User login
ZEUS: Second-generation DES with 30 days DAPT best in bleeding-risk patients
PARIS – The use of a second-generation zotarolimus-eluting coronary stent rather than a bare metal stent in conjunction with 30 days of dual antiplatelet therapy (DAPT) in patients deemed at high bleeding risk results in lower 1-year rates of major adverse cardiovascular events and stent thrombosis, according to a prespecified analysis of the ZEUS trial presented at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
Asked if the ZEUS results mean it’s time to take bare-metal stents (BMS) out of the cupboard and get rid of them, the study presenter, Dr. Marco Valgimigli, replied “I did so already.”
ZEUS (Zotarolimus-Eluting Endeavor Sprint Stent in Uncertain DES Candidates Study) was an open-label, prospective study in which 1,606 patients undergoing urgent or emergent percutaneous coronary intervention were randomized to a thin-strut BMS or the zotarolimus-eluting Endeavor Sprint stent, a second-generation hydrophilic polymer-based device that, uniquely, elutes 100% of the drug within the first 2 weeks. All participants were placed on an intended 30-day regimen of DAPT. The study was conducted in four European countries, explained Dr. Valgimigli of Erasmus University in Rotterdam.
This prespecified analysis focused on the 828 patients with one or more factors placing them at high bleeding risk, since the use of a drug-eluting stent (DES) with a 30-day DAPT protocol hadn’t been adequately studied in that setting, the cardiologist noted.
High bleeding risk was defined by one or more of the following: age greater than 80, being on oral anticoagulation therapy, a prior bleeding event, need for corticosteroid or NSAID therapy, known anemia, or a bleeding diathesis; 47% of study participants had more than one of these criteria.
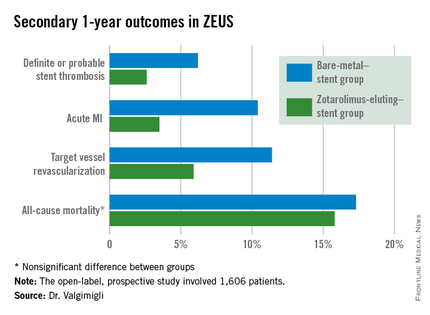
The primary study endpoint was the composite of all-cause mortality, acute MI, or target vessel revascularization through 1 year of follow-up. The rate was 29% in the BMS group, compared with 22.6% in the DES group, for a highly significant 26% relative risk reduction.
The zotarolimus-eluting stent group also fared significantly better in terms of stent thrombosis and the other prespecified secondary endpoints.
Asked how the ZEUS findings have affected his own clinical practice, Dr. Valgimigli replied, “My stent of choice in patients at high bleeding risk is a second-generation DES. Since there aren’t data showing a specific second-generation DES is preferable, basically whatever I have I implant.”
He sticks to the 30-day DAPT regimen featured in the ZEUS protocol except under specific circumstances, which were allowed under the protocol. One involves staged PCI procedures, in which case the 30 days of DAPT begins after the last stent is implanted, even though the patient has been on DAPT in the interim. The other circumstance where he goes beyond 30 days of DAPT in a patient on a second-generation DES is if an ischemic event occurs down the road: “That patient is put back on DAPT and left there,” he said.
In response to another question, Dr. Valgimigli said he doesn’t believe the lower stent thrombosis rate seen in the Endeavor Sprint group in ZEUS is unique to that stent.
“If you look at any BMS versus DES study, taking the first-generation DES out of the picture, it’s quite clear that the second-generation DES are much safer than a BMS,” according to the cardiologist.
The ZEUS study was sponsored by the University of Ferrara (Italy) and funded by Medtronic. Dr. Valgimigli serves as a consultant to and/or on speakers’ bureaus for Medtronic and more than half a dozen other pharmaceutical and medical devices companies.
PARIS – The use of a second-generation zotarolimus-eluting coronary stent rather than a bare metal stent in conjunction with 30 days of dual antiplatelet therapy (DAPT) in patients deemed at high bleeding risk results in lower 1-year rates of major adverse cardiovascular events and stent thrombosis, according to a prespecified analysis of the ZEUS trial presented at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
Asked if the ZEUS results mean it’s time to take bare-metal stents (BMS) out of the cupboard and get rid of them, the study presenter, Dr. Marco Valgimigli, replied “I did so already.”
ZEUS (Zotarolimus-Eluting Endeavor Sprint Stent in Uncertain DES Candidates Study) was an open-label, prospective study in which 1,606 patients undergoing urgent or emergent percutaneous coronary intervention were randomized to a thin-strut BMS or the zotarolimus-eluting Endeavor Sprint stent, a second-generation hydrophilic polymer-based device that, uniquely, elutes 100% of the drug within the first 2 weeks. All participants were placed on an intended 30-day regimen of DAPT. The study was conducted in four European countries, explained Dr. Valgimigli of Erasmus University in Rotterdam.
This prespecified analysis focused on the 828 patients with one or more factors placing them at high bleeding risk, since the use of a drug-eluting stent (DES) with a 30-day DAPT protocol hadn’t been adequately studied in that setting, the cardiologist noted.
High bleeding risk was defined by one or more of the following: age greater than 80, being on oral anticoagulation therapy, a prior bleeding event, need for corticosteroid or NSAID therapy, known anemia, or a bleeding diathesis; 47% of study participants had more than one of these criteria.
The primary study endpoint was the composite of all-cause mortality, acute MI, or target vessel revascularization through 1 year of follow-up. The rate was 29% in the BMS group, compared with 22.6% in the DES group, for a highly significant 26% relative risk reduction.
The zotarolimus-eluting stent group also fared significantly better in terms of stent thrombosis and the other prespecified secondary endpoints.

Asked how the ZEUS findings have affected his own clinical practice, Dr. Valgimigli replied, “My stent of choice in patients at high bleeding risk is a second-generation DES. Since there aren’t data showing a specific second-generation DES is preferable, basically whatever I have I implant.”
He sticks to the 30-day DAPT regimen featured in the ZEUS protocol except under specific circumstances, which were allowed under the protocol. One involves staged PCI procedures, in which case the 30 days of DAPT begins after the last stent is implanted, even though the patient has been on DAPT in the interim. The other circumstance where he goes beyond 30 days of DAPT in a patient on a second-generation DES is if an ischemic event occurs down the road: “That patient is put back on DAPT and left there,” he said.
In response to another question, Dr. Valgimigli said he doesn’t believe the lower stent thrombosis rate seen in the Endeavor Sprint group in ZEUS is unique to that stent.
“If you look at any BMS versus DES study, taking the first-generation DES out of the picture, it’s quite clear that the second-generation DES are much safer than a BMS,” according to the cardiologist.
The ZEUS study was sponsored by the University of Ferrara (Italy) and funded by Medtronic. Dr. Valgimigli serves as a consultant to and/or on speakers’ bureaus for Medtronic and more than half a dozen other pharmaceutical and medical devices companies.
PARIS – The use of a second-generation zotarolimus-eluting coronary stent rather than a bare metal stent in conjunction with 30 days of dual antiplatelet therapy (DAPT) in patients deemed at high bleeding risk results in lower 1-year rates of major adverse cardiovascular events and stent thrombosis, according to a prespecified analysis of the ZEUS trial presented at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
Asked if the ZEUS results mean it’s time to take bare-metal stents (BMS) out of the cupboard and get rid of them, the study presenter, Dr. Marco Valgimigli, replied “I did so already.”
ZEUS (Zotarolimus-Eluting Endeavor Sprint Stent in Uncertain DES Candidates Study) was an open-label, prospective study in which 1,606 patients undergoing urgent or emergent percutaneous coronary intervention were randomized to a thin-strut BMS or the zotarolimus-eluting Endeavor Sprint stent, a second-generation hydrophilic polymer-based device that, uniquely, elutes 100% of the drug within the first 2 weeks. All participants were placed on an intended 30-day regimen of DAPT. The study was conducted in four European countries, explained Dr. Valgimigli of Erasmus University in Rotterdam.
This prespecified analysis focused on the 828 patients with one or more factors placing them at high bleeding risk, since the use of a drug-eluting stent (DES) with a 30-day DAPT protocol hadn’t been adequately studied in that setting, the cardiologist noted.
High bleeding risk was defined by one or more of the following: age greater than 80, being on oral anticoagulation therapy, a prior bleeding event, need for corticosteroid or NSAID therapy, known anemia, or a bleeding diathesis; 47% of study participants had more than one of these criteria.
The primary study endpoint was the composite of all-cause mortality, acute MI, or target vessel revascularization through 1 year of follow-up. The rate was 29% in the BMS group, compared with 22.6% in the DES group, for a highly significant 26% relative risk reduction.
The zotarolimus-eluting stent group also fared significantly better in terms of stent thrombosis and the other prespecified secondary endpoints.

Asked how the ZEUS findings have affected his own clinical practice, Dr. Valgimigli replied, “My stent of choice in patients at high bleeding risk is a second-generation DES. Since there aren’t data showing a specific second-generation DES is preferable, basically whatever I have I implant.”
He sticks to the 30-day DAPT regimen featured in the ZEUS protocol except under specific circumstances, which were allowed under the protocol. One involves staged PCI procedures, in which case the 30 days of DAPT begins after the last stent is implanted, even though the patient has been on DAPT in the interim. The other circumstance where he goes beyond 30 days of DAPT in a patient on a second-generation DES is if an ischemic event occurs down the road: “That patient is put back on DAPT and left there,” he said.
In response to another question, Dr. Valgimigli said he doesn’t believe the lower stent thrombosis rate seen in the Endeavor Sprint group in ZEUS is unique to that stent.
“If you look at any BMS versus DES study, taking the first-generation DES out of the picture, it’s quite clear that the second-generation DES are much safer than a BMS,” according to the cardiologist.
The ZEUS study was sponsored by the University of Ferrara (Italy) and funded by Medtronic. Dr. Valgimigli serves as a consultant to and/or on speakers’ bureaus for Medtronic and more than half a dozen other pharmaceutical and medical devices companies.
AT EUROPCR 2015
Key clinical point: High bleeding risk patients fare significantly better with a second-generation drug-eluting stent and 30 days of dual antiplatelet therapy than with a bare-metal stent.
Major finding: The 1-year incidence of major adverse cardiovascular events was 29.6% in high bleeding risk patients who received a bare-metal stent and 22.6% in those who got a second-generation zotarolimus-eluting stent with 30 days of dual antiplatelet therapy.
Data source: This was a prespecified analysis of 828 high bleeding risk patients randomized to a bare-metal stent or a second-generation zotarolimus-eluting stent in conjunction with 30 days of DAPT and then followed prospectively for 1 year.
Disclosures: The ZEUS study was sponsored by the University of Ferrara (Italy) and funded by Medtronic. The presenter serves as a consultant and/or on speakers’ bureaus for Medtronic and more than half a dozen other pharmaceutical and medical devices companies.
Reports of TAVI leaflet thickening downplayed – for now
PARIS – Thought leaders in interventional cardiology have been quick to throw cold water on recent reports of valve leaflet thickening and abnormal leaflet motion being detected in roughly 10% of patients after transcatheter aortic valve implantation (TAVI) or surgical aortic valve replacement.
“The take home message for the interventional community is there is no need for clinicians to modify their practice in relation to patient selection, TAVI implantation, or follow-up protocols. Specifically, there is no role for systematic CT or transesophageal echocardiographic follow-up of asymptomatic TAVI patients because they’re not at clinical risk, and these additional procedures carry risk in themselves,” Dr. Bernard Prendergast said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“Whilst there is room here for speculation and conjecture, I think most of us are confident that some of these findings may represent imaging artifact or reflect the natural history of biological valve leaflets, which has never been examined in such detail in the past,” added Dr. Prendergast, director of the cardiac structural intervention program at Guys and St. Thomas’ Hospital in London and cochair of the special EuroPCR session devoted to the emerging data on valve leaflet abnormalities.
The session featured three separate studies totaling 345 patients who underwent sophisticated, high-resolution 4D CT imaging or transesophageal echocardiography 5 days or more following TAVI or, less frequently, after surgical aortic valve replacement. Roughly 10% of patients showed a spectrum of leaflet abnormalities: thickening, mildly impaired motion, and/or thin films believed to be thrombi.
The leaflet abnormalities weren’t associated with any particular valve. And, as was emphasized by Dr. Prendergast and other speakers, to date these abnormalities haven’t been associated with a single case of stroke, systemic embolism, or valve failure.
Indeed, more than 100,000 TAVI procedures have been performed worldwide, and stroke rates in contemporary randomized trials and large registries are in the 1%-2% range. That’s better than with surgical valve replacement, Dr. Prendergast observed.
“Nowadays we can see much more than we could in the past, when we worked with 2D echocardiography,” observed discussant Dr. Jeroen J. Bax, professor of cardiology and director of noninvasive cardiology imaging at Leiden (the Netherlands) University Medical Center. “We see things that we do not completely understand. We could say that technology has outpaced our clinical understanding. But although we see things, at the moment there is no consequence in terms of hemodynamic performance or clinical outcomes. And this phenomenon of leaflet thickening has been occurring with surgical aortic valve replacement for many, many years and we simply didn’t realize it.”
Dr. Franz-Josef Neumann, who led one of the three studies, reported that 4D CT on day 5 post TAVI revealed leaflet abnormalities, all completely asymptomatic, in 16 of 154 patients. Two-thirds of the study population were on dual antiplatelet therapy at the time, the rest on a single antiplatelet agent. Dual antiplatelet therapy didn’t protect against leaflet thickening or other abnormalities.
All 16 affected patients were placed on an oral vitamin K antagonist with a target international normalized ratio (INR) of 2.5-3.5. To date, 11 of the 16 have undergone follow-up high-resolution CT after a median of 77 days. The leaflet thickening was resolved in all instances, according to Dr. Neumann, medical director of the department of cardiology and angiology at the University of Freiburg (Germany).
Dr. Raj R. Makkar presented a study of 125 patients who underwent high-resolution imaging after TAVI. Importantly, none of those who were on warfarin as part of their post-TAVI regimen developed leaflet abnormalities.
But he cautioned his colleagues against overreacting to the studies he and Dr. Neumann presented by placing all of their TAVI patients on an oral anticoagulant. He noted that the current TAVI population is elderly and laden with many comorbid conditions, placing them at high risk for bleeding complications.
There is at present no standard, guideline-recommended antiplatelet/antithrombotic regimen for before, during, or after TAVI. Working out the optimal protective drug regimen in this population is now a priority in light of the leaflet abnormality findings, but it will take time and require careful study, said Dr. Makkar, associate director of the Cedars Sinai Heart Institute in Los Angeles.
“Everyone is talking about anticoagulation as the imminent solution. But I want to emphasize that it comes with a price in terms of bleeding. These images are beautiful in terms of spatial resolution, but we must resist our temptation while the industry works on designing less thrombogenic valves,” the cardiologist added.
Discussant Dr. Christoph K. Naber confessed he was “stunned” by the images of leaflet abnormalities.
“Although we haven’t seen any clinical consequences, we have to keep in mind that the group of patients is still small. We have very good experience with TAVI; it has saved the lives of many patients. We know it’s a very good therapy. But if we believe we can go further and offer it to younger, lower-risk patients who will have their device for a longer time, then we should take the time and money to understand what is going on here and what consequences it could have. It’s something we should closely watch, especially if we want to extend the indication,” said Dr. Naber, director of the department of cardiology and angiology at the Contilia Cardiovascular Center in Essen, Germany.
He disclosed that he serves as a consultant to Abbott Vascular, Biotronik, Medtronic, and The Medicines Company. Dr. Makkar has received research grants from Edwards Lifesciences, St. Jude Medical, and Boston Scientific. Dr. Prendergast is on the speakers’ bureau for Edwards Lifesciences.
PARIS – Thought leaders in interventional cardiology have been quick to throw cold water on recent reports of valve leaflet thickening and abnormal leaflet motion being detected in roughly 10% of patients after transcatheter aortic valve implantation (TAVI) or surgical aortic valve replacement.
“The take home message for the interventional community is there is no need for clinicians to modify their practice in relation to patient selection, TAVI implantation, or follow-up protocols. Specifically, there is no role for systematic CT or transesophageal echocardiographic follow-up of asymptomatic TAVI patients because they’re not at clinical risk, and these additional procedures carry risk in themselves,” Dr. Bernard Prendergast said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“Whilst there is room here for speculation and conjecture, I think most of us are confident that some of these findings may represent imaging artifact or reflect the natural history of biological valve leaflets, which has never been examined in such detail in the past,” added Dr. Prendergast, director of the cardiac structural intervention program at Guys and St. Thomas’ Hospital in London and cochair of the special EuroPCR session devoted to the emerging data on valve leaflet abnormalities.
The session featured three separate studies totaling 345 patients who underwent sophisticated, high-resolution 4D CT imaging or transesophageal echocardiography 5 days or more following TAVI or, less frequently, after surgical aortic valve replacement. Roughly 10% of patients showed a spectrum of leaflet abnormalities: thickening, mildly impaired motion, and/or thin films believed to be thrombi.
The leaflet abnormalities weren’t associated with any particular valve. And, as was emphasized by Dr. Prendergast and other speakers, to date these abnormalities haven’t been associated with a single case of stroke, systemic embolism, or valve failure.
Indeed, more than 100,000 TAVI procedures have been performed worldwide, and stroke rates in contemporary randomized trials and large registries are in the 1%-2% range. That’s better than with surgical valve replacement, Dr. Prendergast observed.
“Nowadays we can see much more than we could in the past, when we worked with 2D echocardiography,” observed discussant Dr. Jeroen J. Bax, professor of cardiology and director of noninvasive cardiology imaging at Leiden (the Netherlands) University Medical Center. “We see things that we do not completely understand. We could say that technology has outpaced our clinical understanding. But although we see things, at the moment there is no consequence in terms of hemodynamic performance or clinical outcomes. And this phenomenon of leaflet thickening has been occurring with surgical aortic valve replacement for many, many years and we simply didn’t realize it.”
Dr. Franz-Josef Neumann, who led one of the three studies, reported that 4D CT on day 5 post TAVI revealed leaflet abnormalities, all completely asymptomatic, in 16 of 154 patients. Two-thirds of the study population were on dual antiplatelet therapy at the time, the rest on a single antiplatelet agent. Dual antiplatelet therapy didn’t protect against leaflet thickening or other abnormalities.
All 16 affected patients were placed on an oral vitamin K antagonist with a target international normalized ratio (INR) of 2.5-3.5. To date, 11 of the 16 have undergone follow-up high-resolution CT after a median of 77 days. The leaflet thickening was resolved in all instances, according to Dr. Neumann, medical director of the department of cardiology and angiology at the University of Freiburg (Germany).
Dr. Raj R. Makkar presented a study of 125 patients who underwent high-resolution imaging after TAVI. Importantly, none of those who were on warfarin as part of their post-TAVI regimen developed leaflet abnormalities.
But he cautioned his colleagues against overreacting to the studies he and Dr. Neumann presented by placing all of their TAVI patients on an oral anticoagulant. He noted that the current TAVI population is elderly and laden with many comorbid conditions, placing them at high risk for bleeding complications.
There is at present no standard, guideline-recommended antiplatelet/antithrombotic regimen for before, during, or after TAVI. Working out the optimal protective drug regimen in this population is now a priority in light of the leaflet abnormality findings, but it will take time and require careful study, said Dr. Makkar, associate director of the Cedars Sinai Heart Institute in Los Angeles.
“Everyone is talking about anticoagulation as the imminent solution. But I want to emphasize that it comes with a price in terms of bleeding. These images are beautiful in terms of spatial resolution, but we must resist our temptation while the industry works on designing less thrombogenic valves,” the cardiologist added.
Discussant Dr. Christoph K. Naber confessed he was “stunned” by the images of leaflet abnormalities.
“Although we haven’t seen any clinical consequences, we have to keep in mind that the group of patients is still small. We have very good experience with TAVI; it has saved the lives of many patients. We know it’s a very good therapy. But if we believe we can go further and offer it to younger, lower-risk patients who will have their device for a longer time, then we should take the time and money to understand what is going on here and what consequences it could have. It’s something we should closely watch, especially if we want to extend the indication,” said Dr. Naber, director of the department of cardiology and angiology at the Contilia Cardiovascular Center in Essen, Germany.
He disclosed that he serves as a consultant to Abbott Vascular, Biotronik, Medtronic, and The Medicines Company. Dr. Makkar has received research grants from Edwards Lifesciences, St. Jude Medical, and Boston Scientific. Dr. Prendergast is on the speakers’ bureau for Edwards Lifesciences.
PARIS – Thought leaders in interventional cardiology have been quick to throw cold water on recent reports of valve leaflet thickening and abnormal leaflet motion being detected in roughly 10% of patients after transcatheter aortic valve implantation (TAVI) or surgical aortic valve replacement.
“The take home message for the interventional community is there is no need for clinicians to modify their practice in relation to patient selection, TAVI implantation, or follow-up protocols. Specifically, there is no role for systematic CT or transesophageal echocardiographic follow-up of asymptomatic TAVI patients because they’re not at clinical risk, and these additional procedures carry risk in themselves,” Dr. Bernard Prendergast said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“Whilst there is room here for speculation and conjecture, I think most of us are confident that some of these findings may represent imaging artifact or reflect the natural history of biological valve leaflets, which has never been examined in such detail in the past,” added Dr. Prendergast, director of the cardiac structural intervention program at Guys and St. Thomas’ Hospital in London and cochair of the special EuroPCR session devoted to the emerging data on valve leaflet abnormalities.
The session featured three separate studies totaling 345 patients who underwent sophisticated, high-resolution 4D CT imaging or transesophageal echocardiography 5 days or more following TAVI or, less frequently, after surgical aortic valve replacement. Roughly 10% of patients showed a spectrum of leaflet abnormalities: thickening, mildly impaired motion, and/or thin films believed to be thrombi.
The leaflet abnormalities weren’t associated with any particular valve. And, as was emphasized by Dr. Prendergast and other speakers, to date these abnormalities haven’t been associated with a single case of stroke, systemic embolism, or valve failure.
Indeed, more than 100,000 TAVI procedures have been performed worldwide, and stroke rates in contemporary randomized trials and large registries are in the 1%-2% range. That’s better than with surgical valve replacement, Dr. Prendergast observed.
“Nowadays we can see much more than we could in the past, when we worked with 2D echocardiography,” observed discussant Dr. Jeroen J. Bax, professor of cardiology and director of noninvasive cardiology imaging at Leiden (the Netherlands) University Medical Center. “We see things that we do not completely understand. We could say that technology has outpaced our clinical understanding. But although we see things, at the moment there is no consequence in terms of hemodynamic performance or clinical outcomes. And this phenomenon of leaflet thickening has been occurring with surgical aortic valve replacement for many, many years and we simply didn’t realize it.”
Dr. Franz-Josef Neumann, who led one of the three studies, reported that 4D CT on day 5 post TAVI revealed leaflet abnormalities, all completely asymptomatic, in 16 of 154 patients. Two-thirds of the study population were on dual antiplatelet therapy at the time, the rest on a single antiplatelet agent. Dual antiplatelet therapy didn’t protect against leaflet thickening or other abnormalities.
All 16 affected patients were placed on an oral vitamin K antagonist with a target international normalized ratio (INR) of 2.5-3.5. To date, 11 of the 16 have undergone follow-up high-resolution CT after a median of 77 days. The leaflet thickening was resolved in all instances, according to Dr. Neumann, medical director of the department of cardiology and angiology at the University of Freiburg (Germany).
Dr. Raj R. Makkar presented a study of 125 patients who underwent high-resolution imaging after TAVI. Importantly, none of those who were on warfarin as part of their post-TAVI regimen developed leaflet abnormalities.
But he cautioned his colleagues against overreacting to the studies he and Dr. Neumann presented by placing all of their TAVI patients on an oral anticoagulant. He noted that the current TAVI population is elderly and laden with many comorbid conditions, placing them at high risk for bleeding complications.
There is at present no standard, guideline-recommended antiplatelet/antithrombotic regimen for before, during, or after TAVI. Working out the optimal protective drug regimen in this population is now a priority in light of the leaflet abnormality findings, but it will take time and require careful study, said Dr. Makkar, associate director of the Cedars Sinai Heart Institute in Los Angeles.
“Everyone is talking about anticoagulation as the imminent solution. But I want to emphasize that it comes with a price in terms of bleeding. These images are beautiful in terms of spatial resolution, but we must resist our temptation while the industry works on designing less thrombogenic valves,” the cardiologist added.
Discussant Dr. Christoph K. Naber confessed he was “stunned” by the images of leaflet abnormalities.
“Although we haven’t seen any clinical consequences, we have to keep in mind that the group of patients is still small. We have very good experience with TAVI; it has saved the lives of many patients. We know it’s a very good therapy. But if we believe we can go further and offer it to younger, lower-risk patients who will have their device for a longer time, then we should take the time and money to understand what is going on here and what consequences it could have. It’s something we should closely watch, especially if we want to extend the indication,” said Dr. Naber, director of the department of cardiology and angiology at the Contilia Cardiovascular Center in Essen, Germany.
He disclosed that he serves as a consultant to Abbott Vascular, Biotronik, Medtronic, and The Medicines Company. Dr. Makkar has received research grants from Edwards Lifesciences, St. Jude Medical, and Boston Scientific. Dr. Prendergast is on the speakers’ bureau for Edwards Lifesciences.
EXPERT ANALYSIS FROM EUROPCR
Elephant stent aorta repair – good outcomes, but is it too complex?
An acute aortic tear can be lethal, and more cardiac surgeons are favoring extended aortic arch replacement in these cases. Cardiac surgeons have tried many different arch replacement techniques, but en bloc repair and double- or triple-branch stent grafting carry significant risks, so a team of cardiac surgeons in Beijing has reported good 2-year results with a novel technique that combines stented elephant-trunk implantation with preservation of key vessels.
The technique accomplishes total arch replacement with the stent while preserving the autologous brachiocephalic vessels.
“This technique simplified hemostasis and anastomosis, reduced the size of the residual aortic patch wall, and preserved the autologous brachiocephalic vessels, yielding satisfactory surgical results,” wrote Dr. Li-Zhong Sun and colleagues at Beijing’s Capital Medical University (J. Thorac. Cardiovasc. Surg. 2015 [doi:10.1016/j.jtcvs.2015.03.002]).
There are four keys to the procedure:
• The use of forceps to grasp the stent-free sewing edge of the stented elephant trunk and straightening of the spiral shaped Dacron graft to approximately 3 cm.
• Preservation of the native brachiocephalic vessels.
• Creating a residual aortic wall containing the innominate artery and LCCA that’s as small as possible.
• An end-to-side anastomosis between the left subclavian artery (LSCA) and the left common carotid artery (LCCA), a key junction in their technique.
The 20 study subjects had surgery within 2 weeks of the onset of pain. All 20 were discharged after the procedure, and in a mean follow-up period of 26 months, 18 had good outcomes while 1 patient had thoracoabdominal aortic replacement 9 months after the initial surgery (1 patient was lost to follow-up).
The researchers used computed tomography to confirm patency of the anastomosis between the LSCA and LCCA.
In 2 of the 20 patients, the aorta was normal with aortic dissection limited to the descending aorta. In the remaining patients, the investigators observed thrombus obliteration of the false lumen around the surgical graft in 16, partial thrombosis in 1 and patency in 1.
The surgical technique exposes the right axillary artery through a right subclavicular incision and a median sternotomy, then dissects and exposes the brachiocephalic vessels and the transverse arch. Dissection of the LSCA and LCCA is the key step in making the end-to-end anastomosis between the two vessels. The researchers accomplished this by partially transecting the sternocleidomastoid muscle and other cervical muscles.
Dr. Sun and coauthors said that a separated graft technique offers a number of advantages over other techniques for aortic arch reconstruction. While en bloc repair preserves the native brachiocephalic vessels and, thus, results in long-term patency, the technique carries risk for postoperative rupture of the aortic patch containing the brachiocephalic vessels. Double- or triple-branched stent grafting has resulted in shifting or kinking of the graft and eventually graft occlusion or aortic disruption.
The authors acknowledged the study’s small sample size, and that the outcomes are “preliminary.” They said long-term follow-up would be required to confirm the outcomes.
They had no disclosures to report.
The Beijing study authors’ excellent postoperative outcomes show that alternative surgical techniques for elephant-trunk implantation can be employed safely, but their technique also raises questions about the use of advanced technology, Dr. Prashanth Vallabhajosyula and Dr. Wilson Y. Szeto of the University of Pennsylvania said in their commentary on the study (J. Thorac. Cardiovasc. Surg. 2015 [doi: 10.1016/j.jtcvs.2015.04.003]).
“But does this mean we should be doing [elephant-trunk] operation on every type A dissection patient?” wrote Dr. Vallabhajosyula and Dr. Szeto. If no primary tear appears in the aortic arch or the proximal descending thoracic aorta (DTA), “then should we empirically dissect the arch vessels and perform total arch replacement in an emergent situation?” They also questioned extensive dissection of the left subclavian artery (LSCA) by cutting into the muscles around the surgical site.
Elephant-trunk implantation is more complex than other aortic repair procedures, they noted. “So, if a total arch replacement is not required, then why do it?”
While they acknowledged advantages of total arch replacement, and elephant-trunk implantation in particular, most operations for type A dissection occur in smaller, community hospitals that are ill equipped to perform the procedure. “This raises the issue of wide clinical application of the [elephant-trunk] technique for acute type A dissection,” they said. The real issue may not be what type of anastomosis for the elephant-trunk technique surgeons should use, but rather what surgical technique – the elephant-trunk technique vs. transverse hemiarch reconstruction, they said. (Dr. Vallabhajosyula and Dr. Szeto mentioned that their institution has advocated for the latter.)
“To address this, a more comprehensive and meticulous approach is warranted based on parameters such as patient clinical picture, acuity, malperfusion, arch and DTA anatomy, and primary tear site location,” they said. But for now, Dr. Vallabhajosyula and Dr. Szeto said, the medical literature does not support total arch replacement over transverse hemiarch reconstruction.
Dr. Vallabhajosyula is assistant professor of surgery at the Hospital of the University of Pennsylvania; Dr. Szeto is associate professor of surgery in the division of cardiovascular surgery at the University of Pennsylvania Medical Center–Penn Presbyterian Medical Center.
The Beijing study authors’ excellent postoperative outcomes show that alternative surgical techniques for elephant-trunk implantation can be employed safely, but their technique also raises questions about the use of advanced technology, Dr. Prashanth Vallabhajosyula and Dr. Wilson Y. Szeto of the University of Pennsylvania said in their commentary on the study (J. Thorac. Cardiovasc. Surg. 2015 [doi: 10.1016/j.jtcvs.2015.04.003]).
“But does this mean we should be doing [elephant-trunk] operation on every type A dissection patient?” wrote Dr. Vallabhajosyula and Dr. Szeto. If no primary tear appears in the aortic arch or the proximal descending thoracic aorta (DTA), “then should we empirically dissect the arch vessels and perform total arch replacement in an emergent situation?” They also questioned extensive dissection of the left subclavian artery (LSCA) by cutting into the muscles around the surgical site.
Elephant-trunk implantation is more complex than other aortic repair procedures, they noted. “So, if a total arch replacement is not required, then why do it?”
While they acknowledged advantages of total arch replacement, and elephant-trunk implantation in particular, most operations for type A dissection occur in smaller, community hospitals that are ill equipped to perform the procedure. “This raises the issue of wide clinical application of the [elephant-trunk] technique for acute type A dissection,” they said. The real issue may not be what type of anastomosis for the elephant-trunk technique surgeons should use, but rather what surgical technique – the elephant-trunk technique vs. transverse hemiarch reconstruction, they said. (Dr. Vallabhajosyula and Dr. Szeto mentioned that their institution has advocated for the latter.)
“To address this, a more comprehensive and meticulous approach is warranted based on parameters such as patient clinical picture, acuity, malperfusion, arch and DTA anatomy, and primary tear site location,” they said. But for now, Dr. Vallabhajosyula and Dr. Szeto said, the medical literature does not support total arch replacement over transverse hemiarch reconstruction.
Dr. Vallabhajosyula is assistant professor of surgery at the Hospital of the University of Pennsylvania; Dr. Szeto is associate professor of surgery in the division of cardiovascular surgery at the University of Pennsylvania Medical Center–Penn Presbyterian Medical Center.
The Beijing study authors’ excellent postoperative outcomes show that alternative surgical techniques for elephant-trunk implantation can be employed safely, but their technique also raises questions about the use of advanced technology, Dr. Prashanth Vallabhajosyula and Dr. Wilson Y. Szeto of the University of Pennsylvania said in their commentary on the study (J. Thorac. Cardiovasc. Surg. 2015 [doi: 10.1016/j.jtcvs.2015.04.003]).
“But does this mean we should be doing [elephant-trunk] operation on every type A dissection patient?” wrote Dr. Vallabhajosyula and Dr. Szeto. If no primary tear appears in the aortic arch or the proximal descending thoracic aorta (DTA), “then should we empirically dissect the arch vessels and perform total arch replacement in an emergent situation?” They also questioned extensive dissection of the left subclavian artery (LSCA) by cutting into the muscles around the surgical site.
Elephant-trunk implantation is more complex than other aortic repair procedures, they noted. “So, if a total arch replacement is not required, then why do it?”
While they acknowledged advantages of total arch replacement, and elephant-trunk implantation in particular, most operations for type A dissection occur in smaller, community hospitals that are ill equipped to perform the procedure. “This raises the issue of wide clinical application of the [elephant-trunk] technique for acute type A dissection,” they said. The real issue may not be what type of anastomosis for the elephant-trunk technique surgeons should use, but rather what surgical technique – the elephant-trunk technique vs. transverse hemiarch reconstruction, they said. (Dr. Vallabhajosyula and Dr. Szeto mentioned that their institution has advocated for the latter.)
“To address this, a more comprehensive and meticulous approach is warranted based on parameters such as patient clinical picture, acuity, malperfusion, arch and DTA anatomy, and primary tear site location,” they said. But for now, Dr. Vallabhajosyula and Dr. Szeto said, the medical literature does not support total arch replacement over transverse hemiarch reconstruction.
Dr. Vallabhajosyula is assistant professor of surgery at the Hospital of the University of Pennsylvania; Dr. Szeto is associate professor of surgery in the division of cardiovascular surgery at the University of Pennsylvania Medical Center–Penn Presbyterian Medical Center.
An acute aortic tear can be lethal, and more cardiac surgeons are favoring extended aortic arch replacement in these cases. Cardiac surgeons have tried many different arch replacement techniques, but en bloc repair and double- or triple-branch stent grafting carry significant risks, so a team of cardiac surgeons in Beijing has reported good 2-year results with a novel technique that combines stented elephant-trunk implantation with preservation of key vessels.
The technique accomplishes total arch replacement with the stent while preserving the autologous brachiocephalic vessels.
“This technique simplified hemostasis and anastomosis, reduced the size of the residual aortic patch wall, and preserved the autologous brachiocephalic vessels, yielding satisfactory surgical results,” wrote Dr. Li-Zhong Sun and colleagues at Beijing’s Capital Medical University (J. Thorac. Cardiovasc. Surg. 2015 [doi:10.1016/j.jtcvs.2015.03.002]).
There are four keys to the procedure:
• The use of forceps to grasp the stent-free sewing edge of the stented elephant trunk and straightening of the spiral shaped Dacron graft to approximately 3 cm.
• Preservation of the native brachiocephalic vessels.
• Creating a residual aortic wall containing the innominate artery and LCCA that’s as small as possible.
• An end-to-side anastomosis between the left subclavian artery (LSCA) and the left common carotid artery (LCCA), a key junction in their technique.
The 20 study subjects had surgery within 2 weeks of the onset of pain. All 20 were discharged after the procedure, and in a mean follow-up period of 26 months, 18 had good outcomes while 1 patient had thoracoabdominal aortic replacement 9 months after the initial surgery (1 patient was lost to follow-up).
The researchers used computed tomography to confirm patency of the anastomosis between the LSCA and LCCA.
In 2 of the 20 patients, the aorta was normal with aortic dissection limited to the descending aorta. In the remaining patients, the investigators observed thrombus obliteration of the false lumen around the surgical graft in 16, partial thrombosis in 1 and patency in 1.
The surgical technique exposes the right axillary artery through a right subclavicular incision and a median sternotomy, then dissects and exposes the brachiocephalic vessels and the transverse arch. Dissection of the LSCA and LCCA is the key step in making the end-to-end anastomosis between the two vessels. The researchers accomplished this by partially transecting the sternocleidomastoid muscle and other cervical muscles.
Dr. Sun and coauthors said that a separated graft technique offers a number of advantages over other techniques for aortic arch reconstruction. While en bloc repair preserves the native brachiocephalic vessels and, thus, results in long-term patency, the technique carries risk for postoperative rupture of the aortic patch containing the brachiocephalic vessels. Double- or triple-branched stent grafting has resulted in shifting or kinking of the graft and eventually graft occlusion or aortic disruption.
The authors acknowledged the study’s small sample size, and that the outcomes are “preliminary.” They said long-term follow-up would be required to confirm the outcomes.
They had no disclosures to report.
An acute aortic tear can be lethal, and more cardiac surgeons are favoring extended aortic arch replacement in these cases. Cardiac surgeons have tried many different arch replacement techniques, but en bloc repair and double- or triple-branch stent grafting carry significant risks, so a team of cardiac surgeons in Beijing has reported good 2-year results with a novel technique that combines stented elephant-trunk implantation with preservation of key vessels.
The technique accomplishes total arch replacement with the stent while preserving the autologous brachiocephalic vessels.
“This technique simplified hemostasis and anastomosis, reduced the size of the residual aortic patch wall, and preserved the autologous brachiocephalic vessels, yielding satisfactory surgical results,” wrote Dr. Li-Zhong Sun and colleagues at Beijing’s Capital Medical University (J. Thorac. Cardiovasc. Surg. 2015 [doi:10.1016/j.jtcvs.2015.03.002]).
There are four keys to the procedure:
• The use of forceps to grasp the stent-free sewing edge of the stented elephant trunk and straightening of the spiral shaped Dacron graft to approximately 3 cm.
• Preservation of the native brachiocephalic vessels.
• Creating a residual aortic wall containing the innominate artery and LCCA that’s as small as possible.
• An end-to-side anastomosis between the left subclavian artery (LSCA) and the left common carotid artery (LCCA), a key junction in their technique.
The 20 study subjects had surgery within 2 weeks of the onset of pain. All 20 were discharged after the procedure, and in a mean follow-up period of 26 months, 18 had good outcomes while 1 patient had thoracoabdominal aortic replacement 9 months after the initial surgery (1 patient was lost to follow-up).
The researchers used computed tomography to confirm patency of the anastomosis between the LSCA and LCCA.
In 2 of the 20 patients, the aorta was normal with aortic dissection limited to the descending aorta. In the remaining patients, the investigators observed thrombus obliteration of the false lumen around the surgical graft in 16, partial thrombosis in 1 and patency in 1.
The surgical technique exposes the right axillary artery through a right subclavicular incision and a median sternotomy, then dissects and exposes the brachiocephalic vessels and the transverse arch. Dissection of the LSCA and LCCA is the key step in making the end-to-end anastomosis between the two vessels. The researchers accomplished this by partially transecting the sternocleidomastoid muscle and other cervical muscles.
Dr. Sun and coauthors said that a separated graft technique offers a number of advantages over other techniques for aortic arch reconstruction. While en bloc repair preserves the native brachiocephalic vessels and, thus, results in long-term patency, the technique carries risk for postoperative rupture of the aortic patch containing the brachiocephalic vessels. Double- or triple-branched stent grafting has resulted in shifting or kinking of the graft and eventually graft occlusion or aortic disruption.
The authors acknowledged the study’s small sample size, and that the outcomes are “preliminary.” They said long-term follow-up would be required to confirm the outcomes.
They had no disclosures to report.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point: Total aortic arch replacement with implantation of an elephant-trunk stent avoids risks of other more conventional approaches.
Major finding: Among 20 patients who had the elephant-trunk procedure, 18 had good results at a mean of 26 months after the operation (one had thoracoabdominal aortic arch replacement at 9 months and one was lost to follow-up).
Data source: Retrospective review of 20 patients with acute type A dissection who had total arch replacement at a single center.
Disclosures: The study authors reported having no financial disclosures.
Two U.S. transcatheter valve approvals reshape TAVR
The nature of U.S. transcatheter aortic valve replacement (TAVR) shifted dramatically in mid-June when the Food and Drug Administration, in two separate actions timed just days apart, approved marketing of next-generation models for the only two transcatheter aortic valve replacement systems on the U.S. market.
For inoperable or high-risk patients in came the Edwards Sapien 3 and the CoreValve Evolut R systems, and out went the Sapien XT and the original CoreValve. In the process the transcatheter aortic valve replacement (TAVR) procedures available to American patients on a routine basis became smaller – meaning more likely to use transfemoral approaches, less likely to cause substantial paravalvular leak, less likely to cause stroke, and in the case of the Evolut system also became repositionable and less likely to result in the need for a pacemaker.
With approvals of these two latest-generation devices, in both cases based on follow-up data of only 30 days, “we have really expanded the types of patients who can be treated with TAVR in the U.S., and we can do it with better products and get better outcomes. I am thrilled this technology will be available to patients,” said Dr. Jeffrey J. Popma, a lead investigator for the Evolut U.S. pivotal trial and professor of medicine at Harvard University in Boston.
The pair of approvals were also notable as clear demonstration that the FDA was willing to base its decisions on 30-day follow-up data, a step closer to the approval model it applies to heart valves placed using open surgery. Perhaps most attention-grabbing of all, the FDA based its approval of the Evolut system on data from a total of 151 patients, with 60 coming from the ex-U.S. trial designed to secure a CE mark in Europe, and the other 91 patients the first ones enrolled in what was designed to be the U.S. pivotal trial for Evolut, with a planned enrollment of 250 patients that had almost fully filled by mid-June.
“This was a really good week for the FDA,” said Dr. Popma. “The FDA approved devices with reasonable assurance of safety and efficacy but without the burden of requiring 1 or more years follow-up. We are seeing a changed FDA,” he declared in an interview.
Although the full dataset for all 151 patients that the FDA used to approve the self-expanding Evolut system has not yet been released, investigators from the U.S. trial say data from their first 91 patients looked similar to the 60 patients in the CE trial, the results of which appeared in a poster in March at the annual scientific sessions of the American College of Cardiology.
The CE trial enrolled 60 symptomatic extreme- or high-risk patients at six centers in Australia, New Zealand, and the United Kingdom, who averaged 83 years old and had an average Society of Thoracic Surgeons predicted mortality estimate of 7%. Fifty-nine of the 60 patients (98%) had their TAVR done via the transfemoral route, and during 30-day follow-up no patients died and none had a stroke. The researchers identified a moderate or severe paravalvular leak in 3% of patients after 30 days, and 12% of patients had a new need for a pacemaker. By comparison, in the CoreValve pivotal trial for the first-generation version of this self-expanding valve, death occurred in 3% of patients after 30 days, major strokes in 4%, moderate or severe paravalvular leaks occurred in 9%, and 20% of patients by that point had required placement of a new pacemaker (N. Engl. J. Med. 2014;370:1790-8).
The striking reductions in more severe paravalvular leaks and in the need for a pacemaker likely related at least in part to the ability to reposition the Evolut valve during placement as long as the valve was not more than 80% deployed and as long as retrieval was not attempted more than three times.
Repositioning is “huge” said Dr. Mathew R. Williams, chief of adult cardiac surgery and director of interventional cardiology at New York University, and one of two lead investigators on the U.S. CoreValve Evolut R pivotal trial. “We don’t need to use it most of the time, but having that ability reduces stress and helps make the operator more comfortable,” he said in an interview. The more optimized positioning allowed by the recapture feature likely helped minimize both paravalvular leaks and the valve’s ability to trigger an arrhythmia and need for pacing. In the CE trial the operator used the repositioning function in 15 patients (25%) for a total of 22 repositioning events.
Like Dr. Popma, Dr. Williams welcomed the June 17 approval of the Sapien 3 TAVR system, based on 30-day outcomes from all 583 patients enrolled in the U.S. pivotal trial. Results from that study became public last March in a late-breaker report at the American College of Cardiology meeting.
Sapien 3 treatment of high-risk, symptomatic patients, who averaged 83 years old and had a STS predicted mortality rate of 8.6%, resulted in a 2% mortality rate, a 1.5% stroke rate, a 3% rate of moderate or severe paravalvular leaks, and 13% of patients had a new need for a pacemaker, Dr. Susheel Kodali reported when he presented these data in March. Trial investigators were able to place the Sapien 3 aortic valve via a transfemoral approach in 491 of the 583 patients (84%) enrolled in the study. The adverse outcomes with Sapien 3 contrasted with rates for the prior Sapien XT TAVR system of 3.5% for 30-day mortality, 4.3% for strokes, and a 24% rate of moderate or severe paravalvular leak, said Dr. Kodali, director of the heart valve program at Columbia University in New York.
While the Sapien 3 and Evolut systems appeared to produce roughly similar outcomes for parameters like 30-day mortality, stroke, and paravalvular leak rates, with both systems outperforming what they displaced, the Evolut has two important differences, compared with Sapien 3. First, the Evolut is slightly smaller, with the ability to traverse arteries as narrow as 5 mm, whereas the minimum vessel size for Sapien 3 passage is 5.5 mm; second, Evolute is repositionable, while Sapien 3 is not.
The CoreValve Evolut R trials were sponsored by Medtronic. Dr. Popma has received research grants from and served as a speaker on behalf of Medtronic. He has also been a speaker for Abbott Vascular, Boston Scientific, Covidien, Cook Medical, and Direct Flow Medical and has received research support from five other companies. Dr. Williams has been a consultant to and received research support from Medtronic and has also been a consultant to Edwards and Direct Flow Medical. The Sapien 3 trial was sponsored by Edwards. Dr. Kodali has received research support from Edwards, has an equity interest in Thubrikar Aortic Valve, has received honoraria from St. Jude, and has served on the steering committee for trials sponsored by Claret Medical and Meril.
On Twitter @mitchelzoler
The nature of U.S. transcatheter aortic valve replacement (TAVR) shifted dramatically in mid-June when the Food and Drug Administration, in two separate actions timed just days apart, approved marketing of next-generation models for the only two transcatheter aortic valve replacement systems on the U.S. market.
For inoperable or high-risk patients in came the Edwards Sapien 3 and the CoreValve Evolut R systems, and out went the Sapien XT and the original CoreValve. In the process the transcatheter aortic valve replacement (TAVR) procedures available to American patients on a routine basis became smaller – meaning more likely to use transfemoral approaches, less likely to cause substantial paravalvular leak, less likely to cause stroke, and in the case of the Evolut system also became repositionable and less likely to result in the need for a pacemaker.
With approvals of these two latest-generation devices, in both cases based on follow-up data of only 30 days, “we have really expanded the types of patients who can be treated with TAVR in the U.S., and we can do it with better products and get better outcomes. I am thrilled this technology will be available to patients,” said Dr. Jeffrey J. Popma, a lead investigator for the Evolut U.S. pivotal trial and professor of medicine at Harvard University in Boston.
The pair of approvals were also notable as clear demonstration that the FDA was willing to base its decisions on 30-day follow-up data, a step closer to the approval model it applies to heart valves placed using open surgery. Perhaps most attention-grabbing of all, the FDA based its approval of the Evolut system on data from a total of 151 patients, with 60 coming from the ex-U.S. trial designed to secure a CE mark in Europe, and the other 91 patients the first ones enrolled in what was designed to be the U.S. pivotal trial for Evolut, with a planned enrollment of 250 patients that had almost fully filled by mid-June.
“This was a really good week for the FDA,” said Dr. Popma. “The FDA approved devices with reasonable assurance of safety and efficacy but without the burden of requiring 1 or more years follow-up. We are seeing a changed FDA,” he declared in an interview.
Although the full dataset for all 151 patients that the FDA used to approve the self-expanding Evolut system has not yet been released, investigators from the U.S. trial say data from their first 91 patients looked similar to the 60 patients in the CE trial, the results of which appeared in a poster in March at the annual scientific sessions of the American College of Cardiology.
The CE trial enrolled 60 symptomatic extreme- or high-risk patients at six centers in Australia, New Zealand, and the United Kingdom, who averaged 83 years old and had an average Society of Thoracic Surgeons predicted mortality estimate of 7%. Fifty-nine of the 60 patients (98%) had their TAVR done via the transfemoral route, and during 30-day follow-up no patients died and none had a stroke. The researchers identified a moderate or severe paravalvular leak in 3% of patients after 30 days, and 12% of patients had a new need for a pacemaker. By comparison, in the CoreValve pivotal trial for the first-generation version of this self-expanding valve, death occurred in 3% of patients after 30 days, major strokes in 4%, moderate or severe paravalvular leaks occurred in 9%, and 20% of patients by that point had required placement of a new pacemaker (N. Engl. J. Med. 2014;370:1790-8).
The striking reductions in more severe paravalvular leaks and in the need for a pacemaker likely related at least in part to the ability to reposition the Evolut valve during placement as long as the valve was not more than 80% deployed and as long as retrieval was not attempted more than three times.
Repositioning is “huge” said Dr. Mathew R. Williams, chief of adult cardiac surgery and director of interventional cardiology at New York University, and one of two lead investigators on the U.S. CoreValve Evolut R pivotal trial. “We don’t need to use it most of the time, but having that ability reduces stress and helps make the operator more comfortable,” he said in an interview. The more optimized positioning allowed by the recapture feature likely helped minimize both paravalvular leaks and the valve’s ability to trigger an arrhythmia and need for pacing. In the CE trial the operator used the repositioning function in 15 patients (25%) for a total of 22 repositioning events.
Like Dr. Popma, Dr. Williams welcomed the June 17 approval of the Sapien 3 TAVR system, based on 30-day outcomes from all 583 patients enrolled in the U.S. pivotal trial. Results from that study became public last March in a late-breaker report at the American College of Cardiology meeting.
Sapien 3 treatment of high-risk, symptomatic patients, who averaged 83 years old and had a STS predicted mortality rate of 8.6%, resulted in a 2% mortality rate, a 1.5% stroke rate, a 3% rate of moderate or severe paravalvular leaks, and 13% of patients had a new need for a pacemaker, Dr. Susheel Kodali reported when he presented these data in March. Trial investigators were able to place the Sapien 3 aortic valve via a transfemoral approach in 491 of the 583 patients (84%) enrolled in the study. The adverse outcomes with Sapien 3 contrasted with rates for the prior Sapien XT TAVR system of 3.5% for 30-day mortality, 4.3% for strokes, and a 24% rate of moderate or severe paravalvular leak, said Dr. Kodali, director of the heart valve program at Columbia University in New York.
While the Sapien 3 and Evolut systems appeared to produce roughly similar outcomes for parameters like 30-day mortality, stroke, and paravalvular leak rates, with both systems outperforming what they displaced, the Evolut has two important differences, compared with Sapien 3. First, the Evolut is slightly smaller, with the ability to traverse arteries as narrow as 5 mm, whereas the minimum vessel size for Sapien 3 passage is 5.5 mm; second, Evolute is repositionable, while Sapien 3 is not.
The CoreValve Evolut R trials were sponsored by Medtronic. Dr. Popma has received research grants from and served as a speaker on behalf of Medtronic. He has also been a speaker for Abbott Vascular, Boston Scientific, Covidien, Cook Medical, and Direct Flow Medical and has received research support from five other companies. Dr. Williams has been a consultant to and received research support from Medtronic and has also been a consultant to Edwards and Direct Flow Medical. The Sapien 3 trial was sponsored by Edwards. Dr. Kodali has received research support from Edwards, has an equity interest in Thubrikar Aortic Valve, has received honoraria from St. Jude, and has served on the steering committee for trials sponsored by Claret Medical and Meril.
On Twitter @mitchelzoler
The nature of U.S. transcatheter aortic valve replacement (TAVR) shifted dramatically in mid-June when the Food and Drug Administration, in two separate actions timed just days apart, approved marketing of next-generation models for the only two transcatheter aortic valve replacement systems on the U.S. market.
For inoperable or high-risk patients in came the Edwards Sapien 3 and the CoreValve Evolut R systems, and out went the Sapien XT and the original CoreValve. In the process the transcatheter aortic valve replacement (TAVR) procedures available to American patients on a routine basis became smaller – meaning more likely to use transfemoral approaches, less likely to cause substantial paravalvular leak, less likely to cause stroke, and in the case of the Evolut system also became repositionable and less likely to result in the need for a pacemaker.
With approvals of these two latest-generation devices, in both cases based on follow-up data of only 30 days, “we have really expanded the types of patients who can be treated with TAVR in the U.S., and we can do it with better products and get better outcomes. I am thrilled this technology will be available to patients,” said Dr. Jeffrey J. Popma, a lead investigator for the Evolut U.S. pivotal trial and professor of medicine at Harvard University in Boston.
The pair of approvals were also notable as clear demonstration that the FDA was willing to base its decisions on 30-day follow-up data, a step closer to the approval model it applies to heart valves placed using open surgery. Perhaps most attention-grabbing of all, the FDA based its approval of the Evolut system on data from a total of 151 patients, with 60 coming from the ex-U.S. trial designed to secure a CE mark in Europe, and the other 91 patients the first ones enrolled in what was designed to be the U.S. pivotal trial for Evolut, with a planned enrollment of 250 patients that had almost fully filled by mid-June.
“This was a really good week for the FDA,” said Dr. Popma. “The FDA approved devices with reasonable assurance of safety and efficacy but without the burden of requiring 1 or more years follow-up. We are seeing a changed FDA,” he declared in an interview.
Although the full dataset for all 151 patients that the FDA used to approve the self-expanding Evolut system has not yet been released, investigators from the U.S. trial say data from their first 91 patients looked similar to the 60 patients in the CE trial, the results of which appeared in a poster in March at the annual scientific sessions of the American College of Cardiology.
The CE trial enrolled 60 symptomatic extreme- or high-risk patients at six centers in Australia, New Zealand, and the United Kingdom, who averaged 83 years old and had an average Society of Thoracic Surgeons predicted mortality estimate of 7%. Fifty-nine of the 60 patients (98%) had their TAVR done via the transfemoral route, and during 30-day follow-up no patients died and none had a stroke. The researchers identified a moderate or severe paravalvular leak in 3% of patients after 30 days, and 12% of patients had a new need for a pacemaker. By comparison, in the CoreValve pivotal trial for the first-generation version of this self-expanding valve, death occurred in 3% of patients after 30 days, major strokes in 4%, moderate or severe paravalvular leaks occurred in 9%, and 20% of patients by that point had required placement of a new pacemaker (N. Engl. J. Med. 2014;370:1790-8).
The striking reductions in more severe paravalvular leaks and in the need for a pacemaker likely related at least in part to the ability to reposition the Evolut valve during placement as long as the valve was not more than 80% deployed and as long as retrieval was not attempted more than three times.
Repositioning is “huge” said Dr. Mathew R. Williams, chief of adult cardiac surgery and director of interventional cardiology at New York University, and one of two lead investigators on the U.S. CoreValve Evolut R pivotal trial. “We don’t need to use it most of the time, but having that ability reduces stress and helps make the operator more comfortable,” he said in an interview. The more optimized positioning allowed by the recapture feature likely helped minimize both paravalvular leaks and the valve’s ability to trigger an arrhythmia and need for pacing. In the CE trial the operator used the repositioning function in 15 patients (25%) for a total of 22 repositioning events.
Like Dr. Popma, Dr. Williams welcomed the June 17 approval of the Sapien 3 TAVR system, based on 30-day outcomes from all 583 patients enrolled in the U.S. pivotal trial. Results from that study became public last March in a late-breaker report at the American College of Cardiology meeting.
Sapien 3 treatment of high-risk, symptomatic patients, who averaged 83 years old and had a STS predicted mortality rate of 8.6%, resulted in a 2% mortality rate, a 1.5% stroke rate, a 3% rate of moderate or severe paravalvular leaks, and 13% of patients had a new need for a pacemaker, Dr. Susheel Kodali reported when he presented these data in March. Trial investigators were able to place the Sapien 3 aortic valve via a transfemoral approach in 491 of the 583 patients (84%) enrolled in the study. The adverse outcomes with Sapien 3 contrasted with rates for the prior Sapien XT TAVR system of 3.5% for 30-day mortality, 4.3% for strokes, and a 24% rate of moderate or severe paravalvular leak, said Dr. Kodali, director of the heart valve program at Columbia University in New York.
While the Sapien 3 and Evolut systems appeared to produce roughly similar outcomes for parameters like 30-day mortality, stroke, and paravalvular leak rates, with both systems outperforming what they displaced, the Evolut has two important differences, compared with Sapien 3. First, the Evolut is slightly smaller, with the ability to traverse arteries as narrow as 5 mm, whereas the minimum vessel size for Sapien 3 passage is 5.5 mm; second, Evolute is repositionable, while Sapien 3 is not.
The CoreValve Evolut R trials were sponsored by Medtronic. Dr. Popma has received research grants from and served as a speaker on behalf of Medtronic. He has also been a speaker for Abbott Vascular, Boston Scientific, Covidien, Cook Medical, and Direct Flow Medical and has received research support from five other companies. Dr. Williams has been a consultant to and received research support from Medtronic and has also been a consultant to Edwards and Direct Flow Medical. The Sapien 3 trial was sponsored by Edwards. Dr. Kodali has received research support from Edwards, has an equity interest in Thubrikar Aortic Valve, has received honoraria from St. Jude, and has served on the steering committee for trials sponsored by Claret Medical and Meril.
On Twitter @mitchelzoler
Perioperative factors influenced open TAAA repair
Open thoracoabdominal aortic aneurysm (TAAA) repair produced respectable early outcomes, although preoperative and intraoperative factors were found to influence risk, according to Dr. Joseph S. Coselli, who presented the results of the study he and his colleagues at the Baylor College of Medicine in Houston performed at the annual meeting of the American Association for Thoracic Surgery.
They analyzed data from 3,309 open TAAA repairs performed between October 1986 and December 2014.
“I have been very fortunate to have spent my entire career at Baylor College of Medicine, the epicenter of aortic surgery in the 1950s, ’60s, and ’70s, as well as to have been mentored by Dr. E. Stanley Crawford, who was arguably the finest aortic surgeon of his era. Since transitioning from Dr. Crawford’s surgical practice to my own surgical practice, we have kept his pioneering spirit alive by developing a multimodal strategy for thoracoabdominal aortic aneurysm repair that is based on the Crawford extent of repair and our evolving investigation. We sought to describe our series of over 3,000 TAAA repairs and to identify predictors of early death and other adverse postoperative outcomes,” said Dr. Coselli.
The median patient age was around 67 years, and the repairs involved acute or subacute aortic dissection in about 5% of the cases. Nearly 31% of the case involved chronic dissection, with nearly 22% emergent or urgent repairs and around 5% ruptured aneurysms. Connective tissue disorders were present in roughly 10% of patients. “Operatively, we tend to reserve surgical adjuncts for use in the most-extensive repairs, namely extents I and II TAAA repair; intercostal or lumbar artery reattachment was used in just over half of the repairs, left heart bypass (LHB) was used in around 45% of patients, cold renal perfusion was performed in 58%. and cerebrospinal fluid drainage (CSFD) was used in 45%,” said Dr. Coselli.
There was substantial atherosclerotic disease in older patients, and in nearly 41% of repairs, a visceral vessel procedure was performed.
Unlike many aortic centers that routinely use deep hypothermic circulatory arrest (HCA) for extensive TAAA repair, Dr. Coselli reserved this approach for a small number of highly complex repairs (1.4%) in which the aorta could not be safely clamped.
Of the more than a thousand most extensive (i.e., Crawford extent II) repairs, intercostal/lumbar artery reattachment was used in the vast majority (88%), LHB in 82%, and CSFD in 61%. They used multivariable analysis to identify predictors of operative (30-day or in-hospital) mortality and adverse event, a composite outcome comprising operative death and permanent (present at discharge) spinal cord deficit, renal failure, or stroke, according to Dr. Coselli.
Their results showed an operative mortality rate of 7.5%, a 30-day death rate of 4.8%, with the adverse event outcome occurring in about 14% of repairs. A video of his presentation is available at the AATS website.
The statistically significant predictors of operative death were rupture; renal insufficiency, symptoms, procedures targeting visceral vessels, increasing age, and increasing clamp time, while extent IV repair (the least extensive form of TAAA repair) was inversely associated with death. Their analysis showed that the significant predictors of adverse event were use of HCA, renal insufficiency, rupture, extent II repair, visceral vessel procedures, urgent or emergent repair, increasing age, and increasing clamp time. In addition, they used multivariable analysis to identify predictors of renal failure and paraplegia.
In the 3,060 early survivors, roughly 7% had a life-altering complication at discharge: Nearly 3% of patients had renal failure necessitating dialysis, slightly more than 1% had a unresolved stroke, and about 4% had unresolved paraplegia or paraparesis. Repair failure, primarily pseudoaneurysm, or patch aneurysm, occurred after nearly 3% of repairs, said Dr. Coselli.
Outcomes differed by extent of repair, with the risk being greatest in extent II repair. Actuarial survival was 63.6% at 5 years, 36.8% at 10 years, and 18.3% at 15 years. Freedom from repair failure was nearly 98% at 5 years, around 95% at 10 years, and 94% at 15 years.
“Along with respectable early outcomes, after repair, patients have acceptable long-term survival, and late repair failure was uncommon. Notably, there are several subgroups of patients that do exceedingly well. Paraplegia in young patients with connective tissue disorders, even in the most-extensive repair (extent II), is remarkably rare – these patients do extremely well across the board,” he concluded.
Dr. Cosselli reported that he is a principal investigator and consultant for Medtronic and W.L. Gore & Assoc., as well as being a principal investigator, consultant, and having various financial relationships with Vascutek.
Open thoracoabdominal aortic aneurysm (TAAA) repair produced respectable early outcomes, although preoperative and intraoperative factors were found to influence risk, according to Dr. Joseph S. Coselli, who presented the results of the study he and his colleagues at the Baylor College of Medicine in Houston performed at the annual meeting of the American Association for Thoracic Surgery.
They analyzed data from 3,309 open TAAA repairs performed between October 1986 and December 2014.
“I have been very fortunate to have spent my entire career at Baylor College of Medicine, the epicenter of aortic surgery in the 1950s, ’60s, and ’70s, as well as to have been mentored by Dr. E. Stanley Crawford, who was arguably the finest aortic surgeon of his era. Since transitioning from Dr. Crawford’s surgical practice to my own surgical practice, we have kept his pioneering spirit alive by developing a multimodal strategy for thoracoabdominal aortic aneurysm repair that is based on the Crawford extent of repair and our evolving investigation. We sought to describe our series of over 3,000 TAAA repairs and to identify predictors of early death and other adverse postoperative outcomes,” said Dr. Coselli.
The median patient age was around 67 years, and the repairs involved acute or subacute aortic dissection in about 5% of the cases. Nearly 31% of the case involved chronic dissection, with nearly 22% emergent or urgent repairs and around 5% ruptured aneurysms. Connective tissue disorders were present in roughly 10% of patients. “Operatively, we tend to reserve surgical adjuncts for use in the most-extensive repairs, namely extents I and II TAAA repair; intercostal or lumbar artery reattachment was used in just over half of the repairs, left heart bypass (LHB) was used in around 45% of patients, cold renal perfusion was performed in 58%. and cerebrospinal fluid drainage (CSFD) was used in 45%,” said Dr. Coselli.
There was substantial atherosclerotic disease in older patients, and in nearly 41% of repairs, a visceral vessel procedure was performed.
Unlike many aortic centers that routinely use deep hypothermic circulatory arrest (HCA) for extensive TAAA repair, Dr. Coselli reserved this approach for a small number of highly complex repairs (1.4%) in which the aorta could not be safely clamped.
Of the more than a thousand most extensive (i.e., Crawford extent II) repairs, intercostal/lumbar artery reattachment was used in the vast majority (88%), LHB in 82%, and CSFD in 61%. They used multivariable analysis to identify predictors of operative (30-day or in-hospital) mortality and adverse event, a composite outcome comprising operative death and permanent (present at discharge) spinal cord deficit, renal failure, or stroke, according to Dr. Coselli.
Their results showed an operative mortality rate of 7.5%, a 30-day death rate of 4.8%, with the adverse event outcome occurring in about 14% of repairs. A video of his presentation is available at the AATS website.
The statistically significant predictors of operative death were rupture; renal insufficiency, symptoms, procedures targeting visceral vessels, increasing age, and increasing clamp time, while extent IV repair (the least extensive form of TAAA repair) was inversely associated with death. Their analysis showed that the significant predictors of adverse event were use of HCA, renal insufficiency, rupture, extent II repair, visceral vessel procedures, urgent or emergent repair, increasing age, and increasing clamp time. In addition, they used multivariable analysis to identify predictors of renal failure and paraplegia.
In the 3,060 early survivors, roughly 7% had a life-altering complication at discharge: Nearly 3% of patients had renal failure necessitating dialysis, slightly more than 1% had a unresolved stroke, and about 4% had unresolved paraplegia or paraparesis. Repair failure, primarily pseudoaneurysm, or patch aneurysm, occurred after nearly 3% of repairs, said Dr. Coselli.
Outcomes differed by extent of repair, with the risk being greatest in extent II repair. Actuarial survival was 63.6% at 5 years, 36.8% at 10 years, and 18.3% at 15 years. Freedom from repair failure was nearly 98% at 5 years, around 95% at 10 years, and 94% at 15 years.
“Along with respectable early outcomes, after repair, patients have acceptable long-term survival, and late repair failure was uncommon. Notably, there are several subgroups of patients that do exceedingly well. Paraplegia in young patients with connective tissue disorders, even in the most-extensive repair (extent II), is remarkably rare – these patients do extremely well across the board,” he concluded.
Dr. Cosselli reported that he is a principal investigator and consultant for Medtronic and W.L. Gore & Assoc., as well as being a principal investigator, consultant, and having various financial relationships with Vascutek.
Open thoracoabdominal aortic aneurysm (TAAA) repair produced respectable early outcomes, although preoperative and intraoperative factors were found to influence risk, according to Dr. Joseph S. Coselli, who presented the results of the study he and his colleagues at the Baylor College of Medicine in Houston performed at the annual meeting of the American Association for Thoracic Surgery.
They analyzed data from 3,309 open TAAA repairs performed between October 1986 and December 2014.
“I have been very fortunate to have spent my entire career at Baylor College of Medicine, the epicenter of aortic surgery in the 1950s, ’60s, and ’70s, as well as to have been mentored by Dr. E. Stanley Crawford, who was arguably the finest aortic surgeon of his era. Since transitioning from Dr. Crawford’s surgical practice to my own surgical practice, we have kept his pioneering spirit alive by developing a multimodal strategy for thoracoabdominal aortic aneurysm repair that is based on the Crawford extent of repair and our evolving investigation. We sought to describe our series of over 3,000 TAAA repairs and to identify predictors of early death and other adverse postoperative outcomes,” said Dr. Coselli.
The median patient age was around 67 years, and the repairs involved acute or subacute aortic dissection in about 5% of the cases. Nearly 31% of the case involved chronic dissection, with nearly 22% emergent or urgent repairs and around 5% ruptured aneurysms. Connective tissue disorders were present in roughly 10% of patients. “Operatively, we tend to reserve surgical adjuncts for use in the most-extensive repairs, namely extents I and II TAAA repair; intercostal or lumbar artery reattachment was used in just over half of the repairs, left heart bypass (LHB) was used in around 45% of patients, cold renal perfusion was performed in 58%. and cerebrospinal fluid drainage (CSFD) was used in 45%,” said Dr. Coselli.
There was substantial atherosclerotic disease in older patients, and in nearly 41% of repairs, a visceral vessel procedure was performed.
Unlike many aortic centers that routinely use deep hypothermic circulatory arrest (HCA) for extensive TAAA repair, Dr. Coselli reserved this approach for a small number of highly complex repairs (1.4%) in which the aorta could not be safely clamped.
Of the more than a thousand most extensive (i.e., Crawford extent II) repairs, intercostal/lumbar artery reattachment was used in the vast majority (88%), LHB in 82%, and CSFD in 61%. They used multivariable analysis to identify predictors of operative (30-day or in-hospital) mortality and adverse event, a composite outcome comprising operative death and permanent (present at discharge) spinal cord deficit, renal failure, or stroke, according to Dr. Coselli.
Their results showed an operative mortality rate of 7.5%, a 30-day death rate of 4.8%, with the adverse event outcome occurring in about 14% of repairs. A video of his presentation is available at the AATS website.
The statistically significant predictors of operative death were rupture; renal insufficiency, symptoms, procedures targeting visceral vessels, increasing age, and increasing clamp time, while extent IV repair (the least extensive form of TAAA repair) was inversely associated with death. Their analysis showed that the significant predictors of adverse event were use of HCA, renal insufficiency, rupture, extent II repair, visceral vessel procedures, urgent or emergent repair, increasing age, and increasing clamp time. In addition, they used multivariable analysis to identify predictors of renal failure and paraplegia.
In the 3,060 early survivors, roughly 7% had a life-altering complication at discharge: Nearly 3% of patients had renal failure necessitating dialysis, slightly more than 1% had a unresolved stroke, and about 4% had unresolved paraplegia or paraparesis. Repair failure, primarily pseudoaneurysm, or patch aneurysm, occurred after nearly 3% of repairs, said Dr. Coselli.
Outcomes differed by extent of repair, with the risk being greatest in extent II repair. Actuarial survival was 63.6% at 5 years, 36.8% at 10 years, and 18.3% at 15 years. Freedom from repair failure was nearly 98% at 5 years, around 95% at 10 years, and 94% at 15 years.
“Along with respectable early outcomes, after repair, patients have acceptable long-term survival, and late repair failure was uncommon. Notably, there are several subgroups of patients that do exceedingly well. Paraplegia in young patients with connective tissue disorders, even in the most-extensive repair (extent II), is remarkably rare – these patients do extremely well across the board,” he concluded.
Dr. Cosselli reported that he is a principal investigator and consultant for Medtronic and W.L. Gore & Assoc., as well as being a principal investigator, consultant, and having various financial relationships with Vascutek.
AT THE AATS ANNUAL MEETING
AATS: Spare the aortic valve when possible during aortic root replacements
SEATTLE – Aortic valve–sparing procedures have better outcomes than Bentall procedures with biologic or mechanical valves, according to a review of 1,187 aortic root cases at the University of Toronto.
“If the aortic valve can be spared, AVS [aortic valve–sparing] procedures should be considered for patients undergoing aortic root replacement,” concluded the investigators, led by Dr. Maral Ouzounian, a cardiovascular surgeon at the university.
The team reviewed surgical outcomes there from 1990 to 2010; 282 patients had AVS procedures, 562 had Bentall procedures with biologic valve replacements (b-Bentall); and 343 had Bentalls with mechanical valve replacements (m-Bentall). About 7.5% of AVS patients had bicuspid aortic valves, versus 67.6% in the b-Bentall group and 51.7% in the m-Bentall group.
To control for confounders, patients were matched into 185 triads based on age, year of surgery, and stage of heart failure. The surgeries were all elective; patients with endocarditis or aortic dissections were excluded from the analysis.
Early postoperative outcomes and overall long-term survival were similar between the groups, with about 80% of patients in all three alive at 15-year follow-up.
However, AVS procedures had better long-term freedom from cardiac death (95% at 15 years versus 93% in the b-Bentall and 90% in the m-Bentall groups). Also, AVS patients had lower rates of anticoagulant-related hemorrhages, compared with m-Bentall patients (3.2% versus 17.8%), and lower rates of structural valve deterioration (0% versus 6.5%) and reoperations (6.5% versus 13.5%) than b-Bentall patients. The differences were statistically significant.
About 5% of AVS patients had previous cardiac surgery, versus 12.4% in the b-Bentall and 36.2% in the m-Bentall groups. AVS patients were more likely to have Marfan syndrome, and about 8% of AVS patients had preoperative ejection fractions below 40%, versus 9.3% in the b-Bentall and 13% in the m-Bentall groups. There were no between-group differences in the rates of concomitant coronary bypass or mitral valve surgery. Patients were about 50 years old on average, and about 80% were men.
“In Toronto, we are quite aggressive with valve-sparing operations. We believe in this operation, so whenever we can spare the valve, we do.” Although valve-sparing procedures have become more common in large, high-volume surgery centers over the past 20 years, “community surgeons in small-volume centers are still much more likely to do a Bentall because of the complexity of AVS operations and the art it takes to get it right,” Dr. Ouzounian said at the annual meeting of the American Association for Thoracic Surgery.
Previous investigations have found benefits for AVS procedures, as well. One concluded that “there is no significant difference in terms of re-operation between patients, who presented with [bicuspid or tricuspid aortic valves]. Re-operation rates are higher for patients who presented with severe [aortic regurgitation], but these rates do not reach statistical significance. Hence, root replacement with aortic valve sparing should be offered even in the presence of a” bicuspid aortic valve or severe aortic regurgitation (Eur. J. Cardiothorac. Surg. 2010;38:515-22).
Dr. Ouzounian said she has no disclosures.
SEATTLE – Aortic valve–sparing procedures have better outcomes than Bentall procedures with biologic or mechanical valves, according to a review of 1,187 aortic root cases at the University of Toronto.
“If the aortic valve can be spared, AVS [aortic valve–sparing] procedures should be considered for patients undergoing aortic root replacement,” concluded the investigators, led by Dr. Maral Ouzounian, a cardiovascular surgeon at the university.
The team reviewed surgical outcomes there from 1990 to 2010; 282 patients had AVS procedures, 562 had Bentall procedures with biologic valve replacements (b-Bentall); and 343 had Bentalls with mechanical valve replacements (m-Bentall). About 7.5% of AVS patients had bicuspid aortic valves, versus 67.6% in the b-Bentall group and 51.7% in the m-Bentall group.
To control for confounders, patients were matched into 185 triads based on age, year of surgery, and stage of heart failure. The surgeries were all elective; patients with endocarditis or aortic dissections were excluded from the analysis.
Early postoperative outcomes and overall long-term survival were similar between the groups, with about 80% of patients in all three alive at 15-year follow-up.
However, AVS procedures had better long-term freedom from cardiac death (95% at 15 years versus 93% in the b-Bentall and 90% in the m-Bentall groups). Also, AVS patients had lower rates of anticoagulant-related hemorrhages, compared with m-Bentall patients (3.2% versus 17.8%), and lower rates of structural valve deterioration (0% versus 6.5%) and reoperations (6.5% versus 13.5%) than b-Bentall patients. The differences were statistically significant.
About 5% of AVS patients had previous cardiac surgery, versus 12.4% in the b-Bentall and 36.2% in the m-Bentall groups. AVS patients were more likely to have Marfan syndrome, and about 8% of AVS patients had preoperative ejection fractions below 40%, versus 9.3% in the b-Bentall and 13% in the m-Bentall groups. There were no between-group differences in the rates of concomitant coronary bypass or mitral valve surgery. Patients were about 50 years old on average, and about 80% were men.
“In Toronto, we are quite aggressive with valve-sparing operations. We believe in this operation, so whenever we can spare the valve, we do.” Although valve-sparing procedures have become more common in large, high-volume surgery centers over the past 20 years, “community surgeons in small-volume centers are still much more likely to do a Bentall because of the complexity of AVS operations and the art it takes to get it right,” Dr. Ouzounian said at the annual meeting of the American Association for Thoracic Surgery.
Previous investigations have found benefits for AVS procedures, as well. One concluded that “there is no significant difference in terms of re-operation between patients, who presented with [bicuspid or tricuspid aortic valves]. Re-operation rates are higher for patients who presented with severe [aortic regurgitation], but these rates do not reach statistical significance. Hence, root replacement with aortic valve sparing should be offered even in the presence of a” bicuspid aortic valve or severe aortic regurgitation (Eur. J. Cardiothorac. Surg. 2010;38:515-22).
Dr. Ouzounian said she has no disclosures.
SEATTLE – Aortic valve–sparing procedures have better outcomes than Bentall procedures with biologic or mechanical valves, according to a review of 1,187 aortic root cases at the University of Toronto.
“If the aortic valve can be spared, AVS [aortic valve–sparing] procedures should be considered for patients undergoing aortic root replacement,” concluded the investigators, led by Dr. Maral Ouzounian, a cardiovascular surgeon at the university.
The team reviewed surgical outcomes there from 1990 to 2010; 282 patients had AVS procedures, 562 had Bentall procedures with biologic valve replacements (b-Bentall); and 343 had Bentalls with mechanical valve replacements (m-Bentall). About 7.5% of AVS patients had bicuspid aortic valves, versus 67.6% in the b-Bentall group and 51.7% in the m-Bentall group.
To control for confounders, patients were matched into 185 triads based on age, year of surgery, and stage of heart failure. The surgeries were all elective; patients with endocarditis or aortic dissections were excluded from the analysis.
Early postoperative outcomes and overall long-term survival were similar between the groups, with about 80% of patients in all three alive at 15-year follow-up.
However, AVS procedures had better long-term freedom from cardiac death (95% at 15 years versus 93% in the b-Bentall and 90% in the m-Bentall groups). Also, AVS patients had lower rates of anticoagulant-related hemorrhages, compared with m-Bentall patients (3.2% versus 17.8%), and lower rates of structural valve deterioration (0% versus 6.5%) and reoperations (6.5% versus 13.5%) than b-Bentall patients. The differences were statistically significant.
About 5% of AVS patients had previous cardiac surgery, versus 12.4% in the b-Bentall and 36.2% in the m-Bentall groups. AVS patients were more likely to have Marfan syndrome, and about 8% of AVS patients had preoperative ejection fractions below 40%, versus 9.3% in the b-Bentall and 13% in the m-Bentall groups. There were no between-group differences in the rates of concomitant coronary bypass or mitral valve surgery. Patients were about 50 years old on average, and about 80% were men.
“In Toronto, we are quite aggressive with valve-sparing operations. We believe in this operation, so whenever we can spare the valve, we do.” Although valve-sparing procedures have become more common in large, high-volume surgery centers over the past 20 years, “community surgeons in small-volume centers are still much more likely to do a Bentall because of the complexity of AVS operations and the art it takes to get it right,” Dr. Ouzounian said at the annual meeting of the American Association for Thoracic Surgery.
Previous investigations have found benefits for AVS procedures, as well. One concluded that “there is no significant difference in terms of re-operation between patients, who presented with [bicuspid or tricuspid aortic valves]. Re-operation rates are higher for patients who presented with severe [aortic regurgitation], but these rates do not reach statistical significance. Hence, root replacement with aortic valve sparing should be offered even in the presence of a” bicuspid aortic valve or severe aortic regurgitation (Eur. J. Cardiothorac. Surg. 2010;38:515-22).
Dr. Ouzounian said she has no disclosures.
AT THE AATS ANNUAL MEETING
Key clinical point: Your patients will do better with aortic valve–sparing procedures.
Major finding: Aortic valve–sparing procedures are associated with improved long-term freedom from cardiac death (95% at 15 years) when compared with Bentall procedures with biologic valves (93% freedom from cardiac death at 15 years) or mechanical valves (90% freedom at 15 years).
Data source: Review of 1,187 aortic root replacements at the University of Toronto.
Disclosures: The lead investigator has no disclosures.
EuroPCR: CT-derived FFR promising in evaluating chest pain
PARIS – Noninvasive measurement of computed tomography–derived fractional flow reserve is a potential game changer in the management of patients with stable chest pain.
In a 200-patient proof-of-concept study known as FFR-CT RIPCORD, in which three experienced interventional cardiologists initially devised management plans based on coronary anatomy as defined by the results of CT angiography alone, subsequent knowledge of CT-derived fractional flow reserve (FFR-CT) caused them to change their management strategies in fully 36% of cases, Dr. Nick Curzen reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.

“If this novel proof-of-concept result can be confirmed in large-scale trials, this suggests that noninvasive FFR-CT can be used as a clinically relevant tool that mimics the well-described ability of invasive FFR to refine management decisions for patients with chest pain that are made by invasive coronary angiography alone. This would indeed have important implications for routine clinical practice. FFR-CT may have potential as a noninvasive default method for simultaneous assessment of coronary anatomy and physiology in angina patients in order to define their management, which would completely change the way we look after them,” observed Dr. Curzen, professor of interventional cardiology at the University of Southampton (England).
EuroPCR codirector Dr. Williams Wijns was favorably impressed by the FFR-CT RIPCORD findings.
“This, I find just stunning. It’s really far reaching. This is a complete change in paradigm. Many patients that today undergo invasive angiography won’t even be sent to the cath lab. The invasive center becomes only for treatment,” commented Dr. Wijns, codirector of the cardiovascular center in Aalst, Belgium.
In FFR-CT RIPCORD, the cardiologists received information about a patient’s history and nonvasive CT angiography findings and were asked to reach consensus in selecting one of four management options: optimal medical therapy (OMT) alone, PCI plus OMT, CABG surgery and OMT, or ‘more information needed’ in the form of FFR findings, which identify those coronary lesions that are actually causing ischemia. Instead of receiving the results of conventional invasive FFR obtained using a pressure wire, however, the cardiologists were provided with the noninvasive FFR-CT findings in all 200 cases.
The resultant changes in management were substantial. Thirty percent of the patients initially slated for PCI were reallocated to OMT alone because no ischemic lesions were present. Twelve percent of patients assigned to OMT-only got reassigned to coronary revascularization. Moreover, in 18% of the PCI group, FFR-CT data led to a change in the vessel or vessels targeted for intervention.
“What particularly impressed me were two of those figures: that one-third of PCI patients are redirected to medical therapy, and – even more impressive to me – is the 18% of PCI patients who had a change in their target vessel. That’s a problem we often have in patients with multivessel disease and intermediate lesions: Sometimes we think, for example, the target is the LAD when in fact it’s another vessel,” commented Dr. Jean Fajadet, codirector of the interventional cardiovascular group at the Clinique Pasteur in Toulouse, France.
Dr. Curzen said the exciting thing about FFR-CT is that it could provide in one fell swoop a standardized way of obtaining both the anatomic and physiologic data necessary for informed clinical decision making, and without exposing patients needlessly to the risks of contrast and radiation exposure entailed in invasive coronary angiography.
“When we assess people with stable angina, if you have a room full of invasive cardiologists, we all do it differently at the moment. It’s crazy. A lot of us will do noninvasive tests like stress echo or MRI or some kind of exercise test, and then refer them for an invasive angiogram where we’ll also do an FFR. Some people will go straight for an angiogram. It’s a real mess. The thing I love about FFR-CT is it would be so slick for patients and their families: You see them in a chest pain clinic or your office and you put them in for this test. They don’t have to waste their time coming back several times for different tests. It’s a really beautiful concept,” Dr. Curzen continued.
Right now the turnaround time on FFR-CT is about 12 hours. The dataset has to be sent off to a supercomputer for a complex modeling analysis before the results come back.
“Of course, if this ever becomes clinically proven, I’m sure the turnaround time would become very quick,” according to the cardiologist.
A cost-effectiveness analysis of FFR-CT versus current standard care is ongoing and the results aren’t yet available. However, Dr. Curzen observed, “The cost to the patient is a very important issue: Who would want to have this done invasively if you have a test that proves you don’t need to have an invasive procedure?”
The FFR-CT RIPCORD study was sponsored by Heartflow. Dr. Curzen reported receiving research support from Heartflow, Boston Scientific, Haemonetics, and Medtronic.
PARIS – Noninvasive measurement of computed tomography–derived fractional flow reserve is a potential game changer in the management of patients with stable chest pain.
In a 200-patient proof-of-concept study known as FFR-CT RIPCORD, in which three experienced interventional cardiologists initially devised management plans based on coronary anatomy as defined by the results of CT angiography alone, subsequent knowledge of CT-derived fractional flow reserve (FFR-CT) caused them to change their management strategies in fully 36% of cases, Dr. Nick Curzen reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.

“If this novel proof-of-concept result can be confirmed in large-scale trials, this suggests that noninvasive FFR-CT can be used as a clinically relevant tool that mimics the well-described ability of invasive FFR to refine management decisions for patients with chest pain that are made by invasive coronary angiography alone. This would indeed have important implications for routine clinical practice. FFR-CT may have potential as a noninvasive default method for simultaneous assessment of coronary anatomy and physiology in angina patients in order to define their management, which would completely change the way we look after them,” observed Dr. Curzen, professor of interventional cardiology at the University of Southampton (England).
EuroPCR codirector Dr. Williams Wijns was favorably impressed by the FFR-CT RIPCORD findings.
“This, I find just stunning. It’s really far reaching. This is a complete change in paradigm. Many patients that today undergo invasive angiography won’t even be sent to the cath lab. The invasive center becomes only for treatment,” commented Dr. Wijns, codirector of the cardiovascular center in Aalst, Belgium.
In FFR-CT RIPCORD, the cardiologists received information about a patient’s history and nonvasive CT angiography findings and were asked to reach consensus in selecting one of four management options: optimal medical therapy (OMT) alone, PCI plus OMT, CABG surgery and OMT, or ‘more information needed’ in the form of FFR findings, which identify those coronary lesions that are actually causing ischemia. Instead of receiving the results of conventional invasive FFR obtained using a pressure wire, however, the cardiologists were provided with the noninvasive FFR-CT findings in all 200 cases.
The resultant changes in management were substantial. Thirty percent of the patients initially slated for PCI were reallocated to OMT alone because no ischemic lesions were present. Twelve percent of patients assigned to OMT-only got reassigned to coronary revascularization. Moreover, in 18% of the PCI group, FFR-CT data led to a change in the vessel or vessels targeted for intervention.
“What particularly impressed me were two of those figures: that one-third of PCI patients are redirected to medical therapy, and – even more impressive to me – is the 18% of PCI patients who had a change in their target vessel. That’s a problem we often have in patients with multivessel disease and intermediate lesions: Sometimes we think, for example, the target is the LAD when in fact it’s another vessel,” commented Dr. Jean Fajadet, codirector of the interventional cardiovascular group at the Clinique Pasteur in Toulouse, France.
Dr. Curzen said the exciting thing about FFR-CT is that it could provide in one fell swoop a standardized way of obtaining both the anatomic and physiologic data necessary for informed clinical decision making, and without exposing patients needlessly to the risks of contrast and radiation exposure entailed in invasive coronary angiography.
“When we assess people with stable angina, if you have a room full of invasive cardiologists, we all do it differently at the moment. It’s crazy. A lot of us will do noninvasive tests like stress echo or MRI or some kind of exercise test, and then refer them for an invasive angiogram where we’ll also do an FFR. Some people will go straight for an angiogram. It’s a real mess. The thing I love about FFR-CT is it would be so slick for patients and their families: You see them in a chest pain clinic or your office and you put them in for this test. They don’t have to waste their time coming back several times for different tests. It’s a really beautiful concept,” Dr. Curzen continued.
Right now the turnaround time on FFR-CT is about 12 hours. The dataset has to be sent off to a supercomputer for a complex modeling analysis before the results come back.
“Of course, if this ever becomes clinically proven, I’m sure the turnaround time would become very quick,” according to the cardiologist.
A cost-effectiveness analysis of FFR-CT versus current standard care is ongoing and the results aren’t yet available. However, Dr. Curzen observed, “The cost to the patient is a very important issue: Who would want to have this done invasively if you have a test that proves you don’t need to have an invasive procedure?”
The FFR-CT RIPCORD study was sponsored by Heartflow. Dr. Curzen reported receiving research support from Heartflow, Boston Scientific, Haemonetics, and Medtronic.
PARIS – Noninvasive measurement of computed tomography–derived fractional flow reserve is a potential game changer in the management of patients with stable chest pain.
In a 200-patient proof-of-concept study known as FFR-CT RIPCORD, in which three experienced interventional cardiologists initially devised management plans based on coronary anatomy as defined by the results of CT angiography alone, subsequent knowledge of CT-derived fractional flow reserve (FFR-CT) caused them to change their management strategies in fully 36% of cases, Dr. Nick Curzen reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.

“If this novel proof-of-concept result can be confirmed in large-scale trials, this suggests that noninvasive FFR-CT can be used as a clinically relevant tool that mimics the well-described ability of invasive FFR to refine management decisions for patients with chest pain that are made by invasive coronary angiography alone. This would indeed have important implications for routine clinical practice. FFR-CT may have potential as a noninvasive default method for simultaneous assessment of coronary anatomy and physiology in angina patients in order to define their management, which would completely change the way we look after them,” observed Dr. Curzen, professor of interventional cardiology at the University of Southampton (England).
EuroPCR codirector Dr. Williams Wijns was favorably impressed by the FFR-CT RIPCORD findings.
“This, I find just stunning. It’s really far reaching. This is a complete change in paradigm. Many patients that today undergo invasive angiography won’t even be sent to the cath lab. The invasive center becomes only for treatment,” commented Dr. Wijns, codirector of the cardiovascular center in Aalst, Belgium.
In FFR-CT RIPCORD, the cardiologists received information about a patient’s history and nonvasive CT angiography findings and were asked to reach consensus in selecting one of four management options: optimal medical therapy (OMT) alone, PCI plus OMT, CABG surgery and OMT, or ‘more information needed’ in the form of FFR findings, which identify those coronary lesions that are actually causing ischemia. Instead of receiving the results of conventional invasive FFR obtained using a pressure wire, however, the cardiologists were provided with the noninvasive FFR-CT findings in all 200 cases.
The resultant changes in management were substantial. Thirty percent of the patients initially slated for PCI were reallocated to OMT alone because no ischemic lesions were present. Twelve percent of patients assigned to OMT-only got reassigned to coronary revascularization. Moreover, in 18% of the PCI group, FFR-CT data led to a change in the vessel or vessels targeted for intervention.
“What particularly impressed me were two of those figures: that one-third of PCI patients are redirected to medical therapy, and – even more impressive to me – is the 18% of PCI patients who had a change in their target vessel. That’s a problem we often have in patients with multivessel disease and intermediate lesions: Sometimes we think, for example, the target is the LAD when in fact it’s another vessel,” commented Dr. Jean Fajadet, codirector of the interventional cardiovascular group at the Clinique Pasteur in Toulouse, France.
Dr. Curzen said the exciting thing about FFR-CT is that it could provide in one fell swoop a standardized way of obtaining both the anatomic and physiologic data necessary for informed clinical decision making, and without exposing patients needlessly to the risks of contrast and radiation exposure entailed in invasive coronary angiography.
“When we assess people with stable angina, if you have a room full of invasive cardiologists, we all do it differently at the moment. It’s crazy. A lot of us will do noninvasive tests like stress echo or MRI or some kind of exercise test, and then refer them for an invasive angiogram where we’ll also do an FFR. Some people will go straight for an angiogram. It’s a real mess. The thing I love about FFR-CT is it would be so slick for patients and their families: You see them in a chest pain clinic or your office and you put them in for this test. They don’t have to waste their time coming back several times for different tests. It’s a really beautiful concept,” Dr. Curzen continued.
Right now the turnaround time on FFR-CT is about 12 hours. The dataset has to be sent off to a supercomputer for a complex modeling analysis before the results come back.
“Of course, if this ever becomes clinically proven, I’m sure the turnaround time would become very quick,” according to the cardiologist.
A cost-effectiveness analysis of FFR-CT versus current standard care is ongoing and the results aren’t yet available. However, Dr. Curzen observed, “The cost to the patient is a very important issue: Who would want to have this done invasively if you have a test that proves you don’t need to have an invasive procedure?”
The FFR-CT RIPCORD study was sponsored by Heartflow. Dr. Curzen reported receiving research support from Heartflow, Boston Scientific, Haemonetics, and Medtronic.
AT EUROPCR
Key clinical point: Clinically decisive anatomic and physiologic data regarding the coronary arteries of patients with stable angina can be obtained noninvasively with a single test: CT-derived fractional flow reserve.
Major finding: Noninvasive FFR-CT findings resulted in a change in management strategy for 36% of patients with stable angina whose initial treatment plan was based on CT angiography alone.
Data source: A proof-of-concept study involving 200 patients with stable angina and a panel of three experienced interventional cardiologists making consensus decisions regarding their appropriate management.
Disclosures: The FFR-CT RIPCORD study was sponsored by Heartflow. The presenter reported having received research support from the company.
Stricter DVT prophylaxis guidelines needed for cardiac and vascular surgery
Cardiac and vascular surgery patients should receive deep vein thrombosis (DVT) prophylaxis before and after surgery, say researchers who found a high incidence of postoperative DVT in these patients compared to general surgery patients.
The retrospective study of 2,669,772 surgery patients from the American College of Surgeons National Surgical Quality Improvement Program database found that 18,670 patients developed a DVT within 30 days of the operation.
The incidence of DVT according to the type of surgery was 2% for cardiac surgery, 0.99% for vascular surgery and 0.66% for general surgery, reported Dr. Faisal Aziz and his colleagues at Pennsylvania State University (Ann. Vasc. Surg. 2015; 29: 661-9).
Vascular surgery patients were at 1.5 times the risk of a postop DVT and cardiac surgery patients were at 3 times the risk compared with general surgery patients, a significant difference.
Preoperative factors associated with increased risk of developing DVT in the postoperative period included inpatient admission status (OR 7.8), general anesthesia (OR 2), and dyspnea at rest (OR 5).
“Despite the fact that most arterial surgery operations involve administration of therapeutic doses of anticoagulation therapy during the operations, incidence of postoperative DVT is high in these patients,” the study authors wrote.
“Intraoperative anticoagulation is not protective against development of DVT in the postoperative period” they said.
“Physicians should ensure adequate DVT prophylaxis in postoperative vascular surgery and cardiac surgery patients, according to established evidence based guidelines,” they concluded.
The authors did not report any financial disclosures.
Cardiac and vascular surgery patients should receive deep vein thrombosis (DVT) prophylaxis before and after surgery, say researchers who found a high incidence of postoperative DVT in these patients compared to general surgery patients.
The retrospective study of 2,669,772 surgery patients from the American College of Surgeons National Surgical Quality Improvement Program database found that 18,670 patients developed a DVT within 30 days of the operation.
The incidence of DVT according to the type of surgery was 2% for cardiac surgery, 0.99% for vascular surgery and 0.66% for general surgery, reported Dr. Faisal Aziz and his colleagues at Pennsylvania State University (Ann. Vasc. Surg. 2015; 29: 661-9).
Vascular surgery patients were at 1.5 times the risk of a postop DVT and cardiac surgery patients were at 3 times the risk compared with general surgery patients, a significant difference.
Preoperative factors associated with increased risk of developing DVT in the postoperative period included inpatient admission status (OR 7.8), general anesthesia (OR 2), and dyspnea at rest (OR 5).
“Despite the fact that most arterial surgery operations involve administration of therapeutic doses of anticoagulation therapy during the operations, incidence of postoperative DVT is high in these patients,” the study authors wrote.
“Intraoperative anticoagulation is not protective against development of DVT in the postoperative period” they said.
“Physicians should ensure adequate DVT prophylaxis in postoperative vascular surgery and cardiac surgery patients, according to established evidence based guidelines,” they concluded.
The authors did not report any financial disclosures.
Cardiac and vascular surgery patients should receive deep vein thrombosis (DVT) prophylaxis before and after surgery, say researchers who found a high incidence of postoperative DVT in these patients compared to general surgery patients.
The retrospective study of 2,669,772 surgery patients from the American College of Surgeons National Surgical Quality Improvement Program database found that 18,670 patients developed a DVT within 30 days of the operation.
The incidence of DVT according to the type of surgery was 2% for cardiac surgery, 0.99% for vascular surgery and 0.66% for general surgery, reported Dr. Faisal Aziz and his colleagues at Pennsylvania State University (Ann. Vasc. Surg. 2015; 29: 661-9).
Vascular surgery patients were at 1.5 times the risk of a postop DVT and cardiac surgery patients were at 3 times the risk compared with general surgery patients, a significant difference.
Preoperative factors associated with increased risk of developing DVT in the postoperative period included inpatient admission status (OR 7.8), general anesthesia (OR 2), and dyspnea at rest (OR 5).
“Despite the fact that most arterial surgery operations involve administration of therapeutic doses of anticoagulation therapy during the operations, incidence of postoperative DVT is high in these patients,” the study authors wrote.
“Intraoperative anticoagulation is not protective against development of DVT in the postoperative period” they said.
“Physicians should ensure adequate DVT prophylaxis in postoperative vascular surgery and cardiac surgery patients, according to established evidence based guidelines,” they concluded.
The authors did not report any financial disclosures.
FROM ANNALS OF VASCULAR SURGERY
Key clinical point: Intraoperative anticoagulation alone does not prevent DVT in patients undergoing vascular and cardiac surgery.
Major finding: The incidence of DVT according to the type of surgery was 2% for cardiac surgery, 0.99% for vascular surgery and 0.66% for general surgery.
Data source: Retrospective study of 2,669,772 surgery patients from the American College of Surgeons National Surgical Quality Improvement Program database.
Disclosures: The authors did not report any financial disclosures.
AATS: Simplified mitral valve repair effective in children with Marfan’s
NEW YORK – A simplified surgical approach that uses an adult-sized, basic ring annuloplasty with a complete edge-to-edge leaflet repair has been found to yield good outcomes in a group of children with connective tissue disorders like Marfan syndrome and Loeys-Dietz syndrome and mitral regurgitation, based on an evaluation of the technique at four centers.
Cardiac surgeons employed the simplified operation in 18 patients under age 18 (median age 8.2 years) and found that after 2.4 years of follow-up, no patients required another operation for mitral regurgitation, reported Dr. Luca Vricella, pediatrics director, pediatric cardiac surgery and heart transplantation, John Hopkins University, Baltimore.
The patients underwent the simplified mitral valve repair at Johns Hopkins Pediatric Cardiac Surgery in Baltimore and Orlando; Duke University School of Medicine in Durham, N.C.; and University of Pavia Medical School in Italy.
These young patients can be challenging to operate on because of skeletal abnormalities and marginal pulmonary reserve, Dr. Vricella said at the meeting sponsored by the American Association for Thoracic Surgery. Of the 18 children in the study, 15 had Marfan syndrome, a genetic disorder that commonly affects the heart valves and aorta. “The most common mode of early presentation of mitral valve pathology, from a morphological standpoint, is that mitral regurgitation is often characterized as severe bileaflet prolapse and annular and left ventricular dilation,” Dr. Vricella said.
The simplified technique involves an approach through an atriotomy and placing an adult-sized annuloplasty ring in the valve. The next step is to place an Alfieri stitch, named for the Italian cardiac surgeon Dr. Ottavio Alfieri, with a braided suture in the middle of the valve, which opposes the leaflet “very effectively,” Dr. Vricella said.
In the study group, all 18 patients had severe bileaflet prolapse and severe mitral regurgitation of grade 4 or higher, but all patients also had normal ejection fraction, Dr. Vricella said. One infant was being considered for a heart transplant.
The operation was achieved in isolation in less than an hour. Five patients underwent simultaneous valve-sparing aortic root replacement, one of whom died (one of two deaths in a larger 300-plus group of both adults and children who had valve-sparing aortic root repair). No other complications were reported among the 18-patient group. Median length of stay in the hospital was 9 days.
After the operation, all patients had significant reductions in mitral regurgitation. After 2.4 years, 94% of survivors maintained mild regurgitation or better without stenosis. Entering the study, the median left ventricular end-diastolic diameter (LVEDD) score of all patients was 4.9 (range 2.1-11.9), but at 2.4 years after the operation the median LVEDD score had regressed to 1.3 (range –0.51-4.3).
“In pediatric patients with severe mitral regurgitation and connective tissue disorders, a simplified repair can result in intermediate-term competency without systolic anterior motion and with no mitral stenosis,” Dr. Vricella said. “We’ve been pleasantly surprised in seeing this reduction in left ventricular enlargement, and particularly in this group of patients in which you may need to have a longer plant time, simplifying things so you don’t have to intervene on the subvalvular annuloplasty.”
NEW YORK – A simplified surgical approach that uses an adult-sized, basic ring annuloplasty with a complete edge-to-edge leaflet repair has been found to yield good outcomes in a group of children with connective tissue disorders like Marfan syndrome and Loeys-Dietz syndrome and mitral regurgitation, based on an evaluation of the technique at four centers.
Cardiac surgeons employed the simplified operation in 18 patients under age 18 (median age 8.2 years) and found that after 2.4 years of follow-up, no patients required another operation for mitral regurgitation, reported Dr. Luca Vricella, pediatrics director, pediatric cardiac surgery and heart transplantation, John Hopkins University, Baltimore.
The patients underwent the simplified mitral valve repair at Johns Hopkins Pediatric Cardiac Surgery in Baltimore and Orlando; Duke University School of Medicine in Durham, N.C.; and University of Pavia Medical School in Italy.
These young patients can be challenging to operate on because of skeletal abnormalities and marginal pulmonary reserve, Dr. Vricella said at the meeting sponsored by the American Association for Thoracic Surgery. Of the 18 children in the study, 15 had Marfan syndrome, a genetic disorder that commonly affects the heart valves and aorta. “The most common mode of early presentation of mitral valve pathology, from a morphological standpoint, is that mitral regurgitation is often characterized as severe bileaflet prolapse and annular and left ventricular dilation,” Dr. Vricella said.
The simplified technique involves an approach through an atriotomy and placing an adult-sized annuloplasty ring in the valve. The next step is to place an Alfieri stitch, named for the Italian cardiac surgeon Dr. Ottavio Alfieri, with a braided suture in the middle of the valve, which opposes the leaflet “very effectively,” Dr. Vricella said.
In the study group, all 18 patients had severe bileaflet prolapse and severe mitral regurgitation of grade 4 or higher, but all patients also had normal ejection fraction, Dr. Vricella said. One infant was being considered for a heart transplant.
The operation was achieved in isolation in less than an hour. Five patients underwent simultaneous valve-sparing aortic root replacement, one of whom died (one of two deaths in a larger 300-plus group of both adults and children who had valve-sparing aortic root repair). No other complications were reported among the 18-patient group. Median length of stay in the hospital was 9 days.
After the operation, all patients had significant reductions in mitral regurgitation. After 2.4 years, 94% of survivors maintained mild regurgitation or better without stenosis. Entering the study, the median left ventricular end-diastolic diameter (LVEDD) score of all patients was 4.9 (range 2.1-11.9), but at 2.4 years after the operation the median LVEDD score had regressed to 1.3 (range –0.51-4.3).
“In pediatric patients with severe mitral regurgitation and connective tissue disorders, a simplified repair can result in intermediate-term competency without systolic anterior motion and with no mitral stenosis,” Dr. Vricella said. “We’ve been pleasantly surprised in seeing this reduction in left ventricular enlargement, and particularly in this group of patients in which you may need to have a longer plant time, simplifying things so you don’t have to intervene on the subvalvular annuloplasty.”
NEW YORK – A simplified surgical approach that uses an adult-sized, basic ring annuloplasty with a complete edge-to-edge leaflet repair has been found to yield good outcomes in a group of children with connective tissue disorders like Marfan syndrome and Loeys-Dietz syndrome and mitral regurgitation, based on an evaluation of the technique at four centers.
Cardiac surgeons employed the simplified operation in 18 patients under age 18 (median age 8.2 years) and found that after 2.4 years of follow-up, no patients required another operation for mitral regurgitation, reported Dr. Luca Vricella, pediatrics director, pediatric cardiac surgery and heart transplantation, John Hopkins University, Baltimore.
The patients underwent the simplified mitral valve repair at Johns Hopkins Pediatric Cardiac Surgery in Baltimore and Orlando; Duke University School of Medicine in Durham, N.C.; and University of Pavia Medical School in Italy.
These young patients can be challenging to operate on because of skeletal abnormalities and marginal pulmonary reserve, Dr. Vricella said at the meeting sponsored by the American Association for Thoracic Surgery. Of the 18 children in the study, 15 had Marfan syndrome, a genetic disorder that commonly affects the heart valves and aorta. “The most common mode of early presentation of mitral valve pathology, from a morphological standpoint, is that mitral regurgitation is often characterized as severe bileaflet prolapse and annular and left ventricular dilation,” Dr. Vricella said.
The simplified technique involves an approach through an atriotomy and placing an adult-sized annuloplasty ring in the valve. The next step is to place an Alfieri stitch, named for the Italian cardiac surgeon Dr. Ottavio Alfieri, with a braided suture in the middle of the valve, which opposes the leaflet “very effectively,” Dr. Vricella said.
In the study group, all 18 patients had severe bileaflet prolapse and severe mitral regurgitation of grade 4 or higher, but all patients also had normal ejection fraction, Dr. Vricella said. One infant was being considered for a heart transplant.
The operation was achieved in isolation in less than an hour. Five patients underwent simultaneous valve-sparing aortic root replacement, one of whom died (one of two deaths in a larger 300-plus group of both adults and children who had valve-sparing aortic root repair). No other complications were reported among the 18-patient group. Median length of stay in the hospital was 9 days.
After the operation, all patients had significant reductions in mitral regurgitation. After 2.4 years, 94% of survivors maintained mild regurgitation or better without stenosis. Entering the study, the median left ventricular end-diastolic diameter (LVEDD) score of all patients was 4.9 (range 2.1-11.9), but at 2.4 years after the operation the median LVEDD score had regressed to 1.3 (range –0.51-4.3).
“In pediatric patients with severe mitral regurgitation and connective tissue disorders, a simplified repair can result in intermediate-term competency without systolic anterior motion and with no mitral stenosis,” Dr. Vricella said. “We’ve been pleasantly surprised in seeing this reduction in left ventricular enlargement, and particularly in this group of patients in which you may need to have a longer plant time, simplifying things so you don’t have to intervene on the subvalvular annuloplasty.”
AT THE 2015 MITRAL VALVE CONCLAVE
Key clinical point: A simplified approach for mitral valve repair resulted in good outcomes in a group of children with connective tissue disorders.
Major finding: All 18 children in the study avoided reoperation after mitral valve repair.
Data source: A small trial involving 18 patients at four hospitals.
Disclosures: Dr. Vricella had no relevant relationships to disclose.
Stenting before CABG linked to higher mortality for diabetic patients
Since the debut of drug-eluting stents, more high-risk patient groups, namely diabetic patients, have undergone coronary stenting as opposed to coronary artery bypass grafting (CABG) as an option to open blocked arteries; however, diabetic patients with stents who go on to have CABG have significantly higher 5-year death rates than do unstented diabetics who undergo CABG, according to a study published in the May issue of the Journal of Thoracic and Cardiovascular Surgery.
A review of 7,005 CABG procedures performed from 1996 to 2007 at Mercy St. Vincent Medical Center in Toledo, Ohio, found that diabetic patients with triple-vessel disease and a prior percutaneous coronary intervention with stenting (PCI-S) who underwent CABG had a 39% greater risk of death within 5 years of the operation. The findings are significant, according to Dr. Victor Nauffal and his colleagues at the American University of Beirut, because increasing numbers of patients with coronary stents are referred for CABG (J. Thorac. Cardiovasc. Surg. 2015 [doi:10.1016/j.jtcvs.2015.01.051).
Previous studies have linked prior stenting to an increased risk of bleeding and stent thrombosis during CABG, so having a better understanding of anticoagulation during the operation and the timing of the surgery after stenting could decrease complications. Investigations of the long-term outcomes of patients with stents who have CABG, however, have been lacking. This study investigated the premise that diabetics with triple-vessel disease and a stent had poorer outcomes because of endothelial dysfunction and the increased strain that triple-vessel disease places on the heart.
After exclusions, the final study population comprised 1,583 diabetic patients with concomitant triple-vessel disease, 202 (12.8%) of whom had coronary stents. The study defined triple-vessel disease as blockages of 50% or more in all three native coronary vessels or left main artery plus right coronary artery disease.
Early mortality rates – death within 30 days of the procedure – were similar between the two groups: 3.3% overall, 3% in the prior-PCI group, and 3.3% in the no-PCI group; therefore, prior PCI was not a predictor of early mortality.
Five-year cumulative survival was 78.5% in the no-PCI group, compared with 74.8% in the PCI group. When adjusting for a variety of clinical variables before CABG, stenting was associated with a 39% greater mortality at 5 years. The investigators accounted for the emergence of drug-eluting stents during the 10-year study period but found that they did not contribute significantly to overall outcomes.
The cause of death was known for 81.7% (282 of 345) of the deaths in the overall cohort, with 5-year cardiac deaths higher in the PCI-S group: 8.4% vs. 7.5% for the no-PCI group. “Notably, 100% of PCI-S cardiac mortality was categorized as coronary heart disease related compared to 89.3% (92/103) of cardiac mortality in the no-PCI group,” Dr. Nauffal and his associates said.
Careful patient selection for CABG is in order for diabetics with triple-vessel disease, particularly those with a prior stent, the authors advised. “An early team-based approach including a cardiologist and cardiac surgeon should be implemented for optimal revascularization strategy selection in diabetics with triple-vessel disease and for close medical follow-up of those higher risk CABG patients with history of intracoronary stents,” Dr. Nauffal and his colleagues concluded.
The Johns Hopkins Murex Research Award supported Dr. Nauffal. The authors had no other relevant disclosures.
Because diabetes affects vascular physiology and can lead to multivessel disease, surgical revascularization vs. percutaneous coronary intervention has proved more successful in diabetic patients, Dr. Paul Kurlansky said in his invited commentary. However, “the potential impact of newer generation drug-eluting stents on improving these results remains to be seen,” he wrote (J. Thorac. Cardiovasc. Surg. 2015 [doi:10.1016/j.jtcvs.2015.02.007).
Comparing CABG and PCI in diabetic patients has been challenging for a variety of reasons, including the nuances of clinical judgment and different techniques. “It is in this gray zone of clinical ambiguity that many if not most patients actually reside,” he said, giving credit to Dr. Nauffal and colleagues for trying to address this ambiguity.

|
Dr. Paul Kurlansky |
The study data, however, had many limitations, Dr. Kurlansky said. The authors could not specify indications for stent deployment, disease severity at the time of stenting and the choice of procedure among them. “An equally plausible hypothesis might therefore suggest that the appropriate need for prior stenting identified a subset of patients with more aggressive disease who therefore succumbed at an earlier age,” he said.
CABG that utilizes the internal mammary artery has been linked to enhanced physiologic properties that promote vasodilatation, inhibit thrombosis and atherosclerosis, and support the health of the vascular endothelium, he noted. In the diabetic patient, these properties may enhance the ability of CABG to address not only arterial blockages, but also the underlying physiology of atherosclerosis. “With the rising tide of diabetic vasculopathy, it will become increasingly important to consider both clinical utility and underlying physiology in navigating the uncertain path to optimal patient care,” Dr. Kurlansky wrote.
Dr. Kurlansky is with the department of surgery at Columbia University, New York.
Because diabetes affects vascular physiology and can lead to multivessel disease, surgical revascularization vs. percutaneous coronary intervention has proved more successful in diabetic patients, Dr. Paul Kurlansky said in his invited commentary. However, “the potential impact of newer generation drug-eluting stents on improving these results remains to be seen,” he wrote (J. Thorac. Cardiovasc. Surg. 2015 [doi:10.1016/j.jtcvs.2015.02.007).
Comparing CABG and PCI in diabetic patients has been challenging for a variety of reasons, including the nuances of clinical judgment and different techniques. “It is in this gray zone of clinical ambiguity that many if not most patients actually reside,” he said, giving credit to Dr. Nauffal and colleagues for trying to address this ambiguity.

|
Dr. Paul Kurlansky |
The study data, however, had many limitations, Dr. Kurlansky said. The authors could not specify indications for stent deployment, disease severity at the time of stenting and the choice of procedure among them. “An equally plausible hypothesis might therefore suggest that the appropriate need for prior stenting identified a subset of patients with more aggressive disease who therefore succumbed at an earlier age,” he said.
CABG that utilizes the internal mammary artery has been linked to enhanced physiologic properties that promote vasodilatation, inhibit thrombosis and atherosclerosis, and support the health of the vascular endothelium, he noted. In the diabetic patient, these properties may enhance the ability of CABG to address not only arterial blockages, but also the underlying physiology of atherosclerosis. “With the rising tide of diabetic vasculopathy, it will become increasingly important to consider both clinical utility and underlying physiology in navigating the uncertain path to optimal patient care,” Dr. Kurlansky wrote.
Dr. Kurlansky is with the department of surgery at Columbia University, New York.
Because diabetes affects vascular physiology and can lead to multivessel disease, surgical revascularization vs. percutaneous coronary intervention has proved more successful in diabetic patients, Dr. Paul Kurlansky said in his invited commentary. However, “the potential impact of newer generation drug-eluting stents on improving these results remains to be seen,” he wrote (J. Thorac. Cardiovasc. Surg. 2015 [doi:10.1016/j.jtcvs.2015.02.007).
Comparing CABG and PCI in diabetic patients has been challenging for a variety of reasons, including the nuances of clinical judgment and different techniques. “It is in this gray zone of clinical ambiguity that many if not most patients actually reside,” he said, giving credit to Dr. Nauffal and colleagues for trying to address this ambiguity.

|
Dr. Paul Kurlansky |
The study data, however, had many limitations, Dr. Kurlansky said. The authors could not specify indications for stent deployment, disease severity at the time of stenting and the choice of procedure among them. “An equally plausible hypothesis might therefore suggest that the appropriate need for prior stenting identified a subset of patients with more aggressive disease who therefore succumbed at an earlier age,” he said.
CABG that utilizes the internal mammary artery has been linked to enhanced physiologic properties that promote vasodilatation, inhibit thrombosis and atherosclerosis, and support the health of the vascular endothelium, he noted. In the diabetic patient, these properties may enhance the ability of CABG to address not only arterial blockages, but also the underlying physiology of atherosclerosis. “With the rising tide of diabetic vasculopathy, it will become increasingly important to consider both clinical utility and underlying physiology in navigating the uncertain path to optimal patient care,” Dr. Kurlansky wrote.
Dr. Kurlansky is with the department of surgery at Columbia University, New York.
Since the debut of drug-eluting stents, more high-risk patient groups, namely diabetic patients, have undergone coronary stenting as opposed to coronary artery bypass grafting (CABG) as an option to open blocked arteries; however, diabetic patients with stents who go on to have CABG have significantly higher 5-year death rates than do unstented diabetics who undergo CABG, according to a study published in the May issue of the Journal of Thoracic and Cardiovascular Surgery.
A review of 7,005 CABG procedures performed from 1996 to 2007 at Mercy St. Vincent Medical Center in Toledo, Ohio, found that diabetic patients with triple-vessel disease and a prior percutaneous coronary intervention with stenting (PCI-S) who underwent CABG had a 39% greater risk of death within 5 years of the operation. The findings are significant, according to Dr. Victor Nauffal and his colleagues at the American University of Beirut, because increasing numbers of patients with coronary stents are referred for CABG (J. Thorac. Cardiovasc. Surg. 2015 [doi:10.1016/j.jtcvs.2015.01.051).
Previous studies have linked prior stenting to an increased risk of bleeding and stent thrombosis during CABG, so having a better understanding of anticoagulation during the operation and the timing of the surgery after stenting could decrease complications. Investigations of the long-term outcomes of patients with stents who have CABG, however, have been lacking. This study investigated the premise that diabetics with triple-vessel disease and a stent had poorer outcomes because of endothelial dysfunction and the increased strain that triple-vessel disease places on the heart.
After exclusions, the final study population comprised 1,583 diabetic patients with concomitant triple-vessel disease, 202 (12.8%) of whom had coronary stents. The study defined triple-vessel disease as blockages of 50% or more in all three native coronary vessels or left main artery plus right coronary artery disease.
Early mortality rates – death within 30 days of the procedure – were similar between the two groups: 3.3% overall, 3% in the prior-PCI group, and 3.3% in the no-PCI group; therefore, prior PCI was not a predictor of early mortality.
Five-year cumulative survival was 78.5% in the no-PCI group, compared with 74.8% in the PCI group. When adjusting for a variety of clinical variables before CABG, stenting was associated with a 39% greater mortality at 5 years. The investigators accounted for the emergence of drug-eluting stents during the 10-year study period but found that they did not contribute significantly to overall outcomes.
The cause of death was known for 81.7% (282 of 345) of the deaths in the overall cohort, with 5-year cardiac deaths higher in the PCI-S group: 8.4% vs. 7.5% for the no-PCI group. “Notably, 100% of PCI-S cardiac mortality was categorized as coronary heart disease related compared to 89.3% (92/103) of cardiac mortality in the no-PCI group,” Dr. Nauffal and his associates said.
Careful patient selection for CABG is in order for diabetics with triple-vessel disease, particularly those with a prior stent, the authors advised. “An early team-based approach including a cardiologist and cardiac surgeon should be implemented for optimal revascularization strategy selection in diabetics with triple-vessel disease and for close medical follow-up of those higher risk CABG patients with history of intracoronary stents,” Dr. Nauffal and his colleagues concluded.
The Johns Hopkins Murex Research Award supported Dr. Nauffal. The authors had no other relevant disclosures.
Since the debut of drug-eluting stents, more high-risk patient groups, namely diabetic patients, have undergone coronary stenting as opposed to coronary artery bypass grafting (CABG) as an option to open blocked arteries; however, diabetic patients with stents who go on to have CABG have significantly higher 5-year death rates than do unstented diabetics who undergo CABG, according to a study published in the May issue of the Journal of Thoracic and Cardiovascular Surgery.
A review of 7,005 CABG procedures performed from 1996 to 2007 at Mercy St. Vincent Medical Center in Toledo, Ohio, found that diabetic patients with triple-vessel disease and a prior percutaneous coronary intervention with stenting (PCI-S) who underwent CABG had a 39% greater risk of death within 5 years of the operation. The findings are significant, according to Dr. Victor Nauffal and his colleagues at the American University of Beirut, because increasing numbers of patients with coronary stents are referred for CABG (J. Thorac. Cardiovasc. Surg. 2015 [doi:10.1016/j.jtcvs.2015.01.051).
Previous studies have linked prior stenting to an increased risk of bleeding and stent thrombosis during CABG, so having a better understanding of anticoagulation during the operation and the timing of the surgery after stenting could decrease complications. Investigations of the long-term outcomes of patients with stents who have CABG, however, have been lacking. This study investigated the premise that diabetics with triple-vessel disease and a stent had poorer outcomes because of endothelial dysfunction and the increased strain that triple-vessel disease places on the heart.
After exclusions, the final study population comprised 1,583 diabetic patients with concomitant triple-vessel disease, 202 (12.8%) of whom had coronary stents. The study defined triple-vessel disease as blockages of 50% or more in all three native coronary vessels or left main artery plus right coronary artery disease.
Early mortality rates – death within 30 days of the procedure – were similar between the two groups: 3.3% overall, 3% in the prior-PCI group, and 3.3% in the no-PCI group; therefore, prior PCI was not a predictor of early mortality.
Five-year cumulative survival was 78.5% in the no-PCI group, compared with 74.8% in the PCI group. When adjusting for a variety of clinical variables before CABG, stenting was associated with a 39% greater mortality at 5 years. The investigators accounted for the emergence of drug-eluting stents during the 10-year study period but found that they did not contribute significantly to overall outcomes.
The cause of death was known for 81.7% (282 of 345) of the deaths in the overall cohort, with 5-year cardiac deaths higher in the PCI-S group: 8.4% vs. 7.5% for the no-PCI group. “Notably, 100% of PCI-S cardiac mortality was categorized as coronary heart disease related compared to 89.3% (92/103) of cardiac mortality in the no-PCI group,” Dr. Nauffal and his associates said.
Careful patient selection for CABG is in order for diabetics with triple-vessel disease, particularly those with a prior stent, the authors advised. “An early team-based approach including a cardiologist and cardiac surgeon should be implemented for optimal revascularization strategy selection in diabetics with triple-vessel disease and for close medical follow-up of those higher risk CABG patients with history of intracoronary stents,” Dr. Nauffal and his colleagues concluded.
The Johns Hopkins Murex Research Award supported Dr. Nauffal. The authors had no other relevant disclosures.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point: Diabetic triple vessel–disease patients with prior percutaneous coronary intervention stenting (PCI-S) have poorer long-term outcomes after coronary artery bypass grafting than do patients who had no prior PCI-S.
Major finding: After adjusting for preoperative clinical characteristics and other factors, prior PCI-S was associated with 39% increased risk of mortality 5 years after surgery. Further adjustment for date of surgery or operative parameters did not alter the association.
Data source: Single-center review of 7,005 CABG cases performed from 1996 to 2007.
Disclosures: The Johns Hopkins Murex Research Award supported the lead author. The authors had no other relevant disclosures.