User login
Conference News Roundup—European Society of Cardiology
Patients With High Blood Pressure Should Undergo Clock Drawing Test
A clock drawing test for detecting cognitive dysfunction should be conducted routinely in patients with high blood pressure, according to researchers.
Patients with high blood pressure who have impaired cognitive function are at increased risk of developing dementia within five years. Despite this known link, cognitive function is not routinely measured in patients with high blood pressure.
“The ability to draw the numbers of a clock and a particular time is an easy way to find out if a patient with high blood pressure has cognitive impairment,” said Augusto Vicario, MD, a cardiologist at the Cardiovascular Institute of Buenos Aires. “Identifying these patients provides the opportunity to intervene before dementia develops.”
The Heart-Brain Study in Argentina evaluated the usefulness of the clock drawing test, compared with the Mini-Mental State Examination (MMSE), to detect cognitive impairment in 1,414 adults with high blood pressure recruited from 18 cardiology centers in Argentina. The population’s average blood pressure was 144/84 mm Hg, average age was 60, and 62% were women.
For the clock drawing test, patients were given a piece of paper with a circle on it (diameter, 10 cm). They were asked to write the numbers of the clock in the correct positions inside the circle and draw hands on the clock indicating the time “20 to four.” Patients were scored as having normal cognition or moderate or severe cognitive impairment.
The researchers found a higher prevalence of cognitive impairment with the clock drawing test (36%), compared with the MMSE (21%). Three of 10 patients who had a normal MMSE score had an abnormal clock drawing result. The disparity in results between the two tests was greatest in middle-aged patients.
“Untreated high blood pressure silently and progressively damages the arteries in the subcortex of the brain and stops communication between the subcortex and frontal lobe,” said Dr. Vicario. “This disconnect leads to impaired executive functions such as planning, visuospatial abilities, remembering details, and decision-making. The clock drawing test is known to evaluate executive functions. The MMSE evaluates several other cognitive abilities but is weakly correlated with executive functions.
“Our study suggests that the clock drawing test should be preferred over the MMSE for early detection of executive dysfunction in patients with high blood pressure, particularly in middle age. We think the score on the clock drawing test can be considered a surrogate measure of silent vascular damage in the brain and identifies patients at greater risk of developing dementia. In our study, more than one-third of patients were at risk.
“The clock drawing test should be adopted as a routine screening tool for cognitive decline in patients with high blood pressure. Further studies are needed to determine whether lowering blood pressure can prevent progression to dementia,” Dr. Vicario concluded.
Short Sleep Associated With Doubled Risk of Cardiovascular Disease
Middle-aged men who sleep for five hours or fewer per night have twice the risk of developing a major cardiovascular event during the following two decades, compared with men who sleep for seven to eight hours, according to a study.
“For people with busy lives, sleeping may feel like a waste of time, but our study suggests that short sleep could be linked with future cardiovascular disease,” said Moa Bengtsson of the University of Gothenburg, Sweden.
Previous studies have generated conflicting evidence on whether short sleep is associated with a greater chance of having a future cardiovascular event. This study investigated this relationship in 50-year-old men.
In 1993, 50% of all men born in 1943 and living in Gothenburg were randomly selected to participate in the study. Of the 1,463 invited, 798 (55%) men agreed to take part. Participants underwent a physical examination and completed a questionnaire on current health conditions, average sleep duration, physical activity, and smoking. The men were divided into four groups according to their self-estimated average sleep duration at the start of the study: five or fewer hours, six hours, seven to eight hours (considered normal sleep duration), and more than eight hours.
Participants were followed up for 21 years for the occurrence of major cardiovascular events, which included heart attack, stroke, hospitalization due to heart failure, coronary revascularization, or death from cardiovascular disease. Data on cardiovascular events were collected from medical records, the Swedish Hospital Discharge Registry, and the Swedish Cause of Death Register.
Men with incomplete data on sleep duration, incomplete follow-up information, or who had a major cardiovascular event before the start of the study were excluded, leaving a total of 759 men for the analyses.
High blood pressure, diabetes, obesity, current smoking, low physical activity, and poor sleep quality were more common in men who slept for five or fewer hours per night, compared with those who slept for seven to eight hours.
Compared with those with normal sleep duration, men who slept for five or fewer hours per night had a twofold higher risk of having a major cardiovascular event by age 71. The risk remained doubled after adjusting for cardiovascular risk factors at the start of the study, including obesity, diabetes, and smoking.
“Men with the shortest sleep duration at the age of 50 were twice as likely to have had a cardiovascular event by age 71 as those who slept a normal amount, even when other risk factors were taken into account,” said Ms. Bengtsson.
“In our study, the magnitude of increased cardiovascular risk associated with insufficient sleep is similar to that of smoking or having diabetes at age 50. This was an observational study, so, based on our findings, we cannot conclude that short sleep causes cardiovascular disease or say definitively that sleeping more will reduce risk. However, the findings do suggest that sleep is important, and that should be a wake-up call to all of us.”
How Long Is a Good Night’s Sleep?
Researchers have found that a sweet spot of six to eight hours’ sleep per night is most beneficial for heart health. More or less sleep is detrimental.
“We spend one-third of our lives sleeping, yet we know little about the impact of this biologic need on the cardiovascular system,” said Epameinondas Fountas, MD, of the Onassis Cardiac Surgery Centre in Athens.
The study investigated the relationship between sleep duration and cardiovascular disease using a meta-analysis that included 11 prospective studies published within the past five years of more than one million adults without cardiovascular disease.
Two groups, one with short (ie, fewer than six hours) and another with long (ie, more than eight hours) nightly sleep duration, were compared with the reference group (ie, six to eight hours’ sleep).
Short and long sleepers had a greater risk of developing or dying from coronary artery disease or stroke. Compared with adults who slept for six to eight hours per night, short and long sleepers had 11% and 33% greater risks, respectively, of developing or dying from coronary artery disease or stroke during an average follow-up of 9.3 years.
“Our findings suggest that too much or too little sleep may be bad for the heart,” said Dr. Fountas. “More research is needed to clarify exactly why, but we do know that sleep influences biologic processes like glucose metabolism, blood pressure, and inflammation, all of which have an impact on cardiovascular disease.”
A strength of the current analysis is that only prospective studies were included, noted Dr. Fountas. This technique avoids recall bias.
“Having the odd short night or lie-in is unlikely to be detrimental to health, but evidence is accumulating that prolonged nightly sleep deprivation or excessive sleeping should be avoided,” said Dr. Fountas. “The good news is that there are plenty of ways to get into the habit of getting six to eight hours a night: for example, by going to bed and getting up at the same time every day, avoiding alcohol and caffeine before bed, eating healthily, and being physically active. Getting the right amount of sleep is an important part of a healthy lifestyle.”
Four in 10 Patients With Atrial Fibrillation Have Unrecognized Brain Damage
Four out of 10 patients with atrial fibrillation but no history of stroke or transient ischemic attack have previously unrecognized brain damage, according to the first results of the Swiss Atrial Fibrillation Cohort Study (Swiss-AF).
“Our results suggest that clinically unrecognized brain damage may explain the association between dementia and atrial fibrillation in patients without prior stroke,” said David Conen, MD, MPH, Associate Professor of Cardiology at McMaster University in Hamilton, Canada.
Patients with atrial fibrillation have a significantly increased risk of stroke, which is why most are treated with oral anticoagulation. This increased stroke risk is probably the main reason why patients with atrial fibrillation also face an increased risk of cognitive dysfunction and dementia. The relationship between atrial fibrillation and dementia has also been shown among patients without prior strokes, however, meaning that additional mechanisms must be involved. Clarifying the mechanisms by which atrial fibrillation increases the risk of cognitive dysfunction and dementia is a first step towards developing preventive measures.
Swiss-AF is a prospective, observational study designed to pinpoint the mechanisms of cognitive decline in patients with atrial fibrillation. Dr. Conen’s analysis investigated the prevalence of silent brain damage in patients with atrial fibrillation.
The study enrolled 2,415 patients older than 65 with atrial fibrillation between 2014 and 2017 from 14 centers in Switzerland. All patients without contraindications underwent standardized brain MRI, and the images were analyzed in a central core laboratory. Scans were available in 1,736 patients. Of those patients, 347 (20%) had a history of stroke or transient ischemic attack and were excluded from the analysis.
The final analysis included 1,389 patients with atrial fibrillation but no history of stroke or transient ischemic attack. The average age of participants was 72, and 26% were women. The scans showed that 569 (41%) patients had at least one type of previously unrecognized brain damage: 207 (15%) had a cerebral infarct, 269 (19%) had microbleeds, and 222 (16%) had lacunes.
“Four in 10 patients with atrial fibrillation but no history of stroke or transient ischemic attack had clinically unrecognized silent brain lesions,” said Dr. Conen. “This brain damage could trigger cognitive decline.”
Most study participants (1,234; 89%) were treated with oral anticoagulants. Stefan Osswald, MD, Chief of Cardiology at University Hospital Basel in Switzerland, noted that the cross-sectional analysis looked at the data at a single point in time and cannot address the question of whether the cerebral infarcts and other brain lesions occurred before or after initiation of oral anticoagulation. “The findings nevertheless raise the issue that oral anticoagulation might not prevent all brain damage in patients with atrial fibrillation,” he said.
“All Swiss-AF participants underwent extensive cognitive testing. These data will be analyzed to see whether patients with silent brain lesions also have impaired cognitive function,” said Dr. Conen. Collaborations with other study groups will help determine whether these findings are specific to patients with atrial fibrillation.
Impaired Mental Status Doubles Elderly’s Risk of Death After Heart Attack
Impaired mental status is associated with a doubled risk of death one year after a heart attack in elderly patients, according to researchers.
“Cardiologists should consider conducting simple tests to assess mental status in elderly people after a heart attack,” said Farzin Beygui, MD, hospital practitioner at Caen University Hospital in France. “Patients with reduced mental status can then receive more intensive management such as regular follow-up appointments with their general practitioners or nurses, more specific assessment for an early diagnosis of dementia, and tailored therapy.”
The risks of dementia, Alzheimer’s disease, confusion, and delirium increase with age. Elderly people are also at higher risk of having a heart attack and dying afterwards. People aged 75 and older account for approximately one-third of heart attack admissions and more than half of those dying in the hospital after admission for a heart attack. Until now, it was not known whether impaired mental status affected the prognosis of elderly patients with heart attack.
This study assessed the impact of mental status on the risk of death in 600 patients age 75 and above consecutively admitted for heart attack and followed up for at least one year. Mental status was assessed using the Mini-Mental State Examination (MMSE) and the Confusion Assessment Method (CAM).Cognitive impairment was detected in 174 (29%) patients. Patients with impaired mental function were more than twice as likely to be dead one year after their heart attack than those with healthy mental function. The association was independent of other potential predictors of death such as age, sex, invasive treatment, type of myocardial infarction, heart failure, and severity of the heart attack.
Impaired mental status was also associated with a nearly fourfold higher rate of bleeding complications while in the hospital and a more than twofold higher risk of being readmitted to the hospital for cardiovascular causes within three months after discharge.
“Almost one-third of elderly heart attack patients in our study had reduced mental capacity,” said Dr. Beygui. “These patients had higher risks of bleeding, rehospitalization, and death. This may be because they forget to take their medicines or take them more than prescribed, rather than because of poor cognitive function itself.
“Assessing mental status is a simple way to identify elderly patients at particularly high risk of poor outcomes following a heart attack. Identifying these patients may help us target treatment to those who need it most.”
Patients With High Blood Pressure Should Undergo Clock Drawing Test
A clock drawing test for detecting cognitive dysfunction should be conducted routinely in patients with high blood pressure, according to researchers.
Patients with high blood pressure who have impaired cognitive function are at increased risk of developing dementia within five years. Despite this known link, cognitive function is not routinely measured in patients with high blood pressure.
“The ability to draw the numbers of a clock and a particular time is an easy way to find out if a patient with high blood pressure has cognitive impairment,” said Augusto Vicario, MD, a cardiologist at the Cardiovascular Institute of Buenos Aires. “Identifying these patients provides the opportunity to intervene before dementia develops.”
The Heart-Brain Study in Argentina evaluated the usefulness of the clock drawing test, compared with the Mini-Mental State Examination (MMSE), to detect cognitive impairment in 1,414 adults with high blood pressure recruited from 18 cardiology centers in Argentina. The population’s average blood pressure was 144/84 mm Hg, average age was 60, and 62% were women.
For the clock drawing test, patients were given a piece of paper with a circle on it (diameter, 10 cm). They were asked to write the numbers of the clock in the correct positions inside the circle and draw hands on the clock indicating the time “20 to four.” Patients were scored as having normal cognition or moderate or severe cognitive impairment.
The researchers found a higher prevalence of cognitive impairment with the clock drawing test (36%), compared with the MMSE (21%). Three of 10 patients who had a normal MMSE score had an abnormal clock drawing result. The disparity in results between the two tests was greatest in middle-aged patients.
“Untreated high blood pressure silently and progressively damages the arteries in the subcortex of the brain and stops communication between the subcortex and frontal lobe,” said Dr. Vicario. “This disconnect leads to impaired executive functions such as planning, visuospatial abilities, remembering details, and decision-making. The clock drawing test is known to evaluate executive functions. The MMSE evaluates several other cognitive abilities but is weakly correlated with executive functions.
“Our study suggests that the clock drawing test should be preferred over the MMSE for early detection of executive dysfunction in patients with high blood pressure, particularly in middle age. We think the score on the clock drawing test can be considered a surrogate measure of silent vascular damage in the brain and identifies patients at greater risk of developing dementia. In our study, more than one-third of patients were at risk.
“The clock drawing test should be adopted as a routine screening tool for cognitive decline in patients with high blood pressure. Further studies are needed to determine whether lowering blood pressure can prevent progression to dementia,” Dr. Vicario concluded.
Short Sleep Associated With Doubled Risk of Cardiovascular Disease
Middle-aged men who sleep for five hours or fewer per night have twice the risk of developing a major cardiovascular event during the following two decades, compared with men who sleep for seven to eight hours, according to a study.
“For people with busy lives, sleeping may feel like a waste of time, but our study suggests that short sleep could be linked with future cardiovascular disease,” said Moa Bengtsson of the University of Gothenburg, Sweden.
Previous studies have generated conflicting evidence on whether short sleep is associated with a greater chance of having a future cardiovascular event. This study investigated this relationship in 50-year-old men.
In 1993, 50% of all men born in 1943 and living in Gothenburg were randomly selected to participate in the study. Of the 1,463 invited, 798 (55%) men agreed to take part. Participants underwent a physical examination and completed a questionnaire on current health conditions, average sleep duration, physical activity, and smoking. The men were divided into four groups according to their self-estimated average sleep duration at the start of the study: five or fewer hours, six hours, seven to eight hours (considered normal sleep duration), and more than eight hours.
Participants were followed up for 21 years for the occurrence of major cardiovascular events, which included heart attack, stroke, hospitalization due to heart failure, coronary revascularization, or death from cardiovascular disease. Data on cardiovascular events were collected from medical records, the Swedish Hospital Discharge Registry, and the Swedish Cause of Death Register.
Men with incomplete data on sleep duration, incomplete follow-up information, or who had a major cardiovascular event before the start of the study were excluded, leaving a total of 759 men for the analyses.
High blood pressure, diabetes, obesity, current smoking, low physical activity, and poor sleep quality were more common in men who slept for five or fewer hours per night, compared with those who slept for seven to eight hours.
Compared with those with normal sleep duration, men who slept for five or fewer hours per night had a twofold higher risk of having a major cardiovascular event by age 71. The risk remained doubled after adjusting for cardiovascular risk factors at the start of the study, including obesity, diabetes, and smoking.
“Men with the shortest sleep duration at the age of 50 were twice as likely to have had a cardiovascular event by age 71 as those who slept a normal amount, even when other risk factors were taken into account,” said Ms. Bengtsson.
“In our study, the magnitude of increased cardiovascular risk associated with insufficient sleep is similar to that of smoking or having diabetes at age 50. This was an observational study, so, based on our findings, we cannot conclude that short sleep causes cardiovascular disease or say definitively that sleeping more will reduce risk. However, the findings do suggest that sleep is important, and that should be a wake-up call to all of us.”
How Long Is a Good Night’s Sleep?
Researchers have found that a sweet spot of six to eight hours’ sleep per night is most beneficial for heart health. More or less sleep is detrimental.
“We spend one-third of our lives sleeping, yet we know little about the impact of this biologic need on the cardiovascular system,” said Epameinondas Fountas, MD, of the Onassis Cardiac Surgery Centre in Athens.
The study investigated the relationship between sleep duration and cardiovascular disease using a meta-analysis that included 11 prospective studies published within the past five years of more than one million adults without cardiovascular disease.
Two groups, one with short (ie, fewer than six hours) and another with long (ie, more than eight hours) nightly sleep duration, were compared with the reference group (ie, six to eight hours’ sleep).
Short and long sleepers had a greater risk of developing or dying from coronary artery disease or stroke. Compared with adults who slept for six to eight hours per night, short and long sleepers had 11% and 33% greater risks, respectively, of developing or dying from coronary artery disease or stroke during an average follow-up of 9.3 years.
“Our findings suggest that too much or too little sleep may be bad for the heart,” said Dr. Fountas. “More research is needed to clarify exactly why, but we do know that sleep influences biologic processes like glucose metabolism, blood pressure, and inflammation, all of which have an impact on cardiovascular disease.”
A strength of the current analysis is that only prospective studies were included, noted Dr. Fountas. This technique avoids recall bias.
“Having the odd short night or lie-in is unlikely to be detrimental to health, but evidence is accumulating that prolonged nightly sleep deprivation or excessive sleeping should be avoided,” said Dr. Fountas. “The good news is that there are plenty of ways to get into the habit of getting six to eight hours a night: for example, by going to bed and getting up at the same time every day, avoiding alcohol and caffeine before bed, eating healthily, and being physically active. Getting the right amount of sleep is an important part of a healthy lifestyle.”
Four in 10 Patients With Atrial Fibrillation Have Unrecognized Brain Damage
Four out of 10 patients with atrial fibrillation but no history of stroke or transient ischemic attack have previously unrecognized brain damage, according to the first results of the Swiss Atrial Fibrillation Cohort Study (Swiss-AF).
“Our results suggest that clinically unrecognized brain damage may explain the association between dementia and atrial fibrillation in patients without prior stroke,” said David Conen, MD, MPH, Associate Professor of Cardiology at McMaster University in Hamilton, Canada.
Patients with atrial fibrillation have a significantly increased risk of stroke, which is why most are treated with oral anticoagulation. This increased stroke risk is probably the main reason why patients with atrial fibrillation also face an increased risk of cognitive dysfunction and dementia. The relationship between atrial fibrillation and dementia has also been shown among patients without prior strokes, however, meaning that additional mechanisms must be involved. Clarifying the mechanisms by which atrial fibrillation increases the risk of cognitive dysfunction and dementia is a first step towards developing preventive measures.
Swiss-AF is a prospective, observational study designed to pinpoint the mechanisms of cognitive decline in patients with atrial fibrillation. Dr. Conen’s analysis investigated the prevalence of silent brain damage in patients with atrial fibrillation.
The study enrolled 2,415 patients older than 65 with atrial fibrillation between 2014 and 2017 from 14 centers in Switzerland. All patients without contraindications underwent standardized brain MRI, and the images were analyzed in a central core laboratory. Scans were available in 1,736 patients. Of those patients, 347 (20%) had a history of stroke or transient ischemic attack and were excluded from the analysis.
The final analysis included 1,389 patients with atrial fibrillation but no history of stroke or transient ischemic attack. The average age of participants was 72, and 26% were women. The scans showed that 569 (41%) patients had at least one type of previously unrecognized brain damage: 207 (15%) had a cerebral infarct, 269 (19%) had microbleeds, and 222 (16%) had lacunes.
“Four in 10 patients with atrial fibrillation but no history of stroke or transient ischemic attack had clinically unrecognized silent brain lesions,” said Dr. Conen. “This brain damage could trigger cognitive decline.”
Most study participants (1,234; 89%) were treated with oral anticoagulants. Stefan Osswald, MD, Chief of Cardiology at University Hospital Basel in Switzerland, noted that the cross-sectional analysis looked at the data at a single point in time and cannot address the question of whether the cerebral infarcts and other brain lesions occurred before or after initiation of oral anticoagulation. “The findings nevertheless raise the issue that oral anticoagulation might not prevent all brain damage in patients with atrial fibrillation,” he said.
“All Swiss-AF participants underwent extensive cognitive testing. These data will be analyzed to see whether patients with silent brain lesions also have impaired cognitive function,” said Dr. Conen. Collaborations with other study groups will help determine whether these findings are specific to patients with atrial fibrillation.
Impaired Mental Status Doubles Elderly’s Risk of Death After Heart Attack
Impaired mental status is associated with a doubled risk of death one year after a heart attack in elderly patients, according to researchers.
“Cardiologists should consider conducting simple tests to assess mental status in elderly people after a heart attack,” said Farzin Beygui, MD, hospital practitioner at Caen University Hospital in France. “Patients with reduced mental status can then receive more intensive management such as regular follow-up appointments with their general practitioners or nurses, more specific assessment for an early diagnosis of dementia, and tailored therapy.”
The risks of dementia, Alzheimer’s disease, confusion, and delirium increase with age. Elderly people are also at higher risk of having a heart attack and dying afterwards. People aged 75 and older account for approximately one-third of heart attack admissions and more than half of those dying in the hospital after admission for a heart attack. Until now, it was not known whether impaired mental status affected the prognosis of elderly patients with heart attack.
This study assessed the impact of mental status on the risk of death in 600 patients age 75 and above consecutively admitted for heart attack and followed up for at least one year. Mental status was assessed using the Mini-Mental State Examination (MMSE) and the Confusion Assessment Method (CAM).Cognitive impairment was detected in 174 (29%) patients. Patients with impaired mental function were more than twice as likely to be dead one year after their heart attack than those with healthy mental function. The association was independent of other potential predictors of death such as age, sex, invasive treatment, type of myocardial infarction, heart failure, and severity of the heart attack.
Impaired mental status was also associated with a nearly fourfold higher rate of bleeding complications while in the hospital and a more than twofold higher risk of being readmitted to the hospital for cardiovascular causes within three months after discharge.
“Almost one-third of elderly heart attack patients in our study had reduced mental capacity,” said Dr. Beygui. “These patients had higher risks of bleeding, rehospitalization, and death. This may be because they forget to take their medicines or take them more than prescribed, rather than because of poor cognitive function itself.
“Assessing mental status is a simple way to identify elderly patients at particularly high risk of poor outcomes following a heart attack. Identifying these patients may help us target treatment to those who need it most.”
Patients With High Blood Pressure Should Undergo Clock Drawing Test
A clock drawing test for detecting cognitive dysfunction should be conducted routinely in patients with high blood pressure, according to researchers.
Patients with high blood pressure who have impaired cognitive function are at increased risk of developing dementia within five years. Despite this known link, cognitive function is not routinely measured in patients with high blood pressure.
“The ability to draw the numbers of a clock and a particular time is an easy way to find out if a patient with high blood pressure has cognitive impairment,” said Augusto Vicario, MD, a cardiologist at the Cardiovascular Institute of Buenos Aires. “Identifying these patients provides the opportunity to intervene before dementia develops.”
The Heart-Brain Study in Argentina evaluated the usefulness of the clock drawing test, compared with the Mini-Mental State Examination (MMSE), to detect cognitive impairment in 1,414 adults with high blood pressure recruited from 18 cardiology centers in Argentina. The population’s average blood pressure was 144/84 mm Hg, average age was 60, and 62% were women.
For the clock drawing test, patients were given a piece of paper with a circle on it (diameter, 10 cm). They were asked to write the numbers of the clock in the correct positions inside the circle and draw hands on the clock indicating the time “20 to four.” Patients were scored as having normal cognition or moderate or severe cognitive impairment.
The researchers found a higher prevalence of cognitive impairment with the clock drawing test (36%), compared with the MMSE (21%). Three of 10 patients who had a normal MMSE score had an abnormal clock drawing result. The disparity in results between the two tests was greatest in middle-aged patients.
“Untreated high blood pressure silently and progressively damages the arteries in the subcortex of the brain and stops communication between the subcortex and frontal lobe,” said Dr. Vicario. “This disconnect leads to impaired executive functions such as planning, visuospatial abilities, remembering details, and decision-making. The clock drawing test is known to evaluate executive functions. The MMSE evaluates several other cognitive abilities but is weakly correlated with executive functions.
“Our study suggests that the clock drawing test should be preferred over the MMSE for early detection of executive dysfunction in patients with high blood pressure, particularly in middle age. We think the score on the clock drawing test can be considered a surrogate measure of silent vascular damage in the brain and identifies patients at greater risk of developing dementia. In our study, more than one-third of patients were at risk.
“The clock drawing test should be adopted as a routine screening tool for cognitive decline in patients with high blood pressure. Further studies are needed to determine whether lowering blood pressure can prevent progression to dementia,” Dr. Vicario concluded.
Short Sleep Associated With Doubled Risk of Cardiovascular Disease
Middle-aged men who sleep for five hours or fewer per night have twice the risk of developing a major cardiovascular event during the following two decades, compared with men who sleep for seven to eight hours, according to a study.
“For people with busy lives, sleeping may feel like a waste of time, but our study suggests that short sleep could be linked with future cardiovascular disease,” said Moa Bengtsson of the University of Gothenburg, Sweden.
Previous studies have generated conflicting evidence on whether short sleep is associated with a greater chance of having a future cardiovascular event. This study investigated this relationship in 50-year-old men.
In 1993, 50% of all men born in 1943 and living in Gothenburg were randomly selected to participate in the study. Of the 1,463 invited, 798 (55%) men agreed to take part. Participants underwent a physical examination and completed a questionnaire on current health conditions, average sleep duration, physical activity, and smoking. The men were divided into four groups according to their self-estimated average sleep duration at the start of the study: five or fewer hours, six hours, seven to eight hours (considered normal sleep duration), and more than eight hours.
Participants were followed up for 21 years for the occurrence of major cardiovascular events, which included heart attack, stroke, hospitalization due to heart failure, coronary revascularization, or death from cardiovascular disease. Data on cardiovascular events were collected from medical records, the Swedish Hospital Discharge Registry, and the Swedish Cause of Death Register.
Men with incomplete data on sleep duration, incomplete follow-up information, or who had a major cardiovascular event before the start of the study were excluded, leaving a total of 759 men for the analyses.
High blood pressure, diabetes, obesity, current smoking, low physical activity, and poor sleep quality were more common in men who slept for five or fewer hours per night, compared with those who slept for seven to eight hours.
Compared with those with normal sleep duration, men who slept for five or fewer hours per night had a twofold higher risk of having a major cardiovascular event by age 71. The risk remained doubled after adjusting for cardiovascular risk factors at the start of the study, including obesity, diabetes, and smoking.
“Men with the shortest sleep duration at the age of 50 were twice as likely to have had a cardiovascular event by age 71 as those who slept a normal amount, even when other risk factors were taken into account,” said Ms. Bengtsson.
“In our study, the magnitude of increased cardiovascular risk associated with insufficient sleep is similar to that of smoking or having diabetes at age 50. This was an observational study, so, based on our findings, we cannot conclude that short sleep causes cardiovascular disease or say definitively that sleeping more will reduce risk. However, the findings do suggest that sleep is important, and that should be a wake-up call to all of us.”
How Long Is a Good Night’s Sleep?
Researchers have found that a sweet spot of six to eight hours’ sleep per night is most beneficial for heart health. More or less sleep is detrimental.
“We spend one-third of our lives sleeping, yet we know little about the impact of this biologic need on the cardiovascular system,” said Epameinondas Fountas, MD, of the Onassis Cardiac Surgery Centre in Athens.
The study investigated the relationship between sleep duration and cardiovascular disease using a meta-analysis that included 11 prospective studies published within the past five years of more than one million adults without cardiovascular disease.
Two groups, one with short (ie, fewer than six hours) and another with long (ie, more than eight hours) nightly sleep duration, were compared with the reference group (ie, six to eight hours’ sleep).
Short and long sleepers had a greater risk of developing or dying from coronary artery disease or stroke. Compared with adults who slept for six to eight hours per night, short and long sleepers had 11% and 33% greater risks, respectively, of developing or dying from coronary artery disease or stroke during an average follow-up of 9.3 years.
“Our findings suggest that too much or too little sleep may be bad for the heart,” said Dr. Fountas. “More research is needed to clarify exactly why, but we do know that sleep influences biologic processes like glucose metabolism, blood pressure, and inflammation, all of which have an impact on cardiovascular disease.”
A strength of the current analysis is that only prospective studies were included, noted Dr. Fountas. This technique avoids recall bias.
“Having the odd short night or lie-in is unlikely to be detrimental to health, but evidence is accumulating that prolonged nightly sleep deprivation or excessive sleeping should be avoided,” said Dr. Fountas. “The good news is that there are plenty of ways to get into the habit of getting six to eight hours a night: for example, by going to bed and getting up at the same time every day, avoiding alcohol and caffeine before bed, eating healthily, and being physically active. Getting the right amount of sleep is an important part of a healthy lifestyle.”
Four in 10 Patients With Atrial Fibrillation Have Unrecognized Brain Damage
Four out of 10 patients with atrial fibrillation but no history of stroke or transient ischemic attack have previously unrecognized brain damage, according to the first results of the Swiss Atrial Fibrillation Cohort Study (Swiss-AF).
“Our results suggest that clinically unrecognized brain damage may explain the association between dementia and atrial fibrillation in patients without prior stroke,” said David Conen, MD, MPH, Associate Professor of Cardiology at McMaster University in Hamilton, Canada.
Patients with atrial fibrillation have a significantly increased risk of stroke, which is why most are treated with oral anticoagulation. This increased stroke risk is probably the main reason why patients with atrial fibrillation also face an increased risk of cognitive dysfunction and dementia. The relationship between atrial fibrillation and dementia has also been shown among patients without prior strokes, however, meaning that additional mechanisms must be involved. Clarifying the mechanisms by which atrial fibrillation increases the risk of cognitive dysfunction and dementia is a first step towards developing preventive measures.
Swiss-AF is a prospective, observational study designed to pinpoint the mechanisms of cognitive decline in patients with atrial fibrillation. Dr. Conen’s analysis investigated the prevalence of silent brain damage in patients with atrial fibrillation.
The study enrolled 2,415 patients older than 65 with atrial fibrillation between 2014 and 2017 from 14 centers in Switzerland. All patients without contraindications underwent standardized brain MRI, and the images were analyzed in a central core laboratory. Scans were available in 1,736 patients. Of those patients, 347 (20%) had a history of stroke or transient ischemic attack and were excluded from the analysis.
The final analysis included 1,389 patients with atrial fibrillation but no history of stroke or transient ischemic attack. The average age of participants was 72, and 26% were women. The scans showed that 569 (41%) patients had at least one type of previously unrecognized brain damage: 207 (15%) had a cerebral infarct, 269 (19%) had microbleeds, and 222 (16%) had lacunes.
“Four in 10 patients with atrial fibrillation but no history of stroke or transient ischemic attack had clinically unrecognized silent brain lesions,” said Dr. Conen. “This brain damage could trigger cognitive decline.”
Most study participants (1,234; 89%) were treated with oral anticoagulants. Stefan Osswald, MD, Chief of Cardiology at University Hospital Basel in Switzerland, noted that the cross-sectional analysis looked at the data at a single point in time and cannot address the question of whether the cerebral infarcts and other brain lesions occurred before or after initiation of oral anticoagulation. “The findings nevertheless raise the issue that oral anticoagulation might not prevent all brain damage in patients with atrial fibrillation,” he said.
“All Swiss-AF participants underwent extensive cognitive testing. These data will be analyzed to see whether patients with silent brain lesions also have impaired cognitive function,” said Dr. Conen. Collaborations with other study groups will help determine whether these findings are specific to patients with atrial fibrillation.
Impaired Mental Status Doubles Elderly’s Risk of Death After Heart Attack
Impaired mental status is associated with a doubled risk of death one year after a heart attack in elderly patients, according to researchers.
“Cardiologists should consider conducting simple tests to assess mental status in elderly people after a heart attack,” said Farzin Beygui, MD, hospital practitioner at Caen University Hospital in France. “Patients with reduced mental status can then receive more intensive management such as regular follow-up appointments with their general practitioners or nurses, more specific assessment for an early diagnosis of dementia, and tailored therapy.”
The risks of dementia, Alzheimer’s disease, confusion, and delirium increase with age. Elderly people are also at higher risk of having a heart attack and dying afterwards. People aged 75 and older account for approximately one-third of heart attack admissions and more than half of those dying in the hospital after admission for a heart attack. Until now, it was not known whether impaired mental status affected the prognosis of elderly patients with heart attack.
This study assessed the impact of mental status on the risk of death in 600 patients age 75 and above consecutively admitted for heart attack and followed up for at least one year. Mental status was assessed using the Mini-Mental State Examination (MMSE) and the Confusion Assessment Method (CAM).Cognitive impairment was detected in 174 (29%) patients. Patients with impaired mental function were more than twice as likely to be dead one year after their heart attack than those with healthy mental function. The association was independent of other potential predictors of death such as age, sex, invasive treatment, type of myocardial infarction, heart failure, and severity of the heart attack.
Impaired mental status was also associated with a nearly fourfold higher rate of bleeding complications while in the hospital and a more than twofold higher risk of being readmitted to the hospital for cardiovascular causes within three months after discharge.
“Almost one-third of elderly heart attack patients in our study had reduced mental capacity,” said Dr. Beygui. “These patients had higher risks of bleeding, rehospitalization, and death. This may be because they forget to take their medicines or take them more than prescribed, rather than because of poor cognitive function itself.
“Assessing mental status is a simple way to identify elderly patients at particularly high risk of poor outcomes following a heart attack. Identifying these patients may help us target treatment to those who need it most.”
Diagnosis, Pathology, and Treatment of bvFTD Pose Challenges
Certain clinical features may indicate bvFTD, and off-label treatments may provide benefits.
HILTON HEAD, SC—Behavioral variant frontotemporal dementia (bvFTD), a clinically and pathologically heterogenous condition, can be difficult to distinguish from other forms of dementia or frontotemporal disease. “Although clinical symptoms vary based on which part of the brain is affected, there is a significant amount of overlap, with different pathologies causing the same type of syndrome,” said Richard Ryan Darby, MD, Assistant Professor of Neurology at Vanderbilt University Medical School in Nashville. “In a large proportion of patients, behavioral changes can lead to criminal behavior.” Future research examining brain lesion networks may shed light on this condition, said Dr. Darby at the 41st Annual Contemporary Clinical Neurology Symposium.
Clinical Features and Diagnosis
The incidence of bvFTD is equal to that of Alzheimer’s disease. However, patients with bvFTD tend to be younger: between the ages of 45 and 65. “Social and behavioral features predominate,” said Dr. Darby. “If a patient in this age range with no previous psychiatric history presents to you with a new psychiatric diagnosis such as schizophrenia or bipolar disease, this should raise your suspicion of bvFTD.” These psychiatric problems often cause considerable disruptions in patients’ lives. They often have problems at work and lose their source of income. “Patients often have no insight about their symptoms,” said Dr. Darby.
A differential diagnosis of bvFTD is possible in patients with three or more of the following six clinical features: socially inappropriate behavior (eg, eating from the trash or walking around naked at inappropriate times); lack of empathy; apathy; stereotyped or repetitive behavior (eg, saying things repeatedly, pacing); hyperorality (eg, eating uncontrollably, particularly sweet foods); and executive dysfunction, especially when memory is preserved.
“When you talk to caregivers, they often say, ‘This is not the person I married,’ or, ‘This is not my father. He seems like a different person,’” said Dr. Darby. An MRI or a PET scan showing changes in the frontotemporal lobes is a firm basis for a diagnosis of bvFTD, said Dr. Darby. Genetic testing for autosomal dominant mutation or pathology or an autopsy provides a definite diagnosis.
Pathology
Between 40% and 50% of patients with bvFTD have tau pathology, said Dr. Darby. This pathology includes the classic Pick body form of tau, tufted astrocytes (which are associated with clinical symptoms of progressive supranuclear palsy), and astrocytic plaques (which are associated with symptoms of corticobasal degeneration). Similarly, between 40% and 50% of patients with bvFTD have a TAR DNA-binding protein 43 (TDP-43) pathology. TDP-43 Type A pathology is associated with perirolandic seizures, Type B is associated with
Mutations in C9orf72 occur in 13% to 50% of patients with bvFTD who have genetic mutations. ALS and parkinsonism are common clinical presentations in patients with this genetic mutation. MAPT and GRN mutations are present in 5% to 20% of cases, and each can present clinically as parkinsonism.
Treatment
SSRIs, antipsychotics, anticonvulsants, and stimulants have been used to treat apathy, disinhibition, compulsive behaviors, agitation, and inappropriate behavior in patients with bvFTD. “There are no FDA-approved treatments for bvFTD, and the evidence for [these agents] has been mostly reported in case studies and small clinical trials,” said Dr. Darby. Among the SSRIs, paroxetine improved repetitive behavior in open-label trials, but not in a randomized controlled trial. Sertraline and citalopram have been studied in open-label trials, and trazodone was examined in a randomized controlled crossover trial involving 26 patients.
The FDA issued a black box warning against the use of atypical antipsychotics in patients with dementia because they entail a risk of cardiac- and infection-related mortality. “For patients with bvFTD tau pathology in particular, there is a risk of extrapyramidal adverse effects,” said Dr. Darby. A series of case reports of risperidone and aripiprazole provided evidence of symptom improvement, as did an open label study of olanzapine. Quetiapine improved agitation in a case series but failed to show benefit in a double-blind crossover trial of eight patients with FTD.
Case series have shown evidence that antiepileptic drugs (eg, valproic acid, topiramate, and carbamazepine) have a stabilizing effect. “Stimulants are tried in some patients, but should be used with caution,” said Dr. Darby.
Criminality
Between 37% and 57% of patients with bvFTD engage in criminal behavior. “Approximately 10% to 15% of the time, a patient’s getting in trouble with the law is the reason for the initial presentation,” said Dr. Darby. The types of crimes described in case reports include pedophilia, public masturbation, hit and run, traffic violations, and theft.
“Murder and violent crimes occur but are rare. Crimes committed by patients with bvFTD are usually reactive,” said Dr. Darby. “When asked, they can tell you whether a specific act is right or wrong; however, they don’t show remorse for criminal behavior.” It is not clear whether executive dysfunction and the inability to reason, social perception and the inability to empathize, or differences in moral decision making are the reasons for changes in patients’ behavior, he said.
“One idea is that a network of brain regions, not just one part of the brain, is responsible for formulating the complex concept of morality.” In 2017, Dr. Darby and colleagues systematically mapped brain lesions with a documented temporal association with criminal behavior in 17 patients who were identified through a literature search. Criminal behavior included white collar crimes, and 12 of 17 patients had committed violent crimes. Fifteen cases had no history of criminal behavior before the lesion, and the behavior resolved following treatment of the lesion in two cases.
No single brain region had been damaged in all cases. Because lesion-induced symptoms can arise from sites connected to the lesion location, the investigators identified these sites in the cases. The network of these sites included regions involved in morality, value-based decision making, and theory of mind, but not regions involved in cognitive control or empathy. Darby and colleagues replicated these results in a separate cohort of 23 cases in which a temporal relationship between brain lesions and criminal behavior was plausible, but not definite.
Prior research suggests that the areas associated with criminal behavior in patients with brain lesions closely resemble the areas typically affected in patients with bvFTD. Prospective studies are needed to further elucidate these results, Dr. Darby concluded.
—Adriene Marshall
Suggested Reading
Darby RR, Horn A, Cushman F, Fox MD. Lesion network localization of criminal behavior. Proc Natl Acad Sci U S A. 2018;115(3):601-606.
Perry DC, Brown JA, Possin KL, et al. Clinicopathological correlations in behavioural variant frontotemporal dementia. Brain. 2017;140(12):3329-3345.
Certain clinical features may indicate bvFTD, and off-label treatments may provide benefits.
Certain clinical features may indicate bvFTD, and off-label treatments may provide benefits.
HILTON HEAD, SC—Behavioral variant frontotemporal dementia (bvFTD), a clinically and pathologically heterogenous condition, can be difficult to distinguish from other forms of dementia or frontotemporal disease. “Although clinical symptoms vary based on which part of the brain is affected, there is a significant amount of overlap, with different pathologies causing the same type of syndrome,” said Richard Ryan Darby, MD, Assistant Professor of Neurology at Vanderbilt University Medical School in Nashville. “In a large proportion of patients, behavioral changes can lead to criminal behavior.” Future research examining brain lesion networks may shed light on this condition, said Dr. Darby at the 41st Annual Contemporary Clinical Neurology Symposium.
Clinical Features and Diagnosis
The incidence of bvFTD is equal to that of Alzheimer’s disease. However, patients with bvFTD tend to be younger: between the ages of 45 and 65. “Social and behavioral features predominate,” said Dr. Darby. “If a patient in this age range with no previous psychiatric history presents to you with a new psychiatric diagnosis such as schizophrenia or bipolar disease, this should raise your suspicion of bvFTD.” These psychiatric problems often cause considerable disruptions in patients’ lives. They often have problems at work and lose their source of income. “Patients often have no insight about their symptoms,” said Dr. Darby.
A differential diagnosis of bvFTD is possible in patients with three or more of the following six clinical features: socially inappropriate behavior (eg, eating from the trash or walking around naked at inappropriate times); lack of empathy; apathy; stereotyped or repetitive behavior (eg, saying things repeatedly, pacing); hyperorality (eg, eating uncontrollably, particularly sweet foods); and executive dysfunction, especially when memory is preserved.
“When you talk to caregivers, they often say, ‘This is not the person I married,’ or, ‘This is not my father. He seems like a different person,’” said Dr. Darby. An MRI or a PET scan showing changes in the frontotemporal lobes is a firm basis for a diagnosis of bvFTD, said Dr. Darby. Genetic testing for autosomal dominant mutation or pathology or an autopsy provides a definite diagnosis.
Pathology
Between 40% and 50% of patients with bvFTD have tau pathology, said Dr. Darby. This pathology includes the classic Pick body form of tau, tufted astrocytes (which are associated with clinical symptoms of progressive supranuclear palsy), and astrocytic plaques (which are associated with symptoms of corticobasal degeneration). Similarly, between 40% and 50% of patients with bvFTD have a TAR DNA-binding protein 43 (TDP-43) pathology. TDP-43 Type A pathology is associated with perirolandic seizures, Type B is associated with
Mutations in C9orf72 occur in 13% to 50% of patients with bvFTD who have genetic mutations. ALS and parkinsonism are common clinical presentations in patients with this genetic mutation. MAPT and GRN mutations are present in 5% to 20% of cases, and each can present clinically as parkinsonism.
Treatment
SSRIs, antipsychotics, anticonvulsants, and stimulants have been used to treat apathy, disinhibition, compulsive behaviors, agitation, and inappropriate behavior in patients with bvFTD. “There are no FDA-approved treatments for bvFTD, and the evidence for [these agents] has been mostly reported in case studies and small clinical trials,” said Dr. Darby. Among the SSRIs, paroxetine improved repetitive behavior in open-label trials, but not in a randomized controlled trial. Sertraline and citalopram have been studied in open-label trials, and trazodone was examined in a randomized controlled crossover trial involving 26 patients.
The FDA issued a black box warning against the use of atypical antipsychotics in patients with dementia because they entail a risk of cardiac- and infection-related mortality. “For patients with bvFTD tau pathology in particular, there is a risk of extrapyramidal adverse effects,” said Dr. Darby. A series of case reports of risperidone and aripiprazole provided evidence of symptom improvement, as did an open label study of olanzapine. Quetiapine improved agitation in a case series but failed to show benefit in a double-blind crossover trial of eight patients with FTD.
Case series have shown evidence that antiepileptic drugs (eg, valproic acid, topiramate, and carbamazepine) have a stabilizing effect. “Stimulants are tried in some patients, but should be used with caution,” said Dr. Darby.
Criminality
Between 37% and 57% of patients with bvFTD engage in criminal behavior. “Approximately 10% to 15% of the time, a patient’s getting in trouble with the law is the reason for the initial presentation,” said Dr. Darby. The types of crimes described in case reports include pedophilia, public masturbation, hit and run, traffic violations, and theft.
“Murder and violent crimes occur but are rare. Crimes committed by patients with bvFTD are usually reactive,” said Dr. Darby. “When asked, they can tell you whether a specific act is right or wrong; however, they don’t show remorse for criminal behavior.” It is not clear whether executive dysfunction and the inability to reason, social perception and the inability to empathize, or differences in moral decision making are the reasons for changes in patients’ behavior, he said.
“One idea is that a network of brain regions, not just one part of the brain, is responsible for formulating the complex concept of morality.” In 2017, Dr. Darby and colleagues systematically mapped brain lesions with a documented temporal association with criminal behavior in 17 patients who were identified through a literature search. Criminal behavior included white collar crimes, and 12 of 17 patients had committed violent crimes. Fifteen cases had no history of criminal behavior before the lesion, and the behavior resolved following treatment of the lesion in two cases.
No single brain region had been damaged in all cases. Because lesion-induced symptoms can arise from sites connected to the lesion location, the investigators identified these sites in the cases. The network of these sites included regions involved in morality, value-based decision making, and theory of mind, but not regions involved in cognitive control or empathy. Darby and colleagues replicated these results in a separate cohort of 23 cases in which a temporal relationship between brain lesions and criminal behavior was plausible, but not definite.
Prior research suggests that the areas associated with criminal behavior in patients with brain lesions closely resemble the areas typically affected in patients with bvFTD. Prospective studies are needed to further elucidate these results, Dr. Darby concluded.
—Adriene Marshall
Suggested Reading
Darby RR, Horn A, Cushman F, Fox MD. Lesion network localization of criminal behavior. Proc Natl Acad Sci U S A. 2018;115(3):601-606.
Perry DC, Brown JA, Possin KL, et al. Clinicopathological correlations in behavioural variant frontotemporal dementia. Brain. 2017;140(12):3329-3345.
HILTON HEAD, SC—Behavioral variant frontotemporal dementia (bvFTD), a clinically and pathologically heterogenous condition, can be difficult to distinguish from other forms of dementia or frontotemporal disease. “Although clinical symptoms vary based on which part of the brain is affected, there is a significant amount of overlap, with different pathologies causing the same type of syndrome,” said Richard Ryan Darby, MD, Assistant Professor of Neurology at Vanderbilt University Medical School in Nashville. “In a large proportion of patients, behavioral changes can lead to criminal behavior.” Future research examining brain lesion networks may shed light on this condition, said Dr. Darby at the 41st Annual Contemporary Clinical Neurology Symposium.
Clinical Features and Diagnosis
The incidence of bvFTD is equal to that of Alzheimer’s disease. However, patients with bvFTD tend to be younger: between the ages of 45 and 65. “Social and behavioral features predominate,” said Dr. Darby. “If a patient in this age range with no previous psychiatric history presents to you with a new psychiatric diagnosis such as schizophrenia or bipolar disease, this should raise your suspicion of bvFTD.” These psychiatric problems often cause considerable disruptions in patients’ lives. They often have problems at work and lose their source of income. “Patients often have no insight about their symptoms,” said Dr. Darby.
A differential diagnosis of bvFTD is possible in patients with three or more of the following six clinical features: socially inappropriate behavior (eg, eating from the trash or walking around naked at inappropriate times); lack of empathy; apathy; stereotyped or repetitive behavior (eg, saying things repeatedly, pacing); hyperorality (eg, eating uncontrollably, particularly sweet foods); and executive dysfunction, especially when memory is preserved.
“When you talk to caregivers, they often say, ‘This is not the person I married,’ or, ‘This is not my father. He seems like a different person,’” said Dr. Darby. An MRI or a PET scan showing changes in the frontotemporal lobes is a firm basis for a diagnosis of bvFTD, said Dr. Darby. Genetic testing for autosomal dominant mutation or pathology or an autopsy provides a definite diagnosis.
Pathology
Between 40% and 50% of patients with bvFTD have tau pathology, said Dr. Darby. This pathology includes the classic Pick body form of tau, tufted astrocytes (which are associated with clinical symptoms of progressive supranuclear palsy), and astrocytic plaques (which are associated with symptoms of corticobasal degeneration). Similarly, between 40% and 50% of patients with bvFTD have a TAR DNA-binding protein 43 (TDP-43) pathology. TDP-43 Type A pathology is associated with perirolandic seizures, Type B is associated with
Mutations in C9orf72 occur in 13% to 50% of patients with bvFTD who have genetic mutations. ALS and parkinsonism are common clinical presentations in patients with this genetic mutation. MAPT and GRN mutations are present in 5% to 20% of cases, and each can present clinically as parkinsonism.
Treatment
SSRIs, antipsychotics, anticonvulsants, and stimulants have been used to treat apathy, disinhibition, compulsive behaviors, agitation, and inappropriate behavior in patients with bvFTD. “There are no FDA-approved treatments for bvFTD, and the evidence for [these agents] has been mostly reported in case studies and small clinical trials,” said Dr. Darby. Among the SSRIs, paroxetine improved repetitive behavior in open-label trials, but not in a randomized controlled trial. Sertraline and citalopram have been studied in open-label trials, and trazodone was examined in a randomized controlled crossover trial involving 26 patients.
The FDA issued a black box warning against the use of atypical antipsychotics in patients with dementia because they entail a risk of cardiac- and infection-related mortality. “For patients with bvFTD tau pathology in particular, there is a risk of extrapyramidal adverse effects,” said Dr. Darby. A series of case reports of risperidone and aripiprazole provided evidence of symptom improvement, as did an open label study of olanzapine. Quetiapine improved agitation in a case series but failed to show benefit in a double-blind crossover trial of eight patients with FTD.
Case series have shown evidence that antiepileptic drugs (eg, valproic acid, topiramate, and carbamazepine) have a stabilizing effect. “Stimulants are tried in some patients, but should be used with caution,” said Dr. Darby.
Criminality
Between 37% and 57% of patients with bvFTD engage in criminal behavior. “Approximately 10% to 15% of the time, a patient’s getting in trouble with the law is the reason for the initial presentation,” said Dr. Darby. The types of crimes described in case reports include pedophilia, public masturbation, hit and run, traffic violations, and theft.
“Murder and violent crimes occur but are rare. Crimes committed by patients with bvFTD are usually reactive,” said Dr. Darby. “When asked, they can tell you whether a specific act is right or wrong; however, they don’t show remorse for criminal behavior.” It is not clear whether executive dysfunction and the inability to reason, social perception and the inability to empathize, or differences in moral decision making are the reasons for changes in patients’ behavior, he said.
“One idea is that a network of brain regions, not just one part of the brain, is responsible for formulating the complex concept of morality.” In 2017, Dr. Darby and colleagues systematically mapped brain lesions with a documented temporal association with criminal behavior in 17 patients who were identified through a literature search. Criminal behavior included white collar crimes, and 12 of 17 patients had committed violent crimes. Fifteen cases had no history of criminal behavior before the lesion, and the behavior resolved following treatment of the lesion in two cases.
No single brain region had been damaged in all cases. Because lesion-induced symptoms can arise from sites connected to the lesion location, the investigators identified these sites in the cases. The network of these sites included regions involved in morality, value-based decision making, and theory of mind, but not regions involved in cognitive control or empathy. Darby and colleagues replicated these results in a separate cohort of 23 cases in which a temporal relationship between brain lesions and criminal behavior was plausible, but not definite.
Prior research suggests that the areas associated with criminal behavior in patients with brain lesions closely resemble the areas typically affected in patients with bvFTD. Prospective studies are needed to further elucidate these results, Dr. Darby concluded.
—Adriene Marshall
Suggested Reading
Darby RR, Horn A, Cushman F, Fox MD. Lesion network localization of criminal behavior. Proc Natl Acad Sci U S A. 2018;115(3):601-606.
Perry DC, Brown JA, Possin KL, et al. Clinicopathological correlations in behavioural variant frontotemporal dementia. Brain. 2017;140(12):3329-3345.
Stroke Increases the Risk of All-Cause Dementia
Protecting the blood supply to the brain could reduce the risk of incident dementia.
Stroke is a strong independent risk factor for all-cause dementia, according to research published online ahead of print August 25 in Alzheimer’s & Dementia. Clinicians should incorporate stroke-prevention strategies into their health interventions to reduce patients’ risk of dementia, said the authors.
“Around a third of dementia cases are thought to be potentially preventable, though this estimate does not take into account the risk associated with stroke,” said David Llewellyn, PhD, Senior Research Fellow at University of Exeter Medical School in the United Kingdom. “Our findings indicate that this figure could be even higher and reinforce the importance of protecting the blood supply to the brain when attempting to reduce the global burden of dementia.”
Meta-Analysis of Previous Research
Stroke is a recognized risk factor for all-cause dementia, but no researchers had previously performed a meta-analysis to quantify the risk. Dr. Llewellyn and colleagues searched Medline, PsycINFO, and Embase databases for prospective studies that investigated the association between prevalent or incident stroke and incident all-cause dementia. They excluded studies that lacked a comparison group or that had a comparison group other than a stroke-free group. The investigators pooled adjusted estimates across studies using random effects meta-analysis and evaluated potential effect modifiers with meta-regression.
Dr. Llewellyn and colleagues identified 11,129 articles, 26 of which were eligible for analysis. They also included 16 studies from a previous systematic review and four studies identified through backward and forward citation searches. In all, 36 studies examined prevalent stroke (1.9 million participants), and 12 studies examined incident stroke (1.3 million participants). The studies were conducted in America, Europe, Asia, and Australia and included more than three million participants. Follow-up periods ranged from nine months to 25 years.
Stroke Affected Dementia Risk
When the researchers pooled results from 22 cohorts of participants who were cognitively normal at baseline, they found that those with prevalent stroke had a higher adjusted risk of incident dementia, compared with those without stroke (hazard ratio [HR], 1.69). Sensitivity analyses did not change the results significantly. Prevalent stroke was associated with a higher risk of incident dementia among men than among women. Sex explained 50.2% of heterogeneity between studies for prevalent stroke.
After combining the adjusted results from eight studies, Dr. Llewellyn and colleagues found that incident stroke more than doubled the risk of incident all-cause dementia, compared with no incident stroke (risk ratio [RR], 2.18). For a sensitivity analysis, the investigators excluded three studies that combined stroke with transient ischemic attack; this adjustment strengthened the association.
The study’s strengths include the investigators’ search of several major databases and their contacts with authors who provided relevant data. The analysis reflects the limitations of the original studies, however. These limitations include selective samples and differences in stroke assessment and dementia diagnosis criteria. In addition, dementia may develop years before it is diagnosed. “More detailed reporting of the interval between stroke occurrence and dementia diagnosis in future studies will help to better characterize the role of time since stroke in the risk of dementia,” said Dr. Llewellyn.
—Erik Greb
Kuz´ma E, Lourida I, Moore SF, et al. Stroke and dementia risk: a systematic review and meta-analysis. Alzheimers Dement. 2018 Aug 25 [Epub ahead of print].
Protecting the blood supply to the brain could reduce the risk of incident dementia.
Protecting the blood supply to the brain could reduce the risk of incident dementia.
Stroke is a strong independent risk factor for all-cause dementia, according to research published online ahead of print August 25 in Alzheimer’s & Dementia. Clinicians should incorporate stroke-prevention strategies into their health interventions to reduce patients’ risk of dementia, said the authors.
“Around a third of dementia cases are thought to be potentially preventable, though this estimate does not take into account the risk associated with stroke,” said David Llewellyn, PhD, Senior Research Fellow at University of Exeter Medical School in the United Kingdom. “Our findings indicate that this figure could be even higher and reinforce the importance of protecting the blood supply to the brain when attempting to reduce the global burden of dementia.”
Meta-Analysis of Previous Research
Stroke is a recognized risk factor for all-cause dementia, but no researchers had previously performed a meta-analysis to quantify the risk. Dr. Llewellyn and colleagues searched Medline, PsycINFO, and Embase databases for prospective studies that investigated the association between prevalent or incident stroke and incident all-cause dementia. They excluded studies that lacked a comparison group or that had a comparison group other than a stroke-free group. The investigators pooled adjusted estimates across studies using random effects meta-analysis and evaluated potential effect modifiers with meta-regression.
Dr. Llewellyn and colleagues identified 11,129 articles, 26 of which were eligible for analysis. They also included 16 studies from a previous systematic review and four studies identified through backward and forward citation searches. In all, 36 studies examined prevalent stroke (1.9 million participants), and 12 studies examined incident stroke (1.3 million participants). The studies were conducted in America, Europe, Asia, and Australia and included more than three million participants. Follow-up periods ranged from nine months to 25 years.
Stroke Affected Dementia Risk
When the researchers pooled results from 22 cohorts of participants who were cognitively normal at baseline, they found that those with prevalent stroke had a higher adjusted risk of incident dementia, compared with those without stroke (hazard ratio [HR], 1.69). Sensitivity analyses did not change the results significantly. Prevalent stroke was associated with a higher risk of incident dementia among men than among women. Sex explained 50.2% of heterogeneity between studies for prevalent stroke.
After combining the adjusted results from eight studies, Dr. Llewellyn and colleagues found that incident stroke more than doubled the risk of incident all-cause dementia, compared with no incident stroke (risk ratio [RR], 2.18). For a sensitivity analysis, the investigators excluded three studies that combined stroke with transient ischemic attack; this adjustment strengthened the association.
The study’s strengths include the investigators’ search of several major databases and their contacts with authors who provided relevant data. The analysis reflects the limitations of the original studies, however. These limitations include selective samples and differences in stroke assessment and dementia diagnosis criteria. In addition, dementia may develop years before it is diagnosed. “More detailed reporting of the interval between stroke occurrence and dementia diagnosis in future studies will help to better characterize the role of time since stroke in the risk of dementia,” said Dr. Llewellyn.
—Erik Greb
Kuz´ma E, Lourida I, Moore SF, et al. Stroke and dementia risk: a systematic review and meta-analysis. Alzheimers Dement. 2018 Aug 25 [Epub ahead of print].
Stroke is a strong independent risk factor for all-cause dementia, according to research published online ahead of print August 25 in Alzheimer’s & Dementia. Clinicians should incorporate stroke-prevention strategies into their health interventions to reduce patients’ risk of dementia, said the authors.
“Around a third of dementia cases are thought to be potentially preventable, though this estimate does not take into account the risk associated with stroke,” said David Llewellyn, PhD, Senior Research Fellow at University of Exeter Medical School in the United Kingdom. “Our findings indicate that this figure could be even higher and reinforce the importance of protecting the blood supply to the brain when attempting to reduce the global burden of dementia.”
Meta-Analysis of Previous Research
Stroke is a recognized risk factor for all-cause dementia, but no researchers had previously performed a meta-analysis to quantify the risk. Dr. Llewellyn and colleagues searched Medline, PsycINFO, and Embase databases for prospective studies that investigated the association between prevalent or incident stroke and incident all-cause dementia. They excluded studies that lacked a comparison group or that had a comparison group other than a stroke-free group. The investigators pooled adjusted estimates across studies using random effects meta-analysis and evaluated potential effect modifiers with meta-regression.
Dr. Llewellyn and colleagues identified 11,129 articles, 26 of which were eligible for analysis. They also included 16 studies from a previous systematic review and four studies identified through backward and forward citation searches. In all, 36 studies examined prevalent stroke (1.9 million participants), and 12 studies examined incident stroke (1.3 million participants). The studies were conducted in America, Europe, Asia, and Australia and included more than three million participants. Follow-up periods ranged from nine months to 25 years.
Stroke Affected Dementia Risk
When the researchers pooled results from 22 cohorts of participants who were cognitively normal at baseline, they found that those with prevalent stroke had a higher adjusted risk of incident dementia, compared with those without stroke (hazard ratio [HR], 1.69). Sensitivity analyses did not change the results significantly. Prevalent stroke was associated with a higher risk of incident dementia among men than among women. Sex explained 50.2% of heterogeneity between studies for prevalent stroke.
After combining the adjusted results from eight studies, Dr. Llewellyn and colleagues found that incident stroke more than doubled the risk of incident all-cause dementia, compared with no incident stroke (risk ratio [RR], 2.18). For a sensitivity analysis, the investigators excluded three studies that combined stroke with transient ischemic attack; this adjustment strengthened the association.
The study’s strengths include the investigators’ search of several major databases and their contacts with authors who provided relevant data. The analysis reflects the limitations of the original studies, however. These limitations include selective samples and differences in stroke assessment and dementia diagnosis criteria. In addition, dementia may develop years before it is diagnosed. “More detailed reporting of the interval between stroke occurrence and dementia diagnosis in future studies will help to better characterize the role of time since stroke in the risk of dementia,” said Dr. Llewellyn.
—Erik Greb
Kuz´ma E, Lourida I, Moore SF, et al. Stroke and dementia risk: a systematic review and meta-analysis. Alzheimers Dement. 2018 Aug 25 [Epub ahead of print].
Burden of dementia will shift more to minorities by 2060
, according to a study in Alzheimer’s and Dementia.
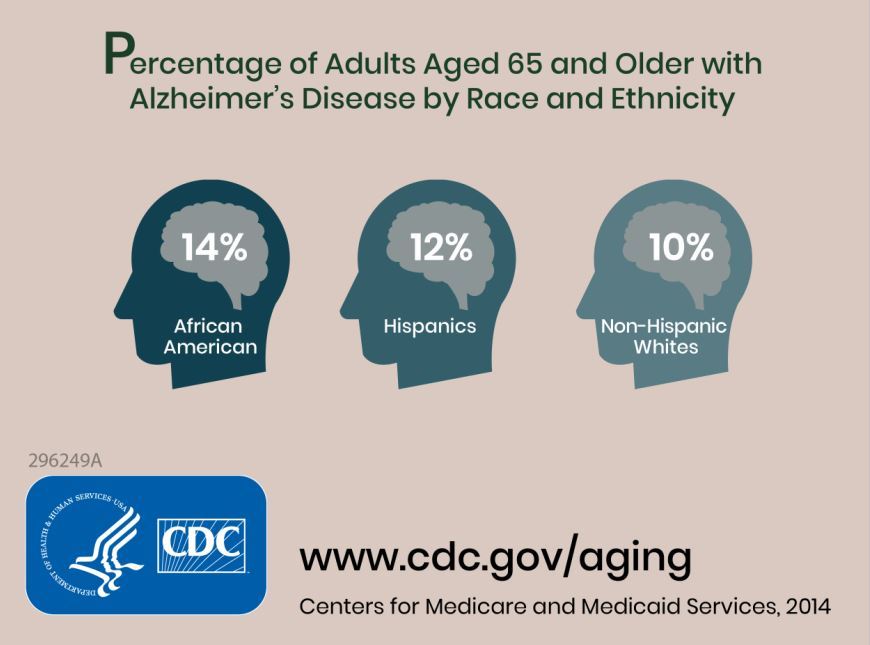
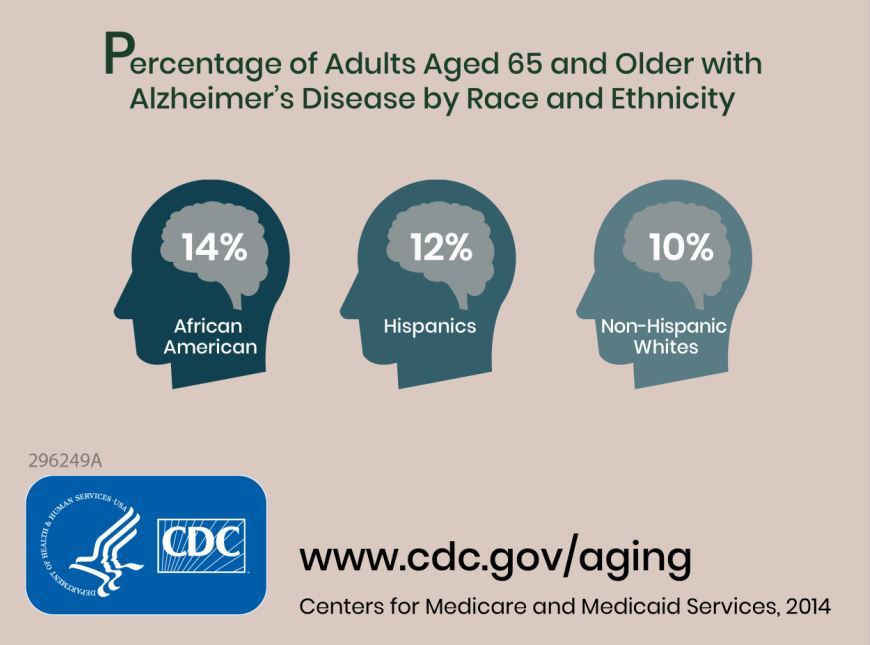
Prior to this study, no research had defined future estimates based on projected changes among demographic groups.
Researchers combined information about the prevalence of Alzheimer’s disease and related dementias (ADRD) by demographic group in 2014 Medicare-Fee-for-Service data with population projections data from the U.S. Census Bureau to assess how existing disparities by demographic group will change as those demographic groups become more or less represented in the U.S. population. They estimated the future prevalence of ADRD for 70 subgroups; these groups were defined by sex, seven racial and ethnic groups, and five age groups. The researchers estimated that in 2014 African Americans had the highest prevalence of ADRD at 13.8%, followed by Hispanics at 12.2%, non-Hispanic whites at 10.3%, American Indian and Alaska Natives at 9.1%, and Asian and Pacific Islanders at 8.4%.
The researchers estimated an overall increase from about 5.0 million people (1.9% of the U.S. population) in 2014 to about 13.9 million (3.3% of the population) in 2060. The non-Hispanic whites group will have the largest total number of cases of ADRD in 2060 because of its relative size, compared with other subgroups, going from about 3.7 million in 2014 to 7.1 million. The ADRD prevalence in non-Hispanic whites will begin to plateau around 2030, whereas the Hispanic population is expected to see the greatest increase, going from 430,000 in 2014 to 3.2 million in 2060.
Some of the limitations of the study include the assumption that the Medicare Fee-for-Service population is representative of the U.S. population and that these prevalences will remain constant over time.
“These estimates can be used for public health planning related to providing culturally competent care for the ADRD population and supporting caregivers from diverse backgrounds,” the researchers concluded.
SOURCE: Matthews KA et al. Alzheimers Dement. 2018 Sep 19. doi: 10.1016/j.jalz.2018.06.3063.
This article was updated 10/4/18.
, according to a study in Alzheimer’s and Dementia.

Prior to this study, no research had defined future estimates based on projected changes among demographic groups.
Researchers combined information about the prevalence of Alzheimer’s disease and related dementias (ADRD) by demographic group in 2014 Medicare-Fee-for-Service data with population projections data from the U.S. Census Bureau to assess how existing disparities by demographic group will change as those demographic groups become more or less represented in the U.S. population. They estimated the future prevalence of ADRD for 70 subgroups; these groups were defined by sex, seven racial and ethnic groups, and five age groups. The researchers estimated that in 2014 African Americans had the highest prevalence of ADRD at 13.8%, followed by Hispanics at 12.2%, non-Hispanic whites at 10.3%, American Indian and Alaska Natives at 9.1%, and Asian and Pacific Islanders at 8.4%.
The researchers estimated an overall increase from about 5.0 million people (1.9% of the U.S. population) in 2014 to about 13.9 million (3.3% of the population) in 2060. The non-Hispanic whites group will have the largest total number of cases of ADRD in 2060 because of its relative size, compared with other subgroups, going from about 3.7 million in 2014 to 7.1 million. The ADRD prevalence in non-Hispanic whites will begin to plateau around 2030, whereas the Hispanic population is expected to see the greatest increase, going from 430,000 in 2014 to 3.2 million in 2060.
Some of the limitations of the study include the assumption that the Medicare Fee-for-Service population is representative of the U.S. population and that these prevalences will remain constant over time.
“These estimates can be used for public health planning related to providing culturally competent care for the ADRD population and supporting caregivers from diverse backgrounds,” the researchers concluded.
SOURCE: Matthews KA et al. Alzheimers Dement. 2018 Sep 19. doi: 10.1016/j.jalz.2018.06.3063.
This article was updated 10/4/18.
, according to a study in Alzheimer’s and Dementia.

Prior to this study, no research had defined future estimates based on projected changes among demographic groups.
Researchers combined information about the prevalence of Alzheimer’s disease and related dementias (ADRD) by demographic group in 2014 Medicare-Fee-for-Service data with population projections data from the U.S. Census Bureau to assess how existing disparities by demographic group will change as those demographic groups become more or less represented in the U.S. population. They estimated the future prevalence of ADRD for 70 subgroups; these groups were defined by sex, seven racial and ethnic groups, and five age groups. The researchers estimated that in 2014 African Americans had the highest prevalence of ADRD at 13.8%, followed by Hispanics at 12.2%, non-Hispanic whites at 10.3%, American Indian and Alaska Natives at 9.1%, and Asian and Pacific Islanders at 8.4%.
The researchers estimated an overall increase from about 5.0 million people (1.9% of the U.S. population) in 2014 to about 13.9 million (3.3% of the population) in 2060. The non-Hispanic whites group will have the largest total number of cases of ADRD in 2060 because of its relative size, compared with other subgroups, going from about 3.7 million in 2014 to 7.1 million. The ADRD prevalence in non-Hispanic whites will begin to plateau around 2030, whereas the Hispanic population is expected to see the greatest increase, going from 430,000 in 2014 to 3.2 million in 2060.
Some of the limitations of the study include the assumption that the Medicare Fee-for-Service population is representative of the U.S. population and that these prevalences will remain constant over time.
“These estimates can be used for public health planning related to providing culturally competent care for the ADRD population and supporting caregivers from diverse backgrounds,” the researchers concluded.
SOURCE: Matthews KA et al. Alzheimers Dement. 2018 Sep 19. doi: 10.1016/j.jalz.2018.06.3063.
This article was updated 10/4/18.
FROM ALZHEIMER’S AND DEMENTIA
Tau PET tracer distinguishes Alzheimer’s from other disorders
PET imaging with an investigational tau tracer, 18F-flortaucipir, distinguished Alzheimer’s disease from other neurodegenerative disorders with a high degree of accuracy in a multicenter study.
PET with 18F-flortaucipir also outperformed structural MRI markers for Alzheimer’s disease (AD), and when compared against amyloid-beta (Abeta) biomarkers, showed higher specificity for an AD dementia diagnosis, researchers wrote in a report published online Sept. 18 in JAMA.
“The accuracy and potential utility of this test in patient care require further research in clinically more representative populations,” they wrote.
The study included 719 participants recruited from memory disorder clinics in Sweden, Korea, and California. That cohort included 179 patients with AD dementia, 254 who had non-AD neurodegenerative diseases, 126 with mild cognitive impairment (MCI), and 160 controls who were cognitively normal. All participants underwent 18F-flortaucipir PET between June 2014 and November 2017.
They found that 18F-flortaucipir showed “high accuracy” for distinguishing AD dementia from non-AD neurodegenerative disorders across all five regions of interest (ROI) evaluated in the study.
For example, 18F-flortaucipir PET had 90.3% accuracy, 89.9% sensitivity, and 90.6% specificity in the temporal meta-ROI, they reported.
By contrast, the accuracy of 18F-flortaucipir was lower for distinguishing MCI caused by AD versus non-AD neurodegenerative conditions. In the temporal meta-ROI, that translated into an 83.4% accuracy, 61.5% sensitivity, and 90.6% specificity, according to the report.
The 18F-flortaucipir tracer outperformed state-of-the-art MRI measures for distinguishing AD dementia from non-AD neurodegenerative disorders, including hippocampal volumes, AD-signature thickness, and whole-brain cortical thickness, Dr. Ossenkoppele and his colleagues found in a secondary analysis.
Likewise, certain analyses showed that specificity for AD dementia versus non-AD neurodegenerative disorders was higher with 18F-flortaucipir than with Abeta status.
While both MRI measures and Abeta status are increasingly used to evaluate cognitive impairment adjunctively in a clinical setting, the utility of 18F-flortaucipir PET as a diagnostic biomarker still must be determined, the investigators wrote in a discussion of their results.
“An intended clinical use of 18F-flortaucipir PET might be to improve the diagnostic work-up as an add-on test to Abeta biomarkers in patients with early-onset dementia and possibly as a triage or even replacement test in patients with late-onset dementia in whom incidental Abeta pathology is common,” they wrote.
The study was sponsored by different sources at each of the clinic sites. Many authors disclosed relationships with companies that are marketing and/or developing PET imaging tracers, including Avid Radiopharmaceuticals/Eli Lilly, GE Healthcare, and Piramal, as well as other pharmaceutical companies.
SOURCE: Ossenkoppele R et al. JAMA. 2018;320(11):1151-62.
PET imaging with an investigational tau tracer, 18F-flortaucipir, distinguished Alzheimer’s disease from other neurodegenerative disorders with a high degree of accuracy in a multicenter study.
PET with 18F-flortaucipir also outperformed structural MRI markers for Alzheimer’s disease (AD), and when compared against amyloid-beta (Abeta) biomarkers, showed higher specificity for an AD dementia diagnosis, researchers wrote in a report published online Sept. 18 in JAMA.
“The accuracy and potential utility of this test in patient care require further research in clinically more representative populations,” they wrote.
The study included 719 participants recruited from memory disorder clinics in Sweden, Korea, and California. That cohort included 179 patients with AD dementia, 254 who had non-AD neurodegenerative diseases, 126 with mild cognitive impairment (MCI), and 160 controls who were cognitively normal. All participants underwent 18F-flortaucipir PET between June 2014 and November 2017.
They found that 18F-flortaucipir showed “high accuracy” for distinguishing AD dementia from non-AD neurodegenerative disorders across all five regions of interest (ROI) evaluated in the study.
For example, 18F-flortaucipir PET had 90.3% accuracy, 89.9% sensitivity, and 90.6% specificity in the temporal meta-ROI, they reported.
By contrast, the accuracy of 18F-flortaucipir was lower for distinguishing MCI caused by AD versus non-AD neurodegenerative conditions. In the temporal meta-ROI, that translated into an 83.4% accuracy, 61.5% sensitivity, and 90.6% specificity, according to the report.
The 18F-flortaucipir tracer outperformed state-of-the-art MRI measures for distinguishing AD dementia from non-AD neurodegenerative disorders, including hippocampal volumes, AD-signature thickness, and whole-brain cortical thickness, Dr. Ossenkoppele and his colleagues found in a secondary analysis.
Likewise, certain analyses showed that specificity for AD dementia versus non-AD neurodegenerative disorders was higher with 18F-flortaucipir than with Abeta status.
While both MRI measures and Abeta status are increasingly used to evaluate cognitive impairment adjunctively in a clinical setting, the utility of 18F-flortaucipir PET as a diagnostic biomarker still must be determined, the investigators wrote in a discussion of their results.
“An intended clinical use of 18F-flortaucipir PET might be to improve the diagnostic work-up as an add-on test to Abeta biomarkers in patients with early-onset dementia and possibly as a triage or even replacement test in patients with late-onset dementia in whom incidental Abeta pathology is common,” they wrote.
The study was sponsored by different sources at each of the clinic sites. Many authors disclosed relationships with companies that are marketing and/or developing PET imaging tracers, including Avid Radiopharmaceuticals/Eli Lilly, GE Healthcare, and Piramal, as well as other pharmaceutical companies.
SOURCE: Ossenkoppele R et al. JAMA. 2018;320(11):1151-62.
PET imaging with an investigational tau tracer, 18F-flortaucipir, distinguished Alzheimer’s disease from other neurodegenerative disorders with a high degree of accuracy in a multicenter study.
PET with 18F-flortaucipir also outperformed structural MRI markers for Alzheimer’s disease (AD), and when compared against amyloid-beta (Abeta) biomarkers, showed higher specificity for an AD dementia diagnosis, researchers wrote in a report published online Sept. 18 in JAMA.
“The accuracy and potential utility of this test in patient care require further research in clinically more representative populations,” they wrote.
The study included 719 participants recruited from memory disorder clinics in Sweden, Korea, and California. That cohort included 179 patients with AD dementia, 254 who had non-AD neurodegenerative diseases, 126 with mild cognitive impairment (MCI), and 160 controls who were cognitively normal. All participants underwent 18F-flortaucipir PET between June 2014 and November 2017.
They found that 18F-flortaucipir showed “high accuracy” for distinguishing AD dementia from non-AD neurodegenerative disorders across all five regions of interest (ROI) evaluated in the study.
For example, 18F-flortaucipir PET had 90.3% accuracy, 89.9% sensitivity, and 90.6% specificity in the temporal meta-ROI, they reported.
By contrast, the accuracy of 18F-flortaucipir was lower for distinguishing MCI caused by AD versus non-AD neurodegenerative conditions. In the temporal meta-ROI, that translated into an 83.4% accuracy, 61.5% sensitivity, and 90.6% specificity, according to the report.
The 18F-flortaucipir tracer outperformed state-of-the-art MRI measures for distinguishing AD dementia from non-AD neurodegenerative disorders, including hippocampal volumes, AD-signature thickness, and whole-brain cortical thickness, Dr. Ossenkoppele and his colleagues found in a secondary analysis.
Likewise, certain analyses showed that specificity for AD dementia versus non-AD neurodegenerative disorders was higher with 18F-flortaucipir than with Abeta status.
While both MRI measures and Abeta status are increasingly used to evaluate cognitive impairment adjunctively in a clinical setting, the utility of 18F-flortaucipir PET as a diagnostic biomarker still must be determined, the investigators wrote in a discussion of their results.
“An intended clinical use of 18F-flortaucipir PET might be to improve the diagnostic work-up as an add-on test to Abeta biomarkers in patients with early-onset dementia and possibly as a triage or even replacement test in patients with late-onset dementia in whom incidental Abeta pathology is common,” they wrote.
The study was sponsored by different sources at each of the clinic sites. Many authors disclosed relationships with companies that are marketing and/or developing PET imaging tracers, including Avid Radiopharmaceuticals/Eli Lilly, GE Healthcare, and Piramal, as well as other pharmaceutical companies.
SOURCE: Ossenkoppele R et al. JAMA. 2018;320(11):1151-62.
FROM JAMA
Key clinical point:
Major finding: PET with 18F-flortaucipir PET had 90.3% accuracy, 89.9% sensitivity, and 90.6% specificity in the temporal metaregion of interest.
Study details: A multicenter, cross-sectional study including 719 participants recruited from memory disorder clinics in Sweden, Korea, and California.
Disclosures: The study was sponsored by different sources at each of the clinic sites. Many authors disclosed relationships with companies marketing and/or developing PET imaging tracers, including Avid Radiopharmaceuticals/Eli Lilly, GE Healthcare, and Piramal, as well as other pharmaceutical companies.
Source: Ossenkoppele R et al. JAMA. 2018;320(11):1151-62.
Pediatric hypertension linked to troubling MRI changes
CHICAGO – There’s another reason to worry about hypertension in children: cognitive decline later in life.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
In a pilot study, Marc Lande, MD, a professor of pediatric nephrology at the University of Rochester (N.Y.), and his colleagues found similar to what’s found in adults with cognitive impairment from hypertension.
The work is ongoing, but it helps explain the subtle deficits on cognitive testing that have been previously demonstrated in children with hypertension.
“The fact that we are finding anything at this very early stage of disease is striking and somewhat bothersome. The hope is that, by improving blood pressure in children, you can improve subsequent cognition and maybe even delay the onset of dementia further down the road,” Dr. Lande said.
For now, the findings underscore the need to diagnose and manage hypertension in children, but there might be additional treatment implications in the future, especially if blood pressure targets are found that ameliorate the problem.
Dr. Lande explained the issues and the emerging evidence in a video interview at the joint scientific sessions of the American Heart Association Council on Hypertension, AHA Council on Kidney in Cardiovascular Disease, and American Society of Hypertension.
CHICAGO – There’s another reason to worry about hypertension in children: cognitive decline later in life.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
In a pilot study, Marc Lande, MD, a professor of pediatric nephrology at the University of Rochester (N.Y.), and his colleagues found similar to what’s found in adults with cognitive impairment from hypertension.
The work is ongoing, but it helps explain the subtle deficits on cognitive testing that have been previously demonstrated in children with hypertension.
“The fact that we are finding anything at this very early stage of disease is striking and somewhat bothersome. The hope is that, by improving blood pressure in children, you can improve subsequent cognition and maybe even delay the onset of dementia further down the road,” Dr. Lande said.
For now, the findings underscore the need to diagnose and manage hypertension in children, but there might be additional treatment implications in the future, especially if blood pressure targets are found that ameliorate the problem.
Dr. Lande explained the issues and the emerging evidence in a video interview at the joint scientific sessions of the American Heart Association Council on Hypertension, AHA Council on Kidney in Cardiovascular Disease, and American Society of Hypertension.
CHICAGO – There’s another reason to worry about hypertension in children: cognitive decline later in life.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
In a pilot study, Marc Lande, MD, a professor of pediatric nephrology at the University of Rochester (N.Y.), and his colleagues found similar to what’s found in adults with cognitive impairment from hypertension.
The work is ongoing, but it helps explain the subtle deficits on cognitive testing that have been previously demonstrated in children with hypertension.
“The fact that we are finding anything at this very early stage of disease is striking and somewhat bothersome. The hope is that, by improving blood pressure in children, you can improve subsequent cognition and maybe even delay the onset of dementia further down the road,” Dr. Lande said.
For now, the findings underscore the need to diagnose and manage hypertension in children, but there might be additional treatment implications in the future, especially if blood pressure targets are found that ameliorate the problem.
Dr. Lande explained the issues and the emerging evidence in a video interview at the joint scientific sessions of the American Heart Association Council on Hypertension, AHA Council on Kidney in Cardiovascular Disease, and American Society of Hypertension.
REPORTING FROM JOINT HYPERTENSION 2018
Intensive Blood Pressure Management May Reduce the Risk of MCI
CHICAGO—Lowering systolic blood pressure to a target of 120 mm Hg or less in people with cardiovascular risk factors reduces the risk of mild cognitive impairment (MCI) by 19% and reduces the risk of probable all-cause dementia by 17%, compared with achieving a less intensive target of lower than 140 mm Hg, according to research presented at AAIC 2018.
The class of antihypertensive did not affect the association. Generic drugs were as effective as branded drugs. Antihypertensive agents provided equal benefits to men, women, whites, blacks, and Hispanics. Furthermore, maintaining systolic blood pressure at 120 mm Hg or lower prevented MCI as well in patients older than 75 as it did in younger patients.
Jeff D. Williamson, MD, Chief of Geriatric Medicine at Wake Forest University in Winston-Salem, North Carolina, presented the results of the four-year SPRINT MIND study. Strict blood pressure control (ie, a systolic target of 120 mm Hg or lower) for 3.2 years reduced the incidence of MCI to a greater extent than any amyloid-targeting investigational drug has done.
“This is the first disease-modifying strategy to reduce the risk of MCI,” said Dr. Williamson. Although the effect of strict blood pressure control on the primary end point (ie, a 17% risk reduction for probable all-cause dementia) was not statistically significant, “it is comforting to see that the benefit went in the same direction and was of the same magnitude,” said Dr. Williamson. “Three years of treatment and 3.2 years of follow-up absolutely reduced the risk.”
Brain imaging underscored the clinical importance of this finding and showed its physiologic pathway. Participants who underwent strict blood pressure control had 18% fewer white matter hyperintensities after four years of follow-up than other participants.
The results may represent a step forward in a field that has seen few of them recently. Generic antihypertensive agents can be inexpensive. They are widely available and confer benefits not only on cardiovascular health, but on kidney health as well, said Dr. Williamson.
“Hypertension is a highly prevalent condition…. The 19% overall risk reduction for MCI will have a huge impact,” he added.
“The most we can say right now is that we are able to reduce risk,” said Maria Carrillo, PhD, Chief Scientific Officer of the Alzheimer’s Association, in an interview. “Reducing the risk of MCI by 19% will have a huge impact on dementia overall. Slowing down the disease progress is a disease modification, versus developing symptoms. So, if that is the definition we are using, then I would say yes, it is disease modifying,” for dementias arising from cerebrovascular pathology.
A Substudy of the SPRINT Trial
SPRINT MIND was a substudy of the Hypertension Systolic Blood Pressure Intervention Trial (SPRINT). It compared two strategies for managing hypertension in older adults. The intensive strategy had a target systolic blood pressure of less than 120 mm Hg, and the standard care strategy had a target of less than 140 mm Hg. SPRINT showed that intensive blood pressure control reduced the risk of cardiovascular events, stroke, and cardiovascular death by 30%. The study results helped inform the 2017 American Heart Association and American College of Cardiology clinical guidelines for treating high blood pressure.
The SPRINT MIND substudy examined whether intensive blood pressure management affected the risk of probable all-cause dementia or MCI
The investigators examined data for 9,361 SPRINT subjects who were age 50 or older (mean age, 68) and had at least one cardiovascular risk factor. Approximately 30% of participants were black, and 10% were Hispanic. The primary outcome was incident probable dementia. Secondary outcomes were MCI and a composite of MCI and probable dementia.
In SPRINT, physicians could choose any appropriate antihypertensive regimen, but were encouraged to use drugs with the strongest evidence of cardiovascular benefit. These drugs included thiazide-type diuretics, loop diuretics, and beta-adrenergic blockers. About 90% of the drugs used during the study were generic.
Subjects were seen monthly for the first three months. During this time, medications were adjusted to achieve the target blood pressure. After the third month, subjects were examined every three months. Medications could be adjusted monthly.
Results Favored Intensive Treatment
At one year, mean systolic blood pressure was 121.4 mm Hg in the intensive-treatment group and 136.2 mm Hg in the standard treatment group. Treatment was stopped early after a median follow-up of 3.26 years because of the observed cardiovascular disease benefit.
The SPRINT MIND study did not meet its primary end point. Incident probable all-cause dementia occurred in 175 people in the standard care group and 147 people in the intensive treatment group. The difference between groups in the rate of 17% risk reduction was not statistically significant.
The results for both secondary end points were significant, however. Incident MCI occurred in 348 participants in the standard treatment group and 285 participants in the intensive treatment group. The difference indicated a statistically significant 19% risk reduction associated with intensive treatment. Furthermore, intensive treatment significantly reduced the risk of the combined secondary end point of MCI and probable dementia by 15%. In all, 463 participants in the standard care group met this end point, compared with 398 in the intensive care group.
The imaging study included 454 subjects who had brain MRI at baseline and at four years after randomization. Total brain volume did not change, said Ilya Nasrallah, MD, Assistant Professor of Radiology at the University of Pennsylvania in Philadelphia. Patients in the intensively managed group, however, had 18% lower white matter lesion load than those in the standard care group.
White matter lesions often indicate small-vessel disease, which is associated with vascular dementia and, perhaps, Alzheimer’s disease. Most patients with Alzheimer’s disease have a mixed dementia that includes a vascular component, said Dr. Carrillo.
The Gravity of MCI
SPRINT MIND did not follow subjects for longer than four years or include follow-up for amyloid positivity or Alzheimer’s disease diagnosis. Nevertheless, preventing MCI is a significant achievement, according to David Knopman, MD, a consultant in the department of neurology at Mayo Clinic in Rochester, Minnesota.
“There is nothing benign about MCI,” said Dr. Knopman. “It is the first sign of overt cognitive dysfunction, and although the rate at which MCI progresses to dementia is slow, the appearance of it is just as important as the appearance of more severe dementia. To be able to see an effect in 3.2 years is quite remarkable. I think this study is going to change clinical practice for people in primary care, and the benefits at the population level are going to be substantial.”
Physicians may want to think about how the SPRINT MIND results might apply to younger patients with hypertension, whether they have other cardiovascular risk factors or not, said Dr. Williamson. “I will adhere to the guidelines we have and keep blood pressure at less than 130 mm Hg and certainly start treating people in their 50s, and probably in their 40s,” he concluded.
—Michele G. Sullivan
Suggested Reading
SPRINT Research Group, Wright JT Jr, Williamson JD, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373(22):2103-2116.
CHICAGO—Lowering systolic blood pressure to a target of 120 mm Hg or less in people with cardiovascular risk factors reduces the risk of mild cognitive impairment (MCI) by 19% and reduces the risk of probable all-cause dementia by 17%, compared with achieving a less intensive target of lower than 140 mm Hg, according to research presented at AAIC 2018.
The class of antihypertensive did not affect the association. Generic drugs were as effective as branded drugs. Antihypertensive agents provided equal benefits to men, women, whites, blacks, and Hispanics. Furthermore, maintaining systolic blood pressure at 120 mm Hg or lower prevented MCI as well in patients older than 75 as it did in younger patients.
Jeff D. Williamson, MD, Chief of Geriatric Medicine at Wake Forest University in Winston-Salem, North Carolina, presented the results of the four-year SPRINT MIND study. Strict blood pressure control (ie, a systolic target of 120 mm Hg or lower) for 3.2 years reduced the incidence of MCI to a greater extent than any amyloid-targeting investigational drug has done.
“This is the first disease-modifying strategy to reduce the risk of MCI,” said Dr. Williamson. Although the effect of strict blood pressure control on the primary end point (ie, a 17% risk reduction for probable all-cause dementia) was not statistically significant, “it is comforting to see that the benefit went in the same direction and was of the same magnitude,” said Dr. Williamson. “Three years of treatment and 3.2 years of follow-up absolutely reduced the risk.”
Brain imaging underscored the clinical importance of this finding and showed its physiologic pathway. Participants who underwent strict blood pressure control had 18% fewer white matter hyperintensities after four years of follow-up than other participants.
The results may represent a step forward in a field that has seen few of them recently. Generic antihypertensive agents can be inexpensive. They are widely available and confer benefits not only on cardiovascular health, but on kidney health as well, said Dr. Williamson.
“Hypertension is a highly prevalent condition…. The 19% overall risk reduction for MCI will have a huge impact,” he added.
“The most we can say right now is that we are able to reduce risk,” said Maria Carrillo, PhD, Chief Scientific Officer of the Alzheimer’s Association, in an interview. “Reducing the risk of MCI by 19% will have a huge impact on dementia overall. Slowing down the disease progress is a disease modification, versus developing symptoms. So, if that is the definition we are using, then I would say yes, it is disease modifying,” for dementias arising from cerebrovascular pathology.
A Substudy of the SPRINT Trial
SPRINT MIND was a substudy of the Hypertension Systolic Blood Pressure Intervention Trial (SPRINT). It compared two strategies for managing hypertension in older adults. The intensive strategy had a target systolic blood pressure of less than 120 mm Hg, and the standard care strategy had a target of less than 140 mm Hg. SPRINT showed that intensive blood pressure control reduced the risk of cardiovascular events, stroke, and cardiovascular death by 30%. The study results helped inform the 2017 American Heart Association and American College of Cardiology clinical guidelines for treating high blood pressure.
The SPRINT MIND substudy examined whether intensive blood pressure management affected the risk of probable all-cause dementia or MCI
The investigators examined data for 9,361 SPRINT subjects who were age 50 or older (mean age, 68) and had at least one cardiovascular risk factor. Approximately 30% of participants were black, and 10% were Hispanic. The primary outcome was incident probable dementia. Secondary outcomes were MCI and a composite of MCI and probable dementia.
In SPRINT, physicians could choose any appropriate antihypertensive regimen, but were encouraged to use drugs with the strongest evidence of cardiovascular benefit. These drugs included thiazide-type diuretics, loop diuretics, and beta-adrenergic blockers. About 90% of the drugs used during the study were generic.
Subjects were seen monthly for the first three months. During this time, medications were adjusted to achieve the target blood pressure. After the third month, subjects were examined every three months. Medications could be adjusted monthly.
Results Favored Intensive Treatment
At one year, mean systolic blood pressure was 121.4 mm Hg in the intensive-treatment group and 136.2 mm Hg in the standard treatment group. Treatment was stopped early after a median follow-up of 3.26 years because of the observed cardiovascular disease benefit.
The SPRINT MIND study did not meet its primary end point. Incident probable all-cause dementia occurred in 175 people in the standard care group and 147 people in the intensive treatment group. The difference between groups in the rate of 17% risk reduction was not statistically significant.
The results for both secondary end points were significant, however. Incident MCI occurred in 348 participants in the standard treatment group and 285 participants in the intensive treatment group. The difference indicated a statistically significant 19% risk reduction associated with intensive treatment. Furthermore, intensive treatment significantly reduced the risk of the combined secondary end point of MCI and probable dementia by 15%. In all, 463 participants in the standard care group met this end point, compared with 398 in the intensive care group.
The imaging study included 454 subjects who had brain MRI at baseline and at four years after randomization. Total brain volume did not change, said Ilya Nasrallah, MD, Assistant Professor of Radiology at the University of Pennsylvania in Philadelphia. Patients in the intensively managed group, however, had 18% lower white matter lesion load than those in the standard care group.
White matter lesions often indicate small-vessel disease, which is associated with vascular dementia and, perhaps, Alzheimer’s disease. Most patients with Alzheimer’s disease have a mixed dementia that includes a vascular component, said Dr. Carrillo.
The Gravity of MCI
SPRINT MIND did not follow subjects for longer than four years or include follow-up for amyloid positivity or Alzheimer’s disease diagnosis. Nevertheless, preventing MCI is a significant achievement, according to David Knopman, MD, a consultant in the department of neurology at Mayo Clinic in Rochester, Minnesota.
“There is nothing benign about MCI,” said Dr. Knopman. “It is the first sign of overt cognitive dysfunction, and although the rate at which MCI progresses to dementia is slow, the appearance of it is just as important as the appearance of more severe dementia. To be able to see an effect in 3.2 years is quite remarkable. I think this study is going to change clinical practice for people in primary care, and the benefits at the population level are going to be substantial.”
Physicians may want to think about how the SPRINT MIND results might apply to younger patients with hypertension, whether they have other cardiovascular risk factors or not, said Dr. Williamson. “I will adhere to the guidelines we have and keep blood pressure at less than 130 mm Hg and certainly start treating people in their 50s, and probably in their 40s,” he concluded.
—Michele G. Sullivan
Suggested Reading
SPRINT Research Group, Wright JT Jr, Williamson JD, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373(22):2103-2116.
CHICAGO—Lowering systolic blood pressure to a target of 120 mm Hg or less in people with cardiovascular risk factors reduces the risk of mild cognitive impairment (MCI) by 19% and reduces the risk of probable all-cause dementia by 17%, compared with achieving a less intensive target of lower than 140 mm Hg, according to research presented at AAIC 2018.
The class of antihypertensive did not affect the association. Generic drugs were as effective as branded drugs. Antihypertensive agents provided equal benefits to men, women, whites, blacks, and Hispanics. Furthermore, maintaining systolic blood pressure at 120 mm Hg or lower prevented MCI as well in patients older than 75 as it did in younger patients.
Jeff D. Williamson, MD, Chief of Geriatric Medicine at Wake Forest University in Winston-Salem, North Carolina, presented the results of the four-year SPRINT MIND study. Strict blood pressure control (ie, a systolic target of 120 mm Hg or lower) for 3.2 years reduced the incidence of MCI to a greater extent than any amyloid-targeting investigational drug has done.
“This is the first disease-modifying strategy to reduce the risk of MCI,” said Dr. Williamson. Although the effect of strict blood pressure control on the primary end point (ie, a 17% risk reduction for probable all-cause dementia) was not statistically significant, “it is comforting to see that the benefit went in the same direction and was of the same magnitude,” said Dr. Williamson. “Three years of treatment and 3.2 years of follow-up absolutely reduced the risk.”
Brain imaging underscored the clinical importance of this finding and showed its physiologic pathway. Participants who underwent strict blood pressure control had 18% fewer white matter hyperintensities after four years of follow-up than other participants.
The results may represent a step forward in a field that has seen few of them recently. Generic antihypertensive agents can be inexpensive. They are widely available and confer benefits not only on cardiovascular health, but on kidney health as well, said Dr. Williamson.
“Hypertension is a highly prevalent condition…. The 19% overall risk reduction for MCI will have a huge impact,” he added.
“The most we can say right now is that we are able to reduce risk,” said Maria Carrillo, PhD, Chief Scientific Officer of the Alzheimer’s Association, in an interview. “Reducing the risk of MCI by 19% will have a huge impact on dementia overall. Slowing down the disease progress is a disease modification, versus developing symptoms. So, if that is the definition we are using, then I would say yes, it is disease modifying,” for dementias arising from cerebrovascular pathology.
A Substudy of the SPRINT Trial
SPRINT MIND was a substudy of the Hypertension Systolic Blood Pressure Intervention Trial (SPRINT). It compared two strategies for managing hypertension in older adults. The intensive strategy had a target systolic blood pressure of less than 120 mm Hg, and the standard care strategy had a target of less than 140 mm Hg. SPRINT showed that intensive blood pressure control reduced the risk of cardiovascular events, stroke, and cardiovascular death by 30%. The study results helped inform the 2017 American Heart Association and American College of Cardiology clinical guidelines for treating high blood pressure.
The SPRINT MIND substudy examined whether intensive blood pressure management affected the risk of probable all-cause dementia or MCI
The investigators examined data for 9,361 SPRINT subjects who were age 50 or older (mean age, 68) and had at least one cardiovascular risk factor. Approximately 30% of participants were black, and 10% were Hispanic. The primary outcome was incident probable dementia. Secondary outcomes were MCI and a composite of MCI and probable dementia.
In SPRINT, physicians could choose any appropriate antihypertensive regimen, but were encouraged to use drugs with the strongest evidence of cardiovascular benefit. These drugs included thiazide-type diuretics, loop diuretics, and beta-adrenergic blockers. About 90% of the drugs used during the study were generic.
Subjects were seen monthly for the first three months. During this time, medications were adjusted to achieve the target blood pressure. After the third month, subjects were examined every three months. Medications could be adjusted monthly.
Results Favored Intensive Treatment
At one year, mean systolic blood pressure was 121.4 mm Hg in the intensive-treatment group and 136.2 mm Hg in the standard treatment group. Treatment was stopped early after a median follow-up of 3.26 years because of the observed cardiovascular disease benefit.
The SPRINT MIND study did not meet its primary end point. Incident probable all-cause dementia occurred in 175 people in the standard care group and 147 people in the intensive treatment group. The difference between groups in the rate of 17% risk reduction was not statistically significant.
The results for both secondary end points were significant, however. Incident MCI occurred in 348 participants in the standard treatment group and 285 participants in the intensive treatment group. The difference indicated a statistically significant 19% risk reduction associated with intensive treatment. Furthermore, intensive treatment significantly reduced the risk of the combined secondary end point of MCI and probable dementia by 15%. In all, 463 participants in the standard care group met this end point, compared with 398 in the intensive care group.
The imaging study included 454 subjects who had brain MRI at baseline and at four years after randomization. Total brain volume did not change, said Ilya Nasrallah, MD, Assistant Professor of Radiology at the University of Pennsylvania in Philadelphia. Patients in the intensively managed group, however, had 18% lower white matter lesion load than those in the standard care group.
White matter lesions often indicate small-vessel disease, which is associated with vascular dementia and, perhaps, Alzheimer’s disease. Most patients with Alzheimer’s disease have a mixed dementia that includes a vascular component, said Dr. Carrillo.
The Gravity of MCI
SPRINT MIND did not follow subjects for longer than four years or include follow-up for amyloid positivity or Alzheimer’s disease diagnosis. Nevertheless, preventing MCI is a significant achievement, according to David Knopman, MD, a consultant in the department of neurology at Mayo Clinic in Rochester, Minnesota.
“There is nothing benign about MCI,” said Dr. Knopman. “It is the first sign of overt cognitive dysfunction, and although the rate at which MCI progresses to dementia is slow, the appearance of it is just as important as the appearance of more severe dementia. To be able to see an effect in 3.2 years is quite remarkable. I think this study is going to change clinical practice for people in primary care, and the benefits at the population level are going to be substantial.”
Physicians may want to think about how the SPRINT MIND results might apply to younger patients with hypertension, whether they have other cardiovascular risk factors or not, said Dr. Williamson. “I will adhere to the guidelines we have and keep blood pressure at less than 130 mm Hg and certainly start treating people in their 50s, and probably in their 40s,” he concluded.
—Michele G. Sullivan
Suggested Reading
SPRINT Research Group, Wright JT Jr, Williamson JD, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373(22):2103-2116.
Nabilone May Reduce Agitation in People With Alzheimer’s Disease
The treatment also improves behavioral symptoms such as delusions, hallucinations, anxiety, and apathy.
CHICAGO—Nabilone, a synthetic cannabinoid, may effectively treat agitation in people with Alzheimer’s disease, according to a randomized, double-blind clinical trial presented at AAIC 2018.
“Agitation, including verbal or physical outbursts, general emotional distress, restlessness, and pacing, is one of the most common behavioral changes associated with Alzheimer’s disease as it progresses and can be a significant cause of caregiver stress,” said Krista L. Lanctôt, PhD, Senior Scientist at Sunnybrook Health Sciences Centre and Professor of Pharmacology and Psychiatry at the University of Toronto.
Dr. Lanctôt and colleagues investigated the potential benefits of nabilone for adults with moderate-to-severe Alzheimer’s dementia and clinically significant agitation. During the 14-week trial, 39 participants (77% male; average age, 87) received nabilone in capsule form (mean therapeutic dose, 1.6 mg) for six weeks, followed by one week without treatment and six weeks of placebo. In addition to measuring agitation, the researchers assessed overall behavioral symptoms, memory, physical changes, and safety.
Dr. Lanctôt’s group found that agitation improved significantly when participants were taking nabilone, compared with when they were receiving placebo, as measured by the Cohen-Mansfield Agitation Inventory. Nabilone also significantly improved overall behavioral symptoms, compared with placebo, as measured by the Neuropsychiatric Inventory.
In addition, the researchers observed small benefits in cognition and nutrition when participants received nabilone during the study. More people in the study experienced sedation when taking nabilone (45%) than when taking placebo (16%).
“Currently prescribed treatments for agitation in Alzheimer’s disease do not work in everybody. And when they do work, the effect is small, and they increase the risk of harmful side effects, including increased risk of death. As a result, there is an urgent need for safer medication options,” said Dr. Lanctôt. “These findings suggest that nabilone may be an effective treatment for agitation; however, the risk of sedation must be carefully monitored. A larger clinical trial would allow us to confirm our findings regarding how effective and safe nabilone is in the treatment of agitation for Alzheimer’s disease.”The FDA has not approved marijuana for the treatment or management of Alzheimer’s disease or other dementias. The use of marijuana as medical treatment is increasingly common, but much about the drug’s use in people with Alzheimer’s disease or other dementias is unknown. No robust, consistent clinical trial data support marijuana for the treatment of Alzheimer’s disease dementia or for related issues.
The treatment also improves behavioral symptoms such as delusions, hallucinations, anxiety, and apathy.
The treatment also improves behavioral symptoms such as delusions, hallucinations, anxiety, and apathy.
CHICAGO—Nabilone, a synthetic cannabinoid, may effectively treat agitation in people with Alzheimer’s disease, according to a randomized, double-blind clinical trial presented at AAIC 2018.
“Agitation, including verbal or physical outbursts, general emotional distress, restlessness, and pacing, is one of the most common behavioral changes associated with Alzheimer’s disease as it progresses and can be a significant cause of caregiver stress,” said Krista L. Lanctôt, PhD, Senior Scientist at Sunnybrook Health Sciences Centre and Professor of Pharmacology and Psychiatry at the University of Toronto.
Dr. Lanctôt and colleagues investigated the potential benefits of nabilone for adults with moderate-to-severe Alzheimer’s dementia and clinically significant agitation. During the 14-week trial, 39 participants (77% male; average age, 87) received nabilone in capsule form (mean therapeutic dose, 1.6 mg) for six weeks, followed by one week without treatment and six weeks of placebo. In addition to measuring agitation, the researchers assessed overall behavioral symptoms, memory, physical changes, and safety.
Dr. Lanctôt’s group found that agitation improved significantly when participants were taking nabilone, compared with when they were receiving placebo, as measured by the Cohen-Mansfield Agitation Inventory. Nabilone also significantly improved overall behavioral symptoms, compared with placebo, as measured by the Neuropsychiatric Inventory.
In addition, the researchers observed small benefits in cognition and nutrition when participants received nabilone during the study. More people in the study experienced sedation when taking nabilone (45%) than when taking placebo (16%).
“Currently prescribed treatments for agitation in Alzheimer’s disease do not work in everybody. And when they do work, the effect is small, and they increase the risk of harmful side effects, including increased risk of death. As a result, there is an urgent need for safer medication options,” said Dr. Lanctôt. “These findings suggest that nabilone may be an effective treatment for agitation; however, the risk of sedation must be carefully monitored. A larger clinical trial would allow us to confirm our findings regarding how effective and safe nabilone is in the treatment of agitation for Alzheimer’s disease.”The FDA has not approved marijuana for the treatment or management of Alzheimer’s disease or other dementias. The use of marijuana as medical treatment is increasingly common, but much about the drug’s use in people with Alzheimer’s disease or other dementias is unknown. No robust, consistent clinical trial data support marijuana for the treatment of Alzheimer’s disease dementia or for related issues.
CHICAGO—Nabilone, a synthetic cannabinoid, may effectively treat agitation in people with Alzheimer’s disease, according to a randomized, double-blind clinical trial presented at AAIC 2018.
“Agitation, including verbal or physical outbursts, general emotional distress, restlessness, and pacing, is one of the most common behavioral changes associated with Alzheimer’s disease as it progresses and can be a significant cause of caregiver stress,” said Krista L. Lanctôt, PhD, Senior Scientist at Sunnybrook Health Sciences Centre and Professor of Pharmacology and Psychiatry at the University of Toronto.
Dr. Lanctôt and colleagues investigated the potential benefits of nabilone for adults with moderate-to-severe Alzheimer’s dementia and clinically significant agitation. During the 14-week trial, 39 participants (77% male; average age, 87) received nabilone in capsule form (mean therapeutic dose, 1.6 mg) for six weeks, followed by one week without treatment and six weeks of placebo. In addition to measuring agitation, the researchers assessed overall behavioral symptoms, memory, physical changes, and safety.
Dr. Lanctôt’s group found that agitation improved significantly when participants were taking nabilone, compared with when they were receiving placebo, as measured by the Cohen-Mansfield Agitation Inventory. Nabilone also significantly improved overall behavioral symptoms, compared with placebo, as measured by the Neuropsychiatric Inventory.
In addition, the researchers observed small benefits in cognition and nutrition when participants received nabilone during the study. More people in the study experienced sedation when taking nabilone (45%) than when taking placebo (16%).
“Currently prescribed treatments for agitation in Alzheimer’s disease do not work in everybody. And when they do work, the effect is small, and they increase the risk of harmful side effects, including increased risk of death. As a result, there is an urgent need for safer medication options,” said Dr. Lanctôt. “These findings suggest that nabilone may be an effective treatment for agitation; however, the risk of sedation must be carefully monitored. A larger clinical trial would allow us to confirm our findings regarding how effective and safe nabilone is in the treatment of agitation for Alzheimer’s disease.”The FDA has not approved marijuana for the treatment or management of Alzheimer’s disease or other dementias. The use of marijuana as medical treatment is increasingly common, but much about the drug’s use in people with Alzheimer’s disease or other dementias is unknown. No robust, consistent clinical trial data support marijuana for the treatment of Alzheimer’s disease dementia or for related issues.
Pregnancy and Years of Reproductive Capability Are Associated With Dementia Risk
Miscarriages and age at menarche and menopause may influence the likelihood of dementia.
CHICAGO—More pregnancies and a longer span of reproductive years appear to protect women against dementia, according to a study presented at AAIC 2018. The results suggest that lifetime estrogen exposure may be an important modulator of long-term cognitive health.
The study by Paola Gilsanz, ScD, staff scientist at Kaiser Permanente in Oakland, California, and colleagues included more than 14,500 women and 50 years of follow-up data. Earlier age of menarche, later menopause, and more completed pregnancies all independently reduced the risk of dementia in women.
Reproductive Years
The researchers analyzed data from a cohort of 14,595 women in the Kaiser Permanente health care database. All of them had completed a comprehensive health checkup between 1964 and 1973 when they were ages 40 to 55. They reported their number of miscarriages, number of children, ages at first and last menstrual period, and the total number of years in their reproductive period. Most of the women in the group (68%) were white, but 16% were black, 6% Asian, and 5% Hispanic. Dr. Gilsanz looked at rates of dementia during 1996 to 2017, when the women were ages 62 to 86.
A multivariate regression model controlled for age, race, education, midlife health issues (eg, hypertension, smoking, and BMI), hysterectomy, and late-life health issues (eg, stroke, heart failure, and diabetes).
Half of the cohort had at least three children, and 75% had at least one miscarriage. The average age at menarche was 13, and the average age at last natural menstrual period was 47. This equated to an average reproductive period of 34 years.
At the end of follow-up, 36% of the cohort had developed dementia.
Women with at least three children were 12% less likely to develop dementia, compared with those with one child. The association remained significant even after researchers controlled for age, race, education, and hysterectomy.
Miscarriages also influenced the risk of dementia. Those who did not report a miscarriage were 20% less likely to develop dementia than were those who had experienced at least one miscarriage. The benefit of no miscarriage was greater among women with at least three children, conferring a 28% reduced risk.
A shorter reproductive period increased the risk of dementia. Those who experienced menarche at age 16 or older had a 31% increased risk of dementia, and those who experienced their last period at age 45 or younger had a 28% greater risk. Each additional year of reproductive capability was associated with a 2% decreased risk.
Women with between 21 and 30 reproductive years were 33% more likely to develop dementia than were those with longer reproductive periods.
Renewed Interest
“Reproductive events that signal different exposures to estrogen, like pregnancy and reproductive period, may play a role in modulating dementia risk,” Dr. Gilsanz said. “Women who are less likely to have a miscarriage may have different hormonal milieus that may be neuroprotective. Underlying health conditions increasing the risk of miscarriages may also elevate risk of dementia.”
Researchers are exploring the link between hormones and cognition with renewed interest, said Suzanne Craft, PhD, of Wake Forest University in Winston-Salem, North Carolina, who moderated a press briefing on the topic. The Women’s Heath Initiative study had a chilling effect on funding for this area of research, Dr. Craft said. “But now I think the pendulum is slowly moving back” toward supporting investigations of hormones and cognition. “It’s clear that something is going on, that there is a link. I am glad we are starting to explore this again.”
—Michele G. Sullivan
Miscarriages and age at menarche and menopause may influence the likelihood of dementia.
Miscarriages and age at menarche and menopause may influence the likelihood of dementia.
CHICAGO—More pregnancies and a longer span of reproductive years appear to protect women against dementia, according to a study presented at AAIC 2018. The results suggest that lifetime estrogen exposure may be an important modulator of long-term cognitive health.
The study by Paola Gilsanz, ScD, staff scientist at Kaiser Permanente in Oakland, California, and colleagues included more than 14,500 women and 50 years of follow-up data. Earlier age of menarche, later menopause, and more completed pregnancies all independently reduced the risk of dementia in women.
Reproductive Years
The researchers analyzed data from a cohort of 14,595 women in the Kaiser Permanente health care database. All of them had completed a comprehensive health checkup between 1964 and 1973 when they were ages 40 to 55. They reported their number of miscarriages, number of children, ages at first and last menstrual period, and the total number of years in their reproductive period. Most of the women in the group (68%) were white, but 16% were black, 6% Asian, and 5% Hispanic. Dr. Gilsanz looked at rates of dementia during 1996 to 2017, when the women were ages 62 to 86.
A multivariate regression model controlled for age, race, education, midlife health issues (eg, hypertension, smoking, and BMI), hysterectomy, and late-life health issues (eg, stroke, heart failure, and diabetes).
Half of the cohort had at least three children, and 75% had at least one miscarriage. The average age at menarche was 13, and the average age at last natural menstrual period was 47. This equated to an average reproductive period of 34 years.
At the end of follow-up, 36% of the cohort had developed dementia.
Women with at least three children were 12% less likely to develop dementia, compared with those with one child. The association remained significant even after researchers controlled for age, race, education, and hysterectomy.
Miscarriages also influenced the risk of dementia. Those who did not report a miscarriage were 20% less likely to develop dementia than were those who had experienced at least one miscarriage. The benefit of no miscarriage was greater among women with at least three children, conferring a 28% reduced risk.
A shorter reproductive period increased the risk of dementia. Those who experienced menarche at age 16 or older had a 31% increased risk of dementia, and those who experienced their last period at age 45 or younger had a 28% greater risk. Each additional year of reproductive capability was associated with a 2% decreased risk.
Women with between 21 and 30 reproductive years were 33% more likely to develop dementia than were those with longer reproductive periods.
Renewed Interest
“Reproductive events that signal different exposures to estrogen, like pregnancy and reproductive period, may play a role in modulating dementia risk,” Dr. Gilsanz said. “Women who are less likely to have a miscarriage may have different hormonal milieus that may be neuroprotective. Underlying health conditions increasing the risk of miscarriages may also elevate risk of dementia.”
Researchers are exploring the link between hormones and cognition with renewed interest, said Suzanne Craft, PhD, of Wake Forest University in Winston-Salem, North Carolina, who moderated a press briefing on the topic. The Women’s Heath Initiative study had a chilling effect on funding for this area of research, Dr. Craft said. “But now I think the pendulum is slowly moving back” toward supporting investigations of hormones and cognition. “It’s clear that something is going on, that there is a link. I am glad we are starting to explore this again.”
—Michele G. Sullivan
CHICAGO—More pregnancies and a longer span of reproductive years appear to protect women against dementia, according to a study presented at AAIC 2018. The results suggest that lifetime estrogen exposure may be an important modulator of long-term cognitive health.
The study by Paola Gilsanz, ScD, staff scientist at Kaiser Permanente in Oakland, California, and colleagues included more than 14,500 women and 50 years of follow-up data. Earlier age of menarche, later menopause, and more completed pregnancies all independently reduced the risk of dementia in women.
Reproductive Years
The researchers analyzed data from a cohort of 14,595 women in the Kaiser Permanente health care database. All of them had completed a comprehensive health checkup between 1964 and 1973 when they were ages 40 to 55. They reported their number of miscarriages, number of children, ages at first and last menstrual period, and the total number of years in their reproductive period. Most of the women in the group (68%) were white, but 16% were black, 6% Asian, and 5% Hispanic. Dr. Gilsanz looked at rates of dementia during 1996 to 2017, when the women were ages 62 to 86.
A multivariate regression model controlled for age, race, education, midlife health issues (eg, hypertension, smoking, and BMI), hysterectomy, and late-life health issues (eg, stroke, heart failure, and diabetes).
Half of the cohort had at least three children, and 75% had at least one miscarriage. The average age at menarche was 13, and the average age at last natural menstrual period was 47. This equated to an average reproductive period of 34 years.
At the end of follow-up, 36% of the cohort had developed dementia.
Women with at least three children were 12% less likely to develop dementia, compared with those with one child. The association remained significant even after researchers controlled for age, race, education, and hysterectomy.
Miscarriages also influenced the risk of dementia. Those who did not report a miscarriage were 20% less likely to develop dementia than were those who had experienced at least one miscarriage. The benefit of no miscarriage was greater among women with at least three children, conferring a 28% reduced risk.
A shorter reproductive period increased the risk of dementia. Those who experienced menarche at age 16 or older had a 31% increased risk of dementia, and those who experienced their last period at age 45 or younger had a 28% greater risk. Each additional year of reproductive capability was associated with a 2% decreased risk.
Women with between 21 and 30 reproductive years were 33% more likely to develop dementia than were those with longer reproductive periods.
Renewed Interest
“Reproductive events that signal different exposures to estrogen, like pregnancy and reproductive period, may play a role in modulating dementia risk,” Dr. Gilsanz said. “Women who are less likely to have a miscarriage may have different hormonal milieus that may be neuroprotective. Underlying health conditions increasing the risk of miscarriages may also elevate risk of dementia.”
Researchers are exploring the link between hormones and cognition with renewed interest, said Suzanne Craft, PhD, of Wake Forest University in Winston-Salem, North Carolina, who moderated a press briefing on the topic. The Women’s Heath Initiative study had a chilling effect on funding for this area of research, Dr. Craft said. “But now I think the pendulum is slowly moving back” toward supporting investigations of hormones and cognition. “It’s clear that something is going on, that there is a link. I am glad we are starting to explore this again.”
—Michele G. Sullivan
Does BAN2401 Benefit Patients With Alzheimer’s Disease?
The study arms did not contain comparable numbers of patients who carried APOE4.
CHICAGO—BAN2401, a monoclonal antibody that targets soluble amyloid-beta oligomers, slows cognitive decline by as much as 47% and clears brain amyloid in 81% of patients with mild cognitive impairment (MCI) and very mild Alzheimer’s disease, according to the results of a phase II study presented at AAIC 2018.
Imbalanced Treatment Groups
The treatment groups were not well balanced in at least one respect, however, which could influence the interpretation of the data. APOE4-positive patients were unequally distributed through the six treatment groups, which included BAN2401 (2.5 mg/kg biweekly, 5 mg/kg monthly, 5 mg/kg biweekly, 10 mg/kg monthly, and 10 mg/kg biweekly) and placebo. This imbalance could have biased cognitive results in the antibody’s favor. APOE4 carriers represented between 70% and 80% of every unsuccessful treatment arm in the trial and approximately 29% of the arm that enjoyed significant cognitive benefits.
“This is a big confound,” said Keith Fargo, PhD, Director of Scientific Programs and Outreach at the Alzheimer’s Association. “According to the trial sponsors, … a regulatory body requested that people with the APOE4 Alzheimer’s risk gene not be included in the highest dose group for safety reasons. Because of this [request], the people in the highest-dose group are different from people in the other groups on an important dimension: they were much less likely to have the APOE4 gene, which is known to be a major risk factor for cognitive decline. So, it is plausible that the people on the highest dose declined differently due to genetic differences, rather than due to being on the highest dose. A planned subgroup analysis will shed more light on this [question] and [on] whether it reduces confidence in the overall findings.”
Regulators Influenced the Trial
Eisai, which is headquartered in Tokyo, and Biogen, which is based in Cambridge, Massachusetts, are developing the antibody. The decision to restructure the randomization was not Eisai’s, according to David Knopman, MD, a consultant in the department of neurology at Mayo Clinic in Rochester, Minnesota.
“European regulators did not allow randomization of [some] APOE4 carriers to the highest dose,” he said in an interview. “I ultimately don’t know what it would do to the results except make them even more difficult to justify as sufficient for registration. In general, in symptomatic Alzheimer’s dementia patients, APOE4 carriage has no substantial impact on rate of decline, but whether [APOE4] status interacted with the treatment is of course completely unknown. Bottom line: just another feature that makes this a phase II study that needs to be followed by a phase III study with a simple design using the high dose.”
“Regardless of who made the decision and why, the data is what it is, and the question remains,” said Michael S. Wolfe, PhD, the Mathias P. Mertes Professor of Medicinal Chemistry at the University of Kansas in Lawrence. “Given the numbers of E4-positive [patients] versus E4-negative [patients] for each treatment group, the interpretation of the results is now seriously thrown into question. The one group that showed a clear slowing of cognitive decline versus placebo—10 mg/kg biweekly—also has far fewer E4-positive [patients] versus E4-negative [patients]. This difference in proportion of E4-positive [patients] could be a major factor in the apparent reduced rate of cognitive decline and confounds the ability to tell if the 10-mg/kg biweekly dose is effective. In contrast, the 10-mg/kg monthly dose group has an E4-positive to E4-negative ratio more comparable to that of placebo, and the effect of the drug on cognitive decline under this dosing regimen is not clearly distinguishable from that seen with placebo.”
Treatment Reduced Amyloid Levels
In addition, BAN2401 failed to meet its 12-month prespecified primary cognitive end points. The investigators reached this conclusion through Bayesian analysis that strove to predict an 80% probability of achieving at least a 25% cognitive benefit. In December 2017, Eisai announced that the treatment had reached 64% probability. Because the company was optimistic about the treatment’s success, it continued with the additional six months of treatment, as the study design allowed, and reanalyzed results with a simpler and more straightforward method. This analysis concluded that the 10-mg/kg biweekly dose slowed decline on the Alzheimer’s Disease Composite Score (ADCOMS), a new tool developed and promoted by Eisai, by 30%. It also found that treatment slowed decline on the Alzheimer’s Disease Assessment Scale-cognitive subscale (ADAS-Cog) by 47%. Treatment cleared brain amyloid in 81% of subjects and had positive effects on CSF biomarkers. CSF amyloid levels increased, and CSF total tau levels decreased, which indicated decreased neuronal injury.
—Michele G. Sullivan
The study arms did not contain comparable numbers of patients who carried APOE4.
The study arms did not contain comparable numbers of patients who carried APOE4.
CHICAGO—BAN2401, a monoclonal antibody that targets soluble amyloid-beta oligomers, slows cognitive decline by as much as 47% and clears brain amyloid in 81% of patients with mild cognitive impairment (MCI) and very mild Alzheimer’s disease, according to the results of a phase II study presented at AAIC 2018.
Imbalanced Treatment Groups
The treatment groups were not well balanced in at least one respect, however, which could influence the interpretation of the data. APOE4-positive patients were unequally distributed through the six treatment groups, which included BAN2401 (2.5 mg/kg biweekly, 5 mg/kg monthly, 5 mg/kg biweekly, 10 mg/kg monthly, and 10 mg/kg biweekly) and placebo. This imbalance could have biased cognitive results in the antibody’s favor. APOE4 carriers represented between 70% and 80% of every unsuccessful treatment arm in the trial and approximately 29% of the arm that enjoyed significant cognitive benefits.
“This is a big confound,” said Keith Fargo, PhD, Director of Scientific Programs and Outreach at the Alzheimer’s Association. “According to the trial sponsors, … a regulatory body requested that people with the APOE4 Alzheimer’s risk gene not be included in the highest dose group for safety reasons. Because of this [request], the people in the highest-dose group are different from people in the other groups on an important dimension: they were much less likely to have the APOE4 gene, which is known to be a major risk factor for cognitive decline. So, it is plausible that the people on the highest dose declined differently due to genetic differences, rather than due to being on the highest dose. A planned subgroup analysis will shed more light on this [question] and [on] whether it reduces confidence in the overall findings.”
Regulators Influenced the Trial
Eisai, which is headquartered in Tokyo, and Biogen, which is based in Cambridge, Massachusetts, are developing the antibody. The decision to restructure the randomization was not Eisai’s, according to David Knopman, MD, a consultant in the department of neurology at Mayo Clinic in Rochester, Minnesota.
“European regulators did not allow randomization of [some] APOE4 carriers to the highest dose,” he said in an interview. “I ultimately don’t know what it would do to the results except make them even more difficult to justify as sufficient for registration. In general, in symptomatic Alzheimer’s dementia patients, APOE4 carriage has no substantial impact on rate of decline, but whether [APOE4] status interacted with the treatment is of course completely unknown. Bottom line: just another feature that makes this a phase II study that needs to be followed by a phase III study with a simple design using the high dose.”
“Regardless of who made the decision and why, the data is what it is, and the question remains,” said Michael S. Wolfe, PhD, the Mathias P. Mertes Professor of Medicinal Chemistry at the University of Kansas in Lawrence. “Given the numbers of E4-positive [patients] versus E4-negative [patients] for each treatment group, the interpretation of the results is now seriously thrown into question. The one group that showed a clear slowing of cognitive decline versus placebo—10 mg/kg biweekly—also has far fewer E4-positive [patients] versus E4-negative [patients]. This difference in proportion of E4-positive [patients] could be a major factor in the apparent reduced rate of cognitive decline and confounds the ability to tell if the 10-mg/kg biweekly dose is effective. In contrast, the 10-mg/kg monthly dose group has an E4-positive to E4-negative ratio more comparable to that of placebo, and the effect of the drug on cognitive decline under this dosing regimen is not clearly distinguishable from that seen with placebo.”
Treatment Reduced Amyloid Levels
In addition, BAN2401 failed to meet its 12-month prespecified primary cognitive end points. The investigators reached this conclusion through Bayesian analysis that strove to predict an 80% probability of achieving at least a 25% cognitive benefit. In December 2017, Eisai announced that the treatment had reached 64% probability. Because the company was optimistic about the treatment’s success, it continued with the additional six months of treatment, as the study design allowed, and reanalyzed results with a simpler and more straightforward method. This analysis concluded that the 10-mg/kg biweekly dose slowed decline on the Alzheimer’s Disease Composite Score (ADCOMS), a new tool developed and promoted by Eisai, by 30%. It also found that treatment slowed decline on the Alzheimer’s Disease Assessment Scale-cognitive subscale (ADAS-Cog) by 47%. Treatment cleared brain amyloid in 81% of subjects and had positive effects on CSF biomarkers. CSF amyloid levels increased, and CSF total tau levels decreased, which indicated decreased neuronal injury.
—Michele G. Sullivan
CHICAGO—BAN2401, a monoclonal antibody that targets soluble amyloid-beta oligomers, slows cognitive decline by as much as 47% and clears brain amyloid in 81% of patients with mild cognitive impairment (MCI) and very mild Alzheimer’s disease, according to the results of a phase II study presented at AAIC 2018.
Imbalanced Treatment Groups
The treatment groups were not well balanced in at least one respect, however, which could influence the interpretation of the data. APOE4-positive patients were unequally distributed through the six treatment groups, which included BAN2401 (2.5 mg/kg biweekly, 5 mg/kg monthly, 5 mg/kg biweekly, 10 mg/kg monthly, and 10 mg/kg biweekly) and placebo. This imbalance could have biased cognitive results in the antibody’s favor. APOE4 carriers represented between 70% and 80% of every unsuccessful treatment arm in the trial and approximately 29% of the arm that enjoyed significant cognitive benefits.
“This is a big confound,” said Keith Fargo, PhD, Director of Scientific Programs and Outreach at the Alzheimer’s Association. “According to the trial sponsors, … a regulatory body requested that people with the APOE4 Alzheimer’s risk gene not be included in the highest dose group for safety reasons. Because of this [request], the people in the highest-dose group are different from people in the other groups on an important dimension: they were much less likely to have the APOE4 gene, which is known to be a major risk factor for cognitive decline. So, it is plausible that the people on the highest dose declined differently due to genetic differences, rather than due to being on the highest dose. A planned subgroup analysis will shed more light on this [question] and [on] whether it reduces confidence in the overall findings.”
Regulators Influenced the Trial
Eisai, which is headquartered in Tokyo, and Biogen, which is based in Cambridge, Massachusetts, are developing the antibody. The decision to restructure the randomization was not Eisai’s, according to David Knopman, MD, a consultant in the department of neurology at Mayo Clinic in Rochester, Minnesota.
“European regulators did not allow randomization of [some] APOE4 carriers to the highest dose,” he said in an interview. “I ultimately don’t know what it would do to the results except make them even more difficult to justify as sufficient for registration. In general, in symptomatic Alzheimer’s dementia patients, APOE4 carriage has no substantial impact on rate of decline, but whether [APOE4] status interacted with the treatment is of course completely unknown. Bottom line: just another feature that makes this a phase II study that needs to be followed by a phase III study with a simple design using the high dose.”
“Regardless of who made the decision and why, the data is what it is, and the question remains,” said Michael S. Wolfe, PhD, the Mathias P. Mertes Professor of Medicinal Chemistry at the University of Kansas in Lawrence. “Given the numbers of E4-positive [patients] versus E4-negative [patients] for each treatment group, the interpretation of the results is now seriously thrown into question. The one group that showed a clear slowing of cognitive decline versus placebo—10 mg/kg biweekly—also has far fewer E4-positive [patients] versus E4-negative [patients]. This difference in proportion of E4-positive [patients] could be a major factor in the apparent reduced rate of cognitive decline and confounds the ability to tell if the 10-mg/kg biweekly dose is effective. In contrast, the 10-mg/kg monthly dose group has an E4-positive to E4-negative ratio more comparable to that of placebo, and the effect of the drug on cognitive decline under this dosing regimen is not clearly distinguishable from that seen with placebo.”
Treatment Reduced Amyloid Levels
In addition, BAN2401 failed to meet its 12-month prespecified primary cognitive end points. The investigators reached this conclusion through Bayesian analysis that strove to predict an 80% probability of achieving at least a 25% cognitive benefit. In December 2017, Eisai announced that the treatment had reached 64% probability. Because the company was optimistic about the treatment’s success, it continued with the additional six months of treatment, as the study design allowed, and reanalyzed results with a simpler and more straightforward method. This analysis concluded that the 10-mg/kg biweekly dose slowed decline on the Alzheimer’s Disease Composite Score (ADCOMS), a new tool developed and promoted by Eisai, by 30%. It also found that treatment slowed decline on the Alzheimer’s Disease Assessment Scale-cognitive subscale (ADAS-Cog) by 47%. Treatment cleared brain amyloid in 81% of subjects and had positive effects on CSF biomarkers. CSF amyloid levels increased, and CSF total tau levels decreased, which indicated decreased neuronal injury.
—Michele G. Sullivan