle of a presidential campaign, ignited a political firestorm concerning her successor. The outcome of selecting and confirming her successor and the political fallout were not immediately apparent. Justice Ginsburg was confirmed just 7 weeks after her nomination by President Clinton, by a vote of 96-3. But those days of Senate consensus are not the current norm.
The next term (called the “October 2020 Term”) will begin on October 5, 2020. The Court will begin with 8 justices and, depending on the nomination process, may operate with 8 justices for some time. When there is a “tie” vote in the Court, the lower court decision is upheld. The Court has been short-handed several times in the past and, with few exceptions, has managed the cases successfully.
The Court has announced that initial arguments will be telephonic. It already has taken a number of cases. The constitutionality of the individual mandate (coverage) in the ACA will once again be before the Court, and that already has produced a flood of amicus briefs from health-related organizations.32 Among other upcoming issues are cases related to state regulation of pharmacy benefit managers, gay rights and foster care, sentencing of juveniles to life in prison without the possibility of parole, a face-off between Google and Oracle on software copyrights, and arbitration. In addition, some of the issues we saw this term will reappear, with more on robocalls, religious freedom and Catholic charities, and immigration and removal cases.
Ruth Bader Ginsburg, as a law student, law professor, lawyer, judge, and justice, was a leading advocate for the rights of women. There were only a few women in law school when she attended, but she graduated tied for first in her class. Although she found it difficult to be hired as a lawyer, as a law professor and lawyer she helped map a strategy to expand legal rights for women, arguing 6 cases before the Supreme Court and winning 5 of them. She served as a federal appeals court judge and then was appointed to the Supreme Court in 1993. She was the second woman to serve on the Court.
As a justice, she was known during much of her tenure on the Court as the leader of the liberal justices, although her jurisprudence was more complex than that simple statement. She was always a strong advocate for the rights of women (and equal rights of men) during her time on the Court. She was a very clear writer; her opinions were direct and easy to understand. She was also fast—she routinely had the record of announcing opinions faster than any of the other current justices. She was 87 when she passed away, having served on the Court for 27 years.
Justice Ginsburg was also something of a cultural phenomenon. In later years she was sometimes known as “the Notorious RBG.” Books, movies, songs, and even workout videos were made about her. In groups she seemed almost shy, but she was thoughtful, kind, and funny (sometimes wickedly so). The outpouring of affection and sympathy at her death was a symbol of the place she held in America. She loved the opera, a passion she shared with her friend, Justice Antonin Scalia. Despite their considerable disagreements on legal matters, Justices Ginsburg and Scalia were close friends. They attended opera with one another, and their families usually spent New Year’s Eves together. They were the 2 most recent justices to pass away while serving on the Court.
The Court heard and ruled on a large number of other significant cases that will have consequences for many years to come. Highlights include:
- In 2 cases involving subpoenas for the President’s personal records, the Court suggested some balance between “nobody is above the law” and not unnecessarily hectoring or interfering with fulfilling the office of President. The Court held that Congress may subpoena a President’s personal and family records, while the President is still in office.1 It instructed lower courts to assess whether the papers are necessary, the subpoena is limited in scope, there is legitimate legislative purpose, whether the burden it imposes on the President is reasonable, and whether the subpoena would unduly interfere with the ability to do the work required as President.
- Similarly, local (state) grand juries may subpoena such personal records, but the President will have the opportunity to raise specific objections to the subpoenas—undue burden, bad faith, or overbreadth. In addition, the respect owed to the office should inform the conduct regarding the subpoena.2
- The Court upheld a federal law that prohibits most robocalls.3 It struck down an amendment that allowed robocalls made to collect debts owed to or guaranteed by the federal government.
- The Court held that a single-director federal agency, whose director cannot be removed by the President (at will), violates the Constitution.4 The Consumer Financial Protection Bureau (created by the Dodd-Frank law) has such a single, no-removal director and that will have to be modified.
- The Court held that the eastern half of Oklahoma (including Tulsa) is part of a Creek Nation reservation.5 This was a question of criminal law jurisdiction, not property ownership. The practical effect is that for crimes involving Native Americans, serious crimes will have to be tried in federal court, while lesser crimes may be tried in tribal courts.
- The Court determined that it was unconstitutional for a state program providing tuition assistance to parents who send their children to private schools, to prohibit students attending religious private schools from participating in the program. That is a burden on the “free exercise” of religion.6
- The Court considered whether there can be civil liability for damages caused by a federal official in the United States harming a foreign national in another country. In this case, a border patrol agent standing in the US shot and killed a Mexican juvenile who was just across the border in Mexico.7 The issue was whether the parents of the Mexican national could sue the US officials for damages. The Court declined to expand liability to include those injured outside the US. Ultimately, the Court was reluctant to impose liability because this liability is not authorized by Congress.
- In a COVID-19 religion case, the Court refused to stop the enforcement of a governor’s COVID-19 order that allowed churches to operate with <100 attendees or 25% occupancy (whichever was lower).8 Meanwhile, businesses, malls, and stores were allowed to reopen without these stringent limitations. The church objected that greater burdens were placed on religion than secular activity. The Court denied the church’s request for an injunction.
- The Court unanimously held that a state may punish or remove a “faithless elector.” Electors cast votes on behalf of their states in the Electoral College—where Presidents are technically selected. Electors are generally pledged to vote for the winner of a state’s vote for President. A few have violated that pledge and voted for someone else. As a practical matter, that could cause real disruption, and the Court upheld state laws that take action against these “faithless” electors.9
- Several days after the Court had officially adjourned for the term, it received several petitions to delay the execution of federal prisoners. One case was based on the method of execution (use of pentobarbital),10 and another was based on the claim that a prisoner had become so mentally incompetent that it was improper to execute him.11 The Court turned down these appeals, allowing the executions to proceed. These were the first federal government executions in 17 years.
References
- Trump v Mazars USA, LLP, 140 S. Ct. 2019 (2020).
- Trump v Vance, 140 S. Ct. 2412 (2020).
- Barr v American Association of Political Consultants, Inc, 140 S. Ct. 2335 (2020).
- Seila Law LLC v Consumer Financial Protection Bureau, 140 S. Ct. 2183 (2020).
- McGirt v Oklahoma, 140 S. Ct. 2452 (2020).
- Espinoza v Montana Department of Revenue, 140 S. Ct. 2246 (2020).
- Hernández v Mesa, 140 S. Ct. 735, 206 L. Ed. 2d 29 (2020).
- South Bay United Pentecostal Church v Newsom, 140 S. Ct. 1613, 207 L. Ed. 2d 154 (2020).
- Chiafalo v Washington, 140 S. Ct. 2316 (2020).
- Barr v Lee, ____ S. Ct. ____ (2020).
- Barr v Purkey, ____ S. Ct. ____ (2020).
1. Liptak A. In a term full of major cases, the Supreme Court tacked to the center. The New York Times. July 10, 2020.
2. June Medical Services LLC v Russo, 591 US 140 S. Ct. 2103, 2112 (2020).
3. Whole Woman’s Health v Hellerstedt, 579 US ___ (2016).
4. Planned Parenthood of Southeastern PA v Casey, 505 US 833, 874 (1992).
5. Brief of the American College of Obstetricians and Gynecologists, the American Academy of Family Physicians, the American Academy of Pediatrics, the American College of Nurse-Midwives, the American College of Osteopathic Obstetricians and Gynecologists, the American College of Physicians, the American Society for Reproductive Medicine, the National Association of Nurse Practitioners in Women’s Health, the North American Society for Pediatric and Adolescent Gynecology, and the Society for Maternal-Fetal Medicine Amici Curiae In Support of Petitioners, June Medical Services v Russo. May 20, 2019. https://www.supremecourt.gov/DocketPDF/18/18-1323/100434/20190520175434029_18-1323%20ACOG%20et%20al.%20cert.%20amicus%20brief.pdf. Accessed August 31, 2020.
6. Brief of Amici Curiae American College of Obstetricians and Gynecologists, American Medical Association, American Academy of Family Physicians, American Academy of Nursing, American Academy of Pediatrics, American College of Nurse-Midwives, American College of Osteopathic Obstetricians and Gynecologists, American College of Physicians, American Osteopathic Association, American Public Health Association, American Society for Reproductive Medicine, North American Society for Pediatric and Adolescent Gynecology, Society for Maternal-Fetal Medicine, and the Society of Ob/Gyn Hospitalists, In Support of June Medical Services, June Medical Services v Russo. December 2, 2019. https://www.supremecourt.gov/DocketPDF/18/18-1323/124091/20191202145531124_18-1323%2018-1460%20tsac%20American%20College%20of%20Obstetricians%20and%20
Gynecologists%20et%20al.pdf [https://perma.cc/8T8V-4D6S]. Accessed August 31, 2020.
7. Brief of Amicus Curiae American Association of Pro-Life Obstetricians and Gynecologists In Support of [Russo] Louisiana Department of Health and Hospitals, June Medical Services v Russo. December 27, 2019. https://www.supremecourt.gov/DocketPDF/18/18-1323/126927/20191227154424488_AAPLOG%20Amicus%20Brief.pdf. Accessed August 31, 2020.
8. Brief of Association of American Physicians and Surgeons as Amicus Curiae in Support of Respondent–Cross-Petitioner, June Medical Services v Russo 2. December 27, 2019. https://www.supremecourt.gov/DocketPDF/18/18-1323/126828/20191227104605915_18-1323%20-1460%20bsac%20AAPS--PDFA.pdf. Accessed August 31, 2020.
9. Ky. Rev. Stat. § 311.727(2).
10. Brief for the American College of Obstetricians and Gynecologists, the American Medical Association, the North American Society for Pediatric and Adolescent Gynecology, the American College of Osteopathic Obstetricians and Gynecologists, and the American Academy of Family Physicians Amici Curiae Supporting Petitioners, EMW Women’s Surgical Center v Meier. October 28, 2019. https://www.supremecourt.gov/DocketPDF/19/19-417/120550/20191028184956458_19-417%20ACOG%20et%20al.%20-%20cert.%20amicus%20brief.pdf. Accessed August 31, 2020.
11. EMW Women’s Surgical Center, PSC v Meier, 140 S. Ct. 655 (2019).
12. Codified at 26 U. S. C. §5000A(f )(2); §§4980H(a), (c)(2) requires employers to provide women with “preventive care and screenings” without “any cost sharing requirements.”
13. Little Sisters of the Poor Saints Peter and Paul Home v Pennsylvania, 140 S. Ct. 2367 (2020).
14. Brief of Amici Curiae American College of Obstetricians and Gynecologists, American Nurses Association, American Academy of Nursing, Physicians for Reproductive Health, and Nurses for Sexual and Reproductive Health, In Support of Respondents and Affirmance, Little Sisters of the Poor Saints Peter and Paul Home v Pennsylvania. April 8, 2020. https://www.supremecourt.gov/DocketPDF/19/19-431/141177/20200408152340136_19-431%20and%2019-454%20Amici%20Curiae.pdf. Accessed August 31, 2020.
15. Little Sisters of the Poor Saints Peter and Paul Home v Pennsylvania, 140 S. Ct. 2367, 2400–12 (2020).
16. Brief for the Association of American Medical Colleges (and more than 30 other organizations, including the American Medical Association, and the American College of Obstetricians and Gynecologists) Amici Curiae, In Support of Respondents, Department of Homeland Security v Regents of University of California. October 4, 2019. https://www.supremecourt.gov/DocketPDF/18/18-587/118129/20191004130646281_Brief%20for%20AAMC%20et%20al%20
Supporting%20Respondents.pdf. Accessed August 31, 2020.
17. Department of Homeland Security v Regents of The University of California, 140 S. Ct. 1891 (2020).
18. United States v Texas, 136 S. Ct. 2271 (2016).
19. Title VII of the Civil Rights Act of 1964, 42 U.S.C §2000e–2(a)(1).
20. Bostock v Clayton County, 140 S. Ct. 1731, 1741 (2020).
21. Brief of the American Medical Association, the American College of Physicians, and 14 additional medical, mental health, and health care organizations as Amici Curiae In Support of the Employees, Bostock v Clayton County. July 3, 2019. https://www.supremecourt.gov/DocketPDF/17/17-1618/107177/20190703172548842_Amicus%20Brief.pdf. Accessed August 31, 2020.
22. Our Lady of Guadalupe School v Morrissey-Berru, 140 S. Ct. 2049 (2020).
23. United States Leadership Against HIV/AIDS, Tuberculosis, and Malaria Act (“the Leadership Act”), 22 U. S. C. §7601 et seq.
24. Agency for International Development v Alliance for Open Society, 140 S. Ct. 2082, 2086 (2020).
25. 42 U.S.C. §1342, §18063.
26. Maine Community Health Options v United States, 140 S. Ct. 1308 (2020).
27. Hague Convention on the Civil Aspects of International Child Abduction (Hague Convention or Convention), implemented in the United States by the International Child Abduction Remedies Act, 22 U. S. C. §9001 et seq.
28. Monasky v Taglieri, 140 S. Ct. 719 (2020).
29. Monasky v Taglieri, 140 S. Ct. 719, 723, 729 (2020).
30. Feldman A. Final stat pack for October term 2019 (upated). July 10, 2020. https://www.scotusblog.com/2020/07/final-stat-pack-for-october-term-2019/. Accessed August 31, 2020.
31. Hejmanowski D. Flush heard around the world. Delaware Gazette. May 8, 2020. https://www.delgazette.com/opinion/columns/83610/flush-heard-around-the-world. Accessed August 31, 2020.
32. SCOTUSblog.com. California v Texas. https://www.scotusblog.com/case-files/cases/california-v-texas/. Accessed August 31, 2020.
1. Liptak A. In a term full of major cases, the Supreme Court tacked to the center. The New York Times. July 10, 2020.
2. June Medical Services LLC v Russo, 591 US 140 S. Ct. 2103, 2112 (2020).
3. Whole Woman’s Health v Hellerstedt, 579 US ___ (2016).
4. Planned Parenthood of Southeastern PA v Casey, 505 US 833, 874 (1992).
5. Brief of the American College of Obstetricians and Gynecologists, the American Academy of Family Physicians, the American Academy of Pediatrics, the American College of Nurse-Midwives, the American College of Osteopathic Obstetricians and Gynecologists, the American College of Physicians, the American Society for Reproductive Medicine, the National Association of Nurse Practitioners in Women’s Health, the North American Society for Pediatric and Adolescent Gynecology, and the Society for Maternal-Fetal Medicine Amici Curiae In Support of Petitioners, June Medical Services v Russo. May 20, 2019. https://www.supremecourt.gov/DocketPDF/18/18-1323/100434/20190520175434029_18-1323%20ACOG%20et%20al.%20cert.%20amicus%20brief.pdf. Accessed August 31, 2020.
6. Brief of Amici Curiae American College of Obstetricians and Gynecologists, American Medical Association, American Academy of Family Physicians, American Academy of Nursing, American Academy of Pediatrics, American College of Nurse-Midwives, American College of Osteopathic Obstetricians and Gynecologists, American College of Physicians, American Osteopathic Association, American Public Health Association, American Society for Reproductive Medicine, North American Society for Pediatric and Adolescent Gynecology, Society for Maternal-Fetal Medicine, and the Society of Ob/Gyn Hospitalists, In Support of June Medical Services, June Medical Services v Russo. December 2, 2019. https://www.supremecourt.gov/DocketPDF/18/18-1323/124091/20191202145531124_18-1323%2018-1460%20tsac%20American%20College%20of%20Obstetricians%20and%20
Gynecologists%20et%20al.pdf [https://perma.cc/8T8V-4D6S]. Accessed August 31, 2020.
7. Brief of Amicus Curiae American Association of Pro-Life Obstetricians and Gynecologists In Support of [Russo] Louisiana Department of Health and Hospitals, June Medical Services v Russo. December 27, 2019. https://www.supremecourt.gov/DocketPDF/18/18-1323/126927/20191227154424488_AAPLOG%20Amicus%20Brief.pdf. Accessed August 31, 2020.
8. Brief of Association of American Physicians and Surgeons as Amicus Curiae in Support of Respondent–Cross-Petitioner, June Medical Services v Russo 2. December 27, 2019. https://www.supremecourt.gov/DocketPDF/18/18-1323/126828/20191227104605915_18-1323%20-1460%20bsac%20AAPS--PDFA.pdf. Accessed August 31, 2020.
9. Ky. Rev. Stat. § 311.727(2).
10. Brief for the American College of Obstetricians and Gynecologists, the American Medical Association, the North American Society for Pediatric and Adolescent Gynecology, the American College of Osteopathic Obstetricians and Gynecologists, and the American Academy of Family Physicians Amici Curiae Supporting Petitioners, EMW Women’s Surgical Center v Meier. October 28, 2019. https://www.supremecourt.gov/DocketPDF/19/19-417/120550/20191028184956458_19-417%20ACOG%20et%20al.%20-%20cert.%20amicus%20brief.pdf. Accessed August 31, 2020.
11. EMW Women’s Surgical Center, PSC v Meier, 140 S. Ct. 655 (2019).
12. Codified at 26 U. S. C. §5000A(f )(2); §§4980H(a), (c)(2) requires employers to provide women with “preventive care and screenings” without “any cost sharing requirements.”
13. Little Sisters of the Poor Saints Peter and Paul Home v Pennsylvania, 140 S. Ct. 2367 (2020).
14. Brief of Amici Curiae American College of Obstetricians and Gynecologists, American Nurses Association, American Academy of Nursing, Physicians for Reproductive Health, and Nurses for Sexual and Reproductive Health, In Support of Respondents and Affirmance, Little Sisters of the Poor Saints Peter and Paul Home v Pennsylvania. April 8, 2020. https://www.supremecourt.gov/DocketPDF/19/19-431/141177/20200408152340136_19-431%20and%2019-454%20Amici%20Curiae.pdf. Accessed August 31, 2020.
15. Little Sisters of the Poor Saints Peter and Paul Home v Pennsylvania, 140 S. Ct. 2367, 2400–12 (2020).
16. Brief for the Association of American Medical Colleges (and more than 30 other organizations, including the American Medical Association, and the American College of Obstetricians and Gynecologists) Amici Curiae, In Support of Respondents, Department of Homeland Security v Regents of University of California. October 4, 2019. https://www.supremecourt.gov/DocketPDF/18/18-587/118129/20191004130646281_Brief%20for%20AAMC%20et%20al%20
Supporting%20Respondents.pdf. Accessed August 31, 2020.
17. Department of Homeland Security v Regents of The University of California, 140 S. Ct. 1891 (2020).
18. United States v Texas, 136 S. Ct. 2271 (2016).
19. Title VII of the Civil Rights Act of 1964, 42 U.S.C §2000e–2(a)(1).
20. Bostock v Clayton County, 140 S. Ct. 1731, 1741 (2020).
21. Brief of the American Medical Association, the American College of Physicians, and 14 additional medical, mental health, and health care organizations as Amici Curiae In Support of the Employees, Bostock v Clayton County. July 3, 2019. https://www.supremecourt.gov/DocketPDF/17/17-1618/107177/20190703172548842_Amicus%20Brief.pdf. Accessed August 31, 2020.
22. Our Lady of Guadalupe School v Morrissey-Berru, 140 S. Ct. 2049 (2020).
23. United States Leadership Against HIV/AIDS, Tuberculosis, and Malaria Act (“the Leadership Act”), 22 U. S. C. §7601 et seq.
24. Agency for International Development v Alliance for Open Society, 140 S. Ct. 2082, 2086 (2020).
25. 42 U.S.C. §1342, §18063.
26. Maine Community Health Options v United States, 140 S. Ct. 1308 (2020).
27. Hague Convention on the Civil Aspects of International Child Abduction (Hague Convention or Convention), implemented in the United States by the International Child Abduction Remedies Act, 22 U. S. C. §9001 et seq.
28. Monasky v Taglieri, 140 S. Ct. 719 (2020).
29. Monasky v Taglieri, 140 S. Ct. 719, 723, 729 (2020).
30. Feldman A. Final stat pack for October term 2019 (upated). July 10, 2020. https://www.scotusblog.com/2020/07/final-stat-pack-for-october-term-2019/. Accessed August 31, 2020.
31. Hejmanowski D. Flush heard around the world. Delaware Gazette. May 8, 2020. https://www.delgazette.com/opinion/columns/83610/flush-heard-around-the-world. Accessed August 31, 2020.
32. SCOTUSblog.com. California v Texas. https://www.scotusblog.com/case-files/cases/california-v-texas/. Accessed August 31, 2020.
Lifting the restrictions on mifepristone during COVID-19: A step in the right direction
Mifepristone is a safe, effective, and well-tolerated medication for managing miscarriage and for medical abortion when combined with misoprostol.1,2 Since the US Food and Drug Administration (FDA) approved its use in 2000, more than 4 million women have used this medication.3 The combination of mifepristone with misoprostol was used for 39% of all US abortions in 2017.4 Approximately 10% of all clinically recognized pregnancies end in miscarriages, and many are safely managed with either misoprostol alone or with the combination of mifepristone and misoprostol.5
The issue
The prescription and distribution of mifepristone is highly regulated by the FDA via requirements outlined in the Risk Evaluation and Mitigation Strategies (REMS) drug safety program. The FDA may determine a REMS is necessary for a specific drug to ensure the benefits of a drug outweigh the potential risks. A REMS may include an informative package insert for patients, follow-up communication to prescribers—including letters, safety protocols or recommended laboratory tests, or Elements to Assure Safe Use (ETASU). ETASU are types of REMS that are placed on medications that have significant potential for serious adverse effects, and without such restrictions FDA approval would be rescinded.
Are mifepristone requirements fairly applied?
The 3 ETASU restrictions on the distribution of mifepristone are in-person dispensation, prescriber certification, and patient signatures on special forms.6 The in-person dispensing requirement is applied to only 16 other medications (one of which is Mifeprex, the brand version of mifepristone), and Mifeprex/mifepristone are the only ones deemed safe for self-administration—meaning that patients receive the drug from a clinic but then may take it at a site of their choosing. The prescriber certification requirement places expectations on providers to account for distribution of doses and keep records of serial numbers (in effect, having clinicians act as both physician and pharmacist, as most medications are distributed and recorded in pharmacies). The patient form was recommended for elimination in 2016 due to its duplicative information and burden on patients—a recommendation that was then overruled by the FDA commissioner.7
These 3 requirements placed on mifepristone specifically target dosages for use related to abortions and miscarriages. Mifepristone is used to treat other medical conditions, with much higher doses, without the same restrictions—in fact, the FDA has allowed much higher doses of mifepristone to be mailed directly to a patient when prescribed for different disorders. The American College of Obstetricians and Gynecologists (ACOG) has long opposed the burdensome REMS requirements on mifepristone for reproductive health indications.8
Arguments regarding the safety of mifepristone must be understood in the context of how the medication is taken, and the unique difference with other medications that must be administered by physicians or in health care facilities. Mifepristone is self-administered, and the desired effect—evacuation of uterine contents—typically occurs after a patient takes the accompanying medication misoprostol, which is some 24 to 72 hours later. This timeframe makes it highly unlikely that any patient would be in the presence of their provider at the time of medication effect, thus an in-person dispensing requirement has no medical bearing on the outcome of the health of the patient.
REMS changes during the COVID-19 pandemic
The coronavirus disease 2019 (COVID-19) pandemic has necessarily changed the structure of REMS and ETASU requirements for many medications, with changes made in order to mitigate viral transmission through the limitation of unnecessary visits to clinics or hospitals. The FDA announced in March of 2020 that it would not enforce pre-prescription requirements, such as laboratory or magnetic resonance imaging results, for many medications (including those more toxic than mifepristone), and that it would lift the requirement for in-person dispensation of several medications.9 Also in March 2020 the Department of Health and Human Services Secretary (HHS) and the Drug Enforcement Agency (DEA) activated a “telemedicine exception” to allow physicians to use telemedicine to satisfy mandatory requirements for prescribing controlled substances, including opioids.10
Despite repeated pleas from organizations, individuals, and physician groups, the FDA continued to enforce the REMS/ETASU for mifepristone as the pandemic decimated communities. Importantly, the pandemic has not had an equal effect on all communities, and the disparities highlighted in outcomes as related to COVID-19 are also reflected in disparities to access to reproductive choices.11 By enforcing REMS/ETASU for mifepristone during a global pandemic, the FDA has placed additional burden on women and people who menstruate. As offices and clinics have closed, and as many jobs have evaporated, additional barriers have emerged, such as lack of childcare, fewer transportation options, and decreased clinic appointments.
As the pandemic continues to affect communities in the United States, ACOG has issued guidance recommending assessment for eligibility for medical abortion remotely, and has encouraged the use of telemedicine and other remote interactions for its members and patients to limit transmission of the virus.
The lawsuit
On May 27, 2020, the American Civil Liberties Union (ACLU) (on behalf of ACOG, the Council of University Chairs of Obstetrics and Gynecology, New York State Academy of Family Physicians, SisterSong, and Honor MacNaughton, MD) filed a civil action against the FDA and HHS challenging the requirement for in-person dispensing of mifepristone and associated ETASU requirements during the COVID-19 pandemic. The plaintiffs sought this injunction based on the claim that these restrictions during the pandemic infringe on the constitutional rights to patients’ privacy and liberty and to equal protection of the law as protected by the Due Process Clause of the Fifth Amendment. Additionally, the ACLU and other organizations said these unnecessary restrictions place patients, providers, and staff at unnecessary risk of viral exposure amidst a global pandemic.
The verdict
On July 13, 2020, a federal court granted the preliminary injunction to suspend FDA’s enforcement of the in-person requirements of mifepristone for abortion during the COVID-19 pandemic. The court denied the motion for suspension of in-person restrictions as applied to miscarriage management. The preliminary injunction applies nationwide without geographic limitation. It will remain in effect until the end of the litigation or for 30 days following the expiration of the public health emergency.
What the outcome means
This injunction is a step in the right direction for patients and providers to allow for autonomy and clinical practice guided by clinician expertise. However, this ruling remains narrow. Patients must be counseled about mifepristone via telemedicine and sign a Patient Agreement Form, which must be returned electronically or by mail. Patients must receive a copy of the mifepristone medication guide, and dispensing of mifepristone must still be conducted by or under the supervision of a certified provider. The medication may not be dispensed by retail pharmacies, thus requiring providers to arrange for mailing of prescriptions to patients. Given state-based legal statutes regarding mailing of medications, this injunction may not lead to an immediate increase in access to care. In addition, patients seeking management for miscarriage must go to clinic to have mifepristone dispensed and thus risk exposure to viral transmission.
What now?
The regulation of mifepristone—in spite of excellent safety and specifically for the narrow purpose of administration in the setting of abortion and miscarriage care—is by definition a discriminatory practice against patients and providers. As clinicians, we are duty-bound to speak out against injustices to our practices and our patients. At a local level, we can work to implement safe practices in the setting of this injunction and continue to work on a national level to ensure this injunction becomes permanent and with more broad scope to eliminate all of the REMS requirements for mifepristone.
ACTION ITEMS
- Act locally! Are you an abortion provider? Contact your local ACLU (find them here) or lawyer in your area for assistance navigating the legal landscape to prescribe after this injunction.
- Act statewide! Press candidates in your state to stand up for science and data. Support legislative acts and bills that address combating discriminatory regulations.
- Act nationally! The President is responsible for appointing the Commissioner of the FDA and the Secretary of Health and Human Services (with Senate advice and consent). Who we elect matters. Seek out opportunities to become involved in increasing access to and awareness of voter registration and Election Day, and speak out against voter suppression. Make sure you are registered to vote here and check your area to review new recommendations amidst the pandemic.
- American College of Obstetricians and Gynecologists. Practice bulletin number 143: medical management of first trimester abortion. Obstet Gynecol. 2014;123:676-692.
- Schreiber CA, Crenin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
- Danco Laboratories. Mifeprex effectiveness and advantages. https://www.earlyoptionpill.com/is-mifeprex-right-for-me/effectiveness-advantages/ Accessed August 2, 2020.
- Jones RK, Witwer E, Jerman J. Abortion incidence and service availability in the United States, 2017. September 2019. https://www.guttmacher.org/report/abortion-incidence-service-availability-us-2017. Accessed September 10, 2020.
- American College of Obstetricians and Gynecologists. Practice bulletin no. 150: early pregnancy loss. Obstet Gynecol. 2015;125:1258-1267.
- US Food and Drug Administration. Risk evaluation and mitigation strategy (REMS) single shared system for mifepristone 200 mg. April 2019. https://www.accessdata.fda.gov/drugsatfda_docs/rems/Mifepristone_2019_04_11_REMS_Full.pdf. Accessed September 10, 2020.
- US Food and Drug Administration; Center for Drug Evaluation and Research. 2016 REMS Review, Summary Review 25. March 29, 2016. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/020687Orig1s020SumR.pdf. Accessed September 10, 2020.
- American College of Obstetricians and Gynecologists. Improving access to mifepristone for reproductive health indications. June 2018. https://www.acog.org/clinical-information/policy-and-position-statements/position-statements/2018/improving-access-to-mifepristone-for-reproductive-health-indications. Accessed August 2, 2020.
- US Food and Drug Administration. Policy for certain REMS requirements during the COVID-19 public health emergency: guidance for industry and health care professionals. March 2020. https://www.fda.gov/media/136317/download. Accessed September 10, 2020.
- US Department of Justice. US Drug Enforcement Administration. COVID-19 Information Page, Telemedicine. https://www.deadiversion.usdoj.gov/coronavirus.html#TELE. Accessed May 25, 2020.
- Centers for Disease Control and Prevention. Coronavirus disease 2019: health equity considerations and racial and ethnic minority groups. https://www.cdc.gov/coronavirus/2019-ncov/community/health-equity/race-ethnicity.html. Updated July 24, 2020. Accessed September 10, 2020.
Mifepristone is a safe, effective, and well-tolerated medication for managing miscarriage and for medical abortion when combined with misoprostol.1,2 Since the US Food and Drug Administration (FDA) approved its use in 2000, more than 4 million women have used this medication.3 The combination of mifepristone with misoprostol was used for 39% of all US abortions in 2017.4 Approximately 10% of all clinically recognized pregnancies end in miscarriages, and many are safely managed with either misoprostol alone or with the combination of mifepristone and misoprostol.5
The issue
The prescription and distribution of mifepristone is highly regulated by the FDA via requirements outlined in the Risk Evaluation and Mitigation Strategies (REMS) drug safety program. The FDA may determine a REMS is necessary for a specific drug to ensure the benefits of a drug outweigh the potential risks. A REMS may include an informative package insert for patients, follow-up communication to prescribers—including letters, safety protocols or recommended laboratory tests, or Elements to Assure Safe Use (ETASU). ETASU are types of REMS that are placed on medications that have significant potential for serious adverse effects, and without such restrictions FDA approval would be rescinded.
Are mifepristone requirements fairly applied?
The 3 ETASU restrictions on the distribution of mifepristone are in-person dispensation, prescriber certification, and patient signatures on special forms.6 The in-person dispensing requirement is applied to only 16 other medications (one of which is Mifeprex, the brand version of mifepristone), and Mifeprex/mifepristone are the only ones deemed safe for self-administration—meaning that patients receive the drug from a clinic but then may take it at a site of their choosing. The prescriber certification requirement places expectations on providers to account for distribution of doses and keep records of serial numbers (in effect, having clinicians act as both physician and pharmacist, as most medications are distributed and recorded in pharmacies). The patient form was recommended for elimination in 2016 due to its duplicative information and burden on patients—a recommendation that was then overruled by the FDA commissioner.7
These 3 requirements placed on mifepristone specifically target dosages for use related to abortions and miscarriages. Mifepristone is used to treat other medical conditions, with much higher doses, without the same restrictions—in fact, the FDA has allowed much higher doses of mifepristone to be mailed directly to a patient when prescribed for different disorders. The American College of Obstetricians and Gynecologists (ACOG) has long opposed the burdensome REMS requirements on mifepristone for reproductive health indications.8
Arguments regarding the safety of mifepristone must be understood in the context of how the medication is taken, and the unique difference with other medications that must be administered by physicians or in health care facilities. Mifepristone is self-administered, and the desired effect—evacuation of uterine contents—typically occurs after a patient takes the accompanying medication misoprostol, which is some 24 to 72 hours later. This timeframe makes it highly unlikely that any patient would be in the presence of their provider at the time of medication effect, thus an in-person dispensing requirement has no medical bearing on the outcome of the health of the patient.
REMS changes during the COVID-19 pandemic
The coronavirus disease 2019 (COVID-19) pandemic has necessarily changed the structure of REMS and ETASU requirements for many medications, with changes made in order to mitigate viral transmission through the limitation of unnecessary visits to clinics or hospitals. The FDA announced in March of 2020 that it would not enforce pre-prescription requirements, such as laboratory or magnetic resonance imaging results, for many medications (including those more toxic than mifepristone), and that it would lift the requirement for in-person dispensation of several medications.9 Also in March 2020 the Department of Health and Human Services Secretary (HHS) and the Drug Enforcement Agency (DEA) activated a “telemedicine exception” to allow physicians to use telemedicine to satisfy mandatory requirements for prescribing controlled substances, including opioids.10
Despite repeated pleas from organizations, individuals, and physician groups, the FDA continued to enforce the REMS/ETASU for mifepristone as the pandemic decimated communities. Importantly, the pandemic has not had an equal effect on all communities, and the disparities highlighted in outcomes as related to COVID-19 are also reflected in disparities to access to reproductive choices.11 By enforcing REMS/ETASU for mifepristone during a global pandemic, the FDA has placed additional burden on women and people who menstruate. As offices and clinics have closed, and as many jobs have evaporated, additional barriers have emerged, such as lack of childcare, fewer transportation options, and decreased clinic appointments.
As the pandemic continues to affect communities in the United States, ACOG has issued guidance recommending assessment for eligibility for medical abortion remotely, and has encouraged the use of telemedicine and other remote interactions for its members and patients to limit transmission of the virus.
The lawsuit
On May 27, 2020, the American Civil Liberties Union (ACLU) (on behalf of ACOG, the Council of University Chairs of Obstetrics and Gynecology, New York State Academy of Family Physicians, SisterSong, and Honor MacNaughton, MD) filed a civil action against the FDA and HHS challenging the requirement for in-person dispensing of mifepristone and associated ETASU requirements during the COVID-19 pandemic. The plaintiffs sought this injunction based on the claim that these restrictions during the pandemic infringe on the constitutional rights to patients’ privacy and liberty and to equal protection of the law as protected by the Due Process Clause of the Fifth Amendment. Additionally, the ACLU and other organizations said these unnecessary restrictions place patients, providers, and staff at unnecessary risk of viral exposure amidst a global pandemic.
The verdict
On July 13, 2020, a federal court granted the preliminary injunction to suspend FDA’s enforcement of the in-person requirements of mifepristone for abortion during the COVID-19 pandemic. The court denied the motion for suspension of in-person restrictions as applied to miscarriage management. The preliminary injunction applies nationwide without geographic limitation. It will remain in effect until the end of the litigation or for 30 days following the expiration of the public health emergency.
What the outcome means
This injunction is a step in the right direction for patients and providers to allow for autonomy and clinical practice guided by clinician expertise. However, this ruling remains narrow. Patients must be counseled about mifepristone via telemedicine and sign a Patient Agreement Form, which must be returned electronically or by mail. Patients must receive a copy of the mifepristone medication guide, and dispensing of mifepristone must still be conducted by or under the supervision of a certified provider. The medication may not be dispensed by retail pharmacies, thus requiring providers to arrange for mailing of prescriptions to patients. Given state-based legal statutes regarding mailing of medications, this injunction may not lead to an immediate increase in access to care. In addition, patients seeking management for miscarriage must go to clinic to have mifepristone dispensed and thus risk exposure to viral transmission.
What now?
The regulation of mifepristone—in spite of excellent safety and specifically for the narrow purpose of administration in the setting of abortion and miscarriage care—is by definition a discriminatory practice against patients and providers. As clinicians, we are duty-bound to speak out against injustices to our practices and our patients. At a local level, we can work to implement safe practices in the setting of this injunction and continue to work on a national level to ensure this injunction becomes permanent and with more broad scope to eliminate all of the REMS requirements for mifepristone.
ACTION ITEMS
- Act locally! Are you an abortion provider? Contact your local ACLU (find them here) or lawyer in your area for assistance navigating the legal landscape to prescribe after this injunction.
- Act statewide! Press candidates in your state to stand up for science and data. Support legislative acts and bills that address combating discriminatory regulations.
- Act nationally! The President is responsible for appointing the Commissioner of the FDA and the Secretary of Health and Human Services (with Senate advice and consent). Who we elect matters. Seek out opportunities to become involved in increasing access to and awareness of voter registration and Election Day, and speak out against voter suppression. Make sure you are registered to vote here and check your area to review new recommendations amidst the pandemic.
Mifepristone is a safe, effective, and well-tolerated medication for managing miscarriage and for medical abortion when combined with misoprostol.1,2 Since the US Food and Drug Administration (FDA) approved its use in 2000, more than 4 million women have used this medication.3 The combination of mifepristone with misoprostol was used for 39% of all US abortions in 2017.4 Approximately 10% of all clinically recognized pregnancies end in miscarriages, and many are safely managed with either misoprostol alone or with the combination of mifepristone and misoprostol.5
The issue
The prescription and distribution of mifepristone is highly regulated by the FDA via requirements outlined in the Risk Evaluation and Mitigation Strategies (REMS) drug safety program. The FDA may determine a REMS is necessary for a specific drug to ensure the benefits of a drug outweigh the potential risks. A REMS may include an informative package insert for patients, follow-up communication to prescribers—including letters, safety protocols or recommended laboratory tests, or Elements to Assure Safe Use (ETASU). ETASU are types of REMS that are placed on medications that have significant potential for serious adverse effects, and without such restrictions FDA approval would be rescinded.
Are mifepristone requirements fairly applied?
The 3 ETASU restrictions on the distribution of mifepristone are in-person dispensation, prescriber certification, and patient signatures on special forms.6 The in-person dispensing requirement is applied to only 16 other medications (one of which is Mifeprex, the brand version of mifepristone), and Mifeprex/mifepristone are the only ones deemed safe for self-administration—meaning that patients receive the drug from a clinic but then may take it at a site of their choosing. The prescriber certification requirement places expectations on providers to account for distribution of doses and keep records of serial numbers (in effect, having clinicians act as both physician and pharmacist, as most medications are distributed and recorded in pharmacies). The patient form was recommended for elimination in 2016 due to its duplicative information and burden on patients—a recommendation that was then overruled by the FDA commissioner.7
These 3 requirements placed on mifepristone specifically target dosages for use related to abortions and miscarriages. Mifepristone is used to treat other medical conditions, with much higher doses, without the same restrictions—in fact, the FDA has allowed much higher doses of mifepristone to be mailed directly to a patient when prescribed for different disorders. The American College of Obstetricians and Gynecologists (ACOG) has long opposed the burdensome REMS requirements on mifepristone for reproductive health indications.8
Arguments regarding the safety of mifepristone must be understood in the context of how the medication is taken, and the unique difference with other medications that must be administered by physicians or in health care facilities. Mifepristone is self-administered, and the desired effect—evacuation of uterine contents—typically occurs after a patient takes the accompanying medication misoprostol, which is some 24 to 72 hours later. This timeframe makes it highly unlikely that any patient would be in the presence of their provider at the time of medication effect, thus an in-person dispensing requirement has no medical bearing on the outcome of the health of the patient.
REMS changes during the COVID-19 pandemic
The coronavirus disease 2019 (COVID-19) pandemic has necessarily changed the structure of REMS and ETASU requirements for many medications, with changes made in order to mitigate viral transmission through the limitation of unnecessary visits to clinics or hospitals. The FDA announced in March of 2020 that it would not enforce pre-prescription requirements, such as laboratory or magnetic resonance imaging results, for many medications (including those more toxic than mifepristone), and that it would lift the requirement for in-person dispensation of several medications.9 Also in March 2020 the Department of Health and Human Services Secretary (HHS) and the Drug Enforcement Agency (DEA) activated a “telemedicine exception” to allow physicians to use telemedicine to satisfy mandatory requirements for prescribing controlled substances, including opioids.10
Despite repeated pleas from organizations, individuals, and physician groups, the FDA continued to enforce the REMS/ETASU for mifepristone as the pandemic decimated communities. Importantly, the pandemic has not had an equal effect on all communities, and the disparities highlighted in outcomes as related to COVID-19 are also reflected in disparities to access to reproductive choices.11 By enforcing REMS/ETASU for mifepristone during a global pandemic, the FDA has placed additional burden on women and people who menstruate. As offices and clinics have closed, and as many jobs have evaporated, additional barriers have emerged, such as lack of childcare, fewer transportation options, and decreased clinic appointments.
As the pandemic continues to affect communities in the United States, ACOG has issued guidance recommending assessment for eligibility for medical abortion remotely, and has encouraged the use of telemedicine and other remote interactions for its members and patients to limit transmission of the virus.
The lawsuit
On May 27, 2020, the American Civil Liberties Union (ACLU) (on behalf of ACOG, the Council of University Chairs of Obstetrics and Gynecology, New York State Academy of Family Physicians, SisterSong, and Honor MacNaughton, MD) filed a civil action against the FDA and HHS challenging the requirement for in-person dispensing of mifepristone and associated ETASU requirements during the COVID-19 pandemic. The plaintiffs sought this injunction based on the claim that these restrictions during the pandemic infringe on the constitutional rights to patients’ privacy and liberty and to equal protection of the law as protected by the Due Process Clause of the Fifth Amendment. Additionally, the ACLU and other organizations said these unnecessary restrictions place patients, providers, and staff at unnecessary risk of viral exposure amidst a global pandemic.
The verdict
On July 13, 2020, a federal court granted the preliminary injunction to suspend FDA’s enforcement of the in-person requirements of mifepristone for abortion during the COVID-19 pandemic. The court denied the motion for suspension of in-person restrictions as applied to miscarriage management. The preliminary injunction applies nationwide without geographic limitation. It will remain in effect until the end of the litigation or for 30 days following the expiration of the public health emergency.
What the outcome means
This injunction is a step in the right direction for patients and providers to allow for autonomy and clinical practice guided by clinician expertise. However, this ruling remains narrow. Patients must be counseled about mifepristone via telemedicine and sign a Patient Agreement Form, which must be returned electronically or by mail. Patients must receive a copy of the mifepristone medication guide, and dispensing of mifepristone must still be conducted by or under the supervision of a certified provider. The medication may not be dispensed by retail pharmacies, thus requiring providers to arrange for mailing of prescriptions to patients. Given state-based legal statutes regarding mailing of medications, this injunction may not lead to an immediate increase in access to care. In addition, patients seeking management for miscarriage must go to clinic to have mifepristone dispensed and thus risk exposure to viral transmission.
What now?
The regulation of mifepristone—in spite of excellent safety and specifically for the narrow purpose of administration in the setting of abortion and miscarriage care—is by definition a discriminatory practice against patients and providers. As clinicians, we are duty-bound to speak out against injustices to our practices and our patients. At a local level, we can work to implement safe practices in the setting of this injunction and continue to work on a national level to ensure this injunction becomes permanent and with more broad scope to eliminate all of the REMS requirements for mifepristone.
ACTION ITEMS
- Act locally! Are you an abortion provider? Contact your local ACLU (find them here) or lawyer in your area for assistance navigating the legal landscape to prescribe after this injunction.
- Act statewide! Press candidates in your state to stand up for science and data. Support legislative acts and bills that address combating discriminatory regulations.
- Act nationally! The President is responsible for appointing the Commissioner of the FDA and the Secretary of Health and Human Services (with Senate advice and consent). Who we elect matters. Seek out opportunities to become involved in increasing access to and awareness of voter registration and Election Day, and speak out against voter suppression. Make sure you are registered to vote here and check your area to review new recommendations amidst the pandemic.
- American College of Obstetricians and Gynecologists. Practice bulletin number 143: medical management of first trimester abortion. Obstet Gynecol. 2014;123:676-692.
- Schreiber CA, Crenin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
- Danco Laboratories. Mifeprex effectiveness and advantages. https://www.earlyoptionpill.com/is-mifeprex-right-for-me/effectiveness-advantages/ Accessed August 2, 2020.
- Jones RK, Witwer E, Jerman J. Abortion incidence and service availability in the United States, 2017. September 2019. https://www.guttmacher.org/report/abortion-incidence-service-availability-us-2017. Accessed September 10, 2020.
- American College of Obstetricians and Gynecologists. Practice bulletin no. 150: early pregnancy loss. Obstet Gynecol. 2015;125:1258-1267.
- US Food and Drug Administration. Risk evaluation and mitigation strategy (REMS) single shared system for mifepristone 200 mg. April 2019. https://www.accessdata.fda.gov/drugsatfda_docs/rems/Mifepristone_2019_04_11_REMS_Full.pdf. Accessed September 10, 2020.
- US Food and Drug Administration; Center for Drug Evaluation and Research. 2016 REMS Review, Summary Review 25. March 29, 2016. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/020687Orig1s020SumR.pdf. Accessed September 10, 2020.
- American College of Obstetricians and Gynecologists. Improving access to mifepristone for reproductive health indications. June 2018. https://www.acog.org/clinical-information/policy-and-position-statements/position-statements/2018/improving-access-to-mifepristone-for-reproductive-health-indications. Accessed August 2, 2020.
- US Food and Drug Administration. Policy for certain REMS requirements during the COVID-19 public health emergency: guidance for industry and health care professionals. March 2020. https://www.fda.gov/media/136317/download. Accessed September 10, 2020.
- US Department of Justice. US Drug Enforcement Administration. COVID-19 Information Page, Telemedicine. https://www.deadiversion.usdoj.gov/coronavirus.html#TELE. Accessed May 25, 2020.
- Centers for Disease Control and Prevention. Coronavirus disease 2019: health equity considerations and racial and ethnic minority groups. https://www.cdc.gov/coronavirus/2019-ncov/community/health-equity/race-ethnicity.html. Updated July 24, 2020. Accessed September 10, 2020.
- American College of Obstetricians and Gynecologists. Practice bulletin number 143: medical management of first trimester abortion. Obstet Gynecol. 2014;123:676-692.
- Schreiber CA, Crenin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
- Danco Laboratories. Mifeprex effectiveness and advantages. https://www.earlyoptionpill.com/is-mifeprex-right-for-me/effectiveness-advantages/ Accessed August 2, 2020.
- Jones RK, Witwer E, Jerman J. Abortion incidence and service availability in the United States, 2017. September 2019. https://www.guttmacher.org/report/abortion-incidence-service-availability-us-2017. Accessed September 10, 2020.
- American College of Obstetricians and Gynecologists. Practice bulletin no. 150: early pregnancy loss. Obstet Gynecol. 2015;125:1258-1267.
- US Food and Drug Administration. Risk evaluation and mitigation strategy (REMS) single shared system for mifepristone 200 mg. April 2019. https://www.accessdata.fda.gov/drugsatfda_docs/rems/Mifepristone_2019_04_11_REMS_Full.pdf. Accessed September 10, 2020.
- US Food and Drug Administration; Center for Drug Evaluation and Research. 2016 REMS Review, Summary Review 25. March 29, 2016. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/020687Orig1s020SumR.pdf. Accessed September 10, 2020.
- American College of Obstetricians and Gynecologists. Improving access to mifepristone for reproductive health indications. June 2018. https://www.acog.org/clinical-information/policy-and-position-statements/position-statements/2018/improving-access-to-mifepristone-for-reproductive-health-indications. Accessed August 2, 2020.
- US Food and Drug Administration. Policy for certain REMS requirements during the COVID-19 public health emergency: guidance for industry and health care professionals. March 2020. https://www.fda.gov/media/136317/download. Accessed September 10, 2020.
- US Department of Justice. US Drug Enforcement Administration. COVID-19 Information Page, Telemedicine. https://www.deadiversion.usdoj.gov/coronavirus.html#TELE. Accessed May 25, 2020.
- Centers for Disease Control and Prevention. Coronavirus disease 2019: health equity considerations and racial and ethnic minority groups. https://www.cdc.gov/coronavirus/2019-ncov/community/health-equity/race-ethnicity.html. Updated July 24, 2020. Accessed September 10, 2020.
Postpartum tubal ligation safe in obese women
Women with a high body mass index who request tubal ligation immediately post partum face no increased risk of complications, compared with normal-weight woman, according to a large, single-institution, retrospective study.
“Our study underscores the overall safety of postpartum tubal ligation among overweight and obese women,” John J. Byrne, MD, MPH, and colleagues at the University of Texas, Dallas, reported in Obstetrics & Gynecology.
“Even among women in the highest BMI category, this procedure is safe and effective,” they noted, despite previous studies identifying body mass index (BMI) higher than 40 kg/m2 “as a significant barrier to this procedure.”
“For the woman who is appropriately counseled and desires permanent contraception, BMI should not impede her access to the procedure,” Dr. Byrne and associates said.
The study included 3,670 women undergoing postpartum tubal ligation after a vaginal delivery between August 2015 and March 2019 at Parkland Hospital, which is operated by the Dallas County Hospital District.
The method used was the Parkland-type tubal ligation – a bilateral midsegment partial salpingectomy performed through a 2-3 cm infraumbilical incision. Women were excluded if they were planning additional surgery, such as ovarian cyst removal or hernia repair at the same time.
Comparing a composite outcome of surgical complications and subsequent pregnancies over a 5-year follow-up, the study found no differences across all maternal BMI categories, which were stratified as: underweight or normal weight (BMI, 24.9 or lower), overweight (25-29.9), class I obesity (30-34.9), class II obesity (35-39.9), and class III obesity (40 or higher).
A full breakdown of the composite morbidity included “blood transfusion, aborted procedure, intraoperative complications (bleeding requiring additional surgery, extension of incision), anesthetic complication (high spinal, bronchospasm, postdural puncture headaches requiring blood patch, and allergic reaction to anesthetic), postoperative complication (deep wound infection, venous thromboembolism, ileus, small bowel obstruction, acute intestinal herniation, peritonitis), return to operating room, incomplete transection of fallopian tube, and subsequent pregnancy,” they reported.
Among the study subjects, the mean BMI was 32.2, with 263 being underweight or normal weight at the time of admission, 1,044 being overweight, 1,371 having class I obesity, 689 having class II obesity, 303 having class III obesity, and 11 patients classified as supermorbidly obese (a BMI of 50 or higher).
Overall, “composite morbidity occurred in 49 (1.3%) women and was not significantly different across BMI categories (P = .07),” noted the authors.
More specifically, there were 19 (1.5%) composite morbidity events in the nonobese cohort and 30 (1.3%) in the obese cohort. “Even among women who had undergone prior abdominal surgery, there was no association of BMI with the rate of procedural complication,” Dr. Byrne and associates added.
The subsequent pregnancy rate was 1.63 per 1,000 procedures performed, which is “significantly lower than previously reported estimates,” they noted. In total, there were six subsequent pregnancies in the cohort: three full term, two ectopic, and one of unknown location.
“Although there was variability in operative time in all BMI categories, this is likely not clinically relevant as the range in operative time overlapped across groups,” reported the authors. “Other surgical metrics, such as estimated blood loss and length of hospitalization after tubal ligation, were found to be no different between BMI categories.”
Their findings “can be generalized to other tubal ligation forms, such as modified Pomeroy and even possibly salpingectomy, if the minilaparotomy incision is the same,” Dr. Byrne and colleagues suggested.
“This innovative study adds an important practical perspective to the literature on postpartum permanent contraception – a finding that should be reassuring for obstetrician/gynecologists,” commented Eve Espey, MD MPH, who was not involved in the research.
“Women with high BMI are significantly less likely to receive desired postvaginal delivery tubal ligation, compared to lower-BMI women, as documented in several prior studies,” said Dr. Espey, who is professor and chair of the department of obstetrics and gynecology at the University of New Mexico, Albuquerque.
“Although those studies did not explore the reasons for nonfulfillment, intuitively concerns about complications or inability to complete the procedure are the most likely explanations,” she added.
“Although this study is limited by its retrospective nature, the smaller number of women in the highest BMI category, and lack of information on patients with unfulfilled requests for tubal ligation, it is overall well designed and should serve to encourage physicians to proceed with postvaginal delivery tubal ligation in patients across all BMI categories,” Dr. Espey concluded.
The study received no external funding; Dr. Byrne and associates reported no relevant financial disclosures. Dr. Espey is a member of the Ob.Gyn. News editorial advisory board, and said she has no relevant financial disclosures.
SOURCE: Byrne JJ et al. Obstet Gynecol. 2020;136:342-8.
Women with a high body mass index who request tubal ligation immediately post partum face no increased risk of complications, compared with normal-weight woman, according to a large, single-institution, retrospective study.
“Our study underscores the overall safety of postpartum tubal ligation among overweight and obese women,” John J. Byrne, MD, MPH, and colleagues at the University of Texas, Dallas, reported in Obstetrics & Gynecology.
“Even among women in the highest BMI category, this procedure is safe and effective,” they noted, despite previous studies identifying body mass index (BMI) higher than 40 kg/m2 “as a significant barrier to this procedure.”
“For the woman who is appropriately counseled and desires permanent contraception, BMI should not impede her access to the procedure,” Dr. Byrne and associates said.
The study included 3,670 women undergoing postpartum tubal ligation after a vaginal delivery between August 2015 and March 2019 at Parkland Hospital, which is operated by the Dallas County Hospital District.
The method used was the Parkland-type tubal ligation – a bilateral midsegment partial salpingectomy performed through a 2-3 cm infraumbilical incision. Women were excluded if they were planning additional surgery, such as ovarian cyst removal or hernia repair at the same time.
Comparing a composite outcome of surgical complications and subsequent pregnancies over a 5-year follow-up, the study found no differences across all maternal BMI categories, which were stratified as: underweight or normal weight (BMI, 24.9 or lower), overweight (25-29.9), class I obesity (30-34.9), class II obesity (35-39.9), and class III obesity (40 or higher).
A full breakdown of the composite morbidity included “blood transfusion, aborted procedure, intraoperative complications (bleeding requiring additional surgery, extension of incision), anesthetic complication (high spinal, bronchospasm, postdural puncture headaches requiring blood patch, and allergic reaction to anesthetic), postoperative complication (deep wound infection, venous thromboembolism, ileus, small bowel obstruction, acute intestinal herniation, peritonitis), return to operating room, incomplete transection of fallopian tube, and subsequent pregnancy,” they reported.
Among the study subjects, the mean BMI was 32.2, with 263 being underweight or normal weight at the time of admission, 1,044 being overweight, 1,371 having class I obesity, 689 having class II obesity, 303 having class III obesity, and 11 patients classified as supermorbidly obese (a BMI of 50 or higher).
Overall, “composite morbidity occurred in 49 (1.3%) women and was not significantly different across BMI categories (P = .07),” noted the authors.
More specifically, there were 19 (1.5%) composite morbidity events in the nonobese cohort and 30 (1.3%) in the obese cohort. “Even among women who had undergone prior abdominal surgery, there was no association of BMI with the rate of procedural complication,” Dr. Byrne and associates added.
The subsequent pregnancy rate was 1.63 per 1,000 procedures performed, which is “significantly lower than previously reported estimates,” they noted. In total, there were six subsequent pregnancies in the cohort: three full term, two ectopic, and one of unknown location.
“Although there was variability in operative time in all BMI categories, this is likely not clinically relevant as the range in operative time overlapped across groups,” reported the authors. “Other surgical metrics, such as estimated blood loss and length of hospitalization after tubal ligation, were found to be no different between BMI categories.”
Their findings “can be generalized to other tubal ligation forms, such as modified Pomeroy and even possibly salpingectomy, if the minilaparotomy incision is the same,” Dr. Byrne and colleagues suggested.
“This innovative study adds an important practical perspective to the literature on postpartum permanent contraception – a finding that should be reassuring for obstetrician/gynecologists,” commented Eve Espey, MD MPH, who was not involved in the research.
“Women with high BMI are significantly less likely to receive desired postvaginal delivery tubal ligation, compared to lower-BMI women, as documented in several prior studies,” said Dr. Espey, who is professor and chair of the department of obstetrics and gynecology at the University of New Mexico, Albuquerque.
“Although those studies did not explore the reasons for nonfulfillment, intuitively concerns about complications or inability to complete the procedure are the most likely explanations,” she added.
“Although this study is limited by its retrospective nature, the smaller number of women in the highest BMI category, and lack of information on patients with unfulfilled requests for tubal ligation, it is overall well designed and should serve to encourage physicians to proceed with postvaginal delivery tubal ligation in patients across all BMI categories,” Dr. Espey concluded.
The study received no external funding; Dr. Byrne and associates reported no relevant financial disclosures. Dr. Espey is a member of the Ob.Gyn. News editorial advisory board, and said she has no relevant financial disclosures.
SOURCE: Byrne JJ et al. Obstet Gynecol. 2020;136:342-8.
Women with a high body mass index who request tubal ligation immediately post partum face no increased risk of complications, compared with normal-weight woman, according to a large, single-institution, retrospective study.
“Our study underscores the overall safety of postpartum tubal ligation among overweight and obese women,” John J. Byrne, MD, MPH, and colleagues at the University of Texas, Dallas, reported in Obstetrics & Gynecology.
“Even among women in the highest BMI category, this procedure is safe and effective,” they noted, despite previous studies identifying body mass index (BMI) higher than 40 kg/m2 “as a significant barrier to this procedure.”
“For the woman who is appropriately counseled and desires permanent contraception, BMI should not impede her access to the procedure,” Dr. Byrne and associates said.
The study included 3,670 women undergoing postpartum tubal ligation after a vaginal delivery between August 2015 and March 2019 at Parkland Hospital, which is operated by the Dallas County Hospital District.
The method used was the Parkland-type tubal ligation – a bilateral midsegment partial salpingectomy performed through a 2-3 cm infraumbilical incision. Women were excluded if they were planning additional surgery, such as ovarian cyst removal or hernia repair at the same time.
Comparing a composite outcome of surgical complications and subsequent pregnancies over a 5-year follow-up, the study found no differences across all maternal BMI categories, which were stratified as: underweight or normal weight (BMI, 24.9 or lower), overweight (25-29.9), class I obesity (30-34.9), class II obesity (35-39.9), and class III obesity (40 or higher).
A full breakdown of the composite morbidity included “blood transfusion, aborted procedure, intraoperative complications (bleeding requiring additional surgery, extension of incision), anesthetic complication (high spinal, bronchospasm, postdural puncture headaches requiring blood patch, and allergic reaction to anesthetic), postoperative complication (deep wound infection, venous thromboembolism, ileus, small bowel obstruction, acute intestinal herniation, peritonitis), return to operating room, incomplete transection of fallopian tube, and subsequent pregnancy,” they reported.
Among the study subjects, the mean BMI was 32.2, with 263 being underweight or normal weight at the time of admission, 1,044 being overweight, 1,371 having class I obesity, 689 having class II obesity, 303 having class III obesity, and 11 patients classified as supermorbidly obese (a BMI of 50 or higher).
Overall, “composite morbidity occurred in 49 (1.3%) women and was not significantly different across BMI categories (P = .07),” noted the authors.
More specifically, there were 19 (1.5%) composite morbidity events in the nonobese cohort and 30 (1.3%) in the obese cohort. “Even among women who had undergone prior abdominal surgery, there was no association of BMI with the rate of procedural complication,” Dr. Byrne and associates added.
The subsequent pregnancy rate was 1.63 per 1,000 procedures performed, which is “significantly lower than previously reported estimates,” they noted. In total, there were six subsequent pregnancies in the cohort: three full term, two ectopic, and one of unknown location.
“Although there was variability in operative time in all BMI categories, this is likely not clinically relevant as the range in operative time overlapped across groups,” reported the authors. “Other surgical metrics, such as estimated blood loss and length of hospitalization after tubal ligation, were found to be no different between BMI categories.”
Their findings “can be generalized to other tubal ligation forms, such as modified Pomeroy and even possibly salpingectomy, if the minilaparotomy incision is the same,” Dr. Byrne and colleagues suggested.
“This innovative study adds an important practical perspective to the literature on postpartum permanent contraception – a finding that should be reassuring for obstetrician/gynecologists,” commented Eve Espey, MD MPH, who was not involved in the research.
“Women with high BMI are significantly less likely to receive desired postvaginal delivery tubal ligation, compared to lower-BMI women, as documented in several prior studies,” said Dr. Espey, who is professor and chair of the department of obstetrics and gynecology at the University of New Mexico, Albuquerque.
“Although those studies did not explore the reasons for nonfulfillment, intuitively concerns about complications or inability to complete the procedure are the most likely explanations,” she added.
“Although this study is limited by its retrospective nature, the smaller number of women in the highest BMI category, and lack of information on patients with unfulfilled requests for tubal ligation, it is overall well designed and should serve to encourage physicians to proceed with postvaginal delivery tubal ligation in patients across all BMI categories,” Dr. Espey concluded.
The study received no external funding; Dr. Byrne and associates reported no relevant financial disclosures. Dr. Espey is a member of the Ob.Gyn. News editorial advisory board, and said she has no relevant financial disclosures.
SOURCE: Byrne JJ et al. Obstet Gynecol. 2020;136:342-8.
FROM OBSTETRICS & GYNECOLOGY
Tamoxifen can reduce bleeding in women with contraceptive implants
A study has found that
“Our data support the use of tamoxifen as an effective option that offers the benefit of a shorter duration of treatment than other approaches such as combined oral contraceptives,” wrote Alison B. Edelman, MD, MPH, of Oregon Health & Science University, Portland, and coauthors. The report is in Obstetrics & Gynecology.
To determine if a short course of tamoxifen – typically used to treat breast cancer – could prove beneficial in reducing bothersome bleeding, the researchers launched a 90-day, double-blind randomized trial of women between the ages of 15 and 45 years who had been using the etonogestrel 68-mg subdermal contraceptive implant for at least 30 days. All participants suffered from frequent or prolonged bleeding or spotting during the previous month; their mean age was 24, and most (n = 62) identified as White.
Of the initial 112 participants, 107 began treatment and were split into two groups: 10 mg of tamoxifen twice a day for 7 days (n = 55) or placebo (n = 52). One hundred and four patients completed treatment one, and 88 completed 90 days. After the first treatment, women in the tamoxifen group experienced 9.8 more consecutive days of amenorrhea (95% confidence interval, 4.6-15.0) compared with the placebo group, as well as more total days of no bleeding in the first 90 days (median 73.5 [24-89] versus 68 [11-81], P = .001).
Afterward, both groups underwent a 90-day, open-label study where all participants took tamoxifen. The differences between the groups mostly disappeared, as they both experienced more amenorrhea days (median 56 [6-81] for tamoxifen and 67.5 [7-83] for placebo) and fewer bleeding days (median 12 [0-63] for tamoxifen and 12 [0-82] for placebo) compared with the placebo group during the initial 90 days. Although no serious adverse events occurred, more women taking tamoxifen reported fluid retention (12 versus 1), headache (19 versus 1), and mood changes (13 versus 2).
“This is a very promising drug for this purpose,” Catherine Cansino, MD, MPH, of the University of California, Davis, said in an interview, adding that it is “a bit unconventional because tamoxifen is traditionally used for cancer or precancer.”
As such, she recognized that young people of reproductive age might be a little wary of the drug. That said, having an effective treatment for troublesome bleeding beyond estrogen-based products should ultimately prove beneficial for clinicians and patients alike.
“Unfortunately, we don’t have long-term data so it’s unclear what the safety outcomes are,” she said, “but having another option to address bothersome bleeding can help women stay on birth control longer. The alternative would be pregnancy, with its own associated risks.”
The authors acknowledged their study’s limitations, including a lack of Black patients and the likelihood that their volunteer cohort “may not reflect the general population of implant users who present for discontinuation owing to bleeding problems.” They also enrolled a small but notable number of women who had been using the implant for less than 3 months, noting that bleeding patterns often change from the first 90 days and so “some of these women would likely experience better (or worse) bleeding irrespective of treatment.”
The study was supported by a Merck Women’s Health Investigator Initiated Studies Program and the Oregon Clinical and Translational Research Institute. Four of the authors acknowledged receiving consulting fees and research support from various organizations and pharmaceutical companies. The remaining three had no relevant financial disclosures. Dr. Cansino is a member of the Ob.Gyn. News editorial advisory board. She said she had no relevant financial disclosures.
SOURCE: Edelman AB et al. Obstet Gynecol. 2020 Jul 9. doi: 10.1097/AOG.0000000000003896.
A study has found that
“Our data support the use of tamoxifen as an effective option that offers the benefit of a shorter duration of treatment than other approaches such as combined oral contraceptives,” wrote Alison B. Edelman, MD, MPH, of Oregon Health & Science University, Portland, and coauthors. The report is in Obstetrics & Gynecology.
To determine if a short course of tamoxifen – typically used to treat breast cancer – could prove beneficial in reducing bothersome bleeding, the researchers launched a 90-day, double-blind randomized trial of women between the ages of 15 and 45 years who had been using the etonogestrel 68-mg subdermal contraceptive implant for at least 30 days. All participants suffered from frequent or prolonged bleeding or spotting during the previous month; their mean age was 24, and most (n = 62) identified as White.
Of the initial 112 participants, 107 began treatment and were split into two groups: 10 mg of tamoxifen twice a day for 7 days (n = 55) or placebo (n = 52). One hundred and four patients completed treatment one, and 88 completed 90 days. After the first treatment, women in the tamoxifen group experienced 9.8 more consecutive days of amenorrhea (95% confidence interval, 4.6-15.0) compared with the placebo group, as well as more total days of no bleeding in the first 90 days (median 73.5 [24-89] versus 68 [11-81], P = .001).
Afterward, both groups underwent a 90-day, open-label study where all participants took tamoxifen. The differences between the groups mostly disappeared, as they both experienced more amenorrhea days (median 56 [6-81] for tamoxifen and 67.5 [7-83] for placebo) and fewer bleeding days (median 12 [0-63] for tamoxifen and 12 [0-82] for placebo) compared with the placebo group during the initial 90 days. Although no serious adverse events occurred, more women taking tamoxifen reported fluid retention (12 versus 1), headache (19 versus 1), and mood changes (13 versus 2).
“This is a very promising drug for this purpose,” Catherine Cansino, MD, MPH, of the University of California, Davis, said in an interview, adding that it is “a bit unconventional because tamoxifen is traditionally used for cancer or precancer.”
As such, she recognized that young people of reproductive age might be a little wary of the drug. That said, having an effective treatment for troublesome bleeding beyond estrogen-based products should ultimately prove beneficial for clinicians and patients alike.
“Unfortunately, we don’t have long-term data so it’s unclear what the safety outcomes are,” she said, “but having another option to address bothersome bleeding can help women stay on birth control longer. The alternative would be pregnancy, with its own associated risks.”
The authors acknowledged their study’s limitations, including a lack of Black patients and the likelihood that their volunteer cohort “may not reflect the general population of implant users who present for discontinuation owing to bleeding problems.” They also enrolled a small but notable number of women who had been using the implant for less than 3 months, noting that bleeding patterns often change from the first 90 days and so “some of these women would likely experience better (or worse) bleeding irrespective of treatment.”
The study was supported by a Merck Women’s Health Investigator Initiated Studies Program and the Oregon Clinical and Translational Research Institute. Four of the authors acknowledged receiving consulting fees and research support from various organizations and pharmaceutical companies. The remaining three had no relevant financial disclosures. Dr. Cansino is a member of the Ob.Gyn. News editorial advisory board. She said she had no relevant financial disclosures.
SOURCE: Edelman AB et al. Obstet Gynecol. 2020 Jul 9. doi: 10.1097/AOG.0000000000003896.
A study has found that
“Our data support the use of tamoxifen as an effective option that offers the benefit of a shorter duration of treatment than other approaches such as combined oral contraceptives,” wrote Alison B. Edelman, MD, MPH, of Oregon Health & Science University, Portland, and coauthors. The report is in Obstetrics & Gynecology.
To determine if a short course of tamoxifen – typically used to treat breast cancer – could prove beneficial in reducing bothersome bleeding, the researchers launched a 90-day, double-blind randomized trial of women between the ages of 15 and 45 years who had been using the etonogestrel 68-mg subdermal contraceptive implant for at least 30 days. All participants suffered from frequent or prolonged bleeding or spotting during the previous month; their mean age was 24, and most (n = 62) identified as White.
Of the initial 112 participants, 107 began treatment and were split into two groups: 10 mg of tamoxifen twice a day for 7 days (n = 55) or placebo (n = 52). One hundred and four patients completed treatment one, and 88 completed 90 days. After the first treatment, women in the tamoxifen group experienced 9.8 more consecutive days of amenorrhea (95% confidence interval, 4.6-15.0) compared with the placebo group, as well as more total days of no bleeding in the first 90 days (median 73.5 [24-89] versus 68 [11-81], P = .001).
Afterward, both groups underwent a 90-day, open-label study where all participants took tamoxifen. The differences between the groups mostly disappeared, as they both experienced more amenorrhea days (median 56 [6-81] for tamoxifen and 67.5 [7-83] for placebo) and fewer bleeding days (median 12 [0-63] for tamoxifen and 12 [0-82] for placebo) compared with the placebo group during the initial 90 days. Although no serious adverse events occurred, more women taking tamoxifen reported fluid retention (12 versus 1), headache (19 versus 1), and mood changes (13 versus 2).
“This is a very promising drug for this purpose,” Catherine Cansino, MD, MPH, of the University of California, Davis, said in an interview, adding that it is “a bit unconventional because tamoxifen is traditionally used for cancer or precancer.”
As such, she recognized that young people of reproductive age might be a little wary of the drug. That said, having an effective treatment for troublesome bleeding beyond estrogen-based products should ultimately prove beneficial for clinicians and patients alike.
“Unfortunately, we don’t have long-term data so it’s unclear what the safety outcomes are,” she said, “but having another option to address bothersome bleeding can help women stay on birth control longer. The alternative would be pregnancy, with its own associated risks.”
The authors acknowledged their study’s limitations, including a lack of Black patients and the likelihood that their volunteer cohort “may not reflect the general population of implant users who present for discontinuation owing to bleeding problems.” They also enrolled a small but notable number of women who had been using the implant for less than 3 months, noting that bleeding patterns often change from the first 90 days and so “some of these women would likely experience better (or worse) bleeding irrespective of treatment.”
The study was supported by a Merck Women’s Health Investigator Initiated Studies Program and the Oregon Clinical and Translational Research Institute. Four of the authors acknowledged receiving consulting fees and research support from various organizations and pharmaceutical companies. The remaining three had no relevant financial disclosures. Dr. Cansino is a member of the Ob.Gyn. News editorial advisory board. She said she had no relevant financial disclosures.
SOURCE: Edelman AB et al. Obstet Gynecol. 2020 Jul 9. doi: 10.1097/AOG.0000000000003896.
FROM OBSTETRICS & GYNECOLOGY