User login
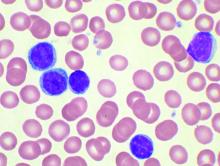
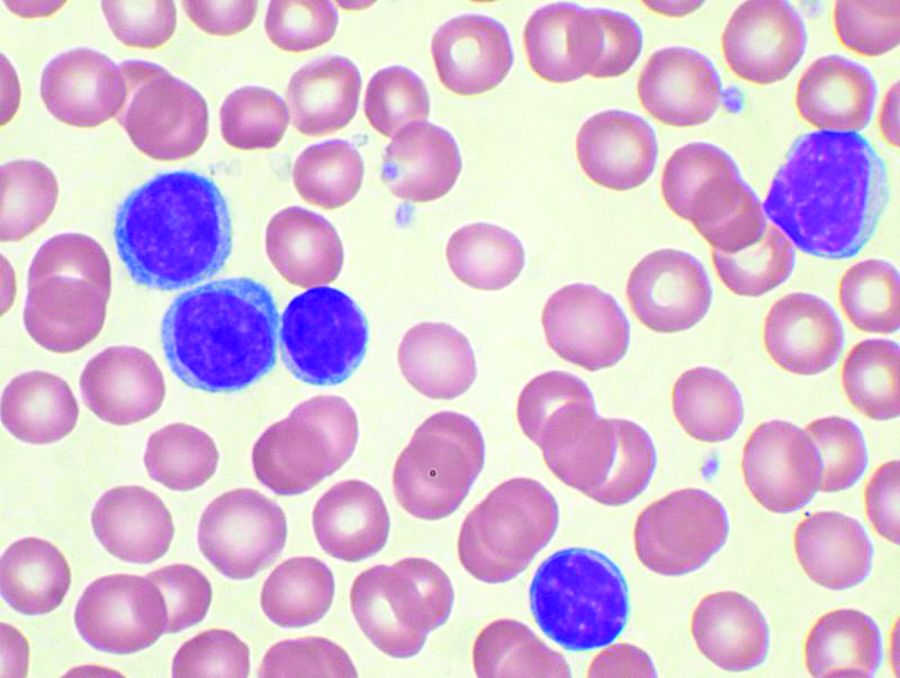
Five prognostic indexes come up short for planning early CLL treatment
Prognostic indexes have been developed recently to assess time to first treatment in early-stage chronic lymphocytic leukemia (CLL) patients. However, none of five indexes evaluated in a study showed more than a moderate prognostic value or were precise enough to permit clinical decisions to be made, according to a report by Spanish researchers.
Their study, published in Clinical Lymphoma, Myeloma and Leukemia, examined the comparative prognostic value of five prognostic indexes – the CLL-IPI, the Barcelona-Brno, the IPS-A, the CLL-01, and the Tailored approach – on evaluating 428 Binet A CLL patients from a multicenter Spanish database which contained the relevant necessary clinical and biological information. The predictive value of the scores was assessed with Harrell´s C index and receiver operating characteristic curve (area under the curve, AUC).
The researchers found a significant association between time to first treatment and risk subgroups for all the indexes used. The most accurate index was the IPS-A (Harrell´s C, 0.72; AUC, 0.76), followed by the CLL-01 (Harrell´s C, 0.69; AUC, 0.70), the CLL-IPI (Harrell´s C, .69; AUC, 0.69), the Barcelona-Brno (Harrell´s C: 0.67, AUC, 0.69) and the Tailored approach (Harrell´s C, 0.61 and 0.58, AUCs, 0.58 and 0.54).
However, the concordance between four of the five indexes (the Tailored approach was not included for technical reasons) compared was low (44%): 146 cases were classified as low risk with all four indexes tested, 36 as intermediate risk, and 4 as high risk. In the remaining 242 patients (56%) at least one discrepancy was detected in the allocation among prognostic subgroups between the indexes. However, only 12 patients (3%) were allocated as low and high risk at the same time with different indexes, showing the extremes of the discordance.
These data suggest that, although all of these indexes “significantly improve clinical staging and help physicians in routine clinical practice, it is necessary to harmonize larger cohorts of patients in order to define the best index for treatment decision making in the real world,” the authors stated.
“All the models had a moderate prognostic value to predict time to first therapy. ... None of them was precise enough to allow clinical decisions based exclusively on it,” the authors concluded.
The study was supported by grants from the Spanish government and a variety of nonprofit institutions. The authors reported no commercial disclosures.
SOURCE: Gascon y Marín IG et al. Clin Lymphoma Myeloma Leuk. 2020 Apr 13. doi: 10.1016/j.clml.2020.03.003.
Prognostic indexes have been developed recently to assess time to first treatment in early-stage chronic lymphocytic leukemia (CLL) patients. However, none of five indexes evaluated in a study showed more than a moderate prognostic value or were precise enough to permit clinical decisions to be made, according to a report by Spanish researchers.
Their study, published in Clinical Lymphoma, Myeloma and Leukemia, examined the comparative prognostic value of five prognostic indexes – the CLL-IPI, the Barcelona-Brno, the IPS-A, the CLL-01, and the Tailored approach – on evaluating 428 Binet A CLL patients from a multicenter Spanish database which contained the relevant necessary clinical and biological information. The predictive value of the scores was assessed with Harrell´s C index and receiver operating characteristic curve (area under the curve, AUC).
The researchers found a significant association between time to first treatment and risk subgroups for all the indexes used. The most accurate index was the IPS-A (Harrell´s C, 0.72; AUC, 0.76), followed by the CLL-01 (Harrell´s C, 0.69; AUC, 0.70), the CLL-IPI (Harrell´s C, .69; AUC, 0.69), the Barcelona-Brno (Harrell´s C: 0.67, AUC, 0.69) and the Tailored approach (Harrell´s C, 0.61 and 0.58, AUCs, 0.58 and 0.54).
However, the concordance between four of the five indexes (the Tailored approach was not included for technical reasons) compared was low (44%): 146 cases were classified as low risk with all four indexes tested, 36 as intermediate risk, and 4 as high risk. In the remaining 242 patients (56%) at least one discrepancy was detected in the allocation among prognostic subgroups between the indexes. However, only 12 patients (3%) were allocated as low and high risk at the same time with different indexes, showing the extremes of the discordance.
These data suggest that, although all of these indexes “significantly improve clinical staging and help physicians in routine clinical practice, it is necessary to harmonize larger cohorts of patients in order to define the best index for treatment decision making in the real world,” the authors stated.
“All the models had a moderate prognostic value to predict time to first therapy. ... None of them was precise enough to allow clinical decisions based exclusively on it,” the authors concluded.
The study was supported by grants from the Spanish government and a variety of nonprofit institutions. The authors reported no commercial disclosures.
SOURCE: Gascon y Marín IG et al. Clin Lymphoma Myeloma Leuk. 2020 Apr 13. doi: 10.1016/j.clml.2020.03.003.
Prognostic indexes have been developed recently to assess time to first treatment in early-stage chronic lymphocytic leukemia (CLL) patients. However, none of five indexes evaluated in a study showed more than a moderate prognostic value or were precise enough to permit clinical decisions to be made, according to a report by Spanish researchers.
Their study, published in Clinical Lymphoma, Myeloma and Leukemia, examined the comparative prognostic value of five prognostic indexes – the CLL-IPI, the Barcelona-Brno, the IPS-A, the CLL-01, and the Tailored approach – on evaluating 428 Binet A CLL patients from a multicenter Spanish database which contained the relevant necessary clinical and biological information. The predictive value of the scores was assessed with Harrell´s C index and receiver operating characteristic curve (area under the curve, AUC).
The researchers found a significant association between time to first treatment and risk subgroups for all the indexes used. The most accurate index was the IPS-A (Harrell´s C, 0.72; AUC, 0.76), followed by the CLL-01 (Harrell´s C, 0.69; AUC, 0.70), the CLL-IPI (Harrell´s C, .69; AUC, 0.69), the Barcelona-Brno (Harrell´s C: 0.67, AUC, 0.69) and the Tailored approach (Harrell´s C, 0.61 and 0.58, AUCs, 0.58 and 0.54).
However, the concordance between four of the five indexes (the Tailored approach was not included for technical reasons) compared was low (44%): 146 cases were classified as low risk with all four indexes tested, 36 as intermediate risk, and 4 as high risk. In the remaining 242 patients (56%) at least one discrepancy was detected in the allocation among prognostic subgroups between the indexes. However, only 12 patients (3%) were allocated as low and high risk at the same time with different indexes, showing the extremes of the discordance.
These data suggest that, although all of these indexes “significantly improve clinical staging and help physicians in routine clinical practice, it is necessary to harmonize larger cohorts of patients in order to define the best index for treatment decision making in the real world,” the authors stated.
“All the models had a moderate prognostic value to predict time to first therapy. ... None of them was precise enough to allow clinical decisions based exclusively on it,” the authors concluded.
The study was supported by grants from the Spanish government and a variety of nonprofit institutions. The authors reported no commercial disclosures.
SOURCE: Gascon y Marín IG et al. Clin Lymphoma Myeloma Leuk. 2020 Apr 13. doi: 10.1016/j.clml.2020.03.003.
FROM CLINICAL LYMPHOMA, MYELOMA AND LEUKEMIA
One-third of high-risk CLL patients received treatment counter to recommendations
Approximately , according to a study based upon data from the informCLL registry. In addition, low levels of prognostic marker testing in these patients was a concern.
Researchers assessed data from 840 enrolled CLL patients, of whom 459 (55%) were previously untreated, and 381 (45%) had relapsed/refractory disease. In terms of therapy, chemoimmunotherapy was more common in previously untreated patients, compared with relapsed/refractory patients (42% vs. 23%), whereas ibrutinib was more frequently used in relapsed/refractory vs. previously untreated patients (51% vs. 39%), according to the researchers.
Fluorescent in situ hybridization (FISH) testing, TP53 mutation, and immunoglobulin heavy chain somatic hypermutation biomarker testing were performed infrequently across all patients at registry enrollment, according to the authors.
Among patients who were tested, the rate of mutated TP53 was the same for previously untreated (14/54; 26%) and relapsed/refractory patients (9/35; 26%). In those patients who were tested, 34% with del(17p), a chromosomal deletion, and 26% of mutated TP53 patients received chemoimmunotherapy combinations. The authors stated that this was concerning in that it contradicts consensus guidelines based on data from several clinical studies. Chemoimmunotherapy is not recommended for these high-risk patients because of poor disease and survival outcomes with this treatment strategy, according to the authors.
“Current clinical practice is not keeping pace with recommendations and guidelines for prognostic
marker testing and subsequent selection of appropriate therapy,” the authors stated.
“Even with the approval of novel agents and updated guidelines, low rates of prognostic biomarker testing may lead to suboptimal therapy choices for patients with unknown risk status. In addition, we note that the presence of high-risk features (del(17p) and TP53) is unfortunately not translating to choosing the optimal therapy for these patients,” the researchers concluded.
The study was sponsored by an AbbVie Company and Janssen. The authors reported consulting and grants from these and other pharmaceutical companies.
SOURCE: Mato AR et al. Clin Lymphoma Myeloma Leuk. 2020;20(3):174-83.
Approximately , according to a study based upon data from the informCLL registry. In addition, low levels of prognostic marker testing in these patients was a concern.
Researchers assessed data from 840 enrolled CLL patients, of whom 459 (55%) were previously untreated, and 381 (45%) had relapsed/refractory disease. In terms of therapy, chemoimmunotherapy was more common in previously untreated patients, compared with relapsed/refractory patients (42% vs. 23%), whereas ibrutinib was more frequently used in relapsed/refractory vs. previously untreated patients (51% vs. 39%), according to the researchers.
Fluorescent in situ hybridization (FISH) testing, TP53 mutation, and immunoglobulin heavy chain somatic hypermutation biomarker testing were performed infrequently across all patients at registry enrollment, according to the authors.
Among patients who were tested, the rate of mutated TP53 was the same for previously untreated (14/54; 26%) and relapsed/refractory patients (9/35; 26%). In those patients who were tested, 34% with del(17p), a chromosomal deletion, and 26% of mutated TP53 patients received chemoimmunotherapy combinations. The authors stated that this was concerning in that it contradicts consensus guidelines based on data from several clinical studies. Chemoimmunotherapy is not recommended for these high-risk patients because of poor disease and survival outcomes with this treatment strategy, according to the authors.
“Current clinical practice is not keeping pace with recommendations and guidelines for prognostic
marker testing and subsequent selection of appropriate therapy,” the authors stated.
“Even with the approval of novel agents and updated guidelines, low rates of prognostic biomarker testing may lead to suboptimal therapy choices for patients with unknown risk status. In addition, we note that the presence of high-risk features (del(17p) and TP53) is unfortunately not translating to choosing the optimal therapy for these patients,” the researchers concluded.
The study was sponsored by an AbbVie Company and Janssen. The authors reported consulting and grants from these and other pharmaceutical companies.
SOURCE: Mato AR et al. Clin Lymphoma Myeloma Leuk. 2020;20(3):174-83.
Approximately , according to a study based upon data from the informCLL registry. In addition, low levels of prognostic marker testing in these patients was a concern.
Researchers assessed data from 840 enrolled CLL patients, of whom 459 (55%) were previously untreated, and 381 (45%) had relapsed/refractory disease. In terms of therapy, chemoimmunotherapy was more common in previously untreated patients, compared with relapsed/refractory patients (42% vs. 23%), whereas ibrutinib was more frequently used in relapsed/refractory vs. previously untreated patients (51% vs. 39%), according to the researchers.
Fluorescent in situ hybridization (FISH) testing, TP53 mutation, and immunoglobulin heavy chain somatic hypermutation biomarker testing were performed infrequently across all patients at registry enrollment, according to the authors.
Among patients who were tested, the rate of mutated TP53 was the same for previously untreated (14/54; 26%) and relapsed/refractory patients (9/35; 26%). In those patients who were tested, 34% with del(17p), a chromosomal deletion, and 26% of mutated TP53 patients received chemoimmunotherapy combinations. The authors stated that this was concerning in that it contradicts consensus guidelines based on data from several clinical studies. Chemoimmunotherapy is not recommended for these high-risk patients because of poor disease and survival outcomes with this treatment strategy, according to the authors.
“Current clinical practice is not keeping pace with recommendations and guidelines for prognostic
marker testing and subsequent selection of appropriate therapy,” the authors stated.
“Even with the approval of novel agents and updated guidelines, low rates of prognostic biomarker testing may lead to suboptimal therapy choices for patients with unknown risk status. In addition, we note that the presence of high-risk features (del(17p) and TP53) is unfortunately not translating to choosing the optimal therapy for these patients,” the researchers concluded.
The study was sponsored by an AbbVie Company and Janssen. The authors reported consulting and grants from these and other pharmaceutical companies.
SOURCE: Mato AR et al. Clin Lymphoma Myeloma Leuk. 2020;20(3):174-83.
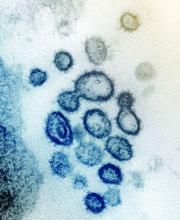
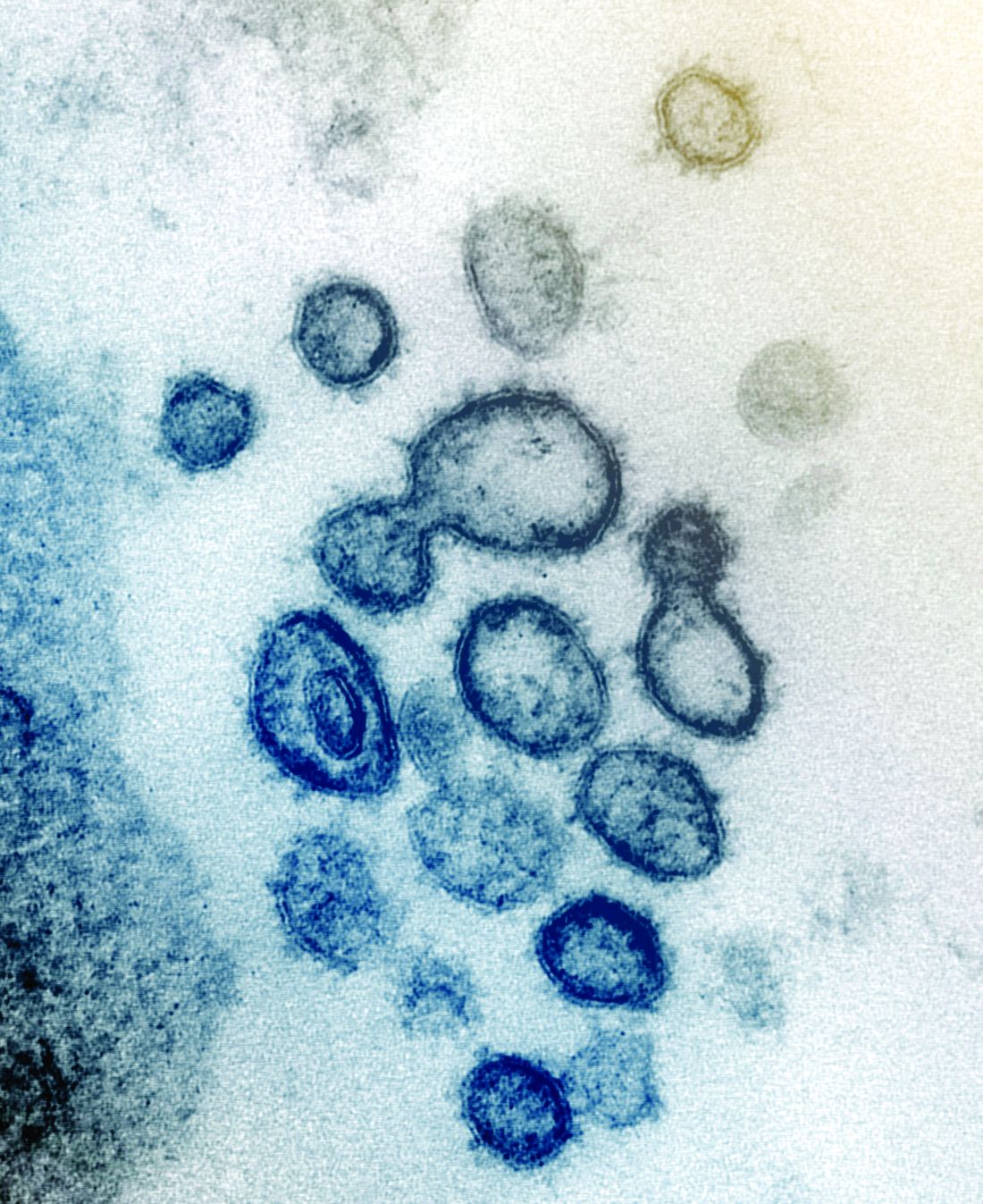
Case study shows CLL may mask COVID-19 infection
Characteristics of patients with chronic lymphocytic leukemia can mask COVID-19 infection, creating a risk for patients, practitioners, and the community, according to a case study published in the Lancet Haematology.
A 39-year-old man with a history of non-Hodgkin lymphoma and chronic lymphocytic leukemia (CLL) presented at a clinic in Wenzhou, China, with symptoms of fever, sore throat, productive cough, and dyspnea, according to the authors. COVID-19 infection was not initially suspected, as his whole blood cell and lymphocyte counts were high, the CLL masked a potential infection, and the patient claimed he had no suspect recent travel history.
However, a CT chest scan showed bilateral ground-glass opacities and a small amount of fluid in the patient’s left pleural cavity, leading the attending physician to suspect COVID-19. Testing was ordered and the real-time reverse-transcription polymerase chain reaction assay result was positive. The patient was immediately transferred to the isolation ward for management and confirmed COVID-19 infection.
Subsequently, the patient admitted travel to the COVID-19 epicenter in Wuhan province, although it was 25 days prior, indicating a longer period of incubation than generally believed, according to the authors. The patient survived treatment and was eventually discharged.
“Clinical and biochemical data of COVID-19 might be partly masked by coexisting chronic lymphocytic leukemia; better diagnostic strategies (i.e., superior CT differential techniques such as radiomics) could be used for diagnosis,” the researchers concluded, speculating that the apparently longer-than-normal COVID-19 incubation period might be the result of the patient’s compromised immune system.
The authors reported that they had no conflicts of interest.
SOURCE: Jin X-H et al. Lancet Haematol. 2020;7(4):E351-2.
Characteristics of patients with chronic lymphocytic leukemia can mask COVID-19 infection, creating a risk for patients, practitioners, and the community, according to a case study published in the Lancet Haematology.
A 39-year-old man with a history of non-Hodgkin lymphoma and chronic lymphocytic leukemia (CLL) presented at a clinic in Wenzhou, China, with symptoms of fever, sore throat, productive cough, and dyspnea, according to the authors. COVID-19 infection was not initially suspected, as his whole blood cell and lymphocyte counts were high, the CLL masked a potential infection, and the patient claimed he had no suspect recent travel history.
However, a CT chest scan showed bilateral ground-glass opacities and a small amount of fluid in the patient’s left pleural cavity, leading the attending physician to suspect COVID-19. Testing was ordered and the real-time reverse-transcription polymerase chain reaction assay result was positive. The patient was immediately transferred to the isolation ward for management and confirmed COVID-19 infection.
Subsequently, the patient admitted travel to the COVID-19 epicenter in Wuhan province, although it was 25 days prior, indicating a longer period of incubation than generally believed, according to the authors. The patient survived treatment and was eventually discharged.
“Clinical and biochemical data of COVID-19 might be partly masked by coexisting chronic lymphocytic leukemia; better diagnostic strategies (i.e., superior CT differential techniques such as radiomics) could be used for diagnosis,” the researchers concluded, speculating that the apparently longer-than-normal COVID-19 incubation period might be the result of the patient’s compromised immune system.
The authors reported that they had no conflicts of interest.
SOURCE: Jin X-H et al. Lancet Haematol. 2020;7(4):E351-2.
Characteristics of patients with chronic lymphocytic leukemia can mask COVID-19 infection, creating a risk for patients, practitioners, and the community, according to a case study published in the Lancet Haematology.
A 39-year-old man with a history of non-Hodgkin lymphoma and chronic lymphocytic leukemia (CLL) presented at a clinic in Wenzhou, China, with symptoms of fever, sore throat, productive cough, and dyspnea, according to the authors. COVID-19 infection was not initially suspected, as his whole blood cell and lymphocyte counts were high, the CLL masked a potential infection, and the patient claimed he had no suspect recent travel history.
However, a CT chest scan showed bilateral ground-glass opacities and a small amount of fluid in the patient’s left pleural cavity, leading the attending physician to suspect COVID-19. Testing was ordered and the real-time reverse-transcription polymerase chain reaction assay result was positive. The patient was immediately transferred to the isolation ward for management and confirmed COVID-19 infection.
Subsequently, the patient admitted travel to the COVID-19 epicenter in Wuhan province, although it was 25 days prior, indicating a longer period of incubation than generally believed, according to the authors. The patient survived treatment and was eventually discharged.
“Clinical and biochemical data of COVID-19 might be partly masked by coexisting chronic lymphocytic leukemia; better diagnostic strategies (i.e., superior CT differential techniques such as radiomics) could be used for diagnosis,” the researchers concluded, speculating that the apparently longer-than-normal COVID-19 incubation period might be the result of the patient’s compromised immune system.
The authors reported that they had no conflicts of interest.
SOURCE: Jin X-H et al. Lancet Haematol. 2020;7(4):E351-2.
FROM THE LANCET HAEMATOLOGY
CLL and breast cancer differ in the expression of regulatory microRNAs
Expression of three microRNAs (miR-155, miR-29a, and miR-27b) was detectable in patients with chronic lymphocytic leukemia (CLL) and in breast cancer (BC) patients, but not in healthy subjects, according to a molecular analysis of patients reported in Molecular Therapy Oncolytics. In addition, circulating microarrays were found to be able to differentiate between both CLL and BC patients and healthy subjects.
The researchers obtained blood samples from 15 CLL patients and tissue samples from 15 BC patients, all from a single center.
The use of quantitative reverse transcription polymerase chain reaction (qRT-PCR) demonstrated a significant increase in the expression of all three miRNAs in patients with BC and CLL, compared with respective healthy groups (P less than .001).
In BC patients, there was a significant difference between the expression of miR-155 and miR-29a (P less than .05), miR-155 and miR-27b (P less than .01), and miR-27b and miR-29a (P less than .001). In CLL patients, the qRT-PCR results showed a significant difference between expression of both miR-27b and miR-29a, compared with expression of miR-155 (P less than .001). In addition, there was a significant association between miR-155 and prevascular invasion (P = .013), but no significant association with other clinical variables (age, tumor grade, nuclear grade, tumor stage, tumor size, area of invasive component, tumor side, margin, or preneural invasion), according to the researchers.
Results also showed that elevated circulating miRNAs were BC specific and could differentiate BC tissues from the controls, and comparing expression of miRNAs between BC and CLL patients, there was also a significant difference for all miRNAs (P less than .001) between them.
“Our results suggest that miR-27b, miR-29a, and miR-155 could be potential new biomarkers for diagnosis, as well as a therapeutic target for CLL and BC,” the researchers concluded.
The authors reported that they had no competing interests.
SOURCE: Raeisi F et al. Mol Ther Oncolytics. 2020;16:230-7.
Expression of three microRNAs (miR-155, miR-29a, and miR-27b) was detectable in patients with chronic lymphocytic leukemia (CLL) and in breast cancer (BC) patients, but not in healthy subjects, according to a molecular analysis of patients reported in Molecular Therapy Oncolytics. In addition, circulating microarrays were found to be able to differentiate between both CLL and BC patients and healthy subjects.
The researchers obtained blood samples from 15 CLL patients and tissue samples from 15 BC patients, all from a single center.
The use of quantitative reverse transcription polymerase chain reaction (qRT-PCR) demonstrated a significant increase in the expression of all three miRNAs in patients with BC and CLL, compared with respective healthy groups (P less than .001).
In BC patients, there was a significant difference between the expression of miR-155 and miR-29a (P less than .05), miR-155 and miR-27b (P less than .01), and miR-27b and miR-29a (P less than .001). In CLL patients, the qRT-PCR results showed a significant difference between expression of both miR-27b and miR-29a, compared with expression of miR-155 (P less than .001). In addition, there was a significant association between miR-155 and prevascular invasion (P = .013), but no significant association with other clinical variables (age, tumor grade, nuclear grade, tumor stage, tumor size, area of invasive component, tumor side, margin, or preneural invasion), according to the researchers.
Results also showed that elevated circulating miRNAs were BC specific and could differentiate BC tissues from the controls, and comparing expression of miRNAs between BC and CLL patients, there was also a significant difference for all miRNAs (P less than .001) between them.
“Our results suggest that miR-27b, miR-29a, and miR-155 could be potential new biomarkers for diagnosis, as well as a therapeutic target for CLL and BC,” the researchers concluded.
The authors reported that they had no competing interests.
SOURCE: Raeisi F et al. Mol Ther Oncolytics. 2020;16:230-7.
Expression of three microRNAs (miR-155, miR-29a, and miR-27b) was detectable in patients with chronic lymphocytic leukemia (CLL) and in breast cancer (BC) patients, but not in healthy subjects, according to a molecular analysis of patients reported in Molecular Therapy Oncolytics. In addition, circulating microarrays were found to be able to differentiate between both CLL and BC patients and healthy subjects.
The researchers obtained blood samples from 15 CLL patients and tissue samples from 15 BC patients, all from a single center.
The use of quantitative reverse transcription polymerase chain reaction (qRT-PCR) demonstrated a significant increase in the expression of all three miRNAs in patients with BC and CLL, compared with respective healthy groups (P less than .001).
In BC patients, there was a significant difference between the expression of miR-155 and miR-29a (P less than .05), miR-155 and miR-27b (P less than .01), and miR-27b and miR-29a (P less than .001). In CLL patients, the qRT-PCR results showed a significant difference between expression of both miR-27b and miR-29a, compared with expression of miR-155 (P less than .001). In addition, there was a significant association between miR-155 and prevascular invasion (P = .013), but no significant association with other clinical variables (age, tumor grade, nuclear grade, tumor stage, tumor size, area of invasive component, tumor side, margin, or preneural invasion), according to the researchers.
Results also showed that elevated circulating miRNAs were BC specific and could differentiate BC tissues from the controls, and comparing expression of miRNAs between BC and CLL patients, there was also a significant difference for all miRNAs (P less than .001) between them.
“Our results suggest that miR-27b, miR-29a, and miR-155 could be potential new biomarkers for diagnosis, as well as a therapeutic target for CLL and BC,” the researchers concluded.
The authors reported that they had no competing interests.
SOURCE: Raeisi F et al. Mol Ther Oncolytics. 2020;16:230-7.
FROM MOLECULAR THERAPY ONCOLYTICS
NMA haploidentical allo-BMT plus post-transplant cyclophosphamide deemed safe, effective for CLL
for patients with chronic lymphocytic leukemia (CLL), according to a study published in Biology of Blood and Marrow Transplantation.
The number of patients undergoing haploidentical allo-BMT has increased substantially, with the advent of new graft-versus-host disease (GVHD) prophylaxis strategies, such as posttransplantation cyclophosphamide (PTCy), that reduce the risk of GVHD complications; however there have been few studies assessing the results of this treatment regimen, according to the authors.
The study assessed 64 consecutive patients with CLL between Jan. 2005 and Aug. 2018 who underwent haploidentical allo-BMT. The median age was 59 years; 4 patients (6.2%) underwent allo-BMT after first-line treatment; 20 patients (31.2%) underwent allo-BMT after second-line treatment, and 40 patients (62.5%) underwent allo-BMT after three or more lines of treatment for relapsed and/or refractory disease.
All patients received a nonmyeloablative (NMA) conditioning regimen consisting of fludarabine, cyclophosphamide, and 200 cGy total body irradiation. Patients received PTCy (i.v. 50 mg/kg per day) on days +3 and +4, along with additional GVHD prophylaxis with mycophenolate mofetil between days +5 and +35 and tacrolimus or sirolimus between days +5 and +180.
For all 64 patients, the median duration of follow-up was 4.4 years based on the reverse Kaplan-Meier method. The 4-year overall survival (OS) was 52%, and the 4-year progression-free survival (PFS) was 37%. The 56 patients with less than 20% marrow CLL involvement before undergoing allo-BMT had a 4-year OS of 61%, a 4-year PFS of 43%, and a median OS of 4.8 years, according to the authors.
Regression analysis demonstrated that donor age, stem cell source, IGHV mutation status, or grade II-III acute GVHD did not affect risk of progression or survival.
“The majority of our patients had unfavorable risk factors, and collectively our data show that haploidentical allo-BMT with PTCy in CLL with less than 20% marrow involvement is a safe treatment option carrying a low risk of serious GVHD and other toxicities,” the researchers concluded.
The study was supported by National Institutes of Health, National Cancer Institute grants. The authors reported that they had no disclosures.
SOURCE: Suman P et al. Biol Blood Marrow Transplant. 2020 Mar 1;26:502-8. doi: 10.1016/j.bbmt.2019.11.008
for patients with chronic lymphocytic leukemia (CLL), according to a study published in Biology of Blood and Marrow Transplantation.
The number of patients undergoing haploidentical allo-BMT has increased substantially, with the advent of new graft-versus-host disease (GVHD) prophylaxis strategies, such as posttransplantation cyclophosphamide (PTCy), that reduce the risk of GVHD complications; however there have been few studies assessing the results of this treatment regimen, according to the authors.
The study assessed 64 consecutive patients with CLL between Jan. 2005 and Aug. 2018 who underwent haploidentical allo-BMT. The median age was 59 years; 4 patients (6.2%) underwent allo-BMT after first-line treatment; 20 patients (31.2%) underwent allo-BMT after second-line treatment, and 40 patients (62.5%) underwent allo-BMT after three or more lines of treatment for relapsed and/or refractory disease.
All patients received a nonmyeloablative (NMA) conditioning regimen consisting of fludarabine, cyclophosphamide, and 200 cGy total body irradiation. Patients received PTCy (i.v. 50 mg/kg per day) on days +3 and +4, along with additional GVHD prophylaxis with mycophenolate mofetil between days +5 and +35 and tacrolimus or sirolimus between days +5 and +180.
For all 64 patients, the median duration of follow-up was 4.4 years based on the reverse Kaplan-Meier method. The 4-year overall survival (OS) was 52%, and the 4-year progression-free survival (PFS) was 37%. The 56 patients with less than 20% marrow CLL involvement before undergoing allo-BMT had a 4-year OS of 61%, a 4-year PFS of 43%, and a median OS of 4.8 years, according to the authors.
Regression analysis demonstrated that donor age, stem cell source, IGHV mutation status, or grade II-III acute GVHD did not affect risk of progression or survival.
“The majority of our patients had unfavorable risk factors, and collectively our data show that haploidentical allo-BMT with PTCy in CLL with less than 20% marrow involvement is a safe treatment option carrying a low risk of serious GVHD and other toxicities,” the researchers concluded.
The study was supported by National Institutes of Health, National Cancer Institute grants. The authors reported that they had no disclosures.
SOURCE: Suman P et al. Biol Blood Marrow Transplant. 2020 Mar 1;26:502-8. doi: 10.1016/j.bbmt.2019.11.008
for patients with chronic lymphocytic leukemia (CLL), according to a study published in Biology of Blood and Marrow Transplantation.
The number of patients undergoing haploidentical allo-BMT has increased substantially, with the advent of new graft-versus-host disease (GVHD) prophylaxis strategies, such as posttransplantation cyclophosphamide (PTCy), that reduce the risk of GVHD complications; however there have been few studies assessing the results of this treatment regimen, according to the authors.
The study assessed 64 consecutive patients with CLL between Jan. 2005 and Aug. 2018 who underwent haploidentical allo-BMT. The median age was 59 years; 4 patients (6.2%) underwent allo-BMT after first-line treatment; 20 patients (31.2%) underwent allo-BMT after second-line treatment, and 40 patients (62.5%) underwent allo-BMT after three or more lines of treatment for relapsed and/or refractory disease.
All patients received a nonmyeloablative (NMA) conditioning regimen consisting of fludarabine, cyclophosphamide, and 200 cGy total body irradiation. Patients received PTCy (i.v. 50 mg/kg per day) on days +3 and +4, along with additional GVHD prophylaxis with mycophenolate mofetil between days +5 and +35 and tacrolimus or sirolimus between days +5 and +180.
For all 64 patients, the median duration of follow-up was 4.4 years based on the reverse Kaplan-Meier method. The 4-year overall survival (OS) was 52%, and the 4-year progression-free survival (PFS) was 37%. The 56 patients with less than 20% marrow CLL involvement before undergoing allo-BMT had a 4-year OS of 61%, a 4-year PFS of 43%, and a median OS of 4.8 years, according to the authors.
Regression analysis demonstrated that donor age, stem cell source, IGHV mutation status, or grade II-III acute GVHD did not affect risk of progression or survival.
“The majority of our patients had unfavorable risk factors, and collectively our data show that haploidentical allo-BMT with PTCy in CLL with less than 20% marrow involvement is a safe treatment option carrying a low risk of serious GVHD and other toxicities,” the researchers concluded.
The study was supported by National Institutes of Health, National Cancer Institute grants. The authors reported that they had no disclosures.
SOURCE: Suman P et al. Biol Blood Marrow Transplant. 2020 Mar 1;26:502-8. doi: 10.1016/j.bbmt.2019.11.008
FROM BIOLOGY OF BLOOD AND MARROW TRANSPLANTATION
Safer CAR uses modified NK cells for advanced CLL, NHL
A chimeric antigen receptor (CAR) construct using transduced natural killer cells instead of T cells was associated with a high complete remission rate without the cytokine release syndrome frequently seen with CAR T cell therapy, early clinical trial results show.
The construct, consisting of natural killer (NK) cells derived from umbilical cord blood that have been transduced to target CD19-expressing cells combined with interleukin 15 and equipped with an “off” switch, offers the prospect of an off-the-shelf CAR product, reported Enli Liu, MD, and colleagues at the University of Texas MD Anderson Cancer Center in Houston.
“We found that allogeneic CAR-NK cells can be delivered in adoptive transfer without the serious cytokine release syndrome and neurologic toxic effects that have been associated with CAR T-cell therapy,” they wrote in The New England Journal of Medicine.
The modified NK cells were delivered to 9 of 11 patients with only partial human leukocyte antigen (HLA) matching, and in 2 patients with no matching, yet there were no cases of graft-versus host disease (GvHD), and no patients had symptoms of cytokine release syndrome (CRS), neurotoxicity, or hemophagocytic lymphohistiocytosis.
CAR T cell production “is a cumbersome process that requires coordination and collection of the cells and there’s several weeks of manufacturing, during which time patients often can have their lymphoma worsen, and so at times it’s a little bit of a race against the clock to get those cells manufactured,” Brian Hill, MD, PhD, director of the lymphoid malignancies program at Taussig Cancer Institute at Cleveland Clinic, said in an interview.
Dr. Hill, who was not involved in the study, said that the proof-of-principle study shows promising early results and offers the prospect of an effective and safe off-the-shelf therapeutic option for patients with lymphoid malignancies.
Advanced B-cell cancers
The investigators conducted a phase 1/2 trial in patients with B-cell lymphoid malignancies, including five patients with chronic lymphocytic leukemia (CLL), one patient with Richter’s transformation and one with accelerated CLL, three with transformed follicular lymphoma, two with diffuse large B-cell lymphoma (DLBCL), and one with follicular lymphoma (focally grade 3B).
The patients were all heavily pretreated, with 3 to as many as 11 prior lines of therapy.
The patients received cord blood-derived NK cells that had been transduced with a retroviral vector expressing genes that encode anti-CD19 CAR, interleukin-15, and inducible caspase 9 as a safety switch.
The cells were expanded in the lab and after the patients underwent lymphodepleting chemotherapy, they received the cells in a single infusion at one of three doses, either 1×105, 1×106, or 1×107 CAR-NK cells per kilogram of body weight.
As noted before, there were no cases of CRS, neurotoxicity, or GvHD and no increase over baseline in inflammatory cytokines, including interleukin-6, a key factor in the development and severity of CRS. The maximum tolerated dose was not reached.
Early efficacy
Of the 11 patients, 8 had a clinical response, and 7 had a complete remission, including 4 patients with lymphomas and 3 with CLL.
The patient with CLL with Richter’s transformation had a remission of the Richter’s component, but not of the CLL itself.
“This is particularly remarkable, because these patients are notoriously very difficult to treat, and the efficacy of autologous CAR T cell therapy in CLL and Richter’s patients has been hampered by lack of fitness of the patient’s own T cells when manufacturing the CAR T cell product, so this approach may obviate the need for autologous T cells in these patients,” Dr. Hill said.
The responses were rapid and occurred within 30 days of infusion at all dose levels. In addition, there was evidence of expansion and persistence of the modified NK cells at low levels for at least 1 year, despite the HLA mismatches between the NK cells and the recipients. The investigators speculated that the inclusion of interleukin-15 in the NL construct may at least partially account for the persistence of the cells and their antitumor activity.
Of the eight patients with a response, five had postremission therapy, including two patients with CLL who had minimal residual disease (MRD), one patient with follicular lymphoma and one with transformed follicular lymphoma who underwent hematopoietic stem-cell transplantation while in complete remission without evidence of MRD, and the patient with CLLL with Richter’s transformation with remission of the lymphoma component, who received a course of venetoclax.
The authors acknowledged that it may be difficult to assess the durability of response after CAR NK therapy in this study because of the allowed consolidation therapy for patients in remission.
They noted that although the patients in the current study each had a fresh CAR NK product manufactured for them, “we have shown that it is possible to produce more than 100 doses of CAR-NK cells from a single cord-blood unit. This capability, together with the apparently minimal HLA-matching requirements between the donor of CAR-NK cells and the patient, may pave the way for a truly off-the-shelf product that could increase treatment accessibility for many more patients.”
The National Institutes of Health supported the study. Dr. Liu disclosed a pending patent for methods of production of CAR-NK cells, and a patent held by MD Anderson for methods of treatment with NK cells. Dr. Hill is a member of the Hematology News editorial advisory board.
SOURCE: Liu E et al. N Engl J Med. 2020 Feb 6;382:545-53.
A chimeric antigen receptor (CAR) construct using transduced natural killer cells instead of T cells was associated with a high complete remission rate without the cytokine release syndrome frequently seen with CAR T cell therapy, early clinical trial results show.
The construct, consisting of natural killer (NK) cells derived from umbilical cord blood that have been transduced to target CD19-expressing cells combined with interleukin 15 and equipped with an “off” switch, offers the prospect of an off-the-shelf CAR product, reported Enli Liu, MD, and colleagues at the University of Texas MD Anderson Cancer Center in Houston.
“We found that allogeneic CAR-NK cells can be delivered in adoptive transfer without the serious cytokine release syndrome and neurologic toxic effects that have been associated with CAR T-cell therapy,” they wrote in The New England Journal of Medicine.
The modified NK cells were delivered to 9 of 11 patients with only partial human leukocyte antigen (HLA) matching, and in 2 patients with no matching, yet there were no cases of graft-versus host disease (GvHD), and no patients had symptoms of cytokine release syndrome (CRS), neurotoxicity, or hemophagocytic lymphohistiocytosis.
CAR T cell production “is a cumbersome process that requires coordination and collection of the cells and there’s several weeks of manufacturing, during which time patients often can have their lymphoma worsen, and so at times it’s a little bit of a race against the clock to get those cells manufactured,” Brian Hill, MD, PhD, director of the lymphoid malignancies program at Taussig Cancer Institute at Cleveland Clinic, said in an interview.
Dr. Hill, who was not involved in the study, said that the proof-of-principle study shows promising early results and offers the prospect of an effective and safe off-the-shelf therapeutic option for patients with lymphoid malignancies.
Advanced B-cell cancers
The investigators conducted a phase 1/2 trial in patients with B-cell lymphoid malignancies, including five patients with chronic lymphocytic leukemia (CLL), one patient with Richter’s transformation and one with accelerated CLL, three with transformed follicular lymphoma, two with diffuse large B-cell lymphoma (DLBCL), and one with follicular lymphoma (focally grade 3B).
The patients were all heavily pretreated, with 3 to as many as 11 prior lines of therapy.
The patients received cord blood-derived NK cells that had been transduced with a retroviral vector expressing genes that encode anti-CD19 CAR, interleukin-15, and inducible caspase 9 as a safety switch.
The cells were expanded in the lab and after the patients underwent lymphodepleting chemotherapy, they received the cells in a single infusion at one of three doses, either 1×105, 1×106, or 1×107 CAR-NK cells per kilogram of body weight.
As noted before, there were no cases of CRS, neurotoxicity, or GvHD and no increase over baseline in inflammatory cytokines, including interleukin-6, a key factor in the development and severity of CRS. The maximum tolerated dose was not reached.
Early efficacy
Of the 11 patients, 8 had a clinical response, and 7 had a complete remission, including 4 patients with lymphomas and 3 with CLL.
The patient with CLL with Richter’s transformation had a remission of the Richter’s component, but not of the CLL itself.
“This is particularly remarkable, because these patients are notoriously very difficult to treat, and the efficacy of autologous CAR T cell therapy in CLL and Richter’s patients has been hampered by lack of fitness of the patient’s own T cells when manufacturing the CAR T cell product, so this approach may obviate the need for autologous T cells in these patients,” Dr. Hill said.
The responses were rapid and occurred within 30 days of infusion at all dose levels. In addition, there was evidence of expansion and persistence of the modified NK cells at low levels for at least 1 year, despite the HLA mismatches between the NK cells and the recipients. The investigators speculated that the inclusion of interleukin-15 in the NL construct may at least partially account for the persistence of the cells and their antitumor activity.
Of the eight patients with a response, five had postremission therapy, including two patients with CLL who had minimal residual disease (MRD), one patient with follicular lymphoma and one with transformed follicular lymphoma who underwent hematopoietic stem-cell transplantation while in complete remission without evidence of MRD, and the patient with CLLL with Richter’s transformation with remission of the lymphoma component, who received a course of venetoclax.
The authors acknowledged that it may be difficult to assess the durability of response after CAR NK therapy in this study because of the allowed consolidation therapy for patients in remission.
They noted that although the patients in the current study each had a fresh CAR NK product manufactured for them, “we have shown that it is possible to produce more than 100 doses of CAR-NK cells from a single cord-blood unit. This capability, together with the apparently minimal HLA-matching requirements between the donor of CAR-NK cells and the patient, may pave the way for a truly off-the-shelf product that could increase treatment accessibility for many more patients.”
The National Institutes of Health supported the study. Dr. Liu disclosed a pending patent for methods of production of CAR-NK cells, and a patent held by MD Anderson for methods of treatment with NK cells. Dr. Hill is a member of the Hematology News editorial advisory board.
SOURCE: Liu E et al. N Engl J Med. 2020 Feb 6;382:545-53.
A chimeric antigen receptor (CAR) construct using transduced natural killer cells instead of T cells was associated with a high complete remission rate without the cytokine release syndrome frequently seen with CAR T cell therapy, early clinical trial results show.
The construct, consisting of natural killer (NK) cells derived from umbilical cord blood that have been transduced to target CD19-expressing cells combined with interleukin 15 and equipped with an “off” switch, offers the prospect of an off-the-shelf CAR product, reported Enli Liu, MD, and colleagues at the University of Texas MD Anderson Cancer Center in Houston.
“We found that allogeneic CAR-NK cells can be delivered in adoptive transfer without the serious cytokine release syndrome and neurologic toxic effects that have been associated with CAR T-cell therapy,” they wrote in The New England Journal of Medicine.
The modified NK cells were delivered to 9 of 11 patients with only partial human leukocyte antigen (HLA) matching, and in 2 patients with no matching, yet there were no cases of graft-versus host disease (GvHD), and no patients had symptoms of cytokine release syndrome (CRS), neurotoxicity, or hemophagocytic lymphohistiocytosis.
CAR T cell production “is a cumbersome process that requires coordination and collection of the cells and there’s several weeks of manufacturing, during which time patients often can have their lymphoma worsen, and so at times it’s a little bit of a race against the clock to get those cells manufactured,” Brian Hill, MD, PhD, director of the lymphoid malignancies program at Taussig Cancer Institute at Cleveland Clinic, said in an interview.
Dr. Hill, who was not involved in the study, said that the proof-of-principle study shows promising early results and offers the prospect of an effective and safe off-the-shelf therapeutic option for patients with lymphoid malignancies.
Advanced B-cell cancers
The investigators conducted a phase 1/2 trial in patients with B-cell lymphoid malignancies, including five patients with chronic lymphocytic leukemia (CLL), one patient with Richter’s transformation and one with accelerated CLL, three with transformed follicular lymphoma, two with diffuse large B-cell lymphoma (DLBCL), and one with follicular lymphoma (focally grade 3B).
The patients were all heavily pretreated, with 3 to as many as 11 prior lines of therapy.
The patients received cord blood-derived NK cells that had been transduced with a retroviral vector expressing genes that encode anti-CD19 CAR, interleukin-15, and inducible caspase 9 as a safety switch.
The cells were expanded in the lab and after the patients underwent lymphodepleting chemotherapy, they received the cells in a single infusion at one of three doses, either 1×105, 1×106, or 1×107 CAR-NK cells per kilogram of body weight.
As noted before, there were no cases of CRS, neurotoxicity, or GvHD and no increase over baseline in inflammatory cytokines, including interleukin-6, a key factor in the development and severity of CRS. The maximum tolerated dose was not reached.
Early efficacy
Of the 11 patients, 8 had a clinical response, and 7 had a complete remission, including 4 patients with lymphomas and 3 with CLL.
The patient with CLL with Richter’s transformation had a remission of the Richter’s component, but not of the CLL itself.
“This is particularly remarkable, because these patients are notoriously very difficult to treat, and the efficacy of autologous CAR T cell therapy in CLL and Richter’s patients has been hampered by lack of fitness of the patient’s own T cells when manufacturing the CAR T cell product, so this approach may obviate the need for autologous T cells in these patients,” Dr. Hill said.
The responses were rapid and occurred within 30 days of infusion at all dose levels. In addition, there was evidence of expansion and persistence of the modified NK cells at low levels for at least 1 year, despite the HLA mismatches between the NK cells and the recipients. The investigators speculated that the inclusion of interleukin-15 in the NL construct may at least partially account for the persistence of the cells and their antitumor activity.
Of the eight patients with a response, five had postremission therapy, including two patients with CLL who had minimal residual disease (MRD), one patient with follicular lymphoma and one with transformed follicular lymphoma who underwent hematopoietic stem-cell transplantation while in complete remission without evidence of MRD, and the patient with CLLL with Richter’s transformation with remission of the lymphoma component, who received a course of venetoclax.
The authors acknowledged that it may be difficult to assess the durability of response after CAR NK therapy in this study because of the allowed consolidation therapy for patients in remission.
They noted that although the patients in the current study each had a fresh CAR NK product manufactured for them, “we have shown that it is possible to produce more than 100 doses of CAR-NK cells from a single cord-blood unit. This capability, together with the apparently minimal HLA-matching requirements between the donor of CAR-NK cells and the patient, may pave the way for a truly off-the-shelf product that could increase treatment accessibility for many more patients.”
The National Institutes of Health supported the study. Dr. Liu disclosed a pending patent for methods of production of CAR-NK cells, and a patent held by MD Anderson for methods of treatment with NK cells. Dr. Hill is a member of the Hematology News editorial advisory board.
SOURCE: Liu E et al. N Engl J Med. 2020 Feb 6;382:545-53.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Novel mutations contribute to progression of venetoclax-treated CLL
Newly discovered gene mutations in the progression of venetoclax-treated relapsed chronic lymphocytic leukemia (CLL) may improve understanding of clinical resistance mechanisms underlying the disease, according to recent research.
“We investigated patients with progressive CLL on venetoclax harboring subclonal BCL2 Gly101Val mutations for the presence of additional acquired BCL2 resistance mutations,” wrote Piers Blombery, MBBS, of the University of Melbourne in Victoria, Australia, and his colleagues in Blood.
Among 67 patients with relapsed disease treated with the BCL2 inhibitor venetoclax, the researchers identified a total of 11 patients with co-occurring BCL2 Gly101Val mutations. Each patient was enrolled in an early phase clinical trial at an institution in Australia.
With respect to testing methods, next-generation sequencing (NGS) and hybridization-based target enrichment technologies were used to detect novel acquired mutations in the BCL2 coding region.
Among those harboring the Gly101Val mutation, additional BCL2 mutations were identified in 10 patients (91%), with a median of three mutations detected per patient (range, 1-7). Previously undescribed mutations included an in-frame insertion mutation (Arg107_Arg110dup), and other substitutions (Asp103/Val156) in the BCL2 gene.
“As with the Gly101Val, these observations support the specificity of these mutations for the context of venetoclax resistance,” they wrote.
The investigators further explained that the BCL2 Asp103Glu mutation could have particular significance in the context of venetoclax sensitivity because of selective targeting of the BCL2 gene.
In comparison to wild-type aspartic acid, the BCL2 Asp103Glu substitution was linked to an approximate 20-fold reduction in affinity for venetoclax, they reported.
“[Our findings] consolidate the paradigm emerging across hematological malignancies of multiple independent molecular mechanisms underpinning an ‘oligoclonal’ pattern of clinical relapse on targeted therapies,” they concluded.
Further studies are needed to fully characterize the relationship between acquired BCL2 mutations and venetoclax resistance.
The study was funded by the Snowdome Foundation, Vision Super and the Wilson Centre for Lymphoma Genomics, the Leukemia and Lymphoma Society, the National Health and Medical Research Council of Australia, and other grant funding sources provided to the study authors. The authors reported financial affiliations with AbbVie, Genentech, and the Walter and Eliza Hall Institute.
Newly discovered gene mutations in the progression of venetoclax-treated relapsed chronic lymphocytic leukemia (CLL) may improve understanding of clinical resistance mechanisms underlying the disease, according to recent research.
“We investigated patients with progressive CLL on venetoclax harboring subclonal BCL2 Gly101Val mutations for the presence of additional acquired BCL2 resistance mutations,” wrote Piers Blombery, MBBS, of the University of Melbourne in Victoria, Australia, and his colleagues in Blood.
Among 67 patients with relapsed disease treated with the BCL2 inhibitor venetoclax, the researchers identified a total of 11 patients with co-occurring BCL2 Gly101Val mutations. Each patient was enrolled in an early phase clinical trial at an institution in Australia.
With respect to testing methods, next-generation sequencing (NGS) and hybridization-based target enrichment technologies were used to detect novel acquired mutations in the BCL2 coding region.
Among those harboring the Gly101Val mutation, additional BCL2 mutations were identified in 10 patients (91%), with a median of three mutations detected per patient (range, 1-7). Previously undescribed mutations included an in-frame insertion mutation (Arg107_Arg110dup), and other substitutions (Asp103/Val156) in the BCL2 gene.
“As with the Gly101Val, these observations support the specificity of these mutations for the context of venetoclax resistance,” they wrote.
The investigators further explained that the BCL2 Asp103Glu mutation could have particular significance in the context of venetoclax sensitivity because of selective targeting of the BCL2 gene.
In comparison to wild-type aspartic acid, the BCL2 Asp103Glu substitution was linked to an approximate 20-fold reduction in affinity for venetoclax, they reported.
“[Our findings] consolidate the paradigm emerging across hematological malignancies of multiple independent molecular mechanisms underpinning an ‘oligoclonal’ pattern of clinical relapse on targeted therapies,” they concluded.
Further studies are needed to fully characterize the relationship between acquired BCL2 mutations and venetoclax resistance.
The study was funded by the Snowdome Foundation, Vision Super and the Wilson Centre for Lymphoma Genomics, the Leukemia and Lymphoma Society, the National Health and Medical Research Council of Australia, and other grant funding sources provided to the study authors. The authors reported financial affiliations with AbbVie, Genentech, and the Walter and Eliza Hall Institute.
Newly discovered gene mutations in the progression of venetoclax-treated relapsed chronic lymphocytic leukemia (CLL) may improve understanding of clinical resistance mechanisms underlying the disease, according to recent research.
“We investigated patients with progressive CLL on venetoclax harboring subclonal BCL2 Gly101Val mutations for the presence of additional acquired BCL2 resistance mutations,” wrote Piers Blombery, MBBS, of the University of Melbourne in Victoria, Australia, and his colleagues in Blood.
Among 67 patients with relapsed disease treated with the BCL2 inhibitor venetoclax, the researchers identified a total of 11 patients with co-occurring BCL2 Gly101Val mutations. Each patient was enrolled in an early phase clinical trial at an institution in Australia.
With respect to testing methods, next-generation sequencing (NGS) and hybridization-based target enrichment technologies were used to detect novel acquired mutations in the BCL2 coding region.
Among those harboring the Gly101Val mutation, additional BCL2 mutations were identified in 10 patients (91%), with a median of three mutations detected per patient (range, 1-7). Previously undescribed mutations included an in-frame insertion mutation (Arg107_Arg110dup), and other substitutions (Asp103/Val156) in the BCL2 gene.
“As with the Gly101Val, these observations support the specificity of these mutations for the context of venetoclax resistance,” they wrote.
The investigators further explained that the BCL2 Asp103Glu mutation could have particular significance in the context of venetoclax sensitivity because of selective targeting of the BCL2 gene.
In comparison to wild-type aspartic acid, the BCL2 Asp103Glu substitution was linked to an approximate 20-fold reduction in affinity for venetoclax, they reported.
“[Our findings] consolidate the paradigm emerging across hematological malignancies of multiple independent molecular mechanisms underpinning an ‘oligoclonal’ pattern of clinical relapse on targeted therapies,” they concluded.
Further studies are needed to fully characterize the relationship between acquired BCL2 mutations and venetoclax resistance.
The study was funded by the Snowdome Foundation, Vision Super and the Wilson Centre for Lymphoma Genomics, the Leukemia and Lymphoma Society, the National Health and Medical Research Council of Australia, and other grant funding sources provided to the study authors. The authors reported financial affiliations with AbbVie, Genentech, and the Walter and Eliza Hall Institute.
FROM BLOOD
Ofatumumab works safely for elderly patients with CLL, comorbidities
For elderly patients with chronic lymphocytic leukemia (CLL) and comorbidities, the anti-CD20 monoclonal antibody ofatumumab may be a safe and effective treatment option, according to a recent phase 2 trial.
Among 32 patients with a median age of 73 years, the overall response rate was 72%, and no grade 4 adverse events occurred, reported lead author Candida Vitale, MD, PhD, of the University of Torino (Italy) and colleagues.
These findings help fill in a knowledge gap created by clinical trial exclusions, which currently make treatment planning “a significant challenge,” the investigators wrote in Journal of Geriatric Oncology.
The study, which was conducted at MD Anderson Cancer Center in Houston, enrolled 34 treatment-naive patients with CLL who were 65 years or older. All patients had an Eastern Cooperative Oncology Group performance status of 2 or 3, or a Charlson comorbidity index of at least 2. Patients with other serious medical conditions and/or primary malignancies were eligible, given that they were not already receiving anticancer therapy.
More than half of the patients (53%) had advanced-stage disease and almost one-third (29%) had at least one other primary cancer diagnosis. Many patients also had high-risk disease characteristics, including a complex karyotype involving three or more chromosomal abnormalities (15%), and/or unmutated immunoglobulin heavy chain variable region (IGHV, 59%).
Among 32 patients eligible for efficacy analysis, the overall response rate was 72%, of which 53% were partial and 19% were complete. Six percent (6%) of patients achieved minimal residual disease negativity. The benefits of ofatumumab extended to patients with high-risk disease characteristics, including unmutated IGHV (65% response rate) and/or a complex karyotype (60% response rate).
Ofatumumab also demonstrated a favorable safety profile, according to the investigators.
With all 34 patients evaluable for safety data, 19 (56%) experienced a grade 1 or 2 infusion-related reaction, and 1 (3%) experienced a grade 3 infusion-related reaction. Twenty-one grade 2 infections were reported, and one grade 3 infection occurred. Other grade 3 treatment-related adverse events included gastrointestinal disturbances, pulmonary embolism, allergic reaction, and hyperglycemia, each of which occurred in one patient. No grade 4 adverse events, or grade 2 or higher hematologic toxicities occurred.
“Our findings show that older patients with poor performance status and comorbidities can safely undergo treatment with ofatumumab,” the investigators concluded. “[The results] also support the possibility of enrolling these patients in clinical trials, so that a larger number of patients will be included and their characteristics will more closely mirror those of typical patients seen in the community.”
The study was funded by Novartis, which markets the antibody. The investigators reported additional relationships with AbbVie, Roche, Celgene, and others.
SOURCE: Vitale et al. J Geriatr Oncol. 2019 Apr 18. doi: 10.1016/j.jgo.2019.04.002.
For elderly patients with chronic lymphocytic leukemia (CLL) and comorbidities, the anti-CD20 monoclonal antibody ofatumumab may be a safe and effective treatment option, according to a recent phase 2 trial.
Among 32 patients with a median age of 73 years, the overall response rate was 72%, and no grade 4 adverse events occurred, reported lead author Candida Vitale, MD, PhD, of the University of Torino (Italy) and colleagues.
These findings help fill in a knowledge gap created by clinical trial exclusions, which currently make treatment planning “a significant challenge,” the investigators wrote in Journal of Geriatric Oncology.
The study, which was conducted at MD Anderson Cancer Center in Houston, enrolled 34 treatment-naive patients with CLL who were 65 years or older. All patients had an Eastern Cooperative Oncology Group performance status of 2 or 3, or a Charlson comorbidity index of at least 2. Patients with other serious medical conditions and/or primary malignancies were eligible, given that they were not already receiving anticancer therapy.
More than half of the patients (53%) had advanced-stage disease and almost one-third (29%) had at least one other primary cancer diagnosis. Many patients also had high-risk disease characteristics, including a complex karyotype involving three or more chromosomal abnormalities (15%), and/or unmutated immunoglobulin heavy chain variable region (IGHV, 59%).
Among 32 patients eligible for efficacy analysis, the overall response rate was 72%, of which 53% were partial and 19% were complete. Six percent (6%) of patients achieved minimal residual disease negativity. The benefits of ofatumumab extended to patients with high-risk disease characteristics, including unmutated IGHV (65% response rate) and/or a complex karyotype (60% response rate).
Ofatumumab also demonstrated a favorable safety profile, according to the investigators.
With all 34 patients evaluable for safety data, 19 (56%) experienced a grade 1 or 2 infusion-related reaction, and 1 (3%) experienced a grade 3 infusion-related reaction. Twenty-one grade 2 infections were reported, and one grade 3 infection occurred. Other grade 3 treatment-related adverse events included gastrointestinal disturbances, pulmonary embolism, allergic reaction, and hyperglycemia, each of which occurred in one patient. No grade 4 adverse events, or grade 2 or higher hematologic toxicities occurred.
“Our findings show that older patients with poor performance status and comorbidities can safely undergo treatment with ofatumumab,” the investigators concluded. “[The results] also support the possibility of enrolling these patients in clinical trials, so that a larger number of patients will be included and their characteristics will more closely mirror those of typical patients seen in the community.”
The study was funded by Novartis, which markets the antibody. The investigators reported additional relationships with AbbVie, Roche, Celgene, and others.
SOURCE: Vitale et al. J Geriatr Oncol. 2019 Apr 18. doi: 10.1016/j.jgo.2019.04.002.
For elderly patients with chronic lymphocytic leukemia (CLL) and comorbidities, the anti-CD20 monoclonal antibody ofatumumab may be a safe and effective treatment option, according to a recent phase 2 trial.
Among 32 patients with a median age of 73 years, the overall response rate was 72%, and no grade 4 adverse events occurred, reported lead author Candida Vitale, MD, PhD, of the University of Torino (Italy) and colleagues.
These findings help fill in a knowledge gap created by clinical trial exclusions, which currently make treatment planning “a significant challenge,” the investigators wrote in Journal of Geriatric Oncology.
The study, which was conducted at MD Anderson Cancer Center in Houston, enrolled 34 treatment-naive patients with CLL who were 65 years or older. All patients had an Eastern Cooperative Oncology Group performance status of 2 or 3, or a Charlson comorbidity index of at least 2. Patients with other serious medical conditions and/or primary malignancies were eligible, given that they were not already receiving anticancer therapy.
More than half of the patients (53%) had advanced-stage disease and almost one-third (29%) had at least one other primary cancer diagnosis. Many patients also had high-risk disease characteristics, including a complex karyotype involving three or more chromosomal abnormalities (15%), and/or unmutated immunoglobulin heavy chain variable region (IGHV, 59%).
Among 32 patients eligible for efficacy analysis, the overall response rate was 72%, of which 53% were partial and 19% were complete. Six percent (6%) of patients achieved minimal residual disease negativity. The benefits of ofatumumab extended to patients with high-risk disease characteristics, including unmutated IGHV (65% response rate) and/or a complex karyotype (60% response rate).
Ofatumumab also demonstrated a favorable safety profile, according to the investigators.
With all 34 patients evaluable for safety data, 19 (56%) experienced a grade 1 or 2 infusion-related reaction, and 1 (3%) experienced a grade 3 infusion-related reaction. Twenty-one grade 2 infections were reported, and one grade 3 infection occurred. Other grade 3 treatment-related adverse events included gastrointestinal disturbances, pulmonary embolism, allergic reaction, and hyperglycemia, each of which occurred in one patient. No grade 4 adverse events, or grade 2 or higher hematologic toxicities occurred.
“Our findings show that older patients with poor performance status and comorbidities can safely undergo treatment with ofatumumab,” the investigators concluded. “[The results] also support the possibility of enrolling these patients in clinical trials, so that a larger number of patients will be included and their characteristics will more closely mirror those of typical patients seen in the community.”
The study was funded by Novartis, which markets the antibody. The investigators reported additional relationships with AbbVie, Roche, Celgene, and others.
SOURCE: Vitale et al. J Geriatr Oncol. 2019 Apr 18. doi: 10.1016/j.jgo.2019.04.002.
FROM THE JOURNAL OF GERIATRIC ONCOLOGY
CAR T-cell therapy may worsen mental health in some patients
Chimeric antigen receptor (CAR) T-cell therapy is generally associated with good long-term neuropsychiatric status, based on a recent patient-reported outcomes study.
But almost one out of five patients may have notably worse cognitive and psychiatric outcomes within 1-5 years of therapy, reported Julia Ruark, MD, of the University of Washington, Seattle, and colleagues. According to Dr. Ruark and associates, this latter finding suggests that CAR T-cell therapy may negatively impact mental health in a subset of patients.
These findings provide clinical insight into a minimally researched patient population.
“At this time, only limited data are available regarding the long-term effects of CAR T-cell therapy,” the investigators wrote in Biology of Blood and Marrow Transplantation. “Thus, it is important to evaluate the late neuropsychiatric effects of CAR T and evaluate their effect on survivors’ quality of life.”
The study involved 40 patients with relapsed or refractory chronic lymphocytic leukemia, non-Hodgkin lymphoma, or acute lymphoblastic leukemia. Before undergoing CAR T-cell therapy, patients underwent standardized mental health screening with validated instruments such as the 7-item Generalized Anxiety Disorder scale. At least 1 year after CAR T-cell therapy, patients completed a questionnaire consisting of the Patient-Reported Outcomes Measurement Information System (PROMIS) Scale v1.2 Global Health and the PROMIS-29 Profile v2.1, and 30 additional questions, 4 of which evaluated cognitive function. These data were converted to T scores for comparative purposes.
Patients who underwent CAR T-cell therapy had statistically similar T scores to the general population mean, suggesting comparable overall neuropsychiatric status. However, a closer look at the data showed that almost one out of five patients who underwent CAR T-cell therapy had global mental health scores that were at least 1 standard deviation lower than the mean for the general population and patients with cancer.
Almost half of the patients (47.5%) who underwent CAR T-cell therapy reported at least one clinically meaningful negative neuropsychiatric outcome. Specifically, 20% reported cognitive difficulties and depression or anxiety, 17.5% reported cognitive difficulties without depression or anxiety, and 10% reported depression or anxiety without cognitive difficulties. One-quarter (25%) of patients reported taking a medication for depression, 20% reported use of anxiolytics, and 15% reported use of sleep medications. Multivariate analysis revealed an association between younger age and depression (P = .01), anxiety (P = .001), and worse long-term global mental health (P = .02). Cognitive difficulties were significantly more common among patients with worse physical and/or mental health.
“[A] subset of patients may experience psychiatric symptoms or cognitive impairment [which may be related to CAR T-cell therapy or other treatments patients have been exposed to], and it is important to identify those patients to assist with intervention strategies,” the investigators concluded.The study was funded by the National Institutes of Health, Life Science Discovery Fund, Juno Therapeutics/Celgene, and others. The investigators reported additional relationships with Nektar Therapeutics, Allogene Therapeutics, T-CURX, and others.
SOURCE: Ruark J et al. Biol Blood Marrow Transplant. 2019 Oct 9. doi: 10.1016/j.bbmt.2019.09.037.
Chimeric antigen receptor (CAR) T-cell therapy is generally associated with good long-term neuropsychiatric status, based on a recent patient-reported outcomes study.
But almost one out of five patients may have notably worse cognitive and psychiatric outcomes within 1-5 years of therapy, reported Julia Ruark, MD, of the University of Washington, Seattle, and colleagues. According to Dr. Ruark and associates, this latter finding suggests that CAR T-cell therapy may negatively impact mental health in a subset of patients.
These findings provide clinical insight into a minimally researched patient population.
“At this time, only limited data are available regarding the long-term effects of CAR T-cell therapy,” the investigators wrote in Biology of Blood and Marrow Transplantation. “Thus, it is important to evaluate the late neuropsychiatric effects of CAR T and evaluate their effect on survivors’ quality of life.”
The study involved 40 patients with relapsed or refractory chronic lymphocytic leukemia, non-Hodgkin lymphoma, or acute lymphoblastic leukemia. Before undergoing CAR T-cell therapy, patients underwent standardized mental health screening with validated instruments such as the 7-item Generalized Anxiety Disorder scale. At least 1 year after CAR T-cell therapy, patients completed a questionnaire consisting of the Patient-Reported Outcomes Measurement Information System (PROMIS) Scale v1.2 Global Health and the PROMIS-29 Profile v2.1, and 30 additional questions, 4 of which evaluated cognitive function. These data were converted to T scores for comparative purposes.
Patients who underwent CAR T-cell therapy had statistically similar T scores to the general population mean, suggesting comparable overall neuropsychiatric status. However, a closer look at the data showed that almost one out of five patients who underwent CAR T-cell therapy had global mental health scores that were at least 1 standard deviation lower than the mean for the general population and patients with cancer.
Almost half of the patients (47.5%) who underwent CAR T-cell therapy reported at least one clinically meaningful negative neuropsychiatric outcome. Specifically, 20% reported cognitive difficulties and depression or anxiety, 17.5% reported cognitive difficulties without depression or anxiety, and 10% reported depression or anxiety without cognitive difficulties. One-quarter (25%) of patients reported taking a medication for depression, 20% reported use of anxiolytics, and 15% reported use of sleep medications. Multivariate analysis revealed an association between younger age and depression (P = .01), anxiety (P = .001), and worse long-term global mental health (P = .02). Cognitive difficulties were significantly more common among patients with worse physical and/or mental health.
“[A] subset of patients may experience psychiatric symptoms or cognitive impairment [which may be related to CAR T-cell therapy or other treatments patients have been exposed to], and it is important to identify those patients to assist with intervention strategies,” the investigators concluded.The study was funded by the National Institutes of Health, Life Science Discovery Fund, Juno Therapeutics/Celgene, and others. The investigators reported additional relationships with Nektar Therapeutics, Allogene Therapeutics, T-CURX, and others.
SOURCE: Ruark J et al. Biol Blood Marrow Transplant. 2019 Oct 9. doi: 10.1016/j.bbmt.2019.09.037.
Chimeric antigen receptor (CAR) T-cell therapy is generally associated with good long-term neuropsychiatric status, based on a recent patient-reported outcomes study.
But almost one out of five patients may have notably worse cognitive and psychiatric outcomes within 1-5 years of therapy, reported Julia Ruark, MD, of the University of Washington, Seattle, and colleagues. According to Dr. Ruark and associates, this latter finding suggests that CAR T-cell therapy may negatively impact mental health in a subset of patients.
These findings provide clinical insight into a minimally researched patient population.
“At this time, only limited data are available regarding the long-term effects of CAR T-cell therapy,” the investigators wrote in Biology of Blood and Marrow Transplantation. “Thus, it is important to evaluate the late neuropsychiatric effects of CAR T and evaluate their effect on survivors’ quality of life.”
The study involved 40 patients with relapsed or refractory chronic lymphocytic leukemia, non-Hodgkin lymphoma, or acute lymphoblastic leukemia. Before undergoing CAR T-cell therapy, patients underwent standardized mental health screening with validated instruments such as the 7-item Generalized Anxiety Disorder scale. At least 1 year after CAR T-cell therapy, patients completed a questionnaire consisting of the Patient-Reported Outcomes Measurement Information System (PROMIS) Scale v1.2 Global Health and the PROMIS-29 Profile v2.1, and 30 additional questions, 4 of which evaluated cognitive function. These data were converted to T scores for comparative purposes.
Patients who underwent CAR T-cell therapy had statistically similar T scores to the general population mean, suggesting comparable overall neuropsychiatric status. However, a closer look at the data showed that almost one out of five patients who underwent CAR T-cell therapy had global mental health scores that were at least 1 standard deviation lower than the mean for the general population and patients with cancer.
Almost half of the patients (47.5%) who underwent CAR T-cell therapy reported at least one clinically meaningful negative neuropsychiatric outcome. Specifically, 20% reported cognitive difficulties and depression or anxiety, 17.5% reported cognitive difficulties without depression or anxiety, and 10% reported depression or anxiety without cognitive difficulties. One-quarter (25%) of patients reported taking a medication for depression, 20% reported use of anxiolytics, and 15% reported use of sleep medications. Multivariate analysis revealed an association between younger age and depression (P = .01), anxiety (P = .001), and worse long-term global mental health (P = .02). Cognitive difficulties were significantly more common among patients with worse physical and/or mental health.
“[A] subset of patients may experience psychiatric symptoms or cognitive impairment [which may be related to CAR T-cell therapy or other treatments patients have been exposed to], and it is important to identify those patients to assist with intervention strategies,” the investigators concluded.The study was funded by the National Institutes of Health, Life Science Discovery Fund, Juno Therapeutics/Celgene, and others. The investigators reported additional relationships with Nektar Therapeutics, Allogene Therapeutics, T-CURX, and others.
SOURCE: Ruark J et al. Biol Blood Marrow Transplant. 2019 Oct 9. doi: 10.1016/j.bbmt.2019.09.037.
FROM BIOLOGY OF BLOOD AND MARROW TRANSPLANTATION