User login
ASH tackles COVID-19 with hematology-related FAQ, promotes new registries
The American Society of Hematology has committed a portion of its website to providing continually updated information addressing specific hematologic disorders in relation to COVID-19.
“As the world grapples with the novel coronavirus, ASH believes that we can help each other be as knowledgeable and prepared as possible,” wrote the society’s president, Stephanie J. Lee, MD, MPH.
On its website, ASH provides relevant COVID-19 information in a series of FAQ divided into malignant and nonmalignant hematologic diseases and disorders. In the malignant category, the various lymphomas and leukemias are individually addressed, as well as other conditions such as myelodysplastic syndromes, myeloproliferative neoplasms, and multiple myeloma. In the nonmalignant category, ASH has provided FAQ on aplastic anemia, thalassemia, sickle cell disease, pulmonary embolism, venous thromboembolism/anticoagulation, coagulopathy, and immune as well as thrombotic thrombocytopenic purpura.
In addition to the continually updated series of relevant FAQ, as part of its response to the pandemic ASH is promoting two unique COVID-19 registries for physicians: the ASH Research Collaborative’s (ASH RC) Data Hub COVID-19 Registry and the Surveillance Epidemiology of Coronavirus (COVID-19) Under Research Exclusion Sickle Cell Disease (SECURE-SCD) Registry.
“The ASH Research Collaborative’s (ASH RC) Data Hub launched the COVID-19 Registry and is currently capturing data on people who test positive for COVID-19 and have been or are currently being treated for hematologic malignancy,” according to the website. The intention is to provide “near real-time observational data summaries,” which will hopefully provide useful information to clinicians treating hematologic malignancies in patients in the midst of the COVID-19 pandemic.
The registry allows clinicians to enter their own cases in a specified format to allow data analysis on clinical practice and patient outcomes that will be aggregated to provide rapid insights for clinicians to help them care for their patients, according to ASH.
The second registry specifically deals with COVID-19 cases in patients with sickle cell disease. It also allows clinicians to add cases with a similar intention of aggregating data to provide near real-time insights into patient care. “We are asking providers caring for these patients to report all of their cases of COVID-19 to this registry,” according to the registry website. The registry is for reporting COVID-19 cases in sickle cell disease patients “after sufficient time has passed to observe the disease course through resolution of acute illness and/or death.”
ASH also provides more generalized information for hematology practitioners dealing with COVID-19 on the topics of conducting their practice and using telemedicine, among others.
Correction, April 15, 2020: This story originally said incorrectly that ASH developed the 2 new registries. The registries are merely being promoted on the ASH website.
The American Society of Hematology has committed a portion of its website to providing continually updated information addressing specific hematologic disorders in relation to COVID-19.
“As the world grapples with the novel coronavirus, ASH believes that we can help each other be as knowledgeable and prepared as possible,” wrote the society’s president, Stephanie J. Lee, MD, MPH.
On its website, ASH provides relevant COVID-19 information in a series of FAQ divided into malignant and nonmalignant hematologic diseases and disorders. In the malignant category, the various lymphomas and leukemias are individually addressed, as well as other conditions such as myelodysplastic syndromes, myeloproliferative neoplasms, and multiple myeloma. In the nonmalignant category, ASH has provided FAQ on aplastic anemia, thalassemia, sickle cell disease, pulmonary embolism, venous thromboembolism/anticoagulation, coagulopathy, and immune as well as thrombotic thrombocytopenic purpura.
In addition to the continually updated series of relevant FAQ, as part of its response to the pandemic ASH is promoting two unique COVID-19 registries for physicians: the ASH Research Collaborative’s (ASH RC) Data Hub COVID-19 Registry and the Surveillance Epidemiology of Coronavirus (COVID-19) Under Research Exclusion Sickle Cell Disease (SECURE-SCD) Registry.
“The ASH Research Collaborative’s (ASH RC) Data Hub launched the COVID-19 Registry and is currently capturing data on people who test positive for COVID-19 and have been or are currently being treated for hematologic malignancy,” according to the website. The intention is to provide “near real-time observational data summaries,” which will hopefully provide useful information to clinicians treating hematologic malignancies in patients in the midst of the COVID-19 pandemic.
The registry allows clinicians to enter their own cases in a specified format to allow data analysis on clinical practice and patient outcomes that will be aggregated to provide rapid insights for clinicians to help them care for their patients, according to ASH.
The second registry specifically deals with COVID-19 cases in patients with sickle cell disease. It also allows clinicians to add cases with a similar intention of aggregating data to provide near real-time insights into patient care. “We are asking providers caring for these patients to report all of their cases of COVID-19 to this registry,” according to the registry website. The registry is for reporting COVID-19 cases in sickle cell disease patients “after sufficient time has passed to observe the disease course through resolution of acute illness and/or death.”
ASH also provides more generalized information for hematology practitioners dealing with COVID-19 on the topics of conducting their practice and using telemedicine, among others.
Correction, April 15, 2020: This story originally said incorrectly that ASH developed the 2 new registries. The registries are merely being promoted on the ASH website.
The American Society of Hematology has committed a portion of its website to providing continually updated information addressing specific hematologic disorders in relation to COVID-19.
“As the world grapples with the novel coronavirus, ASH believes that we can help each other be as knowledgeable and prepared as possible,” wrote the society’s president, Stephanie J. Lee, MD, MPH.
On its website, ASH provides relevant COVID-19 information in a series of FAQ divided into malignant and nonmalignant hematologic diseases and disorders. In the malignant category, the various lymphomas and leukemias are individually addressed, as well as other conditions such as myelodysplastic syndromes, myeloproliferative neoplasms, and multiple myeloma. In the nonmalignant category, ASH has provided FAQ on aplastic anemia, thalassemia, sickle cell disease, pulmonary embolism, venous thromboembolism/anticoagulation, coagulopathy, and immune as well as thrombotic thrombocytopenic purpura.
In addition to the continually updated series of relevant FAQ, as part of its response to the pandemic ASH is promoting two unique COVID-19 registries for physicians: the ASH Research Collaborative’s (ASH RC) Data Hub COVID-19 Registry and the Surveillance Epidemiology of Coronavirus (COVID-19) Under Research Exclusion Sickle Cell Disease (SECURE-SCD) Registry.
“The ASH Research Collaborative’s (ASH RC) Data Hub launched the COVID-19 Registry and is currently capturing data on people who test positive for COVID-19 and have been or are currently being treated for hematologic malignancy,” according to the website. The intention is to provide “near real-time observational data summaries,” which will hopefully provide useful information to clinicians treating hematologic malignancies in patients in the midst of the COVID-19 pandemic.
The registry allows clinicians to enter their own cases in a specified format to allow data analysis on clinical practice and patient outcomes that will be aggregated to provide rapid insights for clinicians to help them care for their patients, according to ASH.
The second registry specifically deals with COVID-19 cases in patients with sickle cell disease. It also allows clinicians to add cases with a similar intention of aggregating data to provide near real-time insights into patient care. “We are asking providers caring for these patients to report all of their cases of COVID-19 to this registry,” according to the registry website. The registry is for reporting COVID-19 cases in sickle cell disease patients “after sufficient time has passed to observe the disease course through resolution of acute illness and/or death.”
ASH also provides more generalized information for hematology practitioners dealing with COVID-19 on the topics of conducting their practice and using telemedicine, among others.
Correction, April 15, 2020: This story originally said incorrectly that ASH developed the 2 new registries. The registries are merely being promoted on the ASH website.
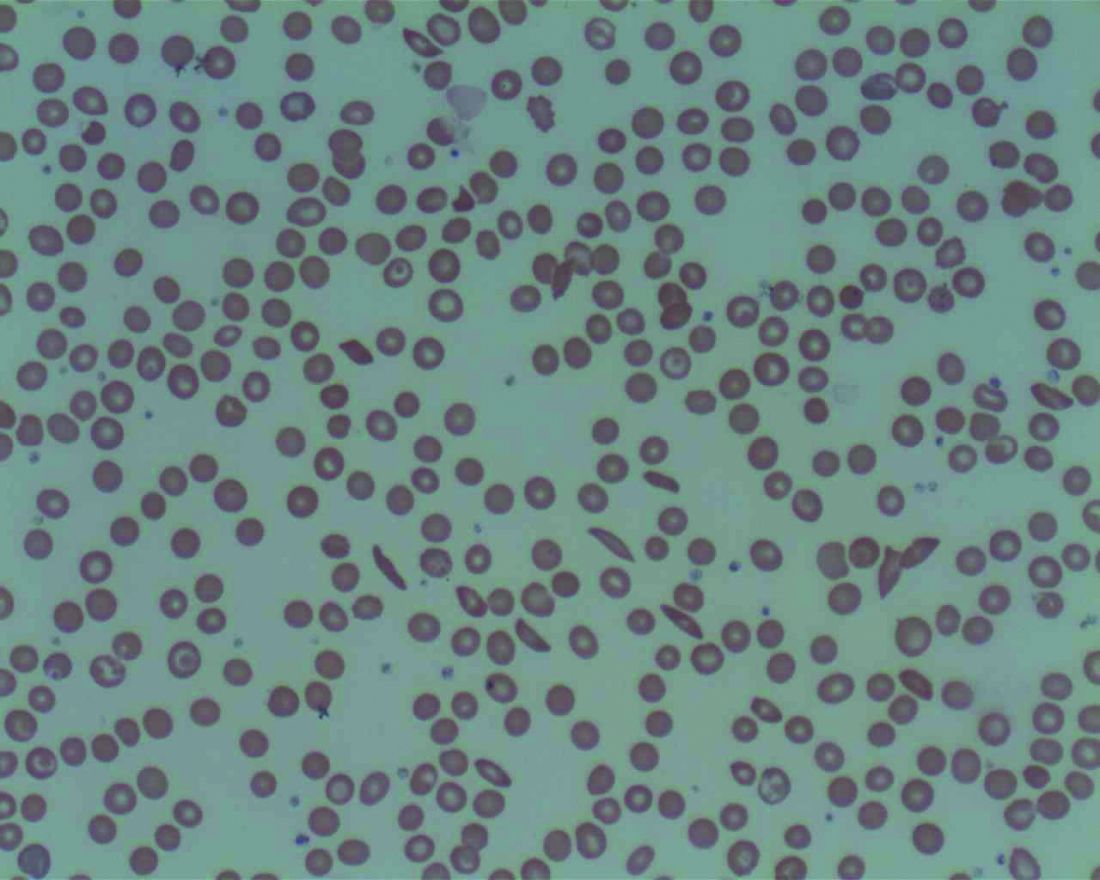
Low plasma sodium levels can predict complications in acute painful episodes of SCD
Although a low blood sodium level has been shown to be a prognostic factor for a number of disorders, it has not been reported on for sickle cell disease, according to French researchers Jean-Simon Rech, MD, and colleagues.
They found that hyponatremia at hospital admission was predictive of complications in initially uncomplicated episodes of painful episodes of sickle cell disease (SCD), according to their study published online in the American Journal of Medicine. Dr. Rech is with the department of internal medicine, Sickle Cell Disease Reference Center, Tenon Hospital, Assistance Publique-Hôpitaux de Paris.
The study assessed 1,218 stays (406 patients) admitted to a single center and the analyses were adjusted for age, sex, hemoglobin genotype and concentration, LDH concentration, and white blood cell count.
The researchers found that hyponatremia (defined as plasma sodium ≤ 135 mmol/L) was significantly associated with the primary endpoint of a composite criterion including acute chest syndrome, intensive care unit transfer, red blood cell transfusion, or inpatient death (P = .001).
With regard to the components of the primary endpoint, hyponatremia was significantly associated with acute chest syndrome (P = .008), as well as with receiving a red blood cell transfusion (P < .001) However, hyponatremia at admission was not significantly associated with intensive care unit transfer (P = .074) and there were no patient deaths.
In addition, hyponatremia was significantly associated with longer stays: 1.1 days adjusted mean length of stay (P < .001).
“Hyponatremia may lead to direct clinical consequences in the management of sickle cell disease patients. Indeed, a plasma sodium concentration ≤ 135mmol/L at admission or a decreasing natremia over the first days of an acute painful episode could be regarded as early signs of incipient acute chest syndrome, prompting clinicians to closely monitor the clinical status of their patients,” the researchers concluded.
The authors declared that they had no conflicts and that there was no funding source.
SOURCE: Rech JS et al. Am J Med. 2020 Mar 18. doi.org/10.1016/j.amjmed.2020.02.017.
Although a low blood sodium level has been shown to be a prognostic factor for a number of disorders, it has not been reported on for sickle cell disease, according to French researchers Jean-Simon Rech, MD, and colleagues.
They found that hyponatremia at hospital admission was predictive of complications in initially uncomplicated episodes of painful episodes of sickle cell disease (SCD), according to their study published online in the American Journal of Medicine. Dr. Rech is with the department of internal medicine, Sickle Cell Disease Reference Center, Tenon Hospital, Assistance Publique-Hôpitaux de Paris.
The study assessed 1,218 stays (406 patients) admitted to a single center and the analyses were adjusted for age, sex, hemoglobin genotype and concentration, LDH concentration, and white blood cell count.
The researchers found that hyponatremia (defined as plasma sodium ≤ 135 mmol/L) was significantly associated with the primary endpoint of a composite criterion including acute chest syndrome, intensive care unit transfer, red blood cell transfusion, or inpatient death (P = .001).
With regard to the components of the primary endpoint, hyponatremia was significantly associated with acute chest syndrome (P = .008), as well as with receiving a red blood cell transfusion (P < .001) However, hyponatremia at admission was not significantly associated with intensive care unit transfer (P = .074) and there were no patient deaths.
In addition, hyponatremia was significantly associated with longer stays: 1.1 days adjusted mean length of stay (P < .001).
“Hyponatremia may lead to direct clinical consequences in the management of sickle cell disease patients. Indeed, a plasma sodium concentration ≤ 135mmol/L at admission or a decreasing natremia over the first days of an acute painful episode could be regarded as early signs of incipient acute chest syndrome, prompting clinicians to closely monitor the clinical status of their patients,” the researchers concluded.
The authors declared that they had no conflicts and that there was no funding source.
SOURCE: Rech JS et al. Am J Med. 2020 Mar 18. doi.org/10.1016/j.amjmed.2020.02.017.
Although a low blood sodium level has been shown to be a prognostic factor for a number of disorders, it has not been reported on for sickle cell disease, according to French researchers Jean-Simon Rech, MD, and colleagues.
They found that hyponatremia at hospital admission was predictive of complications in initially uncomplicated episodes of painful episodes of sickle cell disease (SCD), according to their study published online in the American Journal of Medicine. Dr. Rech is with the department of internal medicine, Sickle Cell Disease Reference Center, Tenon Hospital, Assistance Publique-Hôpitaux de Paris.
The study assessed 1,218 stays (406 patients) admitted to a single center and the analyses were adjusted for age, sex, hemoglobin genotype and concentration, LDH concentration, and white blood cell count.
The researchers found that hyponatremia (defined as plasma sodium ≤ 135 mmol/L) was significantly associated with the primary endpoint of a composite criterion including acute chest syndrome, intensive care unit transfer, red blood cell transfusion, or inpatient death (P = .001).
With regard to the components of the primary endpoint, hyponatremia was significantly associated with acute chest syndrome (P = .008), as well as with receiving a red blood cell transfusion (P < .001) However, hyponatremia at admission was not significantly associated with intensive care unit transfer (P = .074) and there were no patient deaths.
In addition, hyponatremia was significantly associated with longer stays: 1.1 days adjusted mean length of stay (P < .001).
“Hyponatremia may lead to direct clinical consequences in the management of sickle cell disease patients. Indeed, a plasma sodium concentration ≤ 135mmol/L at admission or a decreasing natremia over the first days of an acute painful episode could be regarded as early signs of incipient acute chest syndrome, prompting clinicians to closely monitor the clinical status of their patients,” the researchers concluded.
The authors declared that they had no conflicts and that there was no funding source.
SOURCE: Rech JS et al. Am J Med. 2020 Mar 18. doi.org/10.1016/j.amjmed.2020.02.017.
FROM THE AMERICAN JOURNAL OF MEDICINE
First advance in MDS for decade: Luspatercept for anemia
The US Food and Drug Administration has approved luspatercept (Reblozyl, Bristol-Myers Squibb/Acceleron) for the treatment of anemia in patients with myelodysplastic syndromes (MDS).
The green light represents the first treatment advancement in MDS in more than a decade, says an expert in the field.
Luspatercept is the first and so far only erythroid maturation agent (EMA), and was launched last year when it was approved for the treatment of anemia in adults with beta thalassemia, who require regular red blood cell transfusions.
The new approval is for the treatment of anemia in adult patients with very low- to intermediate-risk MDS with ring sideroblasts and patients with myelodysplastic/myeloproliferative neoplasm with ring sideroblasts and thrombocytosis, after they have progressed on treatment with an erythropoiesis-stimulating agent and who require two or more red blood cell (RBC) units over 8 weeks.
Luspatercept is not a substitute for RBC transfusions in patients who require immediate correction of anemia.
The FDA approval in MDS is based on results from the pivotal, placebo-controlled, phase 3 MEDALIST trial, conducted in 229 patients with very-low–, low- and intermediate-risk non-del(5q) MDS with ring sideroblasts. All patients were RBC transfusion-dependent and had disease that was refractory to, or unlikely to respond to, erythropoiesis-stimulating agents. Results were published in January in the New England Journal of Medicine. The study was funded by Acceleron Pharma and Celgene, which was later acquired by Bristol-Myers Squibb.
These results were first presented at the 2018 annual meeting of the American Society of Hematology (ASH), as reported by Medscape Medical News. At the time, ASH President Alexis Thompson, MD, said it appears that luspatercept can improve the production of endogenous RBCs by enhancing the maturation of these cells in the bone marrow. The drug significantly reduced the need for RBC transfusions, and “this is a very exciting advance for patients who would have few other treatment options,” she said.
“Anemia and the chronic need for transfusions is a very big issue for these patients,” commented lead study author Pierre Fenaux, MD, PhD, from Hôpital Saint-Louis in Paris, France. “With low hemoglobin levels, patients are tired all the time and have an increased risk of falls and cardiovascular events. When you can improve hemoglobin levels, you really see a difference in quality of life.”
The MEDALIST trial is an important milestone for patients with lower-risk, transfusion-dependent MDS, commented Elizabeth Griffiths, MD, associate professor of oncology and director of MDS, Roswell Park Comprehensive Cancer Center, Buffalo, New York.
“No new agents have been approved for MDS in the last 10 years, highlighting this development as a substantial step forward for the MDS community,” she told Medscape Medical News. “Current therapies are time-intensive and only modestly beneficial.”
“The availability of a new, effective drug — particularly relevant to those harboring SF3B1 mutations — is an exciting development and is likely to offer meaningful improvements in quality of life,” Griffiths said. “Since these patients tend to live longer than others with MDS, there are many patients in my clinical practice who would have fit the enrollment criteria for this study. Such patients are eagerly awaiting the opportunity for a decrease in transfusion burden.”
Study Details
In the trial, luspatercept reduced the severity of anemia — 38% of the 153 patients who received luspatercept achieved transfusion independence for 8 weeks or longer compared with 13% of the 76 patients receiving placebo (P < .001).
In the study, patients received luspatercept at a starting dose of 1.0 mg/kg with titration up to 1.75 mg/kg, if needed, or placebo, subcutaneously every 3 weeks for at least 24 weeks.
During the 16 weeks before the initiation of treatment, study patients had received a median of 5 RBC units transfusions during an 8-week period (43.2% of patients had ≥ 6 RBC units, 27.9% had ≥ 4 to < 6 RBC units, and 28.8% had < 4 RBC units). At baseline, 138 (60.3%), 58 (25.3%), and 32 (14%) patients had serum erythropoietin levels less than 200 IU/L, 200-500 IU/L, and greater than 500 IU/L, respectively.
The most common luspatercept-associated adverse events (any grade) in the trial were fatigue, diarrhea, asthenia, nausea, and dizziness. Grade 3 or 4 treatment-emergent adverse events were reported in 42.5% of patients who received luspatercept and 44.7% of patients who received placebo. The incidence of adverse events decreased over time, according to the study authors.
This article first appeared on Medscape.com.
The US Food and Drug Administration has approved luspatercept (Reblozyl, Bristol-Myers Squibb/Acceleron) for the treatment of anemia in patients with myelodysplastic syndromes (MDS).
The green light represents the first treatment advancement in MDS in more than a decade, says an expert in the field.
Luspatercept is the first and so far only erythroid maturation agent (EMA), and was launched last year when it was approved for the treatment of anemia in adults with beta thalassemia, who require regular red blood cell transfusions.
The new approval is for the treatment of anemia in adult patients with very low- to intermediate-risk MDS with ring sideroblasts and patients with myelodysplastic/myeloproliferative neoplasm with ring sideroblasts and thrombocytosis, after they have progressed on treatment with an erythropoiesis-stimulating agent and who require two or more red blood cell (RBC) units over 8 weeks.
Luspatercept is not a substitute for RBC transfusions in patients who require immediate correction of anemia.
The FDA approval in MDS is based on results from the pivotal, placebo-controlled, phase 3 MEDALIST trial, conducted in 229 patients with very-low–, low- and intermediate-risk non-del(5q) MDS with ring sideroblasts. All patients were RBC transfusion-dependent and had disease that was refractory to, or unlikely to respond to, erythropoiesis-stimulating agents. Results were published in January in the New England Journal of Medicine. The study was funded by Acceleron Pharma and Celgene, which was later acquired by Bristol-Myers Squibb.
These results were first presented at the 2018 annual meeting of the American Society of Hematology (ASH), as reported by Medscape Medical News. At the time, ASH President Alexis Thompson, MD, said it appears that luspatercept can improve the production of endogenous RBCs by enhancing the maturation of these cells in the bone marrow. The drug significantly reduced the need for RBC transfusions, and “this is a very exciting advance for patients who would have few other treatment options,” she said.
“Anemia and the chronic need for transfusions is a very big issue for these patients,” commented lead study author Pierre Fenaux, MD, PhD, from Hôpital Saint-Louis in Paris, France. “With low hemoglobin levels, patients are tired all the time and have an increased risk of falls and cardiovascular events. When you can improve hemoglobin levels, you really see a difference in quality of life.”
The MEDALIST trial is an important milestone for patients with lower-risk, transfusion-dependent MDS, commented Elizabeth Griffiths, MD, associate professor of oncology and director of MDS, Roswell Park Comprehensive Cancer Center, Buffalo, New York.
“No new agents have been approved for MDS in the last 10 years, highlighting this development as a substantial step forward for the MDS community,” she told Medscape Medical News. “Current therapies are time-intensive and only modestly beneficial.”
“The availability of a new, effective drug — particularly relevant to those harboring SF3B1 mutations — is an exciting development and is likely to offer meaningful improvements in quality of life,” Griffiths said. “Since these patients tend to live longer than others with MDS, there are many patients in my clinical practice who would have fit the enrollment criteria for this study. Such patients are eagerly awaiting the opportunity for a decrease in transfusion burden.”
Study Details
In the trial, luspatercept reduced the severity of anemia — 38% of the 153 patients who received luspatercept achieved transfusion independence for 8 weeks or longer compared with 13% of the 76 patients receiving placebo (P < .001).
In the study, patients received luspatercept at a starting dose of 1.0 mg/kg with titration up to 1.75 mg/kg, if needed, or placebo, subcutaneously every 3 weeks for at least 24 weeks.
During the 16 weeks before the initiation of treatment, study patients had received a median of 5 RBC units transfusions during an 8-week period (43.2% of patients had ≥ 6 RBC units, 27.9% had ≥ 4 to < 6 RBC units, and 28.8% had < 4 RBC units). At baseline, 138 (60.3%), 58 (25.3%), and 32 (14%) patients had serum erythropoietin levels less than 200 IU/L, 200-500 IU/L, and greater than 500 IU/L, respectively.
The most common luspatercept-associated adverse events (any grade) in the trial were fatigue, diarrhea, asthenia, nausea, and dizziness. Grade 3 or 4 treatment-emergent adverse events were reported in 42.5% of patients who received luspatercept and 44.7% of patients who received placebo. The incidence of adverse events decreased over time, according to the study authors.
This article first appeared on Medscape.com.
The US Food and Drug Administration has approved luspatercept (Reblozyl, Bristol-Myers Squibb/Acceleron) for the treatment of anemia in patients with myelodysplastic syndromes (MDS).
The green light represents the first treatment advancement in MDS in more than a decade, says an expert in the field.
Luspatercept is the first and so far only erythroid maturation agent (EMA), and was launched last year when it was approved for the treatment of anemia in adults with beta thalassemia, who require regular red blood cell transfusions.
The new approval is for the treatment of anemia in adult patients with very low- to intermediate-risk MDS with ring sideroblasts and patients with myelodysplastic/myeloproliferative neoplasm with ring sideroblasts and thrombocytosis, after they have progressed on treatment with an erythropoiesis-stimulating agent and who require two or more red blood cell (RBC) units over 8 weeks.
Luspatercept is not a substitute for RBC transfusions in patients who require immediate correction of anemia.
The FDA approval in MDS is based on results from the pivotal, placebo-controlled, phase 3 MEDALIST trial, conducted in 229 patients with very-low–, low- and intermediate-risk non-del(5q) MDS with ring sideroblasts. All patients were RBC transfusion-dependent and had disease that was refractory to, or unlikely to respond to, erythropoiesis-stimulating agents. Results were published in January in the New England Journal of Medicine. The study was funded by Acceleron Pharma and Celgene, which was later acquired by Bristol-Myers Squibb.
These results were first presented at the 2018 annual meeting of the American Society of Hematology (ASH), as reported by Medscape Medical News. At the time, ASH President Alexis Thompson, MD, said it appears that luspatercept can improve the production of endogenous RBCs by enhancing the maturation of these cells in the bone marrow. The drug significantly reduced the need for RBC transfusions, and “this is a very exciting advance for patients who would have few other treatment options,” she said.
“Anemia and the chronic need for transfusions is a very big issue for these patients,” commented lead study author Pierre Fenaux, MD, PhD, from Hôpital Saint-Louis in Paris, France. “With low hemoglobin levels, patients are tired all the time and have an increased risk of falls and cardiovascular events. When you can improve hemoglobin levels, you really see a difference in quality of life.”
The MEDALIST trial is an important milestone for patients with lower-risk, transfusion-dependent MDS, commented Elizabeth Griffiths, MD, associate professor of oncology and director of MDS, Roswell Park Comprehensive Cancer Center, Buffalo, New York.
“No new agents have been approved for MDS in the last 10 years, highlighting this development as a substantial step forward for the MDS community,” she told Medscape Medical News. “Current therapies are time-intensive and only modestly beneficial.”
“The availability of a new, effective drug — particularly relevant to those harboring SF3B1 mutations — is an exciting development and is likely to offer meaningful improvements in quality of life,” Griffiths said. “Since these patients tend to live longer than others with MDS, there are many patients in my clinical practice who would have fit the enrollment criteria for this study. Such patients are eagerly awaiting the opportunity for a decrease in transfusion burden.”
Study Details
In the trial, luspatercept reduced the severity of anemia — 38% of the 153 patients who received luspatercept achieved transfusion independence for 8 weeks or longer compared with 13% of the 76 patients receiving placebo (P < .001).
In the study, patients received luspatercept at a starting dose of 1.0 mg/kg with titration up to 1.75 mg/kg, if needed, or placebo, subcutaneously every 3 weeks for at least 24 weeks.
During the 16 weeks before the initiation of treatment, study patients had received a median of 5 RBC units transfusions during an 8-week period (43.2% of patients had ≥ 6 RBC units, 27.9% had ≥ 4 to < 6 RBC units, and 28.8% had < 4 RBC units). At baseline, 138 (60.3%), 58 (25.3%), and 32 (14%) patients had serum erythropoietin levels less than 200 IU/L, 200-500 IU/L, and greater than 500 IU/L, respectively.
The most common luspatercept-associated adverse events (any grade) in the trial were fatigue, diarrhea, asthenia, nausea, and dizziness. Grade 3 or 4 treatment-emergent adverse events were reported in 42.5% of patients who received luspatercept and 44.7% of patients who received placebo. The incidence of adverse events decreased over time, according to the study authors.
This article first appeared on Medscape.com.
Low plasma sodium predicts complications in SCD acute pain episodes
Hyponatremia, defined as a plasma sodium concentration ≤ 135 mmol/L at admission, was associated with complications during episodes of acute pain from sickle cell disease, according to a large retrospective analysis.
The French study assessed 1,218 stays of 406 patients admitted for treatment of an acute painful episode of sickle cell disease between 2010-2015. The primary composite endpoint comprised acute chest syndrome, intensive care unit transfer, red blood cell transfusion or inpatient death. The analyses were adjusted for age, sex, hemoglobin genotype and concentration, LDH concentration, and white blood cell count, according to the researchers.
Hyponatremia at admission in the center was associated with the primary endpoint (adjusted odds ratio (OR) 1.95, P = .001). This association was independent from baseline demographic characteristics (age, sex, hemoglobin genotype) and from other prognostic factors examined in the study (lower hemoglobin concentration, higher white blood cell count and LDH concentration).
With regard to individual components of the primary endpoint, hyponatremia was associated with acute chest syndrome (OR 1.95, P = .008) and red blood cell transfusion (OR 2.71, P <.001), but not significantly with intensive care unit transfer (OR 1.83, P = .074). In addition, the adjusted mean length of stay was significantly longer by 1.1 days (P < .001) in patients with hyponatremia at admission.
The study received no funding and the researchers reported that they had no conflicts of interest.
SOURCE: Rech JS et al. Amer J Med. 2020. doi.org/10.1016/j.amjmed.2020.02.017.
Hyponatremia, defined as a plasma sodium concentration ≤ 135 mmol/L at admission, was associated with complications during episodes of acute pain from sickle cell disease, according to a large retrospective analysis.
The French study assessed 1,218 stays of 406 patients admitted for treatment of an acute painful episode of sickle cell disease between 2010-2015. The primary composite endpoint comprised acute chest syndrome, intensive care unit transfer, red blood cell transfusion or inpatient death. The analyses were adjusted for age, sex, hemoglobin genotype and concentration, LDH concentration, and white blood cell count, according to the researchers.
Hyponatremia at admission in the center was associated with the primary endpoint (adjusted odds ratio (OR) 1.95, P = .001). This association was independent from baseline demographic characteristics (age, sex, hemoglobin genotype) and from other prognostic factors examined in the study (lower hemoglobin concentration, higher white blood cell count and LDH concentration).
With regard to individual components of the primary endpoint, hyponatremia was associated with acute chest syndrome (OR 1.95, P = .008) and red blood cell transfusion (OR 2.71, P <.001), but not significantly with intensive care unit transfer (OR 1.83, P = .074). In addition, the adjusted mean length of stay was significantly longer by 1.1 days (P < .001) in patients with hyponatremia at admission.
The study received no funding and the researchers reported that they had no conflicts of interest.
SOURCE: Rech JS et al. Amer J Med. 2020. doi.org/10.1016/j.amjmed.2020.02.017.
Hyponatremia, defined as a plasma sodium concentration ≤ 135 mmol/L at admission, was associated with complications during episodes of acute pain from sickle cell disease, according to a large retrospective analysis.
The French study assessed 1,218 stays of 406 patients admitted for treatment of an acute painful episode of sickle cell disease between 2010-2015. The primary composite endpoint comprised acute chest syndrome, intensive care unit transfer, red blood cell transfusion or inpatient death. The analyses were adjusted for age, sex, hemoglobin genotype and concentration, LDH concentration, and white blood cell count, according to the researchers.
Hyponatremia at admission in the center was associated with the primary endpoint (adjusted odds ratio (OR) 1.95, P = .001). This association was independent from baseline demographic characteristics (age, sex, hemoglobin genotype) and from other prognostic factors examined in the study (lower hemoglobin concentration, higher white blood cell count and LDH concentration).
With regard to individual components of the primary endpoint, hyponatremia was associated with acute chest syndrome (OR 1.95, P = .008) and red blood cell transfusion (OR 2.71, P <.001), but not significantly with intensive care unit transfer (OR 1.83, P = .074). In addition, the adjusted mean length of stay was significantly longer by 1.1 days (P < .001) in patients with hyponatremia at admission.
The study received no funding and the researchers reported that they had no conflicts of interest.
SOURCE: Rech JS et al. Amer J Med. 2020. doi.org/10.1016/j.amjmed.2020.02.017.
FROM THE AMERICAN JOURNAL OF MEDICINE
New sickle cell drugs give hope, but access remains a barrier
Sickle cell disease (SCD) is an incurable genetic blood disorder that reduces patients’ lifespan and quality of life. Many patients live into their 40s or 50s. Yet, throughout their lives, patients are plagued by lethargy, unpredictable painful crises, and frequent hospitalizations. For nearly 20 years, clinicians only had one drug to treat SCD. Since 2017, three new drugs have been approved, but their costs and lack of long-term data have spawned questions regarding access and benefit.
“SCD reduces lifespan by 30 years, and that’s very hard to quantify,” says Ifeyinwa Osunkwo, MD, professor of medicine and pediatrics and director of Sickle Cell Disease Enterprise at the Levine Cancer Institute at Atrium Health in Charlotte, N.C. “If you put a dollar amount on what someone would make working for 30 adult years, that would be more reflective of the true cost of treating the disease.”
In 1984, hydroxyurea (Hydrea, Droxia) became the first drug to treat SCD in adults.
Originally developed as a myelosuppressive antineoplastic, hydroxyurea is used to treat resistant chronic myeloid leukemia and certain head and neck cancers. In SCD, it increases levels of hemoglobin and fetal hemoglobin.
Despite its benefit, hydroxyurea has two major drawbacks: It is only effective in two genotypes – HbSS or HbS/Beta0thal.
HbSS or HbS/Beta0thal genotypes account for 60% of the SCD population, but further studies are required to elucidate hydroxyurea’s effect in other forms of SCD, according to Dr. Osunkwo.
Secondly, hydroxyurea only reduces the frequency of painful episodes by 50% – not enough to ameliorate the pain, said John J. Strouse, MD, associate professor of medicine at Duke University School of Medicine.
Newer therapies offer the potential to enhance the effects of hydroxyurea when used concomitantly. They also give clinicians additional options for patients who either fail hydroxyurea therapy or for whom it is inappropriate.
The amino acid L-glutamine (Endari) became the second drug approved for sickle cell in 2017. Indicated for patients 5 years of age and older, Dr. Osunkwo says many patients were thrilled to have a nonchemotherapeutic option available. However, the medical community received the drug with some skepticism. “The data show Endari is moderately effective at best,” said Dr. Strouse. “Also, the mechanism of action is unclear.”
Additionally, the drug’s powder form and twice-daily dosing regimen make adherence more challenging than swallowing a few hydroxyurea tablets or capsules once a day. In Dr. Osunkwo’s experience, patients who respond best to Endari tend to be those who are naturally motivated individuals who are intentional in their efforts at optimizing their nutrition and self-care.
“It takes a lot to be adherent to Endari,” she said. “You have to work at it.”
On Nov. 15, 2019, the FDA approved crizanlizumab-tmca (Adakveo) for patients 16 years of age and older to decrease the occurrence of vasoocclusive crises.
The drug works by blocking selectin – a protein involved in the painful vascular pathophysiology. Patients receive a loading dose of 5 mg/kg administered via intravenous infusion over 30 minutes at the initiation of therapy, as well as weeks 2 and 4. After that, patients undergo treatment once a month. Nausea, back pain, pyrexia, and arthralgia are the most frequently reported adverse reactions. Clinicians must monitor patients for signs and symptoms of infusion-related reactions.
Ten days later, the FDA approved voxelotor (Oxbryta) for patients ages 12 years and up. The drug inhibits hemoglobin S polymerization and increases hemoglobin levels. Like hydroxyurea, the drug offers the convenience of once-daily dosing, and the tablet can be taken without regard to food. The drug dose requires adjustment for patients with severe hepatic impairment. Headache, fatigue, rash, and gastrointestinal disturbances such as diarrhea, nausea, and abdominal pain fall among the most commonly reported side effects.
Endari, Adakveo, and Oxbryta can all be used as monotherapy. They also provide additional benefits in reducing pain and hospitalizations and improving anemia when used concomitantly with hydroxyurea.
Like so many drugs, these novel therapies are expensive. The cost of these novel treatments has raised some eyebrows.
Annual costs of generic hydroxyurea range in the neighborhood of $1,200. In a 2017 CNBC interview, Endari manufacturer Emmaus stated that it aimed to keep drug costs under $20,000 a year. Annual costs for Adakveo and Oxbryta costs are in the neighborhood of $100,000. Adakveo manufacturer Novartis reportedly priced vials at $2,347. Most patients will require at least three of the maximum four vials per treatment. In a press release, Global Therapeutics stated that Oxbyta would cost $10,417 a month.
However, Dr. Osunkwo says the benefits of these new drugs far exceed the costs from both monetary and quality of life standpoints.
“Sickle cell disease is costly to manage,” she said in an interview. “One hospitalization can cost $10,000.”
Additionally, many SCD patients are publicly insured because of the profound disability and loss of productive work they encounter as a direct consequence of their disease and its complications. Those too sick to complete their high school and postsecondary education find limited employment opportunities.
Those fortunate enough to secure employment face significantly fewer years they can work because their pain, fatigue, frequent hospitalizations, and cumulative organ damage result in permanent disability. Only a smaller number of patients with less severe disease manifestations can secure steady employment and pursue careers that allow them to obtain private insurance.
Even if the newer therapies can help cut some costs, clinicians should be aware that prior authorizations can delay patient access to Adakveo and Oxbyta.
“We wrote the first prescription for Oxbryta in November of 2019, but the prior authorization wasn’t approved until February of 2020,” she said. Adding Adakveo to her institution’s formulary required several months of navigation. Given the arduous process, Dr. Osunkwo anticipates it will take at least year after approval before Adakveo is available for all eligible patients.
The long-term impact of these drugs also remains to be seen, so hydroxyurea will likely remain the drug of choice for many patients, according to Dr. Strouse.
Dr. Osunkwo believes SCD needs more drugs in order to truly optimize outcomes, contain costs, and enhance the patient experience.
Dr. Osunkwo reports consultancy and being on the speaker’s bureau and participating in the advisory board for Novartis, which markets Adakveo, and relationships with a variety of other pharmaceutical companies. She is the editor in chief for Hematology News. Dr. Strouse reports consultancy for Global Therapeutics, which markets Oxbryta.
Sickle cell disease (SCD) is an incurable genetic blood disorder that reduces patients’ lifespan and quality of life. Many patients live into their 40s or 50s. Yet, throughout their lives, patients are plagued by lethargy, unpredictable painful crises, and frequent hospitalizations. For nearly 20 years, clinicians only had one drug to treat SCD. Since 2017, three new drugs have been approved, but their costs and lack of long-term data have spawned questions regarding access and benefit.
“SCD reduces lifespan by 30 years, and that’s very hard to quantify,” says Ifeyinwa Osunkwo, MD, professor of medicine and pediatrics and director of Sickle Cell Disease Enterprise at the Levine Cancer Institute at Atrium Health in Charlotte, N.C. “If you put a dollar amount on what someone would make working for 30 adult years, that would be more reflective of the true cost of treating the disease.”
In 1984, hydroxyurea (Hydrea, Droxia) became the first drug to treat SCD in adults.
Originally developed as a myelosuppressive antineoplastic, hydroxyurea is used to treat resistant chronic myeloid leukemia and certain head and neck cancers. In SCD, it increases levels of hemoglobin and fetal hemoglobin.
Despite its benefit, hydroxyurea has two major drawbacks: It is only effective in two genotypes – HbSS or HbS/Beta0thal.
HbSS or HbS/Beta0thal genotypes account for 60% of the SCD population, but further studies are required to elucidate hydroxyurea’s effect in other forms of SCD, according to Dr. Osunkwo.
Secondly, hydroxyurea only reduces the frequency of painful episodes by 50% – not enough to ameliorate the pain, said John J. Strouse, MD, associate professor of medicine at Duke University School of Medicine.
Newer therapies offer the potential to enhance the effects of hydroxyurea when used concomitantly. They also give clinicians additional options for patients who either fail hydroxyurea therapy or for whom it is inappropriate.
The amino acid L-glutamine (Endari) became the second drug approved for sickle cell in 2017. Indicated for patients 5 years of age and older, Dr. Osunkwo says many patients were thrilled to have a nonchemotherapeutic option available. However, the medical community received the drug with some skepticism. “The data show Endari is moderately effective at best,” said Dr. Strouse. “Also, the mechanism of action is unclear.”
Additionally, the drug’s powder form and twice-daily dosing regimen make adherence more challenging than swallowing a few hydroxyurea tablets or capsules once a day. In Dr. Osunkwo’s experience, patients who respond best to Endari tend to be those who are naturally motivated individuals who are intentional in their efforts at optimizing their nutrition and self-care.
“It takes a lot to be adherent to Endari,” she said. “You have to work at it.”
On Nov. 15, 2019, the FDA approved crizanlizumab-tmca (Adakveo) for patients 16 years of age and older to decrease the occurrence of vasoocclusive crises.
The drug works by blocking selectin – a protein involved in the painful vascular pathophysiology. Patients receive a loading dose of 5 mg/kg administered via intravenous infusion over 30 minutes at the initiation of therapy, as well as weeks 2 and 4. After that, patients undergo treatment once a month. Nausea, back pain, pyrexia, and arthralgia are the most frequently reported adverse reactions. Clinicians must monitor patients for signs and symptoms of infusion-related reactions.
Ten days later, the FDA approved voxelotor (Oxbryta) for patients ages 12 years and up. The drug inhibits hemoglobin S polymerization and increases hemoglobin levels. Like hydroxyurea, the drug offers the convenience of once-daily dosing, and the tablet can be taken without regard to food. The drug dose requires adjustment for patients with severe hepatic impairment. Headache, fatigue, rash, and gastrointestinal disturbances such as diarrhea, nausea, and abdominal pain fall among the most commonly reported side effects.
Endari, Adakveo, and Oxbryta can all be used as monotherapy. They also provide additional benefits in reducing pain and hospitalizations and improving anemia when used concomitantly with hydroxyurea.
Like so many drugs, these novel therapies are expensive. The cost of these novel treatments has raised some eyebrows.
Annual costs of generic hydroxyurea range in the neighborhood of $1,200. In a 2017 CNBC interview, Endari manufacturer Emmaus stated that it aimed to keep drug costs under $20,000 a year. Annual costs for Adakveo and Oxbryta costs are in the neighborhood of $100,000. Adakveo manufacturer Novartis reportedly priced vials at $2,347. Most patients will require at least three of the maximum four vials per treatment. In a press release, Global Therapeutics stated that Oxbyta would cost $10,417 a month.
However, Dr. Osunkwo says the benefits of these new drugs far exceed the costs from both monetary and quality of life standpoints.
“Sickle cell disease is costly to manage,” she said in an interview. “One hospitalization can cost $10,000.”
Additionally, many SCD patients are publicly insured because of the profound disability and loss of productive work they encounter as a direct consequence of their disease and its complications. Those too sick to complete their high school and postsecondary education find limited employment opportunities.
Those fortunate enough to secure employment face significantly fewer years they can work because their pain, fatigue, frequent hospitalizations, and cumulative organ damage result in permanent disability. Only a smaller number of patients with less severe disease manifestations can secure steady employment and pursue careers that allow them to obtain private insurance.
Even if the newer therapies can help cut some costs, clinicians should be aware that prior authorizations can delay patient access to Adakveo and Oxbyta.
“We wrote the first prescription for Oxbryta in November of 2019, but the prior authorization wasn’t approved until February of 2020,” she said. Adding Adakveo to her institution’s formulary required several months of navigation. Given the arduous process, Dr. Osunkwo anticipates it will take at least year after approval before Adakveo is available for all eligible patients.
The long-term impact of these drugs also remains to be seen, so hydroxyurea will likely remain the drug of choice for many patients, according to Dr. Strouse.
Dr. Osunkwo believes SCD needs more drugs in order to truly optimize outcomes, contain costs, and enhance the patient experience.
Dr. Osunkwo reports consultancy and being on the speaker’s bureau and participating in the advisory board for Novartis, which markets Adakveo, and relationships with a variety of other pharmaceutical companies. She is the editor in chief for Hematology News. Dr. Strouse reports consultancy for Global Therapeutics, which markets Oxbryta.
Sickle cell disease (SCD) is an incurable genetic blood disorder that reduces patients’ lifespan and quality of life. Many patients live into their 40s or 50s. Yet, throughout their lives, patients are plagued by lethargy, unpredictable painful crises, and frequent hospitalizations. For nearly 20 years, clinicians only had one drug to treat SCD. Since 2017, three new drugs have been approved, but their costs and lack of long-term data have spawned questions regarding access and benefit.
“SCD reduces lifespan by 30 years, and that’s very hard to quantify,” says Ifeyinwa Osunkwo, MD, professor of medicine and pediatrics and director of Sickle Cell Disease Enterprise at the Levine Cancer Institute at Atrium Health in Charlotte, N.C. “If you put a dollar amount on what someone would make working for 30 adult years, that would be more reflective of the true cost of treating the disease.”
In 1984, hydroxyurea (Hydrea, Droxia) became the first drug to treat SCD in adults.
Originally developed as a myelosuppressive antineoplastic, hydroxyurea is used to treat resistant chronic myeloid leukemia and certain head and neck cancers. In SCD, it increases levels of hemoglobin and fetal hemoglobin.
Despite its benefit, hydroxyurea has two major drawbacks: It is only effective in two genotypes – HbSS or HbS/Beta0thal.
HbSS or HbS/Beta0thal genotypes account for 60% of the SCD population, but further studies are required to elucidate hydroxyurea’s effect in other forms of SCD, according to Dr. Osunkwo.
Secondly, hydroxyurea only reduces the frequency of painful episodes by 50% – not enough to ameliorate the pain, said John J. Strouse, MD, associate professor of medicine at Duke University School of Medicine.
Newer therapies offer the potential to enhance the effects of hydroxyurea when used concomitantly. They also give clinicians additional options for patients who either fail hydroxyurea therapy or for whom it is inappropriate.
The amino acid L-glutamine (Endari) became the second drug approved for sickle cell in 2017. Indicated for patients 5 years of age and older, Dr. Osunkwo says many patients were thrilled to have a nonchemotherapeutic option available. However, the medical community received the drug with some skepticism. “The data show Endari is moderately effective at best,” said Dr. Strouse. “Also, the mechanism of action is unclear.”
Additionally, the drug’s powder form and twice-daily dosing regimen make adherence more challenging than swallowing a few hydroxyurea tablets or capsules once a day. In Dr. Osunkwo’s experience, patients who respond best to Endari tend to be those who are naturally motivated individuals who are intentional in their efforts at optimizing their nutrition and self-care.
“It takes a lot to be adherent to Endari,” she said. “You have to work at it.”
On Nov. 15, 2019, the FDA approved crizanlizumab-tmca (Adakveo) for patients 16 years of age and older to decrease the occurrence of vasoocclusive crises.
The drug works by blocking selectin – a protein involved in the painful vascular pathophysiology. Patients receive a loading dose of 5 mg/kg administered via intravenous infusion over 30 minutes at the initiation of therapy, as well as weeks 2 and 4. After that, patients undergo treatment once a month. Nausea, back pain, pyrexia, and arthralgia are the most frequently reported adverse reactions. Clinicians must monitor patients for signs and symptoms of infusion-related reactions.
Ten days later, the FDA approved voxelotor (Oxbryta) for patients ages 12 years and up. The drug inhibits hemoglobin S polymerization and increases hemoglobin levels. Like hydroxyurea, the drug offers the convenience of once-daily dosing, and the tablet can be taken without regard to food. The drug dose requires adjustment for patients with severe hepatic impairment. Headache, fatigue, rash, and gastrointestinal disturbances such as diarrhea, nausea, and abdominal pain fall among the most commonly reported side effects.
Endari, Adakveo, and Oxbryta can all be used as monotherapy. They also provide additional benefits in reducing pain and hospitalizations and improving anemia when used concomitantly with hydroxyurea.
Like so many drugs, these novel therapies are expensive. The cost of these novel treatments has raised some eyebrows.
Annual costs of generic hydroxyurea range in the neighborhood of $1,200. In a 2017 CNBC interview, Endari manufacturer Emmaus stated that it aimed to keep drug costs under $20,000 a year. Annual costs for Adakveo and Oxbryta costs are in the neighborhood of $100,000. Adakveo manufacturer Novartis reportedly priced vials at $2,347. Most patients will require at least three of the maximum four vials per treatment. In a press release, Global Therapeutics stated that Oxbyta would cost $10,417 a month.
However, Dr. Osunkwo says the benefits of these new drugs far exceed the costs from both monetary and quality of life standpoints.
“Sickle cell disease is costly to manage,” she said in an interview. “One hospitalization can cost $10,000.”
Additionally, many SCD patients are publicly insured because of the profound disability and loss of productive work they encounter as a direct consequence of their disease and its complications. Those too sick to complete their high school and postsecondary education find limited employment opportunities.
Those fortunate enough to secure employment face significantly fewer years they can work because their pain, fatigue, frequent hospitalizations, and cumulative organ damage result in permanent disability. Only a smaller number of patients with less severe disease manifestations can secure steady employment and pursue careers that allow them to obtain private insurance.
Even if the newer therapies can help cut some costs, clinicians should be aware that prior authorizations can delay patient access to Adakveo and Oxbyta.
“We wrote the first prescription for Oxbryta in November of 2019, but the prior authorization wasn’t approved until February of 2020,” she said. Adding Adakveo to her institution’s formulary required several months of navigation. Given the arduous process, Dr. Osunkwo anticipates it will take at least year after approval before Adakveo is available for all eligible patients.
The long-term impact of these drugs also remains to be seen, so hydroxyurea will likely remain the drug of choice for many patients, according to Dr. Strouse.
Dr. Osunkwo believes SCD needs more drugs in order to truly optimize outcomes, contain costs, and enhance the patient experience.
Dr. Osunkwo reports consultancy and being on the speaker’s bureau and participating in the advisory board for Novartis, which markets Adakveo, and relationships with a variety of other pharmaceutical companies. She is the editor in chief for Hematology News. Dr. Strouse reports consultancy for Global Therapeutics, which markets Oxbryta.
Sickle cell patients with vitamin D deficiency prone to more ED visits, longer stays
Patients with sickle cell disease (SCD) plus vitamin D deficiency were found to have more hospitalization outcomes, including number of emergency department (ED) visits, the number of hospital admissions for pain crisis, and the length of hospital admission, according to a study published online by researchers from New York-Presbyterian Brooklyn Methodist Hospital.
The researchers performed a retrospective chart review of all 134 pediatric patients with SCD (aged 1-21 years) from January 2015 to January 2016 in an urban-based hospital setting. Ninety patients with at least one reported vitamin D level who maintained follow-up during the time studied were enrolled. Hospitalization rates were compared between vitamin D deficiency (< 20 ng/mL) and sufficiency (> 20 ng/mL) patients.
When compared to patients with SCD and sufficient vitamin D levels, patients with both SCD and vitamin D deficiency were more likely to have at least one ED visit (P < .01), at least one admission for pain crisis (P < .01), and a longer length of admission (P < .0001), the researchers found.
“Screening and treatment for vitamin D deficiency is generally cost effective and readily available, potentially having a significant impact on the quality of life for those living with sickle cell disease,” the researchers concluded.
The authors reported that there was no study funding and that they had no conflicts of interest.
SOURCE: Brown B et al. Blood Cells Mol Dis. 2020. doi: 10.1016/j.bcmd.2020.102415.
Patients with sickle cell disease (SCD) plus vitamin D deficiency were found to have more hospitalization outcomes, including number of emergency department (ED) visits, the number of hospital admissions for pain crisis, and the length of hospital admission, according to a study published online by researchers from New York-Presbyterian Brooklyn Methodist Hospital.
The researchers performed a retrospective chart review of all 134 pediatric patients with SCD (aged 1-21 years) from January 2015 to January 2016 in an urban-based hospital setting. Ninety patients with at least one reported vitamin D level who maintained follow-up during the time studied were enrolled. Hospitalization rates were compared between vitamin D deficiency (< 20 ng/mL) and sufficiency (> 20 ng/mL) patients.
When compared to patients with SCD and sufficient vitamin D levels, patients with both SCD and vitamin D deficiency were more likely to have at least one ED visit (P < .01), at least one admission for pain crisis (P < .01), and a longer length of admission (P < .0001), the researchers found.
“Screening and treatment for vitamin D deficiency is generally cost effective and readily available, potentially having a significant impact on the quality of life for those living with sickle cell disease,” the researchers concluded.
The authors reported that there was no study funding and that they had no conflicts of interest.
SOURCE: Brown B et al. Blood Cells Mol Dis. 2020. doi: 10.1016/j.bcmd.2020.102415.
Patients with sickle cell disease (SCD) plus vitamin D deficiency were found to have more hospitalization outcomes, including number of emergency department (ED) visits, the number of hospital admissions for pain crisis, and the length of hospital admission, according to a study published online by researchers from New York-Presbyterian Brooklyn Methodist Hospital.
The researchers performed a retrospective chart review of all 134 pediatric patients with SCD (aged 1-21 years) from January 2015 to January 2016 in an urban-based hospital setting. Ninety patients with at least one reported vitamin D level who maintained follow-up during the time studied were enrolled. Hospitalization rates were compared between vitamin D deficiency (< 20 ng/mL) and sufficiency (> 20 ng/mL) patients.
When compared to patients with SCD and sufficient vitamin D levels, patients with both SCD and vitamin D deficiency were more likely to have at least one ED visit (P < .01), at least one admission for pain crisis (P < .01), and a longer length of admission (P < .0001), the researchers found.
“Screening and treatment for vitamin D deficiency is generally cost effective and readily available, potentially having a significant impact on the quality of life for those living with sickle cell disease,” the researchers concluded.
The authors reported that there was no study funding and that they had no conflicts of interest.
SOURCE: Brown B et al. Blood Cells Mol Dis. 2020. doi: 10.1016/j.bcmd.2020.102415.
FROM BLOOD CELLS, MOLECULES, AND DISEASES
Chimerism and the use of fludarabine associated with secondary graft failure in aplastic anemia
Inferior overall survival was observed in patients with aplastic anemia who had mixed or complete recipient-type chimerism or complete donor chimerism after hematopoietic stem cell transplantation (HSCT), according to the results of a registry database study in Japan.
Researchers examined four groups of patients with AA age greater than 15 years who underwent a first allogeneic bone marrow or peripheral blood stem cell transplantation and achieved engraftment.
Group 1 consisted of patients with mixed chimerism (MC) that did not require either granulocyte-colony stimulating factor (G-CSF) or transfusion support; group 2 consisted of MC (with no secondary graft failure (SGF) that required G-CSF and/or transfusion support ; group 3 consisted of patients with SGF with MC or complete recipient-type chimerism; and group 4 consisted of SGF with complete donor-type chimerism.
The overall median follow-up of survivors was 1,727 days. The overall survival (OS) was 90.4% at 1 year and 83.5% at 5 years in patients without MC or SGF (n = 340), which was not different from the OS in groups 1 and 2. However, inferior OS was observed in group 3 (1 year, 52.1%; 5 years, 52.1%) and group 4 (1 year, 82.4%; 5 years, 56.3%). In addition, multivariate analyses showed that the use of fludarabine (Flu) and the absence of irradiation in conditioning were associated with the development of SGF with MC or complete recipient-type chimerism. The use of Flu in conditioning also was associated with SGF with complete donor-type chimerism.
“The occurrence of SGF with both MC/recipient-type and donor-type chimerism after HSCT for AA was associated with inferior OS, and the conditioning regimens influenced the occurrence of SGF,” the researchers concluded.
The Japan Agency for Medical Research and Development funded the research. Two of the authors reported receiving honoraria or research funding from Sanofi KK and one from Shionogi.
SOURCE: Kako S et al. Biol Blood Marrow Transplant 2020. 26:445-50.
Inferior overall survival was observed in patients with aplastic anemia who had mixed or complete recipient-type chimerism or complete donor chimerism after hematopoietic stem cell transplantation (HSCT), according to the results of a registry database study in Japan.
Researchers examined four groups of patients with AA age greater than 15 years who underwent a first allogeneic bone marrow or peripheral blood stem cell transplantation and achieved engraftment.
Group 1 consisted of patients with mixed chimerism (MC) that did not require either granulocyte-colony stimulating factor (G-CSF) or transfusion support; group 2 consisted of MC (with no secondary graft failure (SGF) that required G-CSF and/or transfusion support ; group 3 consisted of patients with SGF with MC or complete recipient-type chimerism; and group 4 consisted of SGF with complete donor-type chimerism.
The overall median follow-up of survivors was 1,727 days. The overall survival (OS) was 90.4% at 1 year and 83.5% at 5 years in patients without MC or SGF (n = 340), which was not different from the OS in groups 1 and 2. However, inferior OS was observed in group 3 (1 year, 52.1%; 5 years, 52.1%) and group 4 (1 year, 82.4%; 5 years, 56.3%). In addition, multivariate analyses showed that the use of fludarabine (Flu) and the absence of irradiation in conditioning were associated with the development of SGF with MC or complete recipient-type chimerism. The use of Flu in conditioning also was associated with SGF with complete donor-type chimerism.
“The occurrence of SGF with both MC/recipient-type and donor-type chimerism after HSCT for AA was associated with inferior OS, and the conditioning regimens influenced the occurrence of SGF,” the researchers concluded.
The Japan Agency for Medical Research and Development funded the research. Two of the authors reported receiving honoraria or research funding from Sanofi KK and one from Shionogi.
SOURCE: Kako S et al. Biol Blood Marrow Transplant 2020. 26:445-50.
Inferior overall survival was observed in patients with aplastic anemia who had mixed or complete recipient-type chimerism or complete donor chimerism after hematopoietic stem cell transplantation (HSCT), according to the results of a registry database study in Japan.
Researchers examined four groups of patients with AA age greater than 15 years who underwent a first allogeneic bone marrow or peripheral blood stem cell transplantation and achieved engraftment.
Group 1 consisted of patients with mixed chimerism (MC) that did not require either granulocyte-colony stimulating factor (G-CSF) or transfusion support; group 2 consisted of MC (with no secondary graft failure (SGF) that required G-CSF and/or transfusion support ; group 3 consisted of patients with SGF with MC or complete recipient-type chimerism; and group 4 consisted of SGF with complete donor-type chimerism.
The overall median follow-up of survivors was 1,727 days. The overall survival (OS) was 90.4% at 1 year and 83.5% at 5 years in patients without MC or SGF (n = 340), which was not different from the OS in groups 1 and 2. However, inferior OS was observed in group 3 (1 year, 52.1%; 5 years, 52.1%) and group 4 (1 year, 82.4%; 5 years, 56.3%). In addition, multivariate analyses showed that the use of fludarabine (Flu) and the absence of irradiation in conditioning were associated with the development of SGF with MC or complete recipient-type chimerism. The use of Flu in conditioning also was associated with SGF with complete donor-type chimerism.
“The occurrence of SGF with both MC/recipient-type and donor-type chimerism after HSCT for AA was associated with inferior OS, and the conditioning regimens influenced the occurrence of SGF,” the researchers concluded.
The Japan Agency for Medical Research and Development funded the research. Two of the authors reported receiving honoraria or research funding from Sanofi KK and one from Shionogi.
SOURCE: Kako S et al. Biol Blood Marrow Transplant 2020. 26:445-50.
FROM BIOLOGY OF BLOOD AND MARROW TRANSPLANTATION
Genetic testing helps avoid false hemoglobinopathy diagnoses in newborns
Confirmatory genetic testing may be useful in the diagnosis of hemoglobinopathies for newborns with an abnormal hemoglobin (Hb) pattern, according to a recent study.
The findings suggest further research is needed to evaluate whether genetic testing programs for newborns could have diagnostic value in the clinical setting.
“We studied a consecutive cohort of newborns with an ‘FSA’ pattern (a suspected diagnosis of HbSbeta+) on the initial newborn screening test,” explained Lisa M. Shook of the University of Cincinnati and colleagues. The results were published in the International Journal of Neonatal Screening.
The retrospective study included a total of 1,151 newborns with an abnormal Hb pattern, 31 of which had an FSA pattern. The newborns were screened for hemoglobinopathies from 2015 to 2018. The findings of the initial newborn screening test (a suspected diagnosis of HbSbeta+) were compared with the diagnosis established using both protein-based and genetic confirmatory testing. Protein-based testing cannot accurately detect several hemoglobinopathies in newborns, especially when beta-thalassemia mutations are involved, according to the authors.
“During this study period, genetic testing was not universally applied in advance; it was used based on clinical suspicion,” the researchers wrote.
Among newborns with an FSA pattern, the mean gestational age was 38.7 weeks. In total, 17 newborns received genetic testing, and 30 had protein-based confirmatory testing.
“In this consecutive cohort of 31 newborns with a suspected diagnosis of HbSbeta+ based on initial newborn screening (an FSA pattern), none actually had HbSbeta+. All had the sickle cell trait (HbAS), instead; that is, we found that an initial FSA pattern was much more likely to indicate a final diagnosis of HbAS than HbSbeta+,” the authors wrote.
This meant that two-thirds of these newborns had a correct diagnosis of HbAS established at 2-4 weeks of age by protein-based confirmatory testing (and confirmed by genetic testing in a subset), but that the remaining one-third still had an incorrect, suspected diagnosis of HbSbeta+. This could lead to unnecessary treatment and testing of infants and incorrect, disease-focused counseling of parents and family members, according to the authors.
Two key limitations of the study were the small sample size and retrospective design.
“Based on this experience in which genetic testing was not universally applied, we now perform simultaneous protein-based and genetic testing as our standard clinical practice,” they concluded.
The study was funded by the National Institutes of Health and the Ohio Department of Health. The authors reported having no conflicts of interest.
SOURCE: Shook LM et al. Int J Neonatal Screen. 2020 Jan 31. doi: 10.3390/ijns6010007
Confirmatory genetic testing may be useful in the diagnosis of hemoglobinopathies for newborns with an abnormal hemoglobin (Hb) pattern, according to a recent study.
The findings suggest further research is needed to evaluate whether genetic testing programs for newborns could have diagnostic value in the clinical setting.
“We studied a consecutive cohort of newborns with an ‘FSA’ pattern (a suspected diagnosis of HbSbeta+) on the initial newborn screening test,” explained Lisa M. Shook of the University of Cincinnati and colleagues. The results were published in the International Journal of Neonatal Screening.
The retrospective study included a total of 1,151 newborns with an abnormal Hb pattern, 31 of which had an FSA pattern. The newborns were screened for hemoglobinopathies from 2015 to 2018. The findings of the initial newborn screening test (a suspected diagnosis of HbSbeta+) were compared with the diagnosis established using both protein-based and genetic confirmatory testing. Protein-based testing cannot accurately detect several hemoglobinopathies in newborns, especially when beta-thalassemia mutations are involved, according to the authors.
“During this study period, genetic testing was not universally applied in advance; it was used based on clinical suspicion,” the researchers wrote.
Among newborns with an FSA pattern, the mean gestational age was 38.7 weeks. In total, 17 newborns received genetic testing, and 30 had protein-based confirmatory testing.
“In this consecutive cohort of 31 newborns with a suspected diagnosis of HbSbeta+ based on initial newborn screening (an FSA pattern), none actually had HbSbeta+. All had the sickle cell trait (HbAS), instead; that is, we found that an initial FSA pattern was much more likely to indicate a final diagnosis of HbAS than HbSbeta+,” the authors wrote.
This meant that two-thirds of these newborns had a correct diagnosis of HbAS established at 2-4 weeks of age by protein-based confirmatory testing (and confirmed by genetic testing in a subset), but that the remaining one-third still had an incorrect, suspected diagnosis of HbSbeta+. This could lead to unnecessary treatment and testing of infants and incorrect, disease-focused counseling of parents and family members, according to the authors.
Two key limitations of the study were the small sample size and retrospective design.
“Based on this experience in which genetic testing was not universally applied, we now perform simultaneous protein-based and genetic testing as our standard clinical practice,” they concluded.
The study was funded by the National Institutes of Health and the Ohio Department of Health. The authors reported having no conflicts of interest.
SOURCE: Shook LM et al. Int J Neonatal Screen. 2020 Jan 31. doi: 10.3390/ijns6010007
Confirmatory genetic testing may be useful in the diagnosis of hemoglobinopathies for newborns with an abnormal hemoglobin (Hb) pattern, according to a recent study.
The findings suggest further research is needed to evaluate whether genetic testing programs for newborns could have diagnostic value in the clinical setting.
“We studied a consecutive cohort of newborns with an ‘FSA’ pattern (a suspected diagnosis of HbSbeta+) on the initial newborn screening test,” explained Lisa M. Shook of the University of Cincinnati and colleagues. The results were published in the International Journal of Neonatal Screening.
The retrospective study included a total of 1,151 newborns with an abnormal Hb pattern, 31 of which had an FSA pattern. The newborns were screened for hemoglobinopathies from 2015 to 2018. The findings of the initial newborn screening test (a suspected diagnosis of HbSbeta+) were compared with the diagnosis established using both protein-based and genetic confirmatory testing. Protein-based testing cannot accurately detect several hemoglobinopathies in newborns, especially when beta-thalassemia mutations are involved, according to the authors.
“During this study period, genetic testing was not universally applied in advance; it was used based on clinical suspicion,” the researchers wrote.
Among newborns with an FSA pattern, the mean gestational age was 38.7 weeks. In total, 17 newborns received genetic testing, and 30 had protein-based confirmatory testing.
“In this consecutive cohort of 31 newborns with a suspected diagnosis of HbSbeta+ based on initial newborn screening (an FSA pattern), none actually had HbSbeta+. All had the sickle cell trait (HbAS), instead; that is, we found that an initial FSA pattern was much more likely to indicate a final diagnosis of HbAS than HbSbeta+,” the authors wrote.
This meant that two-thirds of these newborns had a correct diagnosis of HbAS established at 2-4 weeks of age by protein-based confirmatory testing (and confirmed by genetic testing in a subset), but that the remaining one-third still had an incorrect, suspected diagnosis of HbSbeta+. This could lead to unnecessary treatment and testing of infants and incorrect, disease-focused counseling of parents and family members, according to the authors.
Two key limitations of the study were the small sample size and retrospective design.
“Based on this experience in which genetic testing was not universally applied, we now perform simultaneous protein-based and genetic testing as our standard clinical practice,” they concluded.
The study was funded by the National Institutes of Health and the Ohio Department of Health. The authors reported having no conflicts of interest.
SOURCE: Shook LM et al. Int J Neonatal Screen. 2020 Jan 31. doi: 10.3390/ijns6010007
FROM THE INTERNATIONAL JOURNAL OF NEONATAL SCREENING
Flow-mediated dilation of brachial artery predicts renal dysfunction in sickle cell disease
Sonographic flow-mediated dilation (FMD) of the brachial artery predicts renal dysfunction in patients with sickle cell disease (SCD), according to investigators.
This is the first study to show that FMD – a surrogate biomarker for endothelial dysfunction – inversely correlates with renal artery resistivity index (RARI) and serum cystatin C, reported lead author Oluwagbemiga Oluwole Ayoola, MBChB, of Obafemi Awolowo University in Ile-Ife, Nigeria, and colleagues.
“[B]rachial artery FMD is an essential test in the management of SCD patients for noninvasive assessment of the vascular endothelium,” the investigators wrote in Kidney360. They went on to suggest that FMD could be used to detect early renal impairment in sickle cell disease.
The study involved 44 patients with steady-state, homozygous SCD (HbSS) and 33 age- and sex-matched controls (HbAA). Eligibility criteria excluded individuals with risk factors for endothelial dysfunction, such as obesity, diabetes, and hypertension, as well as those with thalassemia carrier traits.
For each participant, various data were gathered, including demographic and clinical characteristics, serum assays, FMD measurement of the brachial artery, and RARI.
Results showed that patients with sickle cell disease had a significantly lower median FMD value than that of healthy controls (3.44 vs. 5.35; P = .043).
Among patients with SCD, FMD was negatively and independently correlated with RARI (r = -.307; P = .042) and serum cystatin C (r = -.372; P = .013), correlations that the investigators described as “modest.” FMD was not associated with any other biomarkers of SCD severity, such as homocysteine, fetal hemoglobin, or soluble platelet selectin.
Patients in the SCD cohort were further subdivided into two groups based on an FMD cut-off value of 5.35, which was the median measurement among healthy controls. This revealed that median cystatin C level was significantly higher in patients with an FMD value less than 5.35, compared with those who had an FMD value of 5.35 or more.
“[The study] findings suggest that SCD patients with impaired FMD are more likely to have impaired renal function,” the investigators wrote. The results support previous research, they added.
“Even though our findings show relationships rather than causation, we believe it is still a step forward in the ongoing quest to unravel the mysteries of this genetic disease,” they concluded. “Determining the exact age at which FMD impairment [begins] in children with sickle cell disease could be the subject of a future study.”
The study was funded by the Obafemi Awolowo University Teaching Hospital. The investigators reported no conflicts of interest.
SOURCE: Ayoola et al. Kidney360. 2020 Jan 30. doi: 10.34067/KID.0000142019.
Sonographic flow-mediated dilation (FMD) of the brachial artery predicts renal dysfunction in patients with sickle cell disease (SCD), according to investigators.
This is the first study to show that FMD – a surrogate biomarker for endothelial dysfunction – inversely correlates with renal artery resistivity index (RARI) and serum cystatin C, reported lead author Oluwagbemiga Oluwole Ayoola, MBChB, of Obafemi Awolowo University in Ile-Ife, Nigeria, and colleagues.
“[B]rachial artery FMD is an essential test in the management of SCD patients for noninvasive assessment of the vascular endothelium,” the investigators wrote in Kidney360. They went on to suggest that FMD could be used to detect early renal impairment in sickle cell disease.
The study involved 44 patients with steady-state, homozygous SCD (HbSS) and 33 age- and sex-matched controls (HbAA). Eligibility criteria excluded individuals with risk factors for endothelial dysfunction, such as obesity, diabetes, and hypertension, as well as those with thalassemia carrier traits.
For each participant, various data were gathered, including demographic and clinical characteristics, serum assays, FMD measurement of the brachial artery, and RARI.
Results showed that patients with sickle cell disease had a significantly lower median FMD value than that of healthy controls (3.44 vs. 5.35; P = .043).
Among patients with SCD, FMD was negatively and independently correlated with RARI (r = -.307; P = .042) and serum cystatin C (r = -.372; P = .013), correlations that the investigators described as “modest.” FMD was not associated with any other biomarkers of SCD severity, such as homocysteine, fetal hemoglobin, or soluble platelet selectin.
Patients in the SCD cohort were further subdivided into two groups based on an FMD cut-off value of 5.35, which was the median measurement among healthy controls. This revealed that median cystatin C level was significantly higher in patients with an FMD value less than 5.35, compared with those who had an FMD value of 5.35 or more.
“[The study] findings suggest that SCD patients with impaired FMD are more likely to have impaired renal function,” the investigators wrote. The results support previous research, they added.
“Even though our findings show relationships rather than causation, we believe it is still a step forward in the ongoing quest to unravel the mysteries of this genetic disease,” they concluded. “Determining the exact age at which FMD impairment [begins] in children with sickle cell disease could be the subject of a future study.”
The study was funded by the Obafemi Awolowo University Teaching Hospital. The investigators reported no conflicts of interest.
SOURCE: Ayoola et al. Kidney360. 2020 Jan 30. doi: 10.34067/KID.0000142019.
Sonographic flow-mediated dilation (FMD) of the brachial artery predicts renal dysfunction in patients with sickle cell disease (SCD), according to investigators.
This is the first study to show that FMD – a surrogate biomarker for endothelial dysfunction – inversely correlates with renal artery resistivity index (RARI) and serum cystatin C, reported lead author Oluwagbemiga Oluwole Ayoola, MBChB, of Obafemi Awolowo University in Ile-Ife, Nigeria, and colleagues.
“[B]rachial artery FMD is an essential test in the management of SCD patients for noninvasive assessment of the vascular endothelium,” the investigators wrote in Kidney360. They went on to suggest that FMD could be used to detect early renal impairment in sickle cell disease.
The study involved 44 patients with steady-state, homozygous SCD (HbSS) and 33 age- and sex-matched controls (HbAA). Eligibility criteria excluded individuals with risk factors for endothelial dysfunction, such as obesity, diabetes, and hypertension, as well as those with thalassemia carrier traits.
For each participant, various data were gathered, including demographic and clinical characteristics, serum assays, FMD measurement of the brachial artery, and RARI.
Results showed that patients with sickle cell disease had a significantly lower median FMD value than that of healthy controls (3.44 vs. 5.35; P = .043).
Among patients with SCD, FMD was negatively and independently correlated with RARI (r = -.307; P = .042) and serum cystatin C (r = -.372; P = .013), correlations that the investigators described as “modest.” FMD was not associated with any other biomarkers of SCD severity, such as homocysteine, fetal hemoglobin, or soluble platelet selectin.
Patients in the SCD cohort were further subdivided into two groups based on an FMD cut-off value of 5.35, which was the median measurement among healthy controls. This revealed that median cystatin C level was significantly higher in patients with an FMD value less than 5.35, compared with those who had an FMD value of 5.35 or more.
“[The study] findings suggest that SCD patients with impaired FMD are more likely to have impaired renal function,” the investigators wrote. The results support previous research, they added.
“Even though our findings show relationships rather than causation, we believe it is still a step forward in the ongoing quest to unravel the mysteries of this genetic disease,” they concluded. “Determining the exact age at which FMD impairment [begins] in children with sickle cell disease could be the subject of a future study.”
The study was funded by the Obafemi Awolowo University Teaching Hospital. The investigators reported no conflicts of interest.
SOURCE: Ayoola et al. Kidney360. 2020 Jan 30. doi: 10.34067/KID.0000142019.
FROM KIDNEY360
Deferiprone noninferior to deferoxamine for iron overload in SCD, rare anemias
ORLANDO – The oral iron chelator deferiprone showed noninferiority to deferoxamine for treating iron overload in patients with sickle cell disease and other rare anemias in a randomized open-label trial.
The least squares mean change from baseline in liver iron concentration (LIC) – the primary study endpoint – was –4.04 mg/g dry weight (dw) in 152 patients randomized to receive deferiprone, and –4.45 mg/g dw in 76 who received deferoxamine, Janet L. Kwiatkowski, MD, of the Children’s Hospital of Philadelphia reported at the annual meeting of the American Society of Hematology.
The upper limit of the stringent 96.01% confidence interval used for the evaluation of noninferiority in the study was 1.57, thus the findings demonstrated noninferiority of deferiprone, Dr. Kwiatkowski said.
Deferiprone also showed noninferiority for the secondary endpoints of change in cardiac iron (about –0.02 ms on T2* MRI, log-transformed for both groups) and serum ferritin levels (–415 vs. –750 mcg/L for deferiprone vs. deferoxamine) at 12 months. The difference between the groups was not statistically significant for either endpoint.
Study participants, who had a mean age of 16.9 years, were aged 2 years and older with LIC between 7 and 30 mg/g dw. They were recruited from 33 sites in nine countries and randomized 2:1 to receive deferiprone or deferoxamine for up to 12 months; in patients with lower transfusional iron input and/or less severe iron load, deferiprone was dosed at 75 mg/kg daily and deferoxamine was dosed at 20 mg/kg for children and 40 mg/kg for adults. In those with higher iron input and/or more severe iron load, the deferiprone dose was 99 mg/kg daily and the deferoxamine doses were up to 40 mg/kg in children and up to 50 mg/kg for adults.
“Over the course of the treatment period, the dosage could be adjusted downward if there were side effects, or upward if there was no improvement in iron burden,” Dr Kwiatkowski said, adding that after 12 months, patients had the option of continuing on to a 2-year extension trial in which everyone received deferiprone.
No significant demographic differences were noted between the groups; 84% in both groups had sickle cell disease, and the remaining patients had other, rarer forms of transfusion-dependent anemia. Baseline iron burden was similar in the groups.
The rates of acceptable compliance over the course of the study were also similar at 69% and 79% in the deferiprone and deferoxamine arms, respectively, she noted.
No statistically significant difference between the groups was seen in the overall rate of adverse events, treatment-related AEs, serious AEs, or withdrawals from the study due to AEs. Agranulocytosis occurred in one deferiprone patient and zero deferoxamine patients, and mild or moderate neutropenia occurred in four patients and one patient in the groups, respectively.
All episodes resolved, no difference was seen in the rates of any of the serious AEs, and no unexpected serious adverse events occurred, she said.
Patients with sickle cell disease or other rare anemias whose care includes chronic blood transfusions require iron chelation to prevent iron overload. Currently, only deferoxamine and deferasirox are approved chelators in these patient populations, she said, noting that in 2011 deferiprone received accelerated Food and Drug Administration approval for the treatment of thalassemia.
The current study was conducted because of an FDA requirement for postmarket assessment of deferiprone’s efficacy and safety in patients with sickle cell disease and other anemias who develop transfusional iron overload. It was initiated prior to the approval of deferasirox for the first-line treatment of SCD, therefore it was compared only with deferoxamine, she explained.
Dr. Kwiatkowski reported research funding from Apopharma, bluebird bio, Novartis, and Terumo, and consultancy for Agios, bluebird bio, Celgene, and Imara.
ORLANDO – The oral iron chelator deferiprone showed noninferiority to deferoxamine for treating iron overload in patients with sickle cell disease and other rare anemias in a randomized open-label trial.
The least squares mean change from baseline in liver iron concentration (LIC) – the primary study endpoint – was –4.04 mg/g dry weight (dw) in 152 patients randomized to receive deferiprone, and –4.45 mg/g dw in 76 who received deferoxamine, Janet L. Kwiatkowski, MD, of the Children’s Hospital of Philadelphia reported at the annual meeting of the American Society of Hematology.
The upper limit of the stringent 96.01% confidence interval used for the evaluation of noninferiority in the study was 1.57, thus the findings demonstrated noninferiority of deferiprone, Dr. Kwiatkowski said.
Deferiprone also showed noninferiority for the secondary endpoints of change in cardiac iron (about –0.02 ms on T2* MRI, log-transformed for both groups) and serum ferritin levels (–415 vs. –750 mcg/L for deferiprone vs. deferoxamine) at 12 months. The difference between the groups was not statistically significant for either endpoint.
Study participants, who had a mean age of 16.9 years, were aged 2 years and older with LIC between 7 and 30 mg/g dw. They were recruited from 33 sites in nine countries and randomized 2:1 to receive deferiprone or deferoxamine for up to 12 months; in patients with lower transfusional iron input and/or less severe iron load, deferiprone was dosed at 75 mg/kg daily and deferoxamine was dosed at 20 mg/kg for children and 40 mg/kg for adults. In those with higher iron input and/or more severe iron load, the deferiprone dose was 99 mg/kg daily and the deferoxamine doses were up to 40 mg/kg in children and up to 50 mg/kg for adults.
“Over the course of the treatment period, the dosage could be adjusted downward if there were side effects, or upward if there was no improvement in iron burden,” Dr Kwiatkowski said, adding that after 12 months, patients had the option of continuing on to a 2-year extension trial in which everyone received deferiprone.
No significant demographic differences were noted between the groups; 84% in both groups had sickle cell disease, and the remaining patients had other, rarer forms of transfusion-dependent anemia. Baseline iron burden was similar in the groups.
The rates of acceptable compliance over the course of the study were also similar at 69% and 79% in the deferiprone and deferoxamine arms, respectively, she noted.
No statistically significant difference between the groups was seen in the overall rate of adverse events, treatment-related AEs, serious AEs, or withdrawals from the study due to AEs. Agranulocytosis occurred in one deferiprone patient and zero deferoxamine patients, and mild or moderate neutropenia occurred in four patients and one patient in the groups, respectively.
All episodes resolved, no difference was seen in the rates of any of the serious AEs, and no unexpected serious adverse events occurred, she said.
Patients with sickle cell disease or other rare anemias whose care includes chronic blood transfusions require iron chelation to prevent iron overload. Currently, only deferoxamine and deferasirox are approved chelators in these patient populations, she said, noting that in 2011 deferiprone received accelerated Food and Drug Administration approval for the treatment of thalassemia.
The current study was conducted because of an FDA requirement for postmarket assessment of deferiprone’s efficacy and safety in patients with sickle cell disease and other anemias who develop transfusional iron overload. It was initiated prior to the approval of deferasirox for the first-line treatment of SCD, therefore it was compared only with deferoxamine, she explained.
Dr. Kwiatkowski reported research funding from Apopharma, bluebird bio, Novartis, and Terumo, and consultancy for Agios, bluebird bio, Celgene, and Imara.
ORLANDO – The oral iron chelator deferiprone showed noninferiority to deferoxamine for treating iron overload in patients with sickle cell disease and other rare anemias in a randomized open-label trial.
The least squares mean change from baseline in liver iron concentration (LIC) – the primary study endpoint – was –4.04 mg/g dry weight (dw) in 152 patients randomized to receive deferiprone, and –4.45 mg/g dw in 76 who received deferoxamine, Janet L. Kwiatkowski, MD, of the Children’s Hospital of Philadelphia reported at the annual meeting of the American Society of Hematology.
The upper limit of the stringent 96.01% confidence interval used for the evaluation of noninferiority in the study was 1.57, thus the findings demonstrated noninferiority of deferiprone, Dr. Kwiatkowski said.
Deferiprone also showed noninferiority for the secondary endpoints of change in cardiac iron (about –0.02 ms on T2* MRI, log-transformed for both groups) and serum ferritin levels (–415 vs. –750 mcg/L for deferiprone vs. deferoxamine) at 12 months. The difference between the groups was not statistically significant for either endpoint.
Study participants, who had a mean age of 16.9 years, were aged 2 years and older with LIC between 7 and 30 mg/g dw. They were recruited from 33 sites in nine countries and randomized 2:1 to receive deferiprone or deferoxamine for up to 12 months; in patients with lower transfusional iron input and/or less severe iron load, deferiprone was dosed at 75 mg/kg daily and deferoxamine was dosed at 20 mg/kg for children and 40 mg/kg for adults. In those with higher iron input and/or more severe iron load, the deferiprone dose was 99 mg/kg daily and the deferoxamine doses were up to 40 mg/kg in children and up to 50 mg/kg for adults.
“Over the course of the treatment period, the dosage could be adjusted downward if there were side effects, or upward if there was no improvement in iron burden,” Dr Kwiatkowski said, adding that after 12 months, patients had the option of continuing on to a 2-year extension trial in which everyone received deferiprone.
No significant demographic differences were noted between the groups; 84% in both groups had sickle cell disease, and the remaining patients had other, rarer forms of transfusion-dependent anemia. Baseline iron burden was similar in the groups.
The rates of acceptable compliance over the course of the study were also similar at 69% and 79% in the deferiprone and deferoxamine arms, respectively, she noted.
No statistically significant difference between the groups was seen in the overall rate of adverse events, treatment-related AEs, serious AEs, or withdrawals from the study due to AEs. Agranulocytosis occurred in one deferiprone patient and zero deferoxamine patients, and mild or moderate neutropenia occurred in four patients and one patient in the groups, respectively.
All episodes resolved, no difference was seen in the rates of any of the serious AEs, and no unexpected serious adverse events occurred, she said.
Patients with sickle cell disease or other rare anemias whose care includes chronic blood transfusions require iron chelation to prevent iron overload. Currently, only deferoxamine and deferasirox are approved chelators in these patient populations, she said, noting that in 2011 deferiprone received accelerated Food and Drug Administration approval for the treatment of thalassemia.
The current study was conducted because of an FDA requirement for postmarket assessment of deferiprone’s efficacy and safety in patients with sickle cell disease and other anemias who develop transfusional iron overload. It was initiated prior to the approval of deferasirox for the first-line treatment of SCD, therefore it was compared only with deferoxamine, she explained.
Dr. Kwiatkowski reported research funding from Apopharma, bluebird bio, Novartis, and Terumo, and consultancy for Agios, bluebird bio, Celgene, and Imara.
REPORTING FROM ASH 2019