User login
Hippocampal cerebral blood flow upped with antihypertensive use in Alzheimer’s
according to a new study.
Cerebral blood flow in other regions of the brain did not significantly change in patients who took the antihypertensive drug nilvadipine, according to a report on the trial published in Hypertension. Reduced cerebral blood flow is an early marker of Alzheimer’s disease, and the SPRINT MIND study suggests that intensive blood pressure control may reduce the risk of cognitive impairment.
“These findings [of the new study] not only indicate preserved cerebral autoregulation in Alzheimer’s disease but also point toward beneficial cerebrovascular effects of antihypertensive treatment,” said Jurgen A.H.R. Claassen, MD, PhD of Radboud University Medical Center in Nijmegen, the Netherlands, and coauthors. “An important question is whether this observed increase in [cerebral blood flow] translates to clinical benefits. Unfortunately, sample sizes were too small and follow-up time too short to reliably study the effects ... on structural brain measures and cognitive measures.”
Nilvadipine is a dihydropyridine calcium antagonist used to treat hypertension. In the NILVAD trial, investigators assessed the effects of nilvadipine versus placebo in approximately 500 patients with Alzheimer’s disease. The 18-month trial found no beneficial effects of nilvadipine on cognitive function, but subgroup analyses suggested a potential benefit among patients in the early stages of the disease (PLoS Med. 2018 Sep 24;15[9]:e1002660.).
The cerebral blood flow analysis was a preplanned substudy of NILVAD designed to assess how 6 months of treatment with the drug affects cerebral blood flow as measured using MRI arterial spin labeling. The researchers looked at cerebral blood flow in whole-brain gray matter and in specific regions such as the hippocampus.
The substudy analysis included 22 patients who received nilvadipine and 22 who received placebo during the randomized, double-blind study. Participants had a mean age of 72.8 years and a mean Mini-Mental State Examination score of 20.4.
At 6 months, nilvadipine lowered systolic BP by 11.5 mm Hg, and whole-brain gray matter cerebral blood flow remained stable. Blood flow to the hippocampus increased by approximately 20% among patients treated with nilvadipine – by 24.4 mL/100 g per minute to the left hippocampus and by 20.1 mL/100 g per minute to the right hippocampus.
The increased hippocampal cerebral blood flow could be related to nilvadipine’s antihypertensive effects or its effects on amyloid-beta, the authors noted.
“These findings indicate that the known decrease in [cerebral blood flow] in patients with [Alzheimer’s disease] can in some regions be reversed,” they wrote.
“Even though no medical treatment is without risk, getting treatment for high blood pressure could be important to maintain brain health in patients with Alzheimer’s disease,” Dr. Claassen said in a statement. “In the future, we need to find out whether the improvement in blood flow, especially in the hippocampus, can be used as a supportive treatment to slow down progression of Alzheimer’s disease, especially in earlier states of disease.”
The researchers wrote they lacked biomarkers to confirm Alzheimer’s disease pathology. Most of the study participants were white Europeans, which “limits extrapolation [of the findings] to other populations,” they added.
The Alzheimer’s Drug Discovery Foundation and the Dutch Alzheimer Society funded the NILVAD cerebral blood flow substudy. NILVAD was funded by the European Commission Framework 7 Program Health Theme. Dr. Claassen had no disclosures; one coauthor disclosed a pending patent for nilvadipine.
SOURCE: Claassen JAHR et al. Hypertension. 2019 Jun 17. doi: 10.1161/HYPERTENSIONAHA.119.12892.
according to a new study.
Cerebral blood flow in other regions of the brain did not significantly change in patients who took the antihypertensive drug nilvadipine, according to a report on the trial published in Hypertension. Reduced cerebral blood flow is an early marker of Alzheimer’s disease, and the SPRINT MIND study suggests that intensive blood pressure control may reduce the risk of cognitive impairment.
“These findings [of the new study] not only indicate preserved cerebral autoregulation in Alzheimer’s disease but also point toward beneficial cerebrovascular effects of antihypertensive treatment,” said Jurgen A.H.R. Claassen, MD, PhD of Radboud University Medical Center in Nijmegen, the Netherlands, and coauthors. “An important question is whether this observed increase in [cerebral blood flow] translates to clinical benefits. Unfortunately, sample sizes were too small and follow-up time too short to reliably study the effects ... on structural brain measures and cognitive measures.”
Nilvadipine is a dihydropyridine calcium antagonist used to treat hypertension. In the NILVAD trial, investigators assessed the effects of nilvadipine versus placebo in approximately 500 patients with Alzheimer’s disease. The 18-month trial found no beneficial effects of nilvadipine on cognitive function, but subgroup analyses suggested a potential benefit among patients in the early stages of the disease (PLoS Med. 2018 Sep 24;15[9]:e1002660.).
The cerebral blood flow analysis was a preplanned substudy of NILVAD designed to assess how 6 months of treatment with the drug affects cerebral blood flow as measured using MRI arterial spin labeling. The researchers looked at cerebral blood flow in whole-brain gray matter and in specific regions such as the hippocampus.
The substudy analysis included 22 patients who received nilvadipine and 22 who received placebo during the randomized, double-blind study. Participants had a mean age of 72.8 years and a mean Mini-Mental State Examination score of 20.4.
At 6 months, nilvadipine lowered systolic BP by 11.5 mm Hg, and whole-brain gray matter cerebral blood flow remained stable. Blood flow to the hippocampus increased by approximately 20% among patients treated with nilvadipine – by 24.4 mL/100 g per minute to the left hippocampus and by 20.1 mL/100 g per minute to the right hippocampus.
The increased hippocampal cerebral blood flow could be related to nilvadipine’s antihypertensive effects or its effects on amyloid-beta, the authors noted.
“These findings indicate that the known decrease in [cerebral blood flow] in patients with [Alzheimer’s disease] can in some regions be reversed,” they wrote.
“Even though no medical treatment is without risk, getting treatment for high blood pressure could be important to maintain brain health in patients with Alzheimer’s disease,” Dr. Claassen said in a statement. “In the future, we need to find out whether the improvement in blood flow, especially in the hippocampus, can be used as a supportive treatment to slow down progression of Alzheimer’s disease, especially in earlier states of disease.”
The researchers wrote they lacked biomarkers to confirm Alzheimer’s disease pathology. Most of the study participants were white Europeans, which “limits extrapolation [of the findings] to other populations,” they added.
The Alzheimer’s Drug Discovery Foundation and the Dutch Alzheimer Society funded the NILVAD cerebral blood flow substudy. NILVAD was funded by the European Commission Framework 7 Program Health Theme. Dr. Claassen had no disclosures; one coauthor disclosed a pending patent for nilvadipine.
SOURCE: Claassen JAHR et al. Hypertension. 2019 Jun 17. doi: 10.1161/HYPERTENSIONAHA.119.12892.
according to a new study.
Cerebral blood flow in other regions of the brain did not significantly change in patients who took the antihypertensive drug nilvadipine, according to a report on the trial published in Hypertension. Reduced cerebral blood flow is an early marker of Alzheimer’s disease, and the SPRINT MIND study suggests that intensive blood pressure control may reduce the risk of cognitive impairment.
“These findings [of the new study] not only indicate preserved cerebral autoregulation in Alzheimer’s disease but also point toward beneficial cerebrovascular effects of antihypertensive treatment,” said Jurgen A.H.R. Claassen, MD, PhD of Radboud University Medical Center in Nijmegen, the Netherlands, and coauthors. “An important question is whether this observed increase in [cerebral blood flow] translates to clinical benefits. Unfortunately, sample sizes were too small and follow-up time too short to reliably study the effects ... on structural brain measures and cognitive measures.”
Nilvadipine is a dihydropyridine calcium antagonist used to treat hypertension. In the NILVAD trial, investigators assessed the effects of nilvadipine versus placebo in approximately 500 patients with Alzheimer’s disease. The 18-month trial found no beneficial effects of nilvadipine on cognitive function, but subgroup analyses suggested a potential benefit among patients in the early stages of the disease (PLoS Med. 2018 Sep 24;15[9]:e1002660.).
The cerebral blood flow analysis was a preplanned substudy of NILVAD designed to assess how 6 months of treatment with the drug affects cerebral blood flow as measured using MRI arterial spin labeling. The researchers looked at cerebral blood flow in whole-brain gray matter and in specific regions such as the hippocampus.
The substudy analysis included 22 patients who received nilvadipine and 22 who received placebo during the randomized, double-blind study. Participants had a mean age of 72.8 years and a mean Mini-Mental State Examination score of 20.4.
At 6 months, nilvadipine lowered systolic BP by 11.5 mm Hg, and whole-brain gray matter cerebral blood flow remained stable. Blood flow to the hippocampus increased by approximately 20% among patients treated with nilvadipine – by 24.4 mL/100 g per minute to the left hippocampus and by 20.1 mL/100 g per minute to the right hippocampus.
The increased hippocampal cerebral blood flow could be related to nilvadipine’s antihypertensive effects or its effects on amyloid-beta, the authors noted.
“These findings indicate that the known decrease in [cerebral blood flow] in patients with [Alzheimer’s disease] can in some regions be reversed,” they wrote.
“Even though no medical treatment is without risk, getting treatment for high blood pressure could be important to maintain brain health in patients with Alzheimer’s disease,” Dr. Claassen said in a statement. “In the future, we need to find out whether the improvement in blood flow, especially in the hippocampus, can be used as a supportive treatment to slow down progression of Alzheimer’s disease, especially in earlier states of disease.”
The researchers wrote they lacked biomarkers to confirm Alzheimer’s disease pathology. Most of the study participants were white Europeans, which “limits extrapolation [of the findings] to other populations,” they added.
The Alzheimer’s Drug Discovery Foundation and the Dutch Alzheimer Society funded the NILVAD cerebral blood flow substudy. NILVAD was funded by the European Commission Framework 7 Program Health Theme. Dr. Claassen had no disclosures; one coauthor disclosed a pending patent for nilvadipine.
SOURCE: Claassen JAHR et al. Hypertension. 2019 Jun 17. doi: 10.1161/HYPERTENSIONAHA.119.12892.
FROM HYPERTENSION
A/T/N system predicts cognitive decline
Adding the amyloid/tau/neurodegeneration (A/T/N) model of dementia to a clinical model may give an incremental but still significantly increased ability to predict cognitive decline over nearly 5 years, according to findings from a longitudinal cohort study of patients without dementia at baseline.
Although the A/T/N model is still intended only for research purposes, the study came to another important conclusion: About 50% of the memory change associated with normal aging was, in fact, caused by changes associated with Alzheimer’s disease, Clifford R. Jack Jr., MD, and colleagues wrote in JAMA.
The three groups with the fastest rates of memory decline all had abnormal amyloid and either abnormal tau and/or imaging signs of neurodegeneration. “This illustrated a dominant association of memory decline with amyloidosis but only when present in combination with tauopathy, neurodegeneration, or both,” Dr. Jack of the Mayo Clinic, Rochester, Minn., and coauthors wrote.
A/T/N, also known as the National Institute on Aging and Alzheimer’s Association Research Framework, is based on objective amyloid and tau biomarkers and imaging markers of neurodegeneration and is intended to more accurately differentiate Alzheimer’s from other dementias and, potentially, to stage the disease and predict and track decline. It generates eight clinical profiles that can identify Alzheimer’s, rule it out, or include it as a possible diagnosis.
The study comprised 480 elderly individuals enrolled in the Mayo Clinic Study on Aging. Median age of the participants ranged from 67 years in one of the eight clinical profiles (A–/T–/N–) to 83 years in another (A+/T+/N+). Most (92%) were cognitively normal; the remainder had mild cognitive impairment (MCI). They were followed for a median of 4.8 years.
Both amyloid and tau were measured with PET imaging; neuropathology was represented by MRI scans of cortical thickness. Most (n = 140) were negative for all biomarkers (A–/T–/N–). The group positive for all markers (A+/T+/N+) had the largest proportion of MCI subjects (30%). The apolipoprotein E epsilon 4 (APOE4) genotype was more common among the A+ groups than it was among the A– groups (40% vs. 21%).
The individual cognitive decline trajectories varied considerably by age and within each classification group. Only 7% of the A–/T–/N– group were 80 years or older, and only 2% of the A+/N+/T+ group were younger than 70 years.
In a clinical model, age and APOE4 status were significantly associated with faster rates of memory decline. Sex, education, and a cardiovascular/metabolic model were not, however.
“The estimated rate of memory decline in a 75-year-old individual who was an APOE4 noncarrier was –0.04 z-score units per year,” the authors wrote. “An 85-year-old individual who was also an APOE4 noncarrier could be expected to have a decline of –0.08 units per year, while a 75-year-old E4 carrier could be expected to have a decline of –0.08 units per year.”
Every 10 years of additional age was associated with a significant median worsening of 0.4 on z score for memory. A 4-year difference in education was associated with a 0.6-unit higher memory score, while APOE4 carriers had a 0.3-unit lower memory score.
The addition of the A/T/N model significantly improved the prediction of cognitive decline and memory score, although the rates of decline were still considerably variable. All of the A+ groups had the fastest decline rates.
“To place the predictive utility of biomarkers in clinical context, the decline in rates of memory for A+/T+/N–, A+/T–/N+, A+/T+/N+ [abnormal amyloid plus tau or neurodegeneration] were of similar magnitude to a 20-year increase in age and were twice that associated with APOE4 carriership,” they wrote.
A total of 88 participants had a second imaging visit at a median of 15 months. Most (n = 72) had no change in the A/T/N classification. A and T classifications were more stable (98% and 97%, respectively) than was N classification (84%).
A secondary analysis compared this model with generally accepted clinical and biomarker characteristics. Prior research has shown that prevalence of abnormal A/T/N biomarker groups increased with age in the Mayo Clinic Study on Aging. The mean annual memory z-score in this cohort at 60 years was 0.02, which dropped to 0.11 by age 90.
“Forty-six percent of this increase in decline rate [–0.06] was partitioned to the increasing prevalence of abnormal A/T/N profiles, while the remaining decline [–0.07] was partitioned to age,” the investigators reported.
While A+ subjects were most likely to decline, the A+/T–/N+ group presents a conundrum, the team wrote. “A possible explanation is that these individuals have early Alzheimer’s disease [denoted by A+T–] plus neurodegeneration due to comorbid non–Alzheimer’s disease neuropathic changes.”
This is an important point because the cognitive decline of Alzheimer’s is thought to be largely associated with tauopathy, not amyloidosis. “One possible explanation is an effect of subthreshold tau in A+/T–/N+ individuals, but this is speculative. Clearer understanding of the neuropathologic bases for the A+/T–/N+ group, as well as other A/T/N groups, awaits future biomarker-autopsy correlation studies.”
SOURCE: Jack CR et al. JAMA 2019;321:2316-25.
The findings reported by Jack et al. most immediately affect research cohorts, but they raise an interesting suggestion: Only in the presence of concomitant tau, neuropathology, or both does amyloidosis appear related to an increased rate of cognitive decline when compared with non-Alzheimer’s groups.
Prevention studies lasting only a few years may be more likely to find treatment effects on disease progression in actively treated groups of those patients.
An interesting finding in the study is that A+/T–/N+ subjects showed faster rates of cognitive decline than did the A–/T–/N+ groups even though, in both cases, neurodegeneration is thought to be driven by non-Alzheimer’s pathology. What is causing disease in the A–/T–/N+ group will be unclear until the framework is enriched with other important contributors to age-related cognitive decline.
Currently, A/T/N classification – based on neuroimaging – is costly and impractical on a large scale, and so far lacks data on the added value of each specific A/T/N measure and generalizability to more diverse patient populations.
Despite these concerns, the study by Jack et al. represents an important contribution in conceptualizing Alzheimer’s disease and testing the research framework in a relatively large sample of participants.
David Wolk, MD, of the University of Pennsylvania Memory Center, Philadelphia, and colleagues’ comments here are paraphrased from an accompanying editorial (JAMA. 2019;321[23]:2289-91). Dr. Wolk reported receiving grants and personal fees from Avid/Eli Lilly and Merck; personal fees from Janssen, GE Healthcare, and Neuronix; and grants from Biogen and Functional Neuromodulation.
The findings reported by Jack et al. most immediately affect research cohorts, but they raise an interesting suggestion: Only in the presence of concomitant tau, neuropathology, or both does amyloidosis appear related to an increased rate of cognitive decline when compared with non-Alzheimer’s groups.
Prevention studies lasting only a few years may be more likely to find treatment effects on disease progression in actively treated groups of those patients.
An interesting finding in the study is that A+/T–/N+ subjects showed faster rates of cognitive decline than did the A–/T–/N+ groups even though, in both cases, neurodegeneration is thought to be driven by non-Alzheimer’s pathology. What is causing disease in the A–/T–/N+ group will be unclear until the framework is enriched with other important contributors to age-related cognitive decline.
Currently, A/T/N classification – based on neuroimaging – is costly and impractical on a large scale, and so far lacks data on the added value of each specific A/T/N measure and generalizability to more diverse patient populations.
Despite these concerns, the study by Jack et al. represents an important contribution in conceptualizing Alzheimer’s disease and testing the research framework in a relatively large sample of participants.
David Wolk, MD, of the University of Pennsylvania Memory Center, Philadelphia, and colleagues’ comments here are paraphrased from an accompanying editorial (JAMA. 2019;321[23]:2289-91). Dr. Wolk reported receiving grants and personal fees from Avid/Eli Lilly and Merck; personal fees from Janssen, GE Healthcare, and Neuronix; and grants from Biogen and Functional Neuromodulation.
The findings reported by Jack et al. most immediately affect research cohorts, but they raise an interesting suggestion: Only in the presence of concomitant tau, neuropathology, or both does amyloidosis appear related to an increased rate of cognitive decline when compared with non-Alzheimer’s groups.
Prevention studies lasting only a few years may be more likely to find treatment effects on disease progression in actively treated groups of those patients.
An interesting finding in the study is that A+/T–/N+ subjects showed faster rates of cognitive decline than did the A–/T–/N+ groups even though, in both cases, neurodegeneration is thought to be driven by non-Alzheimer’s pathology. What is causing disease in the A–/T–/N+ group will be unclear until the framework is enriched with other important contributors to age-related cognitive decline.
Currently, A/T/N classification – based on neuroimaging – is costly and impractical on a large scale, and so far lacks data on the added value of each specific A/T/N measure and generalizability to more diverse patient populations.
Despite these concerns, the study by Jack et al. represents an important contribution in conceptualizing Alzheimer’s disease and testing the research framework in a relatively large sample of participants.
David Wolk, MD, of the University of Pennsylvania Memory Center, Philadelphia, and colleagues’ comments here are paraphrased from an accompanying editorial (JAMA. 2019;321[23]:2289-91). Dr. Wolk reported receiving grants and personal fees from Avid/Eli Lilly and Merck; personal fees from Janssen, GE Healthcare, and Neuronix; and grants from Biogen and Functional Neuromodulation.
Adding the amyloid/tau/neurodegeneration (A/T/N) model of dementia to a clinical model may give an incremental but still significantly increased ability to predict cognitive decline over nearly 5 years, according to findings from a longitudinal cohort study of patients without dementia at baseline.
Although the A/T/N model is still intended only for research purposes, the study came to another important conclusion: About 50% of the memory change associated with normal aging was, in fact, caused by changes associated with Alzheimer’s disease, Clifford R. Jack Jr., MD, and colleagues wrote in JAMA.
The three groups with the fastest rates of memory decline all had abnormal amyloid and either abnormal tau and/or imaging signs of neurodegeneration. “This illustrated a dominant association of memory decline with amyloidosis but only when present in combination with tauopathy, neurodegeneration, or both,” Dr. Jack of the Mayo Clinic, Rochester, Minn., and coauthors wrote.
A/T/N, also known as the National Institute on Aging and Alzheimer’s Association Research Framework, is based on objective amyloid and tau biomarkers and imaging markers of neurodegeneration and is intended to more accurately differentiate Alzheimer’s from other dementias and, potentially, to stage the disease and predict and track decline. It generates eight clinical profiles that can identify Alzheimer’s, rule it out, or include it as a possible diagnosis.
The study comprised 480 elderly individuals enrolled in the Mayo Clinic Study on Aging. Median age of the participants ranged from 67 years in one of the eight clinical profiles (A–/T–/N–) to 83 years in another (A+/T+/N+). Most (92%) were cognitively normal; the remainder had mild cognitive impairment (MCI). They were followed for a median of 4.8 years.
Both amyloid and tau were measured with PET imaging; neuropathology was represented by MRI scans of cortical thickness. Most (n = 140) were negative for all biomarkers (A–/T–/N–). The group positive for all markers (A+/T+/N+) had the largest proportion of MCI subjects (30%). The apolipoprotein E epsilon 4 (APOE4) genotype was more common among the A+ groups than it was among the A– groups (40% vs. 21%).
The individual cognitive decline trajectories varied considerably by age and within each classification group. Only 7% of the A–/T–/N– group were 80 years or older, and only 2% of the A+/N+/T+ group were younger than 70 years.
In a clinical model, age and APOE4 status were significantly associated with faster rates of memory decline. Sex, education, and a cardiovascular/metabolic model were not, however.
“The estimated rate of memory decline in a 75-year-old individual who was an APOE4 noncarrier was –0.04 z-score units per year,” the authors wrote. “An 85-year-old individual who was also an APOE4 noncarrier could be expected to have a decline of –0.08 units per year, while a 75-year-old E4 carrier could be expected to have a decline of –0.08 units per year.”
Every 10 years of additional age was associated with a significant median worsening of 0.4 on z score for memory. A 4-year difference in education was associated with a 0.6-unit higher memory score, while APOE4 carriers had a 0.3-unit lower memory score.
The addition of the A/T/N model significantly improved the prediction of cognitive decline and memory score, although the rates of decline were still considerably variable. All of the A+ groups had the fastest decline rates.
“To place the predictive utility of biomarkers in clinical context, the decline in rates of memory for A+/T+/N–, A+/T–/N+, A+/T+/N+ [abnormal amyloid plus tau or neurodegeneration] were of similar magnitude to a 20-year increase in age and were twice that associated with APOE4 carriership,” they wrote.
A total of 88 participants had a second imaging visit at a median of 15 months. Most (n = 72) had no change in the A/T/N classification. A and T classifications were more stable (98% and 97%, respectively) than was N classification (84%).
A secondary analysis compared this model with generally accepted clinical and biomarker characteristics. Prior research has shown that prevalence of abnormal A/T/N biomarker groups increased with age in the Mayo Clinic Study on Aging. The mean annual memory z-score in this cohort at 60 years was 0.02, which dropped to 0.11 by age 90.
“Forty-six percent of this increase in decline rate [–0.06] was partitioned to the increasing prevalence of abnormal A/T/N profiles, while the remaining decline [–0.07] was partitioned to age,” the investigators reported.
While A+ subjects were most likely to decline, the A+/T–/N+ group presents a conundrum, the team wrote. “A possible explanation is that these individuals have early Alzheimer’s disease [denoted by A+T–] plus neurodegeneration due to comorbid non–Alzheimer’s disease neuropathic changes.”
This is an important point because the cognitive decline of Alzheimer’s is thought to be largely associated with tauopathy, not amyloidosis. “One possible explanation is an effect of subthreshold tau in A+/T–/N+ individuals, but this is speculative. Clearer understanding of the neuropathologic bases for the A+/T–/N+ group, as well as other A/T/N groups, awaits future biomarker-autopsy correlation studies.”
SOURCE: Jack CR et al. JAMA 2019;321:2316-25.
Adding the amyloid/tau/neurodegeneration (A/T/N) model of dementia to a clinical model may give an incremental but still significantly increased ability to predict cognitive decline over nearly 5 years, according to findings from a longitudinal cohort study of patients without dementia at baseline.
Although the A/T/N model is still intended only for research purposes, the study came to another important conclusion: About 50% of the memory change associated with normal aging was, in fact, caused by changes associated with Alzheimer’s disease, Clifford R. Jack Jr., MD, and colleagues wrote in JAMA.
The three groups with the fastest rates of memory decline all had abnormal amyloid and either abnormal tau and/or imaging signs of neurodegeneration. “This illustrated a dominant association of memory decline with amyloidosis but only when present in combination with tauopathy, neurodegeneration, or both,” Dr. Jack of the Mayo Clinic, Rochester, Minn., and coauthors wrote.
A/T/N, also known as the National Institute on Aging and Alzheimer’s Association Research Framework, is based on objective amyloid and tau biomarkers and imaging markers of neurodegeneration and is intended to more accurately differentiate Alzheimer’s from other dementias and, potentially, to stage the disease and predict and track decline. It generates eight clinical profiles that can identify Alzheimer’s, rule it out, or include it as a possible diagnosis.
The study comprised 480 elderly individuals enrolled in the Mayo Clinic Study on Aging. Median age of the participants ranged from 67 years in one of the eight clinical profiles (A–/T–/N–) to 83 years in another (A+/T+/N+). Most (92%) were cognitively normal; the remainder had mild cognitive impairment (MCI). They were followed for a median of 4.8 years.
Both amyloid and tau were measured with PET imaging; neuropathology was represented by MRI scans of cortical thickness. Most (n = 140) were negative for all biomarkers (A–/T–/N–). The group positive for all markers (A+/T+/N+) had the largest proportion of MCI subjects (30%). The apolipoprotein E epsilon 4 (APOE4) genotype was more common among the A+ groups than it was among the A– groups (40% vs. 21%).
The individual cognitive decline trajectories varied considerably by age and within each classification group. Only 7% of the A–/T–/N– group were 80 years or older, and only 2% of the A+/N+/T+ group were younger than 70 years.
In a clinical model, age and APOE4 status were significantly associated with faster rates of memory decline. Sex, education, and a cardiovascular/metabolic model were not, however.
“The estimated rate of memory decline in a 75-year-old individual who was an APOE4 noncarrier was –0.04 z-score units per year,” the authors wrote. “An 85-year-old individual who was also an APOE4 noncarrier could be expected to have a decline of –0.08 units per year, while a 75-year-old E4 carrier could be expected to have a decline of –0.08 units per year.”
Every 10 years of additional age was associated with a significant median worsening of 0.4 on z score for memory. A 4-year difference in education was associated with a 0.6-unit higher memory score, while APOE4 carriers had a 0.3-unit lower memory score.
The addition of the A/T/N model significantly improved the prediction of cognitive decline and memory score, although the rates of decline were still considerably variable. All of the A+ groups had the fastest decline rates.
“To place the predictive utility of biomarkers in clinical context, the decline in rates of memory for A+/T+/N–, A+/T–/N+, A+/T+/N+ [abnormal amyloid plus tau or neurodegeneration] were of similar magnitude to a 20-year increase in age and were twice that associated with APOE4 carriership,” they wrote.
A total of 88 participants had a second imaging visit at a median of 15 months. Most (n = 72) had no change in the A/T/N classification. A and T classifications were more stable (98% and 97%, respectively) than was N classification (84%).
A secondary analysis compared this model with generally accepted clinical and biomarker characteristics. Prior research has shown that prevalence of abnormal A/T/N biomarker groups increased with age in the Mayo Clinic Study on Aging. The mean annual memory z-score in this cohort at 60 years was 0.02, which dropped to 0.11 by age 90.
“Forty-six percent of this increase in decline rate [–0.06] was partitioned to the increasing prevalence of abnormal A/T/N profiles, while the remaining decline [–0.07] was partitioned to age,” the investigators reported.
While A+ subjects were most likely to decline, the A+/T–/N+ group presents a conundrum, the team wrote. “A possible explanation is that these individuals have early Alzheimer’s disease [denoted by A+T–] plus neurodegeneration due to comorbid non–Alzheimer’s disease neuropathic changes.”
This is an important point because the cognitive decline of Alzheimer’s is thought to be largely associated with tauopathy, not amyloidosis. “One possible explanation is an effect of subthreshold tau in A+/T–/N+ individuals, but this is speculative. Clearer understanding of the neuropathologic bases for the A+/T–/N+ group, as well as other A/T/N groups, awaits future biomarker-autopsy correlation studies.”
SOURCE: Jack CR et al. JAMA 2019;321:2316-25.
FROM JAMA
CSF neurofilament light level could aid in diagnosis
according to an analysis published online ahead of print June 17 in JAMA Neurology. The biomarker has the potential to distinguish between frontotemporal dementia (FTD) and other dementia subtypes, as well as between Parkinson’s disease and atypical parkinsonian syndromes, said the investigators. It may be necessary to identify age- and sex-specific reference values for NfL, they added.
Neurologists have long understood CSF levels of NfL to be elevated in neurodegenerative conditions, but researchers previously had not compared these levels systematically among neurologic disorders. Similarly, the literature indicates a positive association between CSF NfL level and age in healthy controls, but this association has not been evaluated systematically in neurologic disorders. The resulting lack of clarity has impeded the use of NfL as a diagnostic biomarker.
A meta-analysis of CSF samples
Claire Bridel, MD, PhD, of the department of clinical chemistry at the VU University Medical Centre in Amsterdam and colleagues conducted a systematic review and meta-analysis to compare CSF levels of NfL among diagnoses, assess the associations of age and sex with NfL, and evaluate the potential of NfL as a diagnostic biomarker. The investigators searched PubMed for studies published between Jan. 1, 2006, and Jan. 1, 2016, that reported CSF levels of NfL in neurologic or psychiatric conditions or in healthy controls. They included only studies that used the same commercially available immunoassay that has been used in most studies since 2006. The literature indicates that this enzyme-linked immunosorbent assay is sensitive and robust. Dr. Bridel and colleagues contacted study authors and requested their individual-level data.
The investigators sorted the most common neurologic conditions into three groups of similar disorders. The first group included inflammatory conditions of the CNS, such as multiple sclerosis, clinically isolated syndrome (CIS), and optic neuritis. The second group included dementia syndromes (such as Alzheimer’s disease, FTD, vascular dementia, and dementia with Lewy bodies) and amyotrophic lateral sclerosis (ALS). The third category included parkinsonian syndromes such as Parkinson’s disease, Parkinson’s disease dementia, multiple system atrophy (MSA), progressive supranuclear palsy (PSP), and corticobasal syndrome (CBS). The authors used generalized linear mixed-effects models to estimate the fixed effects of age, sex, and diagnosis on log-transformed NfL levels. They modeled cohort of origin as a random intercept.
NfL increased with age
Dr. Bridel and colleagues identified 153 relevant investigations, of which 44 met their inclusion criteria. The original investigators provided data sets for these studies, along with three previously unpublished data sets. The data sets included information from 10,059 participants (mean age, 59.7 years; 54.1% female). After excluding diagnostic categories with fewer than five observations per sex, Dr. Bridel and colleagues included data for 10,012 people in the analysis. In this population, the researchers identified 2,795 patients with inflammatory diseases of the CNS, 4,284 patients with dementia or predementia, 984 patients with parkinsonian disorders, and 1,332 healthy controls.
CSF level of NfL was elevated in most neurologic conditions, compared with healthy controls. The largest effect sizes were in cognitively impaired patients with HIV (21.36), patients with FTD/ALS (10.48), patients with ALS (7.58), and patients with Huntington’s disease (5.88).
In healthy controls, the level of NfL in CSF increased by 3.30% annually. The investigators also observed an association between age and CSF NfL level in people with subjective complaints, bipolar disorder, and most neurodegenerative conditions. They found no association, however, in patients with MS, HIV and cognitive impairment, and rapidly progressive neurodegenerative conditions (such as FTD, ALS, FTD/ALS, MSA, PSP, CBS, and Huntington’s disease). CSF level of NfL was 26.0% higher in men among healthy controls. This discrepancy also was observed in a minority of neurologic conditions, including MS, Alzheimer’s disease, vascular dementia, and Parkinson’s disease.
Mean CSF levels of NfL were similar between patients with inflammatory conditions of the CNS. Among dementias and related disorders, mean CNS level of NfL was significantly higher in FTD than in Alzheimer’s disease (2.08), vascular dementia (1.56), and dementia with Lewy bodies (2.50). Among parkinsonian syndromes, the mean CSF levels of NfL were higher in MSA, PSP, and CBS, compared with Parkinson’s disease.
Many factors influence NfL level in CSF
The association between CNS level of NfL with age among healthy controls “implies that age-specific reference values may be needed and that the diagnostic potential of CSF NfL may decrease with age,” said the researchers. The finding that CSF NfL level was higher in men in a minority of diagnoses has uncertain clinical significance, they added. Sex-specific reference values may be needed.
Dr. Bridel and colleagues found that age, sex, and cohort explained 46% of variation in CSF level of NfL, which suggests that many factors that determine this level have yet to be identified. Disease duration and disease severity could influence the CSF level of NfL, but the data sets that the investigators analyzed did not include this information.
Because CSF NfL level did not differ significantly between relapsing/remitting MS, secondary progressive MS, and primary progressive MS, this biomarker “may not differentiate acute inflammation-induced neuronal damage in the context of relapses from progressive neurodegeneration if the consequences of recent relapses or novel lesion formation are not considered,” said Dr. Bridel and colleagues. The findings do suggest, however, that CSF level of NfL can distinguish FTD from other dementias, as well as Parkinson’s disease from atypical parkinsonian syndromes. Furthermore, it is possible that the findings of this study can be translated to serum level of NfL, said the authors.
One of the study’s limitations was that diagnosis was based on clinical criteria, said Dr. Bridel and colleagues. In addition, the authors were unable to identify dementia of multifactorial origin, which might have reduced the differences in CSF NfL level distributions between dementia subtypes. Finally, the authors only analyzed studies that relied on a specific immunoassay for CSF NfL level.
The authors reported receiving funding from various pharmaceutical and biopharmaceutical companies, as well as from grants and research foundations. The funders did not influence the study design, data analysis, or interpretation, however.
SOURCE: Bridel C et al. JAMA Neurol. 2019 June 17. doi: 10.1001/jamaneurol.2019.1534.
according to an analysis published online ahead of print June 17 in JAMA Neurology. The biomarker has the potential to distinguish between frontotemporal dementia (FTD) and other dementia subtypes, as well as between Parkinson’s disease and atypical parkinsonian syndromes, said the investigators. It may be necessary to identify age- and sex-specific reference values for NfL, they added.
Neurologists have long understood CSF levels of NfL to be elevated in neurodegenerative conditions, but researchers previously had not compared these levels systematically among neurologic disorders. Similarly, the literature indicates a positive association between CSF NfL level and age in healthy controls, but this association has not been evaluated systematically in neurologic disorders. The resulting lack of clarity has impeded the use of NfL as a diagnostic biomarker.
A meta-analysis of CSF samples
Claire Bridel, MD, PhD, of the department of clinical chemistry at the VU University Medical Centre in Amsterdam and colleagues conducted a systematic review and meta-analysis to compare CSF levels of NfL among diagnoses, assess the associations of age and sex with NfL, and evaluate the potential of NfL as a diagnostic biomarker. The investigators searched PubMed for studies published between Jan. 1, 2006, and Jan. 1, 2016, that reported CSF levels of NfL in neurologic or psychiatric conditions or in healthy controls. They included only studies that used the same commercially available immunoassay that has been used in most studies since 2006. The literature indicates that this enzyme-linked immunosorbent assay is sensitive and robust. Dr. Bridel and colleagues contacted study authors and requested their individual-level data.
The investigators sorted the most common neurologic conditions into three groups of similar disorders. The first group included inflammatory conditions of the CNS, such as multiple sclerosis, clinically isolated syndrome (CIS), and optic neuritis. The second group included dementia syndromes (such as Alzheimer’s disease, FTD, vascular dementia, and dementia with Lewy bodies) and amyotrophic lateral sclerosis (ALS). The third category included parkinsonian syndromes such as Parkinson’s disease, Parkinson’s disease dementia, multiple system atrophy (MSA), progressive supranuclear palsy (PSP), and corticobasal syndrome (CBS). The authors used generalized linear mixed-effects models to estimate the fixed effects of age, sex, and diagnosis on log-transformed NfL levels. They modeled cohort of origin as a random intercept.
NfL increased with age
Dr. Bridel and colleagues identified 153 relevant investigations, of which 44 met their inclusion criteria. The original investigators provided data sets for these studies, along with three previously unpublished data sets. The data sets included information from 10,059 participants (mean age, 59.7 years; 54.1% female). After excluding diagnostic categories with fewer than five observations per sex, Dr. Bridel and colleagues included data for 10,012 people in the analysis. In this population, the researchers identified 2,795 patients with inflammatory diseases of the CNS, 4,284 patients with dementia or predementia, 984 patients with parkinsonian disorders, and 1,332 healthy controls.
CSF level of NfL was elevated in most neurologic conditions, compared with healthy controls. The largest effect sizes were in cognitively impaired patients with HIV (21.36), patients with FTD/ALS (10.48), patients with ALS (7.58), and patients with Huntington’s disease (5.88).
In healthy controls, the level of NfL in CSF increased by 3.30% annually. The investigators also observed an association between age and CSF NfL level in people with subjective complaints, bipolar disorder, and most neurodegenerative conditions. They found no association, however, in patients with MS, HIV and cognitive impairment, and rapidly progressive neurodegenerative conditions (such as FTD, ALS, FTD/ALS, MSA, PSP, CBS, and Huntington’s disease). CSF level of NfL was 26.0% higher in men among healthy controls. This discrepancy also was observed in a minority of neurologic conditions, including MS, Alzheimer’s disease, vascular dementia, and Parkinson’s disease.
Mean CSF levels of NfL were similar between patients with inflammatory conditions of the CNS. Among dementias and related disorders, mean CNS level of NfL was significantly higher in FTD than in Alzheimer’s disease (2.08), vascular dementia (1.56), and dementia with Lewy bodies (2.50). Among parkinsonian syndromes, the mean CSF levels of NfL were higher in MSA, PSP, and CBS, compared with Parkinson’s disease.
Many factors influence NfL level in CSF
The association between CNS level of NfL with age among healthy controls “implies that age-specific reference values may be needed and that the diagnostic potential of CSF NfL may decrease with age,” said the researchers. The finding that CSF NfL level was higher in men in a minority of diagnoses has uncertain clinical significance, they added. Sex-specific reference values may be needed.
Dr. Bridel and colleagues found that age, sex, and cohort explained 46% of variation in CSF level of NfL, which suggests that many factors that determine this level have yet to be identified. Disease duration and disease severity could influence the CSF level of NfL, but the data sets that the investigators analyzed did not include this information.
Because CSF NfL level did not differ significantly between relapsing/remitting MS, secondary progressive MS, and primary progressive MS, this biomarker “may not differentiate acute inflammation-induced neuronal damage in the context of relapses from progressive neurodegeneration if the consequences of recent relapses or novel lesion formation are not considered,” said Dr. Bridel and colleagues. The findings do suggest, however, that CSF level of NfL can distinguish FTD from other dementias, as well as Parkinson’s disease from atypical parkinsonian syndromes. Furthermore, it is possible that the findings of this study can be translated to serum level of NfL, said the authors.
One of the study’s limitations was that diagnosis was based on clinical criteria, said Dr. Bridel and colleagues. In addition, the authors were unable to identify dementia of multifactorial origin, which might have reduced the differences in CSF NfL level distributions between dementia subtypes. Finally, the authors only analyzed studies that relied on a specific immunoassay for CSF NfL level.
The authors reported receiving funding from various pharmaceutical and biopharmaceutical companies, as well as from grants and research foundations. The funders did not influence the study design, data analysis, or interpretation, however.
SOURCE: Bridel C et al. JAMA Neurol. 2019 June 17. doi: 10.1001/jamaneurol.2019.1534.
according to an analysis published online ahead of print June 17 in JAMA Neurology. The biomarker has the potential to distinguish between frontotemporal dementia (FTD) and other dementia subtypes, as well as between Parkinson’s disease and atypical parkinsonian syndromes, said the investigators. It may be necessary to identify age- and sex-specific reference values for NfL, they added.
Neurologists have long understood CSF levels of NfL to be elevated in neurodegenerative conditions, but researchers previously had not compared these levels systematically among neurologic disorders. Similarly, the literature indicates a positive association between CSF NfL level and age in healthy controls, but this association has not been evaluated systematically in neurologic disorders. The resulting lack of clarity has impeded the use of NfL as a diagnostic biomarker.
A meta-analysis of CSF samples
Claire Bridel, MD, PhD, of the department of clinical chemistry at the VU University Medical Centre in Amsterdam and colleagues conducted a systematic review and meta-analysis to compare CSF levels of NfL among diagnoses, assess the associations of age and sex with NfL, and evaluate the potential of NfL as a diagnostic biomarker. The investigators searched PubMed for studies published between Jan. 1, 2006, and Jan. 1, 2016, that reported CSF levels of NfL in neurologic or psychiatric conditions or in healthy controls. They included only studies that used the same commercially available immunoassay that has been used in most studies since 2006. The literature indicates that this enzyme-linked immunosorbent assay is sensitive and robust. Dr. Bridel and colleagues contacted study authors and requested their individual-level data.
The investigators sorted the most common neurologic conditions into three groups of similar disorders. The first group included inflammatory conditions of the CNS, such as multiple sclerosis, clinically isolated syndrome (CIS), and optic neuritis. The second group included dementia syndromes (such as Alzheimer’s disease, FTD, vascular dementia, and dementia with Lewy bodies) and amyotrophic lateral sclerosis (ALS). The third category included parkinsonian syndromes such as Parkinson’s disease, Parkinson’s disease dementia, multiple system atrophy (MSA), progressive supranuclear palsy (PSP), and corticobasal syndrome (CBS). The authors used generalized linear mixed-effects models to estimate the fixed effects of age, sex, and diagnosis on log-transformed NfL levels. They modeled cohort of origin as a random intercept.
NfL increased with age
Dr. Bridel and colleagues identified 153 relevant investigations, of which 44 met their inclusion criteria. The original investigators provided data sets for these studies, along with three previously unpublished data sets. The data sets included information from 10,059 participants (mean age, 59.7 years; 54.1% female). After excluding diagnostic categories with fewer than five observations per sex, Dr. Bridel and colleagues included data for 10,012 people in the analysis. In this population, the researchers identified 2,795 patients with inflammatory diseases of the CNS, 4,284 patients with dementia or predementia, 984 patients with parkinsonian disorders, and 1,332 healthy controls.
CSF level of NfL was elevated in most neurologic conditions, compared with healthy controls. The largest effect sizes were in cognitively impaired patients with HIV (21.36), patients with FTD/ALS (10.48), patients with ALS (7.58), and patients with Huntington’s disease (5.88).
In healthy controls, the level of NfL in CSF increased by 3.30% annually. The investigators also observed an association between age and CSF NfL level in people with subjective complaints, bipolar disorder, and most neurodegenerative conditions. They found no association, however, in patients with MS, HIV and cognitive impairment, and rapidly progressive neurodegenerative conditions (such as FTD, ALS, FTD/ALS, MSA, PSP, CBS, and Huntington’s disease). CSF level of NfL was 26.0% higher in men among healthy controls. This discrepancy also was observed in a minority of neurologic conditions, including MS, Alzheimer’s disease, vascular dementia, and Parkinson’s disease.
Mean CSF levels of NfL were similar between patients with inflammatory conditions of the CNS. Among dementias and related disorders, mean CNS level of NfL was significantly higher in FTD than in Alzheimer’s disease (2.08), vascular dementia (1.56), and dementia with Lewy bodies (2.50). Among parkinsonian syndromes, the mean CSF levels of NfL were higher in MSA, PSP, and CBS, compared with Parkinson’s disease.
Many factors influence NfL level in CSF
The association between CNS level of NfL with age among healthy controls “implies that age-specific reference values may be needed and that the diagnostic potential of CSF NfL may decrease with age,” said the researchers. The finding that CSF NfL level was higher in men in a minority of diagnoses has uncertain clinical significance, they added. Sex-specific reference values may be needed.
Dr. Bridel and colleagues found that age, sex, and cohort explained 46% of variation in CSF level of NfL, which suggests that many factors that determine this level have yet to be identified. Disease duration and disease severity could influence the CSF level of NfL, but the data sets that the investigators analyzed did not include this information.
Because CSF NfL level did not differ significantly between relapsing/remitting MS, secondary progressive MS, and primary progressive MS, this biomarker “may not differentiate acute inflammation-induced neuronal damage in the context of relapses from progressive neurodegeneration if the consequences of recent relapses or novel lesion formation are not considered,” said Dr. Bridel and colleagues. The findings do suggest, however, that CSF level of NfL can distinguish FTD from other dementias, as well as Parkinson’s disease from atypical parkinsonian syndromes. Furthermore, it is possible that the findings of this study can be translated to serum level of NfL, said the authors.
One of the study’s limitations was that diagnosis was based on clinical criteria, said Dr. Bridel and colleagues. In addition, the authors were unable to identify dementia of multifactorial origin, which might have reduced the differences in CSF NfL level distributions between dementia subtypes. Finally, the authors only analyzed studies that relied on a specific immunoassay for CSF NfL level.
The authors reported receiving funding from various pharmaceutical and biopharmaceutical companies, as well as from grants and research foundations. The funders did not influence the study design, data analysis, or interpretation, however.
SOURCE: Bridel C et al. JAMA Neurol. 2019 June 17. doi: 10.1001/jamaneurol.2019.1534.
FROM JAMA NEUROLOGY
Cognitive decline sped up after CHD
according to the results of a large prospective study with a median of 12 years of follow-up.
“We found that incident CHD was significantly associated with faster post–CHD-diagnosis cognitive decline, but not pre–CHD-diagnosis or short-term cognitive decline after the event,” Wuxiang Xie, PhD, of Peking University Health Science Center, Beijing, and associates wrote in the Journal of the American College of Cardiology. Linear mixed models showed that cognitive decline sped up during the year after incident CHD.
Past research had suggested a link between accelerated cognitive decline and CHD, but the temporal pattern of the relationship was unclear. For the study, Dr. Xie and associates followed 7,888 adults from the English Longitudinal Study of Aging who were an average of 62 years old and had no history of stroke, MI, angina, or dementia (Alzheimer’s disease or otherwise). All participants underwent a baseline cognitive assessment for verbal memory, semantic fluency, and temporal orientation, plus a median of six follow-up assessments.
In all, 480 (6%) participants developed CHD during follow-up. Their rate of cognitive decline remained constant before and immediately after their CHD diagnosis, but in subsequent years, they experienced significant accelerations in loss of global cognitive function, verbal memory, and temporal orientation even after accounting for time and many demographic and clinical variables. For example, the slope representing temporal change in global cognitive score decreased by a mean of 0.039 per year, compared with the pre-CHD slope (slope difference, –0.039; 95% confidence interval, –0.063 to –0.015; P =. 002). Semantic fluency also declined faster after CHD, but the difference, compared with before CHD, did not reach statistical significance (P = .11).
Individuals without CHD showed no such accelerations in cognitive decline throughout follow-up in adjusted models, the researchers wrote. “Based on repeated cognitive measurements over a long follow-up period, this study revealed a reliable and robust trajectory of cognitive decline [after CHD]. Future studies are warranted to determine the precise mechanisms linking incident CHD to cognitive decline.”
Funders included the National Natural Science Foundation of China, the Beijing Natural Science Foundation, and the Newton International Fellowship from the Academy of Medical Sciences. The researchers reported having no relevant financial disclosures.
SOURCE: Xie W et al. J Amer Coll Cardiol. 2019 Jun 17. doi: 10.1016/j.jacc.2019.04.019.
The findings “highlight the role of cardiovascular risk factors and cardiovascular health as crucial determinants of cognitive trajectories in later life,” wrote Suvi P. Rovio, PhD; Katja Pahkala, PhD; and Olli T. Raitakari, MD, PhD. For example, accelerated declines in verbal memory might indicate a specific vulnerability to vascular changes within the medial temporal lobe and hippocampus.
The fact that cognitive decline did not accelerate immediately after coronary heart disease suggests that CHD itself does not acutely alter the brain, such as by causing microinfarcts, they commented. Instead, CHD might induce longer-term shifts in cerebral vascular function by affecting the blood-brain barrier or perfusion and oxidation in the brain. While these complex relationships need further untangling, the study suggests interventions that cut CHD risk also might help prevent cognitive decline itself and slow the rate of cognitive decline if it occurs.
Dr. Rovio, Dr. Pahkala, and Dr. Raitakari are at the University of Turku (Finland) and Turku University Hospital. These comments are adapted from an editorial accompanying the article by Xie et al. (J Amer Coll Cardiol. 2019 Jun 17. doi: 10.1016/j.jacc.2019.04.020). They reported having no relevant financial disclosures.
The findings “highlight the role of cardiovascular risk factors and cardiovascular health as crucial determinants of cognitive trajectories in later life,” wrote Suvi P. Rovio, PhD; Katja Pahkala, PhD; and Olli T. Raitakari, MD, PhD. For example, accelerated declines in verbal memory might indicate a specific vulnerability to vascular changes within the medial temporal lobe and hippocampus.
The fact that cognitive decline did not accelerate immediately after coronary heart disease suggests that CHD itself does not acutely alter the brain, such as by causing microinfarcts, they commented. Instead, CHD might induce longer-term shifts in cerebral vascular function by affecting the blood-brain barrier or perfusion and oxidation in the brain. While these complex relationships need further untangling, the study suggests interventions that cut CHD risk also might help prevent cognitive decline itself and slow the rate of cognitive decline if it occurs.
Dr. Rovio, Dr. Pahkala, and Dr. Raitakari are at the University of Turku (Finland) and Turku University Hospital. These comments are adapted from an editorial accompanying the article by Xie et al. (J Amer Coll Cardiol. 2019 Jun 17. doi: 10.1016/j.jacc.2019.04.020). They reported having no relevant financial disclosures.
The findings “highlight the role of cardiovascular risk factors and cardiovascular health as crucial determinants of cognitive trajectories in later life,” wrote Suvi P. Rovio, PhD; Katja Pahkala, PhD; and Olli T. Raitakari, MD, PhD. For example, accelerated declines in verbal memory might indicate a specific vulnerability to vascular changes within the medial temporal lobe and hippocampus.
The fact that cognitive decline did not accelerate immediately after coronary heart disease suggests that CHD itself does not acutely alter the brain, such as by causing microinfarcts, they commented. Instead, CHD might induce longer-term shifts in cerebral vascular function by affecting the blood-brain barrier or perfusion and oxidation in the brain. While these complex relationships need further untangling, the study suggests interventions that cut CHD risk also might help prevent cognitive decline itself and slow the rate of cognitive decline if it occurs.
Dr. Rovio, Dr. Pahkala, and Dr. Raitakari are at the University of Turku (Finland) and Turku University Hospital. These comments are adapted from an editorial accompanying the article by Xie et al. (J Amer Coll Cardiol. 2019 Jun 17. doi: 10.1016/j.jacc.2019.04.020). They reported having no relevant financial disclosures.
according to the results of a large prospective study with a median of 12 years of follow-up.
“We found that incident CHD was significantly associated with faster post–CHD-diagnosis cognitive decline, but not pre–CHD-diagnosis or short-term cognitive decline after the event,” Wuxiang Xie, PhD, of Peking University Health Science Center, Beijing, and associates wrote in the Journal of the American College of Cardiology. Linear mixed models showed that cognitive decline sped up during the year after incident CHD.
Past research had suggested a link between accelerated cognitive decline and CHD, but the temporal pattern of the relationship was unclear. For the study, Dr. Xie and associates followed 7,888 adults from the English Longitudinal Study of Aging who were an average of 62 years old and had no history of stroke, MI, angina, or dementia (Alzheimer’s disease or otherwise). All participants underwent a baseline cognitive assessment for verbal memory, semantic fluency, and temporal orientation, plus a median of six follow-up assessments.
In all, 480 (6%) participants developed CHD during follow-up. Their rate of cognitive decline remained constant before and immediately after their CHD diagnosis, but in subsequent years, they experienced significant accelerations in loss of global cognitive function, verbal memory, and temporal orientation even after accounting for time and many demographic and clinical variables. For example, the slope representing temporal change in global cognitive score decreased by a mean of 0.039 per year, compared with the pre-CHD slope (slope difference, –0.039; 95% confidence interval, –0.063 to –0.015; P =. 002). Semantic fluency also declined faster after CHD, but the difference, compared with before CHD, did not reach statistical significance (P = .11).
Individuals without CHD showed no such accelerations in cognitive decline throughout follow-up in adjusted models, the researchers wrote. “Based on repeated cognitive measurements over a long follow-up period, this study revealed a reliable and robust trajectory of cognitive decline [after CHD]. Future studies are warranted to determine the precise mechanisms linking incident CHD to cognitive decline.”
Funders included the National Natural Science Foundation of China, the Beijing Natural Science Foundation, and the Newton International Fellowship from the Academy of Medical Sciences. The researchers reported having no relevant financial disclosures.
SOURCE: Xie W et al. J Amer Coll Cardiol. 2019 Jun 17. doi: 10.1016/j.jacc.2019.04.019.
according to the results of a large prospective study with a median of 12 years of follow-up.
“We found that incident CHD was significantly associated with faster post–CHD-diagnosis cognitive decline, but not pre–CHD-diagnosis or short-term cognitive decline after the event,” Wuxiang Xie, PhD, of Peking University Health Science Center, Beijing, and associates wrote in the Journal of the American College of Cardiology. Linear mixed models showed that cognitive decline sped up during the year after incident CHD.
Past research had suggested a link between accelerated cognitive decline and CHD, but the temporal pattern of the relationship was unclear. For the study, Dr. Xie and associates followed 7,888 adults from the English Longitudinal Study of Aging who were an average of 62 years old and had no history of stroke, MI, angina, or dementia (Alzheimer’s disease or otherwise). All participants underwent a baseline cognitive assessment for verbal memory, semantic fluency, and temporal orientation, plus a median of six follow-up assessments.
In all, 480 (6%) participants developed CHD during follow-up. Their rate of cognitive decline remained constant before and immediately after their CHD diagnosis, but in subsequent years, they experienced significant accelerations in loss of global cognitive function, verbal memory, and temporal orientation even after accounting for time and many demographic and clinical variables. For example, the slope representing temporal change in global cognitive score decreased by a mean of 0.039 per year, compared with the pre-CHD slope (slope difference, –0.039; 95% confidence interval, –0.063 to –0.015; P =. 002). Semantic fluency also declined faster after CHD, but the difference, compared with before CHD, did not reach statistical significance (P = .11).
Individuals without CHD showed no such accelerations in cognitive decline throughout follow-up in adjusted models, the researchers wrote. “Based on repeated cognitive measurements over a long follow-up period, this study revealed a reliable and robust trajectory of cognitive decline [after CHD]. Future studies are warranted to determine the precise mechanisms linking incident CHD to cognitive decline.”
Funders included the National Natural Science Foundation of China, the Beijing Natural Science Foundation, and the Newton International Fellowship from the Academy of Medical Sciences. The researchers reported having no relevant financial disclosures.
SOURCE: Xie W et al. J Amer Coll Cardiol. 2019 Jun 17. doi: 10.1016/j.jacc.2019.04.019.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Restless legs syndrome in MS linked to cognitive impairment
SEATTLE – The results suggest that sleep dysfunction exacerbated by RLS could affect cognition in patients with MS, study lead author Katie L. Cederberg, CPT, a doctoral student in the department of physical therapy at the University of Alabama at Birmingham, said in an interview. She spoke at the annual meeting of the Consortium of Multiple Sclerosis Centers, where she presented the findings.
“RLS severity did predict cognitive impairment,” she said. However, she added, “this is just a snapshot, and we need to do more research.”
Sleep problems, including RLS, are more common in patients with MS than in the general population. “Current research suggests that anywhere from 19% to 67% of individuals with MS experience some sort of sleep difficulty, with rates as high as 80% in some samples,” a 2015 report noted.
As for RLS, a 2018 systematic review and meta-analysis found that “pooled RLS prevalence among MS patients of various ethnicities was 26%, and prevalence was lower in Asia (20%) than outside Asia (27%). Prevalence was higher among cross-sectional studies (30%) than among case-control studies (23%). RLS prevalence was higher among female than among male MS patients (26% vs. 17%), and it was higher among MS patients than among healthy controls (odds ratio, 3.96, 95% confidence interval, 3.29-4.77, P less than .001) (Sleep Med. 2018 Oct;50:97-104).
Ms. Cederberg said the frequency of RLS in patients with MS spurred her and colleagues to explore whether it may affect cognitive function.
For their study, the researchers surveyed 275 patients with MS (mean age = 60, 81% female, 33% employed, 95% white, 66% with relapsing-remitting MS). Of the 275, 75 appeared to have RLS. These patients were similar to the non-RLS patients in multiple areas, but they diverged in scores on the brief Multiple Sclerosis Neuropsychological Questionnaire, which measures self-perception of cognition.
Those with both MS and RLS scored 21.9 (± 11.7) on the test, while those with MS scored 18.0 (± 11.0), P = 0.023.
Analyses linked greater RLS severity to worse self-perceived cognitive impairment and sleep quality. “The diagnosis and treatment of RLS symptoms and other effectors of sleep quality could improve cognitive consequences of MS,” the authors concluded.
The National MS Society funded the study. The study authors reported no relevant disclosures.
SEATTLE – The results suggest that sleep dysfunction exacerbated by RLS could affect cognition in patients with MS, study lead author Katie L. Cederberg, CPT, a doctoral student in the department of physical therapy at the University of Alabama at Birmingham, said in an interview. She spoke at the annual meeting of the Consortium of Multiple Sclerosis Centers, where she presented the findings.
“RLS severity did predict cognitive impairment,” she said. However, she added, “this is just a snapshot, and we need to do more research.”
Sleep problems, including RLS, are more common in patients with MS than in the general population. “Current research suggests that anywhere from 19% to 67% of individuals with MS experience some sort of sleep difficulty, with rates as high as 80% in some samples,” a 2015 report noted.
As for RLS, a 2018 systematic review and meta-analysis found that “pooled RLS prevalence among MS patients of various ethnicities was 26%, and prevalence was lower in Asia (20%) than outside Asia (27%). Prevalence was higher among cross-sectional studies (30%) than among case-control studies (23%). RLS prevalence was higher among female than among male MS patients (26% vs. 17%), and it was higher among MS patients than among healthy controls (odds ratio, 3.96, 95% confidence interval, 3.29-4.77, P less than .001) (Sleep Med. 2018 Oct;50:97-104).
Ms. Cederberg said the frequency of RLS in patients with MS spurred her and colleagues to explore whether it may affect cognitive function.
For their study, the researchers surveyed 275 patients with MS (mean age = 60, 81% female, 33% employed, 95% white, 66% with relapsing-remitting MS). Of the 275, 75 appeared to have RLS. These patients were similar to the non-RLS patients in multiple areas, but they diverged in scores on the brief Multiple Sclerosis Neuropsychological Questionnaire, which measures self-perception of cognition.
Those with both MS and RLS scored 21.9 (± 11.7) on the test, while those with MS scored 18.0 (± 11.0), P = 0.023.
Analyses linked greater RLS severity to worse self-perceived cognitive impairment and sleep quality. “The diagnosis and treatment of RLS symptoms and other effectors of sleep quality could improve cognitive consequences of MS,” the authors concluded.
The National MS Society funded the study. The study authors reported no relevant disclosures.
SEATTLE – The results suggest that sleep dysfunction exacerbated by RLS could affect cognition in patients with MS, study lead author Katie L. Cederberg, CPT, a doctoral student in the department of physical therapy at the University of Alabama at Birmingham, said in an interview. She spoke at the annual meeting of the Consortium of Multiple Sclerosis Centers, where she presented the findings.
“RLS severity did predict cognitive impairment,” she said. However, she added, “this is just a snapshot, and we need to do more research.”
Sleep problems, including RLS, are more common in patients with MS than in the general population. “Current research suggests that anywhere from 19% to 67% of individuals with MS experience some sort of sleep difficulty, with rates as high as 80% in some samples,” a 2015 report noted.
As for RLS, a 2018 systematic review and meta-analysis found that “pooled RLS prevalence among MS patients of various ethnicities was 26%, and prevalence was lower in Asia (20%) than outside Asia (27%). Prevalence was higher among cross-sectional studies (30%) than among case-control studies (23%). RLS prevalence was higher among female than among male MS patients (26% vs. 17%), and it was higher among MS patients than among healthy controls (odds ratio, 3.96, 95% confidence interval, 3.29-4.77, P less than .001) (Sleep Med. 2018 Oct;50:97-104).
Ms. Cederberg said the frequency of RLS in patients with MS spurred her and colleagues to explore whether it may affect cognitive function.
For their study, the researchers surveyed 275 patients with MS (mean age = 60, 81% female, 33% employed, 95% white, 66% with relapsing-remitting MS). Of the 275, 75 appeared to have RLS. These patients were similar to the non-RLS patients in multiple areas, but they diverged in scores on the brief Multiple Sclerosis Neuropsychological Questionnaire, which measures self-perception of cognition.
Those with both MS and RLS scored 21.9 (± 11.7) on the test, while those with MS scored 18.0 (± 11.0), P = 0.023.
Analyses linked greater RLS severity to worse self-perceived cognitive impairment and sleep quality. “The diagnosis and treatment of RLS symptoms and other effectors of sleep quality could improve cognitive consequences of MS,” the authors concluded.
The National MS Society funded the study. The study authors reported no relevant disclosures.
REPORTING FROM CMSC 2019
From sweet to belligerent in the blink of an eye
CASE Combative and agitated
Ms. P, age 87, presents to the emergency department (ED) with her caregiver, who says Ms. P has new-onset altered mental status, agitation, and combativeness.
Ms. P resides at a long-term care (LTC) facility, where according to the nurses she normally is pleasant, well-oriented, and cooperative. Ms. P’s medical history includes major depressive disorder, generalized anxiety disorder, hypertension, chronic kidney disease (CKD) stage III, peptic ulcer disease, gastroesophageal reflux disease, coronary artery disease with 2 past myocardial infarctions requiring stents, chronic obstructive pulmonary disease, hyperlipidemia, bradycardia requiring a pacemaker, paroxysmal atrial fibrillation, asthma, aortic stenosis, peripheral vascular disease, esophageal stricture requiring dilation, deep vein thrombosis, and migraines.
Mr. P’s medication list includes acetaminophen, 650 mg every 6 hours; ipratropium/albuterol nebulized solution, 3 mL 4 times a day; aspirin, 81 mg/d; atorvastatin, 40 mg/d; calcitonin, 1 spray nasally at bedtime; clopidogrel, 75 mg/d; ezetimibe, 10 mg/d; fluoxetine, 20 mg/d; furosemide, 20 mg/d; isosorbide dinitrate, 120 mg/d; lisinopril, 15 mg/d; risperidone, 0.5 mg/d; magnesium oxide, 800 mg/d; pantoprazole, 40 mg/d; polyethylene glycol, 17 g/d; sotalol, 160 mg/d; olanzapine, 5 mg IM every 6 hours as needed for agitation; and tramadol, 50 mg every 8 hours as needed for headache.
Seven days before coming to the ED, Ms. P was started on ceftriaxone, 1 g/d, for suspected community-acquired pneumonia. At that time, the nursing staff noticed behavioral changes. Soon after, Ms. P began refusing all her medications. Two days before presenting to the ED, Ms. P was started on nitrofurantoin, 200 mg/d, for a suspected urinary tract infection, but it was discontinued because of an allergy.
Her caregiver reports that while at the LTC facility, Ms. P’s behavioral changes worsened. Ms. P claimed to be Jesus Christ and said she was talking to the devil; she chased other residents around the facility and slapped medications away from the nursing staff. According to caregivers, this behavior was out of character.
Shortly after arriving in the ED, Ms. P is admitted to the psychiatric unit.
[polldaddy:10332748]
The authors’ observations
Delirium is a complex, acute alteration in a patient’s mental status compared with his/her baseline functioning1 (Table 12). The onset of delirium is quick, happening within hours to days, with fluctuations in mental function. Patients might present with hyperactive, hypoactive, or mixed delirium.3 Patients with hyperactive delirium often have delusions and hallucinations; these patients might be agitated and could become violent with family and caregivers.3 Patients with hypoactive delirium are less likely to experience hallucinations and more likely to show symptoms of sedation.3 Patients with hypoactive delirium can be difficult to diagnose because it is challenging to interview them and understand what might be the cause of their sedated state. Patients also can exhibit a mixed delirium in which they fluctuate between periods of hyperactivity and hypoactivity.3
Continue to: Suspected delirium...
Suspected delirium should be considered a medical emergency because the outcome could be fatal.1 It is important to uncover and treat the underlying cause(s) of delirium rather than solely administering antipsychotics, which might mask the presenting symptoms. In an older study, Francis and Kapoor4 reported that 56% of geriatric patients with delirium had a single definite or probable etiology, while the other 44% had about 2.8 etiologies per patient on average. Delirium risk factors, causes, and factors to consider during patient evaluation are listed in Table 21,3,5-7 and Table 3.1,3,5-7

A synergistic relationship between comorbidities, environment, and medications can induce delirium.5 Identifying irreversible and reversible causes is the key to treating delirium. After the cause has been identified, it can be addressed and the patient could return to his/her previous level of functioning. If the delirium is the result of multiple irreversible causes, it could become chronic.
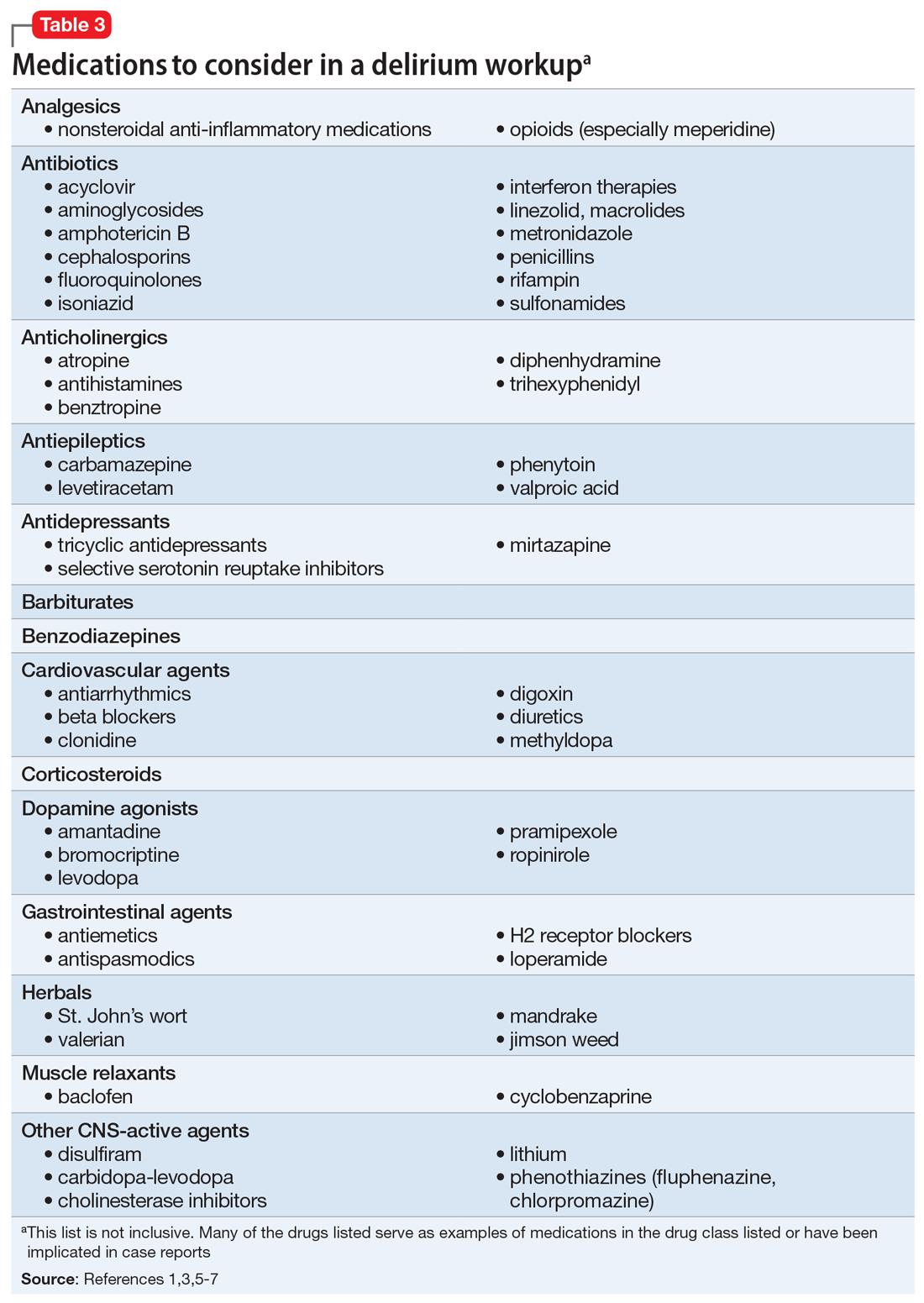
[polldaddy:10332749]
EVALUATION Cardiac dysfunction
Ms. P undergoes laboratory testing. The results include: white blood cell count, 5.9/µL; hemoglobin, 13.6 g/dL; hematocrit, 42.6%; platelets, 304 × 103/µL; sodium,143 mEq/L; potassium, 3.2 mEq/L; chloride, 96 mEq/L; carbon dioxide, 23 mEq/L; blood glucose, 87 mg/dL; creatinine, 1.2 mg/dL; estimated creatinine clearance (eCrCl) level of 33 mL/min/1.73 m2; calcium, 9.5 mg/dL; albumin, 3.6 g/dL; liver enzymes within normal limits; thyroid-stimulating hormone, 0.78 mIU/L; vitamin B12, 995 pg/mL; folic acid, 16.6 ng/mL; vitamin D, 31 pg/mL; and rapid plasma reagin: nonreactive. Urinalysis is unremarkable, and no culture is performed. Urine drug screening/toxicology is positive for the benzodiazepines that she received in the ED (oral alprazolam 0.25 mg given once and oral lorazepam 0.5 mg given once).
Electrocardiogram (ECG) shows atrial flutter/tachycardia with rapid ventricular response, marked left axis deviation, nonspecific ST- and T-wave abnormality, QT/QTC of 301/387 ms, and ventricular rate 151 beats per minute. A CT scan of the head and brain without contrast shows mild atrophy and chronic white matter changes and no acute intracranial abnormality. A two-view chest radiography shows no acute cardiopulmonary findings. Her temperature is 98.4°F; heart rate is 122 beats per minute; respiratory rate is 20 breaths per minute; blood pressure is 161/98 mm Hg; and oxygen saturation is 86% on room air.
Based on this data, Ms. P’s cardiac condition seems to be worsening, which is thought to be caused by her refusal of furosemide, lisinopril, isosorbide, sotalol, clopidogrel, and aspirin. The treatment team plans to work on compliance to resolve these cardiac issues and places Ms. P on 1:1 observation with a sitter and music in attempt to calm her.
Continue to: The authors' observations
The authors’ observations
Many factors can contribute to behavioral or cognitive changes in geriatric patients. Often, a major change noted in an older patient can be attributed to new-onset dementia, dementia with behavioral disturbances, delirium, depression, or acute psychosis. These potential causes should be considered and ruled out in a step-by-step progression. Because patients are unreliable historians during acute distress, a complete history from family or caregivers and exhaustive workup is paramount.
TREATMENT Medication adjustments
In an attempt to resolve Ms. P’s disruptive behaviors, her risperidone dosage is changed to 0.5 mg twice daily. Ms. P is encouraged to use the provided oxygen to raise her saturation level.
On hospital Day 3, a loose stool prompts a Clostridium difficile test as a possible source of delirium; however, the results are negative.
On hospital Day 4, Ms. P is confused and irritable overnight, yelling profanities at staff, refusing care, inappropriately disrobing, and having difficulty falling asleep and staying asleep. Risperidone is discontinued because it appears to have had little or no effect on Ms. P’s disruptive behaviors. Olanzapine, 10 mg/d, is initiated with mirtazapine, 7.5 mg/d, to help with mood, appetite, and sleep. Fluoxetine is also discontinued because of a possible interaction with clopidogrel.
On hospital Days 6 to 8, Ms. P remains upset and unable to follow instructions. Melatonin is initiated to improve her sleep cycle. On Day 9, she continues to decline and is cursing at hospital staff; haloperidol is initiated at 5 mg every morning, 10 mg at bedtime, and 5 mg IM as needed for agitation. Her sleep improves with melatonin and mirtazapine. IV hydration also is initiated. Ms. P has a slight improvement in medication compliance. On Day 11, haloperidol is increased to 5 mg in the morning, 5 mg in the afternoon, and 10 mg at bedtime. On Day 12, haloperidol is changed to 7.5 mg twice daily; a slight improvement in Ms. P’s behavior is noted.
Continue to: On hospital Day 13...
On hospital Day 13, Ms. P’s behavior declines again. She screams profanities at staff and does not recognize the clinicians who have been providing care to her. The physician initiates valproic acid, 125 mg, 3 times a day, to target Ms. P’s behavioral disturbances. A pharmacist notes that the patient’s sotalol could be contributing to Ms. P’s psychiatric presentation, and that based on her eCrCl level of 33 mL/min/1.73 m2, a dosage adjustment or medication change might be warranted.
On Day 14, Ms. P displays erratic behavior and intermittent tachycardia. A cardiac consultation is ordered. A repeat ECG reveals atrial fibrillation with rapid rate and a QT/QTc of 409/432 ms. Ms. P is transferred to the telemetry unit, where the cardiologist discontinues sotalol because the dosage is not properly renally adjusted. Sotalol hydrochloride has been associated with life-threatening ventricular tachycardia.8 Diltiazem, 30 mg every 6 hours is initiated to replace sotalol.
By Day 16, the treatment team notes improved cognition and behavior. On Day 17, the cardiologist reports that Ms. P’s atrial fibrillation is controlled. An ECG reveals mild left ventricular hypertrophy, an ejection fraction of 50% to 55%, no stenosis in the mitral or tricuspid valves, no valvular pulmonic stenosis, and moderate aortic sclerosis. Cardiac markers also are evaluated (creatinine phosphokinase: 105 U/L; creatinine kinase–MB fraction: 2.6 ng/mL; troponin: 0.01 ng/mL; pro-B-type natriuretic peptide: 2,073 pg/mL); and myocardial infarction is ruled out.
On Day 19, Ms. P’s diltiazem is consolidated to a controlled-delivery formulation, 180 mg/d, along with the addition of metoprolol, 12.5 mg twice daily. Ms. P is transferred back to the psychiatric unit.
OUTCOME Gradual improvement
On Days 20 to 23, Ms. P shows remarkable progress, and her mental status, cognition, and behavior slowly return to baseline. Haloperidol and valproic acid are tapered and discontinued. Ms. P is observed to be healthy and oriented to person, place, and time.
Continue to: On Day 25...
On Day 25, she is discharged from the hospital, and returns to the LTC facility.
The authors’ observations
Ms. P’s delirium was a combination of her older age, non-renally adjusted sotalol, and CKD. At admission, the hospital treatment team first thought that pneumonia or antibiotic use could have caused delirium. However, Ms. P’s condition did not improve after antibiotics were stopped. In addition, several chest radiographs found no evidence of pneumonia. It is important to check for any source of infection because infection is a common source of delirium in older patients.1 Urine samples revealed no pathogens, a C. difficile test was negative, and the patient’s white blood cell counts remained within normal limits. Physicians began looking elsewhere for potential causes of Ms. P’s delirium.
Ms. P’s vital signs ruled out a temperature irregularity or hypertension as the cause of her delirium. She has a slightly low oxygen saturation when she first presented, but this quickly returned to normal with administration of oxygen, which ruled out hypoxemia. Laboratory results concluded that Ms. P’s glucose levels were within a normal range and she had no electrolyte imbalances. A head CT scan showed slight atrophy of white matter that is consistent with Ms. P’s age. The head CT scan also showed that Ms. P had no acute condition or head trauma.
In terms of organ function, Ms. P was in relatively healthy condition other than paroxysmal atrial fibrillation and CKD. Chronic kidney disease can interrupt the normal pharmacokinetics of medications. Reviewing Ms. P’s medication list, several agents could have induced delirium, including antidepressants, antipsychotics, cardiovascular medications (beta blocker/antiarrhythmic [sotalol]), and opioid analgesics such as tramadol.5 Ms. P’s condition did not improve after discontinuing fluoxetine, risperidone, or olanzapine, although haloperidol was started in their place. Ms. P scored an 8 on the Naranjo Adverse Drug Reaction Probability Scale, indicating this event was a probable adverse drug reaction.9
Identifying a cause
This was a unique case where sotalol was identified as the culprit for inducing Ms. P’s delirium, because her age and CKD are irreversible. It is important to note that antiarrhythmics can induce arrhythmias when present in high concentrations or administered without appropriate renal dose adjustments. Although Ms. P’s serum levels of sotalol were not evaluated, because of her renal impairment, it is possible that toxic levels of sotalol accumulated and lead to arrhythmias and delirium. Of note, a cardiologist was consulted to safely change Ms. P to a calcium channel blocker so she could undergo cardiac monitoring. With the addition of diltiazem and metoprolol, the patient’s delirium subsided and her arrhythmia was controlled. Once the source of Ms. P’s delirium had been identified, antipsychotics were no longer needed.
Continue to: Bottom Line
Bottom Line
Delirium is a complex disorder that often has multiple causes, both reversible and irreversible. A “process of elimination” approach should be used to accurately identify and manage delirium. If a patient with delirium has little to no response to antipsychotic medications, the underlying cause or causes likely has not yet been addressed, and the evaluation should continue.
Related Resources
- Marcantonio ER. Delirium in hospitalized older adults. N Engl J Med. 2017;377:1456-1466.
- Inouye SK, Westendorp RGJ, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911-922.
Drug Brand Names
Acyclovir • Zovirax
Alprazolam • Niravam, Xanax
Amantadine • Symmetrel
Amphotericin B • Abelcet
Atorvastatin • Lipitor
Atropine • Atropen
Baclofen • EnovaRX-Baclofen
Benztropine • Cogentin
Bromocriptine • Cycloset
Calcitonin • Miacalcin
Carbamazepine • Tegretol
Carbidopa-levodopa • Duopa
Ceftriaxone • Rocephin
Chlorpromazine • Thorazine
Clonidine • Catapres
Clopidogrel • Plavix
Cyclobenzaprine • Amrix
Digoxin • Lanoxin
Diltiazem • Cardizem
Disulfiram • Antabuse
Ezetimibe • Zetia
Fluoxetine • Prozac
Fluphenazine • Prolixin
Furosemide • Lasix
Haloperidol • Haldol
Ipratropium/albuterol nebulized solution • Combivent Respimat
Isoniazid • Isotamine
Isosorbide nitrate • Dilatrate
Levetiracetam • Keppra
Levodopa • Stalevo
Linezolid • Zyvox
Lisinopril • Zestril
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Magnesium Oxide • Mag-200
Meperidine • Demerol
Methyldopa • Aldomet
Metoprolol • Lopressor
Metronidazole • Flagyl
Mirtazapine • Remeron
Nitrofurantoin • Macrobid
Olanzapine • Zyprexa
Pantoprazole • Protonix
Phenytoin • Dilantin
Pramipexole • Mirapex
Rifampin • Rifadin
Risperidone • Risperdal
Ropinirole • Requip
Sotalol hydrochloride • Betapace AF
Tramadol • Ultram
Trihexyphenidyl • Trihexane
Valproic acid • Depakote
1. Fong TG, Tulebaev SR, Inouye SK. Delirium in elderly adults: diagnosis, prevention, and treatment. Nat Rev Neurol. 2009;5(4):210-220.
2. Diagnostic and statistical manual of mental disorders, fifth edition. Washington, DC: American Psychiatric Association; 2013.
3. American Psychiatric Association. Practice guideline for the treatment of patients with delirium. Am J Psychiatry. 1999;156(suppl 5):1-20.
4. Francis J, Kapoor WN. Delirium in hospitalized elderly. J Gen Intern Med. 1990;5(1):65-79.
5. Alagiakrishnan K, Wiens CA. An approach to drug induced delirium in the elderly. Postgrad Med J. 2004;80(945):388-393.
6. Cook IA. Guideline watch: practice guideline for the treatment of patients with delirium. Arlington, VA: American Psychiatric Publishing; 2004.
7. Bourgeois J, Ategan A, Losier B. Delirium in the hospital: emphasis on the management of geriatric patients. Current Psychiatry. 2014;13(8):29,36-42.
8. Betapace AF [package insert]. Zug, Switzerland: Covis Pharma; 2016.
9. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239-245.
CASE Combative and agitated
Ms. P, age 87, presents to the emergency department (ED) with her caregiver, who says Ms. P has new-onset altered mental status, agitation, and combativeness.
Ms. P resides at a long-term care (LTC) facility, where according to the nurses she normally is pleasant, well-oriented, and cooperative. Ms. P’s medical history includes major depressive disorder, generalized anxiety disorder, hypertension, chronic kidney disease (CKD) stage III, peptic ulcer disease, gastroesophageal reflux disease, coronary artery disease with 2 past myocardial infarctions requiring stents, chronic obstructive pulmonary disease, hyperlipidemia, bradycardia requiring a pacemaker, paroxysmal atrial fibrillation, asthma, aortic stenosis, peripheral vascular disease, esophageal stricture requiring dilation, deep vein thrombosis, and migraines.
Mr. P’s medication list includes acetaminophen, 650 mg every 6 hours; ipratropium/albuterol nebulized solution, 3 mL 4 times a day; aspirin, 81 mg/d; atorvastatin, 40 mg/d; calcitonin, 1 spray nasally at bedtime; clopidogrel, 75 mg/d; ezetimibe, 10 mg/d; fluoxetine, 20 mg/d; furosemide, 20 mg/d; isosorbide dinitrate, 120 mg/d; lisinopril, 15 mg/d; risperidone, 0.5 mg/d; magnesium oxide, 800 mg/d; pantoprazole, 40 mg/d; polyethylene glycol, 17 g/d; sotalol, 160 mg/d; olanzapine, 5 mg IM every 6 hours as needed for agitation; and tramadol, 50 mg every 8 hours as needed for headache.
Seven days before coming to the ED, Ms. P was started on ceftriaxone, 1 g/d, for suspected community-acquired pneumonia. At that time, the nursing staff noticed behavioral changes. Soon after, Ms. P began refusing all her medications. Two days before presenting to the ED, Ms. P was started on nitrofurantoin, 200 mg/d, for a suspected urinary tract infection, but it was discontinued because of an allergy.
Her caregiver reports that while at the LTC facility, Ms. P’s behavioral changes worsened. Ms. P claimed to be Jesus Christ and said she was talking to the devil; she chased other residents around the facility and slapped medications away from the nursing staff. According to caregivers, this behavior was out of character.
Shortly after arriving in the ED, Ms. P is admitted to the psychiatric unit.
[polldaddy:10332748]
The authors’ observations
Delirium is a complex, acute alteration in a patient’s mental status compared with his/her baseline functioning1 (Table 12). The onset of delirium is quick, happening within hours to days, with fluctuations in mental function. Patients might present with hyperactive, hypoactive, or mixed delirium.3 Patients with hyperactive delirium often have delusions and hallucinations; these patients might be agitated and could become violent with family and caregivers.3 Patients with hypoactive delirium are less likely to experience hallucinations and more likely to show symptoms of sedation.3 Patients with hypoactive delirium can be difficult to diagnose because it is challenging to interview them and understand what might be the cause of their sedated state. Patients also can exhibit a mixed delirium in which they fluctuate between periods of hyperactivity and hypoactivity.3
Continue to: Suspected delirium...
Suspected delirium should be considered a medical emergency because the outcome could be fatal.1 It is important to uncover and treat the underlying cause(s) of delirium rather than solely administering antipsychotics, which might mask the presenting symptoms. In an older study, Francis and Kapoor4 reported that 56% of geriatric patients with delirium had a single definite or probable etiology, while the other 44% had about 2.8 etiologies per patient on average. Delirium risk factors, causes, and factors to consider during patient evaluation are listed in Table 21,3,5-7 and Table 3.1,3,5-7

A synergistic relationship between comorbidities, environment, and medications can induce delirium.5 Identifying irreversible and reversible causes is the key to treating delirium. After the cause has been identified, it can be addressed and the patient could return to his/her previous level of functioning. If the delirium is the result of multiple irreversible causes, it could become chronic.

[polldaddy:10332749]
EVALUATION Cardiac dysfunction
Ms. P undergoes laboratory testing. The results include: white blood cell count, 5.9/µL; hemoglobin, 13.6 g/dL; hematocrit, 42.6%; platelets, 304 × 103/µL; sodium,143 mEq/L; potassium, 3.2 mEq/L; chloride, 96 mEq/L; carbon dioxide, 23 mEq/L; blood glucose, 87 mg/dL; creatinine, 1.2 mg/dL; estimated creatinine clearance (eCrCl) level of 33 mL/min/1.73 m2; calcium, 9.5 mg/dL; albumin, 3.6 g/dL; liver enzymes within normal limits; thyroid-stimulating hormone, 0.78 mIU/L; vitamin B12, 995 pg/mL; folic acid, 16.6 ng/mL; vitamin D, 31 pg/mL; and rapid plasma reagin: nonreactive. Urinalysis is unremarkable, and no culture is performed. Urine drug screening/toxicology is positive for the benzodiazepines that she received in the ED (oral alprazolam 0.25 mg given once and oral lorazepam 0.5 mg given once).
Electrocardiogram (ECG) shows atrial flutter/tachycardia with rapid ventricular response, marked left axis deviation, nonspecific ST- and T-wave abnormality, QT/QTC of 301/387 ms, and ventricular rate 151 beats per minute. A CT scan of the head and brain without contrast shows mild atrophy and chronic white matter changes and no acute intracranial abnormality. A two-view chest radiography shows no acute cardiopulmonary findings. Her temperature is 98.4°F; heart rate is 122 beats per minute; respiratory rate is 20 breaths per minute; blood pressure is 161/98 mm Hg; and oxygen saturation is 86% on room air.
Based on this data, Ms. P’s cardiac condition seems to be worsening, which is thought to be caused by her refusal of furosemide, lisinopril, isosorbide, sotalol, clopidogrel, and aspirin. The treatment team plans to work on compliance to resolve these cardiac issues and places Ms. P on 1:1 observation with a sitter and music in attempt to calm her.
Continue to: The authors' observations
The authors’ observations
Many factors can contribute to behavioral or cognitive changes in geriatric patients. Often, a major change noted in an older patient can be attributed to new-onset dementia, dementia with behavioral disturbances, delirium, depression, or acute psychosis. These potential causes should be considered and ruled out in a step-by-step progression. Because patients are unreliable historians during acute distress, a complete history from family or caregivers and exhaustive workup is paramount.
TREATMENT Medication adjustments
In an attempt to resolve Ms. P’s disruptive behaviors, her risperidone dosage is changed to 0.5 mg twice daily. Ms. P is encouraged to use the provided oxygen to raise her saturation level.
On hospital Day 3, a loose stool prompts a Clostridium difficile test as a possible source of delirium; however, the results are negative.
On hospital Day 4, Ms. P is confused and irritable overnight, yelling profanities at staff, refusing care, inappropriately disrobing, and having difficulty falling asleep and staying asleep. Risperidone is discontinued because it appears to have had little or no effect on Ms. P’s disruptive behaviors. Olanzapine, 10 mg/d, is initiated with mirtazapine, 7.5 mg/d, to help with mood, appetite, and sleep. Fluoxetine is also discontinued because of a possible interaction with clopidogrel.
On hospital Days 6 to 8, Ms. P remains upset and unable to follow instructions. Melatonin is initiated to improve her sleep cycle. On Day 9, she continues to decline and is cursing at hospital staff; haloperidol is initiated at 5 mg every morning, 10 mg at bedtime, and 5 mg IM as needed for agitation. Her sleep improves with melatonin and mirtazapine. IV hydration also is initiated. Ms. P has a slight improvement in medication compliance. On Day 11, haloperidol is increased to 5 mg in the morning, 5 mg in the afternoon, and 10 mg at bedtime. On Day 12, haloperidol is changed to 7.5 mg twice daily; a slight improvement in Ms. P’s behavior is noted.
Continue to: On hospital Day 13...
On hospital Day 13, Ms. P’s behavior declines again. She screams profanities at staff and does not recognize the clinicians who have been providing care to her. The physician initiates valproic acid, 125 mg, 3 times a day, to target Ms. P’s behavioral disturbances. A pharmacist notes that the patient’s sotalol could be contributing to Ms. P’s psychiatric presentation, and that based on her eCrCl level of 33 mL/min/1.73 m2, a dosage adjustment or medication change might be warranted.
On Day 14, Ms. P displays erratic behavior and intermittent tachycardia. A cardiac consultation is ordered. A repeat ECG reveals atrial fibrillation with rapid rate and a QT/QTc of 409/432 ms. Ms. P is transferred to the telemetry unit, where the cardiologist discontinues sotalol because the dosage is not properly renally adjusted. Sotalol hydrochloride has been associated with life-threatening ventricular tachycardia.8 Diltiazem, 30 mg every 6 hours is initiated to replace sotalol.
By Day 16, the treatment team notes improved cognition and behavior. On Day 17, the cardiologist reports that Ms. P’s atrial fibrillation is controlled. An ECG reveals mild left ventricular hypertrophy, an ejection fraction of 50% to 55%, no stenosis in the mitral or tricuspid valves, no valvular pulmonic stenosis, and moderate aortic sclerosis. Cardiac markers also are evaluated (creatinine phosphokinase: 105 U/L; creatinine kinase–MB fraction: 2.6 ng/mL; troponin: 0.01 ng/mL; pro-B-type natriuretic peptide: 2,073 pg/mL); and myocardial infarction is ruled out.
On Day 19, Ms. P’s diltiazem is consolidated to a controlled-delivery formulation, 180 mg/d, along with the addition of metoprolol, 12.5 mg twice daily. Ms. P is transferred back to the psychiatric unit.
OUTCOME Gradual improvement
On Days 20 to 23, Ms. P shows remarkable progress, and her mental status, cognition, and behavior slowly return to baseline. Haloperidol and valproic acid are tapered and discontinued. Ms. P is observed to be healthy and oriented to person, place, and time.
Continue to: On Day 25...
On Day 25, she is discharged from the hospital, and returns to the LTC facility.
The authors’ observations
Ms. P’s delirium was a combination of her older age, non-renally adjusted sotalol, and CKD. At admission, the hospital treatment team first thought that pneumonia or antibiotic use could have caused delirium. However, Ms. P’s condition did not improve after antibiotics were stopped. In addition, several chest radiographs found no evidence of pneumonia. It is important to check for any source of infection because infection is a common source of delirium in older patients.1 Urine samples revealed no pathogens, a C. difficile test was negative, and the patient’s white blood cell counts remained within normal limits. Physicians began looking elsewhere for potential causes of Ms. P’s delirium.
Ms. P’s vital signs ruled out a temperature irregularity or hypertension as the cause of her delirium. She has a slightly low oxygen saturation when she first presented, but this quickly returned to normal with administration of oxygen, which ruled out hypoxemia. Laboratory results concluded that Ms. P’s glucose levels were within a normal range and she had no electrolyte imbalances. A head CT scan showed slight atrophy of white matter that is consistent with Ms. P’s age. The head CT scan also showed that Ms. P had no acute condition or head trauma.
In terms of organ function, Ms. P was in relatively healthy condition other than paroxysmal atrial fibrillation and CKD. Chronic kidney disease can interrupt the normal pharmacokinetics of medications. Reviewing Ms. P’s medication list, several agents could have induced delirium, including antidepressants, antipsychotics, cardiovascular medications (beta blocker/antiarrhythmic [sotalol]), and opioid analgesics such as tramadol.5 Ms. P’s condition did not improve after discontinuing fluoxetine, risperidone, or olanzapine, although haloperidol was started in their place. Ms. P scored an 8 on the Naranjo Adverse Drug Reaction Probability Scale, indicating this event was a probable adverse drug reaction.9
Identifying a cause
This was a unique case where sotalol was identified as the culprit for inducing Ms. P’s delirium, because her age and CKD are irreversible. It is important to note that antiarrhythmics can induce arrhythmias when present in high concentrations or administered without appropriate renal dose adjustments. Although Ms. P’s serum levels of sotalol were not evaluated, because of her renal impairment, it is possible that toxic levels of sotalol accumulated and lead to arrhythmias and delirium. Of note, a cardiologist was consulted to safely change Ms. P to a calcium channel blocker so she could undergo cardiac monitoring. With the addition of diltiazem and metoprolol, the patient’s delirium subsided and her arrhythmia was controlled. Once the source of Ms. P’s delirium had been identified, antipsychotics were no longer needed.
Continue to: Bottom Line
Bottom Line
Delirium is a complex disorder that often has multiple causes, both reversible and irreversible. A “process of elimination” approach should be used to accurately identify and manage delirium. If a patient with delirium has little to no response to antipsychotic medications, the underlying cause or causes likely has not yet been addressed, and the evaluation should continue.
Related Resources
- Marcantonio ER. Delirium in hospitalized older adults. N Engl J Med. 2017;377:1456-1466.
- Inouye SK, Westendorp RGJ, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911-922.
Drug Brand Names
Acyclovir • Zovirax
Alprazolam • Niravam, Xanax
Amantadine • Symmetrel
Amphotericin B • Abelcet
Atorvastatin • Lipitor
Atropine • Atropen
Baclofen • EnovaRX-Baclofen
Benztropine • Cogentin
Bromocriptine • Cycloset
Calcitonin • Miacalcin
Carbamazepine • Tegretol
Carbidopa-levodopa • Duopa
Ceftriaxone • Rocephin
Chlorpromazine • Thorazine
Clonidine • Catapres
Clopidogrel • Plavix
Cyclobenzaprine • Amrix
Digoxin • Lanoxin
Diltiazem • Cardizem
Disulfiram • Antabuse
Ezetimibe • Zetia
Fluoxetine • Prozac
Fluphenazine • Prolixin
Furosemide • Lasix
Haloperidol • Haldol
Ipratropium/albuterol nebulized solution • Combivent Respimat
Isoniazid • Isotamine
Isosorbide nitrate • Dilatrate
Levetiracetam • Keppra
Levodopa • Stalevo
Linezolid • Zyvox
Lisinopril • Zestril
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Magnesium Oxide • Mag-200
Meperidine • Demerol
Methyldopa • Aldomet
Metoprolol • Lopressor
Metronidazole • Flagyl
Mirtazapine • Remeron
Nitrofurantoin • Macrobid
Olanzapine • Zyprexa
Pantoprazole • Protonix
Phenytoin • Dilantin
Pramipexole • Mirapex
Rifampin • Rifadin
Risperidone • Risperdal
Ropinirole • Requip
Sotalol hydrochloride • Betapace AF
Tramadol • Ultram
Trihexyphenidyl • Trihexane
Valproic acid • Depakote
CASE Combative and agitated
Ms. P, age 87, presents to the emergency department (ED) with her caregiver, who says Ms. P has new-onset altered mental status, agitation, and combativeness.
Ms. P resides at a long-term care (LTC) facility, where according to the nurses she normally is pleasant, well-oriented, and cooperative. Ms. P’s medical history includes major depressive disorder, generalized anxiety disorder, hypertension, chronic kidney disease (CKD) stage III, peptic ulcer disease, gastroesophageal reflux disease, coronary artery disease with 2 past myocardial infarctions requiring stents, chronic obstructive pulmonary disease, hyperlipidemia, bradycardia requiring a pacemaker, paroxysmal atrial fibrillation, asthma, aortic stenosis, peripheral vascular disease, esophageal stricture requiring dilation, deep vein thrombosis, and migraines.
Mr. P’s medication list includes acetaminophen, 650 mg every 6 hours; ipratropium/albuterol nebulized solution, 3 mL 4 times a day; aspirin, 81 mg/d; atorvastatin, 40 mg/d; calcitonin, 1 spray nasally at bedtime; clopidogrel, 75 mg/d; ezetimibe, 10 mg/d; fluoxetine, 20 mg/d; furosemide, 20 mg/d; isosorbide dinitrate, 120 mg/d; lisinopril, 15 mg/d; risperidone, 0.5 mg/d; magnesium oxide, 800 mg/d; pantoprazole, 40 mg/d; polyethylene glycol, 17 g/d; sotalol, 160 mg/d; olanzapine, 5 mg IM every 6 hours as needed for agitation; and tramadol, 50 mg every 8 hours as needed for headache.
Seven days before coming to the ED, Ms. P was started on ceftriaxone, 1 g/d, for suspected community-acquired pneumonia. At that time, the nursing staff noticed behavioral changes. Soon after, Ms. P began refusing all her medications. Two days before presenting to the ED, Ms. P was started on nitrofurantoin, 200 mg/d, for a suspected urinary tract infection, but it was discontinued because of an allergy.
Her caregiver reports that while at the LTC facility, Ms. P’s behavioral changes worsened. Ms. P claimed to be Jesus Christ and said she was talking to the devil; she chased other residents around the facility and slapped medications away from the nursing staff. According to caregivers, this behavior was out of character.
Shortly after arriving in the ED, Ms. P is admitted to the psychiatric unit.
[polldaddy:10332748]
The authors’ observations
Delirium is a complex, acute alteration in a patient’s mental status compared with his/her baseline functioning1 (Table 12). The onset of delirium is quick, happening within hours to days, with fluctuations in mental function. Patients might present with hyperactive, hypoactive, or mixed delirium.3 Patients with hyperactive delirium often have delusions and hallucinations; these patients might be agitated and could become violent with family and caregivers.3 Patients with hypoactive delirium are less likely to experience hallucinations and more likely to show symptoms of sedation.3 Patients with hypoactive delirium can be difficult to diagnose because it is challenging to interview them and understand what might be the cause of their sedated state. Patients also can exhibit a mixed delirium in which they fluctuate between periods of hyperactivity and hypoactivity.3
Continue to: Suspected delirium...
Suspected delirium should be considered a medical emergency because the outcome could be fatal.1 It is important to uncover and treat the underlying cause(s) of delirium rather than solely administering antipsychotics, which might mask the presenting symptoms. In an older study, Francis and Kapoor4 reported that 56% of geriatric patients with delirium had a single definite or probable etiology, while the other 44% had about 2.8 etiologies per patient on average. Delirium risk factors, causes, and factors to consider during patient evaluation are listed in Table 21,3,5-7 and Table 3.1,3,5-7

A synergistic relationship between comorbidities, environment, and medications can induce delirium.5 Identifying irreversible and reversible causes is the key to treating delirium. After the cause has been identified, it can be addressed and the patient could return to his/her previous level of functioning. If the delirium is the result of multiple irreversible causes, it could become chronic.

[polldaddy:10332749]
EVALUATION Cardiac dysfunction
Ms. P undergoes laboratory testing. The results include: white blood cell count, 5.9/µL; hemoglobin, 13.6 g/dL; hematocrit, 42.6%; platelets, 304 × 103/µL; sodium,143 mEq/L; potassium, 3.2 mEq/L; chloride, 96 mEq/L; carbon dioxide, 23 mEq/L; blood glucose, 87 mg/dL; creatinine, 1.2 mg/dL; estimated creatinine clearance (eCrCl) level of 33 mL/min/1.73 m2; calcium, 9.5 mg/dL; albumin, 3.6 g/dL; liver enzymes within normal limits; thyroid-stimulating hormone, 0.78 mIU/L; vitamin B12, 995 pg/mL; folic acid, 16.6 ng/mL; vitamin D, 31 pg/mL; and rapid plasma reagin: nonreactive. Urinalysis is unremarkable, and no culture is performed. Urine drug screening/toxicology is positive for the benzodiazepines that she received in the ED (oral alprazolam 0.25 mg given once and oral lorazepam 0.5 mg given once).
Electrocardiogram (ECG) shows atrial flutter/tachycardia with rapid ventricular response, marked left axis deviation, nonspecific ST- and T-wave abnormality, QT/QTC of 301/387 ms, and ventricular rate 151 beats per minute. A CT scan of the head and brain without contrast shows mild atrophy and chronic white matter changes and no acute intracranial abnormality. A two-view chest radiography shows no acute cardiopulmonary findings. Her temperature is 98.4°F; heart rate is 122 beats per minute; respiratory rate is 20 breaths per minute; blood pressure is 161/98 mm Hg; and oxygen saturation is 86% on room air.
Based on this data, Ms. P’s cardiac condition seems to be worsening, which is thought to be caused by her refusal of furosemide, lisinopril, isosorbide, sotalol, clopidogrel, and aspirin. The treatment team plans to work on compliance to resolve these cardiac issues and places Ms. P on 1:1 observation with a sitter and music in attempt to calm her.
Continue to: The authors' observations
The authors’ observations
Many factors can contribute to behavioral or cognitive changes in geriatric patients. Often, a major change noted in an older patient can be attributed to new-onset dementia, dementia with behavioral disturbances, delirium, depression, or acute psychosis. These potential causes should be considered and ruled out in a step-by-step progression. Because patients are unreliable historians during acute distress, a complete history from family or caregivers and exhaustive workup is paramount.
TREATMENT Medication adjustments
In an attempt to resolve Ms. P’s disruptive behaviors, her risperidone dosage is changed to 0.5 mg twice daily. Ms. P is encouraged to use the provided oxygen to raise her saturation level.
On hospital Day 3, a loose stool prompts a Clostridium difficile test as a possible source of delirium; however, the results are negative.
On hospital Day 4, Ms. P is confused and irritable overnight, yelling profanities at staff, refusing care, inappropriately disrobing, and having difficulty falling asleep and staying asleep. Risperidone is discontinued because it appears to have had little or no effect on Ms. P’s disruptive behaviors. Olanzapine, 10 mg/d, is initiated with mirtazapine, 7.5 mg/d, to help with mood, appetite, and sleep. Fluoxetine is also discontinued because of a possible interaction with clopidogrel.
On hospital Days 6 to 8, Ms. P remains upset and unable to follow instructions. Melatonin is initiated to improve her sleep cycle. On Day 9, she continues to decline and is cursing at hospital staff; haloperidol is initiated at 5 mg every morning, 10 mg at bedtime, and 5 mg IM as needed for agitation. Her sleep improves with melatonin and mirtazapine. IV hydration also is initiated. Ms. P has a slight improvement in medication compliance. On Day 11, haloperidol is increased to 5 mg in the morning, 5 mg in the afternoon, and 10 mg at bedtime. On Day 12, haloperidol is changed to 7.5 mg twice daily; a slight improvement in Ms. P’s behavior is noted.
Continue to: On hospital Day 13...
On hospital Day 13, Ms. P’s behavior declines again. She screams profanities at staff and does not recognize the clinicians who have been providing care to her. The physician initiates valproic acid, 125 mg, 3 times a day, to target Ms. P’s behavioral disturbances. A pharmacist notes that the patient’s sotalol could be contributing to Ms. P’s psychiatric presentation, and that based on her eCrCl level of 33 mL/min/1.73 m2, a dosage adjustment or medication change might be warranted.
On Day 14, Ms. P displays erratic behavior and intermittent tachycardia. A cardiac consultation is ordered. A repeat ECG reveals atrial fibrillation with rapid rate and a QT/QTc of 409/432 ms. Ms. P is transferred to the telemetry unit, where the cardiologist discontinues sotalol because the dosage is not properly renally adjusted. Sotalol hydrochloride has been associated with life-threatening ventricular tachycardia.8 Diltiazem, 30 mg every 6 hours is initiated to replace sotalol.
By Day 16, the treatment team notes improved cognition and behavior. On Day 17, the cardiologist reports that Ms. P’s atrial fibrillation is controlled. An ECG reveals mild left ventricular hypertrophy, an ejection fraction of 50% to 55%, no stenosis in the mitral or tricuspid valves, no valvular pulmonic stenosis, and moderate aortic sclerosis. Cardiac markers also are evaluated (creatinine phosphokinase: 105 U/L; creatinine kinase–MB fraction: 2.6 ng/mL; troponin: 0.01 ng/mL; pro-B-type natriuretic peptide: 2,073 pg/mL); and myocardial infarction is ruled out.
On Day 19, Ms. P’s diltiazem is consolidated to a controlled-delivery formulation, 180 mg/d, along with the addition of metoprolol, 12.5 mg twice daily. Ms. P is transferred back to the psychiatric unit.
OUTCOME Gradual improvement
On Days 20 to 23, Ms. P shows remarkable progress, and her mental status, cognition, and behavior slowly return to baseline. Haloperidol and valproic acid are tapered and discontinued. Ms. P is observed to be healthy and oriented to person, place, and time.
Continue to: On Day 25...
On Day 25, she is discharged from the hospital, and returns to the LTC facility.
The authors’ observations
Ms. P’s delirium was a combination of her older age, non-renally adjusted sotalol, and CKD. At admission, the hospital treatment team first thought that pneumonia or antibiotic use could have caused delirium. However, Ms. P’s condition did not improve after antibiotics were stopped. In addition, several chest radiographs found no evidence of pneumonia. It is important to check for any source of infection because infection is a common source of delirium in older patients.1 Urine samples revealed no pathogens, a C. difficile test was negative, and the patient’s white blood cell counts remained within normal limits. Physicians began looking elsewhere for potential causes of Ms. P’s delirium.
Ms. P’s vital signs ruled out a temperature irregularity or hypertension as the cause of her delirium. She has a slightly low oxygen saturation when she first presented, but this quickly returned to normal with administration of oxygen, which ruled out hypoxemia. Laboratory results concluded that Ms. P’s glucose levels were within a normal range and she had no electrolyte imbalances. A head CT scan showed slight atrophy of white matter that is consistent with Ms. P’s age. The head CT scan also showed that Ms. P had no acute condition or head trauma.
In terms of organ function, Ms. P was in relatively healthy condition other than paroxysmal atrial fibrillation and CKD. Chronic kidney disease can interrupt the normal pharmacokinetics of medications. Reviewing Ms. P’s medication list, several agents could have induced delirium, including antidepressants, antipsychotics, cardiovascular medications (beta blocker/antiarrhythmic [sotalol]), and opioid analgesics such as tramadol.5 Ms. P’s condition did not improve after discontinuing fluoxetine, risperidone, or olanzapine, although haloperidol was started in their place. Ms. P scored an 8 on the Naranjo Adverse Drug Reaction Probability Scale, indicating this event was a probable adverse drug reaction.9
Identifying a cause
This was a unique case where sotalol was identified as the culprit for inducing Ms. P’s delirium, because her age and CKD are irreversible. It is important to note that antiarrhythmics can induce arrhythmias when present in high concentrations or administered without appropriate renal dose adjustments. Although Ms. P’s serum levels of sotalol were not evaluated, because of her renal impairment, it is possible that toxic levels of sotalol accumulated and lead to arrhythmias and delirium. Of note, a cardiologist was consulted to safely change Ms. P to a calcium channel blocker so she could undergo cardiac monitoring. With the addition of diltiazem and metoprolol, the patient’s delirium subsided and her arrhythmia was controlled. Once the source of Ms. P’s delirium had been identified, antipsychotics were no longer needed.
Continue to: Bottom Line
Bottom Line
Delirium is a complex disorder that often has multiple causes, both reversible and irreversible. A “process of elimination” approach should be used to accurately identify and manage delirium. If a patient with delirium has little to no response to antipsychotic medications, the underlying cause or causes likely has not yet been addressed, and the evaluation should continue.
Related Resources
- Marcantonio ER. Delirium in hospitalized older adults. N Engl J Med. 2017;377:1456-1466.
- Inouye SK, Westendorp RGJ, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911-922.
Drug Brand Names
Acyclovir • Zovirax
Alprazolam • Niravam, Xanax
Amantadine • Symmetrel
Amphotericin B • Abelcet
Atorvastatin • Lipitor
Atropine • Atropen
Baclofen • EnovaRX-Baclofen
Benztropine • Cogentin
Bromocriptine • Cycloset
Calcitonin • Miacalcin
Carbamazepine • Tegretol
Carbidopa-levodopa • Duopa
Ceftriaxone • Rocephin
Chlorpromazine • Thorazine
Clonidine • Catapres
Clopidogrel • Plavix
Cyclobenzaprine • Amrix
Digoxin • Lanoxin
Diltiazem • Cardizem
Disulfiram • Antabuse
Ezetimibe • Zetia
Fluoxetine • Prozac
Fluphenazine • Prolixin
Furosemide • Lasix
Haloperidol • Haldol
Ipratropium/albuterol nebulized solution • Combivent Respimat
Isoniazid • Isotamine
Isosorbide nitrate • Dilatrate
Levetiracetam • Keppra
Levodopa • Stalevo
Linezolid • Zyvox
Lisinopril • Zestril
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Magnesium Oxide • Mag-200
Meperidine • Demerol
Methyldopa • Aldomet
Metoprolol • Lopressor
Metronidazole • Flagyl
Mirtazapine • Remeron
Nitrofurantoin • Macrobid
Olanzapine • Zyprexa
Pantoprazole • Protonix
Phenytoin • Dilantin
Pramipexole • Mirapex
Rifampin • Rifadin
Risperidone • Risperdal
Ropinirole • Requip
Sotalol hydrochloride • Betapace AF
Tramadol • Ultram
Trihexyphenidyl • Trihexane
Valproic acid • Depakote
1. Fong TG, Tulebaev SR, Inouye SK. Delirium in elderly adults: diagnosis, prevention, and treatment. Nat Rev Neurol. 2009;5(4):210-220.
2. Diagnostic and statistical manual of mental disorders, fifth edition. Washington, DC: American Psychiatric Association; 2013.
3. American Psychiatric Association. Practice guideline for the treatment of patients with delirium. Am J Psychiatry. 1999;156(suppl 5):1-20.
4. Francis J, Kapoor WN. Delirium in hospitalized elderly. J Gen Intern Med. 1990;5(1):65-79.
5. Alagiakrishnan K, Wiens CA. An approach to drug induced delirium in the elderly. Postgrad Med J. 2004;80(945):388-393.
6. Cook IA. Guideline watch: practice guideline for the treatment of patients with delirium. Arlington, VA: American Psychiatric Publishing; 2004.
7. Bourgeois J, Ategan A, Losier B. Delirium in the hospital: emphasis on the management of geriatric patients. Current Psychiatry. 2014;13(8):29,36-42.
8. Betapace AF [package insert]. Zug, Switzerland: Covis Pharma; 2016.
9. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239-245.
1. Fong TG, Tulebaev SR, Inouye SK. Delirium in elderly adults: diagnosis, prevention, and treatment. Nat Rev Neurol. 2009;5(4):210-220.
2. Diagnostic and statistical manual of mental disorders, fifth edition. Washington, DC: American Psychiatric Association; 2013.
3. American Psychiatric Association. Practice guideline for the treatment of patients with delirium. Am J Psychiatry. 1999;156(suppl 5):1-20.
4. Francis J, Kapoor WN. Delirium in hospitalized elderly. J Gen Intern Med. 1990;5(1):65-79.
5. Alagiakrishnan K, Wiens CA. An approach to drug induced delirium in the elderly. Postgrad Med J. 2004;80(945):388-393.
6. Cook IA. Guideline watch: practice guideline for the treatment of patients with delirium. Arlington, VA: American Psychiatric Publishing; 2004.
7. Bourgeois J, Ategan A, Losier B. Delirium in the hospital: emphasis on the management of geriatric patients. Current Psychiatry. 2014;13(8):29,36-42.
8. Betapace AF [package insert]. Zug, Switzerland: Covis Pharma; 2016.
9. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239-245.
Coding variants in apolipoprotein B may be associated with early-onset Alzheimer’s disease
Variants in the apolipoprotein B gene (APOB), which creates the main protein in low-density and very low-density cholesterol, may be associated with early-onset Alzheimer’s disease, Thomas Wingo, MD, and his colleagues have determined.
The finding may help fill out the genetic risk picture for early-onset Alzheimer’s disease (EOAD), said Dr. Wingo of the Atlanta Veterans Affairs Medical Center. The study found that the already-known genetic markers for EOAD – mutations of the presenilin (PSEN) 1 and 2 genes and amyloid precursor protein (APP) – account for just a small fraction of cases.
“To place the genetic association between APOB and EOAD in context, we note that only 3.4% of all EOAD cases in our combined data set showed a known pathogenic mutation, and we found a stronger association between EOAD and rare coding variants in APOB, compared with PSEN1 in our fully adjusted analysis,” the team wrote. However, “approximately 5.0% of patients with EOAD and 1.7% of controls were found to harbor a rare coding polymorphism in APOB that is likely to disrupt the structure, functions, or abundance of ApoB protein.”
The team conducted genetic analysis on plasma samples from 2,125 EOAD and control subjects included in several research cohorts. They first determined the association between cholesterol and EOAD, and then the frequency of variants in apolipoprotein E epsilon 4 (APOE e4), APP, PSEN1, PSEN2, and ApoB. Gene sequencing revealed that 3.4% of samples showed mutations in APP, PSEN1, or PSEN2.
“Given the strong associations between APOE e4 and EOAD and elevated circulating LDL cholesterol levels, we expected individuals with EOAD to have elevated LDL levels,” the team said. But an analysis of 267 of the samples for lipid levels found that, even after the researchers controlled for APOE e4, EOAD cases had higher total cholesterol, low-density cholesterol, and plasma ApoB, compared with controls. However, they found no association between EOAD and high-density lipoprotein or triglycerides.
“Because total cholesterol largely consists of LDL-C, and ApoB is the main lipoprotein of LDL-C, these findings are consistent with one another.
“From these data, we estimated that LDL-C explains 7.6% of the variance in liability to EOAD, independently of APOE e4 ... These results demonstrate that elevated levels of LDL-C [and ApoB] were significantly associated with increased EOAD risk, and this effect was only partially mediated by APOE e4 genotype.”
The results also raised a question: What was driving the association between LDL and EOAD? Because variants of the ApoB gene can either raise or lower LDL, the team examined variants associated with coding changes. These variants were significantly more common in EOAD cases than in controls (5.0% vs. 1.7%).
“Two affected individuals ... were compound heterozygotes, with the remainder being heterozygotes,” the researchers wrote. “Each compound heterozygote case was heterozygous for two different rare coding sites ... Of these four variants, only [one] has been previously described.”
“Our finding of a significant association between rare coding variants in APOB and EOAD independently of APOE is novel, important, and consistent with multiple genome-wide association studies that revealed strong associations between late-onset AD and common intron markers of genes involved in brain cholesterol metabolism [ABCA7, BIN1, CLU, and SORL1]. Furthermore, mice overexpressing ApoB show hyperlipidemia, neurodegeneration, increases in APP, accumulation of amyloid plaques, and cognitive impairment similar to mice overexpressing wild-type human APP. Collectively, these studies and our findings suggest an important role of cholesterol metabolism in AD pathogenesis.”
This research was supported by grants from the Veterans Health Administration, the National Institutes of Health, the To Remember Foundation, the Douglas French Alzheimer’s Foundation, and a contract with the State of California Department of Health Services. Several authors reported financial ties to pharmaceutical companies outside of this work.
SOURCE: Wingo TS et al. JAMA Neurol. 2019 May 28. doi: 10.1001/jamaneurol.2019.0648.
This important study provides the first evidence that rare genetic coding variants of apolipoprotein B may contribute to the risk of early-onset Alzheimer’s disease, Makoto Ishii, MD, PhD, wrote in an accompanying editorial (JAMA Neurol. 2019 May 28. doi: 10.1001/jamaneurol.2019.0212).
But the study by Wingo et al. doesn’t tell the entire tale, he wrote.
The results from this study “found that there are likely to be additional contributing factors independent of APOB and APOE. These may include rare variants in other genes involved directly in LDL cholesterol metabolism, such as the LDL receptor and proprotein convertase subtilisin/kexin type 913 or factors known to modulate circulating LDL cholesterol levels, such as thyroid hormones.”
Although intriguing, “Clearly, additional studies looking at these factors are needed to fully elucidate the association between LDL cholesterol and EOAD. Furthermore, as the authors of this study note, it is not known if there are protective variants of APOB that would decrease the risk for developing EOAD. Identifying such a protective coding variant of APOB would greatly strengthen the link between APOB and AD pathogenesis.”
Prior studies of circulating APOB levels in humans have reached disparate conclusions. A large population-based study found no association between APOB levels and incident dementia or Alzheimer’s, he noted.
“Therefore, whether these findings can be verified in individuals with late-onset AD remains to be determined.”
Dr. Ishii is with the Feil Family Brain and Mind Research Institute in the department of neurology at Cornell University, New York. He has no relevant disclosures.
This important study provides the first evidence that rare genetic coding variants of apolipoprotein B may contribute to the risk of early-onset Alzheimer’s disease, Makoto Ishii, MD, PhD, wrote in an accompanying editorial (JAMA Neurol. 2019 May 28. doi: 10.1001/jamaneurol.2019.0212).
But the study by Wingo et al. doesn’t tell the entire tale, he wrote.
The results from this study “found that there are likely to be additional contributing factors independent of APOB and APOE. These may include rare variants in other genes involved directly in LDL cholesterol metabolism, such as the LDL receptor and proprotein convertase subtilisin/kexin type 913 or factors known to modulate circulating LDL cholesterol levels, such as thyroid hormones.”
Although intriguing, “Clearly, additional studies looking at these factors are needed to fully elucidate the association between LDL cholesterol and EOAD. Furthermore, as the authors of this study note, it is not known if there are protective variants of APOB that would decrease the risk for developing EOAD. Identifying such a protective coding variant of APOB would greatly strengthen the link between APOB and AD pathogenesis.”
Prior studies of circulating APOB levels in humans have reached disparate conclusions. A large population-based study found no association between APOB levels and incident dementia or Alzheimer’s, he noted.
“Therefore, whether these findings can be verified in individuals with late-onset AD remains to be determined.”
Dr. Ishii is with the Feil Family Brain and Mind Research Institute in the department of neurology at Cornell University, New York. He has no relevant disclosures.
This important study provides the first evidence that rare genetic coding variants of apolipoprotein B may contribute to the risk of early-onset Alzheimer’s disease, Makoto Ishii, MD, PhD, wrote in an accompanying editorial (JAMA Neurol. 2019 May 28. doi: 10.1001/jamaneurol.2019.0212).
But the study by Wingo et al. doesn’t tell the entire tale, he wrote.
The results from this study “found that there are likely to be additional contributing factors independent of APOB and APOE. These may include rare variants in other genes involved directly in LDL cholesterol metabolism, such as the LDL receptor and proprotein convertase subtilisin/kexin type 913 or factors known to modulate circulating LDL cholesterol levels, such as thyroid hormones.”
Although intriguing, “Clearly, additional studies looking at these factors are needed to fully elucidate the association between LDL cholesterol and EOAD. Furthermore, as the authors of this study note, it is not known if there are protective variants of APOB that would decrease the risk for developing EOAD. Identifying such a protective coding variant of APOB would greatly strengthen the link between APOB and AD pathogenesis.”
Prior studies of circulating APOB levels in humans have reached disparate conclusions. A large population-based study found no association between APOB levels and incident dementia or Alzheimer’s, he noted.
“Therefore, whether these findings can be verified in individuals with late-onset AD remains to be determined.”
Dr. Ishii is with the Feil Family Brain and Mind Research Institute in the department of neurology at Cornell University, New York. He has no relevant disclosures.
Variants in the apolipoprotein B gene (APOB), which creates the main protein in low-density and very low-density cholesterol, may be associated with early-onset Alzheimer’s disease, Thomas Wingo, MD, and his colleagues have determined.
The finding may help fill out the genetic risk picture for early-onset Alzheimer’s disease (EOAD), said Dr. Wingo of the Atlanta Veterans Affairs Medical Center. The study found that the already-known genetic markers for EOAD – mutations of the presenilin (PSEN) 1 and 2 genes and amyloid precursor protein (APP) – account for just a small fraction of cases.
“To place the genetic association between APOB and EOAD in context, we note that only 3.4% of all EOAD cases in our combined data set showed a known pathogenic mutation, and we found a stronger association between EOAD and rare coding variants in APOB, compared with PSEN1 in our fully adjusted analysis,” the team wrote. However, “approximately 5.0% of patients with EOAD and 1.7% of controls were found to harbor a rare coding polymorphism in APOB that is likely to disrupt the structure, functions, or abundance of ApoB protein.”
The team conducted genetic analysis on plasma samples from 2,125 EOAD and control subjects included in several research cohorts. They first determined the association between cholesterol and EOAD, and then the frequency of variants in apolipoprotein E epsilon 4 (APOE e4), APP, PSEN1, PSEN2, and ApoB. Gene sequencing revealed that 3.4% of samples showed mutations in APP, PSEN1, or PSEN2.
“Given the strong associations between APOE e4 and EOAD and elevated circulating LDL cholesterol levels, we expected individuals with EOAD to have elevated LDL levels,” the team said. But an analysis of 267 of the samples for lipid levels found that, even after the researchers controlled for APOE e4, EOAD cases had higher total cholesterol, low-density cholesterol, and plasma ApoB, compared with controls. However, they found no association between EOAD and high-density lipoprotein or triglycerides.
“Because total cholesterol largely consists of LDL-C, and ApoB is the main lipoprotein of LDL-C, these findings are consistent with one another.
“From these data, we estimated that LDL-C explains 7.6% of the variance in liability to EOAD, independently of APOE e4 ... These results demonstrate that elevated levels of LDL-C [and ApoB] were significantly associated with increased EOAD risk, and this effect was only partially mediated by APOE e4 genotype.”
The results also raised a question: What was driving the association between LDL and EOAD? Because variants of the ApoB gene can either raise or lower LDL, the team examined variants associated with coding changes. These variants were significantly more common in EOAD cases than in controls (5.0% vs. 1.7%).
“Two affected individuals ... were compound heterozygotes, with the remainder being heterozygotes,” the researchers wrote. “Each compound heterozygote case was heterozygous for two different rare coding sites ... Of these four variants, only [one] has been previously described.”
“Our finding of a significant association between rare coding variants in APOB and EOAD independently of APOE is novel, important, and consistent with multiple genome-wide association studies that revealed strong associations between late-onset AD and common intron markers of genes involved in brain cholesterol metabolism [ABCA7, BIN1, CLU, and SORL1]. Furthermore, mice overexpressing ApoB show hyperlipidemia, neurodegeneration, increases in APP, accumulation of amyloid plaques, and cognitive impairment similar to mice overexpressing wild-type human APP. Collectively, these studies and our findings suggest an important role of cholesterol metabolism in AD pathogenesis.”
This research was supported by grants from the Veterans Health Administration, the National Institutes of Health, the To Remember Foundation, the Douglas French Alzheimer’s Foundation, and a contract with the State of California Department of Health Services. Several authors reported financial ties to pharmaceutical companies outside of this work.
SOURCE: Wingo TS et al. JAMA Neurol. 2019 May 28. doi: 10.1001/jamaneurol.2019.0648.
Variants in the apolipoprotein B gene (APOB), which creates the main protein in low-density and very low-density cholesterol, may be associated with early-onset Alzheimer’s disease, Thomas Wingo, MD, and his colleagues have determined.
The finding may help fill out the genetic risk picture for early-onset Alzheimer’s disease (EOAD), said Dr. Wingo of the Atlanta Veterans Affairs Medical Center. The study found that the already-known genetic markers for EOAD – mutations of the presenilin (PSEN) 1 and 2 genes and amyloid precursor protein (APP) – account for just a small fraction of cases.
“To place the genetic association between APOB and EOAD in context, we note that only 3.4% of all EOAD cases in our combined data set showed a known pathogenic mutation, and we found a stronger association between EOAD and rare coding variants in APOB, compared with PSEN1 in our fully adjusted analysis,” the team wrote. However, “approximately 5.0% of patients with EOAD and 1.7% of controls were found to harbor a rare coding polymorphism in APOB that is likely to disrupt the structure, functions, or abundance of ApoB protein.”
The team conducted genetic analysis on plasma samples from 2,125 EOAD and control subjects included in several research cohorts. They first determined the association between cholesterol and EOAD, and then the frequency of variants in apolipoprotein E epsilon 4 (APOE e4), APP, PSEN1, PSEN2, and ApoB. Gene sequencing revealed that 3.4% of samples showed mutations in APP, PSEN1, or PSEN2.
“Given the strong associations between APOE e4 and EOAD and elevated circulating LDL cholesterol levels, we expected individuals with EOAD to have elevated LDL levels,” the team said. But an analysis of 267 of the samples for lipid levels found that, even after the researchers controlled for APOE e4, EOAD cases had higher total cholesterol, low-density cholesterol, and plasma ApoB, compared with controls. However, they found no association between EOAD and high-density lipoprotein or triglycerides.
“Because total cholesterol largely consists of LDL-C, and ApoB is the main lipoprotein of LDL-C, these findings are consistent with one another.
“From these data, we estimated that LDL-C explains 7.6% of the variance in liability to EOAD, independently of APOE e4 ... These results demonstrate that elevated levels of LDL-C [and ApoB] were significantly associated with increased EOAD risk, and this effect was only partially mediated by APOE e4 genotype.”
The results also raised a question: What was driving the association between LDL and EOAD? Because variants of the ApoB gene can either raise or lower LDL, the team examined variants associated with coding changes. These variants were significantly more common in EOAD cases than in controls (5.0% vs. 1.7%).
“Two affected individuals ... were compound heterozygotes, with the remainder being heterozygotes,” the researchers wrote. “Each compound heterozygote case was heterozygous for two different rare coding sites ... Of these four variants, only [one] has been previously described.”
“Our finding of a significant association between rare coding variants in APOB and EOAD independently of APOE is novel, important, and consistent with multiple genome-wide association studies that revealed strong associations between late-onset AD and common intron markers of genes involved in brain cholesterol metabolism [ABCA7, BIN1, CLU, and SORL1]. Furthermore, mice overexpressing ApoB show hyperlipidemia, neurodegeneration, increases in APP, accumulation of amyloid plaques, and cognitive impairment similar to mice overexpressing wild-type human APP. Collectively, these studies and our findings suggest an important role of cholesterol metabolism in AD pathogenesis.”
This research was supported by grants from the Veterans Health Administration, the National Institutes of Health, the To Remember Foundation, the Douglas French Alzheimer’s Foundation, and a contract with the State of California Department of Health Services. Several authors reported financial ties to pharmaceutical companies outside of this work.
SOURCE: Wingo TS et al. JAMA Neurol. 2019 May 28. doi: 10.1001/jamaneurol.2019.0648.
FROM JAMA NEUROLOGY
Patients with intellectual disability require nuanced care
SAN FRANCISCO – Some physicians are uncomfortable providing mental health care to patients with intellectual disability (ID) because many of the patients’ communications skills are limited. But many resources are available that can help.
In this video, Nita V. Bhatt, MD, MPH, interviews Julie P. Gentile, MD, about some of those resources and discusses how to approach psychiatric treatment interventions for patients with ID.
In addition to the DSM-5, Dr. Gentile said the National Association for the Dually Diagnosed has published the Diagnostic Manual – Intellectual Disability. Another resource is a practical reference manual originally proposed by one of Dr. Gentile’s residents.
“He came into my office for supervision one day and said, ‘You know, there’s all these nuances for psychiatric treatment in this patient population. So we should write a practice, quick reference manual to help clinicians who aren’t able to spend as much time concentrate on this patient population.’ ”
As a result, several residents and faculty members formed a team to produce an 18-chapter book published this year by Springer called the Guide to Intellectual Disabilities: A Clinical Handbook.
Dr. Bhatt is a staff psychiatrist at Twin Valley Behavioral Healthcare, the state psychiatric hospital in Columbus, Ohio. Dr. Gentile is professor and chair of the department of psychiatry at Wright State in Dayton. She is also serves as project director of Ohio’s Telepsychiatry Project for Intellectual Disability and has been awarded more than $7 million in grant funding to support her projects in the field of ID.
Dr. Gentile’s work has been funded by the Ohio Department of Developmental Disabilities and the Ohio Department of Mental Health and Addiction Services.
SAN FRANCISCO – Some physicians are uncomfortable providing mental health care to patients with intellectual disability (ID) because many of the patients’ communications skills are limited. But many resources are available that can help.
In this video, Nita V. Bhatt, MD, MPH, interviews Julie P. Gentile, MD, about some of those resources and discusses how to approach psychiatric treatment interventions for patients with ID.
In addition to the DSM-5, Dr. Gentile said the National Association for the Dually Diagnosed has published the Diagnostic Manual – Intellectual Disability. Another resource is a practical reference manual originally proposed by one of Dr. Gentile’s residents.
“He came into my office for supervision one day and said, ‘You know, there’s all these nuances for psychiatric treatment in this patient population. So we should write a practice, quick reference manual to help clinicians who aren’t able to spend as much time concentrate on this patient population.’ ”
As a result, several residents and faculty members formed a team to produce an 18-chapter book published this year by Springer called the Guide to Intellectual Disabilities: A Clinical Handbook.
Dr. Bhatt is a staff psychiatrist at Twin Valley Behavioral Healthcare, the state psychiatric hospital in Columbus, Ohio. Dr. Gentile is professor and chair of the department of psychiatry at Wright State in Dayton. She is also serves as project director of Ohio’s Telepsychiatry Project for Intellectual Disability and has been awarded more than $7 million in grant funding to support her projects in the field of ID.
Dr. Gentile’s work has been funded by the Ohio Department of Developmental Disabilities and the Ohio Department of Mental Health and Addiction Services.
SAN FRANCISCO – Some physicians are uncomfortable providing mental health care to patients with intellectual disability (ID) because many of the patients’ communications skills are limited. But many resources are available that can help.
In this video, Nita V. Bhatt, MD, MPH, interviews Julie P. Gentile, MD, about some of those resources and discusses how to approach psychiatric treatment interventions for patients with ID.
In addition to the DSM-5, Dr. Gentile said the National Association for the Dually Diagnosed has published the Diagnostic Manual – Intellectual Disability. Another resource is a practical reference manual originally proposed by one of Dr. Gentile’s residents.
“He came into my office for supervision one day and said, ‘You know, there’s all these nuances for psychiatric treatment in this patient population. So we should write a practice, quick reference manual to help clinicians who aren’t able to spend as much time concentrate on this patient population.’ ”
As a result, several residents and faculty members formed a team to produce an 18-chapter book published this year by Springer called the Guide to Intellectual Disabilities: A Clinical Handbook.
Dr. Bhatt is a staff psychiatrist at Twin Valley Behavioral Healthcare, the state psychiatric hospital in Columbus, Ohio. Dr. Gentile is professor and chair of the department of psychiatry at Wright State in Dayton. She is also serves as project director of Ohio’s Telepsychiatry Project for Intellectual Disability and has been awarded more than $7 million in grant funding to support her projects in the field of ID.
Dr. Gentile’s work has been funded by the Ohio Department of Developmental Disabilities and the Ohio Department of Mental Health and Addiction Services.
REPORTING FROM APA 2019
Elderly concussion patients who used statins had lower dementia risk
, compared with similar adults not taking statins.
The findings come from a population-based double cohort study of 28,815 patients in the Ontario Health Insurance Plan. Study patients were enrolled over 20 years, and had a minimum follow-up of 3 years. The study excluded patients hospitalized caused by a severe concussion, those previously diagnosed with delirium or dementia, and those who died within 90 days of their concussions.
Concussions are a common injury in older adults and dementia may be a frequent outcome years afterward, Donald A. Redelmeier, MD, of the University of Toronto and colleagues wrote in a study published in JAMA Neurology. A concussion should not be interpreted as a reason to stop statins, and a potential neuroprotective benefit may encourage medication adherence among patients who are already prescribed a statin.
Of the 28,815 patients studied, 4,727 patients (1 case per 6 patients) developed dementia over the mean follow-up period of 3.9 years. The 7,058 patients who received a statin had a 13% reduced risk of developing dementia, compared with the 21,757 patients who did not (relative risk, 0.87; 95% confidence interval, 0.81-0.93; P less than .001).
Even though statin use was associated with a lower risk, the subsequent incidence of dementia was still twice the population norm in statin users who had concussions, the researchers wrote. The findings indicate concussions are a common injury in older adults and dementia may be a frequent outcome years after concussions.
Statin users who had concussions continued to have a reduced risk of developing dementia after adjustment for patient characteristics, use of other cardiovascular medications, dosage, and depression risk. The statin associated with the greatest risk reduction was rosuvastatin; simvastatin was associated with the least risk reduction. With the possible exception of angiotensin II receptor blockers, no other cardiovascular or noncardiovascular medications were associated with a decreased risk of dementia after a concussion, the researchers wrote.
They also examined data for elderly patients using statins after an ankle sprain and found the risk of dementia was similar for those who did and did not receive statins after the injury.
Factors such as smoking status, exercise, drug adherence, and other unknown aspects of patient health might have influenced the results of the study, the researchers acknowledged. Additionally, a secondary analysis was not statistically powered to distinguish the relative efficacy of statin use before a concussion.
This study was funded in part by a Canada Research Chair in Medical Decision Sciences, the Canadian Institutes of Health Research, the BrightFocus Foundation, and the Comprehensive Research Experience for Medical Students at the University of Toronto. The authors reported no relevant conflicts of interest.
SOURCE: Redelmeier DA et al. JAMA Neurol. 2019 May 20. doi: 10.1001/jamaneurol.2019.1148.
This appears to be the first large study to explore the relationship between statin use, concussions, and the development of dementia. Although statins have anti-inflammatory properties, no trials have linked statins to reduced cognitive impairment. Considering it can be difficult to mitigate against confounding by indication in pharmacologic studies, this observational study included a large group of diverse individuals who developed concussions over a period of 20 years.
Rachel A. Whitmer, PhD, is with the division of epidemiology and department of public health sciences at the University of California, Davis. She made her remarks in a related editorial published with the study, and reported no relevant conflicts of interest.
This appears to be the first large study to explore the relationship between statin use, concussions, and the development of dementia. Although statins have anti-inflammatory properties, no trials have linked statins to reduced cognitive impairment. Considering it can be difficult to mitigate against confounding by indication in pharmacologic studies, this observational study included a large group of diverse individuals who developed concussions over a period of 20 years.
Rachel A. Whitmer, PhD, is with the division of epidemiology and department of public health sciences at the University of California, Davis. She made her remarks in a related editorial published with the study, and reported no relevant conflicts of interest.
This appears to be the first large study to explore the relationship between statin use, concussions, and the development of dementia. Although statins have anti-inflammatory properties, no trials have linked statins to reduced cognitive impairment. Considering it can be difficult to mitigate against confounding by indication in pharmacologic studies, this observational study included a large group of diverse individuals who developed concussions over a period of 20 years.
Rachel A. Whitmer, PhD, is with the division of epidemiology and department of public health sciences at the University of California, Davis. She made her remarks in a related editorial published with the study, and reported no relevant conflicts of interest.
, compared with similar adults not taking statins.
The findings come from a population-based double cohort study of 28,815 patients in the Ontario Health Insurance Plan. Study patients were enrolled over 20 years, and had a minimum follow-up of 3 years. The study excluded patients hospitalized caused by a severe concussion, those previously diagnosed with delirium or dementia, and those who died within 90 days of their concussions.
Concussions are a common injury in older adults and dementia may be a frequent outcome years afterward, Donald A. Redelmeier, MD, of the University of Toronto and colleagues wrote in a study published in JAMA Neurology. A concussion should not be interpreted as a reason to stop statins, and a potential neuroprotective benefit may encourage medication adherence among patients who are already prescribed a statin.
Of the 28,815 patients studied, 4,727 patients (1 case per 6 patients) developed dementia over the mean follow-up period of 3.9 years. The 7,058 patients who received a statin had a 13% reduced risk of developing dementia, compared with the 21,757 patients who did not (relative risk, 0.87; 95% confidence interval, 0.81-0.93; P less than .001).
Even though statin use was associated with a lower risk, the subsequent incidence of dementia was still twice the population norm in statin users who had concussions, the researchers wrote. The findings indicate concussions are a common injury in older adults and dementia may be a frequent outcome years after concussions.
Statin users who had concussions continued to have a reduced risk of developing dementia after adjustment for patient characteristics, use of other cardiovascular medications, dosage, and depression risk. The statin associated with the greatest risk reduction was rosuvastatin; simvastatin was associated with the least risk reduction. With the possible exception of angiotensin II receptor blockers, no other cardiovascular or noncardiovascular medications were associated with a decreased risk of dementia after a concussion, the researchers wrote.
They also examined data for elderly patients using statins after an ankle sprain and found the risk of dementia was similar for those who did and did not receive statins after the injury.
Factors such as smoking status, exercise, drug adherence, and other unknown aspects of patient health might have influenced the results of the study, the researchers acknowledged. Additionally, a secondary analysis was not statistically powered to distinguish the relative efficacy of statin use before a concussion.
This study was funded in part by a Canada Research Chair in Medical Decision Sciences, the Canadian Institutes of Health Research, the BrightFocus Foundation, and the Comprehensive Research Experience for Medical Students at the University of Toronto. The authors reported no relevant conflicts of interest.
SOURCE: Redelmeier DA et al. JAMA Neurol. 2019 May 20. doi: 10.1001/jamaneurol.2019.1148.
, compared with similar adults not taking statins.
The findings come from a population-based double cohort study of 28,815 patients in the Ontario Health Insurance Plan. Study patients were enrolled over 20 years, and had a minimum follow-up of 3 years. The study excluded patients hospitalized caused by a severe concussion, those previously diagnosed with delirium or dementia, and those who died within 90 days of their concussions.
Concussions are a common injury in older adults and dementia may be a frequent outcome years afterward, Donald A. Redelmeier, MD, of the University of Toronto and colleagues wrote in a study published in JAMA Neurology. A concussion should not be interpreted as a reason to stop statins, and a potential neuroprotective benefit may encourage medication adherence among patients who are already prescribed a statin.
Of the 28,815 patients studied, 4,727 patients (1 case per 6 patients) developed dementia over the mean follow-up period of 3.9 years. The 7,058 patients who received a statin had a 13% reduced risk of developing dementia, compared with the 21,757 patients who did not (relative risk, 0.87; 95% confidence interval, 0.81-0.93; P less than .001).
Even though statin use was associated with a lower risk, the subsequent incidence of dementia was still twice the population norm in statin users who had concussions, the researchers wrote. The findings indicate concussions are a common injury in older adults and dementia may be a frequent outcome years after concussions.
Statin users who had concussions continued to have a reduced risk of developing dementia after adjustment for patient characteristics, use of other cardiovascular medications, dosage, and depression risk. The statin associated with the greatest risk reduction was rosuvastatin; simvastatin was associated with the least risk reduction. With the possible exception of angiotensin II receptor blockers, no other cardiovascular or noncardiovascular medications were associated with a decreased risk of dementia after a concussion, the researchers wrote.
They also examined data for elderly patients using statins after an ankle sprain and found the risk of dementia was similar for those who did and did not receive statins after the injury.
Factors such as smoking status, exercise, drug adherence, and other unknown aspects of patient health might have influenced the results of the study, the researchers acknowledged. Additionally, a secondary analysis was not statistically powered to distinguish the relative efficacy of statin use before a concussion.
This study was funded in part by a Canada Research Chair in Medical Decision Sciences, the Canadian Institutes of Health Research, the BrightFocus Foundation, and the Comprehensive Research Experience for Medical Students at the University of Toronto. The authors reported no relevant conflicts of interest.
SOURCE: Redelmeier DA et al. JAMA Neurol. 2019 May 20. doi: 10.1001/jamaneurol.2019.1148.
FROM JAMA NEUROLOGY
Key clinical point: Older adults taking a statin within 90 days after a concussion had a lower rate of dementia.
Major finding: Statin use within 90 days of a concussion in older adults was associated with a 13% reduced risk of dementia (relative risk, 0.87; 95% confidence interval, 0.81-0.93; P less than .001).
Study details: A population-based double cohort study of 28,815 elderly patients who had a concussion between April 1993 and April 2013.
Disclosures: This study was funded in part by a Canada Research Chair in Medical Decision Sciences, the Canadian Institutes of Health Research, the BrightFocus Foundation, and the Comprehensive Research Experience for Medical Students at the University of Toronto. The authors reported no relevant conflicts of interest.
Source: Redelmeier DA et al. JAMA Neurol. 2019 May 20. doi: 10.1001/jamaneurol.2019.1148.
Report on newly recognized cause of dementia should be read widely
Alzheimer’s disease is recognized as the most common cause of dementia, and many in the laity use the two terms almost interchangeably. However, there is increasing recognition that dementia in old age is a complex disorder, with mixed neuropathologies being the norm rather than the exception (Ann Neurol. 2018 Jan;83[1]:74-83).
Alzheimer’s disease (AD) and cerebrovascular pathologies are the most common, but another pathology is receiving increasing attention in relation to cognitive disorders in very old individuals – that related to the transactive response DNA binding protein of 43 kDa (TDP-43). This protein is expressed in most human tissues, including the brain, is localized mostly in nuclei, and binds to RNA and DNA as well as numerous proteins, with the role of regulating gene expression.
It has been known for nearly 2 decades that TDP-43 can become abnormally phosphorylated and translocated to the cytoplasm to produce a proteinopathy that forms the basis of a significant proportion of frontotemporal dementia (FTD) and the majority of amyotrophic lateral sclerosis. More recently, it has also been reported to be common in the brains of older people (over age 80 years) and associated with a cognitive disorder characterized by an amnestic picture that mimics AD. Since the protein deposition is predominantly in the limbic regions (amygdala, hippocampus, insula), it has been termed “‘limbic-predominant, age-related TDP-43 encephalopathy”, or LATE.
A recently convened international working group has published consensus criteria for LATE and provided guidelines for its staging. Community-based autopsy studies suggest that 20%-50% of people aged over 80 years have the neuropathologic change associated with LATE. The clinical presentation resembles amnestic dementia syndrome, much like AD. Both LATE and AD pathologies often occur in the same individual, but the relative predominance of one or the other varies greatly between individuals. The genetic risks of LATE overlap with those for FTD and AD, and other risk factors may also be shared with AD, which remains an area for further investigation. There are at present no specific biomarkers of LATE. It is associated with hippocampal sclerosis in some cases, which may be visible on MRI, but hippocampal sclerosis itself is not specific to TDP-43 pathology.
of LATE and calls for systematic study of the causes of dementia – which may be nearly as common as AD in the very old. The report should be read widely and should remind us of the diverse pathologies that contribute to cognitive disorders, alone and in combination with one another.
Dr. Sachdev is Scientia Professor of Neuropsychiatry and codirector of the Center for Healthy Brain Aging at the University of New South Wales, Sydney; and clinical director of the Neuropsychiatric Institute at the Prince of Wales Hospital, also in Sydney. His major areas of research are drug-induced movement disorders, brain imaging, cognitive aging and dementia. Dr. Sachdev also served on the Neurocognitive Disorders Work Group of the DSM-5.
Alzheimer’s disease is recognized as the most common cause of dementia, and many in the laity use the two terms almost interchangeably. However, there is increasing recognition that dementia in old age is a complex disorder, with mixed neuropathologies being the norm rather than the exception (Ann Neurol. 2018 Jan;83[1]:74-83).
Alzheimer’s disease (AD) and cerebrovascular pathologies are the most common, but another pathology is receiving increasing attention in relation to cognitive disorders in very old individuals – that related to the transactive response DNA binding protein of 43 kDa (TDP-43). This protein is expressed in most human tissues, including the brain, is localized mostly in nuclei, and binds to RNA and DNA as well as numerous proteins, with the role of regulating gene expression.
It has been known for nearly 2 decades that TDP-43 can become abnormally phosphorylated and translocated to the cytoplasm to produce a proteinopathy that forms the basis of a significant proportion of frontotemporal dementia (FTD) and the majority of amyotrophic lateral sclerosis. More recently, it has also been reported to be common in the brains of older people (over age 80 years) and associated with a cognitive disorder characterized by an amnestic picture that mimics AD. Since the protein deposition is predominantly in the limbic regions (amygdala, hippocampus, insula), it has been termed “‘limbic-predominant, age-related TDP-43 encephalopathy”, or LATE.
A recently convened international working group has published consensus criteria for LATE and provided guidelines for its staging. Community-based autopsy studies suggest that 20%-50% of people aged over 80 years have the neuropathologic change associated with LATE. The clinical presentation resembles amnestic dementia syndrome, much like AD. Both LATE and AD pathologies often occur in the same individual, but the relative predominance of one or the other varies greatly between individuals. The genetic risks of LATE overlap with those for FTD and AD, and other risk factors may also be shared with AD, which remains an area for further investigation. There are at present no specific biomarkers of LATE. It is associated with hippocampal sclerosis in some cases, which may be visible on MRI, but hippocampal sclerosis itself is not specific to TDP-43 pathology.
of LATE and calls for systematic study of the causes of dementia – which may be nearly as common as AD in the very old. The report should be read widely and should remind us of the diverse pathologies that contribute to cognitive disorders, alone and in combination with one another.
Dr. Sachdev is Scientia Professor of Neuropsychiatry and codirector of the Center for Healthy Brain Aging at the University of New South Wales, Sydney; and clinical director of the Neuropsychiatric Institute at the Prince of Wales Hospital, also in Sydney. His major areas of research are drug-induced movement disorders, brain imaging, cognitive aging and dementia. Dr. Sachdev also served on the Neurocognitive Disorders Work Group of the DSM-5.
Alzheimer’s disease is recognized as the most common cause of dementia, and many in the laity use the two terms almost interchangeably. However, there is increasing recognition that dementia in old age is a complex disorder, with mixed neuropathologies being the norm rather than the exception (Ann Neurol. 2018 Jan;83[1]:74-83).
Alzheimer’s disease (AD) and cerebrovascular pathologies are the most common, but another pathology is receiving increasing attention in relation to cognitive disorders in very old individuals – that related to the transactive response DNA binding protein of 43 kDa (TDP-43). This protein is expressed in most human tissues, including the brain, is localized mostly in nuclei, and binds to RNA and DNA as well as numerous proteins, with the role of regulating gene expression.
It has been known for nearly 2 decades that TDP-43 can become abnormally phosphorylated and translocated to the cytoplasm to produce a proteinopathy that forms the basis of a significant proportion of frontotemporal dementia (FTD) and the majority of amyotrophic lateral sclerosis. More recently, it has also been reported to be common in the brains of older people (over age 80 years) and associated with a cognitive disorder characterized by an amnestic picture that mimics AD. Since the protein deposition is predominantly in the limbic regions (amygdala, hippocampus, insula), it has been termed “‘limbic-predominant, age-related TDP-43 encephalopathy”, or LATE.
A recently convened international working group has published consensus criteria for LATE and provided guidelines for its staging. Community-based autopsy studies suggest that 20%-50% of people aged over 80 years have the neuropathologic change associated with LATE. The clinical presentation resembles amnestic dementia syndrome, much like AD. Both LATE and AD pathologies often occur in the same individual, but the relative predominance of one or the other varies greatly between individuals. The genetic risks of LATE overlap with those for FTD and AD, and other risk factors may also be shared with AD, which remains an area for further investigation. There are at present no specific biomarkers of LATE. It is associated with hippocampal sclerosis in some cases, which may be visible on MRI, but hippocampal sclerosis itself is not specific to TDP-43 pathology.
of LATE and calls for systematic study of the causes of dementia – which may be nearly as common as AD in the very old. The report should be read widely and should remind us of the diverse pathologies that contribute to cognitive disorders, alone and in combination with one another.
Dr. Sachdev is Scientia Professor of Neuropsychiatry and codirector of the Center for Healthy Brain Aging at the University of New South Wales, Sydney; and clinical director of the Neuropsychiatric Institute at the Prince of Wales Hospital, also in Sydney. His major areas of research are drug-induced movement disorders, brain imaging, cognitive aging and dementia. Dr. Sachdev also served on the Neurocognitive Disorders Work Group of the DSM-5.












