User login
Preventing the psychosocial effects of adult ADHD during the pandemic
As some countries brace for yet another possible surge in the COVID-19 pandemic – particularly among young adults who have not yet been vaccinated – clinicians should remain wary of the cardinal symptoms of adult ADHD.
Research from an Israeli study shows that individuals with unmedicated ADHD are 52% more likely to test positive for the virus.1,2
The symptoms of ADHD, including impulsiveness and inability to follow directions, combined with the tendency to leave adults with ADHD on their own to sort out COVID-19–related protocols – make these individuals susceptible to exposure.
As we know, ADHD is a condition characterized by a pervasive pattern of impulsivity and/or inattention, which greatly reduces organizational capabilities by interfering at the developmental level.3 Other key symptoms include short attention span, hyperactivity, restlessness, difficulty in prioritizing tasks, and an absence of time awareness. Symptom presentation of ADHD is contingent upon the nature of the individual’s overall mental health and etiologic issues that may be traced back to the brain’s development.4
Diagnosing ADHD in adults is relatively difficult, because a formal diagnosis generally requires symptoms to show up between the ages of 6 and 12.5 Also, clinicians can interview parents and family members to assess whether the classical features of ADHD were present in childhood for those suspected of having the condition.
Early vs. late presentation
Among the preschool population, it has been observed that emerging ADHD symptoms may progress with time or remain relatively constant with respect to the activities that children partake in. In some instances, impulsive behavior, especially compared with other symptoms, might be identified quickly by the attentive parent or caregiver. However, when ADHD appears in adulthood, it is possible that prior ADHD symptoms escaped detection – only to be diagnosed later in life because of varying presentations and the increased organizational demands of adulthood.
Meanwhile, diagnosis in adolescence can bring a different set of challenges to the forefront as teenagers face problems with self-management and responsibilities of daily living. These young people must cope with academic6 and social pressures – and a host of new societal expectations.
It is essential to understand how all of those societal factors have affected ADHD and its aspects, especially within the context of COVID-19. The coronavirus has introduced myriad challenges at the global level. Individuals with ADHD exhibit neurodevelopmental and corollary attention deficit issues that make them more susceptible to environmental stressors. Physical distancing practices might aggravate existing behavioral problems.
Distance forced by pandemic offers challenges
Despite the widespread adoption of telemedicine during the pandemic, some physicians think that the delivery of optimal care and the ability to adequately address patients’ health-related concerns have been compromised. Certainly, in the case of addressing the needs of patients with ADHD or related learning disorders, in-person examinations and clinical visits are best.
That is also the case for ADHD patients with comorbid sleep disorders. For those patients, it might be prudent to explore lifestyle changes (for example, improvements in sleep hygiene practices) before resorting to the use of pharmacologic agents such as hypnotics and melatonin. Along similar lines, the European ADHD Guideline Group (EAGG) advises the use of pharmacotherapy after the successful completion of a physical exam; patients already adhering to a treatment plan should continue therapy without interruption. Clinicians caring for patients with adult ADHD have faced a dilemma because treatment breaks increase the likelihood of illnesses resulting from the pandemic. Also, the inability to conduct treatment in person because of the pandemic raises concerns about pharmacotherapy.
The pandemic has affected the course of pediatric care and also has presented new challenges for adolescents as they begin to tackle unique problems related to their health concerns. In prepandemic times, teachers played integral roles in the diagnostic process, because they were able to readily identify children and teenagers with mental and physical challenges. In stark contrast, connecting with students online may not allow teachers to identify skill deficits in young patients or in adults with ADHD.
Furthermore, adults with ADHD and medical comorbidities may be at increased risk of disease exposure directly resulting from an inability to address their social and/or emotional well-being adequately. The social distancing and other mitigation measures advised by public health experts ensure safety and protection but also can present numerous hurdles for children, teenagers, adults – and their respective families.
Individuals with adult ADHD and other psychiatric disorders may downplay their psychological distress7 [for example, sleep dysfunction, issues concerning activities of daily living], and view it as being the natural product of the COVID-19 environment. As a result of their misconceptions, they may avoid increasing their medication dose to control emergent symptoms of hyperactivity and impulsivity, instead opting to manage stress without aid from health care professionals. The absence of patient-provider interactivity and the integration of telemedicine has introduced unnecessary obstacles with respect to medication management and therapy as well as general access to expert advice. It is of utmost importance for clinicians to identify at-risk patients and reeducate the adult ADHD patient on issues concerning medication intake and psychological wellness.
Individuals with developmental disorders may experience numerous setbacks when trying to navigate their environments. The lack of correct feedback, supervision, and guidance may adversely affect adults with ADHD, contributing toward a lack of self-esteem and social awareness.
Individuals with adult ADHD are more likely than are their younger counterparts to have medical comorbidities, such as cardiovascular disease8 and type 2 diabetes,9 so it is crucial to prescribe dietary instructions to patients. Sometimes patients with adult ADHD lack support in the form of acceptance by family and peers, so it is critical for the patient to come to terms with his/her condition and seek professional help, incorporating effective strategies wherever needed to maintain day-to-day functioning.
Other possible comorbidities
There can be risk factors associated with isolation of adults who have depression and/or anxiety, poor eating habits, and maladaptive behaviors. Other adverse health-related issues may include substance use disorder.10 Drug use suppresses developmental growth and may induce ADHD symptom exacerbation. Consistent with Khantzian’s self-medication hypothesis, among individuals with ADHD, including those who lack a formal diagnosis, there is a tendency to gravitate toward illicit substances, in particular, stimulants.11
We also know that adolescents are known to engage in normal risk-taking and social experimentation. Given that, the notion of boundary setting becomes a complicated affair during a pandemic. Adults may no longer be involved in the same types of risk-taking behaviors, but enforced self-isolation coupled with unchecked consumption of various social medial platforms continue to take a toll on personal development. Socialization plays an enormous role in maintaining psychological health, and social media is no substitute for in-person interactions. Such platforms can reduce mental growth opportunities and affect ADHD adults unfavorably.
For instance, it has been reported that women with adult ADHD are more likely to present with negative cognitive biases and symptoms of anxiety as a function of social media use.12,13 As clinicians, we should recommend introducing activities with the aim of enhancing self-acceptance, mindfulness, and the ability to engage in healthy lifestyles. A holistic framework that focuses on psychological wellness and physical fitness will ensure treatment success. Medication management may prove to be a challenge because of differences in dosing, response schedules, and agreed-upon diagnostic criteria used for young patients, compared with those needed for adults.
Treatment strategies
Before the pandemic, researchers were observing an increase globally in the ADHD diagnosis,14 and clinicians have been exploring the efficacy of select medications, sometimes with limited success.15 Stimulant medication, combined with behavioral interventions, is supported by evidenced-based medicine and is the treatment of choice for childhood ADHD.
The stimulant remedy considered to be the most efficacious for adult ADHD is methylphenidate or dextroamphetamine.
However, be sure to proceed with caution and prepare a thorough work-up, because there can be cardiovascular risk factors associated with these medications with a pronounced increase in heart rate and blood pressure.
Stimulant medications are known to increase the risk of stroke or myocardial infarction for individuals with preexisting cardiovascular anomalies or structural anomalies. The American Heart Association no longer recommends a baseline EKG before commencing stimulant therapy with the exception of preexisting cardiac risk. Nonstimulant medications such as atomoxetine are available as alternatives.
The process of finding therapies that will reduce symptoms of ADHD takes considerable time, and individuals may fail to notice improvements, at least initially.
Before prescribing any medicine or therapies, it is important to evaluate for factors that are specific to ADHD and rule out the presence of other learning or developmental disorders, to prevent negative consequences.
Health care professionals can introduce nonspecific interventions as a means of tackling complicated cases of adult ADHD, especially those that coincide with underlying medical conditions (for example, cardiovascular disease, seizure disorders, and/or eating disorders). In such cases, stimulant medications may lead to symptom exacerbation, and the health care professional should carry out a systematic evaluation (risks vs. benefits of drug classes), despite the limitations of online appointments over the course of the pandemic.
Moreover, ADHD symptoms can take on a more severe form within the context of preexisting mood and anxiety disorders. Unfortunately, these comorbidities may have a negative prognostic outcome, too, thereby increasing other health-related risk factors. Psychological interventions can be implemented via online assessments because of recent technological advances worldwide, providing a new level of confidence and social engagement. EEG-assisted biofeedback is an example of new technological modalities that may help improve the overall performance and functionality of individuals with adult ADHD.16 Numerous services and resources are available to patients and their families that can improve mental health and well-being.
Other nonpharmacologic choices also play an instrumental role in bringing harmony and organization into a patient’s life through the use of daily planners, alarms, and to-do lists. In addition, therapists can provide treatment that helps individuals get motivated and reduce their anxiety levels. Behavioral therapies support patient initiatives by increasing their productivity, activity management, and satisfaction. Cognitive-behavioral therapy (CBT), and marriage counseling and family therapy are modalities that may help adults with ADHD change underlying thoughts and perceptions and develop coping skills and self-esteem.
Appropriate to the pandemic situation is another treatment, e-therapy, which includes text-based communication, video calling, and phone calls. It is a low-cost and convenient alternative for people. For some patients, traveling to a particular clinic or counseling center can be difficult, and there is a shortage of counselors worldwide, so it is beneficial to talk with a counselor on a video/phone call or through social media. It is crucial for the ADHD coach to be trained with relevant knowledge to make plans, set goals, and manage a patient’s schedule of activities. For the counselor, sharing tips based on personal experience and making the appropriate suggestions allows adults with ADHD to stay motivated and focus on the task at hand. It should be noted that counselors play an important role in reducing stress levels for those diagnosed with ADHD, allowing patients to lead productive lives and achieve career goals.
Various online support communities can provide patients and their parents with educational resources, to address issues connected to ADHD in a professional manner.17
The road to treatment success
Any delays in treating adults with ADHD can lead to a frustrating situation in which the entire family will be affected. As stated earlier, numerous support groups are available to adults with ADHD, and some of those groups can offer valuable tips about addressing stress management and the diverse roles that parents and family members may play in patient care.18 These groups provide a network for people to exchange ideas and recommend strategies. Online support groups may connect patients directly with key opinion leaders and health care providers.
Individuals with ADHD often experience problems with organization and concentration – especially within the context of the pandemic – and receiving guidance from counselors will provide an opportunity to learn valuable coping strategies and manage symptoms, recognizing and mitigating any mood swings associated with anxiety or depression that emerge alongside their ADHD. Psychotherapy is instrumental in patient care, and individuals with adult ADHD should be taught to acknowledge the role of medications (for example, neglect, divert, or self-medicate). A holistic approach to managing ADHD symptoms is necessary for optimal functioning and independence.
Dr. Aman is a faculty member in the biology department of City Colleges of Chicago. She is a postdoctoral researcher at the International Maternal and Child Health Foundation (IMCHF), Montreal; a fellow, medical staff development, from the American Academy of Medical Management; and a Masters Online Teacher, University of Illinois at Chicago. Dr. Aman disclosed no relevant financial relationships. Dr. Islam is a medical adviser for the IMCHF, Montreal, and is based in New York. He is a postdoctoral fellow, psychopharmacologist, and a board-certified medical affairs specialist. Dr. Islam disclosed no relevant financial relationships. Dr. Karama is a psychiatrist at the Douglas Mental Health University Institute, Montreal. He is an assistant professor at the department of psychiatry, McGill University, also in Montreal. He has no disclosures. Dr. Choudhry is the chief scientific officer and head of the department of mental health and clinical research at the IMCHF. He has no disclosures.
References
1. J Atten Disord. 2020. doi: 10.1177/1087054720943271.
2. ADDitude Magazine. 2020 Jul 23.
3. Management of ADHD in Adults: What the Science Says. 2007 Oct 9. Guilford Press.
4. N Engl J Med. 2013 Nov 14;369(20):1935-44.
5. J Clin Psychiatry. 2002;63 Suppl 12:29-35.
6. J Atten Dis. 2015 Jan 12. doi: 10.1177/1087054144566076.
7. Psychiatry Res. 2020 Oct;292. doi: 10.1016/j.psychres.2020.113345.
8. Case Rep Cardiol. 2016. doi: 10.1155/2016/2343691.
9. Curr Diab Rep. 2019 Jun 27;19(8):46. doi: 10.1007/s11892-019-1174-x.
10. Curr Psychiatry Rep. 2014 Mar;16(3):436. doi: 10.1007/s/11920-013-0436-6.
11. Current Psychiatry. 2014 Dec;13(12):e3-4.
12. BMC Psychiatry. 2020;20(40). doi: 10.1186/s/12888-020-02707-9.
13. Psychol Addict Behav. 2016 Mar;30(2):252–62.
14. ADDitude Magazine. 2017 Apr 6.
15. Harv Mental Health Letter. 2009 Nov.
16. MGM J Med Sci. 2020 Jul 17(3):161-2.
17. J Child Psychol Psychiatry. 2020 Jul 7.
18. Int J Disaster Risk Reduct. 2020 Dec;51. doi: 10.1016/j.ijdrr.2020.101845.
As some countries brace for yet another possible surge in the COVID-19 pandemic – particularly among young adults who have not yet been vaccinated – clinicians should remain wary of the cardinal symptoms of adult ADHD.
Research from an Israeli study shows that individuals with unmedicated ADHD are 52% more likely to test positive for the virus.1,2
The symptoms of ADHD, including impulsiveness and inability to follow directions, combined with the tendency to leave adults with ADHD on their own to sort out COVID-19–related protocols – make these individuals susceptible to exposure.
As we know, ADHD is a condition characterized by a pervasive pattern of impulsivity and/or inattention, which greatly reduces organizational capabilities by interfering at the developmental level.3 Other key symptoms include short attention span, hyperactivity, restlessness, difficulty in prioritizing tasks, and an absence of time awareness. Symptom presentation of ADHD is contingent upon the nature of the individual’s overall mental health and etiologic issues that may be traced back to the brain’s development.4
Diagnosing ADHD in adults is relatively difficult, because a formal diagnosis generally requires symptoms to show up between the ages of 6 and 12.5 Also, clinicians can interview parents and family members to assess whether the classical features of ADHD were present in childhood for those suspected of having the condition.
Early vs. late presentation
Among the preschool population, it has been observed that emerging ADHD symptoms may progress with time or remain relatively constant with respect to the activities that children partake in. In some instances, impulsive behavior, especially compared with other symptoms, might be identified quickly by the attentive parent or caregiver. However, when ADHD appears in adulthood, it is possible that prior ADHD symptoms escaped detection – only to be diagnosed later in life because of varying presentations and the increased organizational demands of adulthood.
Meanwhile, diagnosis in adolescence can bring a different set of challenges to the forefront as teenagers face problems with self-management and responsibilities of daily living. These young people must cope with academic6 and social pressures – and a host of new societal expectations.
It is essential to understand how all of those societal factors have affected ADHD and its aspects, especially within the context of COVID-19. The coronavirus has introduced myriad challenges at the global level. Individuals with ADHD exhibit neurodevelopmental and corollary attention deficit issues that make them more susceptible to environmental stressors. Physical distancing practices might aggravate existing behavioral problems.
Distance forced by pandemic offers challenges
Despite the widespread adoption of telemedicine during the pandemic, some physicians think that the delivery of optimal care and the ability to adequately address patients’ health-related concerns have been compromised. Certainly, in the case of addressing the needs of patients with ADHD or related learning disorders, in-person examinations and clinical visits are best.
That is also the case for ADHD patients with comorbid sleep disorders. For those patients, it might be prudent to explore lifestyle changes (for example, improvements in sleep hygiene practices) before resorting to the use of pharmacologic agents such as hypnotics and melatonin. Along similar lines, the European ADHD Guideline Group (EAGG) advises the use of pharmacotherapy after the successful completion of a physical exam; patients already adhering to a treatment plan should continue therapy without interruption. Clinicians caring for patients with adult ADHD have faced a dilemma because treatment breaks increase the likelihood of illnesses resulting from the pandemic. Also, the inability to conduct treatment in person because of the pandemic raises concerns about pharmacotherapy.
The pandemic has affected the course of pediatric care and also has presented new challenges for adolescents as they begin to tackle unique problems related to their health concerns. In prepandemic times, teachers played integral roles in the diagnostic process, because they were able to readily identify children and teenagers with mental and physical challenges. In stark contrast, connecting with students online may not allow teachers to identify skill deficits in young patients or in adults with ADHD.
Furthermore, adults with ADHD and medical comorbidities may be at increased risk of disease exposure directly resulting from an inability to address their social and/or emotional well-being adequately. The social distancing and other mitigation measures advised by public health experts ensure safety and protection but also can present numerous hurdles for children, teenagers, adults – and their respective families.
Individuals with adult ADHD and other psychiatric disorders may downplay their psychological distress7 [for example, sleep dysfunction, issues concerning activities of daily living], and view it as being the natural product of the COVID-19 environment. As a result of their misconceptions, they may avoid increasing their medication dose to control emergent symptoms of hyperactivity and impulsivity, instead opting to manage stress without aid from health care professionals. The absence of patient-provider interactivity and the integration of telemedicine has introduced unnecessary obstacles with respect to medication management and therapy as well as general access to expert advice. It is of utmost importance for clinicians to identify at-risk patients and reeducate the adult ADHD patient on issues concerning medication intake and psychological wellness.
Individuals with developmental disorders may experience numerous setbacks when trying to navigate their environments. The lack of correct feedback, supervision, and guidance may adversely affect adults with ADHD, contributing toward a lack of self-esteem and social awareness.
Individuals with adult ADHD are more likely than are their younger counterparts to have medical comorbidities, such as cardiovascular disease8 and type 2 diabetes,9 so it is crucial to prescribe dietary instructions to patients. Sometimes patients with adult ADHD lack support in the form of acceptance by family and peers, so it is critical for the patient to come to terms with his/her condition and seek professional help, incorporating effective strategies wherever needed to maintain day-to-day functioning.
Other possible comorbidities
There can be risk factors associated with isolation of adults who have depression and/or anxiety, poor eating habits, and maladaptive behaviors. Other adverse health-related issues may include substance use disorder.10 Drug use suppresses developmental growth and may induce ADHD symptom exacerbation. Consistent with Khantzian’s self-medication hypothesis, among individuals with ADHD, including those who lack a formal diagnosis, there is a tendency to gravitate toward illicit substances, in particular, stimulants.11
We also know that adolescents are known to engage in normal risk-taking and social experimentation. Given that, the notion of boundary setting becomes a complicated affair during a pandemic. Adults may no longer be involved in the same types of risk-taking behaviors, but enforced self-isolation coupled with unchecked consumption of various social medial platforms continue to take a toll on personal development. Socialization plays an enormous role in maintaining psychological health, and social media is no substitute for in-person interactions. Such platforms can reduce mental growth opportunities and affect ADHD adults unfavorably.
For instance, it has been reported that women with adult ADHD are more likely to present with negative cognitive biases and symptoms of anxiety as a function of social media use.12,13 As clinicians, we should recommend introducing activities with the aim of enhancing self-acceptance, mindfulness, and the ability to engage in healthy lifestyles. A holistic framework that focuses on psychological wellness and physical fitness will ensure treatment success. Medication management may prove to be a challenge because of differences in dosing, response schedules, and agreed-upon diagnostic criteria used for young patients, compared with those needed for adults.
Treatment strategies
Before the pandemic, researchers were observing an increase globally in the ADHD diagnosis,14 and clinicians have been exploring the efficacy of select medications, sometimes with limited success.15 Stimulant medication, combined with behavioral interventions, is supported by evidenced-based medicine and is the treatment of choice for childhood ADHD.
The stimulant remedy considered to be the most efficacious for adult ADHD is methylphenidate or dextroamphetamine.
However, be sure to proceed with caution and prepare a thorough work-up, because there can be cardiovascular risk factors associated with these medications with a pronounced increase in heart rate and blood pressure.
Stimulant medications are known to increase the risk of stroke or myocardial infarction for individuals with preexisting cardiovascular anomalies or structural anomalies. The American Heart Association no longer recommends a baseline EKG before commencing stimulant therapy with the exception of preexisting cardiac risk. Nonstimulant medications such as atomoxetine are available as alternatives.
The process of finding therapies that will reduce symptoms of ADHD takes considerable time, and individuals may fail to notice improvements, at least initially.
Before prescribing any medicine or therapies, it is important to evaluate for factors that are specific to ADHD and rule out the presence of other learning or developmental disorders, to prevent negative consequences.
Health care professionals can introduce nonspecific interventions as a means of tackling complicated cases of adult ADHD, especially those that coincide with underlying medical conditions (for example, cardiovascular disease, seizure disorders, and/or eating disorders). In such cases, stimulant medications may lead to symptom exacerbation, and the health care professional should carry out a systematic evaluation (risks vs. benefits of drug classes), despite the limitations of online appointments over the course of the pandemic.
Moreover, ADHD symptoms can take on a more severe form within the context of preexisting mood and anxiety disorders. Unfortunately, these comorbidities may have a negative prognostic outcome, too, thereby increasing other health-related risk factors. Psychological interventions can be implemented via online assessments because of recent technological advances worldwide, providing a new level of confidence and social engagement. EEG-assisted biofeedback is an example of new technological modalities that may help improve the overall performance and functionality of individuals with adult ADHD.16 Numerous services and resources are available to patients and their families that can improve mental health and well-being.
Other nonpharmacologic choices also play an instrumental role in bringing harmony and organization into a patient’s life through the use of daily planners, alarms, and to-do lists. In addition, therapists can provide treatment that helps individuals get motivated and reduce their anxiety levels. Behavioral therapies support patient initiatives by increasing their productivity, activity management, and satisfaction. Cognitive-behavioral therapy (CBT), and marriage counseling and family therapy are modalities that may help adults with ADHD change underlying thoughts and perceptions and develop coping skills and self-esteem.
Appropriate to the pandemic situation is another treatment, e-therapy, which includes text-based communication, video calling, and phone calls. It is a low-cost and convenient alternative for people. For some patients, traveling to a particular clinic or counseling center can be difficult, and there is a shortage of counselors worldwide, so it is beneficial to talk with a counselor on a video/phone call or through social media. It is crucial for the ADHD coach to be trained with relevant knowledge to make plans, set goals, and manage a patient’s schedule of activities. For the counselor, sharing tips based on personal experience and making the appropriate suggestions allows adults with ADHD to stay motivated and focus on the task at hand. It should be noted that counselors play an important role in reducing stress levels for those diagnosed with ADHD, allowing patients to lead productive lives and achieve career goals.
Various online support communities can provide patients and their parents with educational resources, to address issues connected to ADHD in a professional manner.17
The road to treatment success
Any delays in treating adults with ADHD can lead to a frustrating situation in which the entire family will be affected. As stated earlier, numerous support groups are available to adults with ADHD, and some of those groups can offer valuable tips about addressing stress management and the diverse roles that parents and family members may play in patient care.18 These groups provide a network for people to exchange ideas and recommend strategies. Online support groups may connect patients directly with key opinion leaders and health care providers.
Individuals with ADHD often experience problems with organization and concentration – especially within the context of the pandemic – and receiving guidance from counselors will provide an opportunity to learn valuable coping strategies and manage symptoms, recognizing and mitigating any mood swings associated with anxiety or depression that emerge alongside their ADHD. Psychotherapy is instrumental in patient care, and individuals with adult ADHD should be taught to acknowledge the role of medications (for example, neglect, divert, or self-medicate). A holistic approach to managing ADHD symptoms is necessary for optimal functioning and independence.
Dr. Aman is a faculty member in the biology department of City Colleges of Chicago. She is a postdoctoral researcher at the International Maternal and Child Health Foundation (IMCHF), Montreal; a fellow, medical staff development, from the American Academy of Medical Management; and a Masters Online Teacher, University of Illinois at Chicago. Dr. Aman disclosed no relevant financial relationships. Dr. Islam is a medical adviser for the IMCHF, Montreal, and is based in New York. He is a postdoctoral fellow, psychopharmacologist, and a board-certified medical affairs specialist. Dr. Islam disclosed no relevant financial relationships. Dr. Karama is a psychiatrist at the Douglas Mental Health University Institute, Montreal. He is an assistant professor at the department of psychiatry, McGill University, also in Montreal. He has no disclosures. Dr. Choudhry is the chief scientific officer and head of the department of mental health and clinical research at the IMCHF. He has no disclosures.
References
1. J Atten Disord. 2020. doi: 10.1177/1087054720943271.
2. ADDitude Magazine. 2020 Jul 23.
3. Management of ADHD in Adults: What the Science Says. 2007 Oct 9. Guilford Press.
4. N Engl J Med. 2013 Nov 14;369(20):1935-44.
5. J Clin Psychiatry. 2002;63 Suppl 12:29-35.
6. J Atten Dis. 2015 Jan 12. doi: 10.1177/1087054144566076.
7. Psychiatry Res. 2020 Oct;292. doi: 10.1016/j.psychres.2020.113345.
8. Case Rep Cardiol. 2016. doi: 10.1155/2016/2343691.
9. Curr Diab Rep. 2019 Jun 27;19(8):46. doi: 10.1007/s11892-019-1174-x.
10. Curr Psychiatry Rep. 2014 Mar;16(3):436. doi: 10.1007/s/11920-013-0436-6.
11. Current Psychiatry. 2014 Dec;13(12):e3-4.
12. BMC Psychiatry. 2020;20(40). doi: 10.1186/s/12888-020-02707-9.
13. Psychol Addict Behav. 2016 Mar;30(2):252–62.
14. ADDitude Magazine. 2017 Apr 6.
15. Harv Mental Health Letter. 2009 Nov.
16. MGM J Med Sci. 2020 Jul 17(3):161-2.
17. J Child Psychol Psychiatry. 2020 Jul 7.
18. Int J Disaster Risk Reduct. 2020 Dec;51. doi: 10.1016/j.ijdrr.2020.101845.
As some countries brace for yet another possible surge in the COVID-19 pandemic – particularly among young adults who have not yet been vaccinated – clinicians should remain wary of the cardinal symptoms of adult ADHD.
Research from an Israeli study shows that individuals with unmedicated ADHD are 52% more likely to test positive for the virus.1,2
The symptoms of ADHD, including impulsiveness and inability to follow directions, combined with the tendency to leave adults with ADHD on their own to sort out COVID-19–related protocols – make these individuals susceptible to exposure.
As we know, ADHD is a condition characterized by a pervasive pattern of impulsivity and/or inattention, which greatly reduces organizational capabilities by interfering at the developmental level.3 Other key symptoms include short attention span, hyperactivity, restlessness, difficulty in prioritizing tasks, and an absence of time awareness. Symptom presentation of ADHD is contingent upon the nature of the individual’s overall mental health and etiologic issues that may be traced back to the brain’s development.4
Diagnosing ADHD in adults is relatively difficult, because a formal diagnosis generally requires symptoms to show up between the ages of 6 and 12.5 Also, clinicians can interview parents and family members to assess whether the classical features of ADHD were present in childhood for those suspected of having the condition.
Early vs. late presentation
Among the preschool population, it has been observed that emerging ADHD symptoms may progress with time or remain relatively constant with respect to the activities that children partake in. In some instances, impulsive behavior, especially compared with other symptoms, might be identified quickly by the attentive parent or caregiver. However, when ADHD appears in adulthood, it is possible that prior ADHD symptoms escaped detection – only to be diagnosed later in life because of varying presentations and the increased organizational demands of adulthood.
Meanwhile, diagnosis in adolescence can bring a different set of challenges to the forefront as teenagers face problems with self-management and responsibilities of daily living. These young people must cope with academic6 and social pressures – and a host of new societal expectations.
It is essential to understand how all of those societal factors have affected ADHD and its aspects, especially within the context of COVID-19. The coronavirus has introduced myriad challenges at the global level. Individuals with ADHD exhibit neurodevelopmental and corollary attention deficit issues that make them more susceptible to environmental stressors. Physical distancing practices might aggravate existing behavioral problems.
Distance forced by pandemic offers challenges
Despite the widespread adoption of telemedicine during the pandemic, some physicians think that the delivery of optimal care and the ability to adequately address patients’ health-related concerns have been compromised. Certainly, in the case of addressing the needs of patients with ADHD or related learning disorders, in-person examinations and clinical visits are best.
That is also the case for ADHD patients with comorbid sleep disorders. For those patients, it might be prudent to explore lifestyle changes (for example, improvements in sleep hygiene practices) before resorting to the use of pharmacologic agents such as hypnotics and melatonin. Along similar lines, the European ADHD Guideline Group (EAGG) advises the use of pharmacotherapy after the successful completion of a physical exam; patients already adhering to a treatment plan should continue therapy without interruption. Clinicians caring for patients with adult ADHD have faced a dilemma because treatment breaks increase the likelihood of illnesses resulting from the pandemic. Also, the inability to conduct treatment in person because of the pandemic raises concerns about pharmacotherapy.
The pandemic has affected the course of pediatric care and also has presented new challenges for adolescents as they begin to tackle unique problems related to their health concerns. In prepandemic times, teachers played integral roles in the diagnostic process, because they were able to readily identify children and teenagers with mental and physical challenges. In stark contrast, connecting with students online may not allow teachers to identify skill deficits in young patients or in adults with ADHD.
Furthermore, adults with ADHD and medical comorbidities may be at increased risk of disease exposure directly resulting from an inability to address their social and/or emotional well-being adequately. The social distancing and other mitigation measures advised by public health experts ensure safety and protection but also can present numerous hurdles for children, teenagers, adults – and their respective families.
Individuals with adult ADHD and other psychiatric disorders may downplay their psychological distress7 [for example, sleep dysfunction, issues concerning activities of daily living], and view it as being the natural product of the COVID-19 environment. As a result of their misconceptions, they may avoid increasing their medication dose to control emergent symptoms of hyperactivity and impulsivity, instead opting to manage stress without aid from health care professionals. The absence of patient-provider interactivity and the integration of telemedicine has introduced unnecessary obstacles with respect to medication management and therapy as well as general access to expert advice. It is of utmost importance for clinicians to identify at-risk patients and reeducate the adult ADHD patient on issues concerning medication intake and psychological wellness.
Individuals with developmental disorders may experience numerous setbacks when trying to navigate their environments. The lack of correct feedback, supervision, and guidance may adversely affect adults with ADHD, contributing toward a lack of self-esteem and social awareness.
Individuals with adult ADHD are more likely than are their younger counterparts to have medical comorbidities, such as cardiovascular disease8 and type 2 diabetes,9 so it is crucial to prescribe dietary instructions to patients. Sometimes patients with adult ADHD lack support in the form of acceptance by family and peers, so it is critical for the patient to come to terms with his/her condition and seek professional help, incorporating effective strategies wherever needed to maintain day-to-day functioning.
Other possible comorbidities
There can be risk factors associated with isolation of adults who have depression and/or anxiety, poor eating habits, and maladaptive behaviors. Other adverse health-related issues may include substance use disorder.10 Drug use suppresses developmental growth and may induce ADHD symptom exacerbation. Consistent with Khantzian’s self-medication hypothesis, among individuals with ADHD, including those who lack a formal diagnosis, there is a tendency to gravitate toward illicit substances, in particular, stimulants.11
We also know that adolescents are known to engage in normal risk-taking and social experimentation. Given that, the notion of boundary setting becomes a complicated affair during a pandemic. Adults may no longer be involved in the same types of risk-taking behaviors, but enforced self-isolation coupled with unchecked consumption of various social medial platforms continue to take a toll on personal development. Socialization plays an enormous role in maintaining psychological health, and social media is no substitute for in-person interactions. Such platforms can reduce mental growth opportunities and affect ADHD adults unfavorably.
For instance, it has been reported that women with adult ADHD are more likely to present with negative cognitive biases and symptoms of anxiety as a function of social media use.12,13 As clinicians, we should recommend introducing activities with the aim of enhancing self-acceptance, mindfulness, and the ability to engage in healthy lifestyles. A holistic framework that focuses on psychological wellness and physical fitness will ensure treatment success. Medication management may prove to be a challenge because of differences in dosing, response schedules, and agreed-upon diagnostic criteria used for young patients, compared with those needed for adults.
Treatment strategies
Before the pandemic, researchers were observing an increase globally in the ADHD diagnosis,14 and clinicians have been exploring the efficacy of select medications, sometimes with limited success.15 Stimulant medication, combined with behavioral interventions, is supported by evidenced-based medicine and is the treatment of choice for childhood ADHD.
The stimulant remedy considered to be the most efficacious for adult ADHD is methylphenidate or dextroamphetamine.
However, be sure to proceed with caution and prepare a thorough work-up, because there can be cardiovascular risk factors associated with these medications with a pronounced increase in heart rate and blood pressure.
Stimulant medications are known to increase the risk of stroke or myocardial infarction for individuals with preexisting cardiovascular anomalies or structural anomalies. The American Heart Association no longer recommends a baseline EKG before commencing stimulant therapy with the exception of preexisting cardiac risk. Nonstimulant medications such as atomoxetine are available as alternatives.
The process of finding therapies that will reduce symptoms of ADHD takes considerable time, and individuals may fail to notice improvements, at least initially.
Before prescribing any medicine or therapies, it is important to evaluate for factors that are specific to ADHD and rule out the presence of other learning or developmental disorders, to prevent negative consequences.
Health care professionals can introduce nonspecific interventions as a means of tackling complicated cases of adult ADHD, especially those that coincide with underlying medical conditions (for example, cardiovascular disease, seizure disorders, and/or eating disorders). In such cases, stimulant medications may lead to symptom exacerbation, and the health care professional should carry out a systematic evaluation (risks vs. benefits of drug classes), despite the limitations of online appointments over the course of the pandemic.
Moreover, ADHD symptoms can take on a more severe form within the context of preexisting mood and anxiety disorders. Unfortunately, these comorbidities may have a negative prognostic outcome, too, thereby increasing other health-related risk factors. Psychological interventions can be implemented via online assessments because of recent technological advances worldwide, providing a new level of confidence and social engagement. EEG-assisted biofeedback is an example of new technological modalities that may help improve the overall performance and functionality of individuals with adult ADHD.16 Numerous services and resources are available to patients and their families that can improve mental health and well-being.
Other nonpharmacologic choices also play an instrumental role in bringing harmony and organization into a patient’s life through the use of daily planners, alarms, and to-do lists. In addition, therapists can provide treatment that helps individuals get motivated and reduce their anxiety levels. Behavioral therapies support patient initiatives by increasing their productivity, activity management, and satisfaction. Cognitive-behavioral therapy (CBT), and marriage counseling and family therapy are modalities that may help adults with ADHD change underlying thoughts and perceptions and develop coping skills and self-esteem.
Appropriate to the pandemic situation is another treatment, e-therapy, which includes text-based communication, video calling, and phone calls. It is a low-cost and convenient alternative for people. For some patients, traveling to a particular clinic or counseling center can be difficult, and there is a shortage of counselors worldwide, so it is beneficial to talk with a counselor on a video/phone call or through social media. It is crucial for the ADHD coach to be trained with relevant knowledge to make plans, set goals, and manage a patient’s schedule of activities. For the counselor, sharing tips based on personal experience and making the appropriate suggestions allows adults with ADHD to stay motivated and focus on the task at hand. It should be noted that counselors play an important role in reducing stress levels for those diagnosed with ADHD, allowing patients to lead productive lives and achieve career goals.
Various online support communities can provide patients and their parents with educational resources, to address issues connected to ADHD in a professional manner.17
The road to treatment success
Any delays in treating adults with ADHD can lead to a frustrating situation in which the entire family will be affected. As stated earlier, numerous support groups are available to adults with ADHD, and some of those groups can offer valuable tips about addressing stress management and the diverse roles that parents and family members may play in patient care.18 These groups provide a network for people to exchange ideas and recommend strategies. Online support groups may connect patients directly with key opinion leaders and health care providers.
Individuals with ADHD often experience problems with organization and concentration – especially within the context of the pandemic – and receiving guidance from counselors will provide an opportunity to learn valuable coping strategies and manage symptoms, recognizing and mitigating any mood swings associated with anxiety or depression that emerge alongside their ADHD. Psychotherapy is instrumental in patient care, and individuals with adult ADHD should be taught to acknowledge the role of medications (for example, neglect, divert, or self-medicate). A holistic approach to managing ADHD symptoms is necessary for optimal functioning and independence.
Dr. Aman is a faculty member in the biology department of City Colleges of Chicago. She is a postdoctoral researcher at the International Maternal and Child Health Foundation (IMCHF), Montreal; a fellow, medical staff development, from the American Academy of Medical Management; and a Masters Online Teacher, University of Illinois at Chicago. Dr. Aman disclosed no relevant financial relationships. Dr. Islam is a medical adviser for the IMCHF, Montreal, and is based in New York. He is a postdoctoral fellow, psychopharmacologist, and a board-certified medical affairs specialist. Dr. Islam disclosed no relevant financial relationships. Dr. Karama is a psychiatrist at the Douglas Mental Health University Institute, Montreal. He is an assistant professor at the department of psychiatry, McGill University, also in Montreal. He has no disclosures. Dr. Choudhry is the chief scientific officer and head of the department of mental health and clinical research at the IMCHF. He has no disclosures.
References
1. J Atten Disord. 2020. doi: 10.1177/1087054720943271.
2. ADDitude Magazine. 2020 Jul 23.
3. Management of ADHD in Adults: What the Science Says. 2007 Oct 9. Guilford Press.
4. N Engl J Med. 2013 Nov 14;369(20):1935-44.
5. J Clin Psychiatry. 2002;63 Suppl 12:29-35.
6. J Atten Dis. 2015 Jan 12. doi: 10.1177/1087054144566076.
7. Psychiatry Res. 2020 Oct;292. doi: 10.1016/j.psychres.2020.113345.
8. Case Rep Cardiol. 2016. doi: 10.1155/2016/2343691.
9. Curr Diab Rep. 2019 Jun 27;19(8):46. doi: 10.1007/s11892-019-1174-x.
10. Curr Psychiatry Rep. 2014 Mar;16(3):436. doi: 10.1007/s/11920-013-0436-6.
11. Current Psychiatry. 2014 Dec;13(12):e3-4.
12. BMC Psychiatry. 2020;20(40). doi: 10.1186/s/12888-020-02707-9.
13. Psychol Addict Behav. 2016 Mar;30(2):252–62.
14. ADDitude Magazine. 2017 Apr 6.
15. Harv Mental Health Letter. 2009 Nov.
16. MGM J Med Sci. 2020 Jul 17(3):161-2.
17. J Child Psychol Psychiatry. 2020 Jul 7.
18. Int J Disaster Risk Reduct. 2020 Dec;51. doi: 10.1016/j.ijdrr.2020.101845.
Contradictions abound in ‘The End of Mental Illness’
Daniel G. Amen, MD, is an American psychiatrist well-known for his eponymous clinics, television appearances, and series of books on mental health. One of his latest books, “The End of Mental Illness,” summarizes many of his views on the causes of and treatments for mental illnesses.
Dr. Amen’s approaches – such as his advocacy for the widespread use of single photon emission computed tomography (SPECT) imaging – are somewhat controversial and at times fall outside the mainstream of current psychiatric thought. So does “The End of Mental Illness” contain anything of value to the average practicing psychiatrist? (It should be noted that I listened to this as an audiobook and took notes as I listened. This does limit my ability to directly quote portions of the text, but I believe my notes are reliable.)
He begins the book by pointing out that the term “mental illness” might be better replaced with the term “brain illness.” With this shift in terminology, Dr. Amen introduces a theme that recurs throughout the book: That mental illnesses ultimately stem from various ways in which the brain can be harmed. While the suggested change in terminology might help reduce the stigma associated with psychiatric illnesses, Dr. Amen is surprisingly timid about implementing this term in his own book. He repeatedly refers to “brain health/mental health” issues instead of discarding the “mental” term altogether. Even his BRIGHT MINDS acronym for risk factors for mental illnesses includes the term “mind” instead of “brain.”
Continuing the theme of challenging terminology, Dr. Amen goes on to decry the weaknesses of the DSM system of nosology. This is a valid point, because under the current system, the same patient may receive differing diagnoses depending on which provider is seen and how certain symptoms are interpreted. Yet, here again, Dr. Amen does not seem to adhere to his own advice: He uses DSM terminology throughout the book, speaking of depression, anxiety, bipolar disorder, and ADHD. An oddity (which, admittedly, could have been the audiobook reader’s mistake rather than an error in the original text) is that the DSM is referred to as the “Diagnostic and Structural Manual” rather than the Diagnostic and Statistical Manual. He criticizes the DSM for its imprecision, pointing out the variety of symptom combinations that can produce the same diagnoses and how similar symptoms may overlap between differing diagnoses. Yet, his descriptions of common SPECT patterns (his preferred tool to assist in diagnosis) make it clear that here, too, there is a lot of overlap. As an example, ADHD was associated with at least three of the imaging patterns he described. It is also somewhat ironic how Dr. Amen obliquely criticizes the American Psychiatric Association for profiting from the use of the DSM, when SPECT imaging is expensive and profits his own organization.
Dr. Amen repeatedly asserts that psychiatry is unique among medical specialties for making diagnoses based on symptom clusters rather than direct visualization of the affected organ. Yet, psychiatry is not, in fact, unique in making diagnoses in this way. Some examples of diagnoses based on symptom clusters from other medical specialties are systemic lupus erythematosus, fibromyalgia, and chronic fatigue syndrome. Although he asserts that SPECT imaging better demonstrates the root cause of mental illnesses, it is unclear from his book whether this is actually the case.
The descriptions for the ways in which Dr. Amen uses SPECT (which, admittedly, are vague and presumably simplified for a general audience) suggest that he has made observations correlating specific imaging patterns with certain emotional/behavioral outcomes. However, the imaging patterns he describes in the book can be interpreted to represent multiple different mental conditions, making it clear that SPECT is not a laserlike diagnostic tool that produces a single, indisputable diagnosis. Accuracy with SPECT seems especially questionable in light of two case examples he shares where brain imaging was interpreted as representing illness, but the patients were not demonstrating any signs of mental dysfunction. In one case, Dr. Amen opined that the patient’s vibrant spiritual life “overrode” the sick brain, but if this is true,
Patient testimonials are provided, asserting that SPECT imaging helped them know “exactly” what treatment would help them. One cannot help but wonder whether part of the benefit of SPECT imaging is a placebo effect, boosting the confidence of patients that the treatment they are receiving is personalized and scientifically sound. A similar trend is currently seen more broadly in psychiatry with the widespread promotion of pharmacogenetic testing. Such testing may bolster patient confidence in their medication, but its value in improving patient outcomes has not been established.1
Dr. Amen outlines a brief history of mental health care, including differing approaches and therapies from the time of Sigmund Freud up to the present. His outline is somewhat critical of the perceived shortcomings of his psychiatric forebears, yet this seems entirely unnecessary. All scientific disciplines must start somewhere and build from limited knowledge to greater. Is it necessary to belittle Freud for not being able to do SPECT imaging in the 1800s?
Interestingly, Dr. Amen leaves cognitive-behavioral therapy (CBT), a landmark, evidence-based form of psychotherapy, out of his overview of the history of psychiatry. He does go on to mention CBT as part of the treatment offerings of the Amen Clinics, which could leave the lay reader with the incorrect impression that CBT is a treatment unique to Amen Clinics. Similarly, at one point Dr. Amen writes about “what I call automatic negative thoughts.” This phrasing could confuse readers who might not know that automatic thoughts are a concept endemic to CBT.
Dr. Amen writes repeatedly about the Amen Clinics 4 Circles, four key areas of life that can contribute to mental health. These areas are biological, psychological, social, and spiritual. While Amen Clinics may have come up with the term “4 Circles,” the biopsychosocial model of understanding illness was developed by George Engel, MD, in 1977, and current discussions of this model frequently incorporate a spiritual dimension as well.2
Dr. Amen’s writing at times mischaracterizes psychotropic medications in unhelpful ways. He speaks of psychotropic medications generally as being addictive. While this is certainly true for stimulants and benzodiazepines, most would agree that this does not apply to many other commonly used medications in psychiatry, including selective serotonin reuptake inhibitors (SSRIs), tricyclic antidepressants, antipsychotics, and mood stabilizers. He also paints with a broad brush when he states that anxiety medications can cause dementia. A concerning link has been demonstrated between benzodiazepine use and dementia,3 but SSRIs (which are considered first-line medications for anxiety) are not known to cause dementia and may actually delay progression from mild cognitive impairment to Alzheimer’s dementia.4 His mention of medication use affecting a patient’s insurability could have the unfortunate effect of scaring away suffering individuals from seeking help. The one category of psychiatric medication he does not seem concerned about is psychostimulants, which is odd – given the addictive, cardiovascular, and other risks associated with that medication class.
In contrast to his skepticism regarding many psychotropic medications, Dr. Amen expresses significant enthusiasm regarding nutraceutical use. While there has been research in this area supporting a role for some nutraceutical interventions, there is still a need for more rigorous studies.5 To support his endorsement of natural remedies, Dr. Amen mentions that Hippocrates recommended herbs and spices for many health conditions. But Hippocrates lived more than 2,000 years ago, and the state of medicine has advanced significantly since then.
Dr. Amen also mentions that 80% of the developing world relies upon natural or herbal remedies as the primary source of medicine. While he frames this statement as supporting his endorsement of such remedies, it could conversely be said that this is evidence of the need to make pharmacological interventions more widely available in the developing world.
Much of “The End of Mental Illness” is dedicated to reviewing specific risk factors that could cause harm to a person’s mental well-being. One example is head trauma. Dr. Amen documents at least one instance in which he was convinced that his patient had experienced head trauma, and questioned the patient again and again about possible brain injuries. One must wonder whether the positive results of such focused, repetitive questioning might be evidence of confirmation bias, as a search to confirm the preexisting belief of head trauma could lead to overlooking alternative explanations for a patient’s symptoms.
Another risk factor dwelt upon is exposure to toxins. One toxin Dr. Amen rightly recommends avoiding is tobacco smoke. Yet, his approach to advocate for a tobacco-free lifestyle is somewhat problematic. He lists chemicals contained in tobacco smoke, and then names unpleasant items that share those ingredients, such as paint. This smacks of the same sloppy logic manifested in social media memes decrying the use of vaccines by listing their ingredients alongside scary-sounding products that contain identical ingredients (for example, vaccines contain formaldehyde, which is used to embalm dead bodies!). This is analogous to saying that water is bad for you because it contains hydrogen, which is also an ingredient in atomic bombs.
Dr. Amen makes the blanket recommendation to avoid products containing “chemicals.” This is a difficult recommendation to interpret, since literally all matter is made of chemicals. It seems that Dr. Amen is leaning into the vague idea of a “chemical” as something artificially created in a lab, which must, therefore, be dangerous.
Along these lines, Dr. Amen suggests that if a person doesn’t know what is in a specific food item, it should not be eaten. Although this sounds reasonable on the surface, if people were told the names of the proteins and chemical compounds that make up many naturally occurring plants or meats, they would likely not recognize many of them. Dr. Amen dedicates space to list seemingly benign exposures – such as eating nonorganic produce, using two or more beauty products each day, or touching grocery store receipts – as possible “toxins.” By contrast, there is a certain irony in the absence of any mention of the risks associated with radiation from the SPECT imaging he staunchly advocates for. One potential risk of the book listing so many “toxins” to avoid is that patients could waste valuable time and energy eliminating exposures that pose little or no risk, rather than focusing efforts on well-established treatments.
In light of the observations and critiques offered above, one might come away with the impression that I would not recommend “The End of Mental Illness.” However, although one can nitpick details in the book, some of its bigger ideas make it worth commending to readers. Dr. Amen rightfully emphasizes the need for psychiatrists and patients to think more broadly about mental health issues beyond the use of pills. He justifiably criticizes the “15-minute med check” model of practice and the idea that medications are the end-all, be-all of treatment. He demonstrates an appropriate appreciation for the serious risks of reliance on benzodiazepines.6 Dr. Amen points out important contributions from Viktor Frankl, MD, to the field of psychiatry, which may go overlooked today. He also helpfully points out that bipolar disorder may often be misdiagnosed (although he attributes the misdiagnosis to traumatic brain injury, whereas other psychiatrists might say the misdiagnosis is due to borderline personality disorder).
Much of what Dr. Amen writes is sensible, and psychiatrists would do well to adopt the following steps he advocates for: Taking a comprehensive biopsychosocial-spiritual approach to the assessment and treatment of patients; thinking broadly in their differential diagnoses and not forgetting their medical training; understanding that medication alone is often not sufficient to make lasting, positive change in a person’s life; paying attention to healthy habits such as diet, exercise, sleep, and social activity; and knowing that CBT is a valuable tool that can change lives.
There is much to appreciate in “The End of Mental Illness,” especially the overarching idea that psychiatry isn’t just a symptom checklist and a prescription pad. Rather, achieving mental well-being often requires broader thinking and sustained lifestyle changes.
Although I did not agree with everything in the book, it did cause me to think and reflect on my own practice. I read “The End of Mental Illness” with colleagues in my department, and it stimulated a lively discussion. Isn’t that ultimately what a psychiatrist would want from a book like this – the opportunity to reflect, discuss, and potentially improve one’s own practice?
Dr. Weber is physician lead in the department of psychiatry at Intermountain Healthcare Budge Clinic, Logan (Utah) Psychiatry. He disclosed no relevant financial relationships.
References
1. JAMA Netw Open. 2020;3(12). doi: 10.1001/jamanetworkopen.2020.27909.
2. Curr Opin Psychiatry. 2014;27:358-63.
3. BMJ 2014. doi: 10.1136/bmj.g5205.
4. Am J Psychiatry. 2018 Mar 1;175:232-41.
5. Am J Psychiatry. 2016 Jun 1;173:575-87.
6. Current Psychiatry. 2018 Feb;17(2):22-7.
Daniel G. Amen, MD, is an American psychiatrist well-known for his eponymous clinics, television appearances, and series of books on mental health. One of his latest books, “The End of Mental Illness,” summarizes many of his views on the causes of and treatments for mental illnesses.
Dr. Amen’s approaches – such as his advocacy for the widespread use of single photon emission computed tomography (SPECT) imaging – are somewhat controversial and at times fall outside the mainstream of current psychiatric thought. So does “The End of Mental Illness” contain anything of value to the average practicing psychiatrist? (It should be noted that I listened to this as an audiobook and took notes as I listened. This does limit my ability to directly quote portions of the text, but I believe my notes are reliable.)
He begins the book by pointing out that the term “mental illness” might be better replaced with the term “brain illness.” With this shift in terminology, Dr. Amen introduces a theme that recurs throughout the book: That mental illnesses ultimately stem from various ways in which the brain can be harmed. While the suggested change in terminology might help reduce the stigma associated with psychiatric illnesses, Dr. Amen is surprisingly timid about implementing this term in his own book. He repeatedly refers to “brain health/mental health” issues instead of discarding the “mental” term altogether. Even his BRIGHT MINDS acronym for risk factors for mental illnesses includes the term “mind” instead of “brain.”
Continuing the theme of challenging terminology, Dr. Amen goes on to decry the weaknesses of the DSM system of nosology. This is a valid point, because under the current system, the same patient may receive differing diagnoses depending on which provider is seen and how certain symptoms are interpreted. Yet, here again, Dr. Amen does not seem to adhere to his own advice: He uses DSM terminology throughout the book, speaking of depression, anxiety, bipolar disorder, and ADHD. An oddity (which, admittedly, could have been the audiobook reader’s mistake rather than an error in the original text) is that the DSM is referred to as the “Diagnostic and Structural Manual” rather than the Diagnostic and Statistical Manual. He criticizes the DSM for its imprecision, pointing out the variety of symptom combinations that can produce the same diagnoses and how similar symptoms may overlap between differing diagnoses. Yet, his descriptions of common SPECT patterns (his preferred tool to assist in diagnosis) make it clear that here, too, there is a lot of overlap. As an example, ADHD was associated with at least three of the imaging patterns he described. It is also somewhat ironic how Dr. Amen obliquely criticizes the American Psychiatric Association for profiting from the use of the DSM, when SPECT imaging is expensive and profits his own organization.
Dr. Amen repeatedly asserts that psychiatry is unique among medical specialties for making diagnoses based on symptom clusters rather than direct visualization of the affected organ. Yet, psychiatry is not, in fact, unique in making diagnoses in this way. Some examples of diagnoses based on symptom clusters from other medical specialties are systemic lupus erythematosus, fibromyalgia, and chronic fatigue syndrome. Although he asserts that SPECT imaging better demonstrates the root cause of mental illnesses, it is unclear from his book whether this is actually the case.
The descriptions for the ways in which Dr. Amen uses SPECT (which, admittedly, are vague and presumably simplified for a general audience) suggest that he has made observations correlating specific imaging patterns with certain emotional/behavioral outcomes. However, the imaging patterns he describes in the book can be interpreted to represent multiple different mental conditions, making it clear that SPECT is not a laserlike diagnostic tool that produces a single, indisputable diagnosis. Accuracy with SPECT seems especially questionable in light of two case examples he shares where brain imaging was interpreted as representing illness, but the patients were not demonstrating any signs of mental dysfunction. In one case, Dr. Amen opined that the patient’s vibrant spiritual life “overrode” the sick brain, but if this is true,
Patient testimonials are provided, asserting that SPECT imaging helped them know “exactly” what treatment would help them. One cannot help but wonder whether part of the benefit of SPECT imaging is a placebo effect, boosting the confidence of patients that the treatment they are receiving is personalized and scientifically sound. A similar trend is currently seen more broadly in psychiatry with the widespread promotion of pharmacogenetic testing. Such testing may bolster patient confidence in their medication, but its value in improving patient outcomes has not been established.1
Dr. Amen outlines a brief history of mental health care, including differing approaches and therapies from the time of Sigmund Freud up to the present. His outline is somewhat critical of the perceived shortcomings of his psychiatric forebears, yet this seems entirely unnecessary. All scientific disciplines must start somewhere and build from limited knowledge to greater. Is it necessary to belittle Freud for not being able to do SPECT imaging in the 1800s?
Interestingly, Dr. Amen leaves cognitive-behavioral therapy (CBT), a landmark, evidence-based form of psychotherapy, out of his overview of the history of psychiatry. He does go on to mention CBT as part of the treatment offerings of the Amen Clinics, which could leave the lay reader with the incorrect impression that CBT is a treatment unique to Amen Clinics. Similarly, at one point Dr. Amen writes about “what I call automatic negative thoughts.” This phrasing could confuse readers who might not know that automatic thoughts are a concept endemic to CBT.
Dr. Amen writes repeatedly about the Amen Clinics 4 Circles, four key areas of life that can contribute to mental health. These areas are biological, psychological, social, and spiritual. While Amen Clinics may have come up with the term “4 Circles,” the biopsychosocial model of understanding illness was developed by George Engel, MD, in 1977, and current discussions of this model frequently incorporate a spiritual dimension as well.2
Dr. Amen’s writing at times mischaracterizes psychotropic medications in unhelpful ways. He speaks of psychotropic medications generally as being addictive. While this is certainly true for stimulants and benzodiazepines, most would agree that this does not apply to many other commonly used medications in psychiatry, including selective serotonin reuptake inhibitors (SSRIs), tricyclic antidepressants, antipsychotics, and mood stabilizers. He also paints with a broad brush when he states that anxiety medications can cause dementia. A concerning link has been demonstrated between benzodiazepine use and dementia,3 but SSRIs (which are considered first-line medications for anxiety) are not known to cause dementia and may actually delay progression from mild cognitive impairment to Alzheimer’s dementia.4 His mention of medication use affecting a patient’s insurability could have the unfortunate effect of scaring away suffering individuals from seeking help. The one category of psychiatric medication he does not seem concerned about is psychostimulants, which is odd – given the addictive, cardiovascular, and other risks associated with that medication class.
In contrast to his skepticism regarding many psychotropic medications, Dr. Amen expresses significant enthusiasm regarding nutraceutical use. While there has been research in this area supporting a role for some nutraceutical interventions, there is still a need for more rigorous studies.5 To support his endorsement of natural remedies, Dr. Amen mentions that Hippocrates recommended herbs and spices for many health conditions. But Hippocrates lived more than 2,000 years ago, and the state of medicine has advanced significantly since then.
Dr. Amen also mentions that 80% of the developing world relies upon natural or herbal remedies as the primary source of medicine. While he frames this statement as supporting his endorsement of such remedies, it could conversely be said that this is evidence of the need to make pharmacological interventions more widely available in the developing world.
Much of “The End of Mental Illness” is dedicated to reviewing specific risk factors that could cause harm to a person’s mental well-being. One example is head trauma. Dr. Amen documents at least one instance in which he was convinced that his patient had experienced head trauma, and questioned the patient again and again about possible brain injuries. One must wonder whether the positive results of such focused, repetitive questioning might be evidence of confirmation bias, as a search to confirm the preexisting belief of head trauma could lead to overlooking alternative explanations for a patient’s symptoms.
Another risk factor dwelt upon is exposure to toxins. One toxin Dr. Amen rightly recommends avoiding is tobacco smoke. Yet, his approach to advocate for a tobacco-free lifestyle is somewhat problematic. He lists chemicals contained in tobacco smoke, and then names unpleasant items that share those ingredients, such as paint. This smacks of the same sloppy logic manifested in social media memes decrying the use of vaccines by listing their ingredients alongside scary-sounding products that contain identical ingredients (for example, vaccines contain formaldehyde, which is used to embalm dead bodies!). This is analogous to saying that water is bad for you because it contains hydrogen, which is also an ingredient in atomic bombs.
Dr. Amen makes the blanket recommendation to avoid products containing “chemicals.” This is a difficult recommendation to interpret, since literally all matter is made of chemicals. It seems that Dr. Amen is leaning into the vague idea of a “chemical” as something artificially created in a lab, which must, therefore, be dangerous.
Along these lines, Dr. Amen suggests that if a person doesn’t know what is in a specific food item, it should not be eaten. Although this sounds reasonable on the surface, if people were told the names of the proteins and chemical compounds that make up many naturally occurring plants or meats, they would likely not recognize many of them. Dr. Amen dedicates space to list seemingly benign exposures – such as eating nonorganic produce, using two or more beauty products each day, or touching grocery store receipts – as possible “toxins.” By contrast, there is a certain irony in the absence of any mention of the risks associated with radiation from the SPECT imaging he staunchly advocates for. One potential risk of the book listing so many “toxins” to avoid is that patients could waste valuable time and energy eliminating exposures that pose little or no risk, rather than focusing efforts on well-established treatments.
In light of the observations and critiques offered above, one might come away with the impression that I would not recommend “The End of Mental Illness.” However, although one can nitpick details in the book, some of its bigger ideas make it worth commending to readers. Dr. Amen rightfully emphasizes the need for psychiatrists and patients to think more broadly about mental health issues beyond the use of pills. He justifiably criticizes the “15-minute med check” model of practice and the idea that medications are the end-all, be-all of treatment. He demonstrates an appropriate appreciation for the serious risks of reliance on benzodiazepines.6 Dr. Amen points out important contributions from Viktor Frankl, MD, to the field of psychiatry, which may go overlooked today. He also helpfully points out that bipolar disorder may often be misdiagnosed (although he attributes the misdiagnosis to traumatic brain injury, whereas other psychiatrists might say the misdiagnosis is due to borderline personality disorder).
Much of what Dr. Amen writes is sensible, and psychiatrists would do well to adopt the following steps he advocates for: Taking a comprehensive biopsychosocial-spiritual approach to the assessment and treatment of patients; thinking broadly in their differential diagnoses and not forgetting their medical training; understanding that medication alone is often not sufficient to make lasting, positive change in a person’s life; paying attention to healthy habits such as diet, exercise, sleep, and social activity; and knowing that CBT is a valuable tool that can change lives.
There is much to appreciate in “The End of Mental Illness,” especially the overarching idea that psychiatry isn’t just a symptom checklist and a prescription pad. Rather, achieving mental well-being often requires broader thinking and sustained lifestyle changes.
Although I did not agree with everything in the book, it did cause me to think and reflect on my own practice. I read “The End of Mental Illness” with colleagues in my department, and it stimulated a lively discussion. Isn’t that ultimately what a psychiatrist would want from a book like this – the opportunity to reflect, discuss, and potentially improve one’s own practice?
Dr. Weber is physician lead in the department of psychiatry at Intermountain Healthcare Budge Clinic, Logan (Utah) Psychiatry. He disclosed no relevant financial relationships.
References
1. JAMA Netw Open. 2020;3(12). doi: 10.1001/jamanetworkopen.2020.27909.
2. Curr Opin Psychiatry. 2014;27:358-63.
3. BMJ 2014. doi: 10.1136/bmj.g5205.
4. Am J Psychiatry. 2018 Mar 1;175:232-41.
5. Am J Psychiatry. 2016 Jun 1;173:575-87.
6. Current Psychiatry. 2018 Feb;17(2):22-7.
Daniel G. Amen, MD, is an American psychiatrist well-known for his eponymous clinics, television appearances, and series of books on mental health. One of his latest books, “The End of Mental Illness,” summarizes many of his views on the causes of and treatments for mental illnesses.
Dr. Amen’s approaches – such as his advocacy for the widespread use of single photon emission computed tomography (SPECT) imaging – are somewhat controversial and at times fall outside the mainstream of current psychiatric thought. So does “The End of Mental Illness” contain anything of value to the average practicing psychiatrist? (It should be noted that I listened to this as an audiobook and took notes as I listened. This does limit my ability to directly quote portions of the text, but I believe my notes are reliable.)
He begins the book by pointing out that the term “mental illness” might be better replaced with the term “brain illness.” With this shift in terminology, Dr. Amen introduces a theme that recurs throughout the book: That mental illnesses ultimately stem from various ways in which the brain can be harmed. While the suggested change in terminology might help reduce the stigma associated with psychiatric illnesses, Dr. Amen is surprisingly timid about implementing this term in his own book. He repeatedly refers to “brain health/mental health” issues instead of discarding the “mental” term altogether. Even his BRIGHT MINDS acronym for risk factors for mental illnesses includes the term “mind” instead of “brain.”
Continuing the theme of challenging terminology, Dr. Amen goes on to decry the weaknesses of the DSM system of nosology. This is a valid point, because under the current system, the same patient may receive differing diagnoses depending on which provider is seen and how certain symptoms are interpreted. Yet, here again, Dr. Amen does not seem to adhere to his own advice: He uses DSM terminology throughout the book, speaking of depression, anxiety, bipolar disorder, and ADHD. An oddity (which, admittedly, could have been the audiobook reader’s mistake rather than an error in the original text) is that the DSM is referred to as the “Diagnostic and Structural Manual” rather than the Diagnostic and Statistical Manual. He criticizes the DSM for its imprecision, pointing out the variety of symptom combinations that can produce the same diagnoses and how similar symptoms may overlap between differing diagnoses. Yet, his descriptions of common SPECT patterns (his preferred tool to assist in diagnosis) make it clear that here, too, there is a lot of overlap. As an example, ADHD was associated with at least three of the imaging patterns he described. It is also somewhat ironic how Dr. Amen obliquely criticizes the American Psychiatric Association for profiting from the use of the DSM, when SPECT imaging is expensive and profits his own organization.
Dr. Amen repeatedly asserts that psychiatry is unique among medical specialties for making diagnoses based on symptom clusters rather than direct visualization of the affected organ. Yet, psychiatry is not, in fact, unique in making diagnoses in this way. Some examples of diagnoses based on symptom clusters from other medical specialties are systemic lupus erythematosus, fibromyalgia, and chronic fatigue syndrome. Although he asserts that SPECT imaging better demonstrates the root cause of mental illnesses, it is unclear from his book whether this is actually the case.
The descriptions for the ways in which Dr. Amen uses SPECT (which, admittedly, are vague and presumably simplified for a general audience) suggest that he has made observations correlating specific imaging patterns with certain emotional/behavioral outcomes. However, the imaging patterns he describes in the book can be interpreted to represent multiple different mental conditions, making it clear that SPECT is not a laserlike diagnostic tool that produces a single, indisputable diagnosis. Accuracy with SPECT seems especially questionable in light of two case examples he shares where brain imaging was interpreted as representing illness, but the patients were not demonstrating any signs of mental dysfunction. In one case, Dr. Amen opined that the patient’s vibrant spiritual life “overrode” the sick brain, but if this is true,
Patient testimonials are provided, asserting that SPECT imaging helped them know “exactly” what treatment would help them. One cannot help but wonder whether part of the benefit of SPECT imaging is a placebo effect, boosting the confidence of patients that the treatment they are receiving is personalized and scientifically sound. A similar trend is currently seen more broadly in psychiatry with the widespread promotion of pharmacogenetic testing. Such testing may bolster patient confidence in their medication, but its value in improving patient outcomes has not been established.1
Dr. Amen outlines a brief history of mental health care, including differing approaches and therapies from the time of Sigmund Freud up to the present. His outline is somewhat critical of the perceived shortcomings of his psychiatric forebears, yet this seems entirely unnecessary. All scientific disciplines must start somewhere and build from limited knowledge to greater. Is it necessary to belittle Freud for not being able to do SPECT imaging in the 1800s?
Interestingly, Dr. Amen leaves cognitive-behavioral therapy (CBT), a landmark, evidence-based form of psychotherapy, out of his overview of the history of psychiatry. He does go on to mention CBT as part of the treatment offerings of the Amen Clinics, which could leave the lay reader with the incorrect impression that CBT is a treatment unique to Amen Clinics. Similarly, at one point Dr. Amen writes about “what I call automatic negative thoughts.” This phrasing could confuse readers who might not know that automatic thoughts are a concept endemic to CBT.
Dr. Amen writes repeatedly about the Amen Clinics 4 Circles, four key areas of life that can contribute to mental health. These areas are biological, psychological, social, and spiritual. While Amen Clinics may have come up with the term “4 Circles,” the biopsychosocial model of understanding illness was developed by George Engel, MD, in 1977, and current discussions of this model frequently incorporate a spiritual dimension as well.2
Dr. Amen’s writing at times mischaracterizes psychotropic medications in unhelpful ways. He speaks of psychotropic medications generally as being addictive. While this is certainly true for stimulants and benzodiazepines, most would agree that this does not apply to many other commonly used medications in psychiatry, including selective serotonin reuptake inhibitors (SSRIs), tricyclic antidepressants, antipsychotics, and mood stabilizers. He also paints with a broad brush when he states that anxiety medications can cause dementia. A concerning link has been demonstrated between benzodiazepine use and dementia,3 but SSRIs (which are considered first-line medications for anxiety) are not known to cause dementia and may actually delay progression from mild cognitive impairment to Alzheimer’s dementia.4 His mention of medication use affecting a patient’s insurability could have the unfortunate effect of scaring away suffering individuals from seeking help. The one category of psychiatric medication he does not seem concerned about is psychostimulants, which is odd – given the addictive, cardiovascular, and other risks associated with that medication class.
In contrast to his skepticism regarding many psychotropic medications, Dr. Amen expresses significant enthusiasm regarding nutraceutical use. While there has been research in this area supporting a role for some nutraceutical interventions, there is still a need for more rigorous studies.5 To support his endorsement of natural remedies, Dr. Amen mentions that Hippocrates recommended herbs and spices for many health conditions. But Hippocrates lived more than 2,000 years ago, and the state of medicine has advanced significantly since then.
Dr. Amen also mentions that 80% of the developing world relies upon natural or herbal remedies as the primary source of medicine. While he frames this statement as supporting his endorsement of such remedies, it could conversely be said that this is evidence of the need to make pharmacological interventions more widely available in the developing world.
Much of “The End of Mental Illness” is dedicated to reviewing specific risk factors that could cause harm to a person’s mental well-being. One example is head trauma. Dr. Amen documents at least one instance in which he was convinced that his patient had experienced head trauma, and questioned the patient again and again about possible brain injuries. One must wonder whether the positive results of such focused, repetitive questioning might be evidence of confirmation bias, as a search to confirm the preexisting belief of head trauma could lead to overlooking alternative explanations for a patient’s symptoms.
Another risk factor dwelt upon is exposure to toxins. One toxin Dr. Amen rightly recommends avoiding is tobacco smoke. Yet, his approach to advocate for a tobacco-free lifestyle is somewhat problematic. He lists chemicals contained in tobacco smoke, and then names unpleasant items that share those ingredients, such as paint. This smacks of the same sloppy logic manifested in social media memes decrying the use of vaccines by listing their ingredients alongside scary-sounding products that contain identical ingredients (for example, vaccines contain formaldehyde, which is used to embalm dead bodies!). This is analogous to saying that water is bad for you because it contains hydrogen, which is also an ingredient in atomic bombs.
Dr. Amen makes the blanket recommendation to avoid products containing “chemicals.” This is a difficult recommendation to interpret, since literally all matter is made of chemicals. It seems that Dr. Amen is leaning into the vague idea of a “chemical” as something artificially created in a lab, which must, therefore, be dangerous.
Along these lines, Dr. Amen suggests that if a person doesn’t know what is in a specific food item, it should not be eaten. Although this sounds reasonable on the surface, if people were told the names of the proteins and chemical compounds that make up many naturally occurring plants or meats, they would likely not recognize many of them. Dr. Amen dedicates space to list seemingly benign exposures – such as eating nonorganic produce, using two or more beauty products each day, or touching grocery store receipts – as possible “toxins.” By contrast, there is a certain irony in the absence of any mention of the risks associated with radiation from the SPECT imaging he staunchly advocates for. One potential risk of the book listing so many “toxins” to avoid is that patients could waste valuable time and energy eliminating exposures that pose little or no risk, rather than focusing efforts on well-established treatments.
In light of the observations and critiques offered above, one might come away with the impression that I would not recommend “The End of Mental Illness.” However, although one can nitpick details in the book, some of its bigger ideas make it worth commending to readers. Dr. Amen rightfully emphasizes the need for psychiatrists and patients to think more broadly about mental health issues beyond the use of pills. He justifiably criticizes the “15-minute med check” model of practice and the idea that medications are the end-all, be-all of treatment. He demonstrates an appropriate appreciation for the serious risks of reliance on benzodiazepines.6 Dr. Amen points out important contributions from Viktor Frankl, MD, to the field of psychiatry, which may go overlooked today. He also helpfully points out that bipolar disorder may often be misdiagnosed (although he attributes the misdiagnosis to traumatic brain injury, whereas other psychiatrists might say the misdiagnosis is due to borderline personality disorder).
Much of what Dr. Amen writes is sensible, and psychiatrists would do well to adopt the following steps he advocates for: Taking a comprehensive biopsychosocial-spiritual approach to the assessment and treatment of patients; thinking broadly in their differential diagnoses and not forgetting their medical training; understanding that medication alone is often not sufficient to make lasting, positive change in a person’s life; paying attention to healthy habits such as diet, exercise, sleep, and social activity; and knowing that CBT is a valuable tool that can change lives.
There is much to appreciate in “The End of Mental Illness,” especially the overarching idea that psychiatry isn’t just a symptom checklist and a prescription pad. Rather, achieving mental well-being often requires broader thinking and sustained lifestyle changes.
Although I did not agree with everything in the book, it did cause me to think and reflect on my own practice. I read “The End of Mental Illness” with colleagues in my department, and it stimulated a lively discussion. Isn’t that ultimately what a psychiatrist would want from a book like this – the opportunity to reflect, discuss, and potentially improve one’s own practice?
Dr. Weber is physician lead in the department of psychiatry at Intermountain Healthcare Budge Clinic, Logan (Utah) Psychiatry. He disclosed no relevant financial relationships.
References
1. JAMA Netw Open. 2020;3(12). doi: 10.1001/jamanetworkopen.2020.27909.
2. Curr Opin Psychiatry. 2014;27:358-63.
3. BMJ 2014. doi: 10.1136/bmj.g5205.
4. Am J Psychiatry. 2018 Mar 1;175:232-41.
5. Am J Psychiatry. 2016 Jun 1;173:575-87.
6. Current Psychiatry. 2018 Feb;17(2):22-7.
FDA clears nonstimulant for ADHD in children aged 6 years and up
The Food and Drug Administration has approved the nonstimulant medication viloxazine extended-release capsules (Qelbree, Supernus Pharmaceuticals) for the treatment of attention deficit hyperactivity disorder (ADHD) in children aged 6-17 years, the company has announced.
Viloxazine (formerly SPN-812) is a selective norepinephrine reuptake inhibitor. Capsules may be swallowed whole or opened and the entire contents sprinkled onto applesauce, as needed.
The approval of viloxazine is supported by data from four phase 3 clinical trials involving more than 1,000 pediatric patients aged 6-17 years, the company said.
In one randomized, placebo-controlled phase 3 study that included more than 400 children, viloxazine reduced symptoms of ADHD as soon as 1 week after dosing and was well tolerated.
As reported by this news organization, the study was published last July in Clinical Therapeutics.
In addition to its fast onset of action, the fact that it was effective for both inattentive and hyperactive/impulsive clusters of symptoms is “impressive,” study investigator Andrew Cutler, MD, clinical associate professor of psychiatry, SUNY Upstate Medical University, Syracuse, N.Y., said in an interview.
Also noteworthy was the improvement in measures of quality of life and function, “especially function in the areas of school, home life, family relations, and peer relationships, which can be really disrupted with ADHD,” Dr. Cutler said.
The prescribing label for viloxazine includes a boxed warning regarding the potential for suicidal thoughts and behaviors in some children with ADHD treated with the drug, especially within the first few months of treatment or when the dose is changed.
In clinical trials, higher rates of suicidal thoughts and behavior were reported in pediatric patients treated with viloxazine than in patients treated with placebo. Patients taking viloxazine should be closely monitored for any new or sudden changes in mood, behavior, thoughts, and feelings.
Viloxazine has shown promise in a phase 3 trial involving adults with ADHD.
The company plans to submit a supplemental new drug application to the FDA for viloxazine in adults later this year.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved the nonstimulant medication viloxazine extended-release capsules (Qelbree, Supernus Pharmaceuticals) for the treatment of attention deficit hyperactivity disorder (ADHD) in children aged 6-17 years, the company has announced.
Viloxazine (formerly SPN-812) is a selective norepinephrine reuptake inhibitor. Capsules may be swallowed whole or opened and the entire contents sprinkled onto applesauce, as needed.
The approval of viloxazine is supported by data from four phase 3 clinical trials involving more than 1,000 pediatric patients aged 6-17 years, the company said.
In one randomized, placebo-controlled phase 3 study that included more than 400 children, viloxazine reduced symptoms of ADHD as soon as 1 week after dosing and was well tolerated.
As reported by this news organization, the study was published last July in Clinical Therapeutics.
In addition to its fast onset of action, the fact that it was effective for both inattentive and hyperactive/impulsive clusters of symptoms is “impressive,” study investigator Andrew Cutler, MD, clinical associate professor of psychiatry, SUNY Upstate Medical University, Syracuse, N.Y., said in an interview.
Also noteworthy was the improvement in measures of quality of life and function, “especially function in the areas of school, home life, family relations, and peer relationships, which can be really disrupted with ADHD,” Dr. Cutler said.
The prescribing label for viloxazine includes a boxed warning regarding the potential for suicidal thoughts and behaviors in some children with ADHD treated with the drug, especially within the first few months of treatment or when the dose is changed.
In clinical trials, higher rates of suicidal thoughts and behavior were reported in pediatric patients treated with viloxazine than in patients treated with placebo. Patients taking viloxazine should be closely monitored for any new or sudden changes in mood, behavior, thoughts, and feelings.
Viloxazine has shown promise in a phase 3 trial involving adults with ADHD.
The company plans to submit a supplemental new drug application to the FDA for viloxazine in adults later this year.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved the nonstimulant medication viloxazine extended-release capsules (Qelbree, Supernus Pharmaceuticals) for the treatment of attention deficit hyperactivity disorder (ADHD) in children aged 6-17 years, the company has announced.
Viloxazine (formerly SPN-812) is a selective norepinephrine reuptake inhibitor. Capsules may be swallowed whole or opened and the entire contents sprinkled onto applesauce, as needed.
The approval of viloxazine is supported by data from four phase 3 clinical trials involving more than 1,000 pediatric patients aged 6-17 years, the company said.
In one randomized, placebo-controlled phase 3 study that included more than 400 children, viloxazine reduced symptoms of ADHD as soon as 1 week after dosing and was well tolerated.
As reported by this news organization, the study was published last July in Clinical Therapeutics.
In addition to its fast onset of action, the fact that it was effective for both inattentive and hyperactive/impulsive clusters of symptoms is “impressive,” study investigator Andrew Cutler, MD, clinical associate professor of psychiatry, SUNY Upstate Medical University, Syracuse, N.Y., said in an interview.
Also noteworthy was the improvement in measures of quality of life and function, “especially function in the areas of school, home life, family relations, and peer relationships, which can be really disrupted with ADHD,” Dr. Cutler said.
The prescribing label for viloxazine includes a boxed warning regarding the potential for suicidal thoughts and behaviors in some children with ADHD treated with the drug, especially within the first few months of treatment or when the dose is changed.
In clinical trials, higher rates of suicidal thoughts and behavior were reported in pediatric patients treated with viloxazine than in patients treated with placebo. Patients taking viloxazine should be closely monitored for any new or sudden changes in mood, behavior, thoughts, and feelings.
Viloxazine has shown promise in a phase 3 trial involving adults with ADHD.
The company plans to submit a supplemental new drug application to the FDA for viloxazine in adults later this year.
A version of this article first appeared on Medscape.com.
FDA okays novel dual-action stimulant med for ADHD
The Food and Drug Administration has approved a new, once-daily oral stimulant medication for treatment of ADHD in people aged 6 years and older.
Azstarys (KemPharm) combines extended-release serdexmethylphenidate (SDX), KemPharm’s prodrug of dexmethylphenidate (d-MPH), coformulated with immediate-release d-MPH.
Following absorption in the gastrointestinal tract, SDX is converted to d-MPH, which is gradually released throughout the day, providing symptom control both rapidly with the d-MPH and for an extended duration with SDX.
The dual action of Azstarys addresses an unmet need for a medication that has early onset of action and long duration of therapy, with steady ADHD symptom control in one capsule, Corium, the company that will lead U.S. commercialization of the drug, stated in a news release.
“The data documenting the efficacy and safety of this new dual-action medicine, the first ever to use the novel prodrug serdexmethylphenidate together with dexmethylphenidate, is welcome news for clinicians and families to consider when choosing an appropriate ADHD therapy for children,” Ann Childress, MD, president of the Center for Psychiatry and Behavioral Medicine in Las Vegas, who led the phase 3 trial of the drug, said in the release.
The study included 150 children aged 6-12 years with ADHD. Compared with placebo, treatment with Azstarys led to significant improvement in ADHD symptoms, as measured by the primary endpoint, the change from baseline in Swanson, Kotkin, Agler, M-Flynn, and Pelham Rating Scale–Combined scores averaged over 13 hours.
Adverse events seen more often with Azstarys than placebo were headache (5.4% vs. 1.3%), upper abdominal pain (4.1% vs. 1.3%), insomnia (2.7% vs. 1.3%) and pharyngitis (2.7% vs. 0%). No serious adverse events were reported.
The FDA has recommended a schedule II controlled substance classification for Azstarys and the Drug Enforcement Administration will decide on scheduling within 90 days.
Pending the DEA’s action, the launch of Azstarys is anticipated this summer. Azstarys will be available in three once-daily dosage strengths of SDX/d-MPH: 26.1/5.2 mg, 39.2/7.8 mg, and 52.3/10.4 mg.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved a new, once-daily oral stimulant medication for treatment of ADHD in people aged 6 years and older.
Azstarys (KemPharm) combines extended-release serdexmethylphenidate (SDX), KemPharm’s prodrug of dexmethylphenidate (d-MPH), coformulated with immediate-release d-MPH.
Following absorption in the gastrointestinal tract, SDX is converted to d-MPH, which is gradually released throughout the day, providing symptom control both rapidly with the d-MPH and for an extended duration with SDX.
The dual action of Azstarys addresses an unmet need for a medication that has early onset of action and long duration of therapy, with steady ADHD symptom control in one capsule, Corium, the company that will lead U.S. commercialization of the drug, stated in a news release.
“The data documenting the efficacy and safety of this new dual-action medicine, the first ever to use the novel prodrug serdexmethylphenidate together with dexmethylphenidate, is welcome news for clinicians and families to consider when choosing an appropriate ADHD therapy for children,” Ann Childress, MD, president of the Center for Psychiatry and Behavioral Medicine in Las Vegas, who led the phase 3 trial of the drug, said in the release.
The study included 150 children aged 6-12 years with ADHD. Compared with placebo, treatment with Azstarys led to significant improvement in ADHD symptoms, as measured by the primary endpoint, the change from baseline in Swanson, Kotkin, Agler, M-Flynn, and Pelham Rating Scale–Combined scores averaged over 13 hours.
Adverse events seen more often with Azstarys than placebo were headache (5.4% vs. 1.3%), upper abdominal pain (4.1% vs. 1.3%), insomnia (2.7% vs. 1.3%) and pharyngitis (2.7% vs. 0%). No serious adverse events were reported.
The FDA has recommended a schedule II controlled substance classification for Azstarys and the Drug Enforcement Administration will decide on scheduling within 90 days.
Pending the DEA’s action, the launch of Azstarys is anticipated this summer. Azstarys will be available in three once-daily dosage strengths of SDX/d-MPH: 26.1/5.2 mg, 39.2/7.8 mg, and 52.3/10.4 mg.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved a new, once-daily oral stimulant medication for treatment of ADHD in people aged 6 years and older.
Azstarys (KemPharm) combines extended-release serdexmethylphenidate (SDX), KemPharm’s prodrug of dexmethylphenidate (d-MPH), coformulated with immediate-release d-MPH.
Following absorption in the gastrointestinal tract, SDX is converted to d-MPH, which is gradually released throughout the day, providing symptom control both rapidly with the d-MPH and for an extended duration with SDX.
The dual action of Azstarys addresses an unmet need for a medication that has early onset of action and long duration of therapy, with steady ADHD symptom control in one capsule, Corium, the company that will lead U.S. commercialization of the drug, stated in a news release.
“The data documenting the efficacy and safety of this new dual-action medicine, the first ever to use the novel prodrug serdexmethylphenidate together with dexmethylphenidate, is welcome news for clinicians and families to consider when choosing an appropriate ADHD therapy for children,” Ann Childress, MD, president of the Center for Psychiatry and Behavioral Medicine in Las Vegas, who led the phase 3 trial of the drug, said in the release.
The study included 150 children aged 6-12 years with ADHD. Compared with placebo, treatment with Azstarys led to significant improvement in ADHD symptoms, as measured by the primary endpoint, the change from baseline in Swanson, Kotkin, Agler, M-Flynn, and Pelham Rating Scale–Combined scores averaged over 13 hours.
Adverse events seen more often with Azstarys than placebo were headache (5.4% vs. 1.3%), upper abdominal pain (4.1% vs. 1.3%), insomnia (2.7% vs. 1.3%) and pharyngitis (2.7% vs. 0%). No serious adverse events were reported.
The FDA has recommended a schedule II controlled substance classification for Azstarys and the Drug Enforcement Administration will decide on scheduling within 90 days.
Pending the DEA’s action, the launch of Azstarys is anticipated this summer. Azstarys will be available in three once-daily dosage strengths of SDX/d-MPH: 26.1/5.2 mg, 39.2/7.8 mg, and 52.3/10.4 mg.
A version of this article first appeared on Medscape.com.
Does L-methylfolate have a role in ADHD management?
Editor’s note: Readers’ Forum is a new department for correspondence from readers that is not in response to articles published in
Since the completion of the human genome project, the role of pharmacogenomics in treating mental health disorders has become more prevalent. Recently discovered genetic polymorphisms and mutations in the methylenetetrahydrofolate reductase (MTHFR) gene have led clinicians to seek out new therapeutic approaches to personalize mental health care. MTHFR is a key enzyme of folate metabolism, and changes in its gene can result in reduced enzyme activity, which has been associated with psychiatric illnesses such as schizophrenia, major depressive disorder (MDD), attention-deficit/hyperactivity disorder (ADHD), and autism.1 Supplementation with L-methylfolate, the active form of folate, has been found to improve clinical and social recovery in patients with psychiatric illnesses such as schizophrenia and MDD.2 While L-methylfolate is classified as an FDA-approved medicinal food for patients with depression and schizophrenia, its role in ADHD remains controversial.3 L-methylfolate modulates the synthesis of monoamines such as dopamine and norepinephrine, which are pivotal in reducing inattentiveness and hyperactivity in patients with ADHD.4,5 As a result, it could play an important role in the management of ADHD in patients with MTHFR deficiency.
Despite its high prevalence in many children, ADHD can persist into adulthood with impairing symptoms that have long-term social and economic impacts. Conventional methods of treating ADHD include stimulant medications such as methylphenidate, which can increase the levels of dopamine and norepinephrine in the brain. Unfortunately, stimulants’ cost, adverse effect profile, and high potential for abuse can hinder their use and contribute to treatment resistance.6 Because L-methylfolate can cross the blood-brain barrier and lacks the adverse effect profile of stimulants, it represents an alternative that could improve the quality of life for ADHD patients, particularly those with MTHFR polymorphisms or mutations.
Conflicting evidence
Several researchers have investigated the role of L-methylfolate as a supplement or alternative to stimulant therapy for patients with ADHD. While some preliminary studies have found some benefit, others have not. Here we describe 2 studies with differing results.
Quilliin7 (2013). In an open-label study at a children’s hospital in Texas, Quillin7 investigated L-methylfolate for alleviating attention-deficit disorder/ADHD symptoms in 59 patients age 5 to 18. Twenty-seven patients received stimulant therapy. All patients were treated with L-methylfolate, 0.2 mg/kg/d in a chewable tablet form, for 6 weeks. The primary endpoint was change on the average Vanderbilt Assessment Scale Total Symptom Score (TSS), which was 30 at baseline. At the study’s conclusion, the average TSS score was 22, a 27% reduction. Patients who were taking only L-methylfolate had an average score of 21 at the end of the study, which was a 34% improvement, compared with an average TSS score of 23 in those who were taking stimulants.
Surman et al3 (2019). In this 12-week, double-blind, placebo-controlled clinical trial, researchers assessed the efficacy and tolerability of L-methylfolate when added to osmotic-release oral system methylphenidate (OROS-MPH).3 Surman et al3 randomized 44 adult patients (age 18 to 55) who met the DSM-5 criteria for ADHD to a placebo group or an active group. The placebo group was treated with placebo plus OROS-MPH, while the active group received L-methylfolate, 15 mg/d, plus OROS-MPH. OROS-MPH was started at 36 mg/d and titrated to optimal response. The primary endpoint was change in score from baseline on the Adult ADHD Investigator Symptom Report scale. Although it was well tolerated, L-methylfolate was not associated with a significant change in measures of ADHD or mental health function.3 However, researchers noticed that patients who received L-methylfolate needed to receive higher doses of methylphenidate over time. This suggests that supplementation with L-methylfolate could reduce the effectiveness of methylphenidate in adult patients with ADHD.3
While more research is needed, the contradictory results of these studies suggests that the relationship between L-methylfolate and ADHD could be impacted by dosing, as well as by differences in adult and childhood ADHD that are not yet fully understood.
Continue to: An area warranting future research
An area warranting future research
The growth of pharmacogenomics represents an important opportunity to bridge the gap between our understanding of psychiatric illnesses and new ways to treat them. Using L-methylfolate to treat ADHD might help bridge this gap. For this to occur, psychiatrists need to use evidence-based pharmacogenetic research to inform their decision-making. The differing results in studies evaluating the use of L-methylfolate in adult and pediatric patients pose interesting questions that will require more robust research to answer. Clinicians should be cautious in the use of L-methylfolate and recognize the importance of evaluating every patient with ADHD for MTHFR deficiency. This could help personalize care in ways that may improve the quality of life for patients and their families.
1. Wan L, Li Y, Zhang Z, et al. Methylenetetrahydrofolate reductase and psychiatric diseases. Transl Psychiatry. 2018;8. doi: 10.1038/s41398-018-0276-6
2. Godfrey PSA, Toone BK, Bottiglien T, et al. Enhancement of recovery from psychiatric illness by methylfolate. Lancet. 1990;336(8712):392-395.
3. Surman C, Ceranoglu A, Vaudreuil C, et al. Does L-methylfolate supplement methylphenidate pharmacotherapy in attention-deficit/hyperactivity disorder?: evidence of lack of benefit from a double-blind, placebo-controlled, randomized clinical trial. J Clin Psychopharmacol. 2019;39(1):28-38.
4. Stahl SM. L-methylfolate: a vitamin for your monoamines. J Clin Psychiatry. 2008;69(9):1352-1353.
5. Arnsten AFT. Stimulants: therapeutic actions in ADHD. Neuropsychopharmacology. 2006;31(11):2376-2383.
6. Childress A, Tran C. Current investigational drugs for the treatment of attention-deficit/hyperactivity disorder. Expert Opin Investig Drugs. 2016;25(4):463-474.
7. Quillin R. High dose L-methylfolate as novel therapy in ADHD. Abstract presented at: 2013 American Academy of Pediatrics National Conference and Exhibition; October 28, 2013; Orlando, FL.
Editor’s note: Readers’ Forum is a new department for correspondence from readers that is not in response to articles published in
Since the completion of the human genome project, the role of pharmacogenomics in treating mental health disorders has become more prevalent. Recently discovered genetic polymorphisms and mutations in the methylenetetrahydrofolate reductase (MTHFR) gene have led clinicians to seek out new therapeutic approaches to personalize mental health care. MTHFR is a key enzyme of folate metabolism, and changes in its gene can result in reduced enzyme activity, which has been associated with psychiatric illnesses such as schizophrenia, major depressive disorder (MDD), attention-deficit/hyperactivity disorder (ADHD), and autism.1 Supplementation with L-methylfolate, the active form of folate, has been found to improve clinical and social recovery in patients with psychiatric illnesses such as schizophrenia and MDD.2 While L-methylfolate is classified as an FDA-approved medicinal food for patients with depression and schizophrenia, its role in ADHD remains controversial.3 L-methylfolate modulates the synthesis of monoamines such as dopamine and norepinephrine, which are pivotal in reducing inattentiveness and hyperactivity in patients with ADHD.4,5 As a result, it could play an important role in the management of ADHD in patients with MTHFR deficiency.
Despite its high prevalence in many children, ADHD can persist into adulthood with impairing symptoms that have long-term social and economic impacts. Conventional methods of treating ADHD include stimulant medications such as methylphenidate, which can increase the levels of dopamine and norepinephrine in the brain. Unfortunately, stimulants’ cost, adverse effect profile, and high potential for abuse can hinder their use and contribute to treatment resistance.6 Because L-methylfolate can cross the blood-brain barrier and lacks the adverse effect profile of stimulants, it represents an alternative that could improve the quality of life for ADHD patients, particularly those with MTHFR polymorphisms or mutations.
Conflicting evidence
Several researchers have investigated the role of L-methylfolate as a supplement or alternative to stimulant therapy for patients with ADHD. While some preliminary studies have found some benefit, others have not. Here we describe 2 studies with differing results.
Quilliin7 (2013). In an open-label study at a children’s hospital in Texas, Quillin7 investigated L-methylfolate for alleviating attention-deficit disorder/ADHD symptoms in 59 patients age 5 to 18. Twenty-seven patients received stimulant therapy. All patients were treated with L-methylfolate, 0.2 mg/kg/d in a chewable tablet form, for 6 weeks. The primary endpoint was change on the average Vanderbilt Assessment Scale Total Symptom Score (TSS), which was 30 at baseline. At the study’s conclusion, the average TSS score was 22, a 27% reduction. Patients who were taking only L-methylfolate had an average score of 21 at the end of the study, which was a 34% improvement, compared with an average TSS score of 23 in those who were taking stimulants.
Surman et al3 (2019). In this 12-week, double-blind, placebo-controlled clinical trial, researchers assessed the efficacy and tolerability of L-methylfolate when added to osmotic-release oral system methylphenidate (OROS-MPH).3 Surman et al3 randomized 44 adult patients (age 18 to 55) who met the DSM-5 criteria for ADHD to a placebo group or an active group. The placebo group was treated with placebo plus OROS-MPH, while the active group received L-methylfolate, 15 mg/d, plus OROS-MPH. OROS-MPH was started at 36 mg/d and titrated to optimal response. The primary endpoint was change in score from baseline on the Adult ADHD Investigator Symptom Report scale. Although it was well tolerated, L-methylfolate was not associated with a significant change in measures of ADHD or mental health function.3 However, researchers noticed that patients who received L-methylfolate needed to receive higher doses of methylphenidate over time. This suggests that supplementation with L-methylfolate could reduce the effectiveness of methylphenidate in adult patients with ADHD.3
While more research is needed, the contradictory results of these studies suggests that the relationship between L-methylfolate and ADHD could be impacted by dosing, as well as by differences in adult and childhood ADHD that are not yet fully understood.
Continue to: An area warranting future research
An area warranting future research
The growth of pharmacogenomics represents an important opportunity to bridge the gap between our understanding of psychiatric illnesses and new ways to treat them. Using L-methylfolate to treat ADHD might help bridge this gap. For this to occur, psychiatrists need to use evidence-based pharmacogenetic research to inform their decision-making. The differing results in studies evaluating the use of L-methylfolate in adult and pediatric patients pose interesting questions that will require more robust research to answer. Clinicians should be cautious in the use of L-methylfolate and recognize the importance of evaluating every patient with ADHD for MTHFR deficiency. This could help personalize care in ways that may improve the quality of life for patients and their families.
Editor’s note: Readers’ Forum is a new department for correspondence from readers that is not in response to articles published in
Since the completion of the human genome project, the role of pharmacogenomics in treating mental health disorders has become more prevalent. Recently discovered genetic polymorphisms and mutations in the methylenetetrahydrofolate reductase (MTHFR) gene have led clinicians to seek out new therapeutic approaches to personalize mental health care. MTHFR is a key enzyme of folate metabolism, and changes in its gene can result in reduced enzyme activity, which has been associated with psychiatric illnesses such as schizophrenia, major depressive disorder (MDD), attention-deficit/hyperactivity disorder (ADHD), and autism.1 Supplementation with L-methylfolate, the active form of folate, has been found to improve clinical and social recovery in patients with psychiatric illnesses such as schizophrenia and MDD.2 While L-methylfolate is classified as an FDA-approved medicinal food for patients with depression and schizophrenia, its role in ADHD remains controversial.3 L-methylfolate modulates the synthesis of monoamines such as dopamine and norepinephrine, which are pivotal in reducing inattentiveness and hyperactivity in patients with ADHD.4,5 As a result, it could play an important role in the management of ADHD in patients with MTHFR deficiency.
Despite its high prevalence in many children, ADHD can persist into adulthood with impairing symptoms that have long-term social and economic impacts. Conventional methods of treating ADHD include stimulant medications such as methylphenidate, which can increase the levels of dopamine and norepinephrine in the brain. Unfortunately, stimulants’ cost, adverse effect profile, and high potential for abuse can hinder their use and contribute to treatment resistance.6 Because L-methylfolate can cross the blood-brain barrier and lacks the adverse effect profile of stimulants, it represents an alternative that could improve the quality of life for ADHD patients, particularly those with MTHFR polymorphisms or mutations.
Conflicting evidence
Several researchers have investigated the role of L-methylfolate as a supplement or alternative to stimulant therapy for patients with ADHD. While some preliminary studies have found some benefit, others have not. Here we describe 2 studies with differing results.
Quilliin7 (2013). In an open-label study at a children’s hospital in Texas, Quillin7 investigated L-methylfolate for alleviating attention-deficit disorder/ADHD symptoms in 59 patients age 5 to 18. Twenty-seven patients received stimulant therapy. All patients were treated with L-methylfolate, 0.2 mg/kg/d in a chewable tablet form, for 6 weeks. The primary endpoint was change on the average Vanderbilt Assessment Scale Total Symptom Score (TSS), which was 30 at baseline. At the study’s conclusion, the average TSS score was 22, a 27% reduction. Patients who were taking only L-methylfolate had an average score of 21 at the end of the study, which was a 34% improvement, compared with an average TSS score of 23 in those who were taking stimulants.
Surman et al3 (2019). In this 12-week, double-blind, placebo-controlled clinical trial, researchers assessed the efficacy and tolerability of L-methylfolate when added to osmotic-release oral system methylphenidate (OROS-MPH).3 Surman et al3 randomized 44 adult patients (age 18 to 55) who met the DSM-5 criteria for ADHD to a placebo group or an active group. The placebo group was treated with placebo plus OROS-MPH, while the active group received L-methylfolate, 15 mg/d, plus OROS-MPH. OROS-MPH was started at 36 mg/d and titrated to optimal response. The primary endpoint was change in score from baseline on the Adult ADHD Investigator Symptom Report scale. Although it was well tolerated, L-methylfolate was not associated with a significant change in measures of ADHD or mental health function.3 However, researchers noticed that patients who received L-methylfolate needed to receive higher doses of methylphenidate over time. This suggests that supplementation with L-methylfolate could reduce the effectiveness of methylphenidate in adult patients with ADHD.3
While more research is needed, the contradictory results of these studies suggests that the relationship between L-methylfolate and ADHD could be impacted by dosing, as well as by differences in adult and childhood ADHD that are not yet fully understood.
Continue to: An area warranting future research
An area warranting future research
The growth of pharmacogenomics represents an important opportunity to bridge the gap between our understanding of psychiatric illnesses and new ways to treat them. Using L-methylfolate to treat ADHD might help bridge this gap. For this to occur, psychiatrists need to use evidence-based pharmacogenetic research to inform their decision-making. The differing results in studies evaluating the use of L-methylfolate in adult and pediatric patients pose interesting questions that will require more robust research to answer. Clinicians should be cautious in the use of L-methylfolate and recognize the importance of evaluating every patient with ADHD for MTHFR deficiency. This could help personalize care in ways that may improve the quality of life for patients and their families.
1. Wan L, Li Y, Zhang Z, et al. Methylenetetrahydrofolate reductase and psychiatric diseases. Transl Psychiatry. 2018;8. doi: 10.1038/s41398-018-0276-6
2. Godfrey PSA, Toone BK, Bottiglien T, et al. Enhancement of recovery from psychiatric illness by methylfolate. Lancet. 1990;336(8712):392-395.
3. Surman C, Ceranoglu A, Vaudreuil C, et al. Does L-methylfolate supplement methylphenidate pharmacotherapy in attention-deficit/hyperactivity disorder?: evidence of lack of benefit from a double-blind, placebo-controlled, randomized clinical trial. J Clin Psychopharmacol. 2019;39(1):28-38.
4. Stahl SM. L-methylfolate: a vitamin for your monoamines. J Clin Psychiatry. 2008;69(9):1352-1353.
5. Arnsten AFT. Stimulants: therapeutic actions in ADHD. Neuropsychopharmacology. 2006;31(11):2376-2383.
6. Childress A, Tran C. Current investigational drugs for the treatment of attention-deficit/hyperactivity disorder. Expert Opin Investig Drugs. 2016;25(4):463-474.
7. Quillin R. High dose L-methylfolate as novel therapy in ADHD. Abstract presented at: 2013 American Academy of Pediatrics National Conference and Exhibition; October 28, 2013; Orlando, FL.
1. Wan L, Li Y, Zhang Z, et al. Methylenetetrahydrofolate reductase and psychiatric diseases. Transl Psychiatry. 2018;8. doi: 10.1038/s41398-018-0276-6
2. Godfrey PSA, Toone BK, Bottiglien T, et al. Enhancement of recovery from psychiatric illness by methylfolate. Lancet. 1990;336(8712):392-395.
3. Surman C, Ceranoglu A, Vaudreuil C, et al. Does L-methylfolate supplement methylphenidate pharmacotherapy in attention-deficit/hyperactivity disorder?: evidence of lack of benefit from a double-blind, placebo-controlled, randomized clinical trial. J Clin Psychopharmacol. 2019;39(1):28-38.
4. Stahl SM. L-methylfolate: a vitamin for your monoamines. J Clin Psychiatry. 2008;69(9):1352-1353.
5. Arnsten AFT. Stimulants: therapeutic actions in ADHD. Neuropsychopharmacology. 2006;31(11):2376-2383.
6. Childress A, Tran C. Current investigational drugs for the treatment of attention-deficit/hyperactivity disorder. Expert Opin Investig Drugs. 2016;25(4):463-474.
7. Quillin R. High dose L-methylfolate as novel therapy in ADHD. Abstract presented at: 2013 American Academy of Pediatrics National Conference and Exhibition; October 28, 2013; Orlando, FL.
Tips offered for treating co-occurring ADHD and SUDs
When Frances R. Levin, MD, began her clinical psychiatry career in the mid-1990s, she spent a lot of time educating colleagues about the validity of an ADHD diagnosis in adults.
“That’s no longer an issue,” Dr. Levin, the Kennedy-Leavy Professor of Psychiatry at Columbia University, New York, said during an annual psychopharmacology update held by the Nevada Psychiatric Association. “But at the time, we often thought, ‘ADHD is something that’s specific to people who are stimulant users.’ In fact, what we found over the years was that these rates are elevated in a range of substance use populations.”
According to National Comorbidity Survey, a nontreatment sample of more than 3,000 adults, individuals who have SUD have two to three times the risk of having ADHD, while individuals who have ADHD have about three times the rate of having an SUD, compared with those who don’t (Am J Psychiatry. 2006;163[4]:716-23). “When you move to treatment samples, the rates also remain quite high,” said Dr. Levin, who is also chief of the division of substance use disorders at the medical center.
“In the general population, the rates of ADHD are 2%-4%. When we look at people who are coming in specifically for treatment of their SUD, the rates are substantially higher, ranging from 10% to 24%.”
According to a 2014 review of medical literature, potential reasons for the association between ADHD and SUD vary and include underlying biologic deficits, such as parental SUDs and genetics; conduct disorder symptoms, such as defiance, rule breaking, and delinquency; poor performance in school, such as low grades, grade retention, or drop-out; and social difficulties, such as rejection from conventional groups or few quality friendships (Annu Rev Clin Psychol. 2014;10:607-39). Other potential pathways include neurocognitive deficits, stress-negative affect models, impulsive anger, and other underlying traits.
One key reason to treat ADHD in patients with SUDs is that they tend to develop the SUD earlier when the ADHD is present, Dr. Levin said. They’re also less likely to be retained in treatment and have a reduced likelihood of going into remission if dependence develops. “Even when they do achieve remission, it seems to take longer for people to reach remission,” she said. “They have more treatment exposure yet do less well in treatment. This can make it more challenging to treat this population.”
One common assumption from clinicians regarding patients with ADHD and a concomitant SUD is that standard treatments for ADHD do not work in active substance users. Another is that, even if treatments work for ADHD, they do not affect the substance use disorder. “Understandably, there is also concern that active substance abusers will misuse and divert their medications,” she said. “Finally, there are often additional psychiatric comorbidities that may make it harder to effectively treat individuals with ADHD and SUD.”
Since 2002, 15 double-blind outpatient studies using stimulants/atomoxetine to treat substance abusers with ADHD have appeared in the medical literature, Dr. Levin said. Only three have included adolescents. “That’s surprising, because up to 40% of kids who come in for treatment, often for cannabis use disorder, will have ADHD, yet there is very little guidance from empirical studies as to how to best treat them,” she said. “There have been several studies looking at atomoxetine to treat substance abusers with ADHD, but results have been mixed. In the cannabis use populations, atomoxetine has not been shown to be effective in treating the substance use disorder, and results are mixed regarding superiority in reducing ADHD symptoms. There is one study showing that ADHD is more likely to be improved in adults with alcohol use disorders with mixed results regarding the alcohol use.”
Overall, most of the outpatient and inpatient studies conducted in this population have demonstrated some signal in terms of reducing ADHD, she said, while a minority of the outpatient studies suggest some benefit in terms of substance use. “What’s interesting is that when you see a response in terms of the ADHD, you often see an improvement in the substance use as well,” Dr. Levin said. “This potentially suggests that patients may be self-medicating their ADHD symptoms or that if the ADHD responds to treatment, then the patient may benefit from the psychosocial interventions that targets the SUD.”
A separate meta-analysis involving more than 1,000 patients found mixed results from pharmacologic interventions and concluded that, while they modestly improved ADHD symptoms, no beneficial effect was seen on drug abstinence or on treatment discontinuation (J Psychopharmacol. 2015 Jan;29[1]:15-23). “I would argue that you don’t need to be as nihilistic about this as the meta-analysis might suggest, because the devil’s in the details,” said Dr. Levin, whose own research was included in the work.
“First of all, many of the studies had high drop-out rates. The outcome measures were variable, and some of the studies used formulations with poor bioavailability. Also, trials that evaluated atomoxetine or stimulants were combined, which may be problematic given the different mechanisms of action. Further, the meta-analysis did not include two recent placebo-controlled trials in adults with stimulant-use disorders that both found that higher dosing of a long-acting stimulant resulted in greater improvements in ADHD symptoms and stimulant use” (Addict. 2014;109[3]:440-9 and JAMA Psychiatry. 2015;72[6]:593-602).
Dr. Levin went on to note that there are few empirical data to guide treatment for those who have multiple psychiatric disorders, let alone treatment for ADHD and SUDs without additional psychiatric disorders. The challenge is what to treat first and/or how to treat the concomitant conditions safely.
“Generally, if possible, treat what is most clinically impairing first,” she said. “Overall, both stimulants and atomoxetine may work for ADHD even in the presence of additional depression, anxiety disorders, and substance use disorders.”
She cautioned against treating a patient with ADHD medication if there is a preexisting psychosis or bipolar illness. “If you start a stimulant or atomoxetine and psychosis or mania occurs, you clearly want to stop the medication and reassess,” she said. Researchers found that the risk of precipitating mania with a stimulant is uncommon if you alleviate symptoms first with a mood stabilizer. “This is a situation where you probably want to treat the bipolar illness first, but it does not preclude the treatment of ADHD once the mood stabilization has occurred,” she said.
In patients with ADHD and anxiety, she often treats the ADHD first, “because oftentimes the anxiety is driven by the procrastination and the inability to get things done,” she explained. “It’s important to determine whether the anxiety is an independent disorder rather than symptoms of ADHD. Inner restlessness can be described as anxiety.”
When there are concerns that preclude the use of a controlled medication, there are medications, in addition to atomoxetine, that might be considered. While bupropion is not Food and Drug Administration approved for ADHD, it might be useful in comorbid mood disorders for nicotine dependence. Other off-label medications that may help include guanfacine, modafinil, and tricyclic antidepressants.
“To date, robust dosing of long-acting amphetamine or methylphenidate formulations have been shown to be effective for patients with stimulant-use disorder, but as mentioned earlier, the data only come from two studies,” she said.
In order to determine whether stimulant treatment is yielding a benefit in a patient with co-occurring ADHD and SUD, she recommends carrying out a structured assessment of ADHD symptoms. Monitoring for functional improvement is also key.
“If there is no improvement in social, occupational, or academic settings and the patient is still actively using drugs, then there is no reason to keep prescribing,” she said. Close monitoring for cardiovascular or other psychiatric symptoms are key as well. Further, for those individuals with both ADHD and a substance-use disorder, it is critical that both are targeted for treatment.
Dr. Levin reported that she has received research, training, or salary support from the National Institute on Drug Abuse, New York state, and the Substance Abuse and Mental Health Services Administration. She has also received or currently receives industry support from Indivior and U.S. World Meds and for medication and from Major League Baseball. In addition, Dr. Levin has been an unpaid scientific advisory board member for Alkermes, Indivior, and Novartis.
When Frances R. Levin, MD, began her clinical psychiatry career in the mid-1990s, she spent a lot of time educating colleagues about the validity of an ADHD diagnosis in adults.
“That’s no longer an issue,” Dr. Levin, the Kennedy-Leavy Professor of Psychiatry at Columbia University, New York, said during an annual psychopharmacology update held by the Nevada Psychiatric Association. “But at the time, we often thought, ‘ADHD is something that’s specific to people who are stimulant users.’ In fact, what we found over the years was that these rates are elevated in a range of substance use populations.”
According to National Comorbidity Survey, a nontreatment sample of more than 3,000 adults, individuals who have SUD have two to three times the risk of having ADHD, while individuals who have ADHD have about three times the rate of having an SUD, compared with those who don’t (Am J Psychiatry. 2006;163[4]:716-23). “When you move to treatment samples, the rates also remain quite high,” said Dr. Levin, who is also chief of the division of substance use disorders at the medical center.
“In the general population, the rates of ADHD are 2%-4%. When we look at people who are coming in specifically for treatment of their SUD, the rates are substantially higher, ranging from 10% to 24%.”
According to a 2014 review of medical literature, potential reasons for the association between ADHD and SUD vary and include underlying biologic deficits, such as parental SUDs and genetics; conduct disorder symptoms, such as defiance, rule breaking, and delinquency; poor performance in school, such as low grades, grade retention, or drop-out; and social difficulties, such as rejection from conventional groups or few quality friendships (Annu Rev Clin Psychol. 2014;10:607-39). Other potential pathways include neurocognitive deficits, stress-negative affect models, impulsive anger, and other underlying traits.
One key reason to treat ADHD in patients with SUDs is that they tend to develop the SUD earlier when the ADHD is present, Dr. Levin said. They’re also less likely to be retained in treatment and have a reduced likelihood of going into remission if dependence develops. “Even when they do achieve remission, it seems to take longer for people to reach remission,” she said. “They have more treatment exposure yet do less well in treatment. This can make it more challenging to treat this population.”
One common assumption from clinicians regarding patients with ADHD and a concomitant SUD is that standard treatments for ADHD do not work in active substance users. Another is that, even if treatments work for ADHD, they do not affect the substance use disorder. “Understandably, there is also concern that active substance abusers will misuse and divert their medications,” she said. “Finally, there are often additional psychiatric comorbidities that may make it harder to effectively treat individuals with ADHD and SUD.”
Since 2002, 15 double-blind outpatient studies using stimulants/atomoxetine to treat substance abusers with ADHD have appeared in the medical literature, Dr. Levin said. Only three have included adolescents. “That’s surprising, because up to 40% of kids who come in for treatment, often for cannabis use disorder, will have ADHD, yet there is very little guidance from empirical studies as to how to best treat them,” she said. “There have been several studies looking at atomoxetine to treat substance abusers with ADHD, but results have been mixed. In the cannabis use populations, atomoxetine has not been shown to be effective in treating the substance use disorder, and results are mixed regarding superiority in reducing ADHD symptoms. There is one study showing that ADHD is more likely to be improved in adults with alcohol use disorders with mixed results regarding the alcohol use.”
Overall, most of the outpatient and inpatient studies conducted in this population have demonstrated some signal in terms of reducing ADHD, she said, while a minority of the outpatient studies suggest some benefit in terms of substance use. “What’s interesting is that when you see a response in terms of the ADHD, you often see an improvement in the substance use as well,” Dr. Levin said. “This potentially suggests that patients may be self-medicating their ADHD symptoms or that if the ADHD responds to treatment, then the patient may benefit from the psychosocial interventions that targets the SUD.”
A separate meta-analysis involving more than 1,000 patients found mixed results from pharmacologic interventions and concluded that, while they modestly improved ADHD symptoms, no beneficial effect was seen on drug abstinence or on treatment discontinuation (J Psychopharmacol. 2015 Jan;29[1]:15-23). “I would argue that you don’t need to be as nihilistic about this as the meta-analysis might suggest, because the devil’s in the details,” said Dr. Levin, whose own research was included in the work.
“First of all, many of the studies had high drop-out rates. The outcome measures were variable, and some of the studies used formulations with poor bioavailability. Also, trials that evaluated atomoxetine or stimulants were combined, which may be problematic given the different mechanisms of action. Further, the meta-analysis did not include two recent placebo-controlled trials in adults with stimulant-use disorders that both found that higher dosing of a long-acting stimulant resulted in greater improvements in ADHD symptoms and stimulant use” (Addict. 2014;109[3]:440-9 and JAMA Psychiatry. 2015;72[6]:593-602).
Dr. Levin went on to note that there are few empirical data to guide treatment for those who have multiple psychiatric disorders, let alone treatment for ADHD and SUDs without additional psychiatric disorders. The challenge is what to treat first and/or how to treat the concomitant conditions safely.
“Generally, if possible, treat what is most clinically impairing first,” she said. “Overall, both stimulants and atomoxetine may work for ADHD even in the presence of additional depression, anxiety disorders, and substance use disorders.”
She cautioned against treating a patient with ADHD medication if there is a preexisting psychosis or bipolar illness. “If you start a stimulant or atomoxetine and psychosis or mania occurs, you clearly want to stop the medication and reassess,” she said. Researchers found that the risk of precipitating mania with a stimulant is uncommon if you alleviate symptoms first with a mood stabilizer. “This is a situation where you probably want to treat the bipolar illness first, but it does not preclude the treatment of ADHD once the mood stabilization has occurred,” she said.
In patients with ADHD and anxiety, she often treats the ADHD first, “because oftentimes the anxiety is driven by the procrastination and the inability to get things done,” she explained. “It’s important to determine whether the anxiety is an independent disorder rather than symptoms of ADHD. Inner restlessness can be described as anxiety.”
When there are concerns that preclude the use of a controlled medication, there are medications, in addition to atomoxetine, that might be considered. While bupropion is not Food and Drug Administration approved for ADHD, it might be useful in comorbid mood disorders for nicotine dependence. Other off-label medications that may help include guanfacine, modafinil, and tricyclic antidepressants.
“To date, robust dosing of long-acting amphetamine or methylphenidate formulations have been shown to be effective for patients with stimulant-use disorder, but as mentioned earlier, the data only come from two studies,” she said.
In order to determine whether stimulant treatment is yielding a benefit in a patient with co-occurring ADHD and SUD, she recommends carrying out a structured assessment of ADHD symptoms. Monitoring for functional improvement is also key.
“If there is no improvement in social, occupational, or academic settings and the patient is still actively using drugs, then there is no reason to keep prescribing,” she said. Close monitoring for cardiovascular or other psychiatric symptoms are key as well. Further, for those individuals with both ADHD and a substance-use disorder, it is critical that both are targeted for treatment.
Dr. Levin reported that she has received research, training, or salary support from the National Institute on Drug Abuse, New York state, and the Substance Abuse and Mental Health Services Administration. She has also received or currently receives industry support from Indivior and U.S. World Meds and for medication and from Major League Baseball. In addition, Dr. Levin has been an unpaid scientific advisory board member for Alkermes, Indivior, and Novartis.
When Frances R. Levin, MD, began her clinical psychiatry career in the mid-1990s, she spent a lot of time educating colleagues about the validity of an ADHD diagnosis in adults.
“That’s no longer an issue,” Dr. Levin, the Kennedy-Leavy Professor of Psychiatry at Columbia University, New York, said during an annual psychopharmacology update held by the Nevada Psychiatric Association. “But at the time, we often thought, ‘ADHD is something that’s specific to people who are stimulant users.’ In fact, what we found over the years was that these rates are elevated in a range of substance use populations.”
According to National Comorbidity Survey, a nontreatment sample of more than 3,000 adults, individuals who have SUD have two to three times the risk of having ADHD, while individuals who have ADHD have about three times the rate of having an SUD, compared with those who don’t (Am J Psychiatry. 2006;163[4]:716-23). “When you move to treatment samples, the rates also remain quite high,” said Dr. Levin, who is also chief of the division of substance use disorders at the medical center.
“In the general population, the rates of ADHD are 2%-4%. When we look at people who are coming in specifically for treatment of their SUD, the rates are substantially higher, ranging from 10% to 24%.”
According to a 2014 review of medical literature, potential reasons for the association between ADHD and SUD vary and include underlying biologic deficits, such as parental SUDs and genetics; conduct disorder symptoms, such as defiance, rule breaking, and delinquency; poor performance in school, such as low grades, grade retention, or drop-out; and social difficulties, such as rejection from conventional groups or few quality friendships (Annu Rev Clin Psychol. 2014;10:607-39). Other potential pathways include neurocognitive deficits, stress-negative affect models, impulsive anger, and other underlying traits.
One key reason to treat ADHD in patients with SUDs is that they tend to develop the SUD earlier when the ADHD is present, Dr. Levin said. They’re also less likely to be retained in treatment and have a reduced likelihood of going into remission if dependence develops. “Even when they do achieve remission, it seems to take longer for people to reach remission,” she said. “They have more treatment exposure yet do less well in treatment. This can make it more challenging to treat this population.”
One common assumption from clinicians regarding patients with ADHD and a concomitant SUD is that standard treatments for ADHD do not work in active substance users. Another is that, even if treatments work for ADHD, they do not affect the substance use disorder. “Understandably, there is also concern that active substance abusers will misuse and divert their medications,” she said. “Finally, there are often additional psychiatric comorbidities that may make it harder to effectively treat individuals with ADHD and SUD.”
Since 2002, 15 double-blind outpatient studies using stimulants/atomoxetine to treat substance abusers with ADHD have appeared in the medical literature, Dr. Levin said. Only three have included adolescents. “That’s surprising, because up to 40% of kids who come in for treatment, often for cannabis use disorder, will have ADHD, yet there is very little guidance from empirical studies as to how to best treat them,” she said. “There have been several studies looking at atomoxetine to treat substance abusers with ADHD, but results have been mixed. In the cannabis use populations, atomoxetine has not been shown to be effective in treating the substance use disorder, and results are mixed regarding superiority in reducing ADHD symptoms. There is one study showing that ADHD is more likely to be improved in adults with alcohol use disorders with mixed results regarding the alcohol use.”
Overall, most of the outpatient and inpatient studies conducted in this population have demonstrated some signal in terms of reducing ADHD, she said, while a minority of the outpatient studies suggest some benefit in terms of substance use. “What’s interesting is that when you see a response in terms of the ADHD, you often see an improvement in the substance use as well,” Dr. Levin said. “This potentially suggests that patients may be self-medicating their ADHD symptoms or that if the ADHD responds to treatment, then the patient may benefit from the psychosocial interventions that targets the SUD.”
A separate meta-analysis involving more than 1,000 patients found mixed results from pharmacologic interventions and concluded that, while they modestly improved ADHD symptoms, no beneficial effect was seen on drug abstinence or on treatment discontinuation (J Psychopharmacol. 2015 Jan;29[1]:15-23). “I would argue that you don’t need to be as nihilistic about this as the meta-analysis might suggest, because the devil’s in the details,” said Dr. Levin, whose own research was included in the work.
“First of all, many of the studies had high drop-out rates. The outcome measures were variable, and some of the studies used formulations with poor bioavailability. Also, trials that evaluated atomoxetine or stimulants were combined, which may be problematic given the different mechanisms of action. Further, the meta-analysis did not include two recent placebo-controlled trials in adults with stimulant-use disorders that both found that higher dosing of a long-acting stimulant resulted in greater improvements in ADHD symptoms and stimulant use” (Addict. 2014;109[3]:440-9 and JAMA Psychiatry. 2015;72[6]:593-602).
Dr. Levin went on to note that there are few empirical data to guide treatment for those who have multiple psychiatric disorders, let alone treatment for ADHD and SUDs without additional psychiatric disorders. The challenge is what to treat first and/or how to treat the concomitant conditions safely.
“Generally, if possible, treat what is most clinically impairing first,” she said. “Overall, both stimulants and atomoxetine may work for ADHD even in the presence of additional depression, anxiety disorders, and substance use disorders.”
She cautioned against treating a patient with ADHD medication if there is a preexisting psychosis or bipolar illness. “If you start a stimulant or atomoxetine and psychosis or mania occurs, you clearly want to stop the medication and reassess,” she said. Researchers found that the risk of precipitating mania with a stimulant is uncommon if you alleviate symptoms first with a mood stabilizer. “This is a situation where you probably want to treat the bipolar illness first, but it does not preclude the treatment of ADHD once the mood stabilization has occurred,” she said.
In patients with ADHD and anxiety, she often treats the ADHD first, “because oftentimes the anxiety is driven by the procrastination and the inability to get things done,” she explained. “It’s important to determine whether the anxiety is an independent disorder rather than symptoms of ADHD. Inner restlessness can be described as anxiety.”
When there are concerns that preclude the use of a controlled medication, there are medications, in addition to atomoxetine, that might be considered. While bupropion is not Food and Drug Administration approved for ADHD, it might be useful in comorbid mood disorders for nicotine dependence. Other off-label medications that may help include guanfacine, modafinil, and tricyclic antidepressants.
“To date, robust dosing of long-acting amphetamine or methylphenidate formulations have been shown to be effective for patients with stimulant-use disorder, but as mentioned earlier, the data only come from two studies,” she said.
In order to determine whether stimulant treatment is yielding a benefit in a patient with co-occurring ADHD and SUD, she recommends carrying out a structured assessment of ADHD symptoms. Monitoring for functional improvement is also key.
“If there is no improvement in social, occupational, or academic settings and the patient is still actively using drugs, then there is no reason to keep prescribing,” she said. Close monitoring for cardiovascular or other psychiatric symptoms are key as well. Further, for those individuals with both ADHD and a substance-use disorder, it is critical that both are targeted for treatment.
Dr. Levin reported that she has received research, training, or salary support from the National Institute on Drug Abuse, New York state, and the Substance Abuse and Mental Health Services Administration. She has also received or currently receives industry support from Indivior and U.S. World Meds and for medication and from Major League Baseball. In addition, Dr. Levin has been an unpaid scientific advisory board member for Alkermes, Indivior, and Novartis.
FROM NPA 2021
Brain connectivity patterns reliably identify ADHD
Functional brain connectivity patterns are a stable biomarker of attention-deficit/hyperactivity disorder, new research suggests.
By applying a machine-learning approach to brain-imaging data, investigators were able to identify with 99% accuracy the adult study participants who had been diagnosed with ADHD in childhood.
“Even though the symptoms of ADHD may be less apparent in adulthood, the brain-wiring signature seems to be persistent,” study investigator Christopher McNorgan, PhD, of the department of psychology, State University of New York at Buffalo told this news organization.
The findings were published online Dec. 17, 2020, in Frontiers of Psychology.
Deep-learning neural networks
The researchers analyzed archived functional magnetic resonance imaging (fMRI) and behavioral data for 80 adults (mean age, 24 years; 64 male). Of these participants, 55 were diagnosed with ADHD in childhood and 25 were not.
The fMRI data were obtained during a response inhibition task that tested the individual’s ability to not respond automatically; for example, not saying “Simon Says” after someone else makes the comment.
The behavioral data included scores on the Iowa Gambling Task (IGT), which is used to measure impulsivity and risk taking.
“Usually, but not always, people with ADHD make riskier choices on this task,” Dr. McNorgan noted.
The investigators measured the amount of interconnectedness among different brain regions during the response inhibition task, which was repeated four times.
Patterns of interconnectivity were then fed into a deep-learning neural network that learned which patterns belonged to the ADHD group vs. those without ADHD (control group) and which patterns belonged to the high vs. low scorers on the IGT.
Caveats, cautionary notes
“The trained models are then tested on brain patterns they had never seen before, and we found the models would make the correct ADHD diagnosis and could tell apart the high and low scorers on the IGT 99% of the time,” Dr. McNorgan reported.
“The trained classifiers make predictions by calculating probabilities, and the neural networks learned how each of the brain connections contributes towards the final classification probability. We identified the set of brain connections that had the greatest influence on these probability calculations,” he noted.
Because the network classified both ADHD diagnosis and gambling task performance, the researchers were able to distinguish between connections that predicted ADHD when gambling performance was poor, as is typical for patients with ADHD, and those predicting ADHD when gambling performance was uncharacteristically good.
While more work is needed, the findings have potential clinical relevance, Dr. McNorgan said.
“ADHD can be difficult to diagnose reliably. If expense wasn’t an issue, fMRI may be able to help make diagnosis more reliable and objective,” he added.
However, because individuals with ADHD have different behavioral profiles, such as scoring atypically well on the IGT, additional studies using this approach may help identify brain networks “that are more or less active in those with ADHD that show a particular diagnostic trait,” he said.
“This could help inform what treatments might be more effective for those individuals,” Dr. McNorgan said.
Of course, he added, “clinicians’ diagnostic expertise is still required, as I would not base an ADHD diagnosis solely on the results of a single brain scan.”
No cross-validation
Commenting on the findings for this news organization, Vince Calhoun, PhD, neuroscientist and founding director of the Center for Translational Research in Neuroimaging and Data Science, Atlanta, a joint effort between Georgia State, Georgia Tech, and Emory University, noted some study limitations.
One cautionary note is that the investigators “appear to select relevant regions to include in the model based on activation to the task, then computed the predictions using the subset of regions that showed strong activation. The issue is this was done on the same data, so there was no cross-validation of this ‘feature selection’ step,” said Dr. Calhoun, who was not involved with the research. “This is a type of circularity which can lead to inflated accuracies,” he added.
Dr. Calhoun also noted that “multiple ADHD classification studies” have reported accuracies above 90%. In addition, there were only 80 participants in the current dataset.
“That’s relatively small for making strong claims about high accuracies as has been reported elsewhere,” he said.
Dr. McNorgan and Dr. Calhoun have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Functional brain connectivity patterns are a stable biomarker of attention-deficit/hyperactivity disorder, new research suggests.
By applying a machine-learning approach to brain-imaging data, investigators were able to identify with 99% accuracy the adult study participants who had been diagnosed with ADHD in childhood.
“Even though the symptoms of ADHD may be less apparent in adulthood, the brain-wiring signature seems to be persistent,” study investigator Christopher McNorgan, PhD, of the department of psychology, State University of New York at Buffalo told this news organization.
The findings were published online Dec. 17, 2020, in Frontiers of Psychology.
Deep-learning neural networks
The researchers analyzed archived functional magnetic resonance imaging (fMRI) and behavioral data for 80 adults (mean age, 24 years; 64 male). Of these participants, 55 were diagnosed with ADHD in childhood and 25 were not.
The fMRI data were obtained during a response inhibition task that tested the individual’s ability to not respond automatically; for example, not saying “Simon Says” after someone else makes the comment.
The behavioral data included scores on the Iowa Gambling Task (IGT), which is used to measure impulsivity and risk taking.
“Usually, but not always, people with ADHD make riskier choices on this task,” Dr. McNorgan noted.
The investigators measured the amount of interconnectedness among different brain regions during the response inhibition task, which was repeated four times.
Patterns of interconnectivity were then fed into a deep-learning neural network that learned which patterns belonged to the ADHD group vs. those without ADHD (control group) and which patterns belonged to the high vs. low scorers on the IGT.
Caveats, cautionary notes
“The trained models are then tested on brain patterns they had never seen before, and we found the models would make the correct ADHD diagnosis and could tell apart the high and low scorers on the IGT 99% of the time,” Dr. McNorgan reported.
“The trained classifiers make predictions by calculating probabilities, and the neural networks learned how each of the brain connections contributes towards the final classification probability. We identified the set of brain connections that had the greatest influence on these probability calculations,” he noted.
Because the network classified both ADHD diagnosis and gambling task performance, the researchers were able to distinguish between connections that predicted ADHD when gambling performance was poor, as is typical for patients with ADHD, and those predicting ADHD when gambling performance was uncharacteristically good.
While more work is needed, the findings have potential clinical relevance, Dr. McNorgan said.
“ADHD can be difficult to diagnose reliably. If expense wasn’t an issue, fMRI may be able to help make diagnosis more reliable and objective,” he added.
However, because individuals with ADHD have different behavioral profiles, such as scoring atypically well on the IGT, additional studies using this approach may help identify brain networks “that are more or less active in those with ADHD that show a particular diagnostic trait,” he said.
“This could help inform what treatments might be more effective for those individuals,” Dr. McNorgan said.
Of course, he added, “clinicians’ diagnostic expertise is still required, as I would not base an ADHD diagnosis solely on the results of a single brain scan.”
No cross-validation
Commenting on the findings for this news organization, Vince Calhoun, PhD, neuroscientist and founding director of the Center for Translational Research in Neuroimaging and Data Science, Atlanta, a joint effort between Georgia State, Georgia Tech, and Emory University, noted some study limitations.
One cautionary note is that the investigators “appear to select relevant regions to include in the model based on activation to the task, then computed the predictions using the subset of regions that showed strong activation. The issue is this was done on the same data, so there was no cross-validation of this ‘feature selection’ step,” said Dr. Calhoun, who was not involved with the research. “This is a type of circularity which can lead to inflated accuracies,” he added.
Dr. Calhoun also noted that “multiple ADHD classification studies” have reported accuracies above 90%. In addition, there were only 80 participants in the current dataset.
“That’s relatively small for making strong claims about high accuracies as has been reported elsewhere,” he said.
Dr. McNorgan and Dr. Calhoun have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Functional brain connectivity patterns are a stable biomarker of attention-deficit/hyperactivity disorder, new research suggests.
By applying a machine-learning approach to brain-imaging data, investigators were able to identify with 99% accuracy the adult study participants who had been diagnosed with ADHD in childhood.
“Even though the symptoms of ADHD may be less apparent in adulthood, the brain-wiring signature seems to be persistent,” study investigator Christopher McNorgan, PhD, of the department of psychology, State University of New York at Buffalo told this news organization.
The findings were published online Dec. 17, 2020, in Frontiers of Psychology.
Deep-learning neural networks
The researchers analyzed archived functional magnetic resonance imaging (fMRI) and behavioral data for 80 adults (mean age, 24 years; 64 male). Of these participants, 55 were diagnosed with ADHD in childhood and 25 were not.
The fMRI data were obtained during a response inhibition task that tested the individual’s ability to not respond automatically; for example, not saying “Simon Says” after someone else makes the comment.
The behavioral data included scores on the Iowa Gambling Task (IGT), which is used to measure impulsivity and risk taking.
“Usually, but not always, people with ADHD make riskier choices on this task,” Dr. McNorgan noted.
The investigators measured the amount of interconnectedness among different brain regions during the response inhibition task, which was repeated four times.
Patterns of interconnectivity were then fed into a deep-learning neural network that learned which patterns belonged to the ADHD group vs. those without ADHD (control group) and which patterns belonged to the high vs. low scorers on the IGT.
Caveats, cautionary notes
“The trained models are then tested on brain patterns they had never seen before, and we found the models would make the correct ADHD diagnosis and could tell apart the high and low scorers on the IGT 99% of the time,” Dr. McNorgan reported.
“The trained classifiers make predictions by calculating probabilities, and the neural networks learned how each of the brain connections contributes towards the final classification probability. We identified the set of brain connections that had the greatest influence on these probability calculations,” he noted.
Because the network classified both ADHD diagnosis and gambling task performance, the researchers were able to distinguish between connections that predicted ADHD when gambling performance was poor, as is typical for patients with ADHD, and those predicting ADHD when gambling performance was uncharacteristically good.
While more work is needed, the findings have potential clinical relevance, Dr. McNorgan said.
“ADHD can be difficult to diagnose reliably. If expense wasn’t an issue, fMRI may be able to help make diagnosis more reliable and objective,” he added.
However, because individuals with ADHD have different behavioral profiles, such as scoring atypically well on the IGT, additional studies using this approach may help identify brain networks “that are more or less active in those with ADHD that show a particular diagnostic trait,” he said.
“This could help inform what treatments might be more effective for those individuals,” Dr. McNorgan said.
Of course, he added, “clinicians’ diagnostic expertise is still required, as I would not base an ADHD diagnosis solely on the results of a single brain scan.”
No cross-validation
Commenting on the findings for this news organization, Vince Calhoun, PhD, neuroscientist and founding director of the Center for Translational Research in Neuroimaging and Data Science, Atlanta, a joint effort between Georgia State, Georgia Tech, and Emory University, noted some study limitations.
One cautionary note is that the investigators “appear to select relevant regions to include in the model based on activation to the task, then computed the predictions using the subset of regions that showed strong activation. The issue is this was done on the same data, so there was no cross-validation of this ‘feature selection’ step,” said Dr. Calhoun, who was not involved with the research. “This is a type of circularity which can lead to inflated accuracies,” he added.
Dr. Calhoun also noted that “multiple ADHD classification studies” have reported accuracies above 90%. In addition, there were only 80 participants in the current dataset.
“That’s relatively small for making strong claims about high accuracies as has been reported elsewhere,” he said.
Dr. McNorgan and Dr. Calhoun have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cannabis tied to self-harm, death in youth with mood disorders
Adolescents and young adults with mood disorders and cannabis use disorder (CUD) are at significantly increased risk for self-harm, all-cause mortality, homicide, and death by unintentional overdose, new research suggests.
Investigators found the risk for self-harm was three times higher, all-cause mortality was 59% higher, unintentional overdose was 2.5 times higher, and homicide was more than three times higher in those with versus without CUD.
“The take-home message of these findings is that we need to be aware of the perception that cannabis use is harmless, when it’s actually not,” lead author Cynthia Fontanella, PhD, associate professor of psychiatry, Ohio State University Wexner Medical Center, Columbus, said in an interview.
“We need to educate parents and clinicians that there are risks associated with cannabis, including increased risk for self-harm and death, and we need to effectively treat both cannabis use disorder and mood disorders,” she said.
The study was published online Jan. 19, 2021, in JAMA Pediatrics.
Little research in youth
“There has been very little research conducted on CUD in the adolescent population, and most studies have been conducted with adults,” Dr. Fontanella said.
Research on adults has shown that, even in people without mood disorders, cannabis use is associated with the early onset of mood disorders, psychosis, and anxiety disorders and has also been linked with suicidal behavior and increased risk for motor vehicle accidents, Dr. Fontanella said.
“We were motivated to conduct this study because we treat kids with depression and bipolar disorder and we noticed a high prevalence of CUD in this population, so we were curious about what its negative effects might be,” Dr. Fontanella recounted.
The researchers analyzed 7-year data drawn from Ohio Medicaid claims and linked to data from death certificates in 204,780 youths between the ages of 10 and 24 years (mean age was 17.2 years at the time of mood disorder diagnosis). Most were female, non-Hispanic White, enrolled in Medicaid because of poverty, and living in a metropolitan area (65.0%, 66.9%, 87.6%, and 77.1%, respectively).
Participants were followed up to 1 year from diagnosis until the end of enrollment, a self-harm event, or death.
Researchers included demographic, clinical, and treatment factors as covariates.
Close to three-quarters (72.7%) of the cohort had a depressive disorder, followed by unspecified/persistent mood disorder and bipolar disorder (14.9% and 12.4%, respectively). Comorbidities included ADHD (12.4%), anxiety disorder (12.3%), and other mental disorders (13.1%).
One -tenth of the cohort (10.3%) were diagnosed with CUD.
CUD treatment referrals
“Although CUD was associated with suicide in the unadjusted model, it was not significantly associated in adjusted models,” the authors reported.
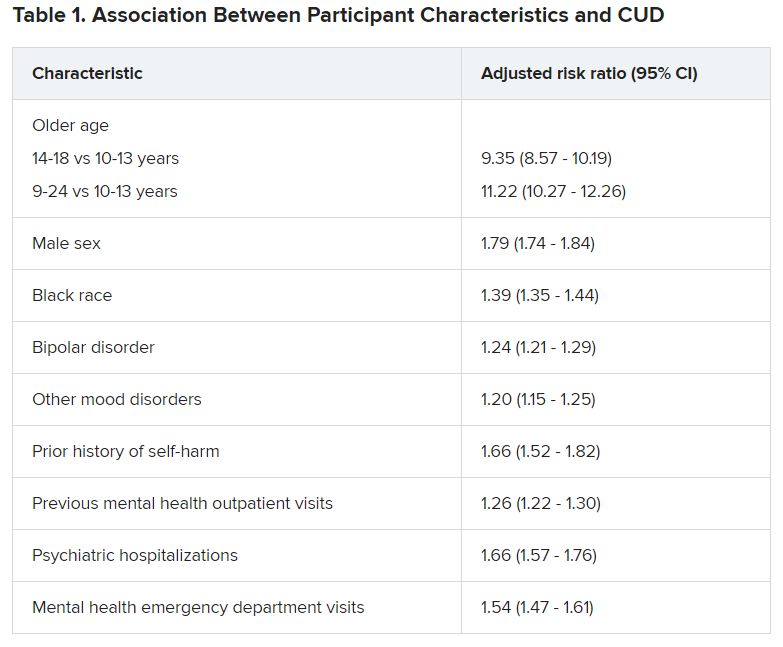
Dr. Fontanella noted that the risk for these adverse outcomes is greater among those who engage in heavy, frequent use or who use cannabis that has higher-potency tetrahydrocannabinol (THC) content.
Reasons why CUD might be associated with these adverse outcomes are that it can increase impulsivity, poor judgment, and clouded thinking, which may in turn increase the risk for self-harm behaviors, she said.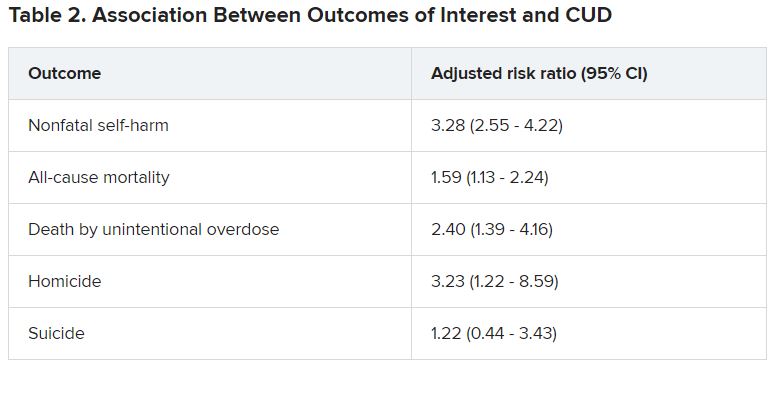
She recommended that clinicians refer youth with CUD for “effective treatments,” including family-based models and individual approaches, such as cognitive behavioral therapy and motivational enhancement therapy.
Open dialogue
In a comment, Wilfrid Noel Raby, MD, PhD, adjunct clinical professor, Albert Einstein College of Medicine, New York, noted that psychosis can occur in patients with CUD and mood disorders – especially bipolar disorder – but was not included as a study outcome. “I would have liked to see more data about that,” he said.
However, “The trend is that cannabis use is starting at younger and younger ages, which has all kinds of ramifications in terms of cerebral development.”
Christopher Hammond, MD, PhD, assistant professor of psychiatry, Johns Hopkins University, Baltimore, said: “Three major strengths of the study are the size of the sample, its longitudinal analysis, and that the authors controlled for a number of potential confounding variables.”
In light of the findings, Dr. Hammond recommended clinicians and other health professionals who work with young people “should screen for cannabis-related problems in youth with mood disorders.”
Dr. Hammond, who is the director of the Co-occurring Disorders in Adolescents and Young Adults Clinical and Research Program, Johns Hopkins Bayview Medical Center, Baltimore, and was not involved with the study, recommended counseling youth with mood disorders and their parents and families “regarding the potential adverse health effects related to cannabis use.”
He also recommended “open dialogue with youth with and without mental health conditions about misleading reports in the national media and advertising about cannabis’ health benefits.”
The study was funded by the National Institute of Mental Health. Dr. Fontanella reported receiving grants from the National Institute of Mental Health during the conduct of the study. Dr. Raby reported no relevant financial relationships. Dr. Hammond reported receiving research grant funding from the National Institutes of Health, the American Academy of Child & Adolescent Psychiatry, Substance Abuse Mental Health Services Administration, the National Network of Depression Centers, and the Armstrong Institute at Johns Hopkins Bayview and serves as a scientific adviser for the National Courts and Science Institute and as a subject matter expert for SAMHSA related to co-occurring substance use disorders and severe emotional disturbance in youth.
A version of this article first appeared on Medscape.com.
Adolescents and young adults with mood disorders and cannabis use disorder (CUD) are at significantly increased risk for self-harm, all-cause mortality, homicide, and death by unintentional overdose, new research suggests.
Investigators found the risk for self-harm was three times higher, all-cause mortality was 59% higher, unintentional overdose was 2.5 times higher, and homicide was more than three times higher in those with versus without CUD.
“The take-home message of these findings is that we need to be aware of the perception that cannabis use is harmless, when it’s actually not,” lead author Cynthia Fontanella, PhD, associate professor of psychiatry, Ohio State University Wexner Medical Center, Columbus, said in an interview.
“We need to educate parents and clinicians that there are risks associated with cannabis, including increased risk for self-harm and death, and we need to effectively treat both cannabis use disorder and mood disorders,” she said.
The study was published online Jan. 19, 2021, in JAMA Pediatrics.
Little research in youth
“There has been very little research conducted on CUD in the adolescent population, and most studies have been conducted with adults,” Dr. Fontanella said.
Research on adults has shown that, even in people without mood disorders, cannabis use is associated with the early onset of mood disorders, psychosis, and anxiety disorders and has also been linked with suicidal behavior and increased risk for motor vehicle accidents, Dr. Fontanella said.
“We were motivated to conduct this study because we treat kids with depression and bipolar disorder and we noticed a high prevalence of CUD in this population, so we were curious about what its negative effects might be,” Dr. Fontanella recounted.
The researchers analyzed 7-year data drawn from Ohio Medicaid claims and linked to data from death certificates in 204,780 youths between the ages of 10 and 24 years (mean age was 17.2 years at the time of mood disorder diagnosis). Most were female, non-Hispanic White, enrolled in Medicaid because of poverty, and living in a metropolitan area (65.0%, 66.9%, 87.6%, and 77.1%, respectively).
Participants were followed up to 1 year from diagnosis until the end of enrollment, a self-harm event, or death.
Researchers included demographic, clinical, and treatment factors as covariates.
Close to three-quarters (72.7%) of the cohort had a depressive disorder, followed by unspecified/persistent mood disorder and bipolar disorder (14.9% and 12.4%, respectively). Comorbidities included ADHD (12.4%), anxiety disorder (12.3%), and other mental disorders (13.1%).
One -tenth of the cohort (10.3%) were diagnosed with CUD.
CUD treatment referrals
“Although CUD was associated with suicide in the unadjusted model, it was not significantly associated in adjusted models,” the authors reported.

Dr. Fontanella noted that the risk for these adverse outcomes is greater among those who engage in heavy, frequent use or who use cannabis that has higher-potency tetrahydrocannabinol (THC) content.
Reasons why CUD might be associated with these adverse outcomes are that it can increase impulsivity, poor judgment, and clouded thinking, which may in turn increase the risk for self-harm behaviors, she said.
She recommended that clinicians refer youth with CUD for “effective treatments,” including family-based models and individual approaches, such as cognitive behavioral therapy and motivational enhancement therapy.
Open dialogue
In a comment, Wilfrid Noel Raby, MD, PhD, adjunct clinical professor, Albert Einstein College of Medicine, New York, noted that psychosis can occur in patients with CUD and mood disorders – especially bipolar disorder – but was not included as a study outcome. “I would have liked to see more data about that,” he said.
However, “The trend is that cannabis use is starting at younger and younger ages, which has all kinds of ramifications in terms of cerebral development.”
Christopher Hammond, MD, PhD, assistant professor of psychiatry, Johns Hopkins University, Baltimore, said: “Three major strengths of the study are the size of the sample, its longitudinal analysis, and that the authors controlled for a number of potential confounding variables.”
In light of the findings, Dr. Hammond recommended clinicians and other health professionals who work with young people “should screen for cannabis-related problems in youth with mood disorders.”
Dr. Hammond, who is the director of the Co-occurring Disorders in Adolescents and Young Adults Clinical and Research Program, Johns Hopkins Bayview Medical Center, Baltimore, and was not involved with the study, recommended counseling youth with mood disorders and their parents and families “regarding the potential adverse health effects related to cannabis use.”
He also recommended “open dialogue with youth with and without mental health conditions about misleading reports in the national media and advertising about cannabis’ health benefits.”
The study was funded by the National Institute of Mental Health. Dr. Fontanella reported receiving grants from the National Institute of Mental Health during the conduct of the study. Dr. Raby reported no relevant financial relationships. Dr. Hammond reported receiving research grant funding from the National Institutes of Health, the American Academy of Child & Adolescent Psychiatry, Substance Abuse Mental Health Services Administration, the National Network of Depression Centers, and the Armstrong Institute at Johns Hopkins Bayview and serves as a scientific adviser for the National Courts and Science Institute and as a subject matter expert for SAMHSA related to co-occurring substance use disorders and severe emotional disturbance in youth.
A version of this article first appeared on Medscape.com.
Adolescents and young adults with mood disorders and cannabis use disorder (CUD) are at significantly increased risk for self-harm, all-cause mortality, homicide, and death by unintentional overdose, new research suggests.
Investigators found the risk for self-harm was three times higher, all-cause mortality was 59% higher, unintentional overdose was 2.5 times higher, and homicide was more than three times higher in those with versus without CUD.
“The take-home message of these findings is that we need to be aware of the perception that cannabis use is harmless, when it’s actually not,” lead author Cynthia Fontanella, PhD, associate professor of psychiatry, Ohio State University Wexner Medical Center, Columbus, said in an interview.
“We need to educate parents and clinicians that there are risks associated with cannabis, including increased risk for self-harm and death, and we need to effectively treat both cannabis use disorder and mood disorders,” she said.
The study was published online Jan. 19, 2021, in JAMA Pediatrics.
Little research in youth
“There has been very little research conducted on CUD in the adolescent population, and most studies have been conducted with adults,” Dr. Fontanella said.
Research on adults has shown that, even in people without mood disorders, cannabis use is associated with the early onset of mood disorders, psychosis, and anxiety disorders and has also been linked with suicidal behavior and increased risk for motor vehicle accidents, Dr. Fontanella said.
“We were motivated to conduct this study because we treat kids with depression and bipolar disorder and we noticed a high prevalence of CUD in this population, so we were curious about what its negative effects might be,” Dr. Fontanella recounted.
The researchers analyzed 7-year data drawn from Ohio Medicaid claims and linked to data from death certificates in 204,780 youths between the ages of 10 and 24 years (mean age was 17.2 years at the time of mood disorder diagnosis). Most were female, non-Hispanic White, enrolled in Medicaid because of poverty, and living in a metropolitan area (65.0%, 66.9%, 87.6%, and 77.1%, respectively).
Participants were followed up to 1 year from diagnosis until the end of enrollment, a self-harm event, or death.
Researchers included demographic, clinical, and treatment factors as covariates.
Close to three-quarters (72.7%) of the cohort had a depressive disorder, followed by unspecified/persistent mood disorder and bipolar disorder (14.9% and 12.4%, respectively). Comorbidities included ADHD (12.4%), anxiety disorder (12.3%), and other mental disorders (13.1%).
One -tenth of the cohort (10.3%) were diagnosed with CUD.
CUD treatment referrals
“Although CUD was associated with suicide in the unadjusted model, it was not significantly associated in adjusted models,” the authors reported.

Dr. Fontanella noted that the risk for these adverse outcomes is greater among those who engage in heavy, frequent use or who use cannabis that has higher-potency tetrahydrocannabinol (THC) content.
Reasons why CUD might be associated with these adverse outcomes are that it can increase impulsivity, poor judgment, and clouded thinking, which may in turn increase the risk for self-harm behaviors, she said.
She recommended that clinicians refer youth with CUD for “effective treatments,” including family-based models and individual approaches, such as cognitive behavioral therapy and motivational enhancement therapy.
Open dialogue
In a comment, Wilfrid Noel Raby, MD, PhD, adjunct clinical professor, Albert Einstein College of Medicine, New York, noted that psychosis can occur in patients with CUD and mood disorders – especially bipolar disorder – but was not included as a study outcome. “I would have liked to see more data about that,” he said.
However, “The trend is that cannabis use is starting at younger and younger ages, which has all kinds of ramifications in terms of cerebral development.”
Christopher Hammond, MD, PhD, assistant professor of psychiatry, Johns Hopkins University, Baltimore, said: “Three major strengths of the study are the size of the sample, its longitudinal analysis, and that the authors controlled for a number of potential confounding variables.”
In light of the findings, Dr. Hammond recommended clinicians and other health professionals who work with young people “should screen for cannabis-related problems in youth with mood disorders.”
Dr. Hammond, who is the director of the Co-occurring Disorders in Adolescents and Young Adults Clinical and Research Program, Johns Hopkins Bayview Medical Center, Baltimore, and was not involved with the study, recommended counseling youth with mood disorders and their parents and families “regarding the potential adverse health effects related to cannabis use.”
He also recommended “open dialogue with youth with and without mental health conditions about misleading reports in the national media and advertising about cannabis’ health benefits.”
The study was funded by the National Institute of Mental Health. Dr. Fontanella reported receiving grants from the National Institute of Mental Health during the conduct of the study. Dr. Raby reported no relevant financial relationships. Dr. Hammond reported receiving research grant funding from the National Institutes of Health, the American Academy of Child & Adolescent Psychiatry, Substance Abuse Mental Health Services Administration, the National Network of Depression Centers, and the Armstrong Institute at Johns Hopkins Bayview and serves as a scientific adviser for the National Courts and Science Institute and as a subject matter expert for SAMHSA related to co-occurring substance use disorders and severe emotional disturbance in youth.
A version of this article first appeared on Medscape.com.
Kids already coping with mental disorders spiral as pandemic topples vital support systems
A bag of Doritos, that’s all Princess wanted.
Her mom calls her Princess, but her real name is Lindsey. She’s 17 and lives with her mom, Sandra, a nurse, outside Atlanta. On May 17, 2020, a Sunday, Lindsey decided she didn’t want breakfast; she wanted Doritos. So she left home and walked to Family Dollar, taking her pants off on the way, while her mom followed on foot, talking to the police on her phone as they went.
Lindsey has autism. It can be hard for her to communicate and navigate social situations. She thrives on routine and gets special help at school. Or got help, before the coronavirus pandemic closed schools and forced tens of millions of children to stay home. Sandra said that’s when their living hell started.
“It’s like her brain was wired,” she said. “She’d just put on her jacket, and she’s out the door. And I’m chasing her.”
On May 17, Sandra chased her all the way to Family Dollar. Hours later, Lindsey was in jail, charged with assaulting her mom. (KHN and NPR are not using the family’s last name.)
Lindsey is 1 of almost 3 million children in the United States who have a serious emotional or behavioral health condition. When the pandemic forced schools and doctors’ offices to close last spring, it also cut children off from the trained teachers and therapists who understand their needs.
As a result, many, like Lindsey, spiraled into EDs and even police custody. Federal data shows a nationwide surge of children in mental health crisis during the pandemic – a surge that’s further taxing an already overstretched safety net.
‘Take her’
Even after schools closed, Lindsey continued to wake up early, get dressed and wait for the bus. When she realized it had stopped coming, Sandra said, her daughter just started walking out of the house, wandering, a few times a week.
In those situations, Sandra did what many families in crisis report they’ve had to do since the pandemic began: Race through the short list of places she could call for help.
First, her state’s mental health crisis hotline. But they often put Sandra on hold.
“This is ridiculous,” she said of the wait. “It’s supposed to be a crisis team. But I’m on hold for 40, 50 minutes. And by the time you get on the phone, [the crisis] is done!”
Then there’s the local hospital’s ED, but Sandra said she had taken Lindsey there for previous crises and been told there isn’t much they can do.
That’s why, on May 17, when Lindsey walked to Family Dollar in just a red T-shirt and underwear to get that bag of Doritos, Sandra called the last option on her list: the police.
Sandra arrived at the store before the police and paid for the chips. According to Sandra and police records, when an officer approached, Lindsey grew agitated and hit her mom on the back, hard.
Sandra said she explained to the officer: “‘She’s autistic. You know, I’m okay. I’m a nurse. I just need to take her home and give her her medication.’ ”
Lindsey takes a mood stabilizer, but because she left home before breakfast, she hadn’t taken it that morning. The officer asked if Sandra wanted to take her to the nearest hospital.
The hospital wouldn’t be able to help Lindsey, Sandra said. It hadn’t before. “They already told me: ‘Ma’am, there’s nothing we can do.’ They just check her labs, it’s fine, and they ship her back home. There’s nothing [the hospital] can do,” she recalled telling the officer.
Sandra asked if the police could drive her daughter home so the teen could take her medication, but the officer said no, they couldn’t. The only other thing they could do, the officer said, was take Lindsey to jail for hitting her mom.
“I’ve tried everything,” Sandra said, exasperated. She paced the parking lot, feeling hopeless, sad and out of options. Finally, in tears, she told the officers: “Take her.”
Lindsey does not like to be touched and fought back when authorities tried to handcuff her. Several officers wrestled her to the ground. At that point, Sandra protested and said an officer threatened to arrest her, too, if she didn’t back away. Lindsey was taken to jail, where she spent much of the night until Sandra was able to post bail.
Clayton County Solicitor-General Charles Brooks denied that Sandra was threatened with arrest and said that, while Lindsey’s case is still pending, his office “is working to ensure that the resolution in this matter involves a plan for medication compliance and not punitive action.”
Sandra isn’t alone in her experience. Multiple families interviewed for this story reported similar experiences of calling in the police when a child was in crisis because caretakers didn’t feel they had any other option.
‘The whole system is really grinding to a halt’
Roughly 6% of U.S. children ages 6-17 years are living with serious emotional or behavioral difficulties, including children with autism, severe anxiety, depression and trauma-related mental health conditions.
Many of these children depend on schools for access to vital therapies. When schools and doctors’ offices stopped providing in-person services last spring, kids were untethered from the people and supports they rely on.
“The lack of in-person services is really detrimental,” said Susan Duffy, MD,a pediatrician and professor of emergency medicine at Brown University, Providence, R.I.
Marjorie, a mother in Florida, said her 15-year-old son has suffered during these disruptions. He has ADHD and oppositional defiant disorder, a condition marked by frequent and persistent hostility. Little things – like being asked to do schoolwork – can send him into a rage, leading to holes punched in walls, broken doors and violent threats. (The family’s last name or her son’s first name are not used to protect her son’s privacy and future prospects.)
The pandemic has shifted both school and her son’s therapy sessions online. But Marjorie said virtual therapy isn’t working because her son doesn’t focus well during sessions and tries to watch television instead. Lately, she has simply been canceling them.
“I was paying for appointments and there was no therapeutic value,” Marjorie said.
The issues cut across socioeconomic lines – affecting families with private insurance, like Marjorie, as well as those who receive coverage through Medicaid, a federal-state program that provides health insurance to low-income people and those with disabilities.
In the first few months of the pandemic, between March and May, children on Medicaid received 44% fewer outpatient mental health services – including therapy and in-home support – compared with the same time period in 2019, according to the Centers for Medicare & Medicaid Services. That’s even after accounting for increased telehealth appointments.
And while the nation’s EDs have seen a decline in overall visits, there was a relative increase in mental health visits for kids in 2020, compared with 2019.
The Centers for Disease Control and Prevention found that, from April to October 2020, hospitals across the United States saw a 24% increase in the proportion of mental health emergency visits for children aged 5-11 years, and a 31% increase for children aged 12-17.
“Not only are we seeing more children, more children are being admitted” to inpatient care.
That’s because there are fewer outpatient services now available to children, she said, and because the conditions of the children showing up at EDs “are more serious.”
This crisis is not only making life harder for these kids and their families, but it’s also stressing the entire health care system.
Child and adolescent psychiatrists working in hospitals around the country said children are increasingly “boarding” in EDs for days, waiting for inpatient admission to a regular hospital or psychiatric hospital.
Before the pandemic, there was already a shortage of inpatient psychiatric beds for children, said Christopher Bellonci, MD, a child psychiatrist at Judge Baker Children’s Center in Boston. That shortage has only gotten worse as hospitals cut capacity to allow for more physical distancing within psychiatric units.
“The whole system is really grinding to a halt at a time when we have unprecedented need,” Dr. Bellonci said.
‘A signal that the rest of your system doesn’t work’
Psychiatrists on the front lines share the frustrations of parents struggling to find help for their children.
Part of the problem is there have never been enough psychiatrists and therapists trained to work with children, intervening in the early stages of their illness, said Jennifer Havens, MD, a child psychiatrist at New York University.
“Tons of people showing up in emergency rooms in bad shape is a signal that the rest of your system doesn’t work,” she said.
Too often, Dr. Havens said, services aren’t available until children are older – and in crisis. “Often for people who don’t have access to services, we wait until they’re too big to be managed.”
While the pandemic has made life harder for Marjorie and her son in Florida, she said it has always been difficult to find the support and care he needs. Last fall, he needed a psychiatric evaluation, but the nearest specialist who would accept her commercial insurance was 100 miles away, in Alabama.
“Even when you have the money or you have the insurance, it is still a travesty,” Marjorie said. “You cannot get help for these kids.”
Parents are frustrated, and so are psychiatrists on the front lines. C.J. Glawe, MD, who leads the psychiatric crisis department at Nationwide Children’s Hospital in Columbus, Ohio, said that once a child is stabilized after a crisis it can be hard to explain to parents that they may not be able to find follow-up care anywhere near their home.
“Especially when I can clearly tell you I know exactly what you need, I just can’t give it to you,” Dr. Glawe said. “It’s demoralizing.”
When states and communities fail to provide children the services they need to live at home, kids can deteriorate and even wind up in jail, like Lindsey. At that point, Dr. Glawe said, the cost and level of care required will be even higher, whether that’s hospitalization or long stays in residential treatment facilities.
That’s exactly the scenario Sandra, Lindsey’s mom, is hoping to avoid for her Princess.
“For me, as a nurse and as a provider, that will be the last thing for my daughter,” she said. “It’s like [state and local leaders] leave it to the school and the parent to deal with, and they don’t care. And that’s the problem. It’s sad because, if I’m not here...”
Her voice trailed off as tears welled.
“She didn’t ask to have autism.”
To help families like Sandra’s and Marjorie’s, advocates said, all levels of government need to invest in creating a mental health system that’s accessible to anyone who needs it.
But given that many states have seen their revenues drop because of the pandemic, there’s a concern services will instead be cut – at a time when the need has never been greater.
This story is part of a reporting partnership that includes NPR, Illinois Public Media and Kaiser Health News. Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
A bag of Doritos, that’s all Princess wanted.
Her mom calls her Princess, but her real name is Lindsey. She’s 17 and lives with her mom, Sandra, a nurse, outside Atlanta. On May 17, 2020, a Sunday, Lindsey decided she didn’t want breakfast; she wanted Doritos. So she left home and walked to Family Dollar, taking her pants off on the way, while her mom followed on foot, talking to the police on her phone as they went.
Lindsey has autism. It can be hard for her to communicate and navigate social situations. She thrives on routine and gets special help at school. Or got help, before the coronavirus pandemic closed schools and forced tens of millions of children to stay home. Sandra said that’s when their living hell started.
“It’s like her brain was wired,” she said. “She’d just put on her jacket, and she’s out the door. And I’m chasing her.”
On May 17, Sandra chased her all the way to Family Dollar. Hours later, Lindsey was in jail, charged with assaulting her mom. (KHN and NPR are not using the family’s last name.)
Lindsey is 1 of almost 3 million children in the United States who have a serious emotional or behavioral health condition. When the pandemic forced schools and doctors’ offices to close last spring, it also cut children off from the trained teachers and therapists who understand their needs.
As a result, many, like Lindsey, spiraled into EDs and even police custody. Federal data shows a nationwide surge of children in mental health crisis during the pandemic – a surge that’s further taxing an already overstretched safety net.
‘Take her’
Even after schools closed, Lindsey continued to wake up early, get dressed and wait for the bus. When she realized it had stopped coming, Sandra said, her daughter just started walking out of the house, wandering, a few times a week.
In those situations, Sandra did what many families in crisis report they’ve had to do since the pandemic began: Race through the short list of places she could call for help.
First, her state’s mental health crisis hotline. But they often put Sandra on hold.
“This is ridiculous,” she said of the wait. “It’s supposed to be a crisis team. But I’m on hold for 40, 50 minutes. And by the time you get on the phone, [the crisis] is done!”
Then there’s the local hospital’s ED, but Sandra said she had taken Lindsey there for previous crises and been told there isn’t much they can do.
That’s why, on May 17, when Lindsey walked to Family Dollar in just a red T-shirt and underwear to get that bag of Doritos, Sandra called the last option on her list: the police.
Sandra arrived at the store before the police and paid for the chips. According to Sandra and police records, when an officer approached, Lindsey grew agitated and hit her mom on the back, hard.
Sandra said she explained to the officer: “‘She’s autistic. You know, I’m okay. I’m a nurse. I just need to take her home and give her her medication.’ ”
Lindsey takes a mood stabilizer, but because she left home before breakfast, she hadn’t taken it that morning. The officer asked if Sandra wanted to take her to the nearest hospital.
The hospital wouldn’t be able to help Lindsey, Sandra said. It hadn’t before. “They already told me: ‘Ma’am, there’s nothing we can do.’ They just check her labs, it’s fine, and they ship her back home. There’s nothing [the hospital] can do,” she recalled telling the officer.
Sandra asked if the police could drive her daughter home so the teen could take her medication, but the officer said no, they couldn’t. The only other thing they could do, the officer said, was take Lindsey to jail for hitting her mom.
“I’ve tried everything,” Sandra said, exasperated. She paced the parking lot, feeling hopeless, sad and out of options. Finally, in tears, she told the officers: “Take her.”
Lindsey does not like to be touched and fought back when authorities tried to handcuff her. Several officers wrestled her to the ground. At that point, Sandra protested and said an officer threatened to arrest her, too, if she didn’t back away. Lindsey was taken to jail, where she spent much of the night until Sandra was able to post bail.
Clayton County Solicitor-General Charles Brooks denied that Sandra was threatened with arrest and said that, while Lindsey’s case is still pending, his office “is working to ensure that the resolution in this matter involves a plan for medication compliance and not punitive action.”
Sandra isn’t alone in her experience. Multiple families interviewed for this story reported similar experiences of calling in the police when a child was in crisis because caretakers didn’t feel they had any other option.
‘The whole system is really grinding to a halt’
Roughly 6% of U.S. children ages 6-17 years are living with serious emotional or behavioral difficulties, including children with autism, severe anxiety, depression and trauma-related mental health conditions.
Many of these children depend on schools for access to vital therapies. When schools and doctors’ offices stopped providing in-person services last spring, kids were untethered from the people and supports they rely on.
“The lack of in-person services is really detrimental,” said Susan Duffy, MD,a pediatrician and professor of emergency medicine at Brown University, Providence, R.I.
Marjorie, a mother in Florida, said her 15-year-old son has suffered during these disruptions. He has ADHD and oppositional defiant disorder, a condition marked by frequent and persistent hostility. Little things – like being asked to do schoolwork – can send him into a rage, leading to holes punched in walls, broken doors and violent threats. (The family’s last name or her son’s first name are not used to protect her son’s privacy and future prospects.)
The pandemic has shifted both school and her son’s therapy sessions online. But Marjorie said virtual therapy isn’t working because her son doesn’t focus well during sessions and tries to watch television instead. Lately, she has simply been canceling them.
“I was paying for appointments and there was no therapeutic value,” Marjorie said.
The issues cut across socioeconomic lines – affecting families with private insurance, like Marjorie, as well as those who receive coverage through Medicaid, a federal-state program that provides health insurance to low-income people and those with disabilities.
In the first few months of the pandemic, between March and May, children on Medicaid received 44% fewer outpatient mental health services – including therapy and in-home support – compared with the same time period in 2019, according to the Centers for Medicare & Medicaid Services. That’s even after accounting for increased telehealth appointments.
And while the nation’s EDs have seen a decline in overall visits, there was a relative increase in mental health visits for kids in 2020, compared with 2019.
The Centers for Disease Control and Prevention found that, from April to October 2020, hospitals across the United States saw a 24% increase in the proportion of mental health emergency visits for children aged 5-11 years, and a 31% increase for children aged 12-17.
“Not only are we seeing more children, more children are being admitted” to inpatient care.
That’s because there are fewer outpatient services now available to children, she said, and because the conditions of the children showing up at EDs “are more serious.”
This crisis is not only making life harder for these kids and their families, but it’s also stressing the entire health care system.
Child and adolescent psychiatrists working in hospitals around the country said children are increasingly “boarding” in EDs for days, waiting for inpatient admission to a regular hospital or psychiatric hospital.
Before the pandemic, there was already a shortage of inpatient psychiatric beds for children, said Christopher Bellonci, MD, a child psychiatrist at Judge Baker Children’s Center in Boston. That shortage has only gotten worse as hospitals cut capacity to allow for more physical distancing within psychiatric units.
“The whole system is really grinding to a halt at a time when we have unprecedented need,” Dr. Bellonci said.
‘A signal that the rest of your system doesn’t work’
Psychiatrists on the front lines share the frustrations of parents struggling to find help for their children.
Part of the problem is there have never been enough psychiatrists and therapists trained to work with children, intervening in the early stages of their illness, said Jennifer Havens, MD, a child psychiatrist at New York University.
“Tons of people showing up in emergency rooms in bad shape is a signal that the rest of your system doesn’t work,” she said.
Too often, Dr. Havens said, services aren’t available until children are older – and in crisis. “Often for people who don’t have access to services, we wait until they’re too big to be managed.”
While the pandemic has made life harder for Marjorie and her son in Florida, she said it has always been difficult to find the support and care he needs. Last fall, he needed a psychiatric evaluation, but the nearest specialist who would accept her commercial insurance was 100 miles away, in Alabama.
“Even when you have the money or you have the insurance, it is still a travesty,” Marjorie said. “You cannot get help for these kids.”
Parents are frustrated, and so are psychiatrists on the front lines. C.J. Glawe, MD, who leads the psychiatric crisis department at Nationwide Children’s Hospital in Columbus, Ohio, said that once a child is stabilized after a crisis it can be hard to explain to parents that they may not be able to find follow-up care anywhere near their home.
“Especially when I can clearly tell you I know exactly what you need, I just can’t give it to you,” Dr. Glawe said. “It’s demoralizing.”
When states and communities fail to provide children the services they need to live at home, kids can deteriorate and even wind up in jail, like Lindsey. At that point, Dr. Glawe said, the cost and level of care required will be even higher, whether that’s hospitalization or long stays in residential treatment facilities.
That’s exactly the scenario Sandra, Lindsey’s mom, is hoping to avoid for her Princess.
“For me, as a nurse and as a provider, that will be the last thing for my daughter,” she said. “It’s like [state and local leaders] leave it to the school and the parent to deal with, and they don’t care. And that’s the problem. It’s sad because, if I’m not here...”
Her voice trailed off as tears welled.
“She didn’t ask to have autism.”
To help families like Sandra’s and Marjorie’s, advocates said, all levels of government need to invest in creating a mental health system that’s accessible to anyone who needs it.
But given that many states have seen their revenues drop because of the pandemic, there’s a concern services will instead be cut – at a time when the need has never been greater.
This story is part of a reporting partnership that includes NPR, Illinois Public Media and Kaiser Health News. Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
A bag of Doritos, that’s all Princess wanted.
Her mom calls her Princess, but her real name is Lindsey. She’s 17 and lives with her mom, Sandra, a nurse, outside Atlanta. On May 17, 2020, a Sunday, Lindsey decided she didn’t want breakfast; she wanted Doritos. So she left home and walked to Family Dollar, taking her pants off on the way, while her mom followed on foot, talking to the police on her phone as they went.
Lindsey has autism. It can be hard for her to communicate and navigate social situations. She thrives on routine and gets special help at school. Or got help, before the coronavirus pandemic closed schools and forced tens of millions of children to stay home. Sandra said that’s when their living hell started.
“It’s like her brain was wired,” she said. “She’d just put on her jacket, and she’s out the door. And I’m chasing her.”
On May 17, Sandra chased her all the way to Family Dollar. Hours later, Lindsey was in jail, charged with assaulting her mom. (KHN and NPR are not using the family’s last name.)
Lindsey is 1 of almost 3 million children in the United States who have a serious emotional or behavioral health condition. When the pandemic forced schools and doctors’ offices to close last spring, it also cut children off from the trained teachers and therapists who understand their needs.
As a result, many, like Lindsey, spiraled into EDs and even police custody. Federal data shows a nationwide surge of children in mental health crisis during the pandemic – a surge that’s further taxing an already overstretched safety net.
‘Take her’
Even after schools closed, Lindsey continued to wake up early, get dressed and wait for the bus. When she realized it had stopped coming, Sandra said, her daughter just started walking out of the house, wandering, a few times a week.
In those situations, Sandra did what many families in crisis report they’ve had to do since the pandemic began: Race through the short list of places she could call for help.
First, her state’s mental health crisis hotline. But they often put Sandra on hold.
“This is ridiculous,” she said of the wait. “It’s supposed to be a crisis team. But I’m on hold for 40, 50 minutes. And by the time you get on the phone, [the crisis] is done!”
Then there’s the local hospital’s ED, but Sandra said she had taken Lindsey there for previous crises and been told there isn’t much they can do.
That’s why, on May 17, when Lindsey walked to Family Dollar in just a red T-shirt and underwear to get that bag of Doritos, Sandra called the last option on her list: the police.
Sandra arrived at the store before the police and paid for the chips. According to Sandra and police records, when an officer approached, Lindsey grew agitated and hit her mom on the back, hard.
Sandra said she explained to the officer: “‘She’s autistic. You know, I’m okay. I’m a nurse. I just need to take her home and give her her medication.’ ”
Lindsey takes a mood stabilizer, but because she left home before breakfast, she hadn’t taken it that morning. The officer asked if Sandra wanted to take her to the nearest hospital.
The hospital wouldn’t be able to help Lindsey, Sandra said. It hadn’t before. “They already told me: ‘Ma’am, there’s nothing we can do.’ They just check her labs, it’s fine, and they ship her back home. There’s nothing [the hospital] can do,” she recalled telling the officer.
Sandra asked if the police could drive her daughter home so the teen could take her medication, but the officer said no, they couldn’t. The only other thing they could do, the officer said, was take Lindsey to jail for hitting her mom.
“I’ve tried everything,” Sandra said, exasperated. She paced the parking lot, feeling hopeless, sad and out of options. Finally, in tears, she told the officers: “Take her.”
Lindsey does not like to be touched and fought back when authorities tried to handcuff her. Several officers wrestled her to the ground. At that point, Sandra protested and said an officer threatened to arrest her, too, if she didn’t back away. Lindsey was taken to jail, where she spent much of the night until Sandra was able to post bail.
Clayton County Solicitor-General Charles Brooks denied that Sandra was threatened with arrest and said that, while Lindsey’s case is still pending, his office “is working to ensure that the resolution in this matter involves a plan for medication compliance and not punitive action.”
Sandra isn’t alone in her experience. Multiple families interviewed for this story reported similar experiences of calling in the police when a child was in crisis because caretakers didn’t feel they had any other option.
‘The whole system is really grinding to a halt’
Roughly 6% of U.S. children ages 6-17 years are living with serious emotional or behavioral difficulties, including children with autism, severe anxiety, depression and trauma-related mental health conditions.
Many of these children depend on schools for access to vital therapies. When schools and doctors’ offices stopped providing in-person services last spring, kids were untethered from the people and supports they rely on.
“The lack of in-person services is really detrimental,” said Susan Duffy, MD,a pediatrician and professor of emergency medicine at Brown University, Providence, R.I.
Marjorie, a mother in Florida, said her 15-year-old son has suffered during these disruptions. He has ADHD and oppositional defiant disorder, a condition marked by frequent and persistent hostility. Little things – like being asked to do schoolwork – can send him into a rage, leading to holes punched in walls, broken doors and violent threats. (The family’s last name or her son’s first name are not used to protect her son’s privacy and future prospects.)
The pandemic has shifted both school and her son’s therapy sessions online. But Marjorie said virtual therapy isn’t working because her son doesn’t focus well during sessions and tries to watch television instead. Lately, she has simply been canceling them.
“I was paying for appointments and there was no therapeutic value,” Marjorie said.
The issues cut across socioeconomic lines – affecting families with private insurance, like Marjorie, as well as those who receive coverage through Medicaid, a federal-state program that provides health insurance to low-income people and those with disabilities.
In the first few months of the pandemic, between March and May, children on Medicaid received 44% fewer outpatient mental health services – including therapy and in-home support – compared with the same time period in 2019, according to the Centers for Medicare & Medicaid Services. That’s even after accounting for increased telehealth appointments.
And while the nation’s EDs have seen a decline in overall visits, there was a relative increase in mental health visits for kids in 2020, compared with 2019.
The Centers for Disease Control and Prevention found that, from April to October 2020, hospitals across the United States saw a 24% increase in the proportion of mental health emergency visits for children aged 5-11 years, and a 31% increase for children aged 12-17.
“Not only are we seeing more children, more children are being admitted” to inpatient care.
That’s because there are fewer outpatient services now available to children, she said, and because the conditions of the children showing up at EDs “are more serious.”
This crisis is not only making life harder for these kids and their families, but it’s also stressing the entire health care system.
Child and adolescent psychiatrists working in hospitals around the country said children are increasingly “boarding” in EDs for days, waiting for inpatient admission to a regular hospital or psychiatric hospital.
Before the pandemic, there was already a shortage of inpatient psychiatric beds for children, said Christopher Bellonci, MD, a child psychiatrist at Judge Baker Children’s Center in Boston. That shortage has only gotten worse as hospitals cut capacity to allow for more physical distancing within psychiatric units.
“The whole system is really grinding to a halt at a time when we have unprecedented need,” Dr. Bellonci said.
‘A signal that the rest of your system doesn’t work’
Psychiatrists on the front lines share the frustrations of parents struggling to find help for their children.
Part of the problem is there have never been enough psychiatrists and therapists trained to work with children, intervening in the early stages of their illness, said Jennifer Havens, MD, a child psychiatrist at New York University.
“Tons of people showing up in emergency rooms in bad shape is a signal that the rest of your system doesn’t work,” she said.
Too often, Dr. Havens said, services aren’t available until children are older – and in crisis. “Often for people who don’t have access to services, we wait until they’re too big to be managed.”
While the pandemic has made life harder for Marjorie and her son in Florida, she said it has always been difficult to find the support and care he needs. Last fall, he needed a psychiatric evaluation, but the nearest specialist who would accept her commercial insurance was 100 miles away, in Alabama.
“Even when you have the money or you have the insurance, it is still a travesty,” Marjorie said. “You cannot get help for these kids.”
Parents are frustrated, and so are psychiatrists on the front lines. C.J. Glawe, MD, who leads the psychiatric crisis department at Nationwide Children’s Hospital in Columbus, Ohio, said that once a child is stabilized after a crisis it can be hard to explain to parents that they may not be able to find follow-up care anywhere near their home.
“Especially when I can clearly tell you I know exactly what you need, I just can’t give it to you,” Dr. Glawe said. “It’s demoralizing.”
When states and communities fail to provide children the services they need to live at home, kids can deteriorate and even wind up in jail, like Lindsey. At that point, Dr. Glawe said, the cost and level of care required will be even higher, whether that’s hospitalization or long stays in residential treatment facilities.
That’s exactly the scenario Sandra, Lindsey’s mom, is hoping to avoid for her Princess.
“For me, as a nurse and as a provider, that will be the last thing for my daughter,” she said. “It’s like [state and local leaders] leave it to the school and the parent to deal with, and they don’t care. And that’s the problem. It’s sad because, if I’m not here...”
Her voice trailed off as tears welled.
“She didn’t ask to have autism.”
To help families like Sandra’s and Marjorie’s, advocates said, all levels of government need to invest in creating a mental health system that’s accessible to anyone who needs it.
But given that many states have seen their revenues drop because of the pandemic, there’s a concern services will instead be cut – at a time when the need has never been greater.
This story is part of a reporting partnership that includes NPR, Illinois Public Media and Kaiser Health News. Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Maternal autoimmune disease raises children’s risk of ADHD
Maternal autoimmune diseases significantly increased the risk of ADHD in children, based on data from a large cohort study of more than 800,000 mothers and children and a subsequent meta-analysis.
“There is growing evidence that immune-related cells and proteins play a role in brain development and function and that maternal immune activation, including infection, autoimmune disease, and chronic inflammation during pregnancy, increases the risk of neurodevelopmental disorders among children,” wrote Timothy C. Nielsen, MPH, of the University of Sydney, and colleagues.
Previous research has examined a link between maternal autoimmune disorders and autism spectrum disorders in children, but associations with ADHD have not been well studied, they said.
In a population-based cohort study published in JAMA Pediatrics, the researchers identified 831,718 mothers and their 831,718 singleton infants in Australia. A total of 12,787 infants were born to mothers with an autoimmune diagnosis; 12,610 of them were matched to 50,440 control infants. ADHD was determined based on prescription for a stimulant treatment or a hospital diagnosis; children with a first ADHD event younger than 3 years were excluded.
In the total cohort of 63,050 infants, the presence of any maternal autoimmune disease was associated with a significantly increased risk of ADHD (hazard ratio, 1.30) as was the presence of several specific conditions: type 1 diabetes (HR, 2.23), psoriasis (HR, 1.66), and rheumatic fever or rheumatic carditis (HR, 1.75).
In addition, the researchers conducted a meta-analysis of the current study and four additional studies that yielded similar results. In the meta-analysis, the risk of ADHD was significantly associated with any maternal autoimmune disease in two studies (HR, 1.20); with maternal type 1 diabetes in four studies (HR, 1.53); with maternal hyperthyroidism in three studies (HR 1.15); and with maternal psoriasis in two studies (HR, 1.31).
Type 1 diabetes (T1D) had the highest HR and was the most often studied condition. However, “the observed association may also be related to nonimmune aspects of T1D, such as glycemic control, as nonautoimmune diabetes has been associated with ADHD among children,” the researchers wrote.
The study findings were limited by several factors, including the lack of outpatient and primary care records to identify maternal autoimmune disease, and lack of data on any medication used to managed diseases during pregnancy, as well as a lack of data on children with ADHD who might not have been treated with medication, the researchers noted. In addition, “given differences in study design and definitions, the pooled HRs presented in the meta-analysis need to be treated cautiously.”
However, the results were strengthened by the hybrid study design and large study population, and were generally consistent with previous research supporting an effect of maternal immune function on fetal neurodevelopment, they noted.
“Our study provides justification for future studies that examine the effect of maternal autoimmune diseases, including biomarkers, condition severity, and management in pregnancy and in the periconception period, on neurodevelopmental disorders in children,” they concluded.
Studies need to explore mechanism of action
The current study, with its hybrid design, adds support to the evidence of an association between any maternal autoimmune disease and ADHD in children, as well as an association between the specific conditions of type 1 diabetes, hyperthyroidism, and psoriasis in mothers and ADHD in children, Søren Dalsgaard, MD, of Aarhus (Denmark) University, wrote in an accompanying editorial.
“Importantly, Nielsen et al. emphasized in their article that, for the many different autoimmune diseases, different underlying mechanisms for the associations with disorders of the central nervous system were likely. They mentioned that, for T1D, low glycemic control may play a role, as type 2 diabetes has been associated with ADHD,” said Dr. Dalsgaard.
“Overall, these mechanisms are thought to include shared genetic and environmental risk factors or direct effects of maternal autoantibodies or cytokines crossing the placenta and altering the fetal immune response, which in turns leads to changes in the central nervous system,” Dr. Dalsgaard explained. However, the current study and previous studies have not identified the mechanisms to explain the association between ADHD in children and maternal autoimmune disease.
“To understand more about these associations, future studies should include researchers and data from different scientific disciplines, such as epidemiology, animal modeling, genetics, and neuroimmunology,” he concluded.
Association is not causality
Overall, the study findings add to the evidence of a correlation between autoimmune diseases and neurologic disease, said Herschel Lessin, MD, of Children’s Medical Group, Poughkeepsie, N.Y., in an interview. “Anything that might contribute to behavioral problems is worth investigating.” However, it is important to remember that association is not causation.
“There is some literature and evidence that autoimmune disease is associated with mental health issues, but the mechanisms of action are unknown,” said Dr. Lessin. ADHD is highly heritable, so the association may be caused by a similar genetic predisposition, or it may be something related to autoimmunity that is impacting the fetus by passing through the placenta.
The current study’s strengths include the large size and hybrid design, but limitations such as the identification of ADHD based on medication prescriptions may have led to underreporting, and identifying maternal autoimmune disease via inpatient hospital diagnosis could have selected for more severe disease, he said.
From a clinical standpoint, the study suggests a correlation that should be noted in a family history and potentially used to inform a diagnosis, especially in cases of type 1 diabetes where the association was strongest, Dr. Lessin said. The findings also support the value of further research to look for mechanisms that might explain whether the association between autoimmune disease and ADHD is autoimmune system causality or shared genetic susceptibility.
The study received no outside funding. One coauthor disclosed receiving grants from the National Blood Authority Australia and the Australian National Health and Medical Research Council during the conduct of the study. Dr. Dalsgaard had no financial conflicts to disclose. Dr. Lessin disclosed serving as editor of the ADHD toolkit for the American Academy of Pediatrics and coauthor of the current ADHD clinical guidelines. He also serves in advisory capacity to Cognoa, a company involved in diagnosis of autism, and Corium/KemPharm, companies involved in the development of ADHD treatments.
Maternal autoimmune diseases significantly increased the risk of ADHD in children, based on data from a large cohort study of more than 800,000 mothers and children and a subsequent meta-analysis.
“There is growing evidence that immune-related cells and proteins play a role in brain development and function and that maternal immune activation, including infection, autoimmune disease, and chronic inflammation during pregnancy, increases the risk of neurodevelopmental disorders among children,” wrote Timothy C. Nielsen, MPH, of the University of Sydney, and colleagues.
Previous research has examined a link between maternal autoimmune disorders and autism spectrum disorders in children, but associations with ADHD have not been well studied, they said.
In a population-based cohort study published in JAMA Pediatrics, the researchers identified 831,718 mothers and their 831,718 singleton infants in Australia. A total of 12,787 infants were born to mothers with an autoimmune diagnosis; 12,610 of them were matched to 50,440 control infants. ADHD was determined based on prescription for a stimulant treatment or a hospital diagnosis; children with a first ADHD event younger than 3 years were excluded.
In the total cohort of 63,050 infants, the presence of any maternal autoimmune disease was associated with a significantly increased risk of ADHD (hazard ratio, 1.30) as was the presence of several specific conditions: type 1 diabetes (HR, 2.23), psoriasis (HR, 1.66), and rheumatic fever or rheumatic carditis (HR, 1.75).
In addition, the researchers conducted a meta-analysis of the current study and four additional studies that yielded similar results. In the meta-analysis, the risk of ADHD was significantly associated with any maternal autoimmune disease in two studies (HR, 1.20); with maternal type 1 diabetes in four studies (HR, 1.53); with maternal hyperthyroidism in three studies (HR 1.15); and with maternal psoriasis in two studies (HR, 1.31).
Type 1 diabetes (T1D) had the highest HR and was the most often studied condition. However, “the observed association may also be related to nonimmune aspects of T1D, such as glycemic control, as nonautoimmune diabetes has been associated with ADHD among children,” the researchers wrote.
The study findings were limited by several factors, including the lack of outpatient and primary care records to identify maternal autoimmune disease, and lack of data on any medication used to managed diseases during pregnancy, as well as a lack of data on children with ADHD who might not have been treated with medication, the researchers noted. In addition, “given differences in study design and definitions, the pooled HRs presented in the meta-analysis need to be treated cautiously.”
However, the results were strengthened by the hybrid study design and large study population, and were generally consistent with previous research supporting an effect of maternal immune function on fetal neurodevelopment, they noted.
“Our study provides justification for future studies that examine the effect of maternal autoimmune diseases, including biomarkers, condition severity, and management in pregnancy and in the periconception period, on neurodevelopmental disorders in children,” they concluded.
Studies need to explore mechanism of action
The current study, with its hybrid design, adds support to the evidence of an association between any maternal autoimmune disease and ADHD in children, as well as an association between the specific conditions of type 1 diabetes, hyperthyroidism, and psoriasis in mothers and ADHD in children, Søren Dalsgaard, MD, of Aarhus (Denmark) University, wrote in an accompanying editorial.
“Importantly, Nielsen et al. emphasized in their article that, for the many different autoimmune diseases, different underlying mechanisms for the associations with disorders of the central nervous system were likely. They mentioned that, for T1D, low glycemic control may play a role, as type 2 diabetes has been associated with ADHD,” said Dr. Dalsgaard.
“Overall, these mechanisms are thought to include shared genetic and environmental risk factors or direct effects of maternal autoantibodies or cytokines crossing the placenta and altering the fetal immune response, which in turns leads to changes in the central nervous system,” Dr. Dalsgaard explained. However, the current study and previous studies have not identified the mechanisms to explain the association between ADHD in children and maternal autoimmune disease.
“To understand more about these associations, future studies should include researchers and data from different scientific disciplines, such as epidemiology, animal modeling, genetics, and neuroimmunology,” he concluded.
Association is not causality
Overall, the study findings add to the evidence of a correlation between autoimmune diseases and neurologic disease, said Herschel Lessin, MD, of Children’s Medical Group, Poughkeepsie, N.Y., in an interview. “Anything that might contribute to behavioral problems is worth investigating.” However, it is important to remember that association is not causation.
“There is some literature and evidence that autoimmune disease is associated with mental health issues, but the mechanisms of action are unknown,” said Dr. Lessin. ADHD is highly heritable, so the association may be caused by a similar genetic predisposition, or it may be something related to autoimmunity that is impacting the fetus by passing through the placenta.
The current study’s strengths include the large size and hybrid design, but limitations such as the identification of ADHD based on medication prescriptions may have led to underreporting, and identifying maternal autoimmune disease via inpatient hospital diagnosis could have selected for more severe disease, he said.
From a clinical standpoint, the study suggests a correlation that should be noted in a family history and potentially used to inform a diagnosis, especially in cases of type 1 diabetes where the association was strongest, Dr. Lessin said. The findings also support the value of further research to look for mechanisms that might explain whether the association between autoimmune disease and ADHD is autoimmune system causality or shared genetic susceptibility.
The study received no outside funding. One coauthor disclosed receiving grants from the National Blood Authority Australia and the Australian National Health and Medical Research Council during the conduct of the study. Dr. Dalsgaard had no financial conflicts to disclose. Dr. Lessin disclosed serving as editor of the ADHD toolkit for the American Academy of Pediatrics and coauthor of the current ADHD clinical guidelines. He also serves in advisory capacity to Cognoa, a company involved in diagnosis of autism, and Corium/KemPharm, companies involved in the development of ADHD treatments.
Maternal autoimmune diseases significantly increased the risk of ADHD in children, based on data from a large cohort study of more than 800,000 mothers and children and a subsequent meta-analysis.
“There is growing evidence that immune-related cells and proteins play a role in brain development and function and that maternal immune activation, including infection, autoimmune disease, and chronic inflammation during pregnancy, increases the risk of neurodevelopmental disorders among children,” wrote Timothy C. Nielsen, MPH, of the University of Sydney, and colleagues.
Previous research has examined a link between maternal autoimmune disorders and autism spectrum disorders in children, but associations with ADHD have not been well studied, they said.
In a population-based cohort study published in JAMA Pediatrics, the researchers identified 831,718 mothers and their 831,718 singleton infants in Australia. A total of 12,787 infants were born to mothers with an autoimmune diagnosis; 12,610 of them were matched to 50,440 control infants. ADHD was determined based on prescription for a stimulant treatment or a hospital diagnosis; children with a first ADHD event younger than 3 years were excluded.
In the total cohort of 63,050 infants, the presence of any maternal autoimmune disease was associated with a significantly increased risk of ADHD (hazard ratio, 1.30) as was the presence of several specific conditions: type 1 diabetes (HR, 2.23), psoriasis (HR, 1.66), and rheumatic fever or rheumatic carditis (HR, 1.75).
In addition, the researchers conducted a meta-analysis of the current study and four additional studies that yielded similar results. In the meta-analysis, the risk of ADHD was significantly associated with any maternal autoimmune disease in two studies (HR, 1.20); with maternal type 1 diabetes in four studies (HR, 1.53); with maternal hyperthyroidism in three studies (HR 1.15); and with maternal psoriasis in two studies (HR, 1.31).
Type 1 diabetes (T1D) had the highest HR and was the most often studied condition. However, “the observed association may also be related to nonimmune aspects of T1D, such as glycemic control, as nonautoimmune diabetes has been associated with ADHD among children,” the researchers wrote.
The study findings were limited by several factors, including the lack of outpatient and primary care records to identify maternal autoimmune disease, and lack of data on any medication used to managed diseases during pregnancy, as well as a lack of data on children with ADHD who might not have been treated with medication, the researchers noted. In addition, “given differences in study design and definitions, the pooled HRs presented in the meta-analysis need to be treated cautiously.”
However, the results were strengthened by the hybrid study design and large study population, and were generally consistent with previous research supporting an effect of maternal immune function on fetal neurodevelopment, they noted.
“Our study provides justification for future studies that examine the effect of maternal autoimmune diseases, including biomarkers, condition severity, and management in pregnancy and in the periconception period, on neurodevelopmental disorders in children,” they concluded.
Studies need to explore mechanism of action
The current study, with its hybrid design, adds support to the evidence of an association between any maternal autoimmune disease and ADHD in children, as well as an association between the specific conditions of type 1 diabetes, hyperthyroidism, and psoriasis in mothers and ADHD in children, Søren Dalsgaard, MD, of Aarhus (Denmark) University, wrote in an accompanying editorial.
“Importantly, Nielsen et al. emphasized in their article that, for the many different autoimmune diseases, different underlying mechanisms for the associations with disorders of the central nervous system were likely. They mentioned that, for T1D, low glycemic control may play a role, as type 2 diabetes has been associated with ADHD,” said Dr. Dalsgaard.
“Overall, these mechanisms are thought to include shared genetic and environmental risk factors or direct effects of maternal autoantibodies or cytokines crossing the placenta and altering the fetal immune response, which in turns leads to changes in the central nervous system,” Dr. Dalsgaard explained. However, the current study and previous studies have not identified the mechanisms to explain the association between ADHD in children and maternal autoimmune disease.
“To understand more about these associations, future studies should include researchers and data from different scientific disciplines, such as epidemiology, animal modeling, genetics, and neuroimmunology,” he concluded.
Association is not causality
Overall, the study findings add to the evidence of a correlation between autoimmune diseases and neurologic disease, said Herschel Lessin, MD, of Children’s Medical Group, Poughkeepsie, N.Y., in an interview. “Anything that might contribute to behavioral problems is worth investigating.” However, it is important to remember that association is not causation.
“There is some literature and evidence that autoimmune disease is associated with mental health issues, but the mechanisms of action are unknown,” said Dr. Lessin. ADHD is highly heritable, so the association may be caused by a similar genetic predisposition, or it may be something related to autoimmunity that is impacting the fetus by passing through the placenta.
The current study’s strengths include the large size and hybrid design, but limitations such as the identification of ADHD based on medication prescriptions may have led to underreporting, and identifying maternal autoimmune disease via inpatient hospital diagnosis could have selected for more severe disease, he said.
From a clinical standpoint, the study suggests a correlation that should be noted in a family history and potentially used to inform a diagnosis, especially in cases of type 1 diabetes where the association was strongest, Dr. Lessin said. The findings also support the value of further research to look for mechanisms that might explain whether the association between autoimmune disease and ADHD is autoimmune system causality or shared genetic susceptibility.
The study received no outside funding. One coauthor disclosed receiving grants from the National Blood Authority Australia and the Australian National Health and Medical Research Council during the conduct of the study. Dr. Dalsgaard had no financial conflicts to disclose. Dr. Lessin disclosed serving as editor of the ADHD toolkit for the American Academy of Pediatrics and coauthor of the current ADHD clinical guidelines. He also serves in advisory capacity to Cognoa, a company involved in diagnosis of autism, and Corium/KemPharm, companies involved in the development of ADHD treatments.
FROM JAMA PEDIATRICS



















