User login
Immunotherapies under investigation in newly diagnosed B-ALL
SAN FRANCISCO – Positive results with blinatumomab and inotuzumab ozogamicin in the relapsed/refractory setting have prompted trials of these immunotherapies in newly diagnosed B-cell acute lymphoblastic leukemia (B-ALL).
Blinatumomab and inotuzumab have been shown to improve overall survival, compared with chemotherapy, in patients with relapsed/refractory B-ALL. However, most adults with relapsed/refractory B-ALL still die, so the initial therapy patients receive is “critical,” according to Jae Park, MD, of Memorial Sloan Kettering Cancer Center in New York.
“Ideally, we do not want to deal with the relapse,” Dr. Park said. “It’s better to cure the disease the first time ... which is the reason clinical trials are incorporating these agents earlier.”
Dr. Park discussed these points at the National Comprehensive Cancer Network Hematologic Malignancies Annual Congress.
Blinatumomab
Dr. Park cited the phase 3 TOWER trial, which showed that blinatumomab produced better response rates and overall survival compared with standard chemotherapy. The trial enrolled 405 patients with Ph-negative relapsed/refractory B-ALL who were randomized to blinatumomab (n = 271) or chemotherapy (n = 134).
The rate of complete response (CR) with full, partial, or incomplete hematologic recovery was 44% with blinatumomab and 25% with chemotherapy (P less than .001). The median overall survival was 7.7 months and 4.0 months, respectively (P = .01; N Engl J Med 2017; 376:836-47).
Based on these data, researchers decided to test blinatumomab in newly diagnosed, elderly patients (65 years and older) with Ph-negative B-ALL in the phase 2 SWOG 1318 study. The study enrolled 31 patients, and 29 were eligible. Their median age at baseline was 75 years (range 66‐84 years).
The patients received blinatumomab for two to five cycles, followed by 18 months of maintenance with prednisone, vincristine, 6-mercaptopurine, and methotrexate. One patient went on to transplant.
In all, 66% of patients achieved a CR or CR with incomplete count recovery. The estimated overall survival was 79% at 6 months and 65% at 1 year. These results were presented at the 2018 annual meeting of the American Society of Hematology (Blood. 2018;132:33).
Another study of blinatumomab as frontline treatment is the ECOG-E1910 trial. In this phase 3 study, researchers are testing chemotherapy, with or without blinatumomab, in adults (aged 30-70 years) with newly diagnosed, BCR-ABL-negative B-ALL. Results from this study are not yet available.
Inotuzumab ozogamicin
Dr. Park also discussed the INOVATE trial, in which inotuzumab ozogamicin bested standard chemotherapy. The trial enrolled patients with Ph-positive or negative, relapsed/refractory B-ALL.
The patients were randomized to inotuzumab (n = 141) or investigator’s choice of chemotherapy (n = 138). Some patients, 41% in the inotuzumab arm and 11% in the chemotherapy arm, went on to transplant.
The CR rate was 80.7% in the inotuzumab arm and 29.4% in the chemotherapy arm (P less than .001). The median progression-free survival was 5 months and 1.8 months, respectively (P less than .001). The median overall survival was 7.7 months and 6.7 months, respectively (P = .04; N Engl J Med 2016; 375:740-53).
Based on these results, researchers are testing inotuzumab as frontline therapy in young adults (aged 18-39 years) with CD22-positive, Ph-negative B-ALL. In the phase 3 A041501 trial, patients are receiving inotuzumab after the first and second courses of treatment with the CALGB 10403 chemotherapy regimen. Results from this trial are not yet available.
Dr. Park reported relationships with Allogene Therapeutics, Amgen, AstraZeneca, Incyte, Kite Pharma, Novartis, and Takeda.
SAN FRANCISCO – Positive results with blinatumomab and inotuzumab ozogamicin in the relapsed/refractory setting have prompted trials of these immunotherapies in newly diagnosed B-cell acute lymphoblastic leukemia (B-ALL).
Blinatumomab and inotuzumab have been shown to improve overall survival, compared with chemotherapy, in patients with relapsed/refractory B-ALL. However, most adults with relapsed/refractory B-ALL still die, so the initial therapy patients receive is “critical,” according to Jae Park, MD, of Memorial Sloan Kettering Cancer Center in New York.
“Ideally, we do not want to deal with the relapse,” Dr. Park said. “It’s better to cure the disease the first time ... which is the reason clinical trials are incorporating these agents earlier.”
Dr. Park discussed these points at the National Comprehensive Cancer Network Hematologic Malignancies Annual Congress.
Blinatumomab
Dr. Park cited the phase 3 TOWER trial, which showed that blinatumomab produced better response rates and overall survival compared with standard chemotherapy. The trial enrolled 405 patients with Ph-negative relapsed/refractory B-ALL who were randomized to blinatumomab (n = 271) or chemotherapy (n = 134).
The rate of complete response (CR) with full, partial, or incomplete hematologic recovery was 44% with blinatumomab and 25% with chemotherapy (P less than .001). The median overall survival was 7.7 months and 4.0 months, respectively (P = .01; N Engl J Med 2017; 376:836-47).
Based on these data, researchers decided to test blinatumomab in newly diagnosed, elderly patients (65 years and older) with Ph-negative B-ALL in the phase 2 SWOG 1318 study. The study enrolled 31 patients, and 29 were eligible. Their median age at baseline was 75 years (range 66‐84 years).
The patients received blinatumomab for two to five cycles, followed by 18 months of maintenance with prednisone, vincristine, 6-mercaptopurine, and methotrexate. One patient went on to transplant.
In all, 66% of patients achieved a CR or CR with incomplete count recovery. The estimated overall survival was 79% at 6 months and 65% at 1 year. These results were presented at the 2018 annual meeting of the American Society of Hematology (Blood. 2018;132:33).
Another study of blinatumomab as frontline treatment is the ECOG-E1910 trial. In this phase 3 study, researchers are testing chemotherapy, with or without blinatumomab, in adults (aged 30-70 years) with newly diagnosed, BCR-ABL-negative B-ALL. Results from this study are not yet available.
Inotuzumab ozogamicin
Dr. Park also discussed the INOVATE trial, in which inotuzumab ozogamicin bested standard chemotherapy. The trial enrolled patients with Ph-positive or negative, relapsed/refractory B-ALL.
The patients were randomized to inotuzumab (n = 141) or investigator’s choice of chemotherapy (n = 138). Some patients, 41% in the inotuzumab arm and 11% in the chemotherapy arm, went on to transplant.
The CR rate was 80.7% in the inotuzumab arm and 29.4% in the chemotherapy arm (P less than .001). The median progression-free survival was 5 months and 1.8 months, respectively (P less than .001). The median overall survival was 7.7 months and 6.7 months, respectively (P = .04; N Engl J Med 2016; 375:740-53).
Based on these results, researchers are testing inotuzumab as frontline therapy in young adults (aged 18-39 years) with CD22-positive, Ph-negative B-ALL. In the phase 3 A041501 trial, patients are receiving inotuzumab after the first and second courses of treatment with the CALGB 10403 chemotherapy regimen. Results from this trial are not yet available.
Dr. Park reported relationships with Allogene Therapeutics, Amgen, AstraZeneca, Incyte, Kite Pharma, Novartis, and Takeda.
SAN FRANCISCO – Positive results with blinatumomab and inotuzumab ozogamicin in the relapsed/refractory setting have prompted trials of these immunotherapies in newly diagnosed B-cell acute lymphoblastic leukemia (B-ALL).
Blinatumomab and inotuzumab have been shown to improve overall survival, compared with chemotherapy, in patients with relapsed/refractory B-ALL. However, most adults with relapsed/refractory B-ALL still die, so the initial therapy patients receive is “critical,” according to Jae Park, MD, of Memorial Sloan Kettering Cancer Center in New York.
“Ideally, we do not want to deal with the relapse,” Dr. Park said. “It’s better to cure the disease the first time ... which is the reason clinical trials are incorporating these agents earlier.”
Dr. Park discussed these points at the National Comprehensive Cancer Network Hematologic Malignancies Annual Congress.
Blinatumomab
Dr. Park cited the phase 3 TOWER trial, which showed that blinatumomab produced better response rates and overall survival compared with standard chemotherapy. The trial enrolled 405 patients with Ph-negative relapsed/refractory B-ALL who were randomized to blinatumomab (n = 271) or chemotherapy (n = 134).
The rate of complete response (CR) with full, partial, or incomplete hematologic recovery was 44% with blinatumomab and 25% with chemotherapy (P less than .001). The median overall survival was 7.7 months and 4.0 months, respectively (P = .01; N Engl J Med 2017; 376:836-47).
Based on these data, researchers decided to test blinatumomab in newly diagnosed, elderly patients (65 years and older) with Ph-negative B-ALL in the phase 2 SWOG 1318 study. The study enrolled 31 patients, and 29 were eligible. Their median age at baseline was 75 years (range 66‐84 years).
The patients received blinatumomab for two to five cycles, followed by 18 months of maintenance with prednisone, vincristine, 6-mercaptopurine, and methotrexate. One patient went on to transplant.
In all, 66% of patients achieved a CR or CR with incomplete count recovery. The estimated overall survival was 79% at 6 months and 65% at 1 year. These results were presented at the 2018 annual meeting of the American Society of Hematology (Blood. 2018;132:33).
Another study of blinatumomab as frontline treatment is the ECOG-E1910 trial. In this phase 3 study, researchers are testing chemotherapy, with or without blinatumomab, in adults (aged 30-70 years) with newly diagnosed, BCR-ABL-negative B-ALL. Results from this study are not yet available.
Inotuzumab ozogamicin
Dr. Park also discussed the INOVATE trial, in which inotuzumab ozogamicin bested standard chemotherapy. The trial enrolled patients with Ph-positive or negative, relapsed/refractory B-ALL.
The patients were randomized to inotuzumab (n = 141) or investigator’s choice of chemotherapy (n = 138). Some patients, 41% in the inotuzumab arm and 11% in the chemotherapy arm, went on to transplant.
The CR rate was 80.7% in the inotuzumab arm and 29.4% in the chemotherapy arm (P less than .001). The median progression-free survival was 5 months and 1.8 months, respectively (P less than .001). The median overall survival was 7.7 months and 6.7 months, respectively (P = .04; N Engl J Med 2016; 375:740-53).
Based on these results, researchers are testing inotuzumab as frontline therapy in young adults (aged 18-39 years) with CD22-positive, Ph-negative B-ALL. In the phase 3 A041501 trial, patients are receiving inotuzumab after the first and second courses of treatment with the CALGB 10403 chemotherapy regimen. Results from this trial are not yet available.
Dr. Park reported relationships with Allogene Therapeutics, Amgen, AstraZeneca, Incyte, Kite Pharma, Novartis, and Takeda.
EXPERT ANALYSIS FROM NCCN HEMATOLOGIC MALIGNANCIES
Anakinra treatment for pediatric ‘cytokine storms’: Does one size fit all?
The biologic drug anakinra appears to be effective in treating children with secondary hemophagocytic lymphohistiocytosis (sHLH)/macrophage activation syndrome (MAS), a dangerous “cytokine storm” that can emerge from infections, cancer, and rheumatic diseases.
Children with systematic juvenile idiopathic arthritis (sJIA) and sHLH/MAS are especially good candidates for treatment with the interleukin-1 receptor antagonist anakinra (Kineret), in whom its safety and benefits have been more widely explored than in pediatric patients with sHLH/MAS related to non-sJIA underlying conditions.
In a study published in Arthritis & Rheumatology, Esraa Eloseily, MD, and colleagues at the University of Alabama at Birmingham, looked at hospitalization records for 44 children (mean age, 10 years; n = 25 females) with sHLH/MAS. The children in the study had heterogeneous underlying conditions including leukemias, infections, and rheumatic diseases. About one-third of patients had no known rheumatic or autoimmune disorder.
Dr. Eloseily and colleagues found that early initiation of anakinra (within 5 days of hospitalization) was significantly associated with improved survival across the cohort, for which mortality was 27%. Thrombocytopenia (less than 100,000/mcL) and STXBP2 mutations were both seen significantly associated with mortality.
Patients with blood cancers – even those in remission at the time of treatment – did poorly. None of the three patients in the cohort with leukemia survived.
Importantly, no deaths were seen among the 13 patients with underlying SJIA who were treated with anakinra, suggesting particular benefit for this patient group.
“In addition to the 10% risk of developing overt MAS as part of sJIA, another 30%-40% of sJIA patients may have occult or subclinical MAS during a disease flare that can eventually lead to overt MAS,” Dr. Eloseily and colleagues wrote. “This association of MAS with sJIA suggested that anakinra would also be a valuable treatment for sJIA-MAS.”
The investigators acknowledged that their study was limited by its retrospective design and “nonuniform approach to therapy, lack of treatment controls, and variable follow-up period.” The authors also acknowledged the potential for selection bias favoring anakinra use in patients who are less severely ill.
In a comment accompanying Dr. Eloseily and colleagues’ study, Sarah Nikiforow, MD, PhD, of the Dana-Farber Cancer Institute in Boston, and Nancy Berliner, MD, of Brigham & Women’s Hospital in Boston, urged clinicians not to interpret the study results as supporting anakinra as “a carte blanche approach to hyperinflammatory syndromes.”
While the study supported the use of anakinra in sJIA with MAS or sHLH, “we posit that patients [with sHLH/MAS] in sepsis, cytokine release syndrome following chimeric antigen receptor T-cell therapy, and other hyperinflammatory syndromes still require individualized approaches to therapy,” Dr. Nikiforow and Dr. Berliner wrote, adding that, “in several studies and anecdotally in our institutional practice, cytotoxic chemotherapy was/is preferred over biologic agents in patients with evidence of more severe inflammatory activity.”
Outside sJIA, Dr. Nikiforow and Dr. Berliner wrote, “early anakinra therapy should be extended to treatment of other forms of sHLH with extreme caution. Specifically, the authors’ suggestion that cytotoxic therapy should be ‘considered’ only after anakinra therapy may be dangerous for some patients.”
Two of Dr. Eloseily’s coinvestigators reported financial and research support from Sobi, the manufacturer of anakinra. No other conflicts of interest were reported.
SOURCES: Eloseily E et al. Arthritis Rheumatol. 2019 Sep 12. doi: 10.1002/art.41103; Nikiforow S, Berliner N. Arthritis Rheumatol. 2019 Sep 16. doi: 10.1002/art.41106.
The biologic drug anakinra appears to be effective in treating children with secondary hemophagocytic lymphohistiocytosis (sHLH)/macrophage activation syndrome (MAS), a dangerous “cytokine storm” that can emerge from infections, cancer, and rheumatic diseases.
Children with systematic juvenile idiopathic arthritis (sJIA) and sHLH/MAS are especially good candidates for treatment with the interleukin-1 receptor antagonist anakinra (Kineret), in whom its safety and benefits have been more widely explored than in pediatric patients with sHLH/MAS related to non-sJIA underlying conditions.
In a study published in Arthritis & Rheumatology, Esraa Eloseily, MD, and colleagues at the University of Alabama at Birmingham, looked at hospitalization records for 44 children (mean age, 10 years; n = 25 females) with sHLH/MAS. The children in the study had heterogeneous underlying conditions including leukemias, infections, and rheumatic diseases. About one-third of patients had no known rheumatic or autoimmune disorder.
Dr. Eloseily and colleagues found that early initiation of anakinra (within 5 days of hospitalization) was significantly associated with improved survival across the cohort, for which mortality was 27%. Thrombocytopenia (less than 100,000/mcL) and STXBP2 mutations were both seen significantly associated with mortality.
Patients with blood cancers – even those in remission at the time of treatment – did poorly. None of the three patients in the cohort with leukemia survived.
Importantly, no deaths were seen among the 13 patients with underlying SJIA who were treated with anakinra, suggesting particular benefit for this patient group.
“In addition to the 10% risk of developing overt MAS as part of sJIA, another 30%-40% of sJIA patients may have occult or subclinical MAS during a disease flare that can eventually lead to overt MAS,” Dr. Eloseily and colleagues wrote. “This association of MAS with sJIA suggested that anakinra would also be a valuable treatment for sJIA-MAS.”
The investigators acknowledged that their study was limited by its retrospective design and “nonuniform approach to therapy, lack of treatment controls, and variable follow-up period.” The authors also acknowledged the potential for selection bias favoring anakinra use in patients who are less severely ill.
In a comment accompanying Dr. Eloseily and colleagues’ study, Sarah Nikiforow, MD, PhD, of the Dana-Farber Cancer Institute in Boston, and Nancy Berliner, MD, of Brigham & Women’s Hospital in Boston, urged clinicians not to interpret the study results as supporting anakinra as “a carte blanche approach to hyperinflammatory syndromes.”
While the study supported the use of anakinra in sJIA with MAS or sHLH, “we posit that patients [with sHLH/MAS] in sepsis, cytokine release syndrome following chimeric antigen receptor T-cell therapy, and other hyperinflammatory syndromes still require individualized approaches to therapy,” Dr. Nikiforow and Dr. Berliner wrote, adding that, “in several studies and anecdotally in our institutional practice, cytotoxic chemotherapy was/is preferred over biologic agents in patients with evidence of more severe inflammatory activity.”
Outside sJIA, Dr. Nikiforow and Dr. Berliner wrote, “early anakinra therapy should be extended to treatment of other forms of sHLH with extreme caution. Specifically, the authors’ suggestion that cytotoxic therapy should be ‘considered’ only after anakinra therapy may be dangerous for some patients.”
Two of Dr. Eloseily’s coinvestigators reported financial and research support from Sobi, the manufacturer of anakinra. No other conflicts of interest were reported.
SOURCES: Eloseily E et al. Arthritis Rheumatol. 2019 Sep 12. doi: 10.1002/art.41103; Nikiforow S, Berliner N. Arthritis Rheumatol. 2019 Sep 16. doi: 10.1002/art.41106.
The biologic drug anakinra appears to be effective in treating children with secondary hemophagocytic lymphohistiocytosis (sHLH)/macrophage activation syndrome (MAS), a dangerous “cytokine storm” that can emerge from infections, cancer, and rheumatic diseases.
Children with systematic juvenile idiopathic arthritis (sJIA) and sHLH/MAS are especially good candidates for treatment with the interleukin-1 receptor antagonist anakinra (Kineret), in whom its safety and benefits have been more widely explored than in pediatric patients with sHLH/MAS related to non-sJIA underlying conditions.
In a study published in Arthritis & Rheumatology, Esraa Eloseily, MD, and colleagues at the University of Alabama at Birmingham, looked at hospitalization records for 44 children (mean age, 10 years; n = 25 females) with sHLH/MAS. The children in the study had heterogeneous underlying conditions including leukemias, infections, and rheumatic diseases. About one-third of patients had no known rheumatic or autoimmune disorder.
Dr. Eloseily and colleagues found that early initiation of anakinra (within 5 days of hospitalization) was significantly associated with improved survival across the cohort, for which mortality was 27%. Thrombocytopenia (less than 100,000/mcL) and STXBP2 mutations were both seen significantly associated with mortality.
Patients with blood cancers – even those in remission at the time of treatment – did poorly. None of the three patients in the cohort with leukemia survived.
Importantly, no deaths were seen among the 13 patients with underlying SJIA who were treated with anakinra, suggesting particular benefit for this patient group.
“In addition to the 10% risk of developing overt MAS as part of sJIA, another 30%-40% of sJIA patients may have occult or subclinical MAS during a disease flare that can eventually lead to overt MAS,” Dr. Eloseily and colleagues wrote. “This association of MAS with sJIA suggested that anakinra would also be a valuable treatment for sJIA-MAS.”
The investigators acknowledged that their study was limited by its retrospective design and “nonuniform approach to therapy, lack of treatment controls, and variable follow-up period.” The authors also acknowledged the potential for selection bias favoring anakinra use in patients who are less severely ill.
In a comment accompanying Dr. Eloseily and colleagues’ study, Sarah Nikiforow, MD, PhD, of the Dana-Farber Cancer Institute in Boston, and Nancy Berliner, MD, of Brigham & Women’s Hospital in Boston, urged clinicians not to interpret the study results as supporting anakinra as “a carte blanche approach to hyperinflammatory syndromes.”
While the study supported the use of anakinra in sJIA with MAS or sHLH, “we posit that patients [with sHLH/MAS] in sepsis, cytokine release syndrome following chimeric antigen receptor T-cell therapy, and other hyperinflammatory syndromes still require individualized approaches to therapy,” Dr. Nikiforow and Dr. Berliner wrote, adding that, “in several studies and anecdotally in our institutional practice, cytotoxic chemotherapy was/is preferred over biologic agents in patients with evidence of more severe inflammatory activity.”
Outside sJIA, Dr. Nikiforow and Dr. Berliner wrote, “early anakinra therapy should be extended to treatment of other forms of sHLH with extreme caution. Specifically, the authors’ suggestion that cytotoxic therapy should be ‘considered’ only after anakinra therapy may be dangerous for some patients.”
Two of Dr. Eloseily’s coinvestigators reported financial and research support from Sobi, the manufacturer of anakinra. No other conflicts of interest were reported.
SOURCES: Eloseily E et al. Arthritis Rheumatol. 2019 Sep 12. doi: 10.1002/art.41103; Nikiforow S, Berliner N. Arthritis Rheumatol. 2019 Sep 16. doi: 10.1002/art.41106.
FROM ARTHRITIS & RHEUMATOLOGY
Stem cells gene edited to be HIV resistant treat ALL, but not HIV
Gene editing of donor stem cells prior to transplantation into a patient with both HIV infection and acute lymphoblastic leukemia (ALL) was safe and effectively treated the patient’s leukemia, but failed to resolve his HIV, investigators reported.
The 27-year-old man received an HLA-matched transplant of hematopoietic stem and progenitor cells (HSPCs) that had been genetically engineered to lack CCR5, a key gateway for HIV entry into cells.
Although the transplant resulted in complete remission of leukemia with full donor chimerism, only about 9% of the posttransplant lymphocytes showed disruption of CCR5, and during a brief trial of antiretroviral therapy interruption his HIV viral load rebounded, reported Hongkui Deng, PhD, and colleagues from Peking University in China.
Although the experiment did not meet its goal of a drug-free HIV remission, it serves as a proof of concept for the use of CRISPR-Cas9 (clustered regularly interspaced palindromic repeats/CRISPR-associated protein 9) gene editing to treat HIV infection, the authors contend.
“These results show the proof of principle that transplantation and long-term engraftment of CRISPR-edited allogeneic HSPCs can be achieved; however, the efficiency of the response was not adequate to achieve the target of cure of HIV-1 infection,” they wrote in a brief report published in the New England Journal of Medicine.
As previously reported, other research groups have investigated genetic editing to mimic a naturally occurring mutation that effectively disables the CCR5 HIV coreceptor, preventing the retrovirus from entering healthy cells. The mutation was first identified in a man named Timothy Brown who came to be known as “the Berlin patient” after he was apparently cured of HIV infection after a bone marrow transplant from a donor who had the mutation.
Dr. Deng and colleagues took advantage of HSPC transplantation, a standard therapy for ALL to see whether it could also have beneficial effects on concomitant HIV infection.
They treated donor HSPCs with CRISPR-Cas9 to ablate CCR5 and then delivered them to the patient along with additional CD34-depleted donor cells from mobilized peripheral blood.
The transplant was a success, with neutrophil engraftment on day 13 and platelet engraftment on day 27, and the leukemia was in morphologic complete remission at week 4 following transplantation. The patient remained in complete remission from leukemia throughout the 19-month follow-up period, with full donor chimerism .
However, when a planned interruption of antiretroviral therapy was carried out at 7 months post transplant, the serum viral load increased to 3 × 107 copies/ml at week 4 following interruption, and the patient was restarted on the drug. His viral levels gradually decreased to undetectable level during the subsequent months.
The investigators noted that 2 weeks after the drug interruption trial was started there was a small increase in the percentage of CCR5 insertion/deletions.
“The low efficiency of gene editing in the patient may be due to the competitive engraftment of the coinfused HSPCs in CD34-depleted cells and the persistence of donor T cells. To further clarify the anti-HIV effect of CCR5-ablated HSPCs, it will be essential to increase the gene-editing efficiency of our CRISPR-Cas9 system and improve the transplantation protocol,” they wrote.
The study was funded by the Beijing Municipal Science and Technology Commission and others (unspecified). All authors reported having nothing to disclose.
SOURCE: Xu L et al. N Engl J Med. 2019. doi: 10.1056/NEJMoa1817426.
Gene editing of donor stem cells prior to transplantation into a patient with both HIV infection and acute lymphoblastic leukemia (ALL) was safe and effectively treated the patient’s leukemia, but failed to resolve his HIV, investigators reported.
The 27-year-old man received an HLA-matched transplant of hematopoietic stem and progenitor cells (HSPCs) that had been genetically engineered to lack CCR5, a key gateway for HIV entry into cells.
Although the transplant resulted in complete remission of leukemia with full donor chimerism, only about 9% of the posttransplant lymphocytes showed disruption of CCR5, and during a brief trial of antiretroviral therapy interruption his HIV viral load rebounded, reported Hongkui Deng, PhD, and colleagues from Peking University in China.
Although the experiment did not meet its goal of a drug-free HIV remission, it serves as a proof of concept for the use of CRISPR-Cas9 (clustered regularly interspaced palindromic repeats/CRISPR-associated protein 9) gene editing to treat HIV infection, the authors contend.
“These results show the proof of principle that transplantation and long-term engraftment of CRISPR-edited allogeneic HSPCs can be achieved; however, the efficiency of the response was not adequate to achieve the target of cure of HIV-1 infection,” they wrote in a brief report published in the New England Journal of Medicine.
As previously reported, other research groups have investigated genetic editing to mimic a naturally occurring mutation that effectively disables the CCR5 HIV coreceptor, preventing the retrovirus from entering healthy cells. The mutation was first identified in a man named Timothy Brown who came to be known as “the Berlin patient” after he was apparently cured of HIV infection after a bone marrow transplant from a donor who had the mutation.
Dr. Deng and colleagues took advantage of HSPC transplantation, a standard therapy for ALL to see whether it could also have beneficial effects on concomitant HIV infection.
They treated donor HSPCs with CRISPR-Cas9 to ablate CCR5 and then delivered them to the patient along with additional CD34-depleted donor cells from mobilized peripheral blood.
The transplant was a success, with neutrophil engraftment on day 13 and platelet engraftment on day 27, and the leukemia was in morphologic complete remission at week 4 following transplantation. The patient remained in complete remission from leukemia throughout the 19-month follow-up period, with full donor chimerism .
However, when a planned interruption of antiretroviral therapy was carried out at 7 months post transplant, the serum viral load increased to 3 × 107 copies/ml at week 4 following interruption, and the patient was restarted on the drug. His viral levels gradually decreased to undetectable level during the subsequent months.
The investigators noted that 2 weeks after the drug interruption trial was started there was a small increase in the percentage of CCR5 insertion/deletions.
“The low efficiency of gene editing in the patient may be due to the competitive engraftment of the coinfused HSPCs in CD34-depleted cells and the persistence of donor T cells. To further clarify the anti-HIV effect of CCR5-ablated HSPCs, it will be essential to increase the gene-editing efficiency of our CRISPR-Cas9 system and improve the transplantation protocol,” they wrote.
The study was funded by the Beijing Municipal Science and Technology Commission and others (unspecified). All authors reported having nothing to disclose.
SOURCE: Xu L et al. N Engl J Med. 2019. doi: 10.1056/NEJMoa1817426.
Gene editing of donor stem cells prior to transplantation into a patient with both HIV infection and acute lymphoblastic leukemia (ALL) was safe and effectively treated the patient’s leukemia, but failed to resolve his HIV, investigators reported.
The 27-year-old man received an HLA-matched transplant of hematopoietic stem and progenitor cells (HSPCs) that had been genetically engineered to lack CCR5, a key gateway for HIV entry into cells.
Although the transplant resulted in complete remission of leukemia with full donor chimerism, only about 9% of the posttransplant lymphocytes showed disruption of CCR5, and during a brief trial of antiretroviral therapy interruption his HIV viral load rebounded, reported Hongkui Deng, PhD, and colleagues from Peking University in China.
Although the experiment did not meet its goal of a drug-free HIV remission, it serves as a proof of concept for the use of CRISPR-Cas9 (clustered regularly interspaced palindromic repeats/CRISPR-associated protein 9) gene editing to treat HIV infection, the authors contend.
“These results show the proof of principle that transplantation and long-term engraftment of CRISPR-edited allogeneic HSPCs can be achieved; however, the efficiency of the response was not adequate to achieve the target of cure of HIV-1 infection,” they wrote in a brief report published in the New England Journal of Medicine.
As previously reported, other research groups have investigated genetic editing to mimic a naturally occurring mutation that effectively disables the CCR5 HIV coreceptor, preventing the retrovirus from entering healthy cells. The mutation was first identified in a man named Timothy Brown who came to be known as “the Berlin patient” after he was apparently cured of HIV infection after a bone marrow transplant from a donor who had the mutation.
Dr. Deng and colleagues took advantage of HSPC transplantation, a standard therapy for ALL to see whether it could also have beneficial effects on concomitant HIV infection.
They treated donor HSPCs with CRISPR-Cas9 to ablate CCR5 and then delivered them to the patient along with additional CD34-depleted donor cells from mobilized peripheral blood.
The transplant was a success, with neutrophil engraftment on day 13 and platelet engraftment on day 27, and the leukemia was in morphologic complete remission at week 4 following transplantation. The patient remained in complete remission from leukemia throughout the 19-month follow-up period, with full donor chimerism .
However, when a planned interruption of antiretroviral therapy was carried out at 7 months post transplant, the serum viral load increased to 3 × 107 copies/ml at week 4 following interruption, and the patient was restarted on the drug. His viral levels gradually decreased to undetectable level during the subsequent months.
The investigators noted that 2 weeks after the drug interruption trial was started there was a small increase in the percentage of CCR5 insertion/deletions.
“The low efficiency of gene editing in the patient may be due to the competitive engraftment of the coinfused HSPCs in CD34-depleted cells and the persistence of donor T cells. To further clarify the anti-HIV effect of CCR5-ablated HSPCs, it will be essential to increase the gene-editing efficiency of our CRISPR-Cas9 system and improve the transplantation protocol,” they wrote.
The study was funded by the Beijing Municipal Science and Technology Commission and others (unspecified). All authors reported having nothing to disclose.
SOURCE: Xu L et al. N Engl J Med. 2019. doi: 10.1056/NEJMoa1817426.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Donor cells depleted of the HIV coreceptor CCR5 effectively treated ALL, but not HIV.
Major finding: The patient had a sustained complete remission of ALL, but HIV persisted after transplantation.
Study details: Case report of a 27-year-old man with ALL and HIV.
Disclosures: The study was funded by the Beijing Municipal Science and Technology Commission and others (unspecified). All authors reported having nothing to disclose.
Source: Xu L et al. N Engl J Med. 2019. doi: 10.1056/NEJMoa1817426.
Predicting outcomes in acute leukemia, NSCLC
In this edition of “How I will treat my next patient,” I take a look at recent studies that examined ways to predict important outcomes in two very different settings, acute leukemia and advanced non–small cell lung cancer (NSCLC). They share the virtue of helping cancer specialists to increase their vigilance for clinically relevant complications and situations and to educate patients and families.
VTE risk in acute leukemia
The risk of venous thromboembolism (VTE) in cancer patients depends upon multiple patient-, tumor-, anatomic-, and treatment-related factors. The Khorana score has become an accepted standard for predicting the risks of VTE and assessing the relative value of various anticoagulants in cancer patients. However, the only hematologic malignancy that is specifically listed among the primary cancer sites in the Khorana score is “lymphoma.” VTE can develop during treatment for acute leukemia, especially among patients with acute lymphoblastic leukemia (ALL).
At the 2019 annual congress of the European Hematology Association, Alejandro Lazo-Langer, MD, and his colleagues proposed a scoring system to quantify the risks of VTE based on a retrospective cohort study of more than 500 acute leukemia patients, diagnosed from 2006-2017. They identified 77 patients with a VTE event, with a median time from diagnosis to VTE of 64 days. Among 20 possible predictive factors, 3 emerged in the final multivariate model – platelet count greater than 50,000 (1 point), ALL (2 points), and prior history of VTE (3 points).
Over a period of 12 months, patients with a score of more than 3 points had a cumulative incidence of VTE of 44%, in comparison with 10.5% among patients with lower scores. They were unable to discern whether particular antineoplastic regimens or drugs enhanced the risk.
The authors proposed that, if verified in a validation cohort study, the scoring system could lead to better patient education about signs and symptoms, more intensive surveillance for high-risk patients, and preventive interventions.
What this means in practice
Although a large number of patient records were reviewed for Dr. Lazo-Langer’s study, there were just 74 ALL patients, and it is unclear whether particular treatment regimens or drugs (such as L-asparaginase in ALL) enhance risk. Further study with a validation cohort (as was performed for the Khorana score for patients with other malignancies), is warranted. The study is thought provoking, but for now, in my opinion, standard clinical vigilance, surveillance, and education regarding VTE in leukemia patients remain appropriate.
Steroid impact in NSCLC with ICI therapy
Patients with autoimmune disease and individuals requiring active treatment with steroids (prednisone at 10 mg/day or more or the equivalent) were excluded from clinical trials that led to Food and Drug Administration approval of immune checkpoint inhibitor (ICI) agents. Recently published data indicate that treatment with 10 mg or more of daily prednisone correlates with poor outcome in NSCLC patients receiving ICI therapy (J Clin Oncol. 2018;36:2872-8; J Thoracic Oncol. 2018;13:1771-5). However, at the 2019 annual meeting of the American Society of Clinical Oncology, analyses of the CancerLinQ database showed that, among NSCLC patients, autoimmune disease and treatment for autoimmune disease are surprisingly prevalent. Should oncologists refuse to treat these patients with ICI agents, alone and in combination with chemotherapy or CTLA4 inhibitors?
Biagio Ricciuti, MD, and colleagues published a retrospective, single-institution record review of 650 advanced NSCLC patients who were treated with ICI plus or minus CTLA-4 inhibition on a correlative intramural research study. Patients who received ICI with concurrent cytotoxic chemotherapy were excluded. They gathered clinical-pathologic information about whether patients received concurrent corticosteroids (10 mg/day or more vs. less than 10 mg/day of prednisone or the equivalent) and the reason for steroid use (oncologic vs. cancer-unrelated indications).
Importantly, they gathered information about programmed death-ligand 1 (PD-L1) tumor proportion scores and tumor mutational burden.
Among the 14.3% patients receiving prednisone 10 mg/day or more at the start of ICI therapy, progression-free survival and overall survival were significantly worse – but only among the 66 patients who needed steroids for oncologic reasons (pain, brain metastases, anorexia, cancer-associated dyspnea). Among the 27 patients who received steroids for cancer-unrelated reasons (autoimmune disease, chronic obstructive pulmonary disease, hypersensitivity pneumonitis), progression-free and overall survival were no different than for patients on prednisone 0-9 mg/day. Imbalances in PD-L1 tumor proportion scores among the groups analyzed did not clearly account for the differences in survival.
What this means in practice
The potential for great treatment outcomes with single-agent ICIs in a subset of advanced NSCLC patients, coupled with the lack of an air-tight biomarker for benefit, has changed the timing of discussions between oncologists and patients about stopping antineoplastic treatment. Since we cannot identify the patients for whom ICI use is futile, the default position has been lenient on using these expensive and potentially toxic therapies.
If verified in a multi-institutional setting, with larger numbers of NSCLC patients receiving steroids for cancer-unrelated reasons, the observations of Dr. Ricciuti and colleagues could help clinicians confidently identify the time to focus discussions on supportive care only. In patients with short survival and strong rationale for maximizing supportive care, analyses like this one could help us deliver more appropriate treatment, instead of more treatment, thereby furthering the goals of personalized cancer patient management.
Dr. Lyss has been a community-based medical oncologist and clinical researcher for more than 35 years, practicing in St. Louis. His clinical and research interests are in the prevention, diagnosis, and treatment of breast and lung cancers and in expanding access to clinical trials to medically underserved populations.
In this edition of “How I will treat my next patient,” I take a look at recent studies that examined ways to predict important outcomes in two very different settings, acute leukemia and advanced non–small cell lung cancer (NSCLC). They share the virtue of helping cancer specialists to increase their vigilance for clinically relevant complications and situations and to educate patients and families.
VTE risk in acute leukemia
The risk of venous thromboembolism (VTE) in cancer patients depends upon multiple patient-, tumor-, anatomic-, and treatment-related factors. The Khorana score has become an accepted standard for predicting the risks of VTE and assessing the relative value of various anticoagulants in cancer patients. However, the only hematologic malignancy that is specifically listed among the primary cancer sites in the Khorana score is “lymphoma.” VTE can develop during treatment for acute leukemia, especially among patients with acute lymphoblastic leukemia (ALL).
At the 2019 annual congress of the European Hematology Association, Alejandro Lazo-Langer, MD, and his colleagues proposed a scoring system to quantify the risks of VTE based on a retrospective cohort study of more than 500 acute leukemia patients, diagnosed from 2006-2017. They identified 77 patients with a VTE event, with a median time from diagnosis to VTE of 64 days. Among 20 possible predictive factors, 3 emerged in the final multivariate model – platelet count greater than 50,000 (1 point), ALL (2 points), and prior history of VTE (3 points).
Over a period of 12 months, patients with a score of more than 3 points had a cumulative incidence of VTE of 44%, in comparison with 10.5% among patients with lower scores. They were unable to discern whether particular antineoplastic regimens or drugs enhanced the risk.
The authors proposed that, if verified in a validation cohort study, the scoring system could lead to better patient education about signs and symptoms, more intensive surveillance for high-risk patients, and preventive interventions.
What this means in practice
Although a large number of patient records were reviewed for Dr. Lazo-Langer’s study, there were just 74 ALL patients, and it is unclear whether particular treatment regimens or drugs (such as L-asparaginase in ALL) enhance risk. Further study with a validation cohort (as was performed for the Khorana score for patients with other malignancies), is warranted. The study is thought provoking, but for now, in my opinion, standard clinical vigilance, surveillance, and education regarding VTE in leukemia patients remain appropriate.
Steroid impact in NSCLC with ICI therapy
Patients with autoimmune disease and individuals requiring active treatment with steroids (prednisone at 10 mg/day or more or the equivalent) were excluded from clinical trials that led to Food and Drug Administration approval of immune checkpoint inhibitor (ICI) agents. Recently published data indicate that treatment with 10 mg or more of daily prednisone correlates with poor outcome in NSCLC patients receiving ICI therapy (J Clin Oncol. 2018;36:2872-8; J Thoracic Oncol. 2018;13:1771-5). However, at the 2019 annual meeting of the American Society of Clinical Oncology, analyses of the CancerLinQ database showed that, among NSCLC patients, autoimmune disease and treatment for autoimmune disease are surprisingly prevalent. Should oncologists refuse to treat these patients with ICI agents, alone and in combination with chemotherapy or CTLA4 inhibitors?
Biagio Ricciuti, MD, and colleagues published a retrospective, single-institution record review of 650 advanced NSCLC patients who were treated with ICI plus or minus CTLA-4 inhibition on a correlative intramural research study. Patients who received ICI with concurrent cytotoxic chemotherapy were excluded. They gathered clinical-pathologic information about whether patients received concurrent corticosteroids (10 mg/day or more vs. less than 10 mg/day of prednisone or the equivalent) and the reason for steroid use (oncologic vs. cancer-unrelated indications).
Importantly, they gathered information about programmed death-ligand 1 (PD-L1) tumor proportion scores and tumor mutational burden.
Among the 14.3% patients receiving prednisone 10 mg/day or more at the start of ICI therapy, progression-free survival and overall survival were significantly worse – but only among the 66 patients who needed steroids for oncologic reasons (pain, brain metastases, anorexia, cancer-associated dyspnea). Among the 27 patients who received steroids for cancer-unrelated reasons (autoimmune disease, chronic obstructive pulmonary disease, hypersensitivity pneumonitis), progression-free and overall survival were no different than for patients on prednisone 0-9 mg/day. Imbalances in PD-L1 tumor proportion scores among the groups analyzed did not clearly account for the differences in survival.
What this means in practice
The potential for great treatment outcomes with single-agent ICIs in a subset of advanced NSCLC patients, coupled with the lack of an air-tight biomarker for benefit, has changed the timing of discussions between oncologists and patients about stopping antineoplastic treatment. Since we cannot identify the patients for whom ICI use is futile, the default position has been lenient on using these expensive and potentially toxic therapies.
If verified in a multi-institutional setting, with larger numbers of NSCLC patients receiving steroids for cancer-unrelated reasons, the observations of Dr. Ricciuti and colleagues could help clinicians confidently identify the time to focus discussions on supportive care only. In patients with short survival and strong rationale for maximizing supportive care, analyses like this one could help us deliver more appropriate treatment, instead of more treatment, thereby furthering the goals of personalized cancer patient management.
Dr. Lyss has been a community-based medical oncologist and clinical researcher for more than 35 years, practicing in St. Louis. His clinical and research interests are in the prevention, diagnosis, and treatment of breast and lung cancers and in expanding access to clinical trials to medically underserved populations.
In this edition of “How I will treat my next patient,” I take a look at recent studies that examined ways to predict important outcomes in two very different settings, acute leukemia and advanced non–small cell lung cancer (NSCLC). They share the virtue of helping cancer specialists to increase their vigilance for clinically relevant complications and situations and to educate patients and families.
VTE risk in acute leukemia
The risk of venous thromboembolism (VTE) in cancer patients depends upon multiple patient-, tumor-, anatomic-, and treatment-related factors. The Khorana score has become an accepted standard for predicting the risks of VTE and assessing the relative value of various anticoagulants in cancer patients. However, the only hematologic malignancy that is specifically listed among the primary cancer sites in the Khorana score is “lymphoma.” VTE can develop during treatment for acute leukemia, especially among patients with acute lymphoblastic leukemia (ALL).
At the 2019 annual congress of the European Hematology Association, Alejandro Lazo-Langer, MD, and his colleagues proposed a scoring system to quantify the risks of VTE based on a retrospective cohort study of more than 500 acute leukemia patients, diagnosed from 2006-2017. They identified 77 patients with a VTE event, with a median time from diagnosis to VTE of 64 days. Among 20 possible predictive factors, 3 emerged in the final multivariate model – platelet count greater than 50,000 (1 point), ALL (2 points), and prior history of VTE (3 points).
Over a period of 12 months, patients with a score of more than 3 points had a cumulative incidence of VTE of 44%, in comparison with 10.5% among patients with lower scores. They were unable to discern whether particular antineoplastic regimens or drugs enhanced the risk.
The authors proposed that, if verified in a validation cohort study, the scoring system could lead to better patient education about signs and symptoms, more intensive surveillance for high-risk patients, and preventive interventions.
What this means in practice
Although a large number of patient records were reviewed for Dr. Lazo-Langer’s study, there were just 74 ALL patients, and it is unclear whether particular treatment regimens or drugs (such as L-asparaginase in ALL) enhance risk. Further study with a validation cohort (as was performed for the Khorana score for patients with other malignancies), is warranted. The study is thought provoking, but for now, in my opinion, standard clinical vigilance, surveillance, and education regarding VTE in leukemia patients remain appropriate.
Steroid impact in NSCLC with ICI therapy
Patients with autoimmune disease and individuals requiring active treatment with steroids (prednisone at 10 mg/day or more or the equivalent) were excluded from clinical trials that led to Food and Drug Administration approval of immune checkpoint inhibitor (ICI) agents. Recently published data indicate that treatment with 10 mg or more of daily prednisone correlates with poor outcome in NSCLC patients receiving ICI therapy (J Clin Oncol. 2018;36:2872-8; J Thoracic Oncol. 2018;13:1771-5). However, at the 2019 annual meeting of the American Society of Clinical Oncology, analyses of the CancerLinQ database showed that, among NSCLC patients, autoimmune disease and treatment for autoimmune disease are surprisingly prevalent. Should oncologists refuse to treat these patients with ICI agents, alone and in combination with chemotherapy or CTLA4 inhibitors?
Biagio Ricciuti, MD, and colleagues published a retrospective, single-institution record review of 650 advanced NSCLC patients who were treated with ICI plus or minus CTLA-4 inhibition on a correlative intramural research study. Patients who received ICI with concurrent cytotoxic chemotherapy were excluded. They gathered clinical-pathologic information about whether patients received concurrent corticosteroids (10 mg/day or more vs. less than 10 mg/day of prednisone or the equivalent) and the reason for steroid use (oncologic vs. cancer-unrelated indications).
Importantly, they gathered information about programmed death-ligand 1 (PD-L1) tumor proportion scores and tumor mutational burden.
Among the 14.3% patients receiving prednisone 10 mg/day or more at the start of ICI therapy, progression-free survival and overall survival were significantly worse – but only among the 66 patients who needed steroids for oncologic reasons (pain, brain metastases, anorexia, cancer-associated dyspnea). Among the 27 patients who received steroids for cancer-unrelated reasons (autoimmune disease, chronic obstructive pulmonary disease, hypersensitivity pneumonitis), progression-free and overall survival were no different than for patients on prednisone 0-9 mg/day. Imbalances in PD-L1 tumor proportion scores among the groups analyzed did not clearly account for the differences in survival.
What this means in practice
The potential for great treatment outcomes with single-agent ICIs in a subset of advanced NSCLC patients, coupled with the lack of an air-tight biomarker for benefit, has changed the timing of discussions between oncologists and patients about stopping antineoplastic treatment. Since we cannot identify the patients for whom ICI use is futile, the default position has been lenient on using these expensive and potentially toxic therapies.
If verified in a multi-institutional setting, with larger numbers of NSCLC patients receiving steroids for cancer-unrelated reasons, the observations of Dr. Ricciuti and colleagues could help clinicians confidently identify the time to focus discussions on supportive care only. In patients with short survival and strong rationale for maximizing supportive care, analyses like this one could help us deliver more appropriate treatment, instead of more treatment, thereby furthering the goals of personalized cancer patient management.
Dr. Lyss has been a community-based medical oncologist and clinical researcher for more than 35 years, practicing in St. Louis. His clinical and research interests are in the prevention, diagnosis, and treatment of breast and lung cancers and in expanding access to clinical trials to medically underserved populations.
‘Robust antitumor immune responses’ observed in pediatric ALL
Pediatric acute lymphoblastic leukemia (ALL) may be more vulnerable to immunotherapies than previously thought, according to researchers.
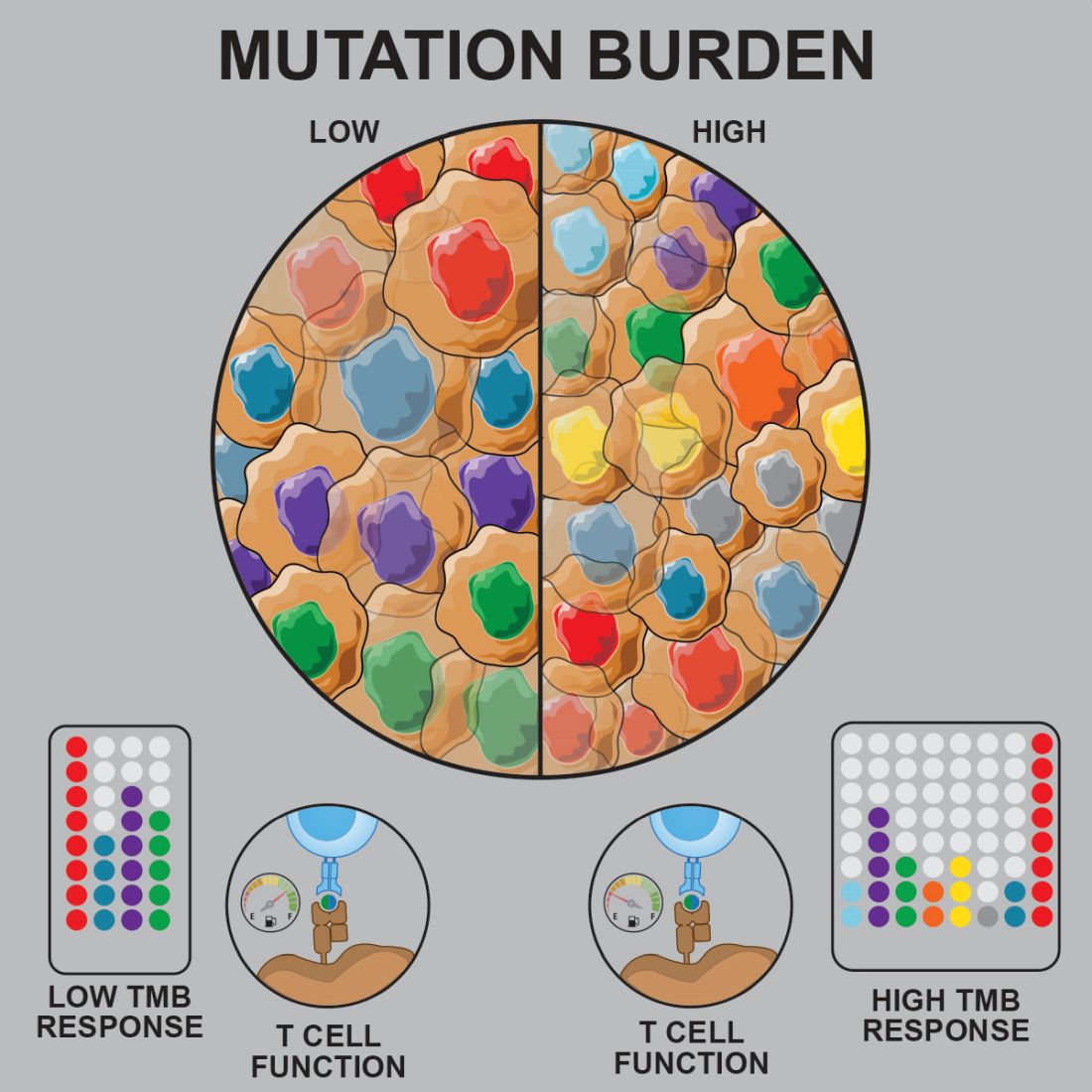
Prior studies suggested that tumors with a low mutational burden don’t elicit strong antitumor responses and therefore aren’t very susceptible to immunotherapy.
Now, researchers have found evidence to suggest that pediatric ALL induces “robust antitumor immune responses” despite a low mutational burden. The investigators identified tumor-associated CD8+ T cells that responded to 86% of neoantigens tested and recognized 68% of neoepitopes tested.
Anthony E. Zamora, PhD, of St. Jude Children’s Research Hospital in Memphis, Tenn., and colleagues recounted these findings in Science Translational Medicine.
The researchers analyzed samples from pediatric patients with ETV-associated ALL (n = 9) or ERG-associated ALL (n = 2) to determine how endogenous CD8+ T cells respond to patient-specific cancer neoantigens.
The investigators first assessed the ability of tumor-specific mutations and gene fusions to generate neoepitopes, or neoantigens predicted to bind patient-specific human leukocyte antigen (HLA) proteins. The team identified 5-28 neoepitopes per patient, including epitopes that spanned the fusion junction in patients with ETV6-RUNX1 fusions.
The researchers then tested whether CD8+ tumor infiltrating lymphocytes (TILs) were directly responsive to mutated neoepitopes. They observed cytokine responses across patient samples, noting that 31 of the 36 putative neoantigens tested (86%) were “immunogenic and capable of inducing robust cytokine responses.”
Next, the investigators mapped TIL responses to specific epitopes using patient-specific tetramers that corresponded to the previously identified neoepitopes. Seventeen of the 25 patient-specific tetramers (68%) bound to TILs above the background set by irrelevant HLA-matched tetramers.
“Within those responses, we observed immunodominance hierarchies among the distinct TIL populations, with a majority of tetramer-bound CD8+ T cells restricted to one or two putative neoepitopes,” the researchers noted.
The team also pointed out that seven of nine patients tested had CD8+ T cells that responded to ETV6-RUNX1.
Finally, the investigators performed transcriptional profiling of ALL-specific CD8+ TILs to assess inter- and intrapatient heterogeneity. The team identified three hierarchical clusters, which were characterized by transcriptional factors and regulators associated with:
- Functional effector CD8+ T cells (TBX21 and EOMES).
- Dysfunctional CD8+ T cells (STAT1/3/4, NR4A2/3, and BCL6).
- Exhausted CD8+ T cells (EOMES, MAF, PRDM1, and BATF).
Considering these findings together, the researchers concluded that “pediatric ALL elicits a potent neoepitope-specific CD8+ T-cell response.” Therefore, adoptive T-cell, monoclonal antibody, and targeted T-cell receptor therapies “should be explored” in pediatric ALL.
This research was supported by the National Institutes of Health, National Cancer Institute, National Institute of General Medical Sciences, Key for a Cure Foundation, and American Lebanese Syrian Associated Charities. The researchers disclosed patent applications and relationships with Pfizer, Amgen, and other companies.
SOURCE: Zamora AE et al. Sci. Transl. Med. 2019 Jun 26. doi: 10.1126/scitranslmed.aat8549.
Pediatric acute lymphoblastic leukemia (ALL) may be more vulnerable to immunotherapies than previously thought, according to researchers.

Prior studies suggested that tumors with a low mutational burden don’t elicit strong antitumor responses and therefore aren’t very susceptible to immunotherapy.
Now, researchers have found evidence to suggest that pediatric ALL induces “robust antitumor immune responses” despite a low mutational burden. The investigators identified tumor-associated CD8+ T cells that responded to 86% of neoantigens tested and recognized 68% of neoepitopes tested.
Anthony E. Zamora, PhD, of St. Jude Children’s Research Hospital in Memphis, Tenn., and colleagues recounted these findings in Science Translational Medicine.
The researchers analyzed samples from pediatric patients with ETV-associated ALL (n = 9) or ERG-associated ALL (n = 2) to determine how endogenous CD8+ T cells respond to patient-specific cancer neoantigens.
The investigators first assessed the ability of tumor-specific mutations and gene fusions to generate neoepitopes, or neoantigens predicted to bind patient-specific human leukocyte antigen (HLA) proteins. The team identified 5-28 neoepitopes per patient, including epitopes that spanned the fusion junction in patients with ETV6-RUNX1 fusions.
The researchers then tested whether CD8+ tumor infiltrating lymphocytes (TILs) were directly responsive to mutated neoepitopes. They observed cytokine responses across patient samples, noting that 31 of the 36 putative neoantigens tested (86%) were “immunogenic and capable of inducing robust cytokine responses.”
Next, the investigators mapped TIL responses to specific epitopes using patient-specific tetramers that corresponded to the previously identified neoepitopes. Seventeen of the 25 patient-specific tetramers (68%) bound to TILs above the background set by irrelevant HLA-matched tetramers.
“Within those responses, we observed immunodominance hierarchies among the distinct TIL populations, with a majority of tetramer-bound CD8+ T cells restricted to one or two putative neoepitopes,” the researchers noted.
The team also pointed out that seven of nine patients tested had CD8+ T cells that responded to ETV6-RUNX1.
Finally, the investigators performed transcriptional profiling of ALL-specific CD8+ TILs to assess inter- and intrapatient heterogeneity. The team identified three hierarchical clusters, which were characterized by transcriptional factors and regulators associated with:
- Functional effector CD8+ T cells (TBX21 and EOMES).
- Dysfunctional CD8+ T cells (STAT1/3/4, NR4A2/3, and BCL6).
- Exhausted CD8+ T cells (EOMES, MAF, PRDM1, and BATF).
Considering these findings together, the researchers concluded that “pediatric ALL elicits a potent neoepitope-specific CD8+ T-cell response.” Therefore, adoptive T-cell, monoclonal antibody, and targeted T-cell receptor therapies “should be explored” in pediatric ALL.
This research was supported by the National Institutes of Health, National Cancer Institute, National Institute of General Medical Sciences, Key for a Cure Foundation, and American Lebanese Syrian Associated Charities. The researchers disclosed patent applications and relationships with Pfizer, Amgen, and other companies.
SOURCE: Zamora AE et al. Sci. Transl. Med. 2019 Jun 26. doi: 10.1126/scitranslmed.aat8549.
Pediatric acute lymphoblastic leukemia (ALL) may be more vulnerable to immunotherapies than previously thought, according to researchers.

Prior studies suggested that tumors with a low mutational burden don’t elicit strong antitumor responses and therefore aren’t very susceptible to immunotherapy.
Now, researchers have found evidence to suggest that pediatric ALL induces “robust antitumor immune responses” despite a low mutational burden. The investigators identified tumor-associated CD8+ T cells that responded to 86% of neoantigens tested and recognized 68% of neoepitopes tested.
Anthony E. Zamora, PhD, of St. Jude Children’s Research Hospital in Memphis, Tenn., and colleagues recounted these findings in Science Translational Medicine.
The researchers analyzed samples from pediatric patients with ETV-associated ALL (n = 9) or ERG-associated ALL (n = 2) to determine how endogenous CD8+ T cells respond to patient-specific cancer neoantigens.
The investigators first assessed the ability of tumor-specific mutations and gene fusions to generate neoepitopes, or neoantigens predicted to bind patient-specific human leukocyte antigen (HLA) proteins. The team identified 5-28 neoepitopes per patient, including epitopes that spanned the fusion junction in patients with ETV6-RUNX1 fusions.
The researchers then tested whether CD8+ tumor infiltrating lymphocytes (TILs) were directly responsive to mutated neoepitopes. They observed cytokine responses across patient samples, noting that 31 of the 36 putative neoantigens tested (86%) were “immunogenic and capable of inducing robust cytokine responses.”
Next, the investigators mapped TIL responses to specific epitopes using patient-specific tetramers that corresponded to the previously identified neoepitopes. Seventeen of the 25 patient-specific tetramers (68%) bound to TILs above the background set by irrelevant HLA-matched tetramers.
“Within those responses, we observed immunodominance hierarchies among the distinct TIL populations, with a majority of tetramer-bound CD8+ T cells restricted to one or two putative neoepitopes,” the researchers noted.
The team also pointed out that seven of nine patients tested had CD8+ T cells that responded to ETV6-RUNX1.
Finally, the investigators performed transcriptional profiling of ALL-specific CD8+ TILs to assess inter- and intrapatient heterogeneity. The team identified three hierarchical clusters, which were characterized by transcriptional factors and regulators associated with:
- Functional effector CD8+ T cells (TBX21 and EOMES).
- Dysfunctional CD8+ T cells (STAT1/3/4, NR4A2/3, and BCL6).
- Exhausted CD8+ T cells (EOMES, MAF, PRDM1, and BATF).
Considering these findings together, the researchers concluded that “pediatric ALL elicits a potent neoepitope-specific CD8+ T-cell response.” Therefore, adoptive T-cell, monoclonal antibody, and targeted T-cell receptor therapies “should be explored” in pediatric ALL.
This research was supported by the National Institutes of Health, National Cancer Institute, National Institute of General Medical Sciences, Key for a Cure Foundation, and American Lebanese Syrian Associated Charities. The researchers disclosed patent applications and relationships with Pfizer, Amgen, and other companies.
SOURCE: Zamora AE et al. Sci. Transl. Med. 2019 Jun 26. doi: 10.1126/scitranslmed.aat8549.
FROM SCIENCE TRANSLATIONAL MEDICINE
Key clinical point: Preclinical research suggests pediatric acute lymphoblastic leukemia (ALL) induces “robust antitumor immune responses” despite a low mutational burden.
Major finding: Investigators identified tumor-associated CD8+ T cells that responded to 86% of neoantigens tested and recognized 68% of neoepitopes tested.
Study details: Analysis of samples from pediatric patients with ETV-associated ALL (n = 9) or ERG-associated ALL (n = 2).
Disclosures: The research was supported by the National Institutes of Health, National Cancer Institute, National Institute of General Medical Sciences, Key for a Cure Foundation, and American Lebanese Syrian Associated Charities. The researchers disclosed patent applications and relationships with Pfizer, Amgen, and other companies.
Source: Zamora AE et al. Sci. Transl. Med. 2019 Jun 26. doi: 10.1126/scitranslmed.aat8549.
NCCN publishes pediatric ALL guidelines
“The cure rate for pediatric ALL in the U.S. has risen from 0% in the 1960s to nearly 90% today. This is among the most profound medical success stories in history,” Patrick Brown, MD, of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University, Baltimore, said in a statement announcing the guidelines. Dr. Brown chairs the NCCN Clinical Practice Guidelines for adult and pediatric ALL.
“Pediatric ALL survivors live a long time; we have to consider long-term effects as well,” Hiroto Inaba, MD, PhD, of St. Jude Children’s Research Hospital, Memphis, and vice chair of the guidelines committee, said in the statement.
The new recommendations highlight the importance of supportive care interventions in an effort to reduce the chances of patients experiencing severe adverse effects.
The pediatric ALL guidelines provide evidence-based recommendations about optimal treatment strategies for ALL to prolong survival in children affected, with a focus on treatment outside of clinical trials (Pediatric Acute Lymphoblastic Leukemia. NCCN.org, Version 1.2019, published May 30, 2019).
While treatment for ALL often includes long-term chemotherapy regimens that involve multiple stages, several novel treatment strategies are summarized in the guidelines, including various types of immunotherapy and targeted therapy.
The guidelines are intended to accompany the NCCN Guidelines for Adult ALL and integrate treatment recommendations for patients in overlapping age categories. The recommendations are organized based on risk level, which may also be associated with age.
“The highest risk [is] associated with those diagnosed within the first 12 months of life or between the ages 10 and 21 years old,” the guideline authors wrote.
Another unique aspect of the guidelines is the recognition of vulnerable populations, such as young infants or children with Down syndrome, who face distinct treatment challenges. The authors provide guidance on the best supportive care measures for these patients.
The NCCN is currently expanding the collection of clinical practice guidelines for additional pediatric malignancies. At present, they are planning to undertake a minimum of 90% of all incident pediatric cancers.
Upcoming guidelines include treatment recommendations for pediatric Burkitt lymphoma, and are scheduled for release later in 2019.
Future efforts include modifying the guidelines for use in low- and middle-income countries, with the goal of providing direction in resource-limited environments.
“We know that many, many children can be cured with inexpensive and widely-available therapies,” Dr. Brown said. “With the increasing global reach of the NCCN Guidelines, we can really pave the way for increasing the cure rates throughout the world.”
“The cure rate for pediatric ALL in the U.S. has risen from 0% in the 1960s to nearly 90% today. This is among the most profound medical success stories in history,” Patrick Brown, MD, of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University, Baltimore, said in a statement announcing the guidelines. Dr. Brown chairs the NCCN Clinical Practice Guidelines for adult and pediatric ALL.
“Pediatric ALL survivors live a long time; we have to consider long-term effects as well,” Hiroto Inaba, MD, PhD, of St. Jude Children’s Research Hospital, Memphis, and vice chair of the guidelines committee, said in the statement.
The new recommendations highlight the importance of supportive care interventions in an effort to reduce the chances of patients experiencing severe adverse effects.
The pediatric ALL guidelines provide evidence-based recommendations about optimal treatment strategies for ALL to prolong survival in children affected, with a focus on treatment outside of clinical trials (Pediatric Acute Lymphoblastic Leukemia. NCCN.org, Version 1.2019, published May 30, 2019).
While treatment for ALL often includes long-term chemotherapy regimens that involve multiple stages, several novel treatment strategies are summarized in the guidelines, including various types of immunotherapy and targeted therapy.
The guidelines are intended to accompany the NCCN Guidelines for Adult ALL and integrate treatment recommendations for patients in overlapping age categories. The recommendations are organized based on risk level, which may also be associated with age.
“The highest risk [is] associated with those diagnosed within the first 12 months of life or between the ages 10 and 21 years old,” the guideline authors wrote.
Another unique aspect of the guidelines is the recognition of vulnerable populations, such as young infants or children with Down syndrome, who face distinct treatment challenges. The authors provide guidance on the best supportive care measures for these patients.
The NCCN is currently expanding the collection of clinical practice guidelines for additional pediatric malignancies. At present, they are planning to undertake a minimum of 90% of all incident pediatric cancers.
Upcoming guidelines include treatment recommendations for pediatric Burkitt lymphoma, and are scheduled for release later in 2019.
Future efforts include modifying the guidelines for use in low- and middle-income countries, with the goal of providing direction in resource-limited environments.
“We know that many, many children can be cured with inexpensive and widely-available therapies,” Dr. Brown said. “With the increasing global reach of the NCCN Guidelines, we can really pave the way for increasing the cure rates throughout the world.”
“The cure rate for pediatric ALL in the U.S. has risen from 0% in the 1960s to nearly 90% today. This is among the most profound medical success stories in history,” Patrick Brown, MD, of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University, Baltimore, said in a statement announcing the guidelines. Dr. Brown chairs the NCCN Clinical Practice Guidelines for adult and pediatric ALL.
“Pediatric ALL survivors live a long time; we have to consider long-term effects as well,” Hiroto Inaba, MD, PhD, of St. Jude Children’s Research Hospital, Memphis, and vice chair of the guidelines committee, said in the statement.
The new recommendations highlight the importance of supportive care interventions in an effort to reduce the chances of patients experiencing severe adverse effects.
The pediatric ALL guidelines provide evidence-based recommendations about optimal treatment strategies for ALL to prolong survival in children affected, with a focus on treatment outside of clinical trials (Pediatric Acute Lymphoblastic Leukemia. NCCN.org, Version 1.2019, published May 30, 2019).
While treatment for ALL often includes long-term chemotherapy regimens that involve multiple stages, several novel treatment strategies are summarized in the guidelines, including various types of immunotherapy and targeted therapy.
The guidelines are intended to accompany the NCCN Guidelines for Adult ALL and integrate treatment recommendations for patients in overlapping age categories. The recommendations are organized based on risk level, which may also be associated with age.
“The highest risk [is] associated with those diagnosed within the first 12 months of life or between the ages 10 and 21 years old,” the guideline authors wrote.
Another unique aspect of the guidelines is the recognition of vulnerable populations, such as young infants or children with Down syndrome, who face distinct treatment challenges. The authors provide guidance on the best supportive care measures for these patients.
The NCCN is currently expanding the collection of clinical practice guidelines for additional pediatric malignancies. At present, they are planning to undertake a minimum of 90% of all incident pediatric cancers.
Upcoming guidelines include treatment recommendations for pediatric Burkitt lymphoma, and are scheduled for release later in 2019.
Future efforts include modifying the guidelines for use in low- and middle-income countries, with the goal of providing direction in resource-limited environments.
“We know that many, many children can be cured with inexpensive and widely-available therapies,” Dr. Brown said. “With the increasing global reach of the NCCN Guidelines, we can really pave the way for increasing the cure rates throughout the world.”
FROM THE NATIONAL COMPREHENSIVE CANCER NETWORK
SC-PEG comparable to pegaspargase in young ALL/LL patients
CHICAGO – Calaspargase pegol (SC-PEG) produces similar outcomes as standard pegaspargase in pediatric and young adult patients with newly diagnosed acute lymphoblastic leukemia (ALL) or lymphoblastic lymphoma (LL), according to a phase 2 trial.
Patients who received SC-PEG every 3 weeks had similar serum asparaginase activity (SAA), toxicities, and survival rates as patients who received standard pegaspargase every 2 weeks.
Lynda M. Vrooman, MD, of Dana-Farber Cancer Institute in Boston, presented these results at the annual meeting of the American Society of Clinical Oncology.
The trial (NCT01574274) enrolled 239 patients, 230 with ALL and 9 with LL. Most patients had B-cell (n = 207) disease. The patients’ median age was 5.2 years (range, 1.0-20.9 years).
“There were no differences in presenting features by randomization,” Dr. Vrooman noted.
The patients were randomized to receive pegaspargase (n = 120) or SC-PEG (n = 119), a pegylated asparaginase formulation with longer half-life. SC-PEG was given at 2,500 IU/m2 every 3 weeks, and pegaspargase was given at 2,500 IU/m2 every 2 weeks.
Either asparaginase product was given as part of a 4-week induction regimen (vincristine, prednisone, doxorubicin, and methotrexate), a 3-week intensification regimen (intrathecal chemotherapy with or without radiotherapy) for central nervous system disease, and a 27-week second consolidation regimen (mercaptopurine, methotrexate, and, in high-risk patients, doxorubicin).
SAA
The researchers observed significantly longer SAA with SC-PEG during induction but not after.
During induction, at 25 days after the first asparaginase dose, 88% of patients on SC-PEG and 17% of those on pegaspargase had SAA of at least 0.10 IU/mL (P less than .001). Post-induction, at week 25, 100% of patients in each group had a nadir SAA of at least 0.10 IU/mL.
“The high nadir serum asparaginase activity levels observed for both preparations suggest dosing strategies could be further optimized,” Dr. Vrooman noted.
Safety
There were no significant differences in adverse events between the SC-PEG and pegaspargase arms during or after induction.
Adverse events during induction (in the SC-PEG and pegaspargase arms, respectively) included grade 2 or higher asparaginase allergy (0% and 1%), grade 2 or higher pancreatitis (3% in both), grade 2 or higher thrombosis (3% and 9%), grade 4 hyperbilirubinemia (3% and 1%), grade 3 or higher bacterial infection (12% and 9%), and grade 3 or higher fungal infection (4% and 5%).
Adverse events after induction (in the SC-PEG and pegaspargase arms, respectively) included grade 2 or higher asparaginase allergy (17% and 14%), grade 2 or higher pancreatitis (15% in both), grade 2 or higher thrombosis (18% and 13%), grade 4 hyperbilirubinemia (4% and 3%), grade 3 or higher bacterial infection (12% and 15%), grade 3 or higher fungal infection (2% and 1%), grade 2 or higher bone fracture (3% and 8%), and grade 2 or higher osteonecrosis (3% and 4%).
Response and survival
The complete response rate was 95% (109/115) in the SC-PEG arm and 99% (114/115) in the pegaspargase arm. Rates of induction failure were 3% (n = 4) and 1% (n = 1), respectively, and rates of relapse were 3% (n = 5) and 8% (n = 10), respectively.
There were two induction deaths and two remission deaths in the SC-PEG arm but no induction or remission deaths in the pegaspargase arm.
The median follow-up was 4 years. The 4-year event-free survival rate was 87.7% with SC-PEG and 90.2% with pegaspargase (P = .78). The 4-year overall survival rate was 94.8% and 95.6%, respectively (P = .74).
In closing, Dr. Vrooman said these data suggest SC-PEG provides similar results as standard pegaspargase. She noted that these data informed the U.S. approval of SC-PEG for pediatric and young adult ALL.
This trial was sponsored by the Dana-Farber Cancer Institute in collaboration with Shire and the National Cancer Institute. Dr. Vrooman said she had no relationships to disclose.
SOURCE: Vrooman LM et al. ASCO 2019. Abstract 10006.
CHICAGO – Calaspargase pegol (SC-PEG) produces similar outcomes as standard pegaspargase in pediatric and young adult patients with newly diagnosed acute lymphoblastic leukemia (ALL) or lymphoblastic lymphoma (LL), according to a phase 2 trial.
Patients who received SC-PEG every 3 weeks had similar serum asparaginase activity (SAA), toxicities, and survival rates as patients who received standard pegaspargase every 2 weeks.
Lynda M. Vrooman, MD, of Dana-Farber Cancer Institute in Boston, presented these results at the annual meeting of the American Society of Clinical Oncology.
The trial (NCT01574274) enrolled 239 patients, 230 with ALL and 9 with LL. Most patients had B-cell (n = 207) disease. The patients’ median age was 5.2 years (range, 1.0-20.9 years).
“There were no differences in presenting features by randomization,” Dr. Vrooman noted.
The patients were randomized to receive pegaspargase (n = 120) or SC-PEG (n = 119), a pegylated asparaginase formulation with longer half-life. SC-PEG was given at 2,500 IU/m2 every 3 weeks, and pegaspargase was given at 2,500 IU/m2 every 2 weeks.
Either asparaginase product was given as part of a 4-week induction regimen (vincristine, prednisone, doxorubicin, and methotrexate), a 3-week intensification regimen (intrathecal chemotherapy with or without radiotherapy) for central nervous system disease, and a 27-week second consolidation regimen (mercaptopurine, methotrexate, and, in high-risk patients, doxorubicin).
SAA
The researchers observed significantly longer SAA with SC-PEG during induction but not after.
During induction, at 25 days after the first asparaginase dose, 88% of patients on SC-PEG and 17% of those on pegaspargase had SAA of at least 0.10 IU/mL (P less than .001). Post-induction, at week 25, 100% of patients in each group had a nadir SAA of at least 0.10 IU/mL.
“The high nadir serum asparaginase activity levels observed for both preparations suggest dosing strategies could be further optimized,” Dr. Vrooman noted.
Safety
There were no significant differences in adverse events between the SC-PEG and pegaspargase arms during or after induction.
Adverse events during induction (in the SC-PEG and pegaspargase arms, respectively) included grade 2 or higher asparaginase allergy (0% and 1%), grade 2 or higher pancreatitis (3% in both), grade 2 or higher thrombosis (3% and 9%), grade 4 hyperbilirubinemia (3% and 1%), grade 3 or higher bacterial infection (12% and 9%), and grade 3 or higher fungal infection (4% and 5%).
Adverse events after induction (in the SC-PEG and pegaspargase arms, respectively) included grade 2 or higher asparaginase allergy (17% and 14%), grade 2 or higher pancreatitis (15% in both), grade 2 or higher thrombosis (18% and 13%), grade 4 hyperbilirubinemia (4% and 3%), grade 3 or higher bacterial infection (12% and 15%), grade 3 or higher fungal infection (2% and 1%), grade 2 or higher bone fracture (3% and 8%), and grade 2 or higher osteonecrosis (3% and 4%).
Response and survival
The complete response rate was 95% (109/115) in the SC-PEG arm and 99% (114/115) in the pegaspargase arm. Rates of induction failure were 3% (n = 4) and 1% (n = 1), respectively, and rates of relapse were 3% (n = 5) and 8% (n = 10), respectively.
There were two induction deaths and two remission deaths in the SC-PEG arm but no induction or remission deaths in the pegaspargase arm.
The median follow-up was 4 years. The 4-year event-free survival rate was 87.7% with SC-PEG and 90.2% with pegaspargase (P = .78). The 4-year overall survival rate was 94.8% and 95.6%, respectively (P = .74).
In closing, Dr. Vrooman said these data suggest SC-PEG provides similar results as standard pegaspargase. She noted that these data informed the U.S. approval of SC-PEG for pediatric and young adult ALL.
This trial was sponsored by the Dana-Farber Cancer Institute in collaboration with Shire and the National Cancer Institute. Dr. Vrooman said she had no relationships to disclose.
SOURCE: Vrooman LM et al. ASCO 2019. Abstract 10006.
CHICAGO – Calaspargase pegol (SC-PEG) produces similar outcomes as standard pegaspargase in pediatric and young adult patients with newly diagnosed acute lymphoblastic leukemia (ALL) or lymphoblastic lymphoma (LL), according to a phase 2 trial.
Patients who received SC-PEG every 3 weeks had similar serum asparaginase activity (SAA), toxicities, and survival rates as patients who received standard pegaspargase every 2 weeks.
Lynda M. Vrooman, MD, of Dana-Farber Cancer Institute in Boston, presented these results at the annual meeting of the American Society of Clinical Oncology.
The trial (NCT01574274) enrolled 239 patients, 230 with ALL and 9 with LL. Most patients had B-cell (n = 207) disease. The patients’ median age was 5.2 years (range, 1.0-20.9 years).
“There were no differences in presenting features by randomization,” Dr. Vrooman noted.
The patients were randomized to receive pegaspargase (n = 120) or SC-PEG (n = 119), a pegylated asparaginase formulation with longer half-life. SC-PEG was given at 2,500 IU/m2 every 3 weeks, and pegaspargase was given at 2,500 IU/m2 every 2 weeks.
Either asparaginase product was given as part of a 4-week induction regimen (vincristine, prednisone, doxorubicin, and methotrexate), a 3-week intensification regimen (intrathecal chemotherapy with or without radiotherapy) for central nervous system disease, and a 27-week second consolidation regimen (mercaptopurine, methotrexate, and, in high-risk patients, doxorubicin).
SAA
The researchers observed significantly longer SAA with SC-PEG during induction but not after.
During induction, at 25 days after the first asparaginase dose, 88% of patients on SC-PEG and 17% of those on pegaspargase had SAA of at least 0.10 IU/mL (P less than .001). Post-induction, at week 25, 100% of patients in each group had a nadir SAA of at least 0.10 IU/mL.
“The high nadir serum asparaginase activity levels observed for both preparations suggest dosing strategies could be further optimized,” Dr. Vrooman noted.
Safety
There were no significant differences in adverse events between the SC-PEG and pegaspargase arms during or after induction.
Adverse events during induction (in the SC-PEG and pegaspargase arms, respectively) included grade 2 or higher asparaginase allergy (0% and 1%), grade 2 or higher pancreatitis (3% in both), grade 2 or higher thrombosis (3% and 9%), grade 4 hyperbilirubinemia (3% and 1%), grade 3 or higher bacterial infection (12% and 9%), and grade 3 or higher fungal infection (4% and 5%).
Adverse events after induction (in the SC-PEG and pegaspargase arms, respectively) included grade 2 or higher asparaginase allergy (17% and 14%), grade 2 or higher pancreatitis (15% in both), grade 2 or higher thrombosis (18% and 13%), grade 4 hyperbilirubinemia (4% and 3%), grade 3 or higher bacterial infection (12% and 15%), grade 3 or higher fungal infection (2% and 1%), grade 2 or higher bone fracture (3% and 8%), and grade 2 or higher osteonecrosis (3% and 4%).
Response and survival
The complete response rate was 95% (109/115) in the SC-PEG arm and 99% (114/115) in the pegaspargase arm. Rates of induction failure were 3% (n = 4) and 1% (n = 1), respectively, and rates of relapse were 3% (n = 5) and 8% (n = 10), respectively.
There were two induction deaths and two remission deaths in the SC-PEG arm but no induction or remission deaths in the pegaspargase arm.
The median follow-up was 4 years. The 4-year event-free survival rate was 87.7% with SC-PEG and 90.2% with pegaspargase (P = .78). The 4-year overall survival rate was 94.8% and 95.6%, respectively (P = .74).
In closing, Dr. Vrooman said these data suggest SC-PEG provides similar results as standard pegaspargase. She noted that these data informed the U.S. approval of SC-PEG for pediatric and young adult ALL.
This trial was sponsored by the Dana-Farber Cancer Institute in collaboration with Shire and the National Cancer Institute. Dr. Vrooman said she had no relationships to disclose.
SOURCE: Vrooman LM et al. ASCO 2019. Abstract 10006.
REPORTING FROM ASCO 2019
Low intensity bridging may be best path to CAR T in adult ALL
CHICAGO – A low intensity chemotherapy regimen may be the best approach to bridge patients waiting for chimeric antigen receptor (CAR) T-cell therapy, according to a retrospective analysis of adults with acute lymphoblastic leukemia (ALL).
Investigators found that high intensity bridging regimens provided no clear outcome benefit, but did produce a greater number of infections.
But the decision on the type of regimen is very much dependent on the individual patient, Karlo Perica, MD, PhD, of Memorial Sloan Kettering Cancer Center in New York, said at the annual meeting of the American Society of Clinical Oncology.
Dr. Perica and his colleagues at Memorial Sloan Kettering examined the effectiveness and toxicity of bridging therapies provided to relapsed or refractory ALL patients waiting to receive CD19 CAR T-cell therapy as part of a phase 1 trial (N Engl J Med. 2018 Feb 1;378[5]:449-59).
Bridging therapy was defined as any therapy given from leukapheresis to cell infusion.
The low-intensity regimens included POMP (6-mercaptopurine, vincristine, methotrexate, and prednisone, or combinations), liposomal vincristine, mini-hyper CVD (reduced cyclophosphamide, dexamethasone, methotrexate, Ara-C), blinatumomab, inotuzumab, oral tyrosine kinase inhibitor-based regimens, or hydroxyurea.
The high-intensity regimens included hyper-CVAD (cyclophosphamide, vincristine, doxorubicin, dexamethasone), high-dose cytarabine, attenuated FLAG/FLAG-IDA (reduced fludarabine, cytarabine, G-CSF plus or minus idarubicin), and pediatric-type induction.
Of the 53 patients who were ultimately infused with CAR T cells, 19 received some type of high intensity regimen, 29 received low intensity regimens, and 5 received no bridging treatment. The group overall was heavily pretreated. Nearly a third of the low intensity and no bridging patients and 42% of the high intensity patients had previously undergone transplant. More than 40% of the low intensity and no bridging patients and about a quarter of the high intensity bridging group had four or more prior lines of therapy.
The use of high intensity bridging therapy was not associated with improved overall response or relapse-free survival to CAR T-cell therapy, the investigators reported. In a subgroup with 23 high disease burden patients with greater than 20% blasts, there was no difference in MRD-negative complete response by intensity (75% versus 60%, Fisher’s P = .65).
High intensity bridging was also not associated with successful CAR T-cell infusion, versus low intensity regimens (63% versus 79%, P greater than .05) or a combined endpoint of CAR T-cell infusion plus transplant or alternative treatment (80% versus 86%, P greater than .05).
In terms of toxicity, the high intensity bridging regimens were associated with a higher rate of grade 3 or 4 infections – 15 versus 11 infections (Fisher’s P = .002). But there was no association with post-infusion grade 3 or 4 cytokine release syndrome or neurotoxicity.
Dr. Perica said the results reflect that the real goal of bridging is not to reduce disease burden but instead to successfully bring patients to the next phase of their treatment. “The goal of the bridging therapy is to get the patient to the CAR infusion,” he said.
Due to the retrospective nature of the study, Dr. Perica said he can’t recommend any single bridging regimen and he emphasized that the decisions are patient-specific.
The original study was funded by several foundations and Juno Therapeutics. Dr. Perica reported royalties from technology licensed to Neximmune.
SOURCE: Perica K et al. ASCO 2019, Abstract 2520.
CHICAGO – A low intensity chemotherapy regimen may be the best approach to bridge patients waiting for chimeric antigen receptor (CAR) T-cell therapy, according to a retrospective analysis of adults with acute lymphoblastic leukemia (ALL).
Investigators found that high intensity bridging regimens provided no clear outcome benefit, but did produce a greater number of infections.
But the decision on the type of regimen is very much dependent on the individual patient, Karlo Perica, MD, PhD, of Memorial Sloan Kettering Cancer Center in New York, said at the annual meeting of the American Society of Clinical Oncology.
Dr. Perica and his colleagues at Memorial Sloan Kettering examined the effectiveness and toxicity of bridging therapies provided to relapsed or refractory ALL patients waiting to receive CD19 CAR T-cell therapy as part of a phase 1 trial (N Engl J Med. 2018 Feb 1;378[5]:449-59).
Bridging therapy was defined as any therapy given from leukapheresis to cell infusion.
The low-intensity regimens included POMP (6-mercaptopurine, vincristine, methotrexate, and prednisone, or combinations), liposomal vincristine, mini-hyper CVD (reduced cyclophosphamide, dexamethasone, methotrexate, Ara-C), blinatumomab, inotuzumab, oral tyrosine kinase inhibitor-based regimens, or hydroxyurea.
The high-intensity regimens included hyper-CVAD (cyclophosphamide, vincristine, doxorubicin, dexamethasone), high-dose cytarabine, attenuated FLAG/FLAG-IDA (reduced fludarabine, cytarabine, G-CSF plus or minus idarubicin), and pediatric-type induction.
Of the 53 patients who were ultimately infused with CAR T cells, 19 received some type of high intensity regimen, 29 received low intensity regimens, and 5 received no bridging treatment. The group overall was heavily pretreated. Nearly a third of the low intensity and no bridging patients and 42% of the high intensity patients had previously undergone transplant. More than 40% of the low intensity and no bridging patients and about a quarter of the high intensity bridging group had four or more prior lines of therapy.
The use of high intensity bridging therapy was not associated with improved overall response or relapse-free survival to CAR T-cell therapy, the investigators reported. In a subgroup with 23 high disease burden patients with greater than 20% blasts, there was no difference in MRD-negative complete response by intensity (75% versus 60%, Fisher’s P = .65).
High intensity bridging was also not associated with successful CAR T-cell infusion, versus low intensity regimens (63% versus 79%, P greater than .05) or a combined endpoint of CAR T-cell infusion plus transplant or alternative treatment (80% versus 86%, P greater than .05).
In terms of toxicity, the high intensity bridging regimens were associated with a higher rate of grade 3 or 4 infections – 15 versus 11 infections (Fisher’s P = .002). But there was no association with post-infusion grade 3 or 4 cytokine release syndrome or neurotoxicity.
Dr. Perica said the results reflect that the real goal of bridging is not to reduce disease burden but instead to successfully bring patients to the next phase of their treatment. “The goal of the bridging therapy is to get the patient to the CAR infusion,” he said.
Due to the retrospective nature of the study, Dr. Perica said he can’t recommend any single bridging regimen and he emphasized that the decisions are patient-specific.
The original study was funded by several foundations and Juno Therapeutics. Dr. Perica reported royalties from technology licensed to Neximmune.
SOURCE: Perica K et al. ASCO 2019, Abstract 2520.
CHICAGO – A low intensity chemotherapy regimen may be the best approach to bridge patients waiting for chimeric antigen receptor (CAR) T-cell therapy, according to a retrospective analysis of adults with acute lymphoblastic leukemia (ALL).
Investigators found that high intensity bridging regimens provided no clear outcome benefit, but did produce a greater number of infections.
But the decision on the type of regimen is very much dependent on the individual patient, Karlo Perica, MD, PhD, of Memorial Sloan Kettering Cancer Center in New York, said at the annual meeting of the American Society of Clinical Oncology.
Dr. Perica and his colleagues at Memorial Sloan Kettering examined the effectiveness and toxicity of bridging therapies provided to relapsed or refractory ALL patients waiting to receive CD19 CAR T-cell therapy as part of a phase 1 trial (N Engl J Med. 2018 Feb 1;378[5]:449-59).
Bridging therapy was defined as any therapy given from leukapheresis to cell infusion.
The low-intensity regimens included POMP (6-mercaptopurine, vincristine, methotrexate, and prednisone, or combinations), liposomal vincristine, mini-hyper CVD (reduced cyclophosphamide, dexamethasone, methotrexate, Ara-C), blinatumomab, inotuzumab, oral tyrosine kinase inhibitor-based regimens, or hydroxyurea.
The high-intensity regimens included hyper-CVAD (cyclophosphamide, vincristine, doxorubicin, dexamethasone), high-dose cytarabine, attenuated FLAG/FLAG-IDA (reduced fludarabine, cytarabine, G-CSF plus or minus idarubicin), and pediatric-type induction.
Of the 53 patients who were ultimately infused with CAR T cells, 19 received some type of high intensity regimen, 29 received low intensity regimens, and 5 received no bridging treatment. The group overall was heavily pretreated. Nearly a third of the low intensity and no bridging patients and 42% of the high intensity patients had previously undergone transplant. More than 40% of the low intensity and no bridging patients and about a quarter of the high intensity bridging group had four or more prior lines of therapy.
The use of high intensity bridging therapy was not associated with improved overall response or relapse-free survival to CAR T-cell therapy, the investigators reported. In a subgroup with 23 high disease burden patients with greater than 20% blasts, there was no difference in MRD-negative complete response by intensity (75% versus 60%, Fisher’s P = .65).
High intensity bridging was also not associated with successful CAR T-cell infusion, versus low intensity regimens (63% versus 79%, P greater than .05) or a combined endpoint of CAR T-cell infusion plus transplant or alternative treatment (80% versus 86%, P greater than .05).
In terms of toxicity, the high intensity bridging regimens were associated with a higher rate of grade 3 or 4 infections – 15 versus 11 infections (Fisher’s P = .002). But there was no association with post-infusion grade 3 or 4 cytokine release syndrome or neurotoxicity.
Dr. Perica said the results reflect that the real goal of bridging is not to reduce disease burden but instead to successfully bring patients to the next phase of their treatment. “The goal of the bridging therapy is to get the patient to the CAR infusion,” he said.
Due to the retrospective nature of the study, Dr. Perica said he can’t recommend any single bridging regimen and he emphasized that the decisions are patient-specific.
The original study was funded by several foundations and Juno Therapeutics. Dr. Perica reported royalties from technology licensed to Neximmune.
SOURCE: Perica K et al. ASCO 2019, Abstract 2520.
FROM ASCO 2019
NGS comparable to FC for minimal residual disease assessment
NEW ORLEANS – Next-generation sequencing of peripheral blood is at least as effective as flow cytometry of bone marrow for assessing minimal residual disease, according to a new study.
Researchers compared bone marrow flow cytometry (FC) and peripheral blood next-generation sequencing (NGS) for minimal residual disease (MRD) assessment in pediatric and young adult patients with B-cell acute lymphoblastic leukemia (B-ALL) who received treatment with tisagenlecleucel. There was a high level of concordance between the assays, but the NGS assay detected more MRD-positive samples and NGS results provided a longer lead time to relapse.
Michael A. Pulsipher, MD, of the Children’s Hospital Los Angeles, presented these results at the annual meeting of the American Society of Pediatric Hematology/Oncology.
The researchers analyzed samples from pediatric and young adult patients aged 2-25 years who had relapsed or refractory B-ALL and received treatment with tisagenlecleucel on the ELIANA or ENSIGN trials.
The patients had received at least two prior lines of therapy and were ineligible for allogeneic transplant. They received a single dose of tisagenlecleucel. MRD was assessed before tisagenlecleucel infusion, at various time points after infusion, and at relapse.
Dr. Pulsipher and his colleagues compared MRD results from an NGS assay – Adaptive Biotechnologies’ clonoSEQ – using peripheral blood and results from FC of bone marrow. NGS and FC results were available for 237 samples from 83 patients.
After treatment, NGS detected more MRD-positive samples at each sensitivity level tested (10-4, 10-5, and 10-6). At 10-6, NGS detected 18% more MRD-positive samples than did FC – 50% and 32%, respectively.
Detection of MRD positivity prior to relapse was faster with NGS than with FC. In 17 of 34 patients with morphological relapse, NGS provided a median lead time of 67 days. FC provided a median lead time of 39 days in 11 of the 34 patients.
About 80% of patients who had an MRD status of zero by NGS at day 28 remained relapse-free for up to 3 years.
Among complete responders (n = 50), the duration of response was significantly longer in patients who had an MRD status of zero at day 28 by NGS than in patients who had an MRD status greater than zero (P = .0003). Overall survival was significantly better among patients with an MRD status of zero as well (P = .0004).
Dr. Pulsipher said additional studies are needed to confirm these findings and determine the best way to know if a patient has been cured or needs additional therapy after tisagenlecleucel.
Dr. Pulsipher reported relationships with Adaptive Biotech, Novartis, Incyte, Amgen, Bellicum Pharmaceuticals, Medac Pharma, and Miltenyi Biotec. ELIANA and ENSIGN were funded by Novartis, which markets tisagenlecleucel as Kymriah.
SOURCE: Pulsipher MA et al. ASPHO 2019, Abstract 2001.
NEW ORLEANS – Next-generation sequencing of peripheral blood is at least as effective as flow cytometry of bone marrow for assessing minimal residual disease, according to a new study.
Researchers compared bone marrow flow cytometry (FC) and peripheral blood next-generation sequencing (NGS) for minimal residual disease (MRD) assessment in pediatric and young adult patients with B-cell acute lymphoblastic leukemia (B-ALL) who received treatment with tisagenlecleucel. There was a high level of concordance between the assays, but the NGS assay detected more MRD-positive samples and NGS results provided a longer lead time to relapse.
Michael A. Pulsipher, MD, of the Children’s Hospital Los Angeles, presented these results at the annual meeting of the American Society of Pediatric Hematology/Oncology.
The researchers analyzed samples from pediatric and young adult patients aged 2-25 years who had relapsed or refractory B-ALL and received treatment with tisagenlecleucel on the ELIANA or ENSIGN trials.
The patients had received at least two prior lines of therapy and were ineligible for allogeneic transplant. They received a single dose of tisagenlecleucel. MRD was assessed before tisagenlecleucel infusion, at various time points after infusion, and at relapse.
Dr. Pulsipher and his colleagues compared MRD results from an NGS assay – Adaptive Biotechnologies’ clonoSEQ – using peripheral blood and results from FC of bone marrow. NGS and FC results were available for 237 samples from 83 patients.
After treatment, NGS detected more MRD-positive samples at each sensitivity level tested (10-4, 10-5, and 10-6). At 10-6, NGS detected 18% more MRD-positive samples than did FC – 50% and 32%, respectively.
Detection of MRD positivity prior to relapse was faster with NGS than with FC. In 17 of 34 patients with morphological relapse, NGS provided a median lead time of 67 days. FC provided a median lead time of 39 days in 11 of the 34 patients.
About 80% of patients who had an MRD status of zero by NGS at day 28 remained relapse-free for up to 3 years.
Among complete responders (n = 50), the duration of response was significantly longer in patients who had an MRD status of zero at day 28 by NGS than in patients who had an MRD status greater than zero (P = .0003). Overall survival was significantly better among patients with an MRD status of zero as well (P = .0004).
Dr. Pulsipher said additional studies are needed to confirm these findings and determine the best way to know if a patient has been cured or needs additional therapy after tisagenlecleucel.
Dr. Pulsipher reported relationships with Adaptive Biotech, Novartis, Incyte, Amgen, Bellicum Pharmaceuticals, Medac Pharma, and Miltenyi Biotec. ELIANA and ENSIGN were funded by Novartis, which markets tisagenlecleucel as Kymriah.
SOURCE: Pulsipher MA et al. ASPHO 2019, Abstract 2001.
NEW ORLEANS – Next-generation sequencing of peripheral blood is at least as effective as flow cytometry of bone marrow for assessing minimal residual disease, according to a new study.
Researchers compared bone marrow flow cytometry (FC) and peripheral blood next-generation sequencing (NGS) for minimal residual disease (MRD) assessment in pediatric and young adult patients with B-cell acute lymphoblastic leukemia (B-ALL) who received treatment with tisagenlecleucel. There was a high level of concordance between the assays, but the NGS assay detected more MRD-positive samples and NGS results provided a longer lead time to relapse.
Michael A. Pulsipher, MD, of the Children’s Hospital Los Angeles, presented these results at the annual meeting of the American Society of Pediatric Hematology/Oncology.
The researchers analyzed samples from pediatric and young adult patients aged 2-25 years who had relapsed or refractory B-ALL and received treatment with tisagenlecleucel on the ELIANA or ENSIGN trials.
The patients had received at least two prior lines of therapy and were ineligible for allogeneic transplant. They received a single dose of tisagenlecleucel. MRD was assessed before tisagenlecleucel infusion, at various time points after infusion, and at relapse.
Dr. Pulsipher and his colleagues compared MRD results from an NGS assay – Adaptive Biotechnologies’ clonoSEQ – using peripheral blood and results from FC of bone marrow. NGS and FC results were available for 237 samples from 83 patients.
After treatment, NGS detected more MRD-positive samples at each sensitivity level tested (10-4, 10-5, and 10-6). At 10-6, NGS detected 18% more MRD-positive samples than did FC – 50% and 32%, respectively.
Detection of MRD positivity prior to relapse was faster with NGS than with FC. In 17 of 34 patients with morphological relapse, NGS provided a median lead time of 67 days. FC provided a median lead time of 39 days in 11 of the 34 patients.
About 80% of patients who had an MRD status of zero by NGS at day 28 remained relapse-free for up to 3 years.
Among complete responders (n = 50), the duration of response was significantly longer in patients who had an MRD status of zero at day 28 by NGS than in patients who had an MRD status greater than zero (P = .0003). Overall survival was significantly better among patients with an MRD status of zero as well (P = .0004).
Dr. Pulsipher said additional studies are needed to confirm these findings and determine the best way to know if a patient has been cured or needs additional therapy after tisagenlecleucel.
Dr. Pulsipher reported relationships with Adaptive Biotech, Novartis, Incyte, Amgen, Bellicum Pharmaceuticals, Medac Pharma, and Miltenyi Biotec. ELIANA and ENSIGN were funded by Novartis, which markets tisagenlecleucel as Kymriah.
SOURCE: Pulsipher MA et al. ASPHO 2019, Abstract 2001.
REPORTING FROM 2019 ASPHO CONFERENCE
Key clinical point: Major finding: At the highest sensitivity level tested, next-generation sequencing detected 18% more minimal residual disease–positive samples than did flow cytometry – 50% and 32%, respectively.
Study details: An analysis of samples from pediatric and young adult patients with B-cell acute lymphoblastic leukemia who received treatment with tisagenlecleucel on the ELIANA and ENSIGN trials.
Disclosures: The speaker reported relationships with Adaptive Biotech, Novartis, Incyte, Amgen, Bellicum Pharmaceuticals, Medac Pharma, and Miltenyi Biotec. The ELIANA and ENSIGN trials were funded by Novartis, which markets tisagenlecleucel as Kymriah.
Source: Pulsipher MA et al. ASPHO 2019, Abstract 2001.
Tagraxofusp produces high response rate in BPDCN
Tagraxofusp demonstrated efficacy in a phase 2 trial of patients with previously treated or untreated blastic plasmacytoid dendritic cell neoplasm (BPDCN).
The overall response rate was 90% in previously untreated patients who received the highest dose of tagraxofusp and 67% in patients with relapsed/refractory BPDCN.
The researchers wrote that capillary leak syndrome (CLS) was an important adverse event in this trial, as it caused two deaths. However, the researchers developed strategies that appear to mitigate the risk of CLS in patients taking tagraxofusp.
Naveen Pemmaraju, MD, of the University of Texas MD Anderson Cancer Center, Houston, and his colleagues conducted the trial and reported the results in the New England Journal of Medicine.
The trial included 47 patients – 32 with previously untreated BPDCN and 15 with relapsed/refractory BPDCN. The patients’ median age at baseline was 70 years and 83% were men.
Three patients (all previously untreated) received tagraxofusp at 7 mcg/kg per day, and 44 patients received a 12 mcg/kg per day dose. All patients were treated on days 1-5 of a 21-day cycle.
Response and survival
In the 29 previously untreated patients who received the 12 mcg/kg dose of tagraxofusp, the overall response rate was 90%. The rate of complete response plus clinical complete response in these patients was 72%.
In the 15 patients with relapsed/refractory BPDCN, the overall response rate was 67%, and the rate of complete response plus clinical complete response was 33%.
A total of 14 patients, 13 of whom had previously untreated BPDCN, went on to transplant.
In the 29 previously untreated patients, the median overall survival was not reached at a median follow-up of 25 months. The overall survival rate was 62% at 12 months, 59% at 18 months, and 52% at 24 months.
In the 15 previously treated patients, the median overall survival was 8.5 months.
Safety
Common adverse events in this trial were ALT increase (64%), AST increase (60%), hypoalbuminemia (55%), peripheral edema (51%), thrombocytopenia (49%), nausea (45%), pyrexia (45%), and fatigue (45%).
Among the 44 patients who received the 12 mcg/kg dose of tagraxofusp, 8 (18%) developed CLS. Six patients had grade 2 CLS, one had grade 4, and one had grade 5. There was an additional CLS-related death in a patient who received tagraxofusp at 7 mcg/kg.
After the first death, the trial protocol was amended to reduce CLS risk. Inclusion criteria were changed so that patients must have normal cardiac function, adequate kidney function, and serum albumin of at least 3.2 g/dL. Additionally, the researchers began monitoring patients’ weight, albumin levels, and kidney function. The team withheld tagraxofusp if patients experienced rapid weight gain or if their serum albumin or systolic blood pressure fell too low.
The trial was sponsored by Stemline Therapeutics. The researchers reported relationships with Stemline and other companies.
Tagraxofusp demonstrated efficacy in a phase 2 trial of patients with previously treated or untreated blastic plasmacytoid dendritic cell neoplasm (BPDCN).
The overall response rate was 90% in previously untreated patients who received the highest dose of tagraxofusp and 67% in patients with relapsed/refractory BPDCN.
The researchers wrote that capillary leak syndrome (CLS) was an important adverse event in this trial, as it caused two deaths. However, the researchers developed strategies that appear to mitigate the risk of CLS in patients taking tagraxofusp.
Naveen Pemmaraju, MD, of the University of Texas MD Anderson Cancer Center, Houston, and his colleagues conducted the trial and reported the results in the New England Journal of Medicine.
The trial included 47 patients – 32 with previously untreated BPDCN and 15 with relapsed/refractory BPDCN. The patients’ median age at baseline was 70 years and 83% were men.
Three patients (all previously untreated) received tagraxofusp at 7 mcg/kg per day, and 44 patients received a 12 mcg/kg per day dose. All patients were treated on days 1-5 of a 21-day cycle.
Response and survival
In the 29 previously untreated patients who received the 12 mcg/kg dose of tagraxofusp, the overall response rate was 90%. The rate of complete response plus clinical complete response in these patients was 72%.
In the 15 patients with relapsed/refractory BPDCN, the overall response rate was 67%, and the rate of complete response plus clinical complete response was 33%.
A total of 14 patients, 13 of whom had previously untreated BPDCN, went on to transplant.
In the 29 previously untreated patients, the median overall survival was not reached at a median follow-up of 25 months. The overall survival rate was 62% at 12 months, 59% at 18 months, and 52% at 24 months.
In the 15 previously treated patients, the median overall survival was 8.5 months.
Safety
Common adverse events in this trial were ALT increase (64%), AST increase (60%), hypoalbuminemia (55%), peripheral edema (51%), thrombocytopenia (49%), nausea (45%), pyrexia (45%), and fatigue (45%).
Among the 44 patients who received the 12 mcg/kg dose of tagraxofusp, 8 (18%) developed CLS. Six patients had grade 2 CLS, one had grade 4, and one had grade 5. There was an additional CLS-related death in a patient who received tagraxofusp at 7 mcg/kg.
After the first death, the trial protocol was amended to reduce CLS risk. Inclusion criteria were changed so that patients must have normal cardiac function, adequate kidney function, and serum albumin of at least 3.2 g/dL. Additionally, the researchers began monitoring patients’ weight, albumin levels, and kidney function. The team withheld tagraxofusp if patients experienced rapid weight gain or if their serum albumin or systolic blood pressure fell too low.
The trial was sponsored by Stemline Therapeutics. The researchers reported relationships with Stemline and other companies.
Tagraxofusp demonstrated efficacy in a phase 2 trial of patients with previously treated or untreated blastic plasmacytoid dendritic cell neoplasm (BPDCN).
The overall response rate was 90% in previously untreated patients who received the highest dose of tagraxofusp and 67% in patients with relapsed/refractory BPDCN.
The researchers wrote that capillary leak syndrome (CLS) was an important adverse event in this trial, as it caused two deaths. However, the researchers developed strategies that appear to mitigate the risk of CLS in patients taking tagraxofusp.
Naveen Pemmaraju, MD, of the University of Texas MD Anderson Cancer Center, Houston, and his colleagues conducted the trial and reported the results in the New England Journal of Medicine.
The trial included 47 patients – 32 with previously untreated BPDCN and 15 with relapsed/refractory BPDCN. The patients’ median age at baseline was 70 years and 83% were men.
Three patients (all previously untreated) received tagraxofusp at 7 mcg/kg per day, and 44 patients received a 12 mcg/kg per day dose. All patients were treated on days 1-5 of a 21-day cycle.
Response and survival
In the 29 previously untreated patients who received the 12 mcg/kg dose of tagraxofusp, the overall response rate was 90%. The rate of complete response plus clinical complete response in these patients was 72%.
In the 15 patients with relapsed/refractory BPDCN, the overall response rate was 67%, and the rate of complete response plus clinical complete response was 33%.
A total of 14 patients, 13 of whom had previously untreated BPDCN, went on to transplant.
In the 29 previously untreated patients, the median overall survival was not reached at a median follow-up of 25 months. The overall survival rate was 62% at 12 months, 59% at 18 months, and 52% at 24 months.
In the 15 previously treated patients, the median overall survival was 8.5 months.
Safety
Common adverse events in this trial were ALT increase (64%), AST increase (60%), hypoalbuminemia (55%), peripheral edema (51%), thrombocytopenia (49%), nausea (45%), pyrexia (45%), and fatigue (45%).
Among the 44 patients who received the 12 mcg/kg dose of tagraxofusp, 8 (18%) developed CLS. Six patients had grade 2 CLS, one had grade 4, and one had grade 5. There was an additional CLS-related death in a patient who received tagraxofusp at 7 mcg/kg.
After the first death, the trial protocol was amended to reduce CLS risk. Inclusion criteria were changed so that patients must have normal cardiac function, adequate kidney function, and serum albumin of at least 3.2 g/dL. Additionally, the researchers began monitoring patients’ weight, albumin levels, and kidney function. The team withheld tagraxofusp if patients experienced rapid weight gain or if their serum albumin or systolic blood pressure fell too low.
The trial was sponsored by Stemline Therapeutics. The researchers reported relationships with Stemline and other companies.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Tagraxofusp produced responses in patients with blastic plasmacytoid dendritic cell neoplasm (BPDCN).
Major finding: The overall response rate was 90% in previously untreated patients who received the highest dose of tagraxofusp and 67% in patients with relapsed/refractory BPDCN.
Study details: A phase 2 trial of 47 patients, 32 with previously untreated BPDCN and 15 with relapsed/refractory BPDCN.
Disclosures: The trial was sponsored by Stemline Therapeutics. The researchers reported relationships with Stemline and other companies.
Source: Pemmaraju N et al. N Engl J Med. 2019;380:1628-37.